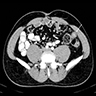
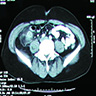
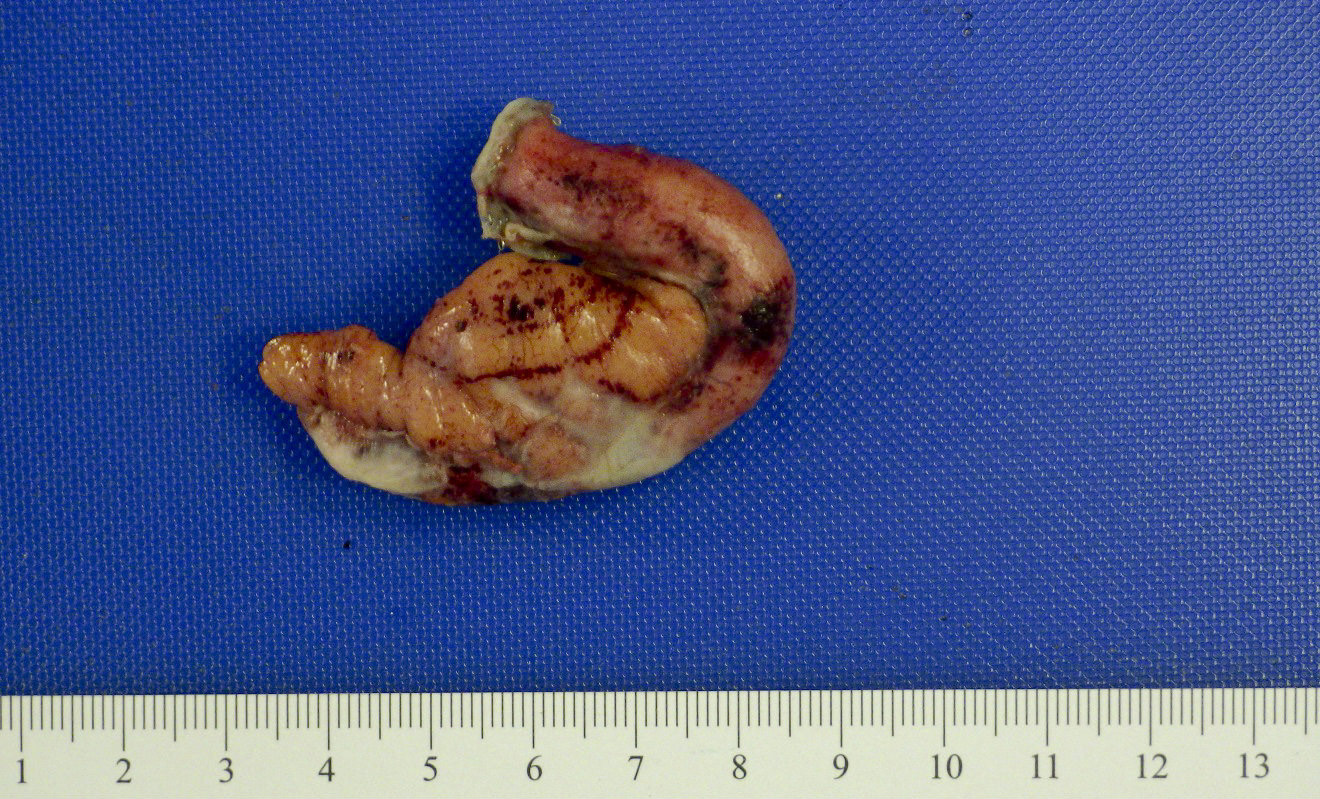
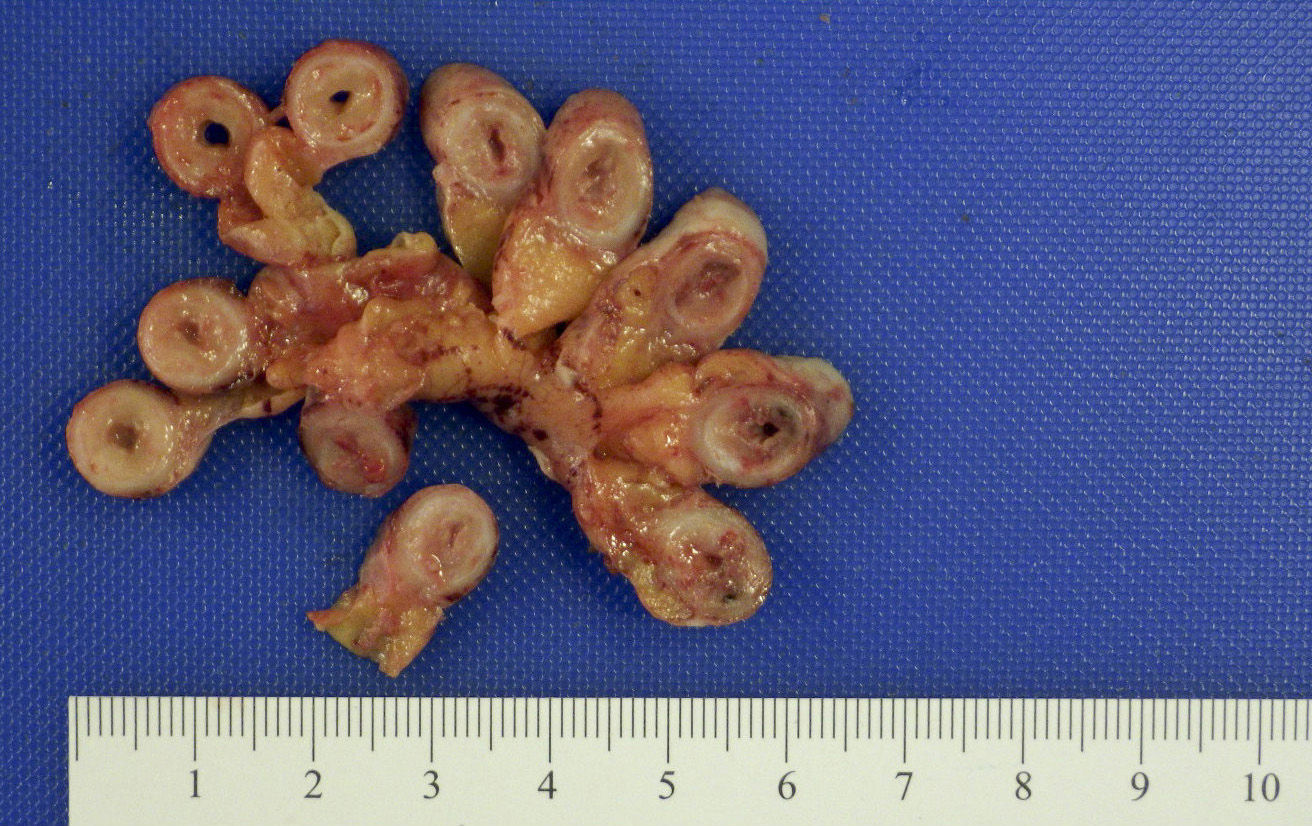
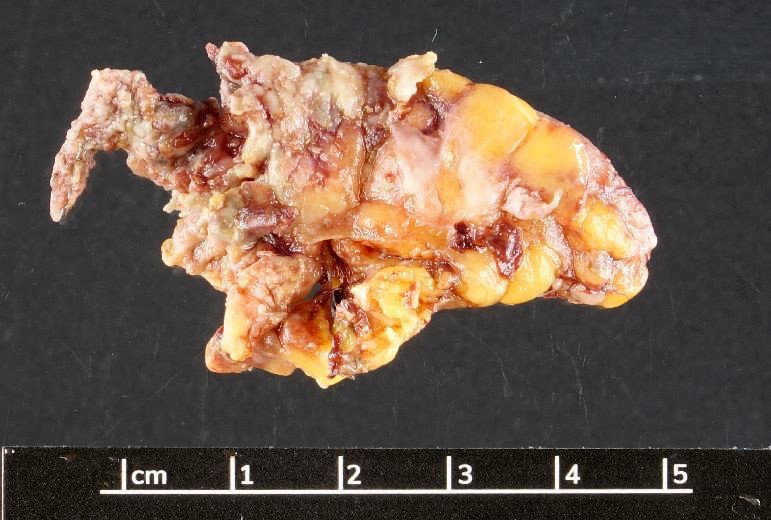
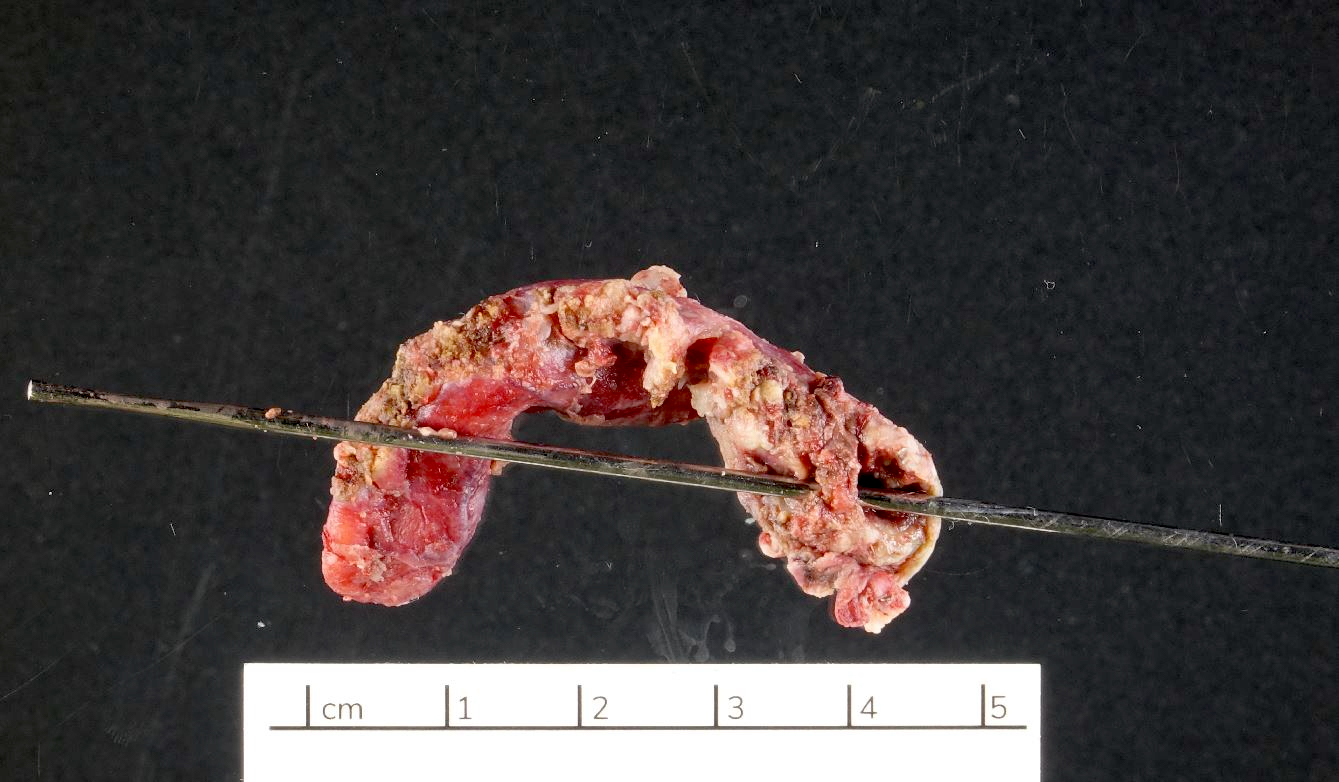
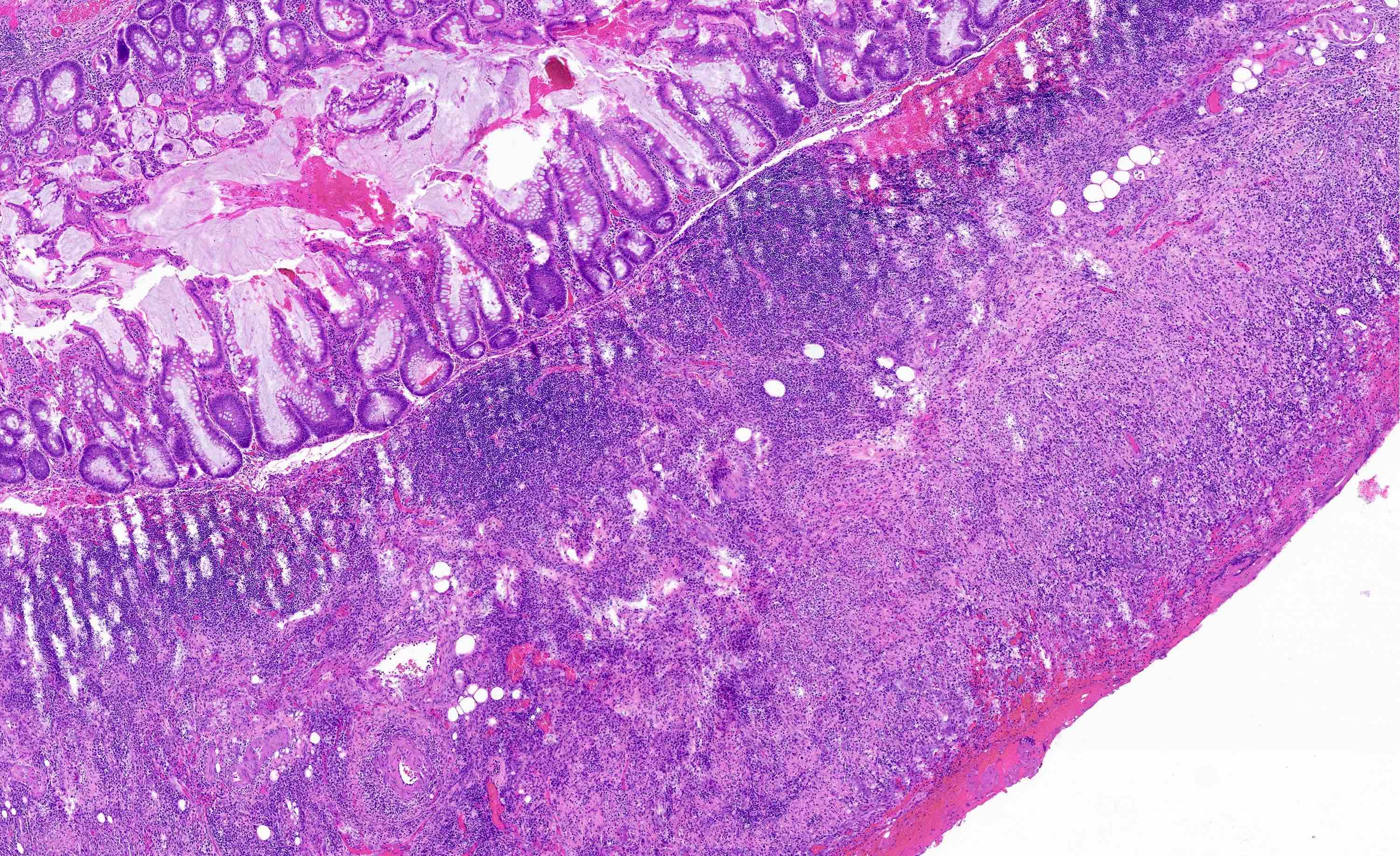
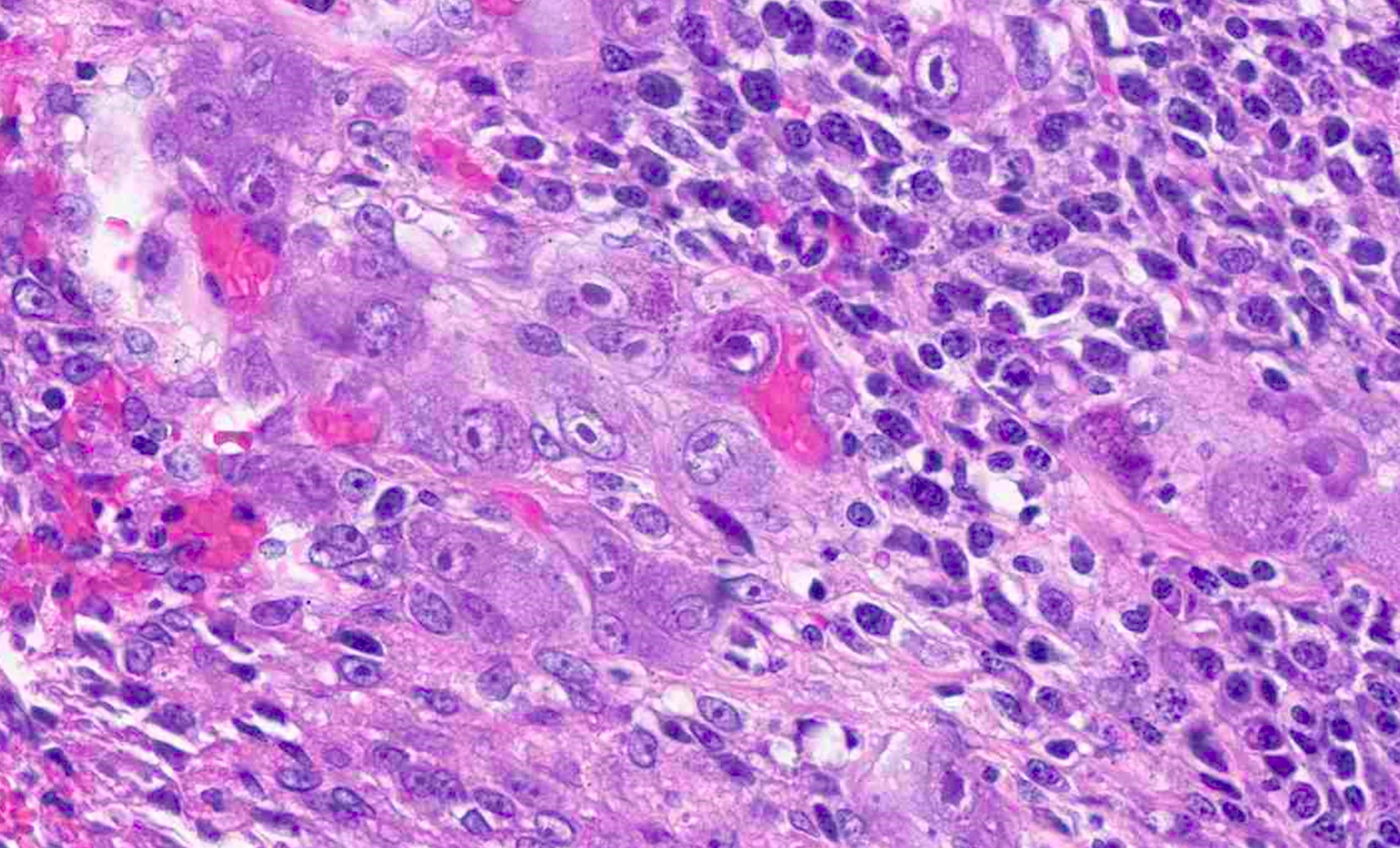
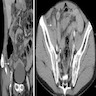
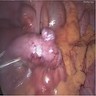
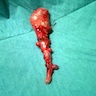
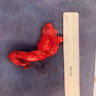
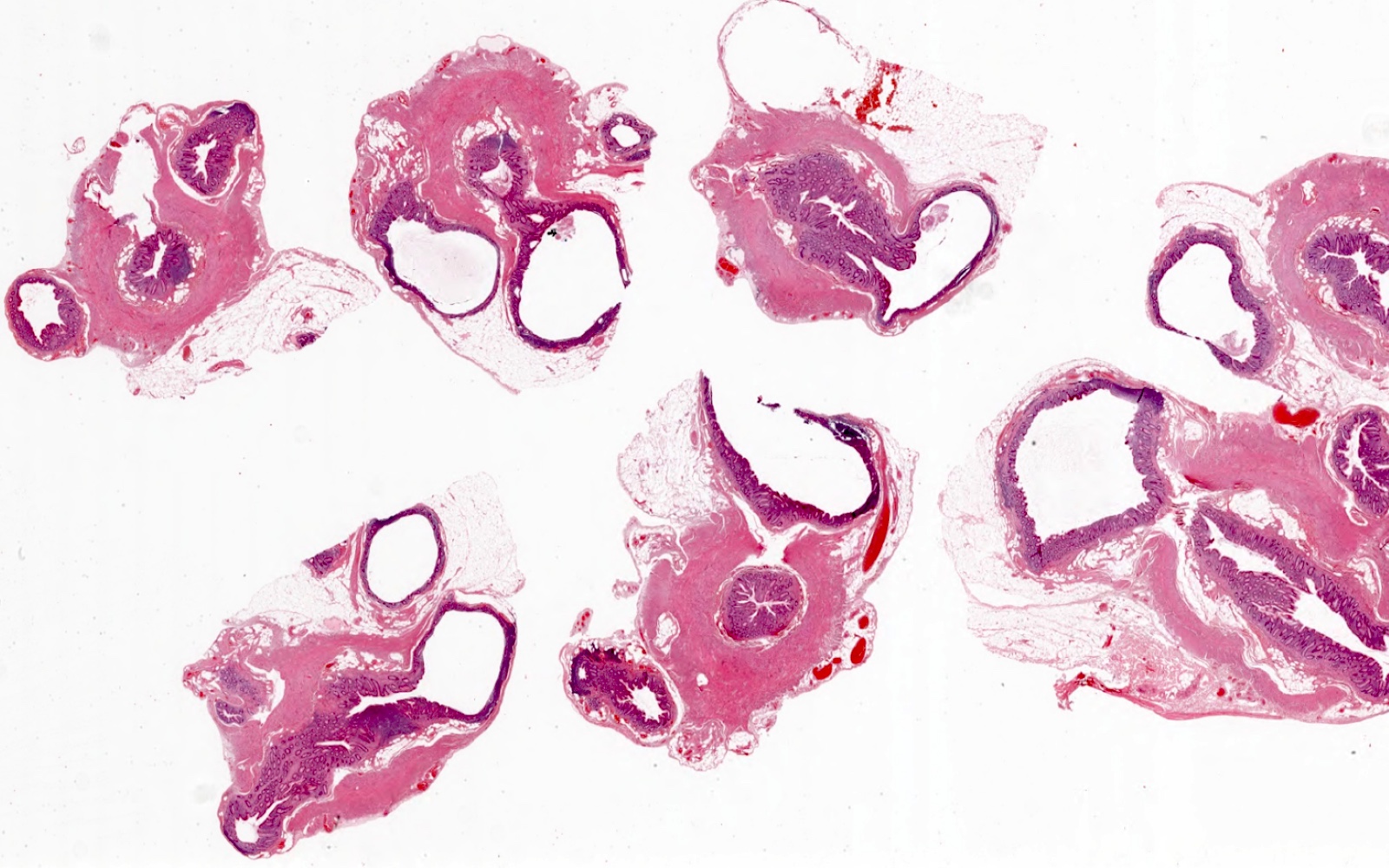
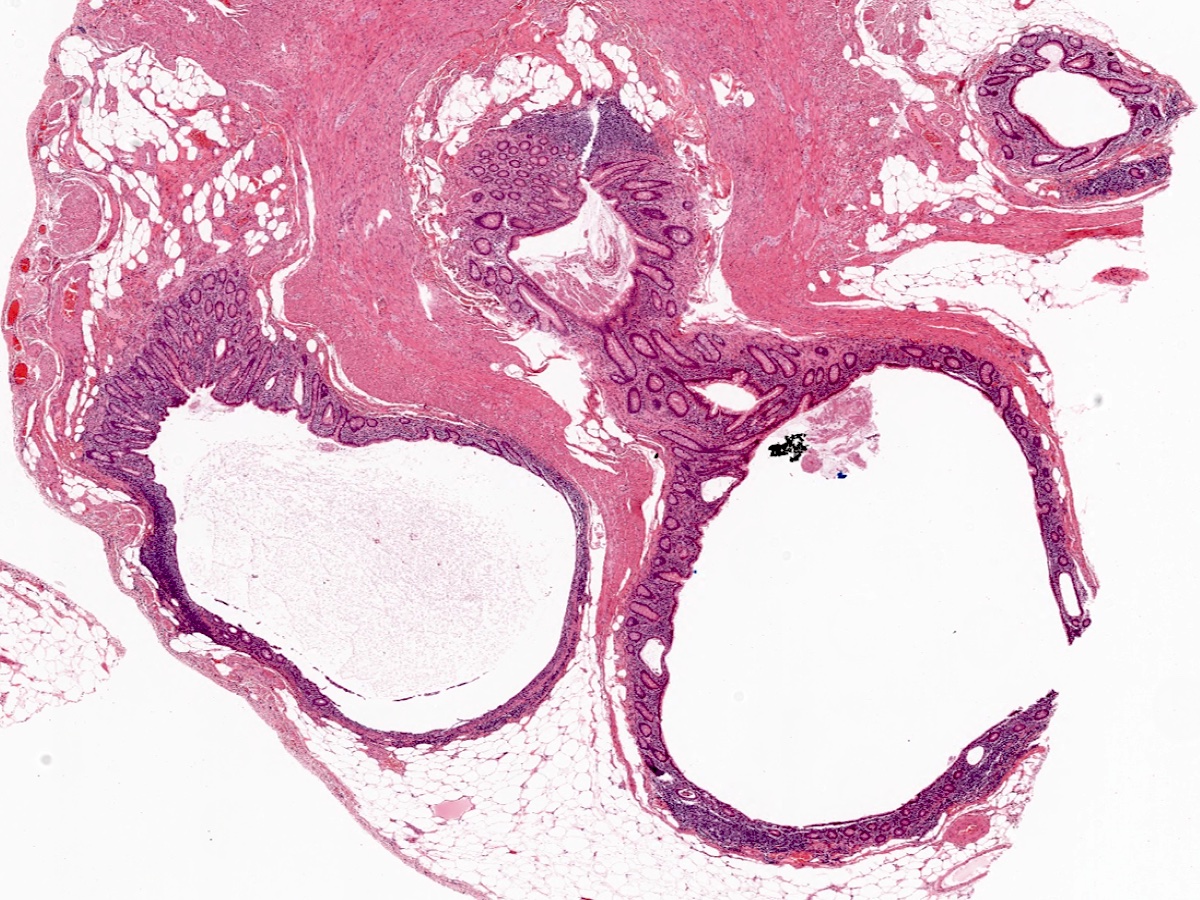
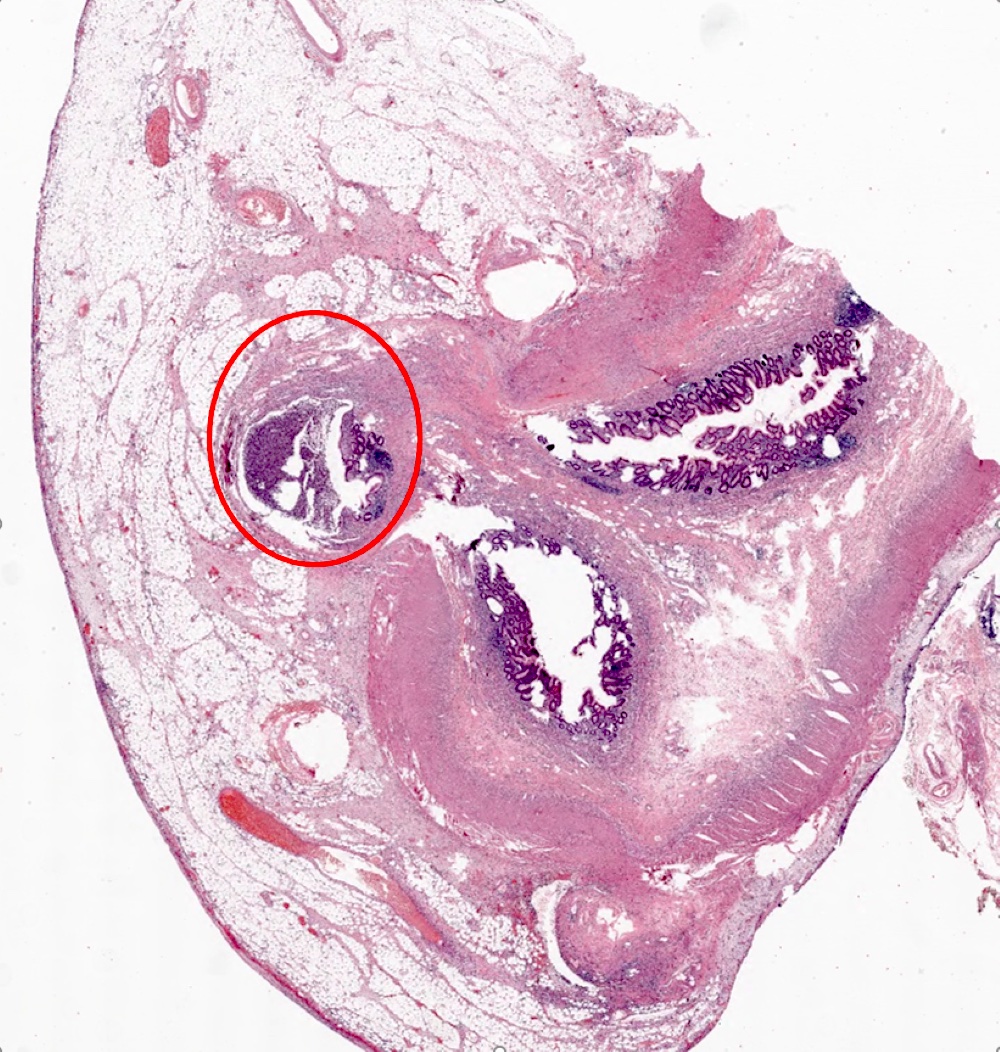
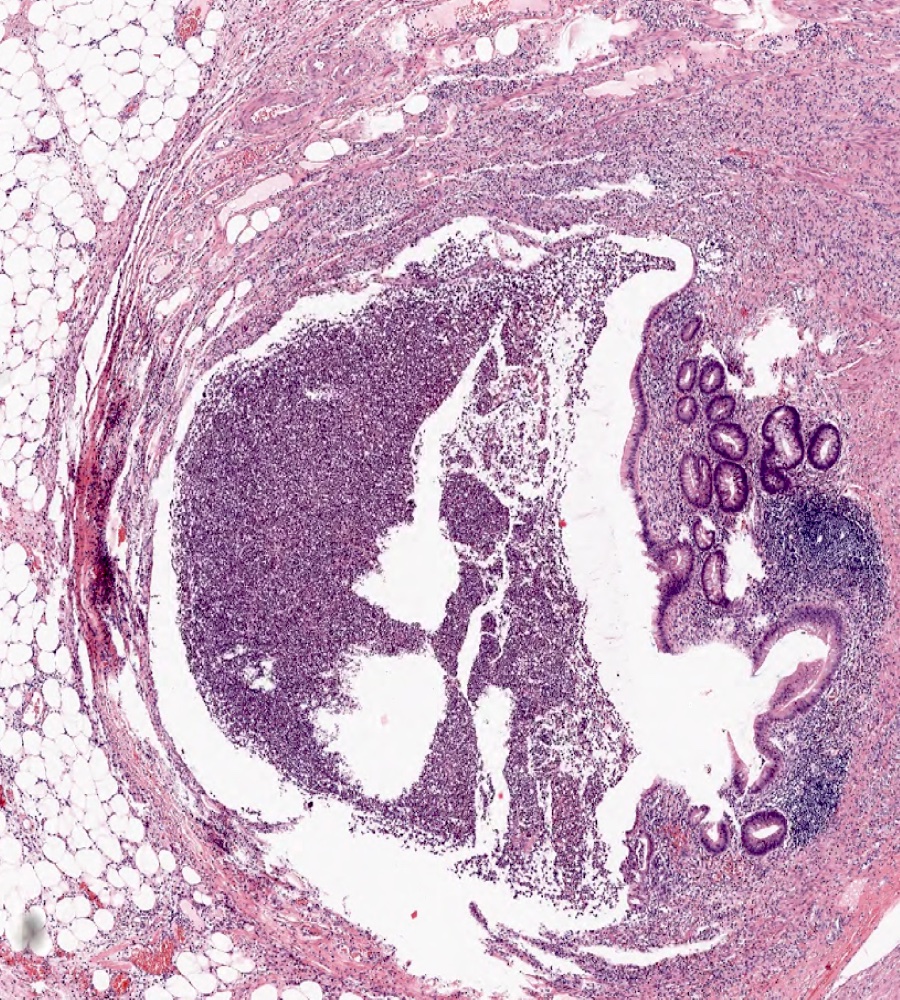
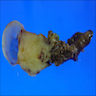
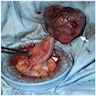
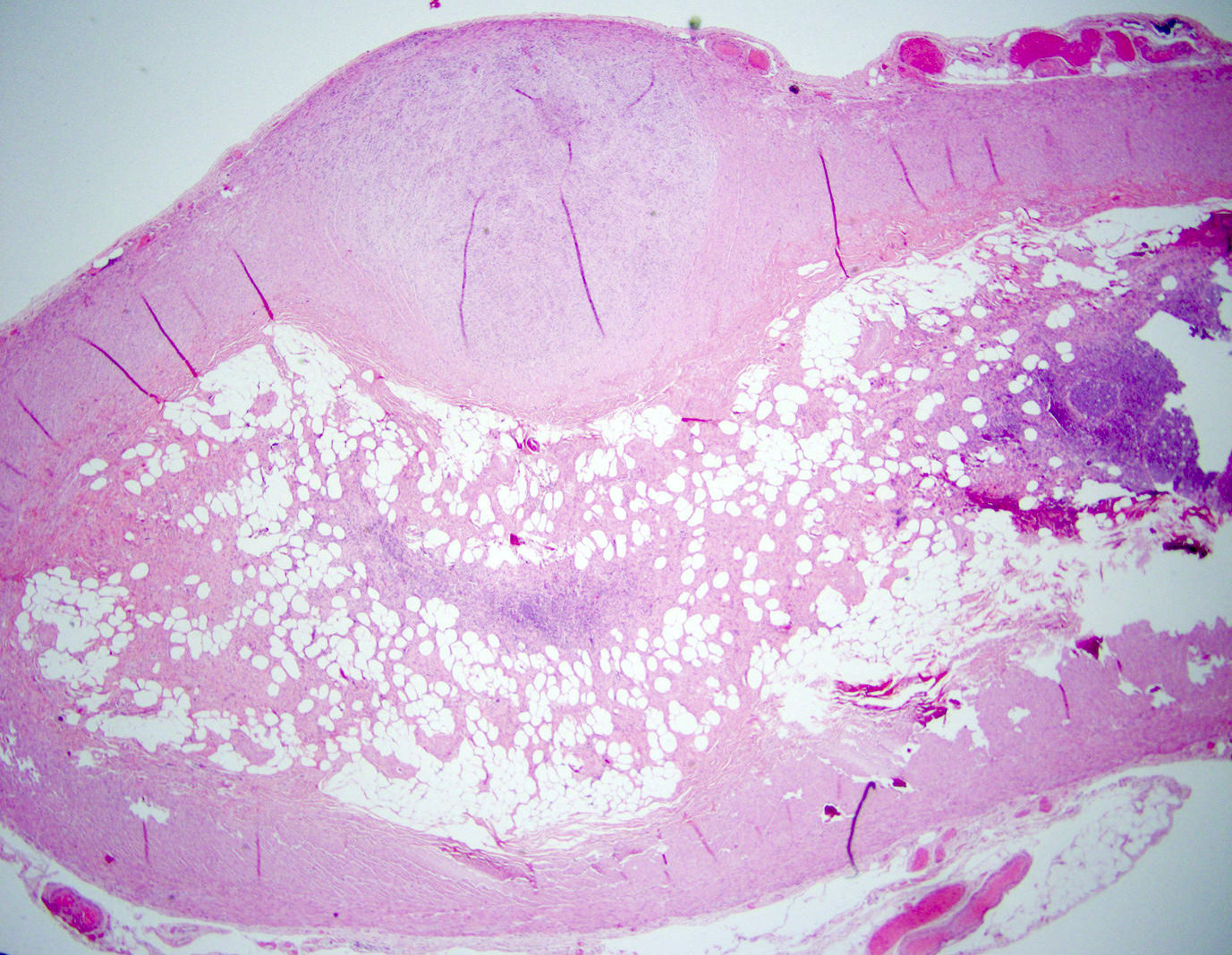
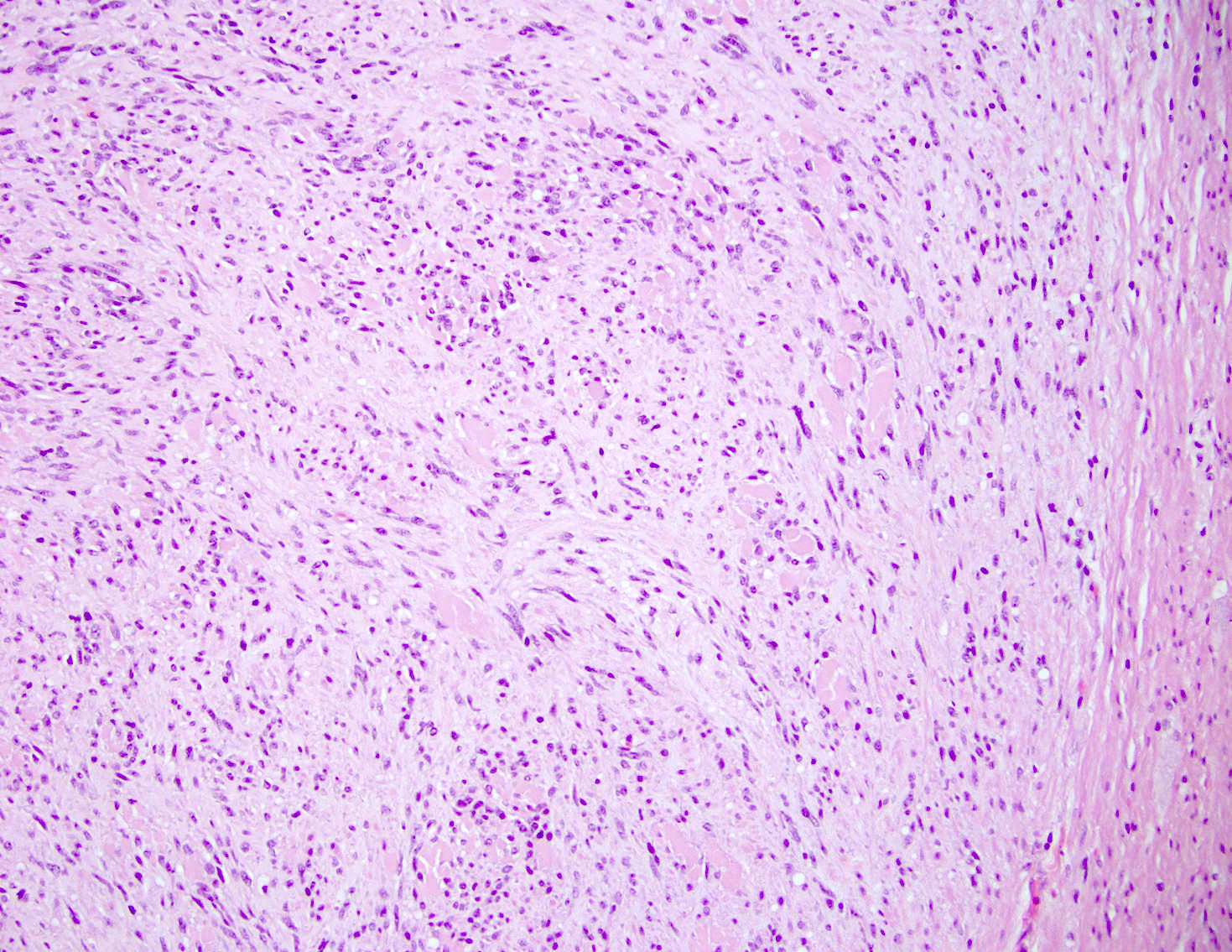
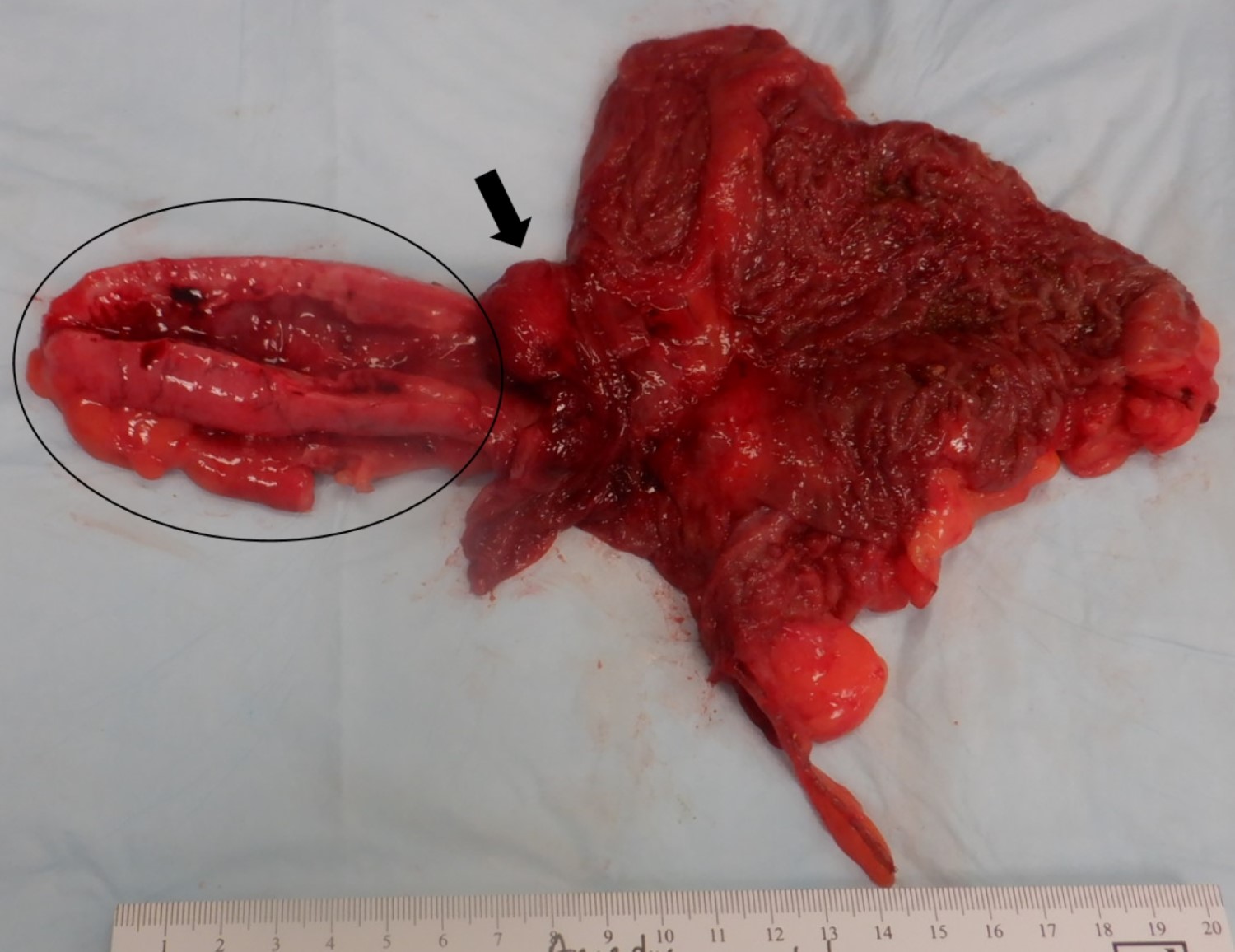
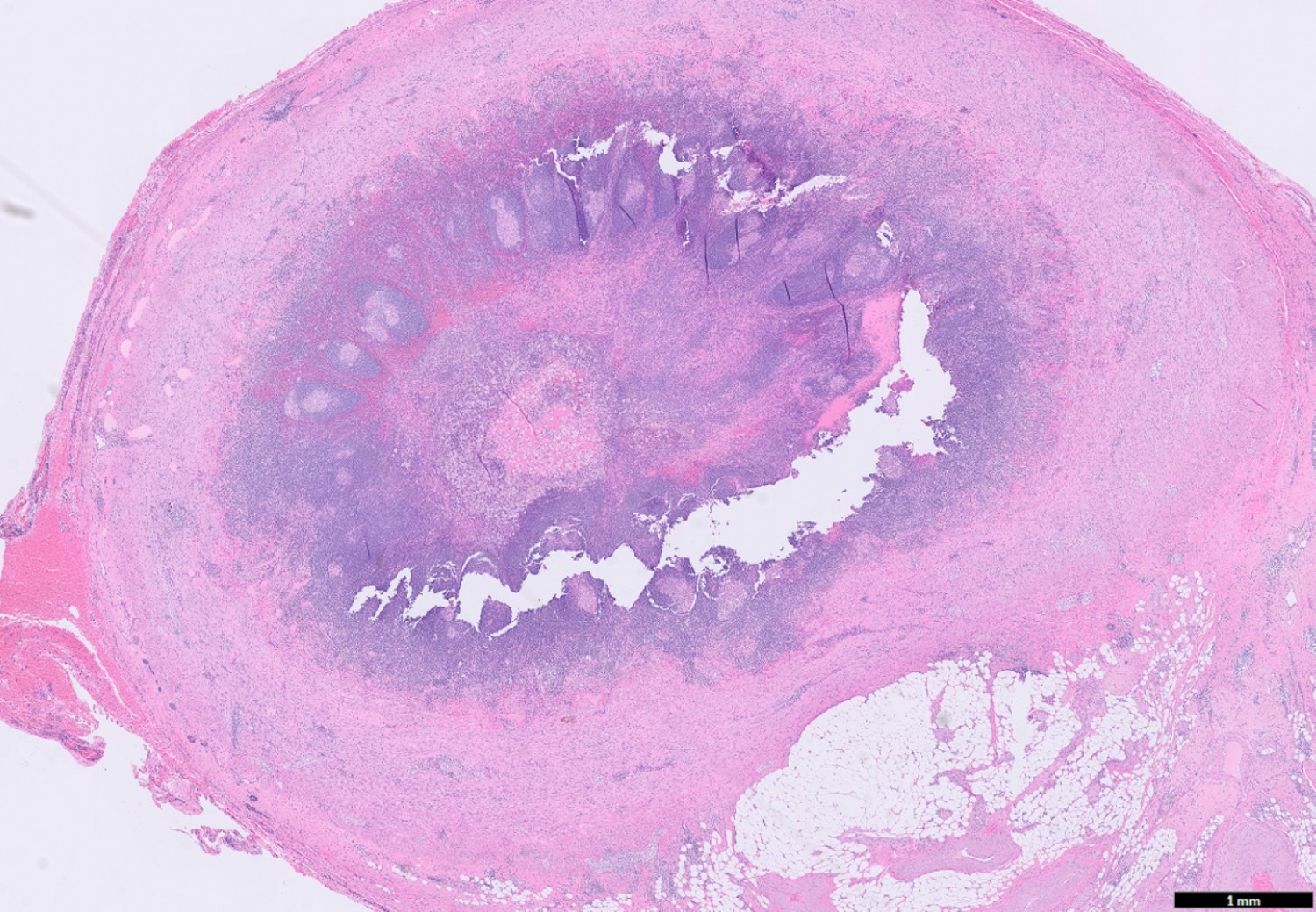
