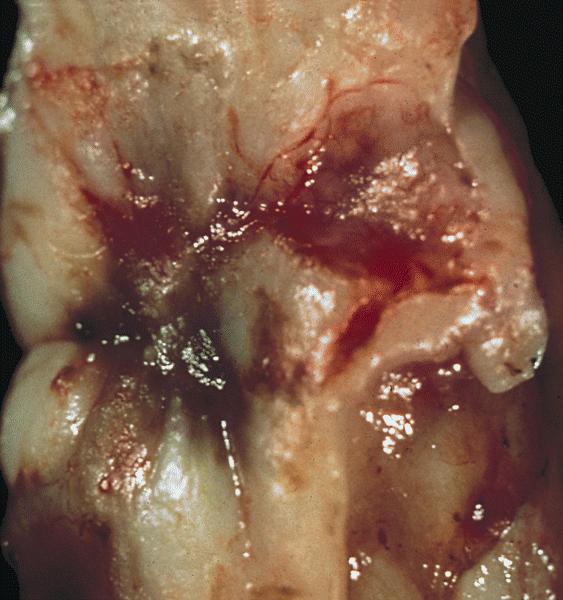
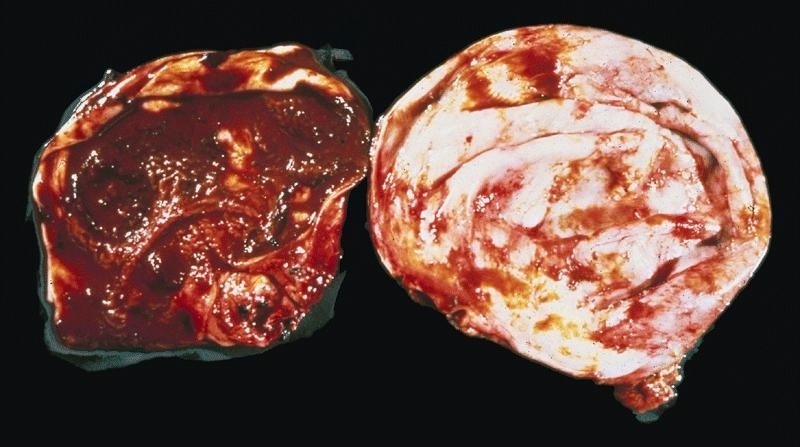
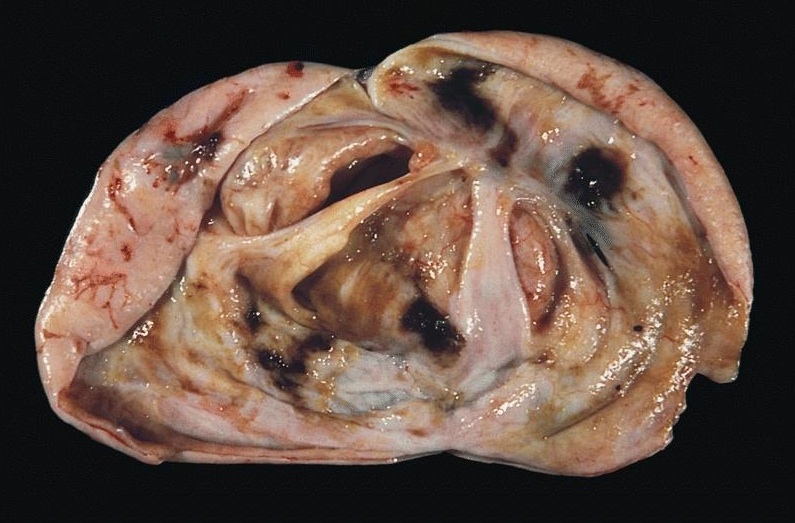
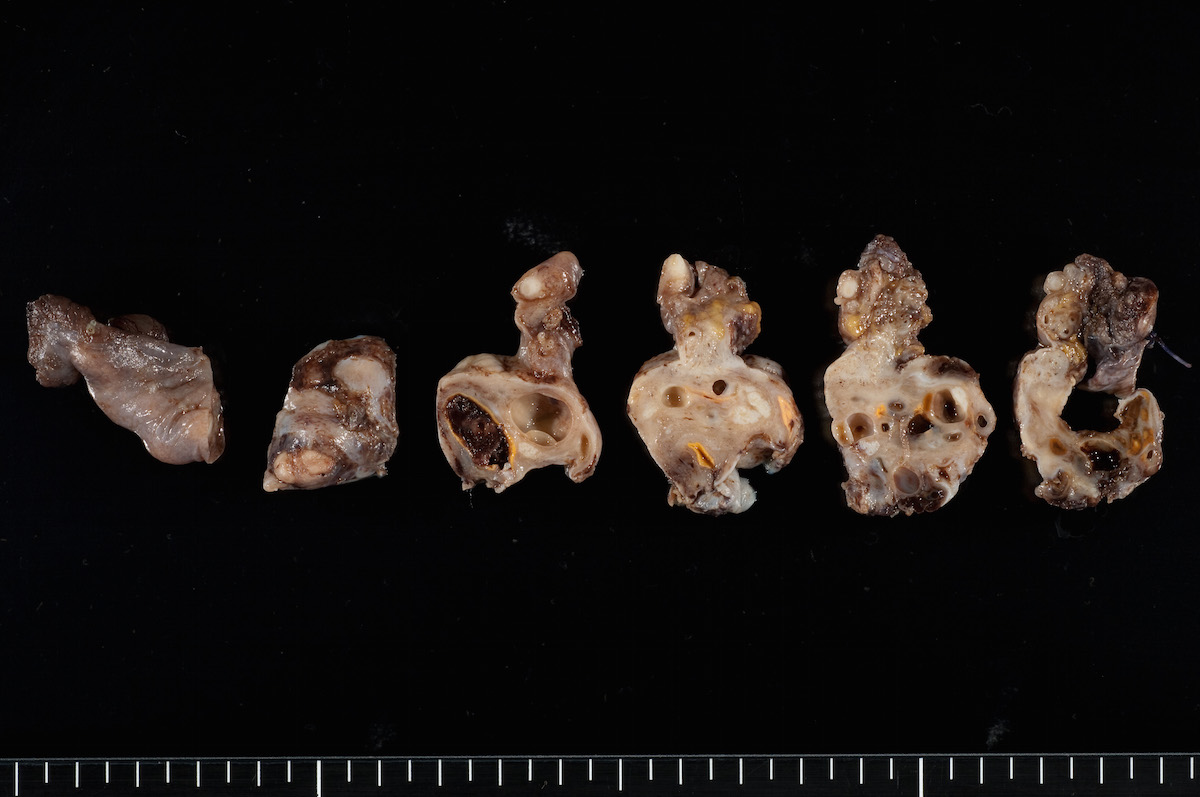
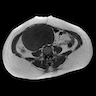
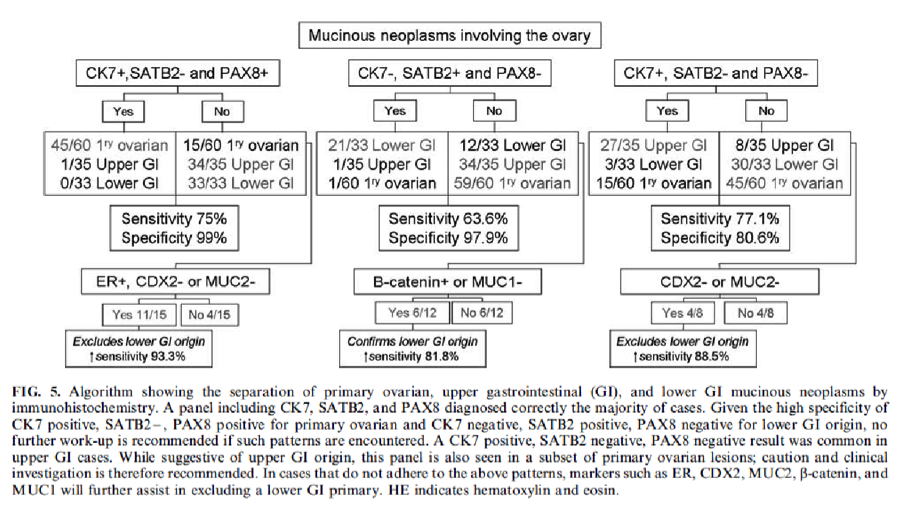
- pTX: primary tumor cannot be assessed
- pT0: no evidence of primary tumor
- pT1a (IA): tumor limited to 1 ovary (capsule intact) or fallopian tube; no tumor on ovarian or fallopian tube surface; no malignant cells in ascites or peritoneal washings
- pT1b (IB): tumor limited to both ovaries (capsules intact) or fallopian tubes; no tumor on ovarian or fallopian tube surface; no malignant cells in ascites or peritoneal washings
- pT1c (IC): tumor limited to 1 or both ovaries or fallopian tubes, with any of following:
- pT1c1 (IC1): surgical spill
- pT1c2 (IC2): capsule ruptured before surgery or tumor on ovarian or fallopian tube surface
- pT1c3 (IC3): malignant cells in ascites or peritoneal washings
- pT2a (IIA): extension or implants on the uterus or fallopian tube(s) or ovaries
- pT2b (IIB): extension to or implants on other pelvic tissues
- pT3a (IIIA2): microscopic extrapelvic (above the pelvic brim) peritoneal involvement with or without positive retroperitoneal lymph nodes
- pT3b (IIIB): macroscopic peritoneal metastasis beyond pelvis ≤ 2 cm with or without retroperitoneal lymph node metastasis
- pT3c (IIIC): macroscopic peritoneal metastasis beyond pelvis > 2 cm with or without retroperitoneal lymph node metastasis (includes extension of tumor to capsule of liver and spleen without parenchymal involvement of either organ)
Superpage
Superpage Topics
46XX DSD
Anatomy & histology
Appendiceal neoplasms
Autoimmune oophoritis (pending)
Benign, borderline and malignant Brenner tumors
Breast carcinoma
Calcification
Carcinoid tumor
Carcinoid tumor metastatic to ovary
Carcinosarcoma
Cervical carcinoma metastatic to ovary
Choriocarcinoma
Clear cell borderline tumor
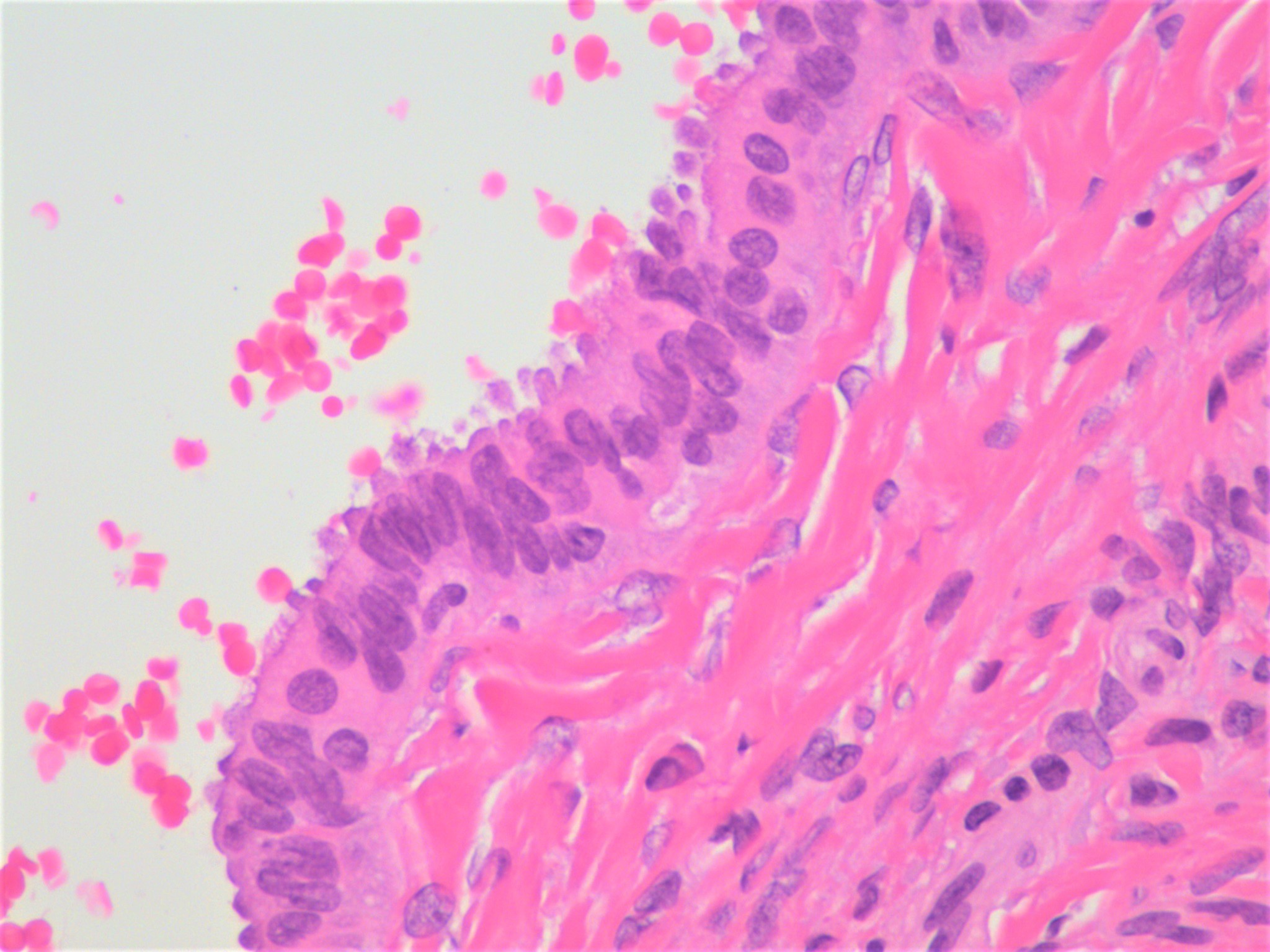
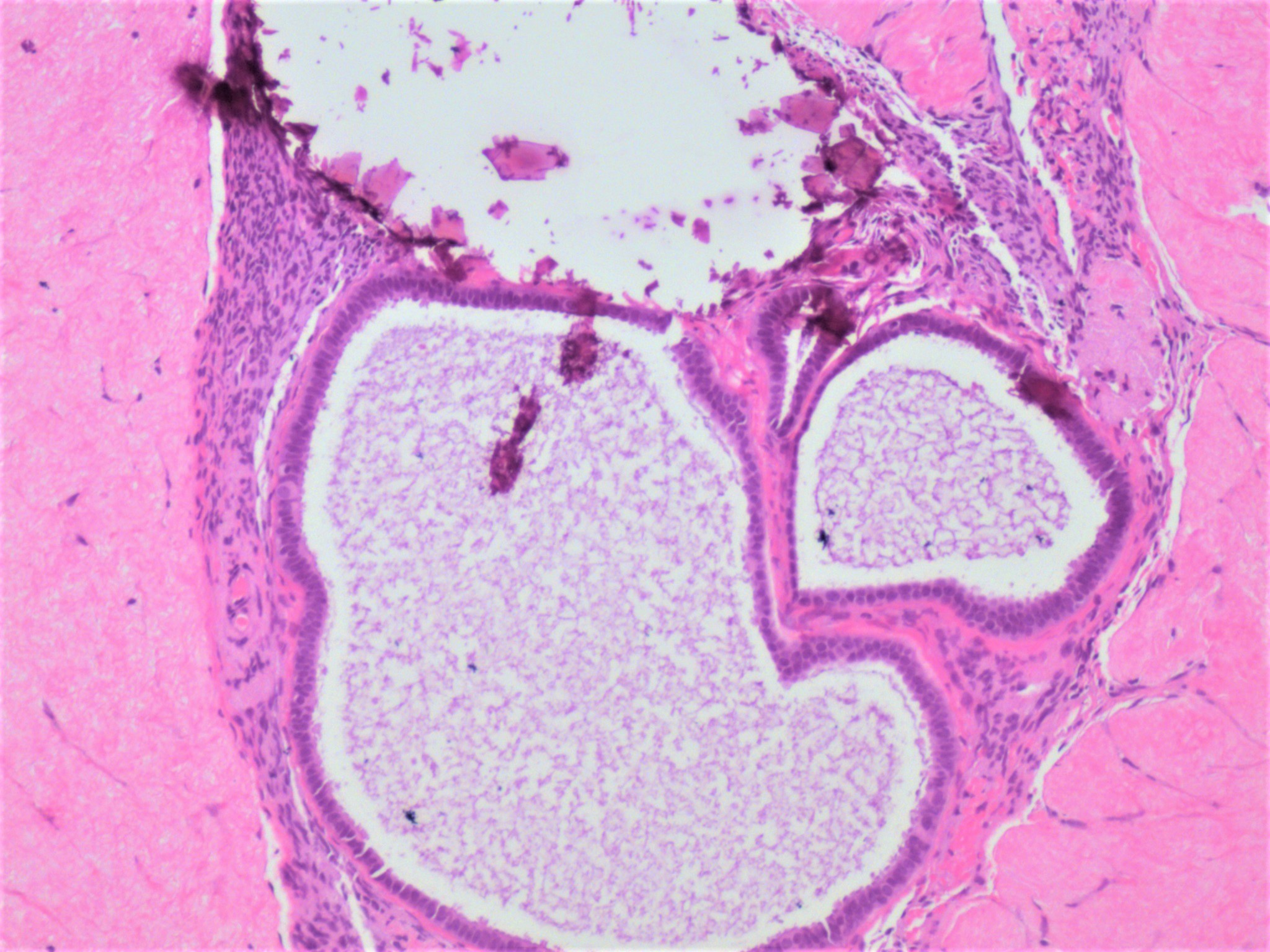
Clear cell carcinoma
Clear cell cystadenoma and adenofibroma
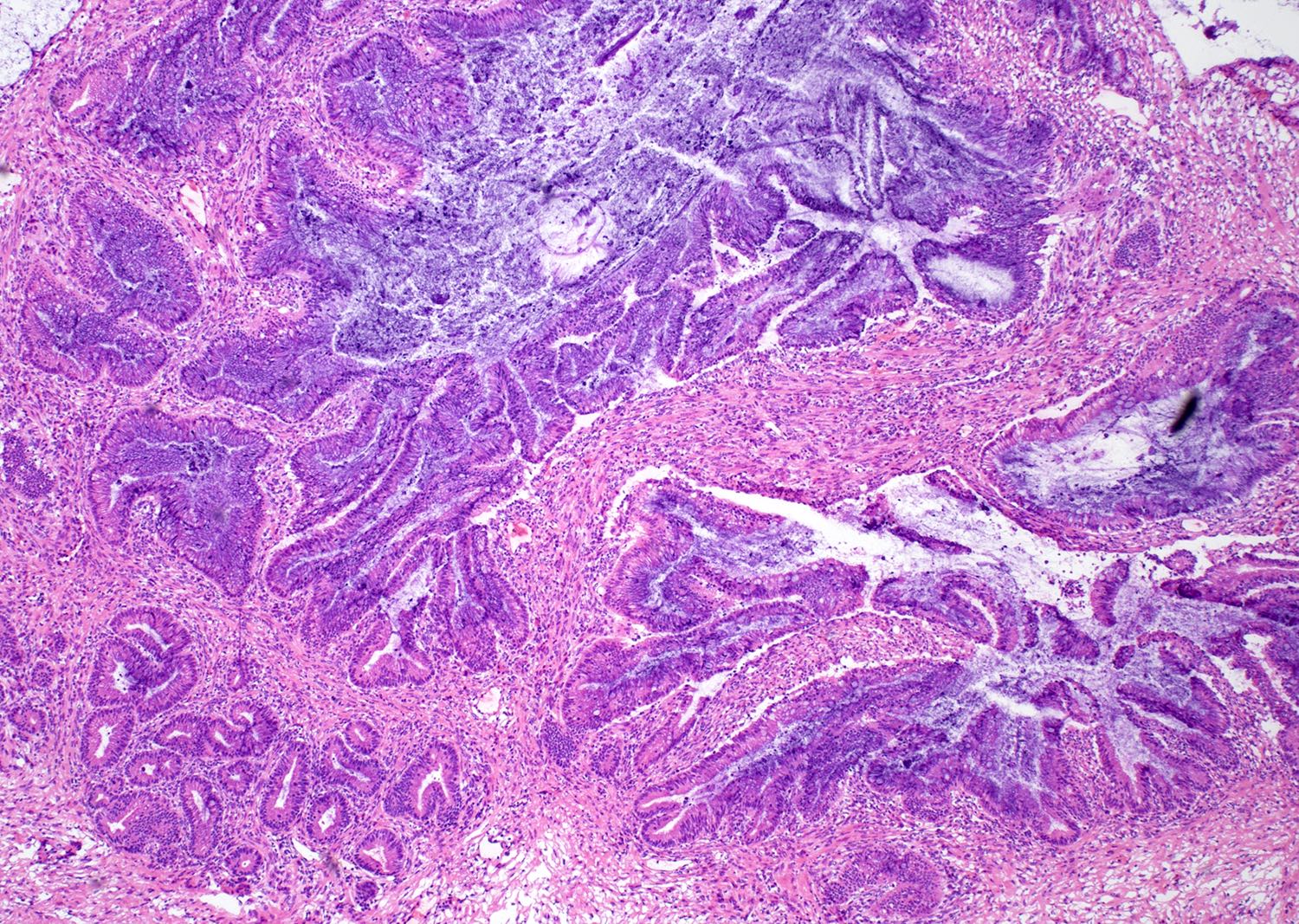
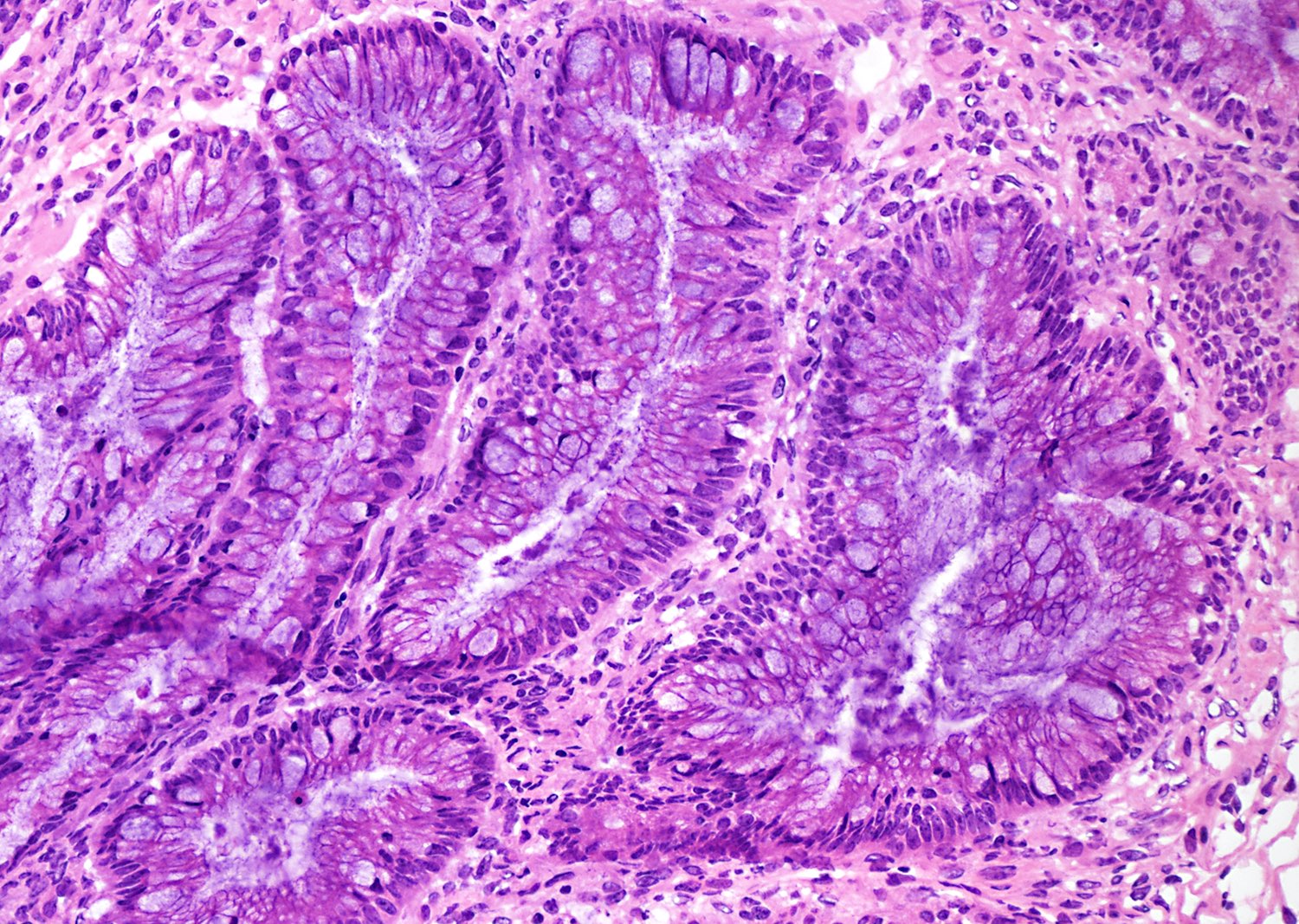
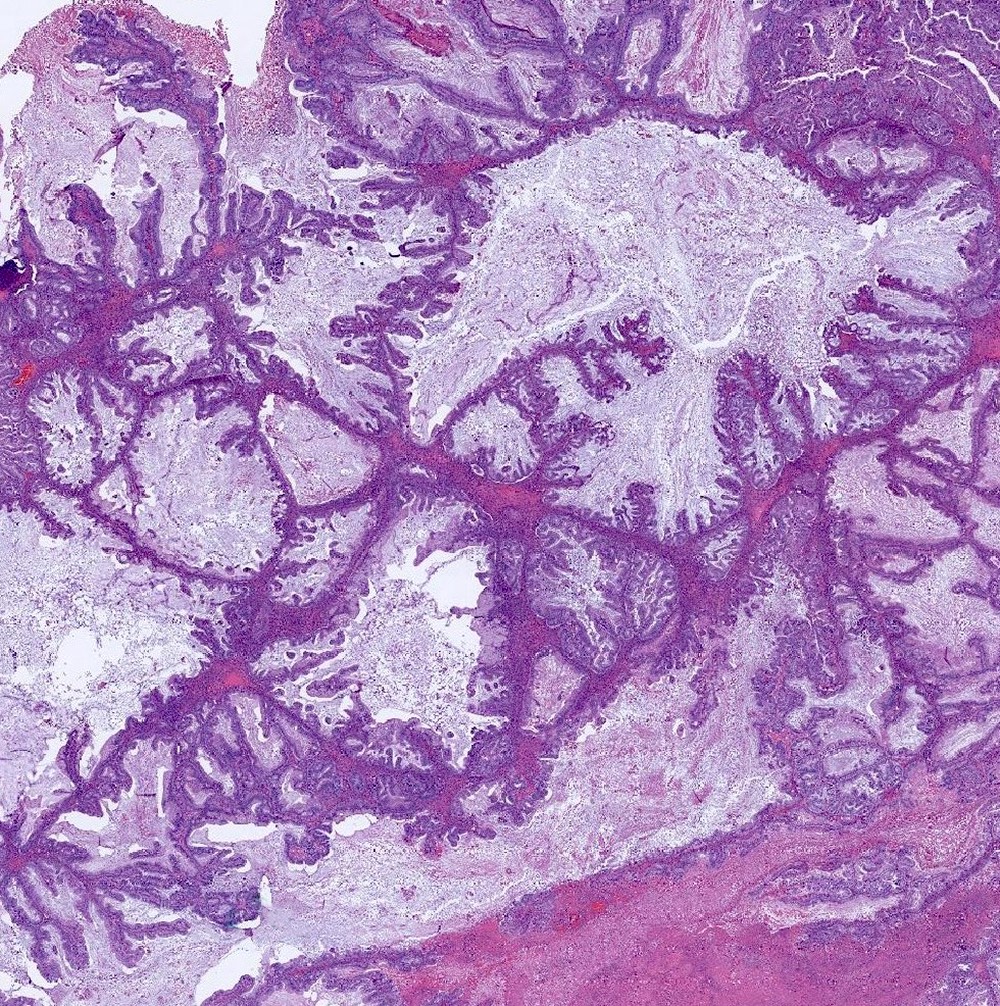
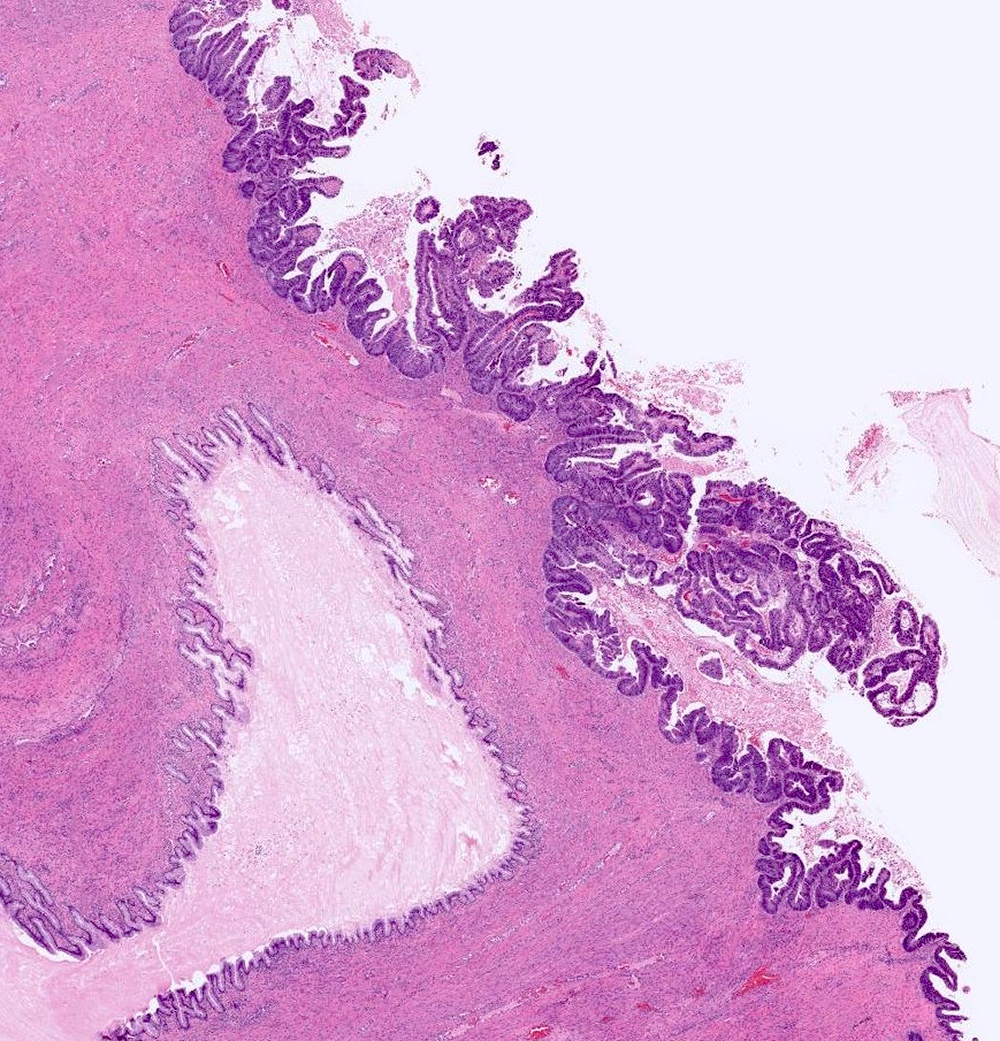
Colorectal adenocarcinoma
Corpus luteum cyst
Cortical inclusion cyst
Disorders of sex development-general
Dysgerminoma
Ectopic decidual reaction
Embryonal carcinoma
Endometrial stromal sarcoma
Endometrioid borderline tumor
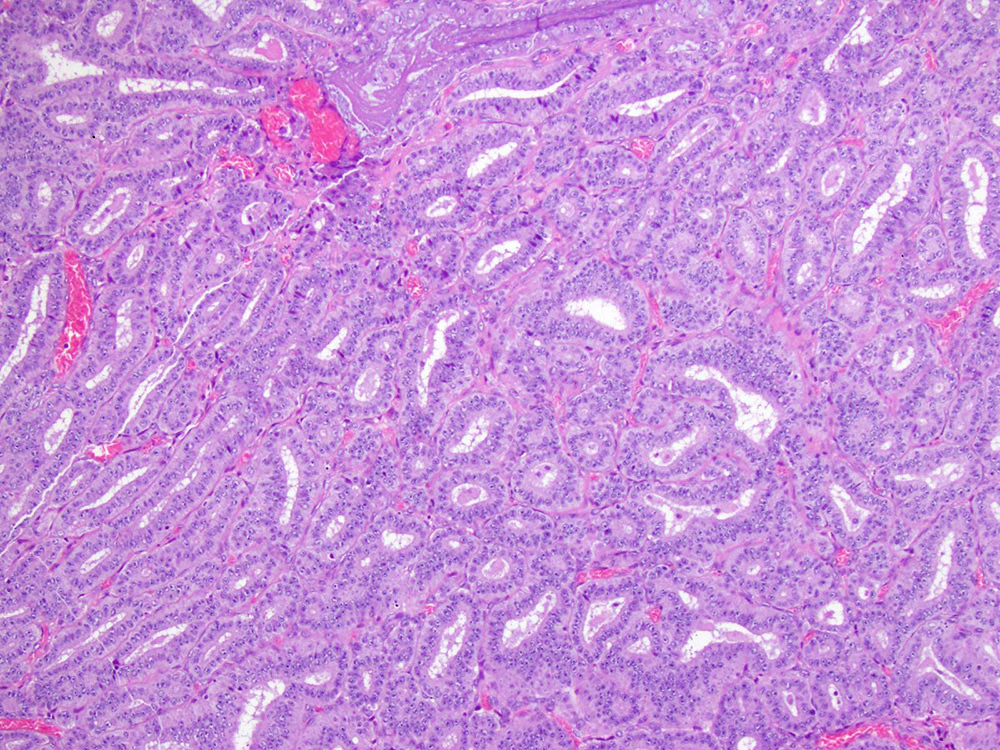
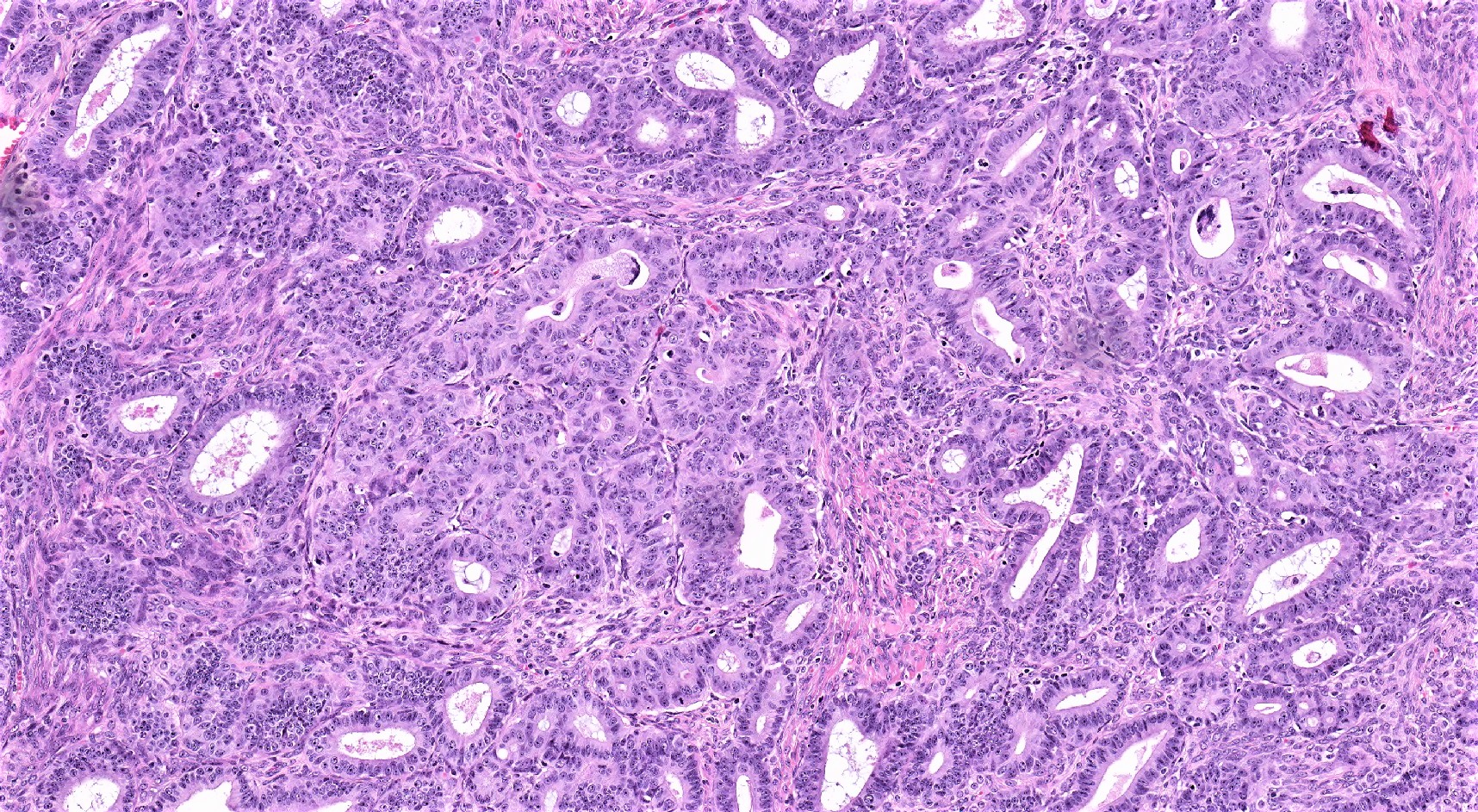
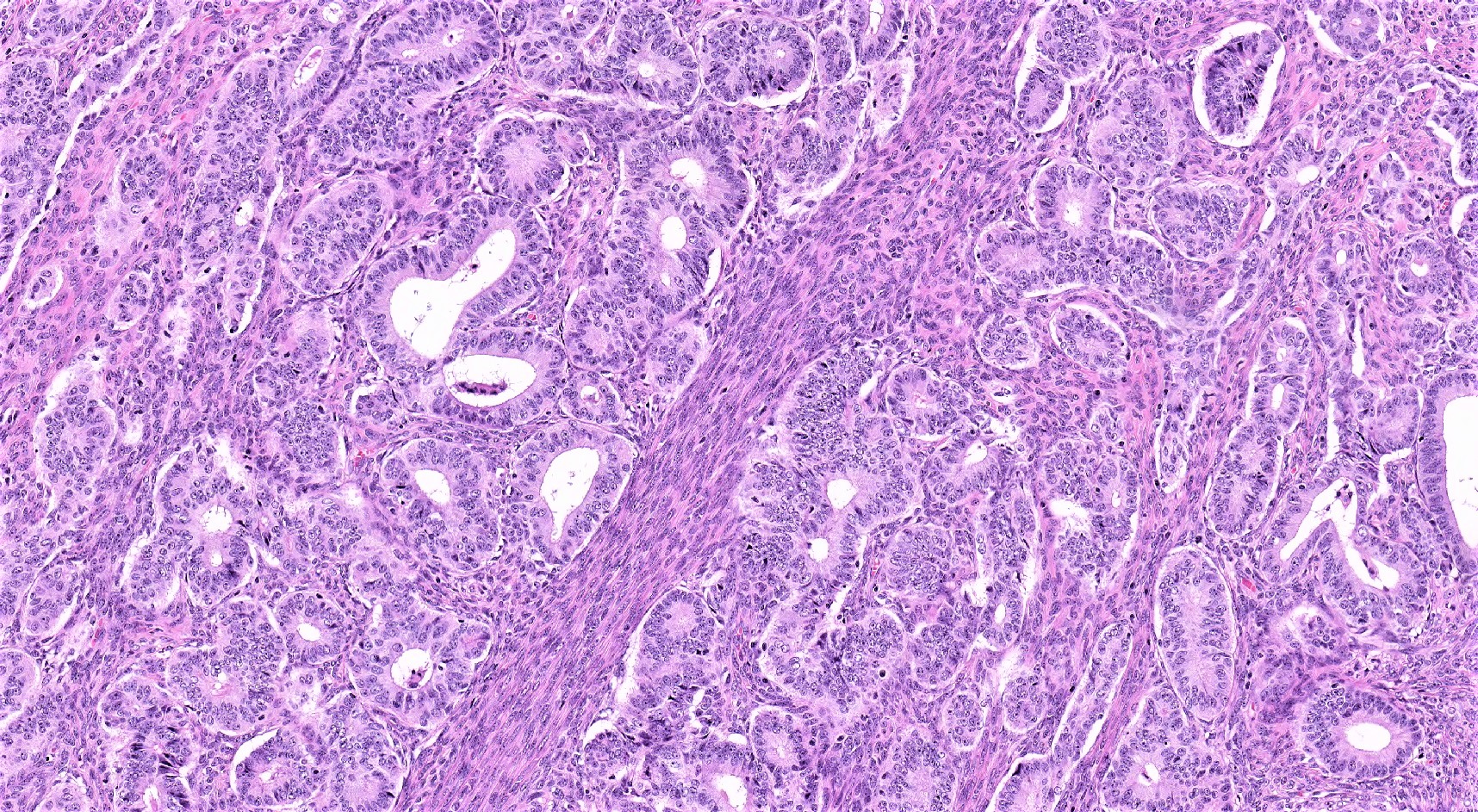
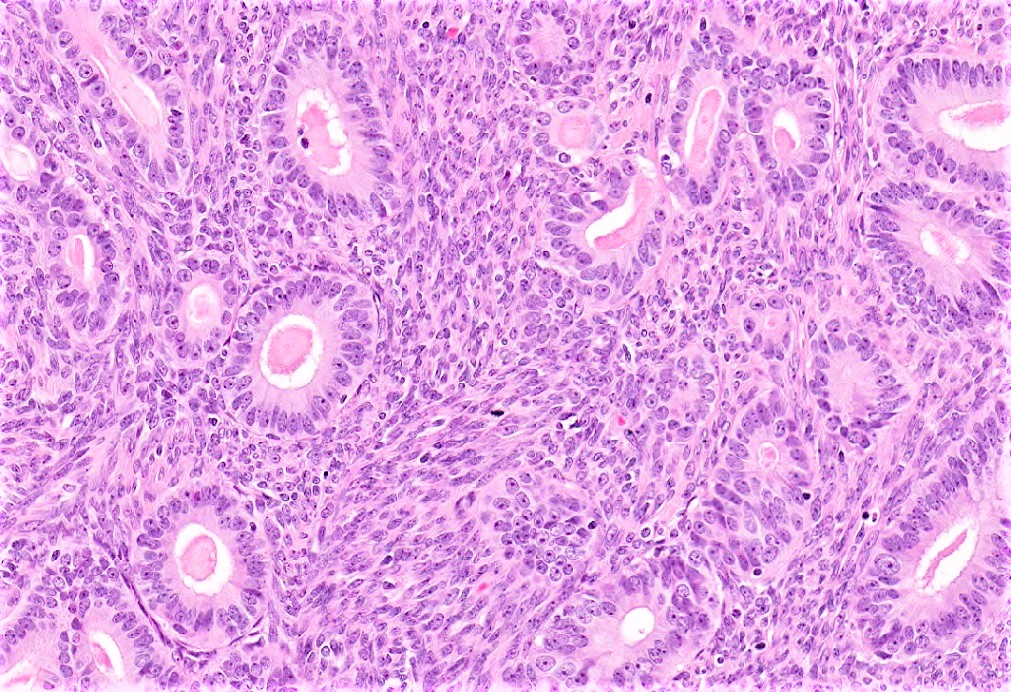
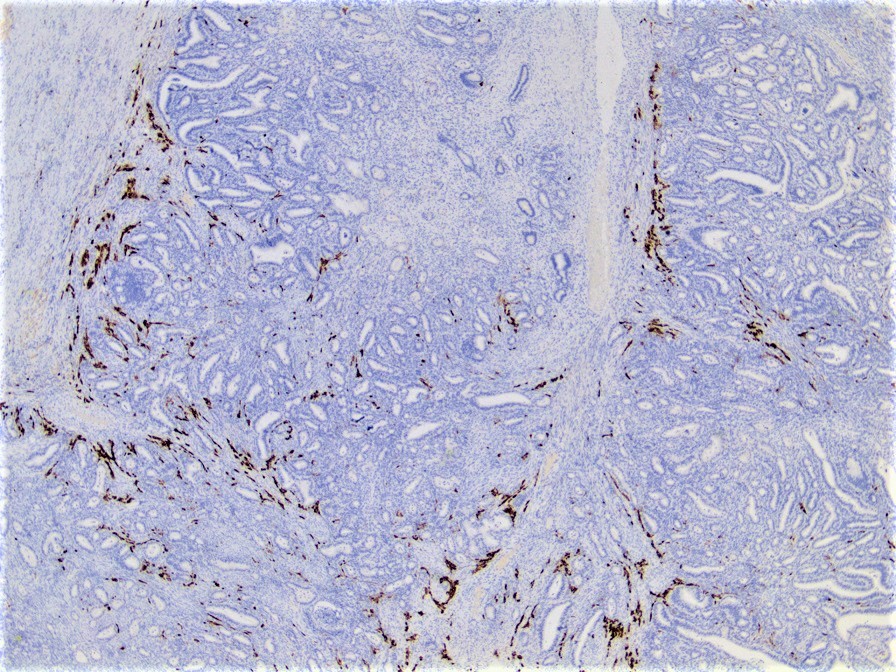
Endometrioid carcinoma
Endometrioid cystadenoma and adenofibroma
Endometriosis
Endosalpingiosis
Epithelial tumors-overview / molecular
Features to report
Fibroma
Fibromatosis and massive edema
Fibrosarcoma
Follicle cyst
Gonadoblastoma
Gonadoblastoma
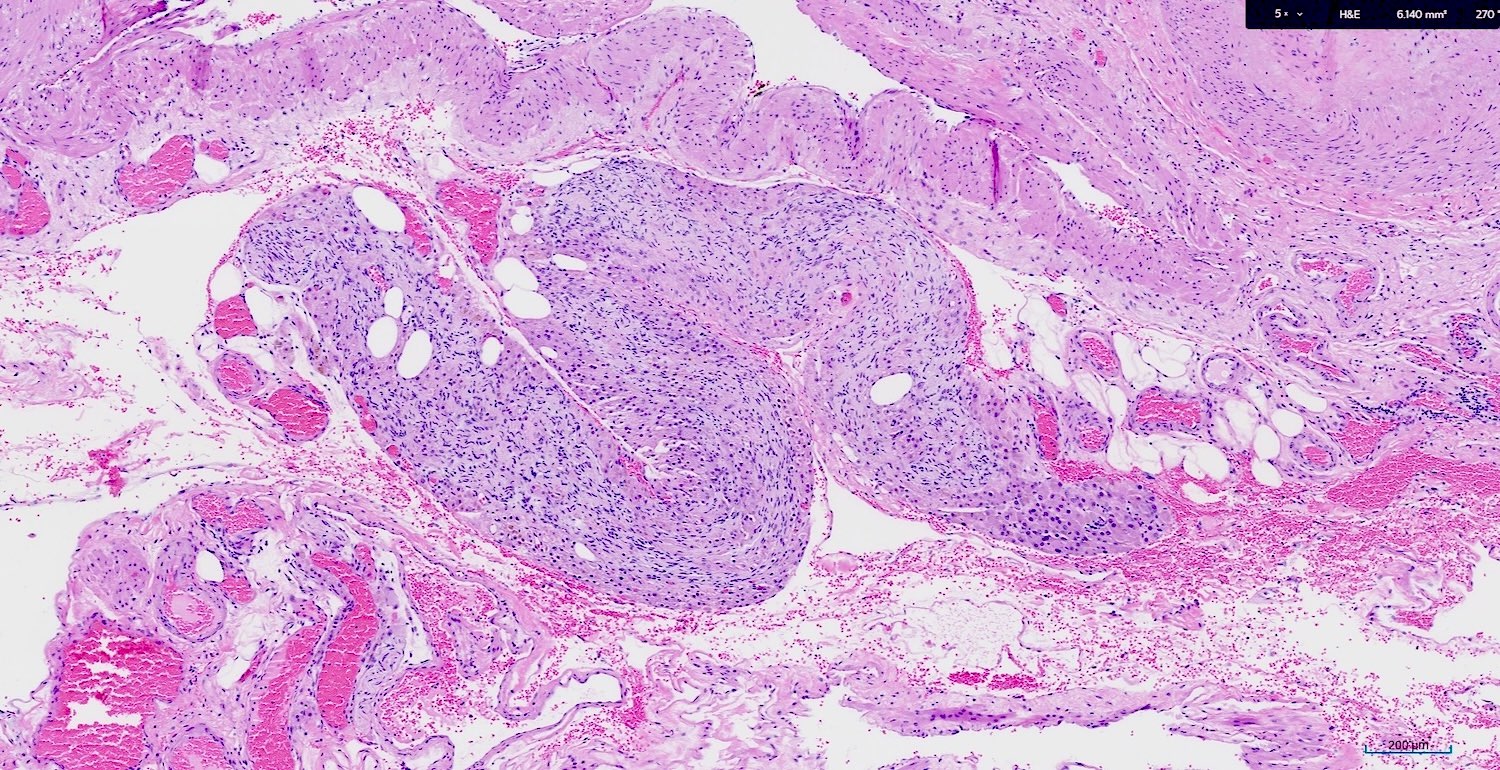
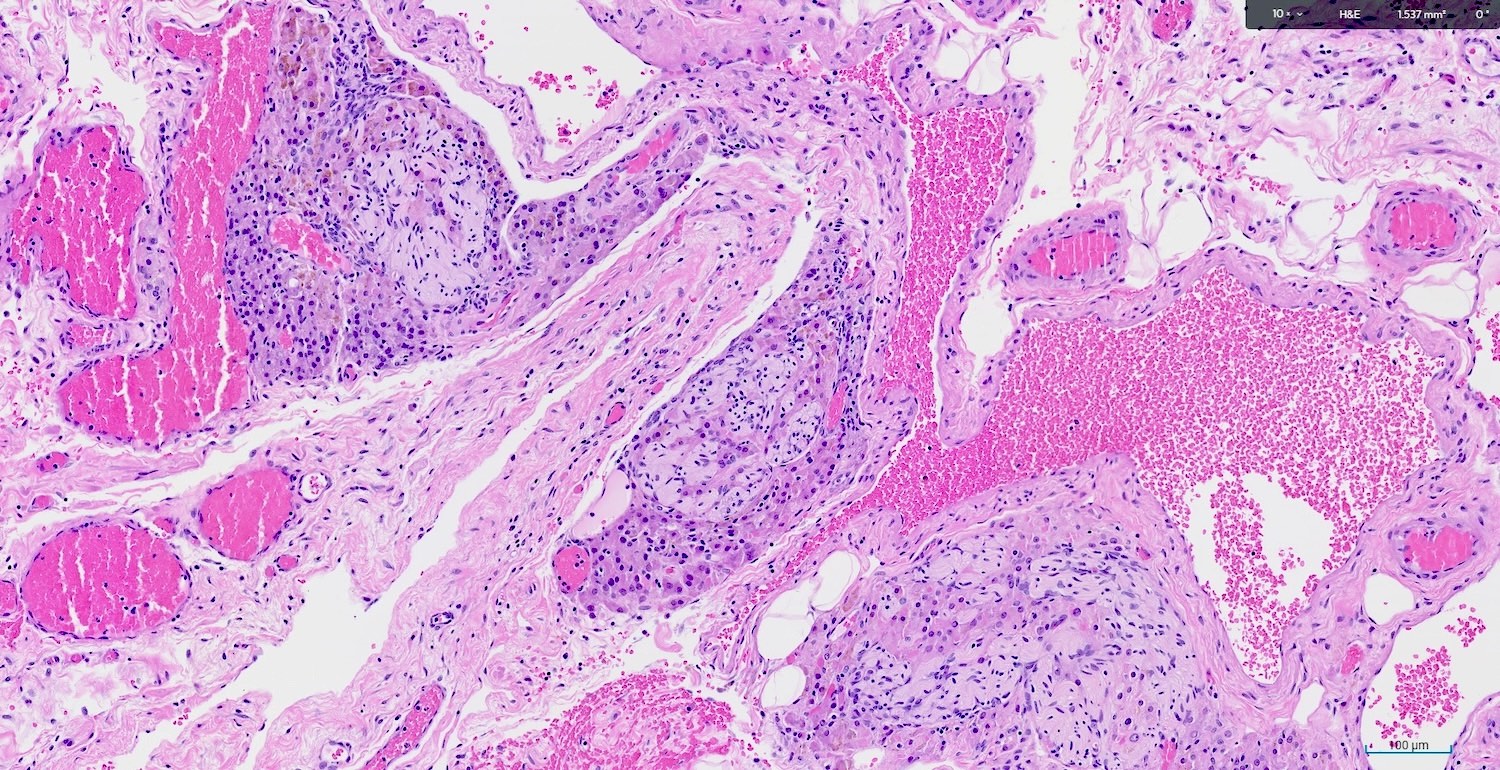
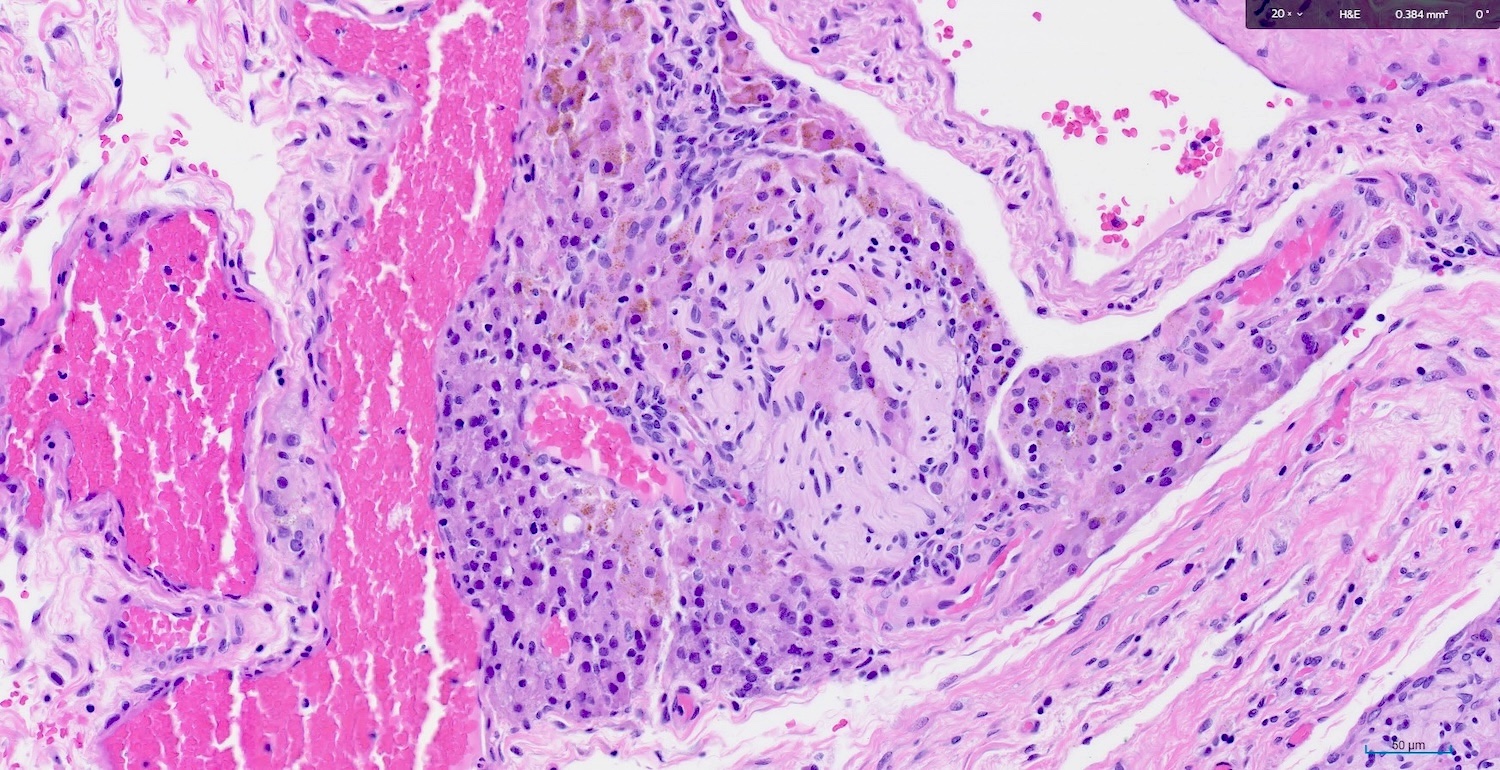
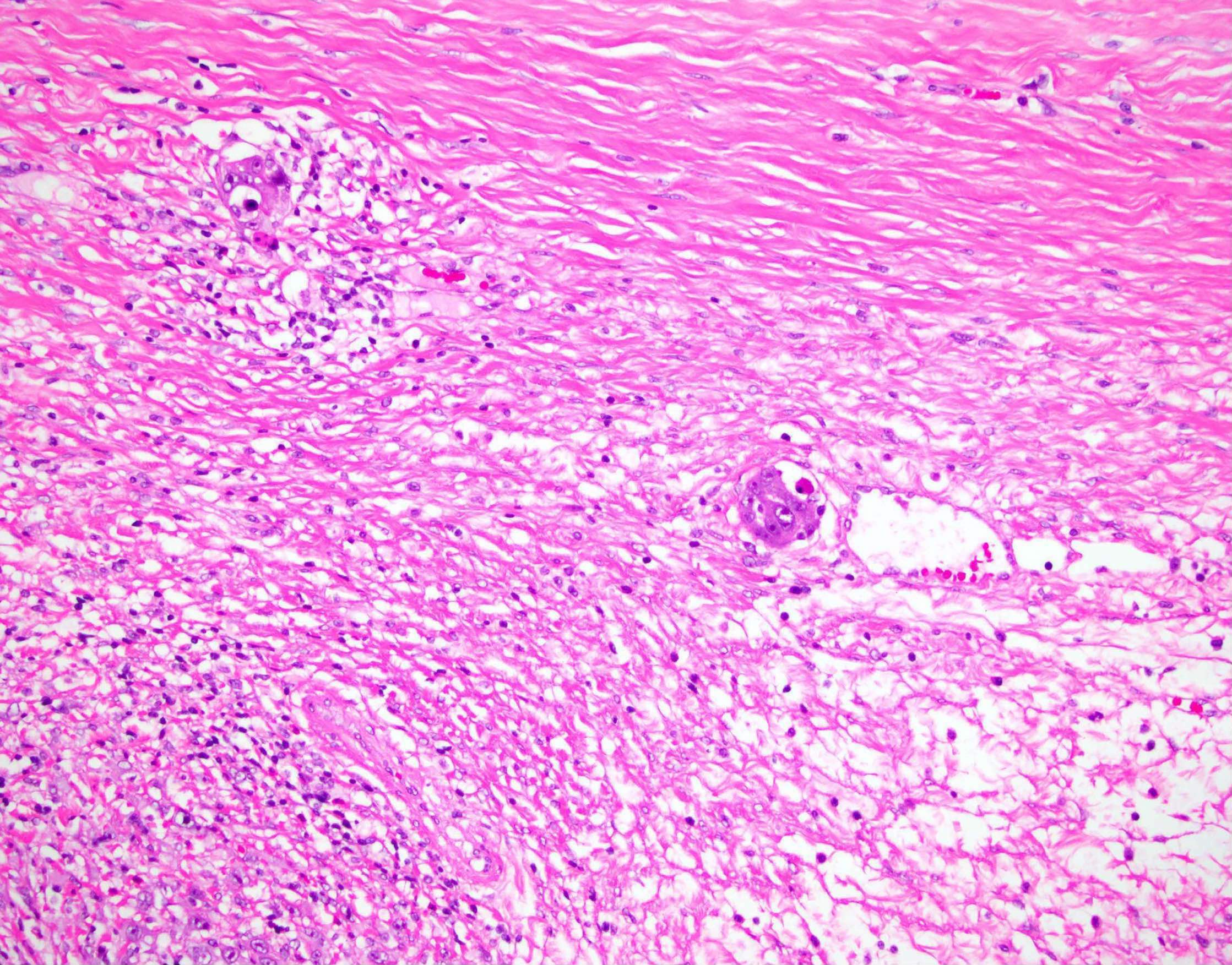
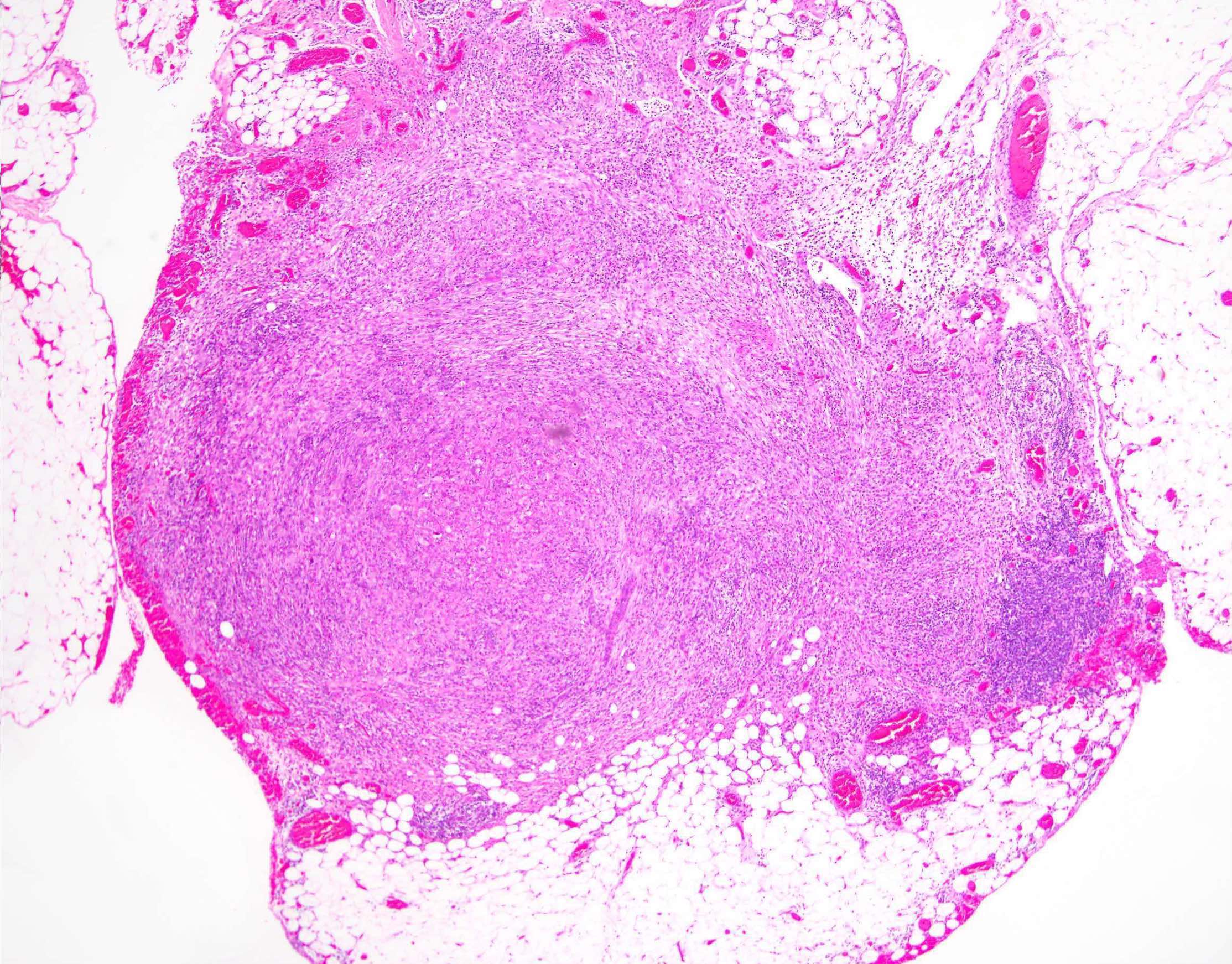
Granulomatous inflammation
Granulosa cell tumor-adult
Granulosa cell tumor-juvenile
Gynandroblastoma
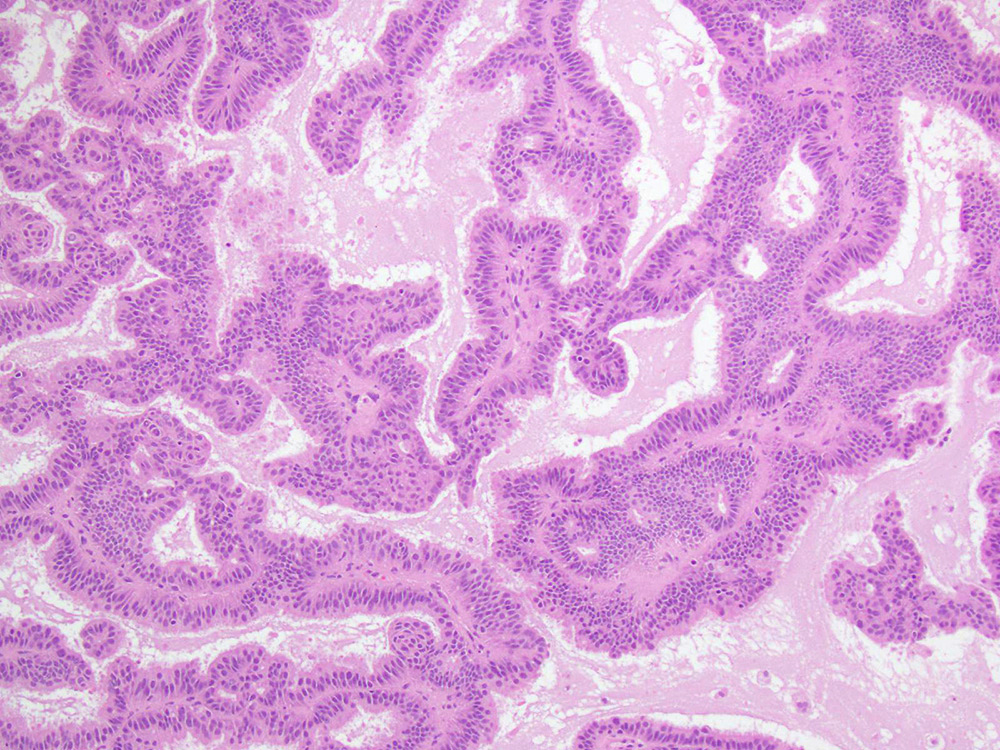
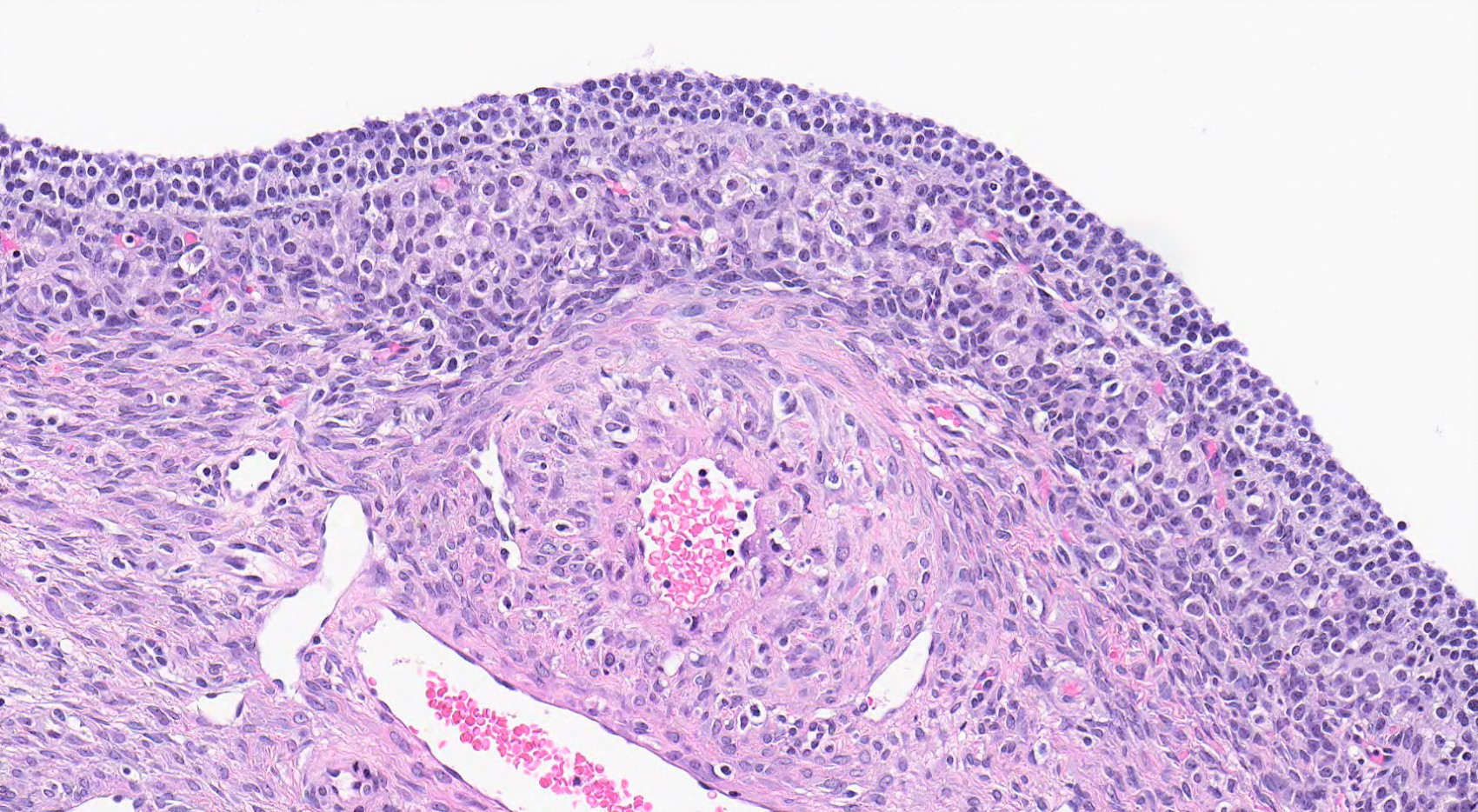
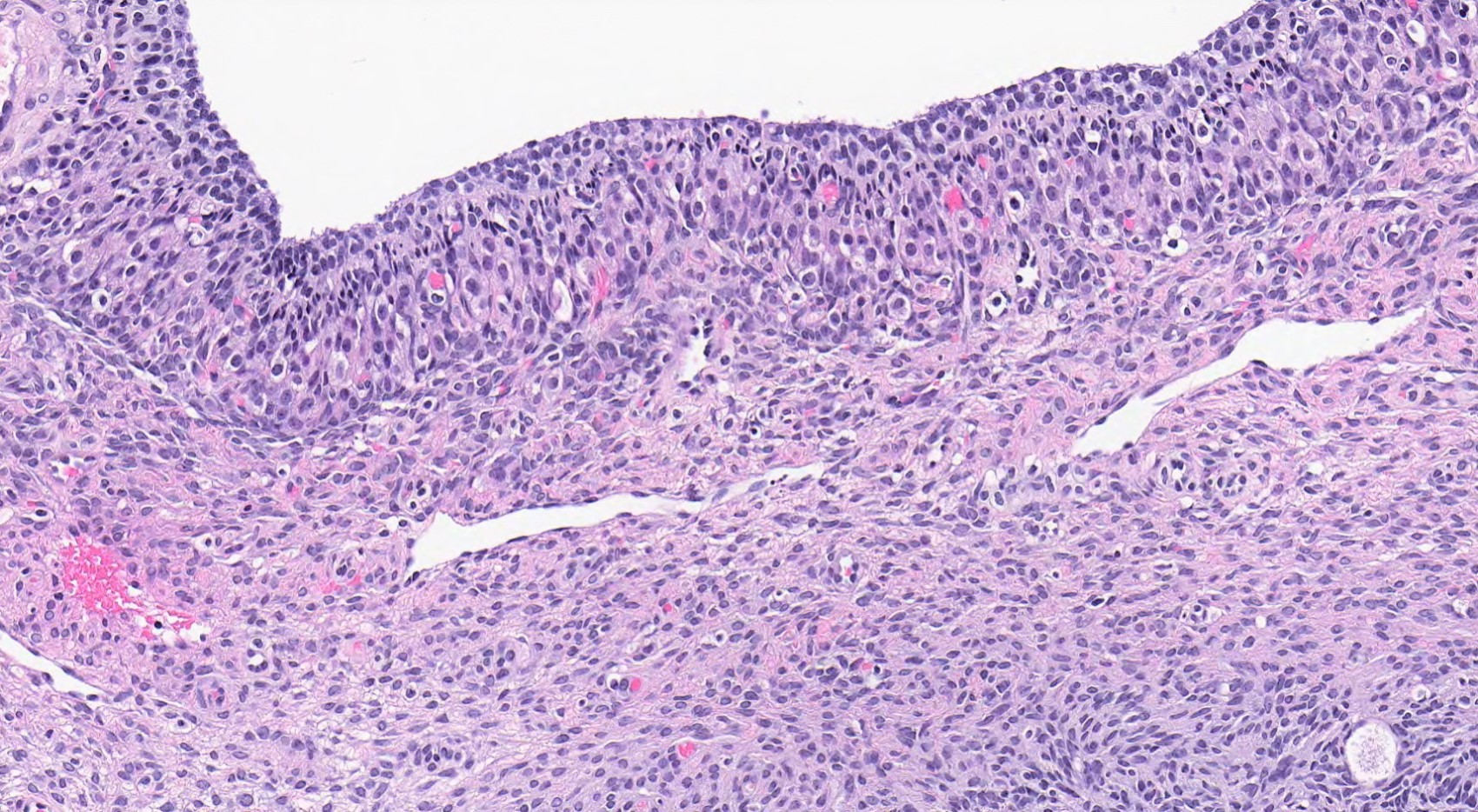
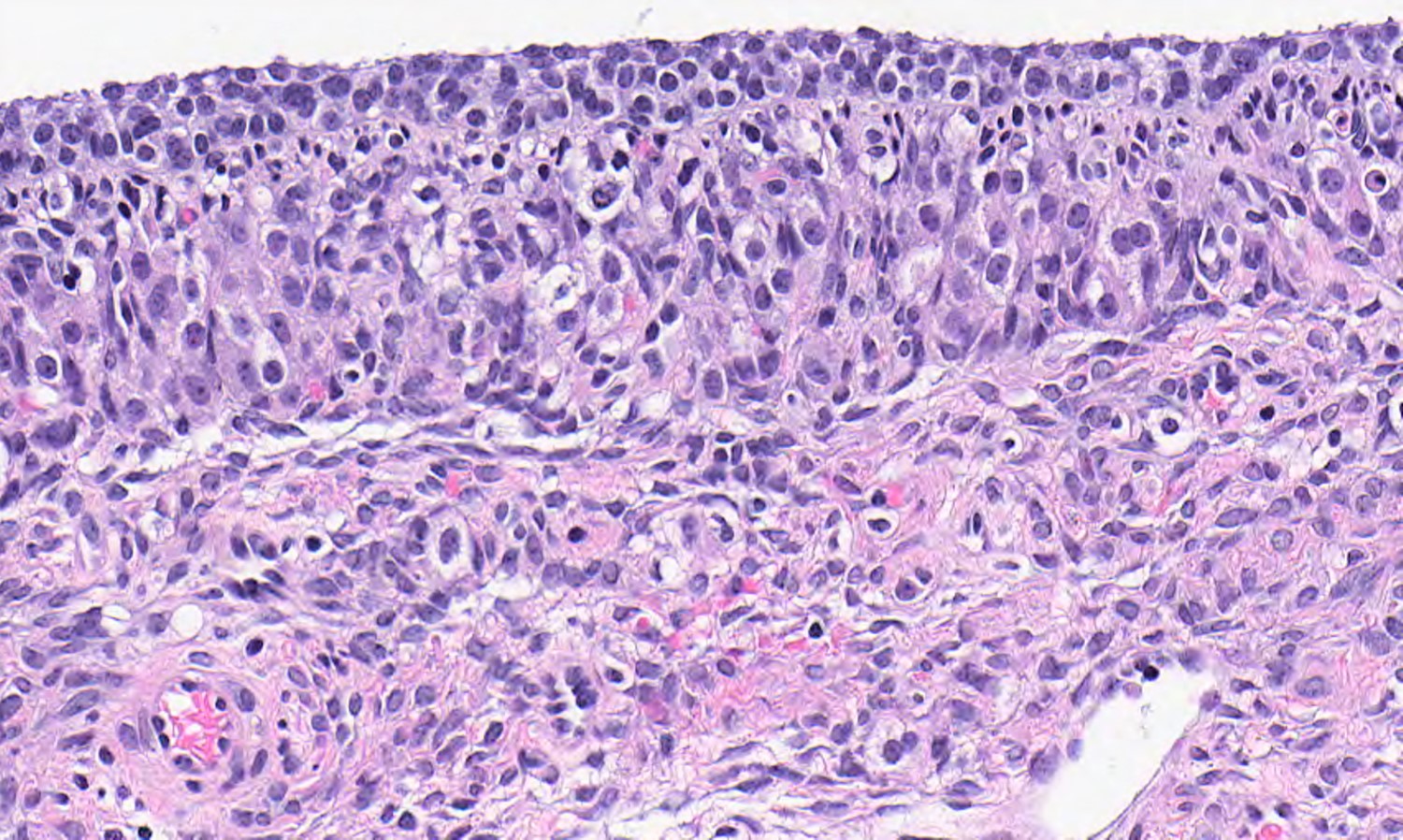
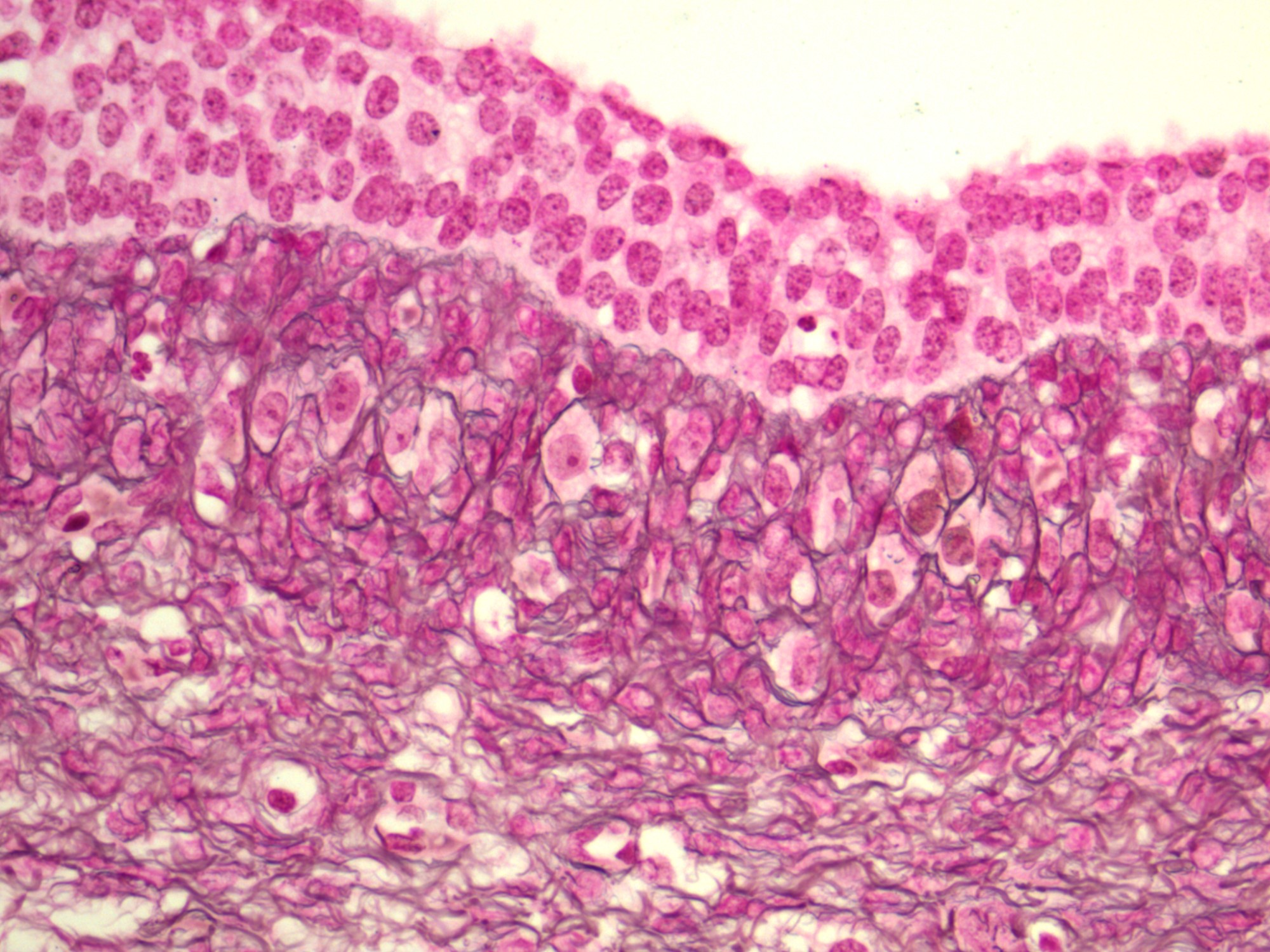
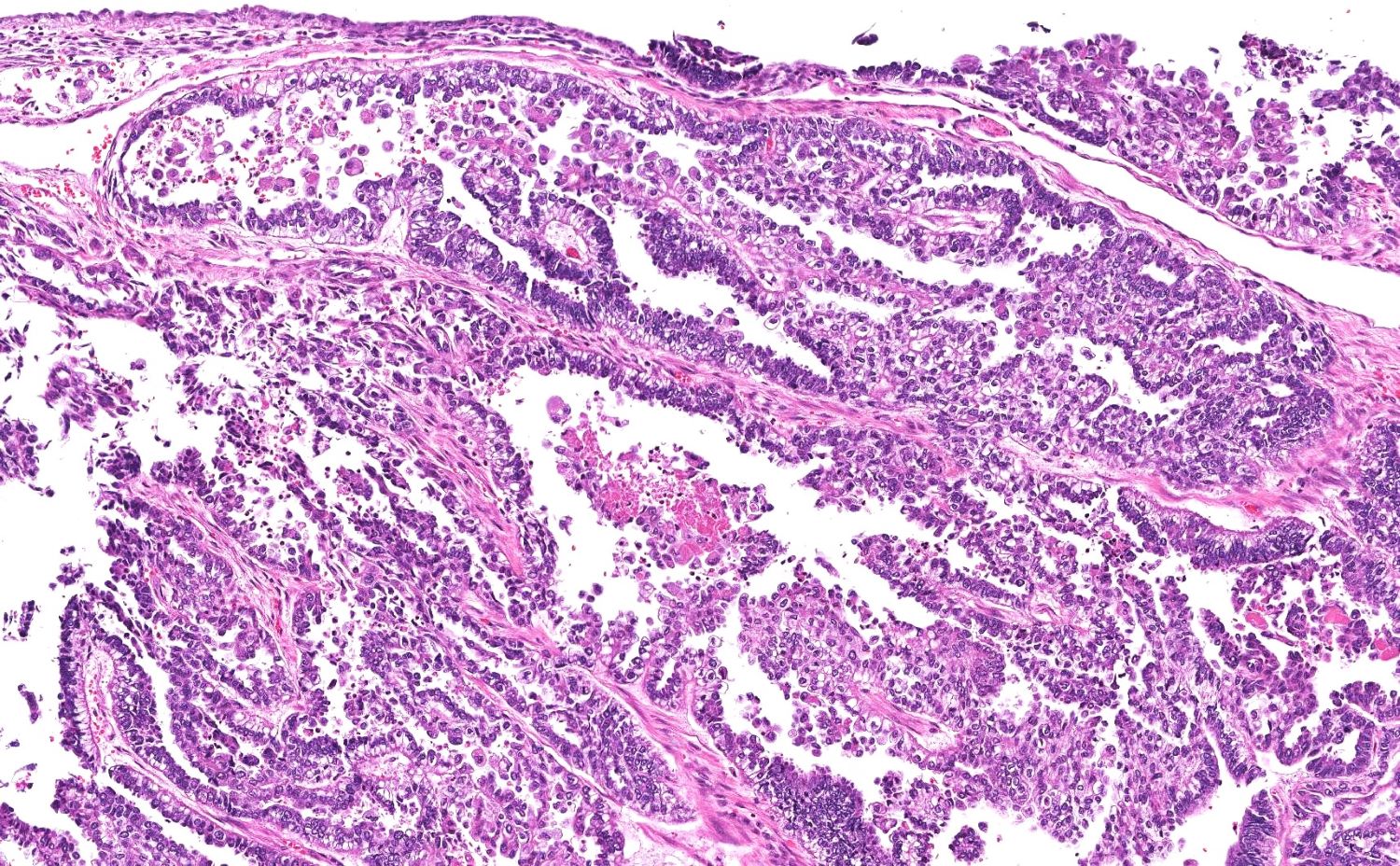
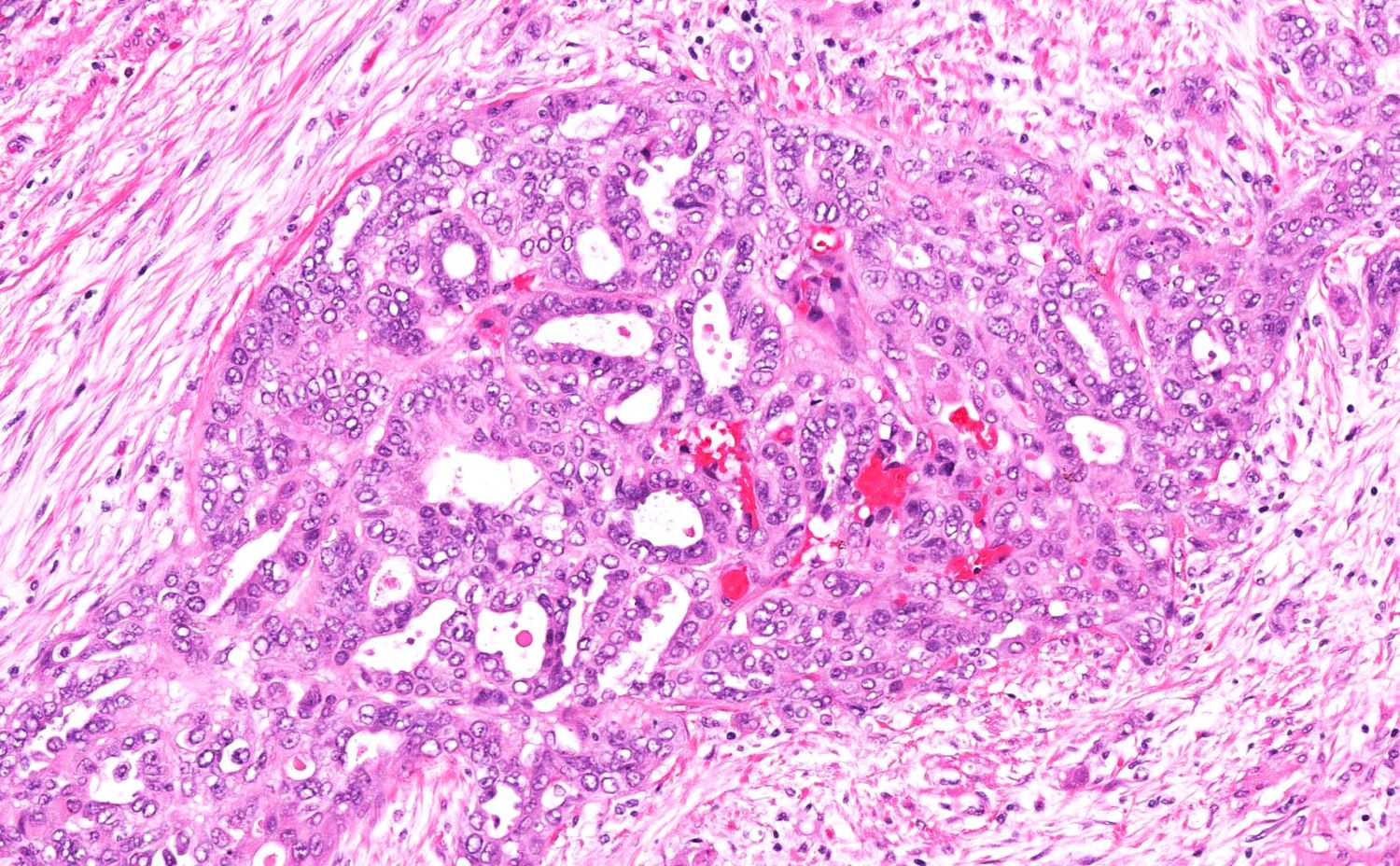
High grade serous carcinoma
Hyperreactio luteinalis
Large solitary luteinized follicular cyst of pregnancy and puerperium
Leiomyoma
Leiomyosarcoma
Leydig cell hyperplasia
Leydig cell tumor
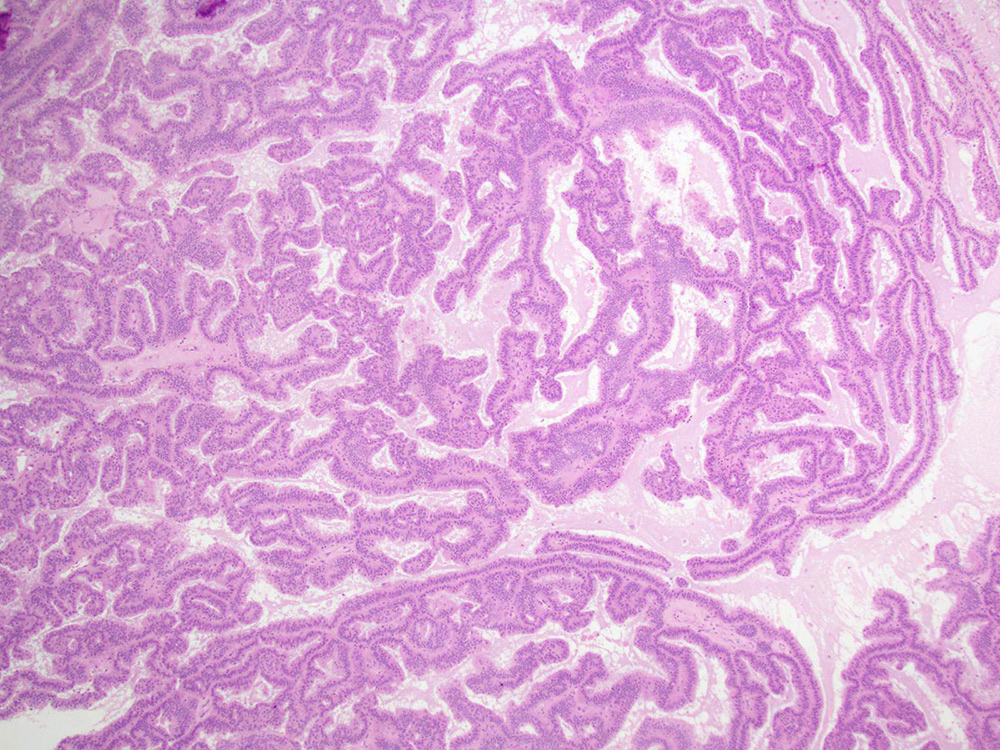
Low grade serous carcinoma
Luteinized thecoma associated with sclerosing peritonitis
Müllerian adenosarcoma
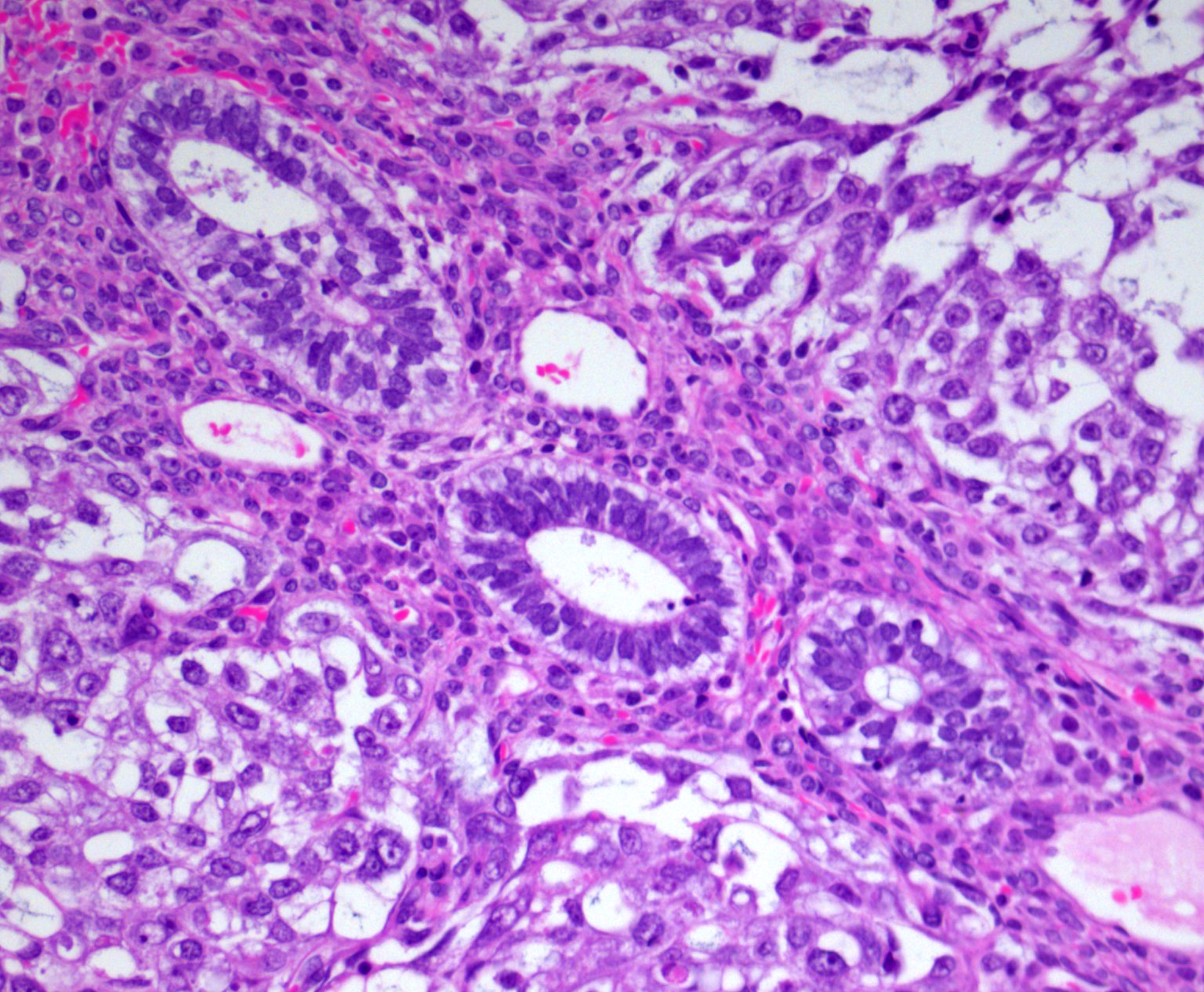
Mesonephric-like adenocarcinoma (uterus / ovary)
Metastases to ovary
Microcystic stromal tumor
Mixed carcinoma
Mixed germ cell - sex cord stromal tumor, unclassified
Mixed germ cell - sex cord stromal tumor, unclassified (pending)
Mixed germ cell tumor
Monodermal cystic teratoma
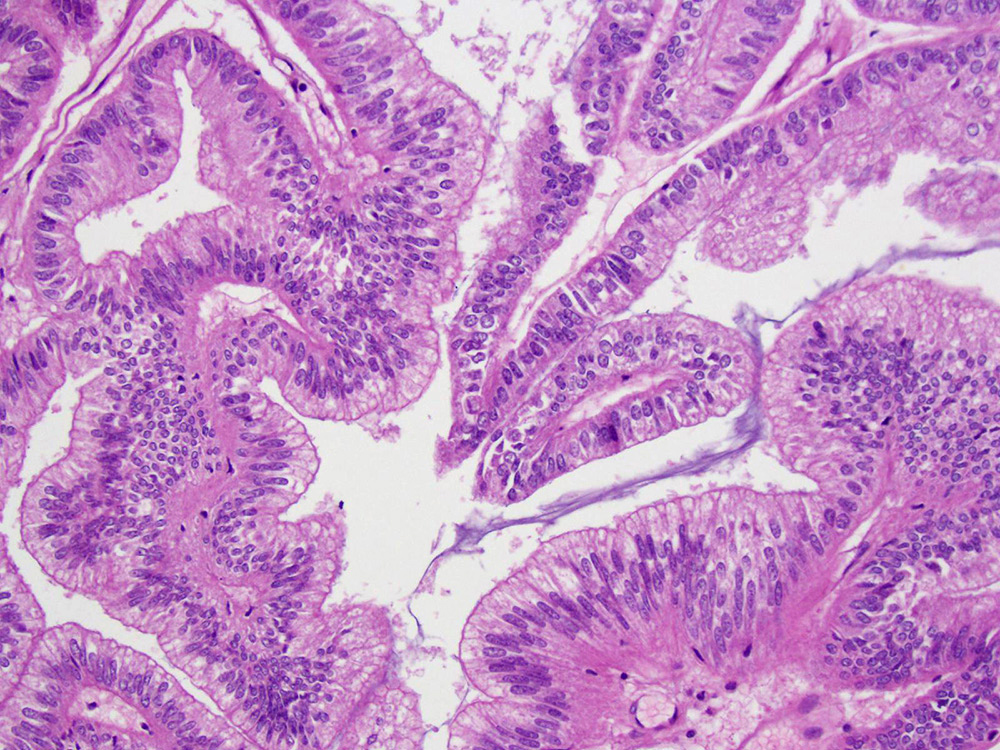
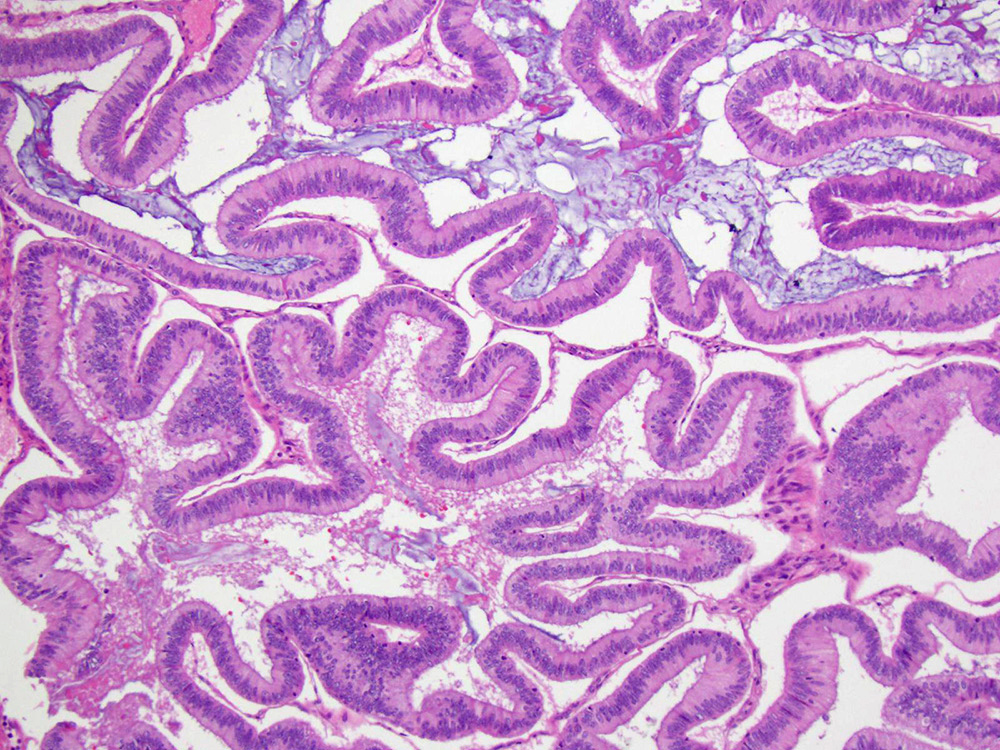
Mucinous borderline tumor
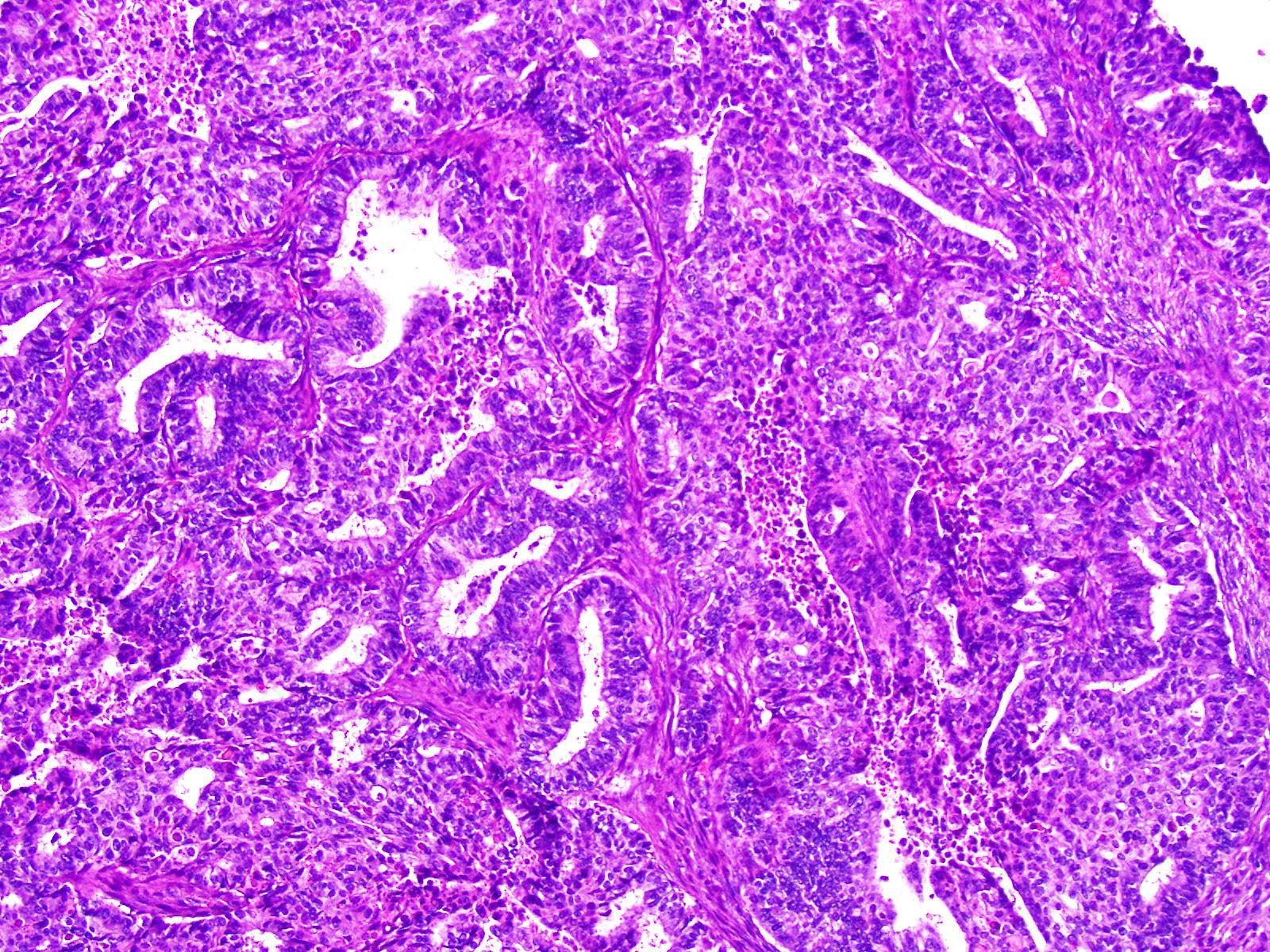
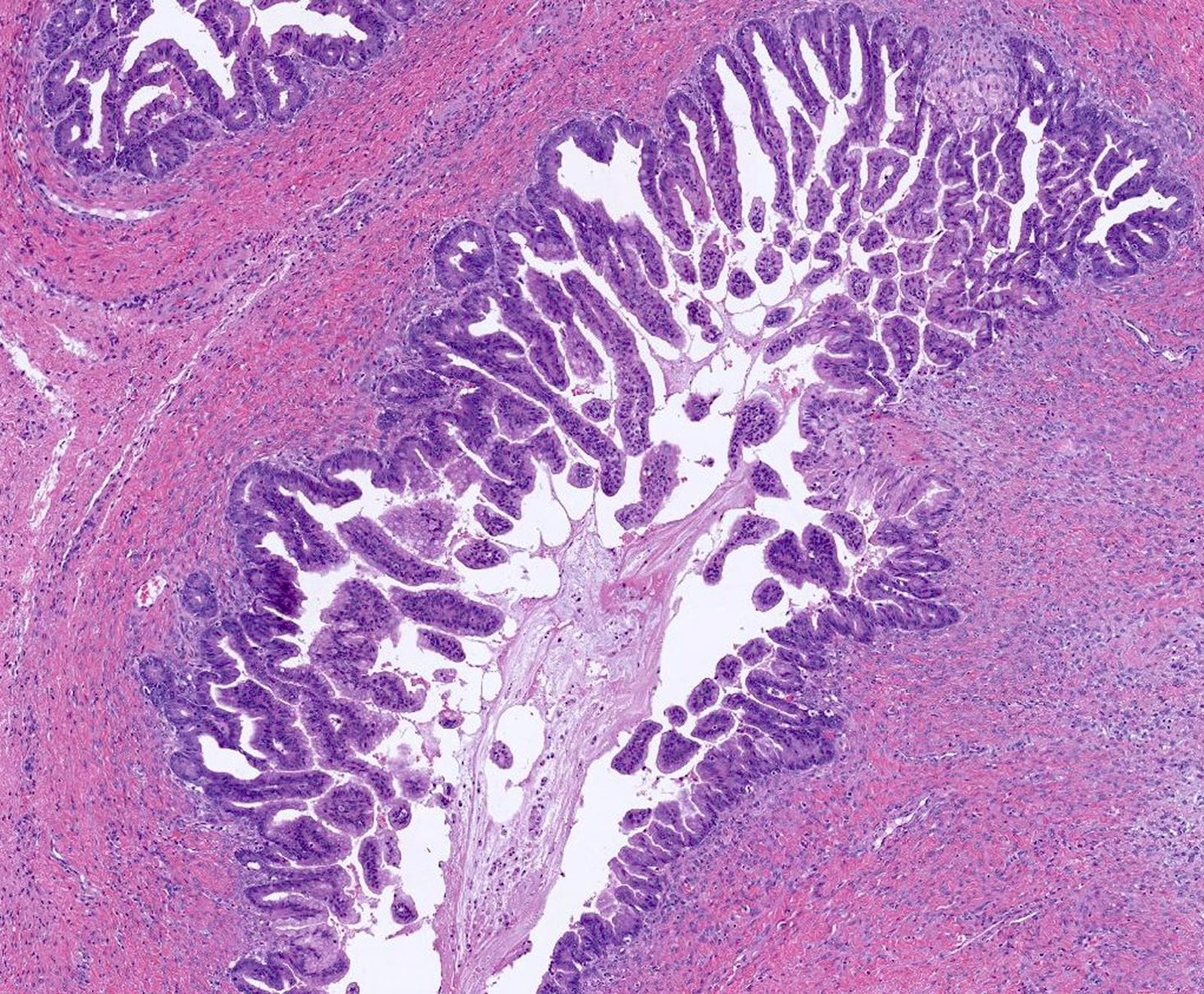
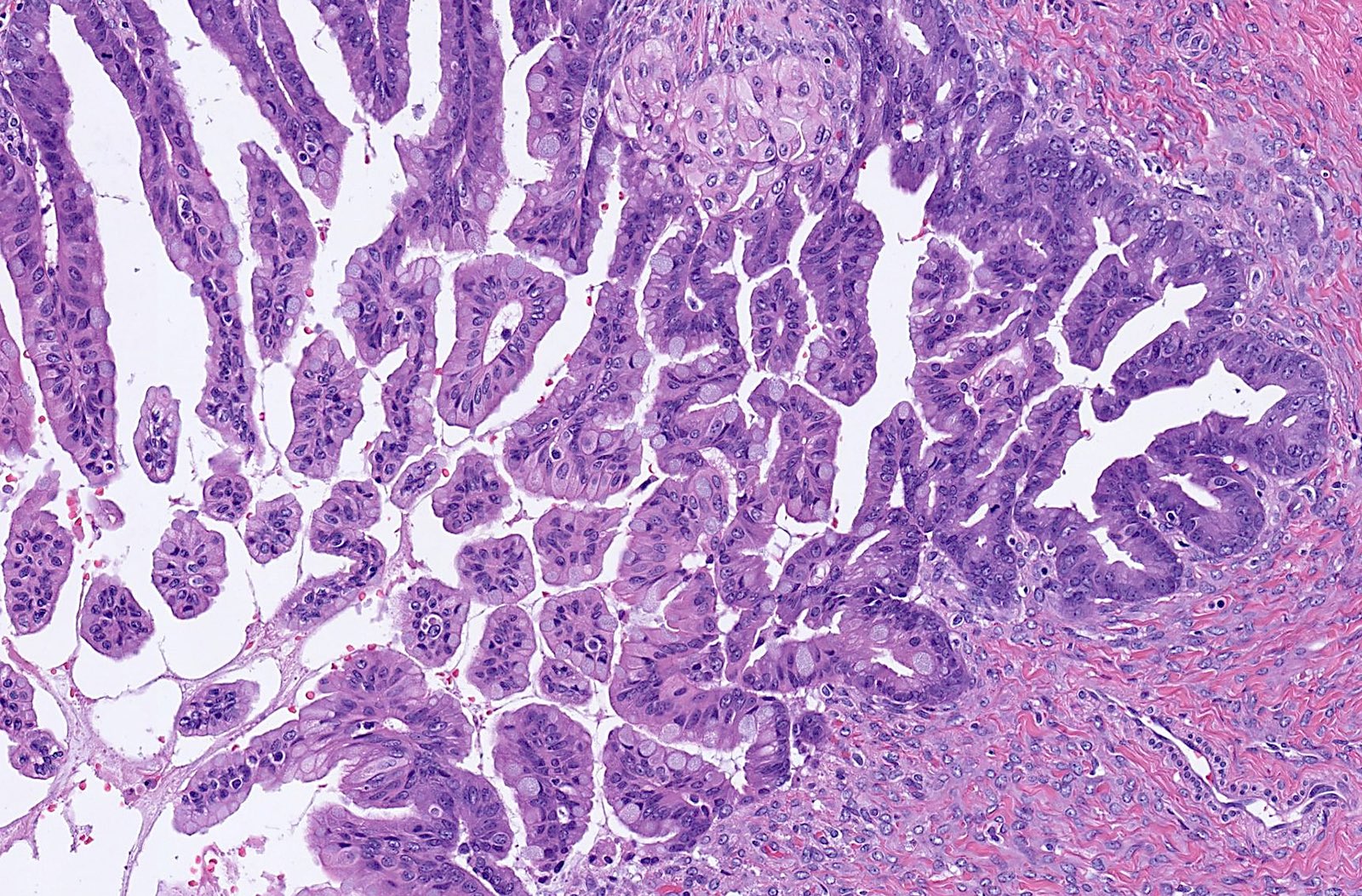
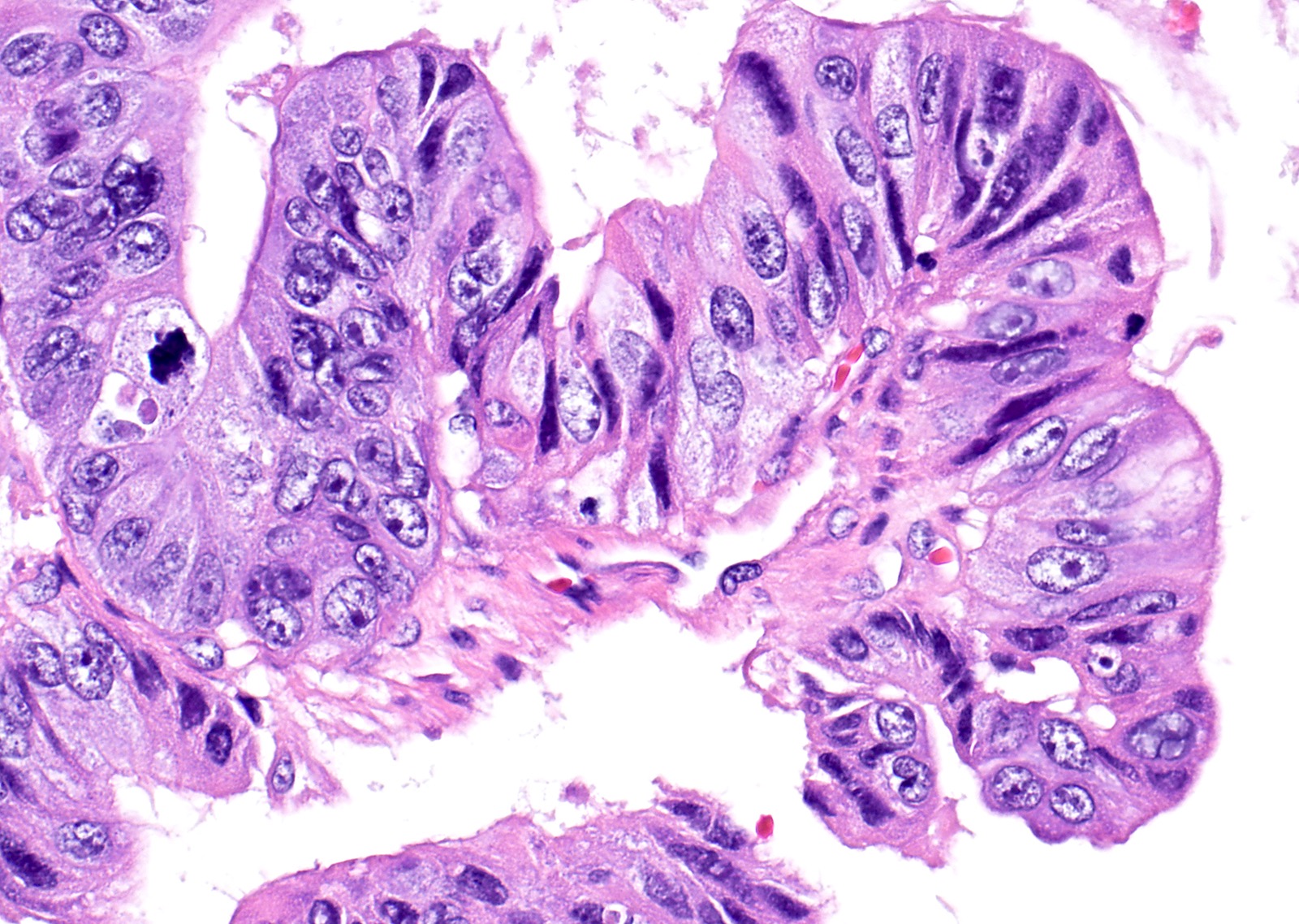
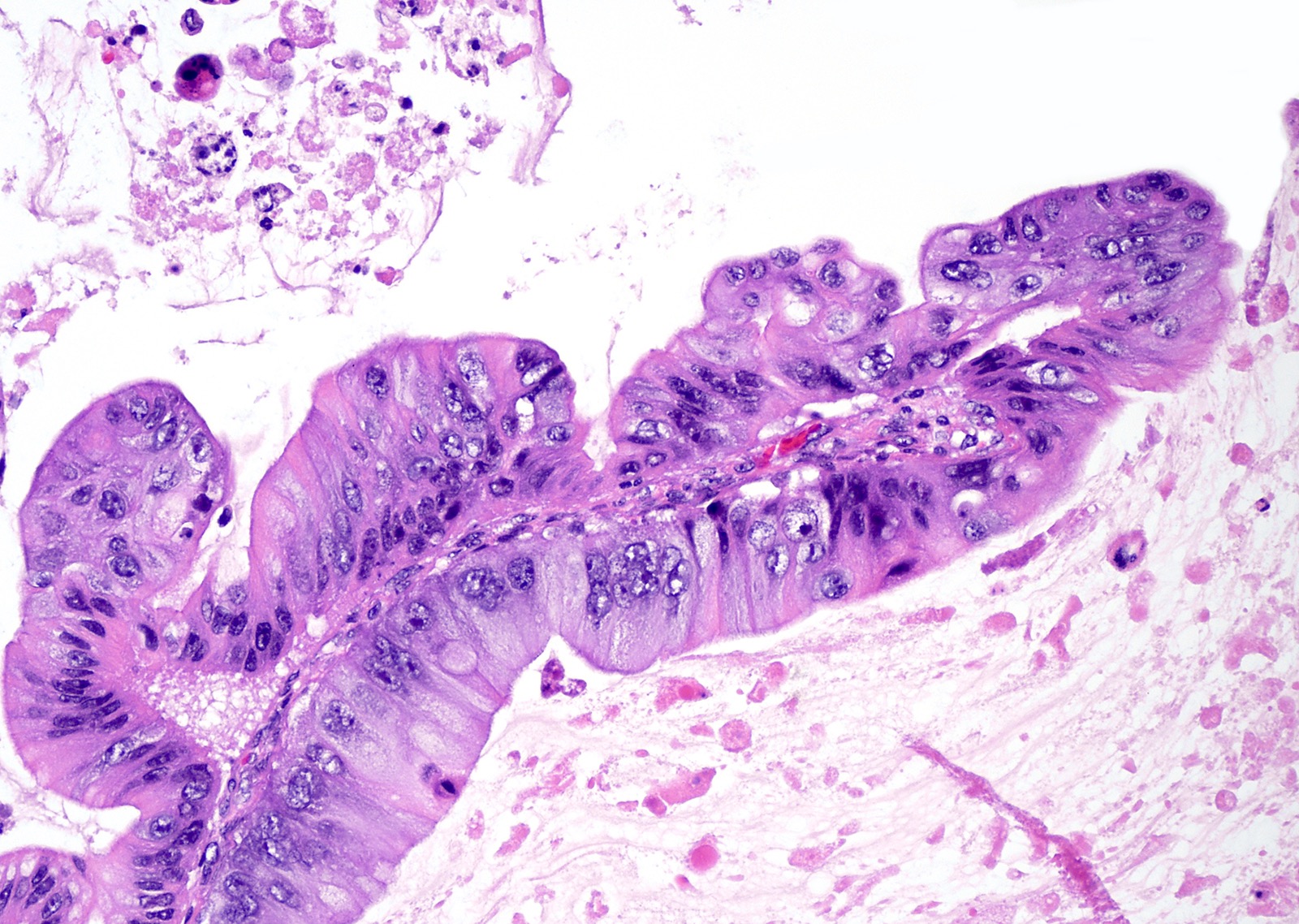
Mucinous carcinoma
Mucinous cystadenoma and adenofibroma
Mural nodules in mucinous cystic neoplasms
Neuroectodermal type tumors (pending)
Olaparib
Ovarian myxoma (pending)
Ovotesticular DSD
Polycystic ovary disease
Pregnancy luteoma
Rete cystadenoma, adenoma and adenocarcinoma
Sclerosing stromal tumor
Seromucinous borderline tumor
Seromucinous cystadenoma and adenofibroma
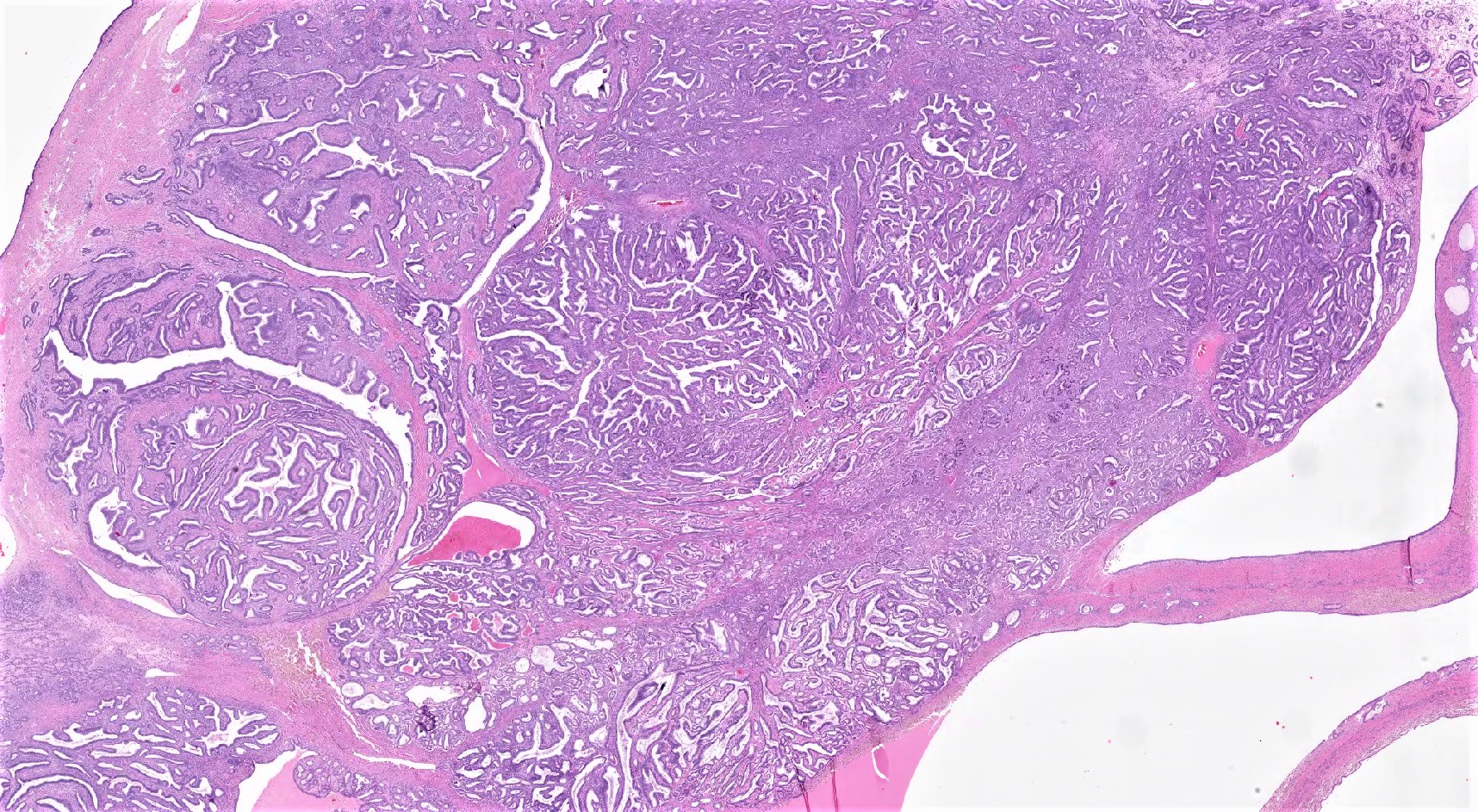
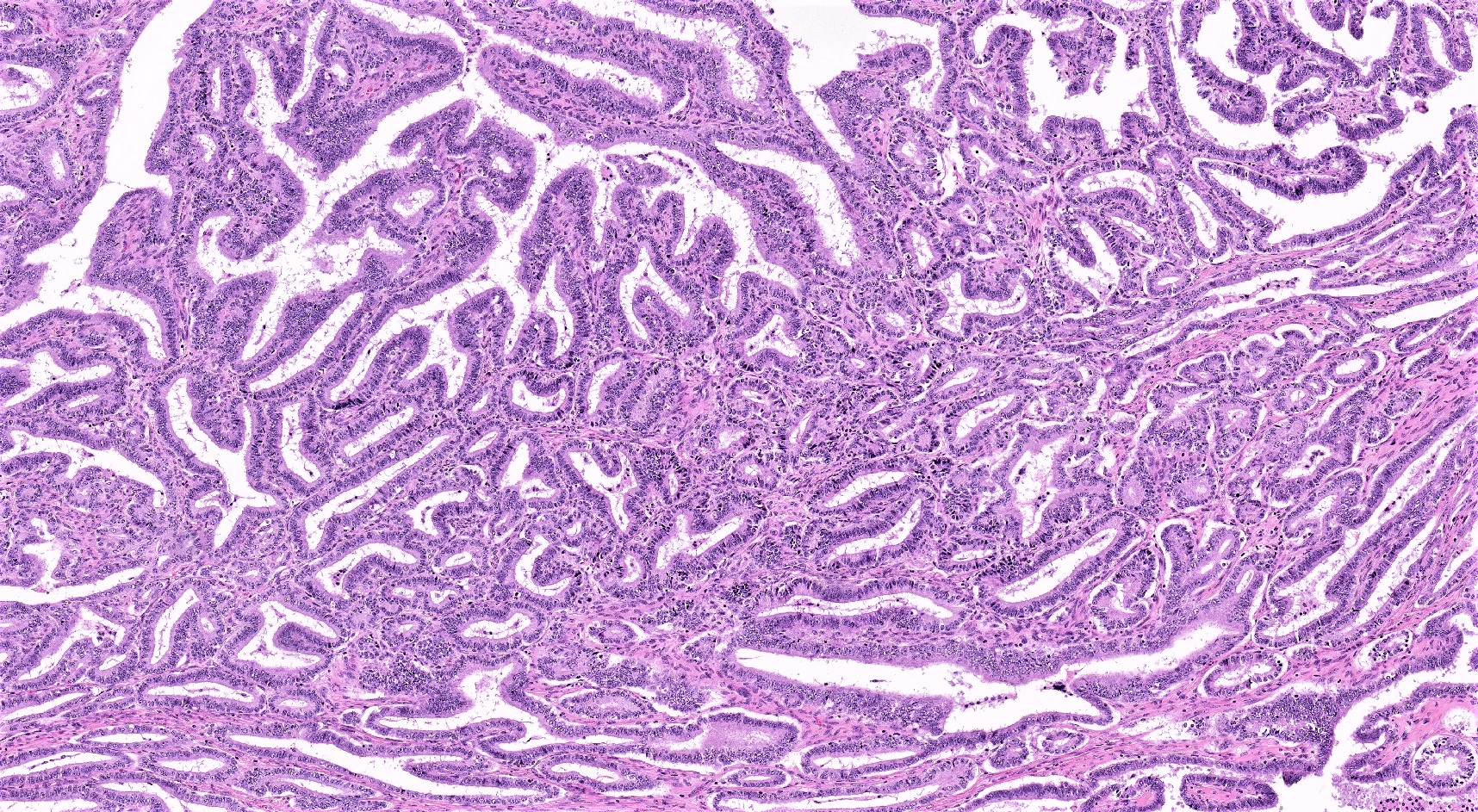
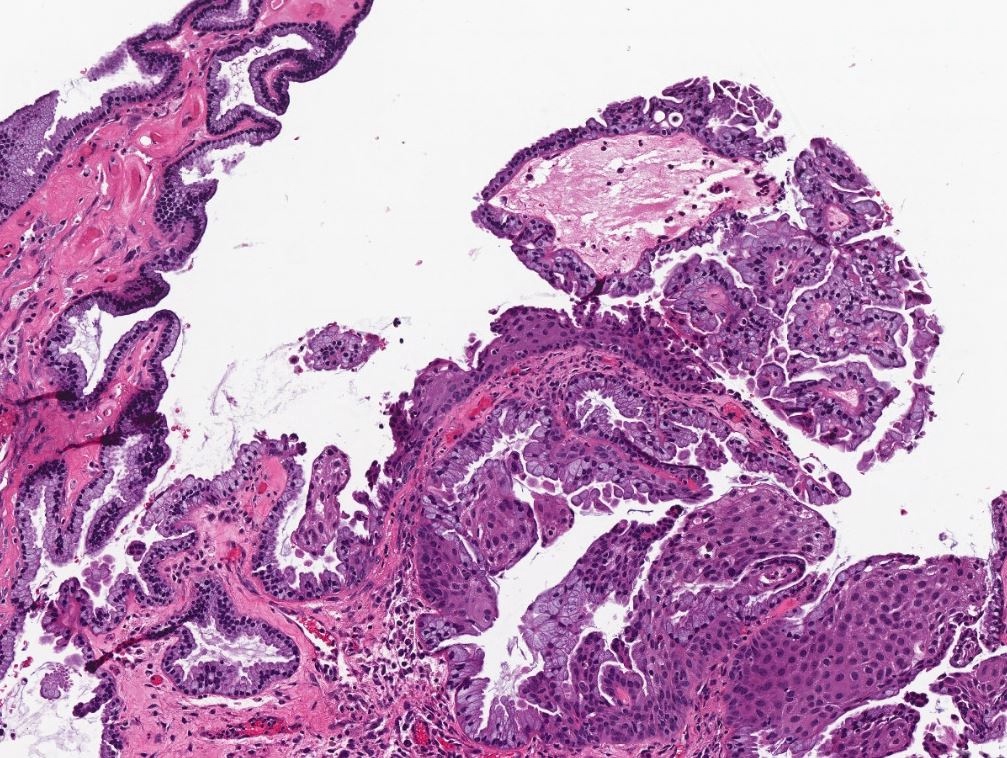
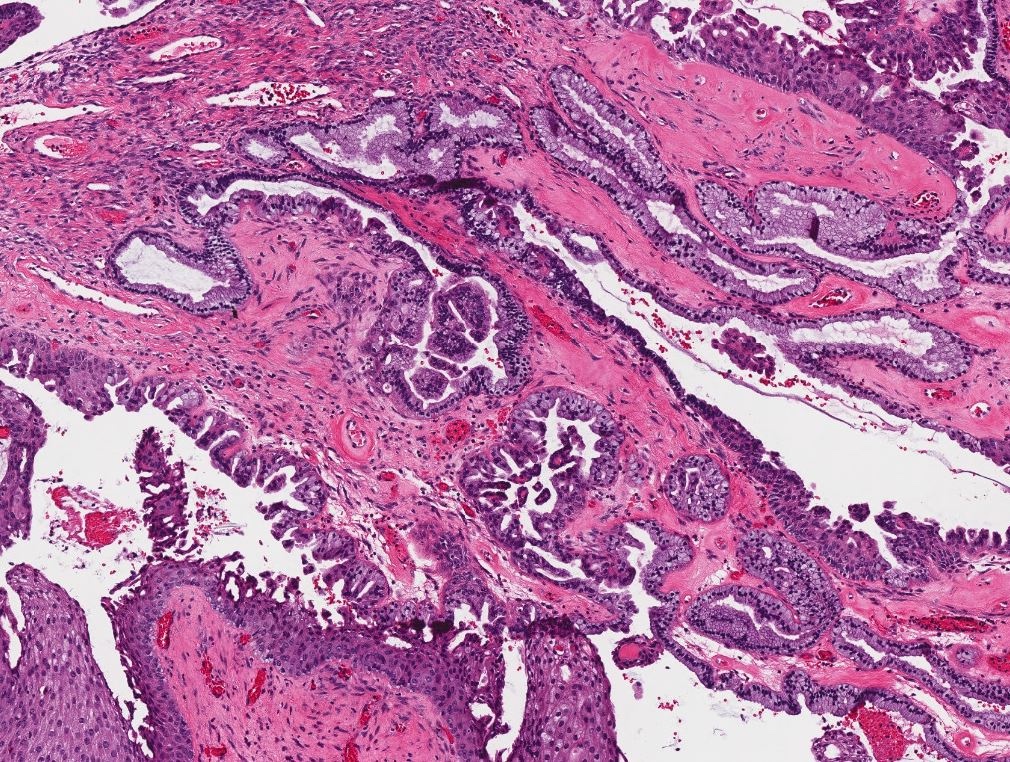
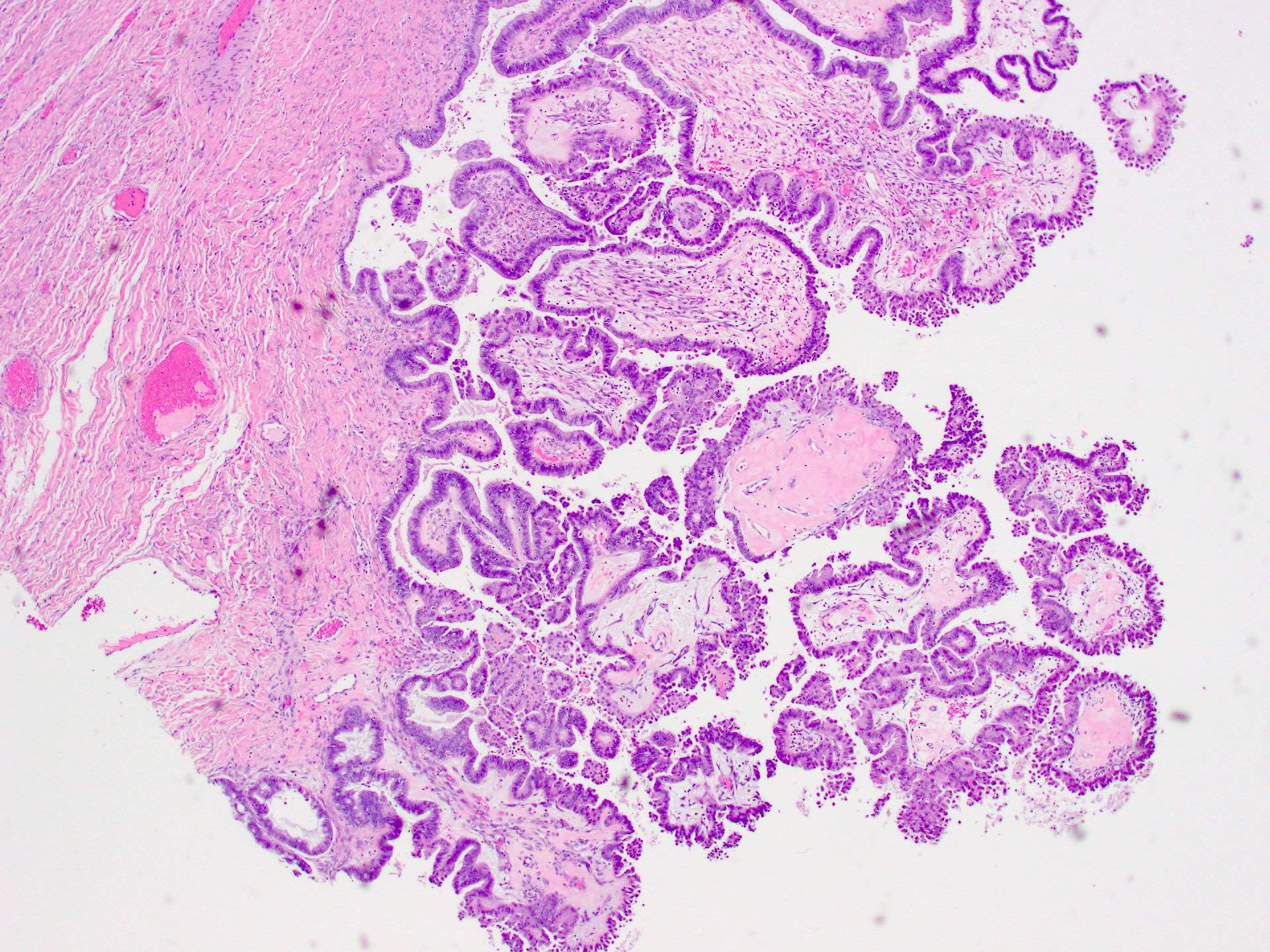
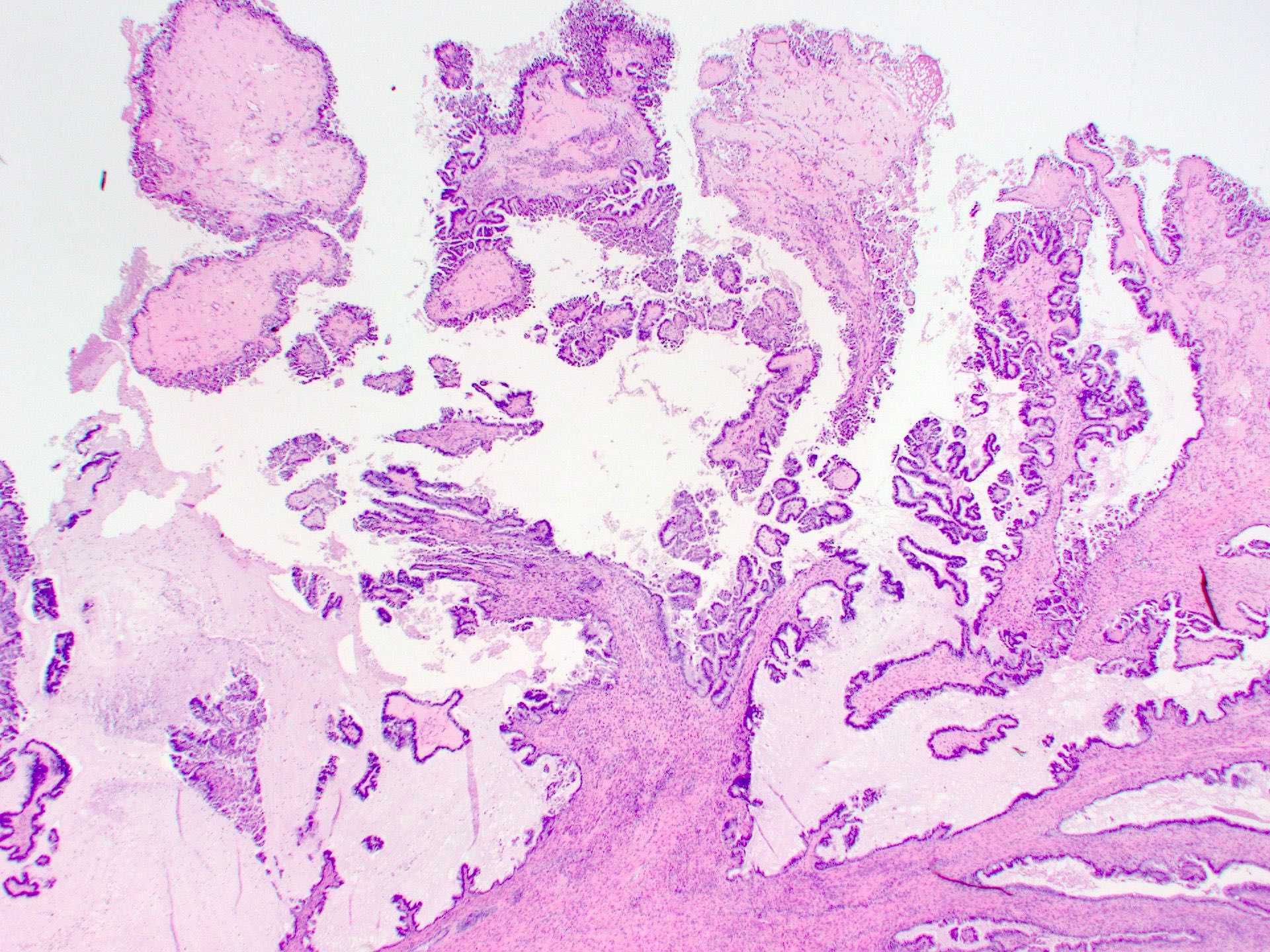
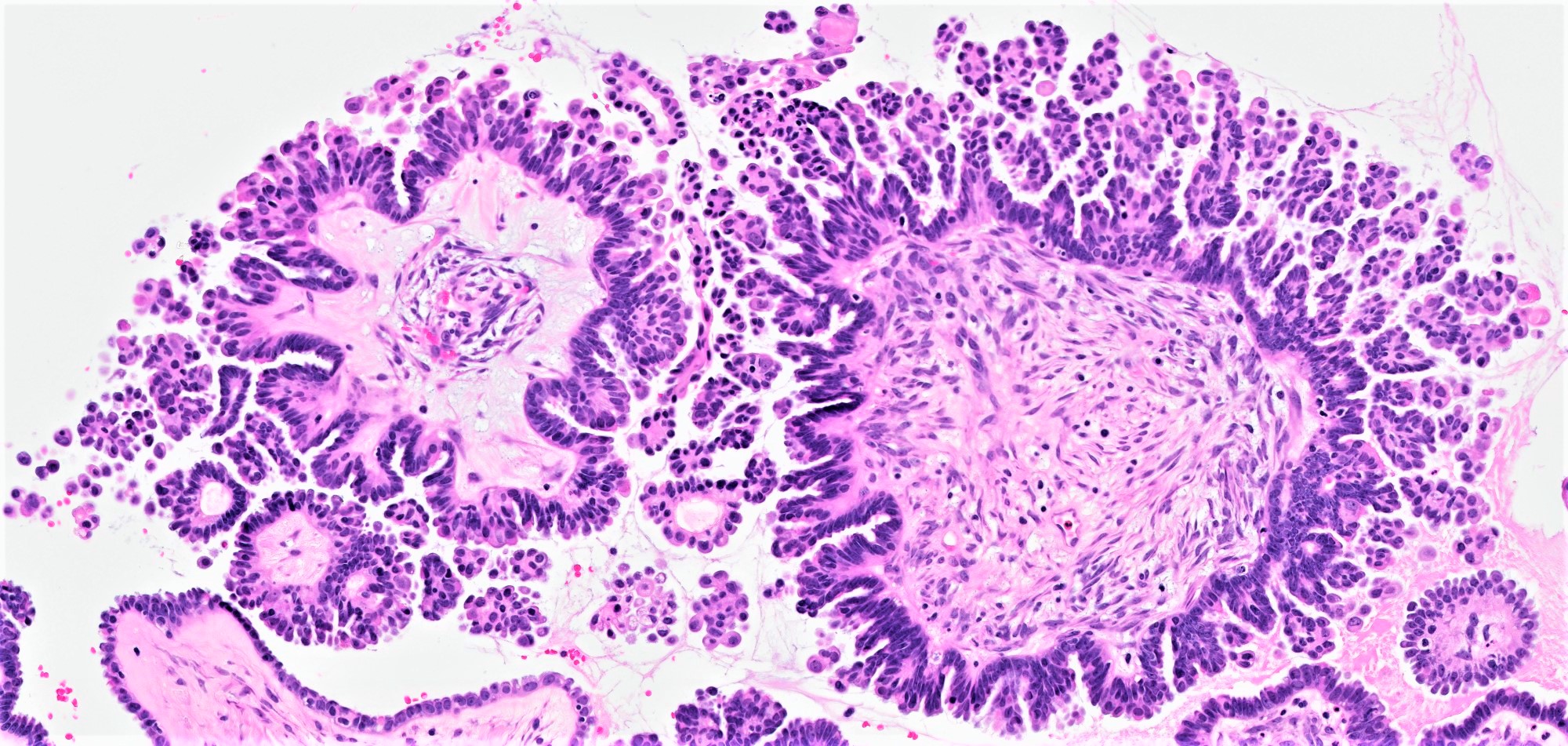
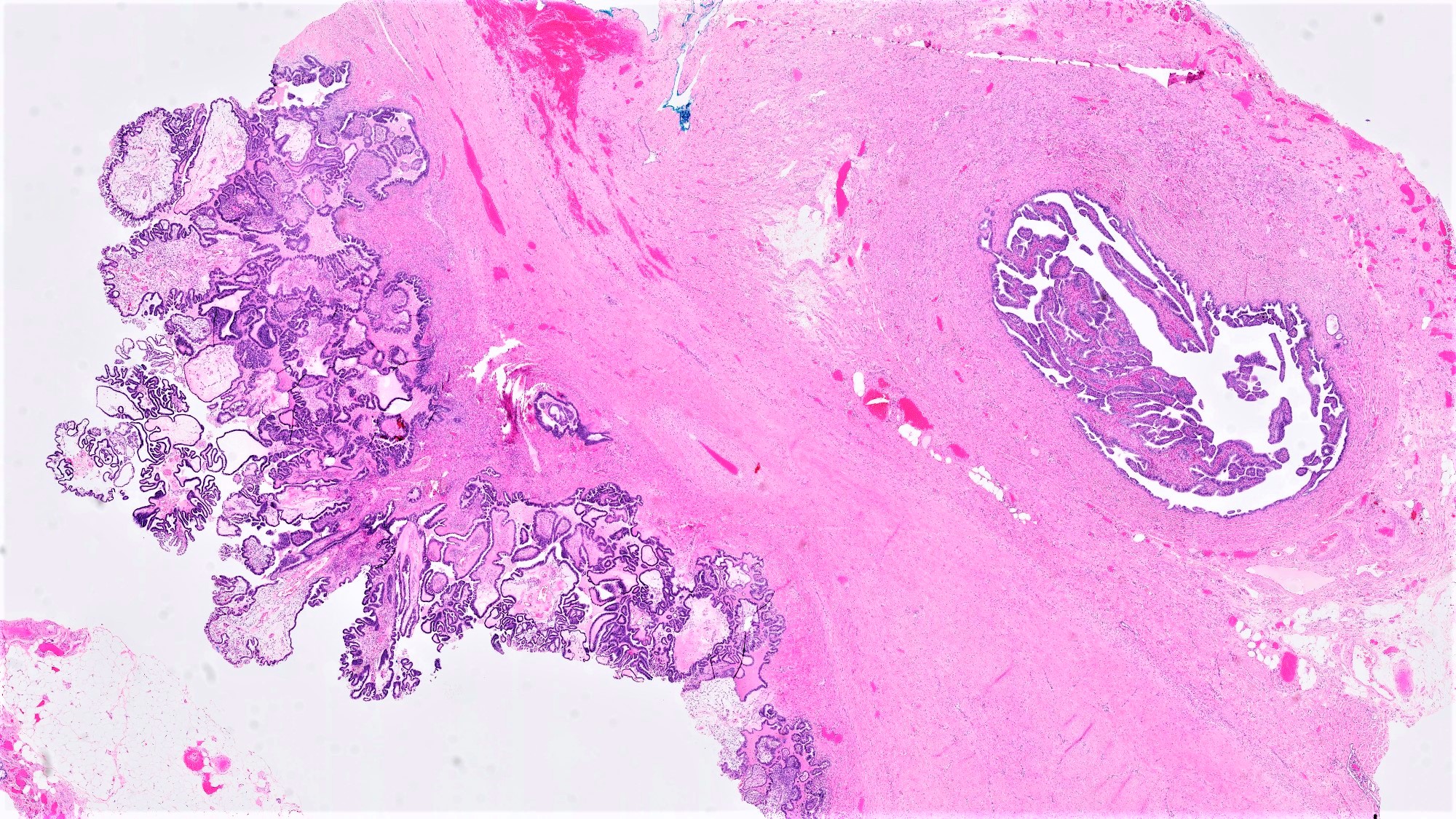
Serous borderline tumor
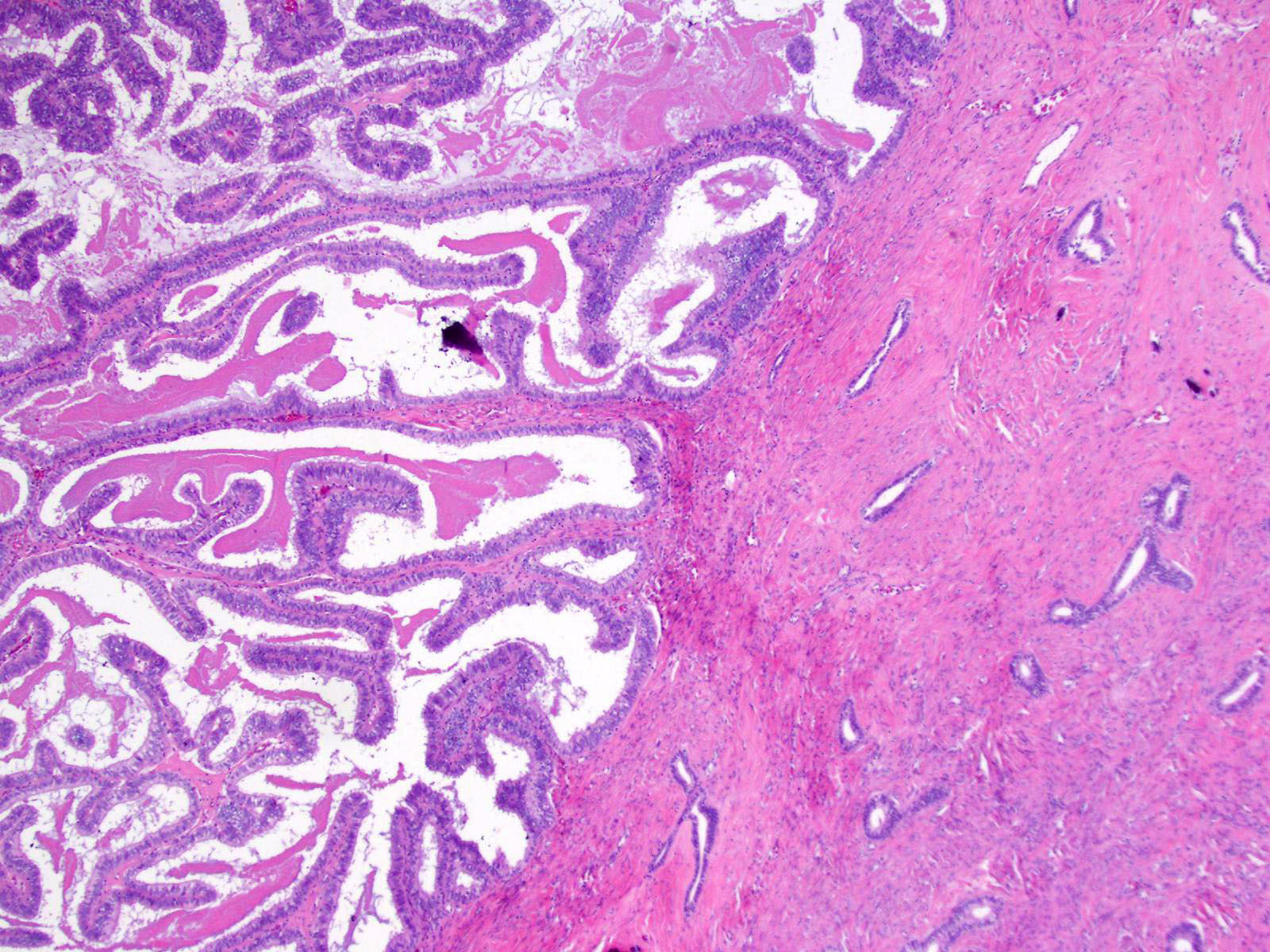
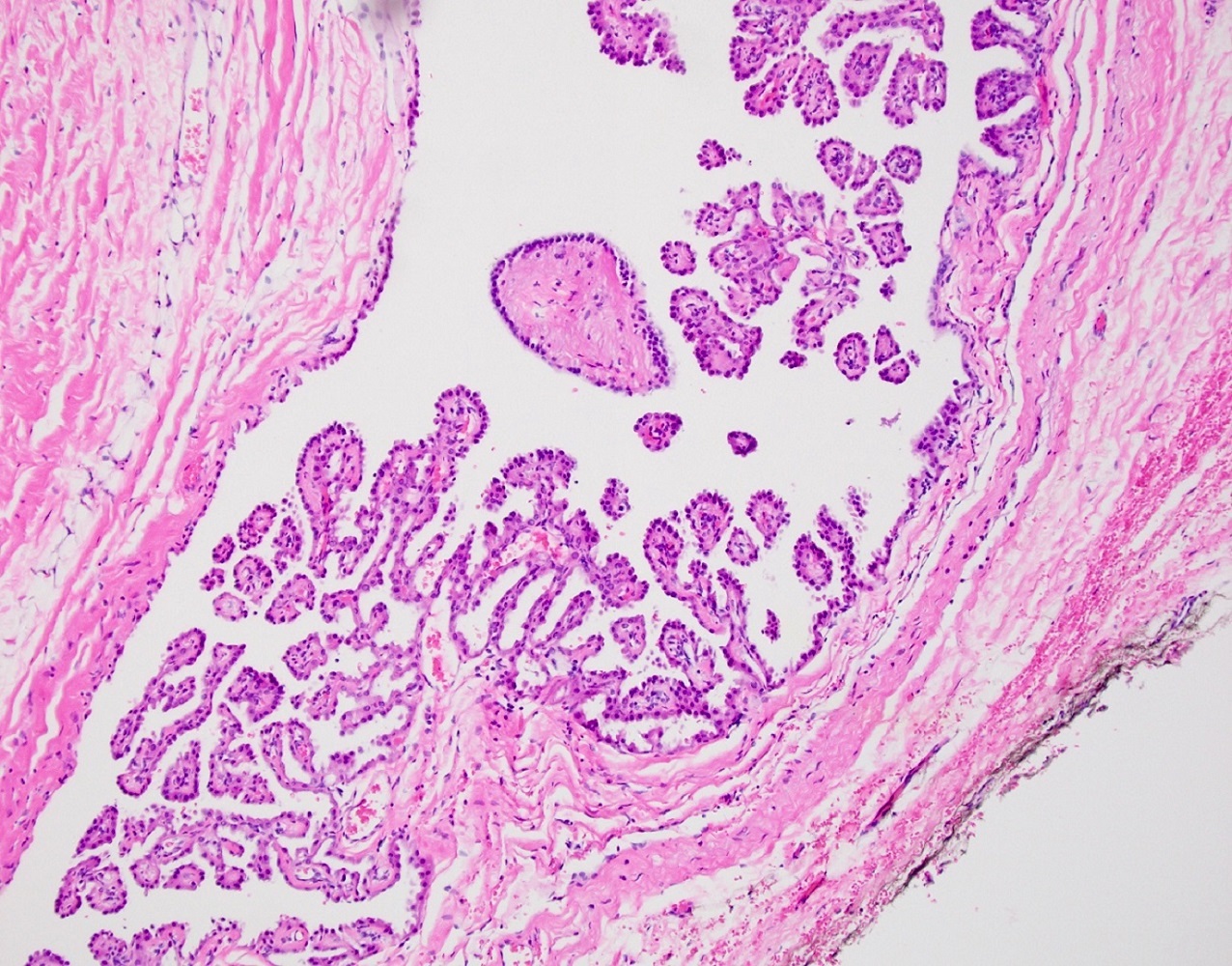
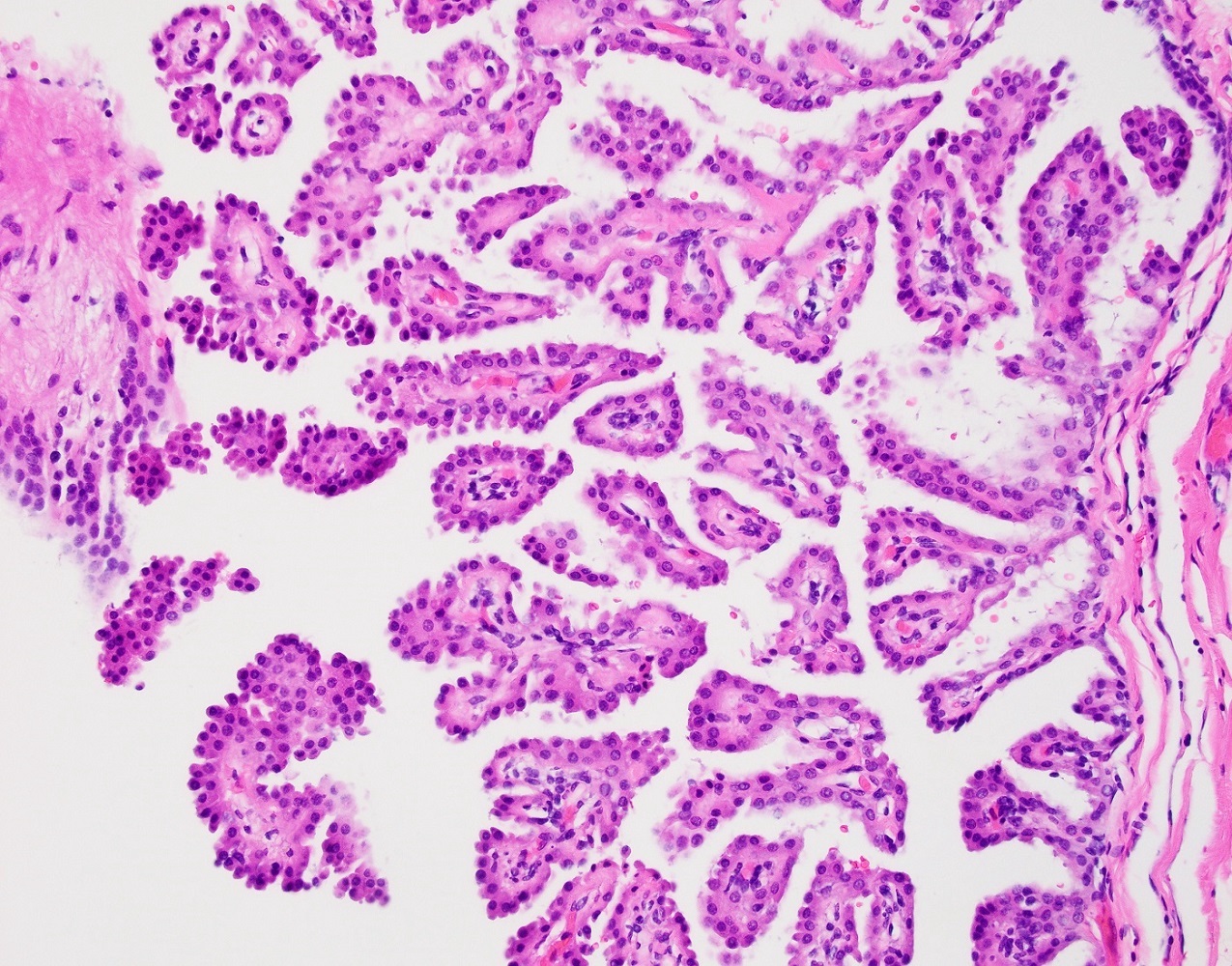
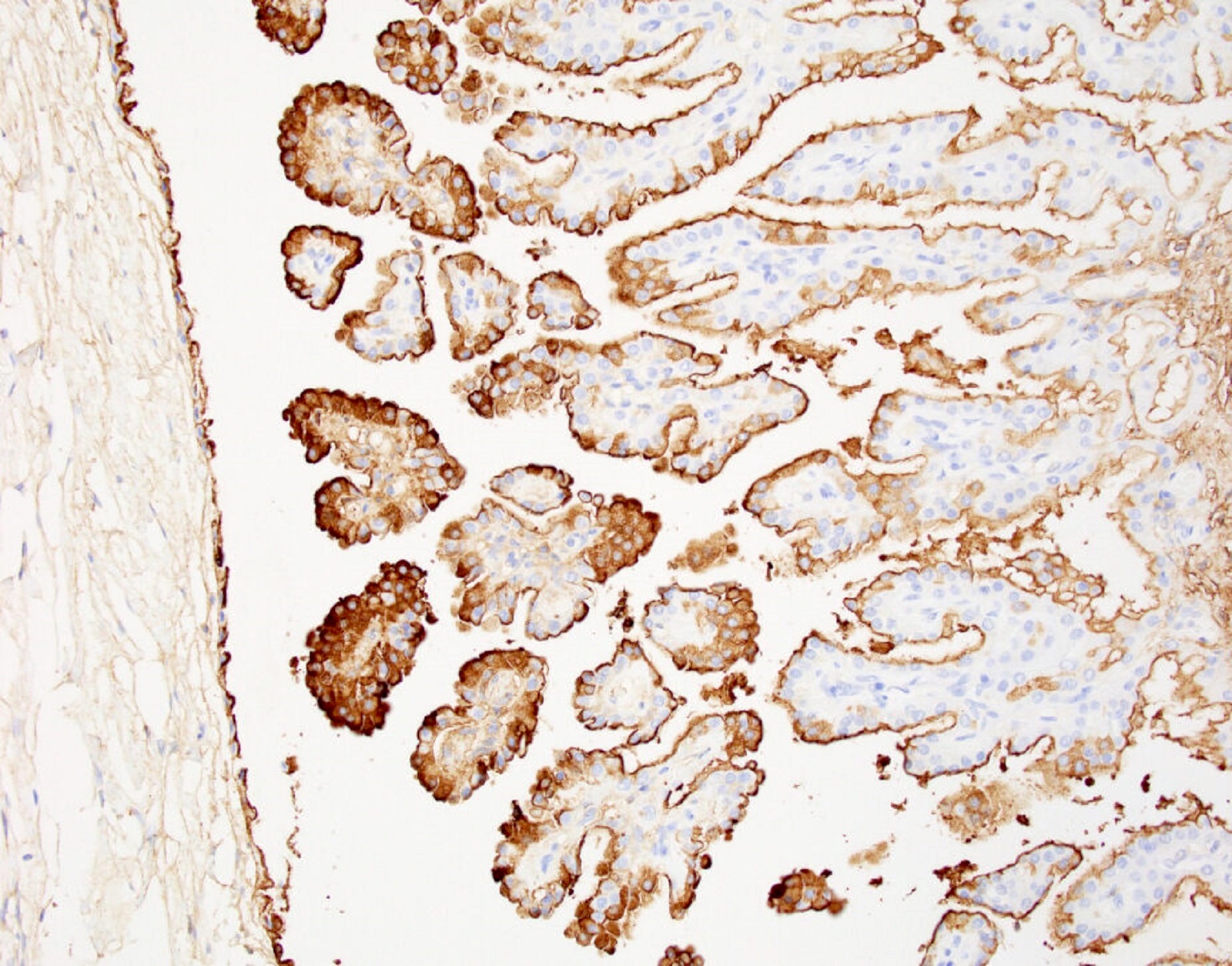
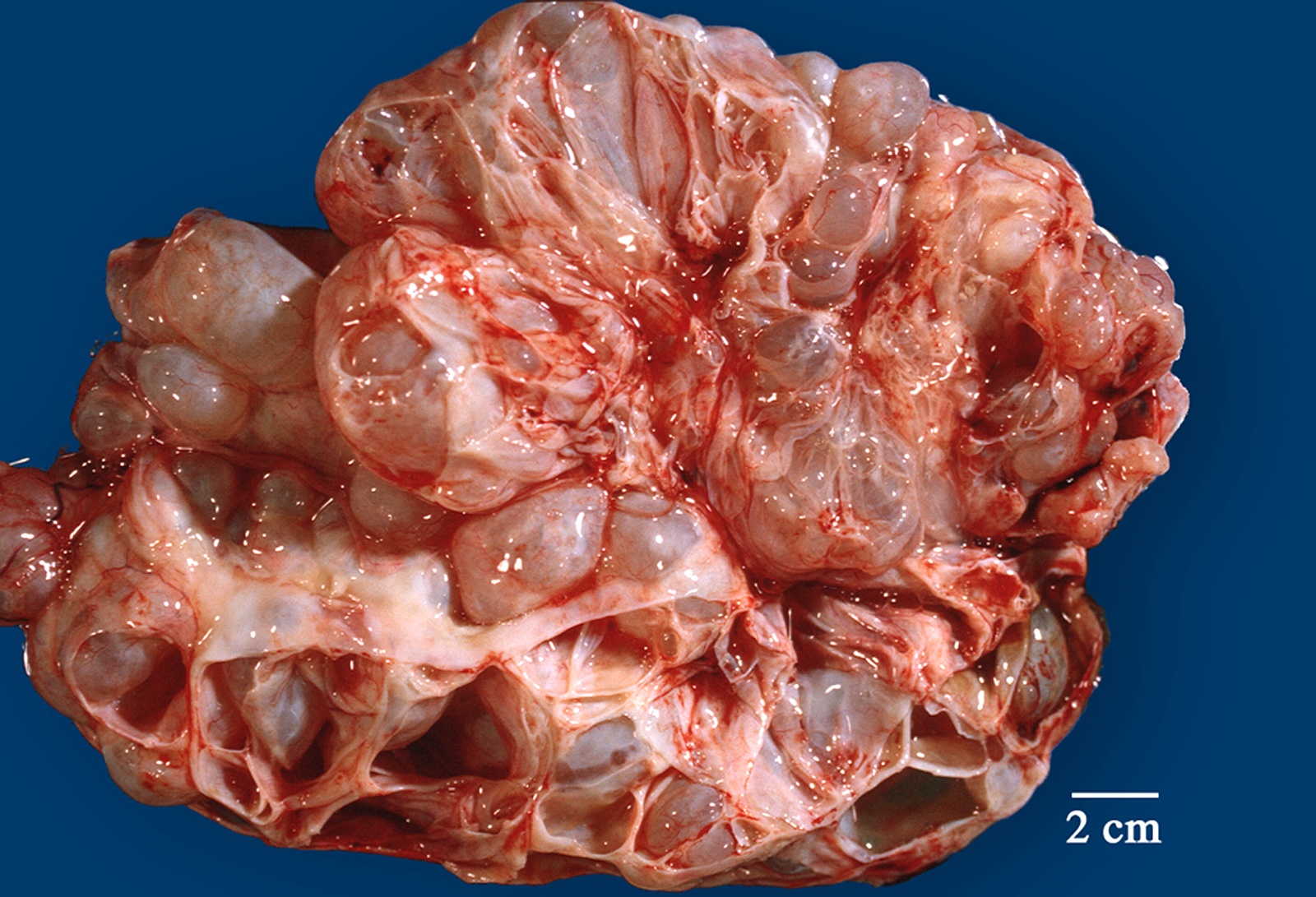
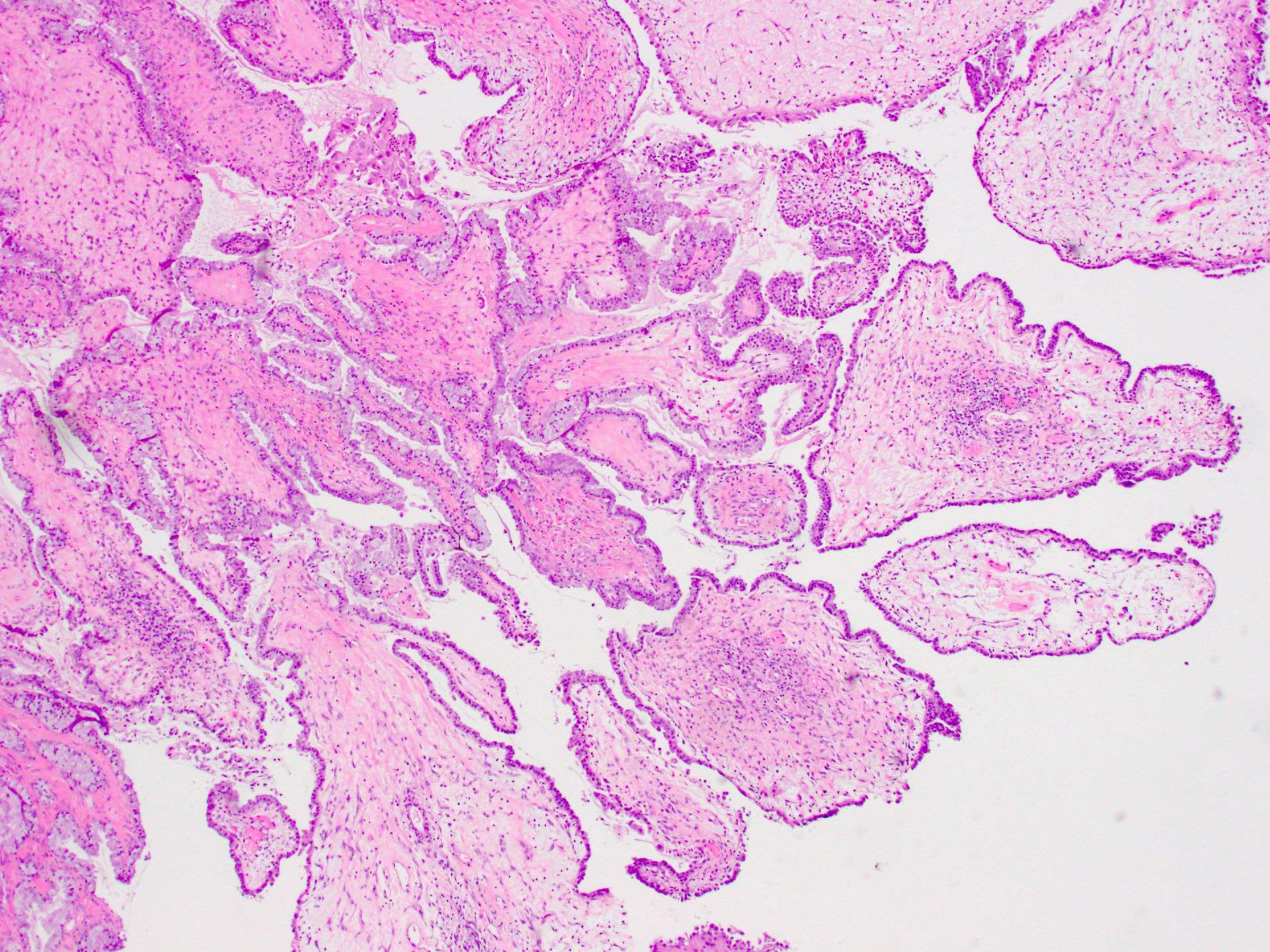
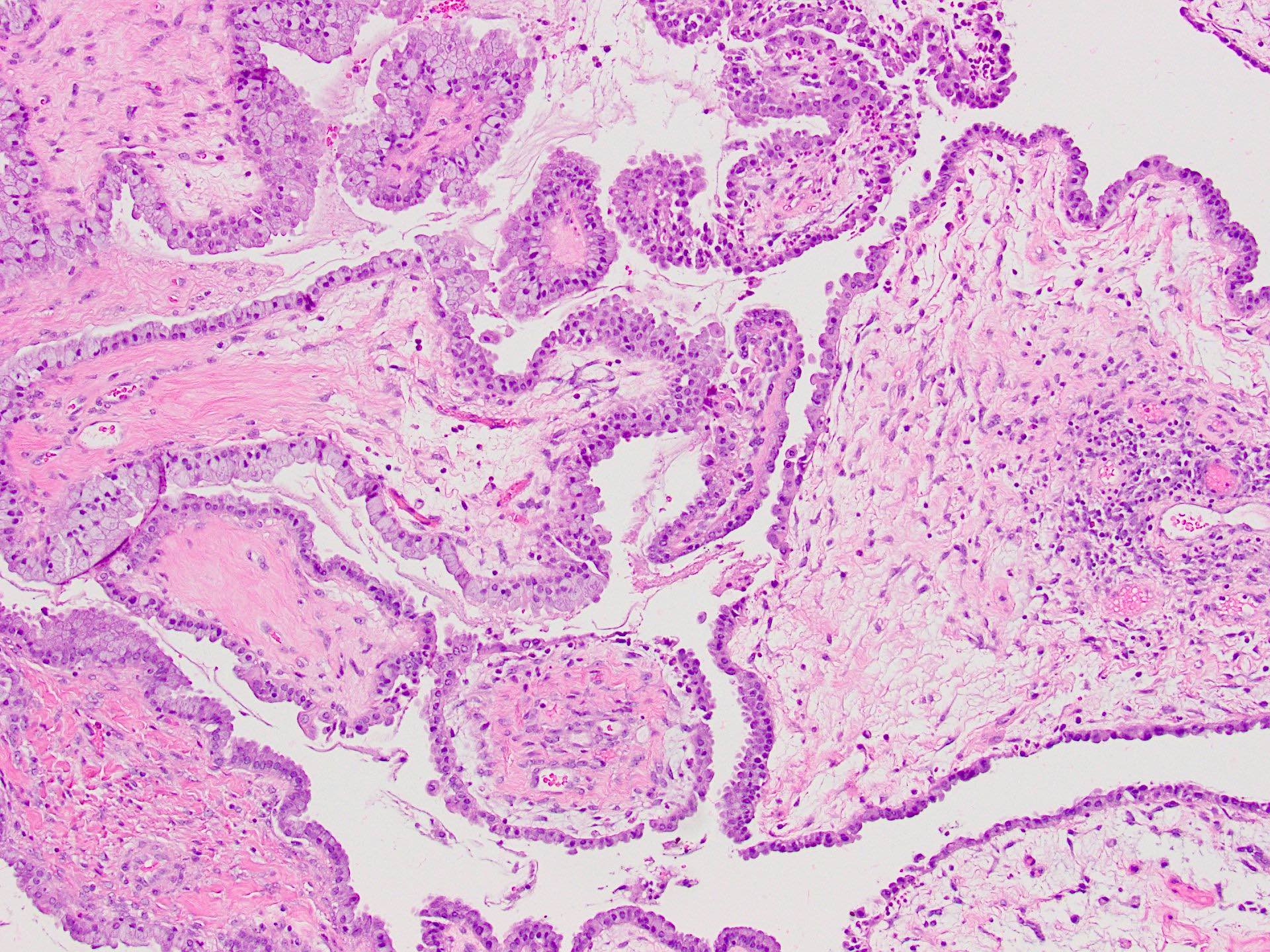
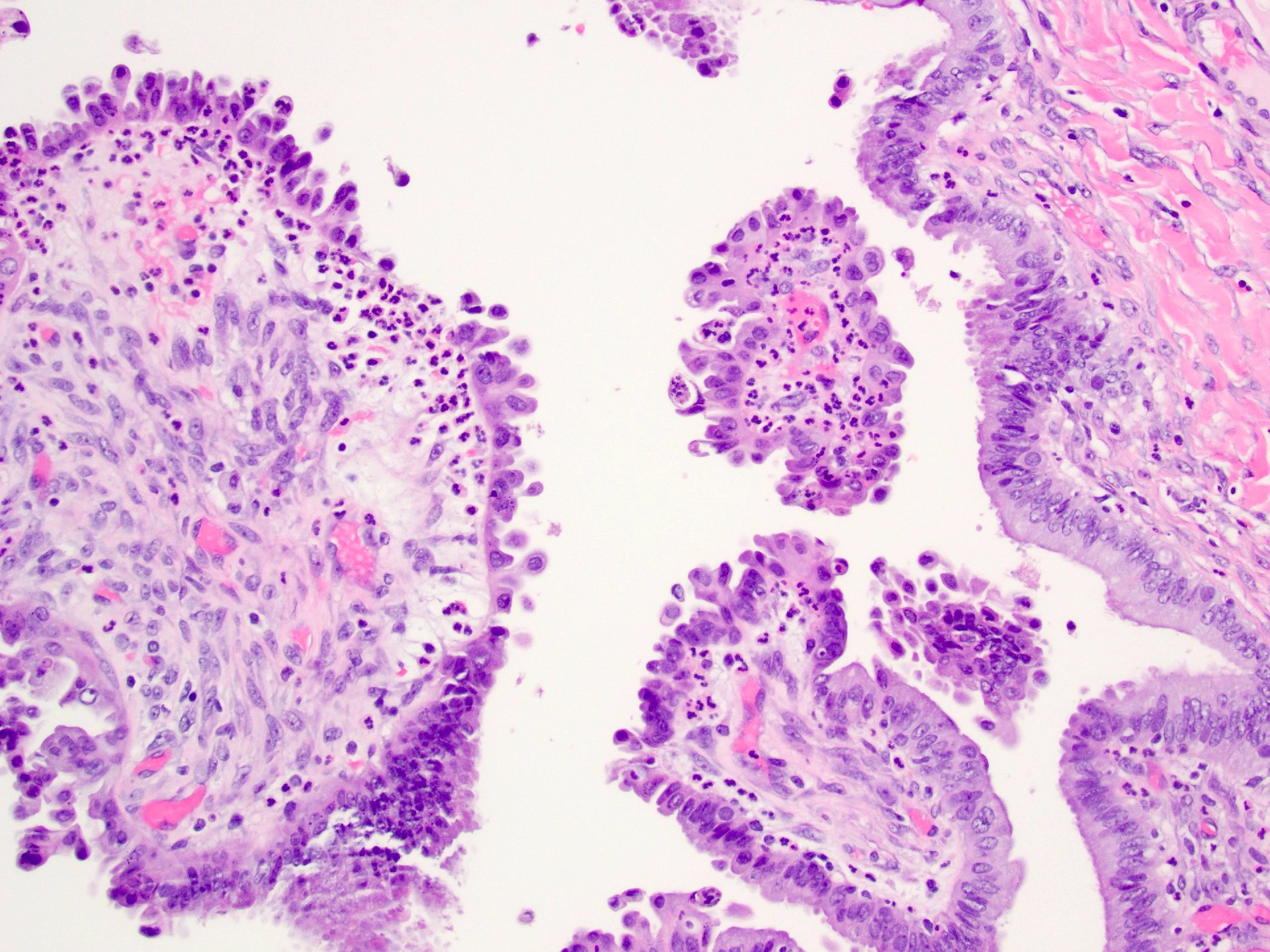
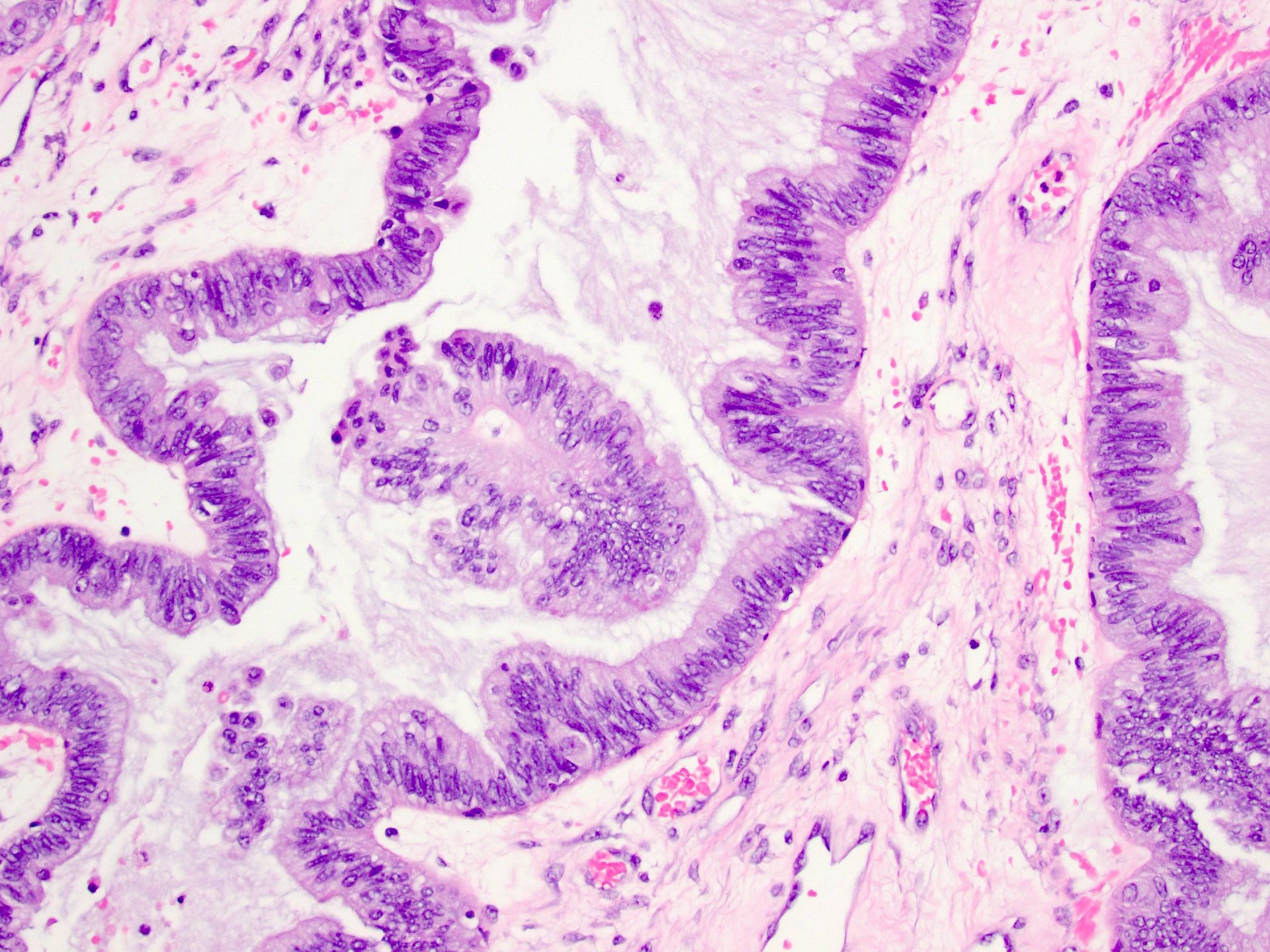
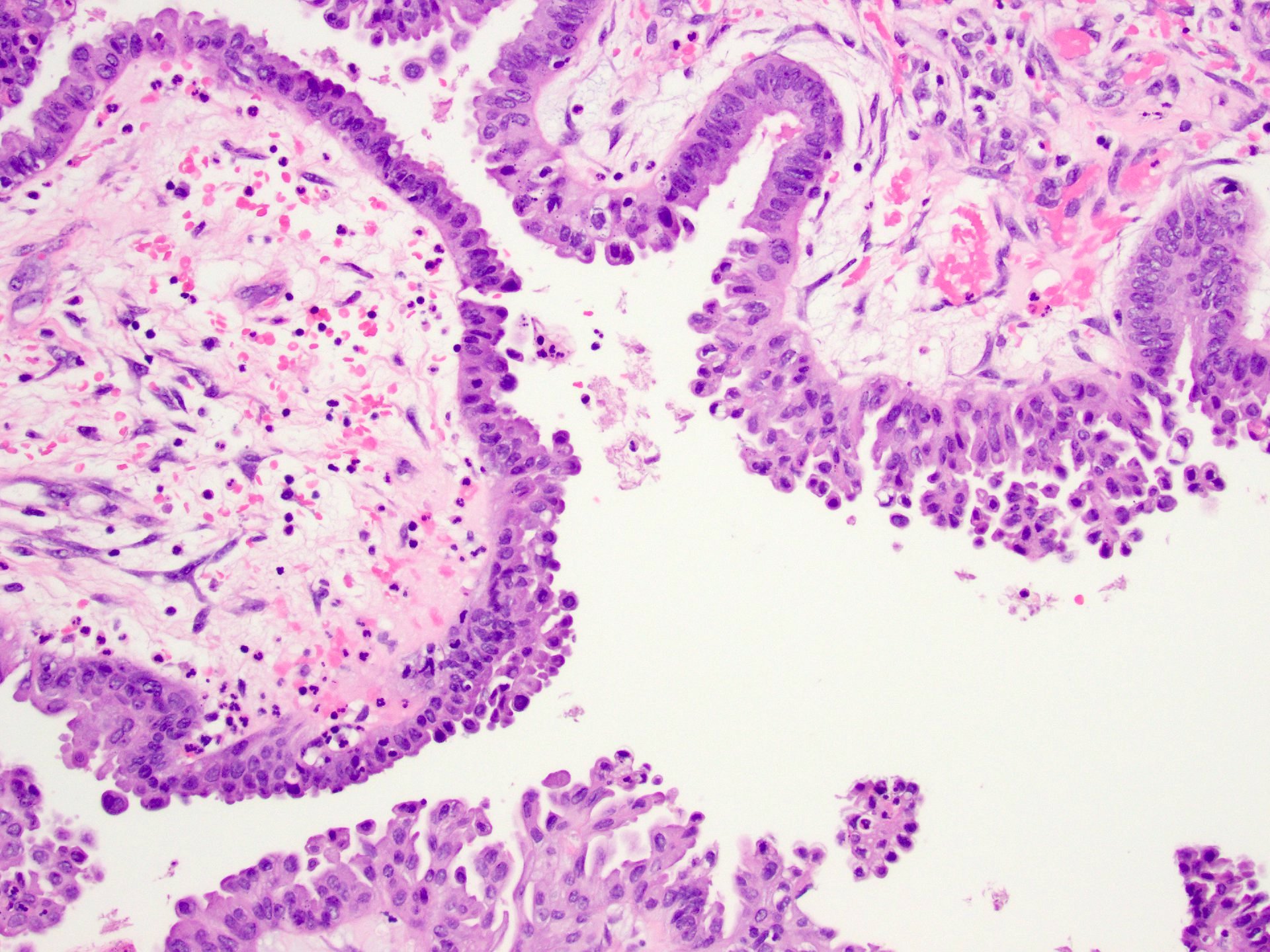
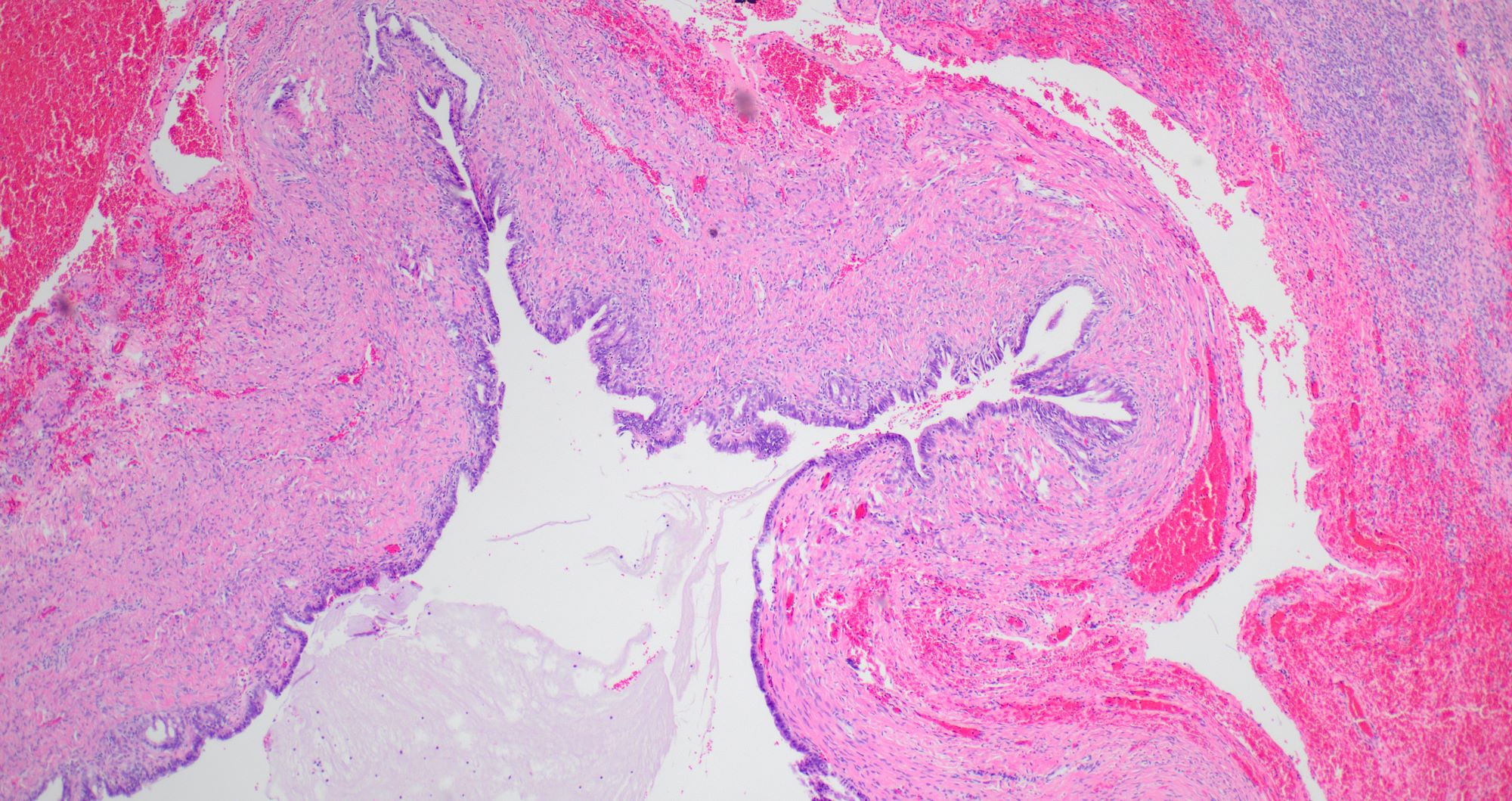
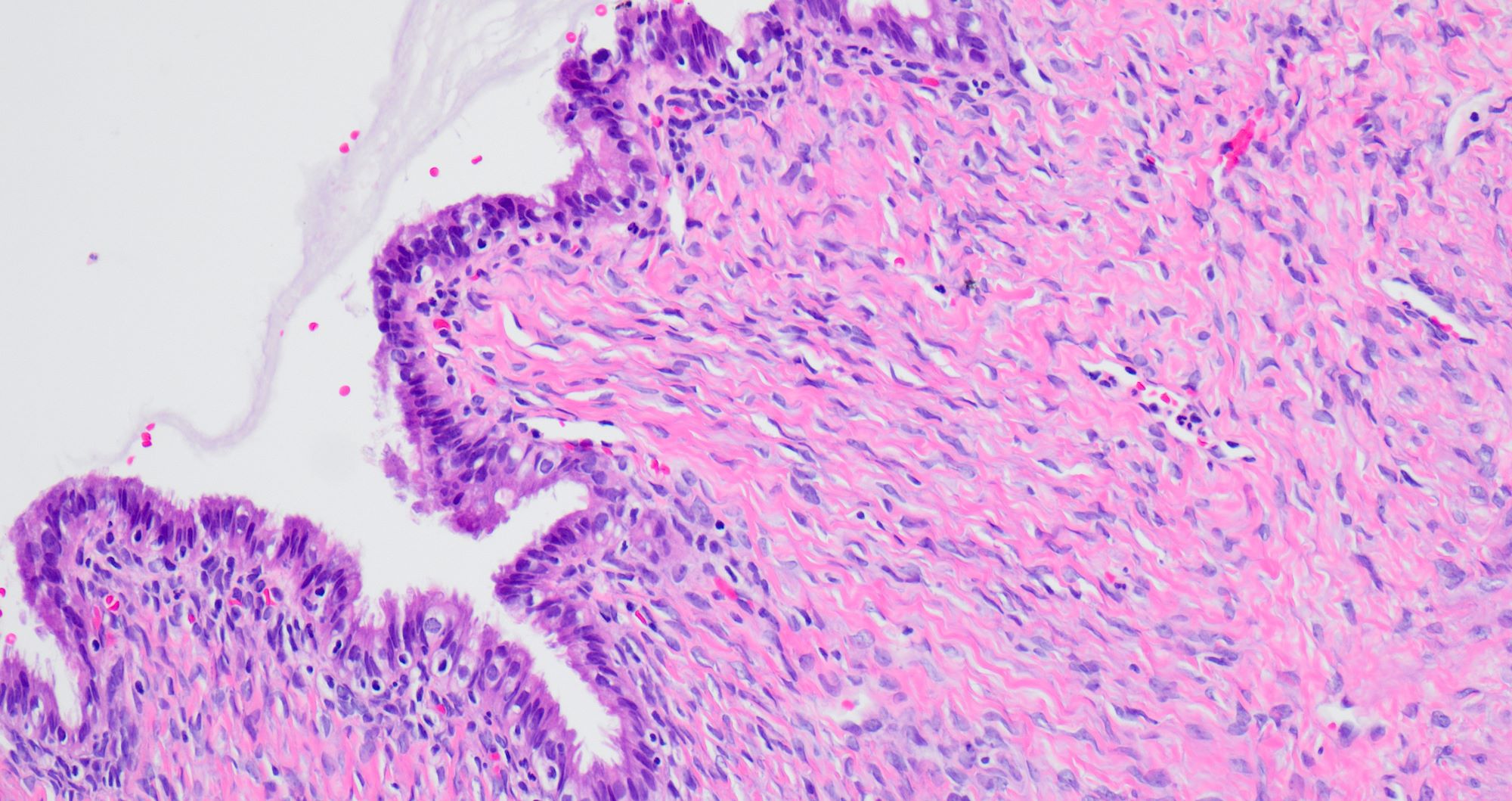
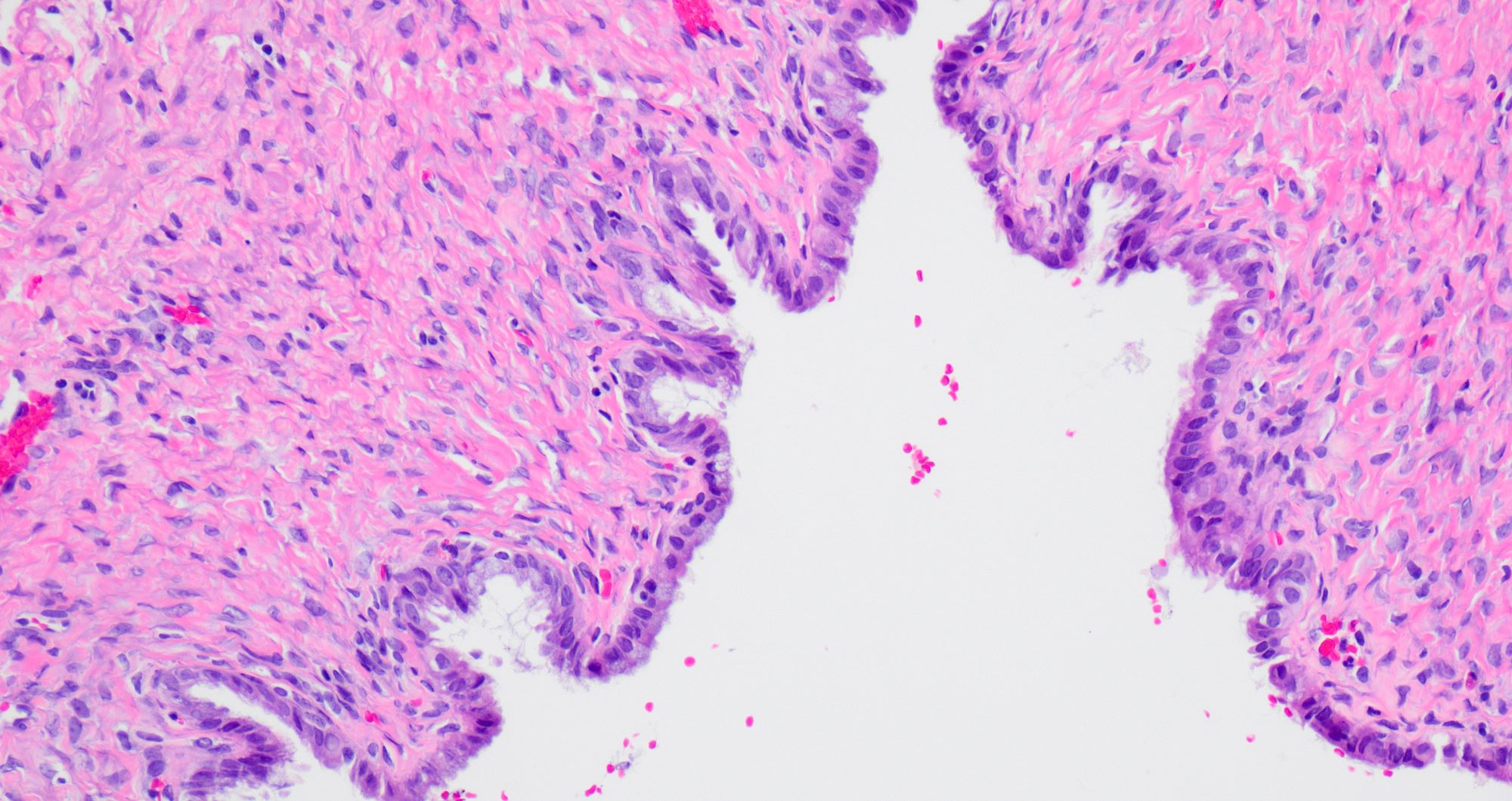
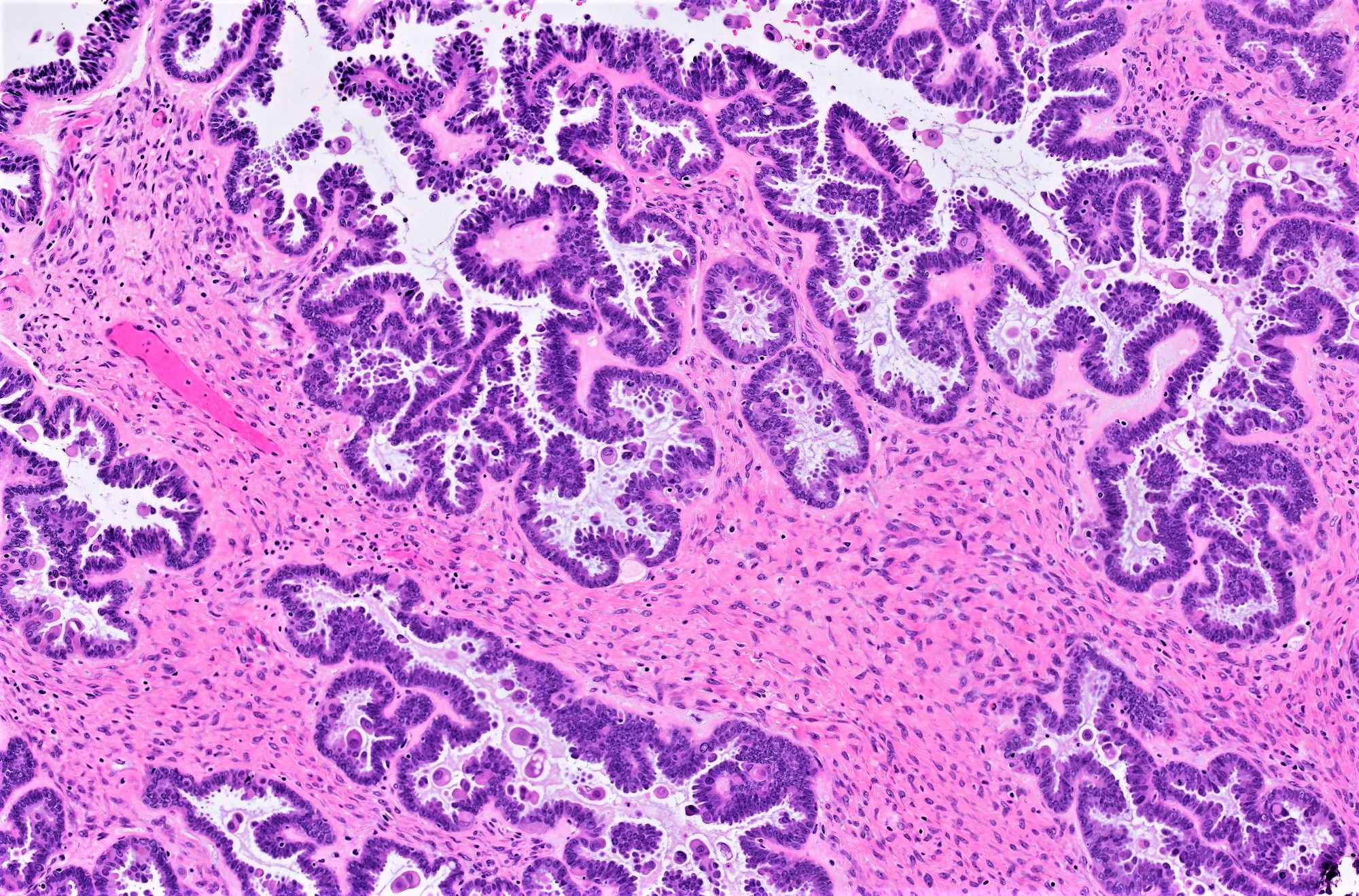
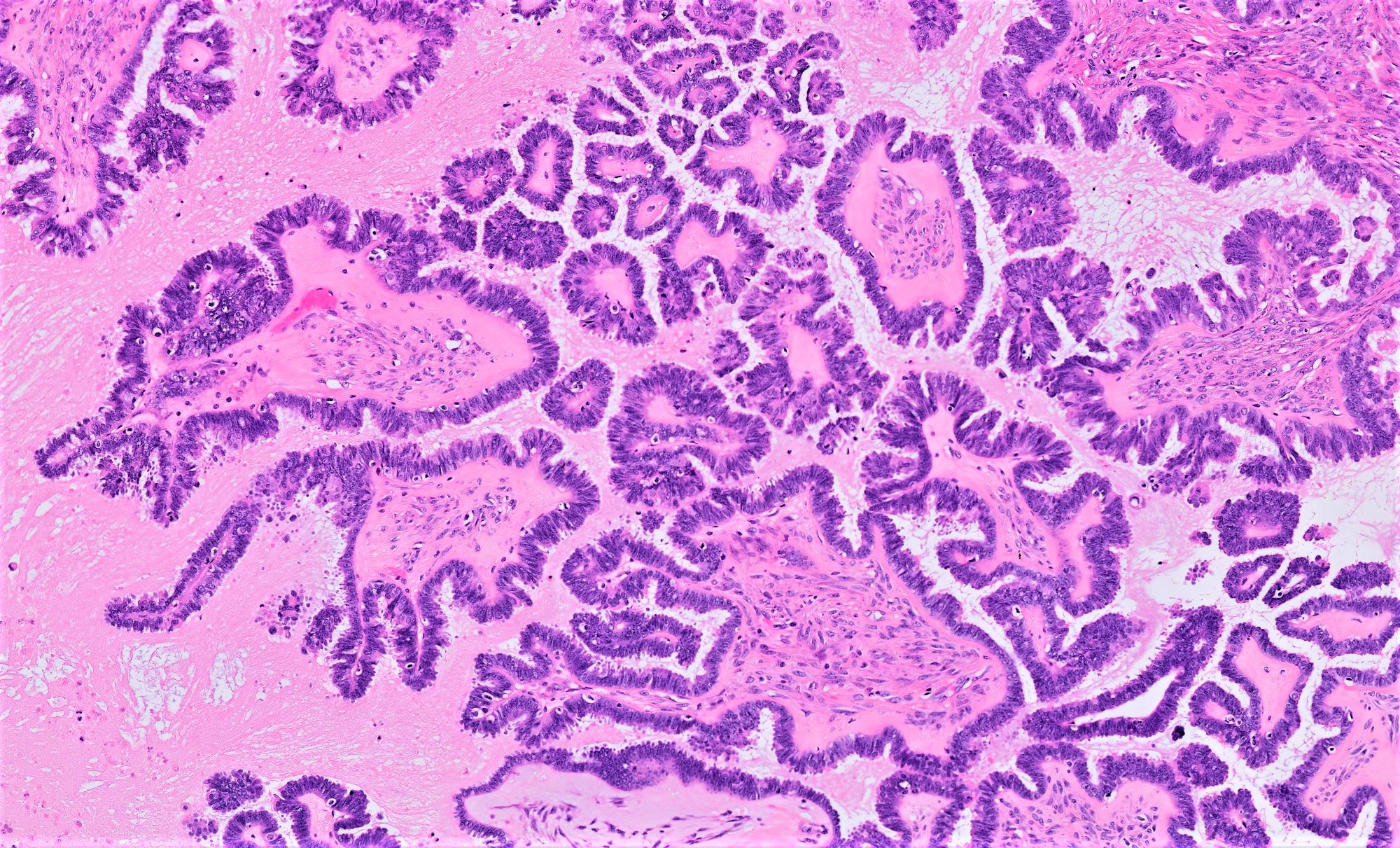
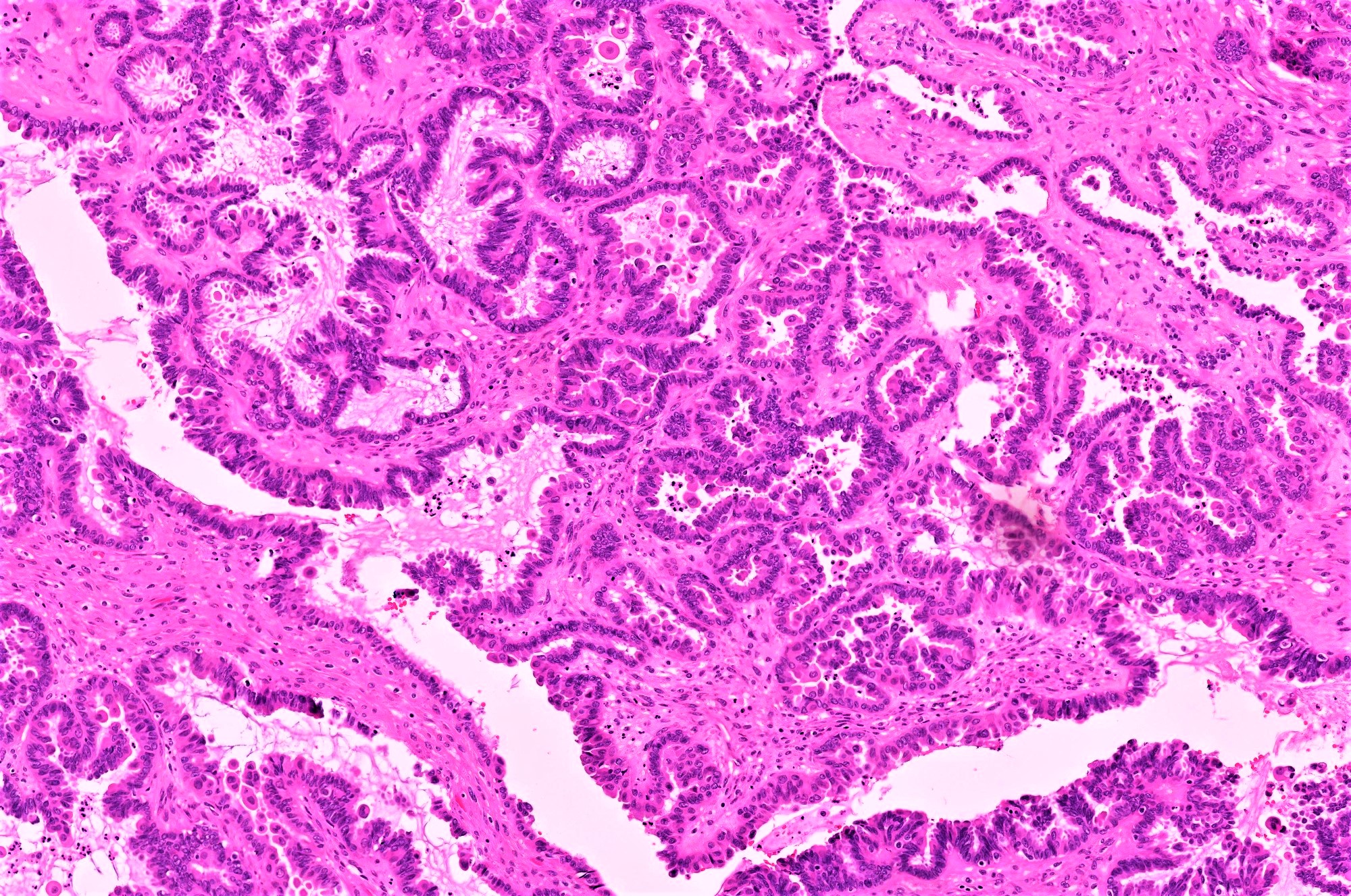
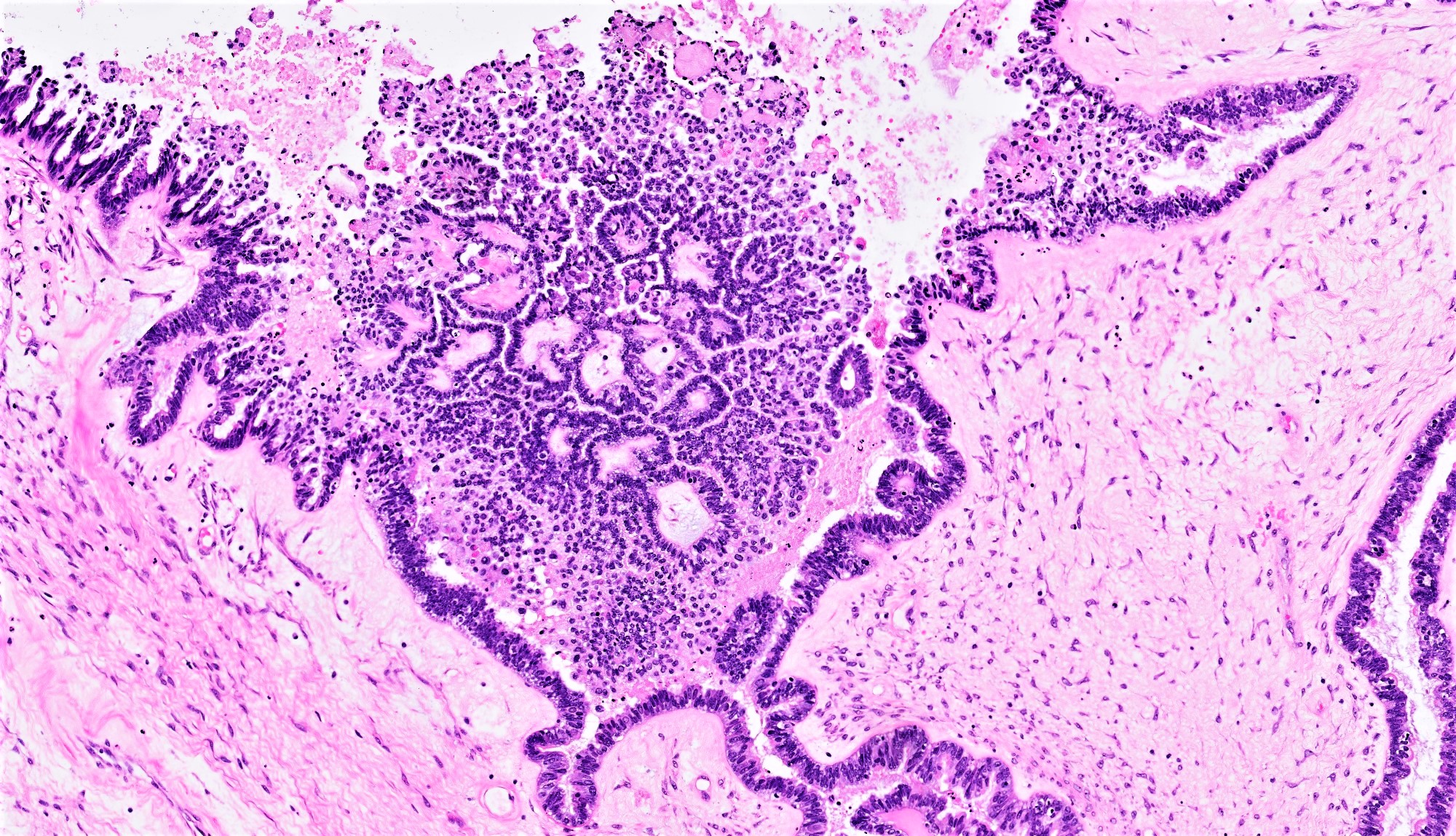
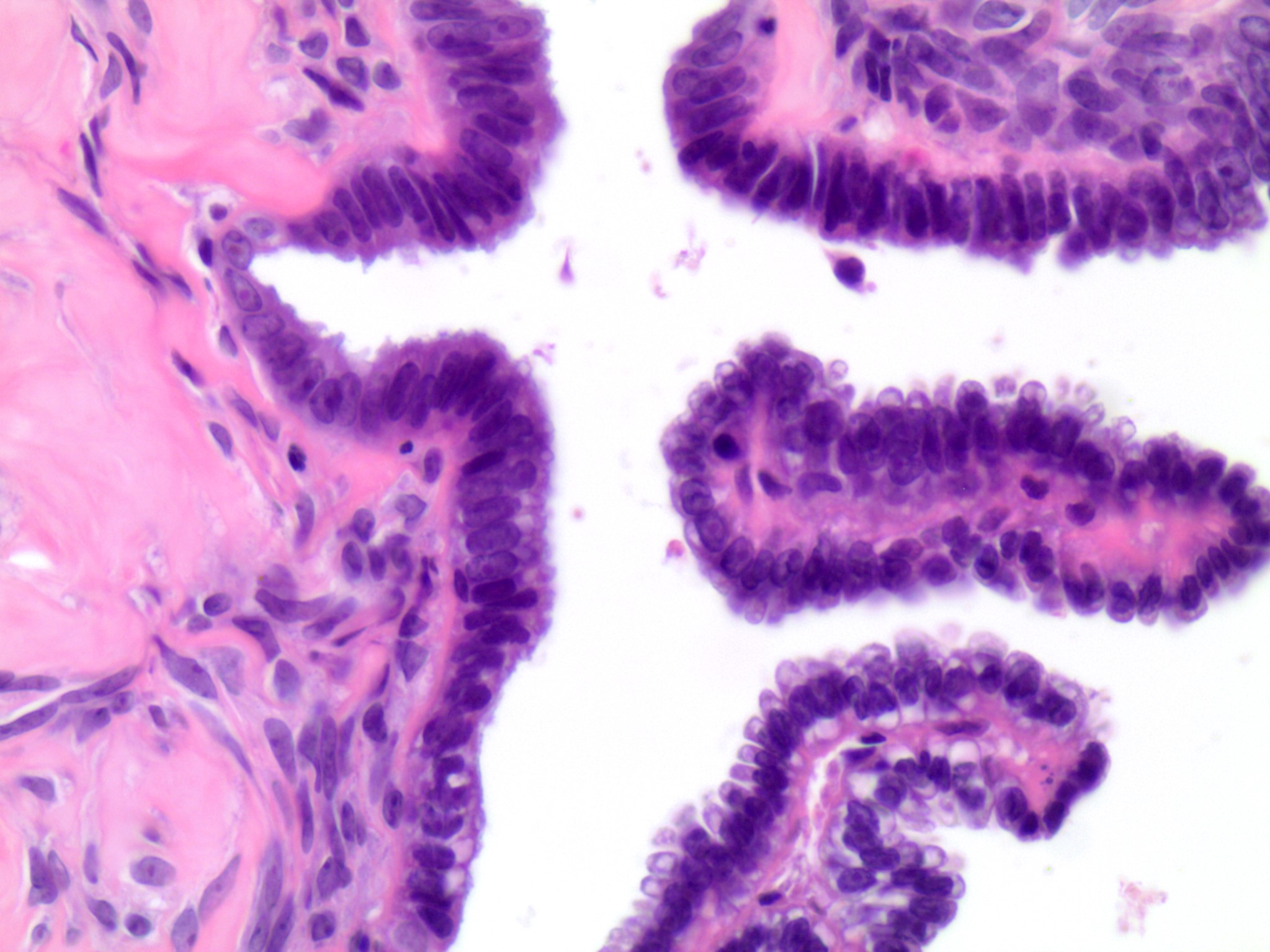
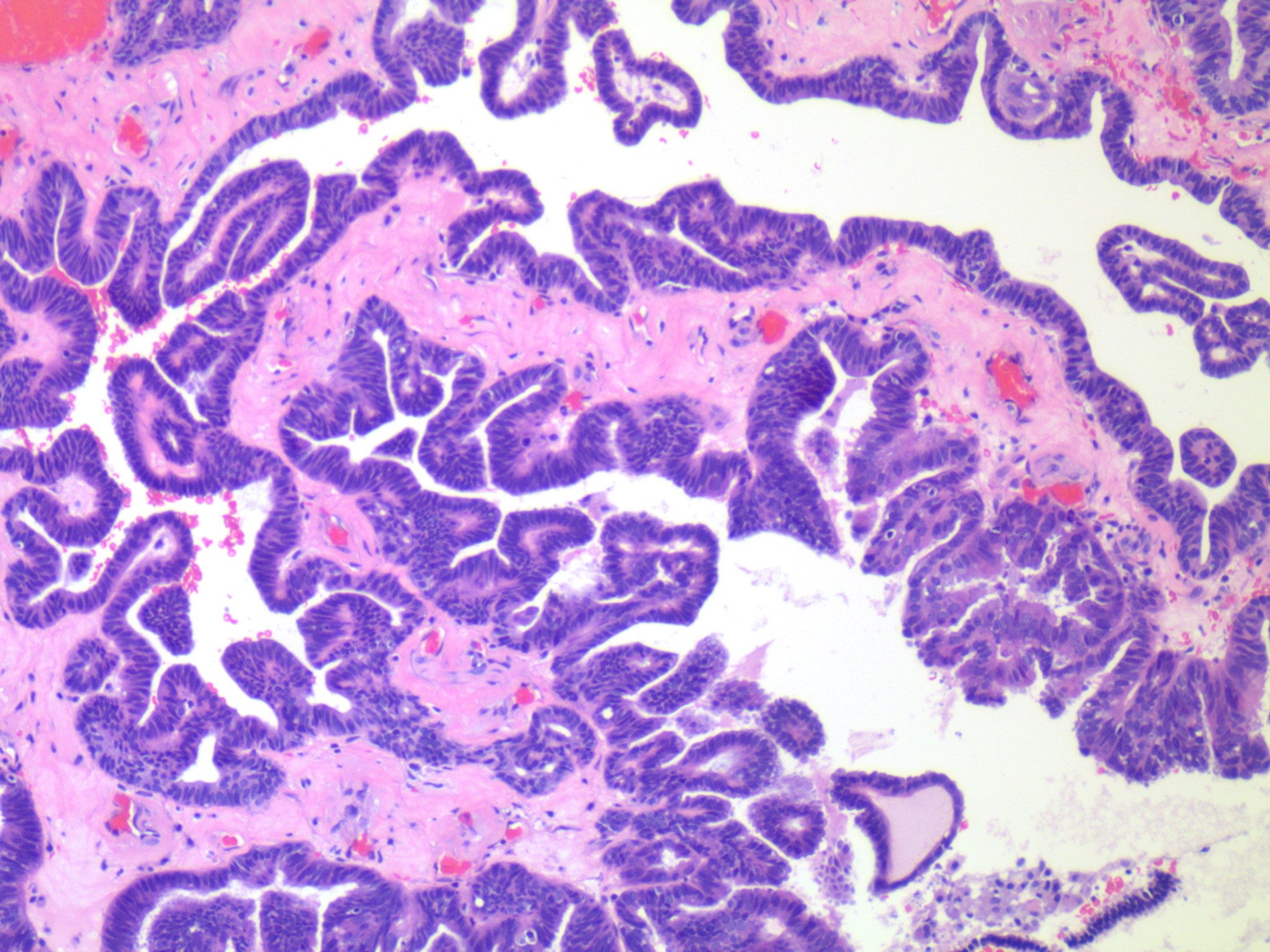
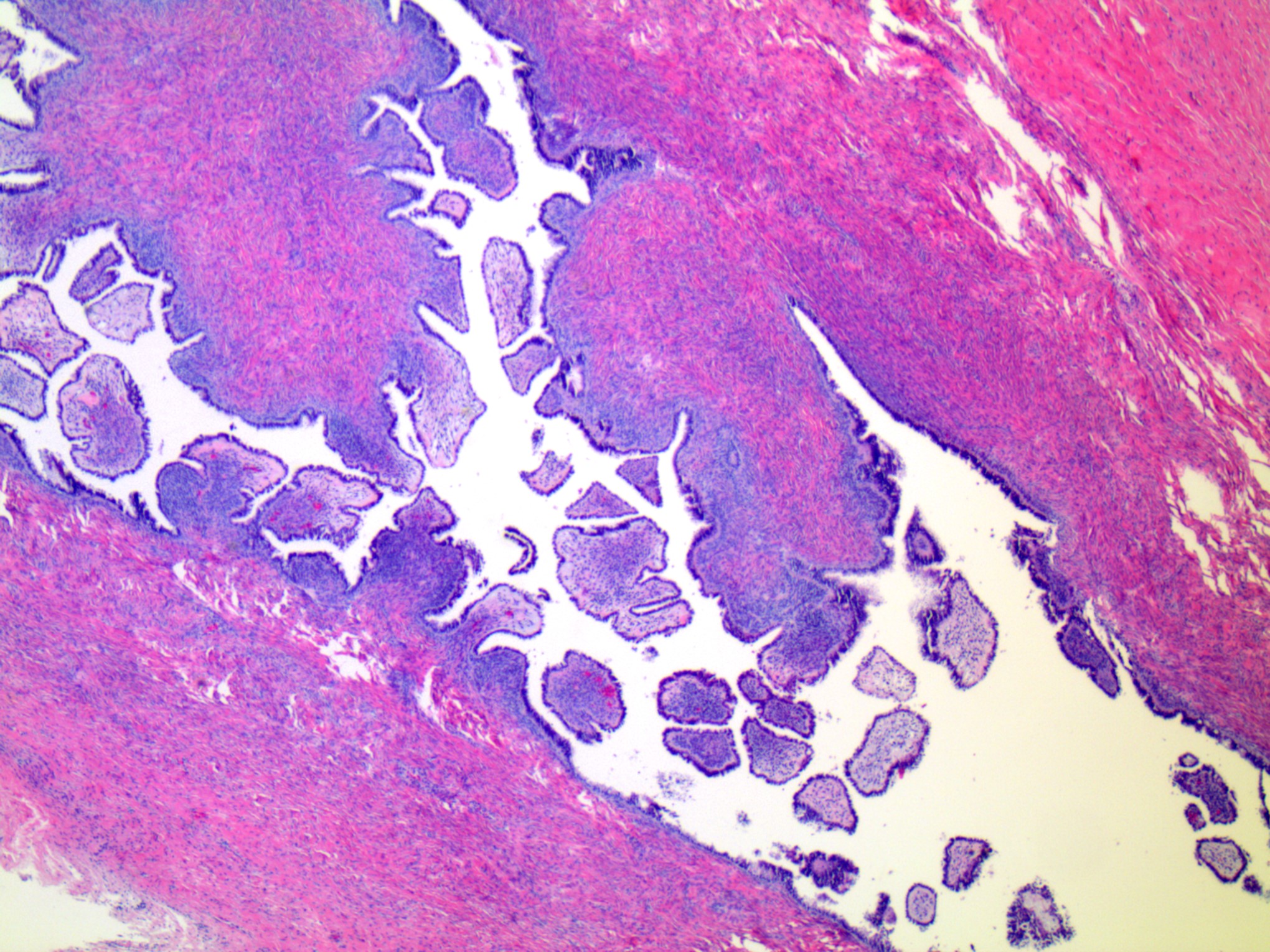
Serous cystadenoma, adenofibroma and surface papilloma
Serous cystadenoma, adenofibroma and surface papilloma
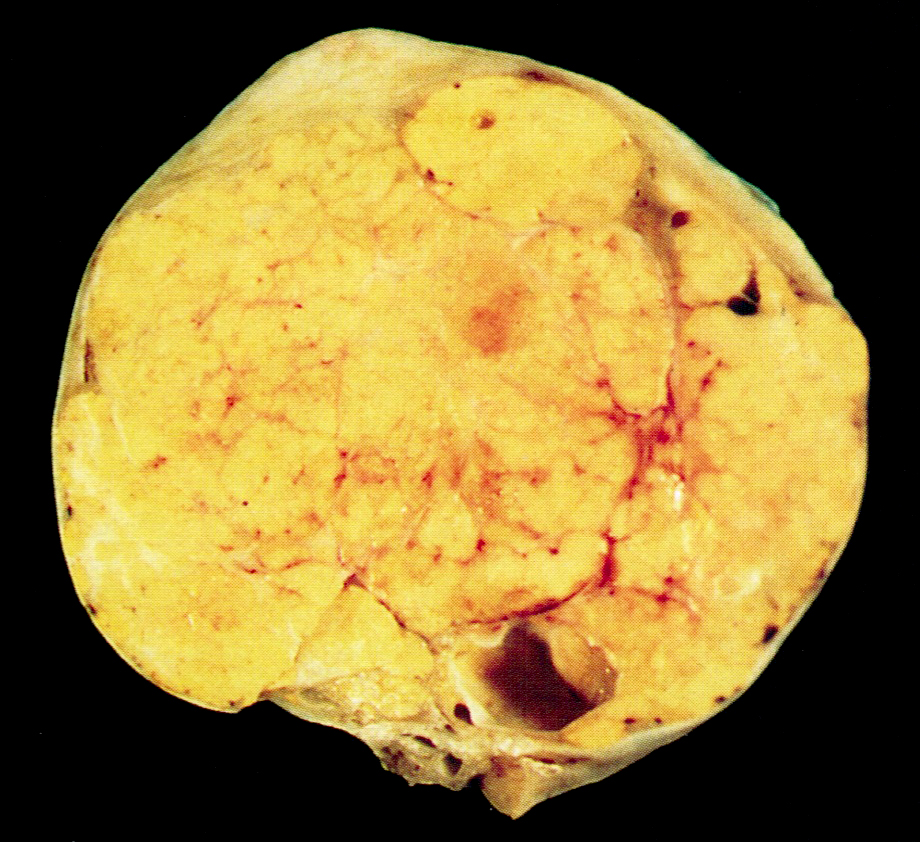
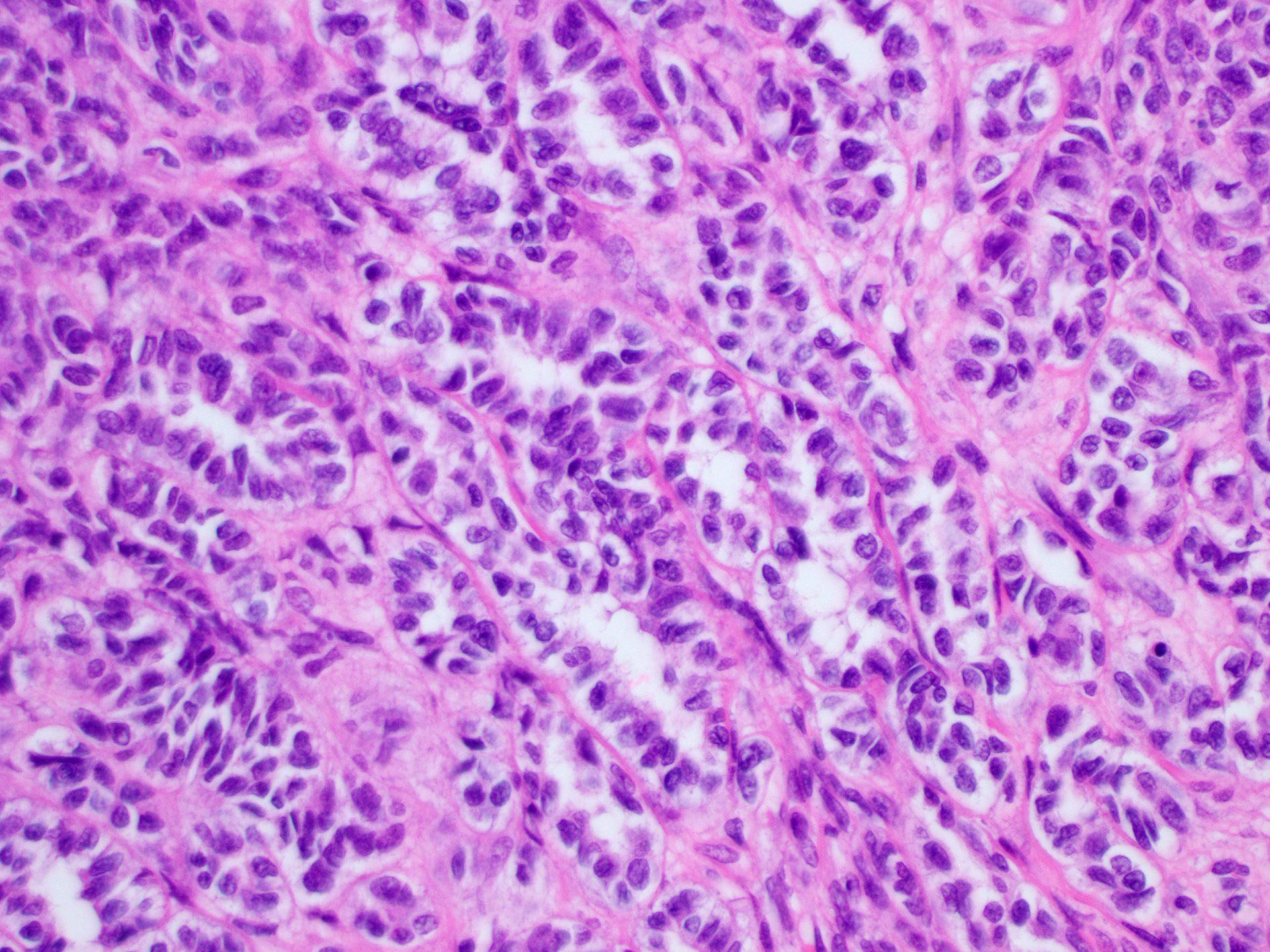
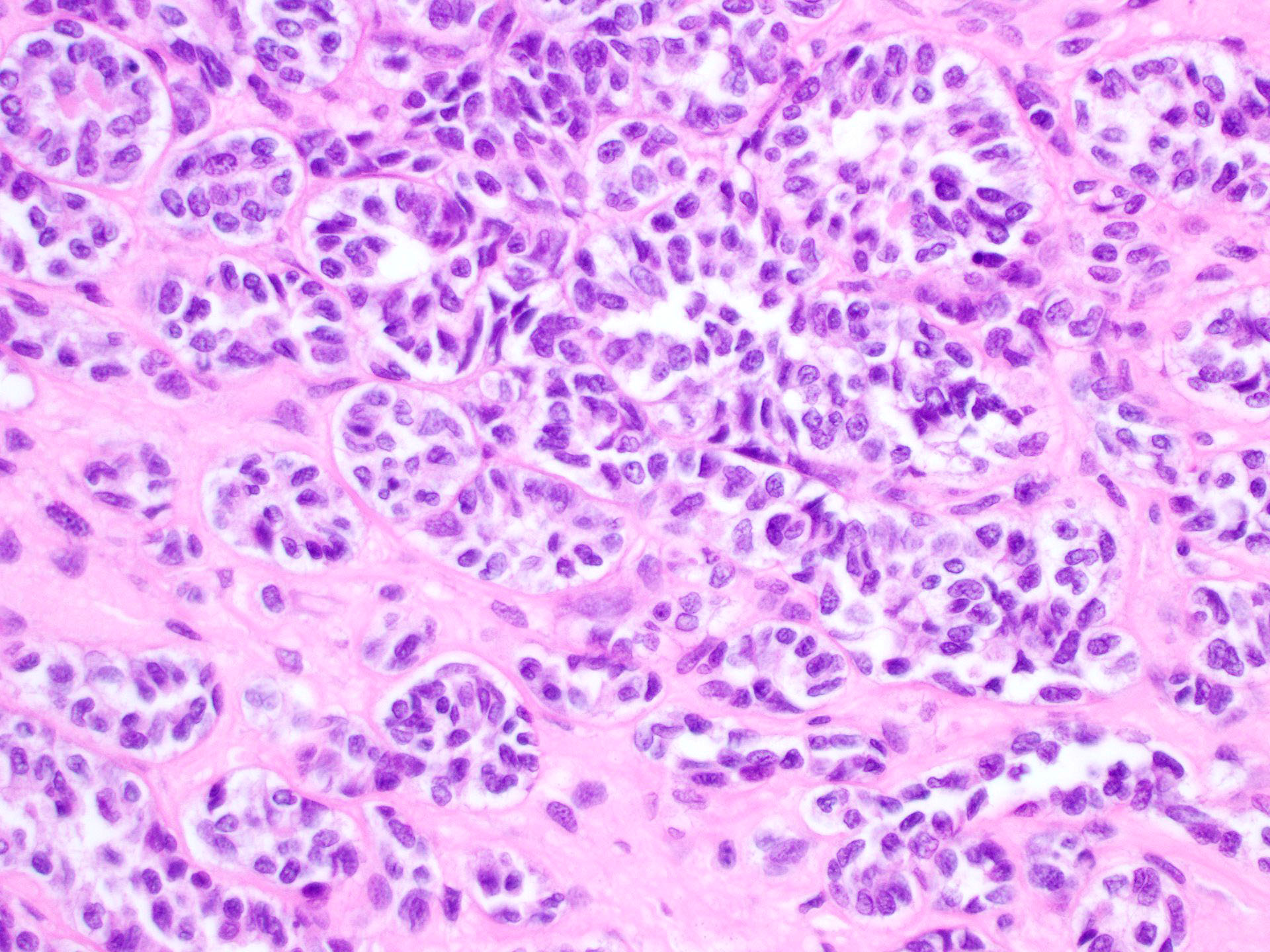
Sertoli cell tumor
Sertoli-Leydig cell tumor
Sex chromosome DSD
Sex cord stromal tumor NOS (pending)
Sex cord tumor with annular tubules
Signet ring stromal tumor
Small cell carcinoma of ovary, hypercalcemic type
Solid pseudopapillary tumor
Somatic neoplasms arising from teratomas
Somatic neoplasms arising from teratomas (pending)
Staging
Steroid cell tumor
Stromal hyperplasia and hyperthecosis
Struma ovarii
Strumal carcinoid
Teratoma-immature
Teratoma-mature
Thecoma
Torsion
Tubo-ovarian abscess
Undifferentiated / dedifferentiated carcinoma (endometrium / ovary)
Upper gastrointestinal tract
WHO classification
Wilms tumor (nephroblastoma)
Xanthogranulomatous oophoritis
Yolk sac tumor46XX DSD
Table of Contents
Female pseudohermaphroditism | Female pseudohermaphroditism associated with congenital adrenal hyperplasia | Female pseudohermaphroditism-nonadrenalFemale pseudohermaphroditism
Definition / general
Terminology
Etiology
Clinical features
Laboratory
Additional references
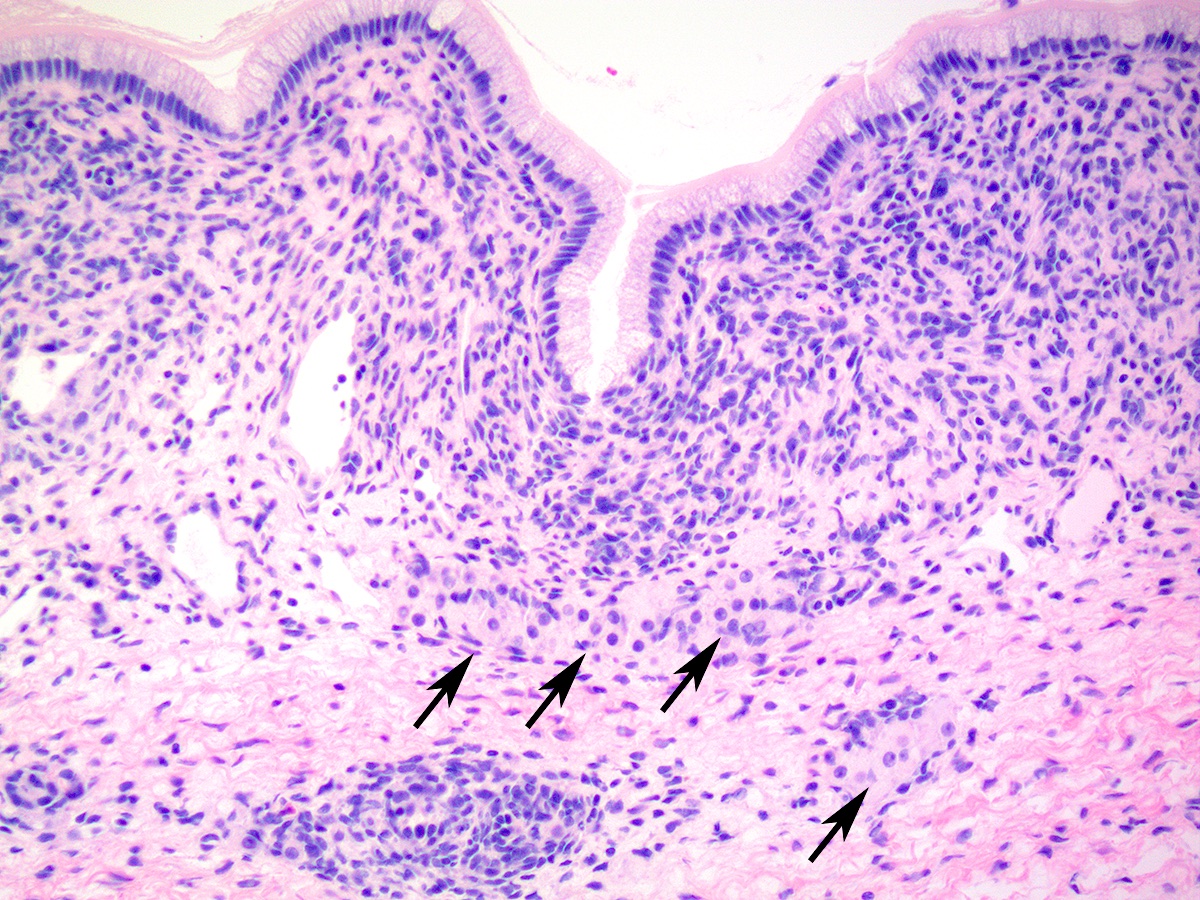
- Disorder of sex development (DSD) with female genotype (46,XX), female internal phenotype (2 ovaries) but variable degrees of virilization
Terminology
- In 2006 the International Consensus Conference on Intersex recommended using the designation "46,XX DSD" instead of the potentially pejorative and confusing term "female pseudohermaphroditism"
Etiology
- Most virilized 46,XX infants have congenital adrenal hyperplasia (CAH)
- Non-CAH etiologies include gestational hyperandrogenism
Clinical features
- In CAH, depending on the site of the steroid biosynthesis defect, there may be under or overproduction of mineralcorticoid, resulting in imbalances in serum electrolytes and blood pressure
Laboratory
- Serum steroid measurements can confirm or rule out congenital adrenal hyperplasia in most 46,XX DSD cases
Additional references
Female pseudohermaphroditism associated with congenital adrenal hyperplasia
Definition / general
Terminology
Epidemiology
Etiology
Diagrams / tables
Images hosted on other servers:
Clinical features
Laboratory
- Most common form of female pseudohermaphroditism in which a female genotype (46,XX) and female internal phenotype (two ovaries) is associated with variable degrees of virilization due to a defect in the steroid biosynthetic pathway
Terminology
- In 2006, the International Consensus Conference on Intersex recommended using the designation "46,XX DSD" to replace the potentially pejorative and confusing term female "pseudohermaphroditism" (Pediatrics 2006;118:e488)
Epidemiology
- CAH is the most common cause of 46,XX DSD
Etiology
- CAH is usually due to 21 alpha hydroxylase or 11 beta hydroxylase deficiency
- There are also other rare mutations (Arg Bras Endocrinol Metabol 2005;49:126)
- Partial 17 alpha hydroxylase / 17,20 lyase deficiency (Gynecol Endocrinol 2008;24:362)
Diagrams / tables
Images hosted on other servers:
Clinical features
- Depending on the site of the steroid biosynthesis defect, there may be under or overproduction of mineralcorticoid, resulting in imbalances in serum electrolytes and blood pressure
- 21 alpha hydroxylase deficiency often leads to hyponatremia, hyperkalemia and hypotension; these patients are at risk for life threatening adrenal crises
Laboratory
- Serum steroid measurements can confirm or rule out CAH in the vast majority of 46,XX DSD cases
- 11 beta hydroxylase deficiency and 3 beta hydroxysteroid dehydrogenase deficiency have characteristic serum steroid patterns
Female pseudohermaphroditism-nonadrenal
Definition / general
Terminology
Epidemiology
Etiology
Clinical features
Laboratory
Case reports
Additional references
- Rarer form of female pseudohermaphroditism in which a female genotype (46,XX) and female internal phenotype (two ovaries) is associated with variable degrees of virilization due to etiologies other than congenital adrenal hyperplasia, including gestational hyperandrogenism (maternal exposure to progestins or androgens)
- 46,XX Barr bodies are chromatin positive
- Ovary with female ducts and variable virilized external genitalia
Terminology
- In 2006, the International Consensus Conference on Intersex recommended using the designation "46,XX DSD" to replace the potentially pejorative and confusing term female "pseudohermaphroditism" (Pediatrics 2006;118:e488)
Epidemiology
- Gestational hyperandrogenism is a rarer cause of 46,XX DSD
Etiology
- May be due to exposure to maternal androgen (maternal luteoma, theca lutein cysts, placental aromatase enzyme deficiency) or synthetic progestational agents
Clinical features
- Diagnosis may be suggested by history of exposure to exogenous progestin or androgen or maternal virilization
Laboratory
- 17 ketosteroids and estrogen levels are normal
Case reports
- With luteoma of pregnancy (Hum Reprod 2002;17:821)
Additional references
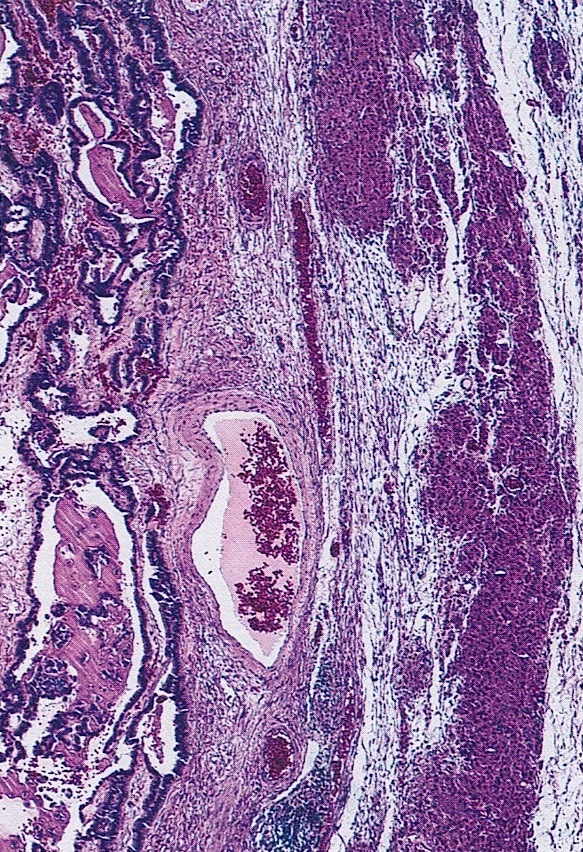
Anatomy & histology
Table of Contents
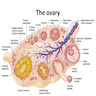
Definition / general | Essential features | Embryology | Physiology | Diagrams / tables | Clinical features | Laboratory | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Negative stains | Videos | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Paired female reproductive glands located on each side of the uterus, adjacent to the lateral pelvic wall, posterior to the broad ligament and anterior to the rectum
- Originate from the genital ridge formed by the thickening of the coelomic epithelium with oogonia later migrating from the yolk sac endoderm (Am J Surg Pathol 1987;11:277)
Essential features
- Paired ovoid organs located on each side of the uterus
- Responsible for the development of the dominant follicle and the production of hormones
- Multiple histologic components can be seen in the normal ovaries and can mimic pathologic findings
Embryology
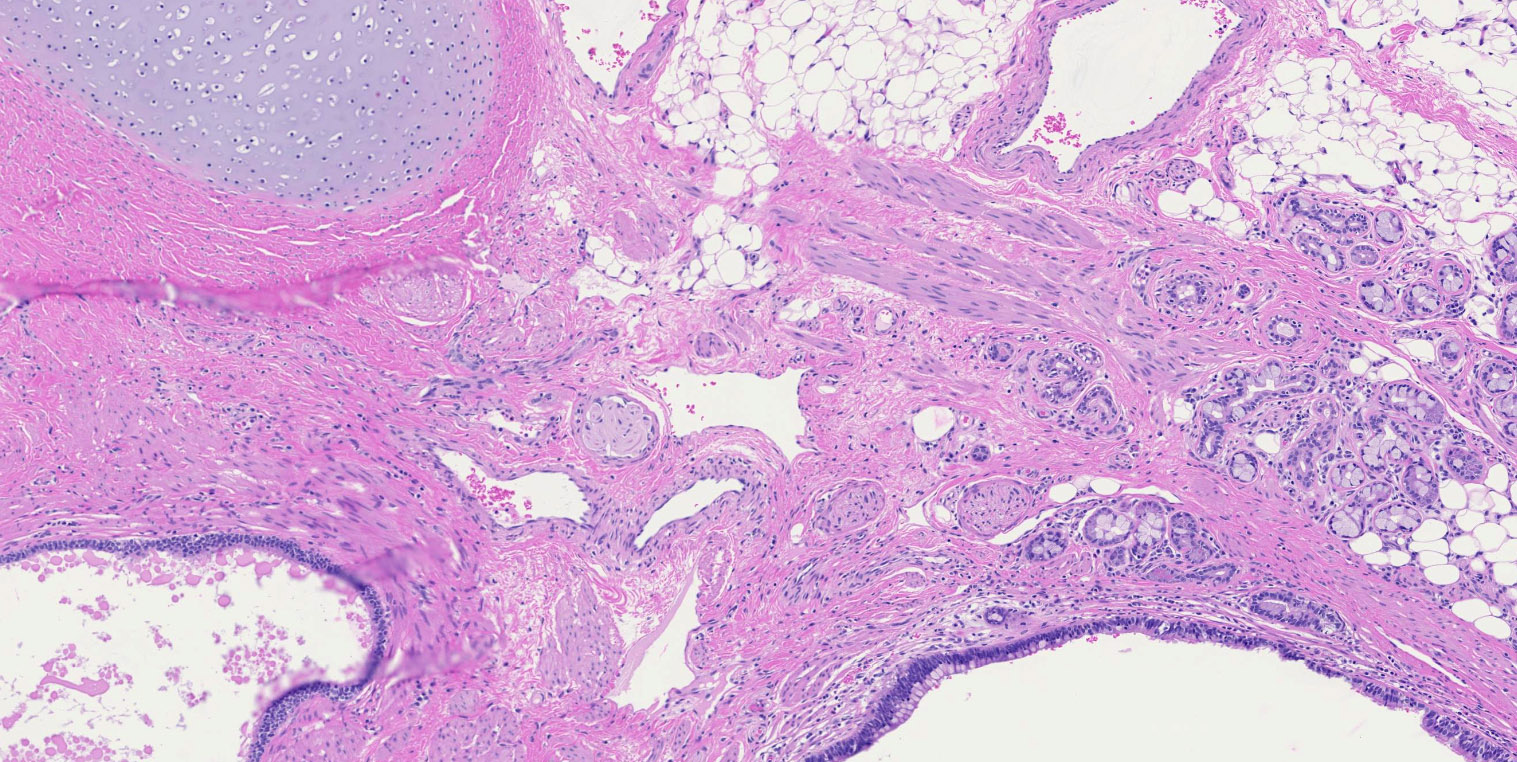
- Ovary is composed of 4 main components, each with different embryologic origins, all of which eventually come together in the developed ovary: surface epithelium, stroma, germ cells and sex cords
- Embryonic coelomic cavity is initially lined by primitive mesothelium (modified coelomic epithelium), which arises from the underlying mesoderm
- At ~5 weeks gestation, the gonadal ridge develops as a coelomic thickening and mesenchymal growth high in the abdominal cavity near where the kidneys develop
- Coelomic epithelium forms the ovarian surface epithelium
- Subcoelomic mesoderm forms the ovarian stroma
- Primordial germ cells migrate from the yolk sac endoderm to the developing ovary
- Invaginations of coelomic epithelium in the superficial ovarian cortex form the sex cords (pregranulosa cells)
- During weeks 12 - 20, the vascular network develops from the hilus towards the cortex and pregranulosa cells encircle germ cells to form primordial follicles, which in turn are encircled by stroma derived theca cells
- Germ cells differentiate into oogonia, which differentiate into primary oocytes and arrest in this stage until puberty
- Each ovary descends into the pelvis along the gubernaculum (attached inferiorly to the inguinal region); the gubernaculum becomes part of the uterine wall at entry of the fallopian tube and persists in adults as ovarian and round ligaments
- Invaginations of the coelomic epithelium at the site of the gonadal ridge also give rise to two types of genital ducts: the mesonephric (Wolffian) ducts and the paramesonephric (Müllerian) ducts
- Müllerian ducts eventually give rise to the fallopian tubes and fuse to form the uterus
- Wolffian (mesonephric) duct remnants may be seen in the vicinity of the Müllerian system later in life
- Gonadal development is influenced by both male and female promoting signals (Mol Endocrinol 2008;22:1)
- References: Wikipedia: Gonadal Ridge [Accessed 26 December 2023], Embryology: Human Embryology [Accessed 26 December 2023]
Physiology
- Function first described by Reinier de Graaf (Arch Pathol Lab Med 2000;124:1115)
- Ovaries play an essential role in the fertility and cycling of reproductive activity in women, mainly by controlling the development of the dominant follicle and producing hormones (estrogen and progesterone) (Endotext: Morphology and Physiology of the Ovary [Accessed 9 September 2021])
- Folliculogenesis starts by recruiting primordial follicles into growing follicles that eventually proceed either to ovulation or death
- Folliculogenesis is divided into preantral (growth and differentiation of follicles) and antral phase (increase in the follicle size)
- Folliculogenesis will result in the production of a single dominant follicle (Graafian follicle), which will eventually ovulate
- ~400,000 primordial follicles containing primary oocytes are present at birth in ovarian stroma (100,000 at gestational age of 15 weeks; 680,000 at 8 months) (Fertil Steril 2007;88:675)
- Follicular decay appears to advance with increasing age; prominent cystic follicles are present at birth and at puberty (Hum Reprod 2008;23:699)
- Germ cells travel from yolk sac endoderm to ovary where they develop into oogonia and oocytes, arresting at prophase of mitosis
- Ovulation: induces cyclic rupture and regenerative repair of the ovarian surface epithelium
- Following ovulation, the dominant follicle becomes the corpus luteum responsible for the production of progesterone during the luteal phase of each menstrual cycle or becomes the corpus luteum of pregnancy to sustain gestation
- Hormones produced by the dominant follicle are essential for the preparation of the uterus for implantation of the embryo (Endotext: Morphology and Physiology of the Ovary [Accessed 9 September 2021])
- Hilus cells produce steroids (predominantly androstenedione); resemble Leydig cells of testis; may produce masculinizing tumors (hilus cell tumors)
Diagrams / tables
Clinical features
- Characteristic streak gonads in patients with Turner syndrome, also known as congenital ovarian hypoplasia, due to monosomy X (45,XO); these are not functional and will subsequently manifest with symptoms of ovarian failure (J Minim Invasive Gynecol 2015;22:S15)
- Primary ovarian insufficiency: a condition diagnosed in women less than 40 years of age and characterized by depletion or dysfunction of the ovarian follicles with subsequent impaired ovarian function (N Engl J Med 2009;360:606)
Laboratory
- Tests that evaluate the ovarian function usually done in the context of primary or secondary amenorrhea or infertility problems (Am Fam Physician 2013;87:781)
- These tests include but are not limited to
- Measurement of follicle stimulating hormone (FSH) and luteinizing hormone (LH) levels
- Estrogen production: serum 17 beta estradiol level
- Serum progesterone level
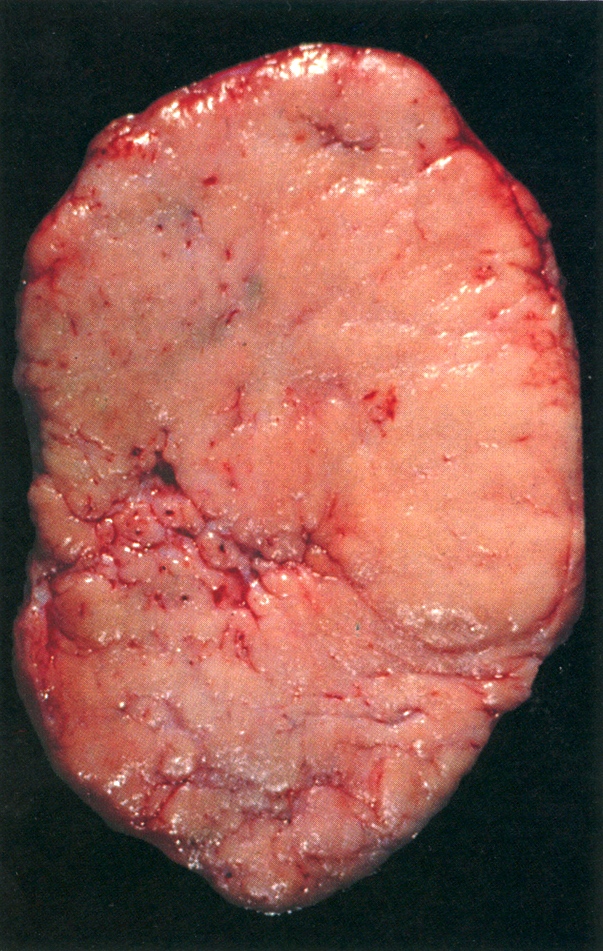
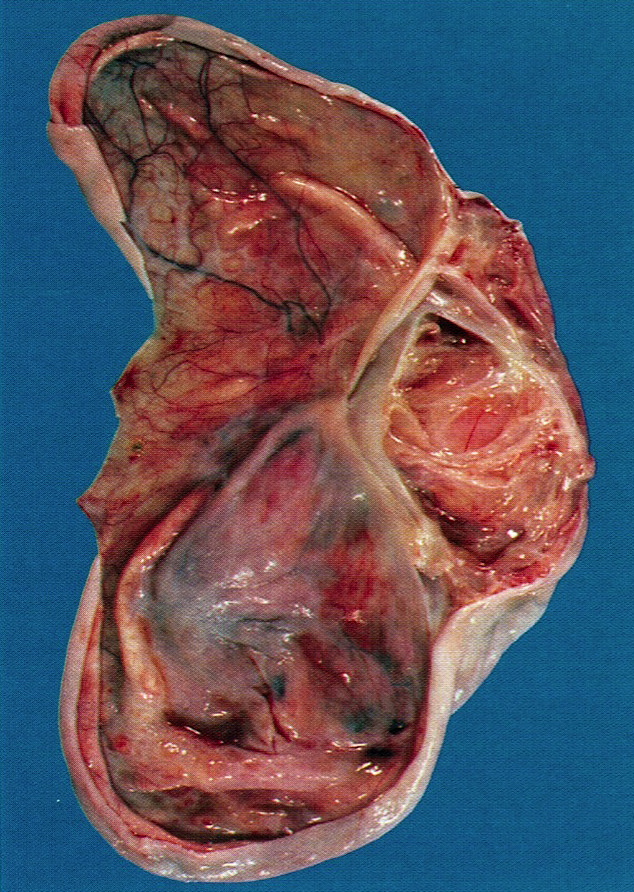
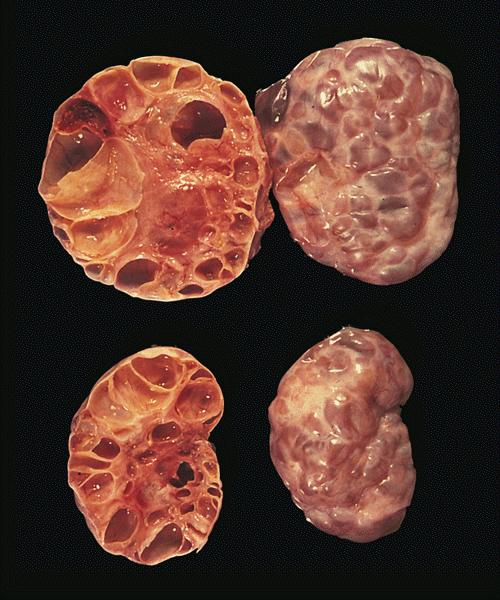
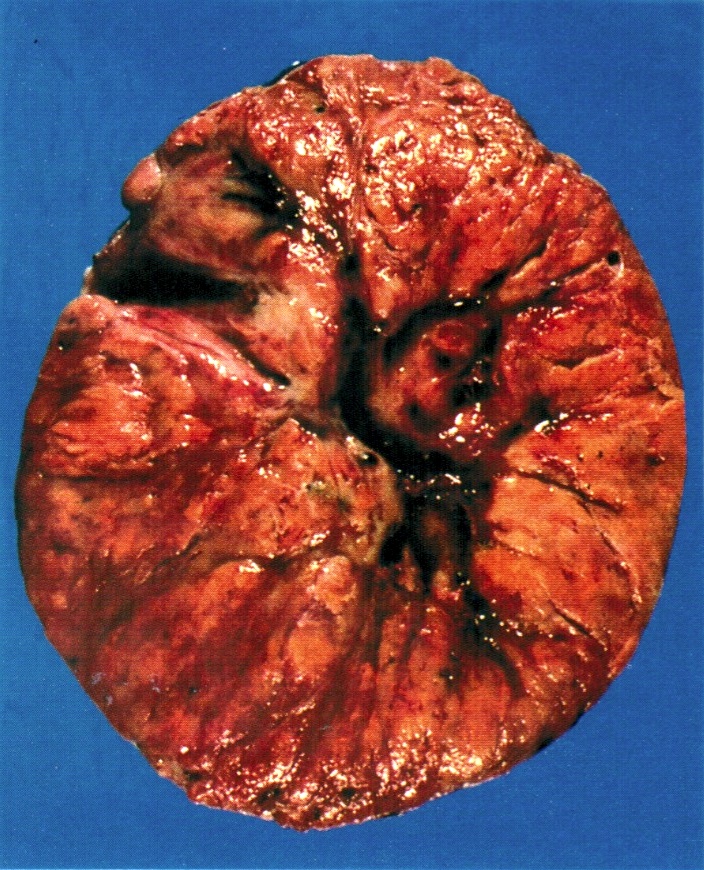
Gross description
- Attachment:
- Along its anterior (hilar) margin to posterior aspect of broad ligament by mesovarium (double fold of peritoneum)
- At its medial pole to ipsilateral uterine cornu by utero-ovarian ligament
- At superior aspect of lateral pole to lateral pelvic side wall by infundibulopelvic (suspensory) ligament
- Laterality in a hysterectomy and bilateral salpingo-oophorectomy specimen established based on the
- Utero-ovarian ligament, which connects each ovary to the ipsilateral uterine cornu and is situated posterolateral and inferior to the attachment of the fallopian tubes
- Posterior peritoneal reflection, which extends over a longer segment of the uterus compared to the anterior surface, which has a longer roughened area
- Appearance of the ovary depends widely on the age / menopausal state:
- Prepubertal ovaries:
- Newborn ovaries are elongated and approximately 1.3 cm in greatest dimension
- Ovaries enlarge during infancy and childhood and reach adult size and shape by the time of puberty
- Neonatal ovaries often have cysts, which resolve spontaneously (J Pediatr Endocrinol Metab 2007;20:397)
- Premenopausal ovaries:
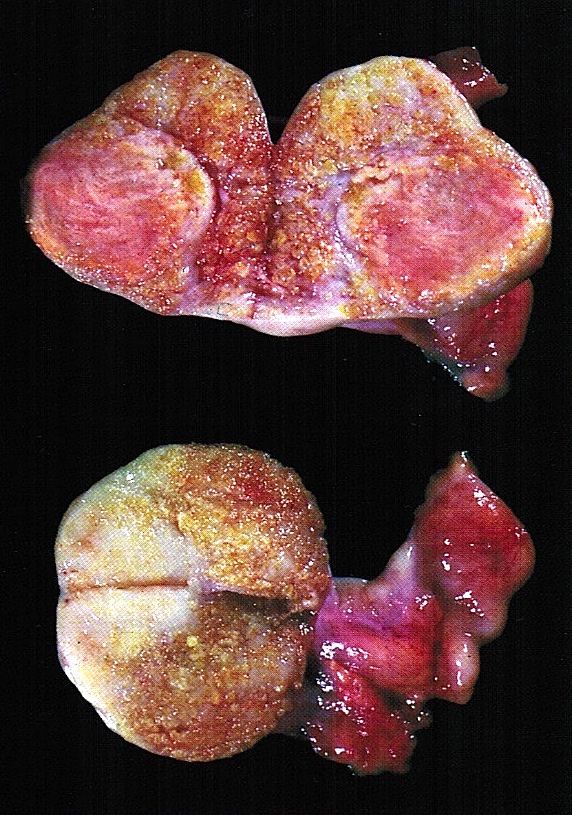
- 3 - 5 cm long and weigh 5 - 8 g; size and weight depend on the amount of follicular derivatives (cysts and corpora albicantia / lutea)
- Pink-white exterior is initially smooth but gradually becomes more convoluted
- Cystic follicles and corpora lutea may be visible from outside
- Cut section may exhibit 3 zones: cortex, medulla and hilus, with follicular derivatives usually in the cortex and medulla
- Postmenopausal ovaries:
- Firm consistency with solid, pale cut surface
- Occasional cysts measuring several millimeters in diameter (inclusion cysts) may be discernible within the cortex
- Small white scars (corpora albicantia) are typically present within the medulla
- Thick walled blood vessels may be appreciable within the medulla and the hilus
- Prepubertal ovaries:
- Blood supply and drainage:
- Arterial supply: approximately 10 arterial branches from anastomotic arcade of ovarian artery (branch of aorta) and ovarian branch of uterine artery penetrate hilus into medulla and cortex
- Venous drainage: left ovarian vein drains to left renal vein, right ovarian vein drains to inferior vena cava
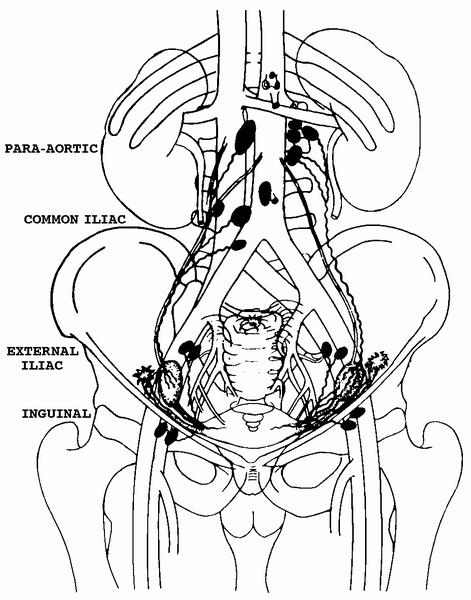
- Lymphatic drainage: originates predominantly from theca layer of follicles, exiting through the hilus, to the mesovarium, along the infundibulopelvic ligament, into upper paraaortic lymph nodes; may bypass to internal iliac, external iliac, common iliac, sacral, obturator, pelvic, retroperitoneal or inguinal nodes
Gross images
Frozen section description
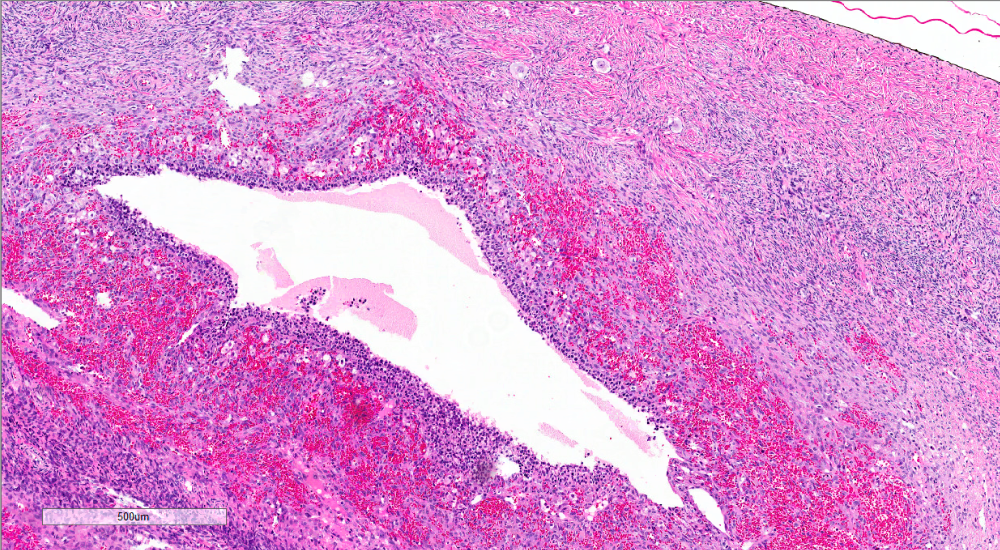
- Enlarged corpus luteum cyst can trigger an intraoperative consultation; the cyst lining is yellow and has a convoluted appearance
- Luteoma of pregnancy can be discovered incidentally during cesarean section or preoperatively on imaging study mimicking an ovarian mass (J Magn Reson Imaging 2009;29:713)
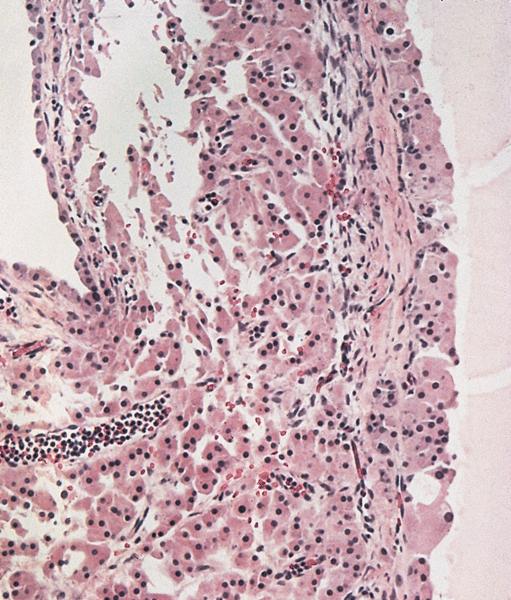
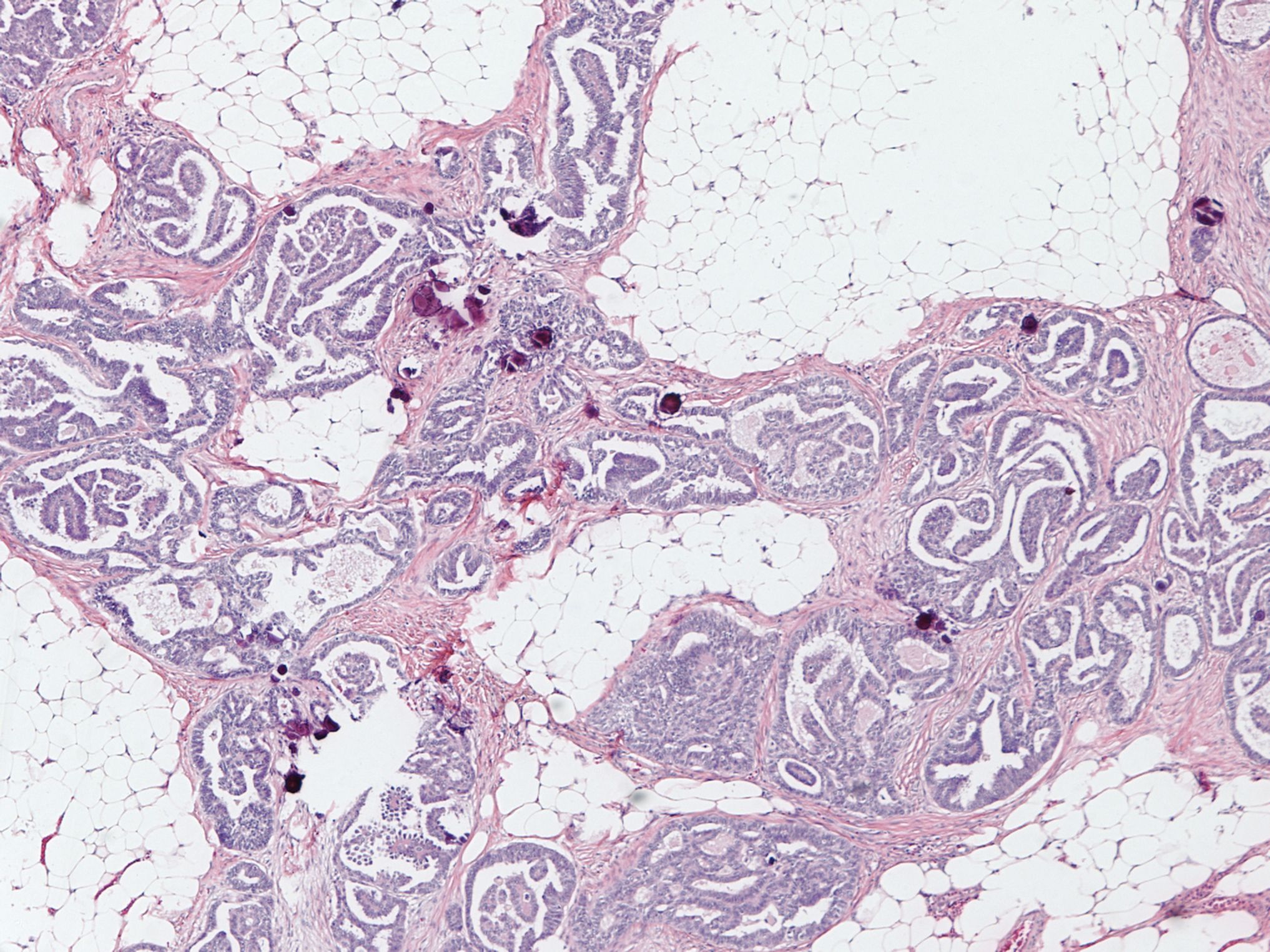
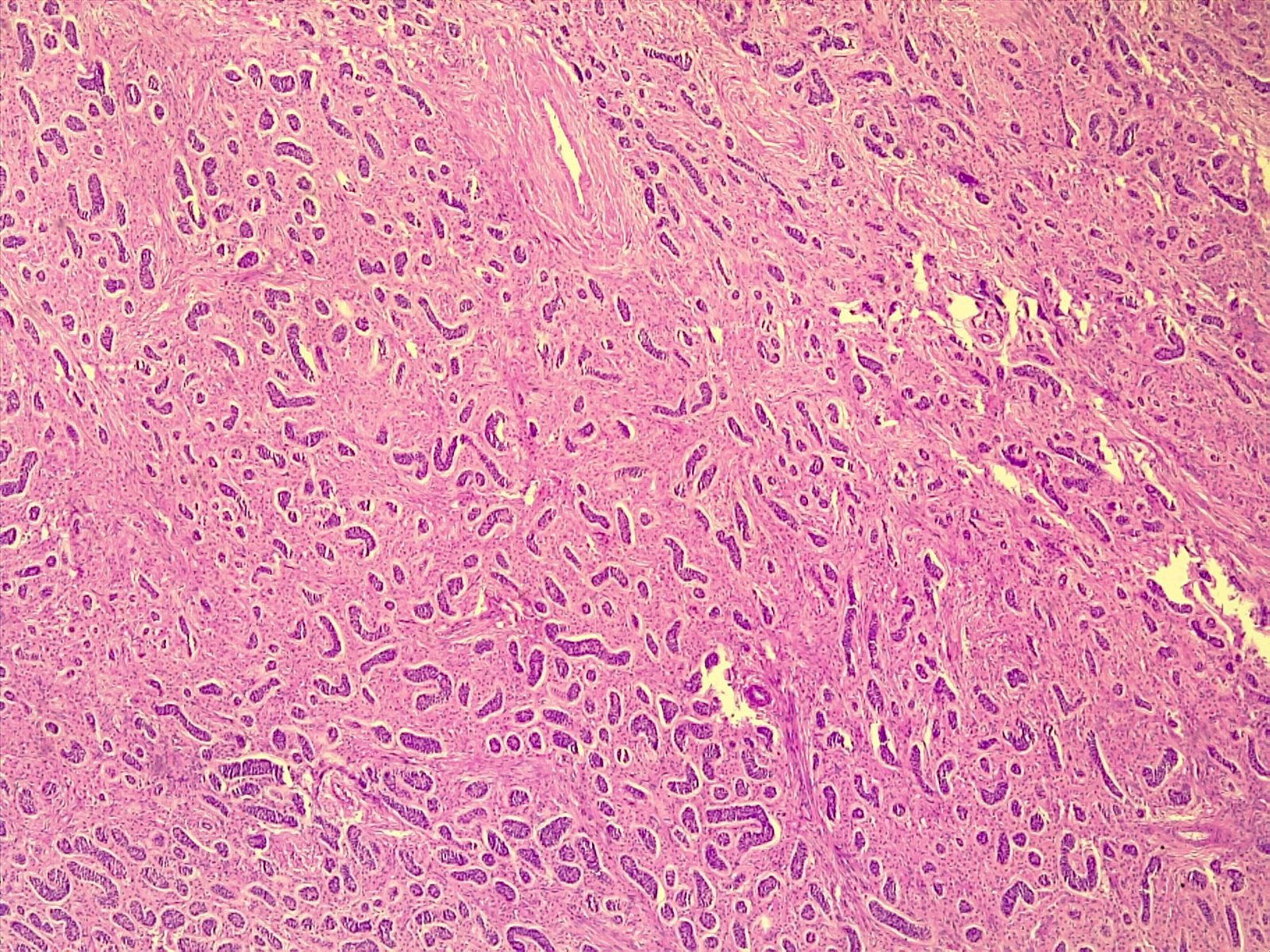
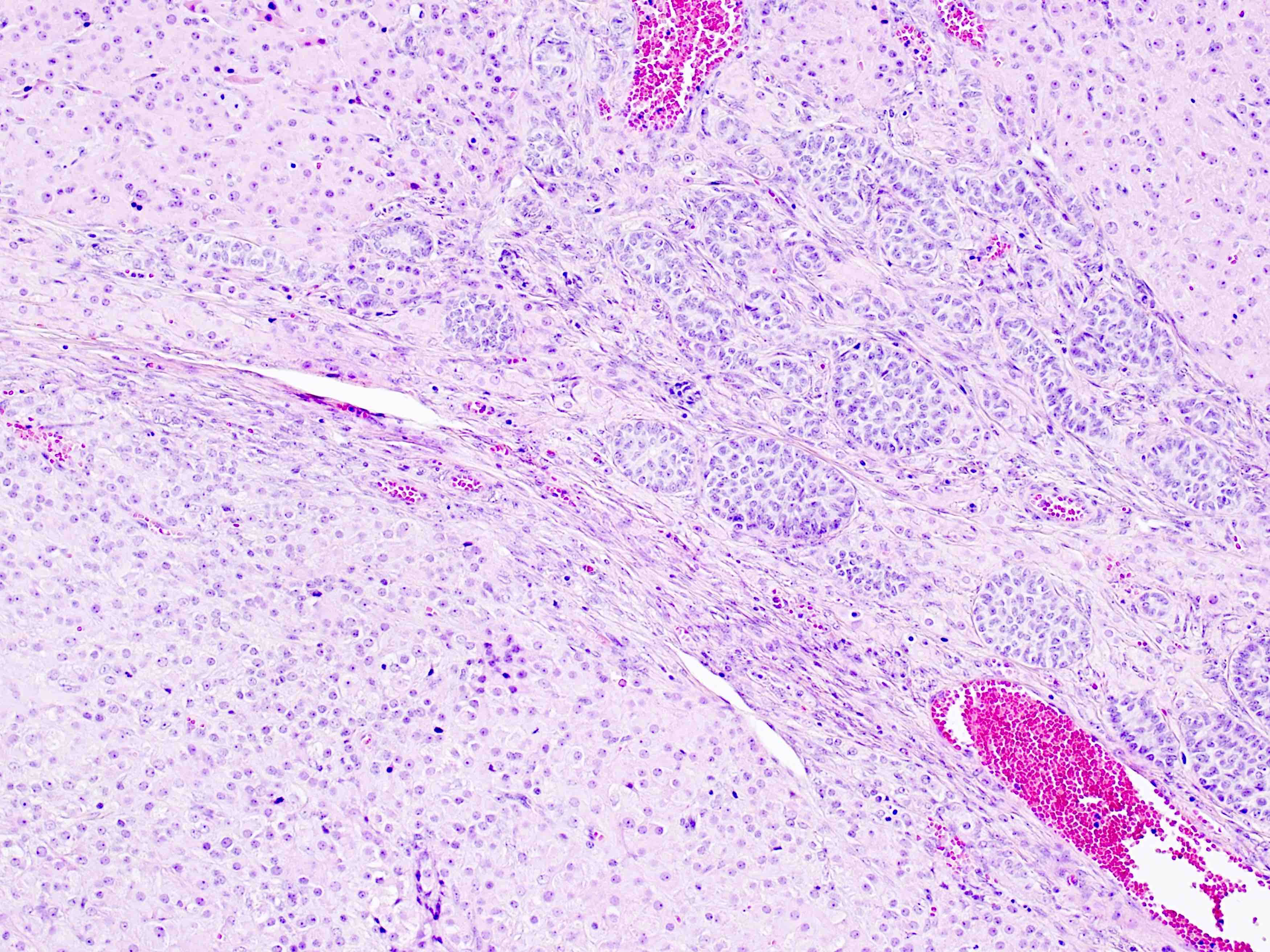
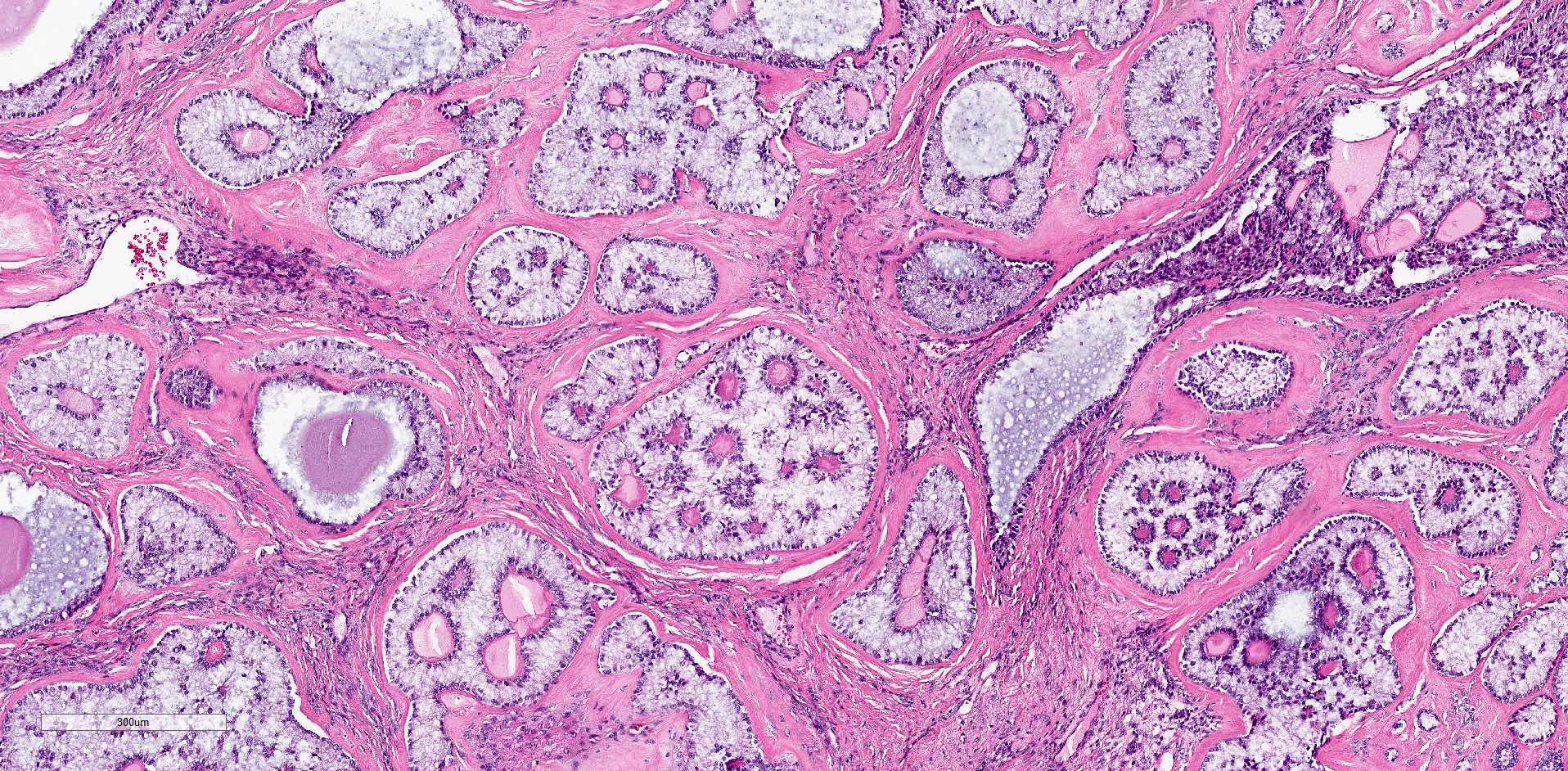
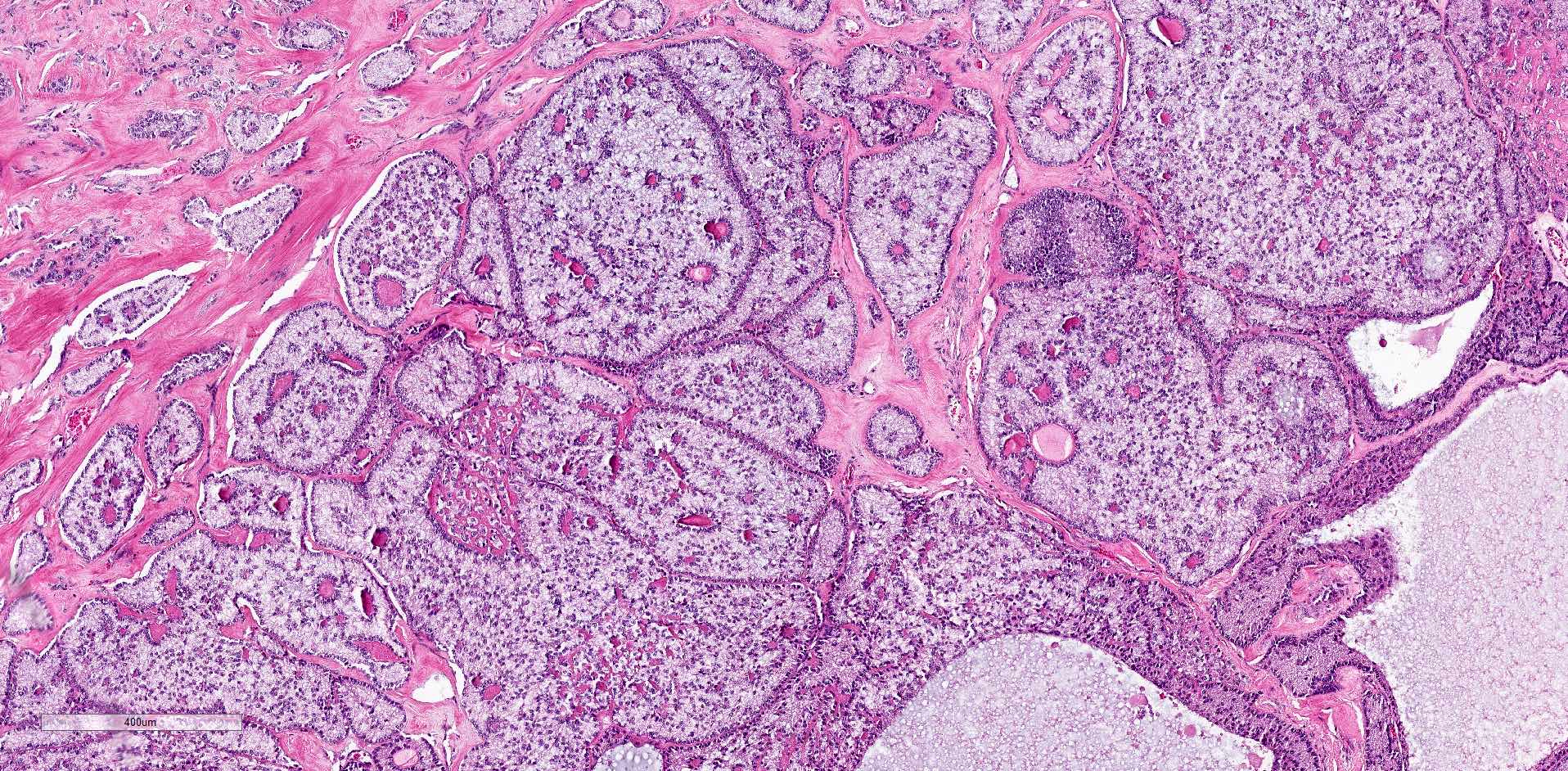
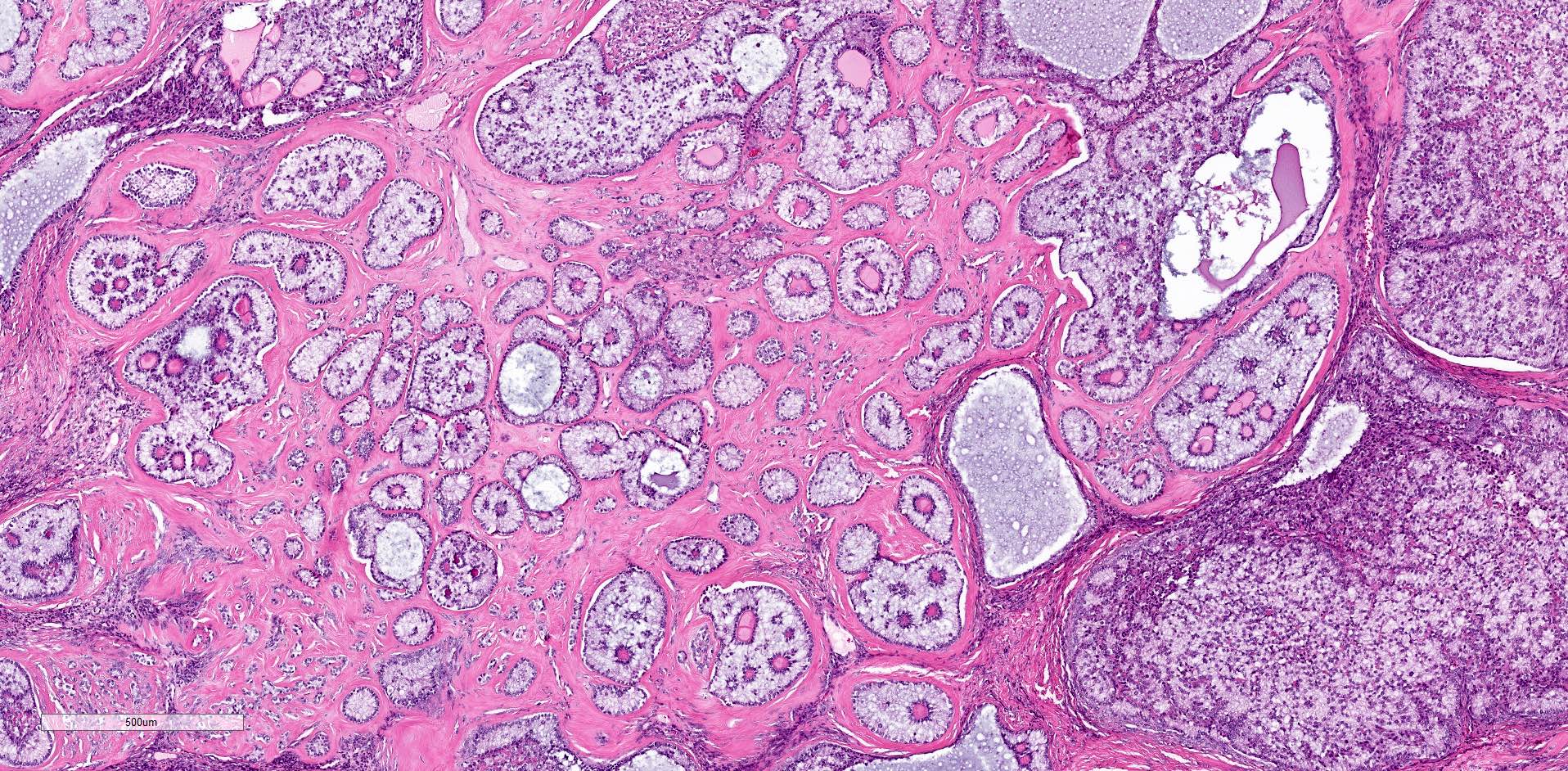
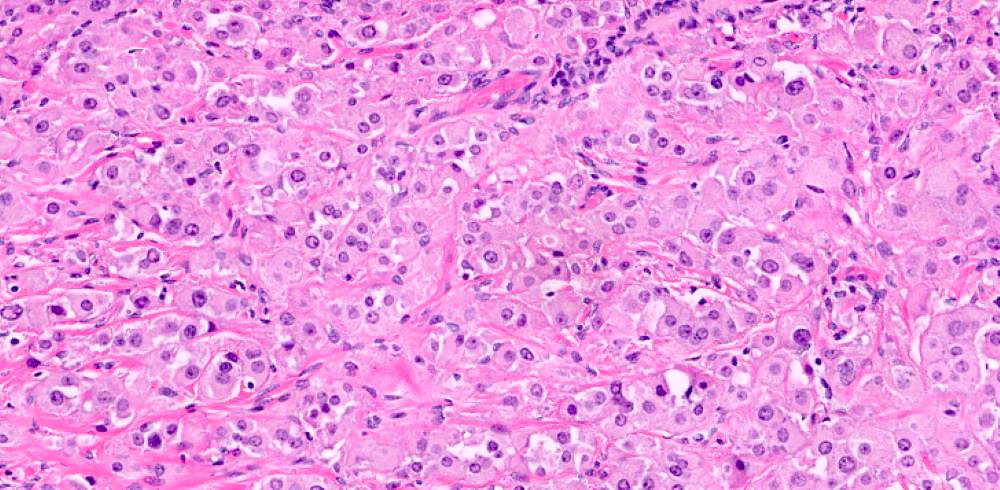
Microscopic (histologic) description
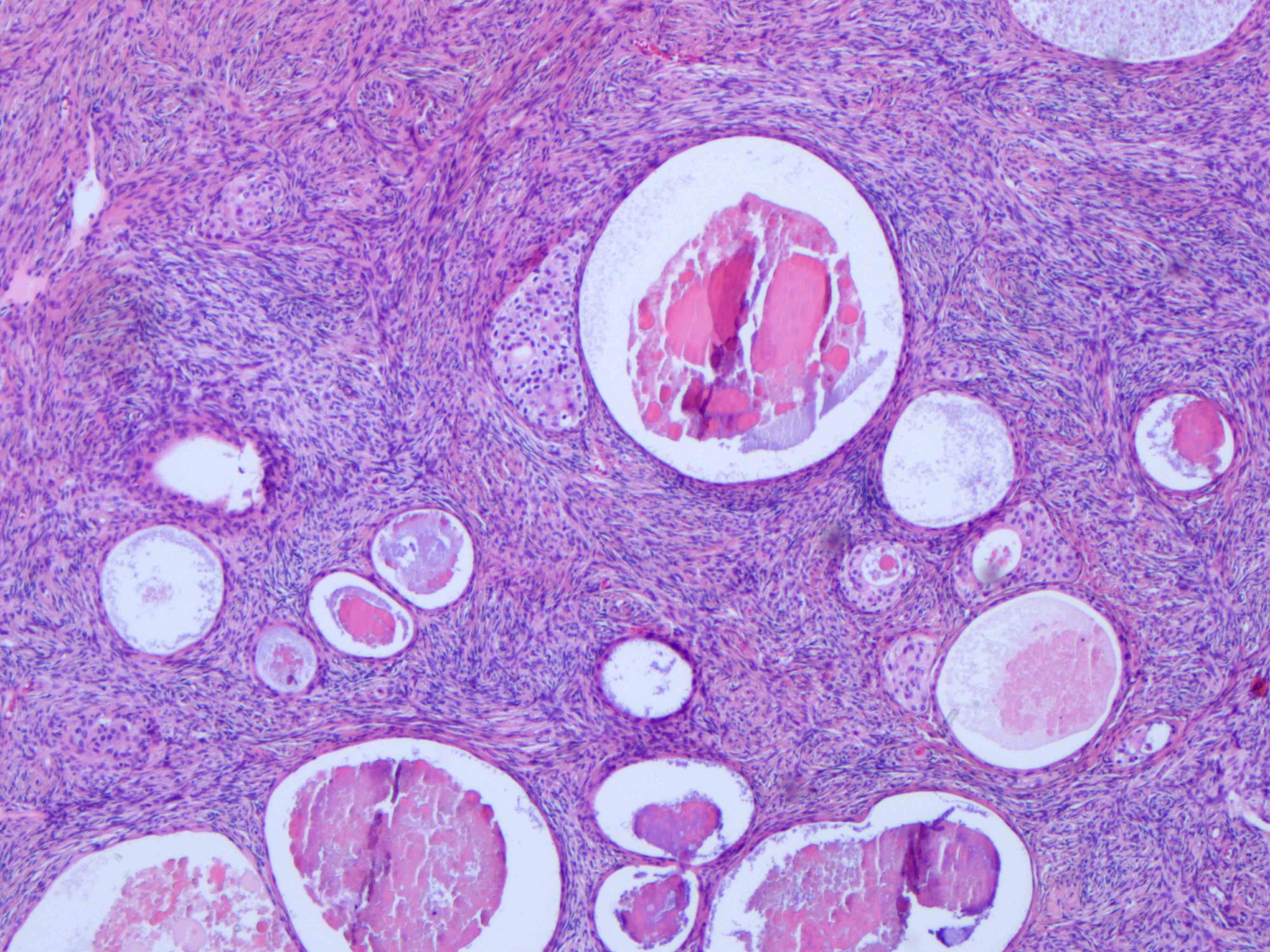
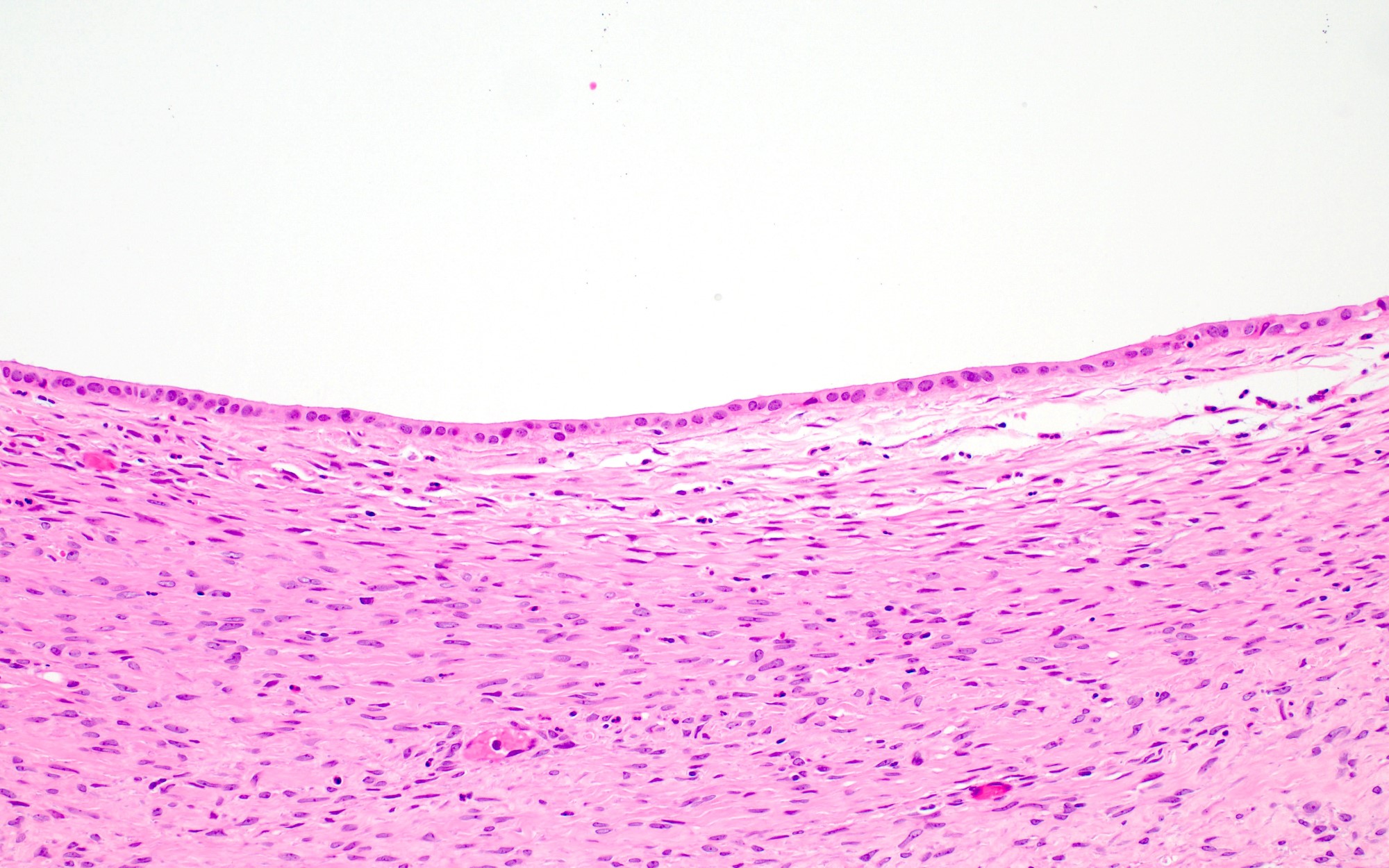
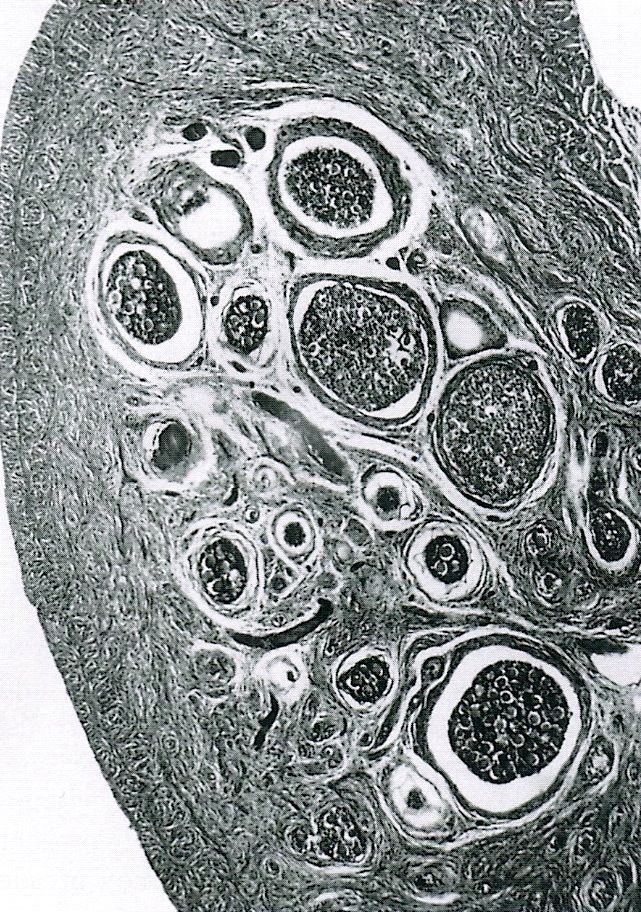
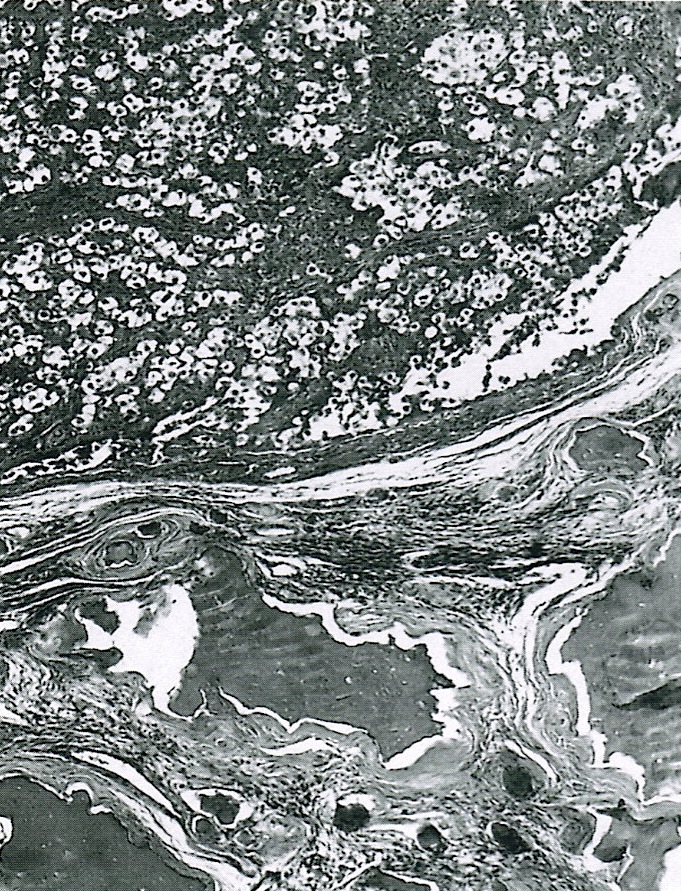
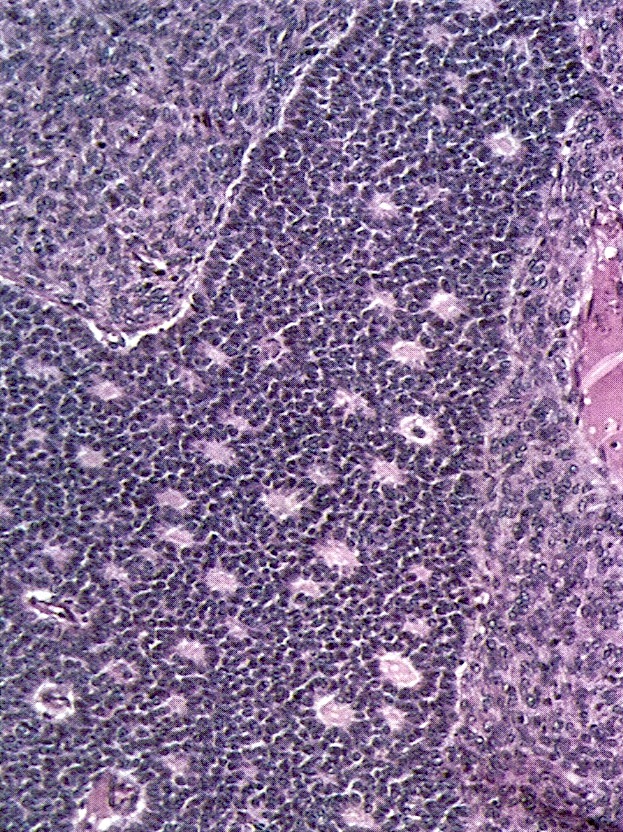
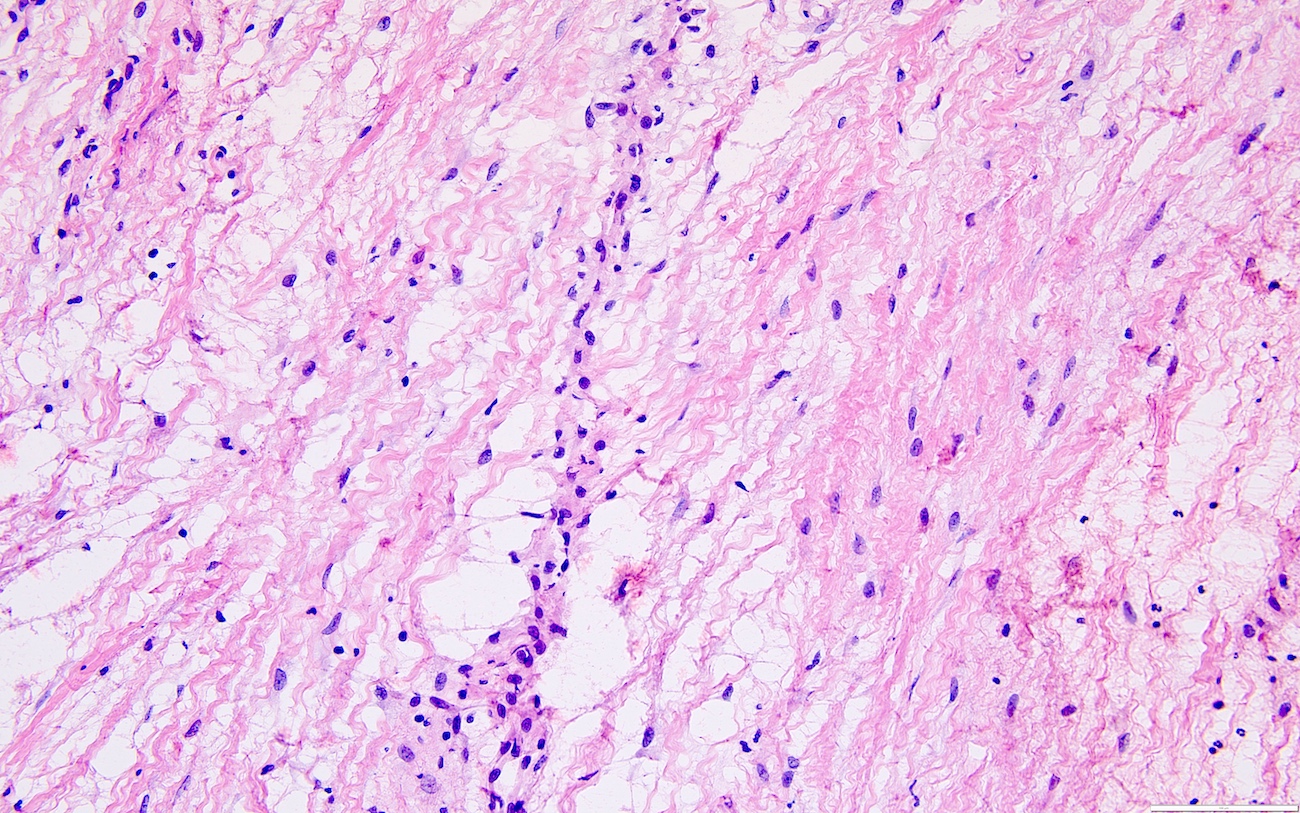
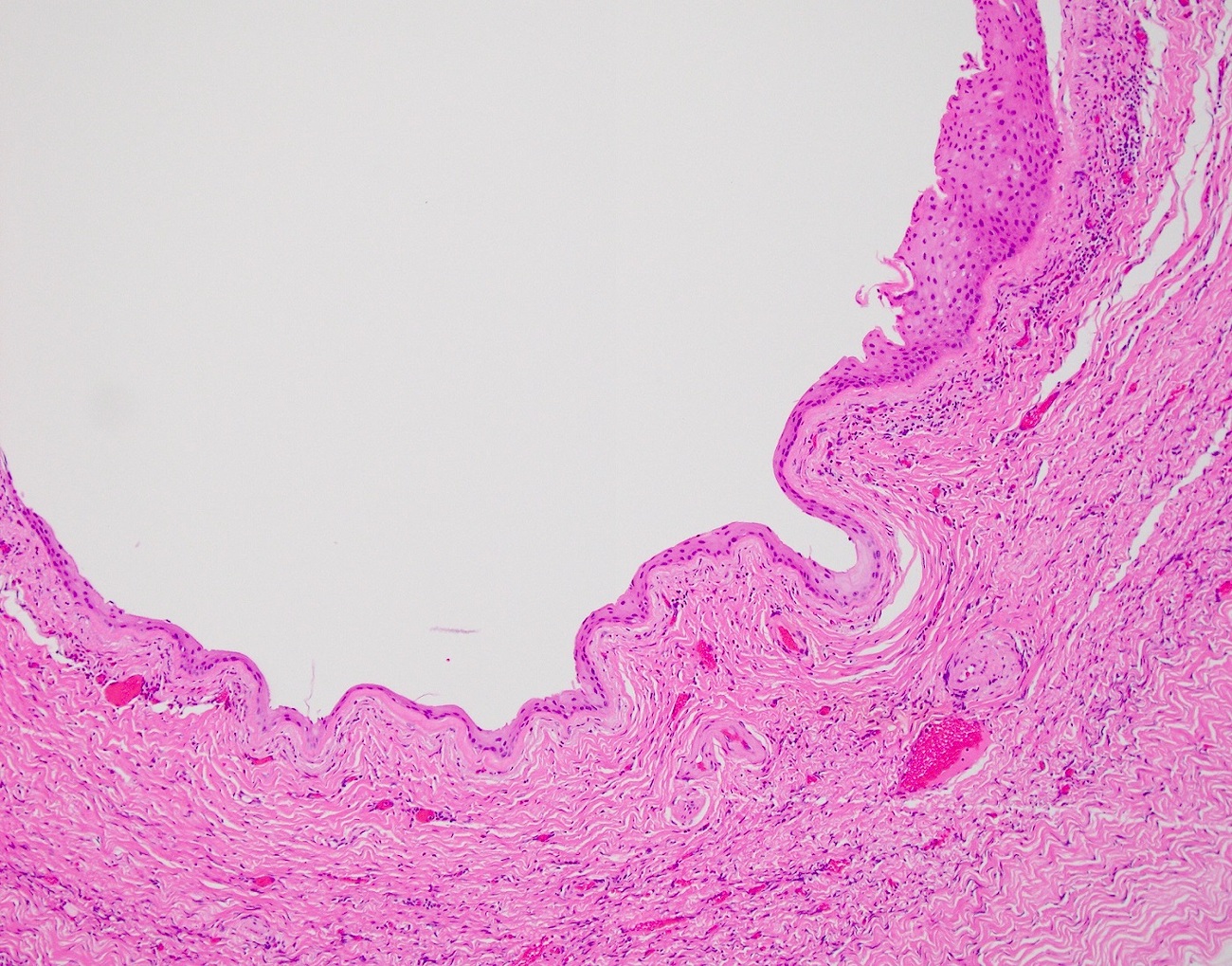
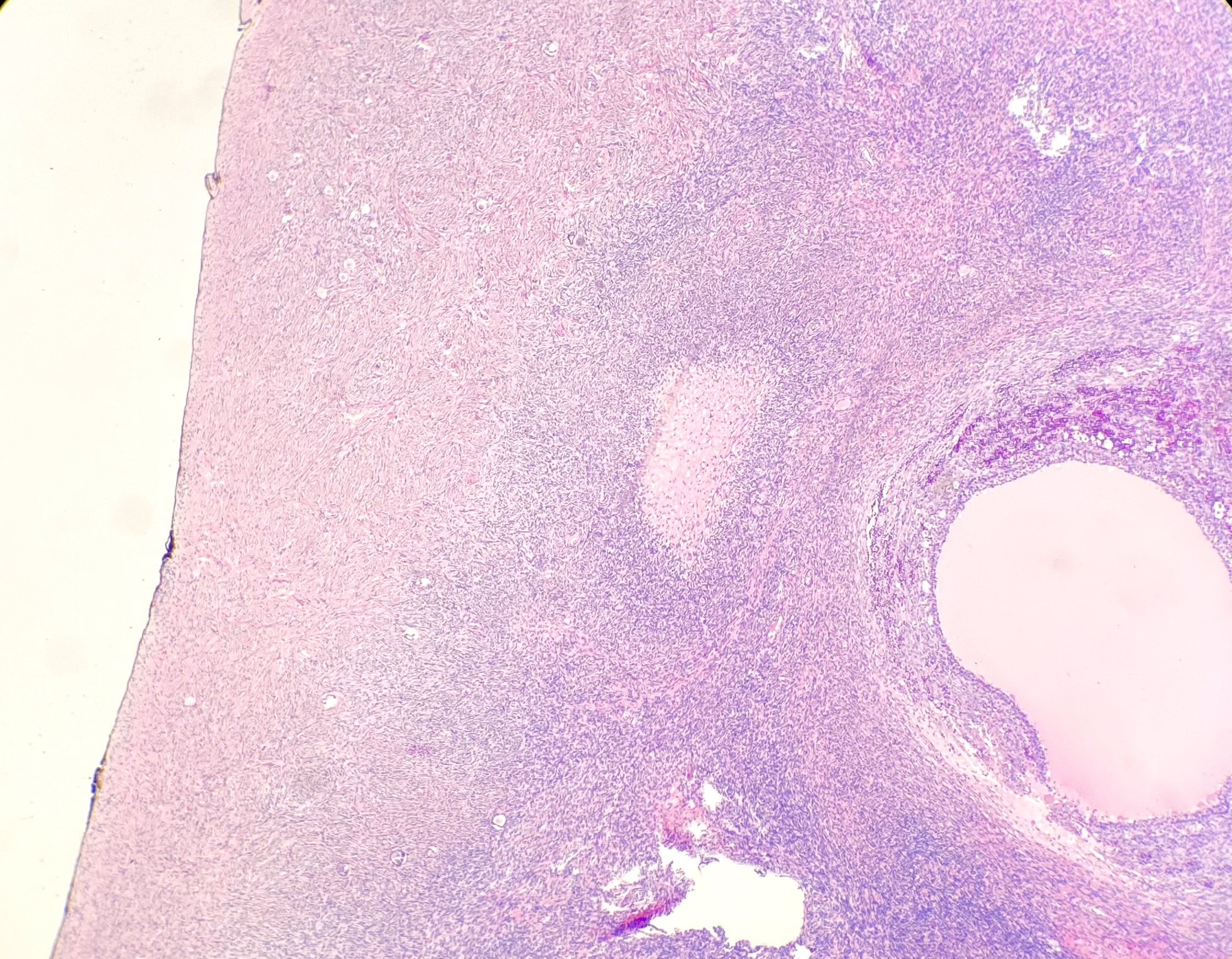
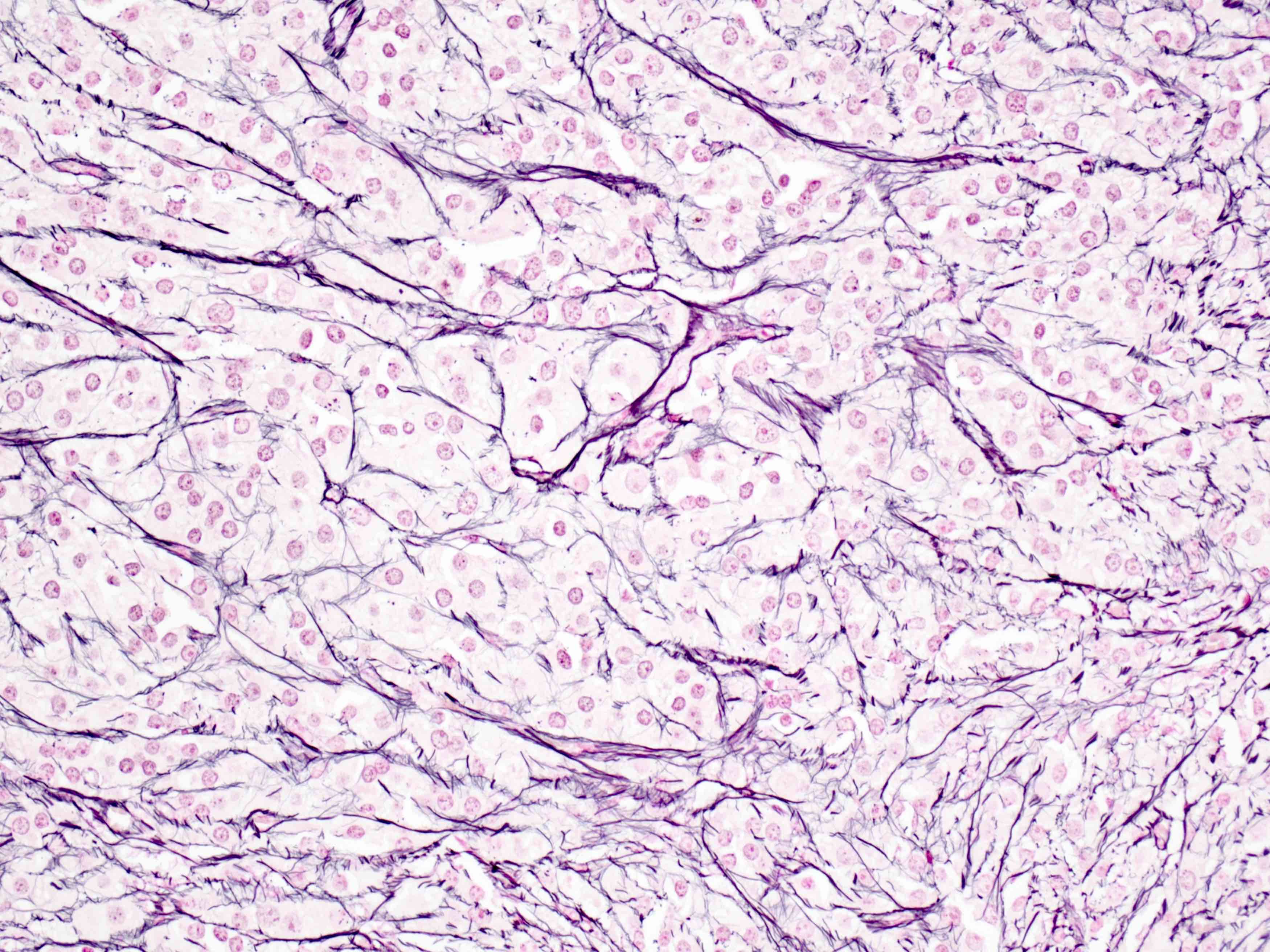
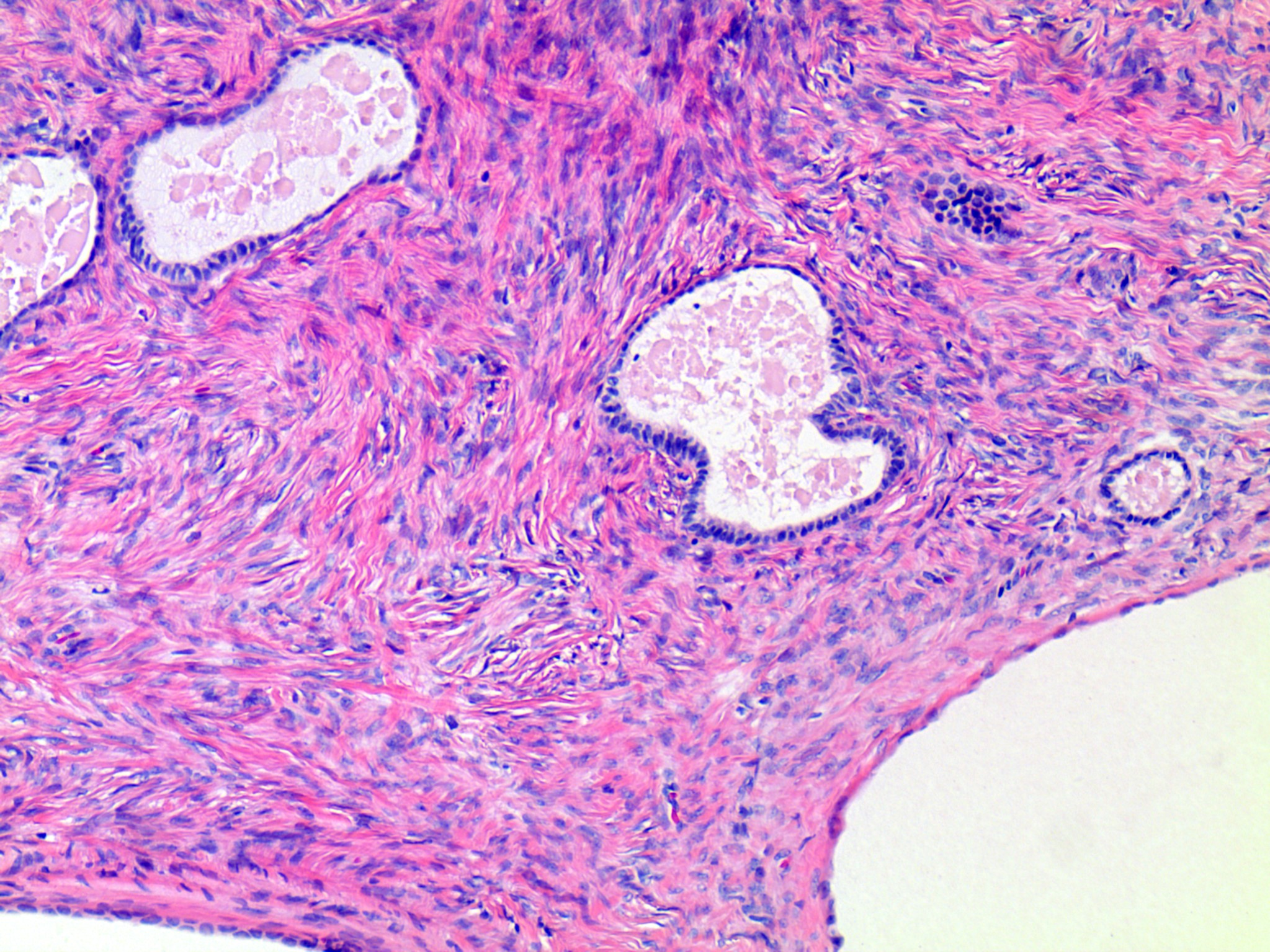
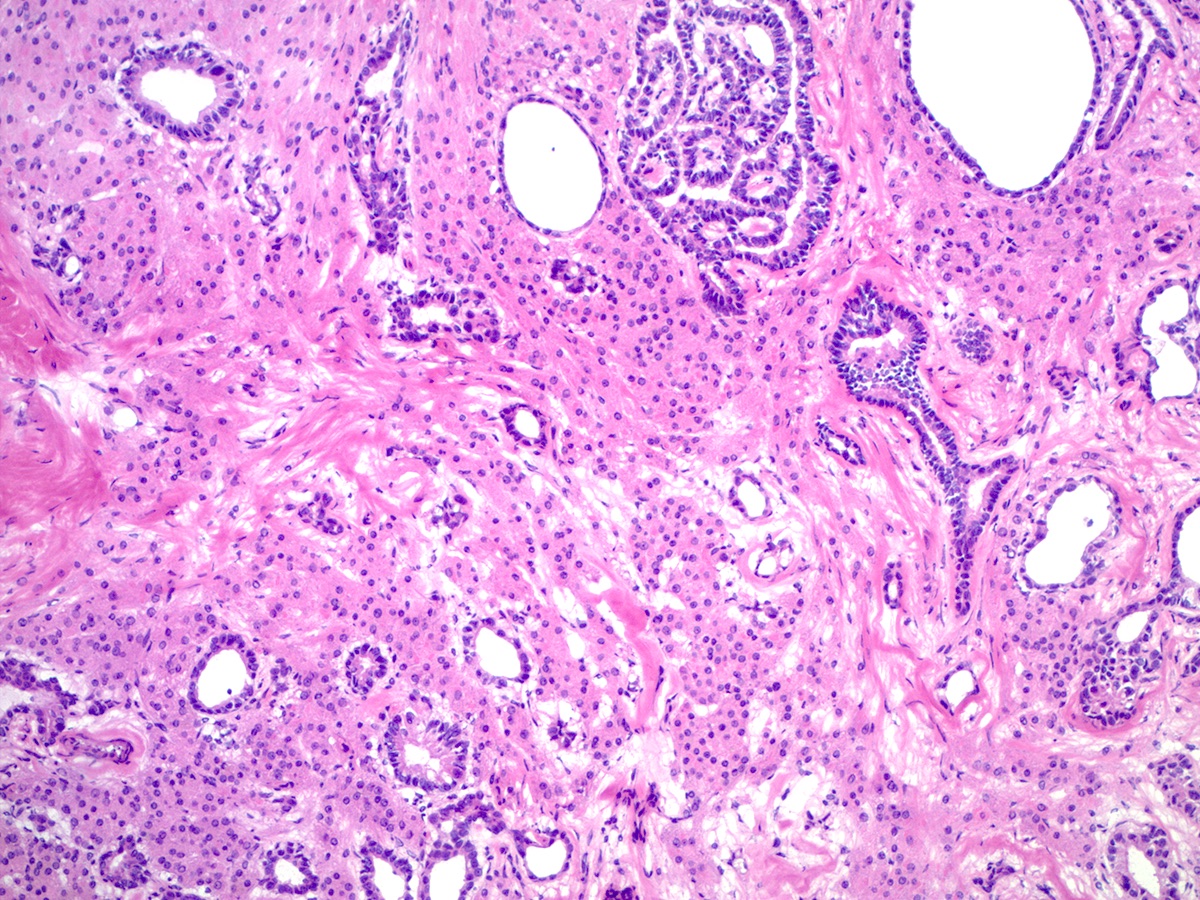
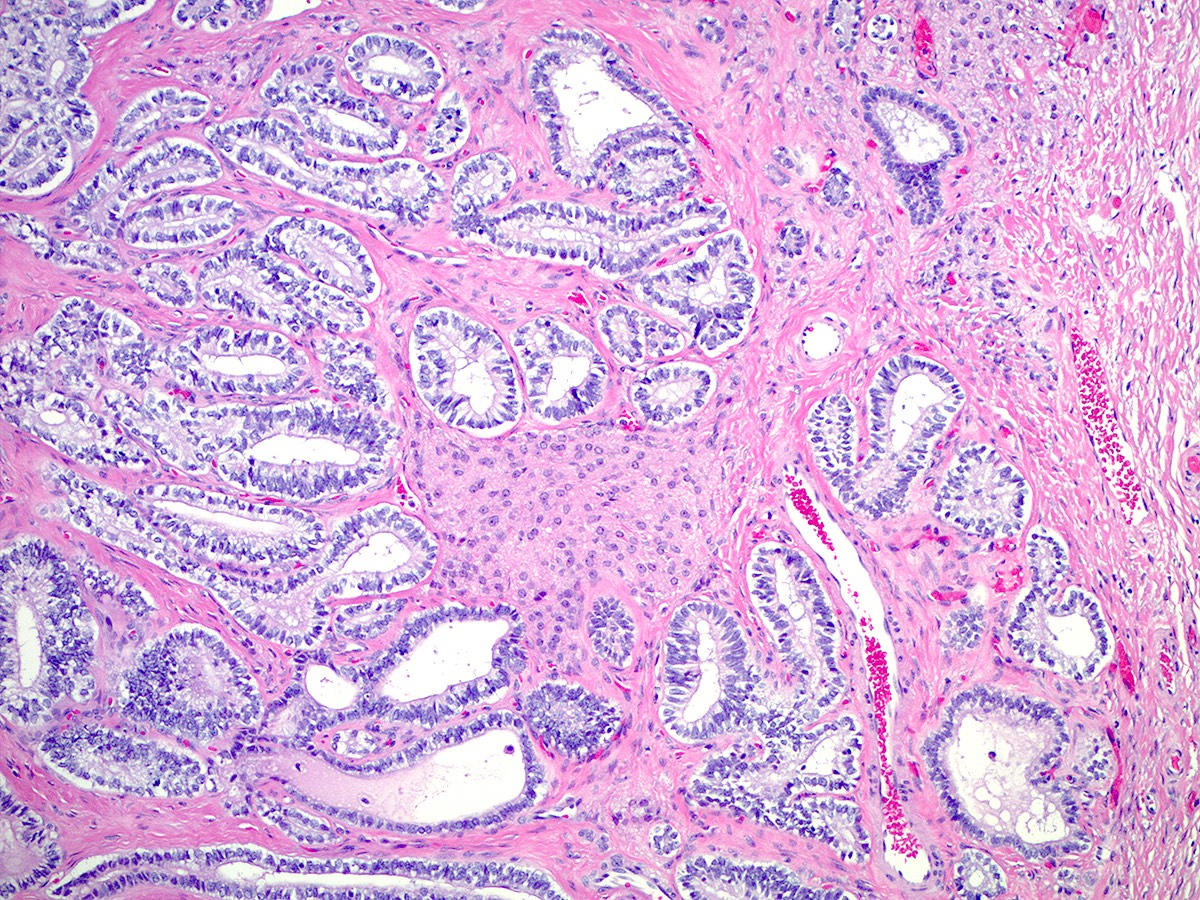
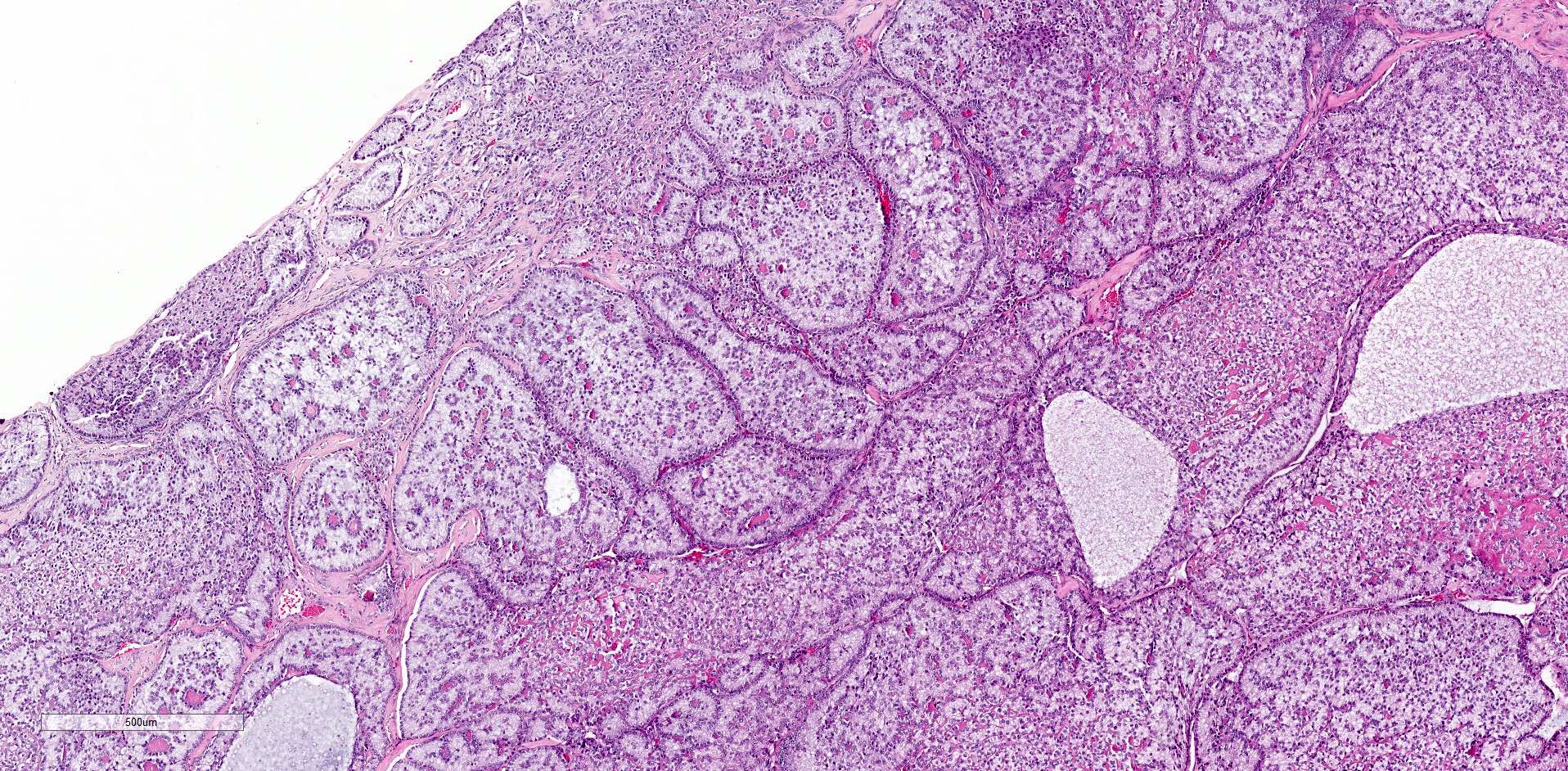
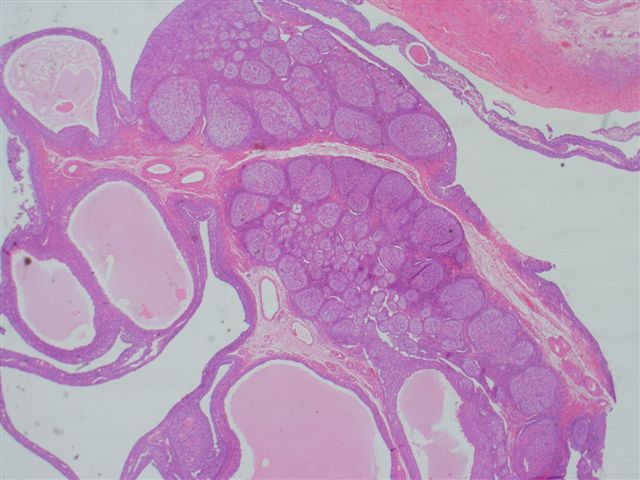
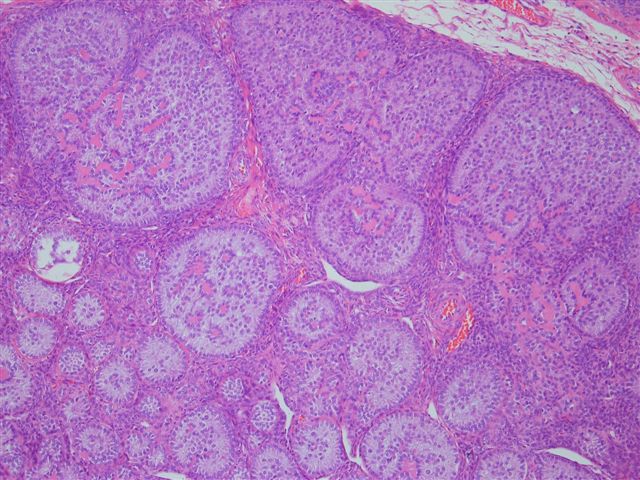
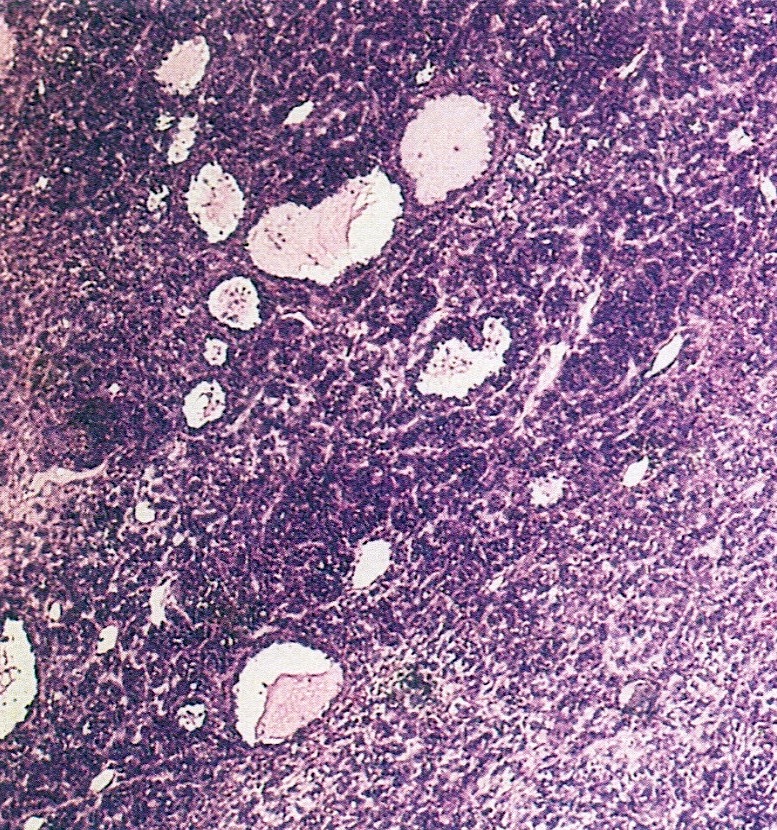
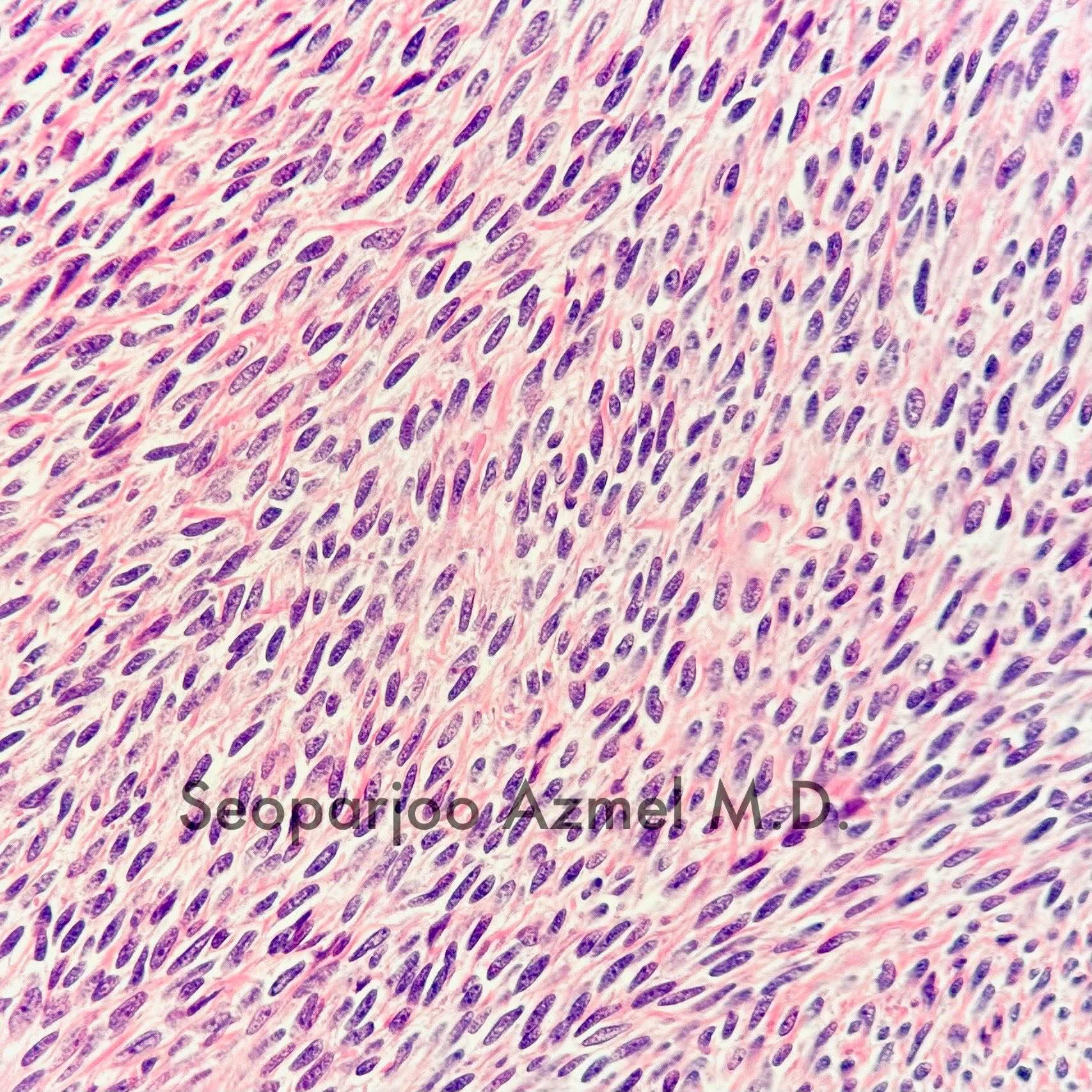
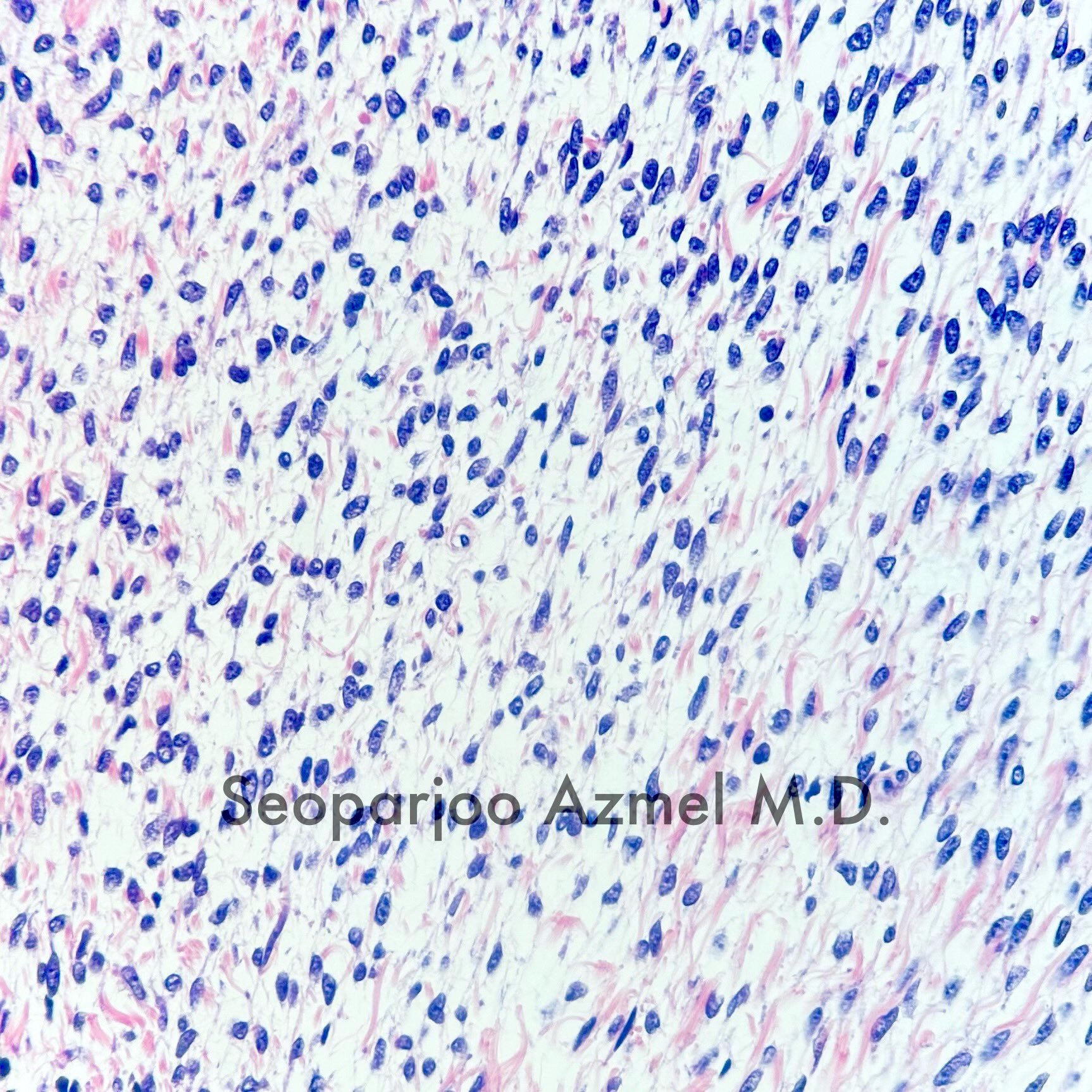
- Ovarian parenchyma can largely be divided into 3 compartments:
- Albuginea: a protective hypocellular compartment, composed of a fibrotic layer measuring approximately 0.3 mm and occupying the most superficial part of the ovary (Obstet Gynecol 1971;37:832, Ann Diagn Pathol 2020;46:151475)
- Cortex: a 0.3 mm hypercellular layer composed of spindle cells arranged parallel to the surface and housing the majority of the follicles in a premenopausal woman (Ann Diagn Pathol 2020;46:151475)
- Medulla: a hypocellular area beneath the cellular cortex and housing abundant blood vessels; nodular or diffuse proliferation of spindle cells can commonly be seen in the medulla (Ann Diagn Pathol 2020;46:151475)
- Stroma:
- Comprises bulk of ovarian tissue
- Resembles fibroblasts in whorls / storiform pattern surrounded by dense reticulin network
- Contains luteinized stromal cells, decidual cells, smooth muscle, fat, neuroendocrine cells and endometrial stroma-like cells
- Ovarian parenchyma can alternatively be defined as the ovarian follicles, given that the follicles are the constituents that perform the function of the ovary with all the other constituents being labeled as ovarian stroma (Reproduction 2020;160:R25)
- Number of primordial follicles and oocytes in premenopausal women can be markedly different between the 2 ovaries (Ann Diagn Pathol 2020;46:151475)
- Within the same ovary, the primordial follicles and oocytes exhibit an uneven distribution in the cortex with clustering in few areas (Ann Diagn Pathol 2020;46:151475)
- In addition to their more common location in the cortex, the primordial follicles can be seen in the medulla within areas of nodular proliferations, especially in women with abundant follicular cysts (Ann Diagn Pathol 2020;46:151475)
- Follicle is designated cystic when it measures 2 mm or more but less than 9 mm; these are very common and can be lateralized to 1 ovary in some individuals (Endocrinol Metab Clin North Am 1998;27:877)
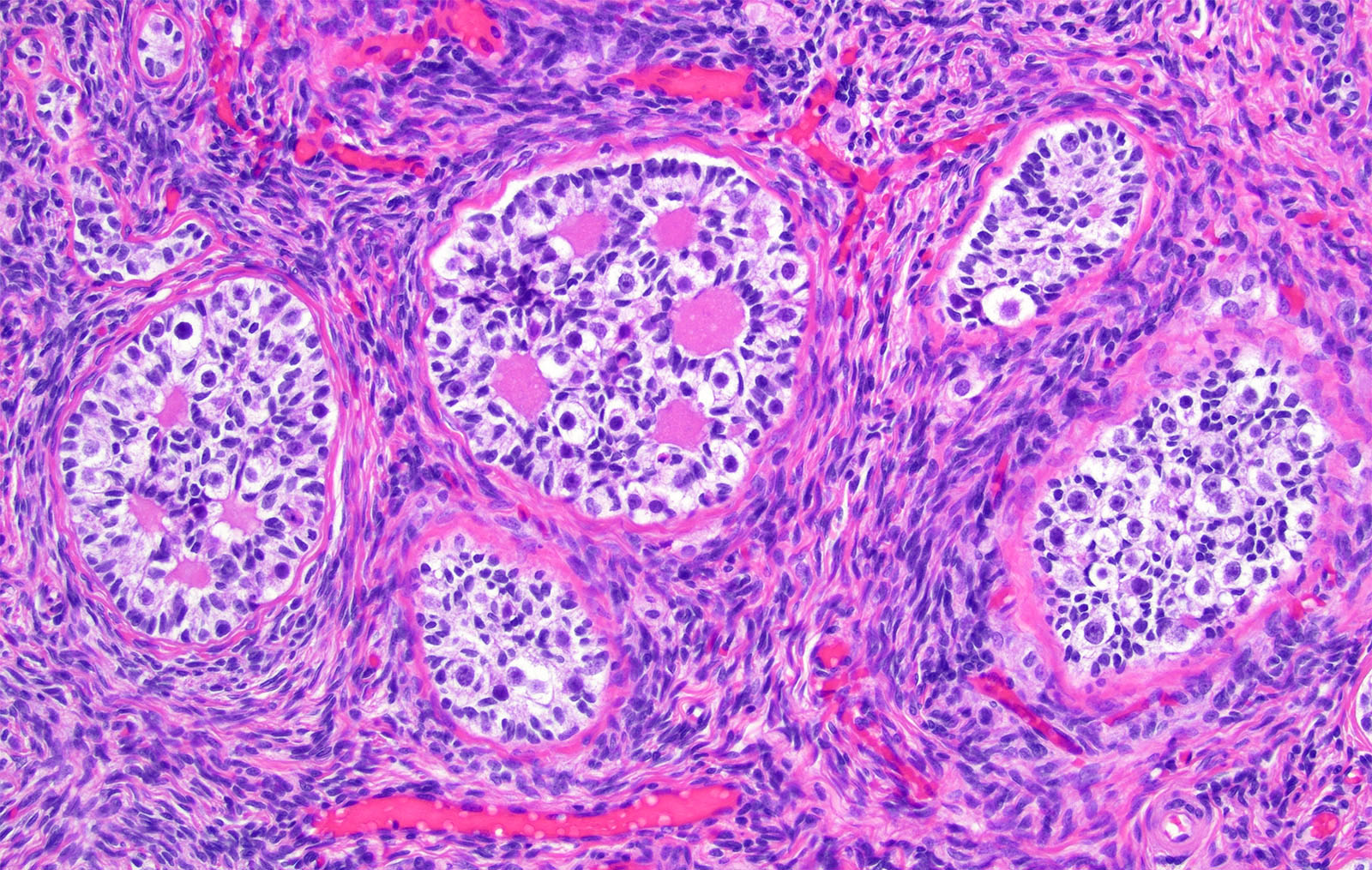
- Stages of follicular development:
- Primordial follicle:
- Diplotene oocyte surrounded by a layer of flattened granulosa cells (Hum Mol Genet 2010;19:397)
- Neo-oogenesis may occur in adults (Endocrine 2005;26:301)
- Maturing follicle:
- Oocyte with surrounding granulosa cell layer
- Lacks reticulum