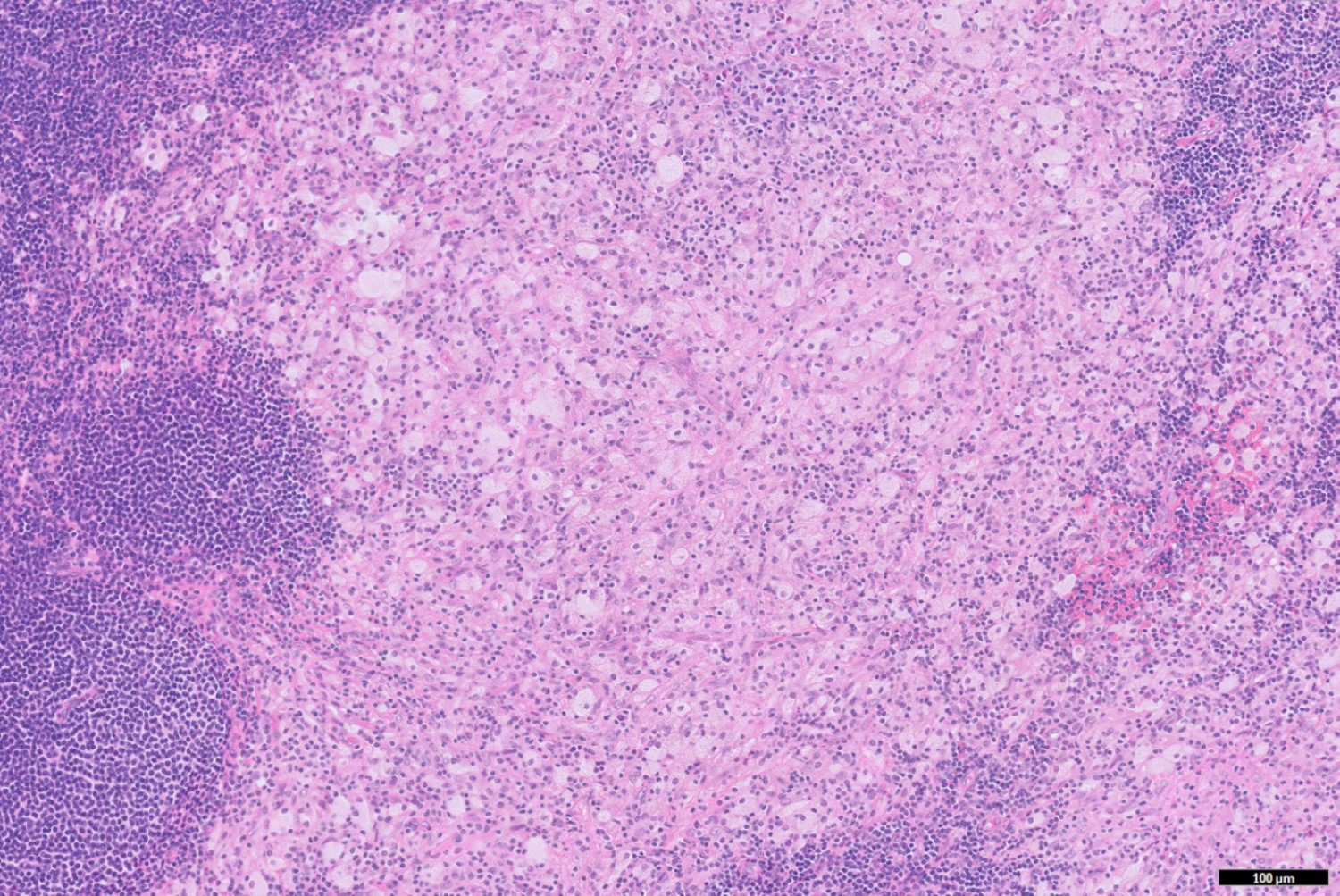
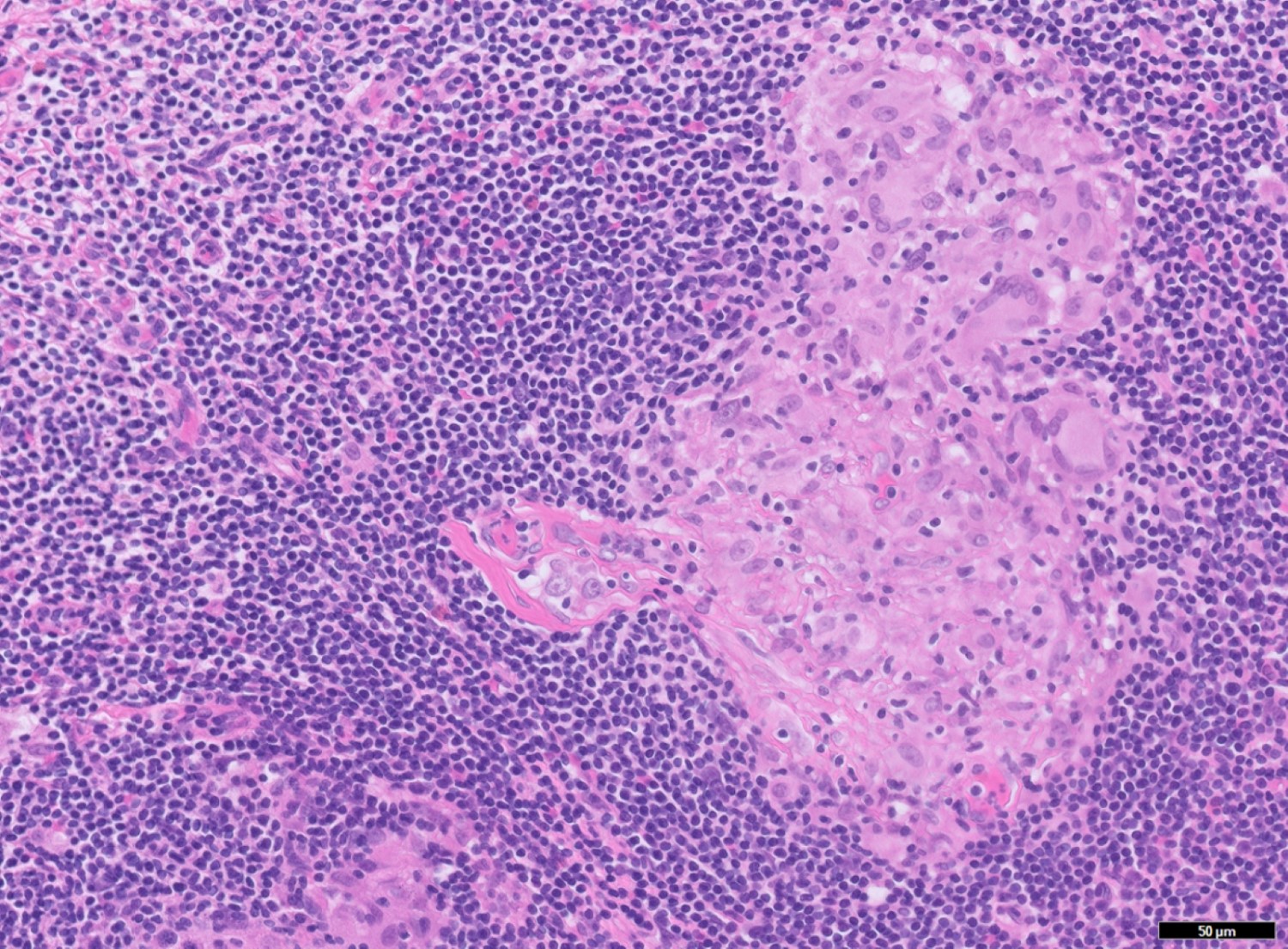
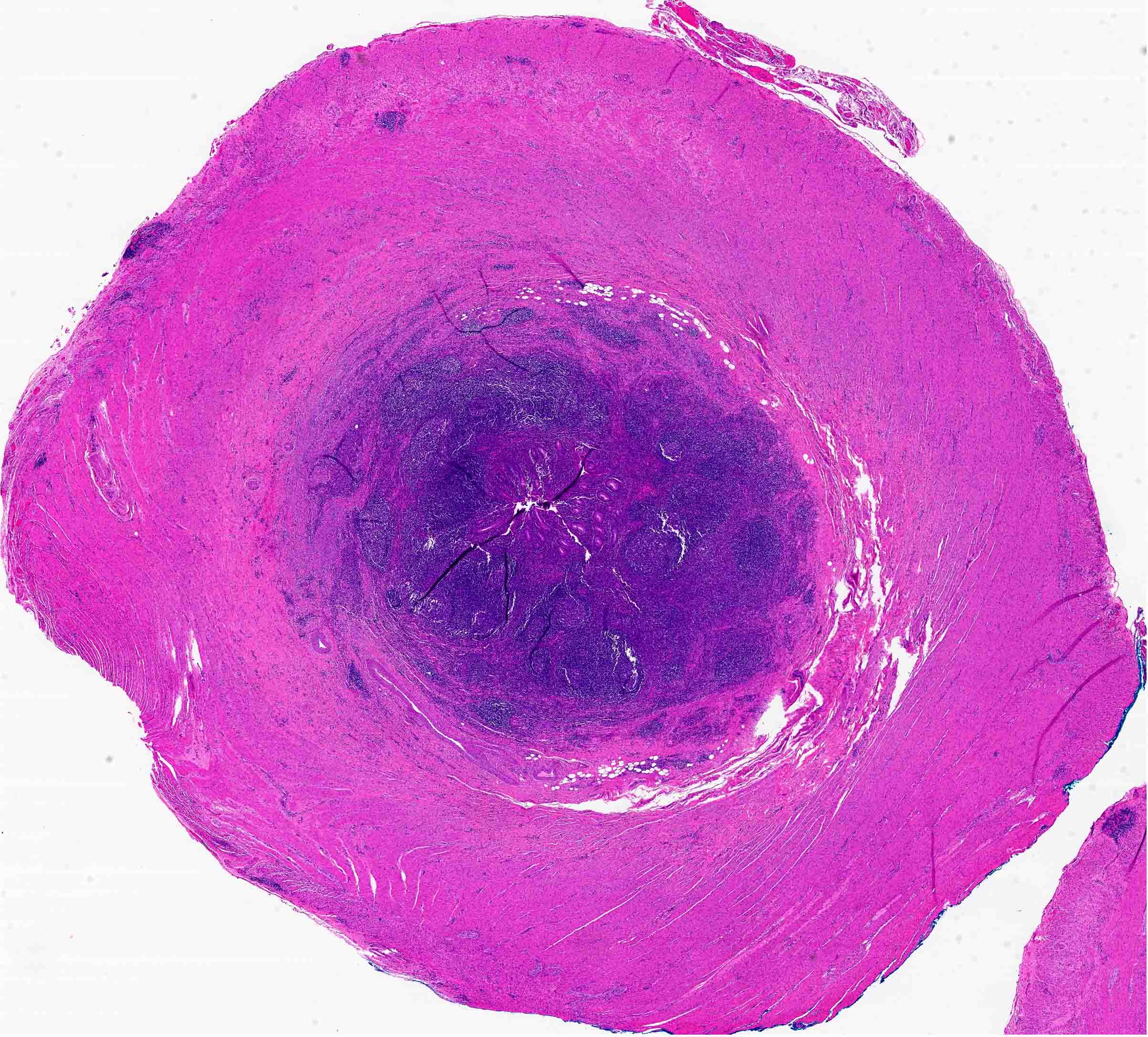
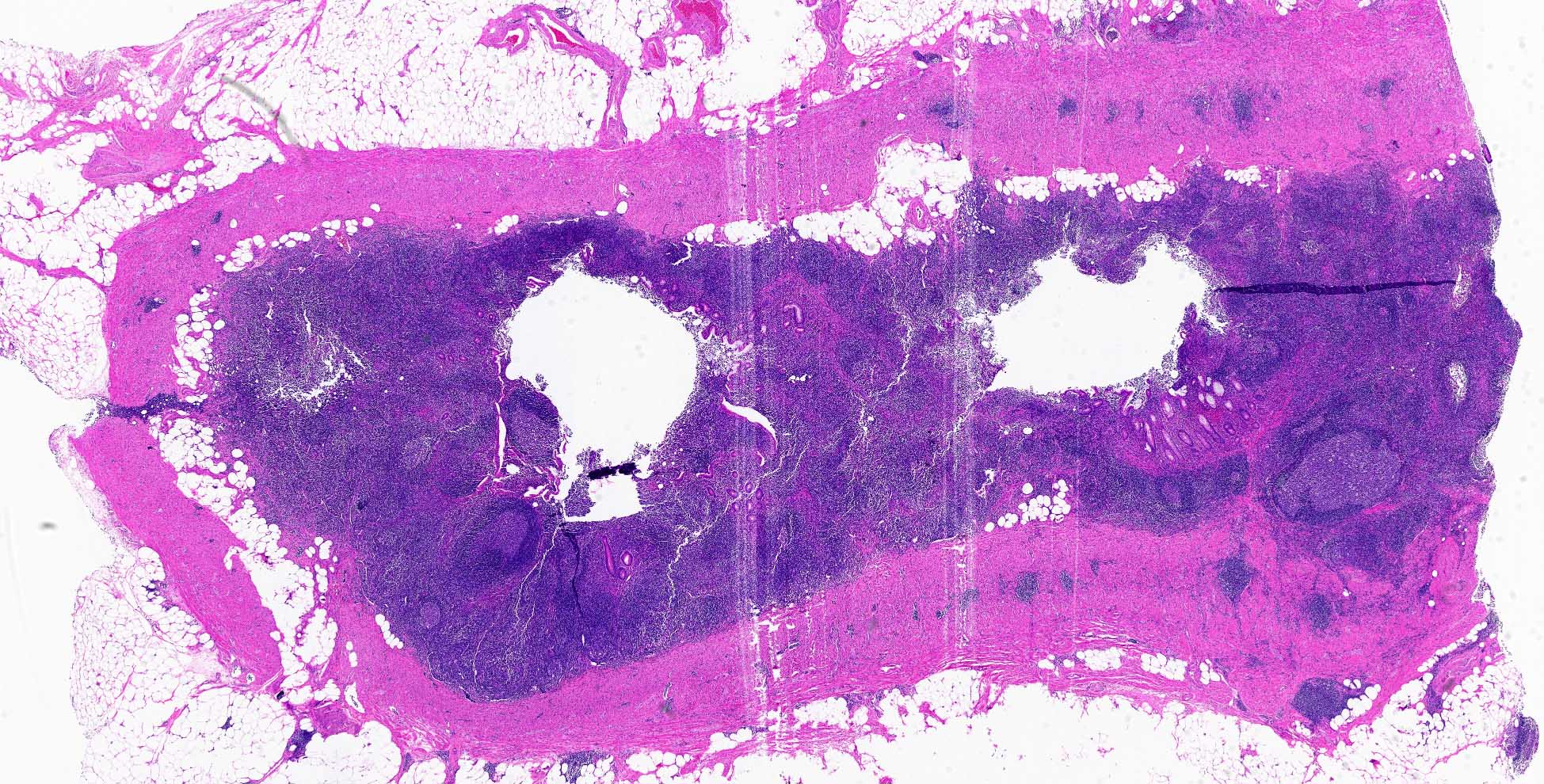
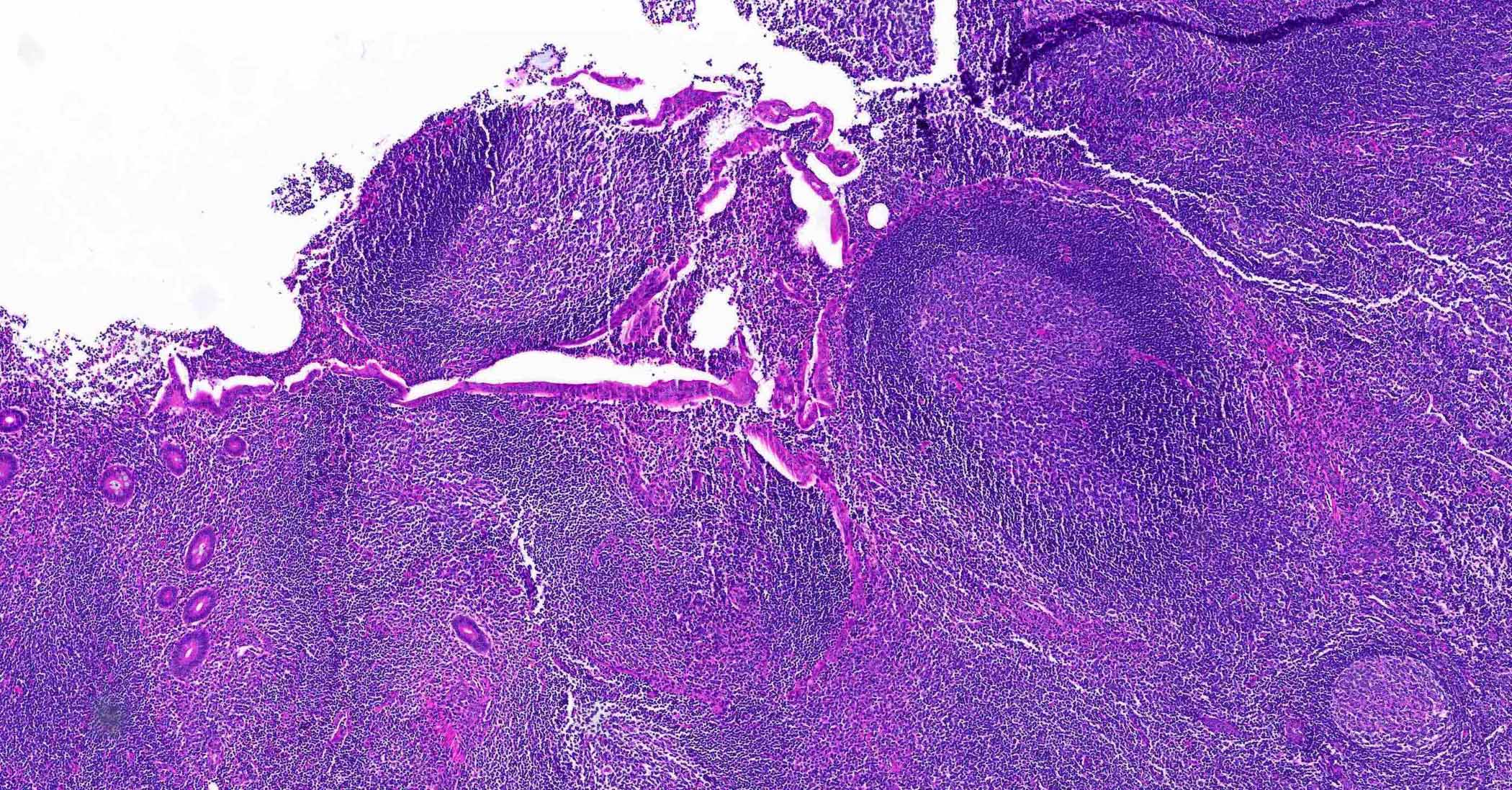
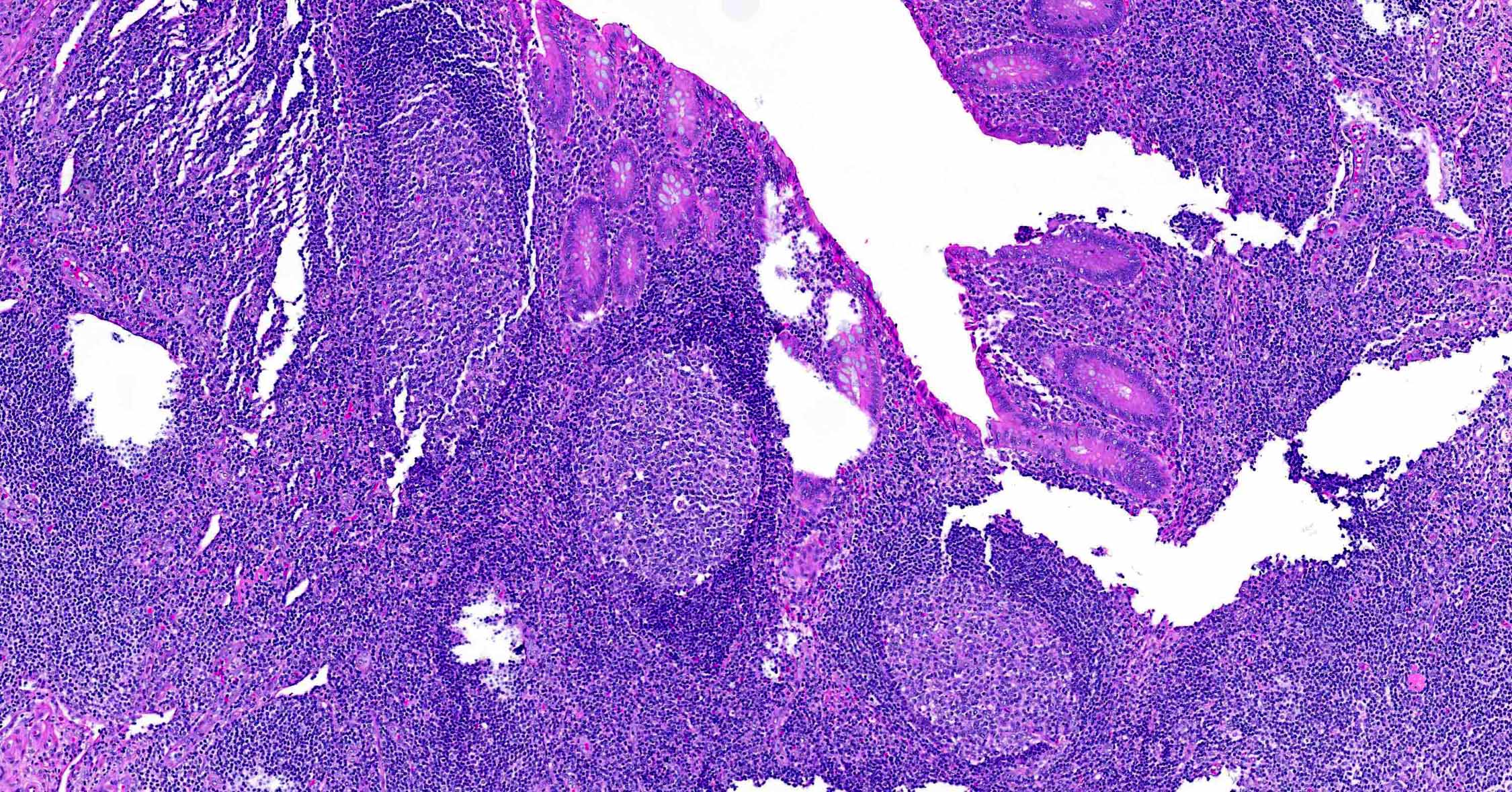
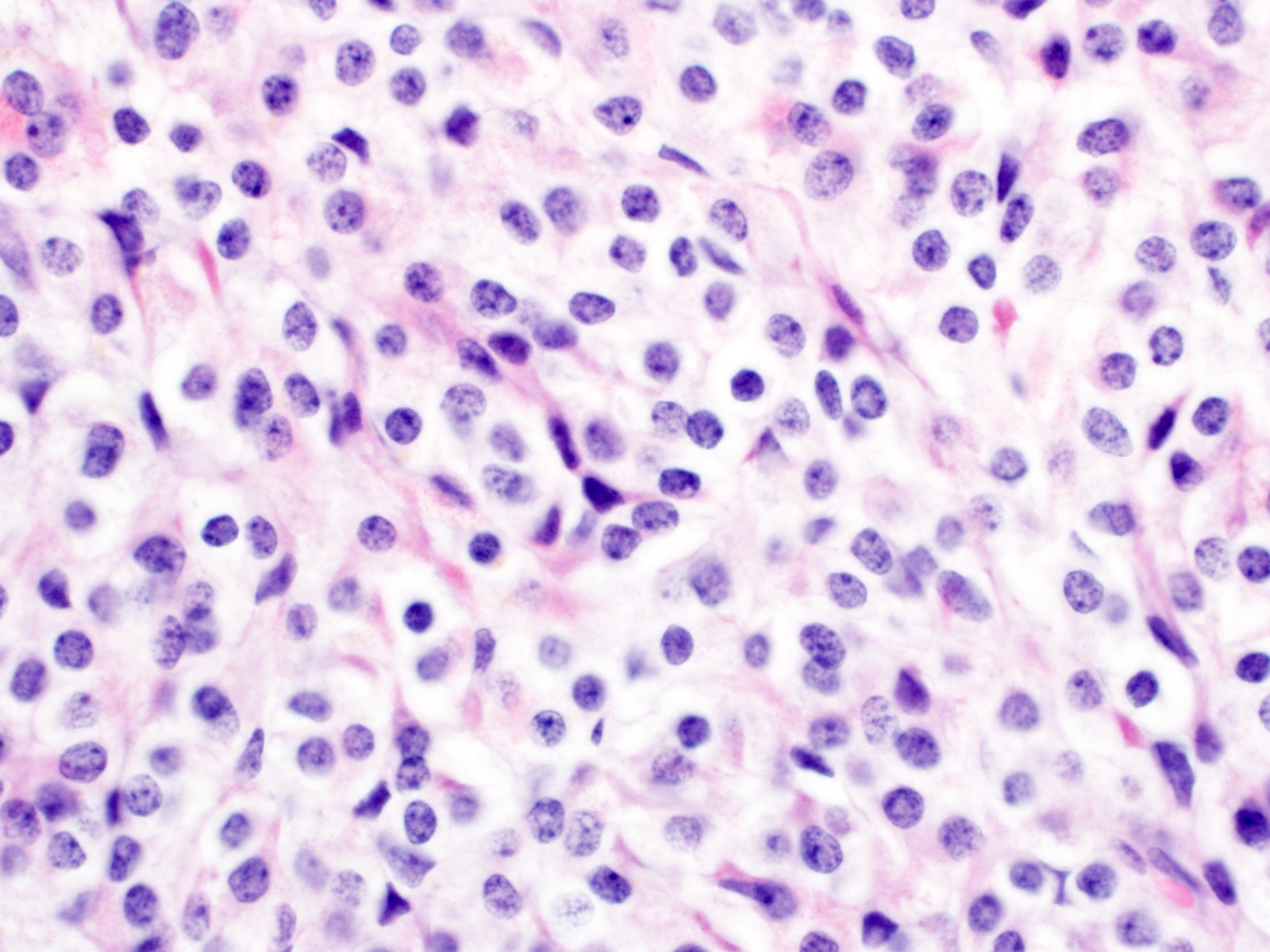
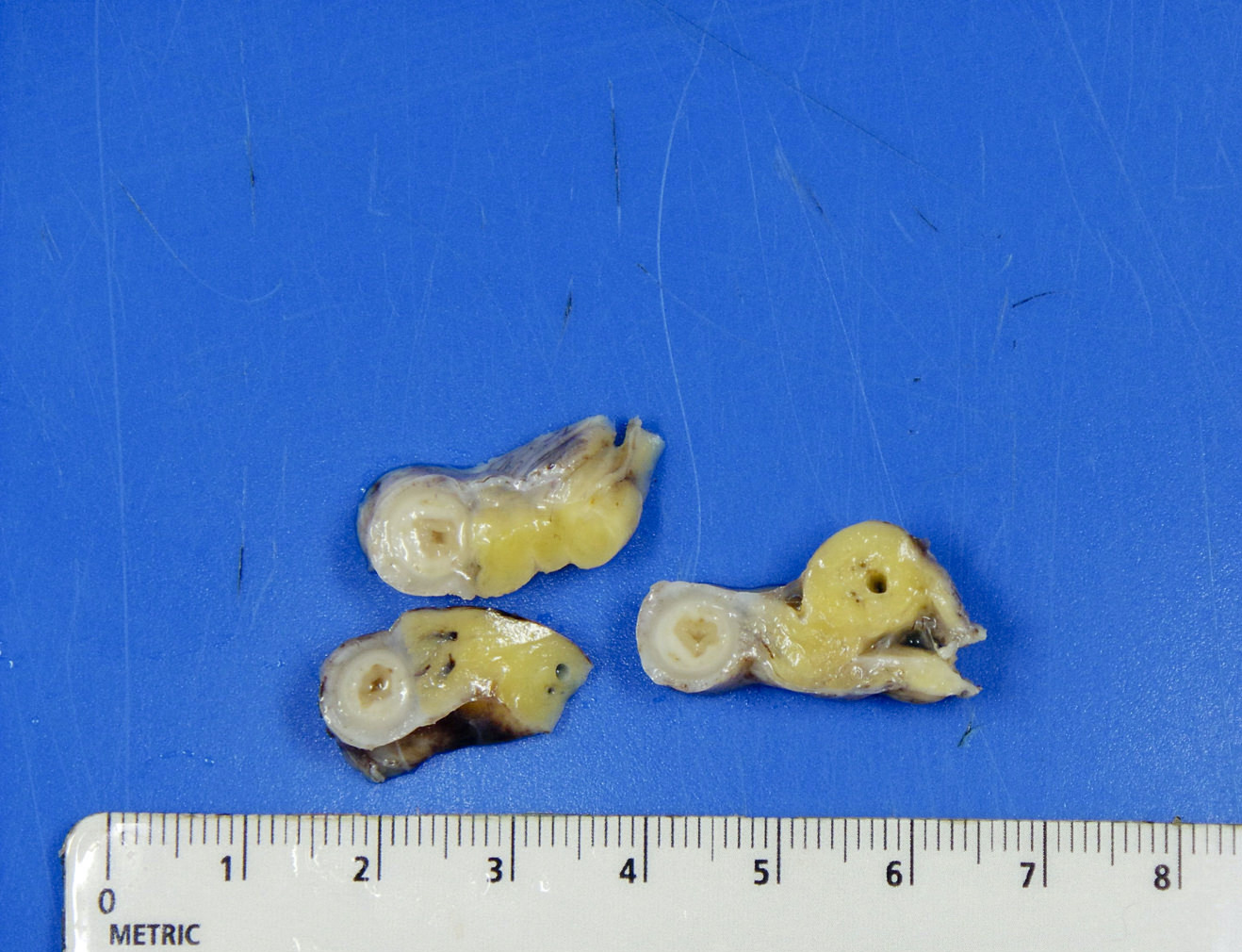
- TX: primary tumor cannot be assessed
- T0: no evidence of primary tumor
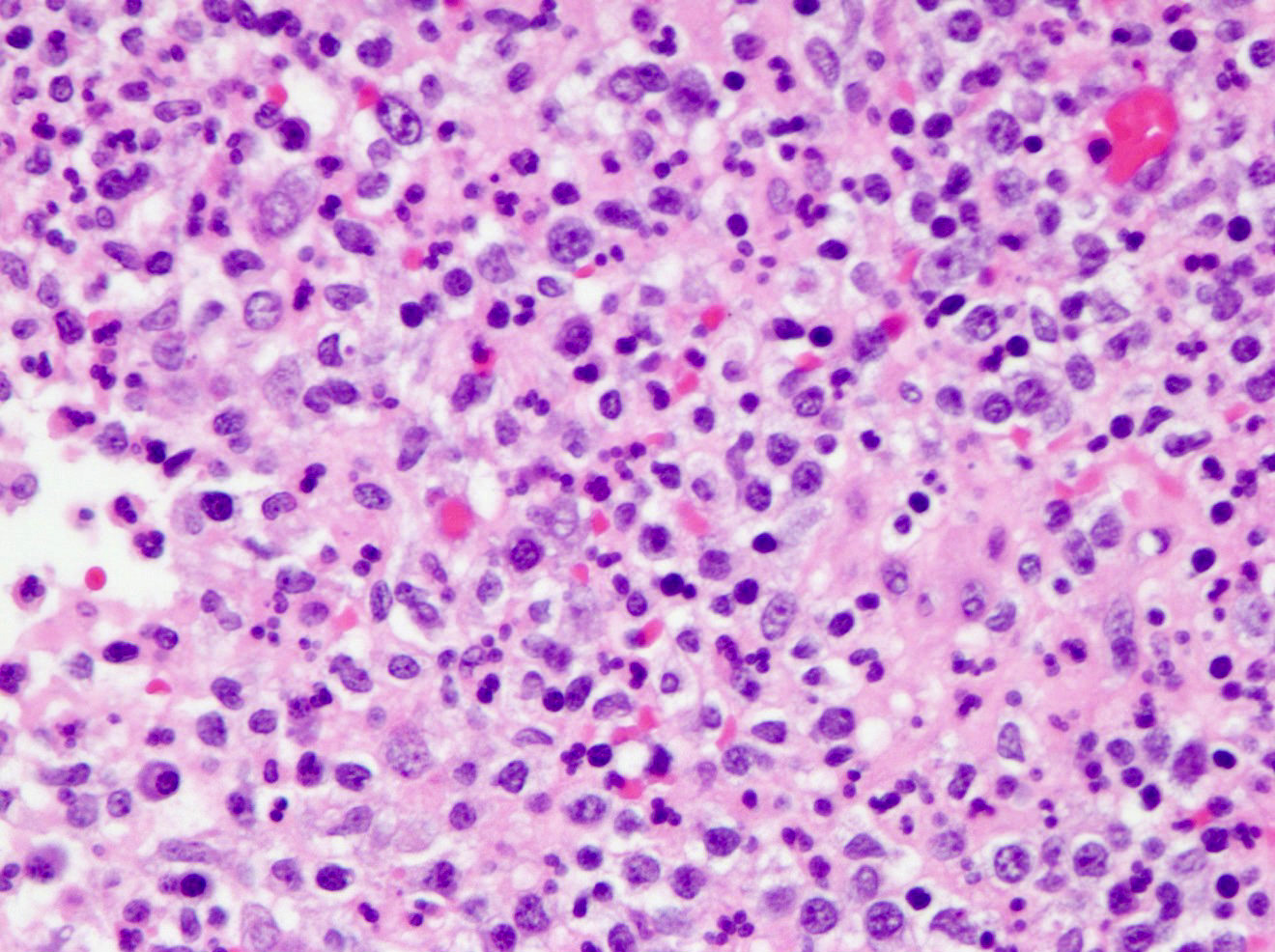
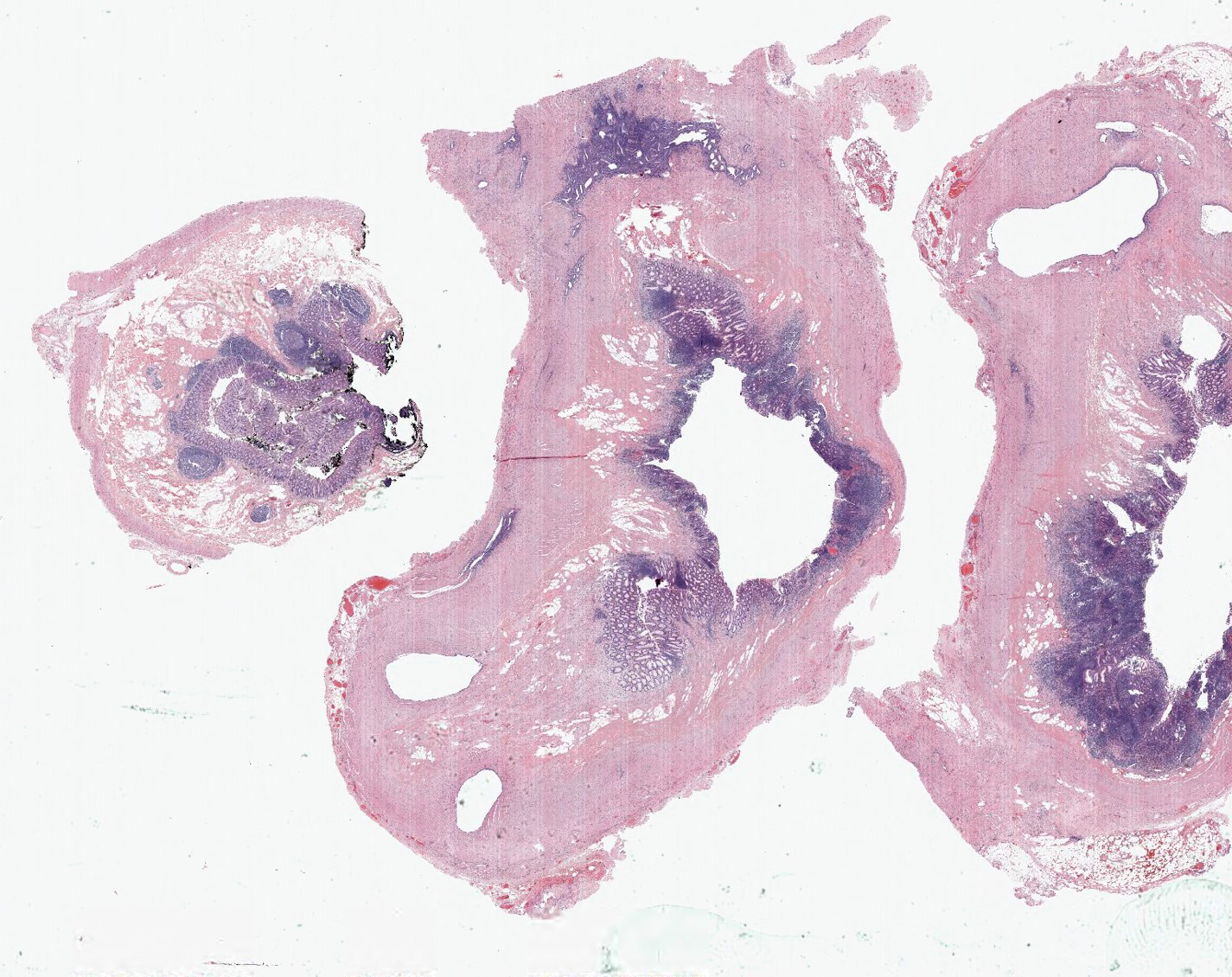
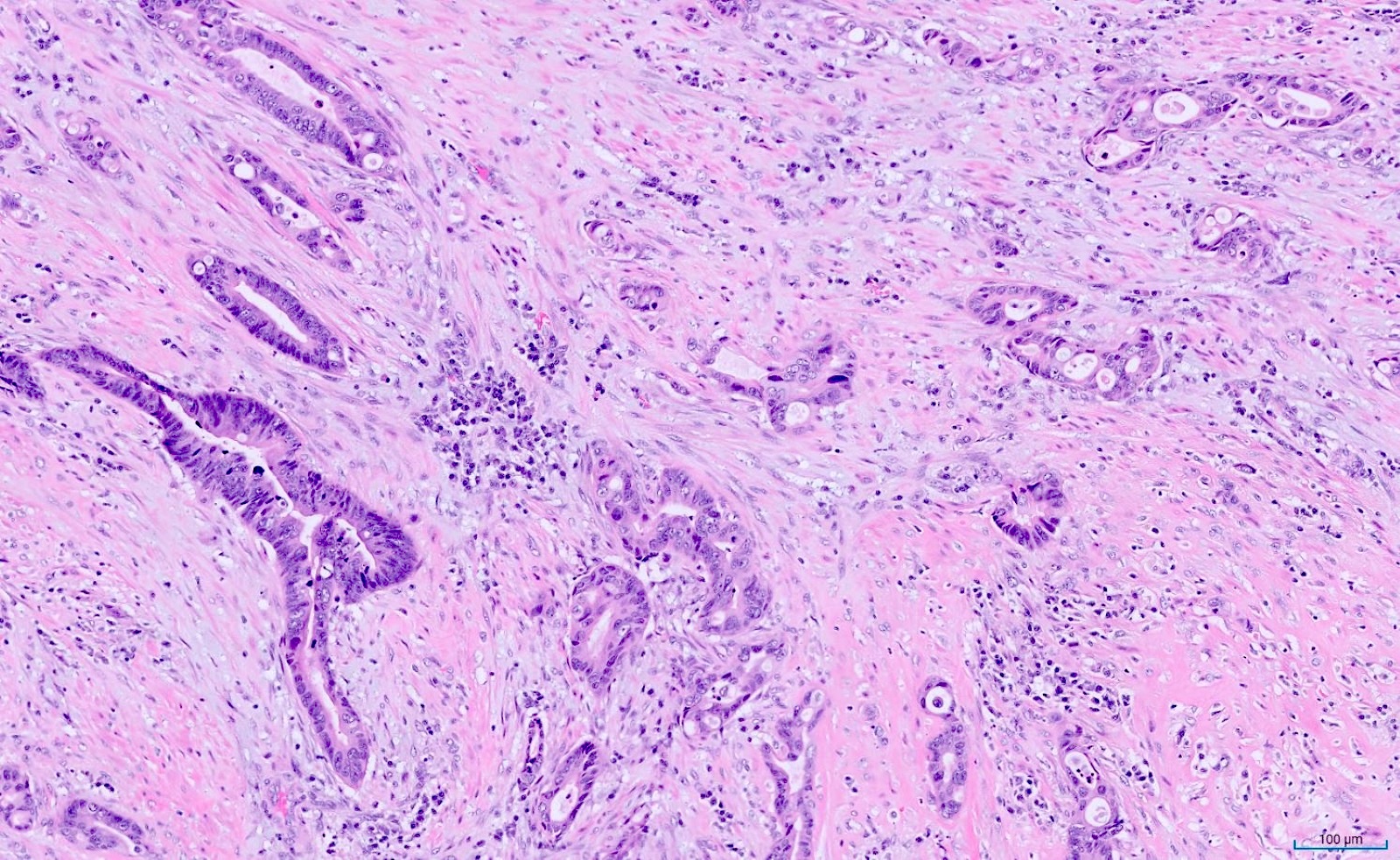
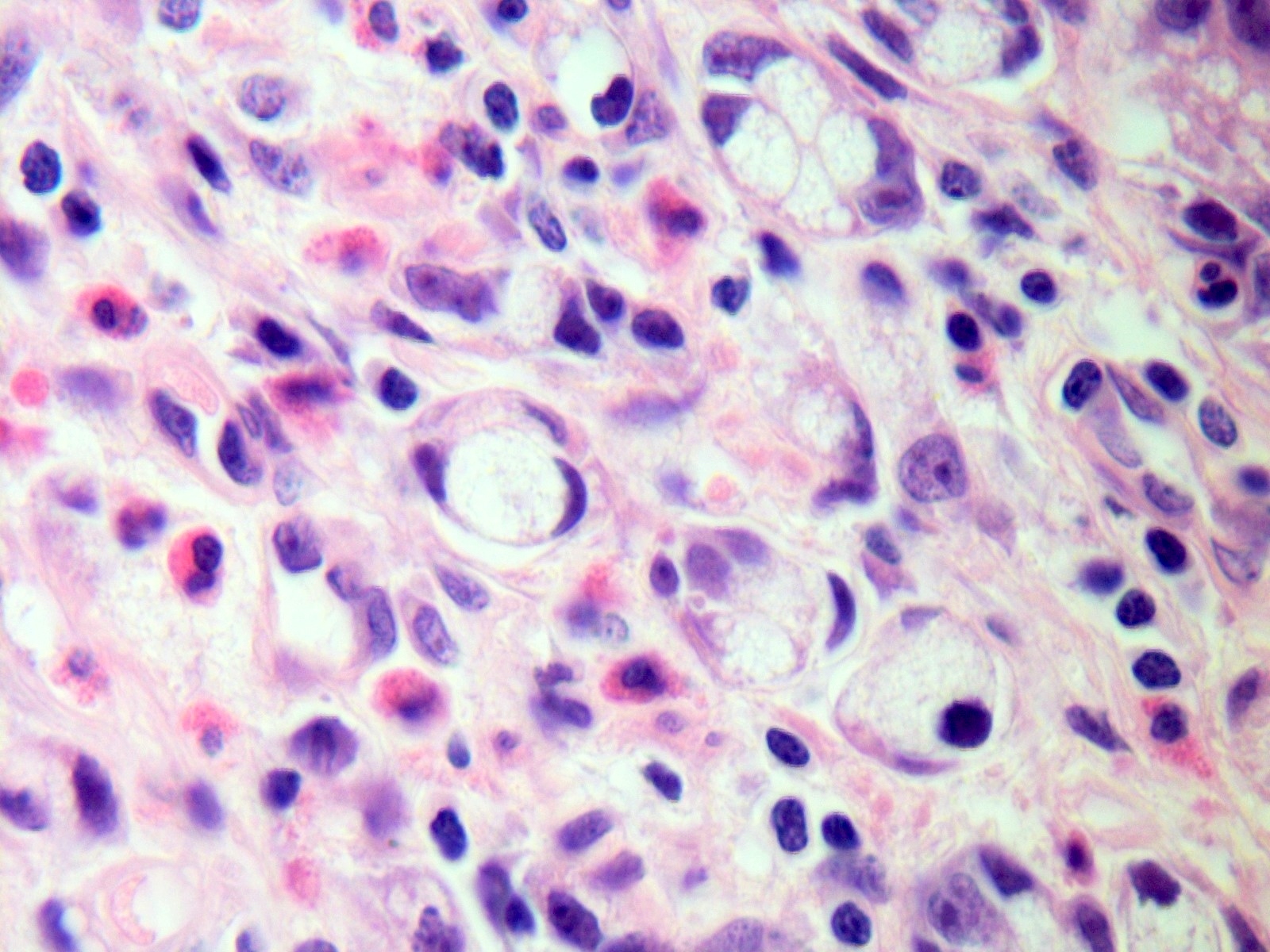
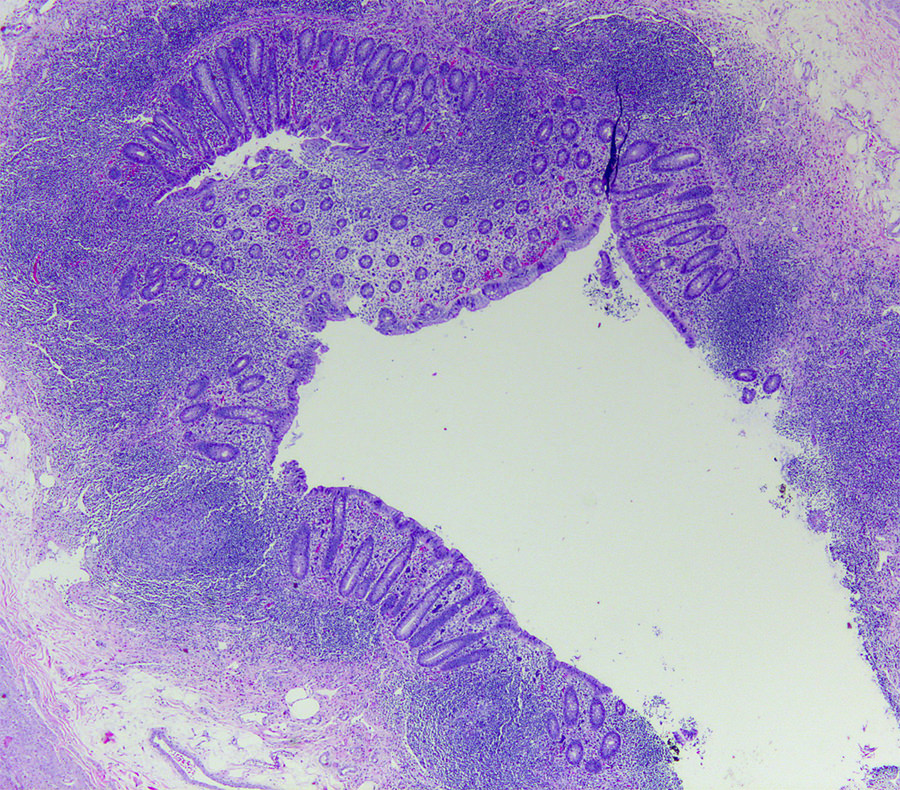
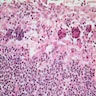
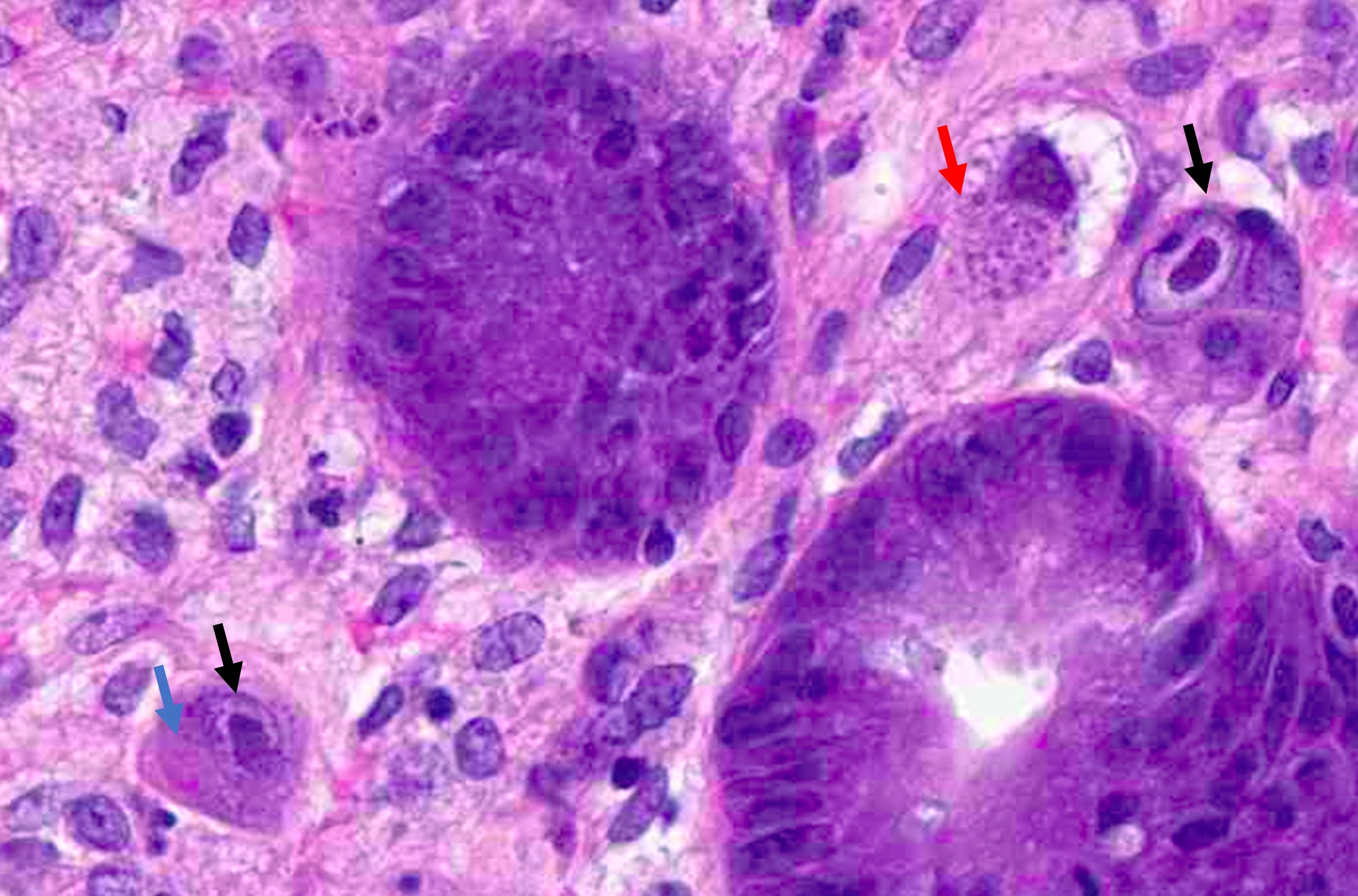
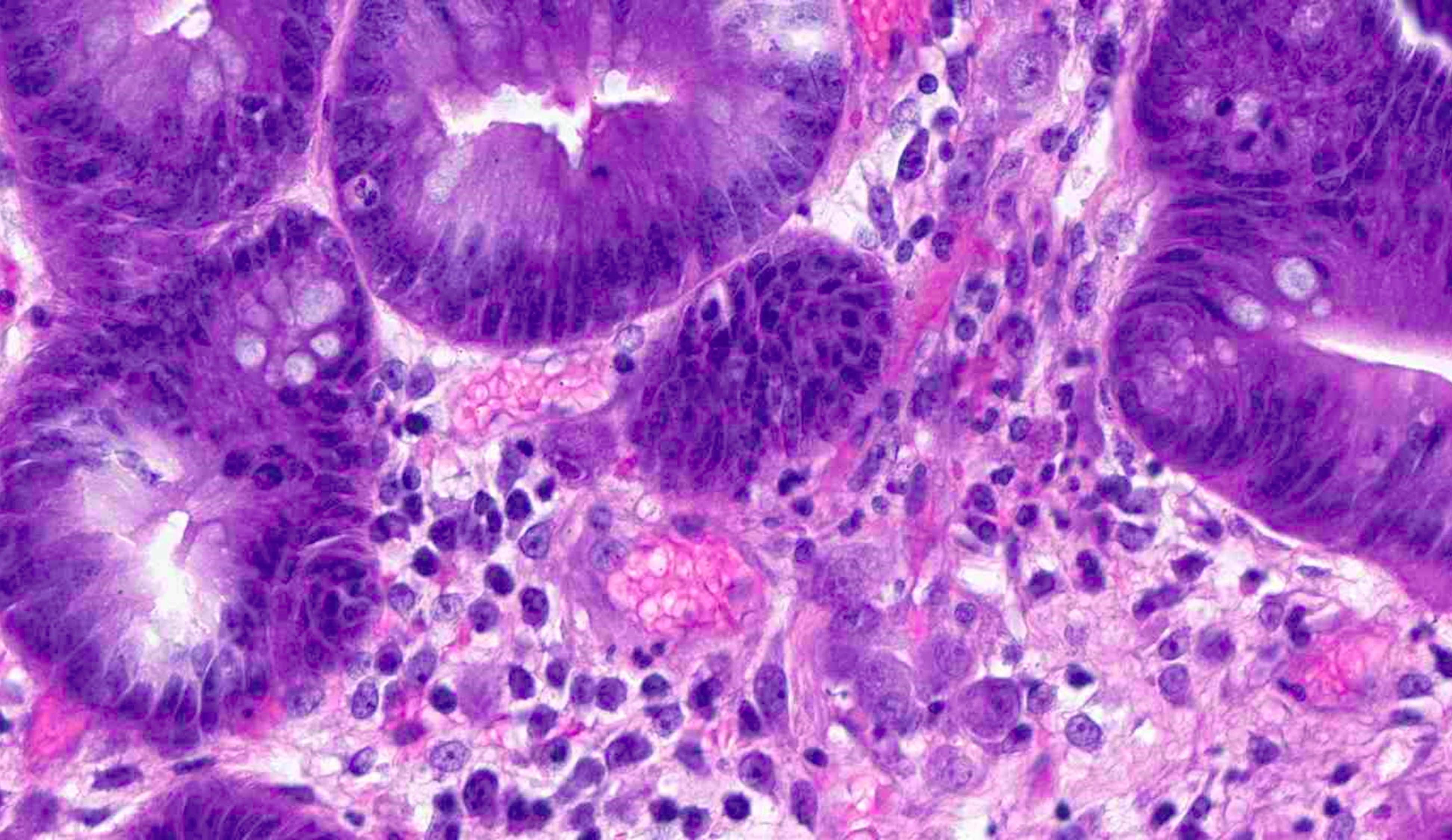
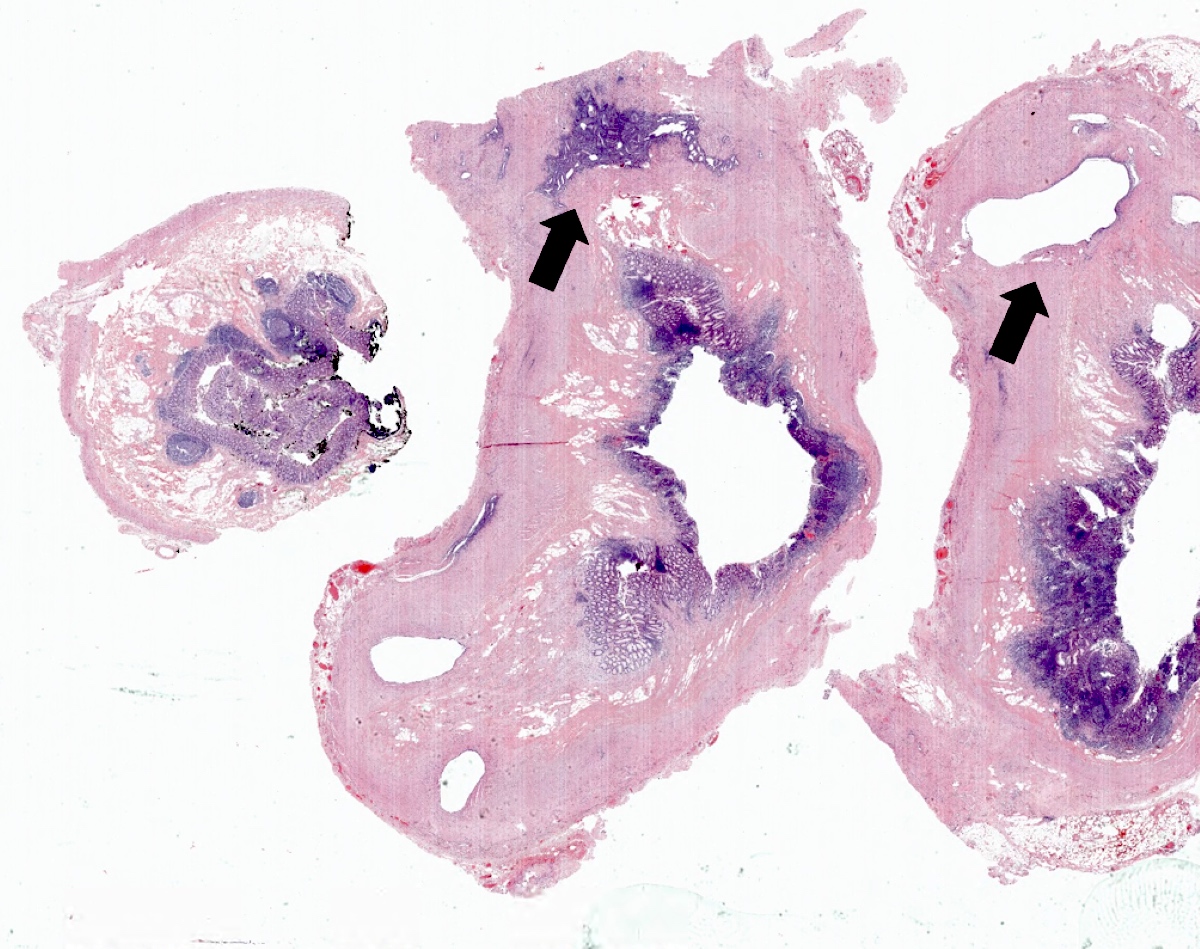
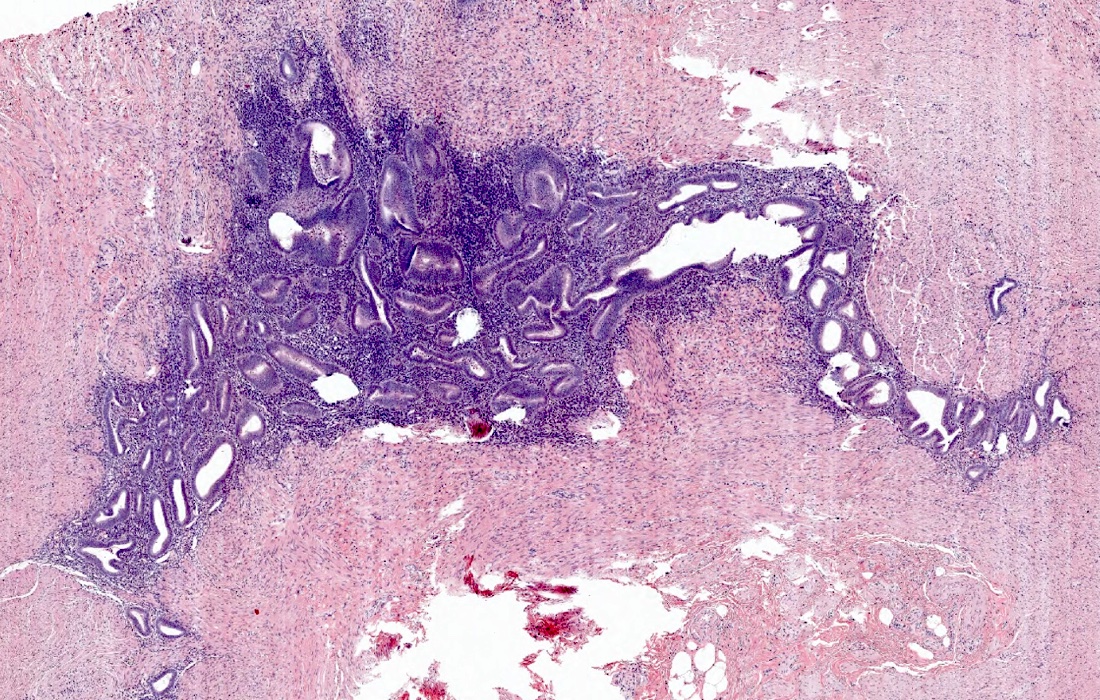
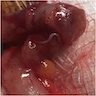
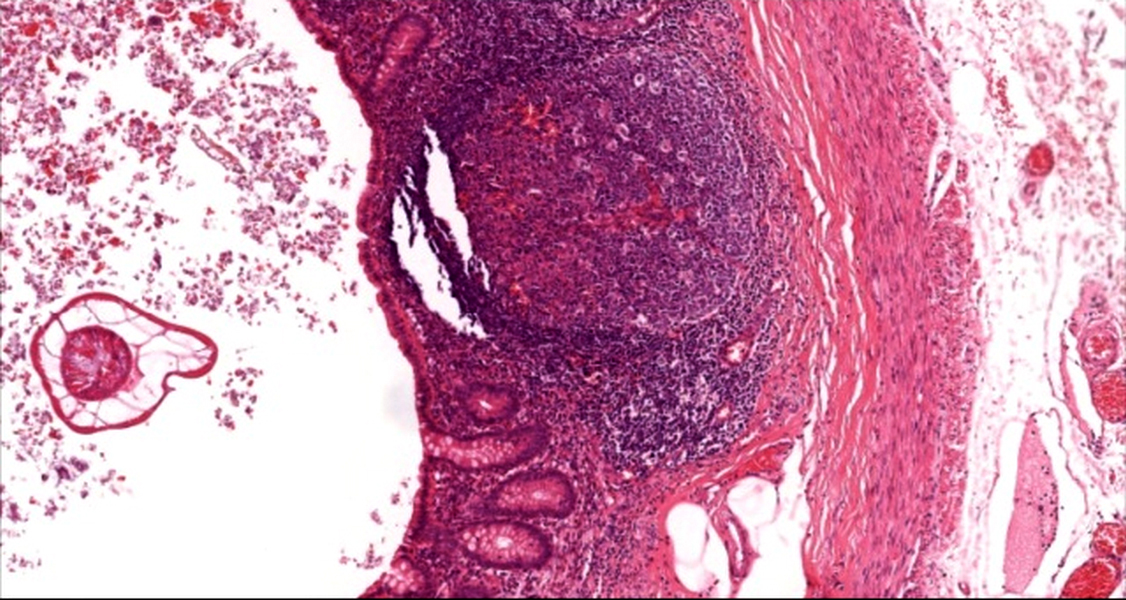
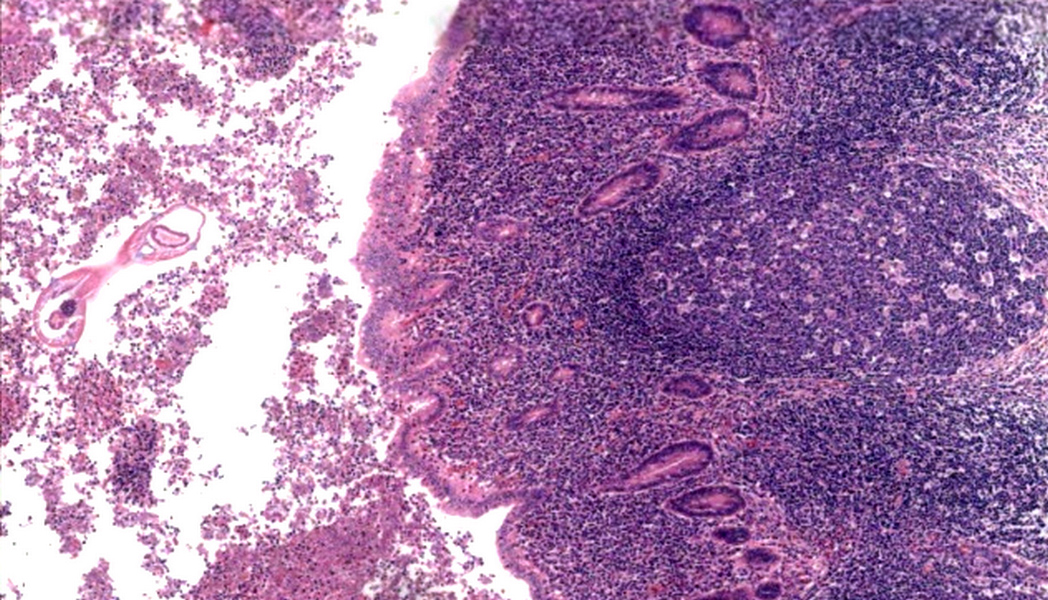