- Contains Call-Exner bodies (rosette-like formations with central filamentous / eosinophilic material consisting of excess basal lamina) and theca cells (within follicle are luteinized and produce sex hormones, external to follicle are very cellular)
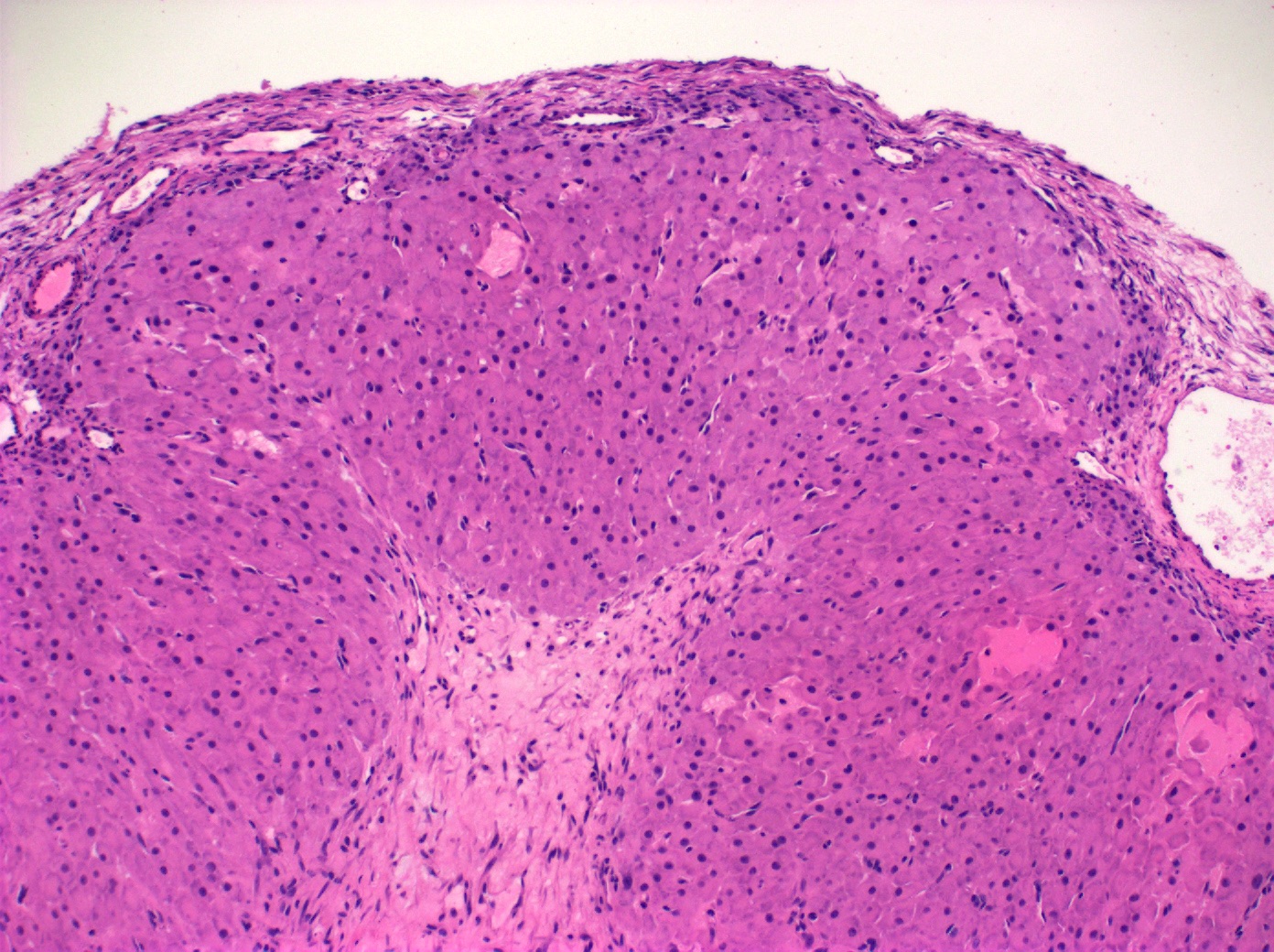
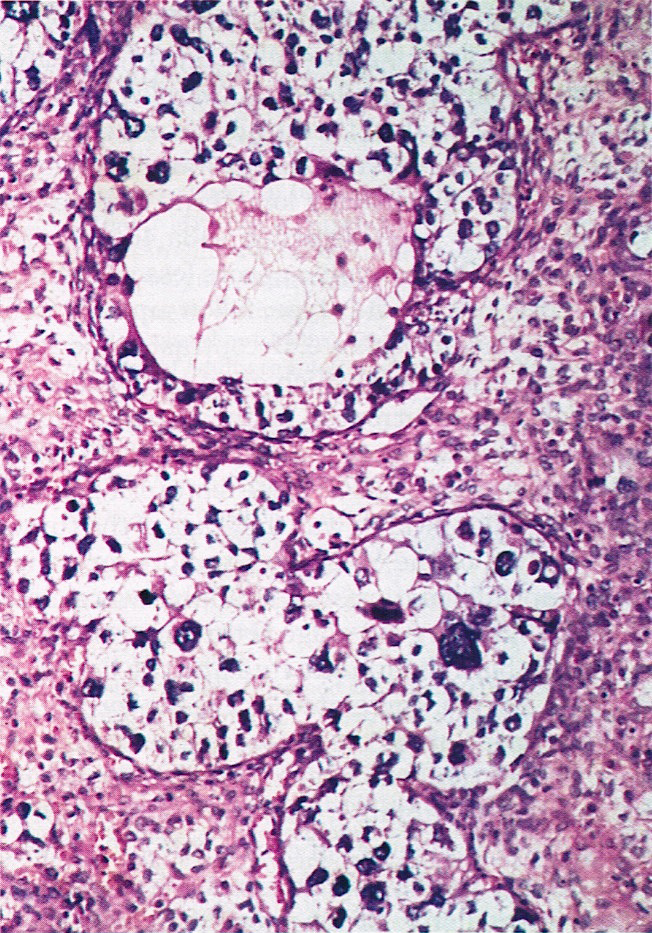
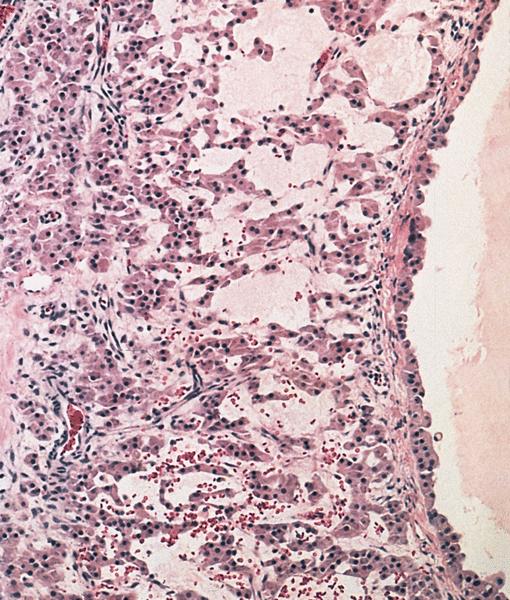
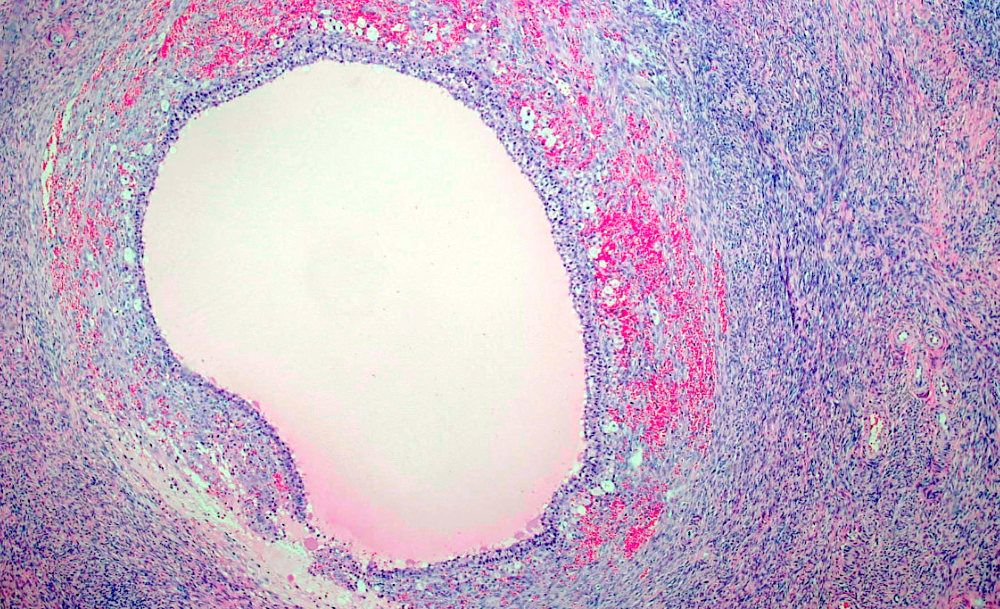
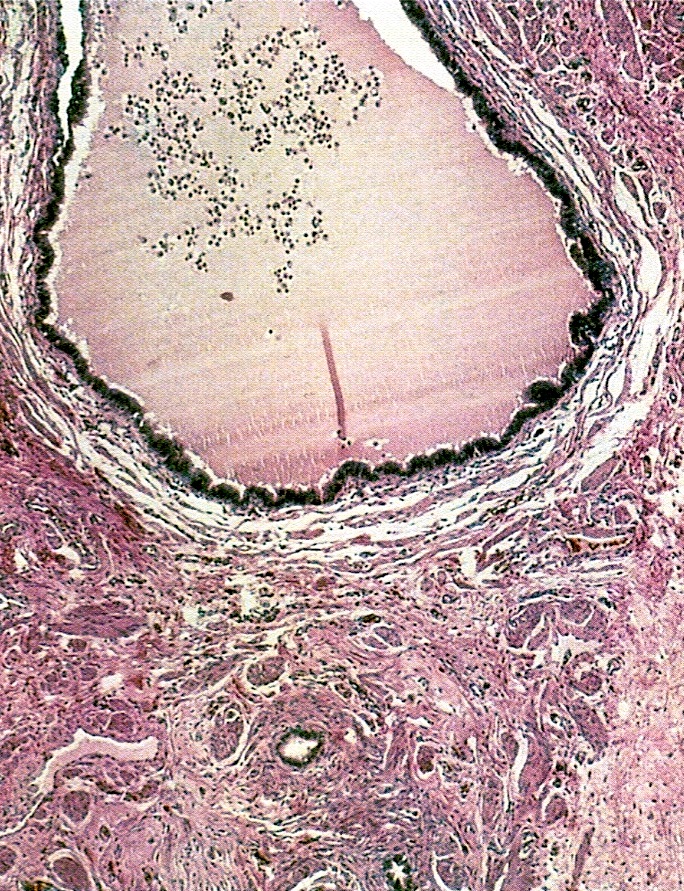
- Corpus luteum:
- 2 cm, round to serpiginous, yellow, lobulated structure with cystic center
- Has luteinized granulosa and theca cells
- In pregnancy, is larger, bright yellow with prominent central cavity, hyaline droplets and calcification
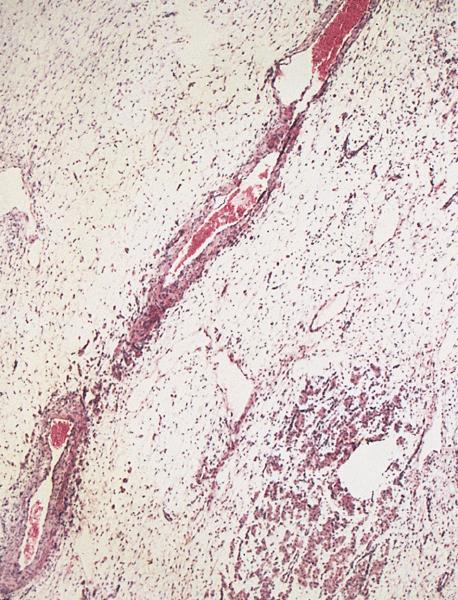
- Corpus albicans: remnant of corpus luteum, hyalinized, paucicellular fibrotic scar with serpiginous contours
- Primordial follicle:
- Other normal components:
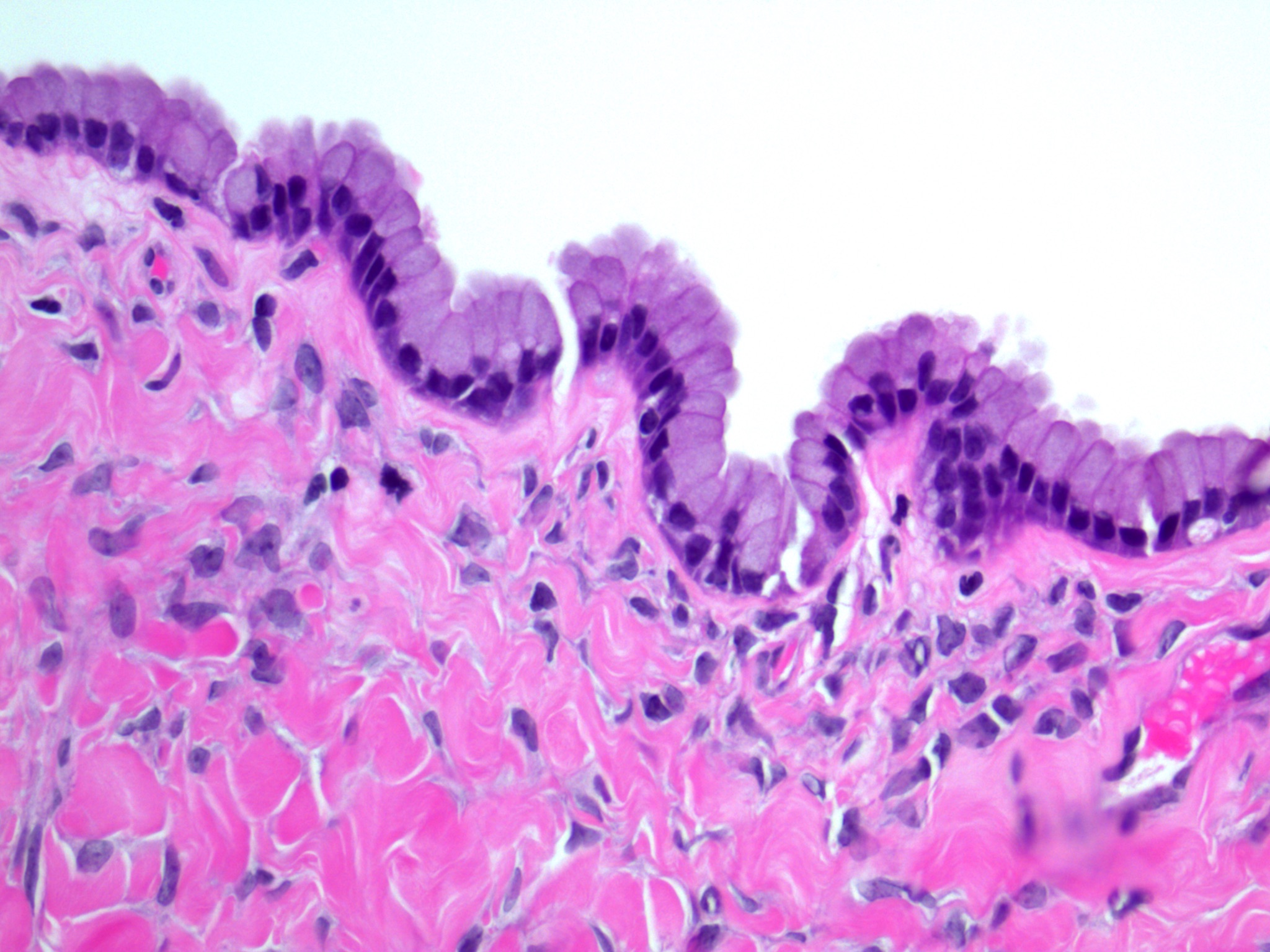
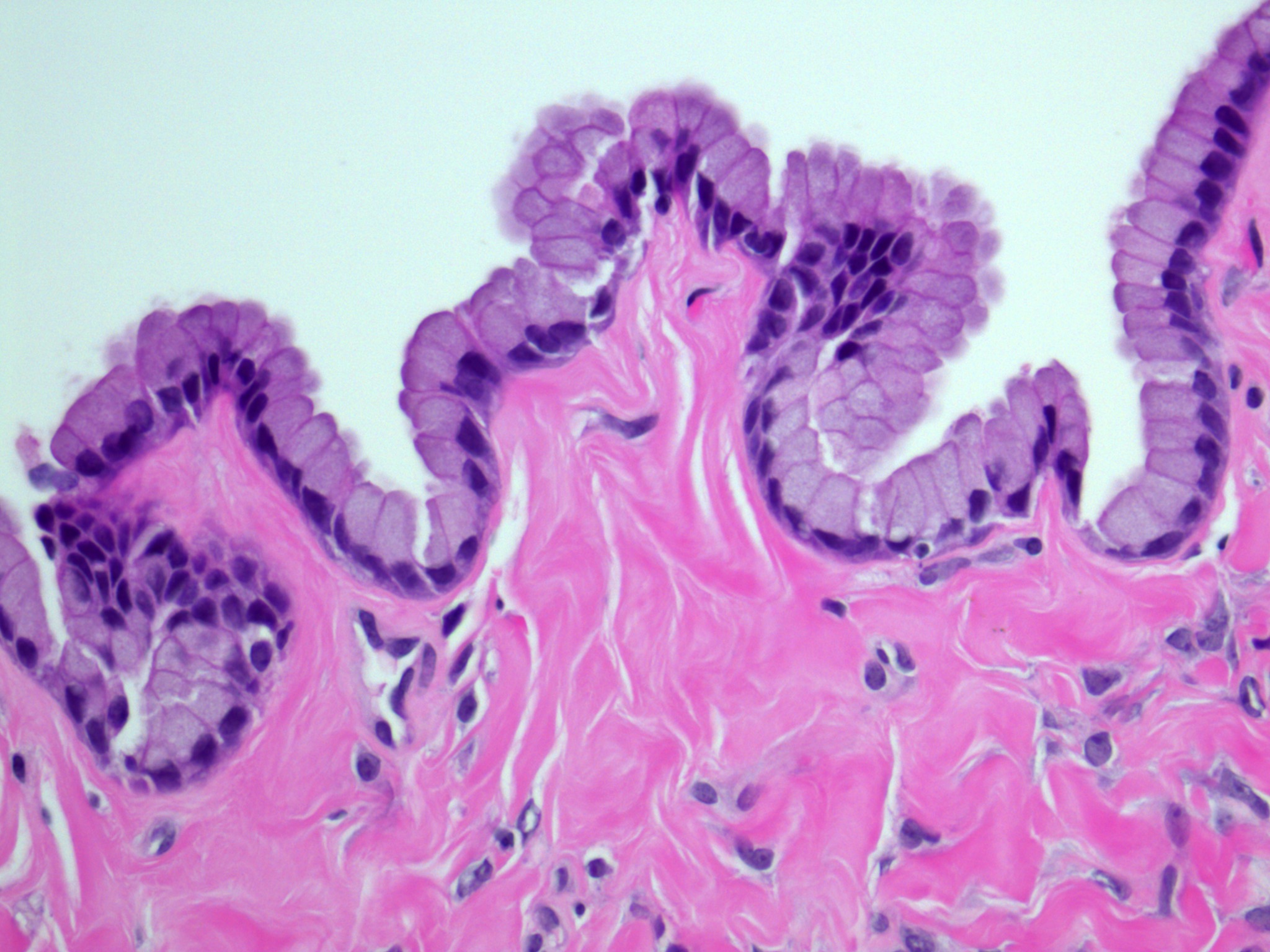
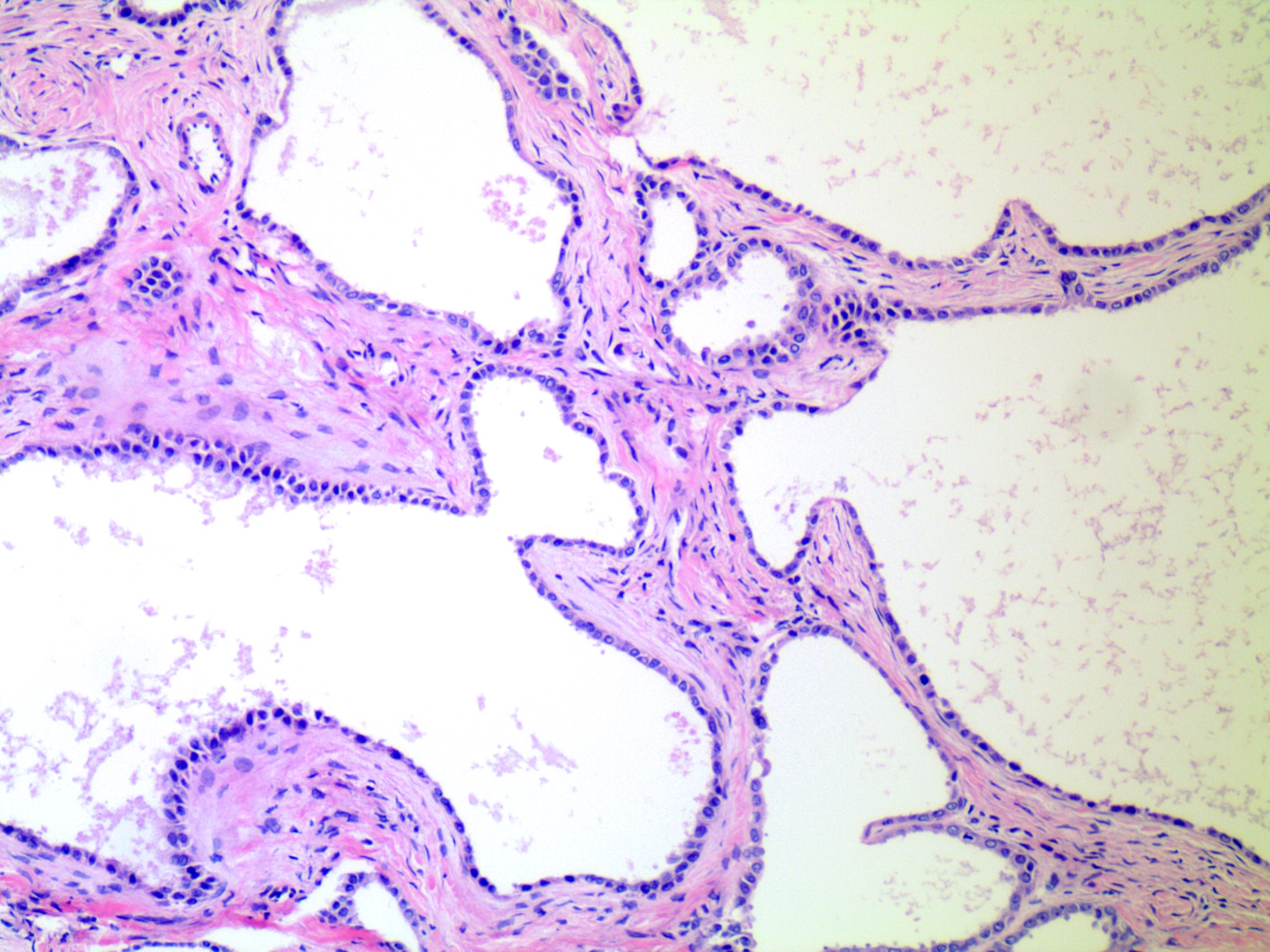
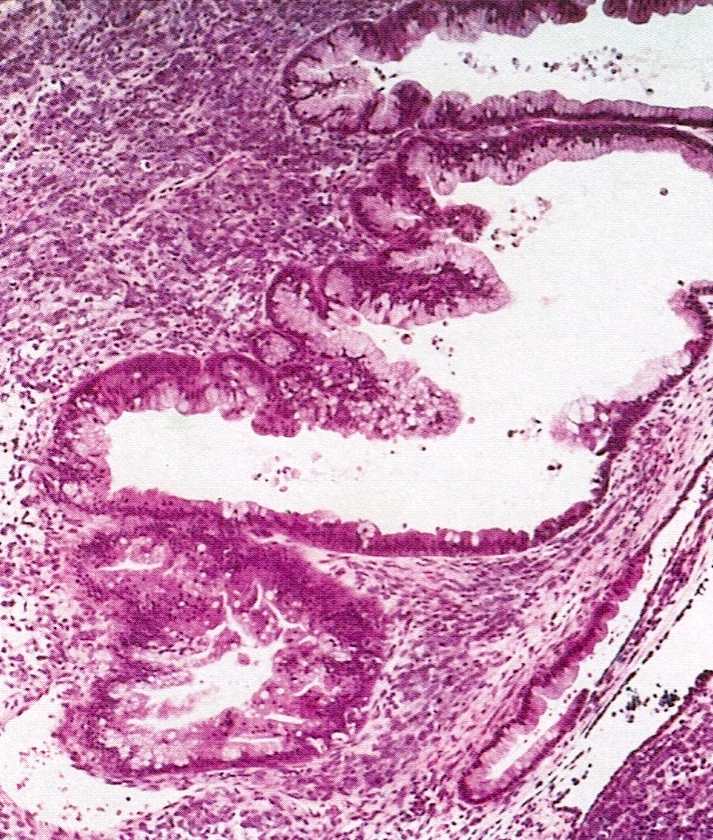
- Surface epithelium:
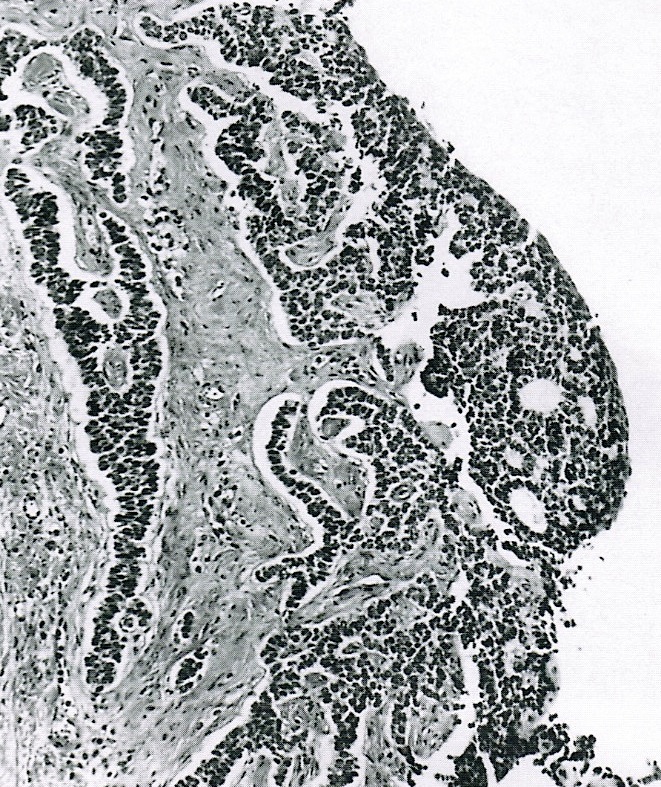
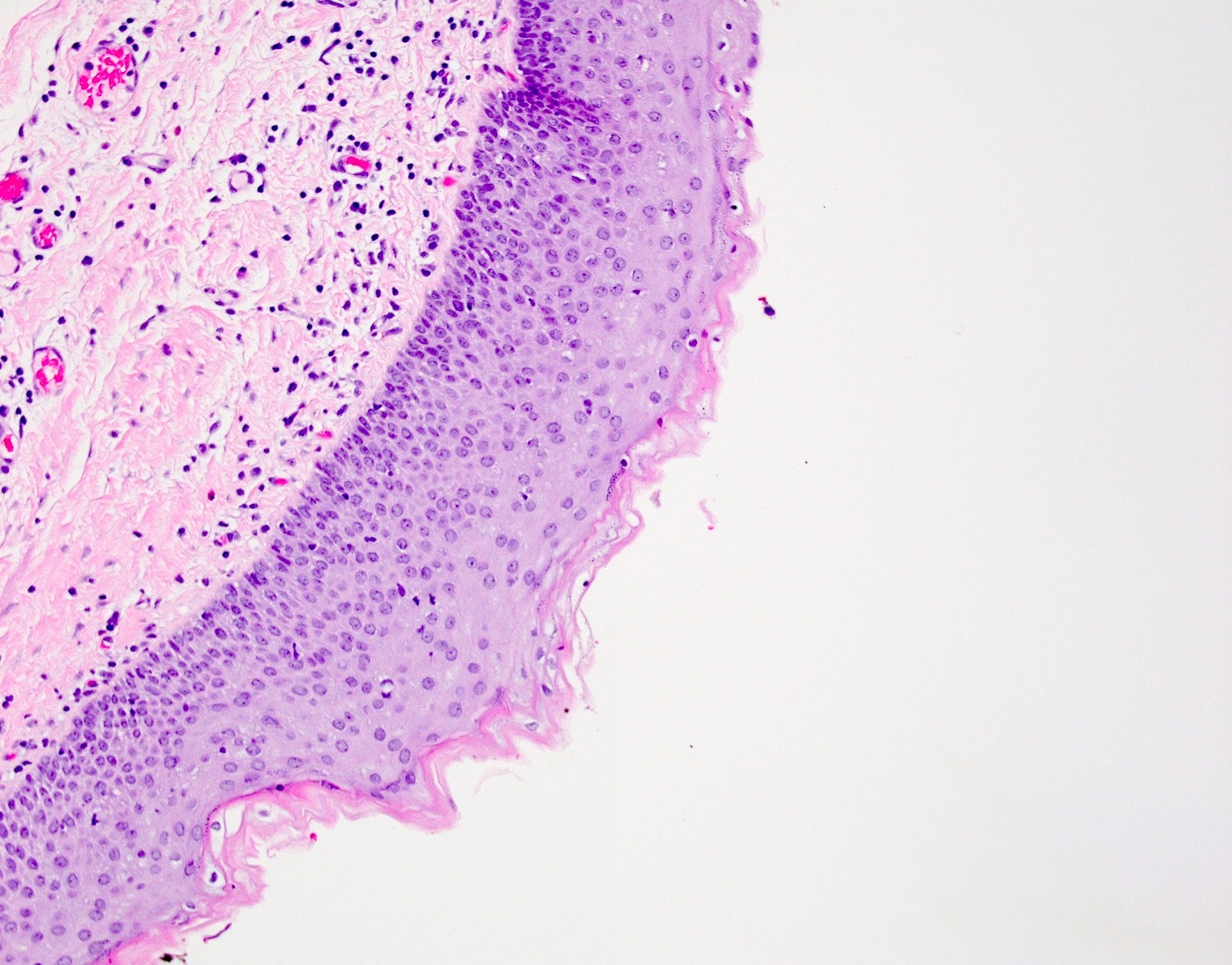
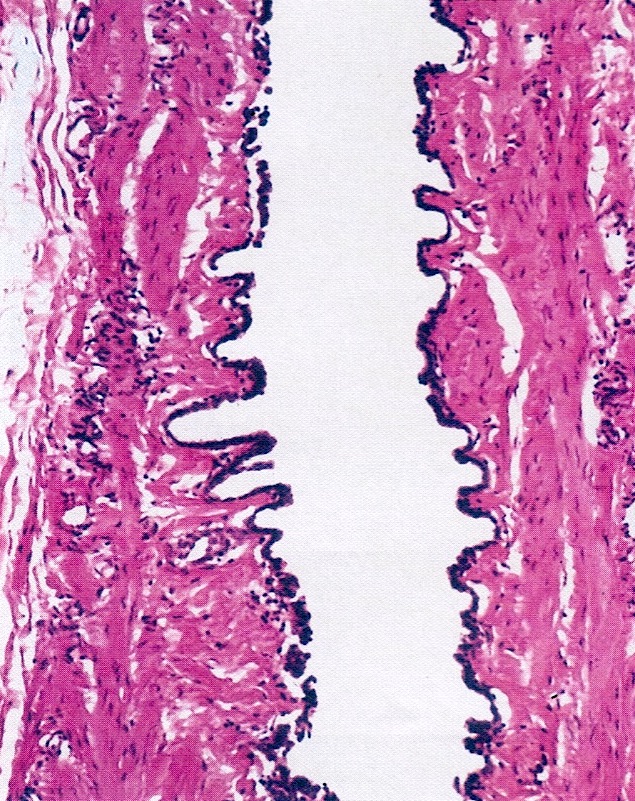
- Ovarian surface epithelium (OSE) is a modified mesothelium, also called coelomic or germinal epithelium (Reprod Biol Endocrinol 2006;4:42)
- Single layer of flat to cuboidal mesothelial type cells, which appear to actively participate in the ovulatory rupture and repair process (J Histochem Cytochem 2020;68:113)
- Closely related to Müllerian duct lining epithelium
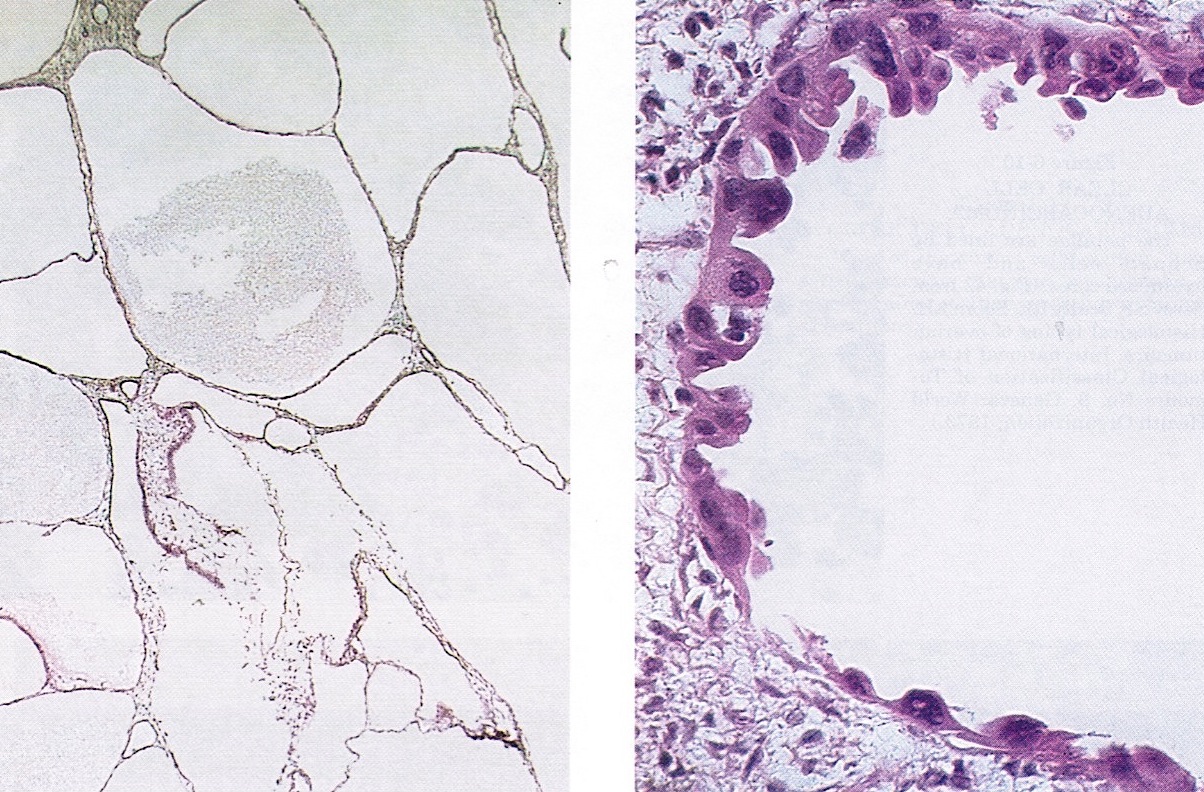
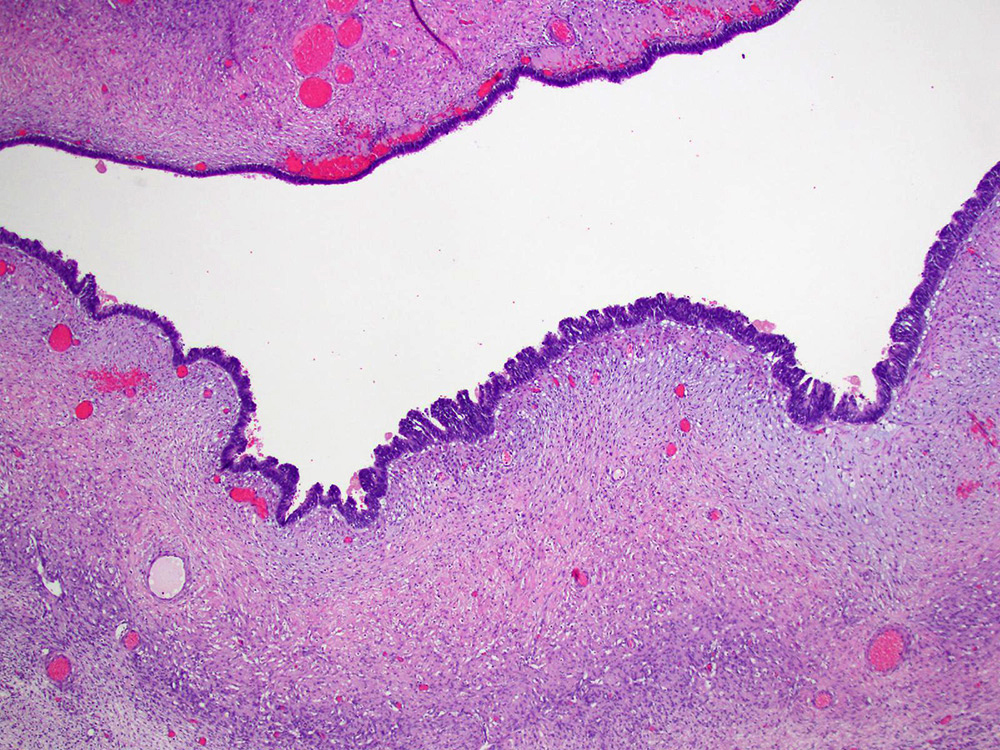
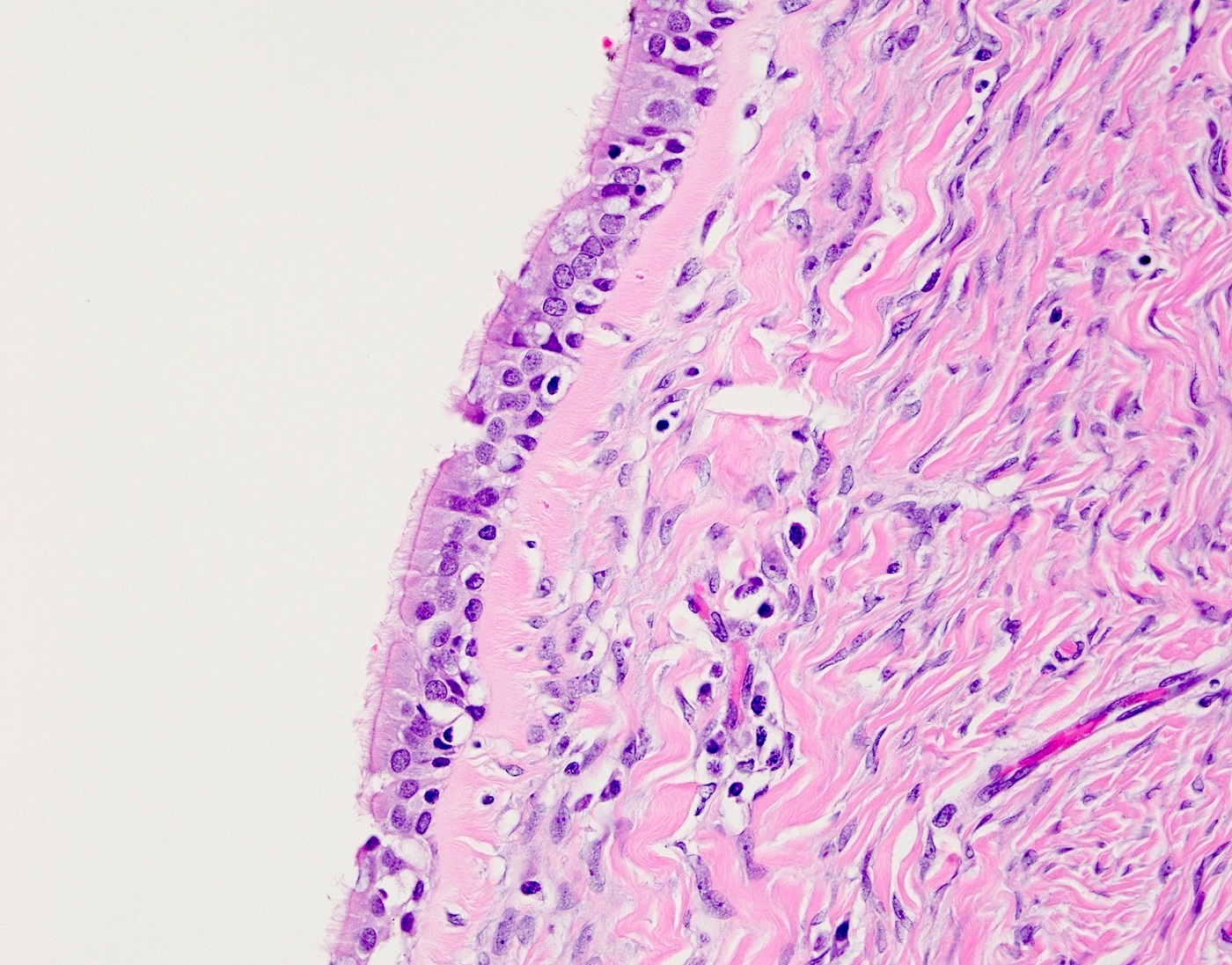
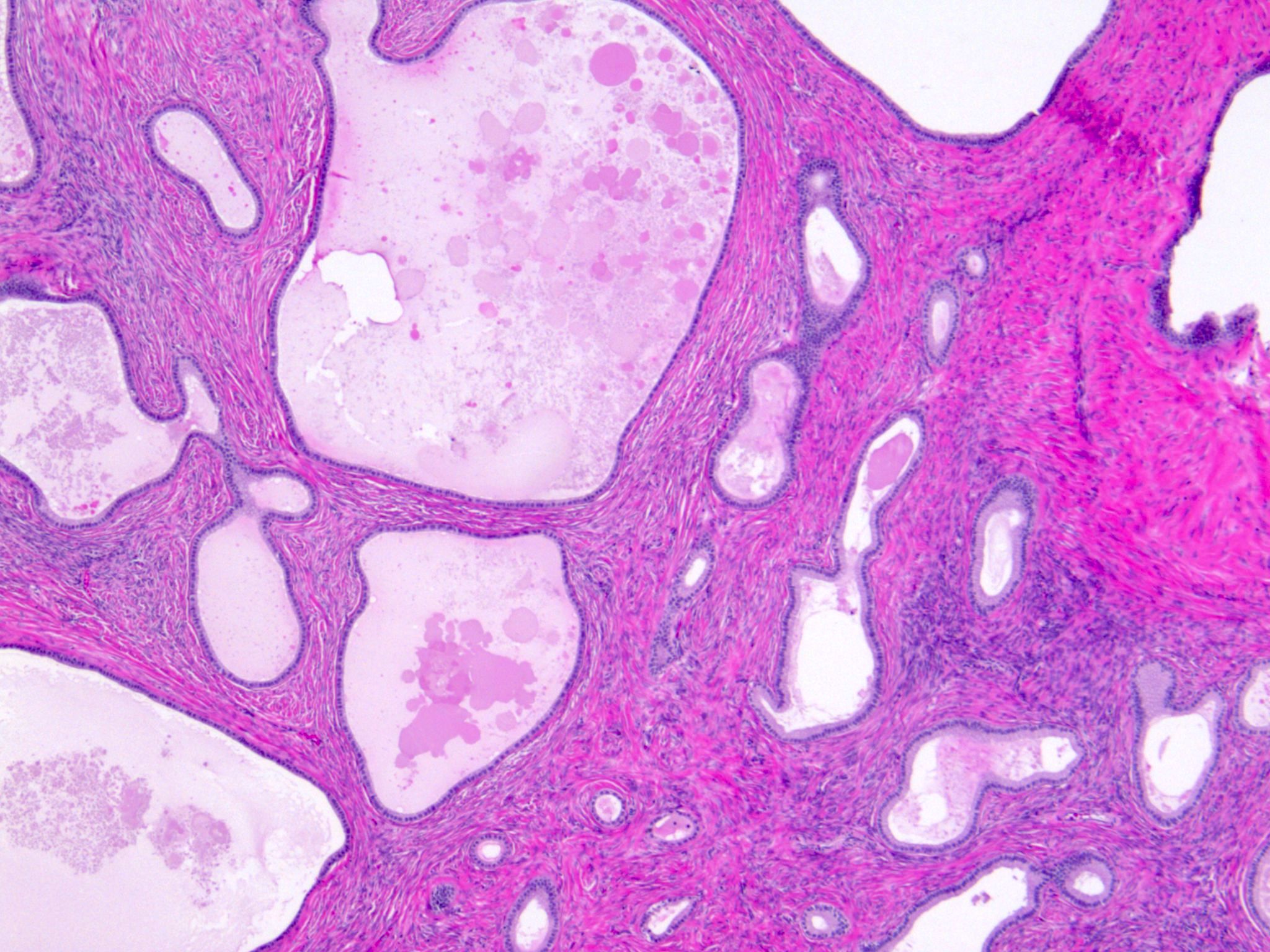
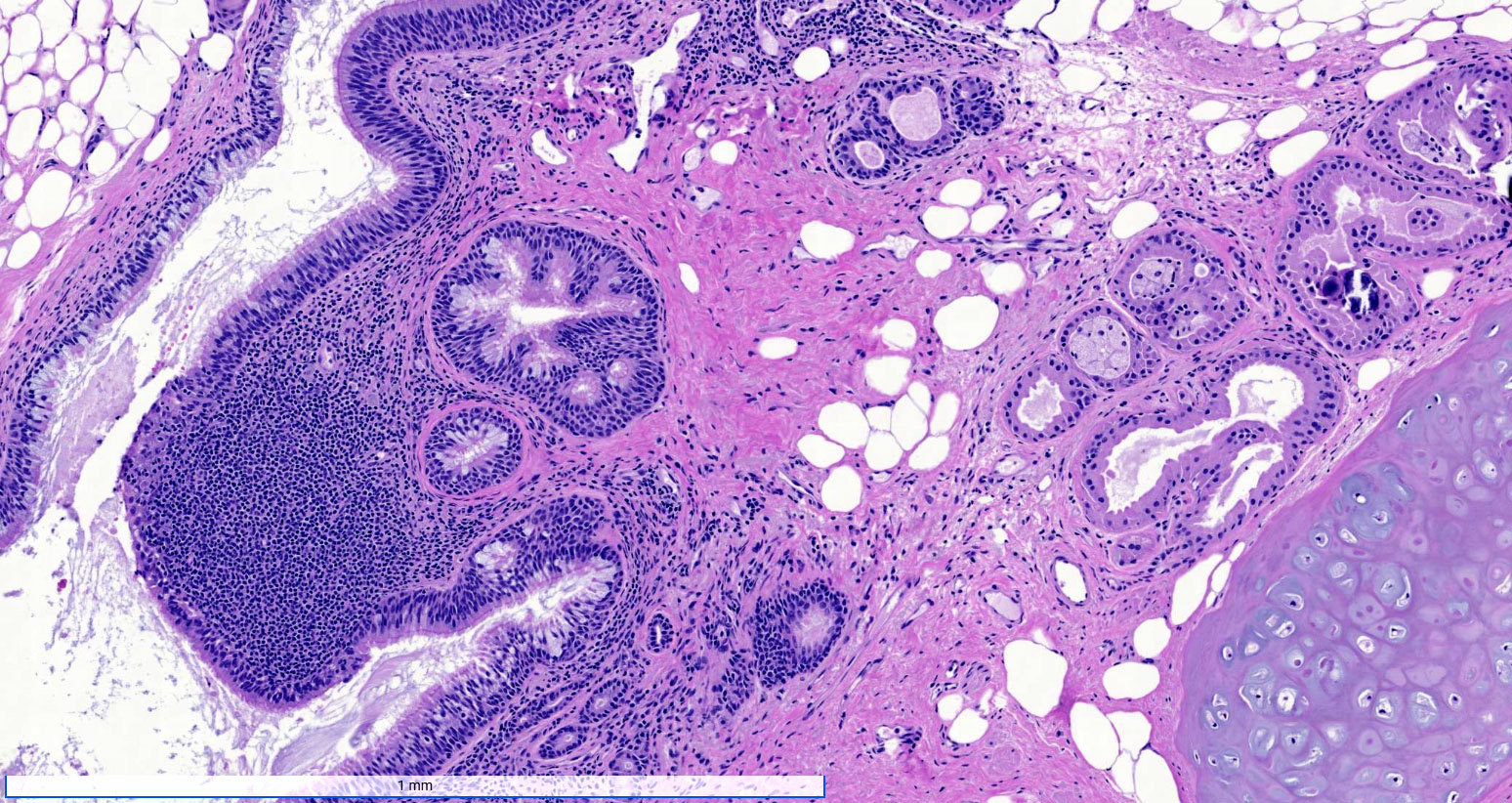
- Mesothelial inclusion cyst:
- Superficial cysts that are lined by mesothelial cells and represent invagination of the surface mesothelial epithelium
- Usually seen in ovaries with clefts (Ann Diagn Pathol 2020;46:151475)
- Endosalpingiosis:
- Group of glands and cysts with a tubal epithelial lining
- Usually seen in ovaries without clefts and commonly associated with calcification
- Rim of fibrotic tissue can surround them (Ann Diagn Pathol 2020;46:151475)
- In contrast to inclusion cysts, their presence can be associated with the recurrence of low grade serous neoplasms (Int J Gynecol Pathol 1998;17:1)
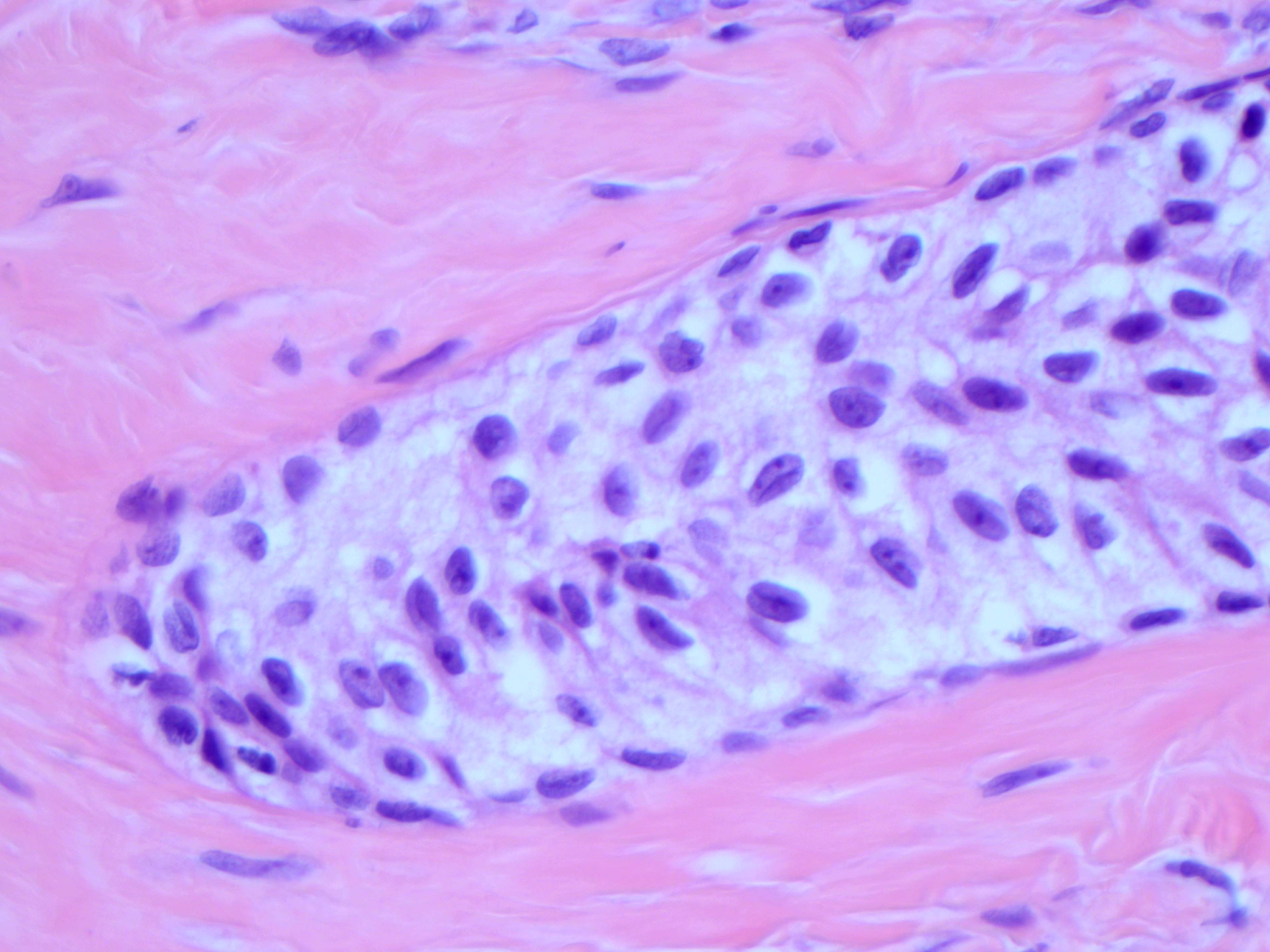
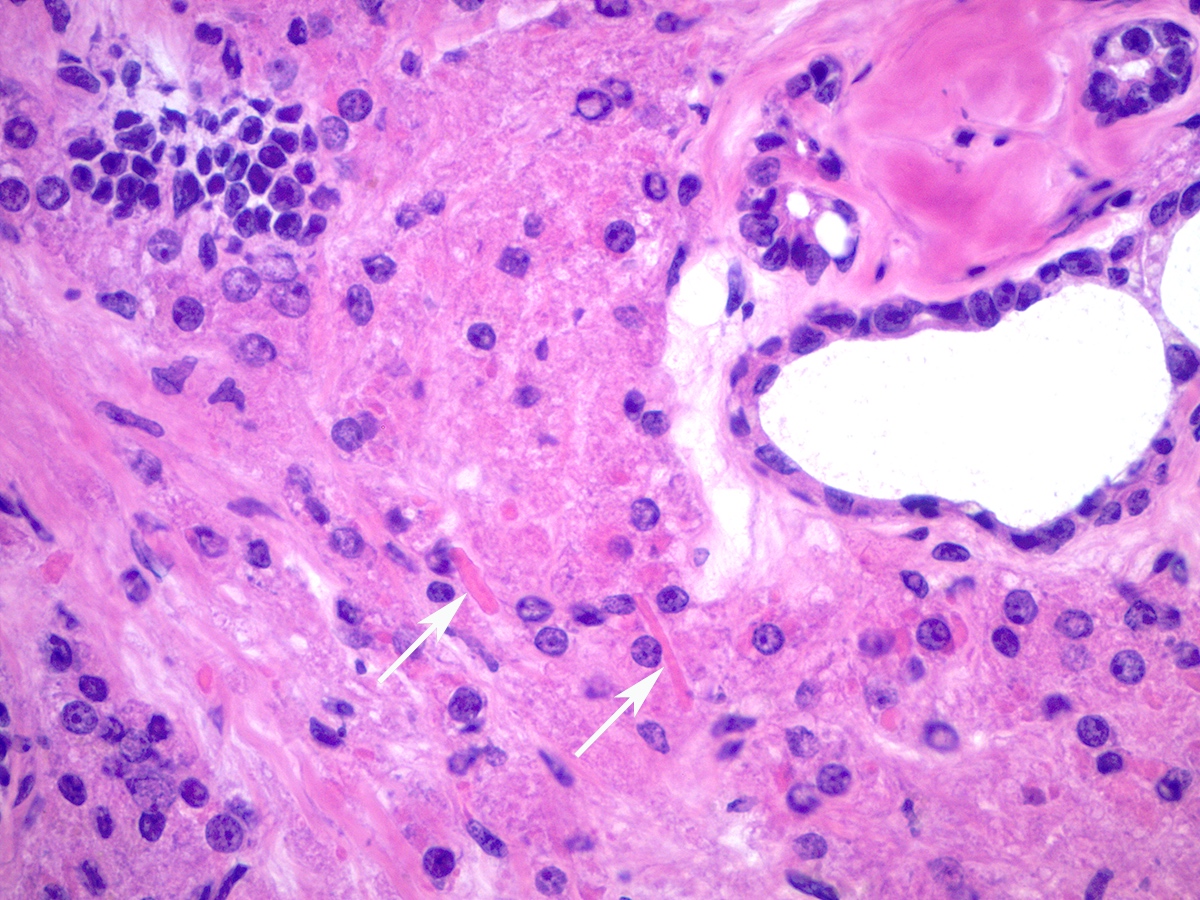
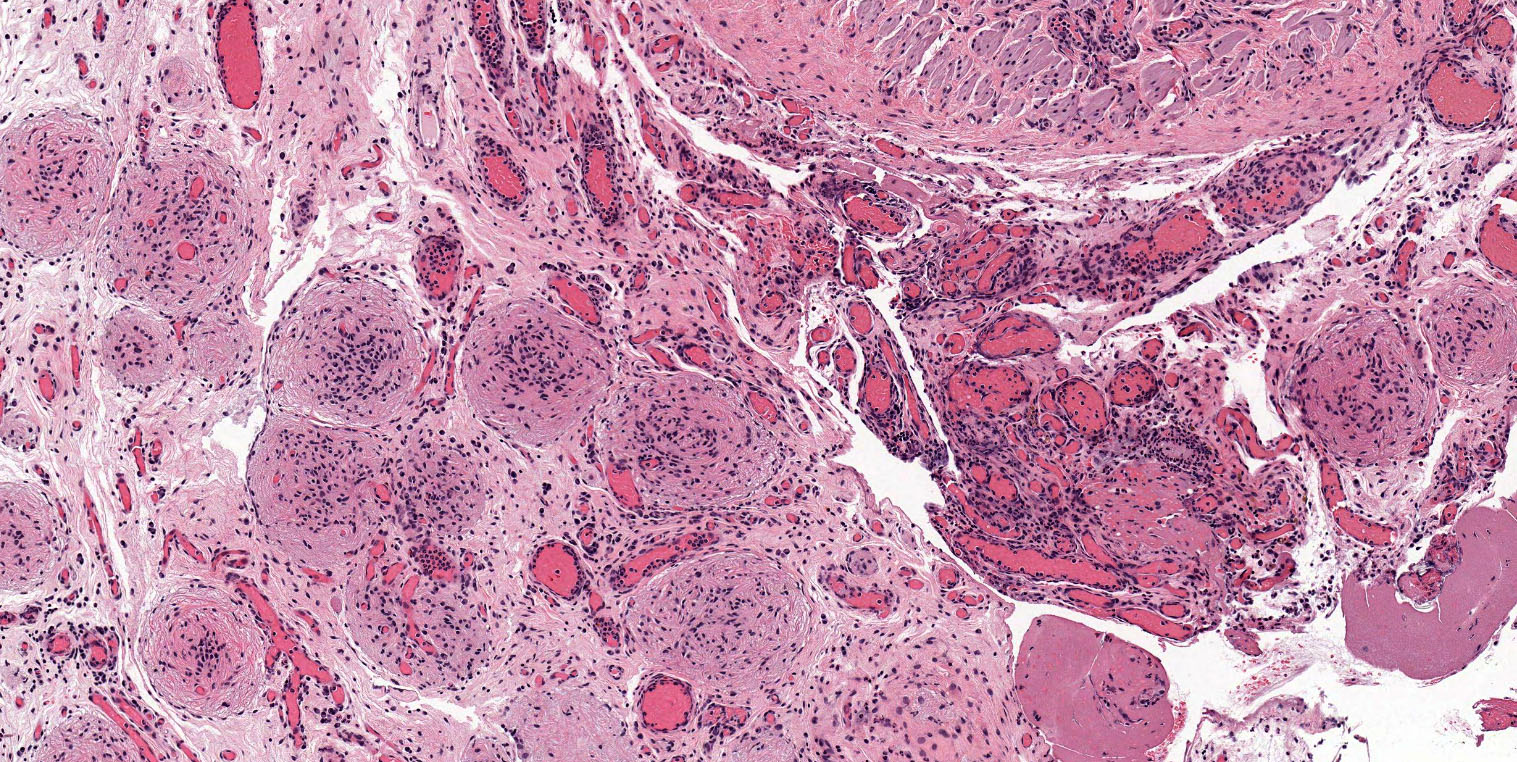
- Hilus (hilar) cells:
- Located in ovarian medulla, rarely within ovarian stroma; located away from the hilum, round to polygonal, epithelial appearing, presumed vestigial remnant of gonad from its ambisexual phase
- Closely associated with large hilar veins and lymphatics and may protrude within their lumina; also associated with nerves
- May contain Reinke crystalloids, lipid, lipochrome pigment
- Resemble steroid cells by electron microscopy with microtubular smooth endoplasmic reticulum, mitochondria with tubular cristae
- Hilar cells are seen in the fetal ovary but not in infancy and childhood; they reappear at puberty
- Hilar cell hyperplasia: associated with hCG administration, pregnancy and choriocarcinoma
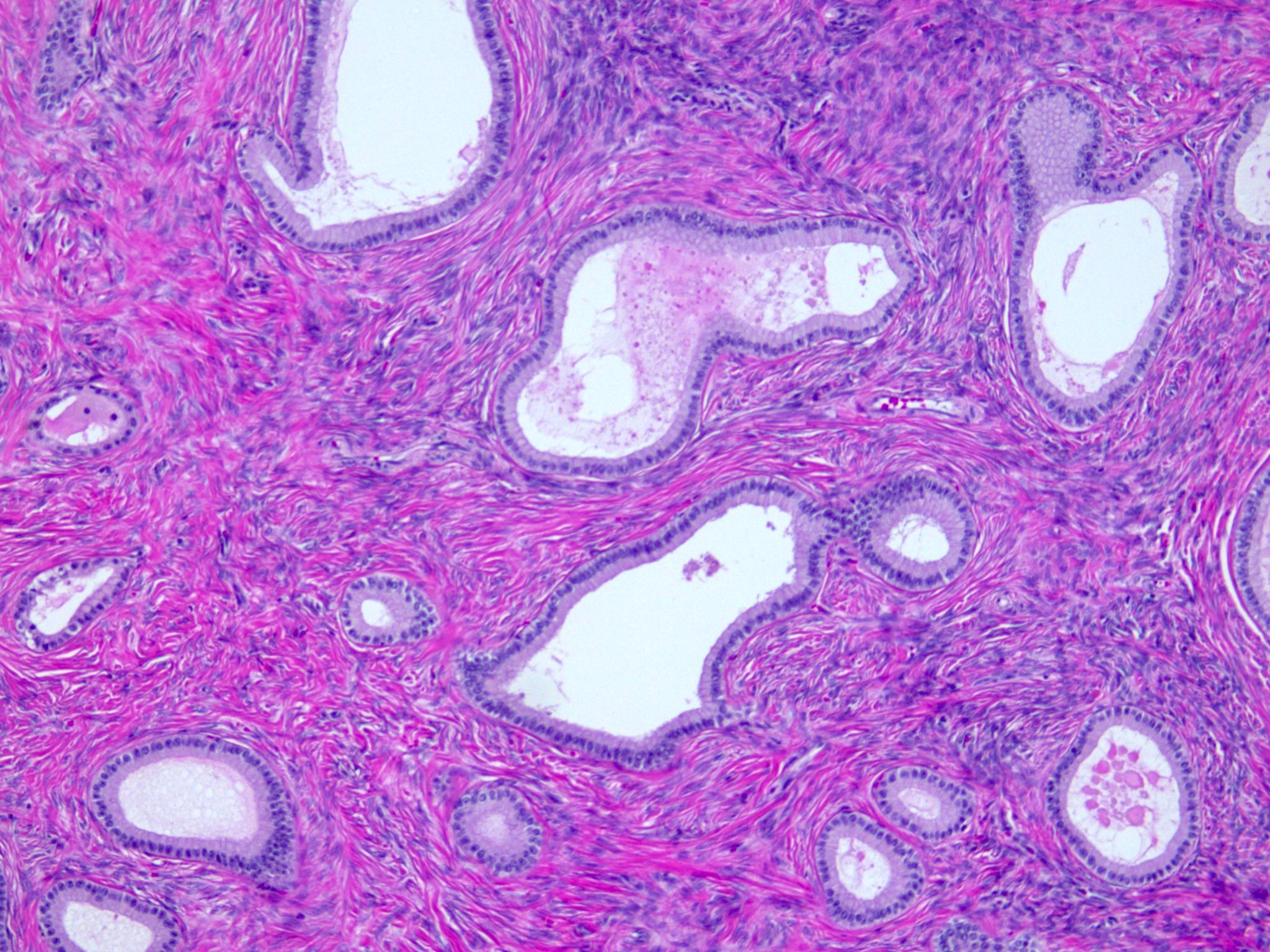
- Rete ovarii:
- Counterpart of rete testis
- Seen as clefts, tubules, cysts, papillae lined by cuboidal or columnar epithelium
- Located usually in the hilum of the ovary, surrounded by spindle cell stroma
- Walthard cell nests:
- Usually microscopic cystic / solid structures with urothelial type epithelium and variable mucin seen in mesovarium, mesosalpinx and ovarian hilus
- Ovarian stem cells:
- Ovary can house stem cells for variable cell types with both somatic and germline stem cells being mentioned
- Somatic stem cells include granulosa cells, surface epithelial cells, thecal cells and stromal cells (Reproduction 2019;157:545)
- Cortical granuloma:
- Common incidental finding in the ovaries of late reproductive and postmenopausal women, composed of an aggregate of epithelioid histiocytes surrounded by a rim of lymphocytes
- They are of uncertain origin but could represent previous areas of endometriosis, luteinized stromal cells or ectopic decidua (Int J Gynecol Pathol 2016;35:544)
- Surface epithelium:
- Can show significant difference in the composition between the right and left side (Int J Gynecol Pathol 1998;17:1)
Microscopic (histologic) images
Virtual slides
Positive stains
Negative stains
Videos
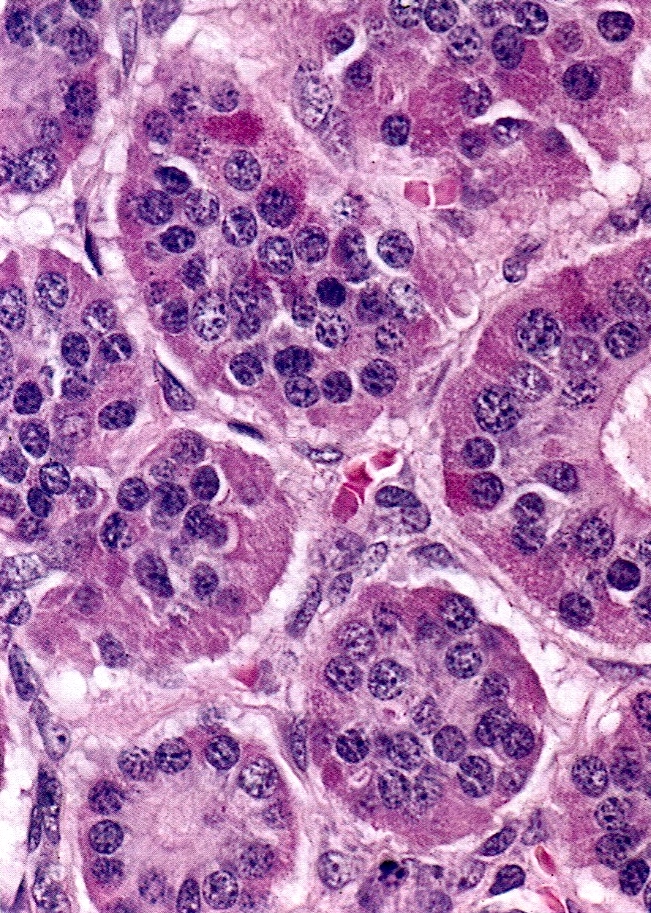
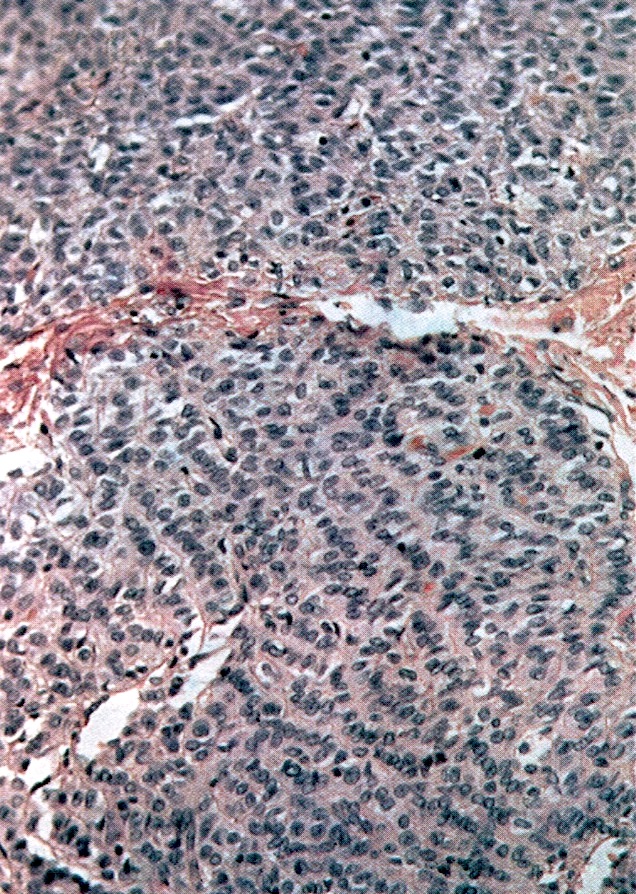
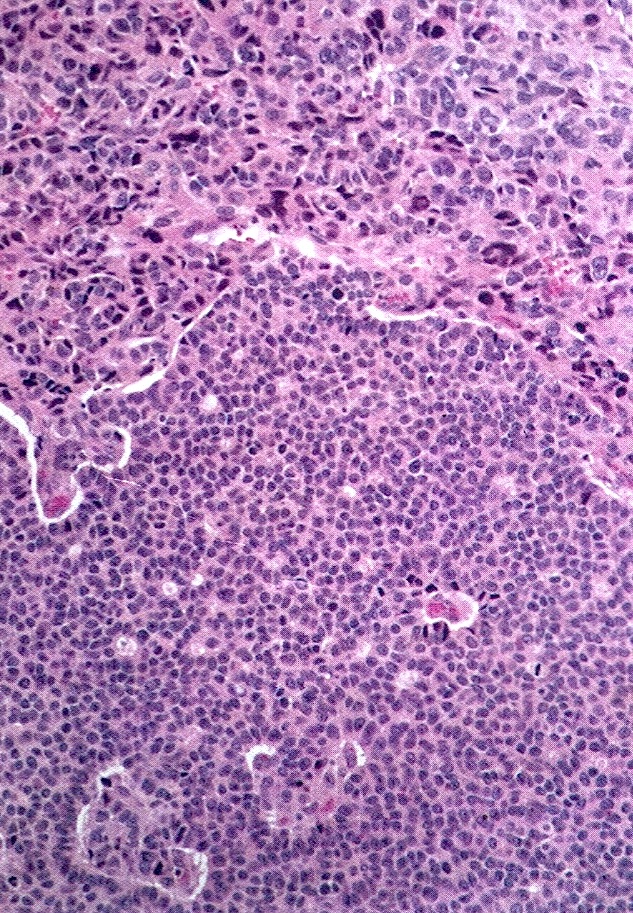
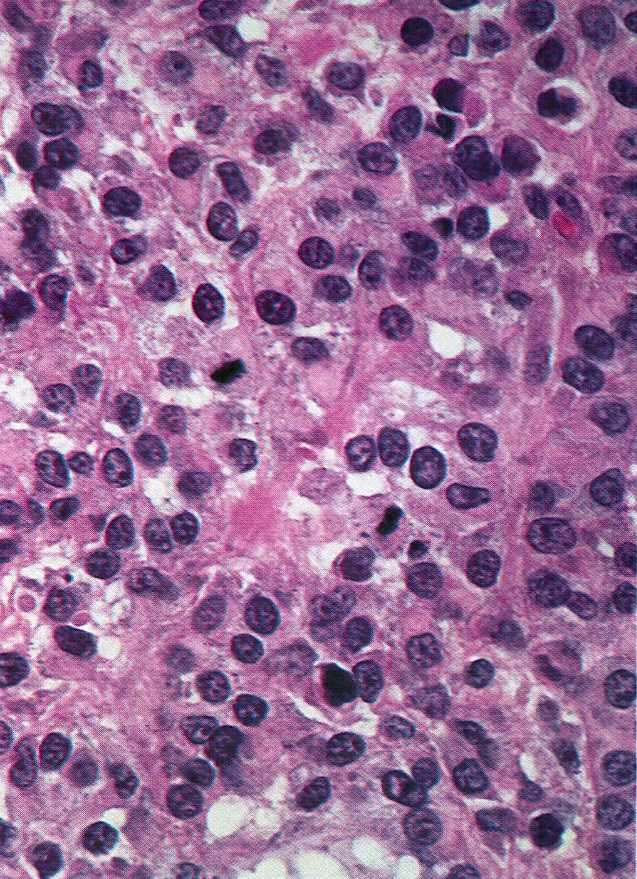
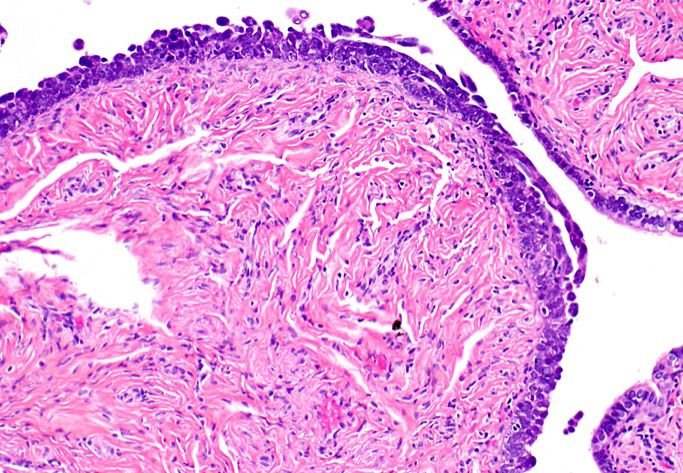
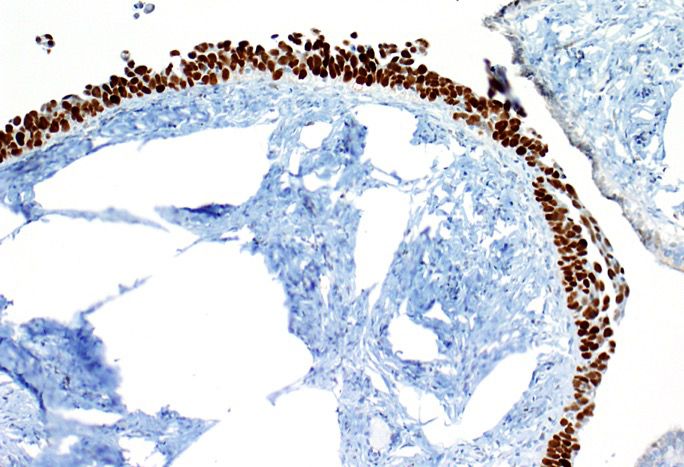
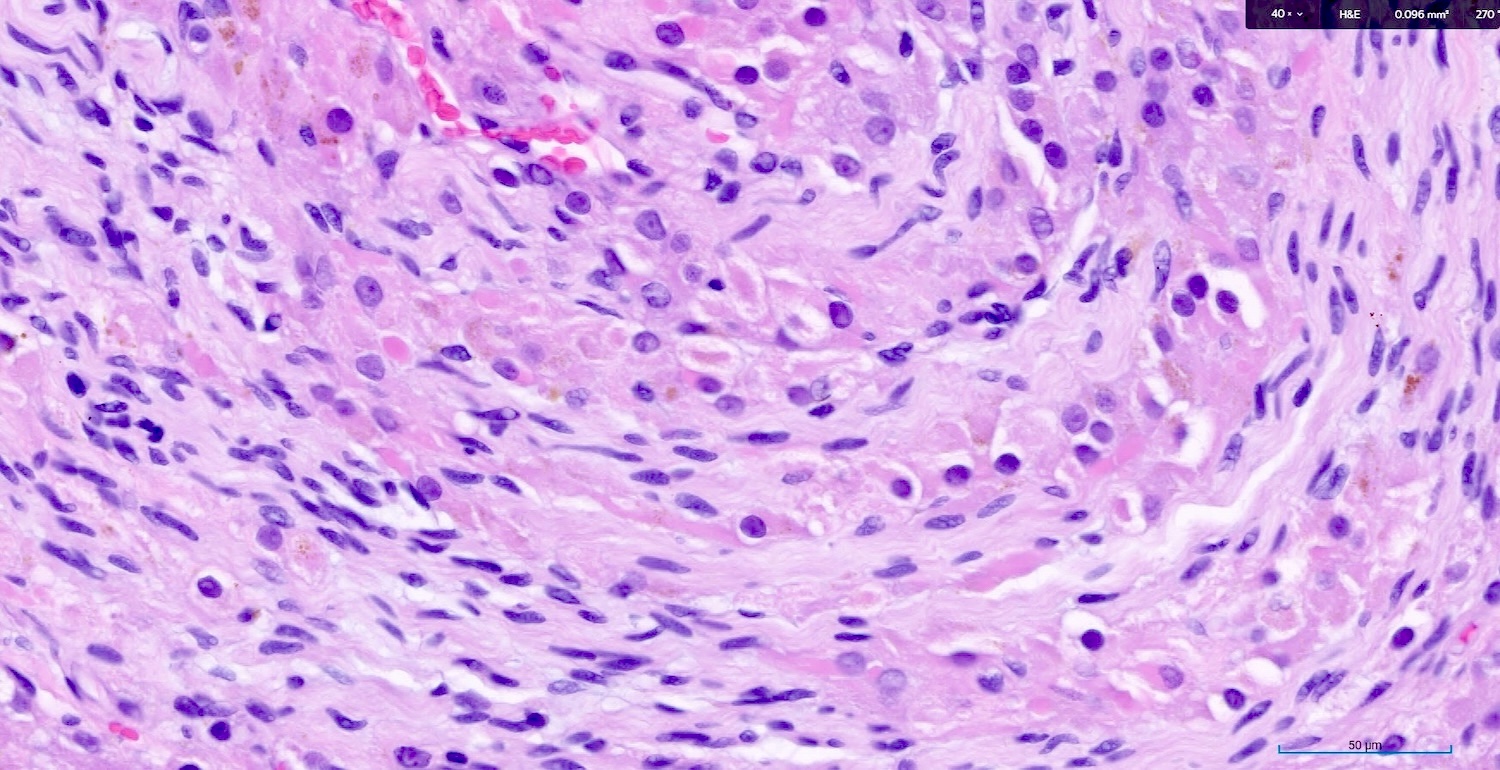
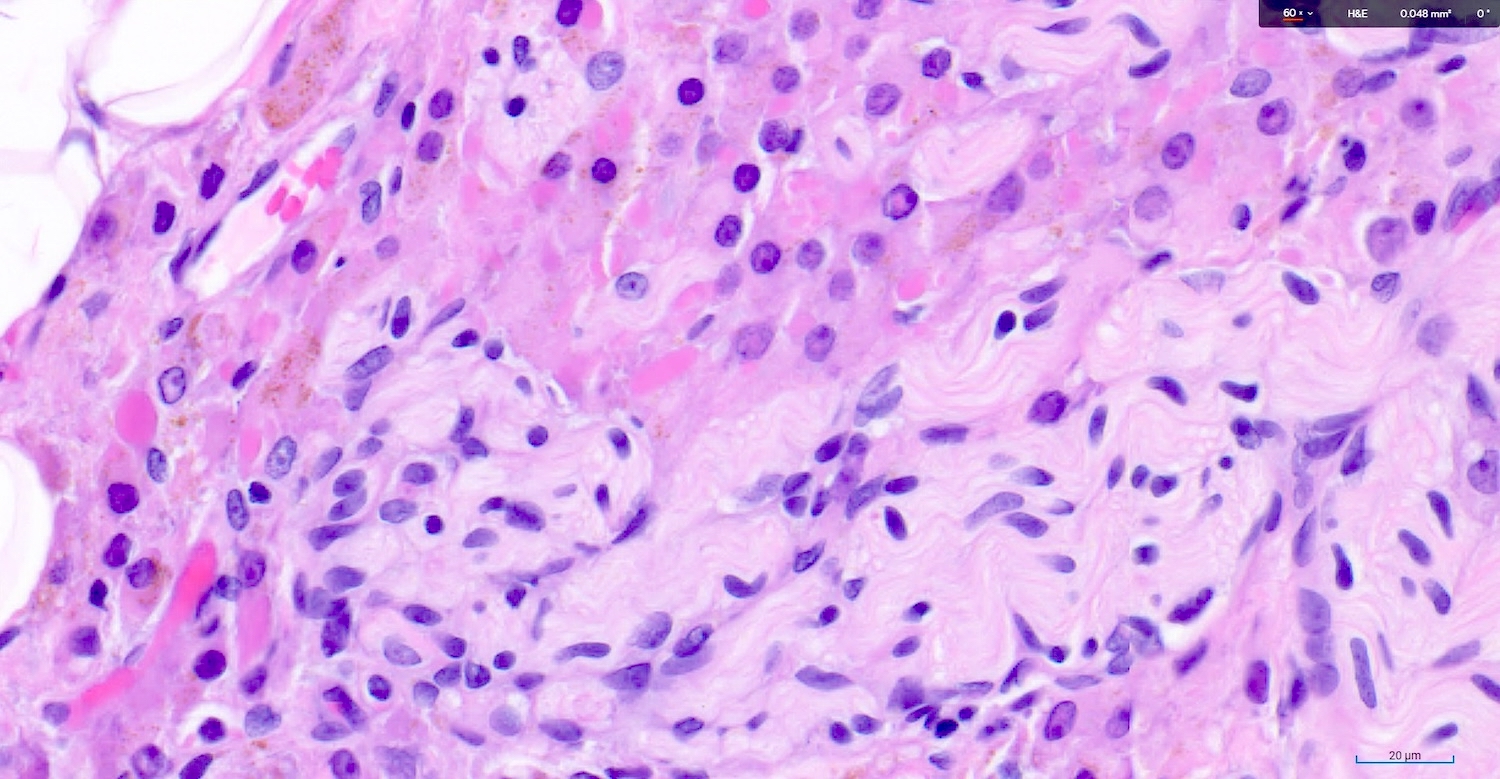
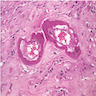
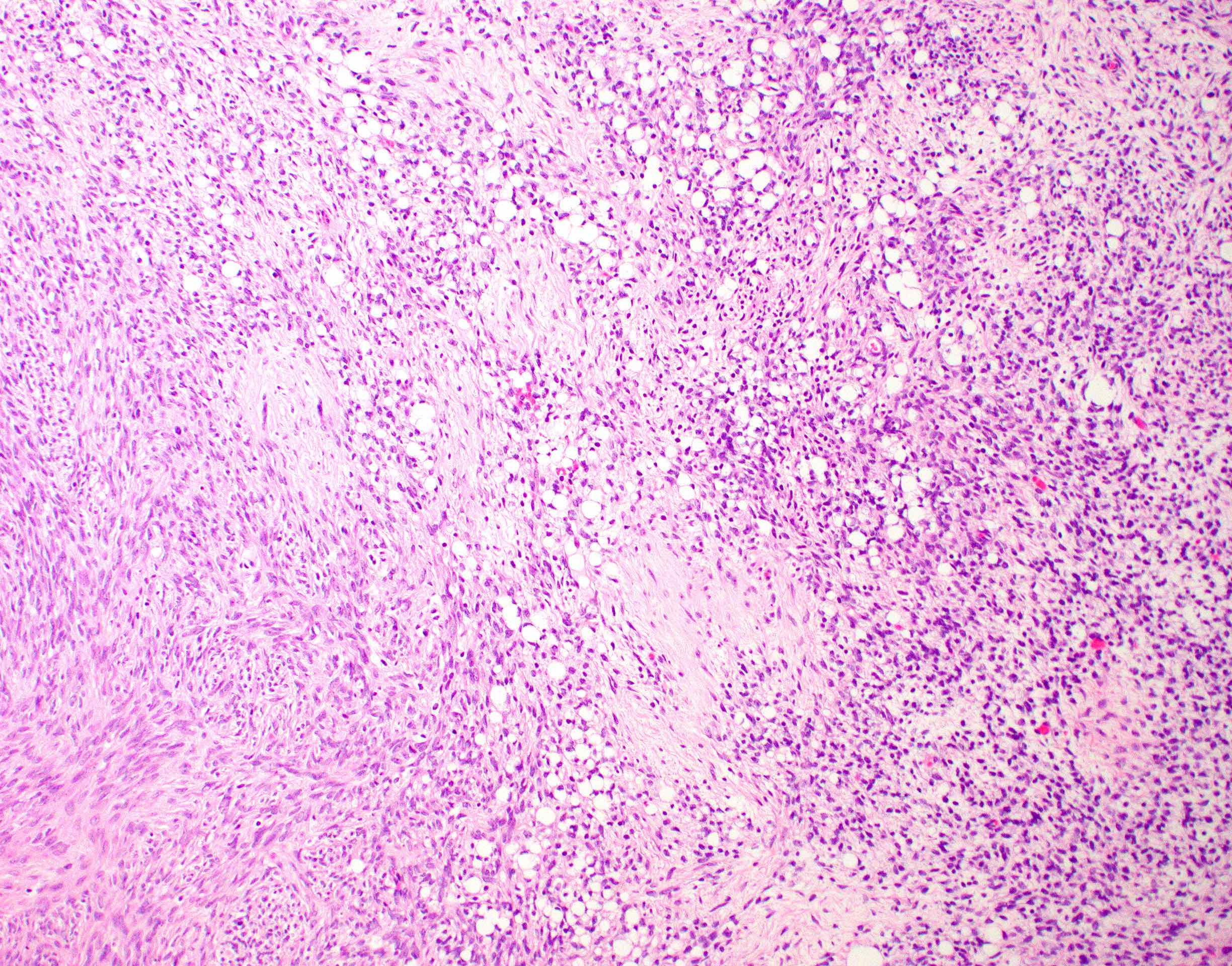
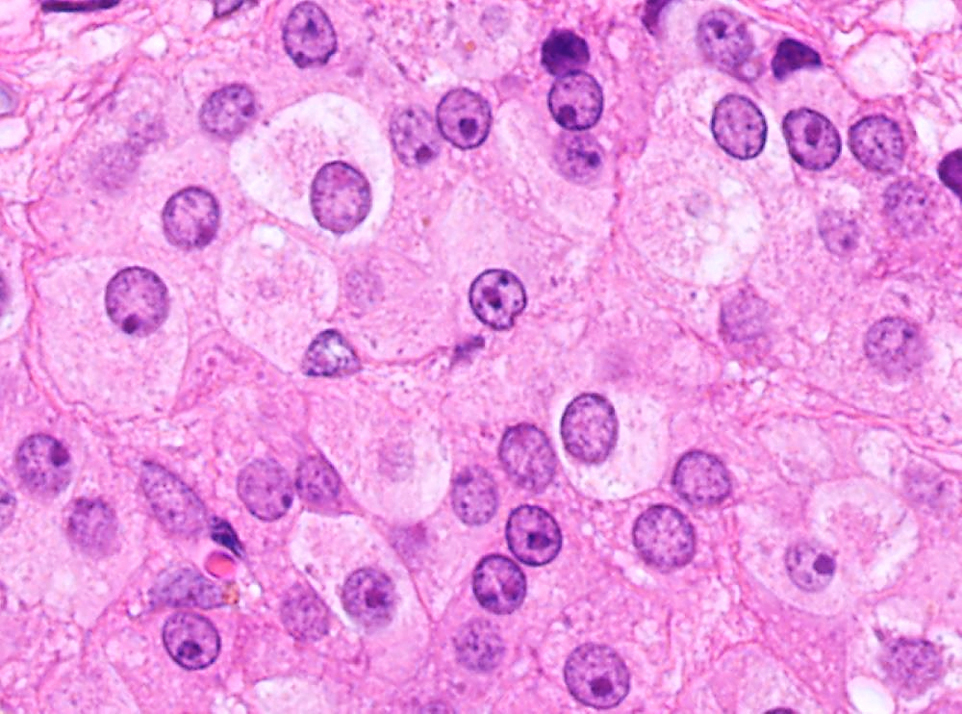
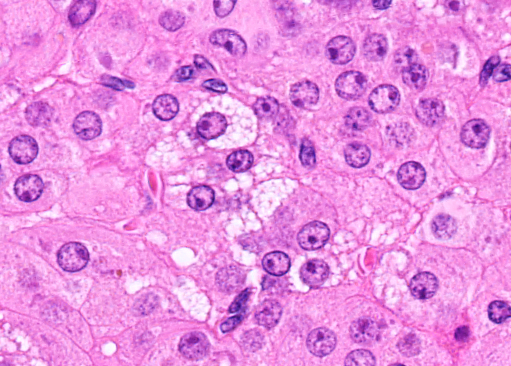
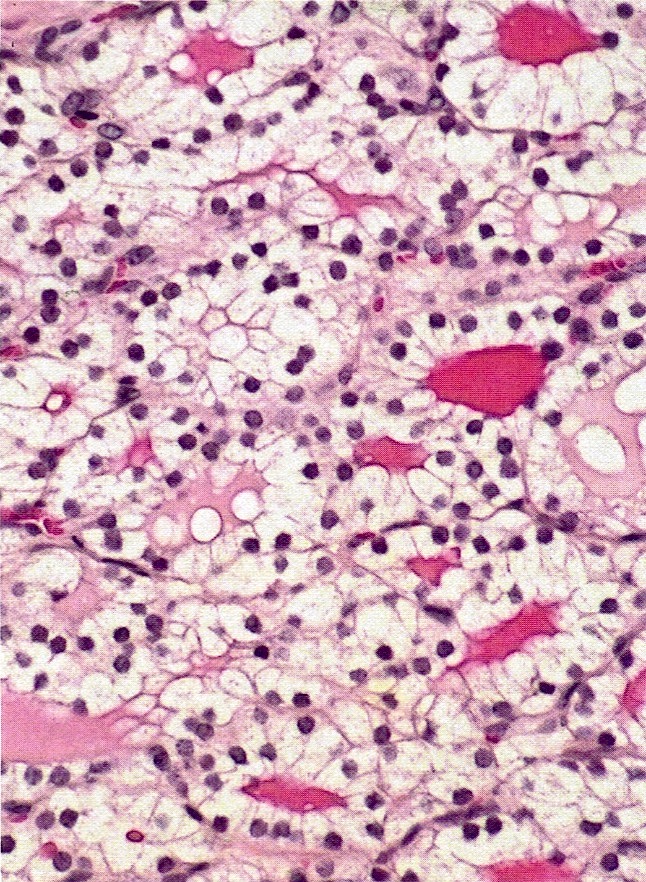
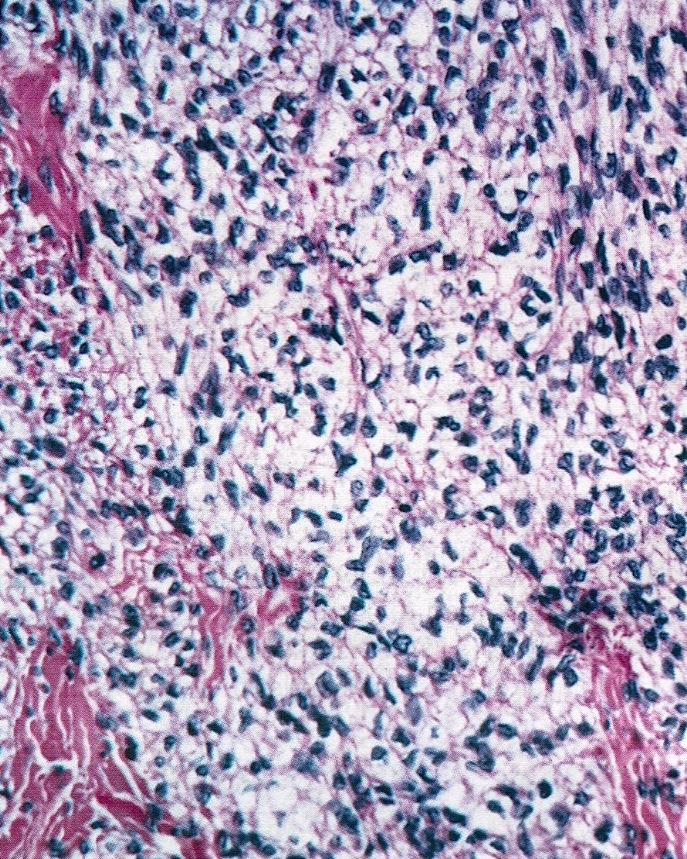
Corpus luteum
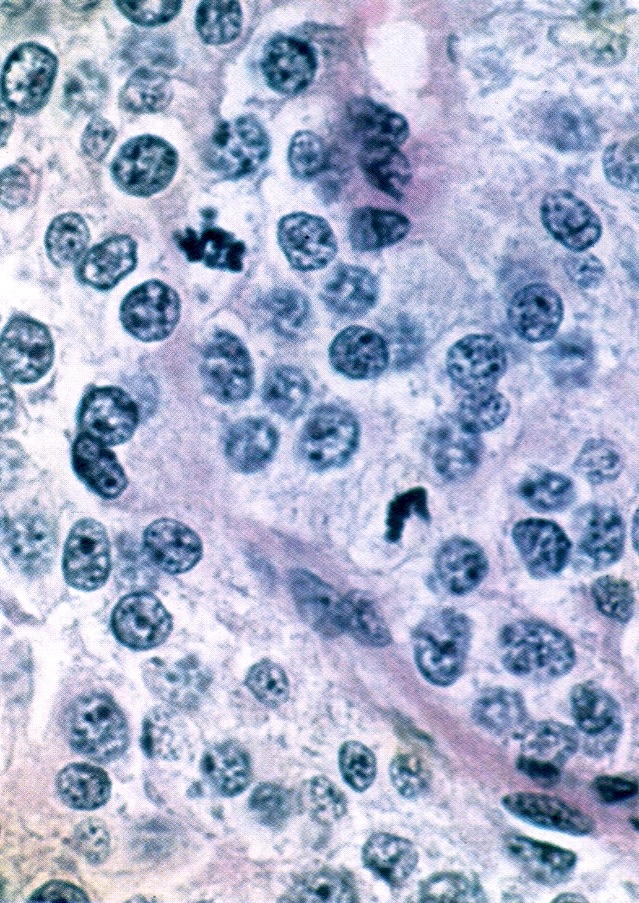
Corpus albicans
Board review style question #1
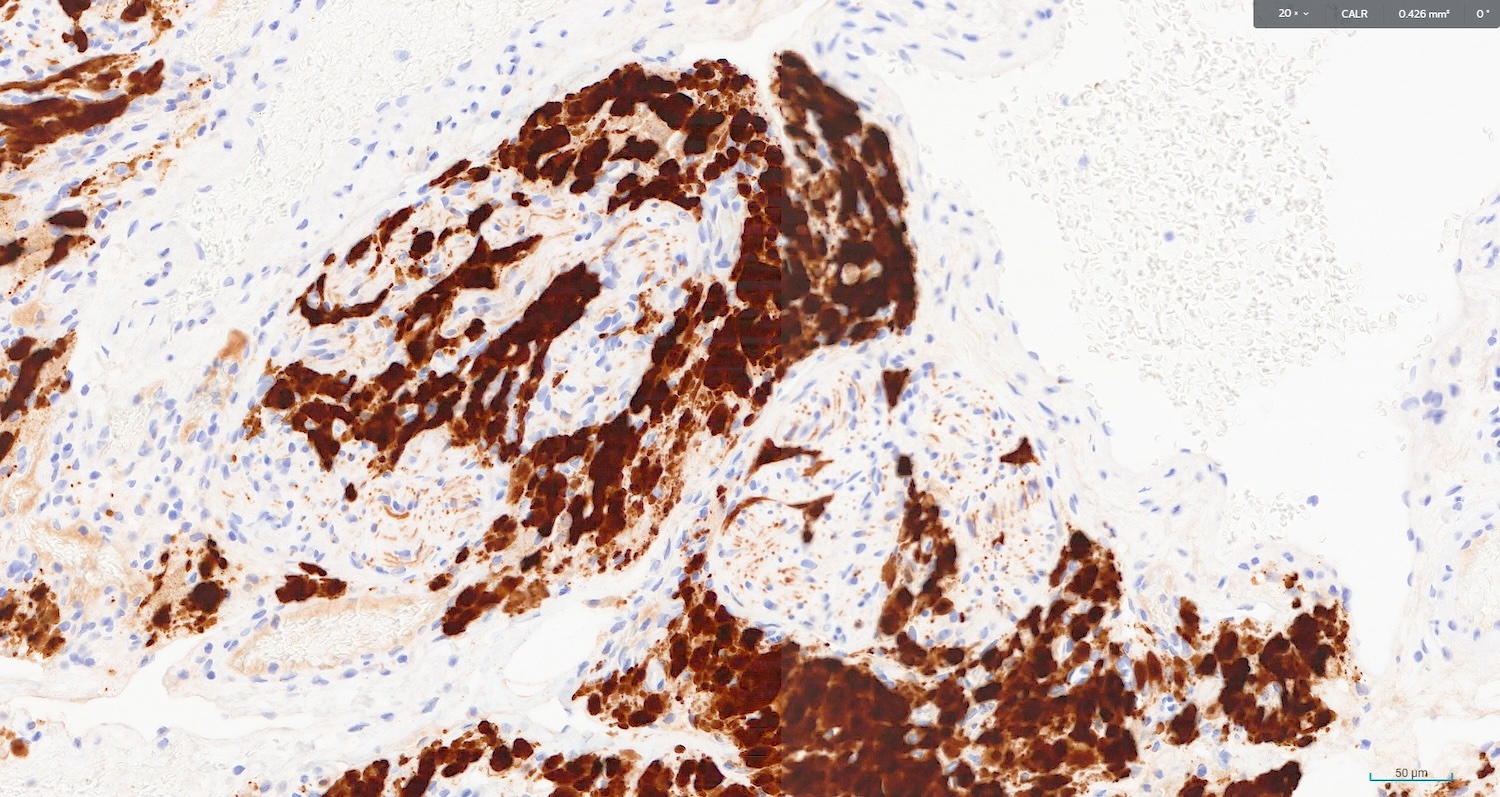
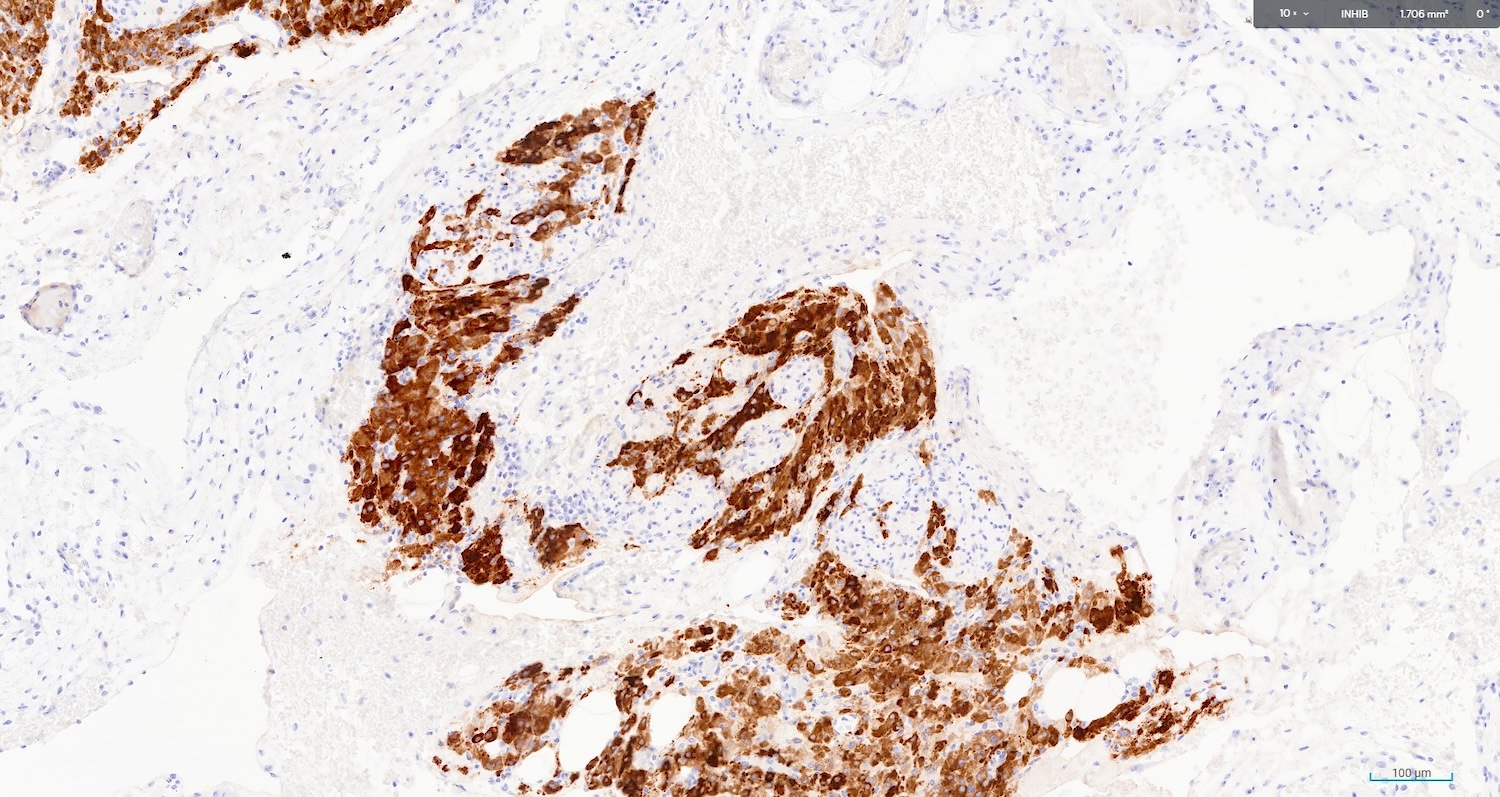
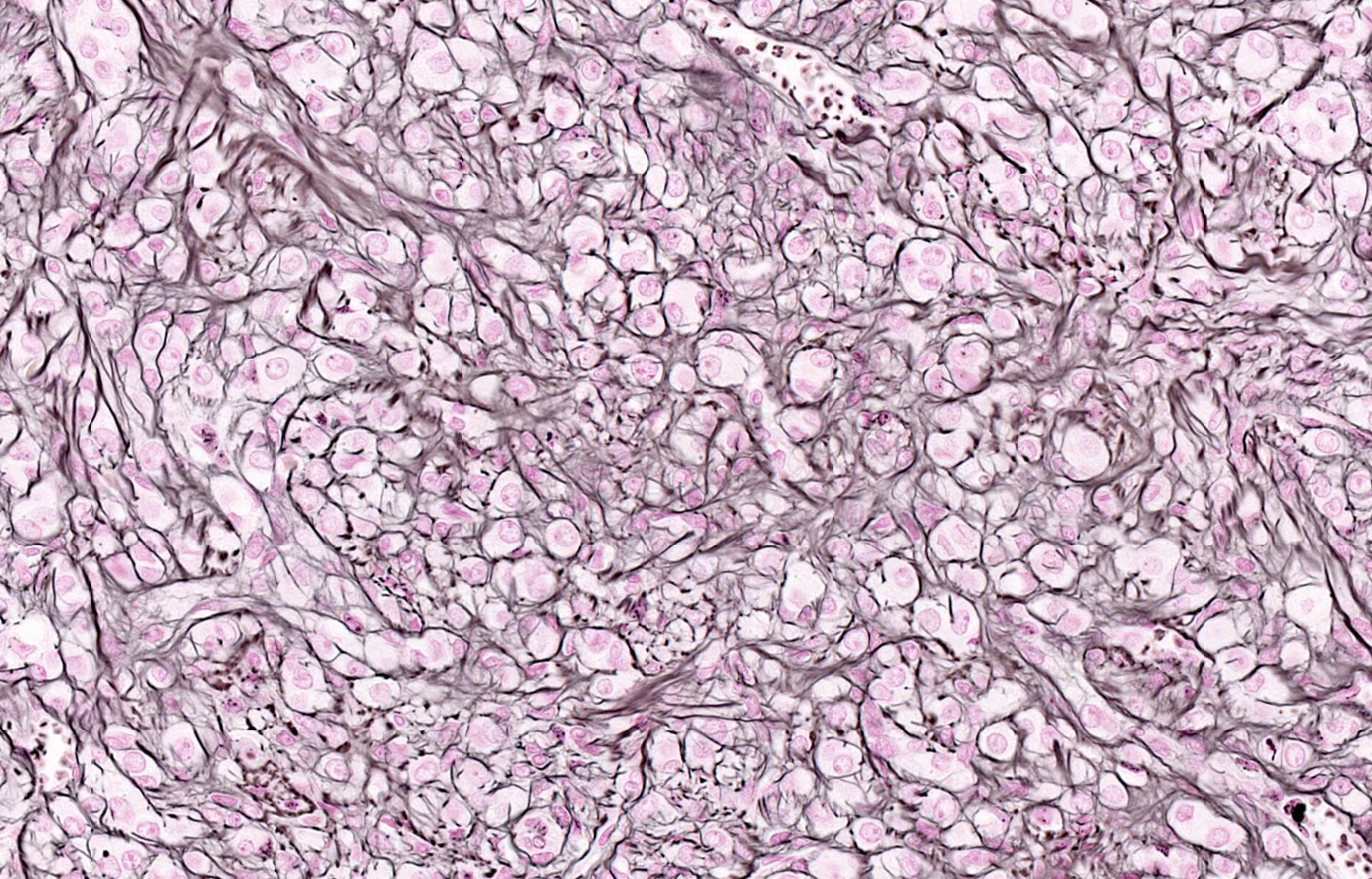
The image above represents a section of a well circumscribed, yellow, cystic nodule seen on gross examination of the ovary. The cells are positive for inhibin and calretinin. Which statement is correct?
- Its presence is permanent and does not depend on the phase of the menstrual cycle or the age of the patient
- Surgical removal of the ovary is indicated to prevent metastasis
- The image represents a physiologic constituent of the ovary
- The image represents a sex cord stromal tumor
Board review style answer #1
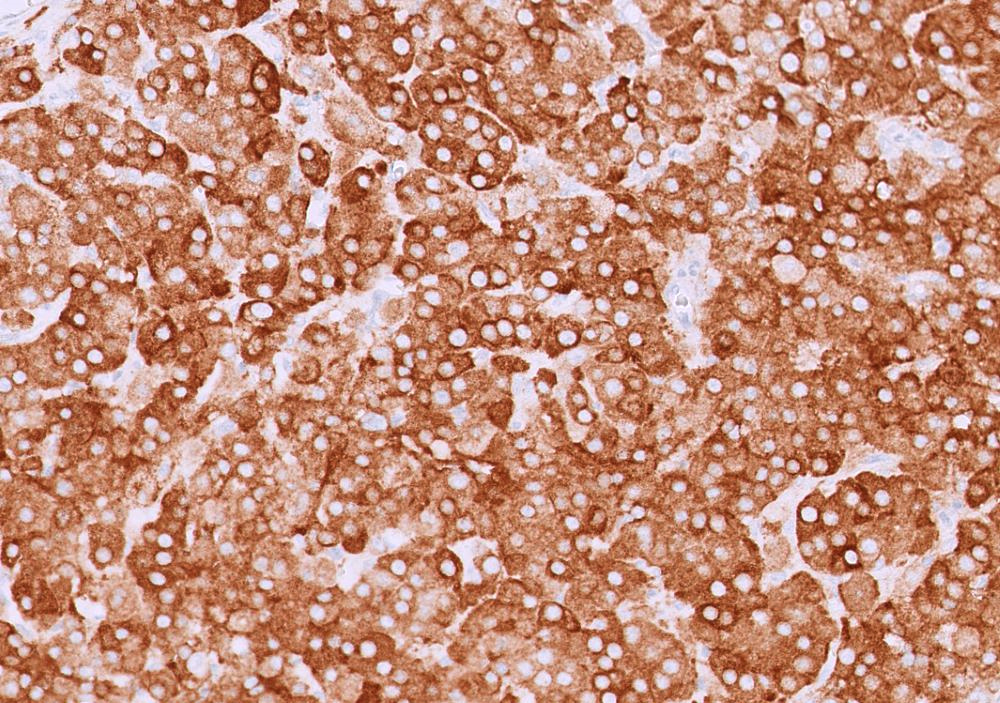
C. The image represents a mature corpus luteum, which is a physiologic structure present in the luteal phase of menstruation. The constituent cells are polygonal with abundant, pale, eosinophilic cytoplasm representing the luteinized granulosa cells. The round nucleus contains 1 or 2 large nucleoli. These cells usually stain similar to steroid hormone producing cells and are typically positive for inhibin and calretinin.
Comment Here
Reference: Ovary - Anatomy & histology
Comment Here
Reference: Ovary - Anatomy & histology
Board review style question #2
Which of the following microscopic findings in an ovary are indicators of a pathologic condition?
- Benign tubular structures located within the ovarian cortex; constituent cells are positive for mesothelial markers
- Benign polygonal cells with clear cytoplasm, round nucleus and positive staining for inhibin and calretinin
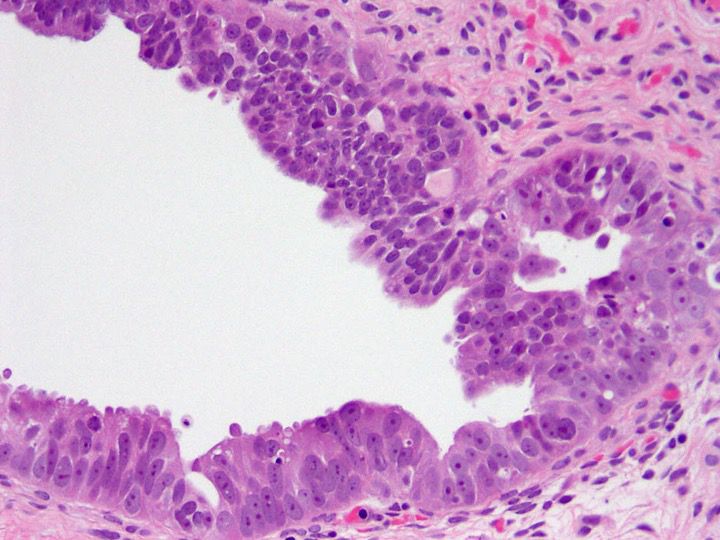
- Epithelial cell proliferation composed of papillary structures lined by cuboidal cells and exhibiting hierarchical branching and tufting of the lining cells
- Urothelial-like nests of cells known as Walthard nests
Board review style answer #2
C. The description is that of a serous neoplasm. Complex architecture including hierarchical branching and tufting of lining epithelial cells should not be seen in normal ovary or in the context of endosalpingiosis.
Comment Here
Reference: Ovary - Anatomy & histology
Comment Here
Reference: Ovary - Anatomy & histology
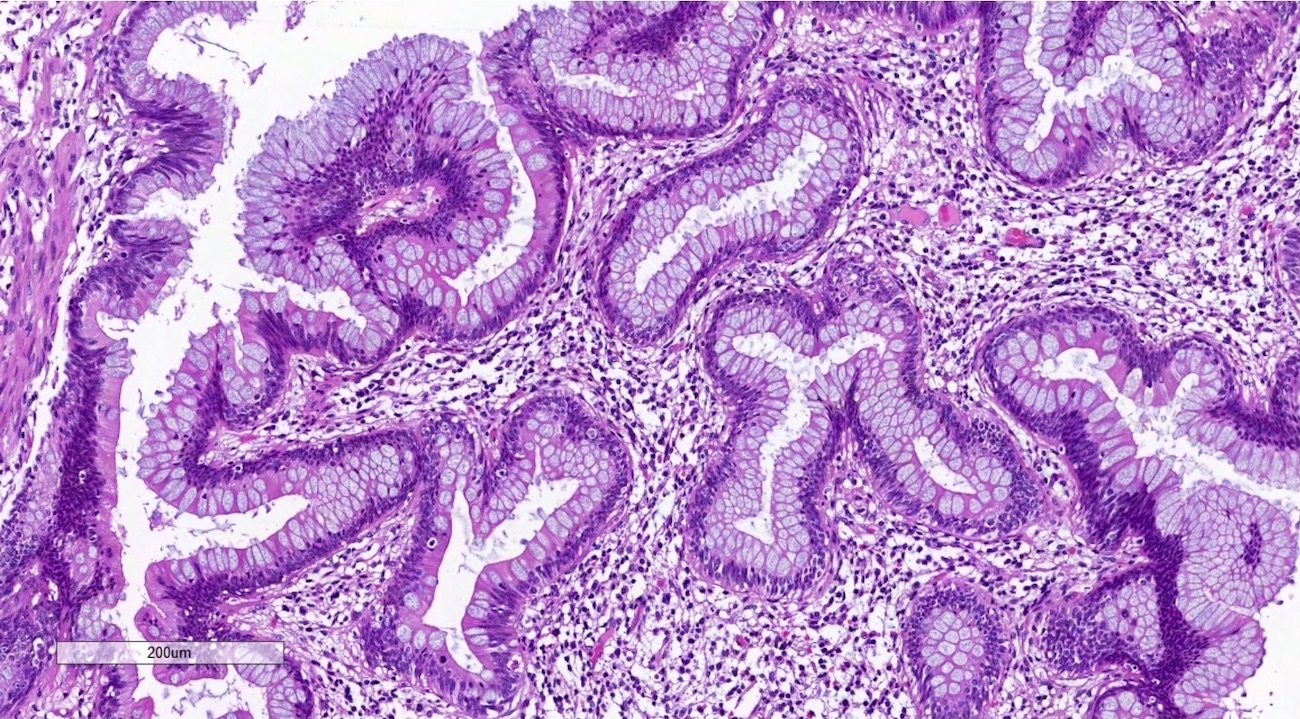
Appendiceal neoplasms
Table of Contents
Definition / general | Terminology | Epidemiology | Pathophysiology | Clinical features | Laboratory | Radiology description | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Differential diagnosisDefinition / general
- Mucinous neoplastic proliferations of the cecal appendix commonly manifest with signs of extra-appendiceal spread, particularly in the form of pseudomyxoma peritonei
- Involvement of other organs such as the ovary may be the first manifestation of the disease
- Current evidence supports that, in the context of pseudomyxoma peritonei, an ovarian mucinous tumor should be regarded as appendiceal in origin, with the exception of a mucinous tumor arising in a mature teratoma (Am J Surg Pathol 1991;15:415, Int J Gynecol Pathol 1997;16:1)
Terminology
- Pseudomyxoma peritonei is a clinicopathologic syndrome characterized by mucinous ascites
- Intra-abdominal mucin is abundant and can be admixed with neoplastic mucinous epithelium (Am Soc Clin Oncol Educ Book 2013;221)
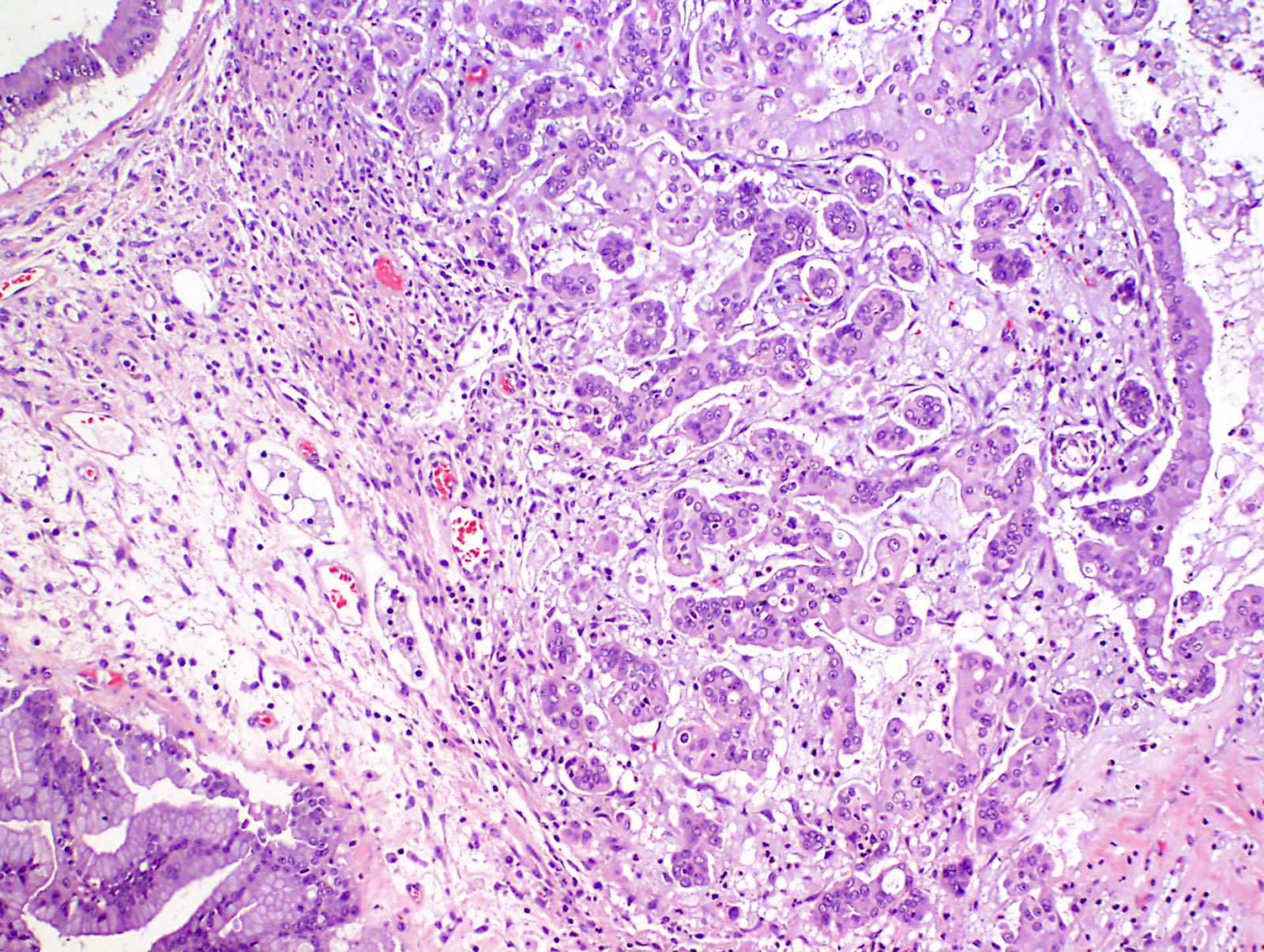
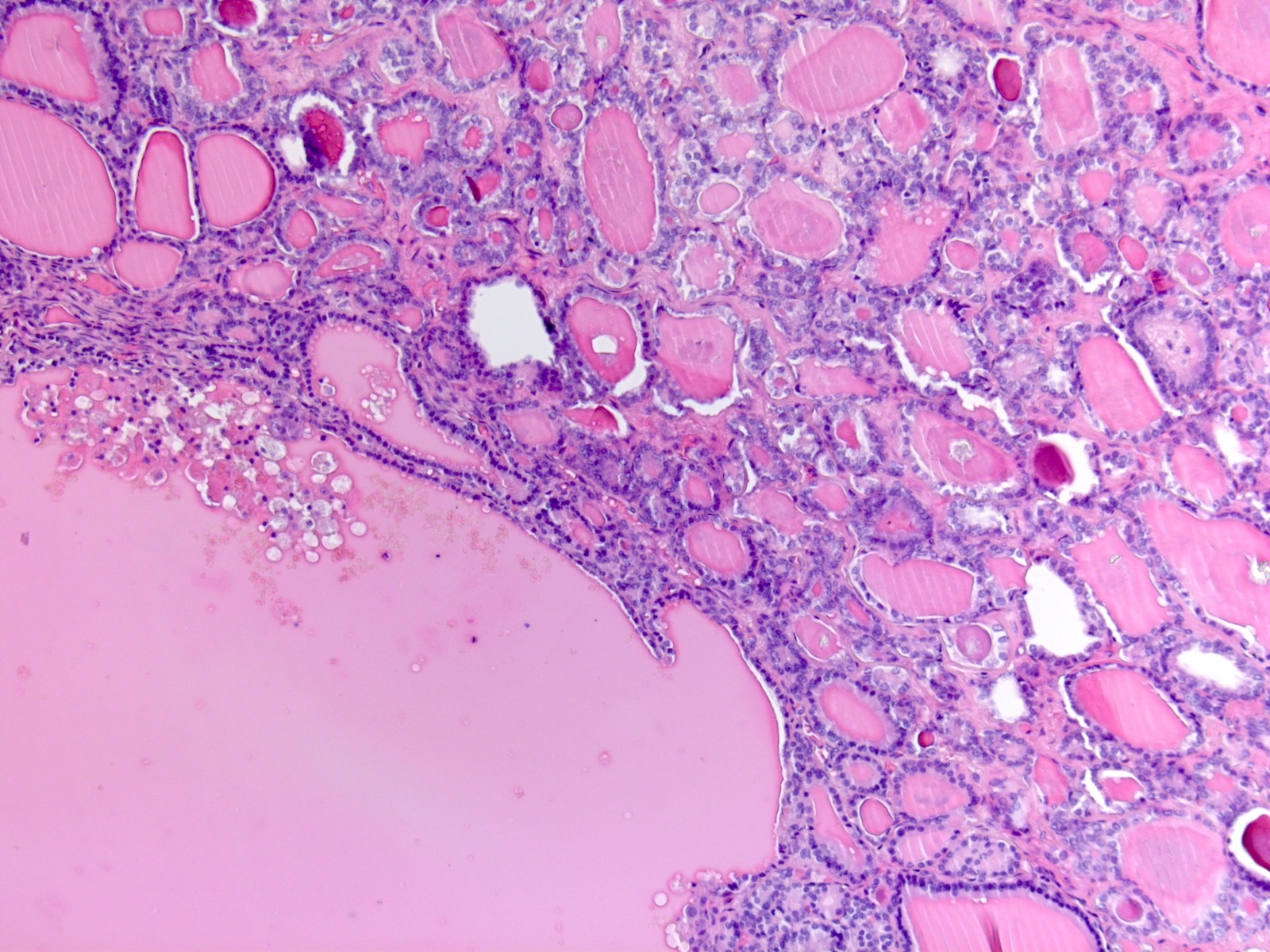
- Although commonly used to refer to all metastatic carcinomas involving the ovary, the term Krukenberg tumor strictly refers to adenocarcinomas with signet-ring cell differentiation, of which most (76%) arise from the stomach (J Clin Pathol 2012;65:585)
Epidemiology
- Primary appendiceal tumors are found in Arch Pathol Lab Med 2011;135:1261)
- 28 - 37% of appendiceal tumors not associated with pseudomyxoma peritoneii present with ovarian metastases (Gynecol Oncol 2014;133:155)
- Among patients with ovarian mucinous tumors showing borderline features, only about 2% have a documented primary appendiceal malignancy
- Despite this low incidence, there is significant overlap of clinical, radiologic and pathologic features between primary and metastatic ovarian adenocarcinoma; in the work-up of a mucinous or endometrioid-like ovarian neoplasm, a secondary malignancy should always be considered pathologically or clinically
- Pseudomyxoma peritonei is 2 - 3 times more common in women than in men
Pathophysiology
- Spread to the ovaries can be hematogenous, lymphatic, transperitoneal or by direct extension (Rev Gastroenterol Mex 1994;59:290)
- The pathogenesis of pseudomyxoma peritonei is still unknown; current theories postulate that the mucin is produced by very low quantities (sometimes undetectable) of neoplastic intestinal-type epithelium, which colonizes the peritoneal cavity presumably after appendiceal tumor rupture
Clinical features
- Patients with metastatic appendiceal adenocarcinoma involving the ovary have a poor prognosis
- Patients with a low grade appendiceal mucinous neoplasm (LAMN) and pseudomyxoma peritonei has a protracted clinical course with multiple recurrences, progressive fibrous adhesions and complications such as fatal obstructive disease
Laboratory
- Elevated CA-125 levels (> 100 U/mL) are common, which has been suggested as a useful feature to distinguish metastatic from primary ovarian mucinous carcinomas (Gynecol Obstet Invest 2011;72:196)
- Elevated CEA levels (> 5 ng/mL) and CA19-9 levels (> 37 U/mL) are seen in a minority of cases
Radiology description
- Bilaterality is seen in up to 83.3% of cases
- On imaging, pseudomyxoma peritonei is suspected if there is irregularly localized or loculated fluid with scalloped appearance within the peritoneal cavity
- The ovarian mass can be cystic, solid or a mixture
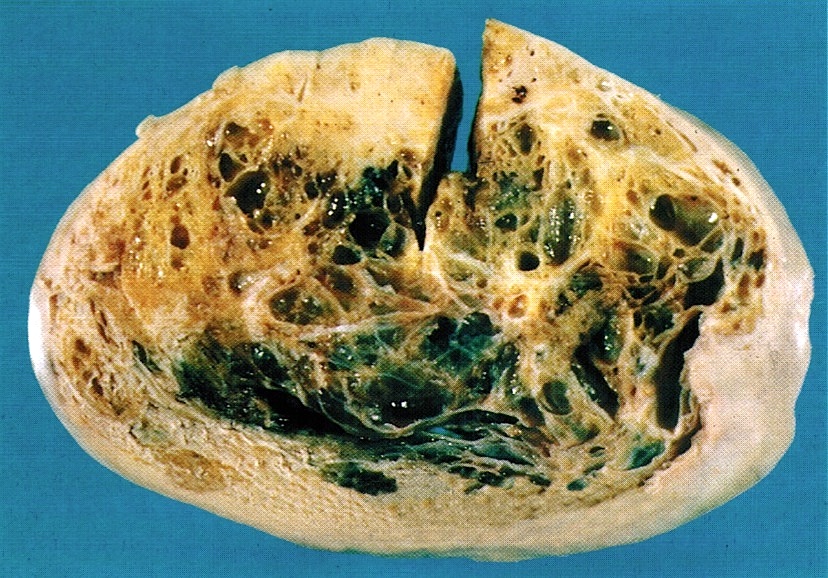
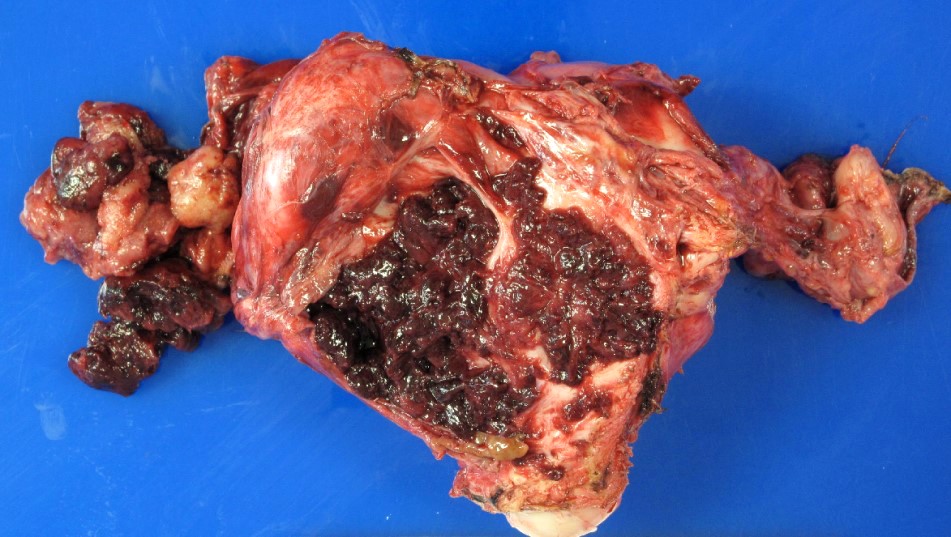
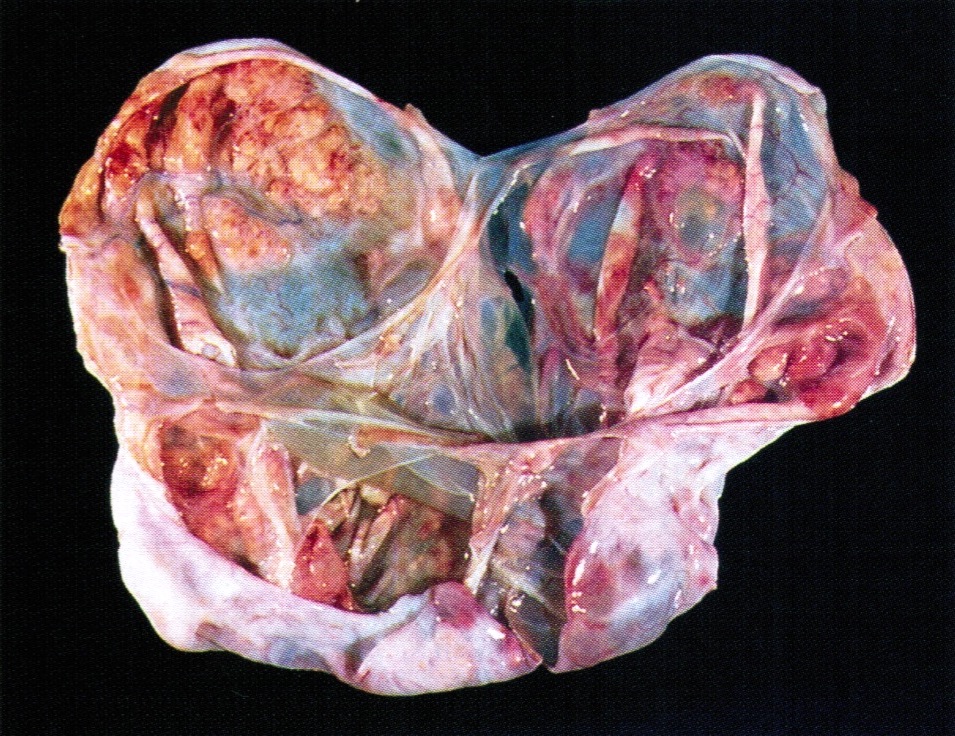
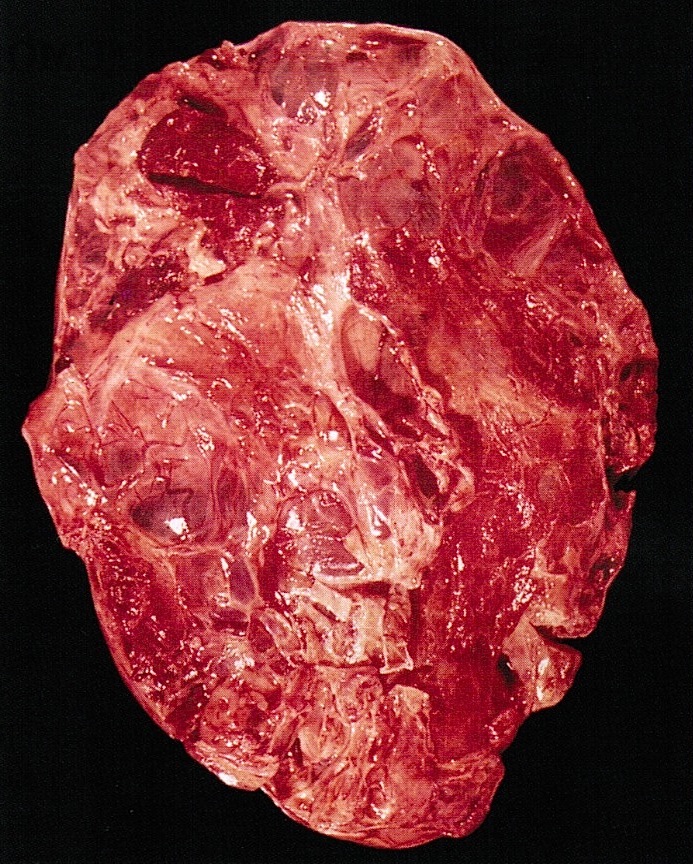
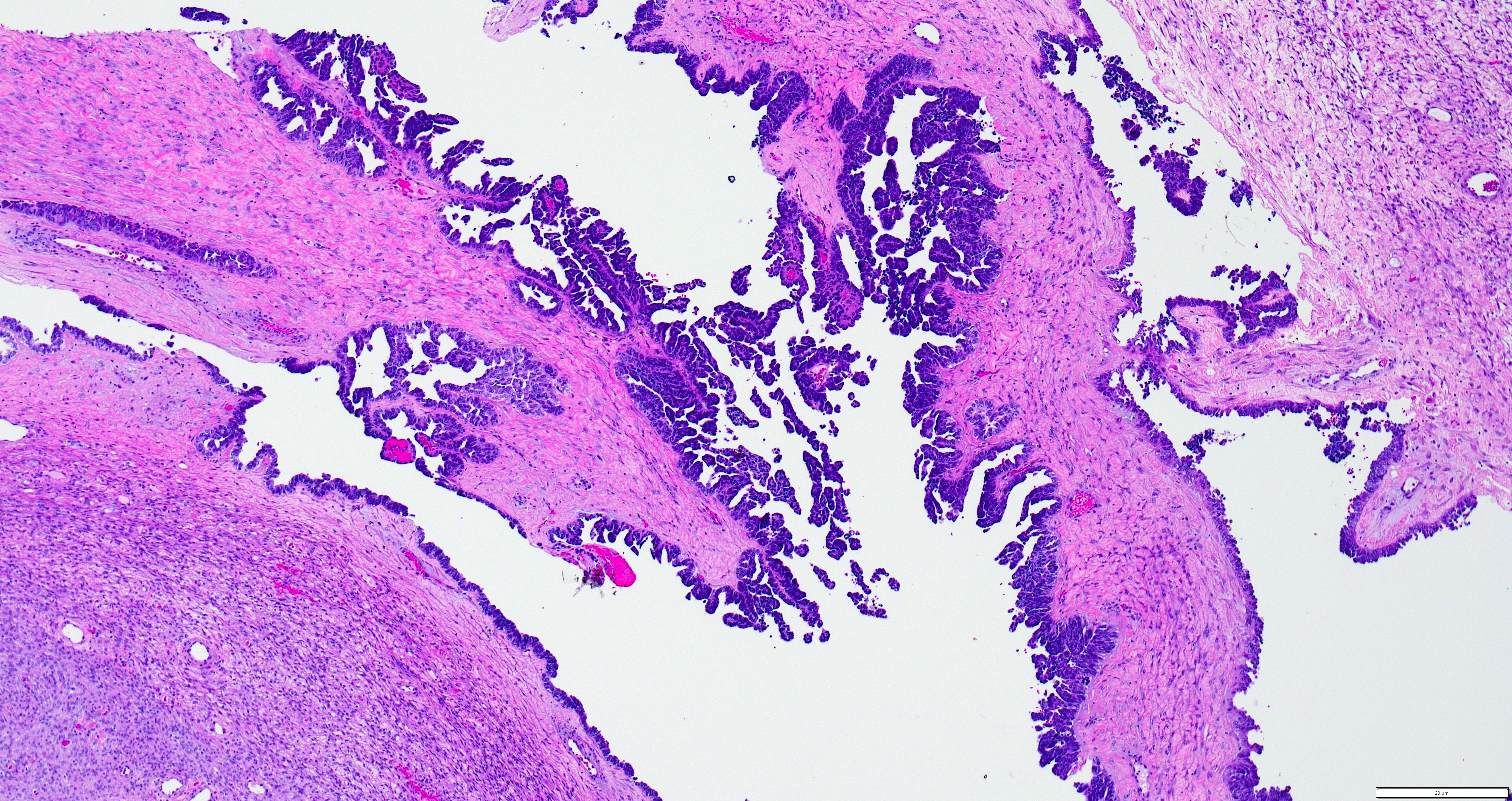
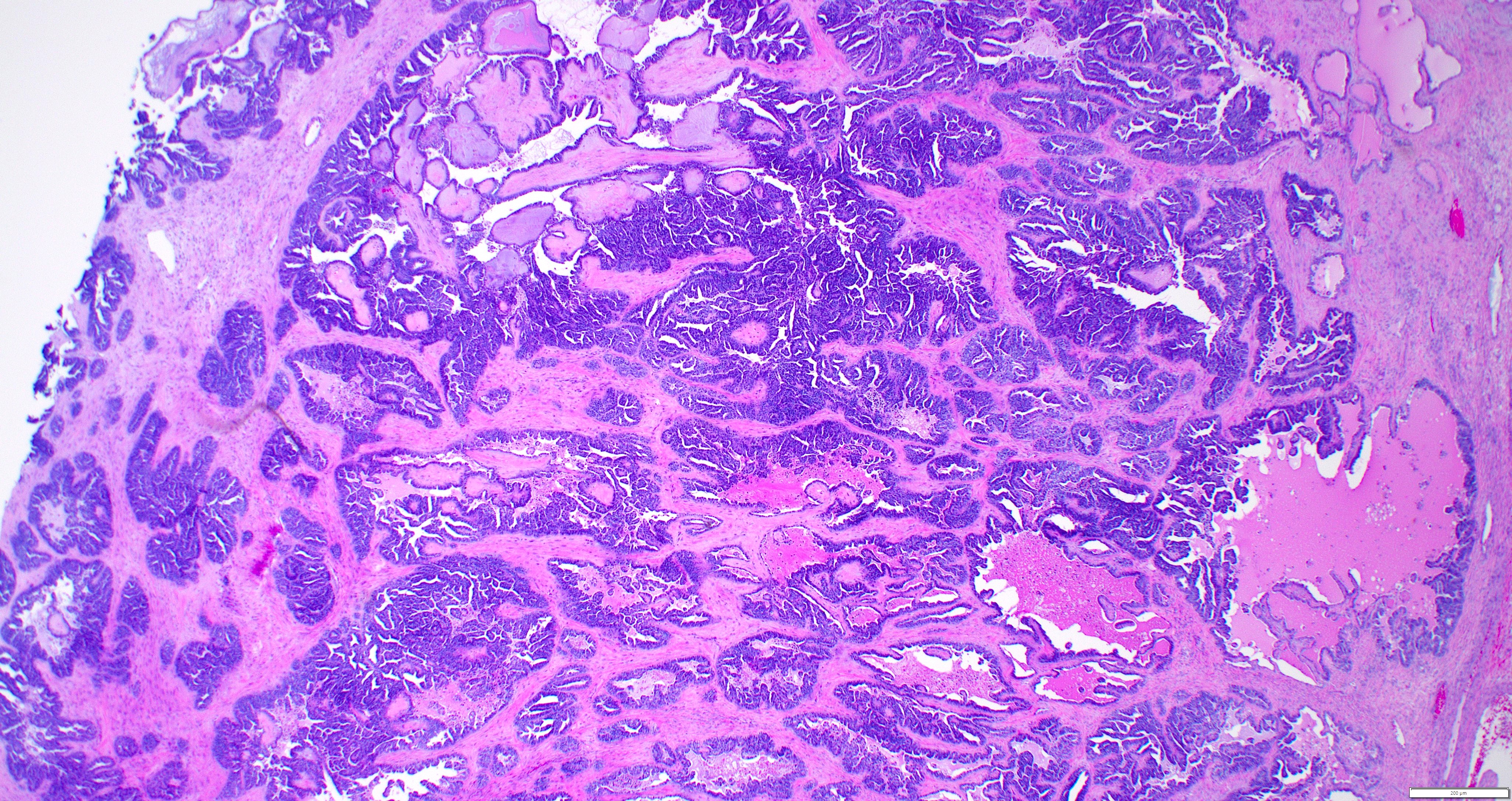
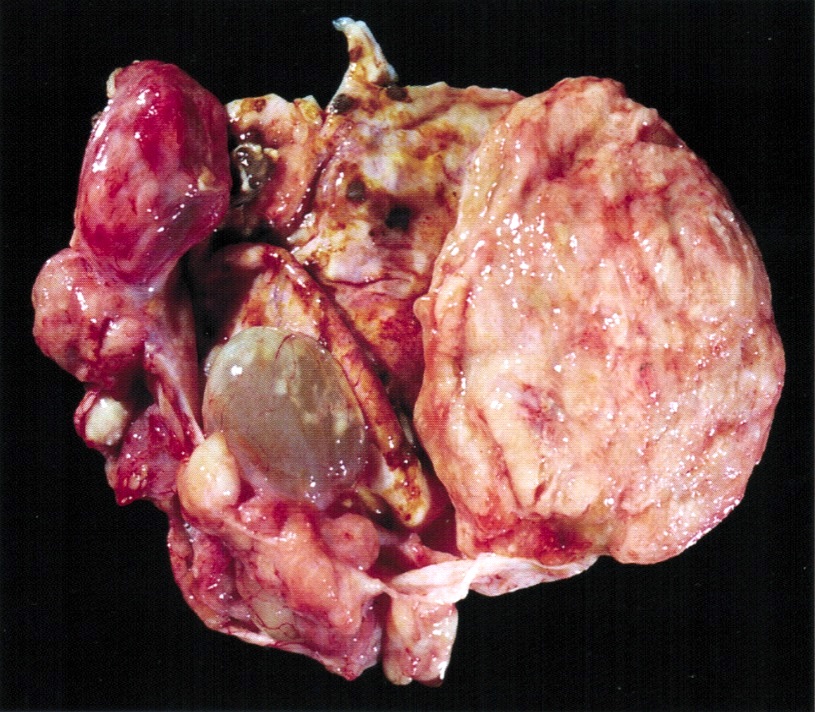
Gross description
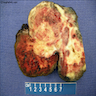
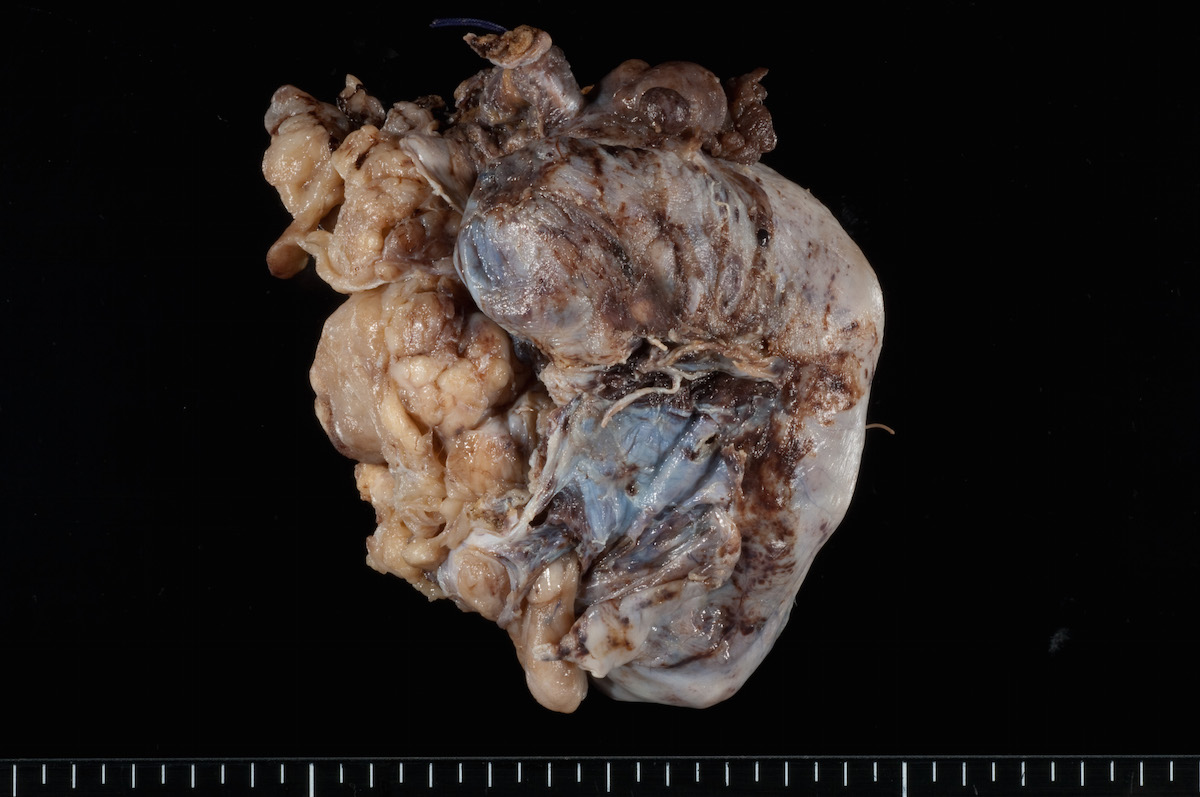
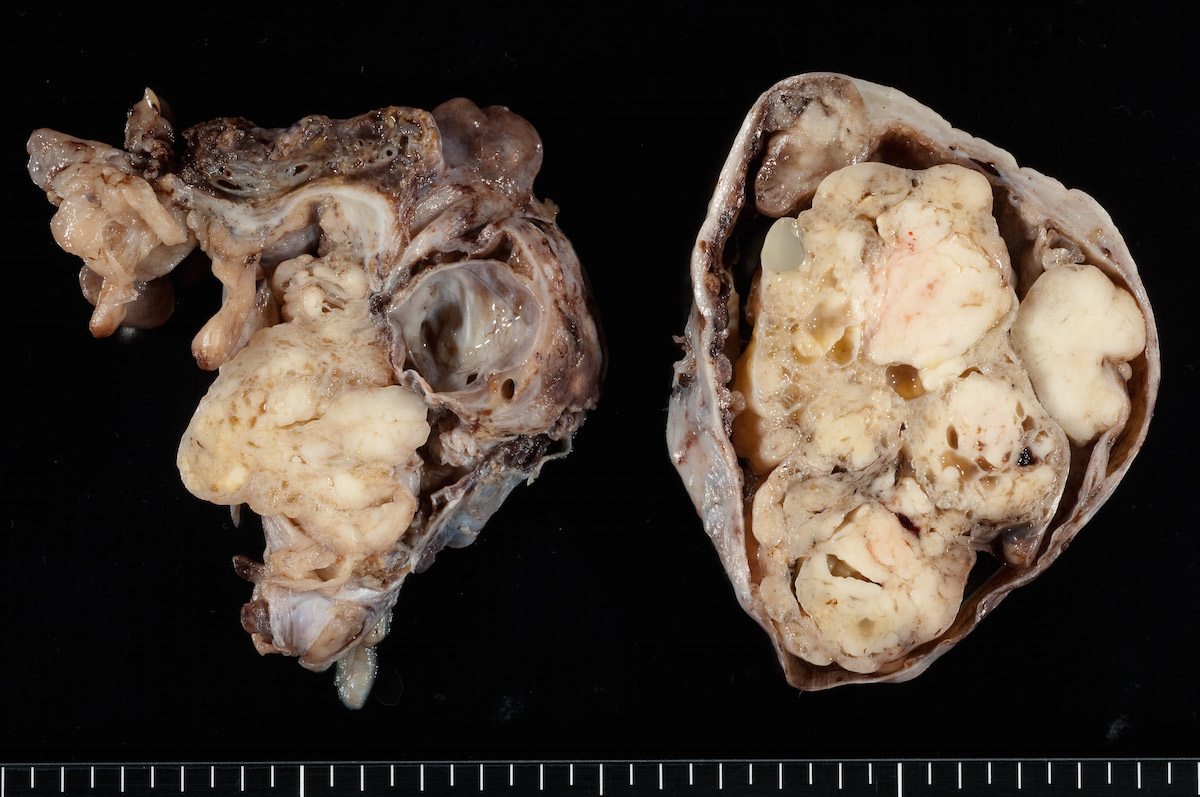
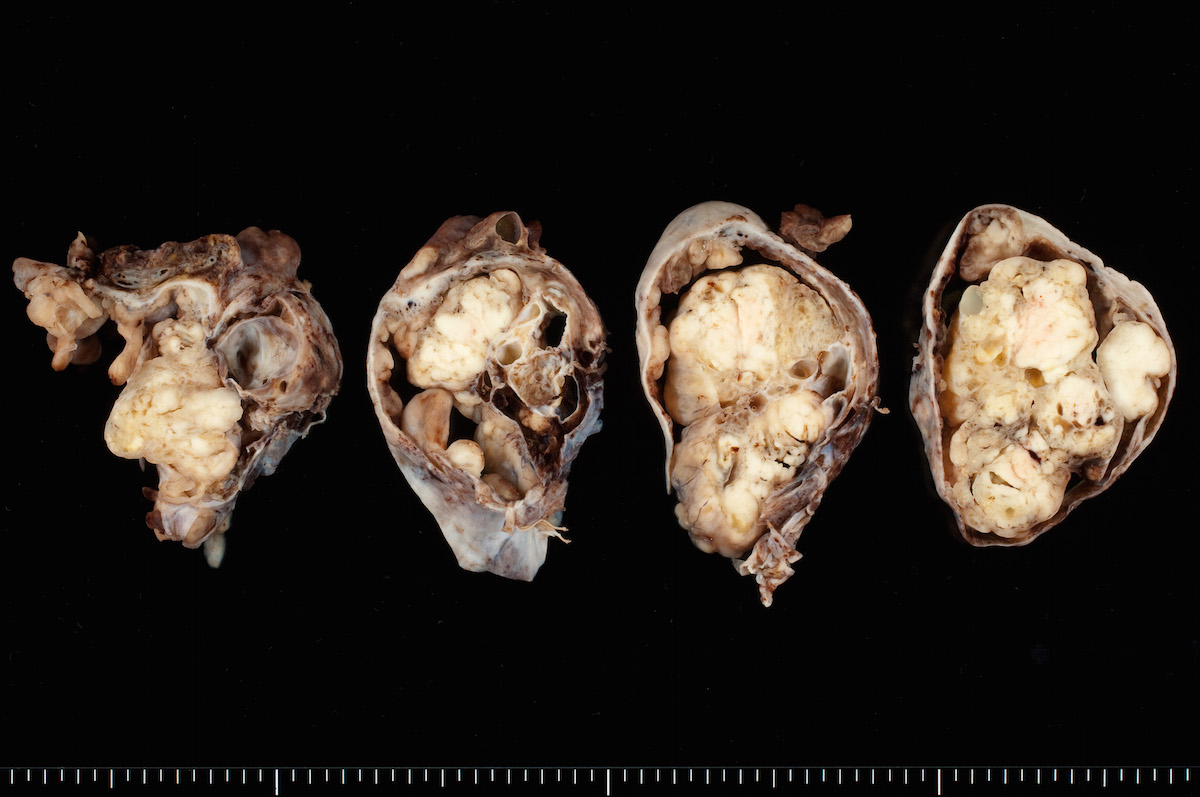
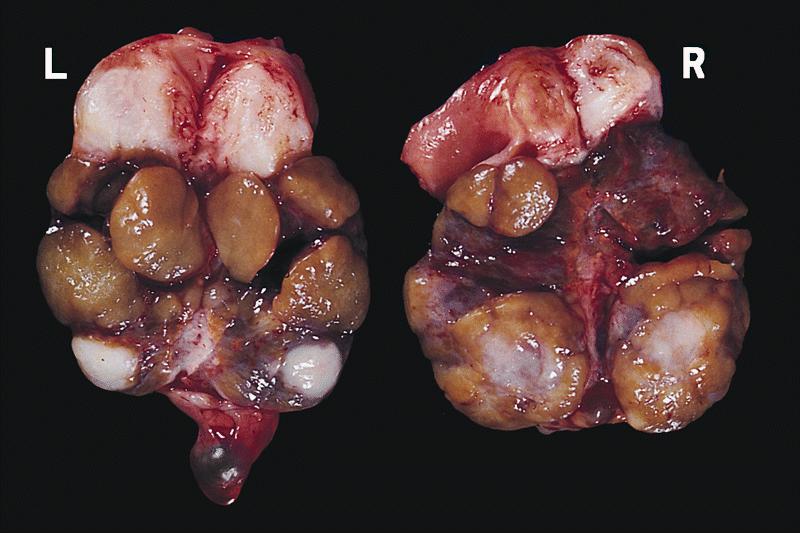
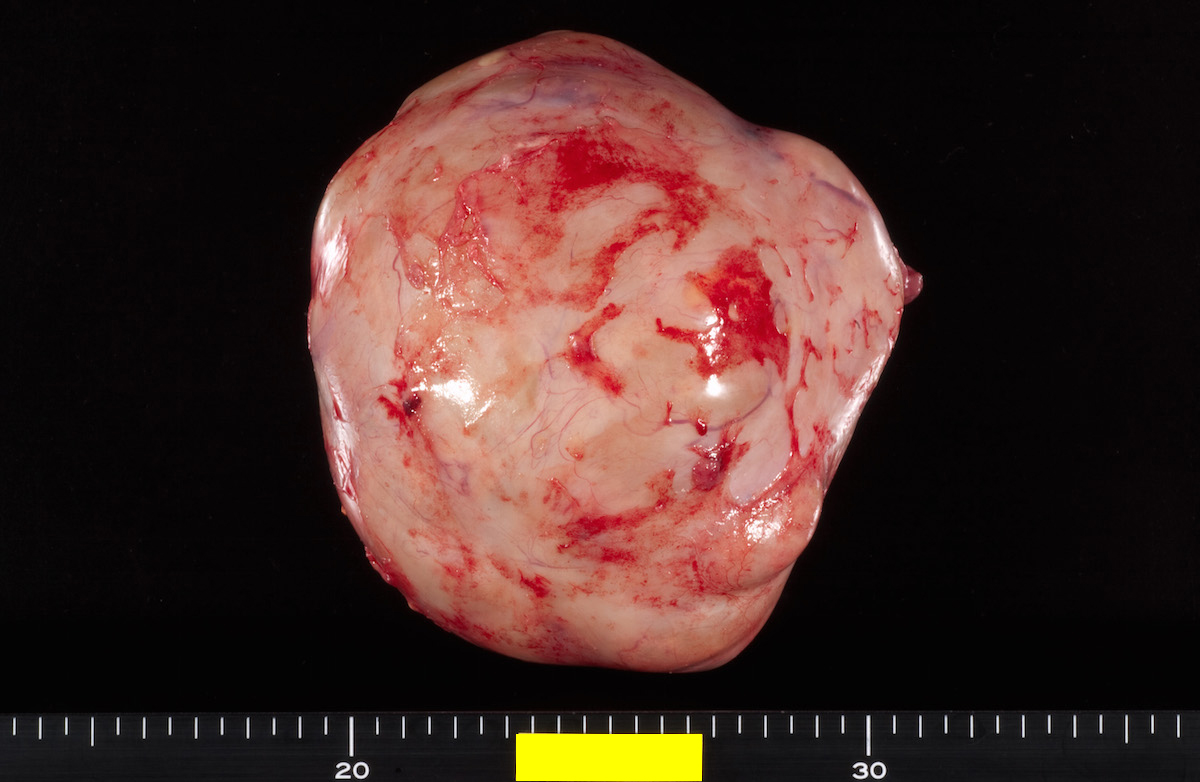
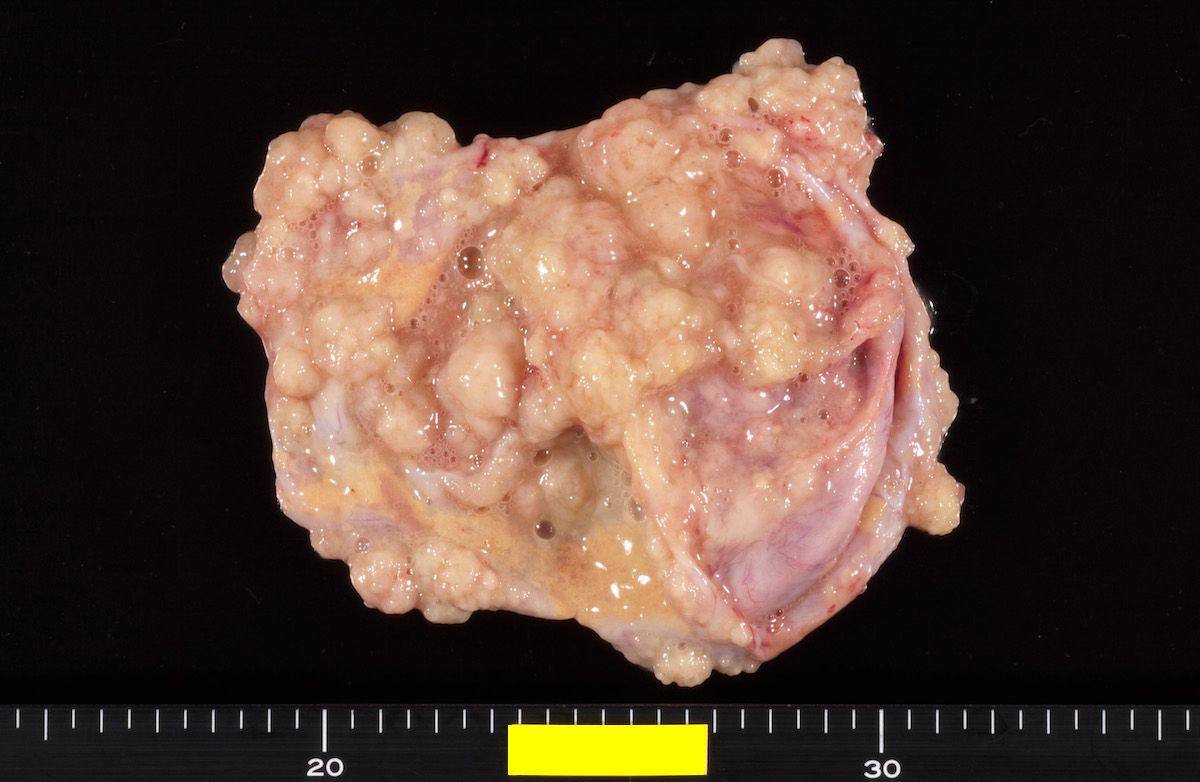
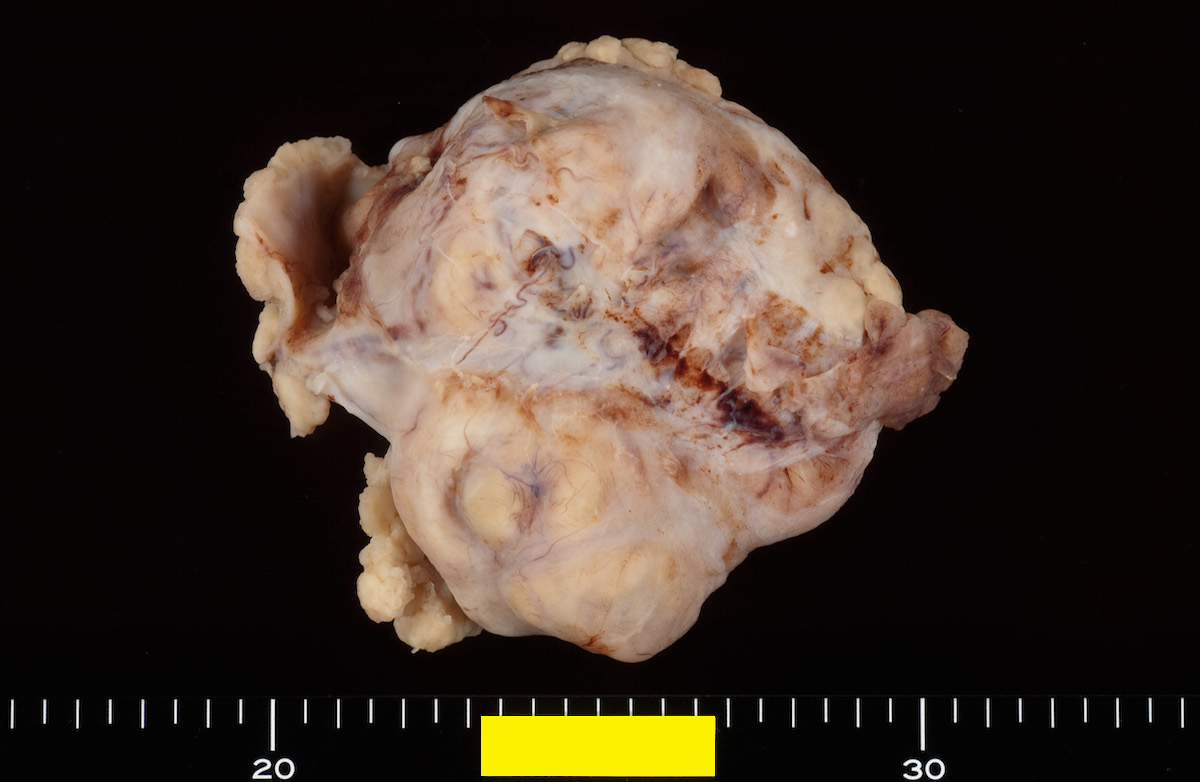
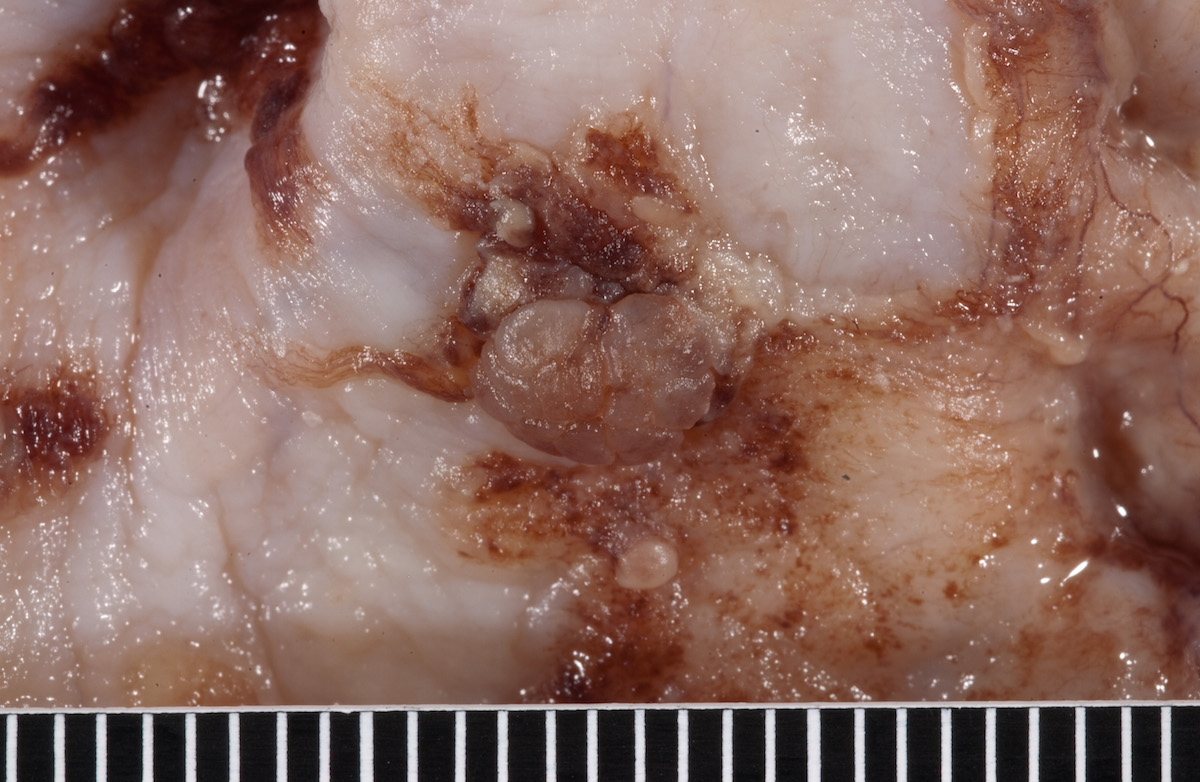
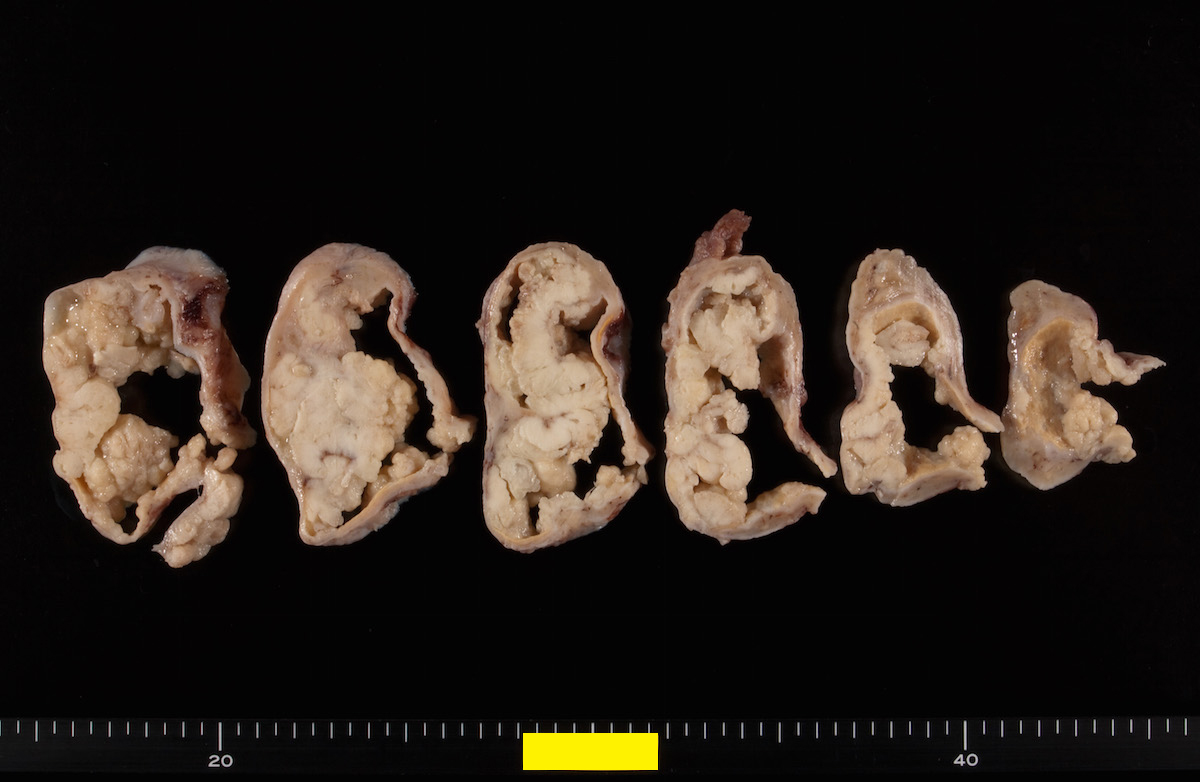
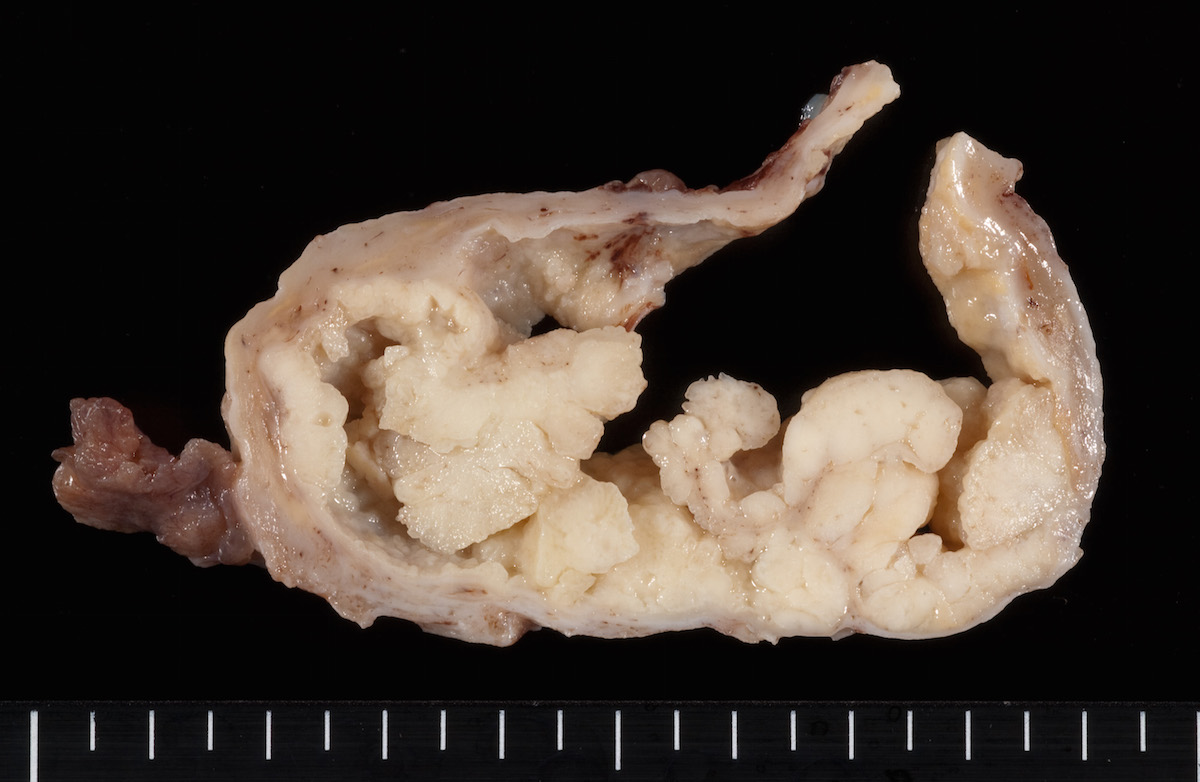
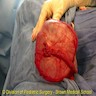
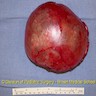
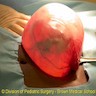
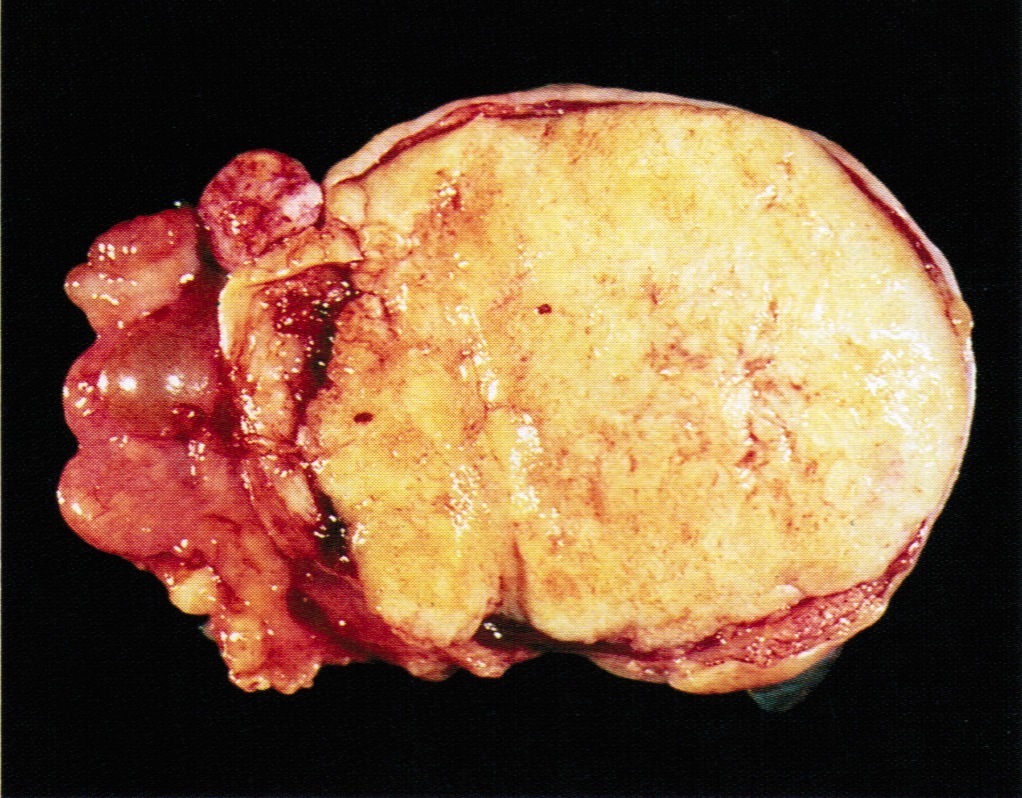
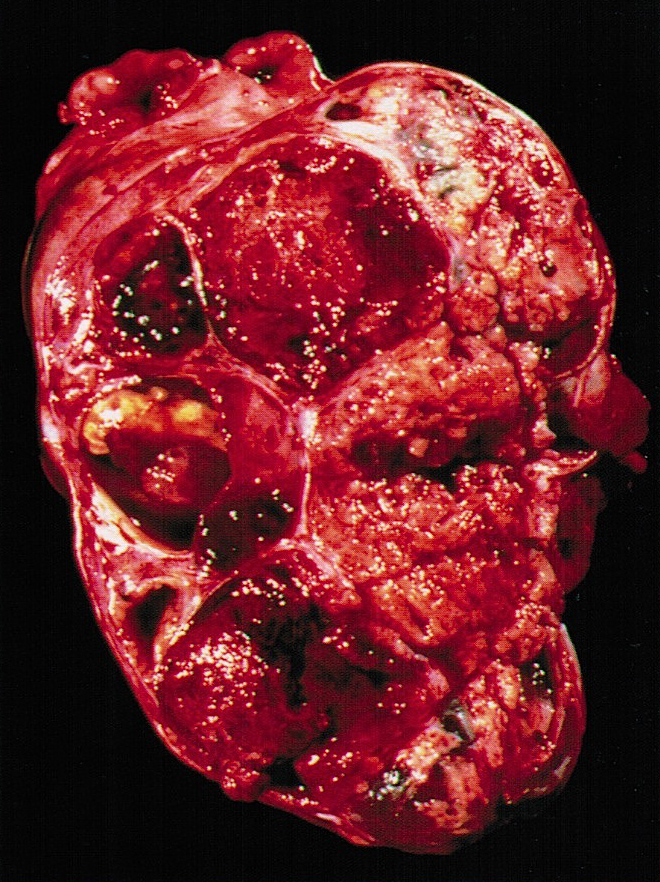
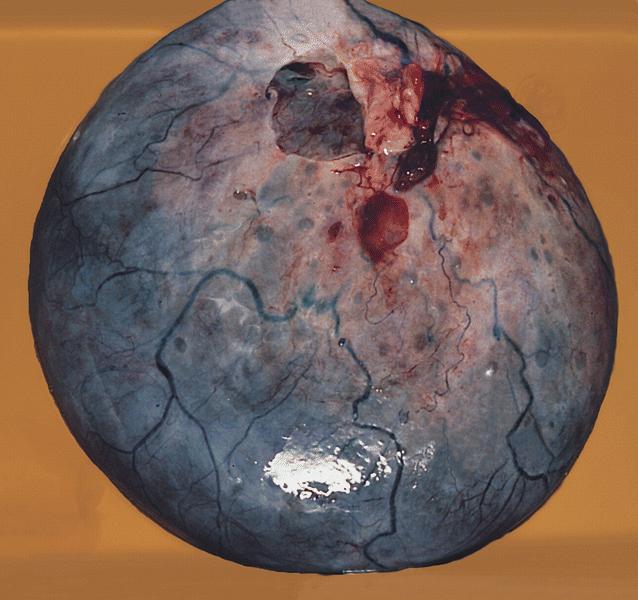
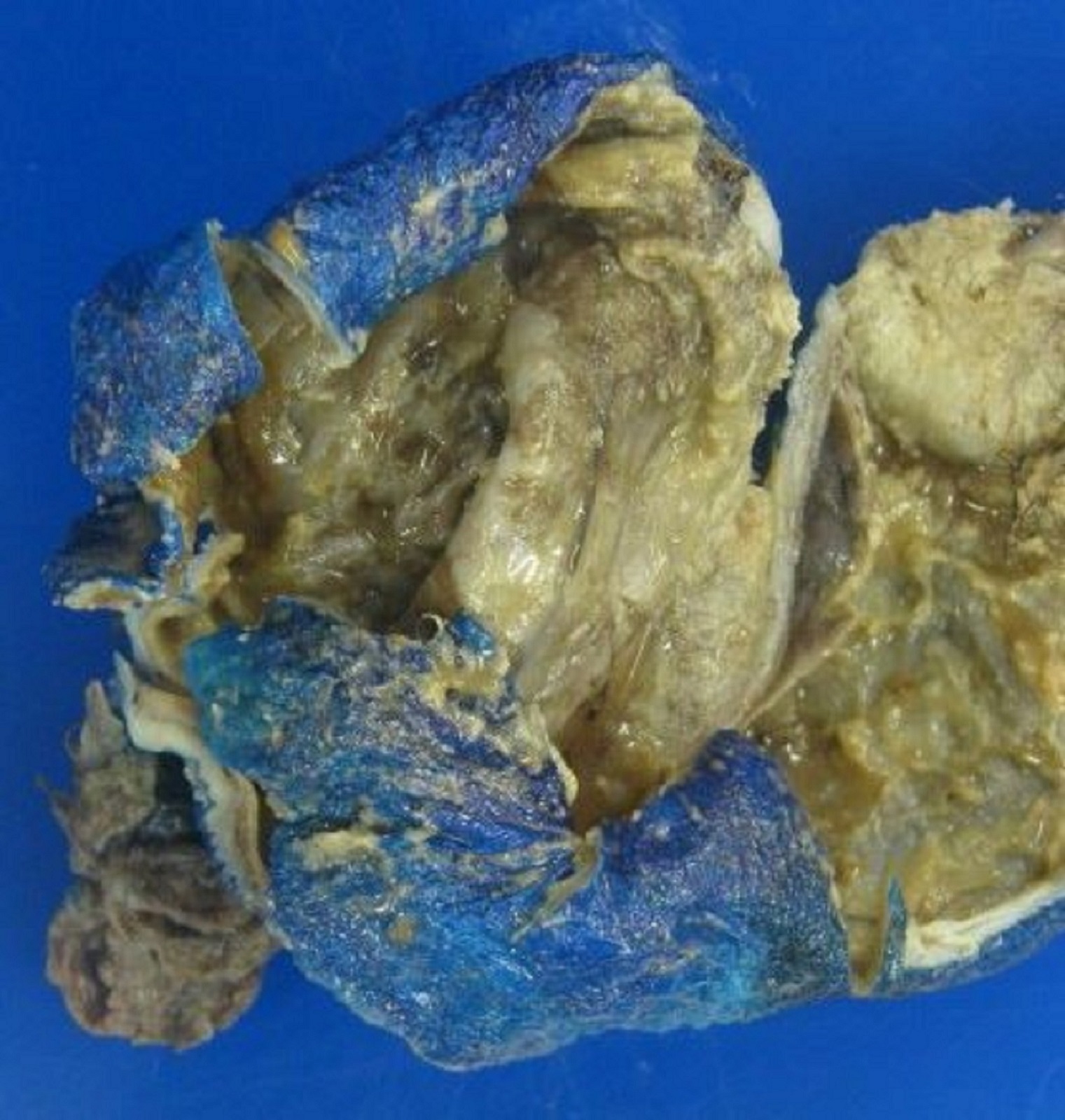
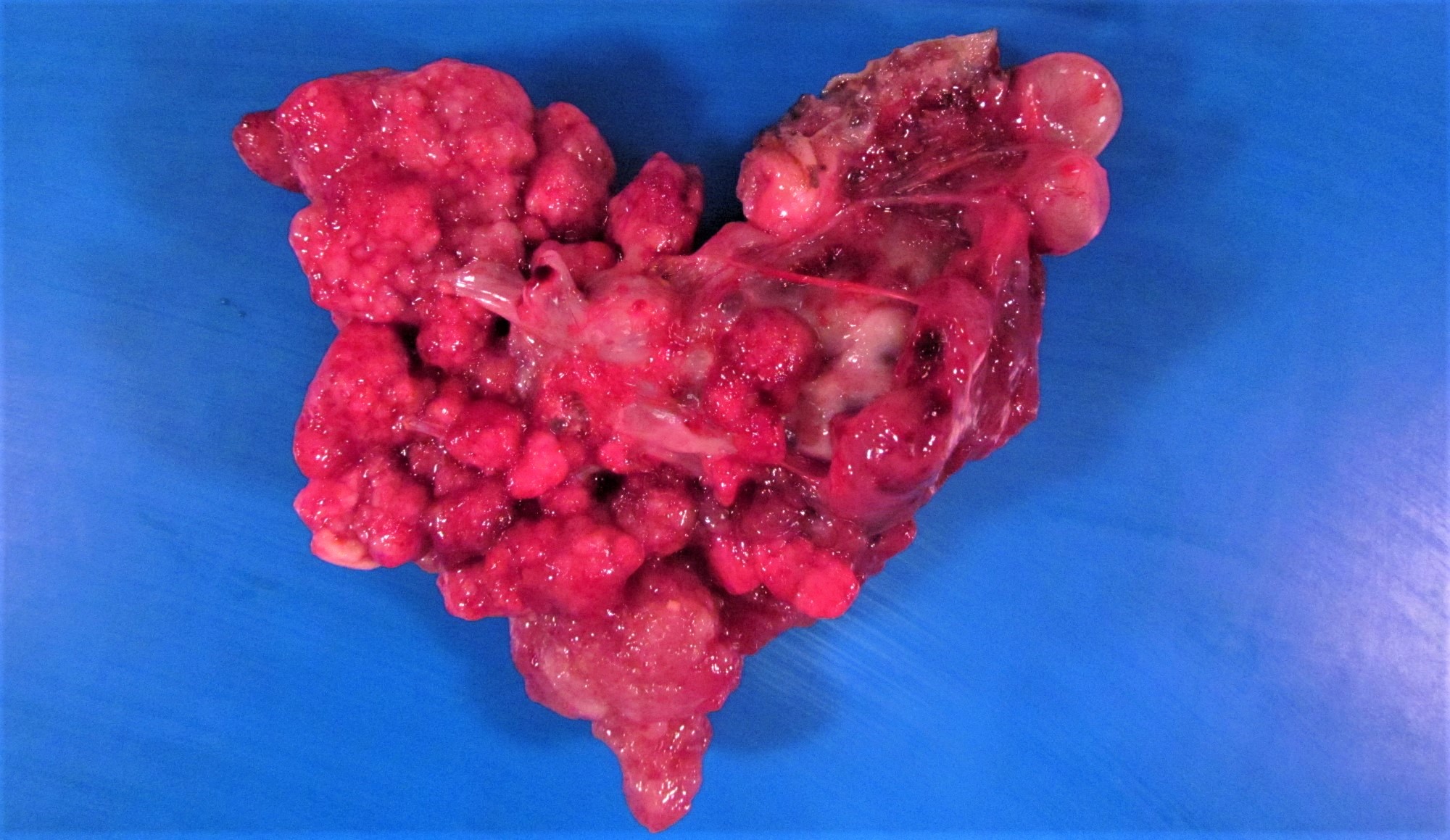
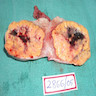
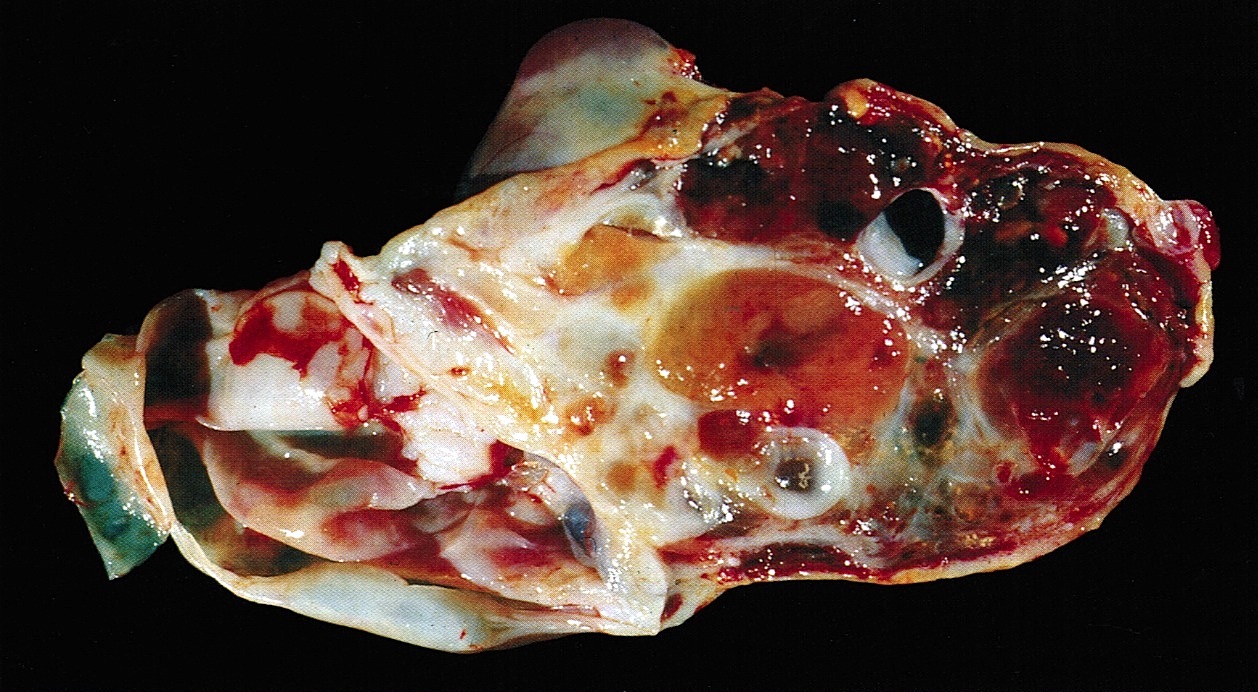
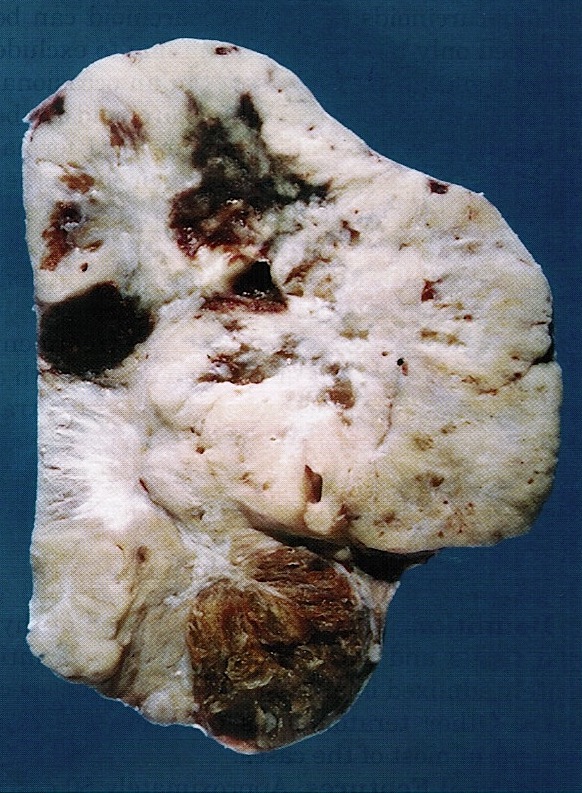
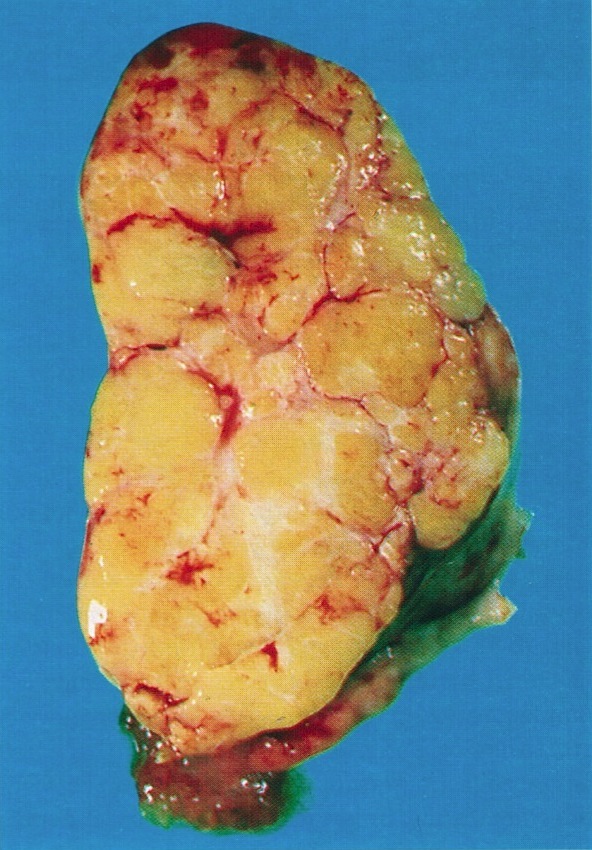
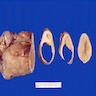
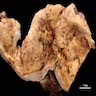
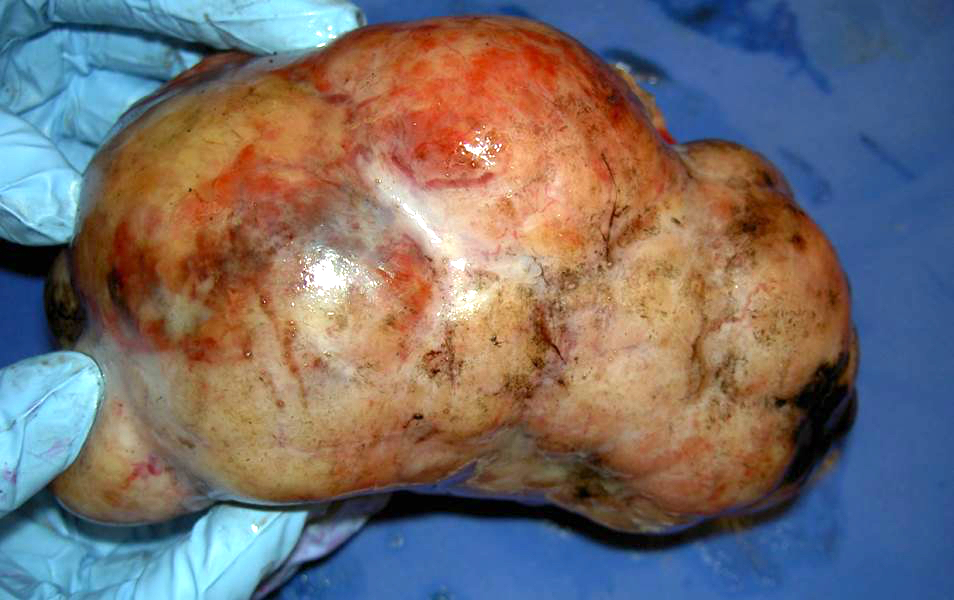
- Ovarian mass is frequently complex (solid and cystic) or purely solid
- Purely cystic unilocular lesions are rare
- Solid tumors have a multinodular appearance and extend to the ovarian / tumor surface
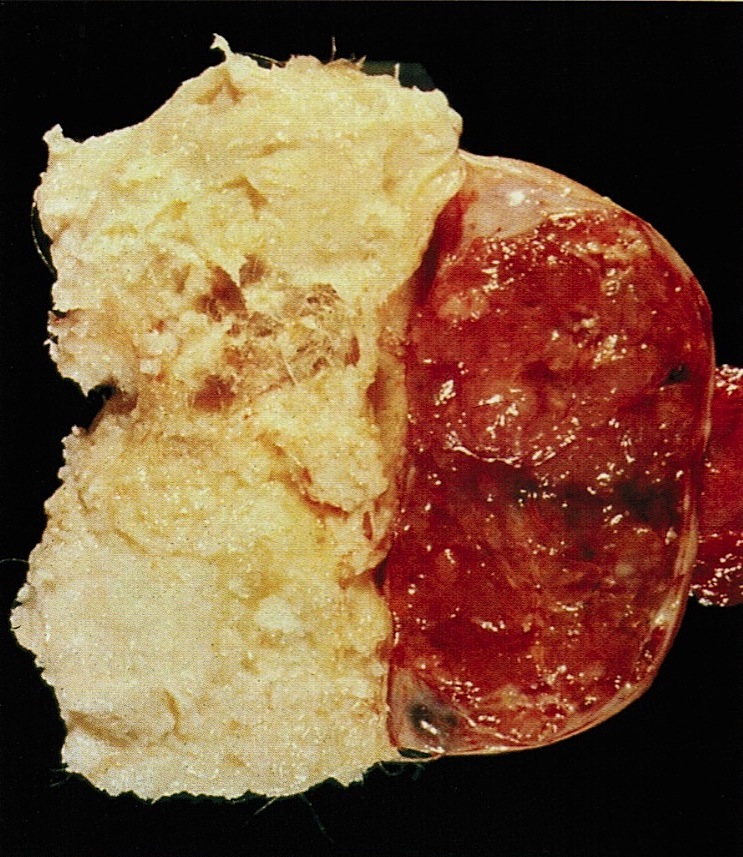
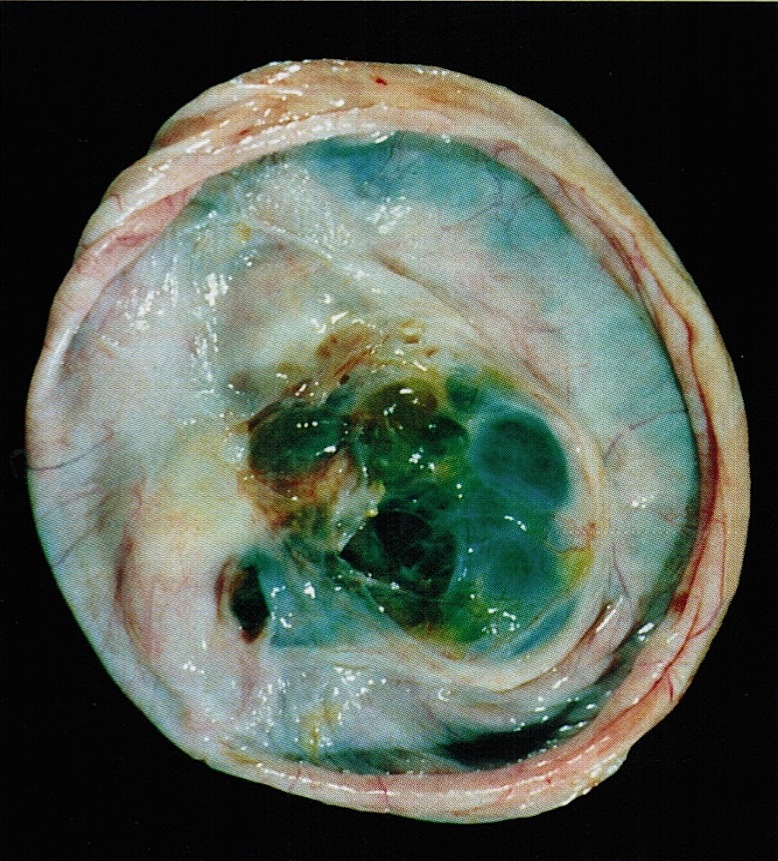
- Cut surface reveals mucinous contents in cystic areas and a soft, glistening appearance of solid areas, sometimes with easily expressed mucinous material from it
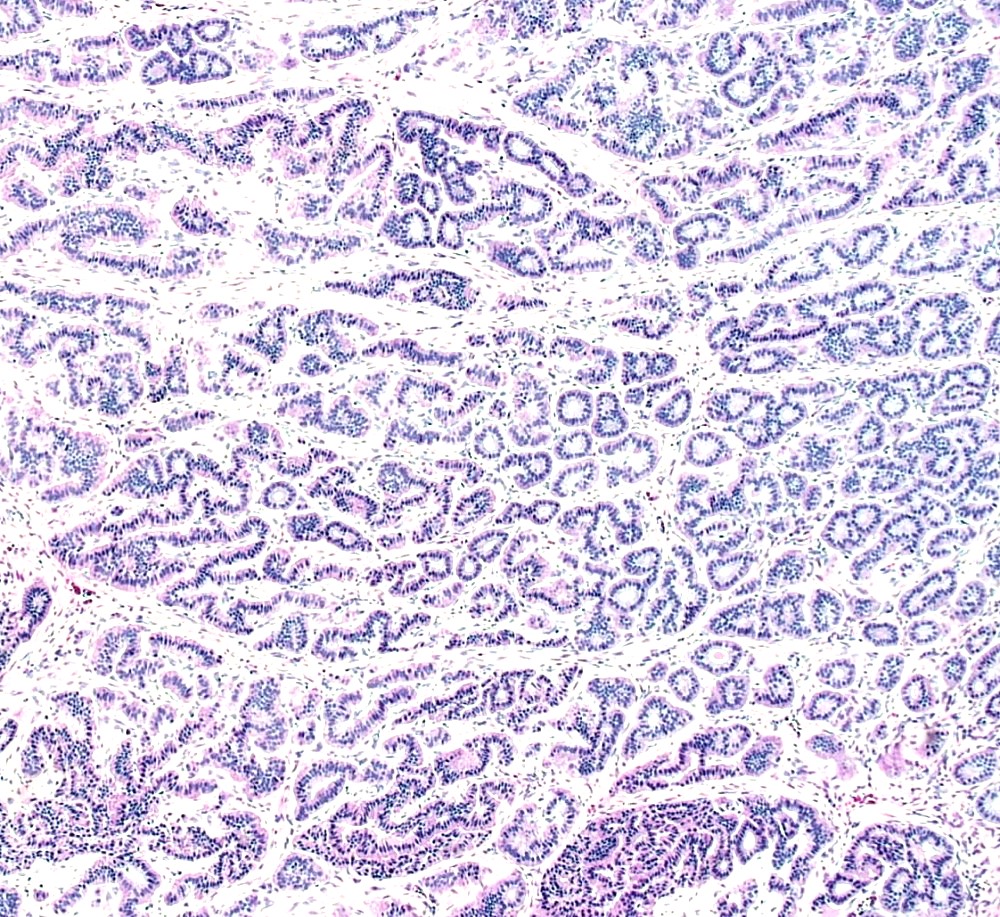
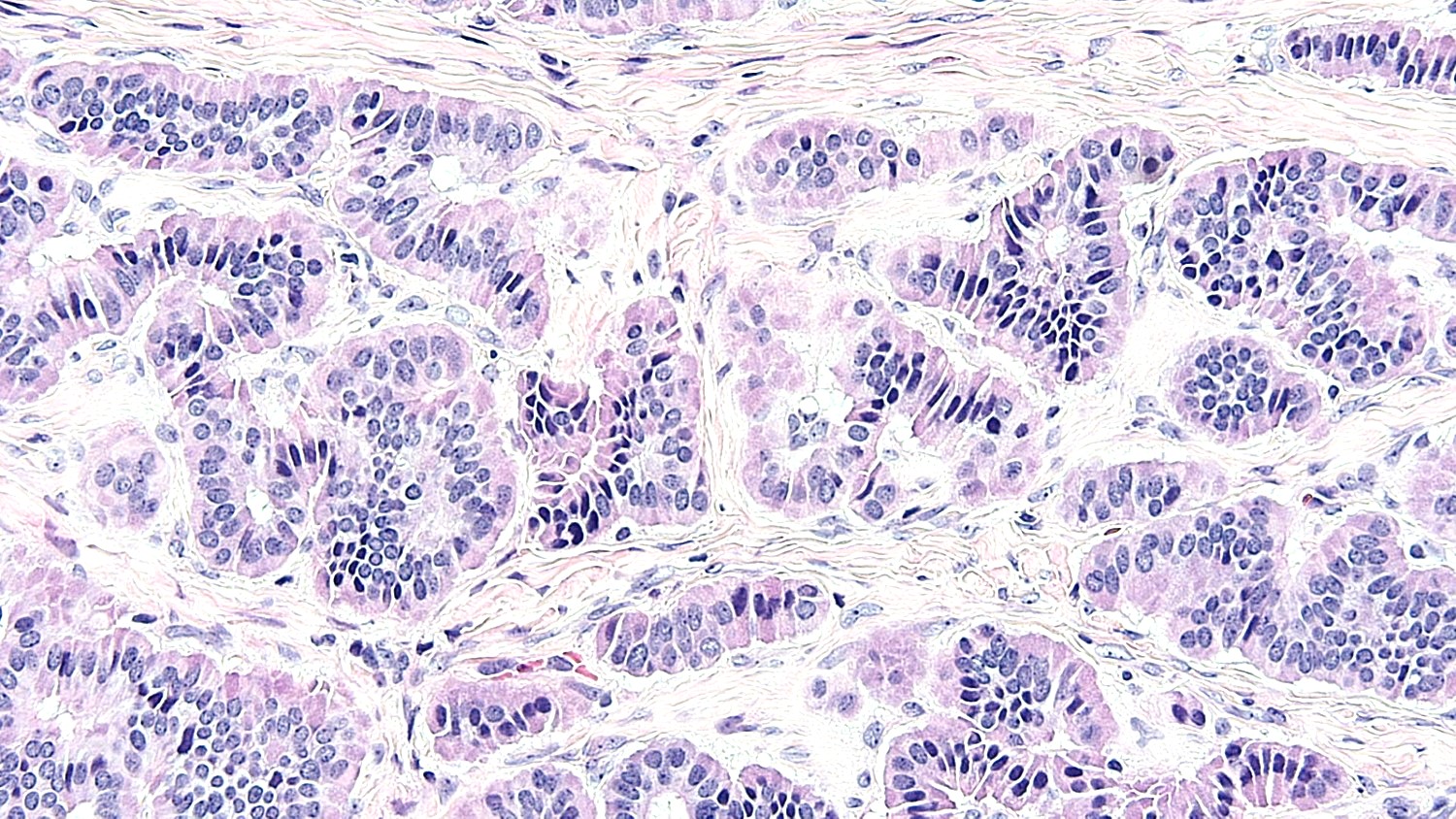
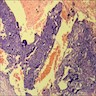
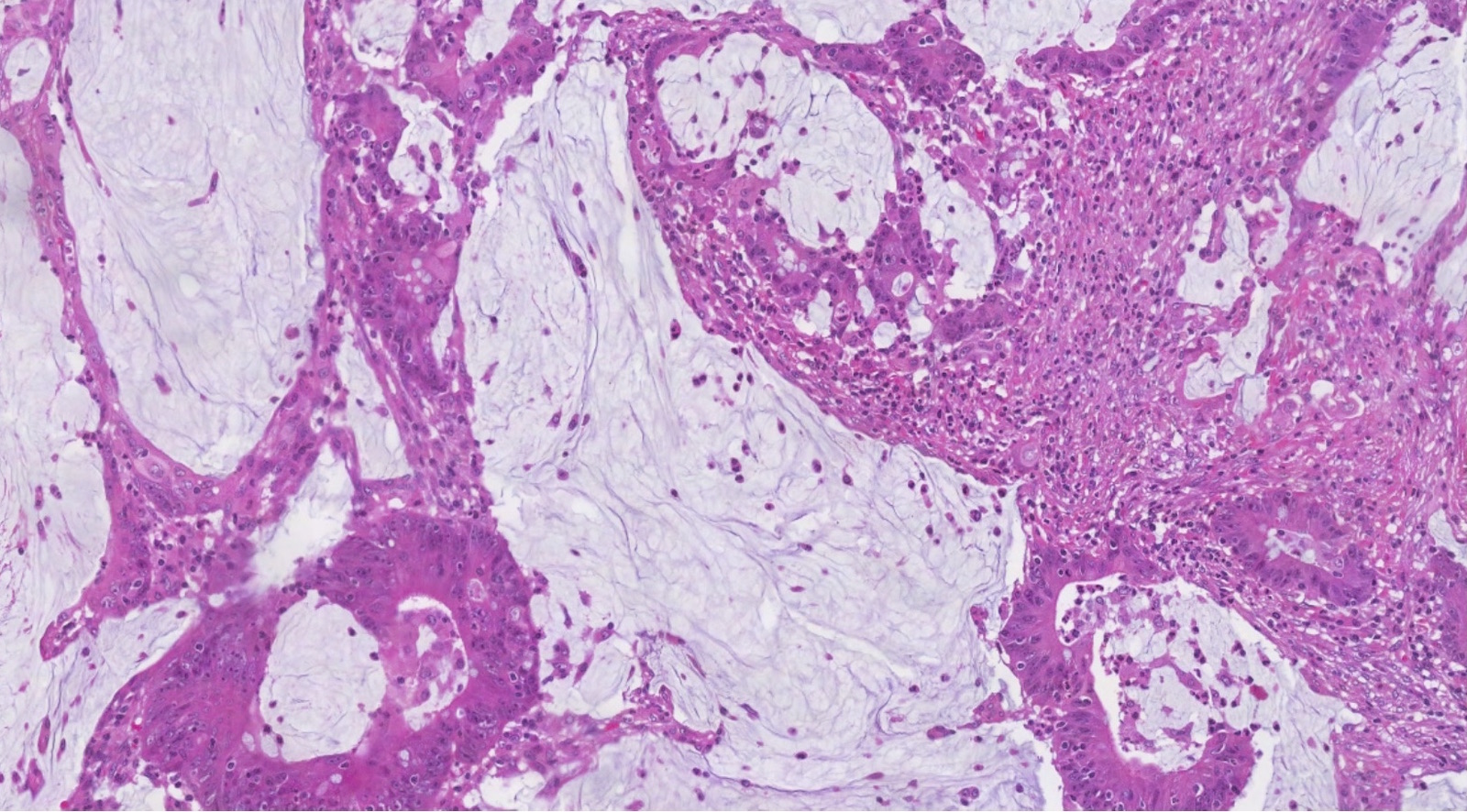
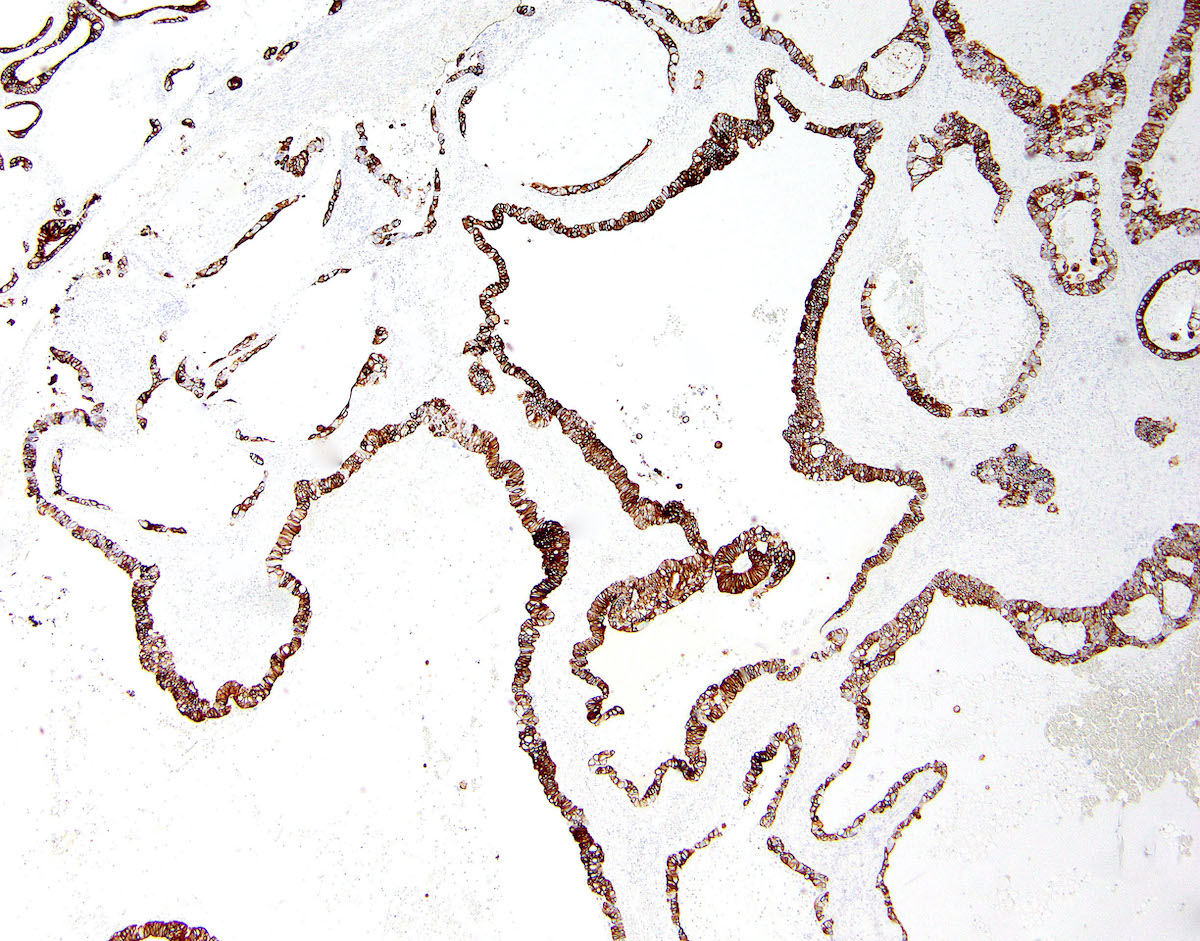
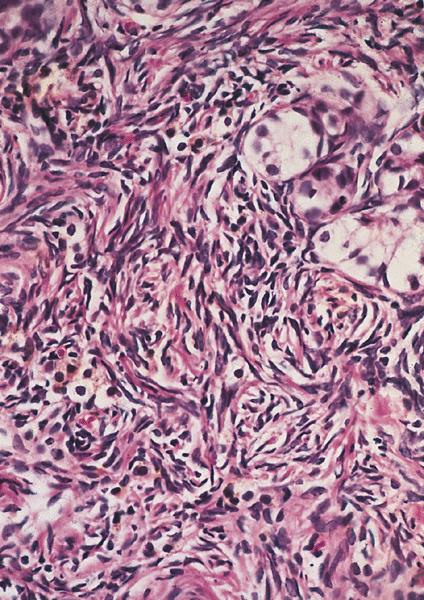
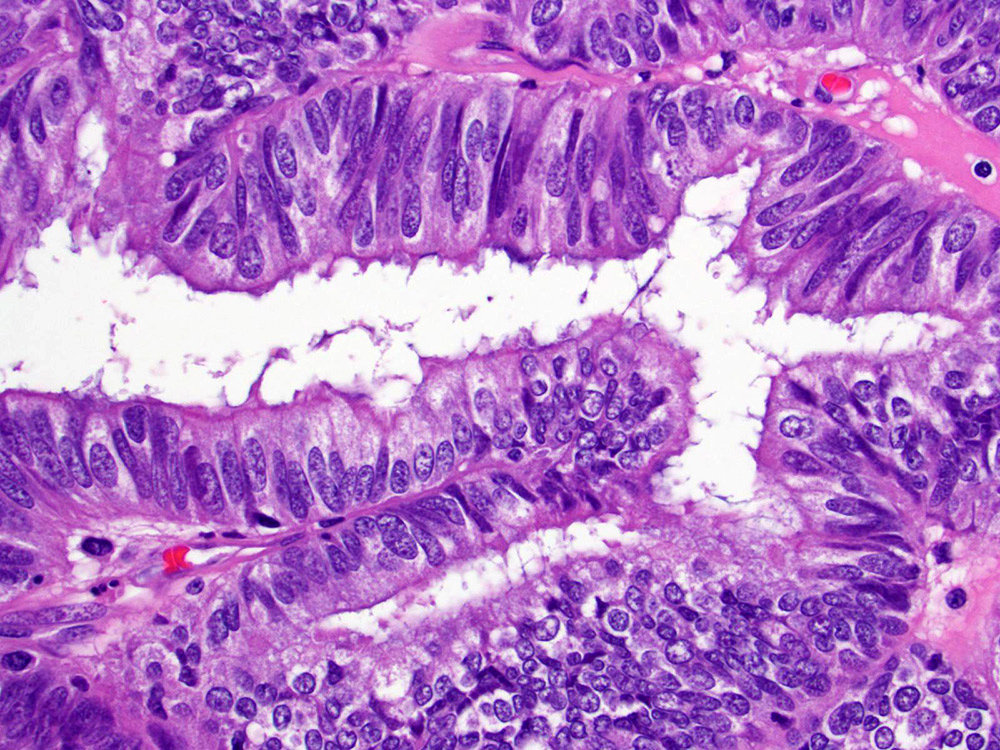
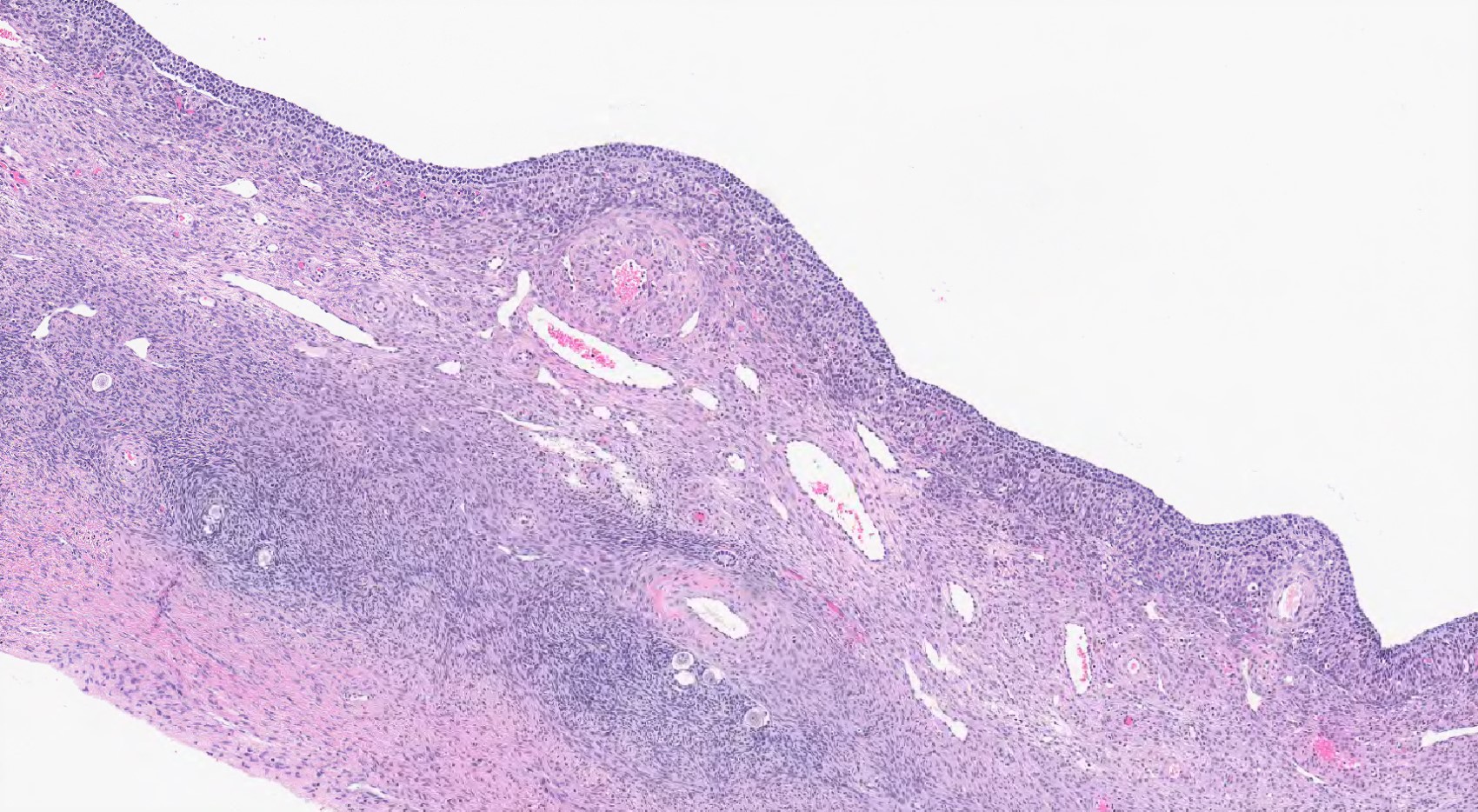
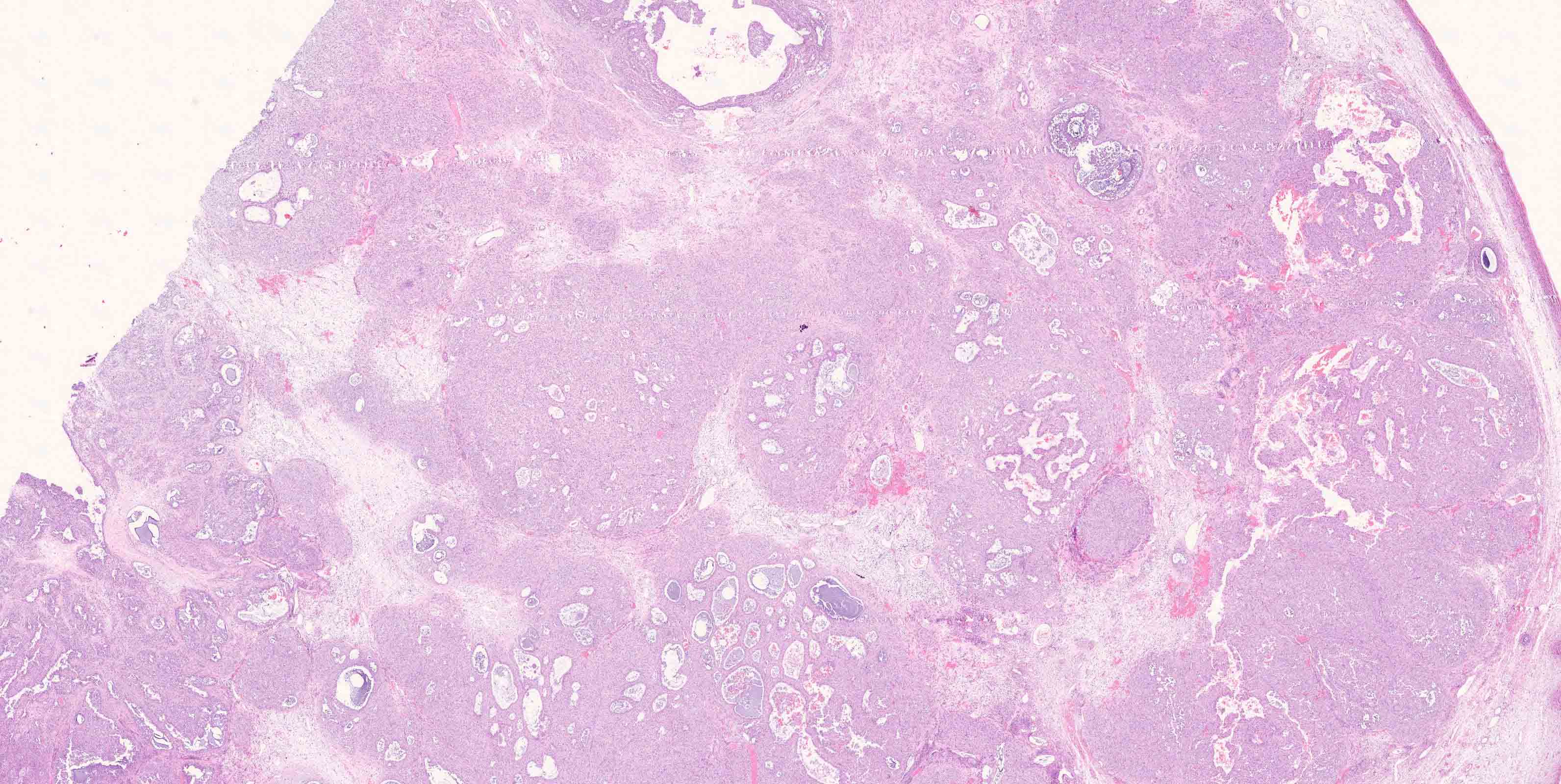
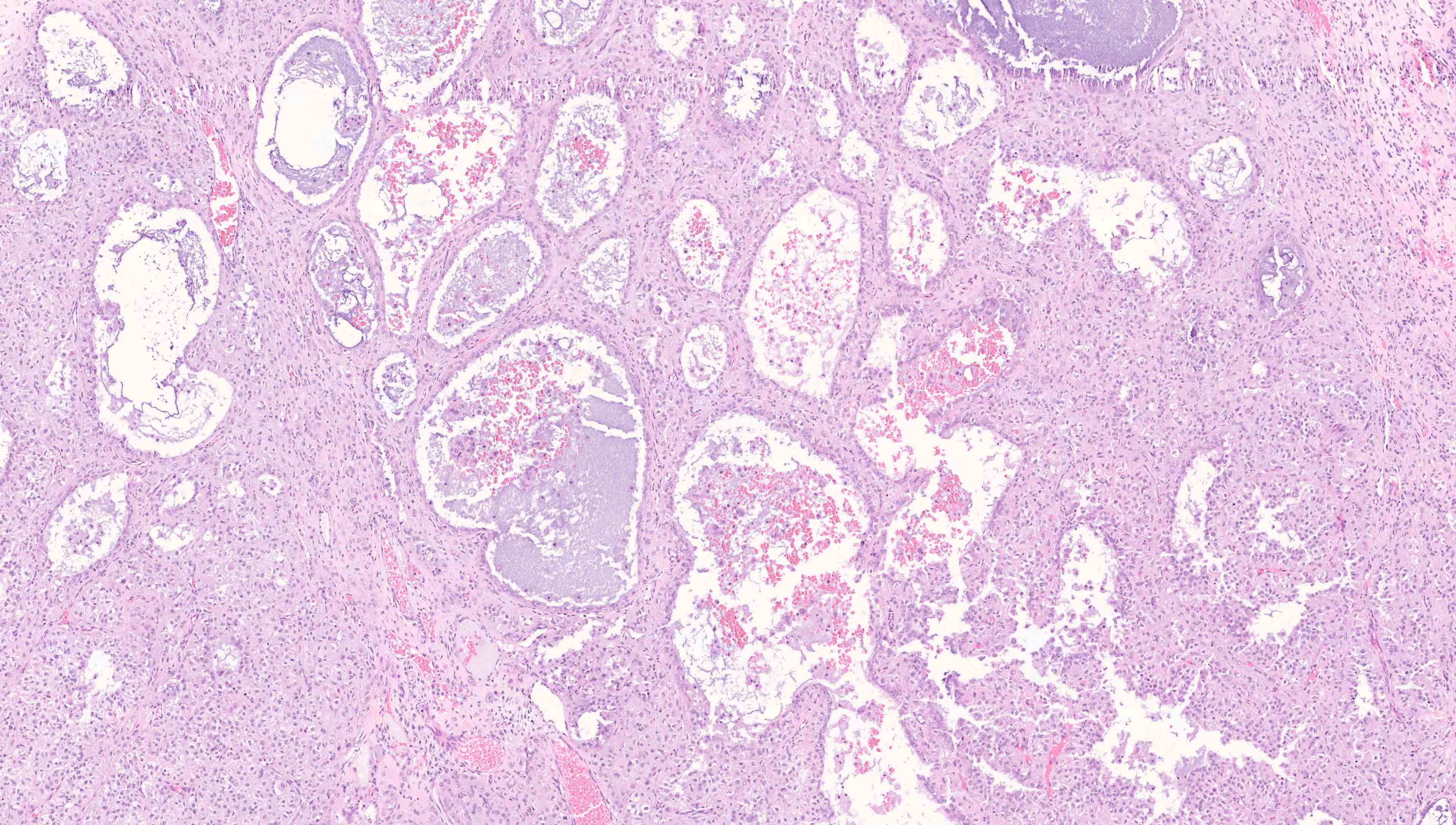
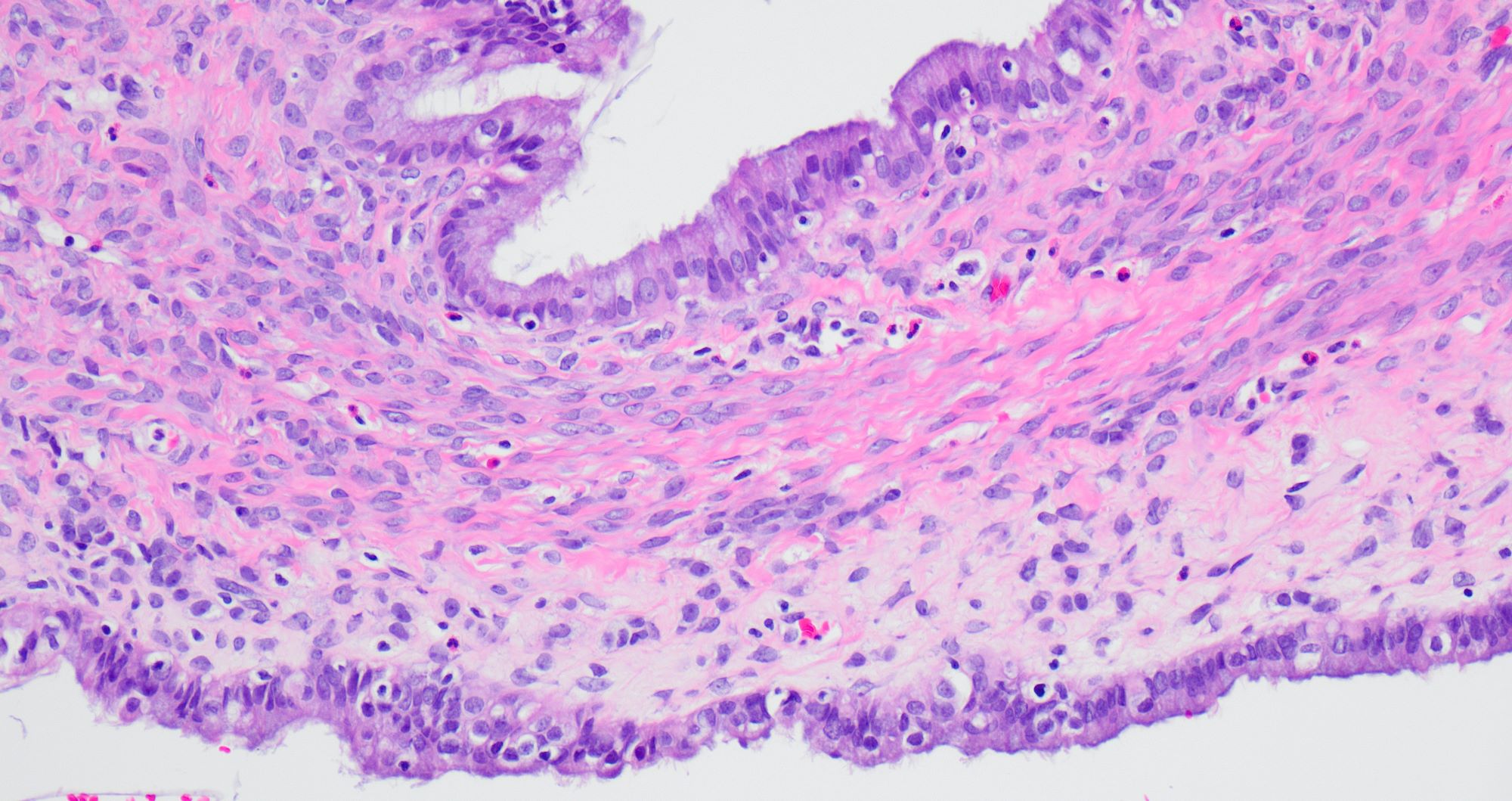
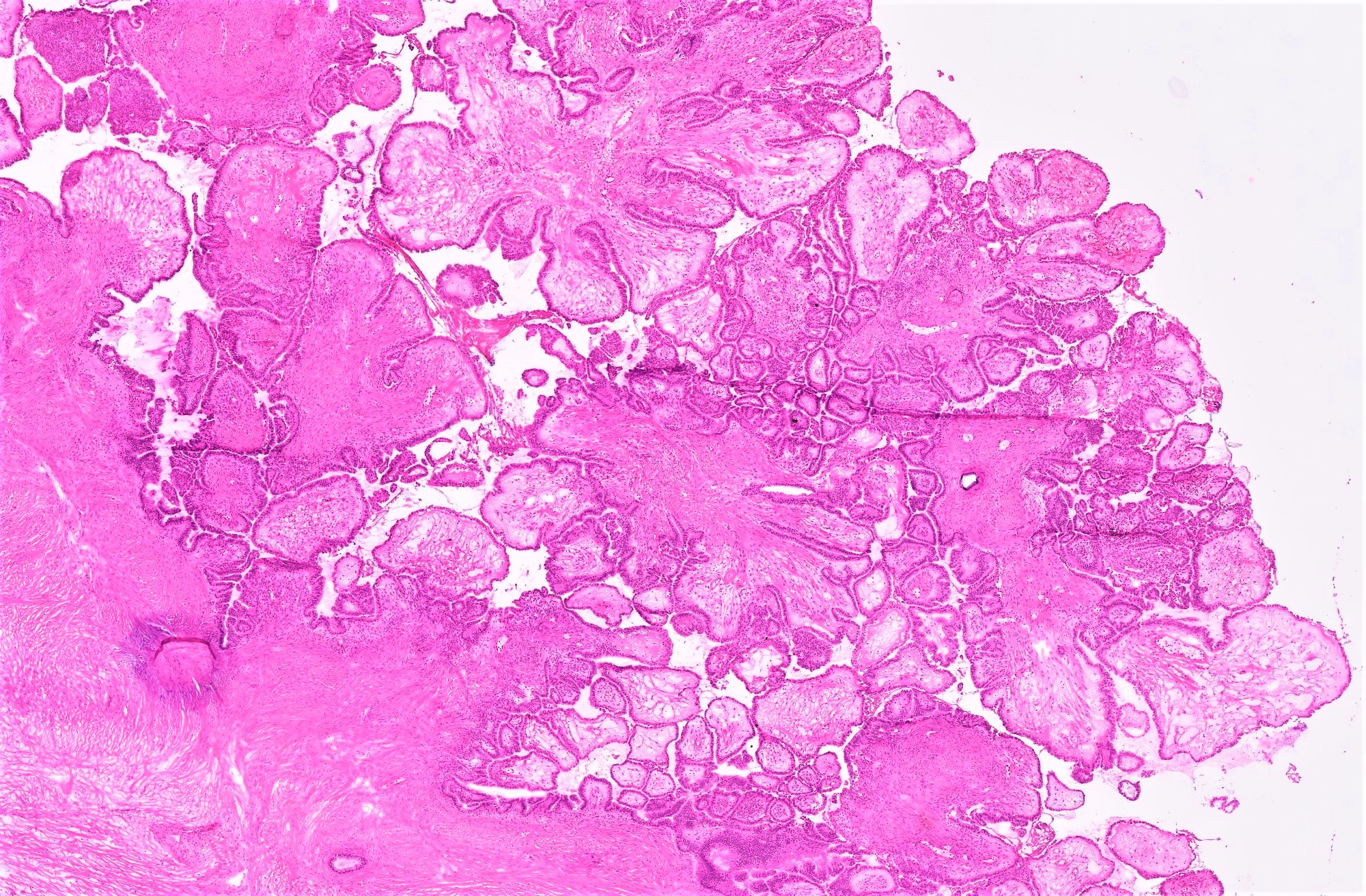
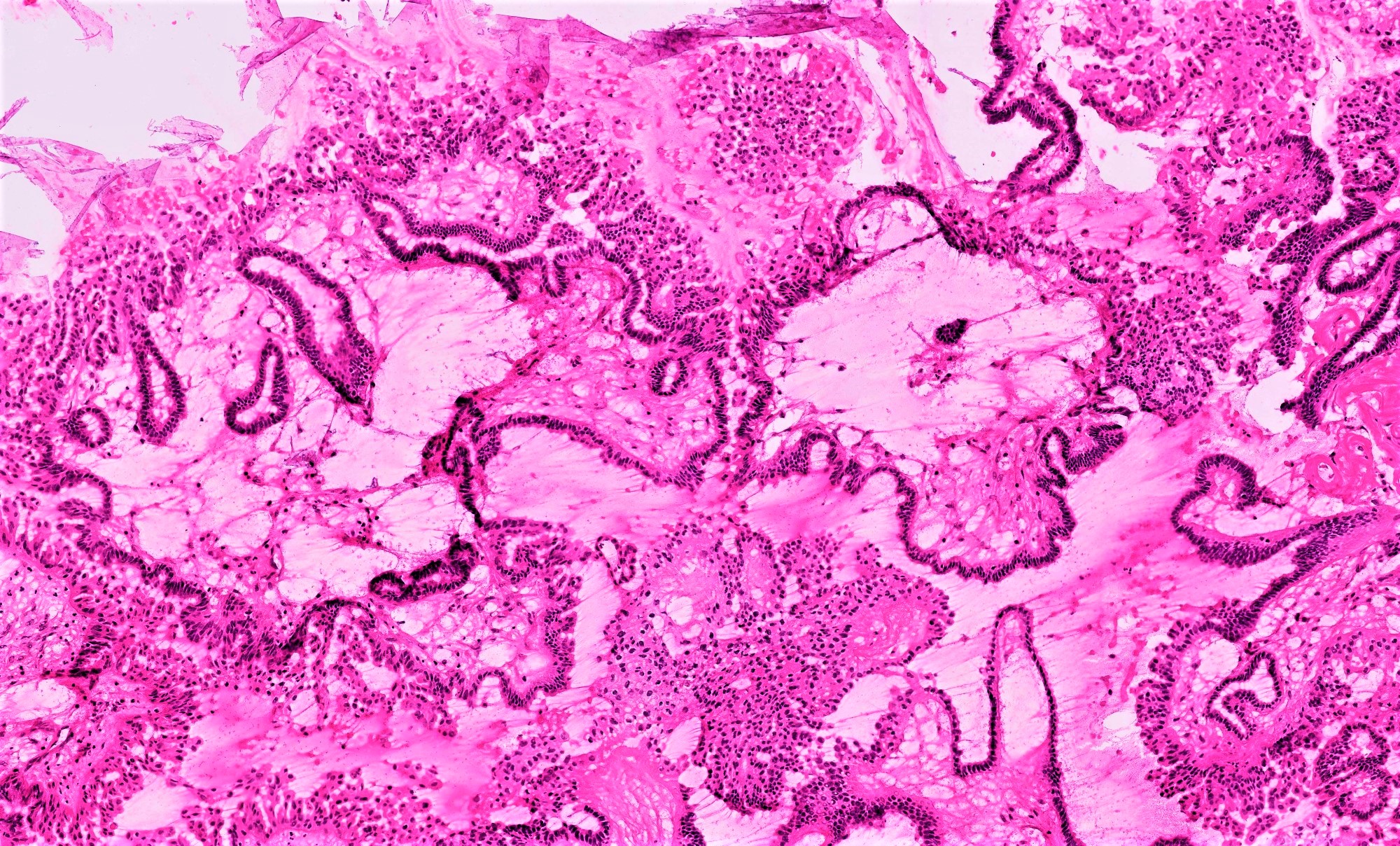
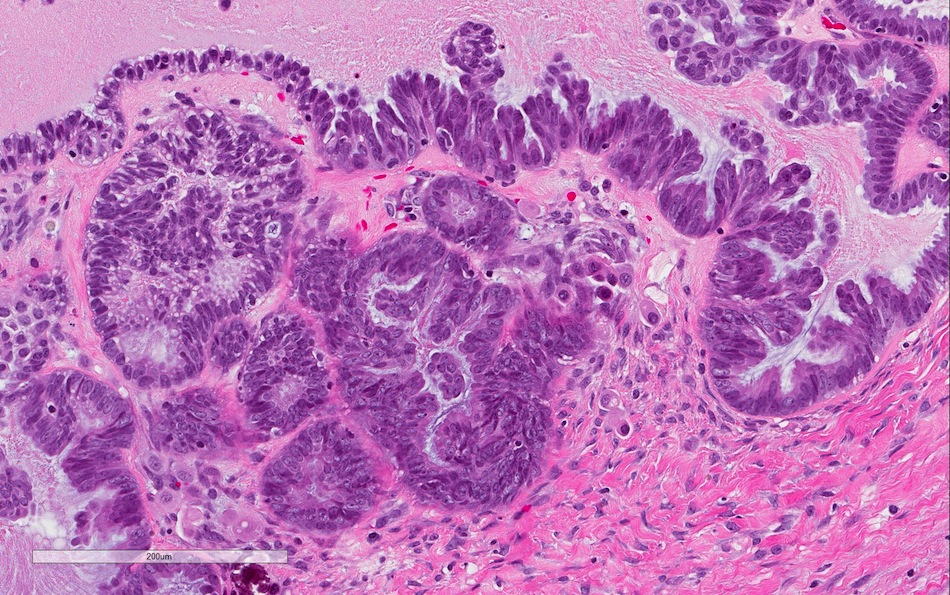
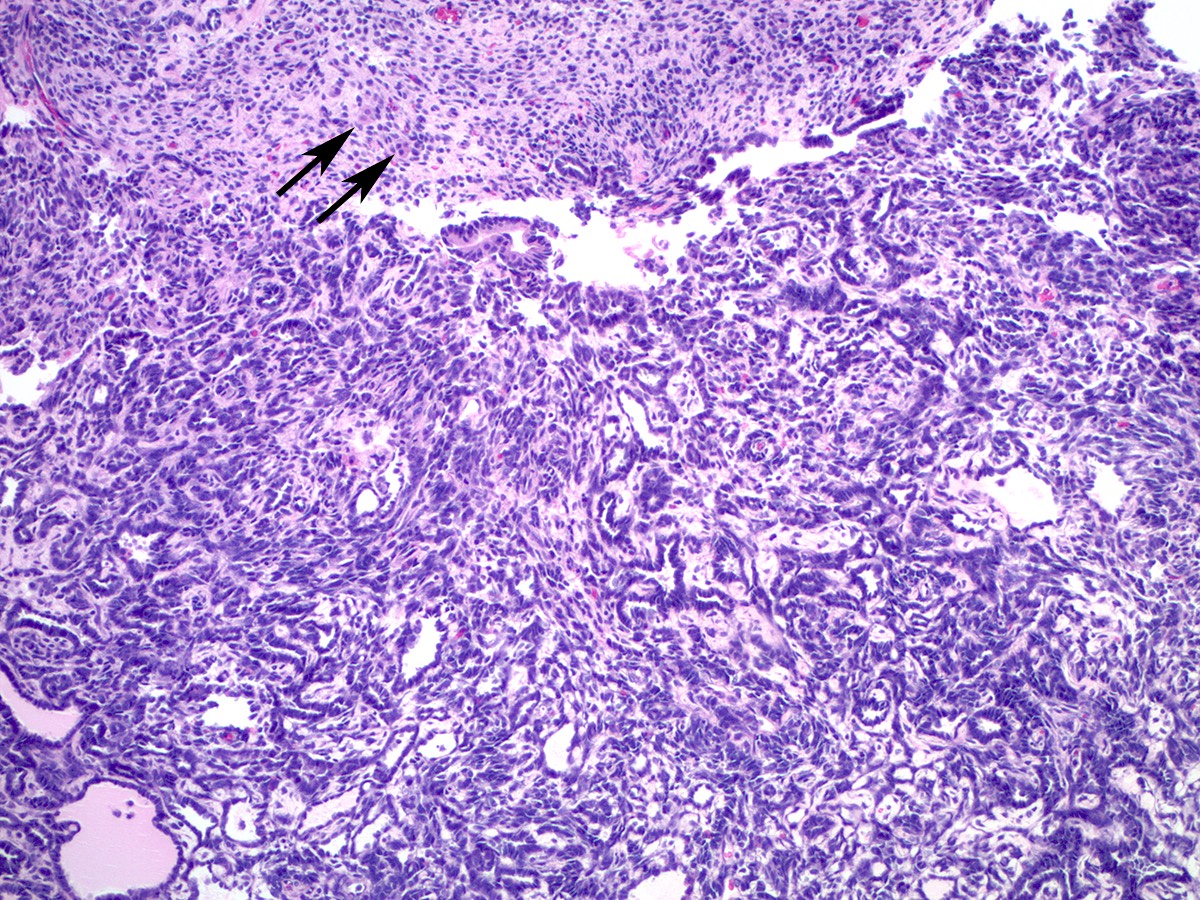
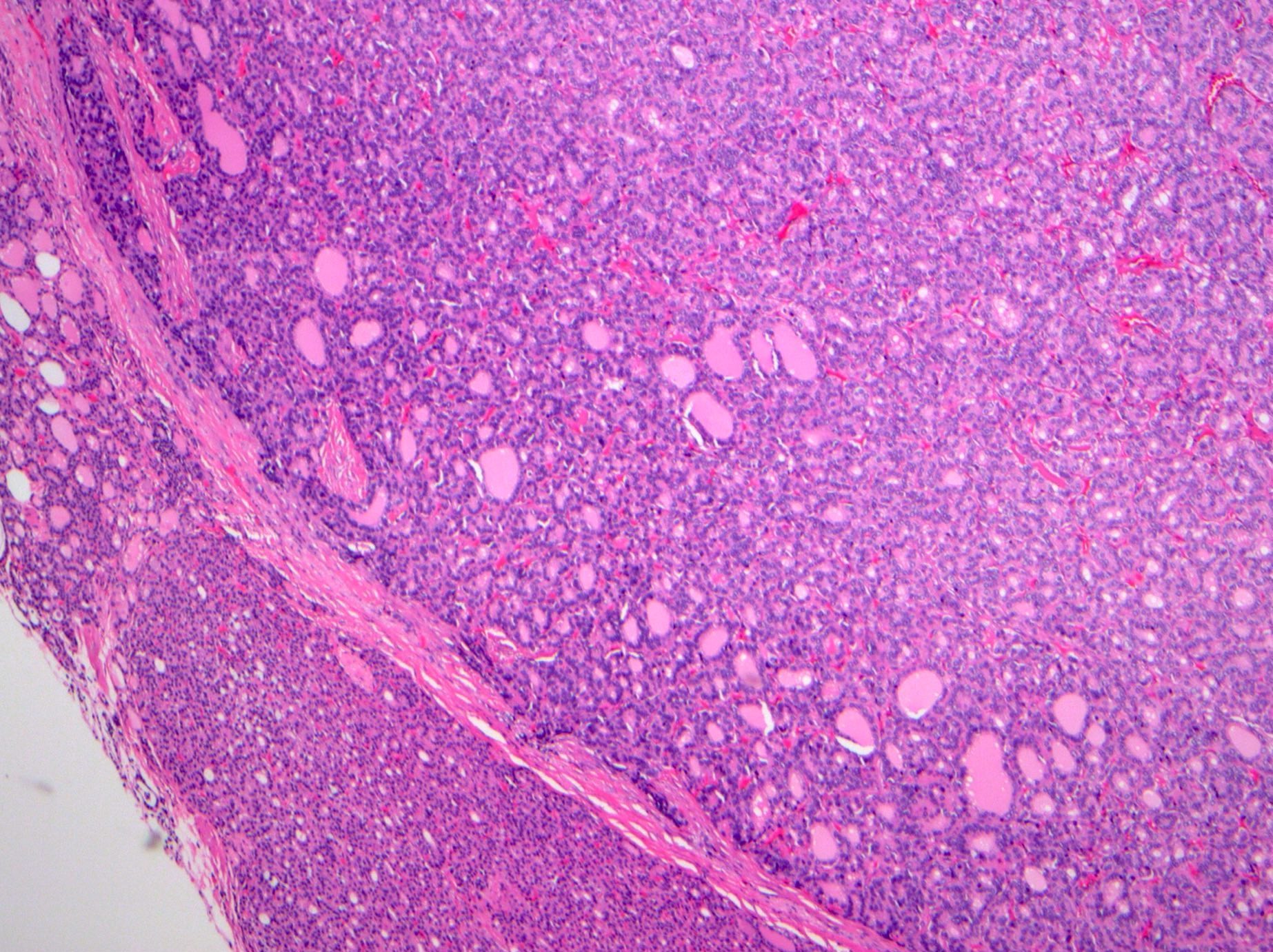
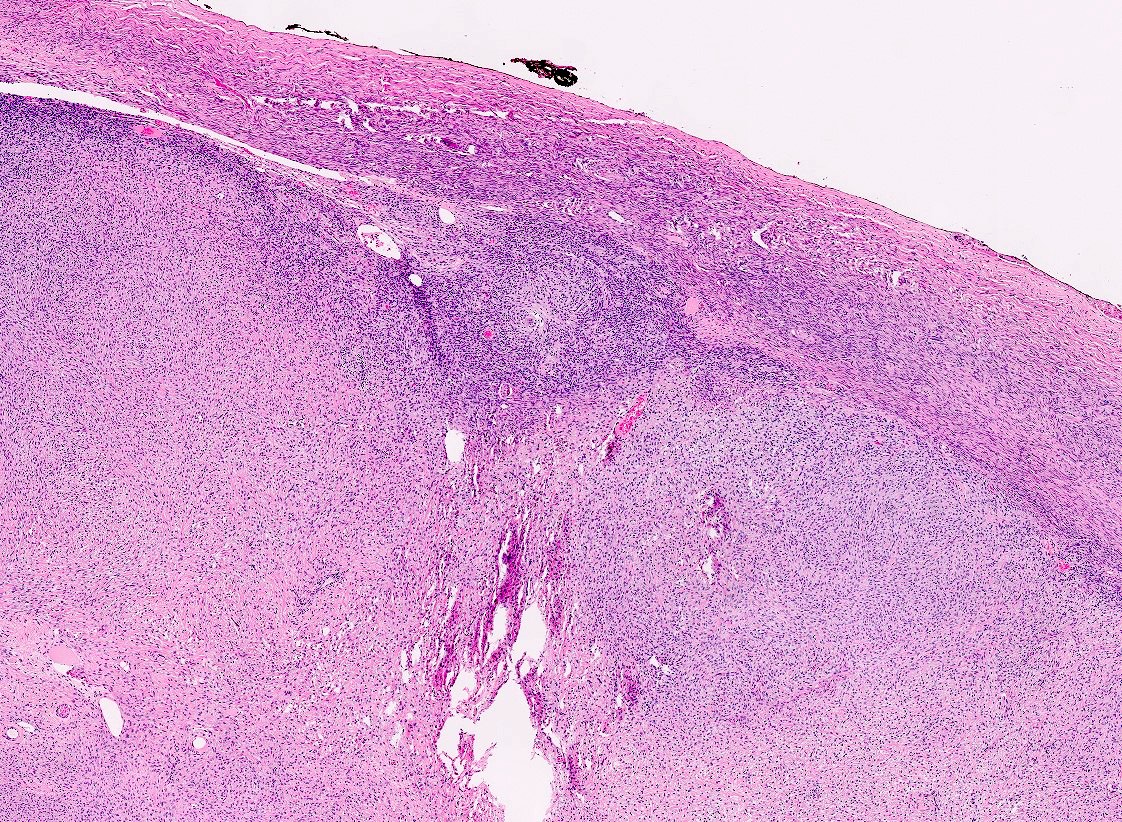
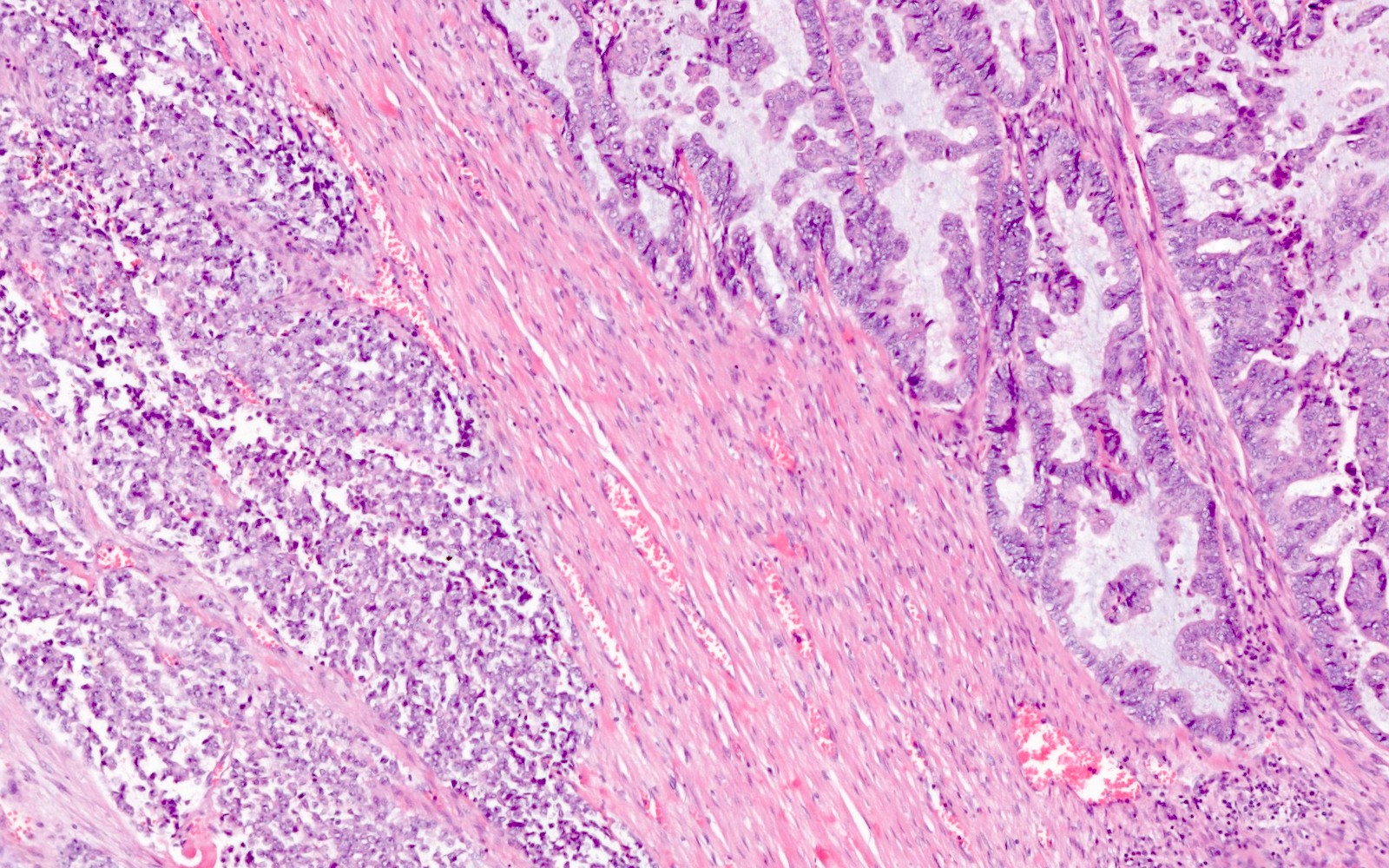
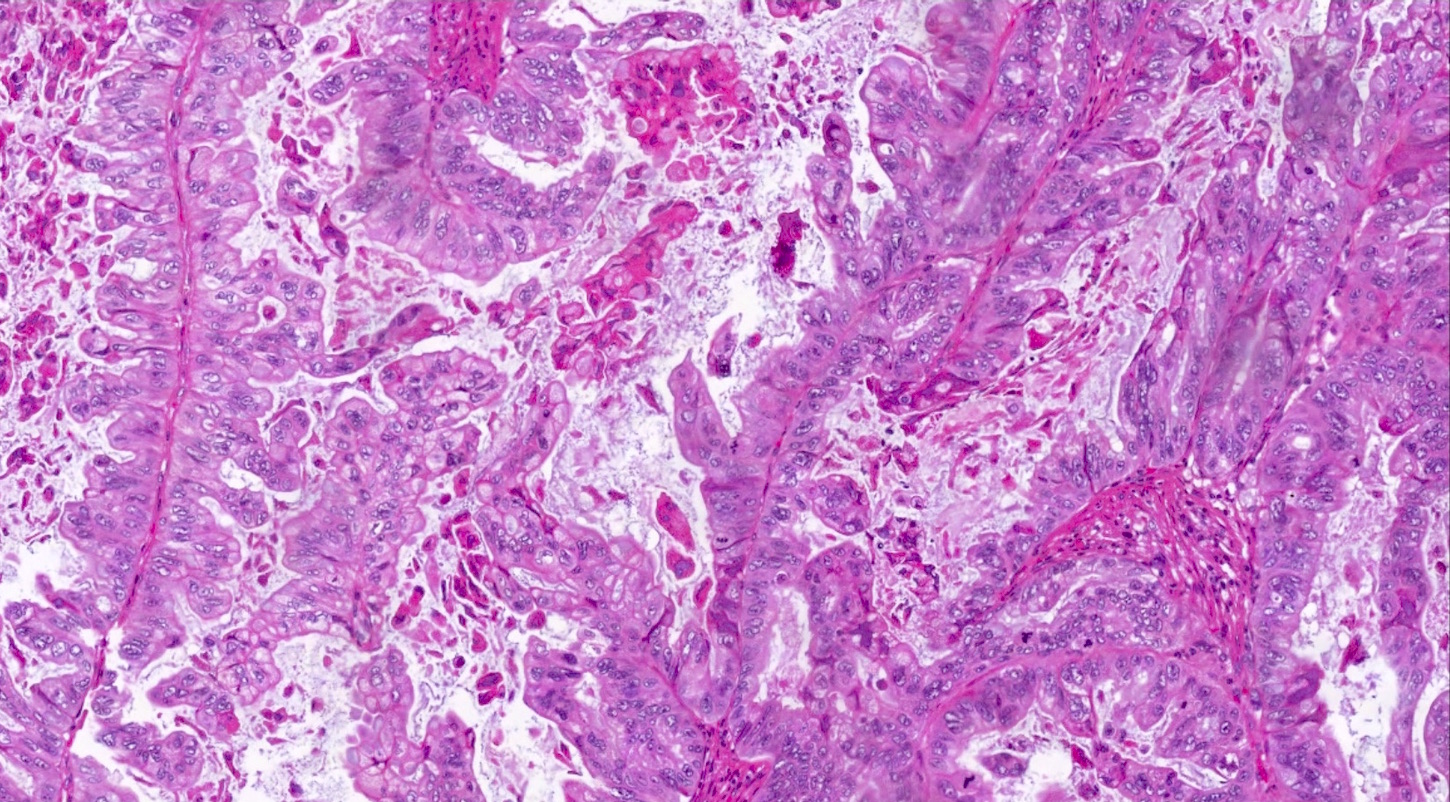
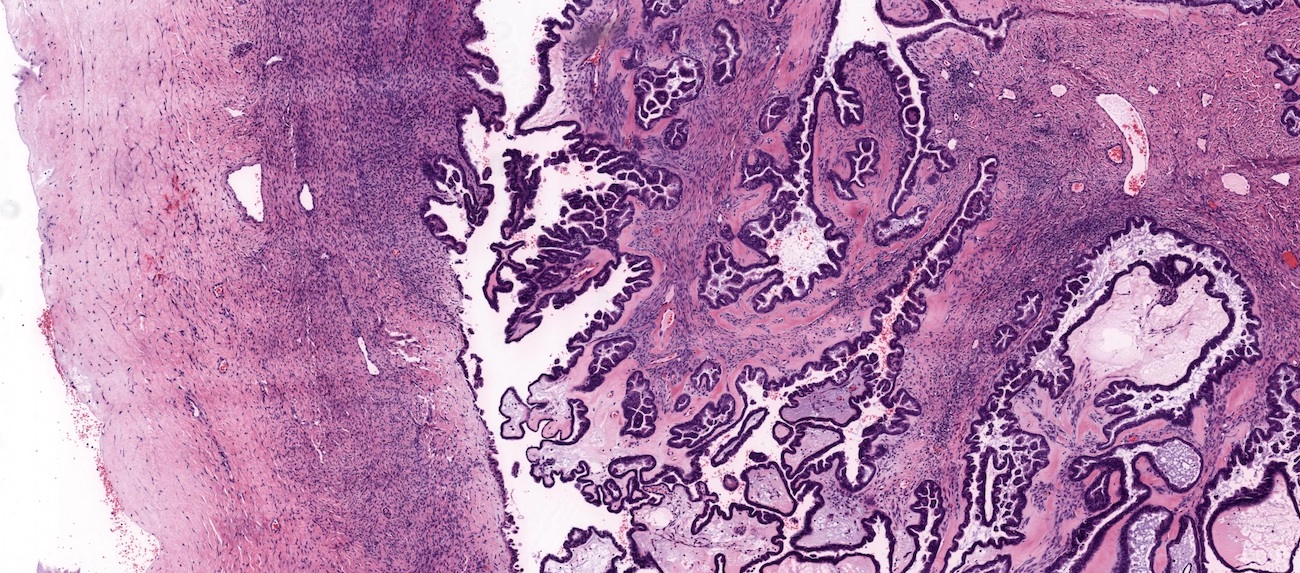
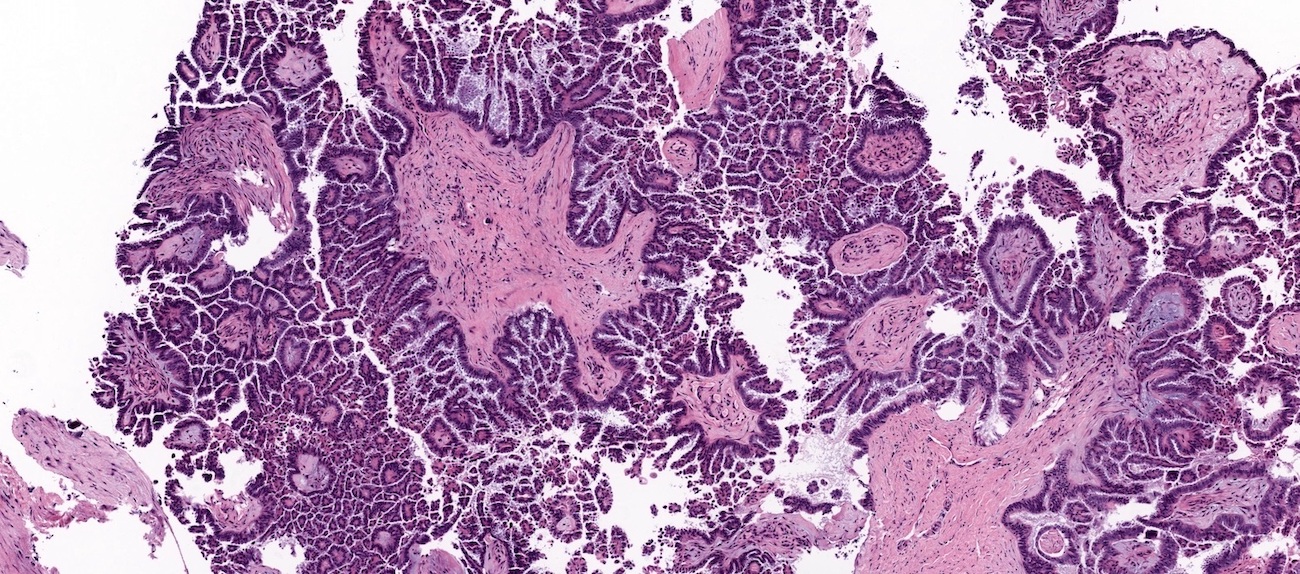
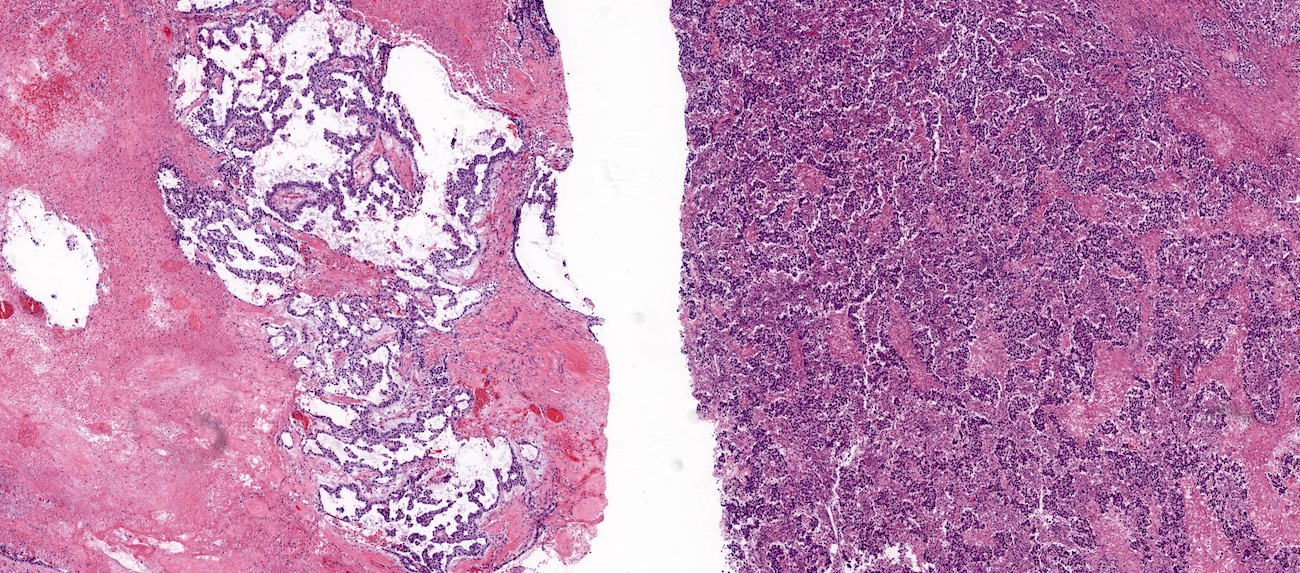
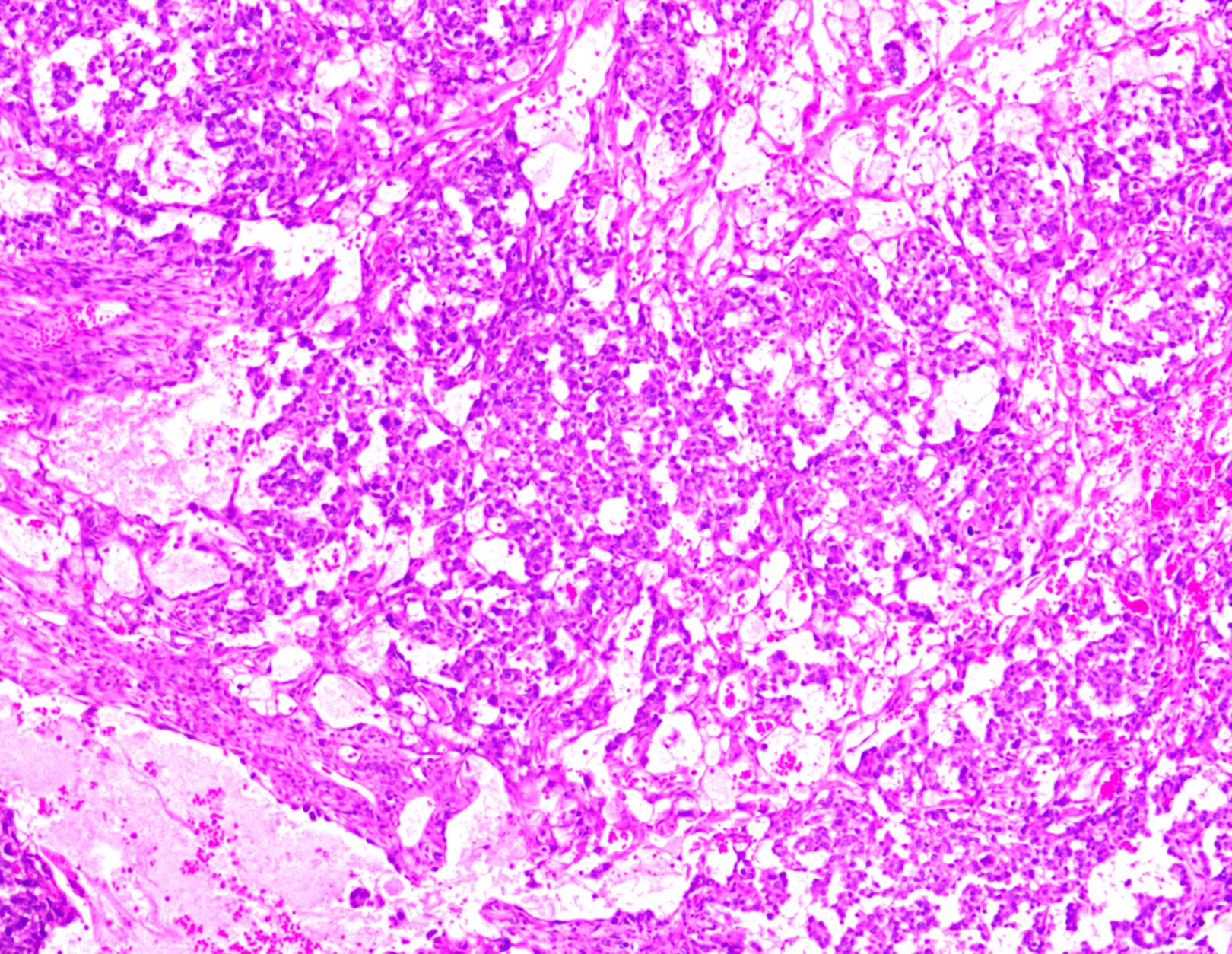
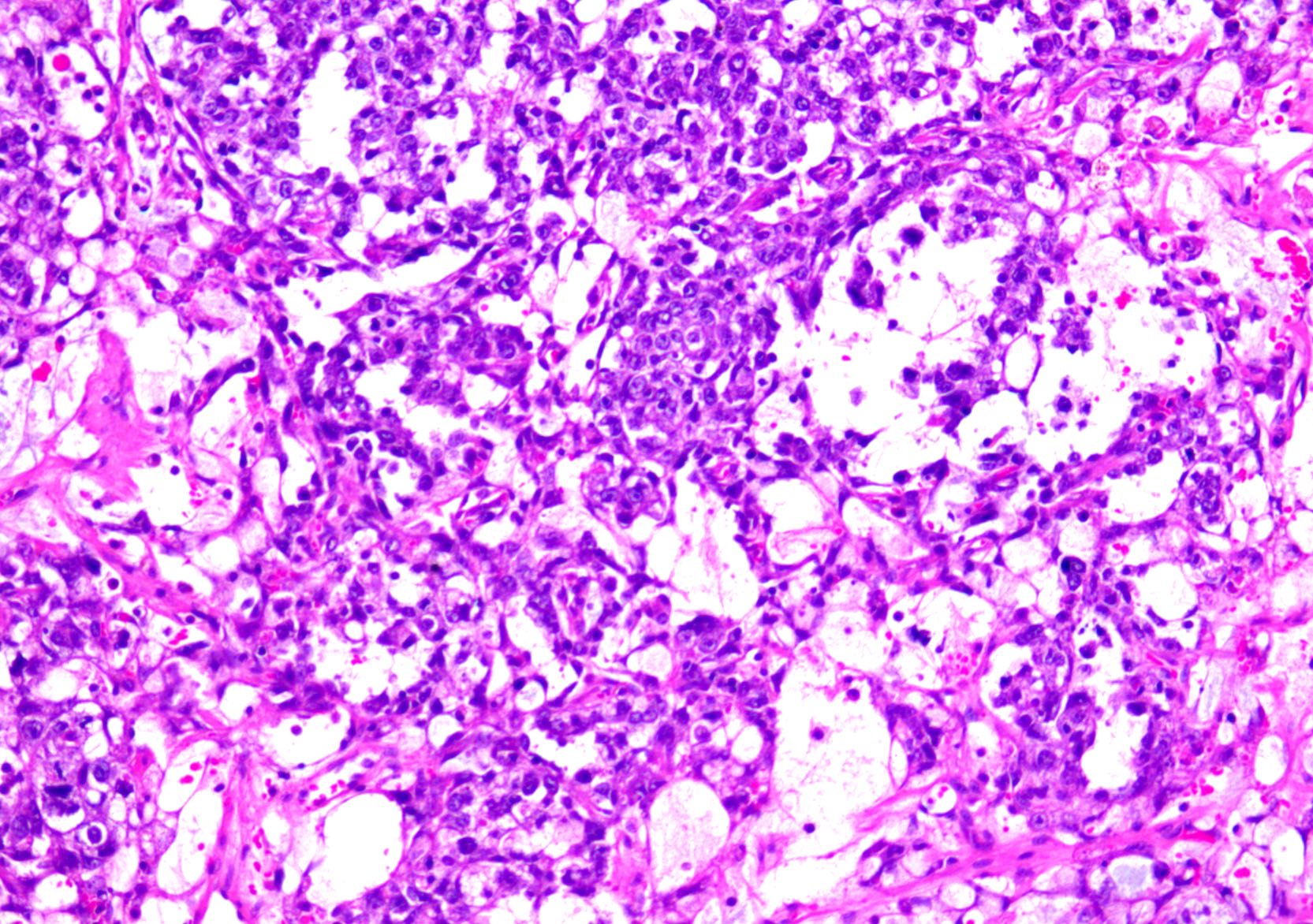
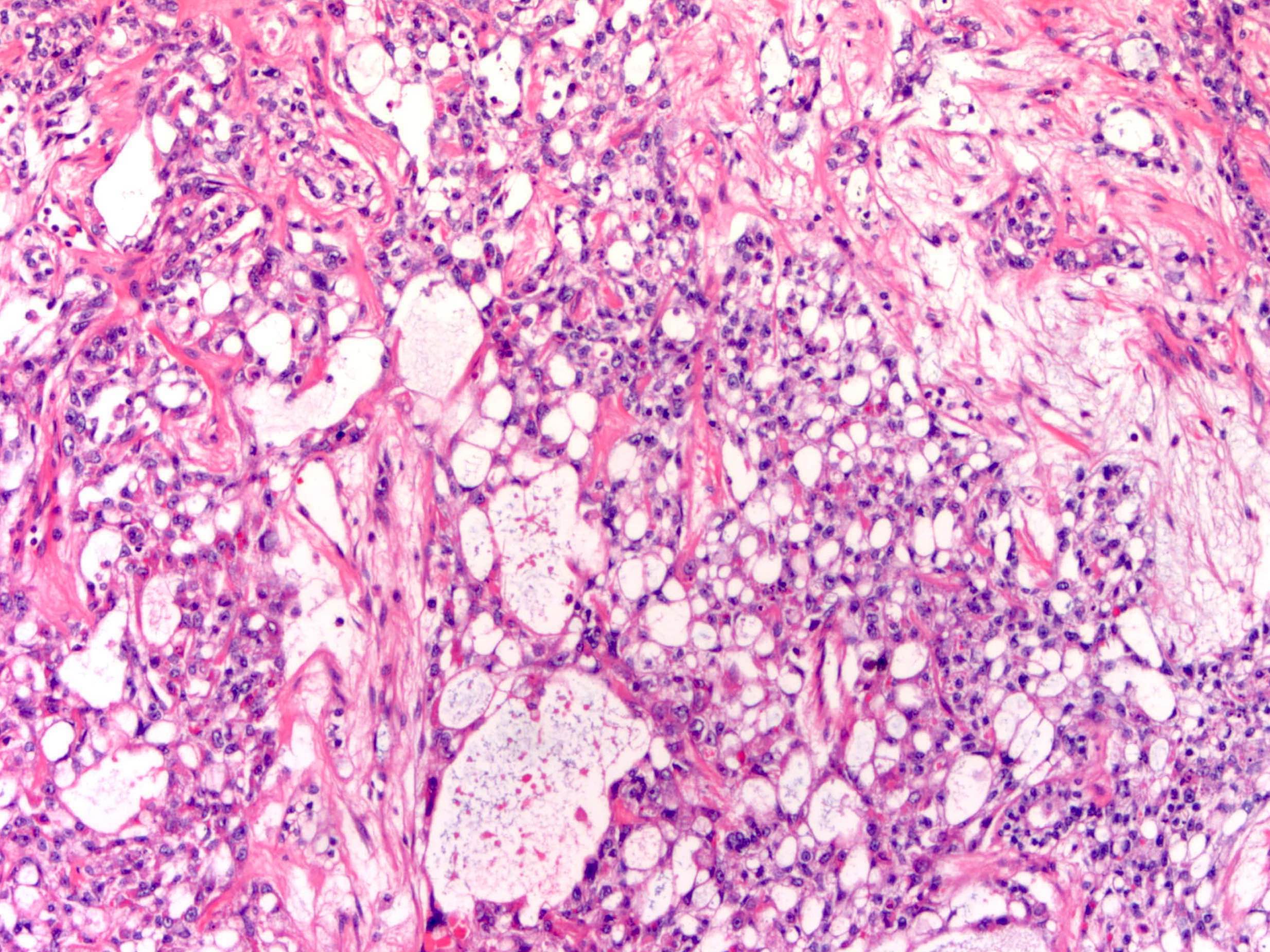
Microscopic (histologic) description
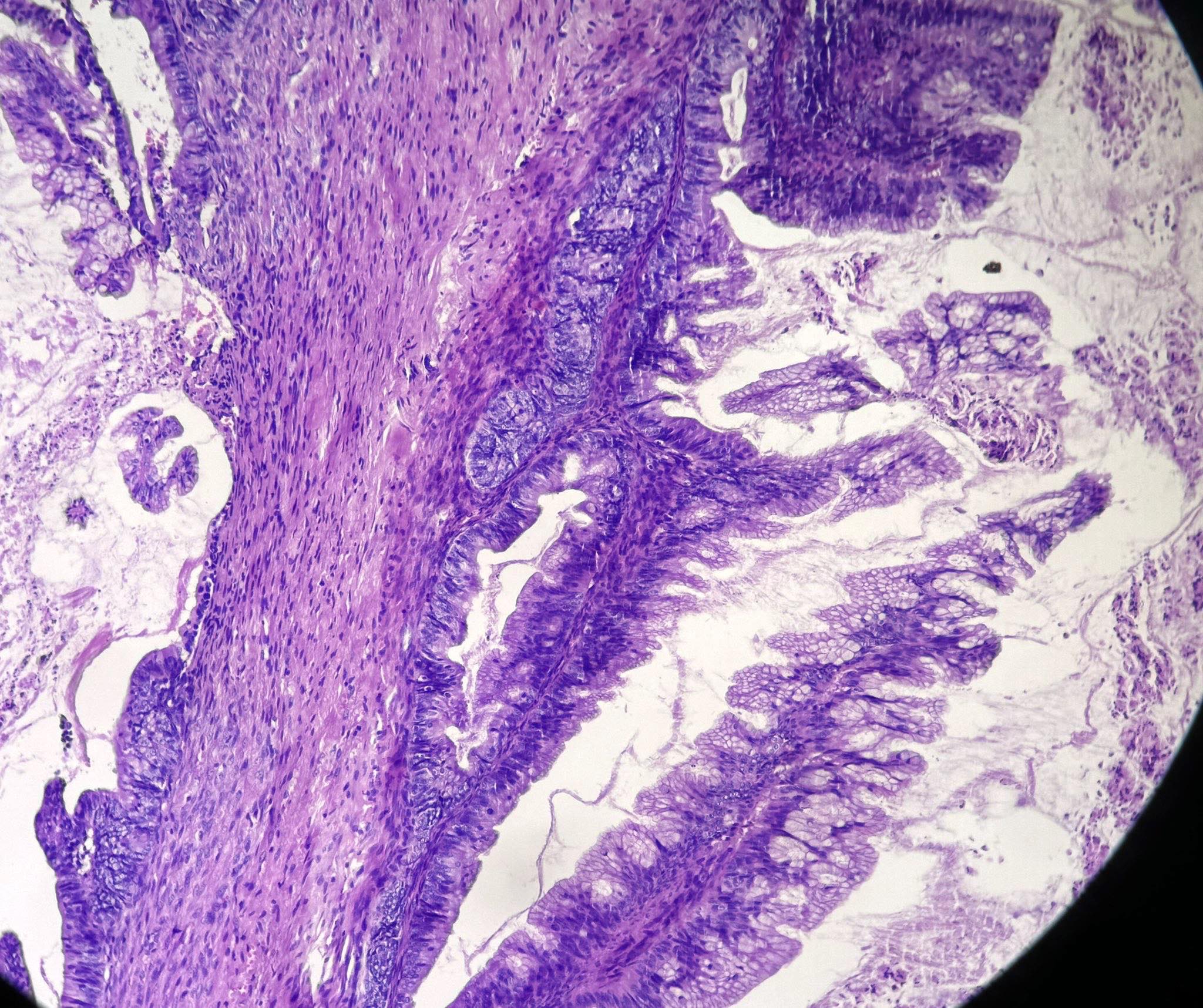
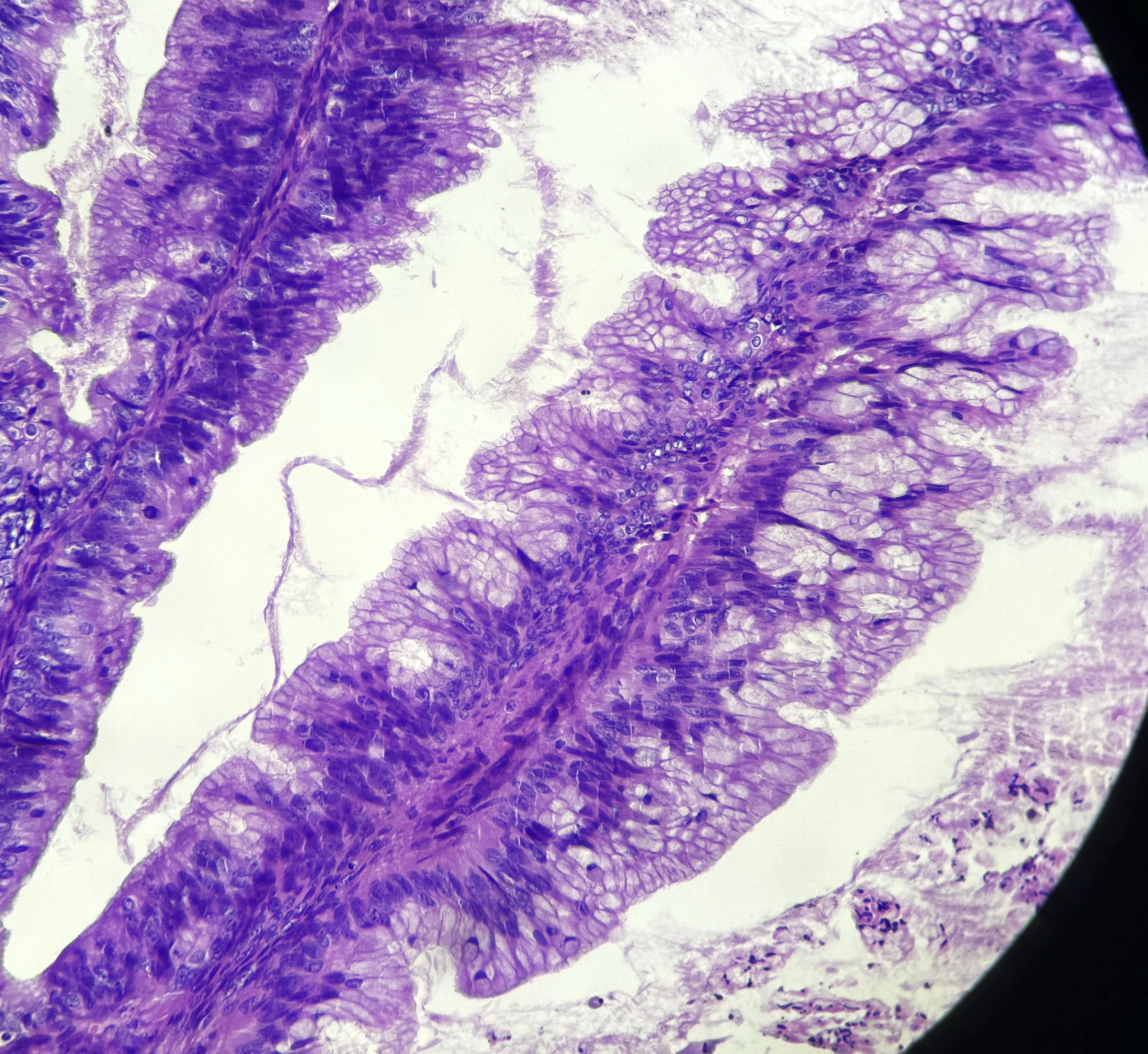
- Low grade appendiceal mucinous neoplasms (LAMN) have bland cytomorphology and mimic benign and borderline ovarian mucinous tumors
- LAMN involving the ovary display certain distinctive microscopic features (Int J Gynecol Pathol 2014;33:1):
- Bilaterality
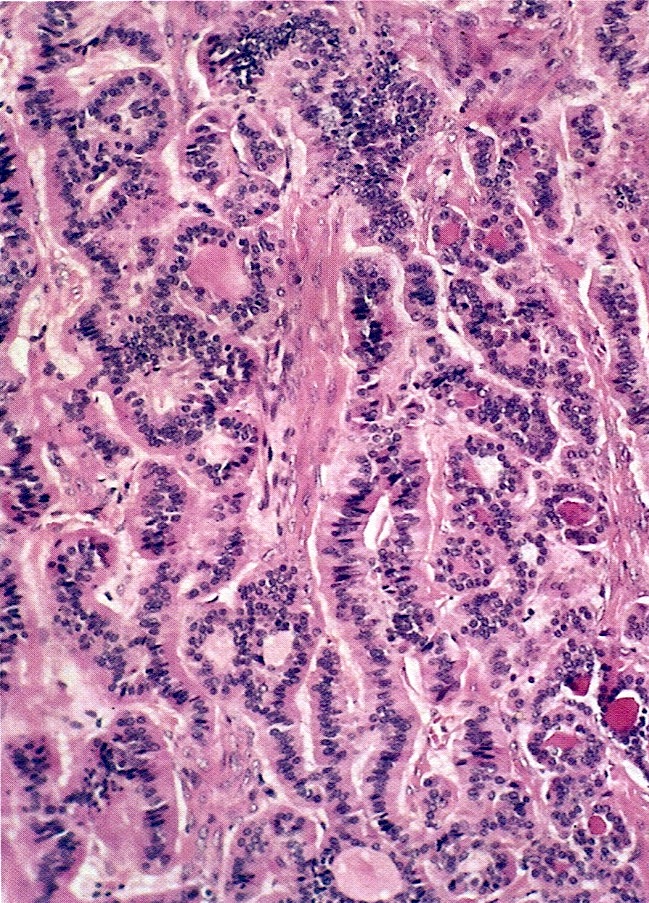
- Tall mucinous cells with indistinct apical borders and lack of cellular stratification
- Elongated glands with shallow epithelial invaginations (scalloping)
- Subepithelial clefts
- These characteristics, however, can also be seen in up to 38.8% of ovarian mucinous borderline tumors
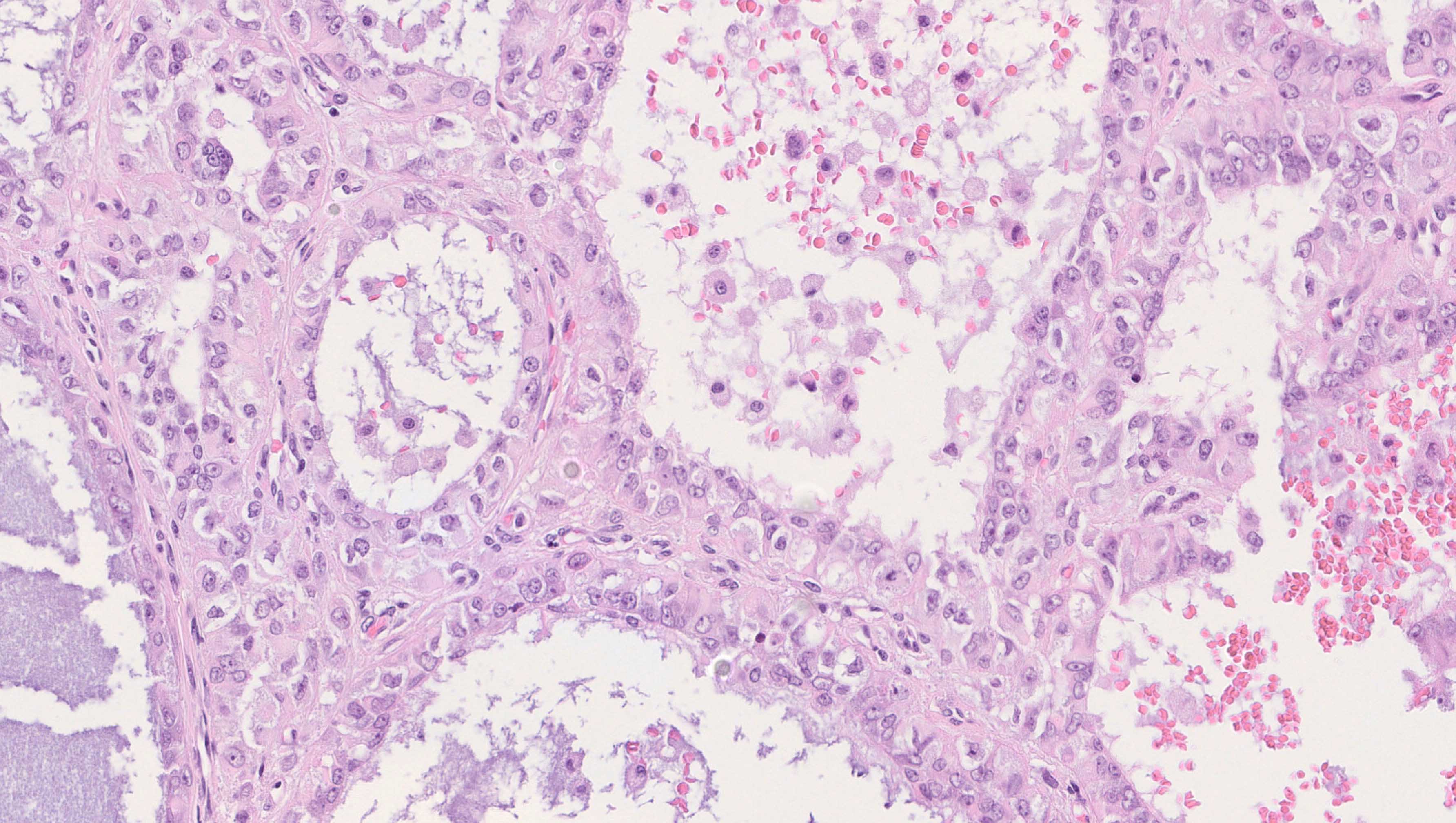
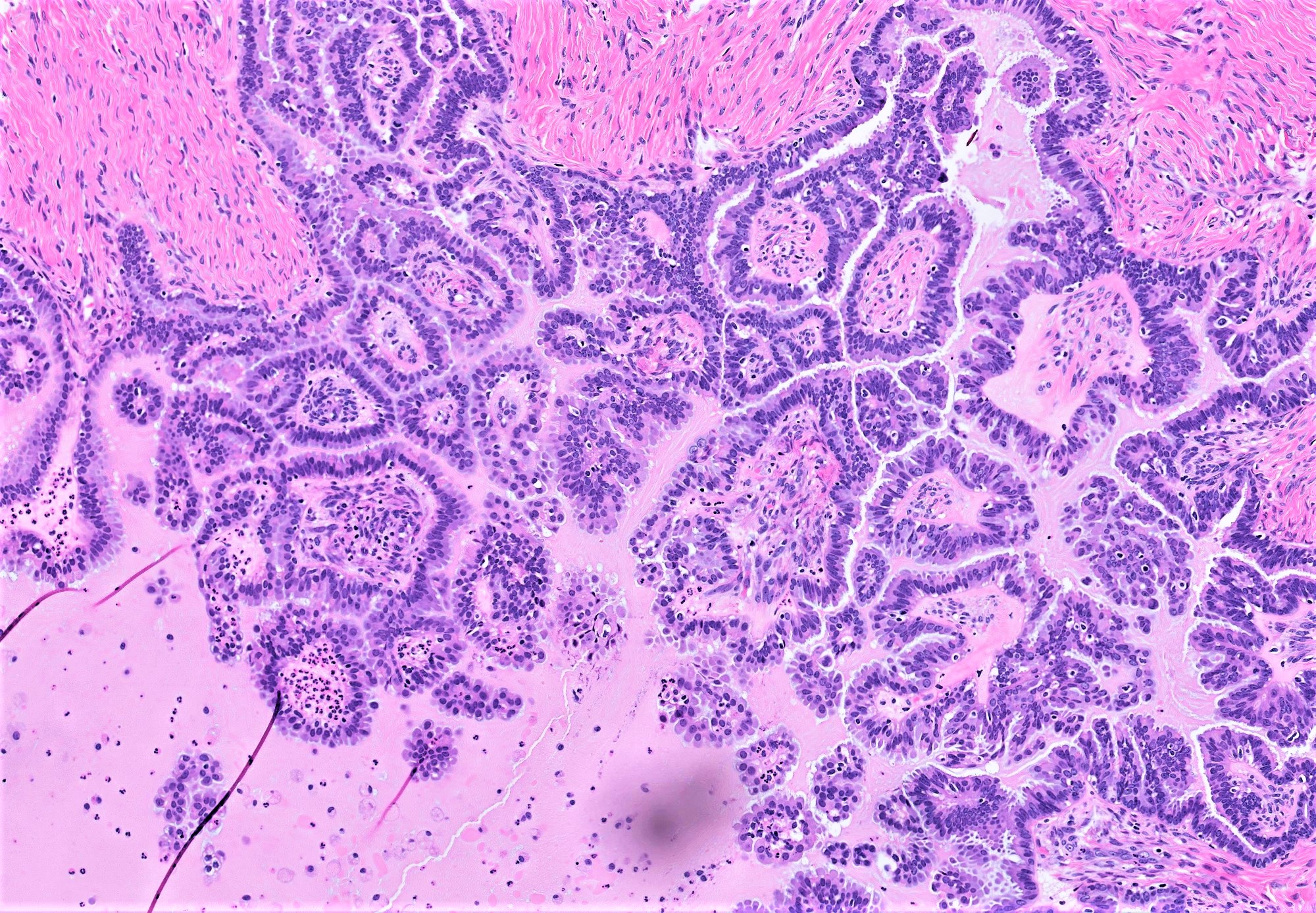
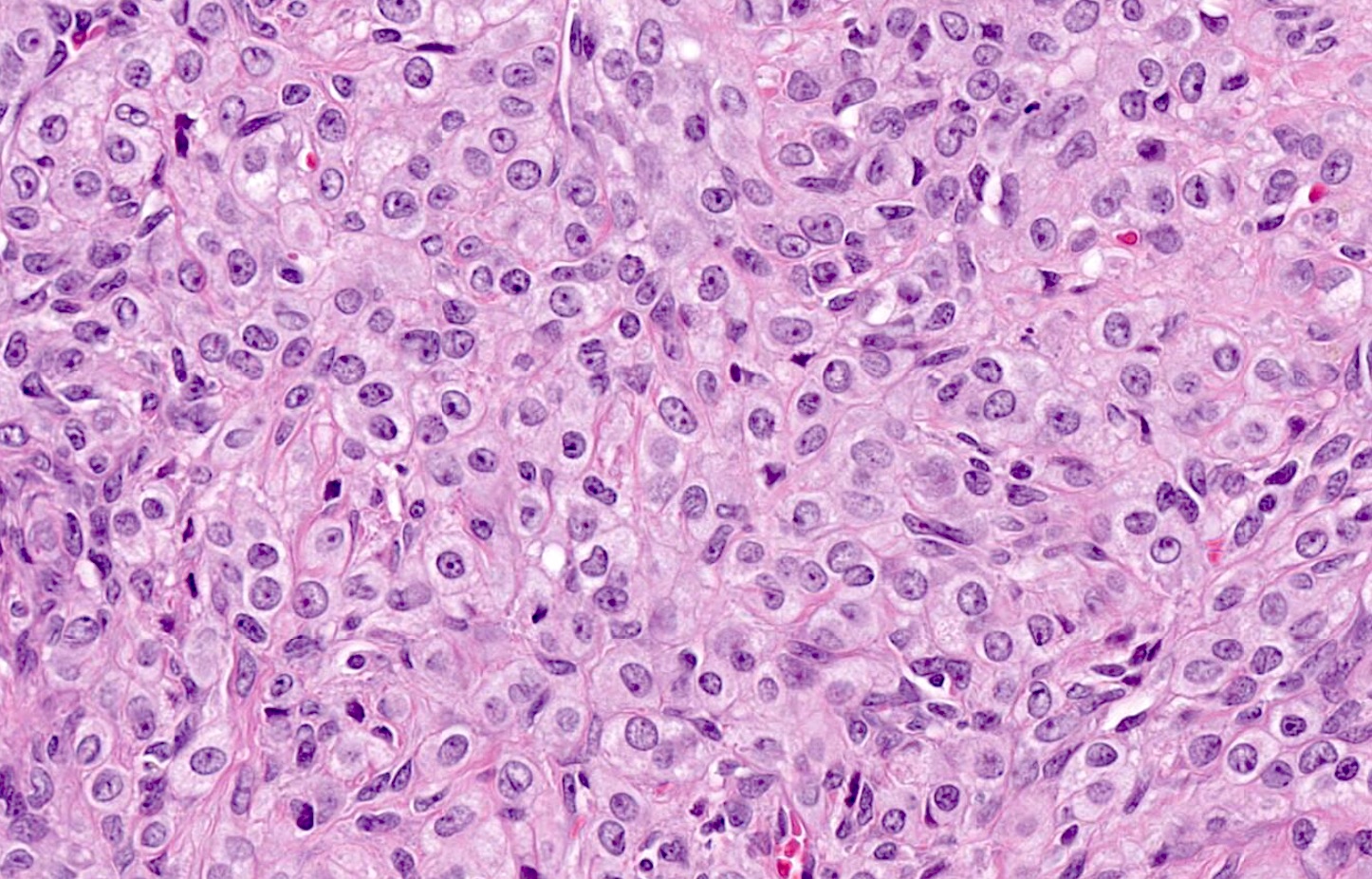
- Appendiceal adenocarcinomas can have a mucinous or a conventional (mucin-depleted) glandular appearance
- Mucinous carcinomas have intestinal (goblet cell) differentiation
- Mucin extravasation and signet-ring cell morphology can be seen
- The term Krukenberg tumor should be reserved to adenocarcinomas involving the ovary with a signet ring cell component > 10% of the tumor volume, regardless of its site of origin (Adv Anat Pathol 2006;13:205)
- Conventional tumor cytomorphology with mucin depletion mimics the architecture and cytoplasmic appearance of primary endometrioid tumors; however, nuclear pleomorphism and hyperchromasia tend to be prominent, and exceed that expected for a primary ovarian tumor
- Central glandular necrosis is also more typical of intestinal tumors, although is not entirely specific
- Several clinical and pathologic features have been described as indicative of secondary (metastatic) origin (Am J Surg Pathol 2003;27:281, J Clin Pathol 2012;65:591), including:
- Bilaterality
- Size less than 10 cm
- Surface involvement
- Infiltrative pattern of invasion
- Presence of signet ring cells
- Extensive lymphovascular space invasion
- Mucin extravasation
- If any of the above features is present, the possibility of a metastasis should be considered and prompt ancillary testing and clinical investigation
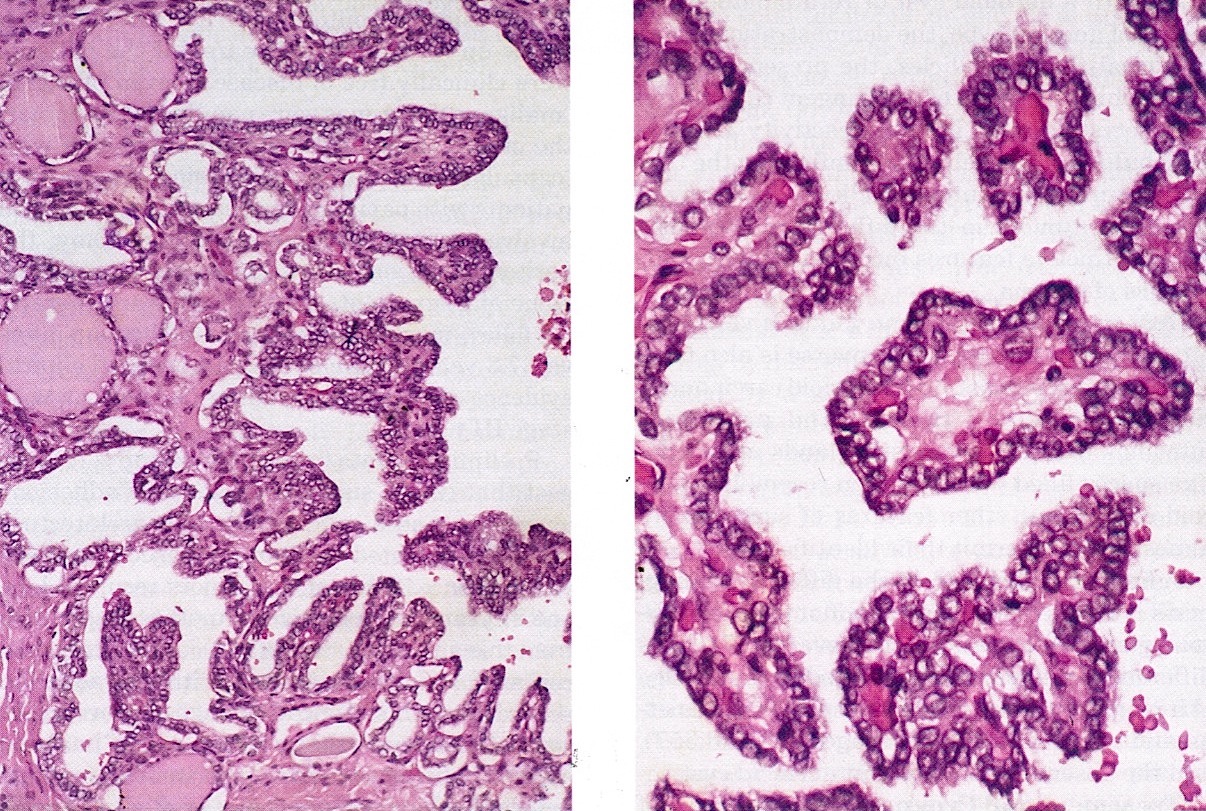
Microscopic (histologic) images
Positive stains
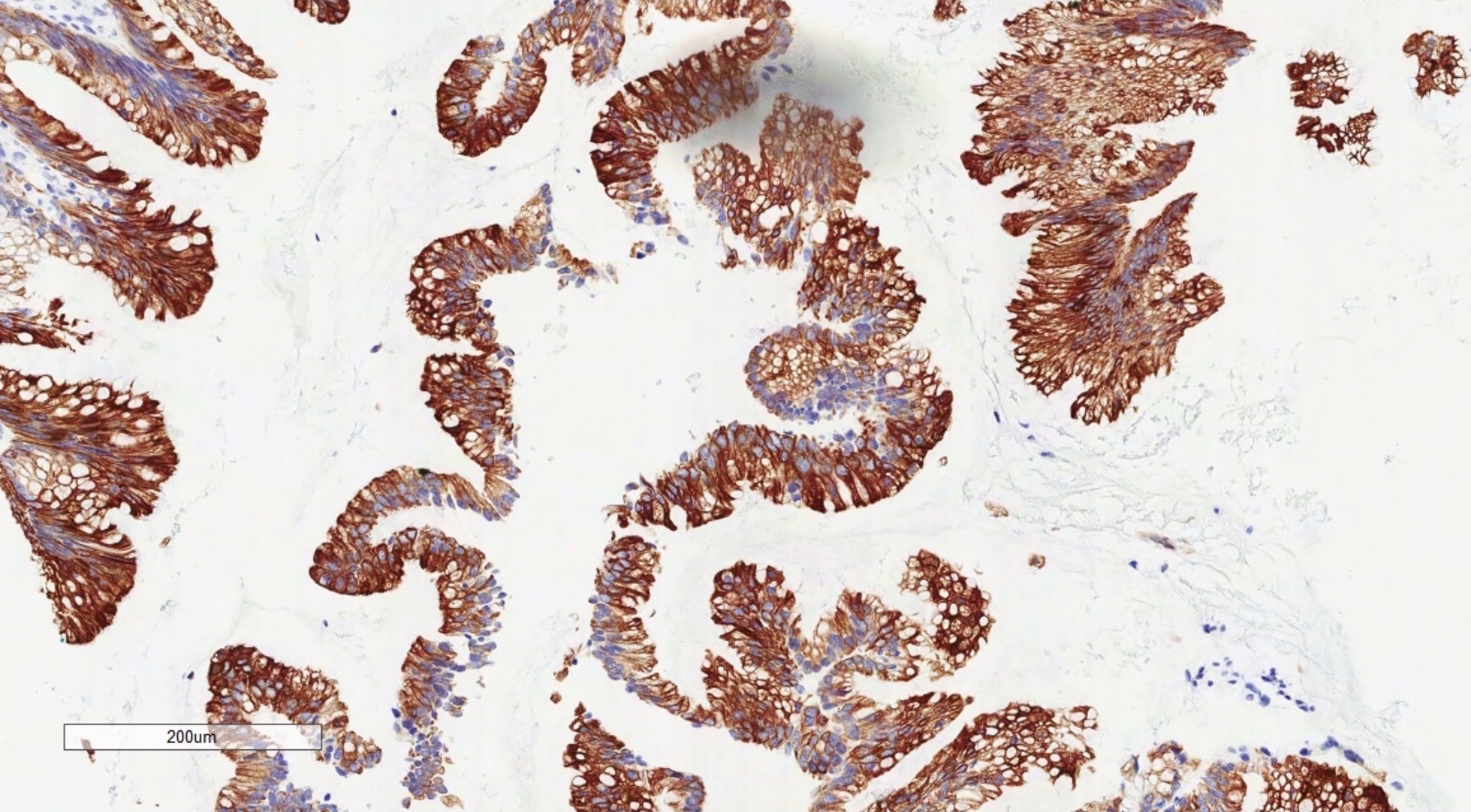
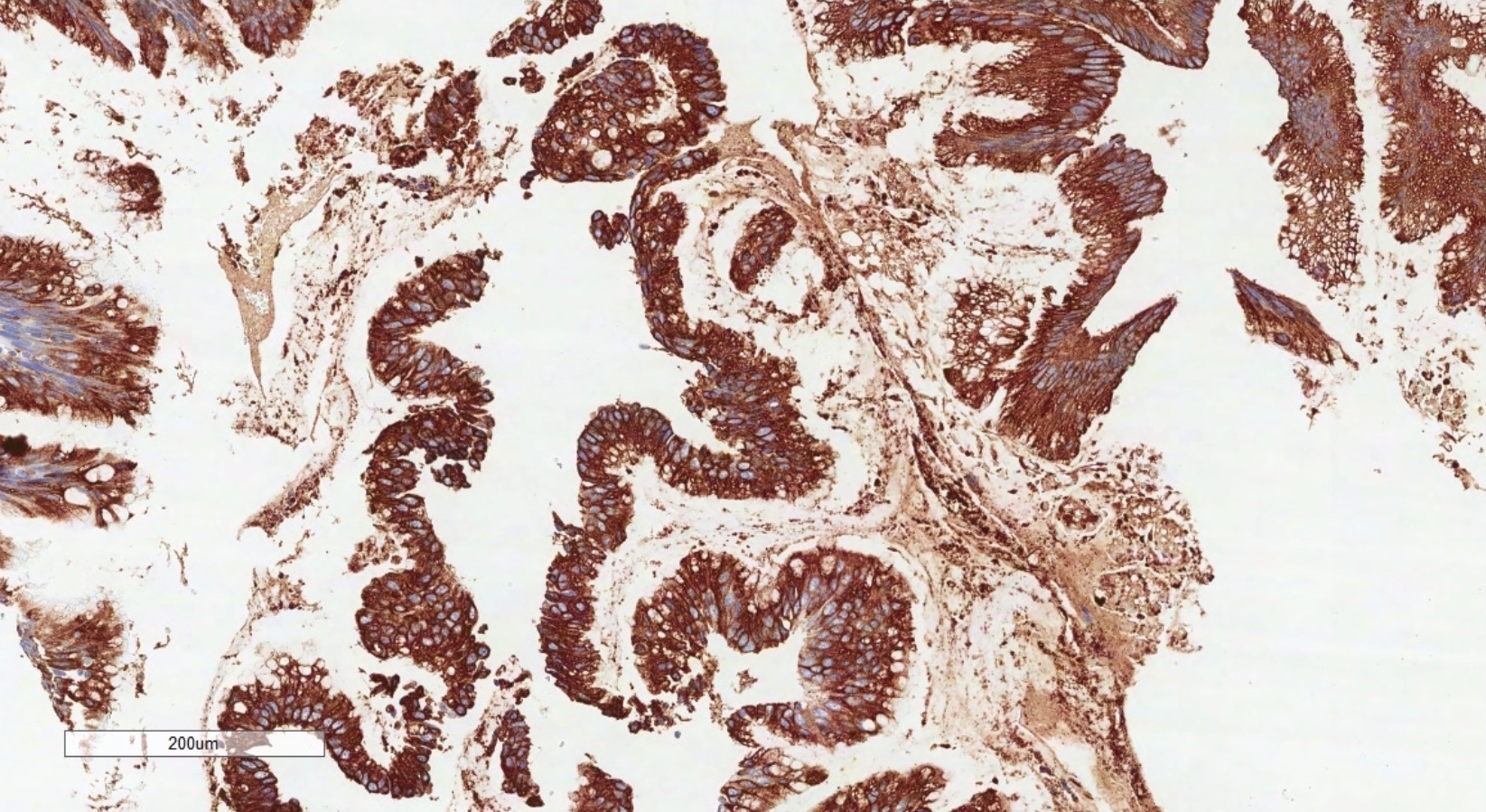
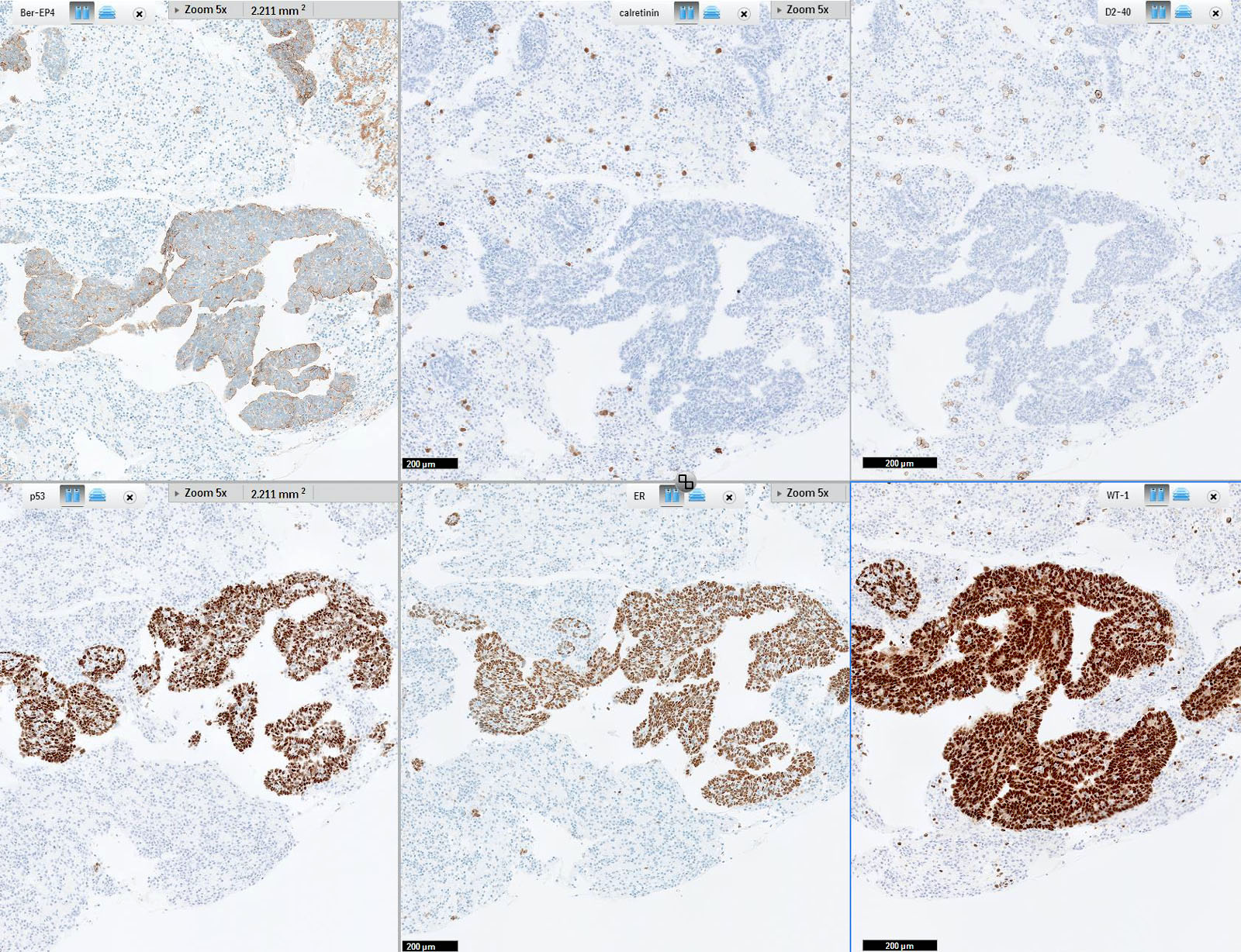
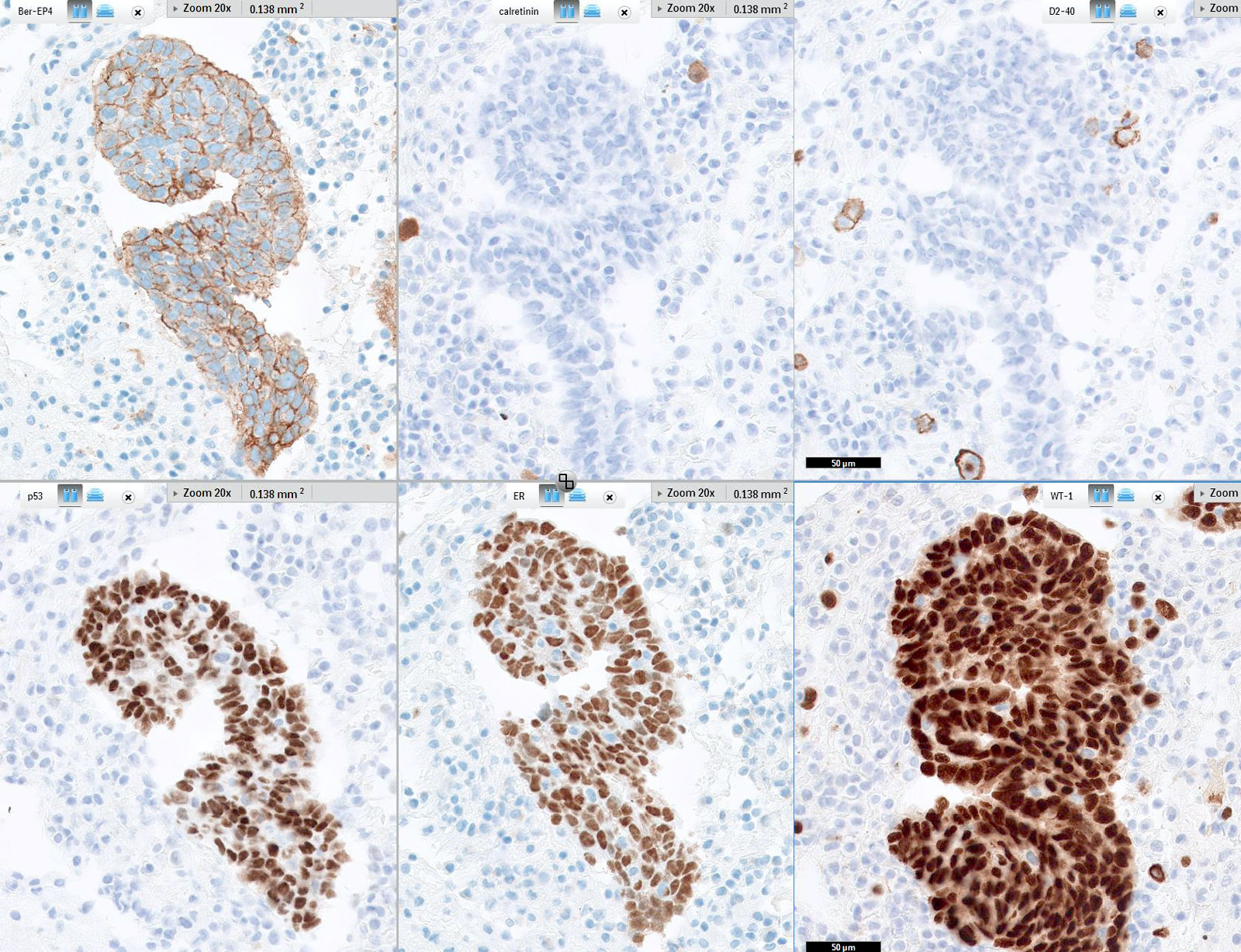
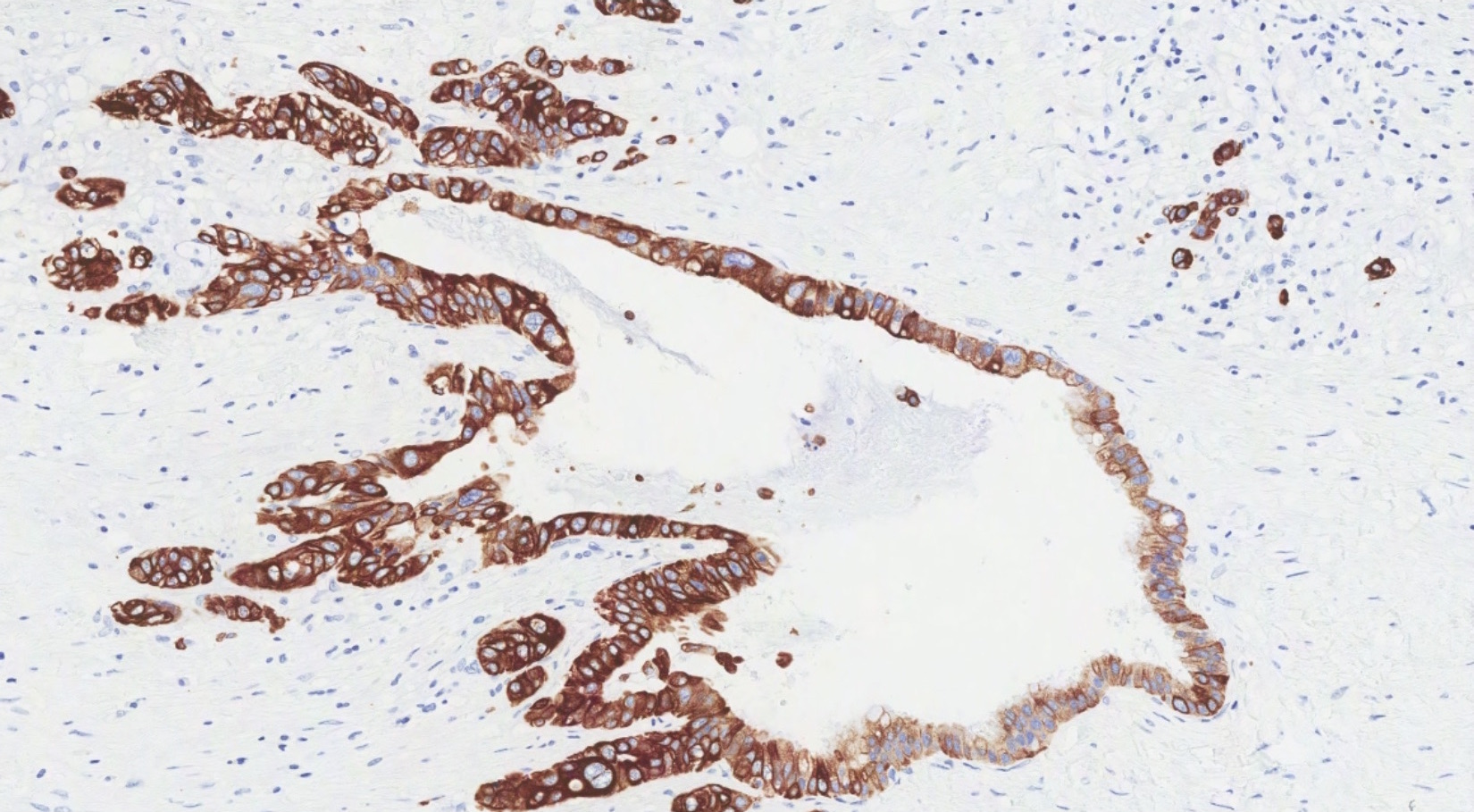
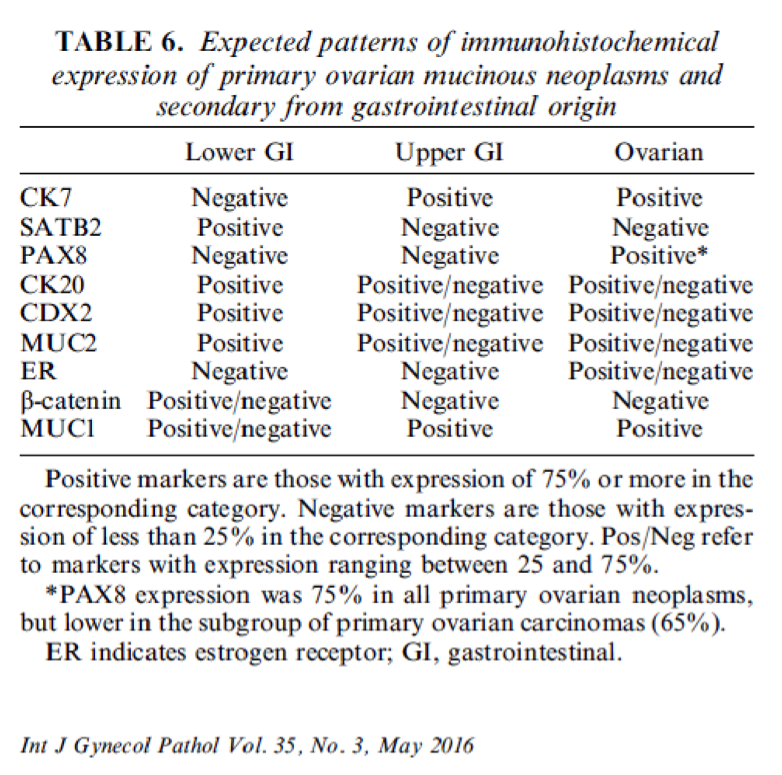
- Immunohistochemistry is a reliable tool in the distinction between primary ovarian tumors and metastases from appendiceal origin
- SATB2 is a sensitive and specific marker of colorectal epithelium
- Also positive in appendiceal epithelium
- This marker has shown 93.8% sensitivity and 97.5% specificity in determining appendiceal origin (Histopathol 2016;68:977)
- CK20, CDX2 and MUC2 are also optimal markers of appendiceal origin; although they are frequently expressed in primary ovarian tumors, such expression is usually patchy / focal
- Diffuse and strong expression for CK20, CDX2 and MUC2 has 90%, 87.5% and 95% specificity for appendiceal origin
Negative stains
Differential diagnosis
- Low grade mucinous neoplasm or mucinous carcinoma with pseudomyxoma peritonei arising in a mature cystic teratoma (Am J Surg Pathol 2007;31:854)
- Metastases from cervix: p16 positive (strong, diffuse)
- Metastases from colon and rectum: mass located in the large intestine, normal appendix (colorectal and appendiceal tumors have a similar IHC profile)
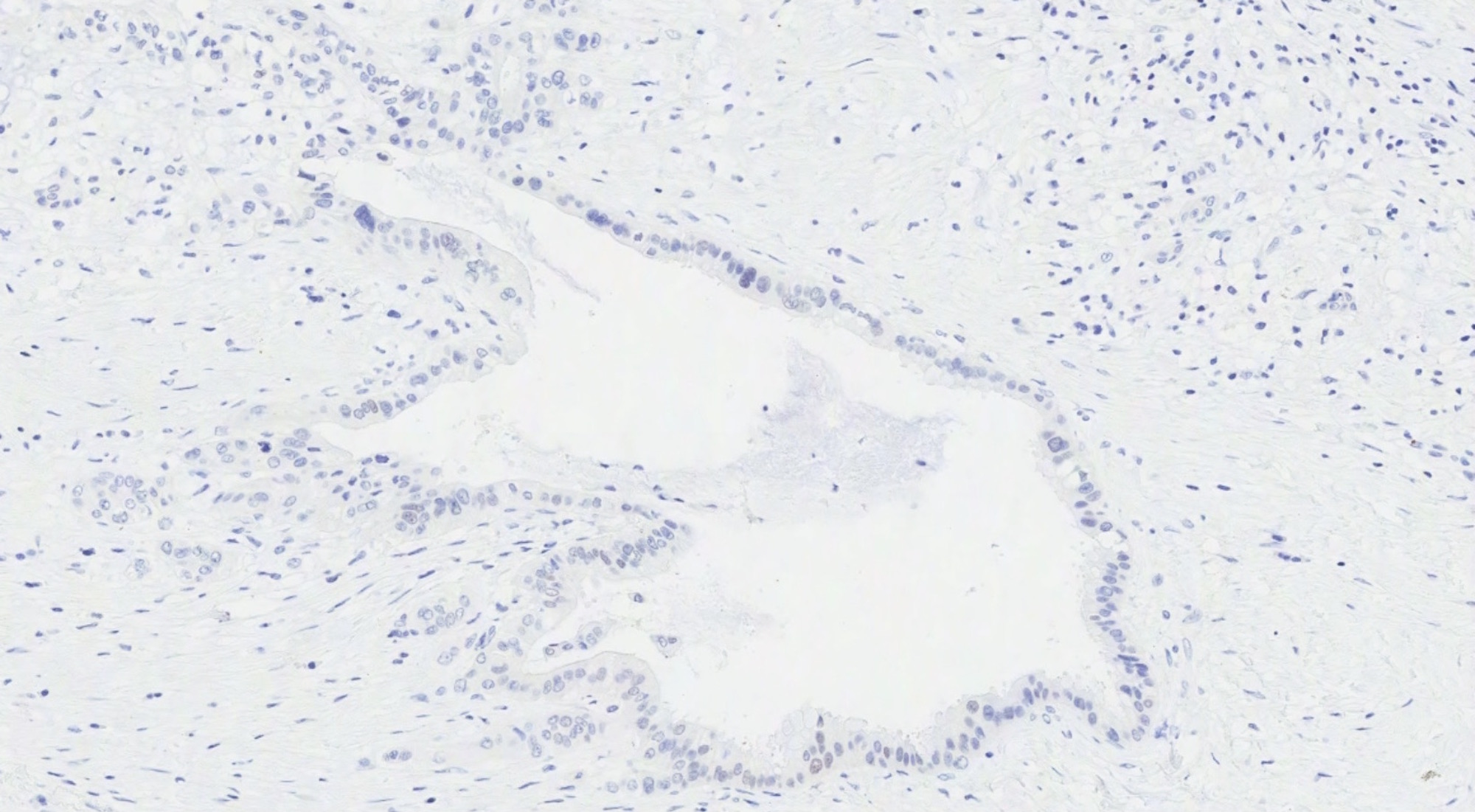
- Metastases from upper GI tract: CK7 positive, CK20 / CDX2 / SATB2 variable (mostly negative)
- Primary ovarian neoplasm (benign, borderline or malignant): absence of suspicious features described above (bilaterality, surface involvement, signet-ring cell morphology, etc), SATB2 negative, CK20 / CDX2 / MUC2 negative or patchy, PAX8 positive
Autoimmune oophoritis (pending)
[Pending]
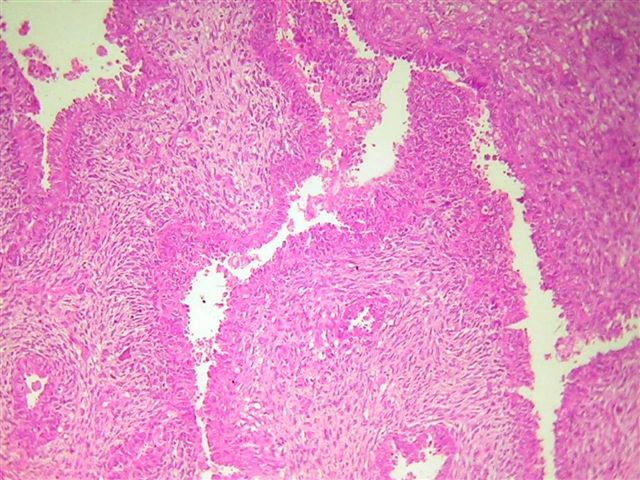
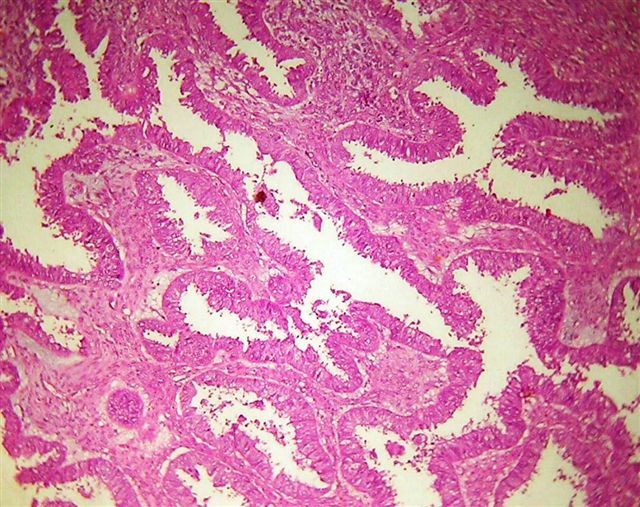
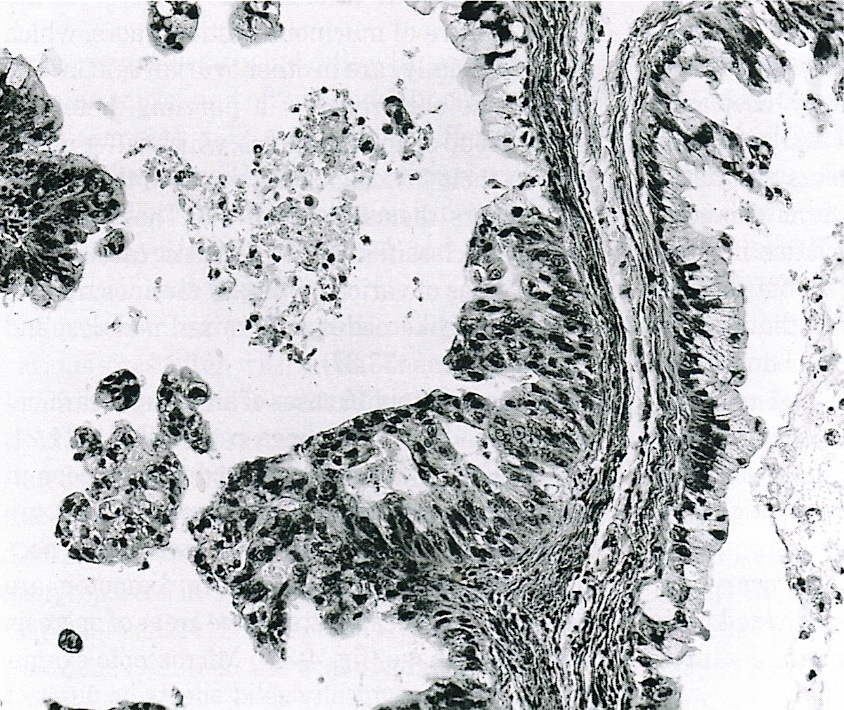
Benign, borderline and malignant Brenner tumors
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
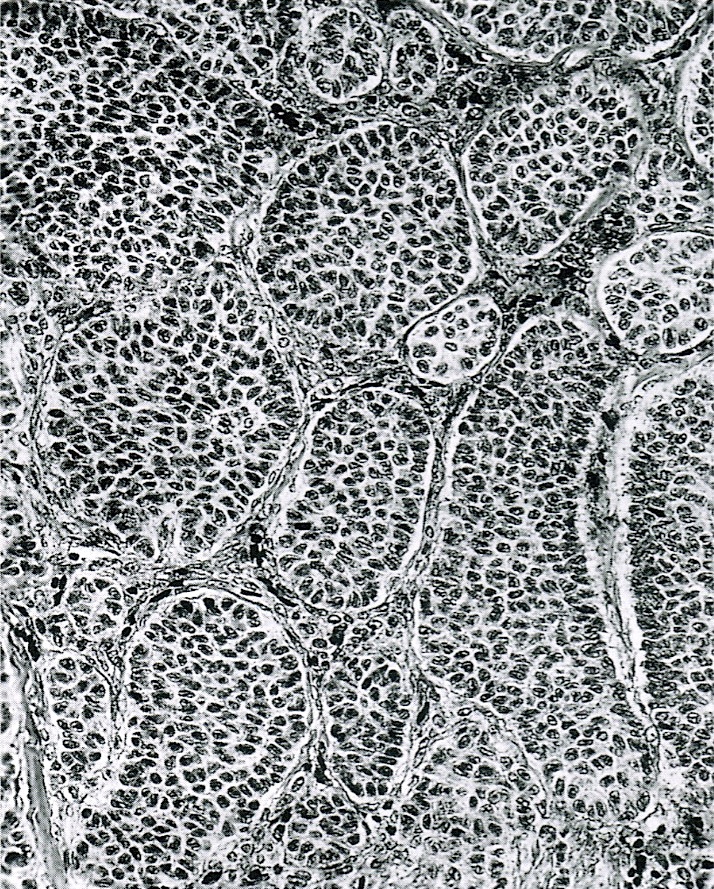
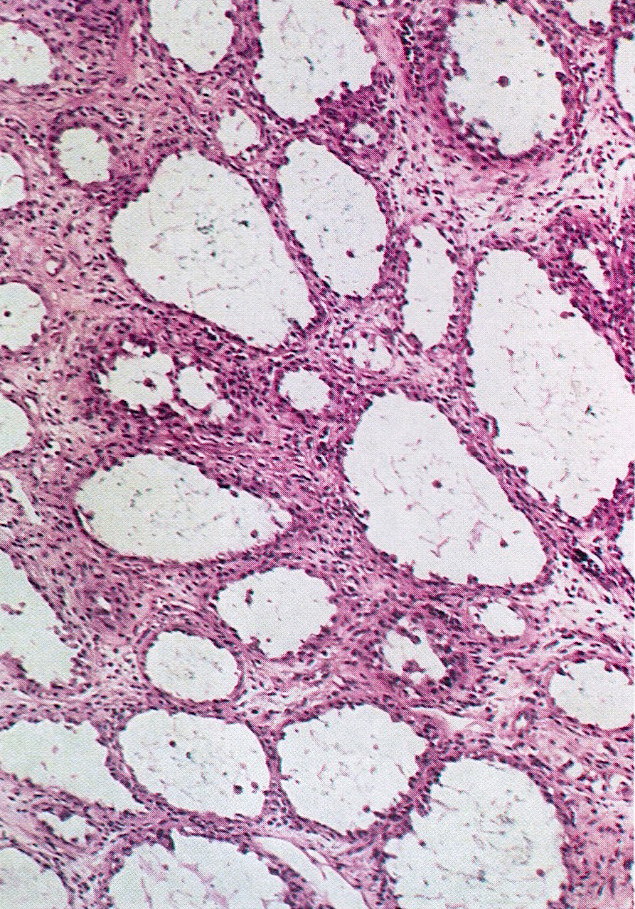
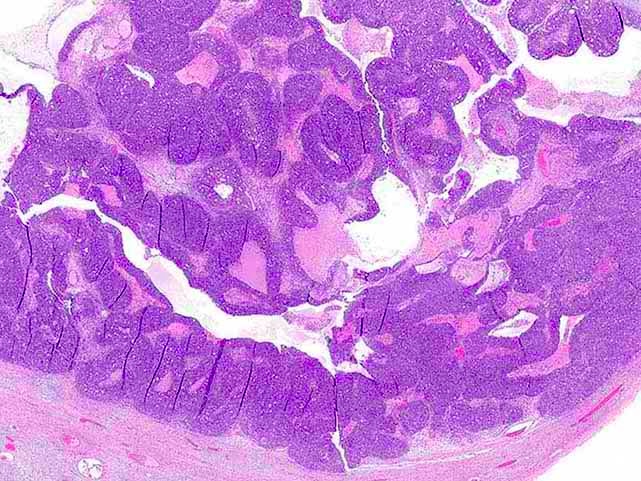
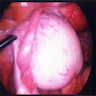
- Tumor composed of transitional / urothelial-like epithelium, typically embedded in fibromatous stroma
- Benign, borderline and malignant variants are recognized, based on the growth pattern and cytological features of the epithelial cells
Essential features
- Benign Brenner tumor:
- Adenofibromatous architecture with nests of bland transitional epithelium present within fibromatous stroma
- Borderline Brenner tumor:
- Papillary architecture with papillae covered by multilayered transitional epithelium
- There is variable but usually low grade cytological atypia
- Malignant Brenner tumor:
- Stromal invasion by carcinoma with transitional cell features, associated with a benign or borderline Brenner tumor
ICD coding
- ICD-O:
- ICD-11:
- 2F32.Y & XH5DX3 - Brenner tumor, NOS
- 2C73.Y & XH2CH8 - Brenner tumor, borderline malignancy
- 2C73.Y & XH6NJ7 - Brenner tumor, malignant
Epidemiology
- Brenner tumors are most common in the fifth and sixth decades but can occur across a wide age range
Sites
- Ovary
- Rare extraovarian Brenner tumors are reported
Pathophysiology
- Cell of origin of Brenner tumors is controversial; they may arise from Walthard rests
Etiology
- Unknown
Clinical features
- Benign Brenner tumors are usually asymptomatic
- Borderline and malignant Brenner tumors are larger and usually present with findings secondary to an adnexal mass
Diagnosis
- Most benign Brenner tumors are an incidental finding in an ovary removed for other reasons
- Borderline and malignant Brenner tumors are usually diagnosed at the time of removal of an adnexal mass
Radiology description
- Nonspecific findings of a solid or solid and cystic ovarian mass
Prognostic factors
- All reported cases of benign and borderline Brenner tumor have had a benign course, although local recurrence has rarely been reported for the latter (Acta Pathol Microbiol Scand Suppl 1972;233:56)
Case reports
- Benign Brenner tumor:
- 58 year old woman with a coexisting benign Brenner tumor and mucinous cystadenoma (Iran J Pathol 2020;15:334)
- 60 year old postmenopausal woman with ovarian mucinous cystic tumor associated with sarcomatous mural nodule and benign Brenner tumor (Medicine (Baltimore) 2019;98:e14066)
- 68 year old woman with a benign Brenner tumor arising in an ectopic ovary (Int J Gynecol Pathol 2020 Sep 17 [Epub ahead of print])
- Borderline Brenner tumor:
- 68 year old woman with borderline Brenner tumor (J Ovarian Res 2014;7:101)
- Malignant Brenner tumor:
- 62 year old woman with malignant Brenner tumor manifesting as bowel obstruction (BMJ Case Rep 2020;13:e235394)
- 77 year old woman with malignant Brenner tumor (Gynecol Oncol Rep 2017;22:26)
Treatment
- Oophorectomy
- No adjuvant treatment for benign or borderline Brenner tumors
- Adjuvant chemotherapy for advanced stage malignant Brenner tumors
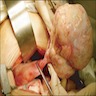
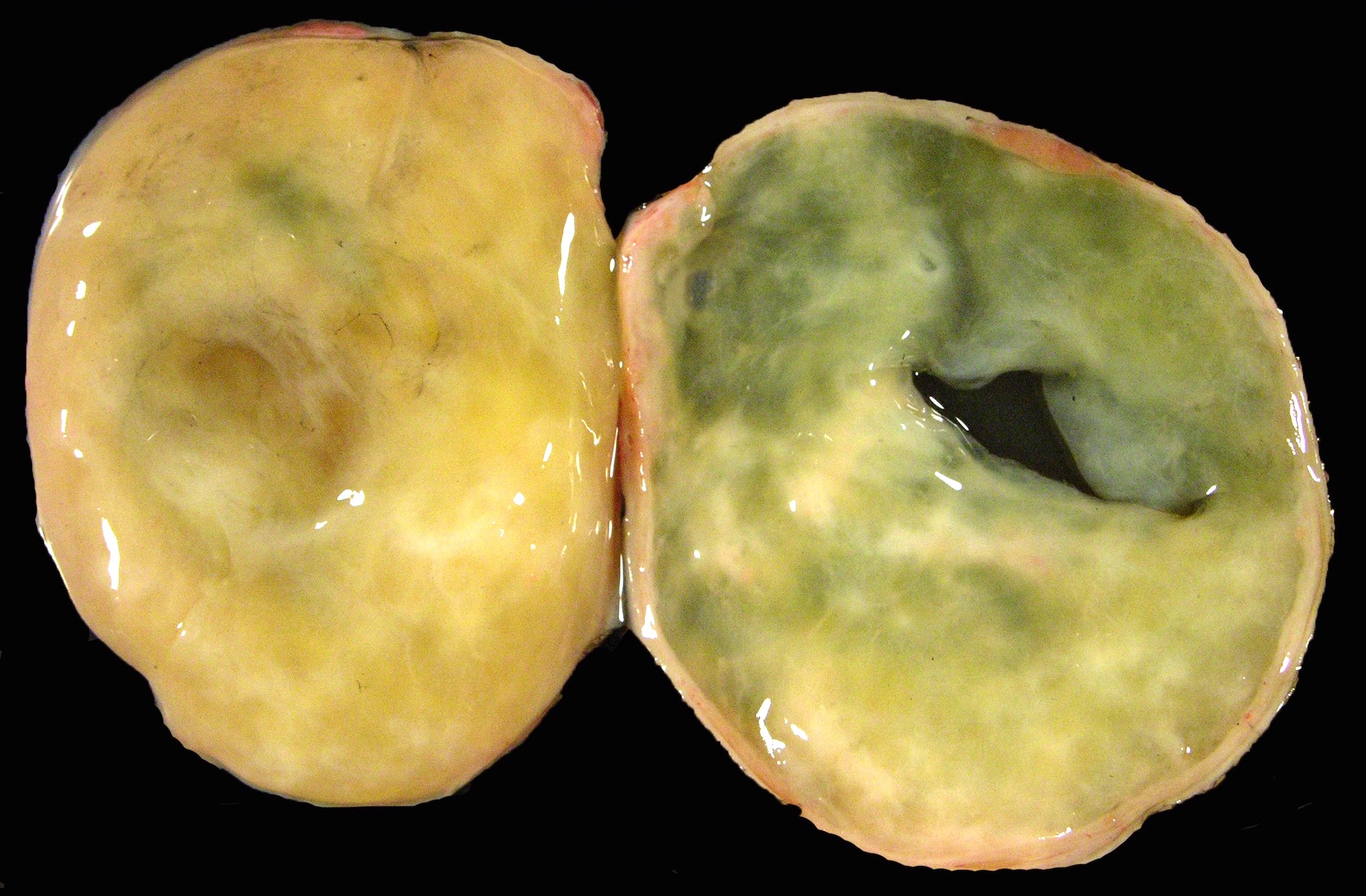
Gross description
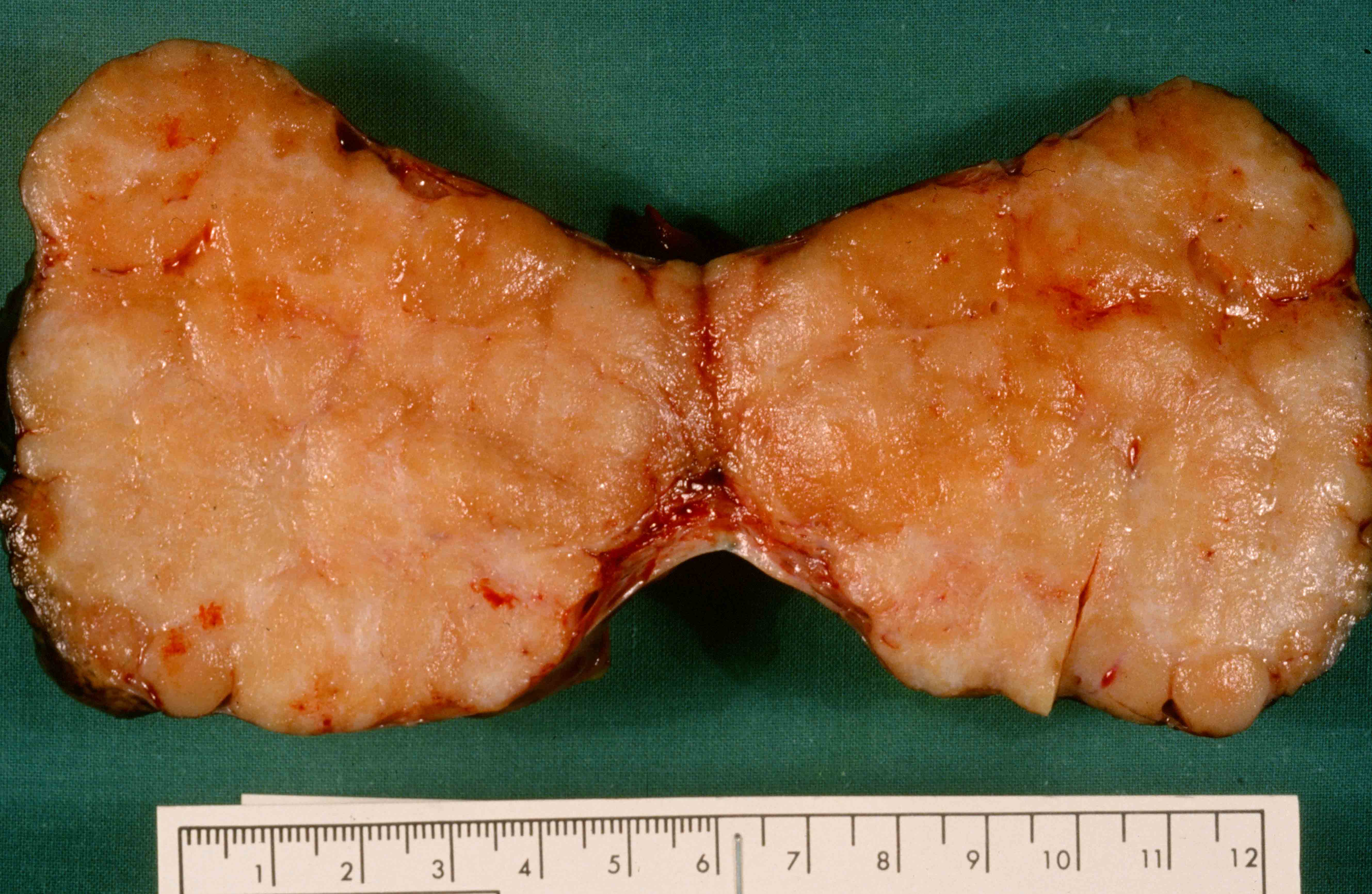
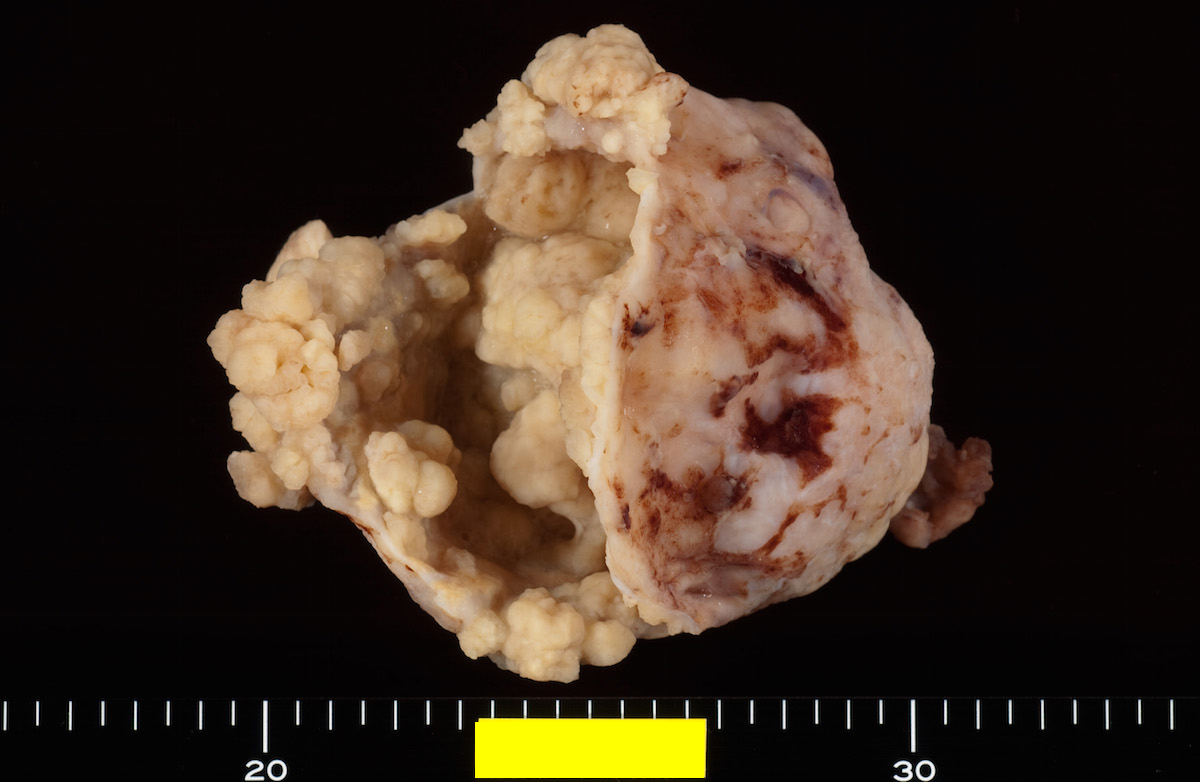
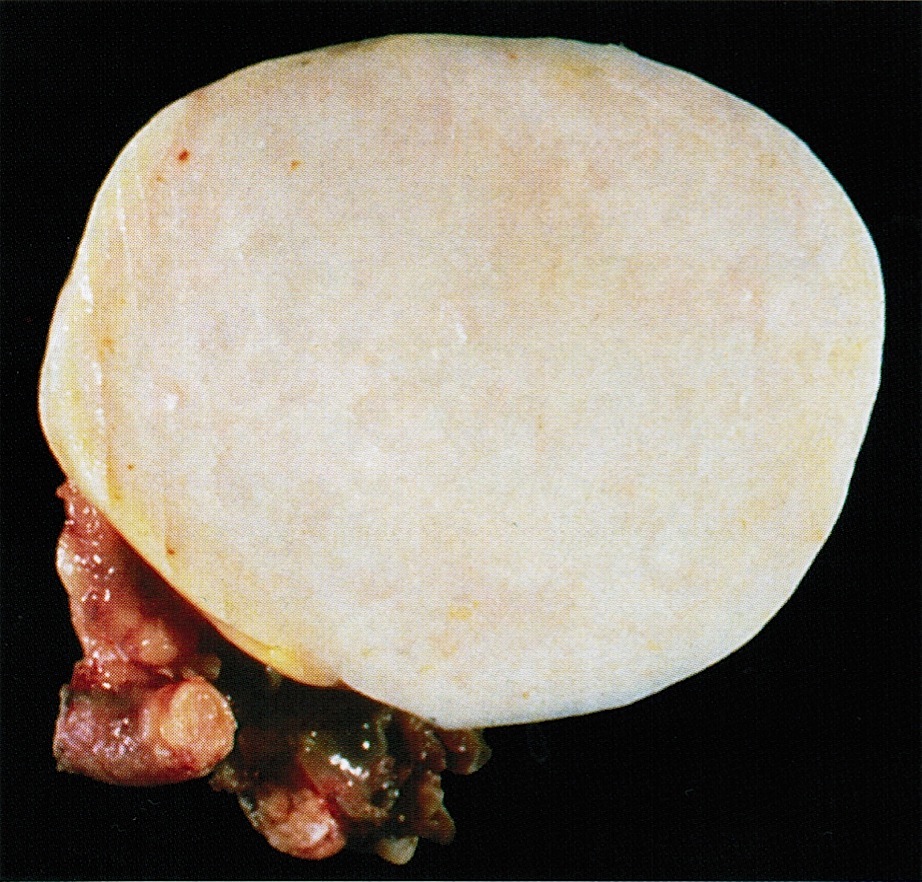
- Benign Brenner tumor:
- Small (usually < 2 cm), circumscribed, fibrous tumor with a uniform cut surface
- Calcifications may be present
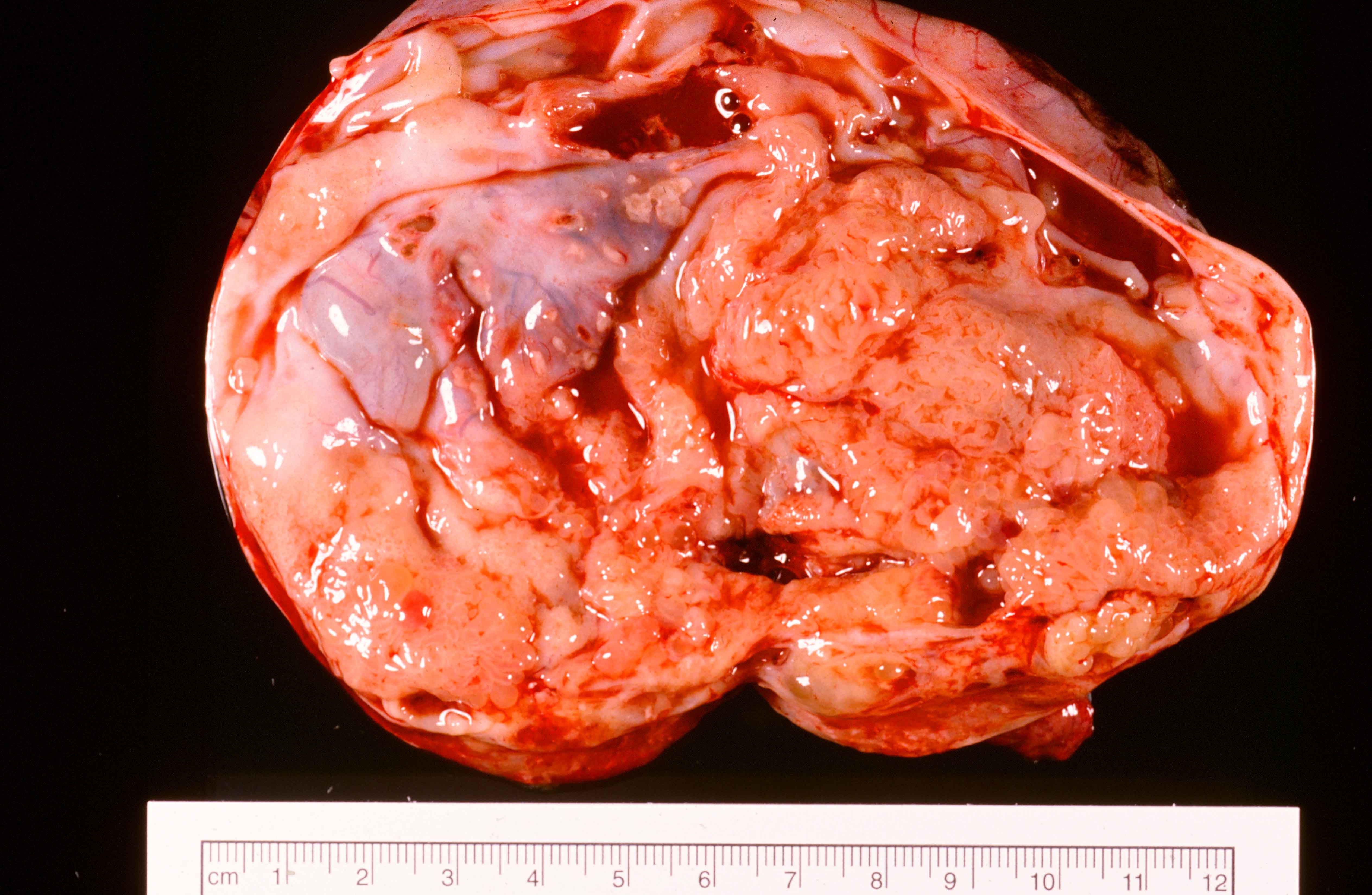
- Borderline and malignant Brenner tumor:
- Smooth surface, larger (usually > 10 cm) with fleshy, polypoid masses projecting into cystic cavity(s)
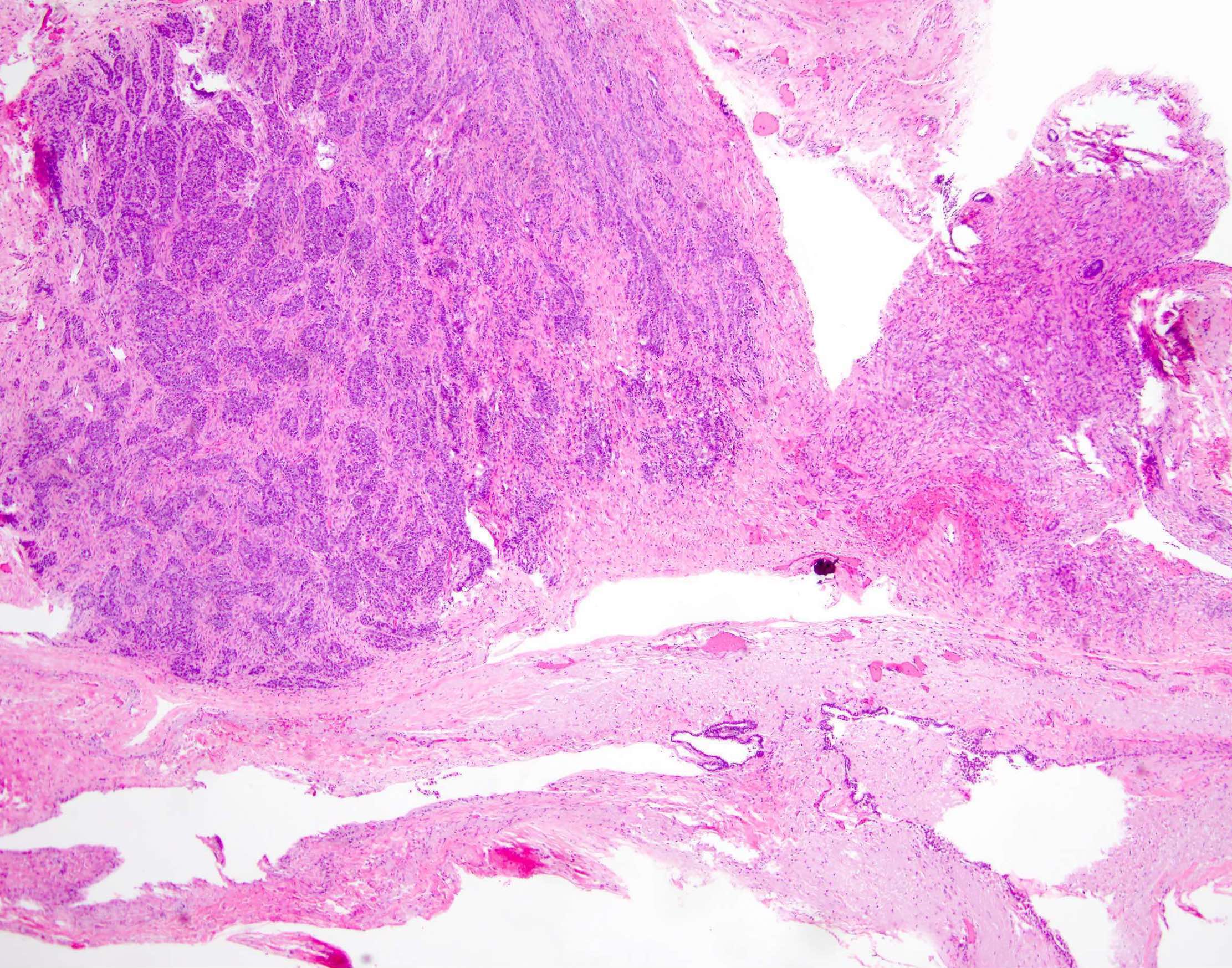
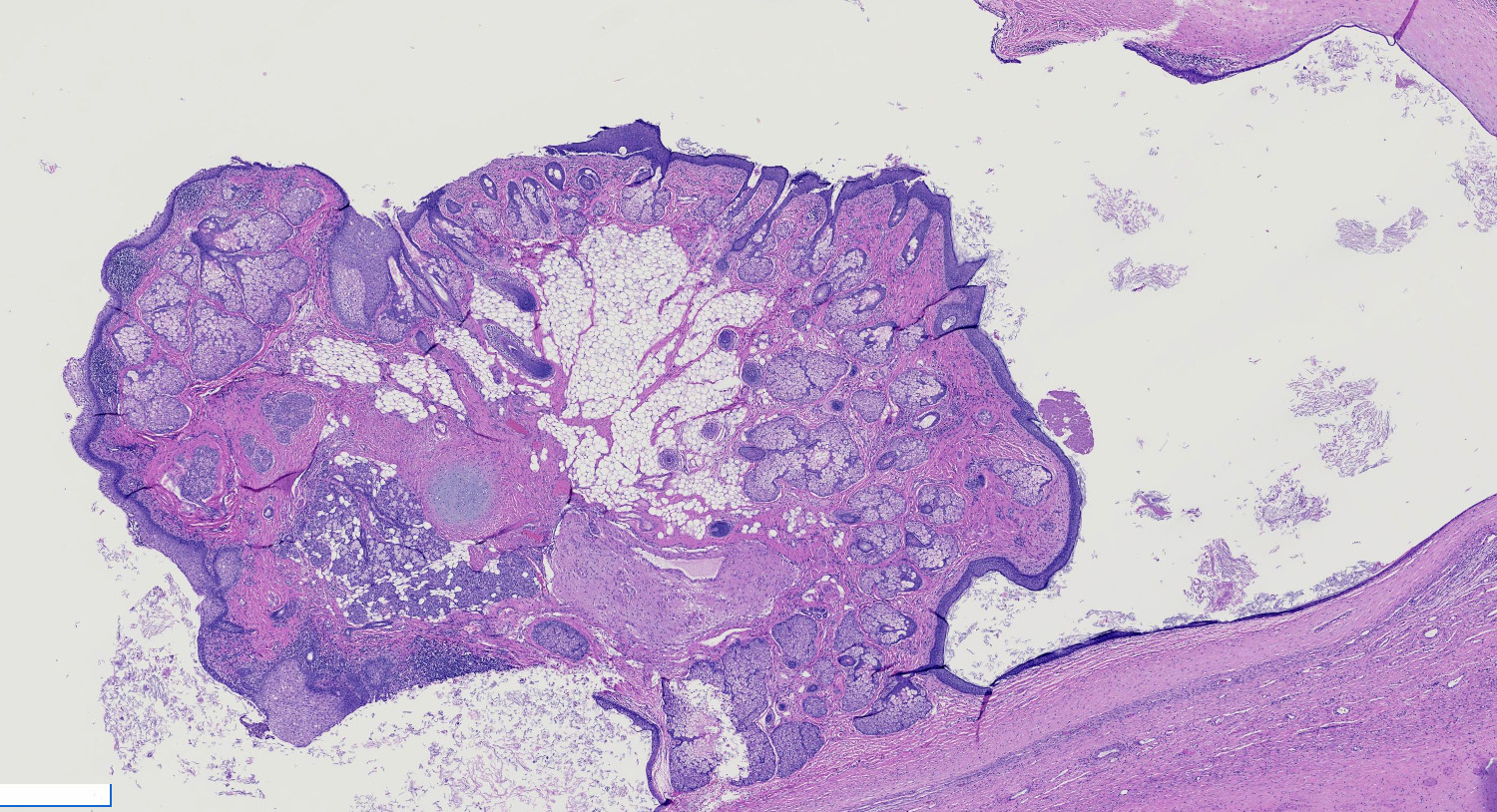
Gross images
Frozen section description
- Benign:
- Adenofibromatous architecture, smooth contoured nests of bland epithelial cells within benign fibromatous stroma
- Borderline or malignant:
- Resembling low grade papillary urothelial tumor of the bladder, with papillary fronds covered by transitional-like epithelium
Frozen section images
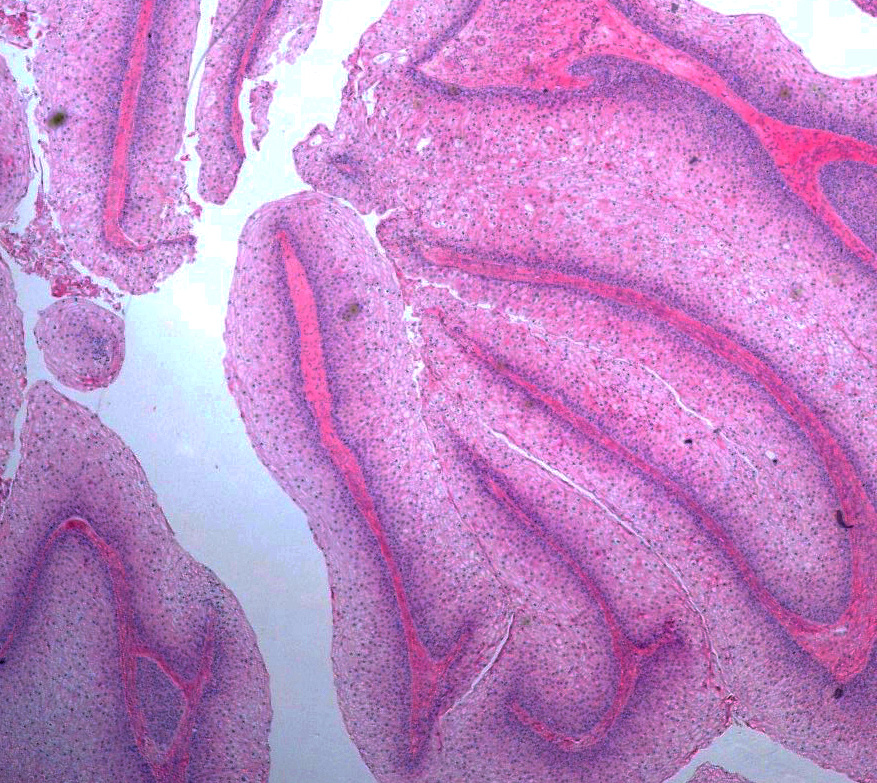
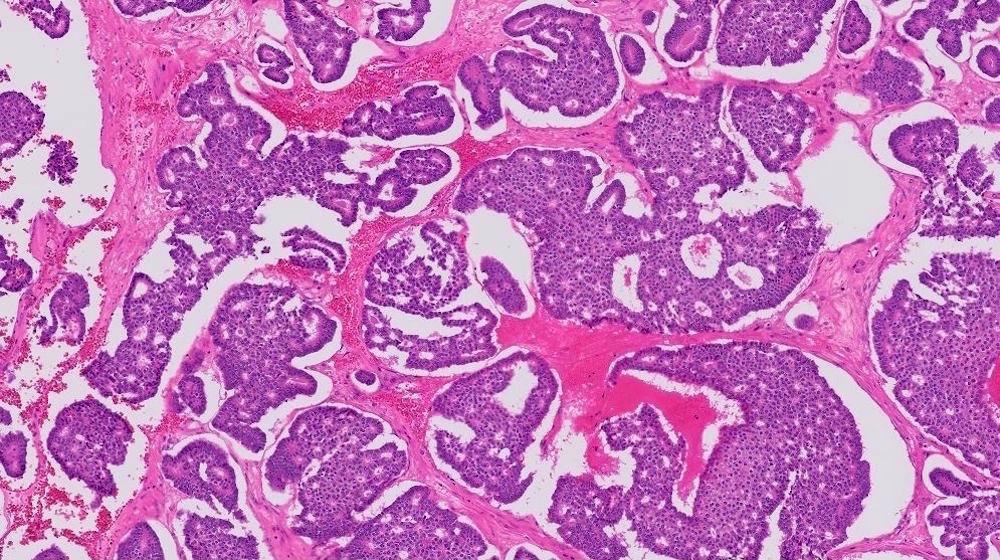
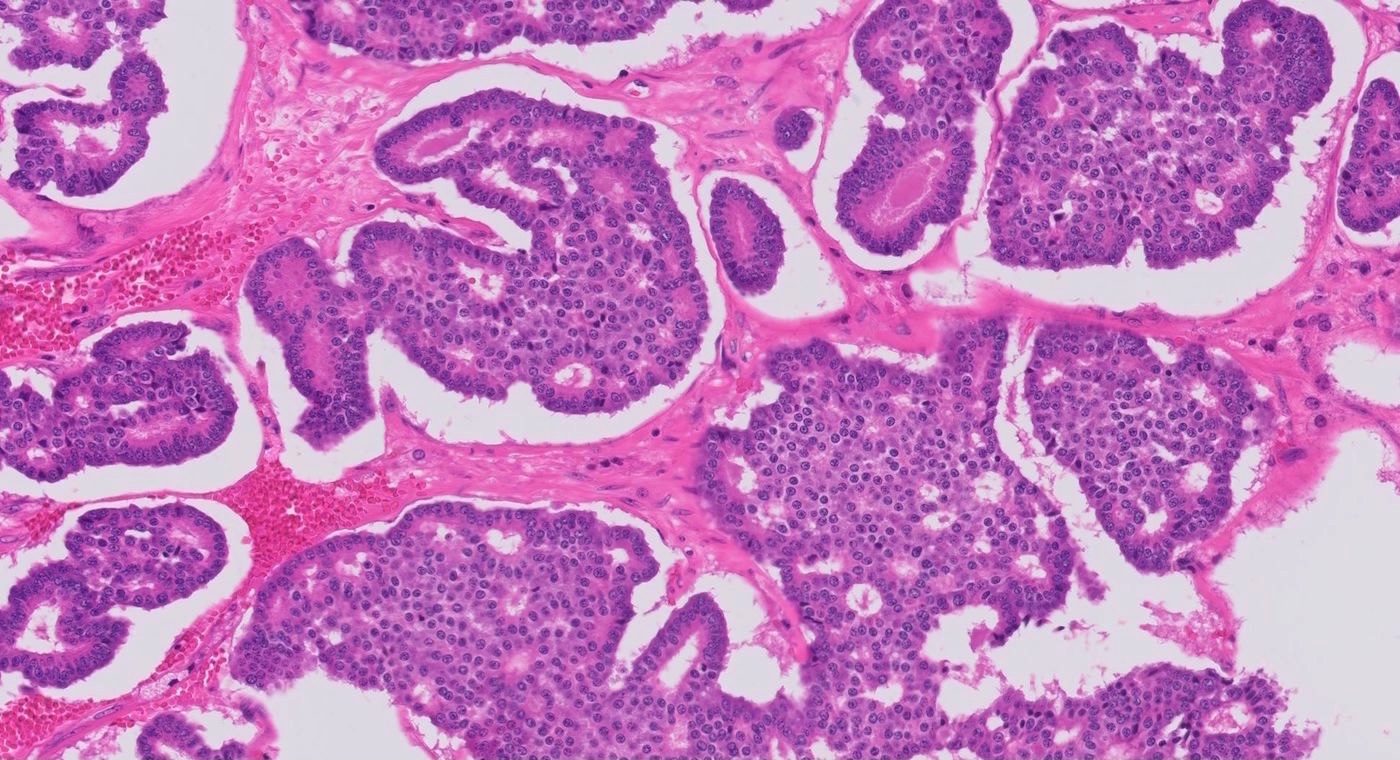
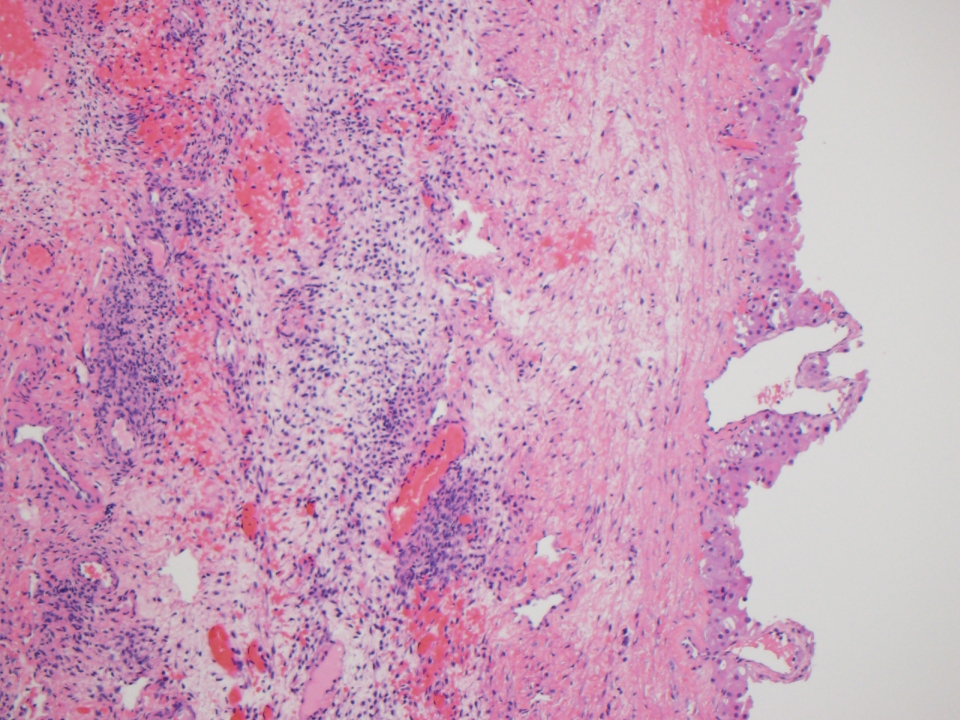
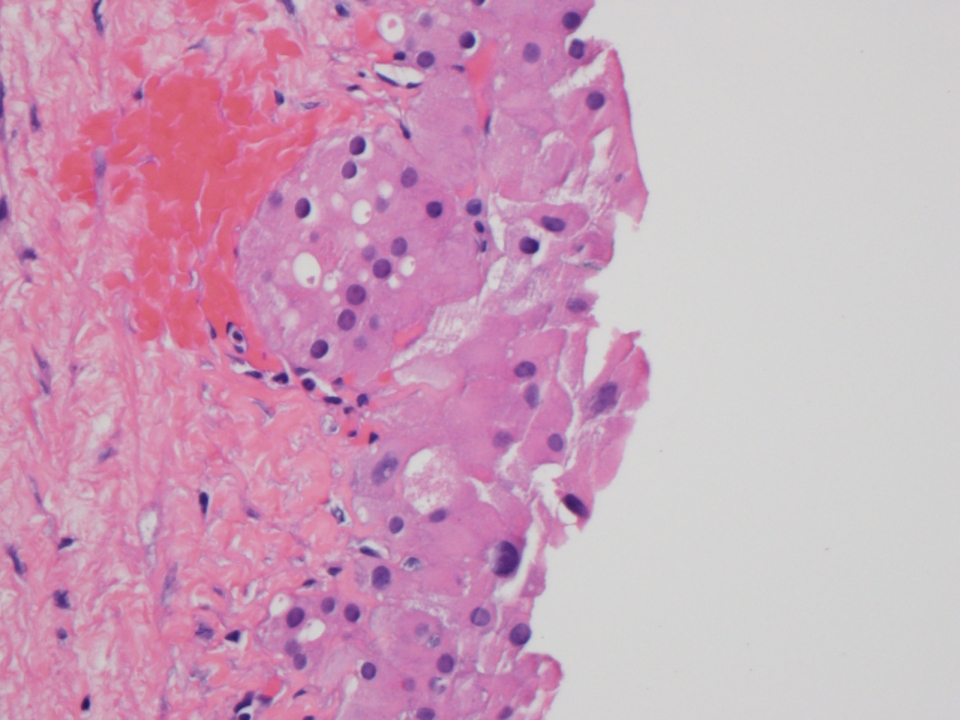
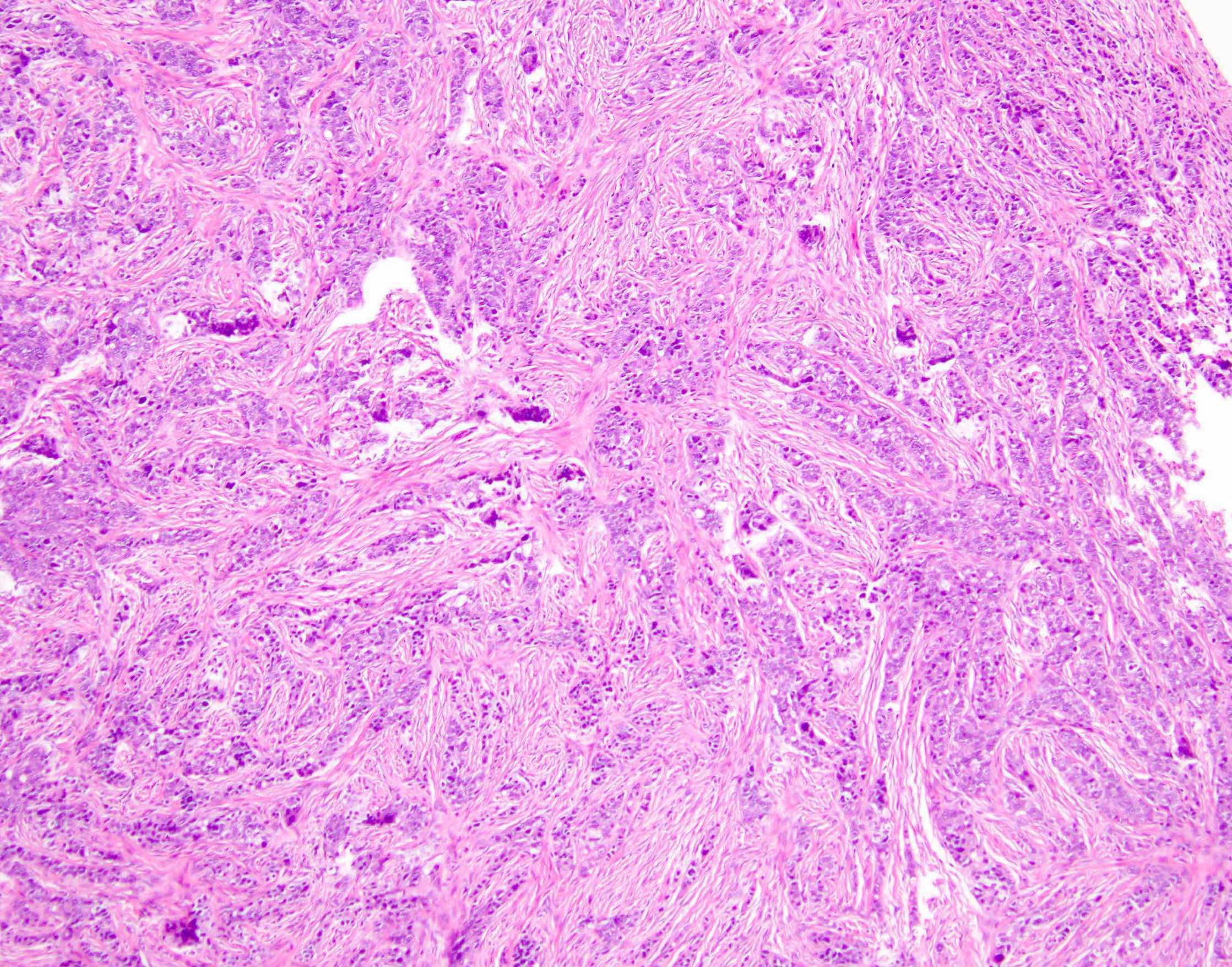
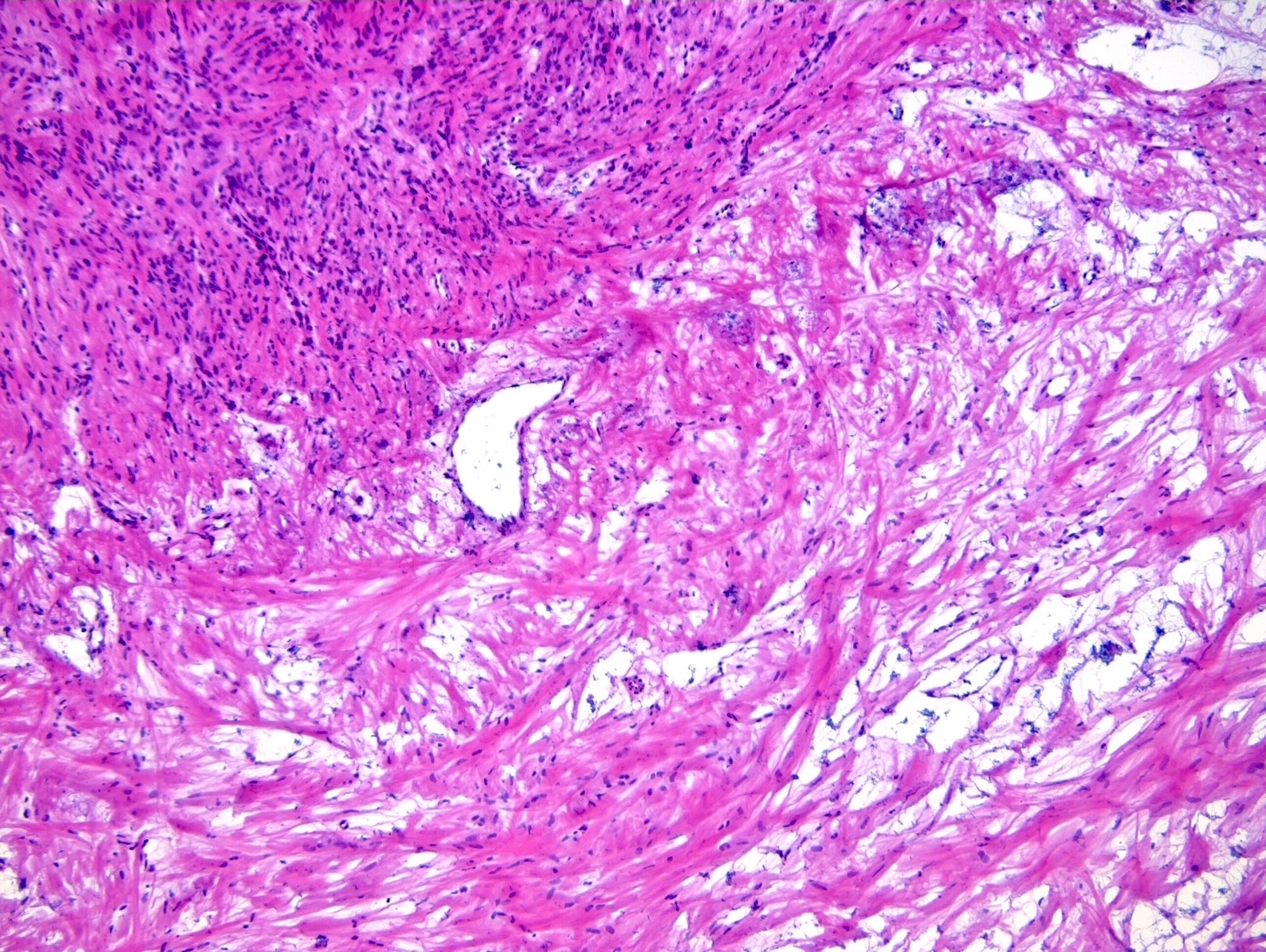
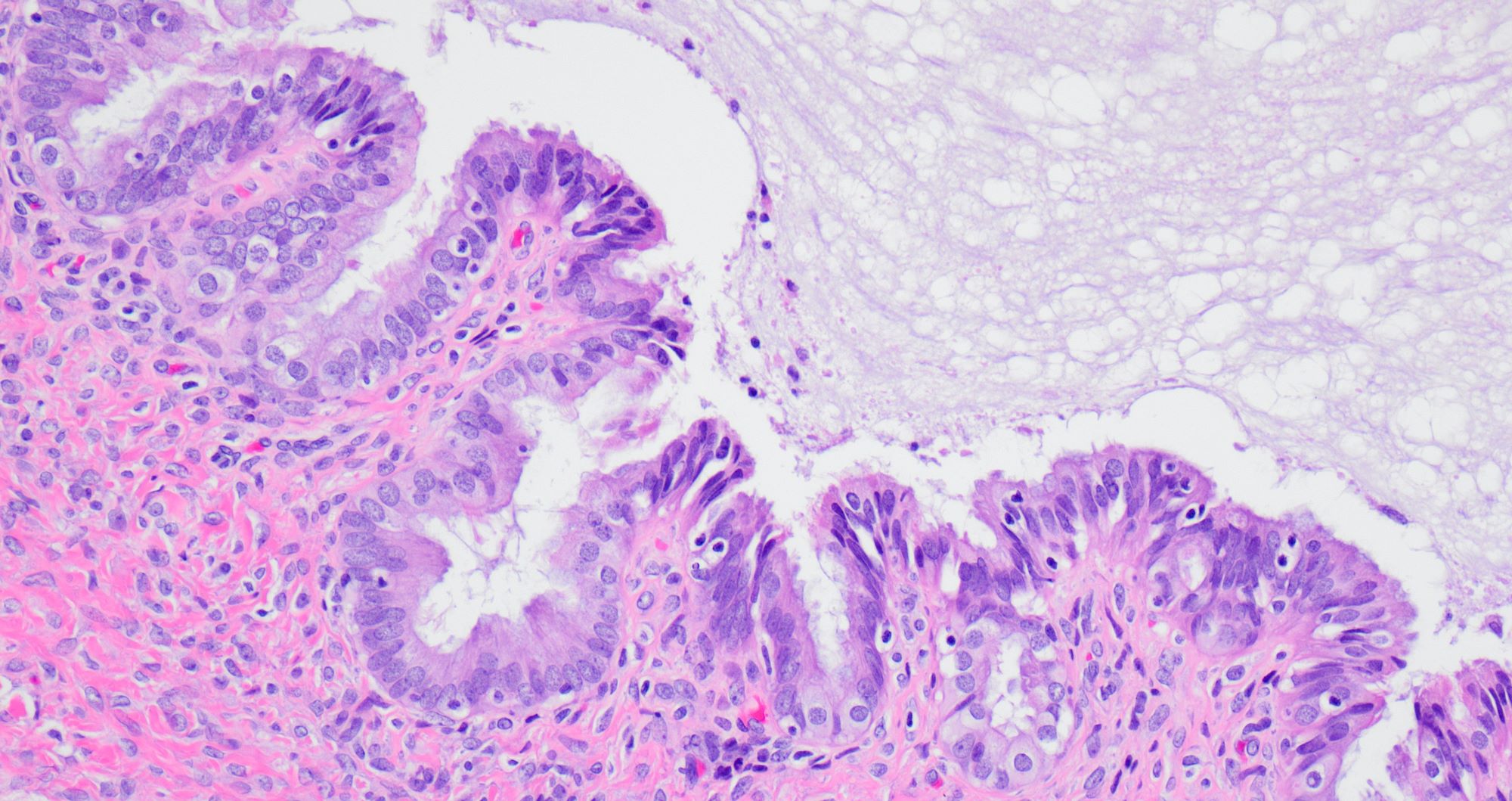
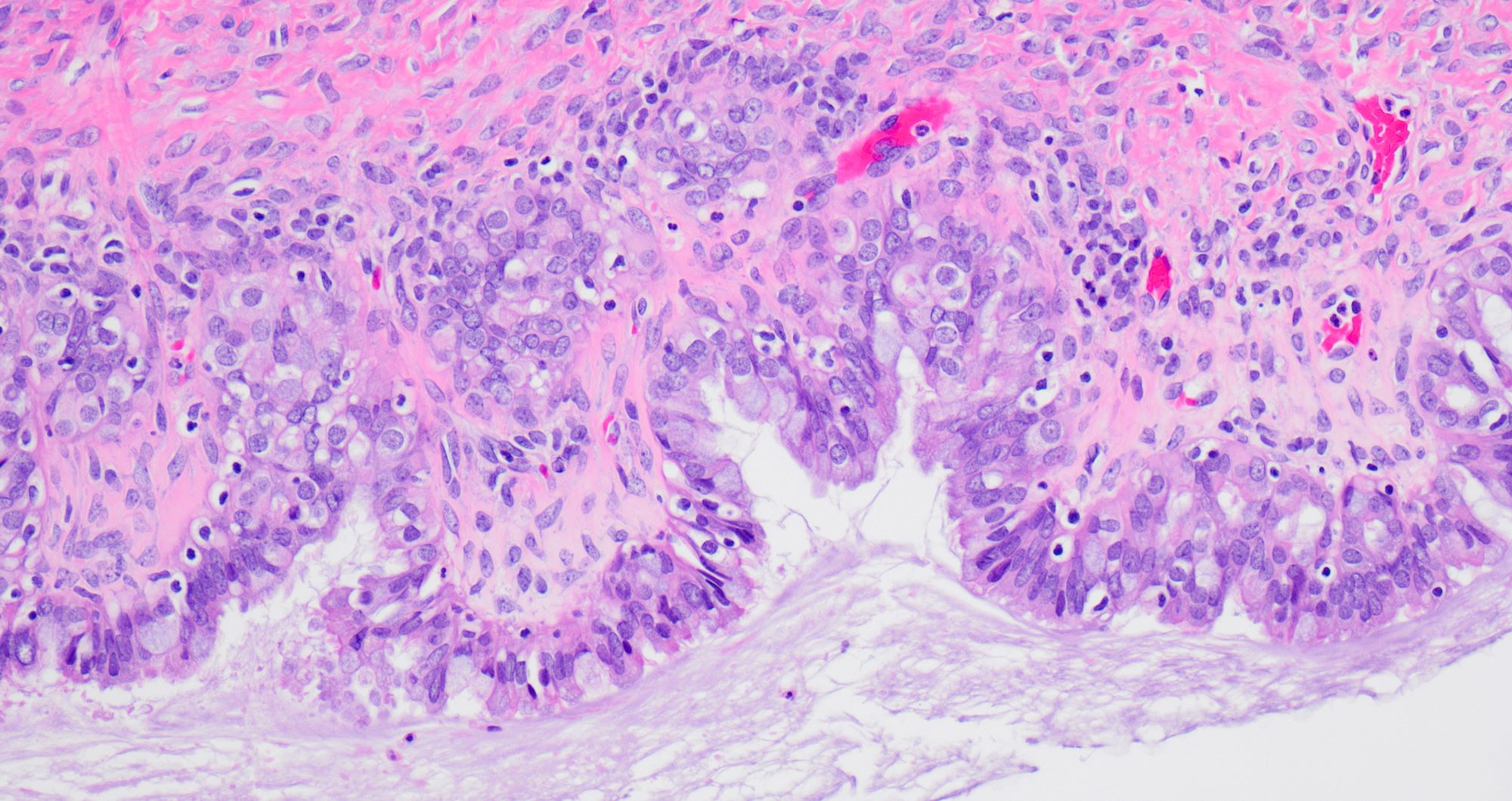
Microscopic (histologic) description
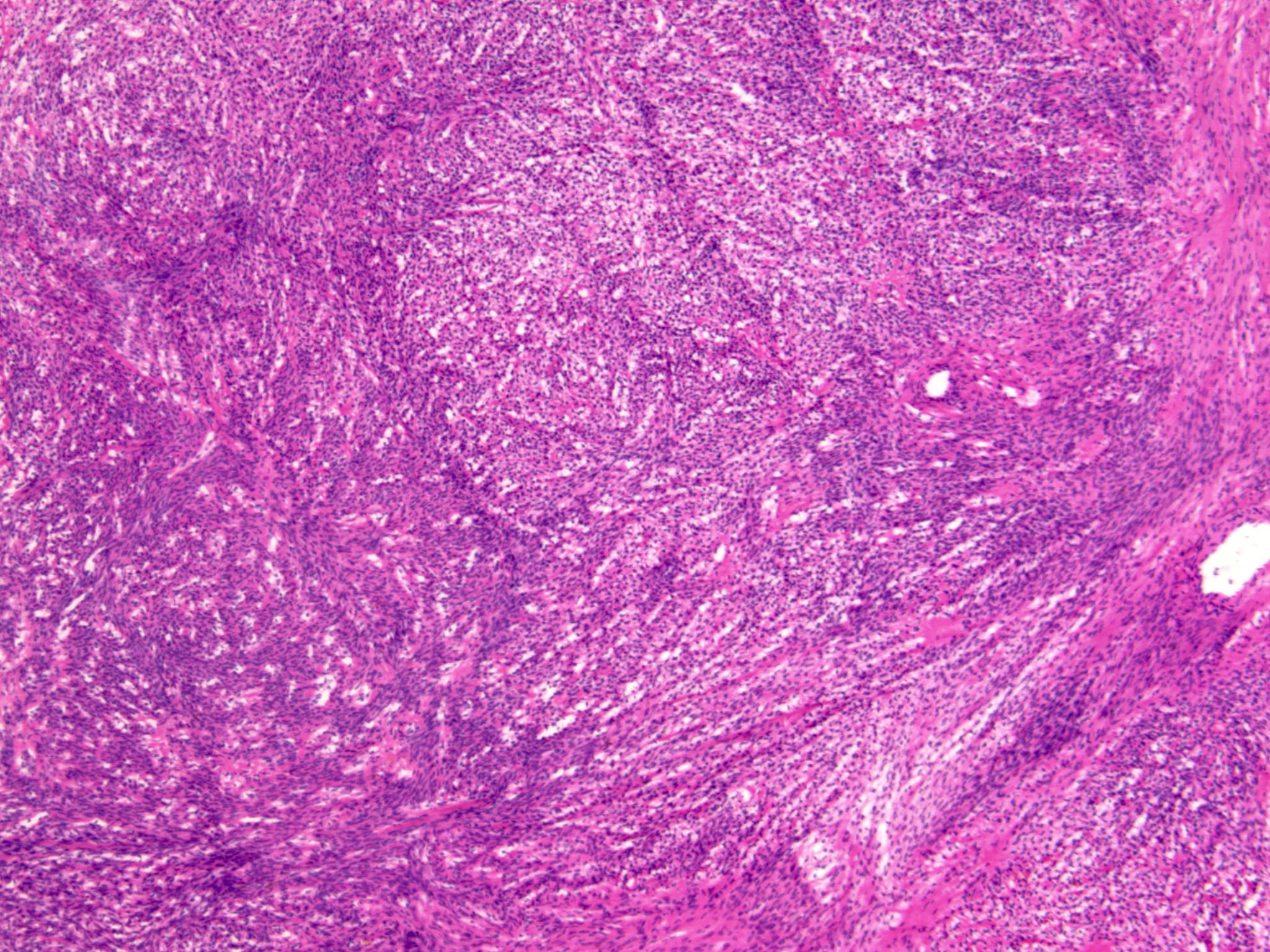
- Benign:
- Smooth contoured nests of bland transitional epithelium within fibromatous stroma
- Transitional cells have uniform oval nuclei and may have a longitudinal nuclear groove
- There may be mucinous epithelium at the center of the nests, with microcyst formation
- Ciliated or nondescript glandular epithelium may be present; a coexistent mucinous cystadenoma is present in 10% of cases
- Calcification is common
- Borderline:
- Papillary architecture with papillae covered by multilayered transitional epithelium
- There is variable cytological atypia; usually low grade but on occasion moderate or marked cytological atypia may be present
- Benign Brenner tumor component is often present
- Malignant:
- Stromal invasion by carcinoma with transitional cell features, with irregular nests of cells and single cells in an infiltrative pattern
- Squamous or mucinous differentiation may be present
- Benign or borderline Brenner tumor component is present
- Reference: Int J Gynecol Pathol 2012;31:499
Microscopic (histologic) images
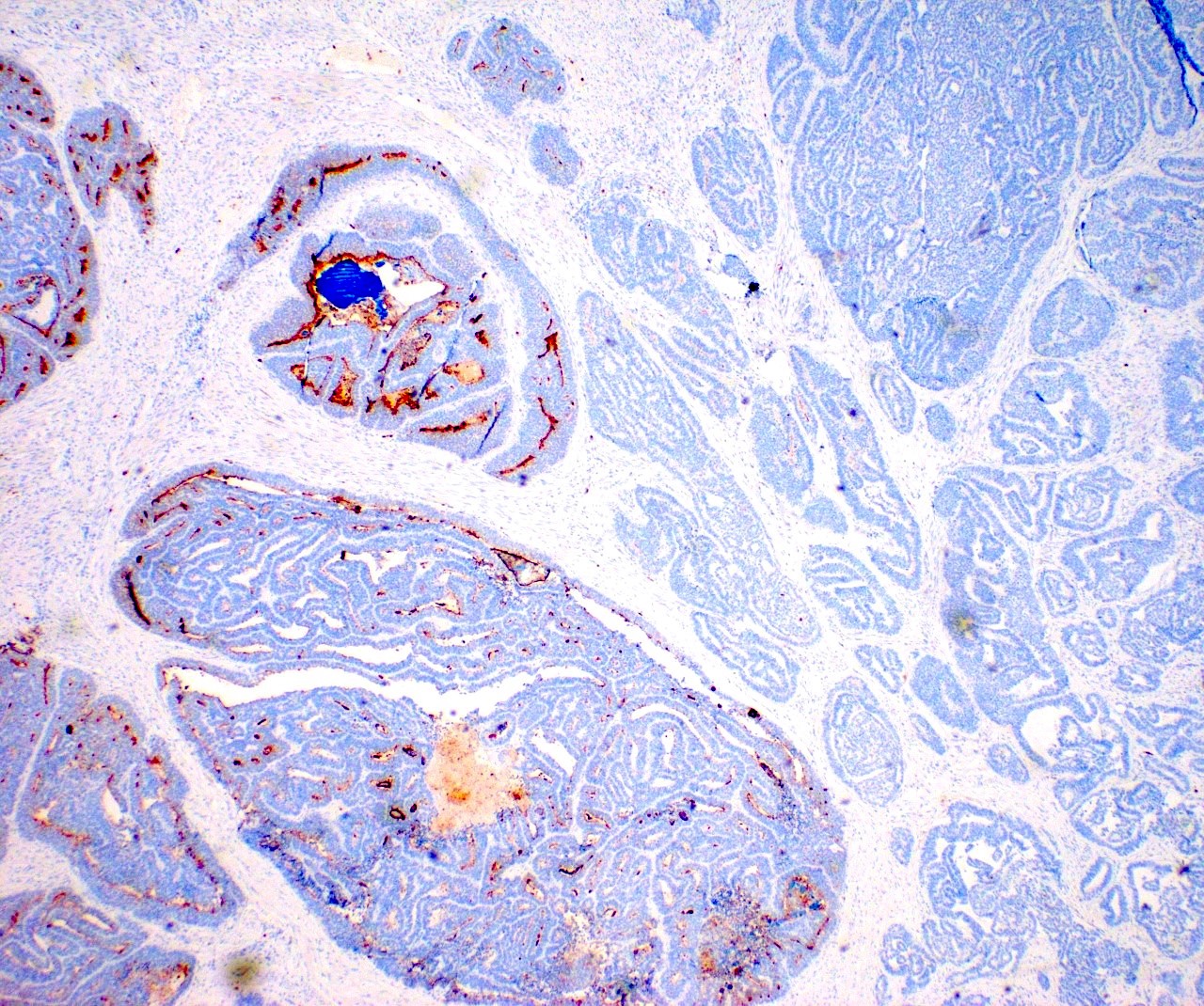
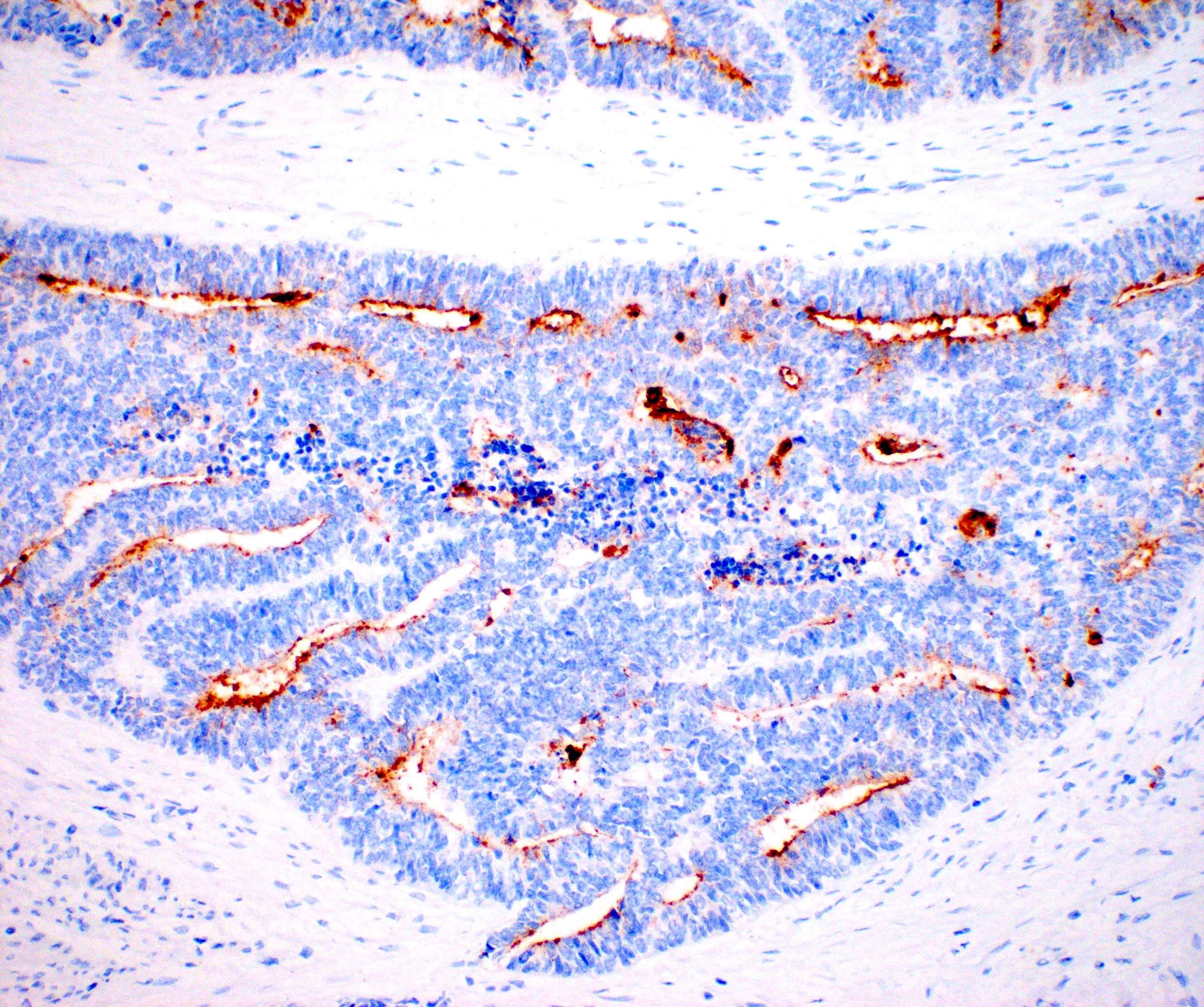
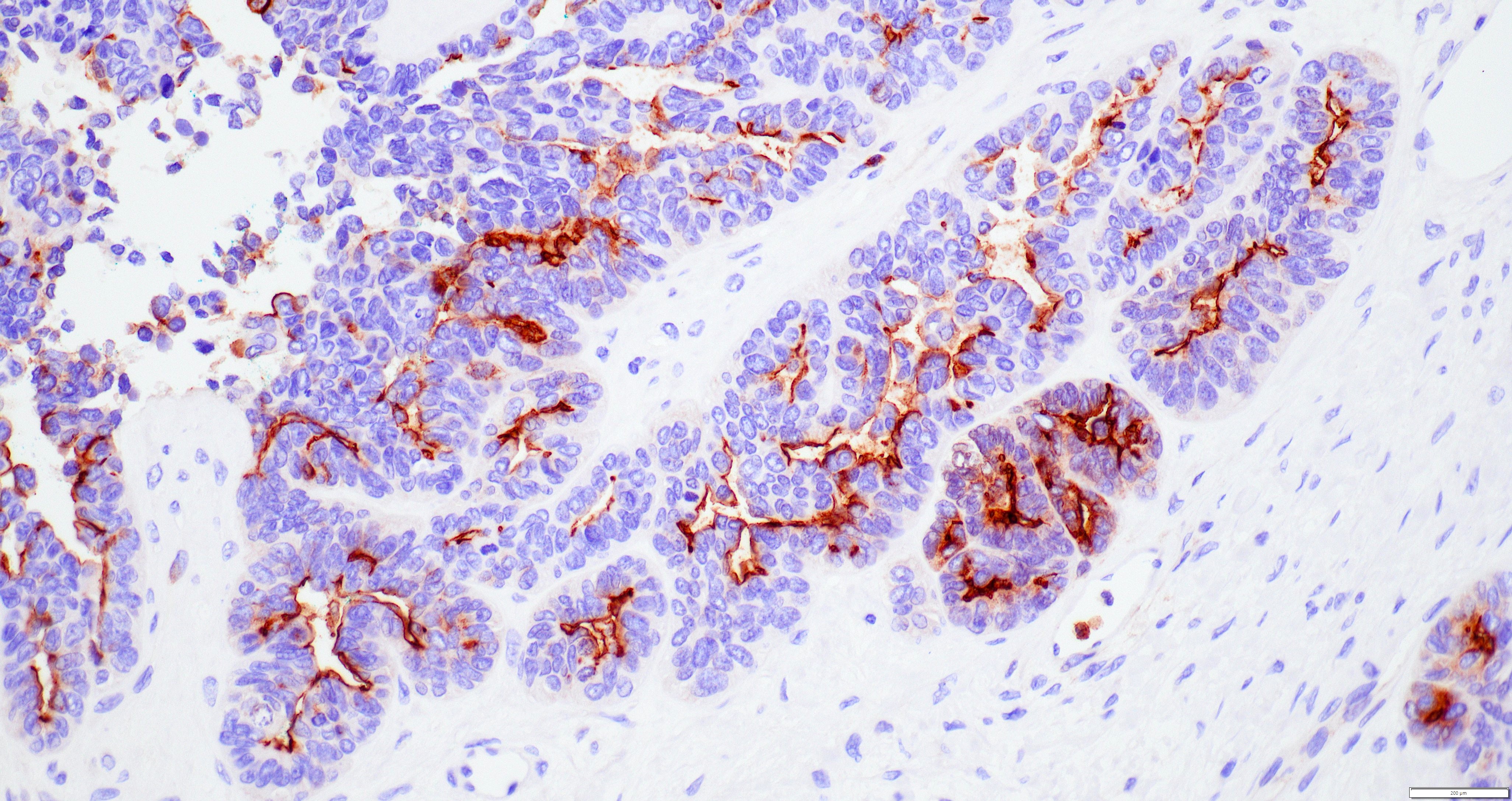
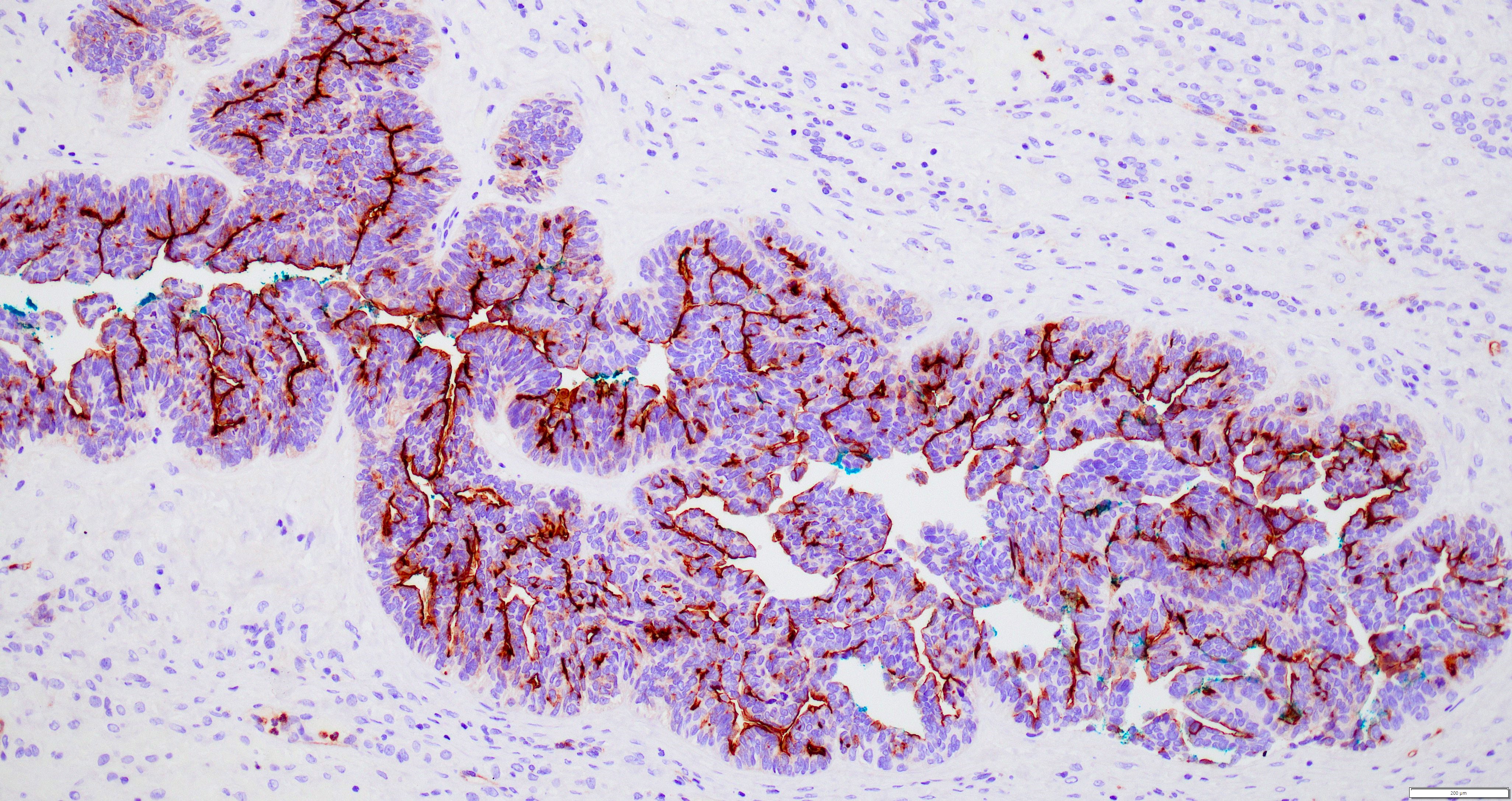
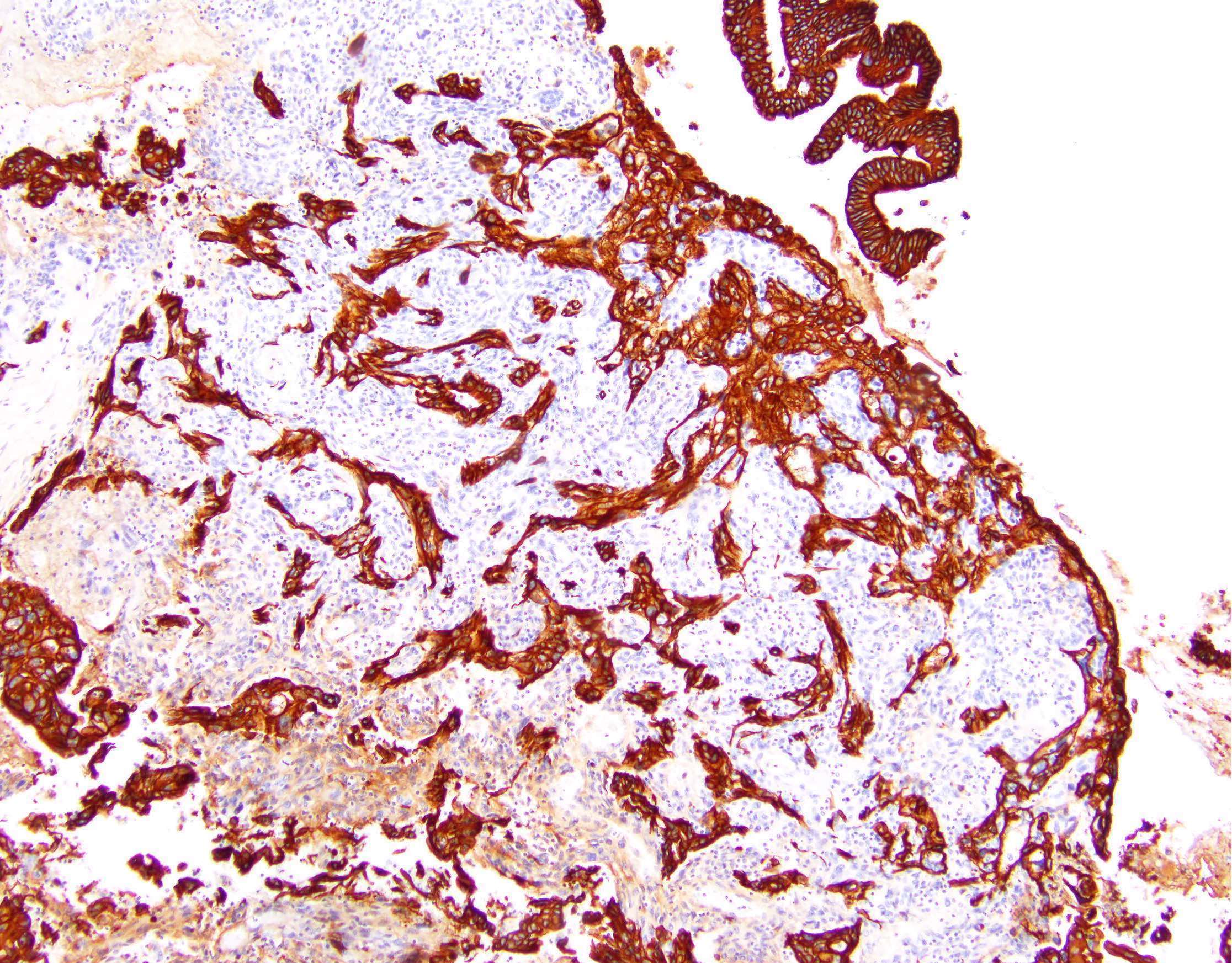
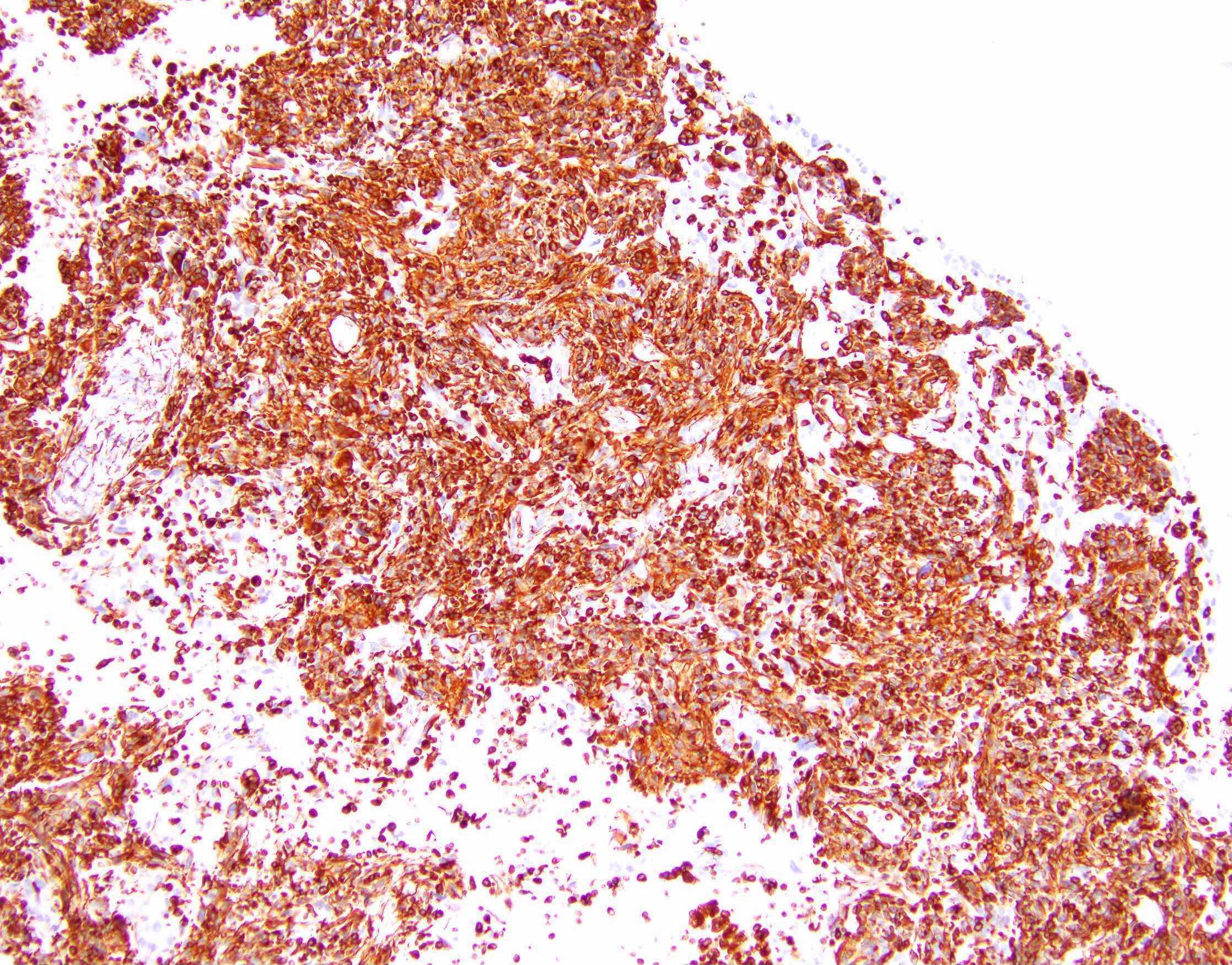
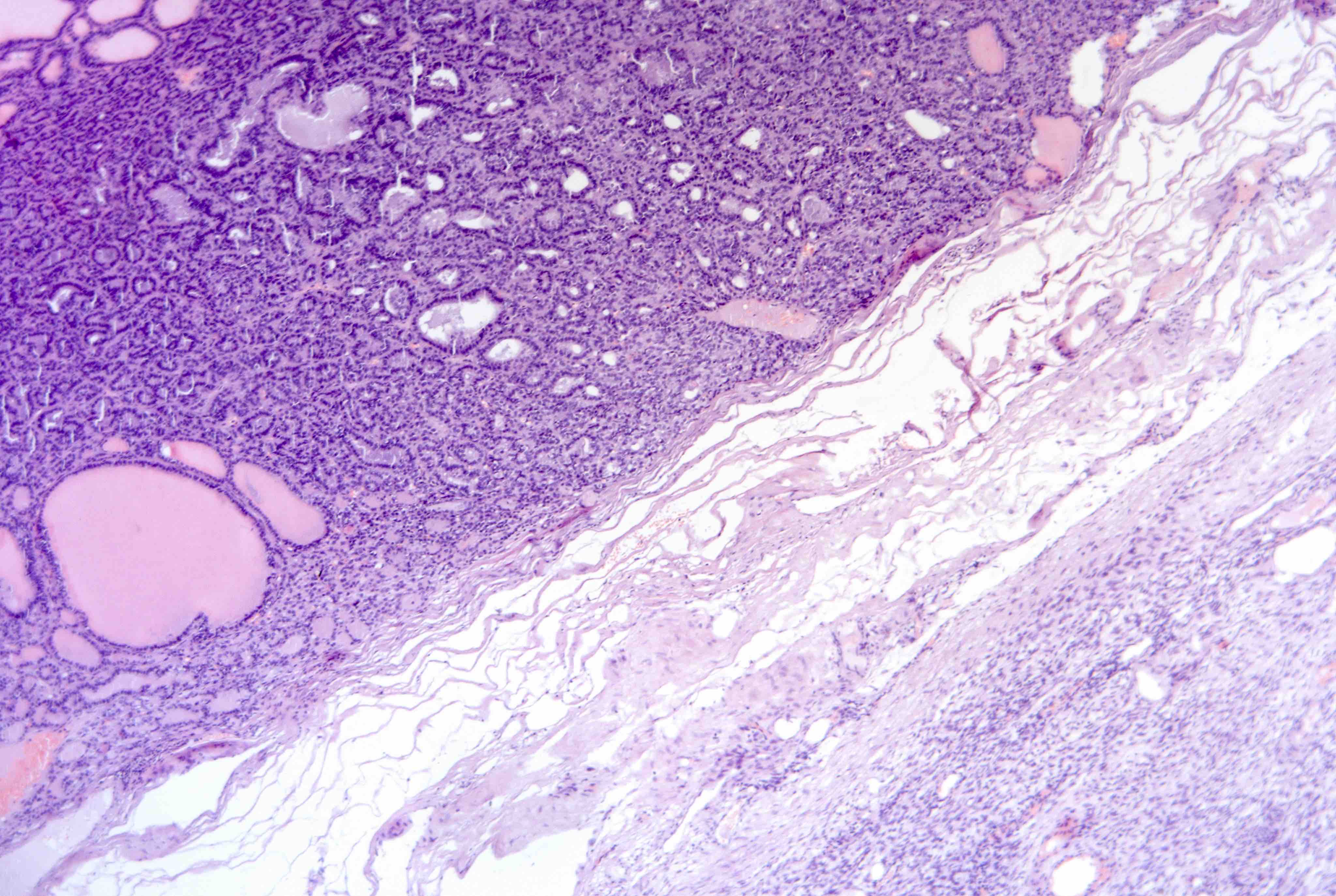
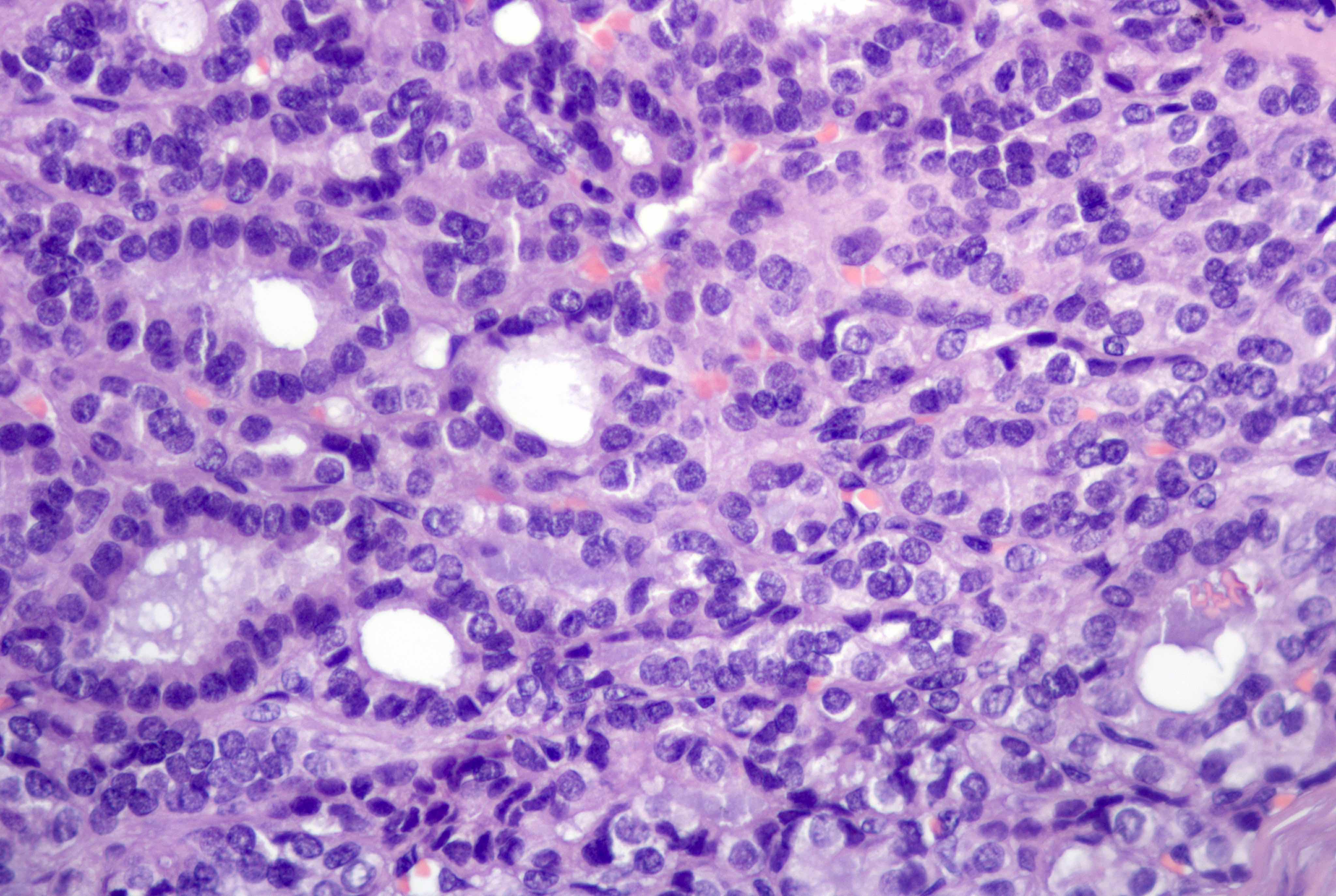
Contributed by Jutta Huvila, M.D., Ph.D. and C. Blake Gilks, M.D.