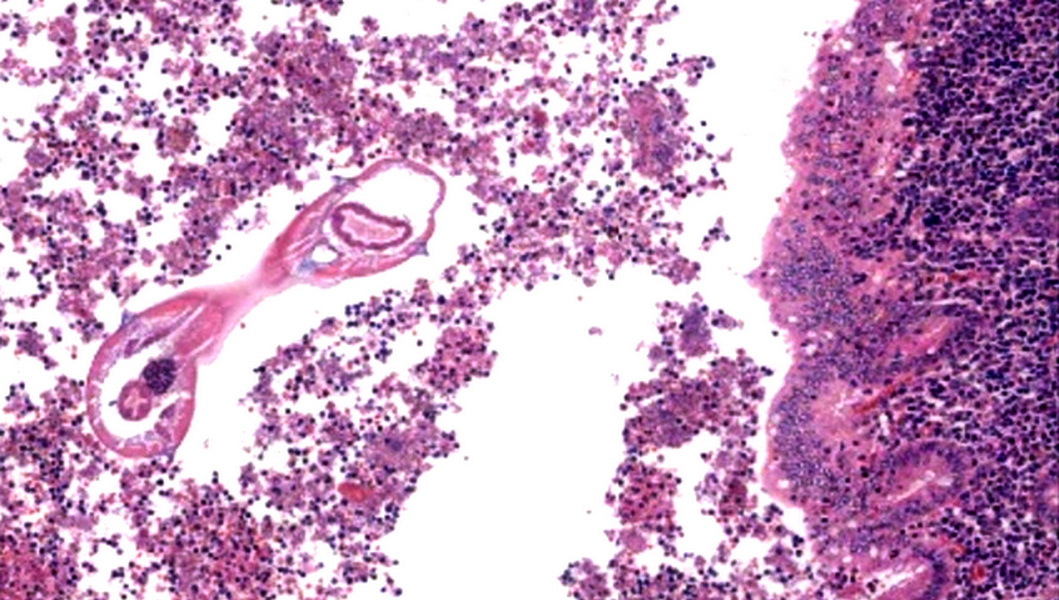
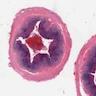
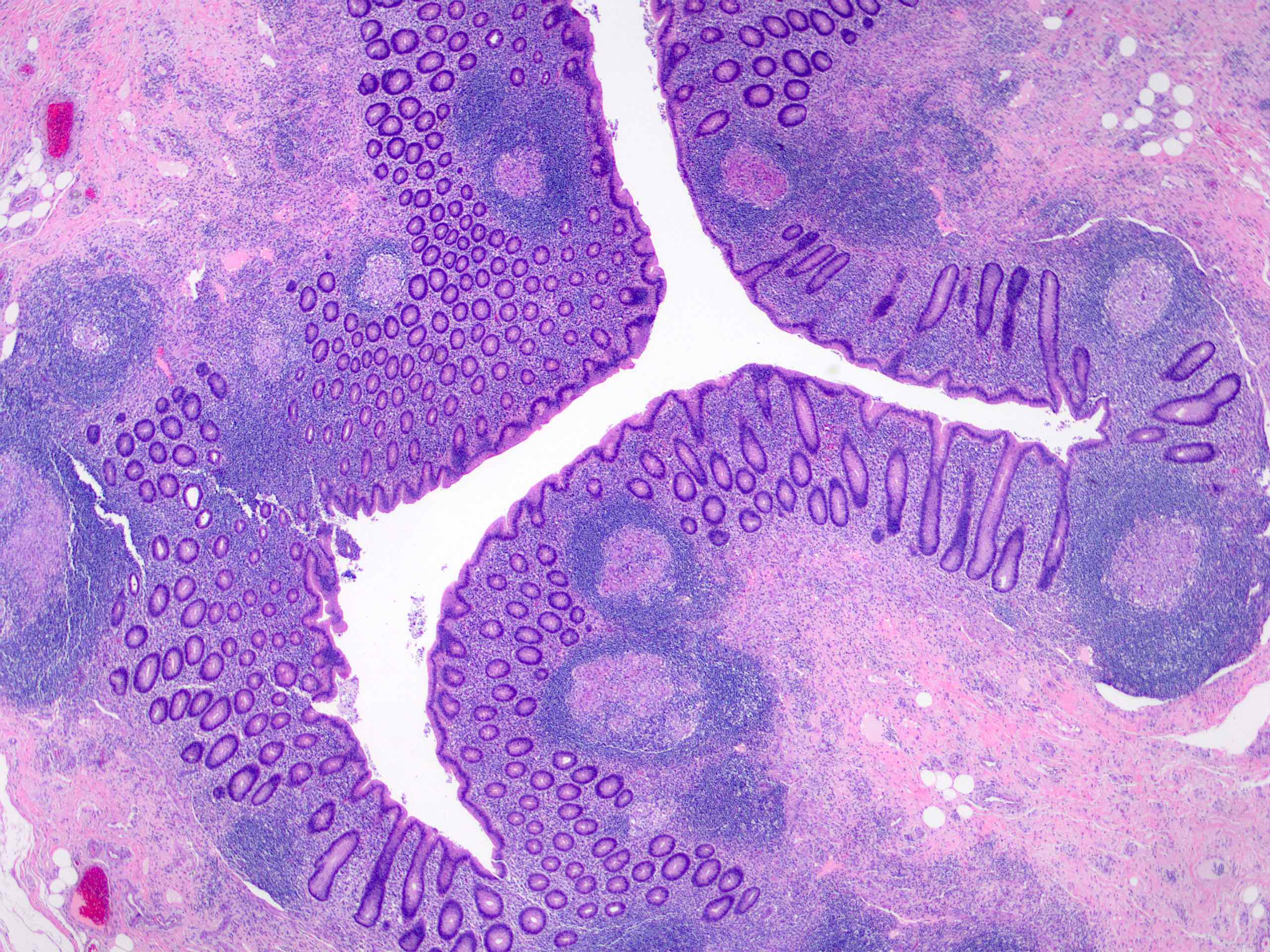
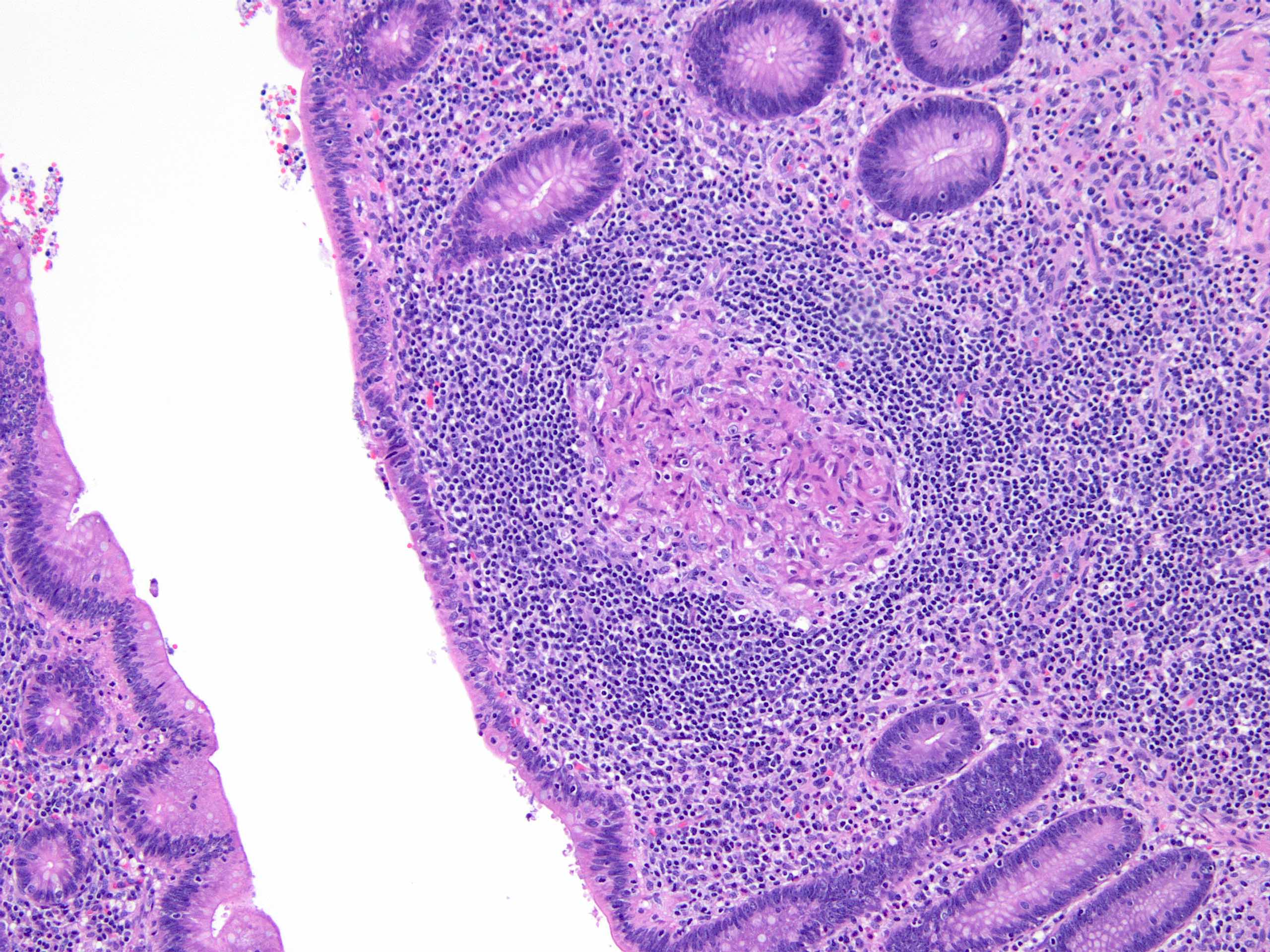
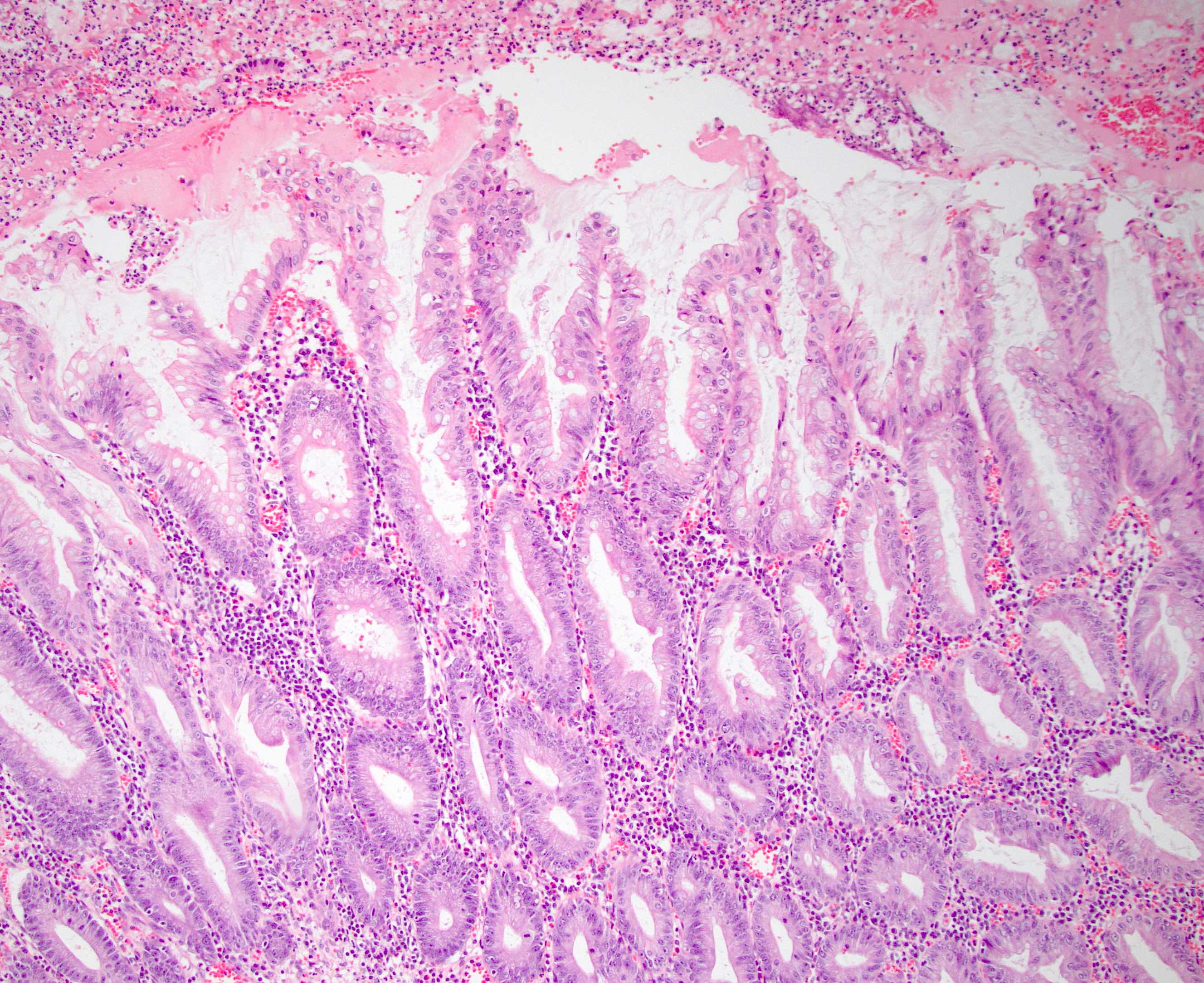
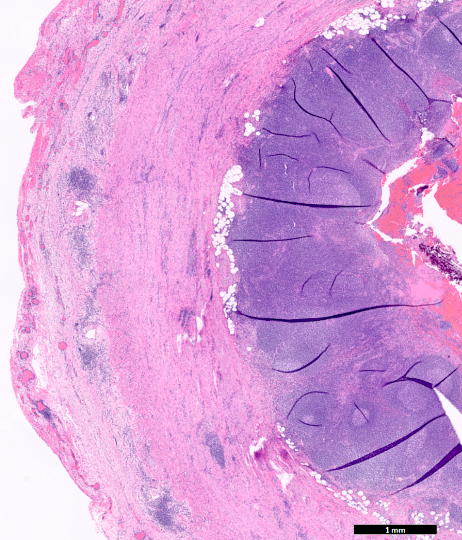
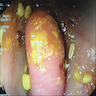
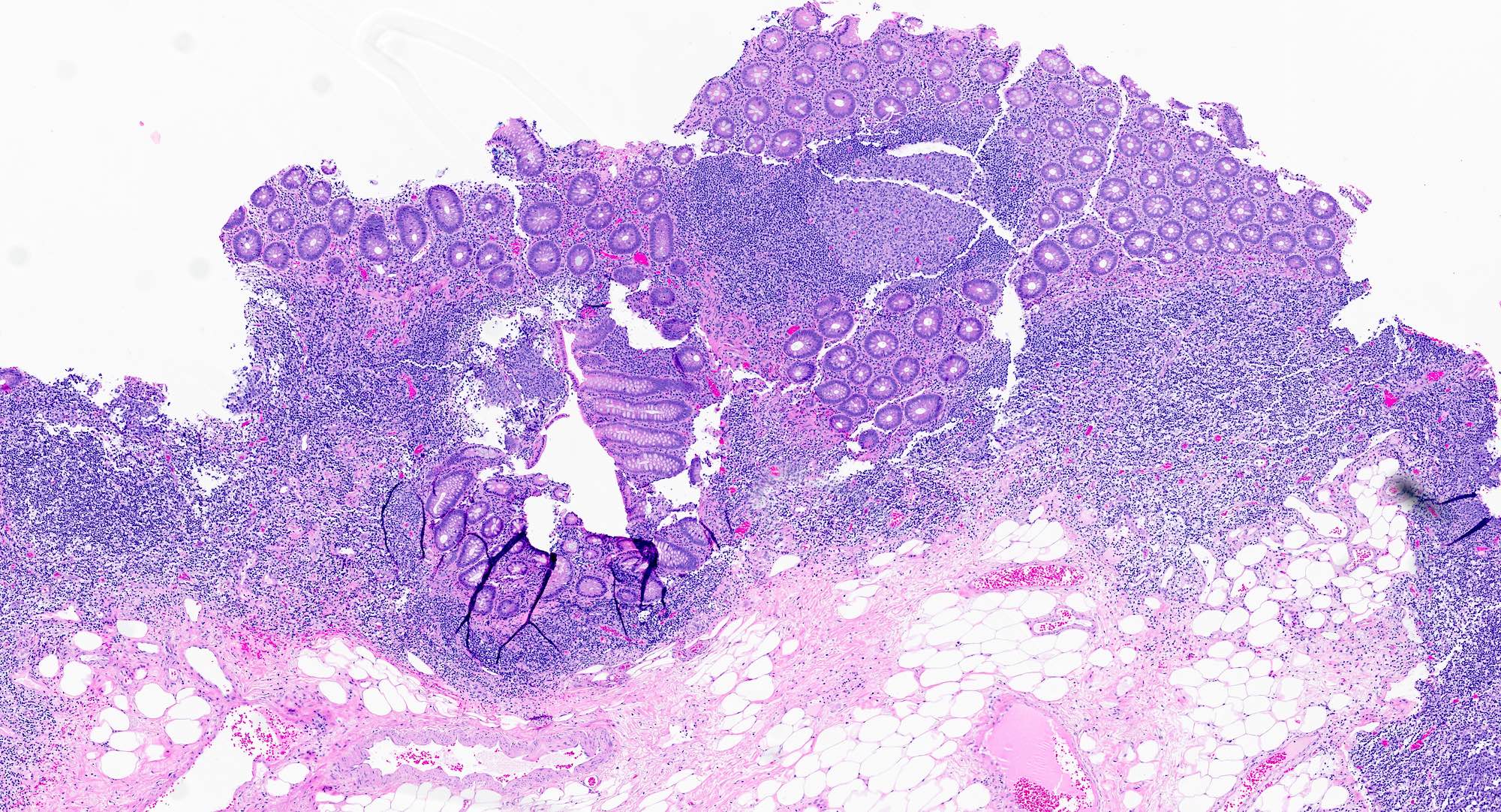
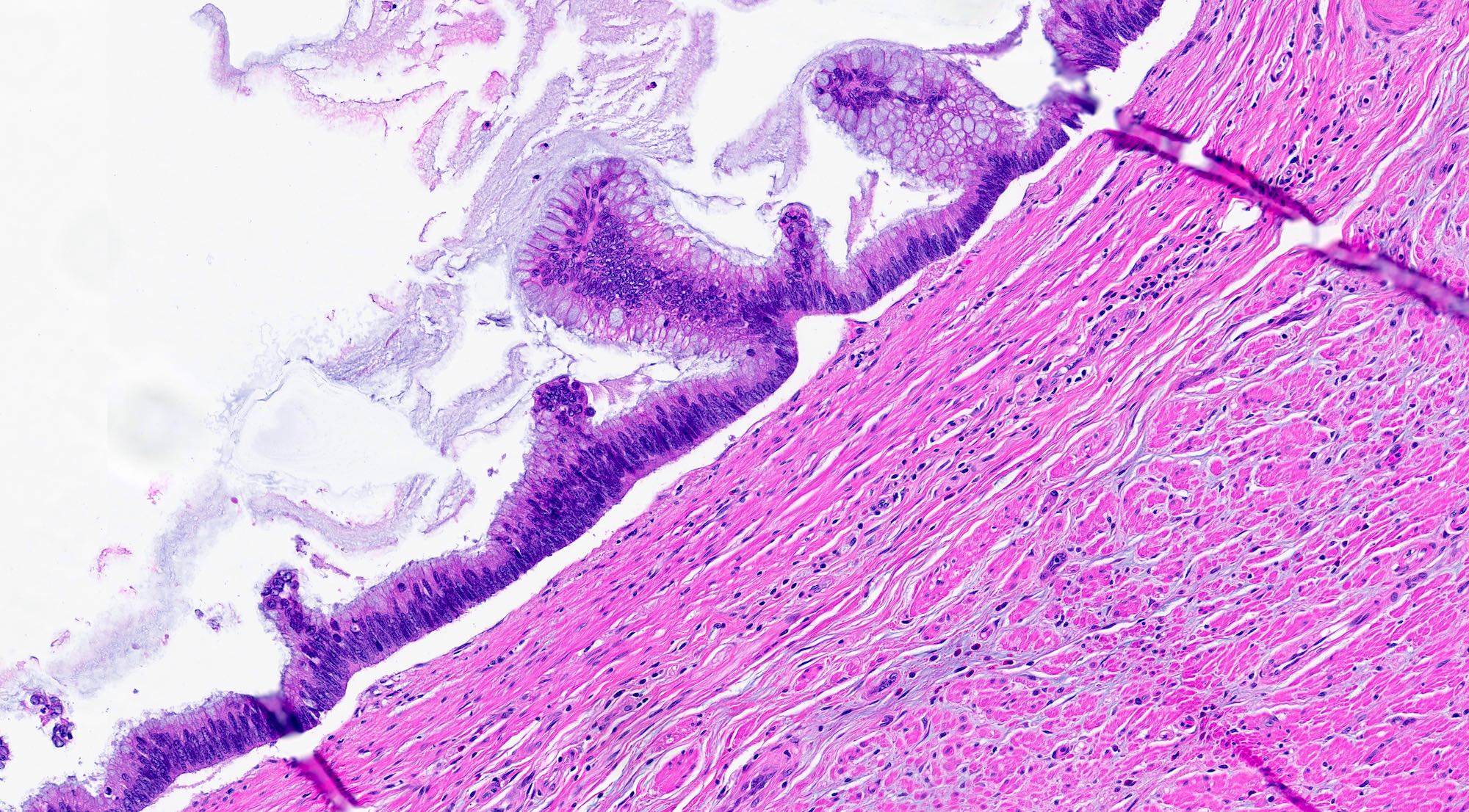
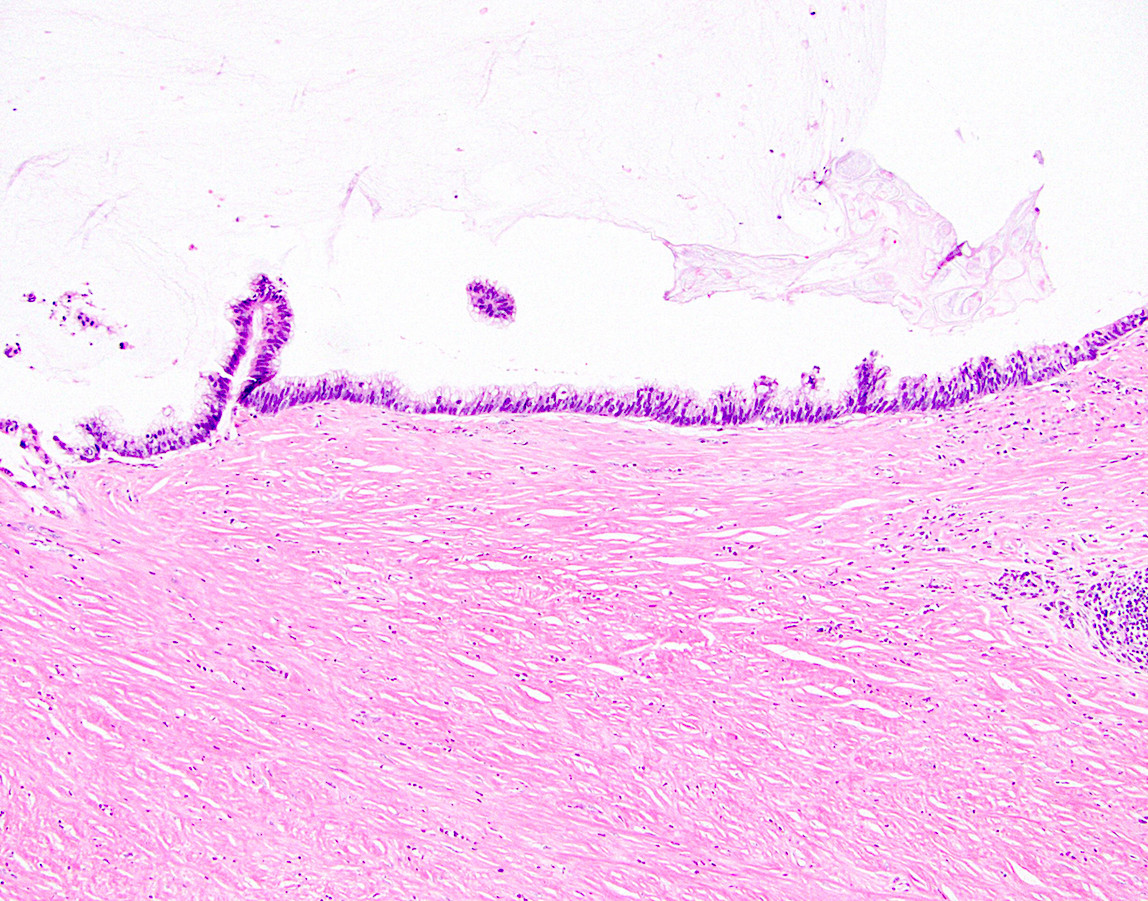
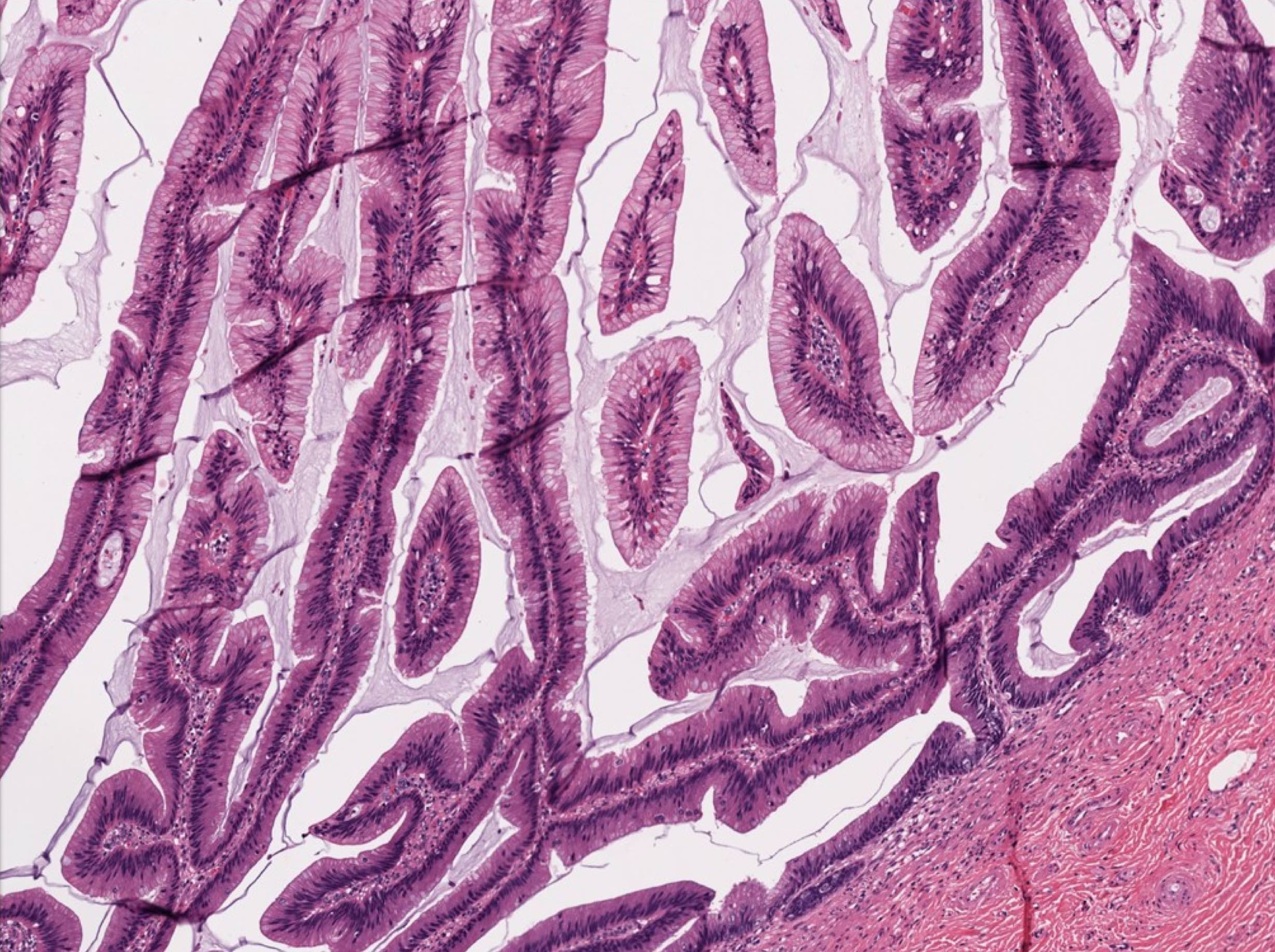
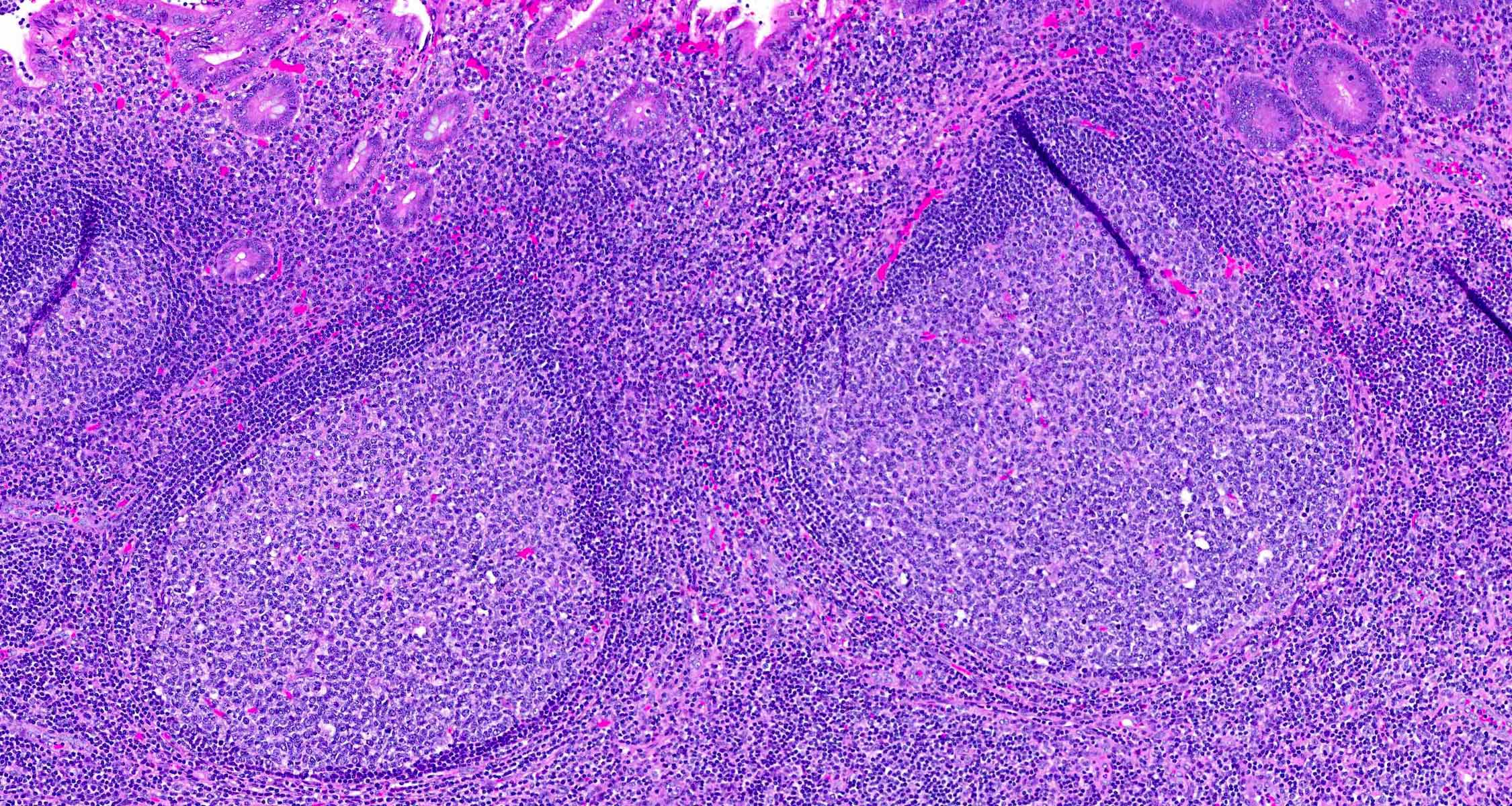
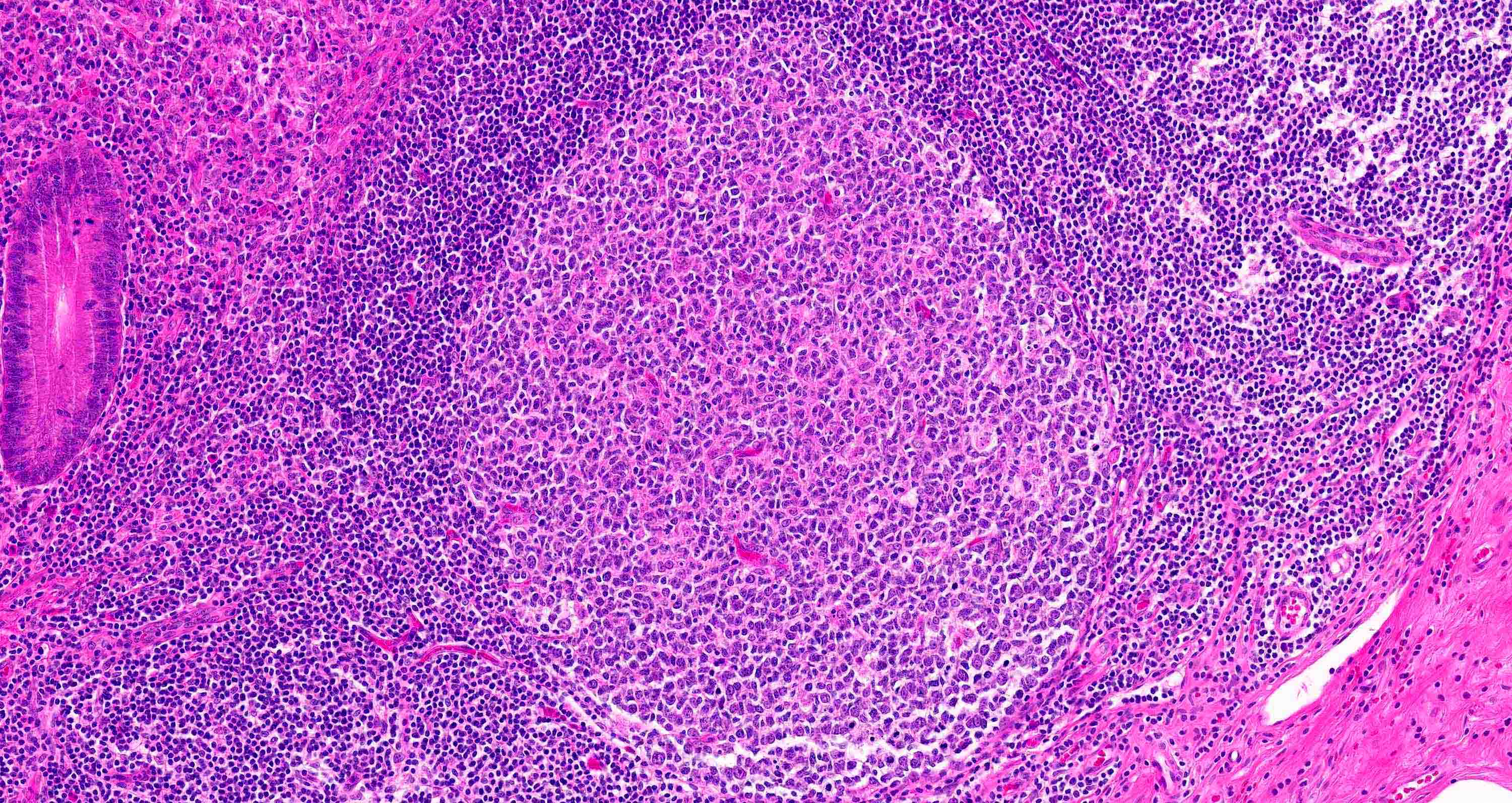
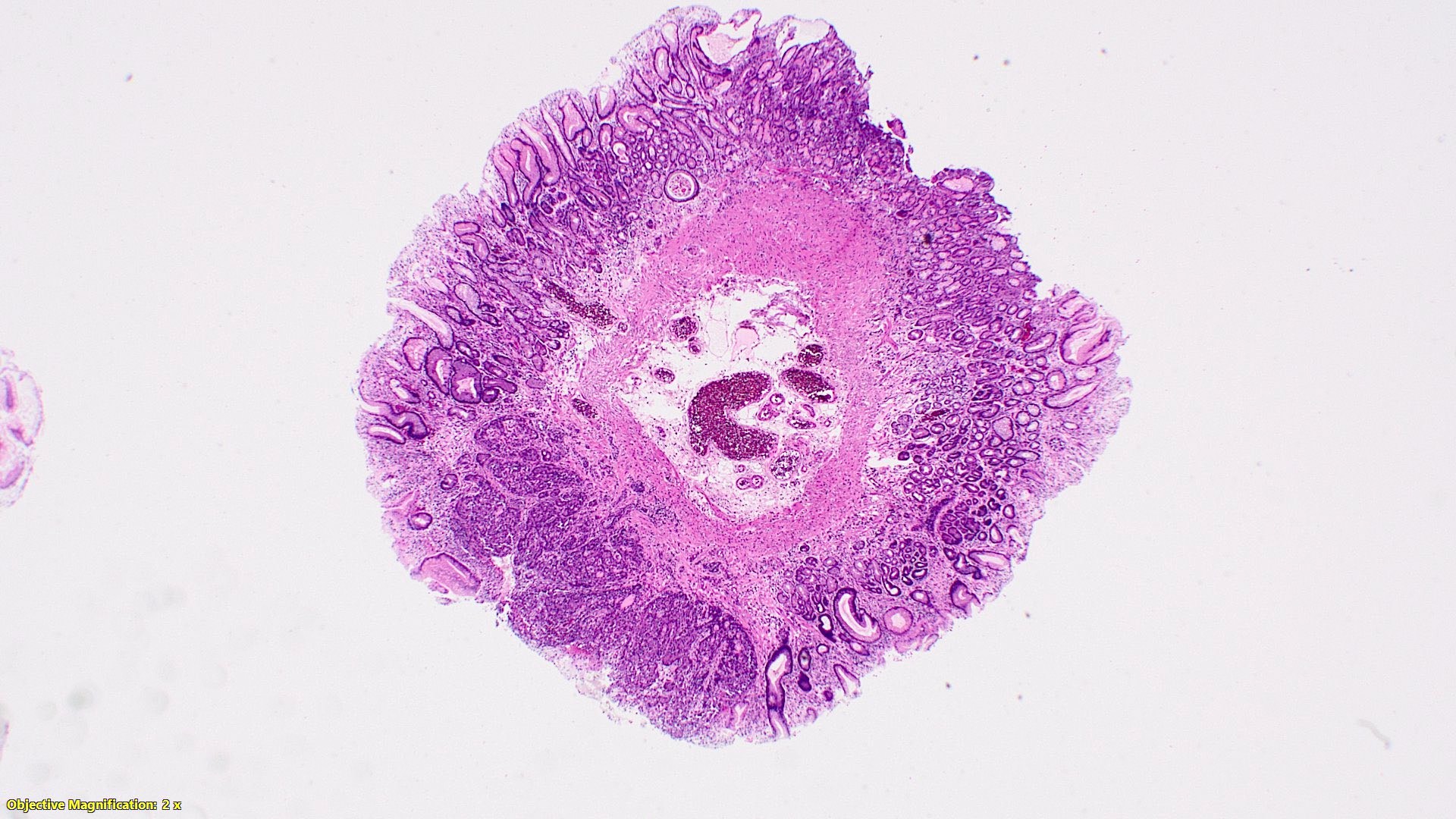
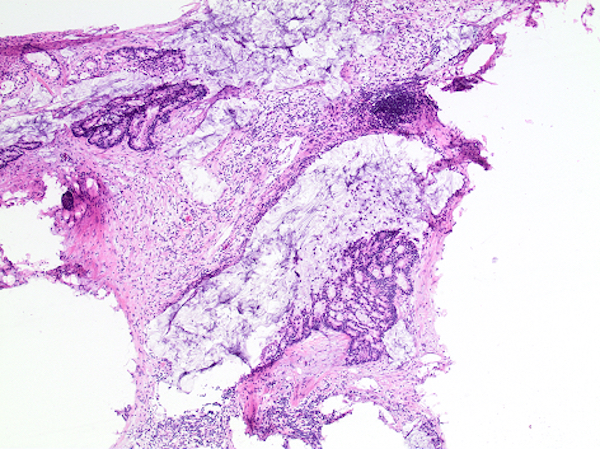
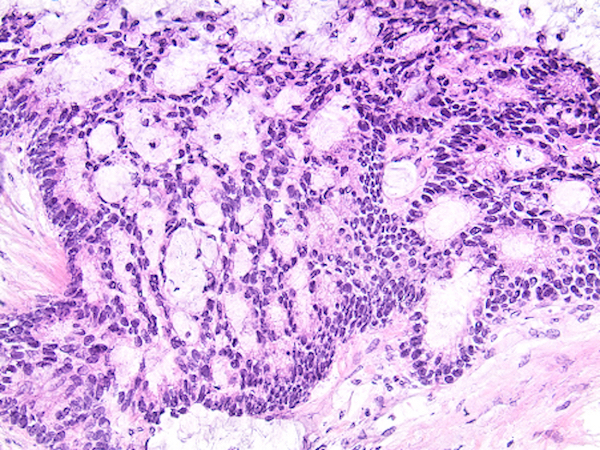
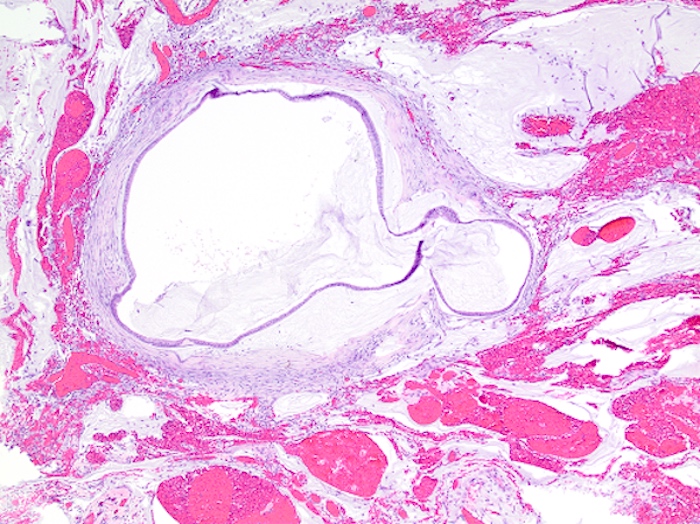
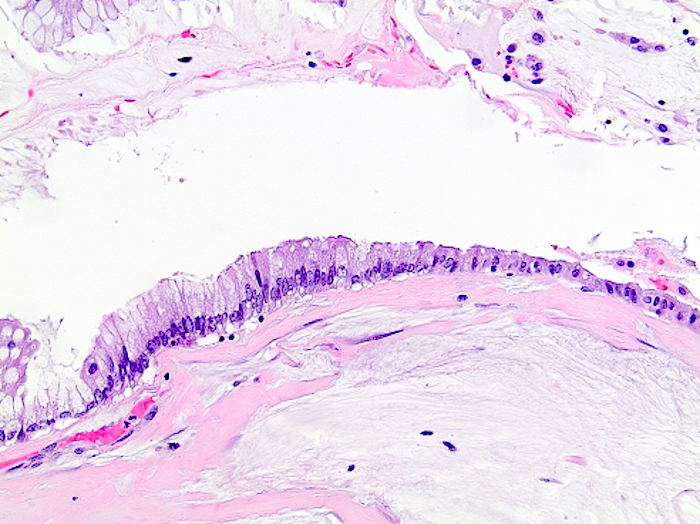
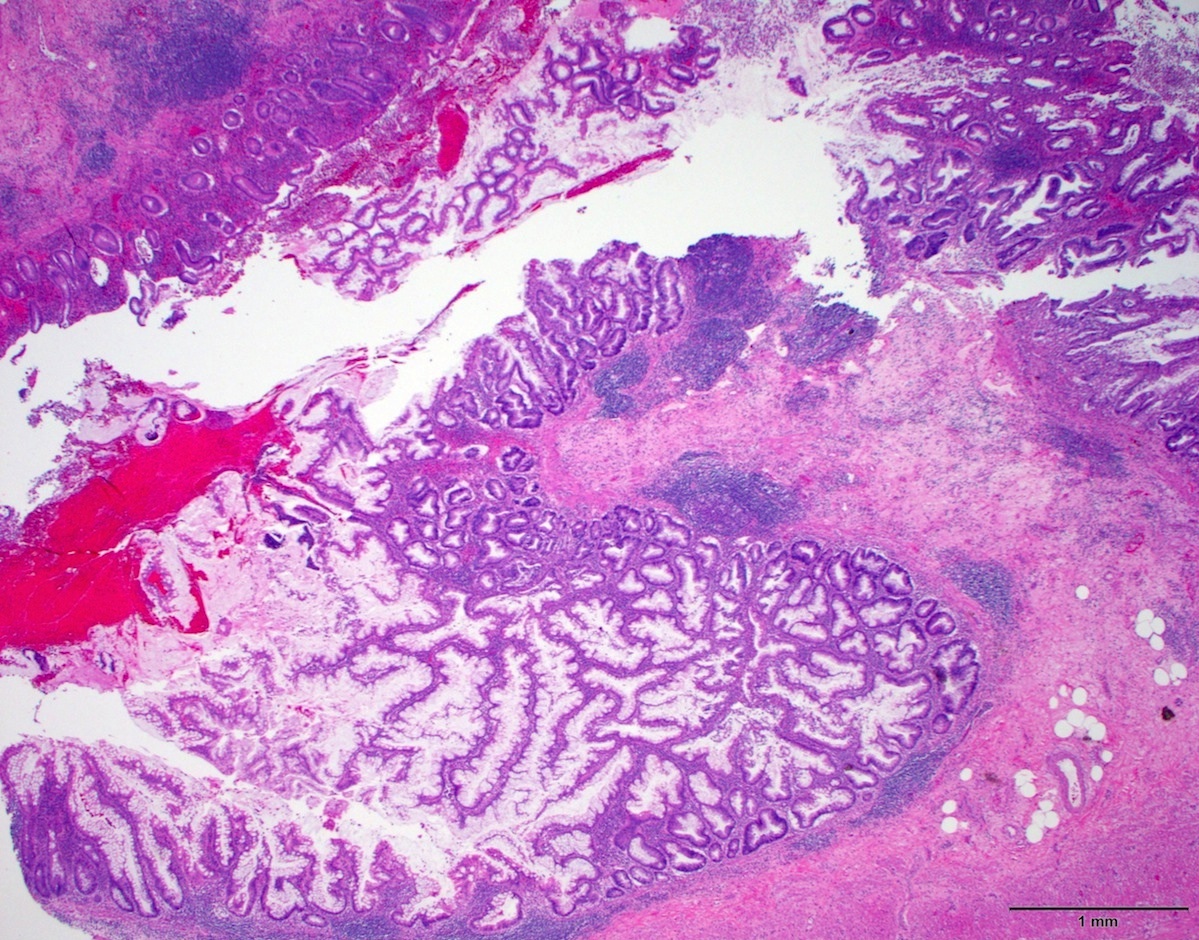
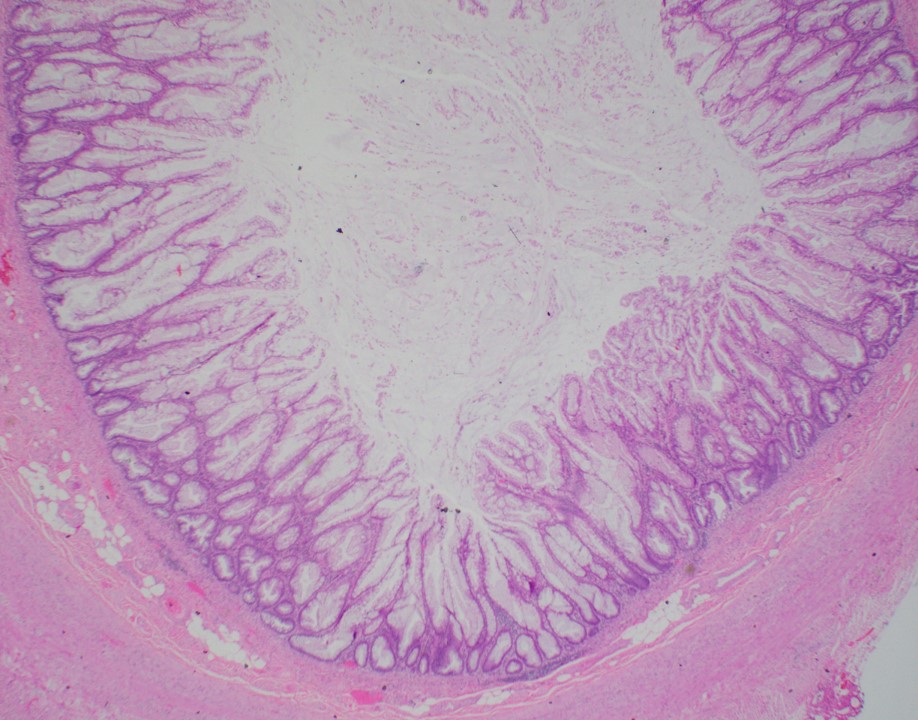
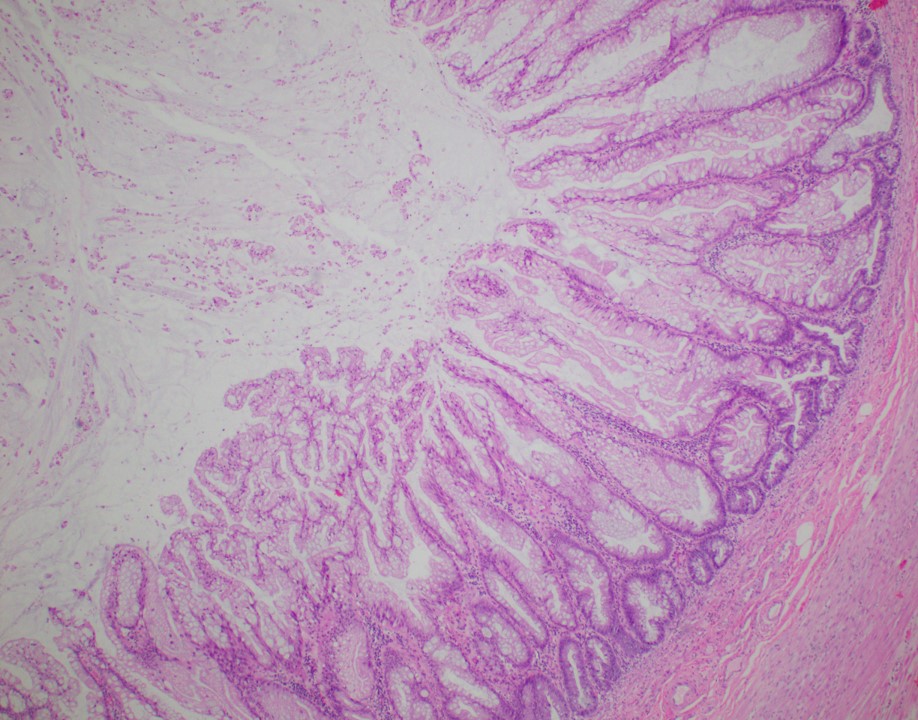
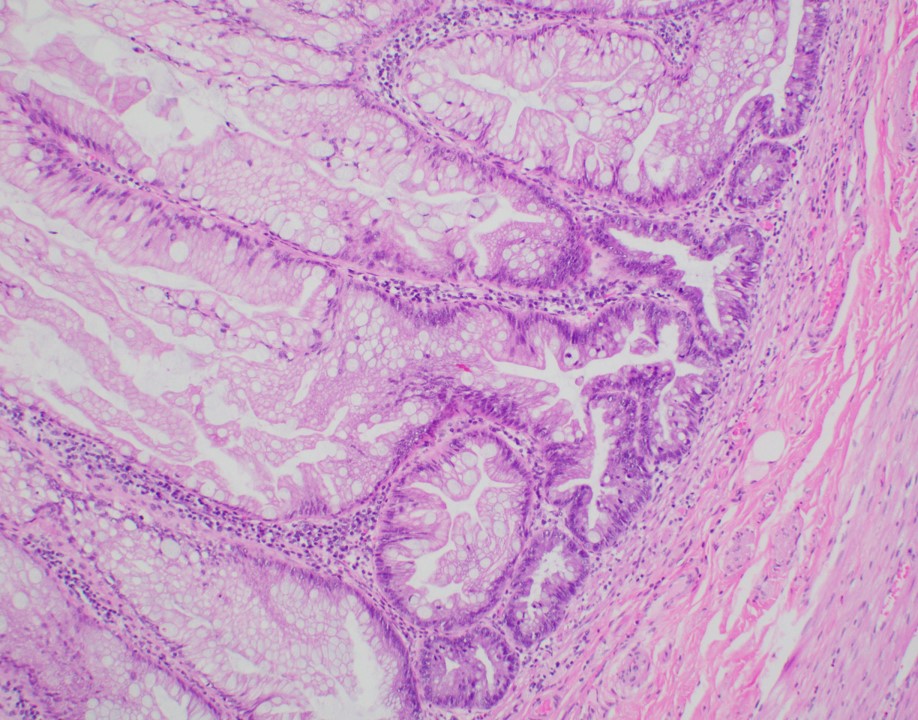