AFIP images
Positive stains
Negative stains
Sample pathology report
- Right ovary, oophorectomy:
- Borderline Brenner tumor (see comment)
- Comment: This borderline Brenner tumor is associated with a component of benign Brenner tumor. Negative for invasive carcinoma.
Differential diagnosis
- For Benign Brenner tumor
- Endometrioid adenofibroma:
- Glandular: lacks multilayered epithelial nests with transitional differentiation
- Adult granulosa cell tumor:
- More characteristic patterns of adult granulosa cell tumor present, such as microfollicular, trabecular or solid growth
- Inhibin immunoreactivity and negative for epithelial markers
- Carcinoid tumor:
- Insular or trabecular architecture
- Lacks prominent fibromatous stroma
- Expression of neuroendocrine markers
- Endometrioid adenofibroma:
- For borderline / malignant Brenner tumors
- High grade serous carcinoma, transitional architectural pattern:
- Areas of conventional high grade serous carcinoma
- High grade nuclear features
- Absence of a benign Brenner component
- WT1 and ER positivity (Int J Gynecol Pathol 2012;31:49)
- Squamous cell carcinoma:
- Keratinization
- High grade cytological features
- Teratoma component
- Endometrioid borderline tumor or carcinoma:
- More prominent glandular component with endometrioid (not mucinous) glands
- ER positive
- Metastatic squamous cell carcinoma:
- History of primary squamous cell carcinoma elsewhere in the body
- Bilateral and multinodular growth
- Lacks prominent papillary architecture
- Metastatic urothelial carcinoma:
- History of urothelial carcinoma
- Bilateral and multinodular growth
- Lacks benign Brenner component
- High grade serous carcinoma, transitional architectural pattern:
Additional references
Board review style question #1
Board review style answer #1
Board review style question #2
Brenner tumors of the ovary typically have which of the following immunophenotypes?
- GATA3 and ER positive
- GATA3 and p63 positive
- GATA3 and WT1 positive
- GATA3 positive and mutant pattern p53 expression
Board review style answer #2
Breast carcinoma
Table of Contents
Definition / general | Pathophysiology | Clinical features | Radiology description | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Differential diagnosisDefinition / general
- Usually incidental finding associated with (a) staging, (b) oophorectomy for patient with BRCA mutation or strong family history of breast cancer, or (c) therapeutic oophorectomy (induced hormone suppression for hormone receptor+ breast carcinoma)
- Ovarian involvement is seen in ~30% of therapeutic oophorectomies for advanced breast cancer, and at autopsy in ~10% of breast cancer cases (Am J Surg Pathol 2006;30:277)
- Involvement by lobular carcinoma (36%, including signet-ring cell type) is more frequent than ductal carcinoma (2.6%) (Br J Cancer 1984;50:23, Adv Anat Pathol 2007;14:149)
- Metastatic disease frequently arises up to 5 years post diagnosis, median 11.5 months, and is related to breast cancer stage (Br J Cancer 1984;50:23)
Pathophysiology
- High rates of hormone receptor+ breast cancer and premenopausal status suggest that hormone regulation is important in metastases to ovary
Clinical features
- Ovarian involvement is usually asymptomatic
- May have GI symptoms of abdominal pain, distension or pressure, abnormal uterine bleeding or mass (Gynecol Oncol 2003;90:397, Gynecol Oncol 2005;98:235)
Radiology description
- USG: mostly bilateral ovarian involvement by solid tumors 10 cm in size or less
Case reports
- 58 year old woman (J Cytol 2009;26:144)
- Two case reports and review of three additional cases (Cancer 1981;48:210)
- Sixteen cases reported as part of a cohort of patients with breast cancer and ovarian disease (Obstet Gynecol 1994;84:449)
Treatment
- Diagnosis of metastatic carcinoma to the ovary is usually done on salpingo-oophorectomy specimens:
- Subsequent treatment is commonly not necessary
- More extensive surgery (contralateral salpingo-oophorectomy, hysterectomy, lymph node dissection) may be considered if obvious or suspected residual tumor and no other sites of metastatic involvement on imaging
- Minimal (e.g., palliative debulking) can also be considered
- Surgery is defined as "optimal" when largest residual tumor mass is J Korean Med Sci 2009;24:114)
Gross description
- 80% are bilateral
- Multiple solid tumor nodules with soft consistency
- Involvement of ovarian surface and superficial cortex is common
Microscopic (histologic) description
- Morphology mirrors the architecture and cytomorphology of the breast primary (including histologic grade)
- Lobular carcinoma: small cords and clusters of tumor cells in ovarian cortex, single cells in between normal cortical stroma; histiocytoid or signet ring cell morphology can be seen
- Ductal carcinoma: tubules / glands or solid nests of tumor cells in a variable fibrous stroma
Microscopic (histologic) images
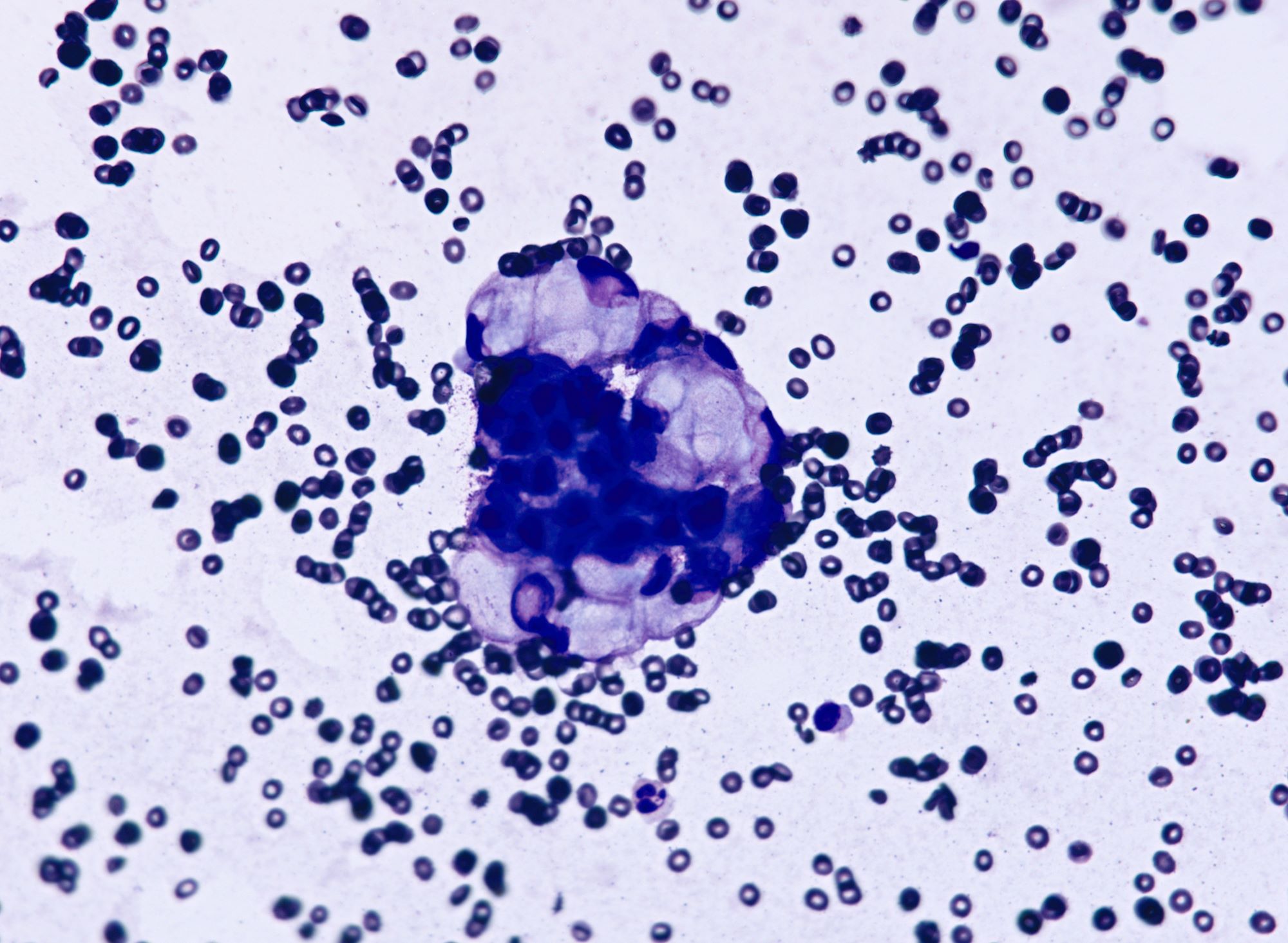
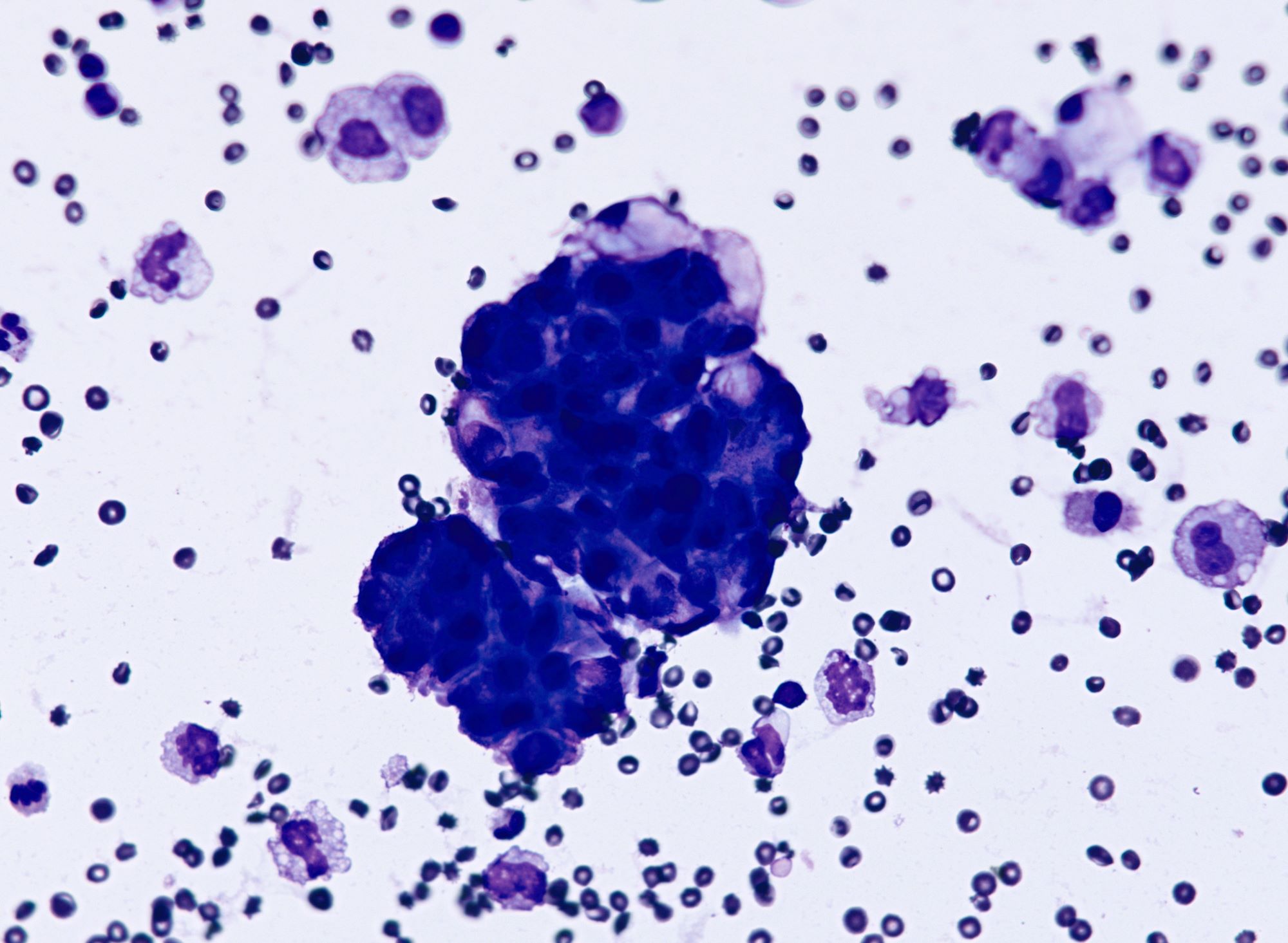
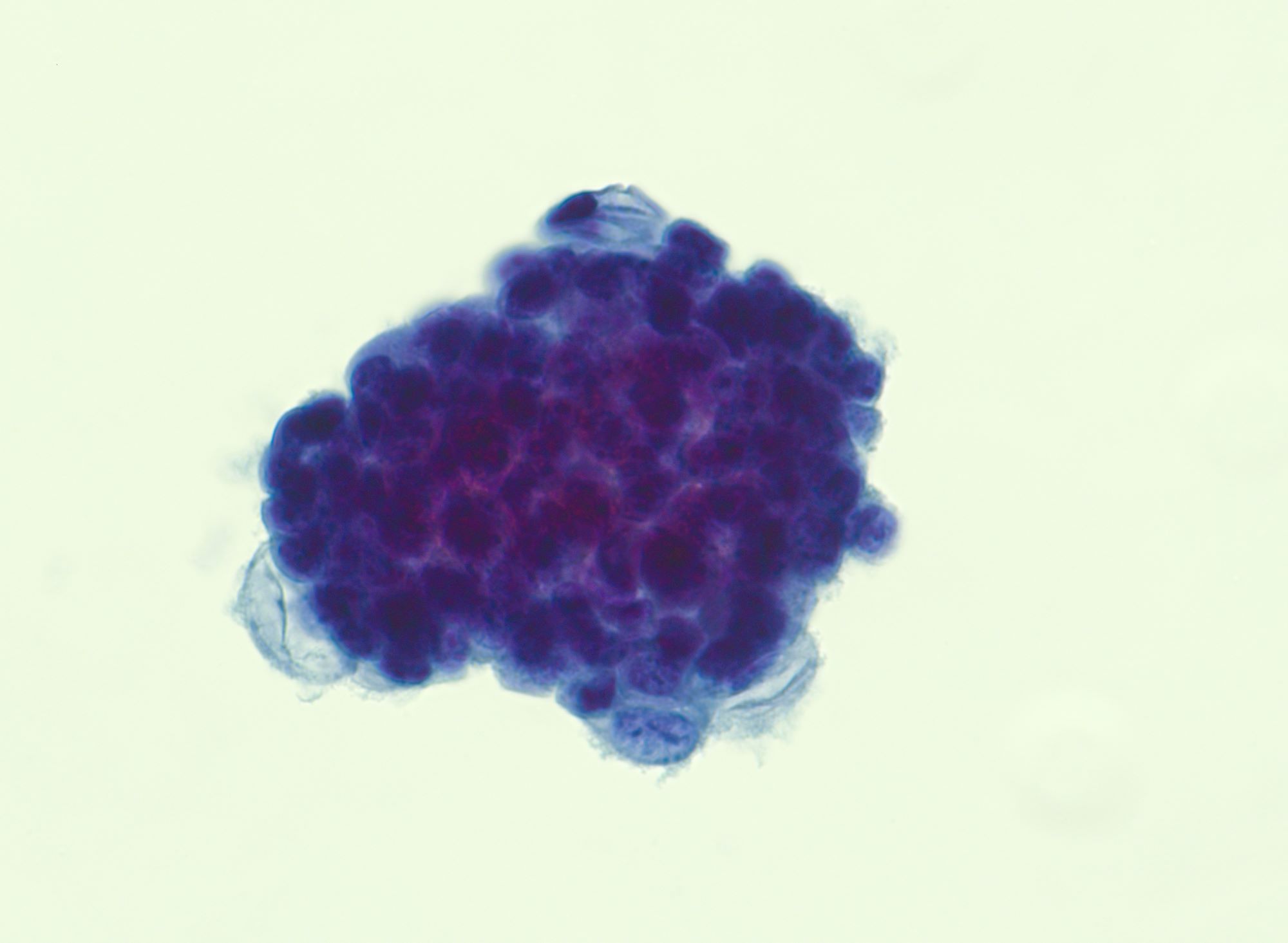
Cytology description
- Cell clusters have high nuclear-cytoplasmic ratio, moderate cytoplasm, vesicular nuclei, conspicuous nucleoli
Positive stains
- GATA3 is consistently positive in mammary carcinomas (Am J Clin Pathol 2012;138:57)
- It is consistently positive in ER positive tumors but is also positive in 69% of ER negative tumors (Am J Clin Pathol 2014;14:648)
- ER, PR and HER2 are not always expressed; expression usually mirrors that of the primary mammary tumor
- Variable (~50%) GCDFP and mammaglobin staining (Hum Pathol 1991;22:368, Mod Pathol 2007;20:208, Am J Clin Pathol 2007;127:103)
Negative stains
- PAX8: usually positive in most ovarian carcinomas subtypes (Am J Surg Pathol 2008;32:1566)
- WT1: positive in only 2% of breast metastases versus 63% of ovarian tumors, but also negative in ovarian clear cell, mucinous and endometroid subtypes
- CA125: weak / negative in breast carcinomas, 90% of ovarian carcinomas are CA125+ (Am J Surg Pathol 2005;29:1482)
Differential diagnosis
- Primary ovarian adenocarcinoma: PAX8+, CA125+
- Metastatic carcinoma from other sites (GI tract): history of known primary and IHC for primary site (CK20, CDX2, TTF1, Napsin A)
Calcification
Table of Contents
Definition / general | Essential features | Terminology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
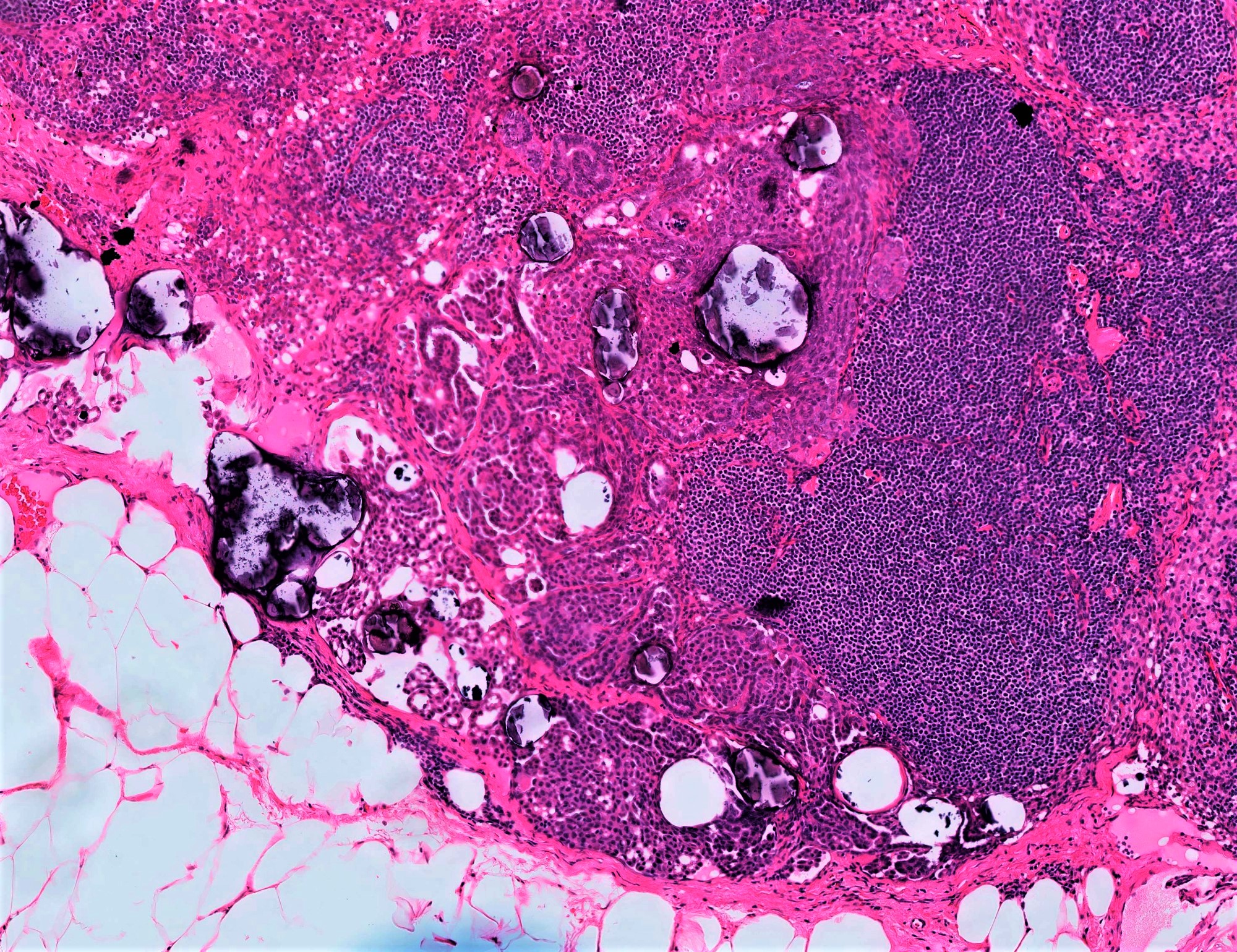
- Ovarian calcifications are commonly found in the context of a neoplasm (mature teratoma, mucinous cystadenoma, serous neoplasia, etc.) or as incidental findings in grossly normal ovaries
Essential features
- Ovarian calcifications can be idiopathic or associated with benign or malignant conditions (endometriosis, teratoma, mucinous lesions, serous neoplasms, etc.)
- Calcifications are divided into psammomatous (psammoma bodies) and nonpsammomatous
- Calcifications associated with a neoplasm do not need to be reported but identifying psammomatous calcifications in an otherwise normal ovarian specimen should prompt additional sampling to rule out the possibility of an underlying serous neoplasm
Terminology
- Calcifications, microcalcifications, osseous metaplasia
Sites
- Ovary
Pathophysiology
- Pathologic calcifications are metastatic (associated with hypercalcemia) or dystrophic (associated with normal calcemia); ovarian calcifications are considered dystrophic, due to calcium deposition in areas of cellular degeneration or necrosis
- Presence of stromal calcifications suggests a paracrine effect in the stromal cells in response to hormonal stimuli (Mod Pathol 2003;16:219)
- Gallstones spilled into the peritoneal cavity during laparoscopic cholecystectomy can lead to secondary cholelithiasis of the ovary (J Reprod Med 2007;52:968)
Etiology
- No known causes
Clinical features
- Calcifications are usually asymptomatic
Diagnosis
- Identified by ultrasound or at the time of histologic examination
Laboratory
- No specific laboratory findings
Radiology description
- Curvilinear or punctate echogenic foci, usually between 1 - 3 mm (Radiology 1996;198:415)
Prognostic factors
- In a study of 28 patients, the presence of large ovarian calcifications (> 5 mm) identified by imaging in otherwise normal ovaries remained stable and was not associated with ovarian neoplasms (Ultrasound Obstet Gynecol 2007;29:438)
Case reports
- 34 year old woman with ossification in the ovary associated with an old hemorrhagic cyst (Int J Clin Exp Pathol 2020;13:2356)
- 46 year old woman with ovarian ossification associated with endometriosis (Clin Exp Obstet Gynecol 2007;34:113)
- 65 year old woman with bilateral ovarian endometriotic cysts showing extensive ossification (Gynecological Surgery 2007;4:191)
Treatment
- Resection if symptomatic, rupture or suspicion for neoplastic process
Gross description
- Microcalcifications usually not apparent grossly
- Areas of bone formation are nodular and calcified
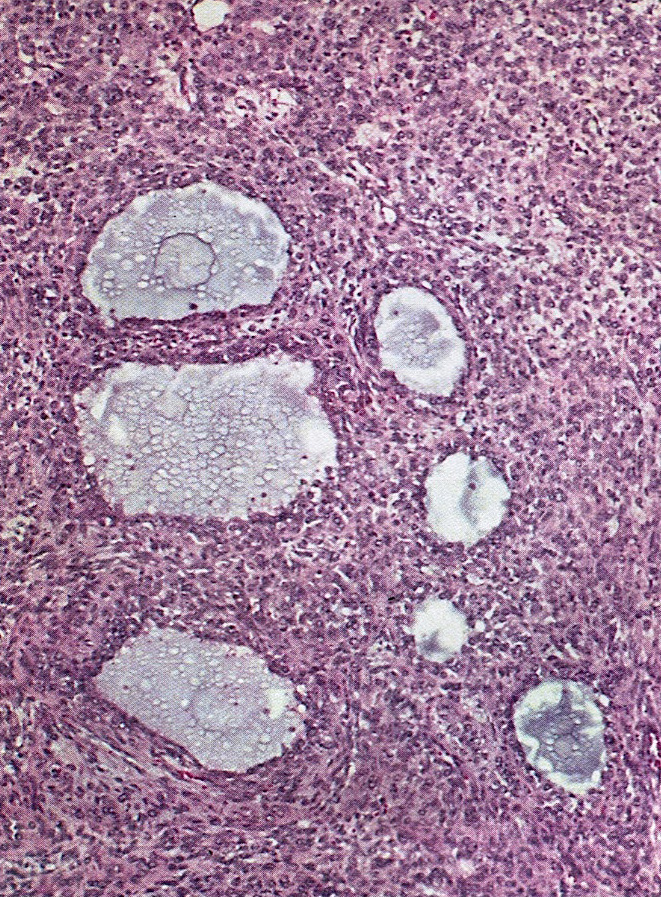
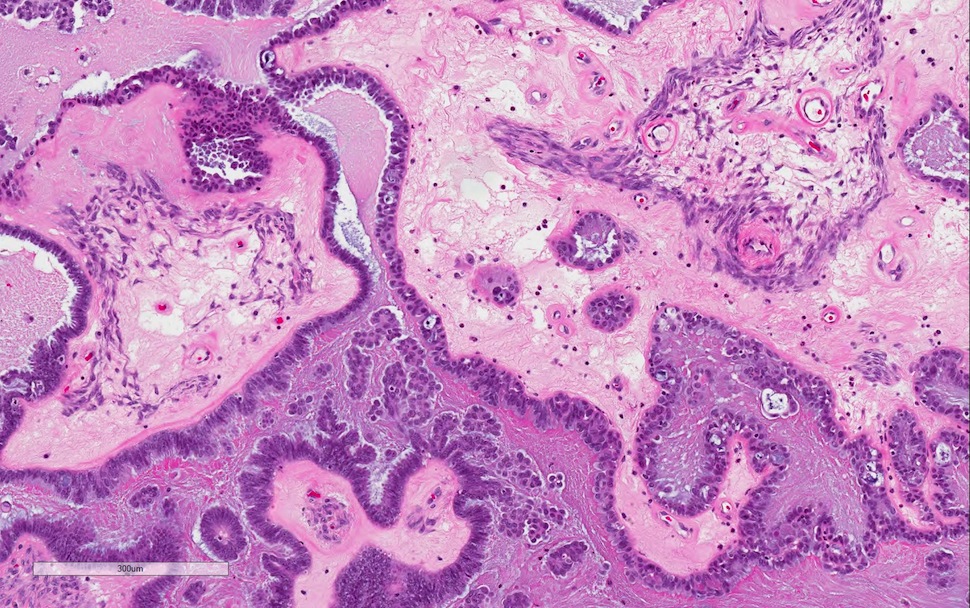
Microscopic (histologic) description
- Most ovarian calcifications consist of calcium phosphate, which stains dark purple on H&E sections
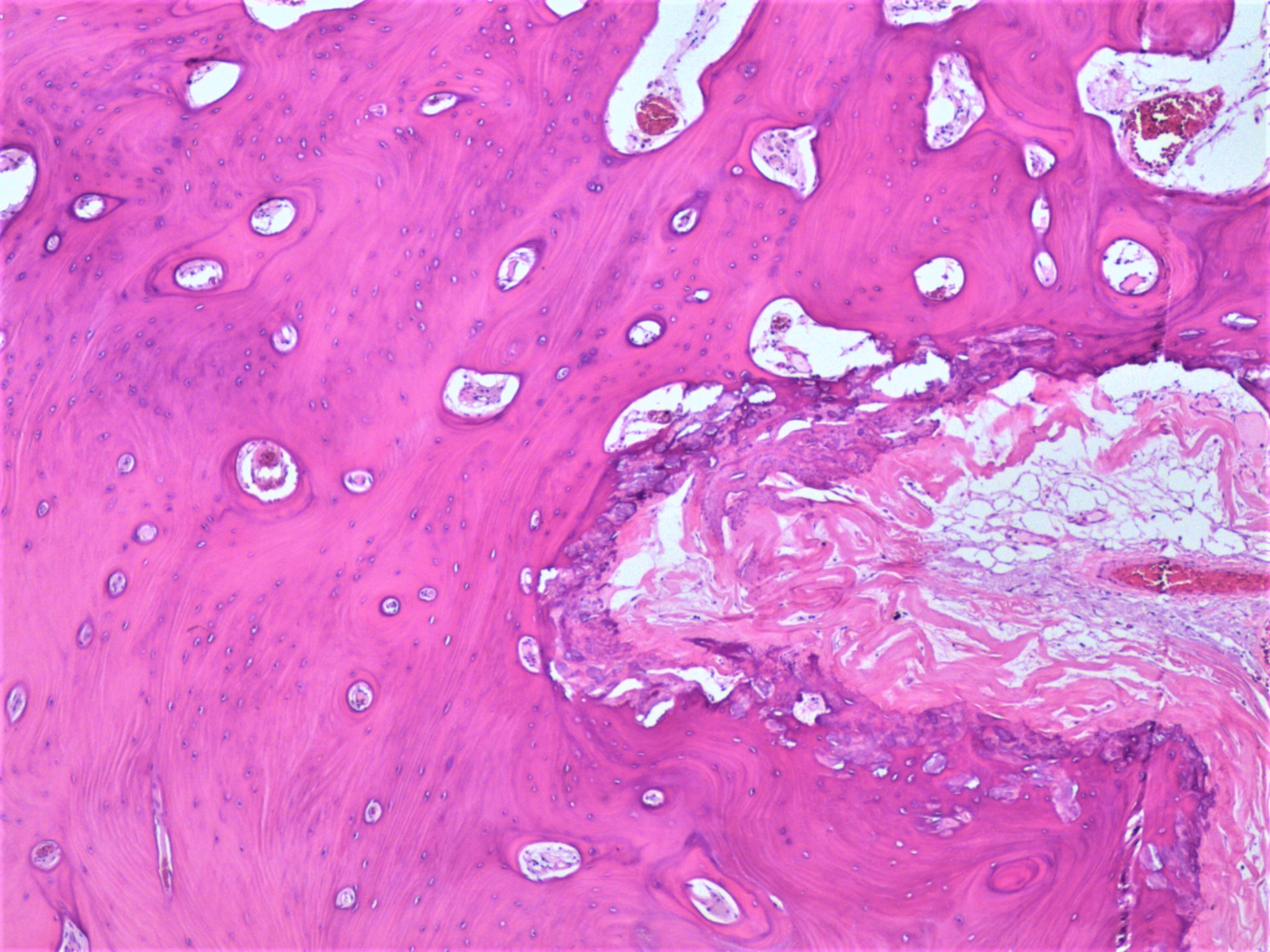
- Psammomatous calcifications: round with concentric laminations (onion-like appearance)
- Nonpsammomatous: irregular shape, dense, nonlaminated
- Bone formation shows well developed bone trabeculae
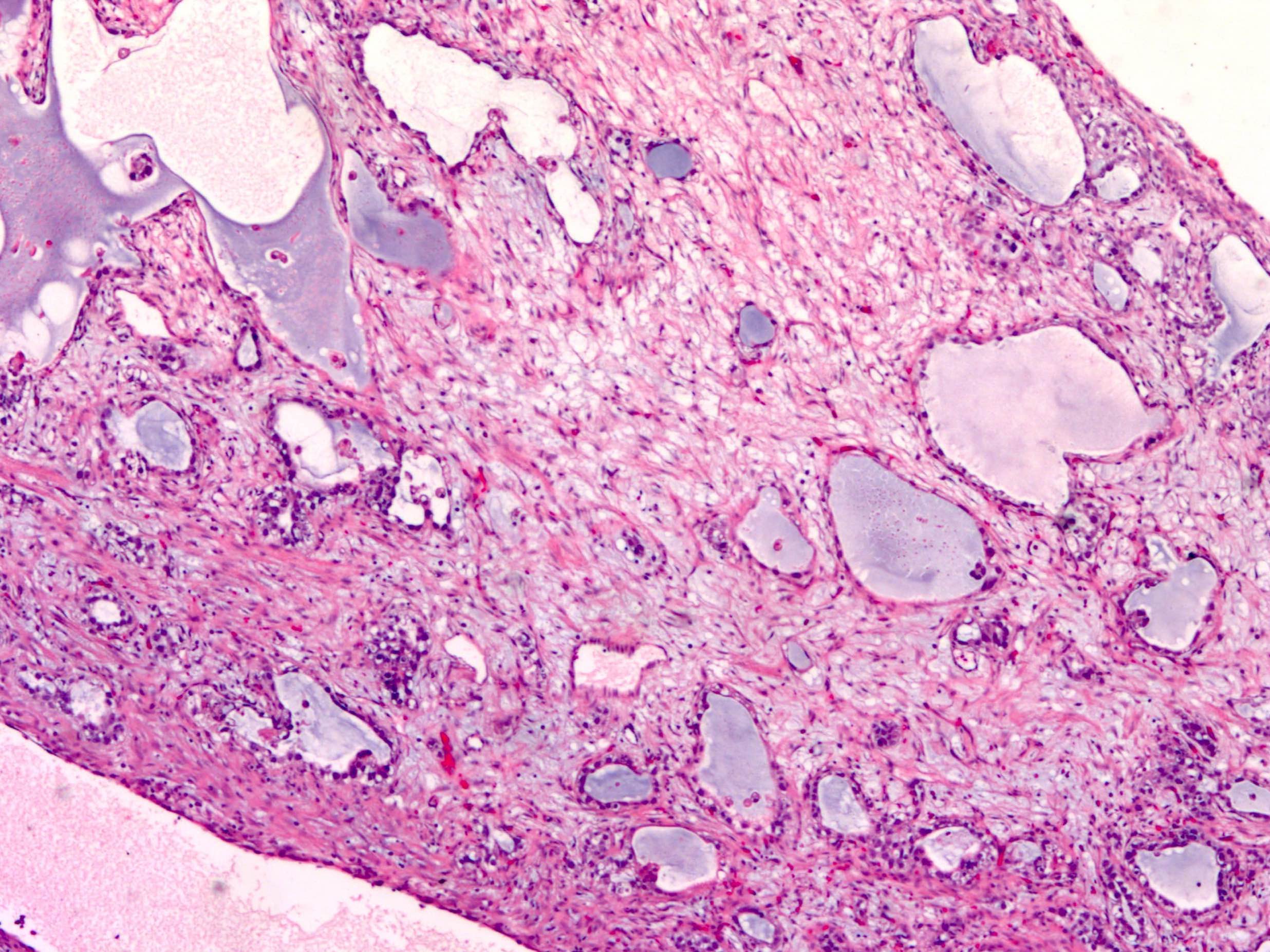
- Background ovary can be otherwise normal or show other associated pathology, such as endometriosis, teratoma, mucinous lesions, serous lesions; calcifications can be stromal or associated with the epithelial component (Mod Pathol 2003;16:219, J Nippon Med Sch 2005;72:29)
- Psammomatous calcifications in the absence of a serous neoplasm should prompt additional sampling to rule out a neoplastic process; their presence should be mentioned in the report if a serous tumor cannot be identified
Microscopic (histologic) images
Sample pathology report
- Ovary, right, oophorectomy:
- Benign ovary with surface adhesions and focal stromal psamommatous calcifications (see comment)
- Comment: The ovary has been extensively sampled, without evidence of a neoplastic process.
Differential diagnosis
- Teratoma:
- Osseous metaplasia versus teratoma: teratoma will have other components apart from bone
Board review style question #1
Board review style answer #1
Board review style question #2
Which of the following is true about calcifications of the ovary?
- Are often symptomatic
- Can be associated with a neoplastic process
- Often arise in the context of hypercalcemia
- Their presence on imaging studies prompts a resection
Board review style answer #2
Carcinoid tumor
Table of Contents
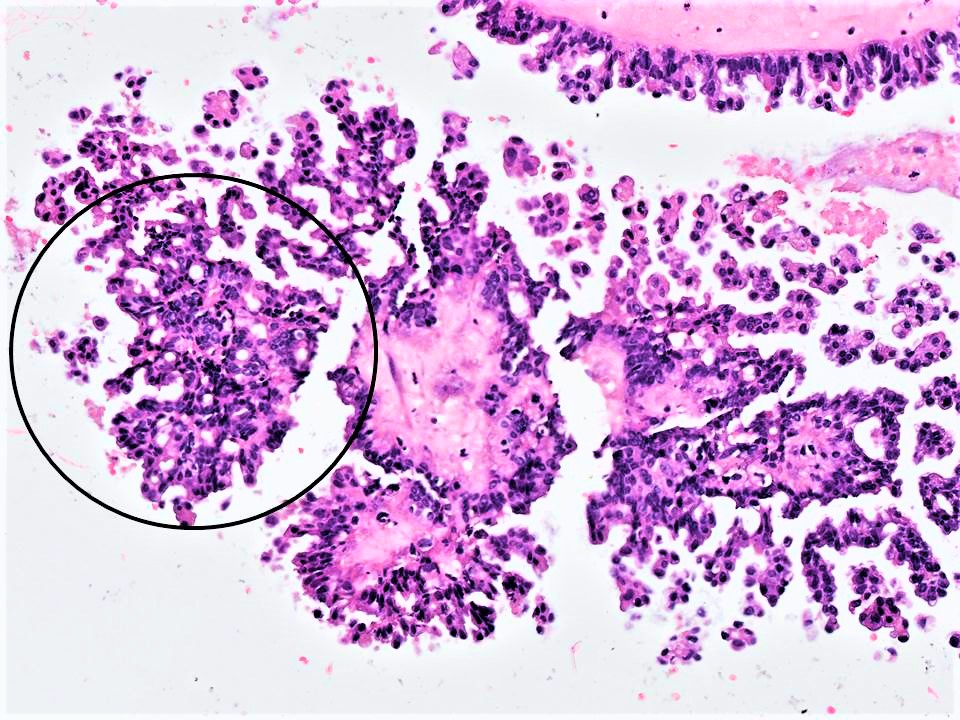
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Negative stains | Electron microscopy description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
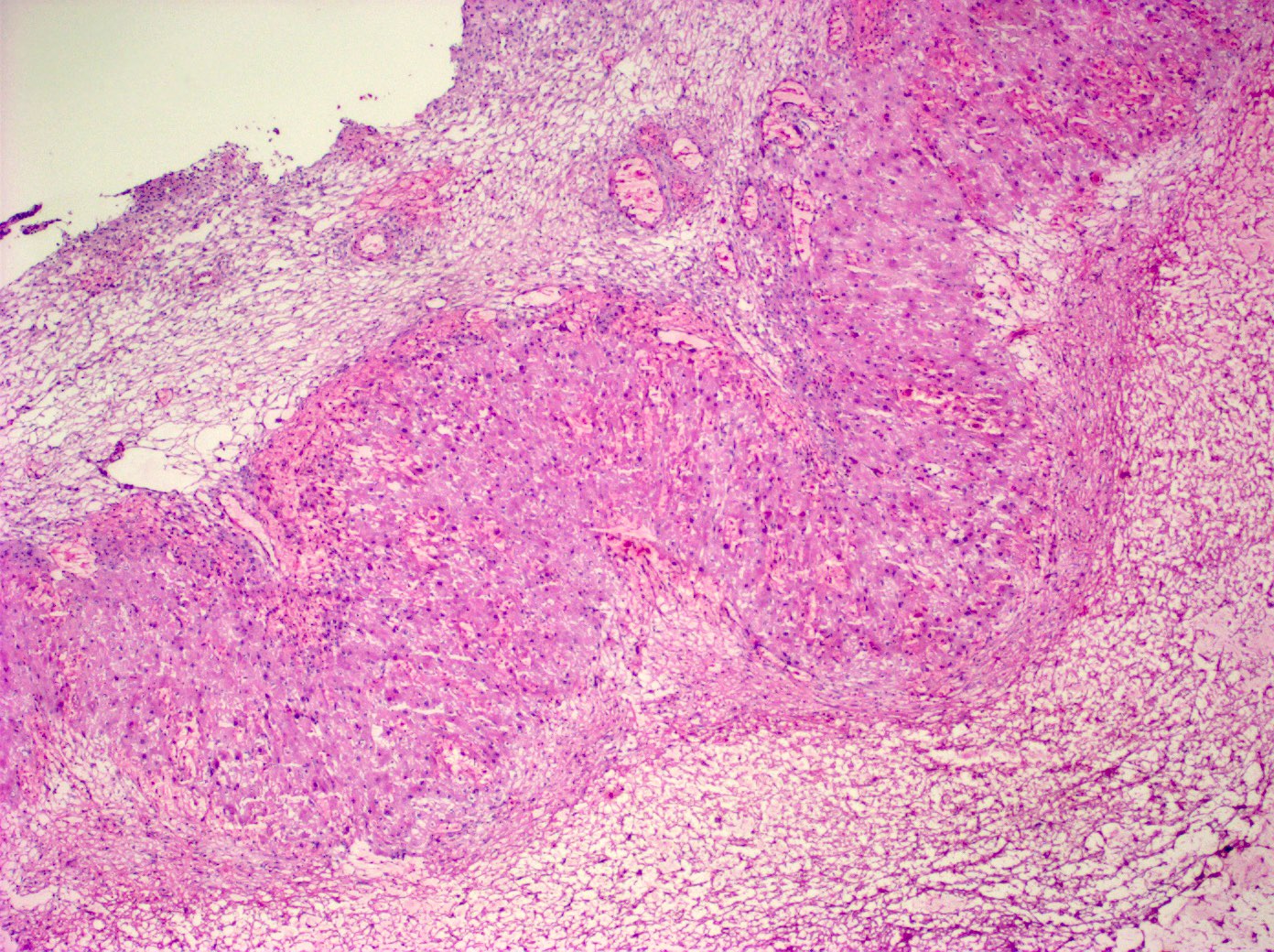
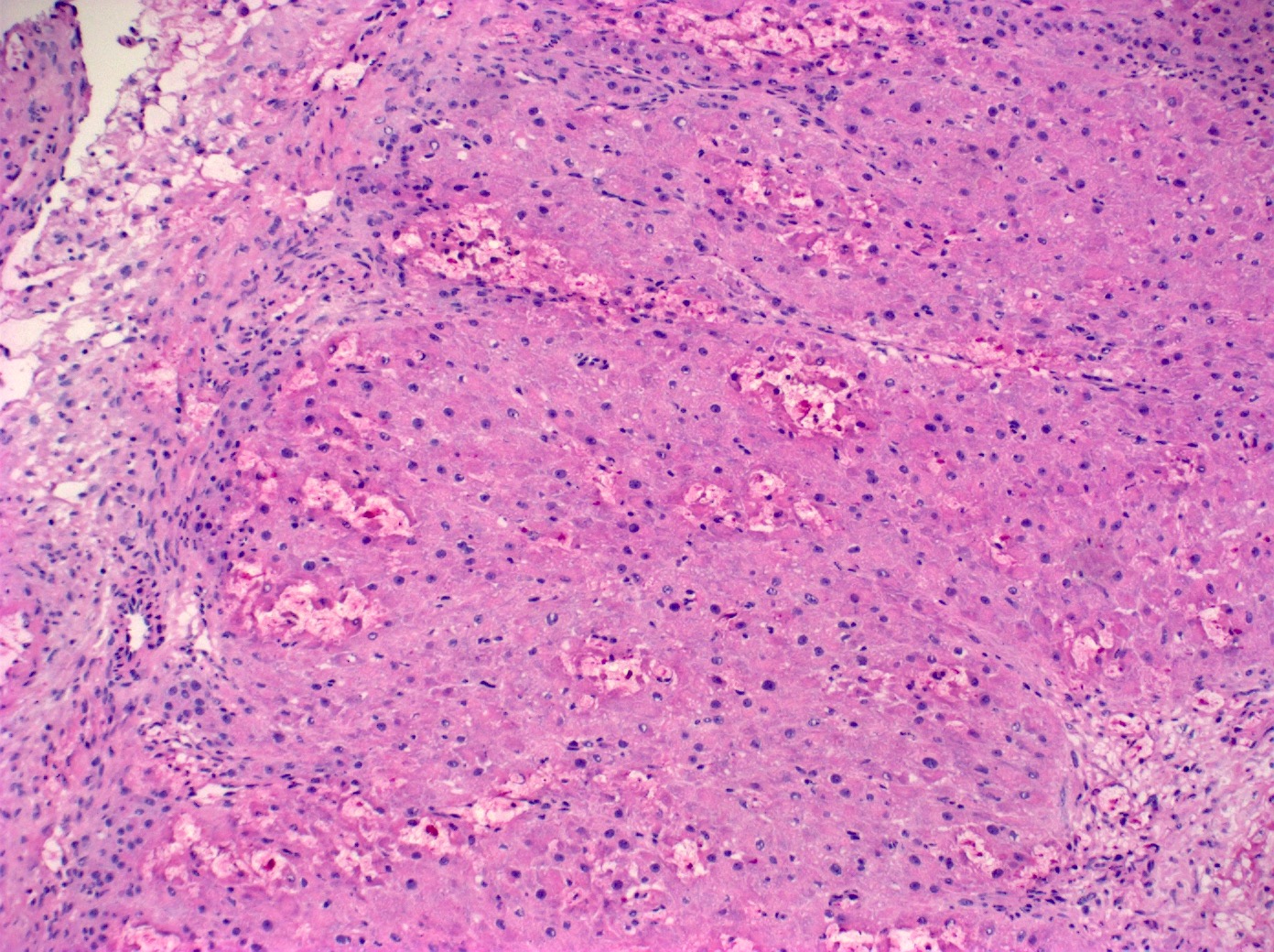
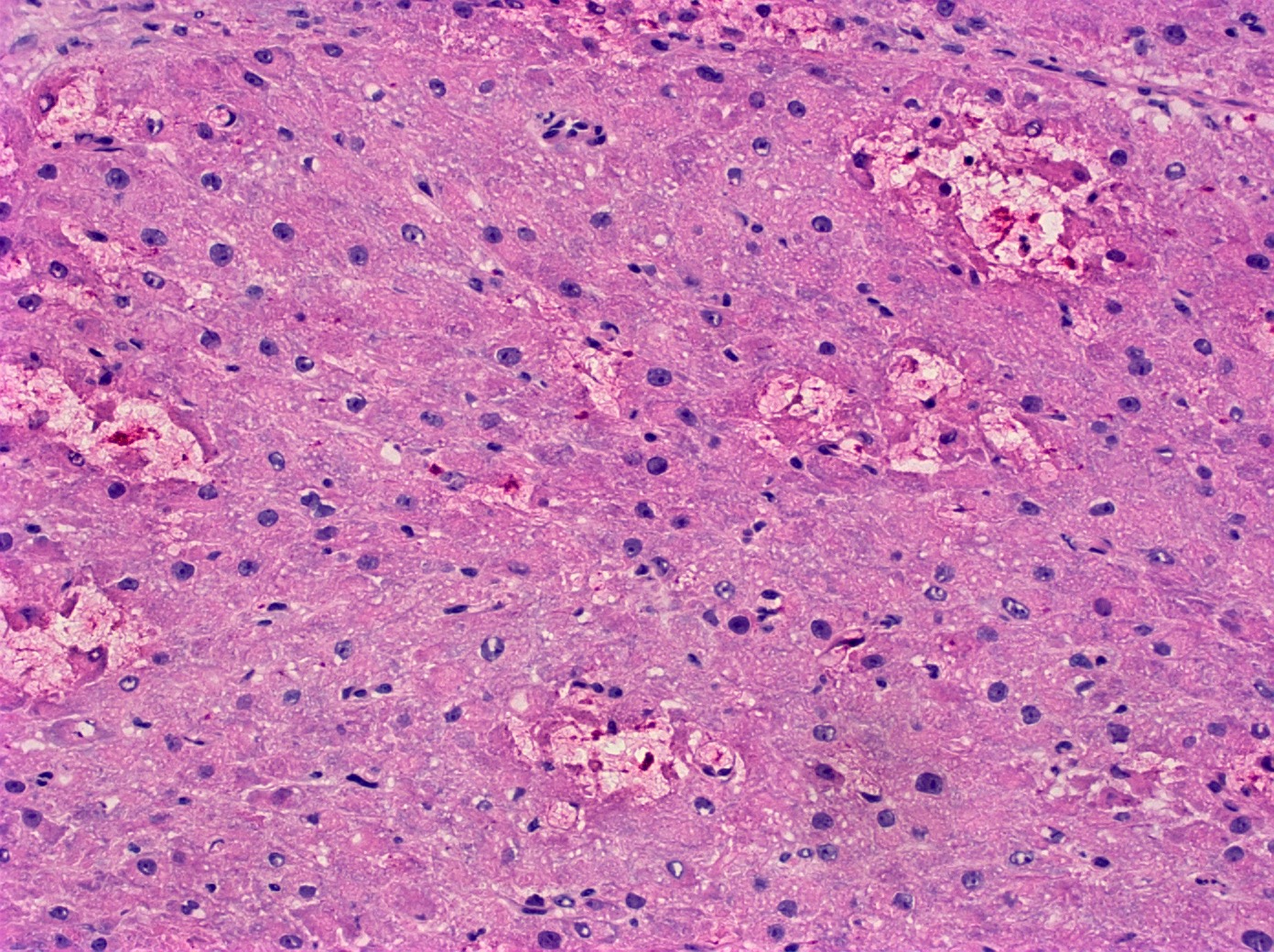
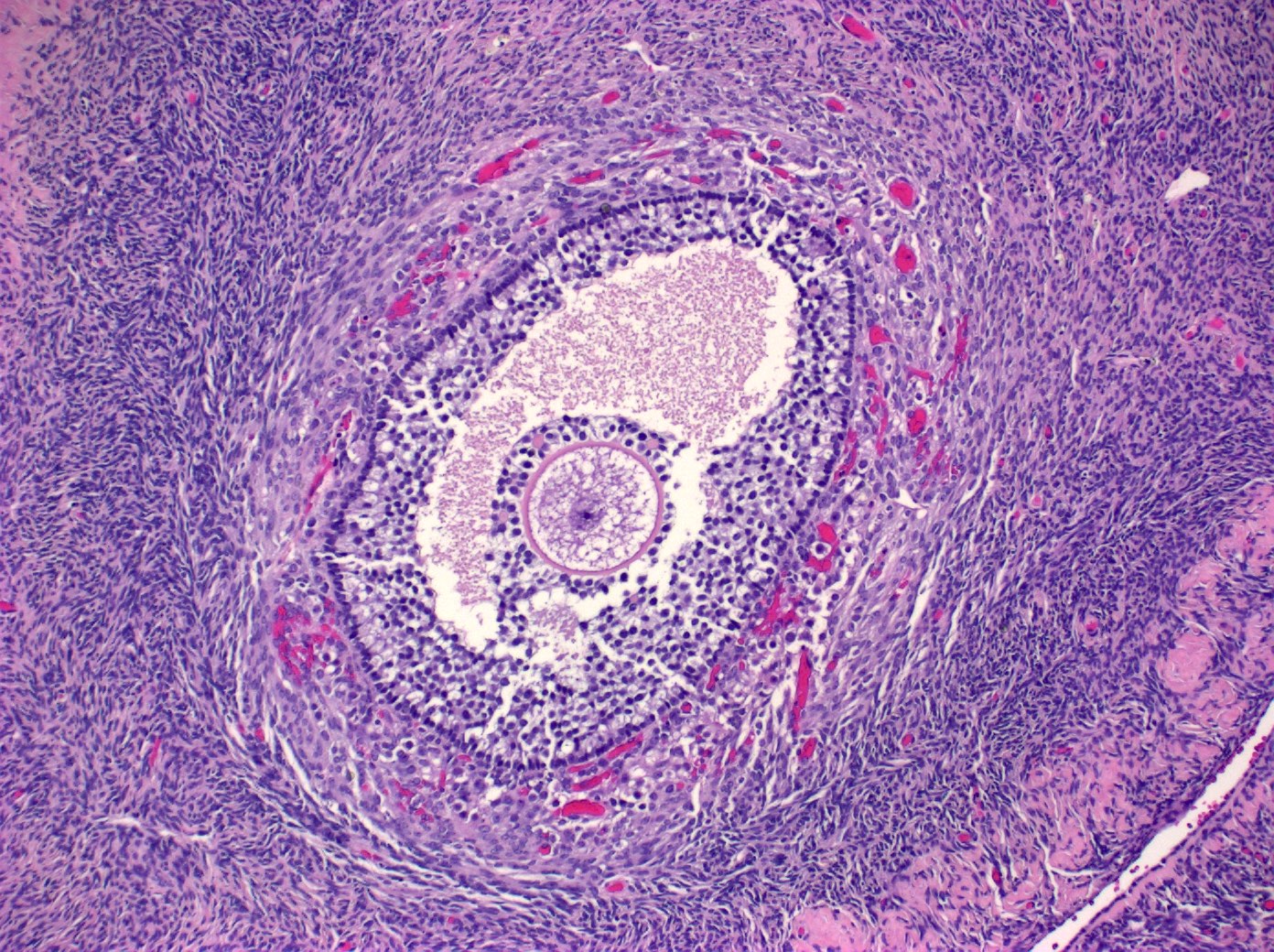
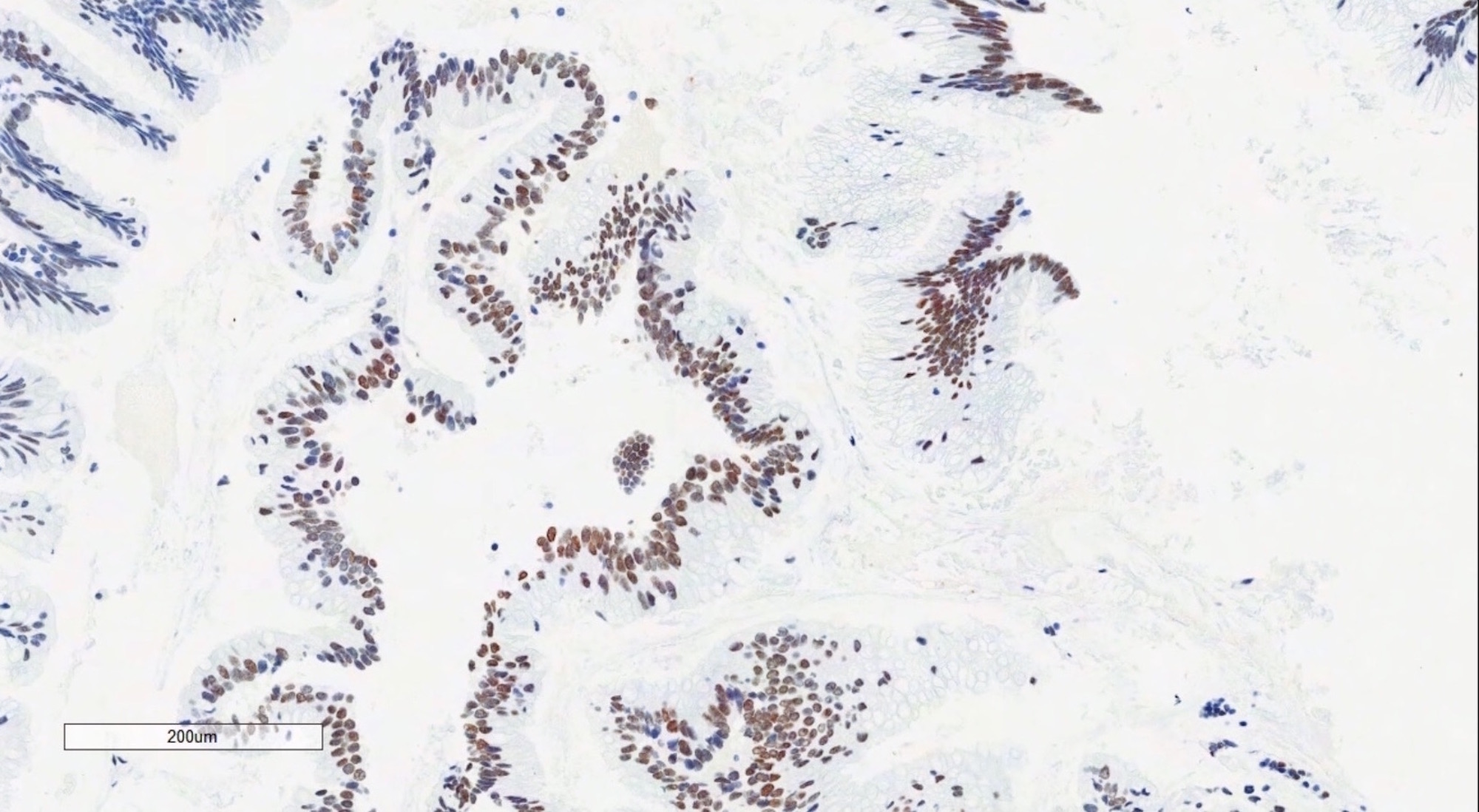
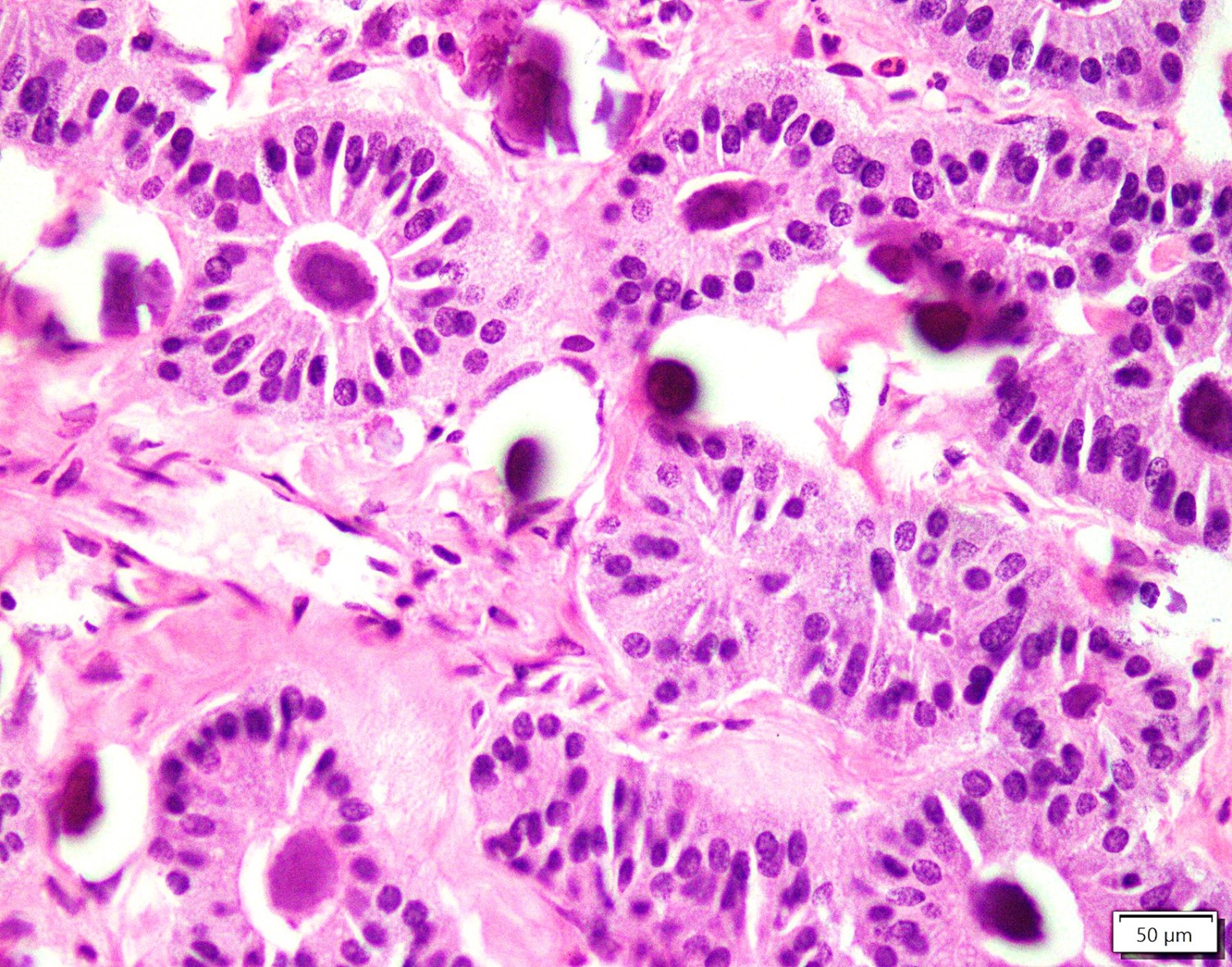
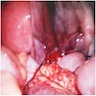
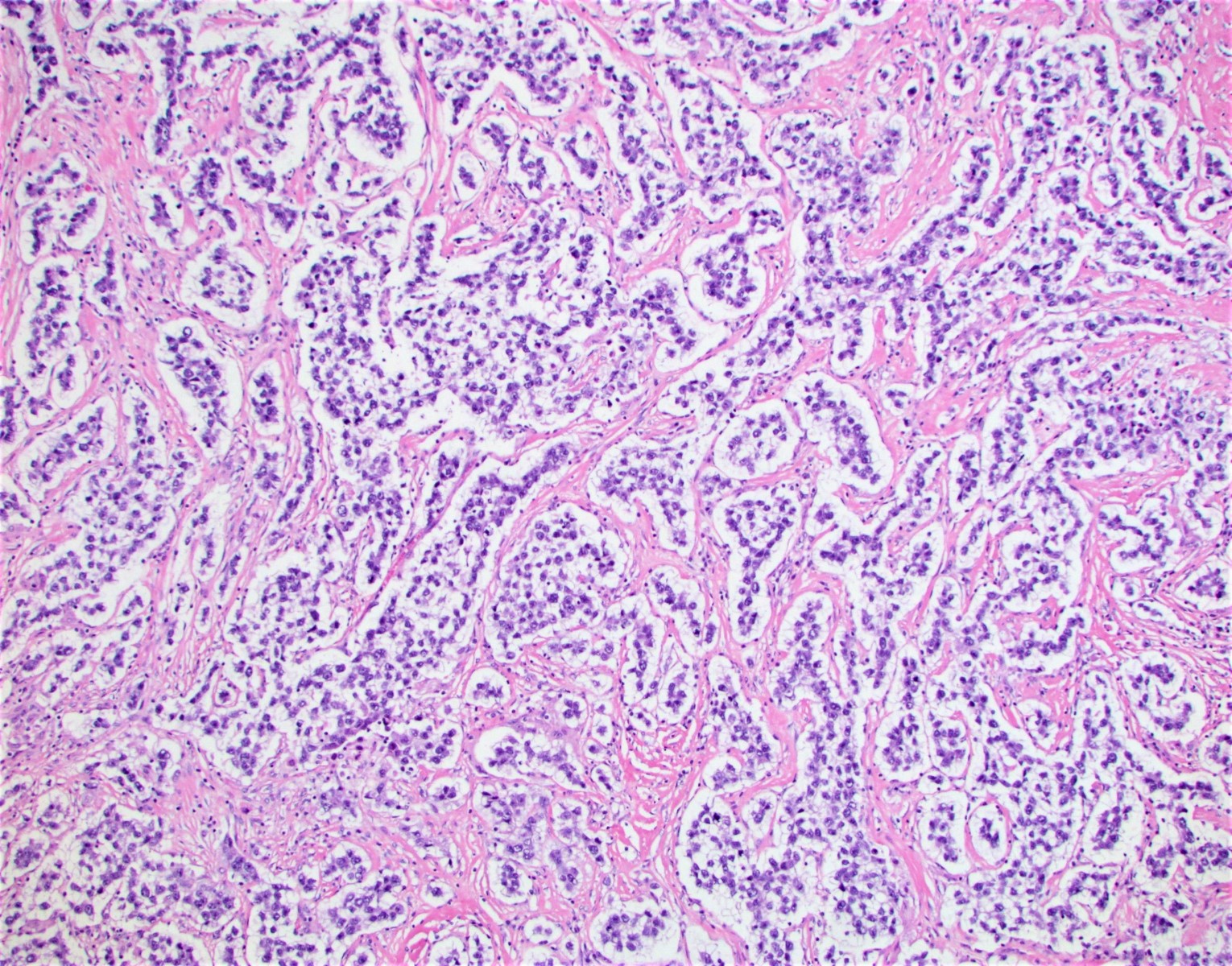
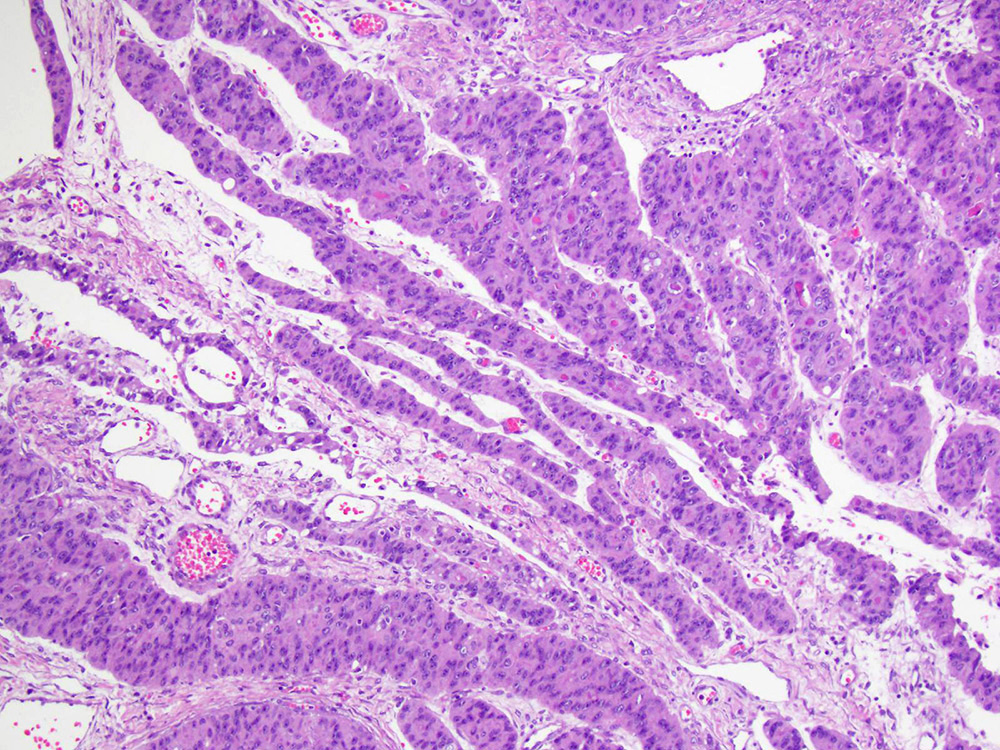
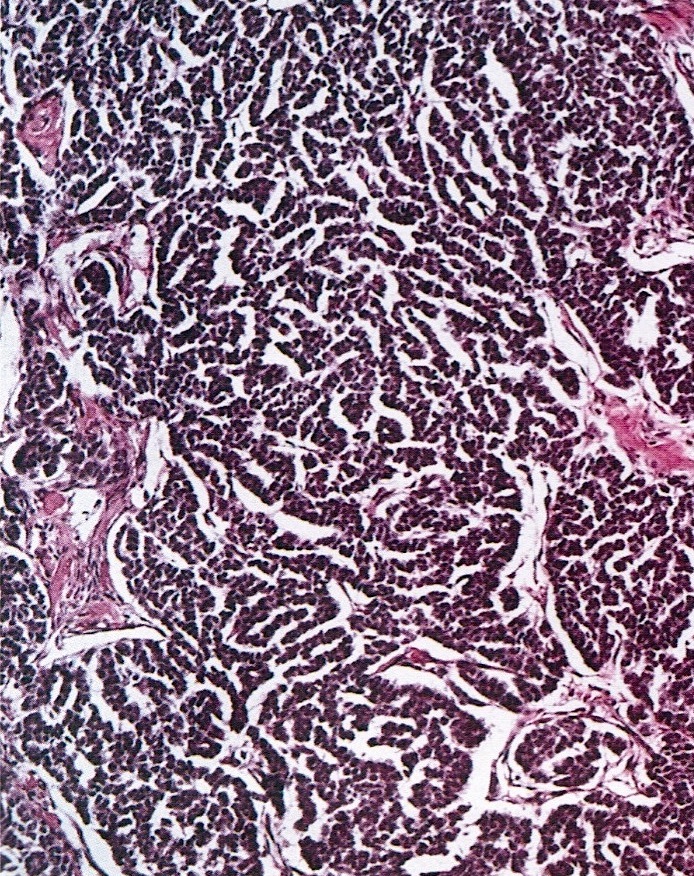
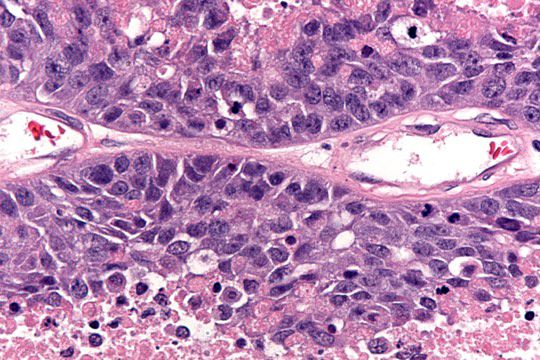
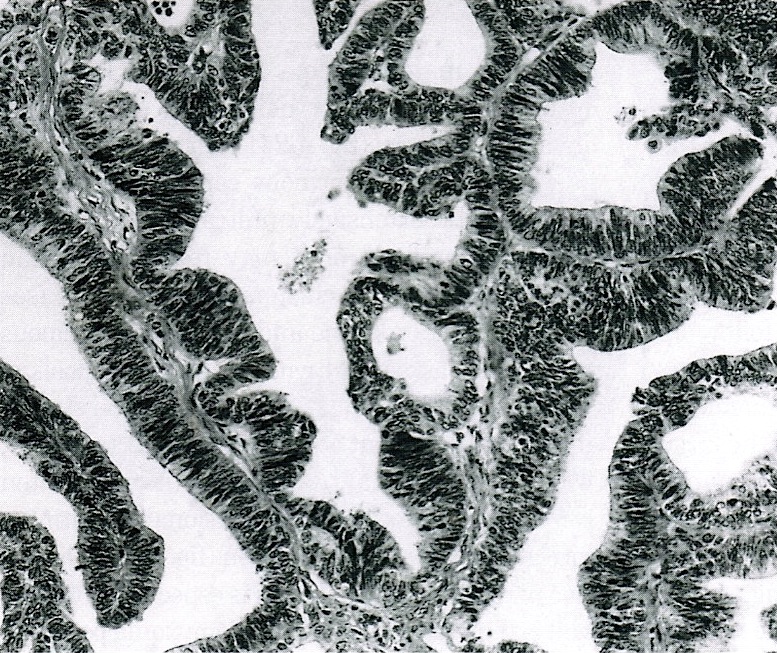
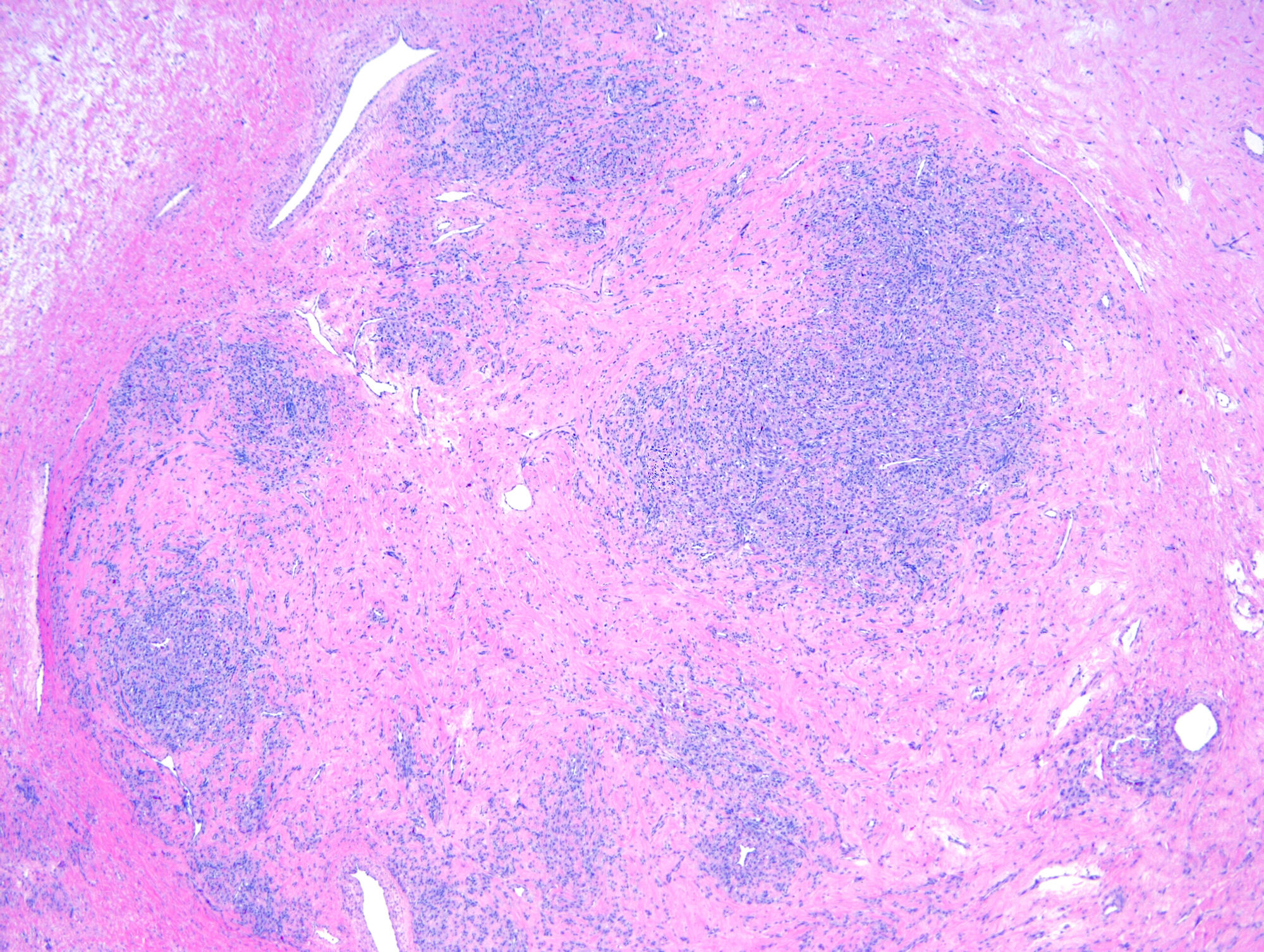
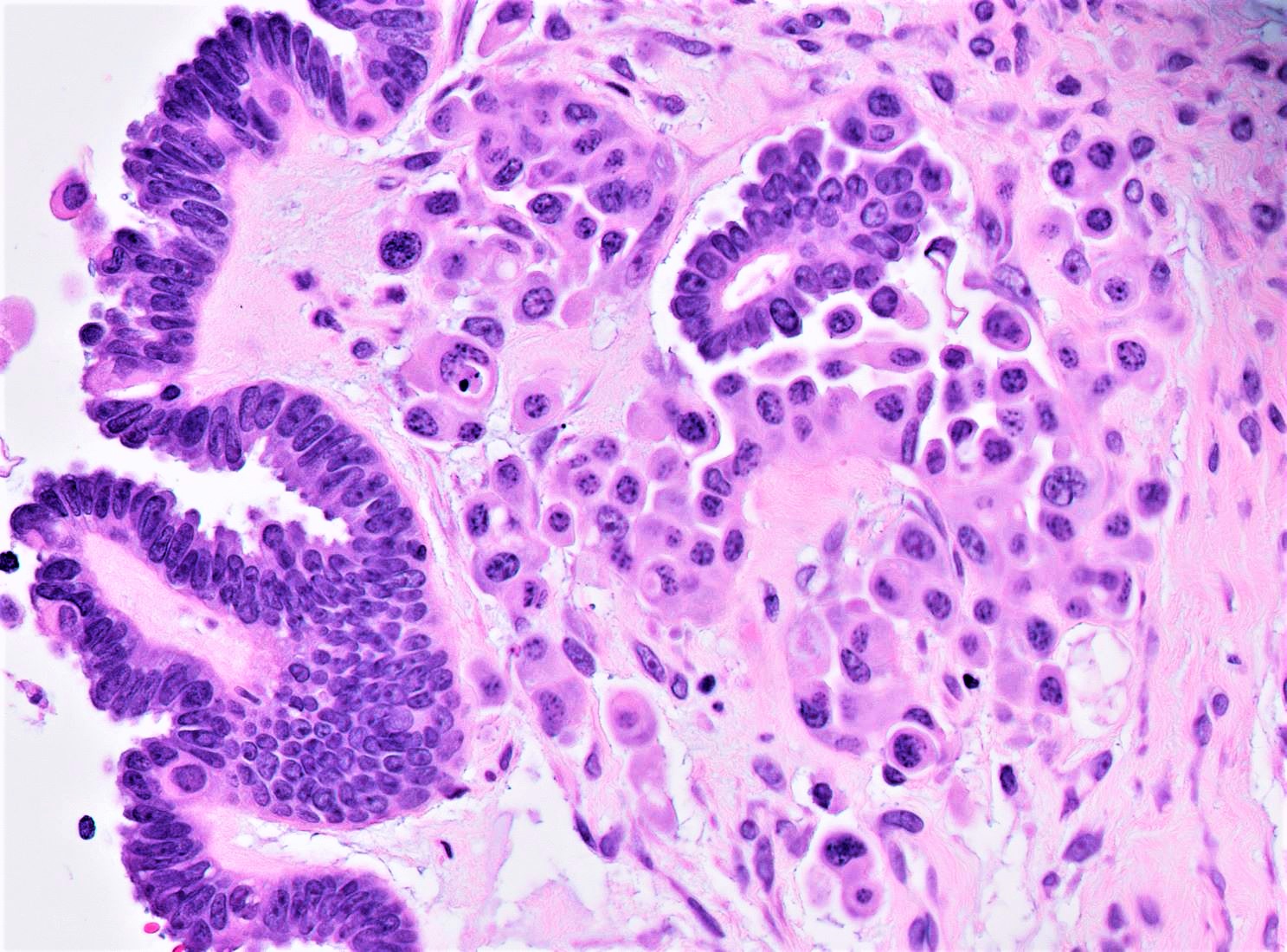
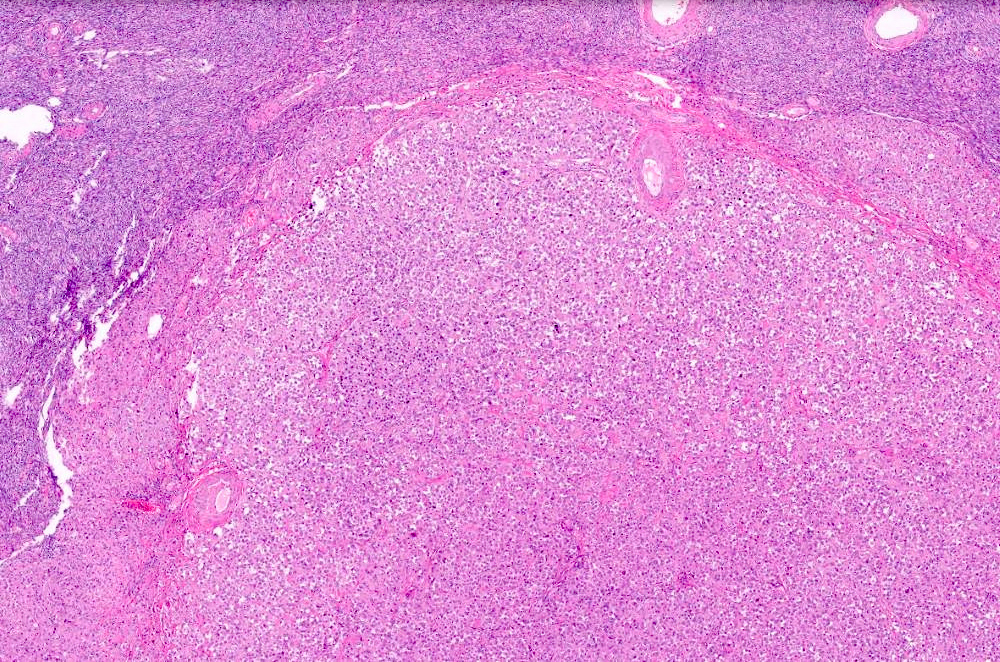
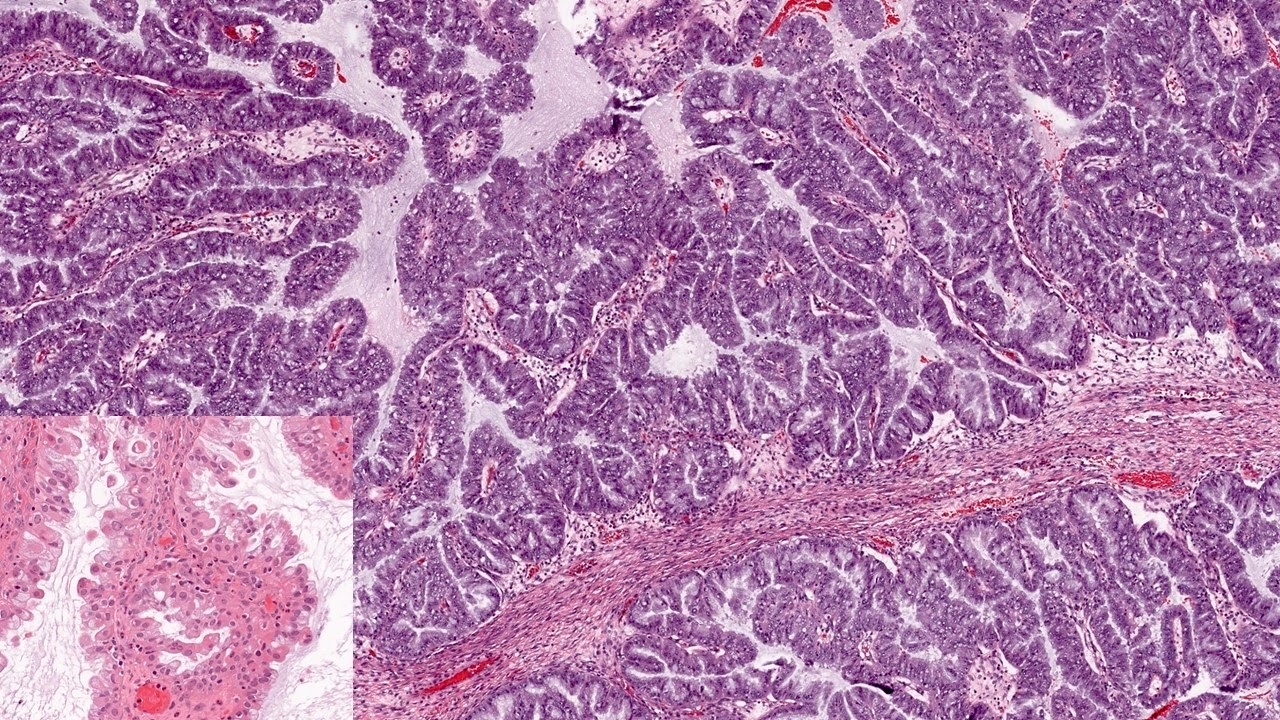
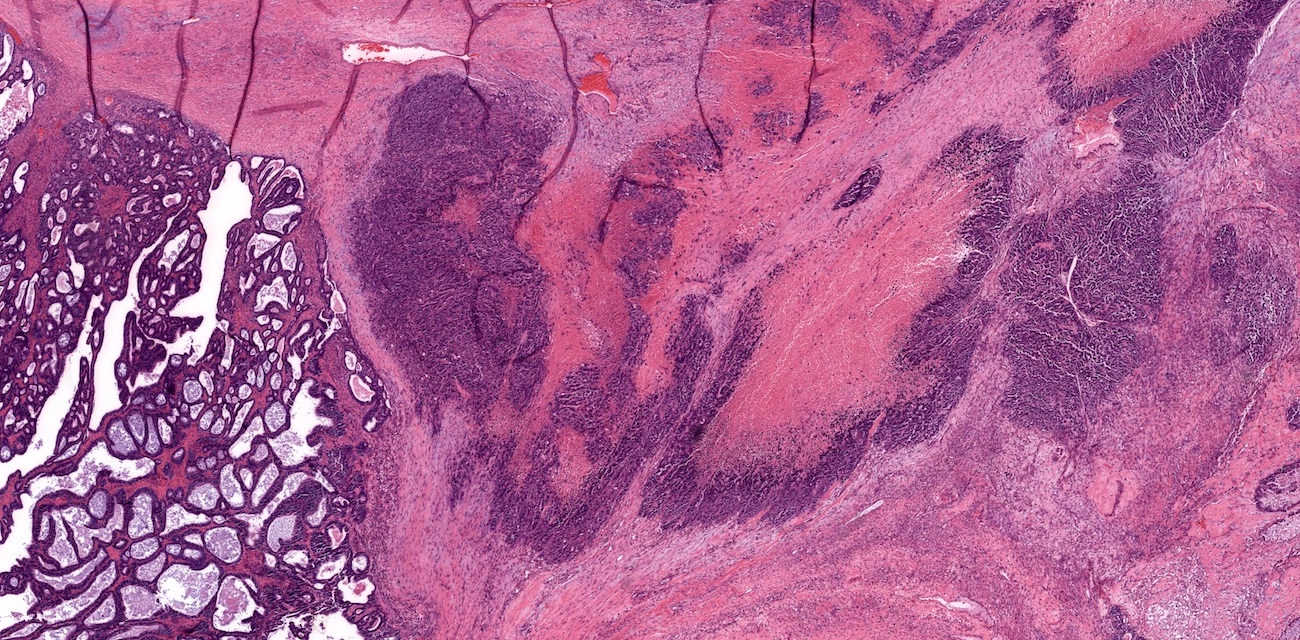
- Primary ovarian carcinoid tumors are well differentiated neuroendocrine tumors resembling those arising in the gastrointestinal tract
- In some cases, teratomatous components are identified (component of mature cystic teratoma)
- 4 types: insular, trabecular, strumal and mucinous
Essential features
- Unilateral
- May be associated with carcinoid syndrome
- Rare
Terminology
- Classified as monodermal teratomas
ICD coding
- ICD-O: 9091/1 - strumal carcinoid
- ICD-10
- ICD-11: 2F76 & XH2XW3 - neoplasms of uncertain behavior of female genital organs & strumal carcinoid
Epidemiology
- Primary ovarian carcinoid is rare and accounts for
- < 0.1% of all ovarian neoplasms (Medicine (Baltimore) 2020;99:e21109)
- < 2% of all carcinoid tumors (Cancer 2003;97:934)
- Patient age at time of presentation ranges from 17 to 83 years with a median of 55 (Gynecol Oncol 1996;61:259)
- Mucinous: observed in a younger group of patients compared to other ovarian carcinoids
- Insular type is the most common (~50%); mucinous is the least common
Sites
- Ovary (Curr Opin Obstet Gynecol 1997;9:44)
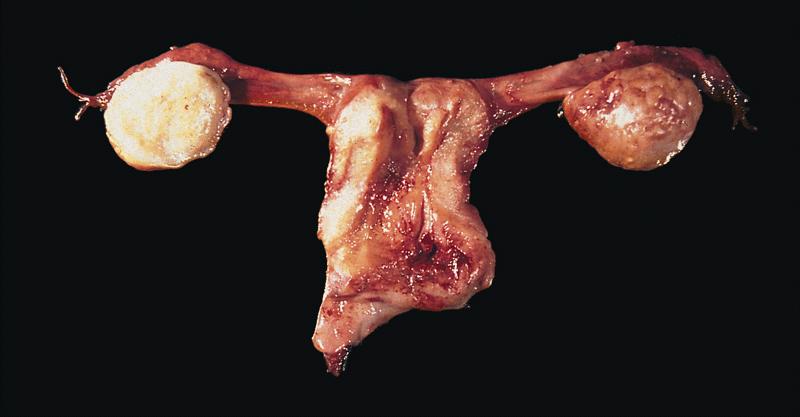
- Primary ovarian carcinoids are unilateral
- Metastatic nearly always bilateral
- Most arise in mature cystic teratoma or in association with mucinous tumor
- Pure form is less frequent; may be associated with struma ovarii
Pathophysiology
- Pathogenesis remains unclear
Etiology
- Arises from neuroendocrine cells within gastrointestinal type epithelium of mature cystic teratoma or rarely other tumors
- A case of carcinoid arising from the teratomatous bronchial mucosa in an ovarian mature cystic teratoma has been reported (Int J Gynecol Pathol 2018;37:123)
Clinical features
- Asymptomatic, incidental finding
- Abdominal mass
- Ascites
- Carcinoid syndrome
- ~33% of patients are > 50 years old
- Presence of carcinoid syndrome correlates with size of the tumor; typically seen in tumors > 7 cm, rarely seen in tumors < 4 cm
- Most common in insular type
- Does not require the presence of liver metastasis since venous drainage bypasses portal venous circulation (Tex Heart Inst J 2019;46:21)
- Carcinoid heart disease may be the initial manifestation
- Occurs in < 10% of cases of primary ovarian carcinoid
- Generally involves right chambers and valves
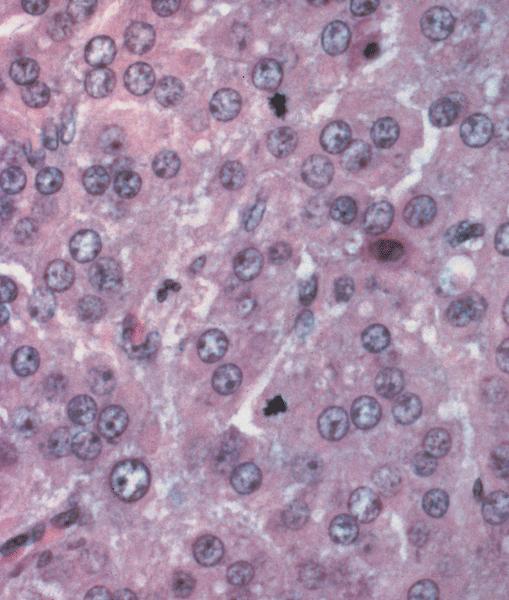
- Endocrine effects thought to occur due to stromal luteinization (Diagnostics (Basel) 2022;12:2706)
- Virilization
- Hirsutism
- Endometrial hyperplasia
- Cushing syndrome
- Constipation due to inhibitory effect on gastrointestinal motility of peptide YY secreted in strumal carcinoid (Obstet Gynecol Sci 2017;60:602)
- Hyperthyroidism in strumal carcinoid
Diagnosis
- Preoperative diagnosis of primary ovarian carcinoid is challenging; typically, the mass is properly classified postoperatively
- May be detected as a mass on physical examination
- Imaging: ovarian mass lacks typical imaging characteristics
- Somatostatin receptor scintigraphy: useful in detection of neuroendocrine tumors that show intensive octreotide intake (Am J Case Rep 2022;23:e937403)
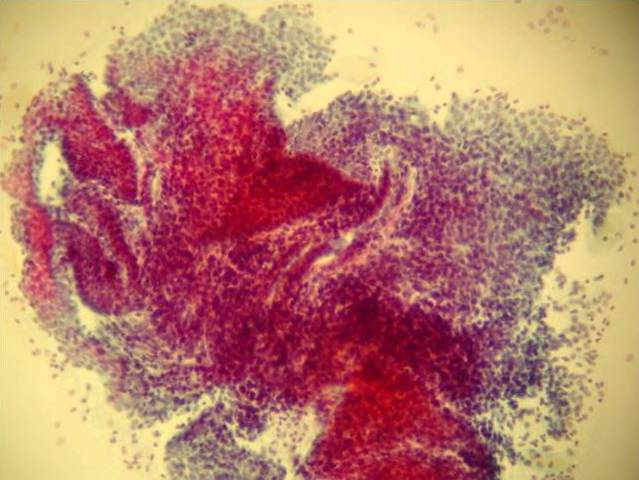
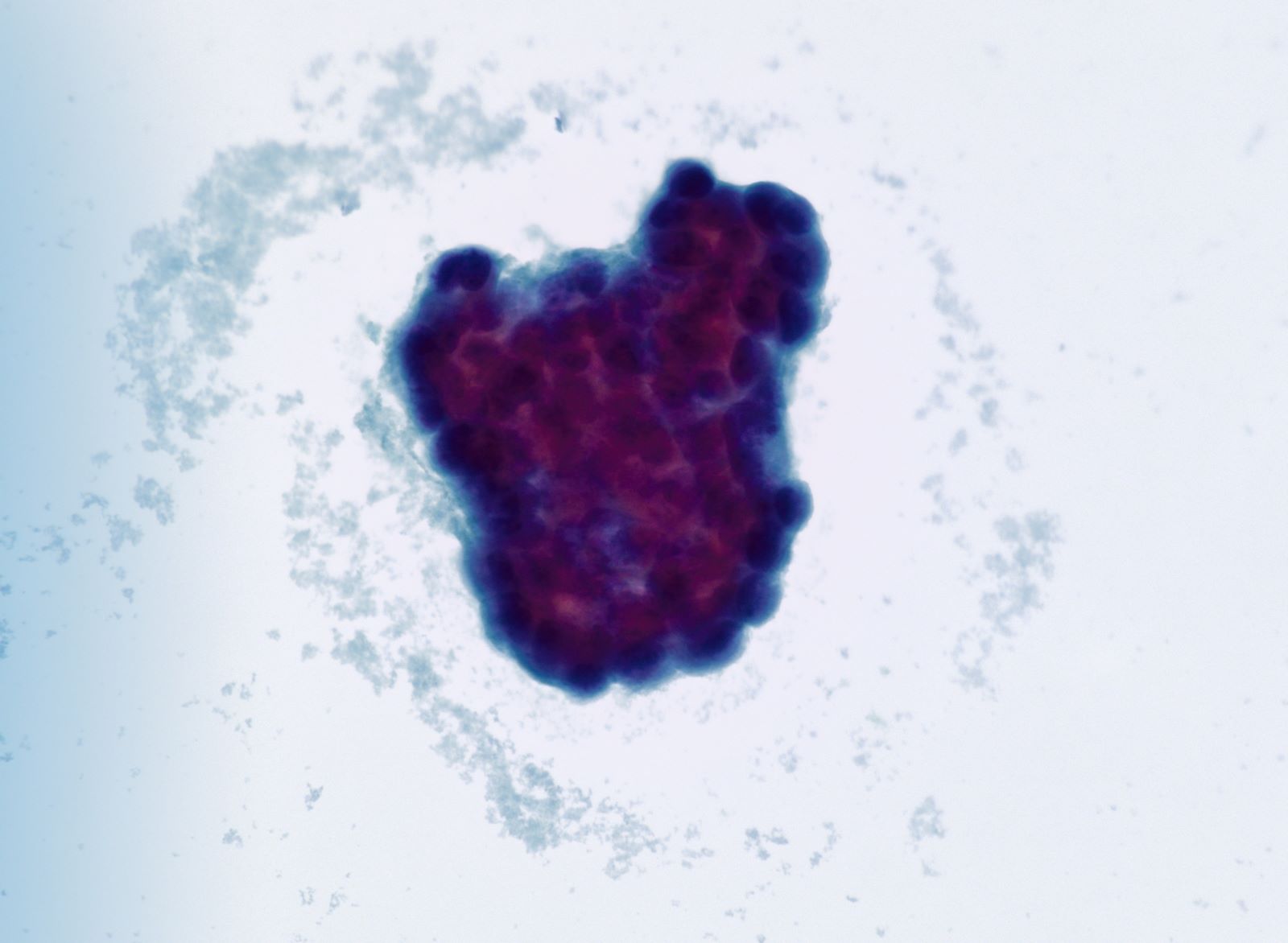
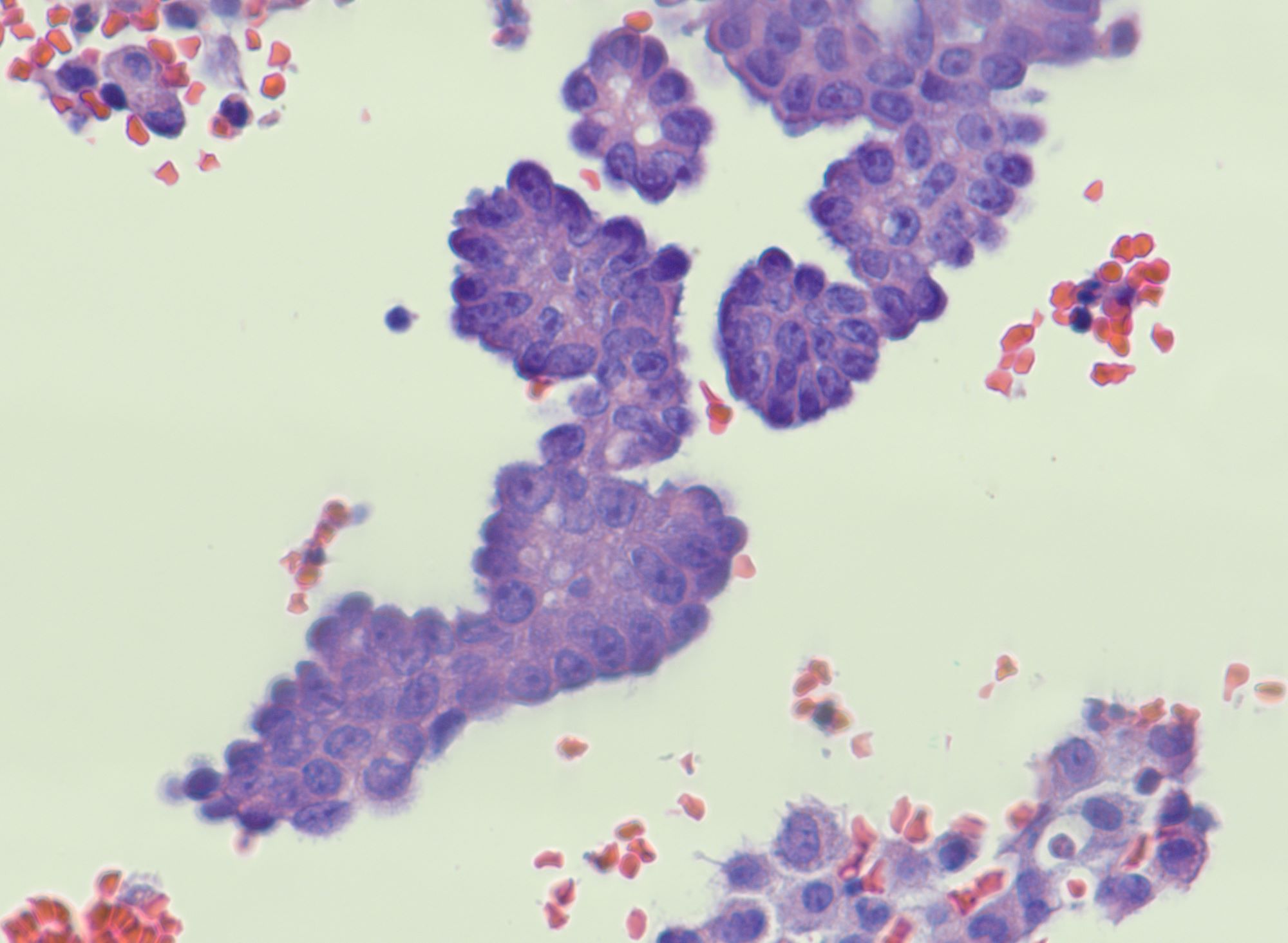
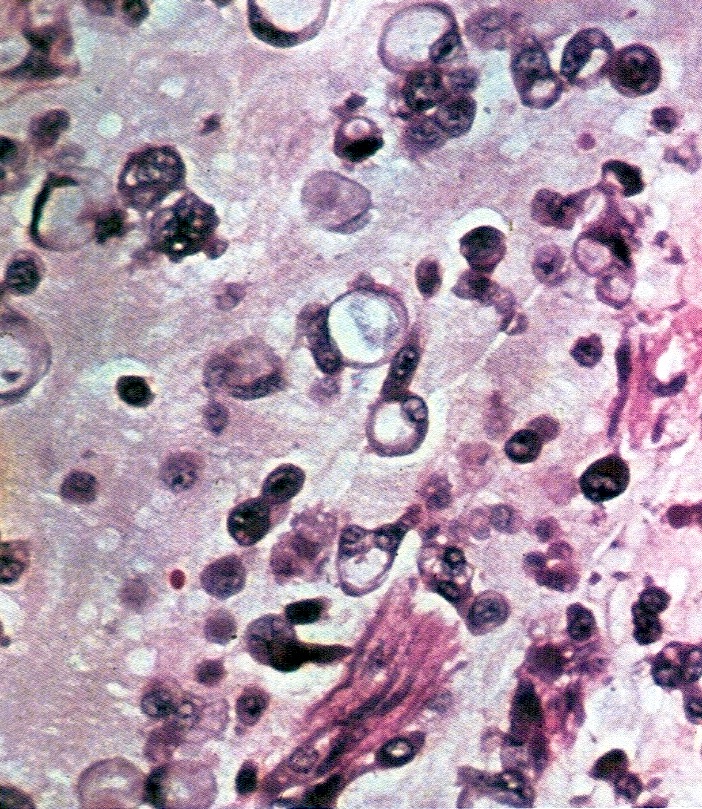
- Cytologic features: polygonal uniform cells, round monotonous nuclei, salt and pepper chromatin, ample to eosinophilic cytoplasm, often containing red to brown argentaffin granules; low mitotic activity
- Mucinous carcinoids cytologically show cells exhibiting neuroendocrine and mucinous differentiation; signet ring cells may be seen in the stroma
- WHO essential and desirable diagnostic criteria
- Essential
- Insular architecture (if insular carcinoid)
- Trabecular or corded architecture (if trabecular carcinoid)
- Thyroid follicles intimately admixed or juxtaposed with carcinoid (if strumal carcinoid)
- Acini or glands with goblet cells free floating in mucin (if mucinous carcinoid)
- Salt and pepper chromatin pattern of the nuclei, with or without cytoplasmic granules
- Desirable: positivity for neuroendocrine markers
- Essential
Laboratory
- Tumor markers may be elevated (Acta Obstet Gynecol Scand 2023;102:935)
- Elevation of CA125 and CA19-9 may mimic ovarian carcinoma
- Neuron specific enolase (NSE)
- Carcinoembryonic antigen (CEA)
- Tumor markers used in carcinoid syndrome to monitor disease activity, response to therapy and early detection of metastasis (Endocrinol Metab Clin North Am 2017;46:669)
- 5-hydroxyindole acetic acid (5-HIAA)
- Chromogranin A
Radiology description
- Ultrasound
- Hypoechoic solid or cystic with solid component adnexal mass (Medicine (Baltimore) 2020;99:e21109)
- Computed tomography (CT)
- Unilateral, lobulated adnexal mass with scattered necrotic areas indistinguishable from other solid ovarian neoplasms
- Solid enhancing nodule in the wall of a mature cystic teratoma or a mucinous neoplasm
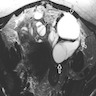
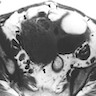
- Magnetic resonance imaging (MRI)
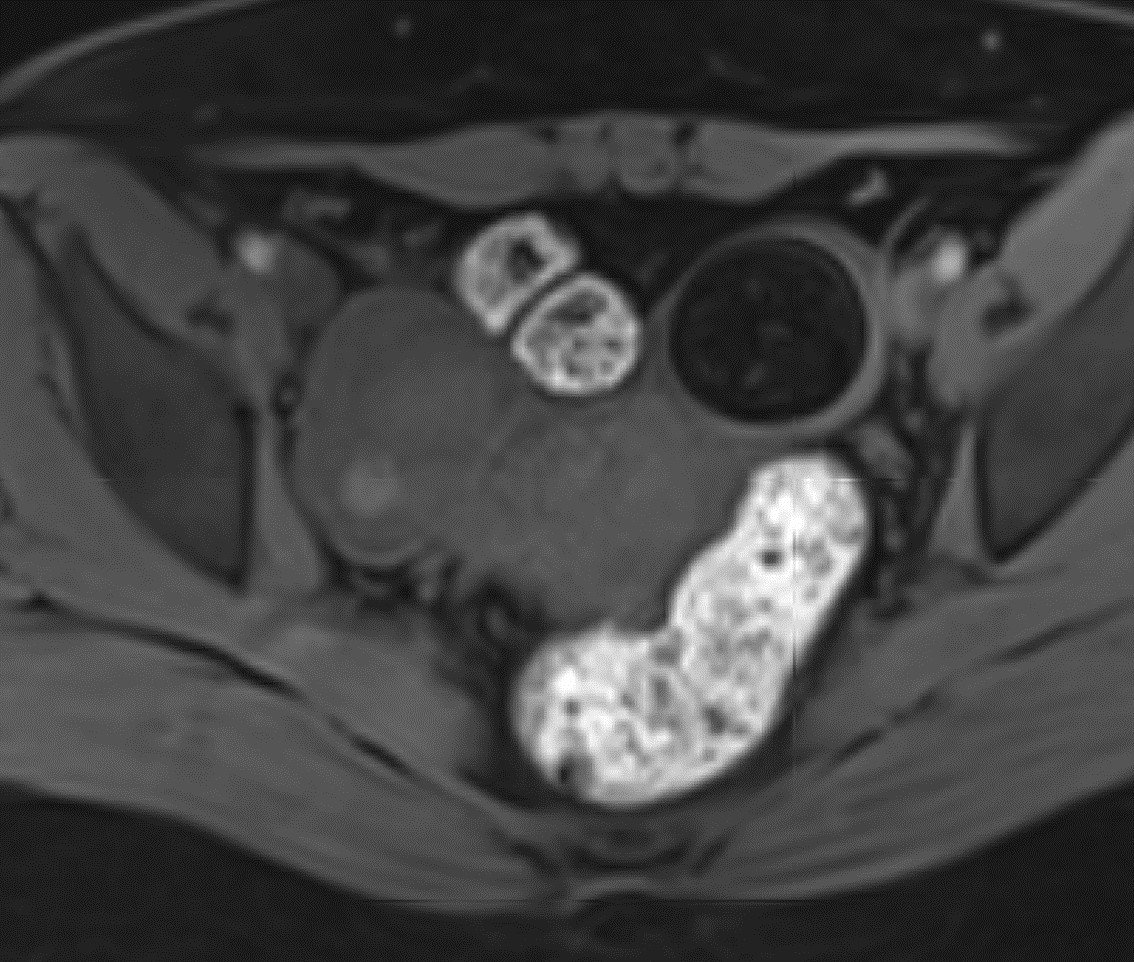
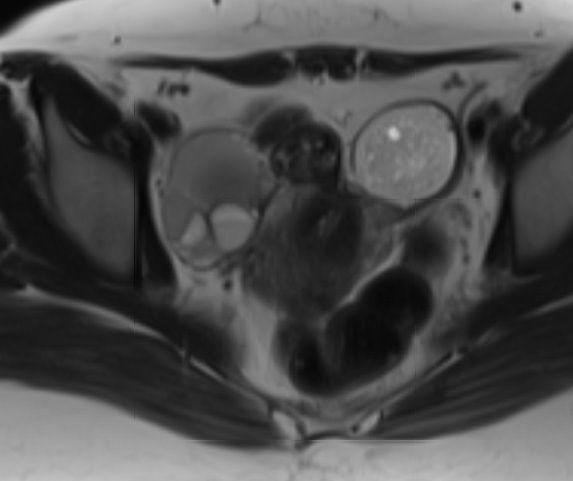
- On T2 weighted MR images, hypointense solid adnexal mass or low intensity focus within a multilocular cystic mass
Radiology images
Prognostic factors
- 5 year survival rate of ovarian carcinoid is 84% and 94% in patients with and without mature cystic teratoma, respectively
- Insular carcinoid
- Favorable prognosis
- Slow growing
- Only occasionally has metastasis
- Trabecular carcinoid
- Favorable prognosis
- Not associated with metastasis
- Strumal carcinoid
- Favorable prognosis
- 15 year recurrence rate is 4.4% (BMC Cancer 2022;22:1090)
- Mucinous
- Behaves more aggressively than other types of primary ovarian carcinoid
- Tends to spread mainly via lymphatics and can metastasize
- No metastases, better prognosis
- Low Ki67 proliferative index; mean index of 2.5% with a maximum of 5% (Acta Obstet Gynecol Scand 2023;102:935)
- Carcinoid heart syndrome has been reported to be associated with overall poor outcome
Case reports
- 18 year old woman with mucinous adenocarcinoma and carcinoid tumor arising within an ovarian mature cystic teratoma (Oman Med J 2023;38:e538)
- 40 year old woman with insular carcinoid with hyperandrogenism and carcinoid heart syndrome (Am J Case Rep 2022;23:e937403)
- 40 year old woman with trabecular carcinoid tumor arising from a mature cystic teratoma (Ci Ji Yi Xue Za Zhi 2019;3:192)
- 51 year old postmenopausal woman with primary ovarian carcinoid exhibiting mixed growth pattern (Medicine (Baltimore) 2023;102:e34391)
- 56 year old woman with ovarian strumal carcinoid (Fukushima J Med Sci 2023;69:51)
Treatment
- Treatment strategies vary (Acta Obstet Gynecol Scand 2023;102:935)
- Surgical
- Conservative
- Cystectomy
- Unilateral salpingo-oophorectomy, especially in reproductive age women
- Radical surgery
- Bilateral salpingo-oophorectomy
- Hysterectomy and bilateral salpingo-oophorectomy
- In mucinous carcinoid, regional lymph node dissection may be required
- Cytoreductive surgery
- Conservative
- Adjuvant treatment restricted to patients with residual or metastatic diseases
- Somatostatin analogs: may be used in metastatic or recurrent primary ovarian carcinoids; treatment of symptoms of excess hormone secretion and for tumor growth control
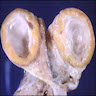
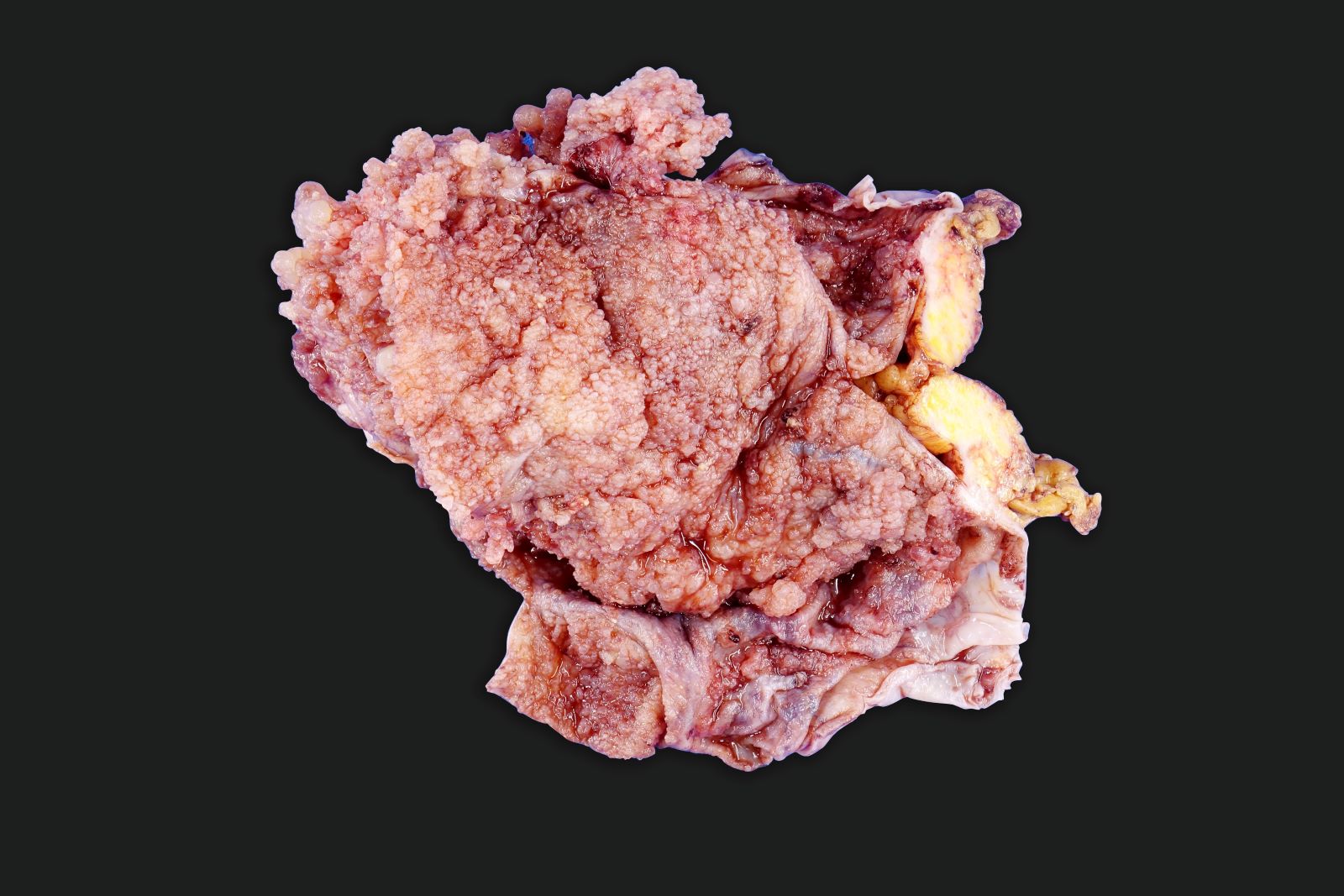
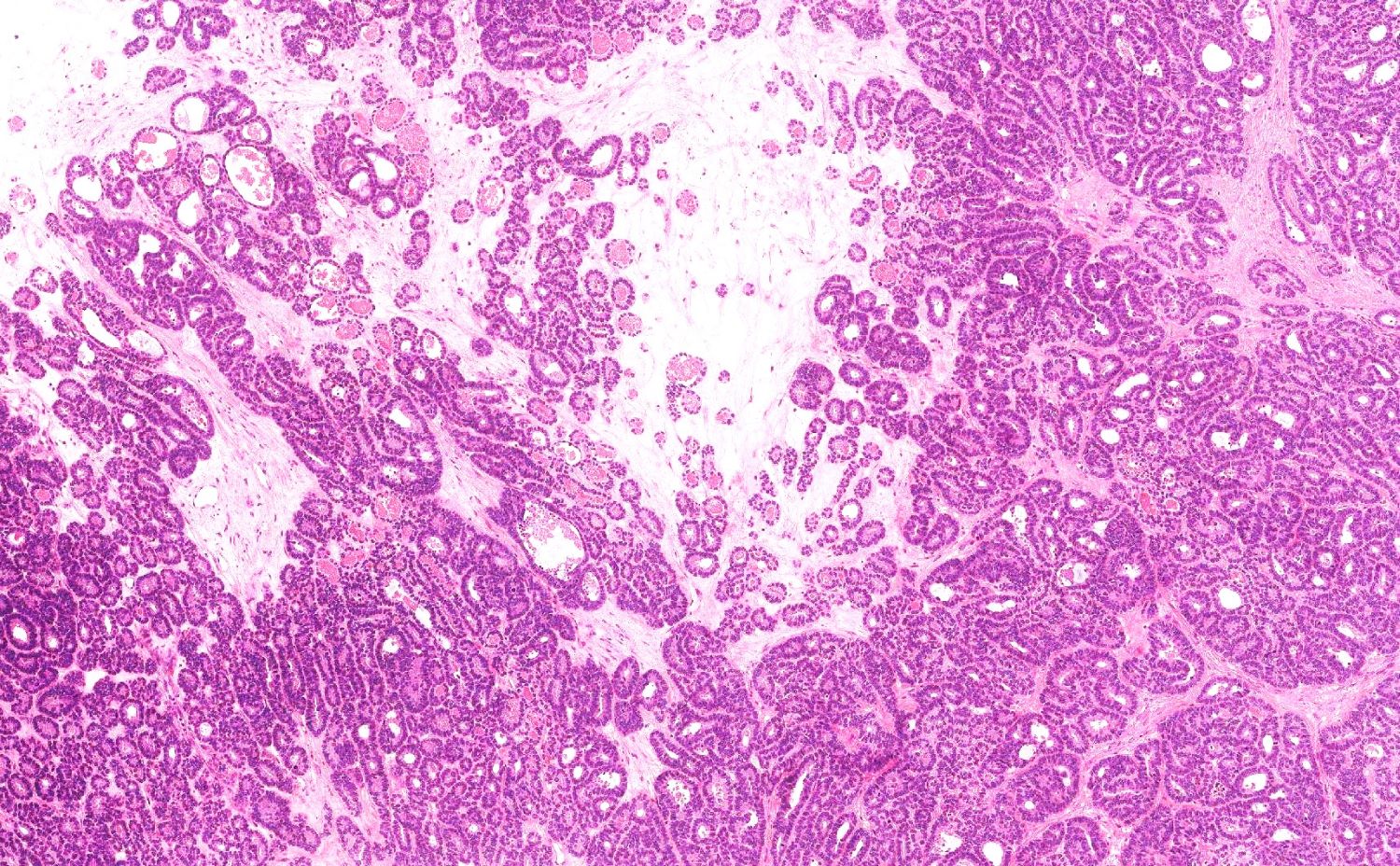
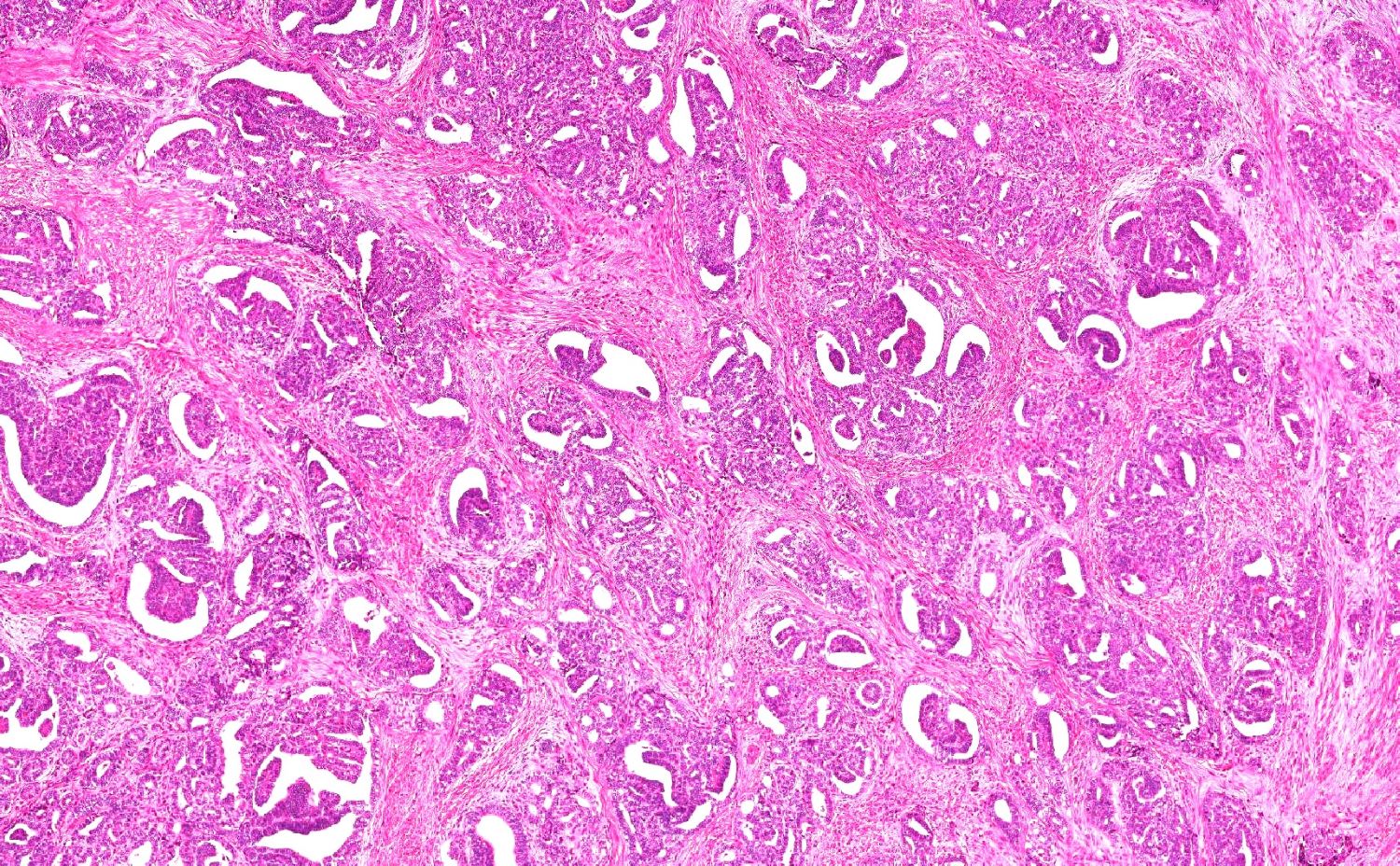
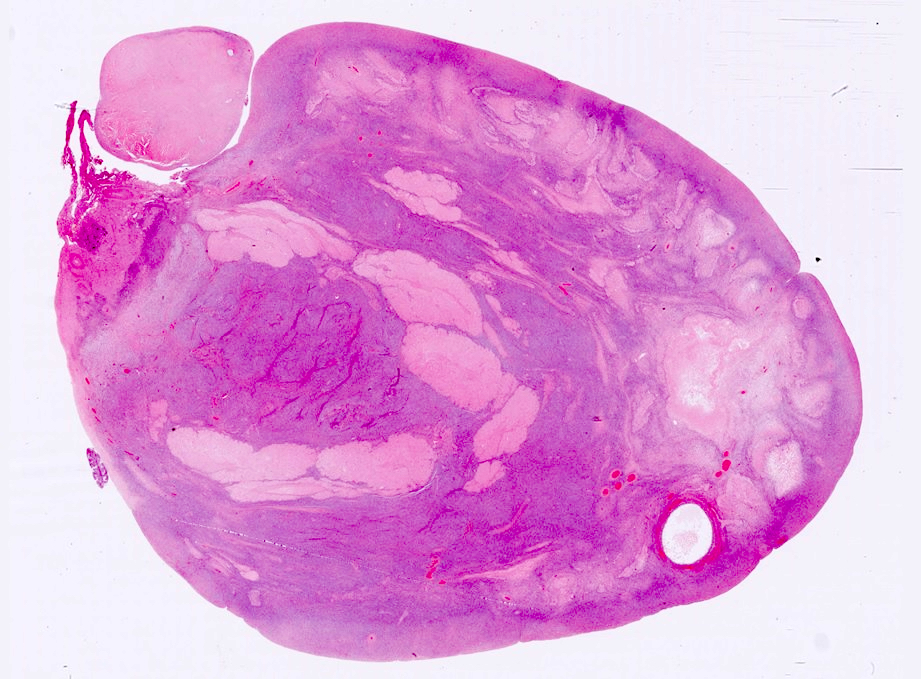
Gross description
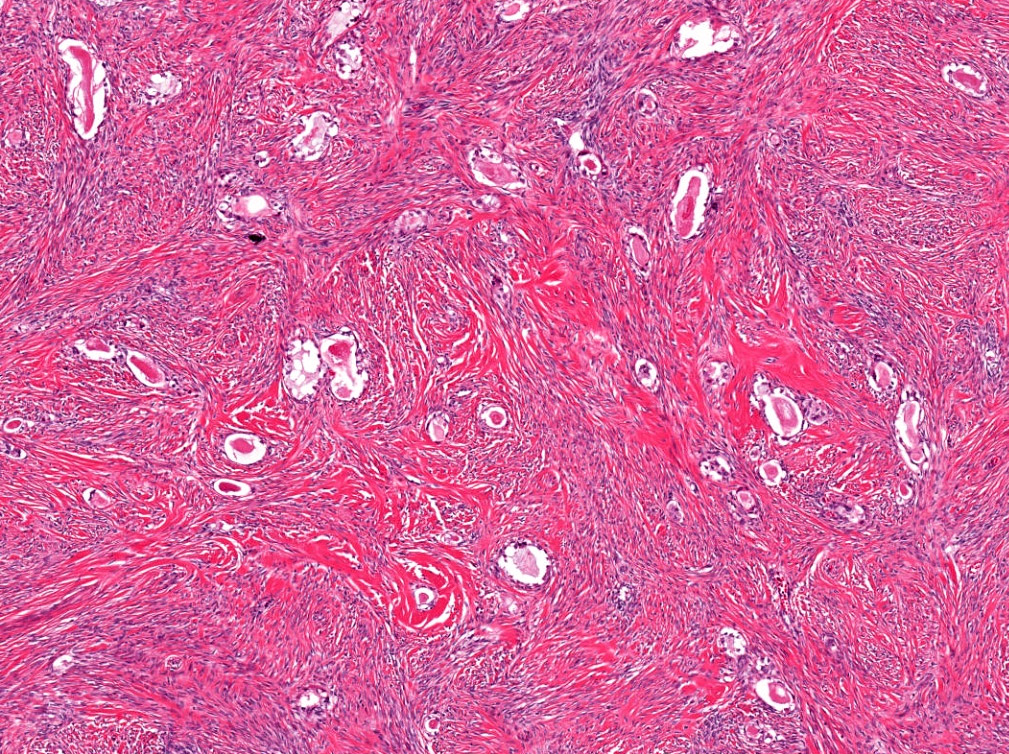
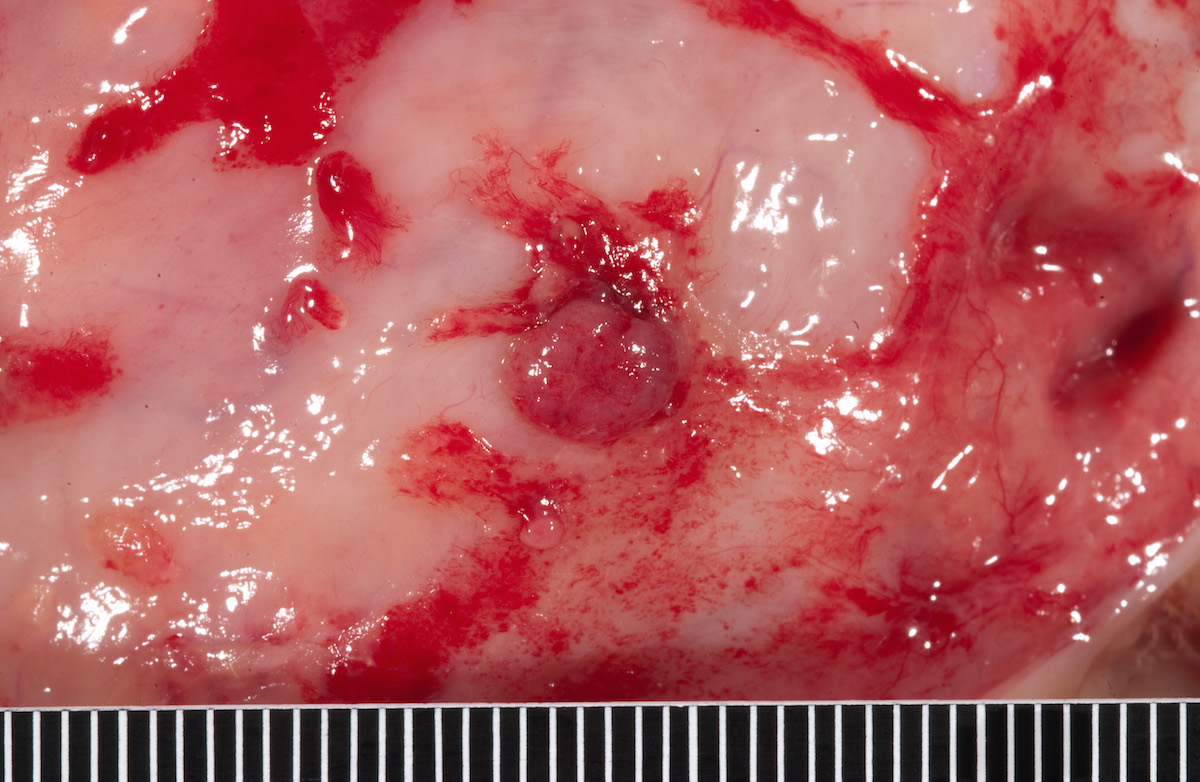
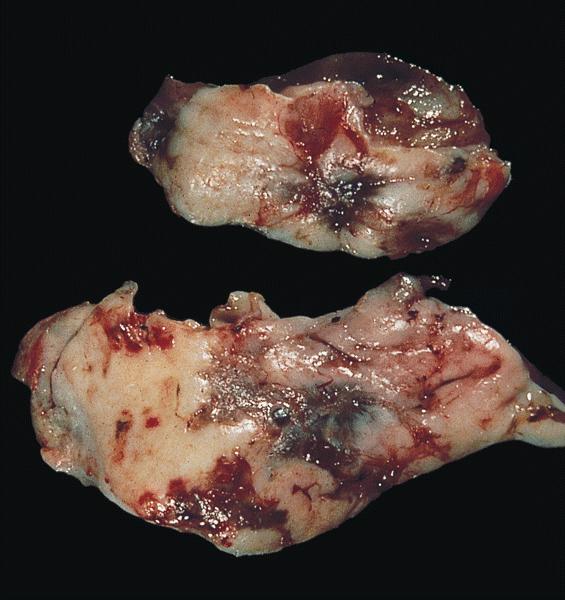
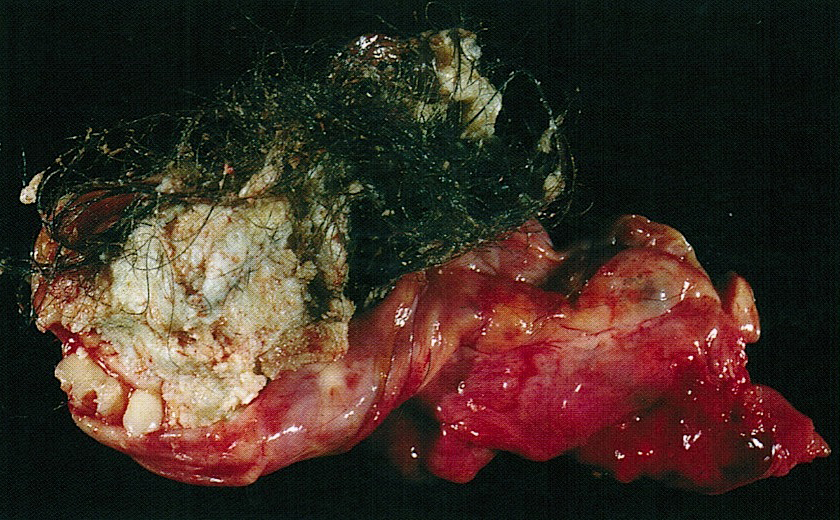
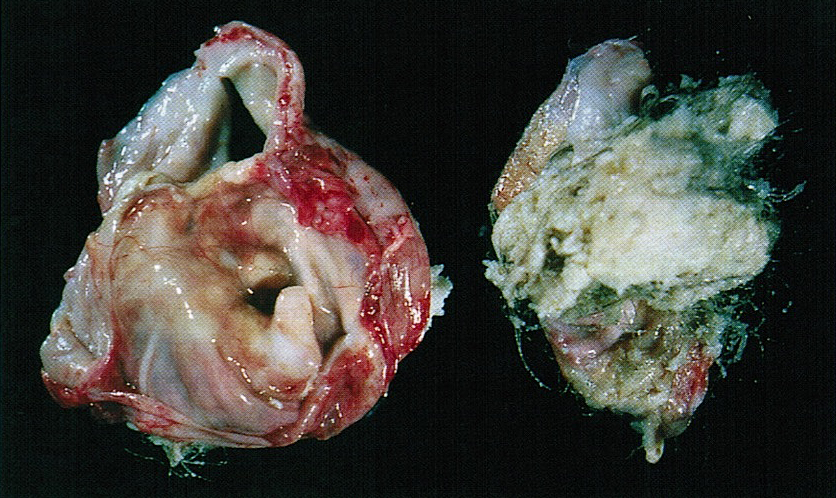
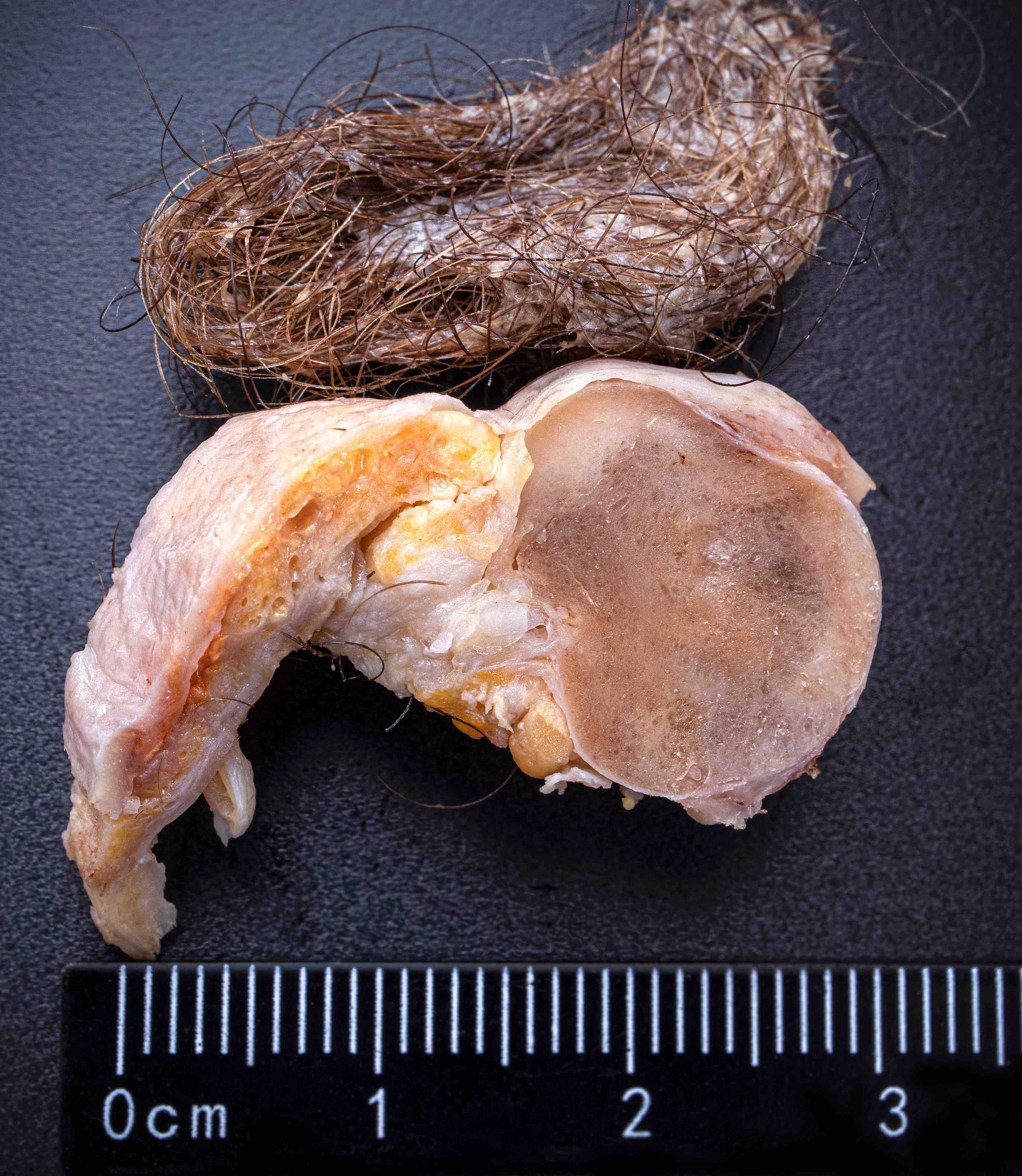
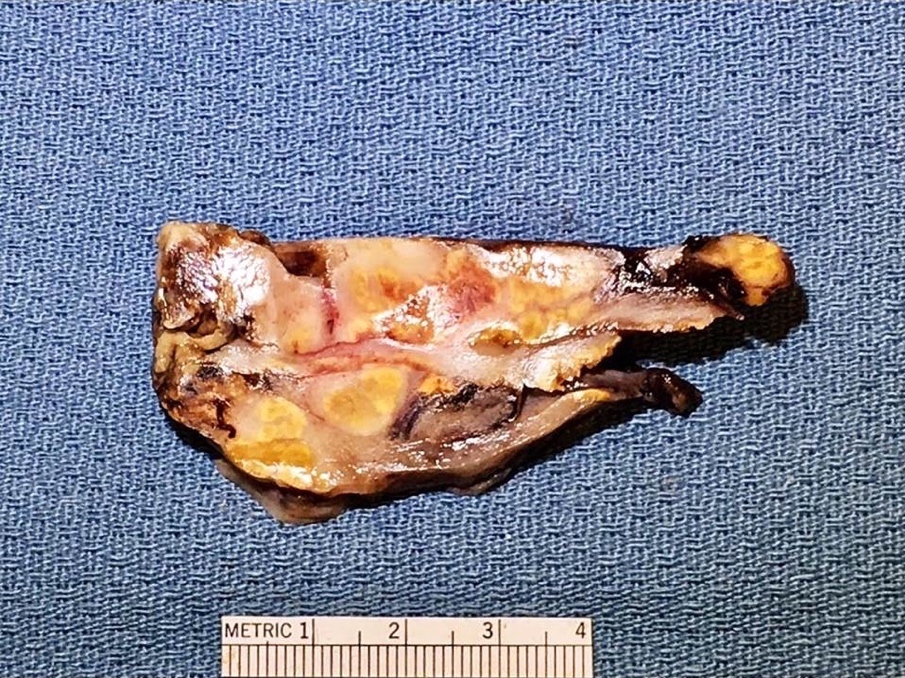
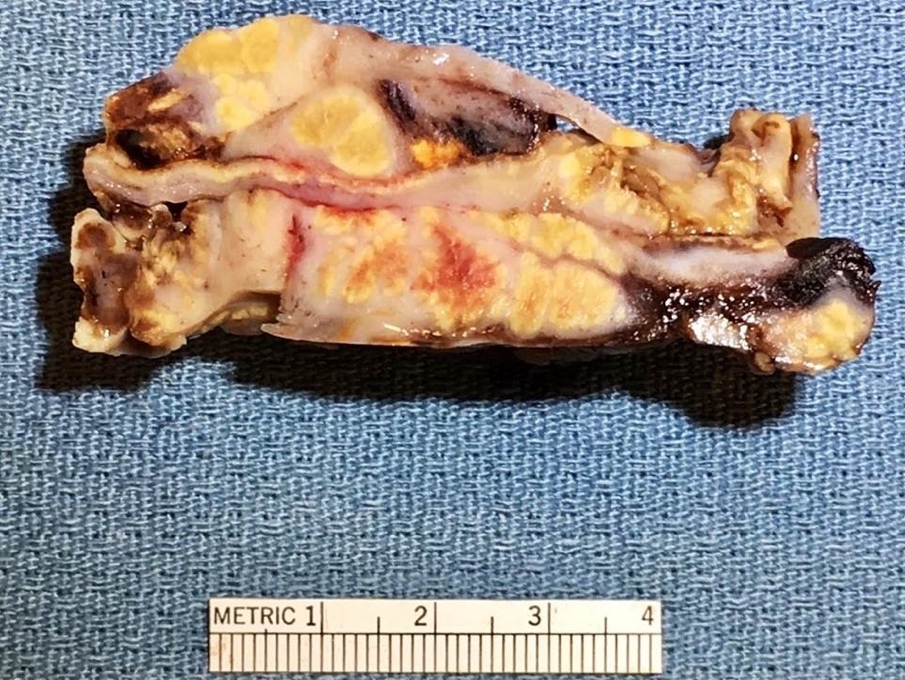
- Usually present as firm, solid and homogeneous gray to yellow masses in association with mature teratoma or mucinous tumor (Curr Opin Obstet Gynecol 1997;9:44)
- If pure (Curr Opin Obstet Gynecol 1997;9:44)
- Strumal carcinoid: solid yellow-brown nodule with fleshy areas
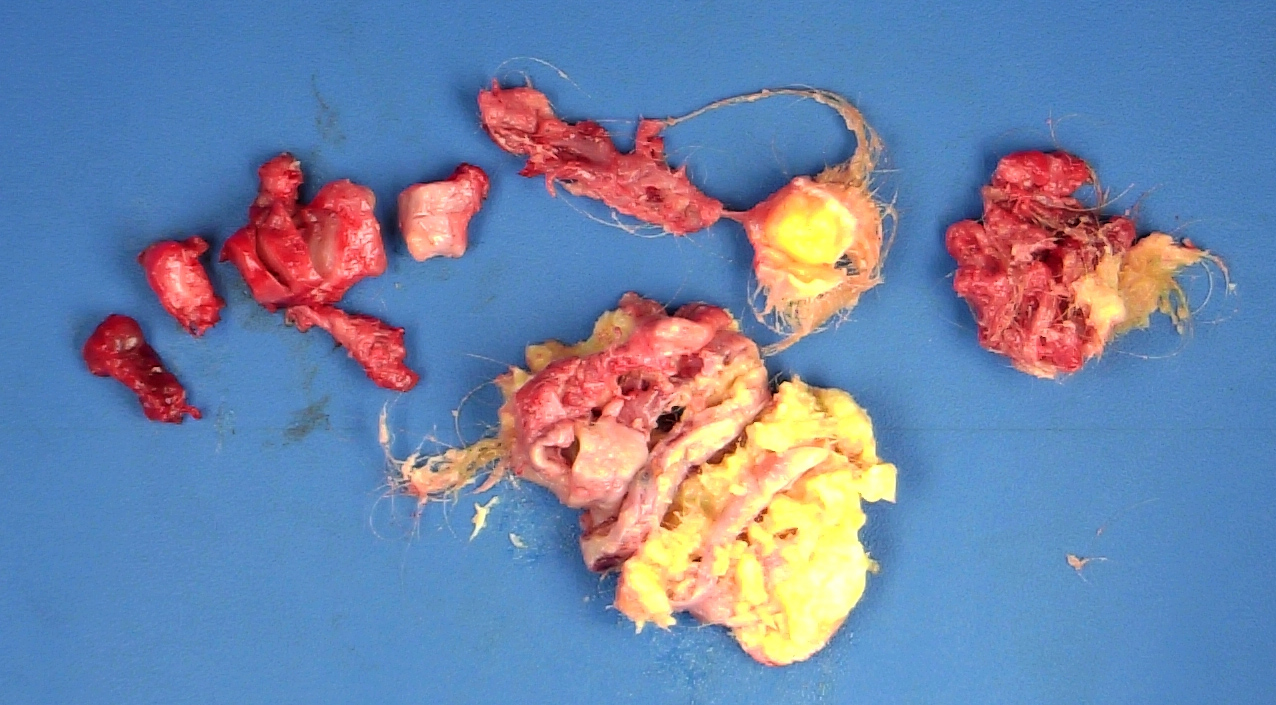
- Mucinous carcinoid: gray-yellow, firm, usually solid but may contain cystic areas even when seen in a pure form; most tumors > 8 cm
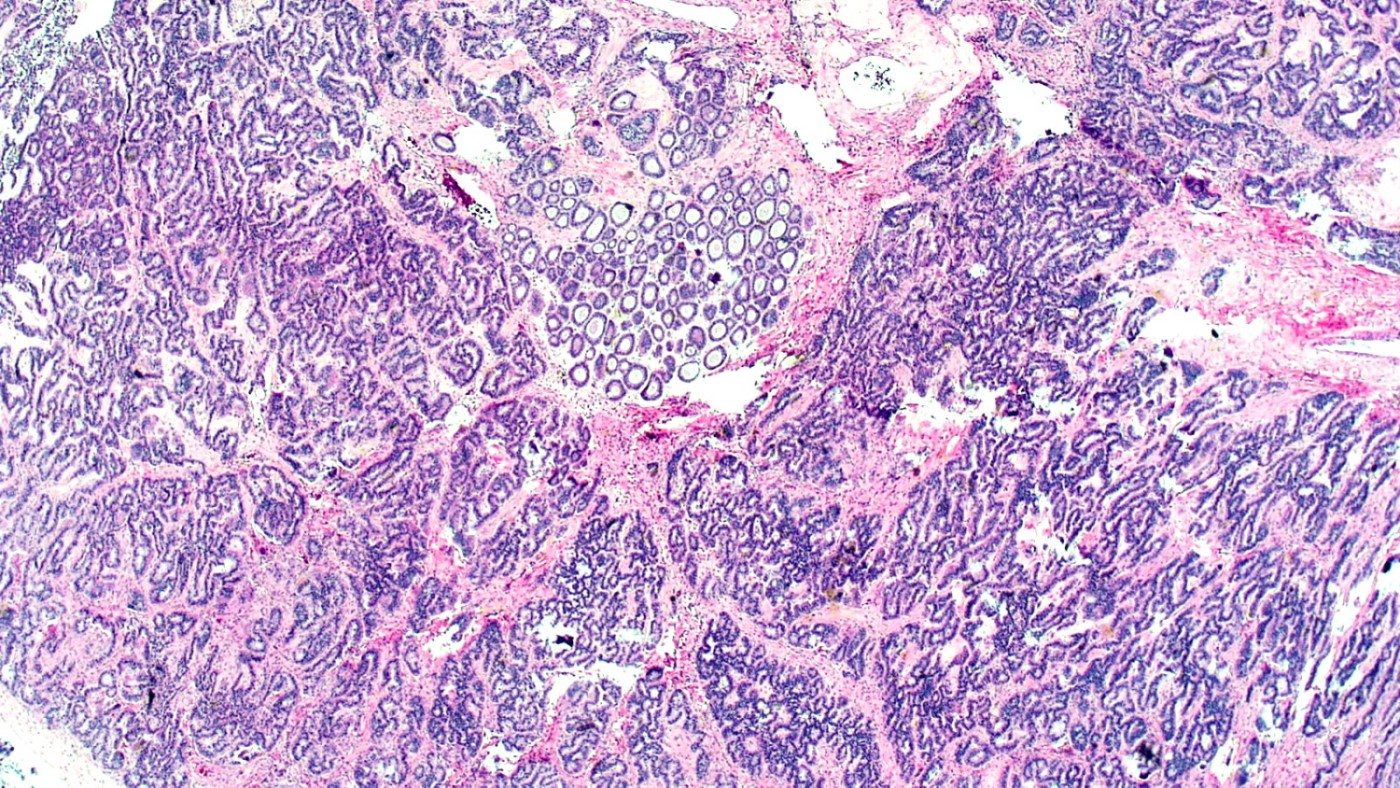
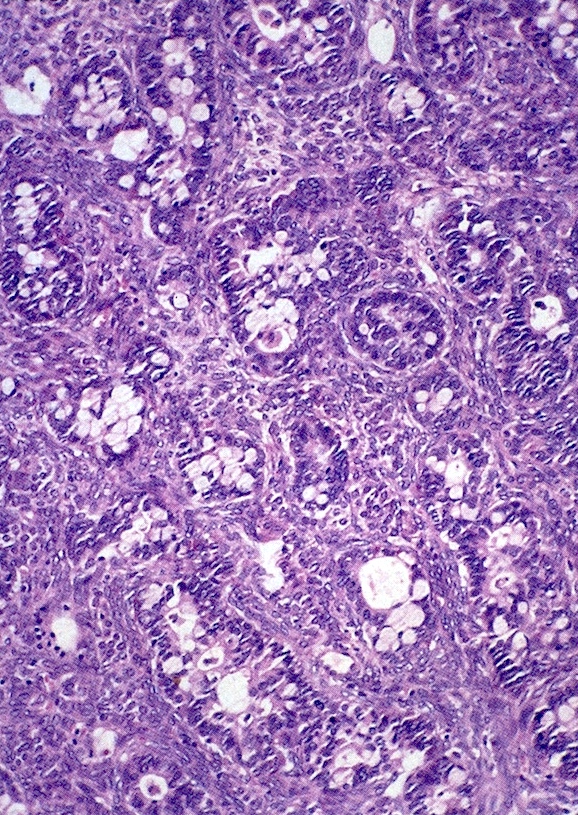
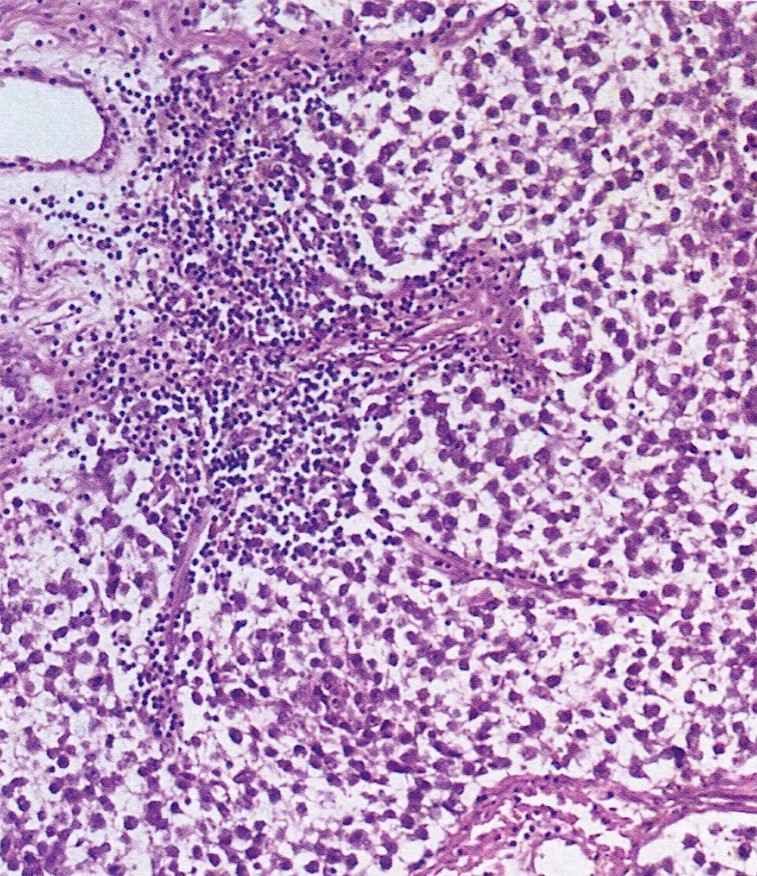
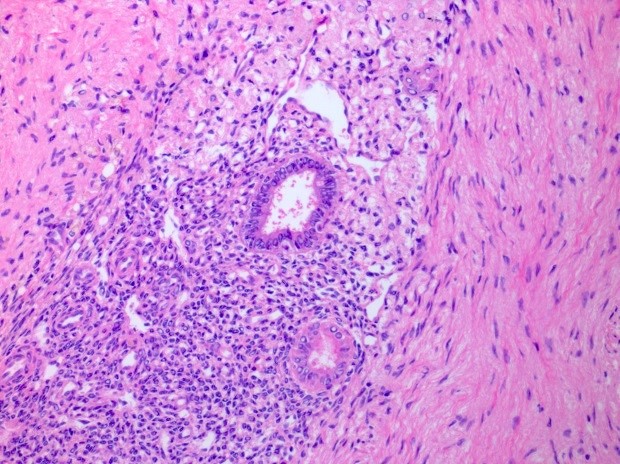
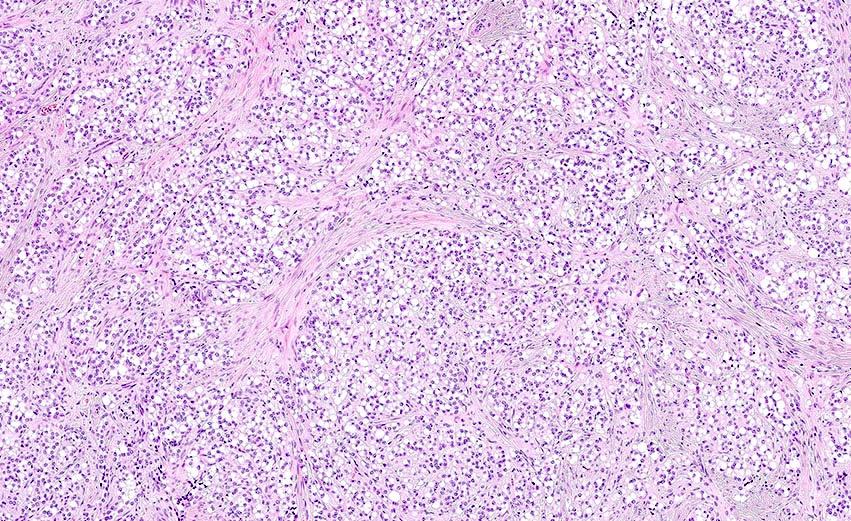
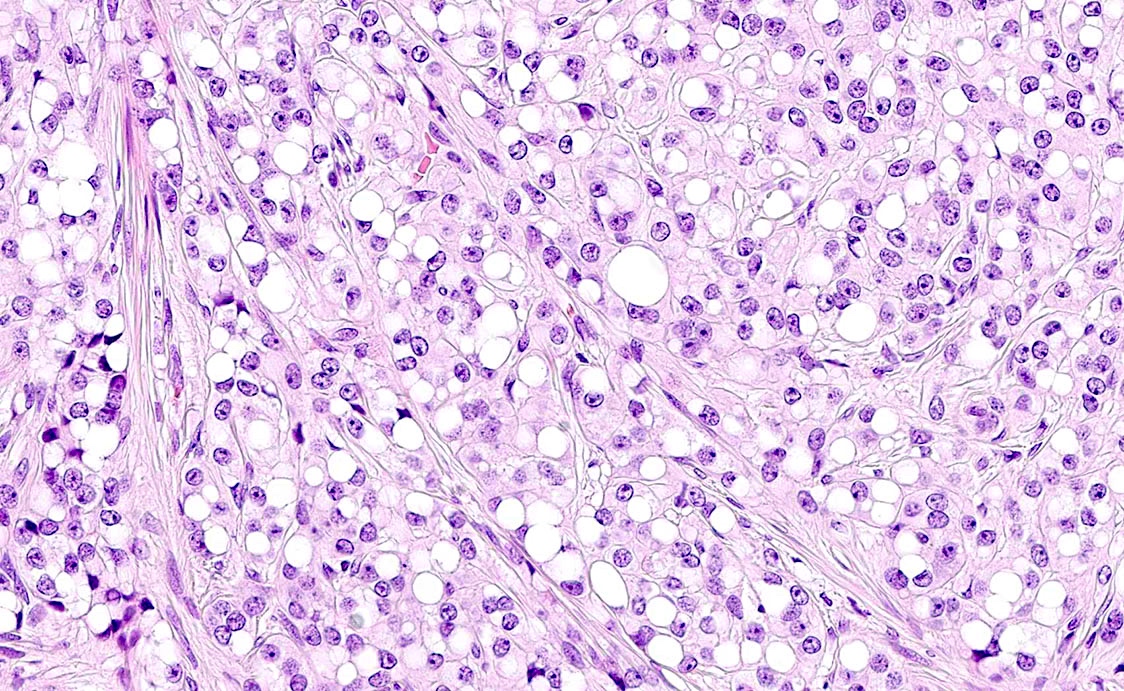
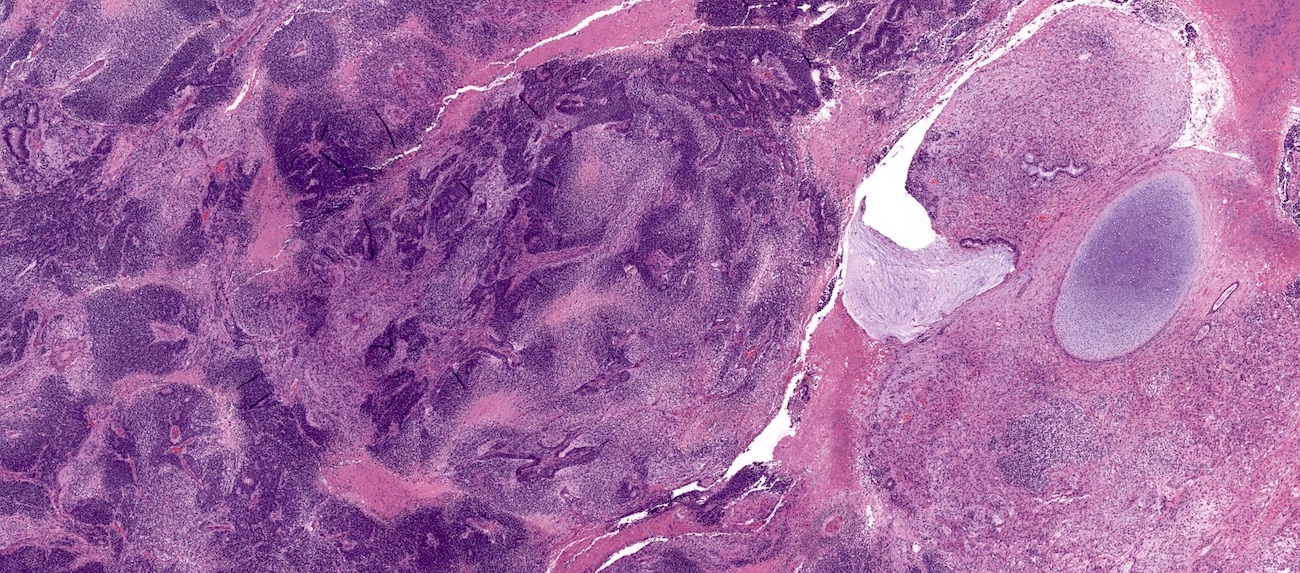
Microscopic (histologic) description
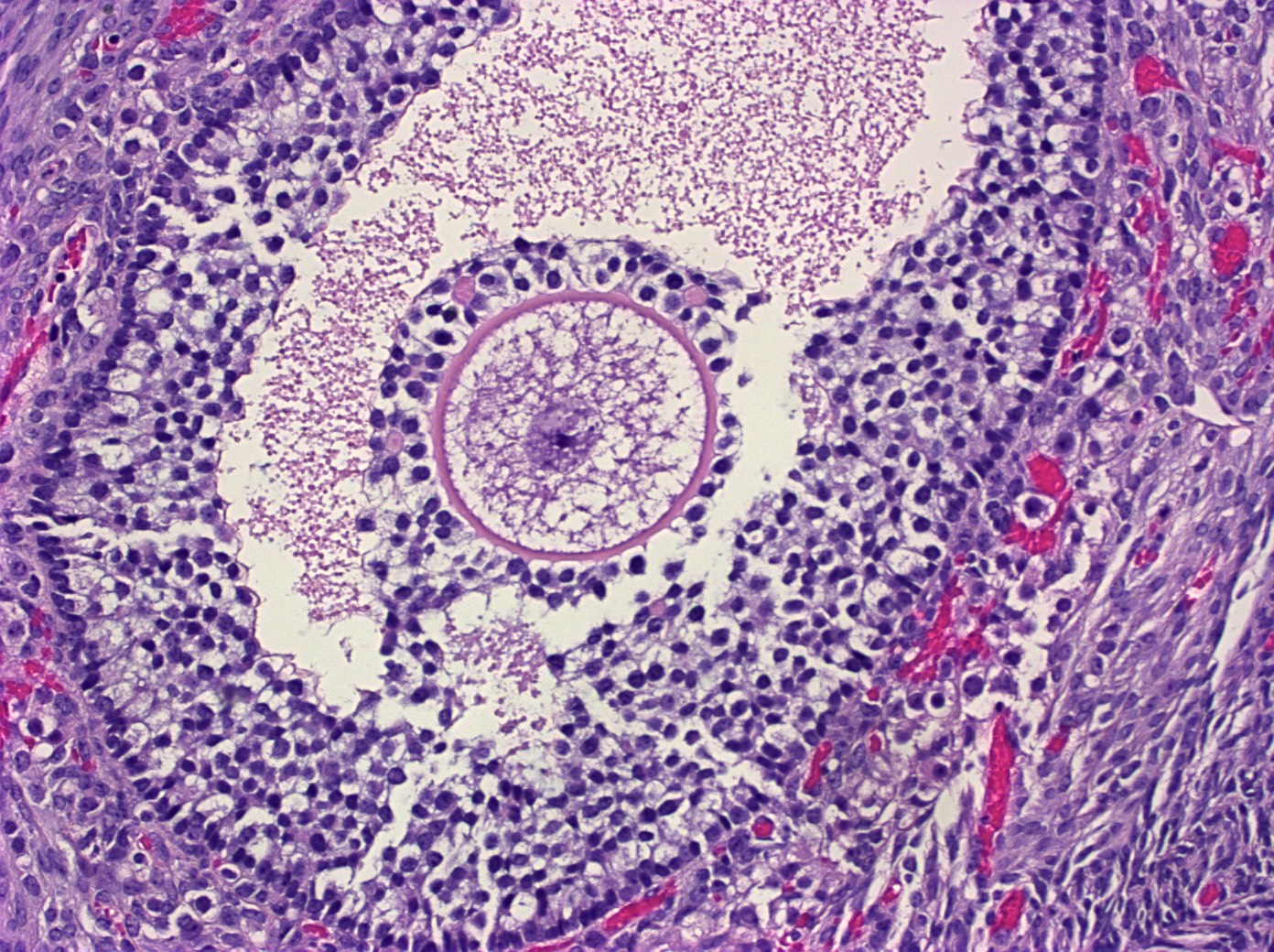
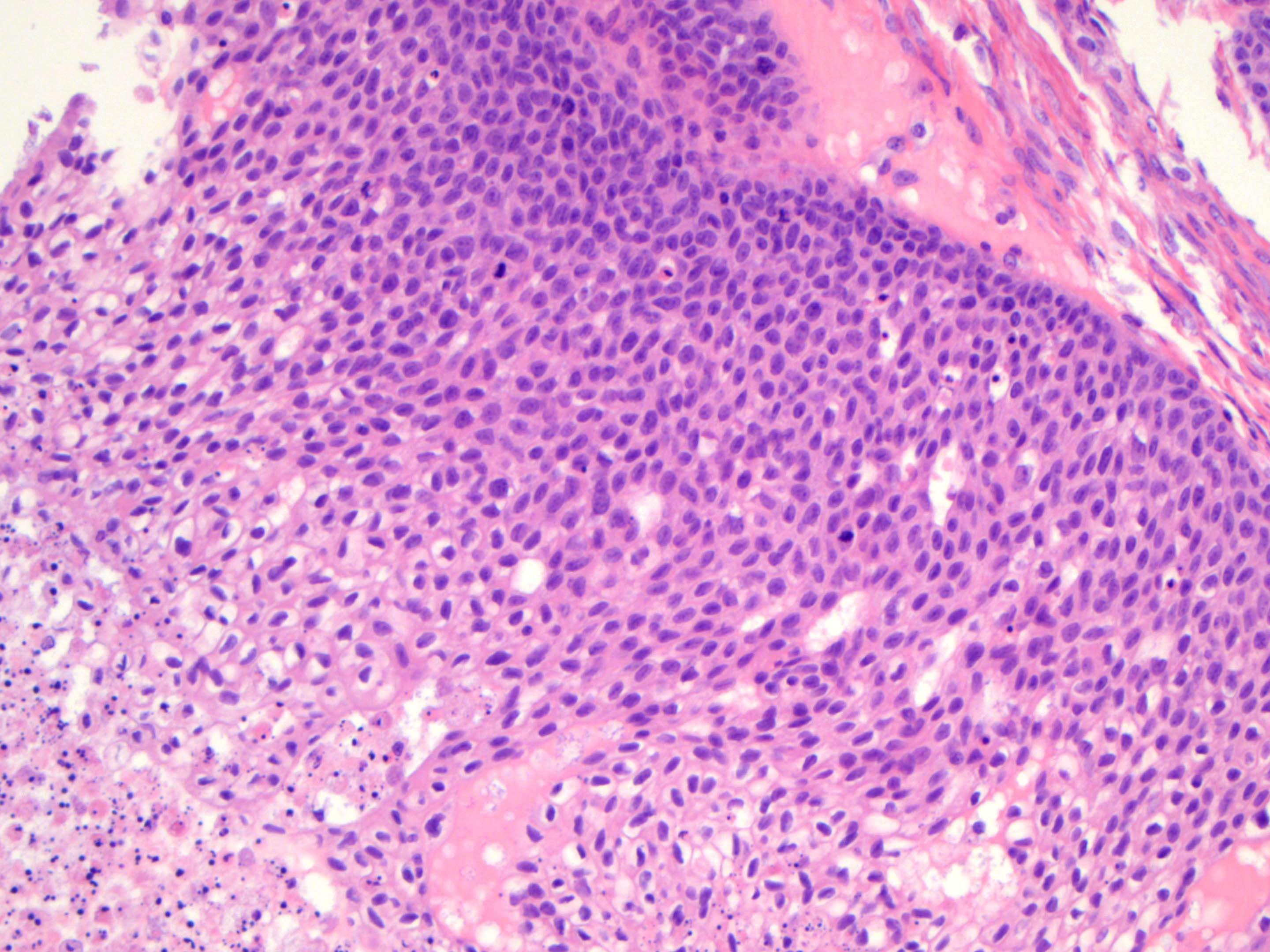
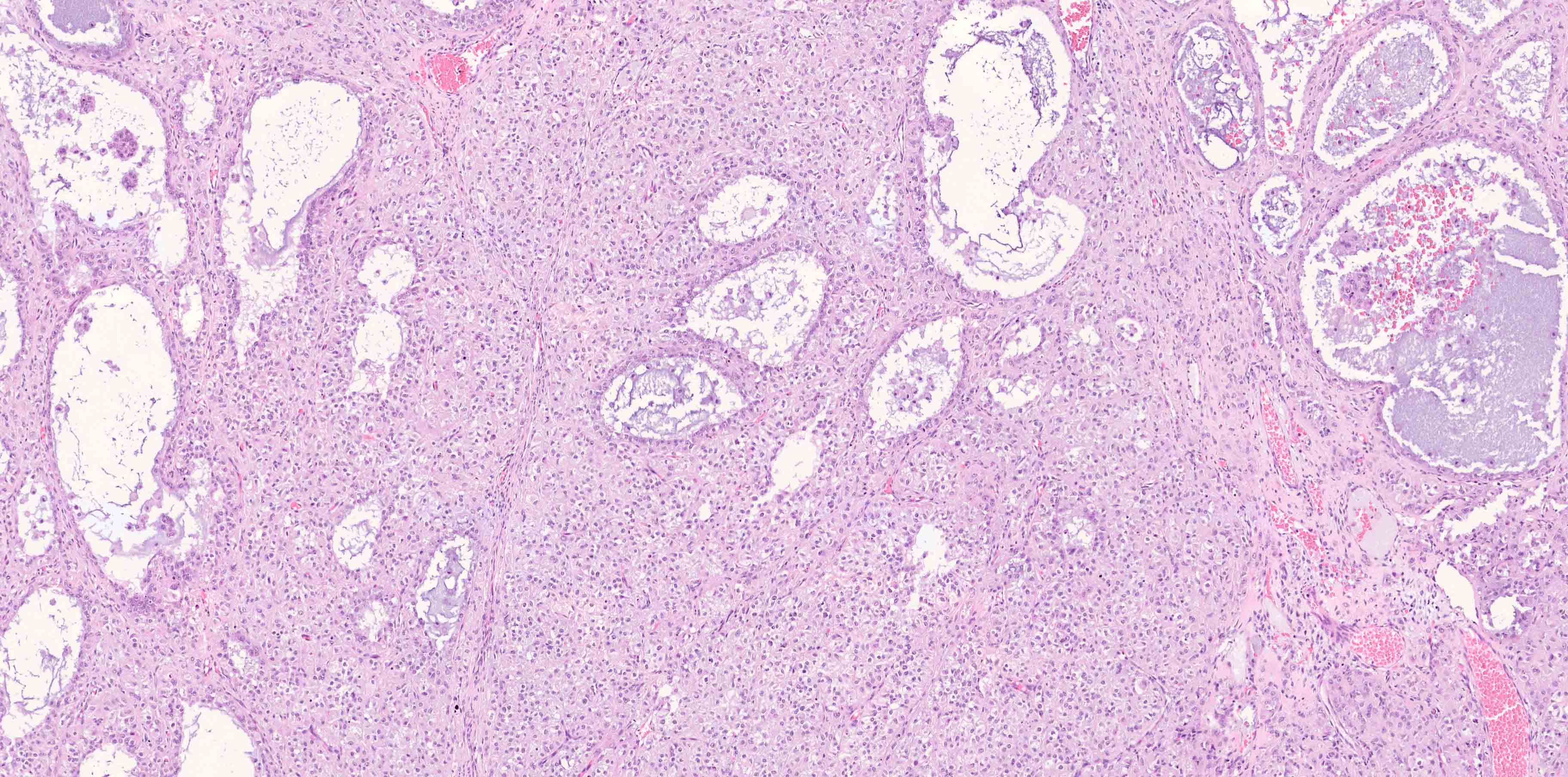
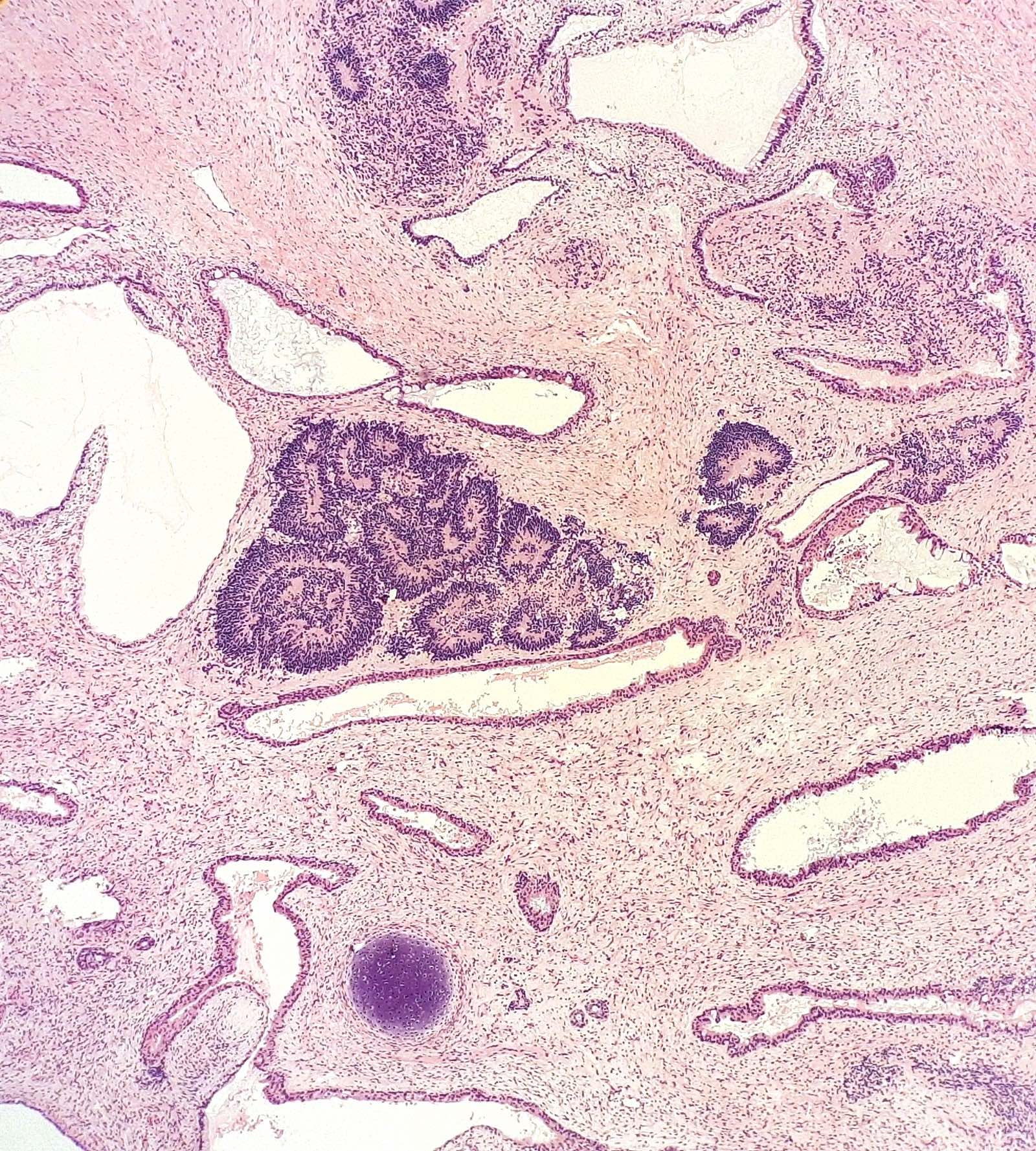
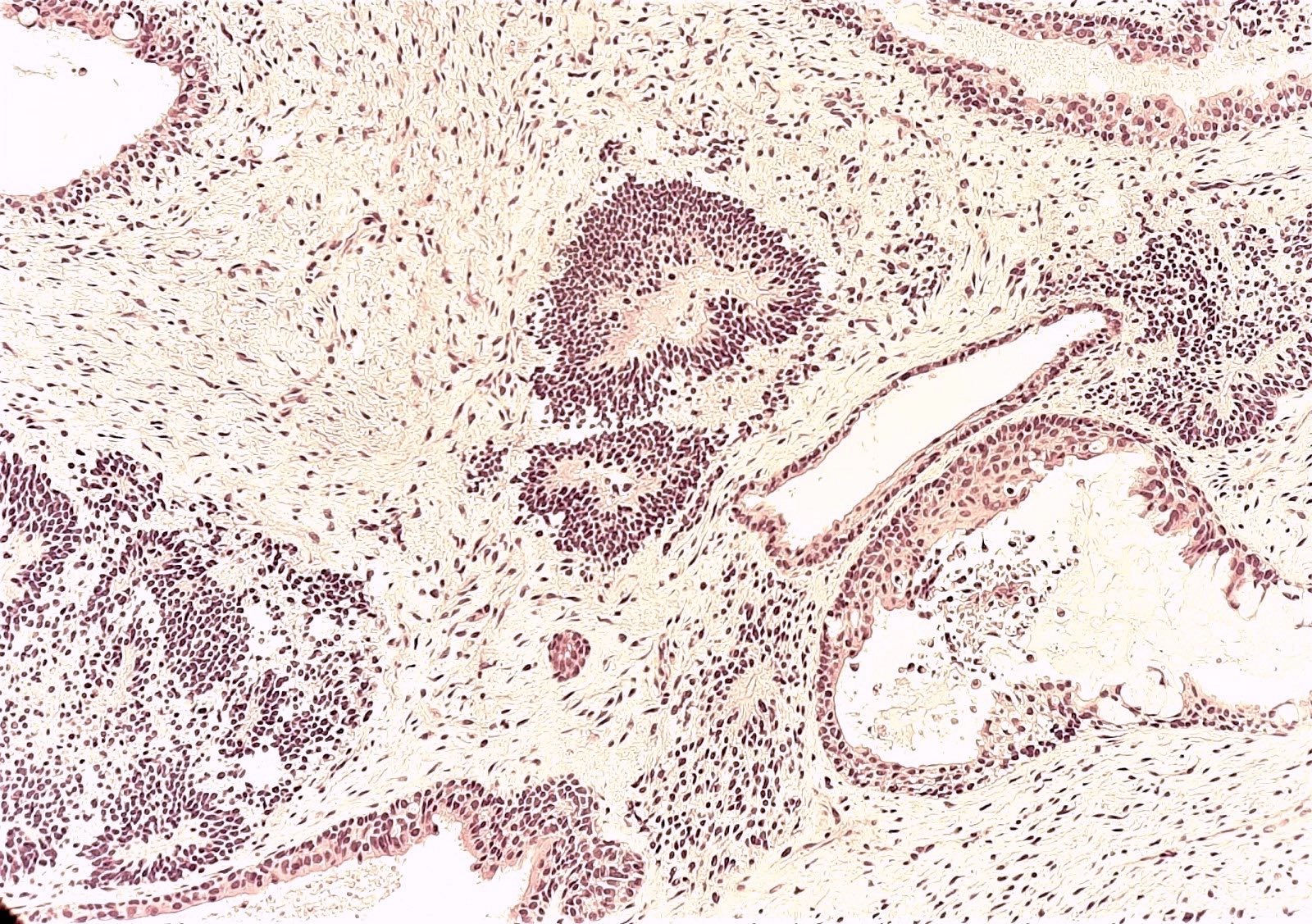
- 4 types (J Int Med Res 2021;49:3000605211034666)
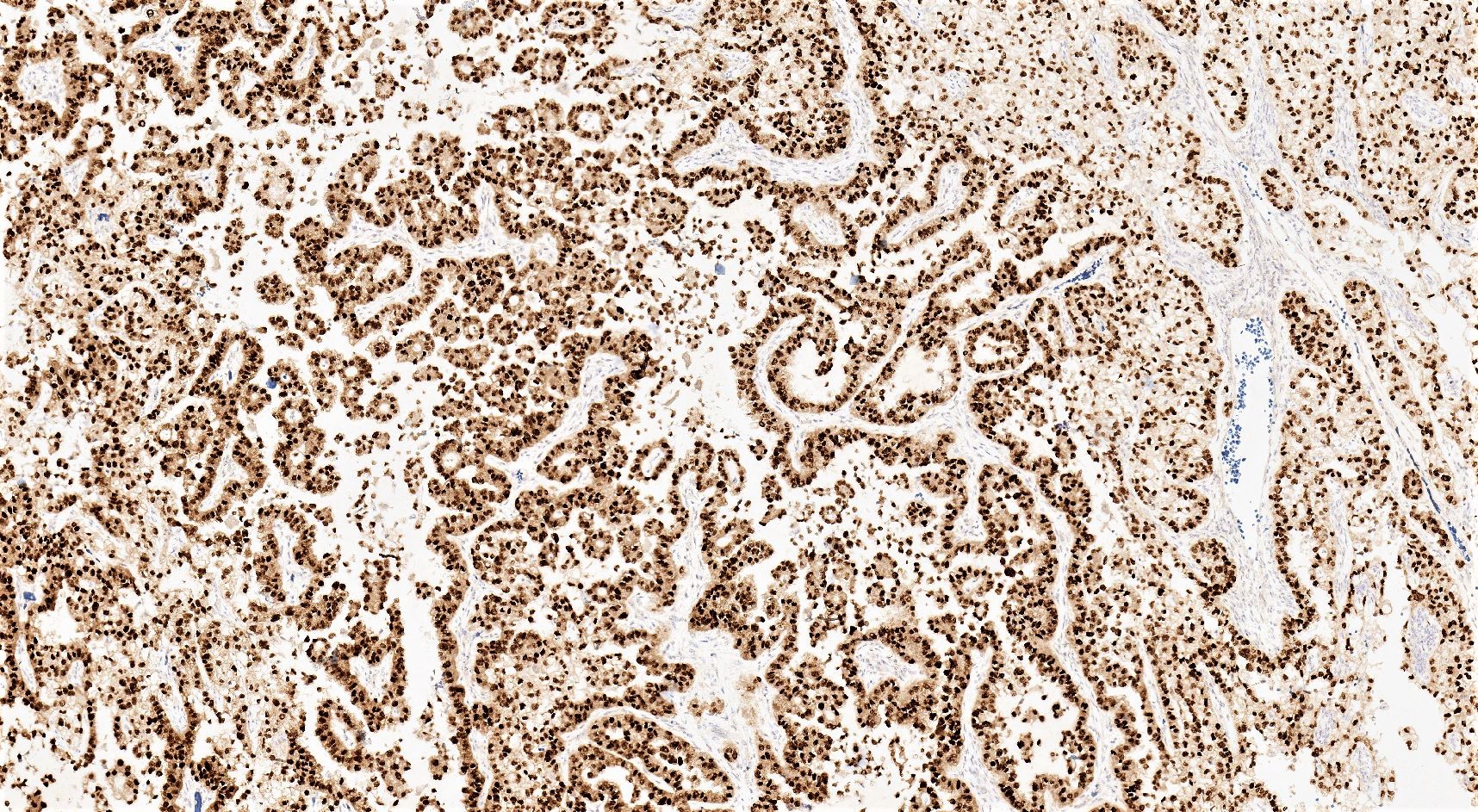
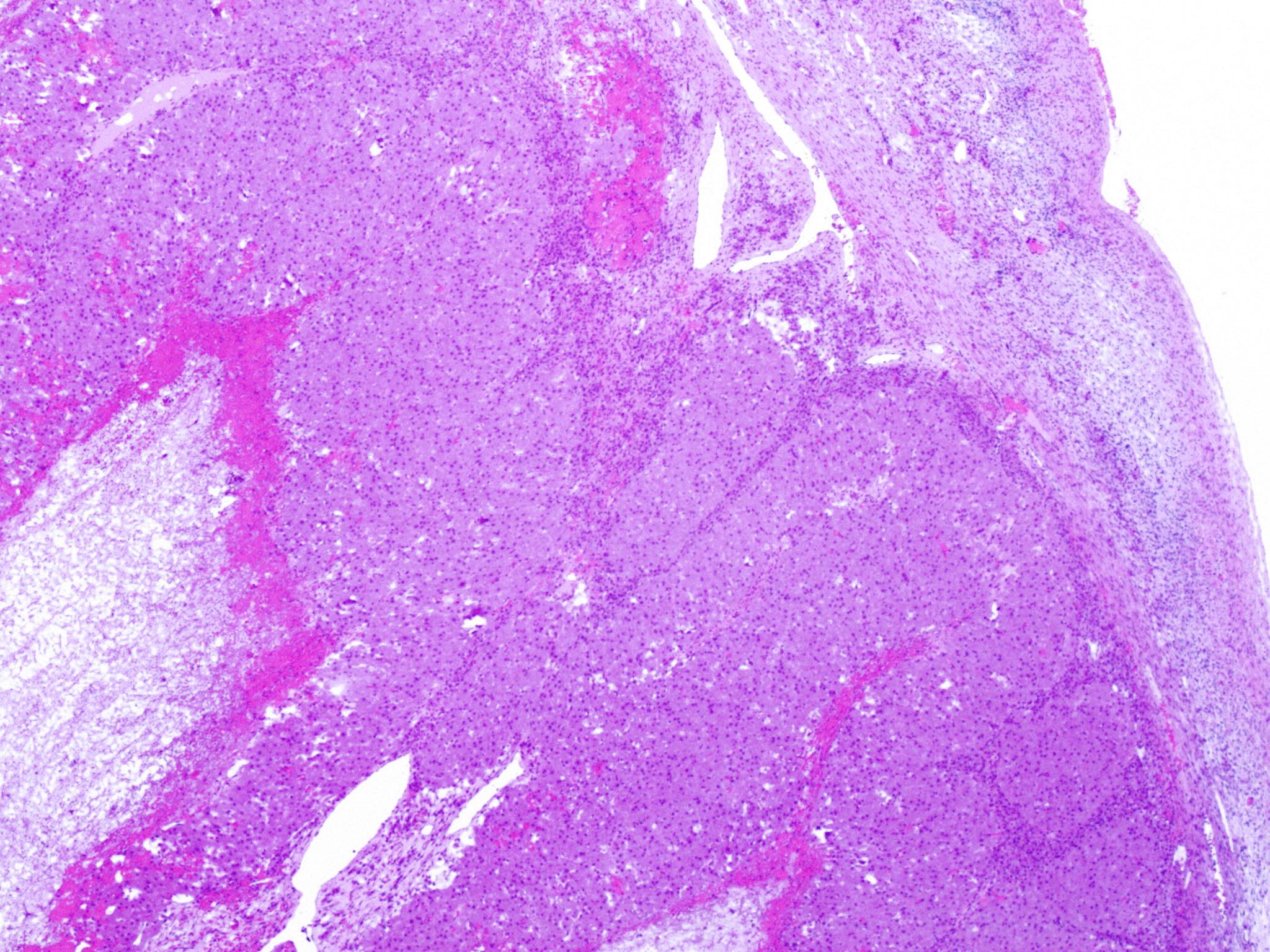
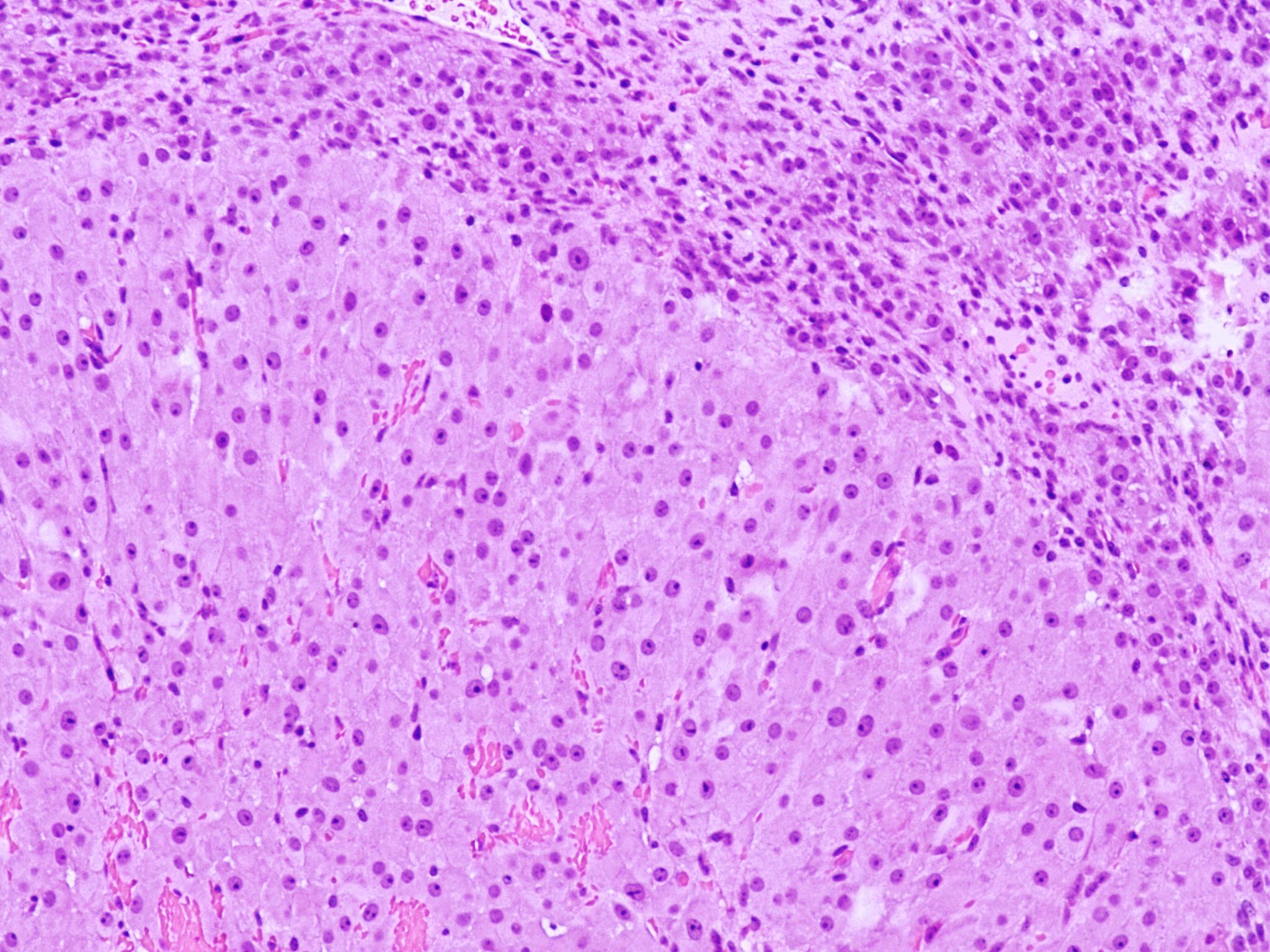
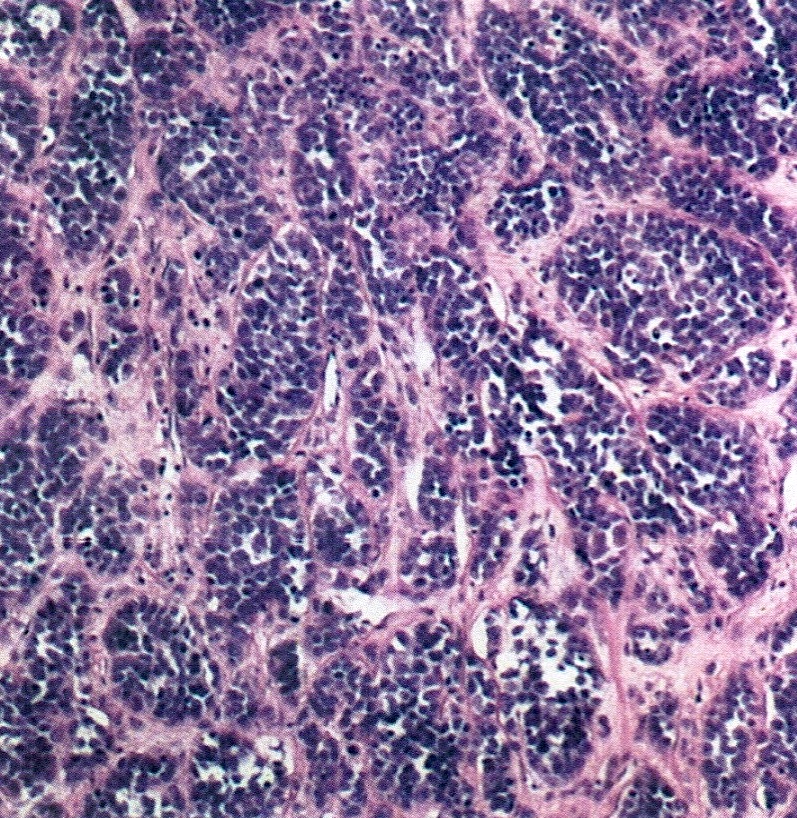
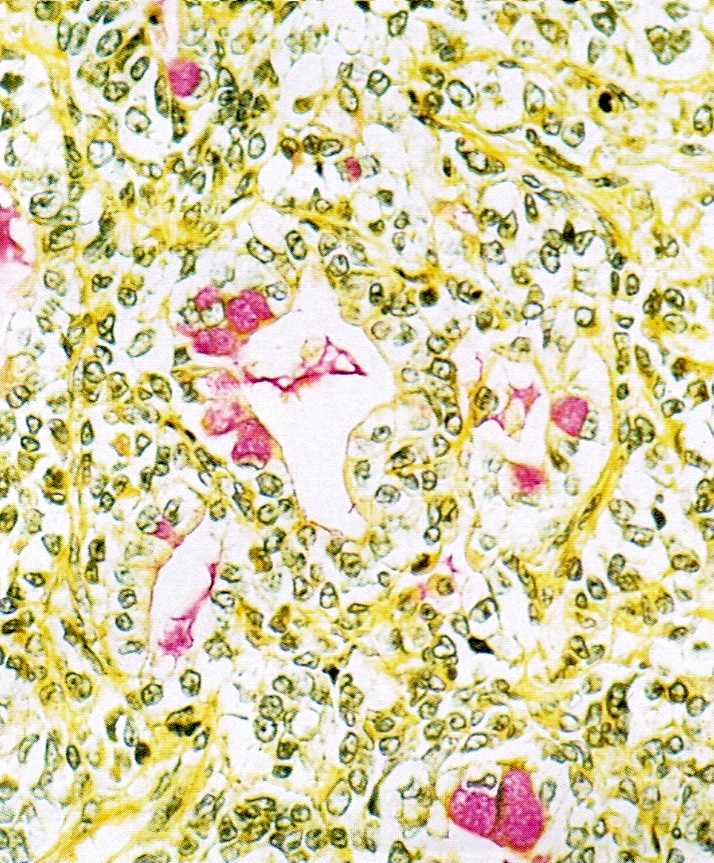
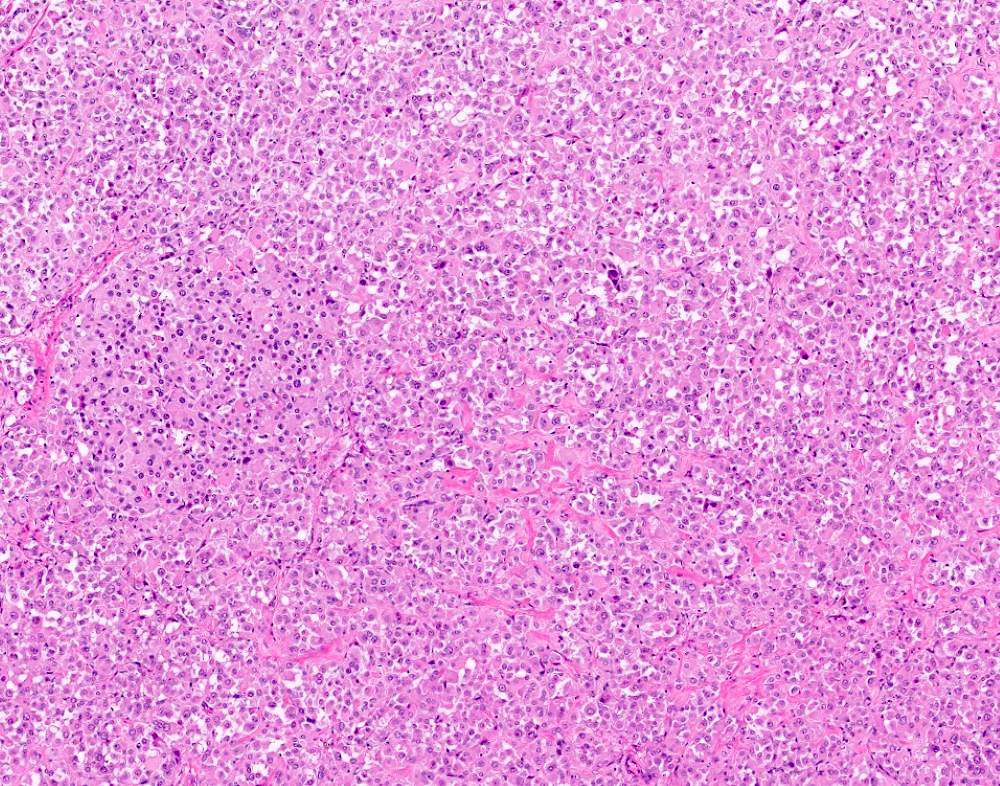
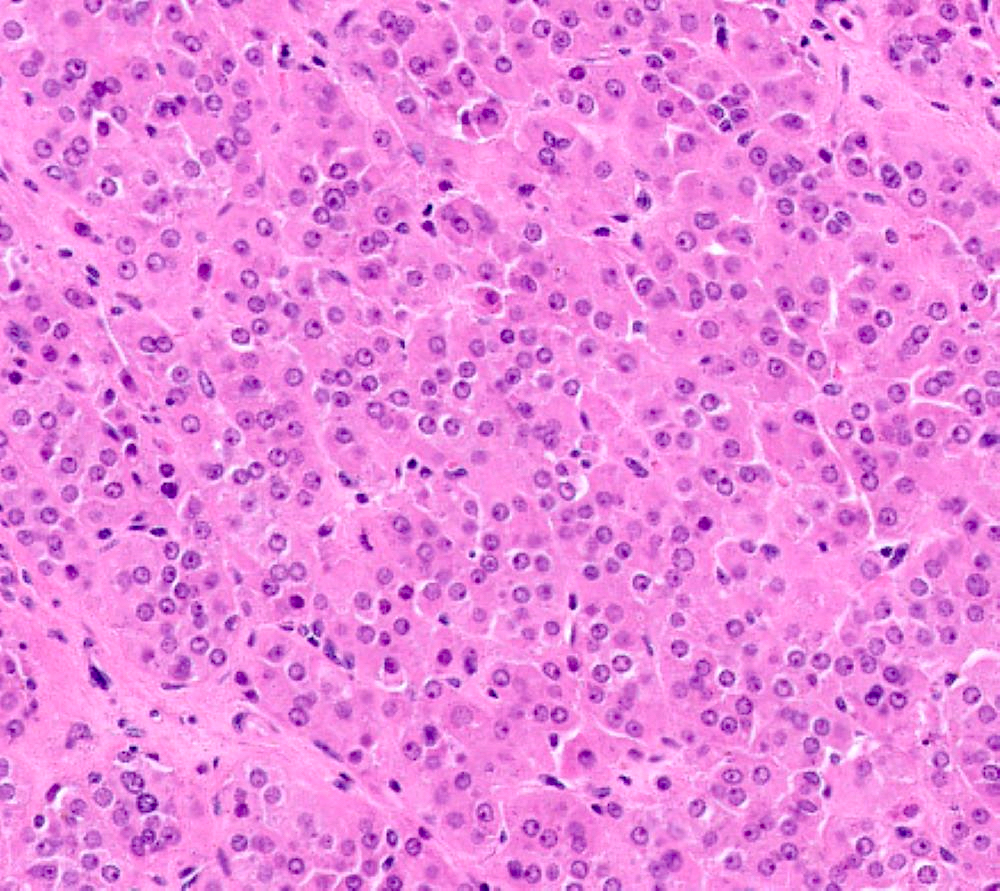
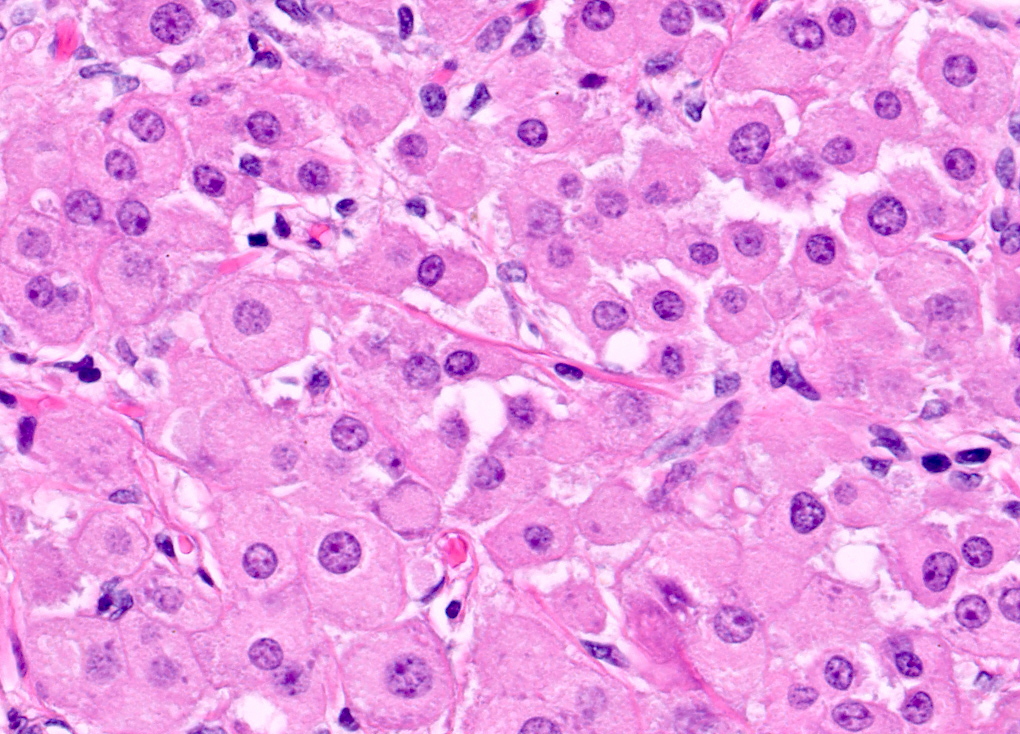
- Insular carcinoid
- Most common type of ovarian carcinoid
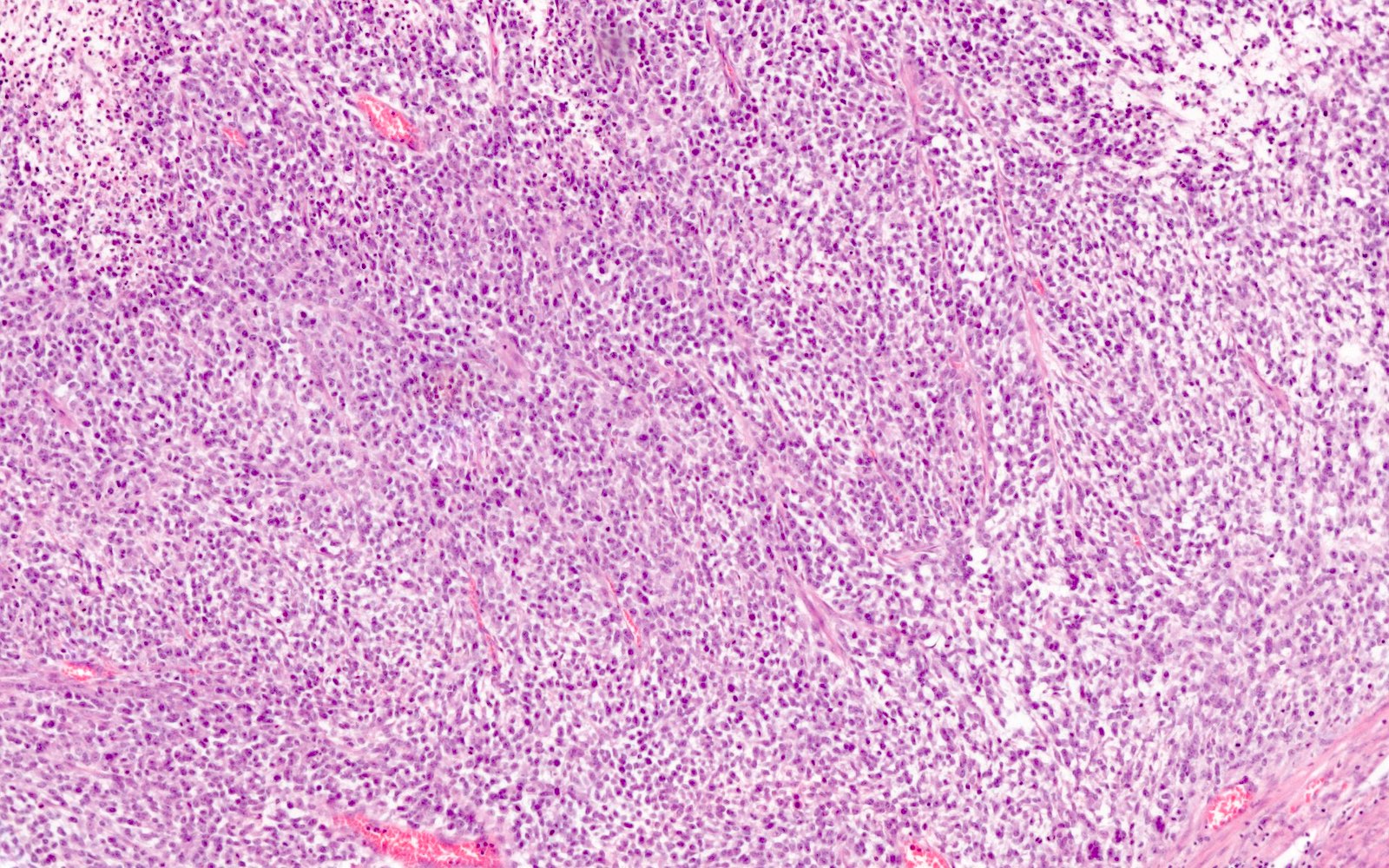
- Growth pattern usually shows large islands, variable sized nests, small acini or glands within the fibrous stroma
- Simple tubular glands, cribriform nests may also be seen
- Retraction artifact around tumor cells is common
- Composed of uniform polygonal epithelial cells with abundant cytoplasm and round, centrally located nuclei; cytoplasm contains red-brown granules, located basally
- Secretions in lumina, psammoma bodies
- Stroma is dense and hyalinized
- Low mitotic activity
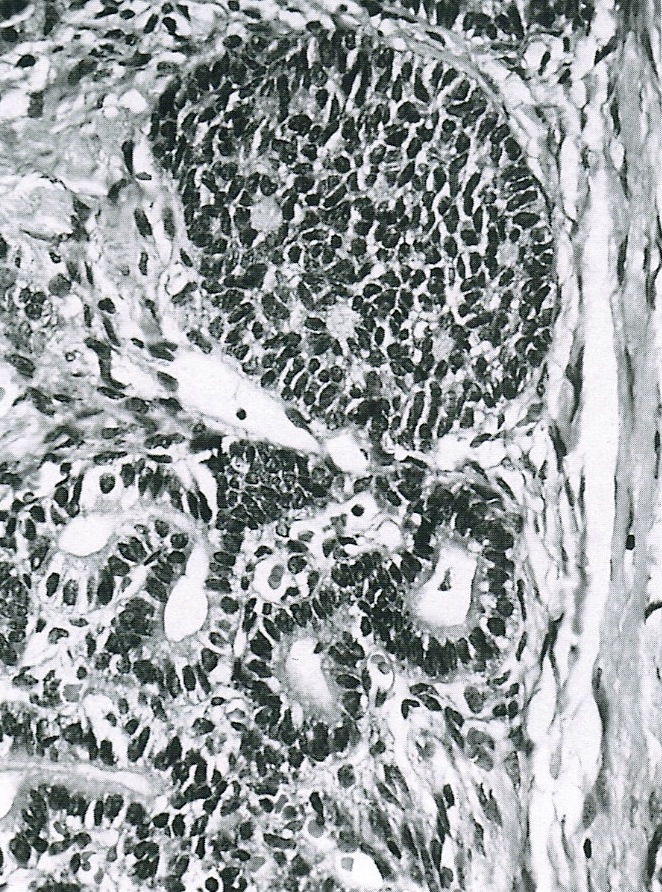
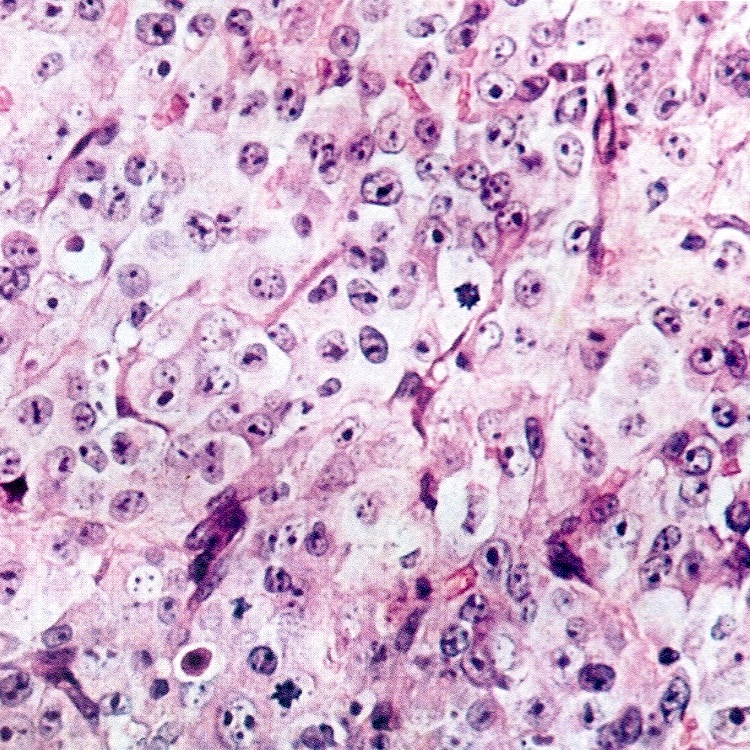
- Trabecular carcinoid
- Associated with mature cystic teratoma, rare in pure form
- 1 or 2 cell layers arranged in long, wavy, branching cords, parallel ribbons or trabeculae
- Occasionally, acinar pattern may be seen admixed with trabecular pattern
- Tumor cells are uniform, round to oval, with amphophilic, slightly granular cytoplasm at the base and prominent, centrally located, ovoid or elongated nuclei with finely stippled chromatin; red-brown granules may be seen at the cell base
- Stroma is represented by dense fibrous connective tissue, which may be hyalinized or luteinized
- Low mitotic activity, only occasional mitoses
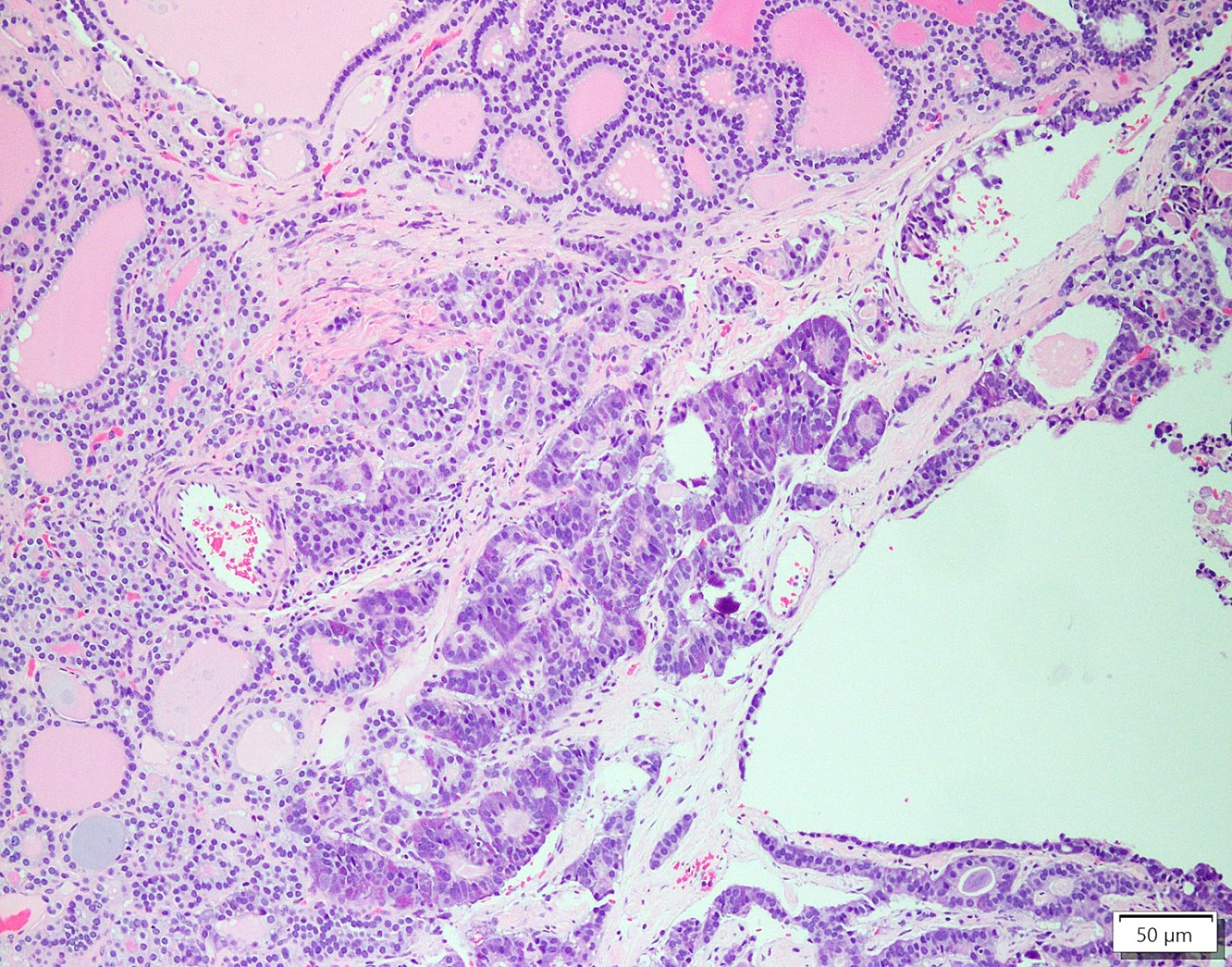
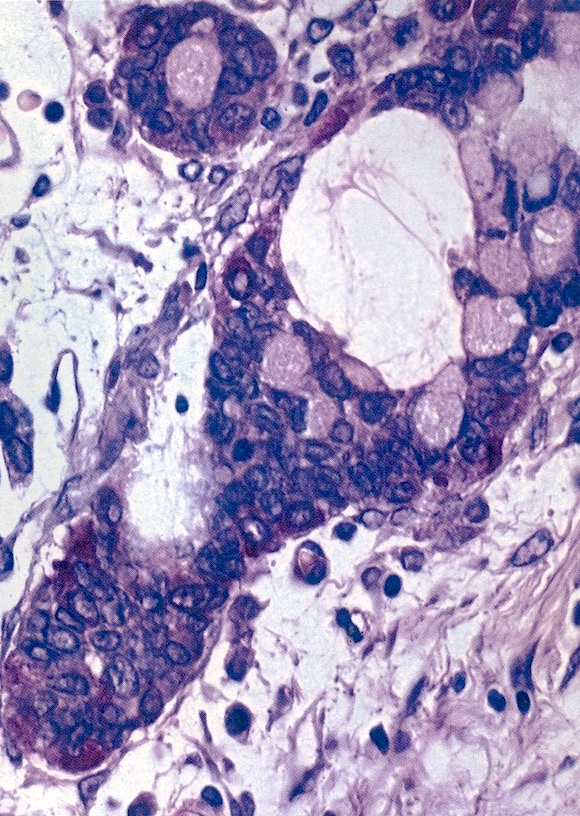
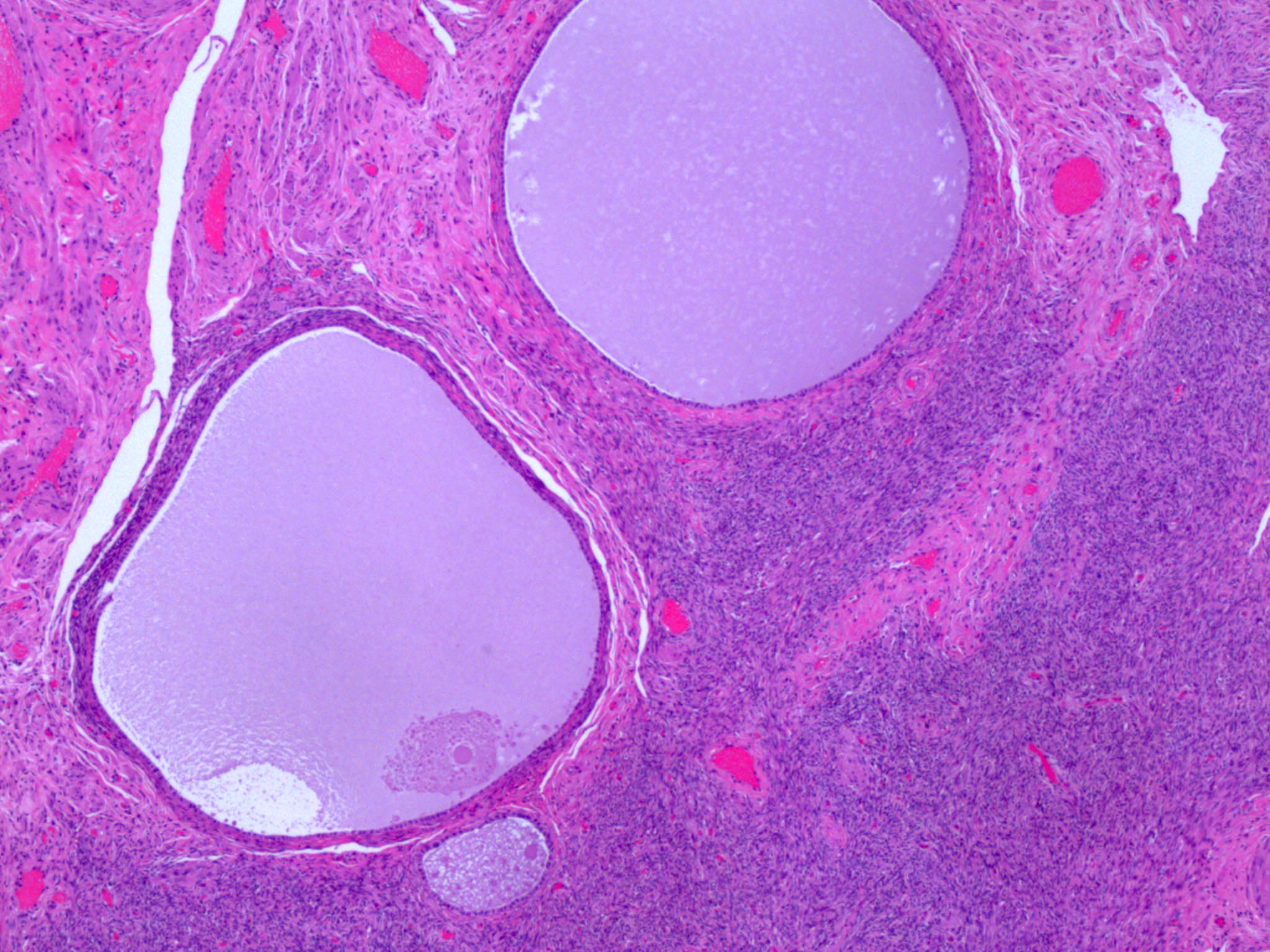
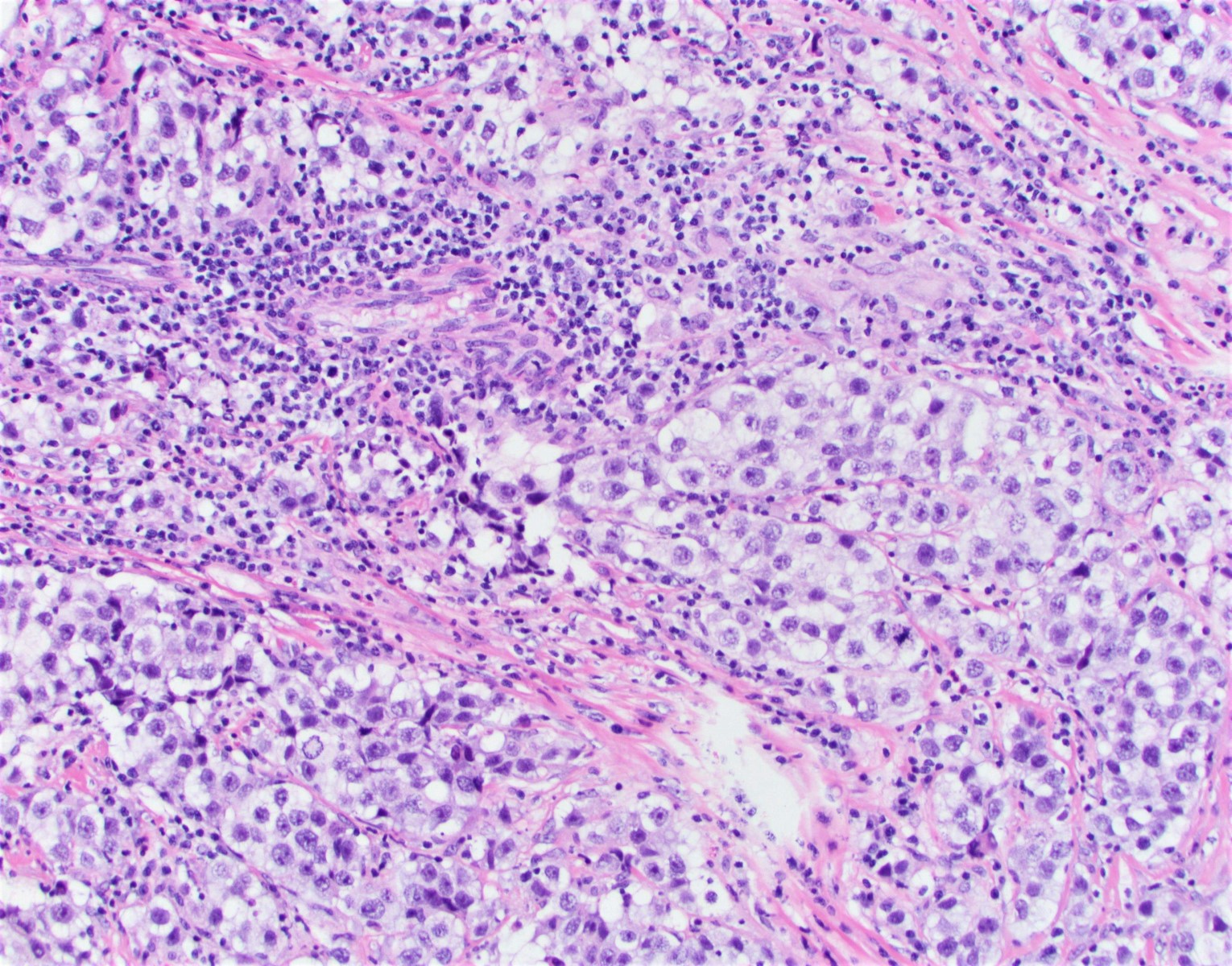
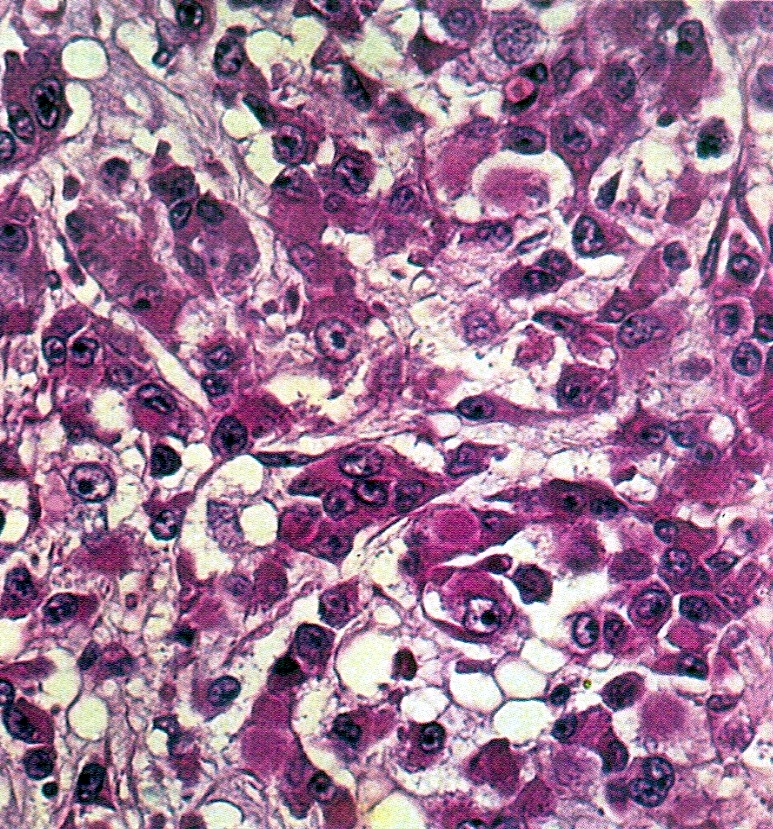
- Strumal carcinoid
- Commonly associated with mature cystic teratoma; often is seen in pure form (40%); rarely can be associated with mucinous tumor (J Cancer Res Clin Oncol 1984;107:125, J Int Med Res 2021;49:3000605211034666, Front Endocrinol (Lausanne) 2022;13:871210)
- Composed of thyroid tissue intimately admixed or juxtaposed with a neuroendocrine neoplasm (struma ovarii and carcinoid)
- Either of the components may be predominant
- Trabecular pattern >> both trabecular and insular patterns > insular only
- Thyroid component resembles normal thyroid tissue; rarely, papillary or follicular carcinoma may be present
- Stroma may show luteinization
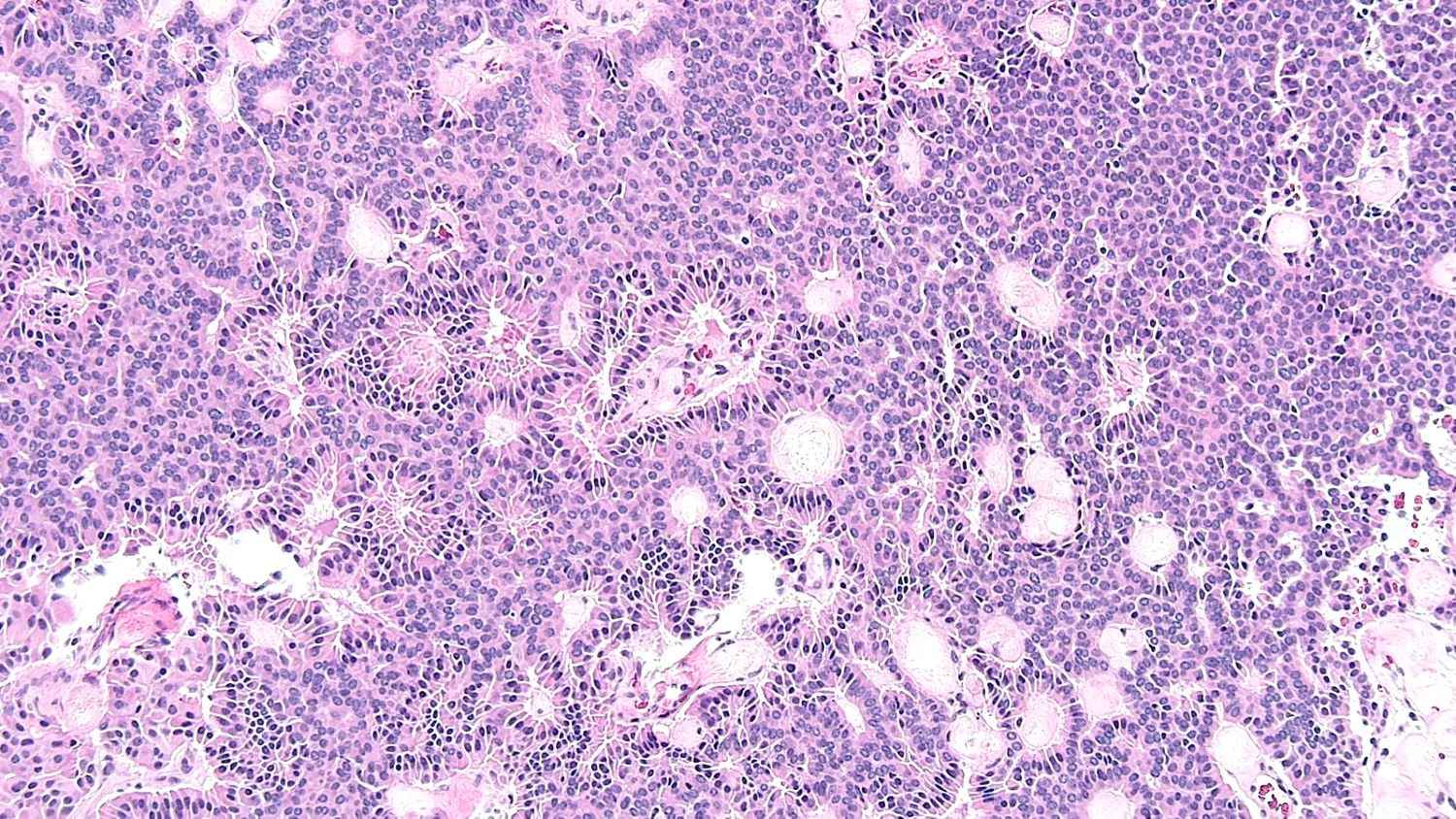
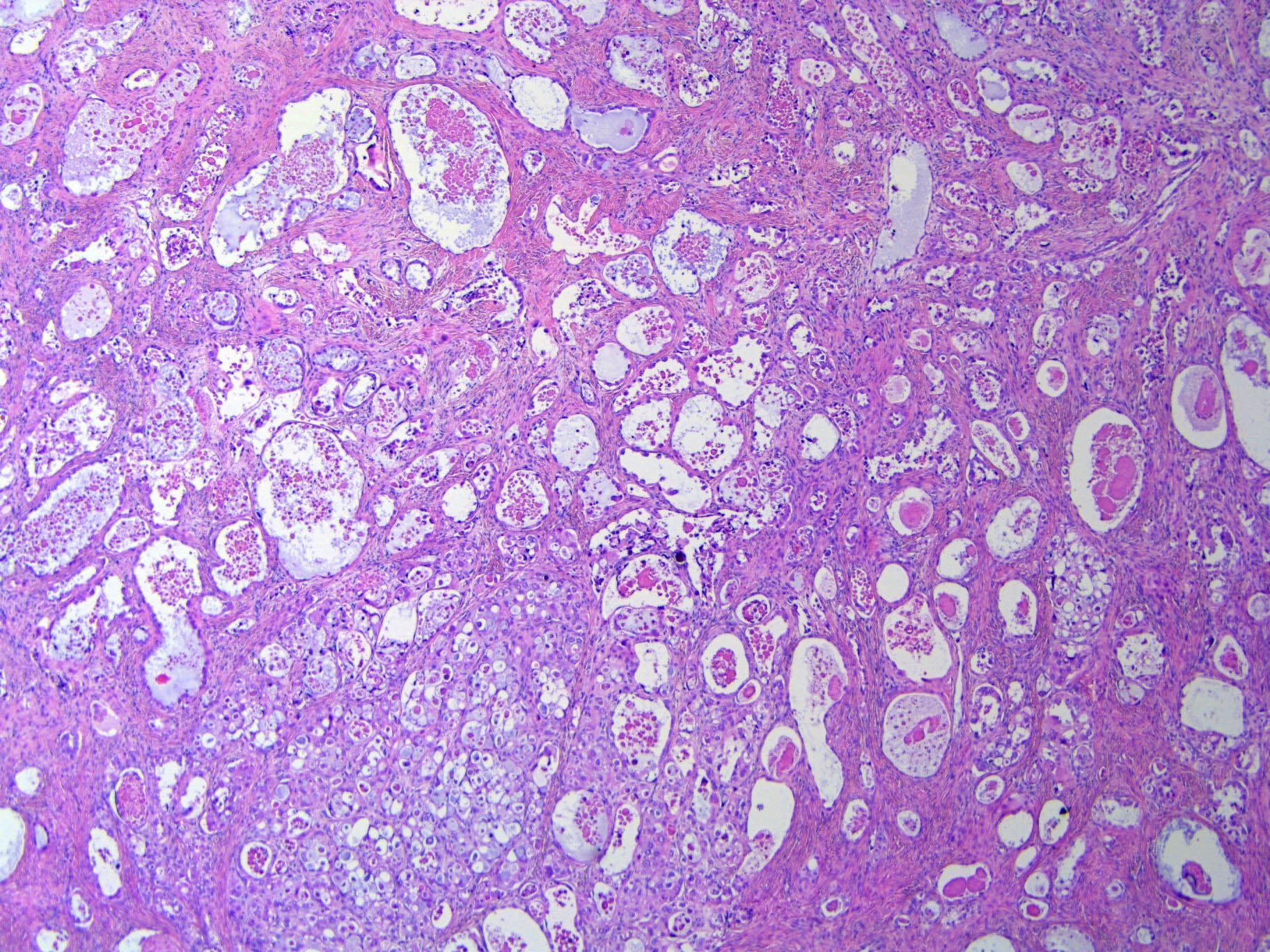
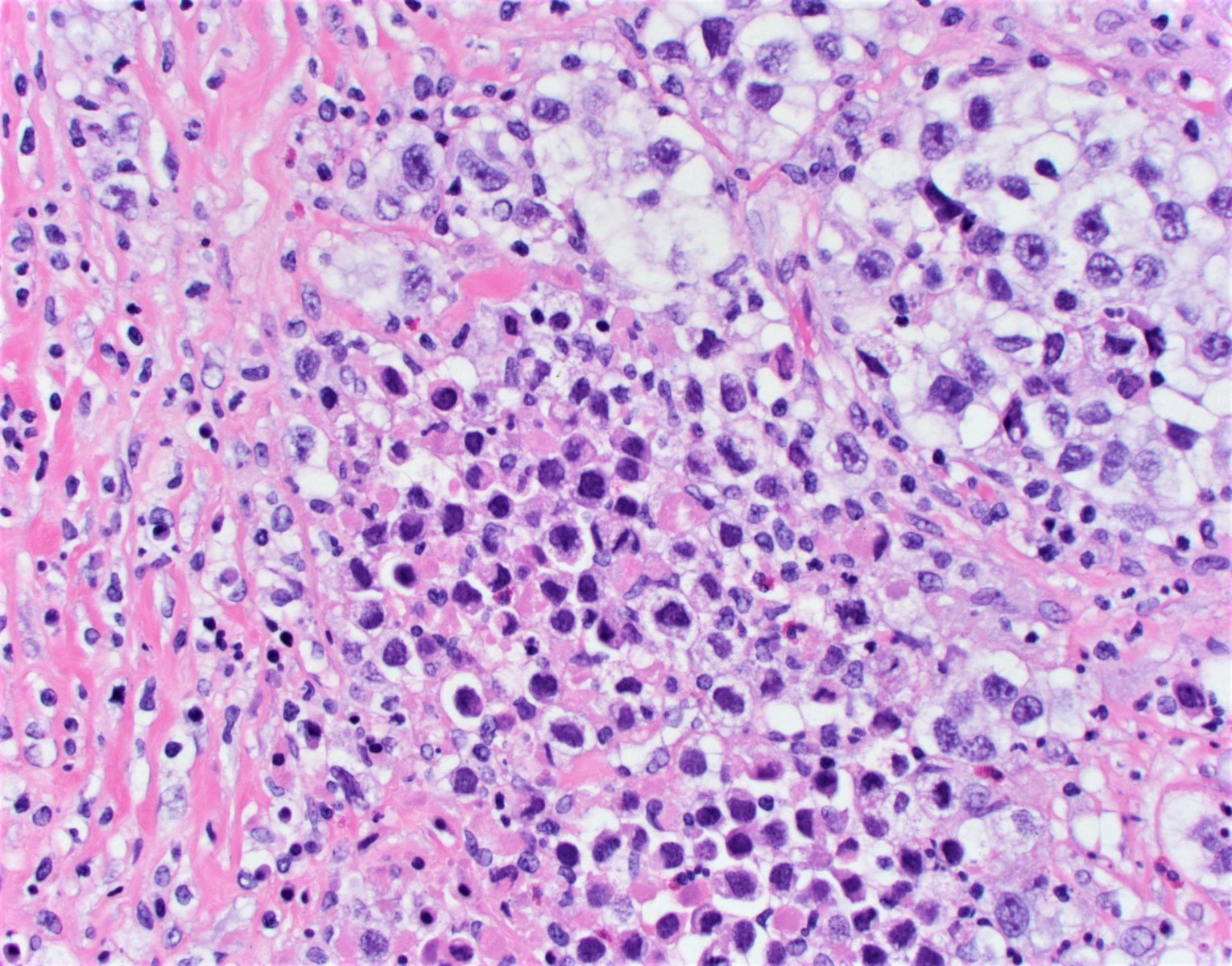
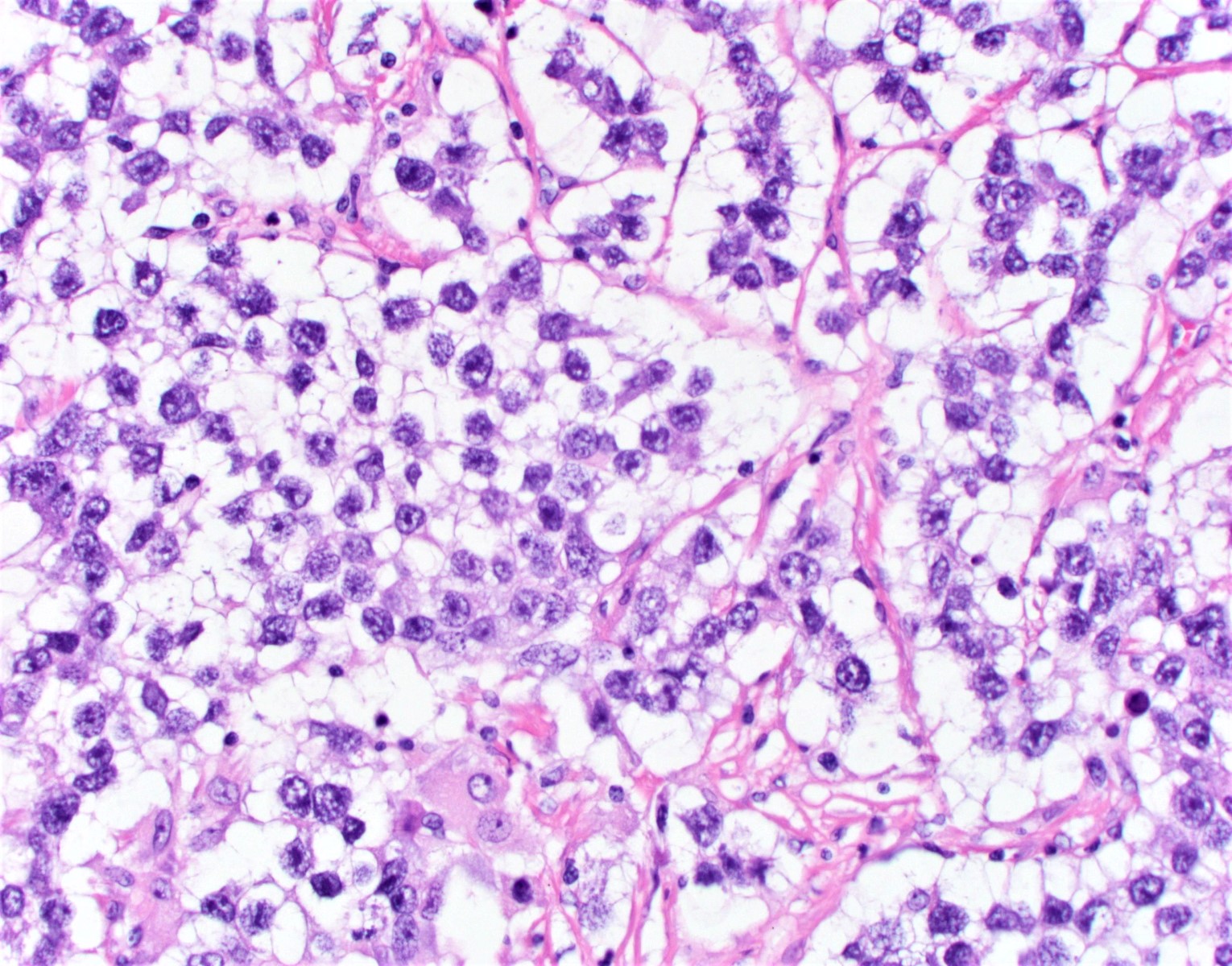
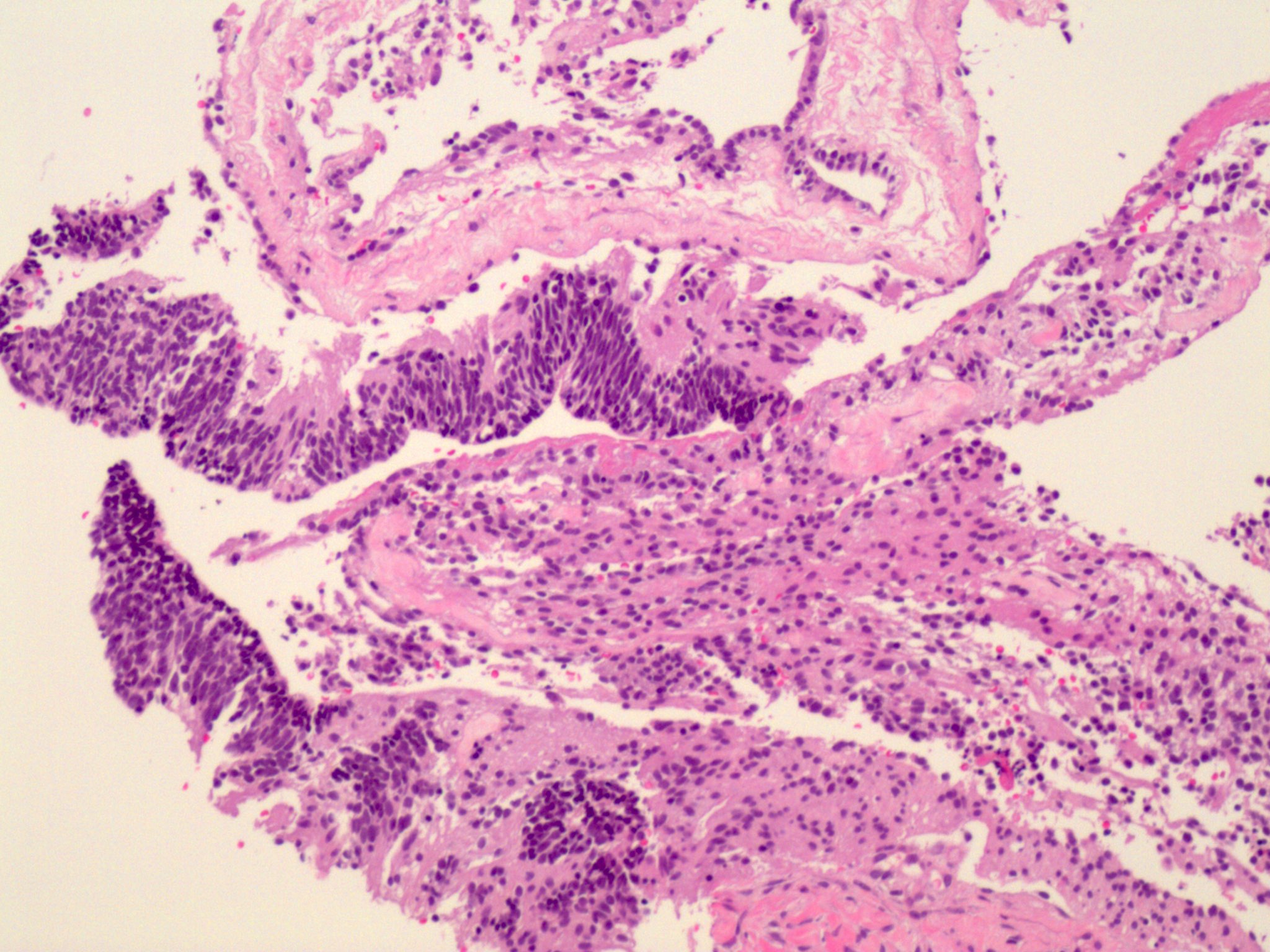
- Mucinous carcinoid
- These morphologically resemble low grade goblet cell adenocarcinoma of the appendix (previously termed goblet cell carcinoid)
- Pure form >> in association with mature cystic teratoma
- Composed of numerous small glands or acini with very small lumina floating in pools of mucin (pseudomyoxoma ovarii); some of the glands or acini may be cystically dilated containing mucin
- Lined by uniform cuboid or columnar and goblet cells and containing small round or oval nuclei and cytoplasmic neuroendocrine granules
- In some areas, the tumor cells tend to invade the surrounding connective tissue, often assuming signet ring appearance
- Tumor may form large solid aggregates, show a less uniform appearance and have more atypical features with large hyperchromatic nuclei and brisk mitotic activity
- Insular carcinoid
- Can also be mixed and present as a combination of 2 or more types of carcinoids
Microscopic (histologic) images
Contributed by Krisztina Hanley, M.D., Rulong Shen, M.D. and AFIP
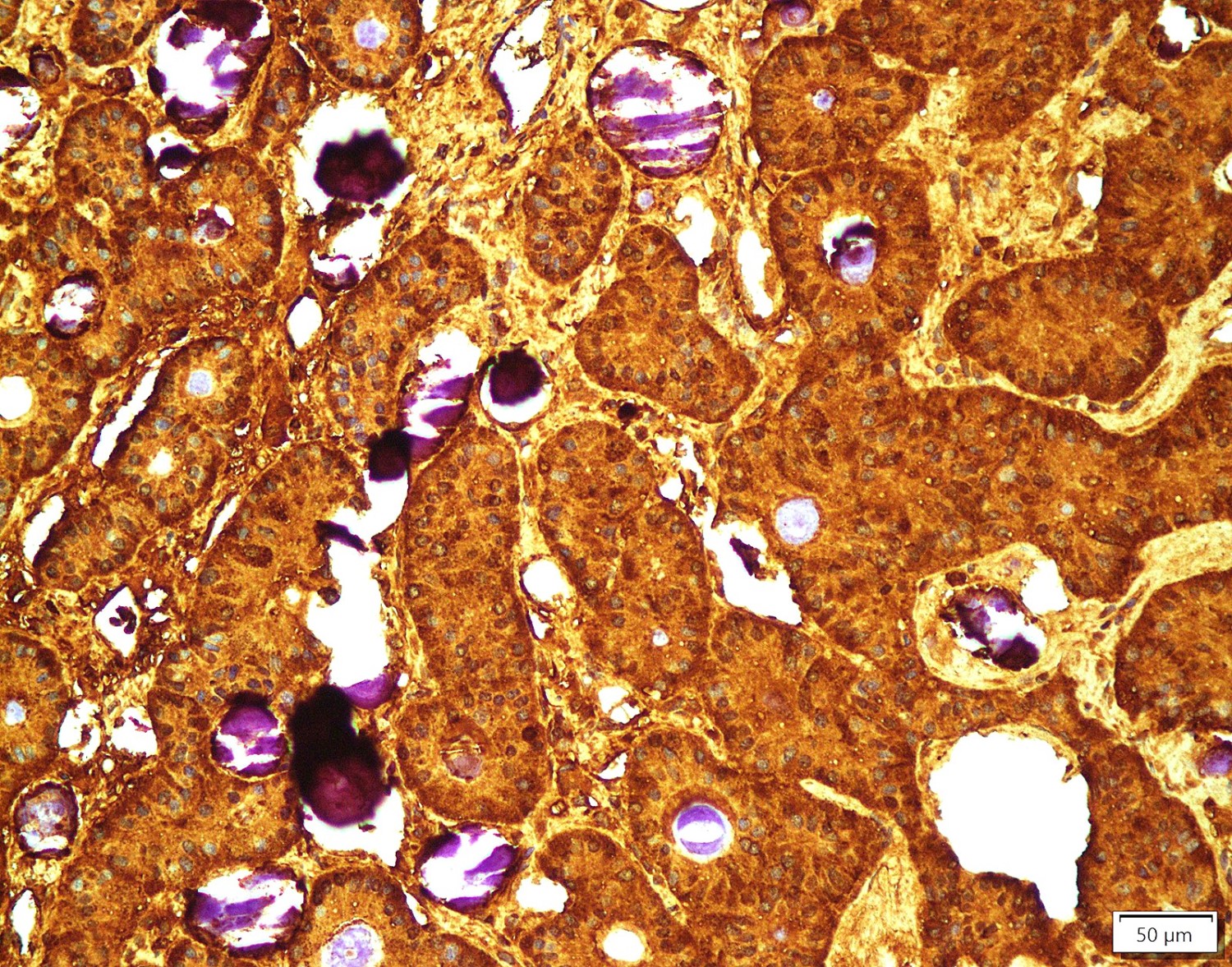
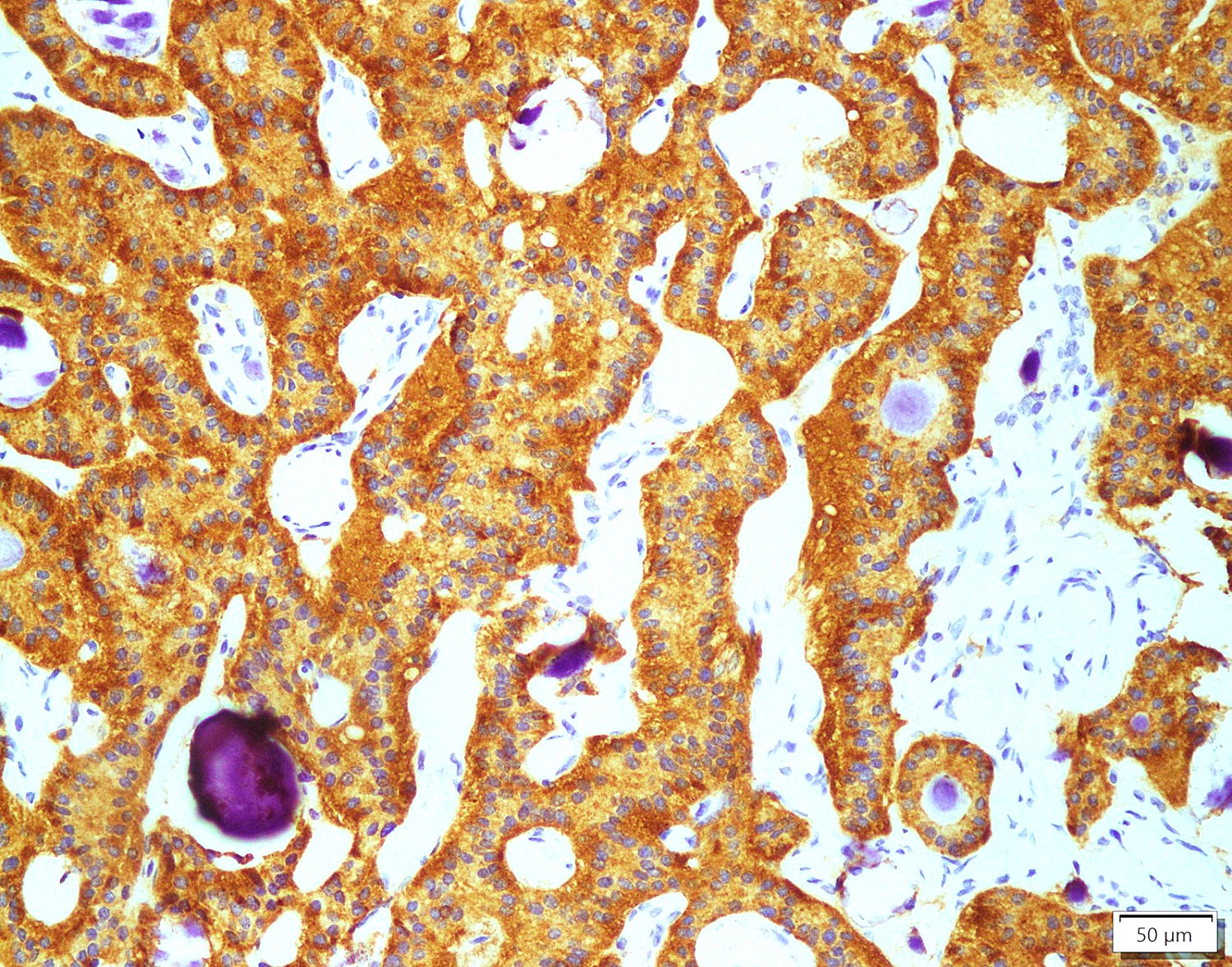
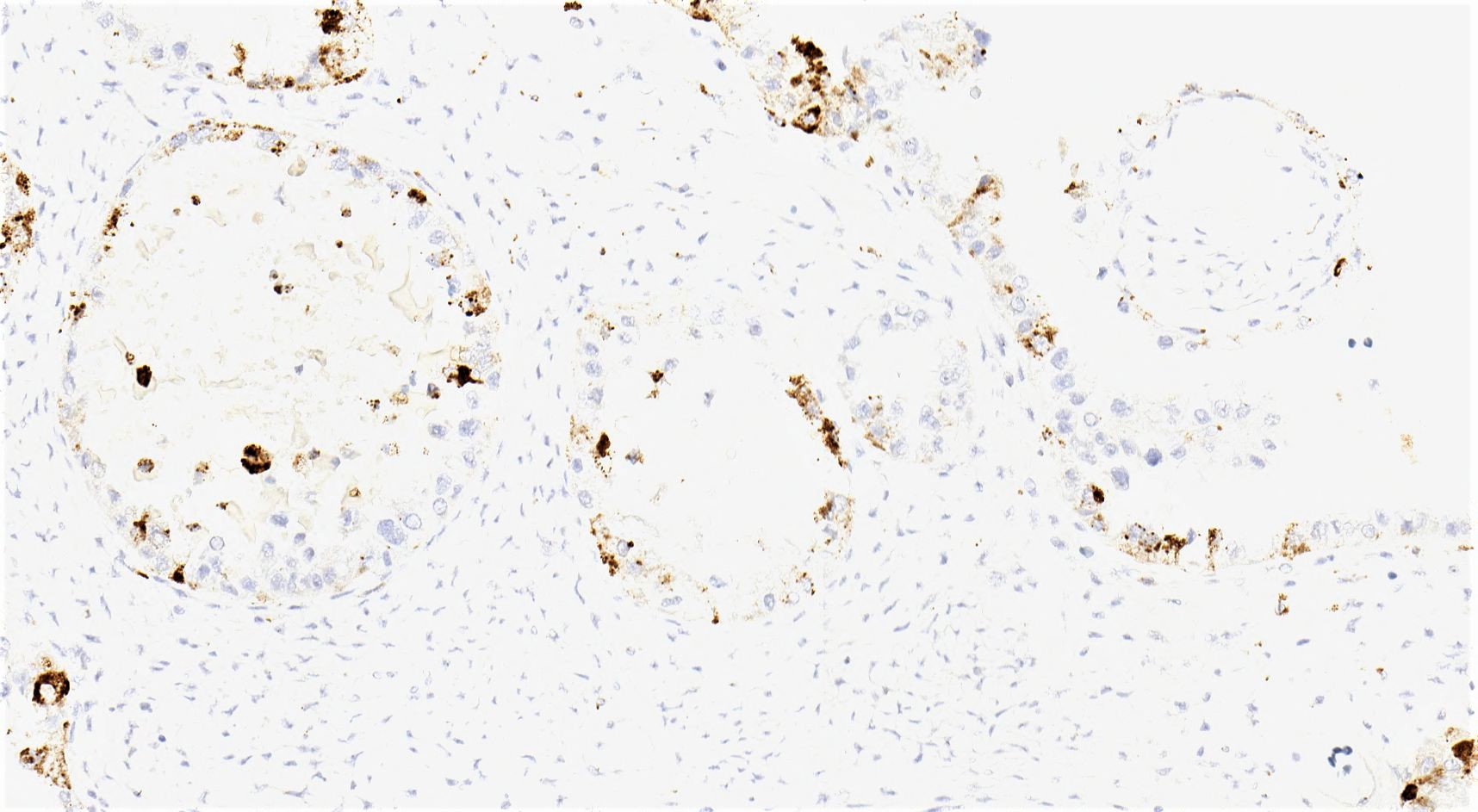
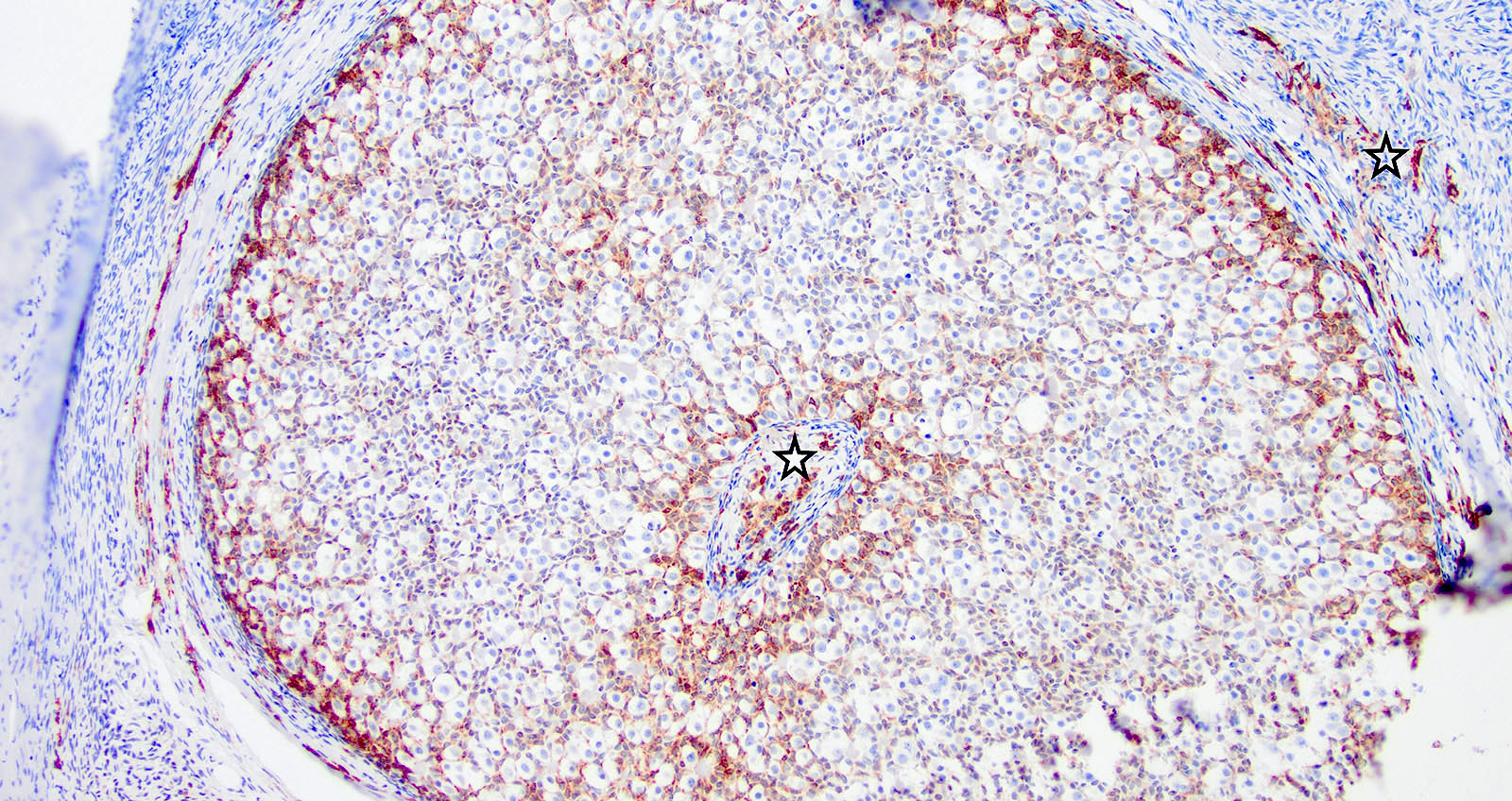
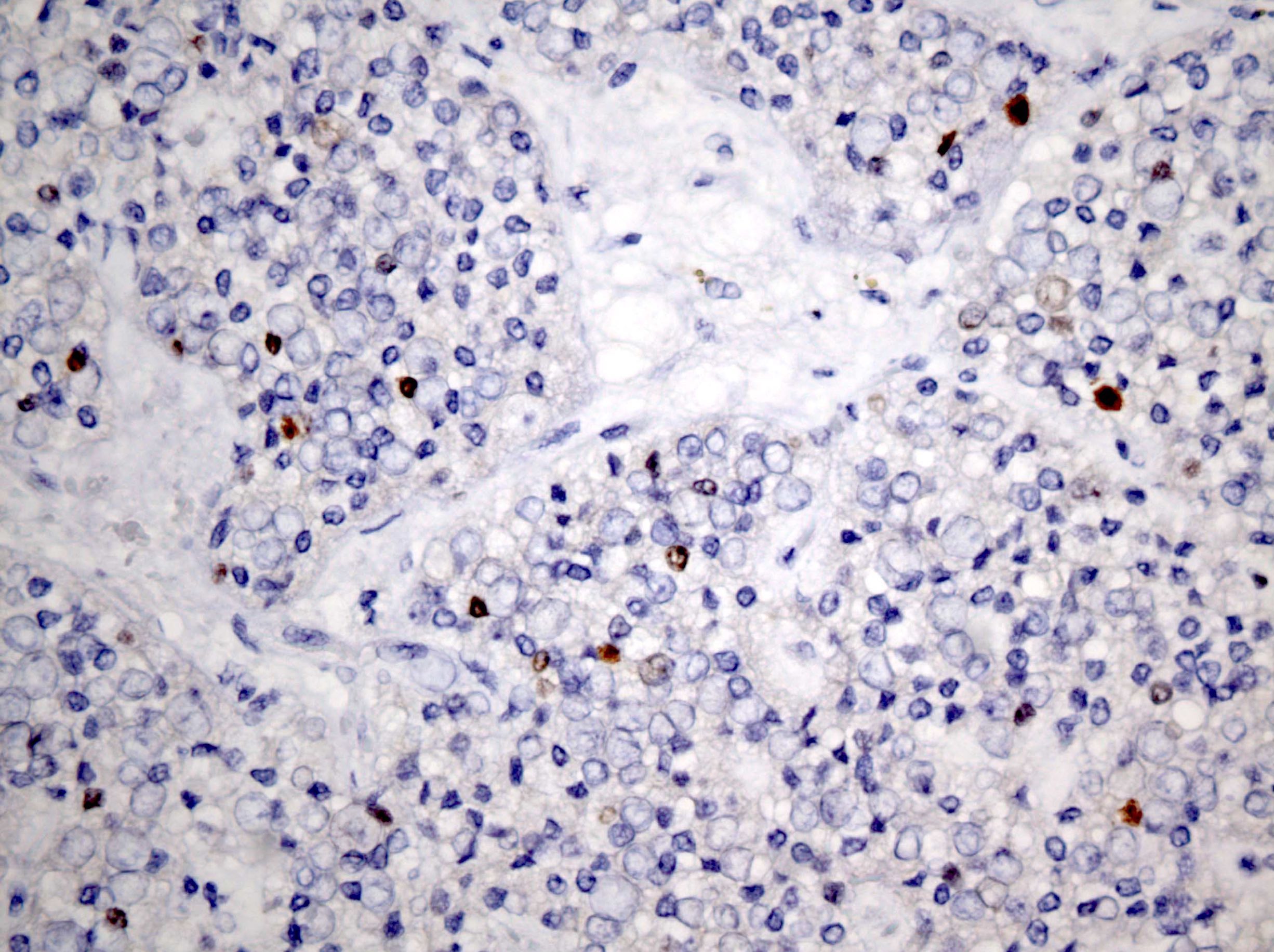
Positive stains
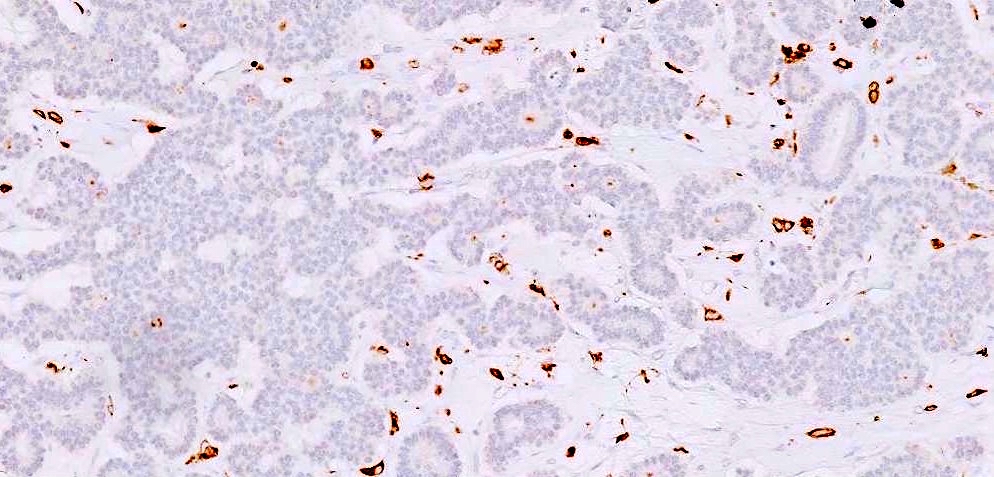
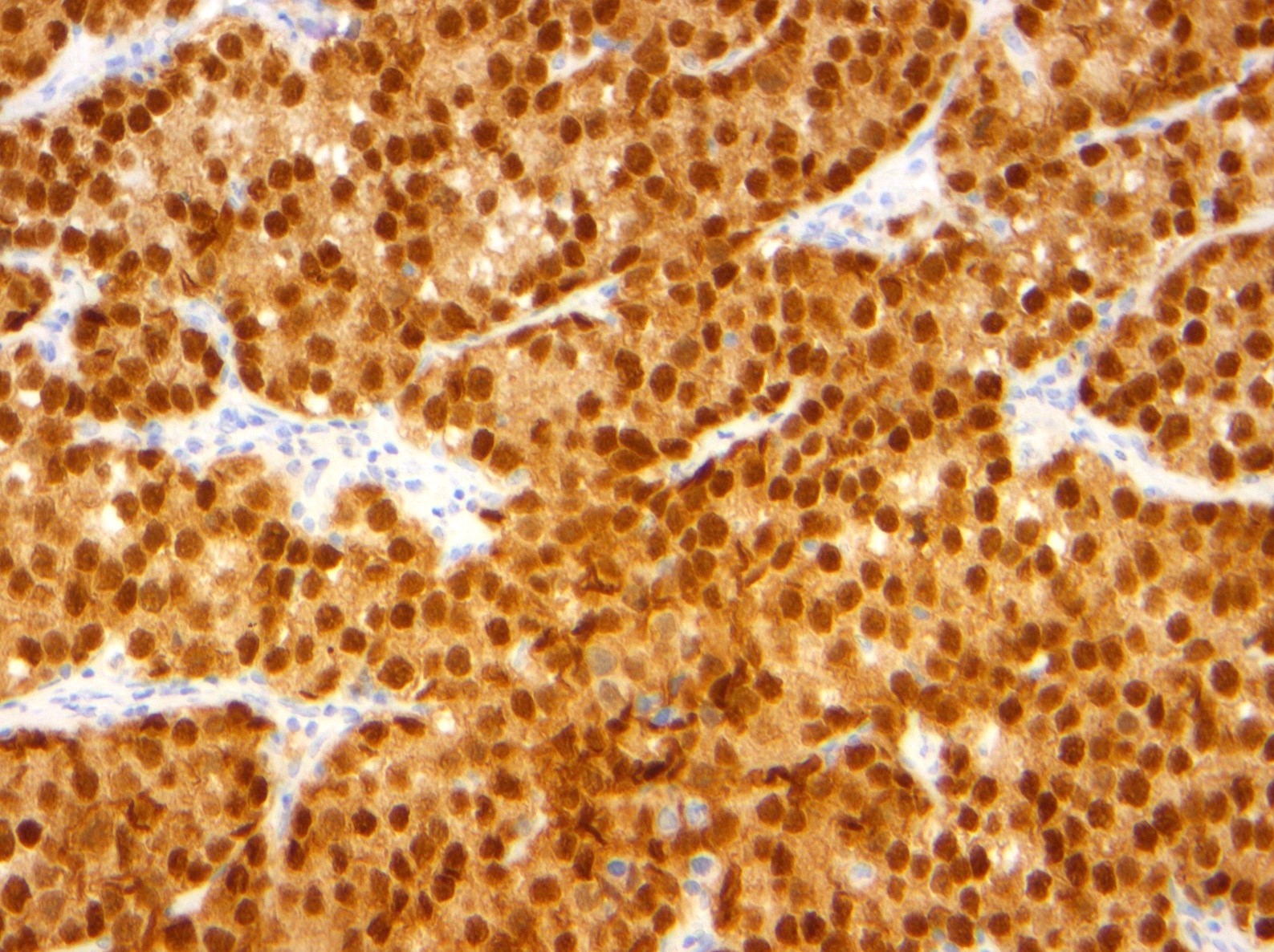
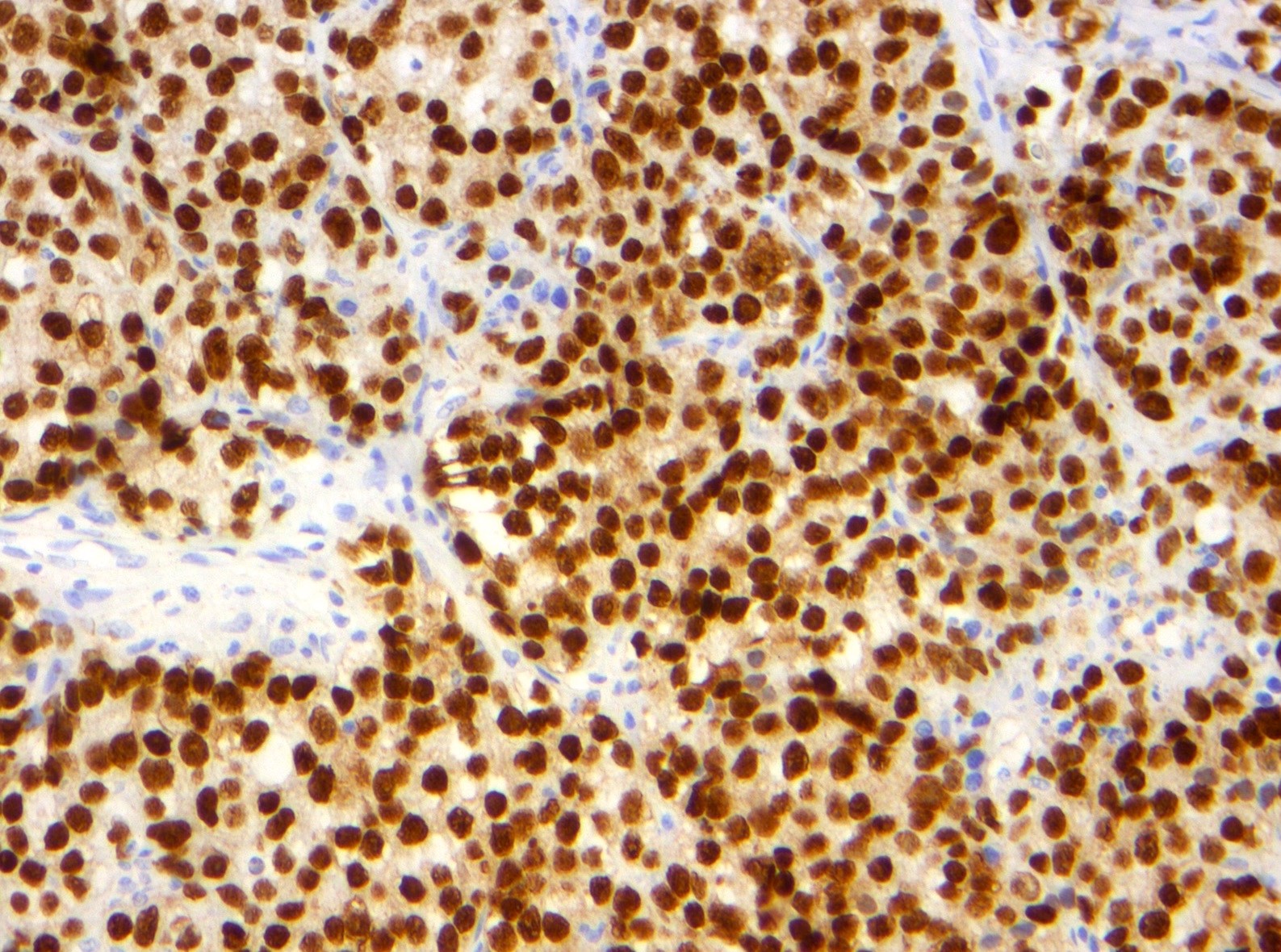
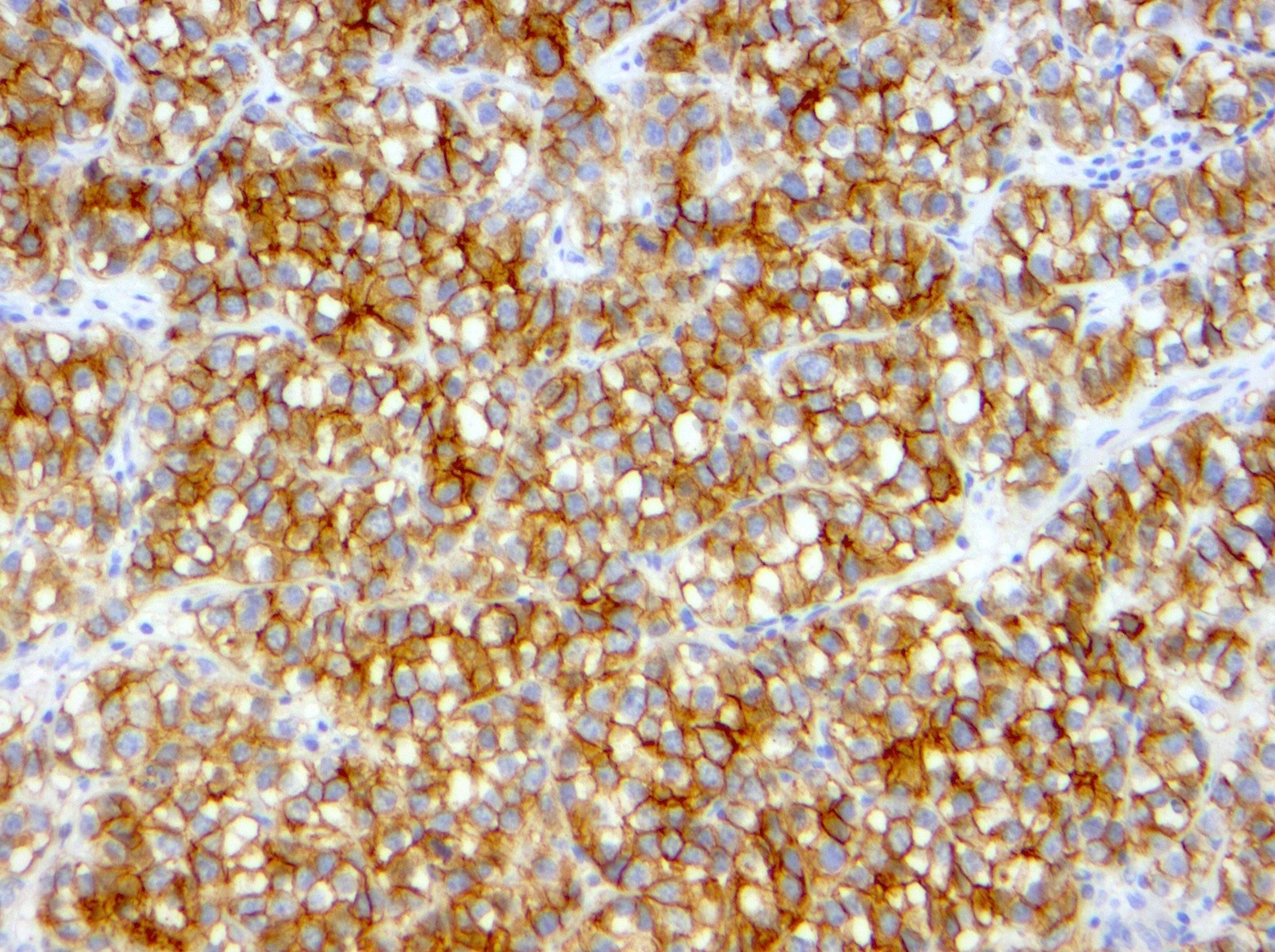
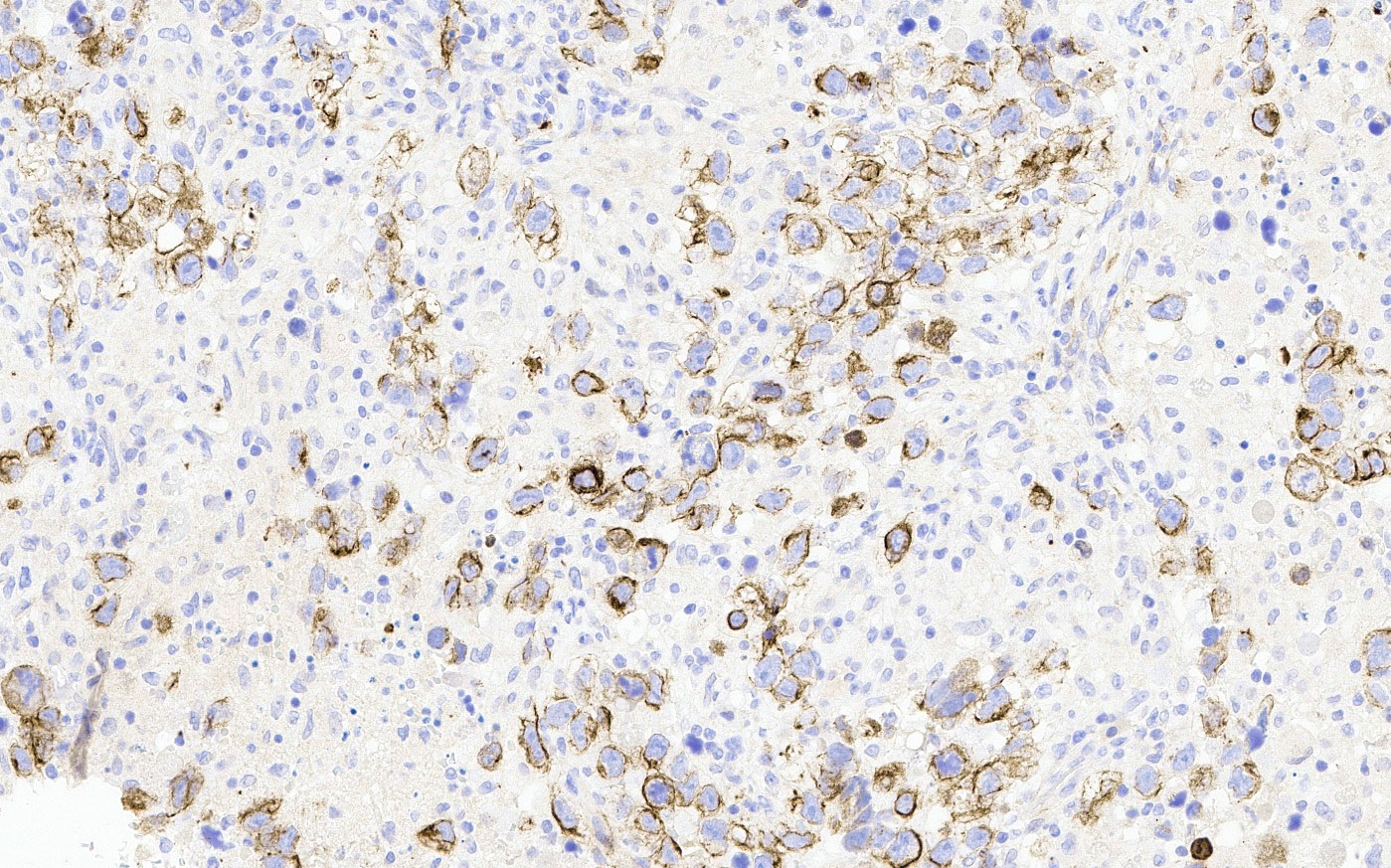
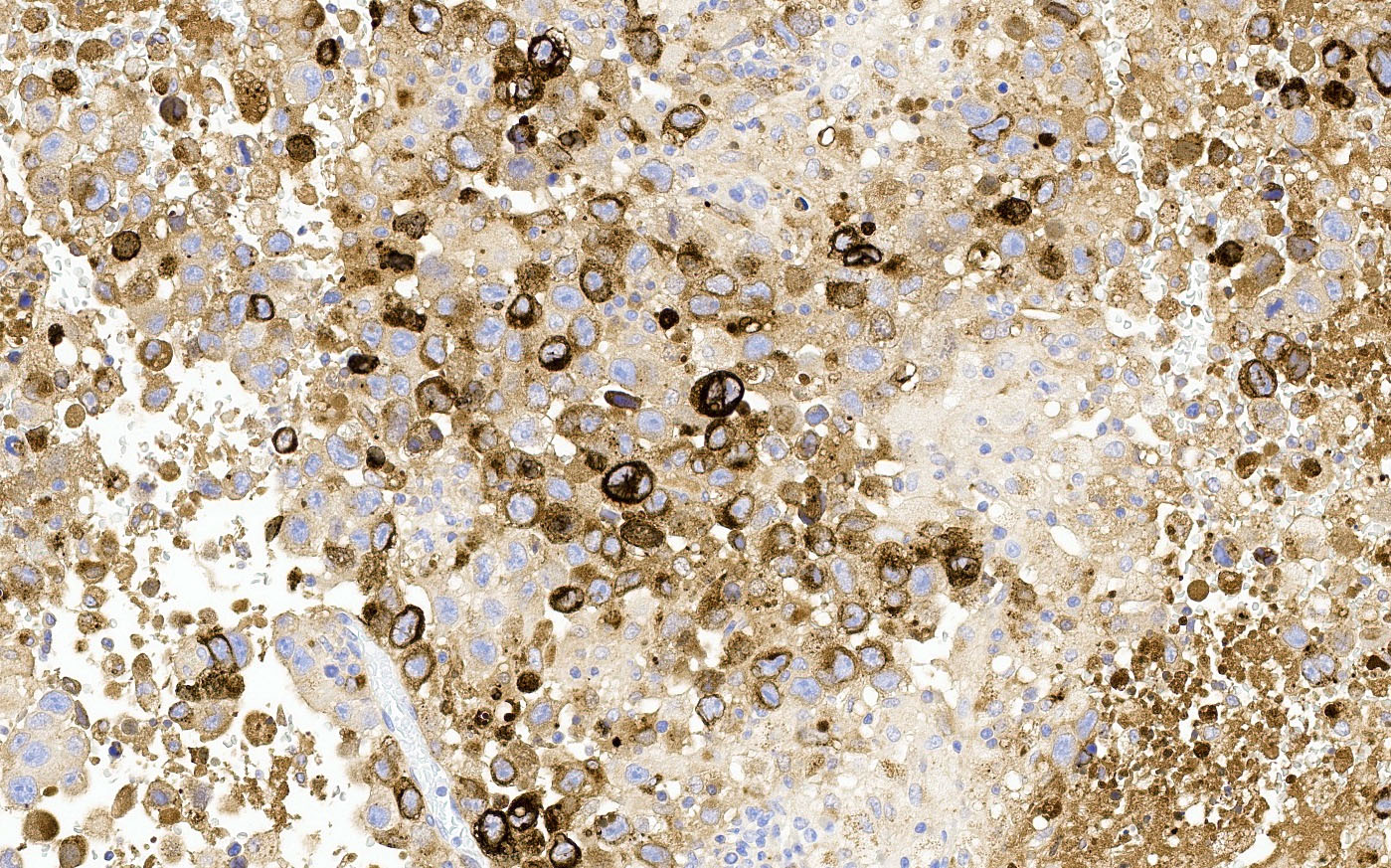
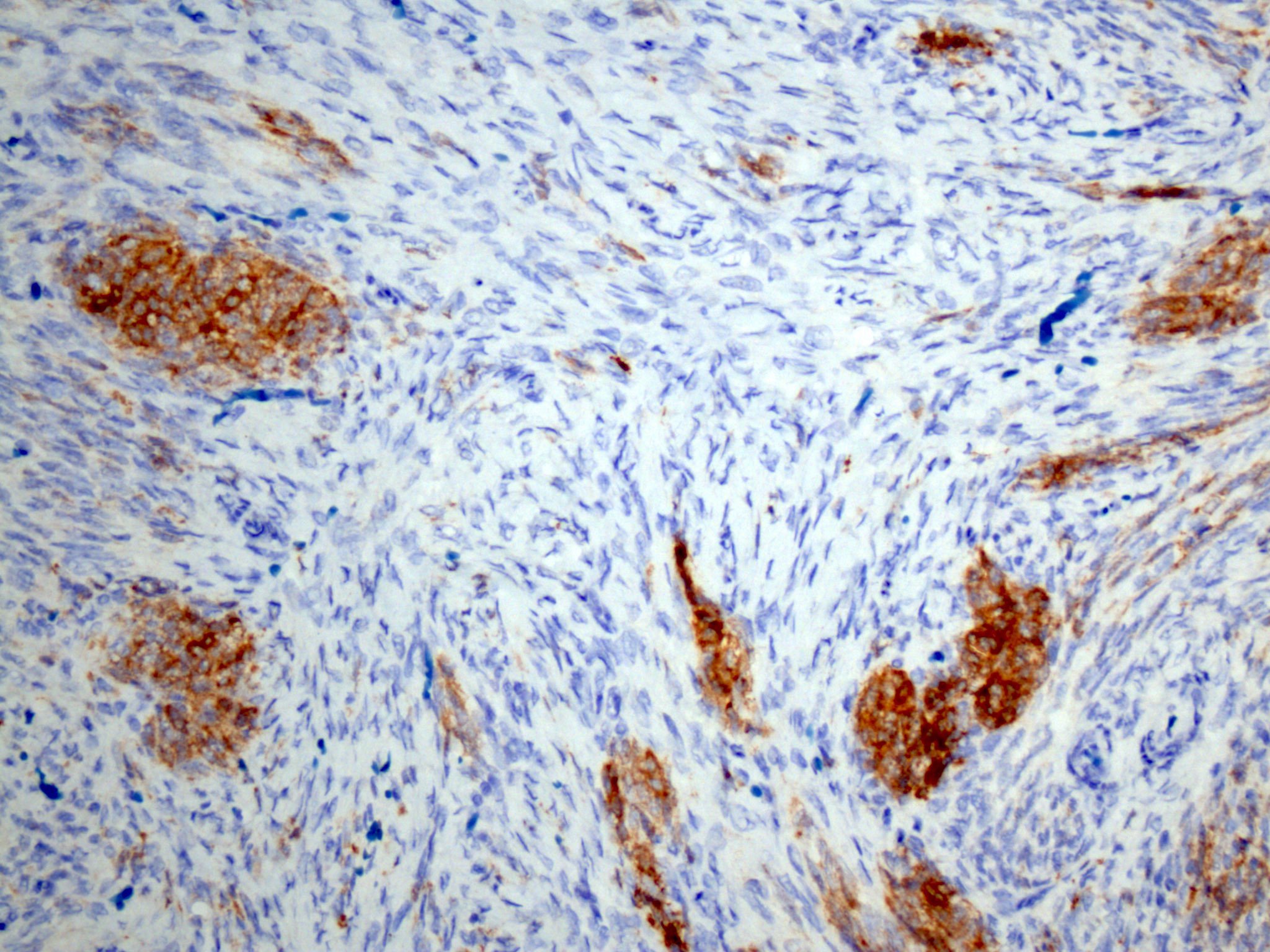
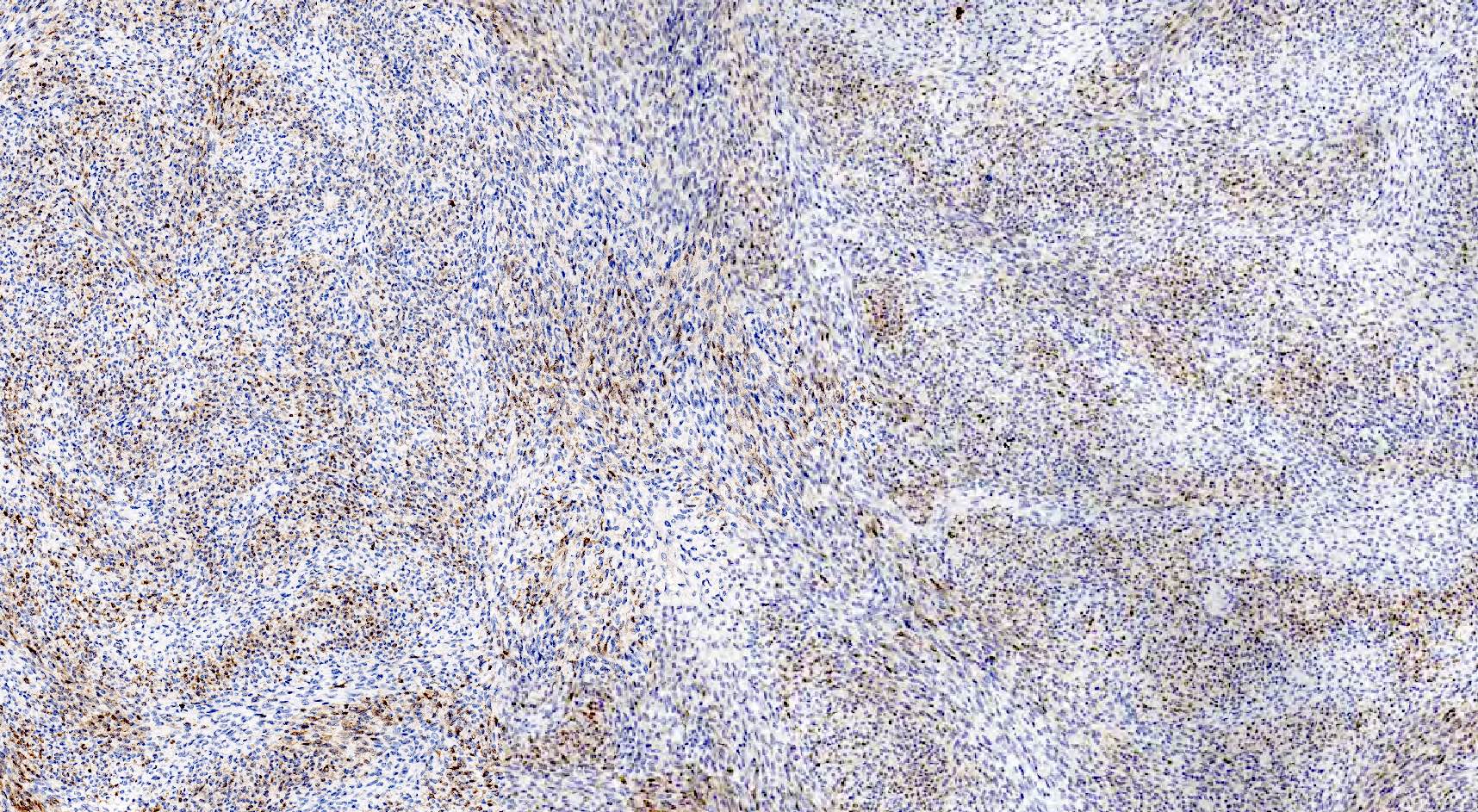
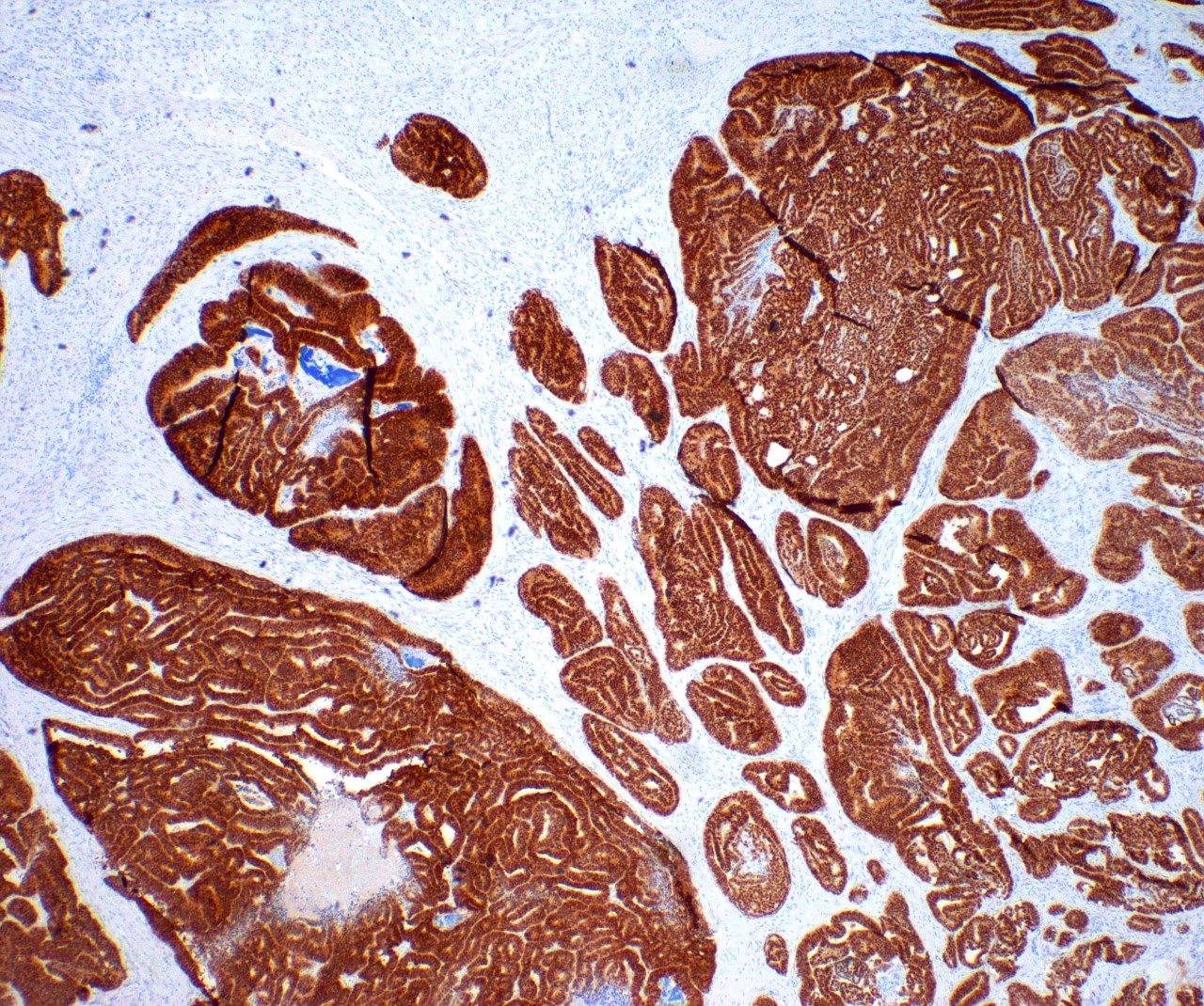
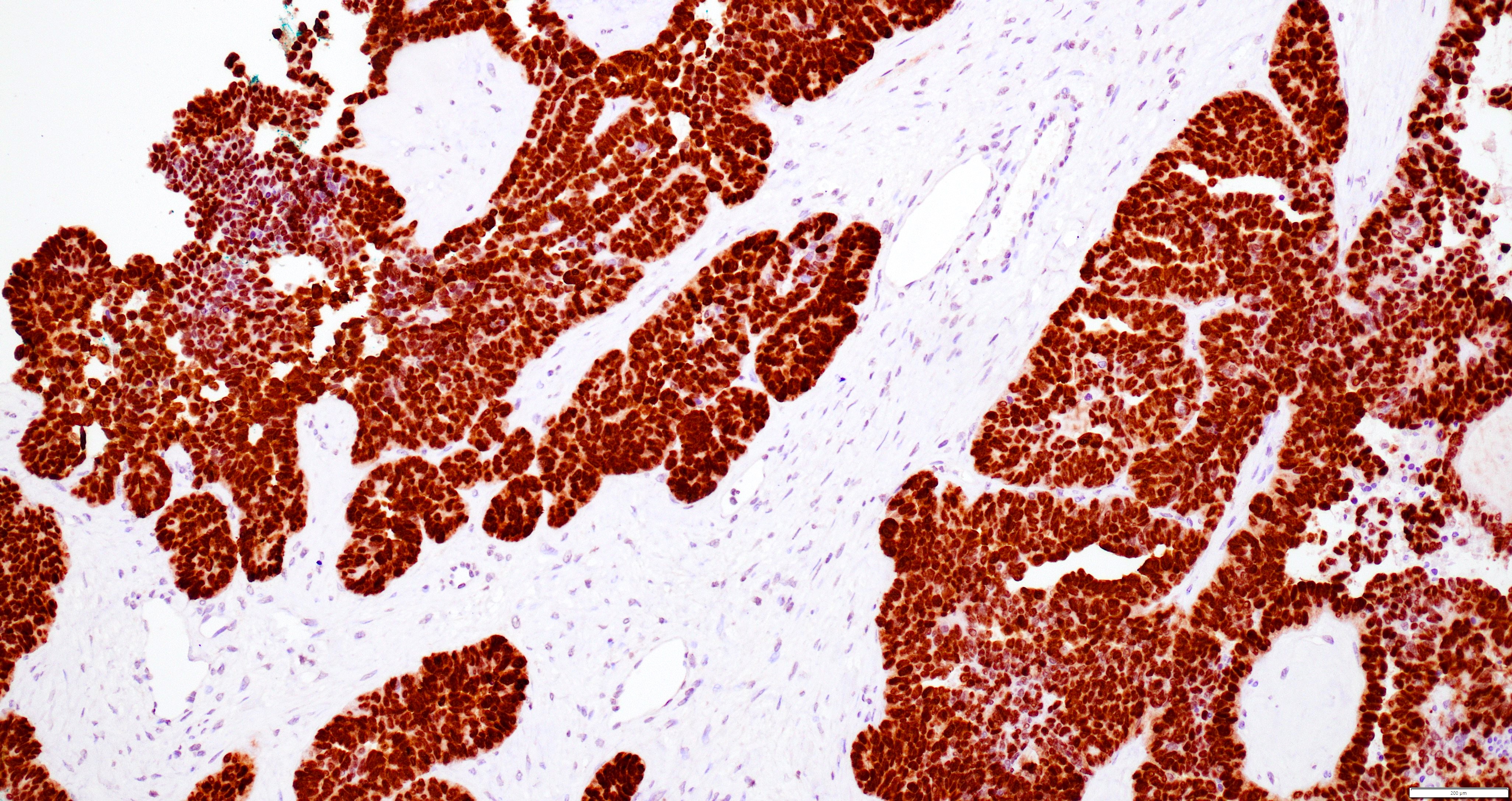
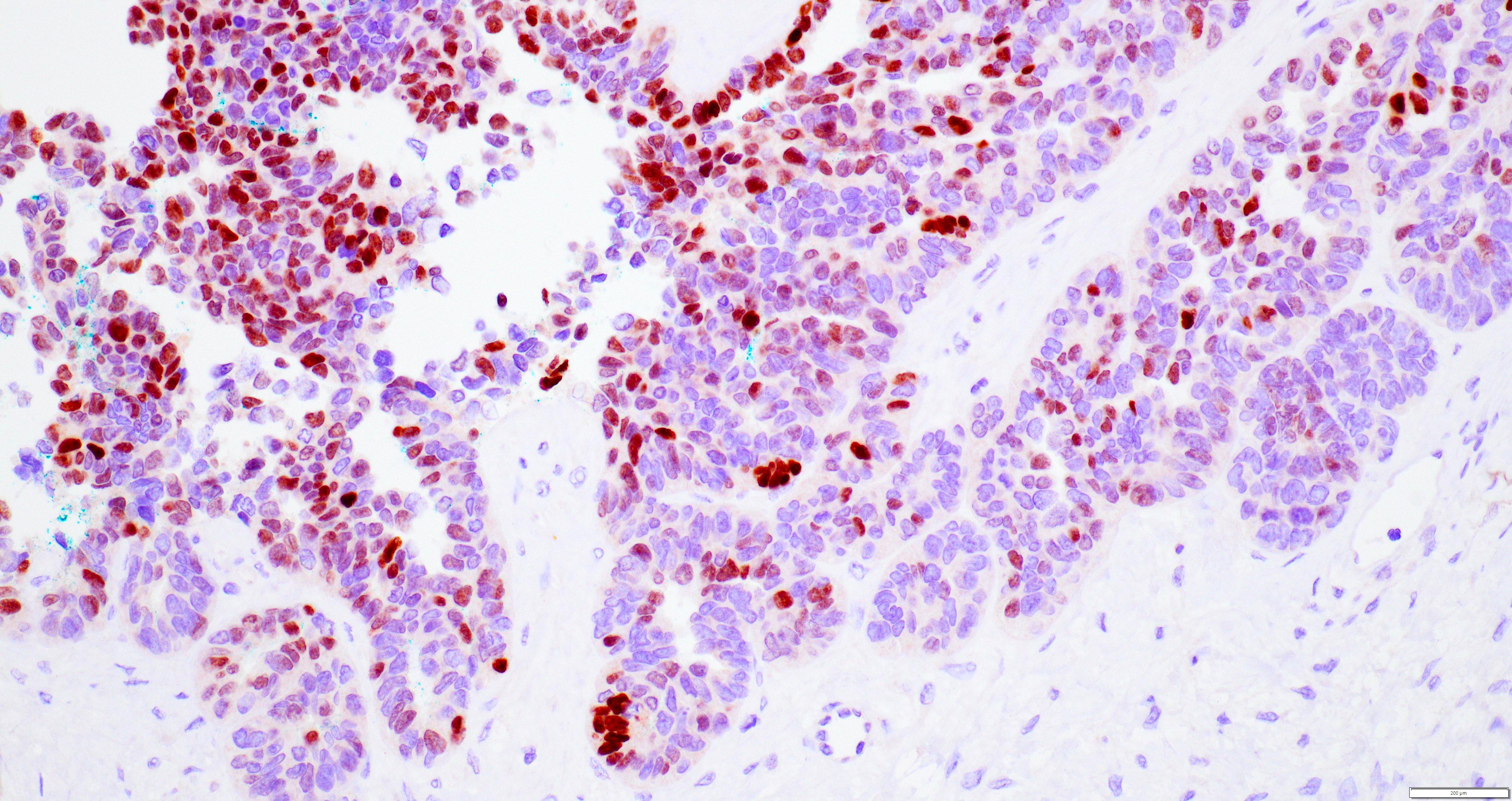
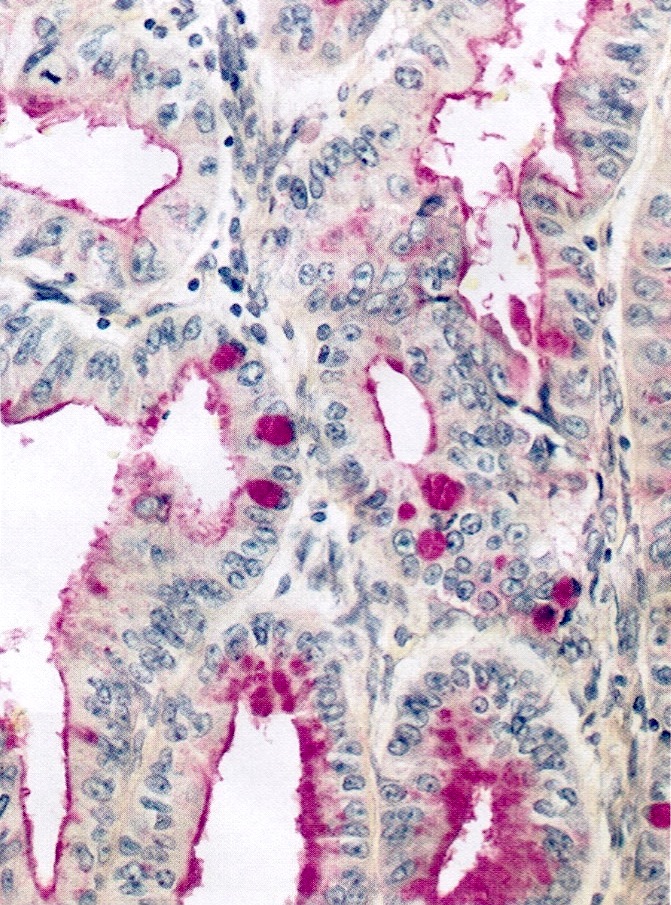
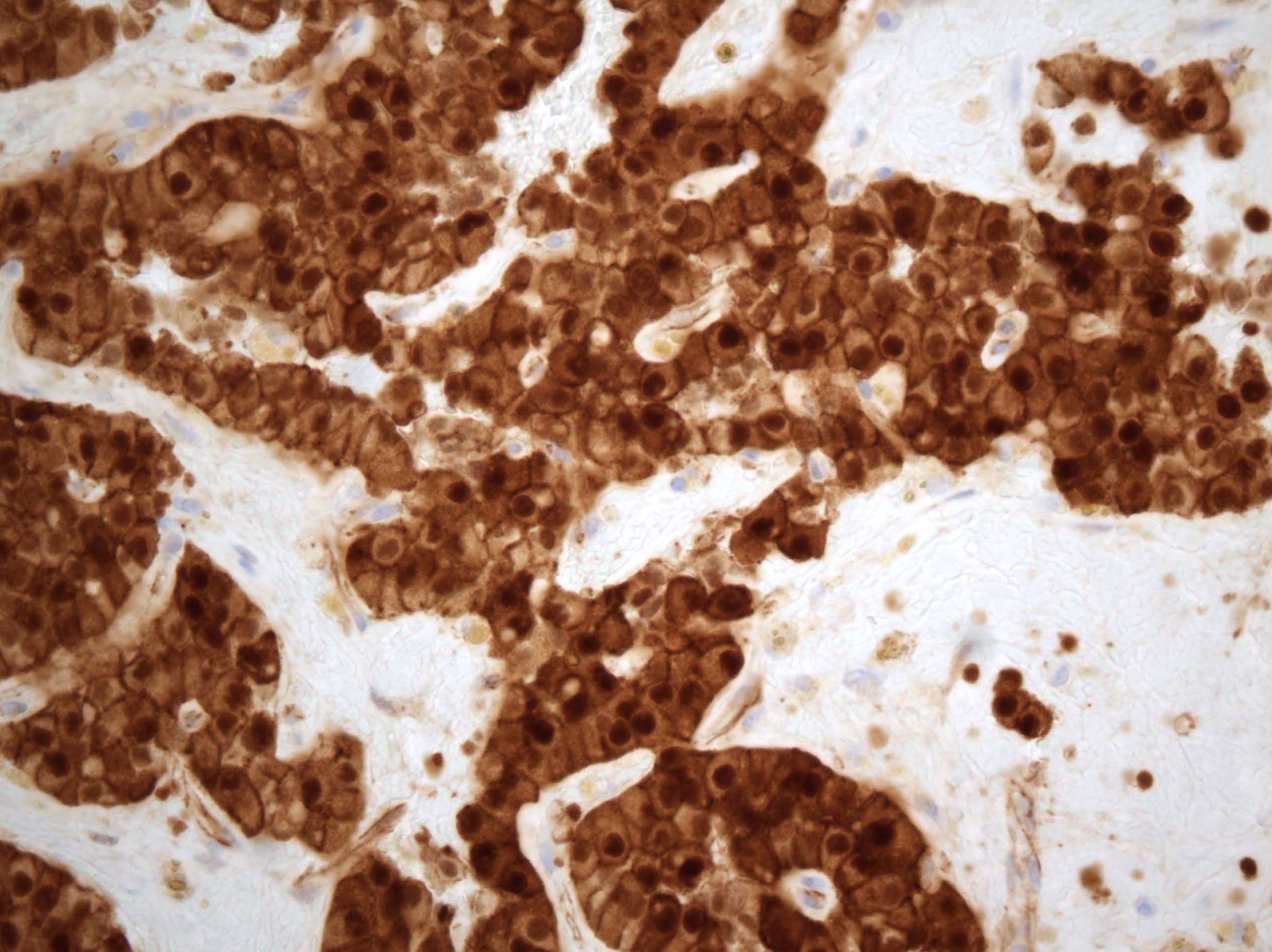
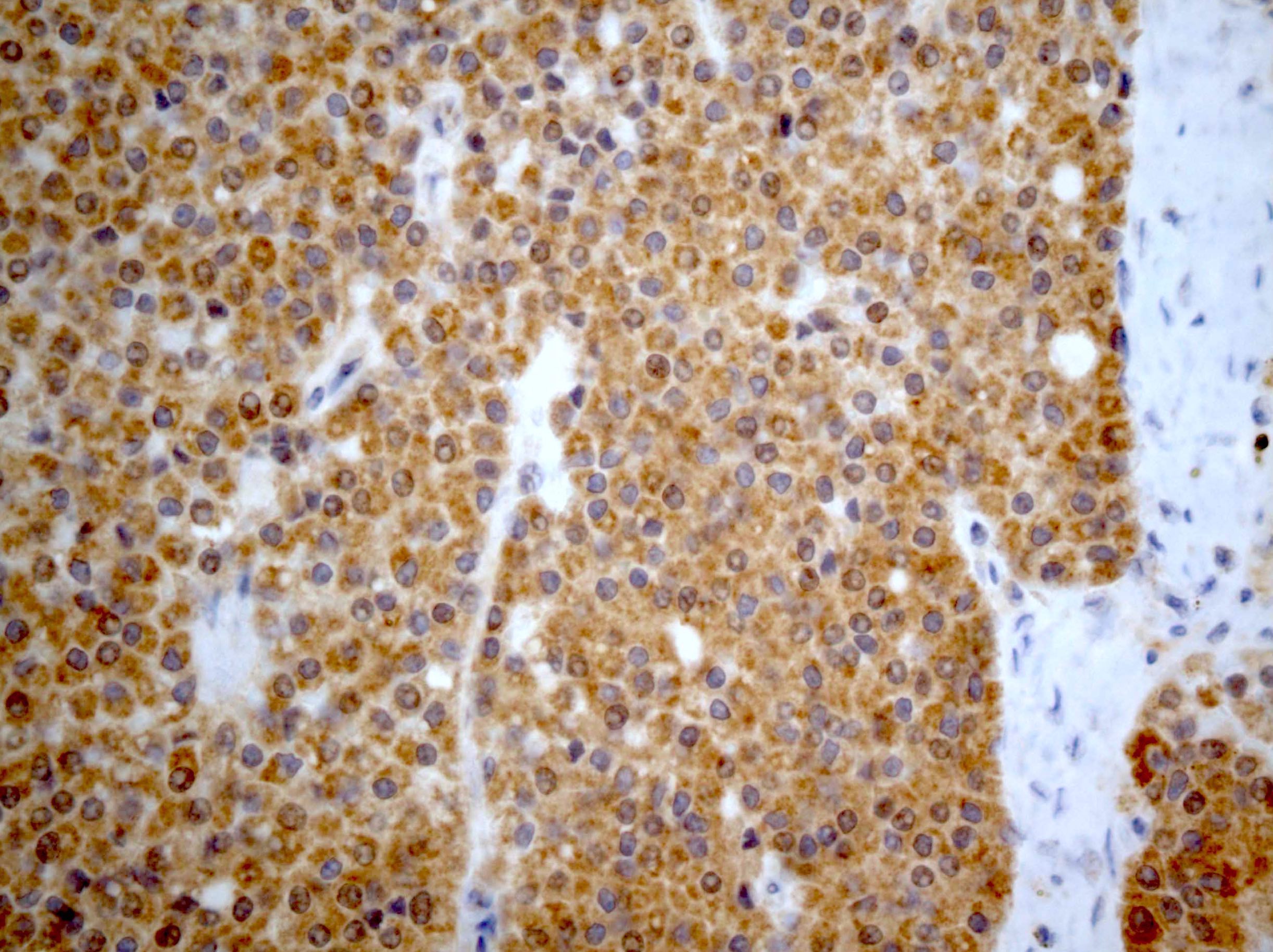
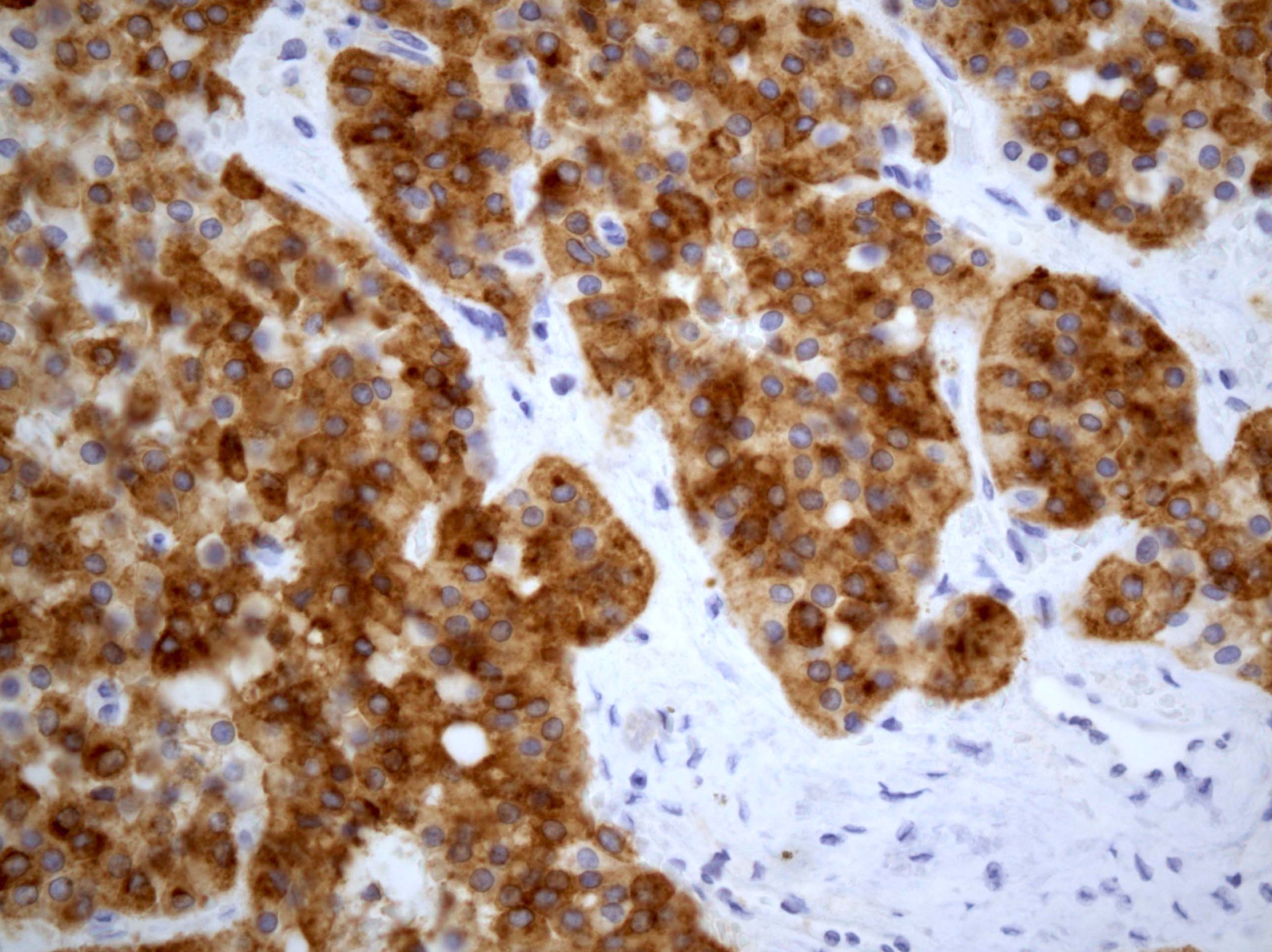
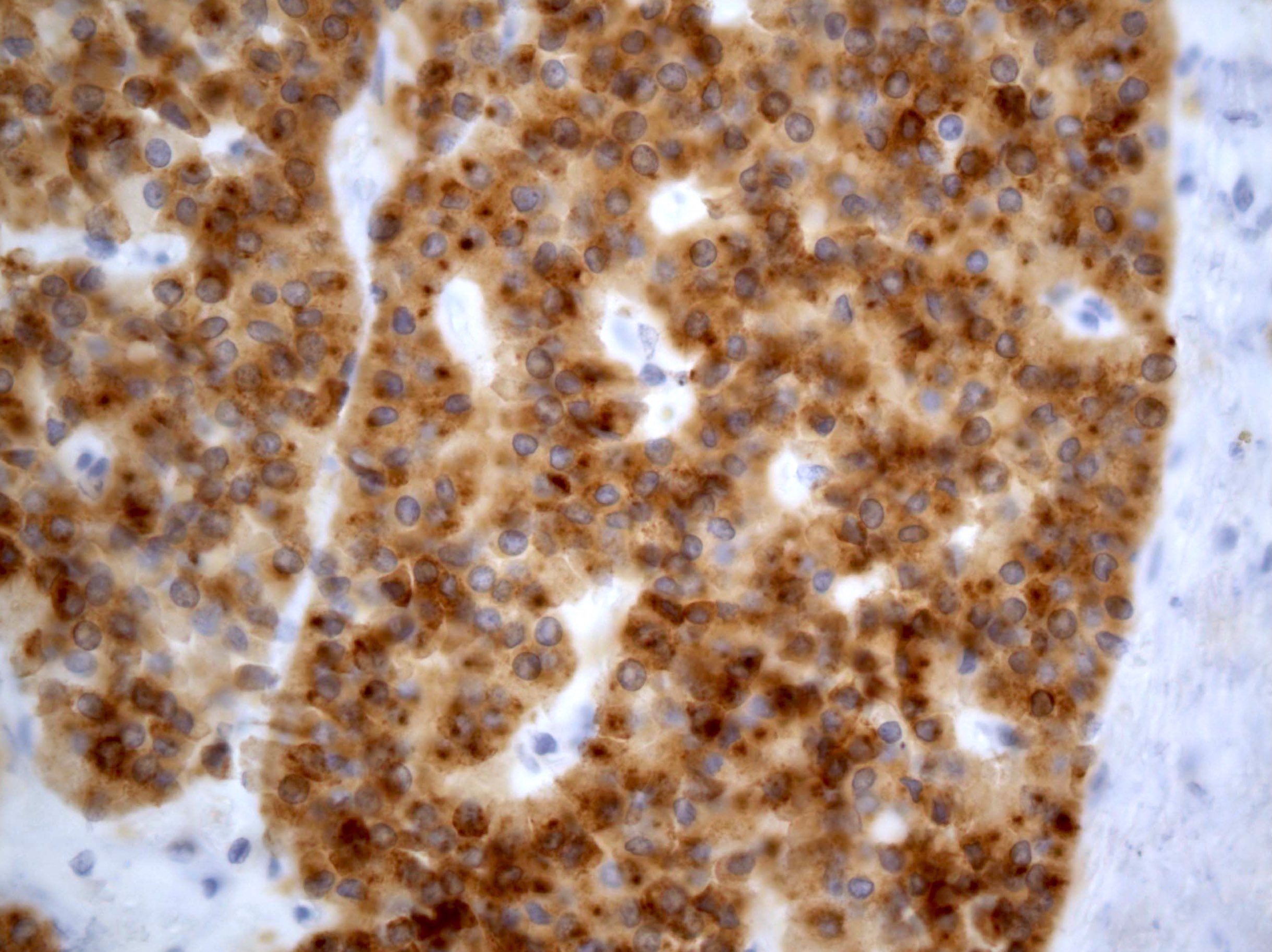
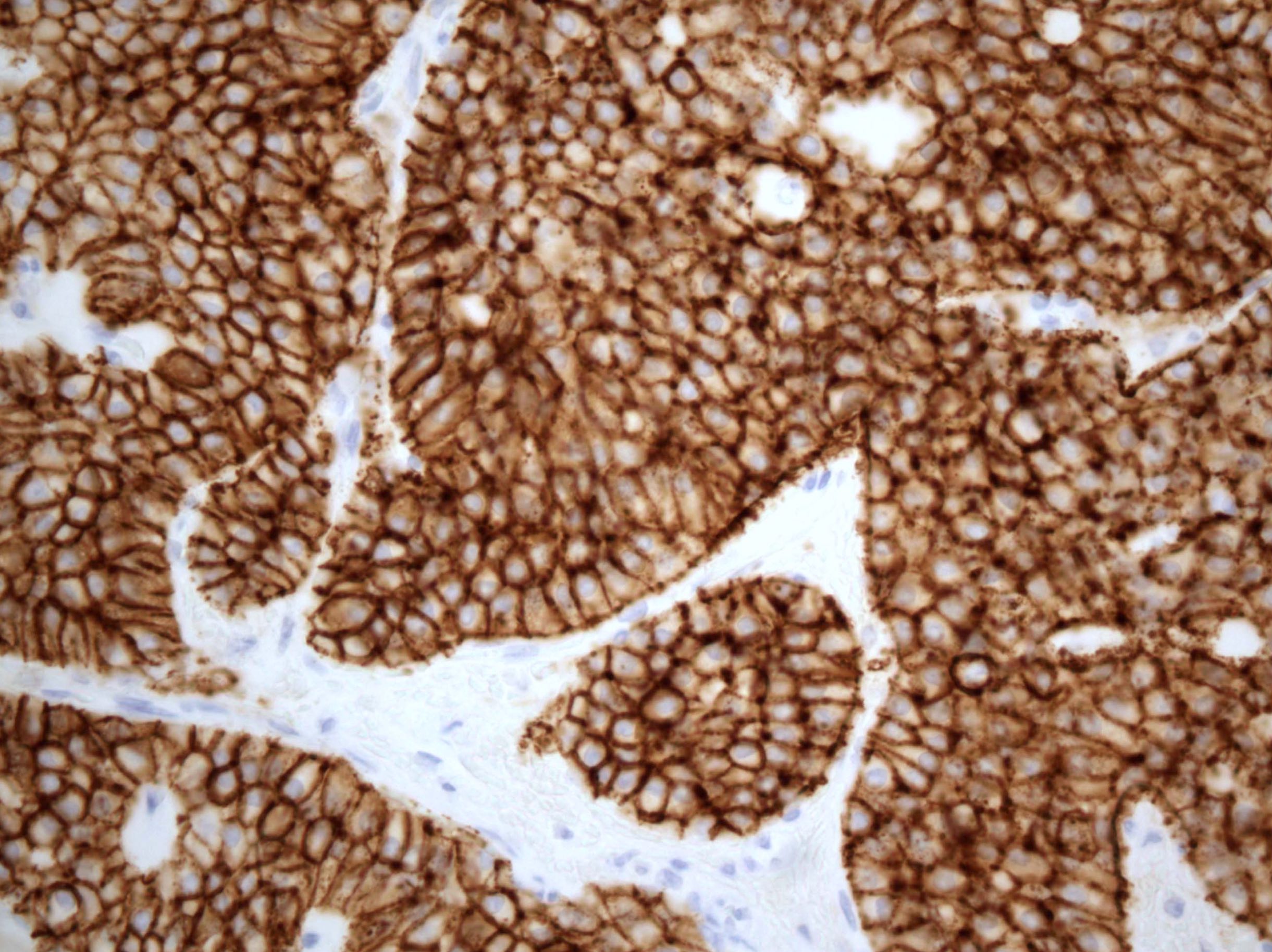
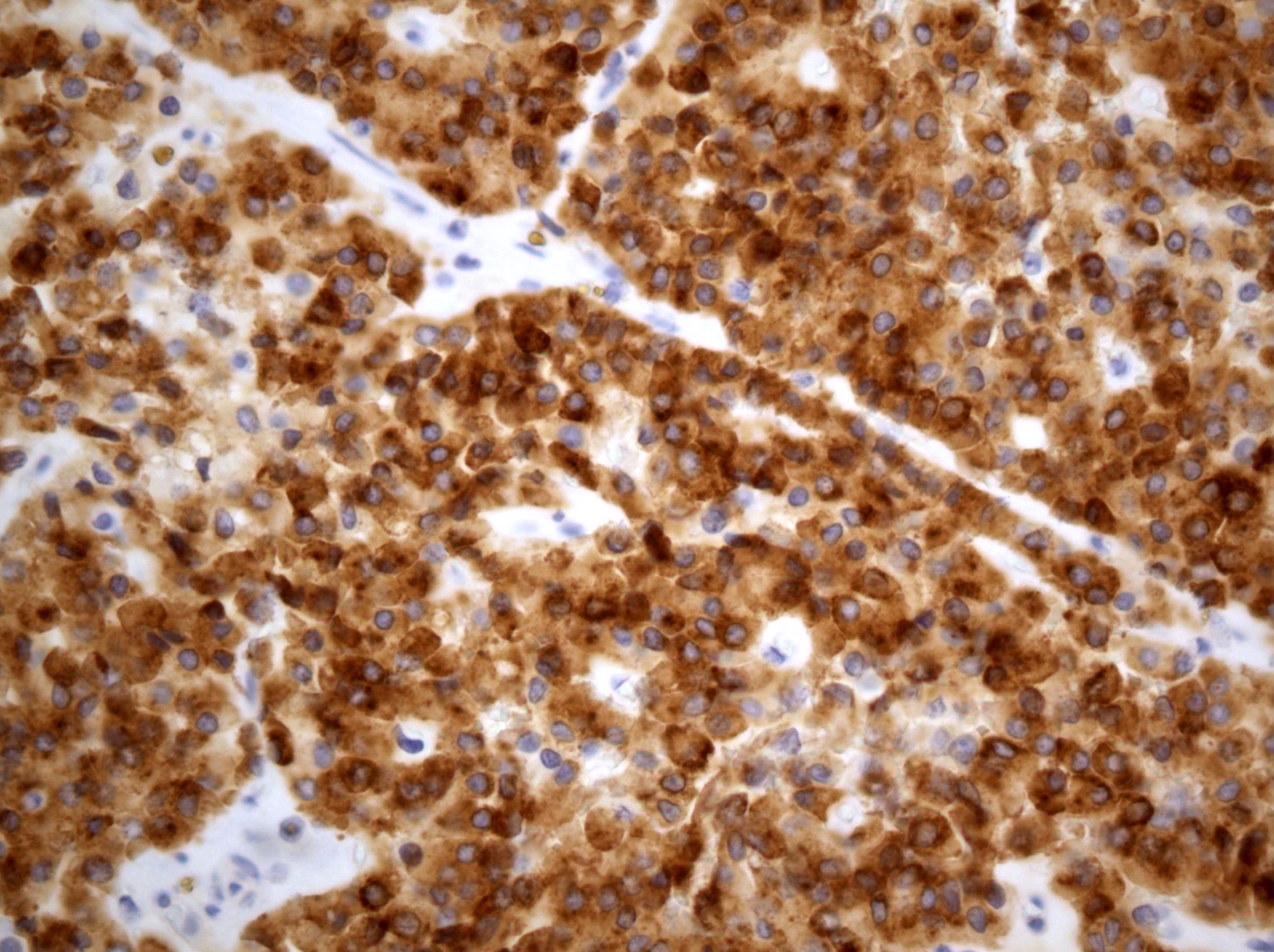
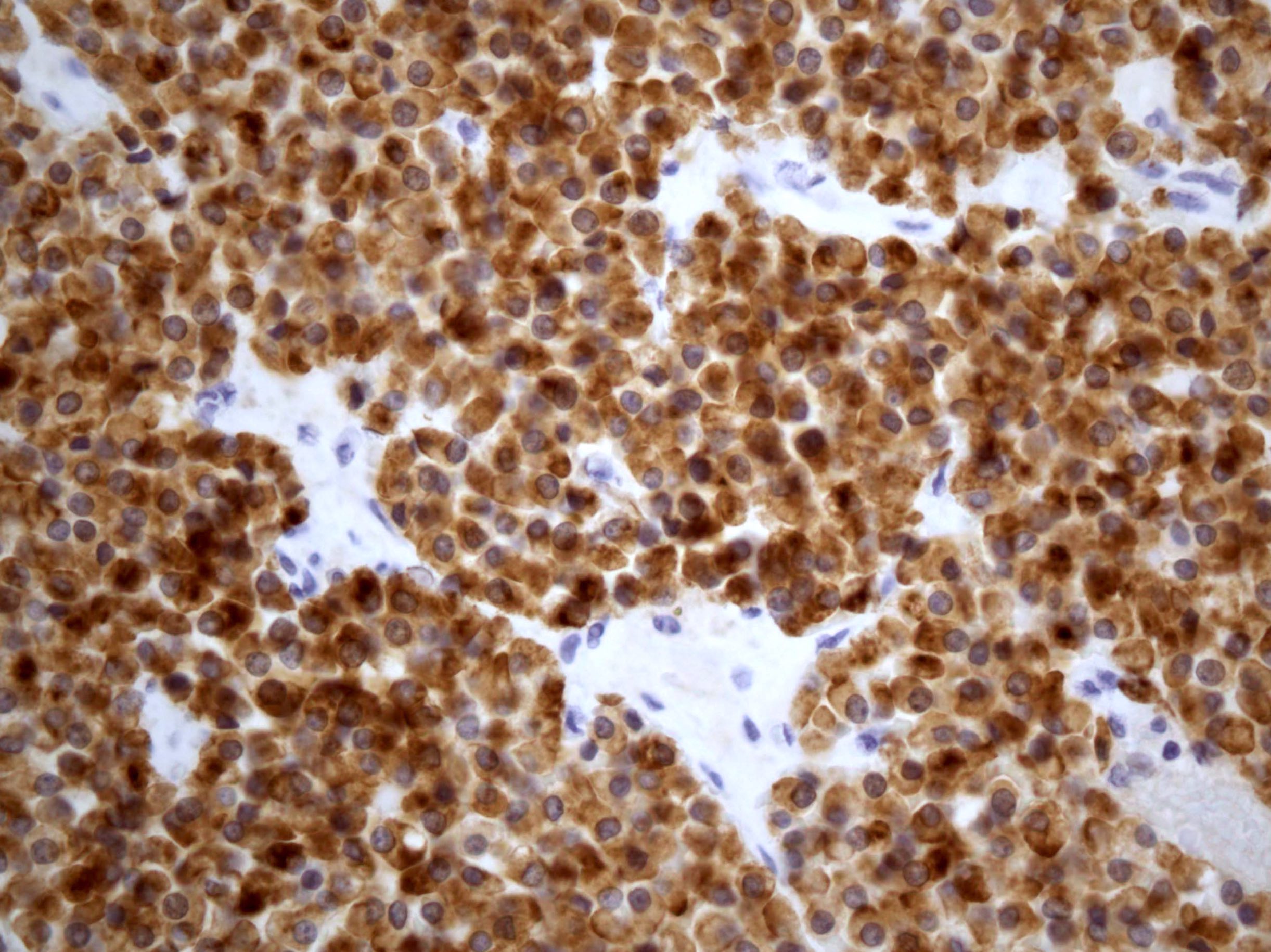
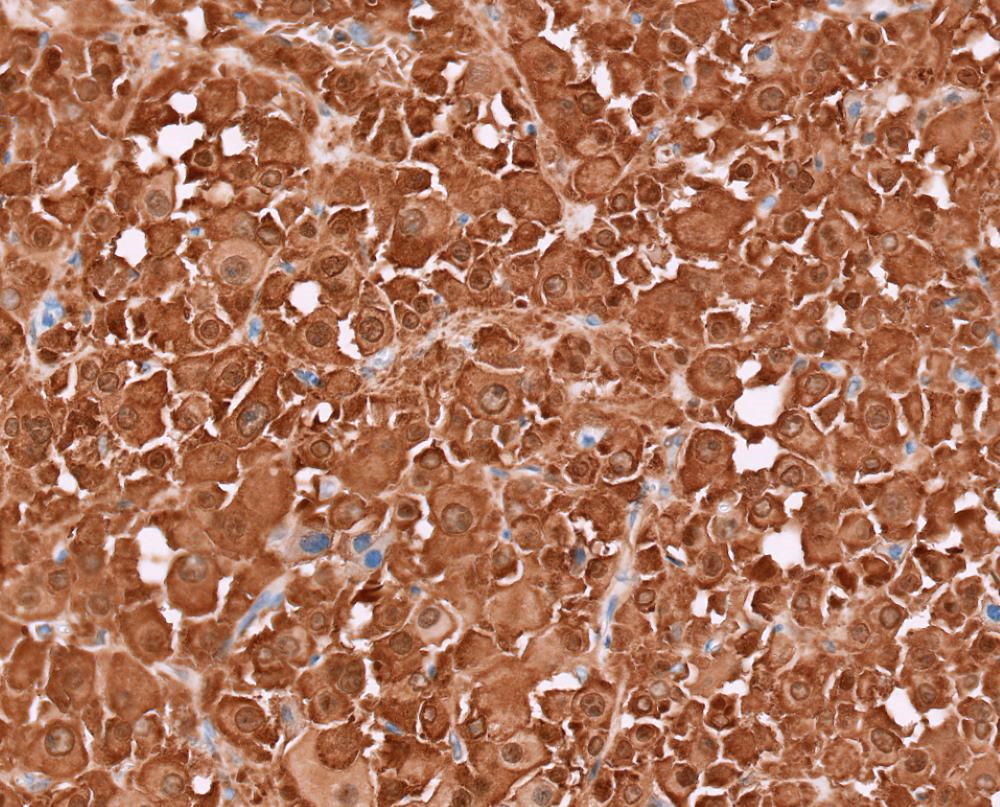
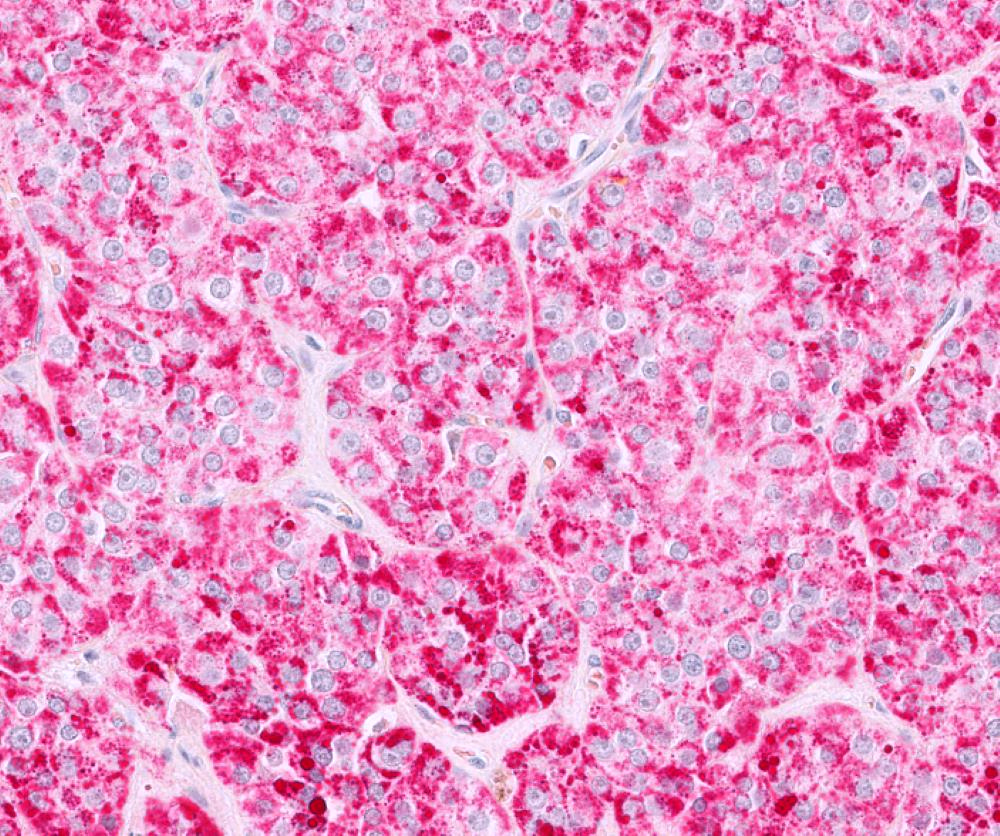
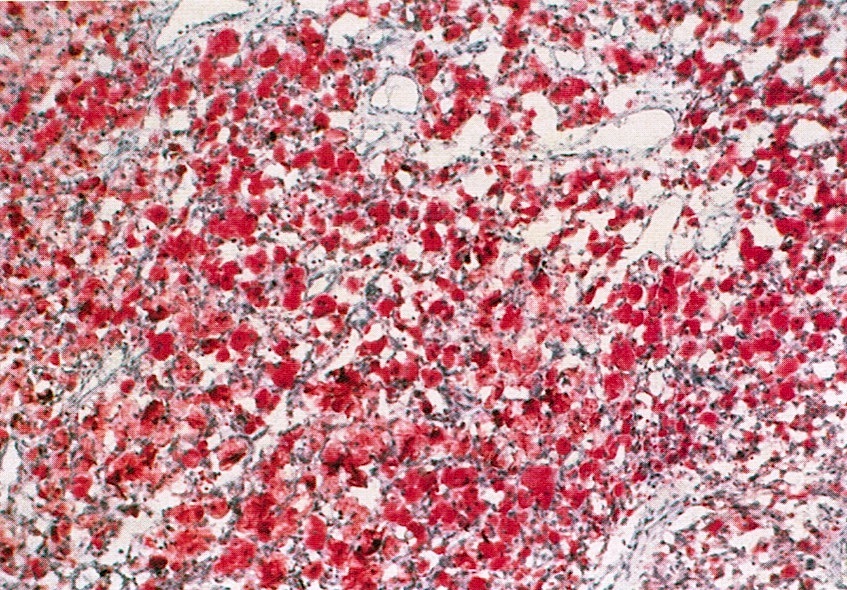
- Insular: chromogranin, synaptophysin, CD56
- Trabecular: synaptophysin, CD56, serotonin
- Strumal: chromogranin, synaptophysin, NSE, galectin3 in carcinoid component; TTF1 in thyroid component (Medicine (Baltimore) 2019;98:e18009)
- Mucinous: pankeratin, synaptophysin, chromogranin, CDX2, CK20
- Reference: Cancers (Basel) 2022;14:1835
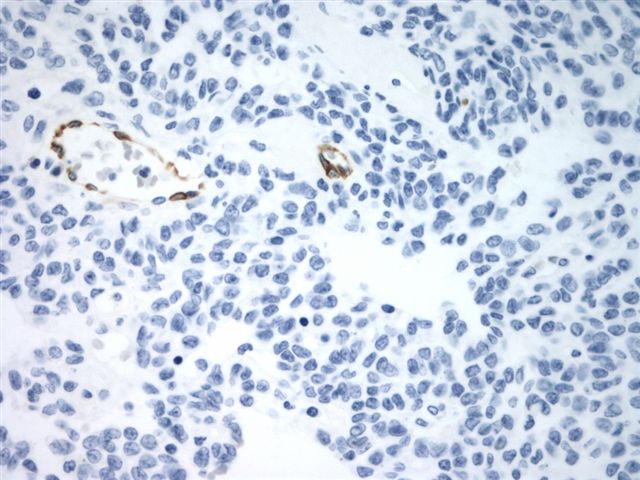
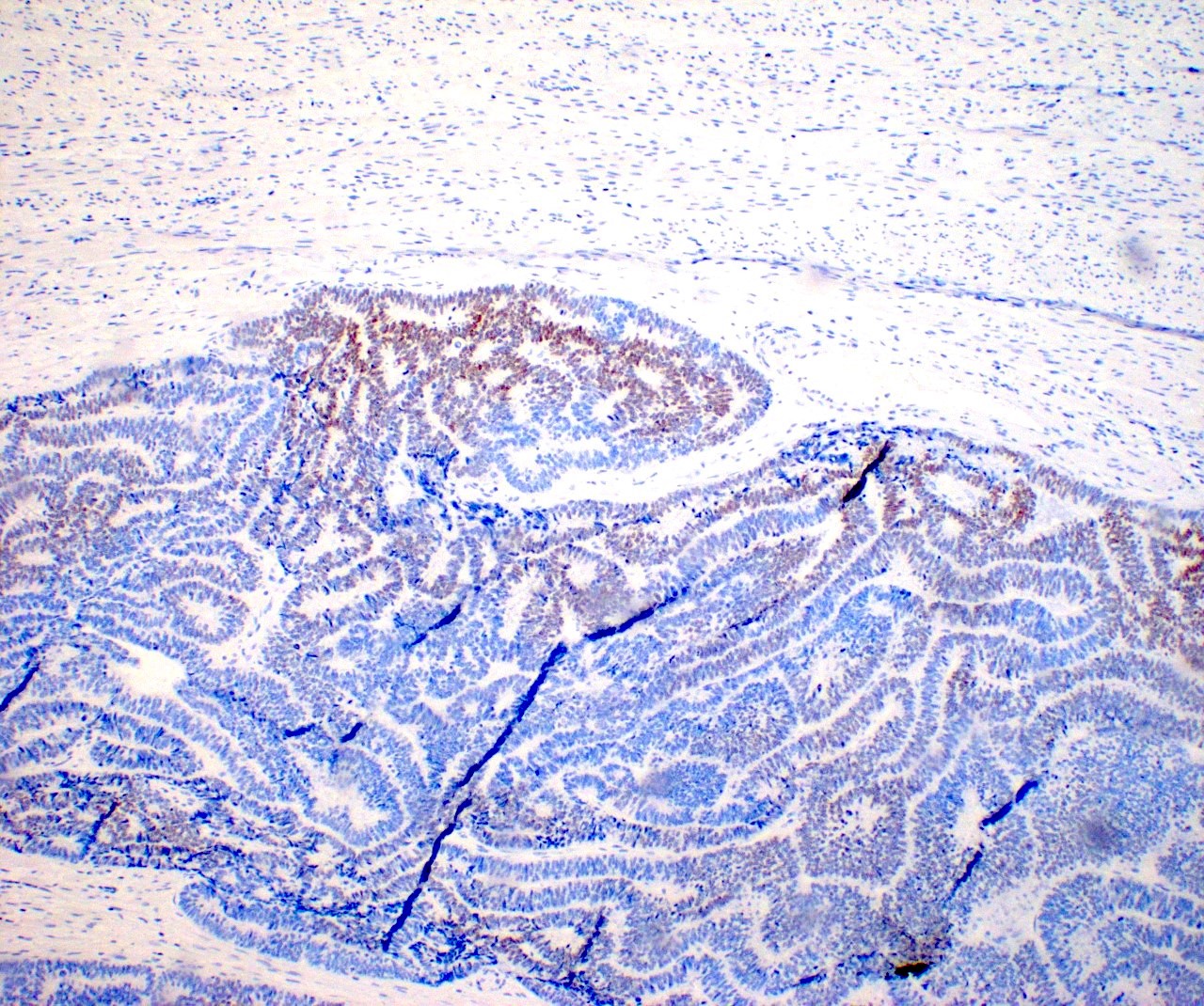
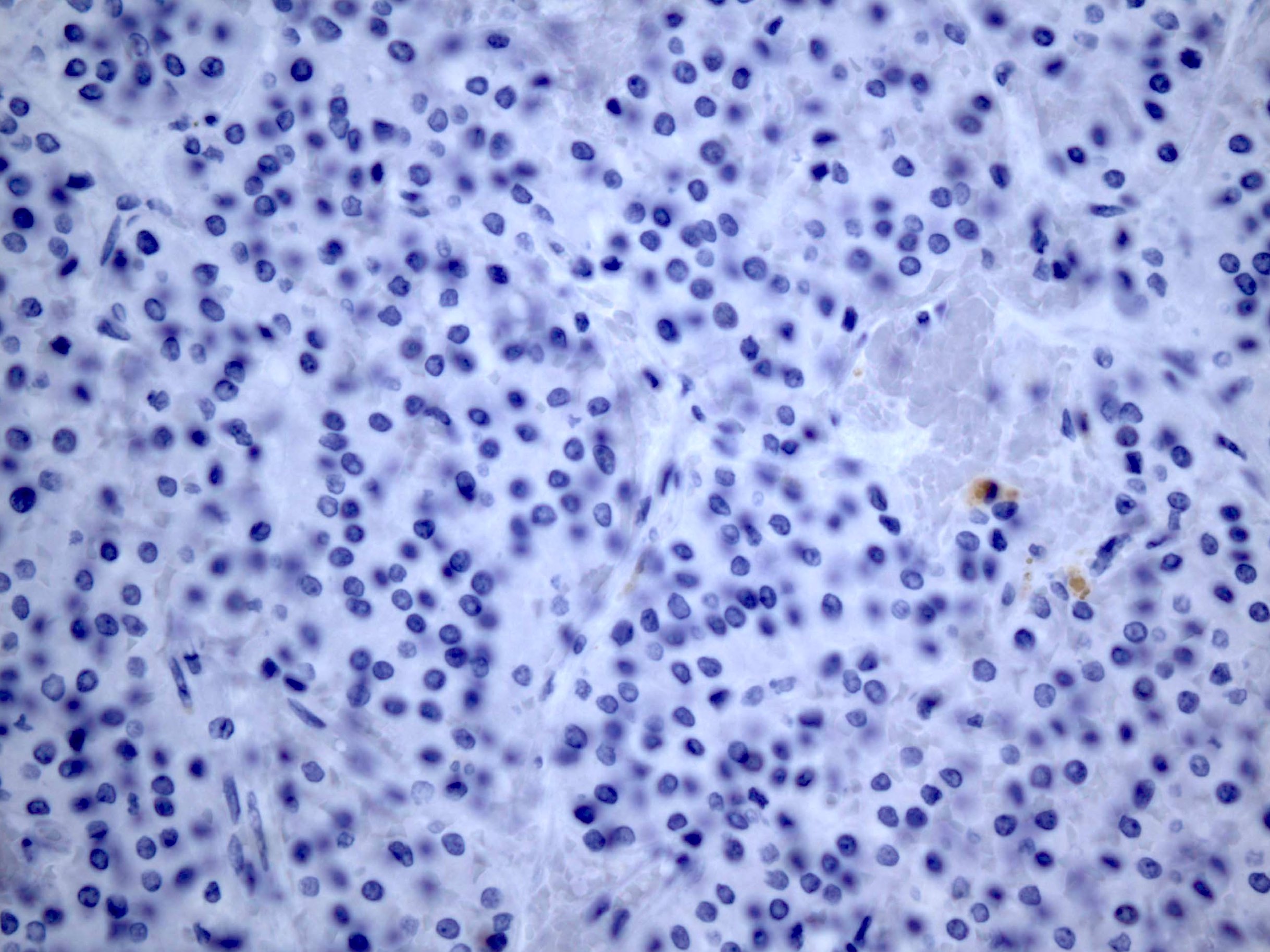
Negative stains
- TTF1, PAX8, thyroglobulin only stain the struma component
Electron microscopy description
- Numerous neurosecretory granules
Videos
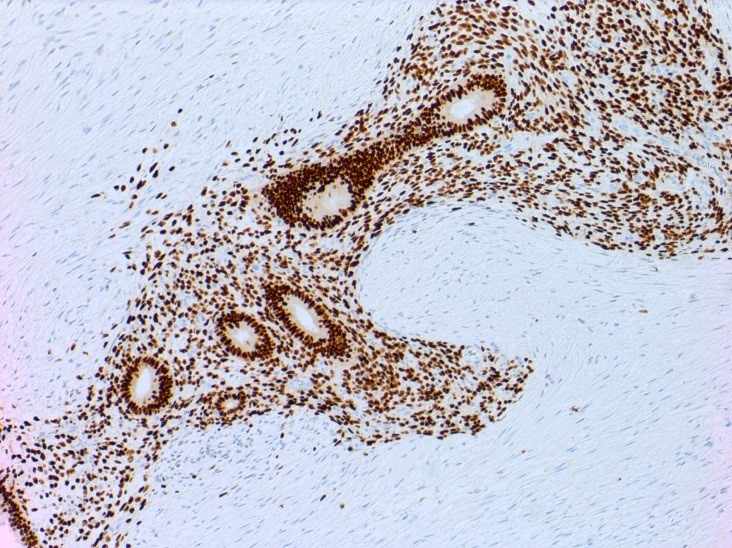
Germ cell neoplasms of the ovary - ovarian carcinoid tumor
by Dr. Wafaey Badawy
Sample pathology report
- Right ovary, oophorectomy:
- Ovarian carcinoid tumor, arising from struma ovarii (see comment)
- Comment: Sections show extensive involvement of the ovary by a well differentiated neuroendocrine tumor, showing acinar, trabecular, tubular, corded and some rare areas of solid growth patterns, confined to the ovary without surface involvement. Background shows benign thyroid tissue. Immunohistochemical stains including chromogranin and synaptophysin support neuroendocrine differentiation. Given the presence of benign thyroid tissue, this neoplasm is classified as a strumal carcinoid. The use of proliferative markers such as Ki67 has not been established in the grading of these tumors and in this case, it is < 1%
- Left ovarian cyst, resection:
- Ovarian carcinoid tumor (4.5 mm), trabecular type, arising in a mature cystic teratoma (dermoid cyst) (see comment)
- Comment: Extensive sampling of this mature teratoma shows a 4.5 mm trabecular carcinoid tumor. Immunohistochemical stains confirm neuroendocrine differentiation as the tumor cells are positive for synaptophysin, chromogranin, CD56, MCK (AE1 / AE3) and Ki67 is ~2%. This small focus of carcinoid tumor shows CDX2 positivity indicating midgut origin, similar to the gastrointestinal counterpart.
Differential diagnosis
- Carcinoid metastasis:
- Bilateral, no associated teratoma or struma ovarii
- Often multinodular growth
- Microscopically indistinguishable from primary
- Most commonly from midgut primary
- Brenner tumor:
- May be confused with insular carcinoid
- Transitional epithelial cells, grooved nuclei, lack of cytoplasmic granules
- Krukenberg tumor:
- Bilateral, marked cellular and nuclear pleomorphism
- Mitotically active, abundant mucin
- Signet ring cells are common
- Lymphovascular invasion is common
- Sertoli cell tumor (SCT) with trabecular architecture:
- SCT is positive for inhibin and calretinin, negative for synaptophysin
- Trabecular carcinoid is positive for synaptophysin, negative for inhibin and calretinin
Additional references
Board review style question #1
Which of the following is true for primary ovarian carcinoid?
- Most cases of primary ovarian carcinoid are associated with mature cystic teratoma
- Mucinous ovarian carcinoid behaves less aggressively than other types
- Primary ovarian carcinoids are poorly differentiated neuroendocrine tumors
- To cause carcinoid syndrome, the presence of liver metastasis is required
Board review style answer #1
A. Most cases of primary ovarian carcinoid are associated with mature cystic teratoma. Despite mucinous ovarian carcinoids more frequently occurring in pure form, 3 of 4 types, including the most common insular type, mostly arise in mature cystic teratoma.
Answer B is incorrect because mucinous ovarian carcinoid is more aggressive than other types of primary ovarian carcinoid.
Answer C is incorrect because per definition, primary ovarian carcinoid tumors are well differentiated neuroendocrine tumors.
Answer D is incorrect because ovarian veins drain directly into systemic circulation: the right ovarian vein drains directly into the inferior vena cava, while the left ovarian vein joins the left renal vein. Therefore, hormones produced by a carcinoid tumor do not undergo hepatic metabolism.
Comment Here
Reference: Carcinoid tumor
Comment Here
Reference: Carcinoid tumor
Board review style question #2
Which of the following indicates poor prognosis of patients with primary ovarian carcinoid?
- Carcinoid heart syndrome
- Insular type
- Low Ki67 proliferative index
- Trabecular type
- Tumor confined to the ovary
Board review style answer #2
A. Carcinoid heart syndrome can be a presenting symptom even in the absence of liver metastases. The 3 year survival has been reported to be 31%.
Answer B is incorrect because insular type is slow growing and only occasionally metastasizes. The prognosis is favorable.
Answer C is incorrect because a high Ki67 proliferative index, not low, is associated with worse outcomes.
Answer D is incorrect because trabecular type is not associated with metastasis and has favorable prognosis.
Answer E is incorrect because the absence of tumor spread is a good prognostic factor.
Comment Here
Reference: Carcinoid tumor
Comment Here
Reference: Carcinoid tumor
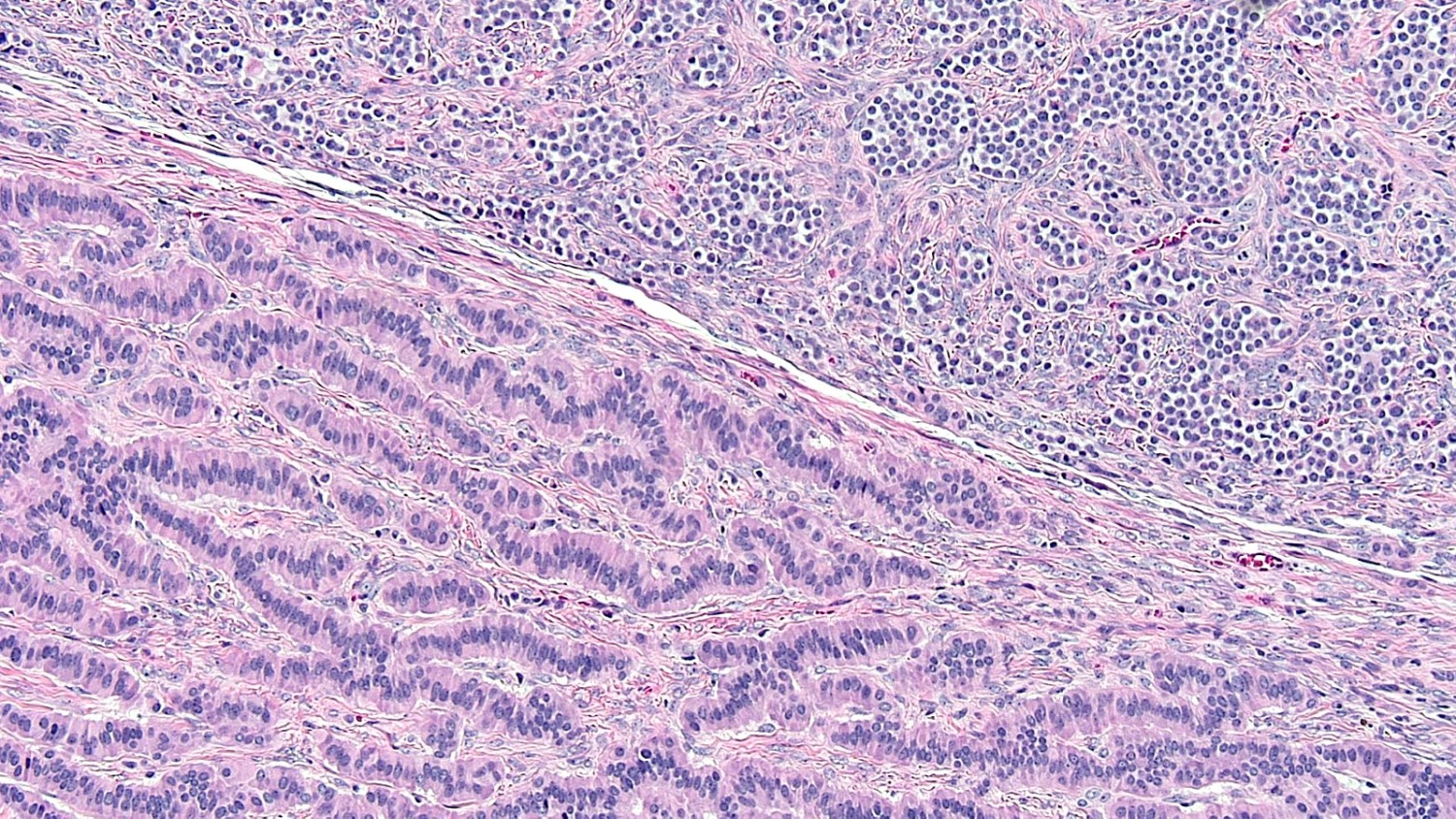
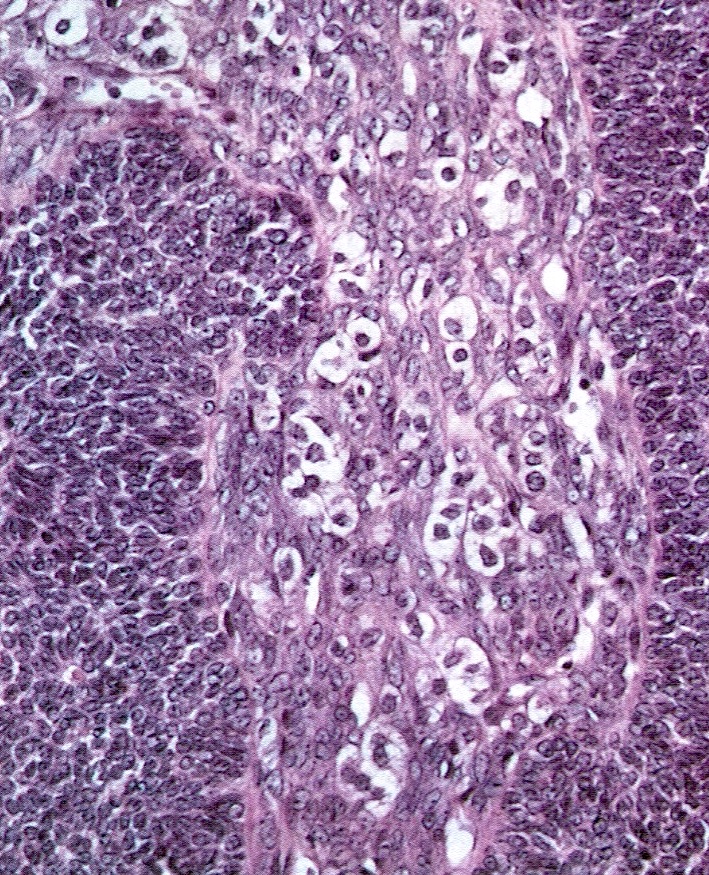
Carcinoid tumor metastatic to ovary
Table of Contents
Definition / general | Epidemiology | Clinical features | Laboratory | Radiology description | Prognostic factors | Case reports | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Differential diagnosisDefinition / general
- Carcinoid tumors involving the ovary can be primary (see Ovary tumor > germ cell tumors > Carcinoid tumors) or metastatic
- Primary ovarian carcinoid tumors are frequently seen in the context of a teratoma
- Robboy et al. reported 35/48 (73%) insular carcinoids were associated with a mature teratoma component
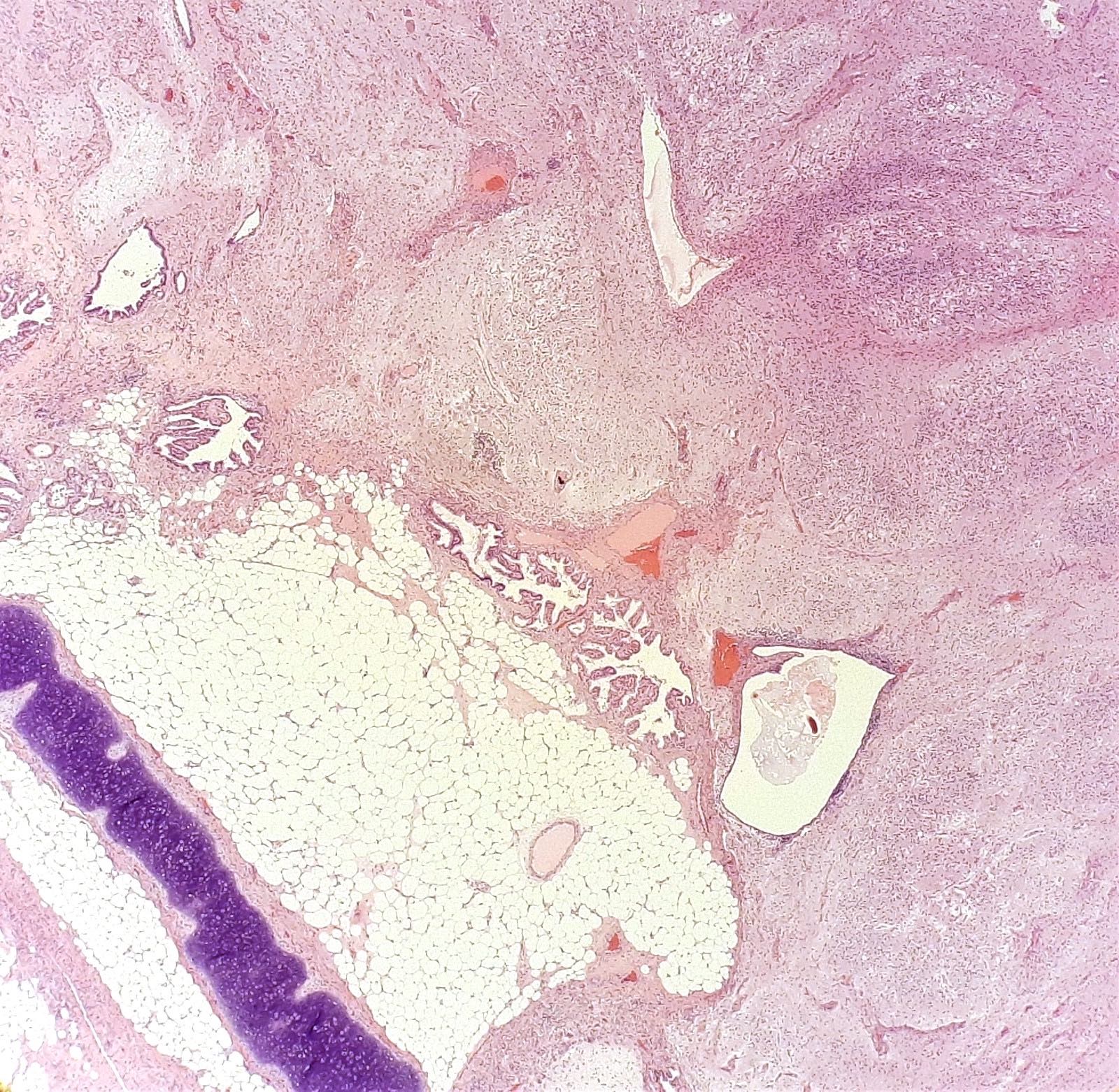
- Also, 2/48 tumors (4%) had a mucinous cystadenoma component (Cancer 1975;36:404)
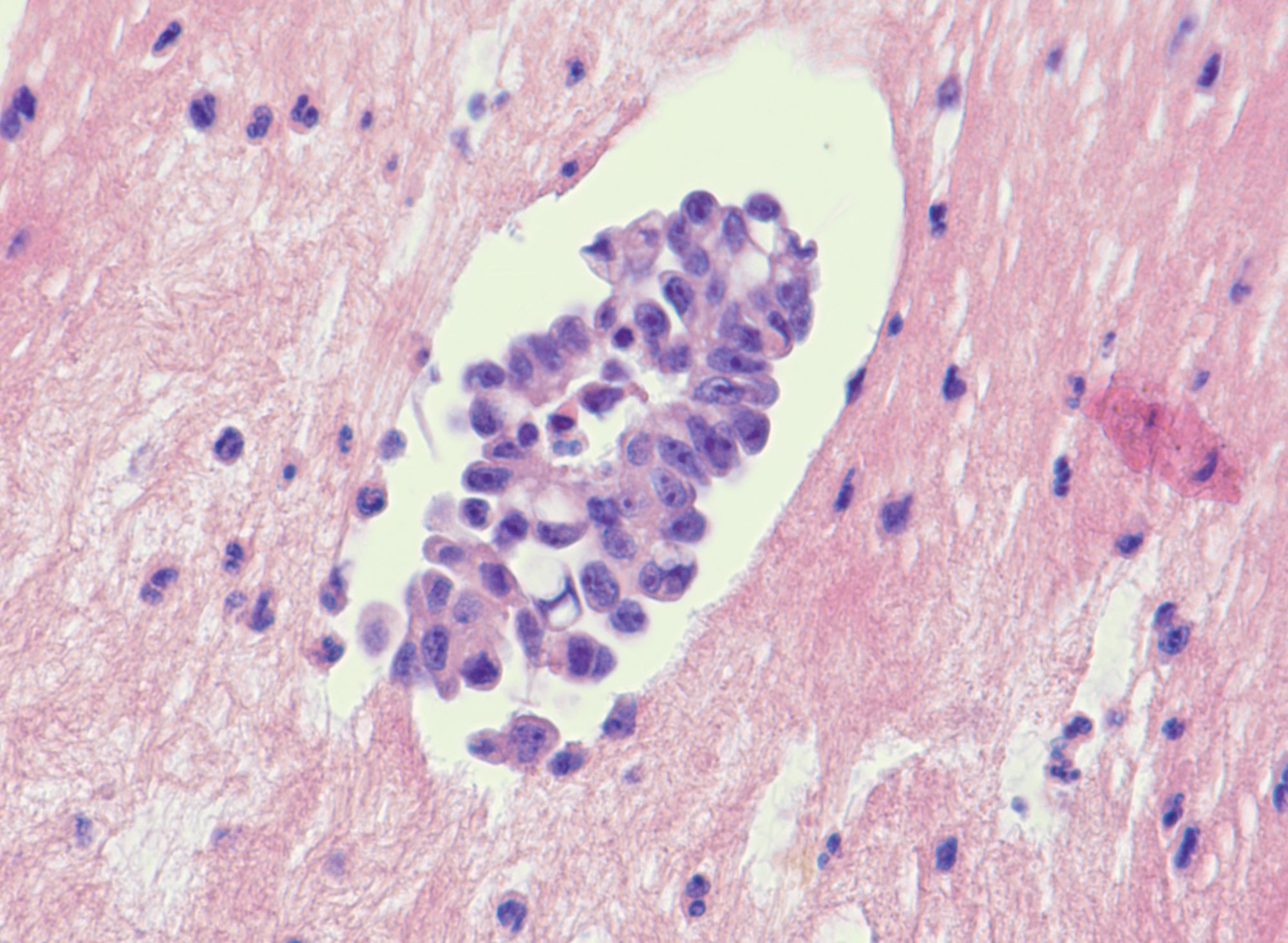
- Metastatic carcinoid tumors originate from the GI tract
- Most common primary site is distal ileum: 20/30 (67%) as reported by Robboy et al. (Cancer 1974;33:798), and 15/17 (88%) as reported by Strosberg et al. (Gynecol Oncol 2007;106:65)
- Less frequent locations are cecum, appendix, jejunum and pancreas
- Synchronous metastases are frequent at time of diagnosis (88 - 100% of cases) (Cancer 1974;33:798, Gynecol Oncol 2007;106:65)
- Involved sites include pelvis (uterine and tubal serosa), abdominal cavity (peritoneum, intestinal serosa, liver) and retroperitoneum (periaortic lymph nodes)
Epidemiology
- Average age 57 to 62 years, range 21 to 82 years (Cancer 1974;33:798, Gynecol Oncol 2007;106:65, Hum Pathol 2013;44:2536, Int J Gynecol Pathol 2008;28:41)
- Most patients (>90%) are Caucasian; a minority are Black and Hispanic (Cancer 1974;33:798, Gynecol Oncol 2007;106:65)
Clinical features
- Symptoms of carcinoid syndrome are seen in 30 - 53% of cases, and include intermittent diarrhea, flushing, ankle edema, involuntary weight loss and cardiac murmurs
- Other symptoms are due to mass effect, including abdominal/pelvic pain and bowel obstruction
- Rarely, tumors are diagnosed incidentally during routine gynecologic examination
Laboratory
- Elevated urinary levels of 5-hydroxyindole acetic acid and serum serotonin are consistently found, as observed by Strosberg et al. (average peak of 24h 5-HIAA urine levels was 60 mg, reference range 0 - 6 mg) (Gynecol Oncol 2007;106:65)
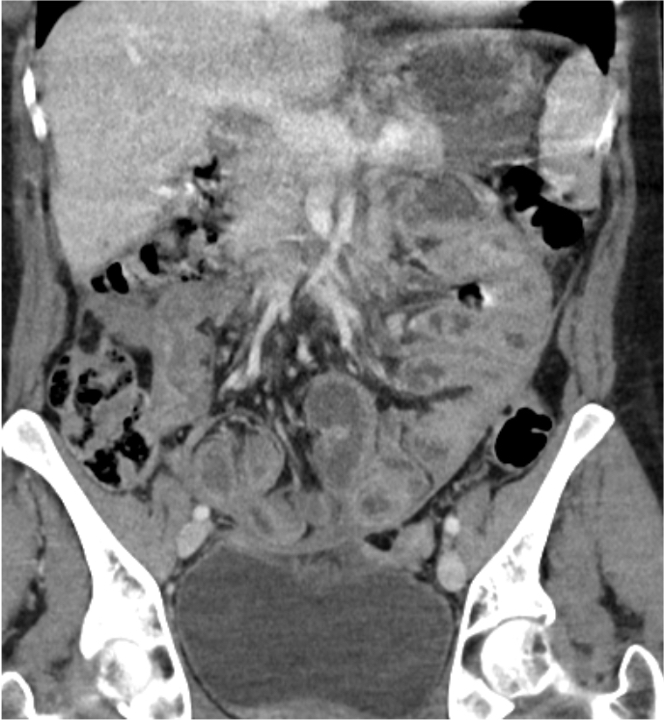
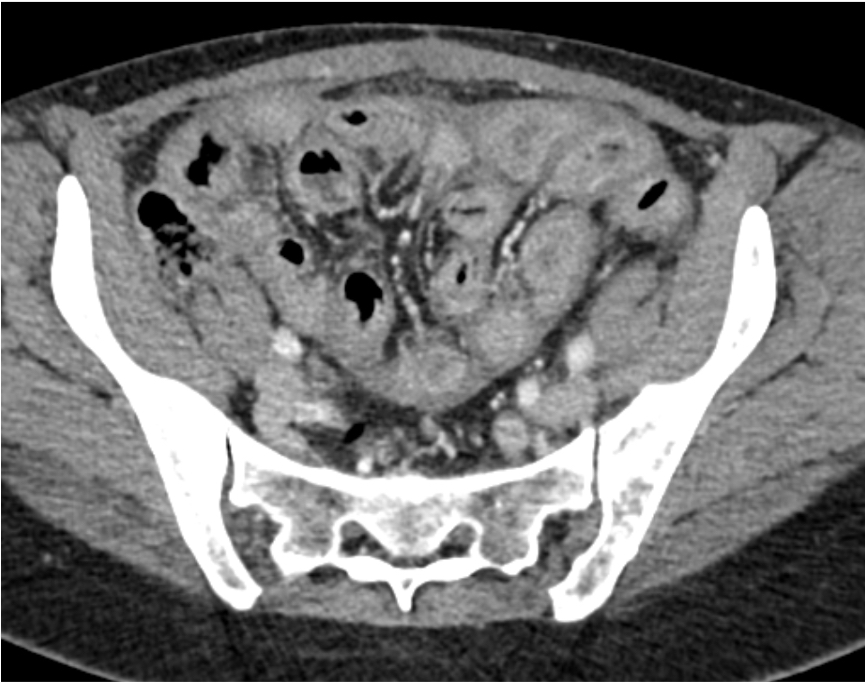
Radiology description
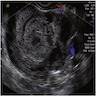
- Pelvic ultrasound confirms the presence of an ovarian mass (and usually detects bilaterality)
- Most patients have abnormal radiotracer uptake on indium-111-pentetreotid scintigraphy
Prognostic factors
- In 1974, the first and largest case series of carcinoid tumors metastatic to the ovary, Robboy et al. reported an overall poor prognosis, with a survival rate of 66% after the first year and 33% at 4 years post-diagnosis (Cancer 1974;33:798)
- A 2007 case series by Strosberg et al. reported an excellent response to long-term Ocreotide treatment and cytoreductive surgery, with only 2 deaths out of 17 patients and a projected 5 year survival rate of 94% (Gynecol Oncol 2007;106:65)
Case reports
- Goblet cell carcinoid of vermiform appendix metastatic to ovary, mimicking primary ovarian mucinous cystadenocarcinoma (Acta Pathol Jpn 1991;41:455)
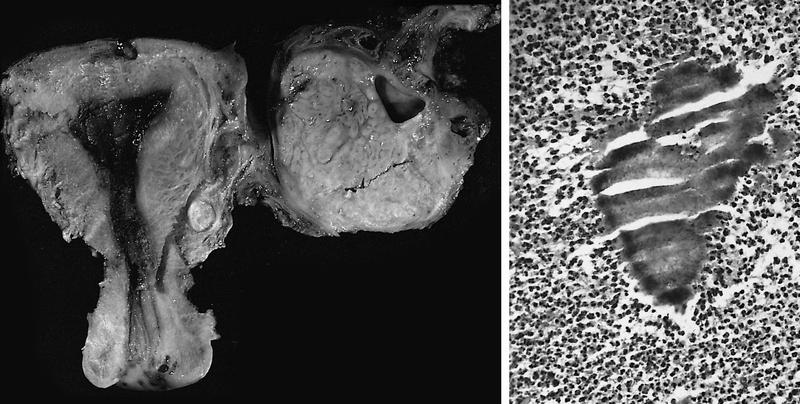
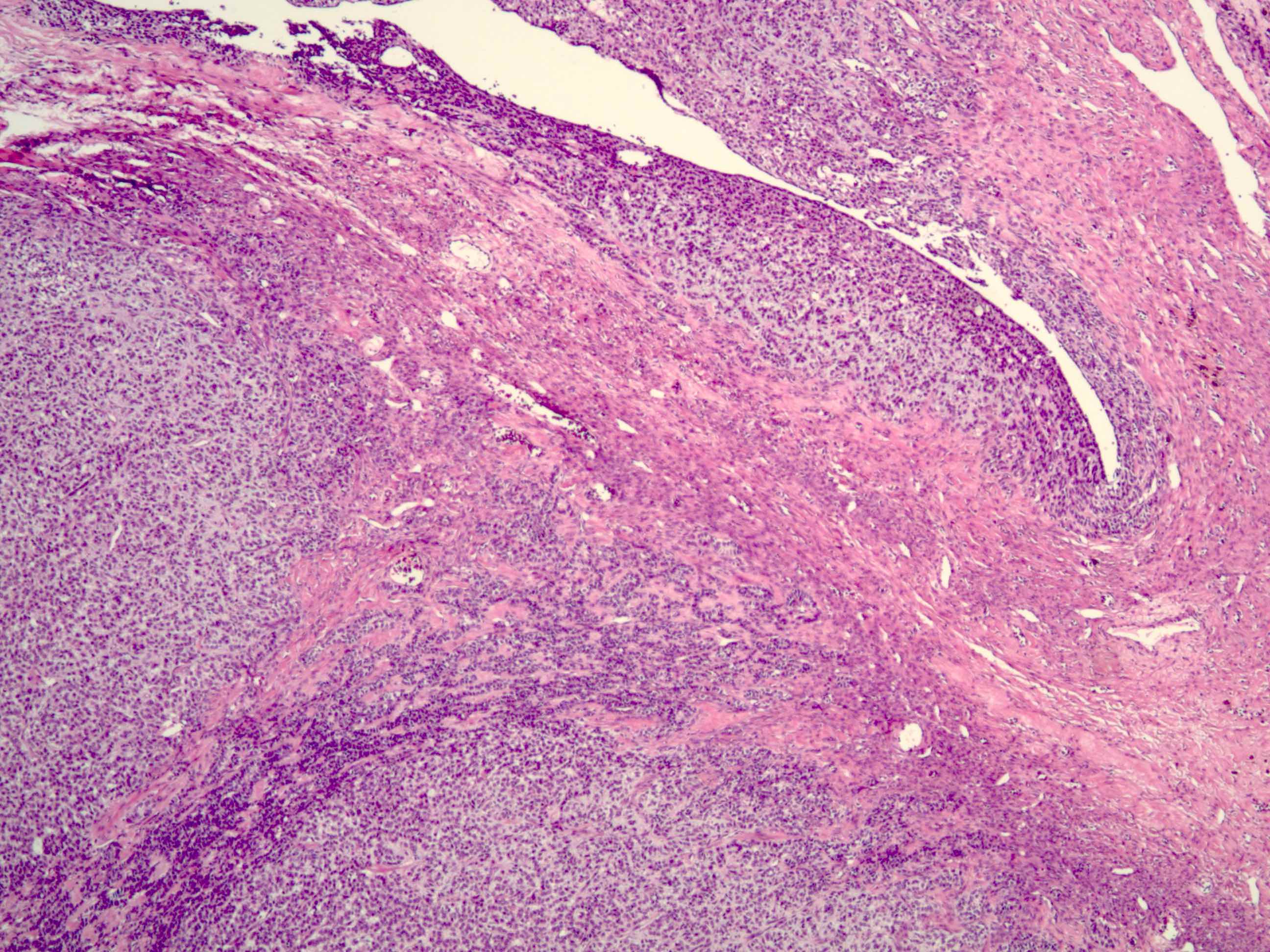
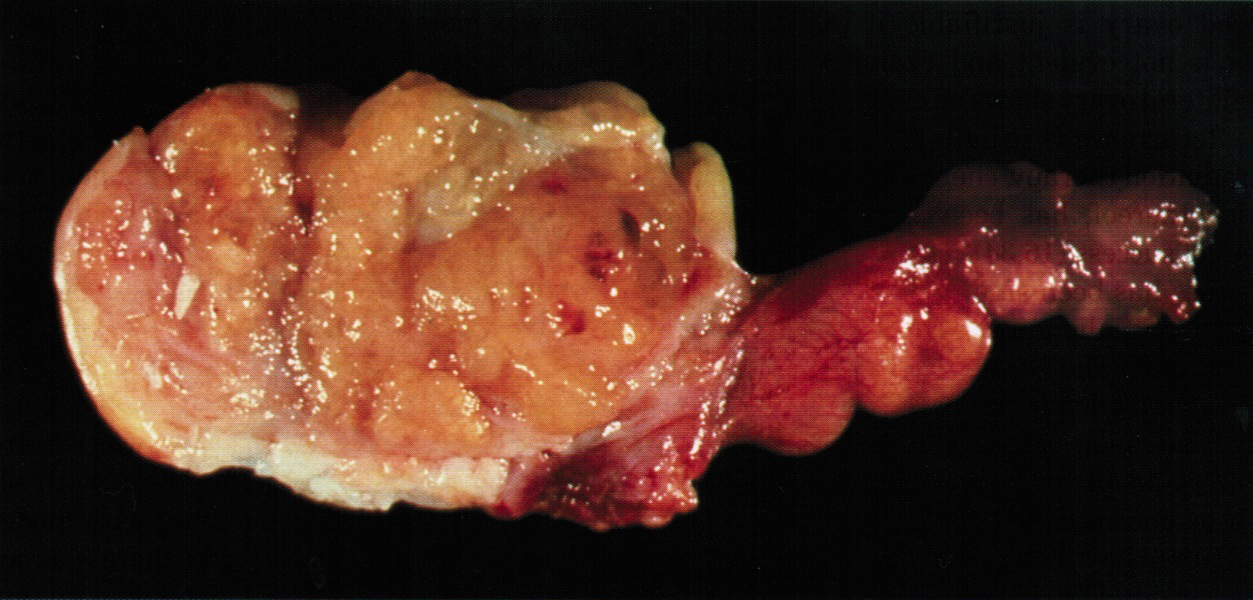
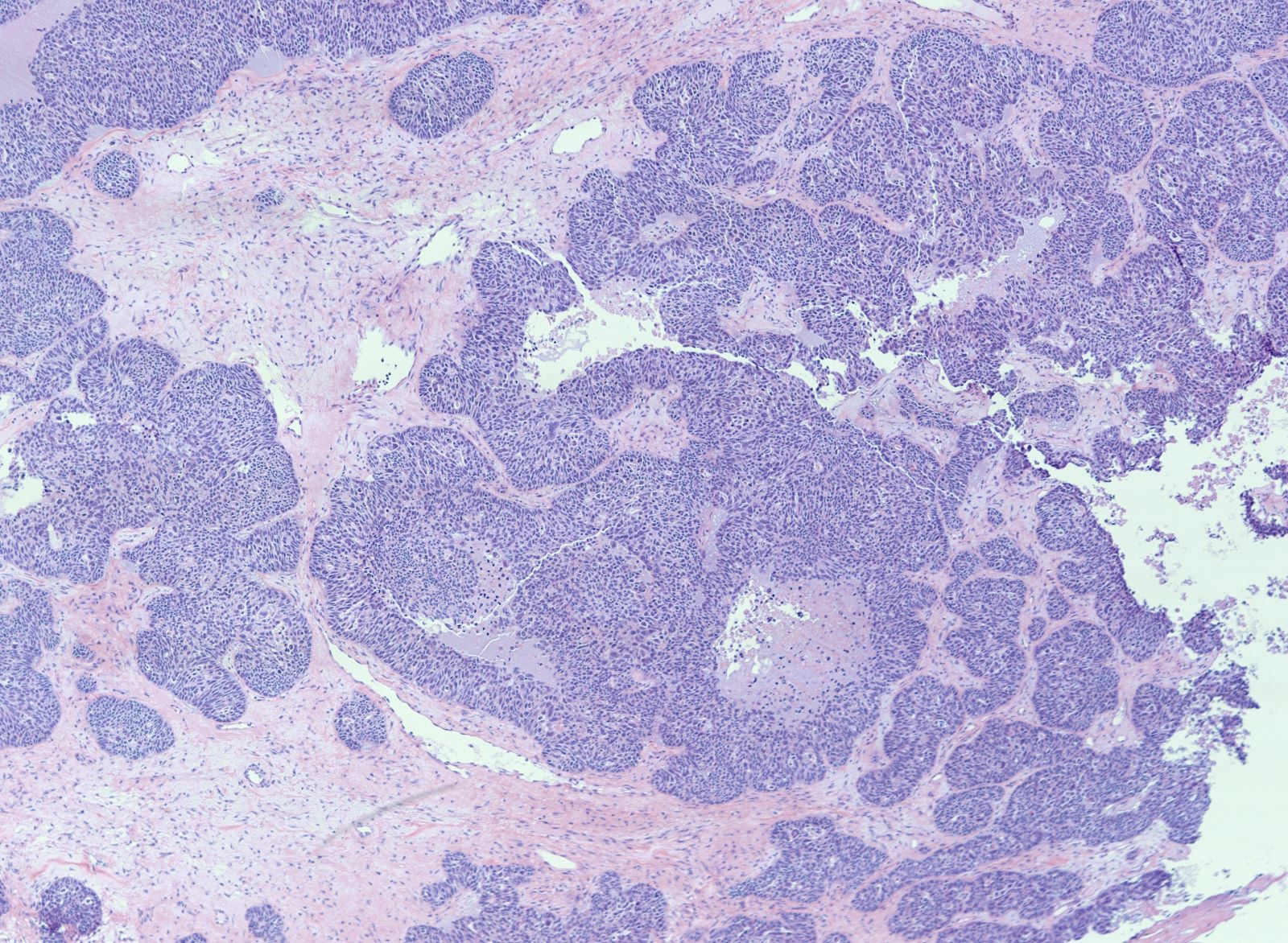
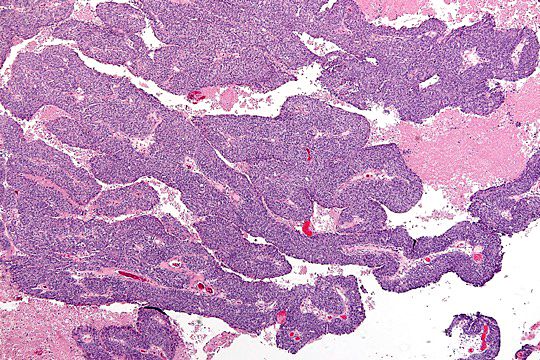
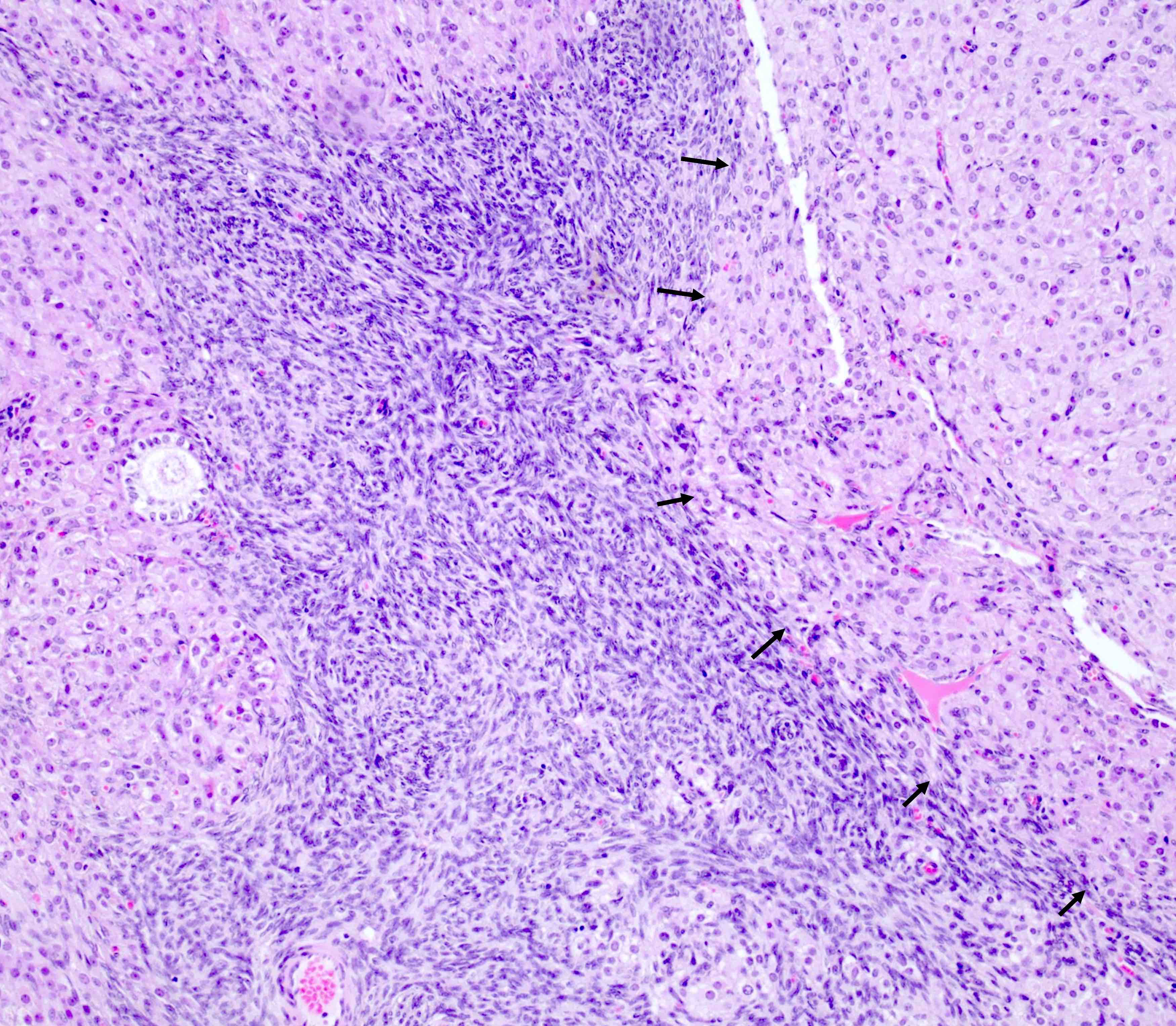
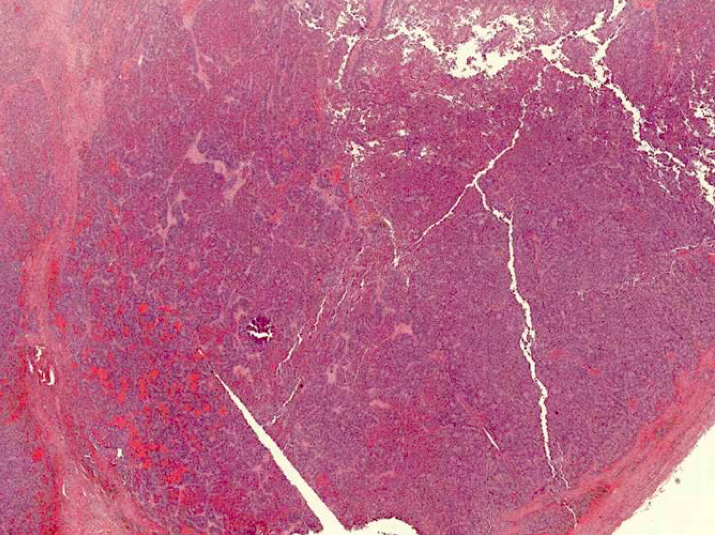
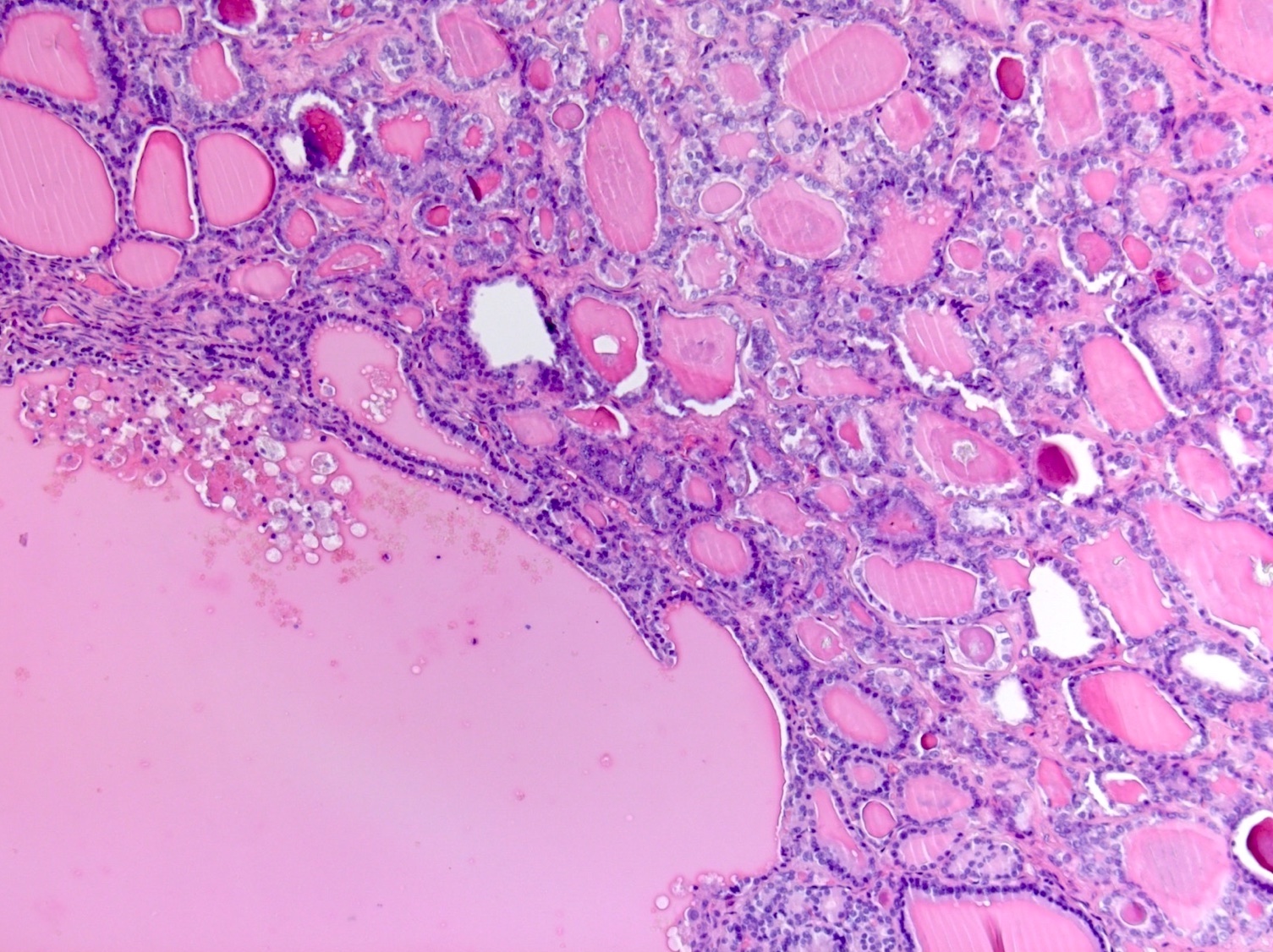
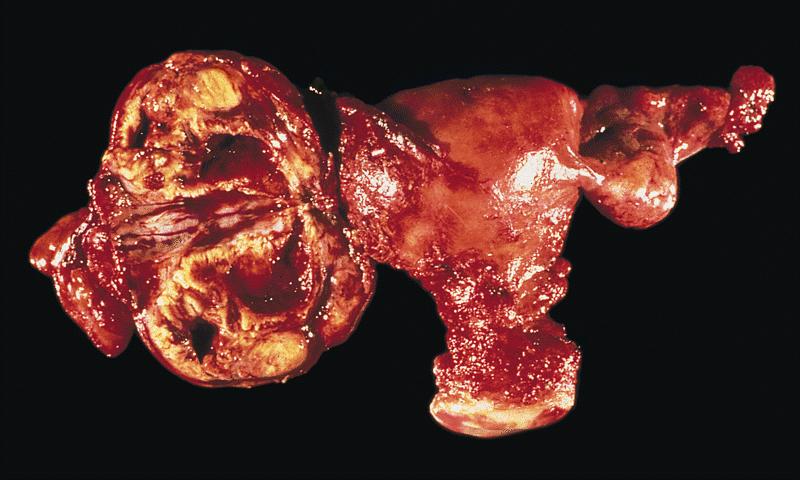
Gross description
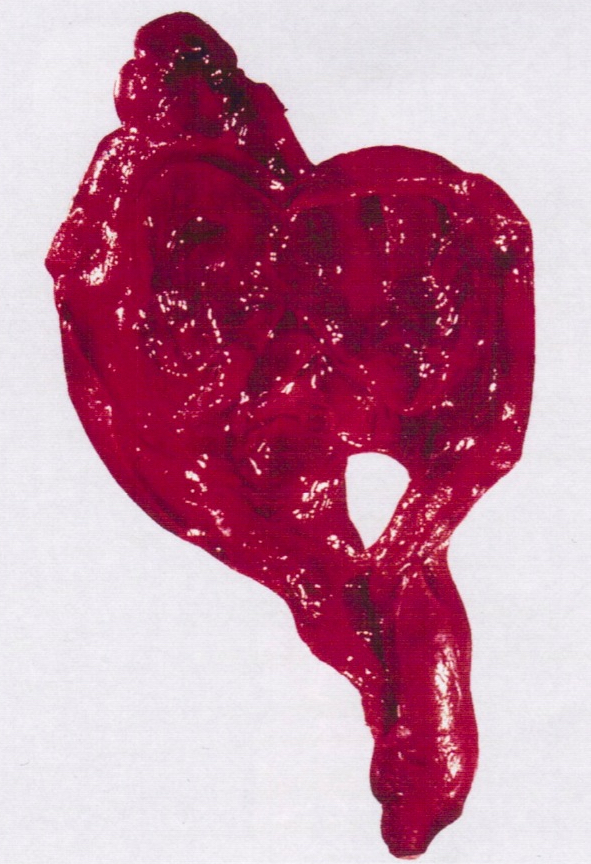
- Tumor size ranges from 4 to 32 cm with an average of 10.2 cm (Int J Gynecol Pathol 2008;28:41)
- Most tumors are purely solid (80% in case series by Rabban et al.) (Int J Gynecol Pathol 2008;28:41); a minority may display a cystic component
- Multinodular growth is frequently observed (60% in case series by Rabban et al.) (Int J Gynecol Pathol 2008;28:41)
- Bilateral ovarian involvement is seen in most cases (60 - 95%) (Cancer 1974;33:798)
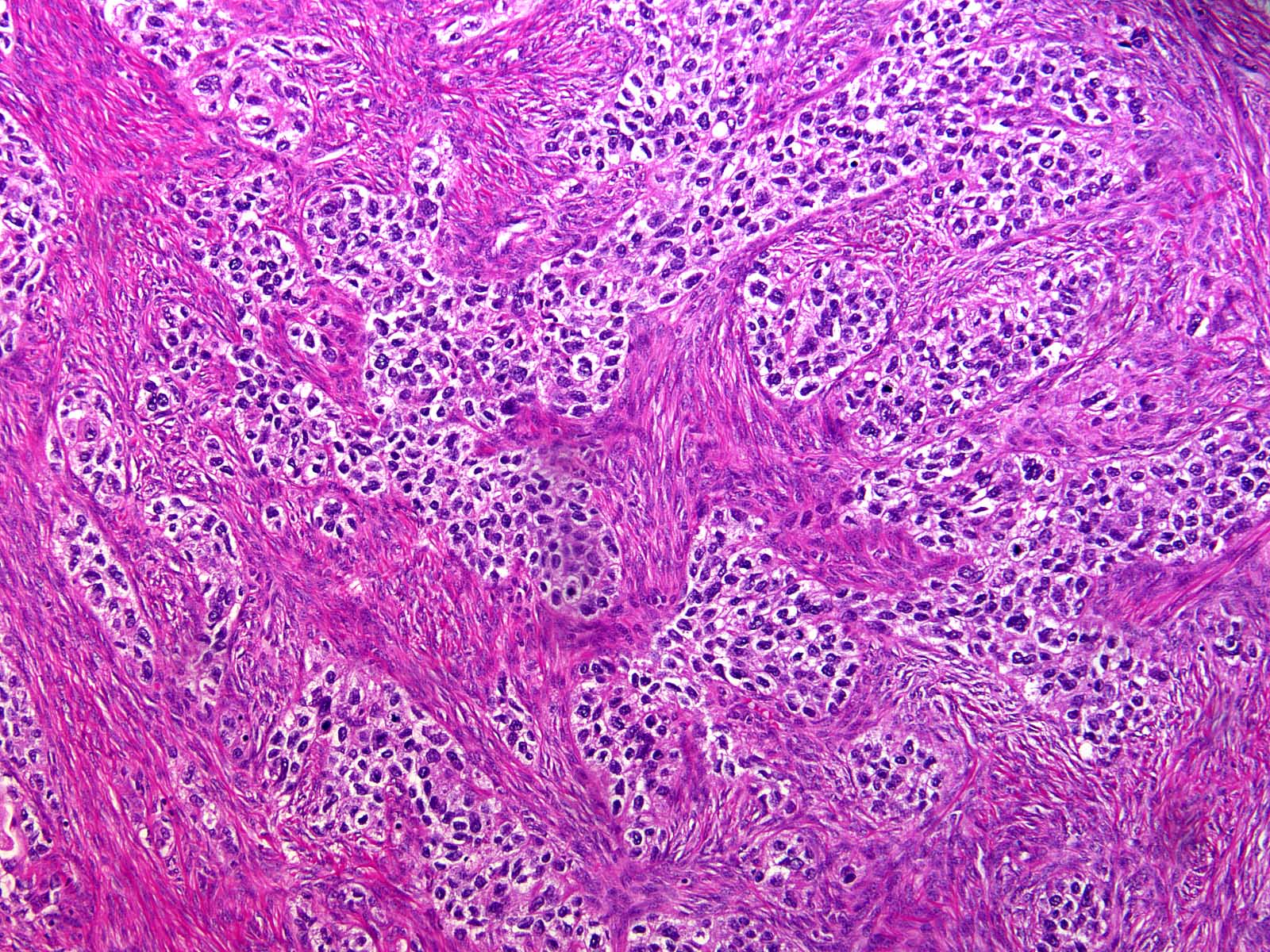
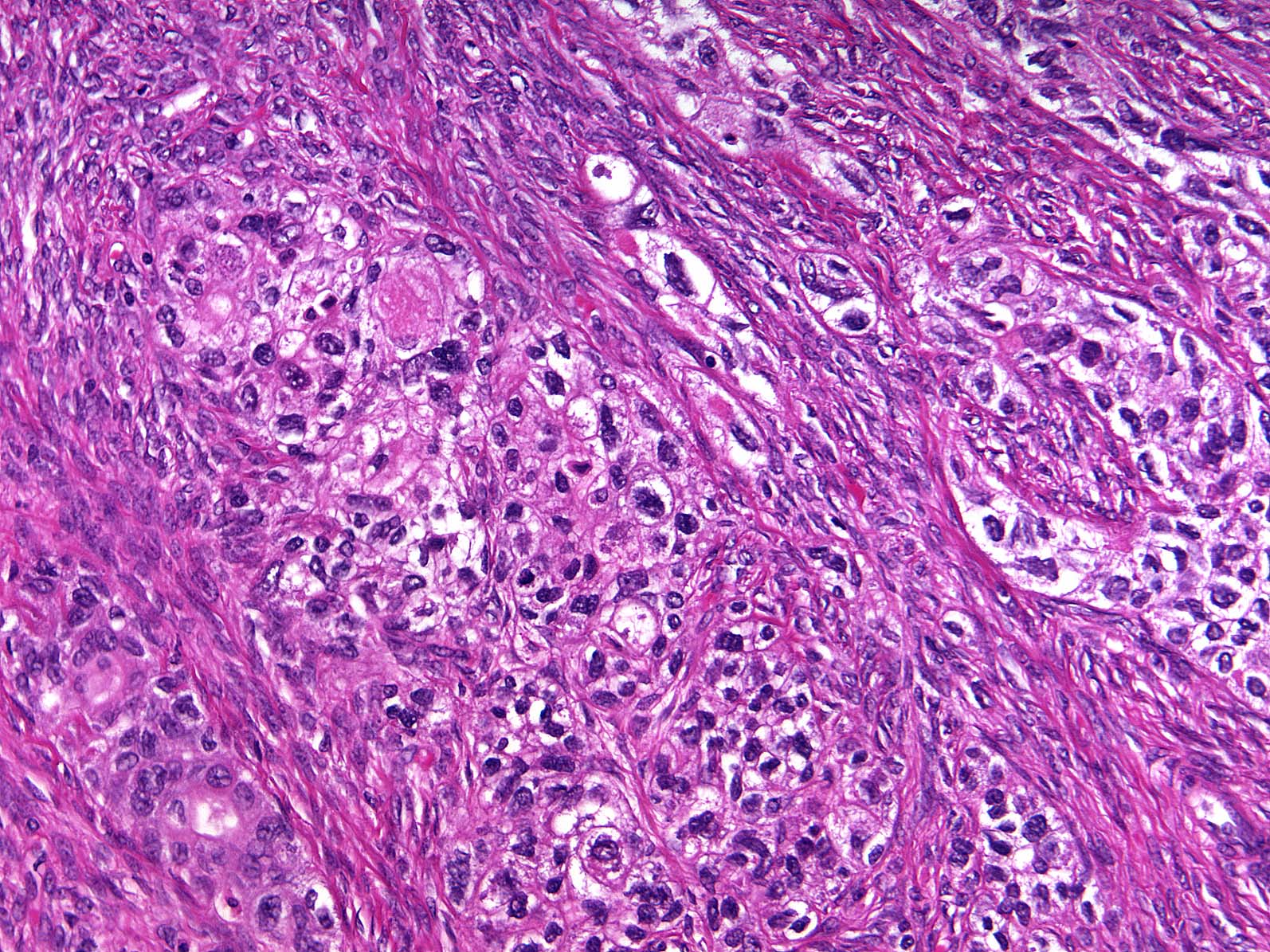
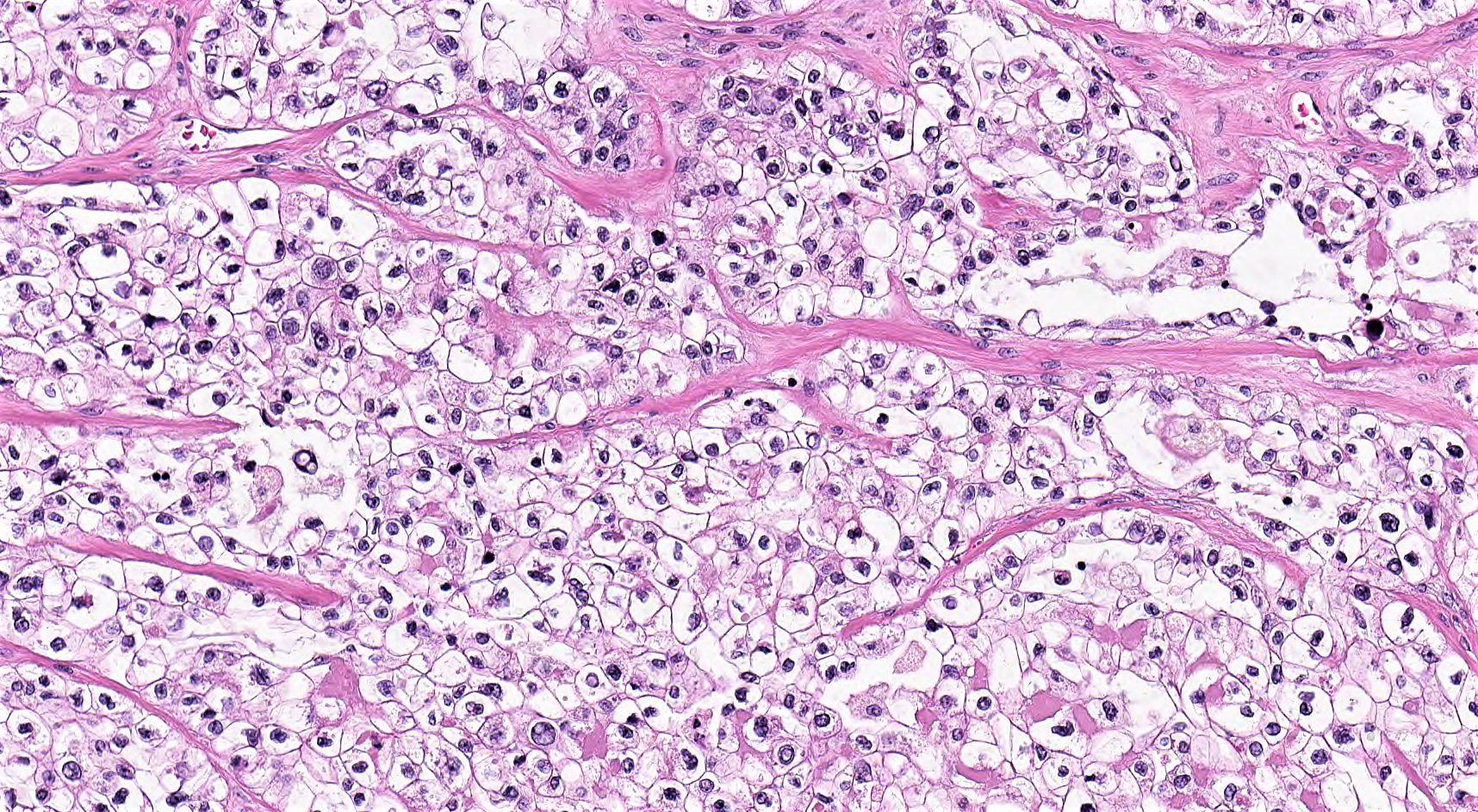
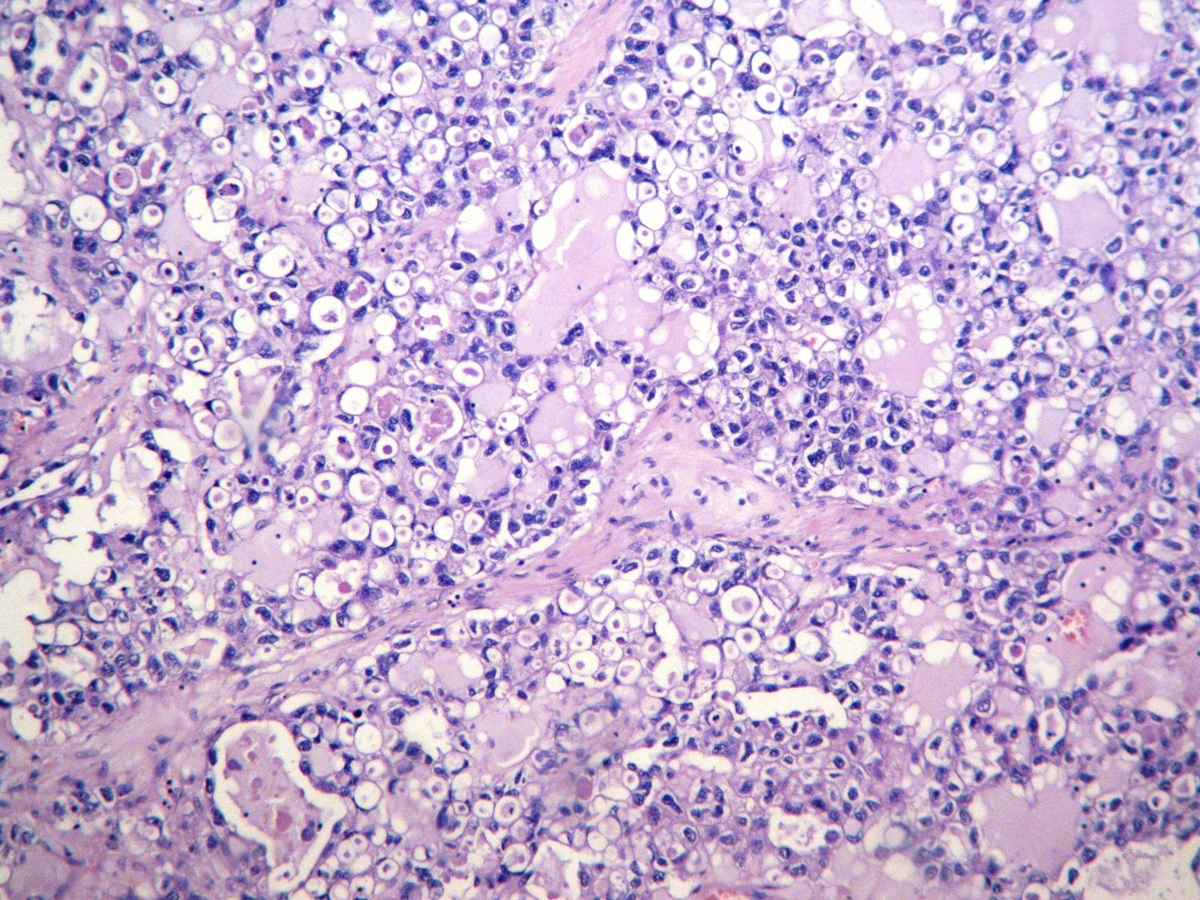
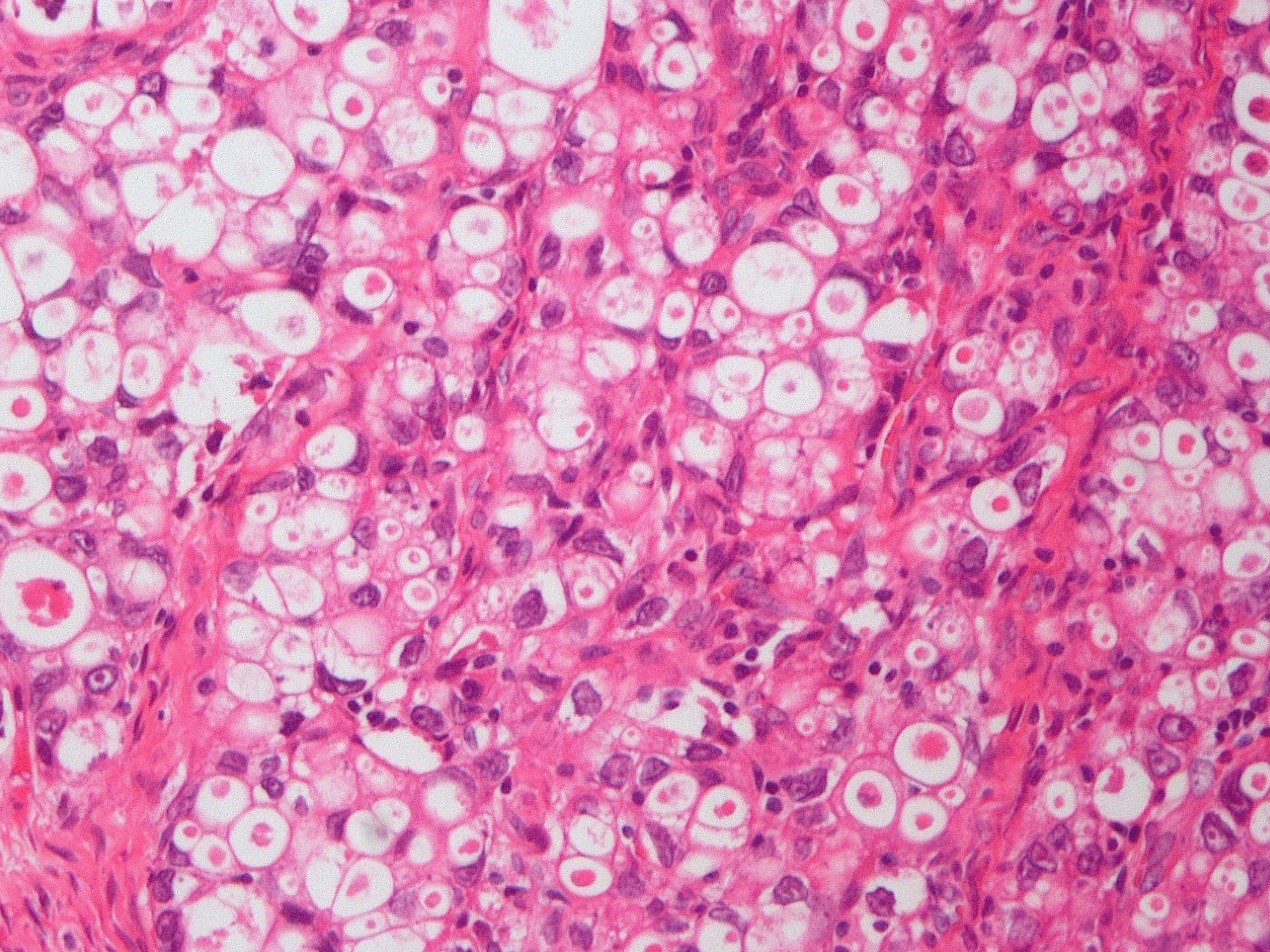
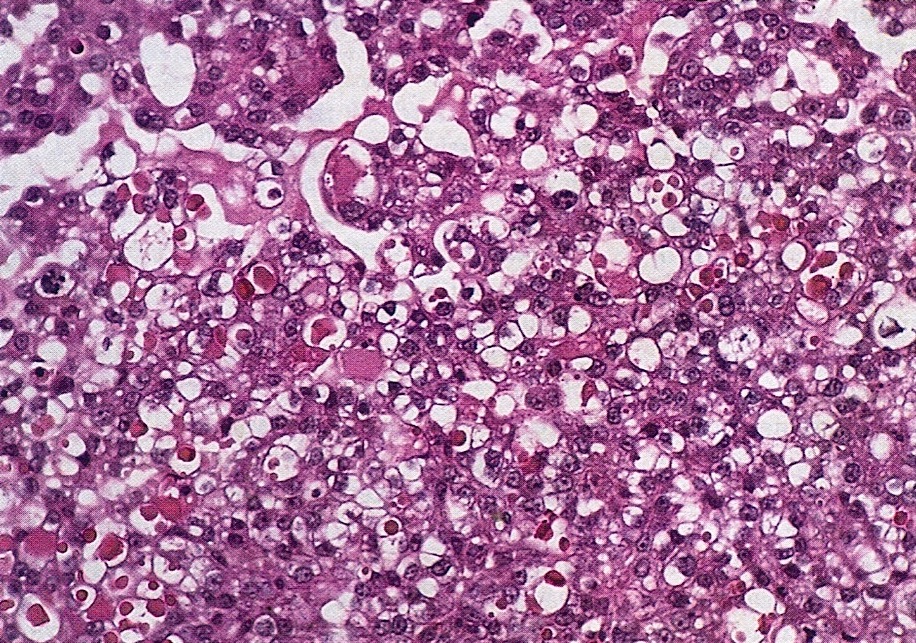
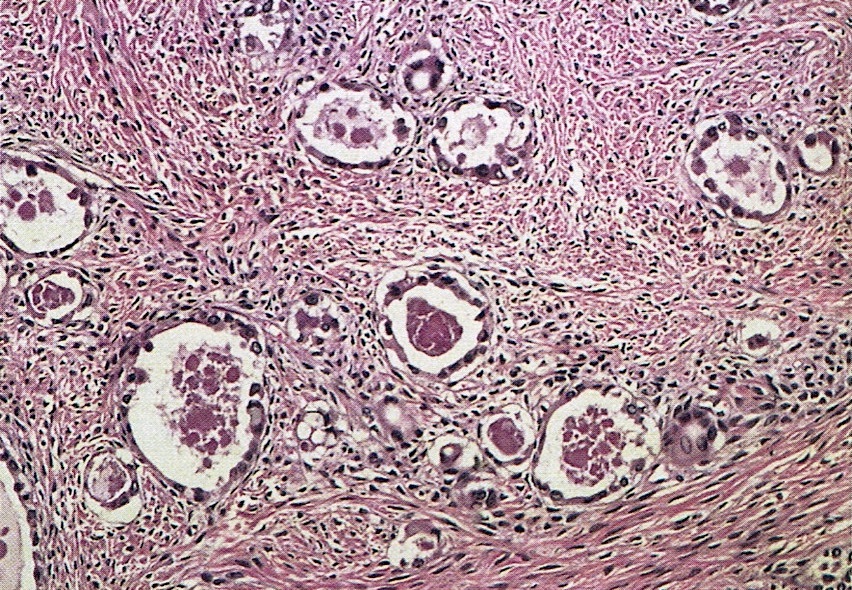
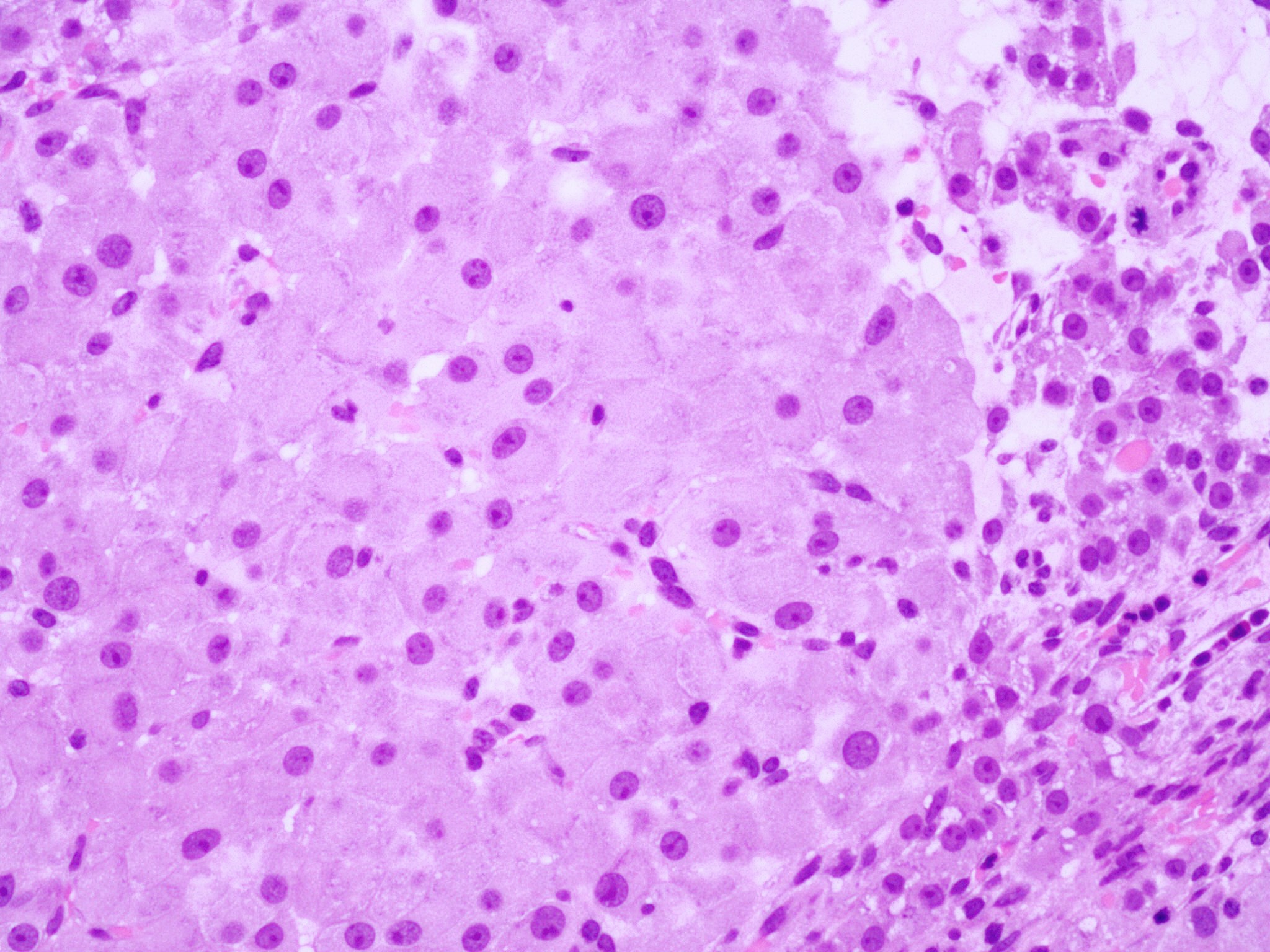
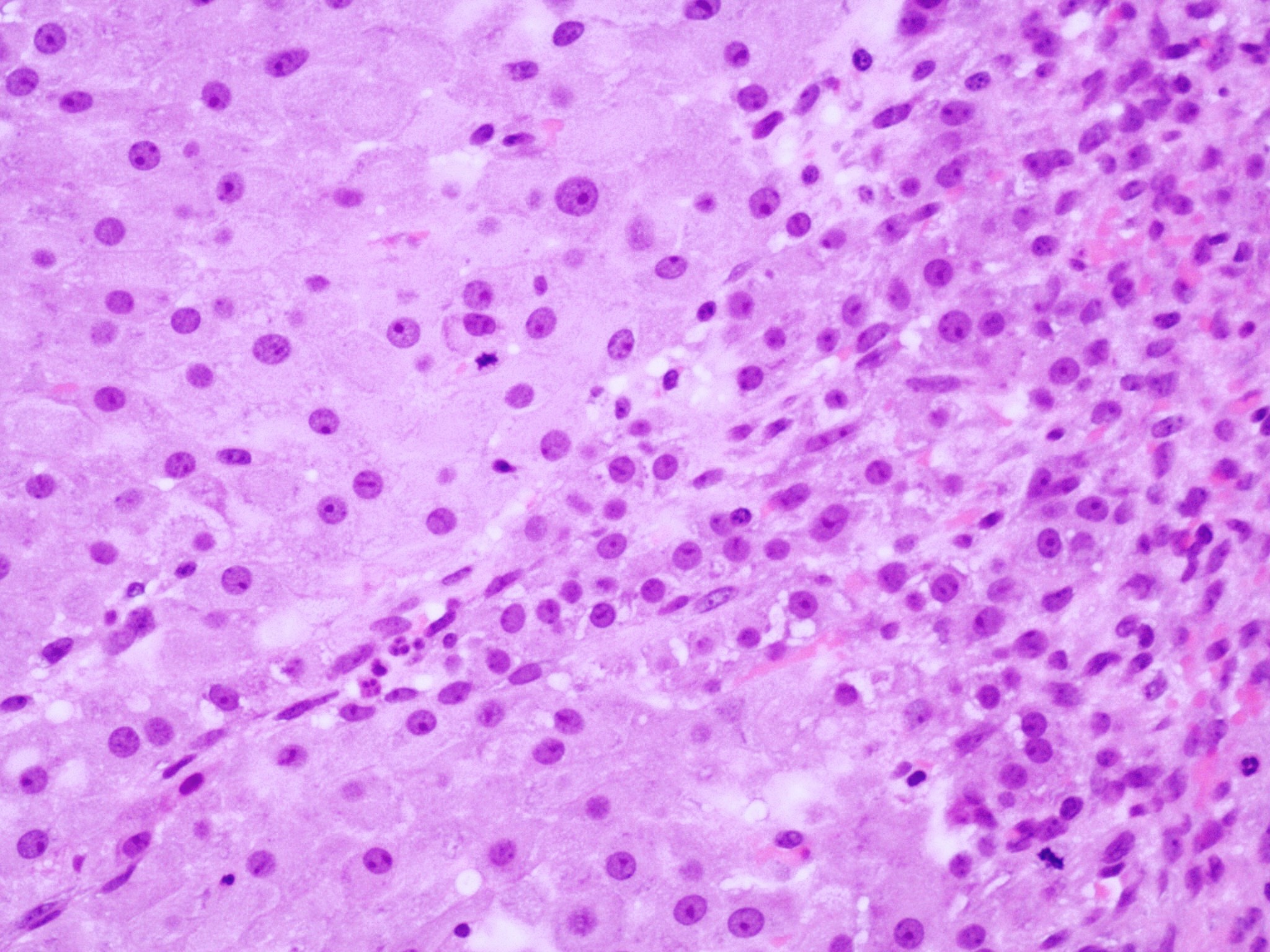
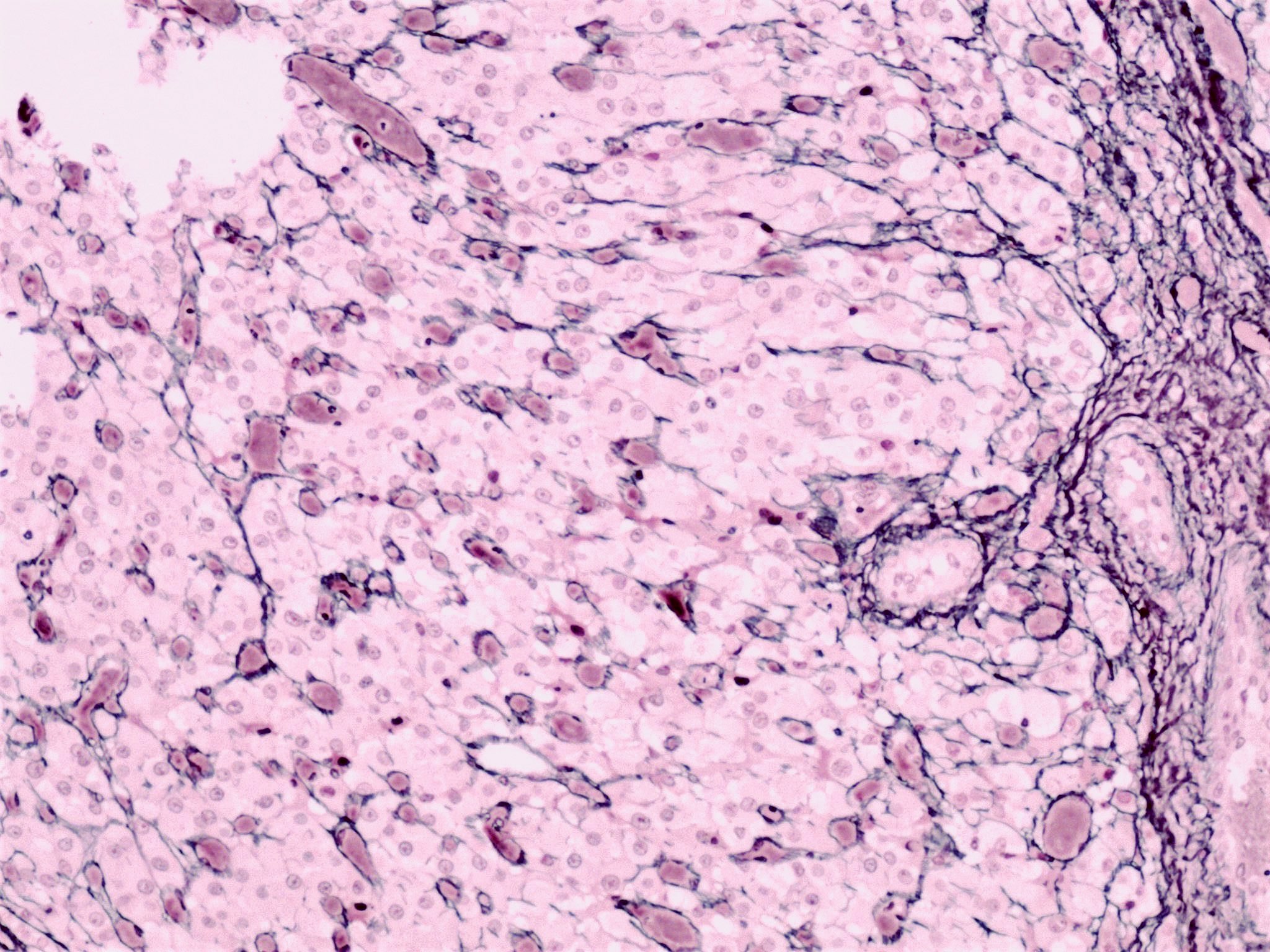
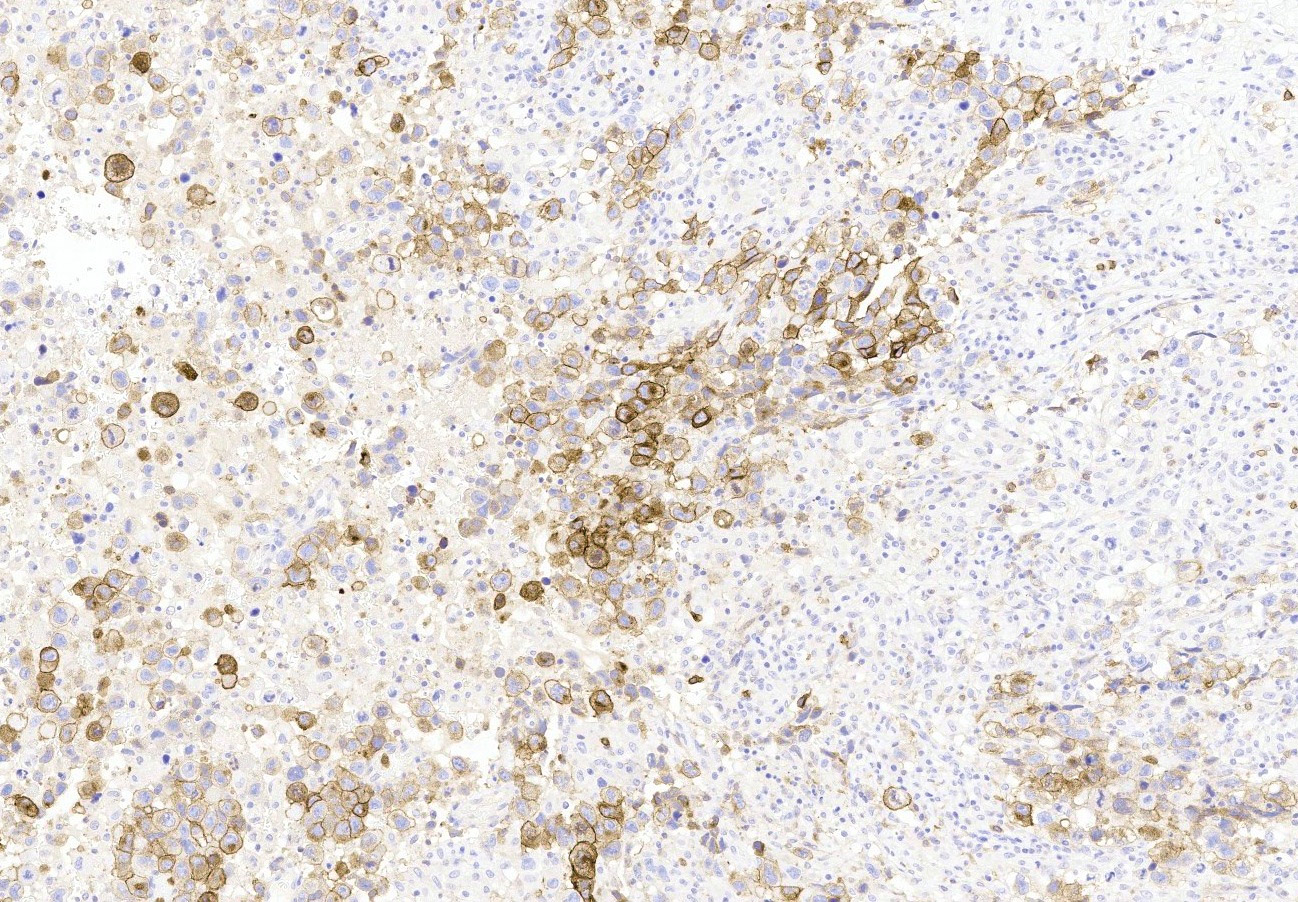
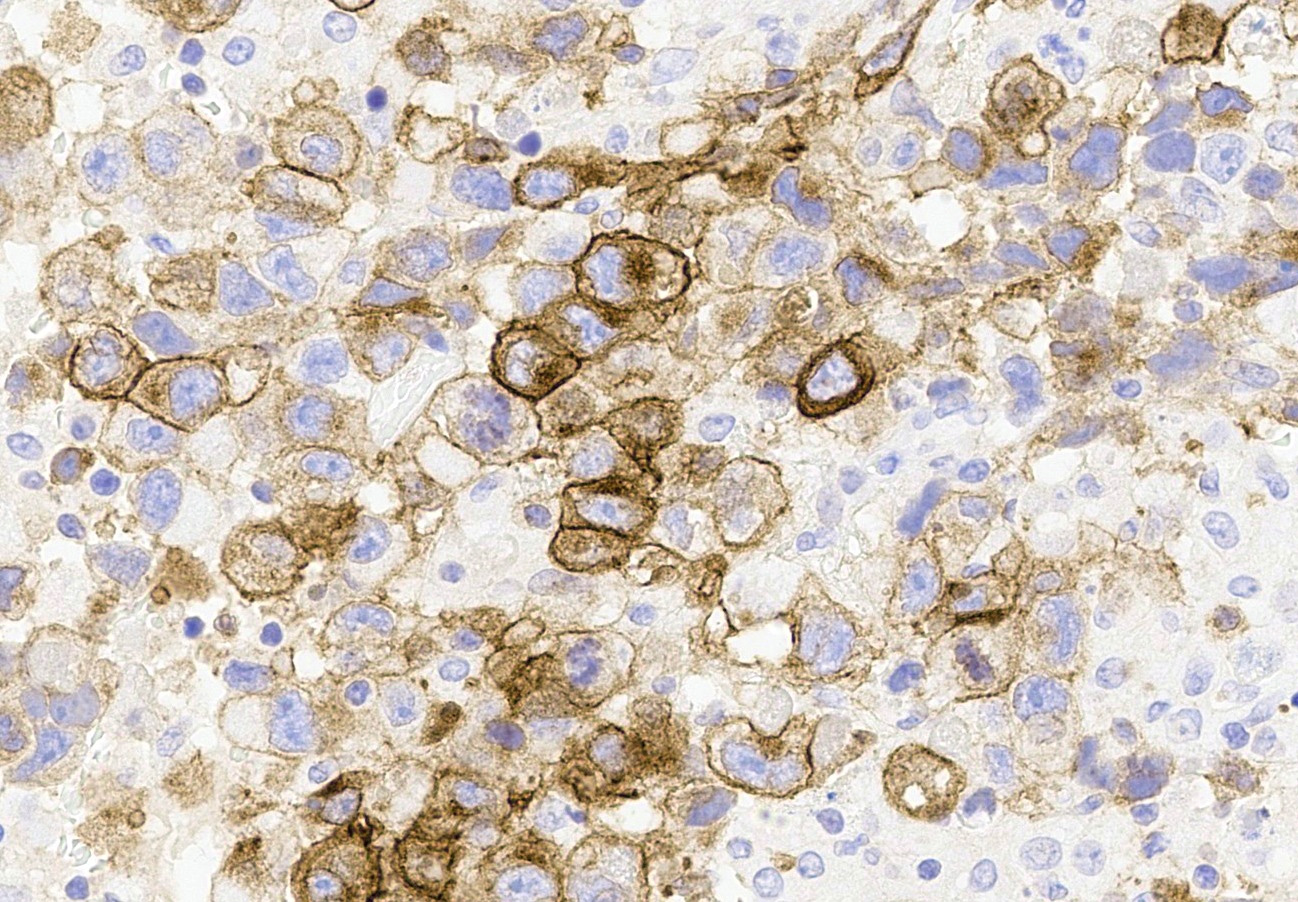
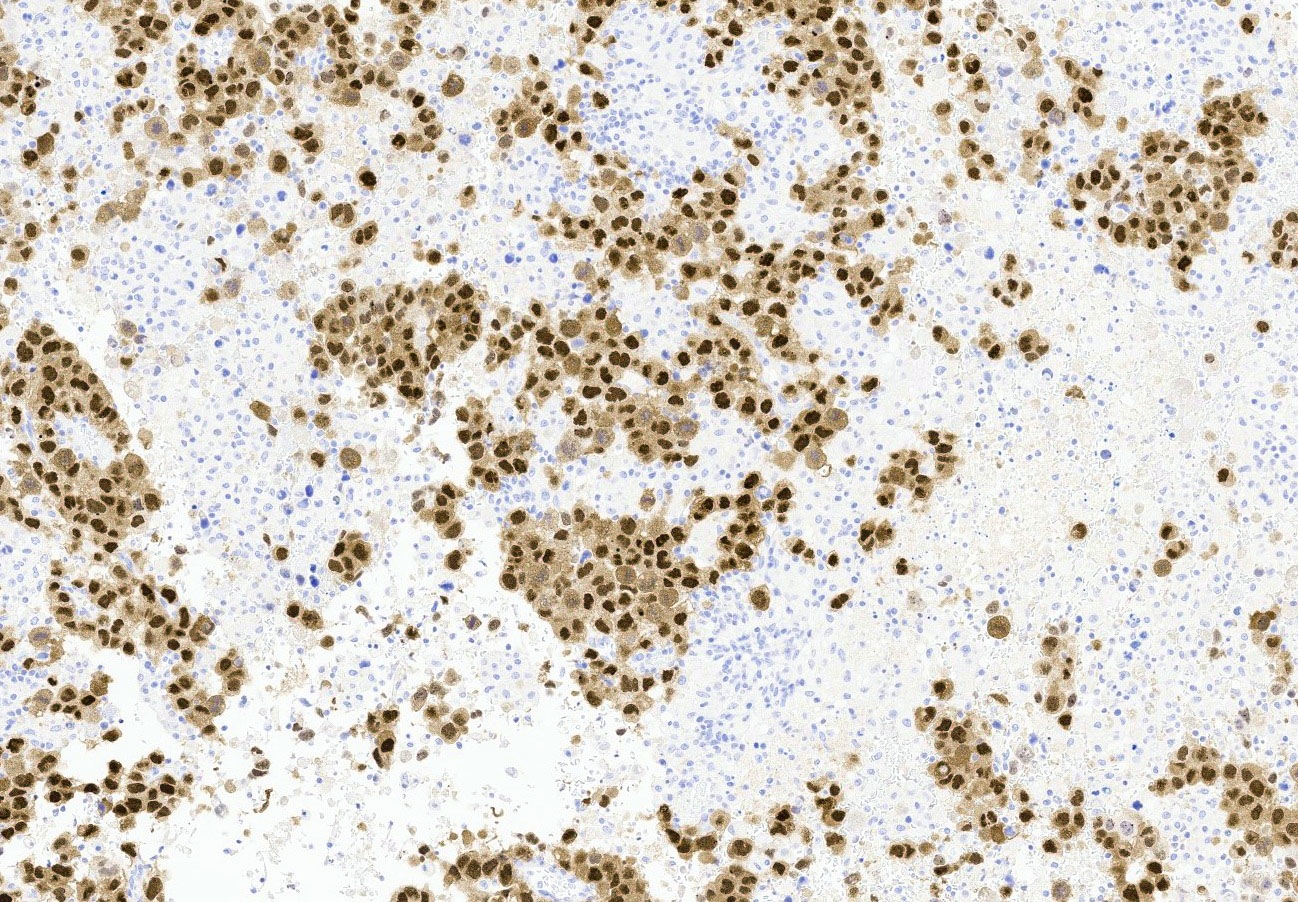
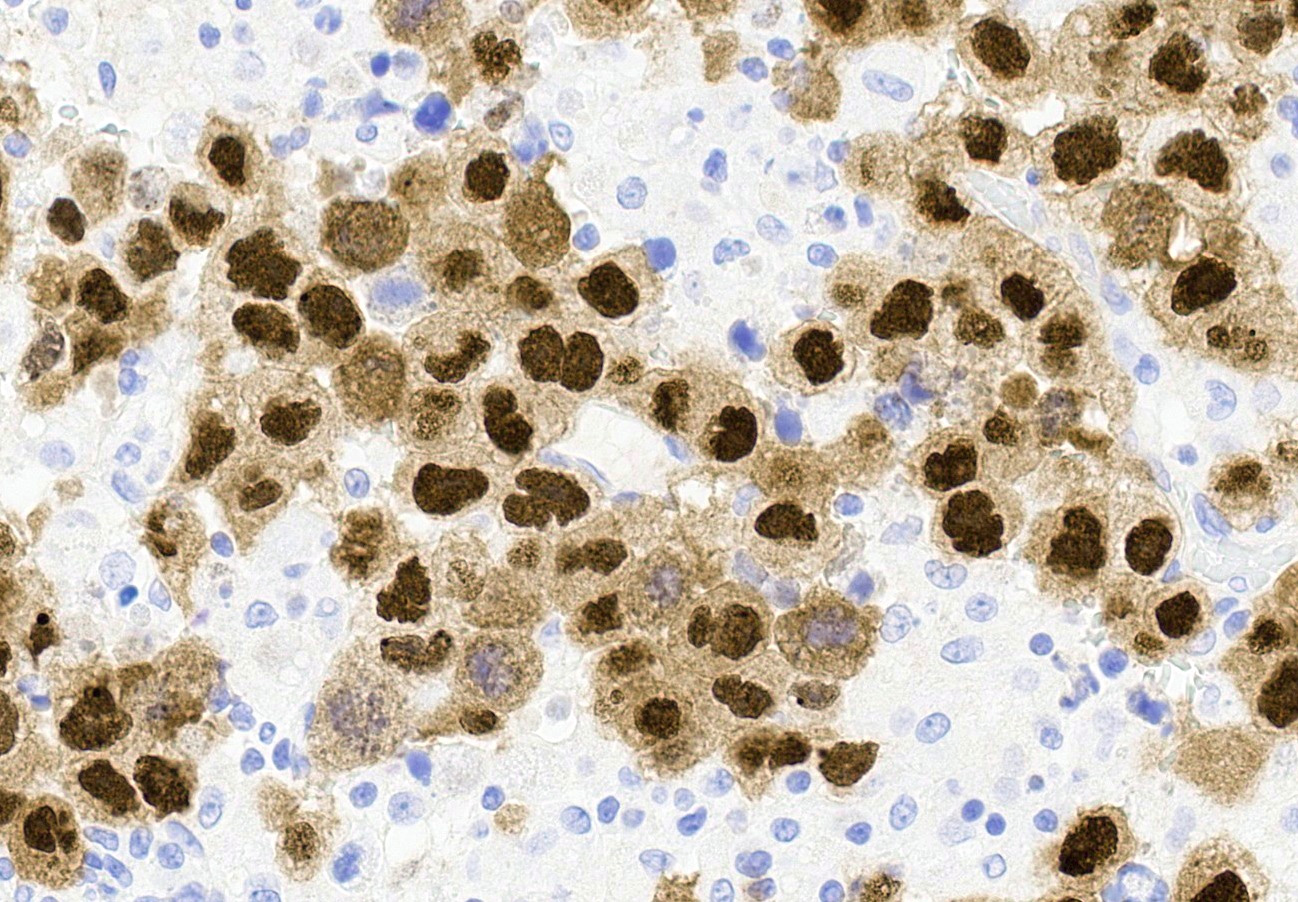
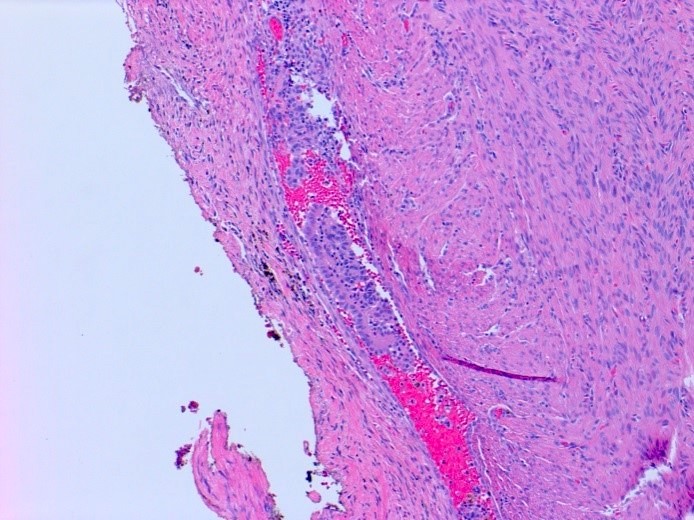
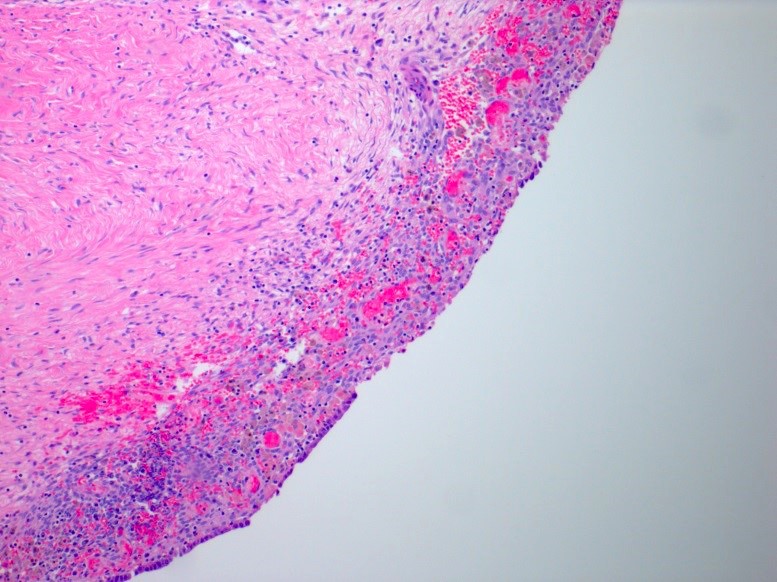
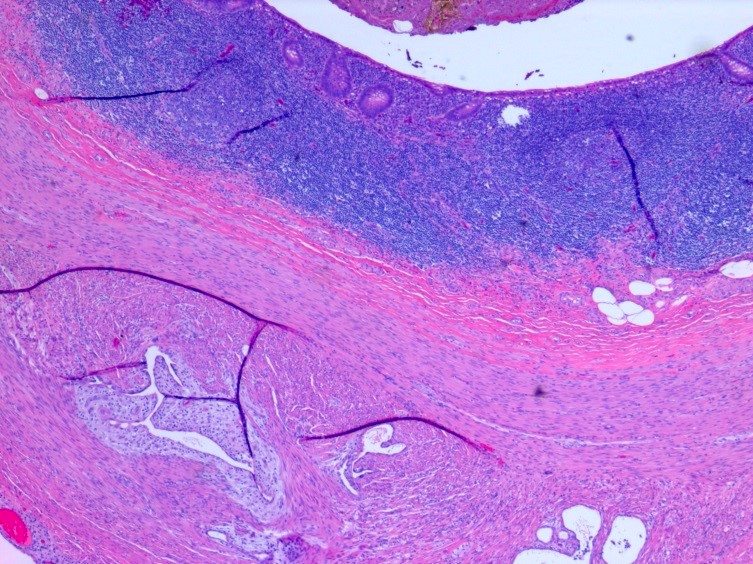
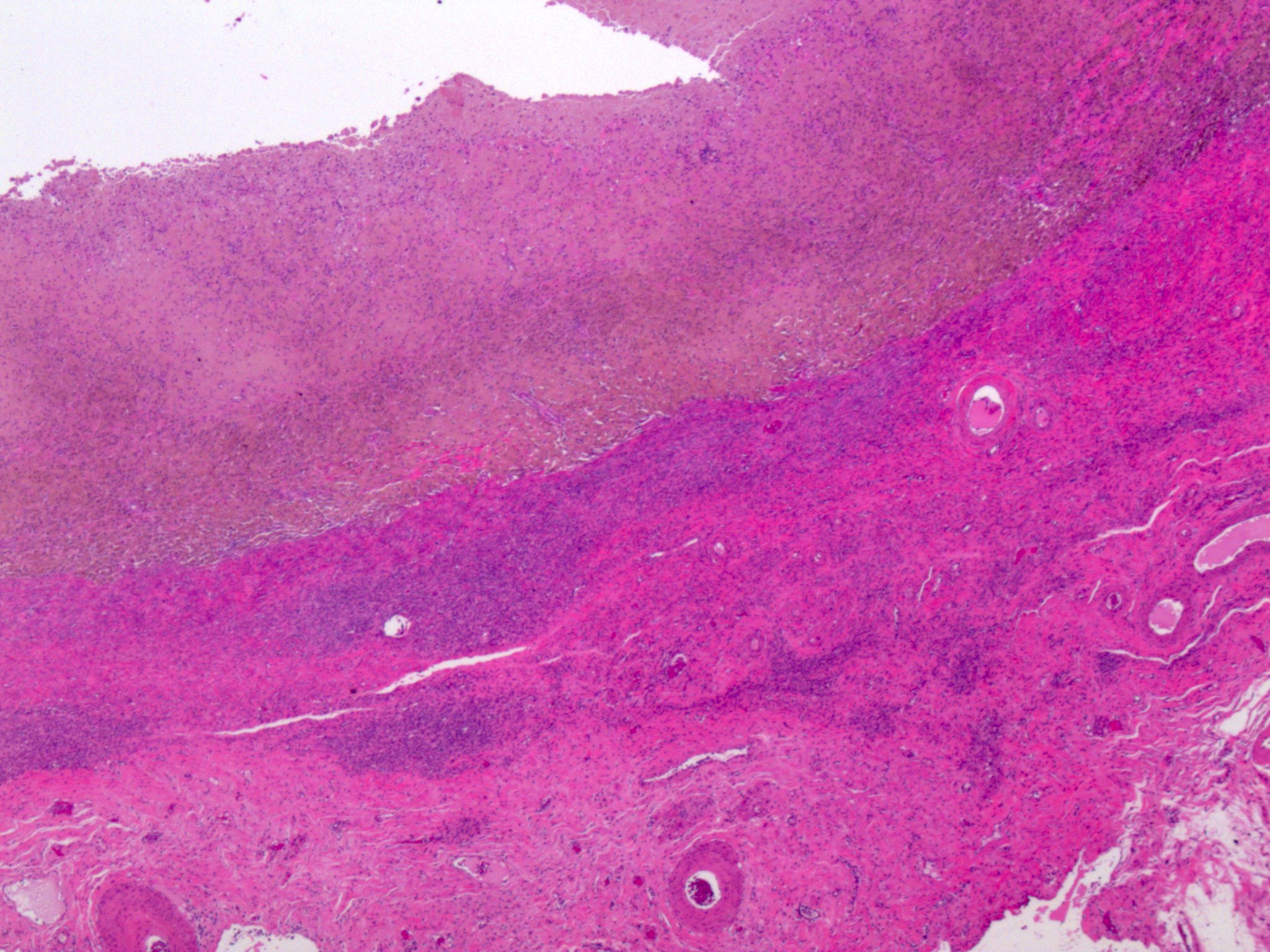
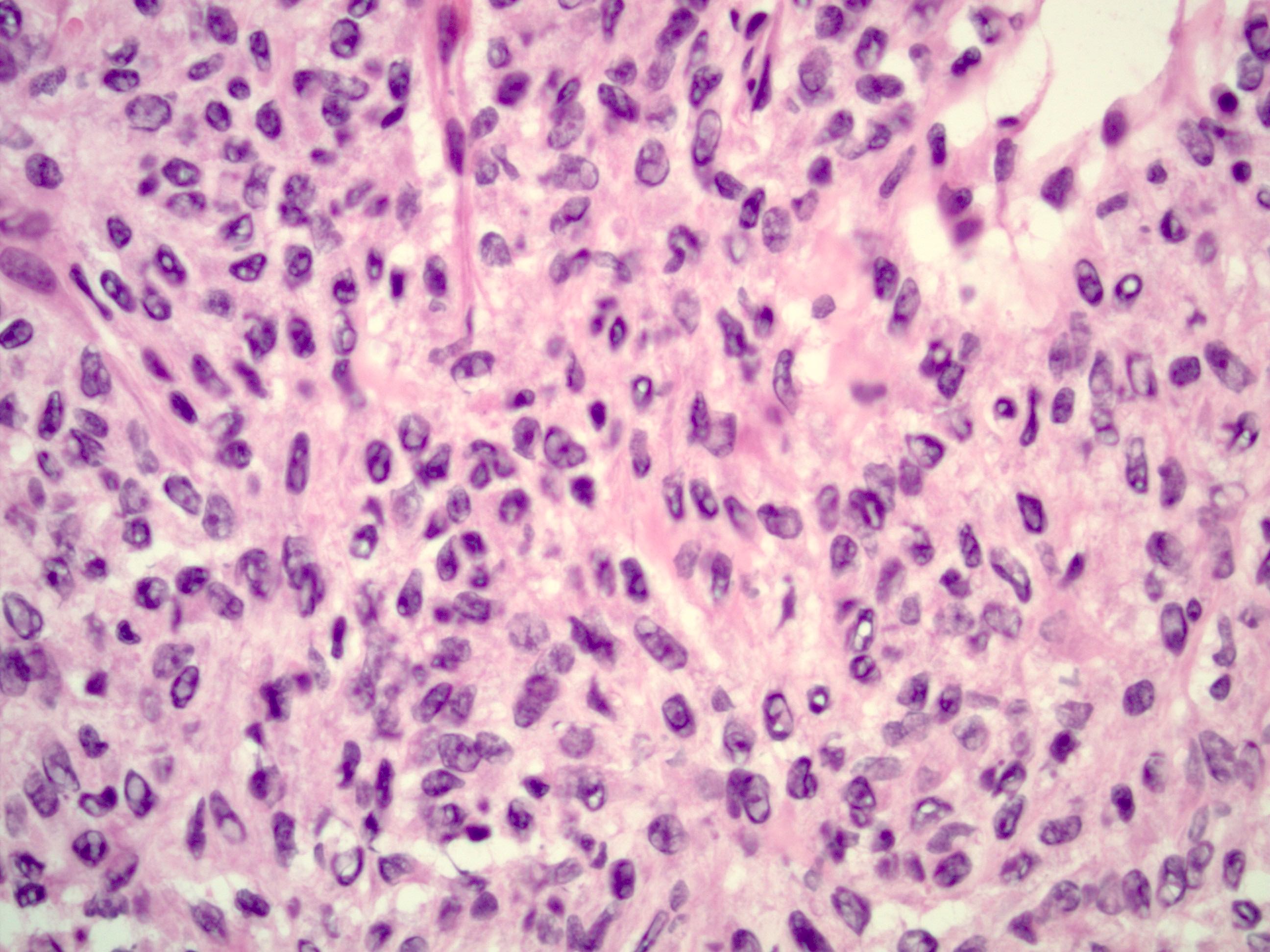
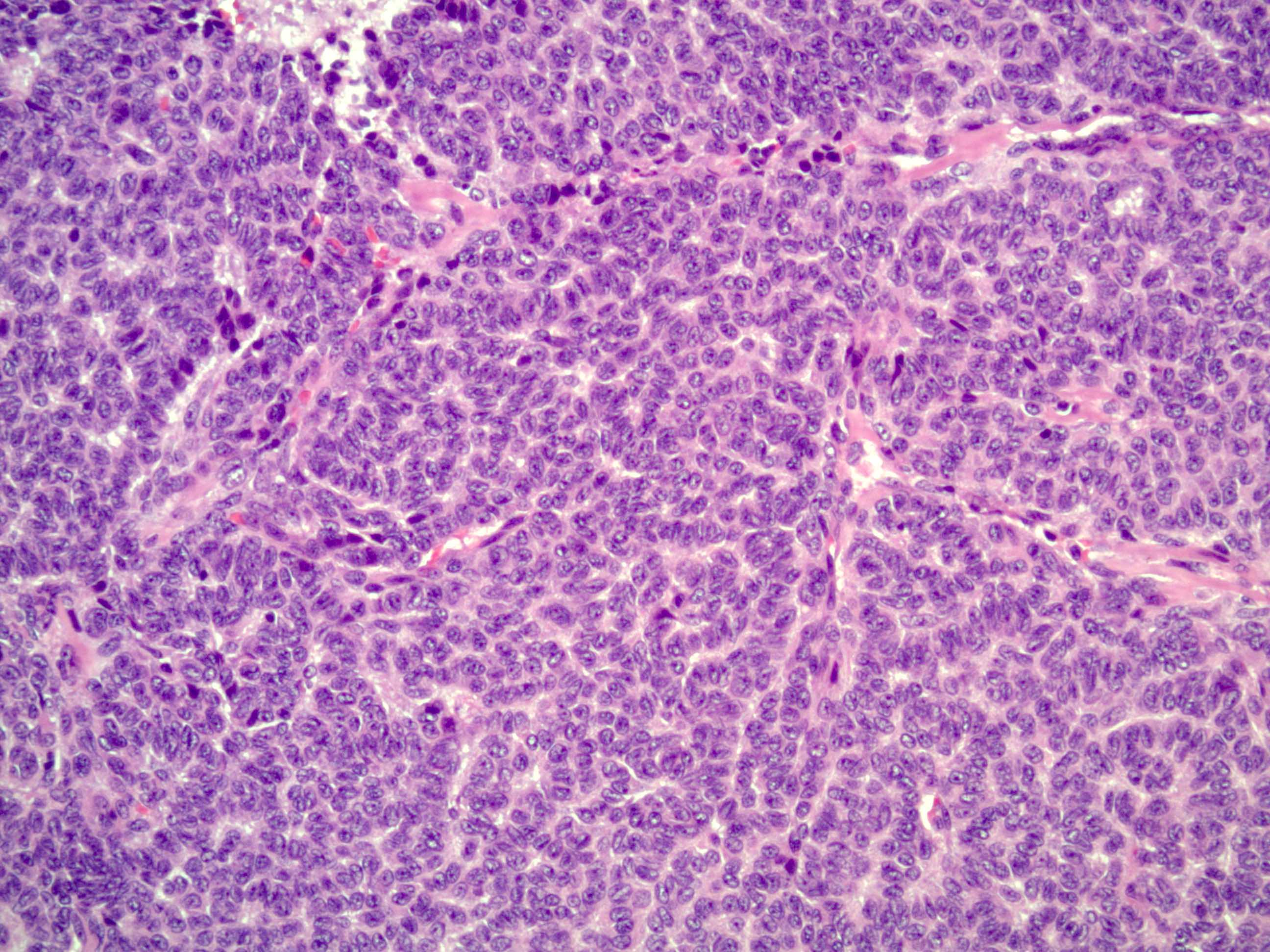
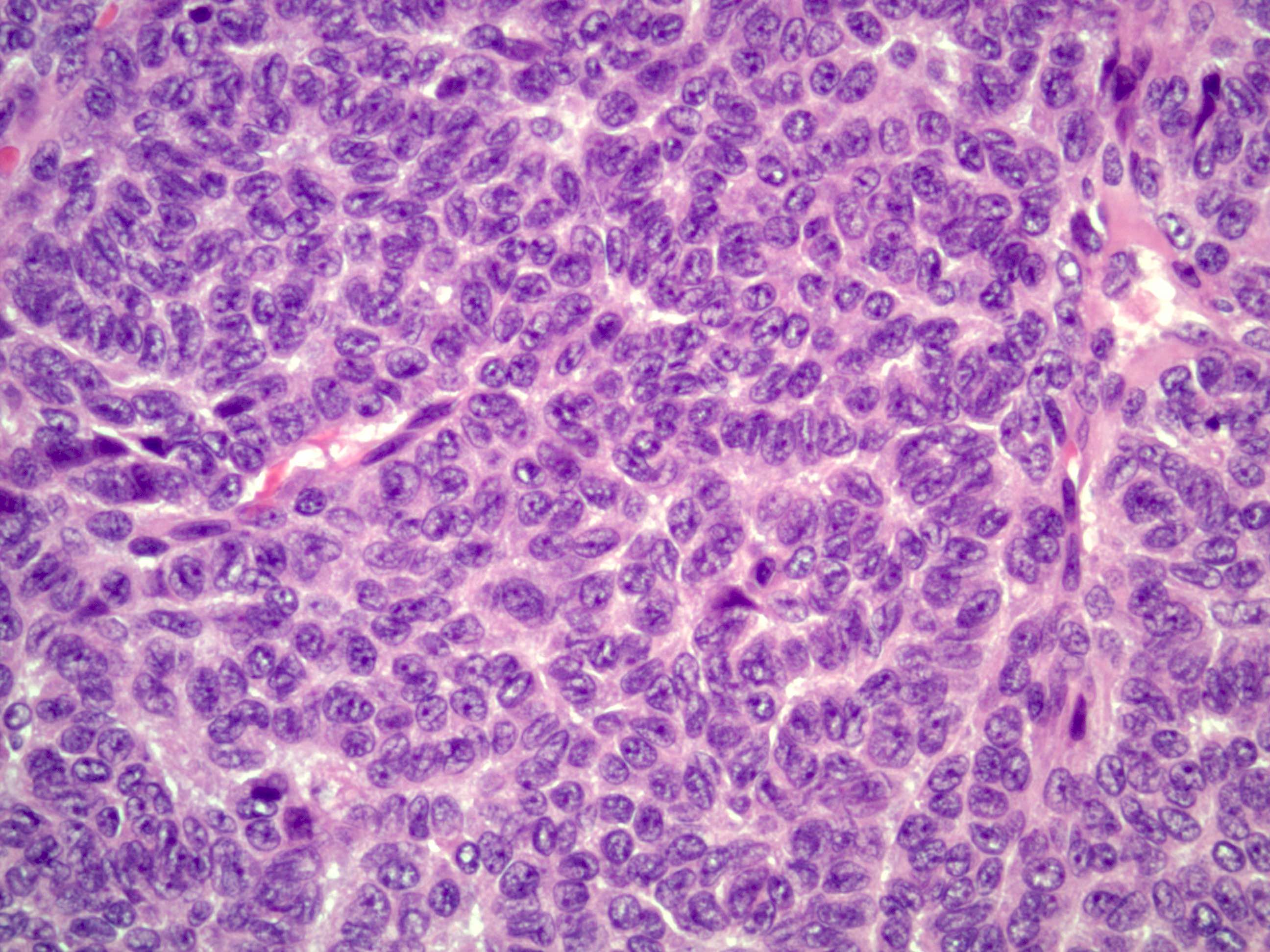
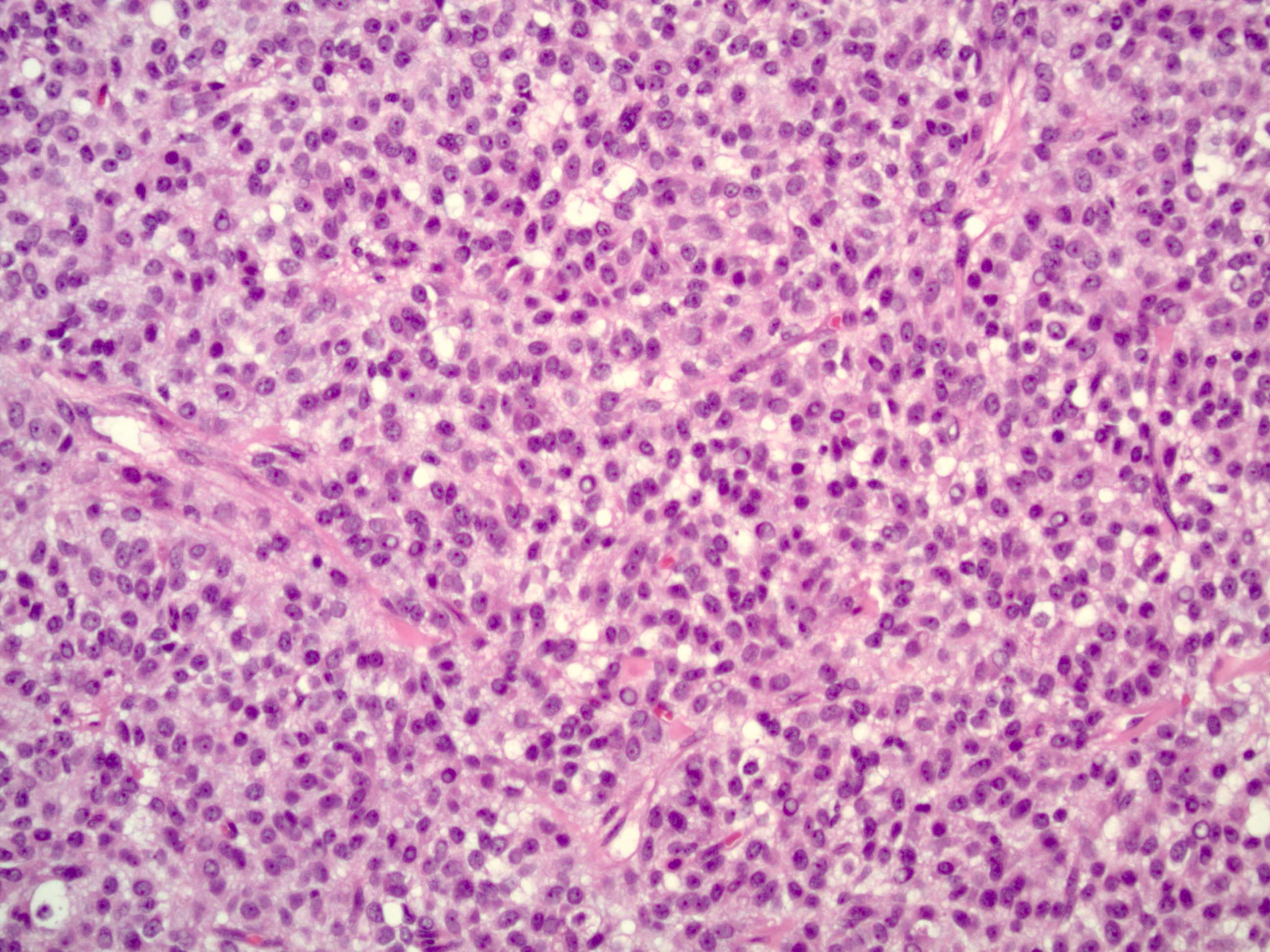
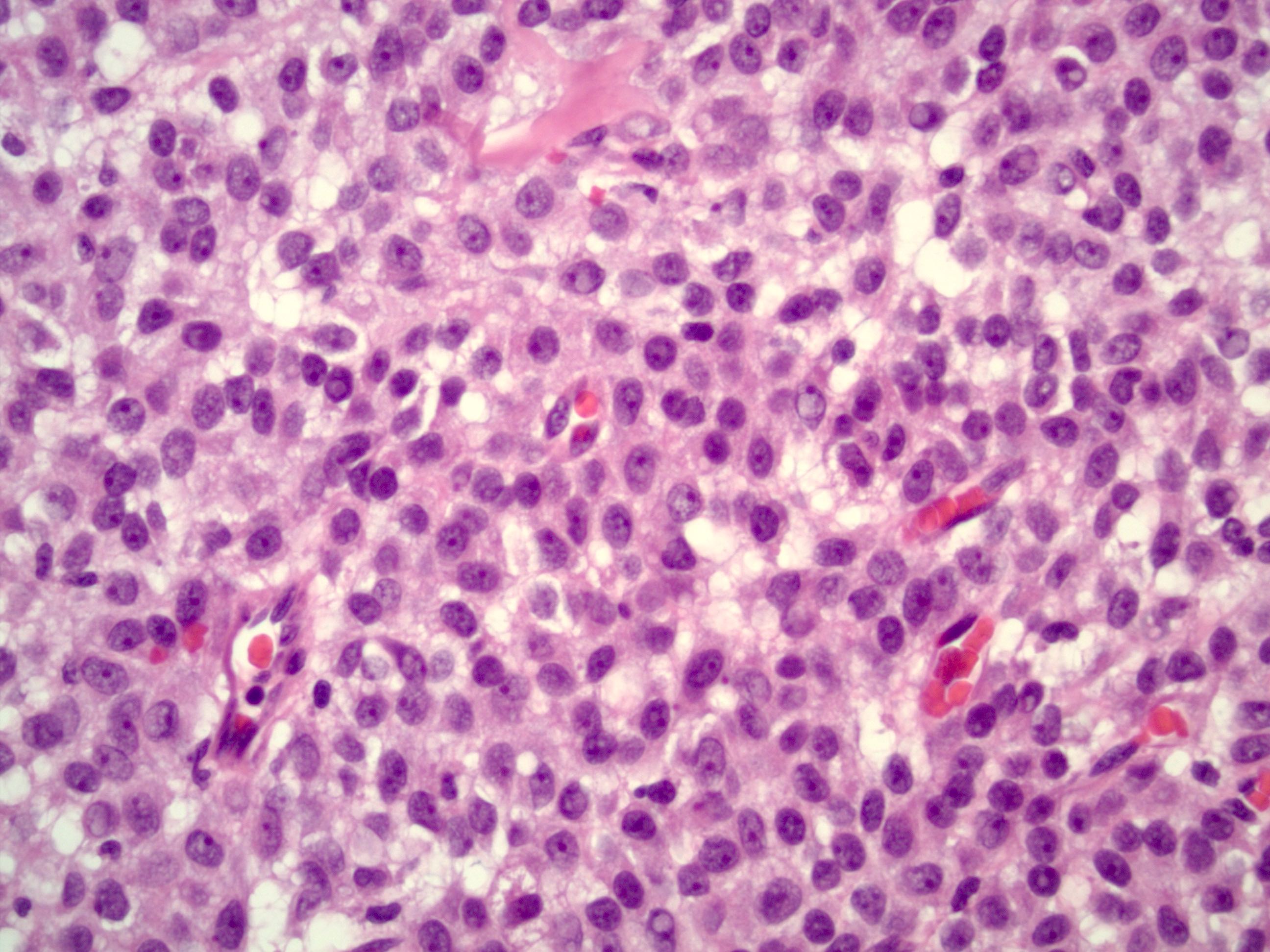
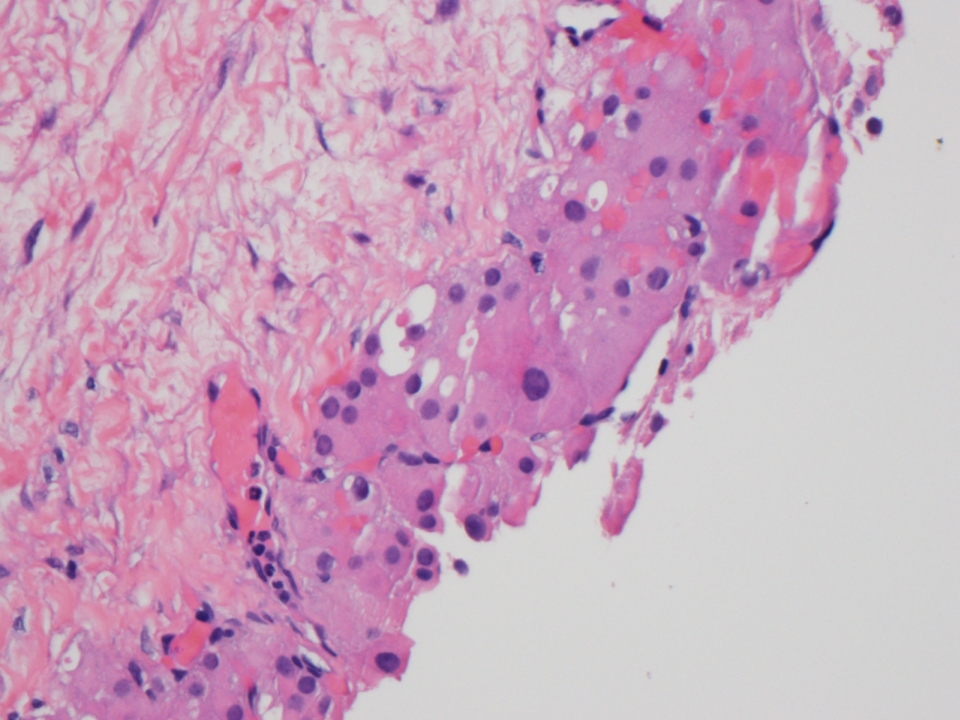
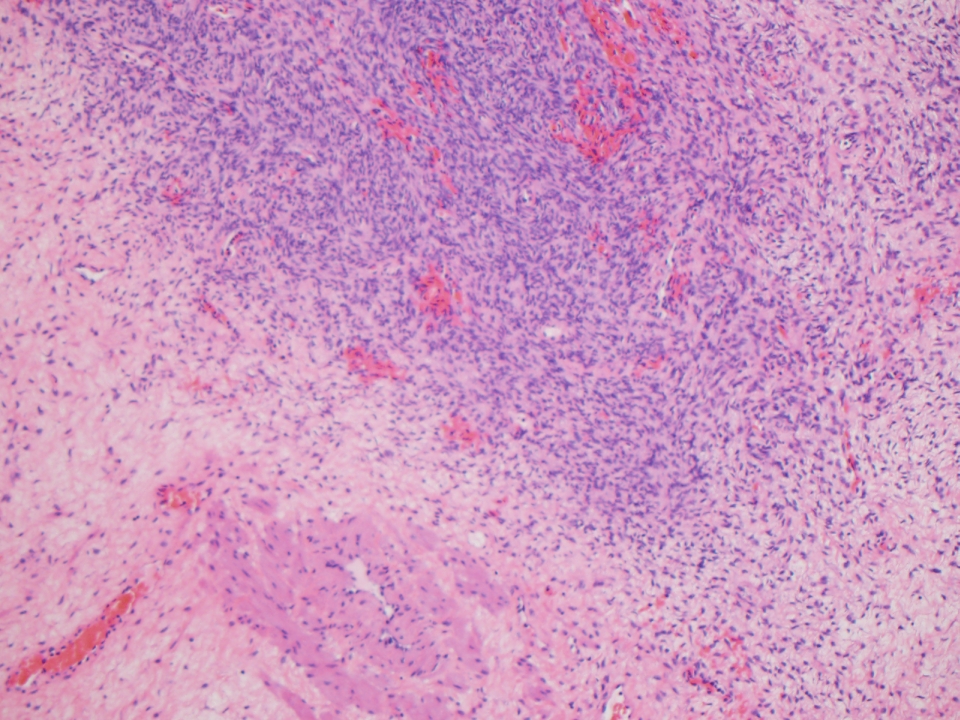
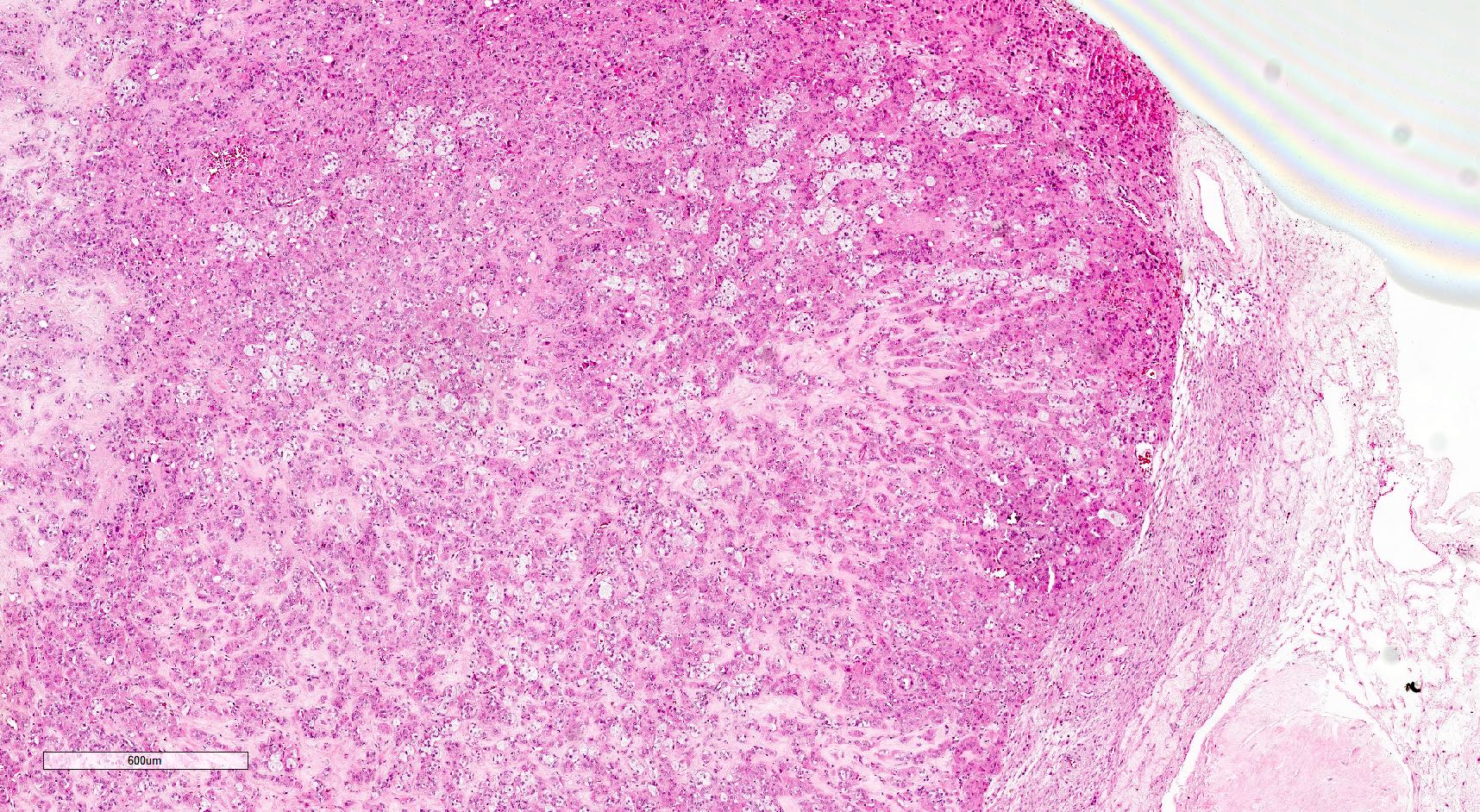
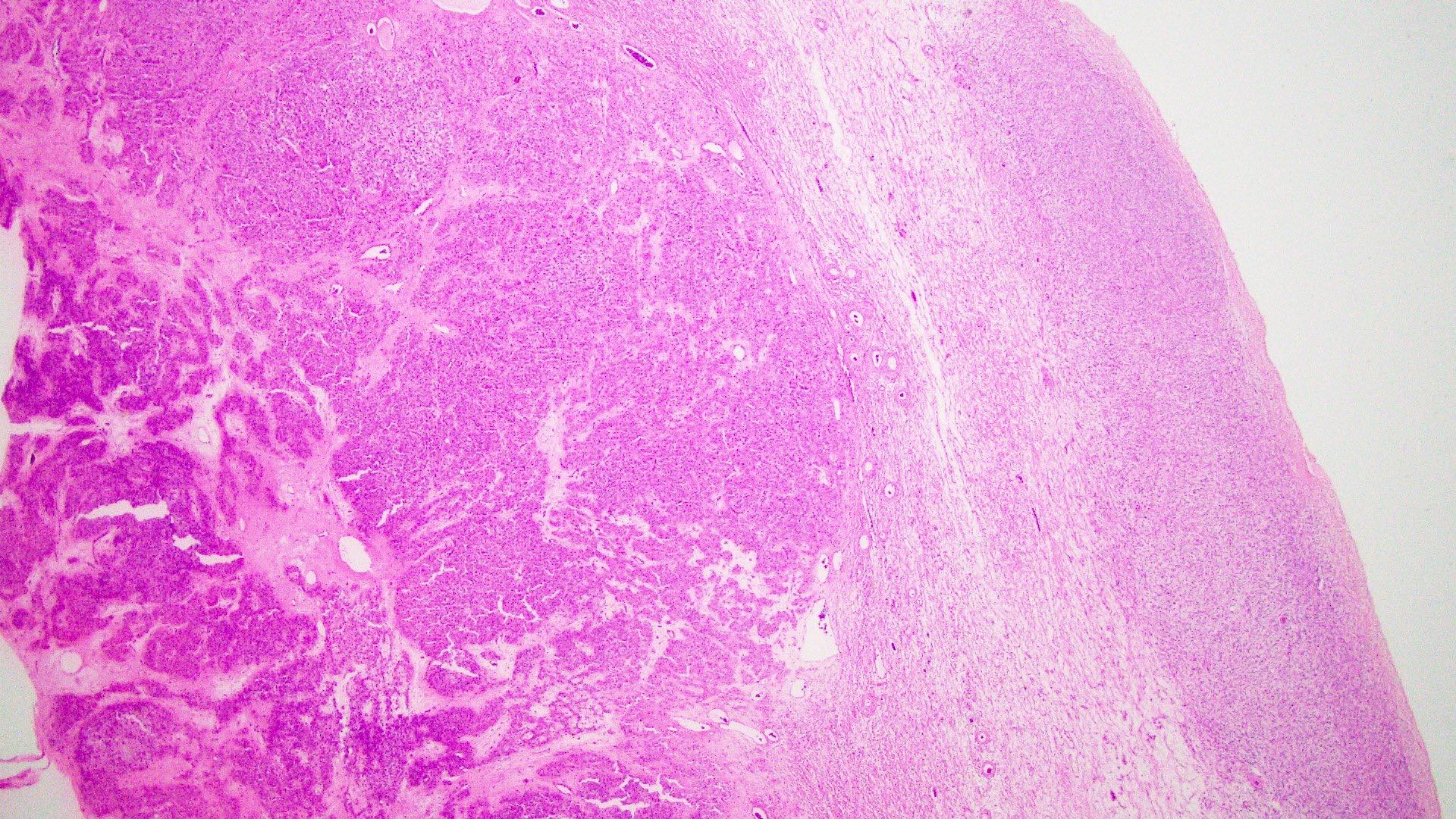
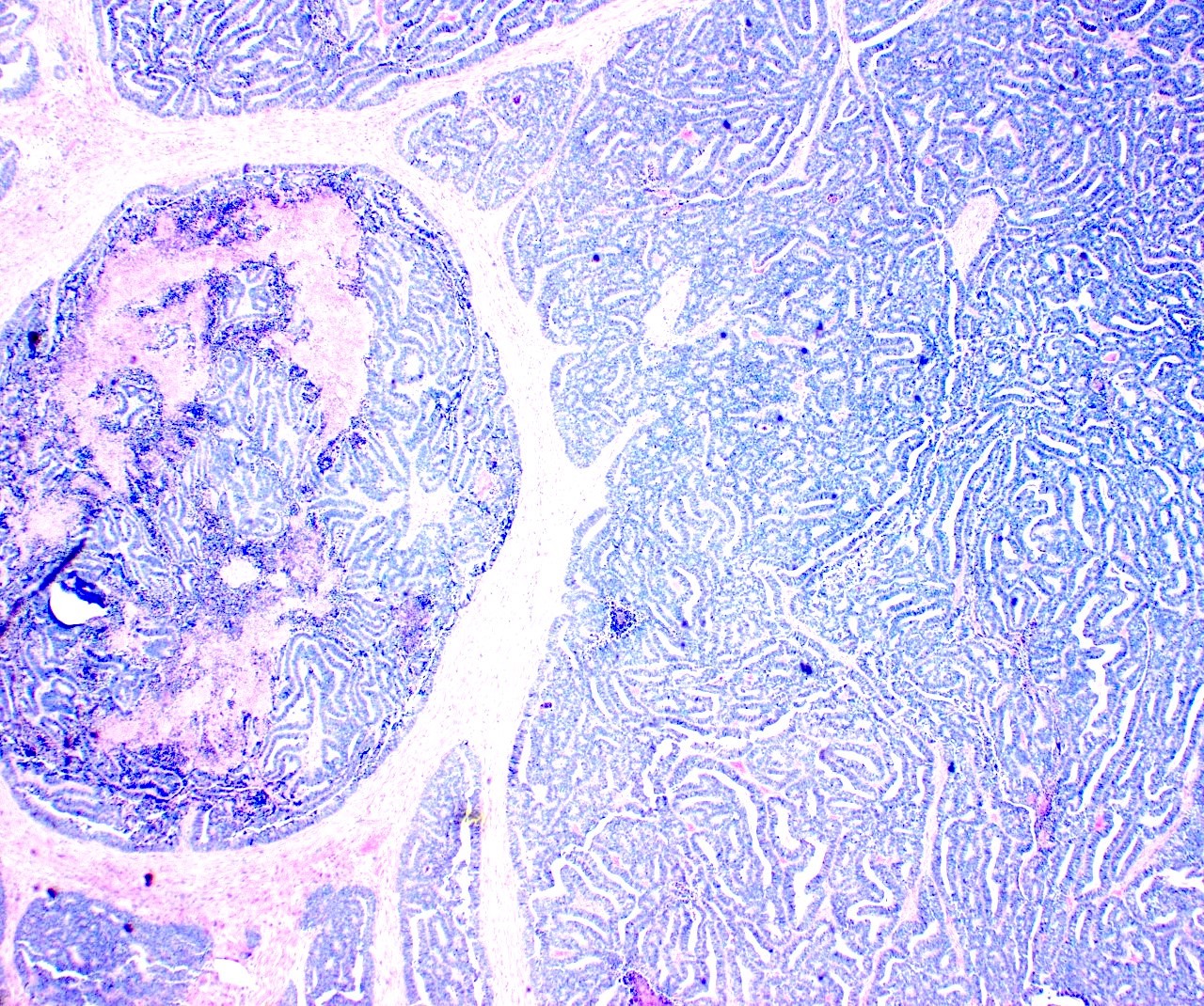
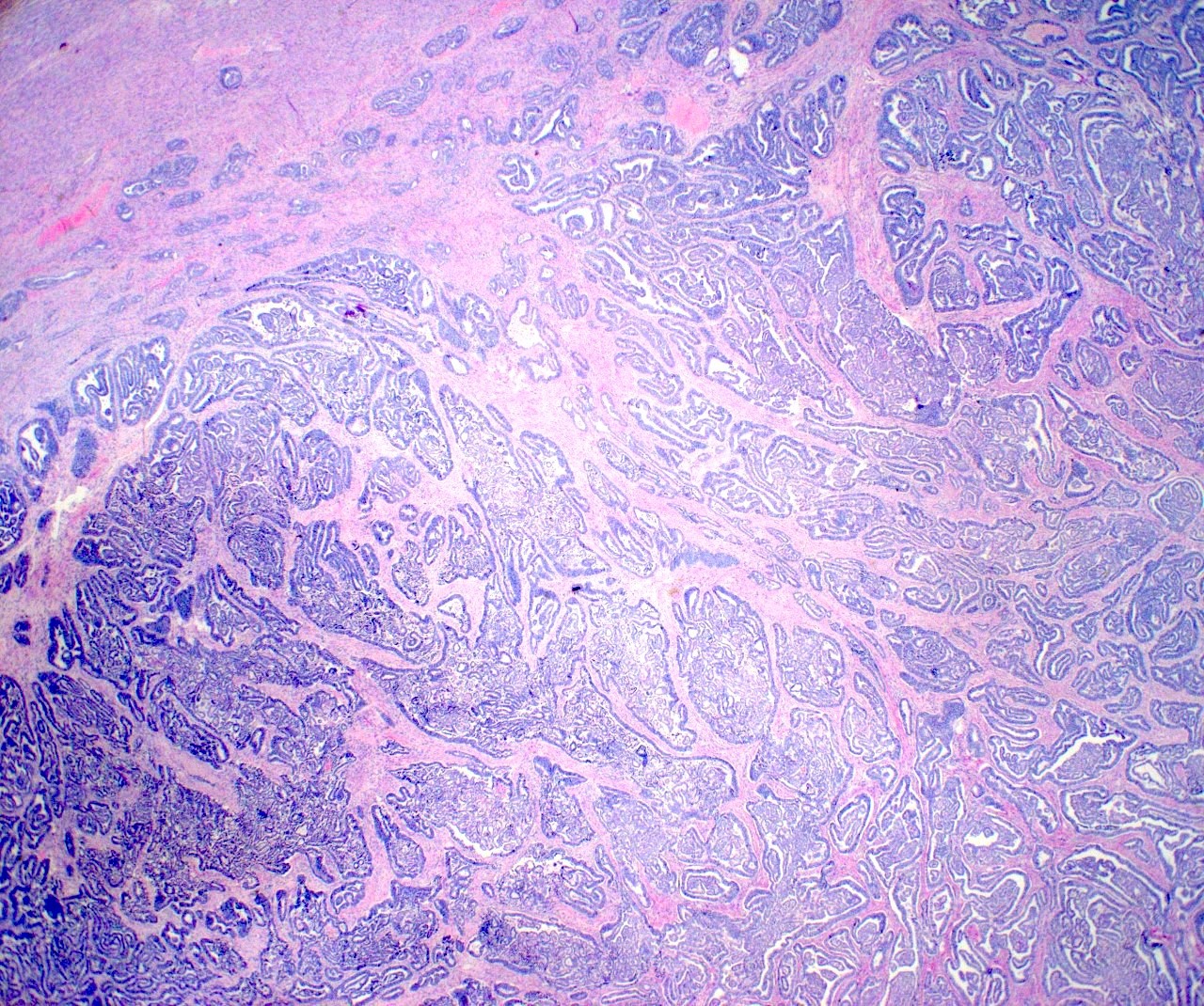
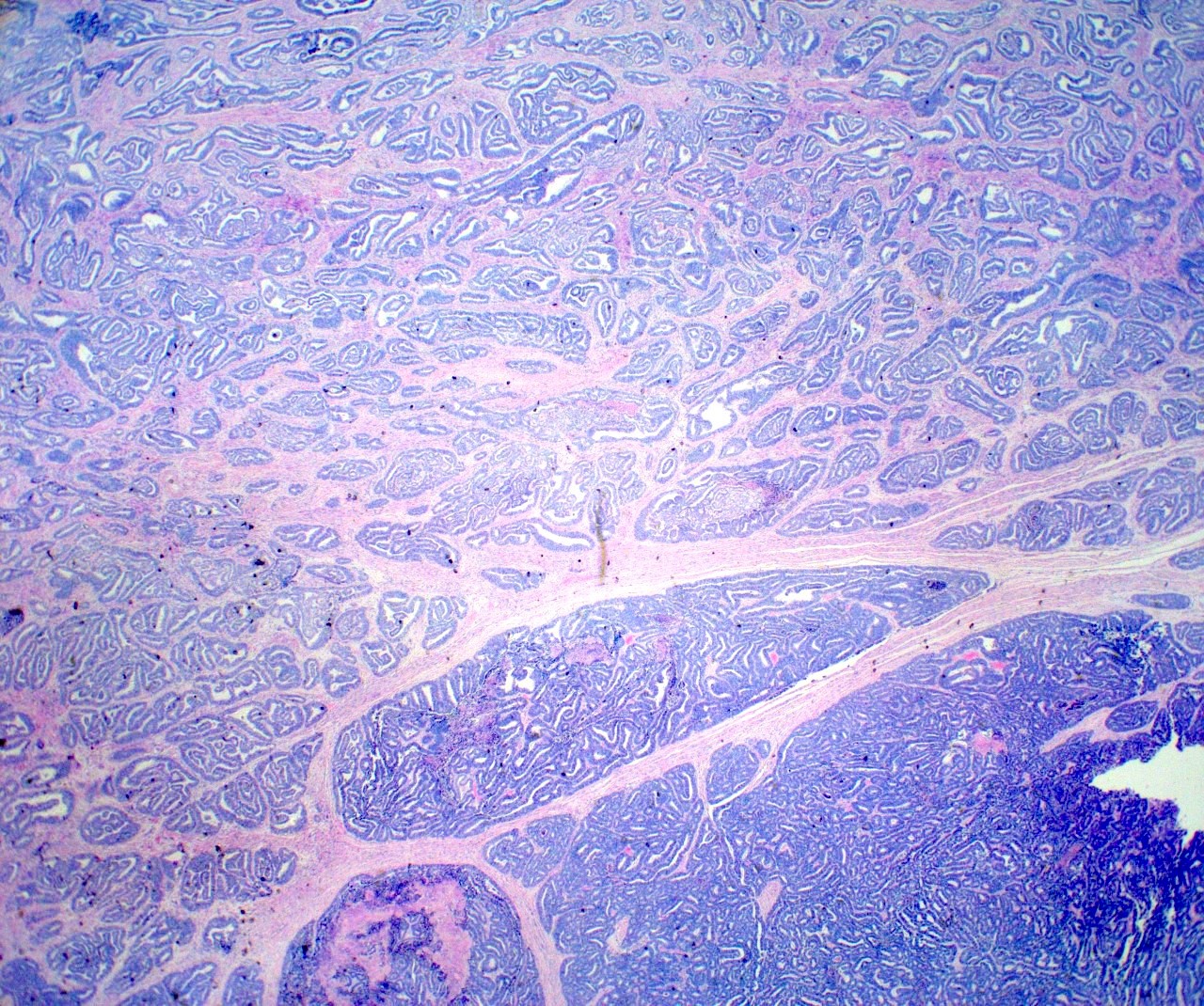
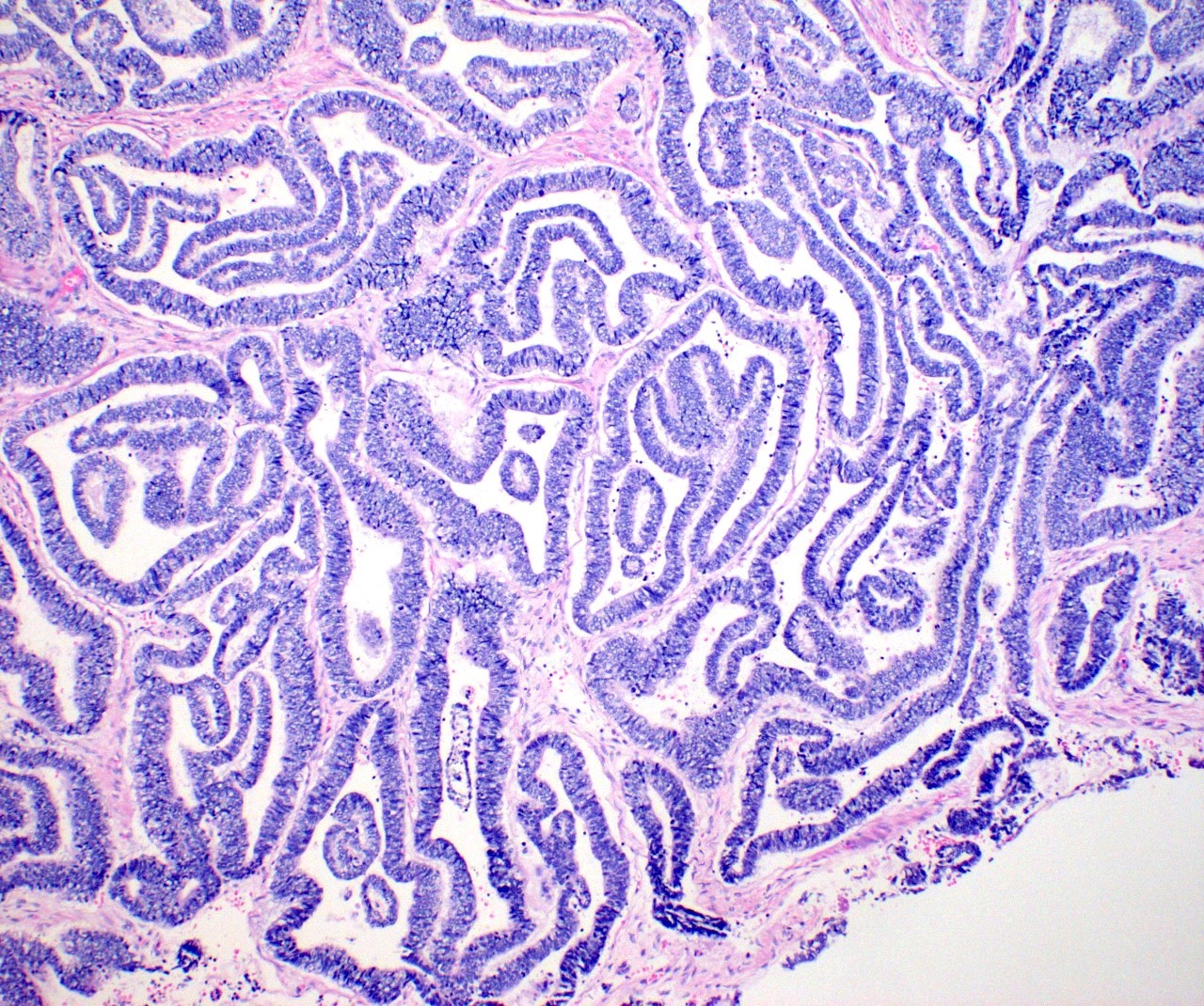
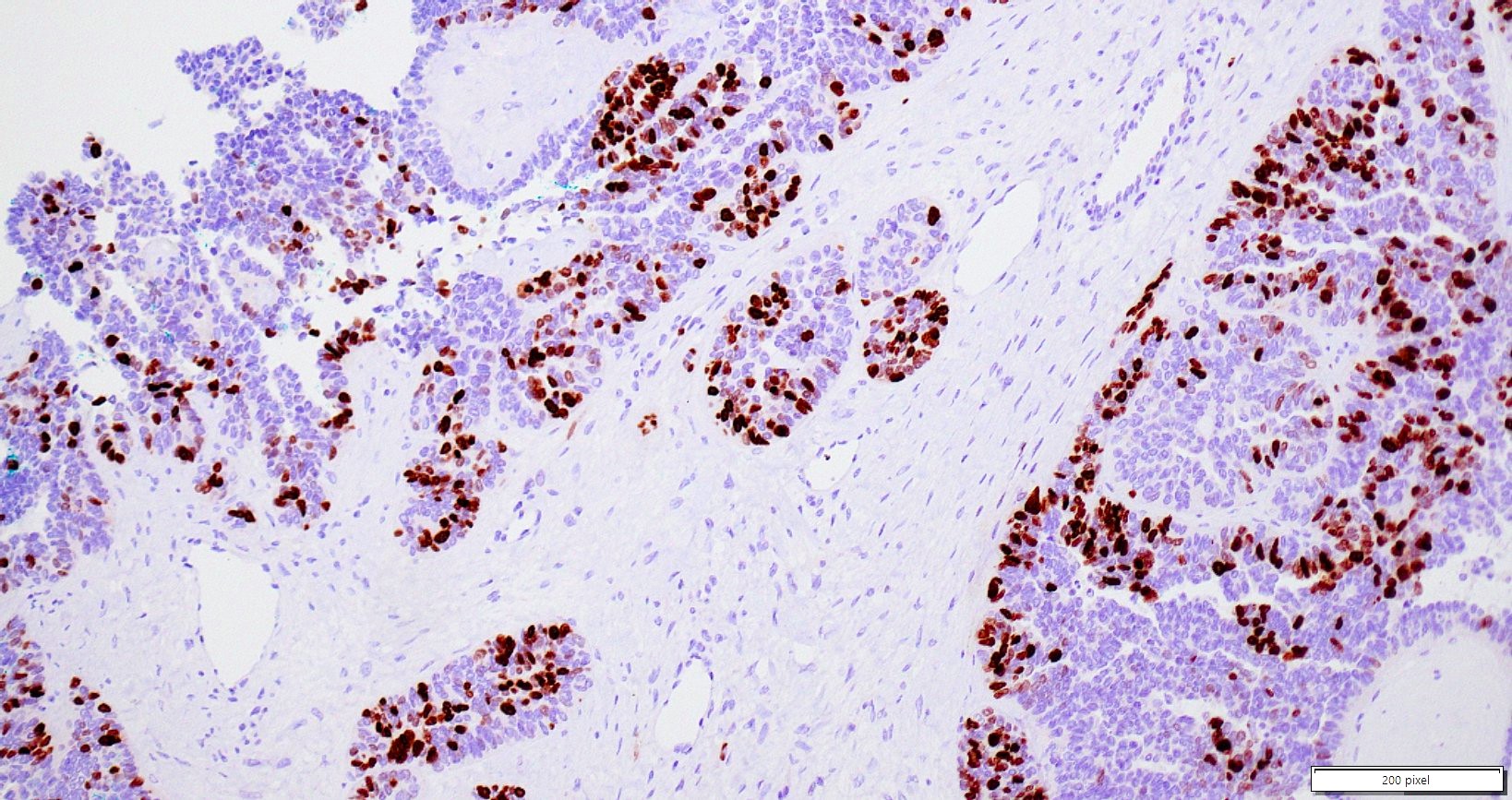
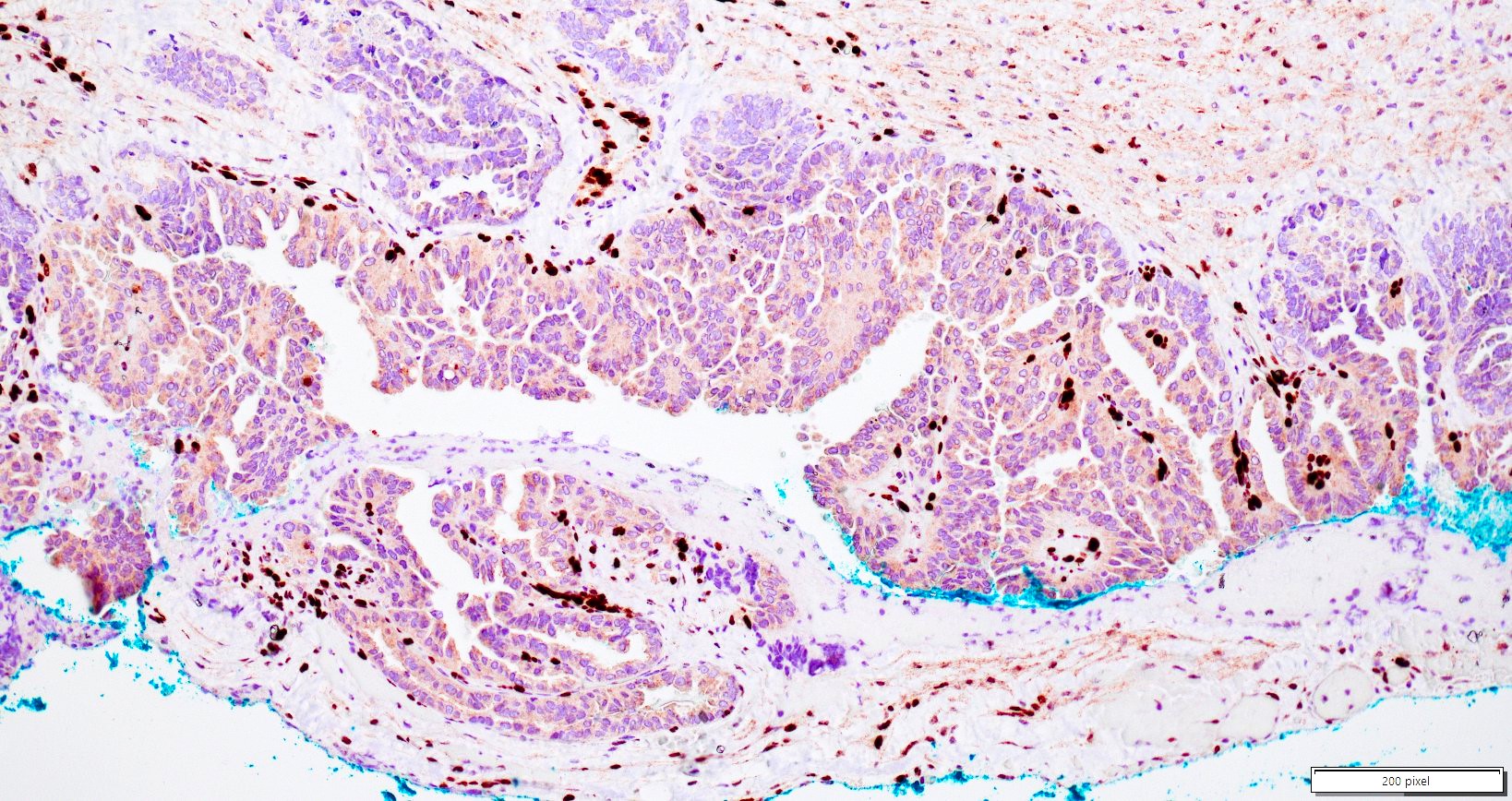
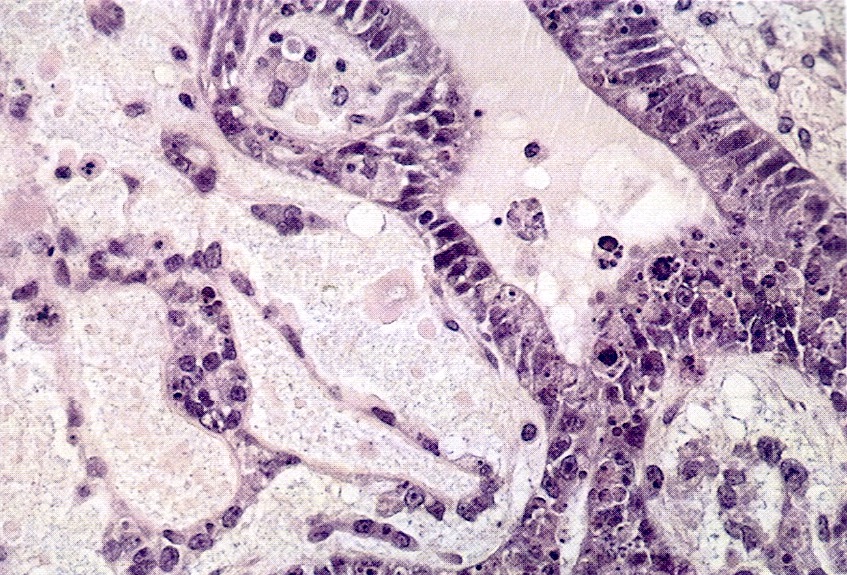
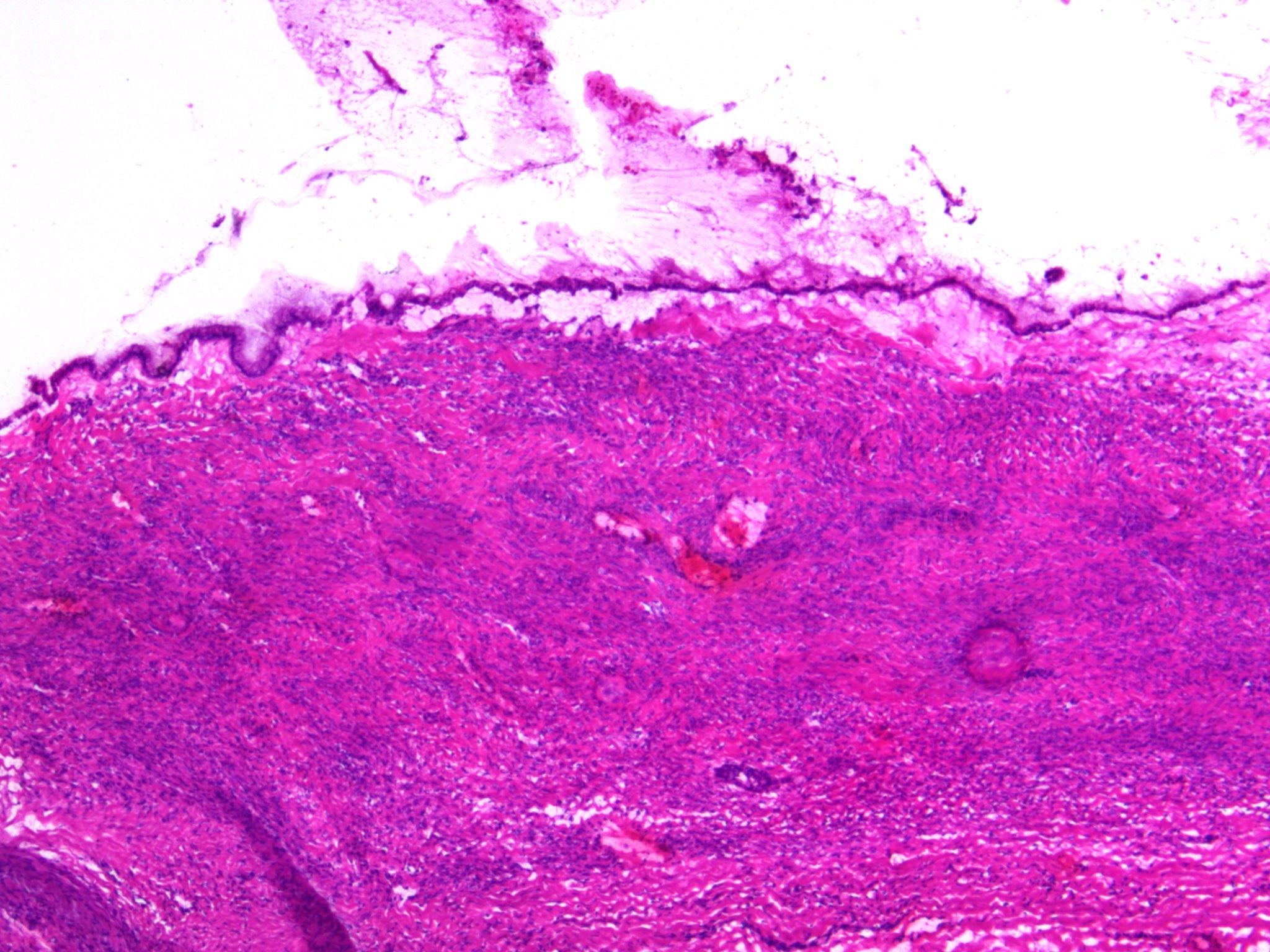
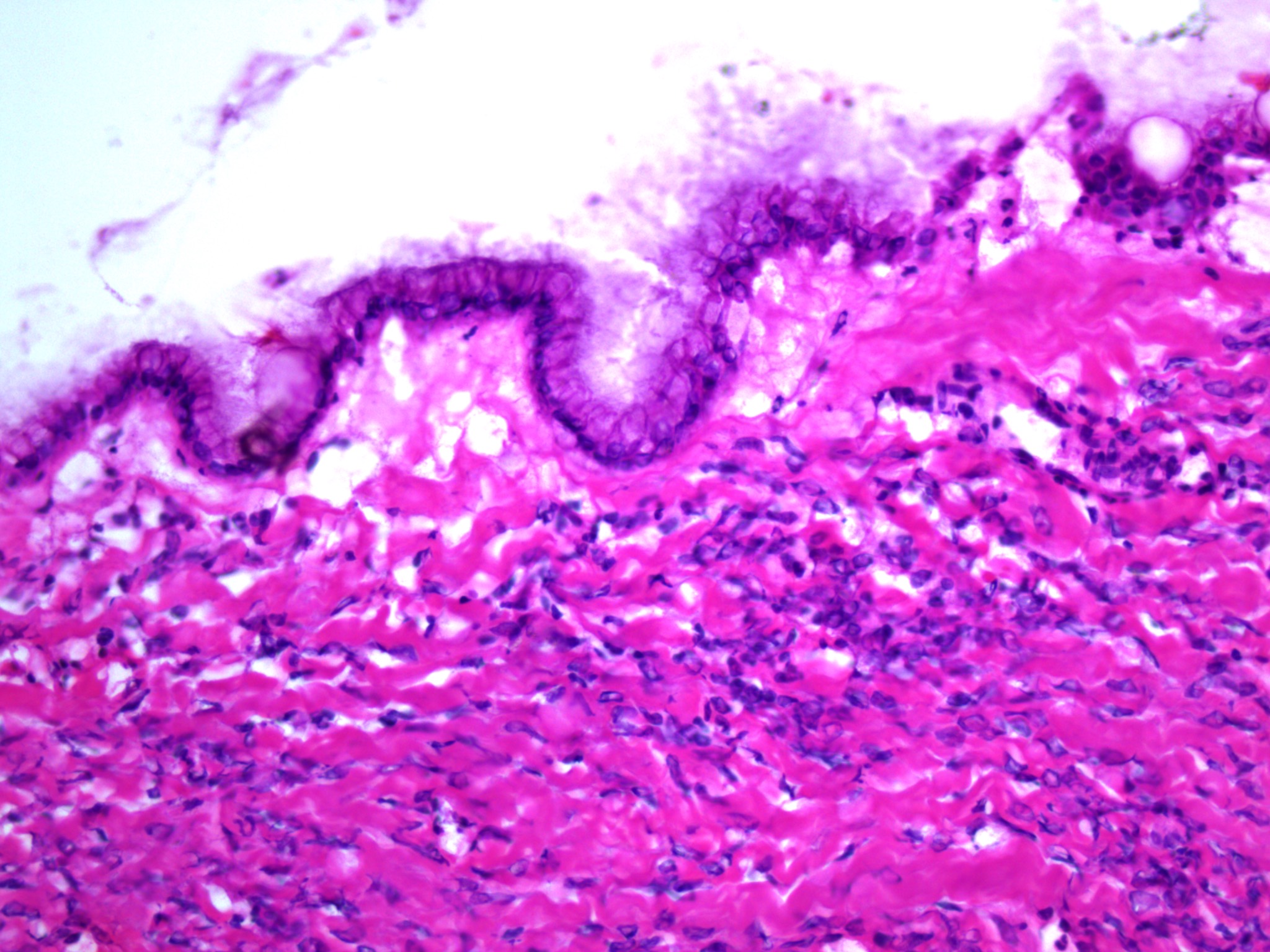
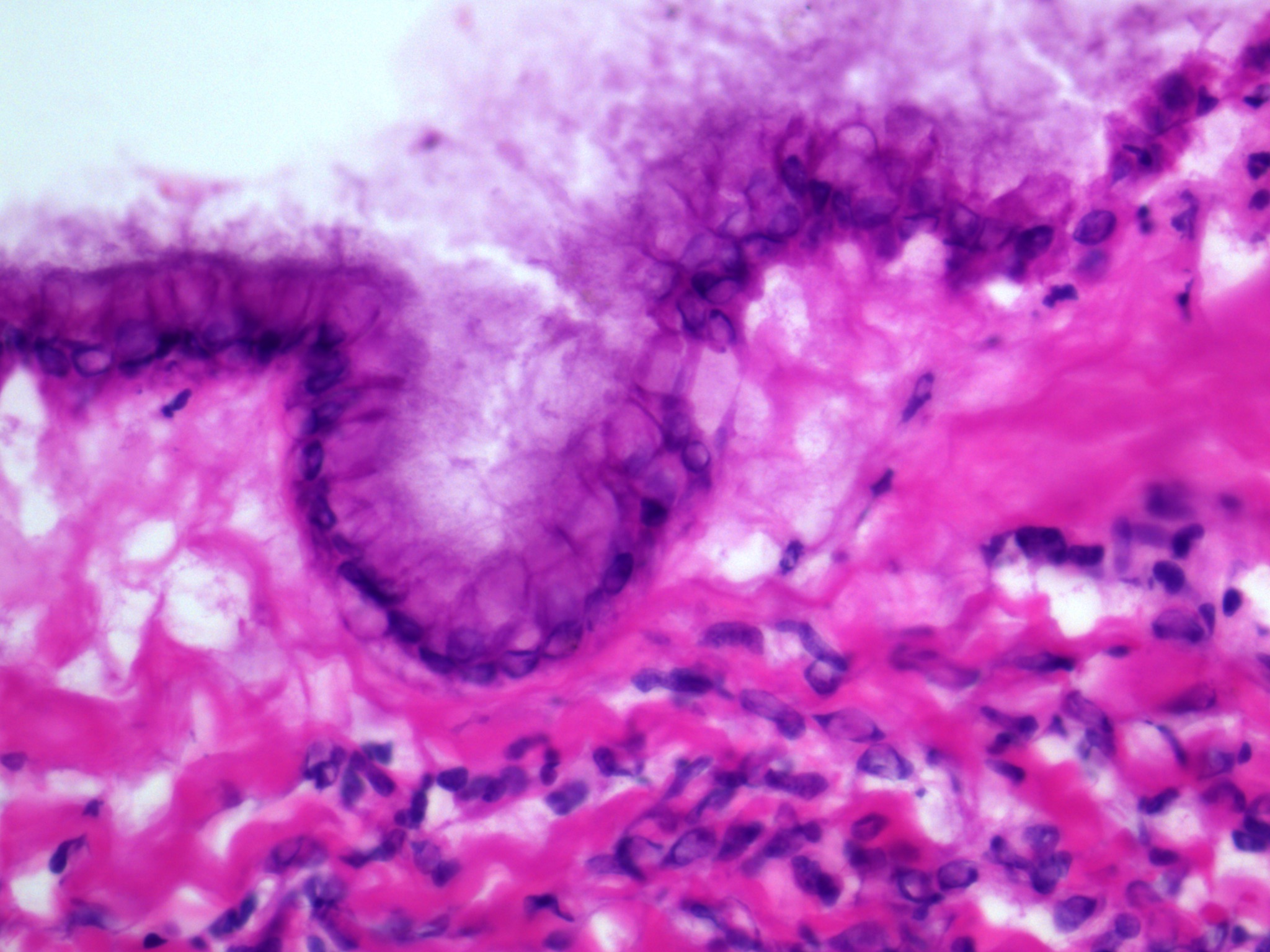
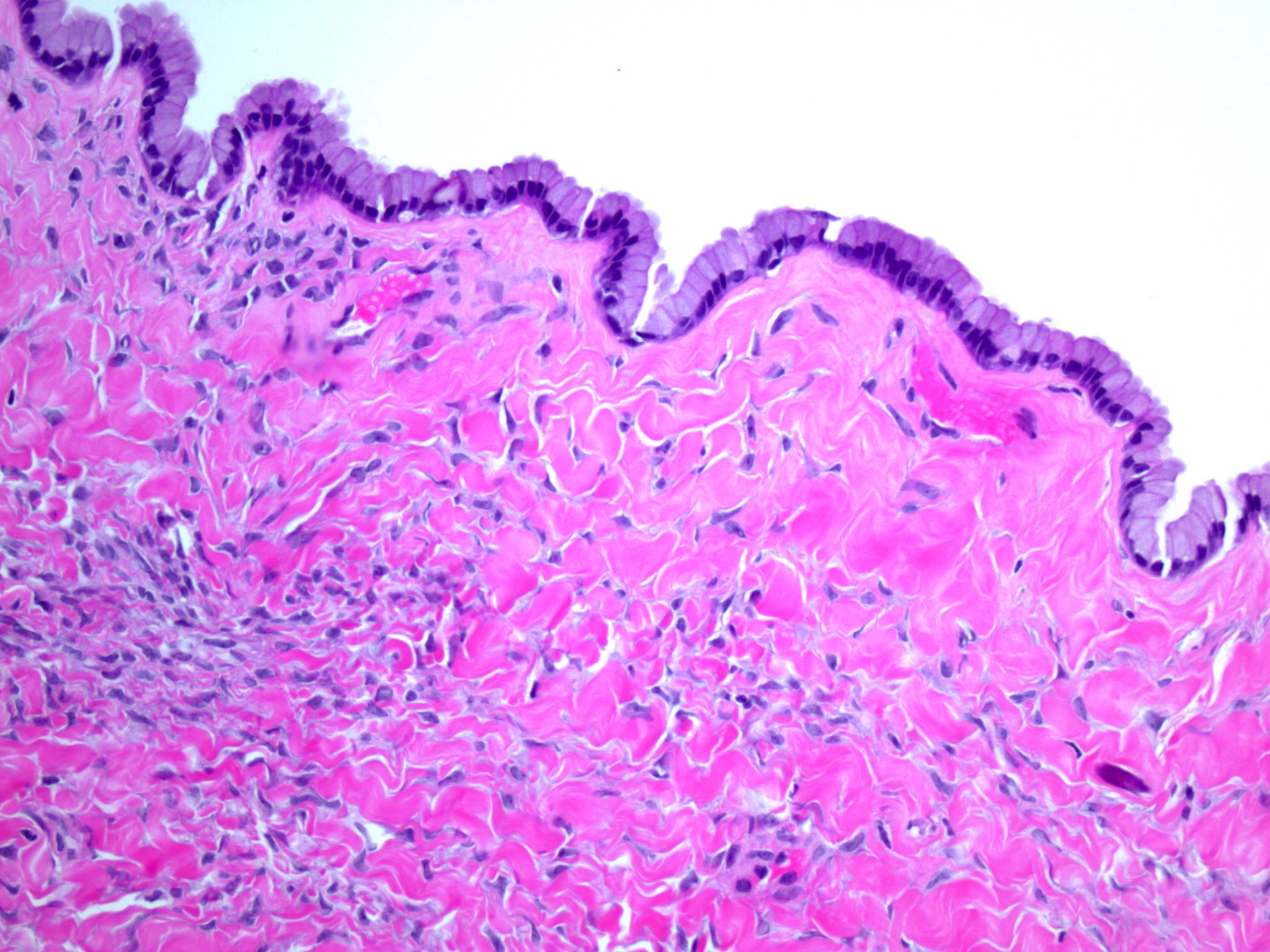
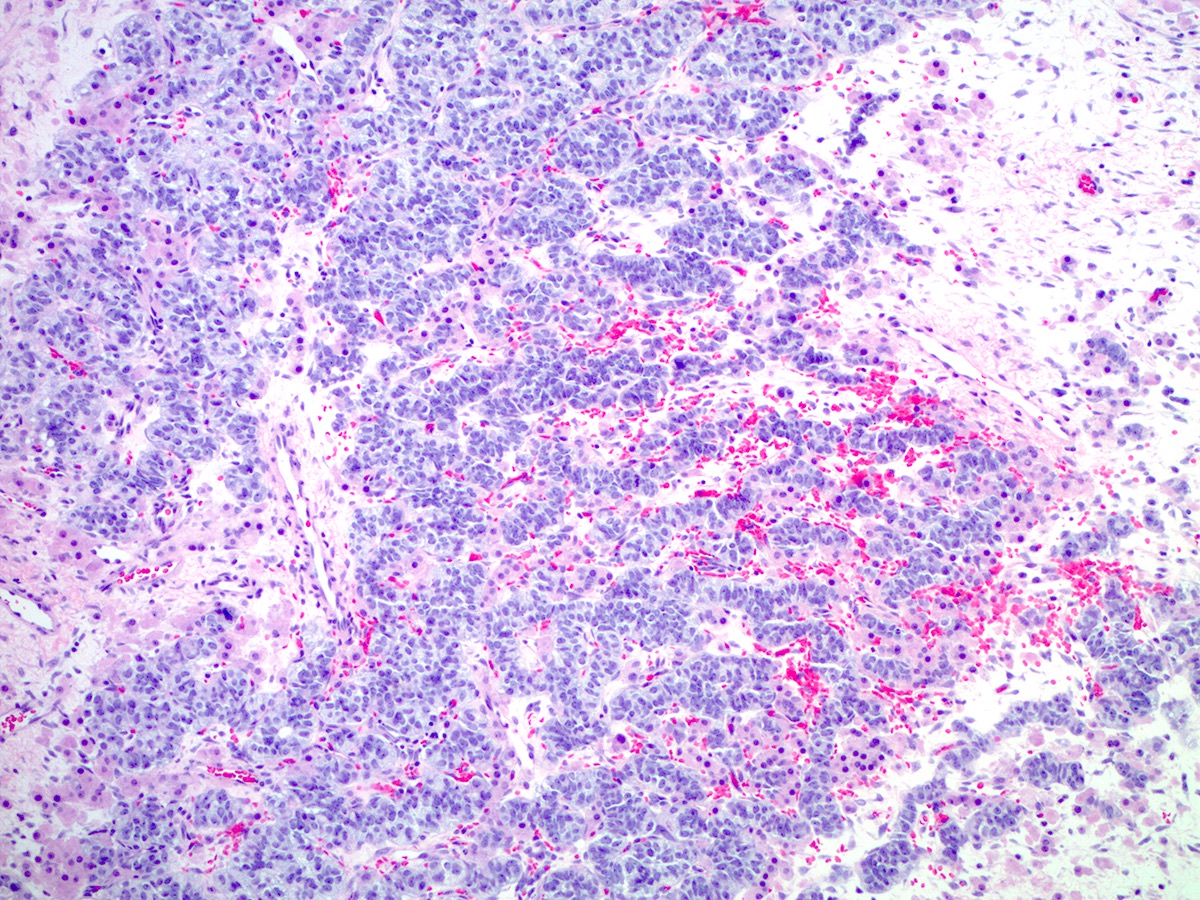
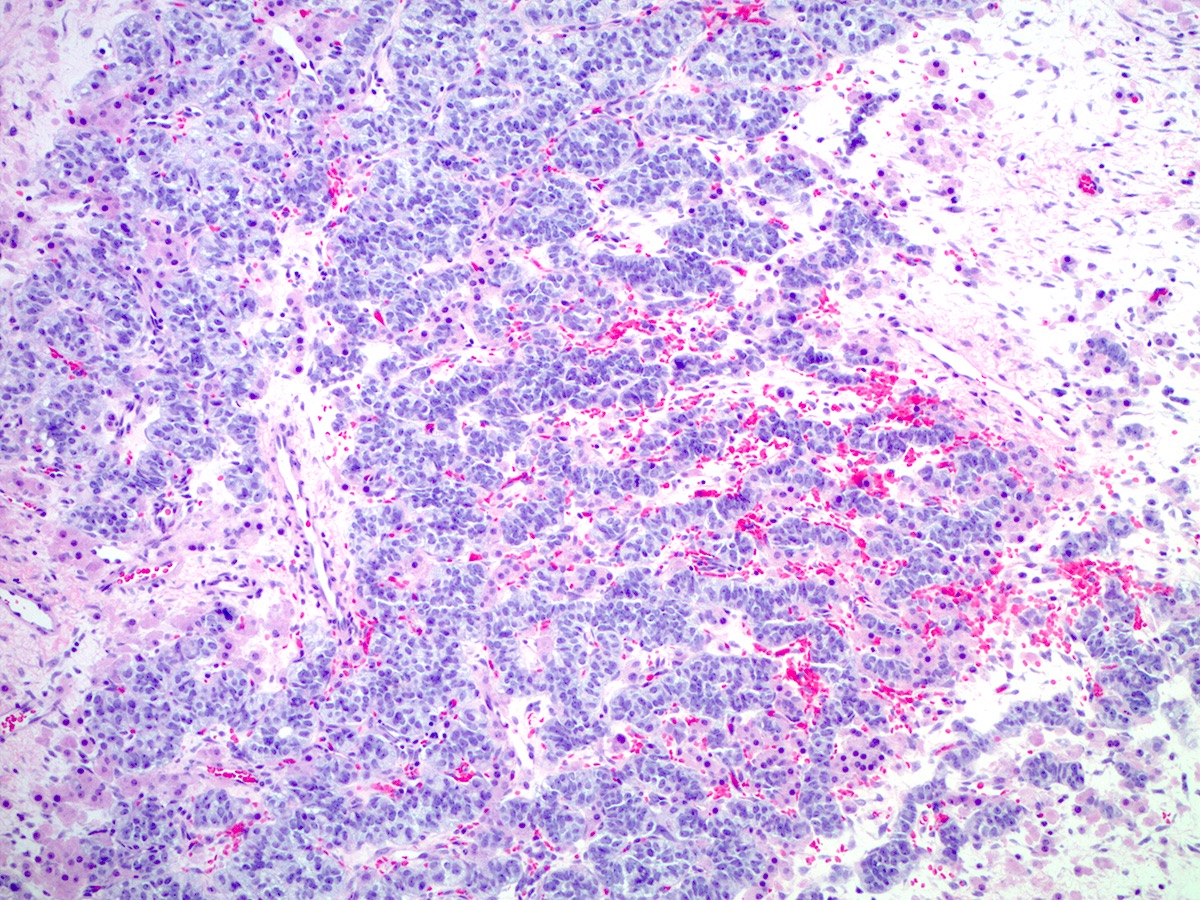
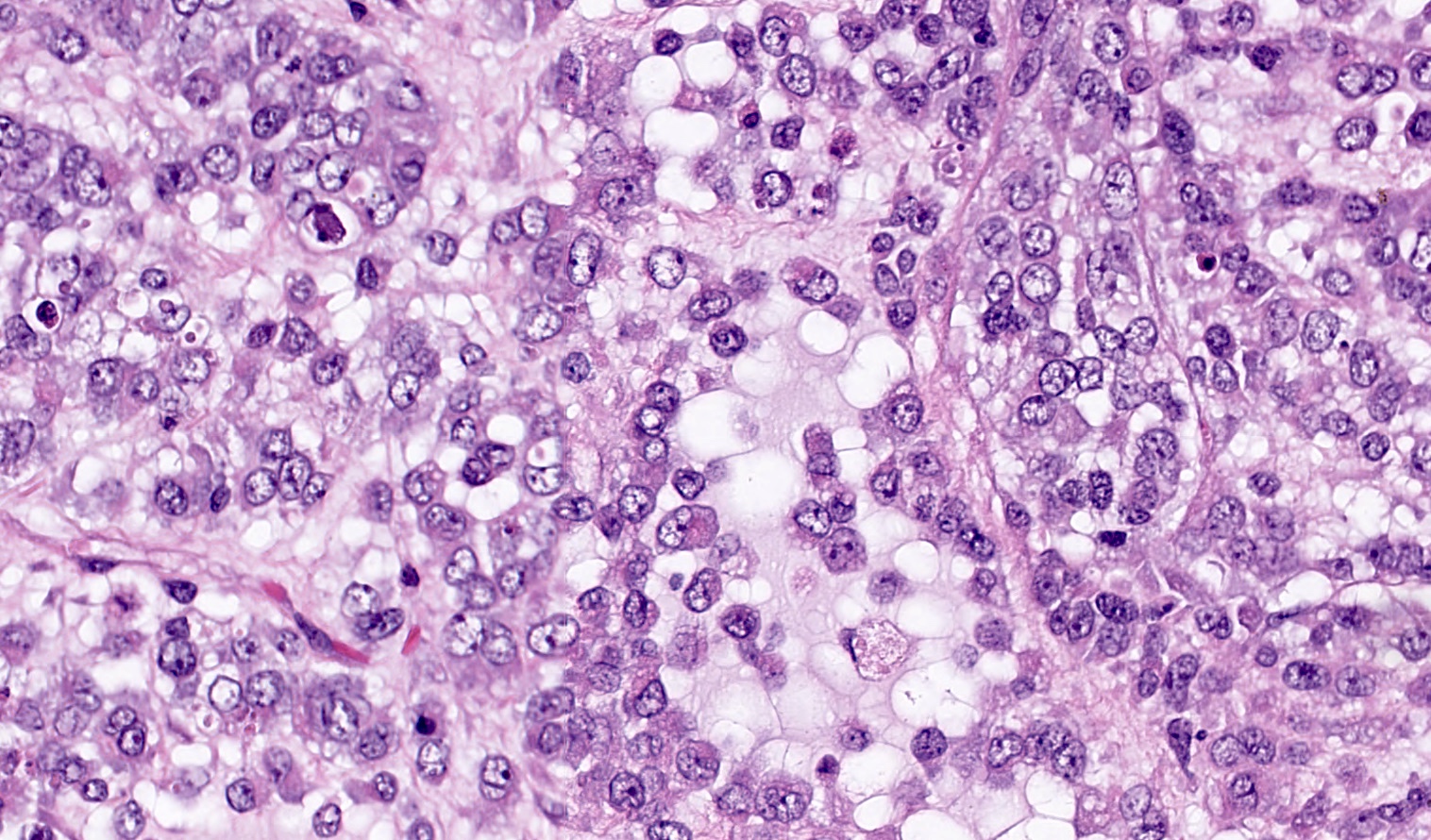
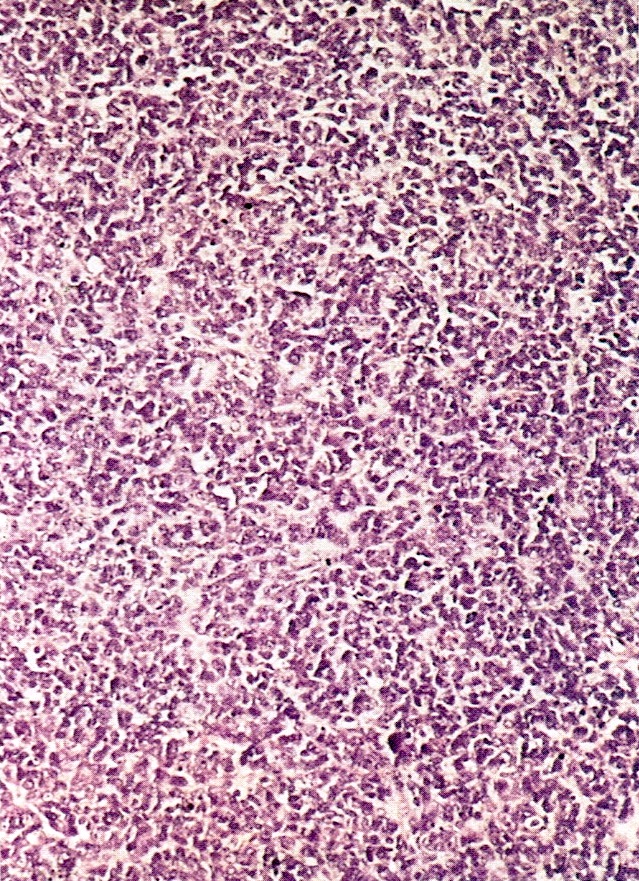
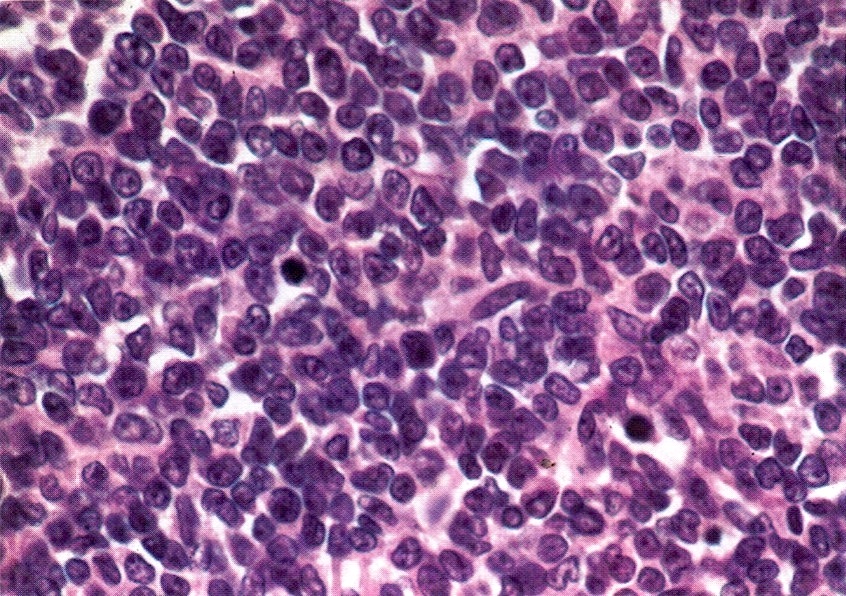
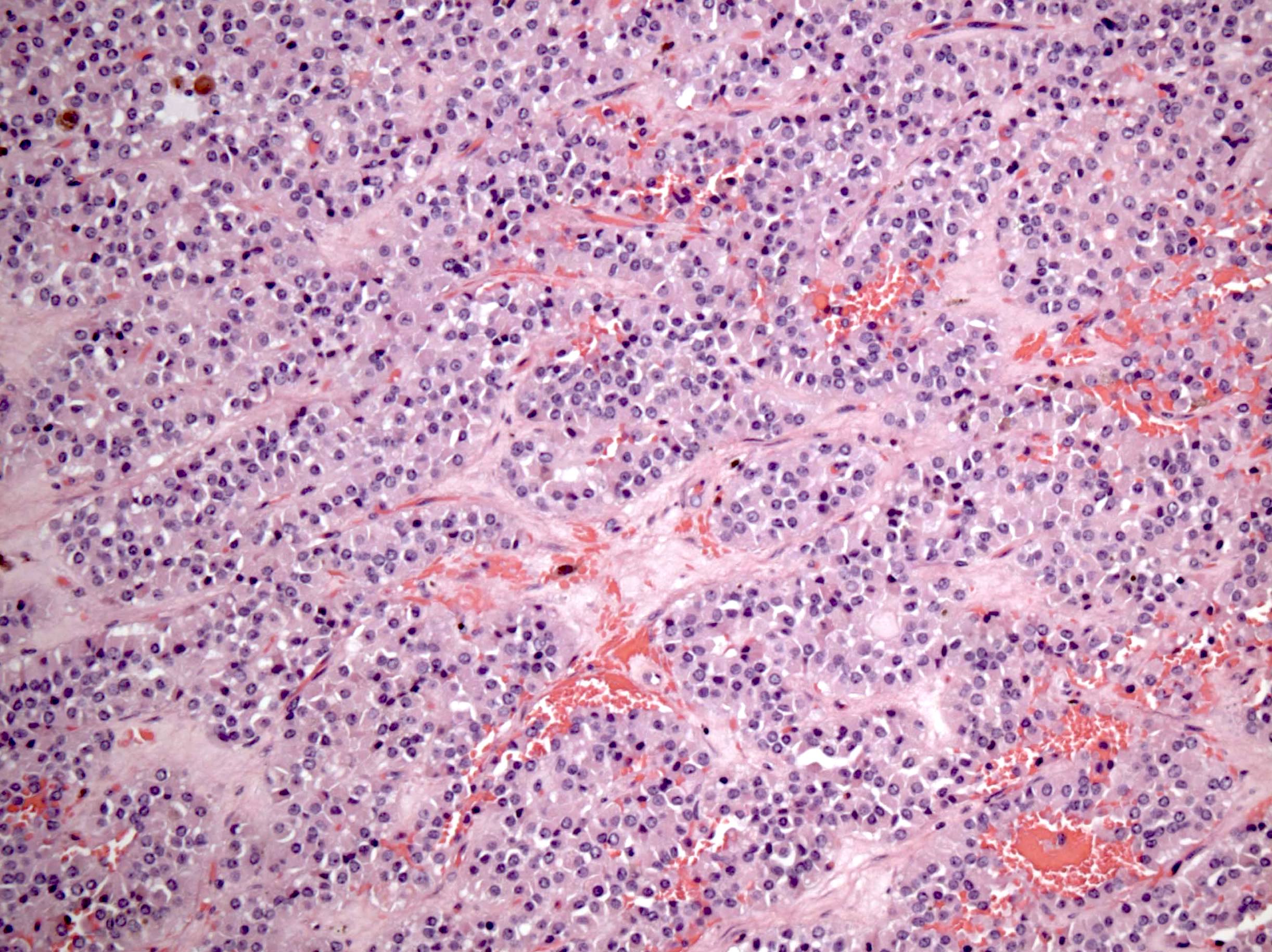
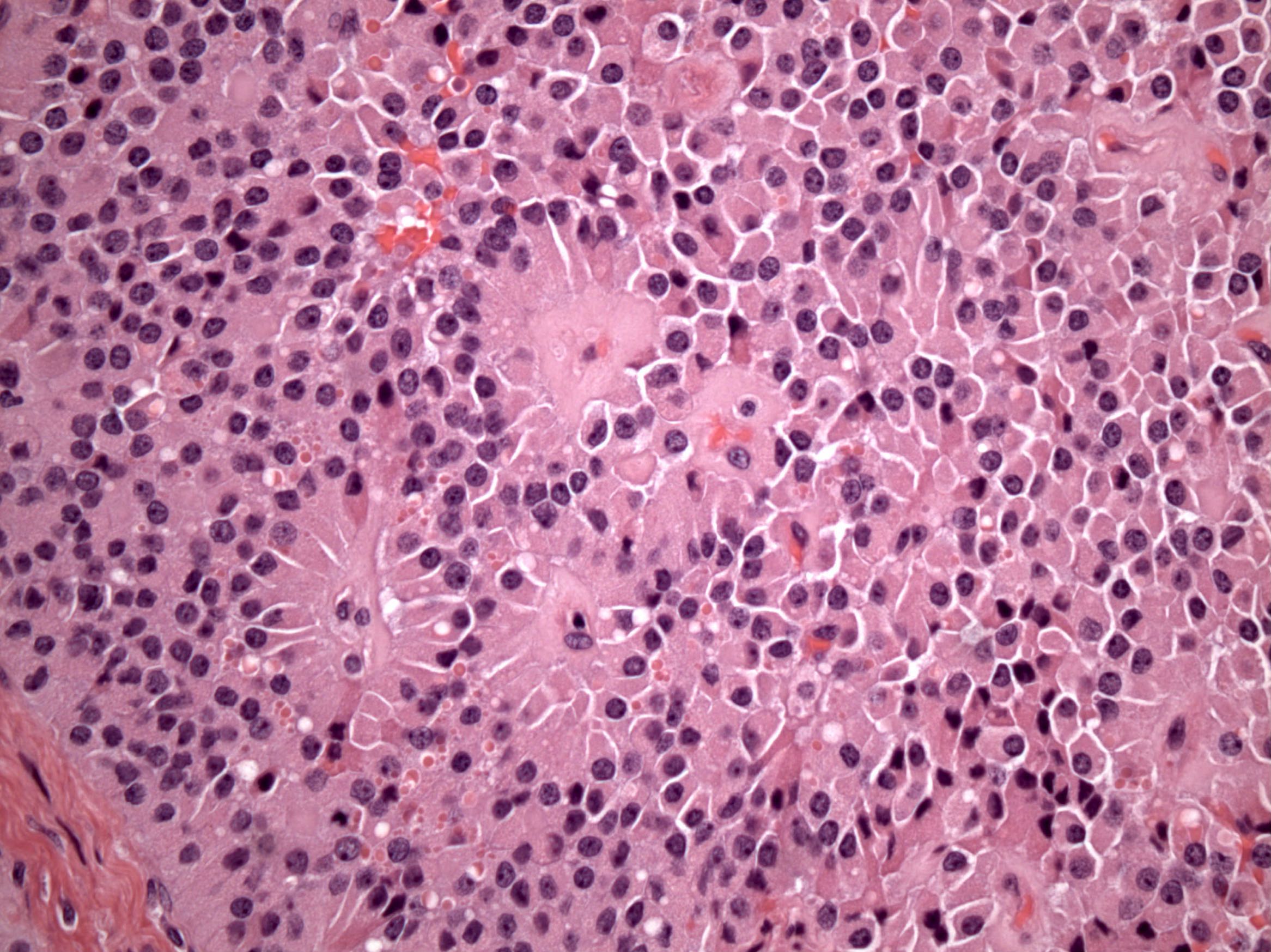
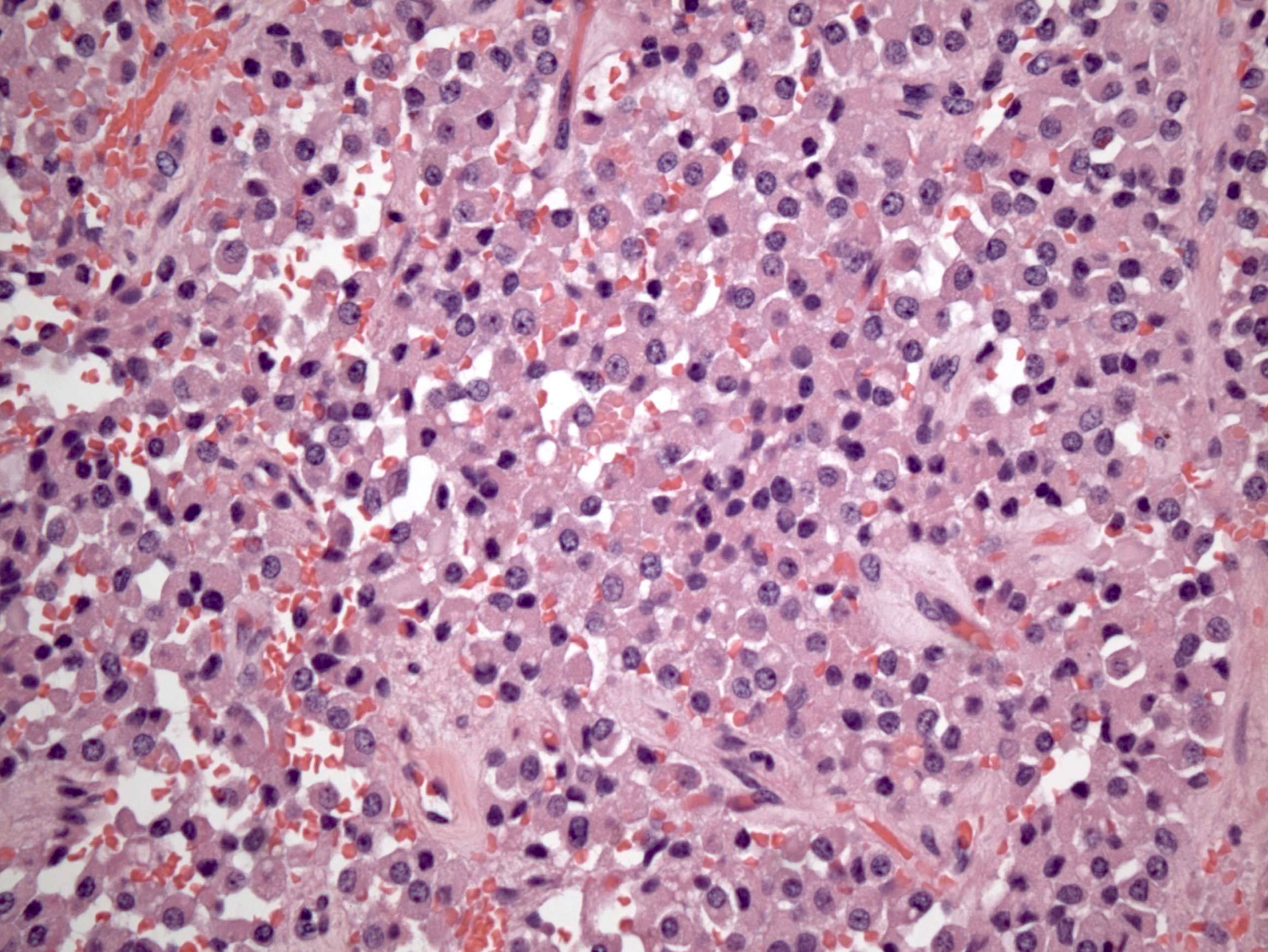
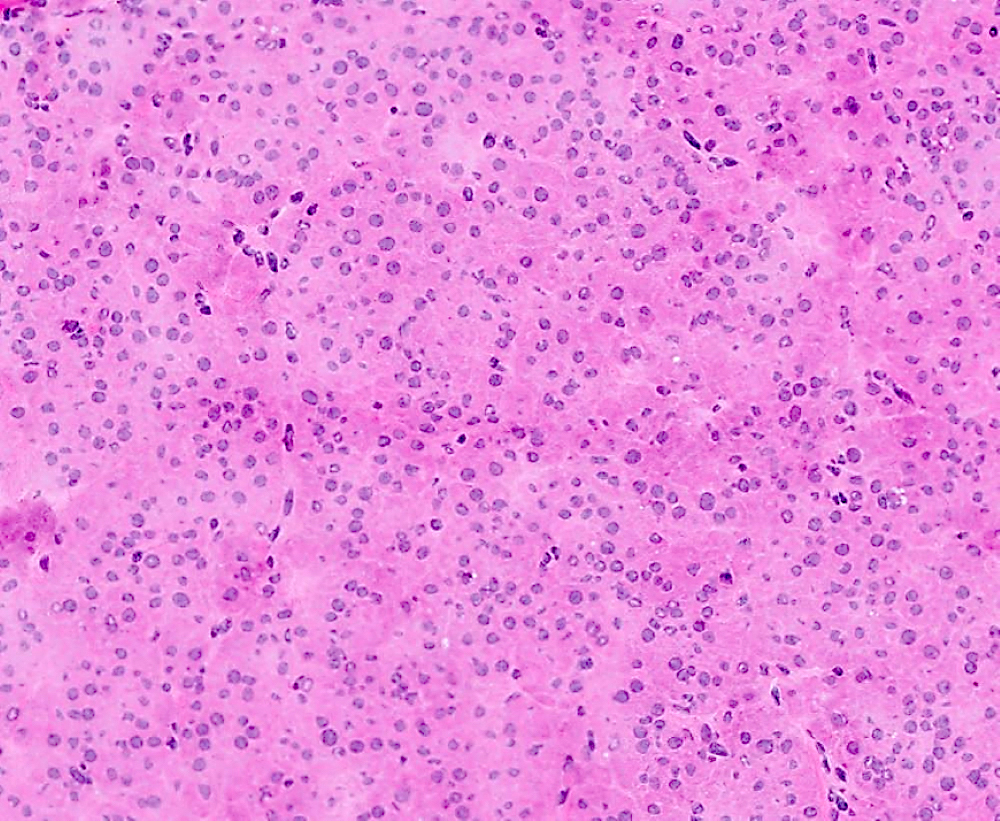
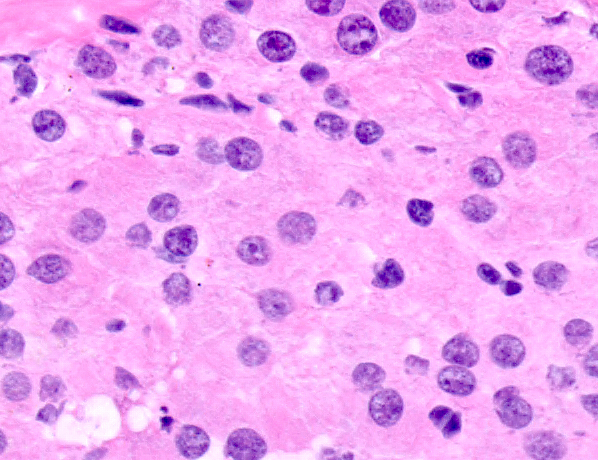
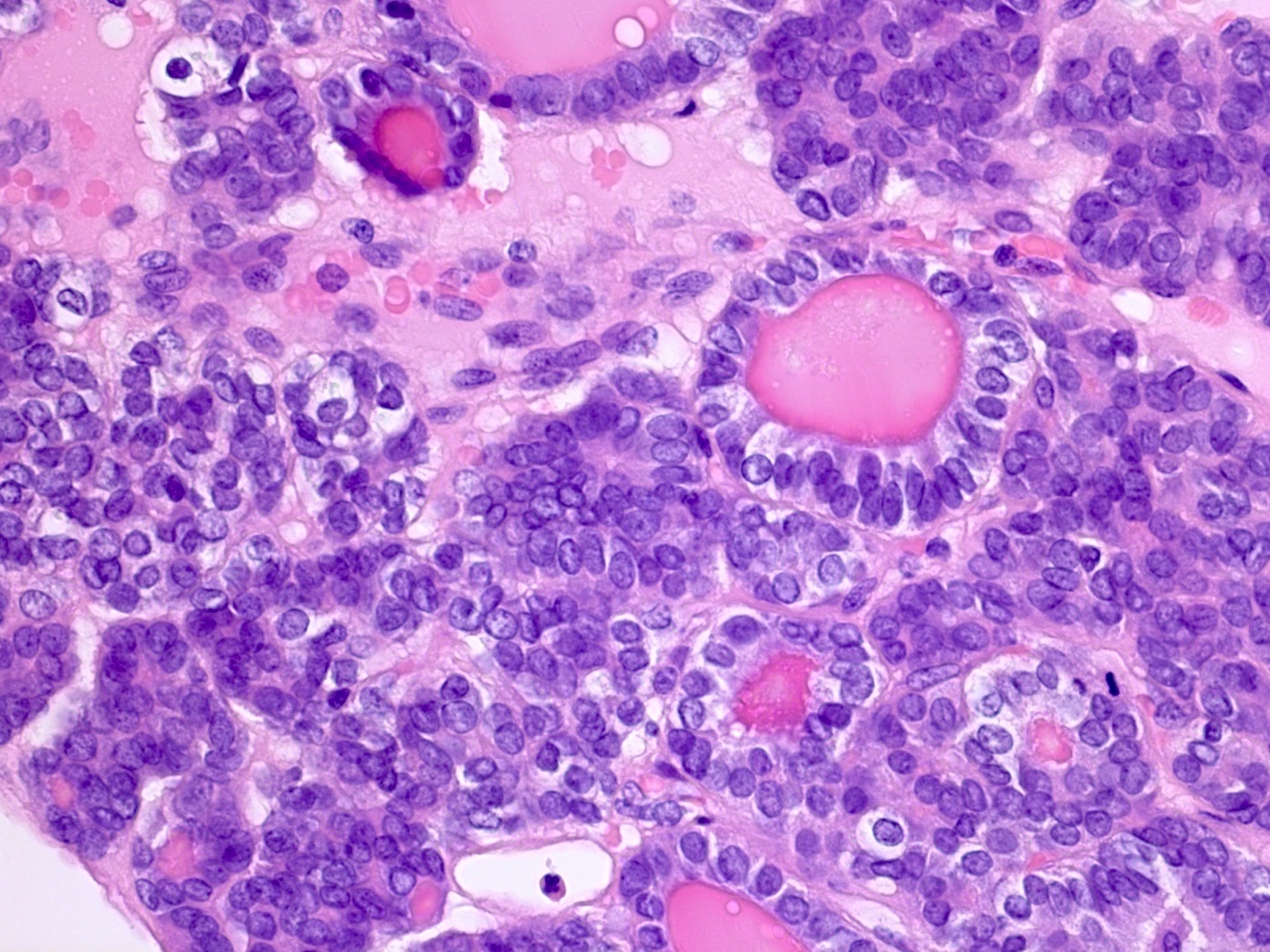
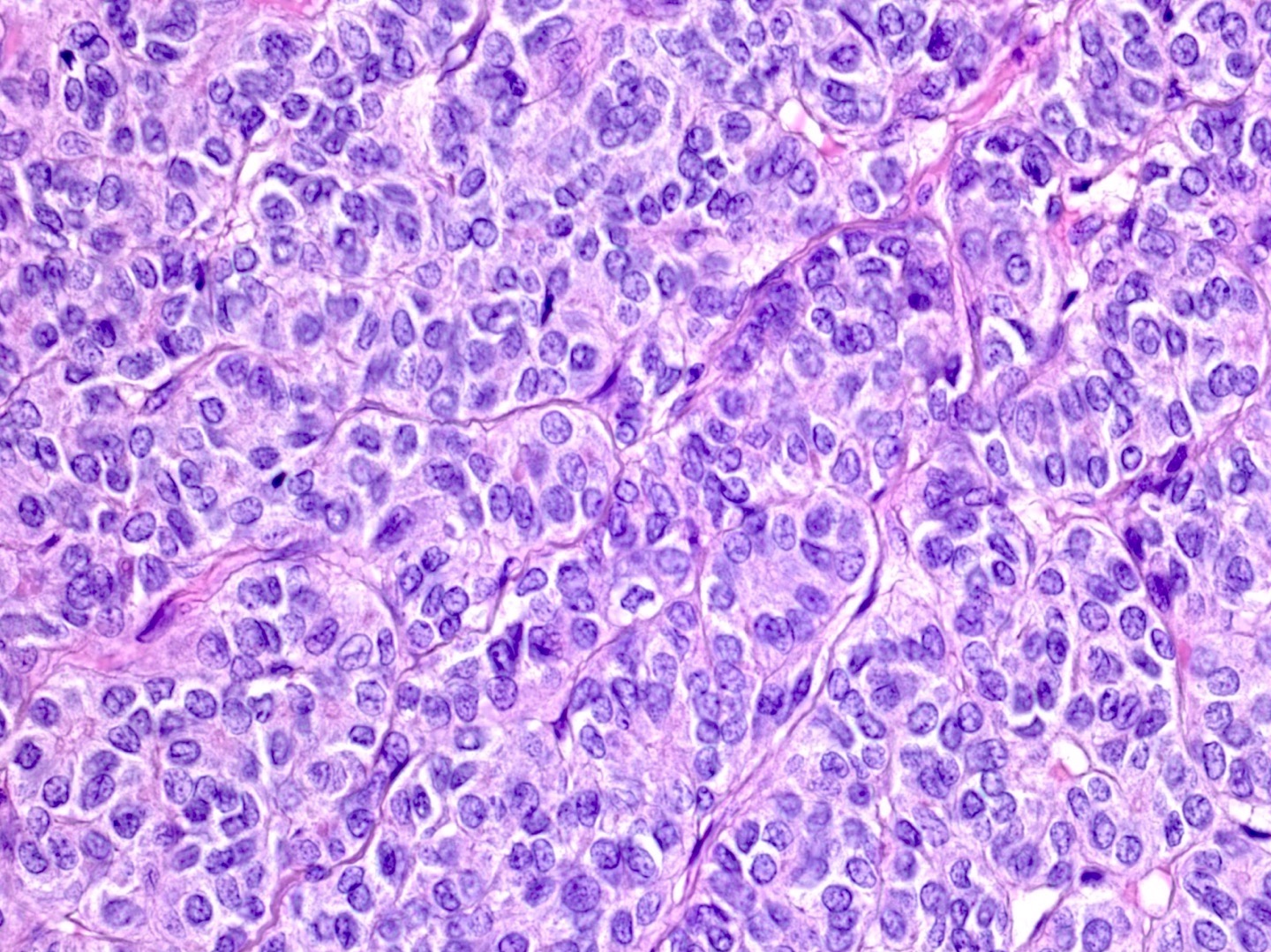
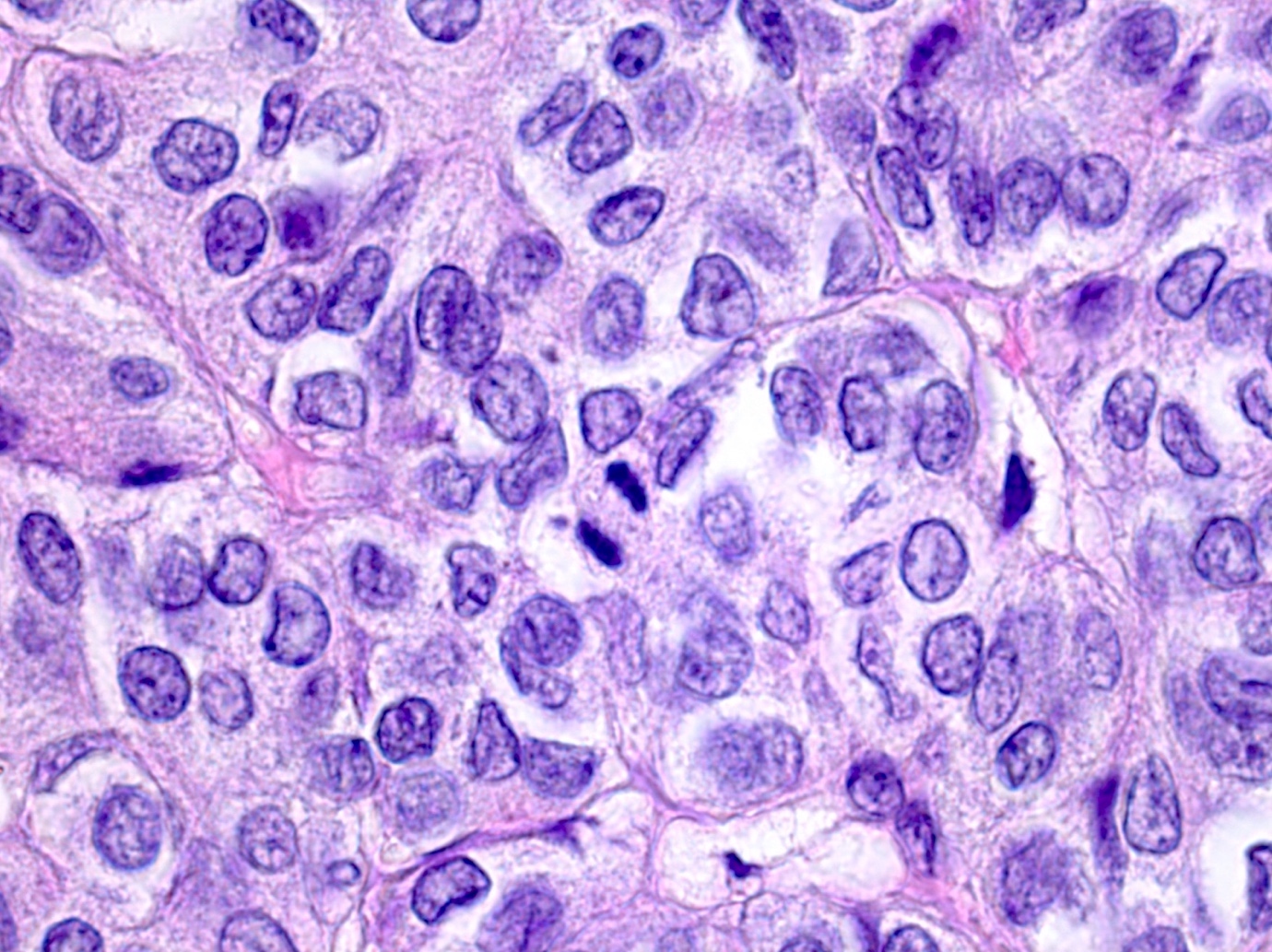
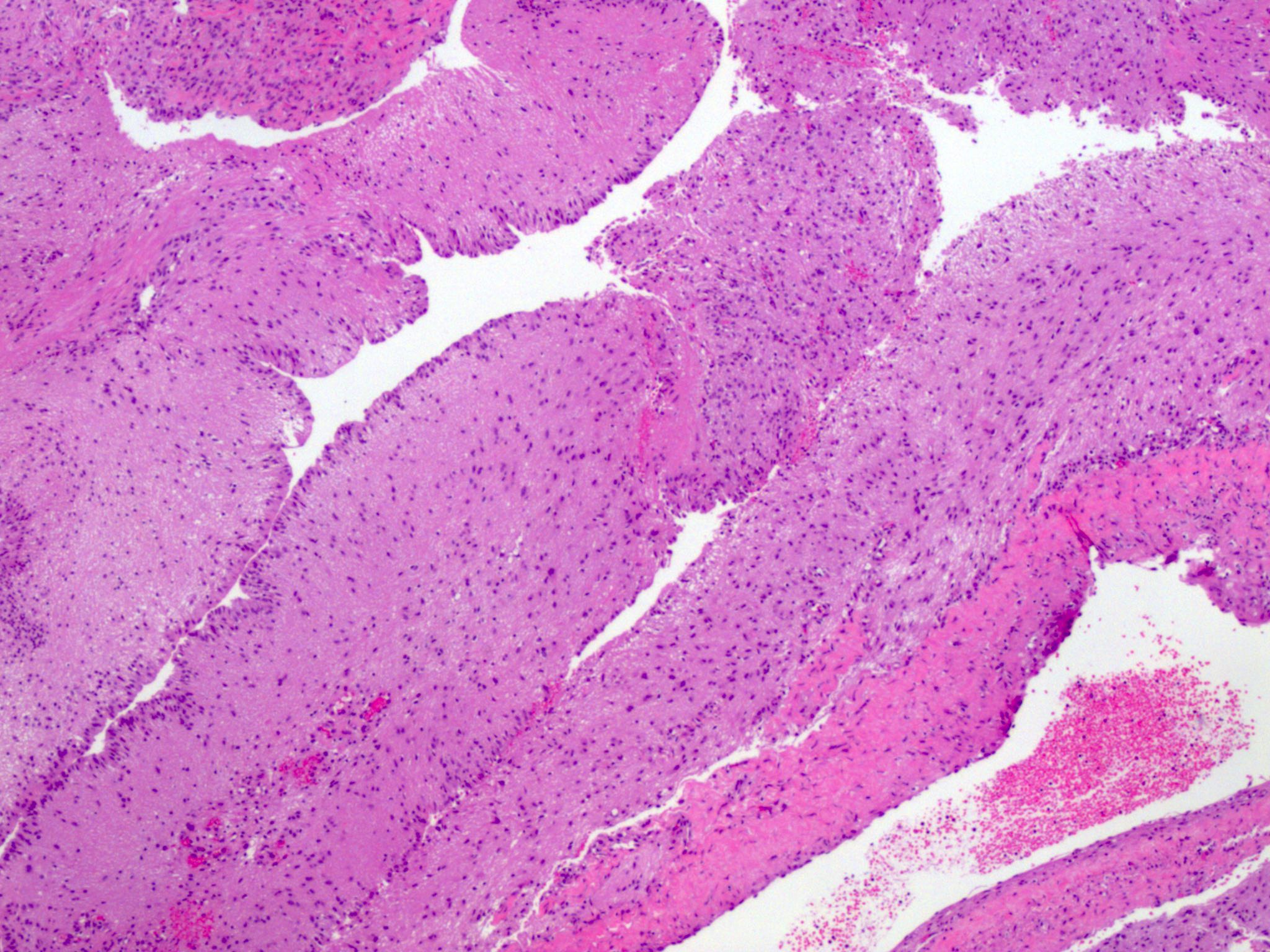
Microscopic (histologic) description
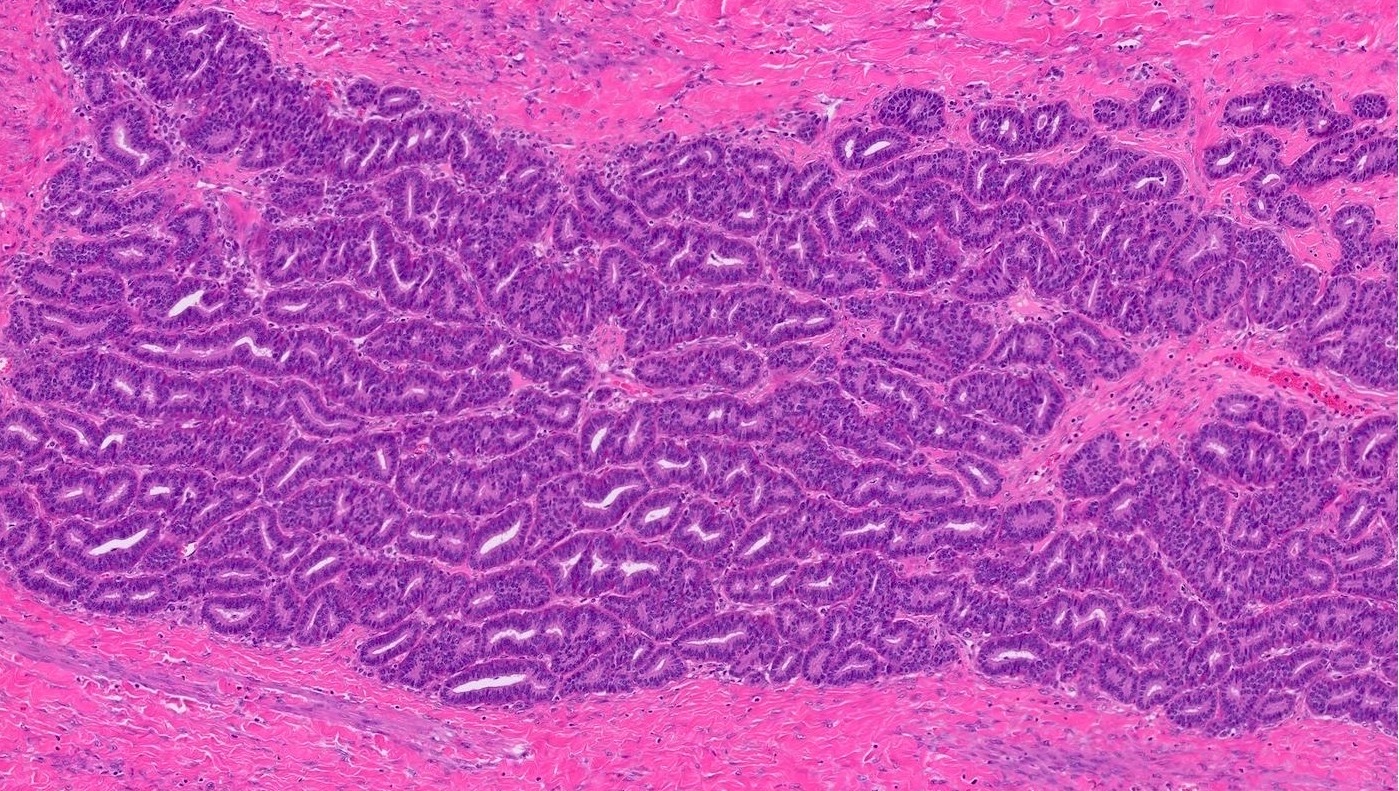
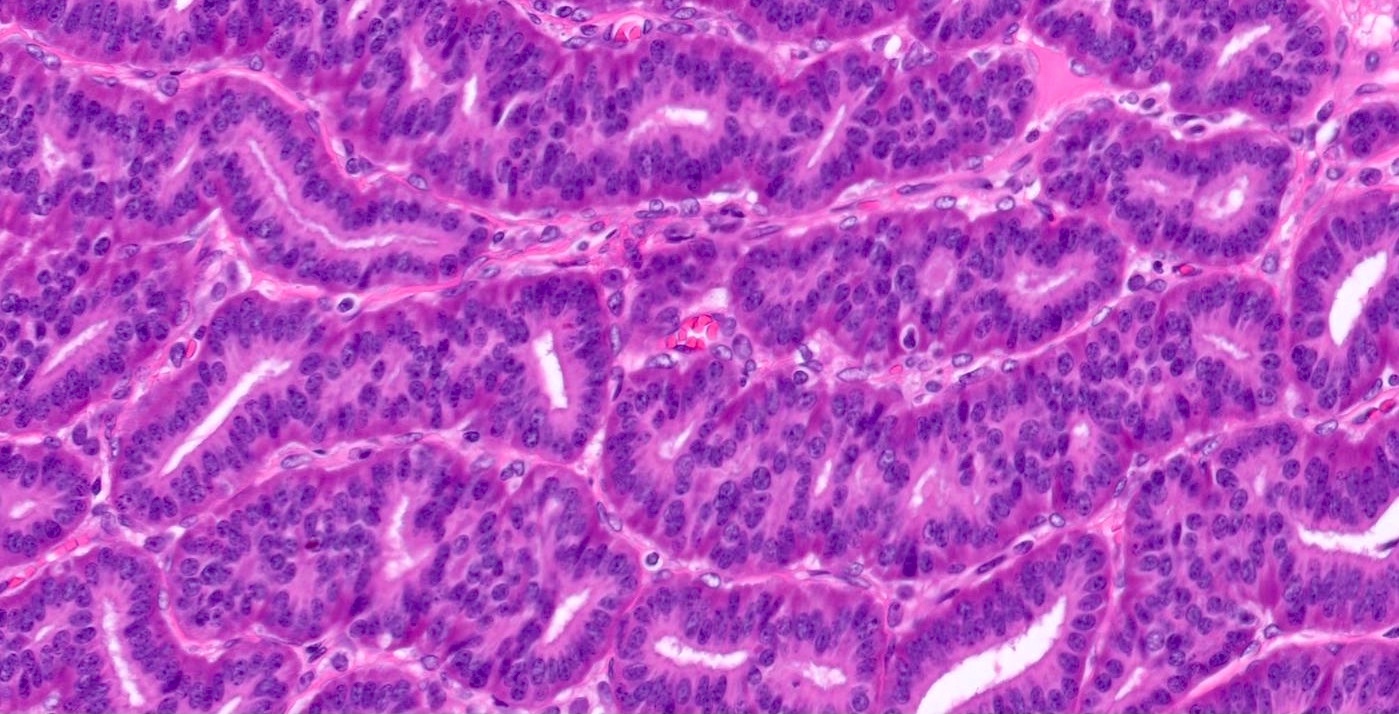
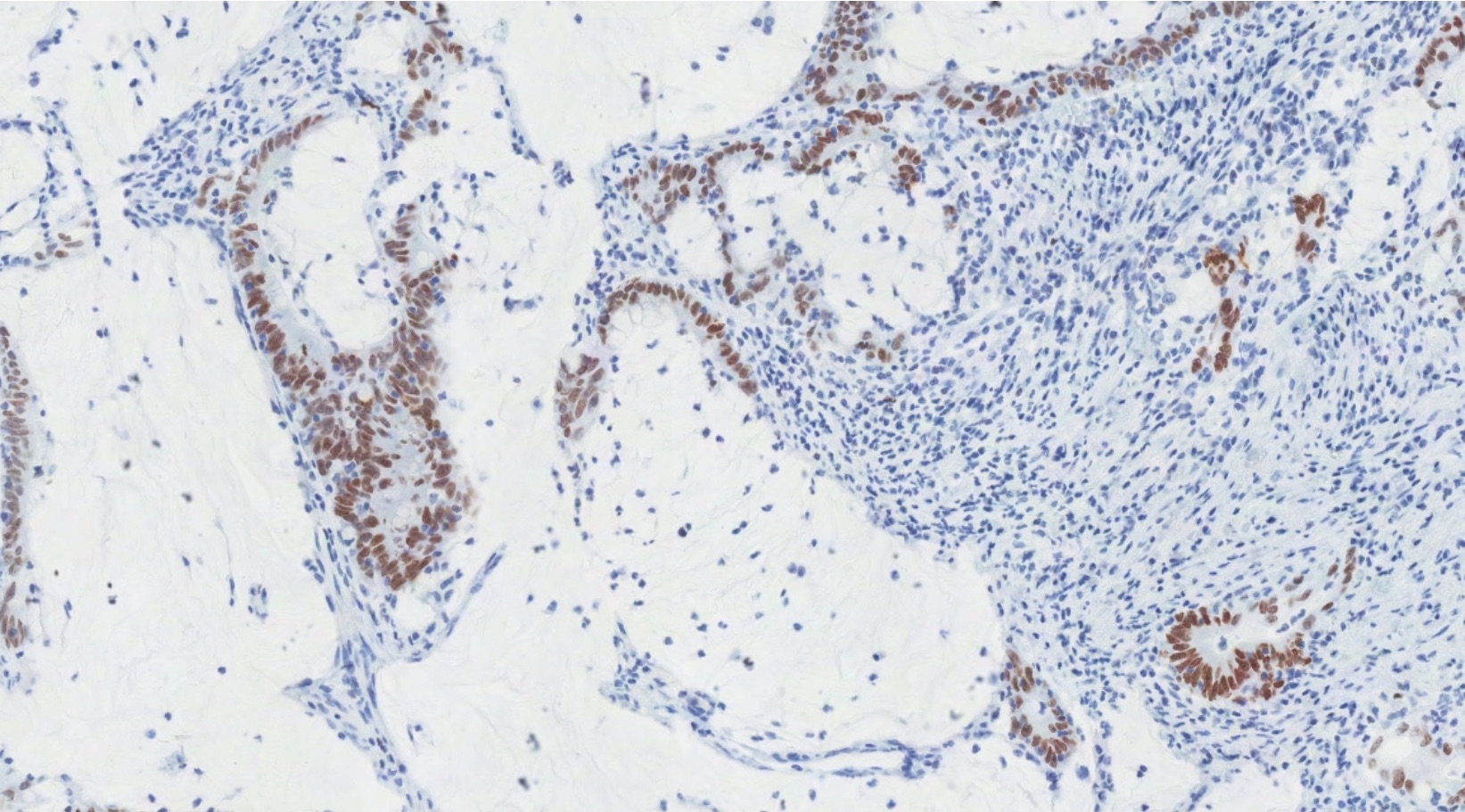
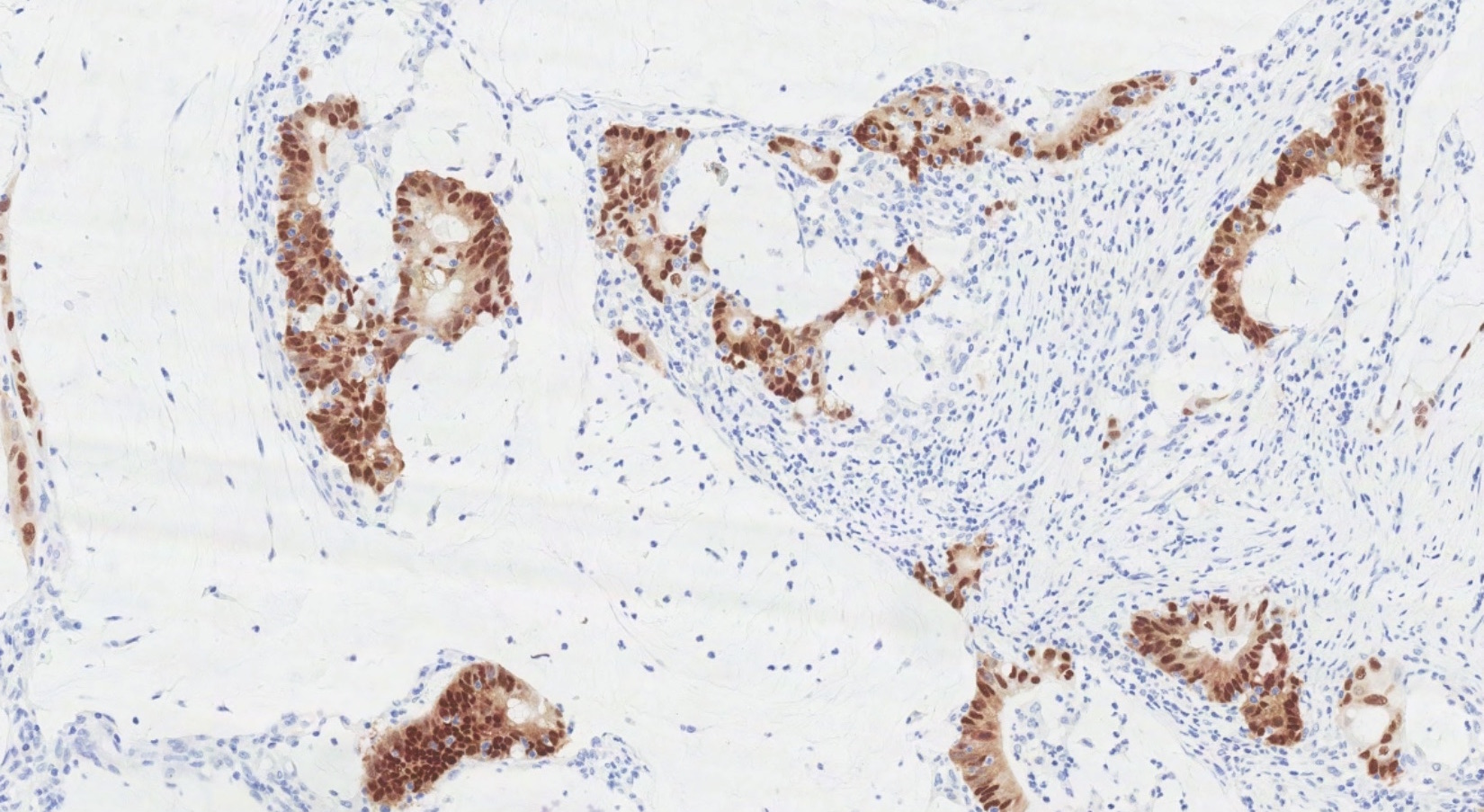
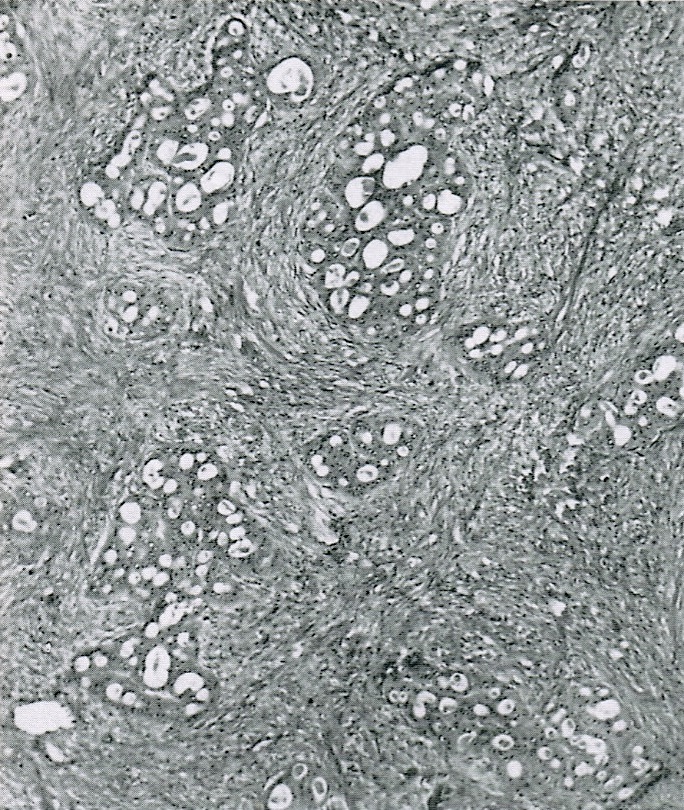
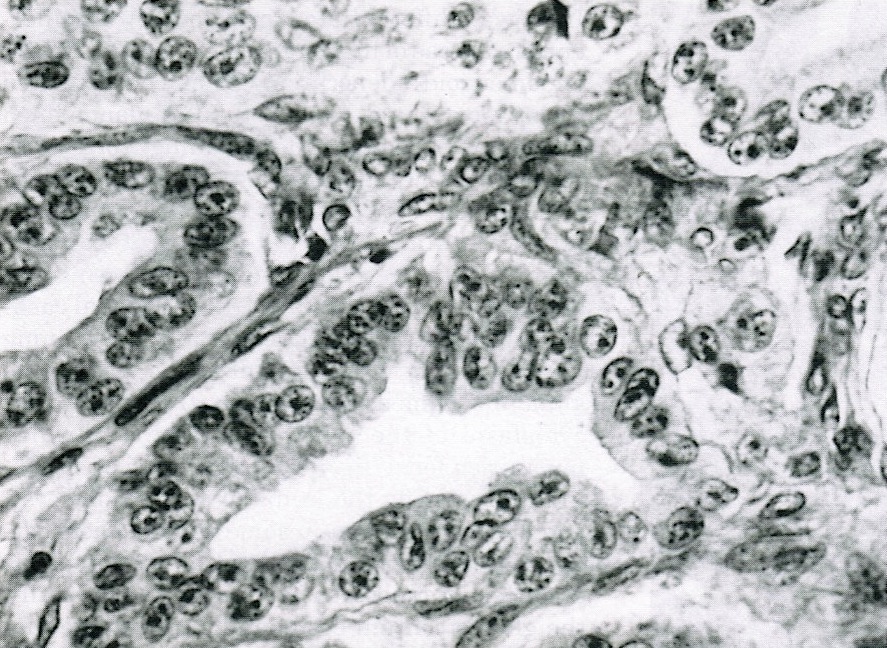
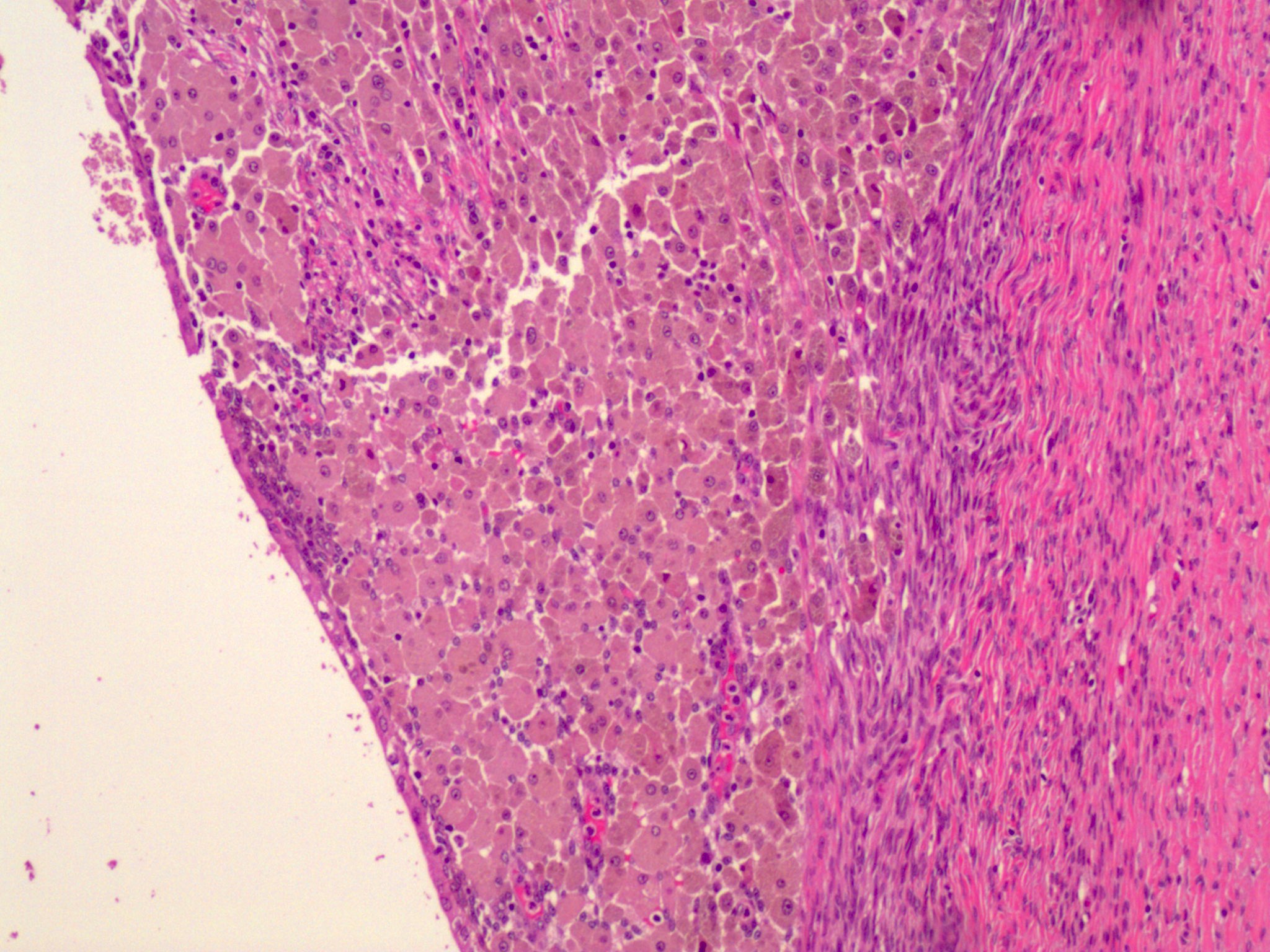
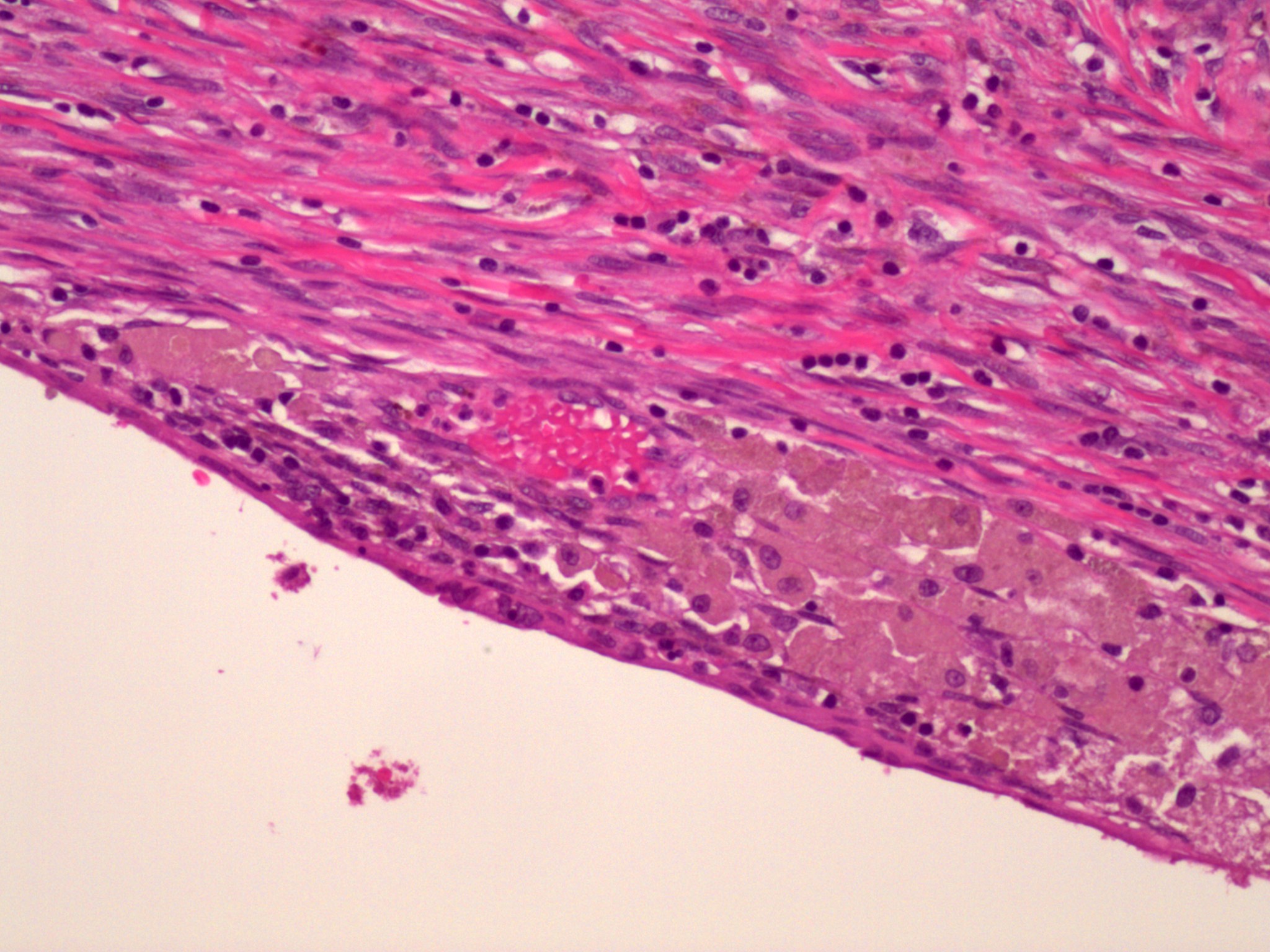
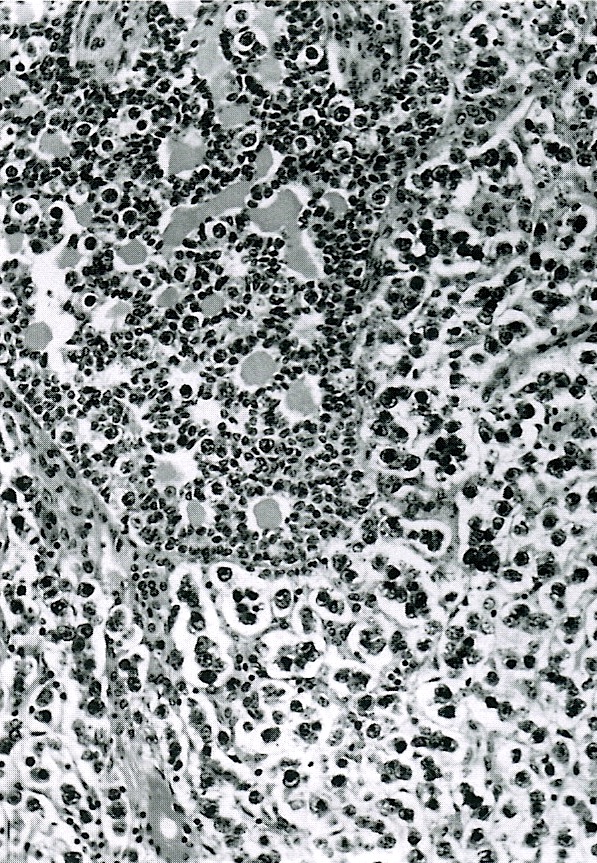
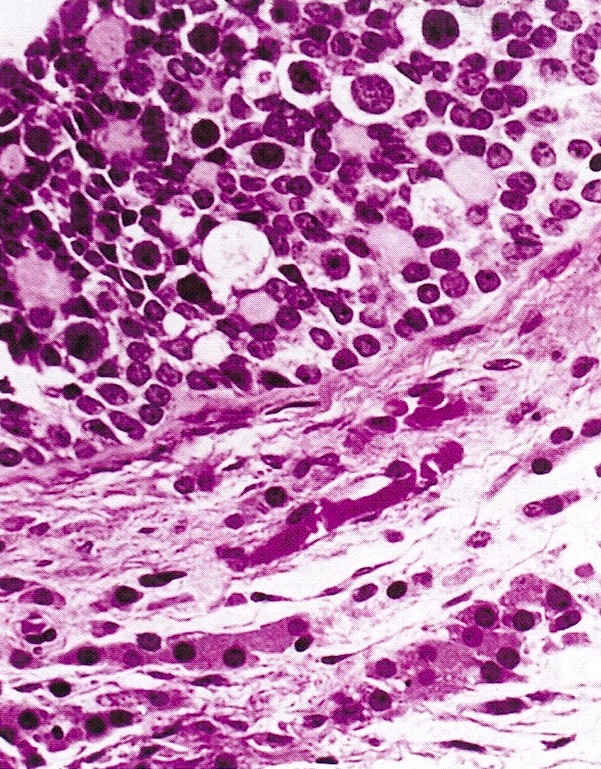
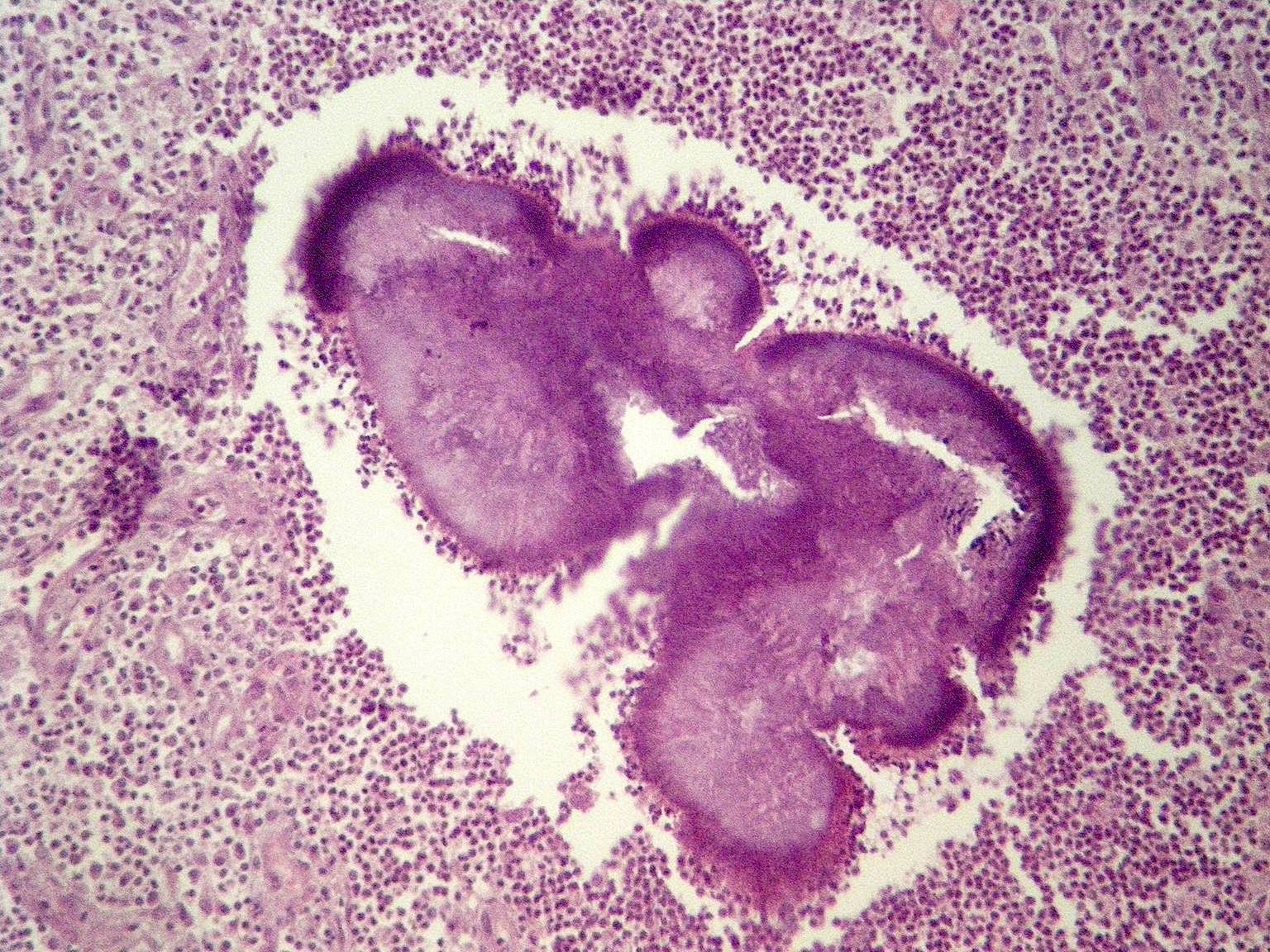
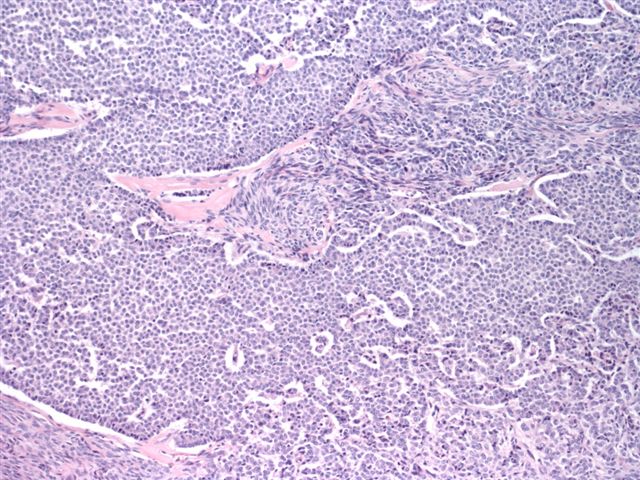
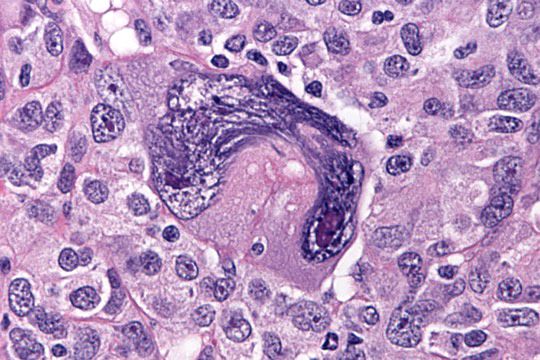
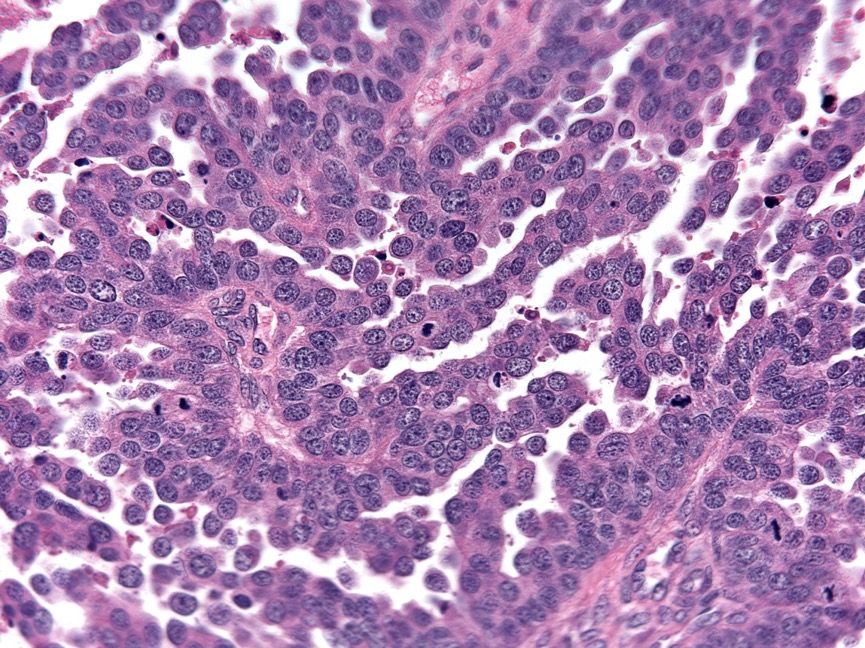
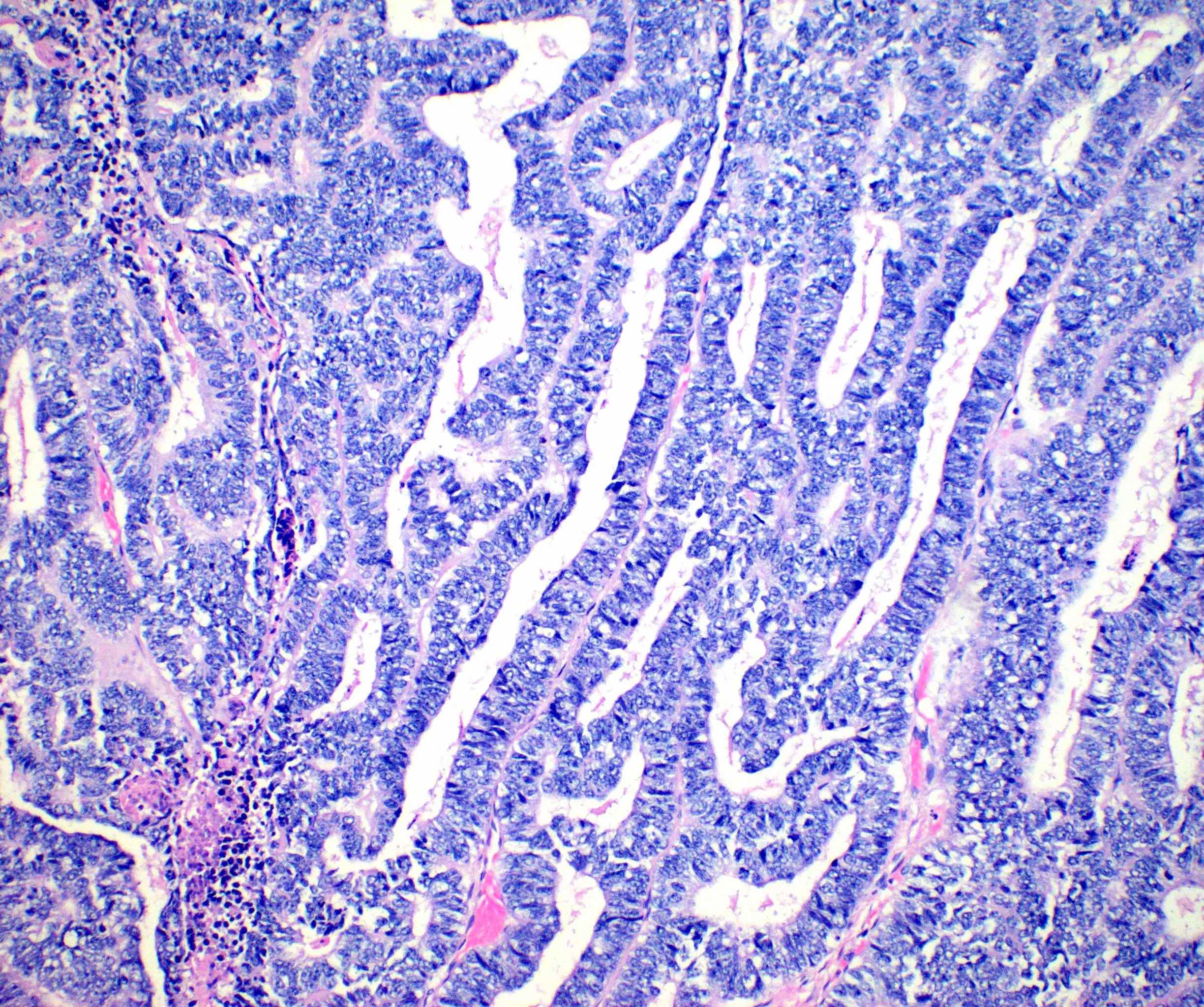
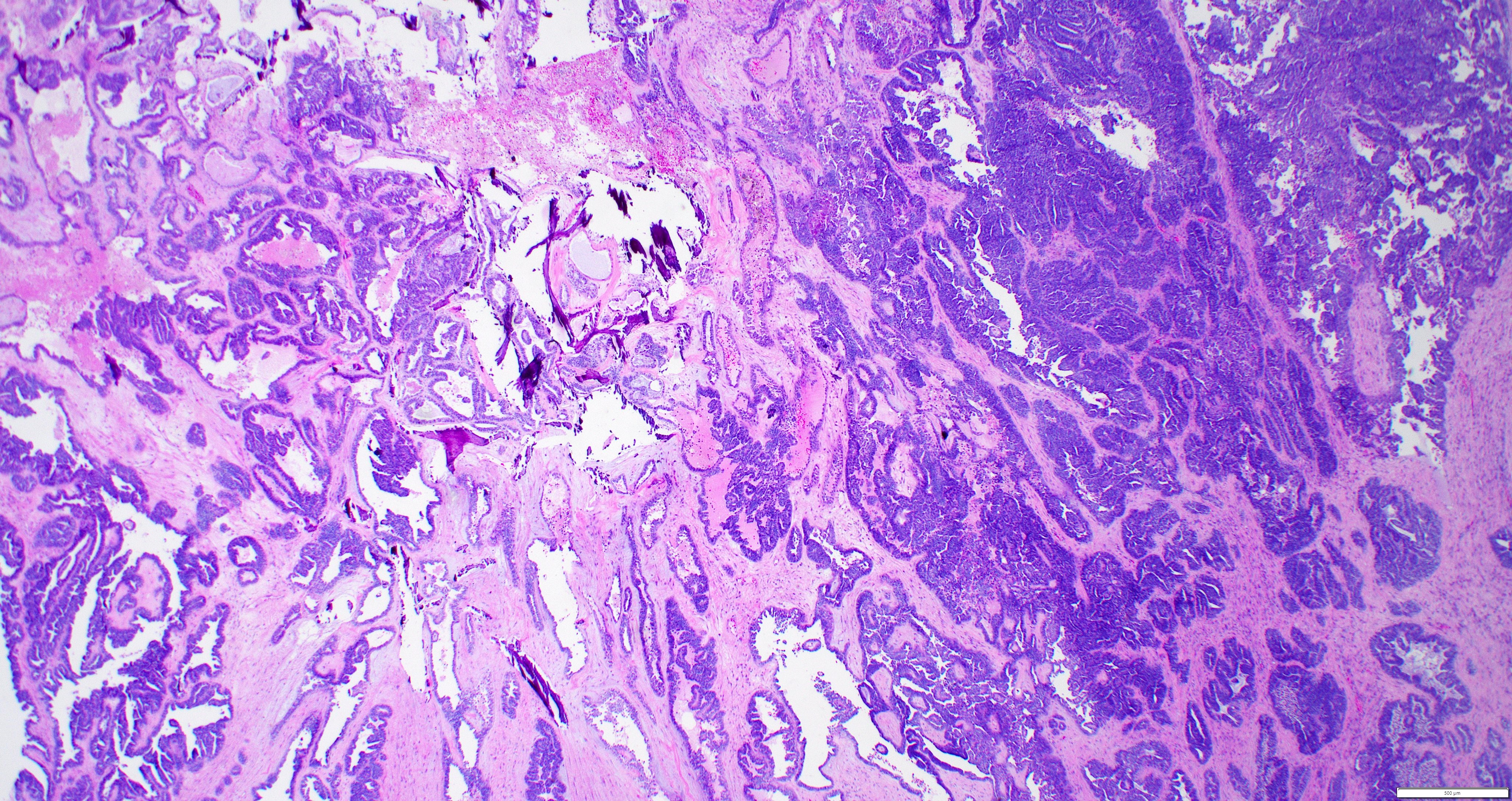
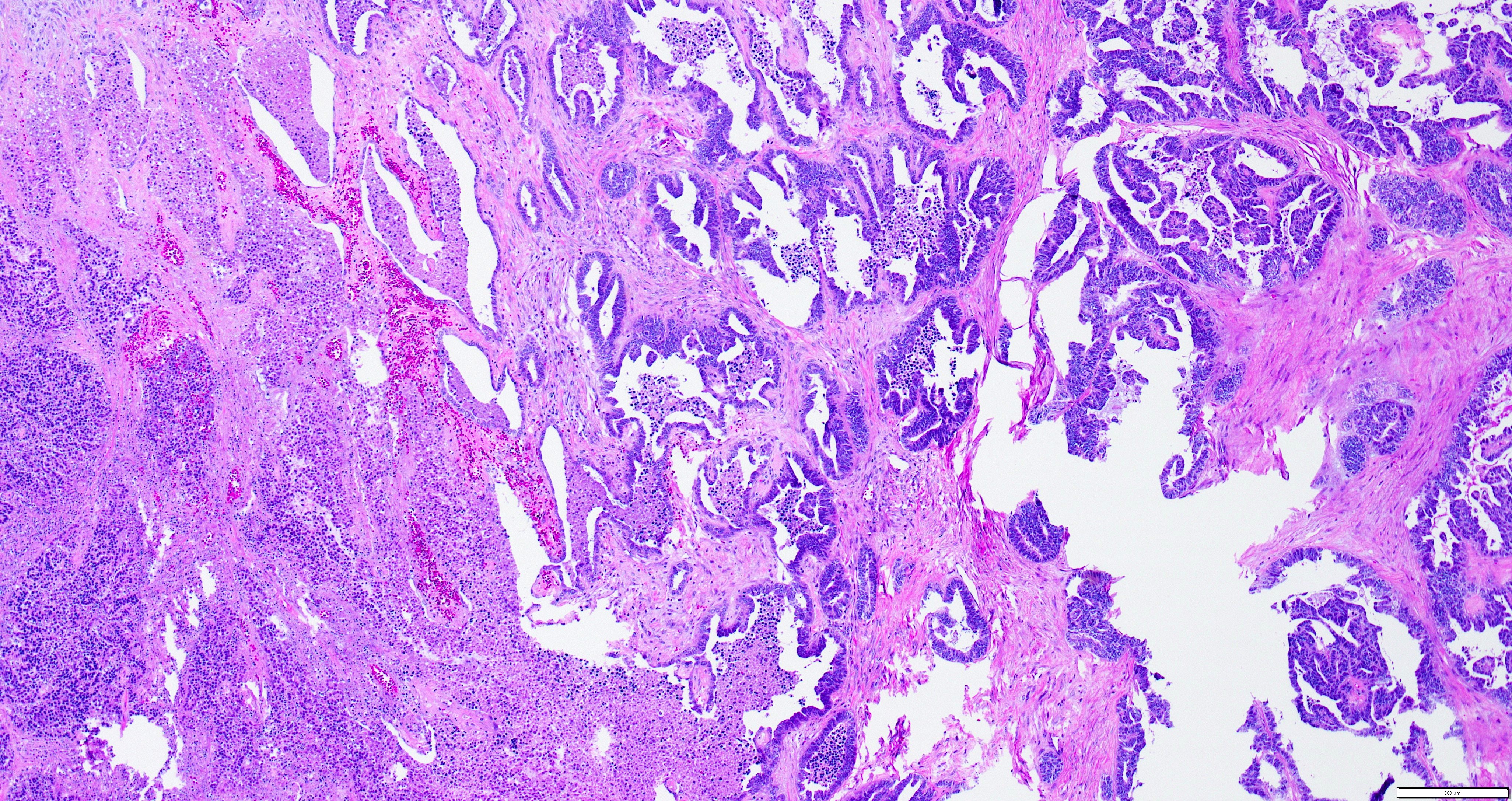
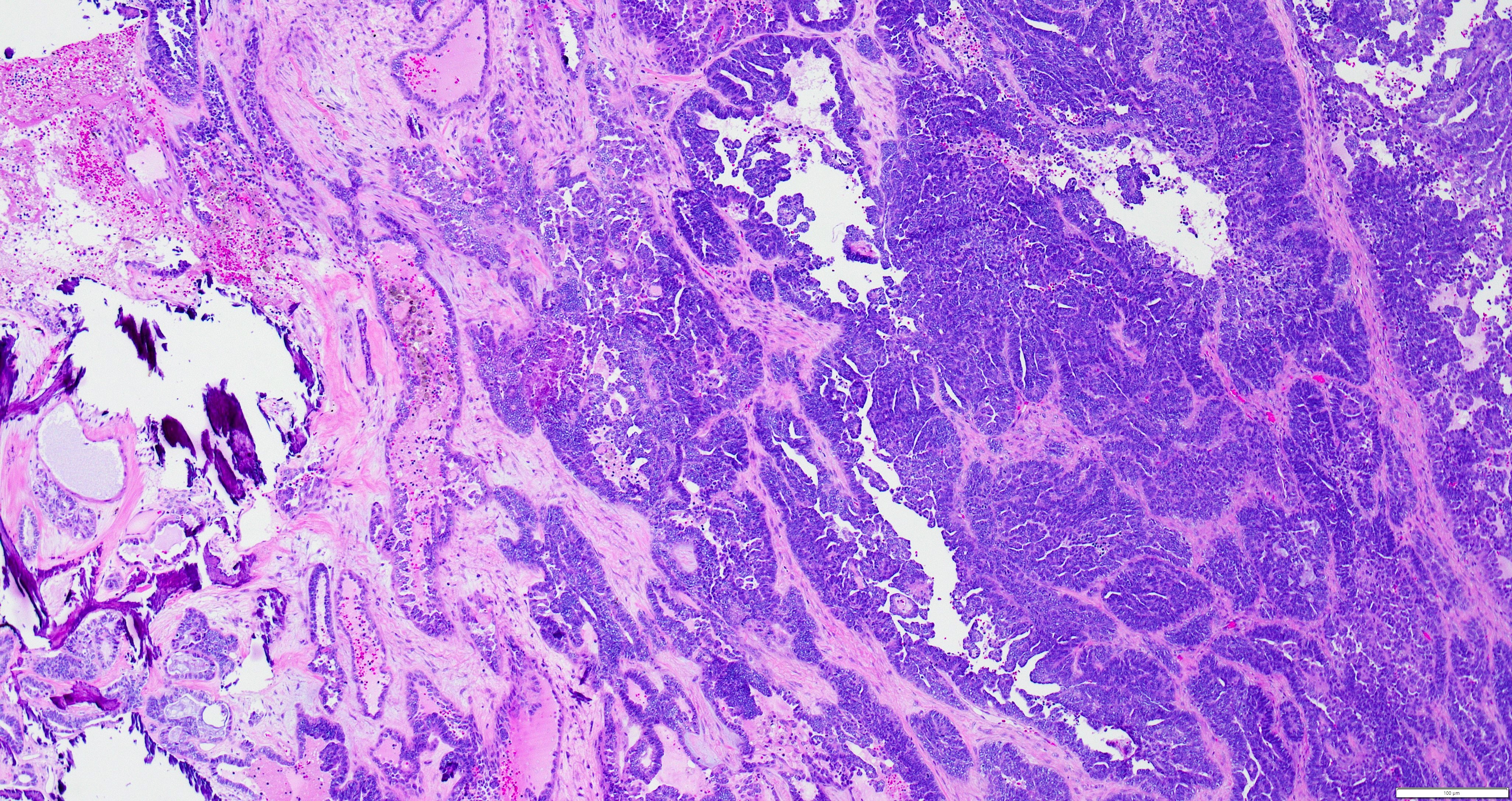
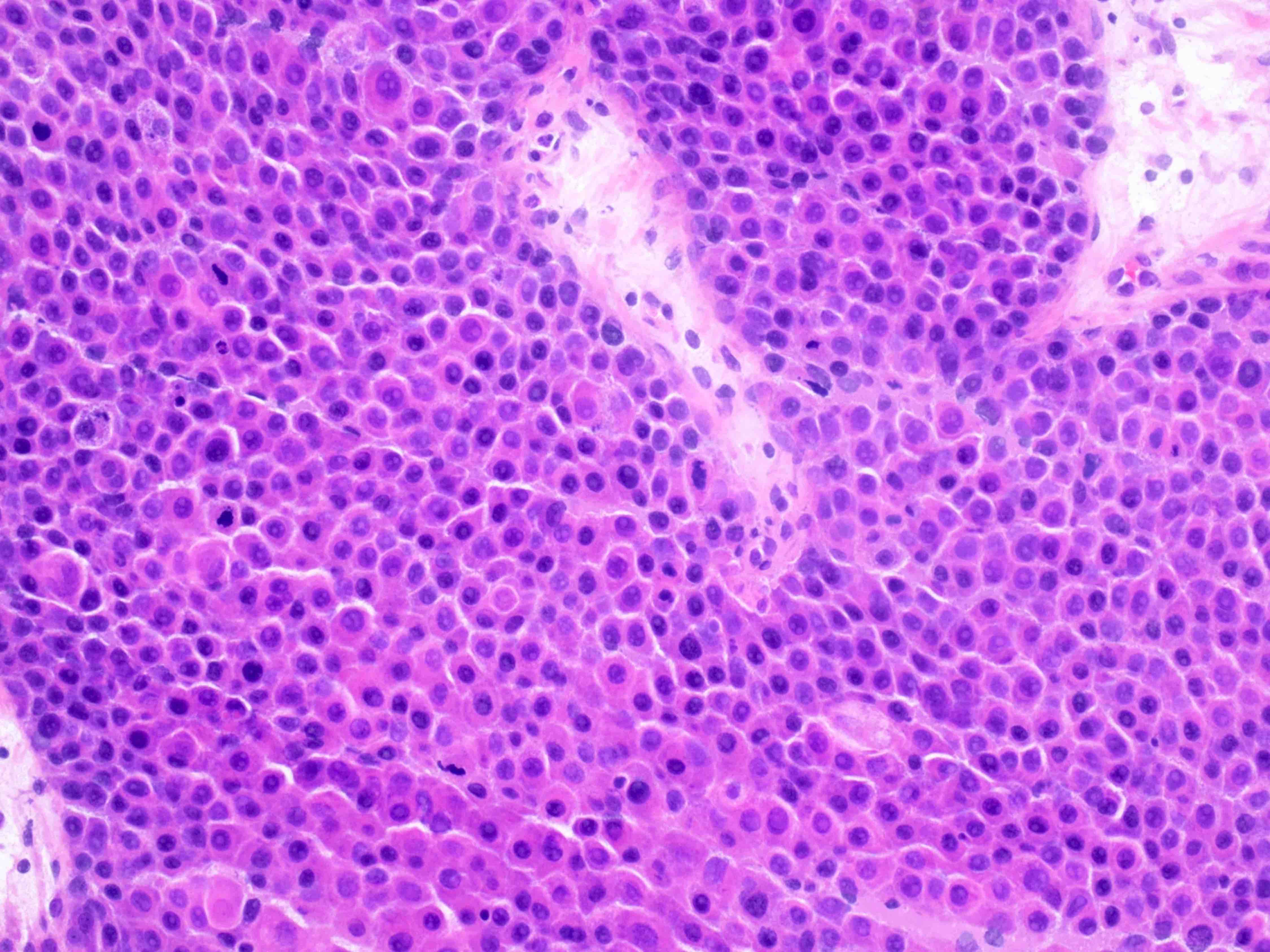
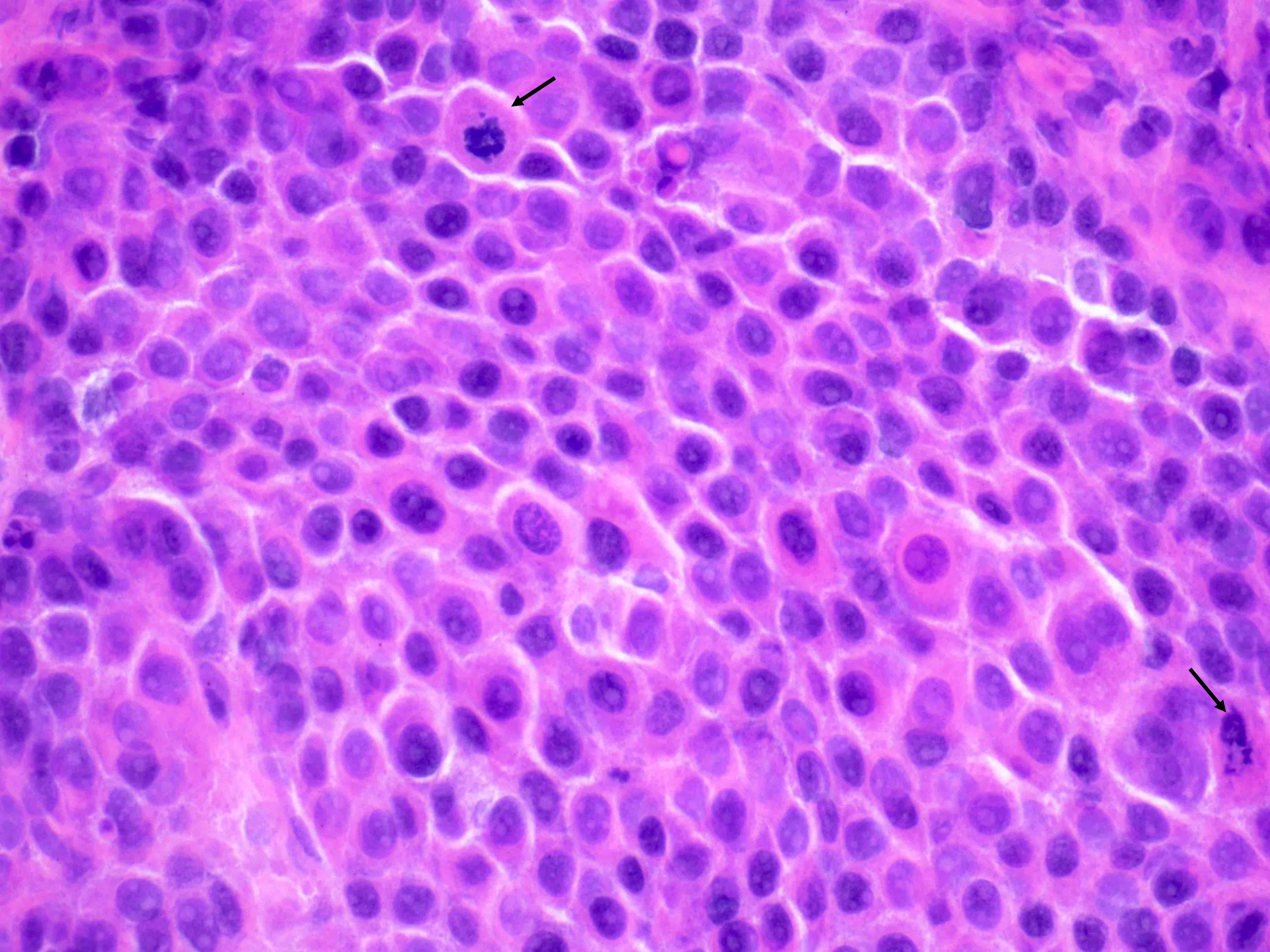
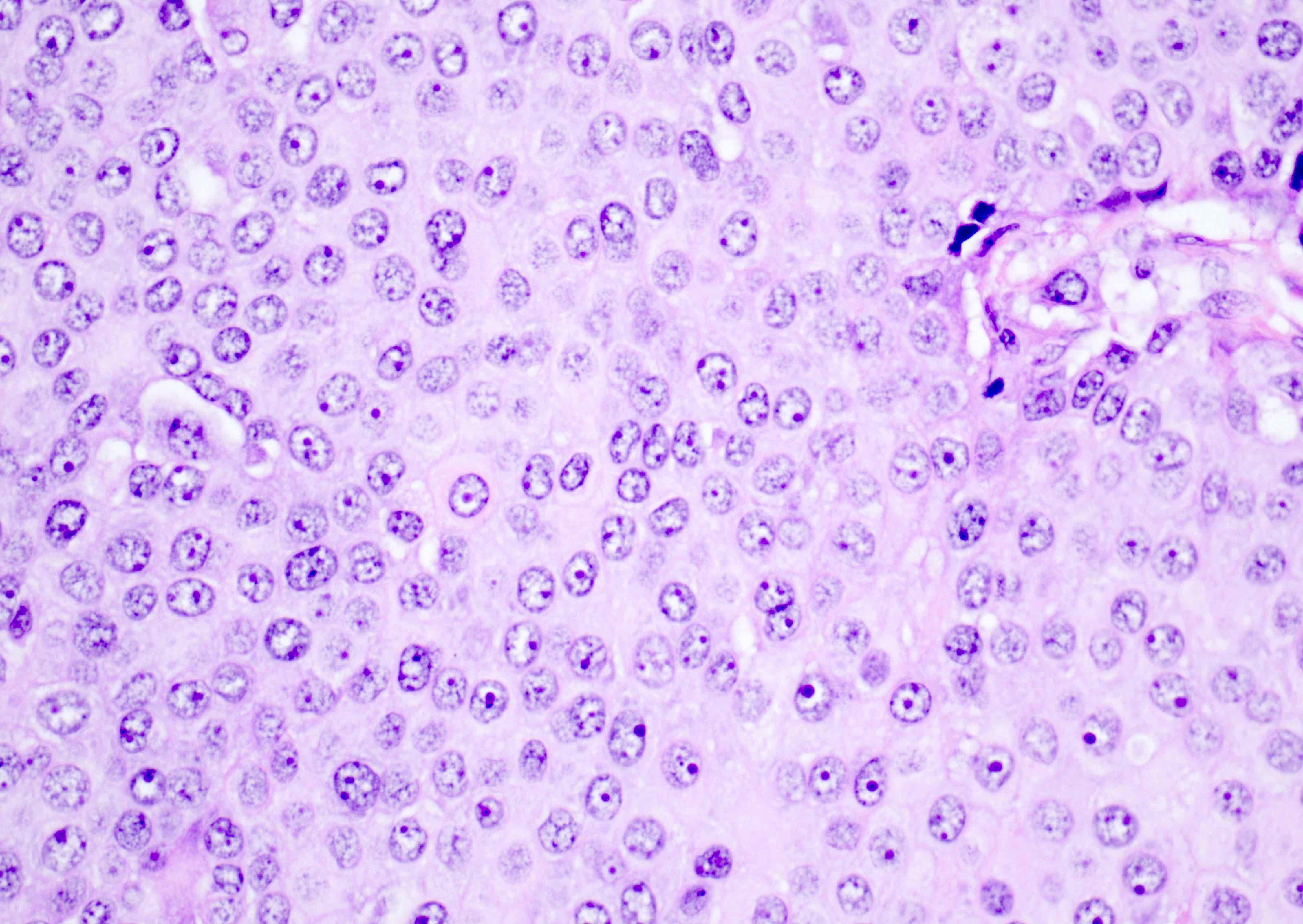
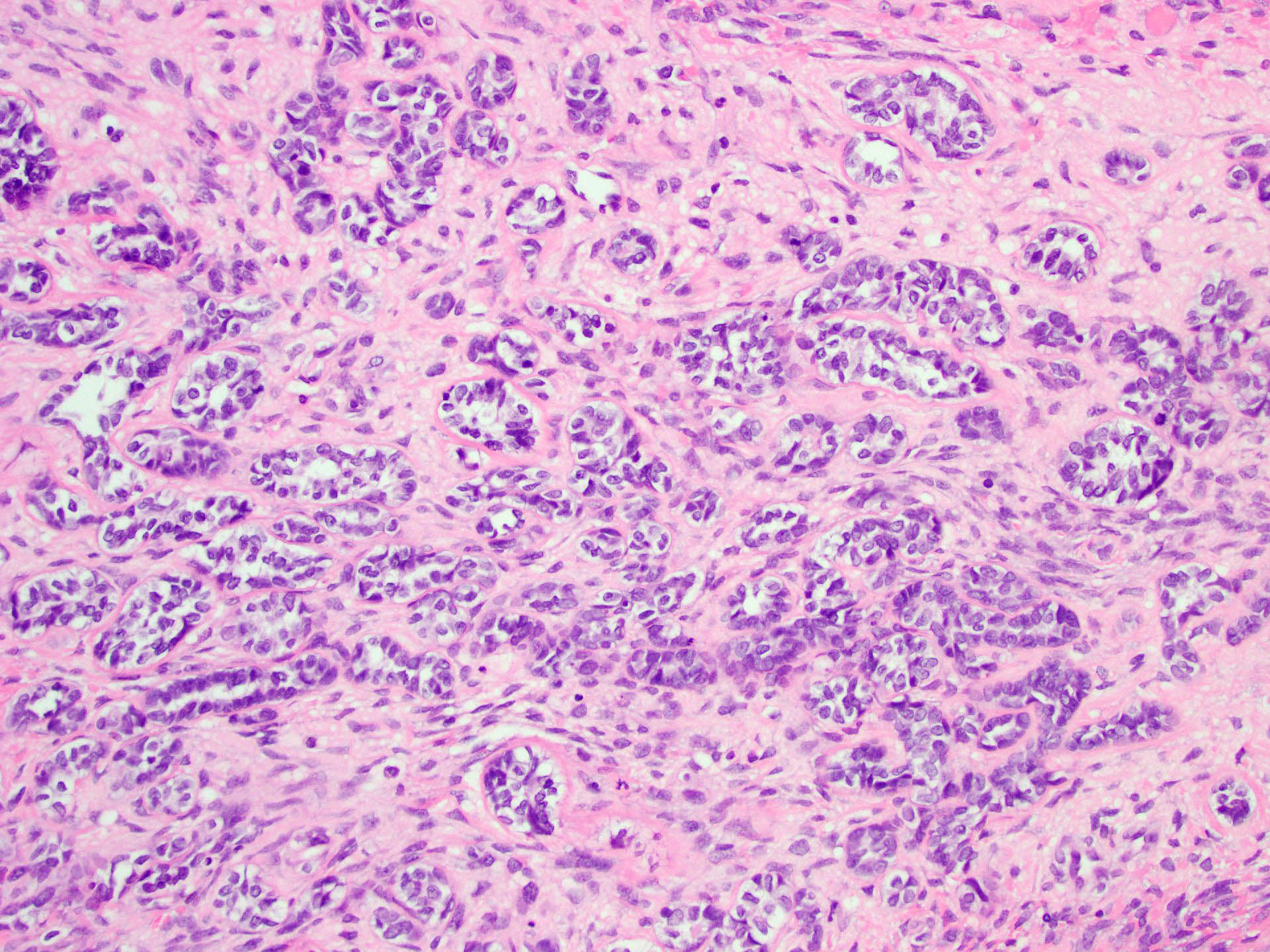
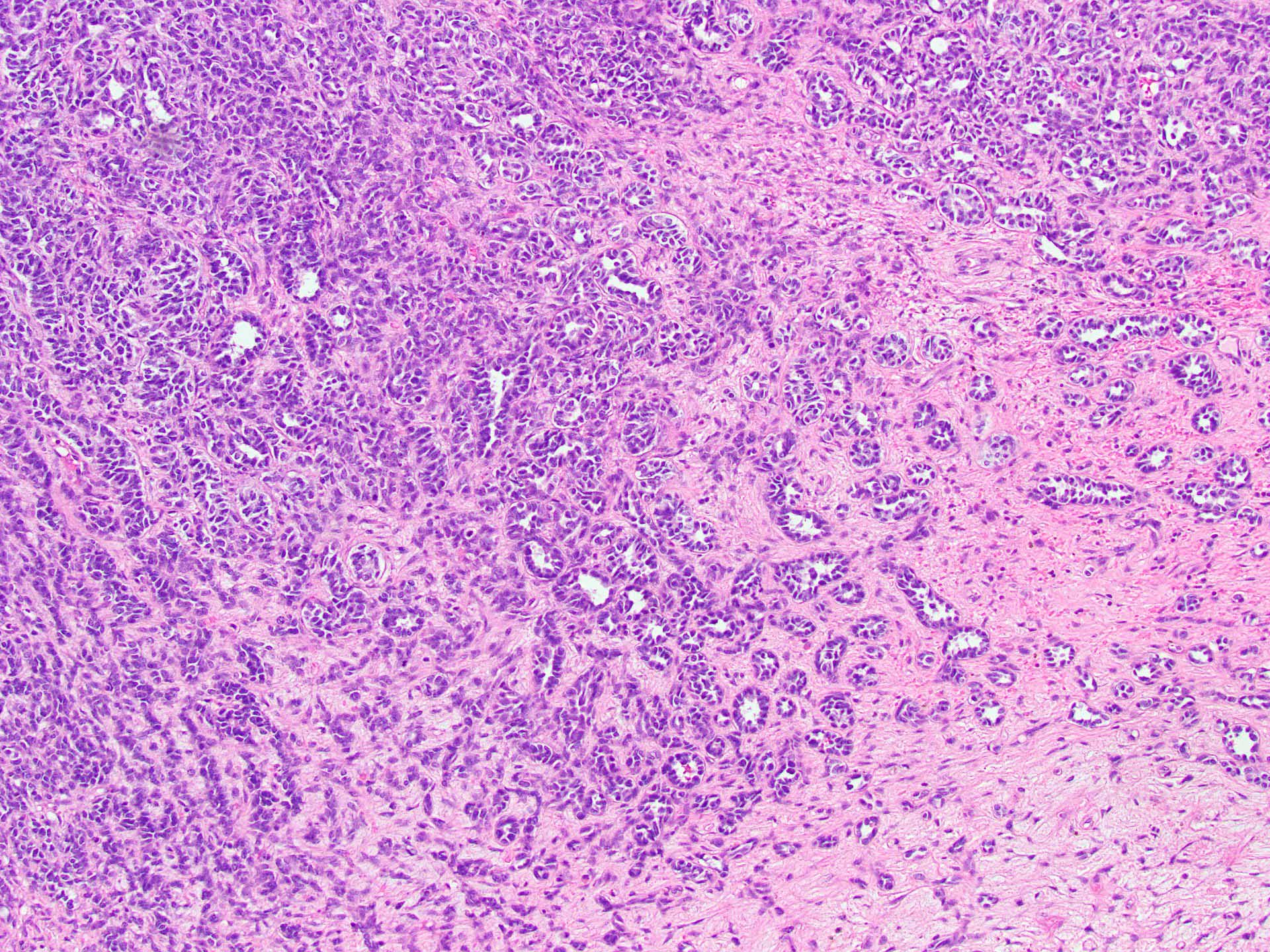
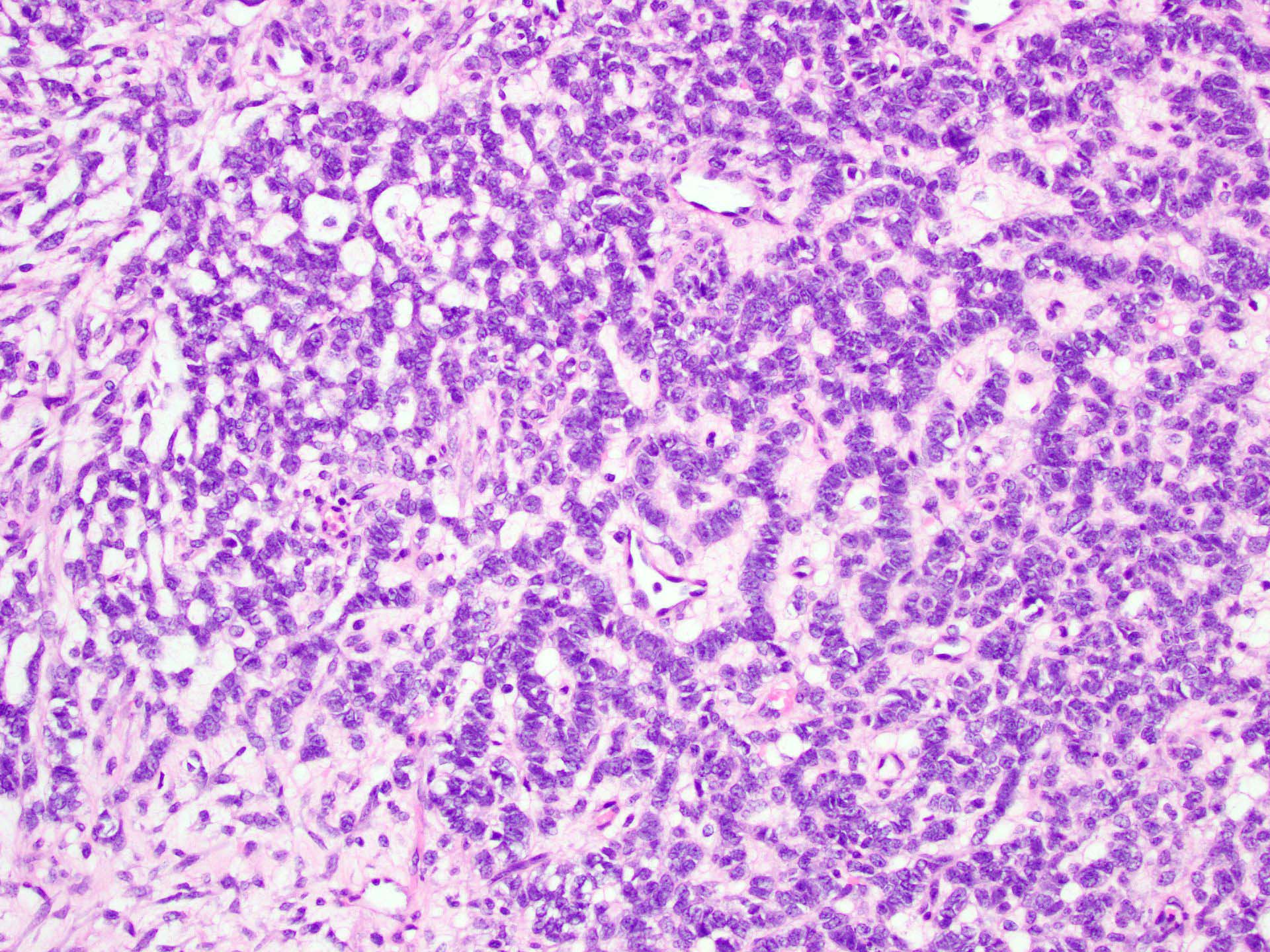
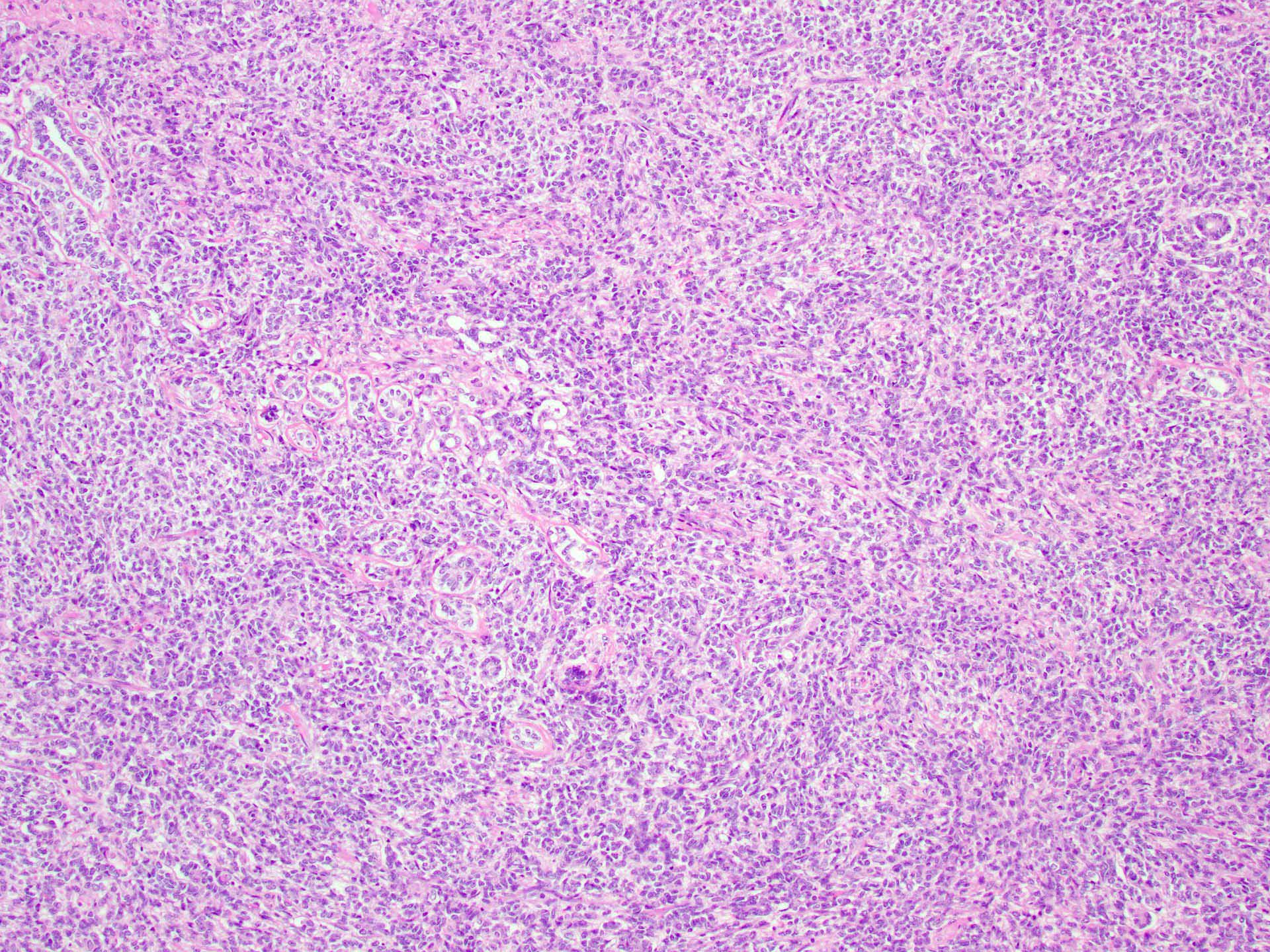
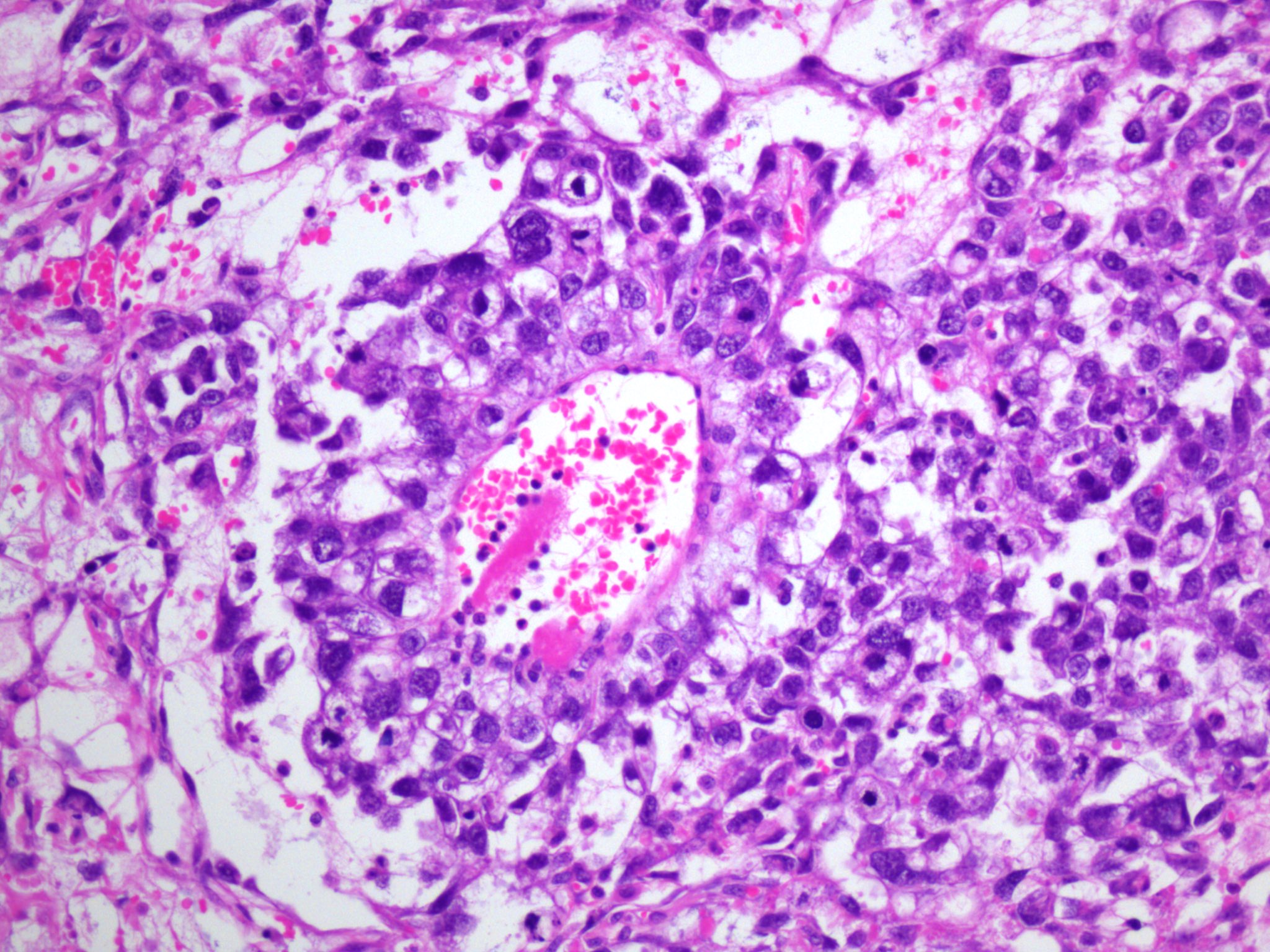
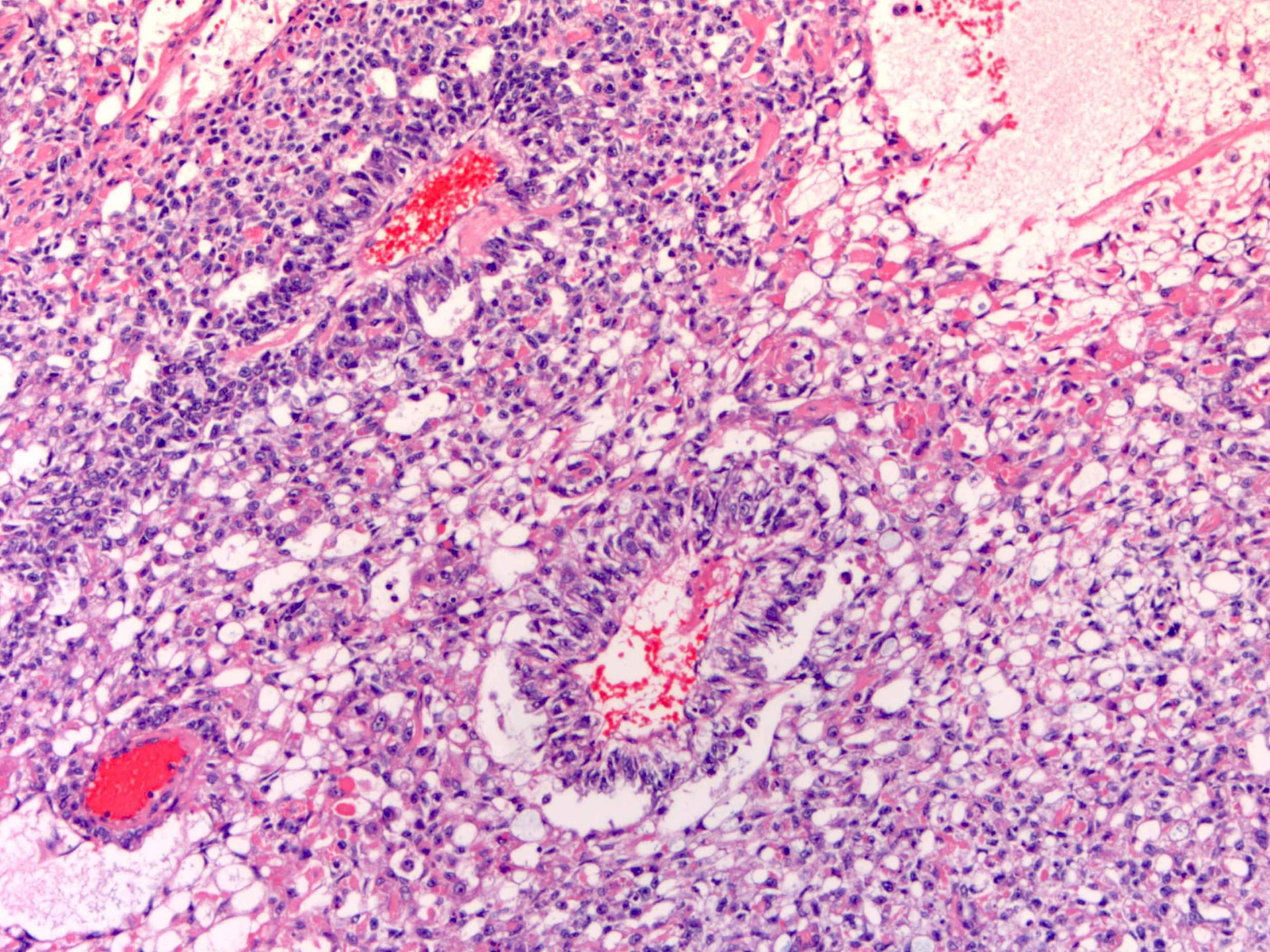
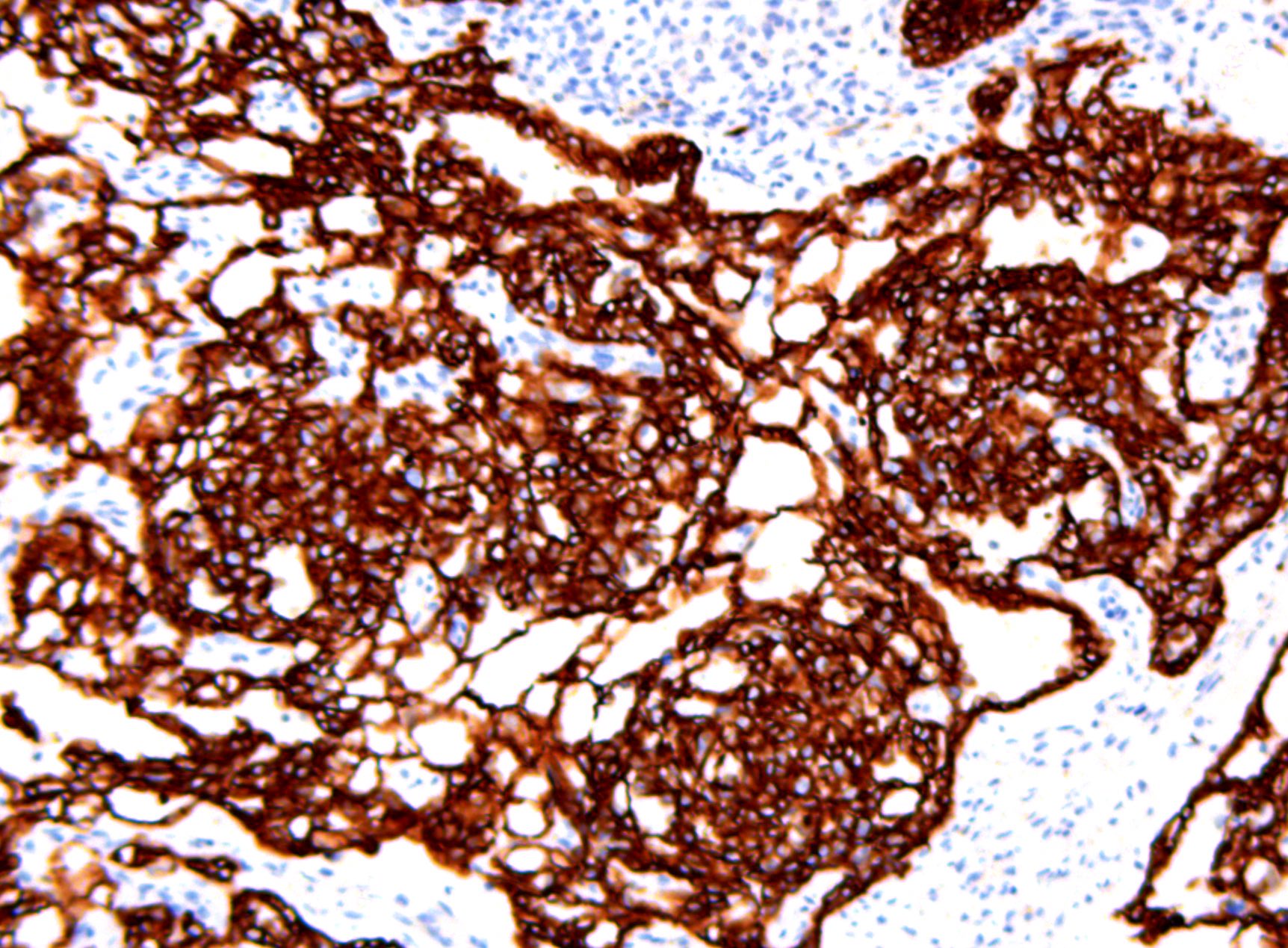
- Insular pattern is the most frequently observed; mucinous and trabecular patterns have also been reported (Cancer 1975;36:157, Int J Gynecol Pathol 2008;28:41)
- Insular architecture is characterized by sharply demarcated nests of tumor cells of varying sizes and shapes in a variable fibromatous stroma
- Acinar configurations and calcifications can also be observed
- Neoplastic cells are typically small and uniform in size, with a central round nucleus containing coarsely and evenly clumped chromatin ("salt and pepper" pattern)
- Cytoplasm is moderate to abundant and eosinophilic, occasionally granular
- Mitotic activity is absent to low (up to 2 mitoses / 10 HPFs)
- Tumor cell necrosis is absent
Microscopic (histologic) images
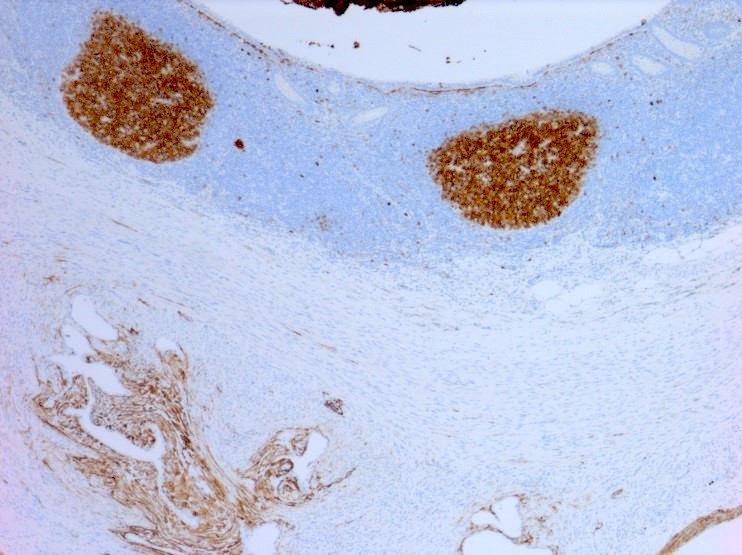
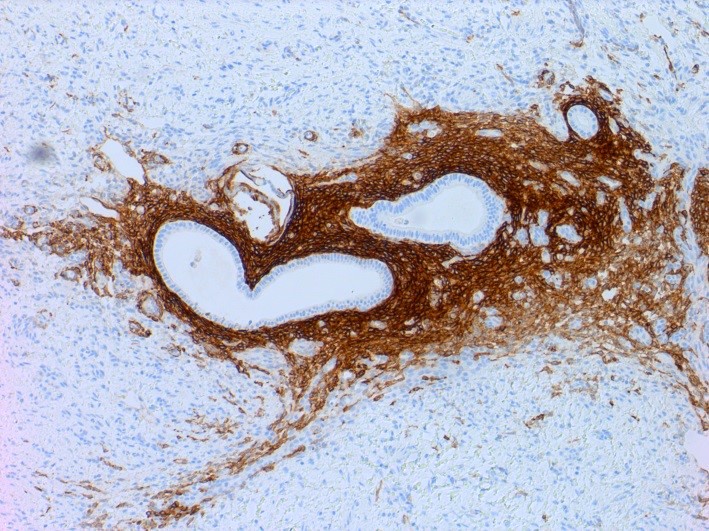
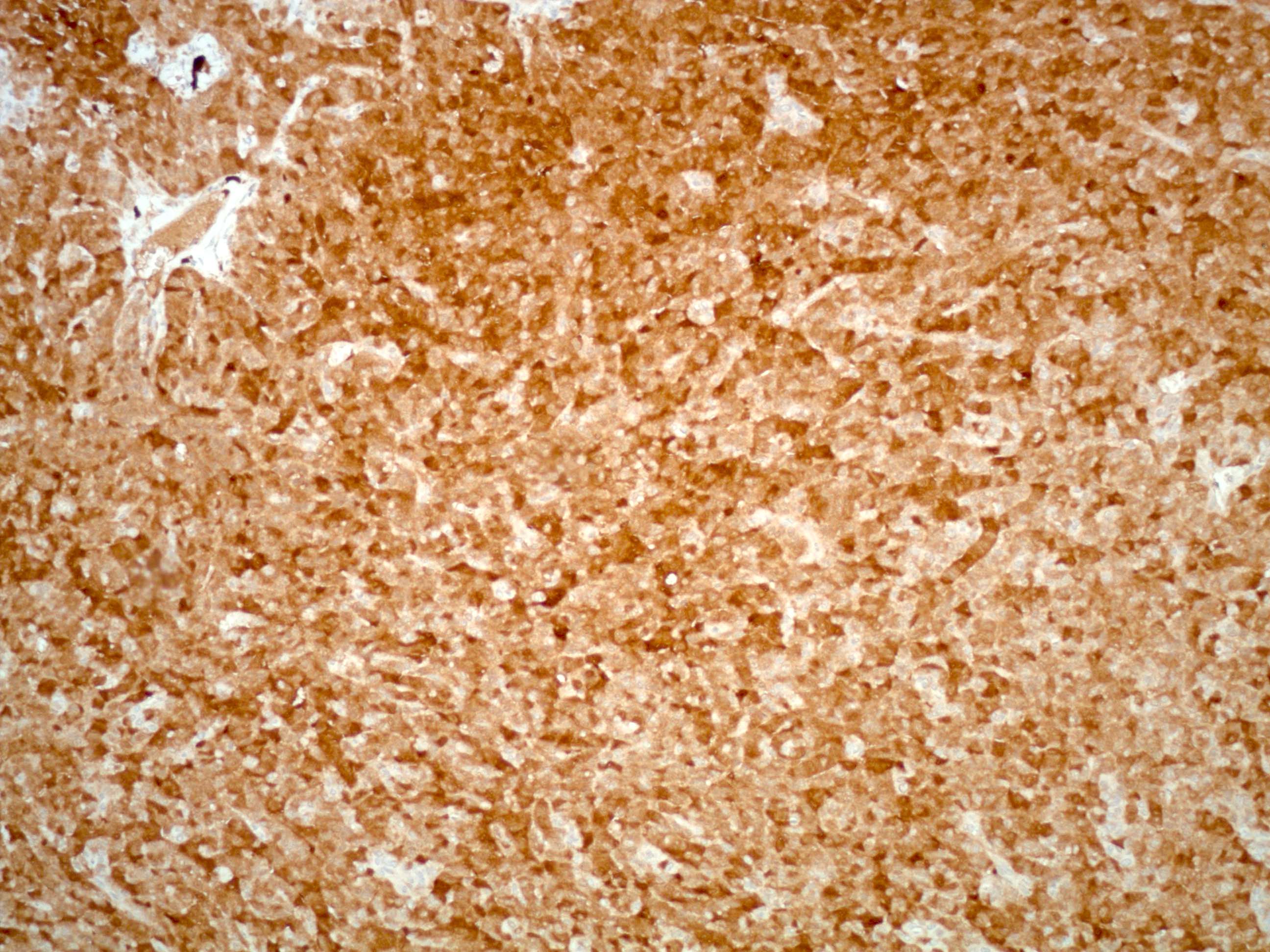
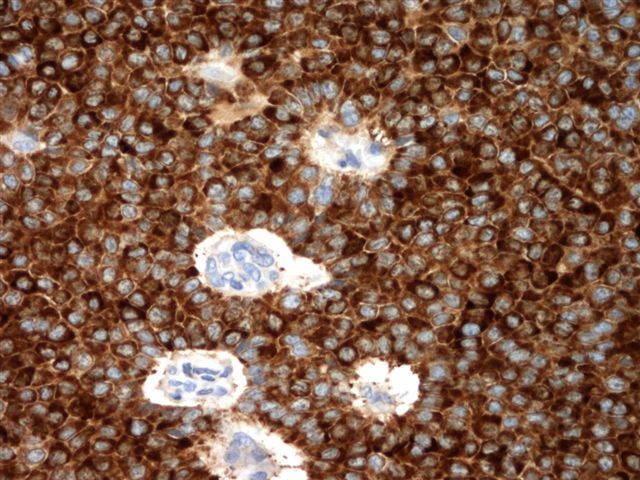
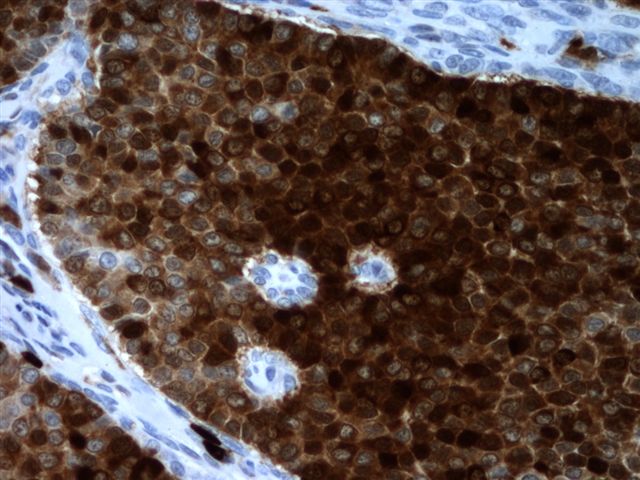
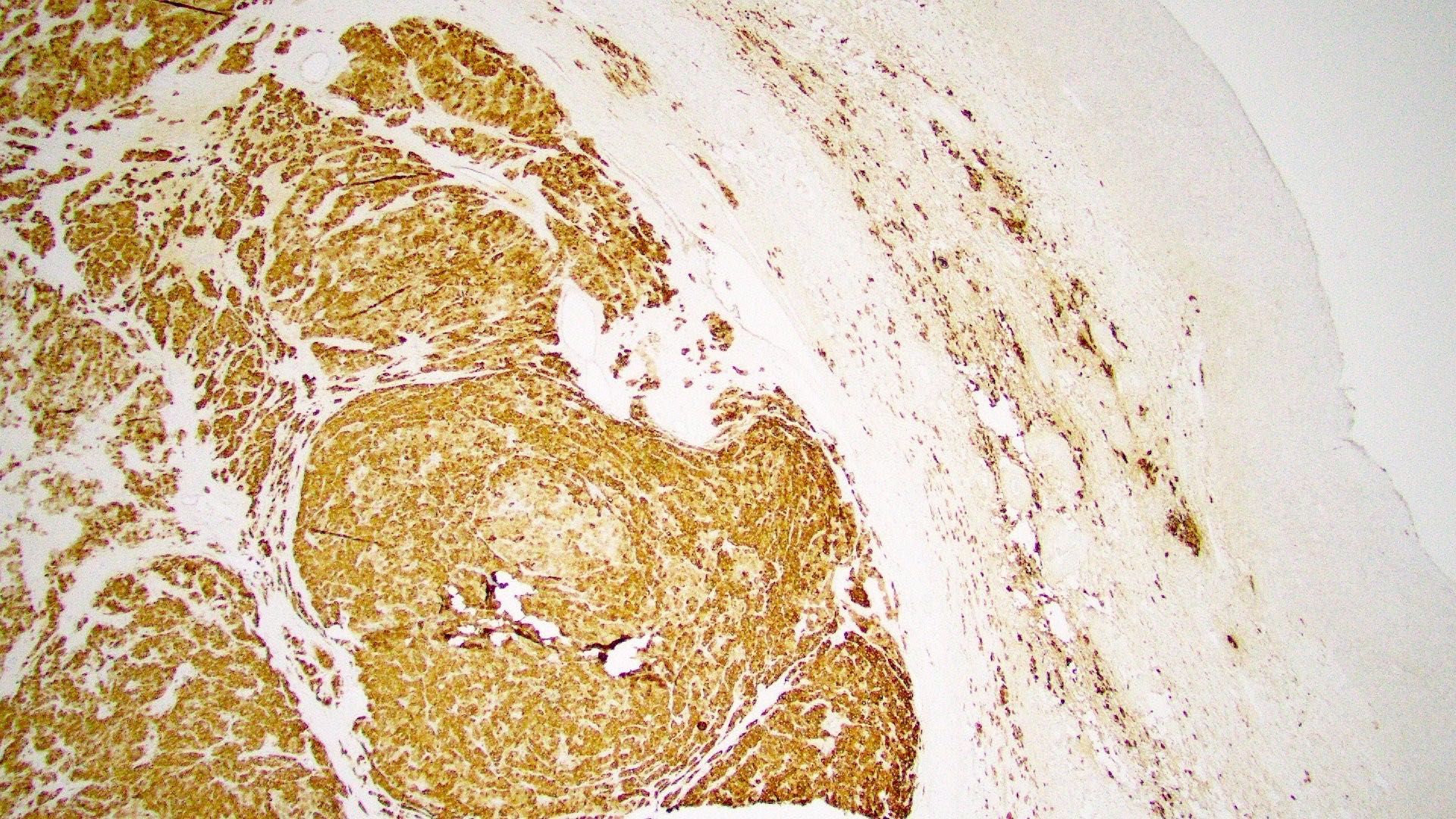
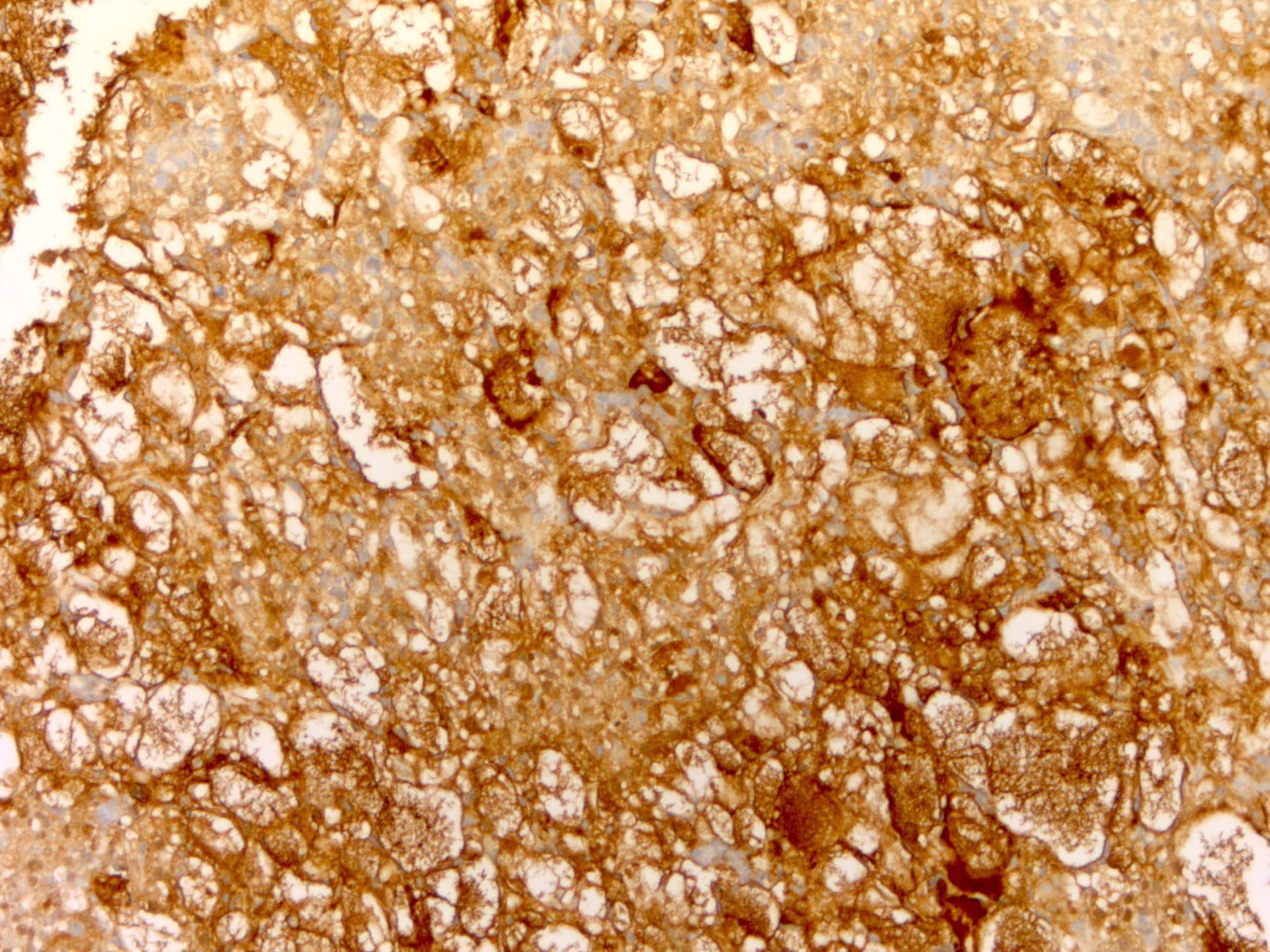
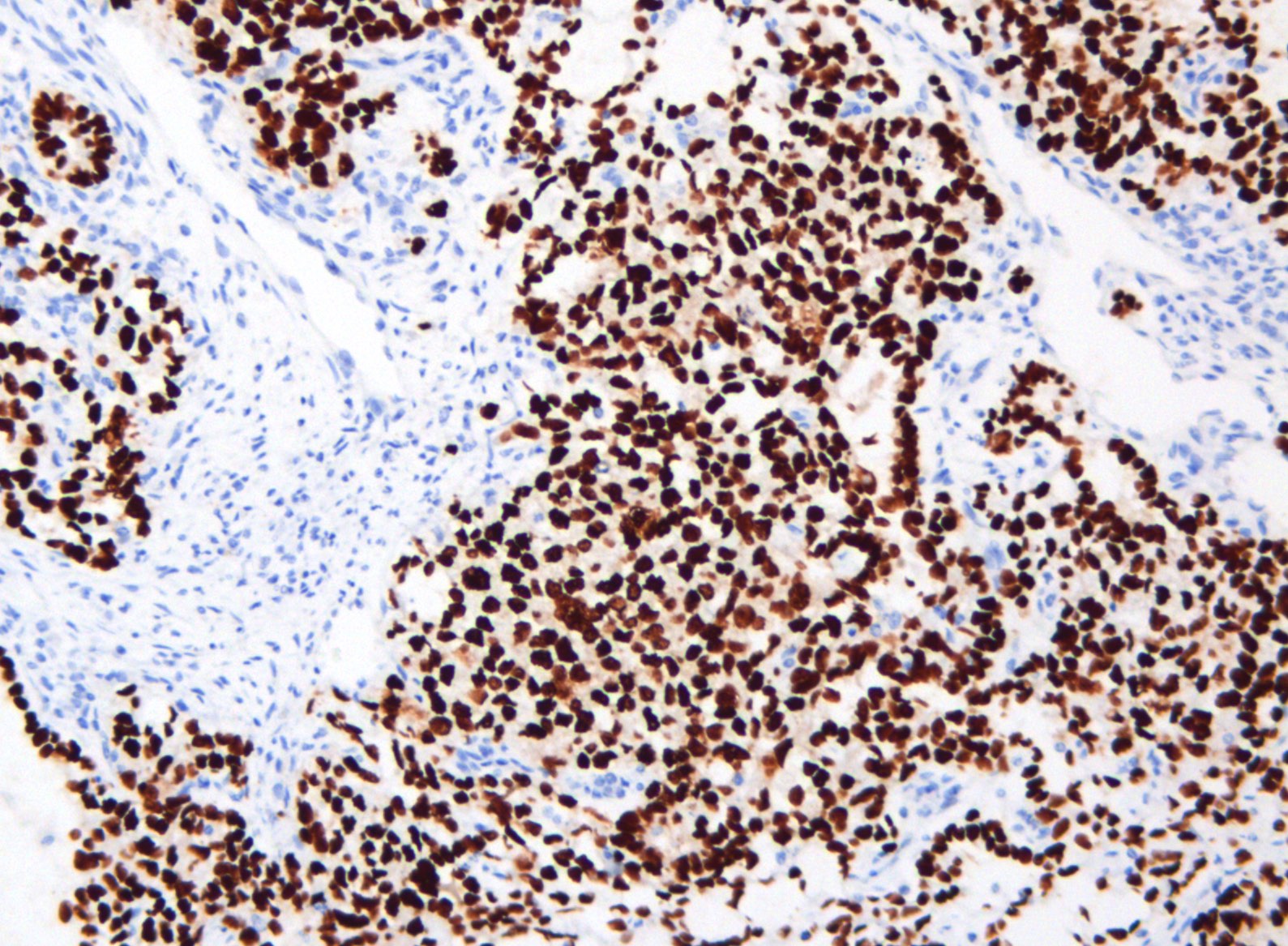
Positive stains
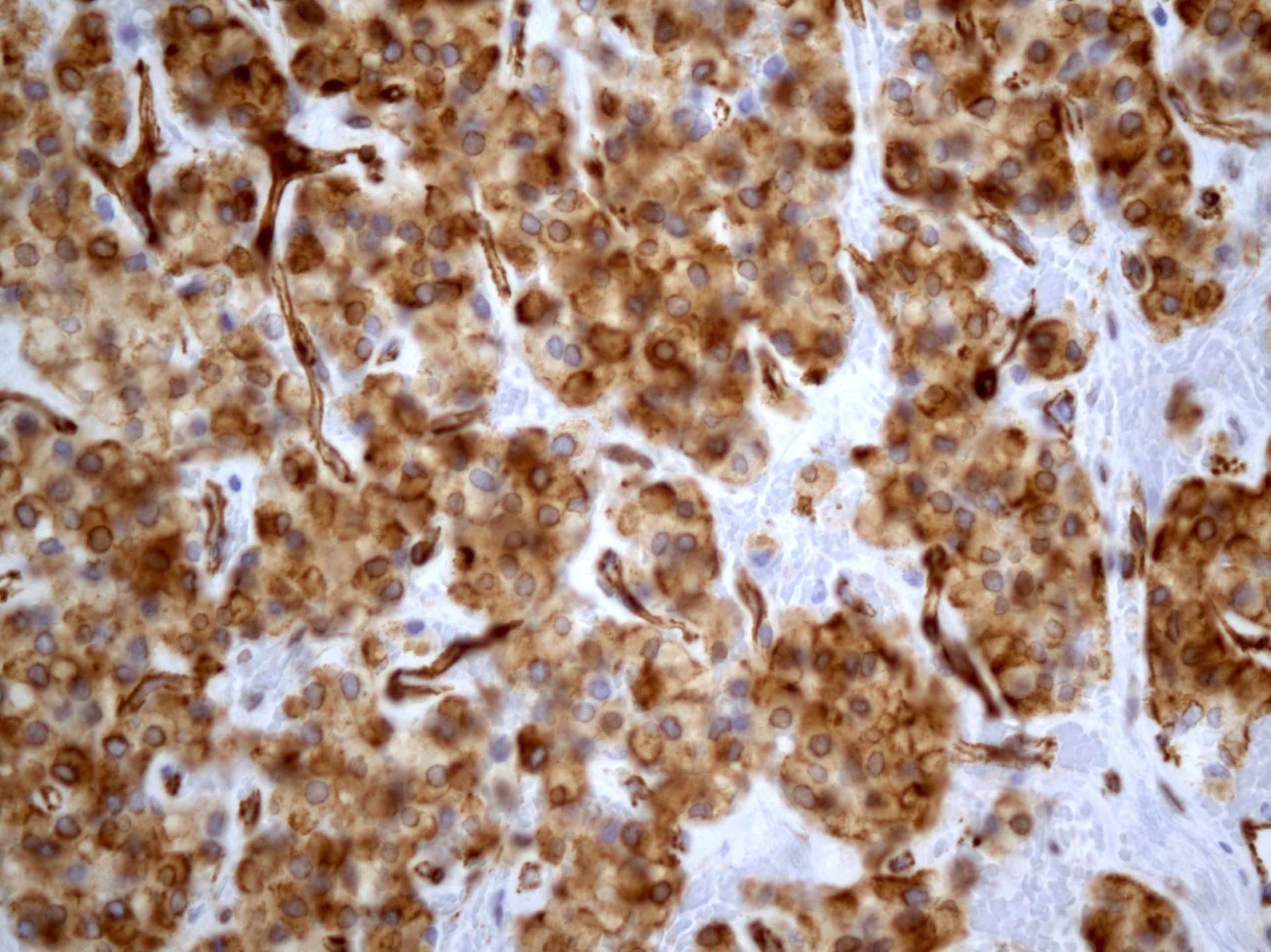
- Chromogranin, synaptophysin, CD56, NSE, pan-cytokeratin (commonly with a perinuclear staining pattern)
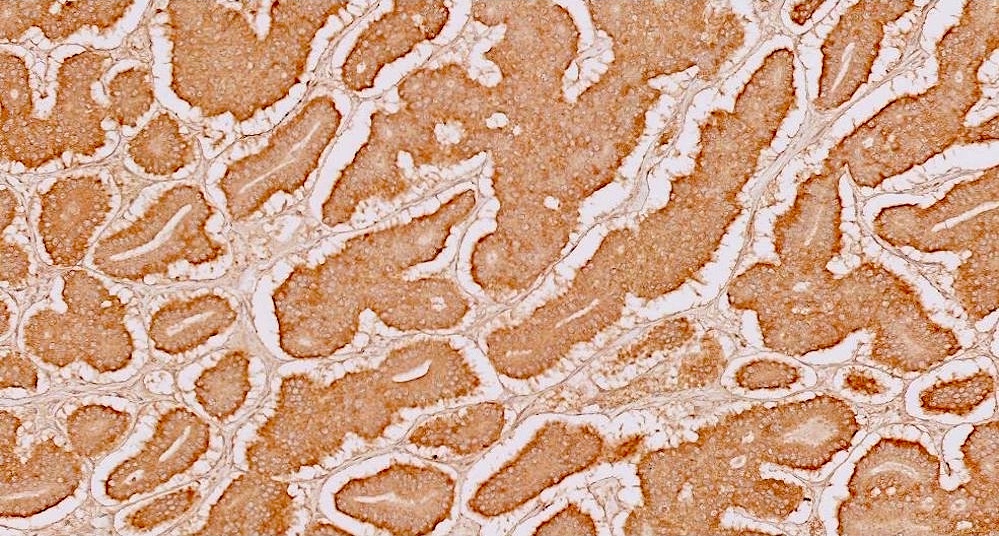