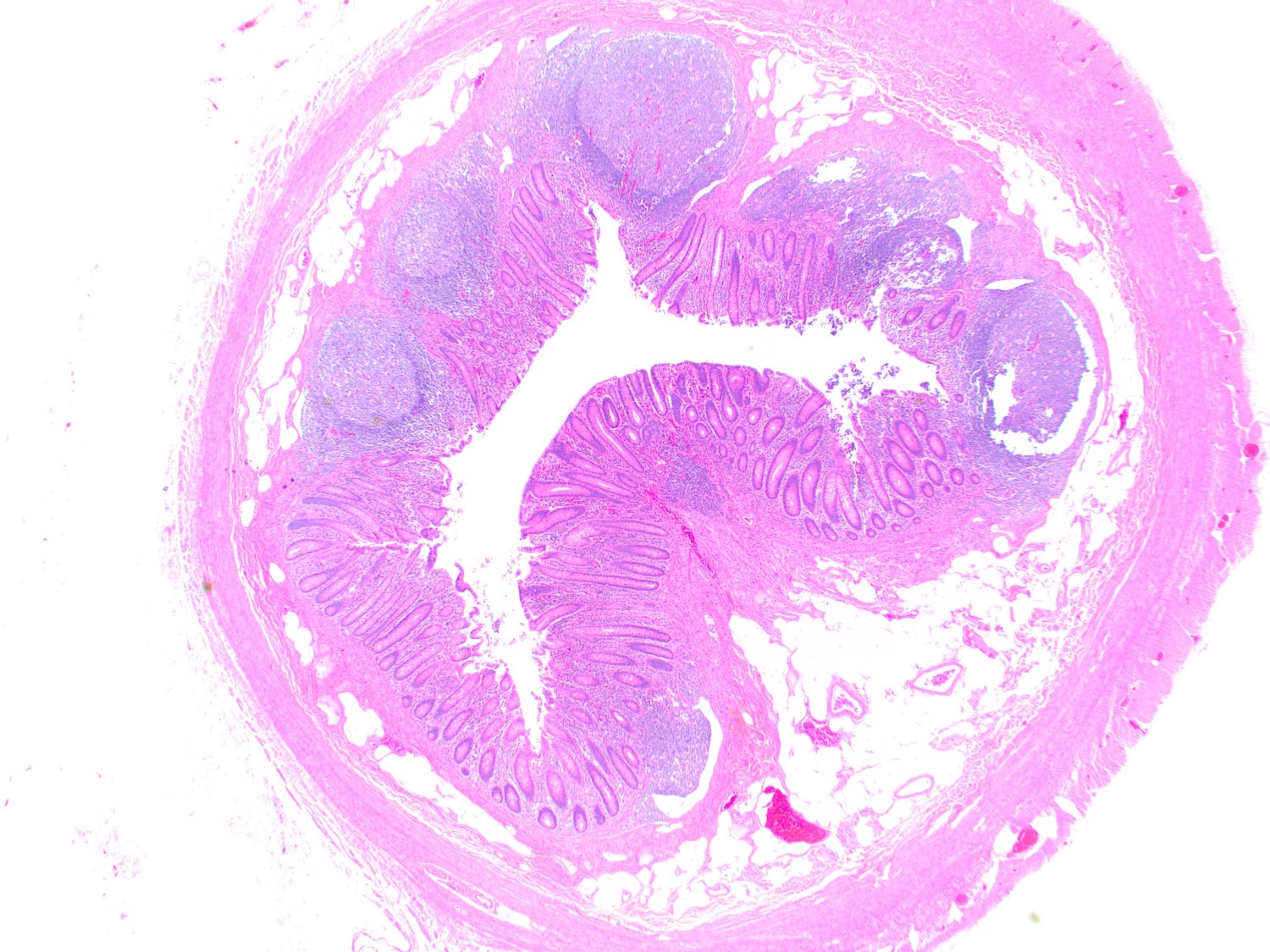
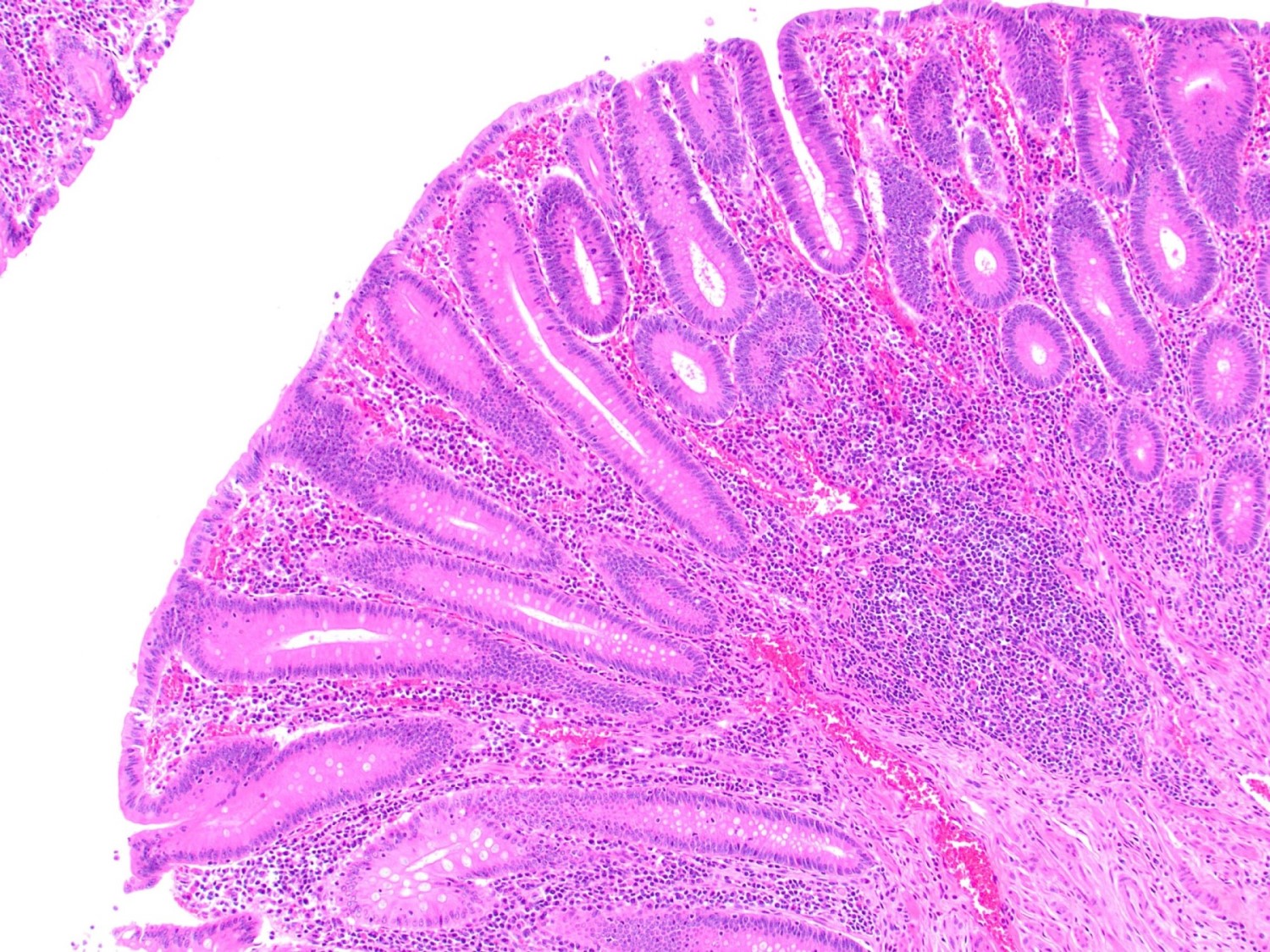
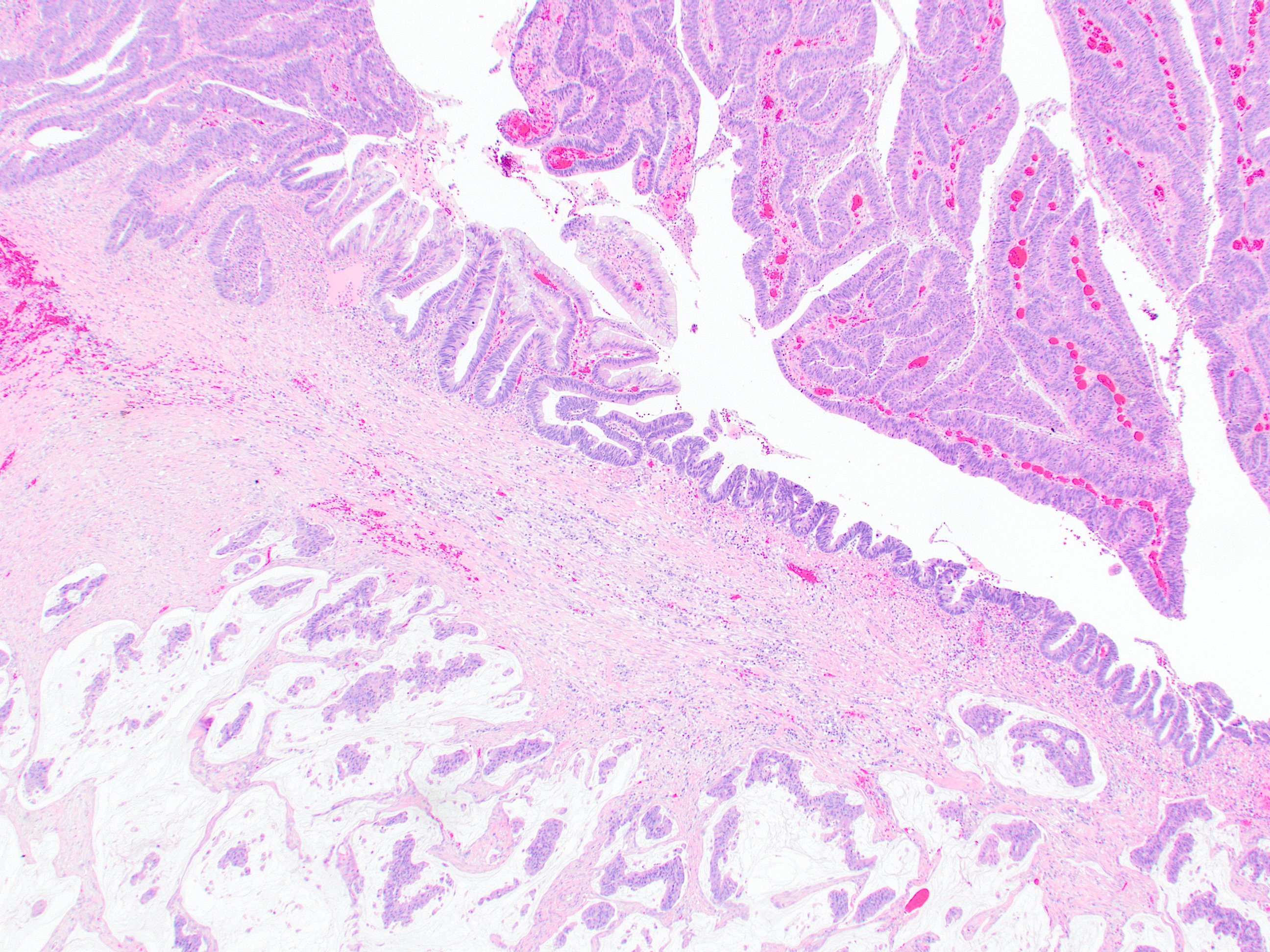
- Tis: carcinoma in situ (intramucosal carcinoma; invasion of the lamina propria or extension into but not through the muscularis mucosae)
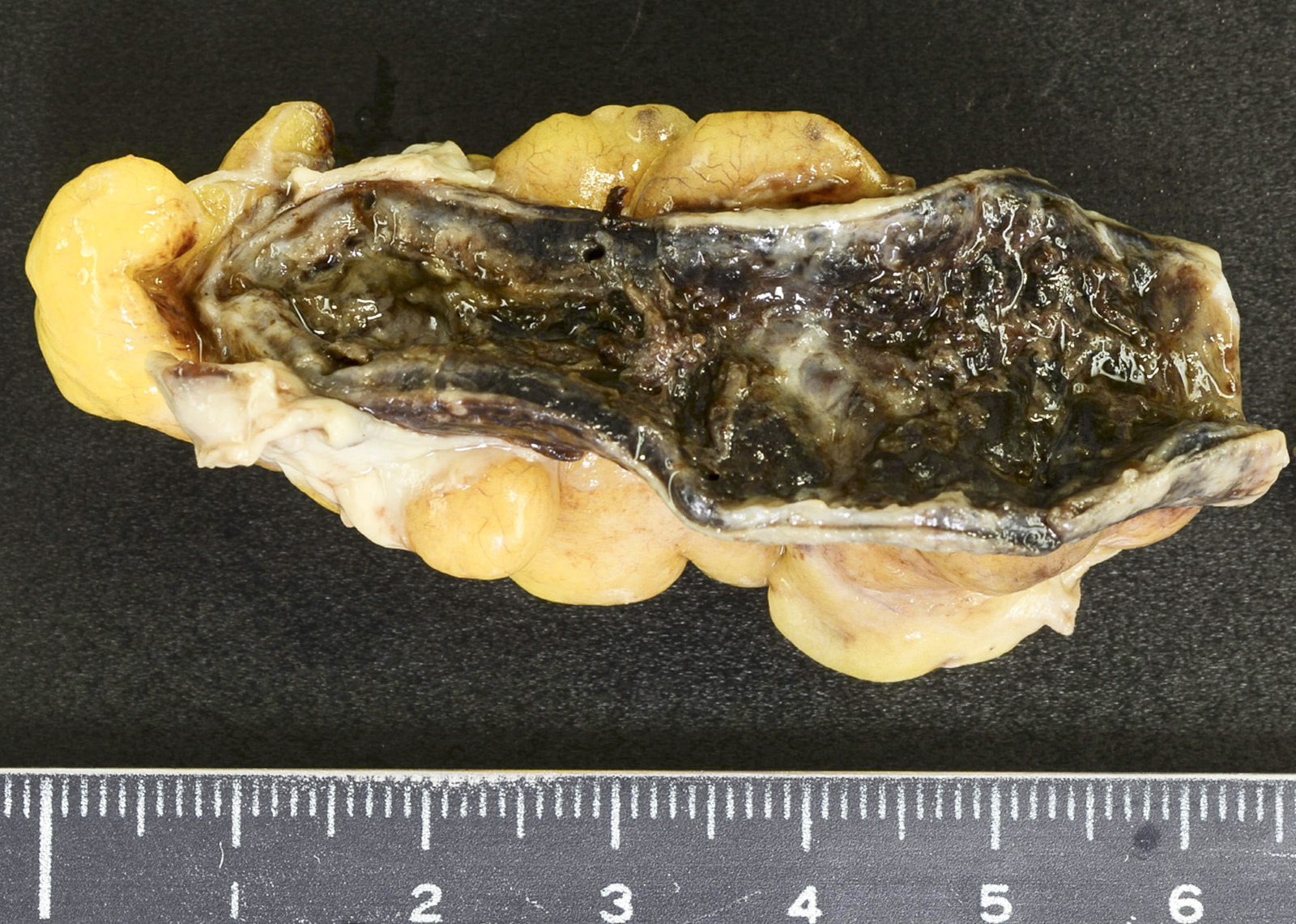
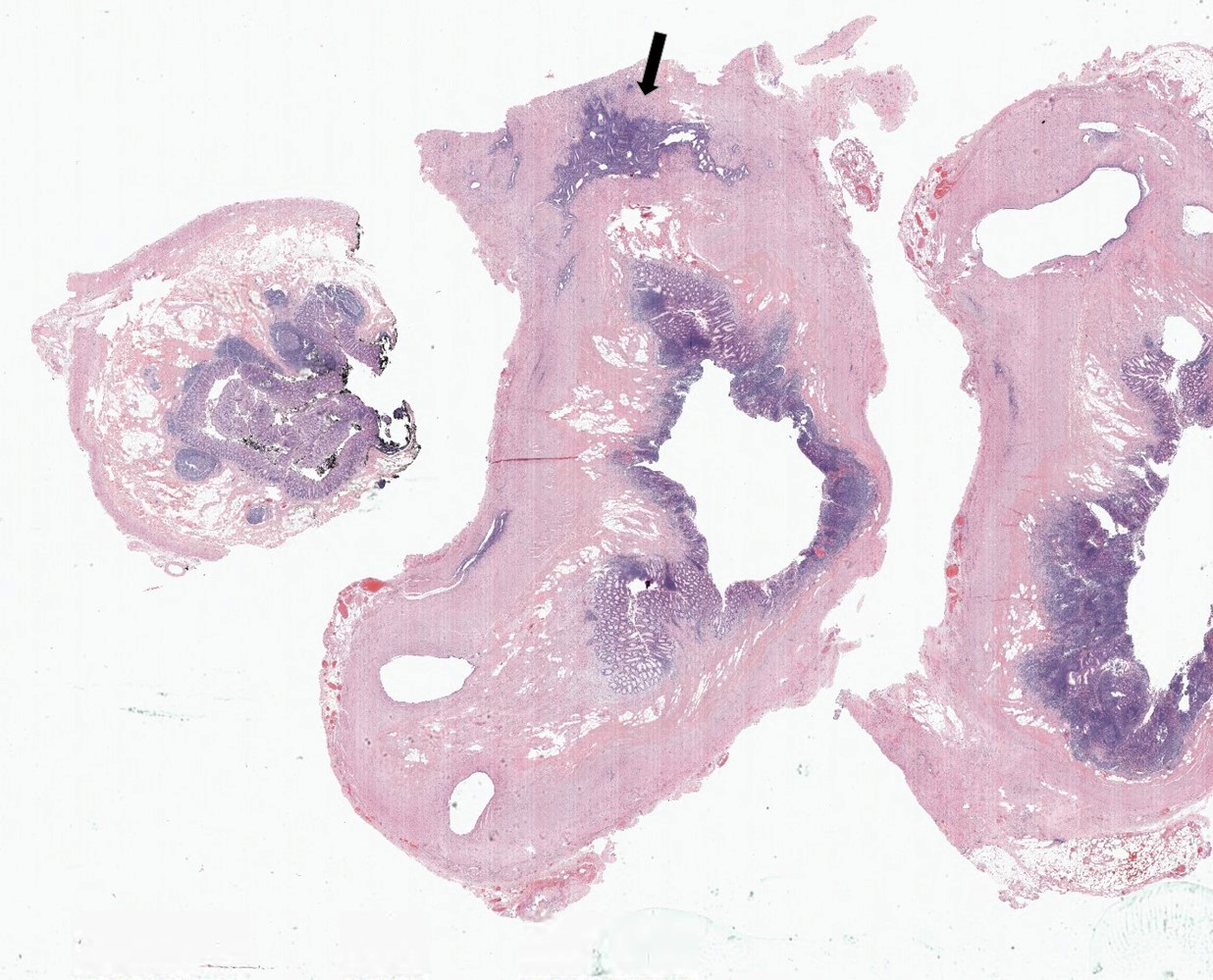
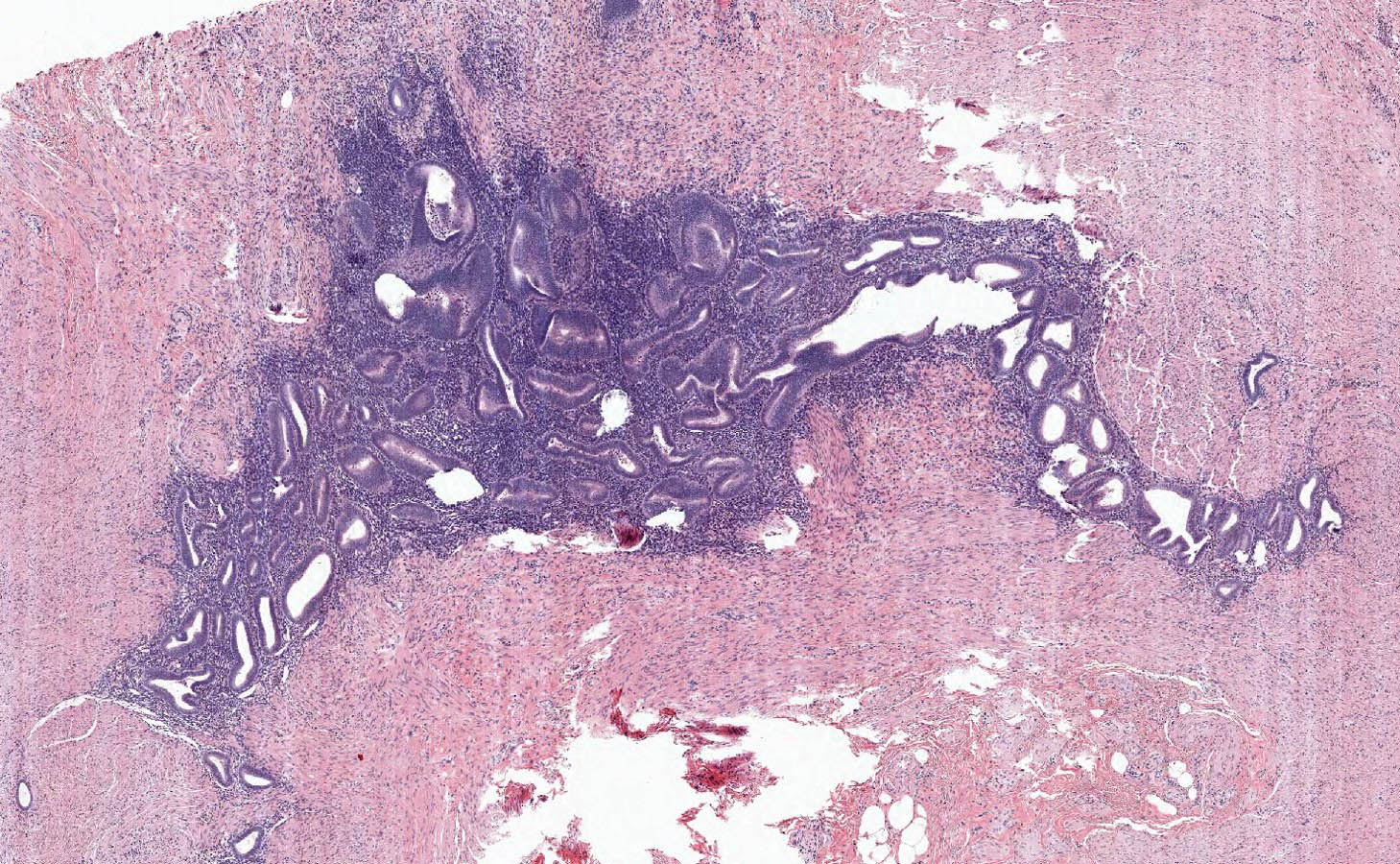
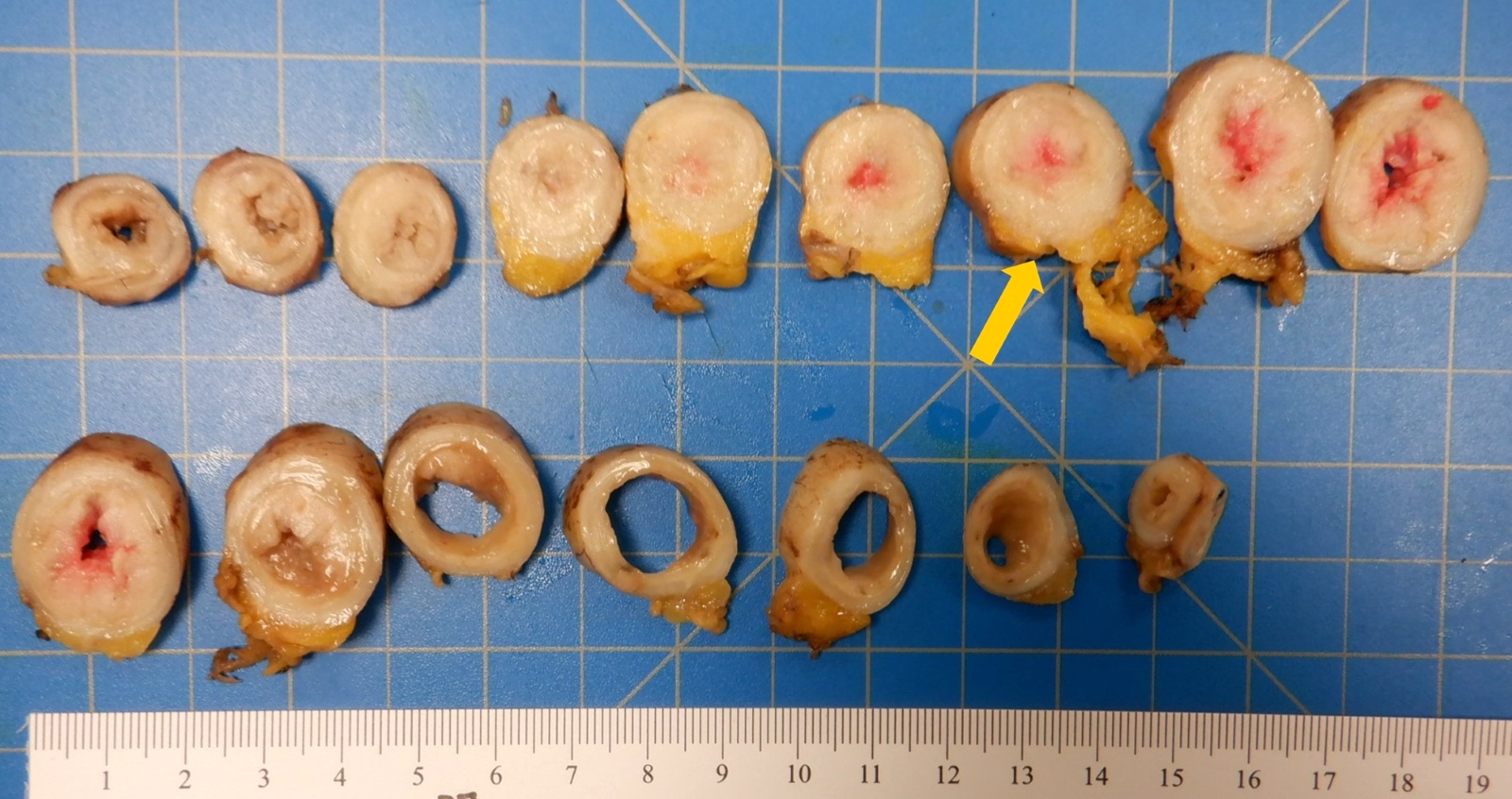
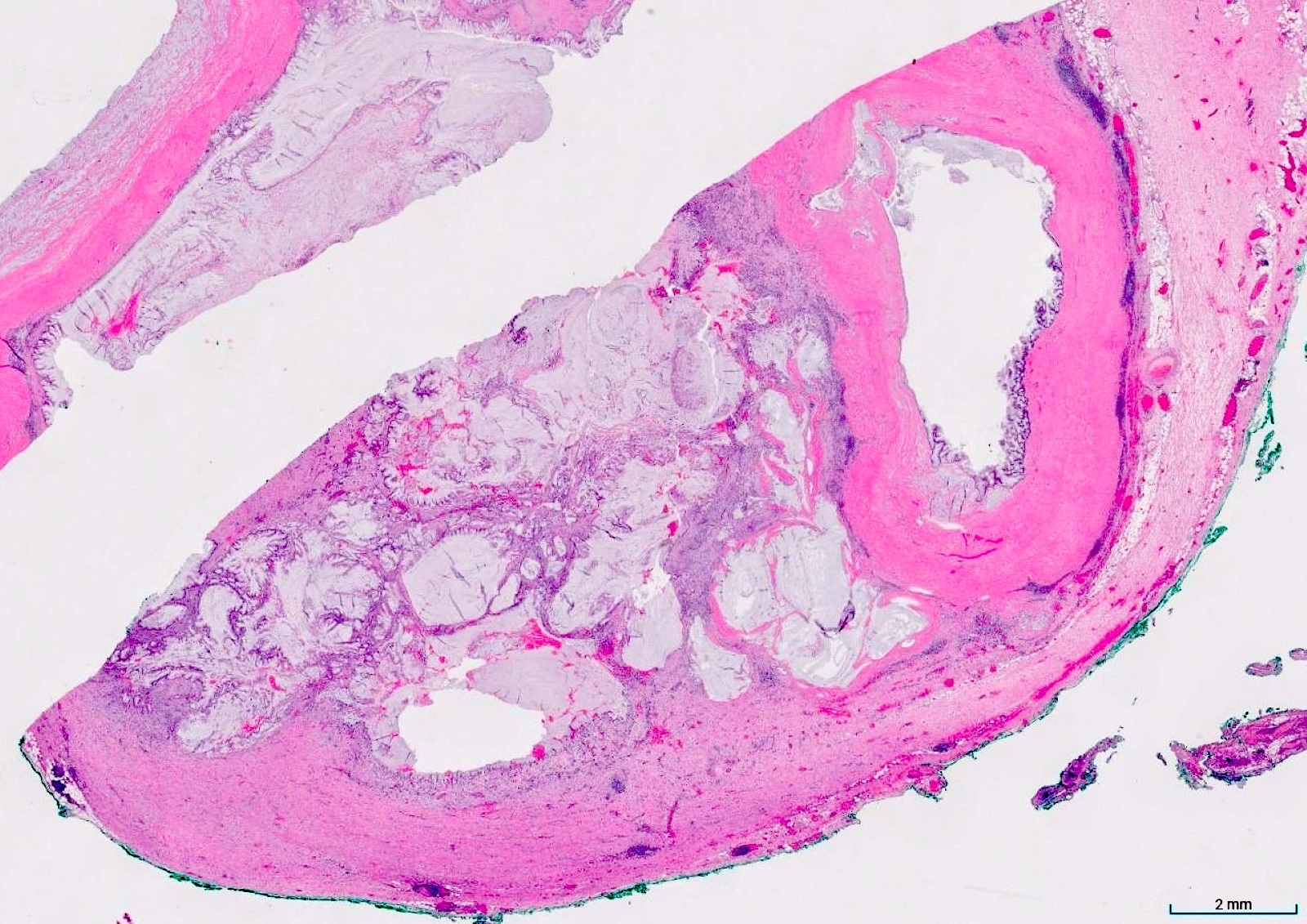
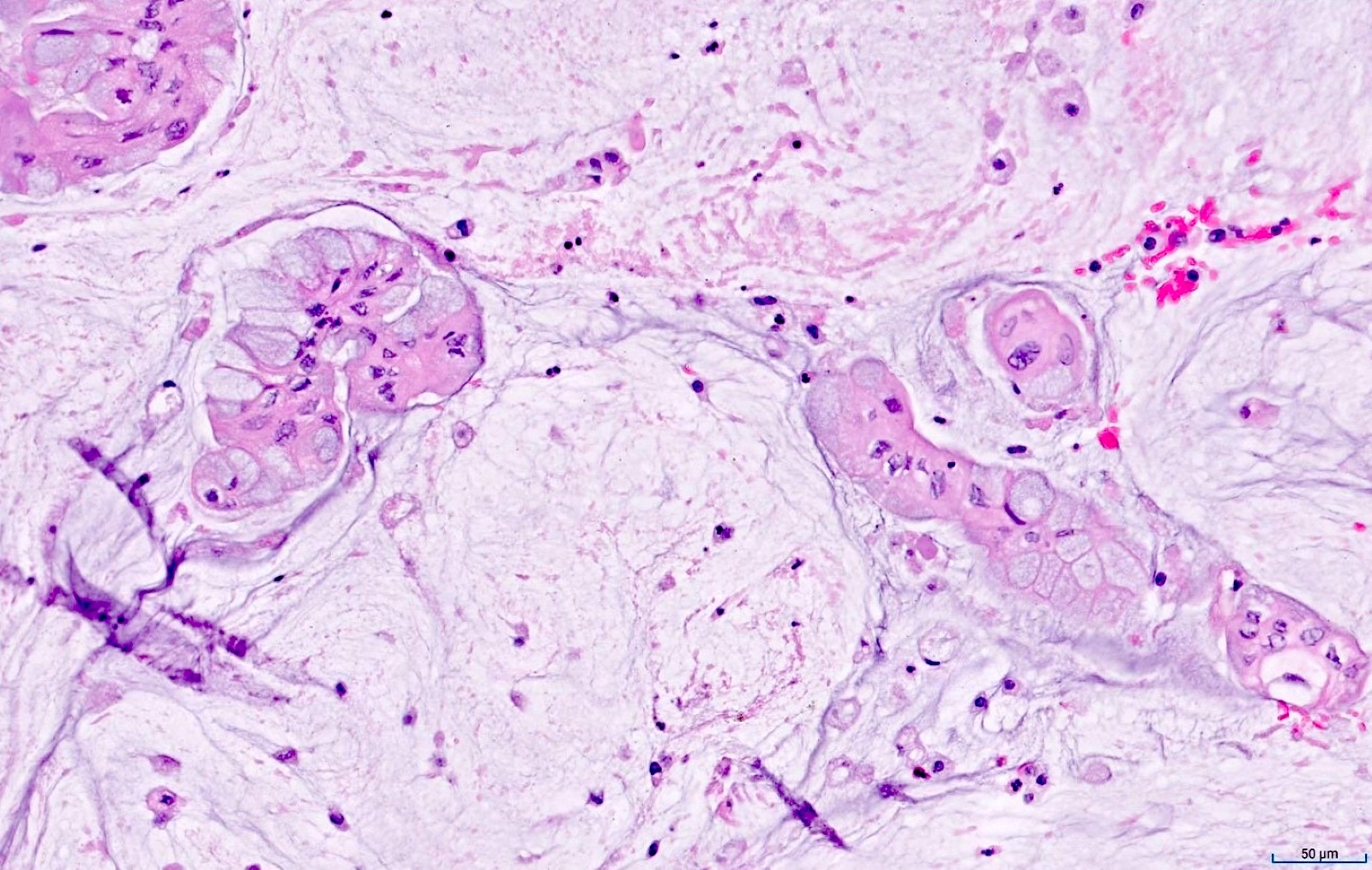
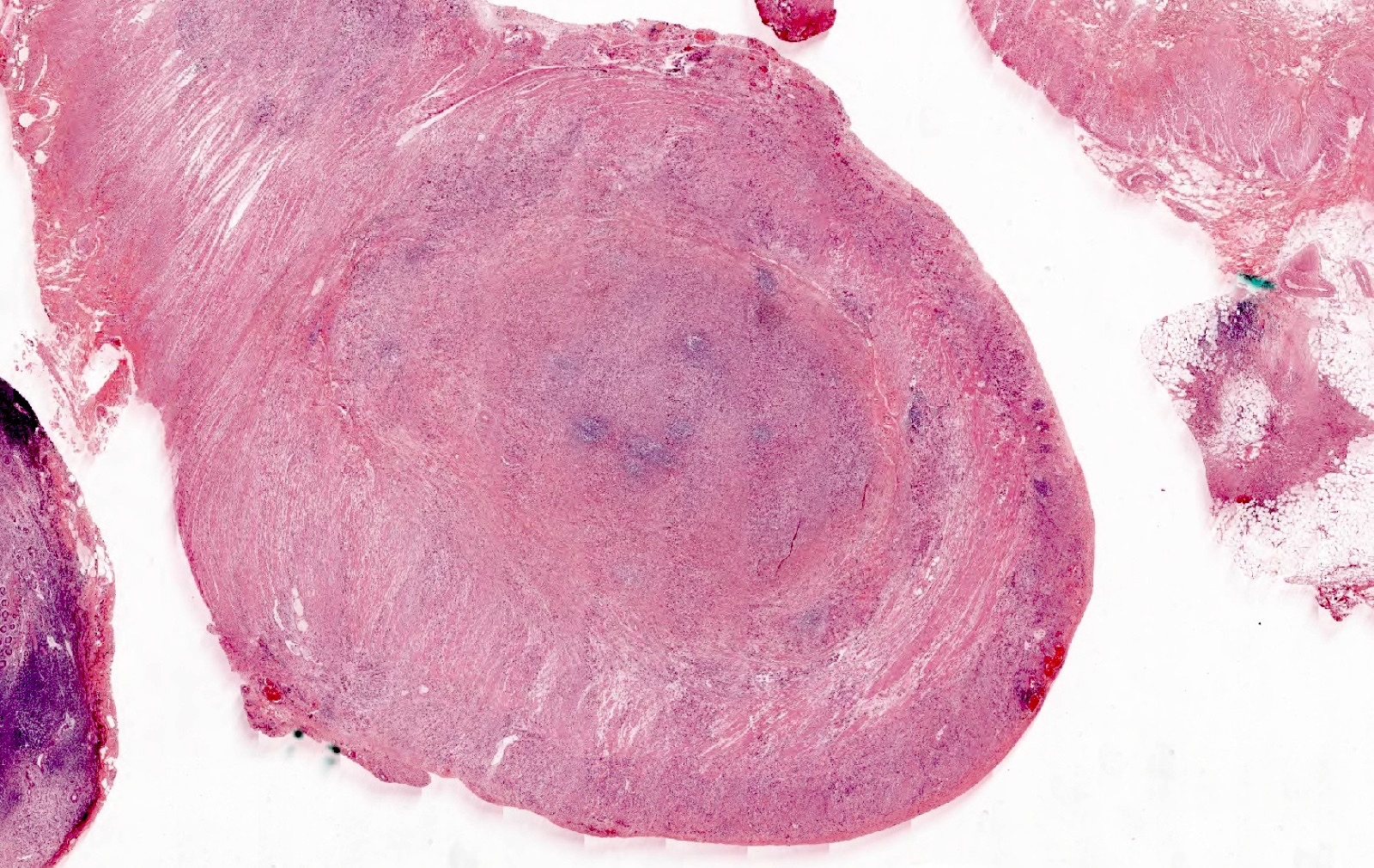
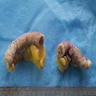
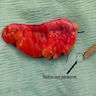
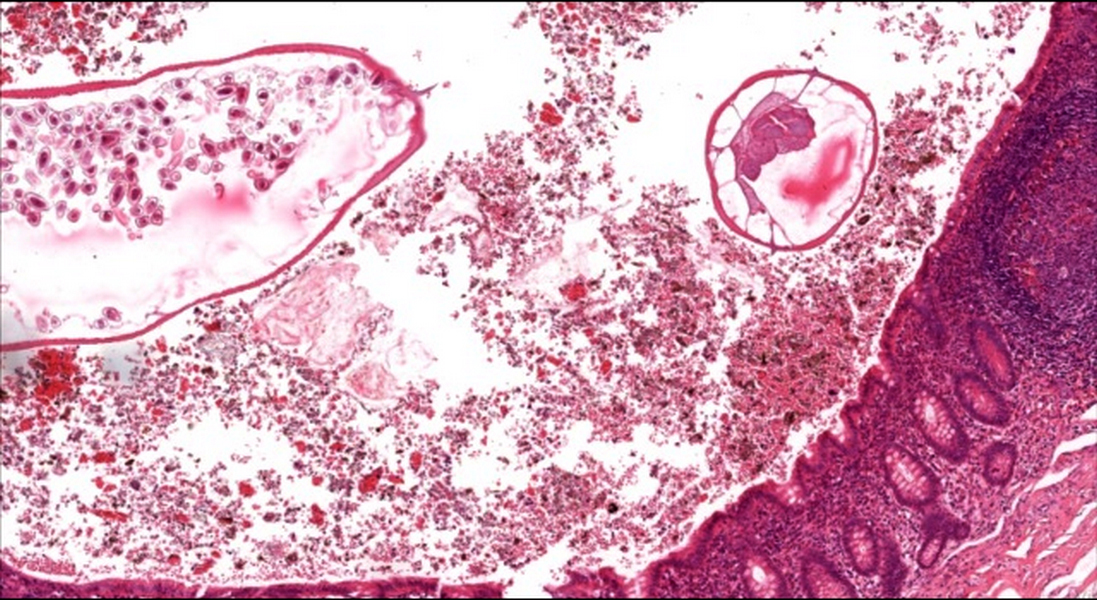
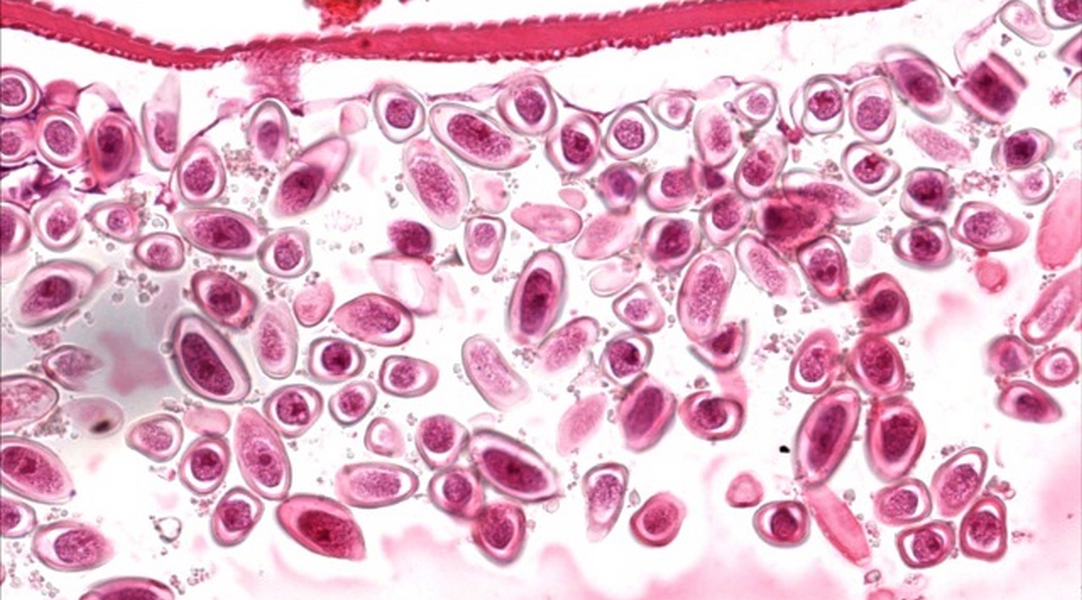
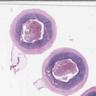
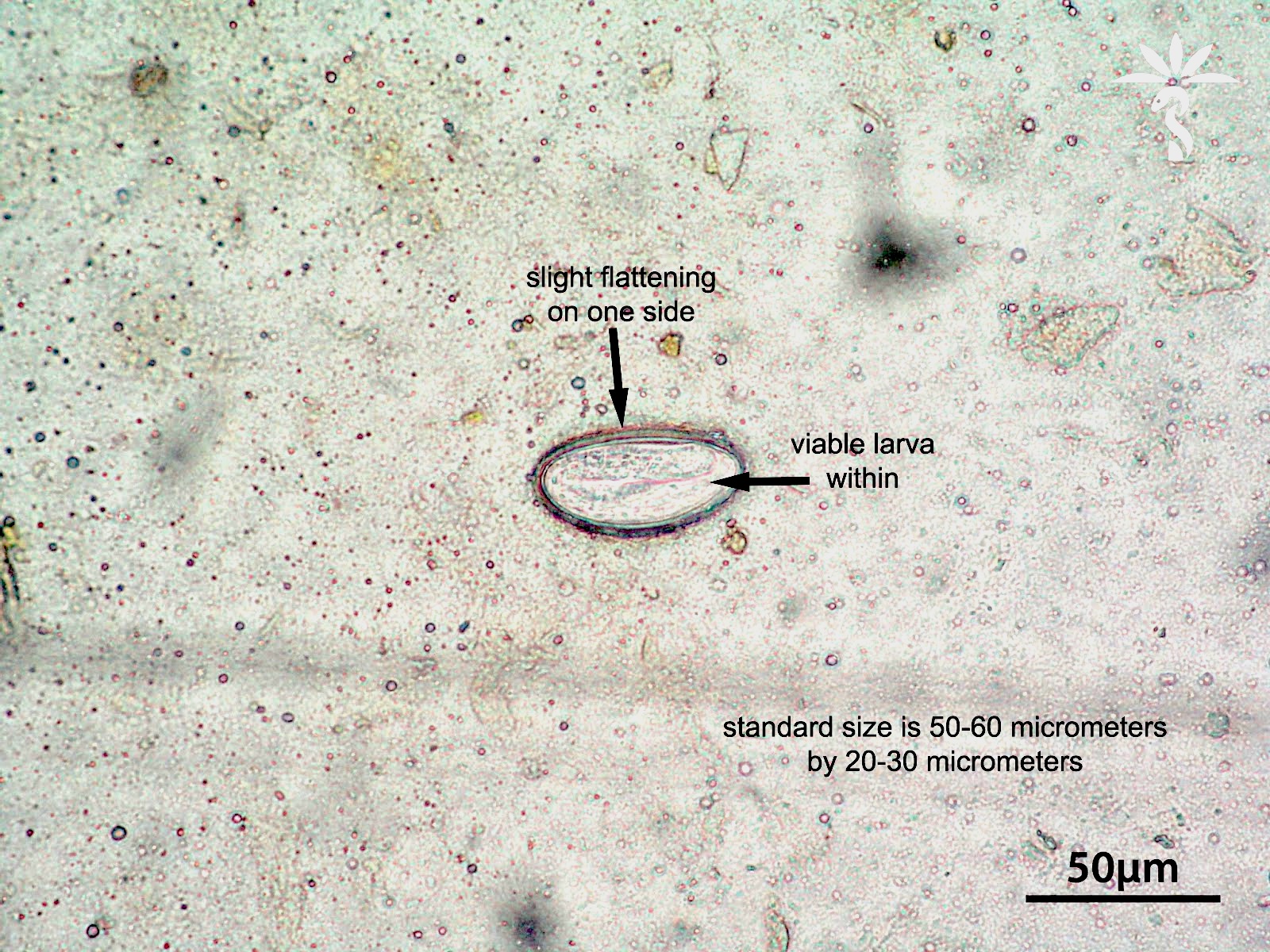
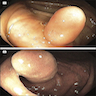
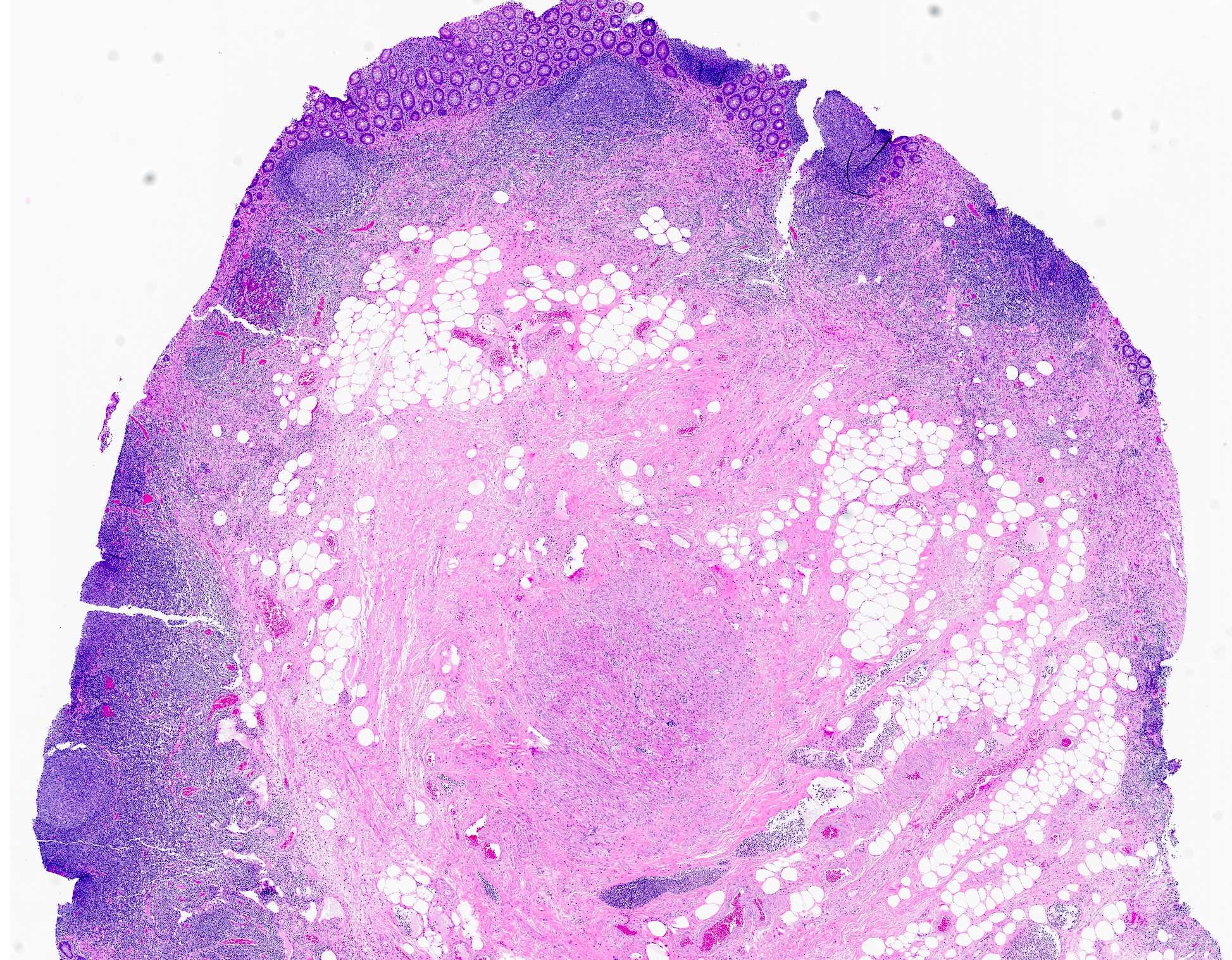
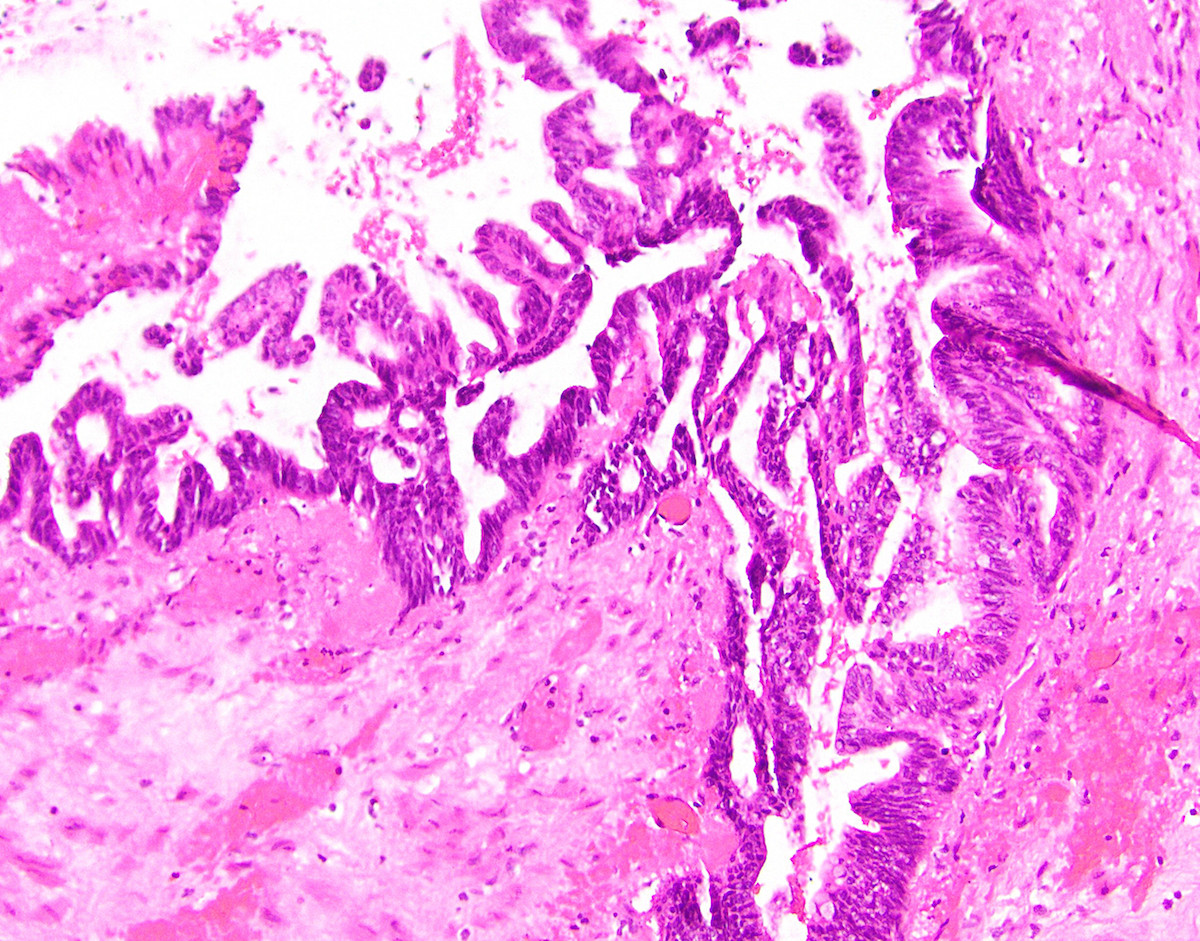