- CDX2: positive in 90 - 100% of carcinoids metastatic to ovary; positivity is frequent in tumors arising in small intestine (95%), appendix (92%) and duodenum (80%), but rare in tumors of colorectum (12%) and stomoach (0%) (Hum Pathol 2013;44:2536)
Differential diagnosis
- Primary ovarian carcinoid tumor: distinction relies on conventional clinicopathologic features, as outlined by Rabban et al. (Int J Gynecol Pathol 2008;28:41):
- Laterality: Frequent in metastatic carcinoid; but all confirmed primary ovarian carcinoids reported have been unilateral
- Multinodular growth: Frequent in metastases, but not seen in primary ovarian carcinoids
- Size: Primary ovarian carcinoids average 3.4 cm in size (excluding any teratomatous element) versus 10.2 cm for metastatic carcinoids (range: 4-32 cm); 75% of primary ovarian carcinoids were 3 cm or less, compared to 0% of metastatic carcinoids to ovary
- Presence of teratomatous elements: supports primary ovarian origin
- Metastates in other sites: as mentioned above, very common in metastatic carcinoid to the ovary
- Nonetheless, primary ovarian carcinoid tumors can rarely present with metastases (J Obstet Gynaecol Res 2010;36:567, Case Rep Obstet Gynecol 2012;2012:961087, Gynecol Oncol 1994;54:222)
- Immunohistochemistry has controversial value in this differential
- Rabban et al. reported 37.5% expression of CDX2 in primary ovarian carcinoids; importantly, most (4/6) insular primary carcinoids expressed this marker and recommended against the use of immunohistochemistry (Int J Gynecol Pathol 2008;28:41)
- In contrast, Desouki et al. found that all 30 primary ovarian carcinoids tested were negative for CDX2, and therefore suggested this marker as sensitive and specific for small intestinal or appendiceal origin (Hum Pathol 2013;44:2536)
- Brenner tumor: nests composed of epithelioid, urothelial type cells with oval, pale, grooved nuclei
- Granulosa cell tumor: Call-Exner bodies, inhibin and calretinin expression
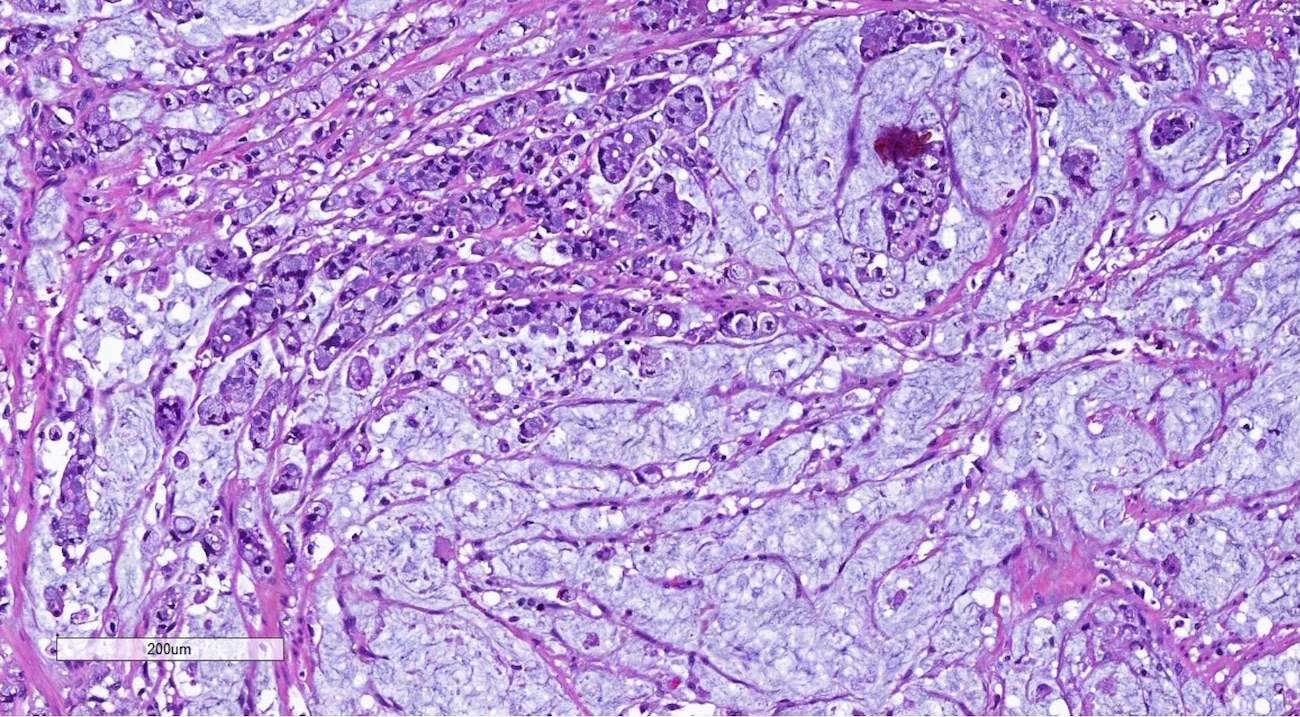
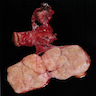
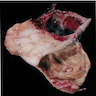
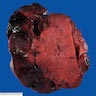
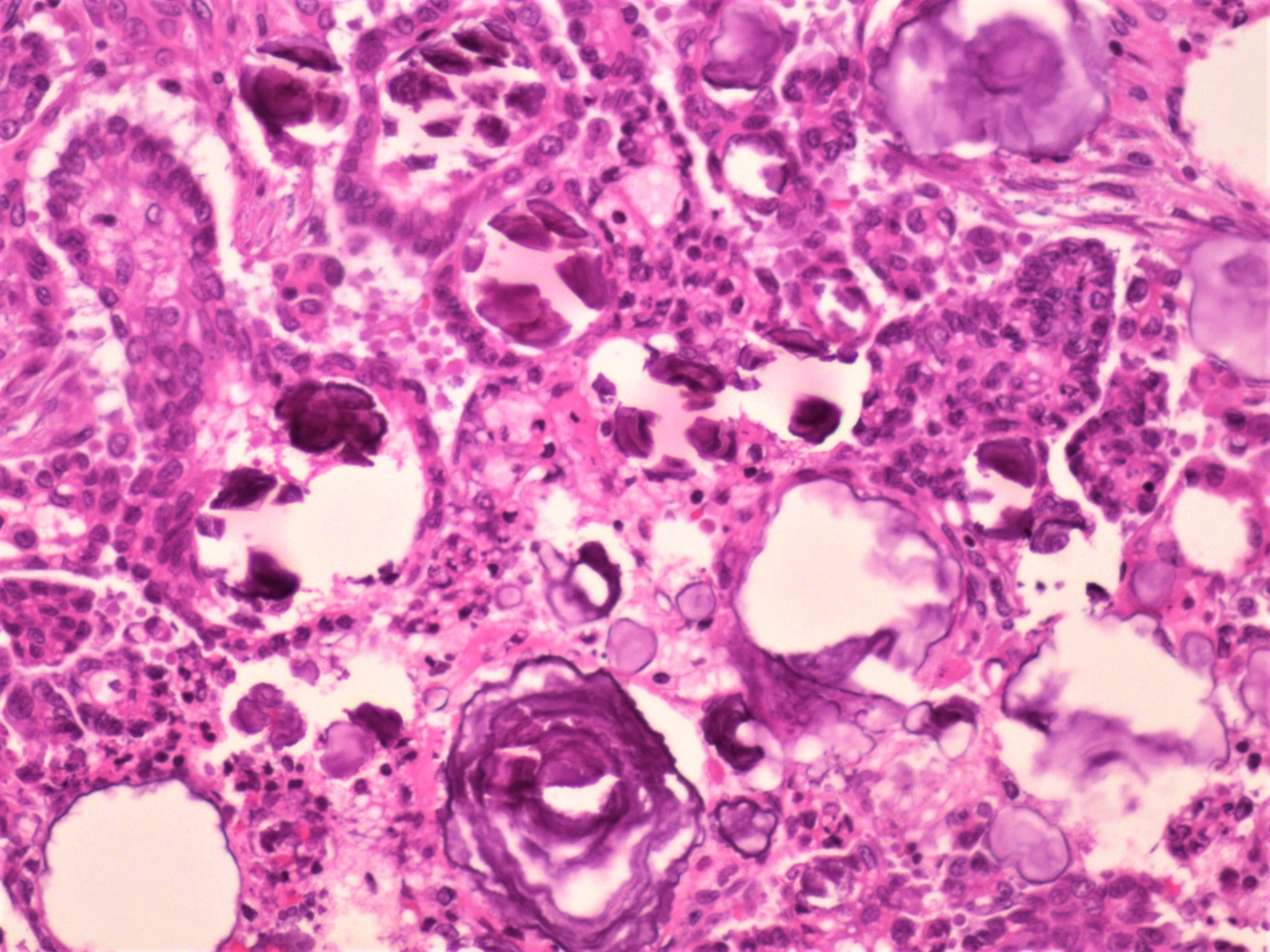
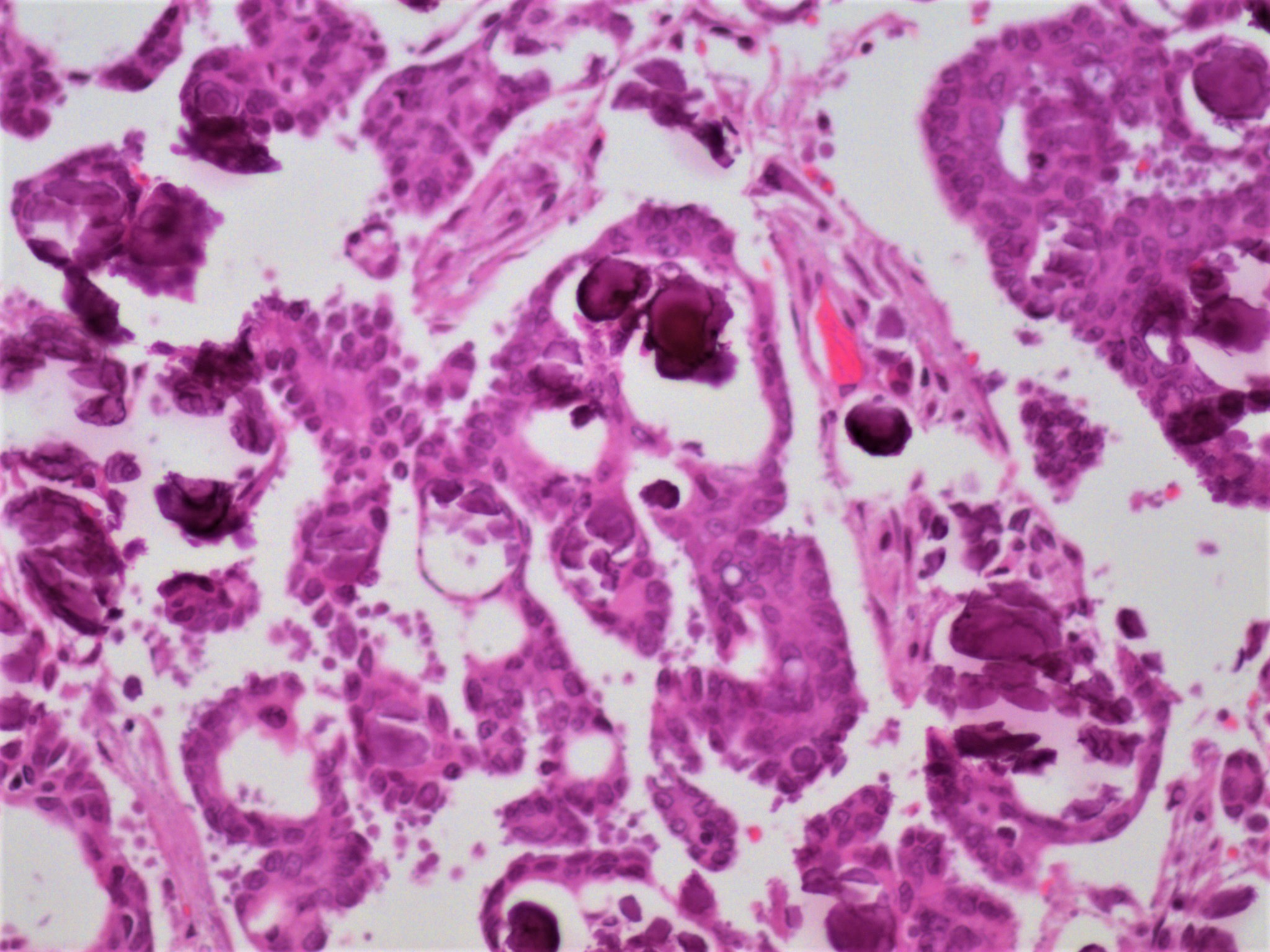
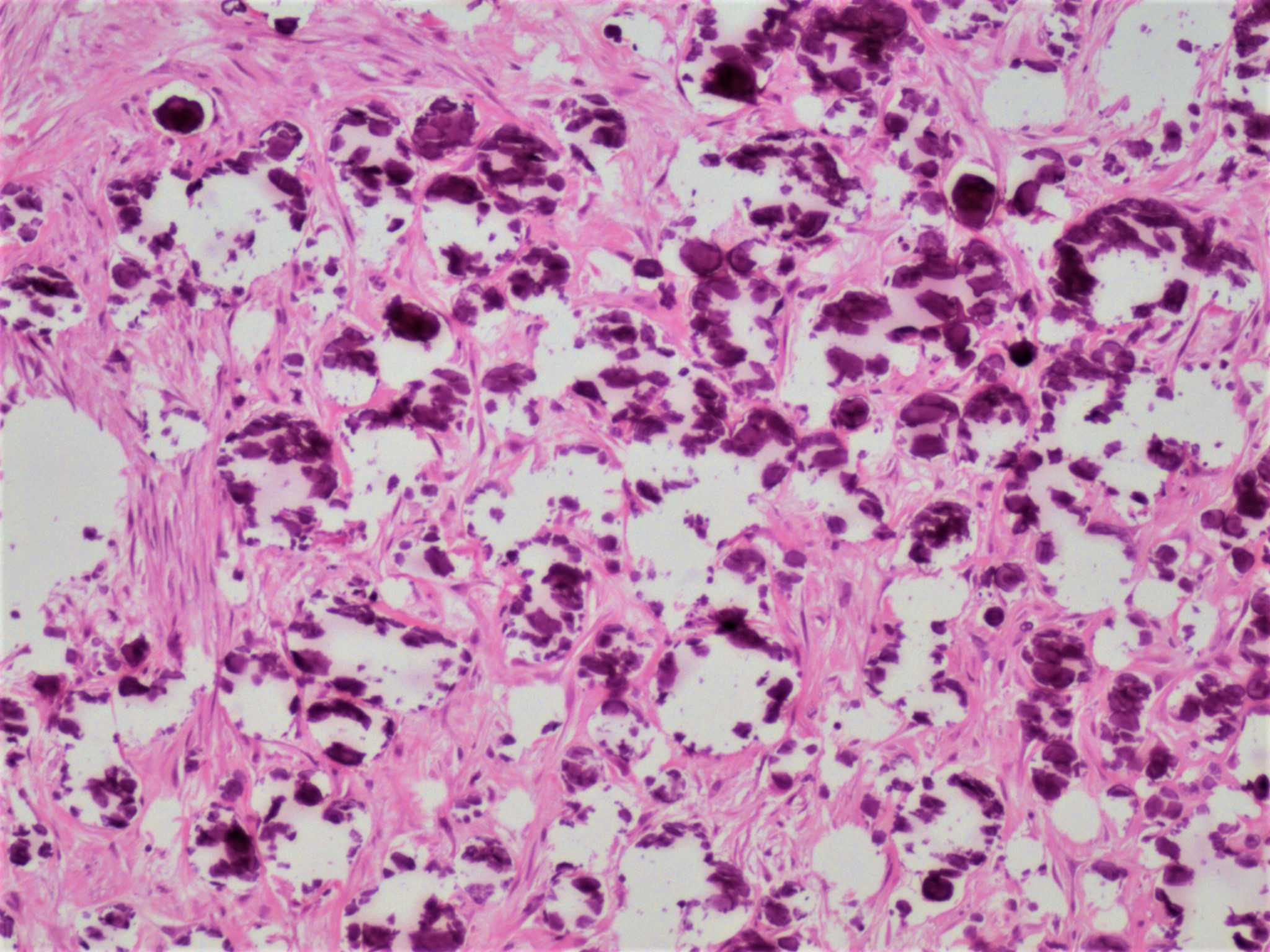
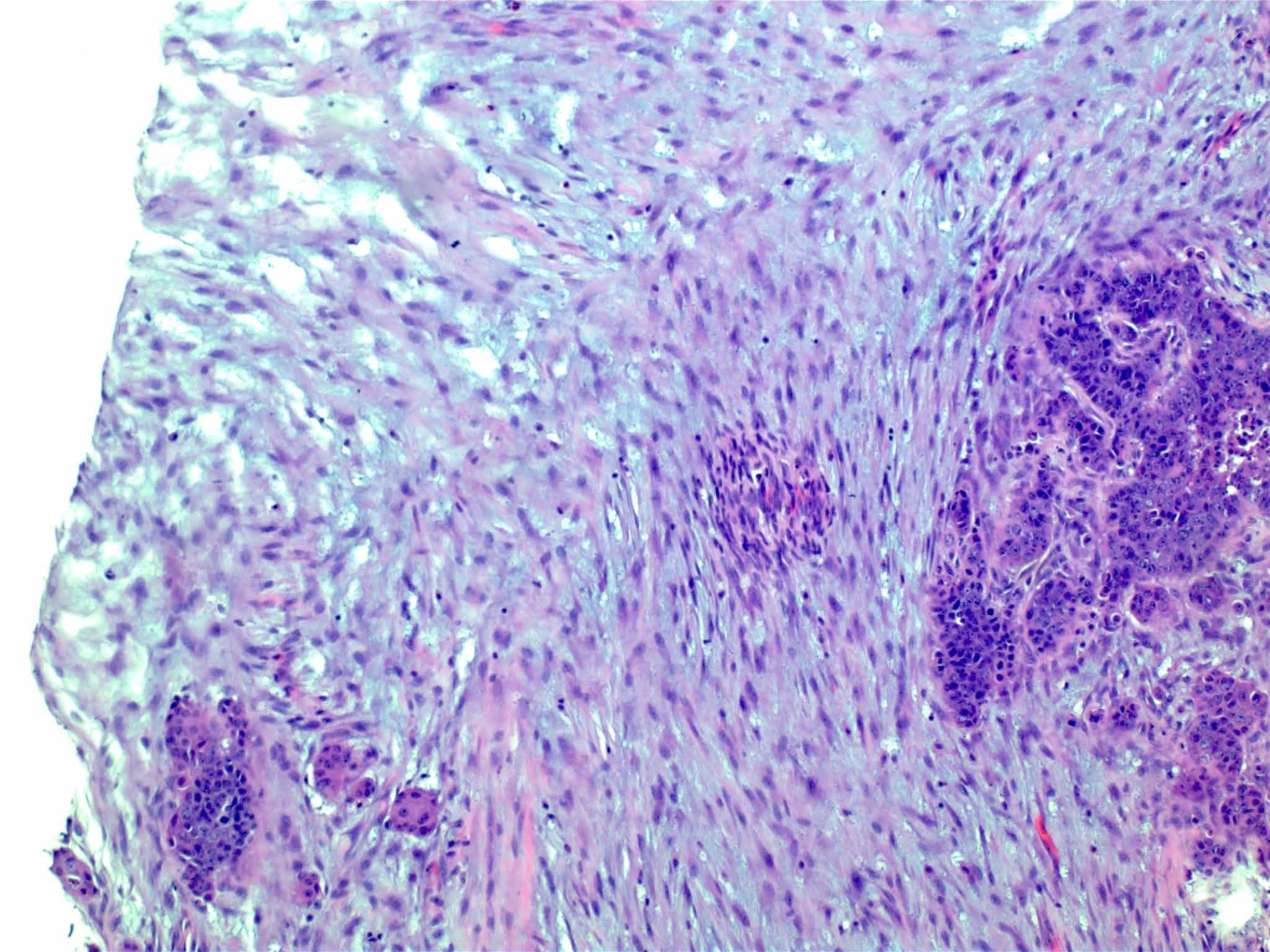
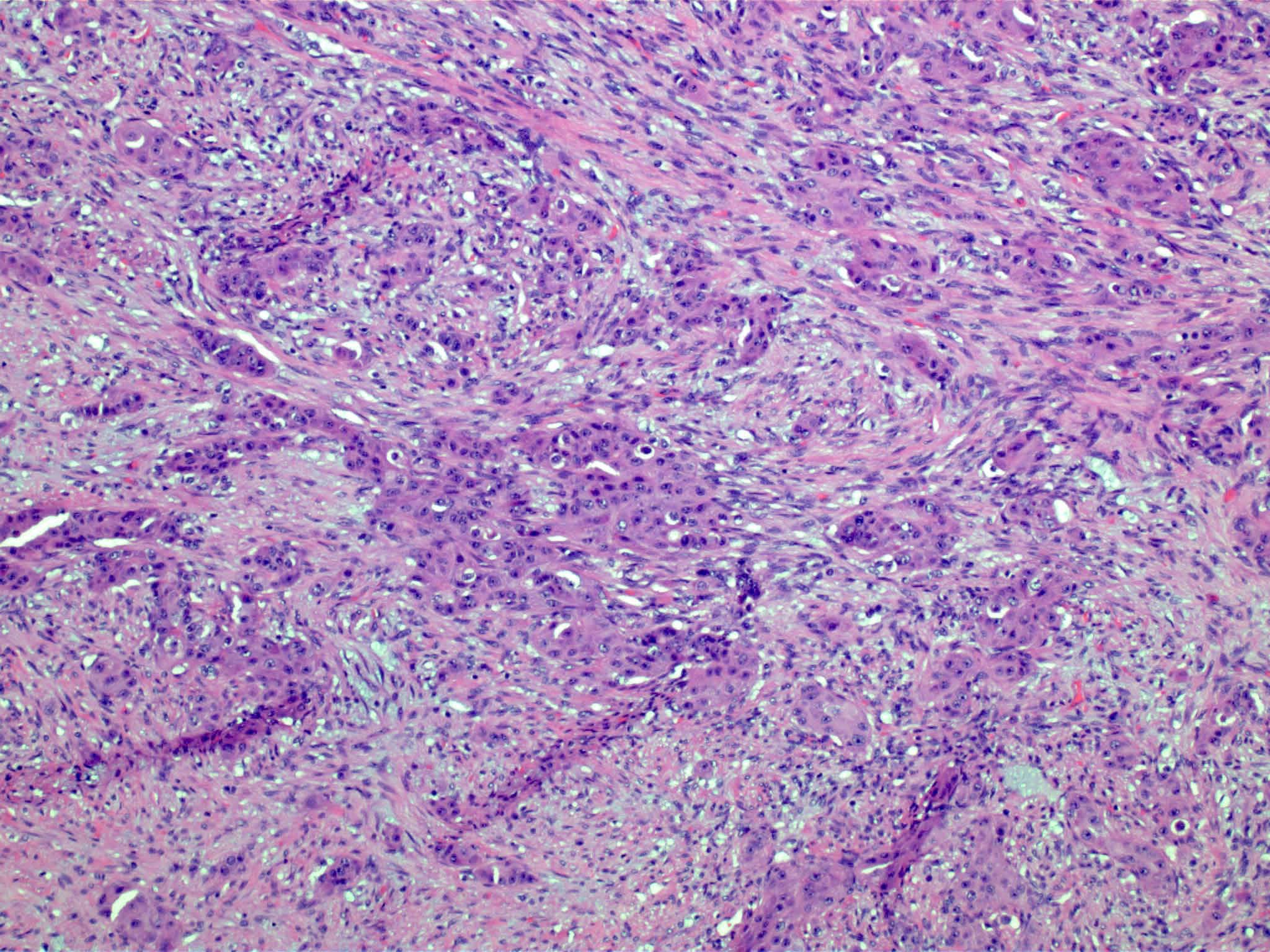
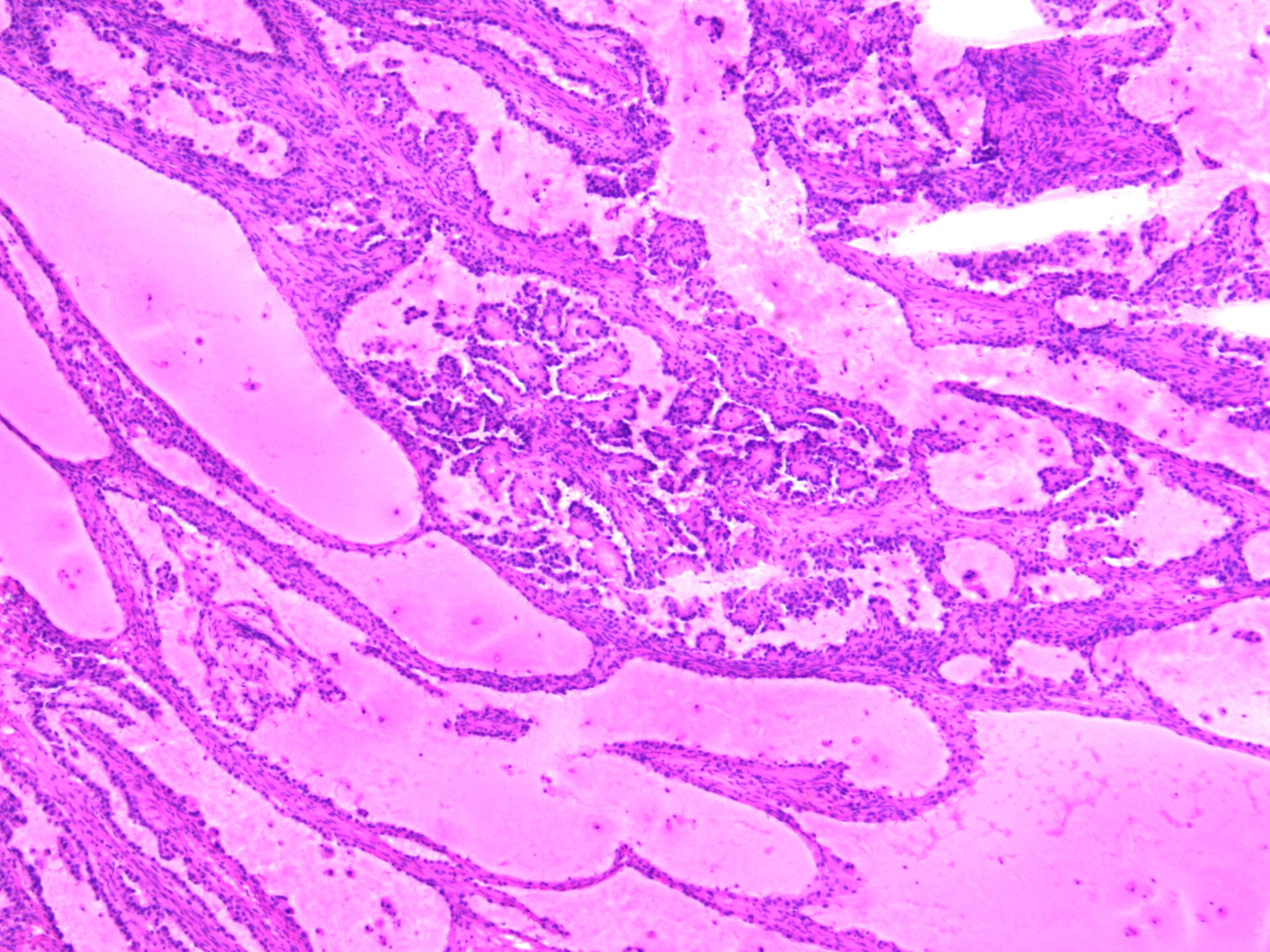
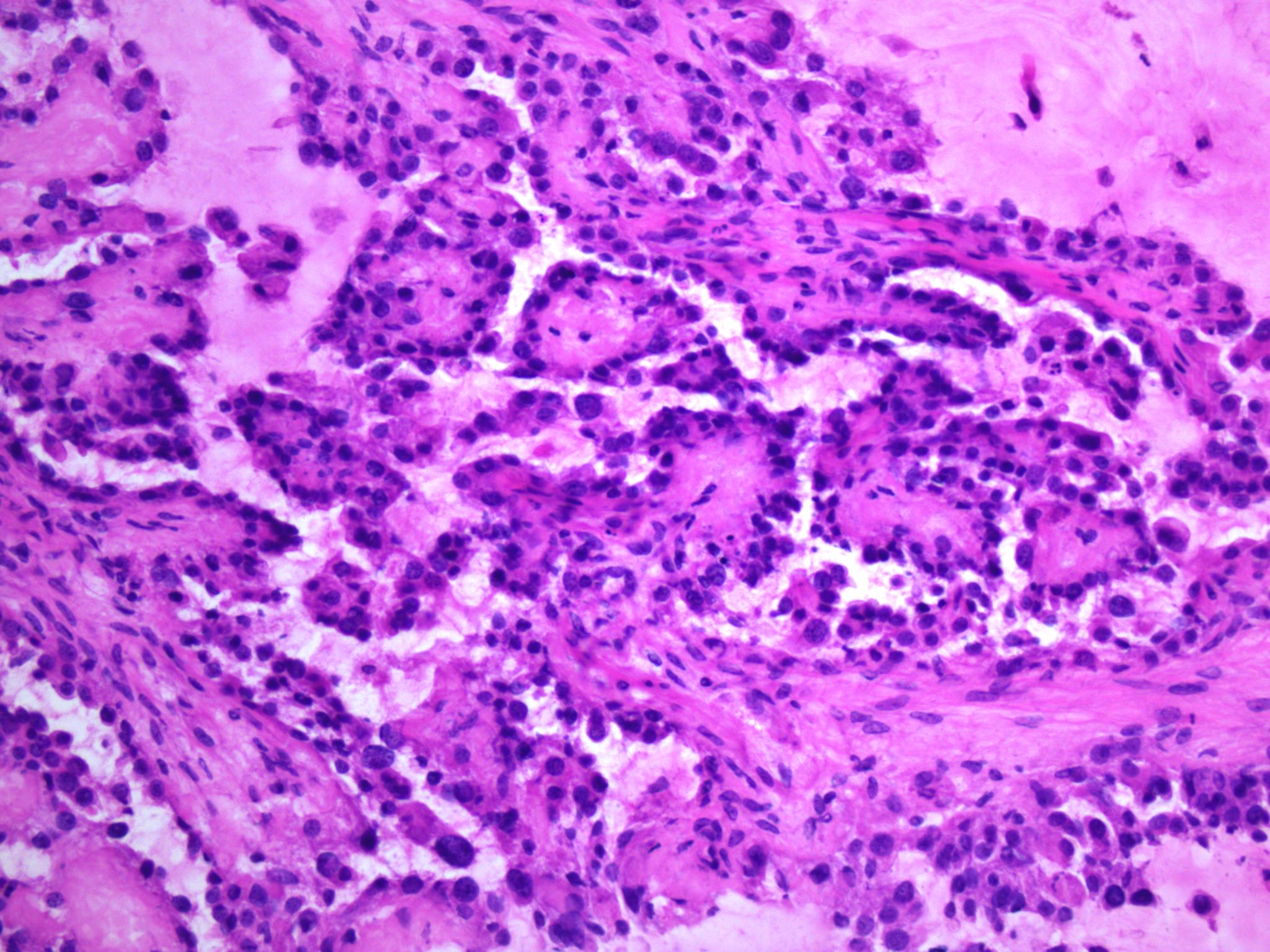
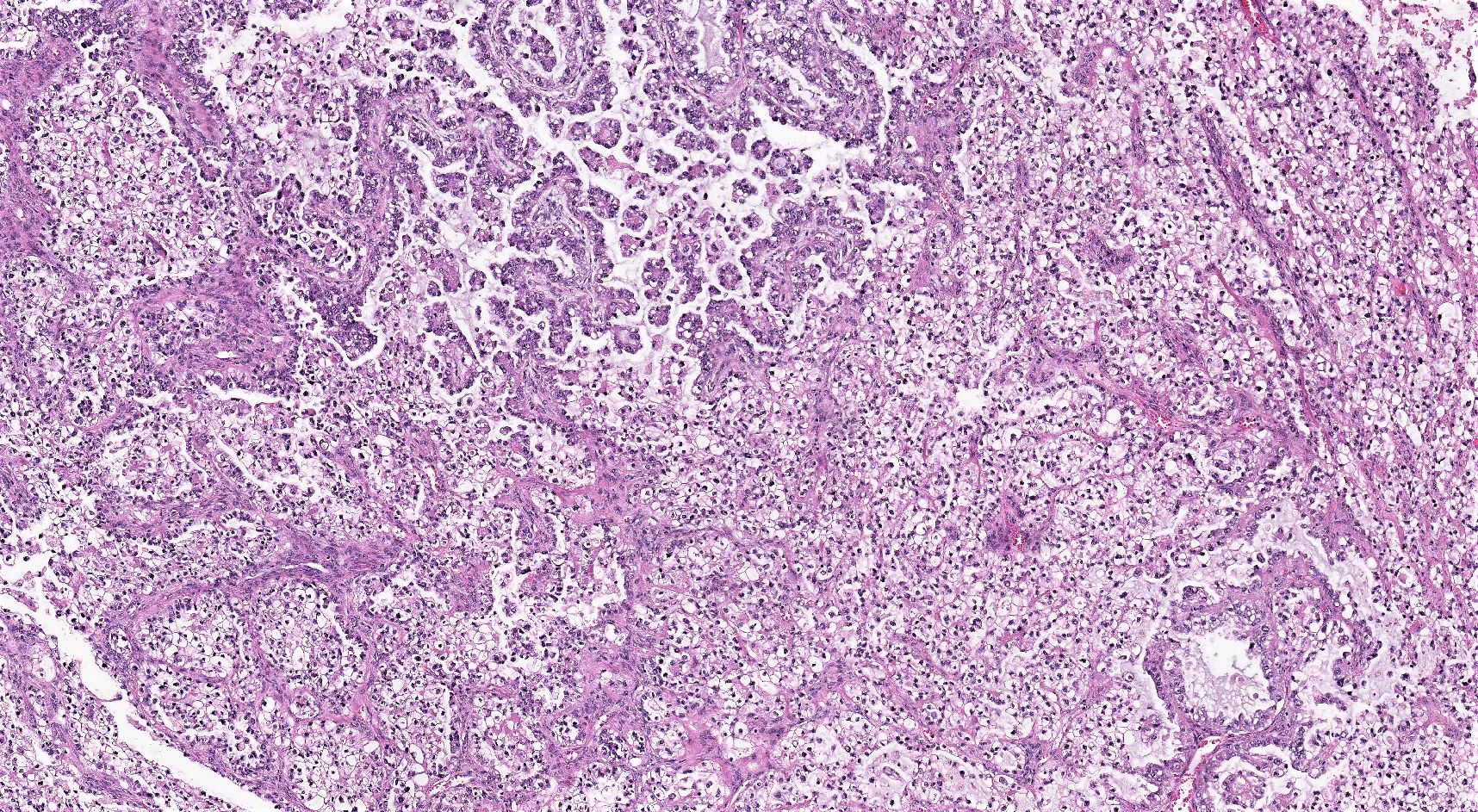
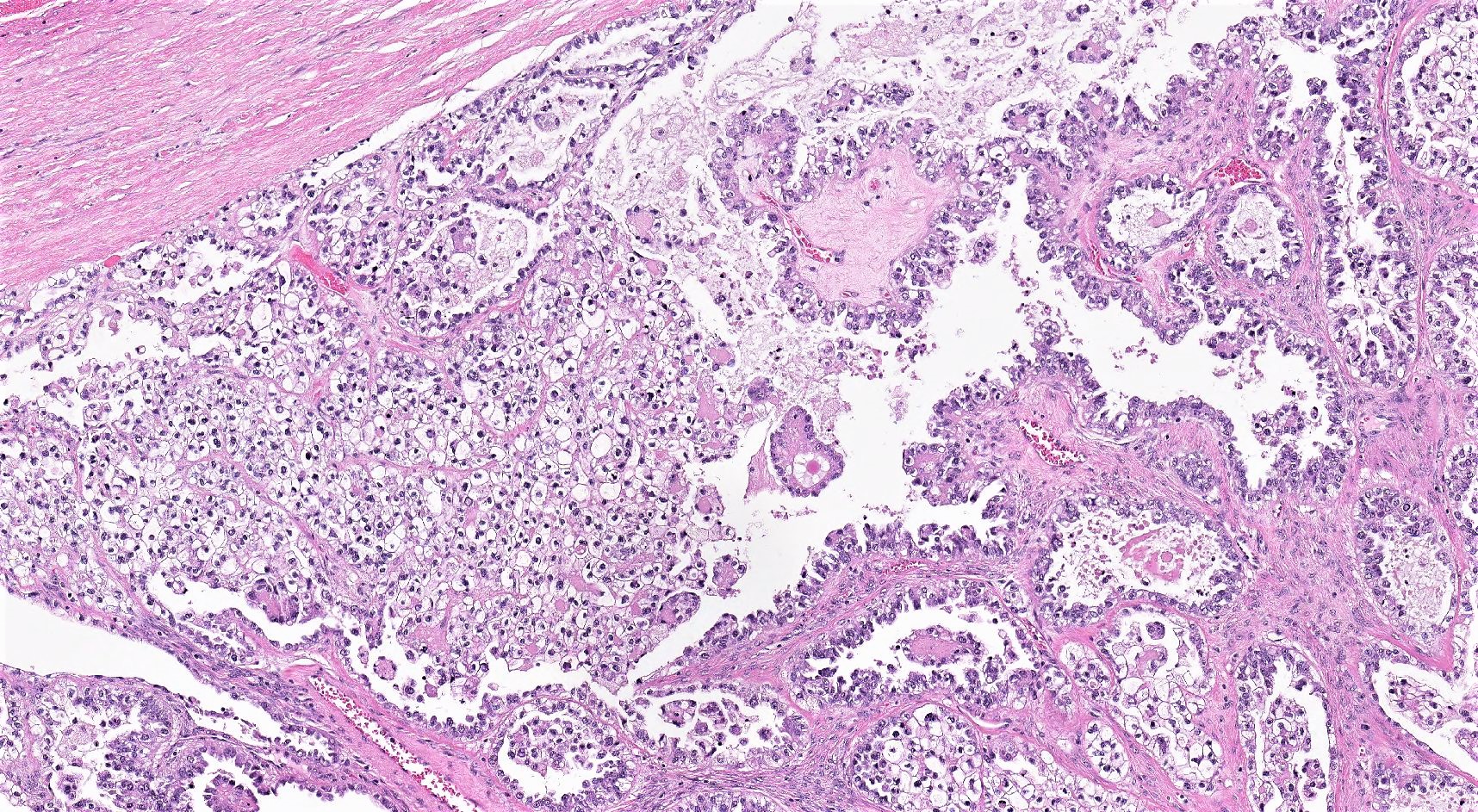
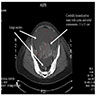
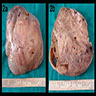
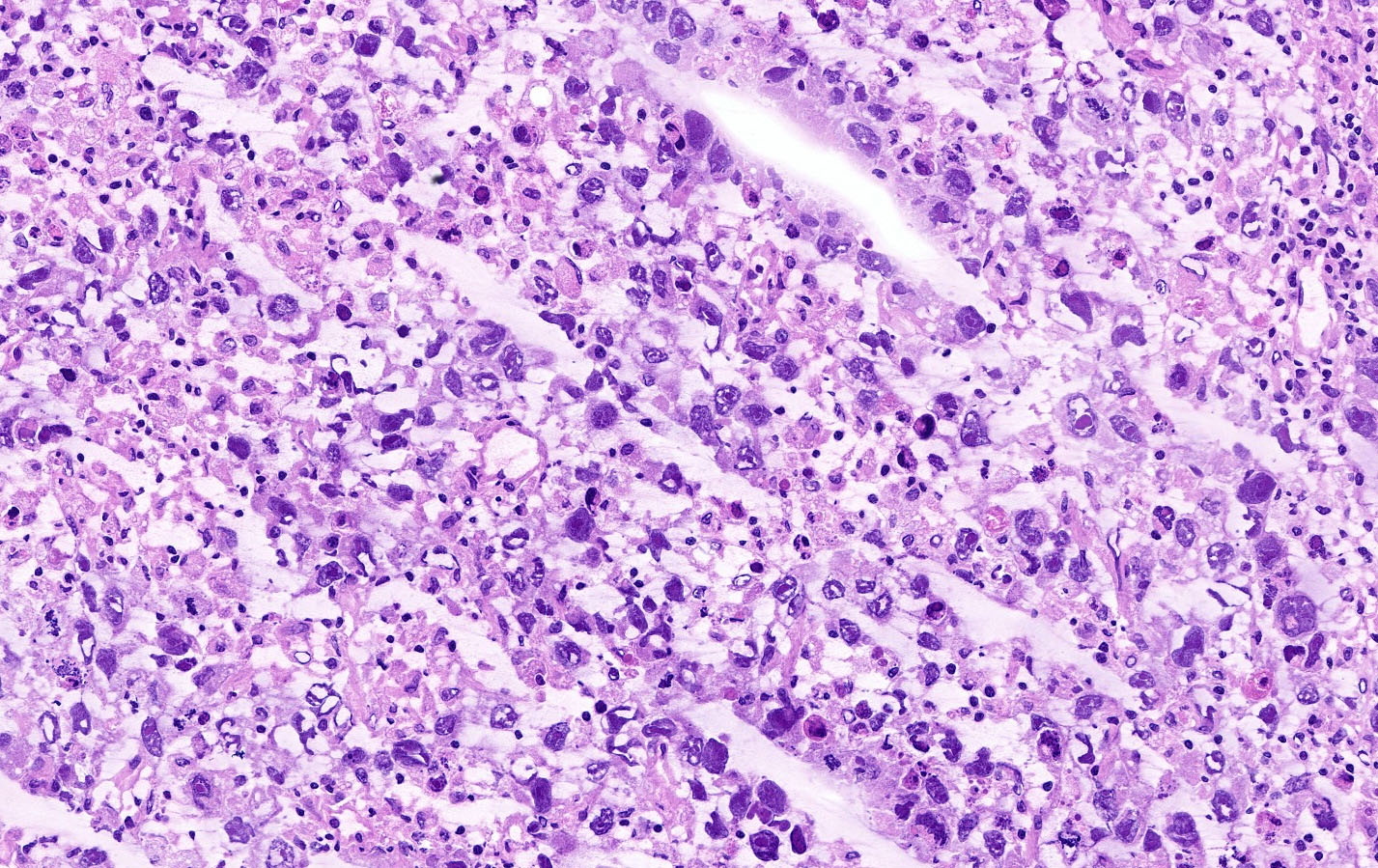
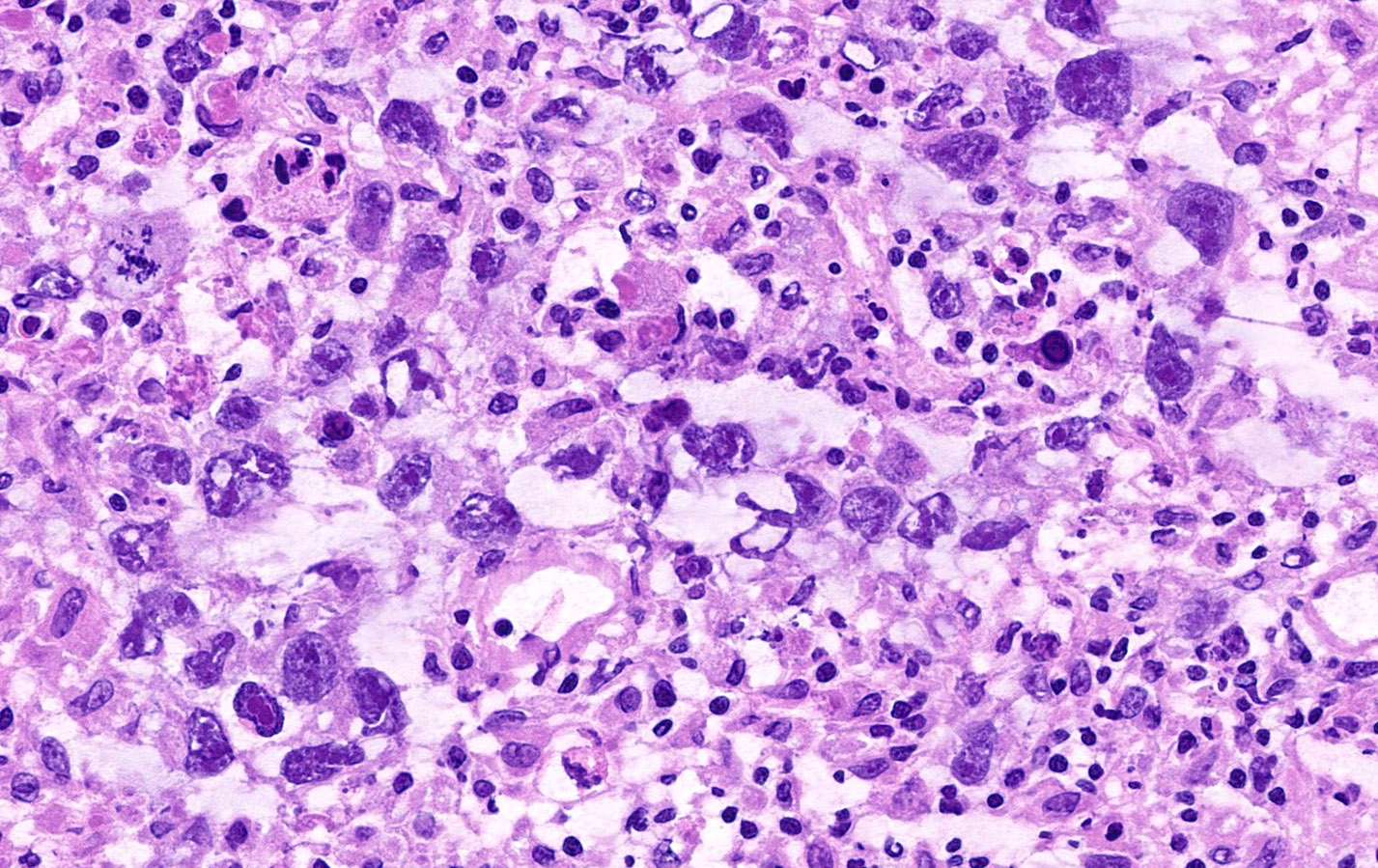
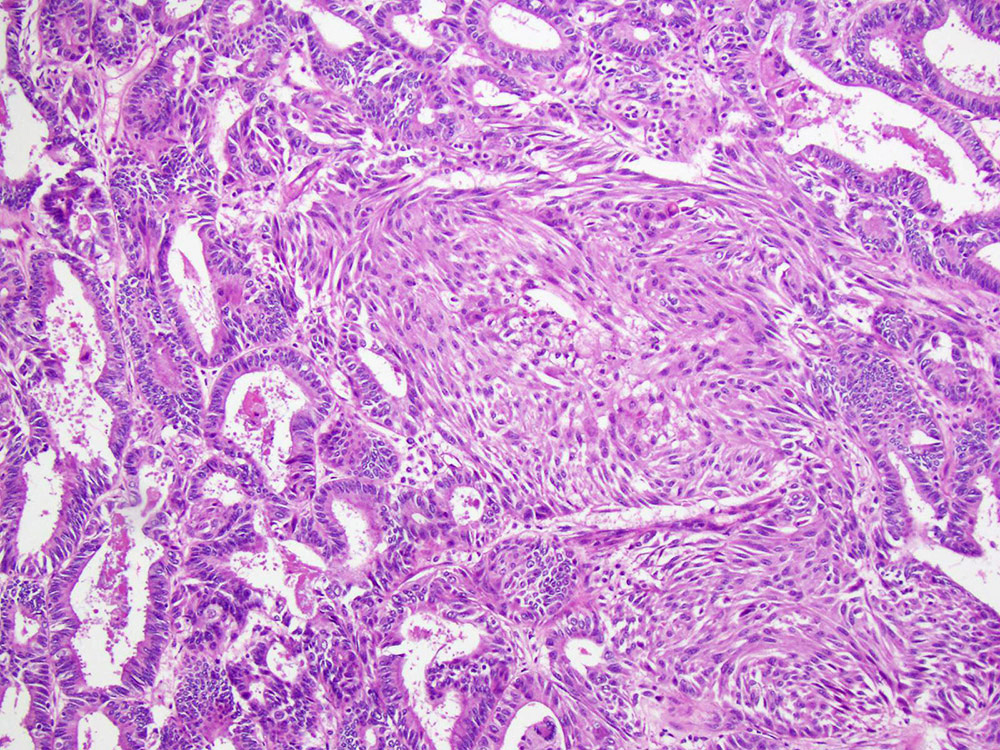
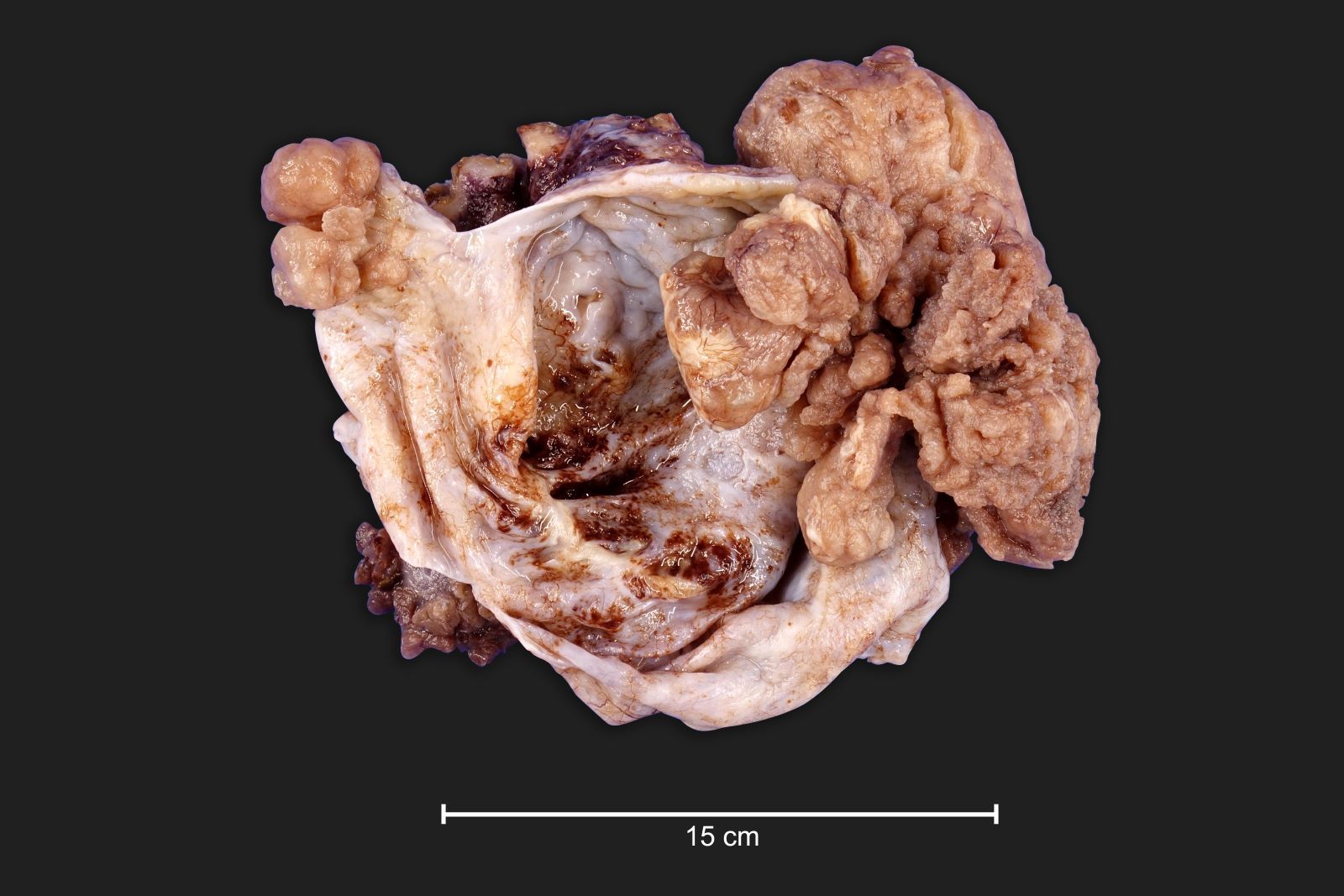
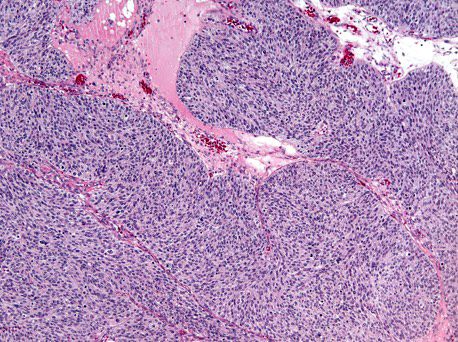
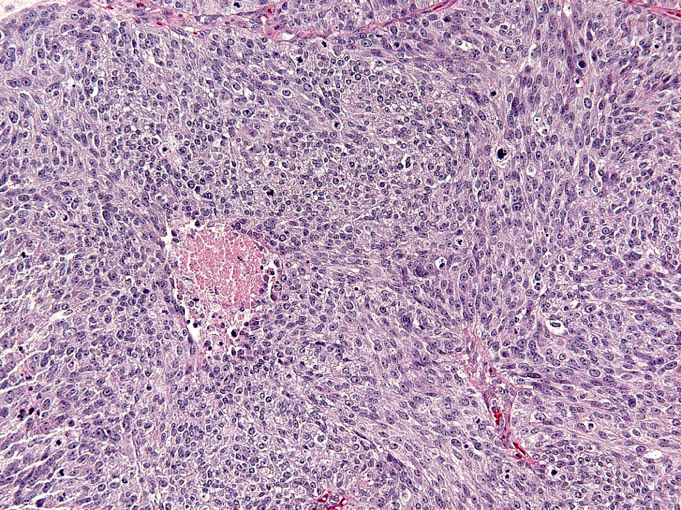
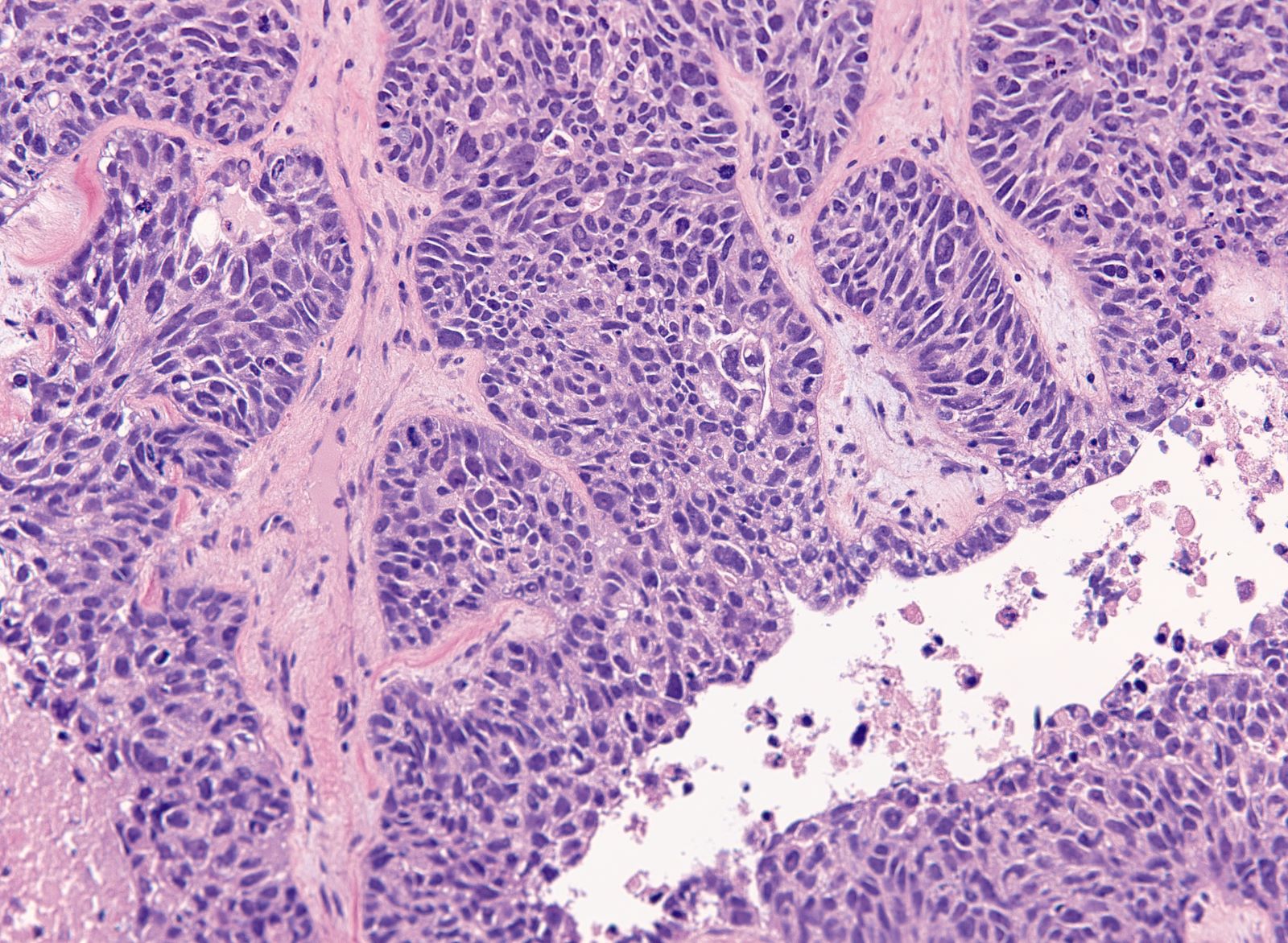
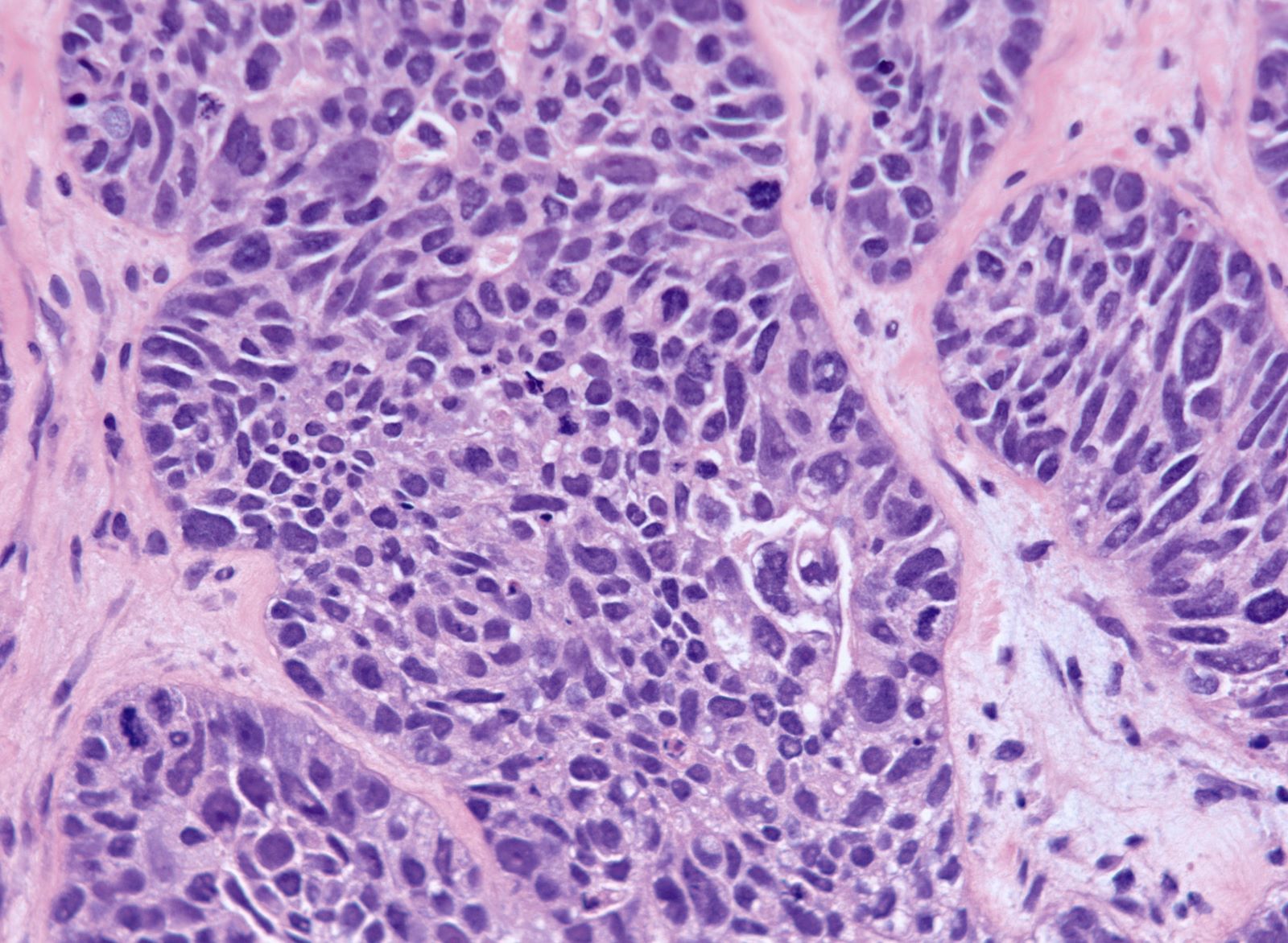
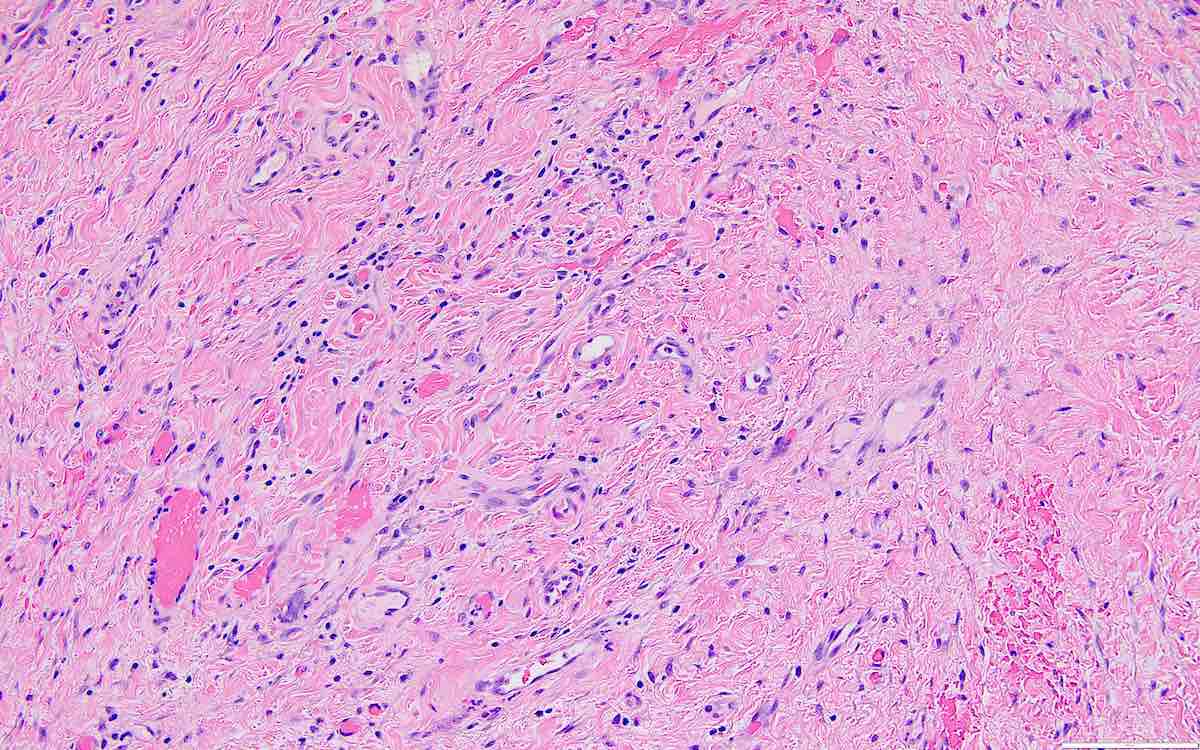
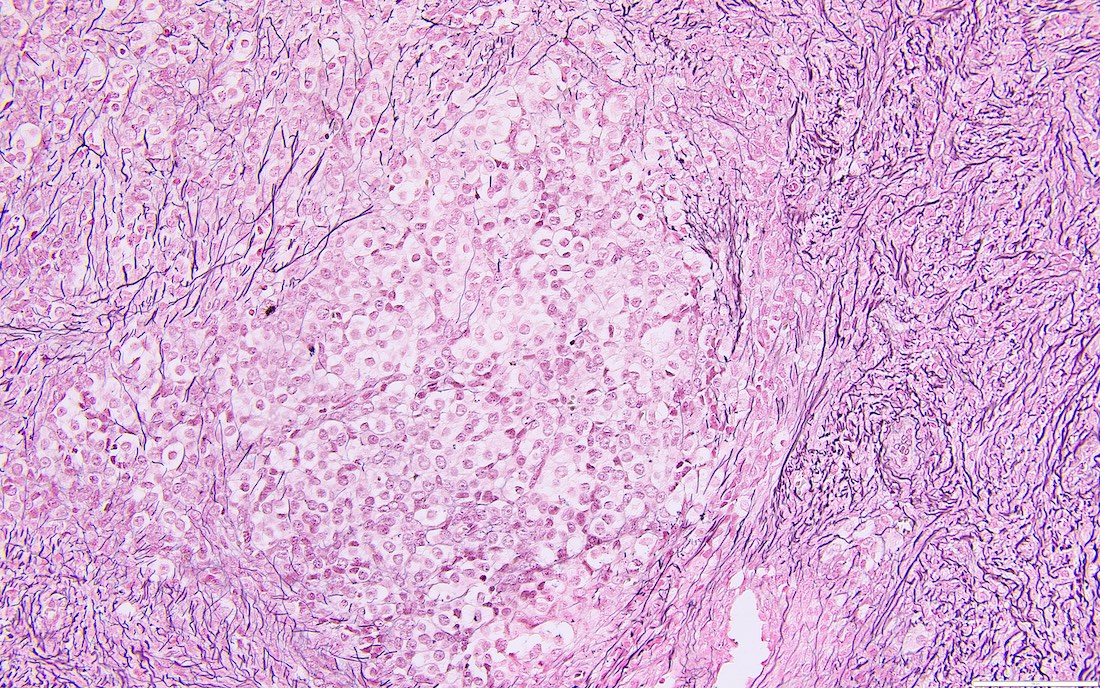
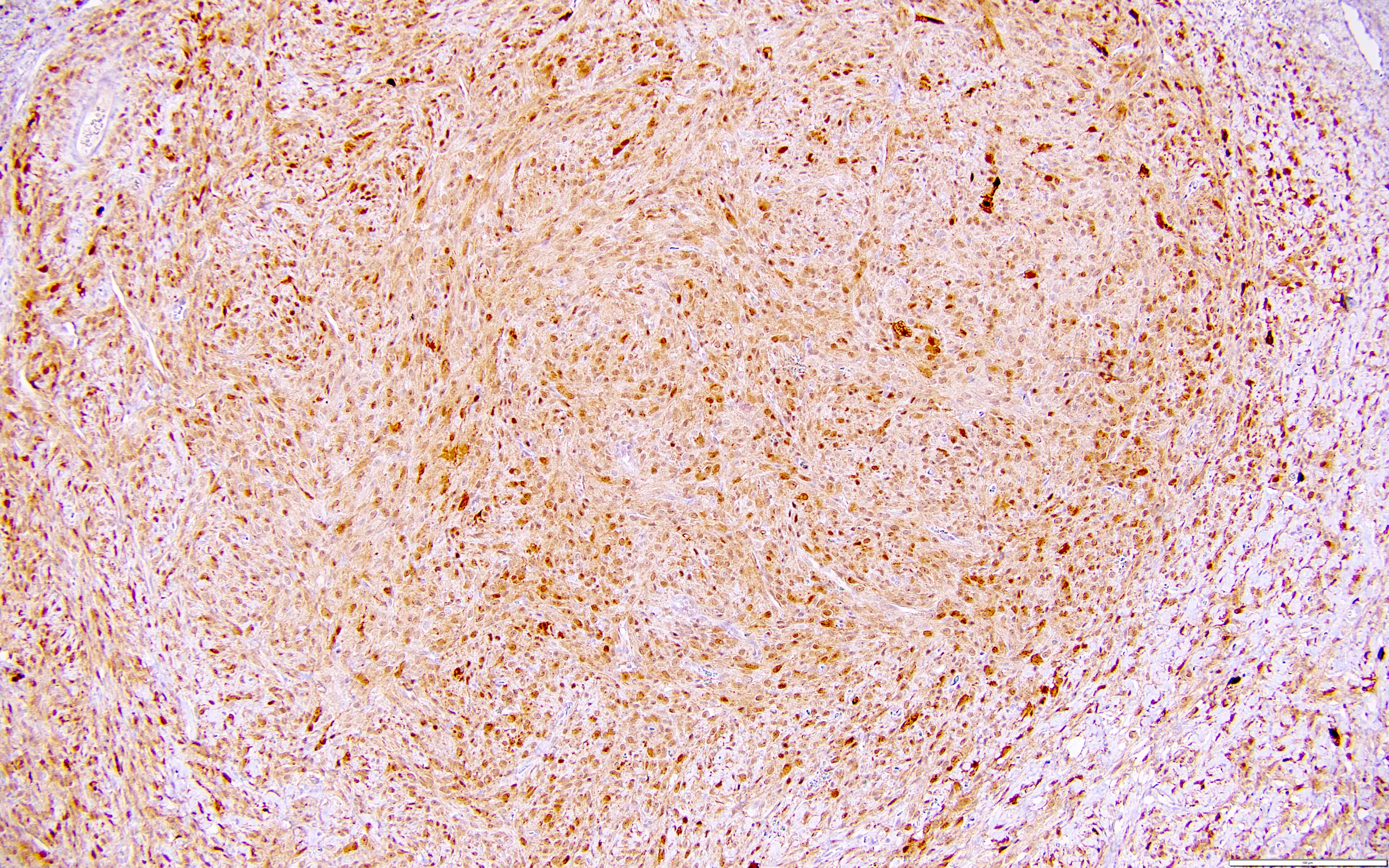
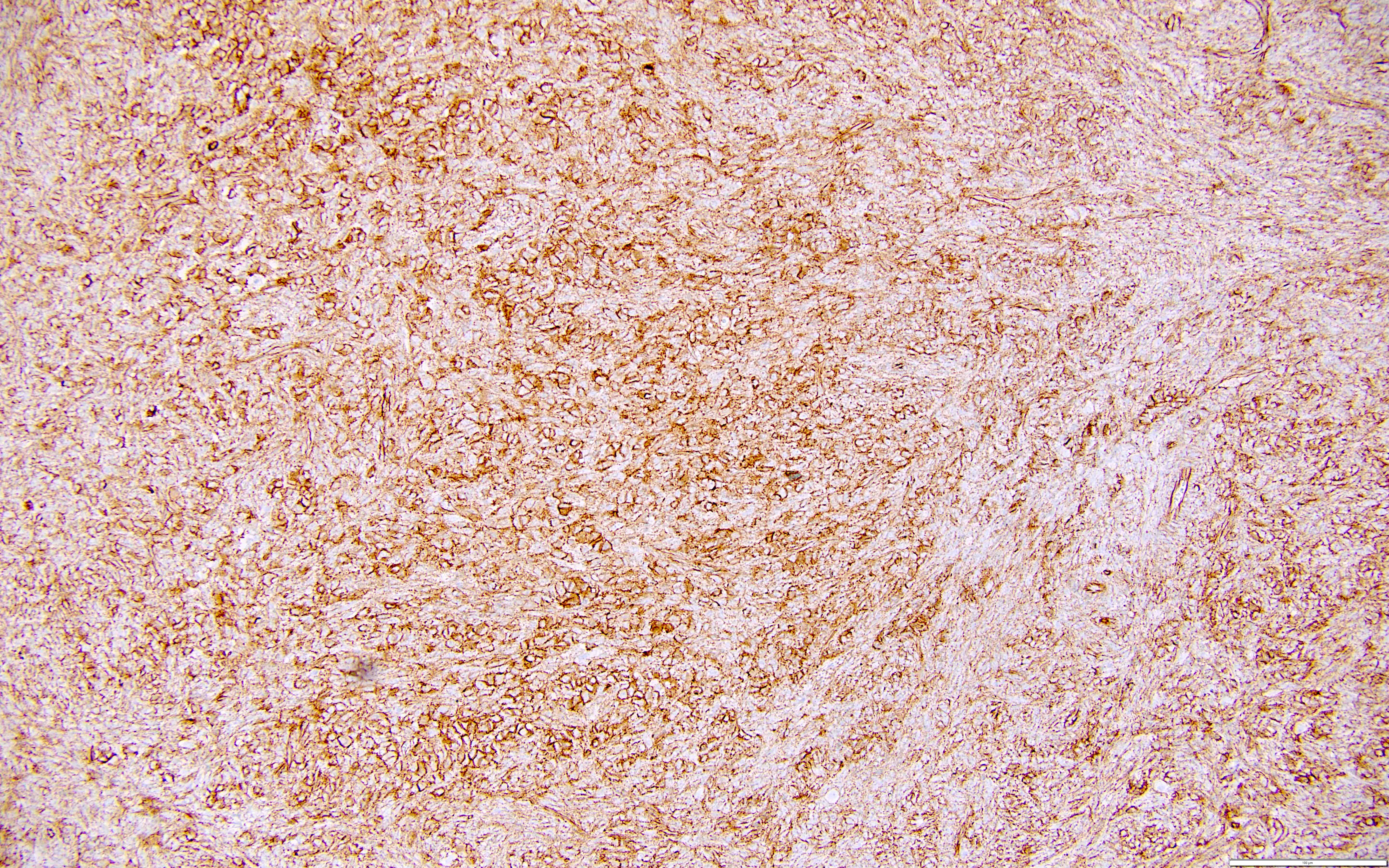
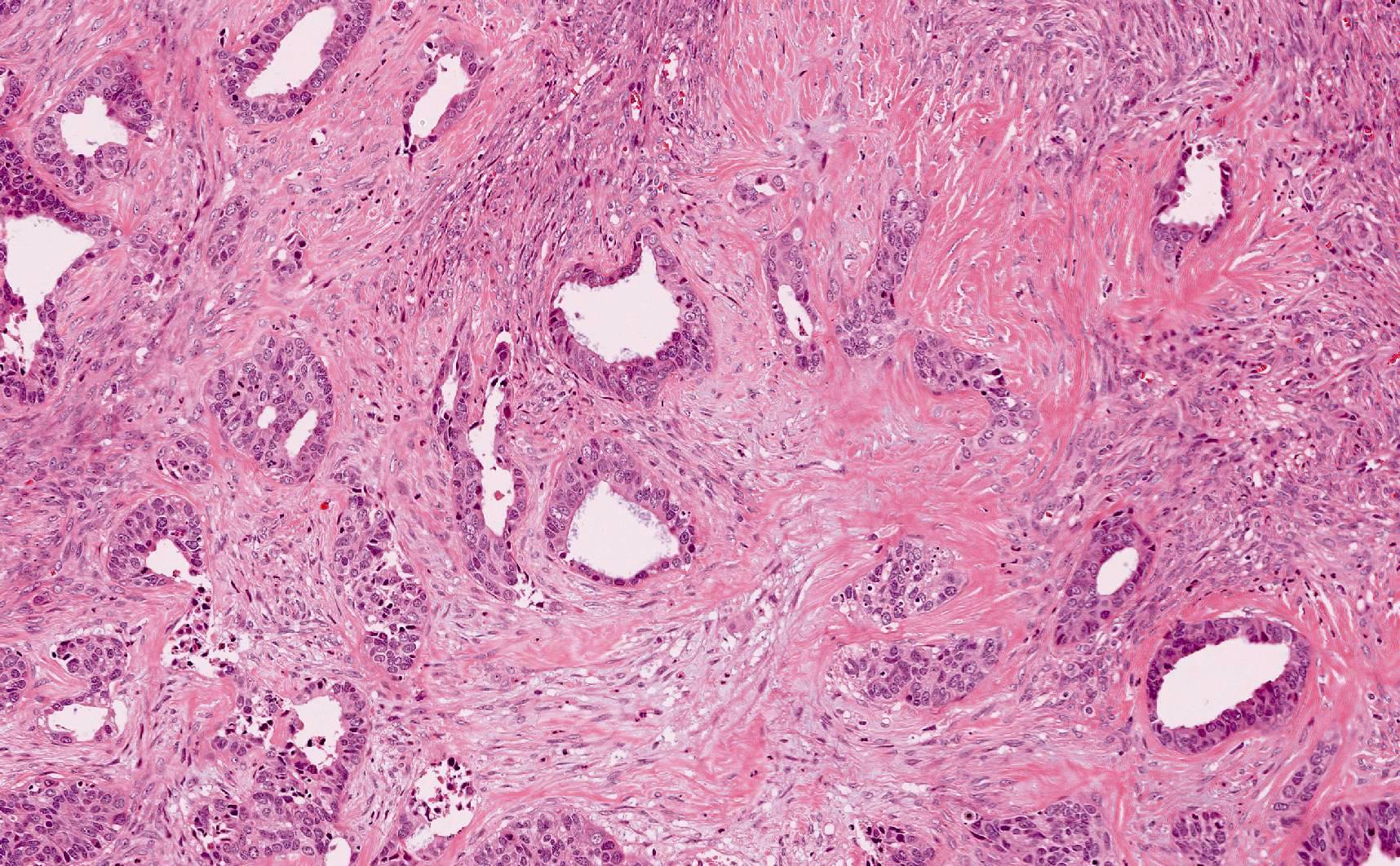
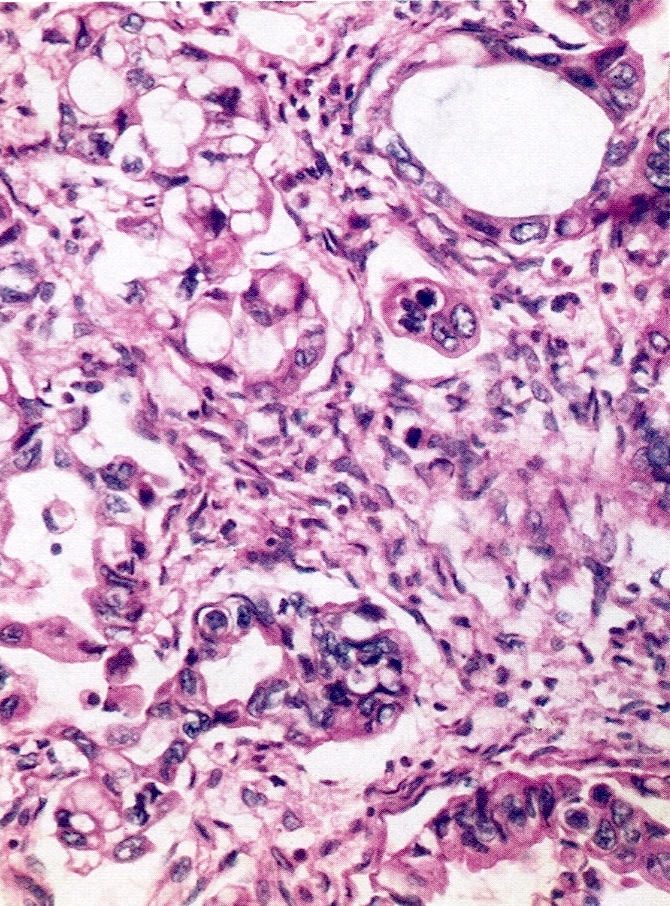
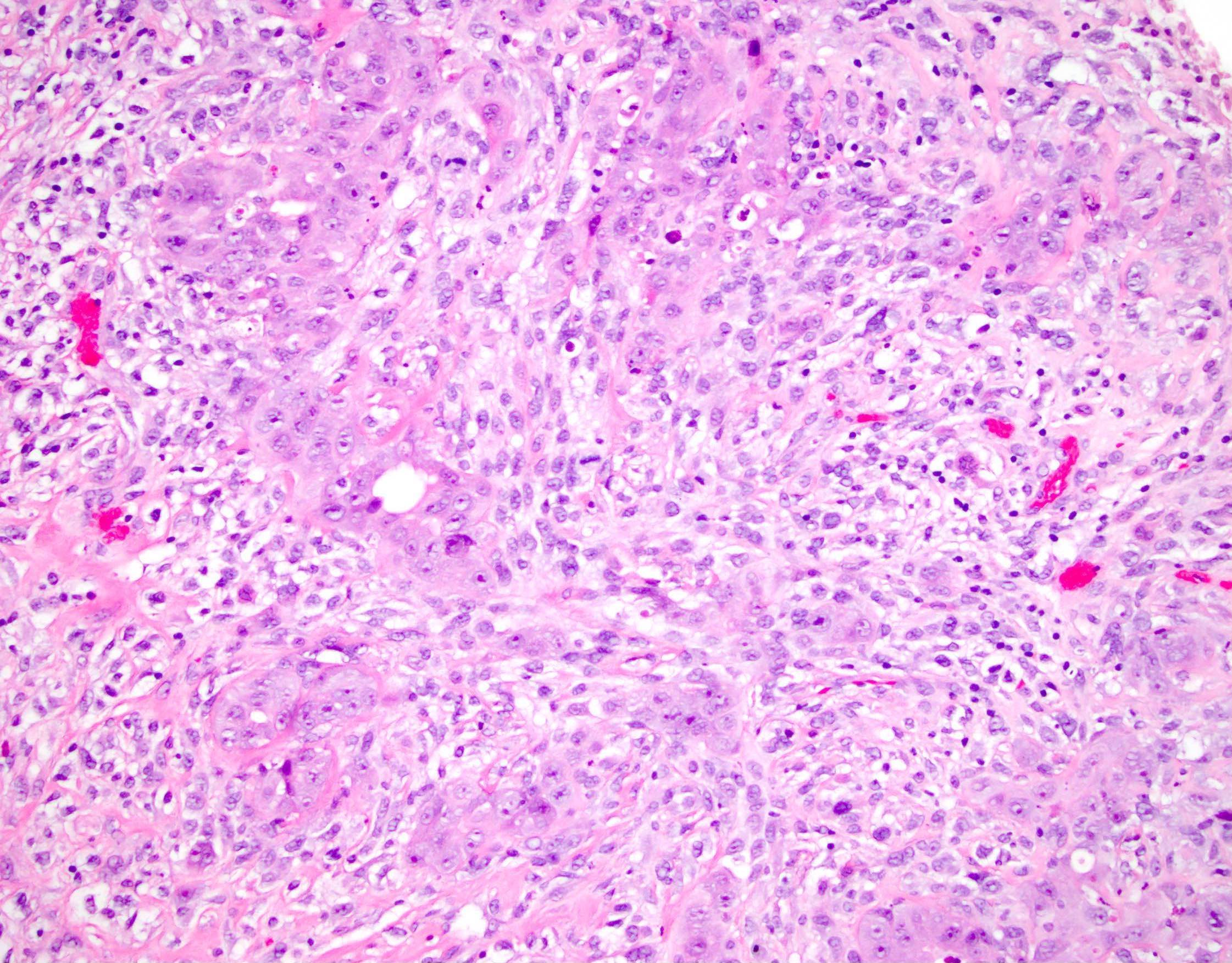
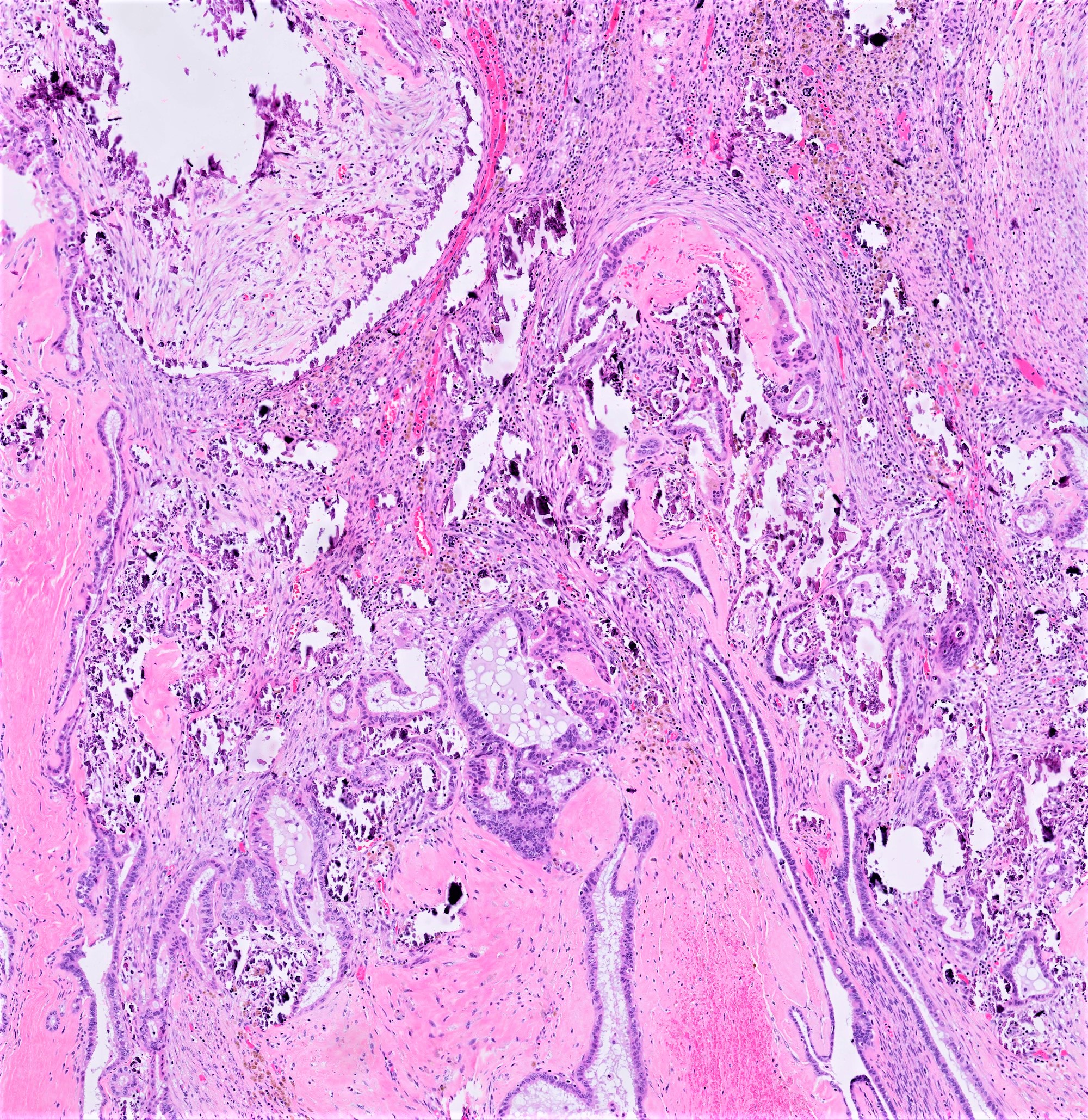
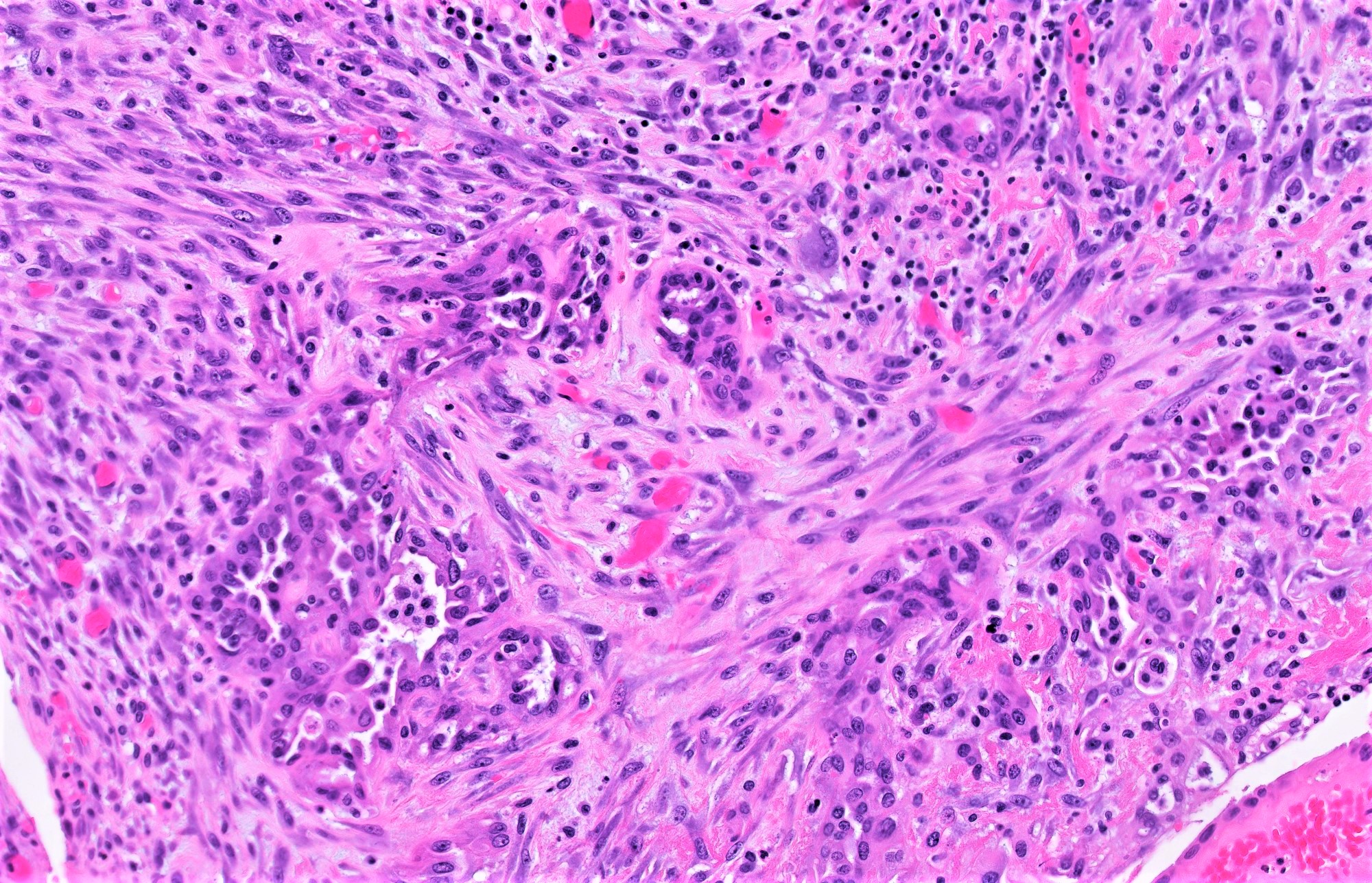
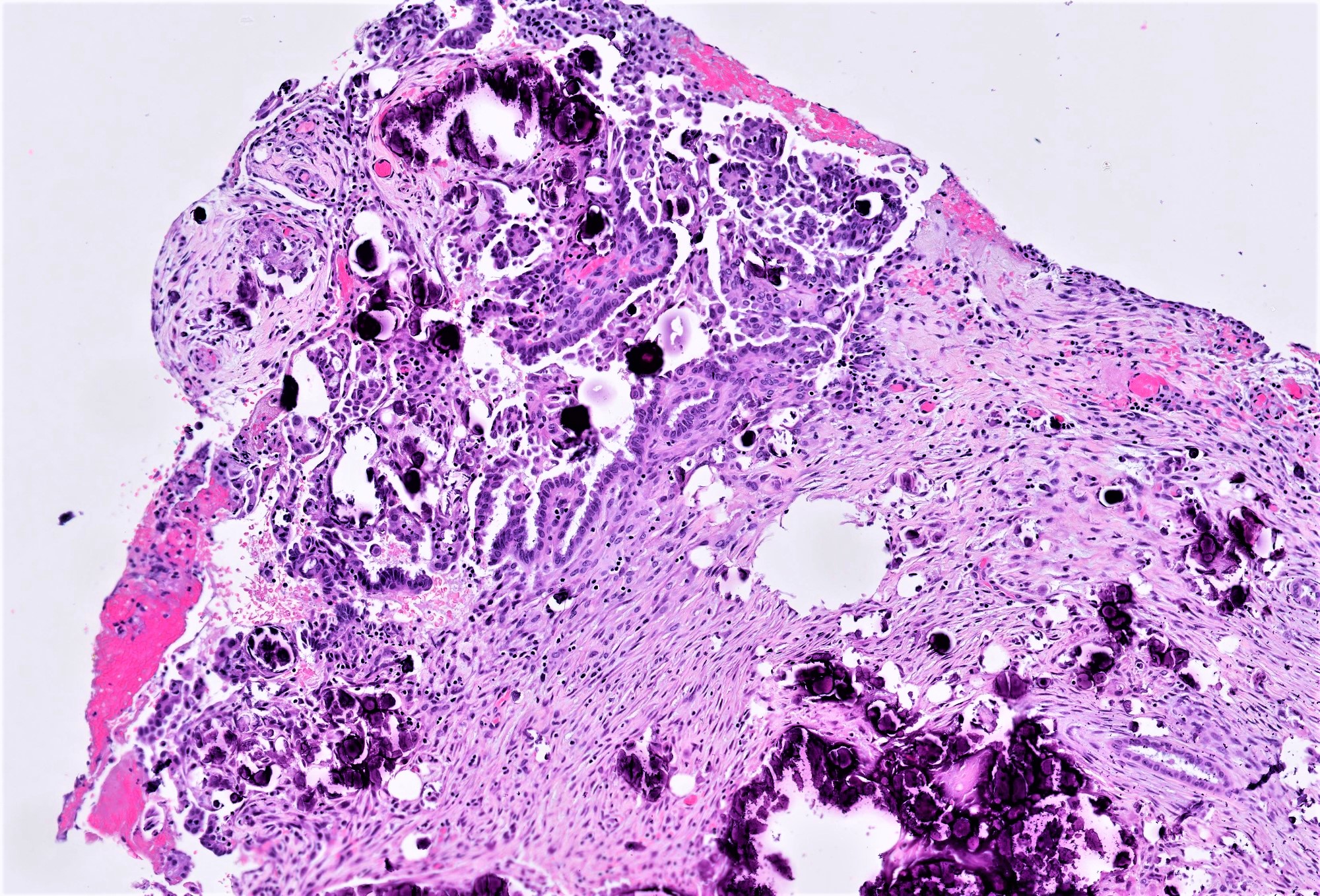
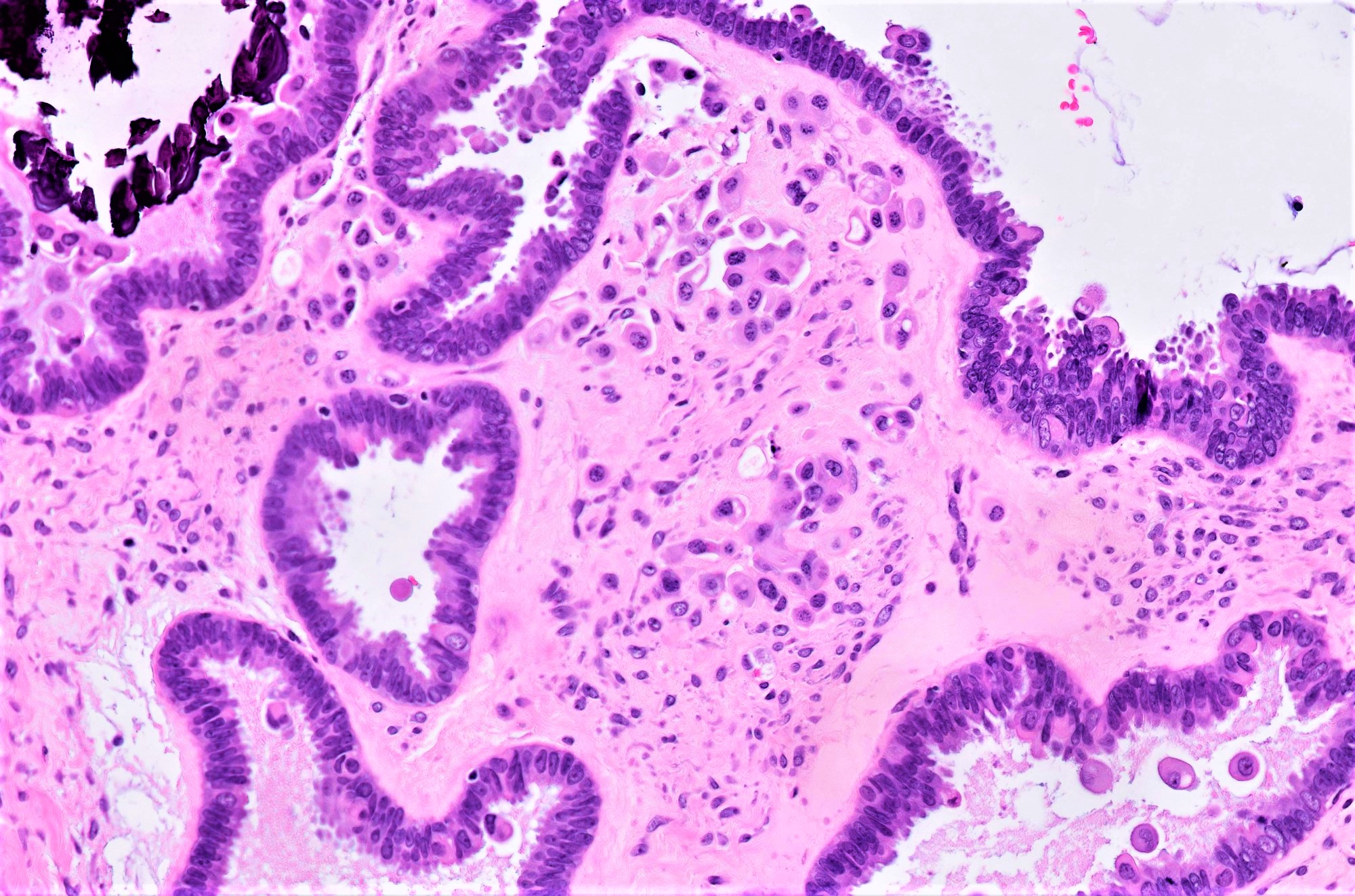
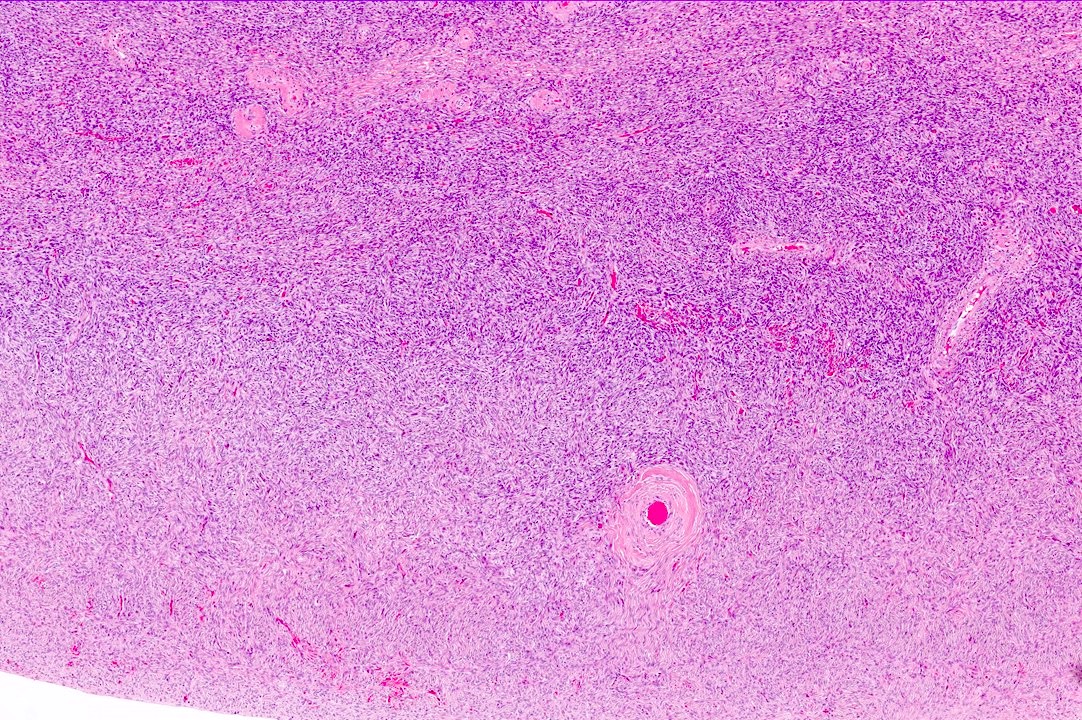
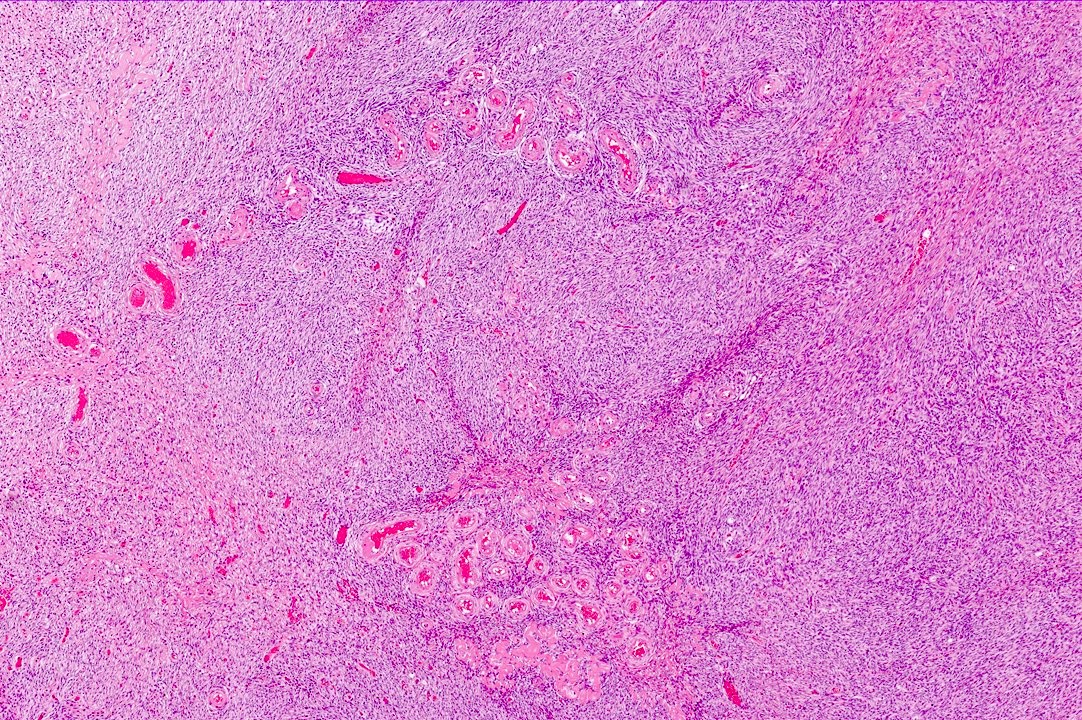
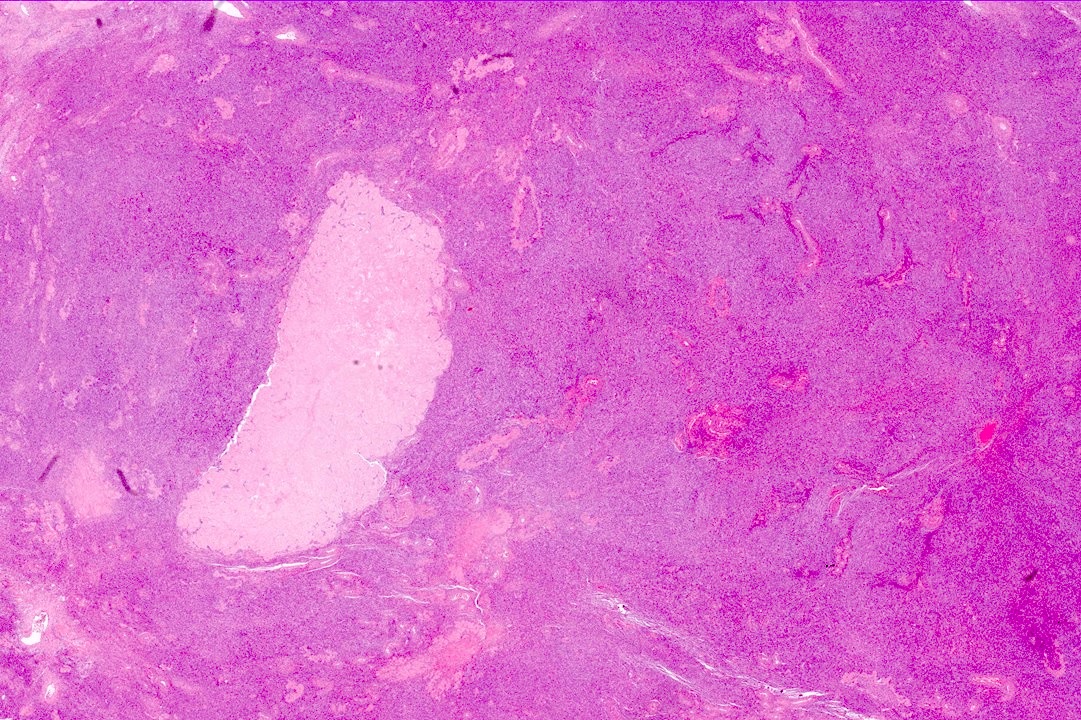
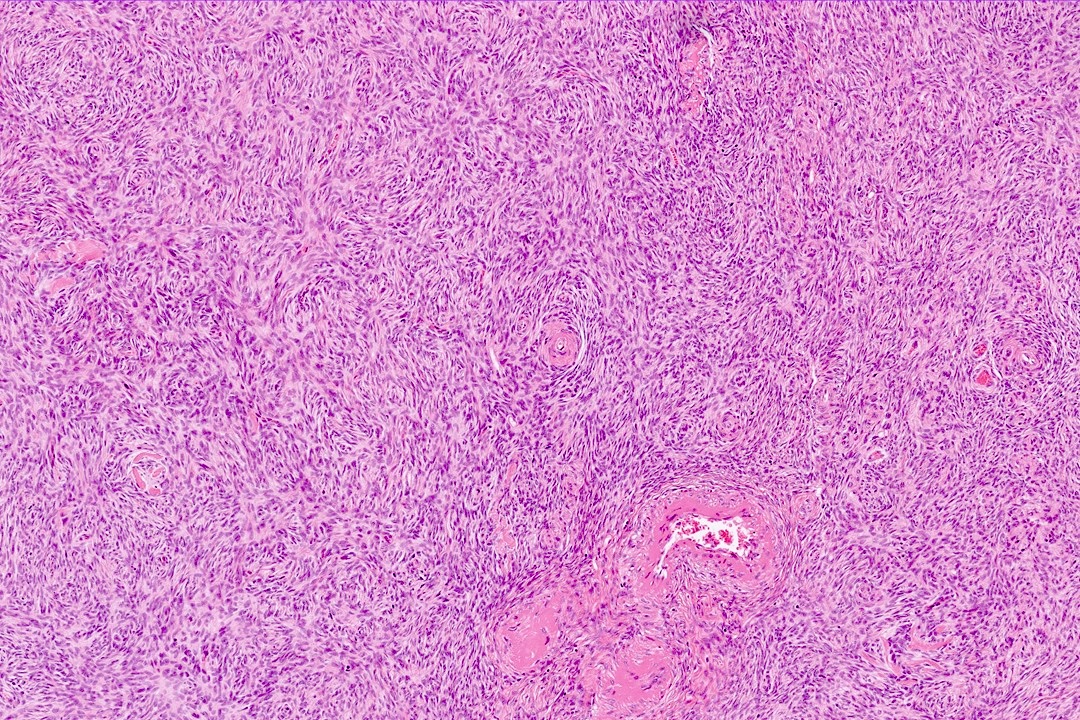
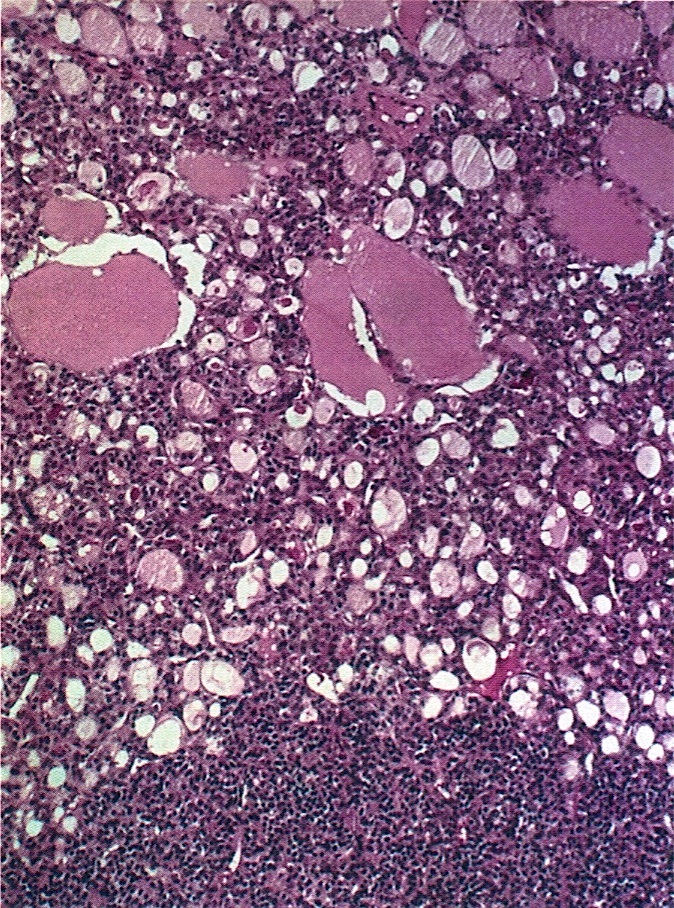
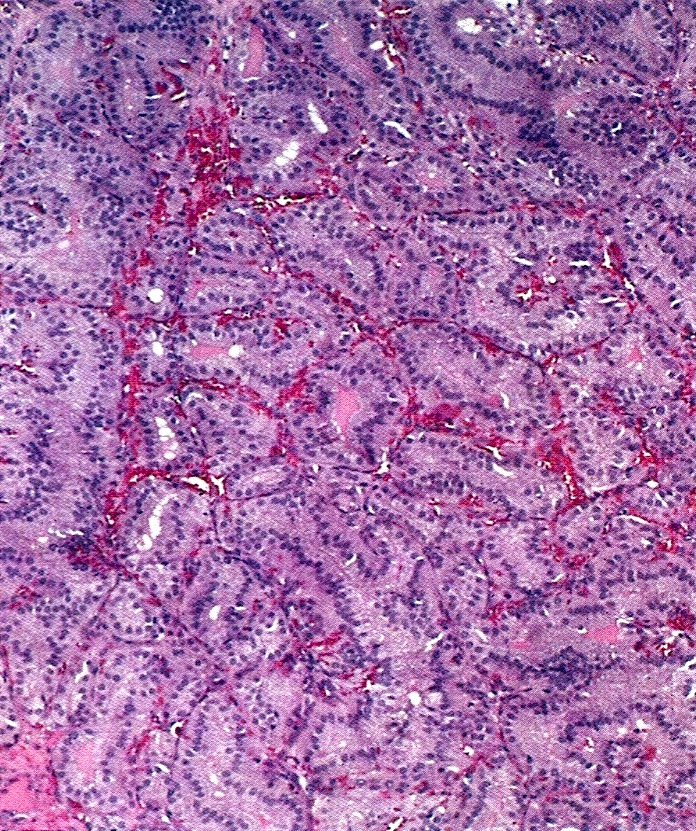
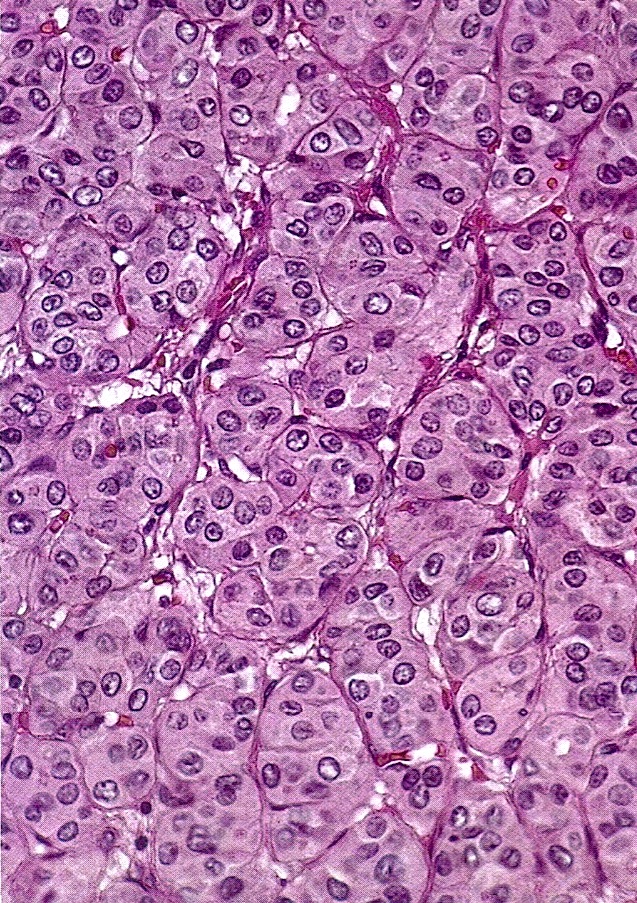
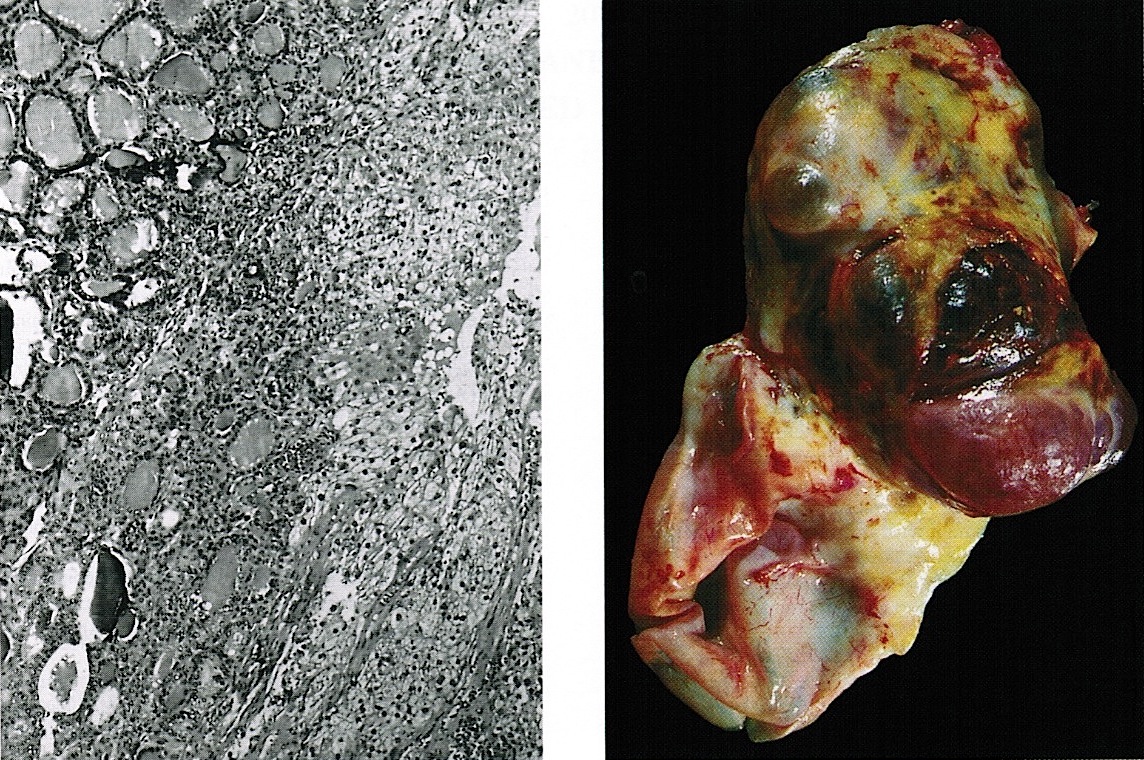
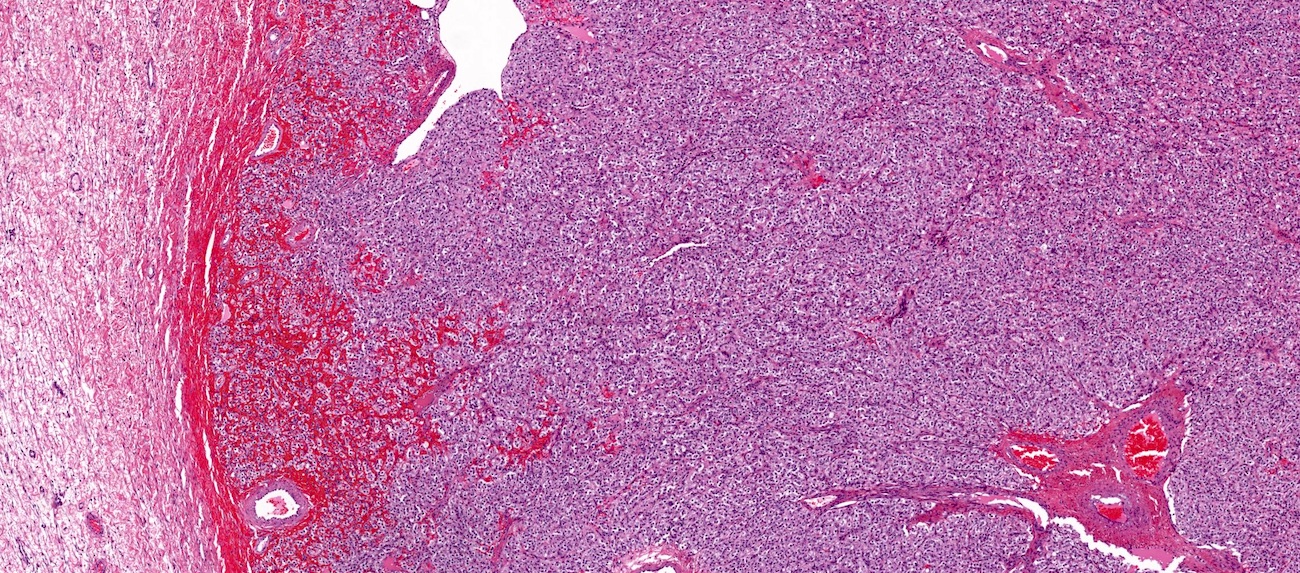
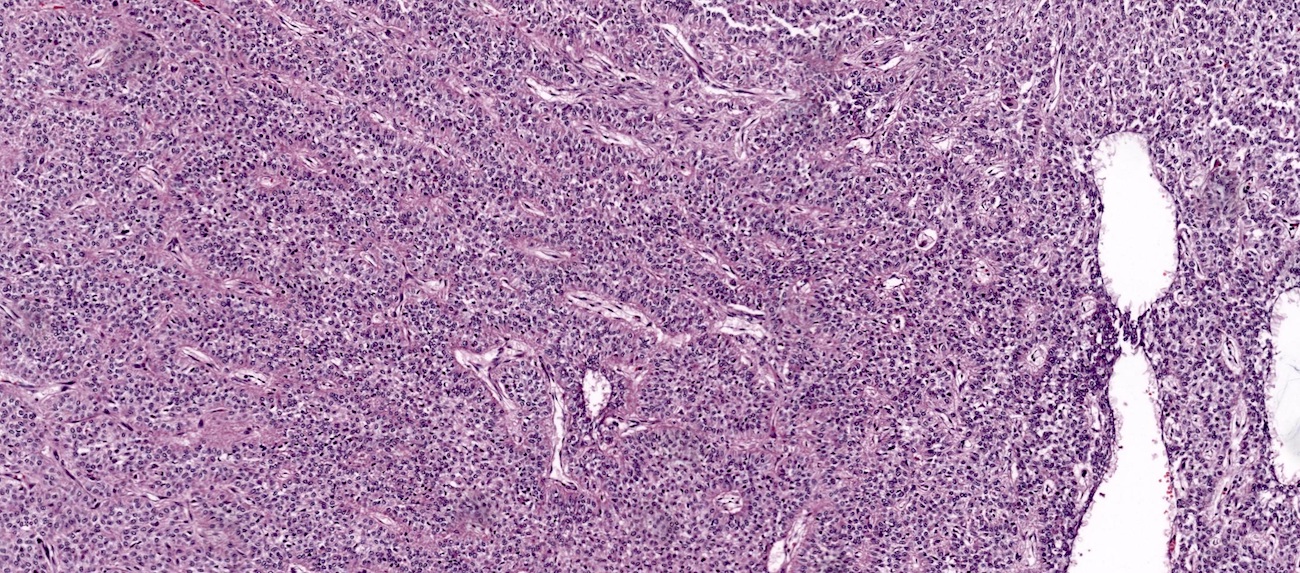
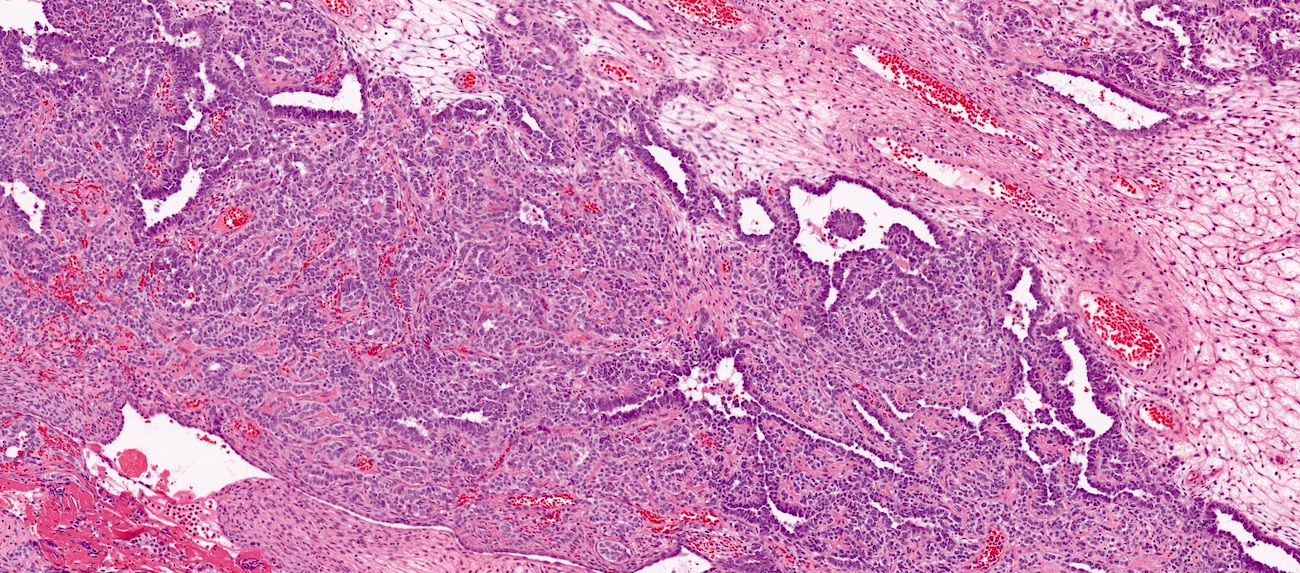
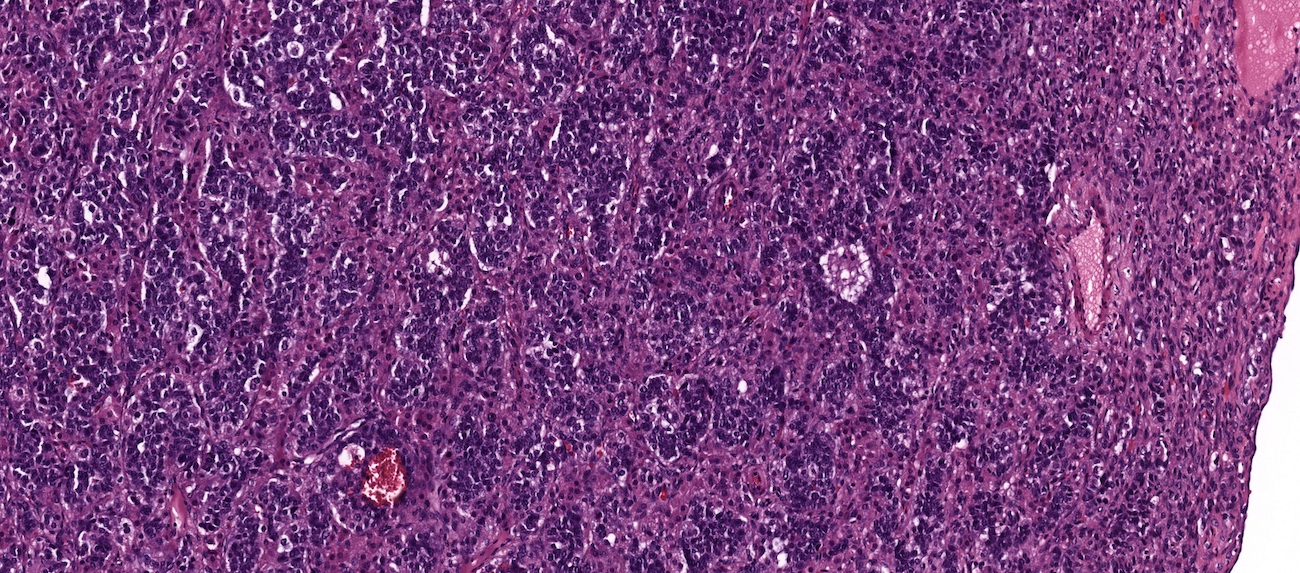
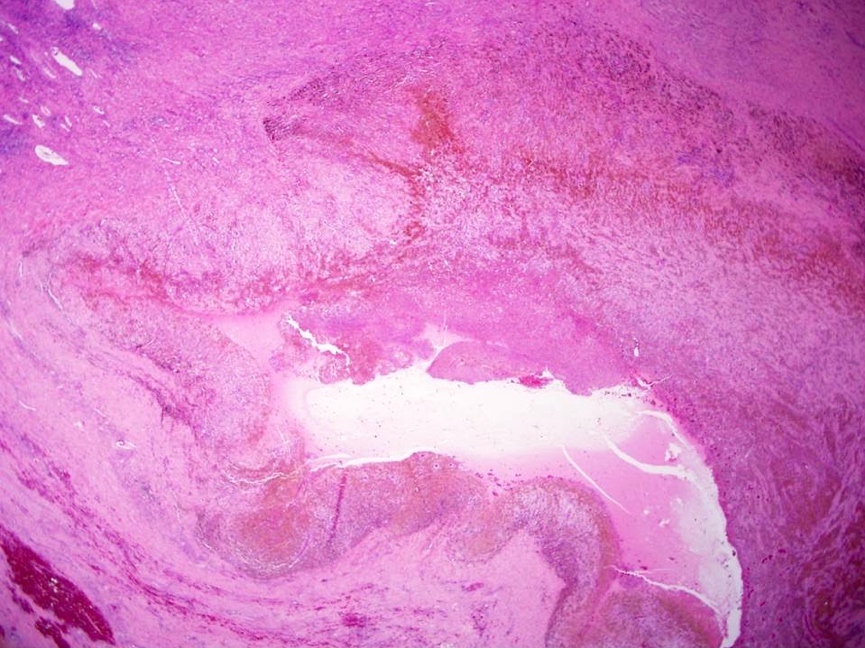
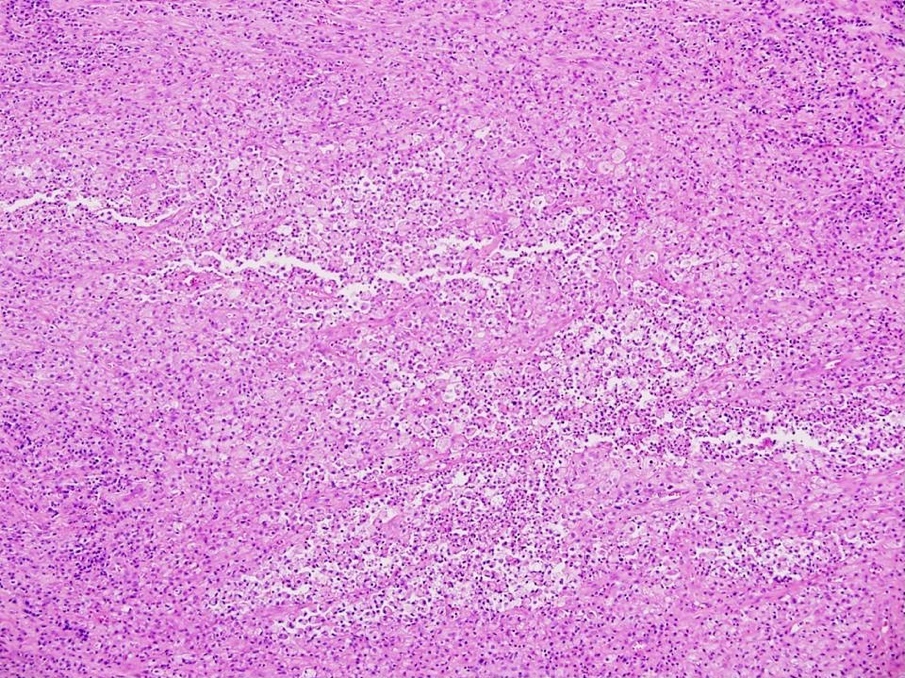
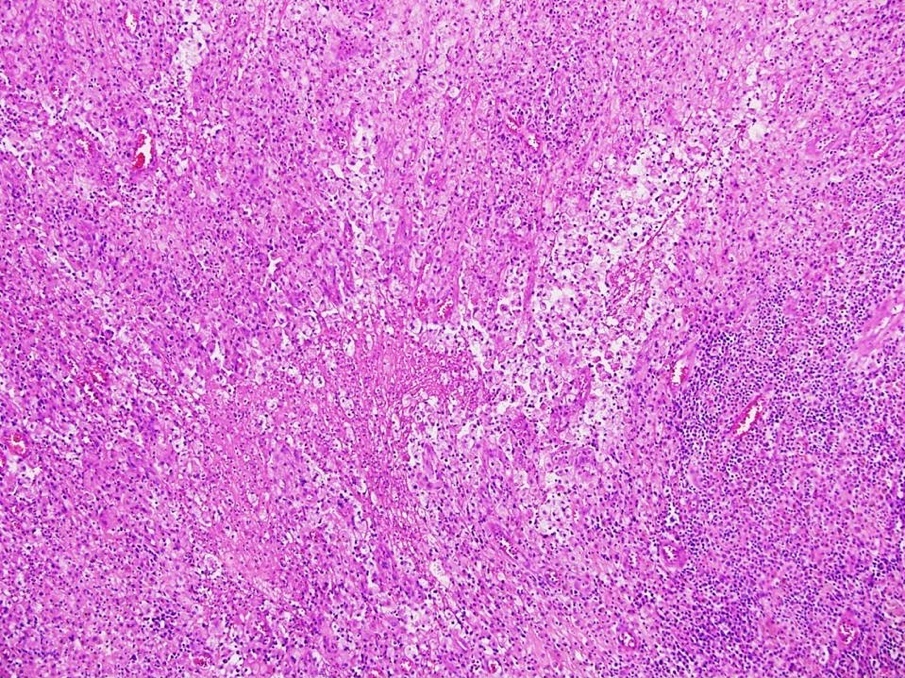
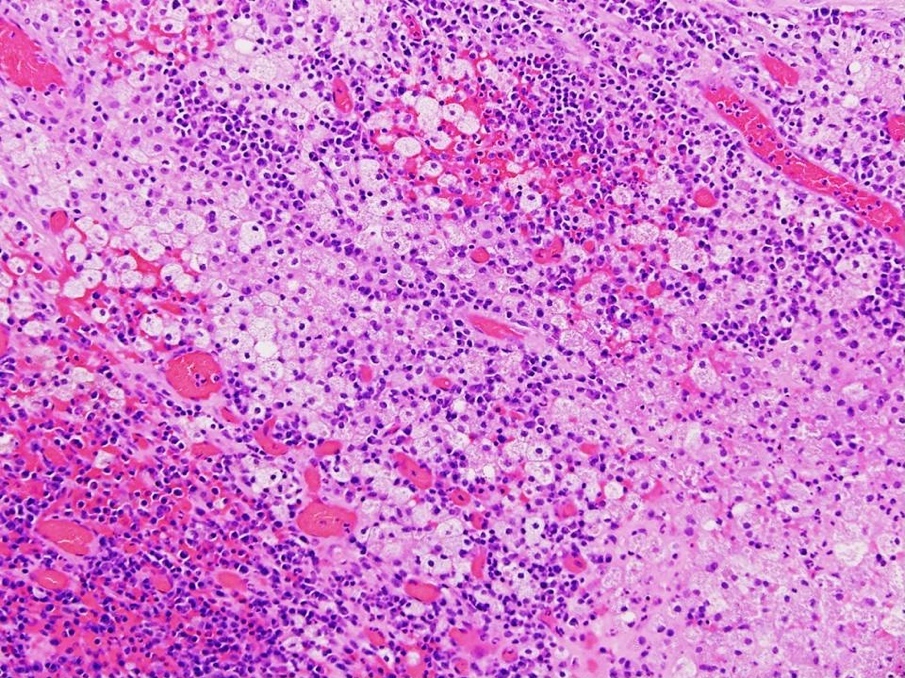
Carcinosarcoma
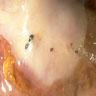
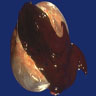
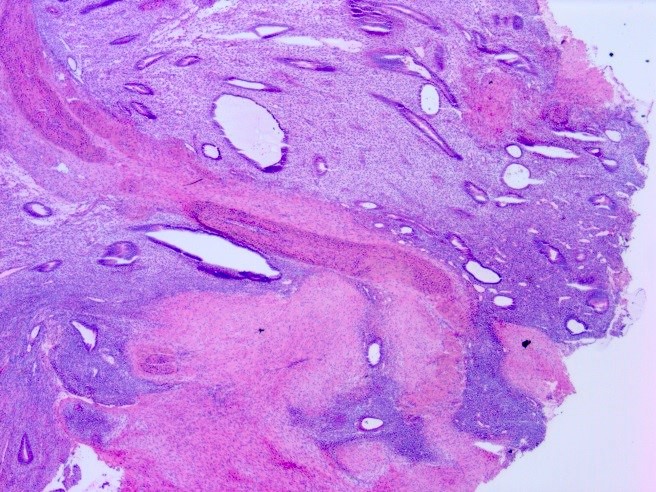
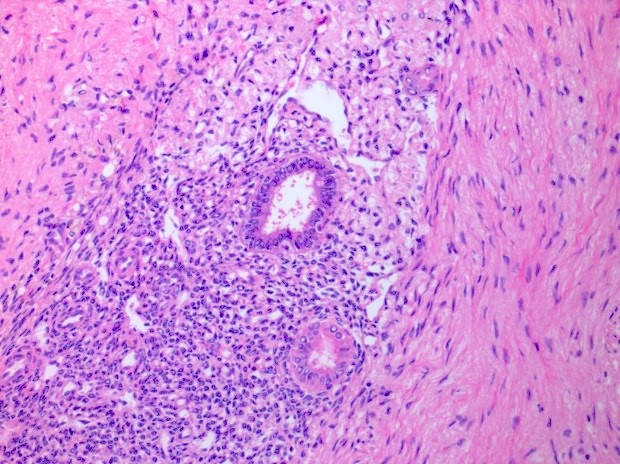
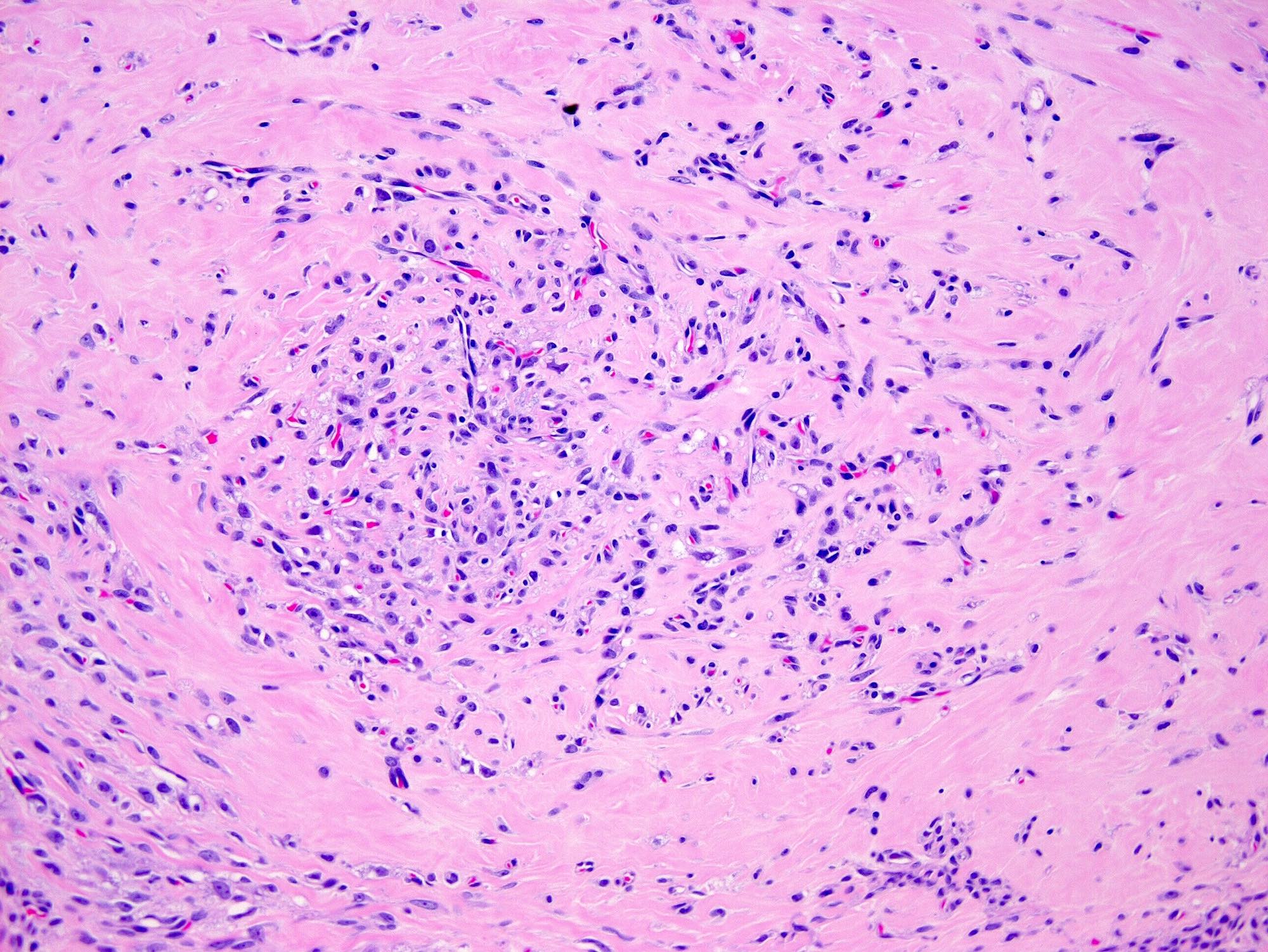
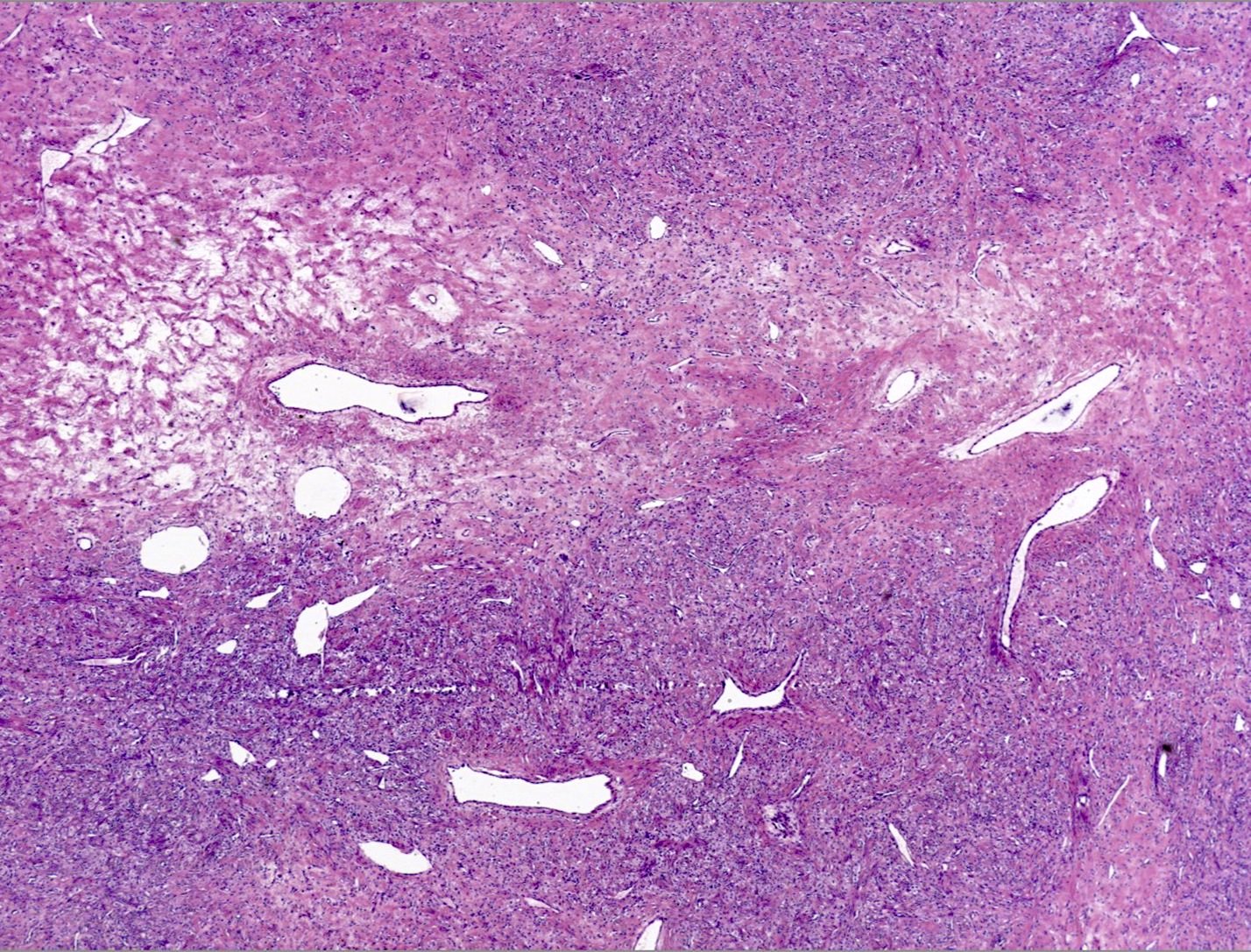
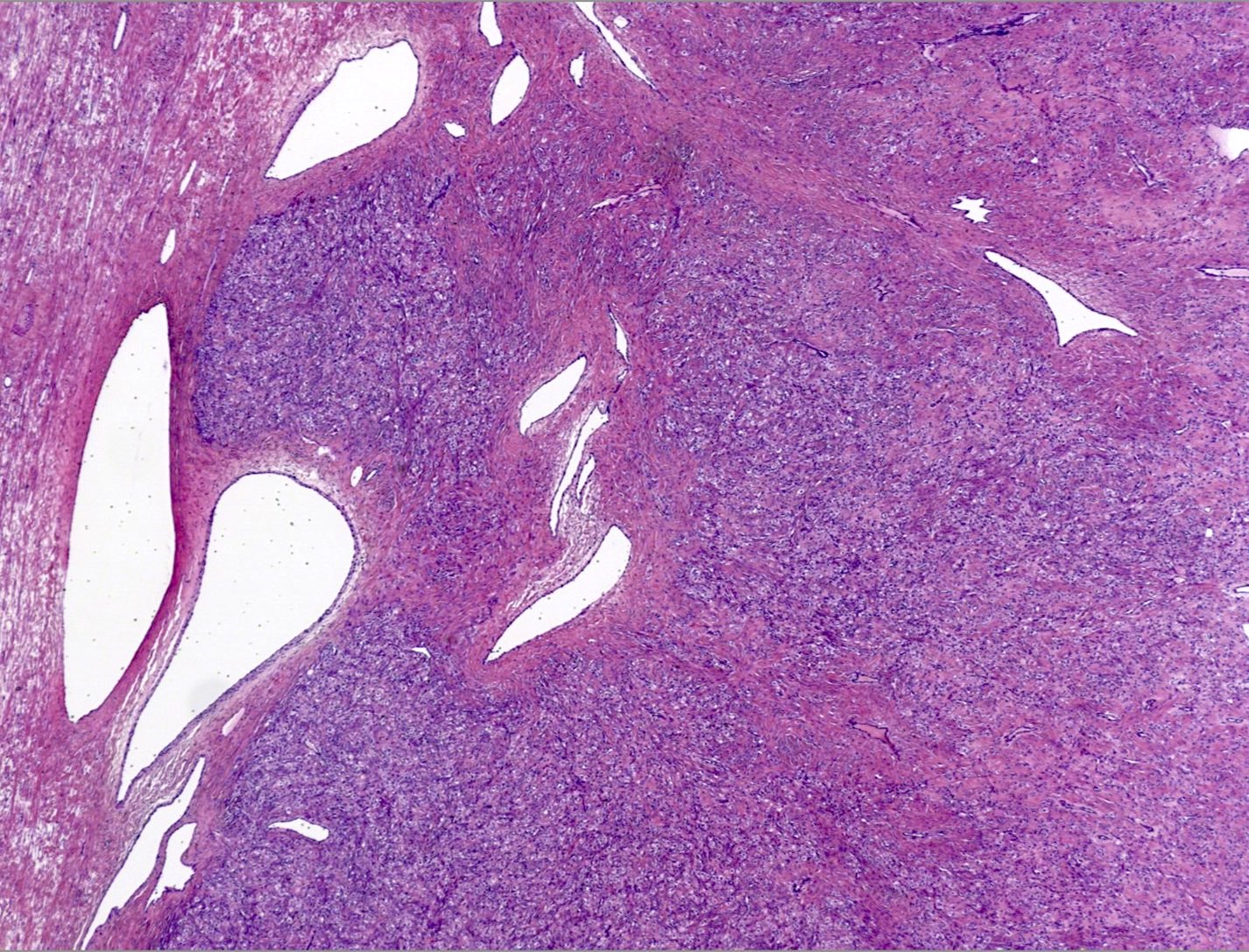
Table of Contents
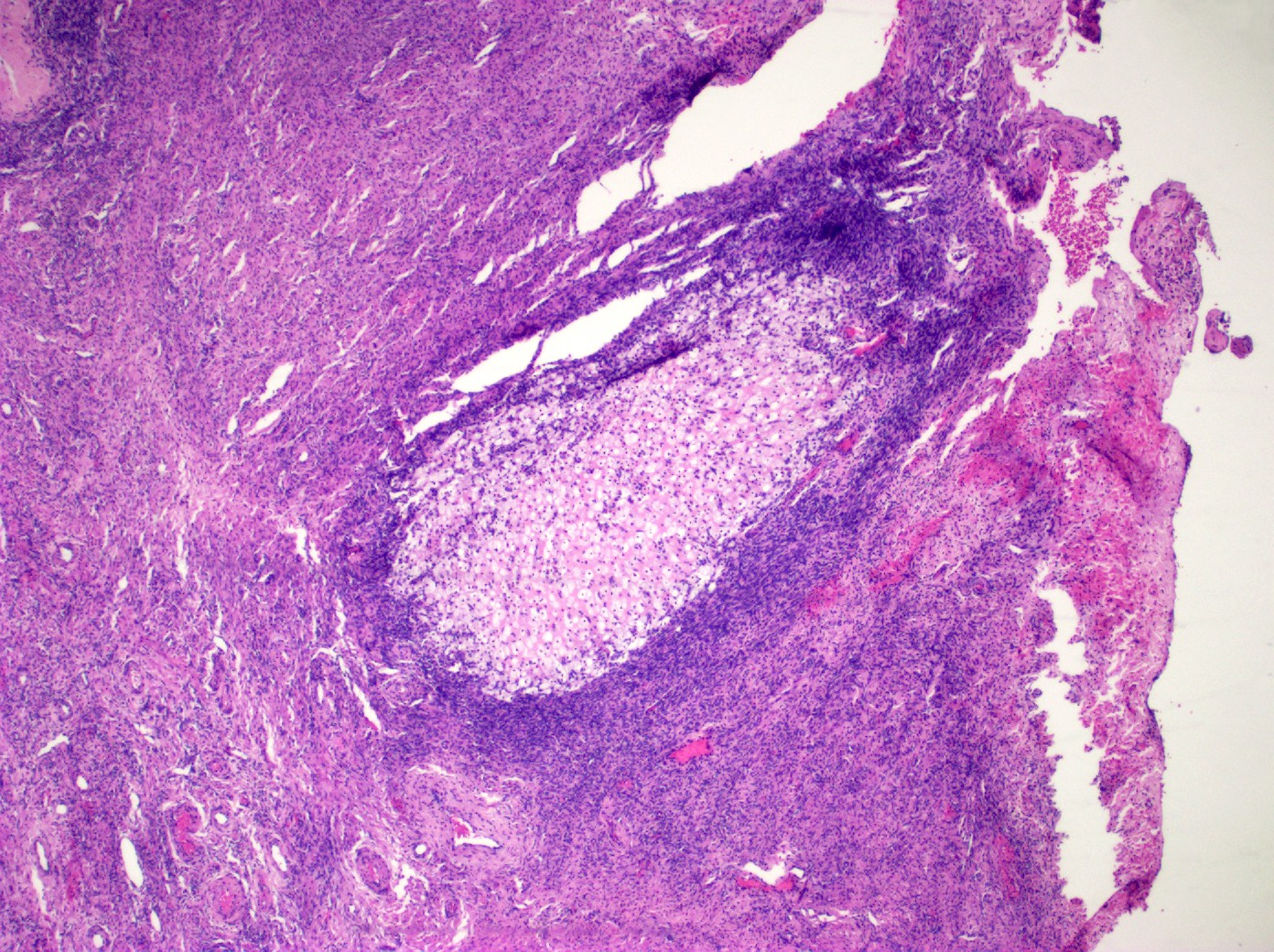
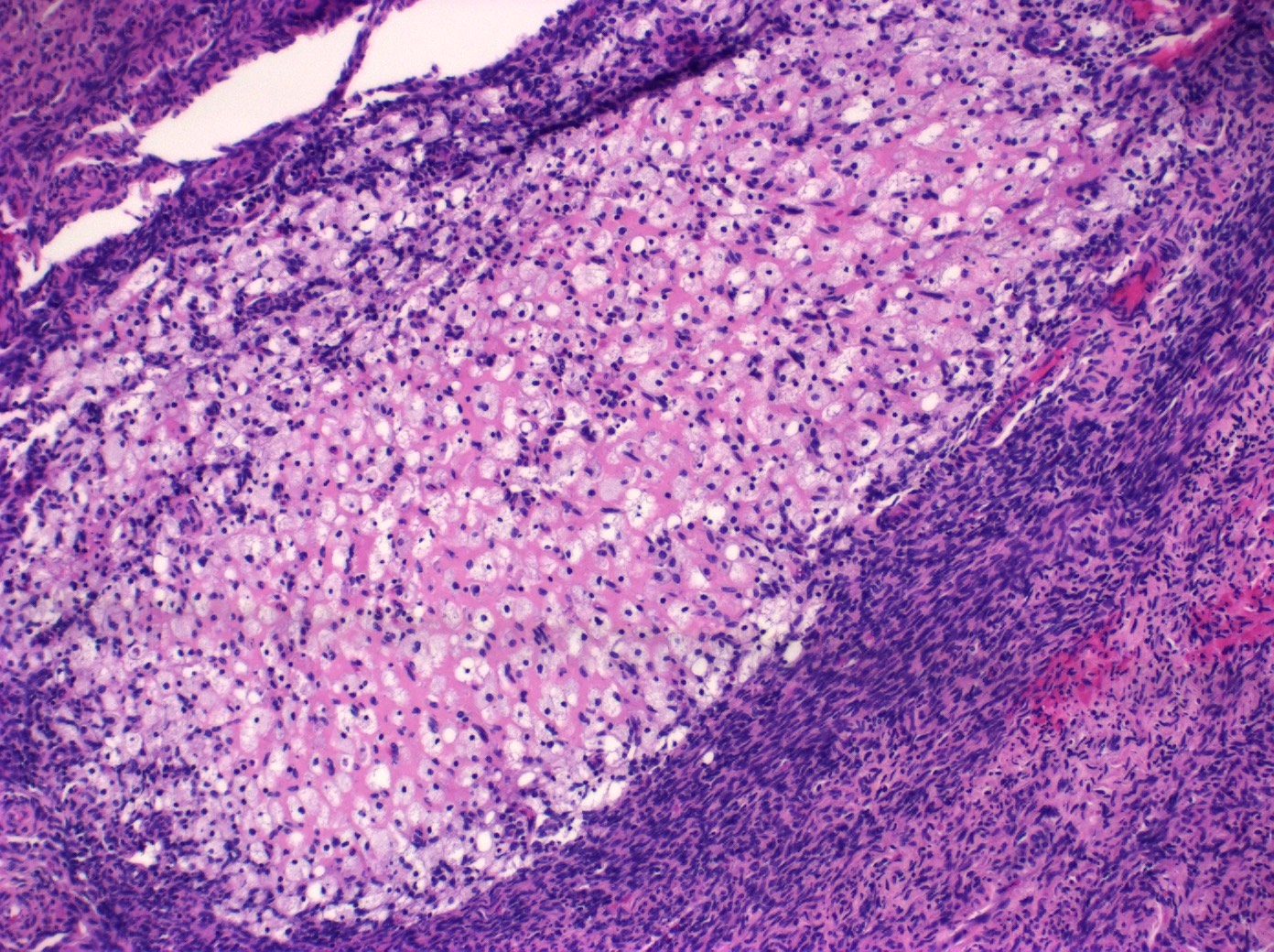
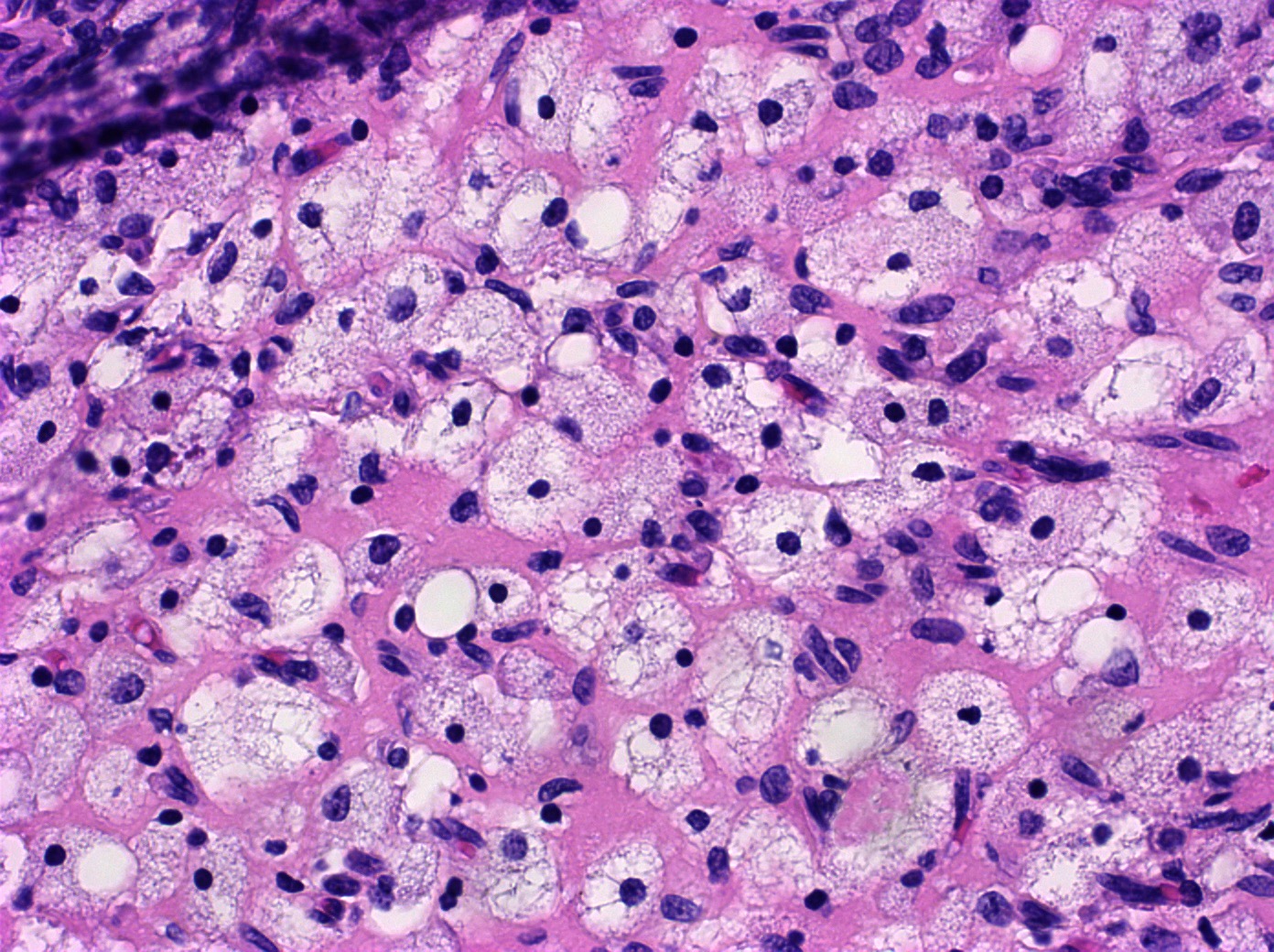
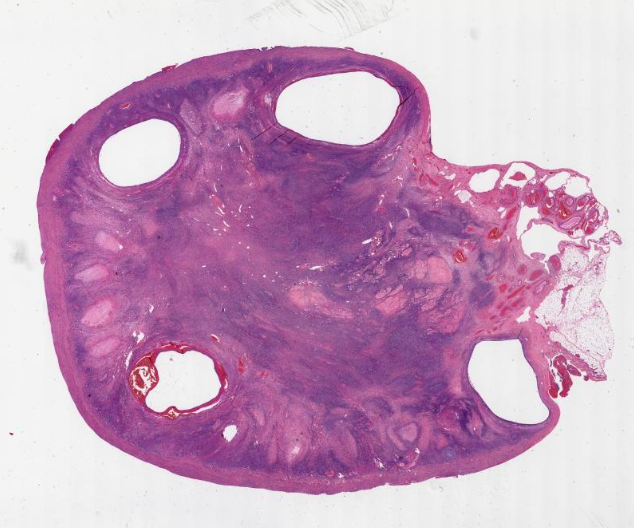
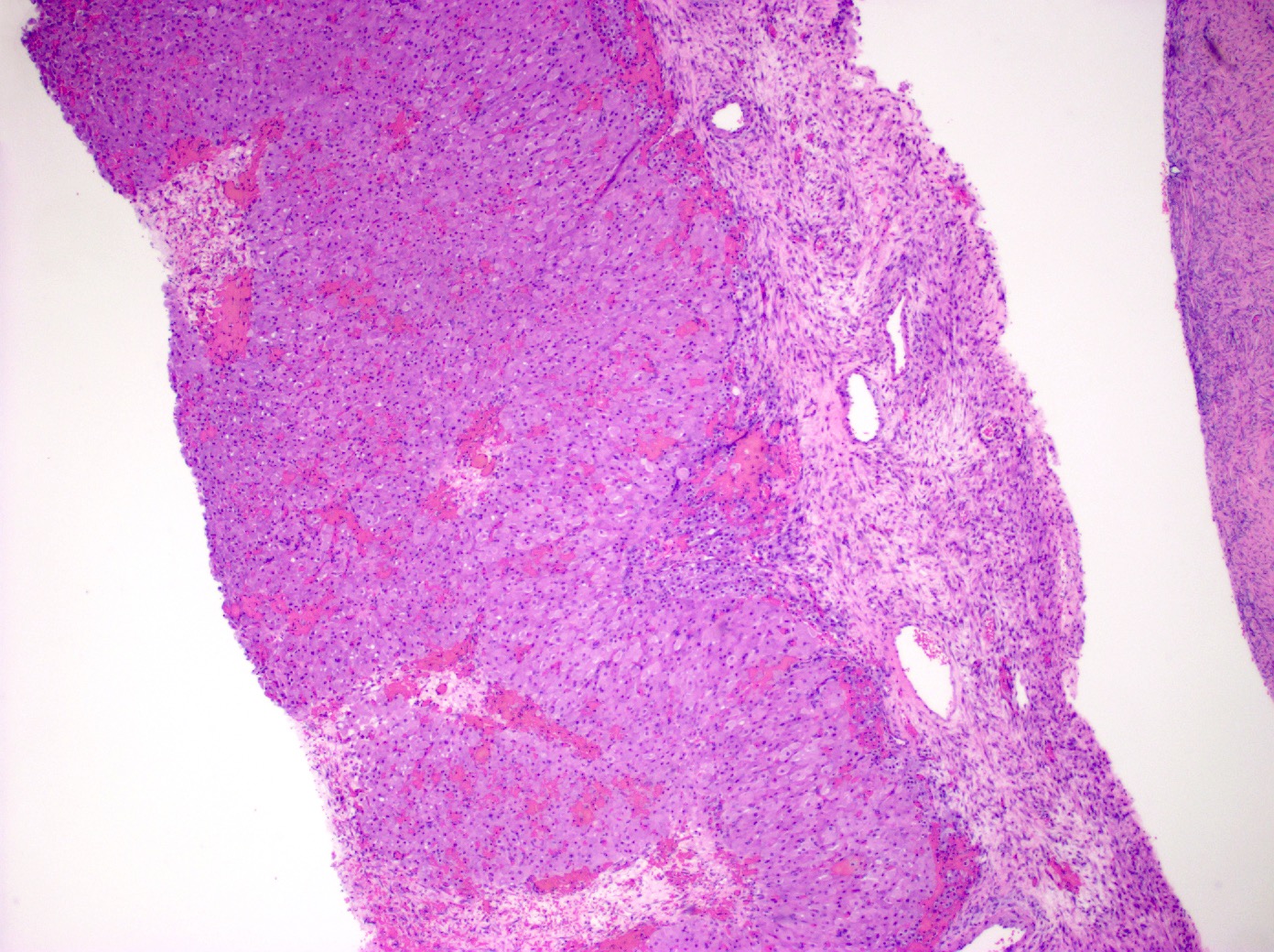
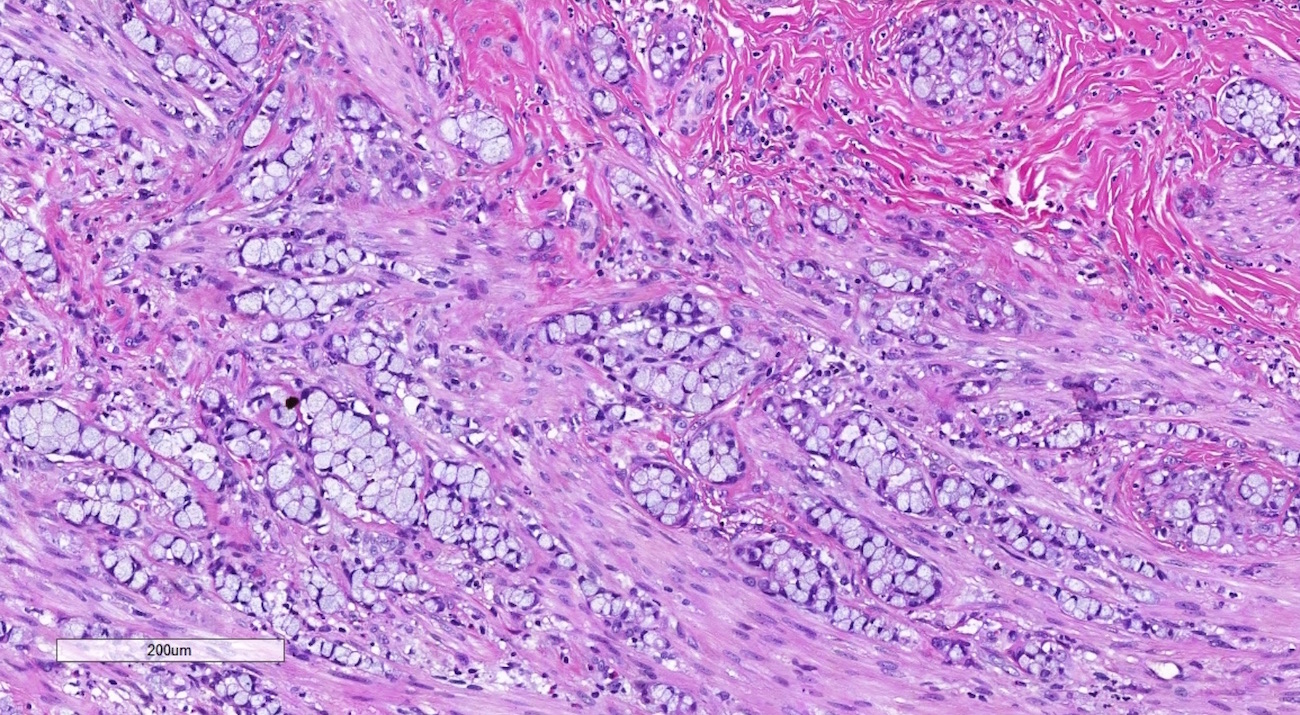
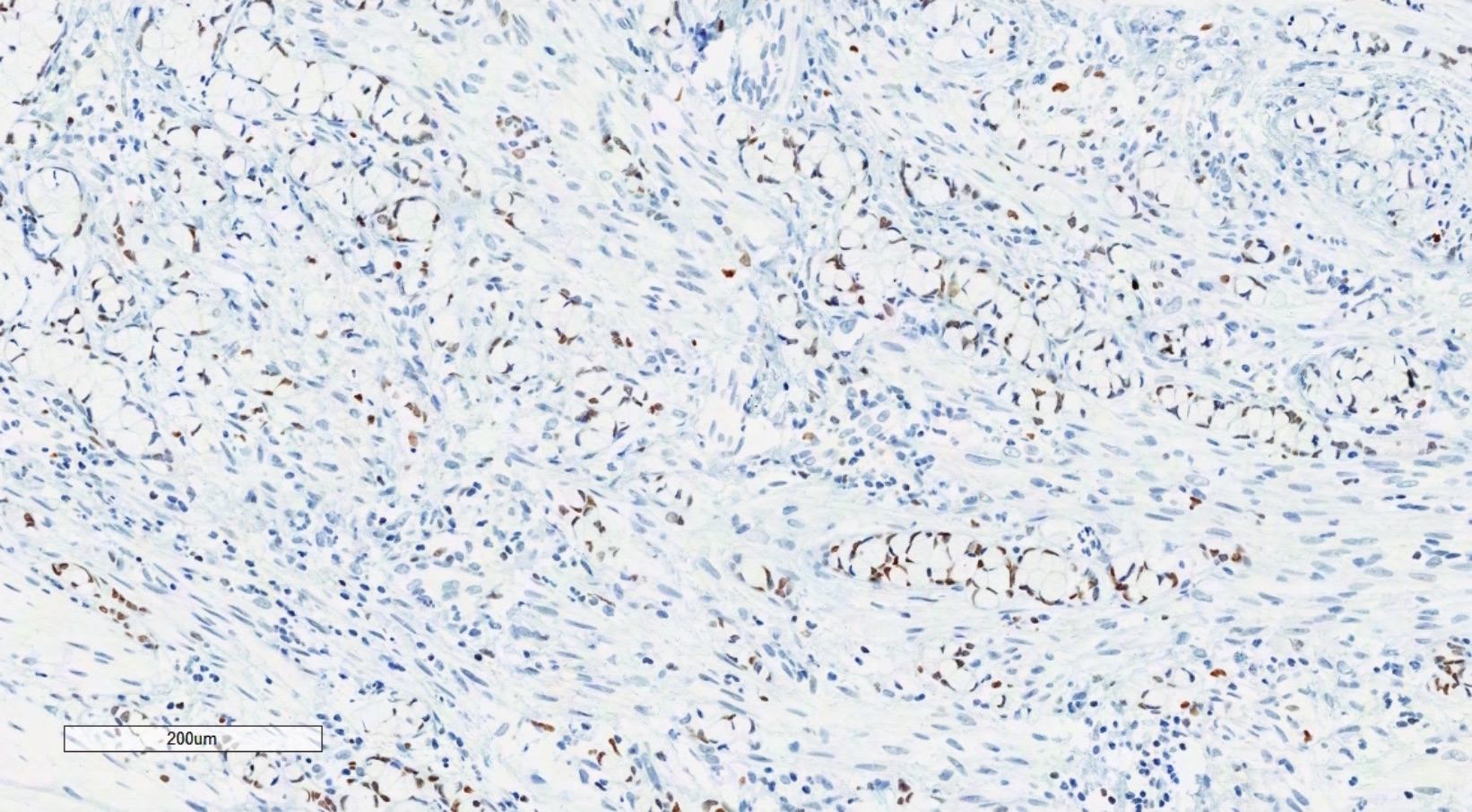
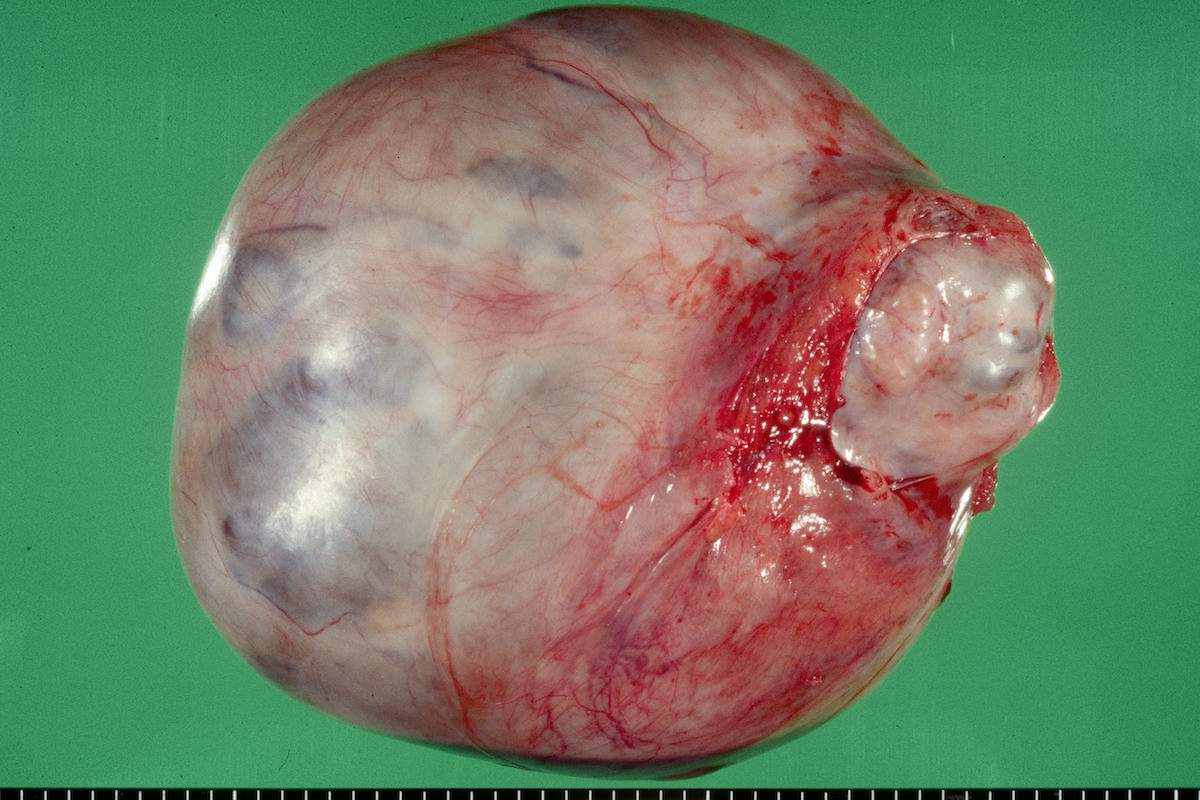
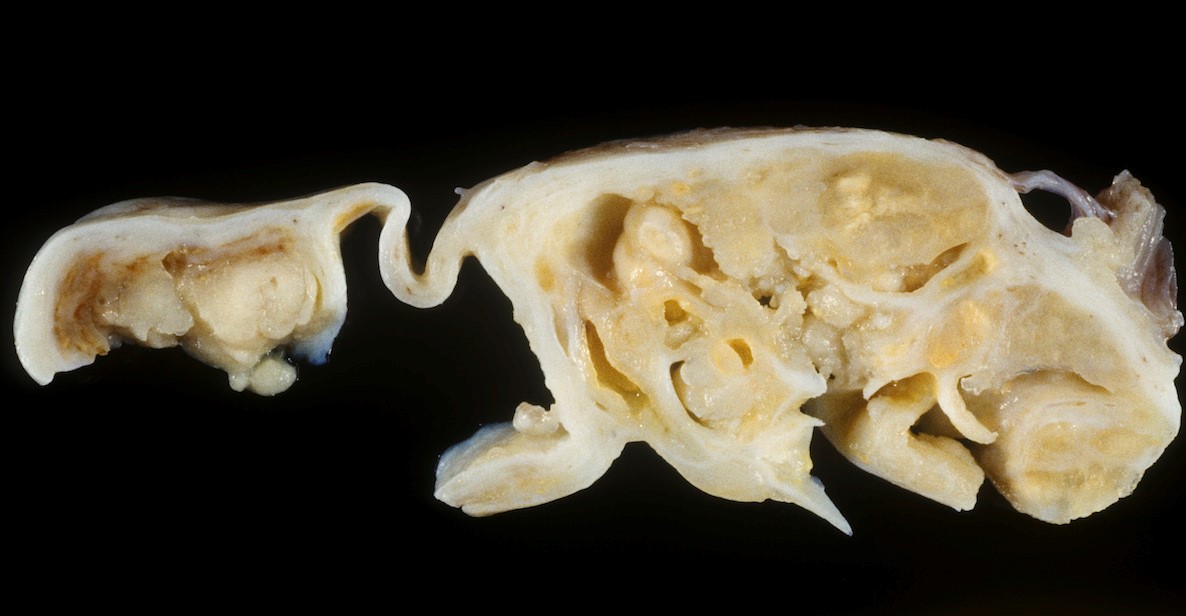
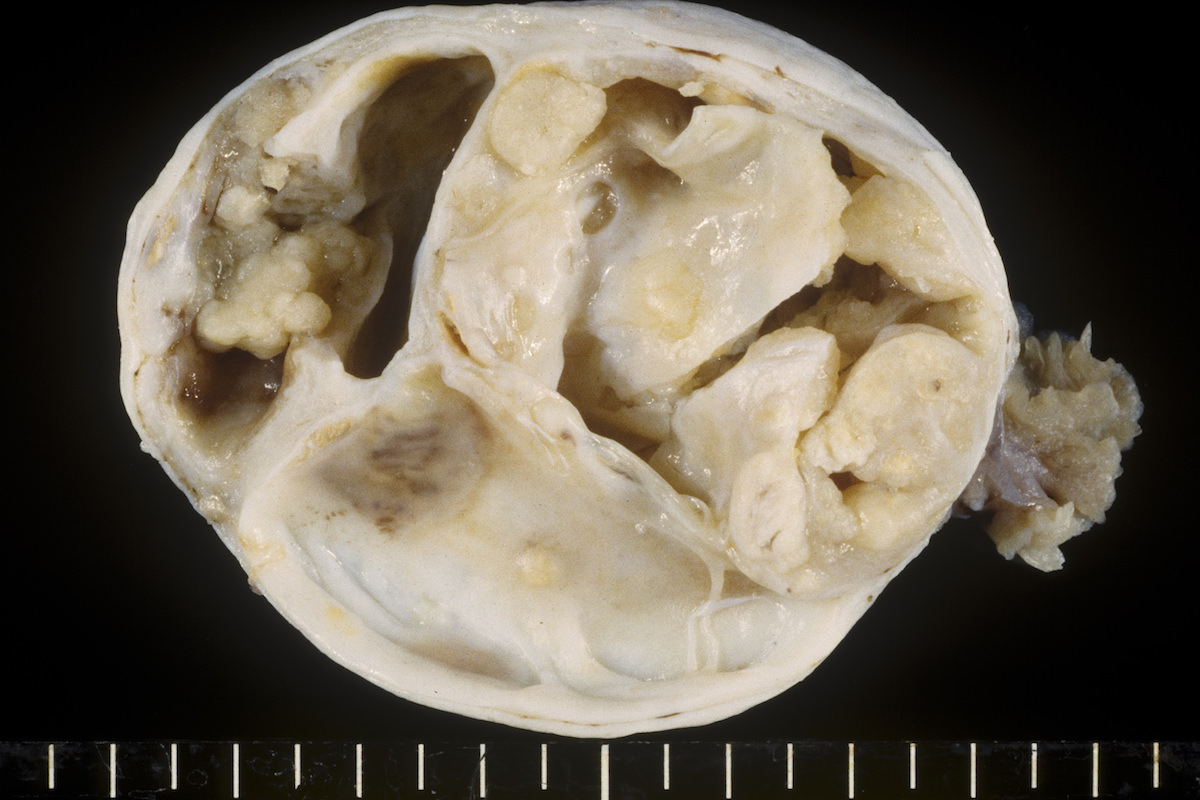
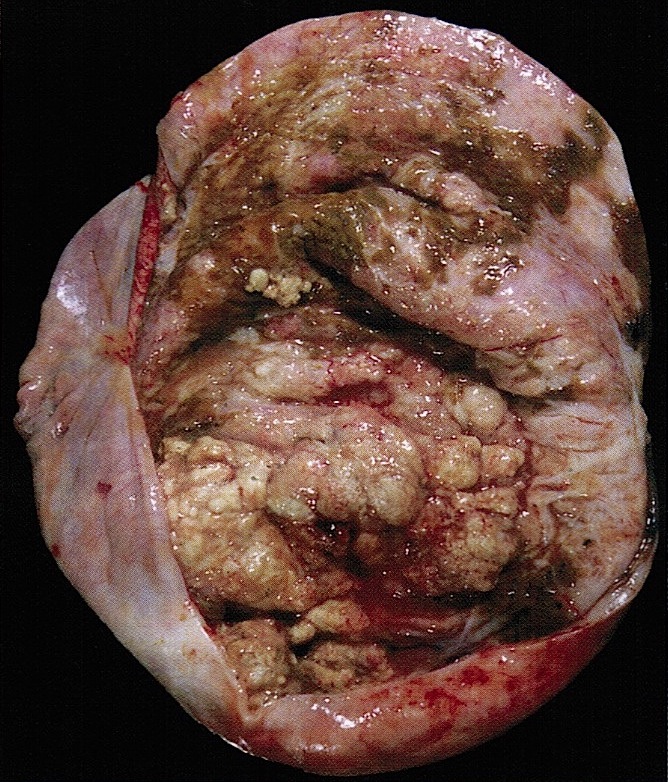
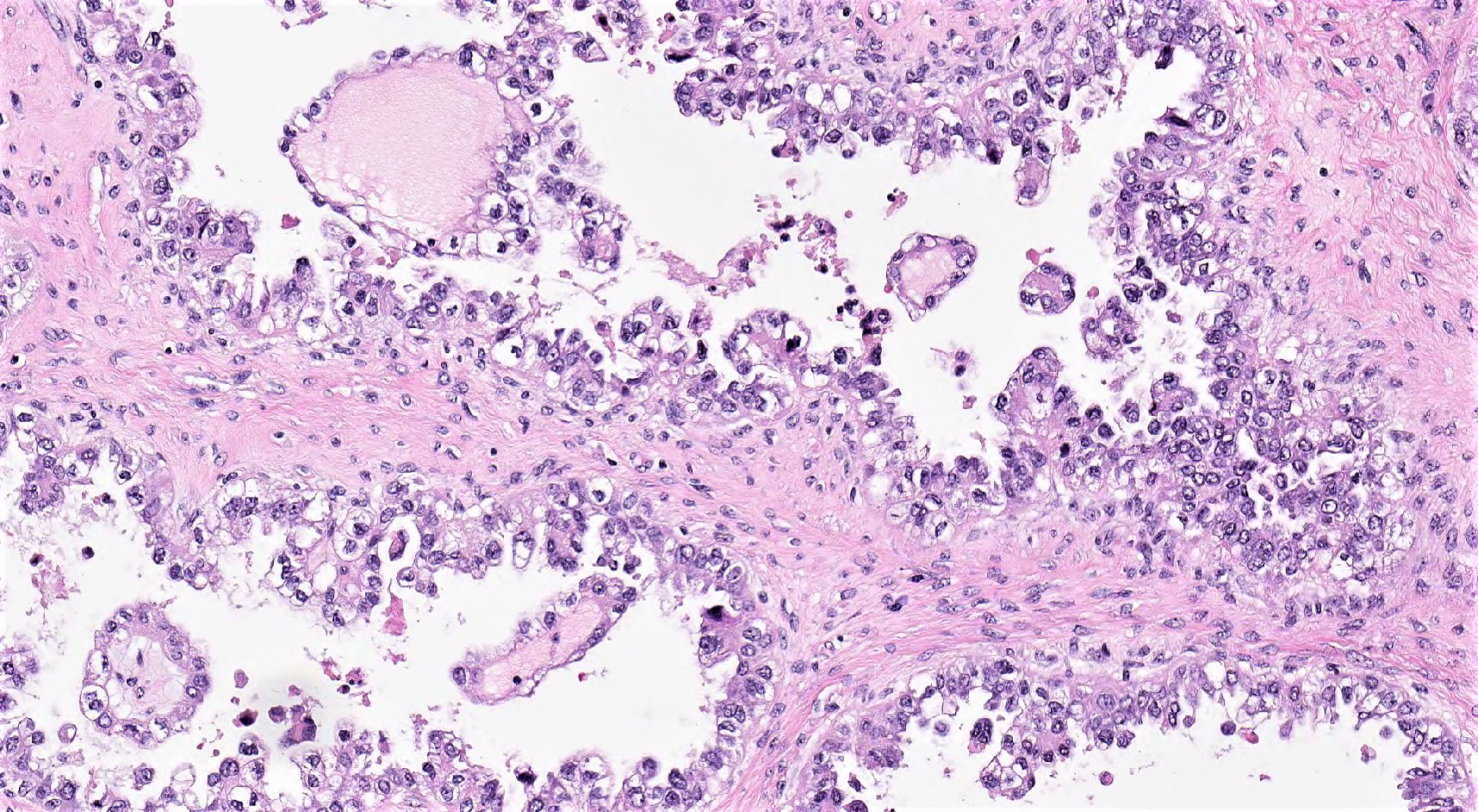
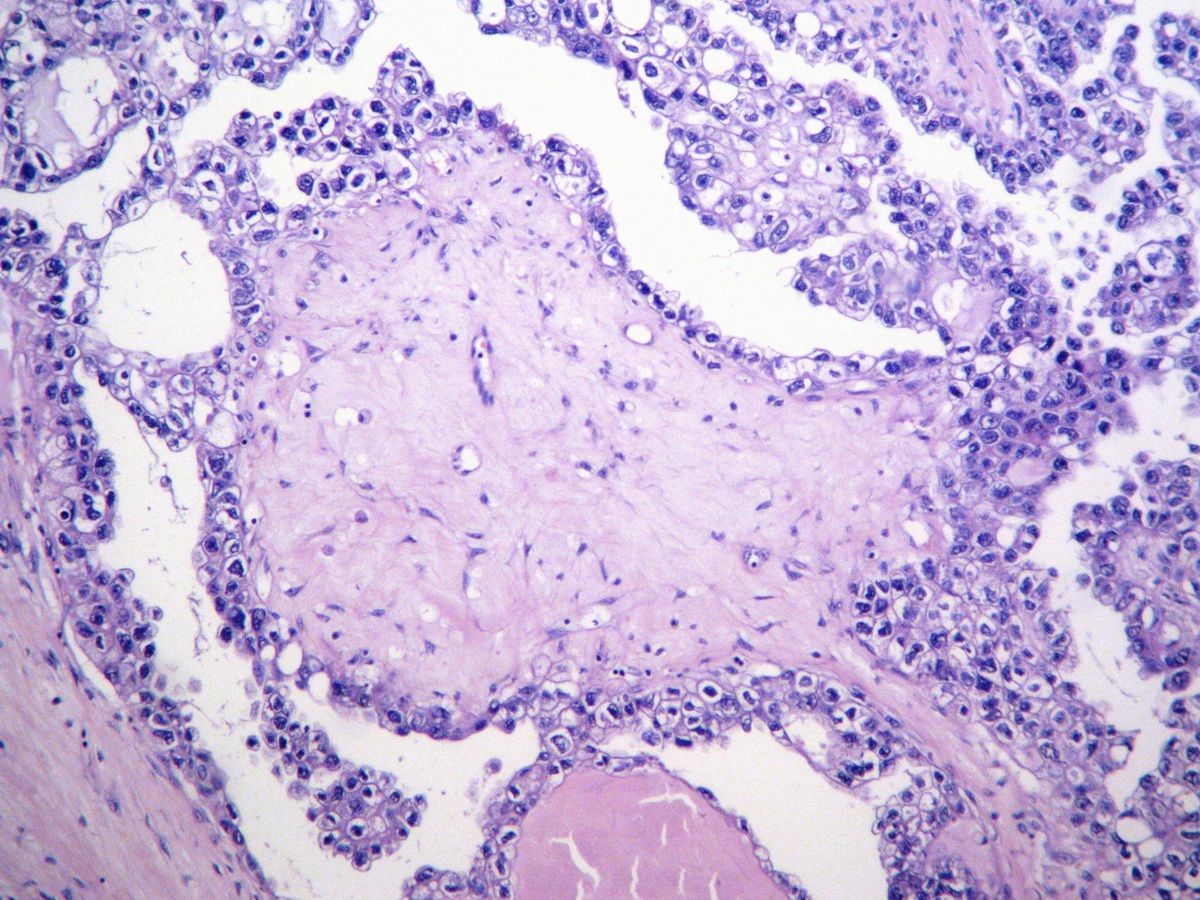
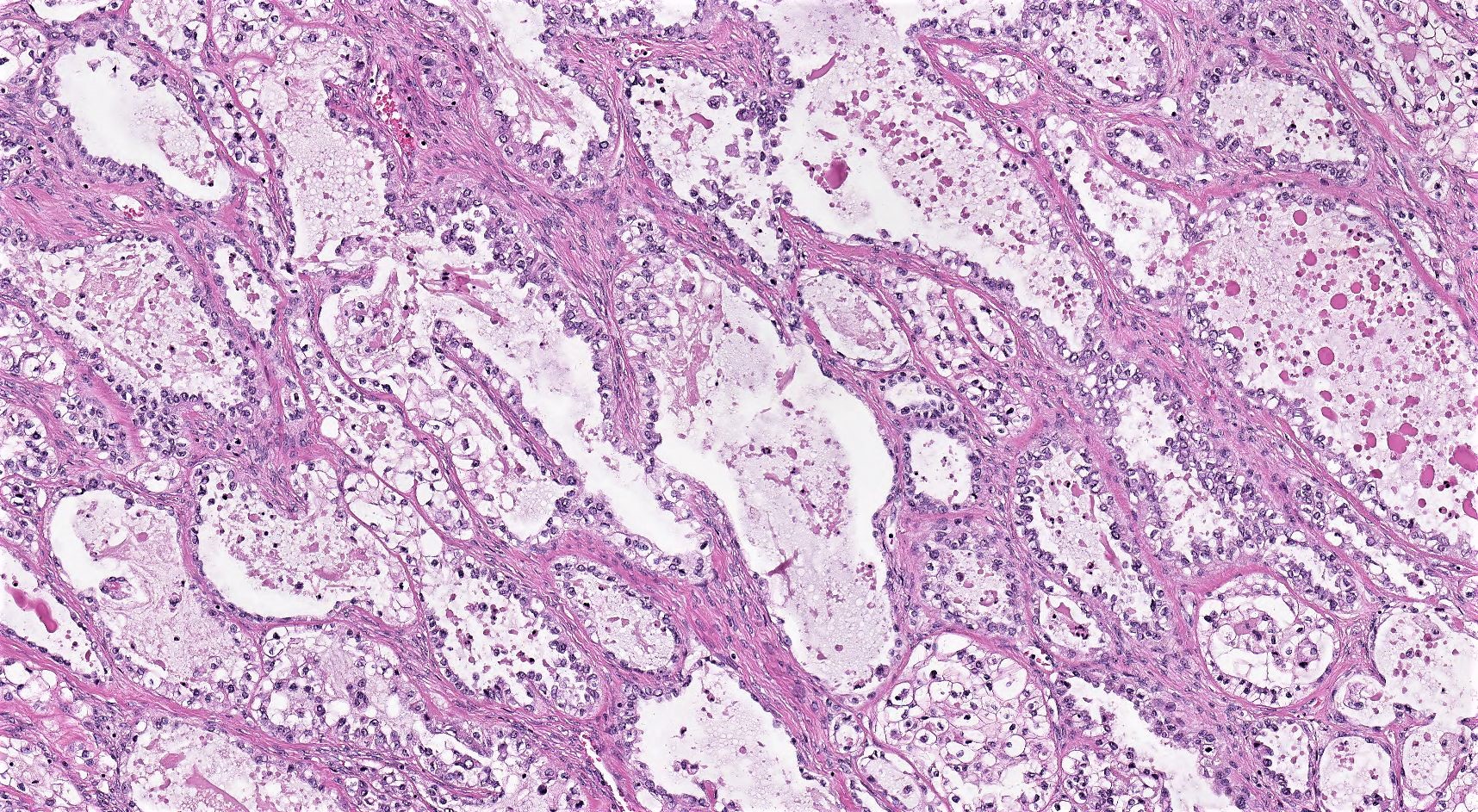
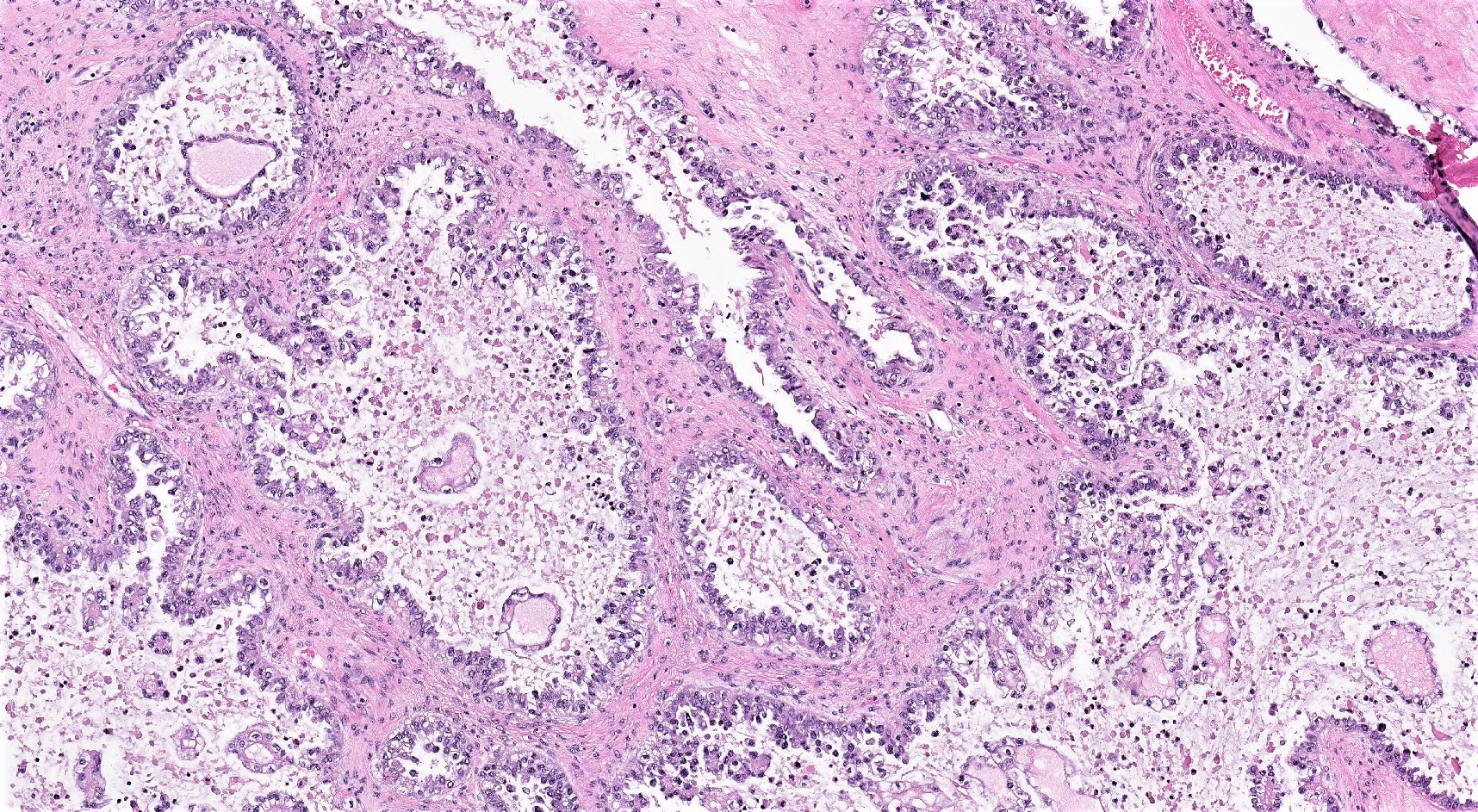
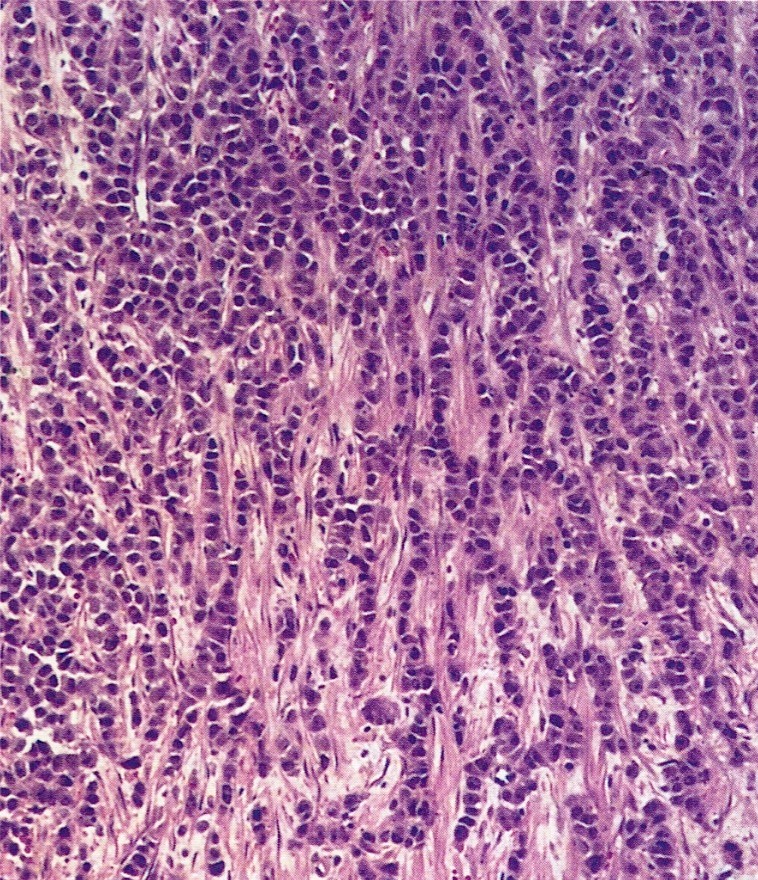
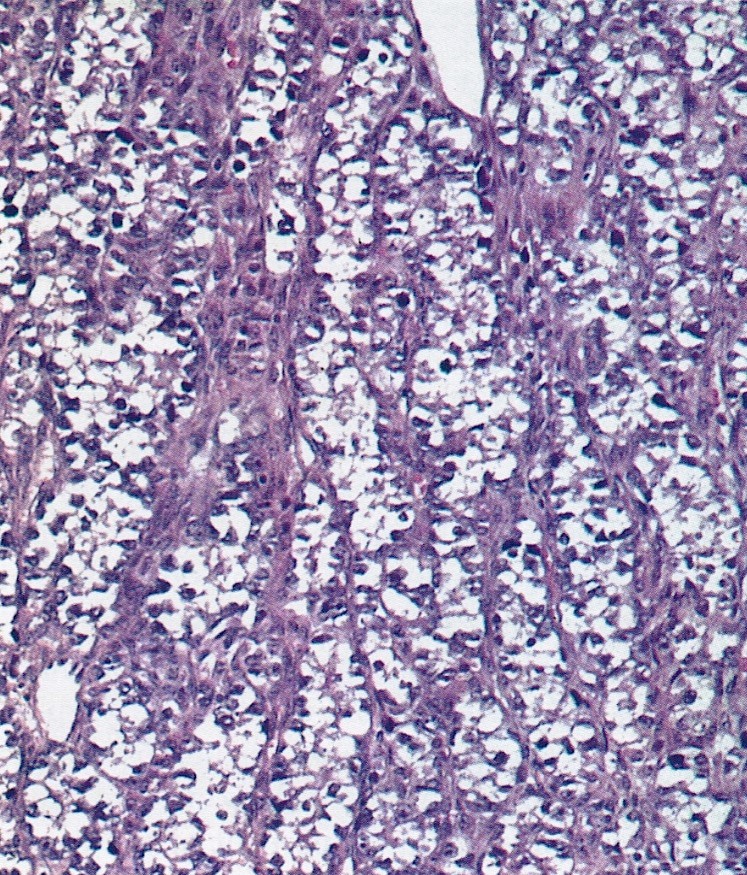
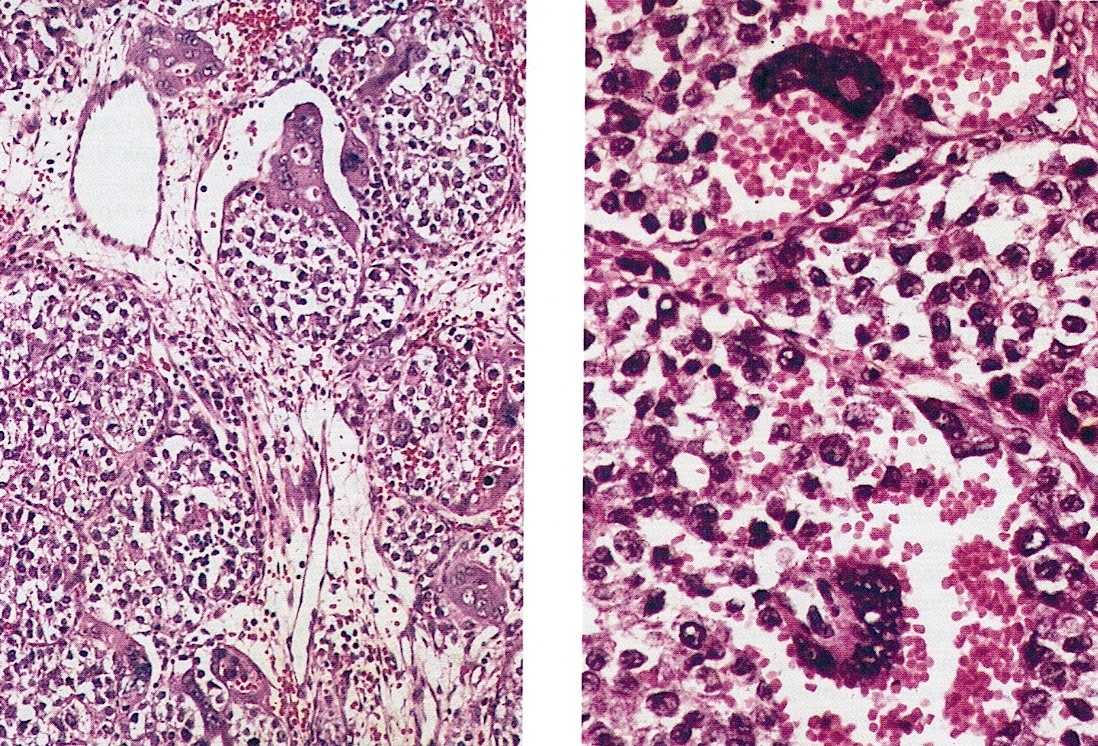
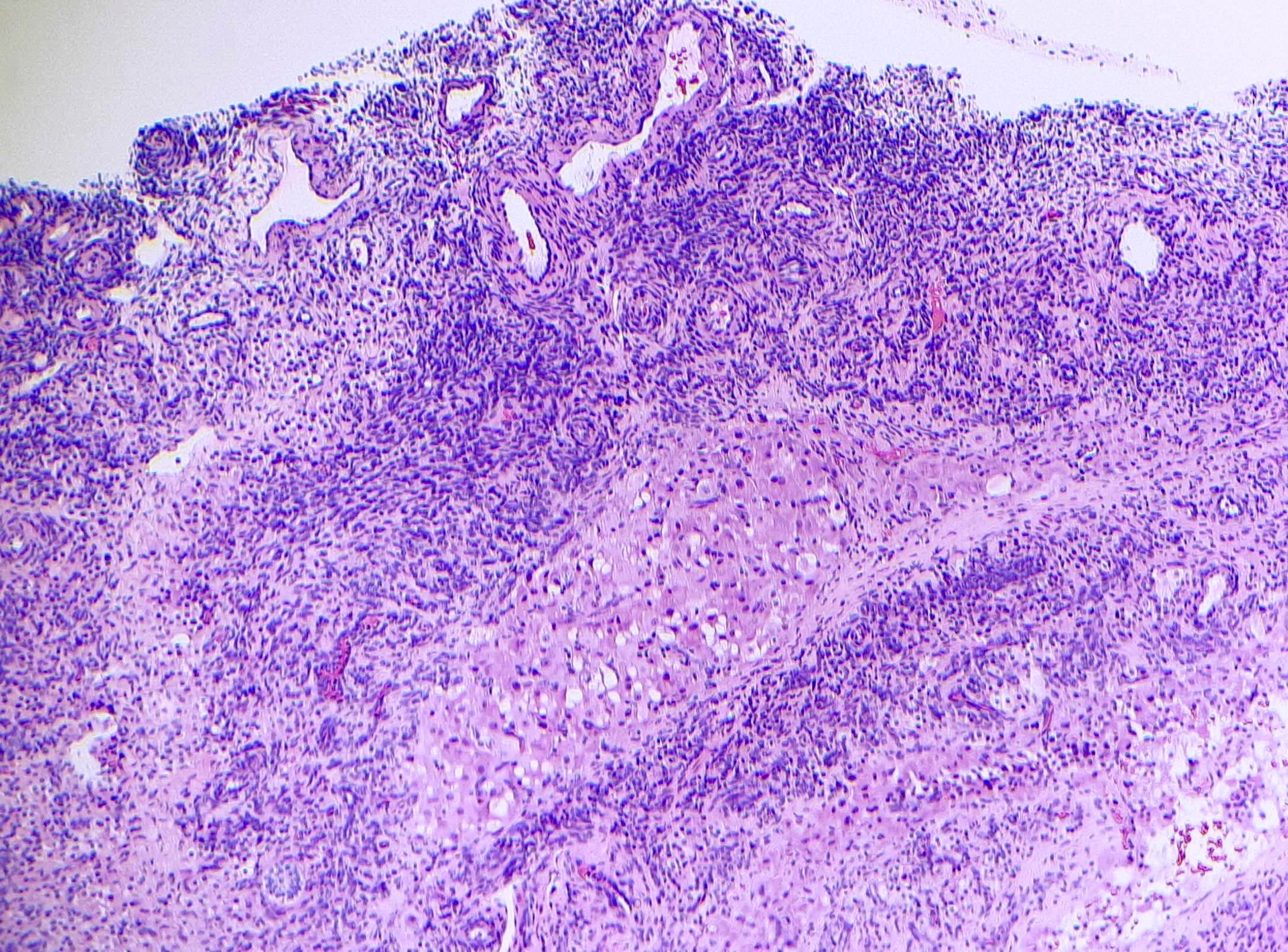
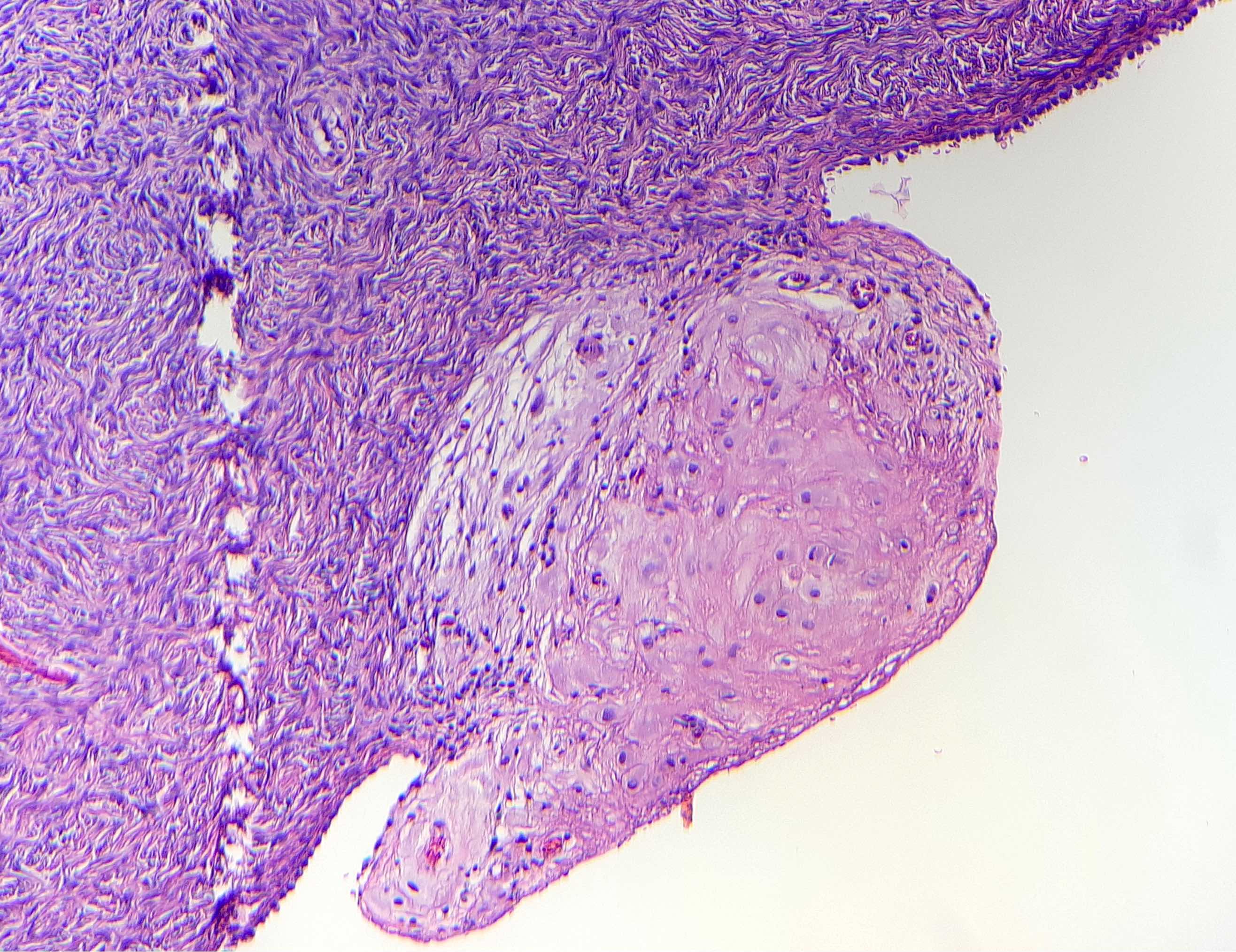
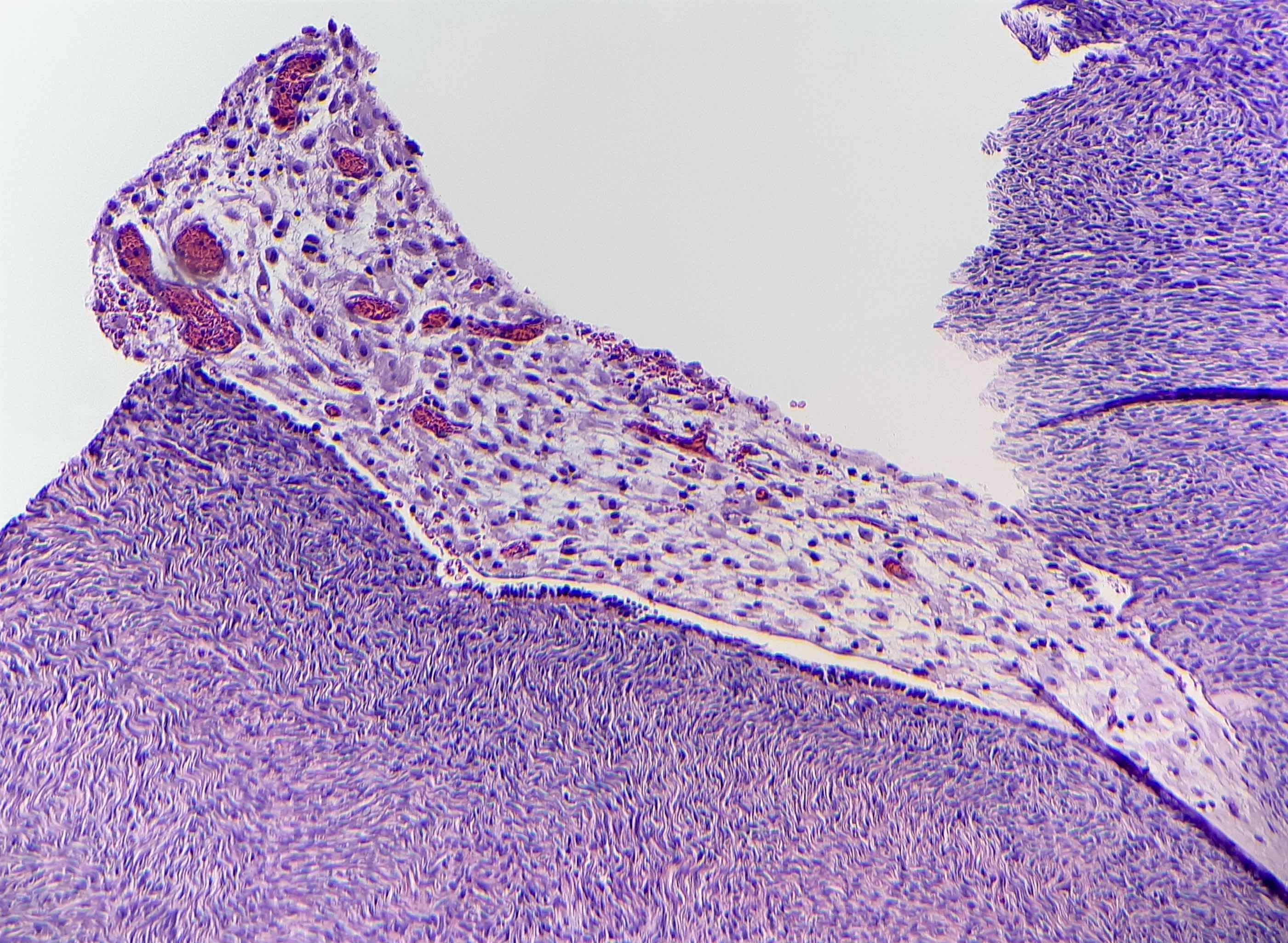
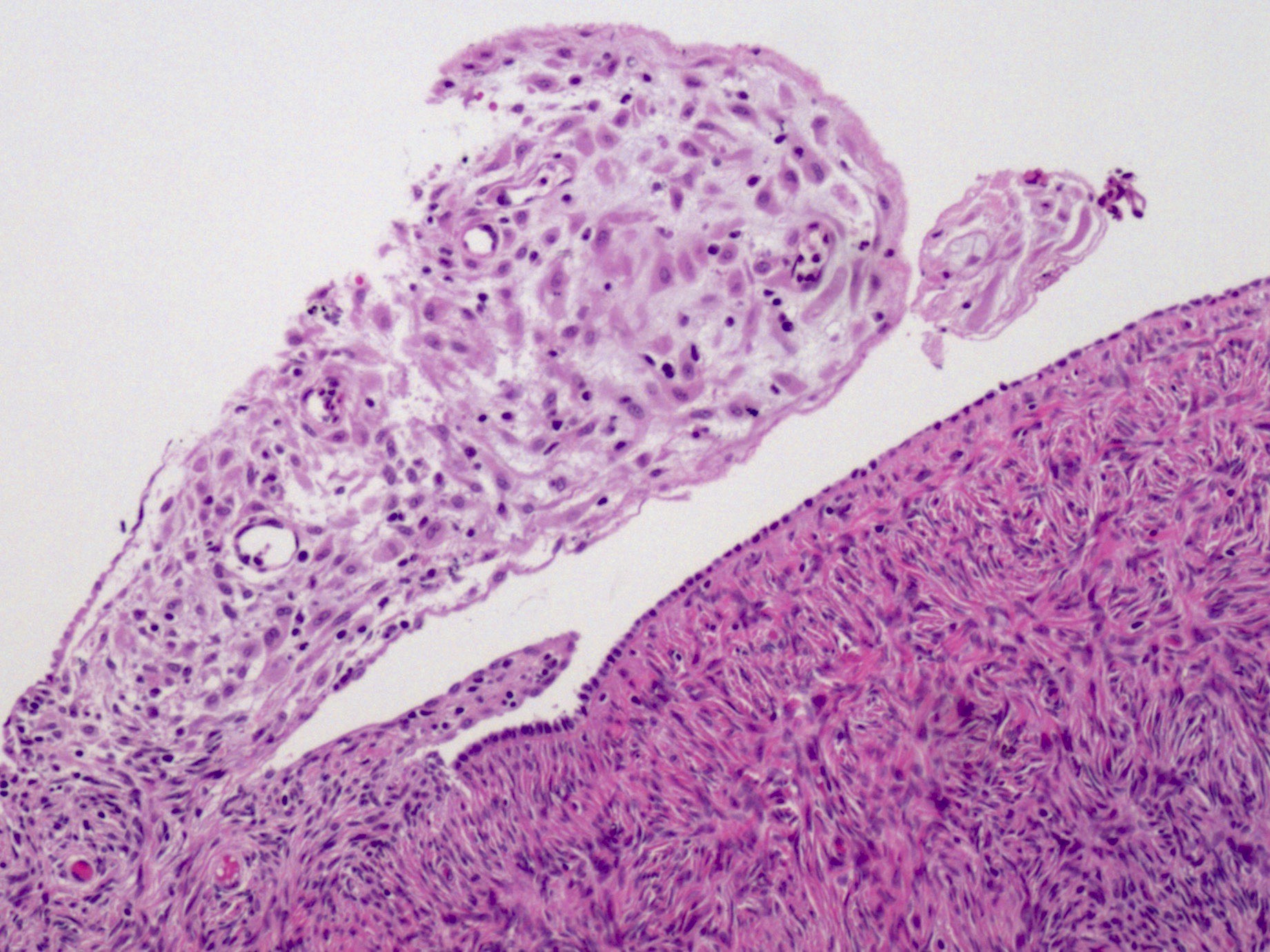
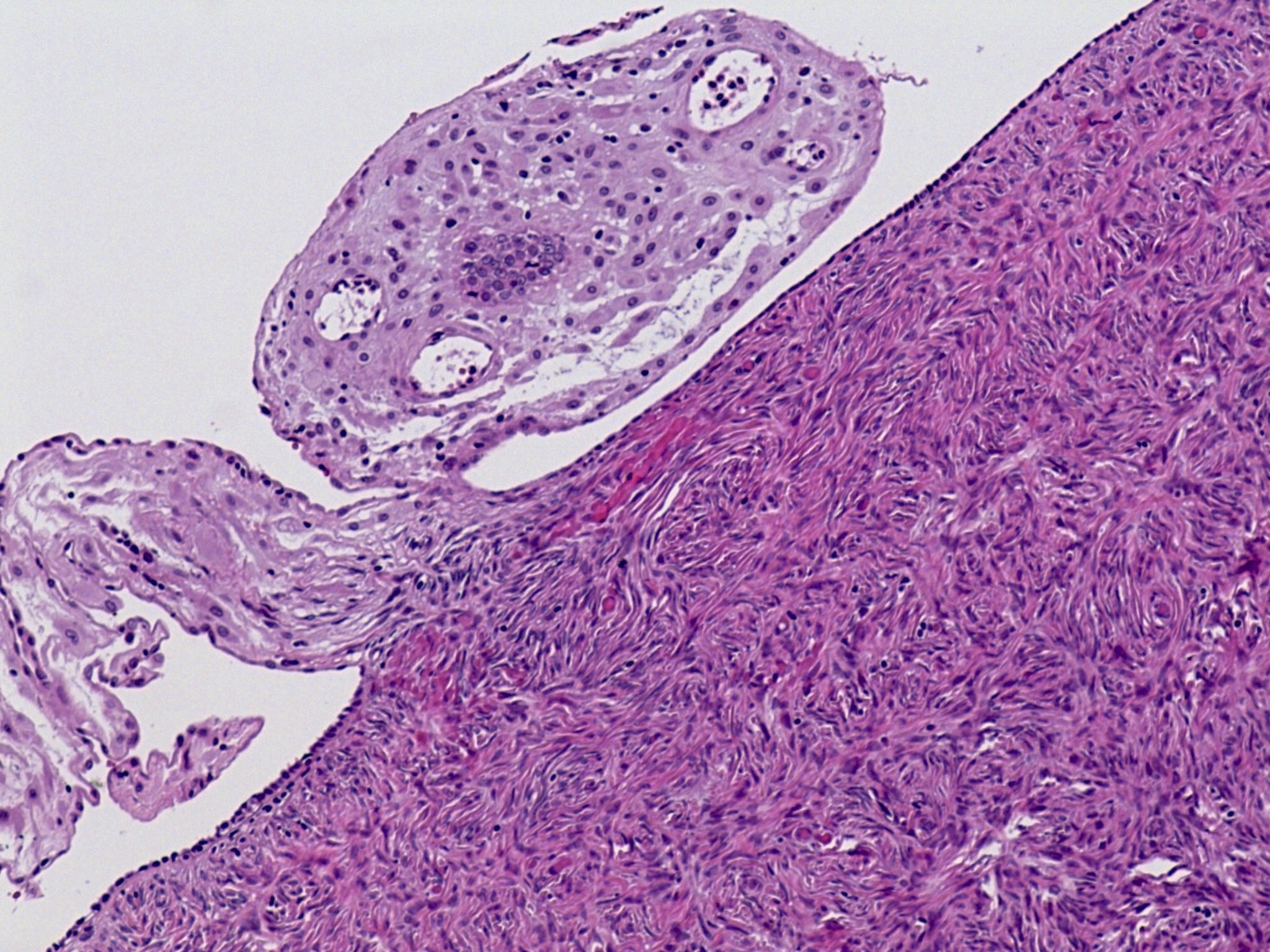
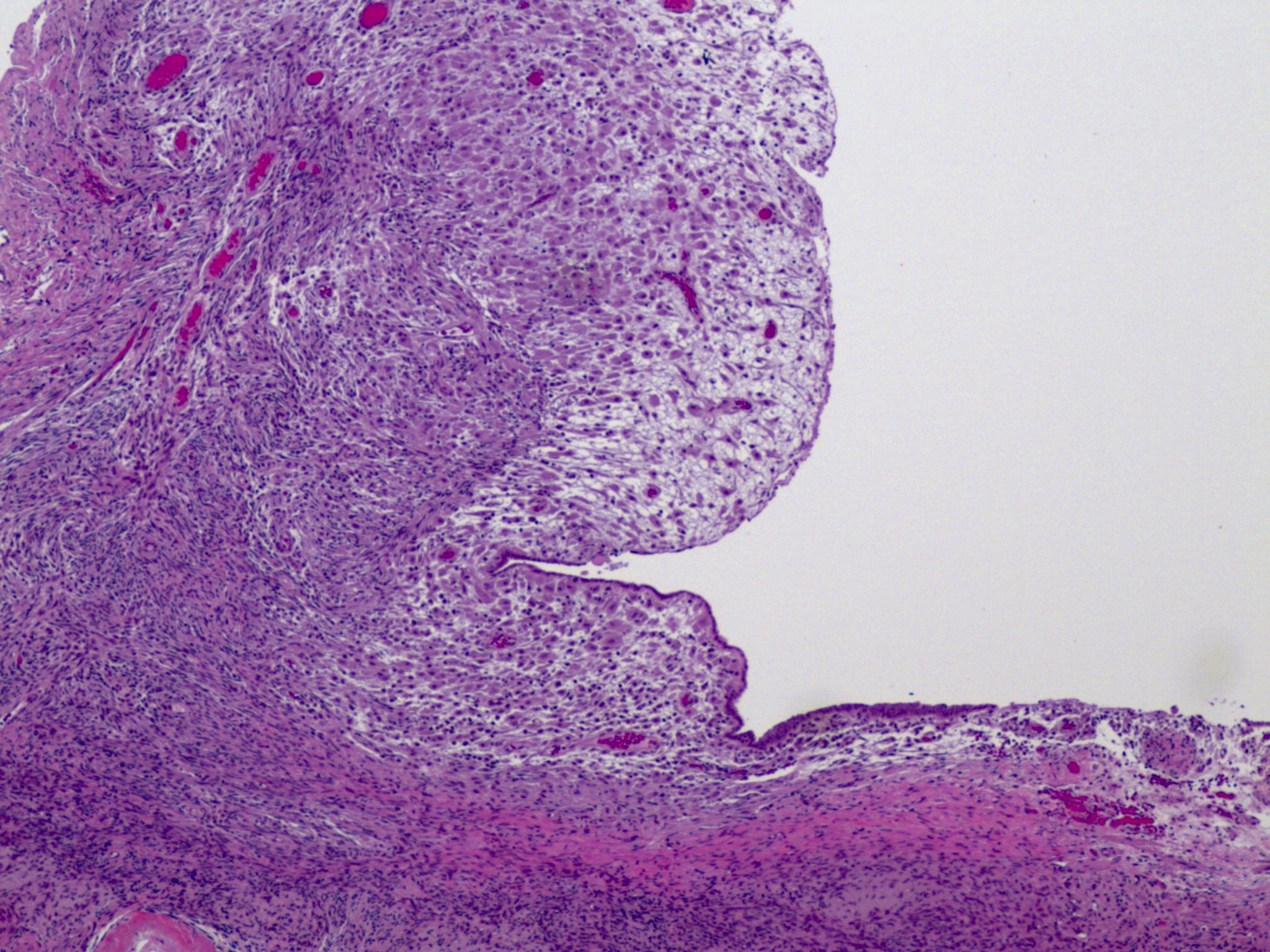
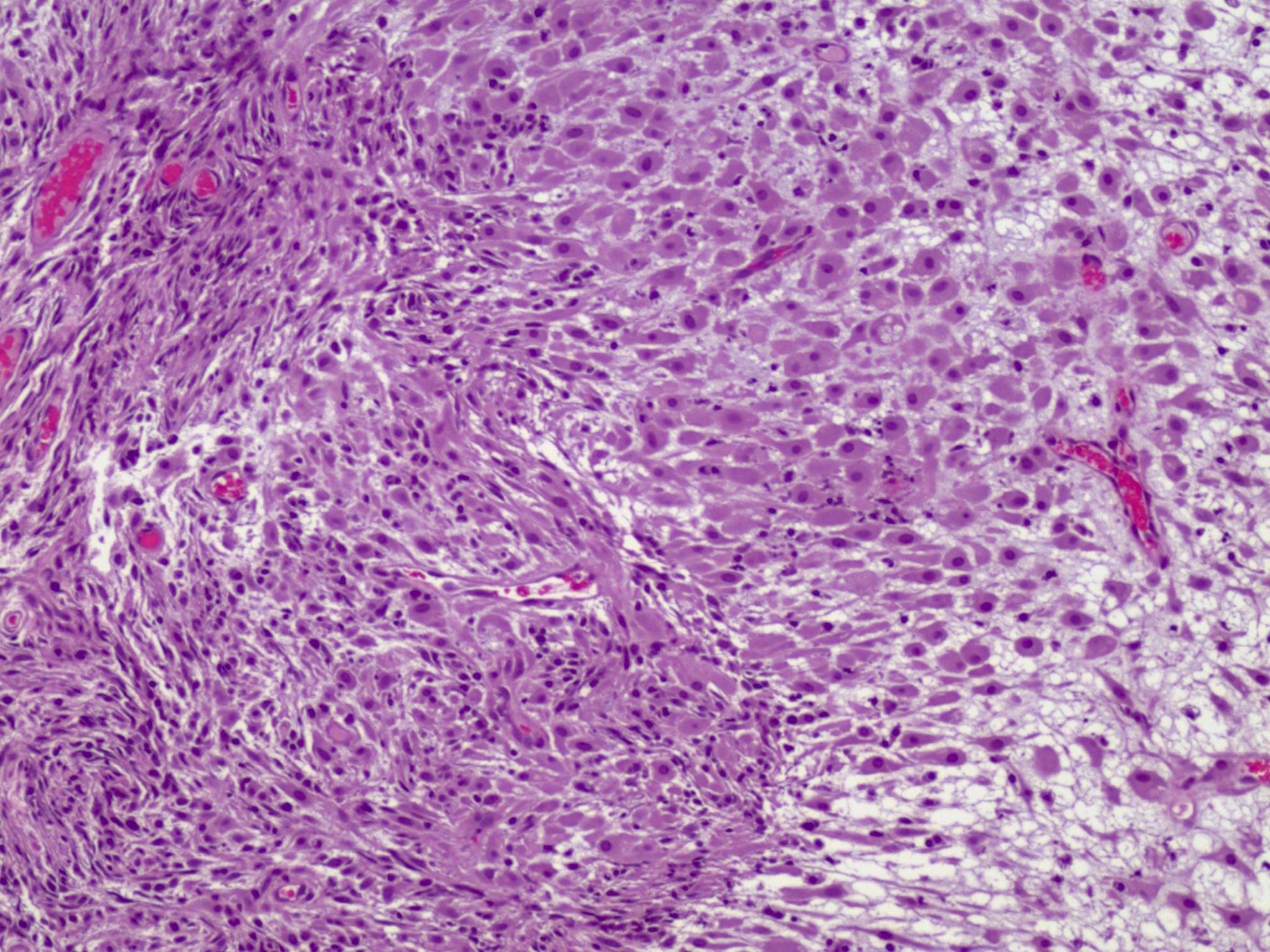
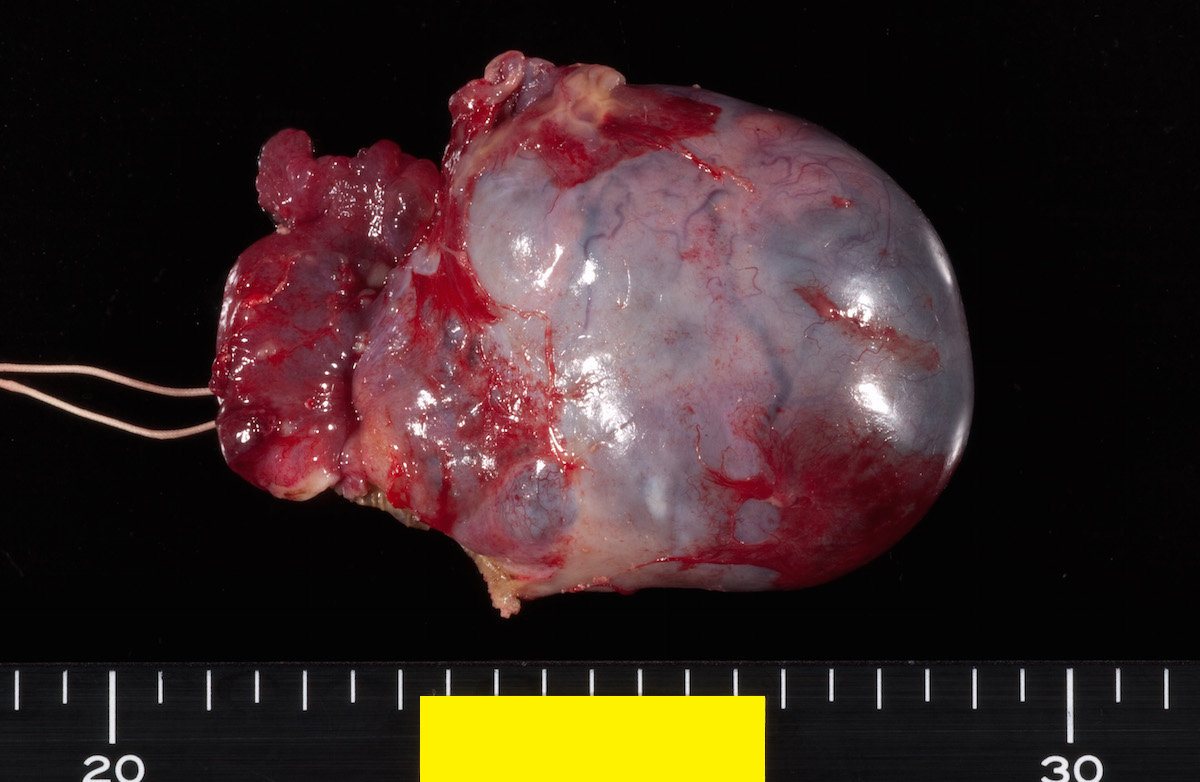
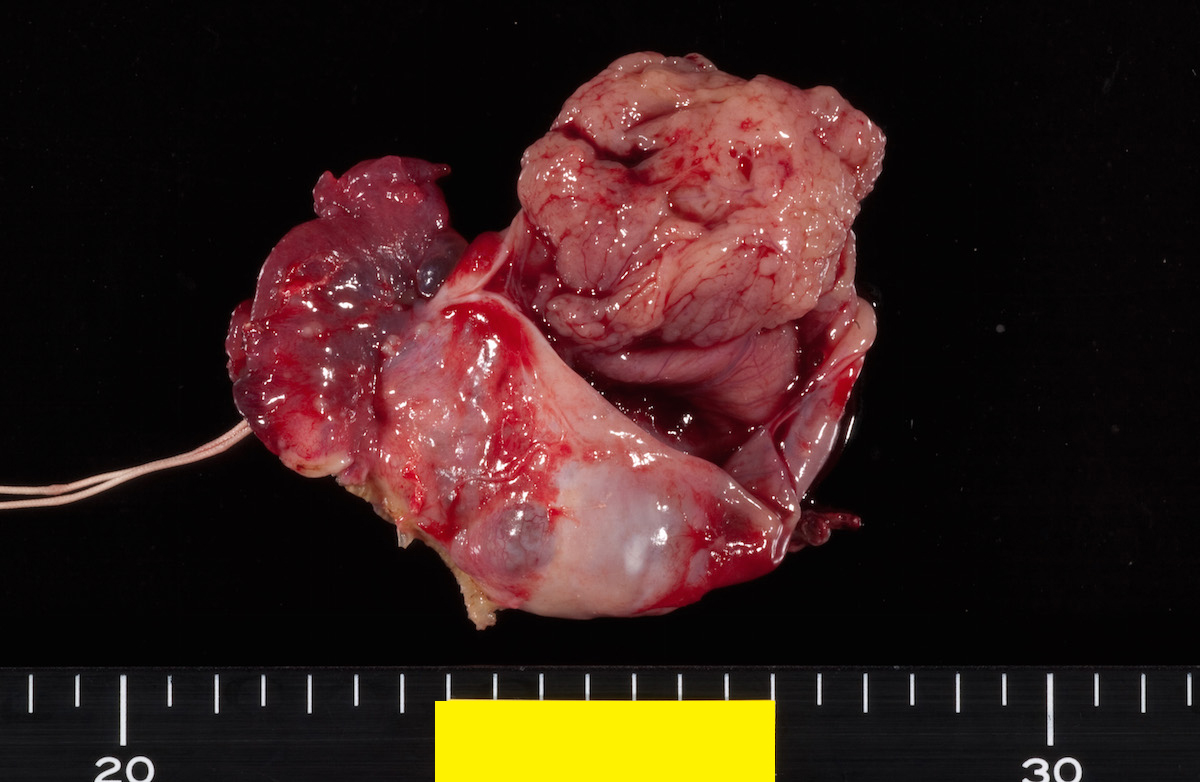
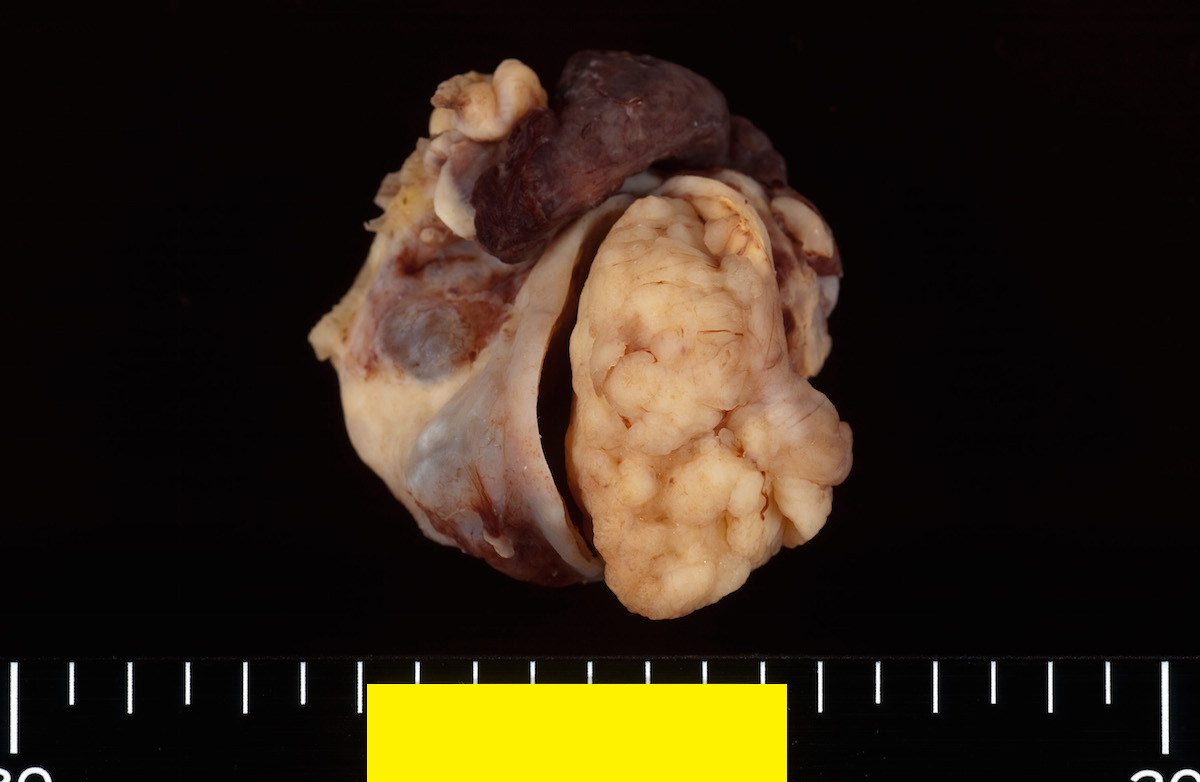
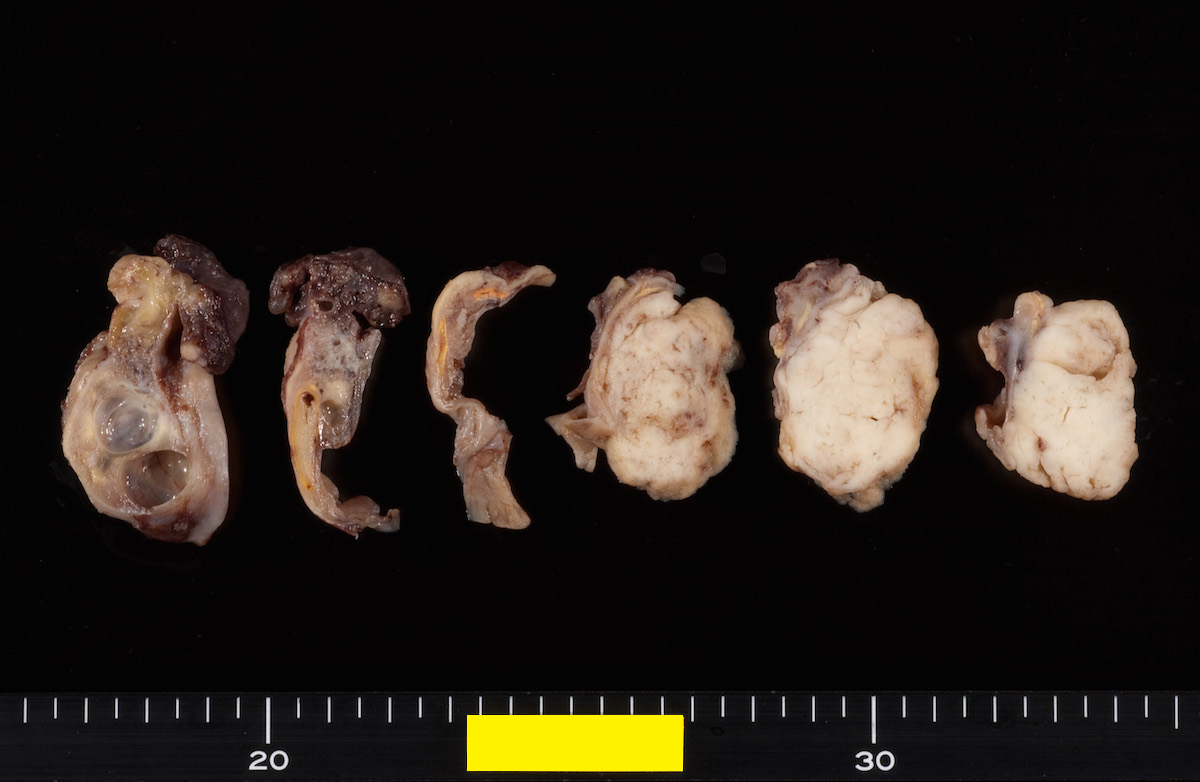
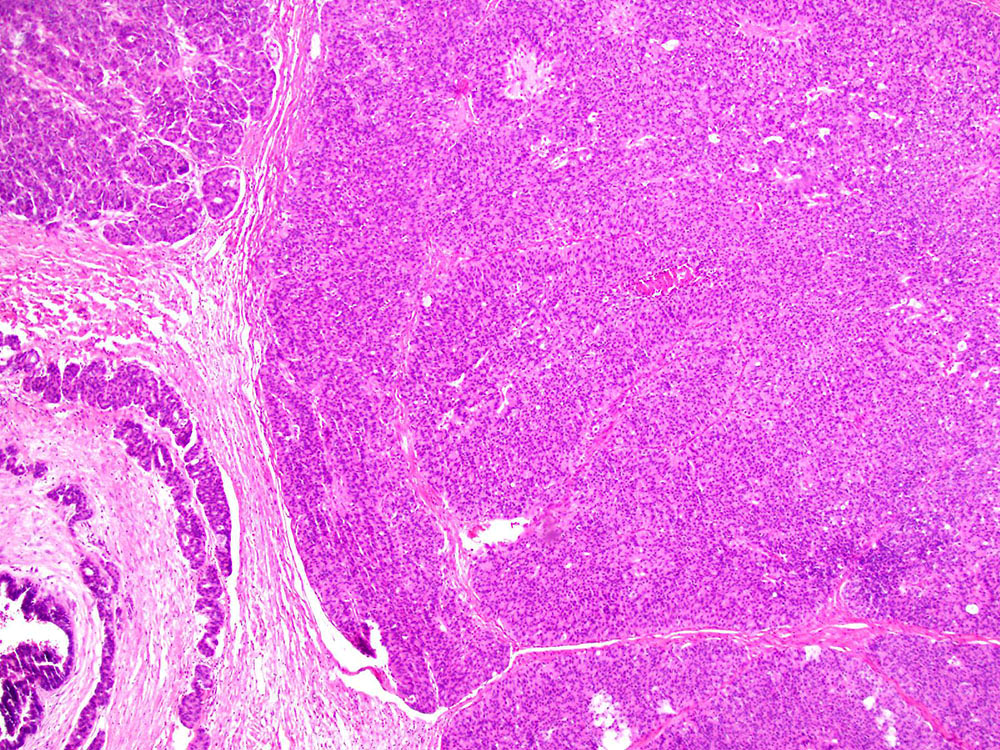
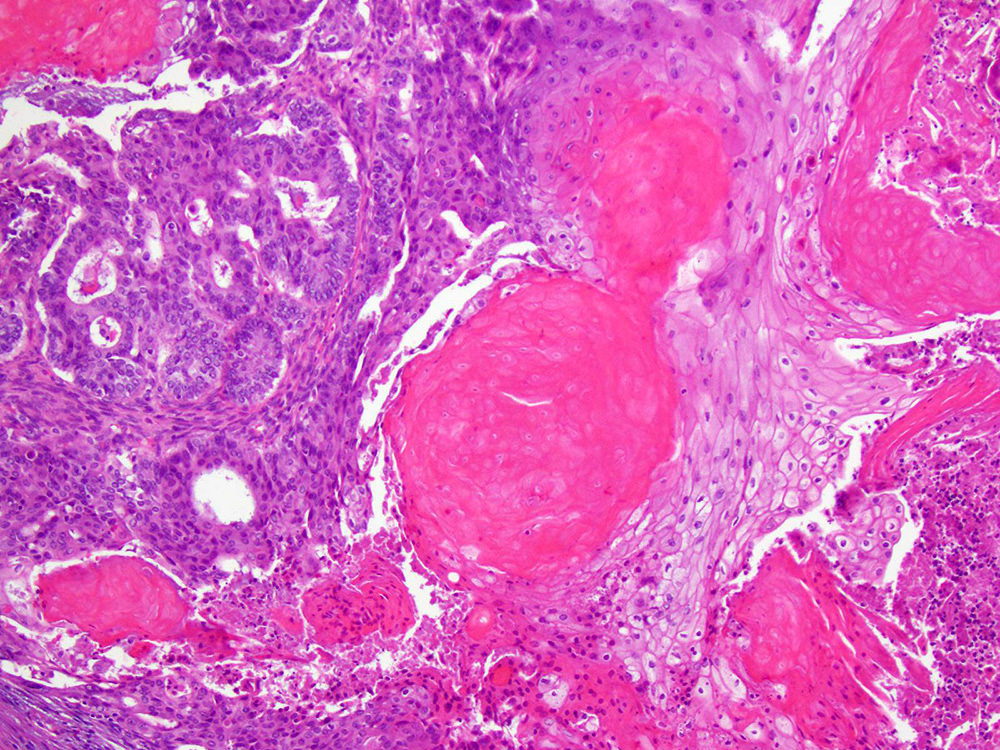
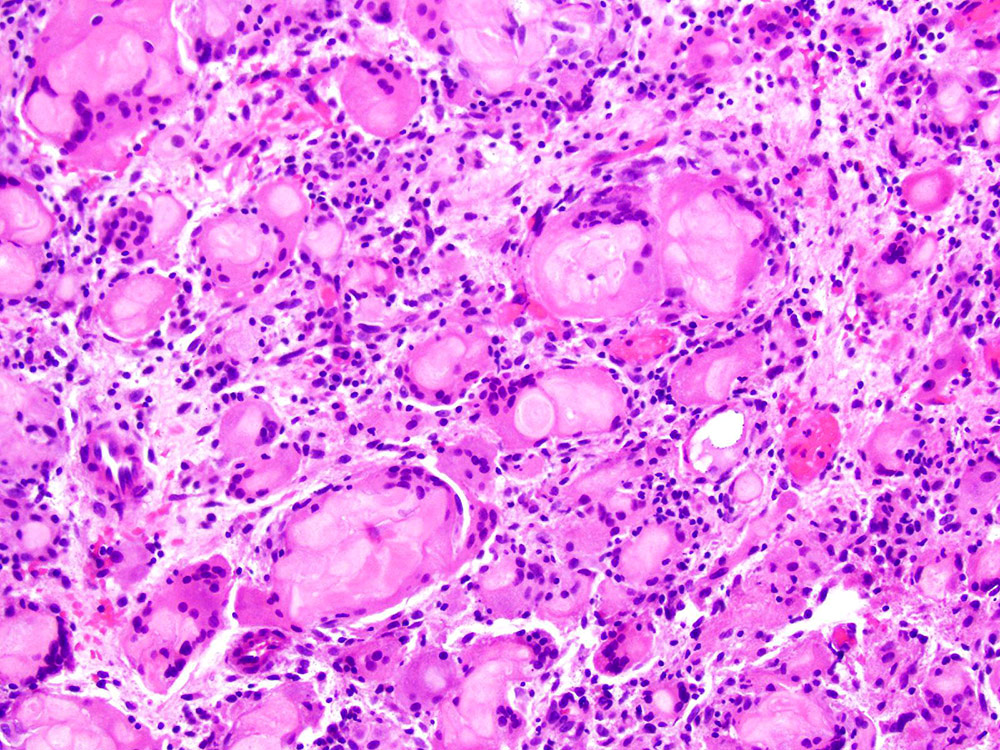
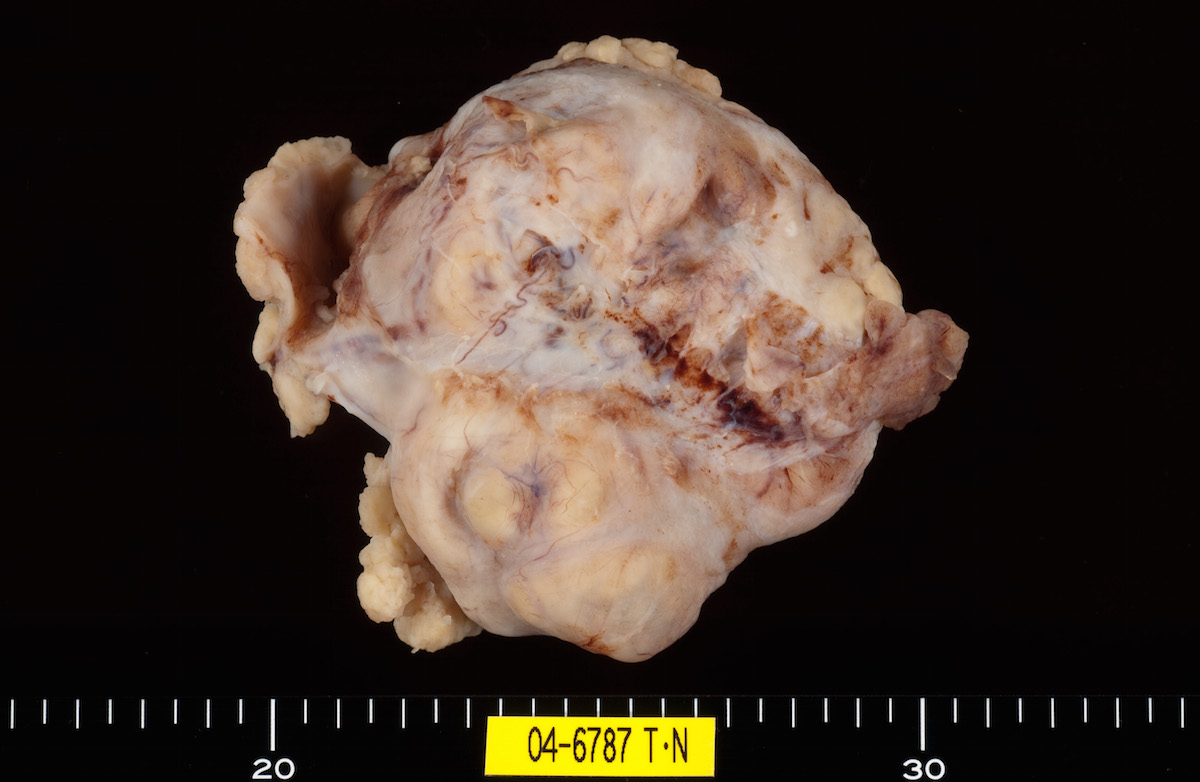
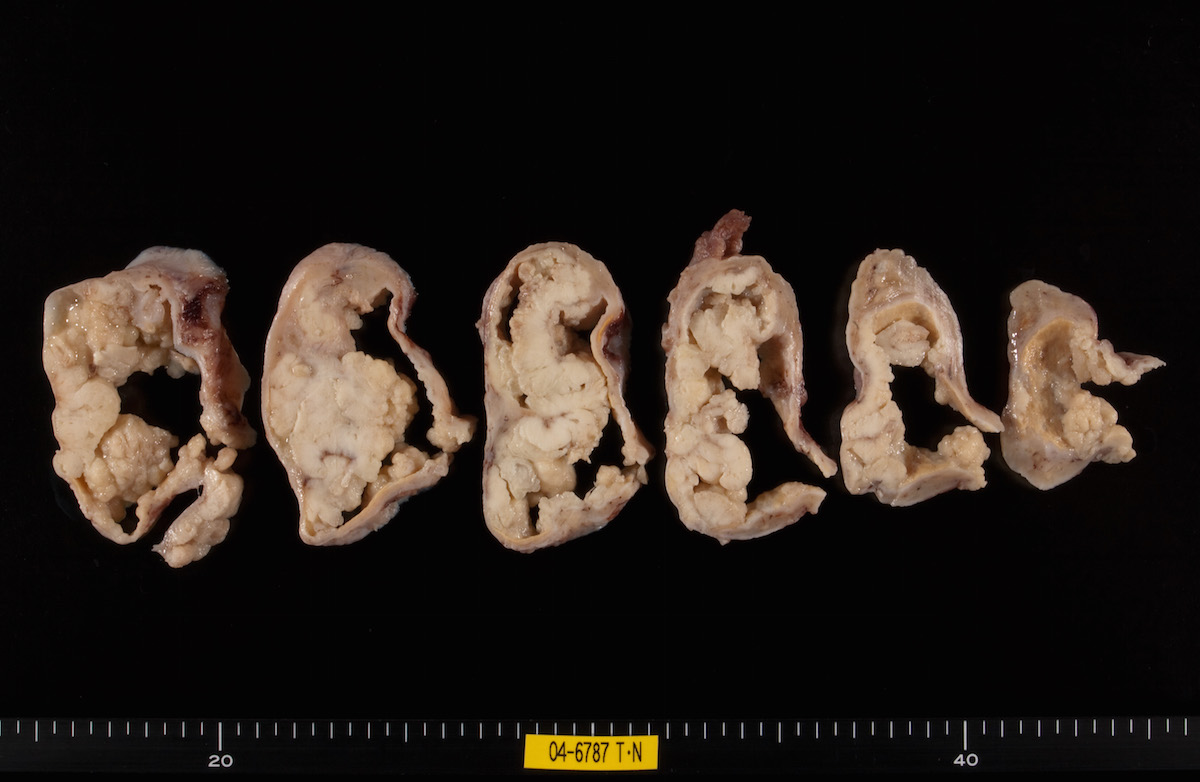
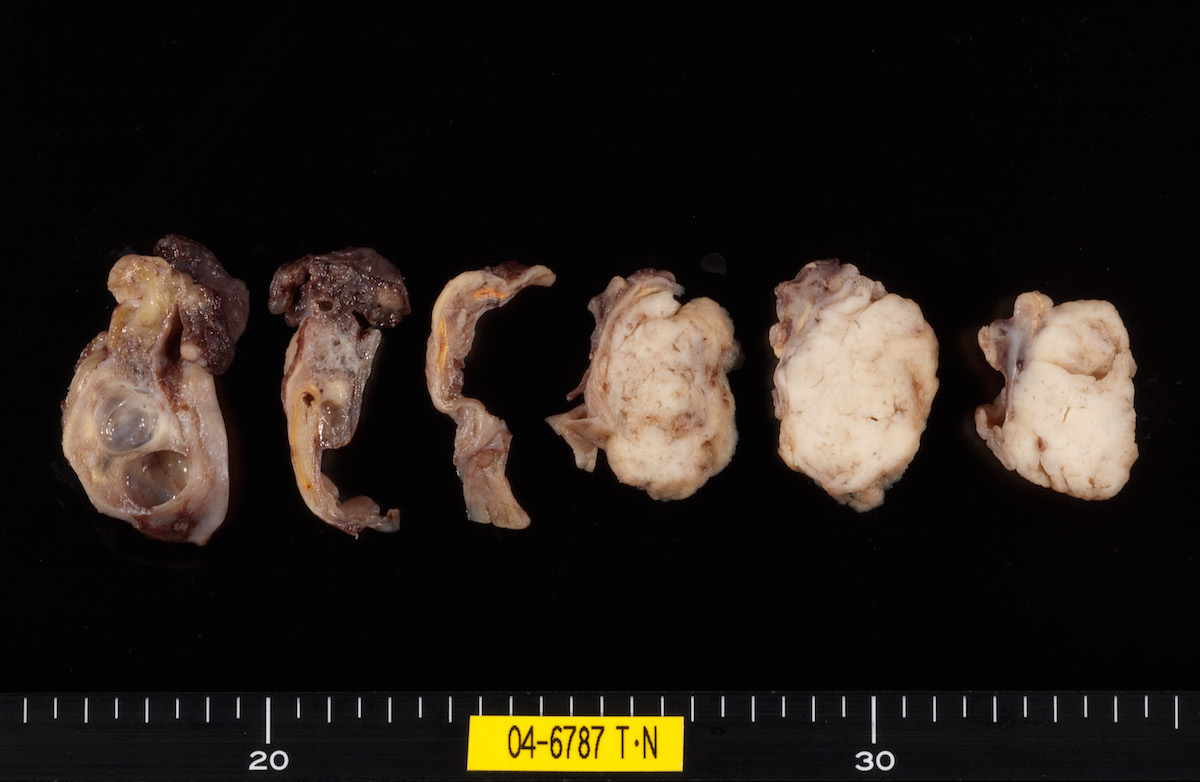
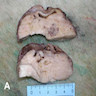
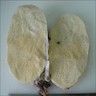
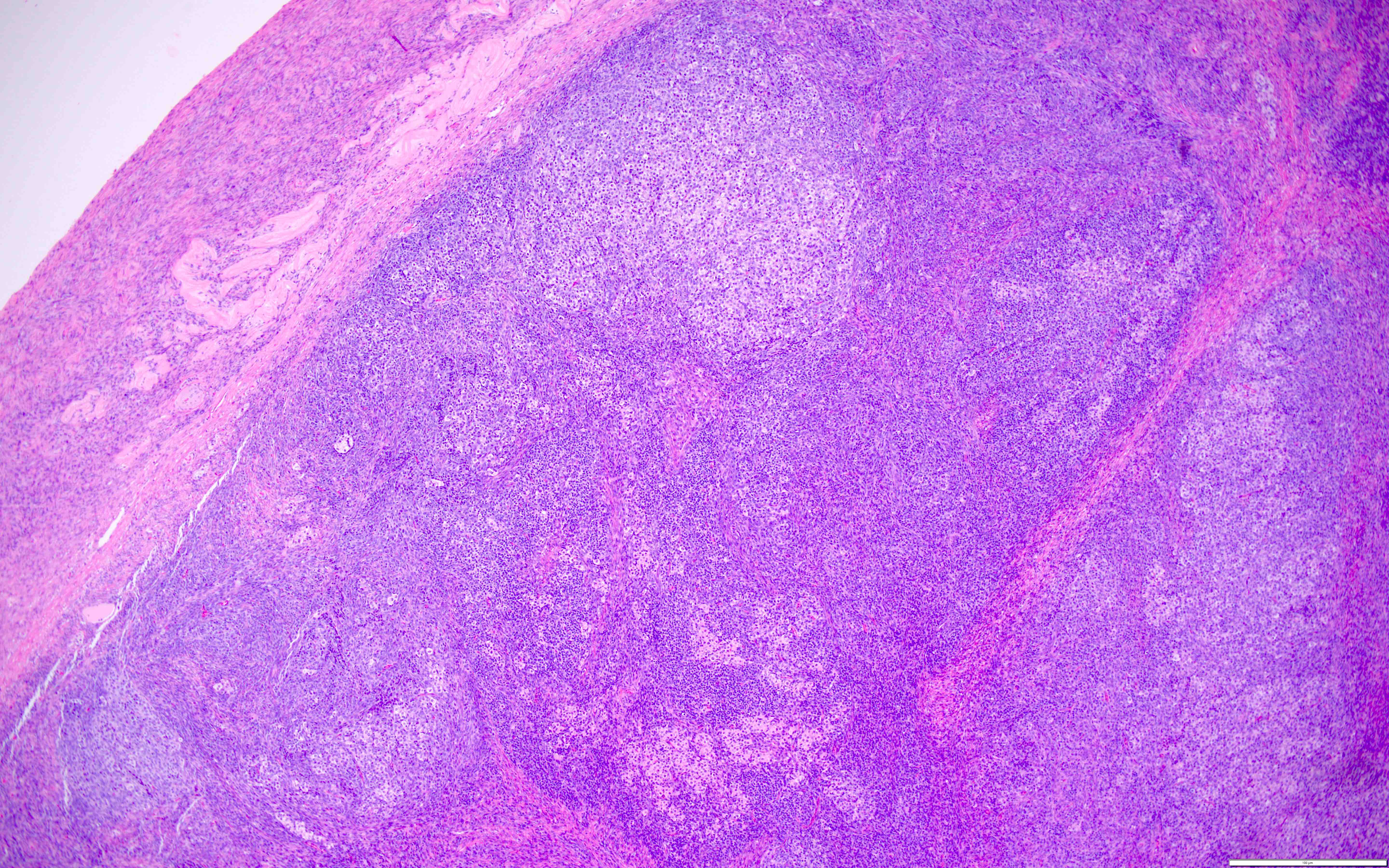
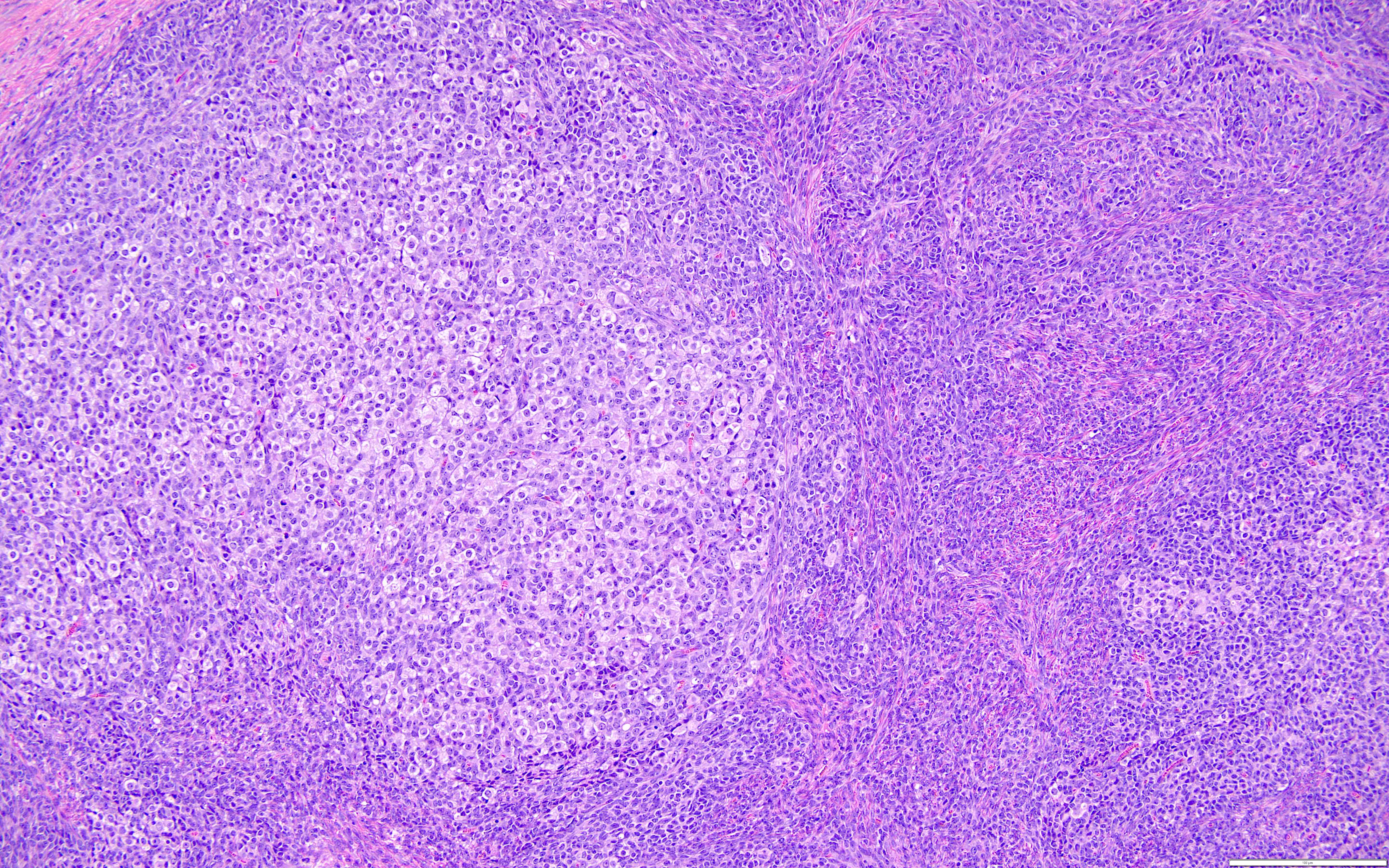
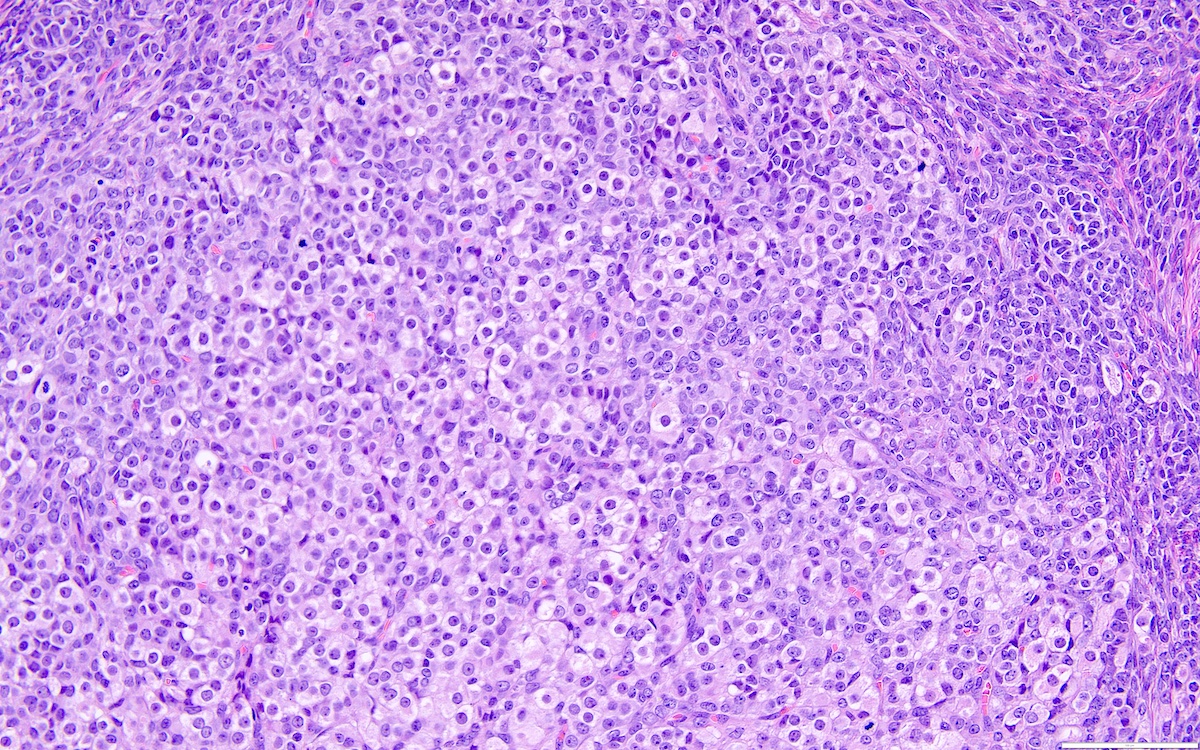
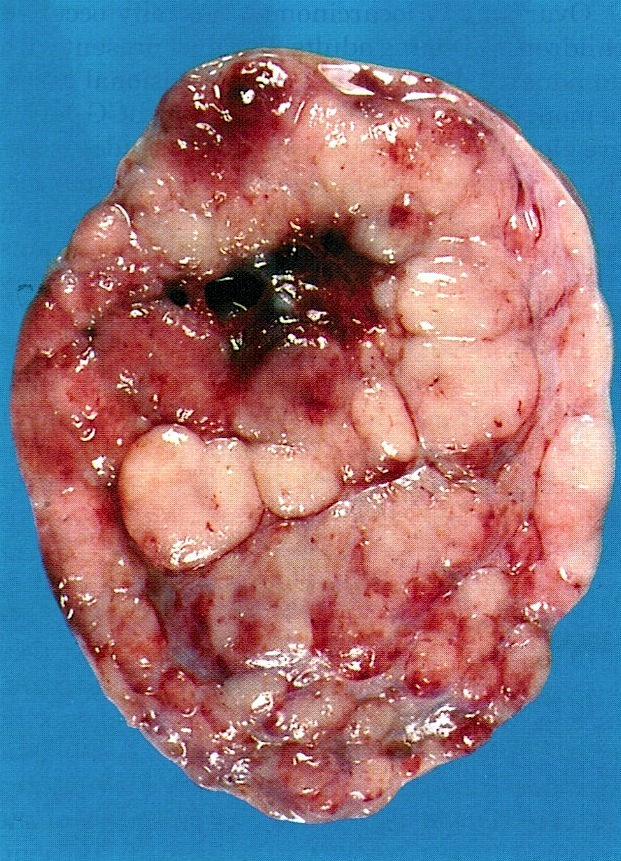
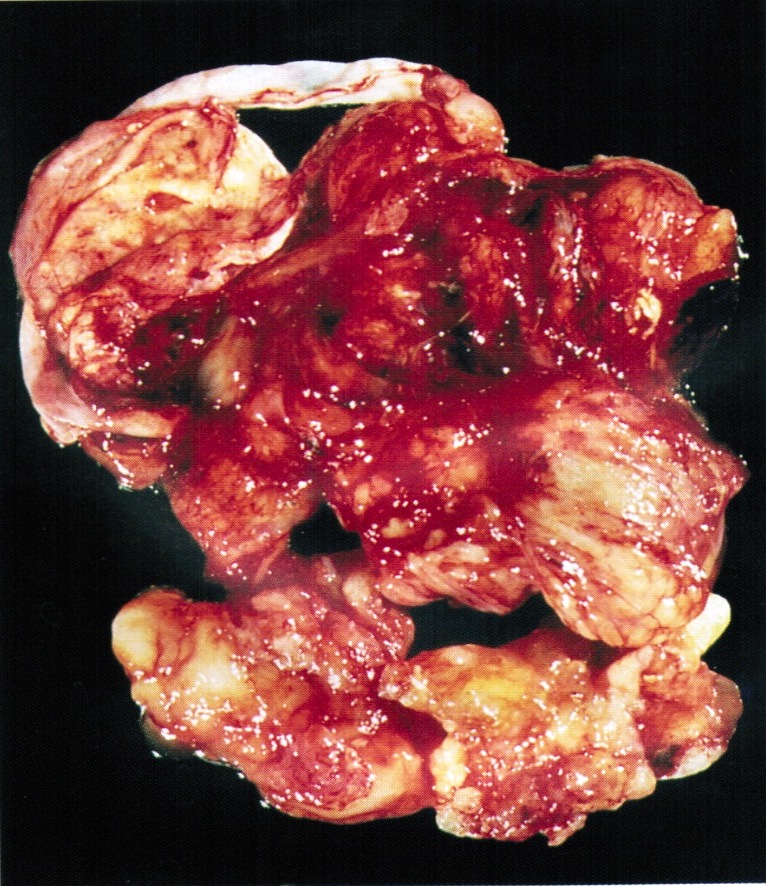
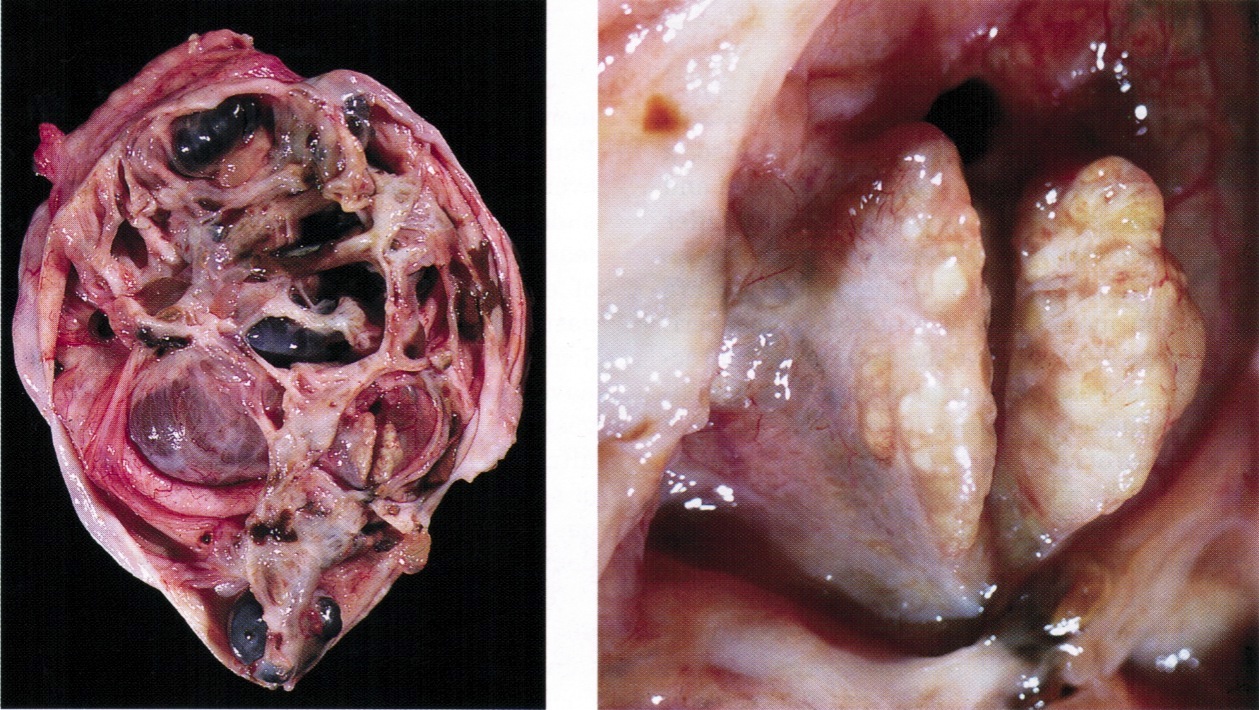
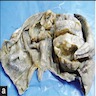
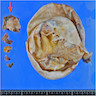
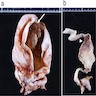
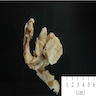
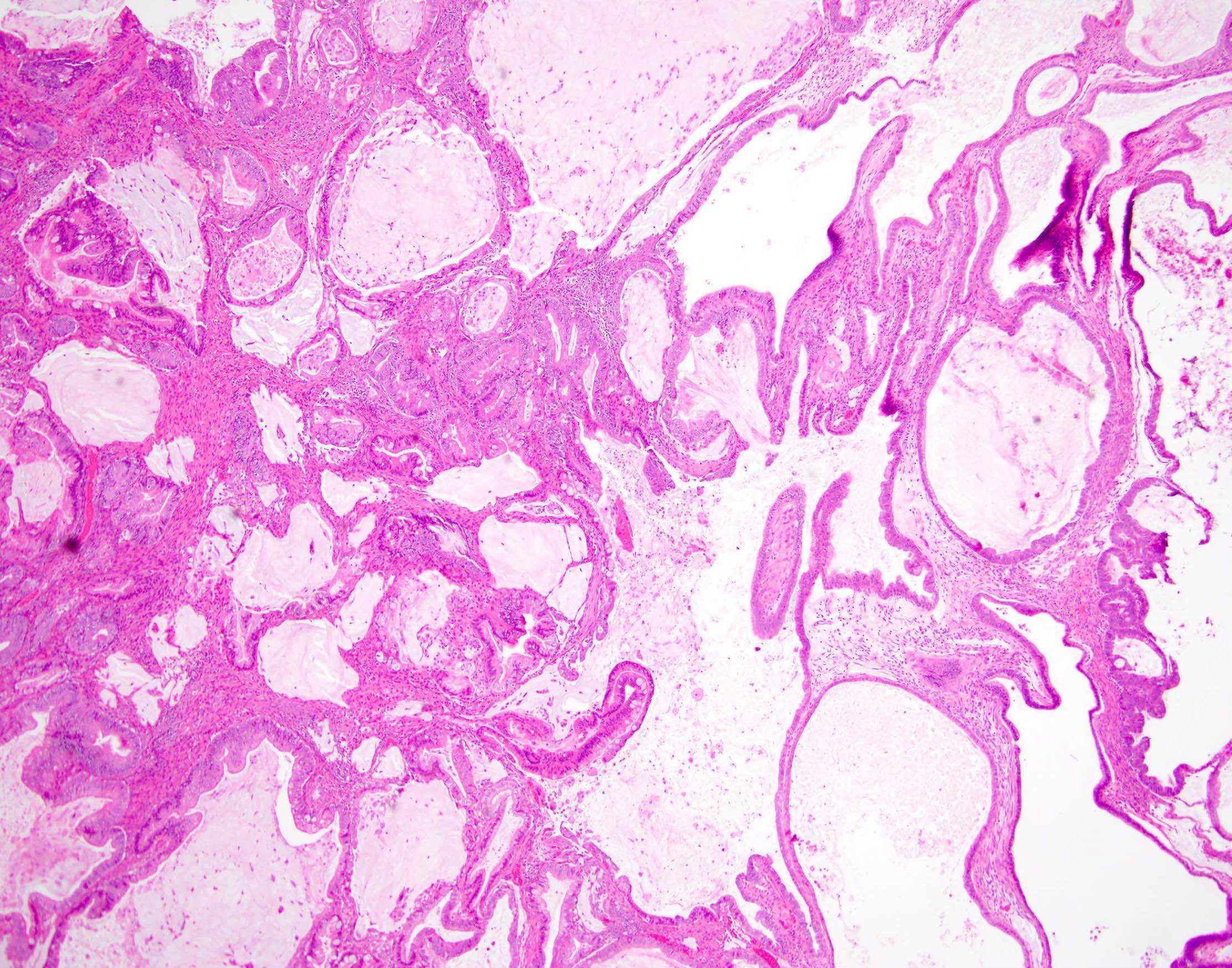
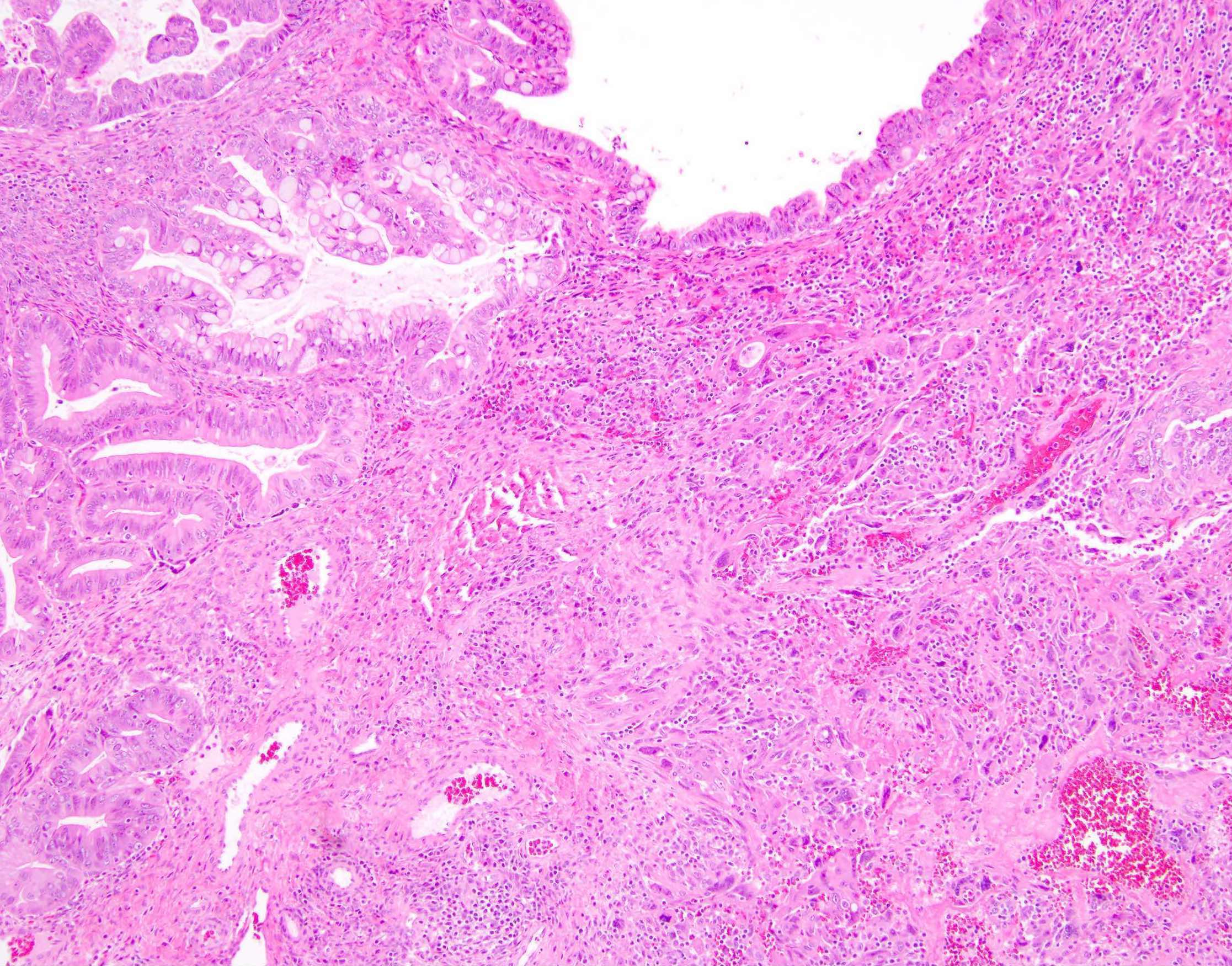
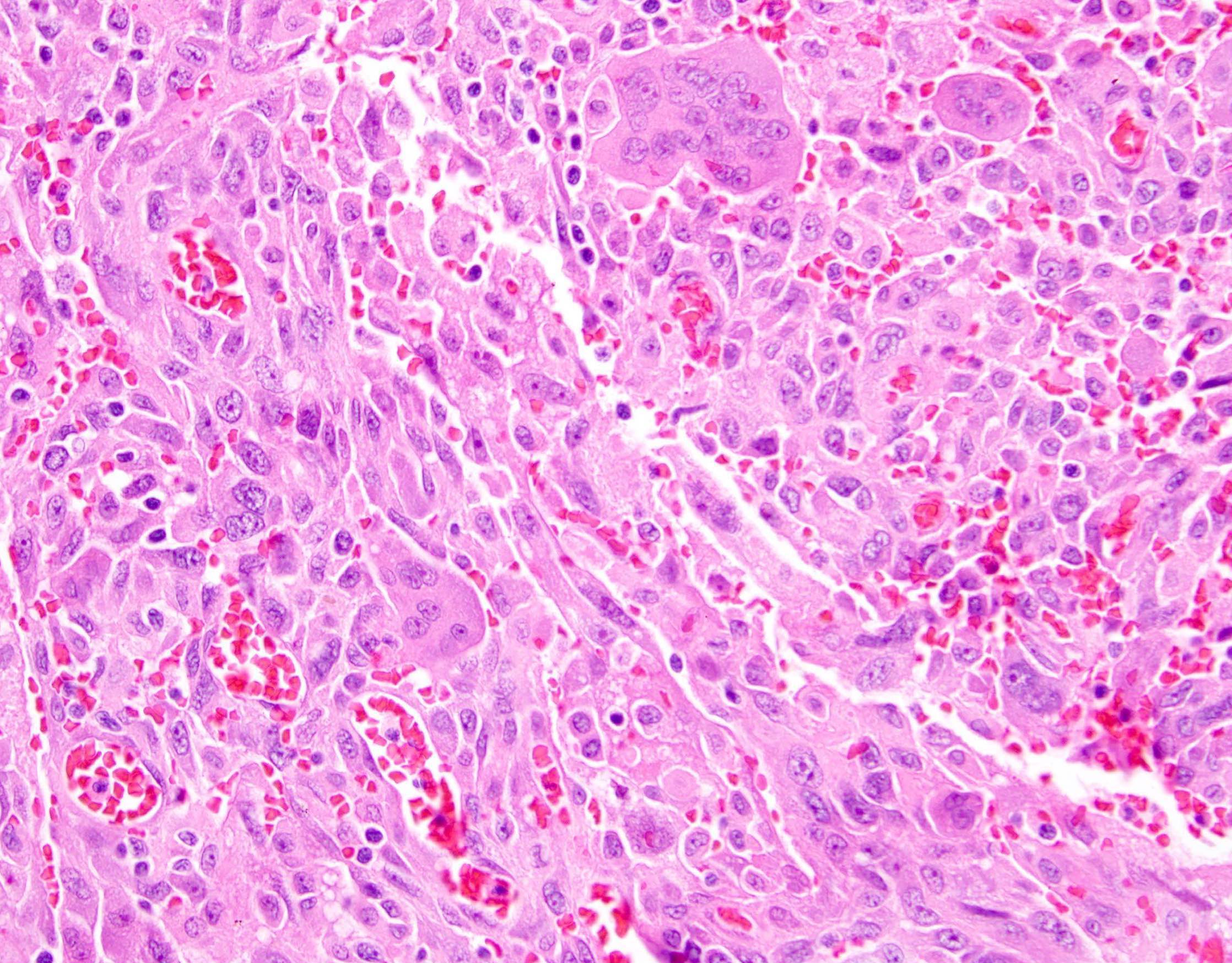
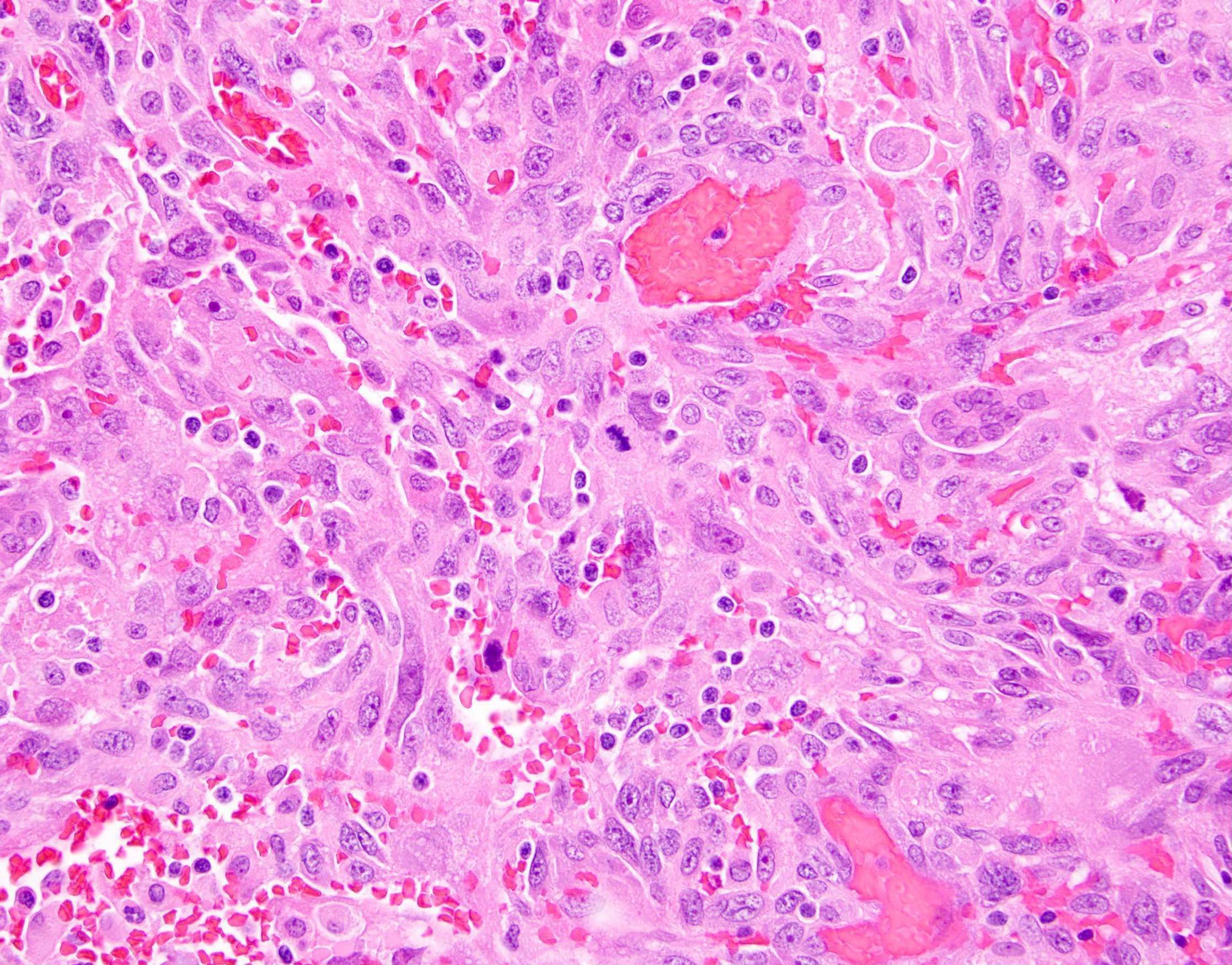
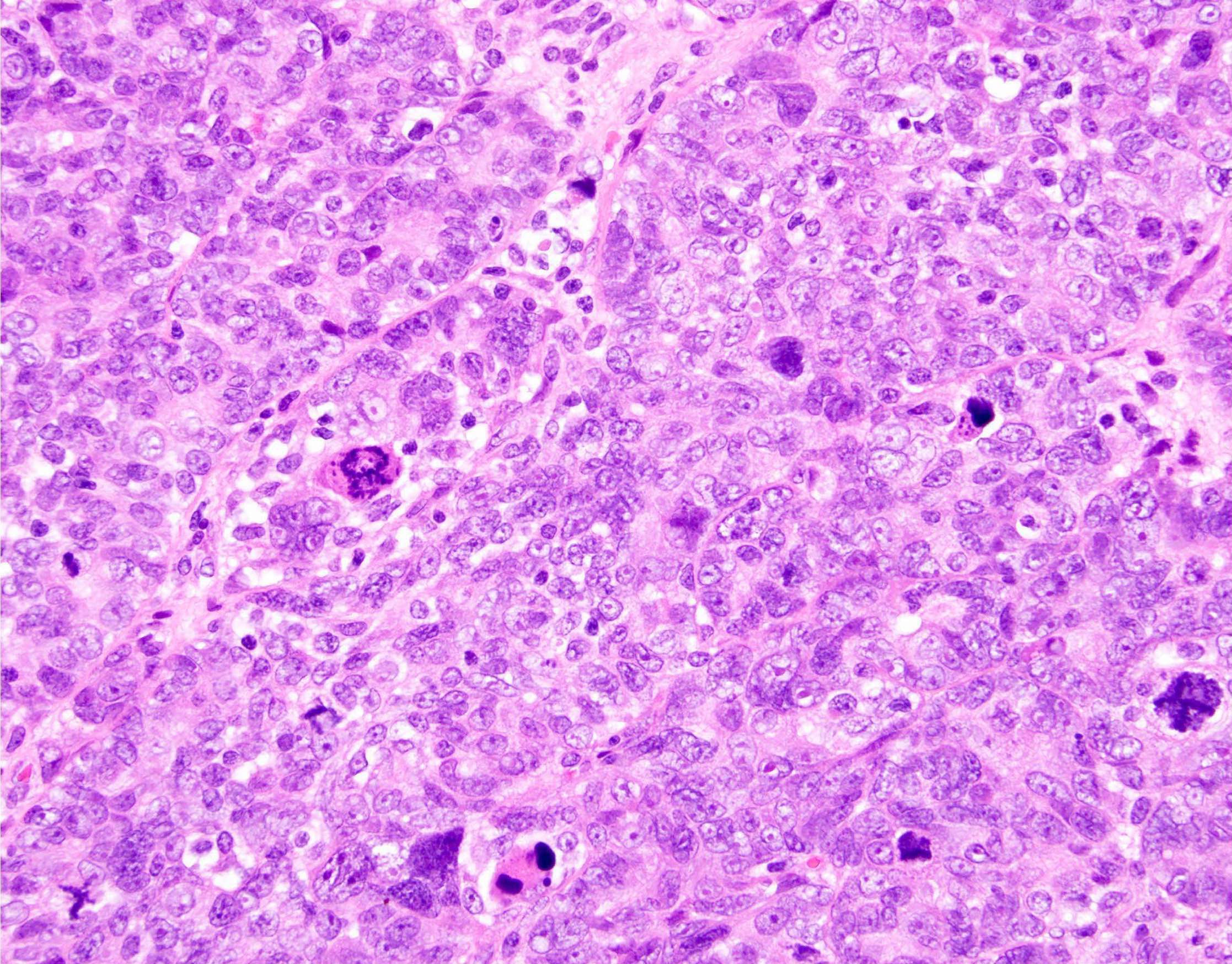
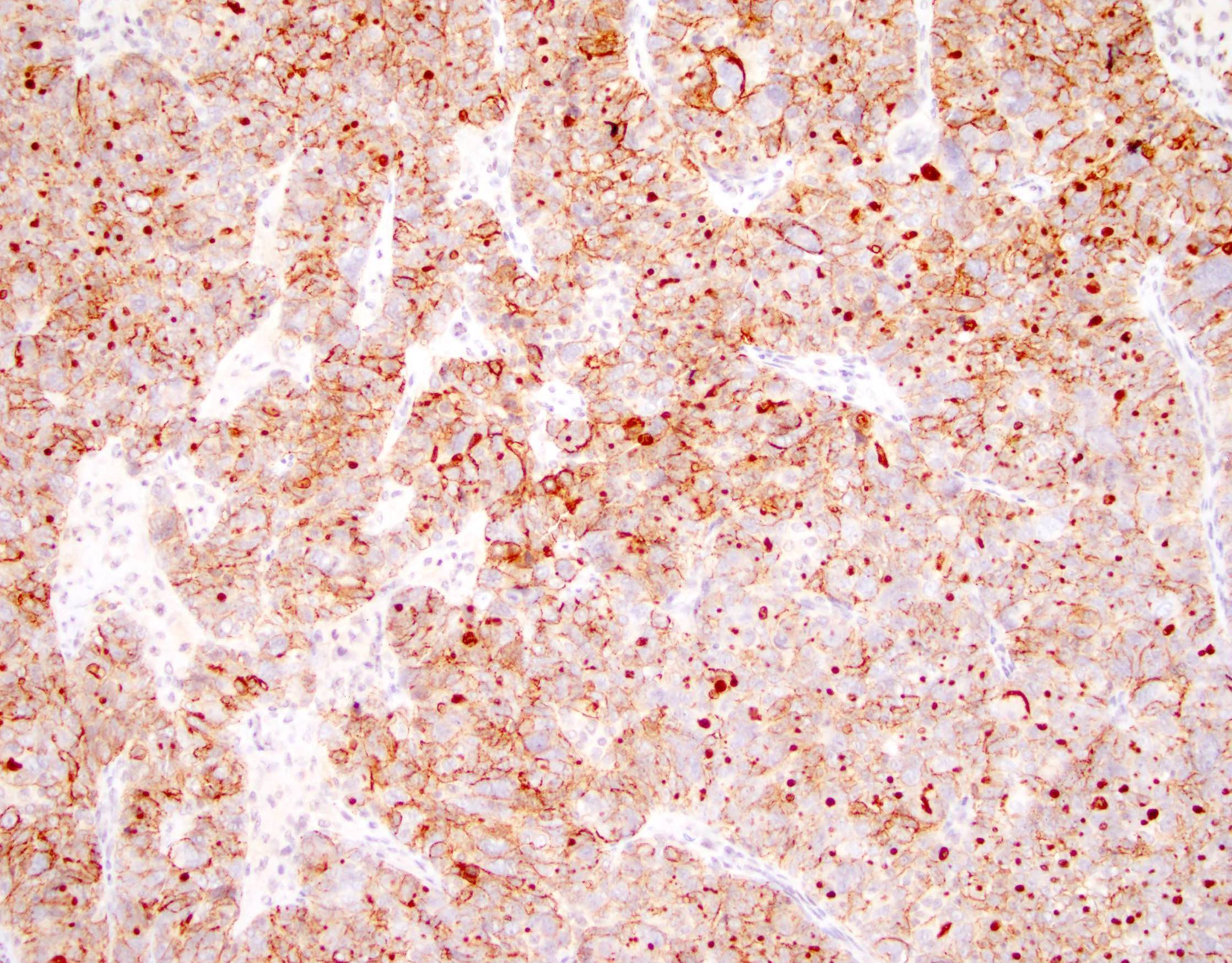
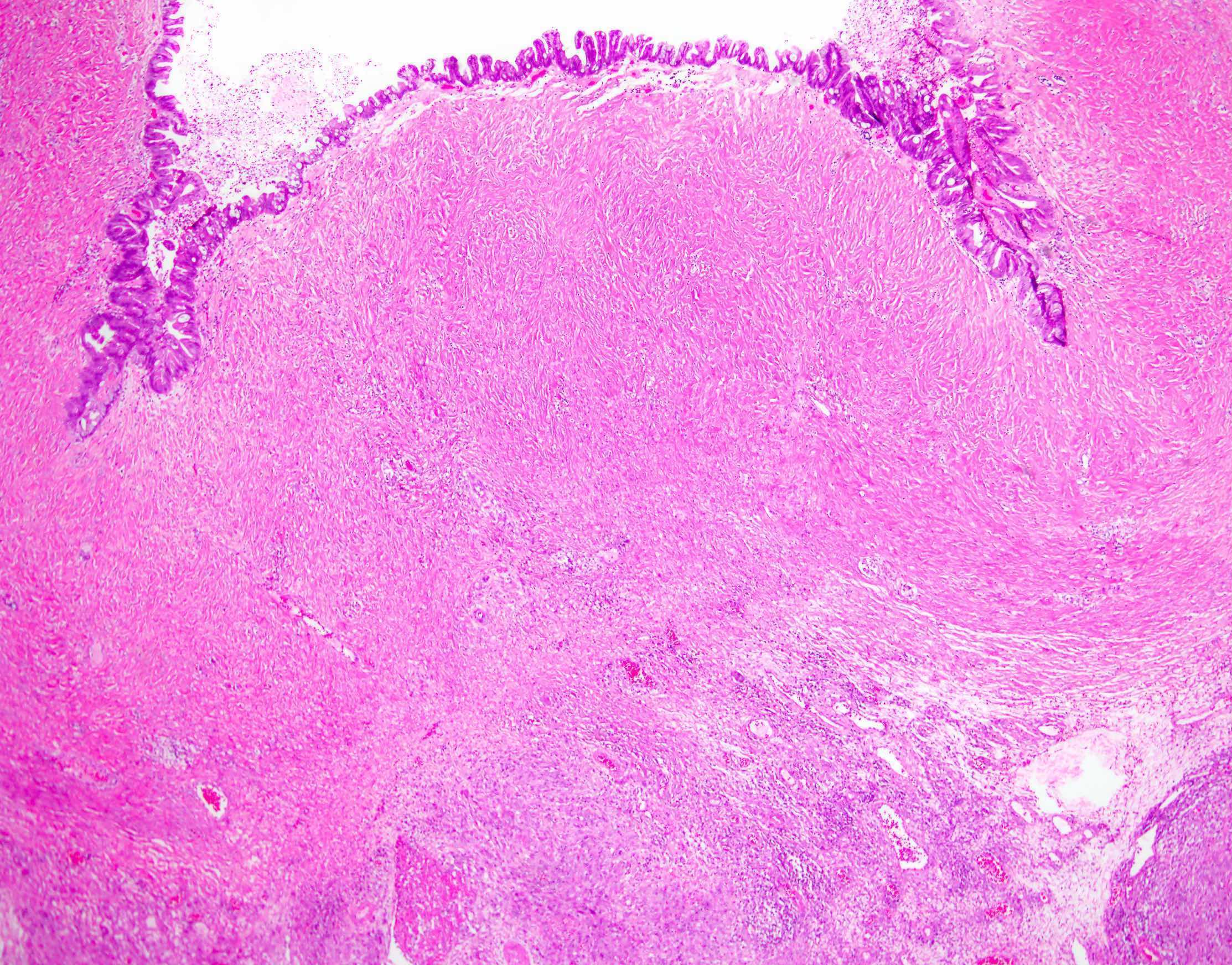
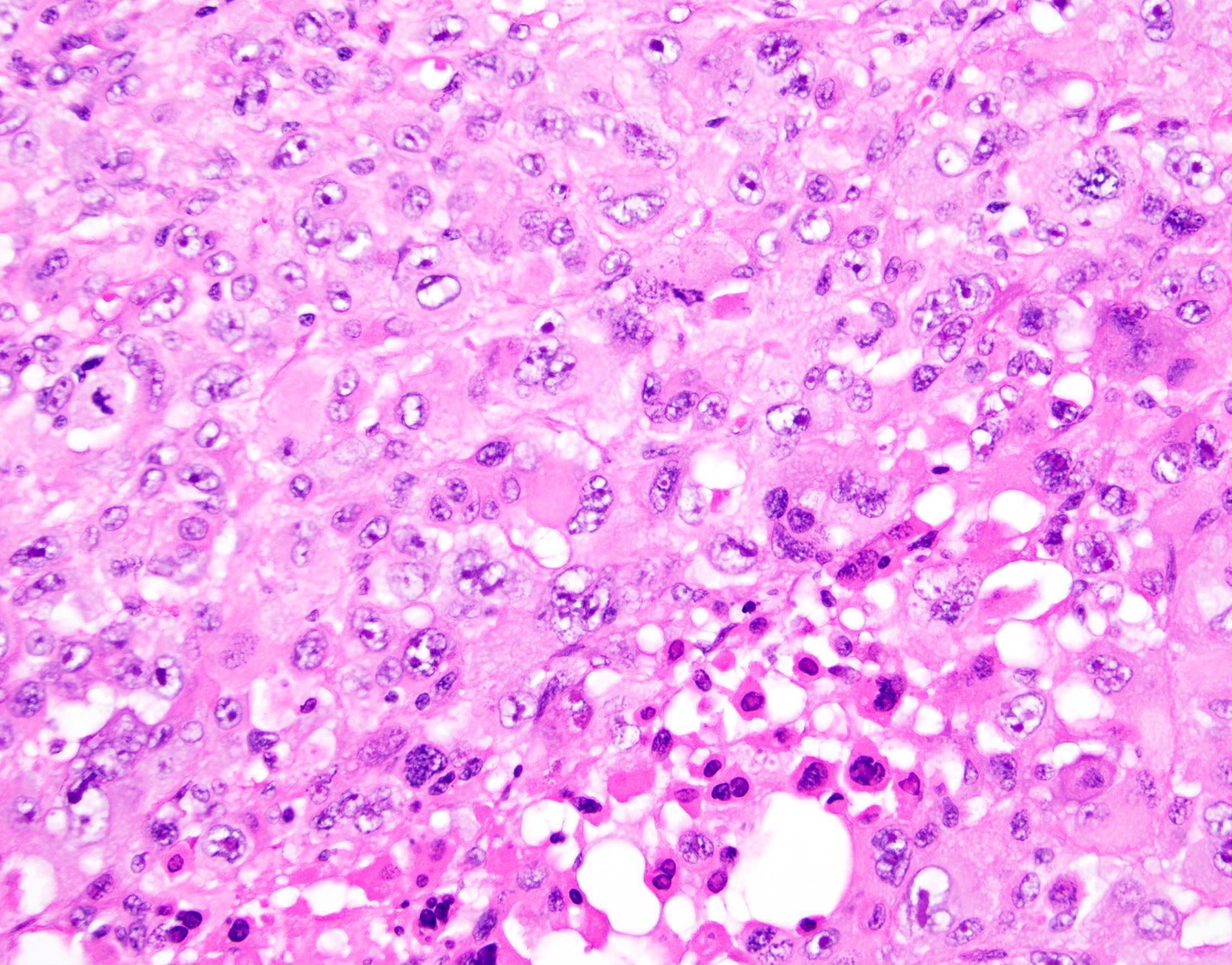
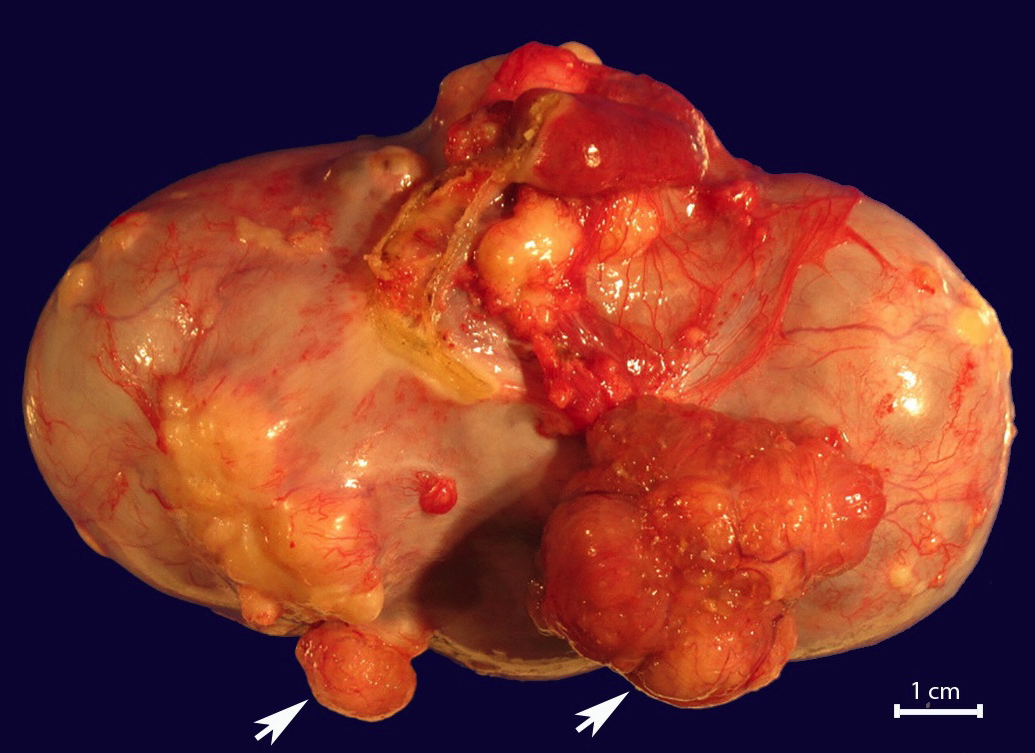
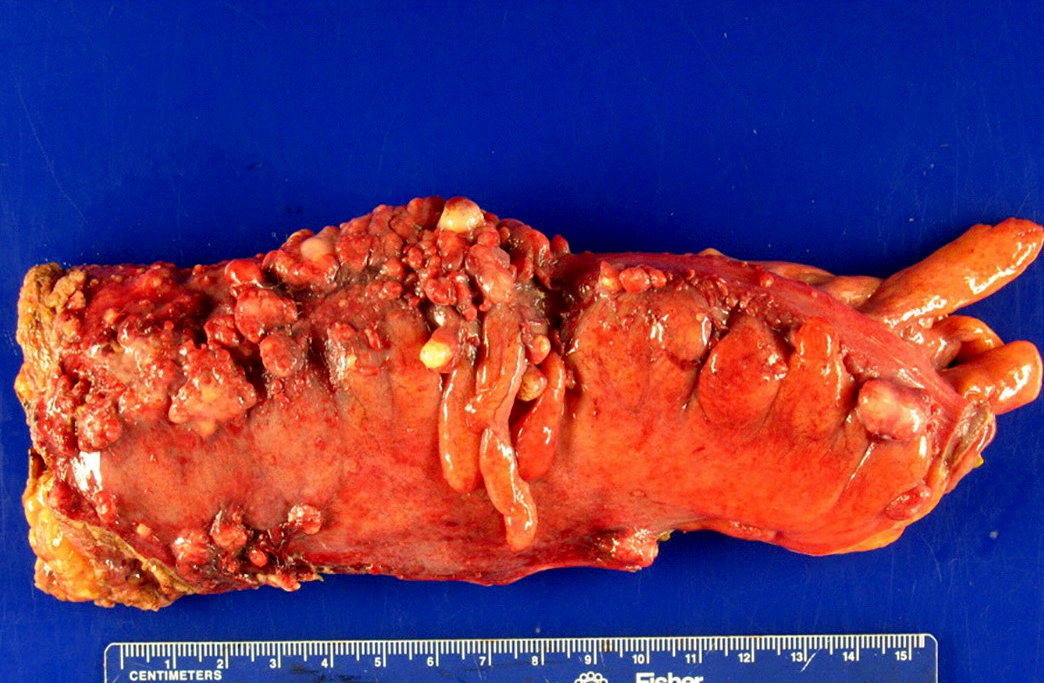
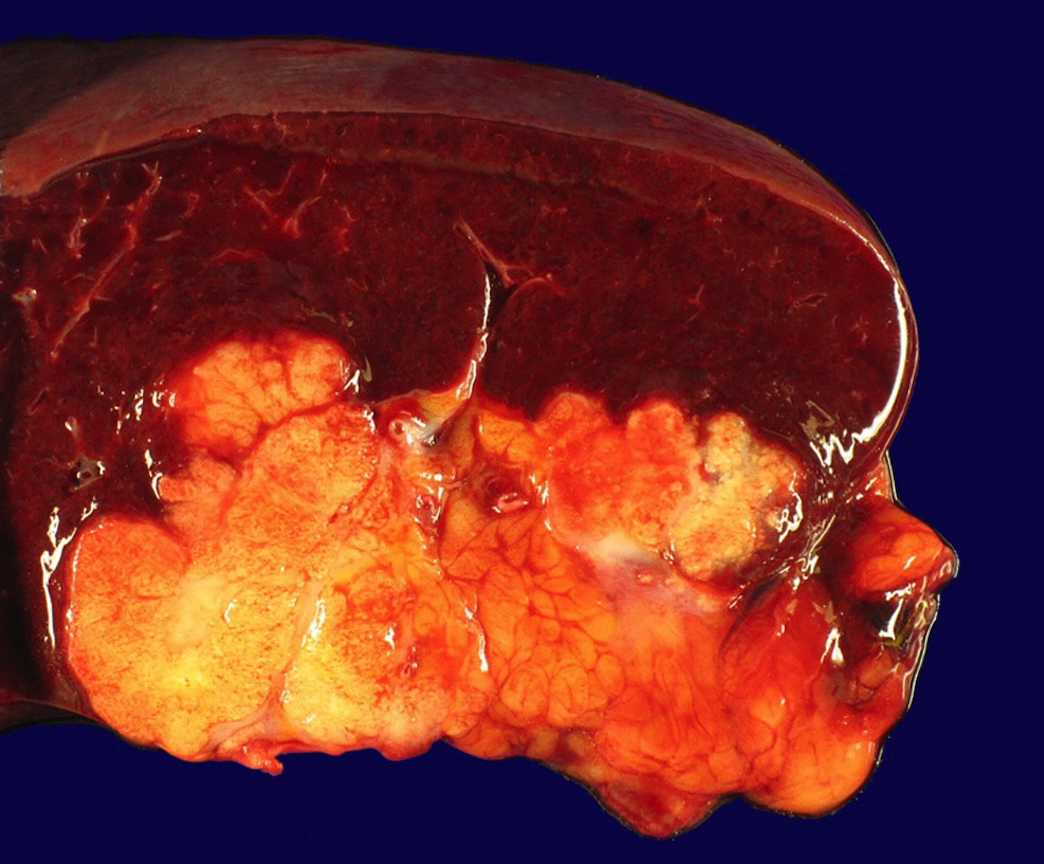
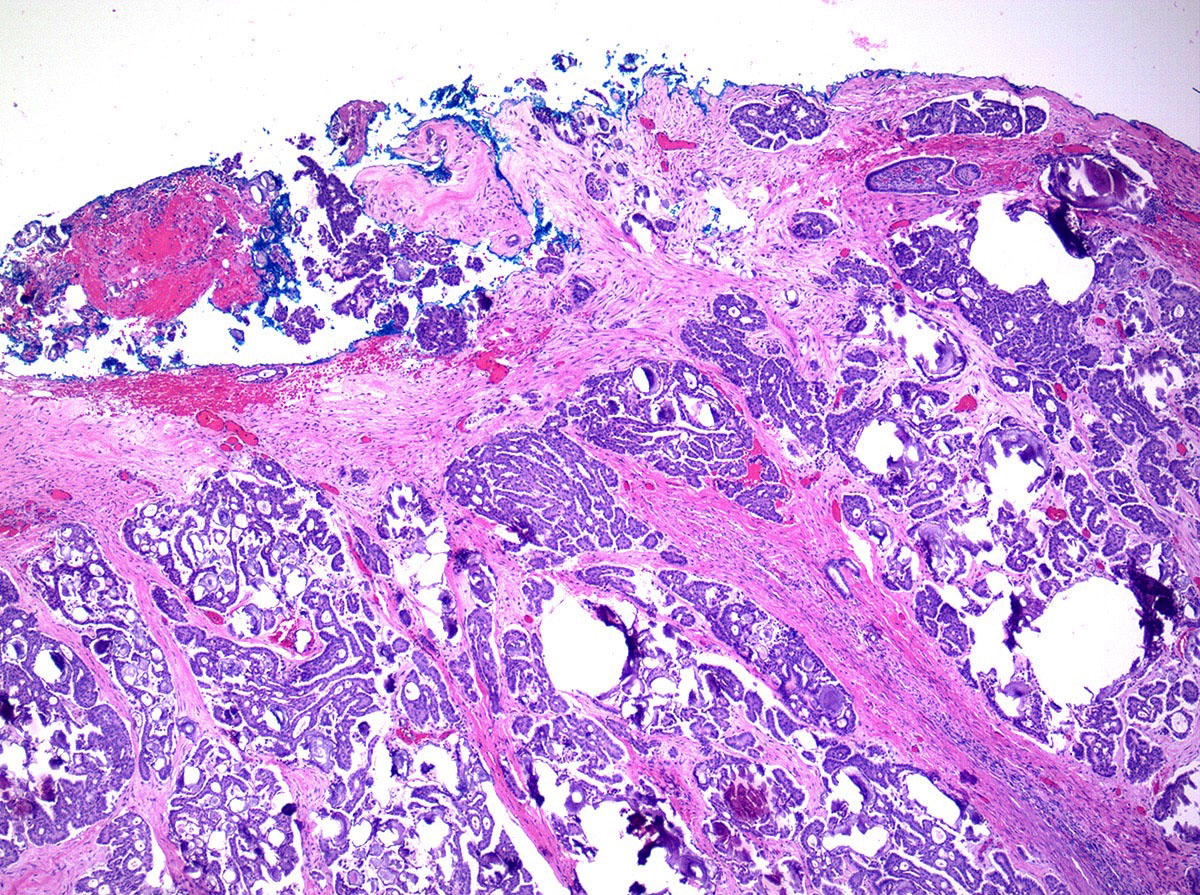
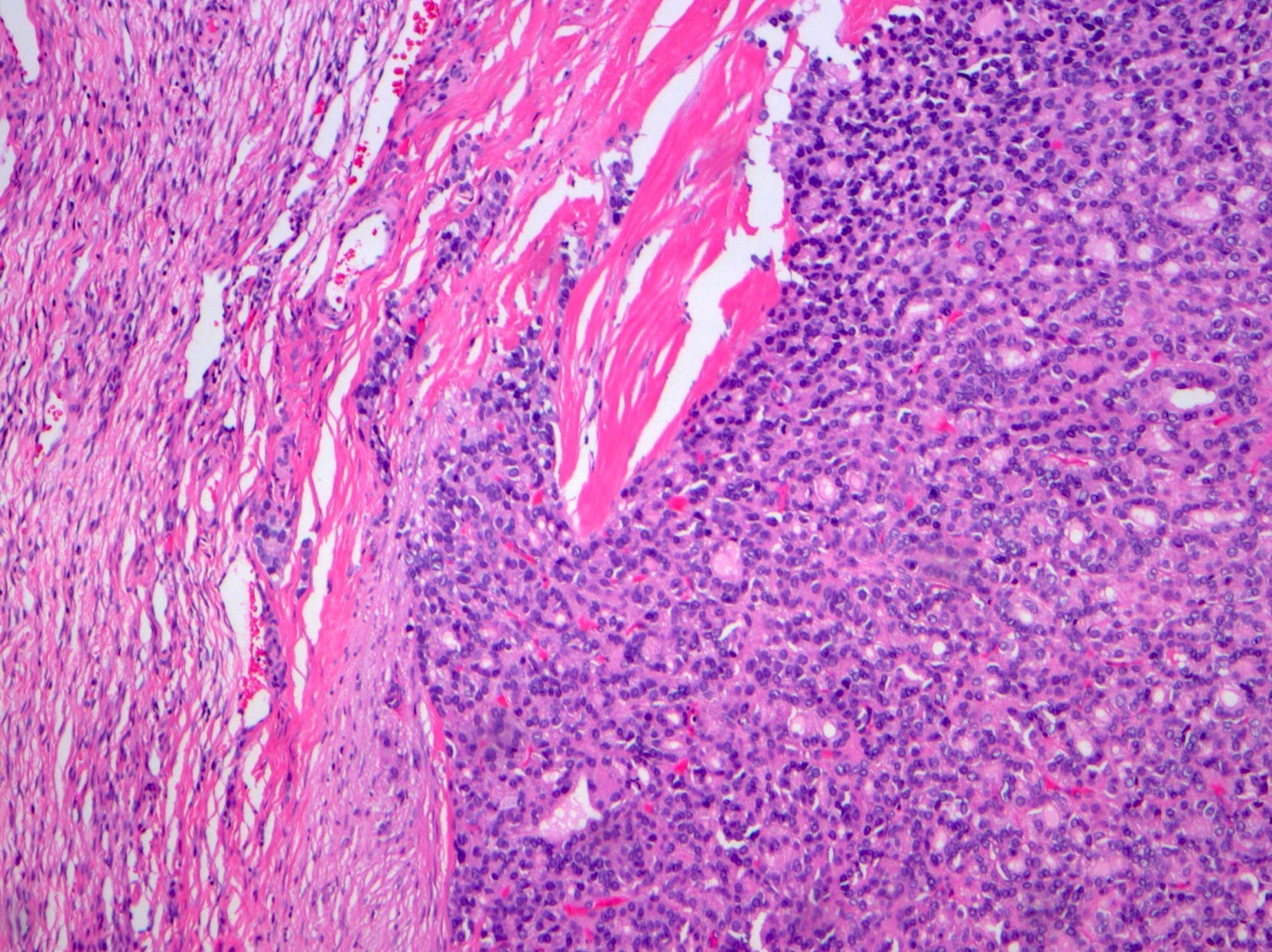
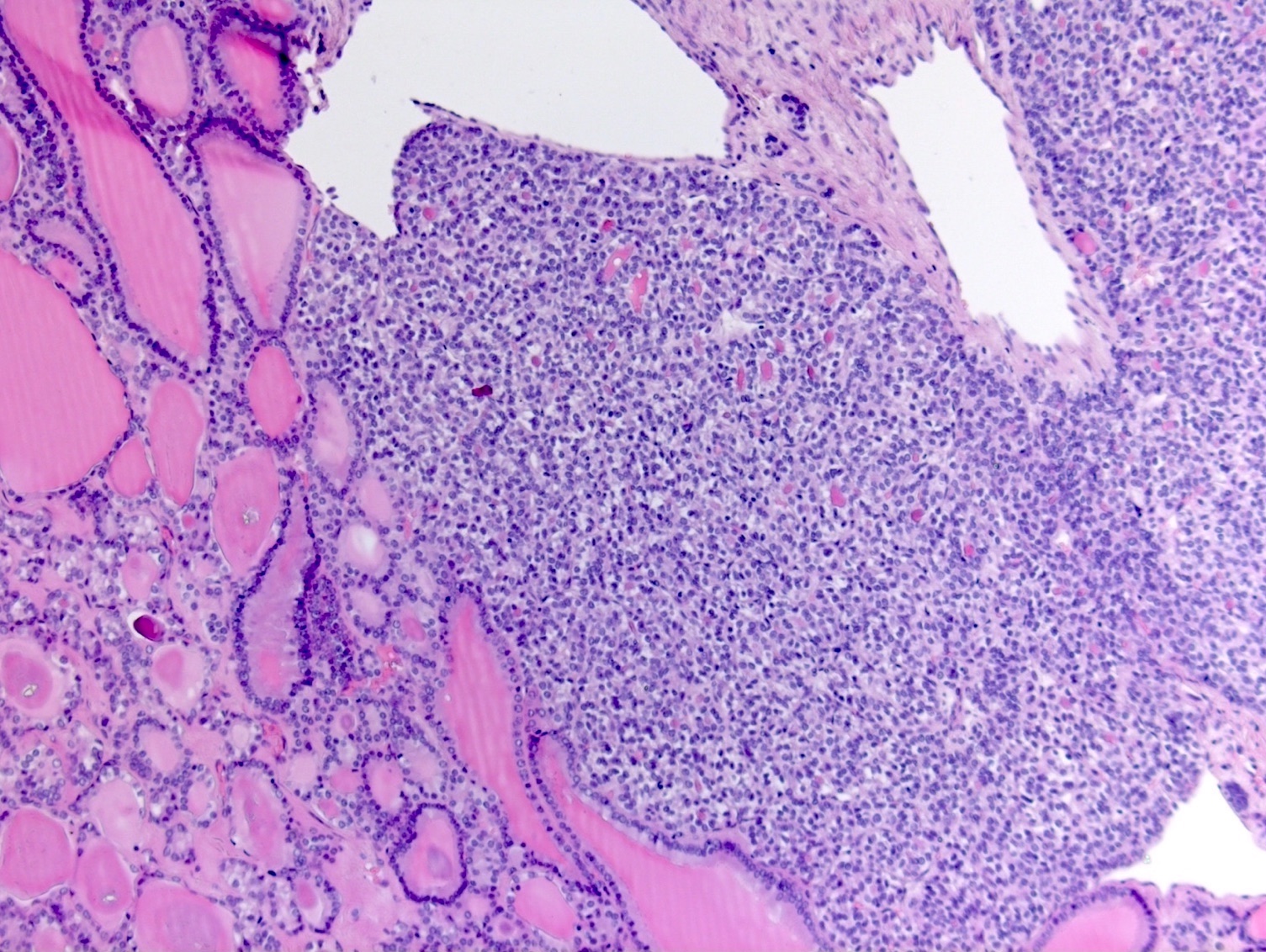
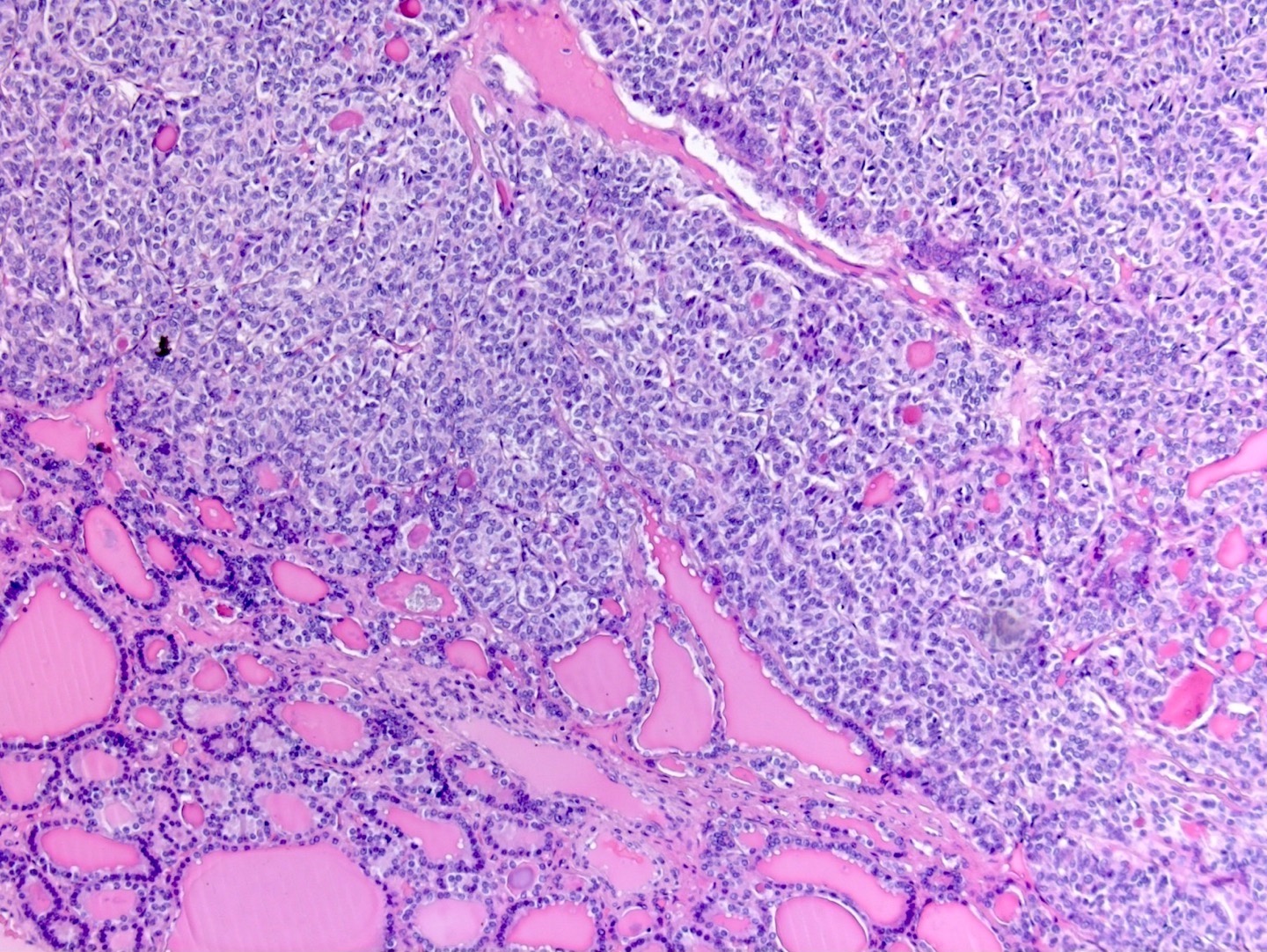
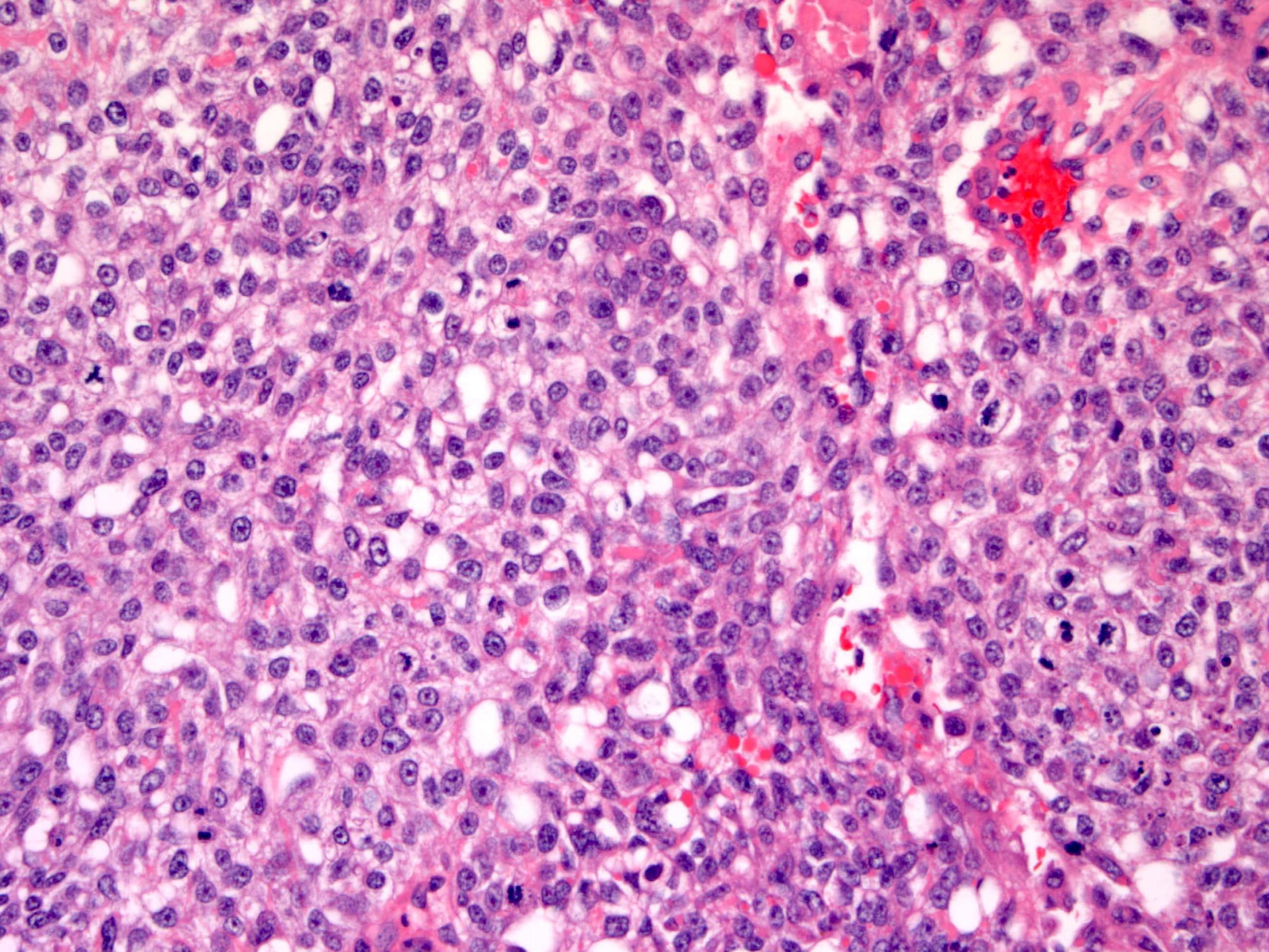
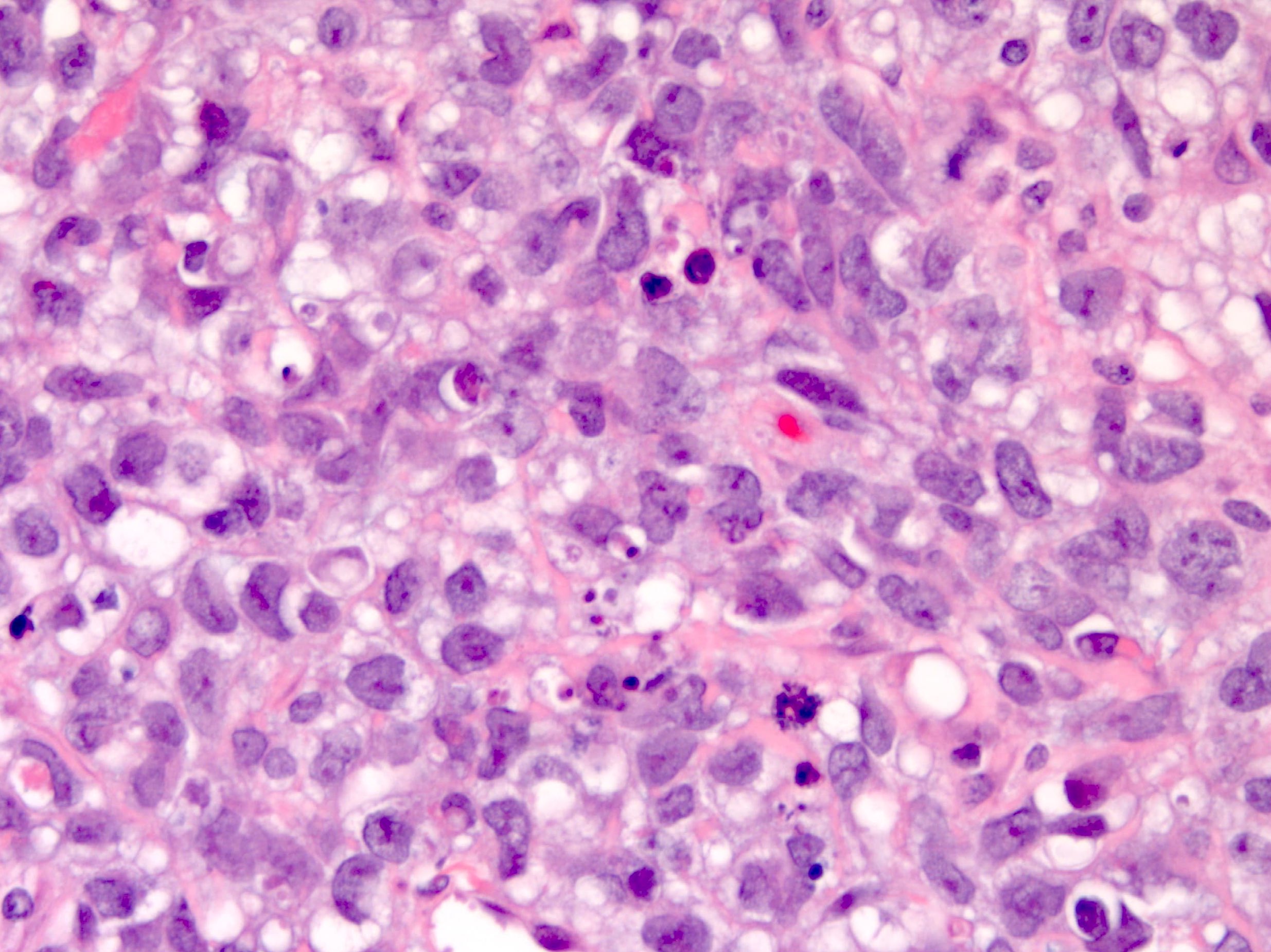
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
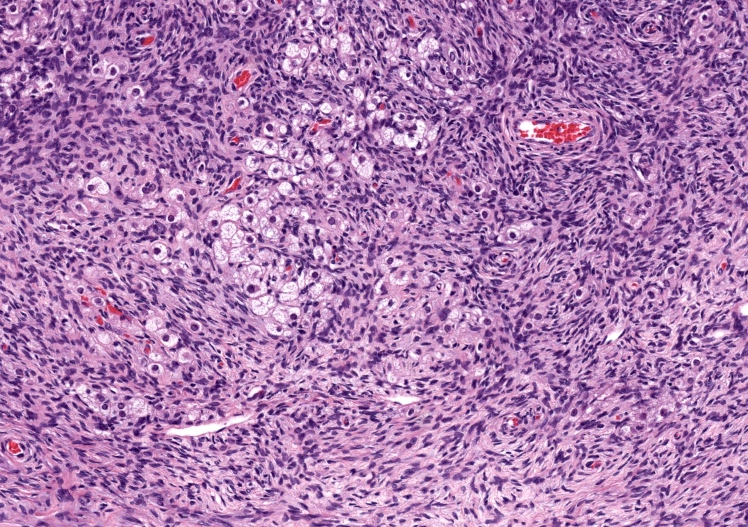
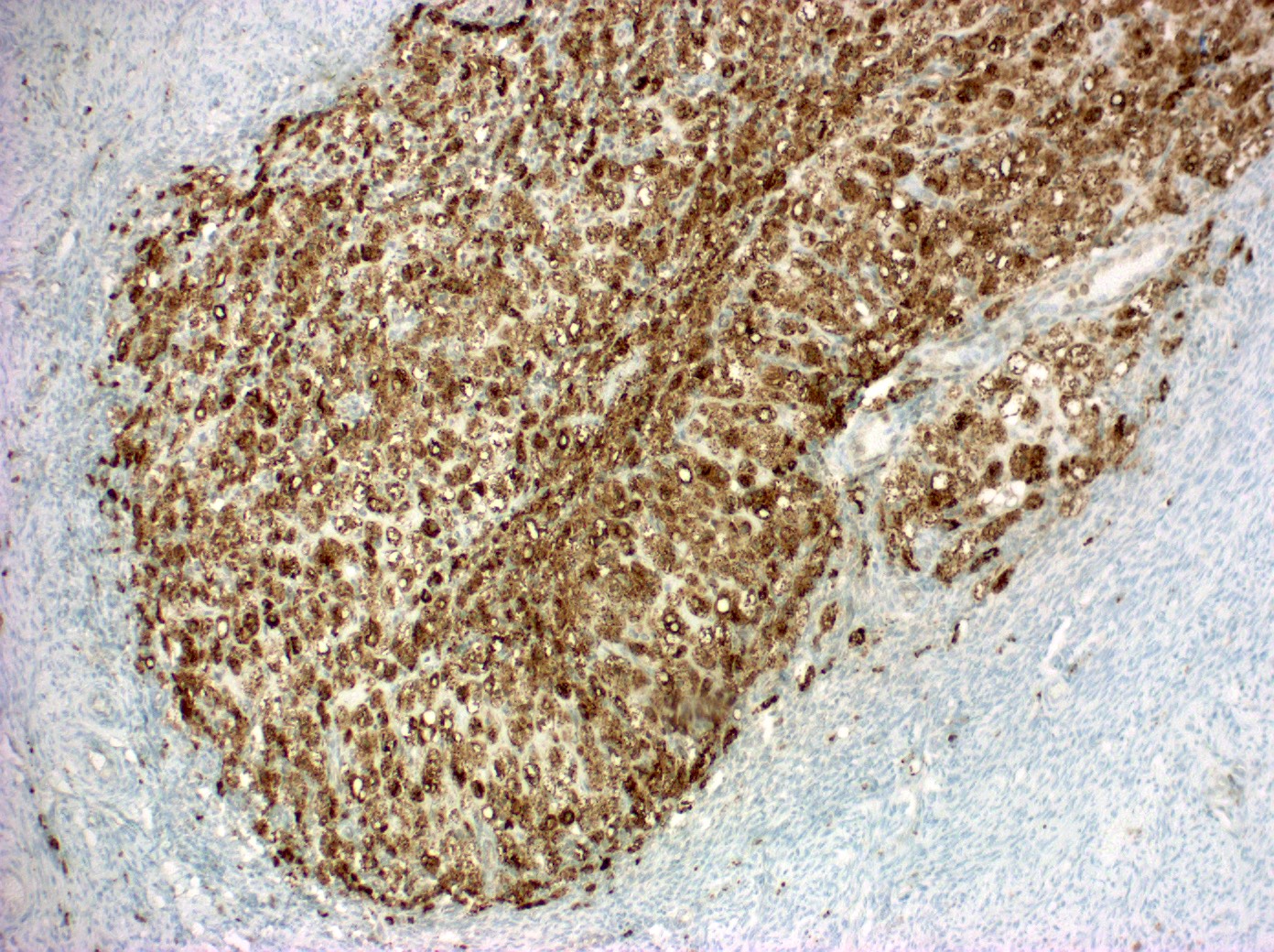
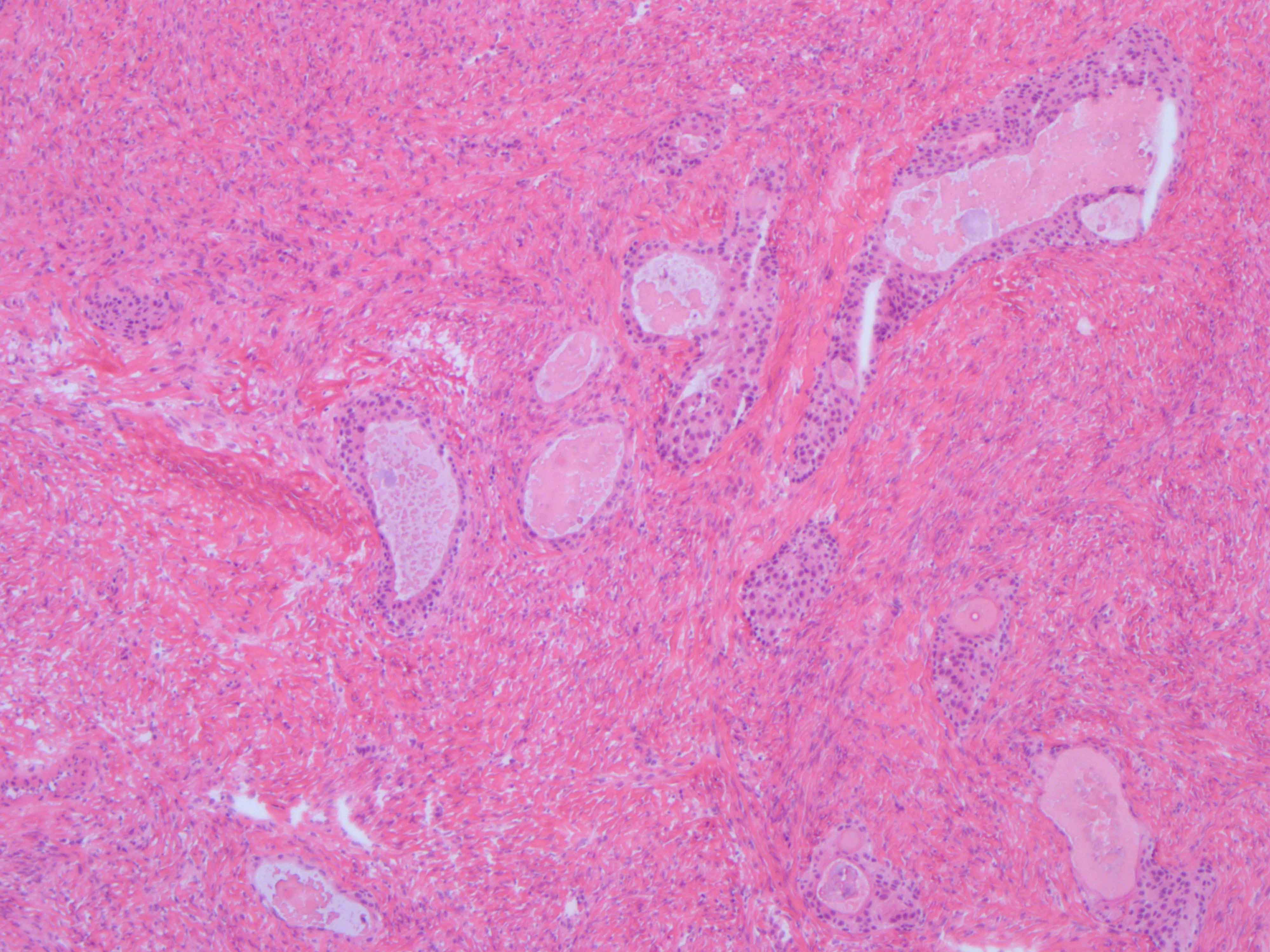
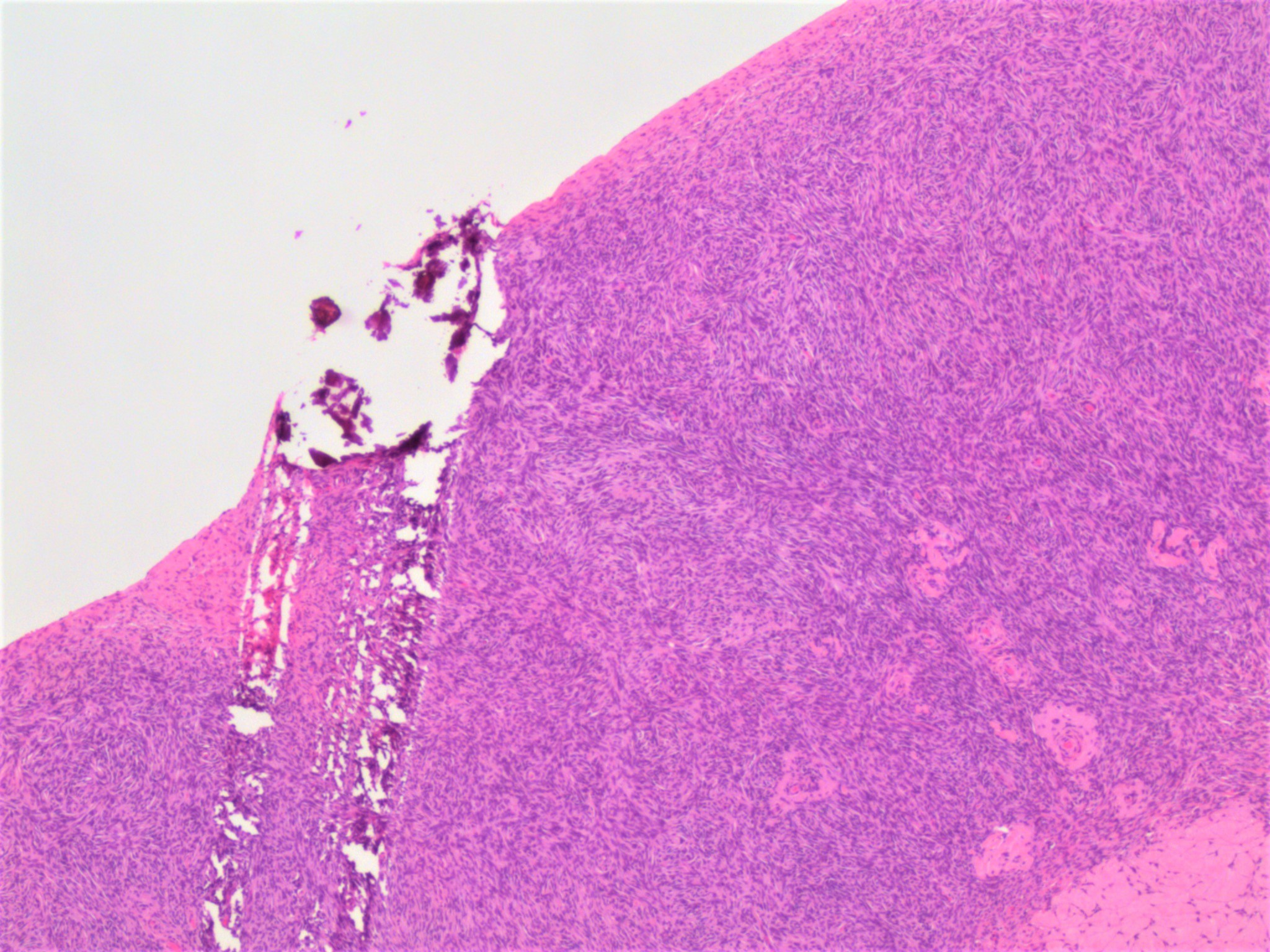
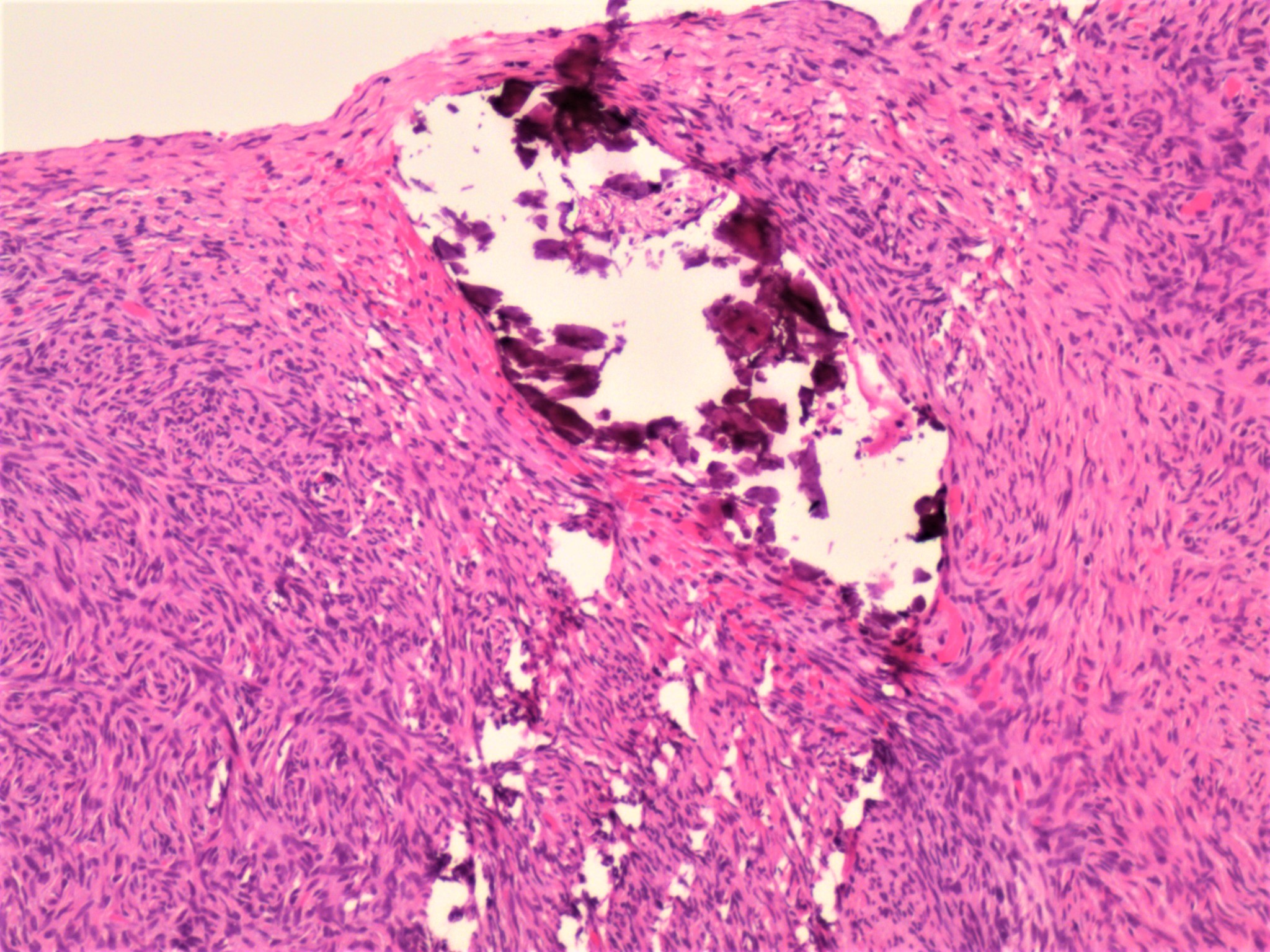
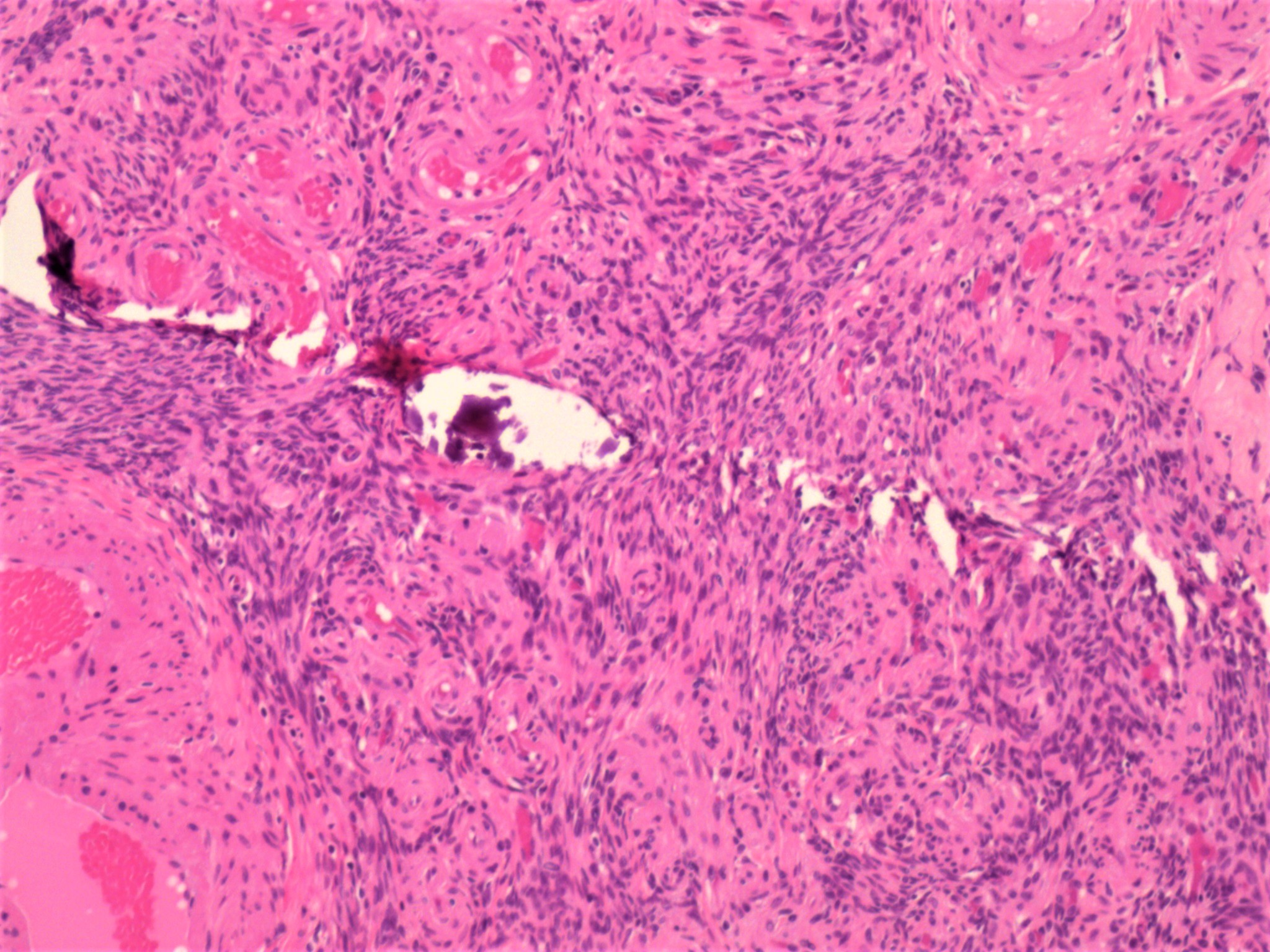
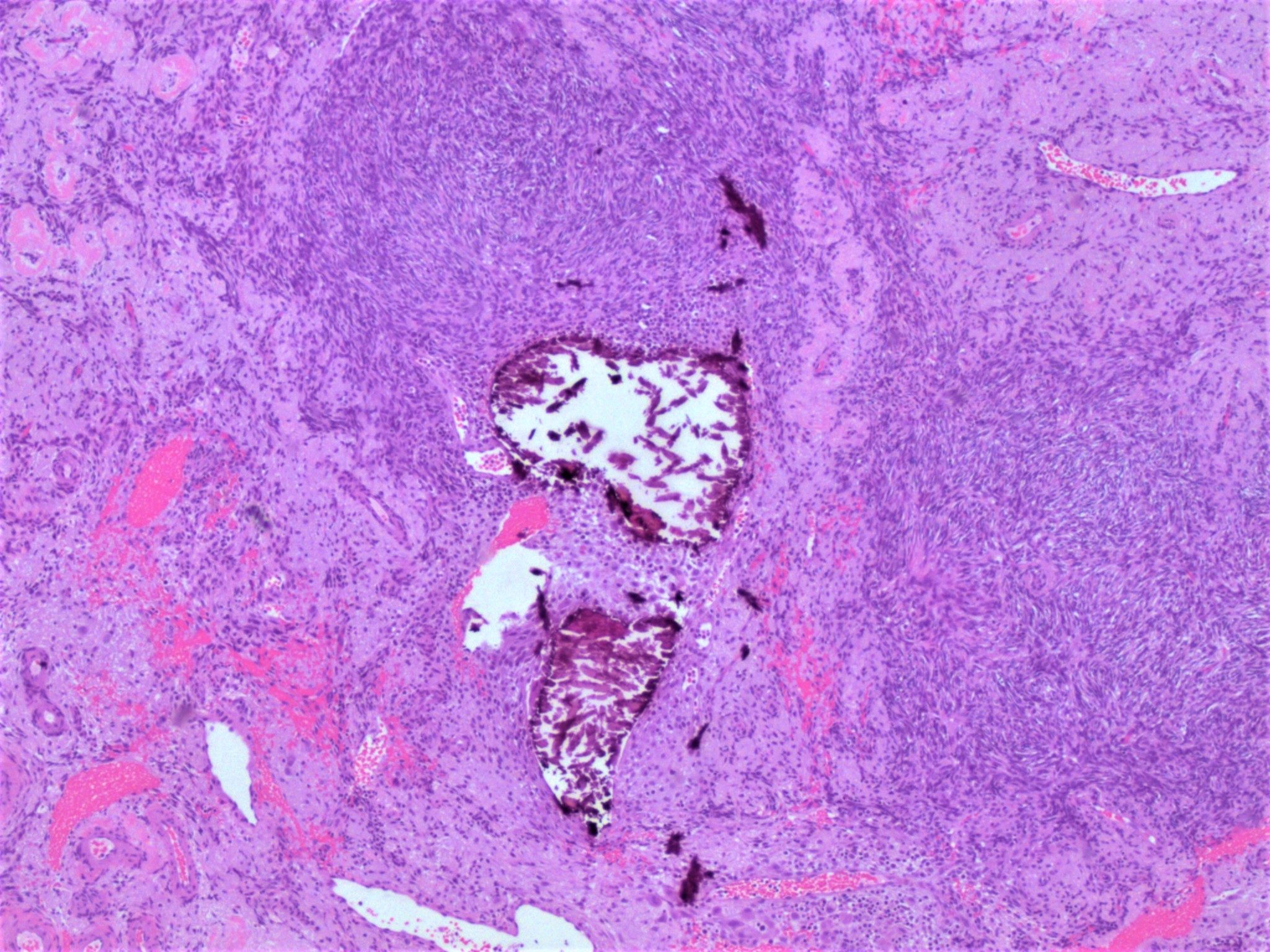
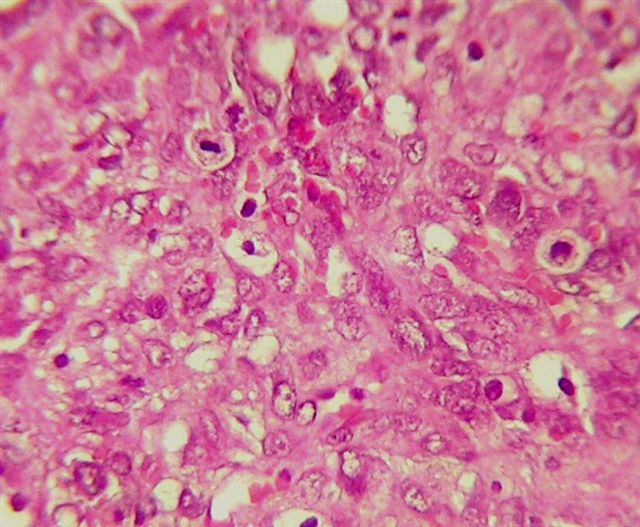
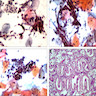
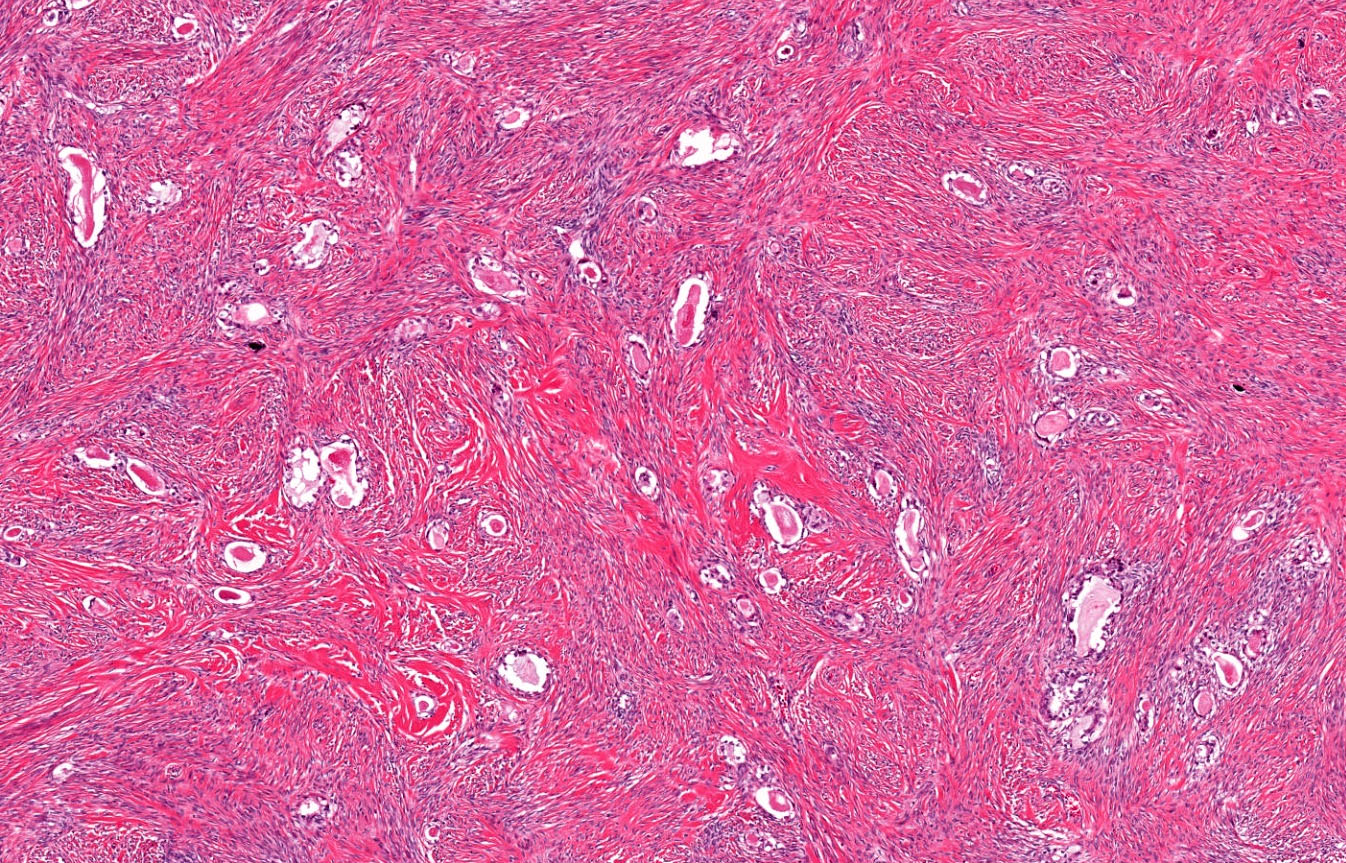
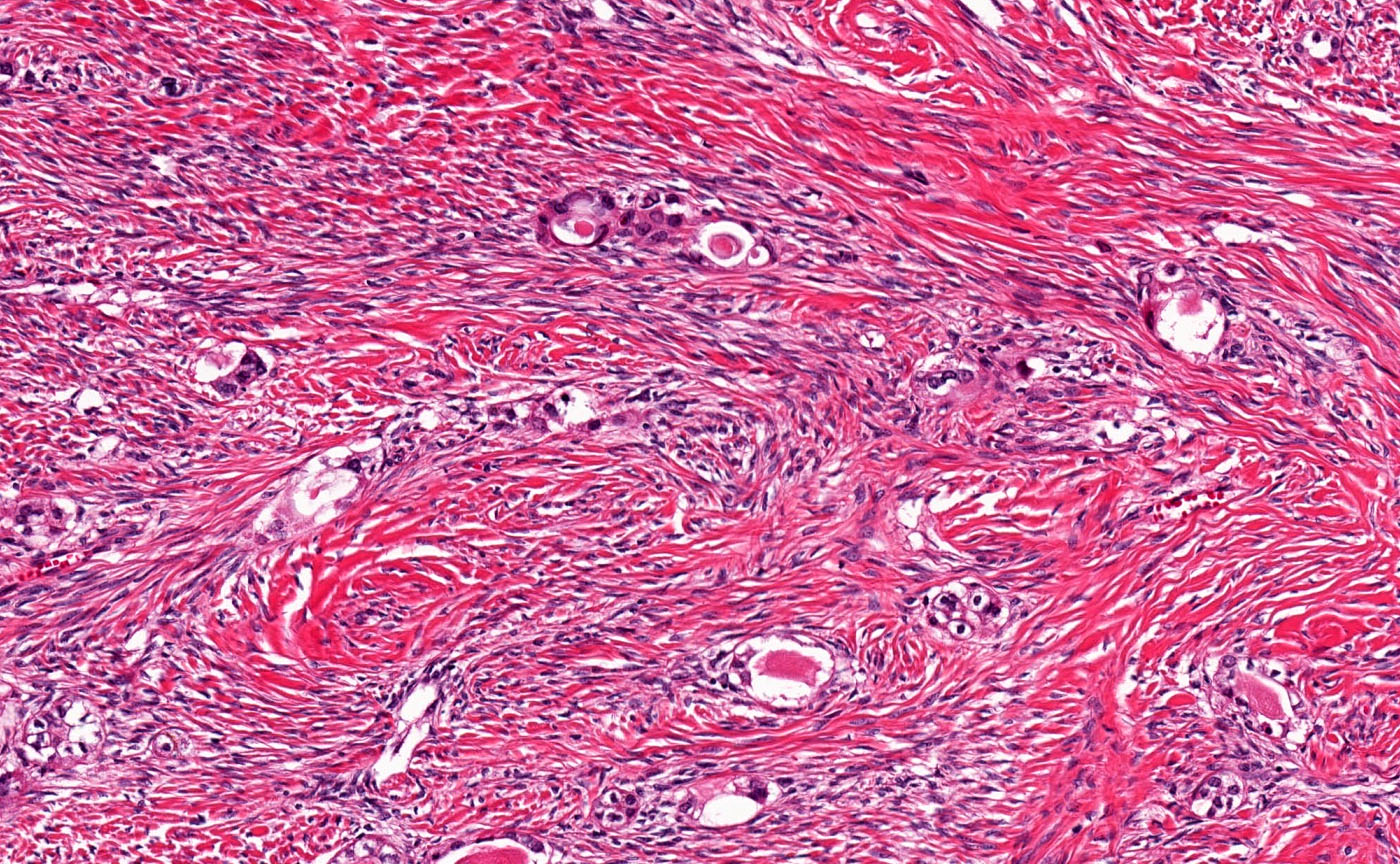
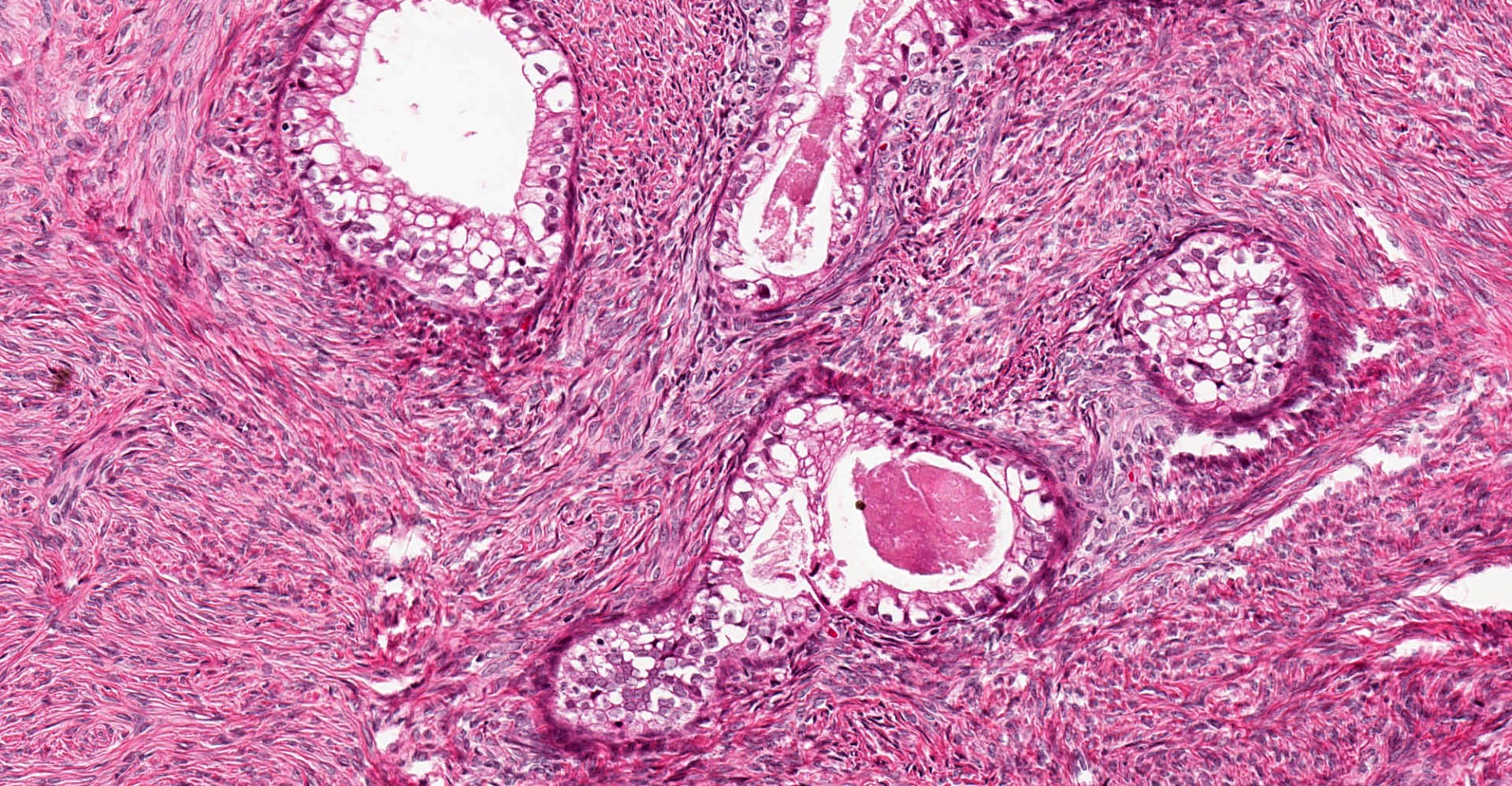
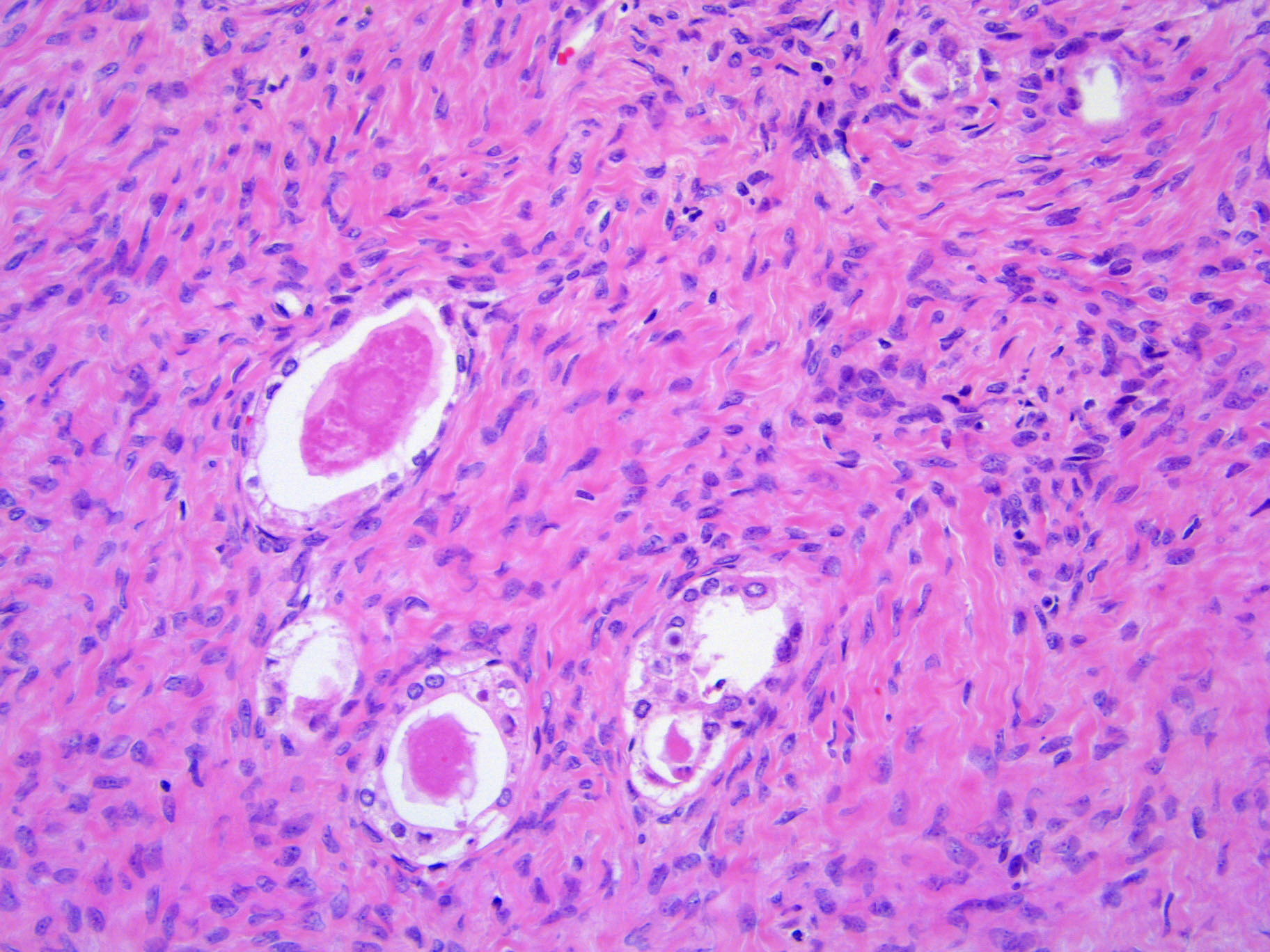
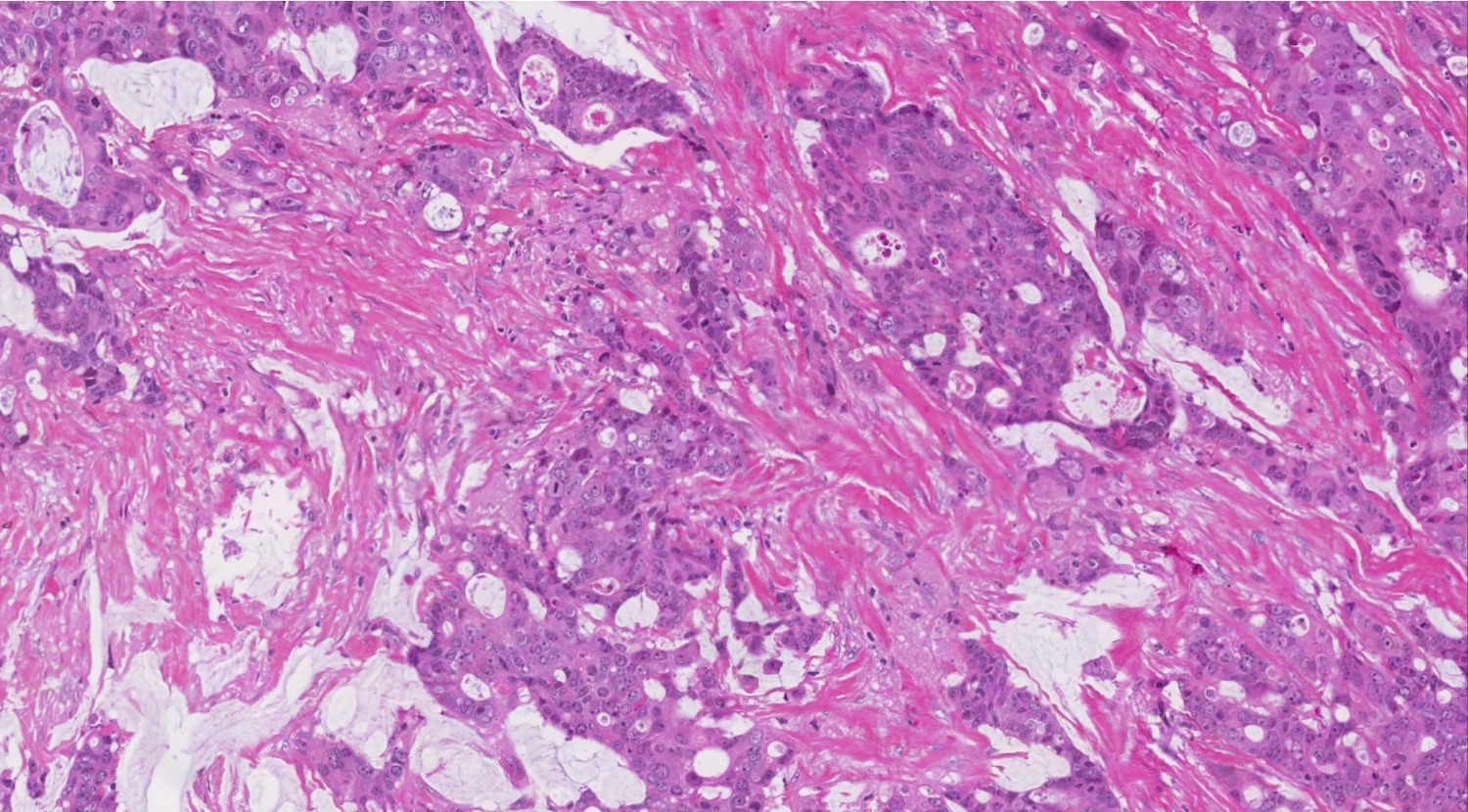
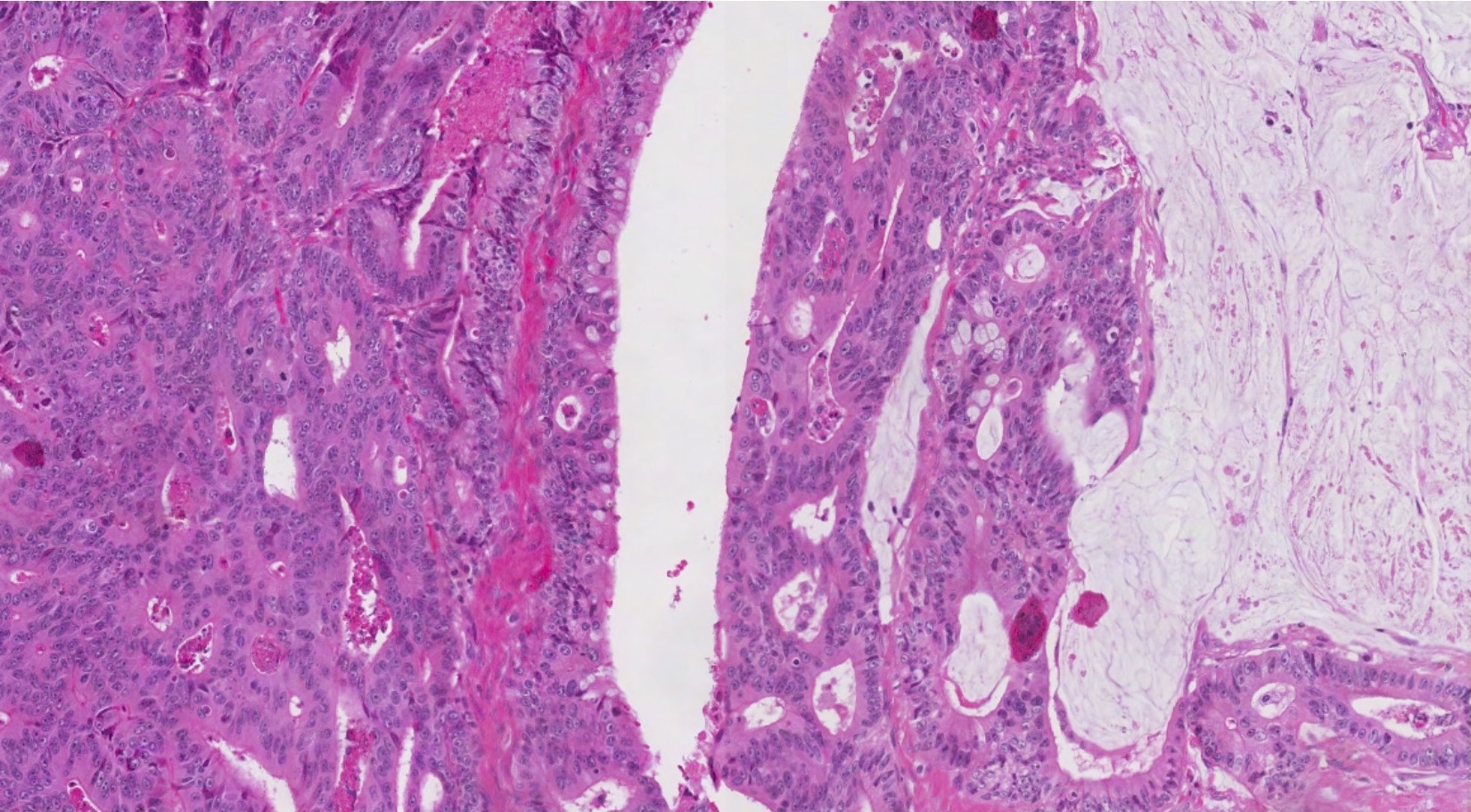
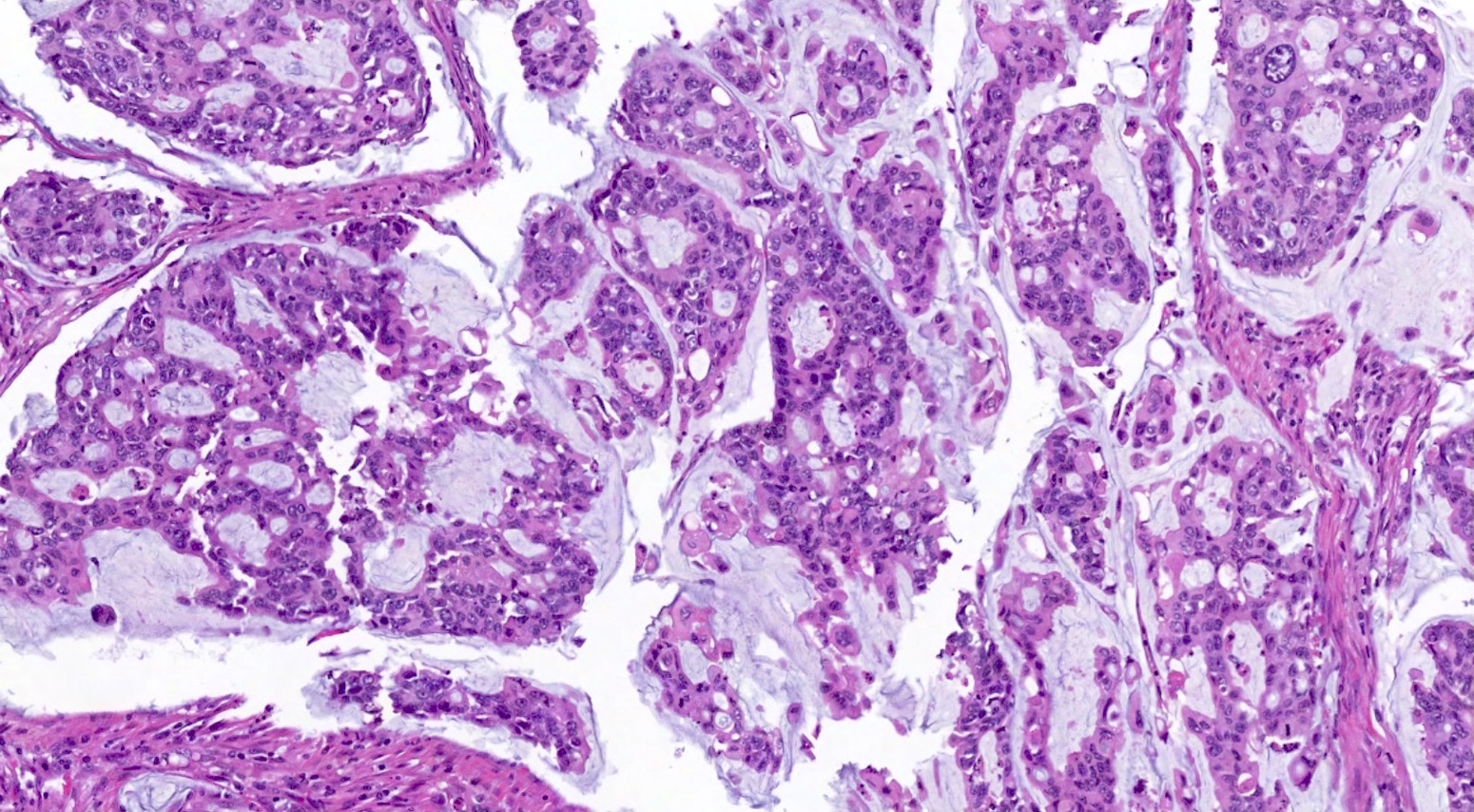
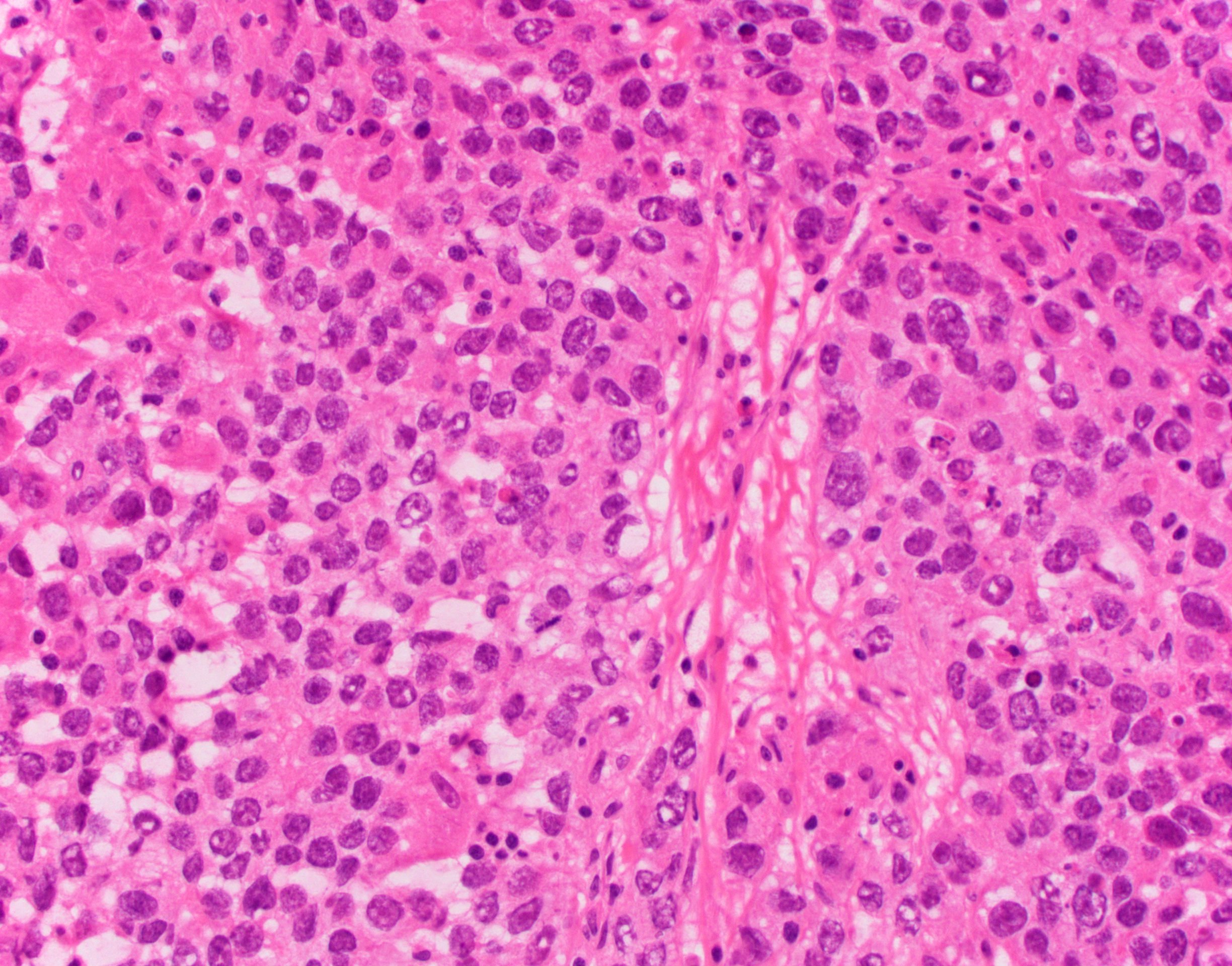
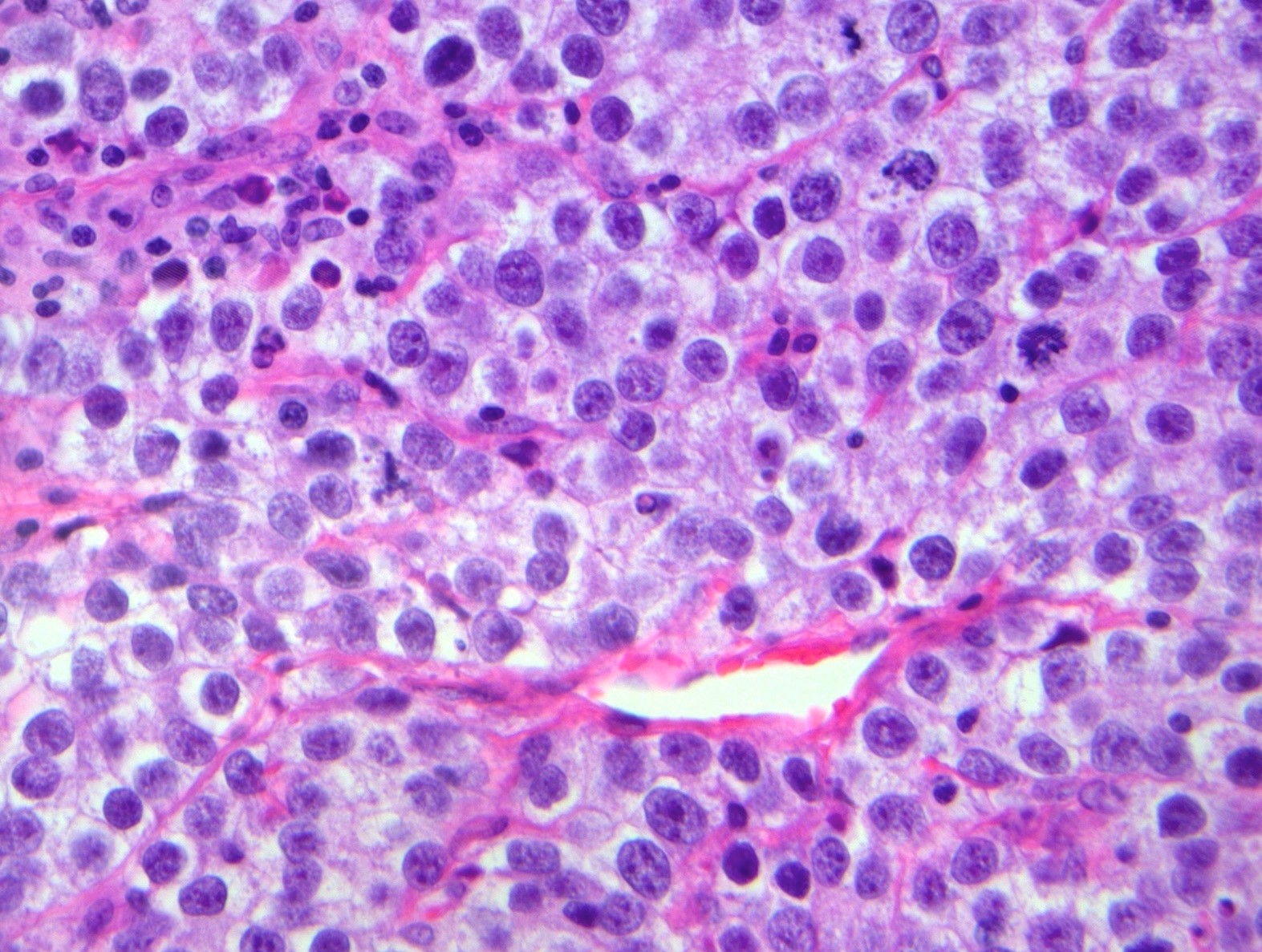
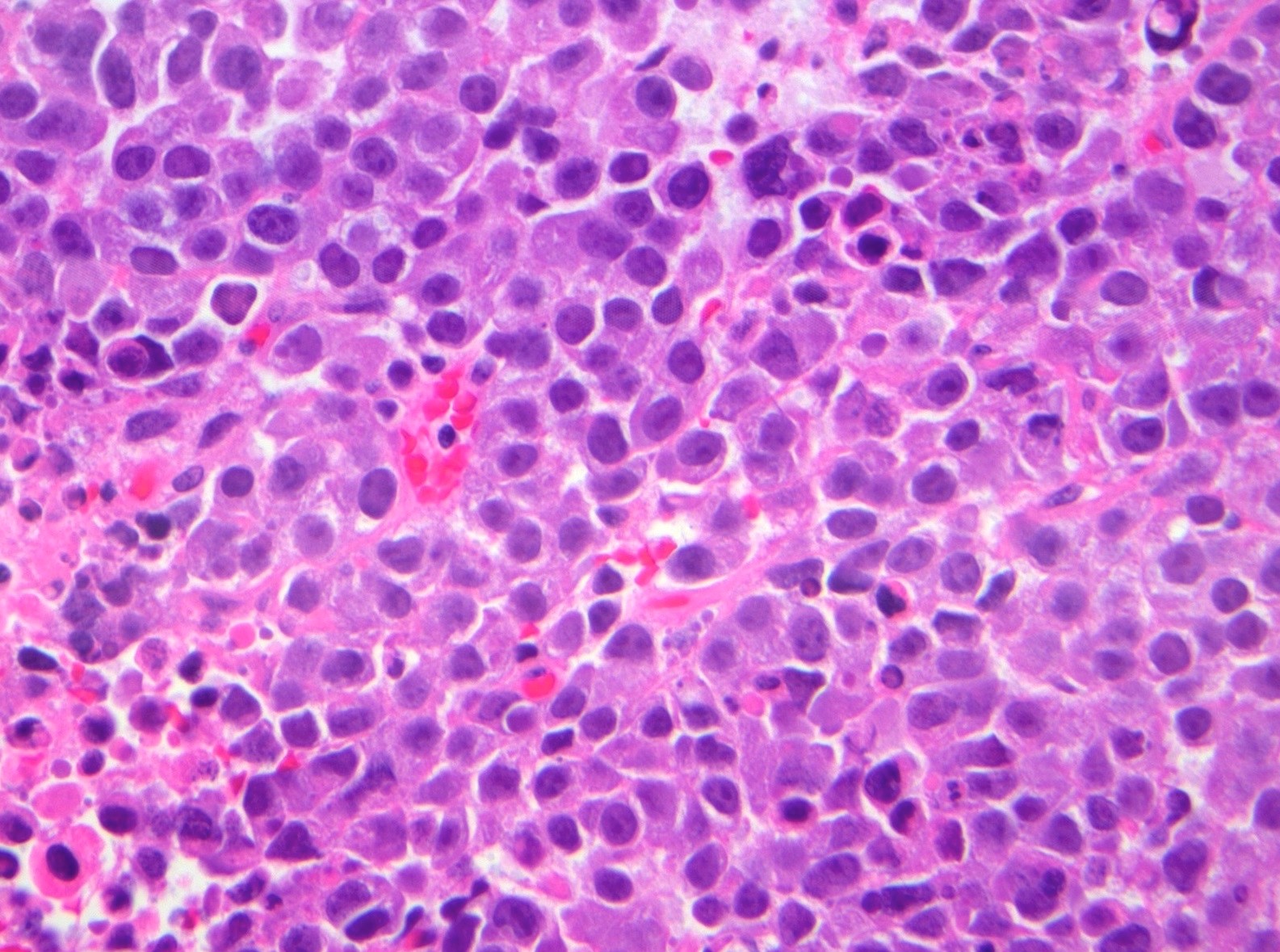
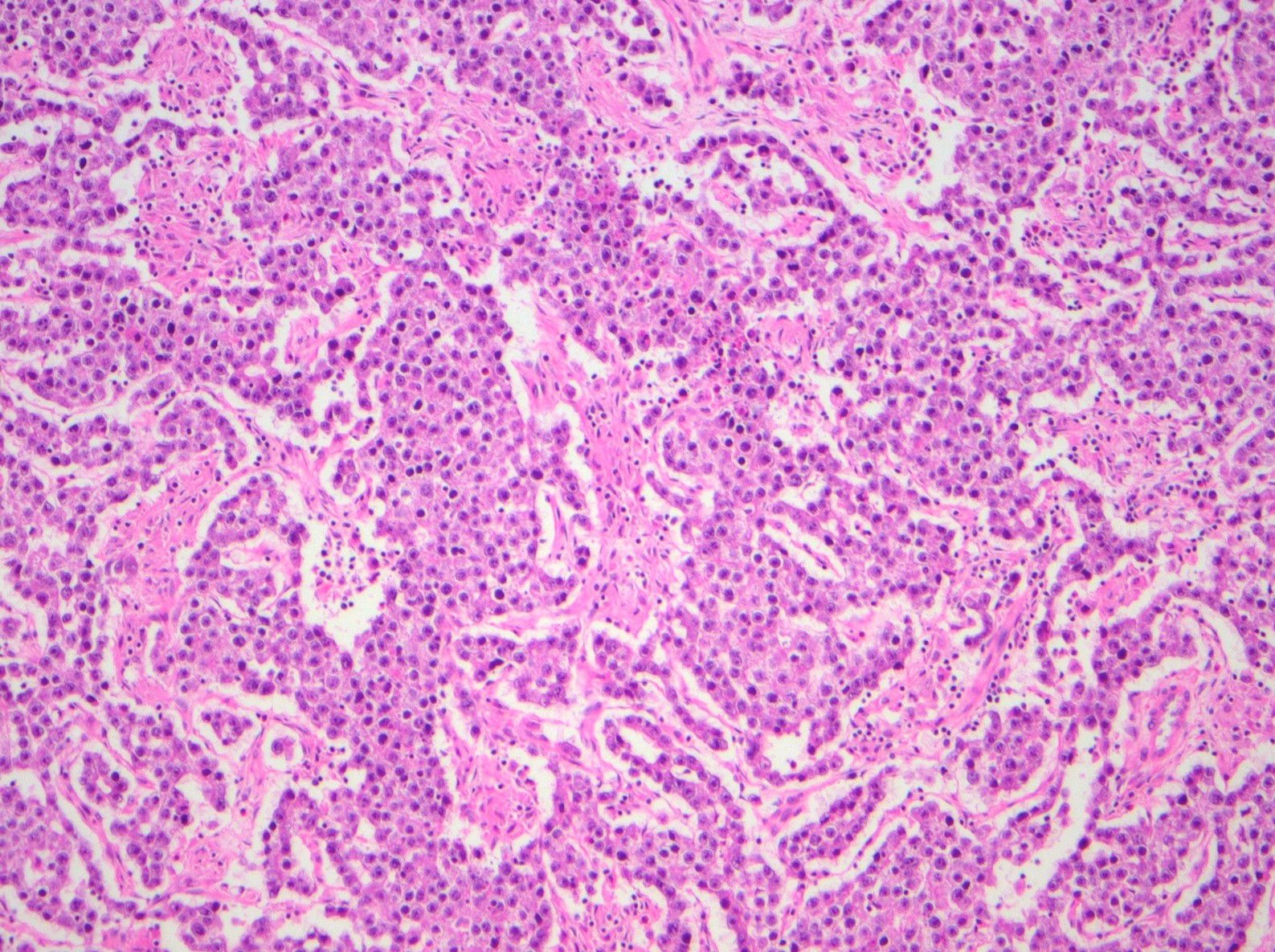
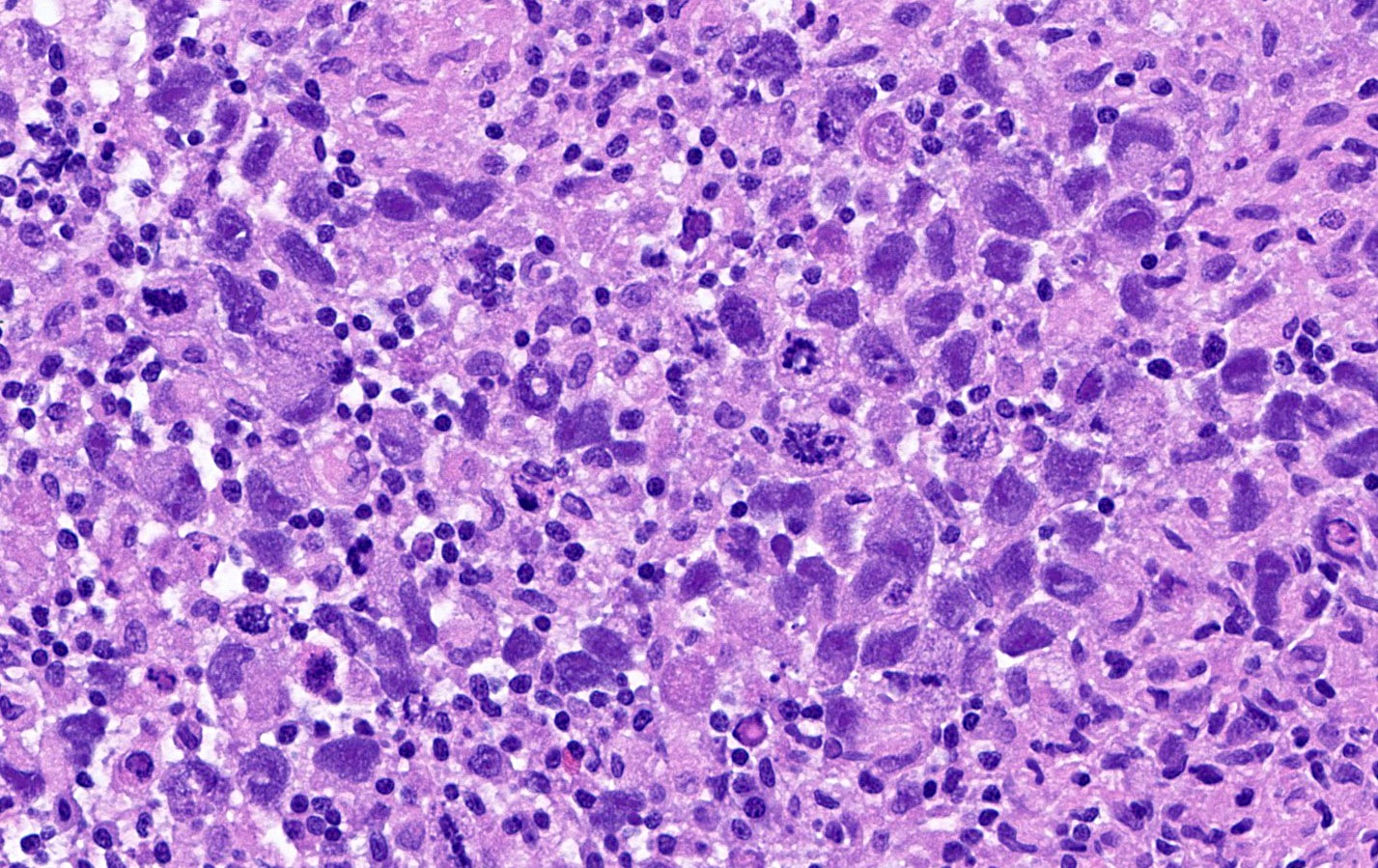
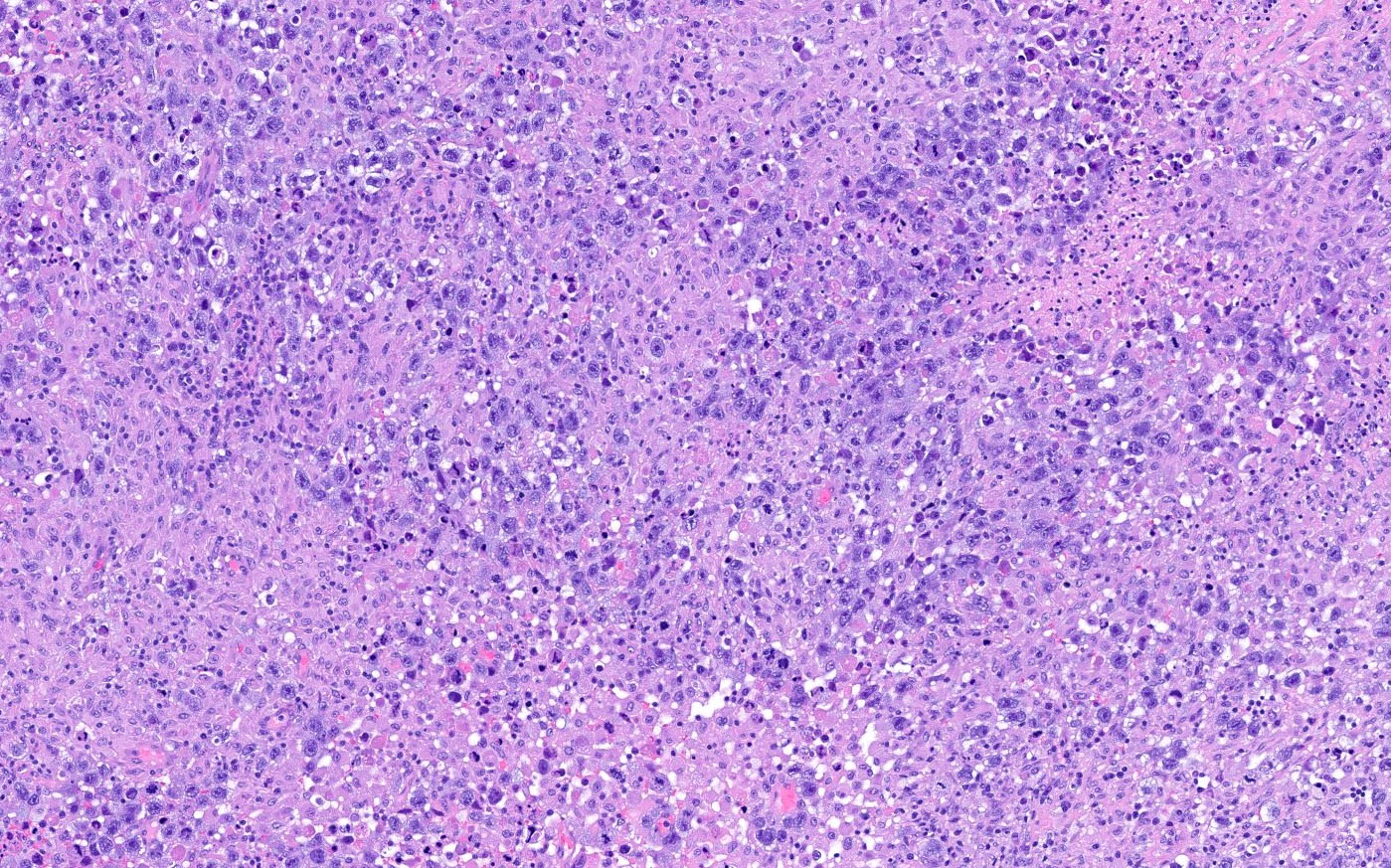
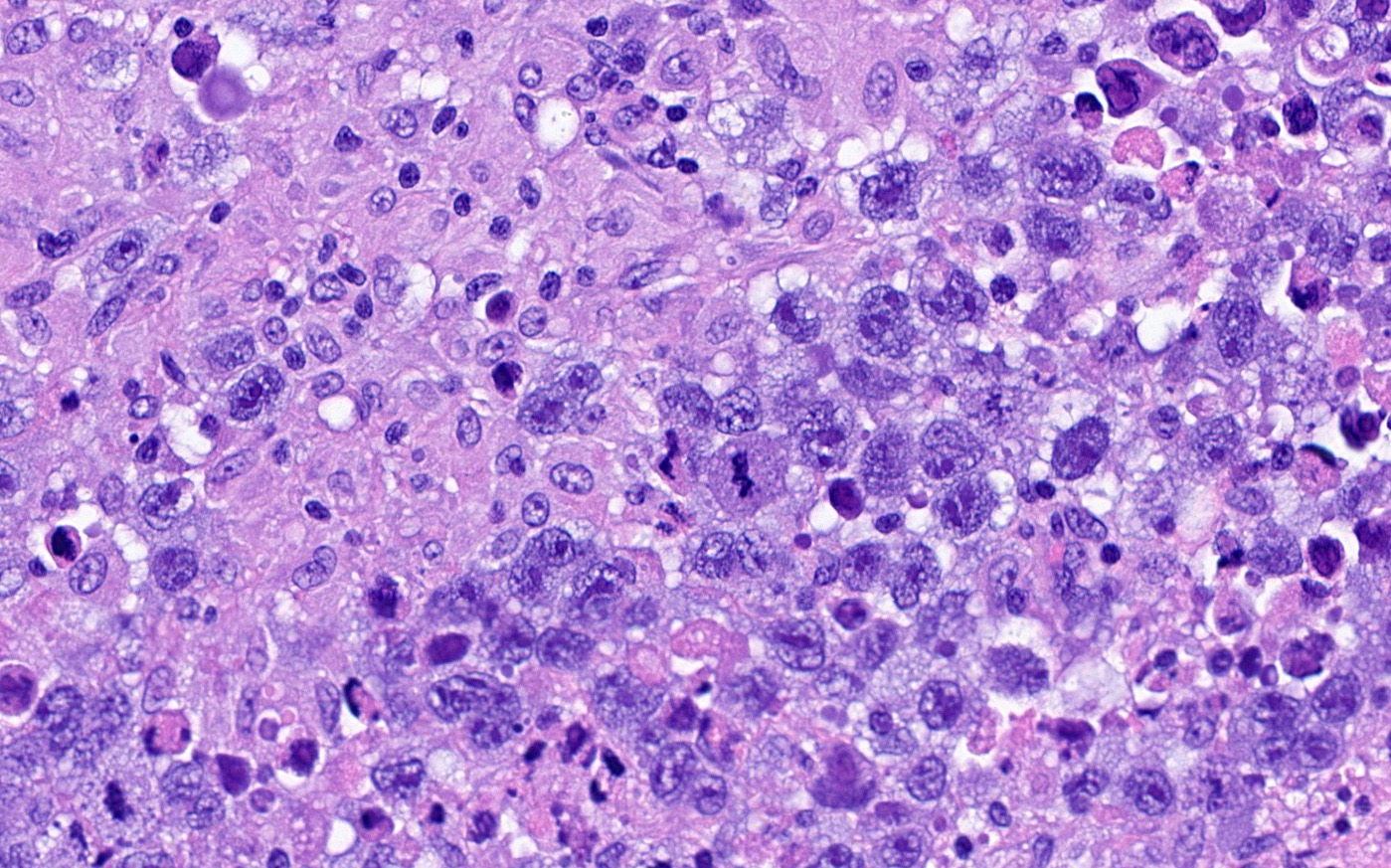
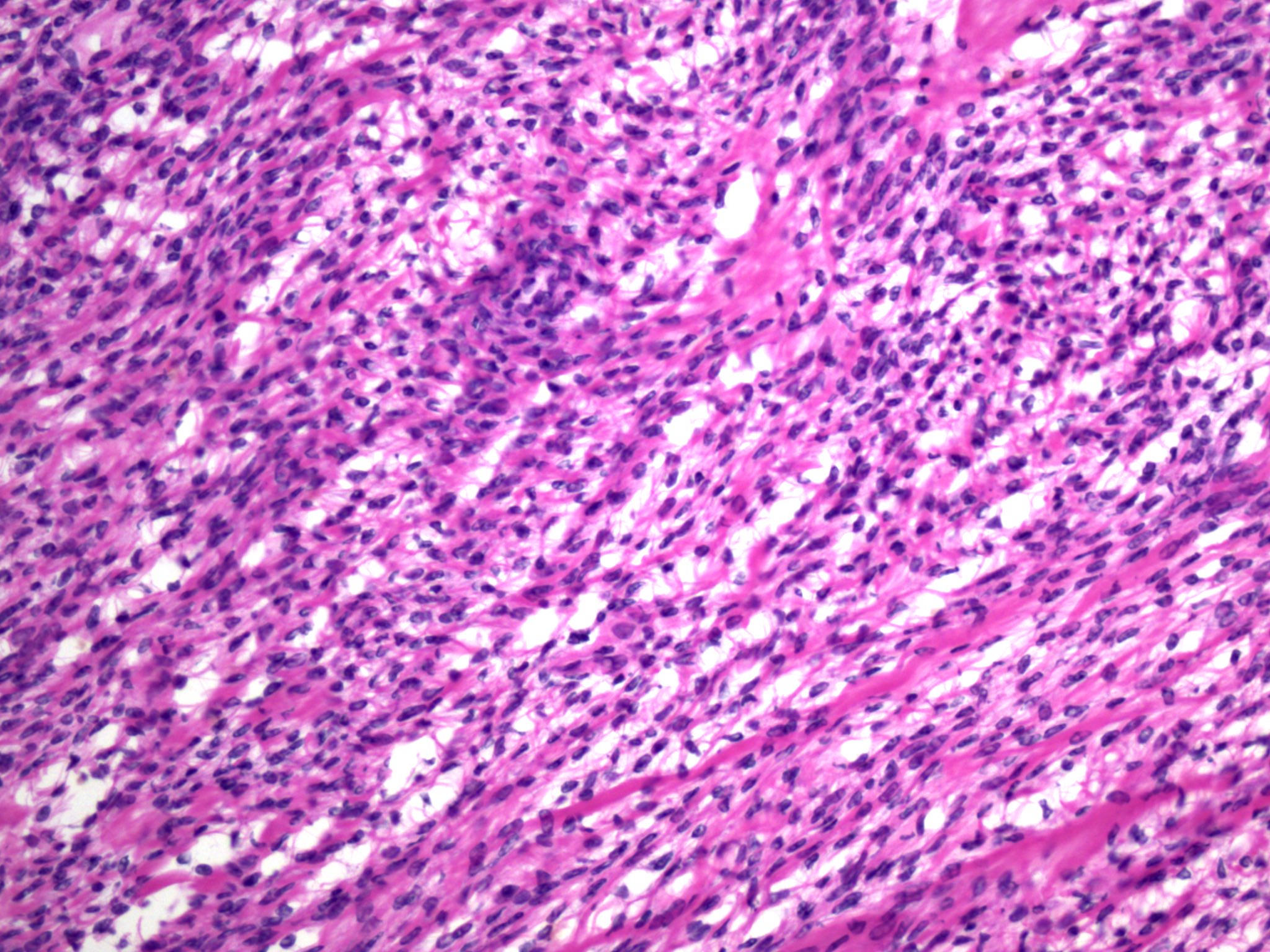
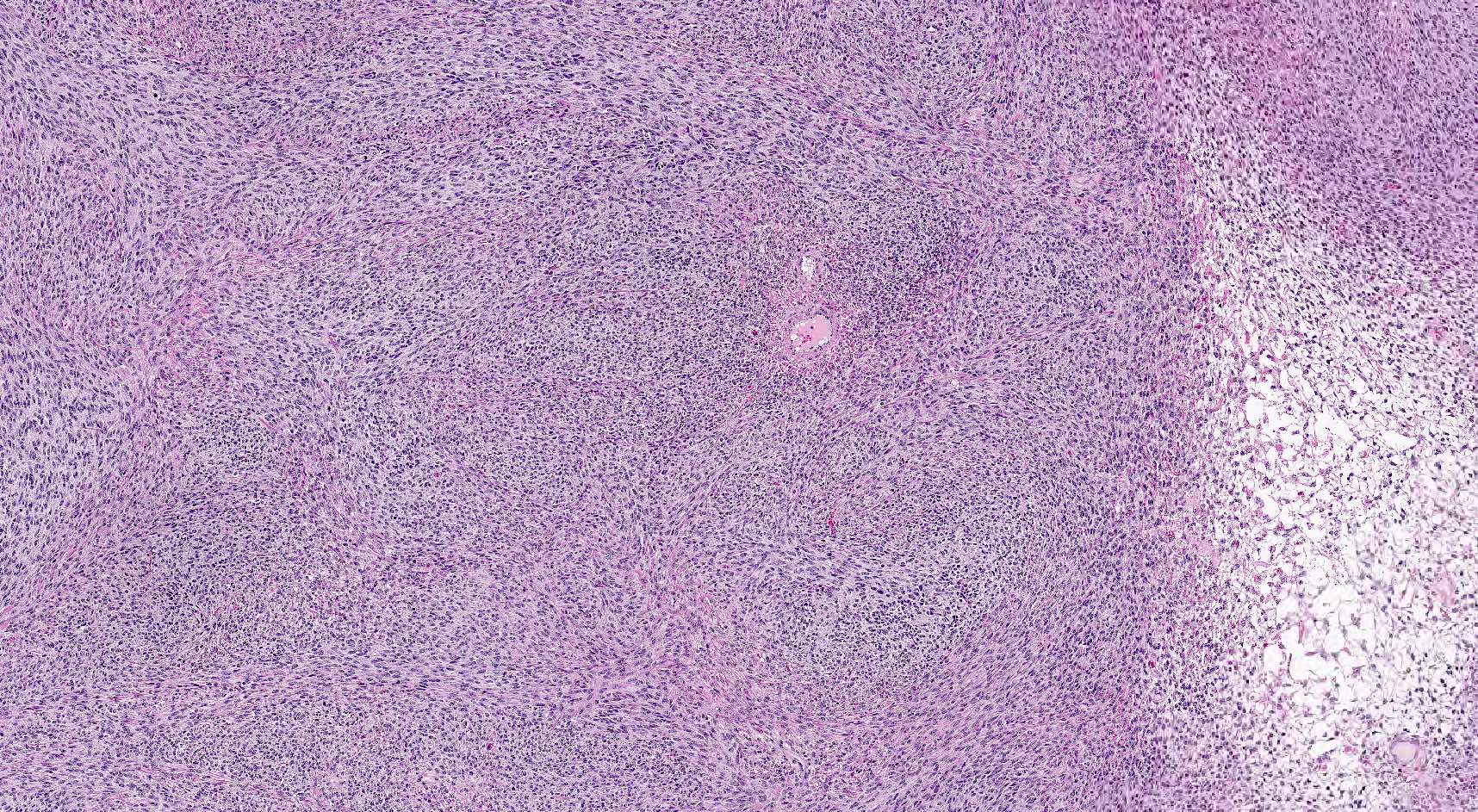
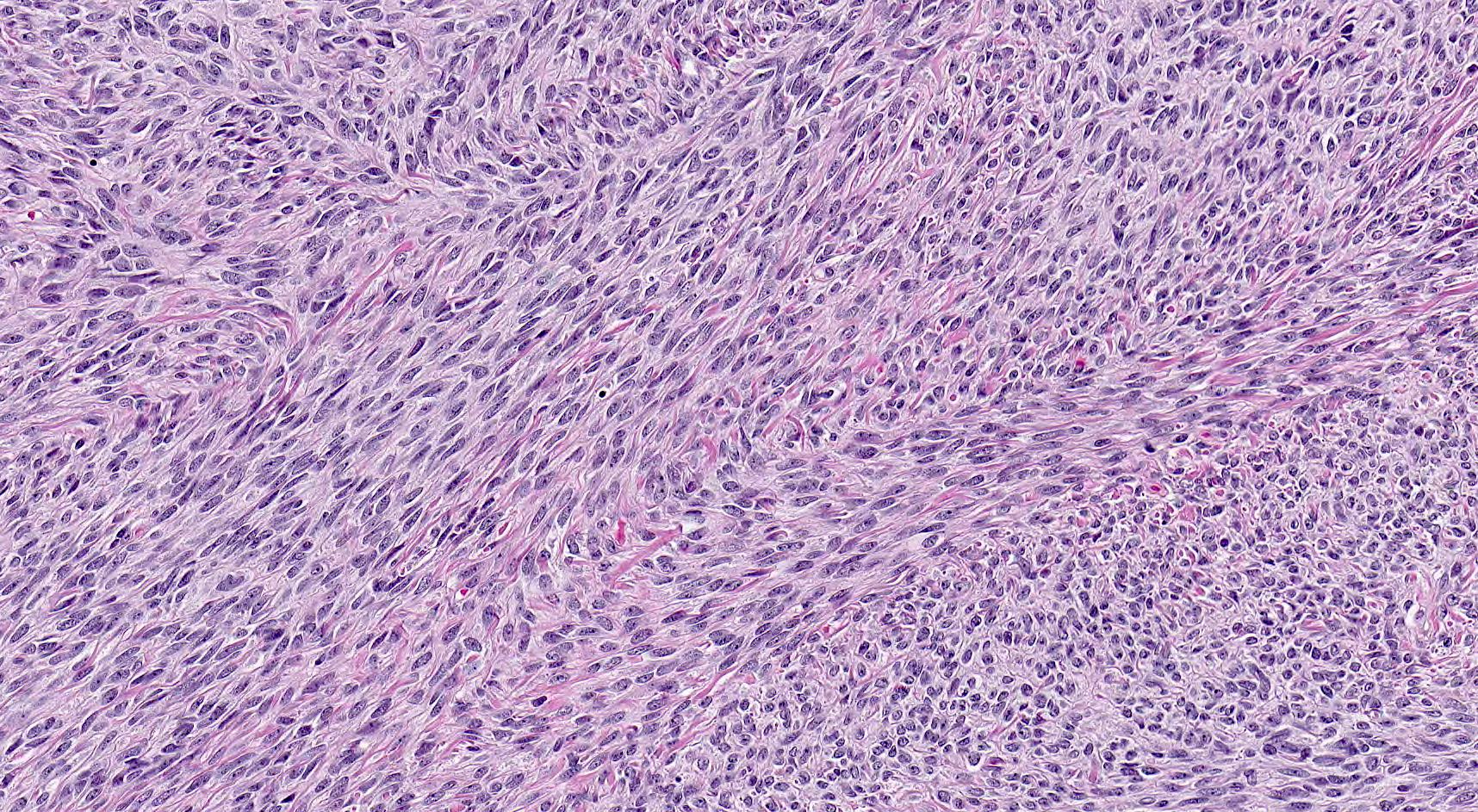
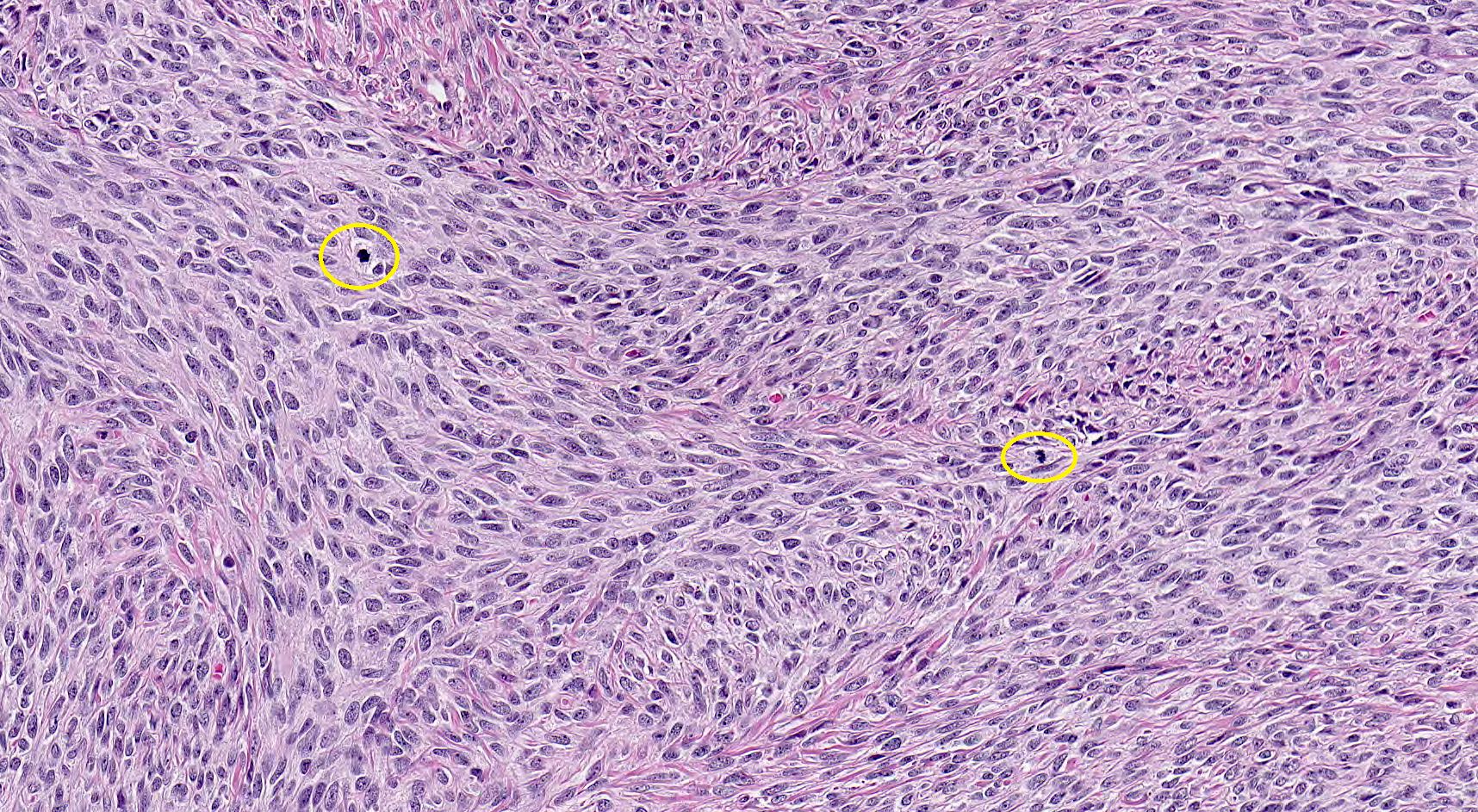
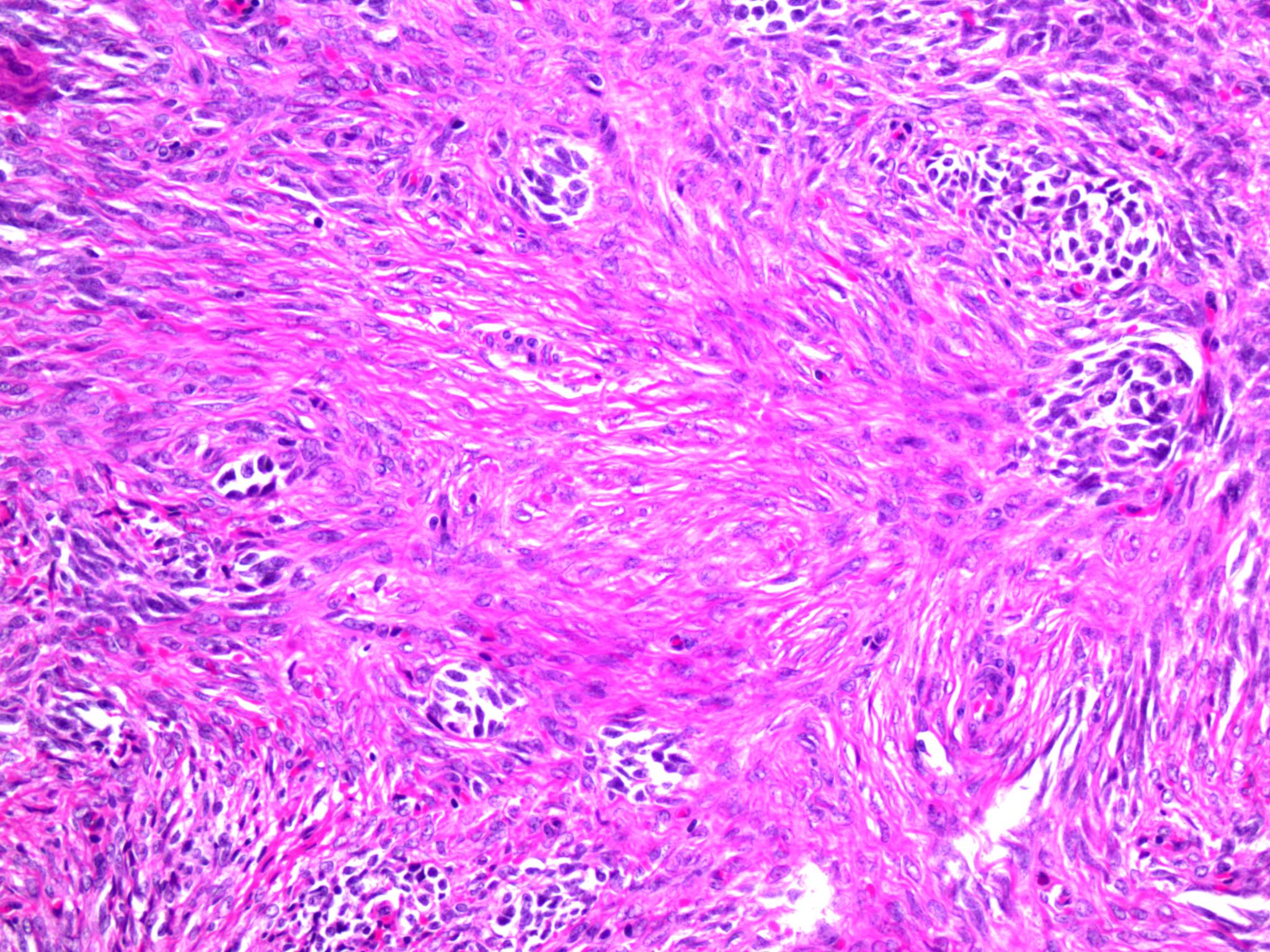
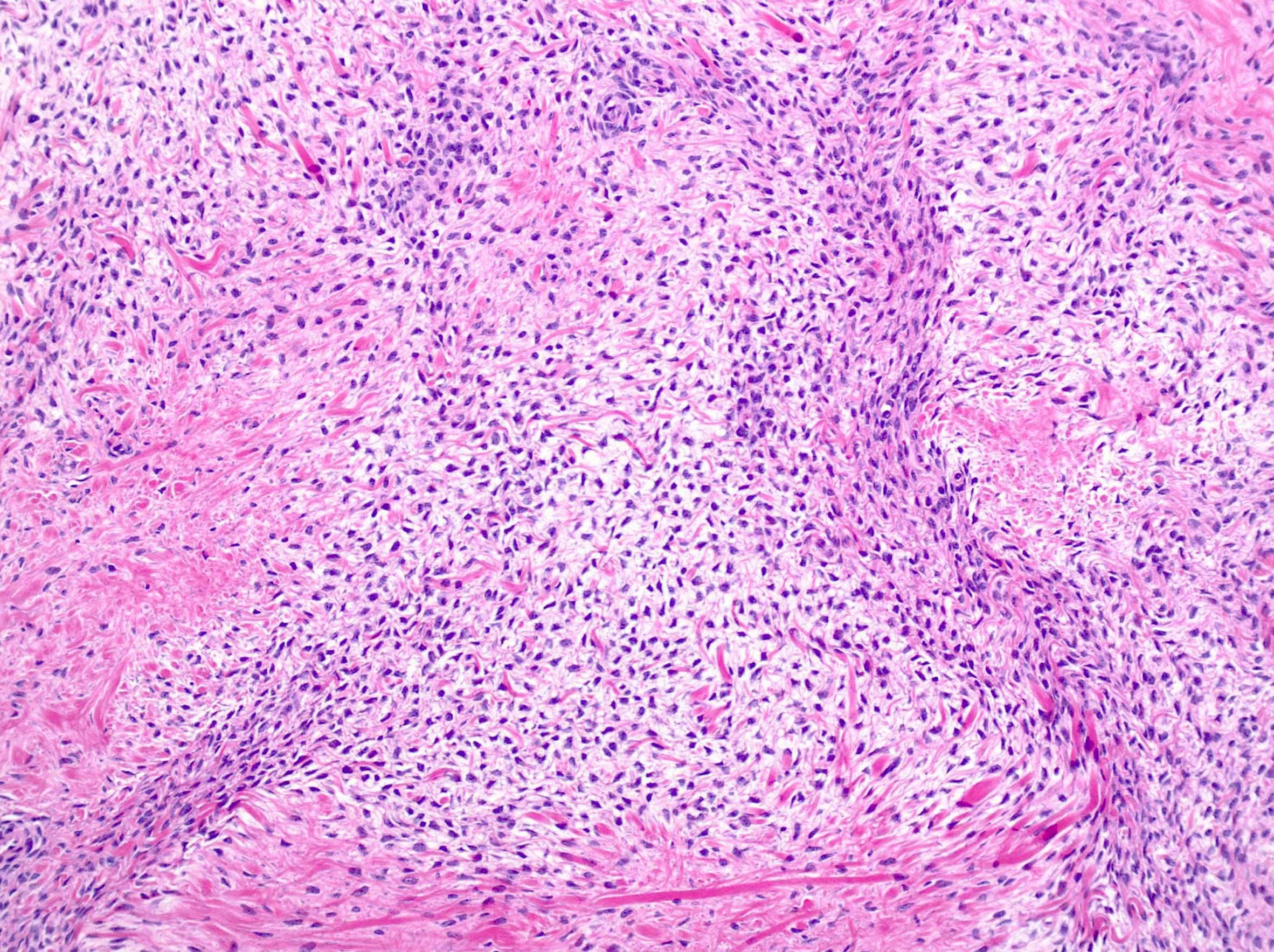
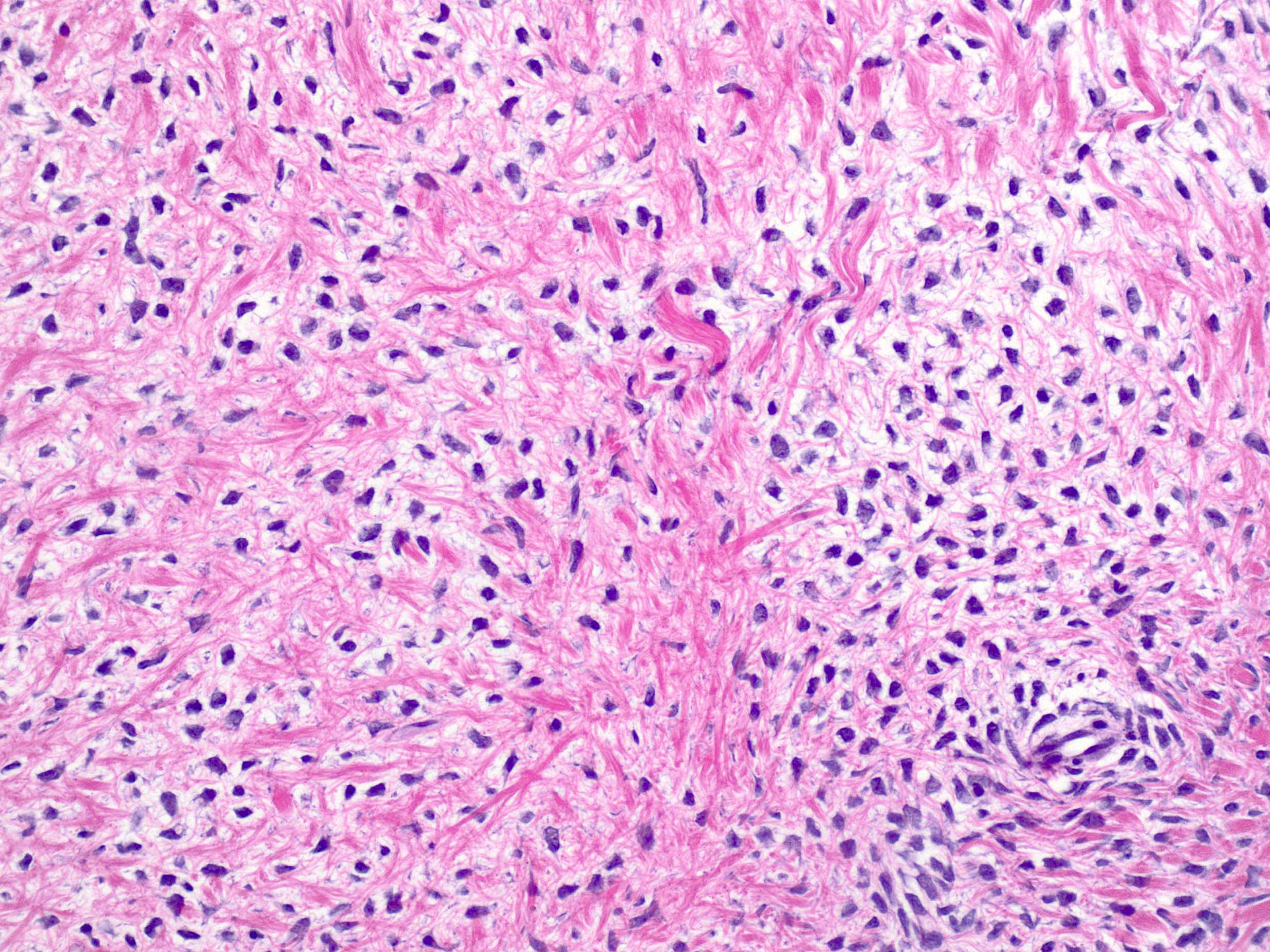
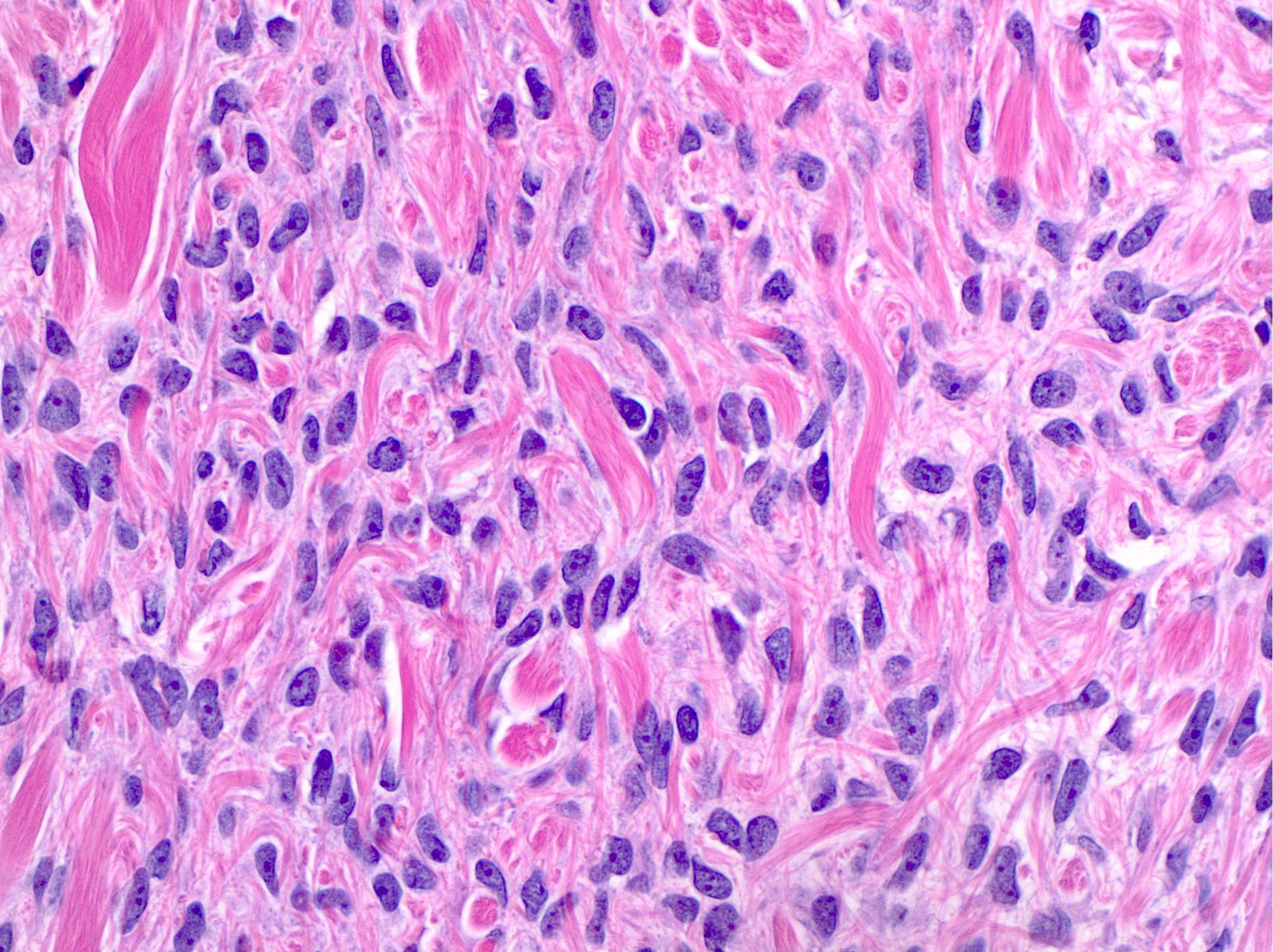
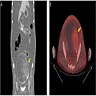
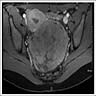
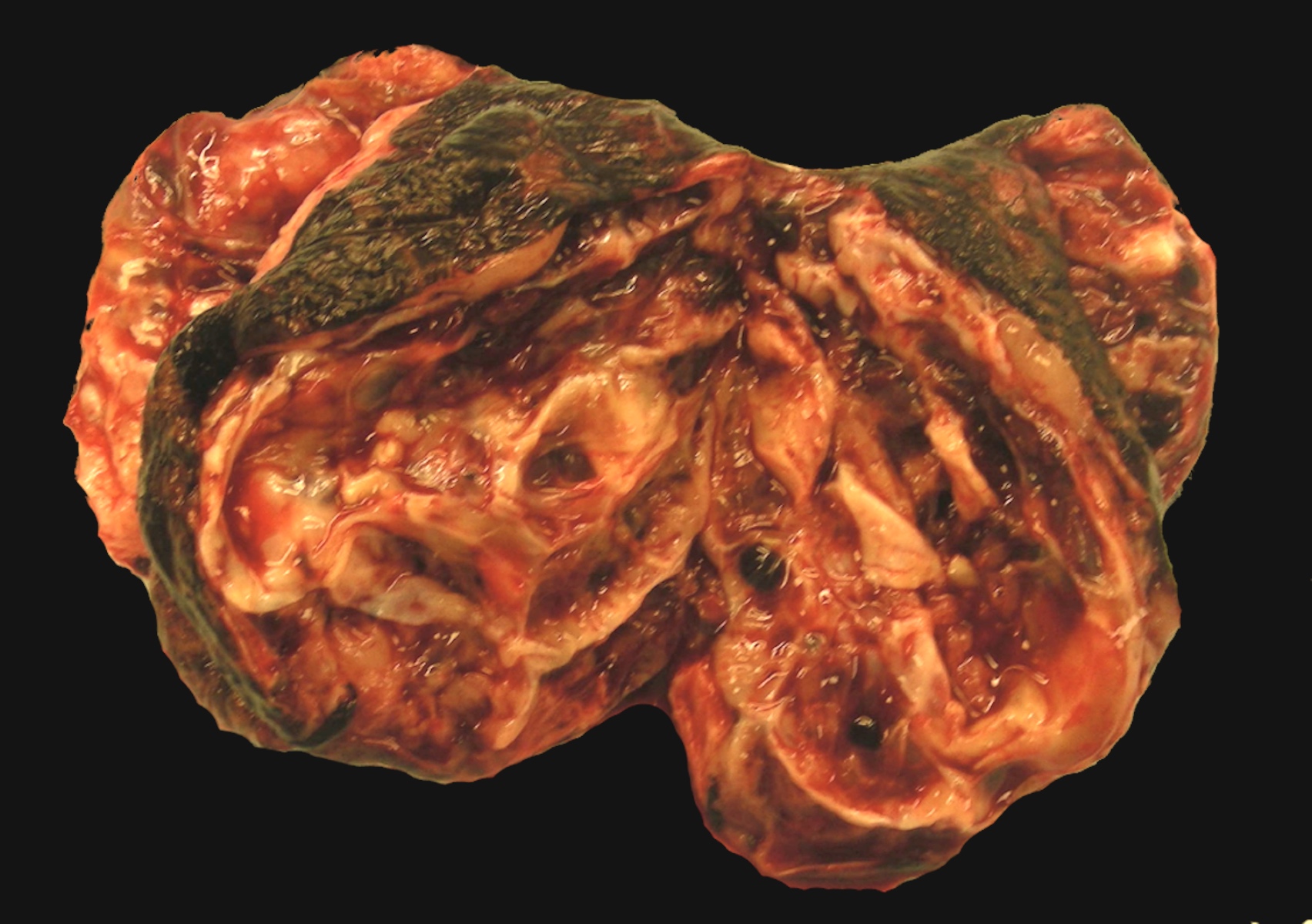
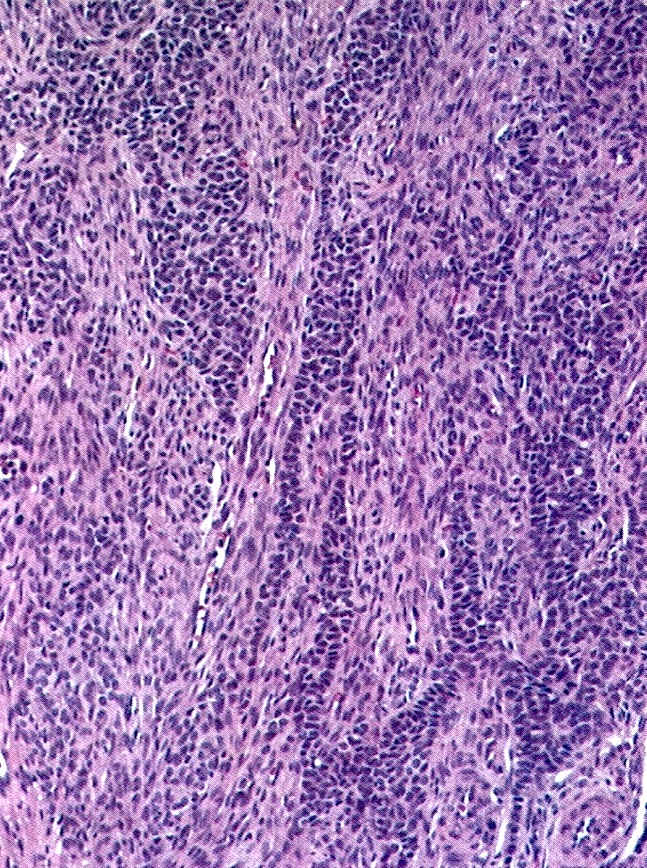
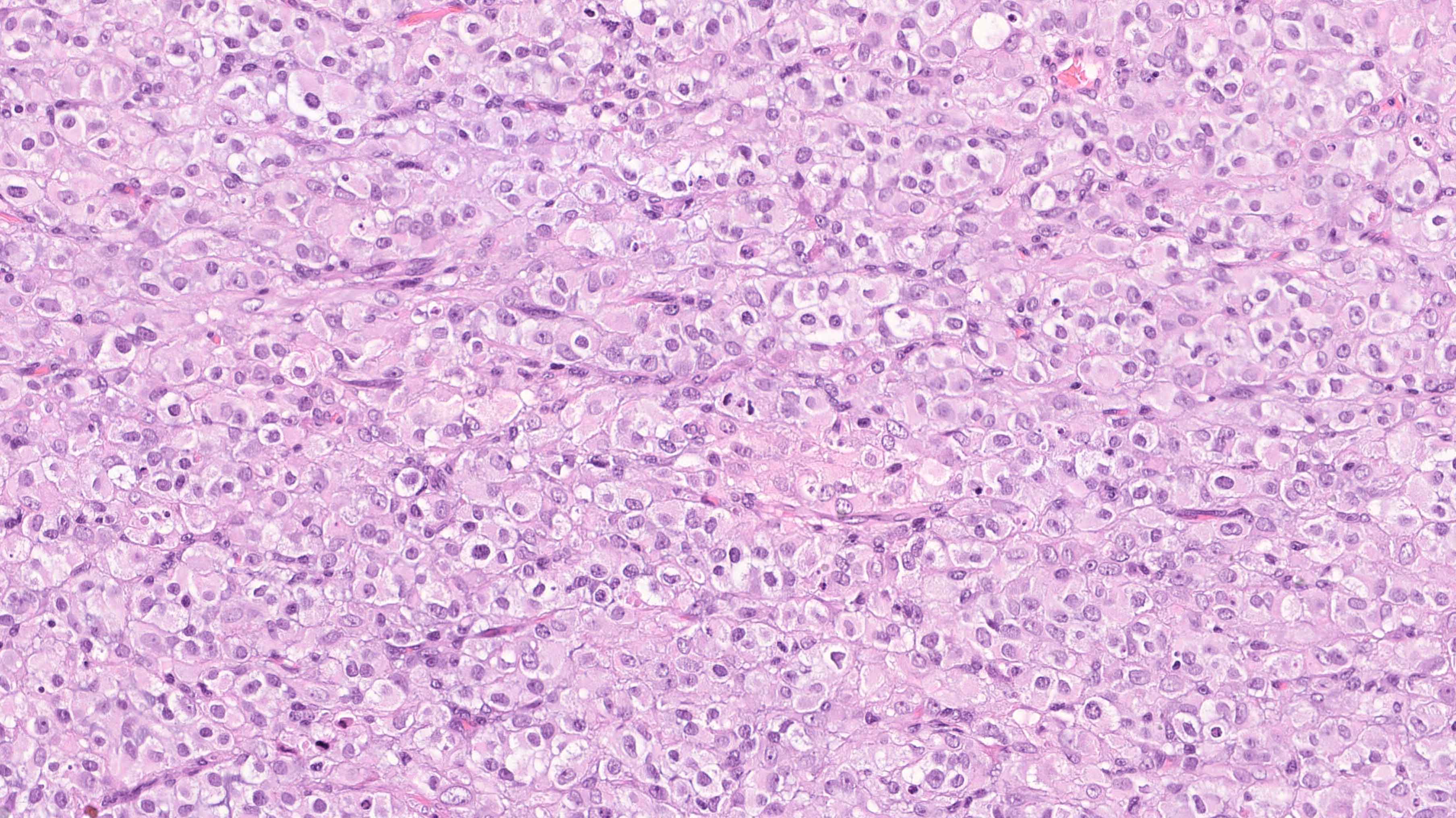
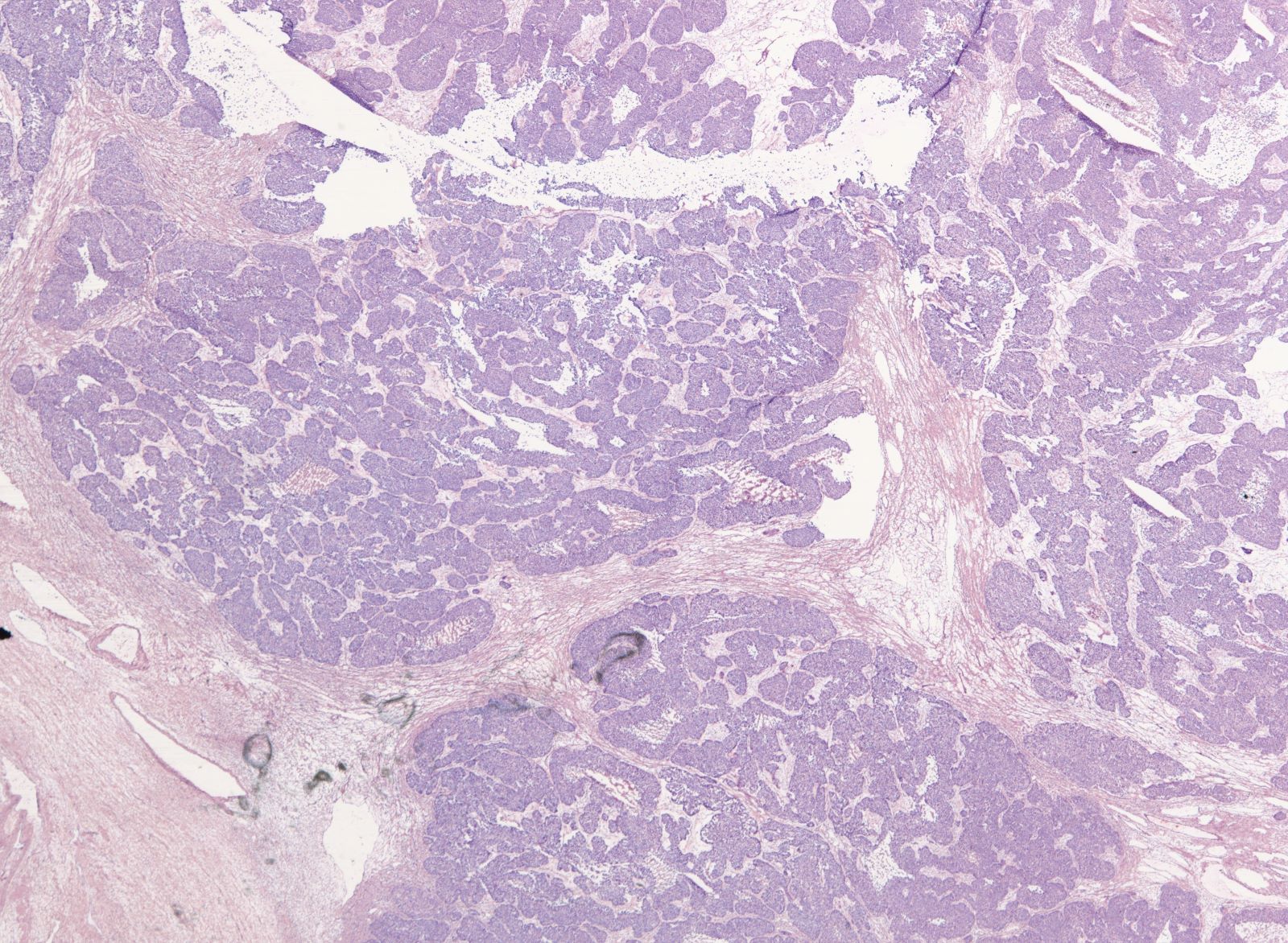
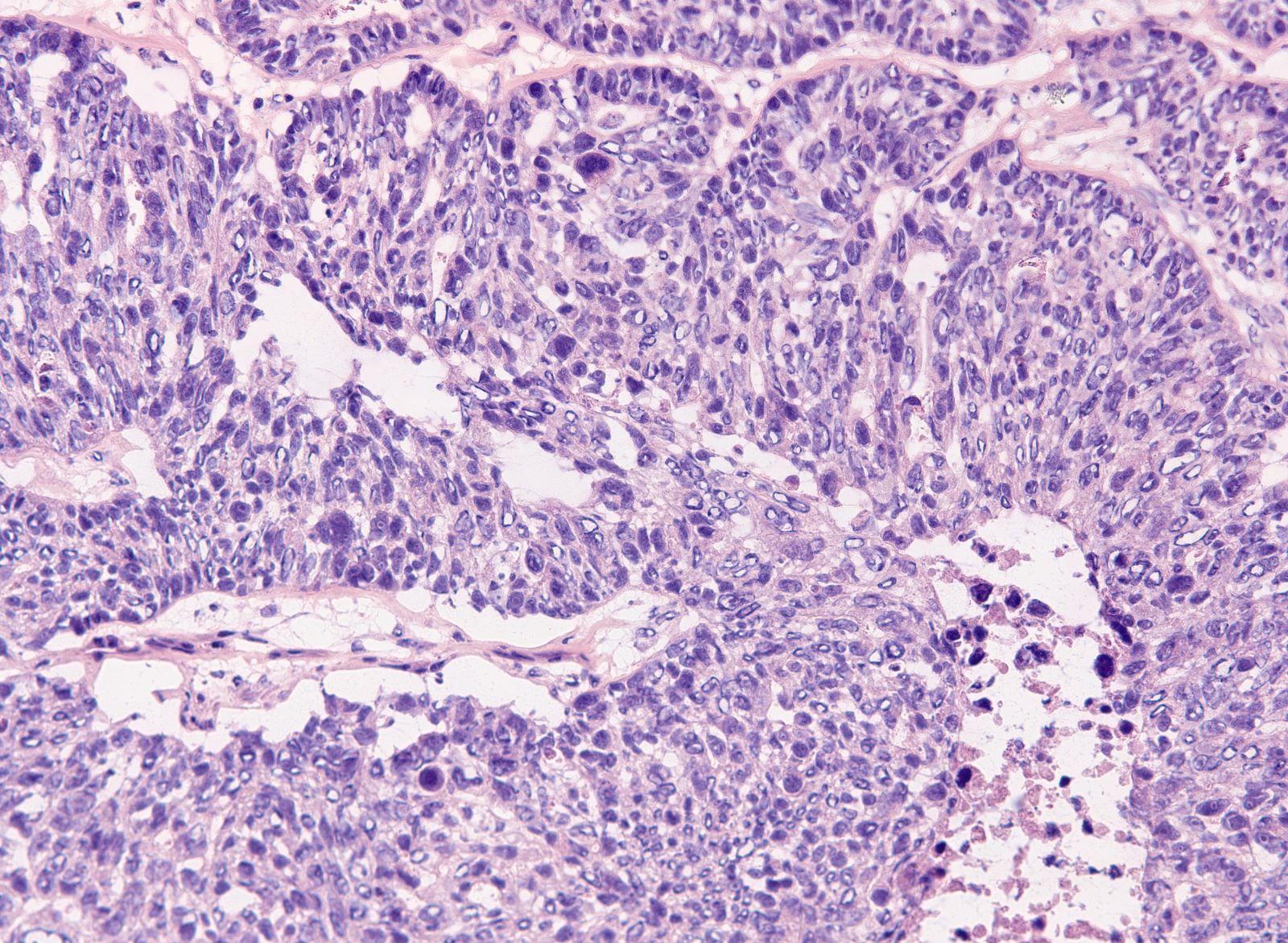
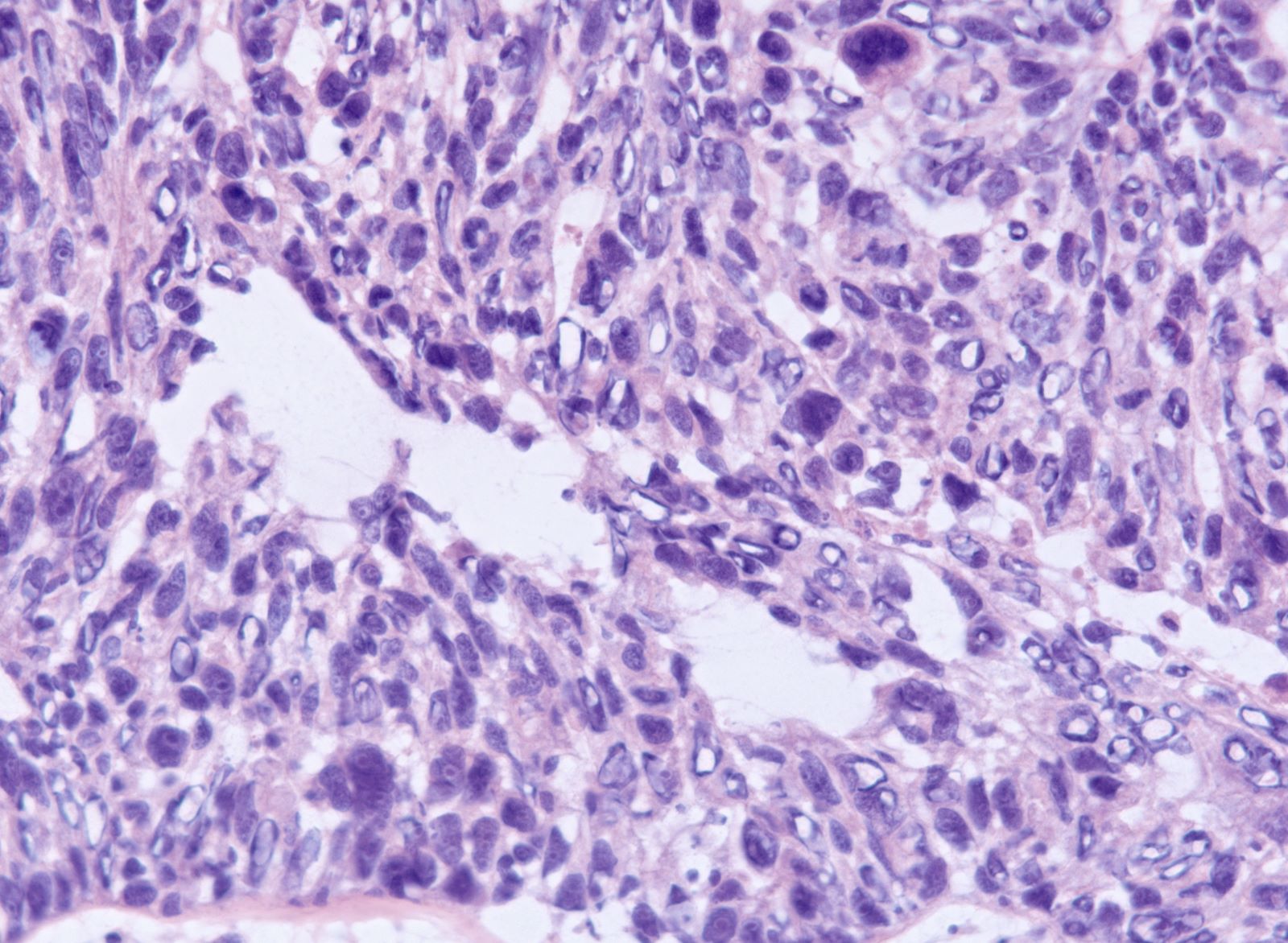
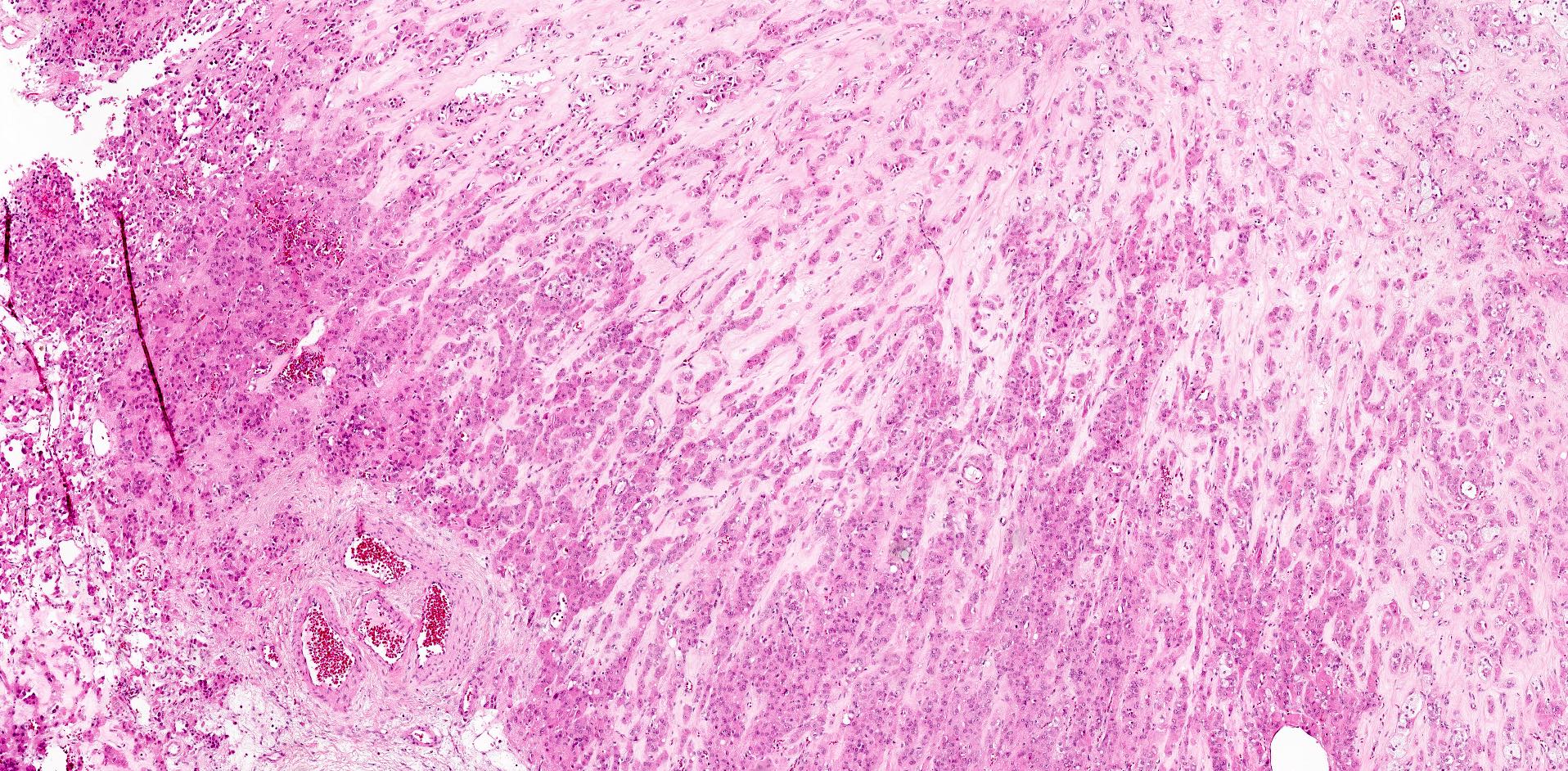
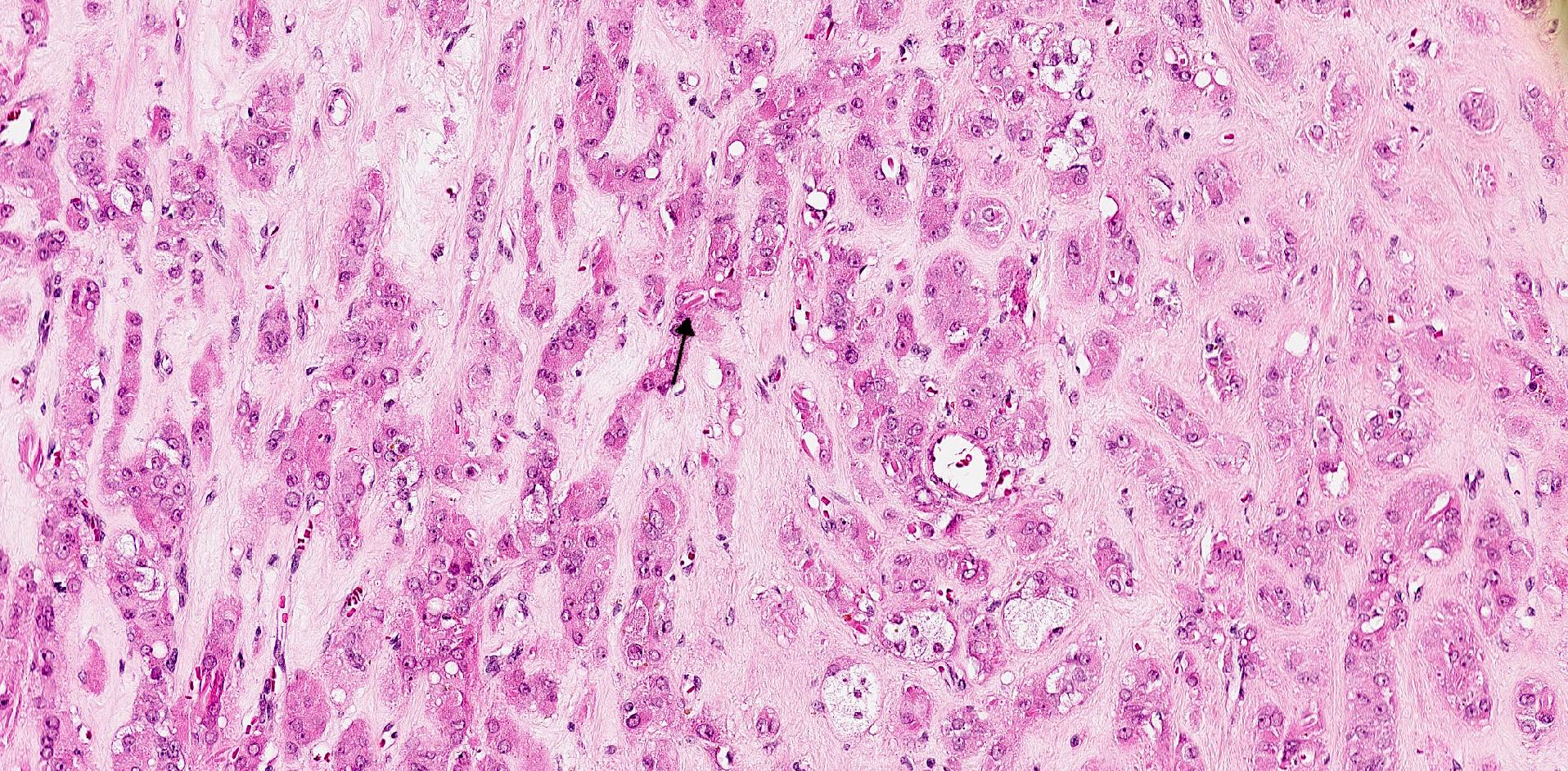
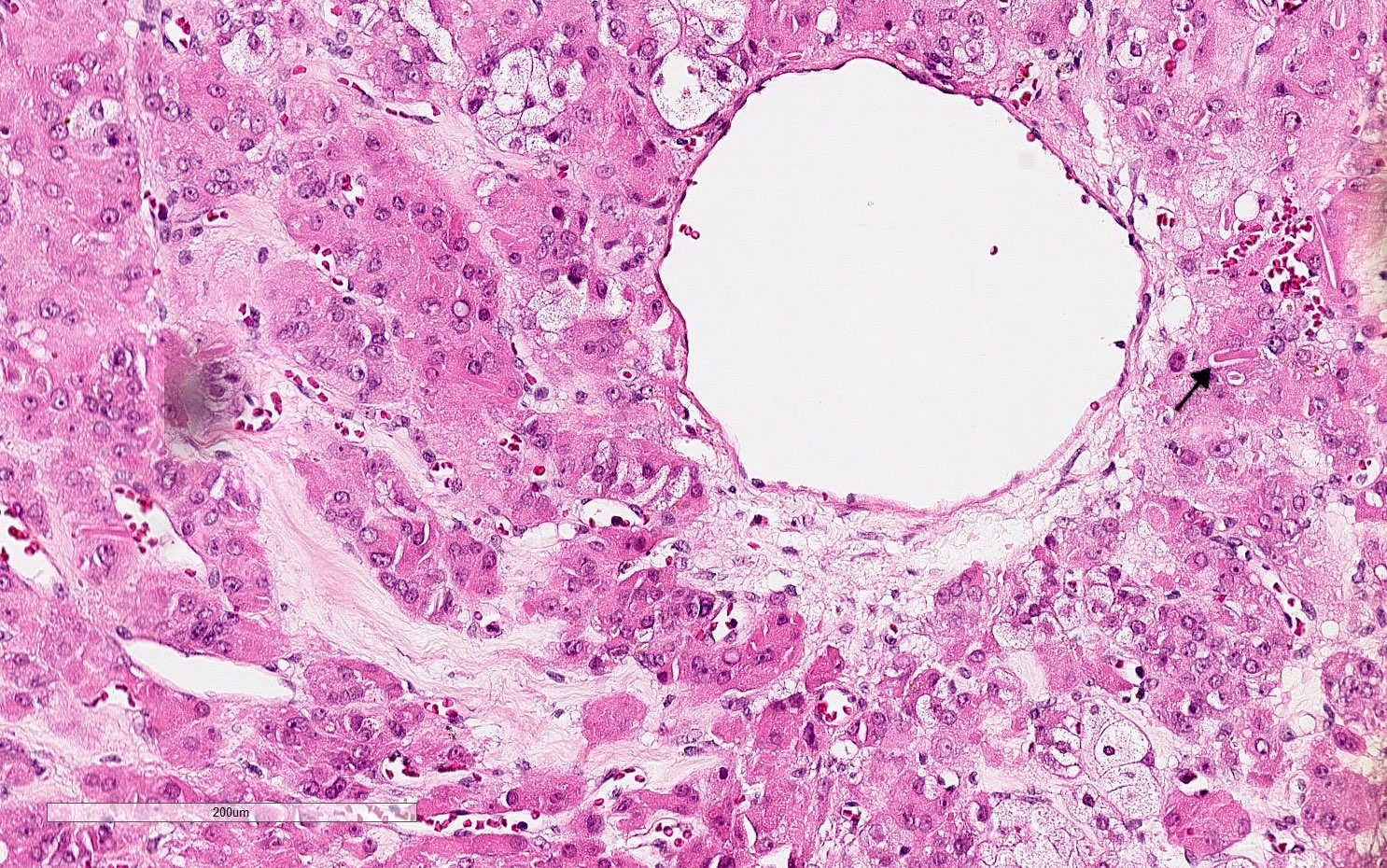
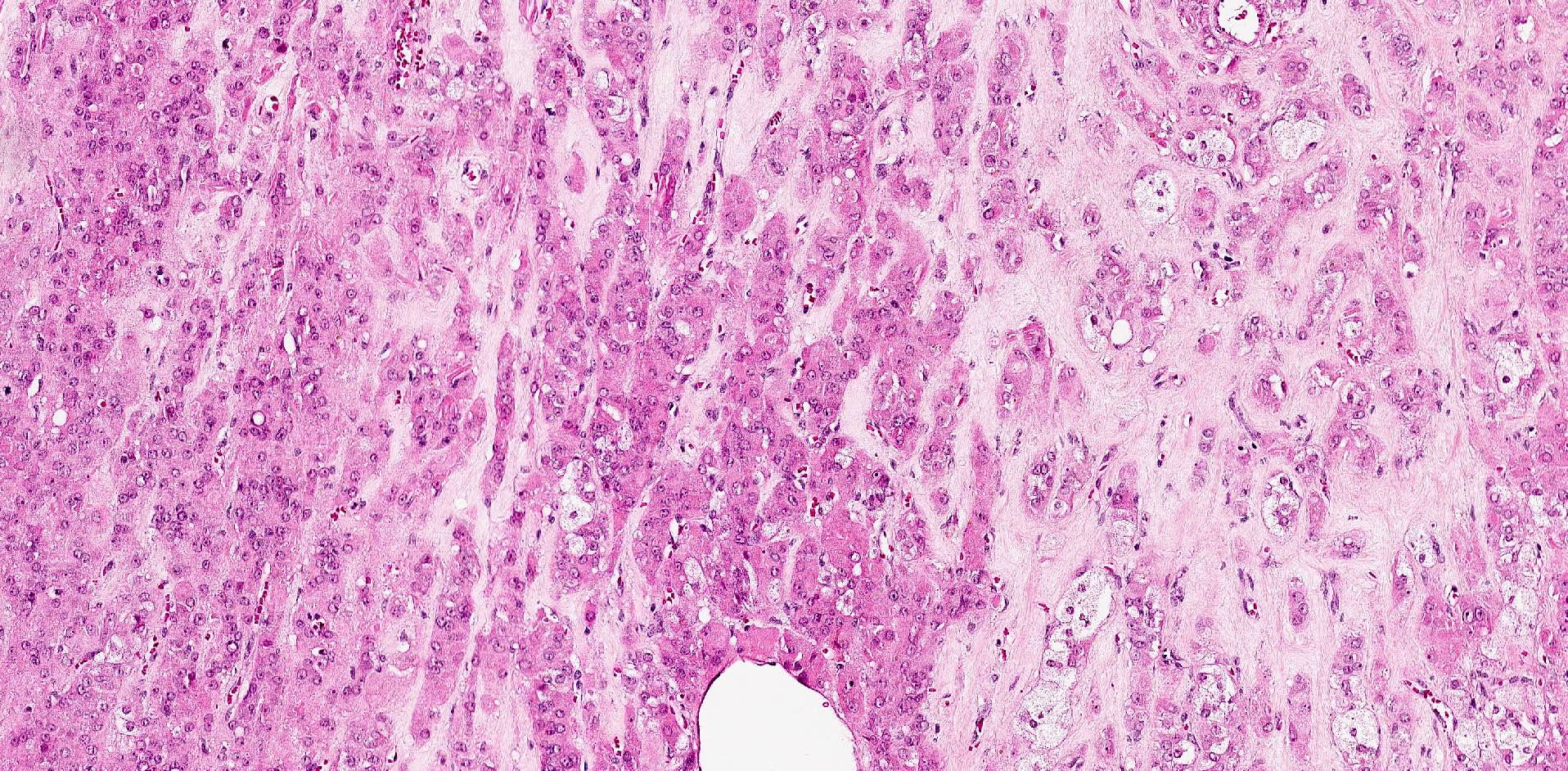
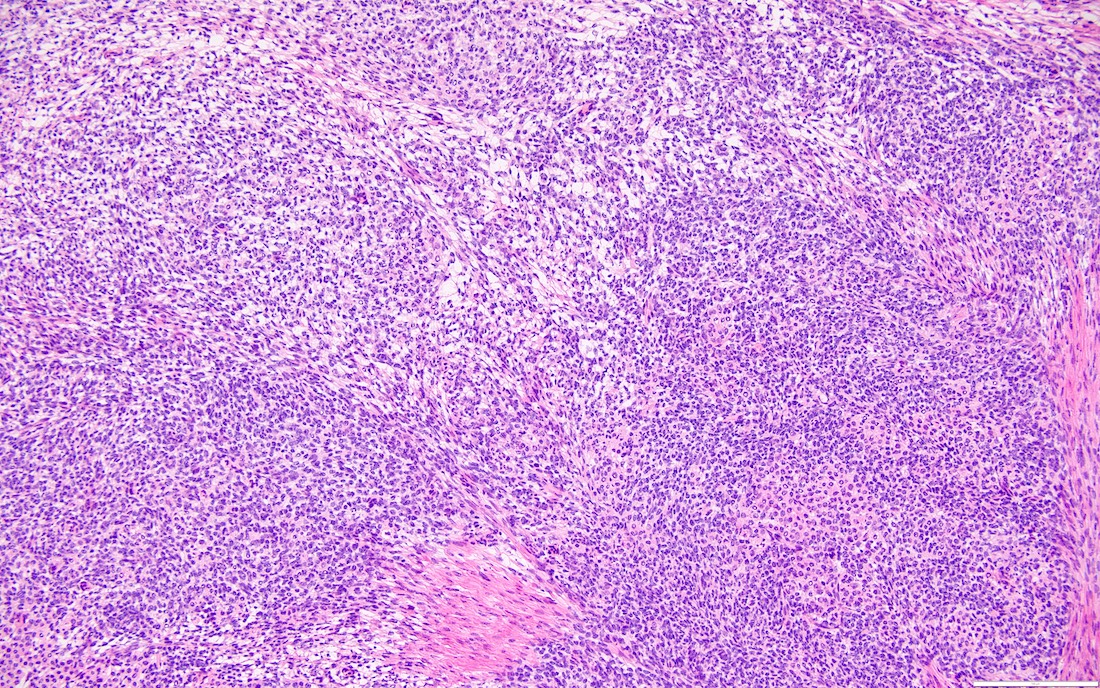
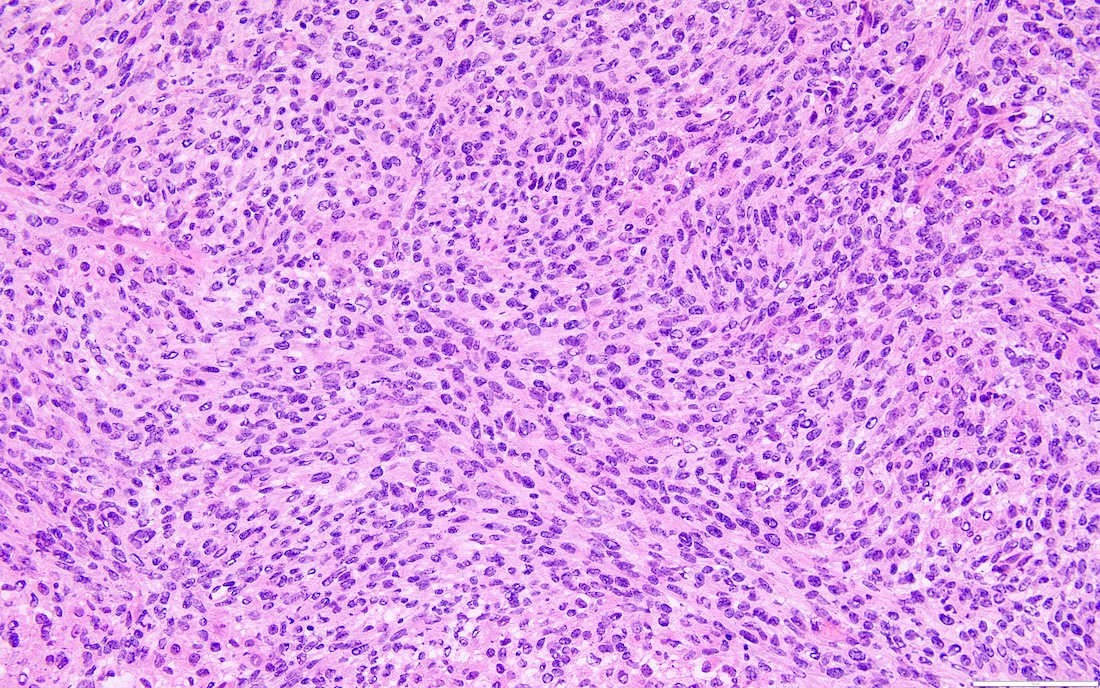
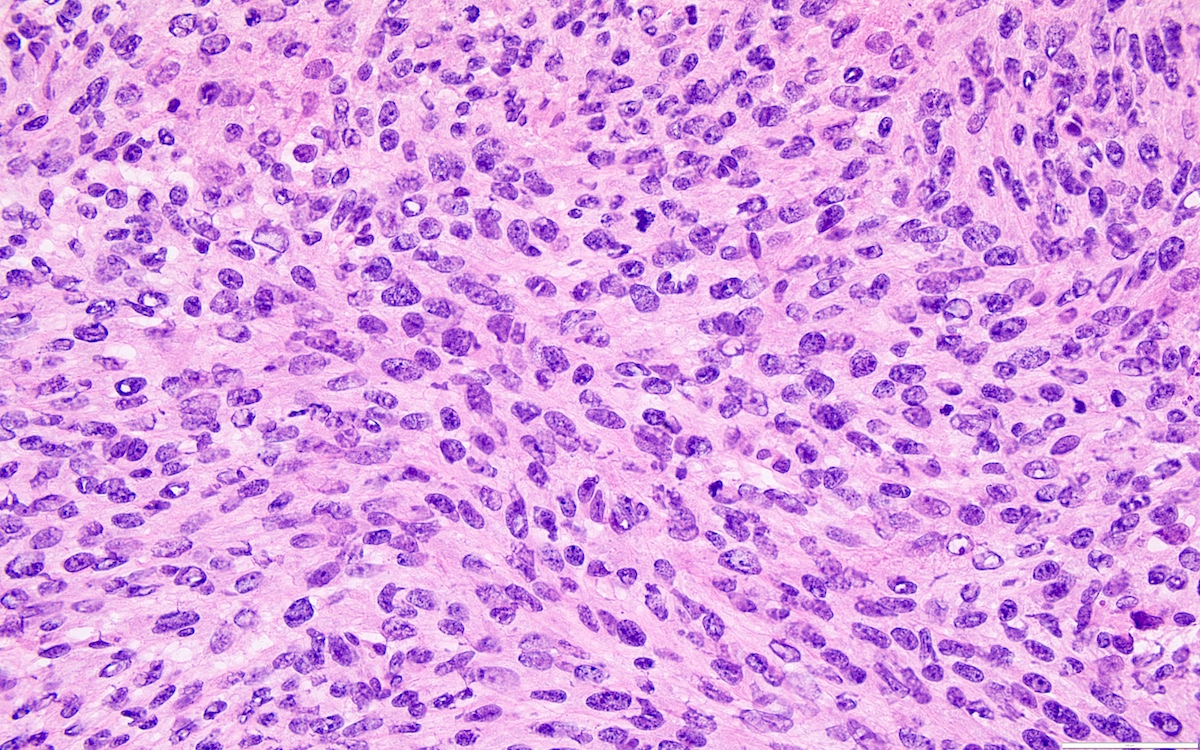
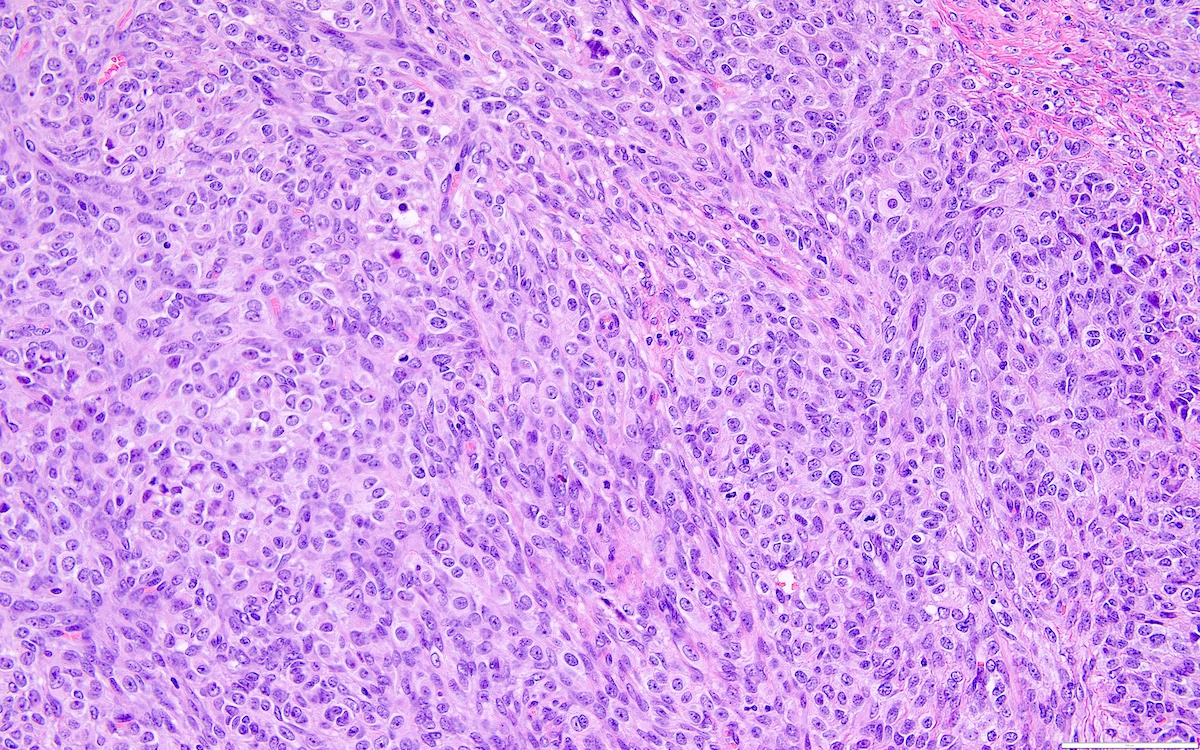
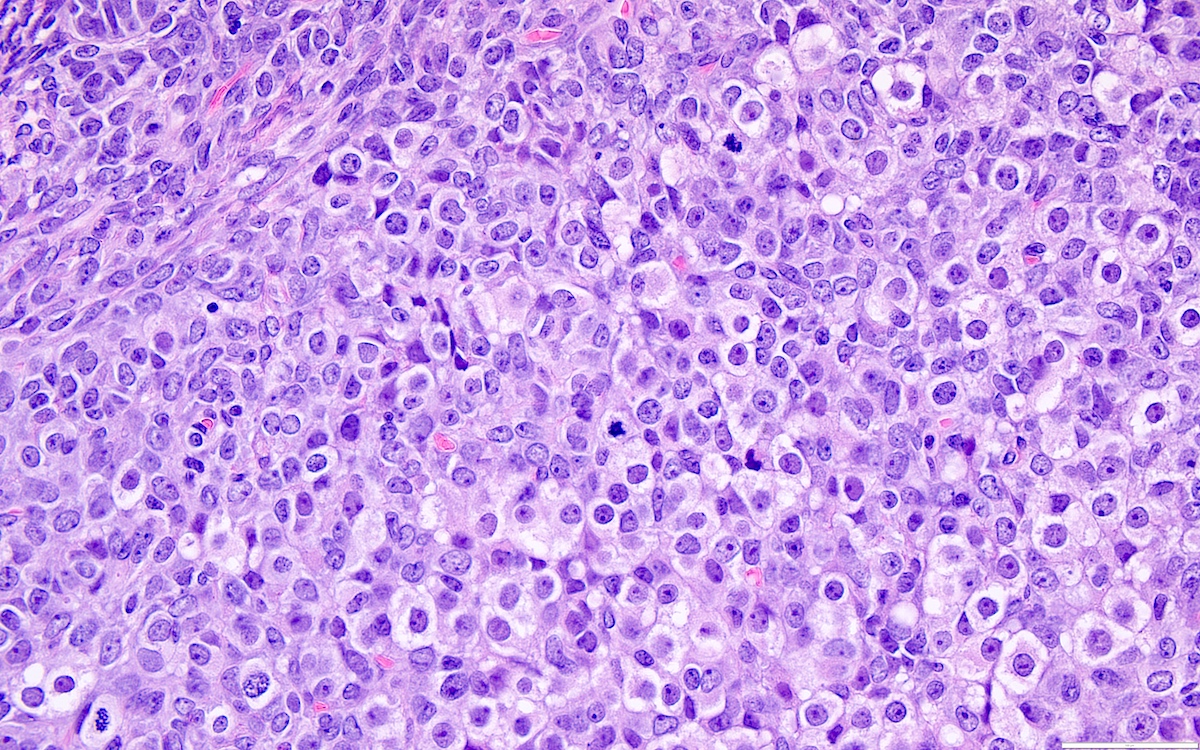
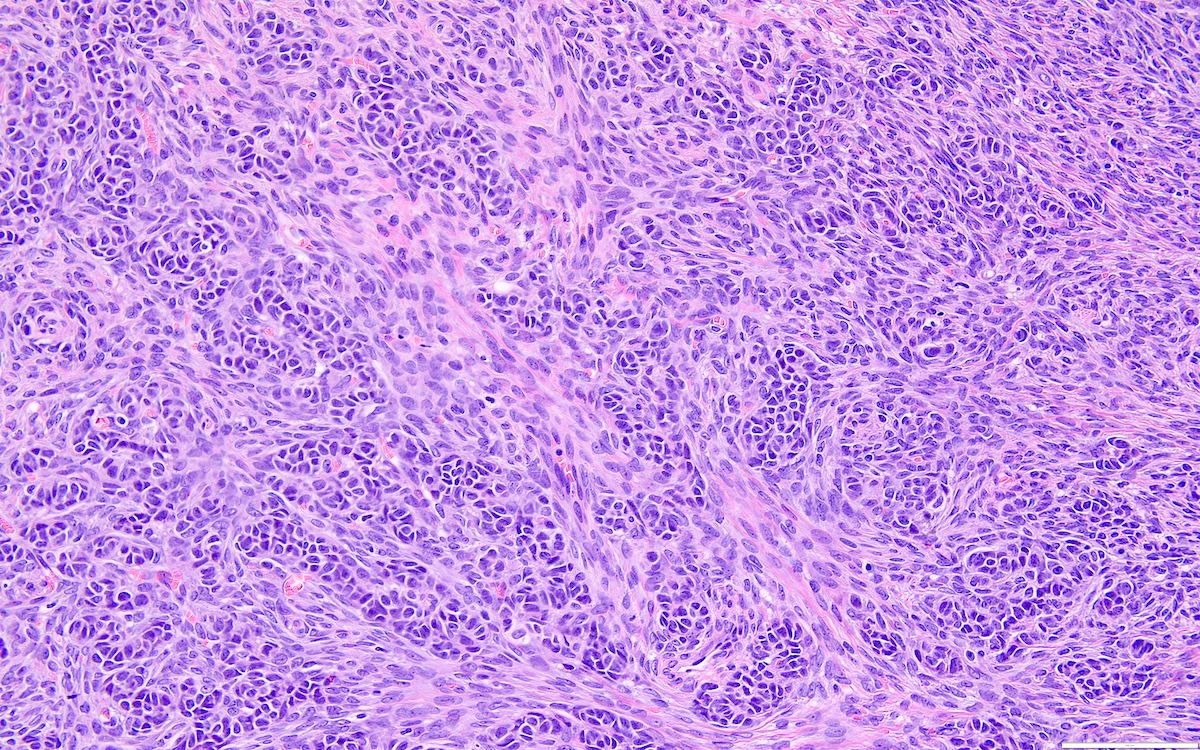
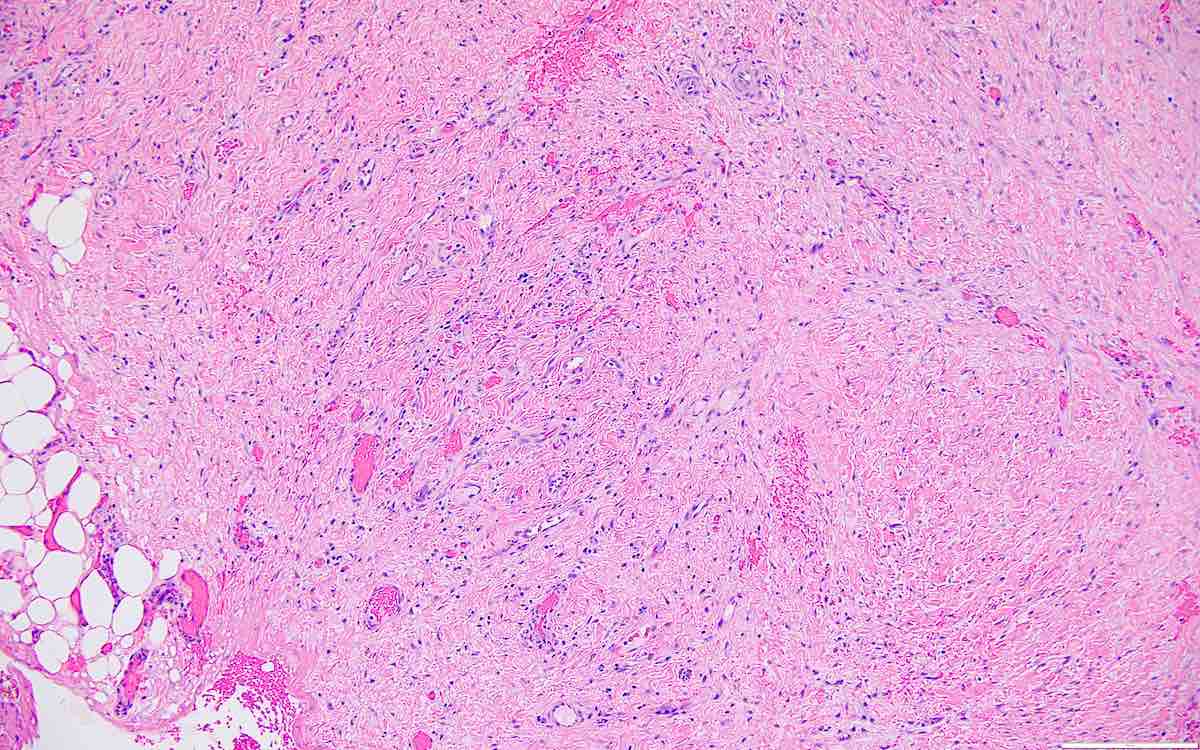
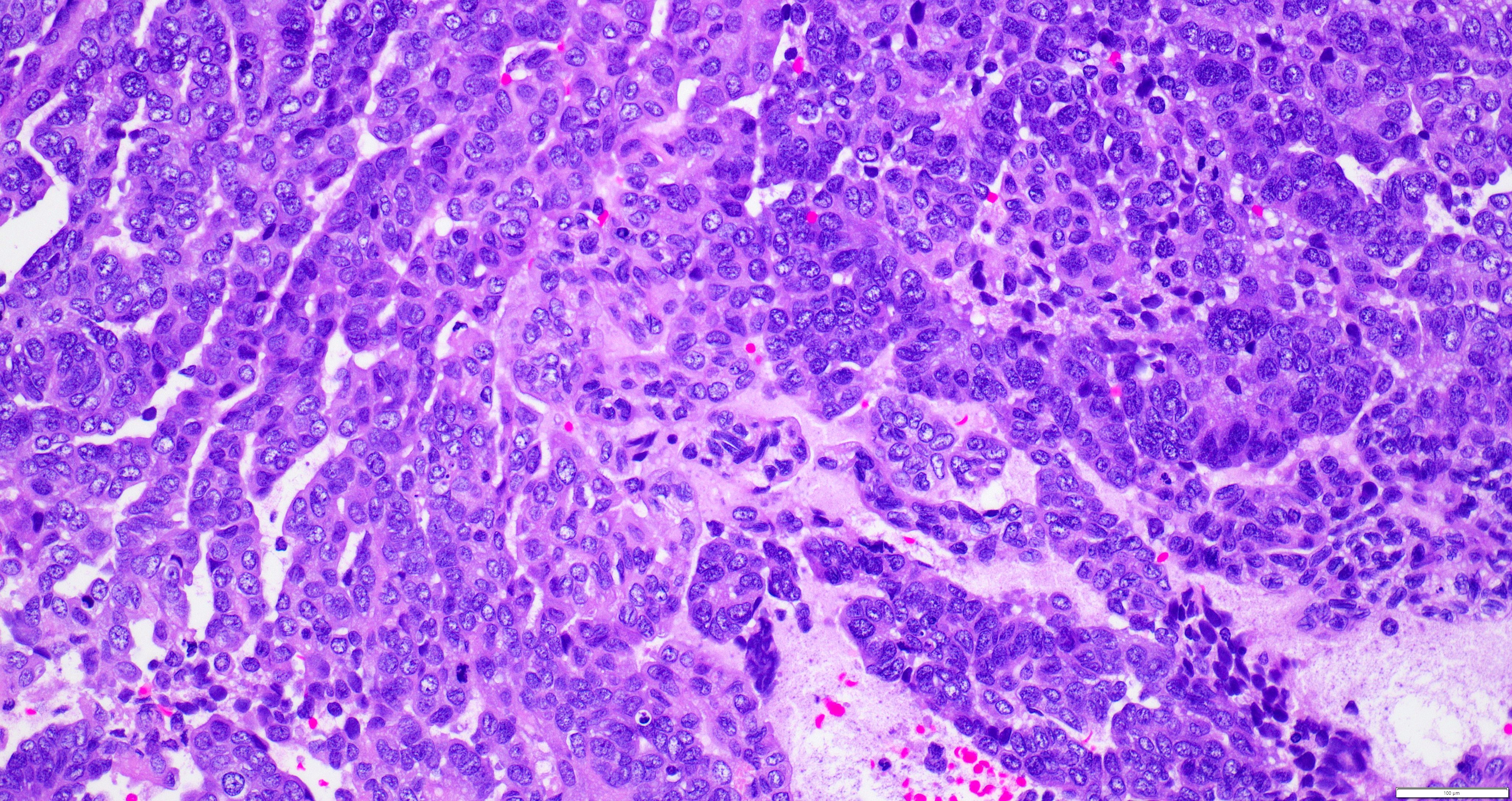
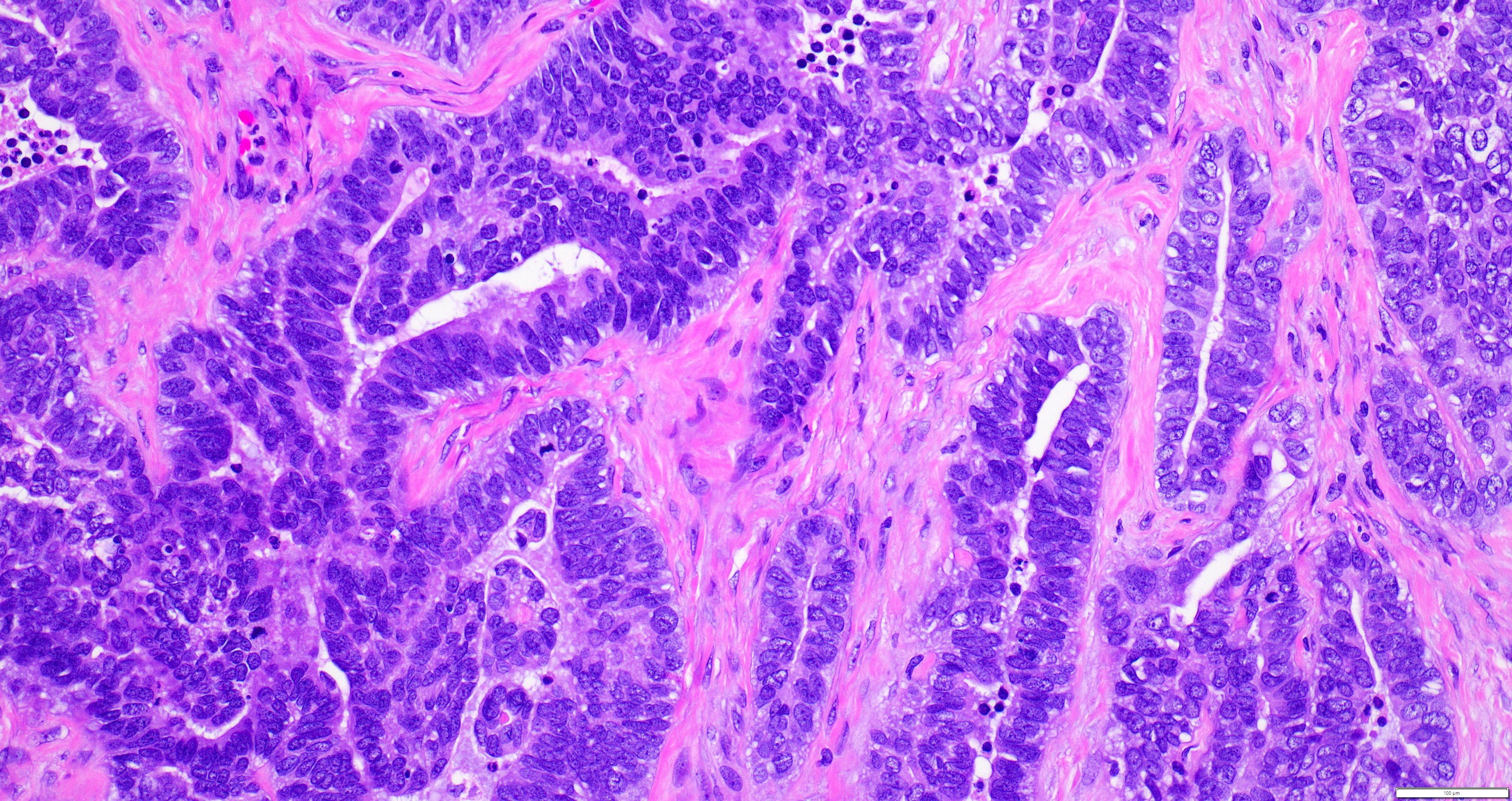
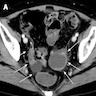
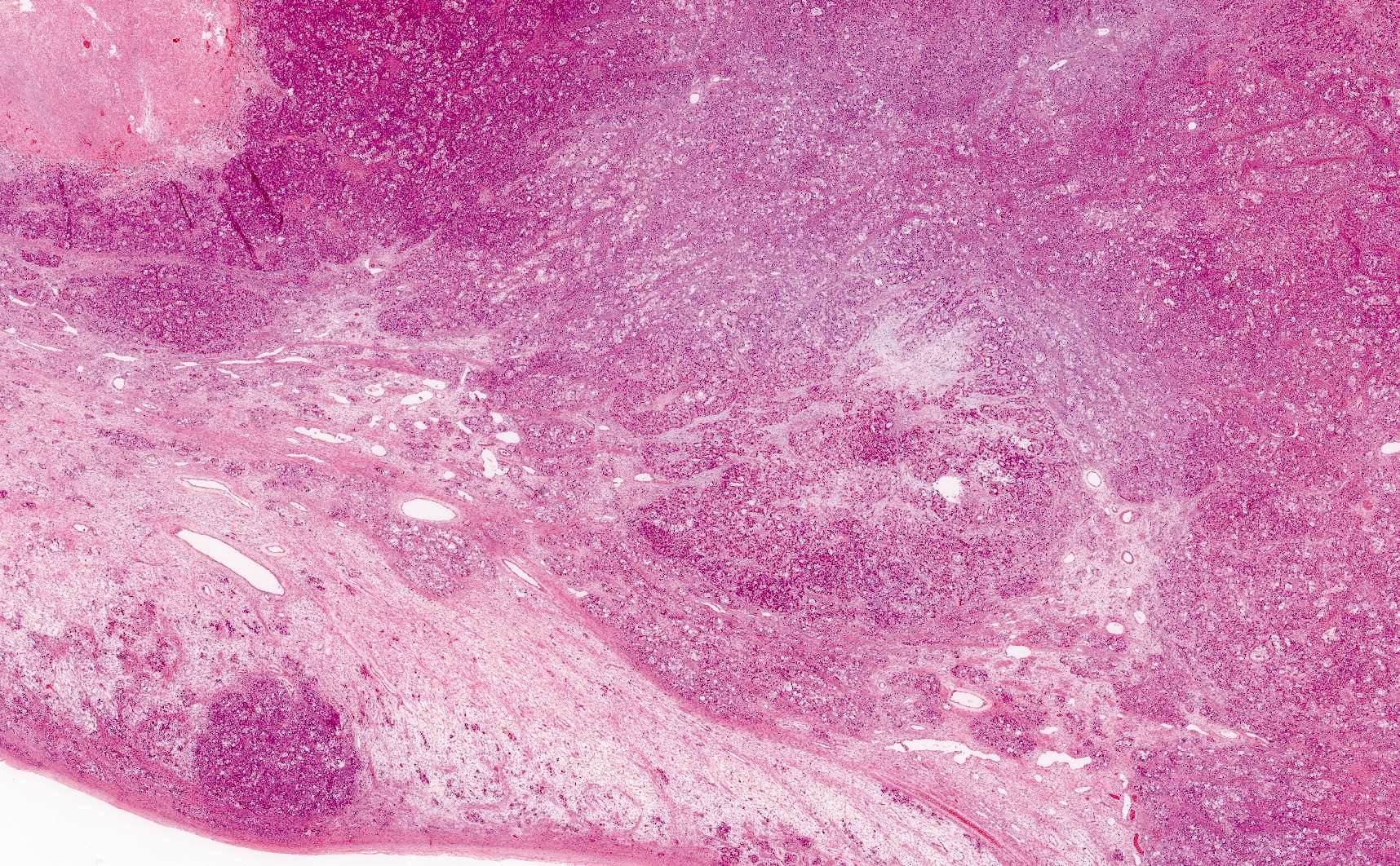
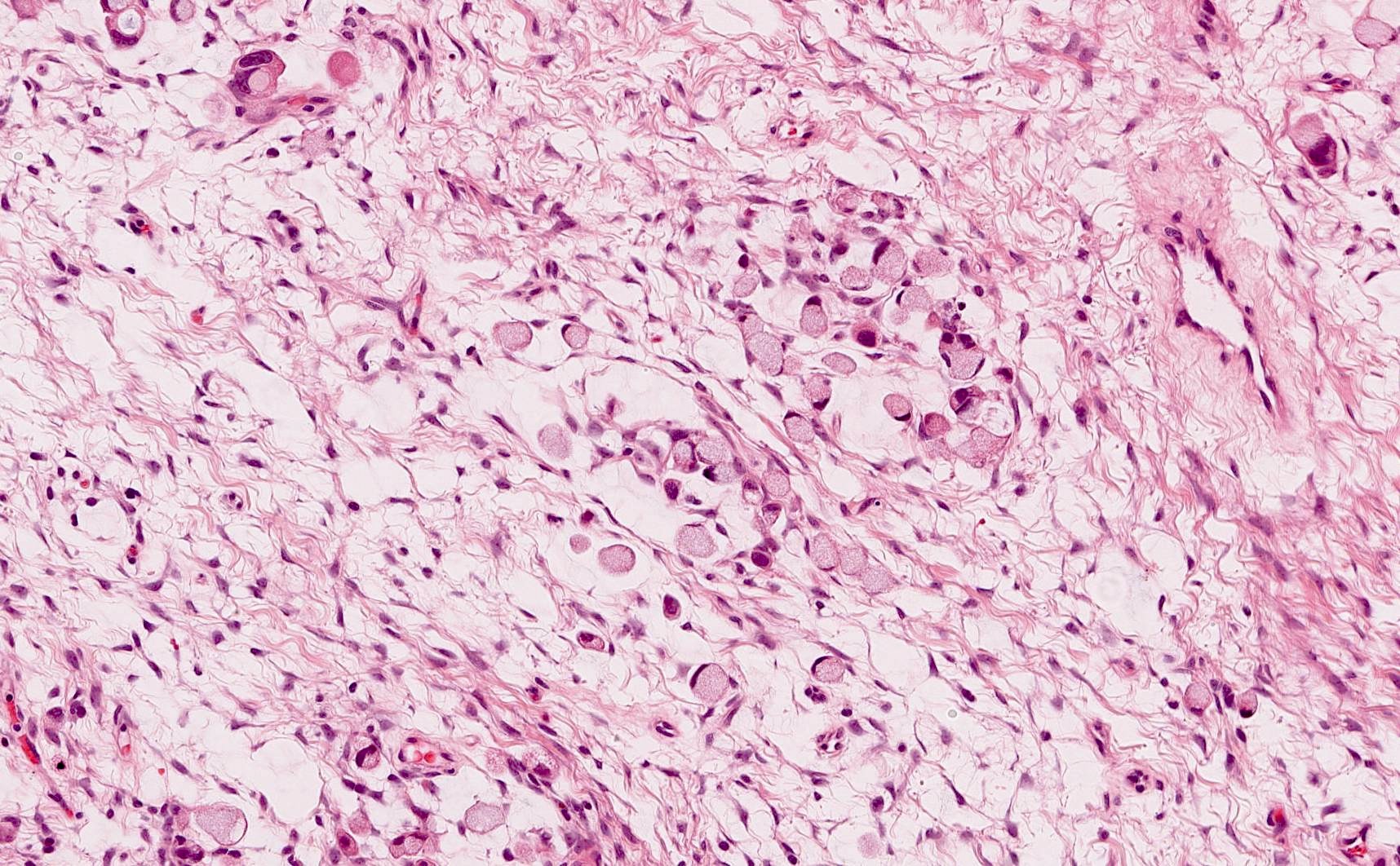
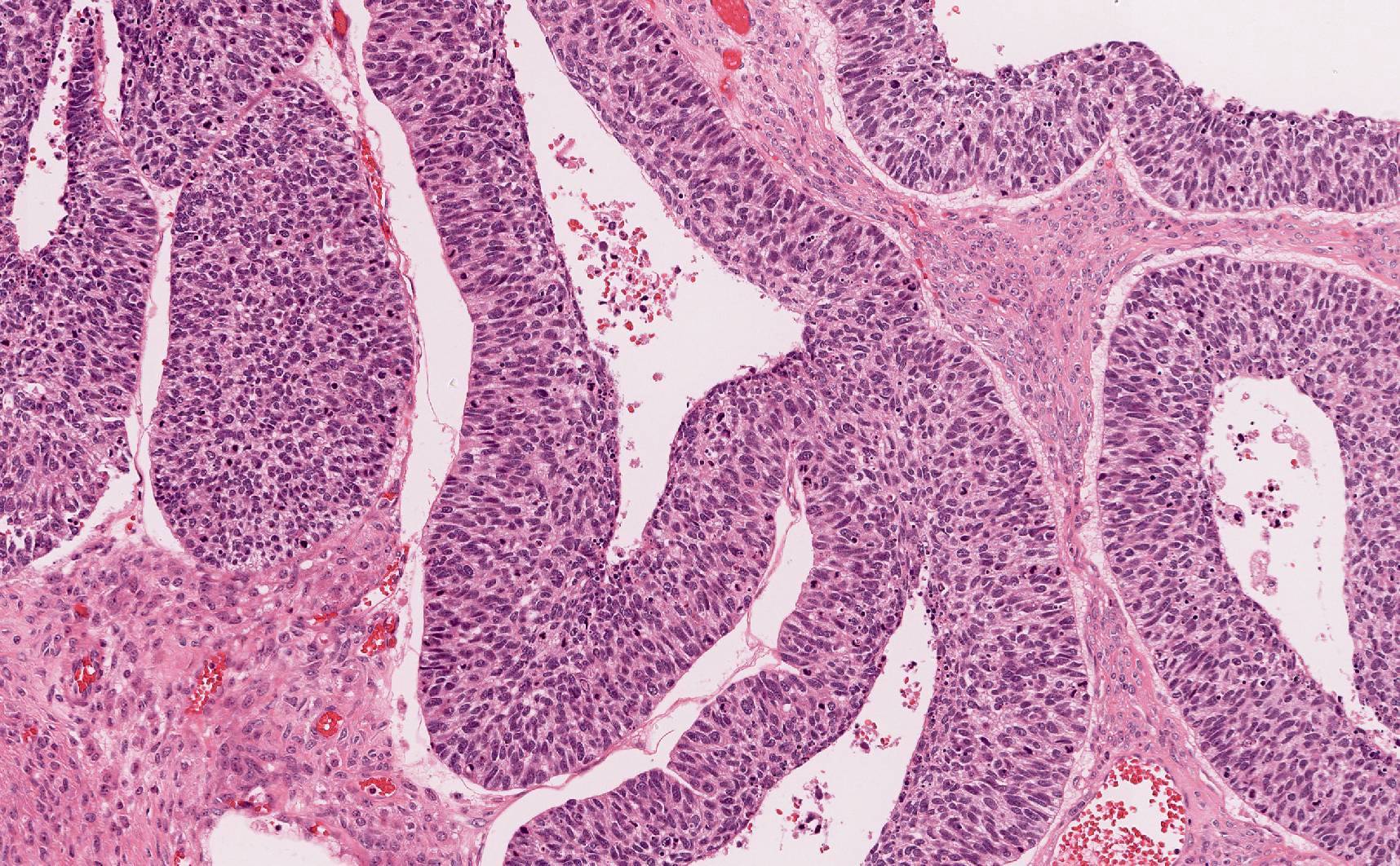
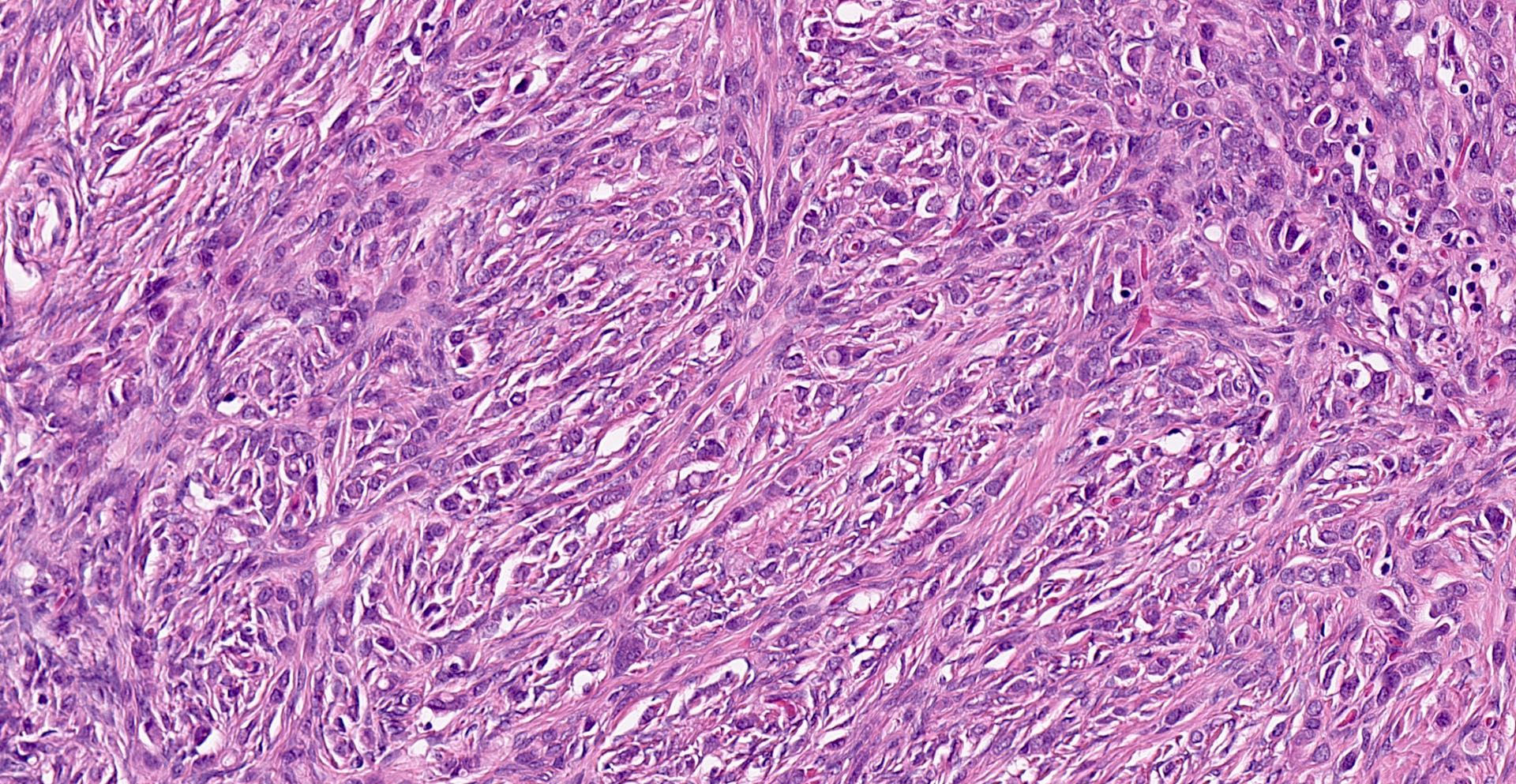
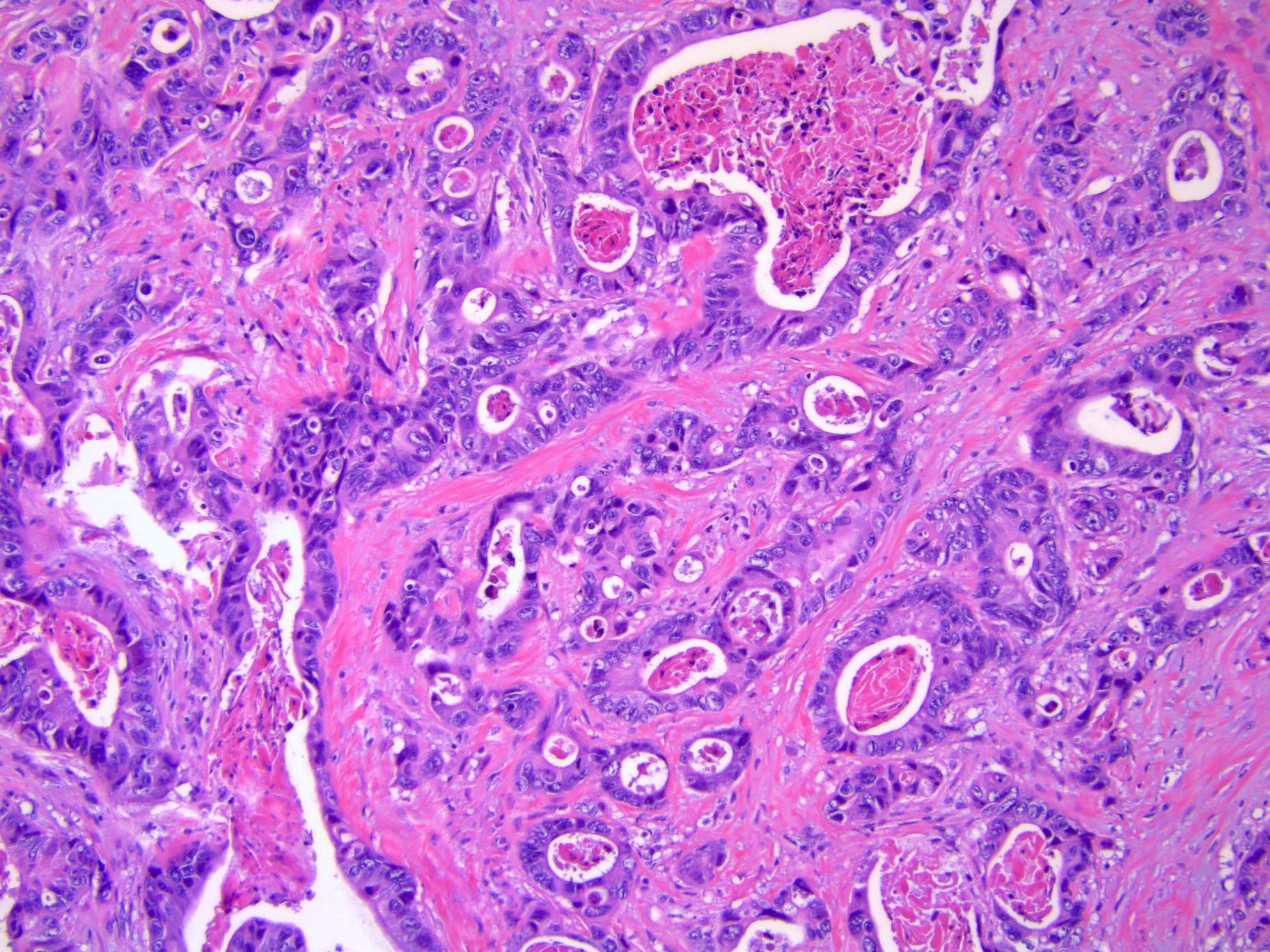
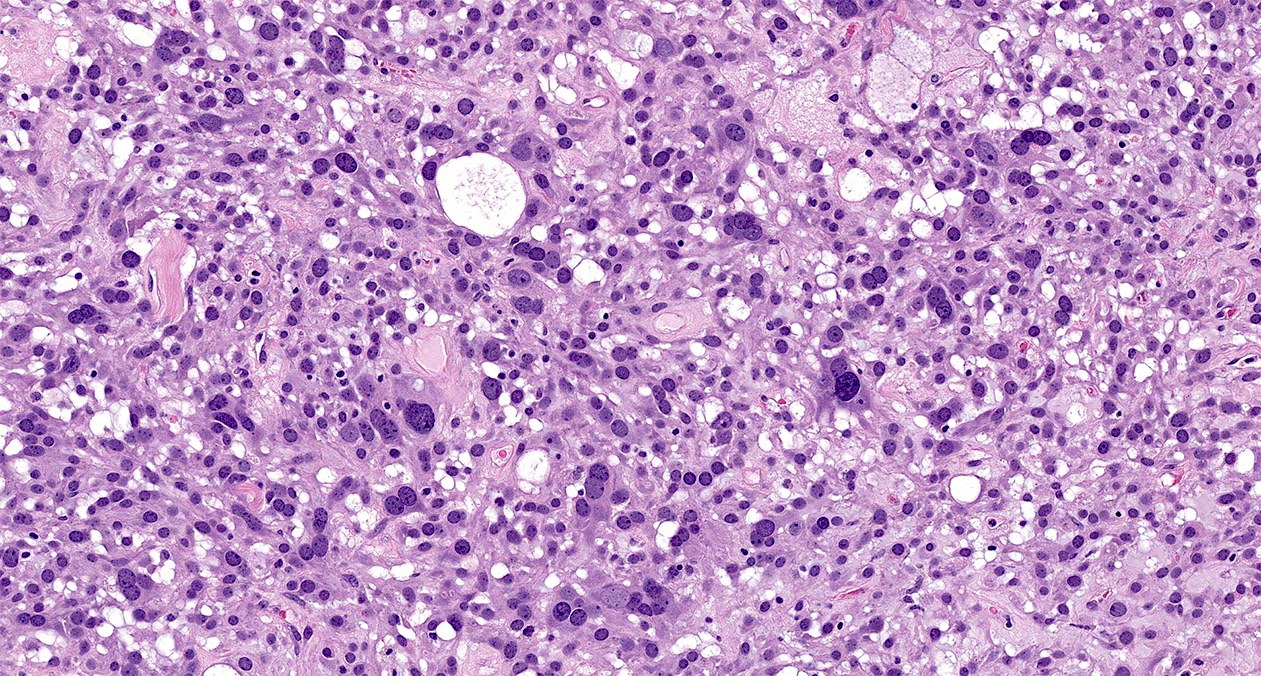
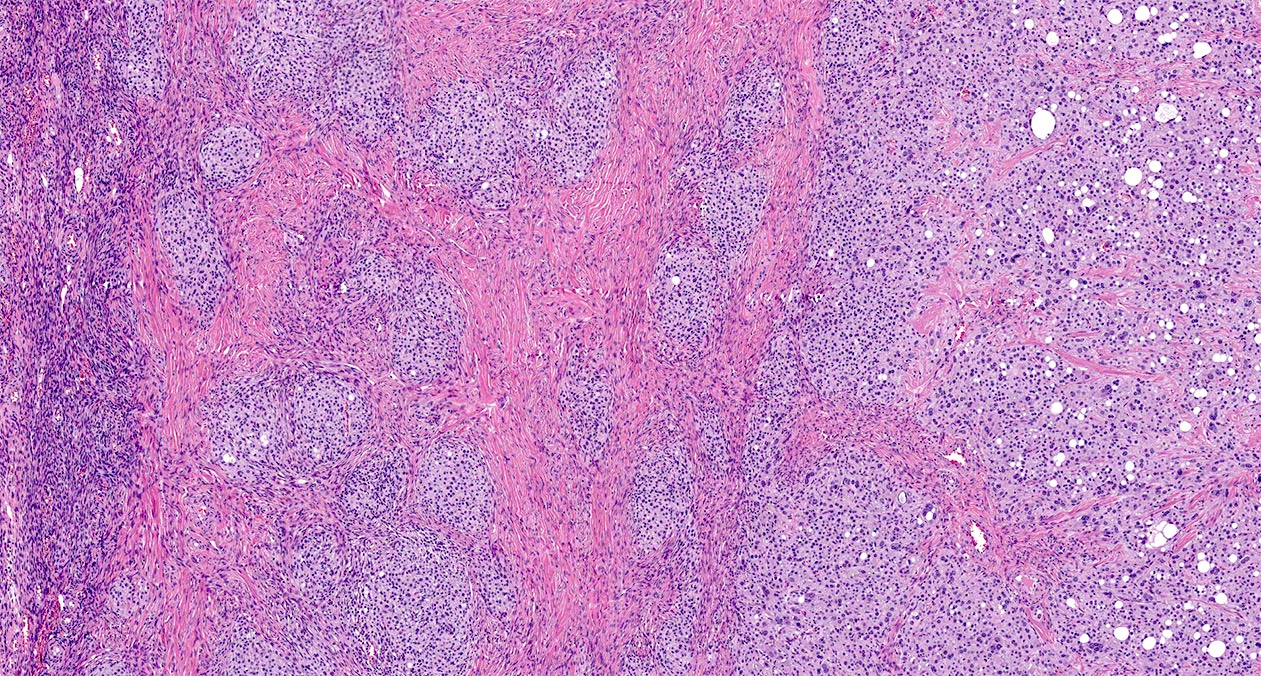
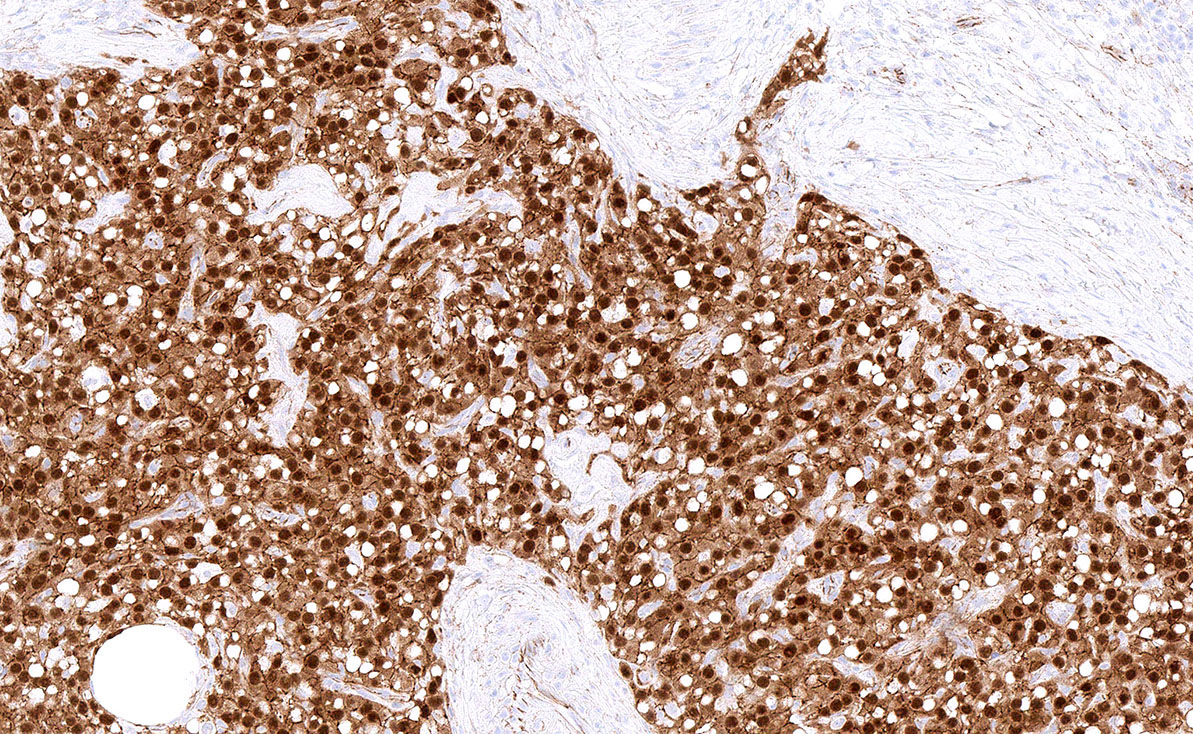
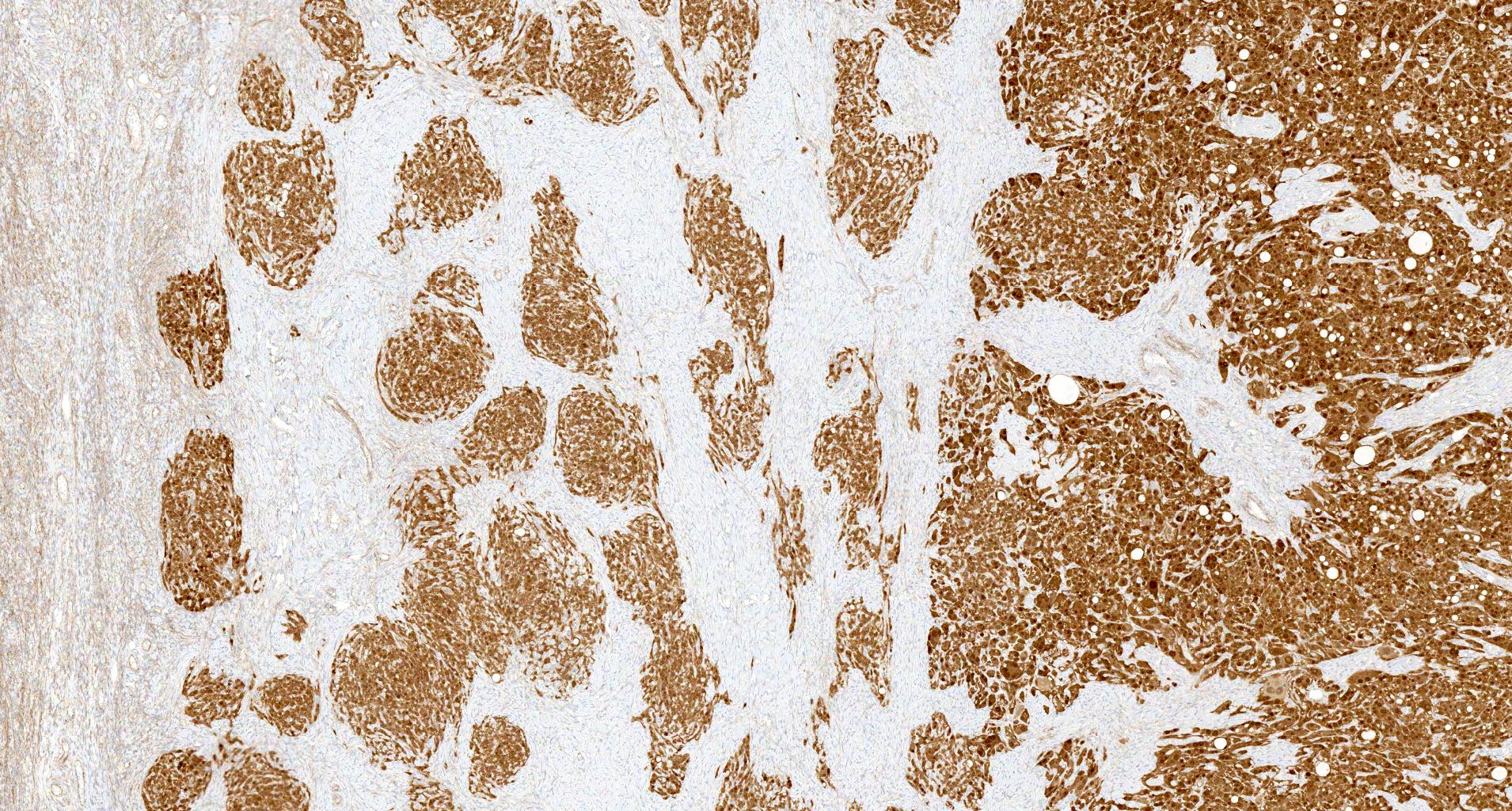
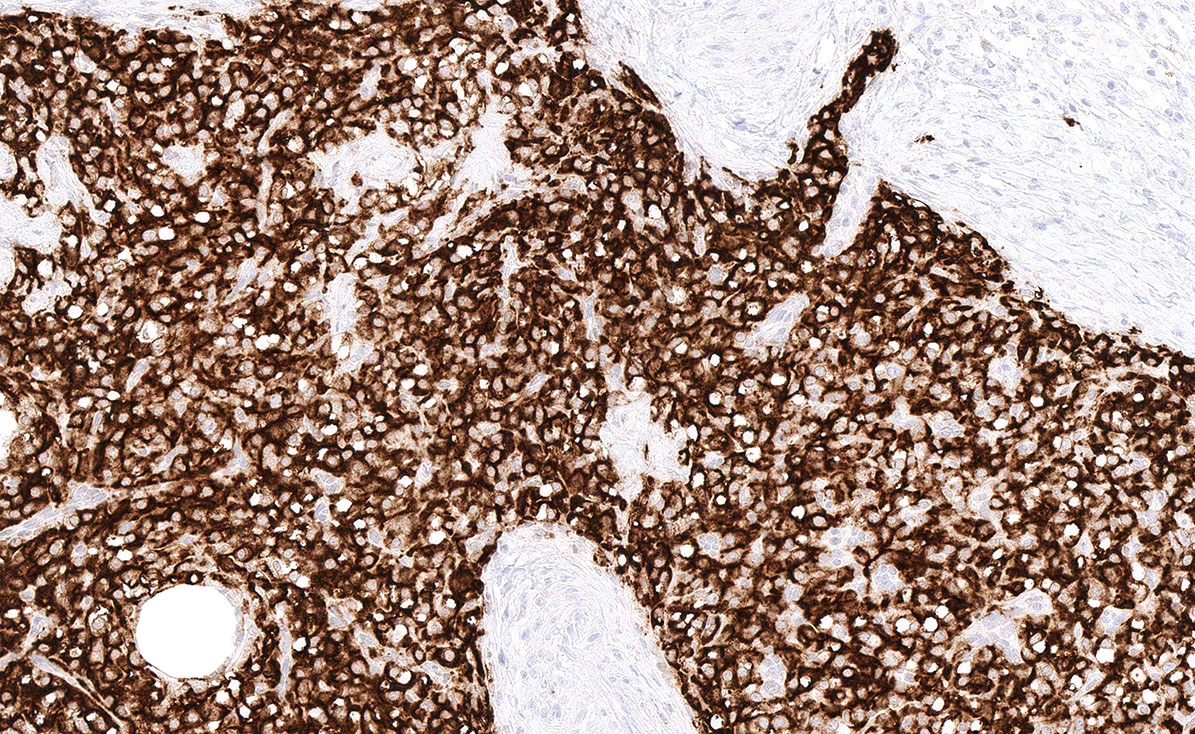
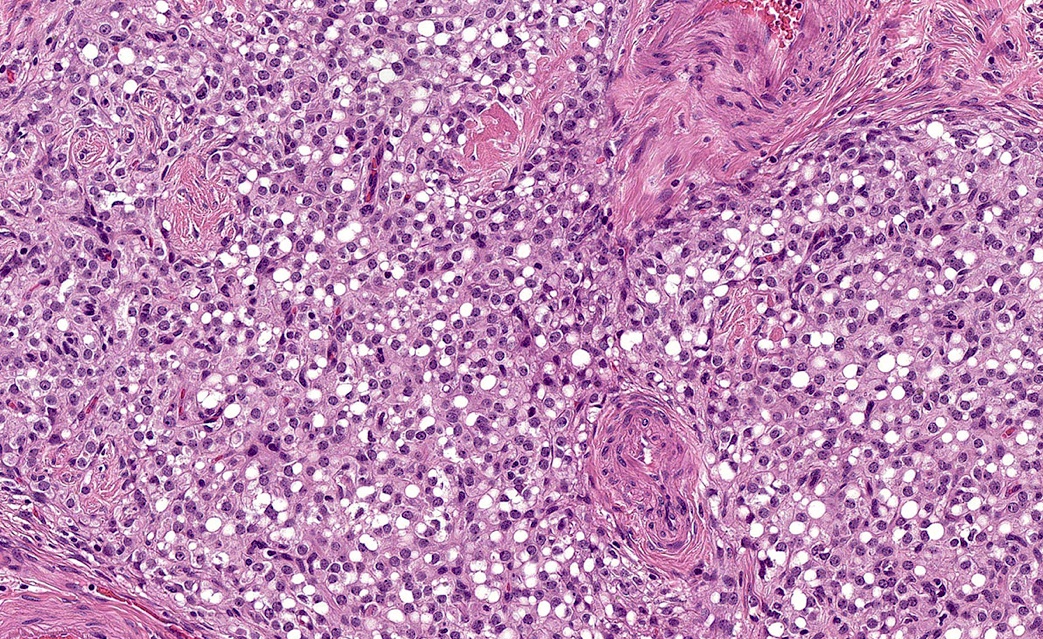
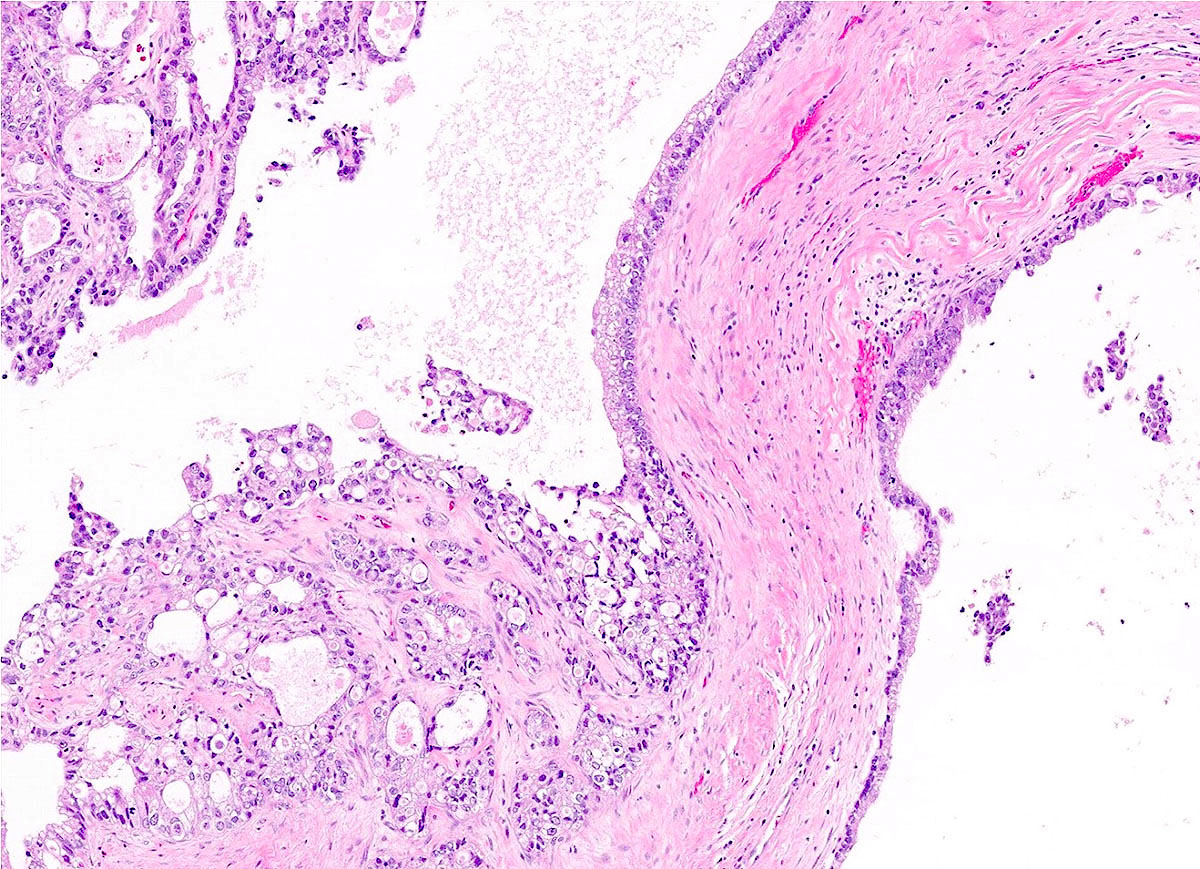
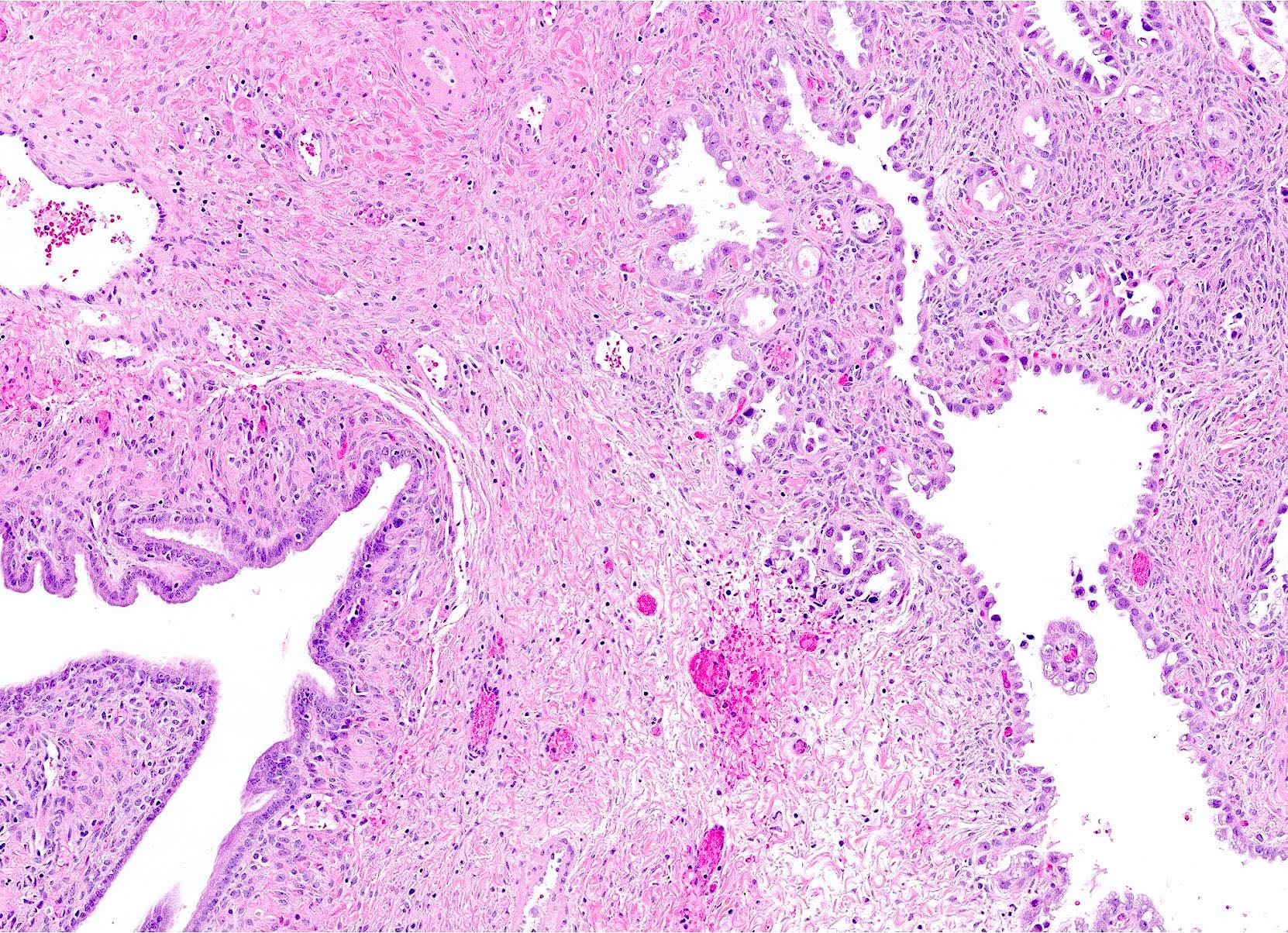
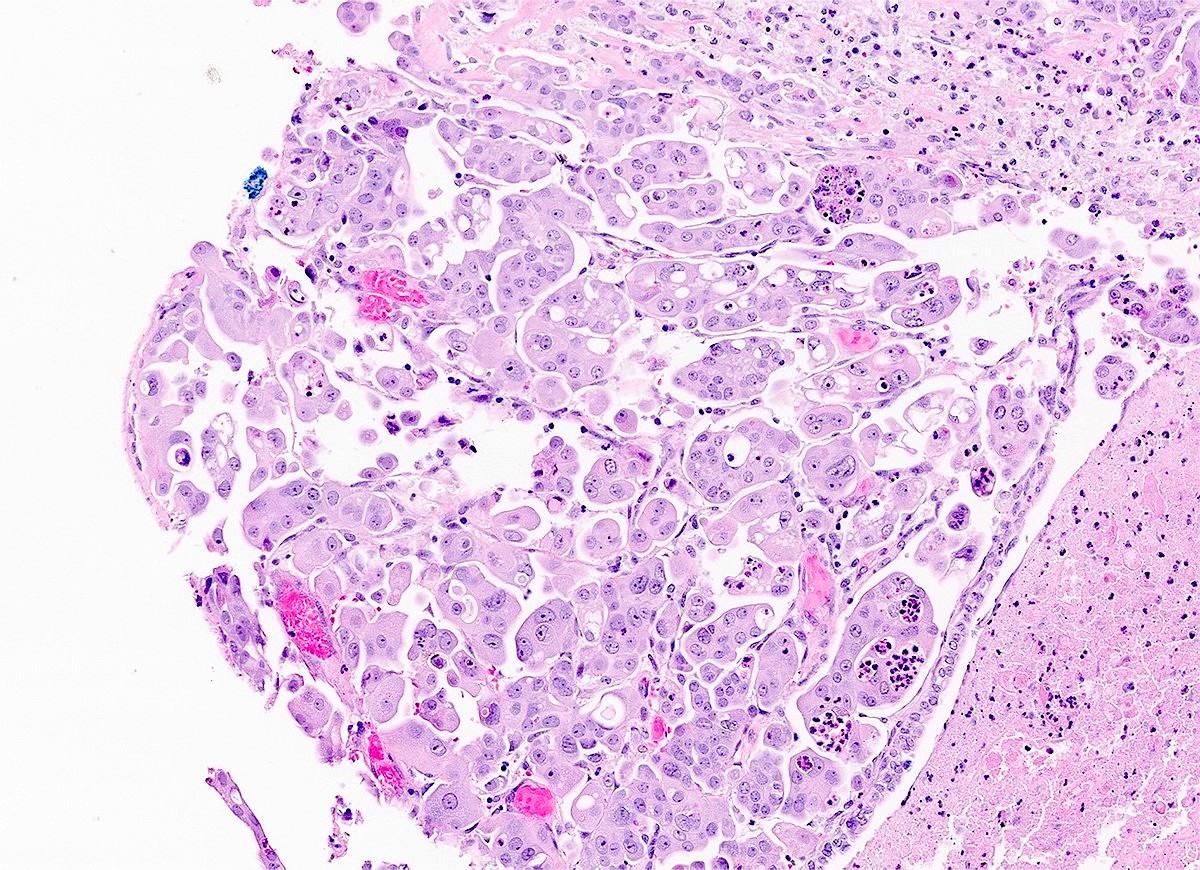
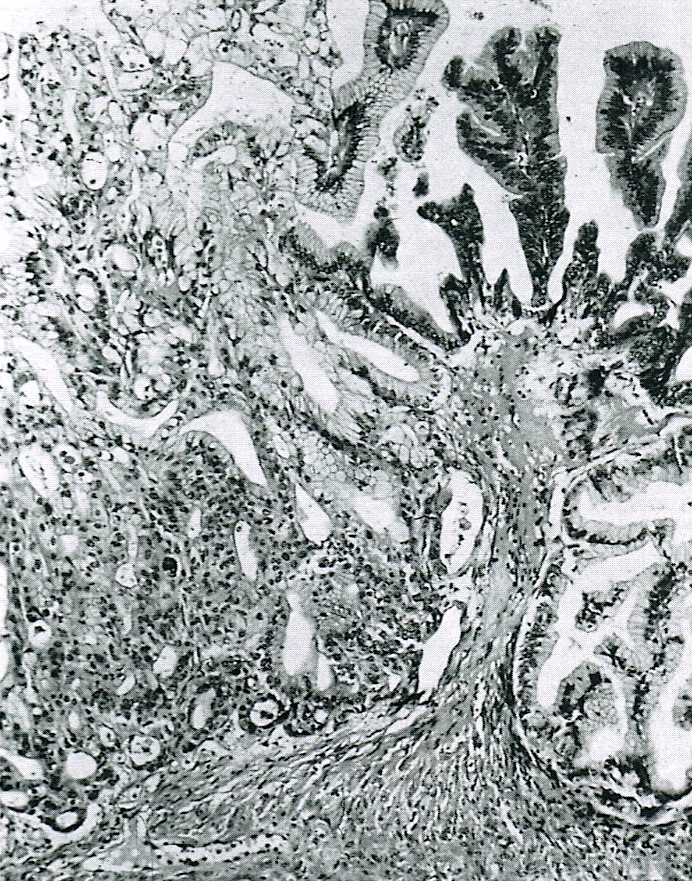
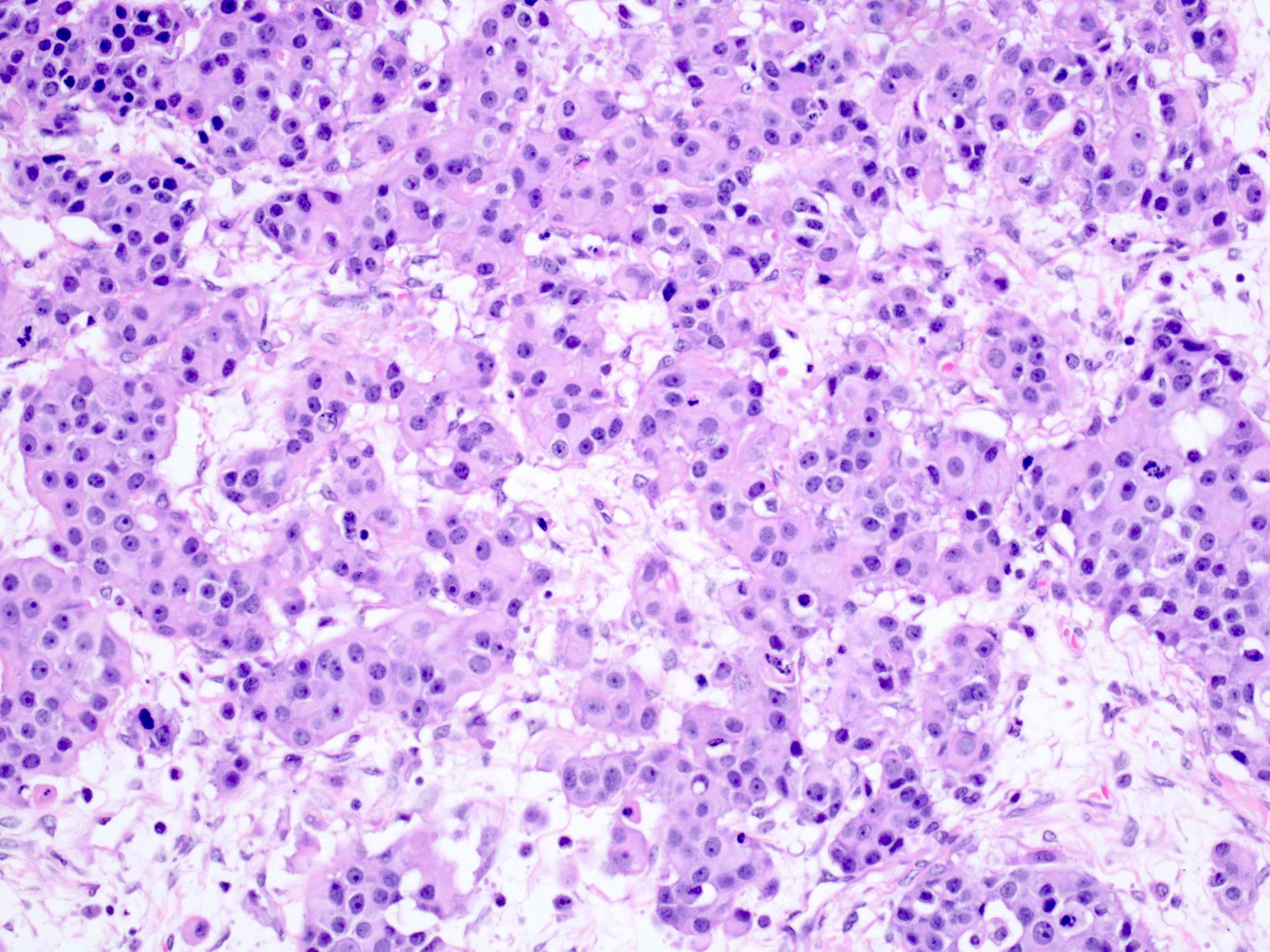
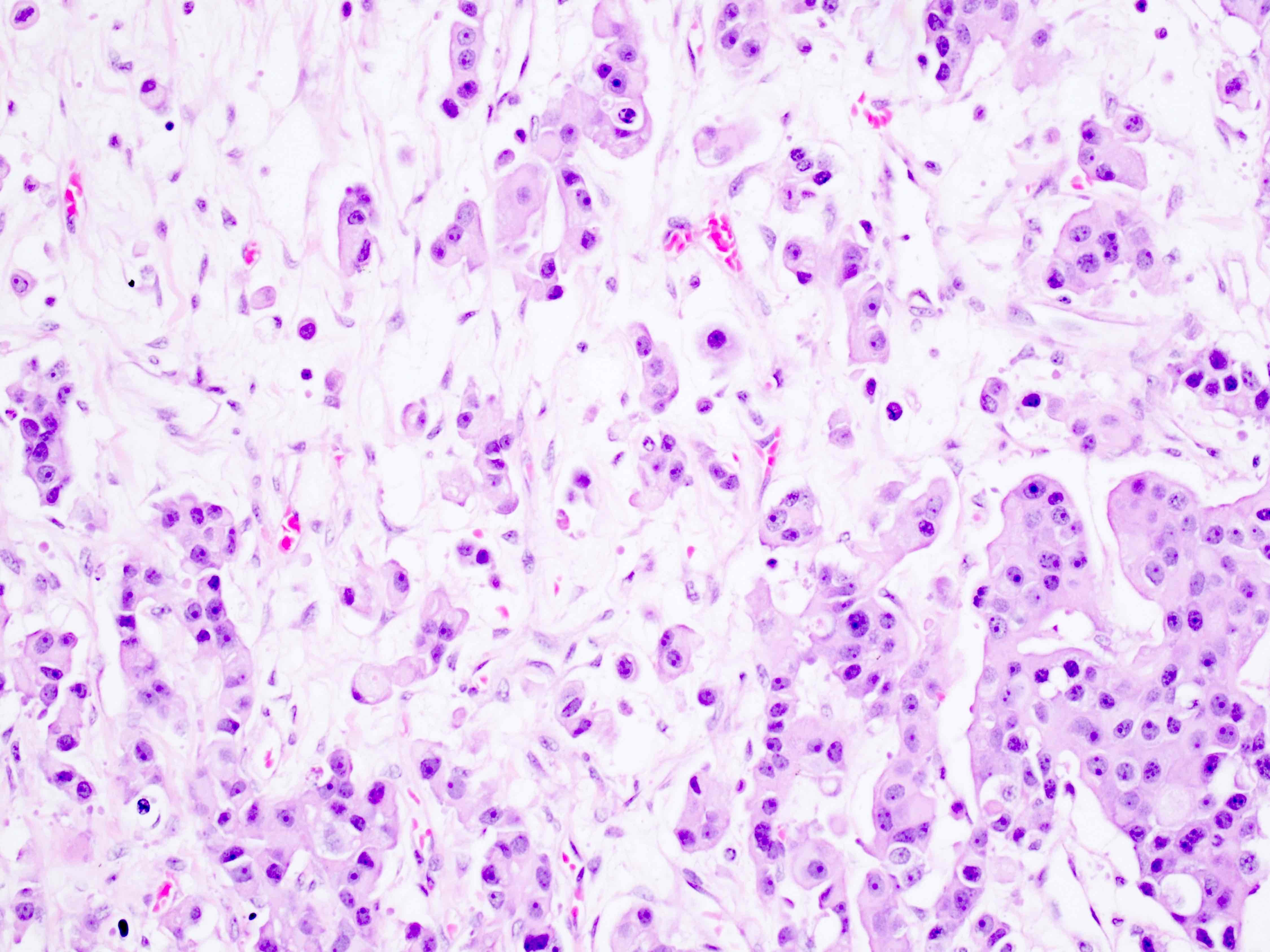
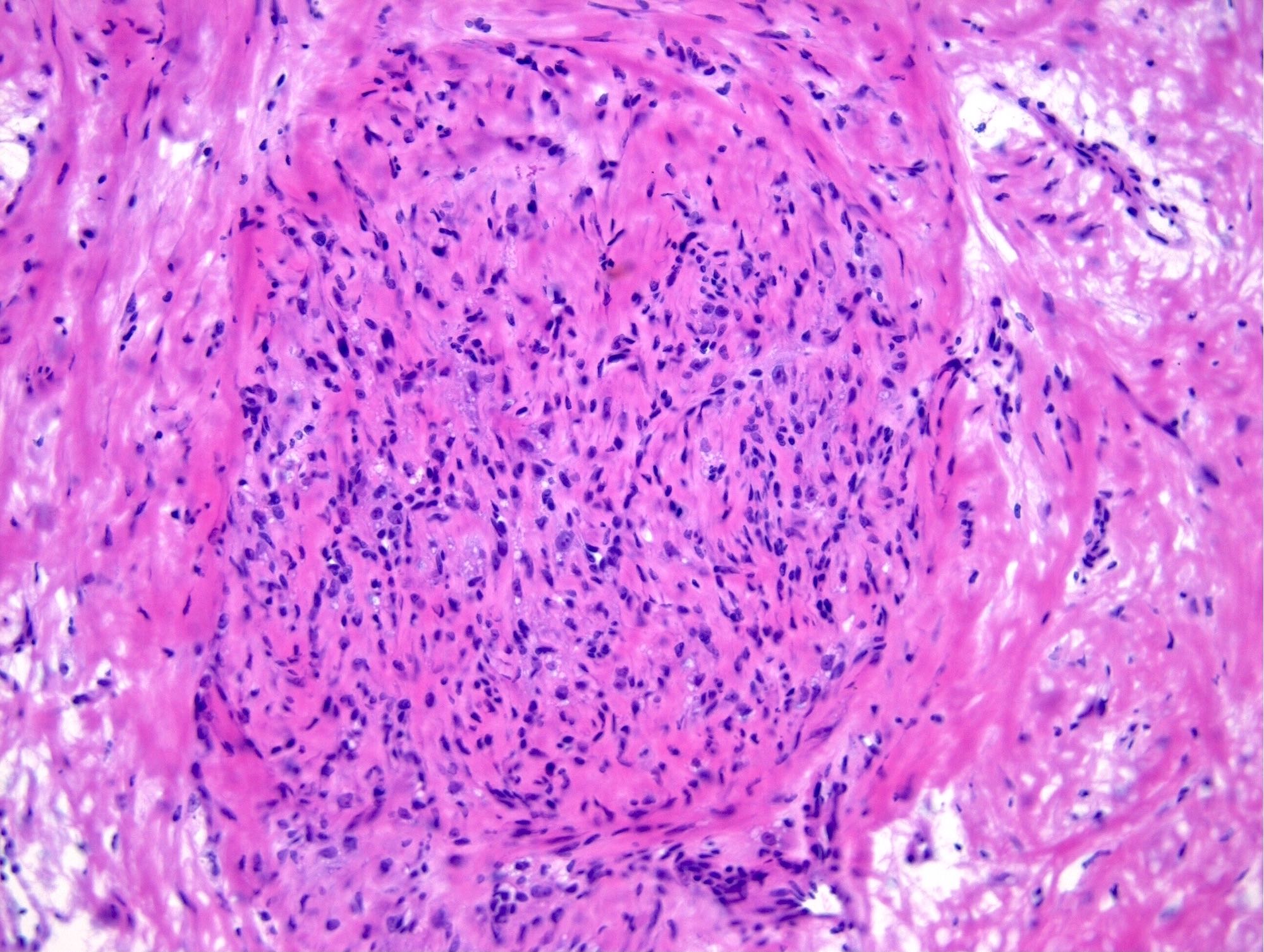
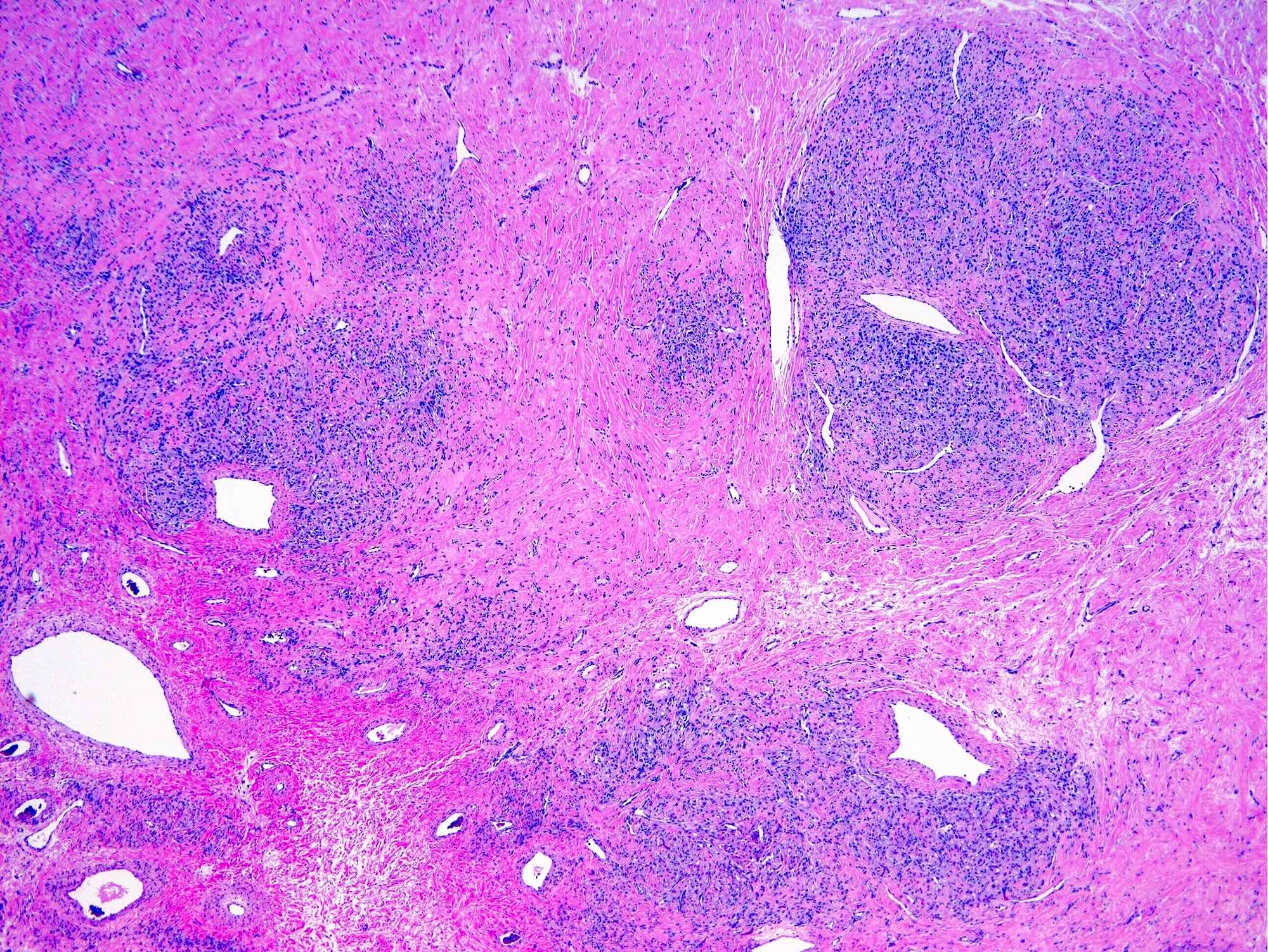
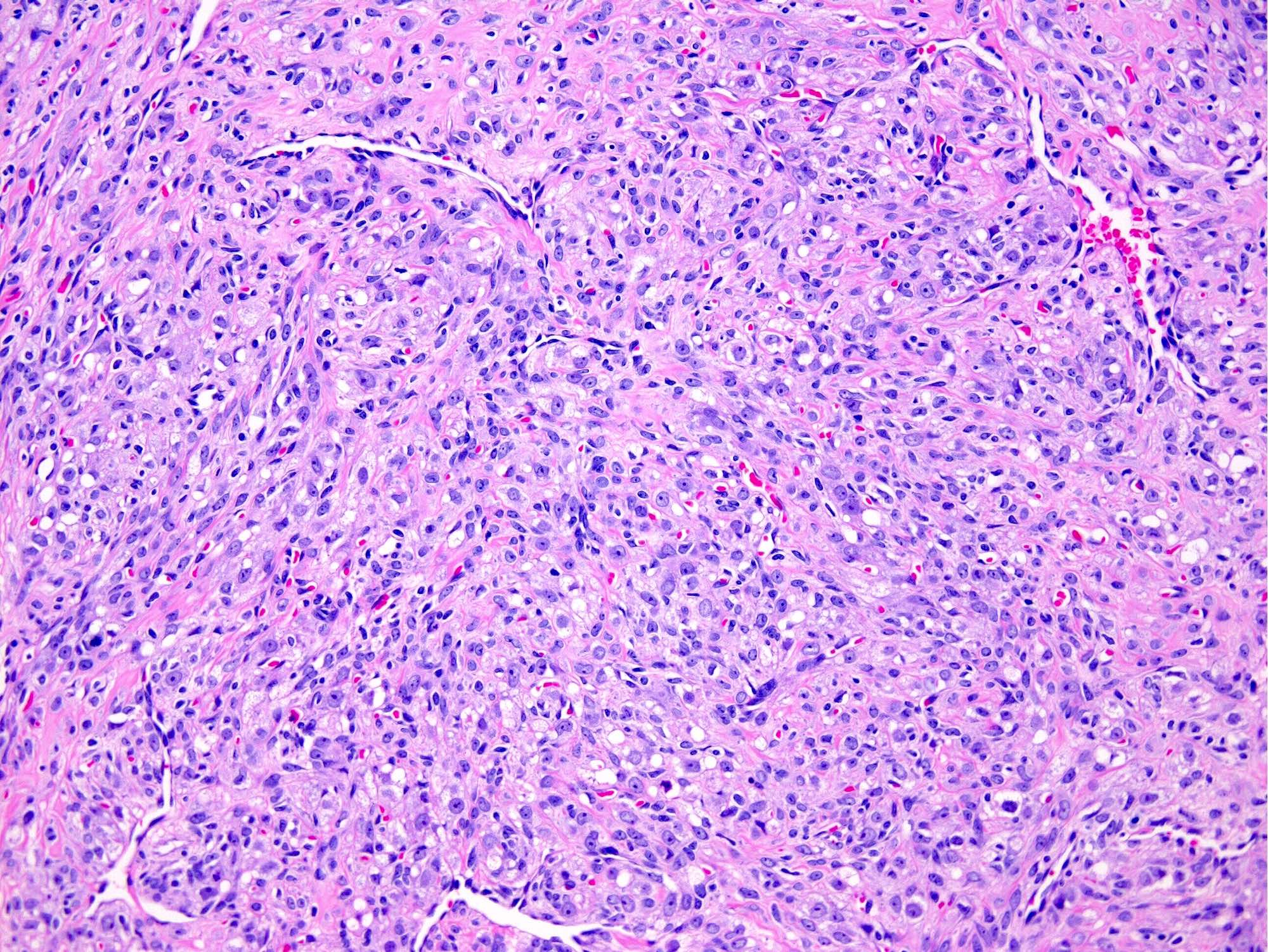
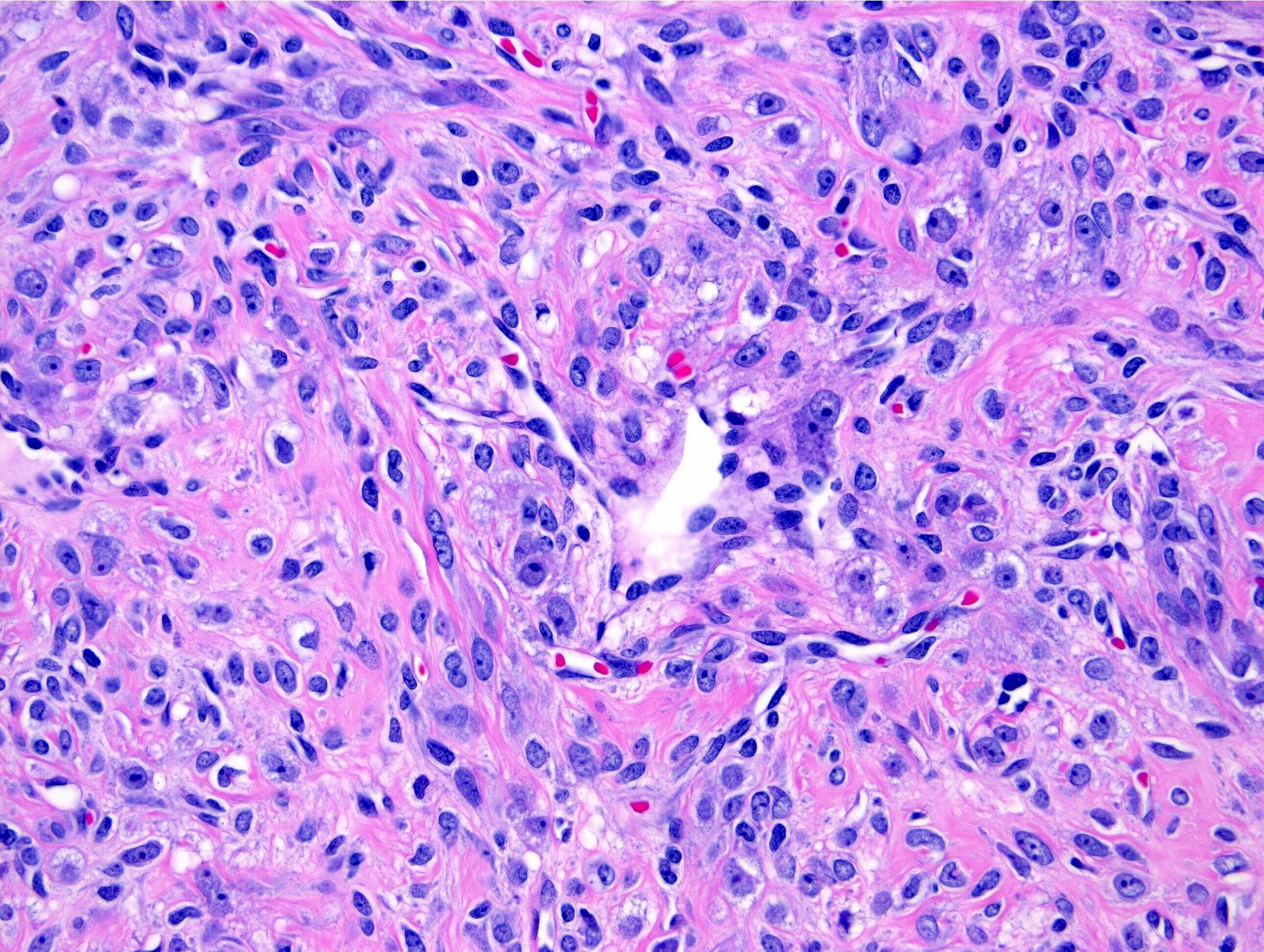
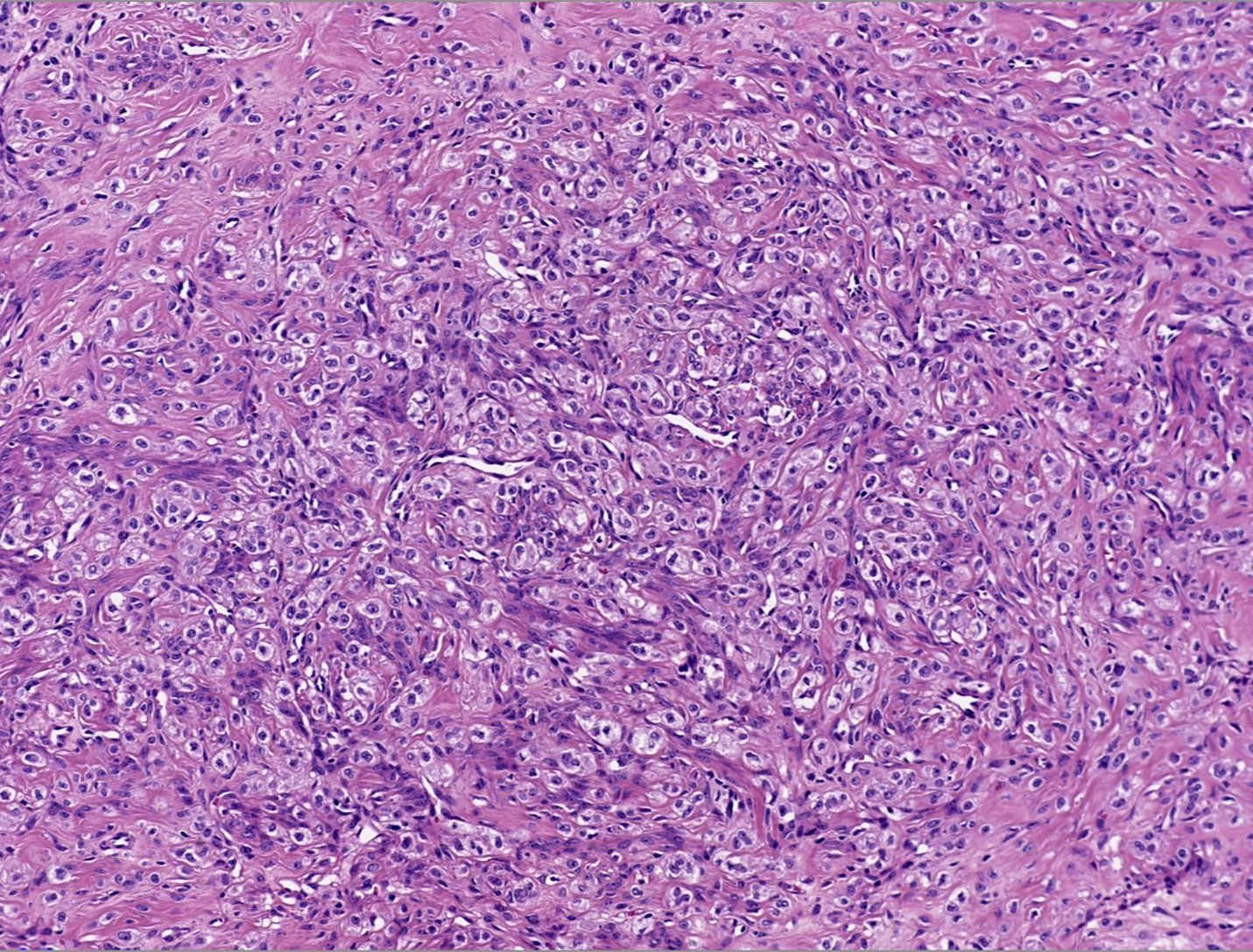
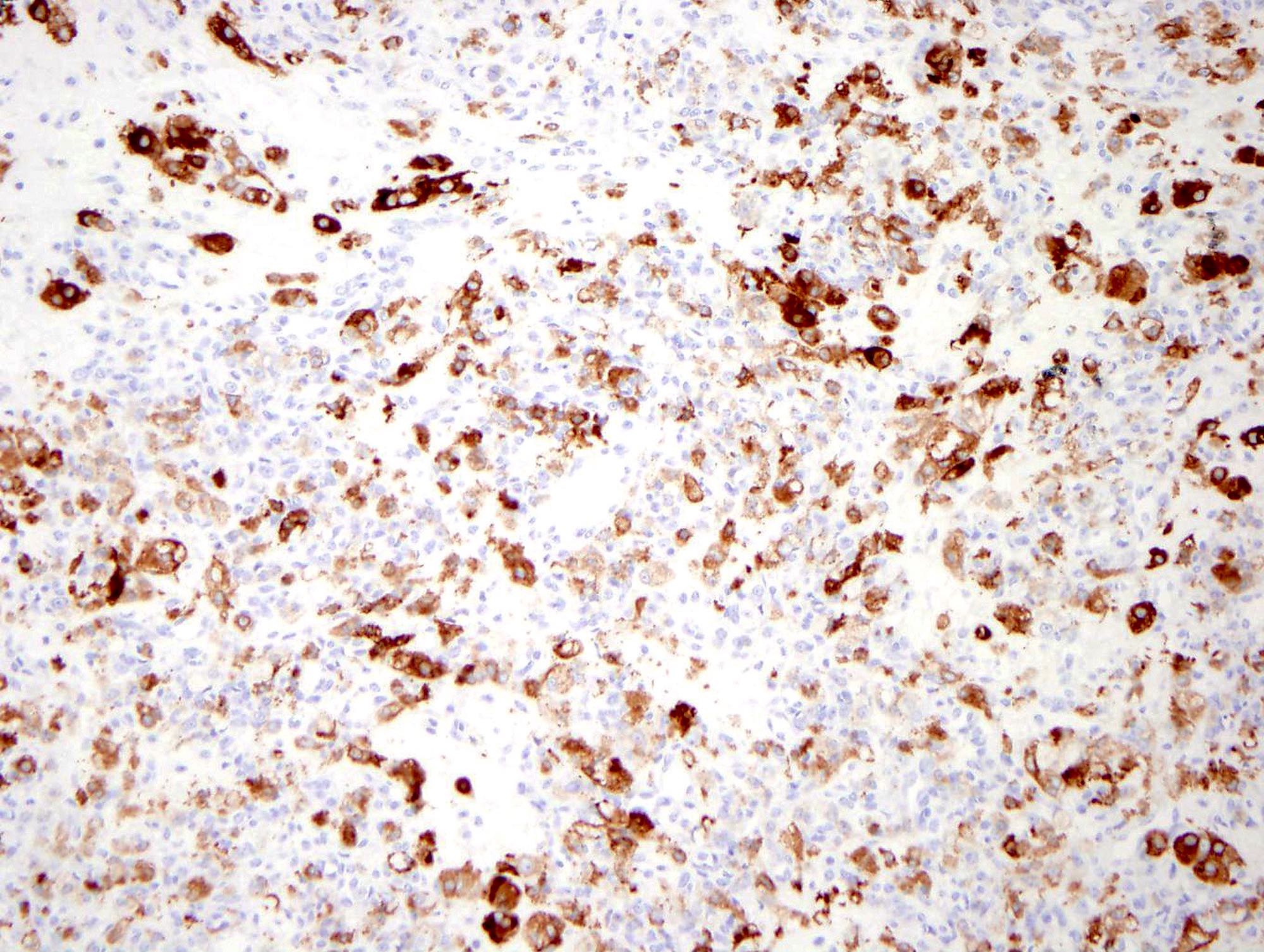
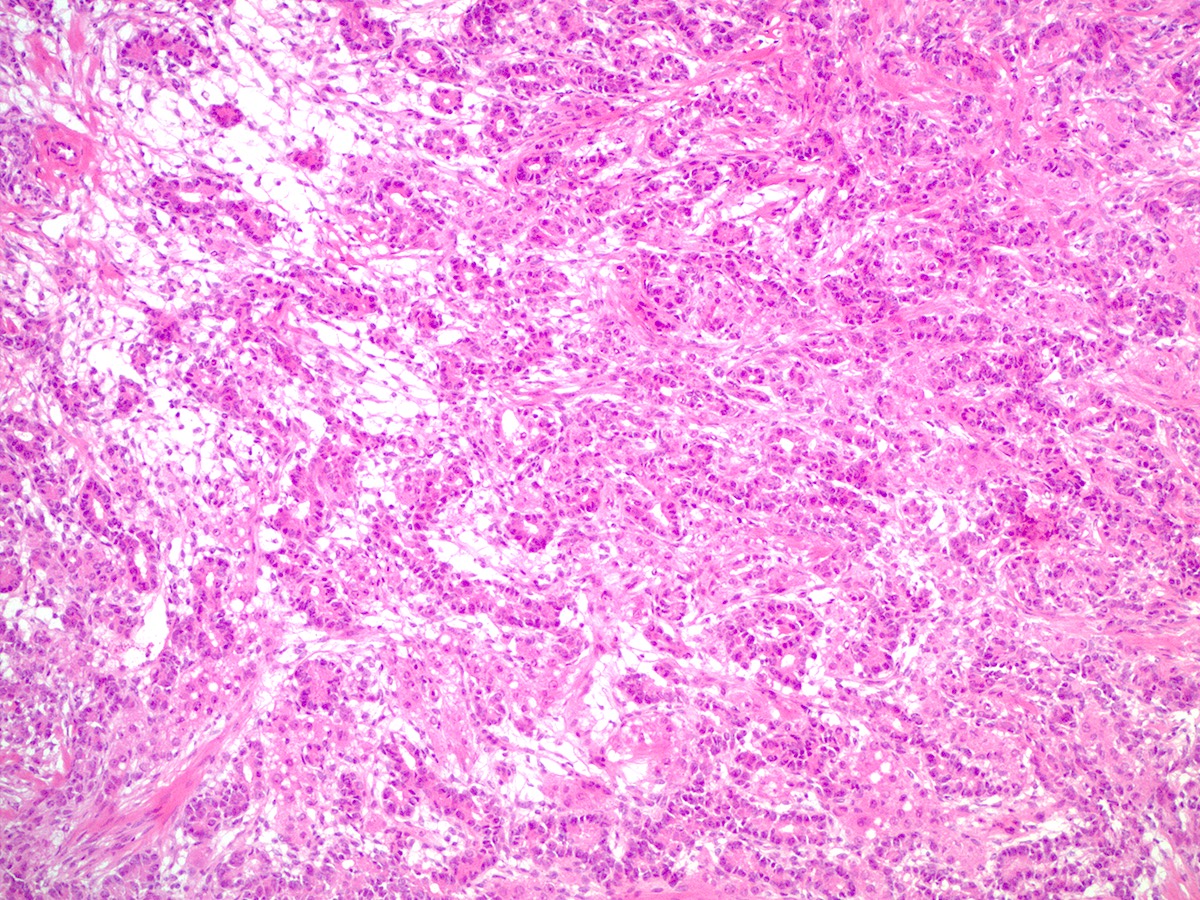
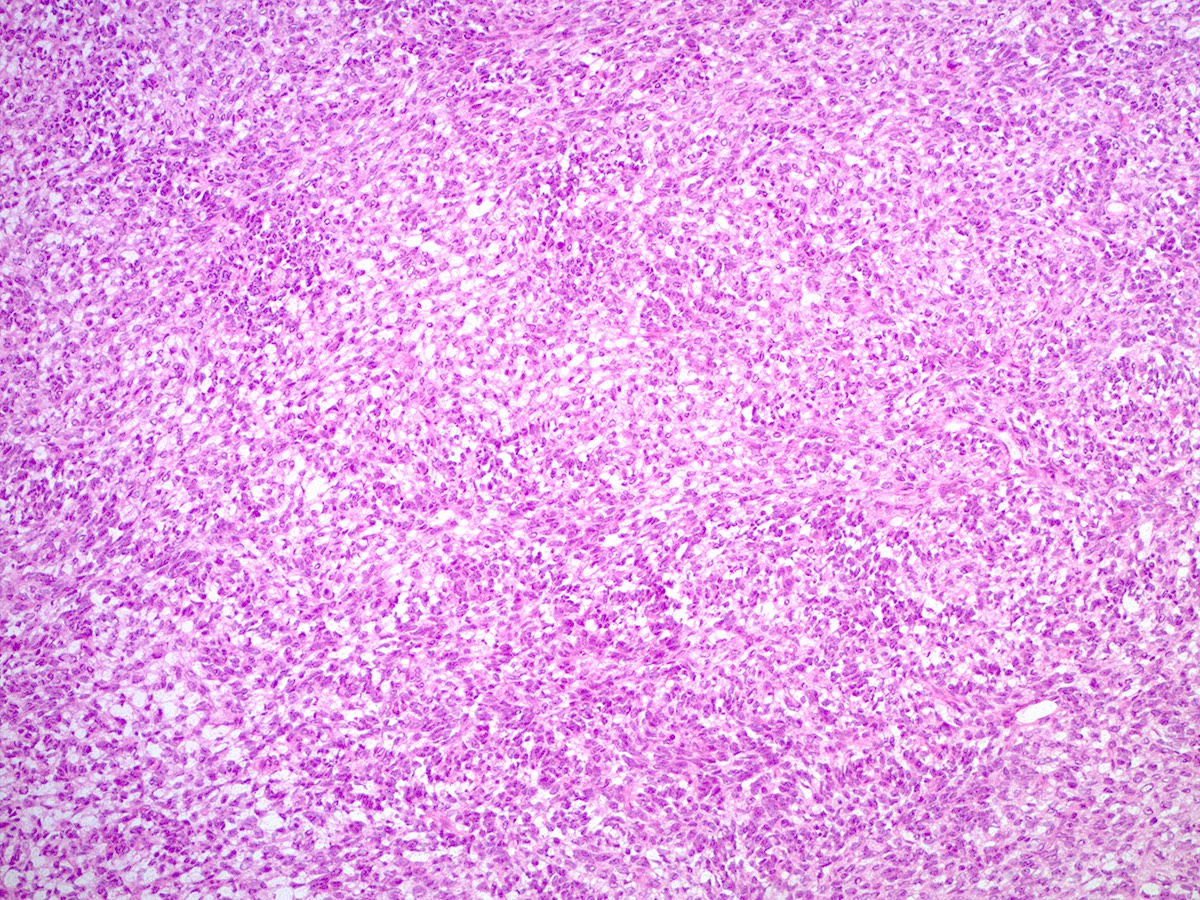
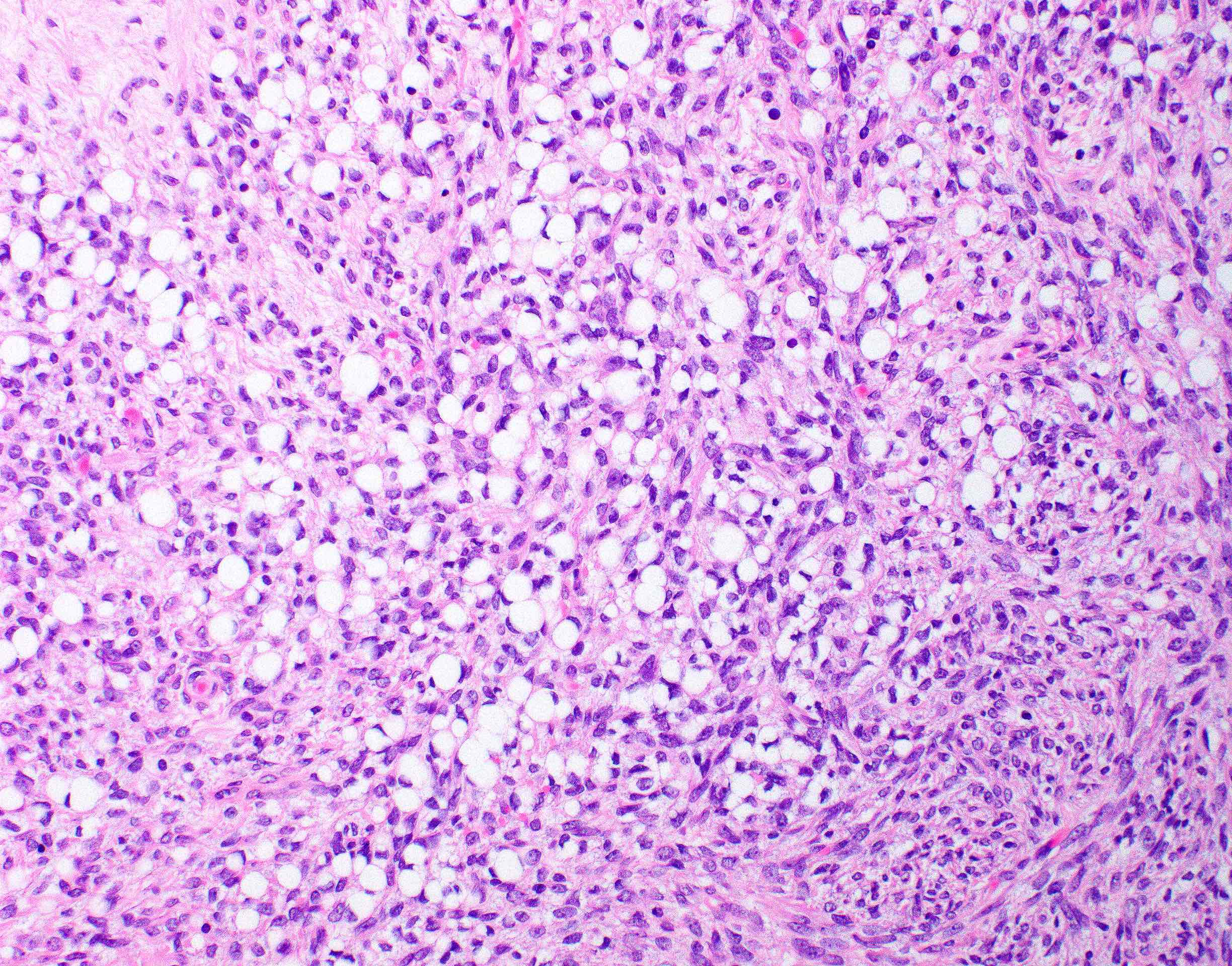
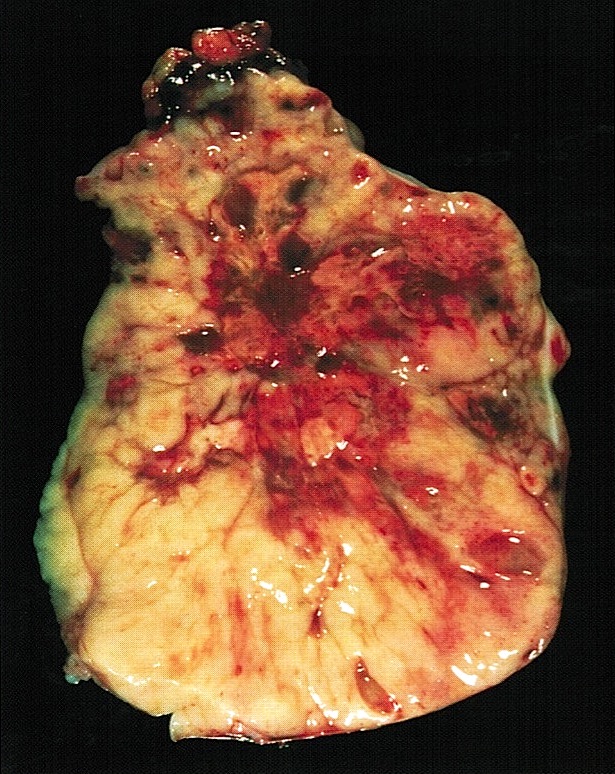
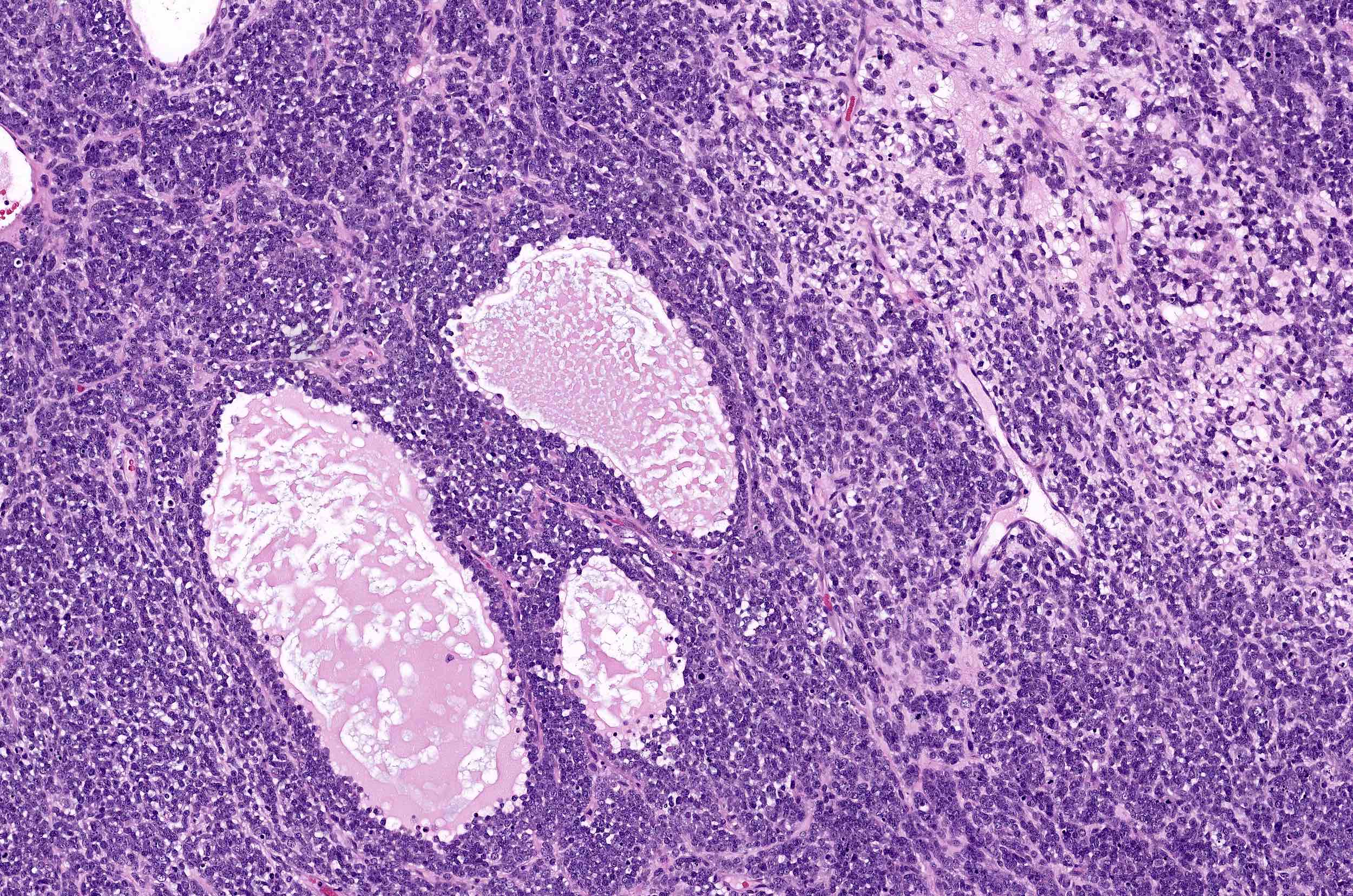
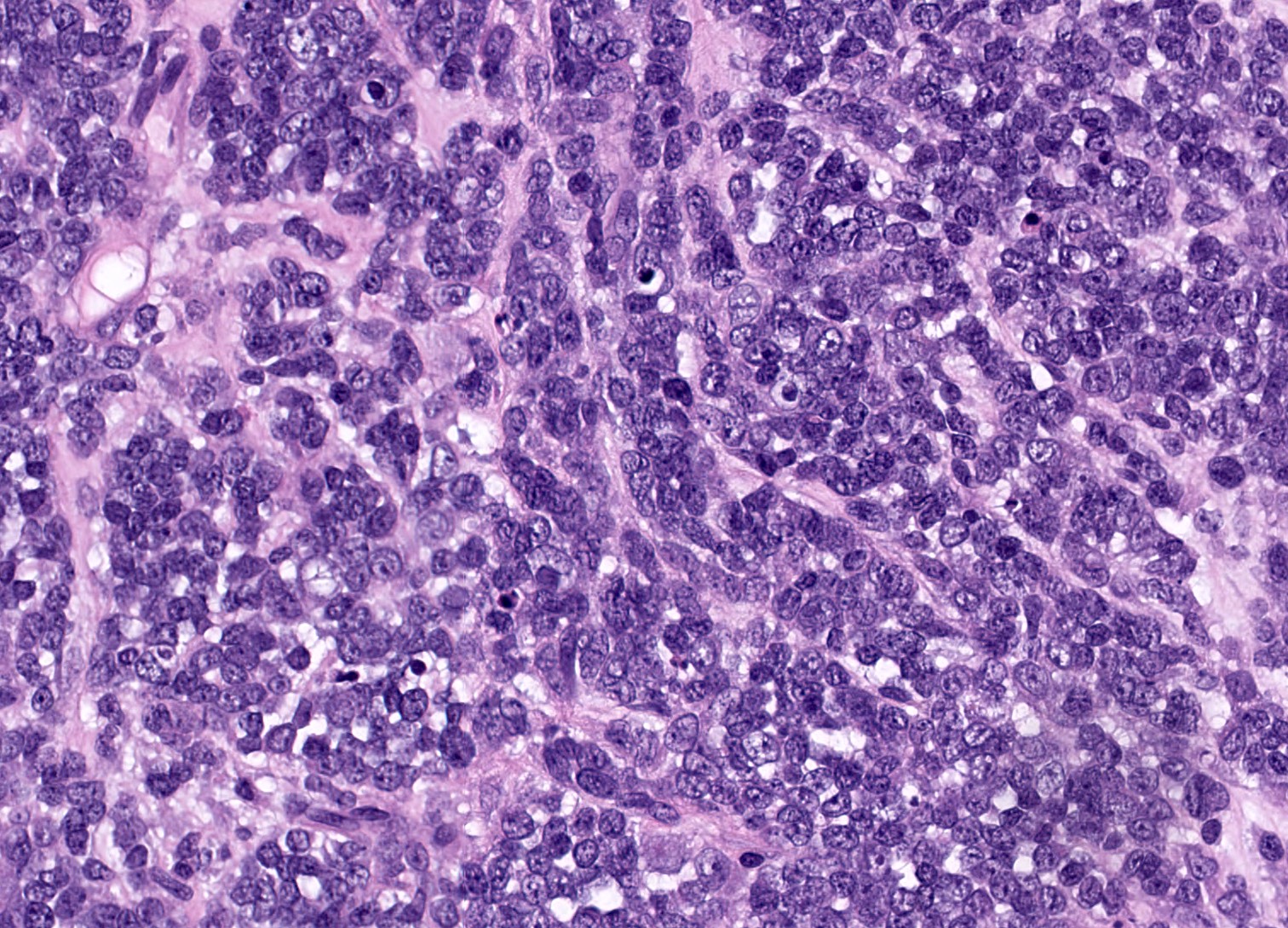
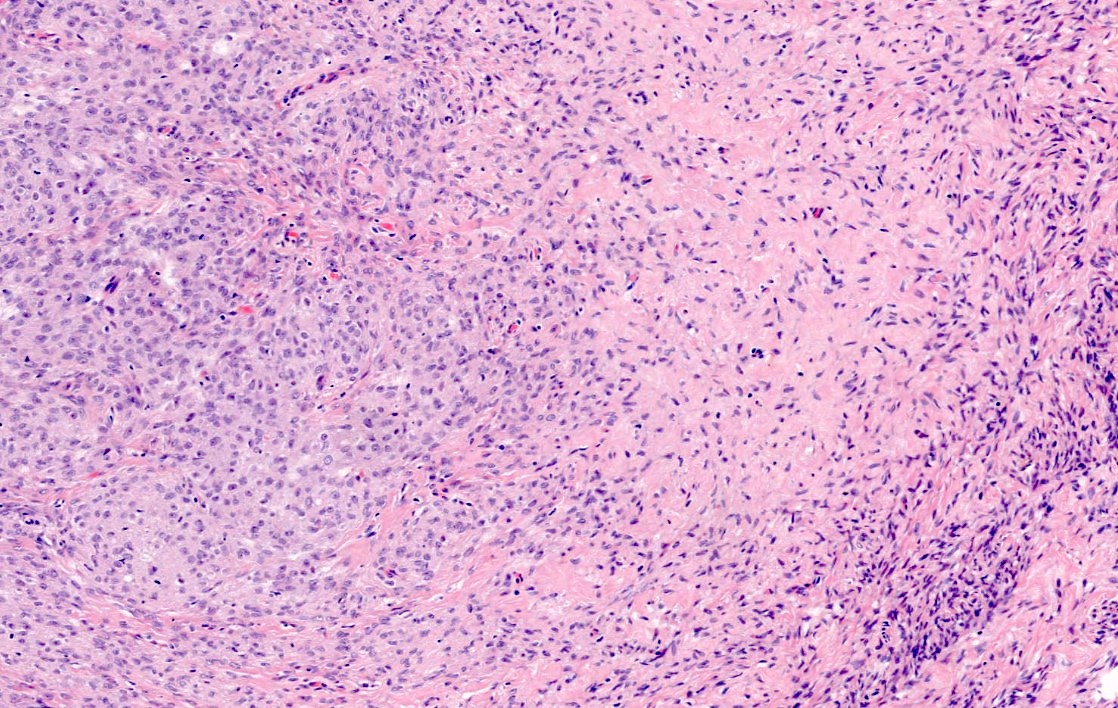
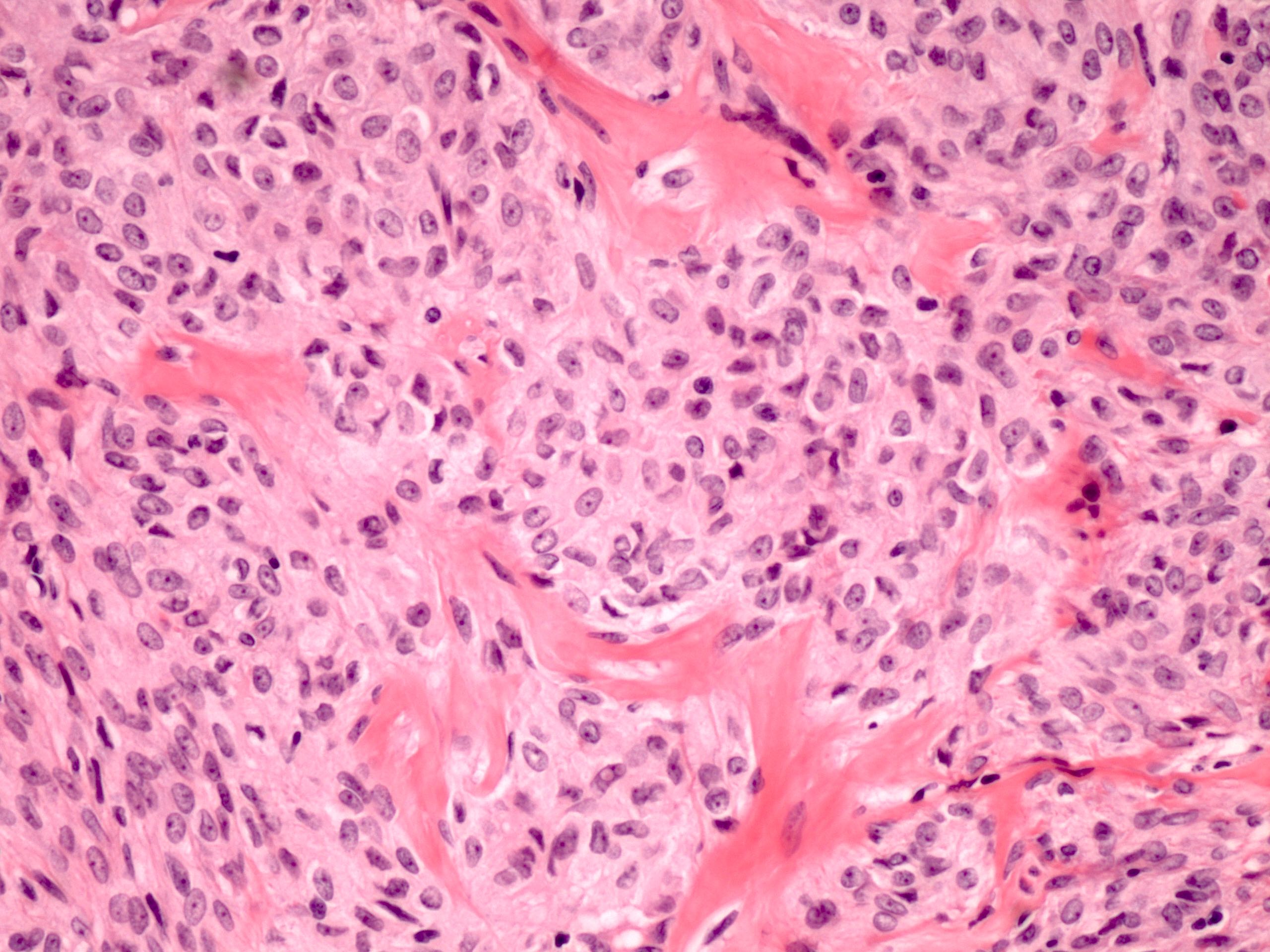
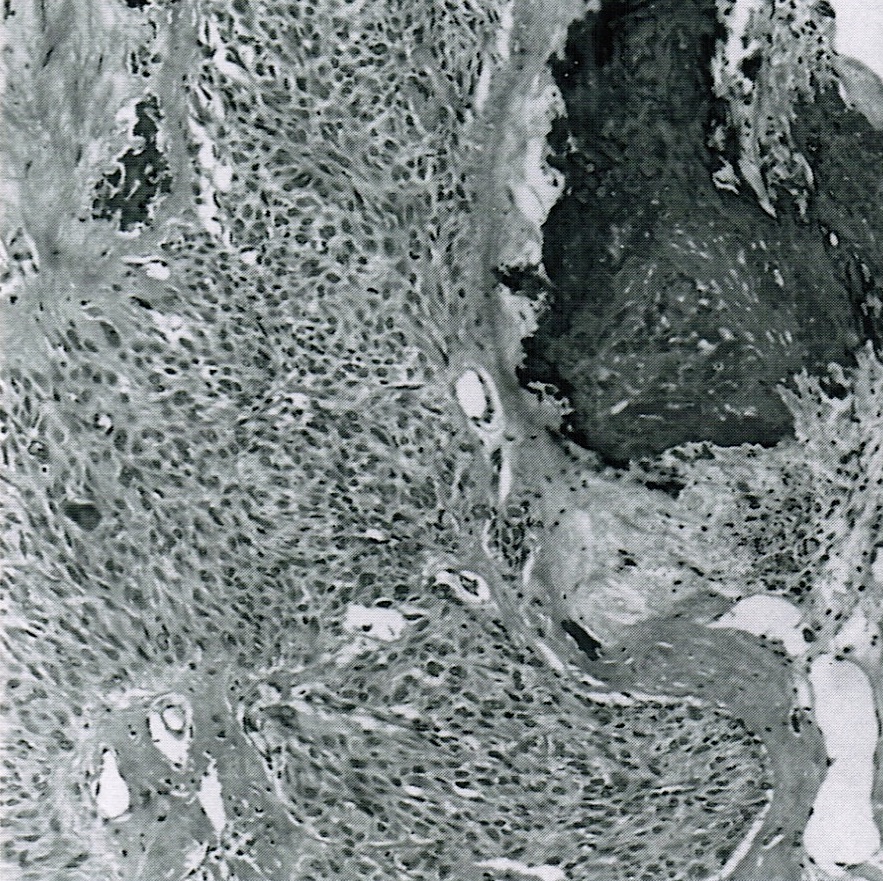
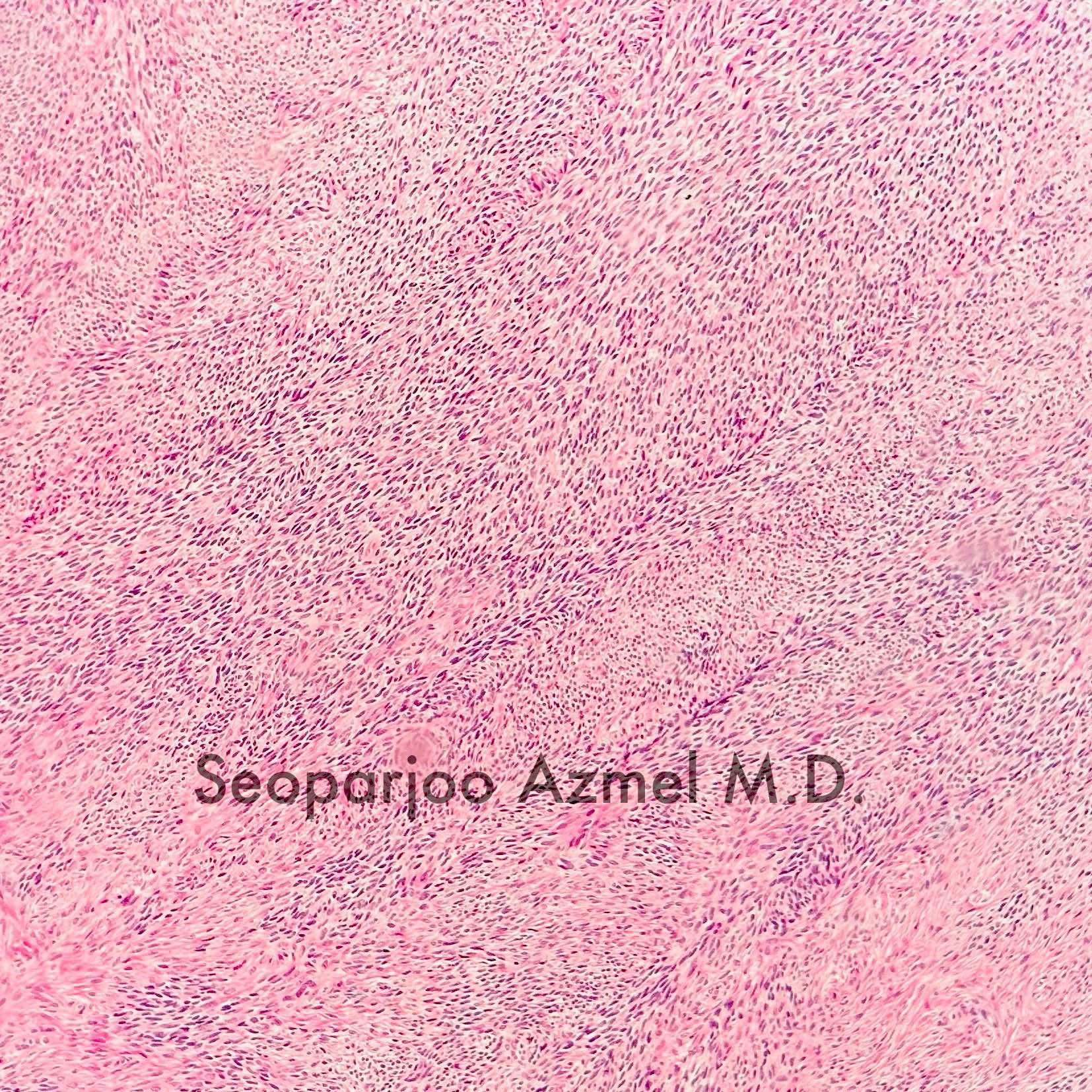
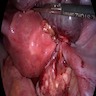
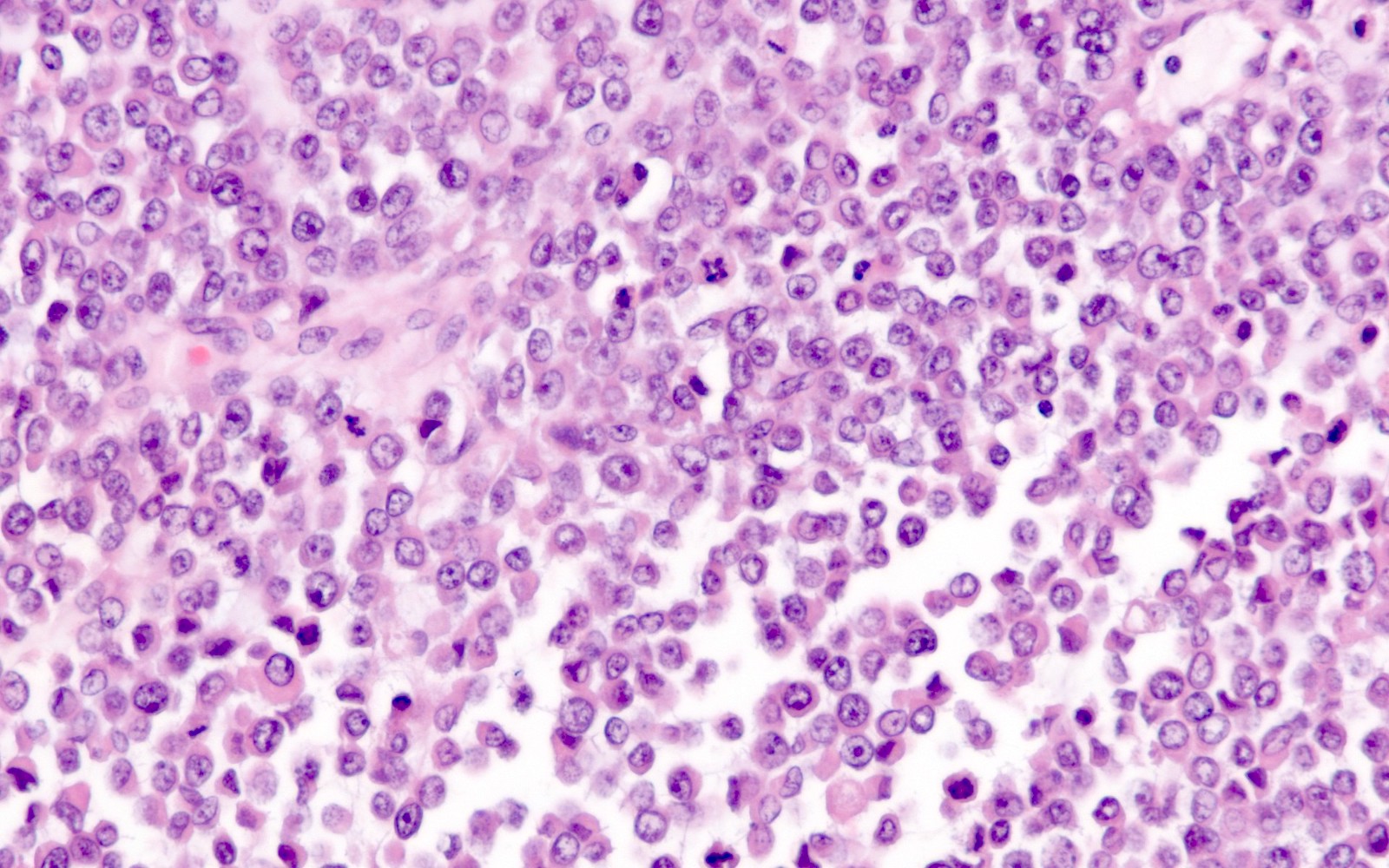
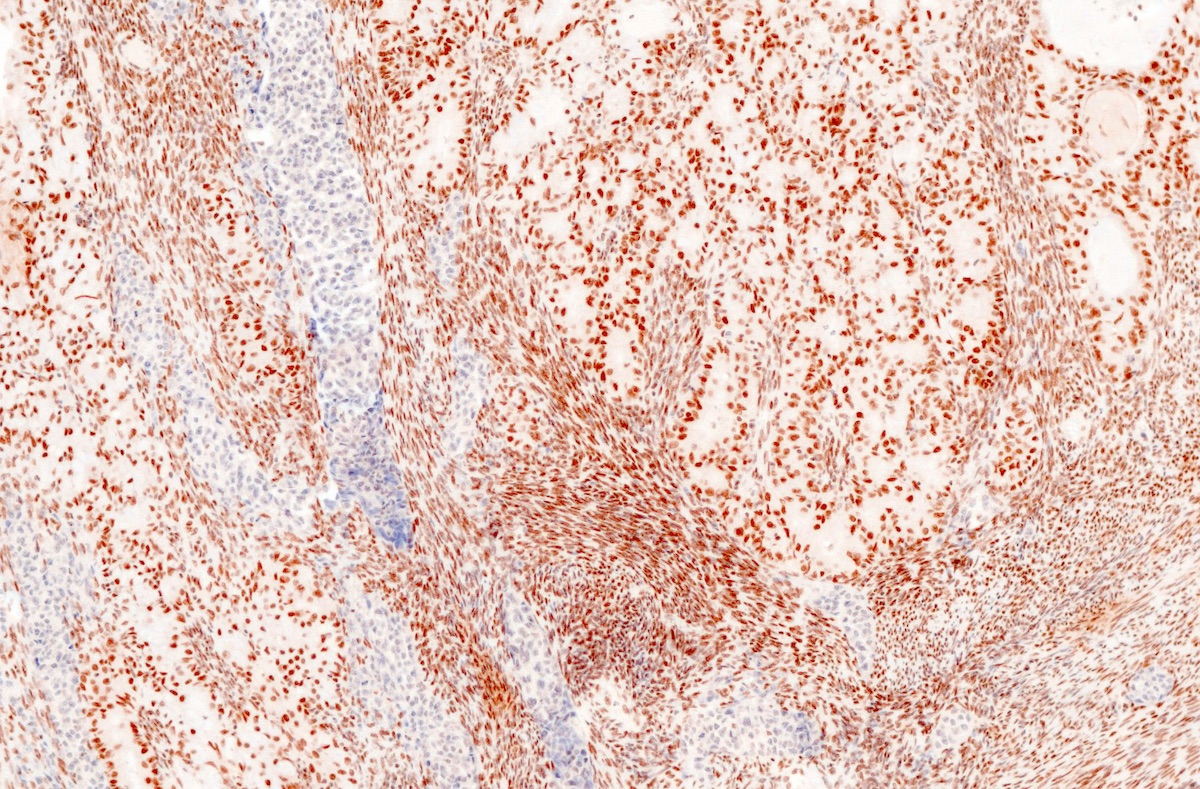
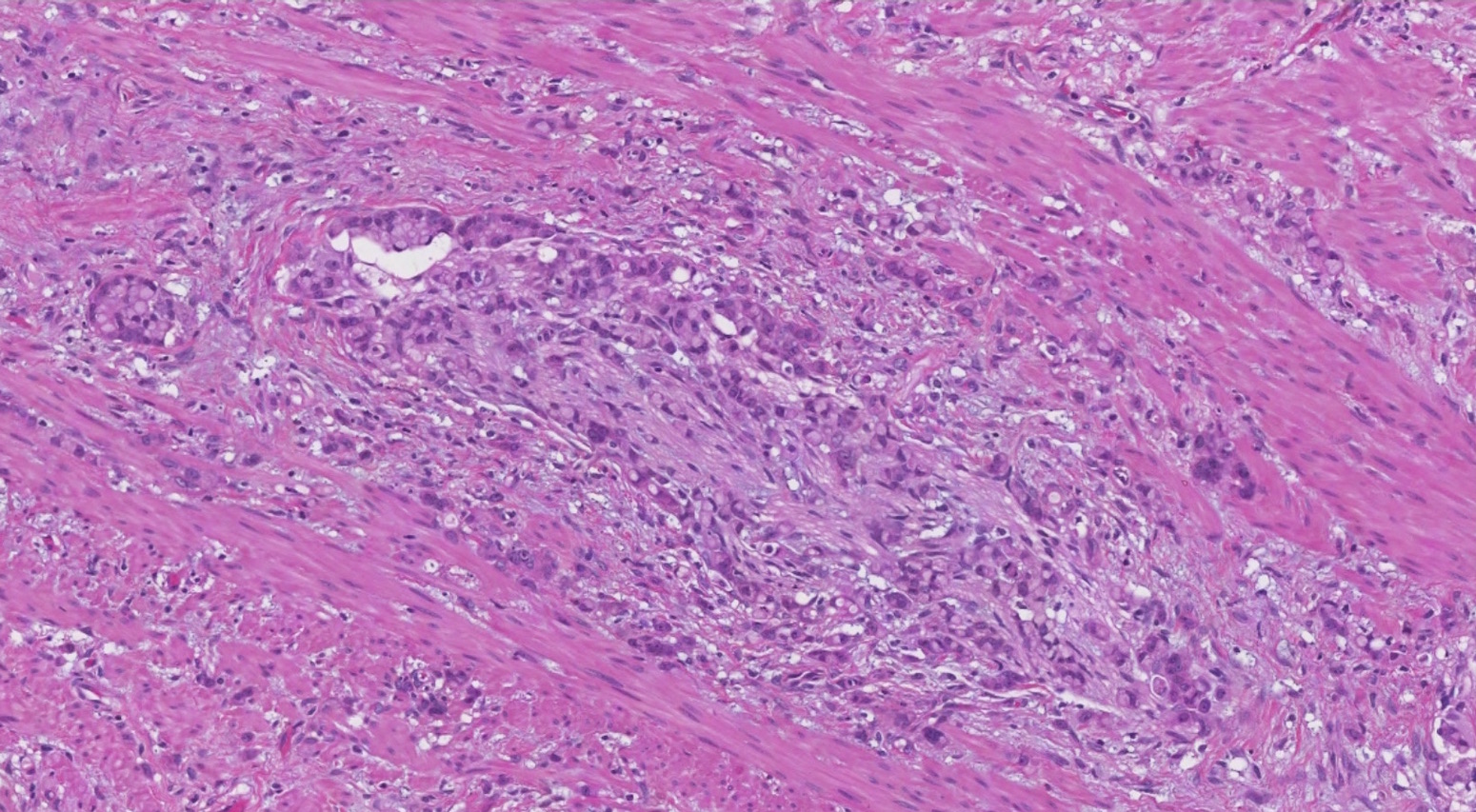
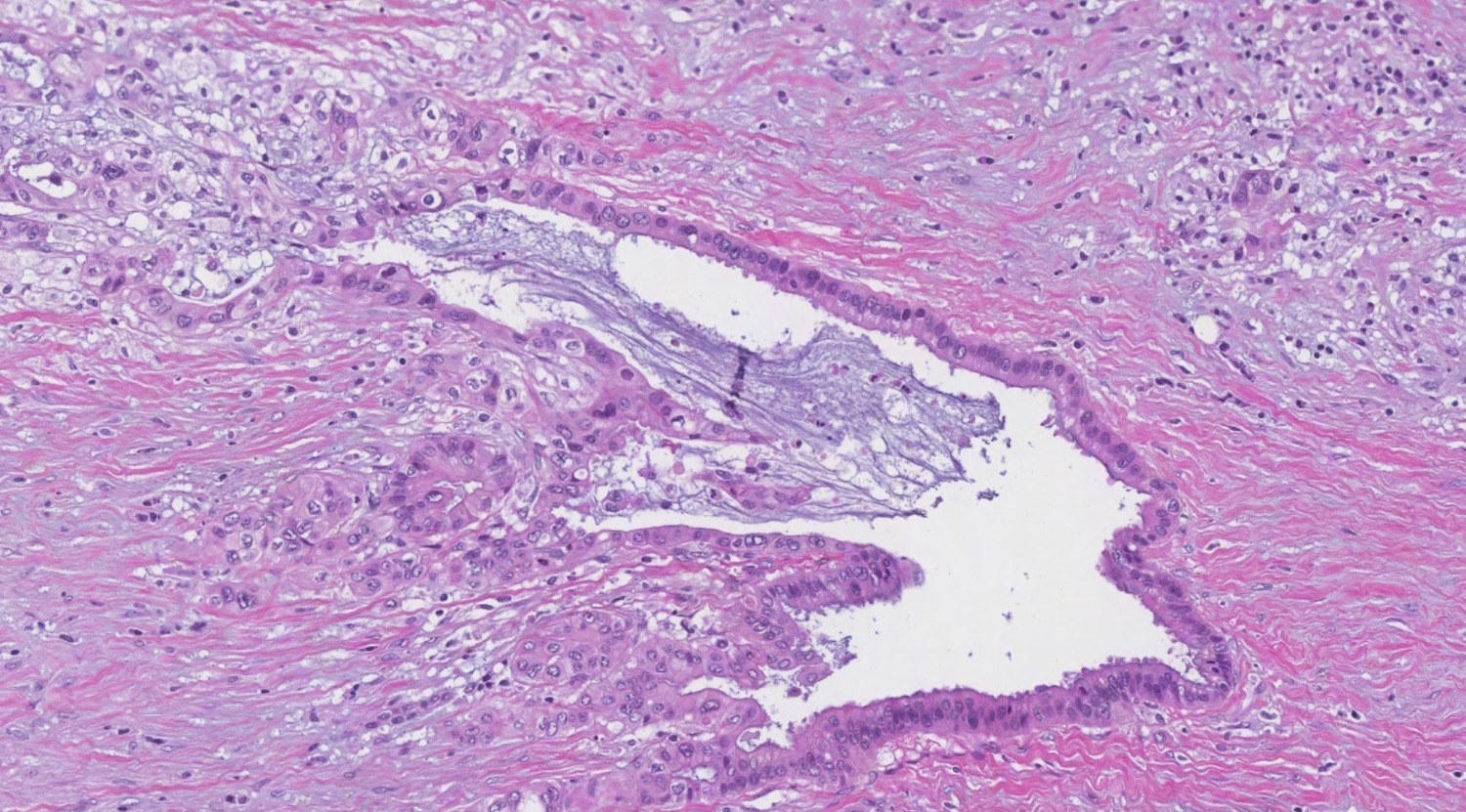
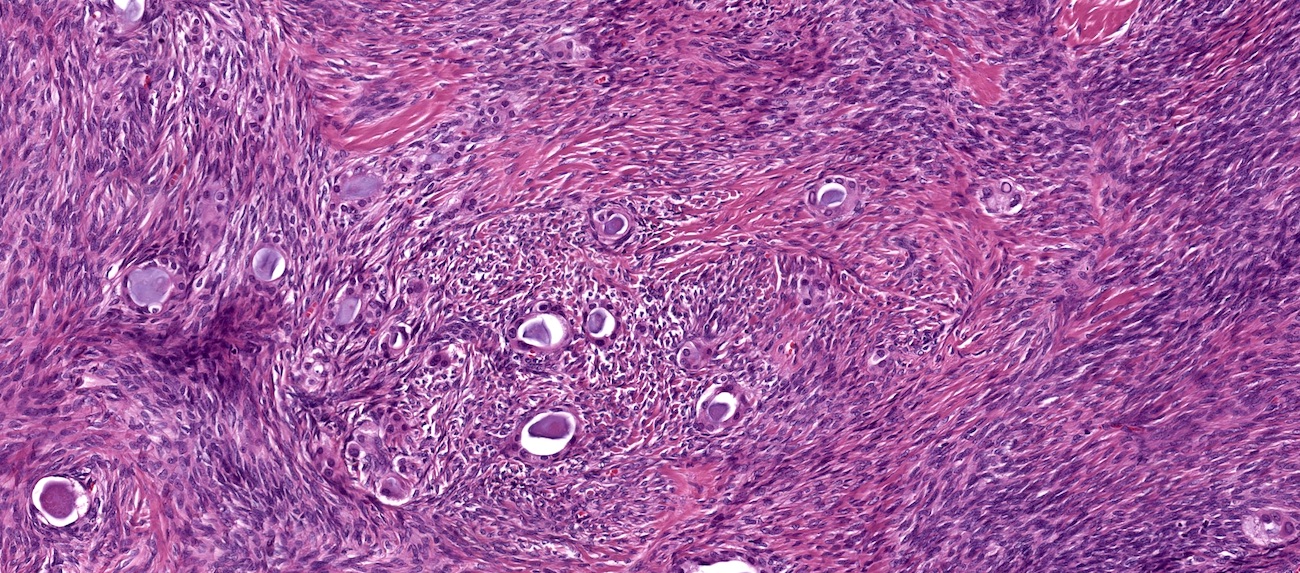
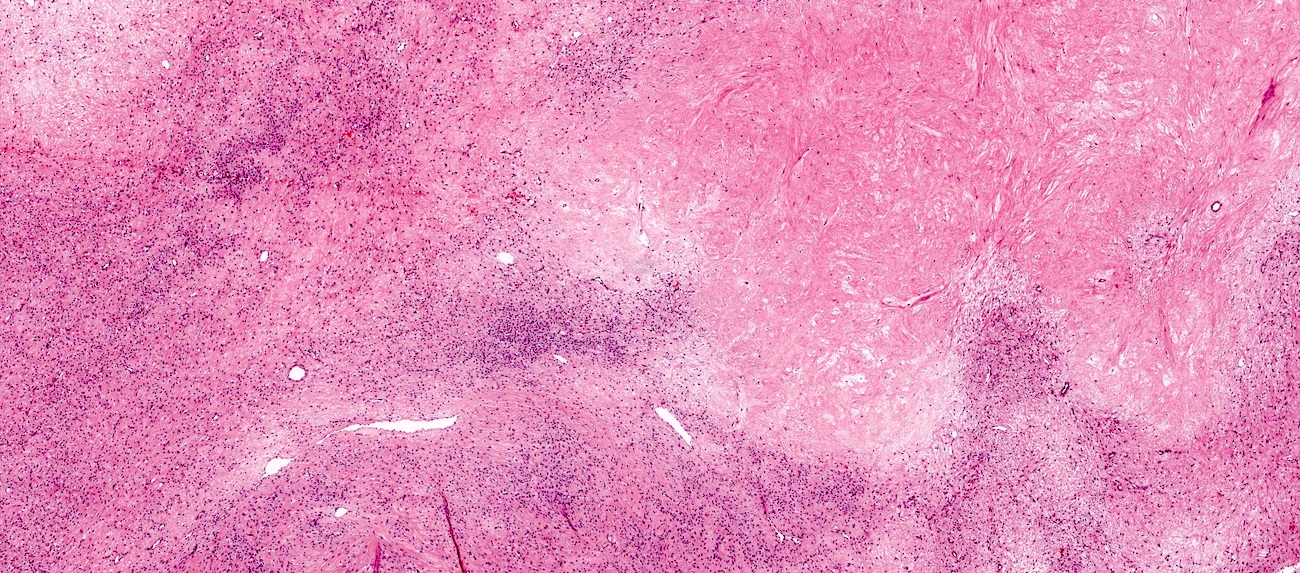
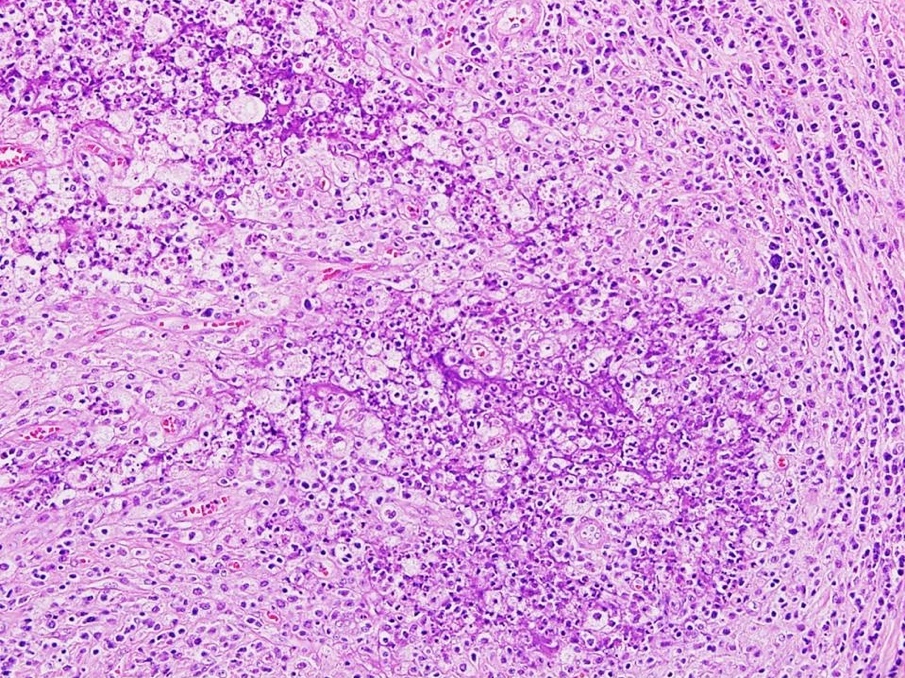
- Biphasic, malignant tumor with high grade epithelial and sarcomatous components
Essential features
- Rare and aggressive ovarian neoplasm (Gynecol Oncol 2016;142:248, Curr Treat Options Oncol 2023;24:1667)
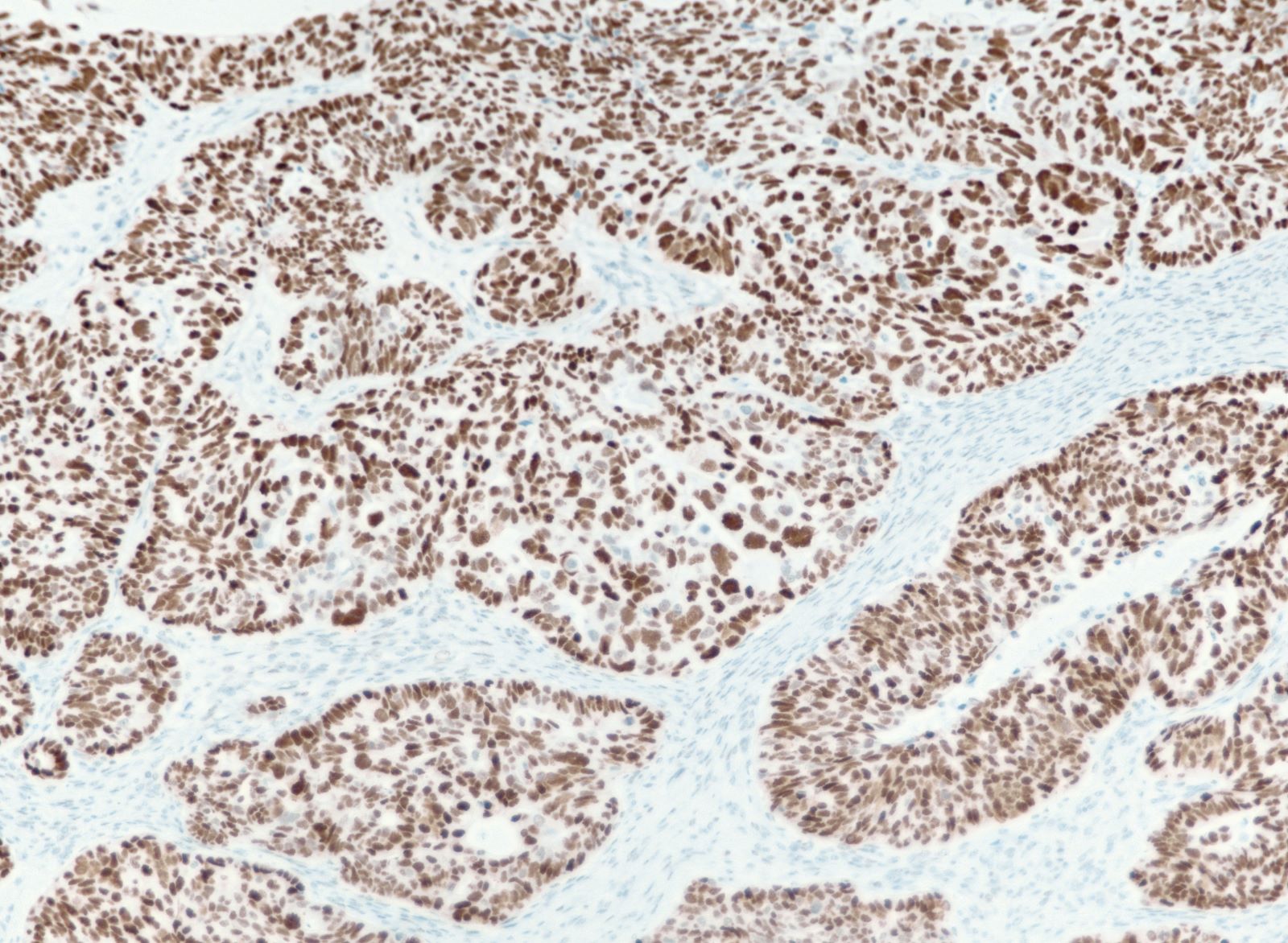
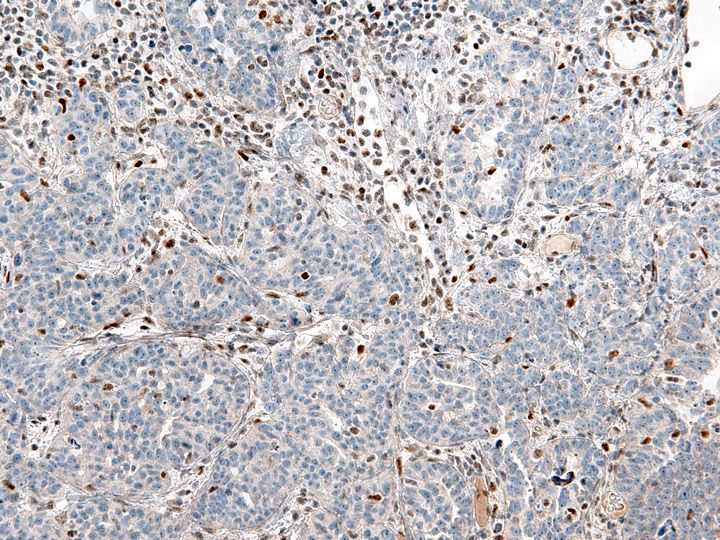
- Carcinoma variant rather than a true mixed epithelial and mesenchymal tumor (WHO, 2020) (Histopathology 2022;80:762)
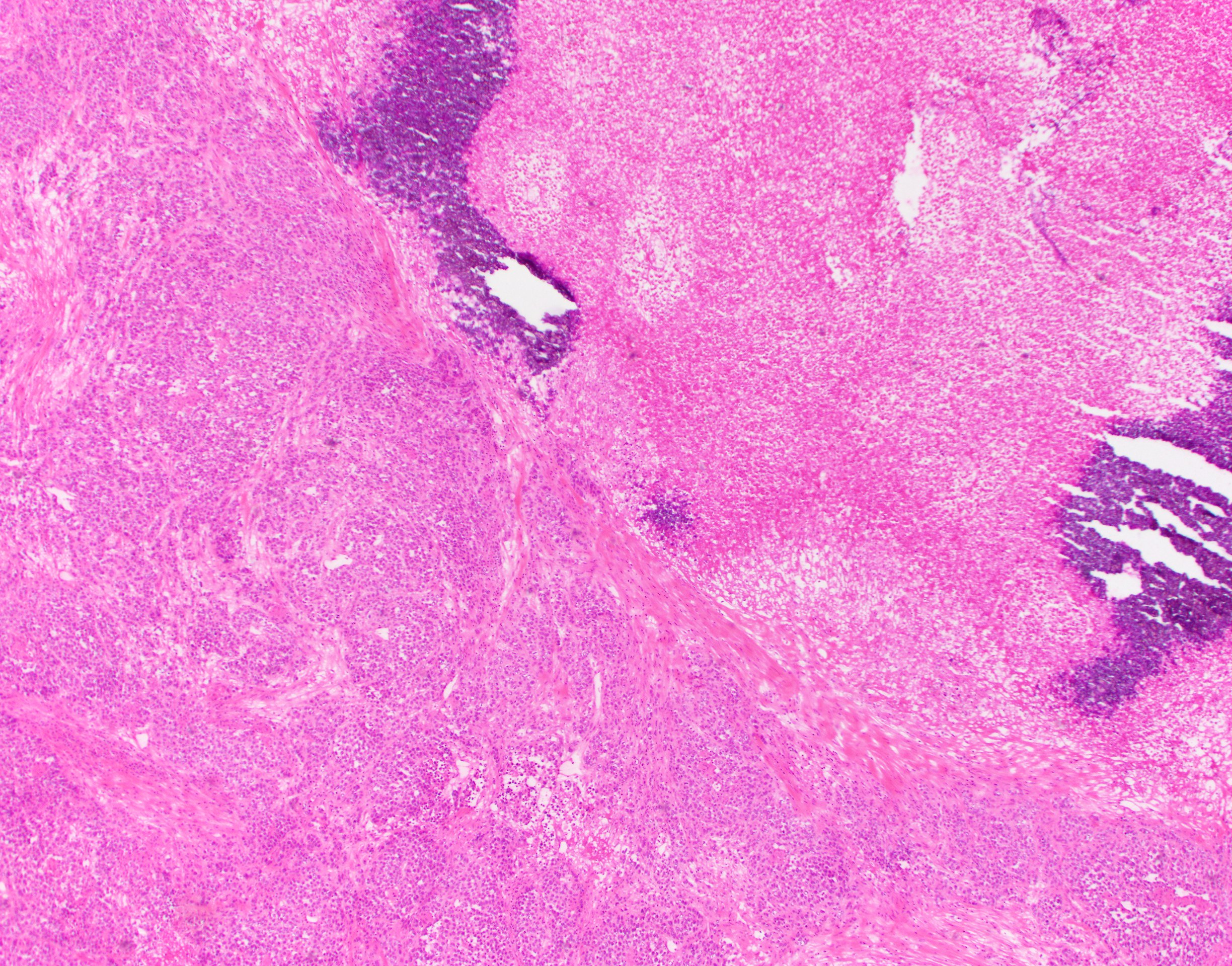
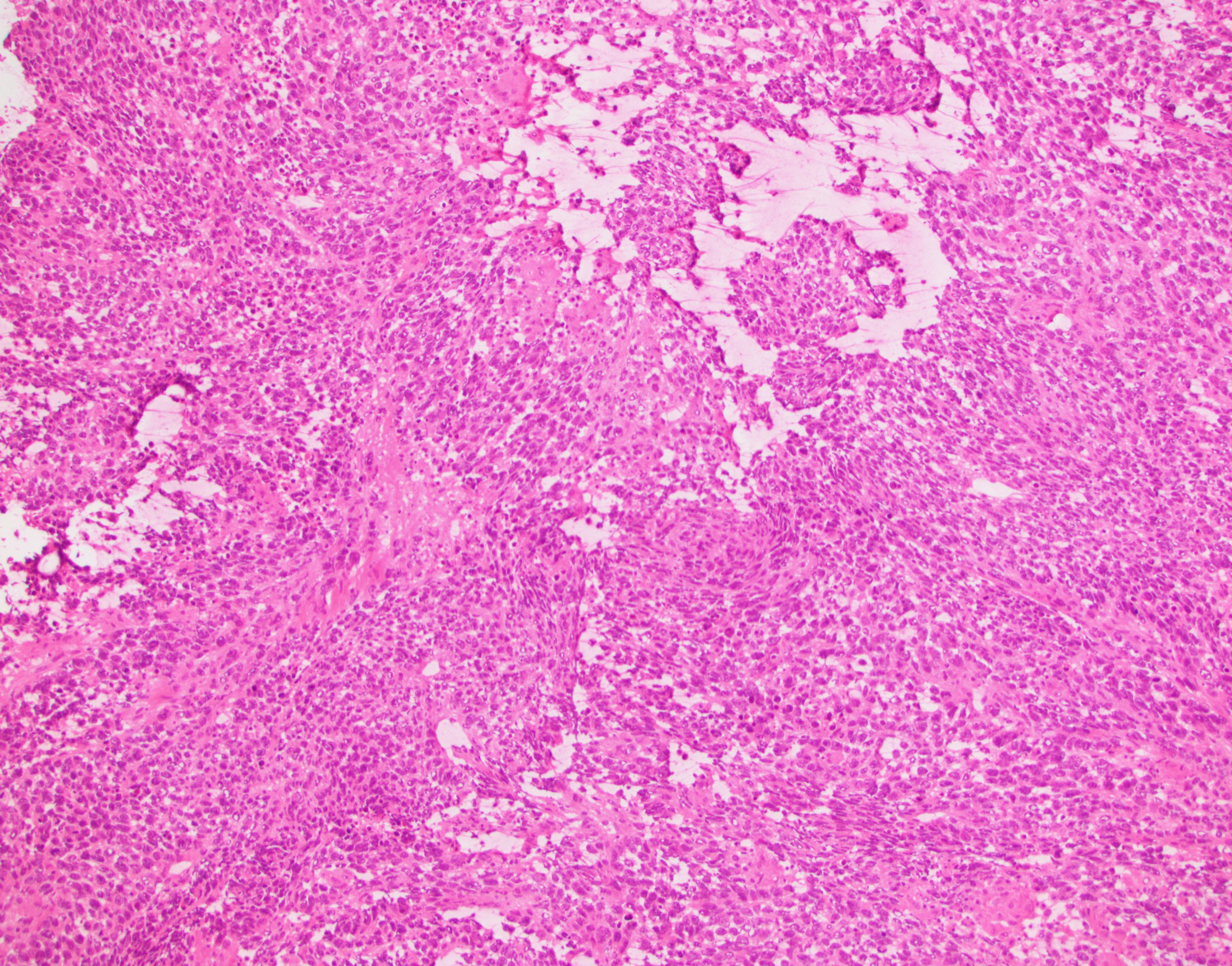
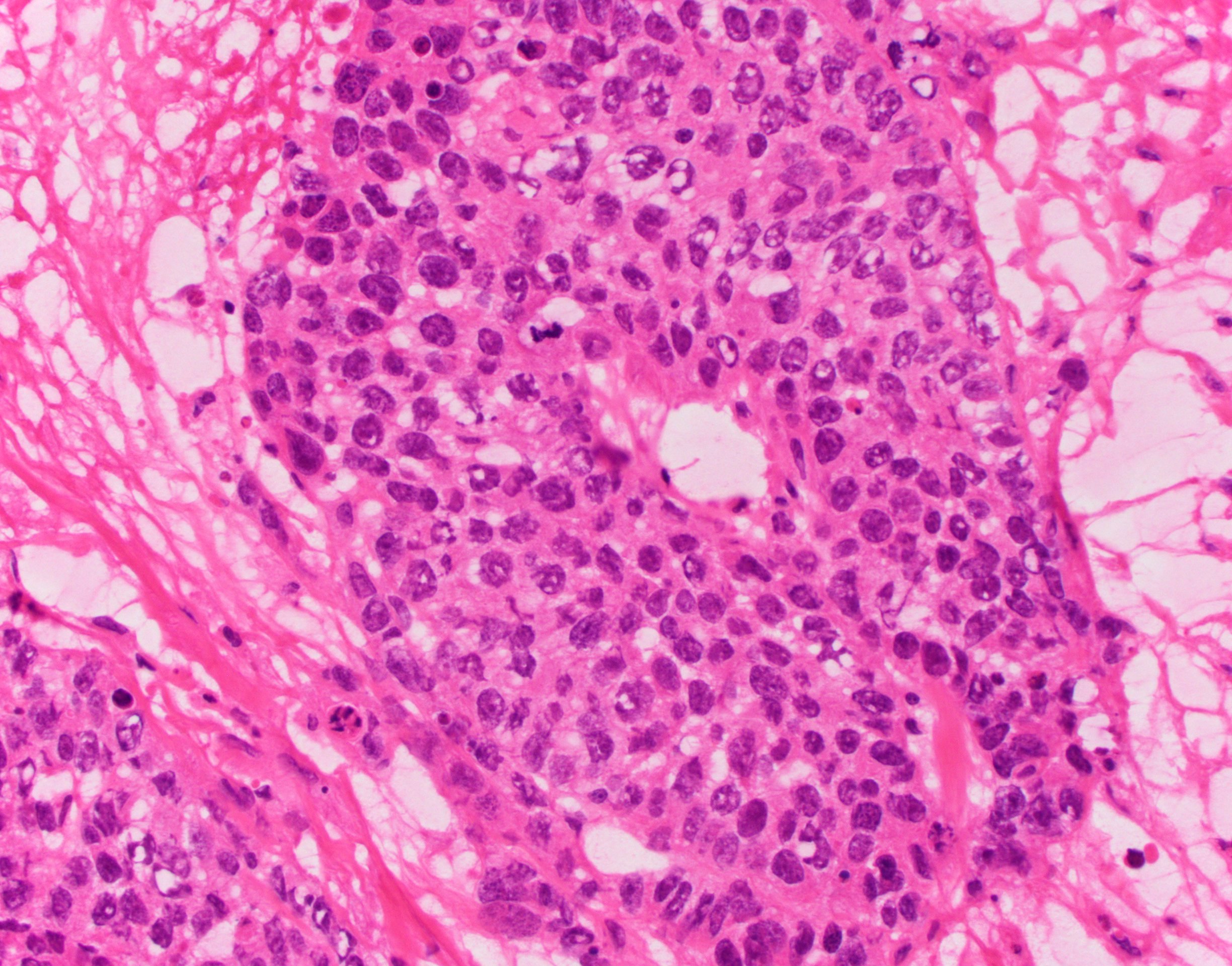
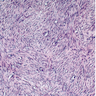
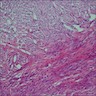
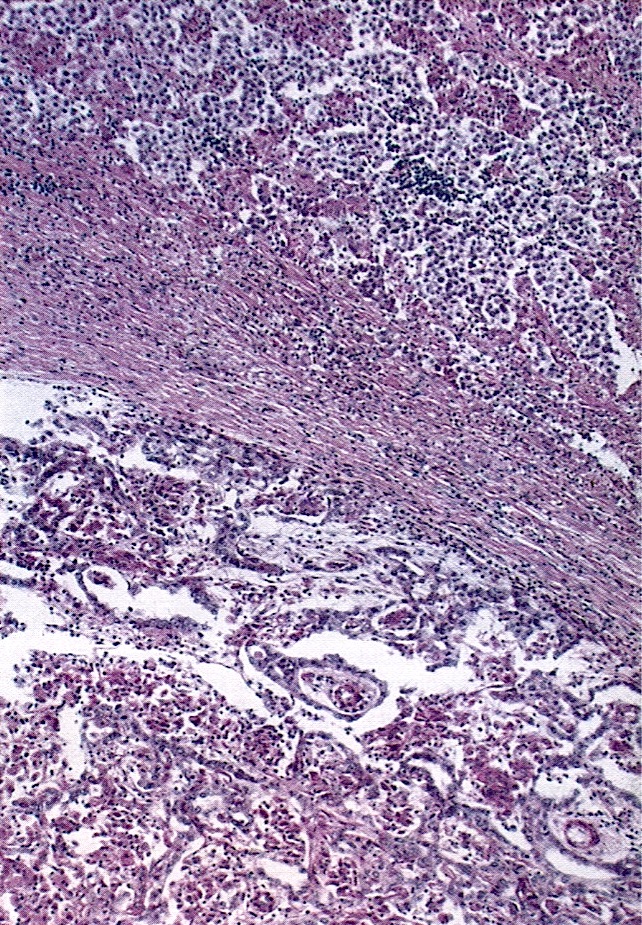
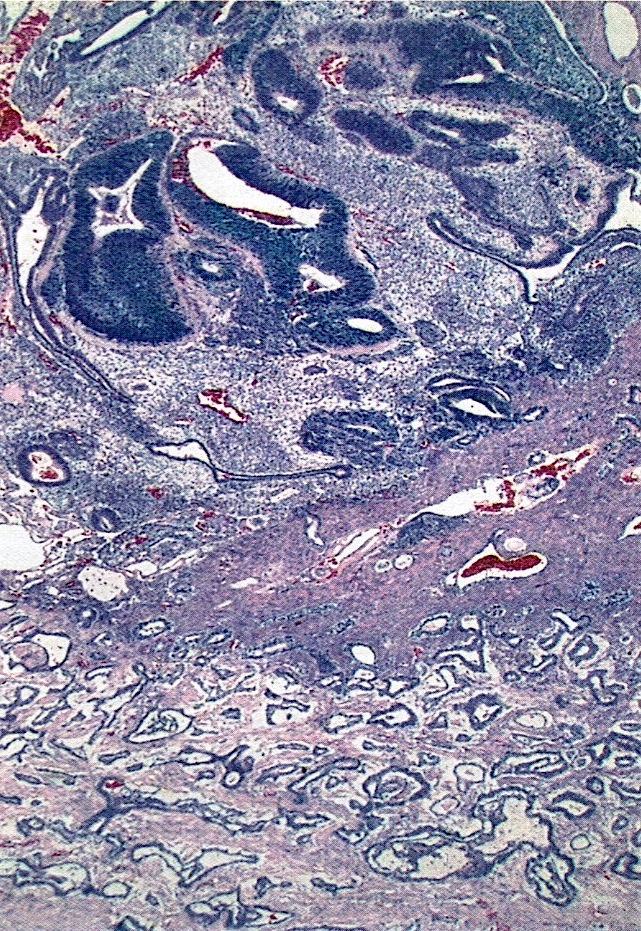
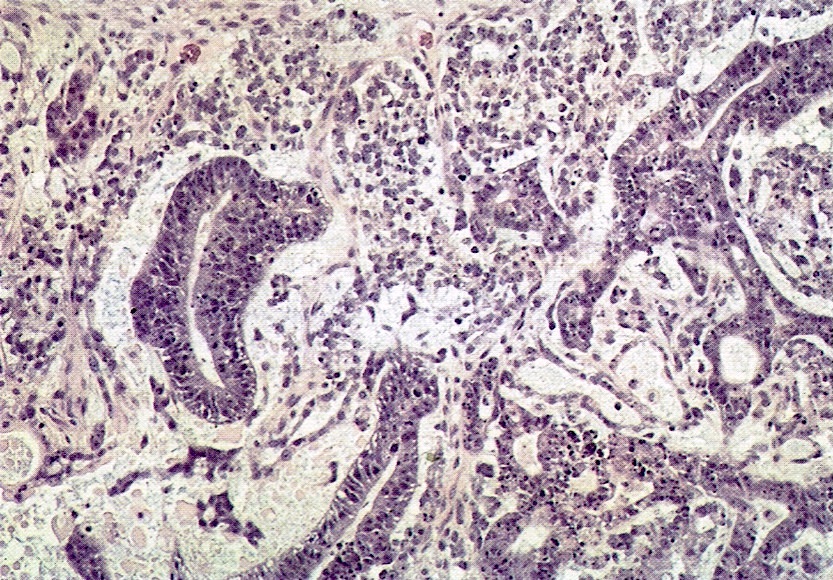
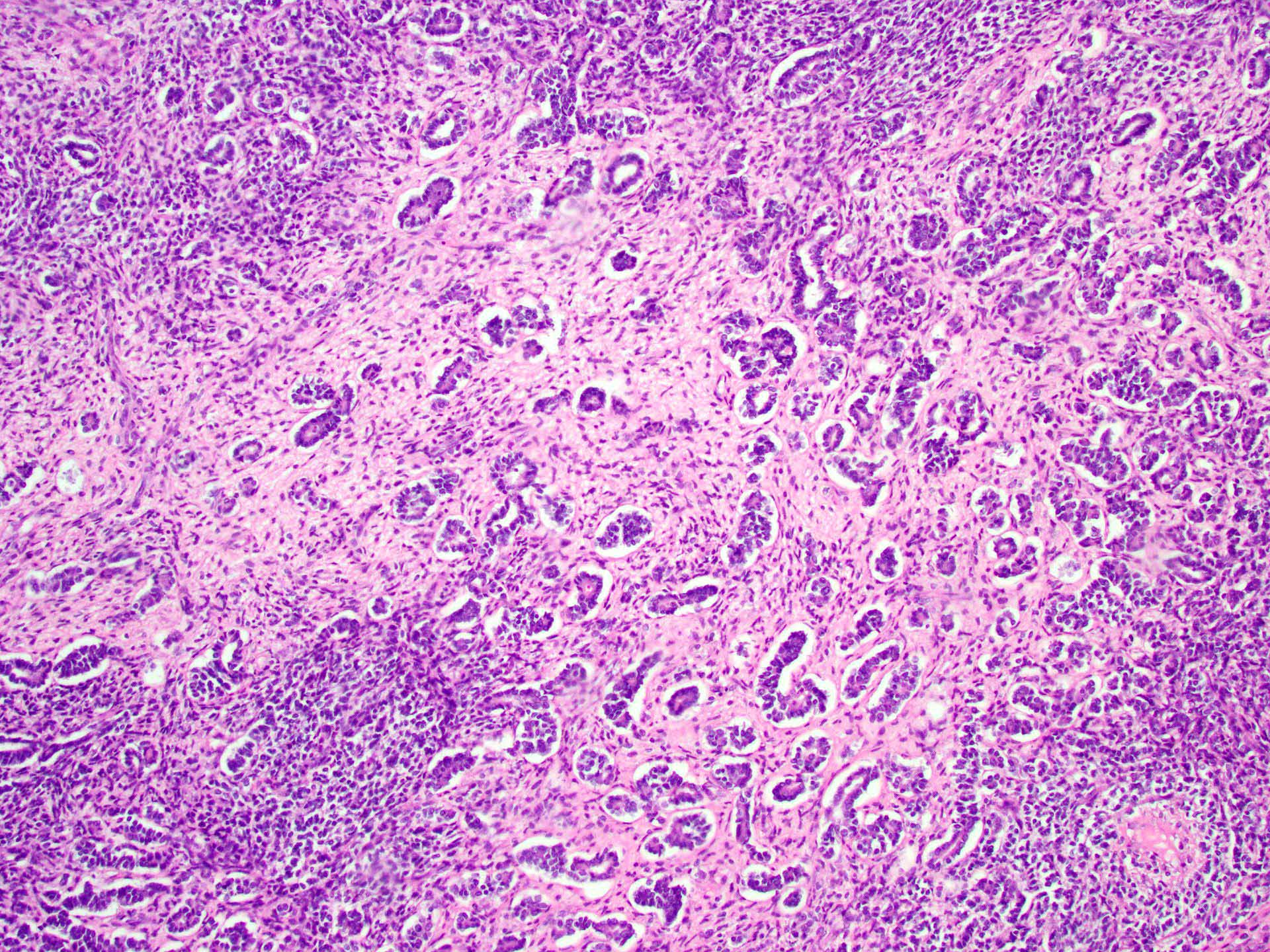
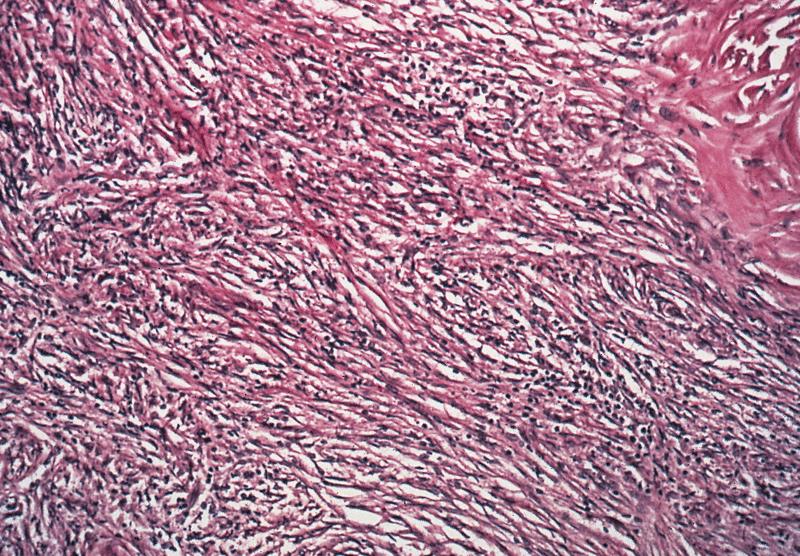
- Histology consists of both high grade carcinomatous and sarcomatous elements