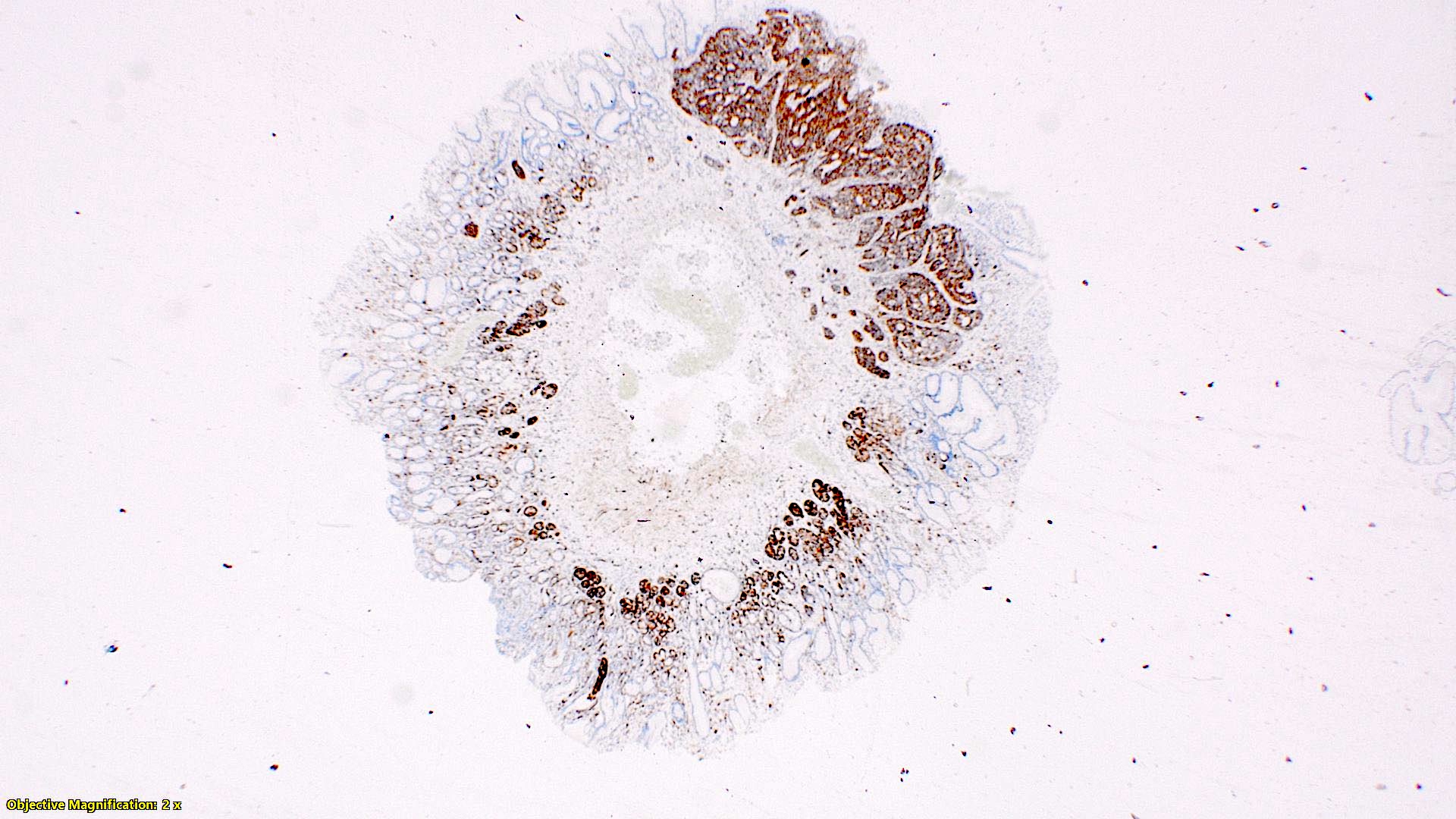
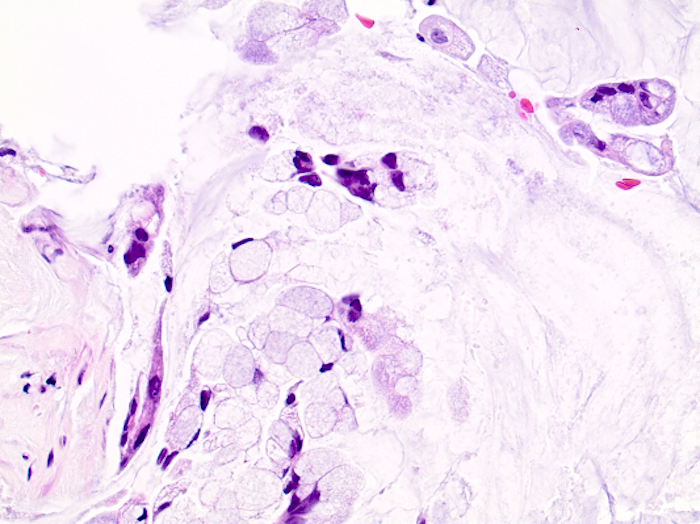
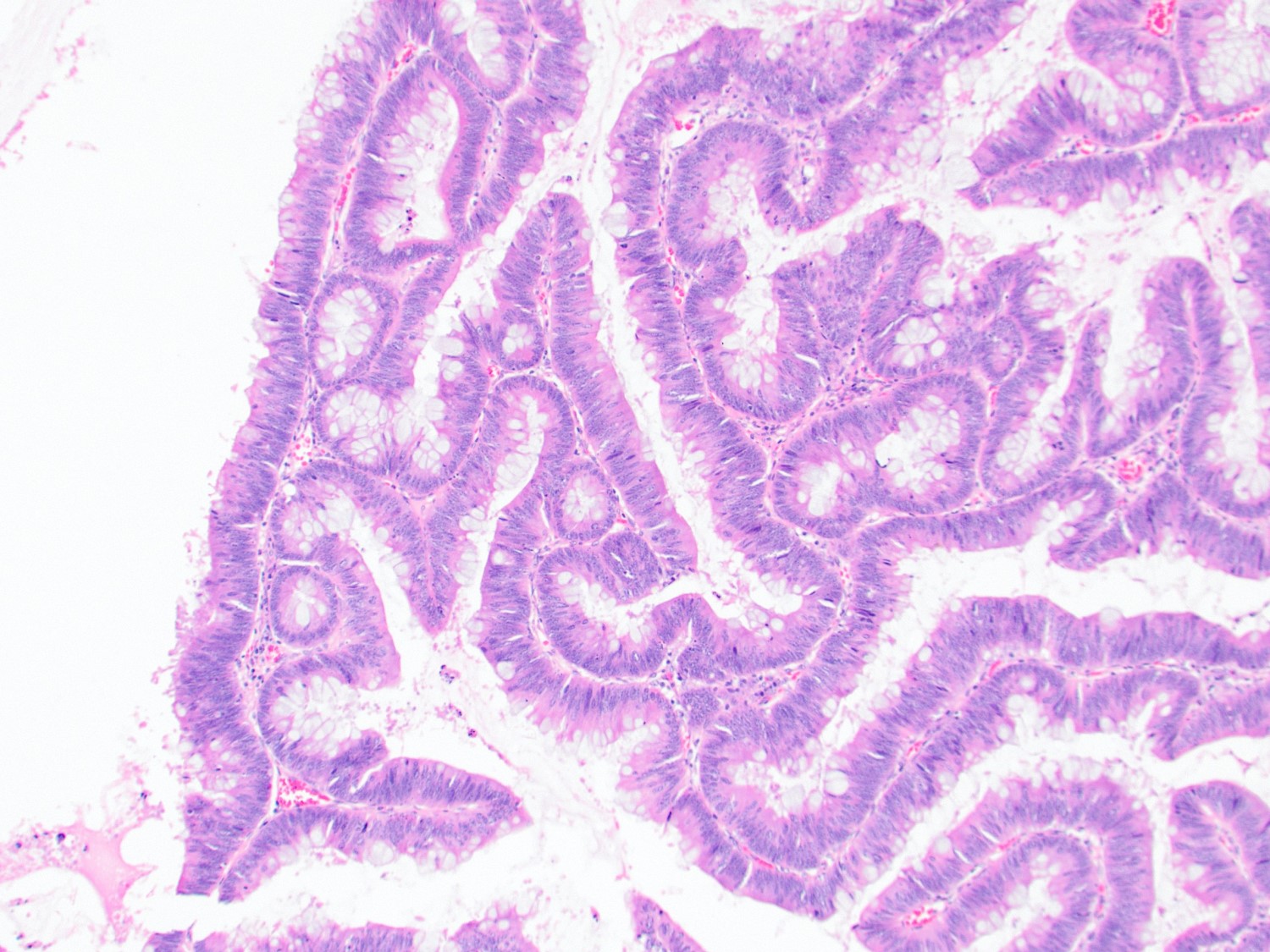
- Tis(LAMN): low grade appendiceal mucinous neoplasm confined by the muscularis propria; acellular mucin or mucinous epithelium may invade into the muscularis propria
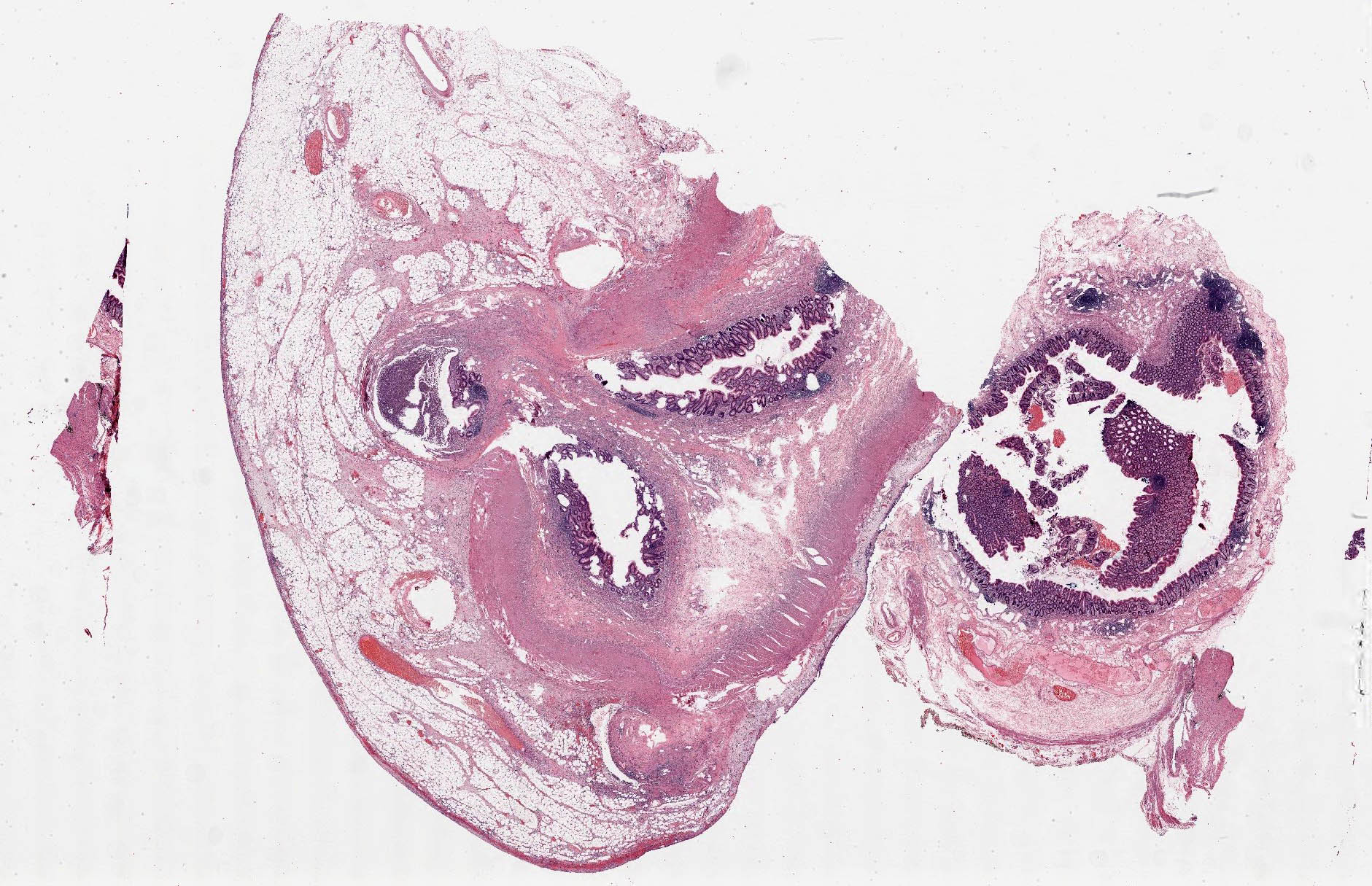
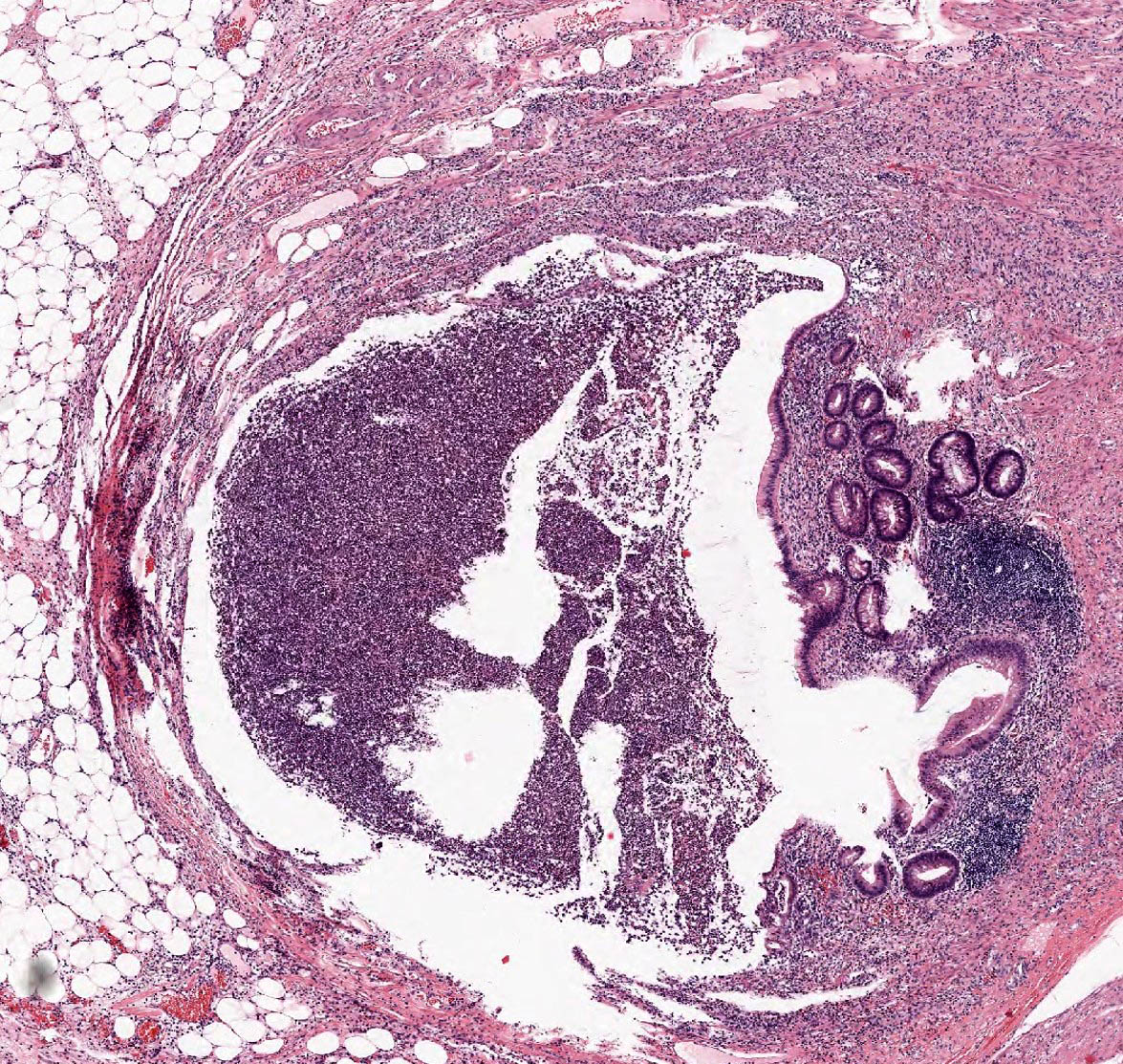
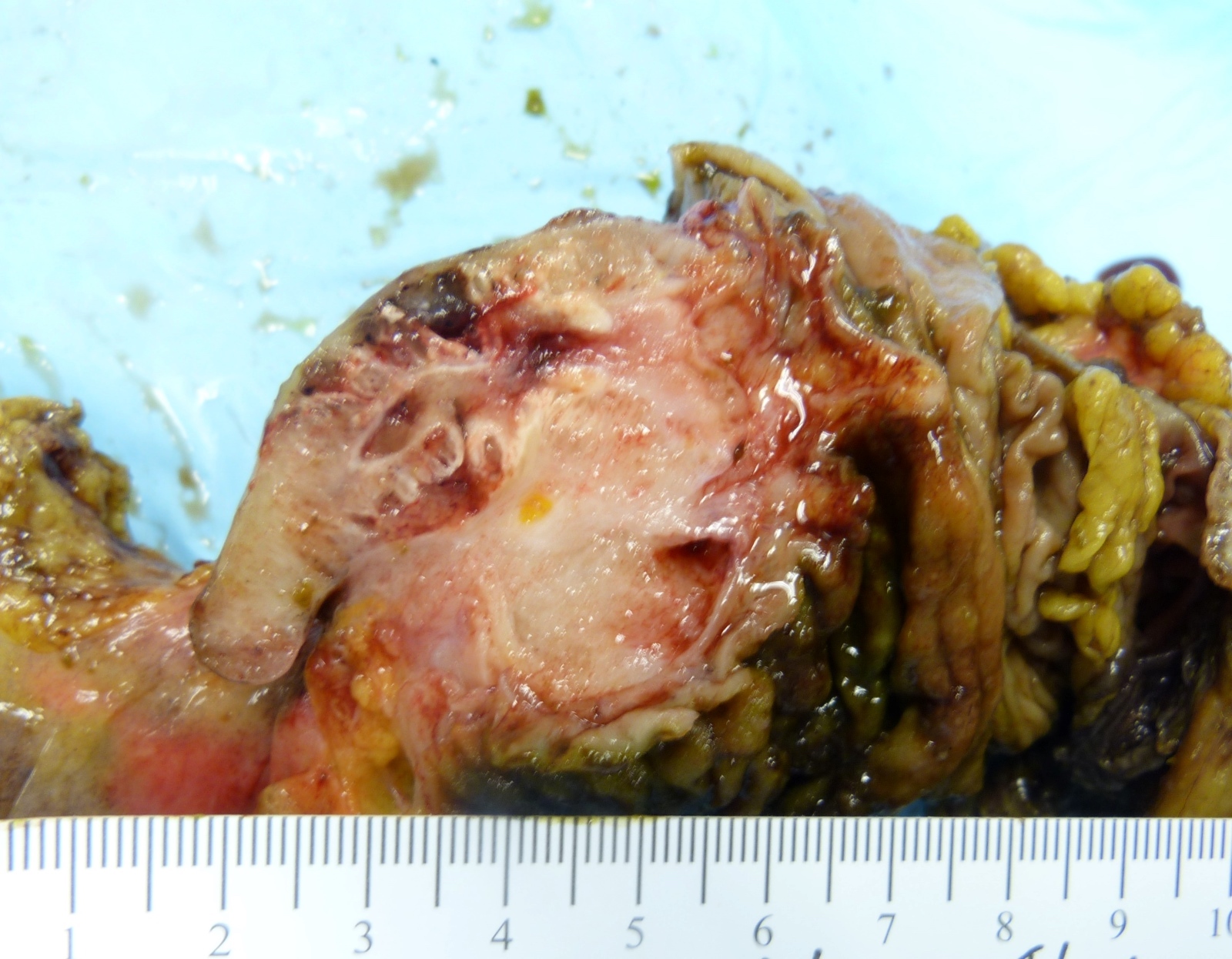
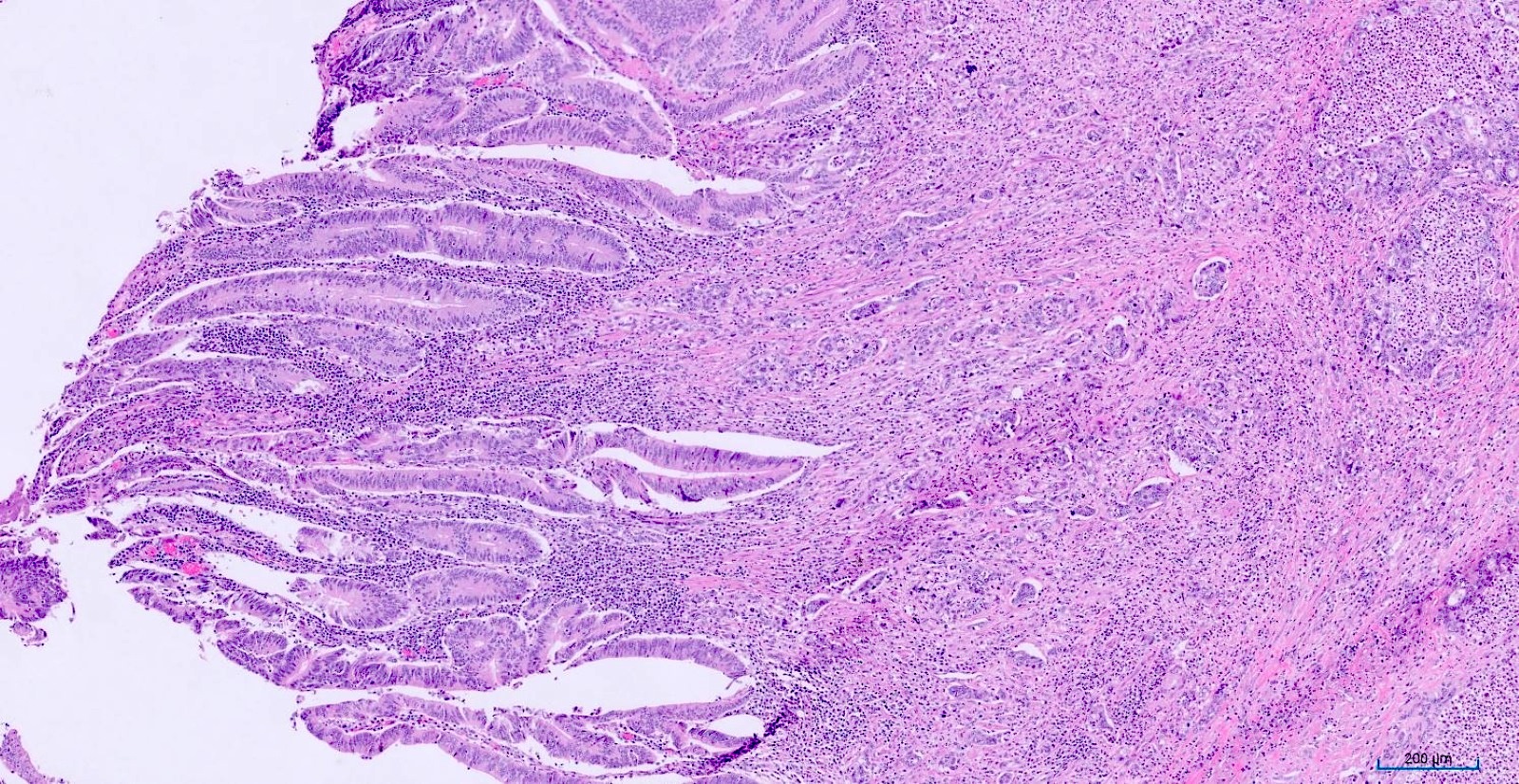
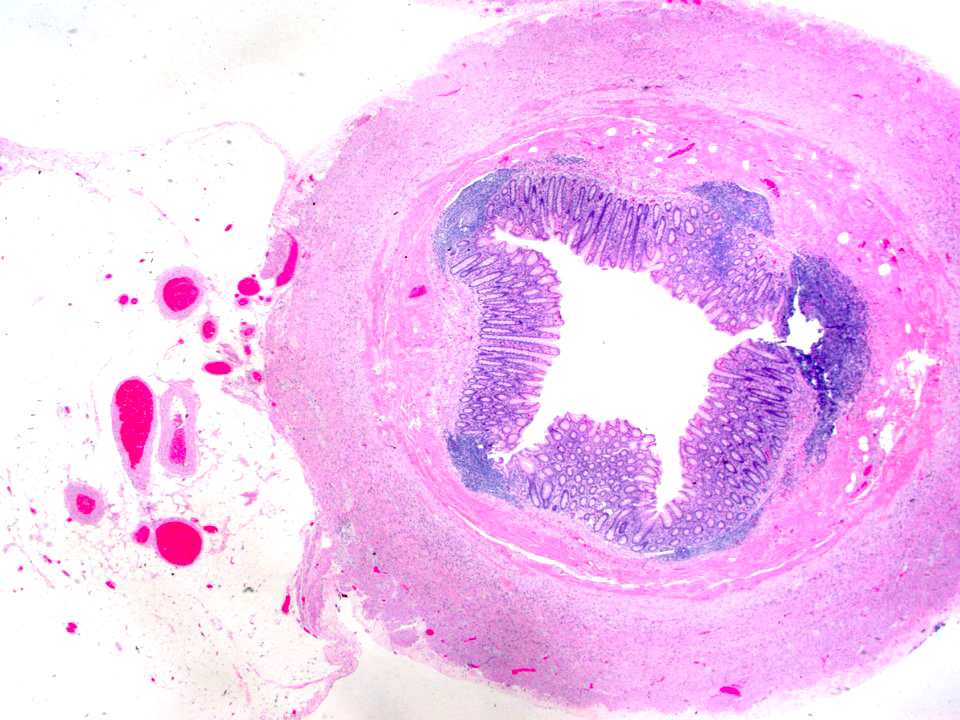
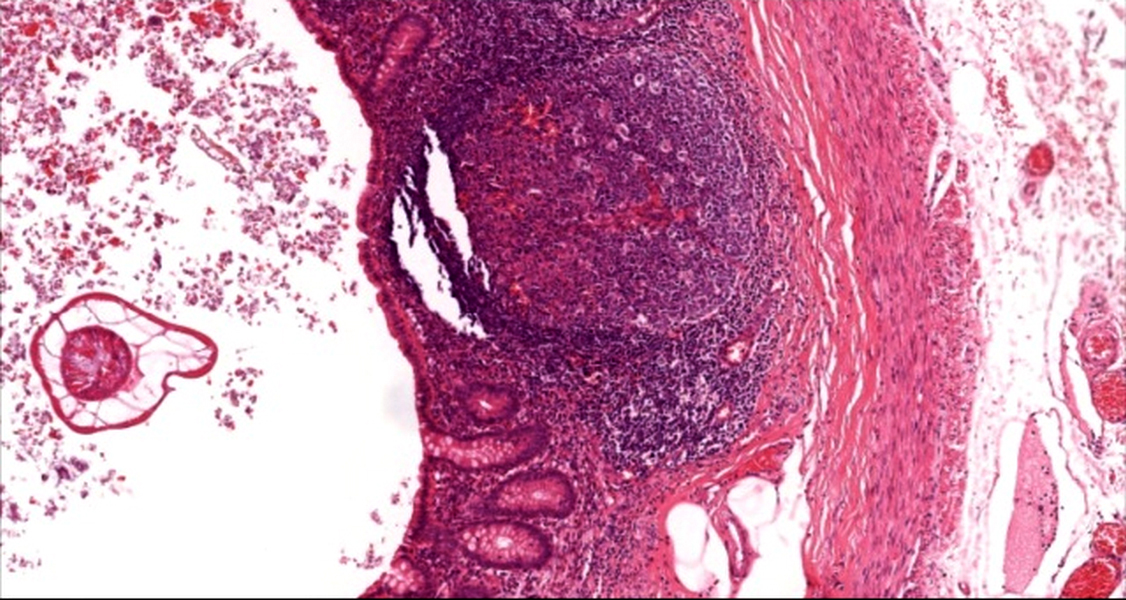
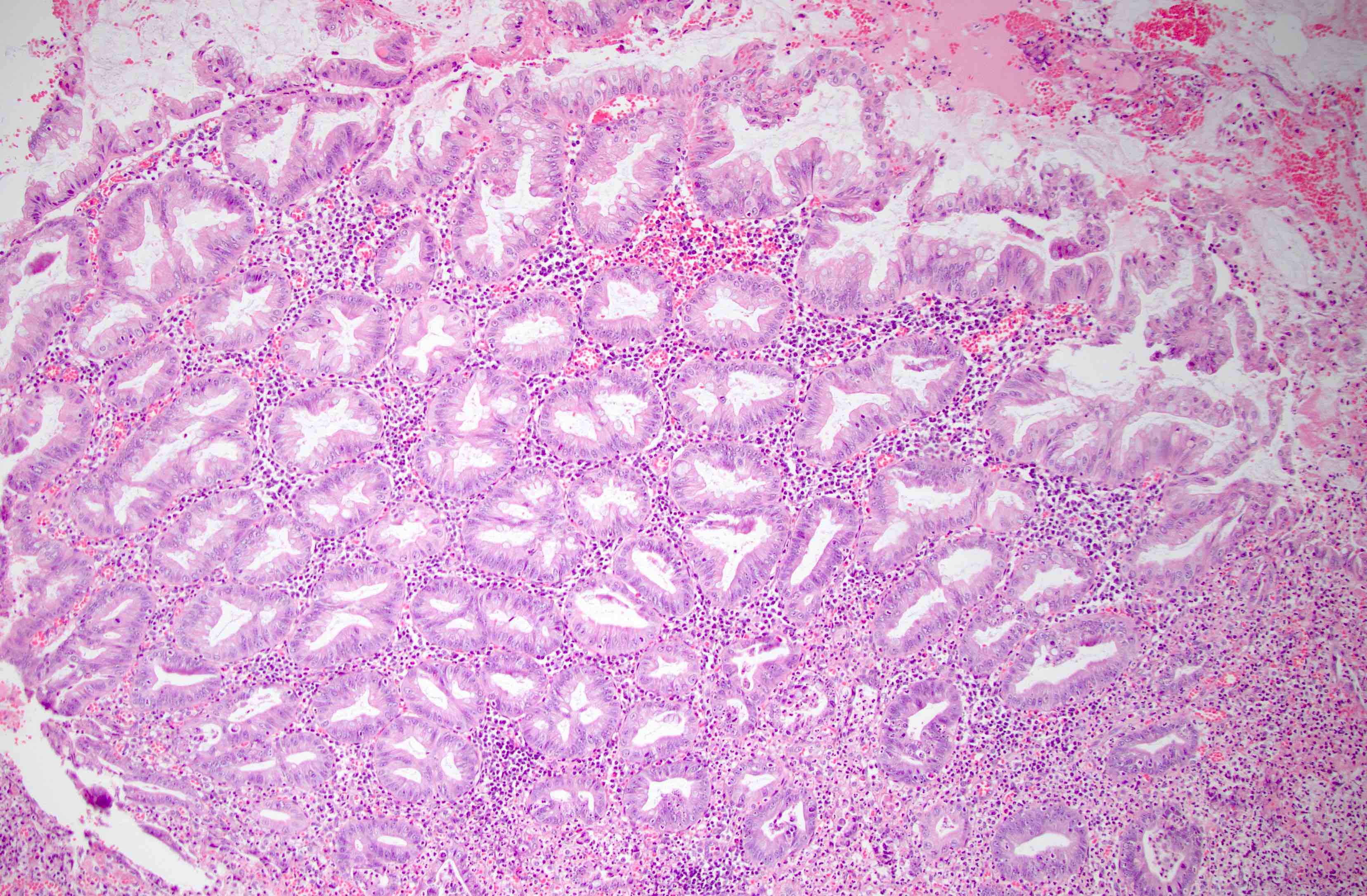
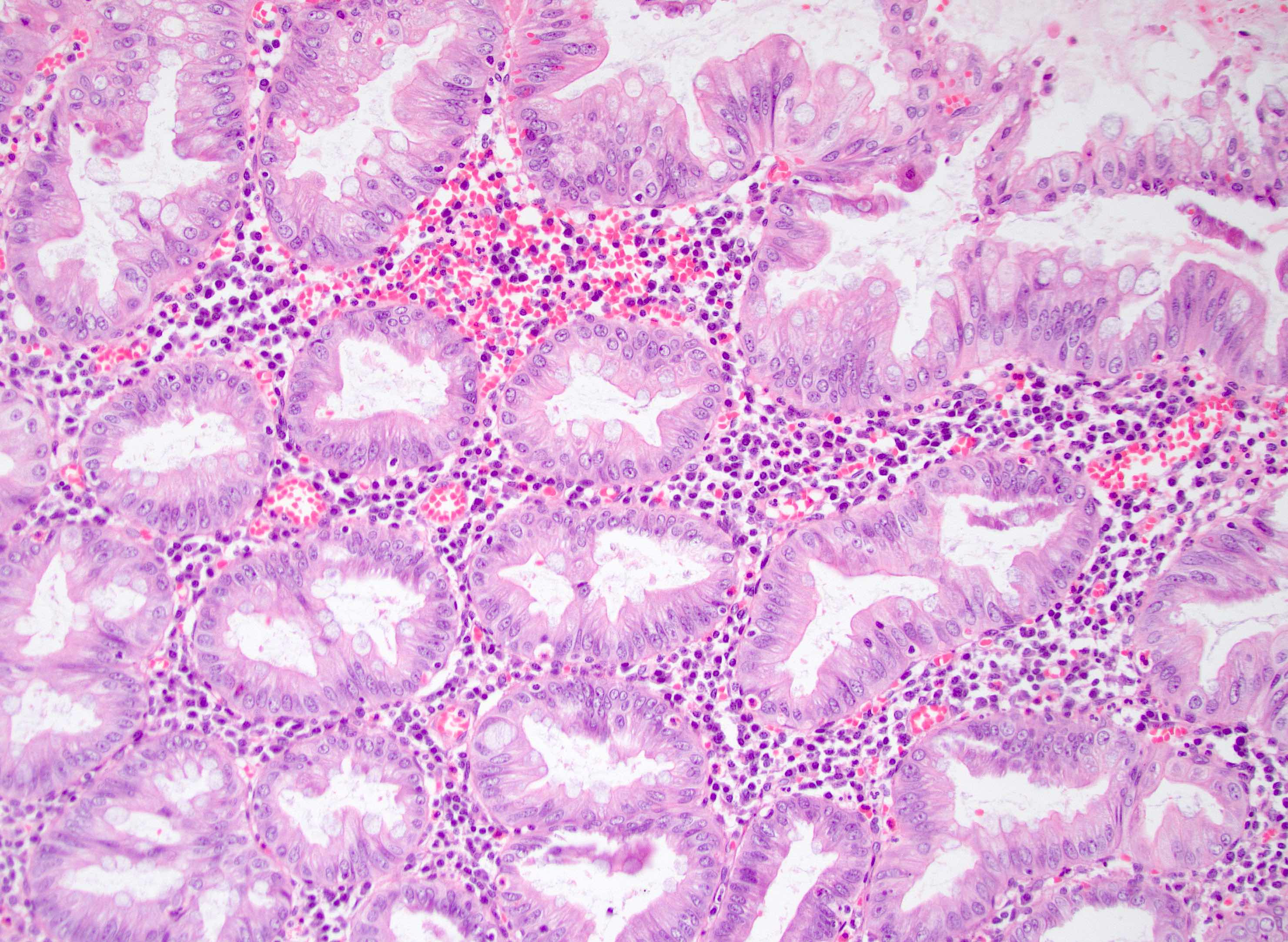
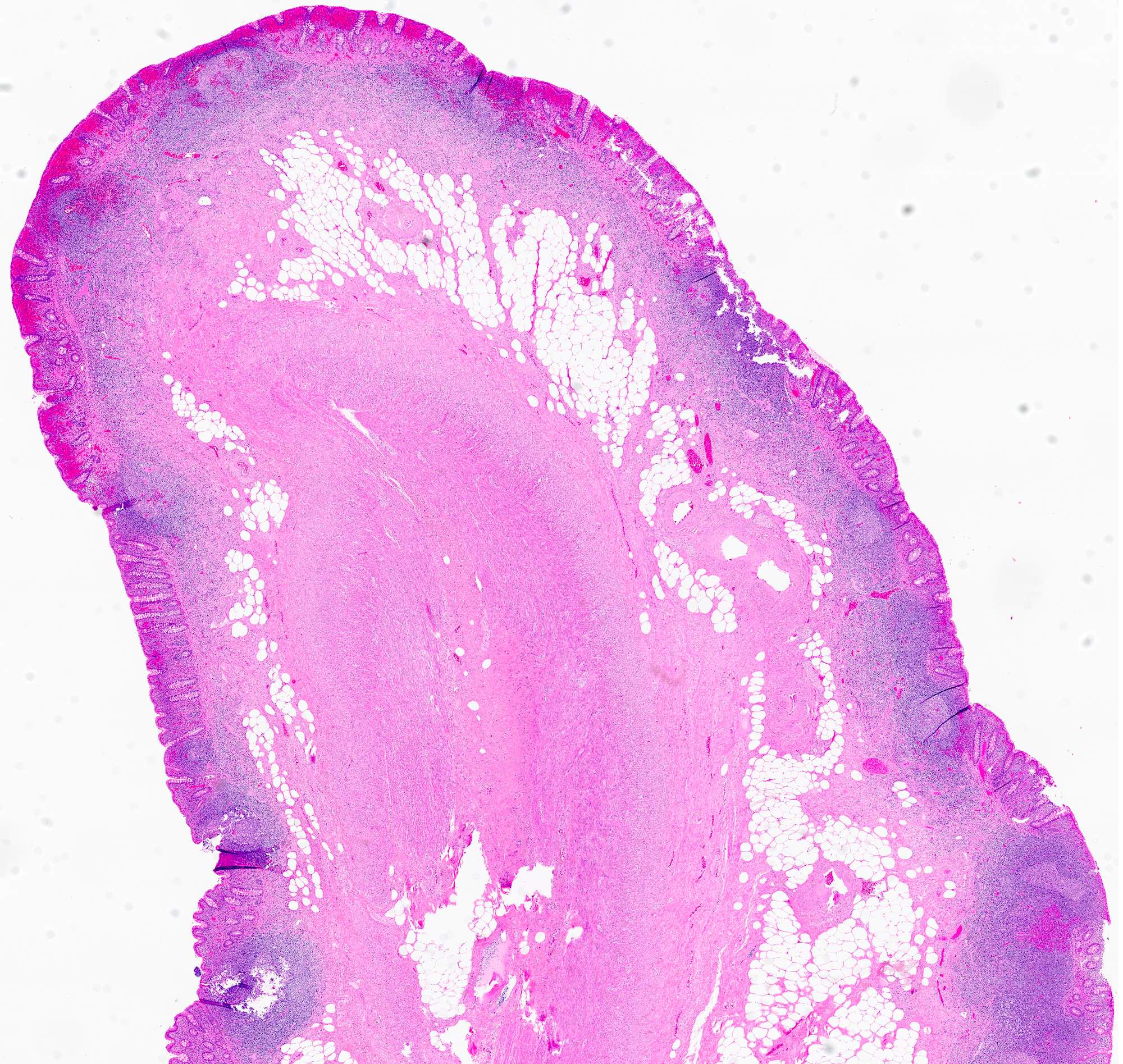
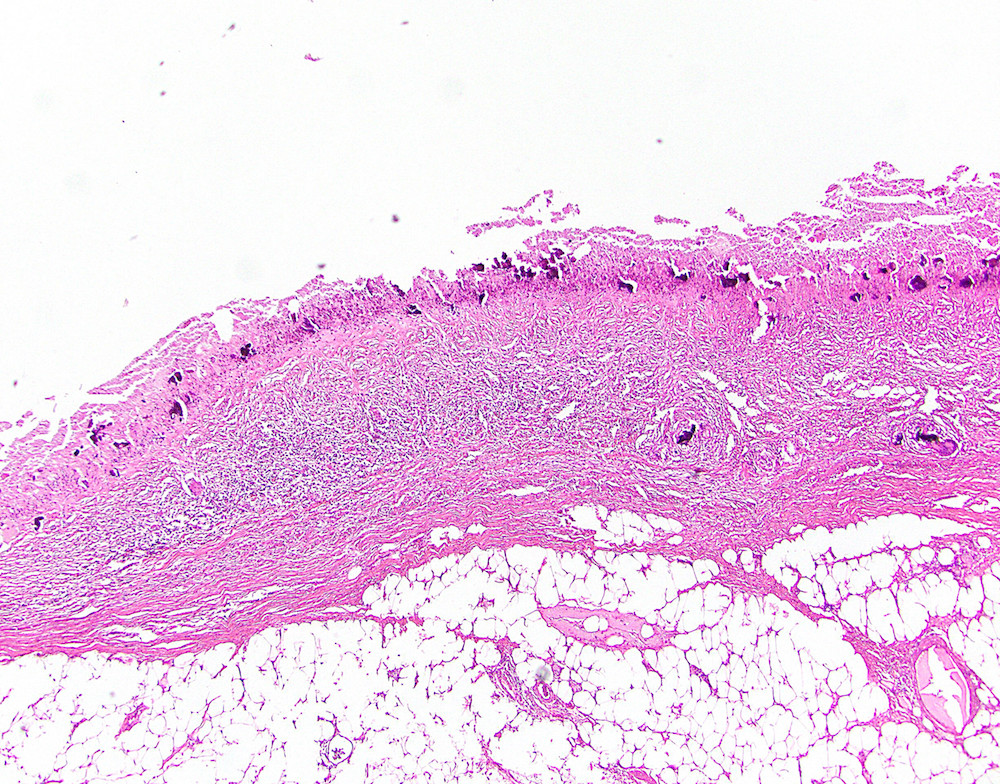
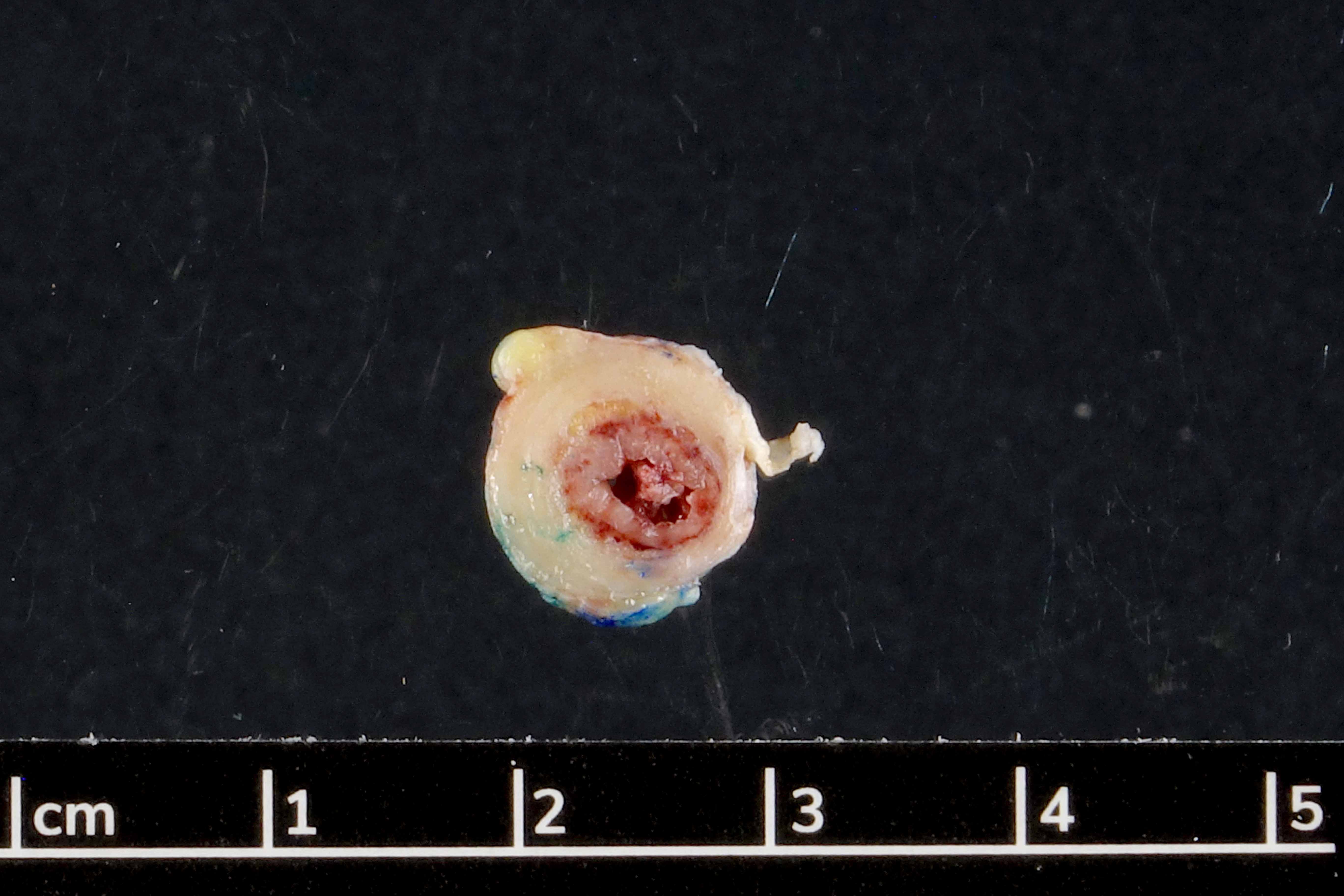
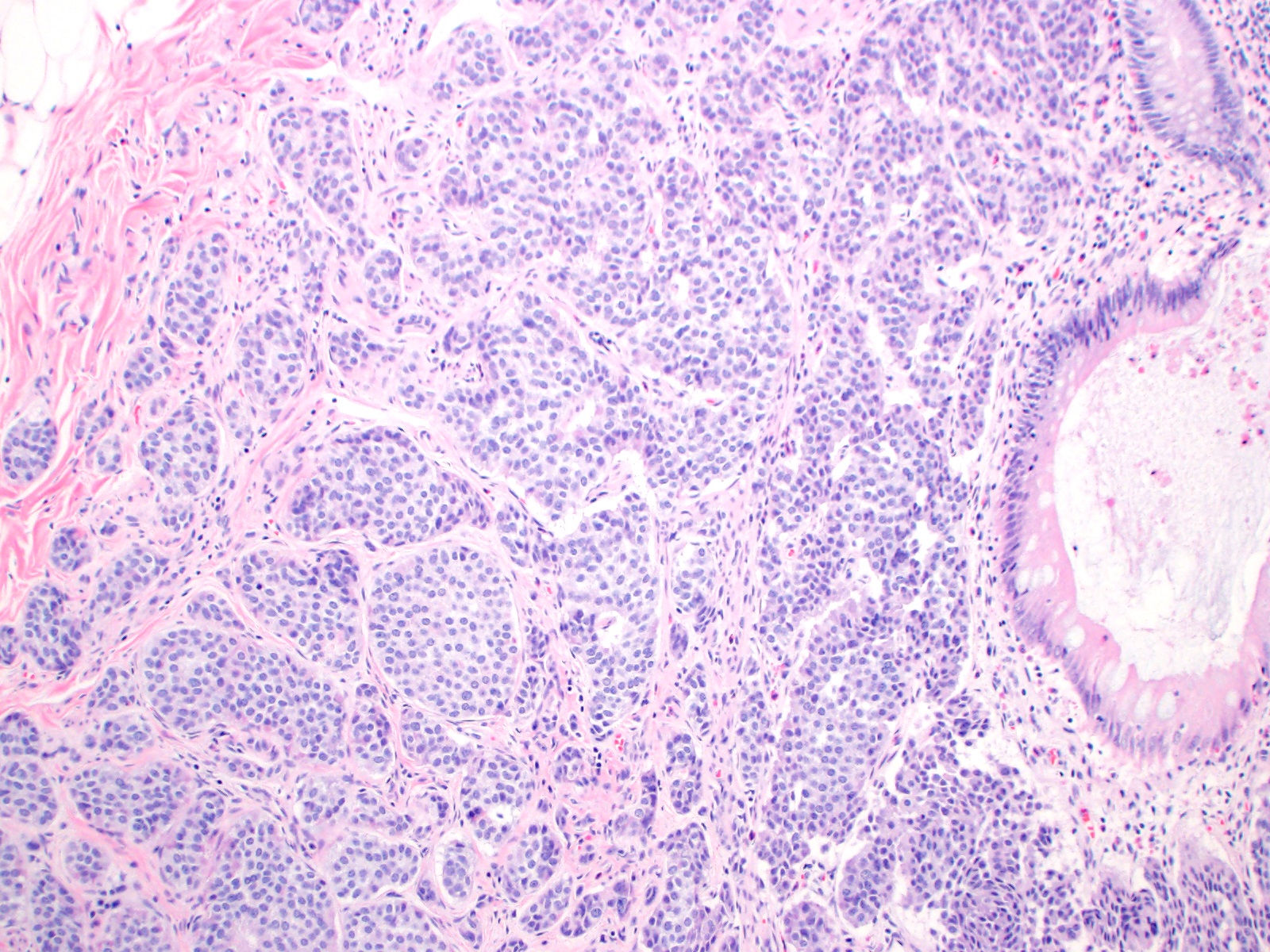
- T1: tumor invades the submucosa (through the muscularis mucosa but not into the muscularis propria)