- High morbidity and mortality rate, with median overall survival of < 2 years (Curr Treat Options Oncol 2023;24:1667)
- Stage is the best predictor of outcome and most patients present at advanced stage
Terminology
- Ovarian carcinosarcoma (OCS)
- Previously called malignant mixed Müllerian tumor (MMMT) (Curr Treat Options Oncol 2023;24:1667)
ICD coding
- ICD-O: 8980/3 - carcinosarcoma, NOS
Epidemiology
- Rare, OCS accounts for < 5% of ovarian malignancies (Curr Treat Options Oncol 2023;24:1667)
- Occurs mainly in postmenopausal, low parity women; median age at diagnosis is typically between 60 and 70 years old (Histopathology 2000;37:427)
- Higher prevalence in Black individuals (African Americans)
- ~90% of OCS cases exhibit malignant spread (Int J Gynecol Cancer 2014;24:S55)
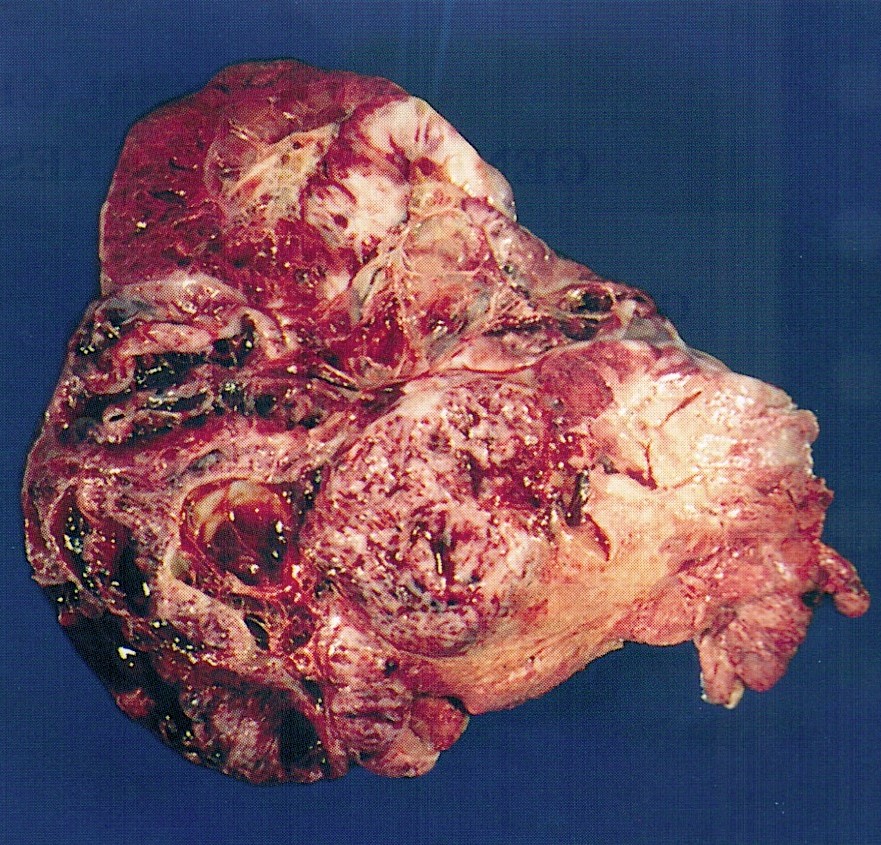
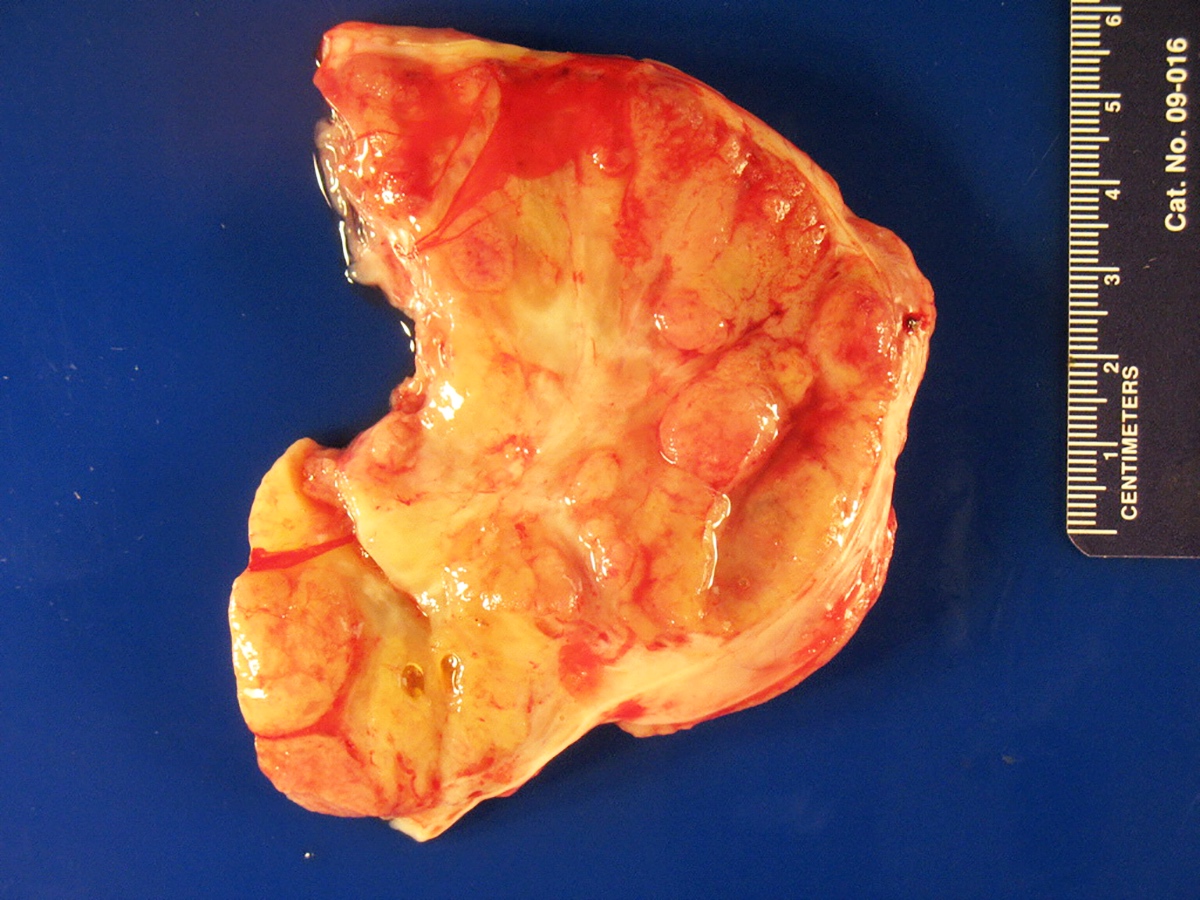
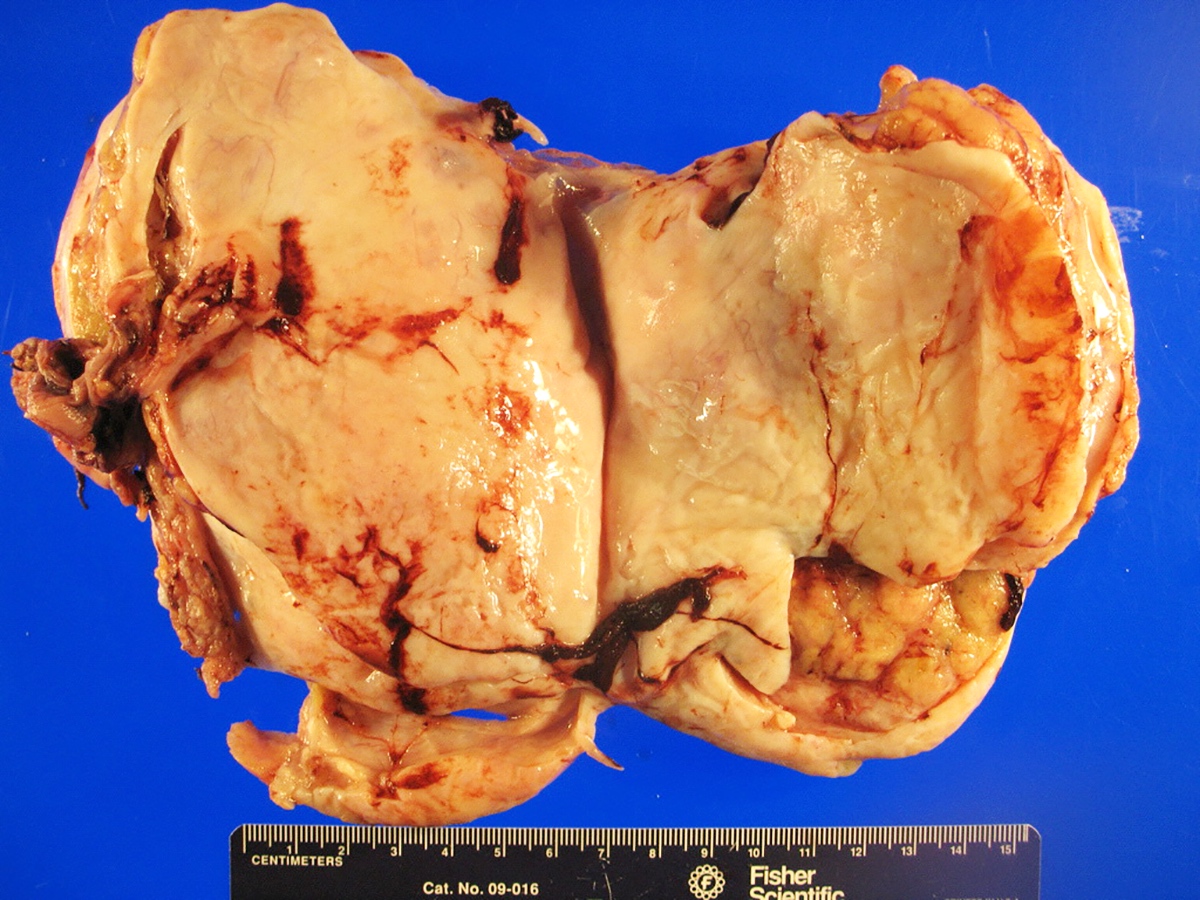
Sites
- More common in other sites such as uterus; rarely involves ovaries, fallopian tubes or cervix
- High rates of lymph node metastasis and vascular invasion
- > 90% of carcinosarcomas spread beyond the ovaries and 33% of cases are associated with peritoneal effusion (Front Oncol 2023;13:1278300)
Pathophysiology
- 3 different theories proposed: conversion theory, the collision theory and the combination theory
- Conversion theory
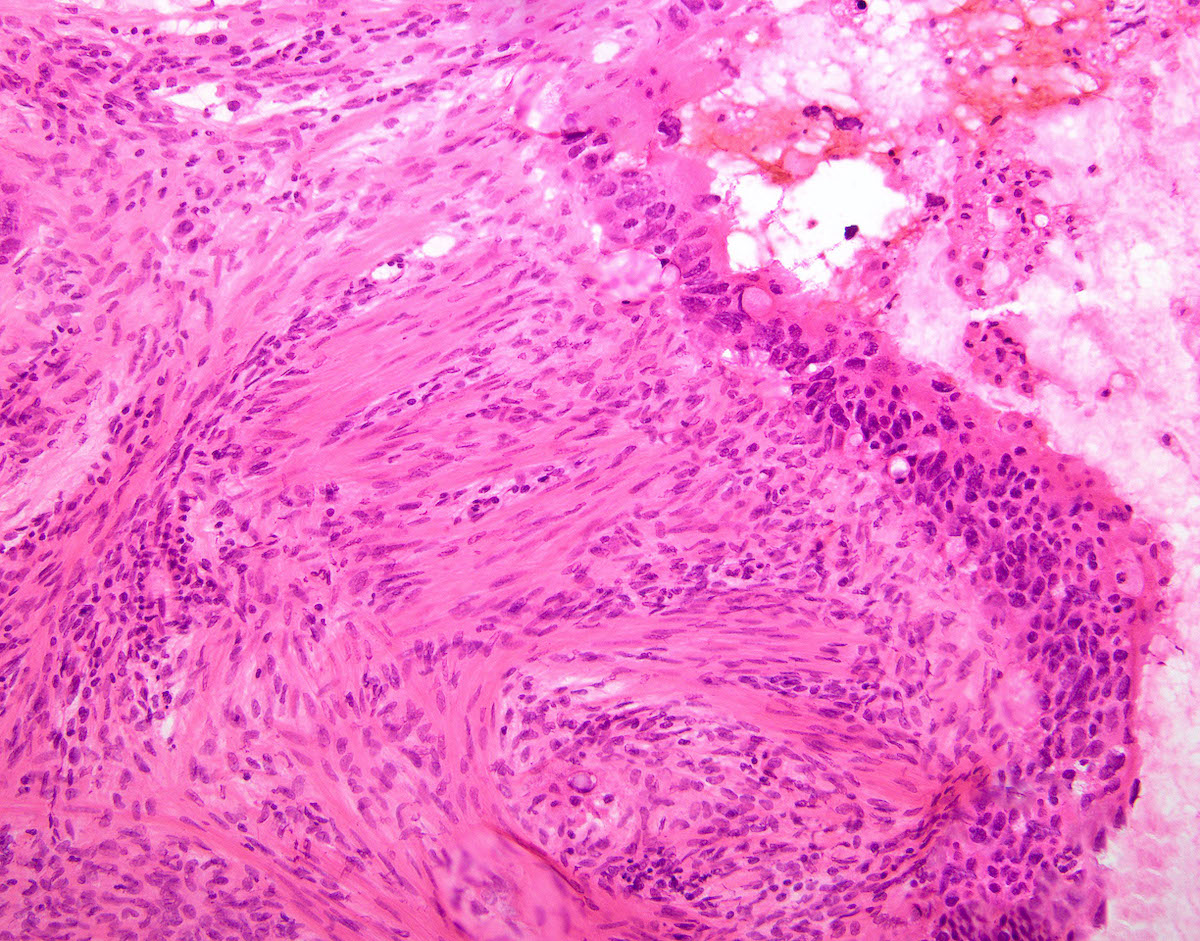
- This is the most accepted theory as shown by studies indicating most carcinosarcomas are monoclonal (Int J Gynecol Pathol 2003;22:368)
- Epithelial origin with epithelial - mesenchymal transition
- Carcinomatous portion arises first, over time differentiating into the sarcomatous portion (Gynecol Oncol 2016;142:248)
- Cells in a carcinomatous component continuously transform into sarcomatous cells during the growth of carcinosarcoma (Anticancer Res 2014;34:7351)
- Supported by the concordant p53 abnormalities seen in both the epithelial and sarcomatous components
- Collision theory
- No longer widely accepted
- States that carcinosarcomas consist of 2 juxtaposed independent tumors: 1 tumor of epithelial origin and 1 of mesenchymal origin merge to form a single carcinosarcoma (Gynecol Oncol 2016;142:248, Int J Gynecol Pathol 2003;22:368)
- Combination theory
- Common stem cell (cells which can give rise to all cell types) gives rise to both the epithelial and mesenchymal components (Cochrane Database Syst Rev 2013;2013:CD006246)
- Majority of carcinosarcomas are monoclonal, making this theory also likely (Int J Gynecol Pathol 2003;22:368)
- Conversion theory
Etiology
- Almost all are sporadic
Clinical features
- Clinical manifestations are nonspecific and related to tumor size and extraovarian extension (J Ovarian Res 2020;13:129)
- May be asymptomatic if lower stage (J Ovarian Res 2020;13:129)
- Symptomatic cases (typically advanced stage) may present with (Gynecol Oncol 2012;125:271)
- Pelvic or abdominal pain / mass
- Early satiety
- Bloating
- Abdominal distention
- Ascites
- Other gastrointestinal complaints
- Tend to manifest at later stages of disease (Curr Opin Obstet Gynecol 2006;18:20)
- Endometriosis significantly more common in ovarian carcinosarcoma than high grade serous carcinoma (Jpn J Radiol 2021;39:357)
Diagnosis
- Like other ovarian tumors, the diagnosis is frequently only rendered after surgical resection
Laboratory
- Many patients may present with elevated CA-125, few with elevated CA-153 levels (Cancer 1998;82:1731, J Ovarian Res 2020;13:129, Cancer 2004;100:2148)
- May be used in the follow up of patients
- However, CA-125 is significantly lower in OCS than in high grade serous carcinoma
- Alpha fetoprotein (AFP) may be elevated in some cases (Gynecol Oncol 2000;77:203)
Radiology description
- Pelvic ultrasound and computed tomography (CT): large, solid heterogeneous pelvic mass, with / without ascites (Ultrasound Obstet Gynecol 2022;59:241)
- Magnetic resonance imaging (MRI) demonstrates stained glass appearance, hemorrhage and necrosis more common in ovarian carcinosarcoma than in high grade serous carcinoma (Jpn J Radiol 2021;39:357)
Prognostic factors
- Prognosis is poor; median overall survival time ranging from 8 to 26 months (Gynecol Oncol 2016;142:248)
- Worst survival among carcinosarcomas of the female genital tract
- Most important prognostic factor: pathological / clinical staging at the time of diagnosis (Gynecol Oncol 2016;142:248)
- At the time of diagnosis, the FIGO stage is usually III - IV (Crit Rev Oncol Hematol 2019;134:46)
- Adverse prognostic factor: presence of sarcomatous component outside the ovary (Am J Surg Pathol 2012;36:831)
- Majority of cases present with extraovarian spread at diagnosis
- Risk factors
- Older age
- Suboptimal surgical resection (Gynecol Oncol 2016;142:248)
- Presence of lymph node metastasis (Curr Treat Options Oncol 2023;24:1667)
Case reports
- 41 year old woman undergoing fertility treatment via in vitro fertilization (Int J Surg Case Rep 2023;104:107937)
- 48 year old woman with cutaneous metastasis of carcinomatous component (Diagn Pathol 2022;17:76)
- 52 year old woman with bilateral OCS (Clin Case Rep 2021;9:e05160)
- 55 year old woman with Cowden syndrome (Gan To Kagaku Ryoho 2022;49:783)
- 77 year old woman with OCS growing into an inguinal hernia sac (Surg Today 2003;33:797)
Treatment
- Mainstay of treatment is multidisciplinary management, including cytoreductive surgery followed by platinum based chemotherapy (usually carboplatin - paclitaxel) (Int J Gynecol Cancer 2014;24:S55)
- Use of ifosfamide / cisplatin combination chemotherapy compared to a carboplatin / taxol combination correlates with better overall survival (Gynecol Oncol 2006;100:128)
- Use of paclitaxel / platinum chemotherapy versus other chemotherapies is optimal for nonprogression and longer survival (Gynecol Obstet Invest 2011;72:208)
- Anthracycline, alkylating agent and cisplatin demonstrated a complete or partial response rate but also high toxicity (Int J Gynecol Cancer 2009;19:1142)
- Similar overall survival to ovarian high grade serous carcinoma when treated in the same manner with optimal cytoreduction and platinum / taxane combination chemotherapy (Int J Clin Oncol 2018;23:329)
Gross description
- Typically, unilateral solid or solid and cystic mass with friable, necrotic / hemorrhagic cut surface (Am J Surg Pathol 2012;36:831)
- Typically unilateral, only 10% bilateral (Clin Case Rep 2021;9:e05160)
- Typically large mass; mean size: 13.6 cm (Am J Surg Pathol 2012;36:831)
- Often spread beyond the ovary and over peritoneal surfaces (Crit Rev Oncol Hematol 2019;134:46)
Microscopic (histologic) description
- High grade malignant carcinomatous and sarcomatous elements admixed
- Carcinomatous element
- Most common carcinomatous element is high serous adenocarcinoma; the second most common is endometrioid (Am J Surg Pathol 2012;36:831)
- Serous > endometrioid > clear cell > squamous > mixed > undifferentiated carcinoma
- Can also be mucinous, mixed high grade serous and endometrioid carcinoma, mixed high grade serous and clear cell carcinoma, or undifferentiated adenocarcinoma (Gynecol Oncol 2016;142:248)
- Most common carcinomatous element is high serous adenocarcinoma; the second most common is endometrioid (Am J Surg Pathol 2012;36:831)
- Sarcomatous element: can be homologous (50%) or heterologous (50%)
- Homologous
- Typically nonspecific malignant mesenchymal cells but may resemble fibrosarcoma, leiomyosarcoma or other sarcomas native to Müllerian organs (Clin Case Rep 2021;9:e05160)
- Heterologous
- Specific malignant elements of a non-Müllerian tissue including cartilaginous, osteosarcomatous or rhabdomyoblastic elements (J Ovarian Res 2020;13:129)
- Incidence of sarcomatous types: chondrosarcoma > rhabdomyosarcoma > osteosarcoma and liposarcoma > neuroectodermal elements
- Most often only 1 type of heterologous sarcoma
- Homologous
- Lymphovascular invasion common
- High grade cytologic atypia and brisk mitotic activity
- Intracytoplasmic hyaline globules in sarcomatous component are frequently seen
Microscopic (histologic) images
Positive stains
- Both carcinomatous and sarcomatous elements are usually positive for p53 abnormal expression and p16 (Histopathology 2000;37:427)
- Both carcinomatous and sarcomatous elements may be positive for
- Tumor cell droplets: PAS+ diastase resistant
- Sarcomatous component
- Variable: CEA, EMA, keratins (CAM 5.2 and AE1 / AE3)
- Vimentin (J Cancer Res Ther 2015;11:1022)
- SALL4, glypican 3 (Patholog Res Int 2012;2012:569609)
- Myogenin, myoD1, muscle specific actin (HHF35) and desmin if rhabdomyoblastic elements (Histopathology 2000;37:427)
- Chondrosarcoma / osteosarcoma areas: S100 protein
- CD99, FLI1 and GFAP may be positive in neuroectodermal component
- SATB2 positive in osteosarcoma and sarcoma, NOS
- Carcinomatous component
- Chromogranin, NSE, CD56 and synaptophysin may be positive
- May show positivity for ER / PR (J Cancer Res Ther 2015;11:1022)
- HER2 / neu
- Variable positivity in OCS (Am J Clin Oncol 2023;46:572)
- Typically expressed in epithelial component (Cancer Sci 2003;94:986)
- Ki67
- Higher expression of Ki67 in carcinomatous element, indicating more aggressive portion of the tumor (Virchows Arch 2008;452:259)
- Immunohistochemical loss of mismatch repair (MMR) proteins may occur (Nat Commun 2014;5:5006)
Negative stains
- AFP, CDX2, desmin, CD34 (epithelioid sarcoma) (Appl Immunohistochem Mol Morphol 2000;8:293)
Molecular / cytogenetics description
- Shown to have mutations in genes commonly associated with uterine and ovarian carcinomas, such as TP53, KRAS and PIK3CA (most commonly observed mutations); others reported include CDKN1B, CTNNB1, FBXW7, PPP2R1A, CDH4, PTEN, PIK3CA, CCNE1, TERT, ARID1A, ARID1B, SPOP, BAZ1A, MLL3 and BCOR (Proc Natl Acad Sci U S A 2016;113:12238, Nat Commun 2014;5:5006)
- TP53 (Am J Clin Oncol 2023;46:572, Histopathology 2000;37:427, Proc Natl Acad Sci U S A 2016;113:12238, Obstet Gynecol 1994;83:118)
- Mutations in TP53 are the most common in OCS, found in > 50% of cases
- Often associated with tumorigenesis, advanced stage and worse survival, though not necessarily prognostic
- Commonly deleted and frequently present in the serous epithelial component (> 50%)
- PIK3CA
- ~40% of OCS exhibit PIK3CA missense mutations; these mutations show no prognostic significance (Nat Commun 2014;5:5006, Histopathology 2000;37:427, J Cancer Res Ther 2015;11:1022, Front Oncol 2023;13:1278300)
- EGFR or KIT
- Overexpression of EGFR or KIT proteins may occur in OCS (Cancer Sci 2003;94:986)
- Mismatch repair (MMR)
- Alterations or losses in MMR proteins are observed in some OCS, with defects more common in uterine than ovarian carcinosarcomas (Nat Commun 2014;5:5006, Am J Clin Oncol 2023;46:572)
- HER2 / Neu, MYC and VEGFA
- Amplification of HER2, MYC and VEGFA genes may be present, potentially impacting tumor growth and progression (Am J Clin Oncol 2023;46:572)
- BRCA1 / BRCA2
- BRCA1 / BRCA2 mutations and associated deficiencies in DNA double strand break repair have been reported, especially in cases where the carcinomatous component is serous carcinoma (Am J Clin Oncol 2023;46:572)
- Tumor mutational burden (TMB)
- TMB in OCS and ovarian tumors is typically low (Am J Clin Oncol 2023;46:572)
Sample pathology report
- Uterus, total hysterectomy and bilateral salpingo-oophorectomy:
- Ovarian carcinosarcoma with a homologous sarcomatous component (see comment)
- Comment: There is a malignant biphasic cell proliferation composed of carcinomatous elements (serous carcinoma) intimately admixed with a sarcomatous element. The constellation of morphological features supports the diagnosis of carcinosarcoma (malignant mixed Müllerian tumor). These are considered malignant epithelial tumors, which behave like a high grade carcinoma.
Differential diagnosis
- Immature teratoma (children):
- Presence of young, immature neuroectodermal elements
- Usually women < 20 years old
- Endometrioid carcinoma with spindle cells:
- Ovaries must be uninvolved or clear metastasis
- Tubal histologic pattern
- Pure carcinomas with desmoplastic stroma:
- Resembles sarcomatous elements
- Sarcomatous components exhibit high grade nuclear atypia and frequent mitotic figures
- Pure carcinoma with desmoplastic stroma is an epithelial tumor with a fibrotic stromal reaction (versus biphasic in OCS)
- Sarcomatoid carcinoma:
- Combination of glandular and spindle components
- Widespread expression of keratin markers
- Dedifferentiated carcinoma:
- Low grade endometrioid carcinoma adjacent to noncohesive poorly differentiated tumor cells
- Occasional rhabdoid morphology
- Mesodermal adenosarcoma with or without sarcomatous overgrowth or mesodermal adenosarcoma with endometrioid carcinoma:
- Benign glandular elements mixed with a sarcomatous stroma
- Presence of periglandular cuffs of cellular stroma
- Lacks high grade malignant epithelial features
- Metastatic tumor from nongynecologic site (e.g., gastric adenocarcinoma [Krukenberg tumor]):
- Highly cellular fibromatous stroma in Krukenberg tumors
- Signet ring cell morphology is common in this disorder but rare in gynecologic primaries (OCS)
- Other tumors showing multilineage differentiation:
- Examples include poorly differentiated Sertoli-Leydig cell tumor with heterologous elements, Müllerian adenosarcoma and immature teratoma
- Do not typically show high grade carcinomatous components (may have benign or low grade neoplastic epithelial elements)
- Relevant immunohistochemical stains for sex cord and germ cell tumors aid in diagnosis
- Ovarian metastasis from a uterine primary:
- Presence of a large tumor in the uterine cavity in patients with both endometrial and ovarian tumors suggests an endometrial primary
Additional references
Board review style question #1
Board review style answer #1
C. CK7+, vimentin+. In ovarian carcinosarcoma, the carcinomatous component (demonstrated here on the right) will be positive for CK7, while the sarcomatous component (on the left) will be positive for vimentin. Answer B is incorrect because at least one component should be positive for CK7 and the other for vimentin. Answers A and D are incorrect because as mentioned above, the carcinomatous component is positive for CK7 and the sarcomatous component is typically positive for vimentin, therefore, the tumor overall should show positivity for CK7 and vimentin in at least one component.
Comment Here
Reference: Carcinosarcoma
Comment Here
Reference: Carcinosarcoma
Board review style question #2
Board review style answer #2
D. TP53 is the most common mutation (> 50%) in carcinosarcomas of the ovary.
Answer B is incorrect because HER2 is amplified in only a small fraction of ovarian carcinosarcoma cases. Answer C is incorrect because KRAS is commonly mutated in ovarian carcinosarcoma cases; however, TP53 is the most commonly reported mutation in ovarian carcinosarcoma. Answer A is incorrect because EGFR is rarely mutated / overexpressed in ovarian carcinosarcoma cases.
Comment Here
Reference: Carcinosarcoma
Comment Here
Reference: Carcinosarcoma
Cervical carcinoma metastatic to ovary
Table of Contents
Definition / general | Epidemiology | Pathophysiology | Clinical features | Radiology description | Prognostic factors | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology images | Negative stains | Molecular / cytogenetics description | Differential diagnosisDefinition / general
- Cervical cancers only rarely metastasize to ovaries
- Proportion of cases presenting with ovarian metastases at the time of surgery ranges from 0.66 to 1.5% (Int J Gynecol Oncol 1997;57:173, Gynecol Oncol 2006;101:234)
- Adenocarcinomas are more likely to metastasize to the ovaries than squamous cell carcinomas
- In different series, 5.3 – 8.2% of cervical adenocarcinomas vs 0.4 - 1.3% of squamous cell carcinomas metastasized to ovary (Gynecol Oncol 2001;82:312, Gynecol Oncol 2006;101:234, Gynecol Oncol 2001;82:504)
- Independent risk factors for ovarian spread include histologic type (adenocarcinoma > squamous cell carcinoma) and uterine corpus involvement
- Other risk factors include vaginal involvement, lymphovascular space invasion and lymph node metastases (J Gynecol Oncol 2008;19:181, Gynecol Oncol 2001;82:312)
- Among HPV negative endocervical adenocarcinomas, risk is low for clear cell carcinomas (Onco Targets Ther 2014;7:111), but high (up to 35%) for gastric type adenocarcinoma (Am J Surg Pathol 2015;39:1449)
Epidemiology
- Mean age of presentation is 57.4 years for squamous cell carcinoma and 50.2 years for adenocarcinoma (Gynecol Oncol 1991;41:46)
Pathophysiology
- Carcinomatous spread from the cervix to the ovaries is hematogenous or lymphatic (Cancer 2000;88:2578)
- Cervical and ovarian tumors are grossly discontinuous with no direct invasion from cervix to ovary reported to date, which also supports the hematogenous spread theory (Diagn Pathol 2014;9:109, Int J Gynecol Pathol 2015;34:551)
- Based on the strong association between uterine corpus involvement and ovarian metastasis, transtubal implantation has also been postulated as a mechanism (Int J Gynaecol Obstet 1997;57:173)
Clinical features
- Mean age of presentation is 45 - 50 years (Gynecol Oncol 2001;82:312, Gynecol Oncol 2006;101:234, Gynecol Oncol 2001;82:504)
- Signs and symptoms are usually related to the cervical lesion (vaginal bleeding, pelvic pain, abnormal cytology)
- Rarely, the presence of an ovarian mass is the only finding at presentation and a cervical primary is initially unsuspected
Radiology description
- USG: enlarged ovaries
Prognostic factors
- Very poor outcome with 5 year overall survival close to 0% (Int J Gynecol Oncol 1997;57:173)
- Outcome not related to tumor stage or histological type (Gynecol Oncol 2006;101:234)
Treatment
- In patients with cervical cancer, ovarian preservation and transposition is recommended in:
- Women 44 years old or younger with squamous cell carcinoma stage IB-II
- Clinically normal ovaries and no history of breast cancer or family history of ovarian cancer (Gynecol Oncol 2001;82:312)
- Oophorectomy is recommended in patients that do not fulfill these criteria, in particular those with adenocarcinoma or locally advanced tumors (Int J Gynaecol Obstet 2007;99:64)
Gross description
- Most ovaries appear grossly normal
- When abnormal, ovaries have a nodular surface and cystic areas (Int J Gynecol Pathol 2015;34:551, Int J Gynecol Oncol 1997;57:173)
- Bilateral involvement and size Am J Surg Pathol 2008;32:128, Int J Gynecol Pathol 2015;34:551-63)
Gross images
Microscopic (histologic) description
- Squamous cell carcinoma:
- Nodular growth of malignant squamous epithelium (keratinizing or non-keratinizing) with confluent and destructive growth (Int J Gynecol Pathol 2015;34:551)
- Adenocarcinoma:
- Malignant glandular proliferation with confluent glandular, cribriform and papillary architecture
- Stromal invasion may be expansile or infiltrative (J Clin Pathol 2012;65:591)
- Usual (endocervical) type:
- Glandular epithelium with mucinous or endometrioid differentiation
- Neoplastic cells have pleomorphic, hyperchromatic, round to elongated nuclei with stratification and conspicuous apical mitoses
- Gastric type:
- Neoplastic cells have columnar shape and granular eosinophilic apical cytoplasm
- Nuclei are round in shape and display significant atypia and pleomorphism
- Importantly, malignant areas usually coexist with regions showing a benign and borderline appearance (architectural and cytologic)
Microscopic (histologic) images
Negative stains
- Adenocarcinoma: CK20, ER, PR, vimentin, SATB2 (Am J Surg Pathol 2015 Nov 5 [Epub ahead of print])
Molecular / cytogenetics description
- Detection of HPV ribonucleic acid by PCR or in situ hybridization is a reliable method to differentiate metastatic HPV related cervical carcinoma (squamous cell and usual type adenocarcinoma) from primary ovarian tumors and other secondary lesions (Int J Gynecol Pathol 2004;23:7)
Differential diagnosis
- Primary mucinous and endometrioid carcinoma of ovary:
- There is significant morphologic and immunophenotypic overlap
- Correlation with clinical history and presentation (laterality, size) is important
- Primary ovarian adenocarcinoma usually has bland cytology; cervical tumors display hyperchromasia, nuclear overlapping and apical mitoses, at least focally
- "Block" positivity for p16 favors a primary HPV+ endocervical adenocarcinoma (Am J Surg Pathol 2007;31:653)
- Metastatic adenocarcinoma from gastrointestinal tract origin:
- Signet ring cell differentiation (uncommon in endocervical primaries), CK20+ and CDX2+ [strong and diffuse staining favors appendiceal and colorectal origin (Histopathology 2015 Nov 6 [Epub ahead of print], Int J Gynecol Pathol 2015 Nov 3 [Epub ahead of print])], SATB2+ (Int J Gynecol Pathol 2015 Nov 3 [Epub ahead of print], Am J Surg Pathol 2015 Nov 5 [Epub ahead of print]), p16- (although gastric type endocervical adenocarcinoma is also p16-)
- Primary squamous cell carcinoma:
- Exceedingly rare and seen in a background of mature teratoma
- Absence of teratomatous elements and "block" positivity for p16 favor a cervical primary
- Metastatic squamous cell carcinoma from skin: p16-
Choriocarcinoma
Table of Contents
Definition / general | Epidemiology | Clinical features | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Differential diagnosis | Additional referencesDefinition / general
- Rare primary ovarian tumor; May develop from ovarian pregnancy (gestational choriocarcinoma), as a germ cell tumor (pure or mixed, non-gestational choriocarcinoma) or as a surface epithelial tumor with choriocarcinomatous differentiation
Epidemiology
- Primary ovarian choriocarcinomas are more common in children and adolescents
- After puberty, origin from an ovarian ectopic pregnancy cannot be excluded
Clinical features
- Presentation similar to ectopic or tubal pregnancy
- Abdominal enlargement
- Pain, sometimes hemoperitoneum
- Isosexual precocity, menstrual abnormalities, androgenic changes
Laboratory
- Markedly elevated serum β hCG
- Monitoring serum levels of β hCG is helpful in predicting recurrence and response to therapy
Radiology description
- On imaging, appears as vascular solid tumor with cystic, hemorrhagic, necrotic areas
Prognostic factors
- In contrast to gestational choriocarcinomas, non-gestational choriocarcinomas are difficult to treat, generally unresponsive to chemotherapy
- Metastases to lungs, liver, bone and viscera are common at diagnosis
Case reports
- 19 year old woman with pure choriocarcinoma of ovary diagnosed by FNA (Indian J Pathol Microbiol 2009;52:417)
- 48 year old woman with pure choriocarcinoma of ovary (J Gynecol Oncol 2011;22:135)
Treatment
- Surgery, chemotherapy
Gross description
- Hemorrhagic, soft, tan with necrosis
Microscopic (histologic) description
- Composed of cytotrophoblast, intermediate trophopblast and multinucleated syncytiotrophoblast around periphery of blood channels
- Cytotrophoblasts have clearly defined cytoplasmic borders, are mononucleated with vesicular nuclei and distinct nucleolus
- Syncytiotrophoblasts contain basophilic to amphophilic cytoplasm with mutiple nuclei
Microscopic (histologic) images
Positive stains
- Cytokeratin, CD10, human placental lactogen, EMA
- Syncytiotrophoblasts are hCG+
Negative stains
- CD31, CD34 (Mod Pathol 2011;24:646)
Molecular / cytogenetics description
- Gestational choriocarcinomas have component of paternal chromosomes, often aneuploid, but hyperploidy and other variations have been described (Atlas of Genetics and Cytogenetics in Oncology and Haematology)
Differential diagnosis
- Adrenocortical carcinoma: see UC Davis-Department of Pathology and Laboratory Medicine
- Malignant pheochromocytoma
- Metastatic choriocarcinoma
- Mixed germ cell tumor with choriocarcinoma component
Additional references
Clear cell borderline tumor
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Gross description | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Adenofibromatous tumor with clear cells demonstrating low grade cytologic atypia and some degree of glandular crowding but without stromal invasion
Essential features
- Abundant adenofibromatous stroma with small glands / cysts with low grade nuclear atypia but without stromal invasion
- No solid, papillary or cribriform growth
- Pure clear cell borderline tumors are rare; more frequently associated with clear cell carcinoma
- If pure clear cell adenofibroma or borderline tumor is present, additional sampling is recommended to exclude clear cell carcinoma
Terminology
- No longer recommended historical terms:
- Atypical proliferative clear cell tumor
- Clear cell tumor of low malignant potential
ICD coding
- ICD-10: D39.10 - neoplasm of uncertain behavior of unspecified ovary
Epidemiology
- Pure clear cell borderline tumors are very rare
- ~21% of ovarian clear cell carcinomas contain a clear cell adenofibromatous component (Am J Surg Pathol 2007;31:999)
- Most patients are postmenopausal (age range of 36 - 82 years old) (Ann Surg Oncol 2022;29:1165)
Sites
- Ovary, almost all unilateral
Pathophysiology
- May be a component of the adenofibromatous pathway of the pathogenesis of ovarian clear cell carcinoma (see Diagrams / tables) (J Cancer 2011;2:94)
- Also see Molecular / cytogenetics description
Etiology
- Associated with endometriosis (~15%) (J Cancer 2011;2:94)
Diagrams / tables
Clinical features
- Incidental imaging finding
- Abdominal / pelvic pain
Diagnosis
- Surgical excision
- Definite diagnosis requires extensive sampling
Prognostic factors
- Good prognosis
- No clinical evidence of aggressive behavior (Cancer 1984;53:1156, J Cancer 2011;2:94)
- Rare recurrences have been reported (Int J Gynaecol Obstet 1996;54:37)
Case reports
- 50 year old woman with 6.5 cm cystic mass in right ovary (Int J Surg Pathol 2018;26:578)
- 53 year old postmenopausal woman with a 12 cm solid ovarian mass (Oman Med J 2012;27:e031)
- 53 year old woman with benign, borderline and clear cell carcinoma of the ovary arising in endometriosis (J Pathol Transl Med 2016;50:155)
- 58 year old woman with 8 cm solid ovarian mass (Case Rep Pathol 2017;2017:3860107)
Treatment
- Surgical resection
- Tumors are typically stage I (Int J Gynecol Cancer 2012;22:993)
Gross description
- Predominantly solid, white-tan mass
- May have small or large cysts, imparting a honeycomb cut surface
Frozen section description
- Small glands or cysts in background of fibromatous stroma
- Lining epithelium comprising 1 - 2 layers of cuboidal, flat or hobnail cells
- Absence of the admixture of growth patterns characteristic of clear cell carcinoma (tubulocystic, papillary and solid)
Microscopic (histologic) description
- Abundant fibromatous stroma
- Small glands or cysts lined by 1 - 2 layers of cuboidal, flat or hobnail cells with clear cytoplasm and low grade nuclear atypia (Cancer 1985;56:2922)
- Atypia: nuclear enlargement, hyperchromasia, small nucleoli
- Presence of atypia may be subjective
- No stromal invasion; lacks architectural complexity
- No confluent, solid, papillary and cribriform architecture
- May show intraluminal eosinophilic secretions
- May show simple cystic outpouchings
- Typically a component of clear cell carcinoma
- Initial sections that demonstrate exclusive clear cell borderline tumor or pure adenofibromatous component warrant additional sampling to exclude clear cell carcinoma
Microscopic (histologic) images
Positive stains
Molecular / cytogenetics description
- Loss of ARID1A is seen in both clear cell carcinoma and its adjacent adenofibromatous component (Mod Pathol 2012;25:615)
- Loss of heterozygosity (LOH) of 5q, 10q and 22q is seen in benign, borderline and malignant clear cell tumors (J Pathol 2008;216:103)
- However, LOH of 1p and 13q is only seen in clear cell carcinoma, suggesting association with malignant transformation
Sample pathology report
- Right ovary and fallopian tube, salpingo-oophorectomy:
- Clear cell borderline tumor, 6 cm
Differential diagnosis
- Clear cell adenofibroma:
- Extremely rare
- No cytologic atypia (Cancer 1984;53:1156)
- No glandular crowding
- Clear cell carcinoma:
- Variety of architectural patterns
- Tubulocystic, papillary, solid
- More often cystic with mural nodule
- Presence of hyalinized stroma
- Variety of architectural patterns
- Endometrioid adenofibroma:
- Clear cell struma ovarii:
- May be associated with a component of mature cystic teratoma
- Areas of classic thyroid tissue typically present, including macrofollicles filled with colloid
- TTF1, thyroglobulin positive
- Napsin A, HNF1β negative
- Yolk sac tumor, polyvesicular vitelline pattern:
- Younger patients; elevated serum AFP levels
- May have prominent cystic component (Am J Surg Pathol 2013;37:393)
- May be associated with other yolk sac tumor patterns
- SALL4, AFP, glypican 3 positive
- Napsin A, PAX8, CK7, EMA negative
Additional references
Board review style question #1
A 52 year old patient undergoes a left salpingo-oophorectomy for a 6 cm ovarian mass. The mass is well sampled and a representative section is shown. Immunohistochemical stains demonstrate that the tumor is positive for PAX8, CK7 and Napsin A, while it is negative for ER, PR, TTF1 and AFP. What is the best diagnosis?
- Clear cell borderline tumor
- Endometrioid adenofibroma
- Struma ovarii
- Yolk sac tumor
Board review style answer #1
A. Clear cell borderline tumor. The image shows an adenofibromatous neoplasm with an immunoprofile consistent with clear cell borderline tumor. Endometrioid adenofibromas are typically ER and PR positive. Yolk sac tumors are typically AFP positive and mostly negative for PAX8 and CK7. While struma ovarii is positive for PAX8 and CK7, it is also positive for TTF1 and negative for Napsin A.
Comment Here
Reference: Clear cell borderline tumor
Comment Here
Reference: Clear cell borderline tumor
Clear cell carcinoma
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Malignant epithelial tumor composed of clear, eosinophilic or hobnail cells with tubulocystic, papillary and solid growth patterns
Essential features
- Admixture of tubulocystic, papillary and solid growth patterns comprised of cells with uniform nuclear atypia without brisk mitotic figures
- Usually positive for CK7, PAX8, napsin A and HNF1β and negative for ER, PR and WT1, with aberrant p53 expression in up to 24% of cases
- May be associated with endometriosis or Lynch syndrome
- Stage is the most important prognostic factor
ICD coding
Epidemiology
- Accounts for 10 - 12% of ovarian carcinomas in North America (Int J Gynecol Pathol 2010;29:203, J Natl Cancer Inst 2019;111:60)
- Racial distribution in United States: 4.8% white, 3.1% black, 11.1% Asian (Gynecol Oncol 2011;121:407)
- Higher incidence in Asia (up to 27% in Japan) (J Obstet Gynaecol Res 2014;40:338, Gynecol Oncol 2019;153:589)
- Mean age is 55 - 56 years (Gynecol Oncol 2008;109:370, J Pathol Clin Res 2018;4:250)
- Increased risk associated with:
- Endometriosis, including endometriotic cysts (50 - 74%) (Lancet Oncol 2012;13:385, Int J Gynecol Cancer 2012;22:1310)
- Less frequently, Lynch syndrome (Eur J Cancer 2016;55:65, Am J Surg Pathol 2008;32:1029, Am J Surg Pathol 2014;38:1173, Clin Cancer Res 2019;25:3962, Int J Clin Exp Pathol 2008;1:502)
- Mismatch repair (MMR) deficiency in 6 - 43.75% of cases
- Younger patients
- 10% develop endometriosis associated carcinomas, including clear cell carcinoma
- Family history of ovarian cancer in a first degree relative (PLoS One 2018;13:e0205000)
- Decreased risk associated with:
- Late menarche (> 15 years), early menopause (< 40 years), tubal ligation, parity, hysterectomy, smoking, oral contraceptives (J Clin Oncol 2016;34:2888)
- Expression of the genetic susceptibility locus (HNF1β) via epigenetic mechanisms (Nat Commun 2013;4:1628)
Sites
- Ovary
- Any extraovarian site with endometriosis
Pathophysiology
- Genetic alterations due to various mutations (see Molecular / cytogenetics description)
Etiology
- Endometriosis (J Pathol 2018;245:265, Int J Gynecol Cancer 2012;22:1310)
- Mutation signatures: age related (40%) and APOBEC mediated (apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like (26%), a family of evolutionarily conserved cytidine deaminases) (Nat Genet 2017;49:856)
Clinical features
- Symptoms related to endometriosis or pelvic mass (Gynecol Oncol 2010;116:374)
- Most common ovarian epithelial neoplasm associated with paraneoplastic syndromes, such as hypercalcemia, thromboembolism, subacute cerebellar degeneration or bilateral diffuse uveal melanocytic proliferation (Onkologie 2009;32:517, Gynecol Oncol 2007;104:406, Semin Diagn Pathol 2019;36:246)
- May be incidental (asymptomatic)
Diagnosis
- Microscopic examination
Laboratory
- May be associated with mildly elevated CA-125 (usually ≤ 200 U/mL) (Medicine (Baltimore) 2018;97:e10881)
Radiology description
- Large unilocular, mainly cystic, smooth marginated mass with 1 or more solid nodular protrusions into the cavity on all imaging modalities
- High attenuated cystic portion on computed tomography (J Comput Assist Tomogr 2006;30:875)
- Variable T1 signal, depending on hemorrhagic components on magnetic resonance imaging
- Blood flow in solid nodules on Doppler ultrasound examination
- In postmenopausal women, ground glass appearance in a cyst ultrasonographically should be evaluated carefully to rule out a component of clear cell carcinoma (Ultrasonography 2015;34:173)
Prognostic factors
- Negative prognostic factors:
- Advanced stage (most important) (Histopathology 2015;66:808, Medicine (Baltimore) 2018;97:e10881)
- Most cases are FIGO stage I (BMC Cancer 2018;18:347, J Natl Cancer Inst 2019;111:60)
- Worse prognosis in stage IC disease compared with stage IA
- Overall 5 year cause specific survival of 66%, including 85% for stage I, 71% for stage II, 35% for stage III and 16% for stage IV (CA Cancer J Clin 2018;68:284)
- Better prognosis than high grade serous carcinoma when confined to the pelvis but worse prognosis if advanced stage except for some MMR deficient tumors (Am J Surg Pathol 2011;35:36)
- Positive lymph nodes (Histopathology 2015;66:808)
- Lymphovascular invasion
- Immunohistochemically, loss of ARID1A, aberrant p53 and block-like p16 staining, expression of IMP3, nuclear expression of beta catenin, fibroblast growth factor receptor 2, chromobox homolog 7 (CBX7), Emi1, CXCR4, HOXA10, glypican 3 and Rsf-1 (HBXAP) (Surg Pathol Clin 2019;12:529)
- Genetic alterations, including amplification of MET or MDM2 (in TP53 wild type cases), CCNE1 copy number gain, somatic copy number variations such as chr20q13.2 harboring the ZNF217 gene (Surg Pathol Clin 2019;12:529)
- Advanced stage (most important) (Histopathology 2015;66:808, Medicine (Baltimore) 2018;97:e10881)
- Positive prognostic factors:
- PIK3CA mutations (Hum Pathol 2012;43:2197)
Case reports
- 28 year old woman with Cowden syndrome diagnosed with PTEN negative ovarian clear cell carcinoma (J Natl Compr Canc Netw 2019;17:7)
- 50 year old woman with cerebellar metastasis of ovarian clear cell carcinoma (Int J Gynecol Pathol 2020;39:68)
- 55 year old woman with primary diaphragmatic clear cell carcinoma associated with endometriosis (Gynecol Oncol Rep 2021;36:100733)
- 61 year old woman with bilateral breast metastases of ovarian clear cell carcinoma (Case Rep Oncol Med 2019;2019:8013913)
- 62 year old woman with ovarian clear cell carcinoma associated with low grade endometrial stromal sarcoma (Gynecol Obstet Invest 2020;85:371)
Treatment
- Hysterectomy, bilateral salpingo-oophorectomy, omentectomy and staging biopsies
- Adjuvant chemoradiation in some patients (Ann Oncol 2016;27:i50)
- Often platinum resistant (Am J Surg Pathol 2011;35:36)
Gross description
- Usually unilateral
- Mean size 13 cm (range 0.8 - 35 cm) (Histopathology 2015;66:808)
- Variable appearance on cut surface:
- Mainly cystic, with fleshy nodules protruding into the cyst lumen
- Solid and cystic (spongy appearance due to small cysts)
- Solid (may be arising from clear cell adenofibroma or borderline clear cell tumor)
- Cyst may contain chocolate brown fluid
- Pale yellow to brown appearance
- Necrosis or hemorrhage
- Ovarian surface involvement in 11% of cases (Histopathology 2015;66:808)
Gross images
Frozen section description
- Admixure of characteristic tubulocystic, papillary and solid growth patterns
- Uniformly atypical cells with clear to eosinophilic cytoplasm, no brisk mitoses
Frozen section images
Microscopic (histologic) description
- 3 classic growth patterns, frequently admixed but 1 pattern (usually papillary or tubulocystic) may predominate (Am J Surg Pathol 2011;35:36, Adv Anat Pathol 2012;19:296, Am J Surg Pathol 2016;40:656, Histopathology 2015;66:808):
- Papillary:
- Most frequent (70%)
- Usually small, round, simple, nonbranching papillae with fibrous / hyalinized, edematous / myxoid or empty (open tumor rings) stromal cores
- Lined by 1 - 2 layers of cuboidal, flattened or hobnail cells
- Micropapillary tufts may be seen
- Rarely irregular, broad papillae with or without hierarchical branching and micropapillae (Am J Surg Pathol 2008;32:269)
- Tubulocystic:
- Frequent (65%)
- Variably sized tubules and cysts, with or without intraluminal dense eosinophilic secretions and outpouchings
- Lined by a single layer of hobnail, cuboidal or flattened cells
- Often associated with an adenofibromatous background
- Solid:
- Least common (62%)
- Diffuse sheets or nested clusters of polyhedral cells separated by delicate septa exhibiting clear to eosinophilic cytoplasm
- Papillary:
- General features:
- Low cuboidal, polyhedral, hobnail or flattened cells with uniform nuclear atypia, prominent nucleoli and variable mitotic activity (usually low at < 6 per 10 high power fields)
- Hobnail cells have hyperchromatic nuclei protruding into lumina of tubulocystic structures or lining papillae
- Focal nuclear pleomorphism may be present in 40%
- Eosinophilic hyaline globules (Am J Surg Pathol 2011;35:36, Adv Anat Pathol 2012;19:296)
- Nuclear pseudoinclusions
- Peritumoural or diffuse lymphoplasmacytic infiltrate may be present
- May be associated with clear cell adenofibroma, clear cell borderline tumor, endometriosis or endometrioid adenocarcinoma
- Not graded (high grade by definition)
- Oxyphilic variant of clear cell carcinoma is composed of tumor cells with variable amounts of clear to granular eosinophilic cytoplasm (oxyphil cells), especially in nested, solid or trabecular growths (Am J Surg Pathol 1987;11:661)
- Rare features:
- Multinucleated cells
- Psammoma bodies
- Diastase resistant intracytoplasmic material imparting a signet ring appearance (so called targetoid cells)
- Intraglandular cellular sloughing mimicking high grade serous carcinoma
- Uncommon growth patterns include glandular, nested, trabecular and reticular-like (myxoid stroma)
Microscopic (histologic) images
Contributed by Gulisa Turashvili, M.D., Ph.D., Sonali Lanjewar, M.D., M.B.B.S., Semir Vranić, M.D., Ph.D. and AFIP images
Cytology description
- Round to oval nuclei with fine chromatin
- Abundant, pale, finely granular to vacuolated cytoplasm and indistinct cytoplasmic membranes
- Hyaline extracellular material forming so called raspberry bodies or globule-like structures (Cytopathology 2016;27:427)
- Psammoma bodies in ascitic fluid (Acta Cytol 2000;44:1005)
Positive stains
- CK7
- EMA
- PAX8
- HNF1β: sensitive but not specific (Mod Pathol 2006;19:83, Pathology 2015;47:105)
- Napsin A: specific with intermediate sensitivity (Mod Pathol 2015;28:111, Pathology 2015;47:105)
- AMACR: specific but not sensitive (Pathology 2015;47:105, Appl Immunohistochem Mol Morphol 2020;28:593)
- p53: aberrant in 7 - 24% of cases (Am J Surg Pathol 2019;43:1591, Am J Surg Pathol 2011;35:36)
- PAS positive diastase sensitive material (glycogen) in clear cells, diastase resistant intracytoplasmic material in targetoid cells
Negative stains
- ARID1A: loss of expression in 15 - 75% (Mod Pathol 2012;25:282, Int J Gynecol Cancer 2012;22:1310)
- MMR: loss of expression in 6 - 43.75% (not adjusted for age) (Am J Surg Pathol 2016;40:656, Int J Clin Exp Pathol 2008;1:502)
- WT1 (Int J Gynecol Pathol 2019;38:353)
- ER
- PR
- AFP: rarely positive
- Glypican 3: rarely positive
- OCT4: rarely positive
- SALL4
- CD10 (Int J Gynecol Pathol 2003;22:272)
- p16: block-like in 20% of cases
Molecular / cytogenetics description
- Loss of function mutations in ARID1A, a tumor suppressor gene from the SWI / SNF chromatin remodelling complex (50%) (N Engl J Med 2010;363:1532, Science 2010;330:228)
- PIK3CA mutations (40%), often associated with ARID1A mutations (Cancer Res 2004;64:7678, Am J Pathol 2009;174:1597, Mod Pathol 2012;25:615, Int J Gynecol Cancer 2016;26:648)
- TERT promoter mutations (16%) (J Pathol 2014;232:473)
- KRAS mutations (10%) (Nat Genet 2017;49:856)
- TP53 mutations (< 10%) (Am J Surg Pathol 2019;43:1591)
- DNA mismatch repair deficiency (up to 6%), most commonly germline mutations in MSH2 (Int J Gynecol Pathol 2012;31:524, Histopathology 2016;69:288, Am J Surg Pathol 2019;43:1591, Clin Cancer Res 2019;25:3962, Am J Surg Pathol 2016;40:656)
- PTEN mutations (5%) (Cancer Res 2000;60:7052)
- Rarely MET, ARID1B, PIK3R1, SMARCA4 and SMARCD3 mutations
Sample pathology report
- Uterus with cervix, fallopian tubes and ovaries, total hysterectomy and bilateral salpingo-oophorectomy:
- Right ovary: clear cell carcinoma, positive for ovarian surface involvement (see synoptic report)
- Left ovary and fallopian tubes: benign
- Endometrium: inactive
- Myometrium: leiomyomata
- Uterine serosa and cervix: benign
Differential diagnosis
- Clear cell adenofibroma:
- Extremely rare
- Scattered tubulocystic structures lined by bland flat to low cuboidal cells, with clear to eosinophilic cytoplasm in a fibromatous background
- Associated with clear cell carcinoma in up to 33% of cases; therefore, extensive sampling is important to rule out clear cell carcinoma (J Cancer 2011;2:94)
- Borderline clear cell tumor:
- Very rare
- Growth pattern similar to clear cell adenofibroma but with cytologic atypia
- Lacks stromal invasion by back to back glands, papillary or solid architecture or solid growth within cysts
- Serous borderline tumor or low grade serous carcinoma:
- High grade serous carcinoma with clear cell features:
- Usually diagnosed at advanced stage
- May be associated with serous tubal intraepithelial carcinoma
- Typical papillary growth pattern
- Lack of small rounded papillae and tubulocystic structures
- Greater cytologic atypia with brisk mitoses
- Usually shows aberrant p53 expression, diffuse WT1, patchy to diffuse ER and PR; lacks HNF1β and napsin A
- Panel of WT1, napsin A, HNF1β and ER recommended for distinction of clear cell carcinoma from high grade serous carcinoma (Am J Surg Pathol 2009;33:14, Int J Gynecol Pathol 2016;35:430)
- Endometrioid carcinoma with clear cell features:
- Metastatic clear cell renal carcinoma:
- Prior history or concurrent renal mass
- Nested alveolar pattern with interalveolar stromal vascularity
- Composed of tumor cells with mostly clear cytoplasm
- Lack of tubulocystic architecture, small rounded papillae and hobnail cells
- Diffuse expression of RCC, carbonic anhydrase IX and CD10 and no reactivity for CK7, ER, PR, napsin A and AMACR (Am J Surg Pathol 2008;32:377)
- Dysgerminoma:
- Yolk sac tumor:
- Young patients
- Elevated serum α fetoprotein
- Variable growth patterns with brisk mitoses
- Schiller-Duval bodies may be seen
- Diffusely positive for SALL4, AFP and glypican 3
- Negative or focally positive for CK7 and EMA
Additional references
Board review style question #1
Board review style answer #1
Board review style question #2
- Which of the following is the most useful immunohistochemical panel for distinguishing between clear cell carcinoma and high grade carcinoma with clear cell features?
- ER, PR, WT1 and p53
- ER, PR, WT1 and SALL4
- PAX8, p16, napsin A and HNF1β
- WT1, ER, napsin A and HNF1β
- WT1, napsin A, p16 and SALL4
Board review style answer #2
Clear cell cystadenoma and adenofibroma
Colorectal adenocarcinoma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Pathophysiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Secondary ovarian involvement by colorectal cancer (CRC) is, by definition, evidence of advanced tumor stage (pM1)
- There is significant overlap of clinical, radiologic and pathologic features between primary and metastatic ovarian adenocarcinoma
- In the workup of mucinous or endometrioid-like ovarian neoplasms, a secondary malignancy should always be excluded, either pathologically or clinically
Essential features
- Mimic of primary ovarian endometrioid or mucinous adenocarcinoma
- Most common metastatic tumor to ovary; usually arising from rectosigmoid colon (Int J Colorectal Dis 2020;35:1035)
- Usual gross morphologic features of ovarian metastasis (bilateral, Variably sized glands, nuclear atypia that is more pronounced than in ovarian primary, dirty necrosis, multinodular / infiltrative growth
- SATB2+, CK20+, CDX2+, CK7-, PAX8-
Terminology
- Metastases to the ovary (OM) are also known as secondary tumors of ovary (STO)
- Although commonly used to refer to all metastatic carcinoma involving the ovary, Krukenberg tumor (KT) strictly refers to adenocarcinoma with signet ring cell differentiation, most of which (76%) arise from the stomach (Am J Surg Pathol 2006;30:277)
ICD coding
Epidemiology
- Rate of OM among all ovarian malignancies varies from 15 - 30% (Int J Gynecol Cancer 2006;16:132, Virchows Arch 2015;467:79, Anal Quant Cytopathol Histpathol 2013;35:241)
- Between 10 - 33% of OM are from CRC, which is the most common primary tumor type (World J Gastrointest Surg 2010;2:109, Virchows Arch 2015;467:79, Anal Quant Cytopathol Histpathol 2013;35:241)
- Median age is 51 - 60 years; 24% are 40 years or younger (World J Gastrointest Surg 2010;2:109, Virchows Arch 2015;467:79, Anal Quant Cytopathol Histpathol 2013;35:241)
- 3.4 - 4.2% of women with CRC present with or will develop OM (Int J Colorectal Dis 2020;35:1035, Int J Colorectal Dis 2021;36:2567)
- Slightly more frequent in premenopausal / perimenopausal (≤ 55 years) women (Int J Colorectal Dis 2021;36:2567)
Pathophysiology
- Spread to the ovaries can be hematogenous, lymphatic, transperitoneal or by direct extension (Clin Exp Metastasis 2017;34:295)
- Hematogenous spread may be more important for colorectal primary than other sites of origin (Obstet Gynecol Int 2011;2011:612817)
Diagrams / tables
- See Figure 5 for algorithm to determine origin using currently available markers: Int J Gynecol Pathol 2016;35:191
Table 1. Most common immunohistochemical expression
patterns seen in primary ovarian mucinous neoplasms and
secondary epithelial tumors from gastrointestinal origin
| Lower GI | Upper GI | Ovarian | |
| CK7 | Negative | Positive | Positive |
| SATB2 | Positive | Negative | Negative |
| PAX8 | Negative | Negative | Positive* |
| CK20 | Positive | Pos / neg | Pos / neg |
| CDX2 | Positive | Pos / neg | Pos / neg |
| MUC2 | Positive | Pos / neg | Pos / neg |
| ER | Negative | Negative | Pos / neg |
| Beta catenin | Pos / neg | Negative | Negative |
| MUC1 | Pos / neg | Positive | Positive |
- GI = gastrointestinal; pos = positive; neg = negative
- In this table, positive markers are those with expression in > 75% and negative markers those with expression in < 25% of tumors in the indicated category
- Expression ranging from 25 to 75% is coded as pos / neg
- *PAX8 expression was 75% in all primary ovarian neoplasms but lower in the subgroup of primary ovarian carcinomas (65%)
- Table contents adapted from Int J Gynecol Pathol 2016;35:191
Clinical features
- Most patients manifest changes in bowel habits, rectal bleeding and bloating; constitutional symptoms are less frequent
- Symptoms related to a pelvic mass include abdominal pain, discomfort or bowel obstruction
- Abnormal vaginal bleeding or even virilization may result from metastasis induced luteinization of the ovarian stroma (Am J Surg Pathol 2006;30:277)
- Mean size of the metastatic lesion usually exceeds the size of the primary tumor
- Right sided CRC with OM is more likely to present with peritoneal carcinomatosis than those with left sided primary (Curr Oncol 2021;28:2914)
- Most cases are stage pT3 (full thickness wall involvement) or pT4 (perforation of visceral peritoneum or direct invasion of adjacent structures); nodal involvement occurs in 87% (Int J Clin Oncol 2020;25:1822)
- Left sided CRC is more common among OM (Curr Oncol 2021;28:2914)
- Rectosigmoid is most common site (Int J Colorectal Dis 2020;35:1035, Virchows Arch 2015;467:79)
Diagnosis
- Imaging is not reliable in distinguishing STO from primary malignancy; definitive diagnosis requires pathologic evaluation
Laboratory
- Elevated CA125 levels (> 100 U/mL) are more characteristic of primary ovarian malignancy but may be seen in OM (J Gastrointest Oncol 2021;12:226)
- In the setting of known CRC, elevated CA125 may suggest OM
- CA125:CEA ratio Indian J Surg Oncol 2021;12:152)
Radiology description
- On imaging, the ovarian mass often has cystic and solid components (Int J Clin Oncol 2020;25:1663)
- Palisading layered structures (mille-feuille sign) were specific for colorectal origin in one study (Eur J Radiol 2020;124:108823)
- Necrosis is common (Transl Cancer Res 2021;10:3248)
- Smooth mass margin (contour) on CT is seen in most lesions (92%), compared to primary ovarian tumors (45%) (Abdom Radiol (NY) 2019;44:1493, J Comput Assist Tomogr 2005;29:69)
Prognostic factors
- Median survival of 25.5 months when ovary is sole site of metastasis (Int J Colorectal Dis 2020;35:1035)
- Poor prognostic factors (Int J Colorectal Dis 2020;35:1035, Int J Clin Oncol 2020;25:1822):
- Additional sites of metastasis
- Residual disease after cytoreductive surgery
- Poorly differentiated or undifferentiated tumor or signet ring cell differentiation
- Age ≥ 70
Case reports
- 29 year old woman with cystic ovarian lesion found 1 month after low anterior resection for pT4bN1b CRC (J Gastrointest Oncol 2021;12:3141)
- 44 year old woman with menorrhagia and a mixed cystic and solid adnexal mass (Radiol Case Rep 2021;16:2799)
- 74 year old woman with pelvic mass, elevated CA125 and CA19-9 (Medicine (Baltimore) 2019;98:e17782)
Treatment
- Given the high frequency of ovarian involvement by CRC and the high proportion of cases that are unsuspected before oophorectomy, preoperative investigation in a patient with an ovarian mass should include consideration for colonoscopy (Int J Gynecol Pathol 2008;27:182)
- Prophylactic oophorectomy is not universally recommended in the setting of known CRC but has been considered in certain contexts (e.g., postmenopausal, synchronous cystic ovarian lesions, ovarian tethering to site of primary tumor, pelvic ascites accumulation) (Updates Surg 2021;73:391)
- Cytoreductive surgery with adjuvant chemotherapy (particularly hyperthermic intraperitoneal chemotherapy [HIPEC]) shows improved survival over primary resection or palliative treatment alone (Int J Colorectal Dis 2020;35:1035, J Gastrointest Oncol 2021;12:226)
Gross description
- Bilateral in 45 - 59% of cases; left sided colorectal primary is most likely to present with unilateral OM (Curr Oncol 2021;28:2914, Int J Clin Oncol 2020;25:1663, Eur J Radiol 2020;124:108823)
- Mass is frequently complex (solid and cystic) or purely solid with multinodular growth (Int J Clin Oncol 2020;25:1822, J Gastrointest Oncol 2021;12:226)
- Purely cystic unilocular lesions are rare
- Surface involvement varies (Curr Oncol 2021;28:2914):
- Right sided primary (58%)
- Left sided primary (25%)
- Although OM are generally smaller (Int J Clin Oncol 2020;25:1822)
- A simple size and laterality algorithm accurately distinguishes OM from primary ovarian malignancy in several metastatic tumor types; however, metastatic CRC appears to routinely violate this model (Am J Surg Pathol 2008;32:128)
Frozen section description
- OM can frequently be mistaken for primary ovarian malignancy on frozen section, particularly in the setting of an occult primary (Int J Gynecol Pathol 2005;24:356)
- Useful features to suggest OM include:
- Bilateral
- Small ( Surface involvement
- Multinodular growth pattern with desmoplasia
- Intervening benign ovarian stroma
- Features suggestive of CRC origin (Am J Surg Pathol 2006;30:177):
- Variably sized glands often with cystic dilation
- Garland pattern
- Intraluminal dirty necrosis
- Diagnostic accuracy of frozen section for CRC with OM specifically, is not well known
Microscopic (histologic) description
- Metastatic colorectal adenocarcinoma to the ovary usually has a mucinous or a conventional glandular appearance
- Mucinous carcinomas have intestinal (goblet cell) differentiation
- Mucin extravasation and signet ring cell morphology can be seen
- Krukenberg tumor is a term that should be reserved for adenocarcinoma involving the ovary with a signet ring cell component > 10% of the tumor volume, regardless of its site of origin (Adv Anat Pathol 2006;13:205)
- Conventional tumor cytomorphology with mucin depletion mimics the architecture and cytoplasmic appearance of a primary ovarian endometrioid tumor
- Nuclear pleomorphism and hyperchromasia tend to be prominent and exceed what is expected for a primary ovarian tumor
- Central glandular necrosis (dirty necrosis) is also more typical of colorectal tumors, although it is not entirely specific
- Desmoplastic stromal response
- Mucinous metastases frequently mimic the appearance of an ovarian mucinous neoplasm and may have areas mimicking a borderline or even benign primary mucinous tumor
- Several clinical and pathologic features have been described as indicative of secondary (metastatic) origin, including (Am J Surg Pathol 2003;27:281, Diagn Pathol 2021;16:20):
- Bilaterality
- Size Surface involvement
- Infiltrative pattern of growth with intervening benign ovarian stroma
- Presence of signet ring cells
- Extensive lymphovascular space invasion
- Mucin extravasation
- If any of the above features is present, the possibility of a metastasis should be considered and prompt ancillary testing and clinical investigation should be performed
Microscopic (histologic) images
Contributed by Carlos Parra-Herran, M.D. and Adam Lechner
Positive stains
- Immunohistochemistry is useful to distinguish primary ovarian tumors and GI metastases but must be interpreted with caution, since there is significant overlap in expression (Int J Gynecol Pathol 2016;35:191)
- CK20, CDX2 and MUC2 are sensitive markers of colorectal origin
- They lack specificity since they are frequently positive in primary ovarian tumors
- SATB2 is a more specific marker of colorectal origin (Int J Gynecol Pathol 2016;35:191, BMC Res Notes 2019;12:770)
- Combination of CK7 and SATB2 using 3 tiered interpretation (absent / focal / diffuse) yields diagnostic accuracy of > 95% (Mod Pathol 2019;32:1834)
- Rates of expression in colorectal tumors are (Am J Surg Pathol 2016;40:419, Am J Surg Pathol 2018;42:160, Int J Gynecol Pathol 2016;35:191, Ann Diagn Pathol 2015;19:249):
- SATB2: 75 - 79% (versus 5% of primary ovarian mucinous tumors and 0% of primary ovarian endometrioid tumors)
- CK20: 68 - 85% (versus 45% of primary ovarian tumors)
- CDX2: 64 - 100% (versus 50% of primary ovarian tumors)
- MUC2: 97% (versus 40% of primary ovarian tumors)
- Beta catenin: 51% (versus 5% of primary ovarian tumors)
- CK7: 21% (versus 95% of primary ovarian tumors)
Negative stains
- PAX8: 0% (versus 30 - 65% of primary ovarian mucinous tumors and 80% of primary ovarian endometrioid tumors)
- ER: 0% (versus 30% of primary ovarian tumors)
- See Diagrams / tables
Molecular / cytogenetics description
- EGFR and HER2 overexpression is more common in OM than other metastatic sites of CRC (BMC Clin Pathol 2019;19:3)
- PIK3CA mutations are significantly less common in CRC with OM (Cancer Biol Ther 2015;16:1726)
- Lower RRM1 protein expression is also seen and may indicate sensitivity to gemcitabine (Cancer Biol Ther 2015;16:1726)
- Reference: Cancer 2017;123:1134
Sample pathology report
- Ovary, oopherectomy:
- Metastatic adenocarcinoma, moderately differentiated (see comment)
- Comment: Immunostaining demonstrates SATB2+, CDX2+, CK20+, CK7- and PAX8- in lesional cells. The cytomorphology and immunohistochemical profile is most consistent with metastatic adenocarcinoma of colorectal primary.
Differential diagnosis
- Metastases from upper GI tract:
- Metastases from cervix:
- p16 positive (strong, diffuse)
- Primary ovarian neoplasm (benign, borderline or malignant):
- Most often mucinous or endometrioid types; less likely high grade serous and clear cell types
- No suspicious features described above (bilaterality, surface involvement, signet ring cell morphology, etc.)
- PAX8+, CK7+, CK20-, CDX2-, SATB2-
- For endometrioid tumors: squamous differentiation, adenofibromatous background, involvement by endometriosis, ER+
- For mucinous tumors: association with teratoma or Brenner tumor, CK7+, PAX8+ (50 - 65% of cases)
- For serous tumors: complex papillary architecture, severe pleomorphism, ER+, WT1+
Board review style question #1
A 51 year old woman presents with a 6 month history of menorrhagia and abdominal discomfort. Pelvic imaging identifies bilateral complex cystic adnexal masses of 7 and 9 centimeters. Whole body PET / CT identifies an additional hypermetabolic lesion in the cecum and in the liver. A representative section taken from a bilateral salpingo-oopherectomy specimen is shown above. Which of the following immunohistochemical results provides the strongest support for the diagnosis?
- CDX2+
- CK7+
- MUC2+
- SATB2+
Board review style answer #1
Board review style question #2
A 60 year old woman is diagnosed with colorectal adenocarcinoma with isolated metastases to the left ovary. She undergoes cytoreductive surgery, including low anterior resection and oophorectomy. Which of the following statements is correct?
- Adjuvant chemotherapy is contraindicated
- The presence of signet ring cell differentiation would indicate better overall survival
- This patient's colorectal cancer likely originated in the cecum
- This patient has a relatively better prognosis compared to those with additional metastases beyond the ovary
Board review style answer #2
D. This patient has a relatively better prognosis compared to those with additional metastases beyond the ovary. Isolated ovarian metastasis in colorectal adenocarcinoma shows improved survival over additional metastases to extraovarian sites. Unilateral ovarian metastasis in CRC most commonly originates from a left sided primary. HIPEC adjuvant therapy at the time of cytoreductive surgery improves survival. Signet ring cell differentiation is a uniformly negative prognostic factor.
Comment Here
Reference: Colorectal adenocarcinoma
Comment Here
Reference: Colorectal adenocarcinoma
Corpus luteum cyst
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Ovarian cyst > 3 cm in diameter, lined by luteinized granulosa and theca cells
Essential features
- Cyst over 3 cm in size
- Cyst lining composed of inner layer of luteinized granulosa cells and outer layer of theca cells
ICD coding
- ICD-11: GA18.1 - Corpus luteum cyst
Epidemiology
- Functional cysts in women of reproductive age, including pregnancy
- Rare in postmenopausal women
Sites
- Ovary
Pathophysiology
- Corpus luteum is a physiological postovulatory structure formed after the dominant follicle releases the ovum
- Its main purpose is to secrete estrogen and progesterone and it normally regresses at the end of the cycle
- Cystic dilation happens when corpus luteum fails to regress and becomes enlarged with fluid / blood
- Reference: StatPearls: Anatomy, Abdomen and Pelvis, Ovary Corpus Luteum [Accessed 20 May 2021]
Clinical features
- Patients can be asymptomatic or present with menstrual irregularities, amenorrhea, abdominal pain, palpable abdominal mass if large size
- If cyst ruptures, patient may present with acute abdomen and hemoperitoneum
- Reference: Turk J Obstet Gynecol 2020;17:300
Diagnosis
- On pelvic ultrasound, appears as simple ovarian cyst, often hemorrhagic; incidental finding or diagnosed during symptomatic workup
Laboratory
- No specific laboratory findings
Radiology description
- On ultrasound: unilocular cyst with prominent peripheral blood flow and thick crenulated vascular walls
- On CT: unilocular structure with crenulated walls and brisk enhancement (Abdom Radiol (NY) 2016;41:2270)
Prognostic factors
- Most cysts resolve spontaneously
- Hemorrhagic cysts over 5 cm or simple cysts between 5 and 7 cm in women of reproductive age require follow up to ensure resolution (Ultrasound Q 2010;26:121)
- In postmenopausal women, consider surgical evaluation of hemorrhagic cysts, as the etiology is more likely neoplastic than functional (Radiology 2010;256:943)
- Vast majority of pregnancy associated simple cysts < 5 cm resolve by weeks 16 - 20 and require no intervention (Clin Obstet Gynecol 2006;49:492)
Case reports
- 15 year old girl with ruptured hemorrhagic corpus luteum cyst of undescended ovary, a rare cause of acute abdomen in an adolescent (J Pediatr Adolesc Gynecol 2016;29:e21)
- 15 year old girl with ruptured corpus luteum cyst of pregnancy with massive hemoperitoneum (J Pediatr Adolesc Gynecol 2007;20:97)
- 16 year old girl with hemoperitoneum from corpus luteum cyst rupture (Case Rep Emerg Med 2014;2014:252657)
- 18 year old woman with congenital hypofibrinogenemia with hemoperitoneum caused by ovulation (Obstet Gynecol Sci 2015;58:427)
Treatment
- Resection if symptomatic, rupture or suspicion for neoplastic process (Radiology 2010;256:943)
- For asymptomatic unilocular cysts with normal CA125, optimal management includes surveillance with ultrasound (Clin Obstet Gynecol 2006;49:506, Clin Exp Obstet Gynecol 2014;41:609)
Gross description
- Single unilocular cyst with smooth surface
- Cyst wall and lining appears yellow and convoluted
- Serous or hemorrhagic contents
- Reference: Kurman: Blaustein's Pathology of the Female Genital Tract, 7th Edition, 2019
Frozen section description
- Convoluted cyst wall lined by cells with abundant eosinophilic cytoplasm and bland nuclear features
- Cytoplasm may appear vacuolated on frozen tissue
Microscopic (histologic) description
- Cyst lining is convoluted, composed of an inner layer of luteinized granulosa cells and outer layer of theca cells
- Granulosa cells are polygonal in shape, with abundant eosinophilic cytoplasm and central round nuclei
- Mitotic figures may be seen in the granulosa cells
- Outer theca cells are smaller in size
- Prominent inner layer of fibrous tissue
- Reference: Kurman: Blaustein's Pathology of the Female Genital Tract, 7th Edition, 2019
Microscopic (histologic) images
Cytology description
- Rarely performed
- Luteinized granulosa cells and hemosiderin laden macrophages in the background of blood and fibrin (Diagn Cytopathol 1990;6:77)
Positive stains
- Usually not necessary for diagnosis
- Calretinin (Histopathology 2001;38:403)
- Inhibin (J Clin Endocrinol Metab 1991;73:470)
- Progesterone receptor (Reproduction 2004;128:423)
- Reticulin preserved within the theca interna layer
Negative stains
- Cytokeratin
- Reticulin diminished / absent in the granulosa layer
Sample pathology report
- Ovary, right, cystectomy:
- Corpus luteum cyst
- Background ovary with cystic follicles and epithelial inclusion cysts
- Negative for malignancy
Differential diagnosis
- Cystic granulosa cell tumor:
- Usually larger
- The 2 cell types in the cyst wall have a more disorderly pattern
- Neoplastic cells can infiltrate the cyst wall
- With or without Call-Exner bodies
- Endometriotic cyst:
- Endometrial glands and stroma, hemosiderin laden macrophages
- Epithelial inclusion cyst:
- Lined by either ciliated (tubal type) or flat (ovarian surface / peritoneal type) epithelium
- Follicular cyst:
- > 3 cm: lined by an inner layer of granulosa cells and an outer layer of theca cells
- Luteinization is either absent or only focal
- Lacks the convoluted appearance on low power magnification
- Cystic corpus luteum:
- Size < 3 cm
Board review style question #1
Board review style answer #1
B. It is a benign finding, expected to undergo regression
Comment Here
Reference: Corpus luteum cyst
Comment Here
Reference: Corpus luteum cyst
Board review style question #2
Which of the following is characteristic of a corpus luteum cyst of the ovary?
- Bilayered lining of granulosa and theca cells
- Presence of endometrial type stroma
- Single layer of ciliated cells with eosinophilic cytoplasm
- Single layer of granulosa cells
Board review style answer #2
Cortical inclusion cyst
Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Prognostic factors | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Cystically dilated glands within the ovarian cortex that result from the invagination of the surface lining or implantation of tubal epithelium
Essential features
- 2 types based on morphology and pathophysiology:
- Peritoneal inclusion cyst: lined by flat epithelium that is invaginated from ovarian surface epithelium; these express a peritoneal phenotype (calretinin, WT1 and D2-40 positive, PAX8 and BerEP4 negative)
- Müllerian inclusion cyst: lined by ciliated tubal epithelium as a result of the implantation of tubal epithelium in the ovarian parenchyma, presumably at the time of ovulation when the ovarian surface epithelium is disrupted; these cysts express a tubal Müllerian phenotype (PAX8, BerEP4 and WT1 positive, calretinin and D2-40 negative)
- Size is less than 1 cm; if more than 1 cm, by convention the lesion is designated as cystadenoma or cystadenofibroma
Terminology
- Cortical inclusion cyst
- Some terminology overlap with endosalpingiosis
Epidemiology
- Can occur at any age but more common postmenopausal
Sites
- Ovary
Pathophysiology
- Via invagination of the ovarian surface epithelium (peritoneal type cyst) or implantation of tubal epithelium at ovulation, when the ovarian surface epithelium is disrupted (Müllerian type cyst) (Histopathology 2018;72:766, Int J Gynecol Pathol 2015;34:3, Mod Pathol 2011;24:1488)
Clinical features
- Incidental findings, usually asymptomatic
Diagnosis
- Histologic examination, usually incidental findings in oophorectomy specimens
Prognostic factors
- Benign entities
- Presence of inclusion cyst in postmenopausal women does not increase the risk of ovarian or other hormone driven cancers (breast and endometrial cancers) (BJOG 2012;119:207)
- Traditionally thought to represent a precursor lesion of ovarian carcinoma; however most ovarian serous carcinomas are now considered to be tubal in origin with serous tubal intraepithelial carcinoma (STIC) as the precursor lesion
- For a subset of ovarian serous carcinomas (in which STIC or tubal involvement is not identified), a potential origin in epithelial inclusion cyst of Müllerian (tubal) phenotype has been postulated (Gynecol Oncol 2013;130:246, Mod Pathol 2011;24:1488, Int J Gynecol Pathol 2015;34:3)
- Use of oral contraceptives for more than 5 years was shown to reduce the number of Müllerian type inclusion cysts (PAX8 positive); this suggests a possible mechanism for reducing the risk of epithelial neoplasms and supports the hypothesis that these cysts constitute a precursor lesion of ovarian serous carcinoma (Diagn Pathol 2016;11:30)
Treatment
- Not required, as these are often incidental findings
Gross description
- Most are not apparent grossly unless very superficial in the cortex
- When visible, they appear as small cysts bulging at the ovarian surface
Microscopic (histologic) description
- Small cysts (Peritoneal inclusion cyst: lined by simple flat epithelium devoid of cilia or mucinous cytoplasm
- Müllerian inclusion cyst: lined by simple cuboidal to columnar epithelium with ciliated cells, sometimes admixed with nonciliated (secretory, peg) cells
- Can have psammomatous calcifications in adjacent stroma
- Reference: Int J Gynecol Pathol 2015;34:3
Positive stains
- Müllerian type cyst: PAX8, BerEP4 and WT1 (Histopathology 2018;72:766, Hum Pathol 2015;46:948, Int J Gynecol Pathol 2000;19:158)
- Peritoneal type cyst: calretinin, WT1 and D2-40 (Int J Gynecol Pathol 2015;34:3)
Negative stains
- Müllerian type cyst: calretinin and D2-40 (Histopathology 2018;72:766, Hum Pathol 2015;46:948, Int J Gynecol Pathol 2000;19:158)
- Peritoneal type cyst: PAX8 and BerEP4 (Int J Gynecol Pathol 2015;34:3)
Sample pathology report
- Left and right ovary, bilateral oophorectomy:
- Benign ovaries with cortical inclusion cysts
- Note: many pathologists do not report the finding of inclusion cysts since they are common and benign
Differential diagnosis
- Endometriosis:
- Presence of endometrial stroma or hemosiderin laden macrophages
- Serous cystadenoma:
- Size: > 1 cm
Board review style question #1
Board review style answer #1
D. Usually is an incidental finding. Epithelial inclusion cysts are incidental findings and can be PAX8 positive (Müllerian type) or negative (peritoneal type). Presence of endometrial stroma distinguishes them from endometriosis, as both can have focal tubal differentiation. Although some relationship with risk of serous neoplasia, they are considered benign findings (not a serous tubal intraepithelial carcinoma [STIC] lesion).
Comment Here
Reference: Epithelial inclusion cyst
Comment Here
Reference: Epithelial inclusion cyst
Board review style question #2
What is the typical immunoprofile of Müllerian type epithelial inclusion cyst of the ovary?
- BerEP4 and D2-40 positive
- BerEP4 and PAX8 positive
- Calretinin and PAX8 positive
- Calretinin and WT1 positive
Board review style answer #2
Disorders of sex development-general
Table of Contents
Definition / general | Terminology | Etiology | Clinical features | Prognostic factors | Treatment | Molecular / cytogenetics description | Pure (complete) gonadal dysgenesis | Pure (complete) gonadal dysgenesis 46XX | Pure (complete) gonadal dysgenesis 46XY | Testicular feminizationDefinition / general
- Disorder of sex development (DSD) in which one or both gonad(s) is / are undeveloped (streak gonad)
- Disorders of sex development are either female pseudohermaphroditism (46XX with 2 ovaries), male pseudohermaphroditism (46XY with two testes), true hermaphroditism (ovotestis present) or gonadal dysgenesis (either pure with normal 46XX or 46XY chromosomes and bilateral streak gonads or mixed with streak gonad)
Terminology
- Pure gonadal dysgenesis (PGD): both gonads are streak gonads (i.e. dysfunctional gonads without germ cells)
- Mixed gonadal dysgenesis (MGD): patient has a testis on one side and a streak gonad on the other
Etiology
- Chromosomal abnormality: may be 46XX, 46XY or 45XO
Clinical features
- Patients with pure gonadal dysgenesis have underdeveloped Müllerian organs (sexual infantilism), sometimes with clitoromegaly, while patients with mixed gonadal dysgenesis have ambiguous or female genitalia
- 46XX and 46XY patients present with primary amenorrhea and delayed secondary sexual development; 45XO present with typical Turner syndrome features
Prognostic factors
- Patients with pure gonadal dysgenesis are raised as female since there is no sexual ambiguity at birth; however, gender assignment in patients with mixed gonadal dysgenesis is variable and depends on the degree of virilization
- Approximately 20% of patients with gonadal dysgenesis develop a gonadal neoplasm within the first two decades of life, usually gonadoblastoma; almost all cases of gonadoblastoma have been reported in patients with pure or mixed gonadal dysgenesis or male pseudohermaphroditism
- Other neoplasms include seminomatous germ cell tumors such as seminoma and dysgerminoma, nonseminomatous germ cell tumors such as embryonal carcinoma and choriocarcinoma, as well as nongerm cell tumors such as Wilms tumor
Treatment
- Given the higher risk of malignancy in dysgenetic gonads, early surgical removal is indicated
Molecular / cytogenetics description
- Pure gonadal dysgenesis: 46XX, 46XY or 45XO
- Mixed gonadal dysgenesis: 45XY / 45XO (mosaic)
Pure (complete) gonadal dysgenesis
Definition / general
Terminology
Etiology
Clinical features
Microscopic (histologic) description
- Presence of undeveloped (streak) gonads in a phenotypic female who may have an either 46,XX female or 46,XY male genotype
Terminology
- Term gonadal dysgenesis originally referred to Turner syndrome but it is now applied to other conditions as well
- Under the new nomenclature, pure (complete) gonadal dysgenesis is considered a type of either 46,XY DSD (disorder of sex development) or 46,XX DSD
Etiology
- For a variety of reasons, known and unknown, primordial germ cells do not form or interact with the gonadal ridge or undergo accelerated atresia, leading to extremely hypoplastic (underdeveloped) and dysfunctioning gonads that are mainly composed of fibrous tissue, hence the name streak gonads
- Gonadal dysfunction leads to deficiency of both Müllerian inhibiting factor and testosterone
Clinical features
- During embryonal development, the human reproductive system has an inherent tendency to give rise to female reproductive organs; therefore, in the absence of hormonal influences from the undeveloped and dysfunctional gonads, patients with complete gonadal dysgenesis are phenotypically female, regardless of genotype
- First clinical manifestation is usually absence of expected female secondary sex characteristic development at puberty
Microscopic (histologic) description
- Streak gonads are mainly composed of fibrous tissue
- Absence of testosterone results in regression of Wolffian ducts; i.e. normal male internal reproductive tracts do not develop
- Absence of Müllerian inhibiting factor allows Müllerian ducts to differentiate into oviducts and the uterus
Pure (complete) gonadal dysgenesis 46XX
Definition / general
Terminology
Etiology
Clinical features
Laboratory
Treatment
Microscopic (histologic) description
Additional references
- Form of gonadal dysgenesis (underdeveloped and dysfunctioning ovaries) associated with female 46,XX genotype and female internal and external phenotype
- Phenotypic female 46,XX but no functional ovaries are present to induce puberty (may have streak ovaries)
Terminology
- Term gonadal dysgenesis originally referred to Turner syndrome but it is now applied to other conditions as well
- Under the new nomenclature, this form of gonadal dysgenesis is considered a type of 46,XX DSD (disorder of sex development)
- Perrault syndrome: 46,XX gonadal dysgenesis with sensorineural hearing loss
Etiology
- May be familial (Am J Med Sci 1980;280:157)
- Occasionally due to mutation in FSH receptor (Cell 1995;82:959)
- For reasons that are not clear, primordial germ cells do not form or interact with the gonadal ridge or undergo accelerated atresia
Clinical features
- First clinical manifestation is usually absence of expected female secondary sex characteristic development at puberty
Laboratory
- Low serum estrogen and progesterone (since no functional ovaries), high serum FSH and LH
Treatment
- Estrogen and progesterone therapy
Microscopic (histologic) description
- Progressive loss of primordial germ cells in the developing gonads of the embryo leads to extremely hypoplastic ovaries mainly composed of fibrous tissue, hence the name streak gonads
Additional references
Pure (complete) gonadal dysgenesis 46XY
Definition / general
Terminology
Etiology
Clinical features
Laboratory
Prognostic factors
Case reports
Treatment
Microscopic (histologic) description
Additional references
- Form of gonadal dysgenesis (underdeveloped and dysfunctioning testes) associated with male 46,XY genotype and female internal and external phenotype
- Phenotypic female, hypoplastic (streak) gonads without germ cells
Terminology
- Term gonadal dysgenesis originally referred to Turner syndrome but it is now applied to other conditions as well
- Under the new nomenclature, this form of gonadal dysgenesis is considered a type of 46,XY DSD (disorder of sex development)
- Also called Swyer syndrome
Etiology
- Mutation in SRY, the sex determining region of the Y chromosome, is reported in 10 - 15%
- Defects in the genetic pathways that lead to development of testes from indifferent gonads result in hypoplastic (streak) testes and lack of testosterone or anti-Müllerian hormone (AMH) production
Clinical features
- In the absence of testosterone, external genitalia fail to virilize and the Wolffian ducts fail to develop, leading to normal female genitalia and absent internal male organs
- Presents with primary amenorrhea (delayed puberty) since no functional gonads are present to induce puberty
- May develop pubic hair through androgens produced from adrenal gland
Laboratory
- Low serum estrogen and progesterone, high serum FSH and LH
Prognostic factors
- High risk of gonadoblastoma or germ cell tumor from gonads (Zhonghua Fu Chan Ke Za Zhi 2008;43:442)
- Dysgerminoma may develop by age 10 (BJOG 2008;115:737)
Case reports
- Successful pregnancy using donor oocytes and oocyte transfer (Fertil Steril 2008;90:2015.e1)
Treatment
- Early excision of gonads (J Gynecol Obstet Biol Reprod (Paris) 2009;38:220)
- Estrogen and progesterone therapy
Microscopic (histologic) description
- Hypoplastic (streak) gonads without primordial germ cells
- In the absence of AMH, Müllerian ducts develop into normal internal female organs (uterus, fallopian tubes, cervix, vagina)
Additional references
Testicular feminization
Definition / general
Terminology
Etiology
Clinical features
Prognostic factors
Case reports
Treatment
Gross images
AFIP images
Microscopic (histologic) description
Microscopic (histologic) images
AFIP images
Additional references
- Male genotype (46,XY) but impaired or absent masculinization of male genitalia or development of male secondary sexual characteristics at puberty due to partial or complete inability of cells to respond to androgens (androgen insensitivity)
- Most common type of male pseudohermaphroditism
- Patients present with amenorrhea or sterility
- Vagina but no uterus
- Also, bilateral cryptorchid testes with nodular masses of immature tubules resembling Sertoli-Leydig tumor
Terminology
- Also called androgen insensitivity syndrome (AIS)
- Divided into three categories:
- Complete androgen insensitivity syndrome (CAIS): normal female external genitalia
- Mild androgen insensitivity syndrome (MAIS): normal male external genitalia
- Partial androgen insensitivity syndrome (PAIS): partially but not fully masculinized external genitalia
Etiology
- Most commonly, a mutation in the androgen receptor (AR) gene
- Other etiologies include: chromosomal abnormalities, dysfunctional androgen biosynthesis, developmental syndromes, teratogens
Clinical features
- Depending on the severity of androgen insensitivity, patients may present with male, female or ambiguous phenotype
- Given the presence of a Y chromosome (more specifically, SRY gene), gonads are testes regardless of phenotype
- Physical exam may reveal a vagina but no ovaries or uterus
Prognostic factors
- 9% develop tumors in cryptorchid testis, which should be removed after puberty
Case reports
- 36 year old with abdominal seminoma (J Obstet Gynaecol Res 2004;30:109)
Treatment
- Currently limited to symptomatic management, including sex assignment, genitoplasty, gonadectomy, hormone replacement, counseling
Gross images
AFIP images
Microscopic (histologic) description
- Bilateral cryptorchid testes with nodular masses of immature tubules resembling Sertoli-Leydig tumor
Microscopic (histologic) images
AFIP images
Additional references
Dysgerminoma
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Malignant primitive germ cell tumor with no specific type of differentiation
Essential features
- Most common malignant ovarian germ cell tumor; female counterpart to testicular seminoma
- Most common in children and young women
- Excellent prognosis with chemotherapy
- Composed of sheets or nests of uniform cells with clear or eosinophilic cytoplasm and distinct cell membranes; nuclei are rounded, often with angulated edges; tumor often separated by fibrous septae containing cytotoxic T cells and epithelioid histiocytes that can extend into tumor
- Positive for SALL4, OCT3/4, D2-40, CD117, PLAP
Epidemiology
- Constitutes 1 - 2% of all malignant ovarian tumors (Am J Surg Pathol 2021;45:1009)
- Most common malignant germ cell tumor of ovary (50%)
- Most common in children and young women
Sites
- Ovary
- Rarely, extraovarian sites, including abdomen, fallopian tube and uterus (J Pediatr Adolesc Gynecol 2021 May 11 [Epub ahead of print], Curr Probl Cancer 2021;45:100667, Indian J Pathol Microbiol 2018;61:590)
Pathophysiology
- Unknown
Etiology
- Arises from primordial germ cells of the ovary
Clinical features
- Most common in children and young women
- May present in pure form or as a component of a malignant mixed germ cell tumor
- Present with abdominal pain or distention
- Bilateral ovarian involvement in 10 - 15% of patients; involvement of contralateral ovary may be microscopic (Cancer Treat Rev 2008;34:427)
- Extraovarian spread in 33% of cases
- One of the most common neoplasms in pregnancy (Cancer Treat Rev 2008;34:427)
- Rare association with gonadal dysgenesis and chromosome Y abnormalities; may arise from gonadoblastoma
- Rare estrogenic manifestations
Diagnosis
- Diagnosis informed by patient's age, radiology and laboratory values (e.g. elevated lactate dehydrogenase [LDH] levels) but ultimately made based on histologic examination of surgical specimen
Laboratory
- Serum LDH often elevated (95%)
- 3 - 5% show low level hCG production related to the presence of multinucleated syncytiotrophoblastic giant cells that are not associated with cytotrophoblasts (Cancer Treat Rev 2008;34:427)
- Serum placental alkaline phosphatase (PLAP) may be elevated (Cancer Treat Rev 2008;34:427)
- Hypercalcemia may develop as a paraneoplastic syndrome, due to tumor production of 1,25-dihydroxyvitamin D3 (active form of vitamin D) (Cureus 2019;11:e6097)
Radiology description
- On ultrasound, tends to appear as a highly vascularized, large, solid, lobulated adnexal mass with irregular internal echogenicity (Ultrasound Obstet Gynecol 2011;37:596)
- Enhancing septa and areas of cystic change that may represent necrosis or hemorrhage may be seen; calcifications may manifest as a speckled pattern (Radiographics 2014;34:777)
Prognostic factors
- Most tumors are stage I at diagnosis
- Recurrence rate is low
- KIT mutations associated with advanced stage at presentation (Cancer 2011;117:2096)
- With treatment, excellent prognosis and overall survival > 90%
- Dysgerminoma patients have the most favorable outcomes compared with those with other malignant germ cell tumors; young age, low grade and surgery are significant predictors for improved survival (Oncol Res Treat 2021;44:145)
Case reports
- 16 year old girl with Swyer syndrome underwent prophylactic bilateral gonadectomy and salpingectomies and was found to have dysgerminoma solely within the fallopian tube (J Pediatr Adolesc Gynecol 2021 May 11 [Epub ahead of print])
- 19 year old woman with 29 cm dysgerminoma and pseudo-Meigs syndrome (Medicine (Baltimore) 2021;100:e26319)
- 22 year old woman with uncommon vaginal metastasis by dysgerminoma (Medicina (Kaunas) 2021;57:534)
- 26 year old pregnant woman with extraovarian (abdominal) dysgerminoma (Curr Probl Cancer 2021;45:100667)
- 27 year old woman with Swyer syndrome and stage IIA dysgerminoma in streak gonad (Gynecol Endocrinol 2018;34:464)
Treatment
- Radiosensitive but responds very well to cisplatin based chemotherapy, with overall survival > 90% (Cancer Treat Rev 2008;34:427)
- Fertility sparing surgery in patients with pure ovarian dysgerminoma with additional staging surgery, including retroperitoneal lymph node dissection to determine stage IA patients exempt from adjuvant chemotherapy (J Adolesc Young Adult Oncol 2021;10:303)
Gross description
- Usually > 10 cm
- Solid and lobulated with fleshy tan-white cut surface (Am J Surg Pathol 2021;45:1009)
- Hemorrhage and necrosis with cystic degeneration may be present
- Presence of calcifications may suggest an associated component of gonadoblastoma but not specific
Gross images
Frozen section description
- May favor diagnosis of high grade malignant germ cell tumor or malignant epithelioid neoplasm on frozen section; however, final diagnosis should be deferred to permanent sections
- Clear cytoplasm or distinct cell membranes may not be appreciated on frozen section due to artifact
- May mimic large cell lymphoma, both of which share similar gross appearance, large neoplastic nuclei and infiltrating background lymphocytes (Int J Gynecol Pathol 2020;39:79, Int J Surg Pathol 2019;27:387)
- Features favoring dysgerminoma include presence of fibrous septae and squared off nuclei
- Thickening of the fallopian tube is more suggestive of lymphoma (Int J Gynecol Pathol 2008;27:353)
- May mimic small cell carcinoma, hypercalcemic type, both of which typically occur in young women and show overlapping features, including paraneoplastic hypercalcemia (Semin Diagn Pathol 2019;36:246)
- Features favoring dysgerminoma include elevated LDH levels, moderately abundant cytoplasm
- In small cell carcinoma, hypercalcemic type, tumor cells usually have scant cytoplasm unless admixed component of large cell variant present
Frozen section images
Microscopic (histologic) description
- Histomorphology identical to that of testicular seminoma
- In a well preserved specimen, characteristic appearance of nests of large, uniform polygonal cells with clear or eosinophilic cytoplasm and distinct cell membranes (alveolar pattern)
- May also show sheets, cords, macronodules, insular growth, microcysts, tubules, pseudoglandular spaces or trabeculae (Arch Pathol Lab Med 2014;138:351, Am J Surg Pathol 2021;45:1009)
- Rarely may show pseudopapillary or macrofollicular growth pattern (Iran J Pathol 2018;13:94)
- Tumor separated by fibrous septae containing cytotoxic T cells and epithelioid histiocytes, often with spillover into tumor; plasma cells, eosinophils, Langhans type giant cells, syncytiotrophoblasts, noncaseating granulomas or lymphoid follicles with germinal centers may be present (Am J Surg Pathol 2021;45:1009)
- Stroma usually loose and delicate but in some cases may be hyalinized, myxoid or luteinized
- Numerous mitotic figures
- Rare cases show syncytiotrophoblastic cells, singly or in clusters, without cytotrophoblastic cells; sample tumor thoroughly to exclude choriocarcinoma
- Extensive hemorrhage and necrosis may be present with dystrophic calcification; if large areas of dystrophic calcification seen in the absence of necrosis, may be a gonadoblastoma
- As gonadoblastomas give rise to dysgerminomas, thorough sampling indicated to rule out its presence
Microscopic (histologic) images
Contributed by Sharon Song, M.D.
AFIP images
Cytology description
- Uniform population of medium to large sized cells (Diagn Cytopathol 2020;48:356)
- Centrally located, round to oval nuclei, often with angulated, squared off borders
- Vesicular, finely granular or coarse chromatin
- Prominent single or multiple nucleoli
- With poor fixation, cells may show loss of cohesion, nuclei can appear hyperchromatic and cytoplasm may appear scant and eosinophilic, mimicking cells of embryonal carcinoma
Positive stains
- OCT3/4 (nuclear), CD117 (KIT; membranous), D2-40 (membranous and cytoplasmic) (Histopathology 2013;62:71)
- NANOG (nuclear) and SALL4 (nuclear) stem cell markers
- PLAP (membranous and cytoplasmic)
- May focally express keratins with cytoplasmic dot or rim-like staining: CAM 5.2 (positive in up to 10% of tumor cells in < 50% of dysgerminomas); AE1 / AE3 and CK7 (positive in up to 10% of tumor cells in < 25% of dysgerminomas)
- hCG, inhibin, glypican 3 and CK7 can highlight syncytiotrophoblastic giant cells; can search for these cells in patients with moderate serum hCG elevation (Histopathology 2013;62:71)
Molecular / cytogenetics description
- Isochromosome 12p in 81% (Pathol Res Pract 2012;208:628, Mod Pathol 2006;19:611)
- KIT mutations in exon 17 codon 816 or KIT amplification detected in approximately 33% of cases and associated with advanced stage at presentation (Cancer 2011;117:2096)
- As this KIT mutation involves exon 17, not exon 11 as in gastrointestinal stromal tumors, these cases are not responsive to KIT receptor inhibitors
Sample pathology report
- Left ovary, left oophorectomy:
- Dysgerminoma (11 cm) (see synoptic report)
- No ovarian surface involvement identified
Differential diagnosis
- Important to rule out other types of mixed germ cell tumor
- Yolk sac tumor:
- Classically variable morphologic patterns (commonly reticular / microcystic)
- Lacks fibrous septae containing lymphocytes
- Glypican 3+, AFP+, OCT3/4-, CD117-, D2-40-
- Yolk sac tumor:
- Lymphoma:
- Lacks fibrous septae
- May be associated with thickened fallopian tube
- Positive lymphoid markers and IgH clonality
Board review style question #1
Board review style answer #1
B. Dysgerminoma is the most common malignant ovarian germ cell tumor.
Comment Here
Reference: Dysgerminoma
Comment Here
Reference: Dysgerminoma
Board review style question #2
- Which of the following immunostains are typically positive in dysgerminoma and can confirm the diagnosis?
- AFP, SALL4, OCT3/4, hCG
- D2-40, CD117, OCT3/4, SALL4
- OCT3/4, EMA, hCG, SALL4
- PLAP, AFP, hCG, AE1 / AE3
Board review style answer #2
B. D2-40, CD117, OCT3/4, SALL4. SALL4 is positive in germ cell tumors. OCT3/4 positivity narrows the differential of germ cell tumors to dysgerminomas and embryonal carcinomas. Dysgerminomas are positive for CD117 and D2-40.
Comment Here
Reference: Dysgerminoma
Comment Here
Reference: Dysgerminoma
Ectopic decidual reaction
Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Sites | Pathophysiology | Diagnosis | Radiology description | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Presence of ectopic decidualized uterine stromal cells in the ovary
Essential features
- Presence of ectopic decidualized uterine stromal cells in the ovary
- Usually an incidental finding associated with pregnancy
- Typically transient, will regress 4 - 6 weeks postpartum
Terminology
- Ectopic decidua or ovarian deciduosis
Epidemiology
- Associated with pregnancy
- In a study of 307 consecutive caesarean sections, macroscopic deciduosis was found in 31 (10.1%) cases (Eur J Obstet Gynecol Reprod Biol 2016;197:54)
- Can also develop as a result of exogenous progesterone effect
Sites
- Ectopic decidua have been described in the cervix, ovary and fallopian tube; also peritoneal surface, appendix, bladder, small and large intestine, mesentery, lymph nodes
Pathophysiology
- High levels of estrogen and progesterone during pregnancy induce mesenchymal fibroblast differentiation into decidual cells (Eur J Obstet Gynecol Reprod Biol 2016;197:54, Pathol Res Pract 1993;189:352, Korean J Obstet Gynecol 2011;54:373)
Diagnosis
- Often an incidental finding in surgical specimens or a discrete nodule / mass discovered during caesarean section
Radiology description
- When the ovarian deciduosis is mass forming, it may be difficult to differentiate from a neoplastic process (Case Rep Obstet Gynecol 2015;2015:217367)
Case reports
- 31 year old woman with decidualized ovarian mass mimicking malignancy (Case Rep Obstet Gynecol 2015;2015:217367)
- 32 year old woman with ovarian deciduosis in pregnancy (Korean J Obstet Gynecol 2011;54:373)
- 33 year old woman with ovarian and peritoneal deciduosis in pregnancy (Diagn Histopathol 2011;17:313)
Treatment
- Benign entity, no further treatment necessary; usually regresses 4 - 6 weeks postpartum
- If mass forming and showing worrisome features for malignancy on imaging, the lesion is excised to establish the correct diagnosis
- Reference: Case Rep Obstet Gynecol 2015;2015:217367
Microscopic (histologic) description
- Large polygonal cells with abundant eosinophilic cytoplasm, bland nuclei and visible nucleoli
- No glands present (to differentiate it from decidualized endometriosis)
- Reference: Case Rep Obstet Gynecol 2015;2015:217367
Microscopic (histologic) images
Positive stains
Negative stains
- Cytokeratin
- Mesothelial markers (WT1, calretinin)
Sample pathology report
- Ovary, oophorectomy:
- Benign ovary with focal deciduosis
Differential diagnosis
- Endometriosis:
- Can also undergo decidualization during pregnancy
- Main difference is that ectopic decidua have no glands (Gynecol Obstet Invest 2008;66:241)
- Epithelial ovarian neoplasm when mass forming:
- Histologic features are bland and mitotic activity is low in deciduosis
- Absence of glands in deciduosis
- Epithelial proliferation with variable atypia and architectural complexity in epithelial neoplasms
- Peritoneal mesothelioma or peritoneal carcinomatosis when there is additional involvement of peritoneal surface:
- Histologic features are bland in deciduosis; also, context of pregnancy is helpful
- Increased atypia, proliferation and architectural complexity in carcinomatosis and mesothelioma
- Metastatic squamous cell carcinoma with keratinization:
- More atypical
- Presence of necrosis
- Mitotically active
- Cytokeratin+
Additional references
Board review style question #1
Which of the following is true about the ovarian change illustrated in the figure shown above?
- It is a malignant process
- It is only seen in the ovary
- It requires lymph node sampling and full staging for management
- More sampling is required to demonstrate the presence of glands
- Typically associated with pregnancy
Board review style answer #1
E. Typically associated with pregnancy. The image shows ovarian deciduosis, which is associated with pregnancy, is typically transient and resolves postpartum. Answer A is incorrect because ovarian deciduosis is a benign process typically associated with pregnancy. Answer D is incorrect because it consists of stromal tissue, without glands. Answer C is incorrect because it typically regresses after pregnancy and does not require treatment. Answer B is incorrect because deciduosis can be seen at other sites (cervix, peritoneal cavity, etc.).
Comment Here
Reference: Ectopic decidual reaction
Comment Here
Reference: Ectopic decidual reaction
Board review style question #2
Which of the following is true about ovarian deciduosis?
- It can be mistaken for endometriosis
- It is CD10 negative by immunohistochemistry
- Presence of nuclear atypia indicates malignant potential
- Responds to chemotherapy
- Treatment includes resection with or without chemotherapy
Board review style answer #2
A. It can be mistaken for endometriosis with stromal decidualization but unlike endometriosis, it does not contain glands. Answer B is incorrect because both have CD10 positive stroma. Answers C, D and E are incorrect because ovarian deciduosis is a benign incidental process and does not require treatment.
Comment Here
Reference: Ectopic decidual reaction
Comment Here
Reference: Ectopic decidual reaction
Embryonal carcinoma
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Immunofluorescence description | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Primitive appearing ovarian tumor that is histologically similar to embryonal carcinoma of the testis
- Often a component in a malignant mixed germ cell tumor of the ovary; the pure form is exceedingly rare
Essential features
- Median age is 14 - 15 years; patients often present with precocious puberty, vaginal bleeding, infertility, amenorrhea and mild hirsutism (Semin Diagn Pathol 2023;40:22)
- Both serum alpha fetoprotein (AFP) and beta human chorionic gonadotropin (βhCG) can be elevated (which can result in a positive pregnancy test) (Semin Diagn Pathol 2023;40:22)
- Positive IHC expression with OCT 3/4, CD30, SOX2, SALL4 and βhCG (if syncytiotrophoblast is present); negative for glypican and CD117
- Worst prognosis among germ cell tumors, even at low stage (Cancer 1976;38:2420)
- Large, primitive pleomorphic cells with abundant cytoplasm arranged in sheets and nests
ICD coding
- ICD-O: 9070/3 - embryonal carcinoma, NOS
- ICD-11: 2C73.Y & XH8MB9 - other specified malignant neoplasms of the ovary & embryonal carcinoma, NOS
Epidemiology
- Primarily in adolescents and young women with average age of 14 - 15 years (Cancer 1976;38:2420, Semin Diagn Pathol 2023;40:22)
- First described in 1976 by Kurman and Norris as a distinct type of malignant germ cell neoplasm to distinguish from endodermal sinus tumor of ovary (Cancer 1976;38:2420)
- Estimated to account for Cancer 1976;38:2420)
Sites
- Ovary
- Metastasis is possible to liver, lungs, retroperitoneal lymph nodes, peritoneal surfaces in up to half of all cases (Cancer 1976;38:2420)
Pathophysiology
- Chromosome 12 abnormalities in 5 of 6 cases analyzed by FISH (iscochromosome 12p or 12p amplification) (Hum Pathol 2010;41:716)
Etiology
- Unknown
Clinical features
- Hormonal manifestation, including precocious puberty, vaginal bleeding, infertility, amenorrhea and mild hirsutism, in approximately half of patients (Cancer 1976;38:2420, Semin Diagn Pathol 2023;40:22)
- May show serum elevation of AFP or βhCG with positive pregnancy test
- Palpable unilateral abdominal or pelvic mass in 80% of patients (Cancer 1976;38:2420)
- May present with abdominal pain, fever, menstrual irregularities or weight loss (Cancer 1976;38:2420)
- Clinical evidence of an endocrinopathy is seen in approximately half of patients (Cancer 1976;38:2420)
Diagnosis
- Large, unilateral abdominal or adnexal mass on imaging (Ultrasound Obstet Gynecol 2021;57:987, Cancer 1976;38:2420)
- Pregnancy test may be positive (increased βhCG) (Cancer 1976;38:2420, Semin Diagn Pathol 2023;40:22)
- AFP may be increased (Cancer 1976;38:2420, Semin Diagn Pathol 2023;40:22)
- Definitive diagnosis made by histologic examination of surgical specimen (Semin Diagn Pathol 2023;40:22)
Laboratory
- Serum βhCG elevation resulting in positive pregnancy test (Semin Diagn Pathol 2023;40:22)
- Serum AFP may be elevated
Radiology description
- Unilateral, solid mass with cystic areas, irregular margins, rich vascularization (Radiographics 2014;34:777, Ultrasound Obstet Gynecol 2021;57:987)
- No reported preference for right or left ovary (Cancer 1976;38:2420)
- 1 embryonal carcinoma of the ovary measured at 216 mm (Ultrasound Obstet Gynecol 2021;57:987)
Prognostic factors
- Worst prognosis among germ cell tumors
- 5 year survival rate for stage I cases: 50% (Semin Diagn Pathol 2023;40:22, Cancer 1976;38:2420)
Case reports
- 11 year old girl with large abdominal mass and ascites (Med Arch 2018;72:371)
- 13 year old girl with abdominal pain and 25 cm mass (Iran J Pathol 2016;11:66)
- 19 year old woman with abdominal pain and massive ascites (J Pediatr Adolesc Gynecol 2011;24:e1)
- 53 year old woman with irregular menses, pelvic pain and positive pregnancy test (Gynecol Oncol 1996;63:133)
Treatment
- Surgery with adjuvant chemotherapy
- Bleomycin, etoposide and cisplatin (BEP) most common chemotherapy regimen (Ann Oncol 2018;29:iv1)
Gross description
- Large, with median size of 17 cm (Cancer 1976;38:2420)
- Cut surface is gray-white or yellow (Cancer 1976;38:2420)
- With or without cysts containing hemorrhage or necrosis (Cancer 1976;38:2420)
Frozen section description
- High grade malignant neoplasm with necrosis
Frozen section images
Microscopic (histologic) description
- Similar to embryonal carcinoma of the testis (Cancer 1976;38:2420)
- Often mixed with other malignant germ cell tumor components (Semin Diagn Pathol 2023;40:22, Cancer 1976;38:2420)
- Sheets and nests of large, primitive pleomorphic cells with abundant cytoplasm (Semin Diagn Pathol 2023;40:22, Cancer 1976;38:2420)
- Round nuclei with coarse membranes, at least 1 prominent nucleolus and brisk mitotic activity (Semin Diagn Pathol 2023;40:22)
- Occasional papillae and gland morphology (Semin Diagn Pathol 2023;40:22)
- Syncytiotrophoblast-like tumor cells are seen (hCG+) around sheets of tumor cells or within stroma (Semin Diagn Pathol 2023;40:22, Cancer 1976;38:2420)
- Hyaline globules, similar to yolk sac tumor, may also be present (Semin Diagn Pathol 2023;40:22)
- Glandular pattern may mimic endometrial carcinoma or glandular morphology of a yolk sac tumor (Semin Diagn Pathol 2023;40:22)
Microscopic (histologic) images
Virtual slides
Immunofluorescence description
- Chromosome 12p overrepresentation
- Isochromosome 12p
Positive stains
- SALL4, OCT 3/4 and SOX2 nuclear positivity (Am J Surg Pathol 2009;33:894, Histopathology 2013;62:71)
- CD30 membranous positivity (Hum Pathol 2010;41:716)
- OCT 3/4 more sensitive than CD30 but can also be expressed in dysgerminoma (Histopathology 2013;62:71)
- AE1 / AE3 (Histopathology 2013;62:71)
- βhCG positive in syncytiotrophoblastic cells (Semin Diagn Pathol 2023;40:22)
- AFP may be expressed in both tumor cells and hyaline globules (Semin Diagn Pathol 2023;40:22)
- CD30 expression might decrease after chemotherapy (Hum Pathol 2006;37:662)
Negative stains
Molecular / cytogenetics description
- Chromosome 12p FISH can be helpful in establishing a diagnosis in conjunction with IHC (Hum Pathol 2010;41:716)
Sample pathology report
- Left ovary and fallopian tube, left salpingo-oophorectomy:
- Malignant mixed germ cell tumor (see comment)
- Comment: The ovarian tumor is compatible with a malignant mixed germ cell tumor composed of embryonal carcinoma (__%) (e.g., yolk sac tumor __%; immature teratoma __%; with or without choriocarcinoma __%). Sheets of pleomorphic, primitive cells with brisk mitotic activity, tumor cell necrosis and positive expression of OCT 3/4 and CD30 are consistent with a component of embryonal carcinoma. Syncytiotrophoblastic cells are present (hCG+), which can raise concern for a component of choriocarcinoma; however, the presence of isolated syncytiotrophoblastic cells has been reported in embryonal carcinoma and there is no evidence of choriocarcinoma in examined sections of this extensively sampled ovarian tumor.
Differential diagnosis
- Yolk sac tumor:
- Microcystic / reticular, polyvesicular vitelline and festoon-like patterns
- Less nuclear pleomorphism
- Positive expression with AFP and glypican 3
- Negative for OCT 3/4 or SOX2
- Dysgerminoma:
- Choriocarcinoma:
- Poorly or undifferentiated carcinoma:
- Juvenile granulosa cell tumor:
- Follicle-like spaces with luteinized tumor cells
- Lack of syncytiotrophoblast
- Positive for inhibin, calretinin and negative for AFP
- Poorly differentiated Sertoli-Leydig cell tumor (SLCT):
- Portion of the tumor will contain a better differentiated component of SLCT
- Positive for inhibin and calretinin
Board review style question #1
Board review style answer #1
C. CD30. CD30 is the most specific marker for embryonal carcinoma of the ovary. Answer A is incorrect because AFP is a marker for yolk sac tumor. Answer D is incorrect because CD117 highlights dysgerminoma. Answer B is incorrect because βhCG is a marker for syncytiotrophoblast and choriocarcinoma. Answer E is incorrect because while OCT 3/4 can stain embryonal carcinoma of the ovary, it also stains dysgerminoma, whereas dysgerminoma should be negative for CD30.
Comment Here
Reference: Embryonal carcinoma
Comment Here
Reference: Embryonal carcinoma
Board review style question #2
The glandular pattern of embryonal carcinoma, particularly if present with hyaline globules, can mimic which of the following tumors?
- Choriocarcinoma
- Dysgerminoma
- Leydig cell tumor
- Sertoli-Leydig cell tumor
- Yolk sac tumor
Board review style answer #2
E. Yolk sac tumor. The glandular pattern of embryonal carcinoma can mimic a yolk sac tumor, particularly in the presence of hyaline globules. Glandular architecture can also mimic a poorly differentiated carcinoma. Answer A is incorrect because choriocarcinoma will have a mixture of syncytiotrophoblast and cytotrophoblast without a prominent glandular architecture. Answer B is incorrect because dysgerminoma will not show a glandular architecture and will have fibrous septa with lymphocytic or granulomatous infiltrate. Answer D is incorrect because Sertoli-Leydig cell tumors will not show a prominent glandular architecture and stain with inhibin / calretinin. Answer C is incorrect because Leydig cell tumors are rare and arise in the hilum and have Reinke crystals.
Comment Here
Reference: Embryonal carcinoma
Comment Here
Reference: Embryonal carcinoma
Endometrial stromal sarcoma
Table of Contents
Definition / general | Sites | Clinical features | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Differential diagnosisDefinition / general
- Composed of cells resembling endometrial stromal cells in the proliferative stage
- Rare in extra-uterine sites
Sites
- Most common site is uterus
- Extrauterine sites rare - includes ovary, fallopian tube, pelvic cavity, abdominal cavity, and retroperitoneum (Int J Gynecol Pathol 1993;12:282)
Clinical features
- Lower abdominal pain
- 20 to 76 years, mean average, 54 years (Cancer 1984;53:1143)
Radiology description
- Ultrasound / USG: solid cystic mass
- CT scan with IV contrast: peripheral enhancement
Prognostic factors
- Behavior resembles high stage primary uterine ESS (Int J Gynecol Pathol 1993;12:282)
Case reports
- 18 month old infant with endometriosis associated serous borderline tumor and endometrial stromal sarcoma of the ovary (Int J Gynecol Pathol 2012;31:98)
- 50 year old woman with endometrial stromal sarcoma presenting as an ovarian cyst (SEAJCRR 2013;2:139)
- 56 year old woman with undifferentiated endometrial sarcoma of ovary (Patholog Res Int 2010;2010:608519)
Treatment
- Salphingo-opherectomy
Gross description
- Solid cystic mass (SEAJCRR 2013;2:139)
Microscopic (histologic) description
- High or low grade
- Low grade: infiltrative and diffuse proliferation of uniform round or oval cells, abundant small vessels
- Undifferentiated: pleomorphic round / oval to spindled cells, high mitotic rate, necrosis
Microscopic (histologic) images
Differential diagnosis
- Malignant mixed Müllerian tumor: smooth / skeletal muscle / carcinoma cells
- Metastatic spread from uterine ESS: primary mass in uterus
- Sex cord stromal tumor
Endometrioid borderline tumor
Table of Contents
Definition / general | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosisDefinition / general
- Rare (Mean age 55 years, range 28-86 years
- 97% present with stage I disease
- 36% associated with endometriosis
- Typically no recurrent disease or metastasis on follow-up, even if intraepithelial carcinoma or microinvasion (Am J Surg Pathol 2003;27:1253, Am J Surg Pathol 2000;24:1465)
Microscopic (histologic) description
- Composed of aggregates, glands or cysts of endometrioid-type epithelium that is atypical or cytologically malignant
- May have architectural atypia including non-branching villous papillae, cribriform glands but lacks destructive stromal invasion, glandular confluence or stromal disappearance
- Often has adenofibromatous pattern (47%) or squamous differentiation (47%)
- May have foci of intraepithelial carcinoma (7%, high grade / grade 3 nuclei are large, pleomorphic with coarse chromatin or hypochromasia, large irregular nucleoli; also many mitotic figures; associated with villoglandular architecture or microinvasion)
- Microinvasion if areas of invasion are 10 mm2 or less (7%)
Microscopic (histologic) images
Differential diagnosis
- Atypical endometriosis: will have endometrial stroma
Endometrioid carcinoma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Definition / general
- Ovarian carcinoma resembling endometrioid adenocarcinoma of the endometrium
Essential features
- Most common ovarian endometrioid tumor
- Usually low grade and diagnosed at early stages
- May be associated with endometriosis and adenofibroma
Terminology
- Seromucinous carcinoma is included as a subtype of endometrioid ovarian carcinoma in the 2020 WHO blue book for female genital tumors
ICD coding
- ICD-11: 2C73.01 - endometrioid adenocarcinoma of ovary
Epidemiology
- 10% of primary ovarian carcinomas (J Natl Cancer Inst 2019;111:60)
- Mean patient age is 55 years
- Associated with endometriosis / endometriotic cyst (15%), endometrioid adenofibroma, synchronous endometrial endometrioid adenocarcinoma or endometrial hyperplasia (15 - 30%) (Eur J Gynaecol Oncol 2015;36:21)
- Risk factors: endometriosis, hormone replacement therapy, first degree family history of breast carcinoma (Lancet Oncol 2012;13:385, J Clin Oncol 2016;34:2888)
- May occur in the setting of Lynch syndrome (Gynecol Oncol 2018;150:92, Am J Surg Pathol 2014;38:1173)
Sites
- Ovaries
Pathophysiology
- Most common molecular alterations: WNT / beta catenin signaling pathway (CTNNB1 - 53%), PI3K pathway (PIK3CA - 40% and PTEN - 17%) (Mod Pathol 2014;27:128)
Etiology
- Unknown
Clinical features
- Most common symptoms are abdominal distention and pain
- Background endometriosis is not associated with survival (J Gynecol Oncol 2018;29:e18)
Laboratory
- Elevated CA-125 (Asian Pac J Cancer Prev 2013;14:4545)
Radiology description
- Most common appearance is a cystic lesion (Acta Radiol 2016;57:758)
Prognostic factors
- Stage is the most important prognostic factor
- Survival rates: > 95% for stage IA and IB and 51% for stage III and IV (J Natl Cancer Inst 2019;111:60)
- Most tumors are confined to the ovary at diagnosis
- CTNNB1 (beta catenin) mutation and low tumor grade are associated with a favorable outcome (Histopathology 2019;74:452, Am J Surg Pathol 2021;45:68)
- Synchronous endometrioid carcinomas of the endometrium and ovaries are often clonally related but typically have indolent clinical behavior (J Natl Cancer Inst 2016;108:djv428, J Natl Cancer Inst 2016;108:djv427, Mod Pathol 2021;34:994)
Case reports
- 39 year old woman with Cowden syndrome incidentally detected from a metachronous ovarian endometrioid carcinoma (BMC Cancer 2019;19:1014)
- 41 year old woman with mixed adenocarcinoma and yolk sac tumor (Int J Clin Exp Pathol 2019;12:3549)
- 45 year old woman with endometrial endometrioid and synchronous bilateral endometrioid ovarian cancer (Anticancer Res 2017;37:969)
- 52 year old woman with ovarian mass on computed tomography imaging and a raised CA-125 (Case of the Month #500)
- 65 year old postmenopausal woman with ovarian endometrioid carcinoma presenting as an abdominal wall abscess (J Midlife Health 2018;9:222)
Treatment
- Surgery for early stage cancers
- Adjuvant chemotherapy is associated with survival benefit for patients with inadequately staged and grade 2 stage I cancers (Gynecol Oncol 2020;156:315)
- Patients with advanced stage disease (FIGO III and IV) might benefit from platinum based chemotherapy (Future Oncol 2018;14:123)
Gross description
- Usually unilateral; only 5% bilateral
- Cystic with solid component and areas of hemorrhage
- With or without polypoid nodule in endometriotic cyst
- Mean tumor size: 11 cm (range: 3 - 22 cm)
- Reference: Arch Gynecol Obstet 2008;278:209
Gross images
AFIP images
Contributed by Ayse Ayhan, M.D.
Frozen section description
- Depending on histologic grade, a combination of glandular and solid areas may be seen
- Squamous differentiation may be present
- Differential diagnosis depends on histologic grade but includes metastatic carcinoma, well differentiated Sertoli-Leydig cell tumor and serous carcinoma (Arch Pathol Lab Med 2019;143:47)
Frozen section images
Microscopic (histologic) description
- Most common morphologic pattern is confluent (back to back) glands
- Stromal invasion is usually by expansion; rarely, destructive stromal invasion can be observed
- Squamous metaplasia (morules or keratin pearls), cytoplasmic mucin, intracytoplasmic vacuoles, oncocytic changes, clear cell changes and cilia and sex cord-like elements (sertoliform) can be observed; none of these morphologic features affect the histologic grade (Am J Surg Pathol 2007;31:1203)
- Histologic grading: same as for endometrial endometrioid adenocarcinoma
- FIGO grade 1: less than 5% solid component
- FIGO grade 2: 6 - 50% solid component
- FIGO grade 3: more than 50% solid component
- Endometriosis or adenofibroma may be present in the background
- Vascular invasion is rare
- Might be associated with serous, undifferentiated carcinoma and yolk sac tumor (mixed carcinoma) (Am J Surg Pathol 1994;18:687, Am J Surg Pathol 1996;20:1056)
- Morphologic features in favor of synchronous endometrial and ovarian tumors:
- Both tumors are low grade
- No myometrial invasion or less than 50% myometrial inivasion
- There is no involvement in any other anatomical sites
- No lymphovascular involvement in either tumor
- Background atypical endometrial hyperplasia in the endometrium
- Unilateral ovarian involvement and unifocal parenchymal distribution of tumor
- Lack of ovarian capsular, multifocal or hilar involvement
- Presence of endometriosis or adenofibroma in the ovary (Int J Gynecol Pathol 2019;38:S75)
Microscopic (histologic) images
Contributed by Ozlen Saglam, M.D.
Contributed by Sakinah A Thiryayi, M.D. and Gulisa Turashvili, M.D., Ph.D. (Case #500)
Cytology description
- Large cohesive cell clusters
- Mild nuclear membrane irregularities
- May see squamous differentiation
- Detection in pelvic washing: sensitivity 58%, specificity 89% Cancer 2004;102:150
Positive stains
- Keratin cocktails, EMA, CK7, PAX8 (15% negative), ER and PR (Semin Diagn Pathol 2005;22:3, Int J Gynecol Pathol 2016;35:430)
- p53: wild / normal type expression (most tumors) (Int J Gynecol Pathol 2016;35:430, Am J Surg Pathol 2018;42:534)
- High grade endometrioid cancers might demonstrate mutational pattern (nuclear staining in > 80% of cells, complete absence of staining, diffuse cytoplasmic staining)
- Mismatch repair protein deficiency has been reported in up to 23% of tumors (Gynecol Oncol 2020;156:669, Am J Surg Pathol 2019;43:235)
Negative stains
Molecular / cytogenetics description
- Ovarian endometrioid cancers and associated endometriosis share mutations in majority of cases (85 - 90%) (J Pathol 2018;245:265)
- CTNNB1 (53%), PIK3CA (40%), KRAS (33%), ARID1A (30%) and PTEN (17%) are common mutations (Cancer Cell 2007;11:321, Mod Pathol 2014;27:128)
- The Cancer Genome Atlas (TCGA) molecular classifiers for endometrial carcinoma has categorized ovarian endometrioid carcinoma into prognostically significant groups (Mod Pathol 2017;30:1748)
Sample pathology report
- Left ovary and fallopian tube, salpingo-oophorectomy:
- Endometrioid adenocarcinoma, FIGO grade 1 (see synoptic report)
- Tumor size: 8.5 cm
- Ovarian surface: not involved by carcinoma
- Fallopian tube: not involved by carcinoma
- Additional findings: background endometriotic cyst
Differential diagnosis
- Adult granulosa cell tumor:
- Sertoli cell tumor / Sertoli-Leydig cell tumor:
- High grade serous carcinoma:
- Architecture: solid, papillary, labyrinthine, glandular
- Nuclei are large
- Nuclear size variability > 3 fold
- High mitotic activity (> 12 mitoses per 10 high power fields)
- Metastatic colonic carcinoma:
- Metastatic endometrial endometrioid adenocarcinoma:
- Metastatic endometrial endometrioid adenocarcinoma is usually high grade (FIGO grade 3)
- Bilateral involvement
- Multinodular growth
- No endometriosis / adenofibroma background
- Clear cell carcinoma:
Board review style question #1
A 48 year old woman with endometrial carcinoma underwent laparoscopic staging procedure and a right ovarian adenocarcinoma was identified. Endometrial and ovarian lesions have identical morphology (see image). The ovarian lesion is confined to the right ovary and endometrial cancer has superficial myometrial invasion. Which immunostain can be useful to rule out metastatic endometrial adenocarcinoma?
- Immunostains are not contributory in differential diagnosis
- Napsin A
- PAX8
- Vimentin
- WT1
Board review style answer #1
A. Immunostains are not contributory in a differential diagnosis
Comment Here
Reference: Endometrioid carcinoma
Comment Here
Reference: Endometrioid carcinoma
Board review style question #2
Which of the following is associated with favorable disease outcome in patients with ovarian endometrioid carcinoma?
- Strong PAX8 expression
- WT1 expression
- Vimentin expression
- Cytoplasmic napsin A expression
- Low grade tumor and nuclear beta catenin expression
Board review style answer #2
E. Low grade tumor and nuclear beta catenin expression
Comment Here
Reference: Endometrioid carcinoma
Comment Here
Reference: Endometrioid carcinoma
Board review style question #3
What clinical manifestations have most commonly been associated with functioning stroma in ovarian tumors?
- Adrenergic manifestations
- Androgenic manifestations
- Estrogenic manifestations
- Progestogenic manifestations
Board review style answer #3
Endometrioid cystadenoma and adenofibroma
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Positive stains | Negative stains | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Benign tumors with endometrioid type glands, sometimes associated with endometriosis
Essential features
- Benign biphasic tumor with endometrioid type glands and fibromatous stroma
- Sometimes associated with endometriosis
- Squamous or mucinous metaplasia or dystrophic stromal calcifications can be seen
ICD coding
- ICD-O:
- ICD-11:
- 2F32.Y & XH1CX5 - other specified benign neoplasm of ovary & endometrioid adenofibroma, NOS
Epidemiology
- Uncommon tumors, most often seen in postmenopausal women (Int J Gynecol Pathol 2000;19:276)
Sites
- Ovary
Pathophysiology
- In some cases, endometrioid cystadenomas may represent endometriotic cysts without obvious endometrial type stroma
Etiology
- Unknown
Clinical features
- Can be an incidental finding
- Symptoms may include abnormal vaginal bleeding, abdominal pain or pelvic mass (J Obstet Gynaecol 2011;31:352)
Diagnosis
- Detected on pelvic imaging (ultrasound, CT, MRI)
- Cystectomy / oophorectomy
Radiology description
- Low T2 weighted signal intensity of solid components (Radiographics 2021;41:289)
Prognostic factors
- These tumors are benign
Case reports
- 46 year old woman with pelvic pain, postmenopausal bleeding and 6.3 cm complex solid and cystic right adnexal mass (J Obstet Gynaecol 2011;31:352)
- 60 year old woman with vaginal bleeding and 14 cm pelvic mass (Diagn Cytopathol 2004;31:38)
- 64 year old woman with solid pelvic tumor (J Obstet Gynaecol Res 1998;24:161)
Treatment
- Not applicable; these tumors are benign
Gross description
- Cystadenoma: usually unilocular cyst
- Adenofibroma: firm, white nodule with honeycomb appearance to cut surface, sometimes in the wall of an endometriotic cyst (J Obstet Gynaecol 2011;31:352)
Microscopic (histologic) description
- Cystadenoma:
- Cyst lined by benign endometrioid epithelium without endometrial stroma
- Endometriosis may be present
- Adenofibroma:
- Widely spaced benign endometrioid glands associated with fibromatous stroma
- Endometriosis may be present
- Areas of mucinous or serous differentiation can be seen (Histopathology 2021;78:445)
Microscopic (histologic) images
Cytology description
- Bland endometrioid cells (epithelial cells with moderate amounts of cytoplasm and bland, eccentrically placed nuclei) and fragments of ovarian type stroma (Diagn Cytopathol 2004 Jul;31:38)
Positive stains
- PAX8, estrogen receptor, progesterone receptor
- Nuclear beta catenin may be seen rarely; beta catenin alterations are better described in endometrioid borderline tumors (Int J Gynecol Pathol 2022 Feb 11 [Epub ahead of print], J Pathol 2006;208:708)
Negative stains
Sample pathology report
- Ovary, right, oophorectomy:
- Right ovary with endometrioid adenofibroma arising in a background of endometriosis
Differential diagnosis
- Endometriosis:
- Has associated endometrial type stroma with or without hemosiderin laden macrophages
- Serous cystadenoma / adenofibroma:
- Glands show only tubal type differentiation
- WT1, ER diffusely positive
- Membranous beta catenin staining
- Seromucinous cystadenoma:
- Glands show admixture of Müllerian epithelia with bland cytological features
- Ciliated, endocervical type, mucinous, endometrioid (which may have squamous or mucinous differentiation)
- Also associated with endometriosis
- Some authors believe that a subset of seromucinous cystadenomas represent benign endometrioid neoplasms with foci of mucinous or serous differentiation (Histopathology 2021;78:445)
- Glands show admixture of Müllerian epithelia with bland cytological features
Additional references
Board review style question #1
Board review style answer #1
Board review style question #2
What feature distinguishes endometrioid cystadenoma / adenofibroma from endometriosis?
- Absence of endometrial type stroma
- Epithelial lining
- Location
- Mucinous differentiation
- Size
Board review style answer #2
A. Absence of endometrial type stroma
Comment Here
Reference: Endometrioid cystadenoma / adenofibroma
Comment Here
Reference: Endometrioid cystadenoma / adenofibroma
Endometriosis
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Positive stains | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Presence of endometrial tissue outside of endometrium and myometrium, consisting of both endometrial glands and stroma
Essential features
- Ectopically located endometrial tissue consisting of at least 2 of the following: endometrial type glands, endometrial type stroma or evidence of chronic hemorrhage
- Endometriosis is associated with ovarian clear cell carcinoma and endometrioid carcinoma and shares similar molecular alterations
- Endometriosis in patients without cancer harbors oncogenic mutations in ARID1A, PIK3CA, KRAS and PPP2R1A, suggesting a neoplastic nature in some cases (N Engl J Med 2017;376:1835)
- CD10 immunohistochemistry can be used to confirm the presence of endometrial stroma
Terminology
- Endometriotic cyst / endometrioma: cystic form of endometriosis
- Atypical endometriosis: endometriosis with cytologic atypia or crowded glands lined by atypical epithelium resembling endometrial atypical hyperplasia (Adv Anat Pathol 2007;14:241)
ICD coding
- ICD-10: N80 - endometriosis of uterus
- N80.0 - endometriosis of uterus
- N80.1 - endometriosis of ovary
- N80.2 - endometriosis of fallopian tube
- N80.3 - endometriosis of pelvic peritoneum
- N80.4 - endometriosis of rectovaginal septum and vagina
- N80.5 - endometriosis of intestine
- N80.6 - endometriosis in cutaneous scar
- N80.8 - other endometriosis
- N80.9 - endometriosis, unspecified
Epidemiology
- Affects 5 - 15% women of reproductive age
- Peak incidence: 30 - 45 years of age
- Estrogen dependent; can rarely affect individuals assigned male at birth taking large doses of estrogen (Fertil Steril 2012;98:511)
Sites
- Ovary (67%) > anterior and posterior cul de sac > posterior broad ligaments, uterosacral ligaments > uterus > fallopian tubes > sigmoid colon and appendix > round ligaments (Eur J Obstet Gynecol Reprod Biol 2018;230:36)
- Also seen in bladder and cervix
- Rarely in remote sites such as lung, regional lymph nodes or skin
Pathophysiology
- Retrograde menstruation hypothesis: endometrial lining cells travel backwards through fallopian tubes during menses to reach peritoneal cavity, proliferate and cause chronic inflammation with formation of adhesions (Nat Rev Endocrinol 2014;10:261)
- Coelomic metaplasia hypothesis: metaplastic transformation of coelomic cells lining the pelvic peritoneum (J Lab Physicians 2010;2:1)
- Induction hypothesis: a combination of the first 2 theories (N Engl J Med 1993;328:1759)
- Development of malignant neoplasm occurs in < 1% of cases; 75% of malignant neoplasms arise in ovarian endometriosis (J Lab Physicians 2010;2:1)
Etiology
- Organochlorine pollutant exposure (Environ Int 2017;108:195)
Clinical features
- Pelvic pain (Arch Gynecol Obstet 2015;292:1295)
- Dyspareunia
- Dysmenorrhea
- Infertility
- Rarely, infection or rupture of an endometriotic cyst with ascites or hemoperitoneum
Diagnosis
- Laparoscopy required for definitive diagnosis, although ~50% laparoscopic biopsy specimens contain microscopic endometriosis (Am J Obstet Gynecol 2001;184:1407)
- Endometriosis in pelvis categorized as superficial peritoneal, ovarian and deeply infiltrating
Radiology description
- Ultrasound is mostly used for ovarian endometriotic cysts
- Typically multilocular cysts with septations and hyperechoic mural nodules (Radiology 1999;210:739)
Prognostic factors
- Risk of development of malignant neoplasm is estimated at 1% for premenopausal women and up to 2.5% for postmenopausal women
- ~75% neoplasms complicating endometriosis arise within the ovary; most common extraovarian site is rectovaginal septum
- Increased risk of endometrioid carcinoma followed by clear cell carcinoma (Clin Chim Acta 2019;493:63)
- Other associated neoplasms include seromucinous neoplasms (mainly borderline), endometrioid adenofibromas and borderline neoplasms, adenosarcomas and endometrial stromal sarcomas (Histopathology 2020;76:76)
- Women with carcinoma arising in endometriosis tend to be premenopausal, obese and with history of unopposed estrogens (Gynecol Oncol 2000;79:18)
- Endometriosis associated carcinomas (other than clear cell) tend to be lower grade and stage than similar ovarian carcinoma without associated endometriosis (Gynecol Oncol 2001;83:100)
Case reports
- 32 year old woman with ruptured endometrioma (J Med Case Rep 2022;16:161)
- 35 year old woman presenting with hematuria and abdominal pain (BMJ Case Rep 2018;11:e226927)
- 37 year old woman with history of pelvic surgery presenting with pelvic pain (Medicine (Baltimore) 2018;97:e0376)
- 37 year old woman with decidualized ovarian endometrioma in pregnancy (J Ovarian Res 2022;15:33)
- 40 year old woman with elevated CA125 and pelvic mass (Medicine (Baltimore) 2019;98:e15741)
- 42 year old woman with supernumerary ovary on rectosigmoid colon with associated endometriosis (Obstet Gynecol Sci 2018;61:702)
- 49 year old Japanese woman with abnormal vaginal bleeding and adenocarcinoma diagnosed by cervical cytology (J Obstet Gynaecol Res 2020;46:536)
- 55 year old woman with peritoneal endometriosis and smooth muscle metaplasia (Int J Clin Exp Pathol 2015;8:3370)
- 62 year old woman presenting with hematuria (Pathology 2017;49:441)
Treatment
- Pain treated with NSAIDs, hormonal contraceptives, GnRH analogues and aromatase inhibitors (Arch Gynecol Obstet 2015;292:1295)
- Surgical resection
Gross description
- Ovarian endometriotic cysts (endometriomas) have fibrotic walls, a smooth lining and dark brown cyst contents (chocolate cyst), often adherent to adjacent organs
- Polypoid endometriosis has a polypoid configuration that raises the differential diagnosis of a neoplasm on gross and intraoperative examination (Am J Surg Pathol 2004;28:285)
- Red, brown, white plaques, sometimes with a gelatinous appearance (Arch Gynecol Obstet 2015;292:1295, Taiwan J Obstet Gynecol 2019;58:328)
Gross images
Frozen section description
- Presence of endometrial glands or endometrial stroma (Taiwan J Obstet Gynecol 2019;58:328)
- Sometimes only macrophages and hemosiderin are present (diagnose as consistent with clinical impression of endometriosis, as other causes are possible)
- Can be associated with fibrous adhesions
- Negative for neoplastic features such as glandular complexity
Frozen section images
Microscopic (histologic) description
- At least 2 of the following 3 features
- Endometrial type glands
- Müllerian type epithelium (can be atrophic to cycling endometrium)
- Can show degenerative atypia (enlarged smudgy nuclei) or metaplasia
- Endometrial type stroma
- Often contains fine capillary network
- May undergo smooth muscle metaplasia, fibrosis (longstanding), decidual change
- May be myxoid (particularly in pregnancy)
- Stroma may be the only identifiable component (stromal endometriosis)
- Evidence of chronic hemorrhage (hemosiderin laden or foamy macrophages)
- Endometrial type glands
- Other rare findings
- Necrotic pseudoxanthomatous nodules: central necrosis surrounded by histiocytes and outer fibrous zone
- Liesegang rings: eosinophilic acellular rings within necrotic tissue (Histopathology 2020;76:76)
- Burnt out endometriosis: this term has been proposed for changes suggestive of endometriosis, such as central necrosis with surrounding fibrosis and pseudoxanthoma cells but lacking confirmatory features as listed above
- Atypical endometriosis: this has been reported in 1.7 - 4.4% of endometriotic lesions and is considered the precursor lesion for endometriosis associated carcinomas (clear cell or endometrioid); may be in continuity with these tumors
- Includes crowded glands lined by atypical epithelium resembling endometrial atypical hyperplasia; nuclear atypia is typically moderate or severe, with hobnailing (Histopathology 1997;30:249, Case Rep Oncol 2013;6:480, Int J Gynecol Pathol 2023 Mar 13 [Epub ahead of print])
- Is associated with synchronous / subsequent neoplasia in 25% of cases and harbors genomic alterations seen in endometriosis associated tumors (Int J Gynecol Pathol 2023 Mar 13 [Epub ahead of print])
Microscopic (histologic) images
Cytology description
- Reported in peritoneal fluid and fine needle aspiration of scar tissue following gynecologic procedure (e.g., Caesarean section) (J Cytol 2017;34:61)
- Variably sized, 3 dimensional spherules with periphery of polygonal endometrial cells with larger, hyperchromatic nuclei and moderate amount of cytoplasm, often with a center of stromal cells with hyperchromatic nuclei, scant cytoplasm and indistinct cytoplasmic borders (Cancer Cytopathol 2013;121:582)
- May have admixed hemosiderin laden macrophages
Positive stains
- CD10 is positive in endometrial stroma
- ER, PR and PAX2 are often positive in endometrial glands and stroma (Am J Surg Pathol 2013;37:1342)
Molecular / cytogenetics description
- Endometriosis and synchronous carcinoma share similar genetic alterations including ARID1A, PTEN and PIK3CA
- Mutations in ARID1A, a tumor suppressor gene, identified in up to 57% of ovarian endometrioid carcinoma and up to 30% of clear cell carcinoma
- Multiple studies suggest ARID1A mutation occurs at early stage of canceration of endometriosis (Oncol Rep 2016;35:607)
- Endometriosis occurring distant from ARID1A deficient carcinomas are more likely to retain ARID1A expression
- Other associated genetic alterations include loss of BAF250a, ER and PR and upregulation of hepatocyte nuclear factor - beta and SKP2
- In one study, loss of DNA mismatch repair protein expression was found in 10% of patients with endometriosis associated ovarian carcinoma (Int J Gynecol Pathol 2012;31:524)
Videos
Causes, symptoms, diagnosis, treatment, pathology
Histopathology - ovary
Sample pathology report
- Uterus, hysterectomy:
- Proliferative endometrium negative for hyperplasia or malignancy
- Unremarkable cervix
- Myometrium with leiomyomata
- Uterine serosa with multifocal endometriosis
- Right ureterosacral peritoneum, excision:
- Fibrous tissue with focal endometrial type stroma and hemorrhage, suggestive of endometriosis
Differential diagnosis
- Endocervicosis:
- Glandular component is endocervical mucinous type, no endometrial stroma, no hemorrhage
- Endosalpingiosis:
- Glandular component is tubal (ciliated with peg / intercalated) cells, no endometrial stroma, no hemorrhage
- Adenomyosis:
- Endometrial glands and stroma in myometrium
- Endometrioid adenocarcinoma:
- Complex glandular growth and cytologic atypia
- Metastatic carcinoma:
- Morphology varies by site of origin; however, no endometrial stroma
- Other features of neoplasm present, including crowded irregular glands, nuclear atypia or elevated mitotic activity
Additional references
Board review style question #1
A 39 year old woman presents with pelvic pain and menorrhagia. Hysterectomy was performed and the histologic findings shown in this photomicrograph were present on the serosal surface and right ovary. What malignant neoplasm is most associated with the lesion?
- Clear cell sarcoma
- Endometrial stromal sarcoma
- High grade serous carcinoma
- Immature teratoma
- Ovarian endometrioid carcinoma
Board review style answer #1
E. Ovarian endometrioid carcinoma. Endometriosis can be associated with endometrioid and clear cell carcinoma, with the greatest association identified in the former neoplasm. Rare endometrial stromal sarcomas and adenosarcomas have also been reported to arise in association with endometriosis. Answer D is incorrect because there is no reported association between germ cell tumors and endometriosis. Answer C is incorrect because there is no reported association between high grade serous carcinoma and endometriosis. Answer A is incorrect because there is no reported association between clear cell sarcoma and endometriosis. Answer B is incorrect because even though some cases of adenosarcomas have been reported to arise in association with endometriosis, the association is less frequent than endometrioid carcinoma.
Comment Here
Reference: Endometriosis
Comment Here
Reference: Endometriosis
Board review style question #2
Which of the following genes is most commonly altered in endometriosis associated carcinomas?
- ARID1A
- BRAF
- CDKN2A (p16)
- PTEN
- TP53
Board review style answer #2
A. ARID1A. Of the 5 genes listed, mutations in tumor suppressor gene ARID1A are most commonly identified in endometriosis and endometriosis associated carcinomas. Many studies suggest that loss of ARID1A expression is an early driver mutation. Other common molecular alterations include mutations in PTEN, PIK3CA, KRAS and to a lesser extent, microsatellite instability with MMR protein loss. Answer D is incorrect because PTEN mutations are less frequent. Answers E and C are incorrect because TP53 and CDKN2A mutations are rare. Answer B is incorrect because BRAF mutations are not associated with endometriosis.
Comment Here
Reference: Endometriosis
Comment Here
Reference: Endometriosis
Endosalpingiosis
Table of Contents
Definition / general | Essential features | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Presence of ectopic glands lined by fallopian tube type ciliated epithelium
Essential features
- Defined as ectopic glands lined by fallopian tube type ciliated epithelium outside the fallopian tube
- Incidental finding in resection specimens
- Diagnosed by microscopic examination
- Dilated glands measure less than 1 cm in size (if more than 1 cm, serous cystadenoma)
Epidemiology
- Typically seen in women of reproductive age
- In 33% of cases, endosalpingiosis is associated with endometriosis (Gynecol Oncol 2016;142:255)
Sites
- Ovaries, omentum, fallopian tube serosa, uterine serosa, bladder, lymph nodes
Pathophysiology
- Multiple hypotheses, including distal fallopian tube implants in the ovarian cortex during ovulation or metaplastic changes of the ovarian surface epithelium or peritoneal cells (Reprod Sci 2013;20:1030)
- Papillary tubal hyperplasia (PTH) is a term that has been proposed to describe small rounded clusters of tubal epithelial cells and small papillae, with or without associated psammoma bodies, that are present within the tubal lumen; PTH has been linked to endosalpingiosis and serous tumor (Am J Surg Pathol 2011;35:1605)
Etiology
- No specific etiology identified
Clinical features
- Mostly asymptomatic and incidentally found in resection specimens
- Rarely mass forming cystic lesion or mass related symptoms (BMJ Case Rep 2014;2014:bcr2013201645)
Diagnosis
- By histopathological examination, with confirmation of morphology and exclusion of endometriosis
Radiology description
- Typically no specific imaging findings
- Multilocular cysts if mass forming (Abdom Imaging 2015;40:471)
Prognostic factors
- Considered a benign finding
- Relationship with development of epithelial malignancies is not clear but patients with endosalpingiosis have been found to be more likely to have concurrent endometriosis as well as uterine and ovarian cancer (Gynecol Oncol 2016;142:255)
Case reports
- 30 year old pregnant woman with cystic endosalpingiosis (Case Rep Obstet Gynecol 2016;2016:8621570)
- 31 year old woman with florid cystic endosalpingiosis, masquerading as a malignancy (BMJ Case Rep 2014;2014:bcr2013201645)
- 55 year old woman with florid cystic endosalpingiosis presenting as an ovarian cyst (Gynecol Minim Invasive Ther 2016;5:170)
Treatment
- Surgery for the mass forming lesions
Gross description
- Simple cysts on ovarian surface
- Florid cystic endosalpingiosis can form a multicystic tumor-like mass (BMJ Case Rep 2014;2014:bcr2013201645)
Microscopic (histologic) description
- Glands lined by ciliated tubal type epithelium
- 3 types of cells:
- Ciliated columnar cells
- Nonciliated columnar secretory mucous cells
- So called intercalated or peg cells
- May have psammoma bodies
- Absence of endometrial stroma
- Glands can be cystically dilated; they are usually found in the superficial ovarian cortex, either scattered or in loose clusters
- Reference: Clement: Atlas of Gynecologic Surgical Pathology, 4th edition, 2019
Microscopic (histologic) images
Positive stains
- PAX2, PAX8 (Am J Surg Pathol 2011;35:1837, Case Rep Obstet Gynecol 2016;2016:8621570)
- WT1, ER, PR (Am J Surg Pathol 2014;38:1612, Abdom Imaging 2015;40:471)
- Endosalpingiosis has a similar biomarker profile to normal fallopian tube and serous neoplasms (phospho-SMAD2, BCL2 and FOXJ1), which is distinct from ovarian surface epithelium (Gynecol Oncol 2014;132:316)
Negative stains
Sample pathology report
- Right and left ovaries, bilateral oophorectomies:
- Benign ovaries with focal endosalpingiosis
- Note: many pathologists do not report the finding of endosalpingiosis since it is common and benign
Differential diagnosis
- Cystic ovarian neoplasms when mass forming:
- Other types of epithelial cysts (mucinous, peritoneal inclusion type, transitional type) have distinct epithelia
- Serous cystadenoma:
- Lined by tubal type epithelium
- Distinction relies (arbitrarily) on size > 1 cm for cystadenomas
- Endometriosis:
- Has endometrial stroma and hemosiderin laden macrophages
Additional references
Board review style question #1
Board review style answer #1
D. Usually an incidental finding. Endosalpingiosis is typically benign incidental finding in resection specimens, Müllerian derived (PAX8 positive). Absence of endometrial stroma distinguishes it from endometriosis, as tubal differentiation can be seen in both entities.
Comment Here
Reference: Endosalpingiosis
Comment Here
Reference: Endosalpingiosis
Board review style question #2
What feature below is most important to distinguish endosalpingiosis from endometriosis?
- Location
- Presence of endometrial stroma
- Presence of tubal epithelium
- Reactivity for PAX8
Board review style answer #2
B. Presence of endometrial stroma. Endometrial stroma is seen in endometriosis but not endosalpingiosis. Endometriosis can have tubal differentiation. It tends to have a similar anatomic distribution as endosalpingiosis and to express markers of Müllerian derivation, such as PAX8.
Comment Here
Reference: Endosalpingiosis
Comment Here
Reference: Endosalpingiosis
Epithelial tumors-overview / molecular
Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Pathophysiology | Etiology | Clinical features | Gross images | Microscopic (histologic) description | Positive stains | Negative stains | Molecular / cytogenetics description | Additional referencesDefinition / general
- The ovary is a highly specialized organ physiologically dedicated to ovum production
- It is composed mainly of stromal cells that support maturing germ cells, covered by a monolayer modified mesothelium (so called ovarian surface epithelium)
- Most ovarian cancers are epithelial, but most testicular tumors derive from germ cells or sex cord stroma
Essential features
- Ovarian carcinoma (previously called ovarian surface epithelial malignant tumors) is not a single entity, but a group of neoplasms with different histotypes, immunohistochemical characteristics, origins and biology
- Although the WHO classification has not substantially changed from the first edition, recent molecular knowledge combined with epidemiological data has caused a considerable reevaluation of the pathogenesis, cell of origin and precursor lesions of ovarian carcinoma
Terminology
- Serous / Endometrioid / Clear cell / Seromucinous / Mucinous / Brenner / Undifferentiated
Epidemiology
- Epithelial cancers represent >90% of ovarian malignant tumors (Pathology 2011 Aug;43:420)
| Tumor type | Acronym | Prevalence |
| High grade serous carcinoma | HGSC | 70% |
| Low grade serous carcinoma | LGSC | 5 % |
| Endometrioid carcinoma | EMC | 10% |
| Clear cell carcinoma | CCC | 10% |
| Seromucinous carcinoma | SMT | rare |
| Mucinous carcinoma | MC | 3% |
| Malignant Brenner tumor | Malignant BT | rare |
| Undifferentiated carcinoma | UC | rare |
Pathophysiology
Traditional pathogenetic view:
Controversies with the traditional view
Realities to consider
Emerging pathogenetic views
- Cells of origin: ovarian surface epithelium (OSE)
- Primary site of origin: ovary
- Mechanism: OSE is a modified mesothelium which undergoes metaplasia, acquiring a Müllerian or non-Müllerian epithelial phenotype and successively undergoes neoplastic transformation
- In 1925, Sampson hypothesized that ovarian carcinoma may arise from malignant transformation of endometriosis, thought to derive from OSE metaplasia or embryonic residuals
- Fathalla’s theory of “incessant ovulation” identifies recurrent ovulation as a transforming event for OSE through direct damage, inflammation and exposure to sex hormone-rich follicular fluid (Lancet 1971;2:163)
- Therefore, incessant ovulation constitutes an index for individual ovarian cancer risk
- This theory assumes that all ovarian surface epithelial tumors have the same origin
- For many years, pathologists diagnosed and classified ovarian epithelial tumors based on histological similarity, size, clinical information and apparent cell of origin; fallopian tubes were inadequately sampled and ignored
Controversies with the traditional view
- No ovarian surface epithelial carcinomas (serous, endometrioid, clear cell, mucinous, seromucinous, transitional) resemble OSE
- Metaplasia of OSE is difficult to demonstrate and almost never observed
- There are no intermediate / hybrid phenotypes of OSE metaplasia tumors
- Real mesotheliomas are rare
- Most ovarian tumors do not express OSE molecular markers
- In the testis, analogous surface (tunica albuginea) tumors are rare
Realities to consider
- Recent advances in molecular genetics have found mutations in BRCA1 and BRCA2 tumor suppressor genes responsible for most hereditary ovarian cancer
- When patients with mutations underwent prophylactic salpingo-oophorectomy, dysplastic precursor lesions were found in fallopian tubes (later named Serous Tubal Intraepithelial Carcinoma, STIC), but not in ovaries
- Tubal ligation reduces the incidence of ovarian endometrioid, clear cell carcinoma and high grade serous carcinoma (Cancer Epidemiol Biomarkers Prev 1996;5:933, Int J Cancer 2016;138:1076)
- Thorough examination of fallopian tubes using the SEE-FIM protocol (Sectioning and Extensively Examining the FIMbriated end), revealed early fallopian tube cancer in sporadic high grade serous carcinomas
- There are overlapping molecular alterations with STIC and high grade serous carcinoma regarding p53, p16, FAS, RSF1, CCNE1 and centrosome amplification, suggesting a clonal relationship (Mod Pathol 2016;29:1254)
- Endometrioid (40%) and clear cell carcinoma (50-90%) are commonly associated with endometriosis, and patients with endometriosis have a 3-10x increased risk of developing ovarian endometrioid and clear cell carcinomas
- Molecular genetic alterations reveal a clonal relationship between endometriosis and endometrioid and clear cell carcinomas (ARID1A, KRAS, MET) (Int J Gynecol Cancer 2012;22:1310)
- Primary mucinous ovarian carcinomas are uncommon: the real incidence of mucinous carcinomas is less than previously thought
Emerging pathogenetic views
- Ovarian carcinoma (so called ovarian surface epithelial tumors) is not a single entity
- Cells of origin: extra-ovarian Müllerian epithelia (endometrium or fallopian tube epithelium)
- Primary site of origin: extra-ovarian Müllerian epithelia (endometrium or fallopian tube epithelium) or ovary (endometriosis or fallopian tube epithelium seeded on the ovary as inclusion cysts)
- Mechanism: genetically normal or initially transformed epithelial cells from fallopian tube or endometrium seed the ovary, whose microenvironment promotes their neoplastic transformation
- Mucinous carcinomas are composed of tumors with different genesis, including mature cystic teratoma and extra-ovarian Müllerian epithelium (Brenner tumors or endometriosis)
Etiology
- The most significant risk factor is family history (Bethesda (MD): National Cancer Institute (US); 2002)
- Contributing factors: excessive gonadotropins, androgen stimulation, pelvic inflammation, ovarian exposure to contaminants and carcinogens (talc and asbestos), nulliparity, refractory infertility, obesity
- Protective factors: multiparity, tubal ligation, hysterectomy, oral contraceptives (use for 5+ years reduces risk 50% even for those with a family history, Cancer Epidemiol Biomarkers Prev. 2009 Feb;18:601)
Clinical features
Genetic syndromes and germline alterations
- Hereditary Breast and Ovary Cancer syndrome from germline mutations in BRCA1 and BRCA2
- Lynch syndrome from germline mutations in DNA mismatch repair genes (MLH1, MSH2, MSH6, PMS2)
- Peutz-Jeghers syndrome from germline mutations in STK11
- Cowden syndrome from germline mutations in PTEN
- Coffin-Siris Syndrome from germline mutations in ARID1A
- Li-Fraumeni syndrome from germline mutations in TP53 / p53
- Other germline mutations that are correlated to ovarian carcinoma affect BRIP1, CHEK2, RAD51
Gross images
Contributed by Ayse Ayhan, M.D.
Clear cell carcinoma, case 1:
Clear cell carcinoma, case 2:
Bilateral endometrioid cancer:
Ovarian tumor:
Microscopic (histologic) description
Putative precursor lesions
- Serous tubal intraepithelial carcinoma / low grade serous carcinoma: for high grade serous carcinoma
- Serous borderline tumor: for low grade serous carcinoma and a few high grade serous carcinomas
- Endometriosis / adenofibroma / borderline tumor: for endometrioid carcinoma, clear cell carcinoma, seromucinous carcinoma, mixed endometrioid-clear cell carcinoma
- Endometriosis / mucinous borderline tumor: for mucinous carcinoma
- Teratoma / Brenner tumor / mucinous borderline tumor: for mucinous carcinoma
- Benign Brenner tumor: for borderline Brenner tumor
Positive stains
- p53 (high grade serous carcinoma, high grade endometrioid carcinoma, mucinous carcinoma)
- nuclear β catenin (endometrioid carcinoma)
- HER2 (mucinous carcinoma)
- Refer to histopathology for lineage specific markers
Negative stains
- p53 (30% of high grade serous carcinoma)
- MLH1, MSH2, MSH6, PMS2 (endometrioid carcinoma)
- PTEN (endometrioid carcinoma)
- ARID1A (endometrioid carcinoma, clear cell carcinoma, seromucinous carcinoma) (Int J Gynecol Cancer 2012;22:1310, Int J Gynecol Pathol 2012;31:297)
- Refer to histopathology for lineage specific markers
Molecular / cytogenetics description
Common mutations
NOTE: High grade serous carcinoma and low grade serous carcinomas have a mutually exclusive mutational signature
| Tumor type | Acronym | Prevalence |
| High grade serous carcinoma | BRCA1 | 12% |
| BRCA2 | 11% | |
| CSMD3 | 6% | |
| FAT3 | 6% | |
| TP53 | 95% | |
| Low grade serous carcinoma | BRAF | 38% |
| KRAS | 19% | |
| Endometrioid carcinoma | ARID1A | 55% |
| CTNNB1 | 60% | |
| KRAS | 7% | |
| MLH1/MSH2/MSH6/PMS2 | 20% | |
| PIK3CA | 35% | |
| PTEN | 16% | |
| PPP2R1A | 10-15% | |
| TP53 | 7% | |
| Clear cell carcinoma | ARID1A | 75% |
| KRAS | 10% | |
| PIK3CA | 35% | |
| PPP2R1A | 10% | |
| TERT | 16% | |
| Seromucinous tumor | ARID1A | 33% |
| KRAS | 69% | |
| Mucinous carcinoma | BRAF | 12% |
| HER2 | 50% | |
| KRAS | 54% | |
| RNF43 | 20% | |
| TP53 | 50% | |
| Borderline Brenner tumors | KRAS | 14% |
| PIK3CA | 28% |
NOTE: High grade serous carcinoma and low grade serous carcinomas have a mutually exclusive mutational signature
- Germline/somatic mutations in BRCA1, BRCA2 and other genes predict:
- Platinum sensitivity and longer survival in women with high grade serous carcinoma
- Benefit from PARP inhibitors (Journal of Cancer 2016;7:1441)
Additional references
- Kuhn E, Ayhan A., The non-ovarian origin and pathogenesis of ovarian carcinomas: update on the pathological and the molecular clues (PDF)
- Kurman RJ, Shih IeM., The Dualistic Model of Ovarian Carcinogenesis: Revisited, Revised, and Expanded (Am J Pathol 2016 Apr;186:733)
- Prat, J., Ovarian carcinomas: five distinct diseases with different origins, genetic alterations, and clinicopathological features (Virchows Arch 2012 Mar;460:237)
- Lim D, Oliva E., Precursors and pathogenesis of ovarian carcinoma (Pathology 2013 Apr;45:229)
Features to report
Definition / general
- Specimen type:
- Ovary (unilateral / bilateral), presence of fallopian tubes, uterus, cervix, omentum, peritoneum
- Procedure done
- Involvement of fallopian tube(s), opposite ovary and other tissues / organs (as mentioned in specimen type)
- Specimen integrity
- Capsule intact, ruptured, fragmented, etc.
- Ovarian surface involvement (present / absent)
- Tumor site (within the ovary)
- Tumor size
- Greatest dimension ____cm
- Additional dimensions ___X ____cm
- Tumor appearance
- Smooth / papillary, solid / cystic / both, necrosis, hemorrhage, calcification
- Histologic type (Refer to WHO classification of Ovarian neoplasms)
- Histologic grade
- Intratumoral T cells
- Lymphovascular space involvement
- Lymph nodes positive / number of nodes examined
- Extranodal extension (present / absent)
- Implants:
- Applicable to serous / seromucinous borderline tumors
- Not sampled / present / absent
- If present: noninvasive epithelial, noninvasive desmoplastic or invasive (mention sites)
- Presence of endometriosis (ovarian / extraovarian), endosalpingosis or other findings
- Treatment effect
- Applicable to carcinomas treated with neoadjuvant therapy
- Poor response / no response / marked response
- Special studies
- Histochemistry, immunohistochemistry, hormone receptor studies, flow cytometry for DNA ploidy, etc.
Additional references
Fibroma
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Benign stromal tumor composed of fibroblastic cells within a variably collagenous stroma
Essential features
- Benign stromal tumor composed of spindled, ovoid to round cells within a variably collagenous stroma
- Variants include fibroma with minor sex cord elements, cellular fibroma and mitotically active cellular fibroma
- Important differential diagnoses for cellular fibroma include diffuse adult type granulosa cell tumor and fibrosarcoma
- Gorlin syndrome should be suspected in young patients with bilateral ovarian fibroma
- Long term follow up is recommended for cellular fibromas associated with rupture and adhesions due to increased risk of recurrence
ICD coding
Epidemiology
- Most common ovarian stromal tumor
- ~4% of all ovarian tumors (Cancer 1981;47:2663)
- Cellular fibromas represent ~10% of all ovarian fibromas
- Mean age 48 years, uncommon before 30
- Occurs in ~25% of patients with nevoid basal cell carcinoma syndrome (Gorlin syndrome) (J Med Genet 1993;30:460)
- Autosomal dominant disease due to mutations of the human homologue of the Drosophila gene PTCH (patched)
Sites
- Ovary
Pathophysiology
- Neoplastic transformation of ovarian stromal cells due to hereditary or sporadic genetic abnormalities (see Molecular / cytogenetics description)
Clinical features
- Most commonly symptoms related to an ovarian mass, such as abdominal pain or distension and increased urinary frequency or may be incidental
- Can be associated with ovarian torsion (BMC Surg 2014;14:38)
- Rarely hormonal manifestations
- Ascites in large (> 10 cm) tumors in 10% of cases (Am J Obstet Gynecol 1970;107:538)
- Syndromic ovarian fibromas:
- Often diagnosed before the age of 30 years, including children
- Usually bilateral (75%), calcified and nodular, often overlapping medially (leading to misdiagnosis as calcified uterine leiomyoma) (Genet Med 2004;6:530)
- May be renin secreting, leading to gestational hypertension (Br J Obstet Gynaecol 1994;101:1015)
- May recur (Hum Reprod 2001;16:1261, Obstet Gynecol 1983;61:95S)
- Meigs syndrome occurs in ~1% of cases (Am J Obstet Gynecol 1937;33:249, Eur J Obstet Gynecol Reprod Biol 2000;92:199)
- Characterized by the triad of benign adnexal mass, ascites and pleural effusion
- Due to seepage of fluid from ovary into peritoneal cavity and then into 1 or both pleural cavities either via lymphatics or through a communication between abdominal and pleural cavity (lumbocostal triangle)
Diagnosis
- Histologic examination for definitive diagnosis
- Tentative diagnosis can be made grossly and at frozen section evaluation (Semin Diagn Pathol 2002;19:237)
Laboratory
- Can lead to nonspecific increase in serum CA125 (Gynecol Oncol 1995;59:405)
Radiology description
- Ultrasonography:
- Usually solid, homogeneous and hypoechoic mass with posterior acoustic shadowing, similar to a pedunculated subserosal uterine leiomyoma (AJR Am J Roentgenol 1985;144:1239)
- Heterogeneous echogenicity in tumors with necrosis, hemorrhage or cystic degeneration
- Computed tomography:
- Slightly hypoattenuating solid mass with poor, very slow contrast enhancement (Radiographics 2002;22:1305)
- Rarely calcifications
- Magnetic resonance imaging (MRI):
- T1: homogeneous low signal intensity
- T2:
- Well circumscribed mass with low signal intensity (J Magn Reson Imaging 1997;7:465)
- Hyperintense areas due to edema or cystic degeneration
- Characteristic feature is a band of T2 hypointensity separating the tumor from the uterus on all imaging planes
- Gadolinium: heterogeneous enhancement
- May mimic malignancy (Radiographics 2002;22:1305)
Radiology images
Prognostic factors
- Excellent in most cases
- Ovarian surface involvement and extraovarian adhesions occur in 6% of cellular fibromas and 10% of mitotically active cellular fibromas (Am J Surg Pathol 2006;30:929)
- Extraovarian spread at surgery in 11% of cellular fibromas and 13% of mitotically active cellular fibromas (Am J Surg Pathol 2006;30:929)
- Cellular fibromas may recur, often after a long interval, warranting long term follow up (Cancer 1981;47:2663)
Case reports
- 15 year old girl with bilateral ovarian fibroma associated with Gorlin syndrome (J Pediatr Adolesc Gynecol 2011;24:e5)
- 19 year old woman with large ovarian fibroma presenting with spontaneous abdominal bleeding (Case Rep Obstet Gynecol 2019;2019:9834915)
- 34 year old woman with mitotically active cellular fibroma that recurred 16 years later (Int J Gynecol Pathol 2020 [Epub ahead of print])
- 40 year old woman with bilateral ovarian fibroma presenting as Meigs syndrome (J Obstet Gynaecol 2013;33:636)
- 66 year old woman with extraovarian fibroma with minor sex cord elements (Int J Surg Pathol 2017;25:472)
Treatment
- Surgical excision (salpingo-oophorectomy, oophorectomy or ovarian sparing procedure with or without hysterectomy depending on patient’s age)
- Cellular fibromas require long term follow up, particularly in the setting of ovarian surface involvement, intraoperative rupture or extraovarian spread (Cancer 1981;47:2663)
Gross description
- Well circumscribed mass with smooth, lobulated surface
- Firm, chalky, solid, white to yellow-white to tan-yellow cut surface that may be whorled
- Frequent edema resulting in softer consistency
- Mean size 6 cm (range 1 - 21.5)
- Usually unilateral (< 10% bilateral)
- Cystic degeneration in ~25% of cases
- Calcifications in ~10% of cases
- ~20% present as pedunculated or polypoid growths on ovarian surface
- With or without hemorrhage or necrosis
- Cellular fibroma:
- Mean size 8 - 9 cm (range 1 - 19)
- Tan-yellow, soft and fleshy cut surface (Cancer 1981;47:2663)
- With or without surface adhesions
- Fibroma associated with Gorlin syndrome:
- Bilateral, multinodular and calcified (J Pediatr Adolesc Gynecol 2011;24:e5)
Gross images
Frozen section description
- Variably cellular spindle cell neoplasm with fascicular or storiform growth with or without hyaline plaques or calcifications
Frozen section images
Microscopic (histologic) description
- Conventional fibroma:
- Recapitulates ovarian cortex
- Usually well circumscribed but nonencapsulated
- Variably cellular fascicular or less frequently, storiform growth of tumor cells within a variably collagenous stroma, sometimes with hyaline plaques (Cancer 1981;47:2663)
- Bland spindled to ovoid nuclei with pointy ends and scant eosinophilic cytoplasm blending with surrounding stroma
- Occasional mitoses (usually up to 3 mitoses per 10 high power fields)
- With or without Verocay-like areas (slightly wavy, parallel arrays of spindled nuclei), calcification, edema, hemorrhage, infarct type necrosis, rare groups of luteinized cells
- Rarely intracytoplasmic lipid (may be diagnosed as thecoma), eosinophilic hyaline globules, melanin pigment or bizarre nuclei (Int J Gynecol Pathol 2009;28:356, Int J Gynecol Pathol 2019;38:92, Int J Surg Pathol 2020 [Epub ahead of print])
- Cellular fibroma (10% of cases):
- Resembles diffuse type of adult granulosa cell tumor
- Densely cellular with little intercellular collagen
- Bland spindled nuclei with up to 3 mitoses per 10 high power fields (Cancer 1981;47:2663)
- "Mitotically active cellular fibroma" has been proposed for cellular fibromas with ≥ 4 mitoses per 10 high power fields (Am J Surg Pathol 2006;30:929)
- Fibroma with minor sex cord elements:
- Sex cord component accounting for < 10% of overall tumor volume
- No prognostic significance
Microscopic (histologic) images
Contributed by Gulisa Turashvili, M.D., Ph.D., Kyle C. Strickland, M.D., Ph.D. and Rex Bentley, M.D.
Virtual slides
Positive stains
- WT1 (Am J Surg Pathol 2008;32:884)
- SF1 (Am J Surg Pathol 2009;33:354)
- FOXL2 (Am J Surg Pathol 2011;35:484)
- Inhibin: 50% of cases, often focal or weak (Mod Pathol 1998;11:656, Mod Pathol 2003;16:584)
- Vimentin (J Pathol 1987;152:253)
- CD56 (Am J Surg Pathol 2008;32:884)
- ER beta
- PR
- SMA (Am J Surg Pathol 2008;32:884)
- Reticulin: differentiates fibroma (individual pericellular reticulin staining pattern) from diffuse type of adult granulosa cell tumor (nested reticulin staining pattern)
Negative stains
- CD10 (Int J Gynecol Pathol 2007;26:359)
- Desmin
- Caldesmon
- CD99 (Am J Surg Pathol 2009;33:354)
- Calretinin: positive in 25% of cases, often focal or weak (Am J Surg Pathol 2009;33:354, Am J Surg Pathol 2011;35:484)
- S100 and CD34: occasionally positive (Am J Surg Pathol 2008;32:884)
Electron microscopy description
- Numerous thin, elongated cells with intercommunicating cytoplasmic processes admixed with abundant collagen fibrils (Cancer 1971;27:438)
- Fibrils appear grouped in bundles with a crossbanding pattern composed of a light band bound by two thin dark bands (recurring at intervals of 600 Å)
- Cells have indistinct cell borders and are compressed by collagenous stroma
- Elongated and thin nuclei with marked peripheral heterochromatin condensation
- Scant cytoplasm containing few mitochondria and scant endoplasmic reticulum
Molecular / cytogenetics description
- Trisomy or tetrasomy 12 (Ann Pathol 2001;21:393, Genes Chromosomes Cancer 1990;2:48, Gynecol Oncol 1990;38:28)
- Rarely, IDH1 mutations (Histopathology 2013;62:667)
- Loss of heterozygosity at 9q22.3 (PTCH1) and 19p13.3 (STK11) in cellular fibromas (Hum Pathol 2005;36:792)
- No or rare FOXL2 mutations (Am J Surg Pathol 2013;37:1450, Int J Gynecol Pathol 2018;37:305)
Sample pathology report
- Right ovary and fallopian tube, salpingo-oophorectomy:
- Ovary: fibroma
- Fallopian tube: unremarkable
Differential diagnosis
- Diffuse adult granulosa cell tumor (GCT):
- Typically see the other patterns of granulosa cell tumor
- Diffusely positive for inhibin and calretinin
- Nested reticulin staining pattern (Int J Gynecol Pathol 2018;37:305)
- FOXL2 mutations (Am J Surg Pathol 2011;35:484, N Engl J Med 2009;360:2719)
- Thecoma:
- May present with estrogenic manifestations, including endometrial carcinoma
- Diffuse or rarely, lobulated or nested growth
- Uniform tumor cells with abundant pale gray cytoplasm and indistinct cell membranes imparting a syncytial appearance
- Ovoid to round nuclei, with or without nucleoli or nuclear grooves
- None to minimal mitotic activity
- With or without hyaline plaques, calcifications, adipose metaplasia, small clusters of steroid type cells with eosinophilic to clear cytoplasm (Am J Surg Pathol 2014;38:1023)
- Rarely, scattered pleomorphic bizarre nuclei due to degenerative changes (Int J Gynecol Pathol 1983;1:325, Am J Surg Pathol 2014;38:1023)
- Usually positive for inhibin, calretinin and other sex cord markers
- Sclerosing stromal tumor:
- Often occurs in women in their third to fourth decades
- Alternating cellular and paucicellular areas with prominent staghorn vasculature
- Pseudolobulation and biphasic population of cells (luteinized and spindled)
- Usually positive for inhibin and calretinin
- Leiomyoma:
- Endometrial stromal tumor, low grade:
- Typical features of low grade endometrial stromal neoplasia, including permeative growth and arteriole-like vessels
- Diffusely positive for CD10
- With or without characteristic gene fusions such as JAZF1-SUZ12 (Orphanet J Rare Dis 2016;11:15)
- Metastatic gastrointestinal stromal tumor:
- Ovarian stromal hyperplasia:
- Bilateral
- Diffuse or multinodular proliferation of stromal cells
- Lacking collagenous stroma and hyaline plaques
- Massive edema:
- Unilateral or bilateral
- No well defined mass
- Proliferation of stromal cells with marked intercellular edema
- Entrapment of preexisting ovarian structures (follicles, corpora lutea or albicantia)
- Fibromatosis:
- Unilateral or bilateral
- No well defined mass
- Proliferation of stromal cells with abundant dense collagen
- Entrapment of preexisting ovarian structures
- Fibrosarcoma:
- Exceptionally rare and no longer considered a distinct WHO entity
- Hypercellular, composed of disorderly fascicles of spindle cells with scant cytoplasm and moderate to marked nuclear atypia, elevated mitotic activity including atypical forms (Cancer 1981;47:2663)
- Presence of ≥ 4 mitoses per 10 high power fields is not sufficient to diagnose fibrosarcoma in the absence of significant cytologic atypia (Am J Surg Pathol 2006;30:929)
- With or without necrosis and hemorrhage
- Focally positive for calretinin or inhibin, negative for CD10 (Am J Surg Pathol 2002;26:1477, Int J Gynecol Pathol 2007;26:359)
Additional references
Board review style question #1
Microscopic examination of an 8 cm unilateral ovarian mass demonstrates a densely cellular neoplasm composed of intersecting fascicles of spindle cells with mild cytologic atypia and approximately 9 mitotic figures per 10 high power fields. Reticulin staining shows pericellular staining pattern and molecular testing is negative for FOXL2 mutation.
What is the most likely diagnosis?
- Fibroma
- Fibrosarcoma
- Leiomyoma
- Leiomyosarcoma
- Mitotically active cellular fibroma
Board review style answer #1
Board review style question #2
You receive an intraoperative consultation for bilateral multinodular ovarian masses in a 16 year old patient. Microscopic examination demonstrates bland, monomorphic spindled cells within a collagenous stroma.
Mutation of which of the following genes is most likely associated with this entity?
Mutation of which of the following genes is most likely associated with this entity?
- FOXL2
- HMGA2
- JAZF1
- PTCH
- Vimentin
Board review style answer #2
D. PTCH. Bilateral ovarian fibroma in a young patient is most likely associated with Gorlin syndrome.
Comment Here
Reference: Fibroma
Comment Here
Reference: Fibroma
Fibromatosis and massive edema
Table of Contents
Definition / general | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosis | Additional referencesDefinition / general
- Tumor-like enlargement of ovary due to edema fluid
- First described in 1969 (Obstet Gynecol 1969;34:564)
- Patients present with pain, abdominal mass, menstrual irregularities, virilization, precocious puberty, Meigs syndrome (with ascites and pleural effusion, eMedicine: Meigs Syndrome)
- May be due to partial torsion of mesovarium leading to interference of venous / lymphatic drainage
- Usually young women
- Right sided involvement is more common
Gross description
- Marked ovarian enlargement, watery cut surface, no necrosis
Microscopic (histologic) description
- Marked edema of stroma surrounding follicles and clusters of luteinized cells
- Stroma around vessels and in superficial cortical zone is normal
- Variable stromal luteinization
Microscopic (histologic) images
Differential diagnosis
- Fibroma: circumscribed, tumor cells replace ovarian architecture
- Krukenberg tumor: usually bilateral, signet ring cells present
Additional references
Fibrosarcoma
Table of Contents
Definition / general | Epidemiology | Clinical features | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Differential diagnosisDefinition / general
- Very rare (More common in bone
Epidemiology
- Median age 49 years, range 20 - 73 years
Clinical features
- Pelvic pain, abdominal enlargement, awareness of abdominal mass (Cancer 1981;47:2663)
Laboratory
- CA125 and CEA in normal range
Radiology description
- MRI: solid mass (Anticancer Res 2007;27:4365)
Prognostic factors
- FIGO stage (BMC Cancer 2010;10:585)
Case reports
- 8 year old girl with nevoid basal cell carcinoma syndrome (Am J Surg Pathol 1984;8:231)
- 32 year old woman (Anticancer Res 2007;27:4365)
- 52 year old woman (Int J Gynecol Cancer 2005;15:1142)
Treatment
- Surgical resection with or without adjuvant chemotherapy or radiation
Gross description
- 5 - 23 cm solid mass, tan-yellow discoloration and partial necrosis (BMC Cancer 2010;10:585)
Microscopic (histologic) description
- Spindle cells arranged in sheets and intersecting fascicles creating a diffuse herringbone appearance
- Mild to moderate nuclear atypia
- Reticulin stain separates individual neoplastic cells
- Mitotic activity > 4/10 HPF is most important criteria for diagnosing fibrosarcoma
Cytology description
- Pleomorphic oval to spindle shaped cells with indistinct cell borders and atypical mitotic figures
Positive stains
Molecular / cytogenetics description
- Trisomy 8 present, may be helpful in distinguishing from cellular fibroma (Am J Surg Pathol 1997;21:52)
Differential diagnosis
- Cellular fibroma: mitotic activity Am J Surg Pathol 1997;21:52)
Follicle cyst
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Benign cyst measuring at least 3 cm and lined by an inner layer of granulosa cells with an outer layer of theca cells
Essential features
- Benign cyst lined by an inner layer of granulosa cells with an outer layer of theca cells
- Measures ≥ 3 cm, as opposed to cystic follicle, which measures < 3 cm
- Should be differentiated from cystic granulosa cell tumor
- May occur at any age, with a variable clinical presentation depending on age and etiology
Terminology
- Follicular cyst
ICD coding
Epidemiology
- Most common in nonpregnant women of reproductive age, especially near menarche or menopause
- May occur at any age, including neonates and children (Acta Paediatr Scand 1987;76:91, Int J Gynecol Pathol 1984;3:318)
- Affects 1 in 2,500 live female births (Obstet Gynecol Surv 1991;46:407)
- Relatively rare in postmenopausal women and may present with hyperestrogenism
- May be associated with McCune-Albright syndrome (Radiology 1988;168:817, Ann Endocrinol (Paris) 2016;77:7, Semin Pediatr Surg 2005;14:78)
Sites
- Ovary
Pathophysiology
- Physiologically:
- Ovarian follicle matures during proliferative phase of menstrual cycle, mature oocyte gets released due to luteinizing hormone (LH) surge at midcycle and follicle transforms to corpus luteum
- If no fertilization, corpus luteum atrophies and forms corpus albicans
- Follicle may become cystic via 2 mechanisms:
- Gonadotropin independent:
- McCune-Albright syndrome (N Engl J Med 1985;312:65)
- Primary hypothyroidism
- Isosexual pseudoprecocity
- Idiopathic central precocious puberty
- Gonadotropin dependent due to hypothalamic pituitary gonadal axis dysfunction:
- Central precocious puberty
- No luteinizing hormone (LH) surge
- Elevated follicle stimulating hormone (FSH)
- No ovulation
- Treatment with gonadotropin releasing hormone analogues, low dose phasic oral contraceptives and tamoxifen (Am J Obstet Gynecol 1987;156:1538, Fertil Steril 1990;53:1091, Gynecol Oncol 1999;72:202)
- Gonadotropin independent:
- Cyst usually disappears within 2 - 3 menstrual cycles but may persist
Clinical features
- Usually asymptomatic and incidental
- May form adnexal or pelvic mass
- Pelvic / abdominal pain, rarely hemoperitoneum, due to rupture or torsion (Am J Obstet Gynecol 1984;149:5)
- May be multiple or bilateral and present with symptoms related to hyperestrogenism (isosexual precocity, pseudoprecocity, menstrual disturbances including amenorrhea and postmenopausal bleeding, endometrial hyperplasia) when associated with McCune-Albright syndrome
- Rarely, central precocious puberty or isosexual precocity not related to McCune-Albright syndrome (Arch Dis Child 1999;81:53)
- Most cysts regress during the first 4 months of life but may undergo torsion, hemorrhage and rupture during the neonatal period or in utero
- May be associated with symptoms of primary hypothyroidism, including Van Wyk-Grumbach syndrome (juvenile hypothyroidism, precocious puberty with delayed bone age and ovarian cysts)
- May be associated with FSH secreting pituitary adenoma and sometimes precedes clinical presentation of adenoma (Int J Gynecol Pathol 2019;38:562)
- May be associated with ovarian remnant syndrome in 7% of cases (Acta Obstet Gynecol Scand 2012;91:965)
- May be associated with autoimmune oophoritis (Obstet Gynecol 1989;74:492)
Diagnosis
- Histologic examination of tissue
Radiology description
- Ultrasound examination (J Ultrasound Med 1988;7:597):
- Thin walled unilocular cyst measuring at least 3 cm
- Posterior acoustic enhancement
- Absence of internal echoes
- No color flow, nodules or any solid components
- Fluid debris level or internal echoes, if torsion
Prognostic factors
- Usually regress spontaneously (Contraception 2002;66:153, Hum Reprod 2000;15:2567)
- May undergo torsion requiring surgery
- May recur when associated with McCune-Albright syndrome
Case reports
- 6 year old girl with precocious pseudopuberty due to ovarian follicle cyst (BMC Res Notes 2013;6:319)
- 13 year old girl with giant ovarian follicle cyst (J Clin Diagn Res 2014;8:OD03)
- 36 year old woman with pituitary adenoma and recurrent ovarian follicle cysts (BMC Res Notes 2013;6:408)
- 47 year old woman with IgG4 related disease associated with ovarian follicle cyst (Am J Case Rep 2020;21:e926803)
Treatment
- Observation
- High dose, combined estrogen progestogen preparations
- LH releasing hormone agonists
- Excision if symptomatic or persistent (Arch Pediatr 1994;1:903)
- Cyst puncture if associated with isosexual pseudoprecocity
Gross description
- Usually solitary
- Ranging from at least 3 cm to up to 18.5 cm (Int J Gynecol Pathol 2021;40:359)
- Larger size during pregnancy and puerperium
- Thin walled cyst with smooth inner surface
- Usually unilocular
- No solid component
- Clear to straw colored fluid contents
- Serosanguinous or hemorrhagic fluid contents or clotted blood if torsion
- Multiple or bilateral if associated with McCune-Albright syndrome
Gross images
Frozen section description
- Benign cystic structure lined by an inner layer of granulosa cells with an outer layer of theca cells
- Either cell type may be luteinized
Microscopic (histologic) description
- Inner layer (1 to several) of granulosa cells with or without luteinization
- May be focally denuded
- Uniform round nuclei lacking grooves (Int J Gynecol Pathol 2021;40:359)
- Moderate amounts of eosinophilic cytoplasm
- Variable mitotic activity ranging from 1 - 36 per 10 high power fields (Int J Gynecol Pathol 2021;40:359)
- Sparse or absent reticulum on reticulin stain
- Outer layer of theca cells with or without luteinization
- Dense reticulum on reticulin stain
- Luteinized cells have eosinophilic to clear cytoplasm and round nuclei with central nucleoli
- Nonluteinized cysts are more common in patients with precocious puberty (Int J Gynecol Pathol 2021;40:359)
- Dystrophic calcifications in neonatal cysts (Int J Gynecol Pathol 2021;40:359)
- Multiple follicle cysts may be associated with eosinophil rich infiltrate (so called eosinophilic perifolliculitis) in autoimmune oophoritis (J Reprod Med 2006;51:141, Int J Gynecol Pathol 2021;40:359)
Microscopic (histologic) images
Positive stains
- Inhibin (J Clin Endocrinol Metab 1993;77:859)
- Calretinin
- SF1
- Immunohistochemistry usually not required for diagnosis
Negative stains
Sample pathology report
- Right ovary, cystectomy:
- Follicle cyst
Differential diagnosis
- Cystic adult type granulosa cell tumor:
- May be virilizing
- Usually larger than follicular cysts
- Multiple layers of granulosa cells
- Typical architectural patterns within cyst wall (Call-Exner bodies, trabecular, corded)
- With or without invagination of granulosa cells into cyst wall (Int J Gynecol Pathol 2021;40:359)
- Nuclear grooves (may be inconspicuous in luteinized forms)
- Extensive sampling may be required
- Cystic juvenile granulosa cell tumor:
- Pale to vacuolated cytoplasm
- Typical noncystic foci usually present
- Cystadenoma:
- Usually 1 layer of epithelial cells with serous, mucinous, clear cell or endometrioid morphology
- Negative for inhibin and calretinin, positive for EMA
- Endometriotic cyst:
- At least focally lined by endometrial-type epithelium
- Surrounded by endometrial stroma with or without hemorrhage or hemosiderin laden macrophages within wall
- Cystic follicle:
- Considered physiologic
- Morphology identical to follicle cyst but measuring < 3 cm
- Simple cyst:
- Denuded cyst without obvious lining or flattened lining
- No theca cells
- Large solitary luteinized follicle cyst (Am J Surg Pathol 1980;4:431):
- Occurs during pregnancy or puerperium
- Larger than follicular cyst (median size 25 cm)
- 1 to several layers of markedly luteinized granulosa cells and theca cells that are usually indistinguishable
- Variable nuclear atypia ranging from small round nuclei with single nucleolus to enlarged nuclei with focal marked pleomorphism, hyperchromasia and smudgy chromatin (degenerative)
- Absent or rare mitotic figures
- Hyperreactio luteinalis:
- Secondary to elevated human chorionic gonadotropin (hCG) levels due to gestational trophoblastic disease, fetal hydrops, multiple gestations, ovarian hyperstimulation syndrome
- Hyperandrogenism in 15%
- Bilateral, multiple, thin walled follicle cysts with distinct granulosa and theca cells
- More extensive luteinization in theca cells compared with granulosa cells
- Markedly edematous ovarian stroma and groups of luteinized stromal cells between cysts
- Corpora lutea in ovarian hyperstimulation syndrome
- Corpus luteum cyst:
- Undulating border and inner fibrous lining with a zone of small blood vessels with involution (Int J Gynecol Pathol 2021;40:359)
- Markedly luteinized polygonal shaped granulosa cells with abundant eosinophilic cytoplasm
- Cortical inclusion cyst:
- < 1 cm
- Lined by serous type ciliated epithelium or nonciliated nonmucinous flat epithelium
Additional references
Board review style question #1
What entities are included in the differential diagnosis of this cystic lesion of the ovary?
- Clear cell carcinoma, cystic follicle and cystic corpus luteum
- Corpus luteum cyst, endometriotic cyst and endometrioid borderline tumor
- Cystic adult type granulosa cell tumor, cystic follicle and corpus luteum cyst
- Endometriotic cyst, corpus luteum cyst and clear cell carcinoma
- Serous borderline tumor, cystic adult type granulosa cell tumor and cystic follicle
Board review style answer #1
C. Cystic adult type granulosa cell tumor, cystic follicle and corpus luteum cyst
Comment Here
Reference: Follicular cyst
Comment Here
Reference: Follicular cyst
Board review style question #2
What features would favor a cystic adult type granulosa cell tumor over a follicle cyst?
- Large size (> 10 cm), 1 - 2 layers of granulosa cells with readily identifiable mitotic figures and without invagination into cyst wall or nuclear grooves
- Large size (> 10 cm), markedly luteinized granulosa cell layer with readily identifiable mitotic figures
- Large size (> 10 cm), thick granulosa cell layer with multiple architectural patterns, invagination into cyst wall and nuclear grooves
- Small size (
- Small size (
Board review style answer #2
C. Large size (> 10 cm), thick granulosa cell layer with multiple architectural patterns, invagination into cyst wall and nuclear grooves
Comment Here
Reference: Follicular cyst
Comment Here
Reference: Follicular cyst
Gonadoblastoma
Table of Contents
Definition / general | Pathophysiology | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Differential diagnosisDefinition / general
- First described by Scully in 1953 (Cancer 1953;6:455)
- Also called dysgenetic gonadoma
- Mixture of germ cell tumor and sex-cord stromal tumor
- Usually occurs in individuals with abnormal sexual development and indeterminate gonads; usually gonadal dysgenesis with Y chromosome (i.e. XY gonadal dysgenesis, XO-XY mosaicism, not XX gonadal dysgenesis); 25% risk of neoplasia in these gonads
- Also present in phenotypically normal women, even during pregnancy, although ovary is never normal
- Y chromosome material appears to participate in gonadoblastoma tumorigenesis (Am J Clin Pathol 1997;108:197, Birth Defects Res C Embryo Today 2009;87:114)
- Associated with ataxia-telangiectasia
- 80% are phenotypic women, 20% are phenotypic men with undescended testicles and female internal secondary organs
- 50% have coexisting dysgerminoma
- Excellent prognosis if completely excised; almost never malignant
- Chromosomal analysis useful to diagnose androgen insensitivity / male pseudohermaphroditism (46,XY) and Turner syndrome (45,XO)
- Imaging:
- Imaging studies useful to diagnose intersexuality and identify patients at risk of developing gonadoblastoma
- Flat abdominal radiograph may reveal gonadal calcification, a classic pathologic finding in gonadoblastoma
Pathophysiology
- Gonadal differentiation starts after 5 weeks of gestation and depends on sex chromosome of fetus
- Errors in this complex multistep process of sexual differentiation may cause dysgenetic gonads
Case reports
- Two young patients with isochromosome 12p in dysgerminoma overgrowth component (Pathol Res Pract 2012;208:628)
- Patient with Denys-Drash syndrome and early presentation of bilateral gonadoblastoma (J Pediatr Endocrinol Metab 2013;26:971)
Treatment
- Pure Gonadoblastoma have excellent prognosis
- Local excision is adequate treatment if no frankly malignant are present
Gross description
- 36% bilateral, tumors usually small and may be microscopic
Gross images
Microscopic (histologic) description
- Primitive germ cells and sex cord stromal cells surrounded by ovarian type stroma
- Nests of dysgerminoma-like germ cells and sex cord derivatives resemble immature Sertoli and granulosa cells
- Arranged in nests surrounded by ovarian stroma containing Leydig or lutein type cells
- Hyalinization and calcification are common
- May be dysgerminoma if overgrowth of this component (Am J Clin Pathol 1997;108:197)
Positive stains
- Anti-Müllerian hormone focally (Hum Pathol 2000;31:1202)
- SALL4 and SF1 (Int J Gynecol Pathol 2013;32:379)
- Occasional c-KIT mutations (PLoS One 2012;7:e43952)
Differential diagnosis
- Incidental finding in ovaries of normal infants / children: associated with follicular cysts, microscopic foci resembling gonadoblastoma or sex cord tumor with annular tubules
Gonadoblastoma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Immunofluorescence description | Immunofluorescence images | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Molecular / cytogenetics description | Molecular / cytogenetics images | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Type of germ cell neoplasia in situ that consists of germ cell neoplasia in situ (GCNIS) / germinoma-like cells and incompletely differentiated sex cord cells
Essential features
- Type of germ cell neoplasia in situ
- Mostly in patients with disorders of sex development (DSD); the presence of the Y chromosome or parts thereof plays a significant role in the pathogenesis
- Rarely in patients with normal peripheral blood karyotype and no evidence of DSD; mostly females
- Classic gonadoblastoma consists of nests or cords of admixed germ cells and small sex cord stromal cells that surround basement membrane-like material, which can be calcified
- Bilateral in 40% of cases; bilateral gonadectomy is practically curative and eliminates the risk of progression to invasive germ cell tumors
Terminology
- Gonadoblastoma
ICD coding
- ICD-10: 9073/1 - gonadoblastoma
- ICD-11
- 2C73.Y & XH0K61 - other specified malignant neoplasms of the ovary & gonadoblastoma
- 2F76 & XH0K61 - neoplasms of uncertain behavior of female genital organs & gonadoblastoma
- 2F77 & XH0K61 - neoplasms of uncertain behavior of male genital organs & gonadoblastoma
Epidemiology
- Almost always in patients with disorders of sex development (DSD)
- Most common karyotypes at risk
- 46,XY complete gonadal dysgenesis
- 45,X / 46,XY partial gonadal dysgenesis (mosaicism)
- 46,XY DSD including partial gonadal dysgenesis and ovotesticular DSD
- Partial androgen insensitivity syndrome (Hum Pathol 2020;100:47, World J Pediatr 2009;5:93)
- May be associated with other chromosomal abnormalities and syndromes
- Turner syndrome with Y chromosomal material (could be cryptic), up to 35% of patients
- Germline or somatic mutations in genes involved in the XY developmental pathway
- WT1 gene: Frasier syndrome, Denys-Drash syndrome (DDS) (Clin Kidney J 2019;12:836)
- SRY gene: some cases of Swyer syndrome (complete gonadal dysgenesis and 46,XY)
- Other genes: SOX9, DHH, ARX, NR5A1 (SF1) and TSPYL1 (see Diagrams / tables) (Semin Diagn Pathol 2014;31:427)
- Infrequently cases have been reported in patients with normal peripheral blood karyotype and no evidence of DSD
- Mostly female (46,XX) and rarely male (46,XY)
- One case in a pregnant woman (J Pathol 2008;215:31)
- Age: majority < 15 years old (1 - 38 years)
- Case of fetus at 28 week gestational age with 46,XY karyotype has been described (Semin Diagn Pathol 2014;31:427)
Sites
- Gonads
Pathophysiology
- Vast majority of gonadoblastomas are associated with disorders of sex development (DSD) and dysgenetic gonads
- Gonadal dysgenesis: incomplete or defective formation of the gonads, due to disturbed migration of germ cells or proper organization in the fetal gonadal ridge
- Many causes: structural or numerical abnormalities of sex chromosomes or mutations in any of the genes involved in the process (J Clin Endocrinol Metab 2006;91:2404)
- Gonadal dysgenesis: incomplete or defective formation of the gonads, due to disturbed migration of germ cells or proper organization in the fetal gonadal ridge
- Formation and differentiation of the normal gonad is a complex process
- Bipotential gonad develops from the urogenital ridge close to the kidney and adrenals (genes like WT1, SF1, DAX1 are involved in the process)
- Germ cells from the yolk sac migrate to the gonads during the fifth week of pregnancy
- If SRY gene in Y chromosome is present
- SRY and SOX9 genes are expressed and initiate a cascade of genetic events; through a stringent time schedule and a balance of activating and inhibiting mechanisms, the gonad is gradually being differentiated into primitive testis with sex cords, first and later into more mature testis with seminiferous tubules
- If SRY gene is absent
- Activation of genes such as FOXL2 leads to ovarian differentiation with initiation of meiosis in the germ cells and formation of primordial follicles
- The earlier this process is interrupted / disturbed, the less the gonad can differentiate into ovary and testis
- Gradual continuum between well differentiated testis and well differentiated ovary
- Combinations of these patterns can be found within the same or opposite gonad of the patient
- Discrete patterns
- Normal testis (with maturation delay of germ cells)
- Dysgenetic testis
- Primitive sex cords containing germ cells
- Streak fibrous tissue (no germ cells) with remnants of testicular or ovarian differentiation
- Undifferentiated gonadal tissue (UGT)
- Normal ovary (World J Pediatr 2009;5:93)
- Suboptimal environments, such as dysgenetic gonads or deficient androgen effects, cause reduced SOX9 expression and increased FOXL2; thus, the germ cells delay their process of maturation and start expressing OCT3/4 (J Pathol 2008;215:31)
- Prolonged expression of OCT3/4, increased KIT / KITL signaling and strong expression of TSPY in the germ cells is hypothesized to be important in the pathogenesis of germ cell neoplasias (J Pathol 2008;216:43)
- TSPY gene is found in the gonadoblastoma susceptibility region on the Y chromosome (GBY region), around centromere of Y chromosome and is necessary for the development of gonadoblastoma
- If the gonad contains well differentiated testicular tissue and seminiferous tubules → carcinoma in situ (CIS)
- If the gonad has primitive sex cords or undifferentiated gonadal tissue → gonadoblastoma (J Pathol 2008;216:43)
- 60% of gonadoblastoma cases have an associated malignant germ cell tumor
- 80% of cases are dysgerminomas / germinomas, while the remaining cases include other tumor types like embryonal carcinoma, teratoma, yolk sac tumor or choriocarcinoma
- Some findings suggest that the other malignant tumors arise from dysgerminoma / germinoma (J Pathol 2008;215:31)
- Pathogenesis of gonadoblastoma is mainly unknown in patients with normal peripheral karyotype and no evidence of DSD (J Pathol 2008;215:31)
Etiology
Repetitive with Pathophysiology
Diagrams / tables
Clinical features
- 80% phenotypically female
- 50% develop in virilized females and 30% in nonvirilized females
- 20% in male patients, usually with hypospadias, cryptorchidism and gynecomastia (Cancer 1970;25:1340)
- Gonadoblastoma can be a steroid secreting tumor and may cause precocious puberty and virilization (Front Horm Res 2019;53:100)
- Most patients present in the neonatal period for ambiguous external genitalia; later in life for primary amenorrhea or investigation of abdominal mass (Semin Diagn Pathol 2014;31:427)
Diagnosis
- History, physical examination, imaging of internal reproductive organs and molecular / cytogenetic studies, including karyotype, fluorescence in situ hybridization (FISH) or polymerase chain reaction (PCR) for evidence of mosaicism and Y material, will aid in the correct diagnosis of DSD subcategory and estimate the risk for the development of germ cell tumor (Int J Mol Sci 2019;20:5017, World J Pediatr 2009;5:93)
- Definite diagnosis is made through the histologic examination of the specimen (biopsy or gonadectomy)
- Diagnostic criteria
- Essential
- Nested to corded arrangement of germ cells (similar to that seen in germinoma / dysgerminoma / seminoma) and small sex cord cells with deposits of basement membrane material
- Immunopositivity for OCT3/4 and PLAP
- Desirable
- Background of a DSD
- Germ cells positive for podoplanin
- Sex cord cells coexpressing SOX9 (weak) and FOXL2 (strong), inhibin, calretinin and SF1
- Essential
Laboratory
- Rise in lactate dehydrogenase (LDH), alpha fetoprotein (AFP), human chorionic gonadotropin (hCG) levels could indicate that a malignant germ cell tumor developed from a pre-existing gonadoblastoma
Radiology description
- Identification of dysgenetic gonads can be challenging due to variations in size and appearance
- Mixed results in studies comparing ultrasound versus magnetic resonance imaging (MRI) as the best imaging modality for identification (J Pediatr Urol 2018;14:154.e1, Semin Diagn Pathol 2014;31:427)
- Abdomen radiographs may identify pelvic calcifications, which could be a sign of gonadoblastoma but need to be differentiated from other causes (AJR Am J Roentgenol 1976;127:1001)
- No distinctive features to detect premalignant transformation (such as development of gonadoblastoma) (Semin Diagn Pathol 2014;31:427)
- Normal ultrasound or MRI does not rule out neoplasm in patients with gonadal dysgenesis (J Pediatr Urol 2018;14:154.e1)
- Surveillance in patients who want to defer gonadectomy can be challenging
Radiology images
Not relevant
Prognostic factors
- Gonadoblastoma is an in situ malignancy and simple excision is curative
- 5 year overall survival (OS) and recurrence free survival (RFS) > 96%, when gonadoblastoma identified during prophylactic gonadectomy (Hum Pathol 2020;100:47)
- If not excised, 60% progress to dysgerminoma / germinoma, 4% embryonal carcinoma or yolk sac tumor and 4% teratoma (Semin Diagn Pathol 2014;31:427)
- If invasive germ cell tumor develops, prognosis depends on the nature and the stage
Case reports
- 1 year old patient with 45,X / 46,XY mixed gonadal dysgenesis and ambiguous genitalia (Acta Med Litu 2022;29:194)
- 10 year old girl with abdominal distention and bilateral ovarian masses on imaging (Cureus 2020;12:e8990)
- 11 year old patient diagnosed with 45,X / 46,XY mosaicism presents for prophylactic bilateral gonadectomy (Ecancermedicalscience 2023;17:1613)
- 15 year old girl with Swyer syndrome presents for evaluation of amenorrhea (J Pediatr Adolesc Gynecol 2020;33:577)
- 21 year old woman with a history of primary amenorrhea presents with abdominal distention (Int Cancer Conf J 2022;11:114)
Treatment
- Simple excision is curative; bilateral gonadectomy is indicated due to the high risk for bilateral disease
- Malignant germ cell tumor such as dysgerminoma → treat accordingly
- Many patient advocacy groups support a more conservative approach whenever possible towards gonadectomy in DSD individuals (Hum Pathol 2020;100:47)
- Ideally, personalized recommendations should be given by an interdisciplinary team consisting of geneticists, endocrinologists, behavioral health specialists and urologists
- Multiple parameters to be considered: gender identity, need for endogenous sex hormone production, desire for fertility and risk of germ cell tumor
- If dysgenetic gonads are retained, proactive surveillance with imaging is insufficient
- Relocating the gonads to accessible areas like inguinal canal or adjacent to the biopsy laparoscopy port site has been suggested (Front Horm Res 2019;53:100)
Clinical images
Not relevant
Gross description
- Bilateral in 40% of cases (Semin Diagn Pathol 2014;31:427)
- Pure gonadoblastomas are rare and typically range from microscopic to usually < 8 cm (Hum Pathol 2020;100:47)
- If associated with germ cell tumors, they can grow significantly in size (Int Cancer Conf J 2022;11:114)
- Firm, tan-yellow to gray, with frequent gritty / granular cut surface (due to calcifications)
- Solid, fleshy, tan colored, lobulated adjacent lesion could represent the development of dysgerminoma (Semin Diagn Pathol 2014;31:427)
Frozen section description
- Unknown at this time
Frozen section images
Not available
Microscopic (histologic) description
- Features of classic gonadoblastoma (Hum Pathol 2020;100:47)
- Noninvasive neoplasm (germ neoplasia in situ) consisting of multiple nests or cords of admixed germ cells and small sex cord stromal cells that surround basement membrane-like material
- Germ cells
- Vary in appearance and number
- Some with round nuclei with prominent nucleoli and clear / pale cytoplasm (dysgerminoma / germinoma-like cells)
- Others with less cytoplasm, small to inconspicuous nucleoli (spermatogonia-like cells)
- Vary in appearance and number
- Sex cord stromal cells
- Bland with angulated, occasionally grooved nuclei and inconspicuous cytoplasm
- Can be arranged at the periphery of the nests (forming palisades), around the eosinophilic material deposits (Call-Exner-like pattern) or around germ cells (follicle-like pattern)
- They are considered incompletely differentiated rather than fully granulosa or Sertoli cells
- Eosinophilic basement membrane-like material
- Can become calcified
- Early calcifications have psammomatous appearance
- Later form larger aggregates, developing mulberry-like shape
- Involuted or burnt out gonadoblastoma: only characteristic calcifications remain due to regression of the cellular components
- Gonadal stroma
- Nonspecific spindle cell stroma with occasional Leydig-like cells, lacking Reinke crystals
- Infiltrative or dissecting gonadoblastoma (DGB)
- Term coined by Dr. Scully and further characterized by Kao et al. to describe unusual patterns of gonadoblastoma (Am J Surg Pathol 2016;40:1417)
- Cord-like pattern: germ cells embedded in cords or small nests of sex cord cells, which are irregularly distributed in the stroma
- Lack of basement membrane deposits and calcifications
- When cords are thin and sparsely distributed → similar picture to undifferentiated gonadal tissue (UGT)
- Anastomosing pattern: the nests are smaller and interconnected
- Solid expansile pattern: large nests often separated by fibrovascular septa, with reduced sex cord cells and deposits of basement membrane-like material that is present but hard to identify
- Cord-like pattern: germ cells embedded in cords or small nests of sex cord cells, which are irregularly distributed in the stroma
- Mixed patterns of dissecting gonadoblastomas are present in most (76%) gonadoblastomas and need to be differentiated from dysgerminoma (solid expansile pattern)
- The following pathogenetic model is hypothesized
- Cord-like / anastomosing DGB (UGT) → classic gonadoblastoma → solid expansile pattern → germinoma (Am J Surg Pathol 2016;40:1417)
- Term coined by Dr. Scully and further characterized by Kao et al. to describe unusual patterns of gonadoblastoma (Am J Surg Pathol 2016;40:1417)
- If not excised, 60% of gonadoblastomas will develop into germinoma and 4% will develop into other germ cell tumors like embryonal carcinoma or yolk sac tumor, etc. (Semin Diagn Pathol 2014;31:427)
Microscopic (histologic) images
Virtual slides
Cytology description
- Intraoperative imprint cytology (touch prep) can be utilized when extensive calcifications are present, hindering frozen sections
- Rare cases of dysgerminoma arising in gonadoblastoma with cytology description in the literature (Diagn Cytopathol 2011;39:42, Diagn Cytopathol 2019;47:1203)
- Gonadoblastoma
- Oval or round clusters with microcystic spaces
- Hyaline globules of metachromatic basement membrane-like material in the spaces, magenta stained; the globules are round and homogenous
- 2 cell types
- Small cells with scant cytoplasm, round to oval nuclei and fine chromatin
- Intermediate cells with elongated nuclei, fine chromatin and occasional grooves
- Calciferous particles may be present in the background
- Dysgerminoma
- Classic features of atypical cells with vesicular nuclei with 1 or 2 nucleoli and pale / vacuolated cytoplasm and admixed lymphocytes
- Gonadoblastoma
- Rare cases of dysgerminoma arising in gonadoblastoma with cytology description in the literature (Diagn Cytopathol 2011;39:42, Diagn Cytopathol 2019;47:1203)
Cytology images
Not available
Immunofluorescence description
Not relevant
Immunofluorescence images
Not relevant
Positive stains
- Germ cells: OCT3/4, PLAP, CD117, podoplanin
- Mature germ cells express TSPY1 (testis specific protein, Y linked 1)
- Immature germ cells express OCT3/4
- Small subpopulation express both (Hum Pathol 2020;100:47)
- Sex cord cells
- Inhibin, cytokeratin, calretinin, FOXL2 (strong), SF1 (Int Cancer Conf J 2022;11:114, Adv Anat Pathol 2021;28:258)
- Müllerian inhibiting substance (MIS) (Adv Anat Pathol 2021;28:258)
- SOX9 (weak to moderate staining) (Hum Pathol 2020;100:47)
- Variable staining for calretinin and WT1
- Leydig-like cells in the stroma: inhibin, calretinin
Negative stains
No pertinent negative stains found in the literature
Electron microscopy description
Not available
Electron microscopy images
Not available
Molecular / cytogenetics description
- Genetic testing plays a major role in the evaluation of patients with suspected DSD
- Peripheral blood karyotype → detect X and Y chromosomes and other chromosomal abnormalities
- FISH analysis with specific X and Y centromere specific probes → assess mosaicism (Best Pract Res Clin Obstet Gynaecol 2018;48:90)
- PCR analysis → identified cryptic Y chromosomal material (Best Pract Res Clin Obstet Gynaecol 2018;48:90)
- DSD patients who carry Y chromosomal DNA segments, especially when the critical gonadoblastoma locus is present, have an increased risk for germ cell neoplasia (Horm Cancer 2017;8:166)
Molecular / cytogenetics images
Videos
Case of gonadoblastoma and Swyer syndrome
Sample pathology report
- Left gonad, left gonadectomy:
- Gonadoblastoma (3 cm)
- No invasive germ cell tumor identified (see comment)
- Comment: The whole specimen (extensively) submitted for microscopic examination.
- Gonadoblastoma is a type of germ cell neoplasia in situ that is considered high risk for progression to invasive germ cell tumor. In 40% of cases, it is bilateral. According to the AJCC 8th edition and UICC system, gonadoblastoma is staged as pTis.
Differential diagnosis
- Sex cord tumor with annular tubules (SCTAT):
- Lacks germ cells
- Not associated with DSD
- Syndromic association with Peutz-Jeghers syndrome (PJS)
- Mixed germ cell - sex cord stromal tumor (MGC SCST):
- Diffuse growth pattern instead of nests
- Basement membrane-like material and calcifications are rare
- Only one type of germ cell; typically benign in testis and malignant in ovaries
- Sertoli cell nodule with intratubular germ cell neoplasia unclassified (IGCNU):
- Phenotypically normal male
- Expanded tubules in nested arrangement, containing immature sex cord cells, IGCNU cells distributed in patchy pattern, round deposits of eosinophilic matrix and characterized by thickened basement membrane
- Sex cord cells have Sertoli differentiation; SOX9 and SF1 diffuse and uniformly strong, FOXL2 consistent negative (Histopathology 1986;10:909)
- Dysgerminoma / germinoma:
- Mimicker of DGB patterns, especially cord-like and solid expansile pattern
- No heterogeneity in the germ cell population; OCT3/4 will be diffusely positive
- No sex cord cell population; inhibin and SF1 can be helpful to exclude the presence of sex cord cells
- No basement membrane-like material deposition
- Granulomas are more frequent; rare in gonadoblastomas
- Gonadoblastoma-like structures in normal fetus or infants:
- Often found in association with follicular cysts (Histopathology 1986;10:909)
- Fetal gonadoblastoid testicular dysplasia:
- Epithelial nodules with spherical eosinophilic globules
- Associated with Walker-Warburg syndrome; not seen in gonadal dysgenesis associated with DSD
- Only rare PLAP positive cells (Pediatr Dev Pathol 2019;22:380, Pediatr Dev Pathol 2007;10:274)
Additional references
Board review style question #1
A newborn is referred to the pediatric endocrinology clinic due to ambiguous genitalia noted at birth. A karyotype reveals a 46,XY genotype. Ultrasound is performed and identifies bilateral gonadal masses in the inguinal canal. Excision of the masses and histopathologic examination reveals the presence of the lesion depicted. What is the diagnosis?
- Adrenal cortical rest
- Dysgerminoma
- Gonadoblastoma
- Sex cord tumor with annular tubules (SCTAT)
Board review style answer #1
C. Gonadoblastoma. The picture shows the classic features of gonadoblastoma i.e. nests of germ cells admixed with sex cord cells, partially differentiated and eosinophilic material deposits. Calcifications are a common feature of gonadoblastoma.
Answer D is incorrect because SCTAT shows a population of sex cord cells and does not have germ cells (large cells with clear cytoplasm and prominent nucleoli).
Answer B is incorrect because although dysgerminoma can arise from gonadoblastoma in dysgenetic gonads, there is no evidence of invasion in the lesion.
Answer A is incorrect because adrenal cortical rests refer to circumscribed nodules of (ectopic) adrenal cortical tissue, frequently found in the paratesticular tissue. However, the morphology is entirely different from the depicted lesion.
Comment Here
Reference: Gonadoblastoma
Comment Here
Reference: Gonadoblastoma
Board review style question #2
Which of the following statements is true regarding this gonadal lesion?
- Gonadoblastoma is a malignant tumor that commonly metastasizes to regional lymph nodes
- Gonadoblastoma typically occurs in individuals with a 46,XY karyotype and dysgenetic gonads
- It consists of a monotonous population of sex cord cells
- Surgical excision is not recommended due to its benign nature
Board review style answer #2
B. Gonadoblastoma typically occurs in individuals with a 46,XY karyotype and dysgenetic gonads. Gonadoblastoma typically presents in patients with disorders of sex development, such as individuals with 46,XY karyotype and dysgenetic gonads.
Answer A is incorrect because gonadoblastoma is a type of germ cell neoplasia in situ and thus cannot metastasize. If not excised, up to 60% of gonadoblastoma can give rise to malignant germ cell tumors, most frequently dysgerminoma.
Answer D is incorrect because gonadoblastoma is a type of carcinoma in situ. Bilateral gonadectomy is often suggested, based on the risk of developing malignant germ cell tumors.
Answer C is incorrect because gonadoblastoma consists of a mixture of germ cells and sex cord cells, not fully differentiated, having features of granulosa / Sertoli cells.
Comment Here
Reference: Gonadoblastoma
Comment Here
Reference: Gonadoblastoma
Granulomatous inflammation
Table of Contents
Definition / general | Epidemiology | Etiology | Clinical features | Laboratory | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Additional referencesDefinition / general
- Necrotizing or nonnecrotizing granulomatous inflammation of the ovary
Epidemiology
- Cortical granulomas are common incidental findings with uncertain clinical significance
- Primary ovarian tuberculosis and other primary ovarian granulomas are uncommon
- Frequency of secondary ovarian granulomas depends on primary cause
Etiology
- Tuberculosis: hematogenous spread to ovary or secondary to tuberculous salpingitis (Arch Gynecol Obstet 2008;278:359); may clinically resemble malignancy (Arch Gynecol Obstet 2010;282:643)
- Actinomyces: associated with IUD (Am J Clin Pathol 1977;68:622)
- Ascaris lumbricoides: J Coll Physicians Surg Pak 2009;19:663
- Bowel contents via colo-ovarian fistula: Obstet Gynecol 1987;69:533
- Crohn disease: usually extension from bowel (Am J Obstet Gynecol 1975;122:527)
- Dysgerminoma: granulomatous reaction
- Enterobius vermicularis: Eur J Gynaecol Oncol 2007;28:513
- Foreign material: often postsurgical, including suture, talc, starch, lubricant and carbon (Obstet Gynecol 1985;66:701, Hum Pathol 1996;27:1008)
- Keratin from cystic teratomas and squamous elements of endometrial and ovarian endometrioid adenocarcinomas
- Malakoplakia: Obstet Gynecol 1987;69:537
- Necrobiotic (palisading) granuloma: often with history of surgery or cauterization months to years previous
- Sarcoidosis: rare (Am J Obstet Gynecol 1990;162:1284)
- Schistosomiasis
Clinical features
- Often an incidental finding or part of a systemic granulomatous disorder
Laboratory
- May be associated with elevated CA 125, raising concern for cancer (Rom J Intern Med 2009;47:297)
Case reports
- Due to microfibrillar collagen hemostat (avitene) used to control bleeding (Arch Pathol Lab Med 1995;119:1161)
Treatment
- In secondary cases, treat primary cause
Gross description
- May present as a mass and simulate a tumor
Microscopic (histologic) description
- Tuberculosis is typically confined to the cortex
Microscopic (histologic) images
Positive stains
- AFB is typically positive in tuberculous oophoritis
Additional references
Granulosa cell tumor-adult
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Positive stains | Negative stains | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Low grade indolent malignant neoplasm originating from granulosa cells of the ovarian follicles, accounting for approximately 10% of all sex cord stromal tumors of the ovary
Essential features
- Low grade indolent sex cord stromal tumor of the ovary with 20 - 30% chance of local recurrence, 5 - 20 years after diagnosis
- Should be considered in the differential diagnosis of solid / cystic and hemorrhagic ovarian mass in postmenopausal patients
- FOXL2 mutation by immunohistochemistry or sequencing identified in > 95% (N Engl J Med 2009;360:2719)
- Immunohistochemical panel could include inhibin, calretinin, FOXL2, SF1, EMA and reticulin special stain
ICD coding
Epidemiology
- Accounts for 2% of all ovarian tumors and is the second most common ovarian sex cord stromal tumors after fibroma / thecomas
- Most granulosa cell tumors are adult type (95%) and 5% are juvenile type
- Wide age range; most common in postmenopausal women with peak age 50 - 55
Sites
- Ovary, often unilateral
Pathophysiology
- Presumed to originate from the granulosa cells of the ovarian follicle; the histogenesis is unknown
- Dimerization of mutant FOXL2 protein (402C → G, C134W) causes negative dominant effect, dysregulates gene expression and plays a major role in tumor pathogenesis (Endocrinology 2011;152:3917)
Clinical features
- Mostly present with symptoms due to adnexal mass or endocrine manifestations
- 10% present with ovarian torsion, tumor rupture and hemoperitoneum
- Most associated with hyperestrogenism, causing metrorrhagia, postmenopausal bleeding, endometrial hyperplasia / carcinoma (5%, usually FIGO grade 1 and superficial) in adults and if in children, associated with precocious puberty
- Rarely associated with hyperandrogenism presenting with virilization or hirsutism
- 10 year survival > 90%; tends to recur locally in the abdomen / pelvis in 20 - 30% of cases, usually 5 years after diagnosis and up to 20 years later
- Most are confined to the ovary (stage I); rarely distant metastasis to lung and liver
- Lymph node metastasis uncommon
Diagnosis
- Surgical specimen: gross, frozen and permanent microscopic examination
- Peritoneal cytology
Laboratory
- Hyperestrogenism
- Hyperandrogenism (rare)
Radiology description
- Large unilateral solid and cystic ovarian mass, with or without torsion / rupture and associated hemoperitoneum
Prognostic factors
- Stage (most important) and tumor rupture
- Large size (> 15 cm) and bilaterality are considered unfavorable prognostic factors; size does not show significant difference if corrected for stage
- No definitive correlation between mitotic activity or nuclear atypia and clinical outcome
- Homozygous FOXL2 mutation and chromosomal imbalance are associated with early relapse and worse outcome (Oncotarget 2020;11:419)
- Higher FOXL2 mRNA expression associated with worse outcome (Mod Pathol 2011;24:1360)
- Higher levels of SMAD3 expression are associated with recurrence in early stage disease (Int J Gynecol Pathol 2017;36:240)
- TERT promoter mutation (C228T) identified in 22% of primary and 41% of recurrent adult GCT (Mod Pathol 2018;31:1107)
Case reports
- 31 year old woman with hypertension and amenorrhea (AACE Clin Case Rep 2018;5:e168)
- 44 year old woman with luteinized adult type granulosa cell tumor and melanin pigment (Int J Gynecol Pathol 2019;38:92)
- 66 year old woman with mixed ovarian tumor composed of Brenner tumor and adult type granulosa cell tumor (Int J Surg Pathol 2018;26:382)
- 67 year old woman with aggressive adult granulosa cell tumor of the ovary without a FOXL2 mutation (J Obstet Gynaecol Res 2019;45:1404)
- 78 year old man with testicular adult type granulosa cell tumor and sarcomatous overgrowth (Diagn Pathol 2014;9:107)
Treatment
- Surgical excision is the main treatment
- Hormone modulator therapy, chemotherapy, radiation therapy, novel targeted treatments can be used in multiply recurrent or metastatic patients
Gross description
- > 95% unilateral and confined to the ovary
- Variable size, average 10 - 12 cm
- Encapsulated with smooth lobulated surface, tan or yellow (depending on the degree of luteinization and lipid content), soft to firm (depending on the amount of fibromatous component), usually solid and cystic with straw colored or mucoid fluid, can have areas of necrosis and hemorrhage
- The more luteinized tumors are more yellow / orange
- May resemble serous cystadenoma
- Rare androgenic tumors tend to be large with thin walled cysts (Arch Pathol Lab Med 1984;108:786)
Frozen section description
- May be mistaken for endometrioid adenocarcinoma on frozen section
- Characteristic nuclear features can be helpful in diagnosis
Microscopic (histologic) description
- Small, bland, cuboidal to polygonal cells with scant cytoplasm and pale, uniform angulated and usually grooved nuclei (coffee bean)
- Various patterns, including diffuse (the most common), trabecular and corded, insular, microfollicular (resembling Call-Exner bodies of the Graafian follicles: small follicle-like structures filled with eosinophilic material) and macrofollicular (the least common)
- Usually a mixed growth pattern is seen
- Rarely can be seen with juvenile type; classification should be based on the predominant histology
- Luteinized adult type (such as during pregnancy): rare (1%) if extensive (> 50%), plump cells with moderate to abundant eosinophilic cytoplasm, conspicuous nucleoli, no nuclear grooves, myxoid or edematous stroma; may resemble steroid cell tumor
- Mitotic activity is usually not brisk (Stroma is usually hypervascular with variable amounts of fibroblasts and theca cells
- Theca cell proliferation is considered a stromal response rather than a second population of tumor cells (granulosa - theca cell tumor)
- Can have a prominent fibrothecomatous stroma; need 10% granulosa cells to be classified as adult granulosa cell tumor, otherwise best classified as thecoma or fibroma with minor sex cord elements
- Rarely show focal hepatic cell differentiation (large cells with abundant eosinophilic, slightly granular cytoplasm); HepPar1+, inhibin- (Am J Surg Pathol 1999;23:1089)
- Rarely (2%) show focal or multifocal areas of atypical cells with enlarged hyperchromatic bizarre, some multinucleated, suggestive of degenerative change and not associated with adverse outcome (Int J Gynecol Pathol 1983;1:325)
- Predominantly cystic granulosa cell tumor or macrofollicular pattern may mimic ovarian follicle
- Small aggregates of nonneoplastic granulosa cells can be seen within vascular spaces adjacent to the follicles: probably a surgery related artifact
Microscopic (histologic) images
Contributed by Shabnam Zarei, M.D. and Sharon Bihlmeyer, M.D.
AFIP images
Cytology description
- Scant cytoplasm, naked nuclei, relatively uniform mildly convoluted nuclei with grooves, vacuolated cytoplasm (Diagn Cytopathol 2008;36:297)
Positive stains
- FOXL2 immunostain is a sensitive (80%) and specific (99%) marker for sex cord stromal tumors (SCST), superior to α inhibin and calretinin and is positive in almost all SCST (98%) with FOXL2 mutation and a large number of those without mutation (67%); majority of adult GCTs (93%) are positive with FOXL2 stain and the immunostain cannot differentiate adult GCT from other SCSTs (Am J Surg Pathol 2011;35:484)
- SF1: most sensitive marker for this as well as most common sex cord stromal tumors (Am J Surg Pathol 2009;33:354)
- Inhibin A: more specific marker
- Calretinin (Am J Surg Pathol 2002;26:1477)
- Reticulin: shows lack of pericellular staining and highlights nests or large groups of granulosa cells and vessels, helps differentiate diffuse pattern from fibrothecoma (Int J Gynecol Pathol 2019;38:143)
- Low molecular weight cytokeratin (CAM5.2, AE1 / AE3): 30 - 60%, usually dot-like or globoid perinuclear pattern (Hum Pathol 1994;25:60, Am J Surg Pathol 1992;16:962)
- S100: 50% (Hum Pathol 1994;25:60)
- CD99: 70% (Histopathology 1995;27:388, Mod Pathol 1998;11:769)
- EGFR: 65% of primary and 85% of recurrent (Gynecol Oncol 2006;101:18)
- Vimentin, WT1, CD56 and SMA
Molecular / cytogenetics description
- FOXL2 402C → G (C134W) somatic mutation is highly sensitive and specific for diagnosis of adult GCT (N Engl J Med 2009;360:2719, PLoS One 2009;4:e7988, Am J Surg Pathol 2011;35:484)
- More than half of cases show chromosomal imbalances (Gynecol Oncol 2005;97:68)
- Most common chromosomal abnormalities are trisomy 12, trisomy 14, monosomy 16 / 16q deletion and monosomy 22 (Gynecol Oncol 2005;97:68)
Videos
Sex cord stromal tumors of the ovary and mimics
Histopathology of adult granulosa cell tumor
Granulosa cell tumor
Sample pathology report
- Left ovary and fallopian tube, left salpingo-oophorectomy:
- Granulosa cell tumor, adult type (see synoptic report)
Differential diagnosis
- Cellular fibroma or cellular thecoma versus diffuse pattern:
- Steroid cell tumor versus extensively luteinized pattern:
- Juvenile granulosa cell tumor (juvenile GCT) versus luteinized type:
- Both with moderate abundant pale cytoplasm, rounded nuclei and no nuclear grooves
- Has follicles with irregular size and shape, no Call-Exner bodies, brisk mitotic activity and can have areas of higher grade atypia
- 90% FOXL2 mutation negative (PLoS One 2009;4:e7988)
- Endometrioid carcinoma (FIGO grade I and those resembling sex cord stromal tumor) versus microfollicular pattern:
- Usually have areas of typical endometrioid carcinoma with mucin producing glands or squamous differentiation
- Cytokeratin+, EMA+, calretinin-, inhibin-
- Carcinoid tumor versus microfollicular and insular pattern:
- Similar insular and nested growth pattern
- May be seen co-existing with teratoma
- Classic salt and pepper chromatin pattern
- Synaptophysin+, chromogranin+
- Sex cord tumors with annular tubules (SCTATs) versus microfollicular pattern:
- Have ring shaped simple and complex tubules, if bilateral and microscopic often associated with Peutz-Jeghers syndrome
- Usually have hyaline deposits larger than Call-Exner bodies and often calcified
- Poorly differentiated ovarian surface epithelial carcinoma versus diffuse pattern:
- Higher grade cytological atypia
- EMA, HMWCK diffusely positive (Virchows Arch A Pathol Anat Histopathol 1989;414:439)
- Endometrial stromal sarcoma, low grade versus diffuse pattern:
- Metastatic breast carcinoma (lobular) versus diffuse pattern:
- Metastatic melanoma versus diffuse pattern:
- Pregnancy related granulosa cell proliferation versus macrofollicular pattern:
- Usually incidental, microscopic, multifocal, lies within atretic follicles surrounded by a thick layer of luteinized theca cells (Hum Pathol 1988;19:657)
Additional references
Board review style question #1
A 59 year old woman underwent abdominal hysterectomy and salpingo-oophorectomy for ovarian torsion and endometrial atypical hyperplasia. You receive a 10 cm hemorrhagic cystic ovarian mass for intraoperative consultation. Frozen section is shown above. What is the most common mutation identified in this ovarian tumor?
- CDH1
- FOXL2
- P53
- STK11
Board review style answer #1
B. The FOXL2 somatic mutation has been identified in 94 - 97% of adult granulosa cell tumors and is thought to play an essential role in the pathogenesis of the disease.
Comment Here
Reference: Granulosa cell tumor - adult
Comment Here
Reference: Granulosa cell tumor - adult
Board review style question #2
Which of the following is associated with worse outcome in adult granulosa cell tumor?
- Cytologic atypia
- FOXL2 heterozygous mutation
- Tumor necrosis
- Tumor rupture
Board review style answer #2
D. The tumor rupture and disease stage are associated with adverse outcome in adult type granulosa cell tumor.
Comment Here
Reference: Granulosa cell tumor - adult
Comment Here
Reference: Granulosa cell tumor - adult
Granulosa cell tumor-juvenile
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Sex cord stromal tumor composed of primitive appearing granulosa cells with follicular and solid growth patterns
Essential features
- Sex cord stromal tumor with primitive granulosa cell differentiation
- Solid and follicular growth
- Almost always occurs in patients younger than 30 years
- Lacks the FOXL2 somatic mutation seen in adult granulosa cell tumor
- Usually stage IA and associated with a favorable prognosis
ICD coding
- ICD-O: 8622/1 - granulosa cell tumor, juvenile
- ICD-10:
- ICD-11: 2F76 & XH2KH2 - granulosa cell tumor, juvenile
Epidemiology
- Mean age 13 years; range 0 - 67 years
- 80% occur before age 20 and 97% before age 30 (Am J Surg Pathol 1984;8:575)
Sites
- Ovary
- Rarely extraovarian (Pediatr Hematol Oncol 2021;38:272)
Pathophysiology
- Unknown
Etiology
- Unknown
Clinical features
- Most prepubertal patients present with sexual precocity due to excessive estrogen production and rarely produce androgens; older patients have nonspecific abdominal swelling and pain (J Endocrinol Invest 2006;29:653, Am J Surg Pathol 1984;8:575)
- Rarely associated with enchondromatosis (Ollier disease), Maffucci syndrome, abnormal karyotype / ambiguous genitalia (Am J Surg Pathol 1984;8:575, Am J Surg Pathol 1985;9:737)
Diagnosis
- Diagnosis is made at the time of removal of an adnexal mass
- Possibility of juvenile granulosa cell tumor may be suspected if there are estrogenic manifestations in a prepubertal patient but histopathological examination is required for diagnosis
Radiology description
- Typically appears on imaging as a large, unilateral, multicystic mass with occasional septae; most contain both solid and cystic components
- Can have a sponge-like appearance on imaging (Radiographics 2014;34:2039)
Prognostic factors
- Of low malignant potential, with a very favorable prognosis for patients with stage IA tumors (which account for a large majority of cases) but a guarded prognosis if there is spread beyond the ovary
Case reports
- Fetus with an androgenic ovarian mass in the third trimester (J Obstet Gynaecol Res 2021;47:2220)
- 2 year old girl with precocious pseudopuberty (J Clin Res Pediatr Endocrinol 2021 Apr 14 [Epub ahead of print])
- 17 year old girl with gender dysphoria (Case Rep Oncol 2020;13:1330)
- 33 year old woman with adnexal mass and Maffucci syndrome (Clin Imaging 2019;56:77)
- 40 year old Japanese woman with late onset juvenile granulosa cell tumor (Acta Med Okayama 2020;74:159)
Treatment
- Large majority of tumors are stage IA and surgical removal, with preservation of fertility if desired, is all that is required for treatment (Acta Obstet Gynecol Scand 2021 May 24 [Epub ahead of print])
- With recurrent or advanced stage tumors, platinum based chemotherapy is typically offered (Acta Obstet Gynecol Scand 2021 May 24 [Epub ahead of print])
Gross description
- Usually unilateral with a smooth surface
- Mean size 12.5 cm (Am J Surg Pathol 1984;8:575)
- Multiloculated, cystic and solid tumor with yellow-white solid areas
- May have hemorrhage and necrosis
Gross images
Frozen section description
- Smooth surfaced ovarian mass in a young patient
- Presence of follicular differentiation is helpful but definitive diagnosis may have to be deferred to permanent sections
Microscopic (histologic) description
- Diffuse or nodular appearance at low power
- Macrofollicle and microfollicle formation containing eosinophilic secretions
- Tumor cells are often luteinized
- Round / oval hyperchromatic nuclei with small nucleoli, irregular nuclear contours
- No / rare nuclear grooves; high mitotic rate (mean 11/10 high power fields) (Arch Pathol Lab Med 1989;113:40)
- Bizarre / atypical nuclei are rarely present
- May have pseudopapillary architecture (Am J Surg Pathol 2008;32:581)
Microscopic (histologic) images
Positive stains
Negative stains
Molecular / cytogenetics description
- Most have AKT1 mutations (EBioMedicine 2015;2:421)
- GNAS mutations reported in 30% of cases (J Clin Endocrinol Metab 2006;91:1842)
- Consistently negative for FOXL2 (402C > G) mutation, while 5 - 10% have a mutation in DICER1; the DICER1 mutations may be germline, i.e. associated with DICER1 syndrome (Am J Surg Pathol 2021;45:223)
- Somatic mutations in IDH1 or IDH2 are associated with Ollier disease or Maffucci syndrome; juvenile granulosa cell tumor is an uncommon manifestation of both diseases (Am J Med Genet A 2020;182:1093)
Sample pathology report
- Right ovary, oophorectomy:
- Juvenile granulosa cell tumor, negative for ovarian surface involvement or extraovarian spread
Differential diagnosis
- Adult granulosa cell tumor:
- Older age at presentation (but there is some overlap in the age ranges)
- Small uniform cells resembling normal granulosa cells
- FOXL2 mutation in 95% of cases (N Engl J Med 2009;360:2719)
- Small cell carcinoma, hypercalcemic type:
- More primitive appearing cells, with scant cytoplasm or rhabdoid cells
- Loss of BRG1 expression (protein encoded by SMARCA4) (J Pathol 2016;238:389)
- Mutation in SMARCA4 (Nat Genet 2014;46:427)
- Sertoli-Leydig cell tumor:
- Tubules (in well differentiated tumors), cords (intermediate differentiation), sarcomatoid growth (poorly differentiated) or retiform architecture
- Follicle-like spaces occasionally seen admixed with typical sertoliform differentiation (Am J Surg Pathol 2021;45:59)
- Gynandroblastoma:
- Typical Sertoli-Leydig cell tumor component coexisting with separate typical juvenile granulosa cell tumor (Am J Surg Pathol 2021;45:59)
- Yolk sac tumor:
- Clear cell carcinoma:
Additional references
Board review style question #1
A 2 year old girl presents with a unilateral ovarian tumor. Histology shows follicle-like spaces lined by cells with moderate amounts of cytoplasm and showing nuclear atypia and prominent nucleoli. What is the diagnosis?
- Juvenile granulosa cell tumor
- Sertoli-Leydig cell tumor
- Teratoma
- Yolk sac tumor
Board review style answer #1
Gynandroblastoma
Definition / general
- Sex cord stromal tumor with equal numbers of granulosa-theca cells and Sertoli-Leydig cells
- Very rare; variable hormones
Case reports
- 24 year old woman with polycystic ovarian syndrome (Arch Pathol Lab Med 2006;130:225)
High grade serous carcinoma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Epithelial carcinoma of serous cell lineage with papillary, glandular and solid growth patterns and high grade cytologic atypia
Essential features
- Often bilateral, solid and cystic ovarian masses
- Solid, pseudoendometrioid, transitional cell carcinoma-like (SET) appearance more common in BRCA1 mutations; need to refer to genetic testing
- 3 patterns of aberrant p53 expression correlate with TP53 mutation
- Most important prognostic factor is stage, ~40% overall 5 year survival
Terminology
- High grade serous adenocarcinoma
ICD coding
- ICD-O: 8461/3 - malignant epithelial serous tumors, high grade serous carcinoma
Epidemiology
- Accounts for majority of ovarian carcinoma diagnoses and related deaths
- Affects women disproportionately in western nations
- Increased risk in Caucasian as compared with African American women
- Decreased risk with several menstrual and reproductive factors:
- Parity
- Later age at menarche
- Earlier age of menopause
- Oral contraceptive use
- Increased risk with estrogen only hormonal therapy, combined estrogen and progestin regimens
Sites
- Tubo-ovarian, peritoneum
Pathophysiology
- Hereditary predisposition in 15 - 20% of cases involving BRCA genes (Am J Surg Pathol 2016;40:404):
- BRCA1 germline mutations cause 50% lifetime risk of ovarian cancer at average age of ~50 years
- BRCA2 germline mutations causes lower lifetime risk (10 - 35%) at a later age (average ~55 years)
- Associated with homologous recombination defects
- Hereditary predisposition in 6% of women with Fanconi anemia
- A significant proportion arise in the fallopian tube and spread to the ovaries and peritoneum
Clinical features
- Mean age: 63 years
- Abdominal distension / bloating / pain
- Nausea
- Anorexia / early satiety
- Back pain
- Urinary incontinence
- Advanced disease at presentation in ~80% of cases
Diagnosis
- CT with contrast is the preferred imaging modality
- Diagnosis is by surgical resection and histologic examination of resected tissue - may be associated with serous intraepithelial carcinoma (STIC) in fallopian tube (Histopathology 2014;65:149, Int J Gynecol Pathol 2017;36:230)
Laboratory
- Elevated serum CA-125 level
Radiology description
- Unilocular or multilocular, often bilateral, cystic and solid ovarian masses
- Papillary projections identifiable
- Peritoneal implantations and lymphadenopathy visible
Prognostic factors
- Most important: stage, ~40% overall 5 year survival
- In advanced stage disease, the amount of residual tumor present after staging and debulking is the most important factor
- Low Ki67 proliferation index associated with platinum resistance and decreased survival
- CD3+ or CD8+ tumor infiltrating lymphocytes confer favorable prognosis
Case reports
- 13 year old girl and 69 year old woman with normal sized ovarian carcinoma syndrome (Horm Mol Biol Clin Investig 2018;35:1)
- 22, 35 and 47 year old women with low grade serous neoplasms of the ovary with transformation to high grade carcinomas (Int J Gynecol Pathol 2012;31:423)
- 51 year old woman with high grade serous ovarian cancer 3 years after bilateral salpingectomy (Mol Clin Oncol 2017;6:201)
- 58 year old woman presented with an adnexal mass (Case of the month #538)
- 73 year old woman with serous carcinoma of the ovary that metastasized to the tail of the pancreas (Mol Clin Oncol 2016;5:41)
Treatment
- Bilateral salpingo-oophorectomy with hysterectomy, debulking and staging biopsies
- Chemotherapy (neoadjuvant and adjuvant)
- Poly (ADP-ribose) polymerase (PARP) inhibitors with best response in patients with BRCA1 / BRCA2 mutations or other homologous recombination deficiency and platinum sensitivity (Cancer Chemother Pharmacol 2018;81:647, Invest New Drugs 2018;36:828)
Gross description
- Often bilateral
- Variable in size; often large, ~30% of cases with grossly normal ovary or surface nodules Exophytic with solid or papillary growth
- Solid areas tan to white with necrosis and hemorrhage
- Serous / bloody fluid filled cysts
- Fallopian tube can be grossly involved at fimbriated end
Gross images
Frozen section description
- Nuclear pleomorphism (> 3x variation in size)
- Moderate to severe nuclear atypia
- Can have large bizarre forms
- Increased mitotic figures, can be atypical
Frozen section images
Microscopic (histologic) description
- Solid masses of columnar to cuboidal cells with eosinophilic cytoplasm and slit-like spaces (fusion of papillae)
- Solid, pseudoendometrioid, transitional cell carcinoma-like (SET) appearance (more common in BRCA1 mutations) (Mod Pathol 2012 Apr;25(4):625)
- Hierarchical papillary branching, glandular and cribriform patterns common
- Significant nuclear atypia
- Significant nuclear pleomorphism (> 3x variation in size) with large, bizarre and multinucleated forms (Hum Pathol 2005;36:1049)
- Prominent nucleolus, often large and eosinophilic
- High mitotic index: ≥ 12 mitotic figures per 10 high power fields, often atypical
- Necrosis is frequent
- Can have increased CD3+ or CD8+ tumor infiltrating lymphocytes (more common in BRCA1 mutation)
- Psammoma bodies are variable
- Serous tubal intraepithelial carcinoma (STIC) (90% fimbria, 10% ampulla / isthmus) exhibits similar histologic features; may be bilateral (10 - 20%) and multifocal
- Findings after neoadjuvant chemotherapy: single cells in a background of histiocytic reaction and fibrosis, clear cell change, decrease in mitotic figures, increased psammoma bodies
Microscopic (histologic) images
Contributed by Erna Forgó, M.D., Teri A. Longacre, M.D., Jijgee Munkhdelger, M.D., Ph.D., Andrey Bychkov, M.D., Ph.D. and Stephanie L. Skala, M.D. (Case #538)
Cytology description
- 3 dimensional clusters or single cells with scant cytoplasm
- Significant nuclear pleomorphism (> 3x variation in size)
- Prominent nucleoli
Cytology images
Positive stains
- PAX8, WT1, p16 (~60%), ER (80%), PR (30%), CK7, D2-40 (variable), calretinin (variable, may be focal)
- Aberrant p53 expression that correlates with TP53 mutation, 3 patterns (J Pathol Clin Res 2016;2:247, Int J Gynecol Pathol 2019;38 Suppl 1:S123):
- Overexpression: strong, diffuse nuclear staining in > 80% of cells (missense mutation)
- Null phenotype: complete absence of staining (loss of function mutation)
- Cytoplasmic expression: Cytoplasmic p53 expression with intensity similar to that of internal nuclear controls (loss of function mutation disrupting nuclear localization domain)
- High Ki67 proliferation index (> 75%) (Oncotarget 2016;8:107877)
Molecular / cytogenetics description
- TP53 alterations in nearly all cases
- Germline, somatic or promoter hypermethylation (inactivation) of BRCA1 and BRCA2 in ~50% of cases (Am J Surg Pathol 2016;40:404)
- Prominent DNA copy number changes, including CCN1, MYC, NOTCH3, PIK3CA, AKT oncogenes
Sample pathology report
- Ovary and fallopian tube, right, salpingo-oophorectomy:
- Ovary
- High grade serous carcinoma (see comment and synoptic report)
- Fallopian tube
- Intravascular involvement by high grade serous carcinoma
- Ovary
- Ovary and fallopian tube, left, salpingo-oophorectomy:
- Ovary
- High grade serous carcinoma
- Fallopian tube
- Intravascular involvement by high grade serous carcinoma
- Paratubal cysts
- Ovary
- Comment: We reviewed the frozen sections and confirm the diagnosis rendered. Histologic sections of the right and left adnexa show a high grade neoplasm growing in a solid trabecular pattern that involves both ovaries without capsular disruption. Abundant tumor associated lymphocytes are present. The tumor cells are positive for CK7 and PAX8 with abnormal (overexpression) of p53 but negative for CK20. Estrogen receptor and progesterone receptor expression is negative. These features are best characterized as high grade serous carcinoma which displays a SET (solid, pseudoendometrioid or transitional) pattern. Ovarian carcinomas which show a SET pattern of high grade serous carcinoma may be associated with BRCA gene mutations (somatic or germline).
Differential diagnosis
- Low grade serous carcinoma:
- Clear cell carcinoma:
- Undifferentiated carcinoma:
- Metastatic endometrioid serous carcinoma:
- Tumor mass, with or without endometrial intraepithelial carcinoma (precursor lesion) in the endometrium
- Usually WT1 negative or patchy
- Endometrioid adenocarcinoma:
- Malignant mesothelioma:
- Simple papillae lined by 1 cell layer without tufting
- Less severe nuclear atypia
- Low mitotic activity
- D2-40, calretinin positive
- ER negative
Board review style question #1
Board review style answer #1
A. BRCA1. SET (solid, pseudoendometrioid or transitional) pattern has a strong correlation between the presence of BRCA1 mutation and ovarian high grade serous carcinoma phenotype. These tumors also tend to have a higher mitotic index, increased tumor infiltrating lymphocytes and necrosis that can be comedonecrosis or geographic necrosis, when compared with unassociated cases. Recognition of this architectural growth pattern allows for the initiation of appropriate genetic testing and referral into necessary screening programs.
Comment Here
Reference: High grade serous carcinoma
Comment Here
Reference: High grade serous carcinoma
Board review style question #2
Which is the most important prognostic factor for overall survival of ovarian high grade serous carcinoma?
- Grade
- Histologic subtype
- Mitotic index
- Stage
Board review style answer #2
D. Stage. Stage is the most important prognostic factor in ovarian high grade serous carcinoma, with a ~40% overall survival at 5 years. In advanced stage disease, the amount of residual tumor present after staging and debulking is the most important factor for survival. Ovarian, tubal and peritoneal high grade serous carcinomas are staged the same. It has been proposed that ovarian disease in absence of serous intraepithelial carcinoma (STIC) be classified as ovarian in origin, ovarian disease in the presence of STIC be classified as tubal in origin and peritoneal disease in absence of ovarian or tubal involvement be classified as peritoneal in origin.
Comment Here
Reference: High grade serous carcinoma
Comment Here
Reference: High grade serous carcinoma
Hyperreactio luteinalis
Table of Contents
Definition / general | Epidemiology | Etiology | Clinical features | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) imagesDefinition / general
- Bilateral, ovarian enlargement due to multiple, luteinized follicle cysts
Epidemiology
- Rare
- Associated with hydatidiform mole, choriocarcinoma, fetal hydrops due to Rh sensitization and multiple gestations
- Rarely seen in uncomplicated, single pregnancy
Etiology
- Due to hCG stimulation or hypersensitivity to hCG
Clinical features
- Usually no symptoms, may have pain from hemorrhage; rarely ascites
- Virilization in 15% of mothers but not female infants in cases without gestational trophoblastic disease
Prognostic factors
- Only rarely recurs (J Clin Pathol 2005;58:439)
Case reports
- Associated with HELLP syndrome (Fertil Steril 2008;90:2008.e13)
- With hirsutism (Gynecol Endocrinol 2007;23:248)
- With marked virilization and hyperglycemia (Am J Perinatol 2006;23:85)
Treatment
- Cysts typically involute within 6 months (Arch Pathol Lab Med 1989;113:921)
- Operate only to remove infarcted tissue or to control hemorrhage
Gross description
- Multiple, bilateral thin walled cysts, filled with clear or hemorrhagic fluid
Microscopic (histologic) description
- Follicular cysts with luteinization of theca interna or granulosa cells, edema of theca layer and stroma
Microscopic (histologic) images
Large solitary luteinized follicular cyst of pregnancy and puerperium
Table of Contents
Definition / general | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosis | Additional referencesDefinition / general
- Rare solitary follicular cyst that occurs during pregnancy and puerperium, may be related to hCG
- Palpable adnexal mass or seen at C section
- No clinical endocrine disturbance
Treatment
- Conservative, most regress during puerperium, may need to excise if symptomatic (Obstet Gynecol 2005;105:1218)
Gross description
- Large, solitary, unilocular cyst, resembles follicular cyst but markedly larger (median size - 25 cm)
Microscopic (histologic) description
- Cyst lined by 1 or more layers of luteinized cells with clear to pink cytoplasm, no theca / granulosa distinction
- Focal nuclear atypia (enlarged, pleomorphic and hyperchromatic nuclei) is probably degenerative
- Also nests of luteinized cells within fibrous tissue of cyst wall (Pathol Res Pract 2006;202:471)
- May have a few mitotic figures (Diagn Pathol 2011;6:3)
Microscopic (histologic) images
Differential diagnosis
- Cystic ovarian neoplasm (low N/C ratio, sporadic atypia and clinical setting favor a solitary follicular cyst)
Additional references
Leiomyoma
Table of Contents
Definition / general | Epidemiology | Pathophysiology | Clinical features | Laboratory | Radiology description | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Differential diagnosisDefinition / general
- Accounts for 0.5 - 1% of all benign ovarian tumors
- Associated with synchronous leiomyomas of uterus
- Do not recur locally or metastasize, even if mitotically active
Epidemiology
- Age varies between 20 and 65 years
- About 16% occur after menopause (J Obstet Gynaecol Res 2005;31:257)
Pathophysiology
- Arises from smooth muscle cells in ovarian hilar blood vessels
- Other possible origins include cells in ovarian ligament, smooth muscle cells or multipotential cells in ovarian stroma, undifferentiated germ cells, or cortical smooth muscle metaplasia (Am J Surg Pathol 2004;28:1436)
Clinical features
- Abdominal pain, mass, vaginal discharge
Laboratory
- CA125, CA19-9 and CEA are within normal range
Radiology description
- Transvaginal ultrasonography: solid mass
- MRI T1 and T2 weighted images: well circumscribed low signal intensity mass (Eur Radiol 1998;8:1444)
Case reports
- 21 year old woman with bilateral massive ovarian leiomyomata (Mod Pathol 1992;5:586)
- 31 year old woman with primary ovarian leiomyoma associated with endometriotic cyst presenting with symptoms of acute appendicitis (Diagn Pathol 2009;4:25)
- 79 year old woman with leiomyoma of the ovary presenting with Meigs' syndrome (J Obstet Gynaecol Res 2005;31:257)
Treatment
- Surgical resection
Gross description
- Solid lobulated mass, median 3 cm (range 0.3 - 20 cm), often in hilar region
- Cut surface is gray-white, whorled
Gross images
Microscopic (histologic) description
- Irregular bundles and whorling of spindle shaped cells with no atypia or pleomorphism
- Degenerative changes like hyaline degeneration and myxomatous changes can be seen
- May be cellular, have prominent mitotic activity or occasionally have bizarre nuclei or myxoid stroma
Microscopic (histologic) images
Positive stains
Negative stains
Differential diagnosis
Leiomyosarcoma
Table of Contents
Definition / general | Sites | Pathophysiology | Clinical features | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Differential diagnosisDefinition / general
- Rare tumor of teratomatous or nonteratomatous origin
- "True" leiomyosarcoma if of nonteratomatous origin
Sites
- Omentum, reteroperitoneum, mesentery
Pathophysiology
- Arises from: malignant degeneration of ovarian leiomyoma; smooth muscle in blood vessel walls in cortical stroma and corpus luteum, muscular attachments of ovarian ligament, wolfian duct remnants, or totipotential ovarian mesenchyme; or from a teratoma
Clinical features
- Mostly postmenopausal patients
- Presents with abdominal pain and mass
- 62% die of disease within mean 24 months (Am J Surg Pathol 2004;28:1436)
Prognostic factors
- Tumor stage, tumor size, grade, mitotic index (Obstet Gynecol Surv 2007;62:480)
Case reports
- 42 year old woman with epithelioid tumor (Gynecol Oncol 2005;97:697)
- 58 year old woman (Arch Pathol Lab Med 1991;115:941)
- 60 year old women (World J Oncol 2001;2:265, Online J Health Allied Scs 2009;8:16)
Treatment
- Multimodality treatment: surgical debulking, postoperative radiotherapy or chemotherapy
- FIGO staging and treatment of ovarian sarcoma is same as for epithelial ovarian carcinoma (Obstet Gynecol Surv 2007;62:480)
Gross description
- Solitary, lobular, soft fleshy solid mass with hemorrhage and cystic degeneration
Microscopic (histologic) description
- Usually 2 of 3: moderate / severe cytologic atypia, 10+ MF/10 HPF, tumor cell necrosis
- Varies from well differentiated to highly pleomorphic sarcoma
Microscopic (histologic) images
Positive stains
Negative stains
Differential diagnosis
- Cellular fibroma: low mitotic activity Krukenberg tumors have stromal reaction, history of GI primary, pan CK+
- Mixed Müllerian tumors: pan CK+ areas
- Sarcomatoid form of sex cord stromal tumors: sex cord elements present
- Undifferentiated carcinomas: pan CK+, H caldesmon-
Leydig cell hyperplasia
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Negative stains | Electron microscopy description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Initially described by Rutgers and Scully (Int J Gynecol Pathol 1986;5:319)
- Benign proliferation of Leydig (hilus) cells in ovarian hilum; polygonal cells with abundant eosinophilic cytoplasm and Reinke crystals, closely associated with neurovasculature
Essential features
- Postmenopausal occurrence
- Predominantly located in the ovarian hilus, closely associated with neurovasculature
- Microscopic aggregates of Leydig (hilus) cells
- Reinke crystals and lipofuscin deposits present
- May present with virilization due to androgen production
- Stain positive for calretinin and inhibin
Terminology
- Acceptable: hilus cell hyperplasia (Int J Gynecol Pathol 1986;5:319)
ICD coding
Epidemiology
- Most common in postmenopausal and pregnant women (Int J Gynecol Pathol 1993;12:108)
- Postmenopausal age range is 72 - 82 years old (average: 75) (Int J Gynecol Pathol 1986;5:319)
Sites
- Usually bilateral in ovarian hilus (Eur J Gynaecol Oncol 2009;30:338)
- May be located in mesovarium, mesosalpinx and in medullar and cortical areas of the ovaries (Histol Histopathol 2017;32:1089)
Pathophysiology
- Fetal Leydig cells differentiate from mesenchymal cells during the eighth week of life; after the eighteenth week, the Leydig cells start to involute (Z Anat Entwicklungsgesch 1971;135:43)
- Fetal Leydig cells disappear completely during the first years of life; new generation of Leydig cells differentiate at puberty (Histopathology 1988;12:307)
- In adult women, the ovarian Leydig cells are possibly derived from the neural crest or the ganglia (Histol Histopathol 2017;32:1089)
Etiology
- Leydig cell proliferation is stimulated by human chorionic gonadotropin (hCG) in pregnancy or luteinizing hormone (LH) in menopause (Case Rep Womens Health 2023;39:e00537)
Clinical features
- Ovarian Leydig cells produce androgens and are associated with increased serum levels of testosterone and androstenedione and eventually virilization (Hum Pathol 2019;85:119)
Diagnosis
- Diagnosis of Leydig cell hyperplasia needs a systematic approach; it is aided by hormone levels rather than imaging and is confirmed by histopathological examination of the ovaries (Case Rep Womens Health 2023;39:e00537)
Laboratory
- Increased serum levels of testosterone and androstenedione (Hum Pathol 2019;85:119)
Radiology description
- Imaging is not helpful; normal findings on imaging can be seen in Leydig cell hyperplasia (Gynecol Endocrinol 2013;29:213)
- Transvaginal ultrasound scan may show prominent ovaries (Case Rep Endocrinol 2022;2022:8804856)
- Nodules can be found on ultrasound or computed tomography (CT) scan (Gynecol Endocrinol 2013;29:213)
Prognostic factors
- Usually, benign; may increase cardiovascular risk due to increased levels of testosterone and androstenedione (Gynecol Endocrinol 2013;29:213)
- Associated with endometrioid endometrial carcinoma due to increased androgen production (Hum Pathol 2019;85:119)
Case reports
- 33 year old virilized woman with pure gonadal dysgenesis and Leydig cell hyperplasia of both gonads (J Clin Endocrinol Metab 1969;29:1429)
- 55 year old postmenopausal woman post neoadjuvant chemotherapy for carcinoma of the right fallopian tube (J Midlife Health 2022;13:247)
- 61 year old woman with hirsutism and virilization (AACE Clin Case Rep 2020;7:26)
- 64 year old postmenopausal woman with gradual onset progressive excessive hair growth (Case Rep Endocrinol 2022;2022:8804856)
- 68 year old woman with hirsutism and temporal balding (Gynecol Endocrinol 2013;29:213)
Treatment
- Medical or surgical treatment
- Surgical treatment consists of uni or bilateral oophorectomy, usually accompanied by salpingectomy
- Cyproterone acetate as an androgen receptor competitive inhibitor
- GnRH analogue followed by surgical treatment (Gynecol Endocrinol 2013;29:213)
Gross description
- Not grossly visible (Case Rep Womens Health 2023;39:e00537)
- Cell clusters do not form large, aggregated nodules leading to either significant ovarian enlargement or shape distortion (Case Rep Endocrinol 2022;2022:8804856)
Microscopic (histologic) description
- Microscopic aggregates measuring < 1 cm in size (Gynecol Endocrinol 2013;29:213)
- Some have suggested a cut off value of > 300 cells in aggregates (Hum Pathol 2019;85:119)
- No evidence of nuclear atypia or increased mitotic activity (Case Rep Endocrinol 2022;2022:8804856)
- Large epithelioid cells are closely associated with nerve fibers and blood vessels or sometimes intermixed with rete varies
- Cells are oval, polygonal or fusiform
- Nucleus is spherical and vesicular but may be ovoid
- Nucleoli are conspicuous, basophilic and variable in number (1 - 3)
- Cytoplasm is granular, with multiple vacuoles or with a clear perinuclear halo
- They contain cytoplasmic lipid and lipofuscin deposits
- In ~30% of the cells, crystalloids of Reinke are present: eosinophilic long, round or rectangular inclusions usually surrounded by a clear halo
- Inclusions can be acidophilic spherical or as hyaline globules that are precursors of the crystal bodies (Histol Histopathol 2017;32:1089)
Microscopic (histologic) images
Positive stains
- Intense cytoplasmic and nuclear calretinin (J Midlife Health 2022;13:247)
- Moderate cytoplasmic for inhibin (Histol Histopathol 2017;32:1089)
- Nuclear staining for CDK4; androgen receptor (Case Rep Womens Health 2023;39:e00537)
Negative stains
Electron microscopy description
- Stacks of rough endoplasmic reticulum, microfilaments, lipid droplets and lipofuscin granules
Videos
Ovarian Leydig cell hyperplasia in woman
Sample pathology report
- Ovary, oophorectomy:
- Leydig (hilus) cell hyperplasia (see comment)
- Comment: Nodular, microscopic aggregates of hilus cells are present in close association with nerve and vascular in the ovarian hilum. The cells are variable in size and polygonal. The nucleus is spherical, conspicuous and variable in number. Acidophilic spherical or hyaline globules and rare crystalloids of Reinke are present. There is no evidence of nuclear atypia or increased mitotic activity.
Differential diagnosis
- Leydig cell tumor:
- Fibrinoid degeneration of vessel walls (characteristic)
- Visible on ultrasound
- Grossly visible, well circumscribed mass, with a mean size of 2 cm
- Adrenal rests:
- Frequently detected in retroperitoneal, pelvic or groin areas
- Vesicular eosinophilic to clear cytoplasm
- Composed of 3 layers of adrenal cortex
- Pregnancy luteoma:
- Occurs during pregnancy and puerperium
- Follicle-like spaces
- Not restricted to ovarian hilum
- Luteinized thecoma:
- Ovarian stromal neoplasm usually not in hilum
- Grossly visible mass composed of theca cells
- Hyaline plaques
Additional references
Board review style question #1
Microscopic perineural clusters of benign looking epithelioid cells containing crystalloids of Reinke have been found incidentally in the hilar area of bilateral ovaries of a 75 year old woman. Positivity for which of the following immunostains would support the diagnosis?
- Calretinin
- Cytokeratin AE1 / AE3
- EMA
- WT1
Board review style answer #1
A. Calretinin. Leydig cell hyperplasia is a benign proliferation of steroid producing Leydig (hilus) cells that show positivity for calretinin, inhibin and androgen receptors and are negative for cytokeratin, EMA and WT1 immunostains. Answer C is incorrect because Leydig cells are considered sex cord stromal cells and they typically do not stain positive for epithelial markers. Answer B is incorrect because Leydig cells are considered sex cord stromal cells and they typically do not stain positive for keratin markers. Answer D is incorrect because WT1 stains Sertoli cells and not Leydig cells.
Comment Here
Reference: Leydig cell hyperplasia
Comment Here
Reference: Leydig cell hyperplasia
Leydig cell tumor
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Etiology | Clinical features | Diagnosis | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Benign steroid cell tumor composed of polygonal cells with abundant eosinophilic cytoplasm and Reinke crystals
Essential features
- Postmenopausal occurrence
- Predominantly located in the ovarian hilus, close to nerve fibers
- Often lobulated or nodular growth
- Reinke cytoplasmic crystals present
Terminology
- Hilus cell tumor (not recommended)
ICD coding
- ICD-10: D27.9 - benign neoplasm of unspecified ovary
- ICD-11: 2F32.Y & XH5XQ2 - other specified benign neoplasm of ovary & Leydig cell tumor of the ovary, NOS
Epidemiology
- 15 - 20% of ovarian steroid cell tumors
- Age range: 32 - 82 years (mean: 58 years) (Int J Gynecol Pathol 1989;8:299)
Etiology
- Arise from ovarian hilus cells that resemble the Leydig cells of the testis
Clinical features
- May cause elevated levels of serum testosterone and urinary 17 ketosteroids
- Androgenic symptoms, rarely estrogenic (Int J Gynecol Pathol 1989;8:299)
- Hirsutism, baldness, voice changes, male pattern musculature, clitoromegaly
Diagnosis
- May be incidental
- Unilateral adnexal mass, may be noted on ultrasound
- WHO (5th edition) criteria
- Essential: well circumscribed tumor centered in the ovarian hilus, consisting of bland steroid cells
- Desirable: cytoplasmic Reinke crystals
Radiology description
- Ultrasound: large mass with heterogenous appearance is commonly seen; however, this is characteristic of sex cord stromal tumors in general (J Oncol Pract 2016;12:940)
Prognostic factors
- Almost always benign
Case reports
- 39 year old woman (gravida 1, para 1) who presented with a high serum testosterone level and secondary amenorrhea, hirsutism and weight gain (Case Rep Womens Health 2021;30:e00298)
- 55 year old postmenopausal woman who presented with a 3 year history of frontal balding and virilization (J Clin Endocrinol Metab 2014;99:12)
- 71 year old woman who presented with postmenopausal hirsutism with 10 years of evolution (BMJ Case Rep 2021;14:e240937)
Treatment
- Surgical removal - oophorectomy
Gross description
- Unilateral, lobulated, solid mass
- Yellow, orange or red-brown soft cut surface
- May have multifocal hemorrhagic areas
- Mostly < 5.0 cm (mean: 2.5 cm) but may be up to 15 cm (Int J Gynecol Pathol 1989;8:299)
Microscopic (histologic) description
- Unencapsulated neoplasms with smooth or irregular borders located in the hilus, next to nonmedullary nerve fibers
- Round to polygonal cells with abundant eosinophilic or vacuolated cytoplasm and round nuclei with small nucleoli
- Reinke crystals: eosinophilic, cytoplasmic rod, rectangular or hexagonal crystals
- Lipochrome pigment is common
- May have cytoplasmic pseudoinclusions or bizarre nuclear atypia
- May have diffuse or lobulated or nodular growth with collagenous septa
- Sheets, nests or rarely cords
- Variably hyalinized or edematous stroma
- May have nuclear clustering with intervening acellular zones
- Fibrinoid degeneration of vessel walls (characteristic)
- May have background hilar cell hyperplasia (Nucci: Gynecologic Pathology - A Volume in Foundations in Diagnostic Pathology Series, 2nd Edition, 2020)
Microscopic (histologic) images
Positive stains
Negative stains
Sample pathology report
- Ovary, right, oophorectomy:
- Leydig cell tumor (see comment)
- Comment: The right ovary shows a well circumscribed tumor centered in the ovarian hilus, consisting of bland steroid cell containing cytoplasmic Reinke crystals and measuring 3 cm. Leydig / hilus cell hyperplasia is identified in the adjacent right ovarian tissue.
Differential diagnosis
- Stromal luteoma:
- More commonly presents with estrogenic manifestations
- Located in the ovarian parenchyma
- Irregularly shaped pseudoacini may be present
- Commonly associated with stromal hyperthecosis
- No Reinke crystals
- Pregnancy luteoma:
- Occurs during pregnancy and puerperium
- Multiple and bilateral
- Follicle-like spaces
- No Reinke crystals
- Stromal hyperthecosis:
- Located in the ovarian cortex
- Nodular aggregates of lutein cells < 1.0 cm
- No Reinke crystals
Additional references
Board review style question #1
A 45 year old woman presents with hirsutism, deepening of the voice and clitoromegaly. Imaging reveals a unilateral solid ovarian mass. Which of the following is the most likely diagnosis?
- Adult granulosa cell tumor
- Dysgerminoma
- Fibroma
- Leydig cell tumor
- Thecoma
Board review style answer #1
D. Leydig cell tumor. Leydig cell tumors usually present in postmenopausal women with androgenic manifestations (hirsutism, voice deepening, clitoromegaly). Answer A is incorrect because adult granulosa cell tumors usually present with estrogenic manifestations such as abnormal uterine bleeding and concomitant endometrial thickening / hyperplasia. Answer E is incorrect because thecoma is an ovarian stromal tumor composed predominantly of cells resembling theca cells and presents more frequently with estrogenic symptoms or with symptoms related to an adnexal mass. Answer C is incorrect because fibromas are associated with Gorlin syndrome (nevoid basal cell carcinoma syndrome) or Meigs syndrome (ascites and pleural effusion). Answer B is incorrect because dysgerminomas arise in children or young women, with abdominal pain and a mass with elevated lactic dehydrogenase (LDH).
Comment Here
Reference: Leydig cell tumor
Comment Here
Reference: Leydig cell tumor
Board review style question #2
A 71 year old woman underwent a total abdominal hysterectomy (TAH) and bilateral salpingo-oophorectomy (BSO) for abnormal uterine bleeding (AUB). She concomitantly reported hirsutism. An incidental finding in the left ovary showed the findings in the image above. What is true about this neoplasm?
- Cytoplasmic Reinke crystals are desirable but not essential for diagnosis of this tumor
- Patients present with estrogenic manifestation
- These neoplasms are frequently bilateral
- This tumor is associated with STK11
Board review style answer #2
A. Cytoplasmic Reinke crystals are desirable but not essential for diagnosis of this tumor. Cytoplasmic Reinke crystals occur rarely but are characteristic in Leydig cell tumor. In the WHO, these are in the desirable category and are not essential for the diagnosis of Leydig cell tumor if the tumor is located in the hilum. Answer D is incorrect because sex cord tumor with annular tubules (SCTAT) are associated with STK11 gene alterations. Answer B is incorrect because adult granulosa cell tumors present with estrogenic manifestations, whereas Leydig cell tumors present most commonly with androgenic manifestations. Answer C is incorrect because Leydig cell tumors most commonly occur unilaterally.
Comment Here
Reference: Leydig cell tumor
Comment Here
Reference: Leydig cell tumor
Low grade serous carcinoma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Epithelial carcinoma of serous cell lineage, usually with distinctive patterns of invasion and low grade malignant cytologic atypia
Essential features
- Often bilateral ovarian masses that are solid or solid and cystic with calcifications
- Extraovarian spread at presentation associated with poor outcome
- Precursor lesion is often a borderline serous tumor
- KRAS and BRAF mutations in 50 - 60% of cases
Terminology
- Low grade serous adenocarcinoma
- Well differentiated serous carcinoma
- Invasive micropapillary carcinoma
ICD coding
- ICD-O: 8460/3 - malignant epithelial serous tumors, low grade serous carcinoma
Epidemiology
- 3.5% of all ovarian carcinomas; 5% of all serous tumors (Int J Gynecol Pathol 2010;29:203)
- Mean age: fifth to sixth decades
- Presents ~10 years earlier than patients with high grade serous carcinoma (Adv Anat Pathol 2009;16:267)
Sites
- Ovary
- Usually bilateral
Pathophysiology
- Precursor lesion is often a borderline serous tumor (Am J Surg Pathol 2004;28:496, Int J Gynecol Pathol 2020;39:43)
- Both tumors can coexist in up to 85% of cases, in variable combinations (Am J Surg Pathol 2016;40:1165)
- Can be synchronous, metachronous
- Can be at same or different site
- Both tumors can coexist in up to 85% of cases, in variable combinations (Am J Surg Pathol 2016;40:1165)
- May rarely progress to high grade serous carcinoma, sarcomatoid carcinoma or carcinosarcoma (Histopathology 2019;74:638, Int J Gynecol Pathol 2012;31:423)
Clinical features
- Abdominal pain / swelling
- Incidental finding
- Most patients present with advanced stage disease
- Can manifest as recurrence following diagnosis of serous borderline tumor
Diagnosis
- CT with contrast is the preferred imaging modality
- Diagnosis is by surgical resection
Laboratory
- Elevated serum CA-125 level (Gynecol Oncol 2014;132:560)
Radiology description
- Ovarian masses are usually solid or solid and cystic
- Can contain thick septae or nodular components with increased vascularity
- Calcifications are often readily identifiable
- Reference: Eur J Radiol Open 2015;2:39
Radiology images
Prognostic factors
- Excellent with surgical excision alone if confined to the ovary
- Extraovarian spread at presentation associated with poor outcome (Int J Gynecol Pathol 2013;32:529)
- 5 and 10 year survival rate in advanced stage is 80% and 50%, respectively
- Better prognosis than high grade serous carcinoma when matched for stage
Case reports
- 29 year old woman with Rubinstein-Taybi syndrome and synchronous ovarian and endometrial carcinomas (Int J Gynecol Pathol 2015;34:132)
- 3 women, ages 33 - 50 years, with serous borderline tumor of the ovary with areas of serous low grade carcinoma and metastases (Am J Surg Pathol 2005;29:496)
- 39 year old woman with platinum resistance and extended response to bevacizumab (Anticancer Drugs 2013;24:986)
- 55 year old Japanese woman with a paraspinal bone metastasis after a 13 year disease free interval (Diagn Pathol 2018;13:43)
- 67 year old woman with BRAF V600E and TERT promoter mutations that transformed to carcinosarcoma in a lymph node (Int J Gynecol Pathol 2019;38:386)
Treatment
- Bilateral salpingo-oophorectomy and hysterectomy with debulking and lymph node biopsies
- Adjuvant chemotherapy or hormonal therapy
- Typically poor response to platinum / Taxol chemotherapy
Gross description
- Often bilateral
- Fine papillary, nodular growth
- Little to no necrosis
- Calcification in the ovary and extraovarian lesions can be extensive
Gross images
Frozen section description
- Uniform population of small cells with scant cytoplasm
- Mild to moderate nuclear atypia at most (grade 1 or 2)
- No nuclear pleomorphism (Hum Pathol 2005;36:1049)
- May have a conspicuous nucleolus
- Minimal necrosis
Microscopic (histologic) description
- Uniform / homogeneous population of small cells with scant cytoplasm
- Mild to moderate nuclear atypia at most (grade 1 or 2)
- No nuclear pleomorphism (Hum Pathol 2005;36:1049)
- May have a conspicuous nucleolus
- Low mitotic index: Little to no necrosis
- Psammoma bodies are frequent
- 2 patterns, noninvasive and invasive:
- Noninvasive: nonhierarchical architecture with micropapillary or cribriform patterns with significant expansile growth
- Invasive (> 5 mm): micropapillary or complex papillae, compact cell nests, inverted macropapillae (with broad fibrovascular cores), cribriform, glandular or cystic, solid sheets with slit-like spaces and single cells
- Multiple different invasive patterns can exist within one tumor
Microscopic (histologic) images
Cytology description
- Malignant glandular cells in clusters and singly
- Moderate amounts of finely vacuolated cytoplasm
- Enlarged hyperchromatic nuclei
- No significant nuclear pleomorphism (Prominent nucleoli
Cytology images
Molecular / cytogenetics description
- KRAS and BRAF mutations in 50 - 60% of cases (Am J Surg Pathol 2010;34:433, J Pathol 2012;226:413, Cancer 2013;119:548)
- NRAS mutations (Histopathology 2019;74:638)
- Few chromosomal abnormalities; 1p36 loss is most significant copy number alteration
- BRAF mutations rare in advanced stage disease (Am J Pathol 2010;177:1611, Cancer 2013;119:548)
Sample pathology report
- Fallopian tube and ovary, right, salpingo-oophorectomy:
- Ovary
- Low grade serous carcinoma (see comment and synoptic report)
- Fallopian tube
- Involved by low grade serous carcinoma
- Comment: Histologic sections show diffuse involvement by carcinoma with low to intermediate grade nuclei, prominent nucleoli, vesicular chromatin and moderate amounts of delicate cytoplasm. Mitotic count is 5 per 10 high power fields. Cells are arranged as solid nests and tightly packed papillary clusters. Immunohistochemical stains show that the tumor cells are positive for WT1, ER (2 - 3+, 90%) and PR (1+, 5%). Tumor cells demonstrate slightly elevated but patchy p53 wild type staining. p16 also demonstrates patchy staining. The findings are consistent with involvement by serous carcinoma of Müllerian origin and the overall morphologic features, low mitotic activity and wild type p53 and p16 staining support the diagnosis of low grade serous carcinoma.
- Ovary
Differential diagnosis
- Serous borderline tumor:
- No or minimal (Absence of prominent nucleoli
- High grade serous carcinoma:
- Increased nuclear pleomorphism with ≥ 12 mitotic figures/10 high power fields (Hum Pathol 2005;36:1049)
- Heterogeneous architecture
- Aberrant p53 expression (overexpression or null phenotype)
- p16 diffusely positive
- Endometrioid carcinoma:
- Glandular architecture
- Squamous differentiation
- WT1 variably expressed
- Malignant peritoneal mesothelioma with secondary involvement of ovary:
- Extensive disease
- D2-40, calretinin positive
- BerEp4, MOC31 negative
Board review style question #1
Board review style answer #1
A. Low grade serous carcinoma. Low grade serous carcinomas of the ovary exhibit minimal necrosis or apoptotic bodies. They demonstrate a low proliferation index and a low mitotic index (
Comment Here
Reference: Low grade serous carcinoma
Comment Here
Reference: Low grade serous carcinoma
Board review style question #2
Which of the following is true about low grade serous carcinoma of the ovary?
- KRAS and BRAF mutations are present in approximately half of the cases
- Most tumors show aberrant p53 expression
- Nearly all the tumors progress to high grade serous carcinomas
- TP53 mutations are present in nearly all of the tumors
Board review style answer #2
A. KRAS and BRAF mutations are present in about half of the cases. Low grade serous carcinomas of the ovary have few point mutations. KRAS and BRAF mutations are present in 50 - 60% of the low grade serous carcinoma of the ovary. BRAF V600E mutation is associated with early stage disease and improved prognosis and it is rarely seen in advanced stage disease.
Comment Here
Reference: Low grade serous carcinoma
Comment Here
Reference: Low grade serous carcinoma
Luteinized thecoma associated with sclerosing peritonitis
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Negative stains | Molecular / cytogenetics description | Molecular / cytogenetics images | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Uncommon syndrome characterized by presence of ovarian (mostly bilateral) stromal tumor(s) resembling theca cells and peritoneal fibrotic lesions
Essential features
- Benign tumor
- Usually bilateral (unilateral cases have been reported)
- Ovarian theca cell proliferation involving the cortex with or without mass formation
- Associated abnormal proliferation of fibroblasts and myofibroblasts in the peritoneum that results in an irreversible fibrosis
- Leads to incarceration and strangulation of the abdominal organs, which is eventually fatal
Terminology
- Luteinized thecomatosis associated with sclerosing (encapsulating) peritonitis
ICD coding
Epidemiology
- Rare
- Age ranges from 10 months to 85 years; median age is 27 (Am J Surg Pathol 2008;32:1273)
Sites
- Ovary, predominantly confined to the ovarian cortex
- Associated peritoneal fibroblastic proliferation
- References: Expert Rev Anticancer Ther 2021;21:23, J Obstet Gynaecol Can 2016;38:41
Pathophysiology
- Still unclear; debatable whether this is a neoplasm or nonneoplastic proliferation
- Suggested that neoplastic theca cells are able to produce substances that, when released into the peritoneal cavity, stimulate fibroblastic proliferation
- Has also been suggested that luteinized theca cells secrete steroid hormones (estrogen and progesterone) that would target submesothelial fibroblasts characterized by hormonal sensitivity
- Among the substances produced are cytokines, in particular transforming growth factor beta (TGFβ), which induce a fibrotic reaction within the peritoneum and, rarely, even in distant anatomical sites
- References: Expert Rev Anticancer Ther 2021;21:23
Etiology
- Unknown
Clinical features
- Abdominal pain
- Ascites
- Symptoms of small bowel obstruction (acute / subacute)
- Hormonal symptoms are absent
- Association with anticonvulsant therapy in some cases
- References: Pathology 2018;50:5, Expert Rev Anticancer Ther 2021;21:23
Diagnosis
- Histologic examination
Laboratory
- Occasionally elevated serum CA125 (Expert Rev Anticancer Ther 2021;21:23)
Radiology description
- Magnetic resonance imaging features
- Cystic, solid adnexal region mass
- Cystic component gives a homogeneous iso or hyperintense signal
- Solid component shows moderate enhancement
- Computed tomography (CT) features
- Thickened contrast material enhanced abnormal peritoneal membrane, dilation of bowel loops with clump appearance
- Thickening or calcification of bowel walls, with or without loculated ascites
- References: J Ovarian Res 2013;6:58, Radiographics 2019;39:62
Radiology images
Prognostic factors
- Ovarian luteinized thecoma itself is a benign tumor without risk of recurrence or metastasis
- However, associated peritoneal fibrosis may result in bowel incarceration and strangulation, causing potentially life threatening bowel obstruction
- References: Expert Rev Anticancer Ther 2021;21:23, Pathology 2018;50:5
Case reports
- 18 year old woman presented with acute abdomen (Gynecol Oncol Rep 2019;28:44)
- 42 year old presented with progressive respiratory distress, increased abdominal girth and sudden weight gain (J Gynecol Obstet Hum Reprod 2021;50:101734)
- 52 year old woman with erythematous rash, coinciding with the rapid onset of ascites and peripheral edema (J Cutan Pathol 2017;44:898)
Treatment
- Surgical management
- Bilateral salpingo-oophorectomy if localized to ovaries
- Oophorectomy or unilateral salpingo-oophorectomy if unilateral and preserved fertility is desired
- Total hysterectomy with bilateral salpingo-oophorectomy is indicated in postmenopausal patients
- Omentectomy in extensive disease
- Peritoneal biopsy in case of peritoneal nodules only
- Appendectomy or small bowel resection in case of involvement
- Medical management (single or combined therapy)
- Cyclophosphamide, thalidomide, leuprorelin, tamoxifen, goserelin and corticosteroids
- References: Expert Rev Anticancer Ther 2021;21:23, Gynecol Oncol Rep 2019;28:44
Gross description
- Usually bilateral
- Dimensions range from 2 - 31 cm
- Outer surface is brown, red, purple, pink or gray
- Cut surface is solid, yellow, pink or gray, very often characterized by cystic hemorrhagic or sometimes soft lobulated, cerebriform or polypoid cut surface
- Peritoneum with brown to white fibrous adhesions
- Omentum with lobulation, nodularities, indurations or thickening
- Thick, white adherent membrane encasing the small bowel (partially or complete)
- Reference: Am J Surg Pathol 2008;32:1273
Frozen section description
- Sheets of bland spindle cell proliferation circumferentially involve the entire cortex, with sparing of the medulla in smaller lesions (Am J Surg Pathol 2008;32:1273)
- Luteinized cells are arranged in small nests or singly with small to moderate amounts of clear or eosinophilic cytoplasm (Am J Surg Pathol 2008;32:1273)
- Mitotic activity is typically prominent (Am J Surg Pathol 2008;32:1273)
- Edema with extravasated erythrocytes, prominent small vessels and microcystic areas
- Peritoneal / omental lesions have proliferation of bland spindled cells with variably fibrillar cytoplasm
- While it is unlikely peritoneal samples are submitted for frozen section examination along with the ovarian tumor, it is very helpful to inquire from the surgeon about the presence of peritoneal lesions or seek this information from the patient's chart as this helps guide diagnosis
Frozen section images
Microscopic (histologic) description
- Highly cellular
- Sheets of spindle cell proliferation circumferentially involves the entire cortex with sparing of the medulla
- Proliferation arranged haphazardly in small fascicles or short cords and frequently includes pre-existing ovarian structures
- Less cellular areas show edema with characteristic microcystic pattern
- Larger lesions show prominent cellular lobules or vaguely nodular cellular aggregates, often highlighted by hemorrhage, separated by less cellular regions with varying amounts of edema which could give a microcystic appearance
- Edematous areas contain extravasated erythrocytes and prominent small vessels
- High power, the cells are rounded, fusiform or stellate and cytoplasm volume ranged from scant to moderate with pale or eosinophilic color
- Ill defined aggregates of luteinized cells with rounded nuclei, eosinophilic to pale cytoplasm, in the background of bland spindle cells
- Brisk mitotic activity may be seen
- Sex cord-like differentiation in rare cases
- Entrapped normal ovarian structures, usually follicles, rarely corpora albicans (Am J Surg Pathol 2008;32:1273)
- Variable amount of hemorrhage, ischemic type necrosis and in rare cases fibrin thrombi in large vessels (Am J Surg Pathol 2008;32:1273)
- Peritoneal lesions composed of a proliferation of fibroblastic spindled cells
- Prominent omental lobulation in which the fibrosis expands between the septa of lobules with or without mild chronic inflammatory lymphocytic infiltrate
Microscopic (histologic) images
Virtual slides
Positive stains
- SF1 (positive in spindle cells) (Am J Surg Pathol 2013;37:1458)
- FOXL2 (positive in spindle cells) (Am J Surg Pathol 2013;37:1458)
- Inhibin (positive in luteinized cells)
- Calretinin (positive in luteinized cells)
- CD56 (positive in luteinized cells)
- Acetylglucosaminyltransferase B (MGAT5B) (positive in luteinized cells) (Medicine (Baltimore) 2023;102:e33911)
- Nuclear receptor coactivator 3 (NCOA3) (positive in luteinized cells) (Medicine (Baltimore) 2023;102:e33911)
- Reticulin stain highlights reticulin fibers separating individual theca cells and around nests in the luteinized cells
Negative stains
- ER (22% positive) (Am J Surg Pathol 2008;32:1273)
- PR (41% positive) (Am J Surg Pathol 2008;32:1273)
- CD117 (33% positive) (Am J Surg Pathol 2008;32:1273)
- Cytokeratin AE1 / AE3
- EMA
- CD34
- Beta catenin (cytoplasmic staining in the luteinized cells)
- TGF beta (Am J Surg Pathol 2008;32:1273)
- WT1 (Medicine (Baltimore) 2023;102:e33911)
- CD99 (Medicine (Baltimore) 2023;102:e33911)
Molecular / cytogenetics description
- MGAT5B::NCOA3 fusion gene recently reported in luteinized thecoma associated with sclerosing peritonitis (LTSP), identified in the luteinized cells (Medicine (Baltimore) 2023;102:e33911)
- Negative for FOXL2 mutation
Sample pathology report
- Right ovary and fallopian tube, right salpingo-oophorectomy:
- Ovarian luteinized thecoma, encased with fibrosis
- Fibrosis involving the surface of the ovary
- Unremarkable right fallopian tube
- Negative for malignancy
- Anterior pelvic peritoneum, excision:
- Peritoneum with fibrosis and mesothelial cell hyperplasia (see comment)
- Negative for malignancy
- Comment: The given picture of peritoneal sclerosis (fibrosis) with hyperplasia of the submesothelial mesenchymal cells can result from a variety of stimuli. Given the finding of luteinized thecoma of the ovary, this favors the diagnosis of luteinized thecoma associated with sclerosing peritonitis (LTSP). LTSP is an uncommon syndrome characterized by the presence of a benign ovarian stromal tumor resembling theca cells and peritoneal fibrotic lesions. In some cases, peritoneal lesions lead to irreversible fibrosis with the incarceration and strangulation of abdominal organs, eventually proving fatal.
- Other causes of peritoneal sclerosis include chronic ambulatory peritoneal dialysis, the use of peritoneovenous shunt, bacterial or mycobacterial infection, sarcoidosis, carcinoid syndrome, familial Mediterranean fever and exposure to fibrogenic foreign materials. Idiopathic sclerosing peritonitis is diagnosed when all known causes are excluded, with supportive intraoperative and imaging findings.
Differential diagnosis
- Thecoma (Am J Surg Pathol 2014;38:1023):
- Unilateral
- Commonly in perimenopausal and postmenopausal women
- No associated peritoneal lesions
- Associated hormonal manifestations and uterine bleeding
- Bland cytologic features with appreciable pale gray cytoplasm and low mitosis (≤ 4 mitoses/10 HPF)
- Reticulin surrounding individual cells
- Fibroma (Am J Surg Pathol 2006;30:929):
- Mostly unilateral
- Fascicles of bland fibrous spindle cells in a variably collagenous stroma
- No or focal luteinized cells
- Focal weak inhibin and calretinin staining
- Sclerosing stromal tumor (Int J Gynecol Pathol 2016;35:549):
- Pseudolobules contain epithelioid and spindled cells
- Alternating hypo and hypercellular areas
- Hemangiopericytoma-like vessels distributed throughout the tumor
- Microcystic stromal tumor (Histopathology 2019;74:443):
- Small round to oval cystic spaces, in areas coalescing to form larger irregular channels
- Lobulated cellular masses with intervening fibrous or fibro-hyaline stroma
- Mutations in β catenin, with associated nuclear immunoreactivity
- Negative for inhibin and calretinin
- Massive ovarian edema (Diagn Pathol 2016;11:18):
- Diffuse edema; uniformly hypocellular fibromatous stroma
- Preservation of the outer cortex
- Stromal hyperthecosis (Gynecol Endocrinol 2021;37:677):
- No ascites or mass; low to absent mitoses
- Commonly in peri and postmenopausal women
- Androgenic manifestations
Additional references
Board review style question #1
A 37 year old woman presents with severe abdominal pain and distention. Laboratory investigations revealed a negative beta hCG and slightly elevated serum CA125. Radiologic images showed thick soft tissue of peritoneal sclerosis encasing the small bowel loops. Exploratory laparotomy reveals a 12.5 cm right ovarian mass and multiple peritoneal nodules, which are resected. Which of the following statements is true regarding the ovarian mass?
- Associated with an unfavorable outcome in the small bowel
- Associated with hormonal manifestations
- Bilateral manifestation indicates metastasis of contralateral ovarian tumor
- Peritoneal nodules are metastasized from the ovarian mass
Board review style answer #1
A. Associated with an unfavorable outcome in the small bowel. Axial abdominopelvic computed tomography (CT) shows peritoneal sclerosis encasing the small bowel loops with a characteristic clumped appearance of small bowel loops within a cocoon. The histopathology picture shows nodules of spindled and luteinized cells. Luteinized thecoma associated with sclerosing peritonitis (LTSP) may result in bowel incarceration and strangulation, causing potentially life threatening bowel obstruction. Answers C and D are incorrect because the ovarian tumor is benign and there is no recurrence or metastasis of the ovarian tumor after excision. Answer B is incorrect because this ovarian tumor usually is not hormonally active.
Comment Here
Reference: Luteinized thecoma associated with sclerosing peritonitis
Comment Here
Reference: Luteinized thecoma associated with sclerosing peritonitis
Müllerian adenosarcoma
Table of Contents
Definition / general | Epidemiology | Pathophysiology | Clinical features | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Differential diagnosisDefinition / general
- Ovarian MMMT is either low grade (Müllerian adenosarcoma) or high grade (carcinosarcoma, mixed mesodermal tumor)
- Müllerian adenonosarcoma is usually unilateral, more commonly seen in endometrium and cervix
- 67% have tumor rupture at or before excision
- Poorer prognosis than uterine adenosarcoma, perhaps due to greater ease of peritoneal spread
- Tends to recur as pure sarcoma or adenosarcoma (Am J Surg Pathol 2002;26:1243)
Epidemiology
- Median age 54 years, range 30 - 84 years (Am J Surg Pathol 2002;26:1243)
Pathophysiology
- Appears to have epithelial origin (Am J Surg Pathol 1990;14:317, Am J Surg Pathol 1995;19:666)
Clinical features
- Abdominal or pelvic pain, abdominal swelling, may present as adnexal mass
Laboratory
- No useful biochemical marker (Curr Opin Obstet Gynecol 2006;18:20)
Radiology description
- MRI: solid pelvic mass, especially in patients with endometriosis
- USG: very low resistive index (Ultrasound Q 2007;23:189)
Prognostic factors
- Poor prognostic factors: extraovarian spread (high stage), tumor rupture, high grade, high grade sarcomatous overgrowth, age < 53 years
- Survival: 5 year - 64%, 10 year - 46%
Case reports
- 34 year old woman with abdominal pain and a large right multicystic ovarian mass (Arch Pathol Lab Med 2004;128:1057)
- Ovarian adenosarcoma with extensive deciduoid morphology arising in endometriosis (Int J Gynecol Pathol 2008;27:398)
- Ovarian adenosarcoma arising from benign cystadenoma and associated intraoperative consultation pitfalls (Int J Gynecol Pathol 2010;29:415)
Treatment
- Salphingopherectomy or panhysterectomy
Gross description
- 97% unilateral, mean 14 cm (range 5 - 50 cm)
- Usually solid with occasional small cysts
Microscopic (histologic) description
- Benign or atypical epithelial component and a low grade malignant stromal component
- Conspicuous noninvasive Müllerian type glands within a predominant malignant stroma, either homologous or heterologous
- Periglandular cuffs of cellular stroma (80%), intraglandular protrusions of cellular stroma (60%) or both
- At least mild stromal atypia (33%)
- Variable stromal mitotic count; usually marked stromal cellularity (resembling endometrial stromal sarcoma or cellular ovarian fibroma) but may also have hypocellular stromal areas
- Other features: glands widely spaced throughout stroma (90%), occasional sarcomatous overgrowth (30%), sex cord-like elements (15%), heterologous elements (12%)
Microscopic (histologic) images
Positive stains
Differential diagnosis
- Benign tumors (adenofibroma or endometriosis): lack periglandular cellular cuffs of cellular stroma, no stromal atypia
- Endometrial stromal sarcoma: sample thoroughly to rule out glandular component
- Immature teratoma: younger patient, usually embryonal neuroectodermal elements, no periglandular cellular cuffs of cellular stroma
- Malignant mixed Müllerian tumor: glandular and stromal epithelium is obviously malignant versus at most atypical glands in stromal sarcoma
Mesonephric-like adenocarcinoma (uterus / ovary)
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Negative stains | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Mesonephric-like adenocarcinoma (MLA) is a rare, recently recognized but frequently misdiagnosed subtype of gynecologic malignancy arising in the ovary and uterine corpus
- MLA shares morphologic, immunophenotypic and molecular characteristics with mesonephric adenocarcinoma (MA) except that mesonephric remnants are not identified in the former
- Entity is included in the 5th edition World Health Organization (WHO) classification of female genital tumors
Essential features
- Rare subtype of gynecologic malignancy with high risk of recurrence and increased tendency to metastasize
- Morphologic hallmark is the combination of architectural patterns in a tumor without squamous or mucinous differentiation; intraluminal dense eosinophilic secretions can be seen
- Low grade morphology with negative ER and PR should prompt the pathologist to perform additional stains
- PAX8, GATA3, TTF1, ER and PR are the main immunohistochemical stains that can be used to support the diagnosis
- Usually mismatch repair (MMR) proficient and p53 wild type
- KRAS mutation is commonly present
ICD coding
Epidemiology
- Endometrial MLA accounts for < 1% of endometrial carcinomas; limited epidemiologic data on ovarian MLA (Am J Surg Pathol 2019;43:389)
- Median age 61 years (range: 36 - 76), more commonly seen in postmenopausal patients (Mod Pathol 2021;34:1570)
Sites
- Uterine corpus and ovary
Pathophysiology
- Controversial whether MLA originates from mesonephric structures or represents Müllerian tumors that have acquired mesonephric characteristics
- Evidence supporting Müllerian origin with mesonephric transdifferentiation
- Endometrial MLA arises from the endometrium rather than myometrium
- MLA is not associated with mesonephric remnants
- Ovarian MLA has been found to coexist with other Müllerian neoplasms such as low grade serous carcinoma (Int J Gynecol Pathol 2020;39:84)
- MA and MLA share KRAS mutations but concurrent PIK3CA mutations, which are described in endometrioid adenocarcinoma, are also documented in MLA
- NRAS mutations may occur (Int J Gynecol Pathol 2018;37:448)
Etiology
- Unknown
Clinical features
- Patients with endometrial MLA may present with abnormal vaginal bleeding
- Patients with ovarian MLA may present with pelvic or abdominal pain and may be associated with endometriosis and other benign, borderline and malignant lesions of Müllerian origin (Am J Surg Pathol 2021;45:498)
Diagnosis
- Definitive diagnosis of MLA requires a surgical specimen
- Limited literature on the cytologic features of these tumors; may be identified on Pap test as atypical glandular cells (AGC) (Am J Surg Pathol 2021;45:498)
- MLA of the uterine corpus was correctly diagnosed on initial biopsy in only 32% of the cases in one study (Am J Surg Pathol 2021;45:498)
Laboratory
- Patient may have elevated tumor markers (CA125, CA19-9) (Int J Gynecol Pathol 2022;41:161)
Prognostic factors
- Factors associated with development of metastasis in mesonephric adenocarcinoma of the uterine corpus (Am J Surg Pathol 2019;43:12)
- Large tumor size (> 4 cm)
- Ill defined tumor border
- Advanced FIGO stages (III - IV)
- Presence of coagulative tumor cell necrosis
- High mitotic activity (> 10/10 high power fields)
- Lymphovascular invasion (Am J Surg Pathol 2019;43:12)
- Associated with aggressive clinical course (J Clin Med 2021;10:698)
- Tends to present with advanced stage (FIGO II or more)
- Increased risk of recurrent disease
- Increased tendency to metastasize to the lungs even for tumors with stage I disease
- Compared with other endometrial adenocarcinomas (Am J Surg Pathol 2021;45:498)
- Better overall survival than carcinosarcoma and serous carcinoma
- Equal overall survival to endometrioid grade 3
- Worse overall survival than endometrioid grade 1 - 2 carcinomas
Case reports
- 32 year old woman with coexistent endometrial mesonephric-like adenocarcinoma and endometrioid carcinoma (Diagn Pathol 2019;14:54)
- 58 year old woman with endometrial mesonephric-like adenocarcinoma presenting as an ocular lesion (Int J Gynecol Pathol 2022;41:161)
- 69 year old woman with synchronous ovarian and uterine mesonephric-like carcinoma that potentially arose from endometrioid adenofibroma (J Obstet Gynaecol Res 2023;49:1052)
- 70 year old woman with mesonephric-like adenocarcinoma arising from the uterine body and mimicking follicular thyroid carcinoma (Histopathology 2019;74:651)
- 71 year old woman with corded and hyalinized mesonephric-like adenocarcinoma of the uterine corpus (Hum Pathol 2019;86:243)
Treatment
- Surgical approach with total hysterectomy and bilateral salpingo-oophorectomy, with or without pelvic and para-aortic lymph node dissection, is the primary therapy
- Adjuvant chemotherapy and radiation therapy have been used
- Hormone therapy has been reported in 2 cases (Am J Surg Pathol 2020;44:429)
- No tumor specific treatment options have been elucidated for MLA
Gross description
- No distinctive macroscopic appearance compared to other endometrial or ovarian tumors
- Areas of necrosis and hemorrhage can be present
- Ovarian MLA is usually unilateral, ranging in size from 4 - 32 cm, with solid or mixed solid cystic, grey-white or yellow-tan appearance (Histopathology 2016;68:1013)
Microscopic (histologic) description
- Variety of histologic patterns that may be present within the same tumor
- Most frequently small tubules with ductal / glandular growth
- Papillary, solid growth, trabecular, retiform, sex cord-like, sieve-like, glomeruloid and spindle cell areas have all been described
- Luminal eosinophilic secretions are characteristic but not always identified
- Tumor cells can be flattened, cuboidal or columnar with mild to moderate cytological atypia
- Clear cell features can be seen but are less common
- High grade cytological atypia is usually not a predominant feature
- Nuclei show vesicular chromatin and nuclear grooves
- Sarcomatoid transformation has been seen in rare instances
- Squamous, ciliated or mucinous differentiation (metaplasia) are not present and there are no associated mesonephric remnants (J Clin Med 2021;10:698)
Microscopic (histologic) images
Contributed by Daniel Graham, M.D., Adele Wong, M.B., B.Ch., B.A.O. and Lucy Ma, M.D.
Positive stains
- PAX8: usually diffusely positive
- GATA3 and TTF1: focal or diffuse with inverse staining pattern described in several studies in the most recent WHO classification; cells positive for GATA3 are negative for TTF1 and vice versa (Am J Surg Pathol 2018;42:1596)
- CD10: focal and apical / luminal
- p53 wild type
- MMR proficient
Negative stains
- ER
- PR (more reliable negative marker than ER)
- Calretinin (usually negative may be focally positive)
- Reference: J Clin Med 2021;10:698
Molecular / cytogenetics description
- Majority of MLA shows KRAS mutation, most commonly G12V and G12D (Am J Surg Pathol 2020;44:429)
- Concurrent ARID1A and PIK3CA mutations are quite common
- PTEN mutation has been reported
- CTNNB1 hotspot mutations have been reported
- Copy number analysis: copy number gain of 1q and 10 are the most common, some have 1p loss (Mod Pathol 2021;34:1570)
- Usually does not have the common molecular abnormalities described in endometrioid (p53, MMR and POLE) (Mod Pathol 2021;34:1570)
- NRAS mutations may occur (Int J Gynecol Pathol 2018;37:448)
Videos
Mesonephric-like adenocarcinoma by Dr. Lewis Hassell
Sample pathology report
- Uterus and cervix, bilateral fallopian tubes and ovaries, total hysterectomy and bilateral salpingo-oophorectomy:
- Endometrial adenocarcinoma, most consistent with mesonephric-like adenocarcinoma (see comment)
- Comment: The tumor exhibits various growth patterns including small tubular, glandular and papillary areas. Tumor cells show diffuse immunoreactivity for PAX8 and focal immunoreactivity for GATA3 and TTF1. Despite the predominant glandular architecture and low grade appearance, the tumor is negative for ER and PR. The cervix is benign and mesonephric remnants are not identified. All these findings are most consistent with endometrial adenocarcinoma, mesonephric-like subtype.
Differential diagnosis
- Low grade endometrioid adenocarcinoma:
- ER and PR positive
- Small tubules with eosinophilic material are not commonly seen
- Lacks the heterogeneity of architectural patterns seen in MLA
- Squamous, ciliated or mucinous differentiation are not seen in MLA
- GATA3 or TTF1 expression are usually negative
- GATA3 expression can be seen in a 6% of endometrial carcinomas including endometrioid type but these cases were always TTF1 negative (Am J Surg Pathol 2015;39:1411)
- Clear cell carcinoma:
- May show variable architectural patterns and cytological atypia
- Positive HNF-1B, napsin A or alpha methyacyl CoA racemase (AMACR)
- Napsin A and HNF-1B usually negative in MLA but can be positive (Int J Gynecol Pathol 2022;41:161)
- Abnormal p53 and MMR can be seen in clear cell carcinoma, in contrast to MLA
- GATA3 and TTF1 are usually negative
- Serous carcinoma:
- Carcinosarcoma:
- Solid areas and spindled cell and sarcomatoid features
- Abnormal p53 expression
- Absence of KRAS mutations (Cancer Genomics Proteomics 2022;19:747)
- Primary lung adenocarcinoma in metastatic setting:
- Cervical mesonephric adenocarcinoma with the involvement of the uterine corpus:
Additional references
Board review style question #1
A 55 year old woman was recently diagnosed with endometrioid intraepithelial neoplasia on endometrial curettage. The entire endometrium is submitted for histologic evaluation. Histologic details from one section are shown in the image above. The tumor cells are diffusely positive for GATA3 and PAX8 and focally positive for TTF1. ER and PR are negative. Which of the following statements is true regarding this entity?
- KRAS mutations, particularly G12V and G12D, are commonly identified
- MMR deficiency is present in half of the cases
- The tumor frequently shows p53 overexpression
- The tumor has better prognosis compared to low grade endometrioid adenocarcinoma
Board review style answer #1
A. KRAS mutations, particularly G12V and G12D, are commonly identified. Despite the low grade morphology commonly encountered in mesonephric-like adenocarcinoma, the tumor has worse prognosis compared to low grade endometrioid adenocarcinoma. They do not show abnormal p53 expression and are usually MMR proficient. KRAS mutations are commonly seen, with G12V and G12D being the most common alterations identified.
Comment Here
Reference: Mesonephric-like adenocarcinoma
Comment Here
Reference: Mesonephric-like adenocarcinoma
Board review style question #2
Which of the following statements is true regarding mesonephric-like adenocarcinoma occurring in the uterus?
- The tumor commonly expresses GATA3 and TTF1 immunohistochemically
- The tumor expresses hormone receptors similar to those seen in conventional endometrioid carcinoma
- The tumor is most often seen in the myometrium without a surface connection to the endometrium
- The tumor often shows DNA polymerase epsilon (POLE) mutation
Board review style answer #2
A. The tumor commonly expresses GATA3 and TTF1 immunohistochemically. The finding of a hormone receptor negative, PAX8, GATA3 and TTF1 positive tumor is highly supportive of the diagnosis of mesonephric-like adenocarcinoma. While mesonephric remnants and mesonephric derived tumors are often found in paracervical or adnexal locations, this is not true of the mesonephric-like tumors in the endometrium, which have a surface component in virtually all cases. Most such tumors are of no specific molecular type.
Comment Here
Reference: Mesonephric-like adenocarcinoma
Comment Here
Reference: Mesonephric-like adenocarcinoma
Metastases to ovary
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Secondary involvement of ovary from malignant neoplasms of extraovarian primary sites
Essential features
- Ovary is the most common site of metastasis within the gynecologic tract
- Colorectal and breast carcinoma are the most common primary tumors to metastasize to the ovary
- Usually bilateral, small (< 10 cm), multinodular tumors with extraovarian spread
Terminology
- Krukenberg tumor
- Designation should be reserved for tumors with an appreciable component (> 10%) of signet ring cells (Gynecol Oncol 2006;101:152)
- Most commonly of gastric primary (Arch Pathol Lab Med 2006;130:1725)
ICD coding
Epidemiology
- ~15% of ovarian malignancies are of metastatic origin (range: 3 - 30%) (Int J Gynecol Cancer 2009;19:1160)
- Ovarian metastasis may present either synchronously or metachronously with primary tumor
- Ovary is the most common site of metastasis within the gynecologic tract (Int J Gynecol Pathol 2019;38:363)
- Colorectal carcinoma is the most common primary tumor to metastasize to the ovary, followed by breast carcinoma (Int J Gynecol Pathol 2023;42:414, Int J Gynecol Cancer 2009;19:1160)
Sites
- Ovary and primary organ site, with or without other organs
Pathophysiology
- Tumors can spread to the ovary by several pathways, depending on the primary
- Direct extension
- Lymphovascular / hematogeneous spread (Obstet Gynecol Int 2011;2011:612817)
- Transcoelomic / transperitoneal dissemination (e.g., Krukenberg tumors of gastric primary)
- Tumor cell exfoliation / transtubal spread (e.g., concurrent low grade endometrioid endometrial and ovarian carcinomas [FIGO stage IIIA endometrial carcinoma] simulating independent primary tumors) (Histopathology 2020;76:37)
Etiology
- Malignant tumor of extraovarian primary site
Clinical features
- Clinical features often relate to the primary tumor
- Pelvic (ovarian) mass may be the first manifestation of the disease from a clinically occult primary (Virchows Arch 2017;470:69)
Diagnosis
- Comprehensive medical history, physical evaluation, intraoperative evaluation, imaging, endoscopy
- Final diagnosis is rendered by histopathology and immunohistochemistry (with or without molecular studies)
Laboratory
- CA-125 is not a useful biomarker in the primary diagnostics of metastases to the ovary (Clin Exp Metastasis 2017;34:295)
- CA-125/CEA ratio may be of clinical use in distinguishing primary ovarian tumors from colorectal carcinoma metastases (Tumour Biol 1992;13:18)
Radiology description
- No specific imaging features differentiate primary ovarian malignancy from metastatic ovarian tumors (Magn Reson Imaging Clin N Am 2023;31:93)
- MRI appearances that suggest a mucinous lesion should trigger a search on the scan for an alternative primary mass in the gastrointestinal tract
Prognostic factors
- Ovarian metastases from colorectal carcinoma are often unresponsive to chemotherapy and are associated with poor survival (Cancer 2017;123:1134)
Case reports
- 37 year old woman with primary jejunal adenocarcinoma presenting as bilateral ovarian metastasis (Gastroenterology Res 2017;10:366)
- 57 year old Japanese woman with malignant phyllodes tumor of the breast metastasizing to the ovary (Int J Surg Pathol 2022;30:427)
- 61 year old woman with metastatic angiosarcoma to an ovarian Brenner tumor (Int J Gynecol Pathol 2023;42:176)
- 64 year old woman with isolated ovarian metastasis from pancreatic adenocarcinoma and the role of molecular analysis in establishing diagnosis (J Pancreat Cancer 2021;7:74)
- 66 year old woman with primary colorectal carcinoma and metachronous ovarian metastasis (Am J Case Rep 2019;20:1515)
Treatment
- Debulking surgery with or without chemotherapy
Gross description
- Bilateral ovarian involvement (overall occurs in 40 - 80% of cases)
- Primary mucinous ovarian tumors, uncommonly bilateral
- Size: usually < 10 cm
- Generally applied to metastatic mucinous tumors, though may be less reliable than previously reported (Gynecol Oncol 2006;101:152)
- Multinodular, solid and cystic, or friable
- Surface involvement
- Hilar involvement (common in hematogenous spread)
Microscopic (histologic) description
- Morphology varies with appearance of primary tumor
- General features
- Involvement of ovarian surface and superficial cortex
- Infiltrative growth pattern with stromal desmoplasia
- Heterogenous nodular invasive growth (Kurman: Blaustein's Pathology of the Female Genital Tract, 7th Edition, 2019)
- Metastatic tumors more often envelop preexisting normal ovarian structures than primary tumors
- Extensive lymphovascular invasion
- Colorectal adenocarcinoma
- Dilated glands with cribriform epithelium draped along the periphery of luminal dirty necrosis (garland pattern)
- Low grade appendiceal mucinous neoplasm
- Hypermucinous columnar epithelium with low grade cytologic atypia
- Retraction of epithelium from underlying stroma is commonly seen
- Abundant pseudomyxoma ovarii
- Gastric carcinoma
- Frequently presents as Krukenberg tumor (composed predominantly of signet ring cells)
- Stroma may alternate from hypercellular to hypocellular (edematous)
- Breast carcinoma
- Lobular or ductal differentiation
- Signet ring cells may be present
- Endocervical adenocarcinoma
- HPV associated
- Variety of glandular patterns
- Epithelium with hyperchromatic nuclei, apical mitoses, basal apoptoses
- HPV independent, gastric type
- Mucinous epithelium with clear / pale cytoplasm with distinct borders
- Cytologic atypia may range from minimal to severe
- HPV associated
- Endometrial adenocarcinoma
- Endometrioid histotype is more common than serous
- Synchronous endometrial and ovarian endometrioid carcinomas
- May apply Scully criteria, though recent studies suggest that these neoplasms are clonally related (J Natl Cancer Inst 2016;108:djv427, J Natl Cancer Inst 2016;108:djv428)
- Melanoma
- Follicle-like spaces are frequently seen
- Diffuse or nodular growth is common
- Intracytoplasmic melanin pigment
Microscopic (histologic) images
Virtual slides
Positive stains
- Depends on the primary tumor
- References: J Clin Pathol 2012;65:596, Int J Gynecol Pathol 2015;34:257
Negative stains
Molecular / cytogenetics description
- KRAS, SMAD4 and NTKR1 mutations are more frequent in cases of ovarian metastasis from colorectal carcinoma than in cases of colorectal carcinoma without ovarian metastasis (Cancer 2017;123:1134)
Sample pathology report
- Fallopian tube and ovary, right and left, bilateral salpingo-oophorectomy:
- Metastatic carcinoma, consistent with patient's known history of breast primary, involving bilateral ovaries (see comment)
- Bilateral fallopian tubes, negative for tumor
- Comment: On immunohistochemistry, the tumor cells are positive for GATA3, mammaglobin and GCDFP-15, while negative for PAX8. These findings support the diagnosis above.
Differential diagnosis
- Primary ovarian mucinous cystic tumor:
- Differential diagnosis for metastatic low grade appendiceal mucinous neoplasm
- Typically unilateral
- Rarely associated with pseudomyxoma peritonei
- May be associated with Brenner tumor or mature cystic teratoma
- CK7+, CK20-, SATB2-
- Primary ovarian endometrioid carcinoma:
- Differential diagnosis for metastatic colorectal adenocarcinoma
- Commonly associated with endometriosis or adenofibromatous component
- Squamous differentiation may be seen
- CK7+, ER+, PAX8+
- Differential diagnosis for metastatic endometrial endometrioid carcinoma in cases of synchronous endometrial and ovarian endometrioid carcinomas
- Scully criteria favoring primary ovarian endometrioid carcinoma (Scully: Tumors of the Ovary, Maldeveloped Gonads, Fallopian Tube, and Broad Ligament: Atlas of Tumor Pathology, 1st Edition, 1998)
- Associated with endometriosis or endometriotic lesions, a subset of which are clonal and demonstrate cancer associated mutations (J Pathol 2015;236:201)
- Low grade (FIGO grade 1 or 2)
- Histologic dissimilarity between ovarian and endometrial tumors
- No or superficial myometrial invasion of endometrial tumor
- No vascular space invasion, surface implants,or predominantly hilar location in ovary
- Ovarian tumor is unilateral
- Regardless of histopathologic criteria, genomic studies suggest that most cases are clonally related (J Natl Cancer Inst 2016;108:djv427, J Natl Cancer Inst 2016;108:djv428)
- Clonal relationship may not apply to Lynch syndrome patients (Mod Pathol 2021;34:994)
- Scully criteria favoring primary ovarian endometrioid carcinoma (Scully: Tumors of the Ovary, Maldeveloped Gonads, Fallopian Tube, and Broad Ligament: Atlas of Tumor Pathology, 1st Edition, 1998)
- Differential diagnosis for metastatic colorectal adenocarcinoma
- Signet ring stromal tumor of the ovary:
- Differential diagnosis for metastatic signet ring cell carcinoma
- Signet ring morphology in a background of fibromatous stroma
- Signet ring cells contain a single large nonmucinous intracytoplasmic vacuole
- Eosinophilic hyaline globules are common
- SF1+, calretinin+, EMA-
Board review style question #1
A 65 year old woman with an ovarian mass undergoes an oophorectomy. A representative section of the mass is shown above. Which of the following immunoprofiles best supports a diagnosis of metastatic colorectal adenocarcinoma?
- CK7+, CK20+, PAX8-, GATA3+, CDX2-, SATB2-
- CK7+, CK20-, PAX8+, GATA3-, CDX2+, SATB2-
- CK7-, CK20+, PAX8-, GATA3-, CDX2+, SATB2+
- CK7-, CK20-, PAX8-, GATA3-, CDX2-, SATB2-
Board review style answer #1
C. CK7-, CK20+, PAX8-, GATA3-, CDX2+, SATB2+. Compared with the other answer choices, this immunoprofile best supports a colorectal adenocarcinoma primary, which is typically CK7-, CK20+, PAX8-, GATA3-, CDX2+ and SATB2+. Answer A is incorrect as it fits the immunoprofile for a metastatic urothelial carcinoma. Answer B is incorrect as it is more consistent with a primary ovarian endometrioid carcinoma. Answer D is incorrect as it is not immunoreactive to any typical adenocarcinoma markers.
Comment Here
Reference: Metastases to ovary
Comment Here
Reference: Metastases to ovary
Board review style question #2
Which of the following organs is the most common site of metastasis within the gynecologic tract?
- Cervix
- Endometrium
- Fallopian tube
- Ovary
- Vulva
Board review style answer #2
D. Ovary. The ovary is the most common site of metastasis within the gynecologic tract. Answers A, B, C and E are incorrect because the vagina is the second most frequent site of metastasis within the gynecologic tract following the ovary (Cancer 1984;53:1978).
Comment Here
Reference: Metastases to ovary
Comment Here
Reference: Metastases to ovary
Microcystic stromal tumor
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Ovarian stromal tumor with variable microcystic morphology
Essential features
- Ovarian stromal tumor with variably prominent microcystic pattern and regions with lobulated cellular masses separated by hyalinized fibrous stroma
- Aberrant nuclear or cytoplasmic localization of beta catenin
- Rare extracolonic manifestation of familial adenomatous polyposis
ICD coding
- ICD-O: 8590/0 - microcystic stromal tumor
- ICD-11: 2F76 & XH35B3 - neoplasms of uncertain behavior of female genital organs & microcystic stromal tumor
Epidemiology
- Mean age of 45 years (range of 26 - 63 years) (Am J Surg Pathol 2009;33:367)
- May rarely occur as a manifestation of familial adenomatous polyposis (Genes Chromosomes Cancer 2015;54:353, Am J Surg Pathol 2018;42:137, Int J Gynecol Pathol 2016;35:561)
Sites
- Ovary
Pathophysiology
- Presumed to arise from ovarian stromal cells
- Mutually exclusive mutations in CTNNB1 and APC result in aberrant nuclear immunoreactivity for beta catenin and cyclin D1 through activation of the Wnt / beta catenin signaling pathway (Histopathology 2020;76:11)
Etiology
- May rarely occur as a manifestation of familial adenomatous polyposis (Genes Chromosomes Cancer 2015;54:353, Am J Surg Pathol 2018;42:137, Int J Gynecol Pathol 2016;35:561)
Clinical features
- Pelvic mass
- Hormonal manifestations are usually absent (Am J Surg Pathol 2009;33:367)
Diagnosis
- WHO diagnostic criteria
- Essential: stromal neoplasm with variable microcystic morphology
- Desirable
- Diffuse nuclear beta catenin and cyclin D1 immunoreactivity
- Lack of expression of inhibin and calretinin
- Reference: Am J Surg Pathol 2009;33:367
Laboratory
- No specific laboratory findings
Radiology description
- Solid cystic mass with low resistance internal vascularity on ultrasound (Int J Gynaecol Obstet 2024;167:455)
Prognostic factors
- Outcome has been uneventful in most reported cases
- Pelvic recurrence has been reported (4 - 9 years after initial diagnosis) (Hum Pathol 2018;78:171, Int J Gynecol Pathol 2023;42:491)
- Omental and peritoneal involvement / metastasis has been described (Int J Gynecol Pathol 2023;42:491, J Ovarian Res 2021;14:73)
Case reports
- 27 year old pregnant woman with ovarian microcystic stromal tumor with predominant bizarre nuclei coexisting with mucinous cystadenoma (Case Rep Pathol 2022;2022:8457901)
- 33 year old woman with bilateral ovarian recurrence of previously treated microcystic stromal tumor (Hum Pathol 2018;78:171)
- 40 year old woman with ovarian microcystic stromal tumor and familial adenomatous polyposis (Genes Chromosomes Cancer 2015;54:353)
- 45, 56, 61 and 71 year old women with variant morphology of microcystic stromal tumor (Histopathology 2019;74:443)
- 47 year old woman with omental metastasis of ovarian microcystic stromal tumor (J Ovarian Res 2021;14:73)
Treatment
- Complete resection is typically curative, though rare recurrences and metastasis have been reported
Gross description
- Usually unilateral
- Typically Am J Surg Pathol 2009;33:367, Am J Surg Pathol 2015;39:1420)
- Well demarcated solid to cystic tumor; solid component with tan-white cut surfaces, cysts with tan-pink lining (Int J Gynaecol Obstet 2024;167:455)
- Foci of hemorrhage or necrosis may be present
Frozen section description
Microscopic (histologic) description
- Distinctive triad of histologic features (Histopathology 2022;80:898)
- Microcysts: small, round / oval, cystic spaces that may coalesce into larger irregular channels, sometimes with eosinophilic or basophilic contents (Virchows Arch 2017;470:225)
- Solid cellular regions
- Hyalinized hypocellular fibrous stroma
- Multinodular growth with fibrous, hyalinized or myxoid stroma separating nodules (Virchows Arch 2017;470:225)
- Cells have a moderate amount of finely granular eosinophilic cytoplasm, bland round / oval nuclei with fine chromatin and indistinct nucleoli (Histopathology 2019;74:443, Front Med (Lausanne) 2020;7:58)
- Multinucleation or enlarged hyperchromatic bizarre nuclei may be seen (Case Rep Pathol 2022;2022:8457901)
- Intracytoplasmic vacuoles are frequently present, sometimes with signet ring morphology (Histopathology 2022;80:898)
- Low mitotic rate (Histopathology 2022;80:898)
- Less common features
- Psammomatous calcifications or osseous metaplasia may be seen in rare cases (Histopathology 2022;80:898)
- Diffuse, nested, tubular, corded or spindle cell growth, sometimes lacking areas with classical morphology (Histopathology 2022;80:898, Histopathology 2019;74:443)
Microscopic (histologic) images
Positive stains
- Beta catenin: aberrant nuclear or cytoplasmic localization (Histopathology 2020;76:11)
- Cyclin D1: diffuse nuclear reactivity (Histopathology 2022;80:898)
- CD10 (Histopathology 2019;74:443)
- WT1 (Histopathology 2019;74:443)
- SF1 (Histopathology 2022;80:898)
- Androgen receptor (Histopathology 2022;80:898, Histopathology 2019;74:443)
- FOXL2 (Histopathology 2020;76:11)
Negative stains
- Estrogen receptor and progesterone receptor are typically negative (Histopathology 2022;80:898)
- Inhibin (Histopathology 2022;80:898)
- Calretinin (Histopathology 2022;80:898)
- Reticulin may show a nested pattern with absence of pericellular staining within nests (Histopathology 2022;80:898)
Electron microscopy description
- Tumor cells with predominantly spindled and stellate morphology with fewer epithelioid cells, arranged in a loose microcystic pattern
- Cytoplasm shows intermediate filaments and variable amounts of other organelles (Ultrastruct Pathol 2014;38:261)
- Centrally placed nuclei are round with thin nuclear membranes and dispersed euchromatin
- Some nuclei have prominent nucleoli
Molecular / cytogenetics description
- Most microcystic stromal tumors harbor somatic mutations (missense or in frame deletion) in CTNNB1 exon 3 (Histopathology 2020;76:11)
- Frameshift, missense and deletion mutations of APC gene described (20%); CTBNN1 and APC mutations thought to be mutually exclusive (Histopathology 2020;76:11, Hum Pathol 2018;78:171)
- Mutations in FOXL2 and DICER1 have been absent in the tumors tested (Virchows Arch 2017;470:225)
Sample pathology report
- Ovary, right, oophorectomy:
- Right ovary with microcystic stromal tumor (6.5 cm) (see comment)
- Comment: Immunohistochemical stains demonstrate that the neoplastic cells are positive for CD10, WT1 and nuclear beta catenin, supporting the morphologic impression of microcystic stromal tumor. While microcystic stromal tumors are usually benign, rare examples of recurrence and metastasis have been reported. Of note, microcystic stromal tumors are sometimes associated with familial adenomatous polyposis syndrome. Clinical correlation is recommended.