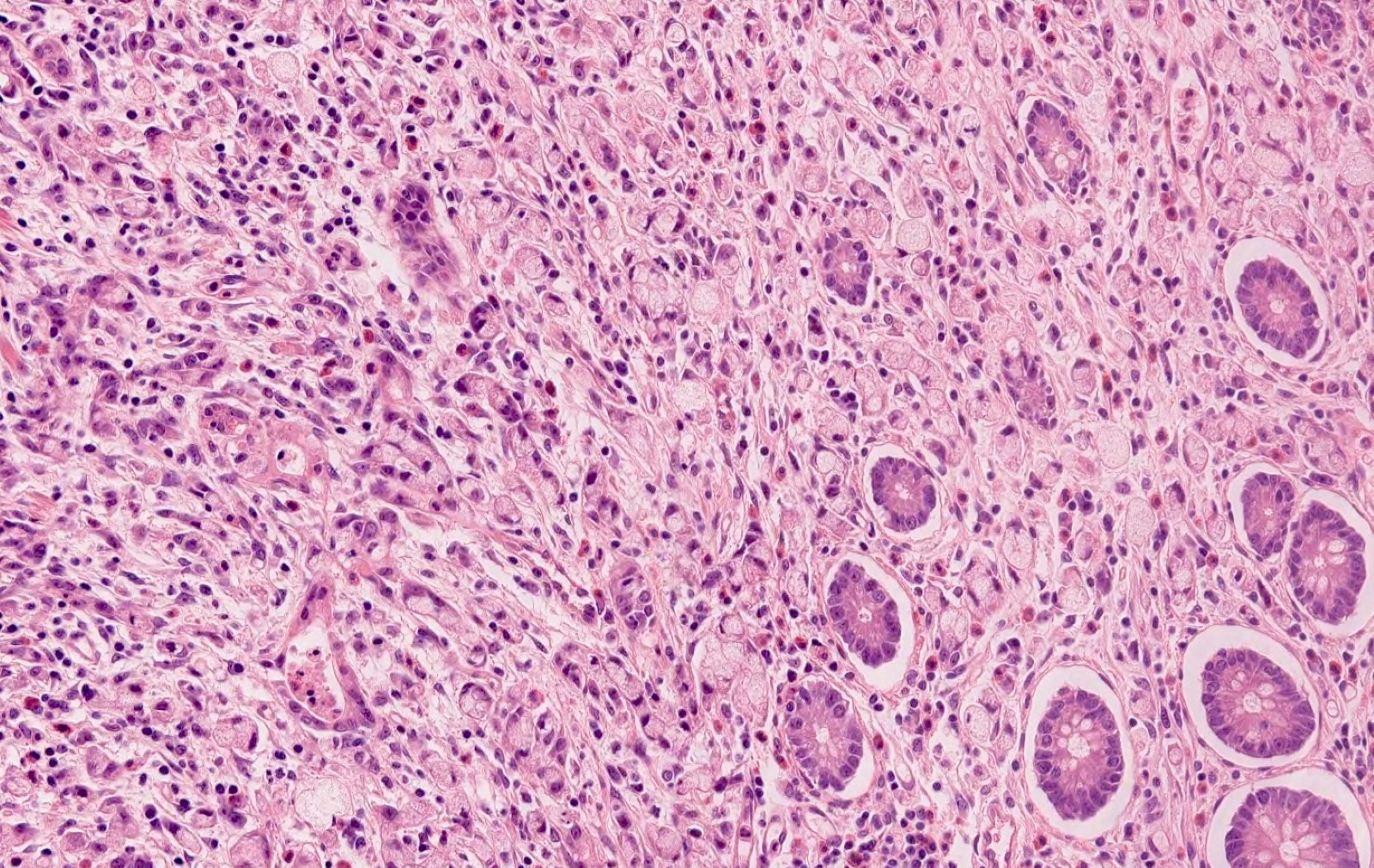
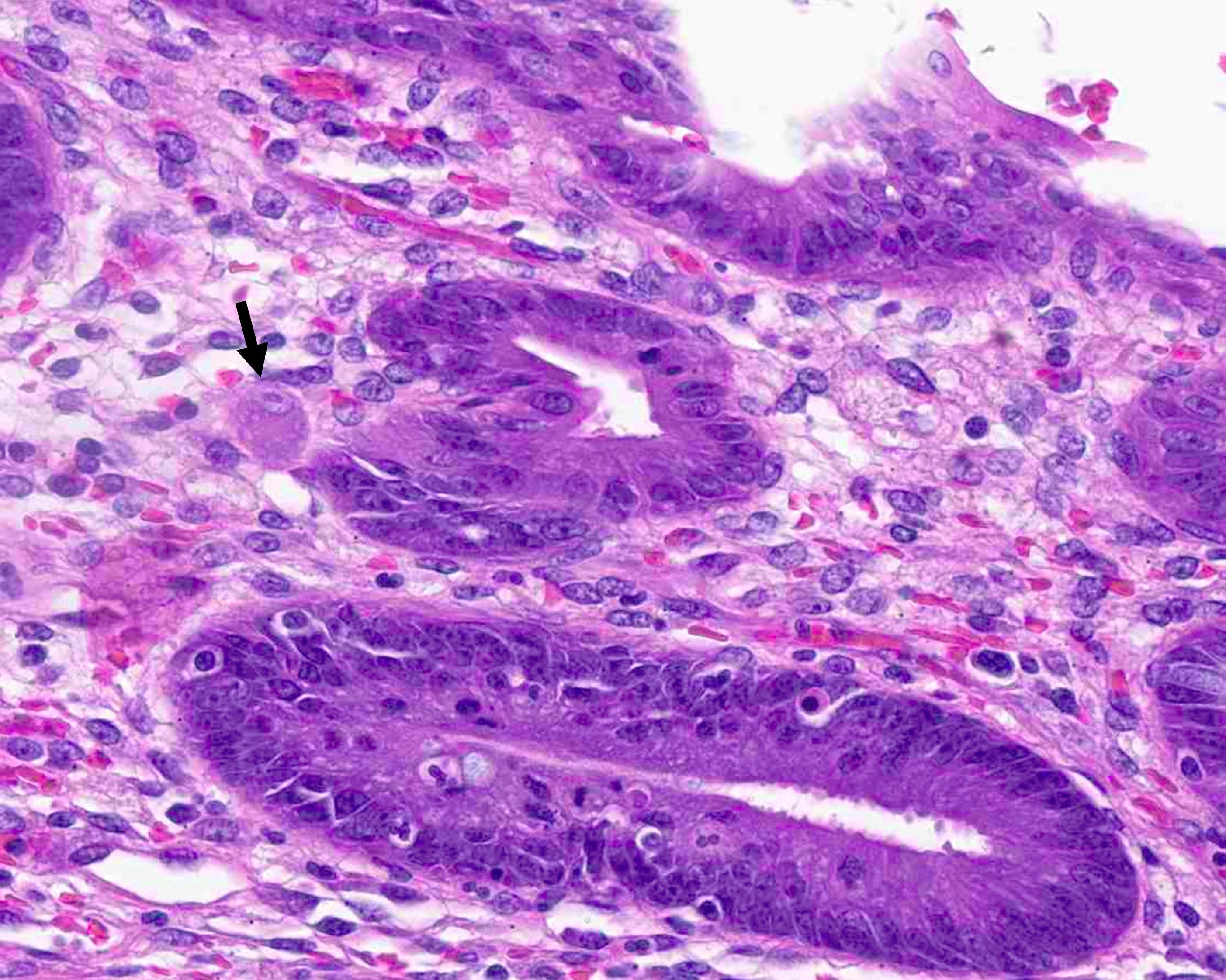
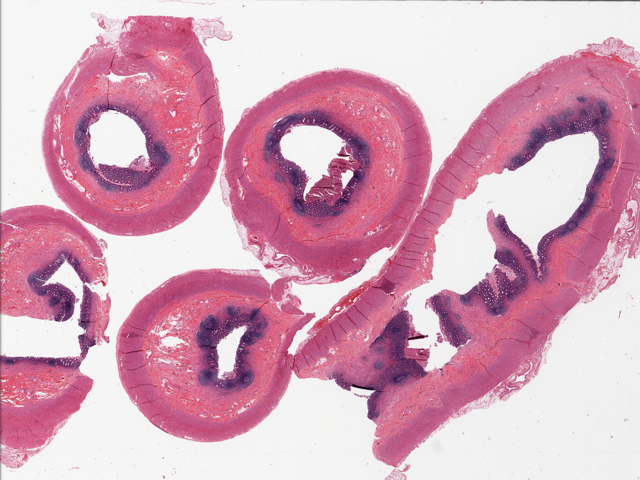
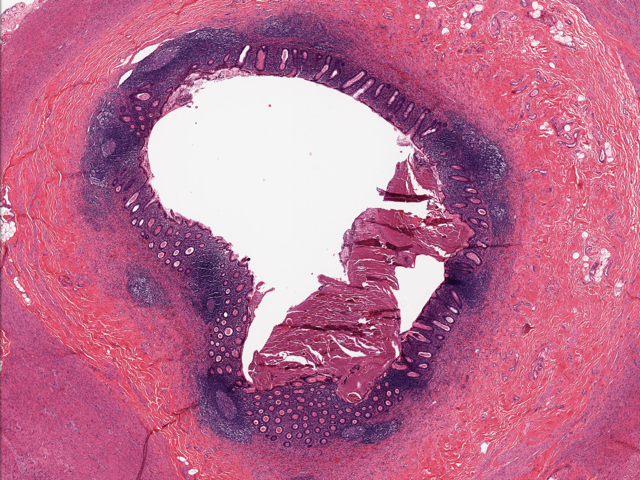
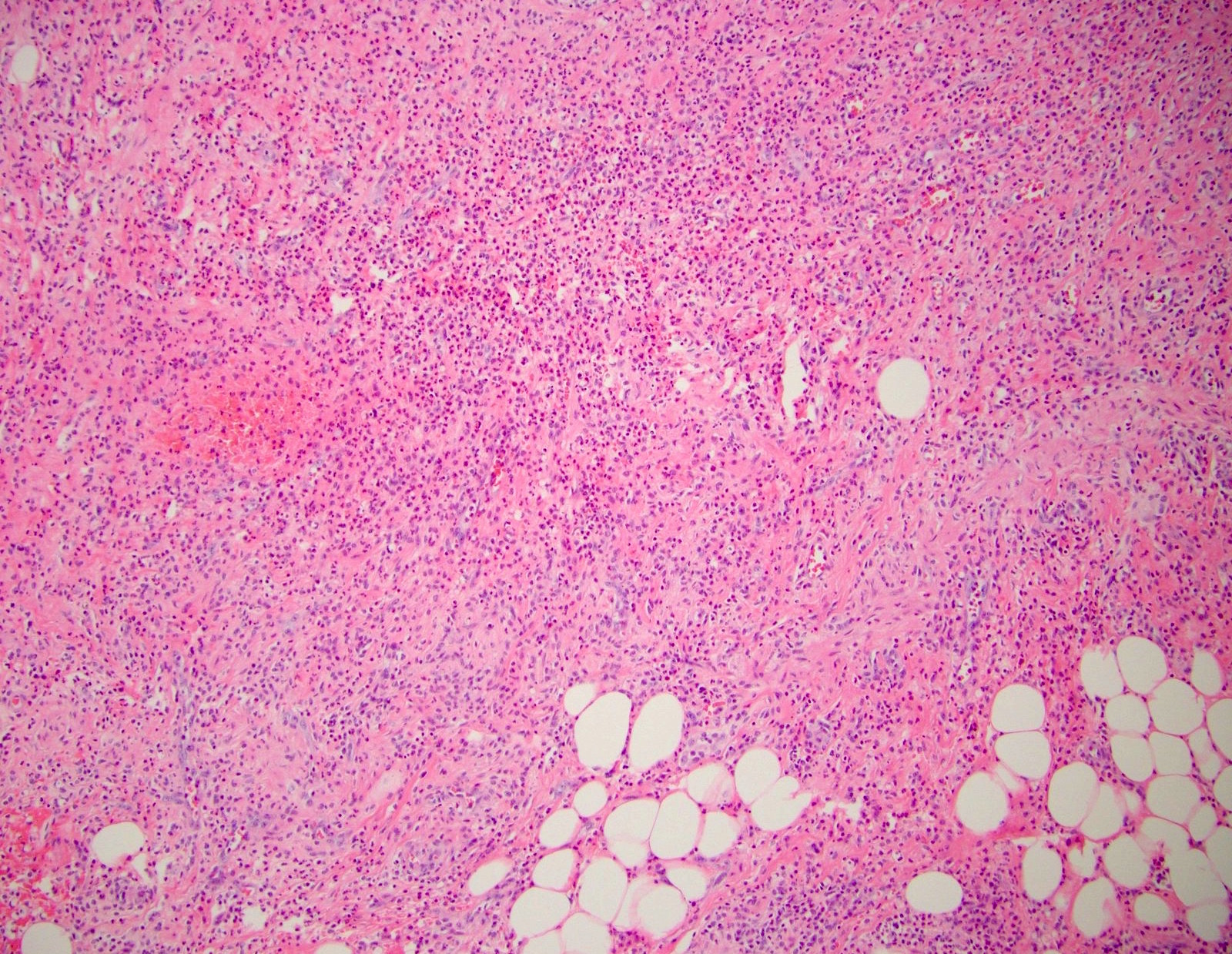
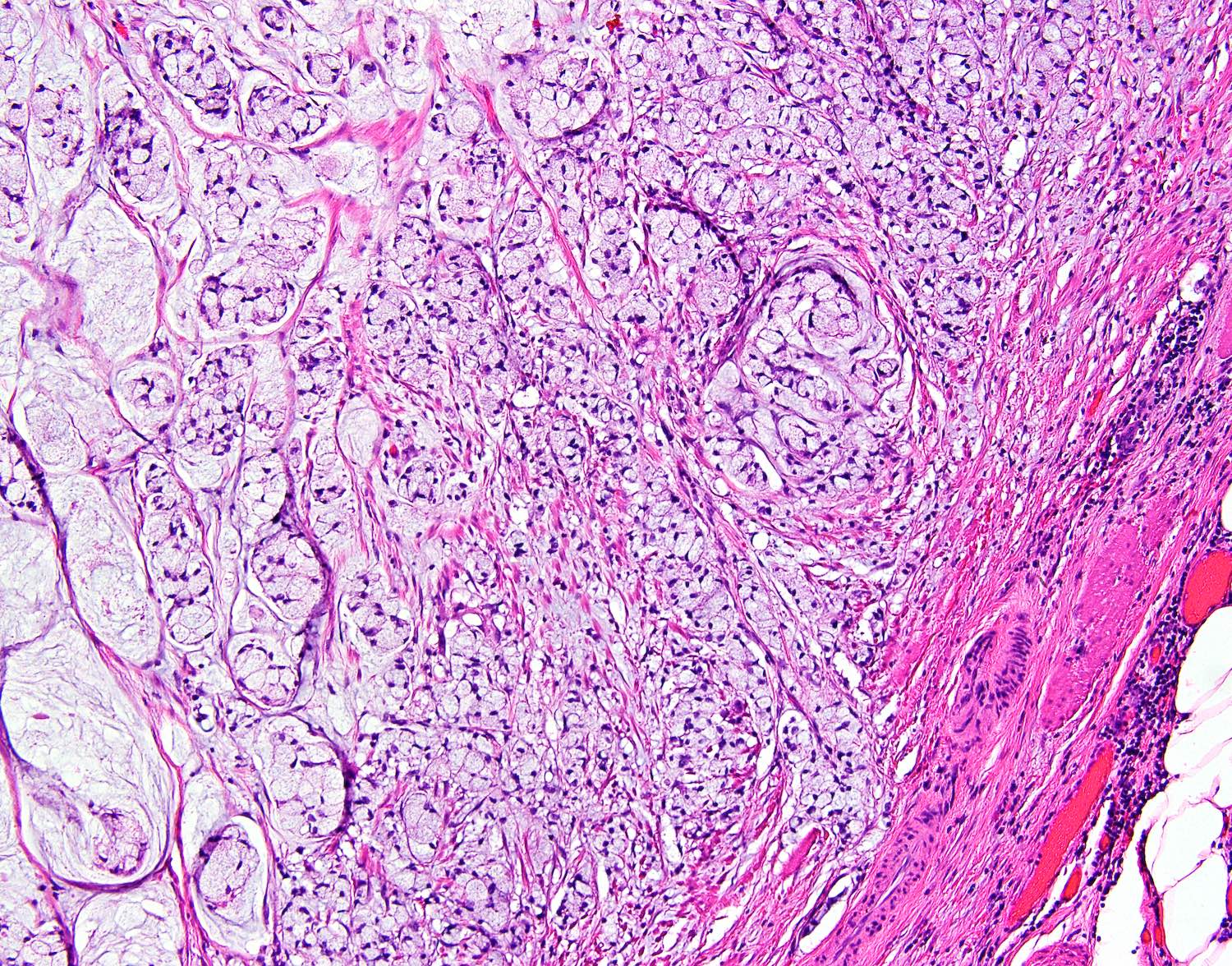
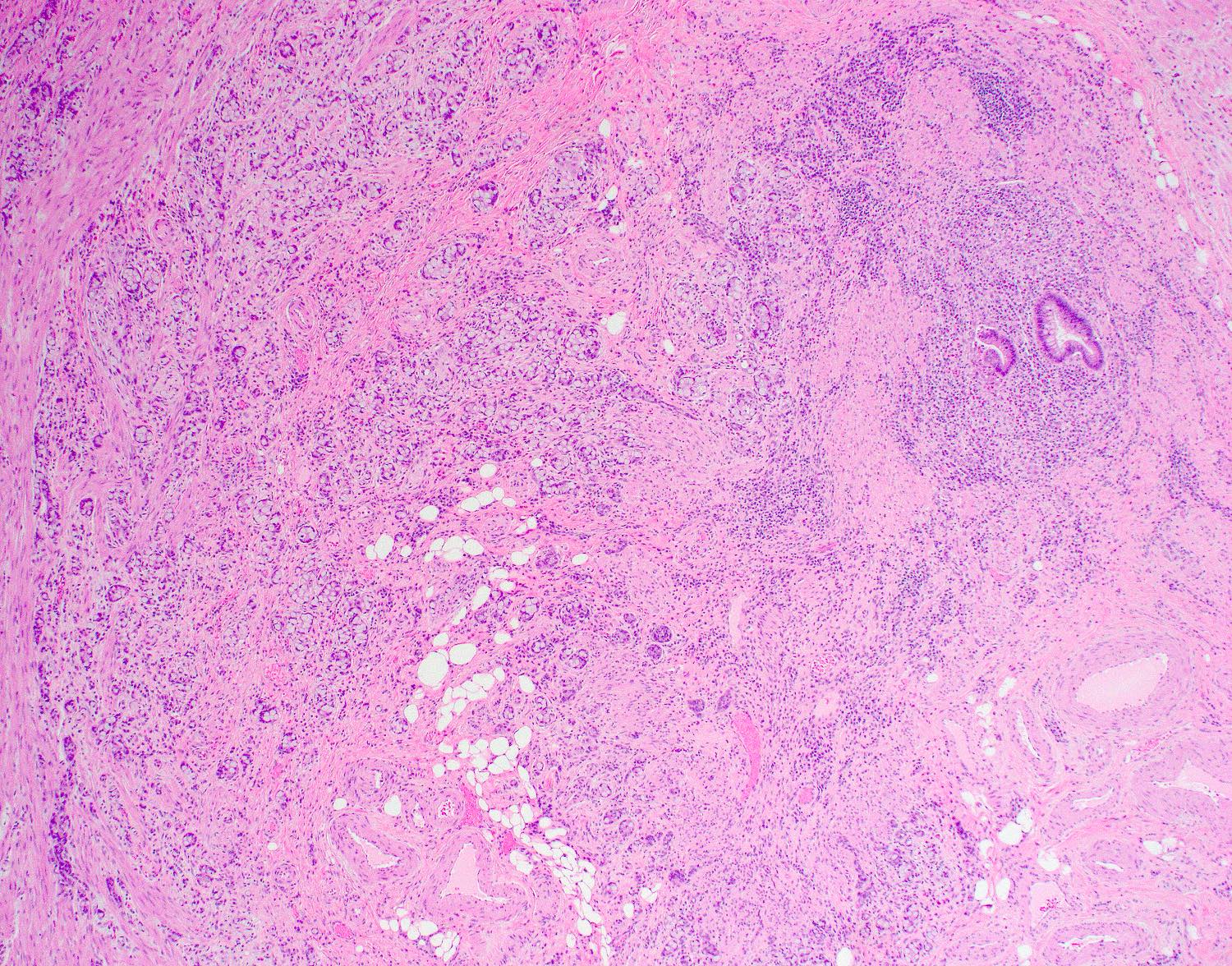
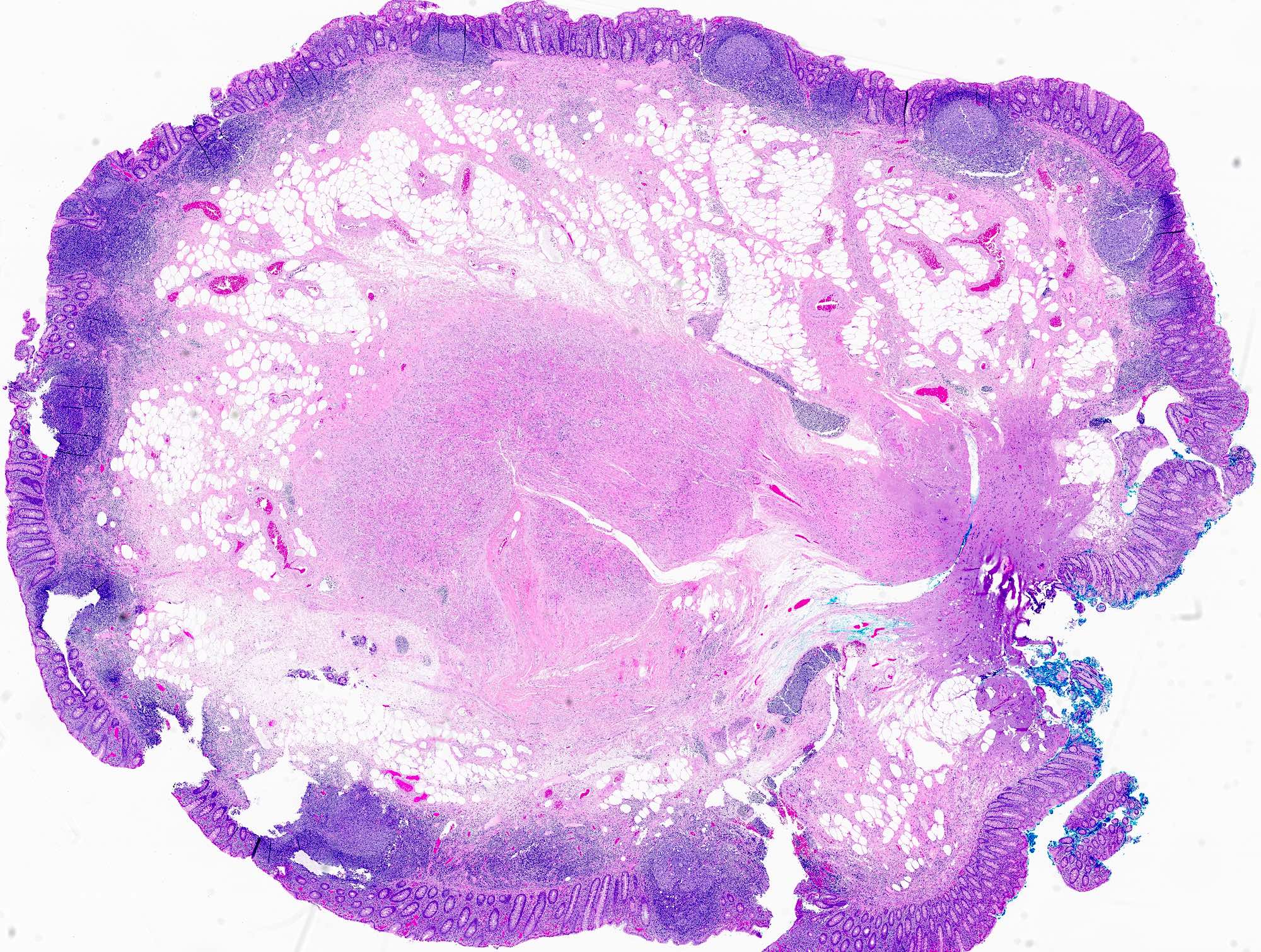
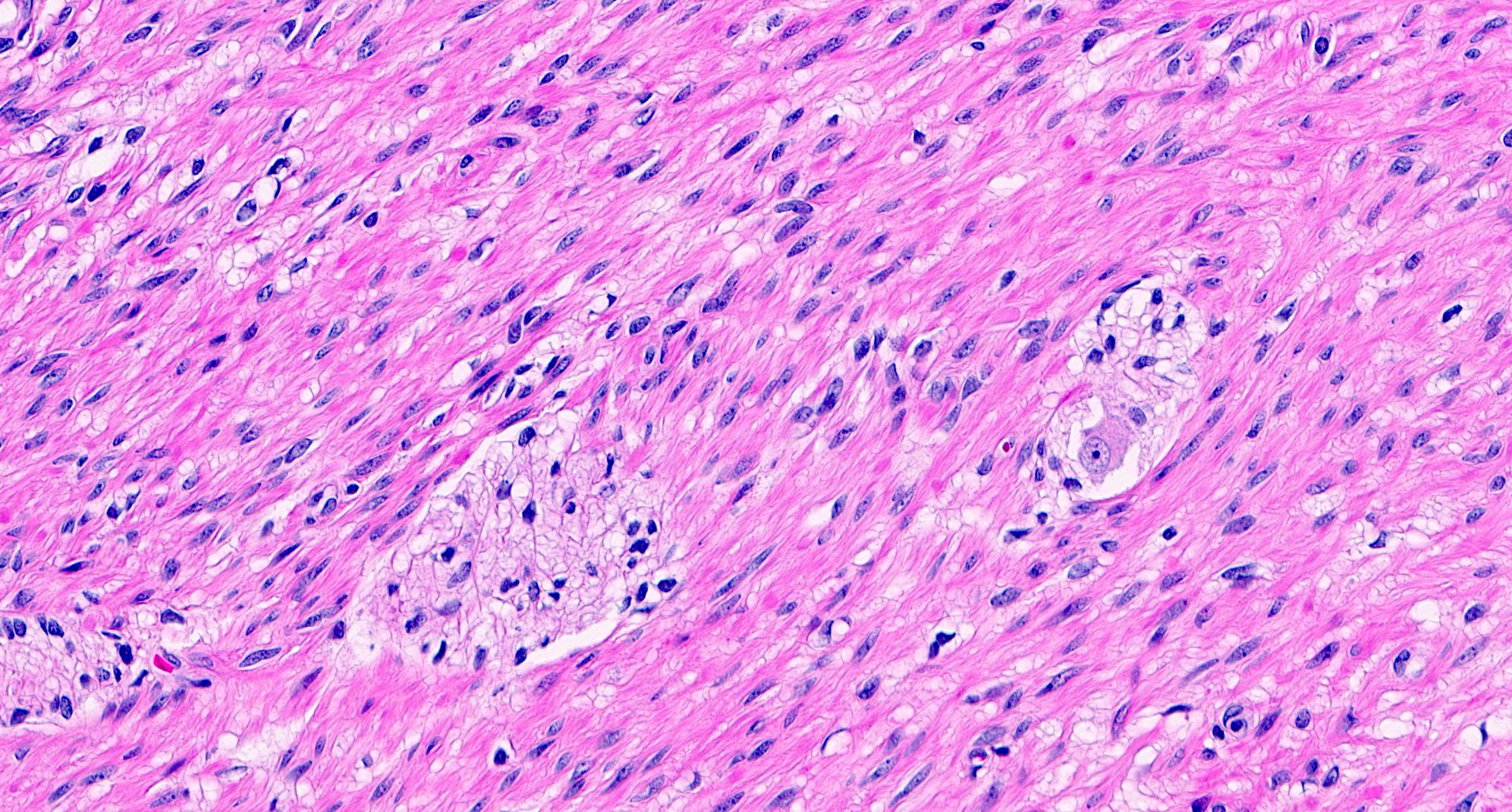
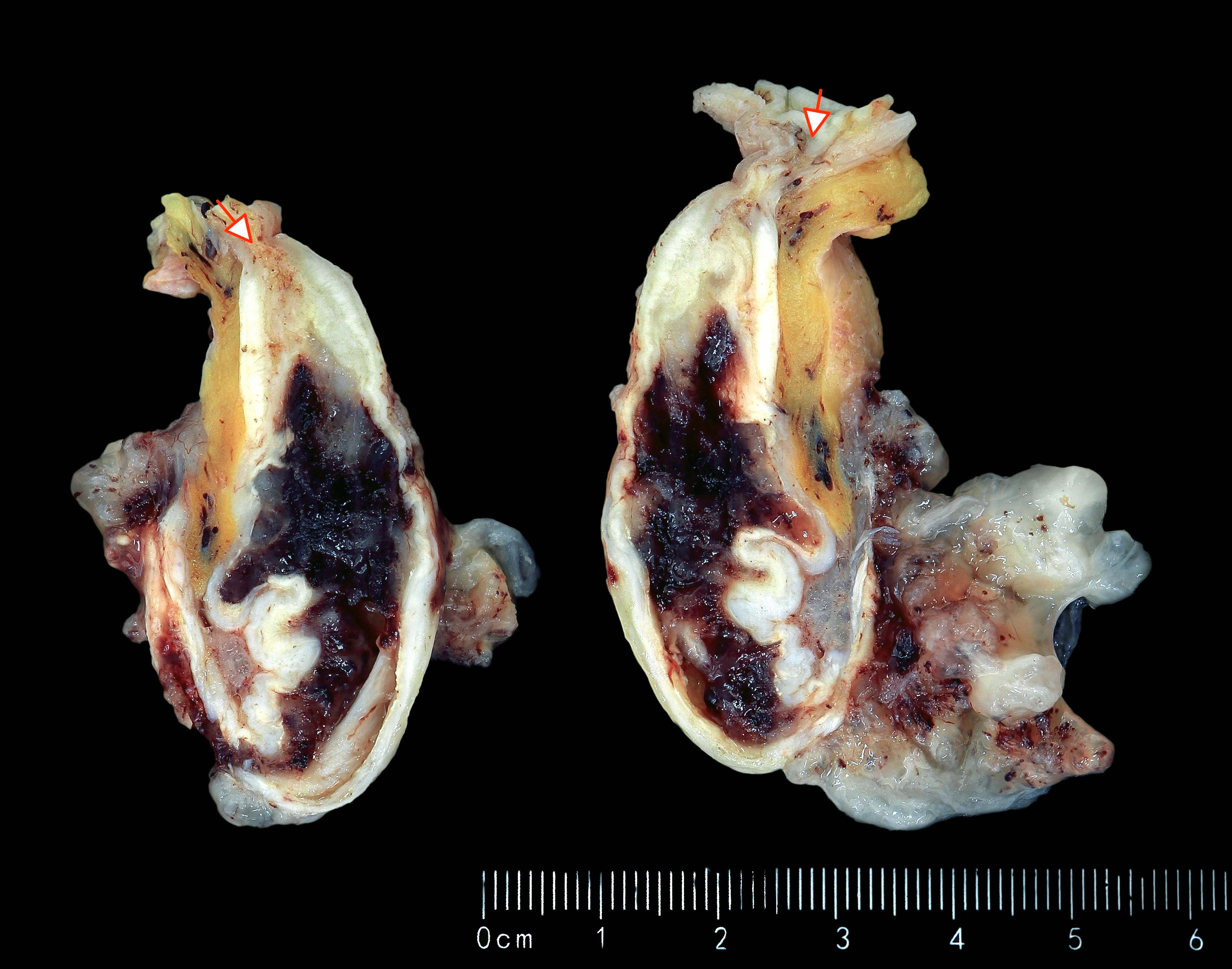
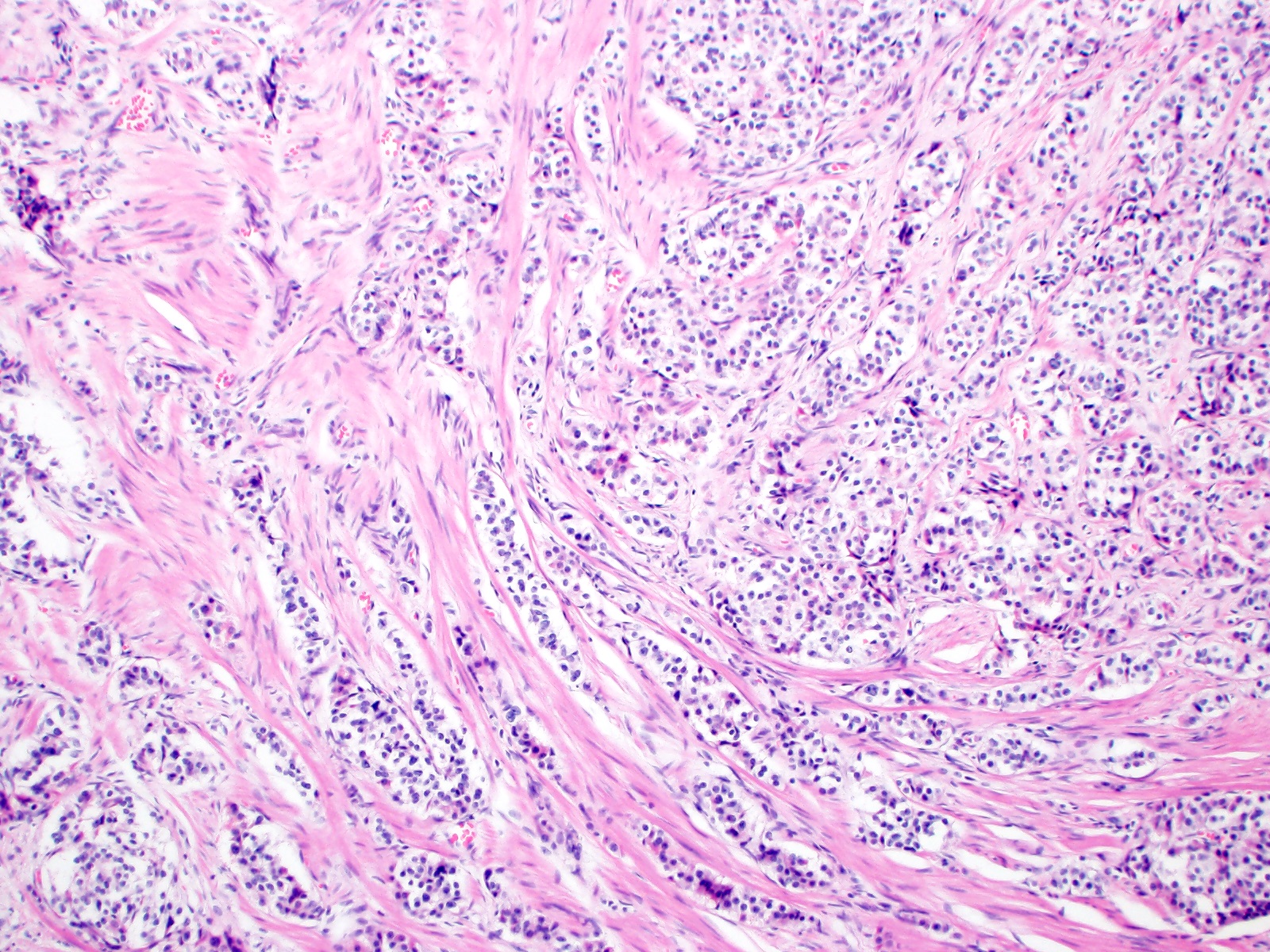
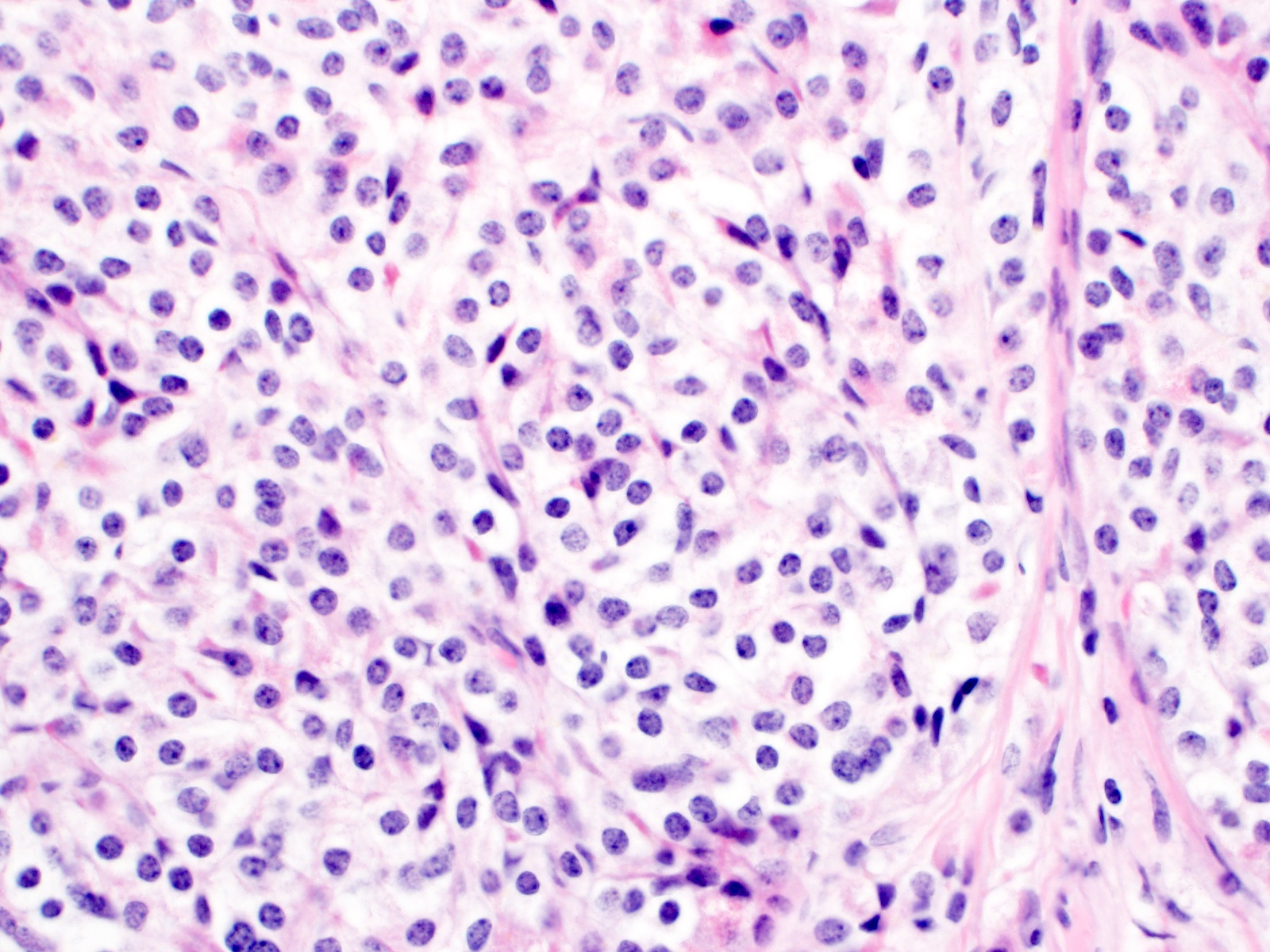
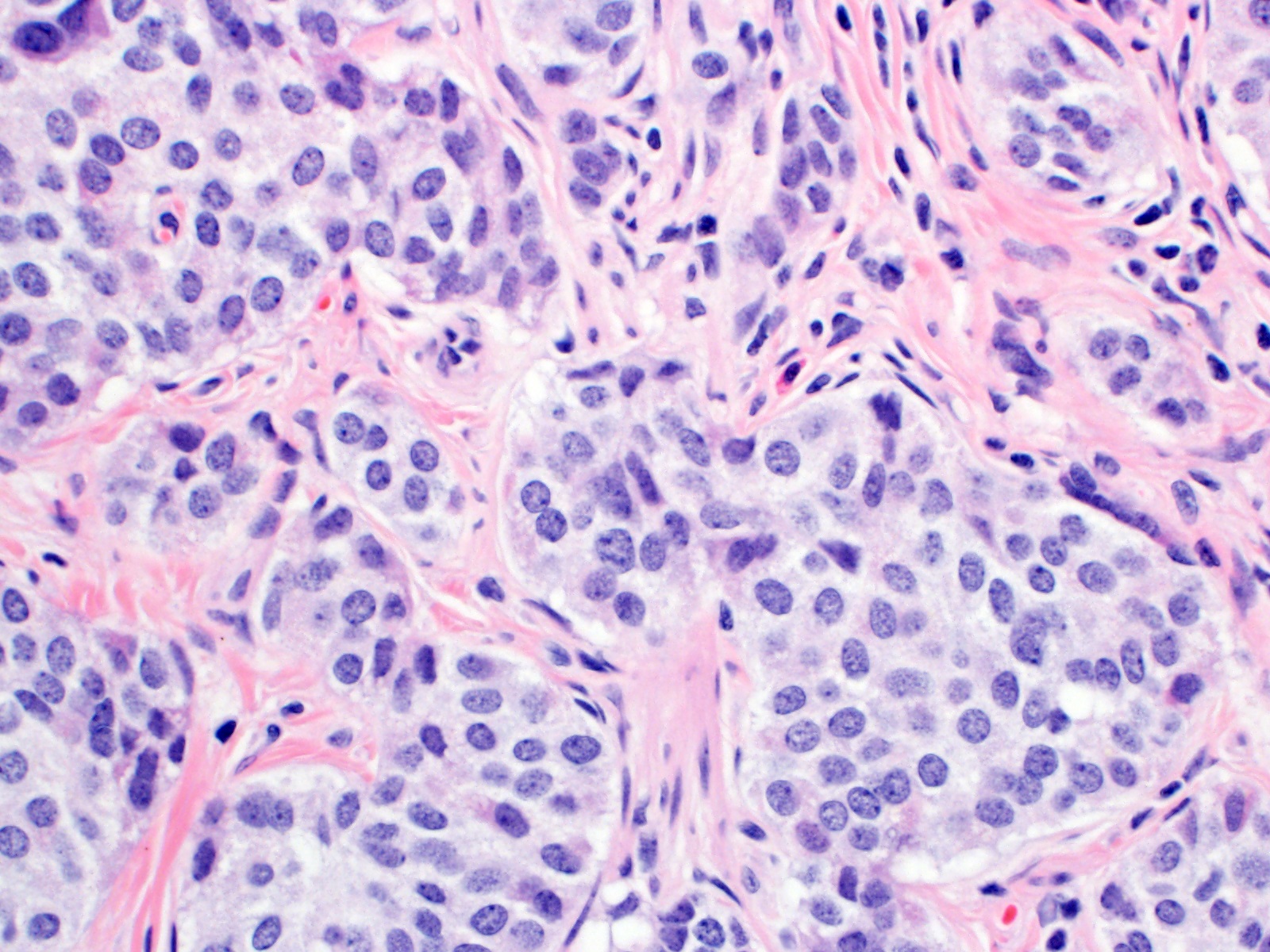
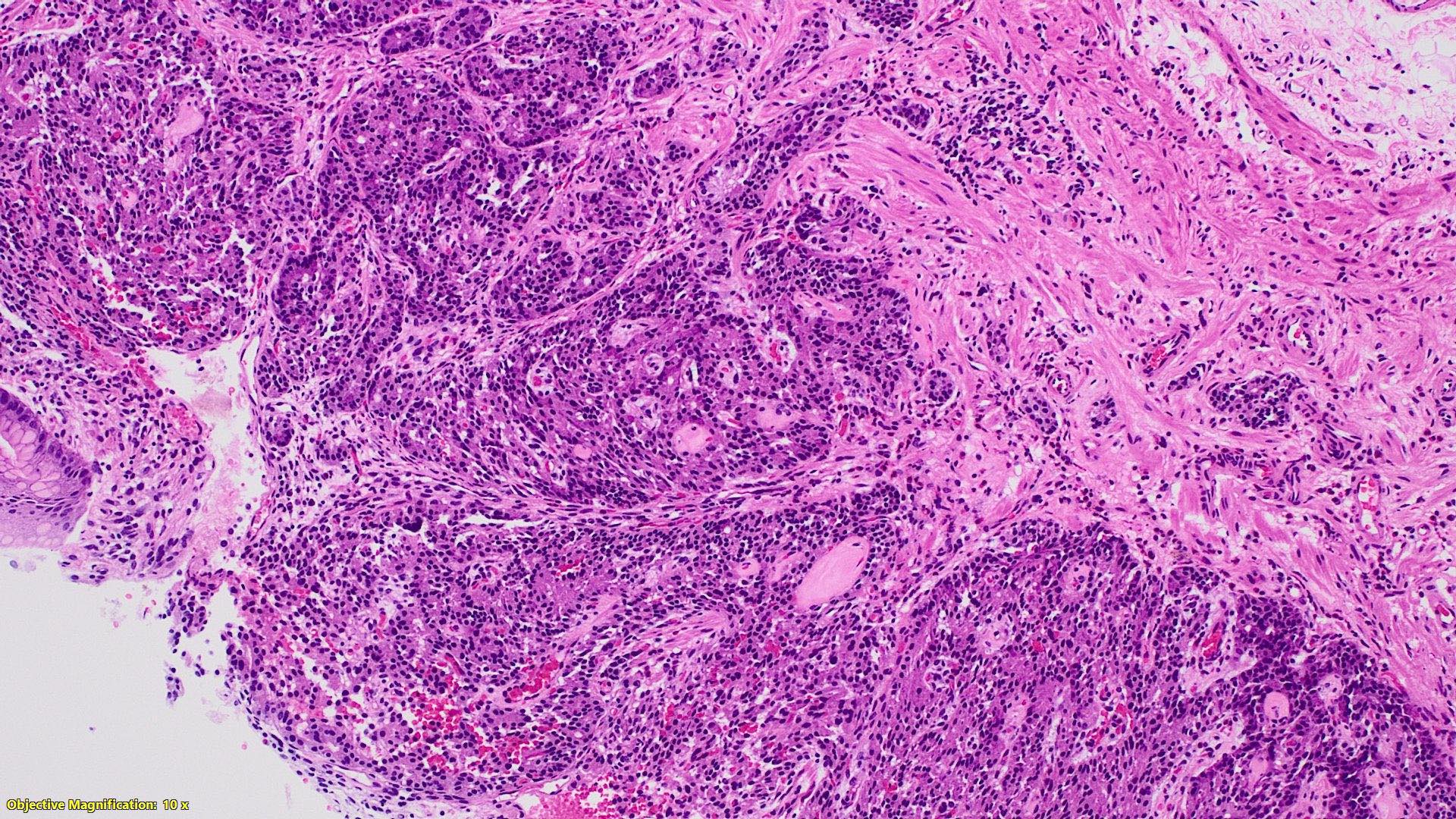
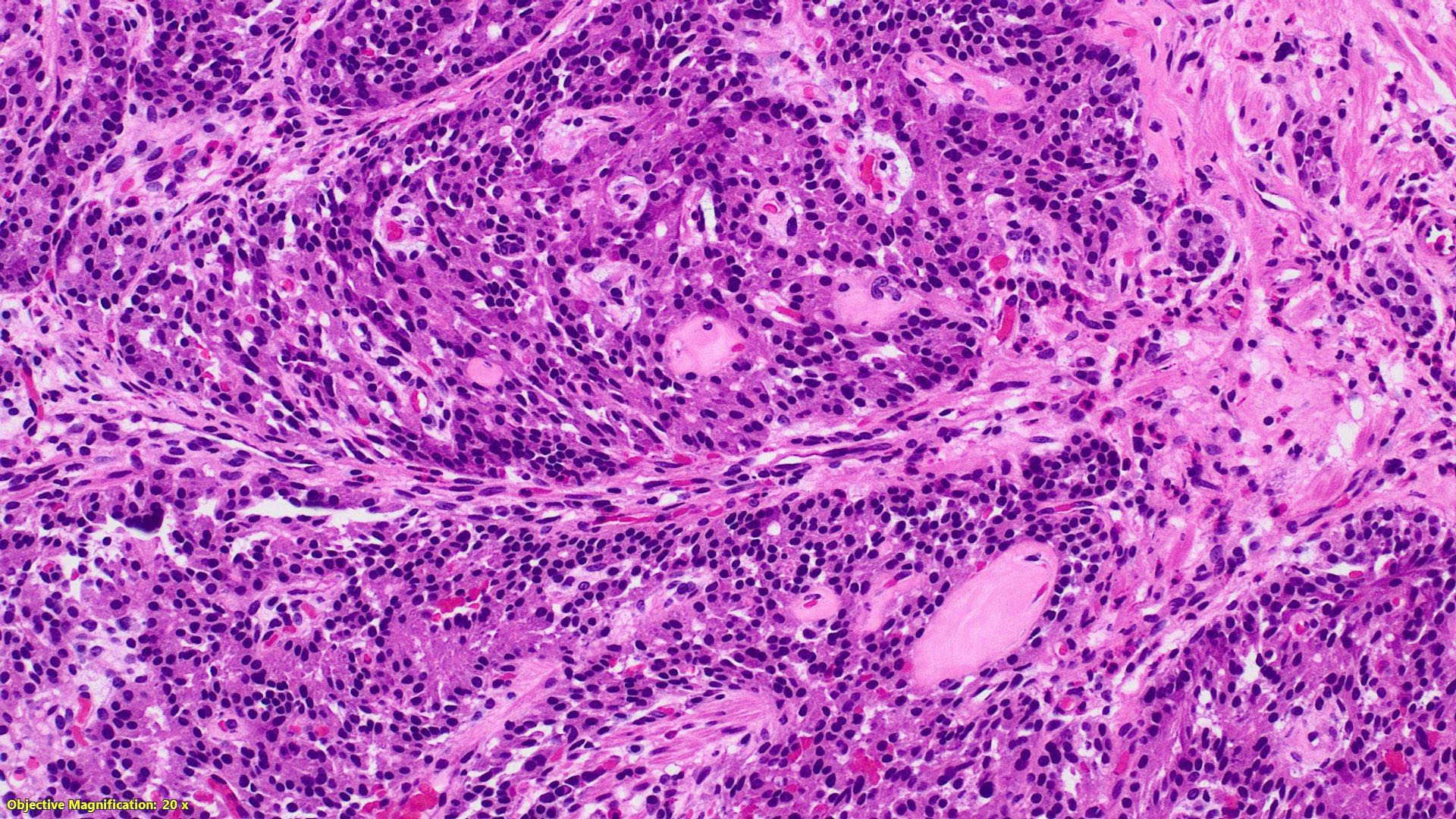
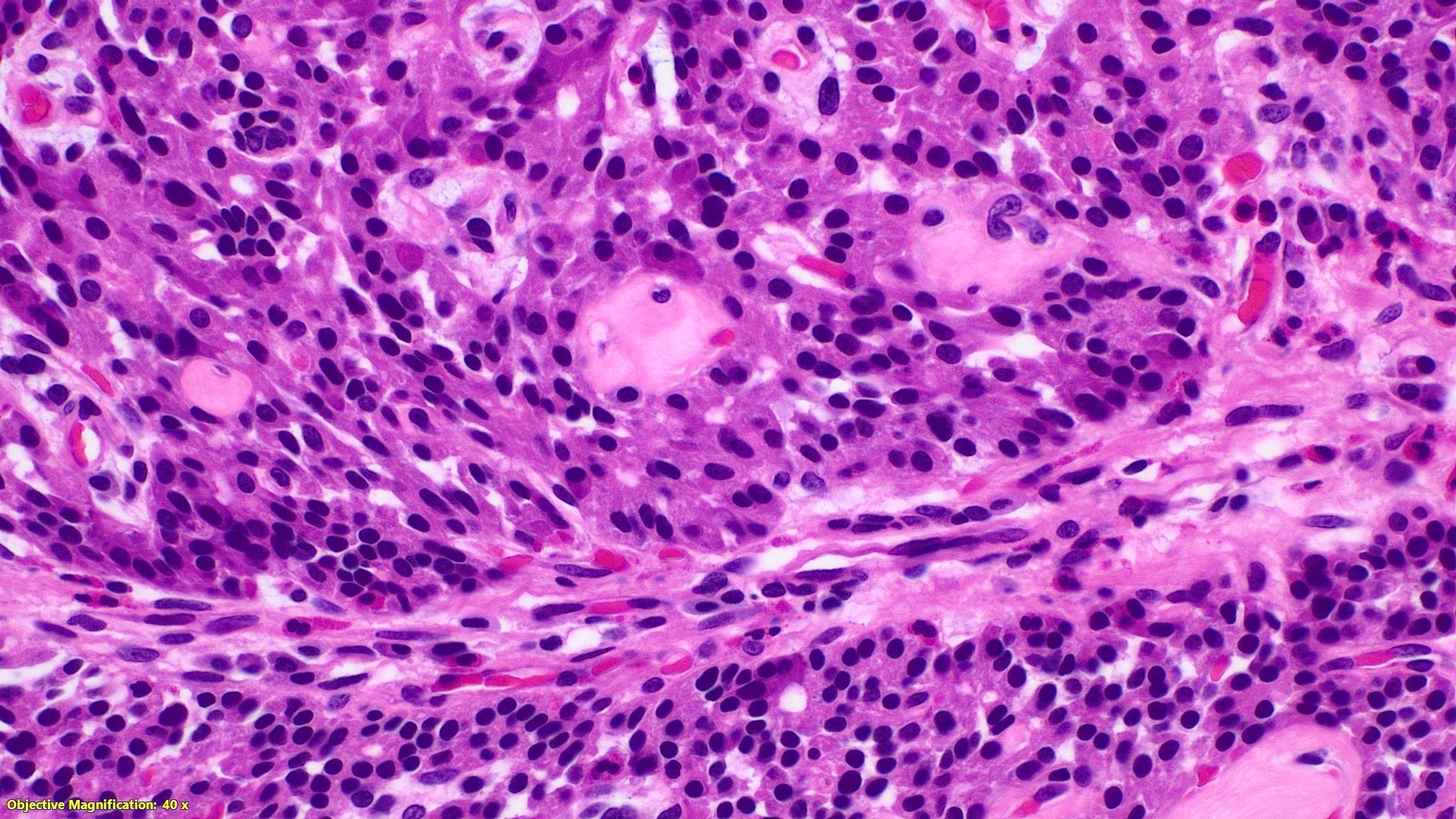
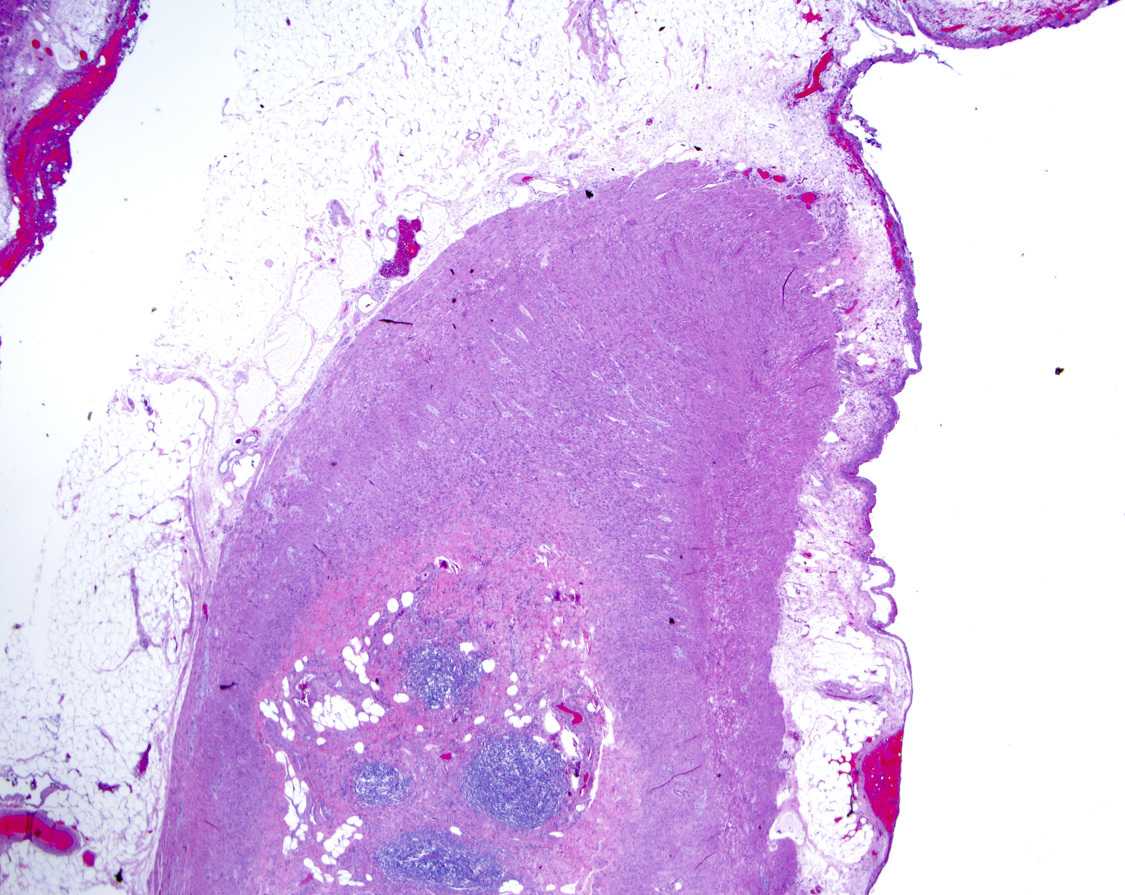
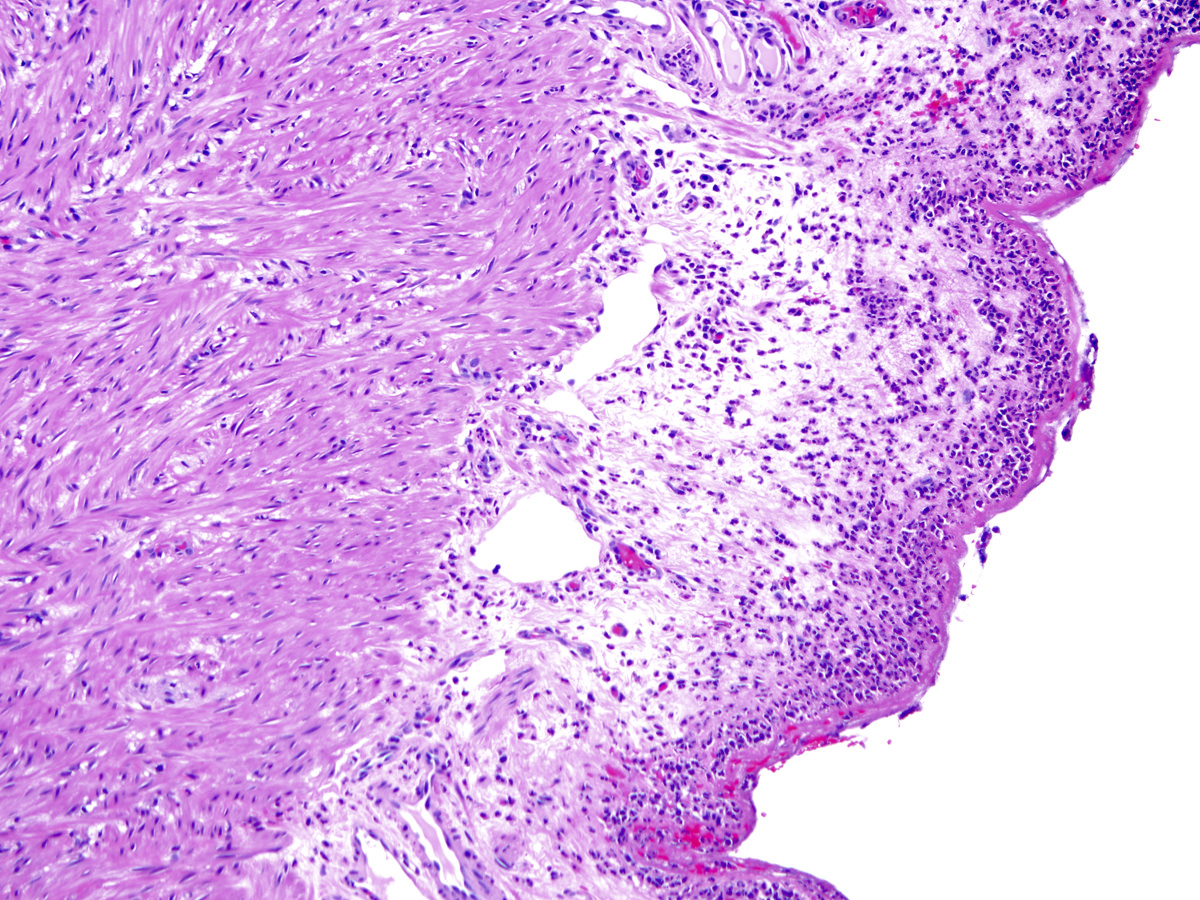
- T2: tumor invades the muscularis propria
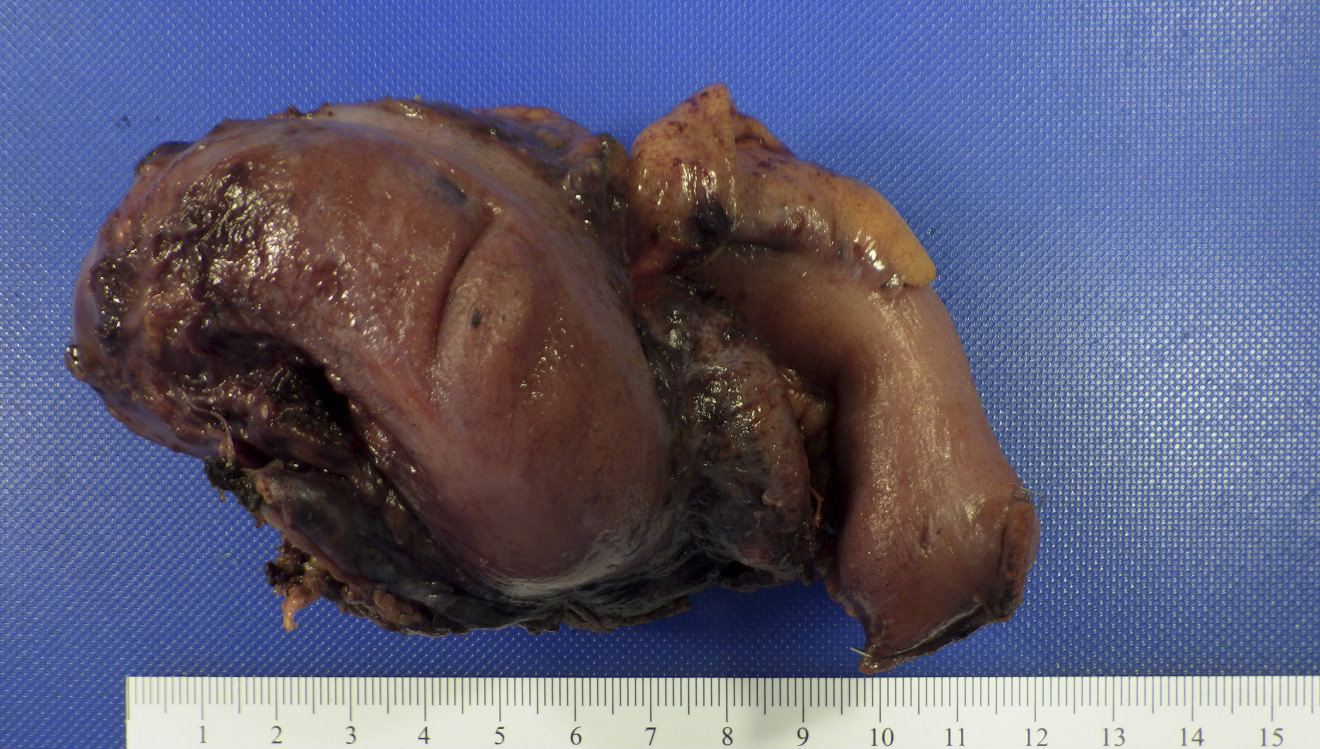
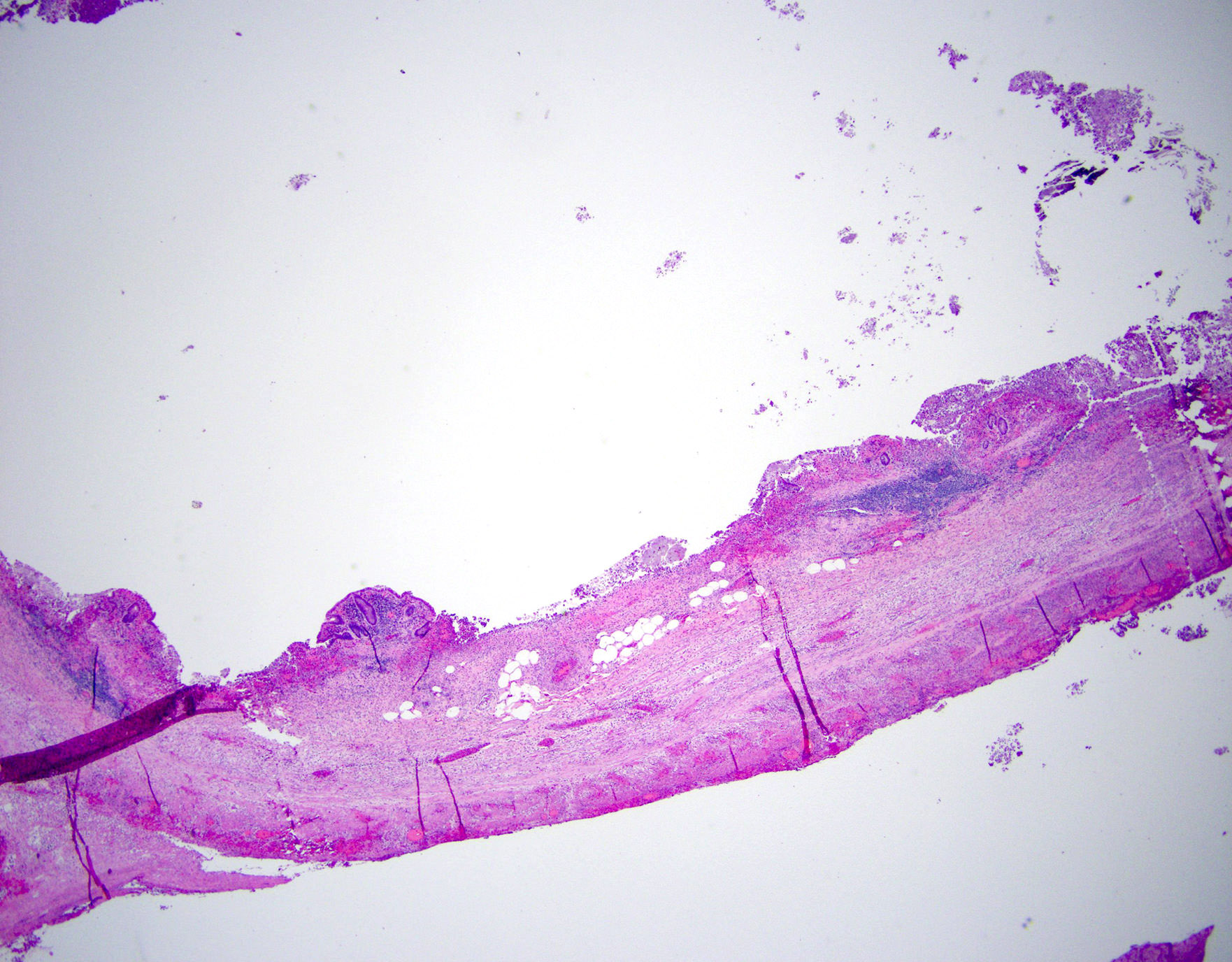
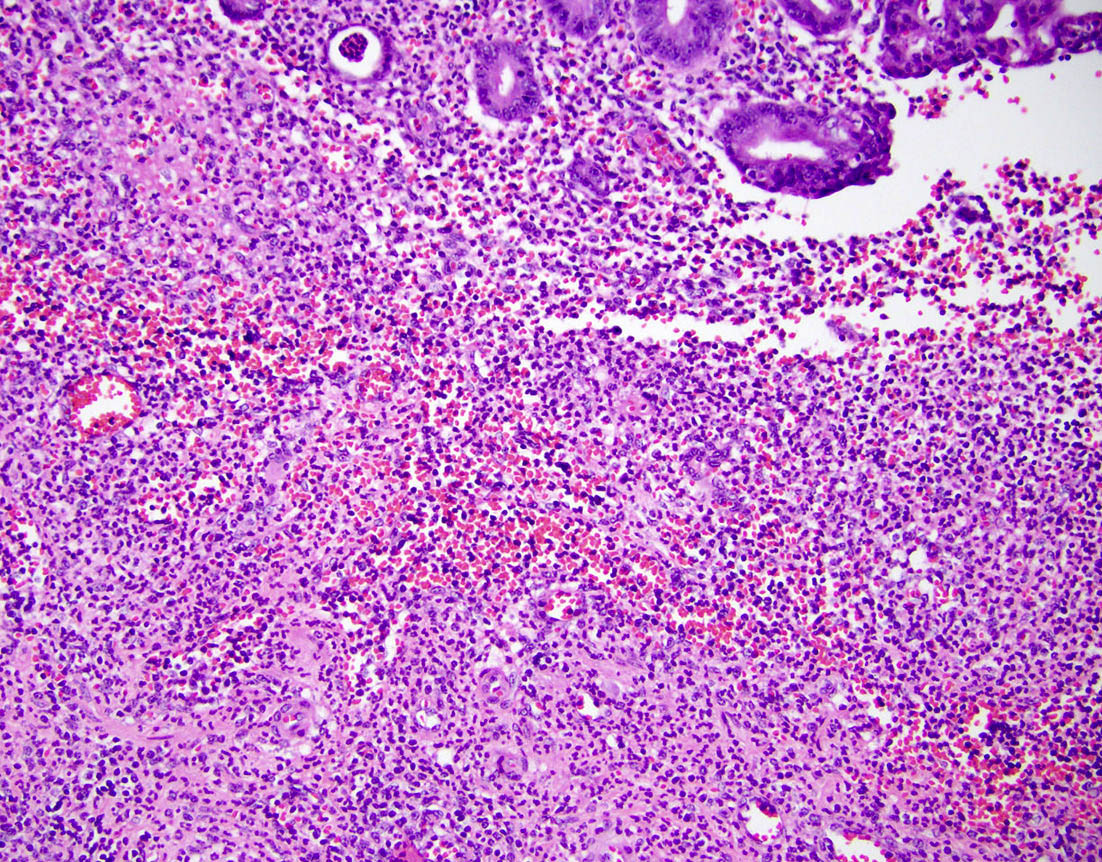
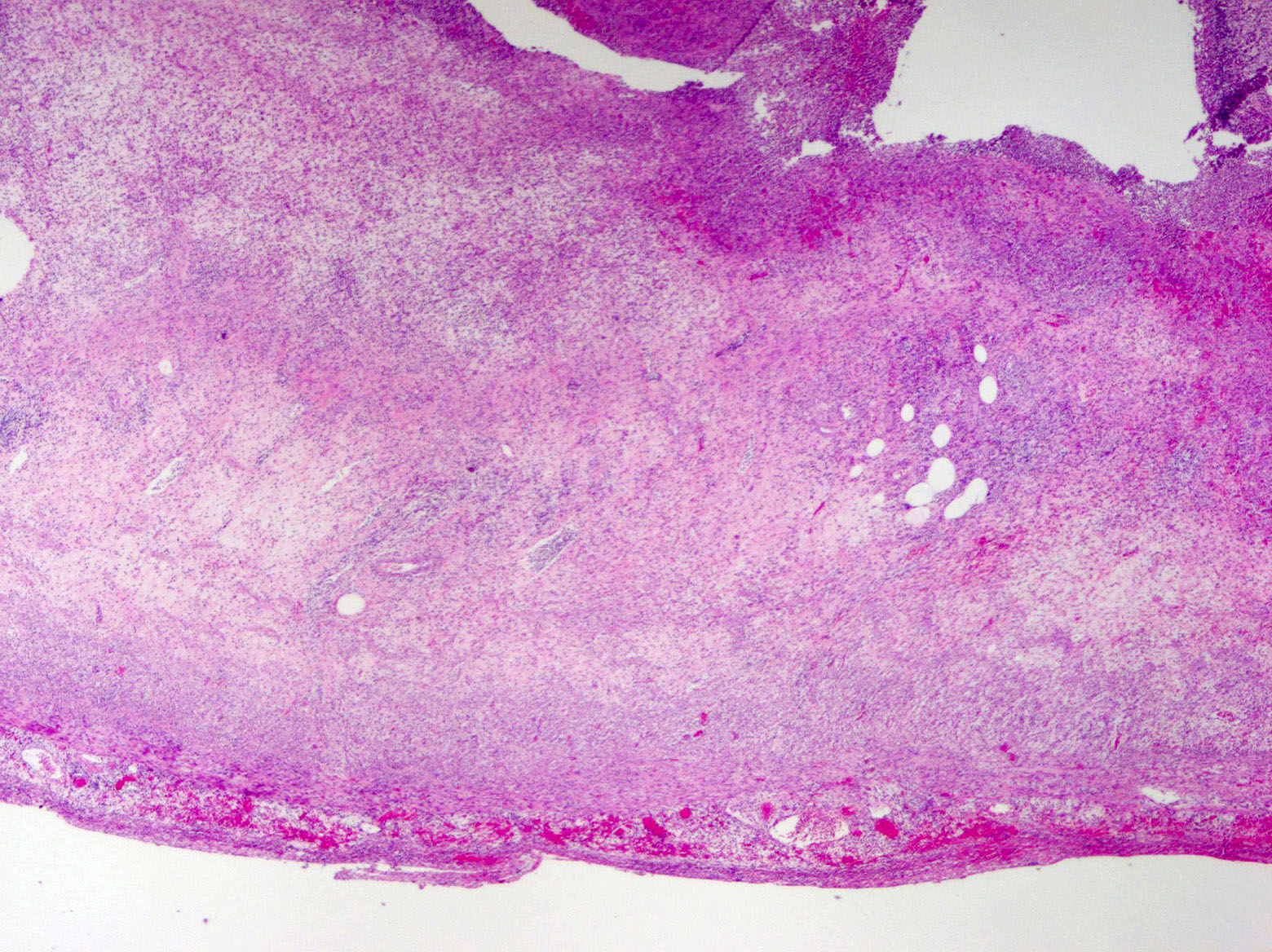