Differential diagnosis
- Adult granulosa cell tumor:
- Nuclear grooves
- Call-Exner bodies
- Positive inhibin, calretinin
- Not expected to show nuclear staining for beta catenin
- Negative (or weak / faint) CD10
- Frequent FOXL2 mutation
- Juvenile granulosa cell tumor:
- Granulosa and theca cells line cysts with variable size
- Negative (or at most faint to moderate) CD10
- Mitoses common
- Thecoma:
- Usually occurs in postmenopausal women
- Associated with estrogenic manifestations
- Solid with yellow cut sections
- Cystic degeneration is rare (Int J Clin Exp Pathol 2019;12:2241)
- Should not show even minor classic microcystic morphology (Arch Pathol Lab Med 2018;142:1459)
- Positive inhibin, calretinin
- Not expected to show nuclear staining for beta catenin
- Negative CD10
- Yolk sac tumor:
- Reticular / cystic pattern with primitive appearance and more mitotic activity (Arch Pathol Lab Med 2018;142:1459)
- Schiller-Duval bodies
- Elevated serum AFP
- Positive SALL4, glypican 3, AFP
- Negative FOXL2, CD10
- Not expected to show nuclear staining for beta catenin
- Steroid cell tumor:
- Often associated with increased levels of androgen
- Should not show even minor classic microcystic morphology (Arch Pathol Lab Med 2018;142:1459)
- Most have at least a minor component of lipid rich cells
- Positive inhibin, MelanA, calretinin
- Struma ovarii:
- Positive TTF1, PAX8
- Negative FOXL2, CD10
- Not expected to show nuclear staining for beta catenin
- Endometrioid carcinoma:
- Positive epithelial membrane antigen
- Pitfall: may show nuclear beta catenin expression
- Negative FOXL2
- Solid pseudopapillary neoplasm:
- Many features shared with microcystic stromal tumor; may exist on a spectrum (Int J Clin Exp Pathol 2015;8:11792, Histopathology 2022;80:898)
- Pseudopapillary structures present
- Positive for CD99, progesterone receptor
- Negative for WT1, FOXL2 by immunohistochemistry
- Sclerosing stromal tumor:
- Staghorn-like blood vessels
- Cells within cellular nodules are rounded to polygonal clear vacuolated cells interspersed with fibroblasts and lutein cells
- Positive for inhibin, calretinin
- Negative for WT1, CD10
- Not expected to show nuclear staining for beta catenin
- Signet ring stromal tumor:
- Predominance of small stromal signet ring cells (usually with a single large vacuole)
- CD10 negative or focally positive
- Variable reactivity for inhibin and calretinin
- Negative for cyclin D1
- No nuclear staining for beta catenin
Additional references
Board review style question #1
Board review style answer #1
B. Microcystic stromal tumor. This tumor shows prominent microcystic features and is not associated with hormonal symptoms. Answer A is incorrect because this tumor does not show the characteristic nuclear grooves or Call-Exner bodies. Answer C is incorrect because this tumor shows prominent microcystic features and is not associated with estrogenic symptoms. Answer D is incorrect because this growth pattern is more consistent with microcystic stromal tumor than yolk sac tumor; Schiller-Duval bodies are not seen in the provided image.
Comment Here
Reference: Microcystic stromal tumor
Comment Here
Reference: Microcystic stromal tumor
Board review style question #2
Which staining pattern below is most consistent with a diagnosis of ovarian microcystic stromal tumor?
- Membranous beta catenin, positive WT1, negative inhibin
- Membranous beta catenin, positive WT1, positive inhibin
- Nuclear beta catenin, negative WT1, negative inhibin
- Nuclear beta catenin, positive WT1, negative inhibin
Board review style answer #2
D. Nuclear beta catenin, positive WT1, negative inhibin. Microcystic stromal tumors typically show nuclear beta catenin staining, positive WT1 and negative inhibin; therefore, answers A - C are incorrect.
Comment Here
Reference: Microcystic stromal tumor
Comment Here
Reference: Microcystic stromal tumor
Mixed carcinoma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Mixed carcinoma of the ovary is a carcinoma comprising 2 or more different histological types, including high grade serous carcinoma (HGSC), endometrioid carcinoma (EC), clear cell carcinoma (CCC), mucinous carcinoma (MC) and low grade serous carcinoma (LGSC)
- Most common combination is mixed endometrioid and clear cell carcinoma
Essential features
- Presence of 2 or more histological types with the components showing distinct morphological differences
- Any percentage of a second histologic type is adequate to label the tumor as a mixed carcinoma according to the WHO classification of female genital tumors (5th edition)
- Immunophenotype of each component mirrors that of the corresponding pure carcinoma
- Majority of mixed carcinomas of the ovary show a common clonal origin (Am J Surg Pathol 2015;39:1548)
- Divergence of 2 histological types (especially mixed clear cell and endometrioid carcinoma) from a common precursor such as endometriosis is common
Terminology
- Mixed cell adenocarcinoma
ICD coding
- ICD-O
- ICD-11
- 2C73.Y & XH2AM6 - other specific malignant neoplasms of the ovary & mixed cell adenocarcinoma
Epidemiology
- Mixed ovarian carcinomas are rare, accounting for ~1% of all ovarian carcinomas (Am J Surg Pathol 2015;39:1548)
- Endometriosis related neoplasms develop in ~1% of patients with endometriosis
- Majority of endometriosis associated tumors are malignant, with endometrioid and clear cell carcinomas being the most common
Sites
- Ovary
Pathophysiology
- Different histological components in most mixed tumors are clonally related and share the same molecular alterations (Am J Surg Pathol 2015;39:1548)
- Endometriosis provides a milieu for neoplastic transformation; the clonal relationship between endometriosis and associated cancers is well established (Cancers (Basel) 2018;10:261)
- Endometrioid and clear cell ovarian cancer with endometriosis have common shared mutations in ARID1A, PIK3CA, CTNNB1, PTEN and KRAS
Etiology
- Endometriosis plays an important role in the development of ovarian mixed carcinoma with endometrioid and clear cell components
Clinical features
- Patients usually present with a pelvic mass or symptoms related to endometriosis or ascites
- Usually occurs in peri and postmenopausal women, although data are limited in the literature
Diagnosis
- In general, imaging studies and serum markers have relatively low specificity and sensitivity for ovarian malignancies
- Definitive diagnosis requires microscopic examination of biopsy or resection specimens
Laboratory
- Serum level measurements of CA125 may be helpful at the time of initial diagnostic work up and for surveillance of disease recurrence
- Rare association with paraneoplastic syndromes with clear cell carcinoma component
Radiology description
- Transvaginal ultrasound is the screening imaging modality of choice and may show a solid or a partially solid and cystic mass with intraluminal papillary projections with increased blood flow (Cancers (Basel) 2022;14:2885)
- Magnetic resonance imaging (MRI) may be used for further work up of inconclusive or indeterminate masses
Prognostic factors
- Prognosis is dependent on the histological components of the tumor and tumor stage
- In one study, there was no significant difference in the median survival between pure ovarian clear cell adenocarcinoma, high grade serous ovarian carcinoma and mixed ovarian carcinomas (clear cell plus serous, clear cell plus endometrioid or serous plus clear cell, plus endometrioid) (Arch Gynecol Obstet 2015;292:923)
- Another study reported no prognostic difference between pure clear cell ovarian carcinoma and mixed ovarian carcinoma with a clear cell component (Int J Gynecol Cancer 2014;24:1590)
- However, further subgroup analysis revealed that patients with mixed clear cell and endometrioid histology had a better prognosis compared to those with mixed clear cell and serous carcinoma
- Endometriosis associated carcinomas are often confined to ovary at presentation and generally have a favorable prognosis (Pathology 2018;50:190)
Case reports
- 48 year old woman with mixed mesonephric-like adenocarcinoma, clear cell carcinoma and endometrioid carcinoma arising from an endometriotic cyst (Int J Surg Pathol 2024;32:1140)
- 49 year old woman with a case of mixed endometrioid and clear cell carcinoma arising from port site endometriosis (J Obstet Gynaecol Res 2019;45:1613)
- 60 year old woman with mixed serous and clear cell adenocarcinoma of the ovary presented with symptomatic hypercalcemia (Perm J 2020;24:19.125)
Treatment
- Complete staging surgery and cytoreductive surgery followed by adjuvant chemotherapy as a standard approach
Gross description
- Macroscopic appearance depends on histological components of the tumor
- Generally, a solid or partially solid and cystic mass with heterogeneous cut surface, hemorrhage and necrosis may be grossly apparent
Microscopic (histologic) description
- At least 2 ovarian carcinoma histological types must be clearly recognizable on morphology (see detailed morphologic descriptions in the corresponding ovarian carcinoma topics)
- Most common combination is mixed endometrioid and clear cell carcinoma
- Any percentage of a second histologic type is sufficient to label the tumor as mixed
- Types present and their percentages should be included in the report
- Immunohistochemistry can be performed to support the presence of different histological components (Am J Surg Pathol 2015;39:1548)
Microscopic (histologic) images
Positive stains
- Immunophenotype of each component reflects that of the corresponding pure carcinoma
- Endometrioid carcinoma (EC) component: PAX8, ER, PR, p53 (wild type pattern)
- HNF-1B is also positive, although usually less intense than in the clear cell carcinoma component
- Clear cell carcinoma (CCC) component: PAX8, napsin A and HNF-1B
- Napsin A (positive in CCC component) and PR (positive in EC component) helps distinguish between the 2 tumor components (Int J Gynecol Pathol 2016;35:430)
Negative stains
Molecular / cytogenetics description
- Majority of the mixed carcinomas of the ovary show a common clonal origin (Am J Surg Pathol 2015;39:1548)
- Mixed ovarian carcinomas with EC and CCC components are often MMR deficient and a subset of cases are associated with Lynch syndrome (Am J Surg Pathol 2014;38:1173)
Sample pathology report
- Uterus, fallopian tubes and ovaries, total hysterectomy and bilateral salpingo-oophorectomy:
- Mixed carcinoma of the right ovary (clear cell carcinoma component 60% and endometrioid carcinoma component 40%) (see comment)
- Background ovarian tissue with endometriosis
- Right ovarian surface not involved by carcinoma
- Left ovary, bilateral fallopian tubes, cervix, endometrium, myometrium and serosa are not involved by carcinoma
- Comment: On immunohistochemistry, the clear cell carcinoma component is positive for PAX8, napsin A and HNF-1B and is negative for ER, PR and WT1. The endometrioid carcinoma component is positive for PAX8, ER and PR and is negative for napsin A and WT1. Both components show loss of MLH1 and PMS2 expression and retained MSH2 and MSH6 immunostaining.
Differential diagnosis
- Pure histologic types of ovarian carcinoma with ambiguous histomorphology and heterogenous morphology:
- Endometrioid carcinomas:
- Can focally display clear cell changes that mimic the solid or papillary patterns of clear cell carcinoma
- High grade serous carcinoma:
- May display a predominantly glandular morphology that mimics endometrioid carcinoma
- BRCA1 mutation associated high grade serous carcinomas have a strong association with a pseudoendometrioid morphology and may be misinterpreted as a mixed endometrioid and high grade serous carcinoma (Mod Pathol 2012;25:625)
- Endometrioid carcinoma with MMR deficiency or POLE exonuclease domain mutation:
- If there is identical MMR deficiency or POLE exonuclease mutation present in different tumor areas, the case should be diagnosed as an endometrioid carcinoma, rather than a mixed carcinoma
- Endometrioid carcinomas:
- Endometrioid carcinoma with extensive mucinous differentiation:
Additional references
Board review style question #1
Board review style answer #1
A. Endometriosis. Divergence of 2 histological types (especially mixed clear cell and endometrioid carcinoma) from a common precursor such as endometriosis is common.
Answer C is incorrect because serous borderline tumor may be associated with low grade serous carcinoma but it is not a precursor lesion of mixed ovarian carcinoma.
Answers B and D are incorrect because mucinous cystadenoma and serous cystadenoma are not precursor lesions of mixed ovarian carcinoma.
Comment Here
Reference: Mixed carcinoma
Comment Here
Reference: Mixed carcinoma
Board review style question #2
Which one of the following statements is true for mixed ovarian carcinomas?
- The most common histologic components are clear cell carcinoma and endometrioid carcinoma
- The most common histologic components are high grade serous carcinoma and endometrioid carcinoma
- They are often associated with germline BRCA1 / 2 mutations
- They are very common, comprising ~30% of all ovarian carcinomas
Board review style answer #2
A. The most common histologic components are clear cell carcinoma and endometrioid carcinoma. Divergence of 2 histological types (especially mixed clear cell and endometrioid carcinoma) from a common precursor such as endometriosis is common.
Answer D is incorrect because mixed ovarian carcinomas are very rare (< 1% of all ovarian carcinomas) (Am J Surg Pathol 2015;39:1548).
Answer B is incorrect because the most common histologic components are clear cell and endometrioid carcinoma.
Answer C is incorrect because mixed ovarian carcinomas are only very rarely associated with BRCA1 / 2 mutations, since those are typically found in high grade serous carcinomas.
Comment Here
Reference: Mixed carcinoma
Comment Here
Reference: Mixed carcinoma
Mixed germ cell - sex cord stromal tumor, unclassified
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Immunofluorescence description | Immunofluorescence images | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Molecular / cytogenetics description | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Definition / general
- Mixed lineage neoplasm composed of germ cells and sex cord cells; occurs in normal gonads in patients without sex chromosomal abnormalities but lacks the distinctive appearance of a gonadoblastoma (Int J Gynecol Pathol 2007;26:313)
- ~10% of tumors have malignant germ cell components (Int J Clin Exp Pathol 2014;7:5259)
Essential features
- Ovarian tumors that are characterized by an irregular mix of dysgerminoma-like germ cells and sex cord cells and lack the characteristic features of gonadoblastoma
- Distinct from gonadoblastoma in macroscopic appearance, histology, absence of regressive changes and occurrence in normal gonads of phenotypically and genetically normal female patients (Int J Gynecol Pathol 1992;11:227)
Terminology
- Pflügerome (Bender: Bulletins Et Mémoires De La Société Anatomique De Paris, 1912)
- Epitheliomas pflugeriens (Maloine et Fils: Diagnostics de laboratoire, 1st Edition, 1923)
- Mixed germ cell sex cord - stromal tumor (MGSCT)
ICD coding
- ICD-O: 8594/1 - mixed germ cell - sex cord stromal tumor, NOS
- ICD-11: 2C73.Y & XH27A8 - other specified malignant neoplasms of the ovary & mixed germ cell - sex cord stromal tumor, unclassified
Epidemiology
- Extremely rare; Less common than gonadoblastoma; true frequency is unknown (Int J Gynecol Pathol 2007;26:313)
- More common in the ovaries than in the testes (Int J Gynecol Pathol 2007;26:313)
- Occurs in female patients with a normal phenotype, normal anatomical and gonadal development and normal 46XX karyotype (Int J Gynecol Pathol 2007;26:313)
- Occurs in male patients with a normal 46XY karyotype (Int J Gynecol Pathol 2007;26:313)
- Typically found in infants or girls younger than 10 years but they may be seen in postmenarchal girls and women of reproductive age (Int J Gynecol Pathol 2007;26:313)
Sites
- Ovary
- Testis (male counterpart)
- Large (> 10 cm) and unilateral (Int J Gynecol Pathol 2007;26:313)
Pathophysiology
- Unknown at this time
Etiology
- Unknown at this time
Diagrams / tables
N/A
Clinical features
- Most tumors occur in infants or children aged Occasionally, tumors are associated with isosexual pseudoprecocity
- References: Int J Gynecol Pathol 2007;26:313, Cancer 1988;61:2122
Diagnosis
- Intraoperative consultation / frozen section
- Resection of tumor
Laboratory
- Elevation of germ cell serological markers
- Alphafetoprotein (AFP)
- Inhibin
- Lactate dehydrogenase (LDH)
- β human chorionic gonadotropin (βhCG)
Radiology description
- Unilateral, solid pelvic / adnexal / abdominal mass
Radiology images
Prognostic factors
- Most of the lesions are clinically benign, with rare cases of dysgerminoma or other malignant germ cell tumors and metastasis; ~10% more frequent in postpubertal patients (Int J Gynecol Pathol 2007;26:313)
- Malignant germ cell tumors are less common in mixed germ cell - sex cord stromal tumors than in gonadoblastoma but metastasis can occur without a secondary malignant component (~20%) (Int J Gynecol Pathol 2007;26:313)
- Female patients with this tumor have had normal full term pregnancies (Fox: Haines & Taylor Obstetrical and Gynaecological Pathology, 5th Edition, 2002)
- Prognosis is favorable without secondary malignant tumors; platinum based chemotherapy is effective when such tumors are present (Int J Gynecol Pathol 2007;26:313)
- 2 ovarian cases had metastasis or recurrence without secondary malignancy, successfully treated with surgery and chemotherapy (Cancer 1988;61:2122, Int J Gynecol Pathol 1998;17:281)
Case reports
- 5 month old girl with 11 cm ovarian mass and monosomy 22 (Genes Chromosomes Cancer 1997;19:192)
- 4 year old girl with precocious puberty and MGSCT with yolk sac tumor (J Med Case Rep 2012;6:162)
- 14 year old girl with precocious puberty and normal phenotype (Cureus 2020;12:e9350)
- 28 year old woman with a right adnexal mass measuring 8 cm in greatest dimension, containing areas with both germ cell and sex cord components (Int J Clin Exp Pathol 2014;7:5259)
Treatment
- Cisplatin based chemotherapy and tumor markers have significantly improved outcomes for patients with secondary malignant germ cell neoplasms (Int J Gynecol Pathol 2007;26:313)
- Since mixed germ cell - sex cord stromal tumors are usually unilateral and occur in normal gonads, removing a normal appearing contralateral gonad is contraindicated; pathologists and surgeons should be aware of the patient's normal phenotype and lack of intersex or chromosomal abnormalities to differentiate this tumor from gonadoblastoma during intraoperative consultation (Int J Gynecol Pathol 2007;26:313)
Clinical images
N/A
Gross description
- Tumors are typically large, unilateral, solid masses with a grayish pink or yellow to pale brown cut surface
Frozen section description
N/A
Frozen section images
N/A
Microscopic (histologic) description
- Variable and irregular mix of germ cells and sex cord cells
- Germ cells are large, with pleomorphic nuclei and clear, PAS positive cytoplasm, resembling dysgerminoma cells
- Sex cord cells may form cords, trabeculae, hollow or solid tubules, cysts or diffuse growth patterns
- Solid, though small spaces or clefts may be present; occasionally, the sex cord element forms a cystic or retiform pattern or annular tubules, similar to features seen in sex cord stromal neoplasms (Int J Gynecol Pathol 1985;4:161, Int J Gynecol Pathol 1998;17:281)
- Germ cell component appears immature with high mitotic activity
- Infrequently, tumors have larger cystic spaces lined by sex cord elements or lack an epithelial lining, sometimes resembling serous neoplasm epithelium (Int J Gynecol Pathol 2007;26:313)
Microscopic (histologic) images
Virtual slides
N/A
Cytology description
N/A
Cytology images
N/A
Immunofluorescence description
N/A
Immunofluorescence images
N/A
Positive stains
- Sex cord elements: inhibin, calretinin, SF1, WT1 (Virchows Arch 2006;448:612)
- Germ cell component
- PLAP, OCT 3/4, CD117 (Virchows Arch 2006;448:612)
- High proliferative index as measured by MIB1 (Virchows Arch 2006;448:612)
- PAS (cytoplasm) (Virchows Arch 2006;448:612)
- Neuron specific enolase (NSE) (Virchows Arch 2006;448:612)
- Cytokeratin AE1 / AE3: perinuclear staining pattern (Virchows Arch 2006;448:612)
Negative stains
- Staining pattern of admixed germ cell and sex cord stromal cell markers highlighting their respective component
Electron microscopy description
N/A
Electron microscopy images
N/A
Molecular / cytogenetics description
- No mutation in the KIT and PDFGRA genes (Virchows Arch 2006;448:612)
Molecular / cytogenetics description
N/A
Videos
N/A
Sample pathology report
- Ovary, right, unilateral oophorectomy:
- Mixed germ cell - sex cord stromal tumor, unclassified (see comment)
- Comment: Mixed germ cell - sex cord stromal tumor components include dysgerminoma (80%) and fibrothecoma (20%).
Differential diagnosis
- Gonadoblastoma:
- Typically occurs in the dysgenetic gonads of intersex patients with a Y chromosome, while mixed germ cell sex cord - stromal tumors develop in normal gonads of patients without sex chromosomal abnormalities (Int J Gynecol Pathol 2007;26:313)
- Anti-Müllerian hormone focally (Hum Pathol 2000;31:1202)
- Positive SALL4 and SF1 (Int J Gynecol Pathol 2013;32:379)
- Occasional KIT mutations (PLoS One 2012;7:e43952)
- Dysgerminoma:
- Mixed germ cell - sex cord stromal tumors with a predominance of germ cells can be mistaken for germinoma, especially when a dysgerminoma component is present (Int J Gynecol Pathol 2007;26:313)
- Positive for CD117 (membranous), OCT 3/4, SALL4, PLAP (PLoS One 2012;7:e43952)
- Isochromosome 12p in 81% (Pathol Res Pract 2012;208:628, Mod Pathol 2006;19:611)
- KIT mutations in exon 17 codon 816 or KIT amplification detected in approximately 33% of cases and associated with advanced stage at presentation (Cancer 2011;117:2096)
- Sex cord stromal tumors:
- In cases where germ cells are few and overlooked, mixed germ cell - sex cord stromal tumors can be misidentified as various types of sex cord stromal tumors (Int J Gynecol Pathol 2007;26:313)
- SF1: sensitive marker (Am J Surg Pathol 2009;33:354)
- Inhibin A: more specific marker
- Adult granulosa cell tumor:
- FOXL2 mutation FOXL2 402C → G (C134W) (N Engl J Med 2009;360:2719, PLoS One 2009;4:e7988, Am J Surg Pathol 2011;35:484)
- Sertoli-Leydig cell tumor:
- DICER hot spot sequences
- 3 molecular subtypes (Am J Surg Pathol 2019;43:628)
- Other mutations identified: MET, CTNNB1, TP53, GNAS (Pathology 2020;52:686)
- DICER hot spot sequences
Additional references
References used elsewhere in topic
Board review style question #1
The tumor shown above is found in the ovary of a 10 year old girl. Which of the following statements accurately distinguishes this tumor from a gonadoblastoma?
- Both mixed germ cell - sex cord stromal tumors and gonadoblastomas typically present with malignant germ cell components in more than 50% of cases
- Gonadoblastomas are characterized by the presence of both germ cells and sex cord stromal cells in patients with normal karyotypes
- Mixed germ cell - sex cord stromal tumors exclusively occur in patients with abnormal sex chromosomes
- Mixed germ cell - sex cord stromal tumors occur in normal gonads of phenotypically and genetically normal patients and lack the characteristic features of a gonadoblastoma
Board review style answer #1
D. Mixed germ cell - sex cord stromal tumors occur in normal gonads of phenotypically and genetically normal patients and lack the characteristic features of a gonadoblastoma. A unique classification of mixed germ cell - sex cord stromal tumor (MGSCT) is that it occurs in patients without chromosomal aberrations, unlike gonadoblastoma. Answer C is incorrect because ovarian MGSCT occurs in patients without chromosomal aberrations. Answer B is incorrect because gonadoblastomas occur in patients with chromosomal aberrations / abnormal karyotypes. Answer A is incorrect because the percentage of MGSCT with a malignant germ cell component is 10%, significantly less than 50%.
Comment Here
Reference: Mixed germ cell - sex cord stromal tumor, unclassified
Comment Here
Reference: Mixed germ cell - sex cord stromal tumor, unclassified
Board review style question #2
Which of the following immunohistochemical stains will display an atypical staining pattern in a mixed germ cell - sex cord stromal tumor?
- Cytokeratin AE1 / AE3
- Inhibin
- OCT 3/4
- PLAP (placental alkaline phosphatase)
Board review style answer #2
A. Cytokeratin AE1 / AE3 will show a unique perinuclear staining pattern in a mixed germ cell - sex cord stromal tumor instead of the typical membranous staining pattern. Answer D is incorrect because PLAP will demonstrate a typical positive staining pattern in the germ cell component. Answer C is incorrect because OCT 3/4 will demonstrate a typical positive staining pattern in the germ cell component. Answer B is incorrect because inhibin will demonstrate a typical positive staining pattern in the sex cord stromal tumor component.
Comment Here
Reference: Mixed germ cell - sex cord stromal tumor, unclassified
Comment Here
Reference: Mixed germ cell - sex cord stromal tumor, unclassified
Board review style question #3
Why is it clinically important to accurately distinguish a mixed germ cell - sex cord stromal tumor from a gonadoblastoma in a patient with an ovarian mass?
- Both tumors have a high risk of metastasis, so aggressive chemotherapy is required in all cases
- Both tumors require removal of the contralateral gonad, even if it appears normal
- Gonadoblastomas typically occur in dysgenetic gonads with a Y chromosome, whereas mixed germ cell - sex cord stromal tumors occur in phenotypically and genetically normal patients, impacting treatment decisions
- Mixed germ cell - sex cord stromal tumors often occur in patients with sex chromosomal abnormalities, influencing genetic counseling
Board review style answer #3
D. Gonadoblastomas typically occur in dysgenetic gonads with a Y chromosome, whereas mixed germ cell - sex cord stromal tumors (MGSCTs) occur in phenotypically and genetically normal patients, impacting treatment decisions. MGSCT occurs in patients without chromosomal aberrations, which does impact treatment decisions. Answer B is incorrect because the typical manage for MGSCT is unilateral oophorectomy with conservation of the contralateral ovary. Answer D is incorrect because MGSCT does not demonstrate typical chromosomal aberrations. Answer A is incorrect because MGSCTs have a low risk of metastasis. Aggressive treatment is not required.
Comment Here
Reference: Mixed germ cell - sex cord stromal tumor, unclassified
Comment Here
Reference: Mixed germ cell - sex cord stromal tumor, unclassified
Mixed germ cell - sex cord stromal tumor, unclassified (pending)
Which of the following is true regarding microinvasion in mucinous borderline tumor?
Which immunohistochemical markers are useful for differentiating this primary ovarian tumor from a low grade appendiceal mucinous neoplasm involving the ovary?
Contributed by Kruti P. Maniar, M.D. and Jian-Jun Wei, M.D.
Images hosted on other servers:
Contributed by Swati Bhardwaj, M.B.B.S., M.D., Tamara Kalir, M.D., Ph.D. and AFIP images
Which of the following is true regarding the ovarian lesion pictured above?
A 35 year old woman is found to have an 8.2 cm right ovarian mass. Histologic sections show a cyst lined by bland columnar epithelium with underlying spindle stroma. What tumors are known to be associated with this type of benign cyst?
A 40 year old woman is seen in the clinic with complaints of irregular uterine bleeding and pelvic fullness. Transvaginal ultrasound shows a 9 cm right ovarian mass. The mass is surgically removed and a pathologic examination shows the features in the picture above. The mass is positive for inhibin and SF1. The tumor is negative for EMA, PAX8, MelanA and chromogranin. In which of the following general categories does this tumor belong?
A 47 year old woman with mucocutaneous oral pigmentations and a history of prior gastrointestinal (GI) polyp resections (multiple hamartomatous polyps) presents for a total abdominal hysterectomy with bilateral salpingo-oophorectomy for menorrhagia. Patient's hysterectomy specimen has no significant histopathologic abnormalities, however, lesion is found in bilateral ovaries. The ovaries were grossly unremarkable. The patient harbors a germline mutation in which of the following genes?
Contributed by Basile Tessier-Cloutier, M.D. and AFIP
A 27 year old woman presented with a 5 cm unilateral ovarian mass. SMARCA4 / BRG1 IHC image is provided above. What is the most likely diagnosis?
Note:
Which of the following is the pTNM stage (per the AJCC 8th edition) of a malignant ovarian tumor involving the right ovarian parenchyma and surface, omentum and splenic capsule (for which the largest tumor focus in the omentum and splenic capsule exceeds 2 cm) and no lymph node or parenchymal spleen metastases?
Contributed by Fatemeh Ghazanfari Amlashi, M.D., Tamara Kalir, M.D., Ph.D. and AFIP
A 57 year old woman presents with postmenopausal bleeding. Endometrial biopsy shows FIGO grade 1 endometrioid type endometrial cancer and hysterectomy reveals stage IA endometrioid type endometrial carcinoma with enlarged bilateral ovaries. Microscopic findings are shown in the image above. What is the diagnosis?
Contributed by Sharon Song, M.S., M.D., Kseniya Korchagina, M.D. and AFIP
Which of the following is true about grading of immature teratoma of the ovary?
A 25 year old pregnant woman is seen in the obstetrics clinic for a routine ultrasound. During the exam, a cystic mass is noted near her left ovary. The mass is eventually surgically removed and a pathologic examination shows the features in the picture shown above. What is an essential feature of this tumor?
Contributed by Victoria Collins, M.D., Tamara Kalir, M.D., Ph.D., AFIP and @SeoparjooAzmel on Twitter
This is a representative image from a solitary ovarian tumor in a 75 year old woman. The tumor shows absent INI1 immunohistochemistry expression. What is the most likely diagnosis?
Contributed by Carlos Parra-Herran, M.D.
Contributed by Lewis A. Hassell, M.D.
Contributed by Gulisa Turashvili, M.D., Ph.D., Sharon Song, M.D. and AFIP images
Back to top
Find related Pathology books: gynecologic
Mixed germ cell tumor
Table of Contents
Gross images | Microscopic (histologic) images | Molecular / cytogenetics descriptionMolecular / cytogenetics description
- Often isochromosome 12p in teratomatous and nonteratomatous components (Mod Pathol 2006;19:766)
Monodermal cystic teratoma
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Benign cystic tumor comprising mature tissues derived from a single germ layer (ectoderm or endoderm), except for struma ovarii, carcinoid and neuroectodermal type tumors (malignant tumors of central neuroectodermal derivation)
Essential features
- Cystic tumor lined by benign keratinizing squamous epithelium or neuroectodermal tissues
- May contain pituitary type adenomas
- Benign with excellent prognosis
Epidemiology
- Monodermal teratomas are rare (Pediatr Dev Pathol 2015;18:341)
- Neuroectodermal cysts have been reported in children and young women
- Epidermoid cysts occur across a wide age range
Sites
- Ovary
Pathophysiology
- Unknown
Etiology
- Unknown
Clinical features
- Abdominal distension or pain
- Amenorrhea due to lactotroph adenoma (Obstet Gynecol 1990;75:540)
- Central obesity and hirsutism due to corticotroph adenoma (Am J Surg Pathol 1987;11:218)
Diagnosis
- Histologic examination
Laboratory
- Elevated prolactin in lactotroph adenoma (JAMA 1990;263:2472)
- Increased cortisol and lack of suppression of either low dose or high dose dexamethasone suppression test in corticotroph adenoma (Am J Surg Pathol 1987;11:218)
Radiology description
- Wide spectrum of radiologic presentation ranging from a purely cystic to a complex cystic mass with a considerable solid component
Radiology images
Prognostic factors
- Benign with excellent prognosis
Case reports
- 2.5 year old girl with ovarian epidermoid cyst torsion associated with appendicitis (Int J Surg Case Rep 2022;100:107731)
- 10 year old girl with neurogenic ovarian cyst (Pediatr Dev Pathol 2015;18:341)
- 36 year old woman with epidermoid cyst treated with laparoscopic cystectomy (Gynecol Minim Invasive Ther 2018;7:40)
- 50 year old woman with a giant (17 cm) epidermoid cyst (J Clin Diagn Res 2012;6:1584)
- 65 year old woman with epidermoid cyst associated with stromal hyperplasia (J Cancer Res Ther 2015;11:1046)
Treatment
- Ovarian cystectomy (Pediatr Dev Pathol 2015;18:341)
- Oophorectomy
Gross description
- Neuroectodermal cysts are thin walled, simple to multilocular cysts filled with clear yellow fluid, ranging from 8 - 15 cm (J Obstet Gynaecol Res 2001;27:21)
- Epidermoid cysts are simple thin walled cysts, with white-grey cheesy material, ranging from 1 - 15 cm (Int J Gynecol Pathol 2009;28:193)
Gross images
Frozen section description
- Rarely performed but usually shows a cystic lesion lined by benign squamous epithelium or neuroectodermal tissues
Microscopic (histologic) description
- Neuroectodermal cyst
- Lined by ependymal cells
- May show choroid plexus-like epithelium along the cyst lining
- Astrocytes, oligodendrocytes, microglia and ganglion cells may be present adjacent to the cyst lining (Histopathology 1994;24:477)
- Epidermoid cyst
- Lined by mature, often keratinizing, stratified squamous epithelium
- Surrounded by a rim of collagenous stroma containing fibroblasts
- Keratinaceous debris fills the cyst lumen, with no evidence of hair or a Rokitansky tubercle (Int J Gynecol Pathol 1996;15:69)
- Rarely, cysts are lined by respiratory type epithelium (Cancer 1979;43:383)
- Lactotroph adenoma
- Closely packed nests of small cells with eosinophilic cytoplasm as seen in the anterior hypophysis (Obstet Gynecol 1990;75:540)
- Corticotroph adenoma
- Monomorphic cells with round nuclei and vacuolated or eosinophilic cytoplasm, growing in nests or cords (Am J Surg Pathol 1987;11:218)
Microscopic (histologic) images
Positive stains
- Immunohistochemistry has a limited role in diagnosis of monodermal cystic teratoma
- Prolactin in lactotroph adenoma (Obstet Gynecol 1990;75:540)
- ACTH in corticotroph adenoma (Am J Surg Pathol 1987;11:218)
- GFAP in neuroectodermal cyst (Histopathology 1994;24:477)
Sample pathology report
- Ovary, right, cystectomy:
- Epidermoid cyst (monodermal cystic teratoma)
Differential diagnosis
- Mature teratoma:
- Mature tissue representing at least 2 germ layers (BMC Cancer 2019;19:217)
- Malignant transformation occurs rarely (most commonly squamous cell carcinoma)
- Immature teratoma:
- Malignant germ cell tumor (Obstet Gynecol 2006;107:1075)
- Contains variable amounts of immature tissue (typically immature neuroepithelium comprising primitive appearing cells with scant cytoplasm, hyperchromatic nuclei and frequent mitoses)
- Steroid cell tumor:
- Pituitary type adenomas may be mistaken for steroid cell tumors with eosinophilic (lipid poor) cells
- Association with ependymal lined neuroectodermal cyst as well as prolactin expression in lactotroph adenoma and ACTH expression in corticotroph adenoma along with negative sex cord stromal markers (inhibin, calretinin, SF1) are helpful clues
Additional references
Board review style question #1
Board review style answer #1
A. Epidermoid cyst. This ovarian cyst is lined by benign keratinizing squamous epithelium, consistent with epidermoid cyst. Answer D is incorrect because struma ovarii is exclusively composed of benign thyroid tissue.
Answer C is incorrect because some monodermal cystic teratomas are lined by respiratory epithelium.
Answer B is incorrect because immature cystic teratoma exhibits immature neuroepithelium in addition to endodermal, mesodermal and ectodermal elements.
Comment Here
Reference: Monodermal cystic teratoma
Comment Here
Reference: Monodermal cystic teratoma
Mucinous borderline tumor
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Noninvasive mucinous neoplasm with complex architecture and gastrointestinal type differentiation
Essential features
- Ovarian tumors with mucinous epithelium of gastrointestinal type and epithelial proliferation (tufting, stratification and villus formation) involving > 10% of tumor
- Mild cytologic atypia, resembling low grade dysplasia of intestinal epithelium
- May be associated with intraepithelial carcinoma (foci with high grade cytologic atypia) and microinvasion (stromal invasion by single cells or small nests, measuring < 5 mm)
- > 90% of tumors are unilateral
- KRAS mutations are the most frequent molecular alterations
Terminology
- Mucinous borderline tumor
- Previously classified as atypical proliferative mucinous tumor or mucinous tumor of low malignant potential
ICD coding
- ICD-O: 8472/1 - mucinous cystic tumor of borderline malignancy
Epidemiology
- Mean age: 45 years (Int J Gynecol Pathol 1995;14:198)
- Second most common subtype of ovarian borderline tumor in Western countries and the most common subtype in Asia (Int J Gynecol Pathol 2011;30:218)
Sites
- Ovary; less frequently in retroperitoneum
Pathophysiology
- May arise from mucinous cystadenoma
- Also associated with Brenner tumor and mature cystic teratoma (Mod Pathol 2020;33:722)
Clinical features
- Symptoms most often are related to pelvic mass
Diagnosis
- May be suggested by pelvic ultrasound
- Definitive diagnosis deferred to oophorectomy
Laboratory
- Patients may have increased CA 125, CEA or CA19-9 levels
Prognostic factors
- Excellent prognosis; stage I at diagnosis
- Mucinous borderline tumor with intraepithelial carcinoma: 95 - 100% overall survival rate (Am J Surg Pathol 2000;24:1447)
- Recurrences may occur following cystectomy
- Tumors with microinvasion are associated with recurrence rate of 5% (Am J Surg Pathol 2007;31:546)
- Anaplastic carcinoma and sarcoma mural nodules are associated with adverse clinical behavior and increased mortality rate; however, anaplastic carcinoma in unruptured stage I tumors does not necessarily carry an adverse prognosis (Am J Surg Pathol 2008;32:383, Int J Gynecol Pathol 1994;13:62)
- Sarcoma-like mural nodule does not have effect on prognosis (Am J Surg Pathol 2002;26:1467)
Case reports
- 42 year old woman with ovarian borderline mucinous tumor accompanied by low grade endometrial stromal sarcoma with myxoid change (Eur J Med Res 2017;22:52)
- 53 year old woman with mucinous borderline tumor with pulmonary and pleural metastasis (Front Med (Lausanne) 2020;7:571348)
- 59 year old postmenopausal woman with primary signet ring cell carcinoma with neuroendocrine differentiation arising in mucinous borderline tumor (Gynecol Oncol Rep 2019;31:100522)
Treatment
- Surgery with staging
Gross description
- > 90% of the tumors are unilateral (Gynecol Oncol 2005;97:80)
- Mean size: 22 cm; some tumors can measure as large as 50 cm (Am J Surg Pathol 2008;32:128)
- Cysts are multiloculated with mucinous contents and smooth external surface
- Solid areas and necrosis may be present
Frozen section description
- Complex architecture with tufting and villus formation
- Glands with luminal mucin
- Mucinous epithelium of gastrointestinal type with goblet cells
- Mild cytologic atypia with nuclear stratification, hyperchromasia and mitotic activity
Frozen section images
Microscopic (histologic) description
- Complex architecture with tufting and villus formation
- Epithelium resembles low grade dysplasia of the intestine with goblet cells, neuroendocrine cells and occasional Paneth cells
- Neoplastic cells have hyperchromasia, crowding, stratification and mitotic activity
- Glands with luminal mucin
- With intraepithelial carcinoma:
- Resembles high grade dysplasia of intestines
- No stromal invasion
- With microinvasion:
- Foci of stromal invasion, measuring < 5 mm in the greatest dimension
- Degree of cytologic atypia is mild and similar to borderline tumor
- Areas of mucin extravasation with inflammatory response are not diagnostic of invasion
- With mural nodule:
- Classified as sarcoma-like, anaplastic carcinoma and sarcoma (Cancer 1979;44:1327)
- Sarcoma-like nodule (reactive): spindled and round cells, multinucleated giant cells and marked inflammation
- Anaplastic carcinoma: rhabdoid, pleomorphic and spindle cells
- Sarcoma: usually without specific differentiation but there are some reports of rhabdomyosarcomatous and leiomyosarcomatous differentiation (Int J Gynecol Pathol 1994;13:62)
- Classified as sarcoma-like, anaplastic carcinoma and sarcoma (Cancer 1979;44:1327)
Microscopic (histologic) images
Cytology description
- Clusters of epithelial cells with nuclear stratification, mild to moderate cytologic atypia and mucinous cytoplasm
Positive stains
- CK7
- CK20 (variable)
- CDX2 (variable) [the extent of expression of CK7 is greater than that of CK20 or CDX2] (Am J Surg Pathol 2006;30:1130)
- PAX8 (focal)
- SATB2 is positive in a small subset of cases, including mucinous tumors associated with teratoma (Ann Diagn Pathol 2015;19:249, Mod Pathol 2019;32:1834)
Molecular / cytogenetics description
- KRAS mutations are identified in 30 - 75% of the tumors (Genome Med 2015;7:87)
- TP53 mutations are present in lower frequency
Sample pathology report
- Ovary, left (oophorectomy):
- Mucinous borderline tumor
- Ovary, right (oophorectomy):
- Mucinous borderline tumor with intraepithelial carcinoma
- Ovary, left (oophorectomy):
- Mucinous borderline tumor with microinvasion
Differential diagnosis
- Seromucinous borderline tumor:
- Admixture of Müllerian type epithelium with endocervical type mucinous epithelium, including serous or ciliated cells
- Associated with endometriosis
- Low grade mucinous neoplasm of appendiceal origin:
- Mucinous epithelium with tall mucinous cells, scalloping, subepithelial clefts, dissecting mucin pools
- Metastatic pancreatobiliary carcinoma:
- Diffuse intraepithelial carcinoma, foci of invasive carcinoma
- Microinvasive carcinoma:
- Stromal invasion morphologically resembles invasive mucinous carcinoma but measuring < 5 mm
- Invasive component has complex architecture and high grade cytologic atypia
- Mucinous cystadenoma:
- Cysts and glands with simple lining composed of nonstratified mucinous epithelium
- Endometrioid adenocarcinoma with mucinous differentiation:
- Mucinous carcinoma with expansile growth pattern:
- Confluent growth pattern with glandular crowding without intervening stroma, measuring ≥ 5 mm
Additional references
Board review style question #1
What is the most common mutation associated with ovarian mucinous borderline tumors?
- BRAF
- CDKN2A
- KRAS
- TP53
Board review style answer #1
C. KRAS. KRAS mutations are present in 30 - 75% of mucinous borderline tumors.
Comment Here
Reference: Mucinous borderline tumor
Comment Here
Reference: Mucinous borderline tumor
Board review style question #2
Which of the following is true regarding microinvasion in mucinous borderline tumor?
- Associated with adverse clinical behavior
- Associated with mucin extravasation with inflammatory response and histiocytes
- Cytologic atypia is severe and similar to intraepithelial carcinoma
- Foci of stromal invasion measuring < 5 mm in the greatest dimension
Board review style answer #2
D. Foci of stromal invasion measuring < 5 mm in the greatest dimension. Cytologic atypia in microinvasion is mild to moderate, similar to adjacent borderline tumor. Presence of severe atypia warrants the diagnosis of microinvasive carcinoma. Mucin extravasation with inflammatory response and histiocytes is associated with gland rupture and not diagnostic of microinvasion. Overall, mucinous borderline tumors have excellent prognosis. Microinvasion is not associated with adverse behavior.
Comment Here
Reference: Mucinous borderline tumor
Comment Here
Reference: Mucinous borderline tumor
Mucinous carcinoma
Table of Contents
Definition / general | Clinical features | Prognostic factors | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Molecular / cytogenetics description | Differential diagnosis | Additional referencesDefinition / general
- 77% of ovarian mucinous carcinomas are metastases, 23% are ovarian primaries (Am J Surg Pathol 2003;27:985)
- Of the ovarian primaries, most arise in a benign or borderline tumor; only 5-10% are pure
- Features favoring primary ovarian carcinoma vs. metastasis are: unilateral, "expansile" pattern of invasion, complex papillary pattern, size > 10 cm, smooth external surface, microscopic cystic glands, necrotic luminal debris, mural nodules and accompanying teratoma, adenofibroma, endometriosis or Brenner tumor (Am J Surg Pathol 2003;27:281)
- Stromal invasion > 10 mm2 distinguishes these tumors from borderline tumors
- Two types of invasion - expansile or infiltrative:
- Expansile tumors are usually stage I and behave "benign"
- Infiltrative tumors may demonstrate malignant behavior and cause death even if stage I (Am J Surg Pathol 2002;26:139)
Clinical features
- Distant metastases are rare
- Survival: 95% for stage I vs. 32% for stages II or greater
Prognostic factors
- Poor prognostic factors for stage I tumors: infiltrative invasion (destructive stromal invasion, Mod Pathol 2005;18:903), high nuclear grade, tumor rupture
- Anaplastic components in intact tumors do not affect prognosis (Am J Surg Pathol 2002;26:139)
Gross description
- Primary tumors are usually unilateral, > 10 cm, smooth capsule, cystic and solid areas of tumor evenly distributed throughout ovary without discrete nodularity
Gross images
Microscopic (histologic) description
- Stromal invasion; also more solid growth, atypia, stratification, papillae, loss of glandular architecture, necrosis (resembles colon carcinoma), greater complexity of glands than borderline tumors
- Stromal invasion may be infiltrative with disorderly penetration of stroma by neoplastic glands, single cells or cell clusters, may have desmoplastic response or expansile (confluent) with complex arrangement of glands, cysts or papillae lined by malignant epithelium with minimal or no intervening stroma with a broad, sharply defined border
- Glands are almost always intestinal type
- Endocervical type usually has other epithelial components (serous, endometrioid, squamous)
- Carcinoma often merges with borderline or benign mucinous tumors
- Rarely has signet ring cells, but differs from Krukenberg tumor
(Am J Surg Pathol 2008;32:1373)
- Grading:
- Not standardized and does not predict prognosis independent of stage (Am J Surg Pathol 2000;24:1447)
- Grade 1-no solid areas
- Grade 2-up to 50% solid foci
- Grade 3-more than 50% solid foci
- Severe nuclear atypia can increase raise grade I or II carcinomas by one grade
Microscopic (histologic) images
Positive stains
- CEA, CK7, CK20, CA125 (weak)
Molecular / cytogenetics description
- K-ras mutations are common
Differential diagnosis
- Endometrioid carcinoma
- Metastatic tumor: bilateral tumors of any size, unilateral tumor Am J Surg Pathol 2008;32:128)
Additional references
Mucinous cystadenoma and adenofibroma
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Benign mucinous neoplasm composed of cysts and glands lined by gastrointestinal or Müllerian type mucinous epithelium lacking architectural complexity or cytologic atypia
Essential features
- Includes cystadenoma and adenofibroma
- Usually unilateral and composed of variable amounts of cysts and glands lined by bland gastrointestinal or Müllerian type mucinous epithelium and variably cellular stroma
- May be associated with mature teratoma or Brenner tumor
- Excellent prognosis, with rare recurrences associated with cystectomy or rupture
ICD coding
- ICD-O:
- ICD-11:
- 2F32.Y & XH6H73 - other specified benign neoplasm of ovary and mucinous cystadenoma, NOS
- 2F32.Y & XH59X8 - other specified benign neoplasm of ovary and mucinous adenofibroma, NOS
Epidemiology
- Accounts for 80% of primary ovarian mucinous neoplasms
- Mucinous cystadenoma > adenofibroma (Int J Gynecol Pathol 2005;24:4, Cancer 1985;55:1958)
- Median age 50 years (range 13 - 79) (Cancer 1985;55:1958)
Sites
- Usually ovary
- Less commonly, retroperitoneum (Int J Surg Case Rep 2012;3:486)
Pathophysiology
- May arise from mature teratoma (possible germ cell origin) or Brenner tumor (Arch Pathol Lab Med 2008;132:1753)
- KRAS mutations in 68% of cases (Cancer 1997;79:1581)
Etiology
- Unknown
Clinical features
- Abdominal distention with or without palpable mass
- Abdominal or pelvic pain
- Other symptoms related to abdominal / pelvic mass
- Rarely, estrogenic or androgenic manifestations secondary to stromal luteinization (Int J Gynecol Pathol 2005;24:4)
Diagnosis
- Microscopic examination
Laboratory
- Rarely, elevated CA-125 (Cancer 1985;55:1958)
Radiology description
- Ultrasonography:
- Usually large multilocular cystic adnexal mass with numerous thin septations
- Various degrees of echogenicity in different locules
- Low level internal echogenicity due to increased mucin content
- Magnetic resonance imaging:
- Usually large multilocular cyst containing fluid of various viscosity resulting in variable signal intensities on both T1 and T2 sequences (stained glass appearance)
- Reference: Radiographics 2000;20:1445, Radiographics 2019;39:982
Prognostic factors
- Excellent prognosis
- Recurrences may occur after cystectomy or rupture and spillage (J Obstet Gynaecol Res 2006;32:615, Am J Obstet Gynecol 2010;202:142.e1)
Case reports
- 13 year old girl with recurrent ovarian mucinous cystadenoma after cystectomy (Int J Surg Case Rep 2021;83:106006)
- 22 year old woman with metachronous ipsilateral recurrent ovarian mucinous cystadenoma in initial pregnancy and mature teratoma in subsequent pregnancy (Cureus 2019;11:e3818)
- 40 year old woman with an ovarian mixed Brenner tumor and mucinous cystadenoma (J Clin Imaging Sci 2020;10:22)
- 71 year old woman with postmenopausal hyperandrogenism associated with ovarian mucinous cystadenoma (BMJ Case Rep 2021;14:e237505)
Treatment
- Oophorectomy or cystectomy
Gross description
- Usually unilateral (95%)
- Smooth or bosselated surface
- Cystadenoma:
- Uni or multilocular cyst with variably sized smooth walled locules
- Filled with dense, viscous, sticky, gelatinous material
- No solid areas or papillary excrescences
- Mean size 10 cm, rarely > 30 cm
- Adenofibroma:
- Usually smaller
- Predominantly white and solid with variable amounts of small cysts (Int J Gynecol Pathol 2005;24:4, Surg Pathol Clin 2019;12:565)
Frozen section description
- Benign cystic neoplasm lined by a single layer of bland mucinous epithelium (cystadenoma) or solid and cystic neoplasm composed of small glands or cysts lined by a single layer of bland mucinous epithelium set in a fibromatous stroma (adenofibroma)
Frozen section images
Microscopic (histologic) description
- Mucinous cystadenoma:
- Uni or multilocular cystic neoplasm composed of multiple cysts and glands lined by a single layer of bland mucinous epithelium
- Mucinous adenofibroma:
- Solid and cystic neoplasm composed of small cysts or glands lined by a single layer of bland mucinous epithelium
- Stroma is fibromatous and variably cellular with or without luteinization
- Focal extravasated mucin or mucin granulomas with numerous histiocytes may be present secondary to cyst / gland rupture
- General features:
- Simple nonstratified mucinous epithelium resembling Müllerian, intestinal or gastric foveolar type epithelium
- > 80% are intestinal type containing goblet cells
- Epithelium may be undulating but usually no epithelial stratification or tufting
- Columnar, cuboidal to flat nonciliated cells
- Variable amounts of mucinous cytoplasm
- Small basally located nuclei lacking cytological atypia
- Absent or minimal mitotic activity and apoptotic bodies
- Spiculated calcifications (62.5%) (Arch Pathol Lab Med 2008;132:1753)
- Rare features:
- Tubular crypt-like outpouchings or papillary infoldings at the periphery of cysts
- Focal mild cytologic atypia and mitoses
- Goblet cells with or without neuroendocrine cells or Paneth cells
- Periglandular stromal condensation and luteinization (more common in pregnancy) (40%) (Arch Pathol Lab Med 2008;132:1753)
- Sex cord-like differentiation
- Necrosis (12.5%) (Arch Pathol Lab Med 2008;132:1753)
- Pseudoxanthoma cells (47.5%) (Arch Pathol Lab Med 2008;132:1753)
- Muciphages (42.5%) (Arch Pathol Lab Med 2008;132:1753)
- Pseudomyxoma ovarii (stromal dissecting mucin) and giant cell reaction (10%) (Arch Pathol Lab Med 2008;132:1753)
- May be associated with:
- Brenner tumor (18%) (Arch Pathol Lab Med 2008;132:1753)
- Mature teratoma (2 - 11%)
- Mural nodules (Indian J Med Sci 2005;59:499, Cancer 1979;44:1327, J Korean Med Sci 1998;13:680)
- Clear cell carcinoma (J Clin Pathol 2000;53:938, J Pak Med Assoc 2007;57:373, Int J Gynecol Pathol 2009;28:584)
- Large cell neuroendocrine carcinoma (Int J Gynecol Pathol 1996;15:167)
- Endometrioid carcinoma (Ann Diagn Pathol 2003;7:300)
- Sex cord stromal tumors, such as granulosa cell tumor and Sertoli-Leydig cell tumor (Arch Pathol Lab Med 1986;110:528, Int J Gynecol Pathol 2005;24:224)
Microscopic (histologic) images
Virtual slides
Cytology description
- Usually negative cytology or reactive mesothelial cells
Positive stains
- CK7 (Am J Clin Pathol 2002;117:944)
- PAX8: variable, may be focal
- CK20: variable (Am J Clin Pathol 2002;117:944)
- CDX2: variable
Negative stains
Molecular / cytogenetics description
- KRAS mutations in 68% of cases (Cancer 1997;79:1581)
Sample pathology report
- Right fallopian tube and ovary, salpingo-oophorectomy:
- Ovary: mucinous cystadenoma
- Fallopian tube: benign
Differential diagnosis
- Mucinous tumor with focal atypia / proliferation (Hum Pathol 2004;35:949, Hum Pathol 2004;35:918, Am J Surg Pathol 1991;15:227):
- Focal (< 10%) architectural complexity, including papillae and tufting or nuclear pseudostratification and crowding
- Mucinous borderline tumor:
- Architectural complexity with variable degrees of epithelial stratification, tufting and villous or slender filiform papillae in at least 10% of tumor
- Metastatic low grade appendiceal mucinous neoplasm:
- Mucin on ovarian and extraovarian surfaces (pseudomyxoma peritonei)
- Bilateral ovarian involvement
- Detachment of mucinous epithelium from stroma (cleft-like space)
- Hypermucinous (highly differentiated, tall, mucin rich) cells
- Positive for CK20, CDX2 and SATB2; negative for PAX8, usually negative for CK7
- Metastatic adenocarcinoma:
- Variable differentiation with high grade nuclear atypia and mitotic activity (gastrointestinal and pancreatico-hepatobiliary tract)
- Nuclear pseudostratification, apical mitoses and apoptotic bodies (human papillomavirus associated endocervical adenocarcinoma)
- Often bilateral, multinodular, with surface involvement, at least focal infiltrative growth and lack of correlation between architecture and cytology (Anticancer Res 2018;38:5465)
Board review style question #1
Which immunohistochemical markers are useful for differentiating this primary ovarian tumor from a low grade appendiceal mucinous neoplasm involving the ovary?
- CK7, CK20, CDX2, SATB2 and PAX8
- CK7, CK20, CDX2, WT1 and SATB2
- CK7, CK20, PAX8, ER and SATB2
- CK7, CK20, SATB2, ER and PR
- CK7, CK20, SATB2, WT1 and PAX8
Board review style answer #1
A. CK7, CK20, CDX2, SATB2 and PAX8. Low grade appendiceal mucinous neoplasm is usually positive for CK20, CDX2 and SATB2 and negative for CK7 and PAX8.
Comment Here
Reference: Mucinous cystadenoma / adenofibroma
Comment Here
Reference: Mucinous cystadenoma / adenofibroma
Board review style question #2
Which of the following is true for ovarian mucinous cystadenoma?
- Always positive for PAX8
- May be associated with mature teratoma or Brenner tumor
- Most common in postmenopausal women
- Usually bilateral ovarian involvement
- Usually positive for ER and PR
Board review style answer #2
B. May be associated with mature teratoma or Brenner tumor
Comment Here
Reference: Mucinous cystadenoma / adenofibroma
Comment Here
Reference: Mucinous cystadenoma / adenofibroma
Mural nodules in mucinous cystic neoplasms
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology / etiology | Clinical features | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Negative stains | Molecular / cytogenetics description | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Grossly circumscribed nodules, most associated with primary ovarian mucinous neoplasms (less frequently other ovarian tumor types)
- May be microscopically invasive but still separate / distinct from the associated mucinous tumor (Int J Gynecol Pathol 1994;13:62)
Essential features
- May be reactive (sarcoma-like), benign neoplastic (leiomyomatous) or malignant (anaplastic carcinoma, sarcoma, carcinosarcoma)
- Associated mucinous tumor may be benign, borderline or malignant
- Can present a diagnostic challenge as the discrimination between truly malignant versus reactive mural nodules can be difficult
Terminology
- General term: ovarian mucinous neoplasm with mural nodule
- Various classification schema used (Int J Gynecol Pathol 1994;13:62, Cancer 1979;44:1332, Am J Surg Pathol 2008;32:383):
- Reactive (sarcoma-like mural nodules)
- Pleomorphic and epulis-like
- Pleomorphic and spindle celled
- Giant cell histiocytic
- Benign neoplastic
- Malignant neoplastic
- Anaplastic carcinoma: rhabdoid, spindled or pleomorphic
- Sarcoma
- Carcinosarcoma
- Reactive (sarcoma-like mural nodules)
ICD coding
Epidemiology
- Females of varying ages and parity
- Sarcomatous nodules generally in older patients compared to sarcoma-like nodules (Am J Surg Pathol 2002;26:1467)
- Sarcoma-like mural nodules: 18 - 83 years (mean 39 years) (Cancer 1979;44:1332, Am J Surg Pathol 2002;26:1467)
- Sarcomatous mural nodules: 49 - 72 years (mean 61 years) (Int J Gynecol Pathol 1994;13:62)
- Anaplastic carcinoma mural nodules: 15 - 93 years (mean 44 years) (Am J Surg Pathol 2008;32:383)
- Carcinosarcoma mural nodules: 27 - 44 years (mean 33 years) (Int J Gynecol Pathol 1994;13:62)
Sites
- Ovary (almost always unilateral)
Pathophysiology / etiology
- Unclear histogenesis; however, may evolve through divergent differentiation of mucinous neoplasm or through a collision phenomenon (Int J Gynecol Pathol 2015;34:19)
- Sarcoma-like mural nodules: likely common histogenesis for different subtypes, favored to be reactive and of histiocytic origin (Cancer 1979;44:1332, Am J Surg Pathol 2002;26:1467)
- Malignant mural nodules clonally related to accompanying mucinous tumor and may represent dedifferentiation (Am J Surg Pathol 2017;41:1261)
- Targeted next generation sequencing has shown a clonal relationship between carcinomatous mural nodules and the ovarian mucinous tumor, indicating a dedifferentiation process (Am J Surg Pathol 2017;41:1261)
Clinical features
- Majority of patients present with abdominal enlargement or pain (Cancer 1979;44:1332, Cancer 1979;44:1327, Am J Surg Pathol 2008;32:383)
- Less common presentations: menometrorrhagia, vomiting, intestinal obstruction, vaginal bleeding (Cancer 1979;44:1332, Am J Surg Pathol 2002;26:1467, Am J Surg Pathol 1991;15:1055)
- Stage at presentation:
- Sarcoma-like mural nodules: all FIGO stage Ia (Cancer 1979;44:1332, Am J Surg Pathol 2002;26:1467)
- Anaplastic carcinoma mural nodules: 68% stage I (Ia or Ic), 32% stage II or higher (Am J Surg Pathol 2008;32:383)
- Sarcomatous mural nodules: 60% stage I, 40% stage III or IV (Int J Gynecol Pathol 1994;13:62)
- Carcinosarcoma mural nodules: 75% stage I, 25% stage III (Int J Gynecol Pathol 1994;13:62)
Prognostic factors
- Sarcoma-like mural nodules behave in benign fashion with no effect on prognosis of associated mucinous tumor (Cancer 1979;44:1332, Am J Surg Pathol 2002;26:1467)
- Overt sarcoma and anaplastic carcinoma carry a much worse prognosis:
- For malignant mural nodules overall: 39% mortality (3 months to 1.5 years) (Am J Surg Pathol 2002;26:1467)
- Anaplastic carcinoma mural nodules: 33% mortality (median 8 months) (Am J Surg Pathol 2008;32:383)
- Sarcoma mural nodules: 67% mortality (within 4 years) (Int J Gynecol Pathol 1994;13:62)
- Among anaplastic carcinomas, FIGO stage is the only significant prognostic factor (stage Ia tumors do not necessarily carry an adverse prognosis) (Am J Surg Pathol 2008;32:383)
Case reports
- 18 and 34 year old women, both with osteosarcomatous mural nodules (Int J Gynecol Pathol 2015;34:369)
- 29 year old woman with solid mural leiomyoma associated with mucinous cystadenoma (Sultan Qaboos Univ Med J 2013;13:127)
- 30 year old woman with borderline mucinous tumor and sarcoma-like mural nodule (J Midlife Health 2014;5:192)
- 36 year old woman with anaplastic carcinoma mural nodule associated with rapid and fatal outcome (Int J Gynecol Pathol 2016;35:348)
- 45 year old woman with mural nodule of anaplastic spindle cell carcinoma within a mucinous borderline tumor (J Ovarian Res 2013;6:86)
- 48 year old woman with multifocal anaplastic carcinoma and sarcoma-like mural nodules (Int J Clin Exp Pathol 2013;6:1688)
- 48 year old woman with anaplastic carcinoma nodule and 60 year old woman with sarcomatous mural nodule (Medicine (Baltimore) 2017;96:e8636)
- 53 year old woman with borderline ovarian seromucinous cystic tumor and anaplastic carcinoma mural nodule (J Ovarian Res 2018;11:77)
Treatment
- Appropriate treatment depends on nature of mural nodule (benign / reactive versus malignant) as well as type of associated mucinous tumor and stage at presentation
- Tumors with sarcoma-like nodules can be followed after oophorectomy
- Staging surgery can be considered in malignant mural nodules, as well as systemic treatment (particularly in patients with extra-ovarian tumor spread)
- Reported cases have been variably treated (Cancer 1979;44:1332, Am J Surg Pathol 2002;26:1467, Am J Surg Pathol 2008;32:383)
- Surgical treatment: unilateral salpingo-oophorectomy, bilateral salpingo-oophorectomy or bilateral salpingo-oophorectomy with hysterectomy
- Subset of patients also underwent omentectomy, appendectomy or lymphadenectomy
- Subset of patients treated with adjuvant chemotherapy, especially in cases of malignant nodules
Gross description
- Typically unilateral, large, unilocular or multilocular cystic mass with smooth external surface (7 - 62 cm, mean ~20 cm) (Am J Surg Pathol 2008;32:383, Cancer 1979;44:1332, Am J Surg Pathol 2002;26:1467)
- 1 or more well circumscribed nodules protruding into lumen
- May be dark brown, hemorrhagic and soft, or firm and yellow-tan; can have necrosis (Am J Surg Pathol 2008;32:383, Cancer 1979;44:1332, Cancer 1982;50:300)
Gross images
Microscopic (histologic) description
- Associated mucinous tumor: endocervical or intestinal type; cystadenoma, borderline tumor or carcinoma (Am J Surg Pathol 2002;26:1467, Cancer 1979;44:1332)
- Mural nodules: sharply circumscribed, separated from overlying cyst lining by a layer of hypocellular stroma (Am J Surg Pathol 2002;26:1467)
- Sarcoma-like mural nodules: 3 types (Cancer 1979;44:1332, Am J Surg Pathol 2002;26:1467)
- Pleomorphic and epulis-like type (resemble giant cell reparative granuloma / giant cell epulis of gingiva):
- Loose network of oval to spindled mononucleate cells with marked nuclear pleomorphism
- Uniformly distributed, multinucleated osteoclast-like giant cells, some with phagocytized red blood cells and debris
- Blood filled spaces
- Variable mitotic activity, can have atypical forms
- Pleomorphic and spindle cell type:
- Fascicles of spindle cells with elongated hyperchromatic nuclei and prominent nucleoli
- Multinucleated giant cells similar to epulis-like type
- Diffuse extravasation of red blood cells; blood filled spaces
- Variable mitotic activity, can have atypical forms
- Mitotic index ranges from 5 to 10/10 HPF (usually in the most cellular areas)
- Giant cell histiocytic type:
- Round mononucleate giant cells in closely packed aggregates separated by delicate fibrous septa
- Cells with vesicular nuclei, ill defined borders, abundant ground glass eosinophilic cytoplasm and minute vacuoles
- No multinucleated giant cells as in other types
- Mitoses rare
- Variable but often marked mixed inflammatory infiltrate in all types of nodules
- Some nodules contain scattered cuboidal epithelium lined glands (likely entrapped)
- Most cases have multiple nodules within the tumor
- Associated mucinous tumor is carcinoma in 50% of cases, the rest being benign and borderline in nature
- Pleomorphic and epulis-like type (resemble giant cell reparative granuloma / giant cell epulis of gingiva):
- Anaplastic carcinoma mural nodules (Am J Surg Pathol 2008;32:383):
- In general, characterized by poor circumscription, tumor infiltration into adjacent stroma and vessels, and necrosis
- Can have scattered multinucleated giant cells of epulis type
- Divided into 3 types:
- Rhabdoid: diffusely arranged cells with abundant bright pink cytoplasm and atypical, eccentrically placed nuclei
- Spindled: spindle cells with atypical vesicular nuclei and moderate eosinophilic cytoplasm, often in herringbone pattern; areas of obvious epithelial differentiation
- Pleomorphic: admixture of above types, more variegated
- Sarcomatous mural nodules: various types of sarcoma described, including fibrosarcoma, rhabdomyosarcoma, undifferentiated sarcoma (Cancer 1979;44:1327, Int J Gynecol Pathol 1994;13:62)
- Other mural nodule types described:
- Multiple or mixed types of nodules may occur in same tumor (Am J Surg Pathol 2002;26:1467)
Microscopic (histologic) images
Contributed by Kruti P. Maniar, M.D. and Jian-Jun Wei, M.D.
Images hosted on other servers:
Positive stains
- Sarcoma-like mural nodules (Am J Surg Pathol 2002;26:1467):
- Vimentin
- CD68 (in most)
- Variable calretinin, smooth muscle actin, muscle specific actin
- Sarcomatous mural nodules: vimentin (Int J Gynecol Pathol 1994;13:62)
- Anaplastic carcinoma mural nodule:
- Pankeratin is positive in many cases (Int J Gynecol Pathol 1994;13:62)
- However, staining can be focal
- Moreover, a negative result does not exclude anaplastic carcinoma
- Leiomyomatous mural nodule: vimentin, desmin, smooth muscle actin, muscle specific actin (Int J Gynecol Pathol 1990;9:80, Am J Surg Pathol 1991;15:1055)
Negative stains
- Sarcoma-like mural nodules (Am J Surg Pathol 2002;26:1467):
- Pankeratin (but can have focal / weak expression)
- ALK, CD34, desmin
- Sarcomatous mural nodule: pankeratin (Int J Gynecol Pathol 1994;13:62)
- Leiomyomatous mural nodule: pankeratin (Int J Gynecol Pathol 1990;9:80, Am J Surg Pathol 1991;15:1055)
Molecular / cytogenetics description
- Recent next generation sequencing study found identical KRAS mutations in paired mucinous tumors and carcinomatous mural nodules (Am J Surg Pathol 2017;41:1261, Int J Gynecol Pathol 2015;34:19)
- Identical CDH1 and p53 mutations may also be seen in both components
- Unpaired p53 and PTEN mutations detected in 2 mural nodules
- One case of different KRAS mutations in sarcomatous mural nodule and associated mucinous tumor - suggests different clones of same tumor (Int J Gynecol Pathol 2014;33:186)
- Overall findings support neoplastic nature of carcinomatous mural nodules and clonal relationship with better differentiated mucinous tumor; acquisition of additional mutations within the mucinous neoplasm may result in transformation to anaplastic morphology (Am J Surg Pathol 2017;41:1261)
- Further larger molecular studies still needed to clearly elucidate the mechanisms and molecular alterations underlying the different types of mural nodules
Differential diagnosis
- Distinguishing different types of mural nodules:
- Sarcoma-like mural nodules versus anaplastic carcinoma:
- Irregular borders, epithelioid and cohesive areas are indicative of carcinoma
- Keratins are useful if positive; however, a negative result should be interpreted with caution (Virchows Arch A Pathol Anat Histopathol 1991;419:89)
- Sarcoma-like mural nodules versus true sarcomatous nodules:
- Immunohistochemistry not useful (both keratin-, vimentin+) (Virchows Arch A Pathol Anat Histopathol 1991;419:89)
- Features favoring true sarcoma: irregular or infiltrative nodule borders, monotonous population of spindle cells, significant nuclear atypia, evidence of vascular or stromal invasion, presence of atypical heterologous elements, older patient age, large nodule size (Virchows Arch A Pathol Anat Histopathol 1991;419:89, Int J Gynecol Pathol 1994;13:62)
- Sarcoma-like mural nodules versus anaplastic carcinoma:
- Other differential diagnoses:
- Carcinosarcoma: carcinoma component usually not mucinous; carcinoma and sarcoma elements more intimately admixed
- Pure sarcoma: no associated epithelial component
- Inflammatory myofibroblastic tumor: larger, less circumscribed, often immunohistochemical positivity for ALK
Board review style question #1
Which of the following features favors diagnosis as a true sarcomatous mural nodule?
- Monotonous spindle cells with infiltration into stroma
- Positivity for CD68
- Positivity for pankeratin AE1 / AE3
- Presence of extravasated red blood cells and blood lakes
- Scattered osteoclast-like multinucleated giant cells
Board review style answer #1
A. Monotonous spindle cells with infiltration into stroma
Comment Here
Reference: Mural nodules in mucinous cystic neoplasms
Comment Here
Reference: Mural nodules in mucinous cystic neoplasms
Board review style question #2
60 year old patient had a 30 cm mucinous ovarian tumor removed. Gross exam demonstrated a 5 cm mural nodule. Based on the histologic image of the mural nodule (right) and the overlying mucinous tumor (left), which of the following is most likely true regarding tumor behavior?

- Absence of vascular invasion indicates that the tumor is benign
- Presence of cytologic atypia within the mural nodule indicates that it is malignant
- Presence of a fibrous layer between the nodule and the mucinous tumor indicates that the mural nodule is benign
- Presence of infiltrative tumor nests between the nodule and the mucinous tumor indicates that the mural nodule is malignant
- Tumor behavior will be driven by the mucinous tumor, which appears benign
Board review style answer #2
D. Presence of infiltrative tumor nests between the nodule and the mucinous tumor indicates that the mural nodule is malignant
All mural nodules are characterized by the presence of a fibrous stromal layer between the nodule and the mucinous tumor. However, tumor invasion into this stroma is indicative of malignancy. Vascular invasion may also be seen in malignant mural nodules but is not required for diagnosis as such. Cytologic atypia may be seen in both malignant mural nodules and benign sarcoma-like mural nodules. A malignant mural nodule can be associated with poor prognosis regardless of the benignity of the associated mucinous tumor.
Comment Here
Reference: Mural nodules in mucinous cystic neoplasms
All mural nodules are characterized by the presence of a fibrous stromal layer between the nodule and the mucinous tumor. However, tumor invasion into this stroma is indicative of malignancy. Vascular invasion may also be seen in malignant mural nodules but is not required for diagnosis as such. Cytologic atypia may be seen in both malignant mural nodules and benign sarcoma-like mural nodules. A malignant mural nodule can be associated with poor prognosis regardless of the benignity of the associated mucinous tumor.
Comment Here
Reference: Mural nodules in mucinous cystic neoplasms
Neuroectodermal type tumors (pending)
[Pending]
Olaparib
Table of Contents
Definition / general | Trade name | Indications | Pathophysiology | Diagrams / tables | Clinical information | Uses by pathologists | Side effects | Drug administration | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Apoly (ADP-ribose) polymerase (PARP) inhibitor blocks PARP1, PARP2, PARP3, which are involved in DNA transcription and DNA repair
- Developed by KuDOS Pharmaceuticals and acquired and developed later by AstraZeneca Pharmaceuticals LP and Merck & Co
- Synonym: AZD2281, MK-7339, Lynparza, PARP inhibitor AZD2281
Trade name
- Lynparza®
Indications
- Advanced ovarian cancer with BRCA1 and BRCA2 germ line mutations, has been treated with other types of chemotherapy (U.S. FDA approved, 12/19/2014) (Drugs.com: Olaparib [Accessed 5 May 2020])
- Previously treated or metastatic breast cancer that is HER2 negative and has germ line mutations in BRCA1 and BRCA2 genes (U.S. FDA approved, 1/12/2018)
- Advanced or recurrent ovarian epithelial, fallopian tube or primary peritoneal cancer with germ line / somatic mutations in BRCA1 and BRCA2 genes and partial or complete response to platinum chemotherapy (U.S. FDA approved, 12/9/2018)
- Metastatic pancreatic cancer with germ line mutations in BRCA1 and BRCA2 genes and has been treated with platinum chemotherapy (U.S. FDA approved, 12/27/2019)
- Reference: National Cancer Institute: Cancer Drugs [Accessed 5 May 2020]
Pathophysiology
- In normal cells, base excision repair and homologous recombination mediated double strand breaks repair responsible for damaged DNA repair (Figure 1, panel A)
- In cells with BRCA1 or BRCA2 mutation, base excision repair and other DNA repair systems compensate for the nonfunctional homologous recombination (Figure 1, panel B)
- In cells with nonfunctioning base excision repair because of PARP1 inhibition and at least one copy of BRCA1 and BRCA2, homologous recombination can repair the DNA (Figure 1, panel C)
- In cancer cells with BRCA1 or BRCA2 mutation, PARP1 inhibitor (olaparib) blocks the base excision repair leading to cell death (Figure 1, panel D)
- Mechanism of drug resistance (Ann Oncol 2019;30:1437)
- Secondary mutations resulting in somatic reversion or restoration of open reading frame of homologous recombination genes including BRCA1/2
- Expression of functional hypomorphic variants of BRCA1/2
- Stabilization of BRCA1/2 variants by HSP90
- Reversion of BRCA1 epigenetic silencing by demethylation process
- Removal of barriers to DNA end resection via loss of 53BP1 or proteins from shieldin complex
- Oncogenic signaling that promotes expression of homologous recombination repair genes
- Protection of the replication fork and decrease in cell cycle progression
- Reference: N Engl J Med 2009;361:189, see diagram below
Clinical information
- BRCA1/2 aberrations occur in ovarian and breast cancers and 5% of other cancers including prostate, skin (nonmelanoma), endometrial, pancreatic and biliary duct cancers (Ann Oncol 2019;30:1437)
- Efficacy of olaparib in BRCA mutated advanced ovarian cancer with prior treatment: objective response rate 34%, complete response 2%, partial response 32% (FDA: LYNPARZA (Olaparib) Tablets [Accessed 6 May 2020])
- Efficacy in BRCA1/2 mutated HER2 negative metastatic breast cancer: objective response rate 52% (olaparib) versus 23% (nonolaparib chemotherapy)
- Olaparib in patients with metastatic castration resistant prostate cancer with DNA repair gene aberrations: TOPARP-B, phase II clinical trial
(Lancet Oncol 2020;21:162)
- 161 of 711 patients with DNA damage repair gene aberrations
- Decrease in PSA of 50%: 37% of patients received 400 mg; 30.2% with 300 mg
- Circulating tumor cell count conversion: 53.6% of patients received 400 mg; 48.1% with 300 mg
- Olaparib in patients with metastatic castration resistant prostate cancer with BRCA1/2 or ATM mutations: PROfound, phase III trial
(Cancer Discov 2019;9:1638)
- 245 patients with BRCA1/2 or ATM mutations
- Objective response rate of 33.3% compared with 2.3% of patients who received enzalutamide or abiraterone plus prednisone
- Restored DNA repair capacities found in resistant clones of carcinoma cells (Clin Cancer Res 2013;19:5485)
- Myelodysplastic syndrome / acute myeloid leukemia occurred in < 1.5% with fatal outcome; discontinue if it is confirmed (Gynecol Oncol 2018;151:190)
- Pneumonitis occurred in < 1% with some fatal outcome; discontinue if it occurs (FDA: LYNPARZA (Olaparib) Tablets [Accessed 6 May 2020])
- Embryo / fetal toxicity: potential hazard to a fetus
- Clinical pharmacokinetics
(FDA: LYNPARZA (Olaparib) Tablets [Accessed 6 May 2020])
- Absorption: rapid, peak median plasma concentrations peak at 1.5 hours after dosing, co-administration of a high fat meal slowed the rate
- Distribution:
- Mean volume of distribution 158 ± 136 L after a single dose of 300 mg
- In vitro protein binding approximately 82%
- Metabolism: mainly by CYP3A4/5 in vitro
- Excretion: 44% via urine and 42% via feces
- Mean terminal plasma half life of 14.9 ± 8.2 hours
- Plasma clearance of 7.4 ± 3.9 L/h, after a single dose of 300 mg
- Drug interactions: avoid concurrent use of strong or moderate CYP3A inhibitors such as itraconazole, clarithromycin, ketoconazole, ritonavir, indinavir, ciprofloxacin, diltiazem, erythromycin, fluconazole (FDA: LYNPARZA (Olaparib) Tablets [Accessed 6 May 2020])
- Olaparib costs about $3,000 per month
Uses by pathologists
- Anti-BRCA1 mouse monoclonal clone MS110, most effective antibody to detect mutated BRCA1
- Positive BRCA1 mutation if < 10% nuclear staining ovarian carcinoma cells (J Clin Pathol 2020;73:191)
- Normal BRCA1: retained BRCA1 staining
- Abnormal BRCA1: equivocal and loss of BRCA1 staining (Am J Surg Pathol 2013;37:138)
Side effects
- Anemia
- Nausea
- Vomiting
- Diarrhea
- Stomatitis
- Infections and infestations
- Nasopharyngitis / sinusitis / rhinitis / influenza
- Fatigue including asthenia
- Metabolism and nutrition disorders
- Decreased appetite
- Arthralgia / myalgia
- Dysgeusia
- Headache
- Neutropenia
- Reference: N Engl J Med 2009;361:123
Drug administration
- 300 mg taken orally, twice daily for 2 years of treatment
- Continued treatment until disease progression or intolerable drug toxicity
Additional references
- J Adv Pract Oncol 2019;10:167, Cells 2019;8:E860, Curr Treat Options Oncol 2018;19:21, Curr Treat Options Oncol 2018;19:1, Genes Dev 2020;34:360, Mol Cancer 2020;19:49, Nature 2012;481:287, N Engl J Med 2012;366:1382, N Engl J Med 2015;373:1697, N Engl J Med 2018;379:2495, N Engl J Med 2019;381:2416, Pancreas (Fairfax) 2019;3:e5
Board review style question #1
Which of the following is true about olaparib?
- Olaparib is used to treat breast and ovarian cancers with BRAF mutation
- Olaparib is used to treat breast and ovarian cancers with BRCA1/2 mutation
- Olaparib is used to treat breast and ovarian cancers with HER2 mutation
- Olaparib is used to treat breast and ovarian cancers with RAS mutation
Board review style answer #1
B. Olaparib is used to treat breast and ovarian cancers with BRCA1/2 mutation
Comment Here
Reference: Olaparib
Comment Here
Reference: Olaparib
Ovarian myxoma (pending)
[Pending]
Ovotesticular DSD
Table of Contents
Definition / general | Terminology | Epidemiology | Clinical features | Laboratory | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Molecular / cytogenetics description | Differential diagnosisDefinition / general
- Ovarian and testicular tissue in the same individual (either bilateral or unilateral ovatestes or separate testis and ovary) with or without abnormal genitalia
Terminology
- Ovotesticular disorder of sex development (DSD) in the new terminology (Best Pract Res Clin Endocrinol Metab 2010;24:187)
Epidemiology
- Very rare, with only about 500 cases identified worldwide
Clinical features
- All have uterus, most have fallopian tubes, some have vas deferens
- Usually ambiguous genitalia (J Pediatr Endocrinol Metab 2001;14:421)
- At puberty, 80% have gynecomastia, 50% menstruate
- May be associated with transexualism (Urologiia 2008;2:14)
- All true hermaphrodites who have successfully completed pregnancy have had male offspring to date (Obstet Gynecol 2009;113:534)
Laboratory
- Hormone levels are typically normal
Prognostic factors
- Important to assign gender as soon as possible (in infants, before age 2 - 4), since: (a) usually no other developmental anomalies and (b) patients will have a better chance for normal psychological, sexual and reproductive lives if gonad inappropriate for gender assignment is removed
- Dysplastic gonads are at risk for developing germ cell malignancy; risk of gonadal malignancy increases if Y chromosome is present
Case reports
- 16 year old phenotypic boy with gynecomastia and pubertal arrest (Horm Res 2007;68:261)
- Due to tetragametic chimerism (fertilization of two ova by two sperm, followed by the fusion of the zygotes and the development of an organism with intermingled cell lines, Urology 2009;73:293)
Treatment
- Plastic surgery is the mainstay after gender is assigned, based on genetic sex, gonadal sex, social sex, psychologic sex and patient request
- Conservative gonadal surgery with long term followup (J Urol 2007;177:726)
- Case series on surgical treatment of 25 cases of hermaphroditism, 4 of whom were true hermaphrodites who had female gender assignment (Ann Plast Surg 2009;63:543)
Microscopic (histologic) description
- Ovary usually normal (prepubertal ovary has primordial follicles with primary oocytes with a few primary or antral follicles)
- Prepubertal testis has immature seminiferous tubules lined by immature Sertoli cells and primitive germ cells, usually without spermatogonia
- Ovotestis often has normal ovarian tissue with atrophic testicular tissue (Obstet Gynecol 2009;113:534)
Microscopic (histologic) images
Positive stains
- Placental-like alkaline phosphatase (PLAP) stained gonocytes indicates higher risk of malignant transformation (Int J Gynecol Pathol 2010;29:33)
Molecular / cytogenetics description
- 70% are 46,XX; 10% are 46,XY; 20% show various forms of mosaicism; less than 1% show 46,XX / 46, XY chimerism or two or more cell lines of different genetic origins
- 80% have chromatin positive Barr bodies
- 60% are 46,XX only in blood cells with Y chromosome present in most of rest of cells
Differential diagnosis
- Mixed gonadal dysgenesis:
- Ovarian tissue, if present, lacks primordial follicles; internal / external genitalia are not relevant for distinguishing these 2 conditions
Polycystic ovary disease
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Pathophysiology | Clinical features | Diagnosis | Radiology description | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Polycystic ovarian syndrome (PCOS) is a clinicopathologic syndrome comprising polycystic ovaries and characteristic clinical features
- Polycystic ovaries resembling PCOS may be seen in prepubertal period or puberty without clinical manifestations
Essential features
- Most common cause of anovulatory infertility
- Presents with a variety of clinical and radiologic findings
- Grossly enlarged, multicystic ovaries, although may not be enlarged in adolescent patients
- Microscopically: ovaries with multiple cysts, hyperthecosis and atretic follicles
- Associated with endometrial hyperplasia and endometrial neoplasia
Terminology
- Formerly called Stein-Leventhal syndrome
Epidemiology
- Affects 4 - 12% of women in the U.S., usually of teenage or childbearing ages (Clin Med Res 2004;2:13)
Pathophysiology
- Development of insulin resistance and hyperandrogenism in polycystic ovary disease is not fully understood
- Familial clustering of cases has been observed, strongly suggesting a genetic basis for polycystic ovary disease; however, no specific genetic abnormality has been shown to be the sole culprit in the development of polycystic ovary disease
Clinical features
- Presents with menstrual disorders (from amenorrhea to menorrhagia) and infertility
- Can cause acne, obesity, hirsutism, insulin resistance and diabetes
- 3 different diagnostic criteria:
- National Institutes of Health (NIH) (1990): androgen excess, oligoovulation and exclusion of other entities that cause polycystic ovaries
- European Society of Human Reproduction and Embryology / American Society of Reproductive Medicine (ESHRE / ASRM) in Rotterdam (2003) (2 of 3 present):
- Oligoovulation or anovulation
- Excess androgen activity (clinical or biochemical signs)
- Polycystic ovaries present (by ultrasound) but no other endocrine disorders
- Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society criteria (2006):
- Hyperandrogenism and any 1 or 2 of:
- Ovulatory dysfunction
- Polycystic ovarian morphology (PCOM)
- Hyperandrogenism and any 1 or 2 of:
- 90% of endometrial cancers arise in a background of chronic estrogen stimulation
- Obesity (associated with PCOS), which encourages the conversion of androstenedione to estrone in adipose tissue via aromatase activity, is strongly associated with this subset of endometrial tumors
- Reference: Crum: Diagnostic Gynecologic and Obstetric Pathology, 3rd Edition, 2017
Diagnosis
- Clinical symptoms:
- Oligo or anovulation
- Androgen excess (hirsutism, acne)
- Radiologic findings:
- Ultrasound is gold standard
- MRI may be used but is not generally indicated
- Reference: Crum: Diagnostic Gynecologic and Obstetric Pathology, 3rd Edition, 2017
Radiology description
- Transvaginal ultrasound:
- Increased ovarian volume with size > 10 mL
- Presence of at least 12 follicles that are between 2 and 9 mm in a single ovary (Hum Reprod 2003;18:598)
- Peripheral distribution of multiple follicles, giving a string of pearls appearance
Case reports
- 18 year old woman with hirsutism and oligomenorrhea (Gynecol Endocrinol 2013;29:273)
- 31 year old woman with polycystic ovary syndrome and increasing ovarian cyst size (Medicine (Baltimore) 2021;100:e28261)
- 32 year old woman with a few year history of hirsutism, menstrual disorders and T1DM (Endocr J 2016;63:193)
Treatment
- Lifestyle modifications, weight loss
- Treatment of hirsutism and insulin resistance
- Infertility:
- Letrozole (aromatase inhibitors) is associated with increased ovulation rate to clomiphene citrate (N Engl J Med 2014;371:119)
- Ovarian drilling puncture of small follicles with electrocautery, although effectiveness has been questioned (Hum Reprod 2002;17:2851, Cochrane Database Syst Rev 2007;3:CD001122)
Gross description
- Enlarged ovaries, with numerous subcortical cysts (cysts may be immature follicles)
Microscopic (histologic) description
- Increased ovarian size, including thickness of cortical and subcortical stroma
- Thickened collagenized tunica
- Normal numbers of primordial follicles
- Twice the expected number of ripening and atretic follicles
- Multiple cystic follicles (1 - 2 mm) with luteinized theca layer (theca lutein hyperplasia)
- Reference: Crum: Diagnostic Gynecologic and Obstetric Pathology, 3rd Edition, 2017
Microscopic (histologic) images
Sample pathology report
- Ovary, left, oophorectomy:
- Benign ovary with collagenized tunica, theca lutein hyperplasia of cystic follicles and atretic follicles with residual theca lutein hyperplasia, consistent with polycystic ovary
Differential diagnosis
- Endocrine disorders:
- Androgen secreting tumors:
- Symptoms of hirsutism, acne, deepening of voice
- Laboratory results: high blood testosterone, DHEA sulfate levels and urine 17 ketosteroids
- Exogenous androgen:
- Laboratory results: high blood testosterone
- Cushing syndrome:
- Adrenal adenoma (ACTH independent Cushing syndrome)
- Symptoms include male pattern baldness, clitoromegaly and deepening of voice
- High cortisol levels, low ACTH level
- Nonclassical congenital adrenal hyperplasia:
- Due to either 21 hydroxylase deficiency (most common), 11β hydroxylase deficiency or 3β hydroxysteroid dehydrogenase deficiency
- Most common biochemical abnormality is adrenal androgen excess
- Measure 17 hydroxyprogesterone, serum cortisol, serum androstenedione
- Analysis of CYP21A2 gene, which encodes 21 hydroxylase, may be used to confirm diagnosis of nonclassic congenital adrenal hyperplasia (Hum Reprod Update 2017;23:580)
- Primary ovarian failure:
- Characterized by high levels of gonadotropins, particularly FSH
- Exceptions are prepubertal age or menopausal transition
- Characterized by high levels of gonadotropins, particularly FSH
- Androgen secreting tumors:
Additional references
Board review style question #1
A 34 year old woman presents with years of irregular menstrual cycles and infertility. On physical examination, she's noted to have hirsutism, central obesity (BMI of 35) and acne. Her bloodwork is noted to have elevated total testosterone and an abnormal glucose tolerance test (GTT). What is she at increased risk of?
- Cardiovascular disease
- Endometrial hyperplasia / adenocarcinoma
- Hypothyroidism
- Primary ovarian failure
Board review style answer #1
B. Endometrial hyperplasia / adenocarcinoma. The vignette describes a patient presenting with polycystic ovary disease (reproductive age woman with hirsutism and infertility). She had biochemical changes indicating polycystic ovary disease, such as elevated testosterone and an abnormal glucose tolerance test suggesting insulin resistance. She is at increased risk for endometrial hyperplasia / adenocarcinoma due to frequent anovulation.
Comment Here
Reference: Polycystic ovary disease
Comment Here
Reference: Polycystic ovary disease
Board review style question #2
Board review style answer #2
C. Fibrotic ovarian cortex. Polycystic ovary syndrome has multiple histologic hallmarks, one of which is the fibrotic ovarian cortex / collagenized tunica. The other features include twice the expected ripening and atretic follicles, multiple cystic follicles with luteinized theca layer (theca lutein hyperplasia).
Comment Here
Reference: Polycystic ovary disease
Comment Here
Reference: Polycystic ovary disease
Pregnancy luteoma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Electron microscopy description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Pregnancy luteoma falls under the tumor-like lesions category of the WHO 2020 classification of tumors
- Self limited, hyperplastic (nonneoplastic) proliferation of large, luteinized ovarian cells during pregnancy, resulting in a tumor-like mass of the ovary that regresses spontaneously during puerperium
Essential features
- Pregnancy luteomas are nonneoplastic (hyperplastic) proliferations of luteinized cells, occurring, by definition, during pregnancy
- Regress spontaneously after delivery
- Microscopic features include diffuse sheets of round cells with abundant eosinophilic cytoplasm and round nuclei
- Some luteomas may show brisk mitoses
- This may be a diagnostic pitfall, especially during intraoperative evaluation
- Presence of uniform bland cells, despite the high mitotic rate and a supportive clinical history, is the key to making a diagnosis in these cases
Terminology
- Luteoma of pregnancy
ICD coding
- ICD-O: 8610/0 - luteoma, NOS
- ICD-11: 2F32.Y & XH40J2 - other specified benign neoplasm of ovary & luteoma, NOS
Epidemiology
- Age group: 15 - 44 years; by definition, during pregnancy (Int J Gynecol Pathol 1993;12:108)
- Commonly seen in mid to late pregnancy (Int J Gynaecol Obstet 2022 Feb 25 [Epub ahead of print])
- 80% of patients are multiparous; luteomas are rare in primiparous women
- 80% of patients are African Americans
- Rarely, may recur in consecutive pregnancies
Sites
- Ovary
Pathophysiology
- Occurs due to nodular hyperplasia of theca interna cells
- Previously, elevated hCG was implicated as a direct causative agent of luteomas, due to the finding of elevated hCG levels in luteoma patients and spontaneous regression after pregnancy
- hCG shares a common alpha subunit with luteinizing hormone (LH) and is thus able to bind to the LH-hCG receptor on the ovarian stromal cells; this causes proliferation and luteinization of ovarian stromal cells
- Factors against hCG being the sole causative agent in luteoma pathogenesis:
- There are other conditions (such as trophoblastic tumors) that are more common, that have elevated hCG levels; luteomas have not been found to be associated with trophoblastic tumors
- Luteomas occur in mid to late pregnancy, while hCG levels are higher in the first trimester
- Therefore, factors other than hCG are also involved in the pathogenesis; exact pathogenesis is unknown (Am J Obstet Gynecol 1969;104:871)
- Pre-existent endocrinopathy, such as stromal hyperthecosis or polycystic ovarian syndrome (PCOS), may predispose to the development of luteomas in some patients
Etiology
- Elevated hCG levels have been implicated in causing hyperplasia of luteinized cells
Clinical features
- Usually asymptomatic, with the mass being incidentally detected at cesarean section or tubal ligation at the end of pregnancy
- Pelvic mass: rarely seen, can rarely cause obstruction of birth canal
- Virilization (Obstet Gynecol 1982;59:105S):
- Seen in 25% of patients, appearing or increasing in the third trimester of pregnancy
- Androgen levels are increased up to 70 times normal
- High androgen levels may be seen in women who do not exhibit virilizing symptoms; this is due to the androgen blunting effect of sex hormone binding globulin (SHBG)
- Virilization symptoms seen with luteomas include:
- Hirsutism
- Deepening of voice
- Acne
- Frontal baldness
- Clitoral hypertrophy, etc.
- Virilization features can be seen in up to 70% of female infants born to women that exhibit virilization features under the influence of androgens, leading to clitoromegaly and labial fusion (Obstet Gynecol 1982;59:105S)
- Androgen levels are high in female infants but lower than maternal levels
- Other uncommon symptoms include:
- Abdominal pain
- Abdominal mass
- Ascites
- Regression of luteomas:
- Regression occurs spontaneously, several weeks after delivery (starts within days after delivery)
- Androgen levels fall to baseline 2 weeks after delivery
- Symptoms of virilization disappear during first 2 weeks after delivery
Diagnosis
- Excisional biopsy, frozen section examination
Laboratory
- Elevated hCG levels
- Elevated androgen levels
Radiology description
- Ultrasound and MRI show a predominantly solid, heterogenous mass
Prognostic factors
- All pregnancy luteomas invariably regress spontaneously after pregnancy
Case reports
- 28 year old woman, gravida 2, para 1 (G2P1), with large ovarian mass causing hirsutism identified intraoperatively (Case Rep Obstet Gynecol 2021;2021:6695117)
- 30 year old woman with pregnancy luteoma associated with ectopic pregnancy (J Reprod Infertil 2017;18:333)
- 33 year old woman with enlarged ovaries discovered incidentally during cesarean section (Int J Appl Basic Med Res 2016;6:282)
Treatment
- Treatment is usually not necessary as these are spontaneously resolving lesions
Gross description
- Mostly unilateral; bilateral in 33% of cases (Am J Surg Pathol 2014;38:239)
- Multiple lesions are seen in 50% of cases
- Usually measure 5 - 10 cm but can be smaller or rarely larger, up to 20 cm
- Cut surface:
- Well circumscribed, multinodular or multiple lesions, brown with focal hemorrhage, without areas of necrosis
- When necrosis is seen (rarely), it is central and due to infarction
Gross images
Frozen section description
- Similar to permanent sections, frozen sections show round to oval cells with abundant eosinophilic cytoplasm, arranged in diffuse sheets
- Caution is advised in interpreting mitoses
- Historically, some luteomas may show brisk mitoses misleading a pathologist into making a diagnosis of malignancy on intraoperative evaluation
- Even with brisk mitoses, cells are uniform and stroma is rarely prominent (Am J Surg Pathol 2014;38:239)
Frozen section images
Microscopic (histologic) description
- Arrangement:
- Lesion is well circumscribed; even when there are multiple nodules, they are sharply circumscribed from the stroma
- Cells are arranged in sheets
- Occasionally, stromal edema may result in separation of cell clusters giving rise to a vaguely nested appearance (Am J Surg Pathol 2014;38:239)
- There may be follicle-like spaces that give rise to a thyroid-like or (when smaller), acinus-like appearance
- Sometimes, a vague reticular or nested pattern may be seen (Am J Surg Pathol 2014;38:239)
- Cells are round to oval with abundant eosinophilic cytoplasm, round nuclei with prominent nucleoli
- Sometimes, cells may have pale, frothy cytoplasm instead of eosinophilic cytoplasm; nuclei are uniform (no nuclear pleomorphism)
- Mitoses: mitoses are infrequent; in some cases, brisk mitoses can be seen, as high as 2 - 3/10 high power fields (Am J Surg Pathol 2014;38:239)
- Necrosis: not usually seen; can be seen in very large tumors, in a central location, due to infarction of the tumor as it outgrows its blood supply
- Stroma: usually scant; some cases may show stromal edema or fibrosis giving rise to a vaguely nested arrangement of tumor cells
- Additional features: some authors have described the presence of granulosa cell proliferations of pregnancy in the ovary uninvolved by pregnancy luteoma (Am J Surg Pathol 2014;38:239)
- Granulosa cell proliferations of pregnancy may be observed in the ovary involved by luteoma
Microscopic (histologic) images
Contributed by Swati Bhardwaj, M.B.B.S., M.D., Tamara Kalir, M.D., Ph.D. and AFIP images
Cytology description
- Moderate to large cells with abundant eosinophilic cytoplasm and uniform, round nuclei
Positive stains
Negative stains
Electron microscopy description
- Electron microscopy shows features of steroid hormone producing cells (Hum Pathol 1976;7:205, Pathol Res Pract 1999;195:859):
- Lipid droplets
- Mitochondria with tubular cristae
- Abundant smooth endoplasmic reticulum
- Dispersed golgi apparatus
- No crystalloid lattice structures are seen
Sample pathology report
- Ovary, oophorectomy specimen:
- Pregnancy luteoma (see comment)
- Comment: Reticulin staining revealed reticulin fibers encircling groups of luteinized cells, consistent with the diagnosis.
- Microscopic description: There are sheets of uniform, round to oval cells with abundant eosinophilic cytoplasm with round nuclei. Background stromal luteosis is seen.
- Ovary, oophorectomy specimen:
- Pregnancy luteoma (see comment)
- Comment: Mitotic activity is brisk but attributed to the hormonal milieu of pregnancy.
- Microscopic description: There are sheets of uniform, round to oval cells with abundant eosinophilic cytoplasm with round nuclei. 5 - 6 mitoses are seen per 10 high power fields. Brisk mitotic activity has been described in pregnancy luteomas. The presence of bland and uniform cells is compatible with a luteoma, despite the brisk mitoses.
Differential diagnosis
- Steroid cell tumor:
- Rarely bilateral
- Grossly, yellow in color
- Cells are smaller than luteoma cells (cells of pregnancy luteoma are 1.5 - 2 times larger than the cells of steroid cell tumor)
- Eosinophilic cells may be accompanied by pale staining lipid rich cells
- Follicle-like spaces are rarely seen
- Distinction between steroid cell tumor and luteomas is important; a subset of steroid cell tumors are clinically malignant
- Dense reticulum pattern and the presence of lipochrome pigment are other features favoring a diagnosis of steroid cell tumor
- In Leydig cell tumors, a hilar location and the presence of Reinke crystals are helpful
- Luteinized thecoma:
- Shows at least focal classical thecoma cells with oval to round cells with spindled nuclei
- Luteinized granulosa cell tumor:
- Shows at least focal areas of granulosa cell tumor with classical morphology
- Can retain evidence of epithelial differentiation
- Even when they are luteinized, adult granulosa cell tumor do not show luteinization to the extent seen in pregnancy luteomas
- FOXL2 402C → G (C134W) somatic mutation is highly sensitive and specific for diagnosis of adult granulosa cell tumor (N Engl J Med 2009;360:2719, PLoS One 2009;4:e7988, Am J Surg Pathol 2011;35:484)
- Malignant melanoma:
- Shows much greater atypia and higher mitotic rate than that seen in pregnancy luteomas
- IHC is positive for MelanA, HMB45, S100, SOX10 (Case Rep Oncol Med 2020;2020:7206786)
Additional references
Board review style question #1
Board review style answer #1
D. Reticulin staining fibers enclosing groups of cells, rather than individual cells
Comment Here
Reference: Pregnancy luteoma
Comment Here
Reference: Pregnancy luteoma
Board review style question #2
Board review style answer #2
D. Lipid rich cells and dense reticulum pattern. Lipid rich cells and dense reticulum patterns favor a diagnosis of steroid cell tumors over pregnancy luteoma. Other features are commonly seen in pregnancy luteomas.
Comment Here
Reference: Pregnancy luteoma
Comment Here
Reference: Pregnancy luteoma
Rete cystadenoma, adenoma and adenocarcinoma
Table of Contents
Definition / general | Case reports | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Additional referencesDefinition / general
- Also called adenoma of rete ovarii
- Rare; mean age 59 years
Case reports
- 11 year old girl (J Pediatr Surg 2005;40:e17)
Gross description
- Mean 9 cm, usually hilar
Microscopic (histologic) description
- Tubulopapillary proliferations of columnar cells with clear cytoplasm; stroma has extensive polygonal, Leydig-like cells
- Rete and hilar mesonephric remnants found in vicinity of the lesion
Positive stains
Additional references
Sclerosing stromal tumor
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Sclerosing stromal tumor is a benign stromal tumor that has a pseudolobular appearance resulting from alternating cellular and hypocellular areas
Essential features
- Alternating cellular and hypocellular areas impart a pseudolobular appearance
- Pseudolobules contain a haphazard arrangement of epithelioid (lutein) and spindled cells
- Hemangiopericytoma-like vessels are conspicuous in both components
- Positive for sex cord markers but negative for EMA and cytokeratin
ICD coding
Epidemiology
- Most common in the first 3 decades (mean: 29 years; median: 26 years) (Histopathology 2022;80:360)
Sites
- Ovary
Pathophysiology
- FHL2-GLI2 fusions activate the Sonic hedgehog (SHH) pathway (Nat Commun 2020;11:44)
Etiology
- Unknown
Clinical features
- Most patients present with menstrual irregularities or abdominopelvic pain; subset is incidental
- Typically nonfunctioning but androgenic or estrogenic manifestations can occur
- Rarely associated with Meigs syndrome (Eur J Gynaecol Oncol 2004;25:528, J Obstet Gynaecol 2010;30:747, World J Clin Cases 2020;8:6364)
Diagnosis
- Mass is often observed on imaging
- Diagnosis is made by histological examination
Laboratory
- CA-125 often within normal limits but elevated levels have been reported (Eur J Gynaecol Oncol 2004;25:528, World J Clin Cases 2020;8:6364)
Radiology description
- CT:
- Plain: nonhomogeneous density
- Contrast: peripheral ring enhancement; may see enhancement of central patch or internal septa
- Venous phase: enhancement decreases but increases with centripetal progression; no enhancement of cystic component (Oncol Lett 2016;11:3817)
- MRI:
- T1 weighted: slight hyperintense periphery, irregular hypointense center
- T2 weighted: hyperintense cystic components or heterogeneous solid mass with intermediate to high intensity
- T1 with contrast: early peripheral enhancement with centripetal progression (AJR Am J Roentgenol 2005;185:207)
Prognostic factors
- Benign tumors; 2 have locally recurred (Int J Gynecol Pathol 2016;35:549, Histopathology 2022;80:360)
Case reports
- 17 year old virilized girl presents with Meigs syndrome (World J Clin Cases 2020;8:6364)
- 22 year old pregnant woman with a 12 cm ovarian mass on imaging (Case Rep Obstet Gynecol 2019;2019:3927971)
- 5 young women (≤ 30 years) with different presentations of an ovarian mass (Indian J Surg Oncol 2018;9:581)
Treatment
- Complete surgical excision (oophorectomy)
Gross description
- Typically unilateral and well circumscribed, ranging from 1.0 - 23 cm (mean: 8.4 cm) (Histopathology 2022;80:360)
- White-yellow variegated solid mass, often edema and cysts
- Hemorrhage, calcifications and rarely necrosis may be seen
Frozen section description
- Alternating cellular and hypocellular areas that impart a pseudolobular appearance may be seen in Krukenberg tumors; carefully examine for incipient metastases
Frozen section images
Microscopic (histologic) description
- Alternating cellular and hypocellular areas impart a pseudolobular appearance
- Hypocellular foci may be ill defined in pregnancy due to expansion of the pseudolobules by lutein cells (Int J Gynecol Pathol 2015;34:357)
- Thin, dilated and branching hemangiopericytoma-like vasculature is often conspicuous in both components
- Pseudolobules comprised of a jumbled admixture of epithelioid (lutein) and spindled cells with minimal atypia
- Epithelioid cells: round nuclei with prominent nucleoli, vesicular chromatin and clear to vacuolated cytoplasm
- Abundant eosinophilic cytoplasm often seen in pregnancy (Int J Gynecol Pathol 2015;34:357)
- Occasionally have a signet ring-like appearance
- Spindled cells: elongated nuclei with indistinct nucleoli, bland chromatin and scant eosinophilic cytoplasm
- Typically round to ovoid but may show angulation if edema is striking
- Epithelioid cells: round nuclei with prominent nucleoli, vesicular chromatin and clear to vacuolated cytoplasm
- Hypocellular areas can be edematous, collagenous (variably keloid-like) or myxoid
- Mitoses are often inconspicuous but rarely can be up to 12/10 high power fields, no atypical forms (Int J Gynecol Pathol 2016;35:549)
- Infarct type necrosis and calcification infrequent
- References: Cancer 1973;31:664, Histopathology 2022;80:360
Microscopic (histologic) images
Positive stains
- Inhibin, calretinin, FOXL2
- CD10, smooth muscle actin
- Diffuse TFE3 expression in a subset of tumors (Anticancer Res 2017;37:5441)
Negative stains
Electron microscopy description
- 3 cell types (Int J Gynecol Pathol 1988;7:280):
- Epithelioid cells: membrane bound cytoplasmic lipid, well developed Golgi
- Spindled cells: fibroblast-like, prominent rough endoplasmic reticulum
- Undifferentiated primitive mesenchymal cells (in hypocellular areas): few organelles, rare cilia and basal lamina
- Smooth muscle differentiation supported by aggregates of cytoplasmic filaments with interspersed dense bodies, pinocytotic vesicles and basal lamina (Ultrastruct Pathol 1992;16:363)
Molecular / cytogenetics description
- GLI2 rearrangements in 81% (Nat Commun 2020;11:44)
- FHL2 is the most common gene partner
Sample pathology report
- Right ovary, oophorectomy:
- Sclerosing stromal tumor (5.0 cm)
Differential diagnosis
- Thecoma (Am J Surg Pathol 2014;38:1023):
- Typically postmenopausal (mean: 50 - 60 years)
- Hormonal manifestations common
- Indistinct cell borders
- Pale gray cytoplasm
- Steroid cell tumor:
- Older (mean: 43 years)
- Hormonal manifestations common
- Staghorn vessels absent
- No spindled cells
- Fibroma:
- Older (mean: 48 years)
- Often have a uniform white cut surface
- Hyalinized plaques
- Metastatic signet ring cell carcinoma (Krukenberg tumor) (Am J Surg Pathol 2006;30:277):
- Clinical history
- Bilateral in > 50%, often with extraovarian disease at presentation
- Staghorn vessels absent
- Admixed with glands, nests or cords of malignant cells (may be very focal)
- Cytokeratin, EMA and mucicarmine positive
- Pregnancy luteoma:
- Often bilateral and multifocal
- Brown and hemorrhagic cut surface
- No admixture of cells
- Solitary fibrous tumor (Histopathology 2018;72:749, Am J Surg Pathol 2022;46:363):
- Microcystic stromal tumor (Histopathology 2022;80:898):
- Triad of microcysts, solid areas and fibrous stroma
- No admixture of cells
- Subset with bizarre nuclei
- Beta catenin (nuclear and cytoplasmic) and cyclin D1 positive
- Inhibin and calretinin negative
- CTNNB1 or APC mutations
Board review style question #1
A 30 year old woman presented with pelvic pain; ultrasound detected a 10 cm mass in the left ovary. She underwent an oophorectomy. You receive the intact specimen with a 10 cm well circumscribed yellow-white edematous lesion with multiple cysts that replaces the entire ovary. Histologic examination shows cellular areas that alternate with hypocellular edematous foci. Staghorn vessels are prominent. What is the likely diagnosis?
- Pregnancy luteoma
- Sclerosing stromal tumor
- Solitary fibrous tumor
- Steroid cell tumor
- Thecoma
Board review style answer #1
Board review style question #2
Which of the following is true regarding the ovarian lesion pictured above?
- It is characterized by a recurring mutation
- It is only seen in pregnancy
- It is typically white, firm and uniform on cut surface
- Marked cytologic atypia, tumor cell necrosis and atypical mitoses are common
- The cellular foci are composed of lutein and spindled cells
Board review style answer #2
E. The cellular foci are composed of lutein and spindled cells
Comment Here
Reference: Sclerosing stromal tumor
Comment Here
Reference: Sclerosing stromal tumor
Seromucinous borderline tumor
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Ovarian seromucinous borderline tumor is a papillary neoplasm of the ovary that is associated with endometriosis and has an excellent prognosis
Essential features
- Papilla with hierarchical branching lined by a mix of Müllerian cell types
- No evidence of stromal invasion
Terminology
- Historic terms that are no longer used: endocervical type mucinous borderline tumor, Müllerian mucinous borderline tumor, Müllerian mucinous papillary borderline tumor, mixed epithelial papillary borderline tumor of Müllerian type
ICD coding
- ICD-O: 8474/1 - seromucinous borderline tumor
- ICD-11: 2C73.Y & XH0RB9 - other specified malignant neoplasms of the ovary & seromucinous borderline tumor
Epidemiology
- Uncommon (Am J Surg Pathol 2002;26:1529)
- Associated with endometriosis (Am J Surg Pathol 2002;26:1529, Am J Surg Pathol 2004;28:1311)
- Presents over a wide age range (mean age is 34 - 39 years) (Cancer 1988;61:546, Cancer 1988;61:340, Am J Surg Pathol 2004;28:1311)
Sites
- Ovary
Pathophysiology
- Subset with loss of ARID1A expression (Int J Gynecol Pathol 2012;31:297)
Etiology
- Associated with endometriosis
- Many arise from endometriotic cysts
Clinical features
- Incidental finding on imaging or may present with signs and symptoms of pelvic mass
- Considered to be endometriosis related ovarian neoplasm, along with endometrioid and clear cell tumors
Diagnosis
- Tumor can be identified via ultrasound or other imaging studies
- Removal of the ovary is then needed for histologic evaluation
Laboratory
- Generally not useful
Radiology description
- Cystic or solid mass
Prognostic factors
- Generally excellent prognosis
Case reports
- 23 year old woman with no history of pregnancy presented to the emergency room with lower abdominal pain (Gynecol Oncol Rep 2021;37:100839)
- 25 year old woman with complex cyst in the left ovary suggesting an endometriotic origin (Int J Womens Health 2020;12:601)
- 39 year old woman who was initially thought to have malignant transformation of endometriosis (Medicine (Baltimore) 2019;98:e15707)
- 65 year old woman with a previous history of bilateral salpingo-oophorectomy had peritoneal cysts increasing in size over 15 years and an increasing CA 19-9 level (BMJ Case Rep 2020;13:e234692)
Treatment
- Surgical management
- Treatment is surgical with the extent of surgery dependent on the stage
- Depending on patient's desire to preserve fertility, a conservative approach may be taken in younger patients (Ann Oncol 2016;27:i20)
Gross description
- Tumors range in size from 1.8 - 18 cm; mean size is 9 cm (Am J Surg Pathol 2015;39:983, Am J Surg Pathol 2002;26:1529)
- Up to 33% of cases are bilateral (Am J Surg Pathol 2002;26:1529)
- Typically a cyst with smooth outer lining and internal papillary excrescences
Frozen section description
- Frozen sections of ovarian tumors are common
- Frozen section will usually show architecture consistent with a borderline tumor with epithelium showing mixed cell types
Microscopic (histologic) description
- Papillary tumor with hierarchical branching
- Epithelial lining composed of cytologically bland cells of mixed Müllerian types: endometrioid, squamous, endocervical type, ciliated, hobnail, clear and indifferent (nondescript) eosinophilic cells
- Stromal cores with edema or fibrosis with conspicuous inflammatory cells (neutrophils or eosinophils)
- Background endometriosis or endometriotic cyst
- Microinvasion has been described
- References: Cancer 1988;61:546, Cancer 1988;61:340, Am J Surg Pathol 2002;26:1529, Am J Surg Pathol 2004;28:1311
Microscopic (histologic) images
Positive stains
Negative stains
- WT1 (can be positive patchy but generally not diffuse)
- CK20, CDX2 (Int J Gynecol Pathol 2016;35:78, In Vivo 2020;34:1341)
Videos
Ovary tumors associated with endometriosis
Sample pathology report
- Right ovary, oophorectomy:
- Seromucinous borderline tumor, size 9 cm (see synoptic report)
Differential diagnosis
- Serous borderline tumor:
- Similar morphology as papillary architecture but has single cell type and lacks other defined Müllerian cell types
- Usually WT1 positive
- Mucinous borderline tumor:
- Gastrointestinal differentiation (Int J Gynecol Pathol 2006;25:83)
- Older age, larger size, less likely to be bilateral, not associated with endometriosis (In Vivo 2020;34:1341)
Additional references
Board review style question #1
A 37 year old woman is found to have a 13.5 cm left ovarian cystic mass with smooth outer surface and internal papillary excrescences. On frozen section, the tumor has papillary, hierarchical branching. The epithelial lining is composed of cytologically bland cells of mixed Müllerian types. Endometriosis background is present but there is no stromal invasion. What is the most likely immunohistochemical profile of this tumor?
- CK7+, CK20-, PAX8-, WT1-
- CK7+, CK20+, PAX8-, WT1-
- CK7+, CK20-, PAX8+, WT1-
- CK7-, CK20+, PAX8-, WT1+
- CK7-, CK20-, PAX8+, WT1-
Board review style answer #1
C. CK7+, CK20-, PAX8+, WT1-. Answers A, B, D and E are incorrect because seromucinous borderline tumors are positive for CK7 and PAX8. They are negative for CK20 and usually negative for WT1. Serous tumors of the ovary are usually positive for WT1.
Comment Here
Reference: Seromucinous borderline tumor
Comment Here
Reference: Seromucinous borderline tumor
Board review style question #2
A 35 year old woman is found to have an 8.2 cm right ovarian mass. Histologic sections show a cyst lined by bland columnar epithelium with underlying spindle stroma. What tumors are known to be associated with this type of benign cyst?
- Clear cell carcinoma, mucinous carcinoma, low grade serous carcinoma
- Endometrioid carcinoma, seromucinous borderline tumor, clear cell carcinoma
- Seromucinous borderline tumor, clear cell tumor, mucinous borderline tumor
- Serous borderline tumor, mucinous borderline tumor, endometrioid carcinoma
Board review style answer #2
B. Endometrioid carcinoma, seromucinous borderline tumor, clear cell carcinoma. The picture shows an endometriotic cyst. Endometriosis associated malignancies include clear cell carcinoma, endometrioid carcinoma and seromucinous borderline tumors. Answers A, C and D are incorrect because serous and mucinous tumors are not associated with endometriosis.
Comment Here
Reference: Seromucinous borderline tumor
Comment Here
Reference: Seromucinous borderline tumor
Seromucinous cystadenoma and adenofibroma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Clinical features | Diagnosis | Radiology description | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Rare, benign ovarian epithelial tumors exhibiting admixture of 2 or more Müllerian type epithelia (mucinous, serous and endometrioid), each accounting for at least 10% of the epithelium
Essential features
- Benign ovarian neoplasms with admixture of 2 or more Müllerian type epithelia, each accounting for 10% or more
- Mucinous, serous and endometrioid cell types
- Mean age 62 years (Histopathology 2021;78:445)
- Association with endometriosis
Terminology
- Müllerian mucinous cystadenoma (preferred by some authors to reflect association with endometriosis, endometrioid and clear cell tumors rather than serous tumors of the ovary)
ICD coding
- ICD-10: D27 - benign neoplasm of ovary
Epidemiology
- Rare tumors
Clinical features
- Detected incidentally during work up of other gynecological diseases
- May present with an abdominal mass, abdominal pain or swelling
- Unilateral / bilateral (bilateral in 40% of cases) (Int J Gynecol Pathol 2022;41:68)
- Mean size is 9 cm, generally smaller than intestinal type neoplasms (Int J Gynecol Pathol 2022;41:68)
Diagnosis
- Microscopic examination
Radiology description
- Unilocular / multilocular cystic masses (J Comput Assist Tomogr 2019;43:119)
Treatment
- Surgery (cystectomy or oophorectomy)
Gross description
- Cystic masses (unilocular or multilocular)
- Seromucinous cystadenofibroma are solid with a fibrous cut surface
Microscopic (histologic) description
- Single layered epithelial lining with occasional stratification
- Cell types: mucinous (endocervical in appearance), serous (ciliated), endometrioid (nonserous, nonciliated), hybrid morphology (serous and mucinous) (Histopathology 2021;78:445)
- Variable degree of papillae (epithelial papillary tufting or broader papillae with fibrovascular core) (Int J Gynecol Pathol 2022;41:68)
- Neutrophilic infiltrate may be present
- Endometrioid epithelium may have squamous metaplasia
- Some tumors have a prominent fibrous stromal component (classified as seromucinous adenofibroma)
- Associated with endometriosis in 27 - 36% of cases (Histopathology 2021;78:445, Int J Gynecol Pathol 2022;41:68)
Microscopic (histologic) images
Sample pathology report
- Right ovary, cystectomy / oophorectomy:
- Seromucinous cystadenoma
Differential diagnosis
- Serous cystadenoma:
- Lined by nonstratified tubal type epithelium
- Mucinous cystadenoma:
- Lined by nonstratified mucinous epithelium with intestinal differentiation (goblet cells)
- Seromucinous borderline tumor:
- Epithelial proliferation > 10%, complex architecture, papillae with hierarchical branching, edematous papillae with neutrophils
- Metastatic tumors:
- Low grade appendiceal mucinous neoplasms, pancreatic adenocarcinoma, endocervical adenocarcinoma, etc.
- Morphology and immunohistochemical panels are helpful
- Low grade appendiceal mucinous neoplasms, pancreatic adenocarcinoma, endocervical adenocarcinoma, etc.
Additional references
Board review style question #1
Seromucinous cystadenoma of ovary is associated with which of the following conditions?
- Endometriosis
- Hypercalcemia
- Isochromosome 12p
- Peutz-Jeghers syndrome
Board review style answer #1
Serous borderline tumor
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Ovarian serous borderline tumor (SBT) is a low grade epithelial neoplasm of generally younger women with a favorable prognosis when diagnosed at an early stage
- Defined, nonobligate precursor to low grade serous carcinoma (LGSC)
- As a borderline tumor, can give rise to extra-ovarian abdominoperitoneal or lymph node implants
Essential features
- 2 morphologic subtypes:
- Conventional SBT shows hierarchically branching papillae lined by stratified, heterogenous epithelium with up to moderate atypia
- Micropapillary / cribriform SBT shows multiple nonbranching filiform structures without fibrovascular cores that are 5 times longer than they are wide, originating directly from bulbous central stalks
- When making distinction between SBT and LGSC in a properly sampled resection, numerous stipulations are in place regarding invasion (size and cytomorphology) and features of extra-ovarian implants (noninvasive versus invasive)
Terminology
- Serous borderline tumor is currently the sole recommended term for primary ovarian tumor (WHO female genital tumors, 2014 and 2020)
- Implant references extra-ovarian nonnodal disease rather than metastasis
- Involvement of lymph node by SBT is preferred over lymph node metastasis
- Micropapillary / cribriform SBT no longer considered definitionally synonymous with noninvasive LGSC per 2020 WHO
- SBT with microinvasion (as defined) is no longer, by definition, synonymous with LGSC per 2020 WHO
- Obsolete terminology no longer recommended:
- Atypical proliferative serous tumor
- Serous tumor of low malignant potential
- Semimalignant serous tumor
- Noninvasive LGSC / micropapillary SBT
ICD coding
- ICD-O: 8442/1 - serous cystadenoma, borderline malignancy (ICD-10: C56.9), serous tumor, NOS, of low malignant potential (C56.9), atypical proliferating serous tumor (C56.9)
- ICD-O: 8462/1 - serous papillary cystic tumor of borderline malignancy (C56.9), atypical proliferative papillary serous tumor (C56.9), papillary serous cystadenoma, borderline malignancy (C56.9), papillary serous tumor of low malignant potential (C56.9)
- ICD-O: 8463/1 - serous surface papillary tumor of borderline malignancy (C56.9)
- ICD-O: 9014/1 - serous adenofibroma of borderline malignancy, serous cystadenofibroma of borderline malignancy
- ICD-10: D39.1 - neoplasm of uncertain behavior of ovary
Epidemiology
- Up to 15% of ovarian serous neoplasms are of the borderline type (Hum Pathol 2000;31:539)
- Median age is fifth decade, with a range from the second to ninth decades; i.e. younger than high grade serous carcinoma (HGSC) demographic
- Nulliparity and unopposed estrogen therapy confer risk, which decreases with increasing parity (Gynecol Oncol 2017;144:571)
- Unlike most other ovarian borderline histotypes (endometrioid, clear cell, seromucinous), SBT is not associated with endometriosis
Sites
- Primary tumor:
- Ovaries, fallopian tube (lumen and paratubal serosa - extremely rare) and peritoneal surfaces
- When ovarian, frequently bilateral (up to 33%) (Gynecol Oncol 2017;144:174)
- Rarely encountered in males; in the paratestis, is thought to derive from either multipotent mesodermal epithelium or Müllerian ductular remnants (Am J Surg Pathol 2001;25:373)
- Implants (all subtypes):
- Ipsilateral ovarian surface (autoimplant), contralateral ovarian surface, omentum, diaphragm, abdominal wall, serosal surface of other abdominopelvic organs
Pathophysiology
- There is data to suggest progression from serous cystadenoma / cystadenofibroma with BRAF / KRAS mutations → SBT → LGSC; however, this is still controversial (Ann Oncol 2016;27:i16)
- Alternative theory suggests that SBT directly arises from papillary hyperplasia of the fallopian tube, which exfoliates and travels through the lumen to implant on ovarian and peritoneal surfaces (Am J Surg Pathol 2011;35:1605)
Etiology
- High incidence of microinvasion in SBTs of pregnant patients
Clinical features
- About a third are asymptomatic; otherwise present with nonspecific pelvic / abdominal pain (Oncologist 2012;17:1515)
- Mass / compression effect on adjacent tissues (constipation, dysuria)
- Uncommonly abdominal bloating, ascites / distention, associated early satiety
Diagnosis
- Definitive diagnosis is made only after both adequate sampling of resection specimen and classification of any synchronous abdominoperitoneal implants (Pathology 2018;50:205)
- Frozen section diagnosis of SBT often dictates subsequent intraoperative staging protocols (ie, more extensive resection, omental and peritoneal biopsies / washings, extent of lymph node dissections)
Laboratory
- Preoperative serum levels of CA 125 are usually elevated (less frequently, CEA and CA19-9)
- Neither sensitive nor specific and cannot distinguish SBT from benign or frankly malignant neoplasms / conditions (Eur J Obstet Gynecol Reprod Biol 2014;183:5, Med Sci Monit 2020;26:e924497)
- Trended postoperatively as qualitative indicator of residual / recurrent disease (not evidence based)
Radiology description
- Radiologically challenging (with any modality) to distinguish between SBT and LGSC
- Pelvic ultrasound (Abdom Radiol (NY) 2020 Aug 29 [Epub ahead of print])
- Primary modality of imaging evaluation - both transvaginal / abdominal components useful to characterize primary mass and appraise extrapelvic disease
- Spectrum of features from serous borderline to LGSC (former has fewer / smaller papillary projections, lower proportion of solid components, thinner septations / wall thicknesses and fewer microcalcifications)
- MRI
- Used to further characterize indeterminate masses or those too large to visualize on ultrasound (Jpn J Radiol 2020;38:782, J Magn Reson Imaging 2014;40:151)
- Variably multicystic to solid, bilateral irregularly enhancing masses with internal septations, fibrous branching and closely packed to loose papillations
- CT or PET / CT
- Used to further characterize enhancing solid components or qualities of extra-ovarian disease (Abdom Radiol (NY) 2020 Aug 29 [Epub ahead of print])
- Presence of nodularity and microcalcifications within peritoneal deposits more likely to indicate invasive (versus noninvasive) implants
Prognostic factors
- Prognosis largely stage dependent (FIGO / TNM):
- Early stage disease (ovary confined, constituting a majority of patients) has survival comparable to that of general population (Gynecol Oncol 2014;134:267)
- Advanced stage at presentation (i.e. extra-ovarian disease) associated with decreased survival; however, prognosis of advanced stage SBT hinges on presence of established poor prognosticators (invasive versus noninvasive implants, micropapillary / cribriform histotype) not reflected in conventional TNM staging
Case reports
- 50 and 61 year old women with early recurrence of ovarian serous borderline tumor as high grade carcinoma (Int J Gynecol Pathol 2004;23:265)
- 61 year old woman with ovarian mesonephric-like adenocarcinoma arising in serous borderline tumor (Diagn Pathol 2020;15:91)
Treatment
- Primary treatment is surgical and subsequently stage dependent:
- Hysterectomy and bilateral salpingo-oophorectomy with complete staging; if fertility desired, then unilateral salpingo-oophorectomy with complete staging (both only if early stage preoperatively)
- With bulky disease or advanced preoperative stage: neoadjuvant chemotherapy followed by cytoreductive surgery
- Advanced postoperative stage, incomplete staging, quality of implants (invasive or noninvasive) and desire for fertility dictates postoperative management
- Observation, completion surgery, adjuvant platinum / taxane based or hormonal chemotherapy (or combination thereof)
- In contrast to high grade serous carcinoma, SBT / LGSC is suboptimally sensitive to platinum based chemotherapy (NCCN: Clinical Practice Guidelines in Oncology (NCCN Guidelines®) [Accessed 11 February 2021])
Gross description
- Received from surgery (often for frozen section) as distended, lobulated bulbous ovarian, adnexal or pelvic mass
- Upon receipt and prior to inking, crucial to document:
- Presence of surface autoimplants (plaque-like deposits, suspicious adhesions)
- Integrity of surface / capsule - intact or focally / diffusely ruptured
- Cut surface:
- Multiloculated cystic to solid mass with variably thick dissecting septations
- Firm to friable tan-yellow edematous papillary outgrowths from inner wall - resembling clusters of small berries (Am J Surg Pathol 2002;26:1111)
- Can be either subtle, inconspicuous foci in an overall smooth walled cyst or solid proliferations overtaking entire cavity
- Serosanguinous fluid contents
- Gross coagulative necrosis rare
- Proper sampling for permanent sections to exclude areas of destructive invasion, atypia or solid growth that would merit promotion to LGSC
- At least 2 representative sections per centimeter of greatest gross tumor dimension (i.e. solid or papillary areas, not the overall simple cyst)
- Grossly normal omentum: 5 - 10 sections total
Gross images
Frozen section description
- Request for frozen section anticipated preoperatively based on imaging and results of peritoneal cytopathology
- Gross intraoperative evaluation:
- Representative sections of any papillary excrescences or solid areas should be evaluated histologically
- Unless focally thickened, uniform smooth walled areas are not grossly concerning and do not merit freezing
- Histologic intraoperative evaluation:
- After concerning histology is judiciously followed up with additional representative sections, report concerning features that would impact subsequent intraoperative course:
- Ambiguous volume of epithelial proliferation
- Micropapillary or cribriform / foci
- True invasion (microinvasion, as defined below, is not an adverse prognosticator and does not influence management)
- After concerning histology is judiciously followed up with additional representative sections, report concerning features that would impact subsequent intraoperative course:
- Correlation between frozen and permanent sections in SBT (up to 93% consistent) better than other borderline histotypes, e.g. endometrioid or mucinous (Gynecol Oncol 2011;123:517, Gynecol Oncol 2007;107:248)
- Risk of underdiagnosis of SBT (frozen) and subsequently LGSC (permanents) increases with larger tumor size (≥ 8 cm)
Frozen section images
Microscopic (histologic) description
- Conventional SBT
- Architecture:
- Numerous slender to bulbous, irregularly contoured papillae with fibrous, hyaline or myxoid cores
- Hierarchical branching pattern - larger papillae arborize to sequentially smaller papillae terminating in epithelial clusters or single cells
- Pseudostratified, crowded lining with hobnailing or epithelial tufting, which exfoliate from papillae
- Psammomatous (concentrically lamellated) or dystrophic calcifications
- Cytologic features:
- Heterogenous cellular population - cuboidal to columnar ciliated, secretory or eosinophilic cells (latter can have baseline atypia, are more numerous during pregnancy)
- Up to moderate atypia - mild cellular enlargement, chromatin coarsening, small nucleoli
- Minimal, nonatypical mitotic activity
- Architecture:
- Micropapillary / cribriform SBT
- Often admixed with areas of conventional SBT
- Micropapillary / cribriform subtype defined as SBT harboring a focus of ≥ 5 mm (confluent linear extent) of pure micropapillary / cribriform growth
- Some experts also cite 10 mm2 (2 dimensional area) towards this definition but this is not recognized in 2020 WHO (Am J Surg Pathol 1999;23:397, Am J Surg Pathol 2002;26:1111)
- Architecture - often combination of both patterns:
- Micropapillary:
- Thread-like extensions lacking true fibrovascular cores, radiating directly from cyst walls or central bulbous and smooth contoured prominences with fibromatous / myxoid stroma
- Micropapillae appear 5 times longer than they are wide, resembling mythical snakes of Medusa’s head
- Low power appearance of labyrinthine spaces created by complex micropapillary interweaving
- Cribriform: confluent sieve-like microacini (occasionally appearing solid), formed by fusion of micropapillae
- Micropapillary:
- Cytologic features distinct from those of conventional SBT:
- Epithelial population is uniform rather than heterogenous - low cuboidal monotonous cells with high nuclear to cytoplasmic (N/C) ratio and small, prominent nucleoli
- Rare to absent ciliation or cytoplasmic eosinophilia
- Minimal, nonatypical mitotic activity and up to moderate atypia
- Often admixed with areas of conventional SBT
- SBT with microinvasion
- Distinction from LGSC in this context is made on size and cytoarchitectural features of the area of concern:
- Foci of microinvasion with cytoarchitecture of SBT actually represent senescent cells and confer no prognostic detriment (Am J Surg Pathol 2014;38:743)
- Individual cells to rounded clusters invading papillary or intracystic stroma with retraction clefting
- Minimally atypical cells with cytoplasmic hypereosinophilia, scant nonatypical mitoses (Surrounding stroma is unremarkable to minimally reactive (fibroblastic reaction, edema)
- Often multiple foci within same tumor (cutoff for progression to LGSC not defined) but any individual one must be
- Foci of microinvasion with cytoarchitecture of LGSC are defined as microinvasive LGSC or SBT with microinvasive LGSC only after proper sampling to exclude frankly invasive LGSC
- Micropapillae, inverted micropapillae or confluent cribriform structures haphazardly infiltrating papillary or intracystic stroma in a fashion similar to conventional invasive LGSC (Am J Surg Pathol 2006;30:1209)
- Monotonous hyperchromatic cells with scant cytoplasm and prominent nucleoli
- Must measure
- Implants
- 2 types of implants: noninvasive and invasive, latter now classified as extra-ovarian LGSC
- Noninvasive implant:
- Grossly, both epithelial and desmoplastic noninvasive implants appear plastered onto peritoneal surface
- 2 histologic subtypes of no clinical bearing but important to recognize each in the differential of invasive implants
- Epithelial:
- Hierarchical papillary proliferations or cellular tufts without associated underlying stroma
- Planted on mesothelial surface or within cystic mesothelial invaginations, with overall smooth epithelial stromal interface
- Cytologic features similar to conventional SBT
- Desmoplastic:
- True papillae with clusters of or single hypereosinophilic cells blending into (compressed by) inflamed fibroblastic, granulation tissue-like stroma
- Despite reactive stroma, neoplastic groups remain serosa based, retain smooth and sharply delineated contours at stromal interface and invade without underlying stromal destruction
- In omentum, desmoplastic implants can create infiltrative inflammatory fibrous septation but do not engulf or extend into adipose tissue
- Autoimplant: deposit of tumor on ovarian surface, often of desmoplastic noninvasive histomorphology
- Peritoneal extra-ovarian LGSC (formerly known as invasive implant) - epithelial predominant lesions:
- Invasive implant defines said focus as extra-ovarian LGSC (even if primary tumor remains as borderline - uncommon situation in which sufficient sampling must be ensured, to exclude histologic features meriting upgrade of the primary)
- Most critical histologic feature of invasion in the context of an SBT implant is presence of destructive stromal growth evident at low magnification (should not be present in desmoplastic noninvasive implants)
- Haphazard infiltration of solid epithelial nests with peripheral retraction clefting / halos resembling lymphatic channels
- In omentum, invasive implants have expansile to infiltrative borders with interruption of normal lobulated fibroadipose architecture
- Lymph node involvement
- Does not portend worse prognosis but retroperitoneal nodes with involvement of SBT still staged per usual (as N1), not as an extra-ovarian implant
- Composed of single cells, small clusters, micropapillae or macropapillae and cribriform glands located in nodal subcapsular sinuses or parenchyma
- Cytologically similar to conventional SBT
- Adjacent endosalpingiosis is common
- Foci of microinvasion with cytoarchitecture of SBT actually represent senescent cells and confer no prognostic detriment (Am J Surg Pathol 2014;38:743)
- Distinction from LGSC in this context is made on size and cytoarchitectural features of the area of concern:
Microscopic (histologic) images
Cytology description
- Peritoneal / pelvic washings for ovarian or pelvic masses are taken immediately post entry into the abdominal cavity (during primary resection operation) to minimize contamination from potential intraoperative tumor rupture
- Rarely can be taken along with preoperative peritoneal or omental biopsies for advanced stage disease
- Positive washings upgrade FIGO / TNM stage of both SBT and LGSC:
- Implies presence of any of the following: ovarian surface involvement, occult extra-ovarian disease (i.e. microscopic invasive / noninvasive implants) or pre/intraoperative tumor rupture
- No reliable cytologic features to distinguish between the above scenarios or between involvement by SBT versus LGSC
- Cytologic features of SBT in washings (Diagn Cytopathol 2004;30:313, Cancer Cytopathol 2012;120:238):
- 3 dimensional clusters with smooth or irregular contours, papillations (more prominent on cell block) or other architecturally complex structures
- 2 cell population of minimally atypical cuboidal nonciliated cells with high N/C ratio and frequent cytoplasmic vacuolization
- Psammomatous calcifications can be seen in any of the following: endosalpingiosis, SBT and serous carcinoma (low or high grade)
Cytology images
Negative stains
- p53 wild type (patchy and weak expression in scattered nuclei)
- To be considered aberrant (aka mutation type), p53 must be either diffusely positive in > 80% of nuclei, completely negative (null type mutant pattern) or any amount of unequivocal cytoplasmic staining (blush does not qualify)
- p16 mosaic (patchy and weak nuclear / cytoplasmic expression)
- To be considered positive, p16 must have strong, diffuse, block-like nuclear and cytoplasmic positivity throughout the area of interest
- CK20, HNF-1B, Napsin A negative
Molecular / cytogenetics description
- KRAS mutations implicated in the progression from SBT to LGSC and are associated with higher risk of recurrence as LGSC (Cancer Res 2004;64:6915, J Pathol 2013;231:449)
- BRAF mutations implicated in the progression from serous cystadenoma to SBT but these are rarely associated with advanced stage SBT or progression to LGSC
- Molecular studies (X inactivation, loss of heterozygosity, KRAS / BRAF sequencing) conflict in regards to clonal versus multifocal versus metaplastic origin of SBT implants (Ann Oncol 2016;27:i16)
Sample pathology report
- Sample report 1:
- Right adnexal mass, excision:
- Ovarian serous borderline tumor (7.5 cm) with surface involvement (see synoptic report)
- Cecal nodule, excision:
- Low grade serous carcinoma (invasive implant)
- Right adnexal mass, excision:
- Sample report 2:
- Left fallopian tube and ovary, left salpingo-oophorectomy:
- Serous ovarian borderline tumor, without ovarian surface involvement (see comment and synoptic report)
- Fallopian tube without diagnostic abnormality
- Comment: Immunohistochemical stains performed with adequate controls on sections of the right ovarian tumor show the tumor cells to be diffusely positive for PAX8, WT1, ER and PR, with focal / weak expression of p53 (wild type expression) and patchy positivity for p16. The combined morphologic and immunohistochemical findings support the above diagnosis.
- Left fallopian tube and ovary, left salpingo-oophorectomy:
Differential diagnosis
- With ovarian SBT
- Serous cystadenoma / cystadenofibroma:
- Should not harbor architectural complexity in the form of papillae, micropapillae, cribriforming or nesting - otherwise:
- If proliferation constitutes If > 10% of overall tumor: serous borderline tumor
- Similar heterogenous population of secretory / ciliated cells but without atypia or mitoses
- Should not harbor architectural complexity in the form of papillae, micropapillae, cribriforming or nesting - otherwise:
- Low grade serous carcinoma:
- Any single focus of invasion with nuclear features of borderline tumor measuring ≥ 5 mm in greatest linear extent (invasive LGSC)
- Any single focus of invasion with nuclear features of low grade serous carcinoma:
- > 5 mm - invasive LGSC
- Noninvasive LGSC and SBT often coexist and can be challenging to distinguish in the absence of invasion
- SBT should not have architecturally confluent cribriform or micropapillary growth - i.e. solid, florid proliferation of low grade cells (amount required to define carcinoma over borderline not defined)
- Endometrioid borderline tumor:
- Arises from / associated with endometriosis (unlike SBT)
- Can have papillary structures but not in hierarchically branching configuration
- Luminal surface of endometrioid glands is nonciliated and crisp / linear, not exfoliative, hobnailing or tufting like SBT
- Squamous, squamous morular or mucinous metaplasia (extremely rare in both low and high grade serous neoplasms)
- Seromucinous borderline tumor (formerly endocervical type mucinous borderline tumor):
- Arises from / associated with endometriosis (unlike SBT)
- Has hierarchically branching edematous papillae but in contrast to SBT, these have a prominent stromal acute inflammatory infiltrate
- Lined by endocervical type mucinous but occasionally ciliated to indifferent cells (unclassifiable cells unique to seromucinous tumors - abundant eosinophilic cytoplasm and prominent nucleoli)
- Serous cystadenoma / cystadenofibroma:
- With SBT autoimplant
- Invasive LGSC arising in SBT (Am J Surg Pathol 2006;30:457):
- As autoimplants are usually identical in morphology to peritoneal noninvasive desmoplastic implants, can mimic invasive LGSC arising in the borderline tumor if they are situated between papillae of exophytic or intracystic tumor
- Autoimplants are more stromal dominant with a more prominent inflammatory infiltrate
- Epithelial component of autoimplants appears compressed by desmoplastic reaction, rigid structural retention like the nests of microinvasive LGSC
- Invasive LGSC arising in SBT (Am J Surg Pathol 2006;30:457):
- With noninvasive peritoneal implant of SBT
- Mesothelial hyperplasia:
- Diffuse sheets to papillary proliferations of monotonous mesothelial cells restricted to serosa and demarcated from underlying stroma
- Variable cellular atypia with rare mitoses
- Both can be PAX8, WT1 positive but SBT is negative for calretinin, D2-40
- Well differentiated papillary mesothelioma:
- Tubulopapillary architecture (uniformly short / blunted rather than hierarchically branching papillae) with bland cuboidal monolayered mesothelial lining
- Psammoma bodies associated with both
- Immunoprofile similar to mesothelial hyperplasia (above)
- Endosalpingiosis:
- Endosalpingiosis is limited to simple glandular structures lined by a monolayer of cells similar to fallopian tube epithelium with neither atypia nor cellular crowding / tufting
- Primary peritoneal SBT:
- Can be multifocal and is histomorphologically identical to either epithelial or desmoplastic noninvasive implants of ovarian SBT
- Defined as primary peritoneal SBT only when a primary ovarian SBT is excluded
- Can be multifocal and is histomorphologically identical to either epithelial or desmoplastic noninvasive implants of ovarian SBT
- Mesothelial hyperplasia:
Additional references
Board review style question #1
Board review style answer #1
B. Increased risk of extra-ovarian peritoneal implants
Comment Here
Reference: Serous borderline tumor
Comment Here
Reference: Serous borderline tumor
Board review style question #2
Which of the following features, if present, increases the TNM stage of a serous borderline tumor?
- Lymphovascular invasion
- Positive pelvic washings
- Presence of frank destructive invasion
- Presence of microinvasion
- Tumor size ≥ 10 cm
Board review style answer #2
Serous cystadenoma, adenofibroma and surface papilloma
Serous cystadenoma, adenofibroma and surface papilloma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Benign partially or completely cystic lesion measuring > 1 cm in size and composed of cells resembling fallopian tube epithelium or cuboidal nonciliated epithelium resembling ovarian surface epithelium
Essential features
- Benign; > 1 cm in size (< 1 cm signifies a cortical inclusion cyst); composed of cells resembling fallopian tube epithelium
- Presents over a broad age range and are generally asymptomatic
- Usually small, uni to multilocular cysts lined by a single layer of tall, columnar, ciliated cells
- Adenofibromas and cystadenofibromas are composed predominantly of fibrous stroma, with glands and cysts forming a minor component
Terminology
- Includes cystadenoma, cystadenofibroma, adenofibroma, papillary cystadenoma, papillary cystadenofibroma, papillary adenofibroma
- Term used depends on the relative amount of fibrous stroma but distinctions are often arbitrary
ICD coding
- ICD-11: 2F32.3 - serous ovarian cystadenoma
Epidemiology
- Patients present over a broad age range
- Most often found in adult women of reproductive age
Sites
- Ovary, less commonly fallopian tube
Pathophysiology
- DNA copy number changes may be seen in stromal fibromatous cells and epithelial cells (Clin Cancer Res 2011;17:7273, Lab Invest 2004;84:778)
Etiology
- Unknown
Clinical features
- Generally asymptomatic
- Symptoms related to an ovarian mass
- One of the more common ovarian tumors to undergo torsion
Diagnosis
- Cystectomy or oophorectomy
Laboratory
- CA-125 levels may be mildly elevated (rarely marked) (Arch Gynecol Obstet 2007;276:559)
Radiology description
- Typically anechoic with thin, smooth walls and posterior acoustic enhancement; unilocular cysts, thin walls, minimal septations and absence of papillary projections (Radiographics 2000;20:1445)
- Imaging modality: pelvic ultrasound or CT scan
Prognostic factors
- May recur after incomplete excision
Case reports
- 63 year old woman with ruptured benign serous ovarian cystadenoma mimicking ovarian malignancy with peritoneal carcinomatosis (Diagn Interv Imaging 2016;97:1187)
- 64 year old woman with bilateral ovarian fibromas and concomitant unilateral serous cystadenoma (J Obstet Gynaecol 2019;39:1027)
- 65 year old woman with ovarian serous cystadenoma with ectopic adrenal tissue (Int J Surg Case Rep 2017;33:89)
Treatment
- Surgery (cystectomy or oophorectomy)
Gross description
- Cystadenoma:
- Usually 3 - 10 cm (but can be up to 30 cm), oval to round, smooth glistening surface
- Usually watery clear to pale yellow cyst fluid but can be viscous and mucoid
- Rarely papillary excrescences are seen on outer surface
- Cystadenofibroma:
- Varies from solid areas with knobby papillae to firm confluent areas
- Adenofibroma:
- Entirely solid with small cysts
- Reference: Kurman: Blaustein's Pathology of the Female Genital Tract (Springer Reference), 7th Edition, 2019
Frozen section description
- Uni or multiloculated cysts with single layer of cuboidal or columnar epithelium and simple papillary projections, if present
- Bland appearing fibrous stroma in varying amounts
- No invasion, architectural complexity or atypia
Microscopic (histologic) description
- Usually small, uni to multilocular cysts lined by a single layer of tall, columnar, ciliated cells resembling normal tubal epithelium or cuboidal nonciliated epithelium resembling ovarian surface epithelium
- Stroma contains spindle fibroblasts
- If papillae are present, they are simple
- Adenofibromas and cystadenofibromas are composed predominantly of fibrous stroma, with glands and cysts forming a minor component
- If < 10% of the total tumor volume shows epithelial proliferation within the cysts that would otherwise qualify as serous borderline tumor, the tumor is designated as serous cystadenoma with focal epithelial proliferation
- Reference: Kurman: Blaustein's Pathology of the Female Genital Tract (Springer Reference), 7th Edition, 2019
Microscopic (histologic) images
Cytology description
- Groups, strips or clusters of epithelial cells with small, bland, round to oval nuclei and variable cytoplasm with or without cilia
- Background cyst contents, including histiocytes and proteinaceous debris
- Cannot definitively diagnose on a cytology specimen; histologic examination is required for classification
Positive stains
Sample pathology report
- Right ovary, oophorectomy:
- Serous cystadenofibroma (3.3 cm)
- Left ovary, oophorectomy:
- Serous cystadenoma with focal epithelial proliferation (see comment)
- Comment: The 8.3 cm cystic ovarian mass was extensively sampled. Focal epithelial proliferation (small, noncomplex papillae) is noted, which represents less than 10% of sampled cyst wall. These finding represent a benign serous cystadenoma with focal epithelial proliferation.
Differential diagnosis
- Rete cyst / cystadenoma:
- Located in ovarian hilus, undulating epithelium, smooth muscle wall, cyst lining is a single layer of flat cuboidal cells
- Nests of Leydig cells may be present in the wall
- Paratubal Müllerian cyst (hydatid cyst of Morgagni):
- Paramesonephric cyst attached to fimbria, thin fallopian tube type epithelium with small epithelial plicae projecting into the lumen, may have smooth muscle in the wall
- Peritoneal cyst:
- Lined by mesothelial cells, often associated with ovarian surface adhesions
- Mesonephric cyst:
- Lined by cuboidal cells and usually surrounded by smooth muscle
- Mucinous cystadenoma:
- Lined by a single layer of mucin containing tall columnar epithelium
- Hydrosalpinx:
- Dilated fallopian tube lumen, lined by ciliated epithelium, attenuated or rare plicae, well developed smooth muscle in the wall
- Cortical / epithelial inclusion cyst:
- Lined by simple cuboidal to columnar epithelium with ciliated cells, sometimes admixed with nonciliated cells
- Serous borderline tumor:
- Epithelial proliferation with architectural complexity, including branching of irregularly shaped papillae
- Seromucinous cystadenoma:
- Lined by epithelium with 2 or more Müllerian cell types each accounting for at least 10% of the epithelium (Histopathology 2021;78:445)
- Frequently associated with endometriosis (Int J Gynecol Pathol 2021 Feb 11 [Epub ahead of print])
Additional references
Board review style question #1
Board review style answer #1
Board review style question #2
What feature distinguishes cystadenoma from cortical inclusion cyst?
- Epithelial lining
- Location
- Presence of psammoma bodies
- Relationship to ovarian serosa
- Size
Board review style answer #2
Sertoli cell tumor
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Pure sex cord neoplasm of the ovary composed of Sertoli cells most commonly arranged in a tubular pattern
- Rare to no Leydig cells present
- Many have hormone manifestations (most commonly estrogenic)
Essential features
- Solid or hollow tubules with or without other histologic patterns containing cuboidal cells that are positive for sex cord markers
ICD coding
- ICD-O: 8640/1 - Sertoli cell tumor, NOS
- ICD-11: 2F32.Z & XH4H24 - benign neoplasm of ovary, unspecified & Sertoli cell tumor, NOS
Epidemiology
- Rare tumor
- Occurs at any age (mean: 30) (Am J Surg Pathol 2005;29:143)
Sites
- Ovary
Pathophysiology
- Subset have DICER1 mutation (Mod Pathol 2015;28:1603)
Etiology
- No known causative agents
Clinical features
- Incidental finding or may present with signs and symptoms of pelvic mass
- Hormonal manifestations
- Most commonly due to estrogen
- Less commonly due to production of androgens, renin, progesterone or aldosterone
- Associations
- Peutz-Jeghers syndrome (lipid rich and oxyphilic variants) (Int J Surg Pathol 2016;24:269)
- DICER mutation
- Reference: Cancer 1980;46:2281
Diagnosis
- Tumor can be identified via ultrasound or other imaging studies
- Removal of the ovary is then needed for histologic evaluation
Laboratory
- Generally not useful although hormonal manifestations are seen and suggest a possible steroid producing tumor
Radiology description
- Predominantly solid mass in the ovarian parenchyma
Prognostic factors
- Excellent in the vast majority of cases
- Features of malignancy
- Severe cytologic atypia
- Necrosis
- Mitotic rate > 5/10 high power fields
- Tumor size > 5 cm
- Reference: Am J Surg Pathol 2005;29:143
Case reports
- 29 year old woman, previously diagnosed with polycystic ovarian syndrome, presented with complaints of amenorrhea for the past 3 years (Medicina (Kaunas) 2022;58:1638)
- 32 year old woman with rapidly growing pelvic tumors and widespread bulky peritoneal disseminations (Gynecol Oncol Rep 2018;24:54)
- 46 year old regularly menstruating woman presented with complaints of dull aching, recurrent pain in the suprapubic region (Clin Case Rep 2022;10:e05892)
Treatment
- Oophorectomy
- Chemotherapy with or without radiation if malignant
Gross description
- Unilateral, solid, tan to yellow
- Mean size is 8 cm
Frozen section description
- Frozen sections of ovarian tumors are common
- Frozen section will usually show a bland tumor composed of tubules
- History of hormone activity, unilaterality and young age are helpful features to recognize the tumor as likely benign
- Reference: Arch Pathol Lab Med 2019;143:47
Microscopic (histologic) description
- Tubular pattern (most common and usually present at least focally) with solid or hollow tubules
- Cuboidal or columnar cells
- Bland oval to round, monotonous nuclei
- Pale cytoplasm
- Lipid rich or oxyphilic variants may be associated with Peutz-Jeghers syndrome
- Other patterns: trabecular, diffuse, alveolar, pseudopapillary, reniform, pseudoendometrioid, spindled
- Absent to very rare Leydig cells
- Pathologic features predictive of malignant behavior include 5 mitoses per 10 high power fields, severe cytologic atypia, necrosis and size > 5 cm
- Reference: Am J Surg Pathol 2005;29:143
Microscopic (histologic) images
Positive stains
- Inhibin, SF1, calretinin
- Frequently positive: CD99, WT1, AE1 / AE3
- Reference: Am J Surg Pathol 2007;31:255
Negative stains
- EMA, CK7, PAX8, GATA3, chromogranin
Molecular / cytogenetics description
- DICER1 mutations can be seen in some of these tumors
Sample pathology report
- Ovary, right, oophorectomy:
- Sertoli cell tumor
Differential diagnosis
- Sertoli-Leydig cell tumor:
- Abundant Leydig cell component (MelanA positive)
- Endometrioid carcinoma:
- Carcinoid tumor:
- Associated teratoma or mucinous tumor
- Chromogranin positive
- Inhibin and SF1 negative
- Female adnexal tumor of probable Wolffian origin:
- Hilar Sertoli cell proliferation:
- Located in the hilum
- Microscopic
Additional references
Board review style question #1
A 40 year old woman is seen in the clinic with complaints of irregular uterine bleeding and pelvic fullness. Transvaginal ultrasound shows a 9 cm right ovarian mass. The mass is surgically removed and a pathologic examination shows the features in the picture above. The mass is positive for inhibin and SF1. The tumor is negative for EMA, PAX8, MelanA and chromogranin. In which of the following general categories does this tumor belong?
- Epithelial
- Neuroendocrine tumor
- Pure sex cord tumor
- Sex cord stromal tumor
Board review style answer #1
C. Pure sex cord tumor. Sertoli cell tumor is a pure sex cord neoplasm of the ovary composed of Sertoli cells most commonly arranged in a tubular pattern. Sertoli cells are positive for inhibin and SF1. Answer D is incorrect because no Leydig cells, which are positive for MelanA and are stromal in nature, are present. Answer B is incorrect because the tumor is negative for neuroendocrine markers. Answer A is incorrect because the tumor is negative for EMA and PAX8.
Comment Here
Reference: Sertoli cell tumor
Comment Here
Reference: Sertoli cell tumor
Sertoli-Leydig cell tumor
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Rare ovarian tumor composed of sex cord (Sertoli cells) and stromal (Leydig cells) elements, accounting for < 0.5% of all ovarian neoplasms
Essential features
- Rare ovarian tumor composed of sex cord (Sertoli cells) and stromal (Leydig cells) elements
- May occur sporadically or in patients with DICER1 syndrome
- 3 molecular subtypes (Am J Surg Pathol 2019;43:628):
- DICER1 mutant: younger age, moderately / poorly differentiated, retiform or heterologous elements
- FOXL2 c.402C>G (p.Cys134Trp) mutant: postmenopausal patients, moderately / poorly differentiated, no retiform or heterologous elements
- DICER1 / FOXL2 wildtype: intermediate age, no retiform or heterologous elements, including all well differentiated tumors
- Express general sex cord markers (inhibin, calretinin, SF1, FOXL2 and CD56); CK7 and EMA immunostains are negative
- Behavior correlates with tumor grade and histologic subtype
Terminology
- Sertoli-Leydig cell tumor
- Well differentiated
- Moderately differentiated / intermediate grade
- Poorly differentiated
- Sertoli-Leydig cell tumor with heterologous elements
- Sertoli-Leydig cell tumor, retiform variant
- Histologic grade and subtype correlates with clinical behavior (see Prognostic factors)
ICD coding
- ICD-O:
- 8631/1 - Sertoli-Leydig cell tumor, NOS
- ICD-11:
- XH0UP7 - Sertoli-Leydig cell tumor of intermediate differentiation
- XH6FQ9 - Sertoli-Leydig cell tumor, NOS
- XH8U56 - Sertoli-Leydig cell tumor, intermediate differentiation, with heterologous elements
- XH6XB6 - Sertoli-Leydig cell tumor, retiform
- XH3PN1 - Sertoli-Leydig cell tumor, retiform, with heterologous elements
Epidemiology
- Rare, accounting for < 0.5% of all ovarian neoplasms and 1 - 2% of pediatric ovarian cancers
- Most common in young patients with a mean age of 25 years (Am J Surg Pathol 1985;9:543)
- May also present after menopause (Eur J Gynaecol Oncol 2017;38:214)
- Tumors with a retiform pattern or germline DICER1 mutation occur at a younger age (Gynecol Oncol 2017;147:521)
- Nearly all pediatric Sertoli-Leydig cell tumors are moderately or poorly differentiated, are DICER1 syndrome associated and often show a retiform pattern or heterologous elements
Sites
- Ovary
Pathophysiology
- May occur sporadically or in patients with DICER1 syndrome (OMIM: Pleuropulmonary Blastoma; PPB [Accessed 16 August 2021])
- Rare tumor predisposition syndrome caused by germline mutations in DICER1, a gene encoding the RNase III enzyme in the microRNA maturation pathway (N Engl J Med 2012;366:234, Br J Cancer 2013;109:2744)
- Other tumors and disorders associated with DICER1 syndrome include pleuropulmonary blastoma, cystic nephroma, multinodular goiter and botryoid embryonal rhabdomyosarcoma, among others (Nat Rev Cancer 2014;14:662)
- Germline mutation is generally a truncating mutation; may occur anywhere in the gene
- Second hit somatic mutation is a focused hotspot missense mutation affecting the RNase IIIb domain of DICER1 (Histopathology 2016;69:903)
- Sporadic Sertoli-Leydig cell tumors (moderately and poorly differentiated) harbor somatic mutations at the same hotspot of DICER1 gene (Am J Surg Pathol 2017;41:1178)
- Somatic hotspot DICER1 mutations are present in approximately half of all cases (range: 15 - 97%), 61 - 69% of which also have germline DICER1 mutations (Am J Surg Pathol 2017;41:1178, Gynecol Oncol 2017;147:521, Histopathology 2016;68:279)
Etiology
- May occur sporadically or in patients with DICER1 syndrome (see Pathophysiology) (OMIM: Pleuropulmonary Blastoma; PPB [Accessed 16 August 2021])
Clinical features
- Clinical presentation may include pelvic pain or a pelvic mass, rarely with ascites and tumor rupture
- Androgenic hormonal symptoms (virilization) are common and present in approximately 50% of patients; they include hirsutism, clitoromegaly, breast atrophy and menstrual irregularity or amenorrhea (Am J Surg Pathol 1985;9:543)
- Estrogenic hormonal manifestations are rare
Diagnosis
- May be suspected clinically in a young patient presenting with a combination of virilization, elevated testosterone levels and ovarian / pelvic mass on imaging studies
- Clinical presentation often overlaps with other, more common entities, as nearly half of patients with Sertoli-Leydig cell tumors lack the characteristic androgenic symptoms and may be older (peri or postmenopausal, expanding the clinical differential diagnosis) (Eur J Gynaecol Oncol 2017;38:214)
- Intraoperative frozen section evaluation is helpful to confirm the clinical suspicion and guide the surgical management
Laboratory
- May have elevated testosterone (Eur J Gynaecol Oncol 2017;38:214)
Radiology description
- Ultrasound, pelvic CT or MRI can show a solid or solid and cystic adnexal mass
Prognostic factors
- Behavior correlates with tumor grade and histologic subtype
- Well differentiated Sertoli-Leydig cell tumors are essentially benign
- Approximately 10% of moderately differentiated and 13 - 59% of poorly differentiated tumors have malignant behavior (Gynecol Oncol 2012;125:673, Gynecol Oncol 2012;127:384, J Obstet Gynaecol Res 2013;39:305)
- Heterologous elements, retiform pattern, tumor rupture and spread outside the ovary (stage II or higher tumor stage) are adverse prognostic factors (J Obstet Gynaecol Res 2013;39:305, Gynecol Oncol 2012;125:673)
- Patients with germline DICER1 mutations have a favorable prognosis compared with those with somatic DICER1 mutations only (Gynecol Oncol 2017;147:521)
Case reports
- 15 year old girl with Sertoli-Leydig cell tumor and DICER1 mutation (Case Rep Obstet Gynecol 2018;2018:7927362)
- 20 and 21 year old sisters with Sertoli-Leydig cell tumor and DICER1 syndrome (Medicine (Baltimore) 2020;99:e20806)
- 68 year old woman with ovarian Sertoli-Leydig cell tumor with heterologous hepatocytes and a hepatocellular carcinomatous element (Int J Gynecol Pathol 2019;38:247)
- 3 cases of bilateral ovarian Sertoli-Leydig cell tumors in patients with DICER1 syndrome (Histopathology 2020;77:223)
- 9 of 16 cases of familial multinodular goiter and Sertoli-Leydig cell tumors are associated with a large intragenic in frame DICER1 deletion (Eur J Endocrinol 2018;178:K11)
Treatment
- Conservative fertility sparing surgery (unilateral salpingo-oophorectomy) and staging procedure (with or without lymphadenectomy) is usually performed in young patients with stage I tumors (Gynecol Oncol 2012;125:673, Gynecol Oncol 2012;127:384, J Obstet Gynaecol Res 2013;39:305)
- Older patients who do not wish to preserve fertility typically undergo bilateral salpingo-oophorectomy, total hysterectomy and complete surgical staging
- Platinum based adjuvant chemotherapy has also been reported in patients with moderately and poorly differentiated tumors, heterologous mesenchymal elements, advanced stage or rupture (J Obstet Gynaecol Res 2013;39:305, Eur J Gynaecol Oncol 2017;38:214)
- Genetic counseling and germline DICER1 mutation testing is recommended (Am J Surg Pathol 2017;41:1178)
Gross description
- Almost always unilateral (Am J Surg Pathol 1985;9:543)
- Tumor size ranges widely from < 1 cm to 35 cm (mean 12 - 14 cm) (Am J Surg Pathol 1985;9:543)
- Cut surface is typically solid, tan-yellow
- Cystic component may also be seen, especially in tumors with heterologous elements or with a retiform morphologic pattern
- Poorly differentiated tumor may show grossly identifiable foci of necrosis
Frozen section description
- Most critical issue is differentiation from epithelial ovarian malignancies
- Unlike epithelial malignancies, Sertoli-Leydig cell tumor is almost always unilateral and typically occurs in younger patients
- Clinical history of hormonal (androgenic) symptoms is helpful and should be actively sought from the surgical team
- Key microscopic features on frozen section include admixture of Sertoli cell tubules or compressed cords with variable amount of Leydig cell clusters
- Intracytoplasmic Reinke crystals may be better preserved compared to formalin fixed paraffin embedded permanent sections
- Sertoli-Leydig cell tumor: fertility sparing surgery is typically performed in younger patients
- Epithelial ovarian malignancies: generally requires complete surgical staging and the role of fertility preserving surgery is more controversial (Hui: Atlas of Intraoperative Frozen Section Diagnosis in Gynecologic Pathology, 1st Edition, 2015, Mod Pathol 1999;12:933, J Obstet Gynaecol Res 2013;39:305, Gynecol Oncol 2012;125:673)
Frozen section images
Microscopic (histologic) description
- Difficulty of histologic diagnosis increases with tumor grade
- While Sertoli and Leydig components can be readily identified in well differentiated tumors, less differentiated tumors lack well formed Sertoli cell tubules and have fewer Leydig cells
- Well differentiated
- Open or compressed Sertoli cell tubules, admixed with clusters of Leydig cells in the intervening stroma
- Sertoli cells are low columnar to cuboidal with oval to round nuclei, which often show nuclear grooves and small nucleoli
- Leydig cells can be recognized by their round nuclei and abundant eosinophilic cytoplasm with characteristic Reinke crystals and lipofuscin pigment
- No significant atypia or mitotic activity
- Moderately differentiated
- Typically has a diffuse or lobulated architectural pattern and may show alternating hypo and hypercellularity on low magnification
- Sertoli cells form compressed tubules, cords or diffuse sheets and have hyperchromatic, oval or spindled nuclei with mild to moderate atypia and occasional mitotic figures (average 5 per 10 high power fields)
- Rare small clusters of Leydig cells are admixed with the Sertoli cell component
- Follicular differentiation may be seen in moderately and poorly differentiated tumors and may resemble juvenile granulosa cell tumor (Am J Surg Pathol 2021;45:59)
- Poorly differentiated
- Diffuse sheets of immature, sarcomatoid Sertoli cells with moderate to marked nuclear atypia and only rare foci of vague cord formation
- Increased mitotic activity, up to 20 mitoses per 10 high power fields
- Leydig cells are difficult to find; a few small clusters are typically located at the periphery of tumor nodules
- Sertoli-Leydig cell tumor with heterologous elements
- Heterologous (epithelial or mesenchymal) elements may be seen in approximately 20% of moderately or poorly differentiated tumors and in retiform Sertoli-Leydig cell tumor (Am J Surg Pathol 1985;9:543)
- Most common heterologous element is mucinous (intestinal or gastric type) epithelium, which may be benign, borderline or malignant (Cancer 1982;50:2448)
- Carcinoid tumor (trabecular or goblet cell) has also been described arising from heterologous mucinous epithelial elements (Cancer 1982;50:2448)
- Heterologous mesenchymal (cartilage or skeletal muscle) elements are less common (Cancer 1982;50:2465)
- Rare focal hepatocyte differentiation has also been reported and may be associated with increased serum AFP (Hum Pathol 1999;30:611)
- Sertoli-Leydig cell tumor, retiform variant
- Approximately 15% of Sertoli-Leydig cell tumors demonstrate focal or diffuse retiform pattern, forming anastomosing, slit-like, irregular spaces or multicystic, sieve-like or papillary architecture (Am J Surg Pathol 1985;9:543, Am J Surg Pathol 1983;7:755)
Microscopic (histologic) images
Positive stains
- General sex cord proteins, such as inhibin, calretinin, SF1, FOXL2, CD56, WT1 and CD99 (Am J Surg Pathol 1997;21:583, Arch Pathol Lab Med 2017;141:1052)
- Vimentin and pancytokeratin (Am J Surg Pathol 2007;31:592)
- MelanA / MART1 in Leydig cells (Am J Surg Pathol 2011;35:484)
- CK20 and CDX2 in heterologous intestinal type mucinous epithelium
- AFP, arginase and HepPar1 in hepatoid elements
- Reticulin histochemical staining may be helpful in differentiating Sertoli-Leydig cell tumor (completely absent or nested pattern) from fibromas and thecomas (individual pericellular reticulin network)
- Absence of reticulin staining is not specific for Sertoli-Leydig cell tumors, as adult granulosa cell tumors and epithelial ovarian tumors also show the same staining pattern (Int J Gynecol Pathol 2018;37:305)
Negative stains
- CK7 and EMA immunostains are negative; helpful in distinguishing Sertoli-Leydig cell tumors from ovarian epithelial tumors (Arch Pathol Lab Med 2017;141:1052)
- Reticulin is absent (highlights the individual pericellular reticulin network in ovarian fibromas / thecomas; also absent in adult granulosa cell tumors and epithelial ovarian tumors) (Int J Gynecol Pathol 2018;37:305)
Molecular / cytogenetics description
- 3 molecular subtypes (Am J Surg Pathol 2019;43:628):
- DICER1 mutant: younger age, moderately / poorly differentiated, retiform or heterologous elements
- FOXL2 c.402C>G (p.Cys134Trp) mutant: postmenopausal patients, moderately / poorly differentiated, no retiform or heterologous elements
- DICER1 / FOXL2 wildtype: intermediate age, no retiform or heterologous elements, including all well differentiated tumors
- Somatic hotspot DICER1 mutations are present in approximately half of all cases (range: 15 - 97%), 61 - 69% of which also have germline DICER1 mutations (Gynecol Oncol 2017;147:521, Am J Surg Pathol 2017;41:1178, Histopathology 2016;68:279, Mod Pathol 2015;28:1603)
- More recent study identified DICER1 mutations in all of their moderately and poorly differentiated tumors, whereas none of the well differentiated tumors had DICER1 mutations, raising the possibility that well differentiated Sertoli-Leydig cell tumors may represent a different pathogenetic entity (Am J Surg Pathol 2017;41:1178)
- FOXL2 mutation has been reported in up to 22% of ovarian Sertoli-Leydig cell tumors and is mutually exclusive with DICER1 mutations (Histopathology 2016;68:279, J Pathol 2010;221:147, Int J Gynecol Pathol 2018;37:305)
Sample pathology report
- Ovary and fallopian tube, right, salpingo-oophorectomy:
- Sertoli-Leydig cell tumor of the ovary, poorly differentiated, with heterologous elements (embryonal rhabdomyosarcoma) (see comment)
- Tumor size: 28 cm
- Tumor ruptured intraoperatively (see surgeon's operative report)
- Ovarian surface involvement: not definitely identified
- Fallopian tube: negative for tumor
- TNM stage (AJCC 8th edition): pT1c1 Nx, at least stage IC1
- Comment: Pelvic wash cytology is negative. Referral for genetic counseling is recommended.
Differential diagnosis
- Sertoli-Leydig cell tumor:
- Endometrioid adenocarcinoma, especially sertoliform variant (Mod Pathol 1999;12:933):
- Positive for CK7 and EMA and negative for sex cord markers (Arch Pathol Lab Med 2017;141:1052)
- Presence of squamous differentiation is helpful
- Adult granulosa cell tumor:
- Lacks Leydig cell component
- Majority of tumors have FOXL2 mutations (Histopathology 2016;68:279, J Pathol 2010;221:147, Int J Gynecol Pathol 2018;37:305)
- Fibroma:
- Lacks Leydig cell component
- Reticulin stain shows individual pericellular network (Int J Gynecol Pathol 2018;37:305)
- Tubular Krukenberg tumor (i.e. tubular variant of metastatic signet ring cell carcinoma):
- Endometrioid adenocarcinoma, especially sertoliform variant (Mod Pathol 1999;12:933):
- Retiform Sertoli-Leydig cell tumors:
- Yolk sac tumor:
- Lacks Leydig cell component
- Positive for AFP and SALL4 (Histopathology 2014;65:51)
- Low grade serous tumors (borderline tumor and low grade serous carcinoma):
- Positive for CK7
- Lacks Leydig cell component
- Often bilateral
- Yolk sac tumor:
- Sertoli-Leydig cell tumors with heterologous elements:
- Carcinosarcoma:
- Mucinous tumors (primary ovarian and metastatic):
- Lack of the surrounding atypical sex cord (Sertoli cell) and Leydig cell component
- Teratoma:
- Lack of atypical sex cord (Sertoli cell) and Leydig cell component
- Usually predominant ectodermal component
Board review style question #1
Board review style answer #1
B. DICER1. Somatic hotspot missense mutation affecting the RNase IIIb domain of DICER1 gene are common in Sertoli-Leydig cell tumors. In a recent large case series, all moderately and poorly differentiated Sertoli-Leydig cell tumors harbored DICER1 mutations.
Comment Here
Reference: Sertoli-Leydig cell tumor
Comment Here
Reference: Sertoli-Leydig cell tumor
Board review style question #2
Sertoli-Leydig cell tumors of the ovary typically show which of the following immunoprofiles?
- CK7+ / EMA+ / inhibin- / calretinin+
- CK7- / EMA+ / inhibin+ / calretinin-
- CK7- / EMA- / inhibin+ / calretinin+
- CK7- / EMA- / inhibin+ / calretinin-
Board review style answer #2
C. CK7- / EMA- / inhibin+ / calretinin+. Sertoli-Leydig cell tumors express general sex cord immunohistochemical markers, such as inhibin and calretinin. CK7 and EMA immunostains are negative and can help differentiate Sertoli-Leydig cell tumors from sertoliform endometrioid adenocarcinoma of the ovary, which is typically positive for both CK7 and EMA.
Comment Here
Reference: Sertoli-Leydig cell tumor
Comment Here
Reference: Sertoli-Leydig cell tumor
Sex chromosome DSD
Mixed gonadal dysgenesis
Definition / general
Terminology
Etiology
Clinical features
Laboratory
Prognostic factors
Case reports
Treatment
Microscopic (histologic) description
Microscopic (histologic) images
AFIP images
Molecular / cytogenetics description
Differential diagnosis
Additional references
- Mixed gonadal dysgenesis refers to an individual who usually has a differentiated gonad on one side and a streak gonad or streak testis on the other side
- Either (a) testis plus contralateral streak gonad, (b) testis and contralateral gonadal agenesis, (c) hypoplastic gonads with tubules in one gonad or (d) streak gonad with contralateral tumor
- A type of asymmetrical gonadal dysgenesis due to chromosomal abnormality
- Often mosaicism: 45X / 46XY, 45X / 46XX or 45X / 47XXY; may also have 46XX but missing part of one X chromosome
Terminology
- In 2005, the Chicago Consensus of Intersex Disorders proposed the term "mixed gonadal dysgenesis" to substitute for disorders of sex development, defined by congenital conditions in which the development of chromosomal, gonadal or anatomical sex is altered
- There is much confusion about the nomenclature for patients with gonadal dysgenesis
- Asymmetrical gonadal dysgenesis is the term used if one gonad displays more complete development and can be identified as a testis or ovary (usually is a testis) and the other gonad is a streak
- Streak testis: streak tissue identified at periphery of differentiated testis
- Streak gonad: ovarian type stroma without differentiated gonadal structures
Etiology
- Reproductive system developmental disorder characterized by progressive loss of primordial cells on the developing glands of an embryo
- This loss leads to extremely hypoplastic and dysfuctioning gonads, which are mainly composed of fibrous tissue, hence the name streak glands
- During embryogenesis, the reproductive system is intrinsically conditioned to give rise to a female, unless altered by hormone production
- As a result, if a gonad cannot express hormones, as occurrs with gonadal dysgenesis, the affected person will still develop both internal and external genitalia
- In gonadal dysgenesis, the accompanying hormonal failure prevents the development of secondary sex characteristics in either sex, resulting in the appearance of a sexually infantile female and infertility
Clinical features
- Phenotype may be ambiguous, male, female, Turner-like syndrome or intersex
- Müllerian structures present since no / minimal anti-Müllerian hormone is produced
- Usually bilateral fallopian tubes are present, occasionally vas deferens
- Females may exhibit clitoromegaly; no breast development except with tumors
- Phenotypic males may have short stature, 90 degree penile torsion, undescended testis, chordae without hypospadias, hypospadias or no obvious abnormalities
- Patients with 45X mosaicism are often considered to have a variant of Turner syndrome
- Phenotypic females may develop virilization at puberty, often complete
- High risk for gonadoblastoma (30%) if Y chromosome material is present, which may obliterate testicular elements and cause incorrect diagnosis
- Higher risk for other medical problems, including deficient immunoglobulin levels, aberrant bony development of inner ear structures, cardiovascular and renal anomalies (horseshoe kidney)
Laboratory
- Elevated FSH
- Chomatin negative Barr bodies
- Low immunoglobulins levels
Prognostic factors
- High risk for gonadoblastoma (30%) if Y chromosome material is present, which may obliterate testicular elements and cause incorrect diagnosis
Case reports
- Short stature in phenotypic male with 45X0 / 46XY mosaicism (Nat Clin Pract Endocrinol Metab 2008;4:524)
- Mistaken diagnosis of testicular dysgenesis (Arq Bras Endocrinol Metabol 2010;54:331)
Treatment
- Excise gonads early to prevent tumors
- Hormone replacement therapy
Microscopic (histologic) description
- Prepubertal patients show normal immature testis
- At / after puberty, tubules exhibit mild hypospermatogenesis to total sclerosis
- Streak gonad has ovarian stroma without primordial ovarian follicles
- Streak ovary is streak gonad with primordial follicles and primitive sex cord-like structures with or without germ cell components within the ovarian type stroma, mimicking gonadoblastoma, granulosa cell tumor or Sertoli cell tumors
Microscopic (histologic) images
AFIP images
Molecular / cytogenetics description
- FISH may be helpful (Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2008;25:570)
- Usually no mutations of SRY (Eur J Obstet Gynecol Reprod Biol 2004;115:55) but see J Med Genet 1993;30:655
Differential diagnosis
- True hermaphroditism:
- Ovary has numerous primordial follicles containing primary oocytes, nature of internal or external genitalia is not relevant (Mod Pathol 2002;15:1013)
Additional references
Turner syndrome
Definition / general
Terminology
Epidemiology
Etiology
Clinical features
Laboratory
Case reports
Treatment
Clinical images
Images hosted on other servers:
Microscopic (histologic) description
Molecular / cytogenetics images
Images hosted on other servers:
- Describes females with a variety of features (most commonly short stature and gonadal insufficiency) associated with loss of an entire X chromosome (45,X0) in about 50%, mosaicism in 15 - 25% and the rest with loss of only a portion of the X chromosome containing the tip of its short arm
Terminology
- Also called Ullrich-Turner syndrome
Epidemiology
- Occurs in about 1:4000 live births
- Increased frequency in abortuses or women with short stature
Etiology
- Haploinsufficiency of genes that are normally expressed by both X chromosomes including:
- "Short stature homeobox containing gene on the X chromosome" (SHOX) in chondrocytes
- Lymphatic gene, leading to absence or hypoplasia of lymphatics, which causes lymphedema, cystic hygroma, webbed neck, low posterior hairline, nail dysplasia and lymphedematous hands and feet at birth
- Multiple genes involved in ovarian function, leading to gonadal dysgenesis and accelerated loss of oocytes at 15 weeks gestation
Clinical features
- Growth failure: short stature with cubitus valgus, genu valgum and short fourth metacarpals (Wikipedia: Cubitus Valgus [Accessed 15 January 2024], Wikipedia: Genu Valgum [Accessed 15 January 2024])
- High palate, prominent ears, chronic otitis media, obstructive sleep apnea, increased sensitivity to noise and problems learning how to suck, blow, eat and articulate (Wikipedia: Turner Syndrome [Accessed 15 January 2024])
- Cardiovascular disease: including bicuspid aortic valve, coarctation of the aorta, partial anomalous pulmonary venous return (PAPVR) and atrial and ventricular septal defects
- Gonadal failure: have female ducts and external genitalia but usually no pubertal development
- Learning disabilities: deficits in visuomotor skills, visual spatial skills, visual and working memory, visual attention, executive functions and social skills
- Associated with nongonadal neoplasms: including atypical polypoid adenomyoma of uterus, endometrial adenocarcinoma, leukemia and soft tissue tumors
Laboratory
- Diagnosis requires a standard 30 cell karyotype
- Markedly elevated serum FSH, reduced serum estrogen
Case reports
- Mother and daughter with nonmosaic Turner syndrome (Fertil Steril 2004;82:923)
Treatment
- Growth hormone (to increase stature) as soon as growth failure occurs
- Cardiac imaging (MRI) at diagnosis and repeated in 5 - 10 year intervals to assess for congenital heart abnormalities and the emergence of aortic dilatation (precursor to aortic dissection)
- Aggressive treatment of hypertension
- Hormone replacement therapy (transdermal estradiol) beginning at normal pubertal age continued to age 50
- Routine neuropsychological testing and family support, including special education
- Also search for hidden Y chromosome mosaicism (J Pediatr Endocrinol Metab 2006;19:1113)
- Follow up medical care for various conditions in adulthood (Endocr Rev 2002;23:120, J Clin Endocrinol Metab 2010;95:1487)
Clinical images
Images hosted on other servers:
Microscopic (histologic) description
- Streak gonad with fibrous tissue resembling ovarian stroma
Molecular / cytogenetics images
Images hosted on other servers:
Sex cord stromal tumor NOS (pending)
[Pending]
Sex cord tumor with annular tubules
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Sex cord tumor with annular tubules (SCTAT) is a distinctive variant of sex cord stromal tumor that histologically exhibits a unique morphology, characterized by sharply circumscribed nests composed of ring-like tubules that encircle basement membrane-like material
Essential features
- Presence of characteristic tubular pattern, with antipodal distribution of nuclei and basement membrane-like material
- Rare tumor, < 1% of all sex cord stromal tumors
- 2 types: sporadic and syndromic, the latter is associated with Peutz-Jeghers syndrome
- Peak incidence between third and fourth decade
- Uncertain clinical behavior: syndromic types usually have a benign clinical course, up to 20% of sporadic type exhibit extraovarian spread
ICD coding
- ICD-O: 8623/1 - sex cord tumor with annular tubules
- ICD-10: D39.1 - neoplasm of uncertain behavior of ovary
- ICD-11: 2F76 & XH5BV8 - neoplasms of uncertain behavior of female genital organs & sex cord tumor with annular tubules
Epidemiology
- Overall rare tumor (accounting for < 1% of all sex cord tumors)
- Syndromic: associated with germline STK11 mutations on chromosome 19p13.3 (Peutz-Jeghers syndrome), incidental finding
- Nonsyndromic: mean age at presentation is 36 years of age (range: 6 - 76 years)
- References: Cancer 1982;50:1384, Crum: Diagnostic Gynecologic and Obstetric Pathology, 3rd Edition, 2017
Sites
- Peutz-Jeghers syndrome associated cases occur as bilateral ovarian lesions, sometimes multifocal
- Nonsyndromic / sporadic cases are unilateral ovarian lesions
Pathophysiology
- Peutz-Jeghers syndrome associated lesions harbor germline STK11 mutations
Etiology
- Unknown
Clinical features
- Lesions may be seen at any age, mainly in the third and fourth decade
- Incidental finding in a patient with Peutz-Jeghers syndrome
- Nonsyndromic
- Associated with nonspecific symptoms and signs
- Menstrual irregularities
- Premenstrual girls often have precocious pseudopuberty (Fletcher: Diagnostic Histopathology of Tumors, 5th Edition, 2020)
- Older women may have menstrual dysfunction or postmenopausal bleeding (Fletcher: Diagnostic Histopathology of Tumors, 5th Edition, 2020)
- Rarely, nonsyndromic cases may be associated with signs related to progesterone production
Diagnosis
- Histologic evaluation
Radiology description
- Ultrasound imaging (Pediatr Radiol 2007;37:1270)
- Multiple small, lobulated, discrete high echogenicity masses or tumorlets
- Occasionally may have calcifications
Prognostic factors
- Syndrome associated tumors are typically benign
- Nonsyndromic / sporadic cases may exhibit extraovarian spread in ~20% of patients
- Malignant potential of SCTAT cannot be reliably assessed on histologic evaluation as high risk features such as mitotic rate, lymphovascular invasion or ovarian surface involvement are not consistently associated with poor outcomes (Crum: Diagnostic Gynecologic and Obstetric Pathology, 3rd Edition, 2017)
- Malignant SCTAT may have early or late recurrences decades after initial presentation (Crum: Diagnostic Gynecologic and Obstetric Pathology, 3rd Edition, 2017)
- Sites of metastasis include pelvic peritoneum, retroperitoneum and lung (Crum: Diagnostic Gynecologic and Obstetric Pathology, 3rd Edition, 2017)
Case reports
- 11 year old girl with Peutz-Jeghers syndrome and unilateral ovarian tumor most consistent with Sertoli cell tumor associated SCTAT (Int J Surg Pathol 2016;24:269)
- 14 year old girl without Peutz-Jeghers syndrome and with stage III A1 malignant SCTAT (J Obstet Gynaecol Res 2016;42:224)
- 35 and 36 year old women with minimal deviation adenocarcinoma of the cervix, tumorlets of sex cord tumor with annular tubules of the ovaries and Peutz-Jeghers syndrome (J Gynecol Oncol 2013;24:92)
- 60 year old woman with primary diagnosis of granulosa cell tumor with SCTAT (J Obstet Gynaecol Can 2021;43:361)
Treatment
- Surgical management (salpingo-oophorectomy) is a feasible treatment option for nonsyndromic cases
- Complete resection in recurrent cases is advised
- Prognosis is overall favorable
- Reference: BMC Cancer 2015;15:270
Gross description
- Nonsyndromic tumors
- Unilateral masses ranging in size from several millimeters to 3 cm
- Solid, tan to yellow
- Cysts may be seen and occasionally may predominate (Pathology 2018;50:5)
- Syndromic tumors
- Bilateral and multifocal lesions
- Often microscopic; may not be grossly visible
- Gritty texture may be noted if there is a mass
Microscopic (histologic) description
- Syndromic and nonsyndromic tumors have similar morphology
- Variably size round nests of sharply delineated simple and complex tubules containing basement membrane-like material, which may also be present around the tubules
- Sertoliform tubules are classified as annular by their arrangement as small concentric secondary ring shaped acini peripherally arranged around the circumference of a larger tubule
- Tall cells with ample amount of pale cytoplasm and basally located round nuclei
- Often with antipodal nuclear distribution within the tubules
- Tumors associated with Peutz-Jeghers syndrome
- Calcifications within the tubules may be seen (Pathology 2018;50:5)
- May be associated with endocervical gastric type adenocarcinoma (adenoma malignum)
- Nonsyndromic / sporadic tumors
- May focally transition to granulosa or Sertoli cell morphology
- Cytologic atypia and mitotic activity may rarely be seen
- May be hyalinized
- Reference: Crum: Diagnostic Gynecologic and Obstetric Pathology, 3rd Edition, 2017
Microscopic (histologic) images
Positive stains
Molecular / cytogenetics description
- If syndromic, associated with a germline STK11 gene mutations on chromosome 19p13.3 (BMC Med Genet 2016;17:77)
Sample pathology report
- Right ovary, oophorectomy:
- Sex cord tumor with annular tubules (SCTAT), single 9 mm focus (see comment)
- Comment: This neoplasm is an incidental finding. There is no mitotic activity, cytologic atypia, lymphovascular invasion or ovarian surface involvement noted.
- Ovaries and fallopian tubes, bilateral salpingo-oophorectomy:
- Ovaries: bilateral multifocal sex cord tumor with annular tubules (SCTAT), ranging in size from 1.2 mm to 28 mm (see comment)
- Fallopian tubes: benign fimbriated, histologically unremarkable
- Comment: Given the presence of bilateral ovarian involvement and multifocal disease, a possibility of Peutz-Jeghers syndrome associated SCTAT should be considered and excluded. Germline testing for STK11 mutation is recommended.
Differential diagnosis
- Gonadoblastoma:
- Multiple variably sized round nests are distributed in a fibrous to cellular gonadal stroma
- Nests contain 3 components
- Germ cells
- Small sex cord cells
- Globular basement membrane deposits
- Sex cord cells may palisade at the periphery of nests and may resemble the appearance of SCTAT
- Adult granulosa cell tumor:
- Tumor cells of round to oval nuclei with irregular nuclear membrane and nuclear grooves (coffee bean nuclei) and scant cytoplasm
- May form small spaces containing eosinophilic material (Call-Exner bodies) in a microfollicular pattern
- Associated with FOXL2 point mutation (N Engl J Med 2009;360:2719)
- Sertoli cell tumor:
- Cuboidal or columnar cells with pale, at times lipid rich to eosinophilic cytoplasm
- Bland oval to round nuclei, with small nucleoli
- Lipid rich and oxyphilic tumors may be associated with Peutz-Jeghers syndrome
- Areas strongly resembling SCTAT may be seen, especially in patients with Peutz-Jeghers syndrome
- Subset may harbor DICER1 mutations (Mod Pathol 2015;28:1603)
Additional references
Board review style question #1
A 47 year old woman with mucocutaneous oral pigmentations and a history of prior gastrointestinal (GI) polyp resections (multiple hamartomatous polyps) presents for a total abdominal hysterectomy with bilateral salpingo-oophorectomy for menorrhagia. Patient's hysterectomy specimen has no significant histopathologic abnormalities, however, lesion is found in bilateral ovaries. The ovaries were grossly unremarkable. The patient harbors a germline mutation in which of the following genes?
- DICER1
- MMR deficiency
- PTEN
- STK11
Board review style answer #1
D. STK11. The patient has characteristic findings and history of Peutz-Jeghers syndrome. This includes mucocutaneous pigmentation, previous hamartomatous gastrointestinal (GI) polyps. The incidental ovarian findings resemble sex cord tumor with annular tubules (SCTAT). This entity is frequently associated with Peutz-Jeghers syndrome and is often an incidental finding. They are grossly unremarkable and usually involve bilateral ovaries.
Answer A is not correct as DICER1 is associated with Sertoli-Leydig cell tumor, sarcoma and gynandroblastoma, as well as other nongynecologic tumors. Answer B is incorrect because MMR loss is associated with Lynch syndrome. Lynch syndrome predisposes to endometrioid endometrial adenocarcinoma, gastric and intestinal cancers to name a few. Answer C is incorrect because PTEN gene mutations are part of Cowden syndrome, which is associated with high prevalance of thyroid, breast and endometrial neoplasias.
Comment Here
Reference: Sex cord tumor with annular tubules
Answer A is not correct as DICER1 is associated with Sertoli-Leydig cell tumor, sarcoma and gynandroblastoma, as well as other nongynecologic tumors. Answer B is incorrect because MMR loss is associated with Lynch syndrome. Lynch syndrome predisposes to endometrioid endometrial adenocarcinoma, gastric and intestinal cancers to name a few. Answer C is incorrect because PTEN gene mutations are part of Cowden syndrome, which is associated with high prevalance of thyroid, breast and endometrial neoplasias.
Comment Here
Reference: Sex cord tumor with annular tubules
Signet ring stromal tumor
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Rare, benign ovarian stromal tumor with signet ring cells
Essential features
- Primary ovarian stromal neoplasm comprised of various proportions of signet ring and spindle cells
- Cytoplasmic vacuoles in signet ring cells are negative for lipid, mucin and glycogen
- Usually positive, at least focally, for 1 or more sex cord markers (SF1, inhibin, calretinin) but typically negative for epithelial membrane antigen (EMA)
Terminology
- Signet ring stromal cell tumor (SRSCT)
- Signet ring stromal tumor (SRST)
ICD coding
Epidemiology
- ~20 cases reported to date
- No age predilection (range: 28 - 70 years; mean: 49 years) (Am J Surg Pathol 2022;46:1599)
Sites
- Ovary
Pathophysiology
- Unknown; hypothesized to arise from ovarian stromal cells or multifocal conversion of a fibroma (Adv Anat Pathol 2014;21:443)
Etiology
- Unknown
Clinical features
- Nonspecific including abdominopelvic pain, abnormal uterine bleeding or incidental finding on imaging
Diagnosis
- Oophorectomy
Laboratory
- CA-125 typically within normal limits
- Mildly elevated (76.1 U/mL; normal < 46 U/mL) in one report (Int J Gynecol Pathol 2020;39:193)
Radiology description
- No defining features to distinguish from other ovarian neoplasms on imaging
- Solid or cystic, may appear complex
Prognostic factors
- No reports of metastasis or recurrence but overall limited follow up data
Case reports
- 44 year old woman with polymenorrhea and 4 cm right adnexal mass (J Turk Ger Gynecol Assoc 2011;12:59)
- 69 year old obese woman with bilateral adnexal masses on imaging (Obstet Gynecol 2010;116:556)
- 70 year old woman with abdominal distention, rectal bleeding and bilateral ovarian masses (Int J Gynecol Pathol 2020;39:193)
- 76 year old woman with incidental complex adnexal mass (Med Mol Morphol 2008;41:165)
Treatment
- Surgical excision (oophorectomy)
Gross description
- Most are unilateral
- Typically well circumscribed and unencapsulated
- Yellow to tan and soft to firm cut surface
- May show white fibromatous areas
- Variable hemorrhage, necrosis and cystic spaces
- Range: 3.0 - 20 cm (mean: 12.8 cm) (Am J Surg Pathol 2022;46:1599)
Microscopic (histologic) description
- 2 cell populations in variable proportions
- Diffuse growth of round cells with single to occasionally multiple intracytoplasmic, optically clear vacuoles, which peripherally displace and indent nuclei into a crescent shape (signet ring cells)
- Fusiform cells with eosinophilic cytoplasm and ovoid nucleus
- May have fibroma-like areas that merge with signet ring component
- Absent to minimal cellular atypia, mitotic activity and necrosis
- Rarely show brisk mitoses (13/10 and 16/10 high power fields) (Ultrastruct Pathol 1995;19:401, Int J Gynecol Pathol 2004;23:45)
- PAS+ intracytoplasmic hyaline globules noted in ~50% (Ultrastruct Pathol 1995;19:401, Virchows Arch A Pathol Anat Histopathol 1993;422:333, Int J Gynecol Pathol 2004;23:45, Int J Surg Pathol 2008;16:180, Pathol Oncol Res 2008;14:333, Med Mol Morphol 2008;41:165, J Turk Ger Gynecol Assoc 2011;12:59, Int J Gynecol Pathol 2020;39:193)
Microscopic (histologic) images
Positive stains
- Usually positive, at least focally for 1 or more sex cord markers (SF1, calretinin, inhibin, WT1) (Am J Surg Pathol 2022;46:1599)
- Variable expression for CD10 (usually focal), AE1 / AE3 and CAM5.2
Negative stains
- Hallmark is negativity of intracytoplasmic vacuoles for
- Mucins (mucicarmine, PASD, Alcian blue)
- Glycogen (PAS, Alcian blue)
- Lipid (Sudan III, Oil Red O)
- Usually negative for cyclin D1, with cytoplasmic beta catenin expression (not nuclear)
- EMA, CK7, CK20
- Reference: Am J Surg Pathol 2022;46:1599
Electron microscopy description
- Cytoplasmic vacuoles result from (Ultrastruct Pathol 1995;19:401)
- Generalized edema of cytoplasmic matrix
- Dilatation of mitochondria
- Invagination of cell membranes (pseudoinclusions) by extracellular matrix
- No basement membrane formation and abundant free ribosomes (Cancer 1976;38:166, Gynecol Oncol 2000;77:323)
- Hyaline globules show features of lysosomes or degenerating erythrocytes (Med Mol Morphol 2008;41:165, Ultrastruct Pathol 1995;19:401)
Molecular / cytogenetics description
- Negative for FOXL2 and CTNNB1 mutations (Int J Gynecol Pathol 2020;39:193, Am J Surg Pathol 2022;46:1599)
Sample pathology report
- Right ovary, oophorectomy:
- Signet ring stromal tumor (5.0 cm)
Differential diagnosis
- Krukenberg tumor (Am J Surg Pathol 2006;30:277):
- Bilateral in > 50%, often with extraovarian disease at presentation
- Nodular growth
- Admixed with glands, nests or cords of malignant cells (may be very focal)
- Cytokeratin strongly and diffusely positive
- Vacuoles are positive for PASD and mucicarmine
- Similar to SRST, it might have prominent fibromatous component
- Microcystic stromal tumor (Am J Surg Pathol 2009;33:367, Am J Surg Pathol 2015;39:1420, Am J Surg Pathol 2018;42:137, Am J Surg Pathol 2022;46:1599):
- Often separated into lobules by hyalinized bands
- Admixture of microcysts, solid cellular areas and fibrous stroma
- Up to 60% have bizarre nuclei
- Diffuse cyclin D1 and CD10
- Aberrant (nuclear) beta catenin
- Negative for calretinin and inhibin
- Characterized by CTNNB1 or APC mutations
- Signet ring cell change (Adv Anat Pathol 2014;21:443):
- Recent study suggests that some signet ring stromal tumors are fibromas with signet ring cell change (Am J Surg Pathol 2022;46:1599)
- Described in adult granulosa cell tumor (n=1), Brenner tumor (n=1) and serous cystadenofibroma (n=2)
Board review style question #1
Board review style answer #1
B. It is negative for EMA. This is a signet ring stromal tumor. Answers A and E are incorrect as this is a benign entity for which excision is curative. Answer D is incorrect because the tumor is usually unilateral but may rarely be bilateral. Answer C is incorrect as it is negative for mucicarmine. Given the signet ring cell morphology and cytokeratin expression, there may be concern for a metastatic mucinous carcinoma with signet ring cells (i.e., Krukenberg tumor). However, EMA helps to distinguish between the 2 entities as it is negative in signet ring stromal tumor but positive in Krukenberg tumor.
Comment Here
Reference: Signet ring stromal tumor
Comment Here
Reference: Signet ring stromal tumor
Board review style question #2
A 32 year old woman without significant medical history presents with abdominal pain. Workup reveals a 2 cm right adnexal mass on ultrasound and she undergoes salpingo-oophorectomy. Histologic examination of the ovarian tumor reveals sheets of cells with crescent shaped nuclei and intracytoplasmic vacuoles. The vacuoles are negative for mucicarmine and periodic acid Schiff (PAS). The cells are positive for inhibin and steroidogenic factor 1 (SF1) but negative for cyclin D1 and epithelial membrane antigen (EMA). Beta catenin shows cytoplasmic expression. What is the likely diagnosis?
- Adult granulosa cell tumor
- Brenner tumor
- Krukenberg tumor
- Microcystic stromal tumor
- Signet ring stromal tumor
Board review style answer #2
E. Signet ring stromal tumor. All of the answers above are reasonable considerations for which clinicopathologic and immunohistochemical features should aid towards the diagnosis; however, in contrast to the other options, signet ring stromal tumors should present as a unilateral mass with expression of sex cord markers and negativity for cyclin D1 and beta catenin (membranous positivity only). Answer A is incorrect because adult granulosa cell tumor usually occurs in postmenopausal females who present with estrogenic manifestations. Histologically, multiple growth patterns are common and cells are usually small with coffee bean nuclei, longitudinal nuclear grooves and scant cytoplasm. Answer C is incorrect because Krukenberg tumor also presents in older females, usually as bilateral ovarian masses, with positivity for cytokeratin and negativity for sex cord markers. Answer D is incorrect because microcystic stromal tumor can show signet ring cells along with variably thick collagenous septations and strong nuclear expression of beta catenin and cyclin D1. Answer B is incorrect because Brenner tumor usually shows a nested architecture of transitional type cells embedded in a fibromatous stroma and should be positive for CK7 and EMA.
Comment Here
Reference: Signet ring stromal tumor
Comment Here
Reference: Signet ring stromal tumor
Small cell carcinoma of ovary, hypercalcemic type
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Small cell carcinoma of the ovary, hypercalcemic type (SCCOHT) is a rare and highly aggressive ovarian malignancy defined by an inactivating mutation of the SMARCA4 gene, that occurs in young women and is commonly associated with hypercalcemia
Essential features
- Small cell neoplasm with undifferentiated morphology (monomorphic, discohesive and mitotically active) with or without follicle-like spaces, rhabdoid morphology or large cell component
- Loss of nuclear SMARCA4 / BRG1 immunohistochemical expression (> 93% of cases)
Terminology
- SMARCA4 deficient carcinoma of ovary
- Malignant rhabdoid tumor of the ovary
- This lesion is unrelated to small cell neuroendocrine (pulmonary) carcinoma, which refers to a neuroendocrine neoplasm of the ovary
ICD coding
- ICD-O: 8044/3 - small cell carcinoma, intermediate cell
- ICD-11: 2C73.0Y & XH8ZR8 - other specified carcinomas of ovary & small cell carcinoma, hypercalcemic type
Epidemiology
- Adolescent and young adult women (median: 25 years) (Gynecol Oncol 2016;141:454, Nat Genet 2014;46:427)
- Cases associated with germline mutations may occur in younger patients (Gynecol Oncol 2016;141:454, Nat Genet 2014;46:427)
- Very rare cases reported in postmenopausal women
Sites
- Ovary
Pathophysiology
- Inactivating mutation (germline or somatic) of the SMARCA4 gene, a core subunit of the SWI / SNF complex, drives oncogenesis (Gynecol Oncol 2016;141:454)
- SWI / SNF complex is an important regulator of the chromatin remodeling process
Etiology
- Predisposition factor: rhabdoid tumor predisposition syndrome 2 (RTPS2)
- Exact cellular origin remains unknown
- Although SCCOHT was initially believed to be of epithelial origin, studies have suggested a primitive germ cell, neural or mesenchymal progenitor (Hum Pathol 1987;18:175, Histopathology 2017;70:1147, J Pathol 2017;242:371)
- Studies have shown distinct DNA methylation signatures in SCCOHT that are distinct from ovarian epithelial neoplasms (J Pathol 2022;257:140)
Clinical features
- Poor clinical outcome (Gynecol Oncol 2016;141:454)
- Most patients present at an advanced stage (Gynecol Oncol 2016;141:454)
- Nonspecific symptoms may include abdominal pain, distention and signs related to intestinal obstruction
- A minority of patients may show symptoms of hypercalcemia (N Engl J Med 2010;362:1031)
- Patients may harbor SMARCA4 germline mutation which is associated with the rhabdoid tumor predisposition syndrome 2 (RTPS2) and also predisposes for other undifferentiated malignancies such as atypical teratoid / rhabdoid tumors (ATRT) and other extracranial malignant rhabdoid tumors (OMIM: Rhabdoid Tumor Predisposition Syndrome 2; RTPS2 [Accessed 22 February 2023])
Diagnosis
- There are no established tests to screen for SCCOHT
- When clinical suspicion arises, abdominal ultrasound and computed tomography scans are useful adjuncts
- Definitive diagnosis requires biopsy
- All patients diagnosed with SCCOHT should be referred to clinical genetics services and offered testing for germline SMARCA4 pathogenic variants
- At risk family member surveillance
- In patient's family members with germline SMARCA4 mutation, surveillance may include clinical examination and ultrasound, as well as whole body and CNS MRI
- Furthermore risk reducing bilateral salpingo-oophorectomy (RRBSO) may be considered; however, the benefits of early detection using imaging and RRBSO remain unproven
- Guidelines are provided by the International SCCOHT Consortium (ISC) (Clin Cancer Res 2020;26:3908)
Laboratory
- Hypercalcemia can be detected in a majority of patients, likely from parathyroid hormone related peptide secretion by the tumor cells
- Most of these patients will not present with symptoms of hypercalcemia (N Engl J Med 2010;362:1031, Cancer 1994;73:1878, Gynecol Oncol 2016;141:454)
Prognostic factors
- Stage is the most important parameter (Gynecol Oncol 2016;141:454)
- FIGO stage I: 55% 5 year overall survival
- FIGO stage II: 40% 5 year overall survival
- FIGO stage III: 29% 5 year overall survival
- FIGO stage IV: 0% 5 year overall survival
- Favorable prognostic factors include
- Older patient age at diagnosis (> 40 years) (Gynecol Oncol 2016;141:454)
- Small tumor size (< 10 cm) and absence of large cell component (Am J Surg Pathol 1994;18:1102)
Case reports
- 18 - 29 year old women with small cell carcinoma of the ovary of hypercalcemic type with responses to anti-PD1 immunotherapy (J Natl Cancer Inst 2018;110:787)
- 21 year old pregnant woman with small cell carcinoma of the ovary of hypercalcemic type (Medicine (Baltimore) 2020;99:e20387)
- 24 year old woman with an abdominal mass (Case Rep Oncol 2016;9:305)
Treatment
- No established standard treatment
- Patients are usually treated by a combination of surgery, chemotherapy and radiation
- Treatment guidelines are provided by the International SCCOHT Consortium (ISC) (Clin Cancer Res 2020;26:3908)
- Following complete response to initial chemotherapy, high dose chemotherapy with autologous stem cell rescue (HDC-aSCR) may be considered (Gynecol Oncol 2016;141:454)
- There is active research looking at epigenetic modulators, kinase inhibitors and immunotherapy as potential treatment alternatives
Gross description
- Unilateral (vast majority of cases), solid or cystic mass with lobulated or nodular surface
- Mean size of 15 cm (range: 6 - 26 cm)
- Fleshy and tan to white to gray cut surface
- Hemorrhage and necrosis are common
- Familial cases are more often bilateral (Arch Pathol Lab Med 1995;119:523, J Med Genet 1996;33:333)
Microscopic (histologic) description
- Small round cells with scant cytoplasm, small nucleoli and brisk mitotic activity
- Diffuse growth of tightly packed cells, sometimes in a nested or corded architecture
- Follicle-like spaces (80%), usually containing eosinophilic or basophilic fluid
- Necrosis seen in most tumors
- Focal myxoid stroma is common
- Most show a large cell component, often with rhabdoid features, of varying extent (if > 50% of the tumor, it is referred to as a small cell carcinoma of the ovary hypercalcemic, large cell variant)
- Intermixed mucinous glands are reported in some cases (Arch Pathol Lab Med 1995;119:523)
Microscopic (histologic) images
Contributed by Basile Tessier-Cloutier, M.D. and AFIP
Positive stains
- WT1 (86 - 93%) (Int J Gynecol Pathol 2004;23:330, Histopathology 2007;51:305)
- CD10 (93%) (Int J Gynecol Pathol 2004;23:330)
- Calretinin (73%) (Int J Gynecol Pathol 2004;23:330)
- EMA (87%) (Int J Gynecol Pathol 2004;23:330)
- AE1 / AE3 (60%) (Int J Gynecol Pathol 2004;23:330)
- p53 wild type pattern (Histopathology 2023;83:154)
Negative stains
- Loss of nuclear SMARCA4 / BRG1 expression (Pol J Pathol 2013;64:238, Nat Genet 2014;46:427, Nat Genet 2014;46:438, Nat Genet 2014;46:424)
- Loss of nuclear SMARCA2 / BRM expression (J Pathol 2016;238:389)
- CAM5.2 (20%) (Int J Gynecol Pathol 2004;23:330)
- Inhibin (Int J Gynecol Pathol 2004;23:330)
- CD99 (Int J Gynecol Pathol 2004;23:330)
- TTF1 (Histopathology 2007;51:305)
- OCT3/4 (Histopathology 2007;51:305)
- Loss of nuclear SMARCB1 / INI1 staining has been reported in rare cases of SMARCA4 proficient tumors (J Pathol 2016;238:389)
- FOXL2
Molecular / cytogenetics description
- Almost all SCCOHTs harbor inactivating somatic or germline mutations in SMARCA4 (Pol J Pathol 2013;64:238, Nat Genet 2014;46:427, Nat Genet 2014;46:438, Nat Genet 2014;46:424)
- SCCOHTs show SMARCA2 loss of expression likely due to transcriptional / posttranscriptional silencing (J Pathol 2016;238:389)
- SCCOHTs are chromosomally stable cancers (Orphanet J Rare Dis 2013;8:33, Oncotarget 2016;7:1732, J Pathol 2022;257:140)
- SCCOHTs harbor distinct DNA methylation profiles, which distinguish them from other SWI / SNF deficient cancers (Oncotarget 2016;7:1732, Acta Neuropathol 2021;141:291, J Pathol 2022;257:140)
Sample pathology report
- Ovary, oophorectomy:
- Small cell carcinoma of the ovary hypercalcemic type (see comment and synoptic report)
- Comment: Immunohistochemical tests for SMARCA4 / BRG1 and SMARCA2 / BRM show loss of expression. Small cell carcinoma of the ovary, hypercalcemic type (SCCOHT) can be associated with the rhabdoid tumor predisposition syndrome 2 (RTPS2); referral to medical genetics is recommended.
Differential diagnosis
- Undifferentiated and dedifferentiated carcinomas of the ovary:
- Older age (median: 53 years)
- May be associated with a differentiated ovarian carcinoma (dedifferentiated carcinoma)
- Mismatch repair deficiency is common
- Loss of nuclear expression of SMARCA4 / BRG1, SMARCB1 / INI1 or ARID1B
- Associated with other genetic alterations (PTEN, ARID1A, PIK3CA, KRAS, CTNNB1, NRAS, TP53)
- Granulosa cell tumor, adult and juvenile:
- Prominent follicle-like spaces (juvenile)
- Small cells usually not present (juvenile)
- Prominent nuclear grooves (adult)
- Usually, no brisk mitotic activity of small cells (adult)
- Intact nuclear expression of SMARCA4 / BRG1 (J Pathol 2016;238:389)
- Positive for FOXL2
- Small cell carcinoma, pulmonary type (primary or metastatic):
- Usually lacks follicle-like spaces
- Positive for TTF1
- May show dot-like positivity for CK20
- Intact nuclear expression of SMARCA4 / BRG1 (J Pathol 2016;238:389)
- Dysgerminoma:
- Usually lacks follicle-like spaces
- Large, uniform cells with clear or eosinophilic cytoplasm and distinct membranes
- May show granulomatous reaction
- Positive for OCT4, PLAP, KIT and SALL4
- Intact nuclear expression of SMARCA4 / BRG1 (J Pathol 2016;238:389)
- Embryonal rhabdomyosarcoma:
- Primitive neuroectodermal tumor (central type or peripheral type):
- Associated with ovarian teratoma (central type)
- Positive for FLI1 and GFAP
- EWSR1 rearrangement (peripheral type)
- Melanoma (primary or metastatic):
- May be associated with ovarian teratoma (primary)
- Often bilateral (metastatic)
- May show cytoplasmatic melanin pigment
- Intact nuclear expression of SMARCA4 / BRG1 (J Pathol 2016;238:389)
- Positive for S100, HMB45, MelanA
- Negative for cytokeratin and WT1
- Lymphoma:
- Lacks follicle-like spaces
- Negative for cytokeratin and WT1
- Positive for lymphoid markers (subtype specific)
Board review style question #1
A 27 year old woman presented with a 5 cm unilateral ovarian mass. SMARCA4 / BRG1 IHC image is provided above. What is the most likely diagnosis?
- Dysgerminoma
- Granulosa cell tumor, adult type
- Granulosa cell tumor, juvenile type
- Metastatic melanoma
- Small cell carcinoma of the ovary, hypercalcemic type
Board review style answer #1
E. Small cell carcinoma of the ovary, hypercalcemic type. The H&E image shows a solid and corded undifferentiated tumor with follicle-like spaces and focal myxoid stroma. The immunohistochemical test for SMARCA4 / BRG1 shows aberrant expression (loss of nuclear staining). The morphology and immunophenotype are consistent with the diagnosis of SCCOHT. All 4 other options may have some overlapping morphologic features with SCCOHT but retain SMARCA4 / BRG1 expression.
Comment Here
Reference: Small cell carcinoma of ovary, hypercalcemic type
Comment Here
Reference: Small cell carcinoma of ovary, hypercalcemic type
Board review style question #2
Which of the following immunohistochemical tests shows aberrant expression in small cell carcinoma of the ovary, hypercalcemic type (SCCOHT)?
- BAP1
- MDM2
- MLH1
- p53
- SMARCA4 / BRG1
Board review style answer #2
E. SMARCA4 / BRG1. SCCOHT is molecularly defined by inactivating SMARCA4 mutations, which are associated with SMARCA4 / BRG1 nuclear loss of expression. The other options are in other entities such as mesothelioma (BAP1 loss of expression), malignant Brenner tumor (MDM2 overexpression), ovarian undifferentiated and dedifferentiated carcinoma (MLH1 loss of expression) and high grade serous carcinoma (aberrant p53 expression).
Comment Here
Reference: Small cell carcinoma of ovary, hypercalcemic type
Comment Here
Reference: Small cell carcinoma of ovary, hypercalcemic type
Solid pseudopapillary tumor
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Ovarian tumor that is morphologically, immunohistochemically and genetically identical to pancreatic solid pseudopapillary neoplasm
- Categorized as miscellaneous tumor of ovary in WHO classification (Kurman: WHO Classification of Tumours of the Female Reproductive Organs, 4th Edition, 2014)
Essential features
- Rare tumor of reproductive age group; 10 reported cases
- Possible origin from genital ridge related cells, presumed epithelial / neuroendocrine nature
- Solid nests with pseudopapillary architecture, cysts with colloid-like material, papillae with myxoid stroma and tumor cell nuclei oriented away from the papillae
- Abnormal nuclear β catenin immunohistochemical staining and E-cadherin (negative NCH-38 clone)
- Limited cases with followup, presumed to be of low malignant potential, like pancreatic solid pseudopapillary neoplasm
Terminology
- Primary ovarian solid pseudopapillary neoplasm
- Extrapancreatic solid pseudopapillary neoplasm
- Miscellaneous ovarian tumors (Kurman: WHO Classification of Tumours of the Female Reproductive Organs, 4th Edition, 2014)
ICD coding
- ICD-10: C56.9 - Malignant neoplasm of unspecified ovary
Epidemiology
- Rare tumor
- Females of reproductive age, range 17 - 57 years
- 10 reported cases: 6 from U.S., 2 from China, 1 each from India and Japan (Am J Surg Pathol 2010;34:1514, Hum Pathol 2012;43:1339, Ann Diagn Pathol 2012;16:498, Int J Gynecol Pathol 2018;37:110, Int J Clin Exp Pathol 2015;8:8645, Int J Gynecol Pathol 2011;30:539, Indian J Pathol Microbiol 2016;59:348, Pathol Int 2014;64:460)
Sites
- Ovary
Clinical features
- Abdominal mass, fullness, swelling or pain (6 cases)
- Pelvic pain (2 cases)
- Incidental radiologic finding (2 cases)
- Postmenopausal bleeding (1 case)
Diagnosis
- Exclude metastasis of solid pseudopapillary neoplasm from extraovarian site
Radiology description
- Ultrasound: solid and cystic complex adnexal mass with high density in cyst (Int J Clin Exp Pathol 2015;8:8645)
- Multiloculated heterogeneous ovarian cystic mass (Hum Pathol 2012;43:1339)
Prognostic factors
- Unknown due to limited number of cases
- Only patient who died of disease was stage IV
- Tumor showed high mitotic rate (62/50 high powered fields), necrosis and lymphovascular invasion (Ann Diagn Pathol 2012;16:498)
- 6 patients had no evidence of disease at followup, including a stage IIIC case at 18 months followup
Case reports
- 17, 21 and 57 year old women; first reported cases (Am J Surg Pathol 2010;34:1514)
- 22 and 47 year old women with metastasis from pancreas to ovary (Mol Clin Oncol 2016;4:845)
- 47 year old woman with overlap of microcystic stromal tumor and SPN (Int J Clin Exp Pathol 2015;8:11792)
- 48 year old woman with clinically aggressive tumor (Hum Pathol 2012;43:1339)
- 49 year old woman with CTNNB1 point mutation (Int J Gynecol Pathol 2018;37:110)
Treatment
- Surgery is mainstay of treatment
Gross description
- Circumscribed ovarian / adnexal mass with solid and cystic hemorrhagic cut surface
Microscopic (histologic) description
- Well circumscribed tumor
- Tumor cell sheets, nests and bands surrounded by fibrous septa
- Thin delicate capillary sized vessels forming myxohyaline pseudopapillary cores; nuclei located away from the papillary core
- Moderate amount of pale eosinophilic cytoplasm with central / eccentric round to oval nuclei with uniform chromatin
- Inconspicuous mitoses, usually no necrosis, no significant nuclear atypia
- Abundant foamy cytoplasm, paranuclear vacuoles, nuclear grooves and intra / extracellular PAS+ diastase resistant eosinophilic globules can be present
- Microcysts with colloid-like material
- Infarction can be present
Microscopic (histologic) images
Positive stains
Negative stains
- E-cadherin: no staining with NCH-38 clone
- Chromogranin: usually negative
- Calretinin, inhibin, S100, desmin, TTF1, GFAP and HMB45
Electron microscopy description
- Tumor cells with numerous pleomorphic mitochondria interspersed among short strands of rough endoplasmic reticulum (Ann Diagn Pathol 2012;16:498)
Molecular / cytogenetics description
- c.110C > T point mutation in CTNNB1 exon 3 (Pathol Int 2014;64:460)
- c.98C > G point mutation in CTNNB1 exon 3 (Int J Gynecol Pathol 2018;37:110)
Differential diagnosis
- Granulosa cell tumor:
- Cuboidal to polygonal cells with Call-Exner bodies arranged in macrofollicular, trabecular, solid and insular patterns
- Inhibin+ and calretinin+
- Steroid cell tumor:
- Sheets and nests of large vacuolated cells with clear or eosinophilic cytoplasm; minimal to no nuclear atypia
- Inhibin+ and calretinin+
- Metastatic pancreatic solid pseudopapillary neoplasm (Mol Clin Oncol 2016;4:845):
- Similar morphologically
- Clinical correlation and pancreatic / abdominal imaging required
- Metastatic neuroendocrine tumor:
- Cells arranged in nests, sheets and ribbons separated by thin walled vessels
- Salt and pepper chromatin and moderate granular cytoplasm
- Keratin+, chromogranin+ and synaptophysin+
- Struma ovarii:
- Mature thyroid tissue with variable sized follicles lined by cuboidal cells
- TTF1+ and thyroglobulin+
- Paraganglioma / pheochromocytoma:
- Zellballen pattern with supporting vessels and spindle sustentacular cells
- S100+ sustentacular cells, chromogranin+ and synaptophysin+
- Melanoma:
- Gastrointestinal stromal tumor:
- Ependymoma:
Board review style question #1
The most characteristic immunohistochemical staining pattern of solid pseudopapillary neoplasm of the ovary is
- c-kit-
- Consistent keratin expression
- Consistent positive expression of both chromogranin and synaptophysin
- Nuclear β catenin+ and E-cadherin-
- S100+ and HMB45+
Board review style answer #1
Board review style question #2
The image below is taken from an H&E stained section of a 3.0 cm circumscribed hemorrhagic left ovarian lesion of a 48 year old woman. Plasma inhibin levels were normal. Tumor cells expressed CAM5.2, vimentin and synaptophysin and were negative for S100, inhibin, FOXL2, E-cadherin and chromogranin. Genetic analysis showed c.98C > G point mutation in CTNNB1 exon 3. The diagnosis is
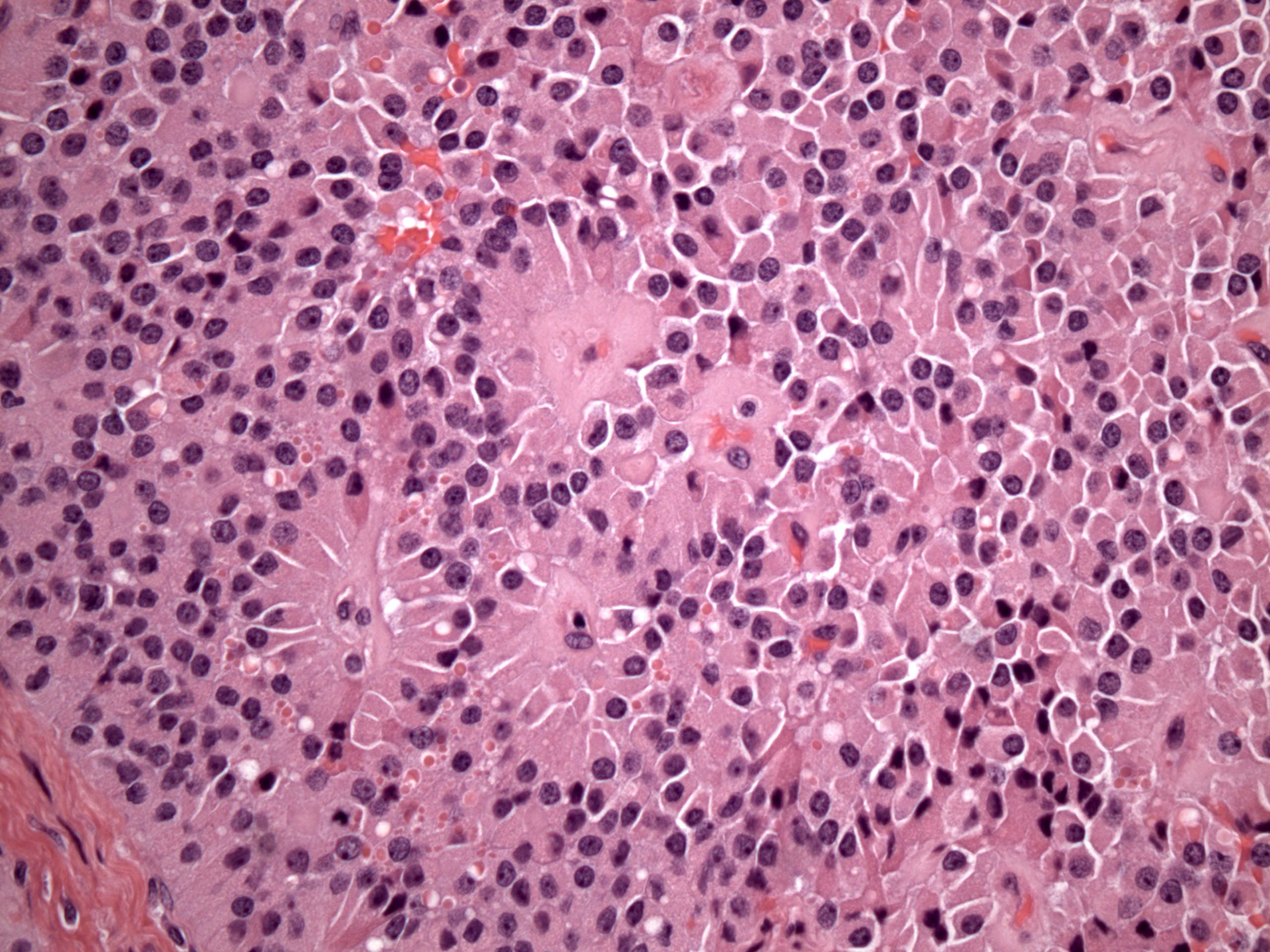

- Ependymoma
- Granulosa cell tumor
- Microcystic stromal tumor
- Solid pseudopapillary neoplasm
- Strumal carcinoid
Board review style answer #2
Somatic neoplasms arising from teratomas
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Immunofluorescence description | Immunofluorescence images | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Molecular / cytogenetics description | Molecular / cytogenetics images | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Any benign or malignant neoplasm arising from any tissue within teratomas, either mature or immature
Essential features
- Any benign or malignant neoplasm arising from any tissue within teratomas, either mature or immature
- Wide variety of somatic malignancies can arise (2% of all teratomas)
- The most common carcinoma is squamous cell carcinoma; the second most common carcinoma is adenocarcinoma
- Sarcomas account for 8%
- Other malignancies are exceedingly rare
- Immunohistochemistry is applied according to the histotype of the somatic lesion
Terminology
- Teratoma with malignant transformation
- Malignant teratoma of ovary
ICD coding
- ICD-O: 9084/3 - teratoma with malignant transformation
- ICD-11: 2C73.3 & XH33E8 - malignant teratoma of ovary & teratoma with malignant transformation
Epidemiology
- Wide age range: 21 - 75 years, mean 53 years
- Mature teratomas account for 20% of all ovarian neoplasms in pathological studies
- Somatic neoplasms may be encountered in up to 2% of mature cystic teratomas (extremely rare in immature teratomas) (J Obstet Gynaecol Res 2022;48:3068)
- Cutaneous neoplasms appear in up to the 2% of dermoid cysts, with squamous cell carcinoma representing the majority; about 80% of malignant tumors in teratomas (BMC Cancer 2019;19:217)
- Adenocarcinoma is the second most common carcinoma in teratomas (7%) (Prz Menopauzalny 2018;17:63)
- Sarcoma accounts for 8%
- Melanomas, melanocytic nevi, basal cell carcinomas and sebaceous neoplasms are much less common (BMC Womens Health 2019;19:149)
- Other benign and malignant tumors have also been reported, including low grade malignant mucinous neoplasms, choroid plexus papilloma, Paget disease, glomus tumor, benign soft tissue tumors and lymphomas
Sites
- Tumors arising in ovarian teratomas are typically unilateral
Pathophysiology
- Ovarian squamous cell carcinoma arising in mature cystic teratoma has a high mutational burden, with TP53 mutation the most common abnormality (Clin Cancer Res 2017;23:7633)
Etiology
- Unknown at this time
Diagrams / tables
Not relevant to this topic
Clinical features
- Typically, unilateral and generally appear later than uncomplicated teratomas (about 20 years later) (Arch Gynecol Obstet 2007;275:179)
- Large or malignant tumors can show signs and symptoms associated with a mass
- Diameter > 10 cm and elevated CEA levels in patients aged 45 and above indicate a potential malignancy (Medicine (Baltimore) 2024;103:e38793)
- Sarcomas are more frequently seen in younger patients compared to carcinomas
Diagnosis
- Incidental findings
- May present with rapid abdominal swelling or acute abdominal pain (Acta Med Philipp 2024;58:55)
- Squamous cell carcinoma shows a varied morphology, ranging from well differentiated and keratinizing to poorly differentiated to anaplastic (including sarcomatoid) (J Gynecol Oncol 2017;28:e69)
- Melanomas may show usual (epithelioid and spindle) or unusual appearances, including pseudopapillary architecture, follicle-like spaces and a myxoid background
- Sebaceous lesions include sebaceous hyperplasia, adenoma, sebaceoma and carcinoma
- Adenocarcinomas most commonly arise from gastrointestinal type epithelium and respiratory type epithelium
- Benign and low grade mucinous epithelial neoplasms with mucin extravasation (more common within the ovary but also seen outside it) resembling appendiceal primaries may arise in teratomas and mimic metastases (Int Cancer Conf J 2018;7:59)
- Sarcomas include the subtypes described in soft tissues (Int J Surg Case Rep 2018;42:90)
- Finding a teratomatous component is critical; an associated appendiceal primary should be excluded (Histopathology 2016;68:977)
- Essential and desirable diagnostic criteria are based on the histological type of the somatic neoplasm
Laboratory
- May present with elevated CEA
Radiology description
- Absence of clinical and radiological specificity of malignant transformation (the diagnosis remains purely histological) (Case Rep Obstet Gynecol 2021;2021:5527467)
- Computed tomography (CT): solid cystic septate pelvic mass
- Magnetic resonance imaging (MRI): well defined solid mass, lobulated
- Ultrasound (US): lobulated ovarian mass, solid (J Cancer Res Ther 2023;19:S623)
Radiology images
Prognostic factors
- Prognosis largely relies on the stage (J Formos Med Assoc 2008;107:857)
- Patients with tumors confined to the ovary tend to have a positive prognosis
- Overall 5 year survival rate for all stages is between 15% and 52%, up to nearly 80% for stage I tumors (Front Oncol 2022;12:842703)
- Advanced disease (extraovarian spread, 50% at diagnosis) have a poorer prognosis compared to more common types of ovarian cancers
Case reports
- 29 year old woman with a history of back pain and amenorrhea (Gynecol Oncol Rep 2022;44:101100)
- 43 year old woman with squamous cell carcinoma transformation in a mature cystic teratoma of the ovary (J Med Case Rep 2024;18:145)
- 45 year old woman with abdominal pain (Radiol Case Rep 2021;16:3275)
- 47 year old woman investigated for diffuse pelvic pain (In Vivo 2020;34:2141)
- 51 year old premenopausal woman with left lower abdomen pain (Fukushima J Med Sci 2020;66:44)
- Middle aged woman with lower abdominal pain (Cureus 2023;15:e44159)
Treatment
- For benign tumors, oophorectomy is the treatment of choice
- In malignant cases, bilateral salpingo-oophorectomy + hysterectomy + eventual debulking
- Chemotherapy or radiotherapy in advanced stage
- Reference: J Obstet Gynaecol Res 2022;48:3068
Gross description
- Dermoid cyst with thickening or nodule protruding from the wall (Indian J Pathol Microbiol 2022;65:369)
- Cut surface with solid and cystic, or multicystic or papillary areas
- Neoplasms may completely obscure the underlying teratoma
- Necrosis or hemorrhage are common
Frozen section description
- Appearance of the frozen section depends on the type of somatic neoplasm encountered
- Must be found in the context of a teratoma
- For this purpose, the nonsolid part of the lesion must also be sampled
- Reference: J Midlife Health 2024;15:115
Microscopic (histologic) description
- Microscopic appearance is that of a mature cystic teratoma with a component of a solid neoplasm, either benign or malignant
- Squamous cell carcinoma: from well to poorly differentiated, different histotypes (conventional, keratinizing, papillary, sarcomatoid, acantholytic)
- They may have cystic degeneration or adjacent areas of dysplasia or carcinoma in situ (Medicine (Baltimore) 2021;100:e24726)
- Benign / low grade mucinous neoplasms, typically originating from the intestinal type epithelium
- It can be associated with mucin extravasation in the ovarian stroma (so called pseudomyxoma ovarii), other (Case Rep Oncol 2016;9:331)
- Other adenocarcinoma (gastric, bronchial), adenosquamous, small cell and adnexal tumors
- Malignant melanoma or other melanocytic proliferations
- Sarcoma: leiomyosarcoma, rhabdomyosarcoma, osteosarcoma, chondrosarcoma, liposarcoma, angiosarcoma, other (Int J Surg Case Rep 2018;42:90)
- Paraganglioma, rhabdomyoma, glomus tumor, choroid plexus papilloma, lymphoma, other
Microscopic (histologic) images
Virtual slides
No virtual slides found
Cytology description
- Not clinically relevant
Cytology images
Not relevant to this topic
Immunofluorescence description
Not relevant to this topic
Immunofluorescence images
Not relevant to this topic
Positive stains
- Depending on the neoplasia encountered, for example
- Adenocarcinoma arising from gastrointestinal epithelium: CDX2, CK20 (Cureus 2023;15:e44159, Int J Gynecol Pathol 2016;35:352)
- Squamous cell carcinoma: p63, p40, CK8 / 18, CK5/6 (variable), p53 mutated pattern (BMC Cancer 2019;19:217)
- Melanomas: HMB45, MelanA (BMC Womens Health 2019;19:149)
Negative stains
- It depends on the neoplasia encountered
- Squamous cell carcinoma: p16 (BMC Cancer 2019;19:217)
- Adenocarcinoma arising from gastrointestinal epithelium: CK7 (Int J Gynecol Pathol 2016;35:352)
Electron microscopy description
Not relevant to this topic
Electron microscopy images
Not relevant to this topic
Molecular / cytogenetics description
- Not clinically relevant
Molecular / cytogenetics images
Not relevant to this topic
Videos
None
Sample pathology report
- Ovary, ovarian cyst, ovariectomy:
- Low grade mucinous adenocarcinoma arising from mature teratoma (see comment)
- Comment: Mature cystic teratoma, comprising solid nodule composed of well differentiated (low grade) adenocarcinomatous glands, immerse in extravasated pool of mucin (i.e., pseudomyxoma ovarii). If not consensually submitted, low grade appendiceal mucinous neoplasia must be ruled out. Bilateral salpingo-oophorectomy recommended.
Differential diagnosis
- Metastatic squamous cell carcinoma from other sites:
- Thoroughly check for prior history
- More frequently bilateral
- Not associated with dermoid cyst
- p16 positive if from the uterine cervix
- Metastatic appendiceal mucinous tumor:
- Appendiceal tumor
- Often associated with pseudomyxoma peritonei
Additional references
Board review style question #1
Board review style answer #1
D. Mucinous low grade carcinoma arising from teratoma. The images are from a mature cystic teratoma with a solid component. The lesion is well differentiated (low grade) mucinous neoplasia. The glands are embedded in extravasated mucin, which dissects the ovarian stroma, forming what is referred to as pseudomyxoma ovarii.
Answer A is incorrect because mucinous low grade tumors from the appendix usually are accompanied by pseudomyxoma peritonei. However, this is the top differential diagnosis. Appendiceal neoplasms must be ruled out.
Answer B is incorrect because immature teratoma contains immature tissue (mostly of neuroectodermal origin), not carcinomatous.
Answer C is incorrect because metastatic carcinoma is usually bilateral, not associated with pseudomyxoma ovarii and has a prior history of colorectal cancer.
Comment Here
Reference: Somatic neoplasms arising from teratomas
Comment Here
Reference: Somatic neoplasms arising from teratomas
Board review style question #2
Which of the following immunohistochemistry profiles would be expected in squamous cell carcinoma arising from teratoma?
- CK7 positive
- ER, PR positive
- HMB45 positive
- p63, p40 positive
Board review style answer #2
D. p63, p40 positive. The immunohistochemistry is expressed accordingly to the neoplasm encountered, Squamous cell carcinoma expresses the classical immunohistochemical markers of squamous differentiation.
Answer A is incorrect because squamous cell carcinoma doesn't express CK7.
Answer B is incorrect because ER and PR are not expressed in squamous cells carcinoma, even if it arises from ovarian teratomatous tissue.
Answer C is incorrect because HMB45 is a marker for melanoma and not for squamous cell carcinoma.
Comment Here
Reference: Somatic neoplasms arising from teratomas
Comment Here
Reference: Somatic neoplasms arising from teratomas
Somatic neoplasms arising from teratomas (pending)
[Pending]
Staging
Table of Contents
Definition / general | Essential features | ICD coding | Primary tumor (pT) and FIGO stages in ( ) | Regional lymph nodes (pN) and FIGO stages in ( ) | Distant metastasis (pM) and FIGO stages in ( ) | Prefixes | AJCC prognostic stage groups | Registry data collection variables | Histologic grade (G) | Histopathologic type | Gross images | Microscopic (histologic) images | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- All primary tumors (malignant or potentially malignant [borderline] of the ovary and fallopian tube) and primary peritoneal carcinoma are covered by this staging system
- The following topics are not covered: metastatic tumors to the ovary and fallopian tube, hematopoietic malignancies, peritoneal primaries other than primary peritoneal carcinoma (i.e., mesothelioma, gastrointestinal stromal tumor)
Essential features
- AJCC 7th edition staging was sunset on December 31, 2017; as of January 1, 2018, use of the 8th edition is mandatory
ICD coding
- ICD-10:
- D39.10 - neoplasm of uncertain behavior of unspecified ovary
- D39.11 - neoplasm of uncertain behavior of right ovary
- D39.12 - neoplasm of uncertain behavior of left ovary
- C56.1 - malignant neoplasm of right ovary
- C56.2 - malignant neoplasm of left ovary
- C56.9 - malignant neoplasm of unspecified ovary
- C57.00 - malignant neoplasm of unspecified fallopian tube
- C57.01 - malignant neoplasm of right fallopian tube
- C57.02 - malignant neoplasm of left fallopian tube
- C48.1 - malignant neoplasm of specified parts of peritoneum
- C48.2 - malignant neoplasm of peritoneum, unspecified
- C48.8 - malignant neoplasm of overlapping sites of retroperitoneum and peritoneum
Primary tumor (pT) and FIGO stages in ( )
Regional lymph nodes (pN) and FIGO stages in ( )
- pNX: regional lymph nodes cannot be assessed
- pN0: no regional lymph node metastasis
- pN0(i+): isolated tumor cells in regional lymph node(s) ≤ 0.2 mm
- pN1a (IIIA1i): lymph node metastatic deposit > 0.2 mm to ≤ 10 mm
- pN1b (IIIA1ii): lymph node metastatic deposit > 10 mm
Note:
- Regional lymph nodes include the following: external iliac, internal iliac (hypogastric), obturator, common iliac, para-aortic, pelvic NOS and retroperitoneal NOS lymph nodes
Distant metastasis (pM) and FIGO stages in ( )
- pM0: no distant metastasis
- pM1a (IVA): pleural effusion with positive cytology
- pM1b (IVB): liver or splenic parenchymal metastases; metastases to extra-abdominal organs (including inguinal lymph nodes and lymph nodes outside the abdominal cavity); transmural involvement of intestine
Prefixes
- cTNM: clinical classification
- pTNM: pathological classification
- ypTNM: post therapy pathological classification
- rTNM: recurrence or retreatment classification
- aTNM: autopsy classification
AJCC prognostic stage groups
| Stage I: | pT1 | N0 | M0 | ||||
| Stage IA: | pT1a | N0 | M0 | ||||
| Stage IB: | pT1b | N0 | M0 | ||||
| Stage IC: | pT1c | N0 | M0 | ||||
| Stage II: | pT2 | N0 | M0 | ||||
| Stage IIA: | pT2a | N0 | M0 | ||||
| Stage IIB: | pT2b | N0 | M0 | ||||
| Stage IIIA1: | pT1/2 | N1 | M0 | ||||
| Stage IIIA2: | pT3a | any N | M0 | ||||
| Stage IIIB: | pT3b | any N | M0 | ||||
| Stage IIIC: | pT3c | any N | M0 | ||||
| Stage IV: | any T | any N | M1 | ||||
| Stage IVA: | any T | any N | M1a | ||||
| Stage IVB: | any T | any N | M1b |
Registry data collection variables
- FIGO stage
- Preoperative CA125 level
- Gross residual tumor after primary cytoreductive surgery
- Residual tumor volume after primary cytoreductive surgery
- Residual tumor location following primary cytoreductive surgery
Histologic grade (G)
- GX: grade cannot be assessed
- GB: borderline tumor
- G1: well differentiated
- G2: moderately differentiated
- G3: poorly differentiated
Histopathologic type
- Epithelial tumors (borderline or malignant)
- Serous tumors
- Serous borderline tumor
- Low grade serous carcinoma
- High grade serous carcinoma
- Mucinous tumors
- Mucinous borderline tumor
- Mucinous carcinoma
- Endometrioid tumors
- Endometrioid borderline tumor
- Endometrioid carcinoma
- Clear cell tumors
- Clear cell borderline tumor
- Clear cell carcinoma
- Seromucinous tumors
- Seromucinous borderline tumor
- (Seromucinous carcinoma - now considered a subtype of endometrioid carcinoma, according to WHO 5th edition)
- Brenner tumors
- Borderline Brenner tumor
- Malignant Brenner tumor
- Other carcinomas
- Mesonephric-like adenocarcinoma
- Undifferentiated and dedifferentiated carcinoma
- Carcinosarcoma
- Mixed carcinoma
- Serous tumors
- Sex cord - stromal tumors (malignant or potentially malignant)
- Adult granulosa cell tumor
- Juvenile granulosa cell tumor
- Sertoli-Leydig cell tumor
- Gynandroblastoma
- Steroid cell tumor, NOS
- Fibrosarcoma
- Germ cell tumors (malignant)
- Dysgerminoma
- Yolk sac tumor
- Immature teratoma
- Choriocarcinoma (nongestational)
- Embryonal carcinoma
- Mixed germ cell tumor
- Somatic neoplasms arising from teratomas
- Miscellaneous tumors
- Small cell carcinoma, hypercalcemic type
- Adenocarcinoma of rete ovarii
Gross images
Microscopic (histologic) images
Additional references
Board review style question #1
Which of the following is the pTNM stage (per the AJCC 8th edition) of a malignant ovarian tumor involving the right ovarian parenchyma and surface, omentum and splenic capsule (for which the largest tumor focus in the omentum and splenic capsule exceeds 2 cm) and no lymph node or parenchymal spleen metastases?
- pT1c N0 M0, stage IC
- pT1c N0 M1b, stage IVB
- pT3b N0 M0, stage IIIB
- pT3c N0 M1b, stage IVB
- pT3c N0 M0, stage IIIC
Board review style answer #1
E. pT3c N0 M0, stage IIIC. Macroscopic peritoneal metastasis beyond the pelvis (including capsule of liver and spleen without parenchymal involvement) measuring more than 2 cm in greatest dimension is pT3c. Parenchymal metastasis to the liver or spleen is pM1b, which would yield stage IVB.
Comment Here
Reference: Staging - ovary
Comment Here
Reference: Staging - ovary
Steroid cell tumor
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Ovarian stromal tumor composed of steroid cells with malignant potential
Essential features
- Mostly unilateral
- Half of cases have androgenic manifestations
- Microscopic features include diffuse sheets of polygonal to rounded tumor cells with moderate to abundant cytoplasm (Am J Surg Pathol 1987;11:835)
Terminology
- Steroid cell tumor, not otherwise specified (NOS)
ICD coding
- ICD-O
- ICD-11: 2C73.Y & XH4L39 - other specified malignant neoplasms of the ovary & steroid cell tumor, malignant
Epidemiology
- Rare (Average age in general: 43 year old
- Average age in von Hippel-Lindau: 27 years old (Int J Clin Exp Pathol 2022;15:332)
- In an analysis of 63 cases, patients with clinically malignant tumors were 16 years older than patients with benign tumor (Am J Surg Pathol 1987;11:835)
Sites
- Ovary
- Extraovarian origin (very rare) (Int J Gynecol Pathol 2019;38:151)
Pathophysiology
- Tumors are presumed to be of ovarian stromal cell origin
Etiology
- Dysregulation in the hypoxia signaling pathway (Endocr Relat Cancer 2023;30:e230179)
- VHL aberration in von Hippel-Lindau (Int J Clin Exp Pathol 2022;15:332)
- Genomic instability, MDM1 / CDK4 coamplification, ATRX rearrangement, MDM2 / CDK4 copy number gain, BAP1 mutation in malignant tumors
- In nonmalignant tumors: mutation in FH, CTTNB1, CASP10 and P53 (Am J Surg Pathol 2023;47:1398)
Clinical features
- Androgenic (~50%), estrogenic changes including rare examples of isosexual pseudoprecocity (~10%), occasional progestogenic changes, Cushing syndrome or elevated cortisol levels (uncommon)
- Rarely with hypercalcemia, erythrocytosis, ascites or acute heart failure
- Reported in von Hippel-Lindau (both types I and II), with the most common presentation including abnormal uterine bleeding, amenorrhea and infertility (Int J Clin Exp Pathol 2022;15:332)
Diagnosis
- Histologic examination
Laboratory
- In patients with androgenic changes, Cushing syndrome or both: elevated urinary levels of 17-ketosteroids and 17-hydroxycorticosteroids, serum testosterone and androstenedione
- In Cushing syndrome: elevated free cortisol in the blood or urine
Radiology description
- Ultrasound: hypoechoic / isoechoic, homogenous or heterogeneous texture, characterized by abundant blood flow signals (Oncol Lett 2022;24:370)
- Computed tomography (CT): low density area, consistent with lipid content within the mass (AJR Am J Roentgenol 2007;188:W393, Magn Reson Med Sci 2019;18:251)
- Magnetic resonance imaging (MRI): intermediate signal intensity on T2 weighted images and avid contrast enhancement (indicating hypervascularity of the tumor); signal loss on out of phase MR imaging, consistent with lipid content within the mass (AJR Am J Roentgenol 2007;188:W393, Magn Reson Med Sci 2019;18:251)
- Overall CT and MRI findings are variable, owing to the amount of lipid component and fibrous stroma (AJR Am J Roentgenol 2007;188:W393, Magn Reson Med Sci 2019;18:251)
Radiology images
Prognostic factors
- Clinically malignant in 25 - 43% of cases (Am J Surg Pathol 1987;11:835)
- Patients with clinically malignant tumor were on average 16 years older than patients with benign tumors in one series (Am J Surg Pathol 1987;11:835)
- No malignant steroid cell tumors have been reported in patients in their first 2 decades
- Rare tumors have recurred up to 19 years postoperatively (Am J Surg Pathol 1987;11:835)
- Extraovarian spread of tumor is present at the time of operation in a small minority of cases; 3 cases with Cushing disease with extensive intra-abdominal spread of tumor (Int J Gynecol Pathol 1987;6:40)
- Features associated with malignancy (Am J Surg Pathol 1987;11:835)
- 2+ mitoses per 10 high power fields (92% malignant)
- Necrosis (86% malignant)
- 7 cm or larger (78% malignant)
- Intratumoral hemorrhage (77% malignant)
- Grade 2 or 3 nuclear atypia (64% malignant)
- Aggressive behavior with 4 or more malignant features (Am J Surg Pathol 2023;47:1398)
Case reports
- 16 year old girl with history of von Hippel-Lindau (VHL) syndrome and a 3 cm well defined nodule in right ovary (Int J Gynecol Pathol 2020;39:473)
- 21 and 23 year old women with malignant and benign steroid cell tumors, NOS (J Ovarian Res 2013;6:53)
- 28 year old woman with acute heart failure, virilization and 7 cm right adnexal mass (J Obstet Gynaecol Res 2020;46:1211)
- 35 year old woman with virilization and a pelvic mass (Arch Pathol Lab Med 2006;130:113)
- 56 and 74 year old women with mixed steroid cell tumor and fibroma in extraovarian and ovarian tissue, respectively (Int J Gynecol Pathol 2019;38:151)
- 64 year old woman with collision signet ring stromal tumor and steroid cell tumor of the ovary (Int J Gynecol Pathol 2017;36:261)
Treatment
- Unilateral salpingo-oophorectomy if fertility sparing is desired; otherwise, total hysterectomy and bilateral salpingo-oophorectomy
- Adjuvant chemotherapy has been used for cases with malignant type; however, there is no consensus (Case Rep Oncol 2020;13:358, Case Rep Obstet Gynecol 2016;2016:6184573)
- Tumor excision, chemotherapy and gonadotropin releasing hormone (GnRH) analog therapy are reported for disease recurrence or progression (BMC Endocr Disord 2022;22:265, Gynecol Oncol Rep 2023;46:101169)
Gross description
- Wide size range (mean size: 8.4 cm) (Am J Surg Pathol 1987;11:835)
- Unilateral (95%)
- Solid, well circumscribed, occasionally lobulated
- Sections from yellow-orange (lipid rich) to red-brown (lipid poor) to dark brown-black (abundant lipochrome pigment)
- Occasionally with necrosis, hemorrhage and cystic degeneration
Gross images
Frozen section description
- Diffuse or nested arrangement of tumor cells
- Cells with abundant eosinophilic or clear, foamy cytoplasm
Frozen section images
Microscopic (histologic) description
- Architectural pattern: diffuse (most common) but occasionally in nests, cords, pseudoglandular and follicle-like arrangements (Am J Surg Pathol 1987;11:835)
- Stroma: usually sparse (85%) but may be fibrotic or hyalinized, rarely edematous or myxoid, exceptionally with calcification and psammoma body formation
- Polygonal to rounded tumor cells with distinct cell borders, central nuclei and moderate to abundant cytoplasm
- Tumor cell cytoplasm: eosinophilic and granular (lipid poor) to vacuolated and spongy (lipid rich); tumors with a mixture of 2 cell types are also present
- More lipid rich cytoplasm in this tumor compared to other subtypes of steroid cell tumor
- Lipochrome pigment present (40%)
- Rarely, signet ring appearance
- Adipocytic metaplasia and hyaline globule (unusual) (Am J Surg Pathol 2023;47:1398)
- Usually absent / slight nuclear atypia along with low mitotic activity (Necrosis and hemorrhage are occasionally present, particularly in tumors with significant cytologic atypia
- Metastatic tumor is similar to the primary tumor in some cases but more poorly differentiated in others
- Stromal hyperthecosis may be seen in the adjacent ovarian stroma and contralateral ovary, particularly in small tumors
Microscopic (histologic) images
Contributed by Fatemeh Ghazanfari Amlashi, M.D., Tamara Kalir, M.D., Ph.D. and AFIP
Cytology description
- Large polygonal to round cells
- Arranged in sheets and attached with vascular stromal tissue fragments (J Cytol 2015;32:284)
- Cells with small central round nuclei with conspicuous nucleoli and abundant granular to pale foamy cytoplasm
- Ascites in malignant steroid cell tumor: isolated tumor cells or clusters with slight overlapping and cannibalism formation (Acta Cytol 2017;61:165)
Positive stains
Negative stains
Molecular / cytogenetics description
- Pathogenic variants in several hypoxia related genes, including HIF1A, VHL, SDHB, SRC, IDH2 and FOXO4 (Endocr Relat Cancer 2023;30:e230179)
- Mutation in FH, CTTNB1, CASP10 and P53 (Am J Surg Pathol 2023;47:1398)
- Malignant steroid cell tumor with genomic instability, MDM1 / CDK4 coamplification, ATRX rearrangement, MDM2 / CDK4 copy number gain, BAP1 mutation (Am J Surg Pathol 2023;47:1398)
Videos
Steroid cell tumor
Sample pathology report
- Right ovary and fallopian tube, salpingo-oophorectomy:
- Ovarian steroid cell tumor, not otherwise specified (6 cm size) (see comment)
- Fallopian tube with no significant pathologic change
- Comment: Sections show a well circumscribed neoplasm composed of abundant clear cytoplasm. There is mild nuclear atypia. No mitosis or necrosis identified. Immunostains show the tumor cells are positive for inhibin, calretinin and negative for CD10, supporting the diagnosis.
- Left ovary and fallopian tube, salpingo-oophorectomy:
- Ovarian steroid cell tumor, not otherwise specified (12 cm size) (see comment)
- Fallopian tube with no significant pathologic change
- Comment: Sections show a well circumscribed neoplasm composed of sheets of round cells with abundant eosinophilic granular cytoplasm. Intratumoral hemorrhage and focal necrosis is present. Moderate nuclear atypia and mitoses (4/10 high power fields) are seen. The ovarian capsule is intact and uninvolved by the tumor cells. Immunostains show the tumor cells are positive for calretinin and inhibin, supporting the diagnosis.
Differential diagnosis
- Ovarian clear cell carcinoma:
- Periodic acid-Schiff (PAS) positive, diastase sensitive, glycogen rich cytoplasm and eccentric nuclei
- Variety of architectural patterns (Int J Gynecol Pathol 2019;38:S40)
- Almost always accompanied by a variable component of clear and hobnail cells
- Metastatic renal cell carcinoma:
- Glycogen rich cytoplasm and eccentric nuclei
- Prominent sinusoidal vascular framework (Int J Gynecol Pathol 2018;37:525)
- Areas with tubular differentiation
- History of a prior or concurrent renal mass
- No endocrine manifestation
- CD10 positive
- Pregnancy luteoma:
- Third trimester of pregnancy
- Bilateral (33%) (Am J Surg Pathol 2014;38:239)
- Multiple (50%)
- Abundant eosinophilic cytoplasm containing little or no lipid
- Mitotic figures may be numerous, up to 2 - 3/10 high power fields
- Leydig cell tumor:
- Reinke crystalloids (Am J Surg Pathol 2023;47:1398)
- Acellular eosinophilic zone
- Nuclear clustering
- Fibrinoid material within vessel walls
- Located in ovarian hilum
- Sertoli cell tumor (lipid rich):
- At least focal tubular differentiation in adequately sampled tumors
- Absence of lipochrome pigments
- Metastatic melanoma:
- Bilateral (40%)
- No endocrinologic manifestation
- More malignant nuclear features than steroid cell tumors
- MelanA, S100 positive (Am J Surg Pathol 2004;28:771)
- Inhibin, calretinin, SF1 negative
- Oxyphilic struma ovarii:
- Association with other teratomatous elements (Endocr Pathol 2015;26:342)
- Presence of colloid
- Thyroglobulin positive
- Hepatoid yolk sac tumor:
- Elevated serum alpha fetoprotein (Am J Surg Pathol 2022;46:309)
- Glandular lumens may be seen
- AFP positive (Cancer 1982;50:2355)
Additional references
Board review style question #1
Board review style answer #1
A. Inhibin+, calretinin+, MelanA+, PAX8-. Steroid cell tumor, NOS shows positive inhibin, calretinin and MelanA and negative PAX8. Note that MelanA (A103 clone) can be expressed in steroid cell tumor and the presence of it should not necessarily prompt the diagnosis of melanoma. Answer B is incorrect because steroid cell tumor has a 30% risk of malignancy. Answer C is incorrect because steroid cell tumors are mostly unilateral. Answer D is incorrect because having various architectural patterns is a feature of ovarian clear cell carcinoma.
Comment Here
Reference: Steroid cell tumor
Comment Here
Reference: Steroid cell tumor
Board review style question #2
A 40 year old woman presented with 5 cm, solid, well defined adnexal mass. Which of the following features is in favor of steroid cell tumor, NOS?
- Acellular eosinophilic material and nuclear clustering
- Location in hilum
- Reinke crystal
- Sheets of tumor cells with distinct cell borders and clear to foamy cytoplasm
Board review style answer #2
D. Sheets of tumor cells with distinct cell borders and clear to foamy cytoplasm. Steroid cell tumor, NOS is mostly seen as sheets of tumor cells with distinct cell borders and can have clear to foamy cytoplasm in lipid rich tumor. Answers A, B and C are incorrect because these are features of Leydig cell tumor.
Comment Here
Reference: Steroid cell tumor
Comment Here
Reference: Steroid cell tumor
Stromal hyperplasia and hyperthecosis
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Ovarian stromal hyperplasia is a nonneoplastic functional disorder resulting in nodular or diffuse proliferation of ovarian stroma
- Hyperthecosis is characterized by proliferation of ovarian stroma along with luteinization of stromal cells (J Turk Ger Gynecol Assoc 2013;14:182)
Essential features
- Ovarian stromal hyperplasia and hyperthecosis are nonneoplastic conditions resulting in diffuse or nodular proliferation of ovarian stroma as single cells, clusters or nodules of luteinized cells
- Patients can present with bleeding due to endometrial pathology or symptoms of hyperandrogenism, principally androgenetic alopecia or hirsutism, along with elevated testosterone levels
- Both show an excess of androgen production leading to increased testosterone
- Ovaries with stromal hyperplasia and hyperthecosis secrete more androstenedione than normal ovaries that can be converted to estradiol by peripheral aromatization (BJOG 2003;110:690)
ICD coding
- ICD-11: 5A80.Y - other specified ovarian dysfunction
Epidemiology
- Generally encountered in postmenopausal women with an average age of 55 years (age range: 51 - 64 years) (J Menopausal Med 2020;26:39)
- Rare in women of reproductive age (< 1%) (Case Rep Obstet Gynecol 2023;2023:2783464)
Sites
- Ovarian stroma
Pathophysiology
- Studies have shown that ovaries with stromal hyperplasia and hyperthecosis secrete more androstenedione and estradiol than normal ovaries
- Most patients with stromal hyperplasia and hyperthecosis have normal gonadotrophin levels, unlike patients with polycystic ovary syndrome (Histopathology 1989;14:369)
- Some in vitro incubation studies show that stromal proliferation in premenopausal and postmenopausal women is increased by luteinizing hormone
- Luteinizing hormone also increases androgen production in ovaries with or without stromal hyperplasia (Am J Obstet Gynecol 1980;136:997, J Clin Endocrinol Metab 1992;74:172)
- Most patients with stromal hyperplasia and hyperthecosis show hyperinsulinemia or insulin resistance that likely plays a role in pathogenesis (Am J Obstet Gynecol 1986;154:384)
Etiology
- Etiology is not completely known (J Menopausal Med 2020;26:39)
- Some studies suggest genetic etiology (Medicina (Kaunas) 2023;59:1097)
- There may be an association with hyperandrogenism, insulin resistance and acanthosis nigricans (HAIR AN) syndrome
Clinical features
- Symptoms and signs of hyperandrogenism, such as deepening voice, acne, hirsutism, frontal alopecia, clitoromegaly
- Other features include obesity, hyperinsulinemia, acanthosis nigricans and metabolic syndrome
- There is an increased risk of vaginal bleeding, endometrial hyperplasia and carcinoma, cardiovascular pathology and diabetes mellitus (J Menopausal Med 2020;26:39)
- May be associated with HAIR AN syndrome in a few patients
Diagnosis
- Initial investigation is usually done to rule out adrenal tumors and androgen secreting ovarian tumors
- Clinical assessment for hirsutism, alopecia, deepening of voice, etc.
- Serological assessment: elevated testosterone, androgen and estrogen levels, normal or suppressed luteinizing hormone, normal dehydroepiandrosterone (DHEA), DHEA sulfate (DHEAS), 17 hydroxyprogesterone (17 OHP), normal progesterone and prolactin levels
- Imaging: enlarged ovaries without hypervascularization on imaging (J Menopausal Med 2020;26:39)
- Histology: the gold standard diagnostic test used for confirmation (and subsequent treatment) consists of histological investigation performed after bilateral salpingo-oophorectomy (J Menopausal Med 2020;26:39)
Laboratory
- Increased androgen, testosterone and estrogen levels
- Normal or suppressed LH and FSH
- Normal serum androstenedione or dehydroepiandrosterone sulfate (DHEAS) concentrations
- Unfavorable lipid profiles (i.e., increase in low density lipoprotein [LDL] and triglycerides, along with decrease in high density lipoprotein [HDL] associated with the hyperandrogenemia)
Radiology description
- Imaging: enlarged ovaries without hypervascularization on ultrasonography (J Menopausal Med 2020;26:39)
- In severe cases, bilateral expansion of ovarian stroma with more solid ovaries (J Turk Ger Gynecol Assoc 2013;14:182)
- Increased ovarian volume for the age group or nodules / cysts, however, unlike polycystic ovary syndrome, the number of follicles tends to be normal (Case Rep Obstet Gynecol 2023;2023:2783464)
- Magnetic resonance can show homogeneous T2 hypointensity and mild enhancement of the ovaries (Endocrinol Diabetes Metab Case Rep 2021;2021:20)
Prognostic factors
- Although it is a benign nonneoplastic condition, ovarian stromal hyperplasia / increased ovarian volume is believed to be associated with an increased risk of endometrial cancer; hence, malignancy should be excluded and patient should be treated to reduce the neoplastic risk (Ann Saudi Med 2012;32:588, Cancer Epidemiol Biomarkers Prev 2006;15:1550, Case Rep Obstet Gynecol 2023;2023:2783464)
Case reports
- 55 year old multiparous woman presented with bilateral ovarian hyperthecosis and endometrial intraepithelial neoplasia (EIN) (J Turk Ger Gynecol Assoc 2013;14:182)
- 62 year woman presented with bilateral ovarian stromal hyperplasia (Endocrinol Diabetes Metab Case Rep 2021;2021:20)
- 64 year old woman presented with serous cystadenoma and hyperthecosis (Case Rep Obstet Gynecol 2023;2023:2783464)
- 64 year old woman presented with virilizing symptoms and ovarian stromal hyperplasia (Postgrad Med J 1991;67:304)
Treatment
- Bilateral oophorectomy in postmenopausal women, however, if the patient has multiple comorbidities and is not fit for surgical option, then long term gonadotropin releasing hormone agonist treatment may be an option (J Turk Ger Gynecol Assoc 2013;14:182)
- In young females, long term gonadotropin releasing hormone agonist is an alternative to preserve fertility (Endocrinol Diabetes Metab Case Rep 2021;2021:20)
Gross description
- Mostly involves bilateral ovaries with increase in size
- May be nodular or diffuse with solid, firm, homogeneous white to yellow cut surface
Microscopic (histologic) description
- Diffuse or nodular proliferation of ovarian stroma in cortex, medulla or both
- Stromal proliferation of spindle cells with scant intervening collagen
- May be diffuse or present as a nodule with coalescent nodules (Endocrinol Diabetes Metab Case Rep 2021;2021:20)
- Entrapped follicular derivatives may be present
- In hyperthecosis, single cells, nests or nodules of polygonal luteinized theca cells with eosinophilic or clear cytoplasm < 1 cm are present
Microscopic (histologic) images
Positive stains
- WT1, ER, PR, CD56 in stromal cells (Am J Surg Pathol 2008;32:884)
- Inhibin and calretinin strongly positive in luteinized cells, negative or weakly positive in stromal cells
- FOXL2
Negative stains
- AE1 / AE3, EMA, cytokeratin MNF 116 and CD10
Sample pathology report
- Right ovary, oophorectomy:
- Ovarian stromal hyperplasia / hyperthecosis (see comment)
- Comment: This ovarian tissue shows expansion of ovarian stroma due to diffuse proliferation of stromal cells. In between these stromal cells, few clusters of eosinophilic luteinized cells are also present. No evidence of epithelial elements, increased mitosis, pleomorphism or necrosis is identified.
- Clinicopathological correlation: Correlation with serological markers and endometrial thickness is recommended as this condition is frequently associated with hyperandrogenism and endometrial hyperplasia.
Differential diagnosis
- Normal ovary:
- Normal appearance of cortex and medulla
- Cortex is the hypercellular outer zone having majority of follicles in premenopausal women; medulla is the hypocellular central area containing blood vessels and diffuse or nodular population of spindle cells
- Young women have thick cortex and contain follicles in various stages of development, while this is atrophied in old age and mainly contain corpus albicans
- Diagnosis of stromal hyperplasia and hyperthecosis is a subjective estimation of cortex and medullary cellularity with respect to age
- Fibroma:
- Mostly unilateral mass
- Usually well circumscribed and composed of variable cellular spindle to oval cells with scant cytoplasm, arranged as intersecting fascicles with intervening collagen; sometimes hyaline plaques are also present
- Cellular fibroma is densely cellular with little intervening collagen that may be mitotically active
- Steroid cell tumor, NOS:
- This tumor is comprised of expansile diffuse proliferation of large polygonal cells with eosinophilic, pale or vacuolated cytoplasm
- Steroid cells nodules > 1 cm arising in a background of stromal hyperthecosis are now best diagnosed as steroid cell tumor, NOS, as the previous diagnostic category of stromal luteoma is no longer recognized as a distinct entity
- Luteinized thecoma associated with sclerosing peritonitis:
- Extremely rare lesion, patient presents with abdominal swelling, ascites and bowel obstruction
- Almost always bilateral, ovaries have tan to red sectioned surface
- Microscopically, ovaries are hypercellular and show microcystic appearance due to edema
- Cells are predominantly spindled with minority component of small luteinized cells
- SF1 and FOXL2 are positive in spindle cells while calretinin and inhibin are positive in luteinized cells
- Sex cord stromal tumor, NOS:
- Both sex cord and stromal components are present
- Stromal component highlighted by reticulin staining
- Pregnancy luteoma:
- Associated with pregnancy
- Comprised of oval to round uniform cells with abundant eosinophilic cytoplasm
- Massive ovarian edema and fibromatosis:
- Prominent edema of stroma sparing cortex
- Clusters of luteinized cells may be present
- Krukenberg tumor:
- Isolated atypical cells with signet ring cell morphology in ovarian stroma
- AE1 / AE3 positive
- Low grade endometrial stromal sarcoma:
- Oval to spindle cells with minimal atypia and associated arterioles
- CD10 positive
Additional references
Board review style question #1
A specimen for bilateral oophorectomy is received with enlarged ovaries. One ovary measures 44 x 30 x 25 mm while the second measures 35 x 30 x 25 mm. Sectioning shows homogeneous solid areas with a single 3 mm cyst. Microscopy shows a diffuse population of spindle cells with no pleomorphism, mitosis or necrosis. What is the likely diagnosis?
- Adult granulosa cell tumor
- Fibroma
- Metastatic tumor
- Sex cord stromal tumor, NOS
- Stromal hyperplasia
Board review style answer #1
E. Stromal hyperplasia. There is a homogeneous population of spindle cells with no atypia. Answer A is incorrect because adult granulosa cell tumor will show atypia with variable patterns and nuclear grooving. Answer B is incorrect because no hyalinized areas with intersecting fascicles are present. Answer C is incorrect because no epithelial elements are present in this tumor. Answer D is incorrect because no sex cord-like areas or biphasic cell populations are identified.
Comment Here
Reference: Stromal hyperplasia and hyperthecosis
Comment Here
Reference: Stromal hyperplasia and hyperthecosis
Board review style question #2
A 57 year old woman presents with postmenopausal bleeding. Endometrial biopsy shows FIGO grade 1 endometrioid type endometrial cancer and hysterectomy reveals stage IA endometrioid type endometrial carcinoma with enlarged bilateral ovaries. Microscopic findings are shown in the image above. What is the diagnosis?
- Fibroma
- Leydig cell tumor
- Metastatic endometrioid type endometrial cancer
- Sex cord stromal tumor
- Stromal hyperplasia and hyperthecosis
Board review style answer #2
E. Stromal hyperplasia and hyperthecosis. Clusters of luteinized cells are present in stromal hyperplasia. Answer A is incorrect because no hyalinized areas with intersecting fascicles are present. Answer B is incorrect because no Leydig cell population is identified and the cells do not contain Reinke crystals. Answer C is incorrect because no epithelial elements are identified. Answer D is incorrect because no tumor-like mass or epithelial elements are identified.
Comment Here
Reference: Stromal hyperplasia and hyperthecosis
Comment Here
Reference: Stromal hyperplasia and hyperthecosis
Struma ovarii
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology / etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Monodermal ovarian teratoma primarily (> 50%) or exclusively composed of benign thyroid tissue
- Any mature teratoma with malignant thyroid tissue
Essential features
- Ovarian teratoma either composed predominantly or exclusively of benign thyroid tissue or with any amount of malignant thyroid tissue
- Most common thyroid malignancy to occur in struma ovarii is papillary thyroid carcinoma, followed by follicular carcinoma
ICD coding
Epidemiology
- Most common type of monodermal teratoma
- Accounts for 3% of ovarian teratomas (Pathol 2007;39:139)
- Usually presents in the fifth decade
Sites
- Ovary
Pathophysiology / etiology
- Unknown at this time
Clinical features
- Most often an incidental and asymptomatic finding but may present as a pelvic mass with abdominal pain (Pathology 2007;39:139)
- Uncommonly presents as hyperthyroidism or pseudo-Meigs syndrome (with ascites and pleural effusion) (Pathology 2007;39:139)
- Seen in association with Brenner tumor, serous and mucinous cystadenoma and fibrothecoma (Endocr Pathol 2015;26:342)
Diagnosis
- Diagnosis is made by microscopic examination of resected tissue
Laboratory
- Mildly increased CA125 levels in up to 30% of cases (J Gynecol Oncol 2008;19:135)
Radiology description
- Typically a lobulated and multiloculated solid cystic lesion on imaging (Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2015;37:309, Abdom Imaging 2012;37:904)
- MRI is more specific, showing high and low signal intensity on T1 and T2 weighted images from the colloid (J Med Imaging Radiat Oncol 2009;53:480)
Prognostic factors
- Most cases have a good prognosis, even when malignancy is present
Case reports
- 10 year old girl with tachycardia, normal thyroid and thyrotoxicosis with papillary thyroid carcinoma arising from a struma ovarii (Int J Surg Case Rep 2018;51:218)
- 48 year old woman with malignant struma ovarii presenting 14 years after oophorectomy and chemotherapy (Medicine (Baltimore) 2018;97:e13867)
- 52 year old woman with clinical features of advanced ovarian carcinoma found to have a struma ovarii (Oncol Lett 2015;9:1739)
- 61 year old woman with insular carcinoma (90%) and adjacent papillary thyroid carcinoma arising in a struma ovarii (Gynecol Oncol Rep 2016;18:1)
- 62 year old woman with 2 different types of RAS mutations in a papillary thyroid carcinoma from a struma ovarii and papillary thyroid microcarcinoma of the thyroid (J Endocr Soc 2018;2:944)
- 74 year old woman with a mixed ovarian tumor composed of elements of Brenner tumor, mucinous cystadenoma and struma ovarii (Int J Gynecol Pathol 2019;38:576)
Treatment
- Oophorectomy
- Surgery for malignant struma ovarii may include total abdominal hysterectomy and bilateral salpingo-oophorectomy with complete staging (Gynecol Oncol 2004;94:835)
Gross description
- Typically unilateral and solid with a gelatinous, red-brown to green cut surface; may show goiter-like multinodular or cystic change (Am J Surg Pathol 1994;18:785)
Gross images
Frozen section description
- Frozen section sample usually shows a mature teratoma or benign thyroid tissue; rarely, a possible malignant component may be detected
Microscopic (histologic) description
- Variably sized macro and microfollicles often containing colloid
- Other architectural patterns include solid areas composed of cells with clear to oxyphilic cytoplasm, trabeculae, cords and pseudotubular structures
- Rarely, stroma in between follicles may be fibrotic or edematous; peripheral stromal leutinization may also be seen
- Adenomatous hyperplasia or proliferative changes may be seen, such as areas of densely packed follicles or papillary formations lacking nuclear features of papillary thyroid carcinoma
- Birefringent calcium oxalate crystals may be seen in colloid
- Most commonly associated malignancy is papillary thyroid carcinoma, cytologically characterized by crowded and overlapping elongated nuclei with irregular contours and chromatin clearing, usually with papillary or follicular architecture
- Follicular carcinoma is the second most common malignancy; since ovarian lesions typically lack capsule, demonstration of tumor invasion into surrounding ovarian tissue, vascular invasion or metastases is needed as evidence of malignancy
- Uncommonly associated with the more recently described highly differentiated follicular carcinoma of ovarian origin, characterized by extraovarian spread of thyroid elements histologically resembling nonneoplastic thyroid tissue (Int J Gynecol Pathol 2008;27:213)
- Undifferentiated (anaplastic) carcinoma and medullary carcinoma have also been described in association with struma ovarii
Microscopic (histologic) images
Contributed by Sharon Song, M.S., M.D., Kseniya Korchagina, M.D. and AFIP
Cytology description
- Flattened to cuboidal / columnar cells with small round to oval nuclei, even chromatin and pale to eosinophilic cytoplasm (Crum: Gynecologic and Obstetric Pathology, 3rd Edition, 2017)
- Clear cell change may occasionally be seen
Positive stains
Molecular / cytogenetics description
- BRAF mutations and RET / PTC rearrangements may be seen in papillary thyroid carcinomas arising from a struma ovarii (Am J Surg Pathol 2007;31:1337, Endocr Pathol 2007;18:182)
Sample pathology report
- Ovary and fallopian tube, left, salpingo-oophorectomy:
- Struma ovarii, 4.5 cm
- Ovary and fallopian tube, left, salpingo-oophorectomy:
- Papillary thyroid carcinoma, follicular and papillary growth patterns, 1.1 cm, arising in a background of struma ovarii
Differential diagnosis
- Metastatic thyroid carcinoma to the ovary:
- Clinical history of primary thyroid carcinoma
- Strumal carcinoid:
- Corded, trabecular or follicular architecture with salt and pepper nuclear chromatin pattern and occasional eosinophilic granules
- Positive staining for TTF1, PAX8, thyroglobulin (in follicular component) and neuroendocrine markers chromogranin, synaptophysin, CD56 and neuron specific enolase
- Ovarian clear cell carcinoma:
- Tubulocystic, papillary and solid growth patterns with cytologically atypical cells, often hobnailing into cysts
- Positive staining for HNF1β, Napsin A and AMACR
- Negative for TTF1, thyroglobulin
- Sex cord stromal tumor:
- Tubular, trabecular or corded growth without eosinophilic secretions
- Positive staining for inhibin A, calretinin and SF1
- Negative for TTF1, PAX8 (usually negative, Appl Immunohistochem Mol Morphol 2011;19:293), thyroglobulin
- Melanoma:
Board review style question #1
What type of crystals may be seen in struma ovarii?
- Calcium carbonate
- Calcium oxalate
- Calcium phosphate
- Triple phosphate
- Tyrosine
Board review style answer #1
Board review style question #2
Board review style answer #2
B. CA125 is a widely accepted biomarker for ovarian cancers that can also be elevated in many other conditions, such as menstruation, endometriosis, pregnancy, pelvic inflammatory disease, fibroids, liver disease and tumors of the endometrium, breasts and lungs. It can be increased in up to 30% of struma ovarii.
Comment Here
Reference: Struma ovarii
Comment Here
Reference: Struma ovarii
Strumal carcinoid
Table of Contents
Definition / general | Case reports | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Electron microscopy descriptionDefinition / general
- Has features of carcinoid tumor and struma ovarii
- Associated with MEN IIA / III
Case reports
- Associated with MEN IIA (Arch Pathol Lab Med 1992;116:200)
Microscopic (histologic) description
- Often other teratomatous elements
Microscopic (histologic) images
Positive stains
- Neuron specific enolase, chromogranin, synaptophysin, thyroglobulin and PAP (similar to rectal carcinoids)
Negative stains
Electron microscopy description
- Numerous electron-dense neurosecretory granules (Am J Clin Pathol 1978;69:356)
Teratoma-immature
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- A malignant germ cell tumor consisting of a teratoma containing variable amounts of immature tissue (typically primitive neuroectodermal)
Essential features
- Malignant germ cell tumor of the ovary composed of cells from the 3 germ layers and containing variable amounts of mature and immature tissue
- Affects mostly young females Grossly solid ovarian tumor mass with necrosis and hemorrhage on cut sections
- Grading is based on the amount of immature neuroepithelium
- Can have associated nodal or peritoneal gliomatosis (presence of mature neural tissue), which increases risk of recurrence
- Grading and stage are the most important factors for prognosis and prognosis is greatly improved after chemotherapy
Terminology
- Immature teratoma
ICD coding
- ICD-O: 9080/3 - teratoma, malignant, NOS
Epidemiology
- Immature teratomas constitute about a third of malignant germ cell tumors (Cancer 1976;37:2359, Obstet Gynecol 2006;107:1075)
- Mean age at diagnosis is 20.6; only 4 of 244 patients with immature teratoma were older than 40 (Int J Gynecol Pathol 1994;13:283)
Sites
- Ovary
Pathophysiology
- Abnormal differentiation of fetal germ cells
- 2 pathogenetic models for ovarian teratomas, similar to their testicular counterparts
- Pure mature and immature teratomas are not derived from transformed ovarian germ cells
- Teratomatous components of mixed germ cell tumors derived from their associated nonteratomatous components
- Pure mature and immature teratomas do not have i(12p) or 12p amplification, abnormalities that are identified in both teratomatous and nonteratomatous components of mixed germ cell tumors; this supports a different pathogenesis for pure versus mixed tumors (Mod Pathol 2006;19:766)
- 25% of cases with immature teratoma have peritoneal implants composed of mature glial tissue (gliomatosis peritonei); this phenomenon is thought to arise from metaplasia of submesothelial cells in response to growth factors secreted by the ovarian tumor, rather than a true metastatic process (Hum Pathol 1976;7:625, Hum Pathol 2004;35:685, Int J Gynecol Cancer 2015;25:244, Mod Pathol 2015;28:1613)
- Rarely, mature glial tissue is also present in lymph nodes (nodal gliomatosis), usually with associated gliomatosis peritonei
- Prognosis is good and the presence of nodal gliomatosis does not warrant chemotherapy (J Ovarian Res 2016;9:45, Mod Pathol 2015;28:1613)
Etiology
- No known causative agents
Clinical features
- Associated with symptoms of rapidly growing abdominal mass, pain, distention and torsion
- Growing teratoma syndrome: immature teratoma status postneoadjuvant chemotherapy consists of persistent or enlarging ovarian tumor with normalization of serum markers
- Thought to represent conversion of deposits of immature teratoma into mature teratoma as a result of chemotherapy (chemotherapeutic retroconversion) (Int J Gynecol Pathol 2015;34:465, Medicine (Baltimore) 2016;95:e2647)
- Histologic examination shows only mature teratoma
Diagnosis
- A combination of clinical, radiological and laboratory findings: rapidly enlarging adnexal mass in a young patient with mildly elevated alpha fetoprotein (AFP) and typical radiological findings raises the possibility of immature teratoma
- Requires histological examination for confirmation of diagnosis and grading
- Reference: Int J Gynecol Pathol 1994;13:283
Laboratory
- Low level increase of AFP
- High levels of AFP suggest the presence of a yolk sac tumor component (mixed germ cell tumor)
Radiology description
- On CT imaging, the solid component appears large and irregular, with coarse calcifications and small foci of fat scattered throughout the lesion rather than localized, as seen in dermoid cyst (J Med Imaging Radiat Oncol 2009;53:480, Eur J Radiol 2009;72:454)
Prognostic factors
- Older age at diagnosis, higher stage and grade confer worse prognosis but overall improved after chemotherapy, with 72% survival for stage IV disease (Gynecol Oncol 2016;142:261, Gynecol Oncol 2016;142:446)
- Grade is the most important risk factor for relapse across all age groups
- In a study of 98 pediatric patients and 81 adult patients, there were no relapses in grade 1 patients, irrespective of stage or age; only 1 adult patient with a grade 2 tumor relapsed (Cancer 2016;122:230)
- In patients with grade 3 tumors, recurrence rate was 21% in the pediatric cohort and 20% in the adult cohort and was significantly associated with stage (Cancer 2016;122:230)
- Higher risk of recurrence with presence of gliomatosis peritonei or lymph node gliomatosis but similar overall survival (Virchows Arch 2012;461:299, J Ovarian Res 2016;9:45)
Case reports
- 5 year old girl with ruptured immature teratoma at initial presentation (IJAR 2017;5:1811)
- 12 year old girl with grade 1 immature teratoma in the left ovary that presented as growing teratoma syndrome of the ovary (Medicine (Baltimore) 2020;99:e22297)
- 12 year old monozygotic twin girls with simultaneous presentation of ovarian mature and immature teratomas (Case Rep Obstet Gynecol 2017;2017:5810515)
- 13 year old girl with growing teratoma syndrome after treatment for immature teratoma (J Obstet Gynaecol India 2017;67:295)
- 31 year old woman with immature teratoma in pregnancy (Open Access Maced J Med Sci 2019;7:1016)
- 46 year old woman presented with simultaneous immature teratoma in the endometrium and mature teratomas in the ovary associated with gliomatosis peritonei (Int J Gynecol Pathol 2017;36:222)
Treatment
- Surgery plus chemotherapy for grade 2 or 3 lesions in adult women (Cancer 2016;122:230, Gynecol Oncol 2011;123:50)
- Patients with stage I, grade 1 tumors can be followed by surveillance (Gynecol Oncol 2011;123:50)
- Primarily surgical treatment for pediatric population (J Clin Oncol 2008;26:3590)
Gross description
- Usually unilateral ovarian masses with a mean size of 16 cm (range: 5 - 42 cm) (Int J Gynecol Pathol 1994;13:283)
- Solid with cystic areas or mostly solid; hemorrhage and necrosis on cut sections
- Contralateral ovary with mature teratoma in 9% of cases
Frozen section description
- Extensive sampling may be needed to include areas of immature teratoma at the time of frozen section in cases where a preoperative diagnosis of immature teratoma is suspected; sample nodular, soft appearing and mass forming areas
Microscopic (histologic) description
- Variable amounts of mature elements from all 3 germ layers, admixed with immature elements, mostly neuroectodermal
- Immature neuroepithelium
- Immature neuroepithelium can be spindled (sarcomatoid) or with rosette, pseudorosette and primitive tubule formation; the latter is easier to recognize
- Cells appear primitive with scant cytoplasm, hyperchromatic nuclei and frequent mitoses, which helps differentiate the immature elements from mature brain tissue
- Only immature neuroepithelium is used for grading, so it is important to recognize as such on histological slides
- Grading is based on the number of low power microscopic fields containing aggregated amounts of immature neuroepithelium in any one slide
- Original grading system (Cancer 1976;37:2359)
- Grade 1: ≤ 1 low power field (using a 4x power objective, which when using a 10x eyepiece, leads to a 40x magnification) in any slide
- Grade 2: > 1 but ≤ 3 low power fields in any slide
- Grade 3: > 3 low power fields in any slide
- Grade 2 and 3 tumors are considered high grade, therefore the updated grading system is 2 tiered, with improved reproducibility (Int J Gynecol Pathol 1994;13:283)
- Low grade: ≤ 1 low power field (using a 4x power objective, which when using a 10x eyepiece, leads to a 40x magnification) in any slide
- High grade: > 1 low power field in any slide
- Neural tissue can have a prominent associated benign vascular proliferation with Wagner-Meissner-like corpuscles, which should not be interpreted as an immature component (Int J Gynecol Pathol 2002;21:16, Int J Surg Pathol 2008;16:320)
- Fetal cartilage can be present and is not considered an immature element
- Small foci of yolk sac tumor or embryonal carcinoma (Int J Gynecol Pathol 1994;13:283, Cancer 1976;37:2359)
- Peritoneal and nodal gliomatosis nodules consist of mature glial tissue, considered grade 0 for grading purposes; thorough sampling is required to identify immature tissue (metastatic immature teratoma component), which has a worse prognosis
Microscopic (histologic) images
Cytology description
- Immature elements are rarely observed in ascites (Diagn Cytopathol 2005;33:39)
Positive stains
- Immunohistochemistry has limited role in diagnosis of immature teratoma
- Immature neuroepithelium
- S100, GFAP, NSE (does not differentiate between mature and immature components)
- OCT4 and PAX6 (OCT4 preferentially expressed in immature neural tissue of advanced stage immature teratomas) (Am J Surg Pathol 2010;34:1842)
- SALL4, SOX2, glypican 3 (Hum Pathol 2010;41:716, Histopathology 2011;58:312)
- Gliomatosis peritonei: SOX2+ (Mod Pathol 2015;28:1613)
Negative stains
- Gliomatosis peritonei: OCT4- / NANOG- (Mod Pathol 2015;28:1613)
- Neuroepithelium is negative for CD30, alpha fetoprotein (AFP), keratin, PAX8
Molecular / cytogenetics description
- i(12p) and 12p amplification is present in immature teratomatous components of mixed germ cell tumors but not in pure immature teratomas (Mod Pathol 2006;19:766)
Sample pathology report
- Left ovary, oophorectomy:
- Immature teratoma (see synoptic report)
- Tumor grade: 1 (low grade)
- Tumor size: 16 cm
- Ovarian surface involvement: present
- Immature teratoma (see synoptic report)
Differential diagnosis
- Mature cystic teratoma with mature neural elements:
- Predominantly cystic appearance grossly
- Neural elements are mature
- Microscopic foci of immature elements do not warrant a diagnosis of immature teratoma (Int J Gynecol Pathol 1987;6:203)
- Mature solid teratoma:
- Grossly similar
- Composed exclusively of mature elements
- Very rare, requires very rigorous sampling to rule out an immature component (Gynecol Oncol 1989;33:212)
- Carcinosarcoma:
- Usually postmenopausal women
- Mixed malignant epithelial and mesenchymal components, usually high grade
- Mesenchymal component is frequently chondrosarcoma (no fetal cartilage)
- Rare cases of ovarian carcinosarcoma with neuroectodermal differentiation have been reported (teratoid carcinosarcoma) (J Obstet Gynaecol Res 2010;36:907, Case Rep Oncol 2019;12:241)
- Mixed germ cell tumor:
- Extensive sampling is required to rule out another germ cell component (usually yolk sac tumor or embryonal carcinoma)
Additional references
Board review style question #1
Which of the following is true about gliomatosis peritonei found in ovarian immature teratoma?
- Considered a metaplastic rather than a true metastatic process
- Consists of immature glial tissue
- Has no impact on prognosis of immature teratoma
- Is used for grading of the immature teratoma
Board review style answer #1
A. Considered a metaplastic rather than a true metastatic process. The current understanding of gliomatosis is as a metaplastic rather than a true metastatic process. Answer B is incorrect because gliomatosis consists of mature neural tissue. Answer C is incorrect because presence of gliomatosis has been linked with increased risk of recurrence. Answer D is incorrect because only immature neuroepithelium component is used for grading.
Comment Here
Reference: Teratoma - immature
Comment Here
Reference: Teratoma - immature
Board review style question #2
Which of the following is true about grading of immature teratoma of the ovary?
- Both grade 1 and grade 2 tumors are considered low grade in the 2 tier classification system
- Grade does not impact prognosis
- Presence of immature elements in more than 1 low power field is in keeping with high grade
- Takes into account all histologic types of immature tissue
Board review style answer #2
C. Presence of immature elements in more than 1 low power field is in keeping with high grade in the 2 tier classification system. Answer B is incorrect because higher grade correlates with worse prognosis. Answer A is incorrect because grade 2 tumors are considered high grade in the 2 tier classification system. Answer D is incorrect because only immature neuroepithelium component is used for grading.
Comment Here
Reference: Teratoma - immature
Comment Here
Reference: Teratoma - immature
Teratoma-mature
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Benign tumor of the ovary composed of mature tissue representing at least 2 embryonic layers (ectoderm, mesoderm or endoderm)
Essential features
- Mature tissue representing at least 2 embryonic layers
- If malignant transformation, prognosis is related to the type of malignancy
Terminology
- Mature cystic teratoma
- Not recommended: dermoid cyst, mature solid teratoma
Epidemiology
- Most common ovarian tumor (20% of all ovarian tumors, 95% of all ovarian germ cell tumors)
- Reproductive age
- 10% bilateral (Cancer 1971;27:343)
Sites
- Ovary
Pathophysiology
- Most widely accepted origin is from the primordial germ cell (Mod Pathol 2017;30:1467)
Etiology
- No known causative agents
Diagrams / tables
Clinical features
- Slow growing, often incidental finding
- Rarely present with or are associated with:
- Torsion or rupture
- Mucinous tumors of the ovary (e.g., mucinous cystadenoma) (Clin Diagn Res 2016;10:ED11)
- Autoimmune hemolytic anemias (Saudi Med J 2019;40:397)
- Paraneoplastic encephalitis (Ann Neurol 2007;61:25)
Diagnosis
- Mainly by ultrasound
- Histologic examination
Radiology description
- Wide variety of presentations, depending on the tissues present (see Diagrams / tables)
Radiology images
Prognostic factors
- Benign tumor
- Malignant transformation occurs rarely
- Usually in older patients
- Most common is squamous cell carcinoma
- Prognosis dependent on stage
- Microscopic foci of immature neuroepithelium does not warrant diagnosis of immature teratoma and will not affect prognosis (Int J Gynecol Pathol 1987;6:203)
- Gliomatosis peritonei (mature glial tissue implanted on peritoneal surface) does not adversely affect prognosis (J Ovarian Res 2016;9:45)
- Anti–n-methyl-D-aspartate receptor (NMDAR) encephalitis will have best outcome with early treatment (immunotherapy and tumor removal) (Lancet Neurol 2013;12:157)
Case reports
- 15 year old girl with meningioma arising in a mature cystic teratoma (Cancer Imaging 2020;20:15)
- 20 year old woman with mature teratoma in ectopic ovary (J Minim Invasive Gynecol 2019;26:348)
- 22 year old woman with secondary retroperitoneal mature teratoma 4 years after resection of mature teratoma of the ovary (Radiol Case Rep 2019;14:692)
- 59 year old woman with squamous cell carcinoma in a mature teratoma of ovary (BMC Cancer 2019;19:217)
- 71 year old woman with ovarian clear cell carcinoma arising in a mature cystic teratoma (J Ovarian Res 2018;11:74)
Treatment
- Cystectomy in young women to preserve fertility
- Salpingo-oophorectomy in older women
Gross description
- Smooth cyst that may contain hair, teeth, cartilage, bone or sebaceous material
- Generally Raised protuberance in cyst wall (Rokitansky nodule)
- Reference: StatPearls: Cystic Teratoma [Accessed 29 July 2021]
Gross images
Frozen section description
- Rarely performed but will show mixture of mature tissues
- Solid areas should be sampled to exclude immature teratoma
Microscopic (histologic) description
- Mixture of mature, benign tissues
- Ectodermal (most common): squamous epithelium, sebaceous glands, hair follicles, brain tissue
- Mesodermal (second most common): bone, cartilage, smooth muscle, fibroadipose tissue
- Endodermal: intestinal or respiratory epithelium, thyroid, salivary gland
- Microscopic foci of immature neuroepithelium (less than or equal to 4 foci or 21 mm2) does not warrant diagnosis of immature teratoma and will not affect prognosis (Int J Gynecol Pathol 1987;6:203)
- Fat necrosis and foreign body reaction may be seen
- Cases associated with NMDAR encephalitis usually show neuroglial tissue associated with lymphoid aggregates with germinal centers, low number of mature neurons and a hypercellular astrocyte population (Am J Surg Pathol 2019;43:949)
- Reference: StatPearls: Cystic Teratoma [Accessed 29 July 2021]
Microscopic (histologic) images
Virtual slides
Molecular / cytogenetics description
- Diploid with normal (46XX) karyotype
Sample pathology report
- Ovary, right, cystectomy:
- Mature teratoma
Differential diagnosis
- Immature teratoma:
- Contains immature tissues (greater than 4 foci or greater than 21 mm2) (Int J Gynecol Pathol 1987;6:203)
- Younger patients
- Larger and fast growing
- Monodermal teratoma:
- Contain a single germ layer
- Neuroectodermal cyst - lined by ependymal cells
- Epidermoid cyst - lined by squamous epithelium (no sebaceous glands present)
- Struma ovarii - thyroid tissue is the dominant or sole component
- Strumal carcinoid - well differentiated neuroendocrine tumor admixed / juxtaposed with thyroid tissue
- Contain a single germ layer
Board review style question #1
A 25 year old pregnant woman is seen in the obstetrics clinic for a routine ultrasound. During the exam, a cystic mass is noted near her left ovary. The mass is eventually surgically removed and a pathologic examination shows the features in the picture shown above. What is an essential feature of this tumor?
- Gliomatosis peritonei
- Immature tissue
- Mature tissue representing at least 2 embryonic layers
- Size greater than 10 cm
Board review style answer #1
C. Mature tissue representing at least 2 embryonic layers. Mature teratoma can contain microscopic foci of immature neuroectodermal tissue in the cyst wall. This finding does not change the prognosis and should not lead to the tumor being classified as immature teratoma. Gliomatosis peritonei is a rare finding associated with teratoma and size of tumor is variable with most less than 10 cm. Mature teratomas must contain at least 2 embryonic layers.
Comment Here
Reference: Teratoma - mature
Comment Here
Reference: Teratoma - mature
Board review style question #2
Board review style answer #2
D. Mature teratoma. The photo shows a mass with yellow sebaceous material and hair. In a 19 year old woman, this most likely represents a mature teratoma. Mature teratomas are the most common ovarian tumor and are especially common in women of reproductive age.
Comment Here
Reference: Teratoma - mature
Comment Here
Reference: Teratoma - mature
Thecoma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Ovarian stromal neoplasm, almost always benign, composed of cells resembling theca cells
Essential features
- Almost always benign
- Usually occurs in postmenopausal women who present with uterine bleeding
- Histology shows a predominant population of cells with ovoid to round nuclei and pale gray cytoplasm
- Reticulin stain and molecular testing for FOXL2 mutation help distinguish thecoma from adult granulosa cell tumor
Terminology
- Theca cell tumor
ICD coding
- ICD-O: 8600/0 - thecoma, NOS
- ICD-10: D27 - benign neoplasm of ovary
- ICD-11: 2F32.Y & XH34A0 - other specified benign neoplasm of ovary & thecoma, NOS
Epidemiology
- Uncommon
- Usually postmenopausal women
- Mean age = 59 years (Ann Diagn Pathol 2008;12:12)
- Age ranges from 16 to 81 (Am J Surg Pathol 2014;38:1023)
Sites
- Ovary
Pathophysiology
- Molecular alterations, such as trisomy 12 and loss of heterozygosity (LOH) at the PTCH gene (see Molecular / cytogenetics description)
Etiology
- Unknown
Clinical features
- May be discovered incidentally
- Most common symptom is postmenopausal bleeding (Am J Surg Pathol 2014;38:1023)
- Symptoms can be related to mass: pelvic pain / pressure
- May present with estrogenic or androgenic manifestations
- Associated with endometrial hyperplasia and malignancy
Diagnosis
- Histologic examination
Laboratory
- Occasionally elevated serum inhibin A and inhibin B (Gynecol Oncol Rep 2020;34:100658, Gynecol Endocrinol 2016;32:872)
Radiology description
- Sonographic features:
- Adnexal hypoechoic mass with clear border and acoustic attenuation
- Minimal Doppler flow signal (J Ovarian Res 2016;9:81)
- Magnetic resonance imaging features:
- Isointense or slightly hyperintense
- Pelvic fluid accumulation (Medicine (Baltimore) 2020;99:e20358)
- Computed tomography features:
- Isodense or hypodense (J Comput Assist Tomogr 2012;36:46)
- Occasionally thecomas have positive F-FDG uptake on PET scan (J Obstet Gynaecol Res 2017;43:599)
Prognostic factors
- Thecomas are almost always benign but may be associated with endometrial malignancy
Case reports
- 57 year old woman with postmenopausal bleeding and elevated serum inhibin B level (Gynecol Oncol Rep 2020;34:100658)
- 58 year old woman with progressive scalp hair loss (Skin Appendage Disord 2019;5:259)
- 61 year old woman with Meigs syndrome and elevated CA-125 level (J Menopausal Med 2015;21:56)
Treatment
- Oophorectomy if fertility sparing is desired
- Total hysterectomy with bilateral salpingo-oophorectomy is indicated in postmenopausal patients
- References: Gynecol Oncol 1994;55:S62, Acta Radiol Oncol 1980;19:241, Gynecol Oncol 1985;21:135
Gross description
- Usually unilateral
- Most are < 5 cm
- Solid, yellow and lobulated or white with focal yellow areas
- Occasionally cystic change and hemorrhage are present
- Necrosis is rare (Am J Surg Pathol 2014;38:1023)
Gross images
Frozen section description
- Sheets of oval to round cells with moderate to abundant pale gray cytoplasm
- Bilaterality should raise suspicion for metastasis
- Signet ring cells may be missed on frozen section and misinterpreted as fibrothecoma (Yeungnam Univ J Med 2019;36:163)
Frozen section images
Microscopic (histologic) description
- Predominant population of cells showing ovoid to round nuclei and pale gray cytoplasm, which can be abundant
- Minor component of the tumor may have spindled nuclei, reflecting overlap between fibroma and thecoma
- Indistinct cell membranes impart a syncytial appearance
- Diffuse or nodular growth pattern
- Absent or minimal nuclear atypia
- Mitotic rate usually < 5/10 high power fields
- Hyaline plaques
- Cytoplasmic lipid vacuoles may be present but are not essential
- May show aggregates of cells with brightly eosinophilic cytoplasm (lutein cells)
- Calcification is more common in young patients (Int J Gynecol Pathol 1988;7:343)
- Uncommon features include keloid-like sclerosis, nuclear grooves, bizarre nuclear atypia (Am J Surg Pathol 2014;38:1023)
- Rarely contains a minor component of sex cord elements (Int J Gynecol Pathol 1983;2:227)
- Malignant thecoma: very rare, diagnosis requires diffuse moderate to severe nuclear atypia and high mitotic rate (> 4/10 high power fields) (Am J Surg Pathol 2011;35:e15)
Microscopic (histologic) images
Contributed by Victoria Collins, M.D., Tamara Kalir, M.D., Ph.D., AFIP and @SeoparjooAzmel on Twitter
Positive stains
- Inhibin
- Calretinin
- Reticulin stain shows a pericellular pattern (Int J Gynecol Pathol 2018;37:305)
- SF1
- FOXL2 (Mod Pathol 2013;26:860)
- WT1 (Am J Surg Pathol 2009;33:354)
- CD56 (Am J Surg Pathol 2008;32:884)
- Vimentin
- Oil red O in lipid rich cells
- GLUT5 in FDG PET positive tumors (J Obstet Gynaecol Res 2017;43:599)
Negative stains
Electron microscopy description
- Cytoplasmic lipid (Ultrastruct Pathol 1992;16:363)
- Type I cells: dispersed chromatin, basal lamina investment of each cell, coiled / branching rough endoplasmic reticulum, sparse smooth endoplasmic reticulum, irregular mitochondria
- Type II cells: degenerative changes, large round mitochondria with incomplete cristae and centers displaced by microfilaments (Hum Pathol 1984;15:153)
Molecular / cytogenetics description
- Trisomy 12 or tetrasomy 12 (Gynecol Oncol 1990;36:413, Cancer Genet Cytogenet 1993;71:180)
- Trisomy 4 (Gynecol Oncol 1992;45:66)
- Loss of heterozygosity (LOH) at 9q22.3 (PTCH1) and 19p13.3 (Hum Pathol 2005;36:792)
- FOXL2 mutation has been reported in a subset of thecomas (3 out of 14 tumors in one series) (N Engl J Med 2009;360:2719)
- However these may represent misdiagnosed granulosa cell tumors (PLoS One 2009;4:e7988)
- Pericellular reticulin staining is 100% specific for absence of FOXL2 mutation (Int J Gynecol Pathol 2018;37:305)
Videos
Thecoma of ovary
Ovarian pathology
Sample pathology report
- Right ovary and fallopian tube, salpingo-oophorectomy:
- Ovarian thecoma
- Fallopian tube with no significant pathologic changes
Differential diagnosis
- Adult granulosa cell tumor:
- Reticulin stain shows nested pattern (Int J Gynecol Pathol 2019;38:143)
- Harbors FOXL2 mutation (Mod Pathol 2013;26:860, Int J Gynecol Pathol 2018;37:305)
- Fibroma:
- Intersecting bundles of spindle cells producing collagen
- A morphologic spectrum between fibroma and thecoma exists
- Indeterminate tumors have been called fibrothecoma
- Luteinized thecoma associated with sclerosing peritonitis:
- Younger age at presentation (median age: 28)
- Symptoms include abdominal pain, ascites and bowel obstruction
- Cutaneous involvement has been reported (Ann N Y Acad Sci 1985;448:231)
- Bland spindle cell proliferation circumferentially involving the ovarian cortex with sparing of the medulla
- Almost always bilateral (Am J Surg Pathol 2008;32:1273)
- Sclerosing stromal tumor:
- Pseudolobular growth pattern with cellular nodules separated by dense collagenous or edematous connective tissue (Cancer 1973;31:664)
- Staghorn vessels
- Microcystic stromal tumor:
- Small, anastomosing cysts
- Inhibin-, calretinin-
- Nuclear beta catenin+
- Cyclin D1+
- CD10+ (Am J Surg Pathol 2015;39:1420)
- Steroid cell tumor, NOS:
- FOXL2- (Am J Surg Pathol 2011;35:484)
- Polygonal cells with abundant eosinophilic cytoplasm
- Typically has a component of lipid rich cells (pale and vacuolated) (Arch Pathol Lab Med 2018;142:1459)
- Leydig cell tumor:
- Tumor centered in ovarian hilus
- Cytoplasmic Reinke crystals
- Nuclei tend to cluster, forming eosinophilic nuclei free zones (Int J Gynecol Pathol 1989;8:299)
- Fibrinoid necrosis of blood vessel walls
Additional references
Board review style question #1
Board review style answer #1
Board review style question #2
Board review style answer #2
Torsion
Table of Contents
Definition / general | Epidemiology | Etiology | Clinical features | Radiology description | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) descriptionDefinition / general
Ovary:
Fallopian tube:
- Rare; partial or complete rotation of ovarian vascular pedicle, causing obstruction to venous outflow and arterial inflow
- In children, ovary is often normal (Arch Pediatr Adolesc Med 2005;159:532)
Fallopian tube:
- "Twisting" of fallopian tube that blocks blood flow, causing ischemia and associated edema and pain
- Often accompanies torsion of adjacent ovary (often with a cyst) but may be isolated to tube
Epidemiology
Fallopian tube:
- Occurs in women of all ages
- 2/3 involve right tube
Etiology
Fallopian tube:
- Usually associated with a mass / cyst causing rotation / obstruction
- Also PID, hydrosalpinx, tubal ligation or adhesions
- Occasionally no identifiable cause
Clinical features
-
Ovary:
- Presents with abdominal pain, patients need emergency ultrasound and laparoscopy (J Pediatr Adolesc Gynecol 2008;21:201)
- Predisposing factors: large cyst, neoplasms (benign or malignant), pelvic inflammatory disease; also associated with in vitro fertilization
- sharp lower abdominal pain, fever, tachycardia, leukocytosis (Acta Obstet Gynecol Scand 2010;89:1354)
Fallopian tube:
Radiology description
- Fallopian tube: ultrasound usually shows adnexal mass with heterogeneous echogenicity and cystic component with fluid levels
Case reports
- 25 year old woman with isolated fallopian tube torsion (AJR Am J Roentgenol 2005;185:1590)
- 32 year old woman at 32 weeks gestation with nausea, pain and vomiting (J Med Case Reports 2008;2:378)
Treatment
Ovary:
Fallopian tube:
- Cases during pregnancy (J Obstet Gynaecol Res 2008;34:683)
- Recommended to attempt to preserve ovary, since most are viable (Clin Obstet Gynecol 2006;49:459)
Fallopian tube:
- May resolve or tube may become necrotic and calcified with autoamputation of tube and ovary
- Often requires surgical excision
Gross description
- Fallopian tube: swollen, dusky fallopian tube, with or without associated mass / cyst
Microscopic (histologic) description
- Fallopian tube: ischemic necrosis of mucosa and wall
Tubo-ovarian abscess
Abscess
Definition / general
Epidemiology
Etiology
Clinical features
Laboratory
Case reports
Treatment
Gross description
Gross images
AFIP images
Microscopic (histologic) description
Microscopic (histologic) images
Contributed by Eman Abdelzaher, M.D., Ph.D. and AFIP
- Nonspecific abscess localized to the ovary
- Primary ovarian abscess is defined as inflammation arising in the ovarian tissue
- Secondary ovarian abscess originates in extraovarian sites
Epidemiology
- Primary ovarian abscesses are quite rare; most cases of ovarian abscess are secondary
Etiology
- Primary abscesses may arise due to disruption of ovarian capsule, as in ovulation or surgical intervention (vaginal hysterectomy, ovarian cystectomy, caesarean section, pregnancy, use of intrauterine device, transvaginal or percutaneous needle aspiration of endometrioma or follicle aspiration as part of in vitro fertilization), which gives bacteria access to the ovarian stroma (Tunis Med 2010;88:285)
- Also occurs due to hematogenous and lymphatic spread
- Secondary ovarian abscess may be associated with tubo-ovarian abscess, salpingitis, ascending infection of lower genital tract (pelvic inflammatory disease) or complication of GI infections (diverticulitis, appendicitis)
Clinical features
- Nonspecific symptoms, making diagnosis difficult
- Time between capsule disruption and clinical presentation depends on bacterial inoculum dose, type and virulence and if infection occurred secondary to direct contamination at surgery or via devitalized tissue
Laboratory
- Most common infectious organisms are Streptococcus type B; other bacteria
Case reports
- 2 cases of Brucella ovarian abscess (J Clin Ultrasound 2007;35:395)
- Enterobius vermicularis infection with tubo-ovarian abscess and peritonitis occurring during pregnancy (Surg Infect (Larchmt) 2009;10:545)
- Tubo-ovarian abscess with Morganella morganii bacteremia (J Microbiol Immunol Infect 2009;42:357)
- Abdominal abscesses with Streptococcus milleri group after laparoscopic chromopertubation (Acta Obstet Gynecol Scand 2010;89:982)
Treatment
- Initial treatment is intravenous antibiotics
- If no response within 72 hours, if abscess ruptures or if surrounding organs are affected, immediate laparoscopy or laparotomy with removal of the ovary is the main treatment
Gross description
- Large, loculated cyst with pus or secretion
Gross images
AFIP images
Microscopic (histologic) description
- Cyst wall often contains ovarian stroma
Microscopic (histologic) images
Contributed by Eman Abdelzaher, M.D., Ph.D. and AFIP
Pelvic inflammatory disease (PID)
Definition / general
Epidemiology
Sites
Etiology
Clinical features
Laboratory
Prognostic factors
Treatment
Gross images
Images hosted on other servers:
Additional references
- Polymicrobial infection in women characterized by inflammation of the upper genital tract, including endometritis, salpingitis, pelvic peritonitis and occasionally leading to tubo-ovarian abscess
Epidemiology
- Primarily affects young, sexually active and reproductive aged women
- Inversely proportional to age with the highest rates in the 15 to 19 year old group
- Incidence steadily increased in 1970s due to rising rates of STDs and peaked in early 1980s
- Although current incidence is lower, estimates of prevalence are not exact and likely underrepresent the true prevalence of disease due to subclinical PID, increasing rates of outpatient diagnosis and inaccuracies in diagnosis
Sites
- Usually begins in endometrium and is associated with tubal involvement and tubo-ovarian abscess or cyst
Etiology
- Most commonly due to gonorrhea, chlamydia, enteric bacteria or puerperal infections (from end of third stage of labor until uterus completely involutes at 3 - 6 weeks)
- Acute, symptomatic forms, previously primarily due to Neisseria gonorrhoeae, have been replaced by mild to moderate cases due to Chlamydia trachomatis or mycoplasmas
- Usually polymicrobial
Clinical features
- No single, subjective complaint, physical examination finding or laboratory finding is highly sensitive or specific for the diagnosis
- Pelvic pain, adnexal tenderness, fever and vaginal discharge are common
- Classified as acute, subacute or subclinical
Laboratory
- Peripheral white blood cell count is nonspecific and elevated in only 44% of cases
- Elevations in C reactive protein or erythrocyte sedimentation rate show good sensitivity and specificity
- Vaginal wet smear with 3+ white blood cells per high power field has a high sensitivity for upper genital tract infection and the absence of WBCs has a high negative predictive value
- Transvaginal ultrasound may demonstrate a swollen, tortuous fallopian tube
- Laparoscopic evaluation of the pelvic organs is considered the gold standard for diagnosing PID
Prognostic factors
- Short and long term sequelae include tubal factor infertility, ectopic pregnancy, chronic pelvic pain, peritonitis, bacteremia or intestinal obstruction due to adhesions
Treatment
- Outpatient treatments, mostly by the oral route, have replaced inpatient, intravenous treatments for uncomplicated PID (Curr Opin Infect Dis 2010;23:83)
Gross images
Images hosted on other servers:
Additional references
Undifferentiated / dedifferentiated carcinoma (endometrium / ovary)
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- This topic covers tumors of the endometrium and ovary
- Undifferentiated carcinomas are characterized by a patternless, sheet-like growth of discohesive cells with an aggressive clinical course (Am J Surg Pathol 2005;29:1316, Mod Pathol 2010;23:781, J Pathol Clin Res 2021;7:144)
- Tumors are often associated with mismatch repair and SWI / SNF protein deficiency (Mod Pathol 2016;29:302, Histopathology 2016;69:560, Mod Pathol 2016;29:1586)
- If areas of a differentiated carcinoma are found, the tumor is called dedifferentiated carcinoma (Int J Gynecol Pathol 2006;25:52)
Essential features
- At least focal sheet-like growth pattern with lack of glandular or papillary differentiation
- High mitotic and proliferation indices
- Absence or significantly diminished expression of markers of differentiation (e.g. CK7, PAX8, ER and claudin4)
- Presence of a differentiated component (usually low grade endometrioid adenocarcinoma) is necessary for the diagnosis of dedifferentiated carcinoma
- Loss of a core SWI / SNF protein is helpful in confirming the diagnosis but not essential to make the diagnosis
ICD coding
- ICD-O:
- ICD-11:
- 2C73.Y & XH1YY4 - other specified malignant neoplasms of the ovary & carcinoma, undifferentiated, NOS
- 2C73.Y & XH5R16 - other specified malignant neoplasms of the ovary & dedifferentiated carcinoma
- 2C76.Y & XH1YY4 - other specified malignant neoplasms of the corpus uteri & carcinoma, undifferentiated, NOS
- 2C76.Y & XH5R16 - other specified malignant neoplasms of the corpus uteri & dedifferentiated carcinoma
Epidemiology
- Median age is 55 years; range 21 - 76 years (Mod Pathol 2010;23:781)
- 2% of endometrial carcinomas (Mod Pathol 2010;23:781)
- 0.5% of ovarian carcinomas (Int J Gynecol Pathol 2010;29:203)
Sites
- Endometrium
- Ovary
Pathophysiology
- Likely evolves from a differentiated endometrial or ovarian carcinoma secondary to disrupted epigenetic modulation
- Inactivation of the SWI / SNF chromatin remodeling complex is common
- Often associated with mismatch repair deficiency
Etiology
- No associated environmental risk factors
Clinical features
- Usually presents at advanced stage
- Abdominal pain and swelling
Diagnosis
- There are no established tests to screen for endometrial and ovarian malignancies where the vast majority of undifferentiated and dedifferentiated carcinomas occur
- When clinical suspicion arises, abdominal ultrasound and computed tomography scans are useful adjunct
- Definitive diagnosis requires biopsy tissue
Radiology description
- Large, solid adnexal mass with variable hemorrhage and necrosis
Prognostic factors
- Undifferentiated and dedifferentiated tumors have a poor prognosis (Int J Gynecol Pathol 2006;25:52, Mod Pathol 2010;23:781, J Pathol Clin Res 2021;7:144)
- Presence of undifferentiated component in a differentiated tumor carries similar prognosis as pure undifferentiated carcinoma
Case reports
- 54 year old woman with dedifferentiated carcinoma of the ovary (Cesk Patol Spring 2018;54:33)
- 55 year old woman with BRG1 deficient dedifferentiated endometrioid adenocarcinoma of the ovary (Pathology 2016;48:82)
- 63 year old postmenopausal woman with an abdominal mass (Int J Clin Exp Pathol 2014;7:4422)
- 73 year old woman with ovarian undifferentiated carcinoma with voluminous mesenteric presentation (Int J Surg Case Rep 2012;3:551)
Treatment
- Primary ovarian tumor treated primarily with chemotherapy followed by surgery
- Total abdominal hysterectomy and bilateral salpingo-oophorectomy (TAH BSO) with or without lymphadenectomy
- Emerging evidence supports that SWI / SNF deficient undifferentiated and dedifferentiated carcinoma does not respond to the conventional platinum based regimens (J Pathol Clin Res 2021;7:144)
Gross description
- Large, solid mass with extensive tumor necrosis
Microscopic (histologic) description
- Undifferentiated component:
- Diffuse, sheet-like growth pattern with lack of glandular or papillary architecture (occasionally glandular or papillary structures from the differentiated component may be entrapped)
- Comprised of a proliferation of monomorphic, medium sized cells
- Abrupt keratinization may be seen
- Cells frequently show discohesion (at least focally)
- Variable amount of cytoplasm, sometimes with rhabdoid features
- Brisk mitoses
- Necrosis frequently present
- Some cases may show focal spindling and myxoid changes
- Differentiated component:
- Typically low grade endometrioid adenocarcinoma
- Rarely high grade endometrioid adenocarcinoma, high grade serous carcinoma or clear cell carcinoma
Microscopic (histologic) images
Positive stains
- In the differentiated component (if present): CK7, PAX8, ER / PR, E-cadherin
- In the undifferentiated component:
- Elevated Ki67 index
- EMA, keratins, CK18 or PAX8, ER / PR may be focally expressed but can be completely absent (Mod Pathol 2010;23:781)
- Neuroendocrine markers (chromogranin, INSM1, synaptophysin and CD56) are frequently expressed; most cases show only focal expression in 1 or 2 neuroendocrine markers
Negative stains
- In the differentiated component (if present):
- p53 wild type pattern (patchy negative to intermediate nuclear staining)
- Uncommonly the differentiated component may be high grade, which can be associated with a mutant p53 IHC pattern
- p16 (patchy negative to weak nuclear and cytoplasmic staining)
- Uncommonly the differentiated component may be high grade, which can be associated with a diffuse p16 IHC pattern
- Mismatch repair deficiency (MLH1, PMS2, MSH2, MSH6) is common (~50%)
- p53 wild type pattern (patchy negative to intermediate nuclear staining)
- In the undifferentiated component:
- CK7, PAX8, ER, WT1, claudin4 (may have weak or focal expression)
- SWI / SNF complex loss of protein expression (BRG1, INI1 or co-loss of ARID1A and ARID1B)
- Usually p53 wild type (negative to patchy intermediate nuclear staining)
- p16 (negative to patchy weak nuclear and cytoplasmic staining)
- Mismatch repair deficiency (MLH1, PMS2, MSH2, MSH6) is common (~50%)
Molecular / cytogenetics description
- Commonly shows an inactivating mutation in one of the core SWI / SNF complex genes, resulting in the loss of expression of BRG1 (SMARCA4 gene), INI1 (SMARCB1 gene) or co-loss of ARID1A and ARID1B
- Most cases are associated with mismatch repair deficiency and of those with MLH1 deficiency, almost all show MLH1 promoter hypermethylation
- Mutations in other genes associated with endometrioid carcinoma, such as PTEN, PIK3CA, KRAS and CTNNB1, are often reported
Sample pathology report
- Uterus, fallopian tubes and ovaries, hysterectomy and bilateral salpingo-oophorectomy:
- Endometrial dedifferentiated carcinoma (see synoptic report)
- Associated with an endometrioid adenocarcinoma FIGO grade 1
- Mismatch repair status: abnormal (MLH1 lost)
- Uterus, fallopian tubes and ovaries, hysterectomy and bilateral salpingo-oophorectomy:
- Undifferentiated carcinoma of the ovary (see synoptic report)
- Mismatch repair status: abnormal (MLH1 lost)
Differential diagnosis
- Endometrial undifferentiated and dedifferentiated carcinoma:
- Endometrial serous carcinoma, solid variant:
- Tumor cells have a cohesive growth pattern
- Mutated p53 IHC pattern
- Intact SWI / SNF immunohistochemistry
- High grade endometrial endometrioid adenocarcinoma:
- Tumor cells have a cohesive growth pattern
- Intact SWI / SNF immunohistochemistry
- May show isolated ARID1A loss of expression
- Carcinosarcoma of the endometrium (malignant mixed Müllerian tumor):
- Homologous or heterologous sarcoma
- Frequently mutated p53 IHC pattern
- SMARCA4 deficient uterine sarcoma:
- Endometrial small cell neuroendocrine carcinoma:
- Neuroendocrine nuclear features (fine chromatin, nuclear molding)
- Diffuse expression of 2 or more neuroendocrine markers (chromogranin, synaptophysin, INSM1, CD56)
- Intact SWI / SNF immunohistochemistry
- Lymphoma:
- CD45 positive
- Intact SWI / SNF immunohistochemistry
- Endometrial serous carcinoma, solid variant:
- Ovarian undifferentiated and dedifferentiated carcinoma:
- High grade serous carcinoma of the ovary, solid variant:
- Tumor cells have a cohesive growth pattern
- Mutated p53 IHC pattern
- Intact SWI / SNF immunohistochemistry
- High grade endometrioid adenocarcinoma of the ovary:
- Tumor cells have a cohesive growth pattern
- Intact SWI / SNF immunohistochemistry
- May show isolated ARID1A loss of expression
- Carcinosarcoma of the ovary (malignant mixed Müllerian tumor):
- Homologous or heterologous sarcoma
- Frequently mutated p53 IHC pattern
- Small cell carcinoma of the ovary hypercalcemic type:
- Small cell neuroendocrine carcinoma (small cell carcinoma of the ovary, pulmonary type):
- Neuroendocrine nuclear features (fine chromatin, nuclear molding)
- Diffuse expression of 2 or more neuroendocrine markers (chromogranin, synaptophysin, INSM1, CD56)
- Intact SWI / SNF immunohistochemistry
- Lymphoma:
- CD45 positive
- Intact SWI / SNF immunohistochemistry
- High grade serous carcinoma of the ovary, solid variant:
Additional references
Board review style question #1
This is a representative image from a solitary ovarian tumor in a 75 year old woman. The tumor shows absent INI1 immunohistochemistry expression. What is the most likely diagnosis?
- Carcinosarcoma
- High grade serous carcinoma of the ovary
- Primary diffuse large B cell lymphoma of the ovary
- Small cell carcinoma of the ovary hypercalcemic type
- Undifferentiated carcinoma of the ovary
Board review style answer #1
E. Undifferentiated carcinoma of the ovary
Comment Here
Reference: Undifferentiated / dedifferentiated carcinoma (endometrium / ovary)
Comment Here
Reference: Undifferentiated / dedifferentiated carcinoma (endometrium / ovary)
Board review style question #2
Board review style answer #2
C. Endometrial dedifferentiated carcinoma
Comment Here
Reference: Undifferentiated / dedifferentiated carcinoma (endometrium / ovary)
Comment Here
Reference: Undifferentiated / dedifferentiated carcinoma (endometrium / ovary)
Upper gastrointestinal tract
Table of Contents
Definition / general | Terminology | Epidemiology | Pathophysiology | Clinical features | Laboratory | Radiology description | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Differential diagnosis | Additional referencesDefinition / general
- Secondary ovarian involvement by an adenocarcinoma of gastric, pancreatic or biliary tract origin is, by definition, evidence of advanced tumor stage (pM1), and carries a poor prognosis
- Significant overlap of clinical, radiologic and pathologic features between primary and metastatic ovarian adenocarcinoma
- In the work up of a mucinous ovarian neoplasm, a secondary malignancy should always be excluded pathologically or clinically
Terminology
- Although commonly used to refer to all metastatic carcinoma involving the ovary, the term Krukenberg tumor strictly refers to adenocarcinoma with signet-ring cell differentiation; most (76%) arise from the stomach (J Clin Pathol 2012;65:585)
Epidemiology
- The rate of secondary (metastatic) ovarian malignancies varies from 6 - 22% (Pathol Int 2005;55:231)
- The rate of metastatic tumors from non-gynecologic organs is 9 - 14% (World J Gastrointest Surg 2010;2:109)
- Gastric carcinoma
- Average age of diagnosis is 45 years
- An ovarian mass is usually the presenting sign, and 70 - 75% of patients have no primary identified at time of diagnosis
- Rates of ovarian involvement by gastric carcinoma depends on:
- Prevalence - more frequent in regions with high prevalence such as Japan and Colombia
- Type of cancer – diffuse (signet ring cell) type is more likely to metastasize
- Young patients (Adv Anat Pathol 2006;13:205)
- Pancreatic and biliary tree carcinoma
- A primary lesion is commonly unsuspected at oophorectomy and only discovered intra- or post-operatively (Am J Surg Pathol 1989;13:748, Int J Gynecol Pathol 2008;27:366)
Pathophysiology
- Poorly differentiated tumors (most with signet-ring cell morphology) are more likely to metastasize to the ovary
Clinical features
- Symptoms related to a pelvic mass include abdominal pain or discomfort, bowel obstruction, abnormal vaginal bleeding
Laboratory
- Elevated CA-125 levels (> 100 U/mL) are common; suggested as a useful feature to distinguish from primary ovarian mucinous carcinoma (Gynecol Obstet Invest 2011;72:196), which have elevated CEA levels (> 5 ng/mL) and CA19-9 levels (> 37 U/mL) in a minority of cases
Radiology description
- Bilaterality is a strong indicator of secondary origin, and occurs more frequently in gastric cancer (~ 80%) than in colon and pancreatobiliary tumors (Ultrasound Obstet Gynecol 2012;39:581)
- Krukenberg tumor: bilateral complex masses with hypodense solid components (due to prominent stromal reaction) and internal hyperdensity (mucin) on T1 and T2 weighted MRI images
- Other types: cystic and solid masses with multinodular and necrotic solid components
Treatment
- Gastric adenocarcinoma is amenable to HER2 targeted therapy (Lancet 2010;376:687)
Gross description
- Mass is frequently complex (solid and cystic) or purely solid
- Purely cystic unilocular lesions are rare
- Solid tumors have a multinodular appearance and extend to the ovarian / tumor surface
- Some authors have reported a predominant small size (Am J Surg Pathol 2003;27:985, Am J Surg Pathol 2008;32:128)
- There is, however, reported evidence of significant overlap of such features: in a recent series, all metastatic carcinoma of upper GI origin were unilateral (2 / 6) or > 10 cm (4 / 6) (Int J Gynecol Pathol 2016;35:191)
Microscopic (histologic) description
- Metastases frequently mimic the appearance of an ovarian mucinous neoplasm
- May have areas mimicking a borderline or even benign primary mucinous tumor
- Several clinical and pathologic features have been described as indicative of secondary (metastatic) origin, including:
- Bilaterality
- Size less than 10 cm
- Surface involvement
- Infiltrative pattern of invasion
- Presence of signet ring cells
- Extensive lymphovascular space invasion
- Mucin extravasation
- If any of the above is present, the possibility of a metastasis should be considered and prompt ancillary testing and clinical investigation should be conducted
- The term Krukenberg tumor should be reserved for adenocarcinoma involving the ovary with a signet ring cell component > 10% of tumor volume
- Krukenberg tumors are usually solid and show a prominent fibromatous background
- Stroma is variably cellular and edematous
- Tumor cell infiltration is subtle, but diffuse
- Carcinoma cells have a dense eosinophilic cytoplasm and hyperchromatic irregular nuclei
- Signet-ring cells are usually dispersed between stromal fibers, but can also aggregate in clusters
- Upper GI adenocarcinoma without a significant (> 10%) signet-ring cell component tends to be moderately to poorly differentiated
- The amount of intracellular mucin varies according to the degree of mucinous differentiation
- Adenocarcinoma with mucin depletion mimics the architecture and cytoplasmic appearance of primary endometrioid tumors; however, nuclear pleomorphism and hyperchromasia tends to be prominent, and exceed what is expected for a primary ovarian tumor
Positive stains
- Immunohistochemistry is useful to distinguish primary ovarian tumors from lower GI tract metastases (colon, rectum, appendix), but its role in identifying upper GI primaries (gastric, pancreatic, biliary tract) is limited given the significant overlap with primary ovarian tumors
- CK7: 88% (vs 95% of primary ovarian tumors)
- CK20: 43% (vs 45% of primary ovarian tumors)
- CDX2: 65% (vs 50% of primary ovarian tumors)
- SATB2: 11.5% (vs 5% of primary ovarian tumors)
- Mucins MUC1, MUC4 and MUC5AC may be useful markers of GI adenocarcinoma:
- MUC1 and MUC5AC may discriminate among different origins (Int J Gynecol Pathol 2014;33:166, Int J Clin Exp Pathol 2013;6:613)
- Pancreatic: MUC1+, MUC5AC+
- Biliary: MUC1+, MUC5AC-
- Ovary: MUC1-, MUC5AC+
- Gastric and colorectal: MUC1-, MUC5AC-
- Keratin 17 (CK17) is expressed in 90% of pancreatic and 50% of pancreatobiliary adenocarcinoma, whereas esophageal and gastric cancers tend to be negative (Am J Surg Pathol 2005 Mar;29:359)
Negative stains
- Rates of expression in upper GI tract tumors are as follows:
- PAX8: 2.8% (vs 30 - 65% of primary ovarian mucinous tumors and 80% of primary ovarian endometrioid tumors)
- Among upper GI tumors, PAX8 expression has only been documented in pancreatic and esophageal adenocarcinoma (Am J Surg Pathol 2011;35:816)
- ER: 0% (vs 30% of primary ovarian tumors)
- Inactivation of DPC4 / SMAD4 tumor suppressor gene is seen in approximately 55% of pancreatic ductal adenocarcinoma (Am J Clin Pathol 2001;116:831)
Contributed by Carlos Parra-Herran, M.D.
Differential diagnosis
- Primary ovarian neoplasm (benign, borderline or malignant): no suspicious features described above (bilaterality, surface involvement, signet-ring cell morphology, etc), PAX8+
- Metastases from lower GI tract: positive for SATB2 / CK20 / CDX2 / MUC2, CK7-
- Metastases from cervix: p16+ (strong, diffuse)
- Sertoli-Leydig cell tumors: main differential with Krukenberg tumors given the solid appearance, the fibromatous background and the presence of cells with dense eosinophilic cytoplasm seen in both entities
- The former lacks signet-ring cells and cytokeratin expression (pan-cytokeratin, CK7, CK20), and will conversely express inhibin and calretinin
Additional references
WHO classification
Table of Contents
Definition / general | Major updates | WHO (2020) | Microscopic (histologic) images | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- This topic covers tumors of ovarian origin (primary) only, with most tumors falling under the categories of epithelial, mesenchymal, sex cord stromal and germ cell
- The remaining (exceptionally rare) tumors are in the miscellaneous category
Major updates
- WHO first classified low and high grade serous carcinoma as different subtypes of the same tumor; now it is evident that these are 2 different types of carcinoma with different molecular characteristics, immunohistochemical profile, morphology, pathogenesis and tumor progression
- Most high grade serous carcinomas of the ovary arise from the distal fimbria of the fallopian tube from a precursor lesion known as serous tubal intraepithelial carcinoma (STIC) (Pathology 2015;47:423, Mod Pathol 2015;28:1101)
- Low grade serous carcinomas of the ovary arise from benign or borderline serous neoplasms
- SET (solid, endometrial-like, transitional) patterns harbor p53 mutations and are now under the category of high grade serous carcinoma
- Many mutations have been found in sex cord stromal tumors and can differentiate subtypes, such as adult granulosa cell tumor (FOXL2) from poorly differentiated Sertoli-Leydig cell tumors (DICER1) and microcystic stromal tumor (CTNNB1 or APC mutation) (N Engl J Med 2009;360:2719, Am J Surg Pathol 2017;41:1178, Genes Chromosomes Cancer 2015;54:353)
- Small cell carcinoma of hypercalcemic type is associated with SMARCA4 mutations (Histopathology 2016;69:727)
- Seromucinous carcinoma has been removed and is now a subtype of endometrioid carcinoma (Am J Surg Pathol 2015;39:983)
- Gynandroblastoma has been reintroduced after being removed from the previous WHO edition
WHO (2020)
Epithelial tumors
Mesenchymal tumors
Mixed epithelial and mesenchymal tumors
Sex cord stromal tumors
Germ cell tumors
Miscellaneous tumors
Tumor-like lesions
- Serous tumors
- Serous cystadenoma, adenofibroma and surface papilloma
- Serous borderline tumor
- Serous borderline tumor, micropapillary variant
- Low grade serous carcinoma
- High grade serous carcinoma
- Mucinous tumors
- Endometrioid tumors
- Clear cell tumors
- Clear cell cystadenoma and adenofibroma
- Clear cell borderline tumor
- Clear cell carcinoma
- Seromucinous tumors
- Seromucinous cystadenoma and adenofibroma
- Seromucinous borderline tumor
- Brenner tumors
- Other carcinomas
Mesenchymal tumors
- Endometrioid stromal sarcoma
- Smooth muscle tumors
- Leiomyoma
- Smooth muscle tumor of uncertain malignant potential
- Leiomyosarcoma
- Ovarian myxoma
Mixed epithelial and mesenchymal tumors
Sex cord stromal tumors
- Pure stromal tumors
- Fibroma, NOS
- Thecoma
- Luteinized thecoma associated with sclerosing peritonitis
- Sclerosing stromal tumor
- Microcystic stromal tumor
- Signet ring stromal tumor
- Leydig cell tumor
- Steroid cell tumor, NOS
- Malignant steroid cell tumor
- Fibrosarcoma
- Pure sex cord tumors
- Mixed sex cord stromal tumors
- Sertoli-Leydig cell tumor
- Sex cord stromal tumor, NOS
- Gynandroblastoma
Germ cell tumors
- Teratoma, benign
- Immature teratoma, NOS
- Dysgerminoma
- Yolk sac tumor
- Embryonal carcinoma
- Choriocarcinoma, NOS
- Mixed germ cell tumor
- Monodermal teratomas and somatic type tumors arising from a dermoid cyst
- Germ cell sex cord stromal tumors
- Gonadoblastoma
- Dissecting gonadoblastoma
- Undifferentiated gonadal tissue
- Mixed germ cell - sex cord stromal tumor, unclassified
- Gonadoblastoma
Miscellaneous tumors
- Rete cystadenoma, adenoma and adenocarcinoma
- Wolffian tumor
- Solid pseudopapillary tumor
- Small cell carcinoma of the ovary, hypercalcemic type
- Wilms tumor
Tumor-like lesions
Microscopic (histologic) images
Contributed by Lewis A. Hassell, M.D.
Additional references
Board review style question #1
The WHO 2020 classification of ovarian tumors defines benign, borderline and malignant versions of which of the following tumor types?
- Brenner tumors
- Endometrial stromal tumors
- Granulosa cell tumors
- Mesonephric tumors
- Seromucinous tumors
Board review style answer #1
A. Brenner tumors are classified into 3 groups: benign, borderline and malignant. Malignant seromucinous tumors were removed from WHO 2019. The other tumors do not include these separations.
Comment Here
Reference: Ovary - WHO classification
Comment Here
Reference: Ovary - WHO classification
Board review style question #2
Board review style answer #2
D. Sex cord tumor with annular tubules is characterized by cells with clear cytoplasm, basal nuclei and eosinophilic tubular material resembling basement membrane. The cells sometimes resemble Sertoli cells and the eosinophilic tubular material can mimic Call-Exner bodies of granulosa cell tumors.
Comment Here
Reference: Ovary - WHO classification
Comment Here
Reference: Ovary - WHO classification
Wilms tumor (nephroblastoma)
Table of Contents
Definition / general | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Radiology description | Prognostic factors | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Negative stains | Differential diagnosisDefinition / general
- See also topics in Kidney tumor chapter: Childhood Wilms, Adult Wilms
- Extrarenal Wilms tumor are very rare, mostly seen in children
- Occur mostly in reteroperitoneum and very rarely in ovary
- Hypothesis for origin of these tumors is still debated
Epidemiology
- To date, limited case reports:
- 14 months (Journal of Gynecologic Surgery 2012;28:306)
- 3.5 years (J Pediatr Surg 2002;37:127)
- 21 years (Hum Pathol 2000;31:761)
- 56 years (Cancer 1988;61:1460)
Sites
- Usually retroperitoneal and inguinal regions (J Pediatr Surg 2002;37:127)
Pathophysiology
- Hypotheses regarding the origin: see J Indian Assoc Pediatr Surg 2007;12:145
- From ectopic metanephric blastema
- From primitive mesodermal tissue
- Connheim's cell rest theory
Clinical features
- Palpable mass
Diagnosis
- The diagnostic criteria necessary to establish the diagnosis include absence of primary kidney tumor and supernumerary kidney (radiologically and surgically)
Radiology description
- USG and CT: solid mass with cystic components (J Pediatr Surg 2002;37:127)
Prognostic factors
- When matched with the appropriate stage, prognosis is similar to its renal counterpart (J Indian Assoc Pediatr Surg 2007;12:145)
Treatment
- Excision and chemotherapy
- Similar staging and treatment protocol to renal Wilms tumor (J Clin Oncol 1991;9:167)
Gross description
- Solid large mass with cystic cavities
Microscopic (histologic) description
- Sheets of undifferentiated blastemal component with or without epithelial, mesenchymal differentiation and anaplastic features
Microscopic (histologic) images
Negative stains
Differential diagnosis
Xanthogranulomatous oophoritis
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Xanthogranulomatous oophoritis is an uncommon, nonneoplastic, chronic process (Iran J Pathol 2018;13:372)
- Destruction by massive cellular infiltration of foamy histiocytes admixed with multinucleated giant cells, plasma cells, fibroblasts, neutrophils and foci of necrosis (Iran J Pathol 2018;13:372)
Essential features
- Xanthogranulomatous oophoritis is a rare form of chronic oophoritis (J Indian Med Assoc 2012;110:653)
- Affects fallopian tubes or ovaries focally or entirely, forming a mass-like lesion in the pelvic cavity that invades the surrounding tissues (J Cancer 2012;3:100)
Terminology
- Xanthogranulomatous oophoritis
- Xanthogranulomatous inflammation of the ovary
- Xanthogranulomatous inflammation of the female genital tract
- Ovarian fibroxanthoma
ICD coding
- ICD-10: N70.92 - oophoritis, unspecified
Epidemiology
- Most frequent in females of reproductive age (range: 23 - 72 years)
- Average age of patients with affected ovaries is 31 years (AJR Am J Roentgenol 2002;178:749)
- Only 13 related cases of xanthogranulomatous inflammation involving fallopian tube or ovary have been described through 2012 (J Cancer 2012;3:100)
Sites
- Xanthogranulomatous inflammation of the female genital tract is not common and usually involves the endometrium; however, xanthogranulomatous inflammation of the ovaries is a rare entity (J Ayub Med Coll Abbottabad 2017;29:162)
Pathophysiology
- Exact pathophysiology is unknown
- Associated with infection, ineffective antibiotic therapy
- Ineffective clearance of bacteria by phagocytes, endometriosis, intrauterine contraceptive device, pelvic inflammatory disease and drugs (antibiotics) is suggested (Iran J Pathol 2018;13:372)
Etiology
- Development can be of multiple predisposing factors, for example, pelvic inflammatory diseases, intrauterine device use, uterine leiomyoma, endometriosis and inappropriate antibiotic intake (BMJ Case Rep 2015 Jun 25;2015)
Clinical features
- Longstanding history of pelvic inflammatory disease
- Symptoms such as anorexia, fever, suprapubic pain, menorrhagia or vaginal bleeding, adnexal tenderness and a pelvic mass are the usual chief complaints (Iran J Pathol 2018;13:372)
Diagnosis
- Careful histopathology by experienced pathologists aided by immunohistochemistry can lead to a definitive diagnosis (J Ayub Med Coll Abbottabad 2017;29:162)
Radiology description
- Ultrasound of the abdomen reveals a thick walled, cystic hyperechoic ovarian mass (Iran J Pathol 2018;13:372)
- Pelvic CT scan shows a peripherally enhancing ovarian cystic lesion, which represents either an inflammatory process or ovarian neoplasm (Iran J Pathol 2018;13:372)
- Presence of nonenhancing intramural nodules in the thickened wall of an ovarian cystic mass may be a unique MRI indicator of xanthogranulomatous oophoritis (AJR Am J Roentgenol 2002;178:749)
Radiology images
Prognostic factors
- Since xanthogranulomatous oophoritis is usually associated with pelvic inflammatory disease, endometriosis, intrauterine death, etc., these patients should be followed up closely (Indian J Pathol Microbiol 2010;53:197)
Case reports
- 20 year old woman presenting with a tubo-ovarian mass (Iran J Pathol 2018;13:372)
- 24 year old woman who was misdiagnosed with an ovarian neoplasm (J Midlife Health 2018;9:41)
- 25 year old woman presented with intermittent fever and lower abdominal pain (Indian J Pathol Microbiol 1999;42:89)
- 26 year old woman with a 10 year history of pelvic inflammatory disease (Int J Gynecol Pathol 1984;3:398)
- 31 year old Lebanese woman, 8 years following an open appendectomy as a reaction to talcum powder present on surgical gloves (J Med Liban 2012;60:169)
- 45 year old woman with heavy vaginal bleeding for 20 days (BMJ Case Rep 2015 Jun 25;2015)
- 47 year old woman with a several month history of intermittent abdominal pain localized to the left suprapubic region (Arch Pathol Lab Med 2001;125:260)
- 48 year old woman with a 3 day history of lower abdominal pain and fever (AJR Am J Roentgenol 2002;178:749)
Treatment
- Treatment of choice is oophorectomy (J Midlife Health 2018;9:41)
Gross description
- Ovarian lesion usually has a solid mass with rugged outer surface
- Serial sectioning mostly reveals tan, grayish white and necrotic cut surfaces (J Cancer 2012;3:100)
Microscopic (histologic) description
- Usually shows characteristic dense fibrosis with infiltration of the ovarian stroma by sheets and nodules of foamy macrophages, many histiocytes, lymphocytes and neutrophils (Iran J Pathol 2018;13:372)
Microscopic (histologic) images
Positive stains
- CD68 in foamy histiocytes
- CD3 in T cells
- CD20 in B cells (J Cancer 2012;3:100)
Sample pathology report
- Left ovary and fallopian tube, left salpingo-oophorectomy:
- Cystic ovary with endometriosis
- Extensive xanthogranulomatous oophoritis
- Fallopian tube with no significant pathologic change
Differential diagnosis
- Ovarian carcinoma:
- May be difficult to differentiate with imaging
- Gross and microscopic pathologic findings will easily differentiate malignant tumors from inflammatory lesions
- Endometrioma:
- Usually filled with chocolate colored fluid
- Histologic sections of cystic wall show hemosiderin laden macrophages without abundant foamy histiocytes
- Tubo-ovarian mass:
- Benign tumors can occur in the tubo-ovarian area, i.e. leiomyoma
- Gross and histologic examinations reveal smooth muscle neoplasm rather than inflammatory process / lesion
Additional references
Board review style question #1
Board review style answer #1
E. Xanthogranulomatous oophoritis (the gross photo shows variably sized yellow nodules, which are
consistent with changes of xanthogranulomatous nodules of ovary).
Comment Here
Reference: Xanthogranulomatous oophoritis
Comment Here
Reference: Xanthogranulomatous oophoritis
Board review style question #2
Board review style answer #2
A. Endometriosis and xanthogranulomatous oophoritis. The microscopic photo shows endometriosis with increased foamy histiocytes in stroma and adjacent soft tissue, consistent with xanthomatous change.
Comment Here
Reference: Xanthogranulomatous oophoritis
Comment Here
Reference: Xanthogranulomatous oophoritis
Yolk sac tumor
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Primitive germ cell tumor with a variety of morphologic patterns, ranging from endodermal extraembryonic structures (secondary yolk sac, allantois) to, less commonly, endodermal somatic tissues (intestine, liver, mesenchyme)
Essential features
- Most common before the age of 30
- Usually occurs as a pure form or rarely as a component of a mixed germ cell tumor
- Numerous morphologic patterns, with the hallmark Schiller-Duval bodies in some cases and immunohistochemical expression of SALL4, glypican 3 and AFP
- Often associated with elevated serum alpha fetoprotein (AFP)
- Usually favorable clinical outcomes due to chemosensitivity
Terminology
- Primitive endodermal tumor (not recommended)
- Endodermal sinus tumor (not recommended)
ICD coding
- ICD-O: 9071/3 - yolk sac tumor
- ICD-10: C56 - malignant neoplasm of ovary
- ICD-11:
- 2C73.5 - endodermal sinus tumor, unspecified site, female
- 2C73.Y & XH09W7 - other specified malignant neoplasms of the ovary, yolk sac tumor
Epidemiology
- ~20% of malignant germ cell tumors of the ovary (Obstet Gynecol 2006;107:1075)
- Second most common malignant ovarian germ cell tumor after dysgerminoma
- Mean age is 19 years; most common in the second and third decades of life (Int J Surg Pathol 2014;22:677)
Sites
- Usually ovary
- Less common in uterus, vagina, vulva and peritoneum (Am J Surg Pathol 2017;41:1)
Pathophysiology
- Chromosome 12 abnormalities, usually isochromosome 12p, in ~75% of patients (Cancer Res 1998;58:3105)
Clinical features
- Abdominal enlargement or pain
- Lower abdominal or pelvic mass (Int J Surg Pathol 2014;22:677)
- Rarely hormonal manifestations
Diagnosis
- Microscopic examination
Laboratory
- Elevated serum levels of AFP (may be used diagnostically and in monitoring therapy)
Radiology description
- Ultrasonography:
- Both echogenic and hypoechoic components (AJR Am J Roentgenol 1996;167:791)
- Computed tomography:
- Usually appears as a unilateral large complex mass with solid and cystic components, heterogeneous enhancement and enlarged intratumoral vessels with hemorrhage and capsular tear (Acta Radiol 2016;57:197)
- Helpful features for differentiating yolk sac tumor from other ovarian tumors include a mixed solid / cystic nature, intratumoral hemorrhage, marked enhancement and dilated intratumoral vessels (Sci Rep 2015;5:11000)
- Intratumoral calcification and fatty tissue if associated with teratoma
- Magnetic resonance imaging:
- Prominent signal voids (J Comput Assist Tomogr 2000;24:605)
- Often with areas of hemorrhage
Prognostic factors
- Usually favorable prognosis due to chemosensitivity, with complete cure in > 80% of cases
- Stage dependent, with 5 year survival rates of > 95% for stage I - II, 70% for stage III and 50% for stage IV (Gynecol Oncol 2017;147:296, Int J Gynecol Cancer 2018;28:77)
- Prominent polyvesicular vitelline pattern associated with more indolent behavior (Am J Surg Pathol 2013;37:393)
- Pure hepatoid and glandular intestinal type yolk sac tumors associated with poorer prognosis
Case reports
- 12 year old girl with ovarian yolk sac tumor presenting with acute abdominal pain and elevated serum AFP (Acta Biomed 2019;90:599)
- 17 year old girl with bilateral metachronous ovarian yolk sac tumors (J Pediatr Adolesc Gynecol 2017;30:259)
- 19 year old woman with mixed germ cell tumor (yolk sac tumor and choriocarcinoma) arising in gonadoblastoma (Int J Surg Pathol 2018;26:287)
- 20 year old woman with fertility sparing surgery for ovarian yolk sac tumor (J Clin Diagn Res 2017;11:QD12)
- 21 year old woman with ovarian yolk sac tumor associated with granulomatous reaction resembling tuberculosis (Turk Patoloji Derg 2016;32:126)
Treatment
- Unilateral salpingo-oophorectomy (Eur J Surg Oncol 2006;32:1063)
- Adjuvant multiagent combination chemotherapy
Gross description
- Usually unilateral ovarian mass with predilection for right ovary
- Encapsulated with smooth and glistening surface, round, oval or globular, may be firm or somewhat lobulated
- Rupture in 25% of cases (Int J Surg Pathol 2014;22:677)
- Mean size 15 cm (range 3 - 30)
- Fleshy, gray-yellow to gray-tan, solid and cystic friable cut surface, often with gelatinous changes and areas of hemorrhage and necrosis (Cancer 1976;38:2404)
- May form adhesions to surrounding structures
- When part of a mixed germ cell tumor, other components, such as a mature cystic teratoma or dysgerminoma, may be grossly recognizable
- Honeycomb appearance (multiple small cysts) on cut surface if polyvesicular vitelline component is present (Am J Surg Pathol 2013;37:393)
Gross images
Frozen section description
- Admixure of characteristic growth patterns and tumor cells with clear to eosinophilic cytoplasm, variable cytologic atypia and mitotic activity, with or without Schiller-Duval bodies
Frozen section images
Microscopic (histologic) description
- Multiple histologic patterns with predominance of 1 or 2 patterns
- Reticular / microcystic pattern:
- Most common pattern
- Loose meshwork of anastomosing channels and variably sized cysts (macro or microcysts) lined by primitive tumor cells with varying amounts of clear to eosinophilic cytoplasm (Cancer 1976;38:2404, Histopathology 2012;60:1023, Int J Surg Pathol 2014;22:677)
- Lining cells may be flattened and deceptively bland
- Tumor cells occasionally contain lipid and have a signet ring-like morphology
- Cysts may contain eosinophilic hyaline globules and amorphous, eosinophilic acellular basement membrane-like material
- Loose, hypocellular or myxoid stroma
- Endodermal sinus pattern:
- Anastomosing network of labyrinthine-like spaces lined by tumor cells
- Formation of vaguely glomeruloid perivascular structures (Schiller-Duval bodies)
- Hallmark of yolk sac tumor but their absence does not rule out the diagnosis
- Variably present, ranging from 20 - 75% of cases (Surg Pathol Clin 2019;12:621)
- Rounded to elongated papillary structures containing a central fibrovascular core with a single central vessel, surrounded by tumor cells projecting into a cystic / sinusoidal space (resembling immature glomeruli) (Histopathology 2012;60:1023)
- Papillary pattern:
- Papillae containing fibrovascular cores lined by pleomorphic tumor cells with brisk mitoses
- Fibrovascular cores may be hyalinized
- May contain tumor giant cells (mono or multinucleated)
- Solid pattern:
- Sheets of polygonal tumor cells with large vesicular or pyknotic nuclei with prominent nucleoli, brisk mitoses, clear to eosinophilic cytoplasm and well defined borders, sometimes with prominent hyaline globules
- Cells may be smaller and more blastema-like with scant cytoplasm
- May contain tumor giant cells (mono or multinucleated)
- Festoon pattern:
- Complex ribbons and undulating cords
- Occasionally with a drape-like arrangement
- Glandular pattern (forming endodermal somatic derivatives):
- Endometrioid type areas with glandular or villoglandular structures lined by single or multiple layers of tall columnar cells containing subnuclear or supranuclear vacuoles resembling secretory endometrium
- Intestinal type areas with glandular structures lined by mucinous columnar or low columnar glands, ranging from primitive cribriform structures to well differentiated glands with goblet cells and rarely Paneth cells
- Both types may occur in pure form and may contain tumor giant cells (mono or multinucleated)
- Polyvesicular vitelline pattern (Am J Surg Pathol 2013;37:393):
- May occur in pure form
- Variably sized cysts or vesicles lined by flat to cuboidal to columnar cells, sometimes with basal or paraluminal vacuolation
- Variably cellular stroma, occasionally with eccentric constriction (resembling subdivision of primary yolk sac vesicle)
- Parietal pattern:
- Tumor cells embedded in extracellular linear bands of PAS positive basement membrane-like material
- Hepatoid pattern:
- Tends to occur in pure form
- Aggregates, clusters or cords of large polygonal cells with abundant uniform or granular eosinophilic cytoplasm, round nuclei and prominent nucleoli, separated by thin fibrous bands (Cancer 1982;50:2355)
- Mesenchyme-like pattern:
- Cords, tubules and gland-like structures of tumor cells scattered in edematous to myxoid stroma
- May be markedly myxoid (magma reticulare)
- General features:
- Variable cytologic atypia and mitotic activity
- Pale eosinophilic to clear cytoplasm
- Prominent nucleoli
- Intracellular hyaline globules
- Tumor cells lining cystic structures can be deceptively bland
- Rarely stromal luteinization
- May show areas of extramedullary hematopoiesis
- May be admixed with other malignant germ cell tumor, usually with dysgerminoma or gonadoblastoma in patients with gonadal dysgenesis
- May be associated with synchronous or metachronous ipsilateral or contralateral mature cystic teratoma
Microscopic (histologic) images
Contributed by Gulisa Turashvili, M.D., Ph.D., Sharon Song, M.D. and AFIP images
Positive stains
- SALL4: marker of primitive germ cell differentiation (Arch Pathol Lab Med 2014;138:351, Am J Surg Pathol 2009;33:894)
- AFP: highly specific but 60% sensitive, often patchy / focal and weak (Histopathology 2013;62:71)
- Glypican 3: less specific but stronger expression (Am J Surg Pathol 2008;32:600)
- PLAP
- HNF1β
- Pancytokeratin
- GATA3: positive in reticular / microcystic, papillary and polyvesicular vitelline patterns but not in the glandular pattern (Histopathology 2016;68:613)
- HepPar1: positive in glandular and hepatoid patterns
- CEA, albumin: positive in hepatoid pattern (Int J Gynecol Pathol 2014;33:365)
- TTF1: positive in foregut / respiratory pattern (Histopathology 2014;65:51)
- CDX2: positive in glandular intestinal type pattern
- Villin: positive in reticular / microcystic and glandular intestinal type patterns
Negative stains
Electron microscopy description
- Glandular intestinal type yolk sac tumor shows large nuclei with prominent nucleolonema, numerous intracytoplasmic ribosomes, rough endoplasmic reticulum, mitochondria and dense amorphous intracellular material (Pathol Res Pract 1987;182:609)
Molecular / cytogenetics description
- Usually isochromosome 12p (Mod Pathol 2006;19:766)
Sample pathology report
- Right fallopian tube and ovary, salpingo-oophorectomy:
- Ovary: yolk sac tumor (see synoptic report)
- Fallopian tube: benign
Differential diagnosis
- Clear cell carcinoma:
- Typical architectural patterns (tubulocystic, papillary, solid) with or without hobnail cells
- Lack of microcysts and Schiller-Duval bodies
- Discordance between mitotic activity and cytologic atypia
- Stromal hyalinization
- Background adenofibroma or endometriosis
- Positive for napsin A, HNF1β, PAX8, CK7, EMA
- Negative for SALL4 and AFP
- Glypican 3 is not helpful in this differential diagnosis
- Endometrioid carcinoma:
- Usually older patients
- Typical cytology (columnar cells with moderately atypical pseudostratified nuclei)
- Lack of histologic patterns of yolk sac tumor, Schiller-Duval bodies and primitive appearing nuclei (Int J Surg Pathol 2014;22:677)
- Squamous differentiation
- May be associated with endometriosis
- Positive for PAX8, ER, PR, CK7, EMA
- Negative for SALL4 and AFP
- Glypican 3 is not helpful in this differential diagnosis
- Dysgerminoma:
- Monomorphic architecture composed of cells with ample pale cytoplasm and large round nuclei
- Lack of histologic patterns of yolk sac tumor, Schiller-Duval bodies and hyaline bodies
- Associated with lymphocytic and granulomatous reactions
- Positive for OCT 3/4 and D2-40
- Negative for AFP and glypican 3
- Embryonal carcinoma:
- Very rare in pure form in the ovary
- Lack of histologic patterns of yolk sac tumor and Schiller-Duval bodies
- Aggregated of primitive cells with more marked nuclear atypia and more granular cytoplasm
- Positive for OCT 3/4, CD30 and SOX2
- Negative for AFP and glypican 3
- Sertoli-Leydig cell tumor:
- Androgenic manifestations
- Morphologic patterns of Sertoli cell tumor or Leydig cells
- Positive for inhibin, calretinin, FOXL2 and SF1
- Negative for SALL4, AFP and glypican 3
- Juvenile granulosa cell tumor:
- Solid or follicular growth composed of polygonal cells with eosinophilic to clear cytoplasm and hyperchromatic nuclei without grooves
- Lack of histologic patterns of yolk sac tumor and Schiller-Duval bodies
- Positive for inhibin, calretinin, FOXL2 and SF1
- Negative for SALL4, AFP and glypican 3
- Metastatic hepatocellular carcinoma:
- Immature teratoma:
- Admixture of endodermal, mesodermal and ectodermal elements
- Somatic yolk sac tumor:
- Yolk sac tumor associated with a somatic epithelial neoplasm, usually high grade (Histopathology 2016;69:739)
- Thought to be due to transdifferentiation of high grade carcinoma to yolk sac tumor, rather than a true mixed or collision tumor (i.e. somatic rather than germ cell origin) (Histopathology 2016;69:739, Int J Gynecol Pathol 2011;30:442)
- Typically displays a reticular pattern (Histopathology 2017;71:562)
- Occurs in postmenopausal women and exhibits poor prognosis (Int J Gynecol Pathol 2011;30:442)
Additional references
Board review style question #1
Board review style answer #1
Board review style question #2
Which of the following is true about yolk sac tumors?
- Call-Exner bodies
- Expression of SALL4, AFP and glypican 3 by immunohistochemistry
- Homer Wright rosettes
- Low serum AFP levels
- Most common in postmenopausal women
Board review style answer #2
B. Expression of SALL4, AFP and glypican 3 by immunohistochemistry
Comment Here
Reference: Yolk sac tumor
Comment Here
Reference: Yolk sac tumor
Recent Ovary Pathology books
Find related Pathology books: gynecologic