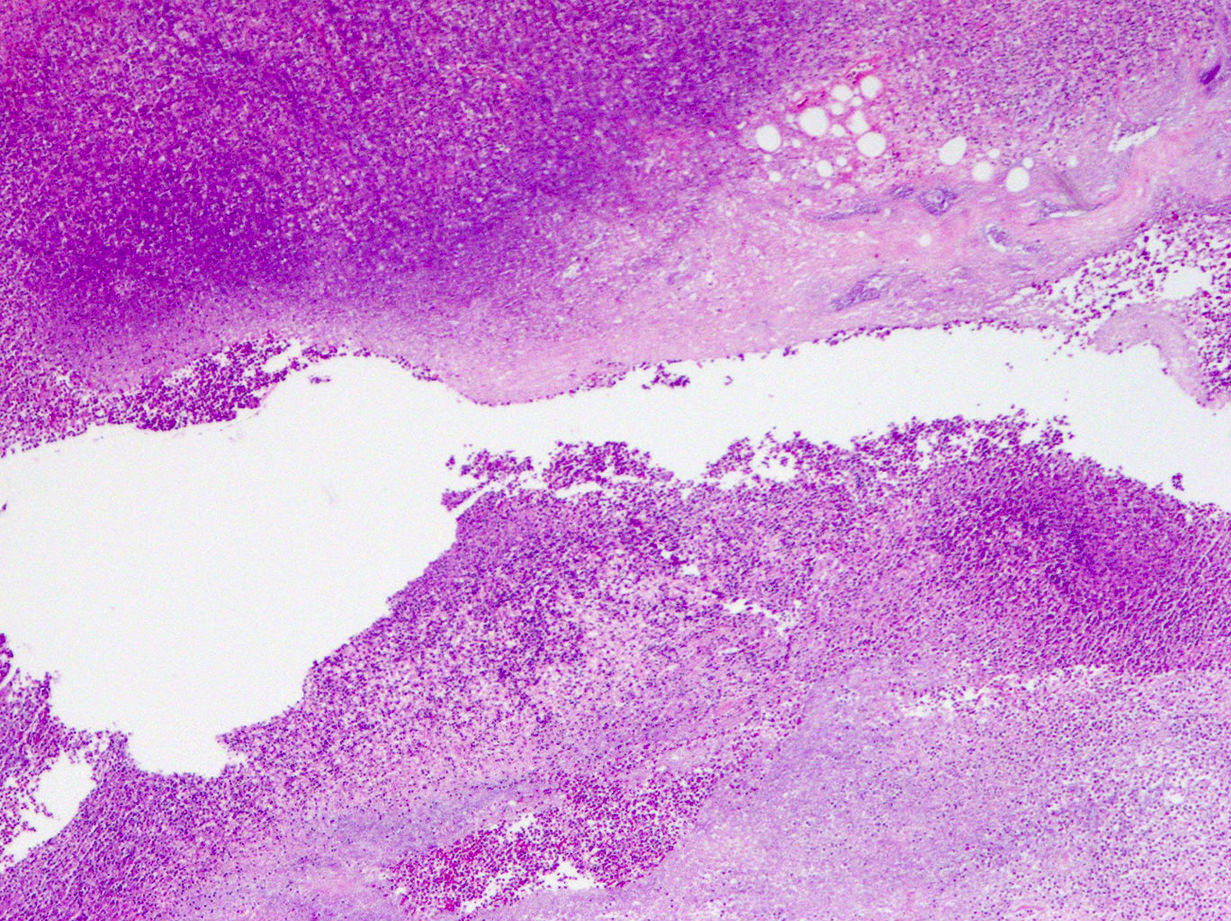
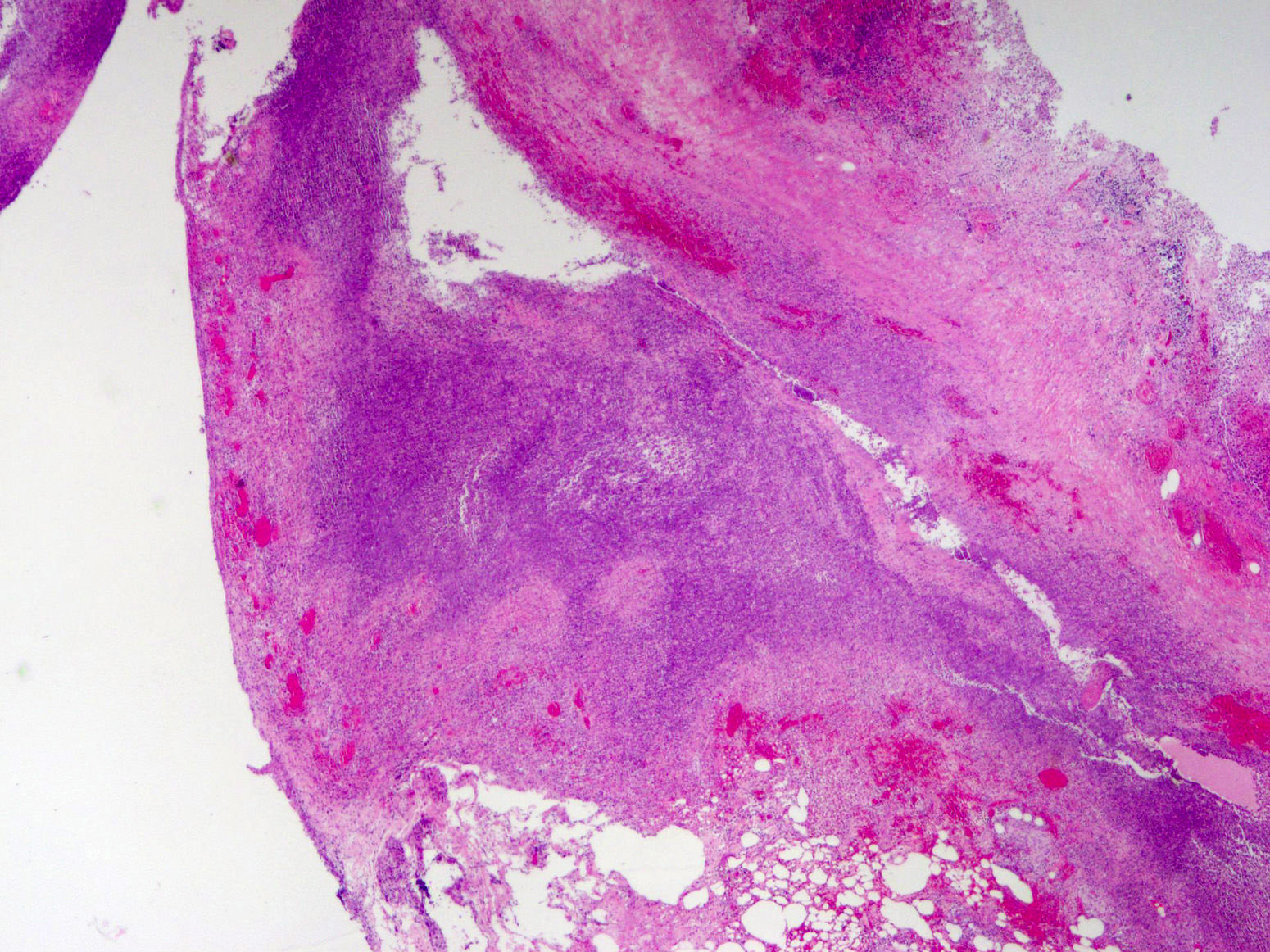
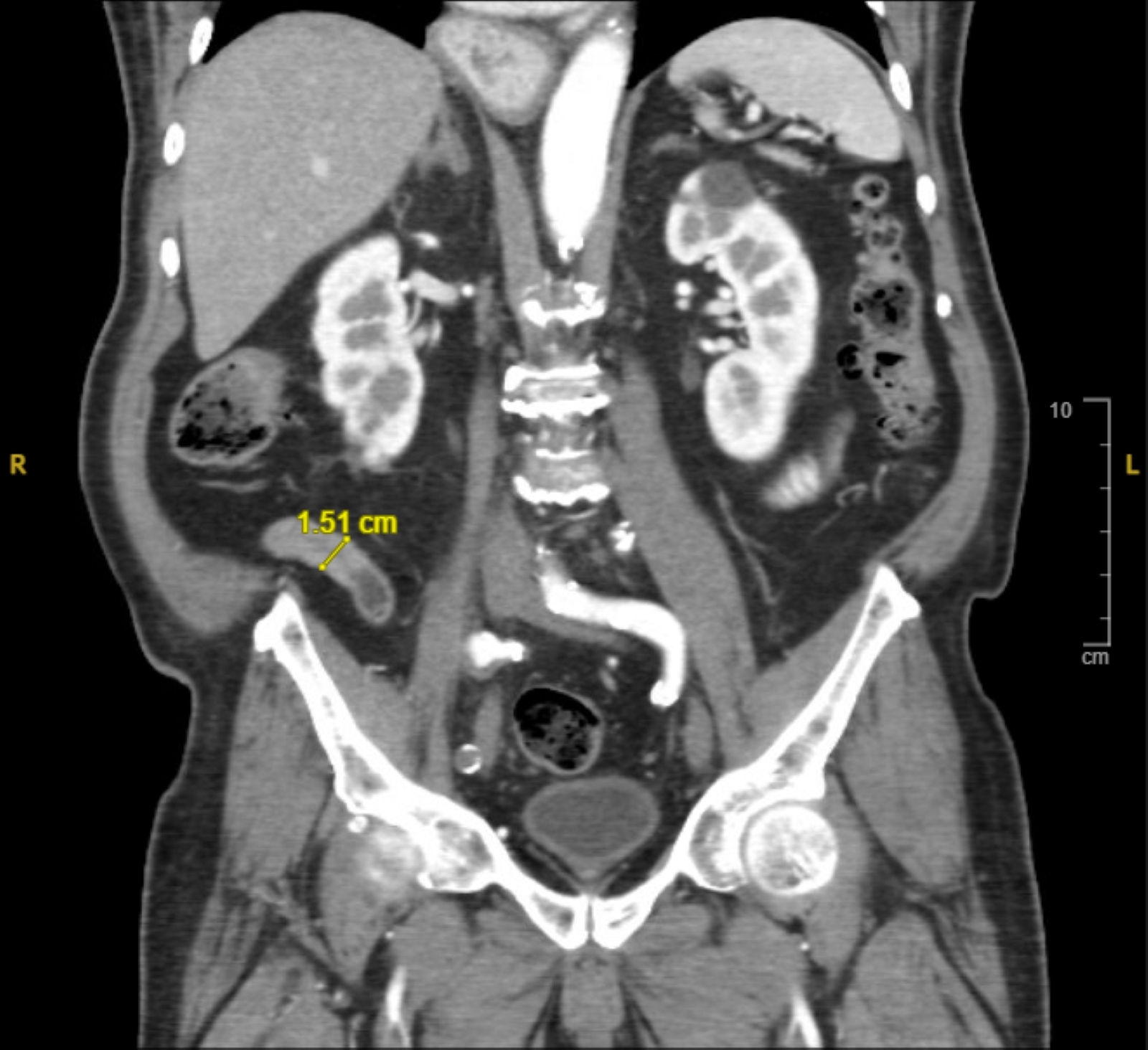
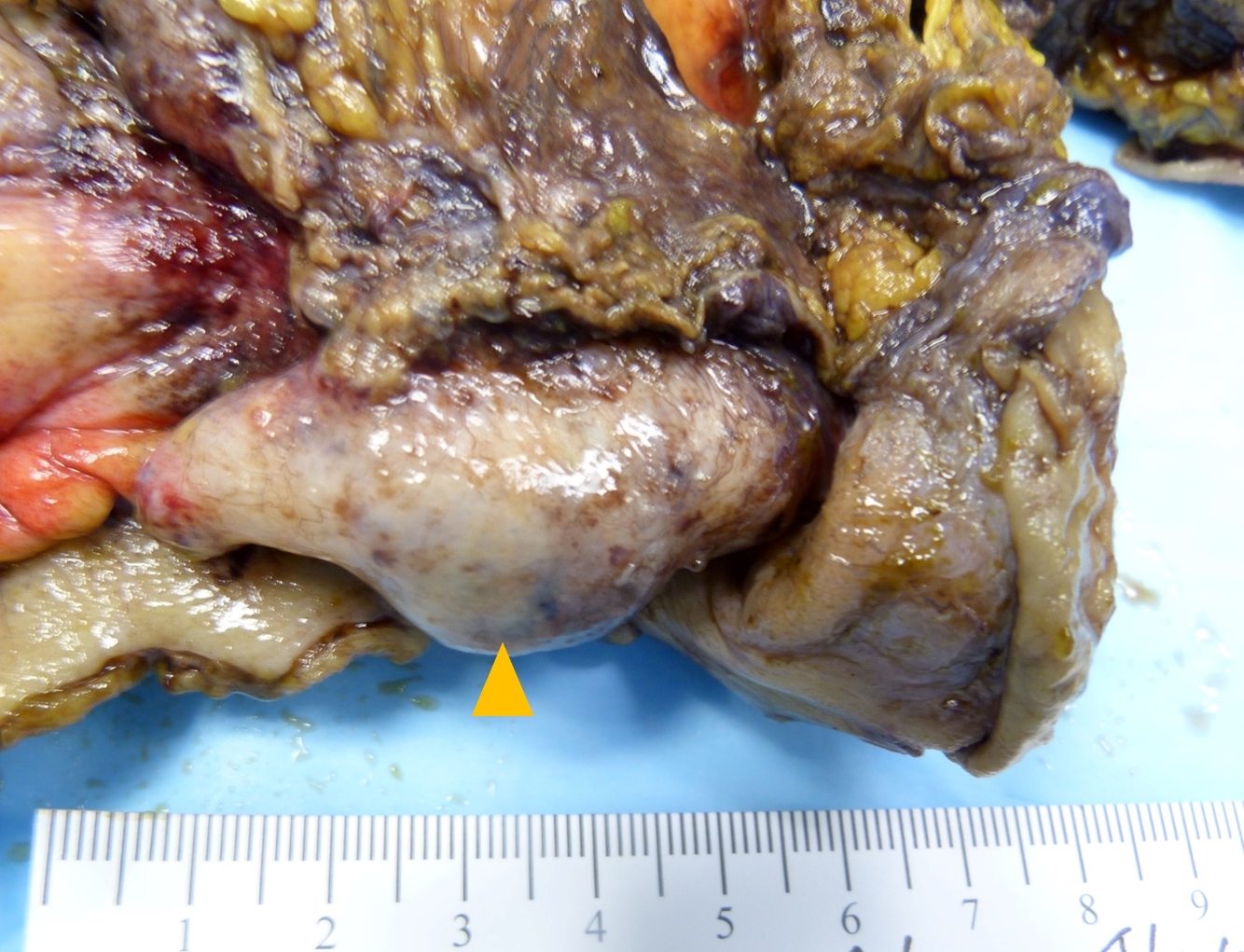
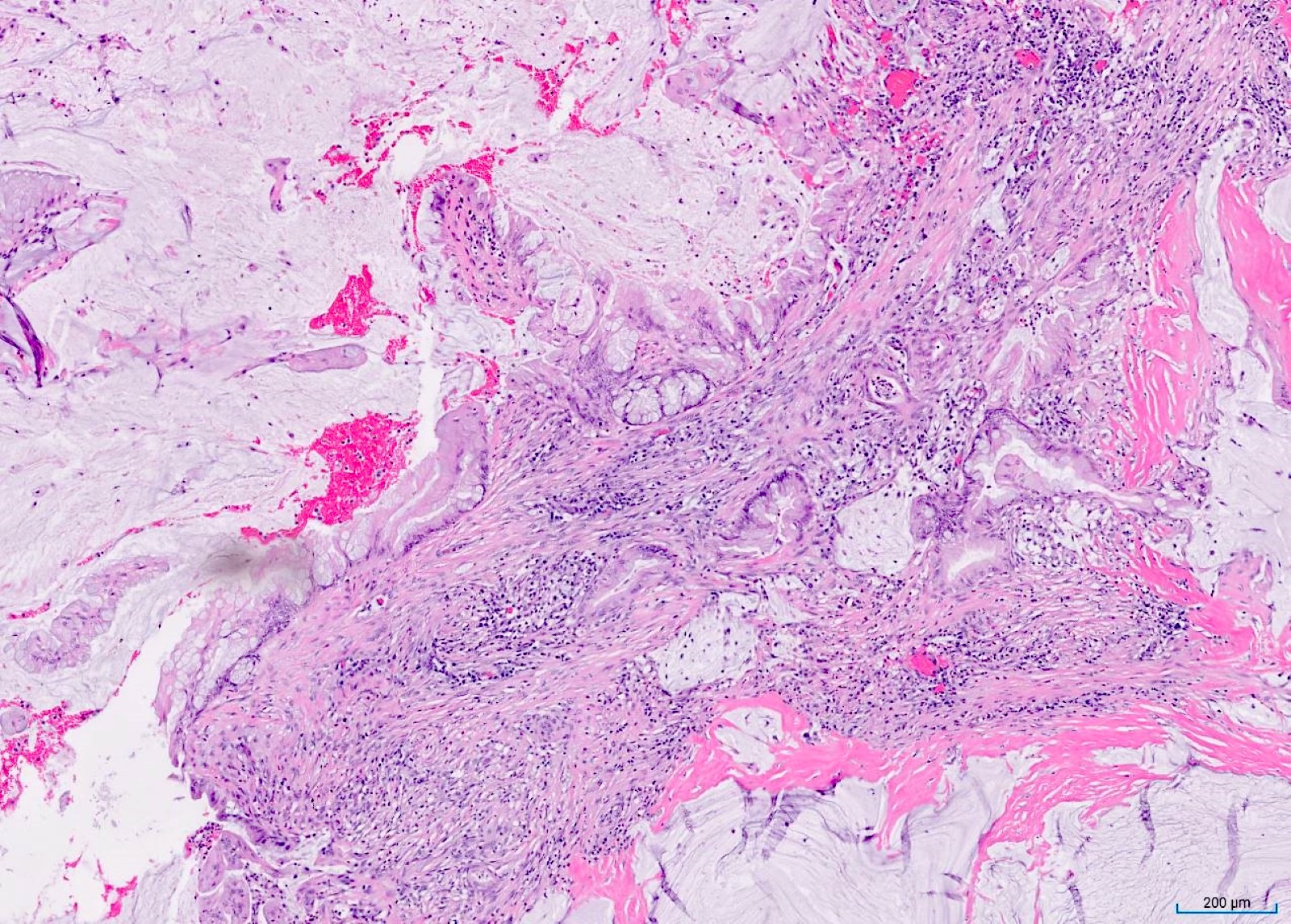
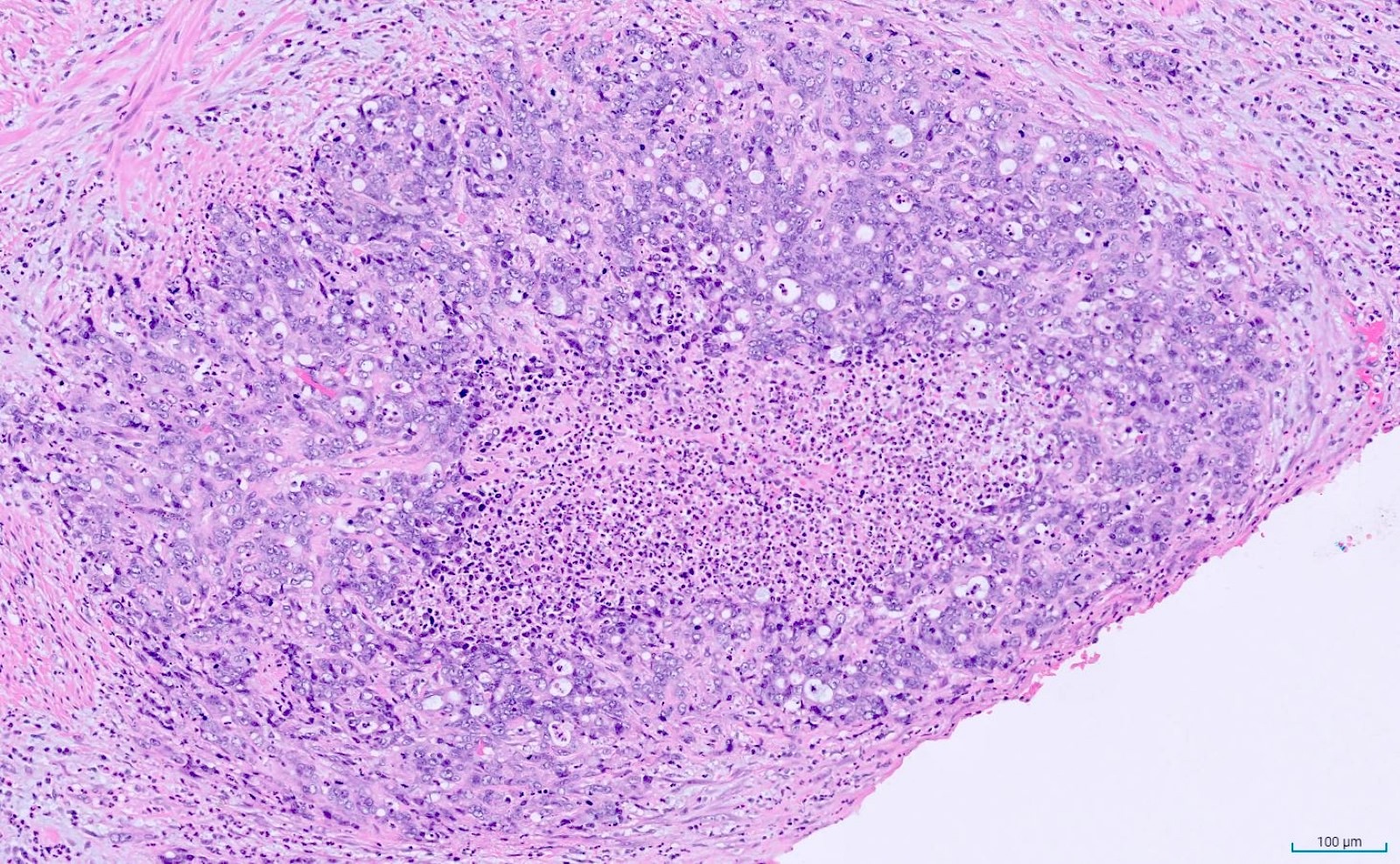
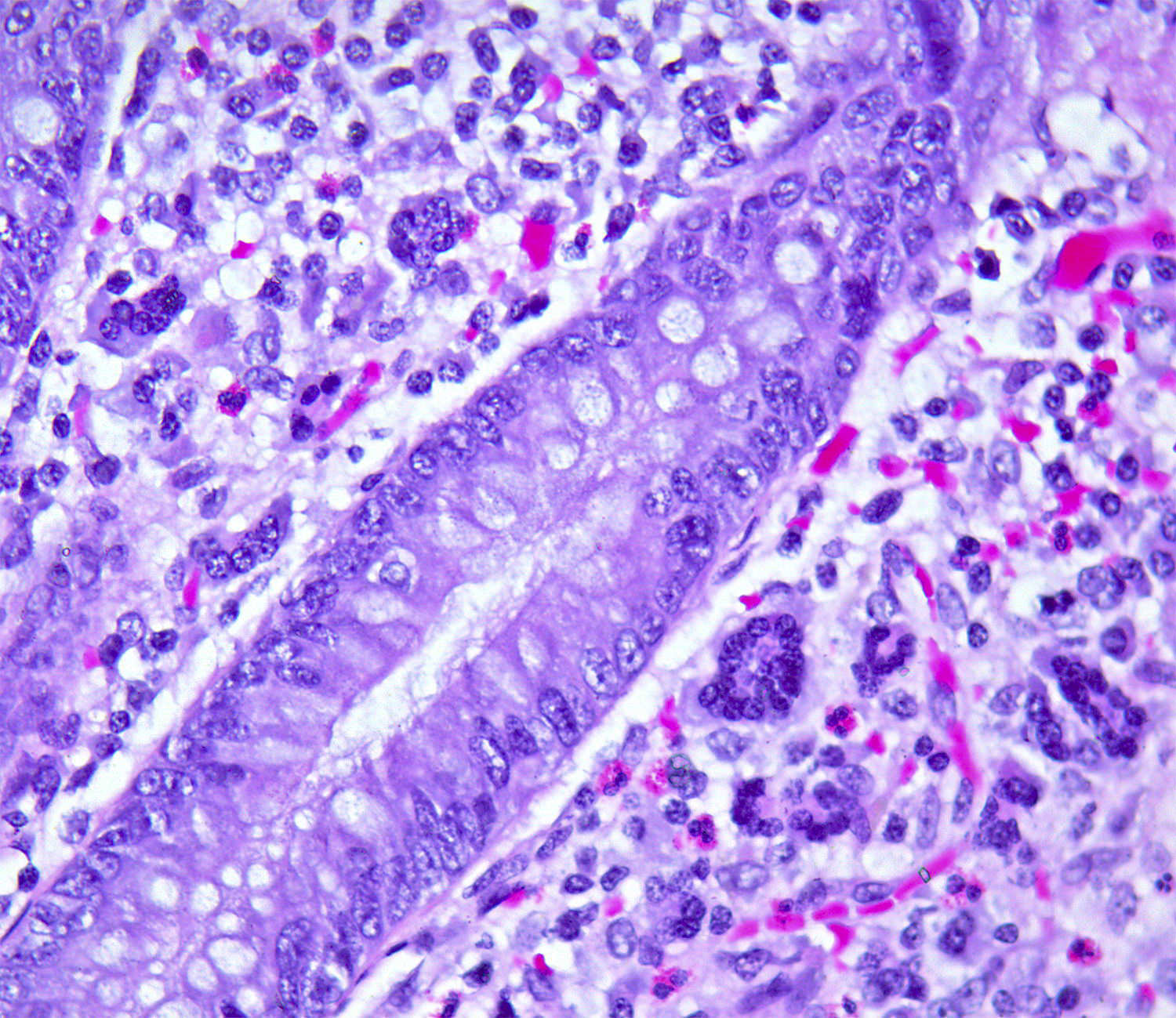
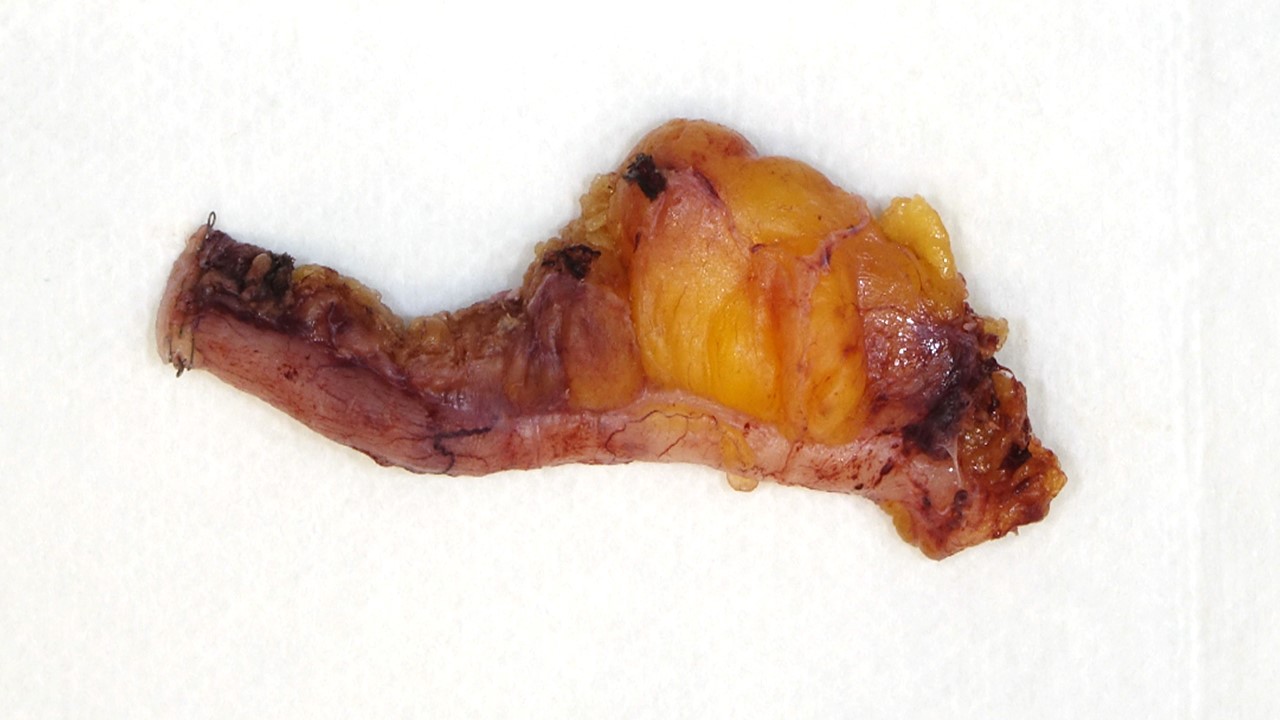
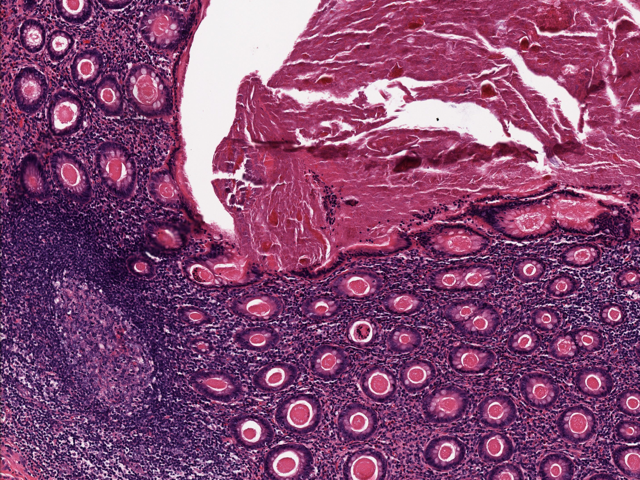
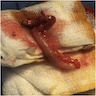
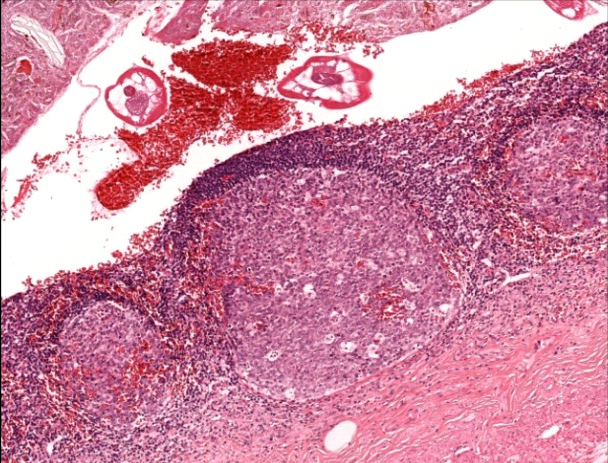
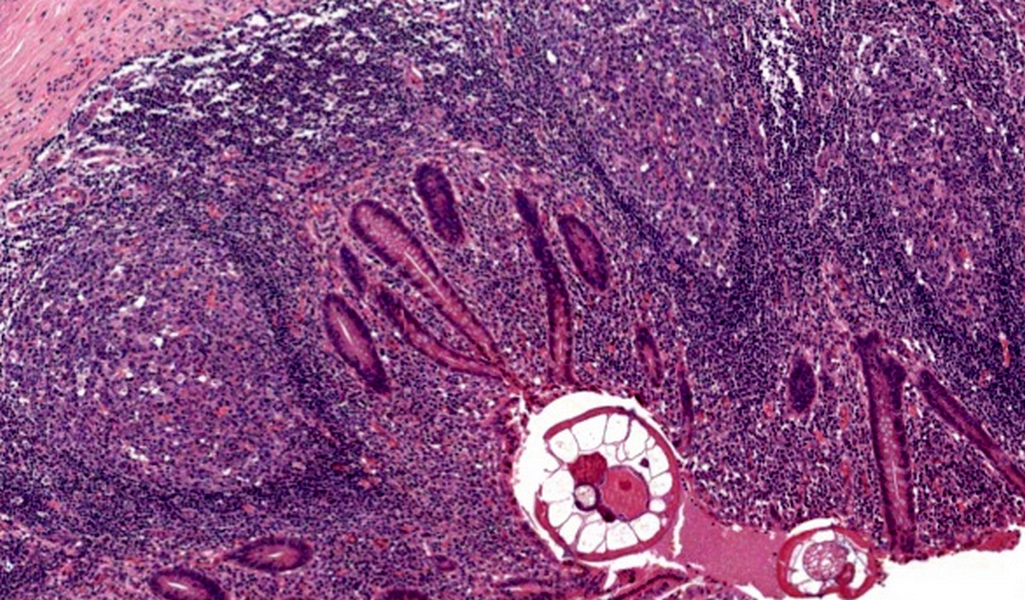
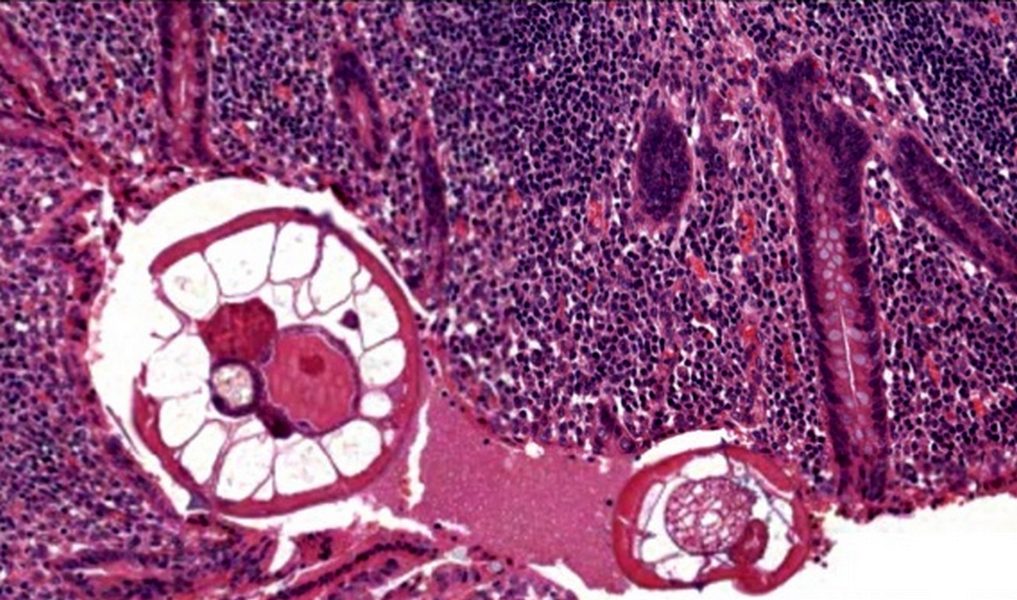
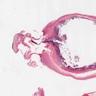
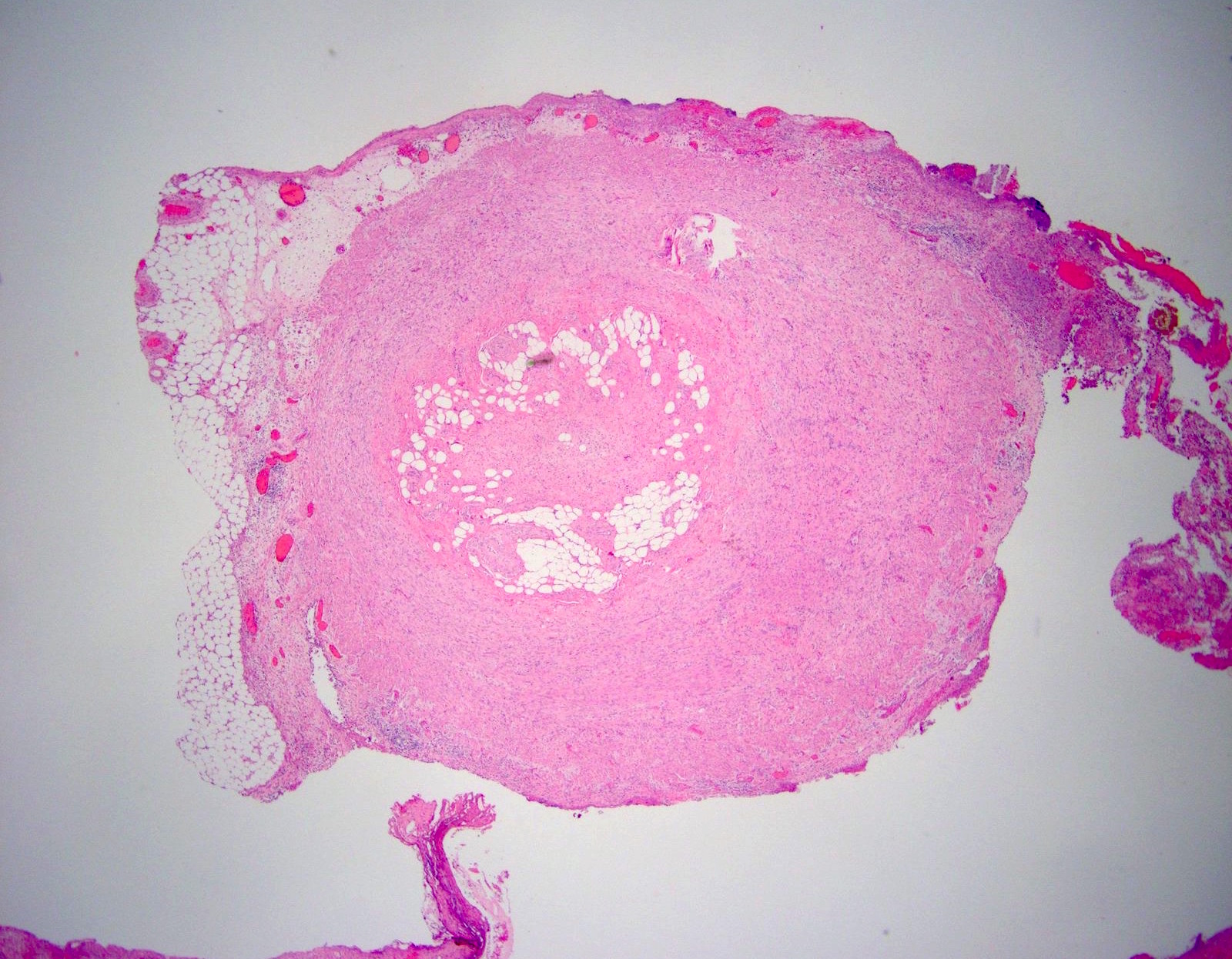
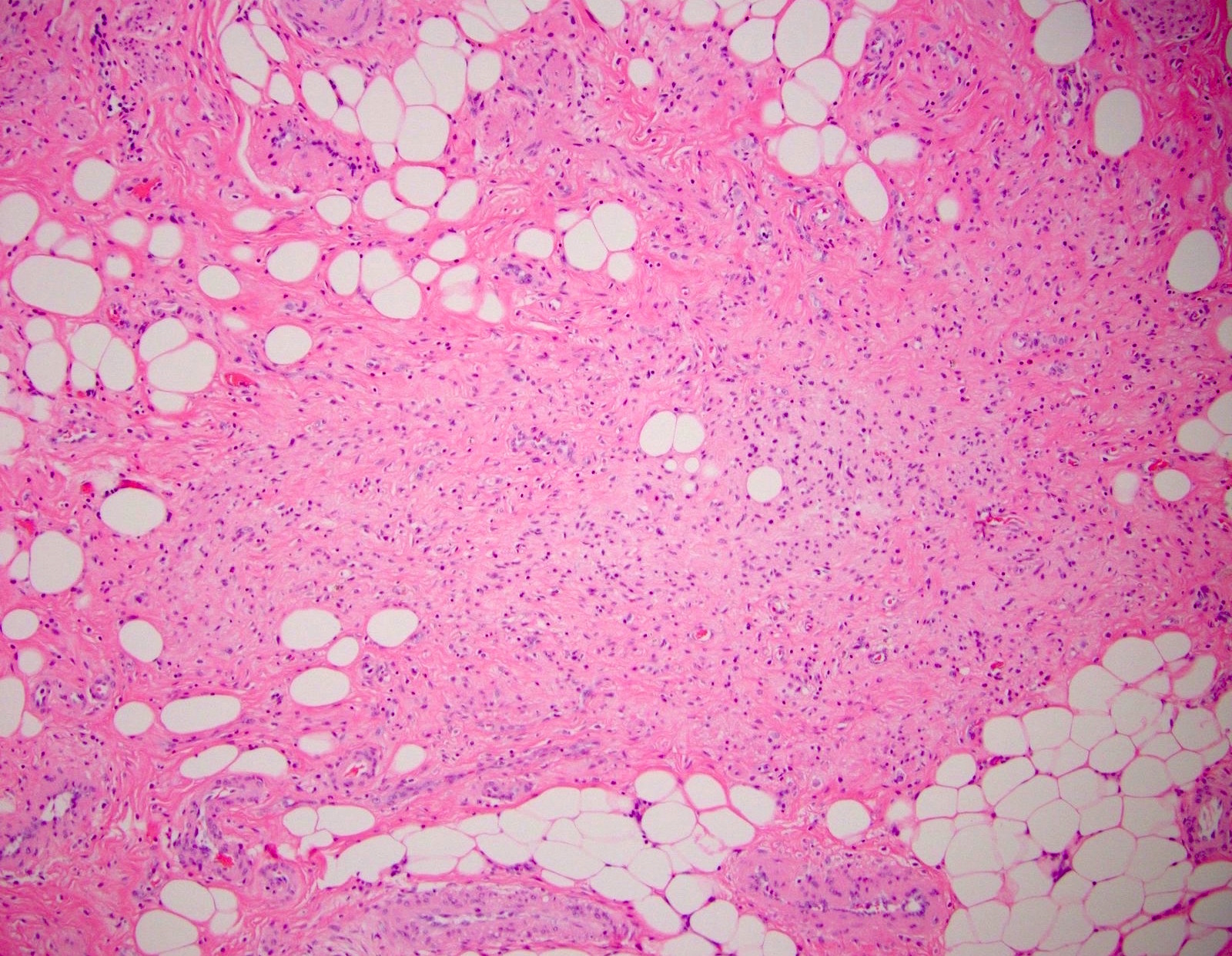
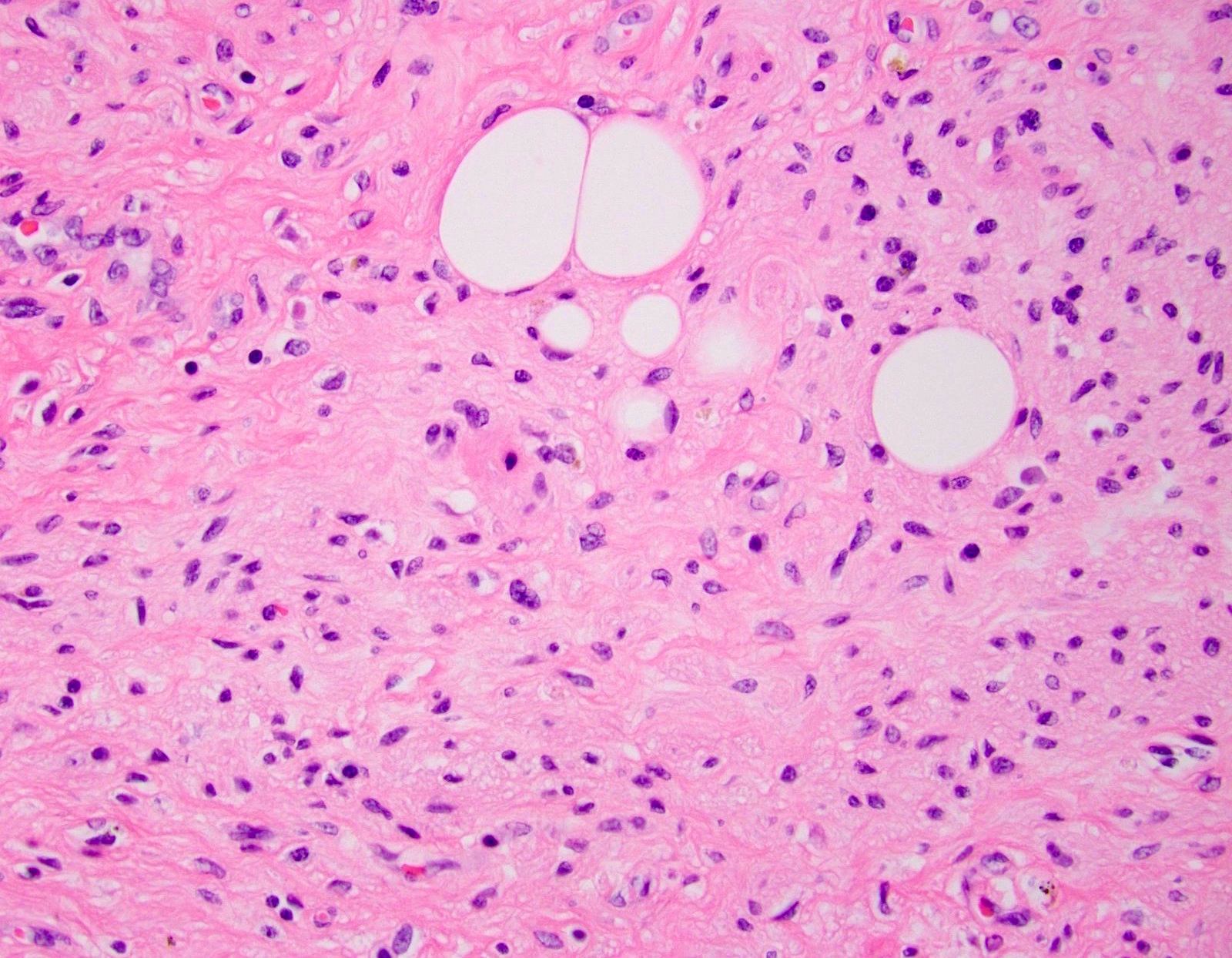
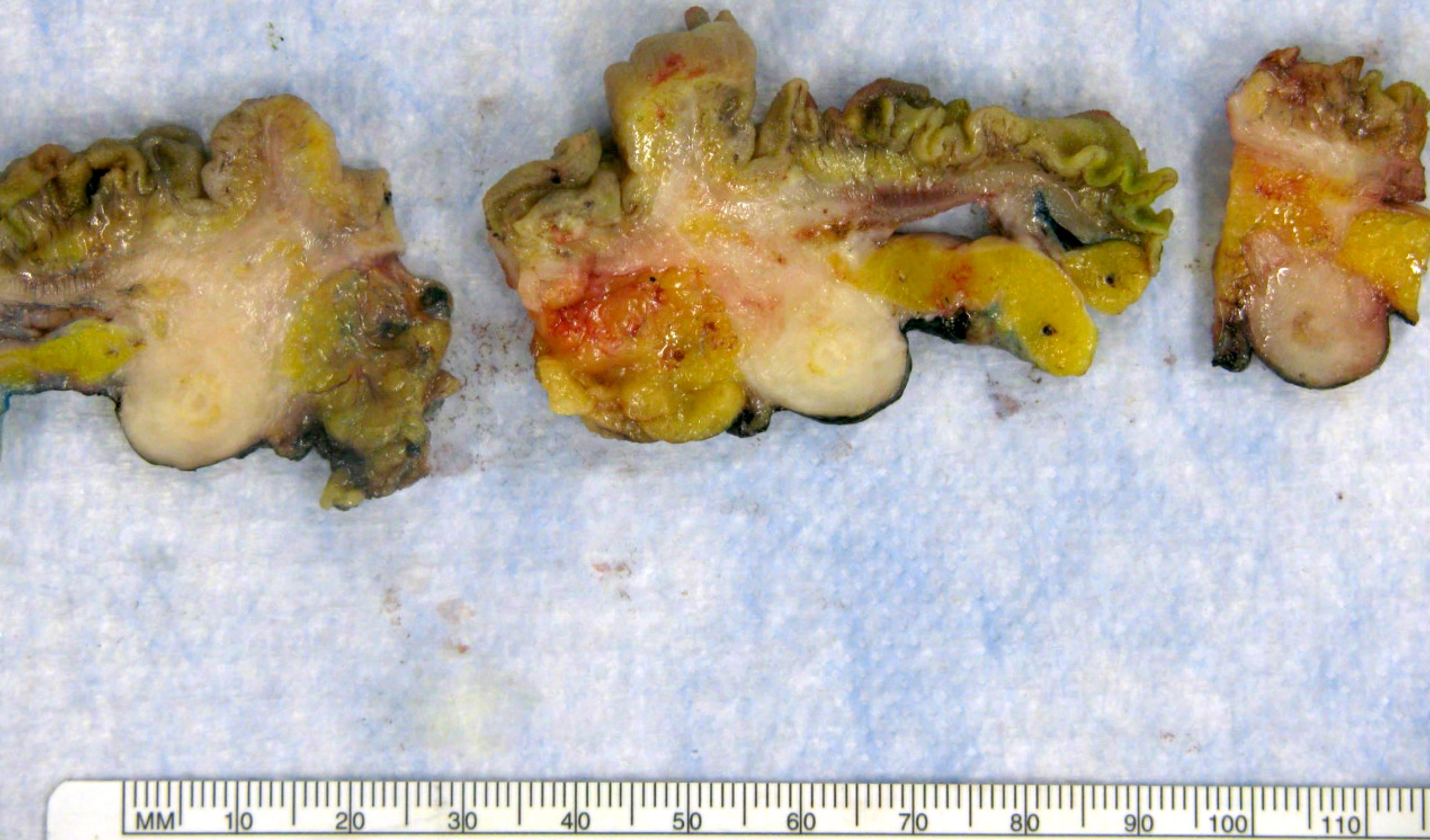
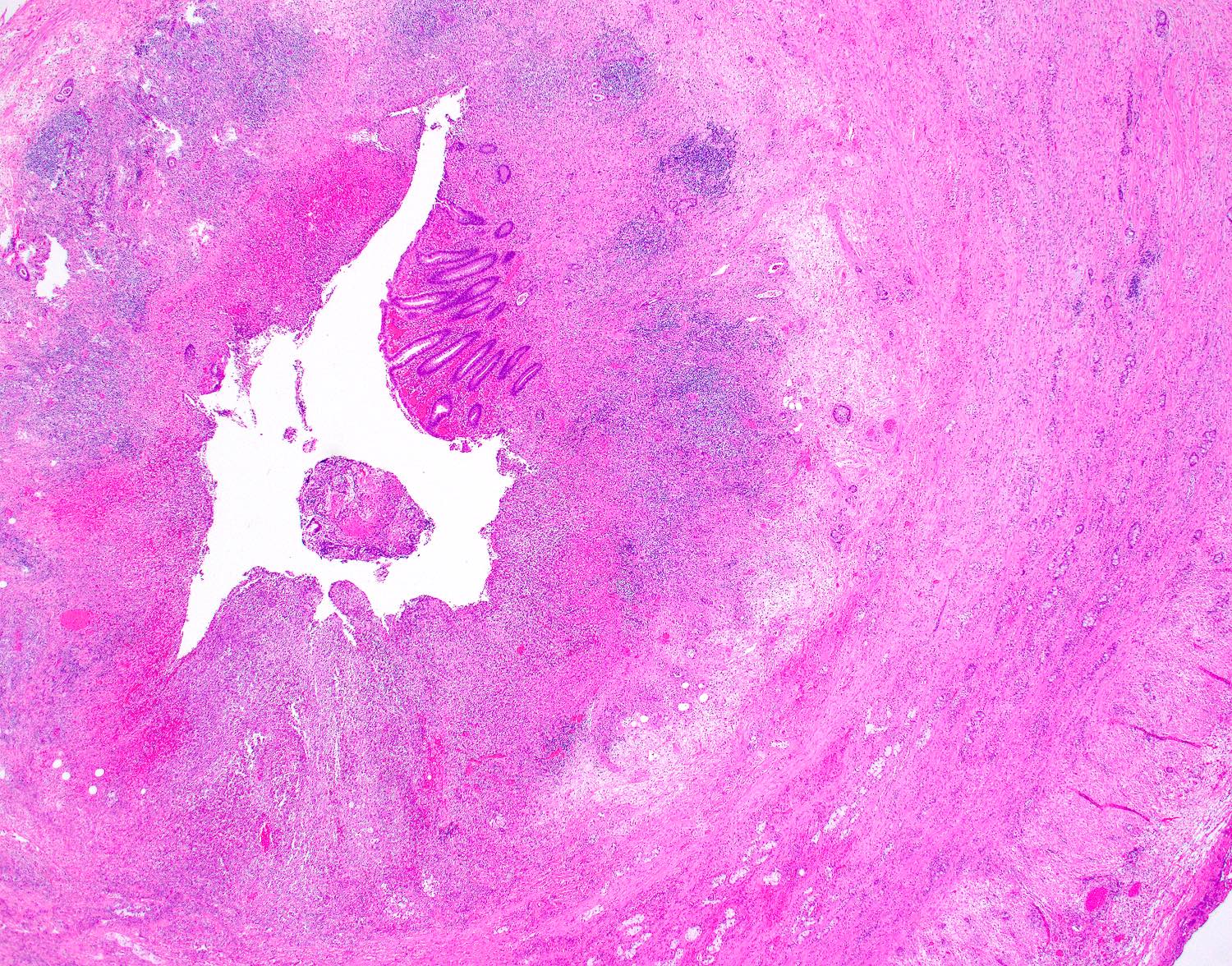
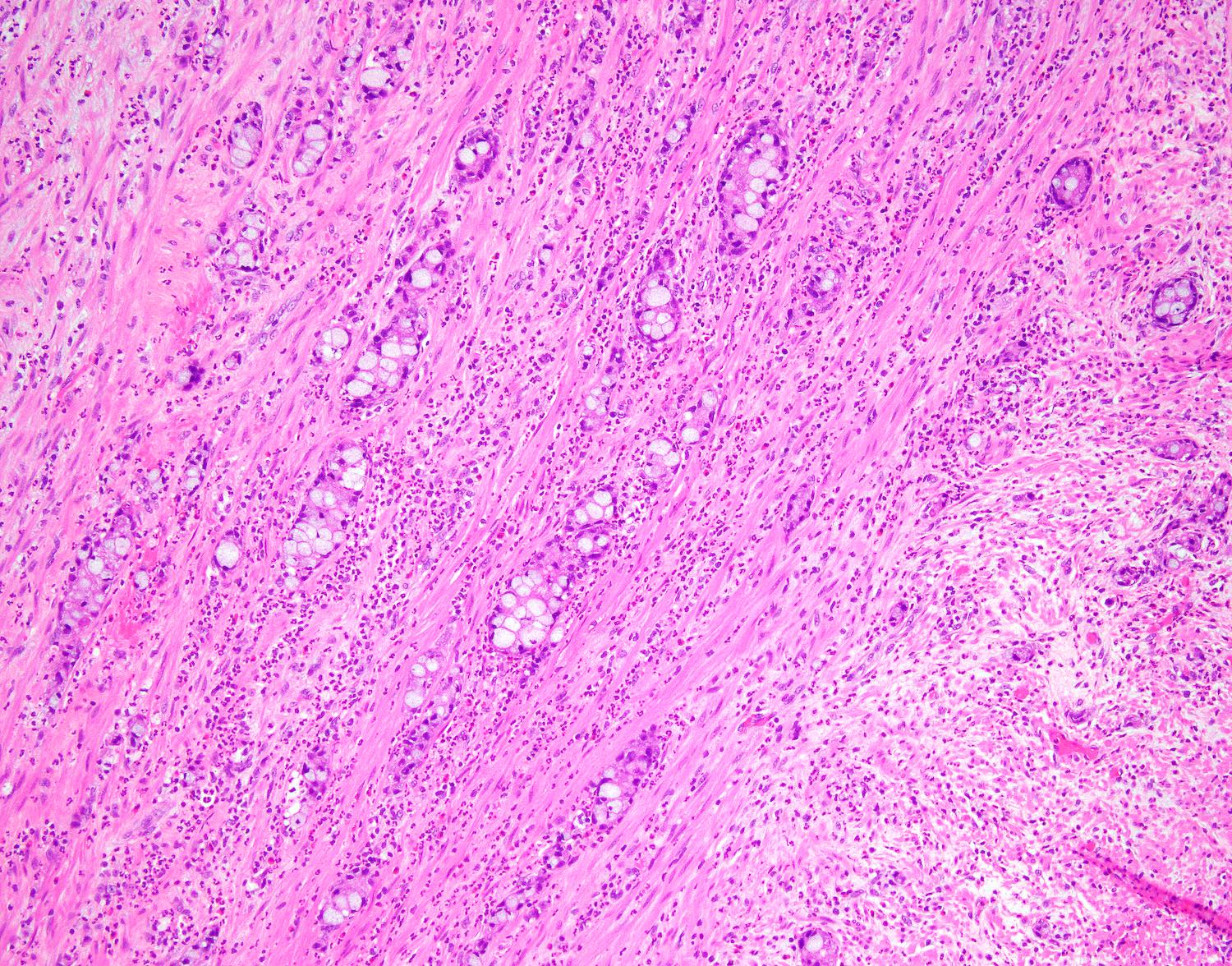
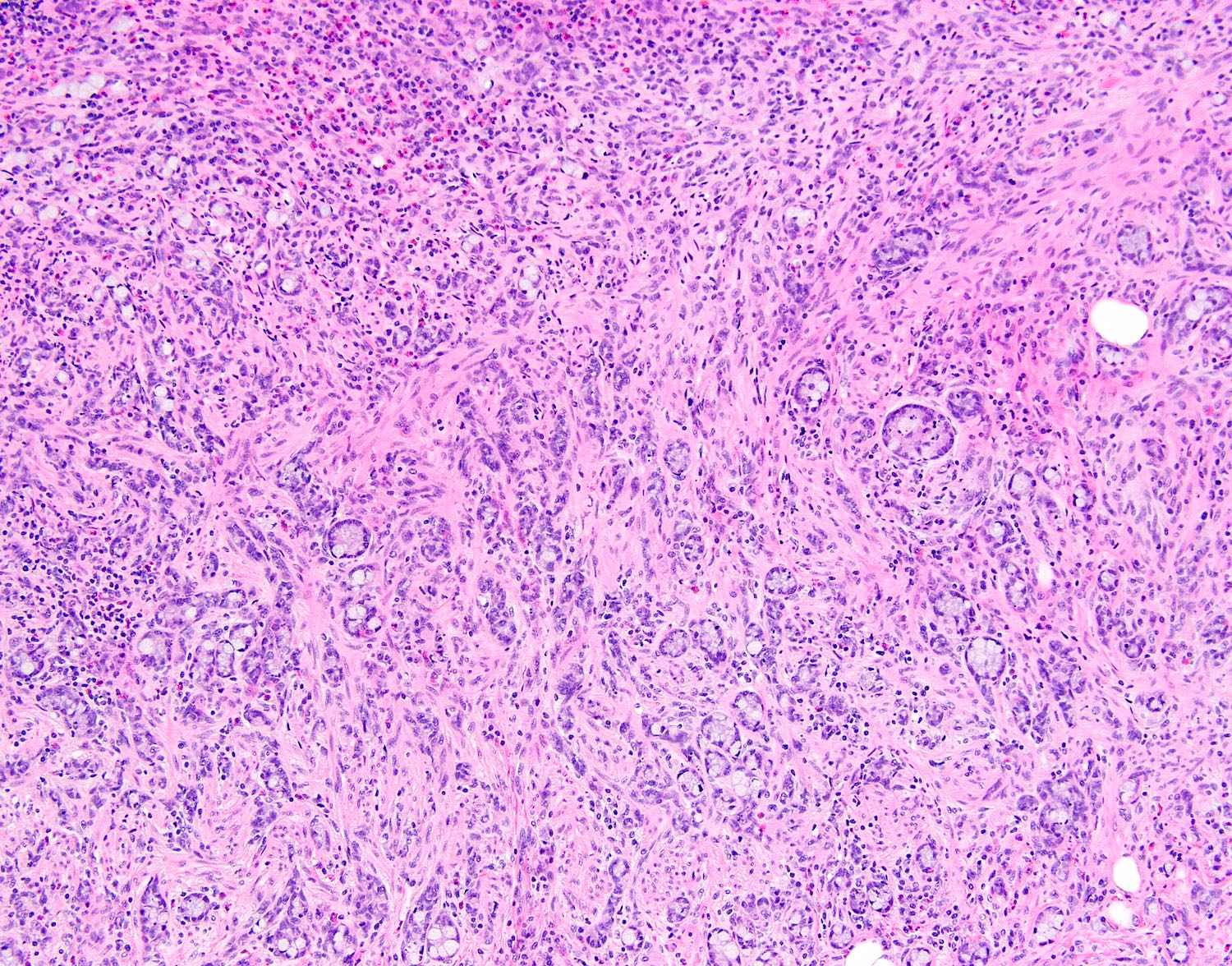
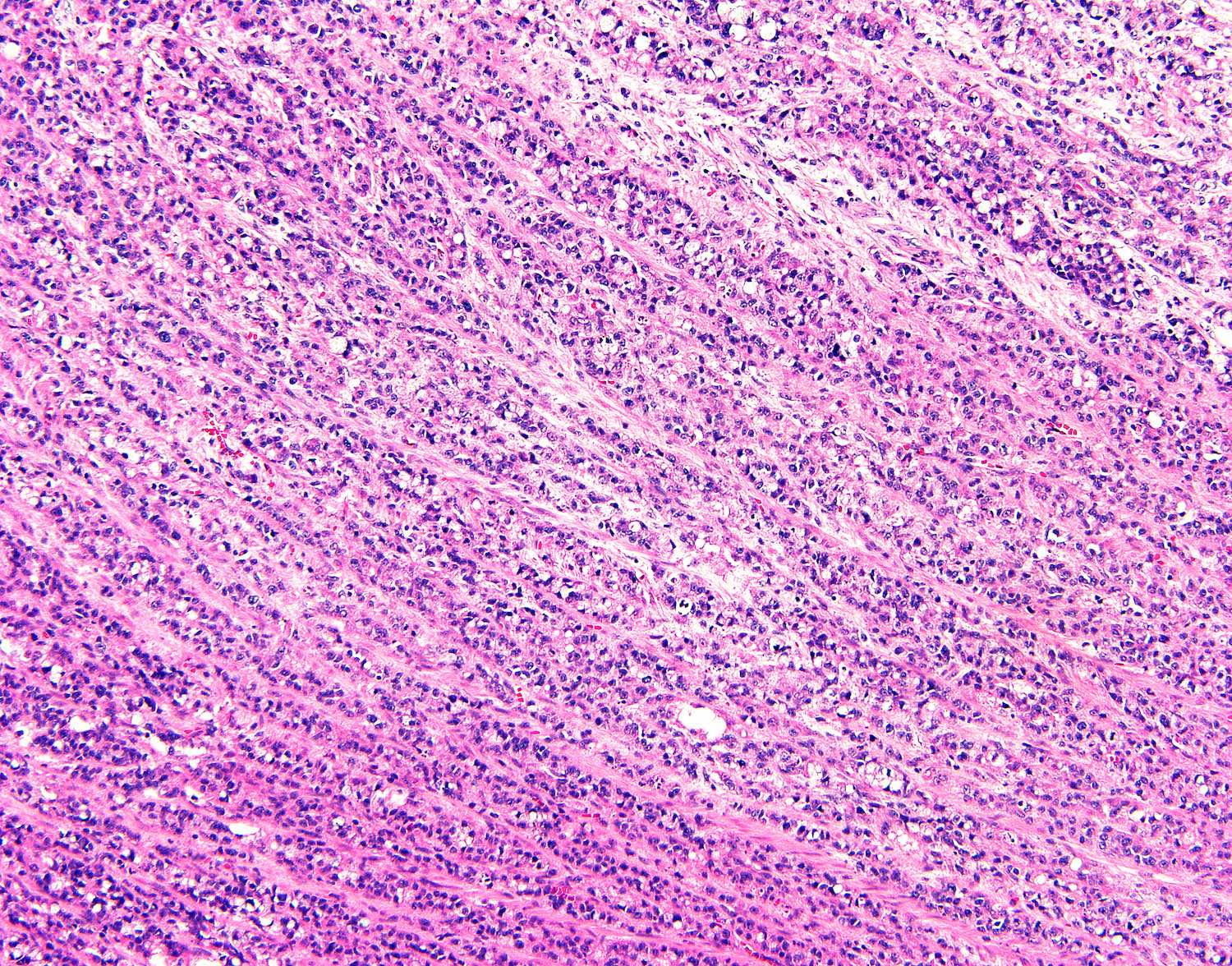
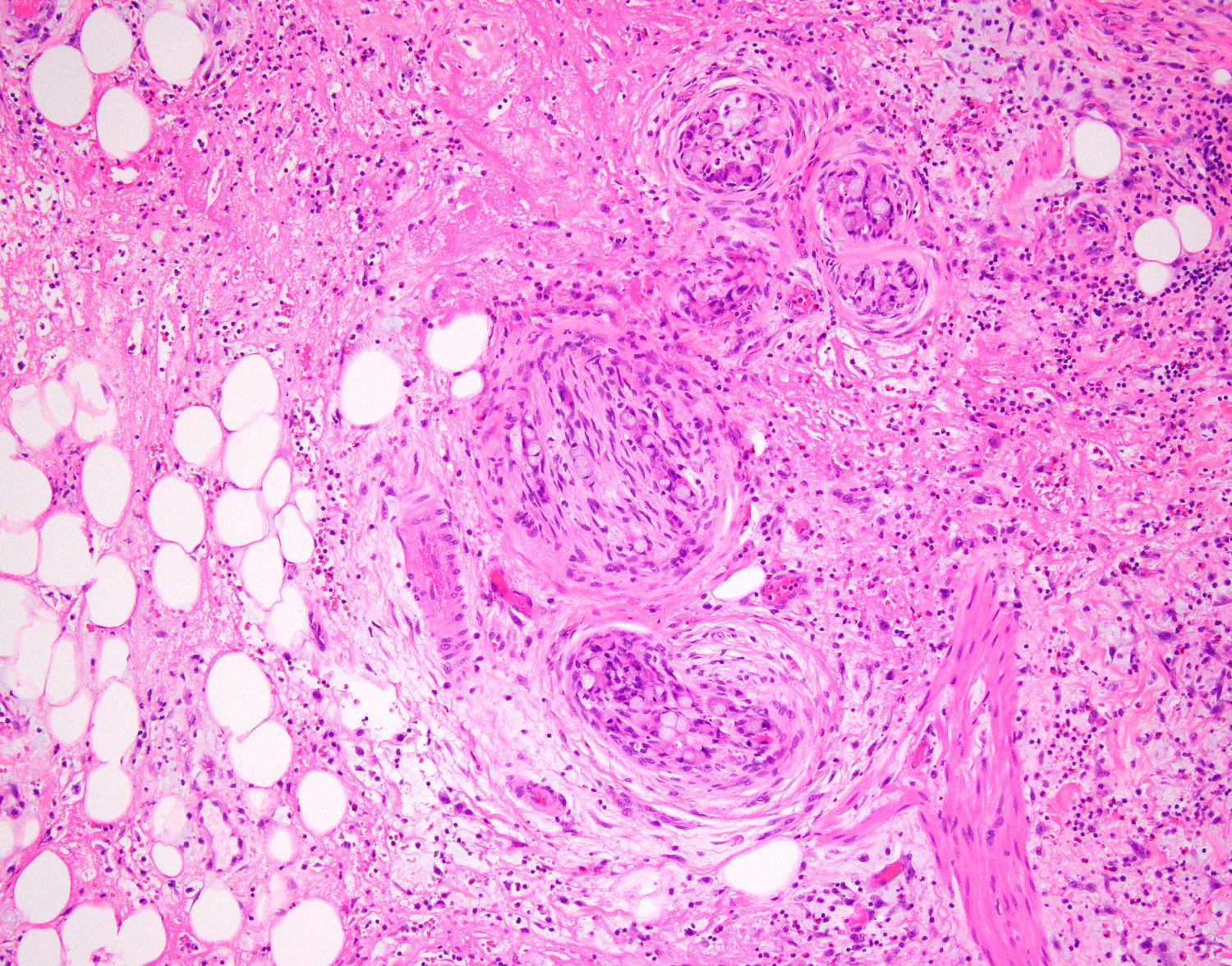
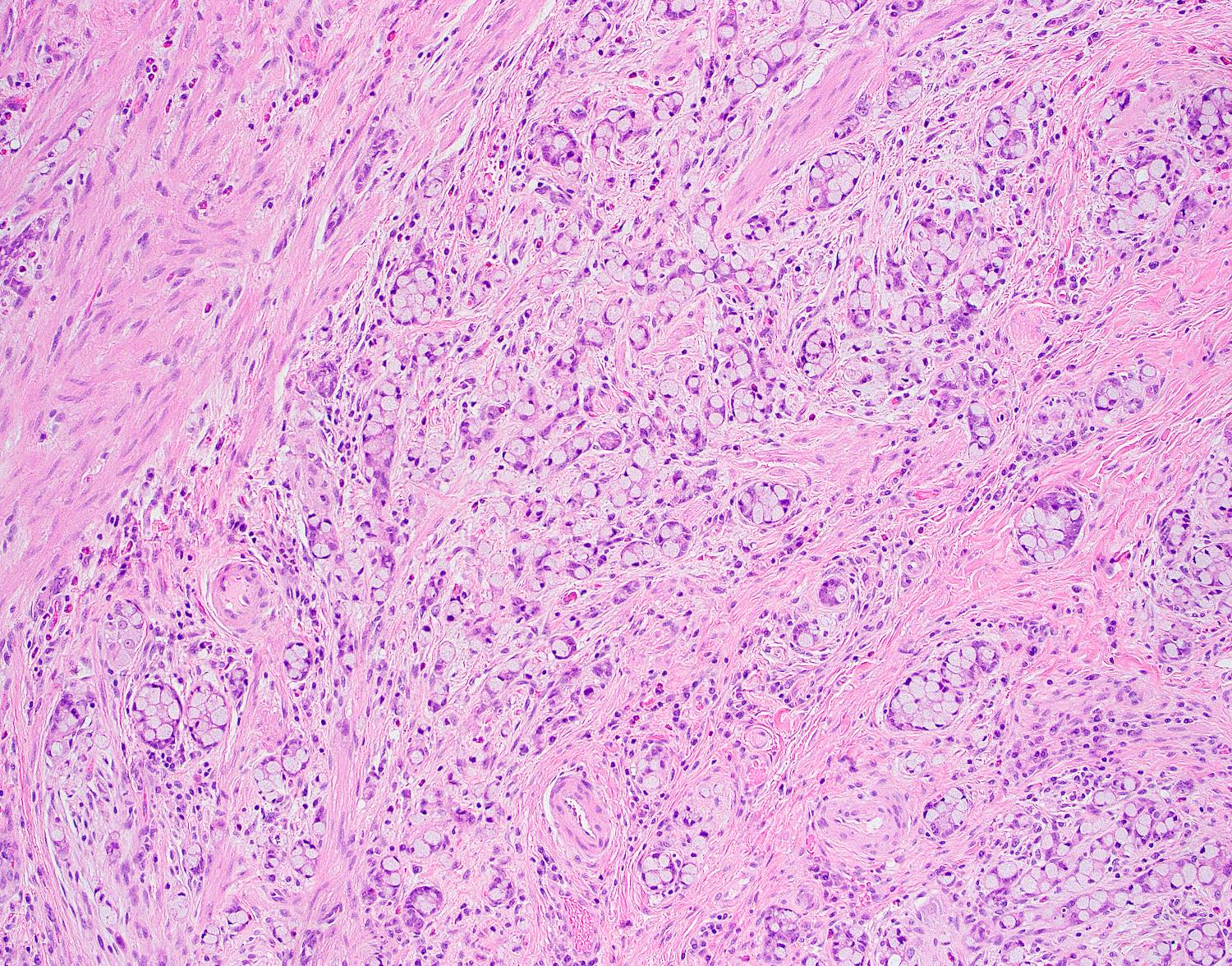
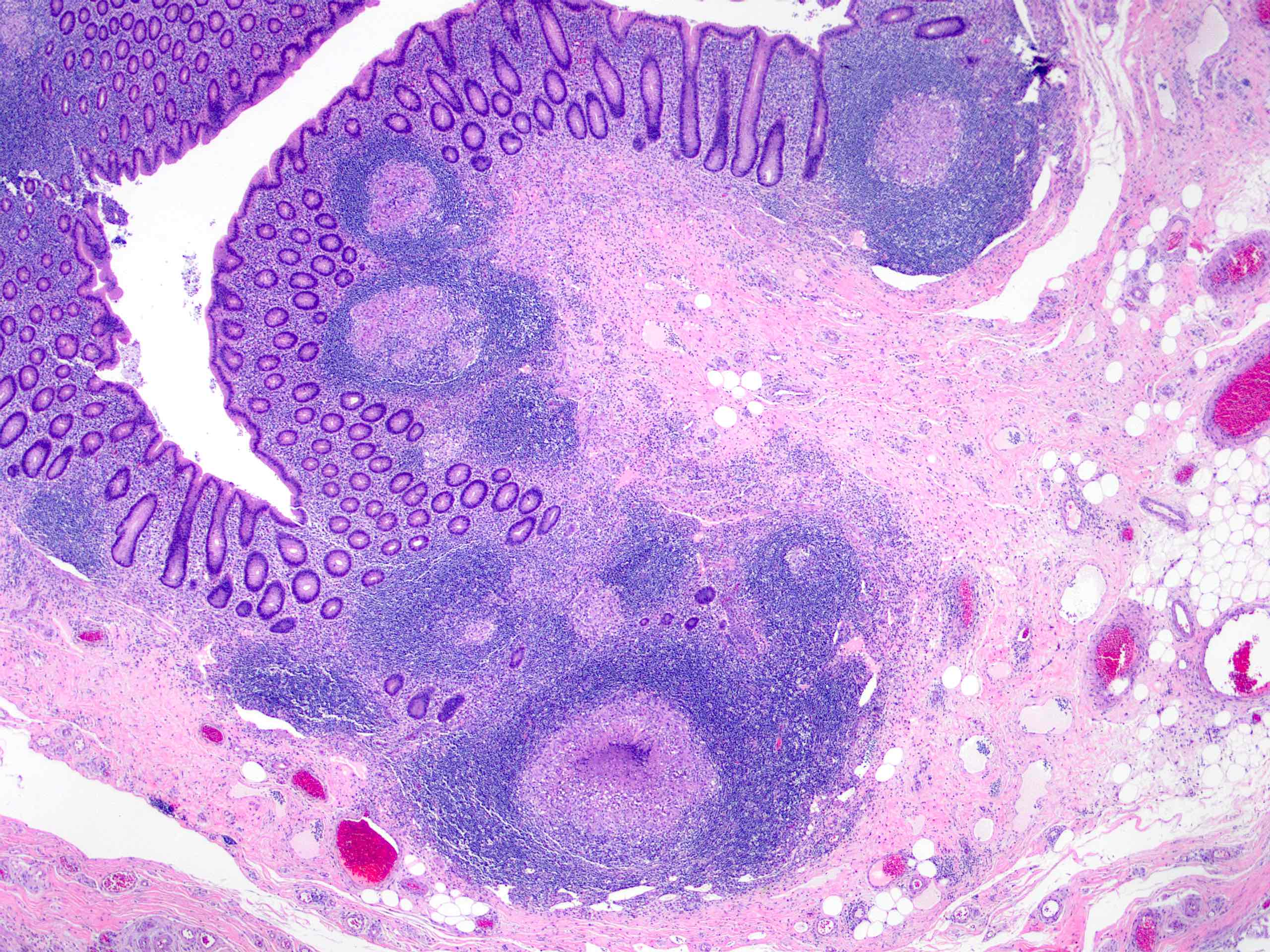
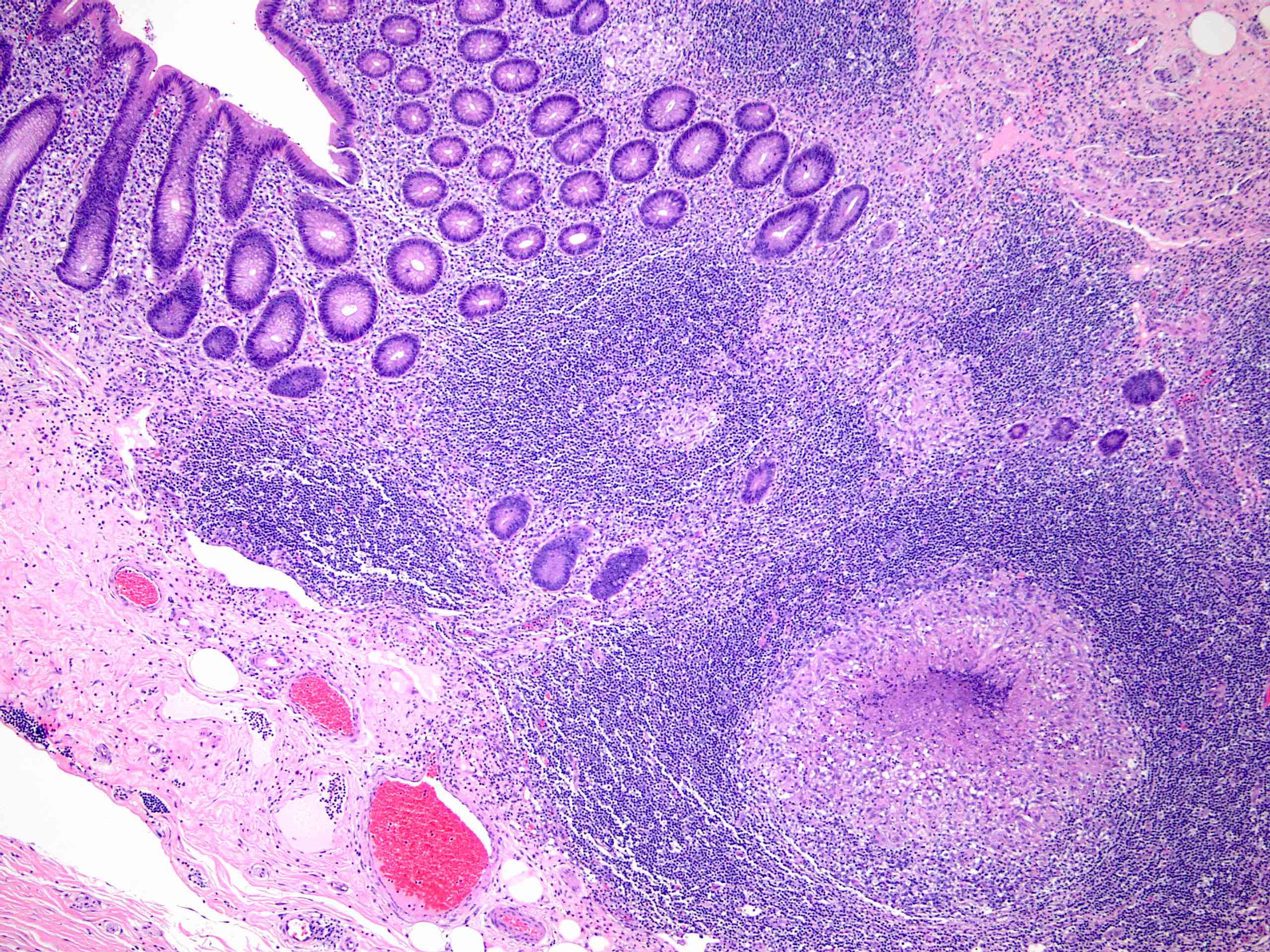
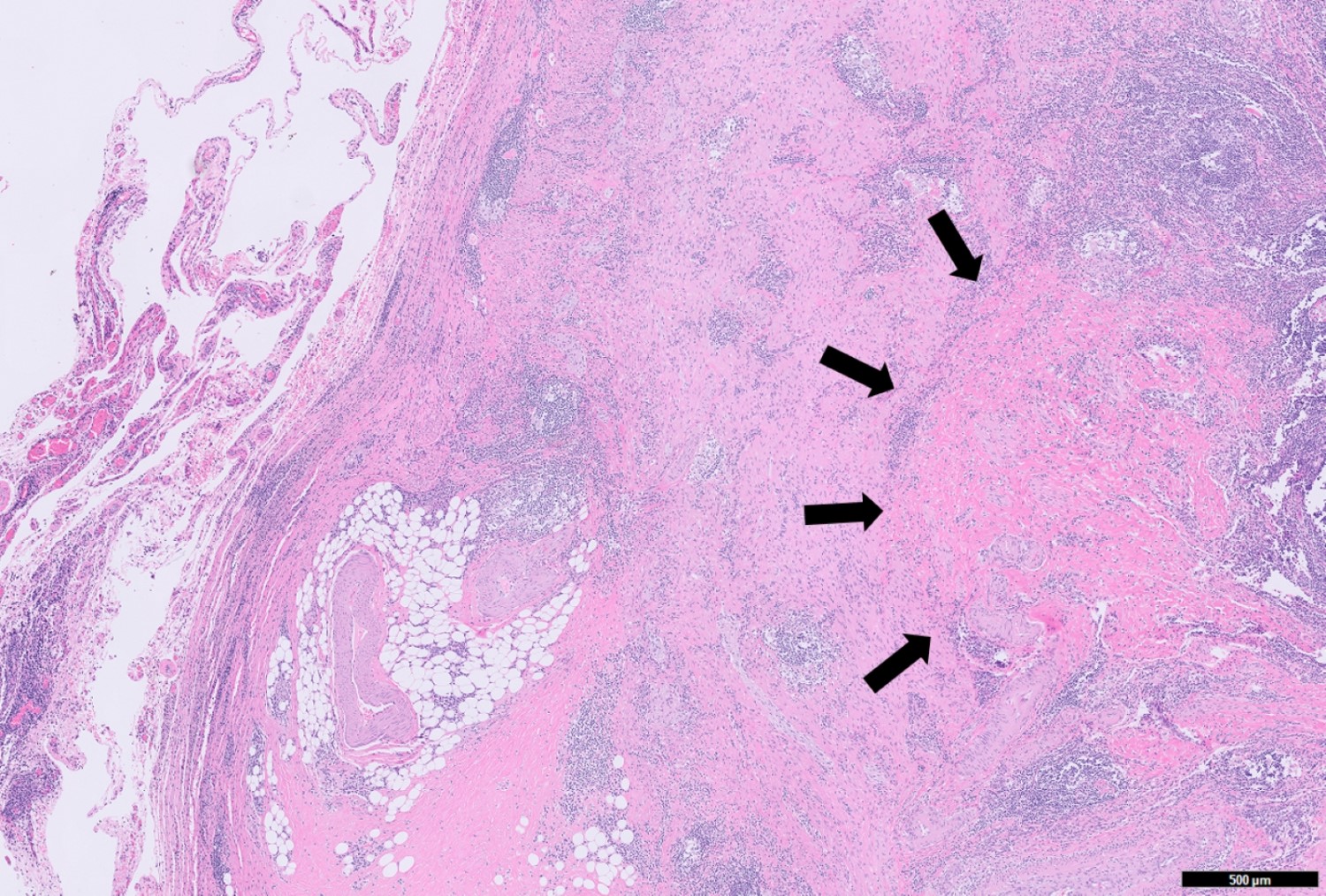
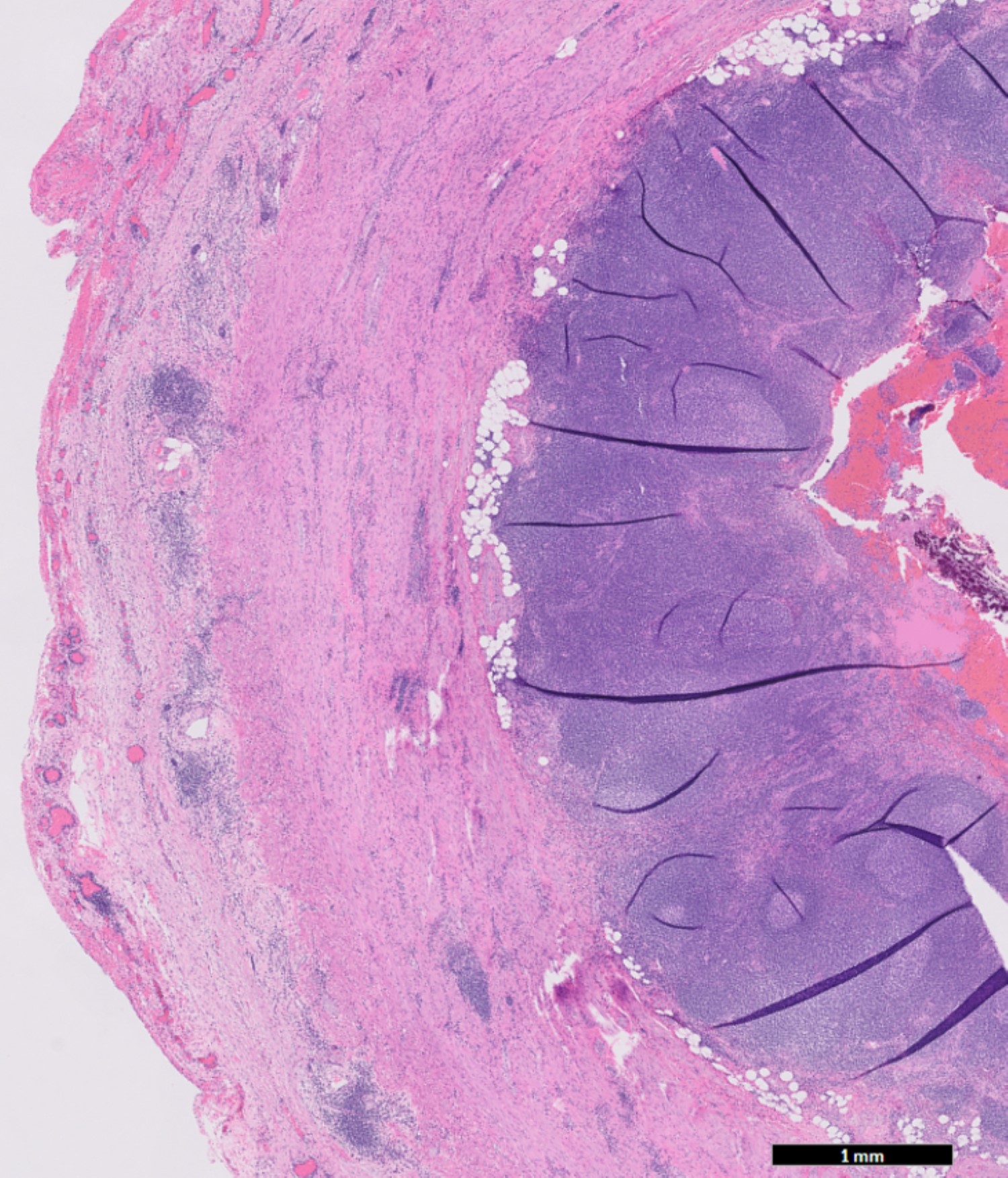
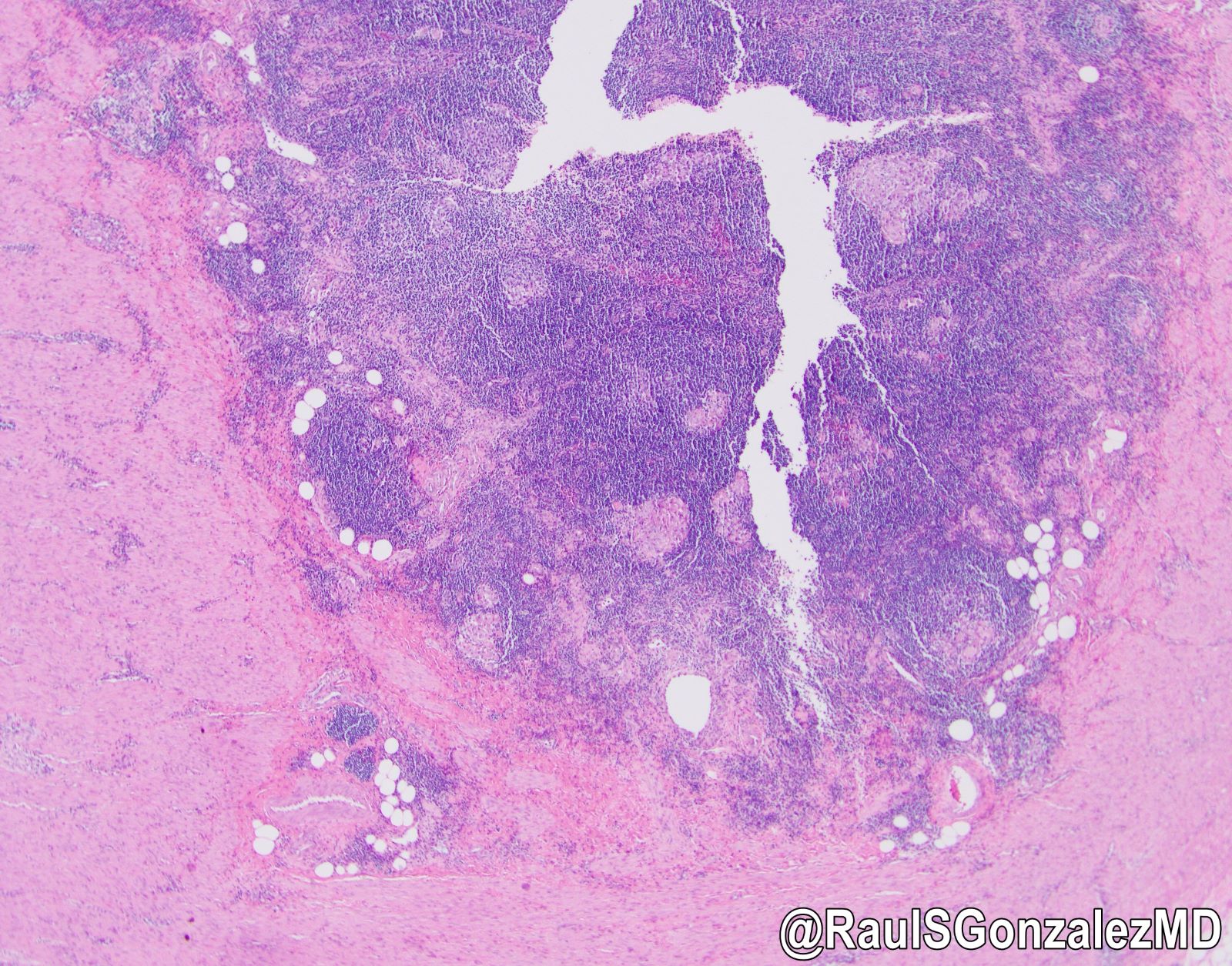
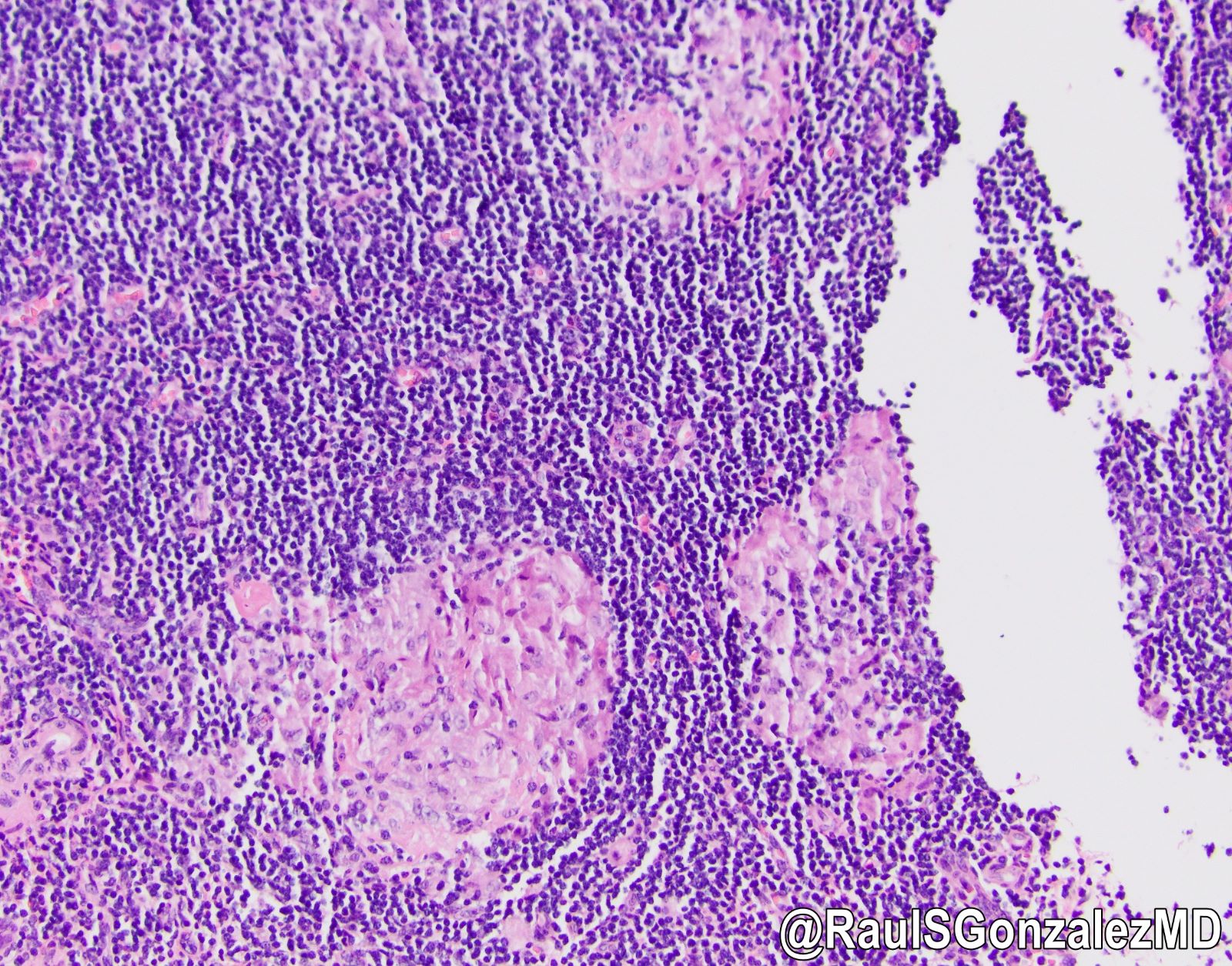
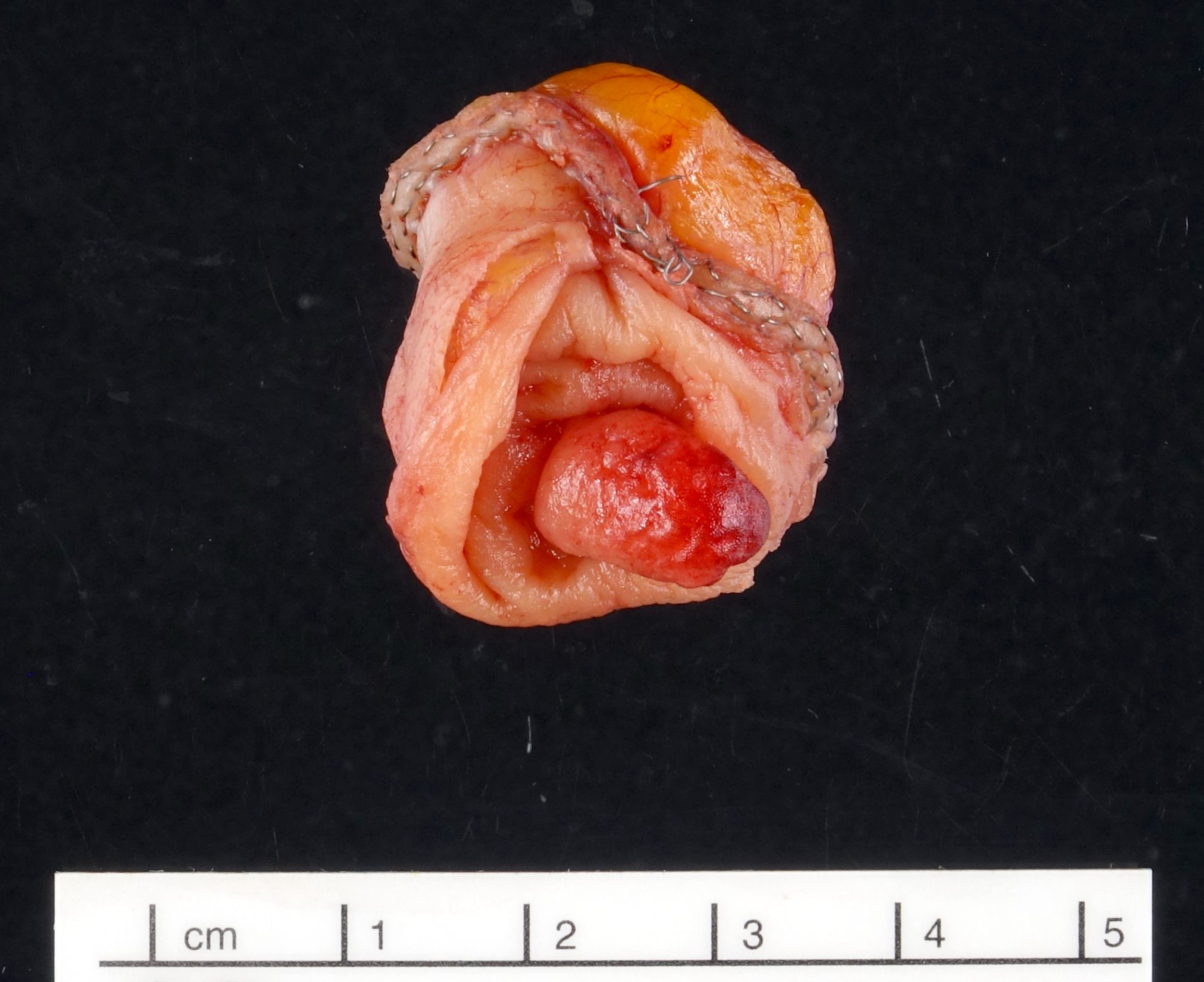
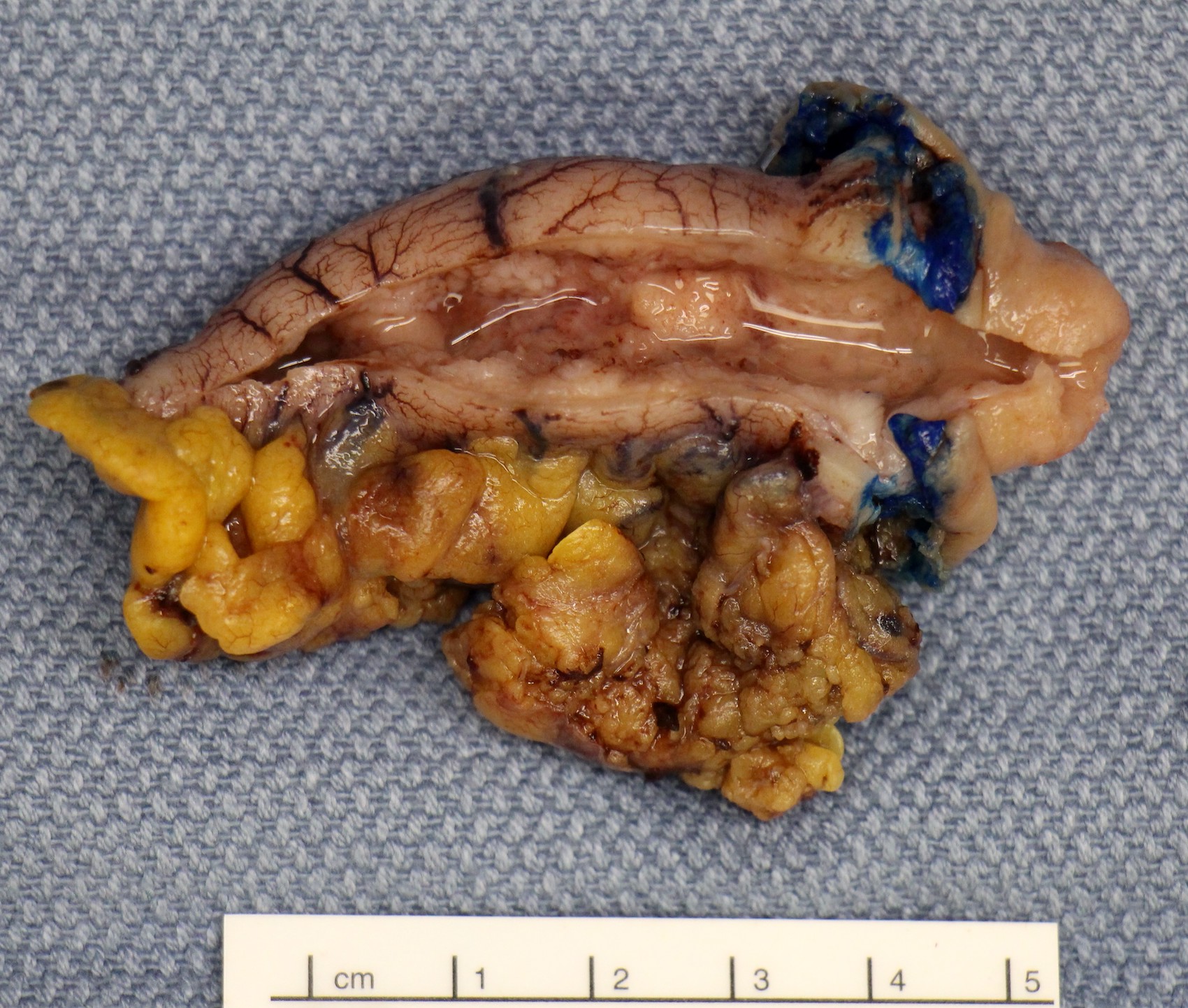
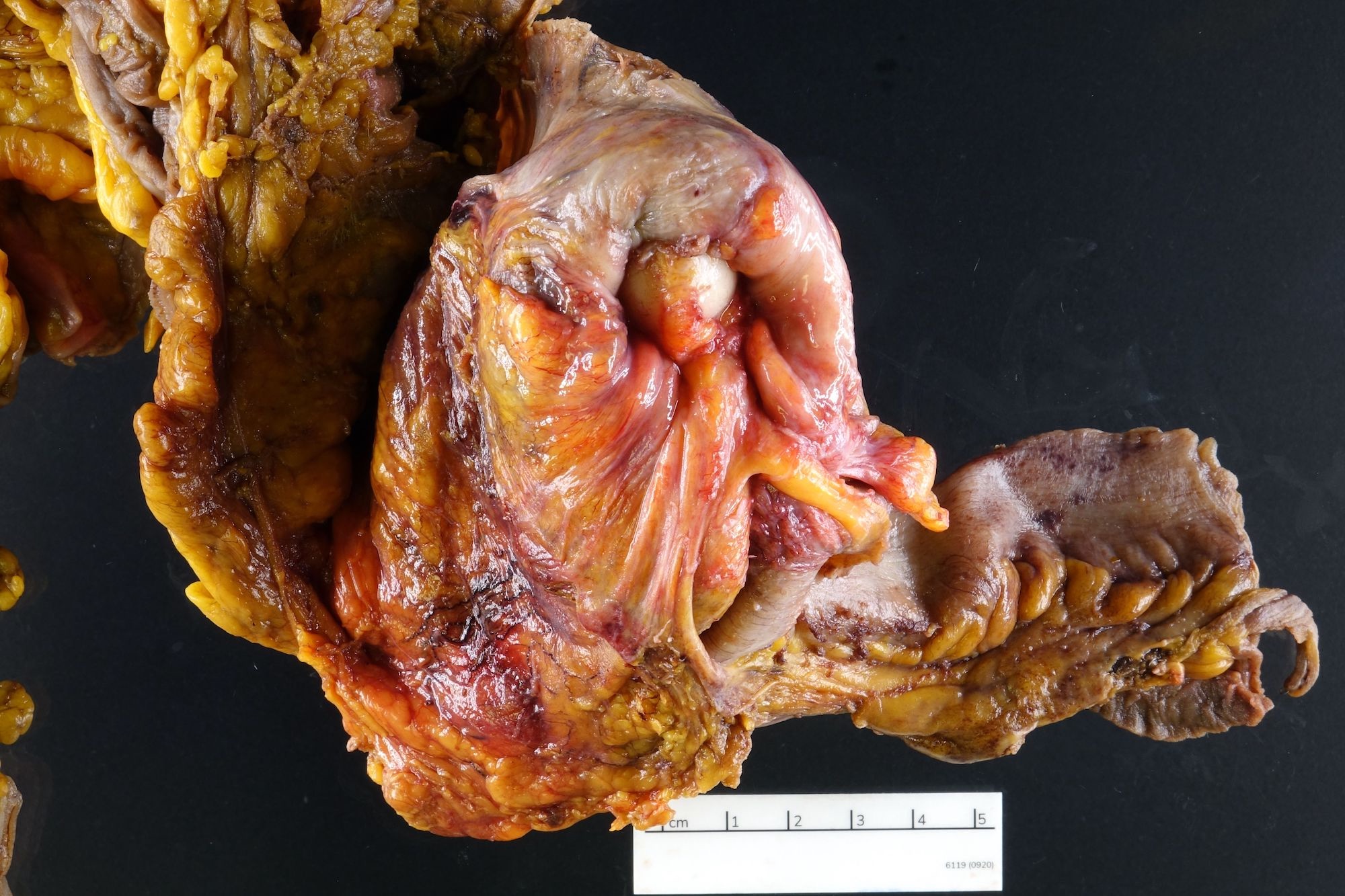
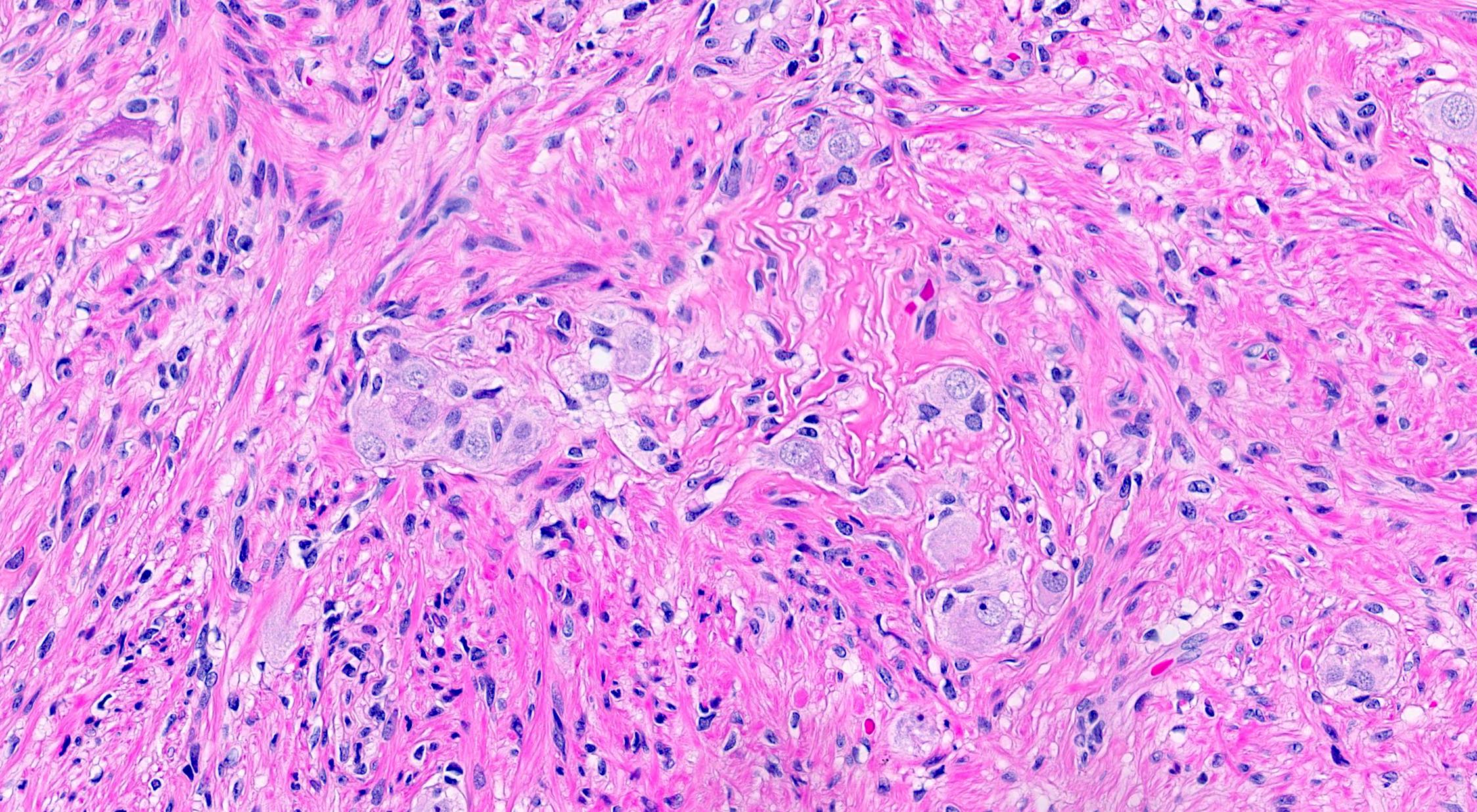
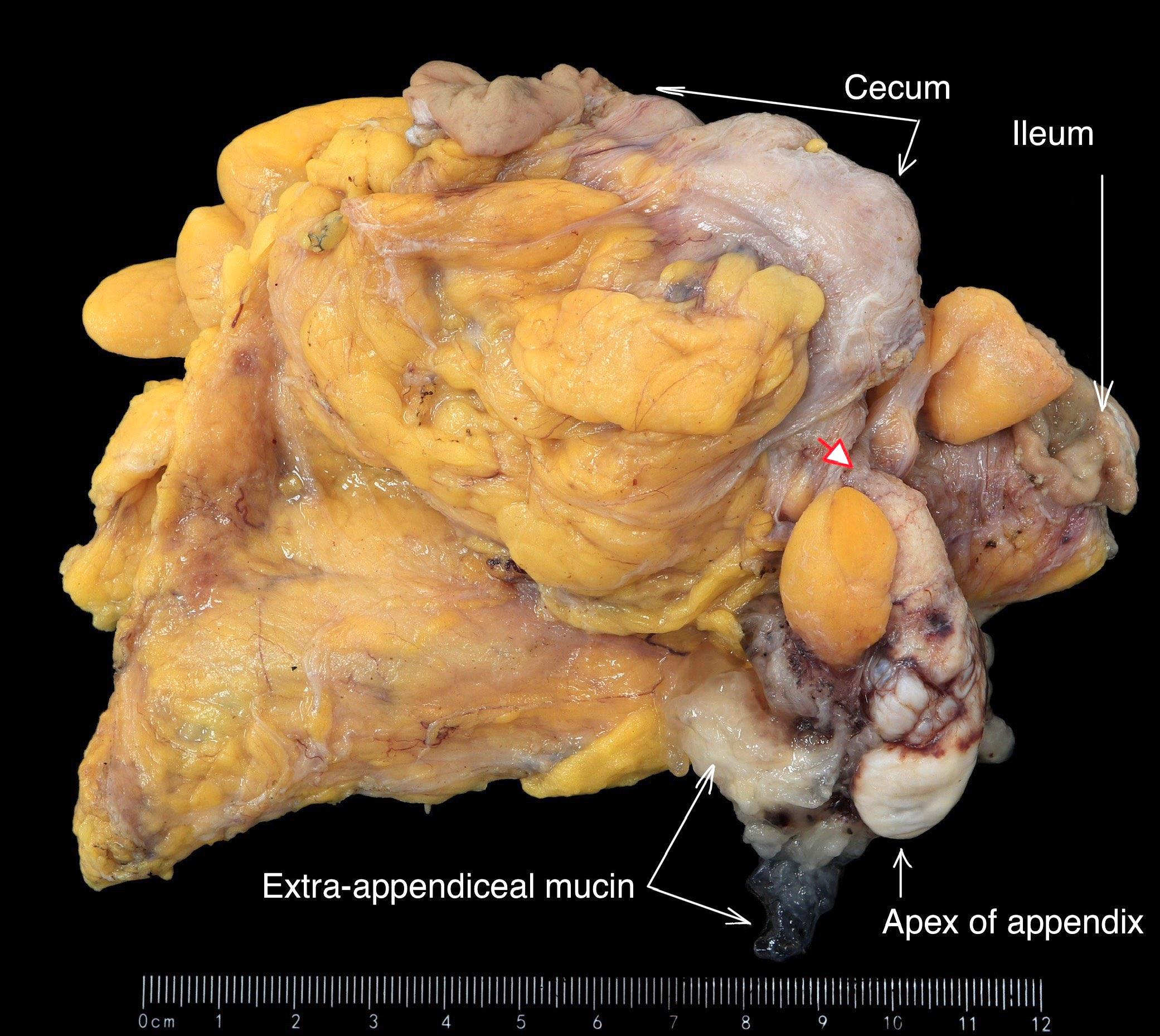
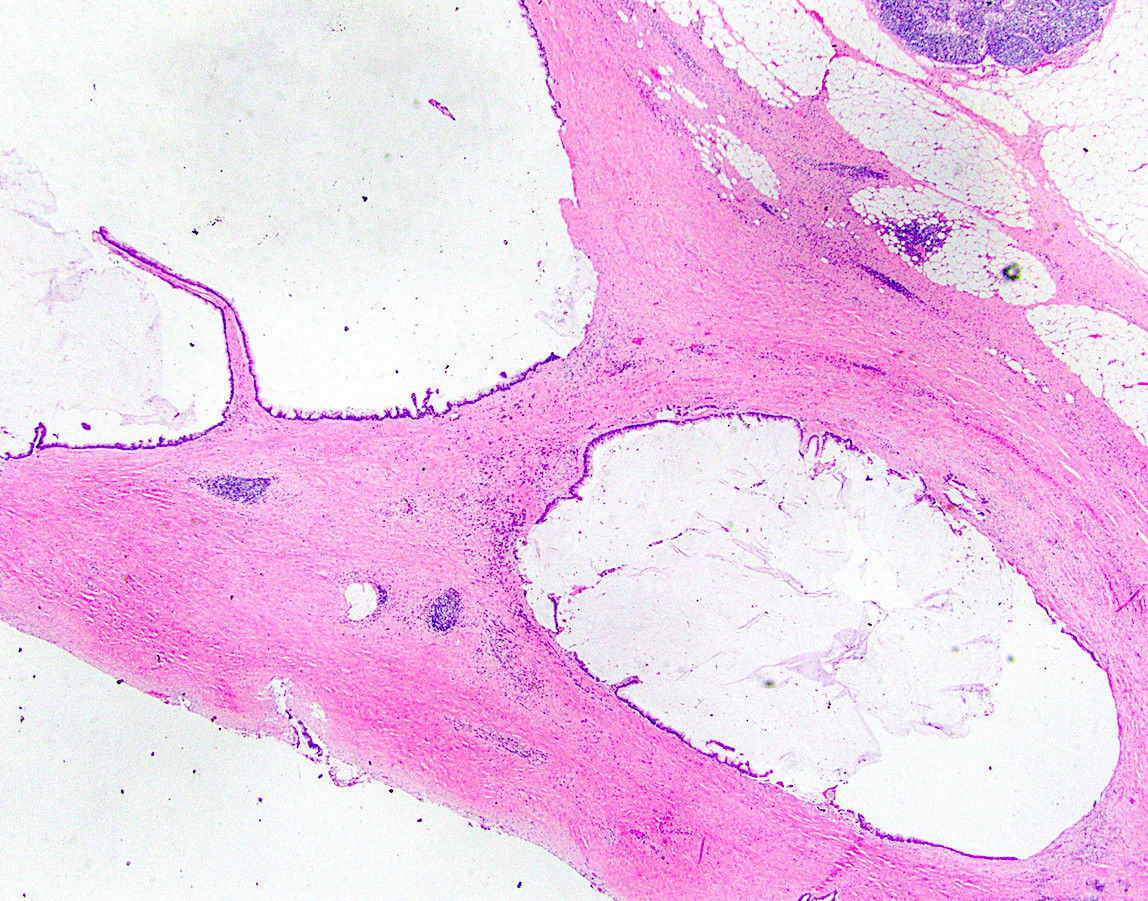
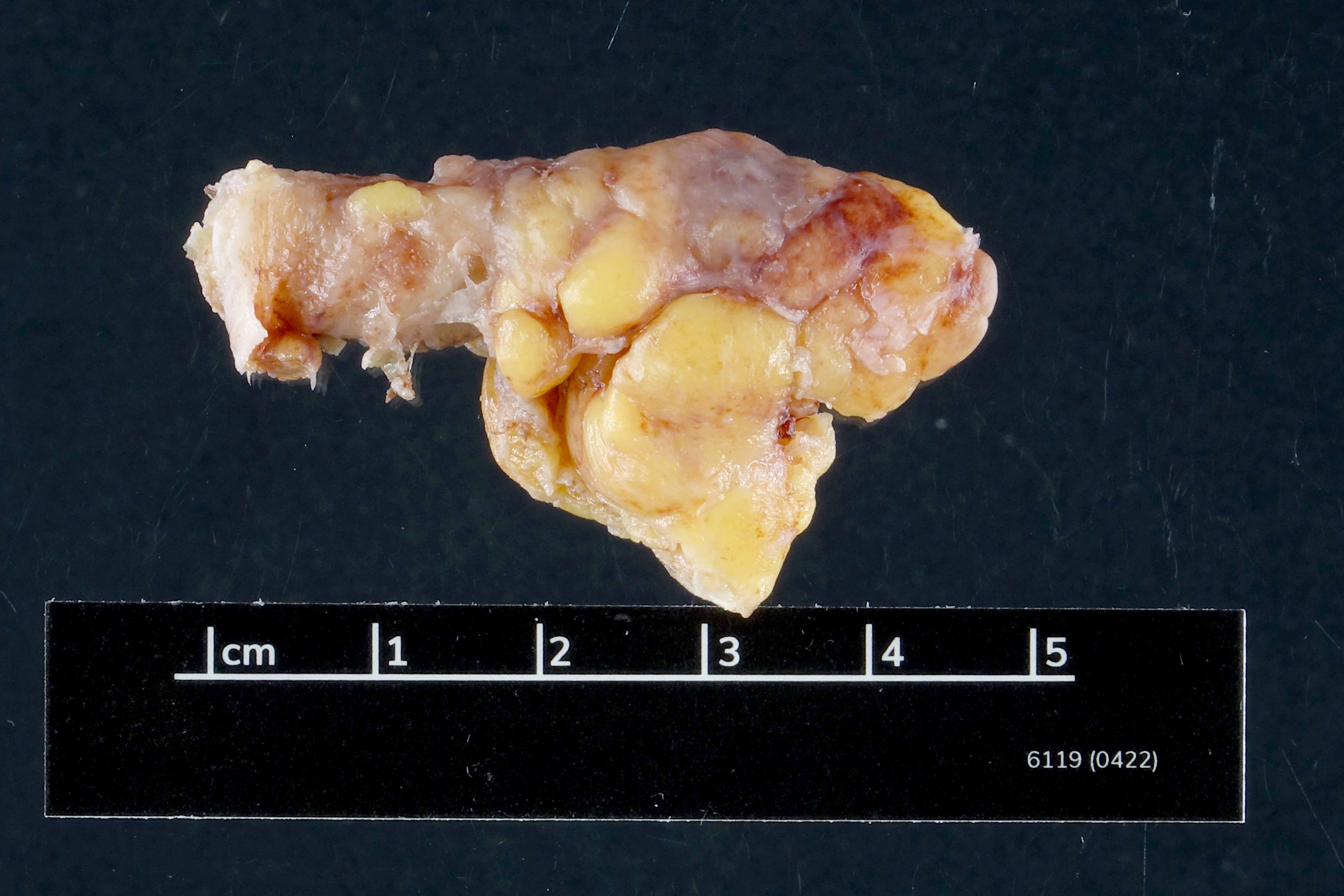
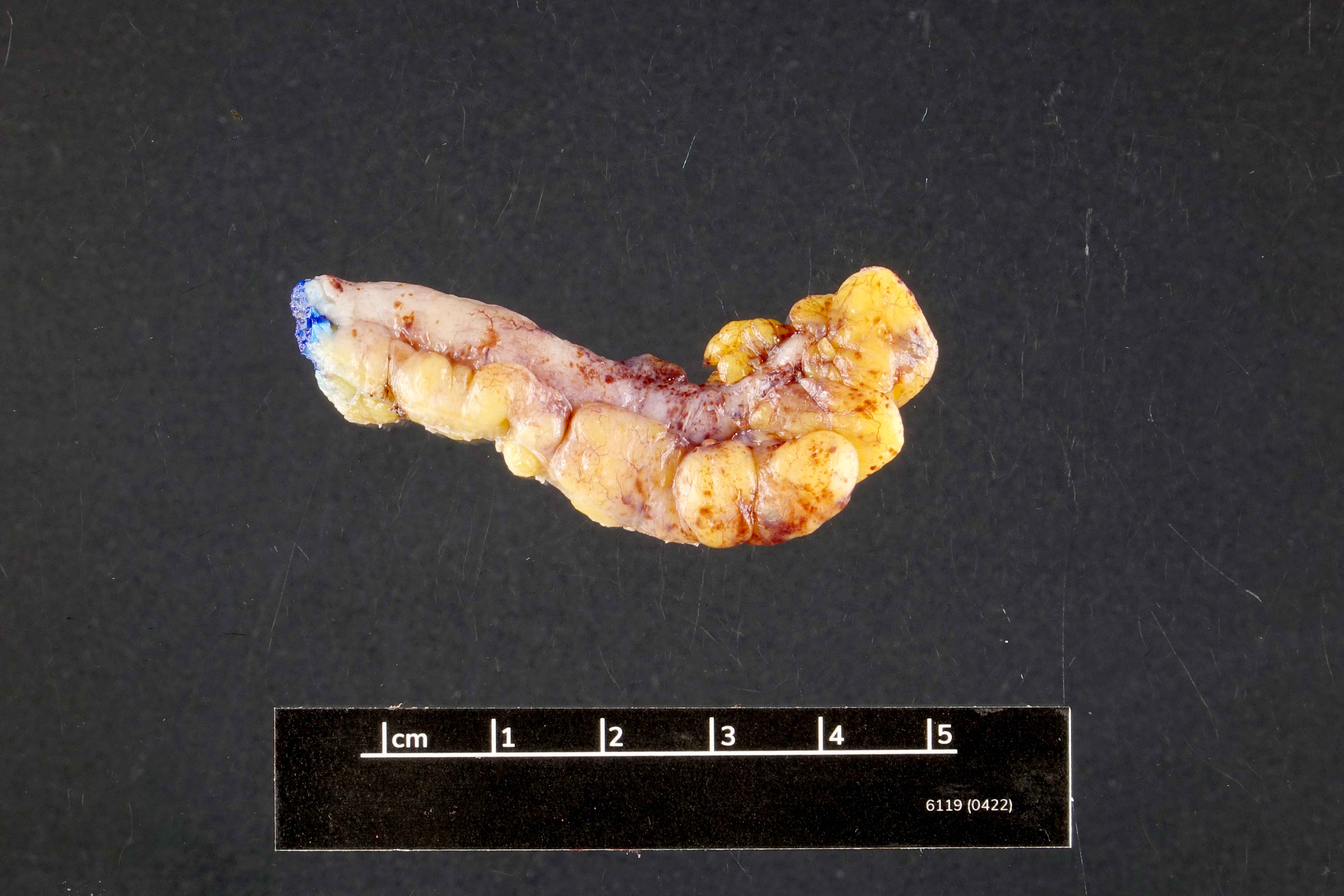
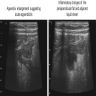
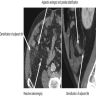
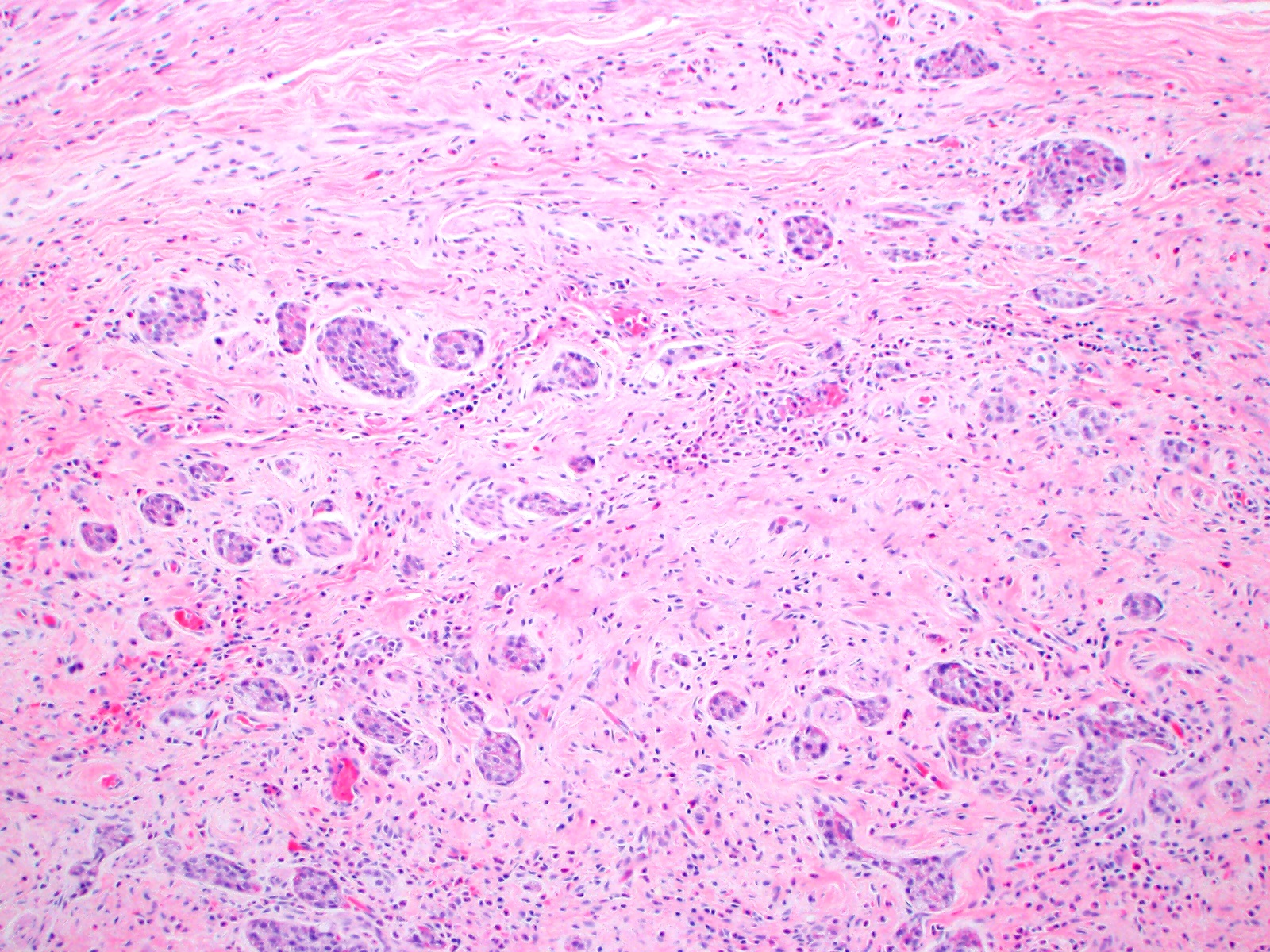
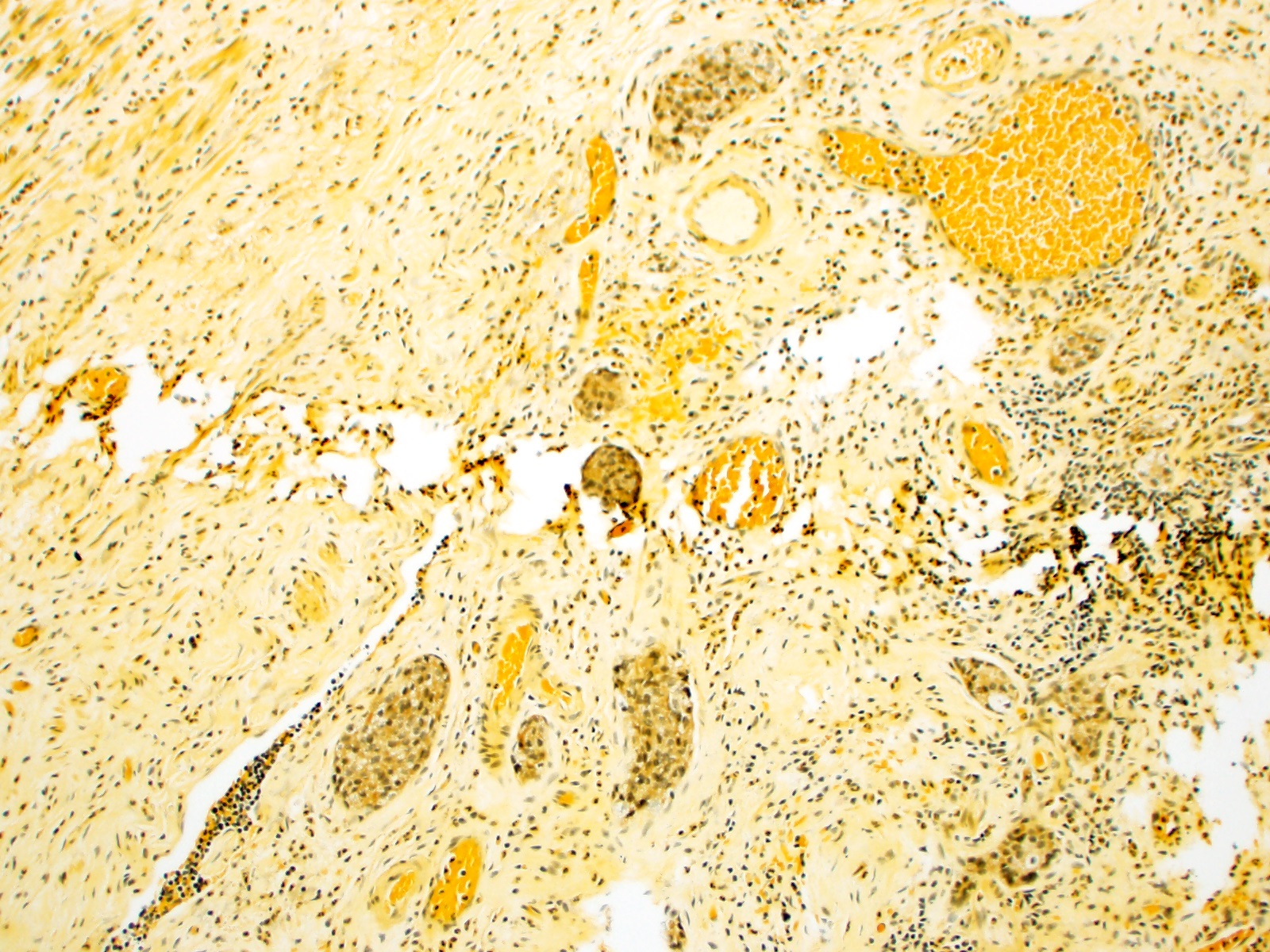
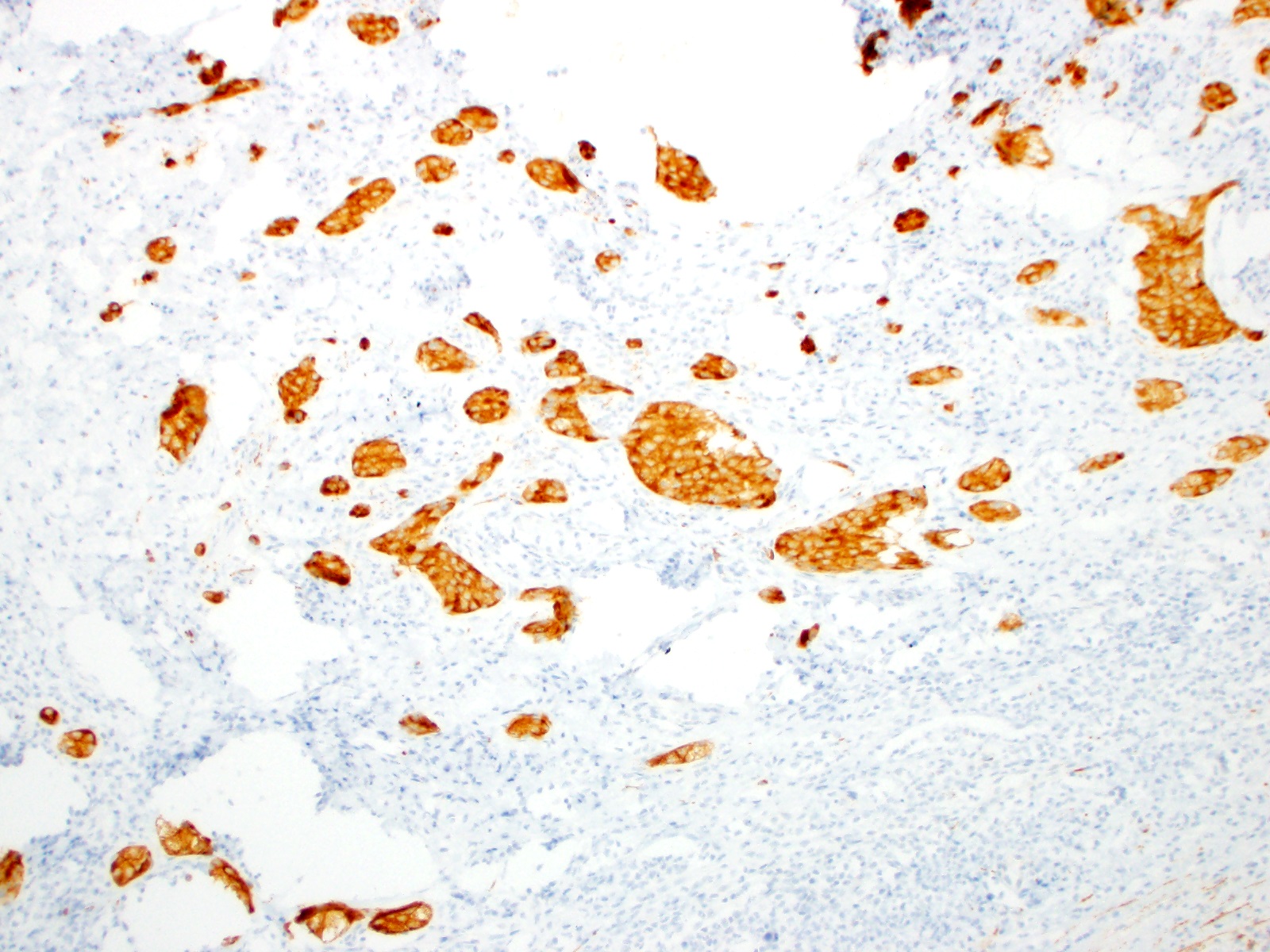
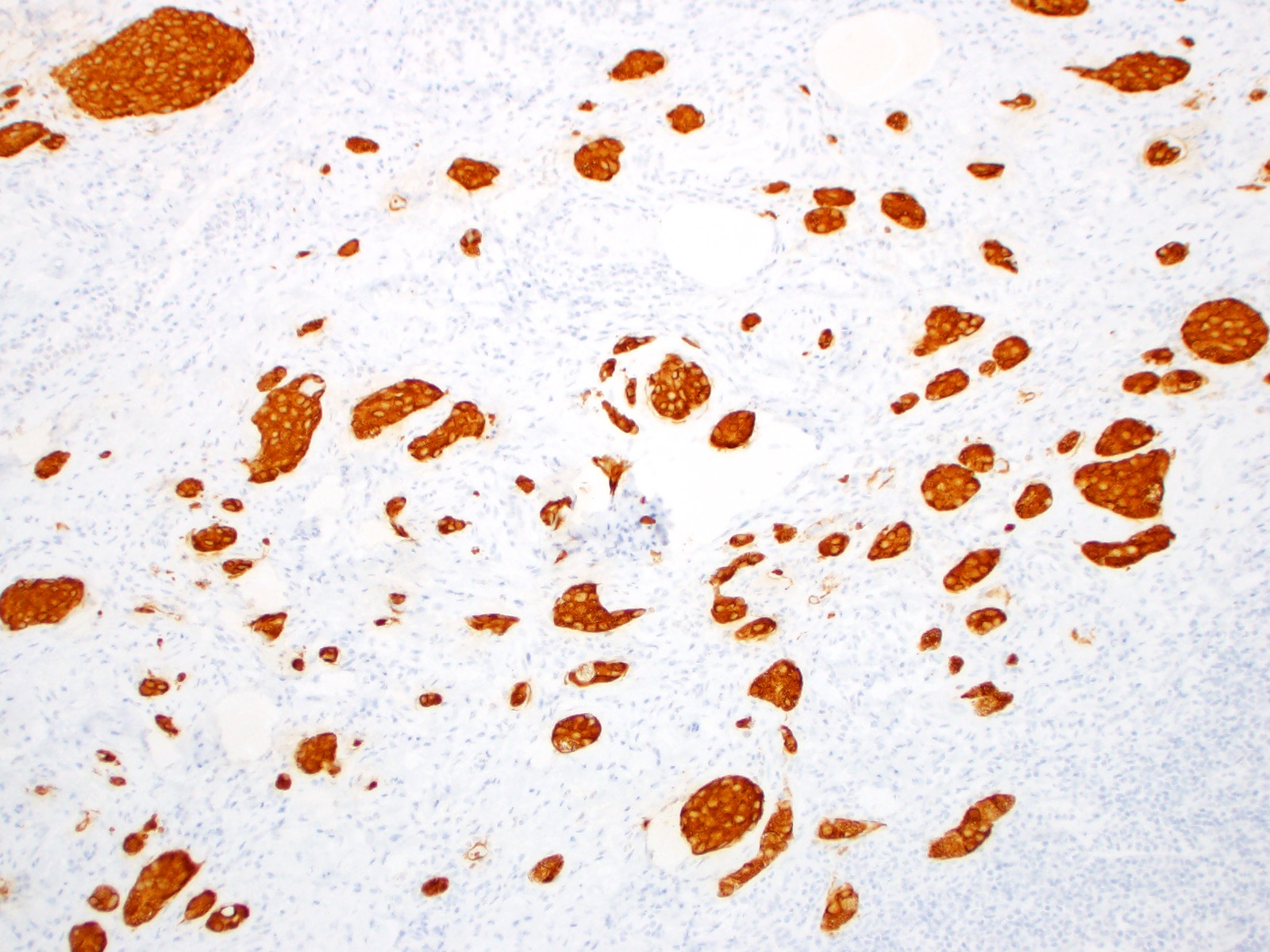
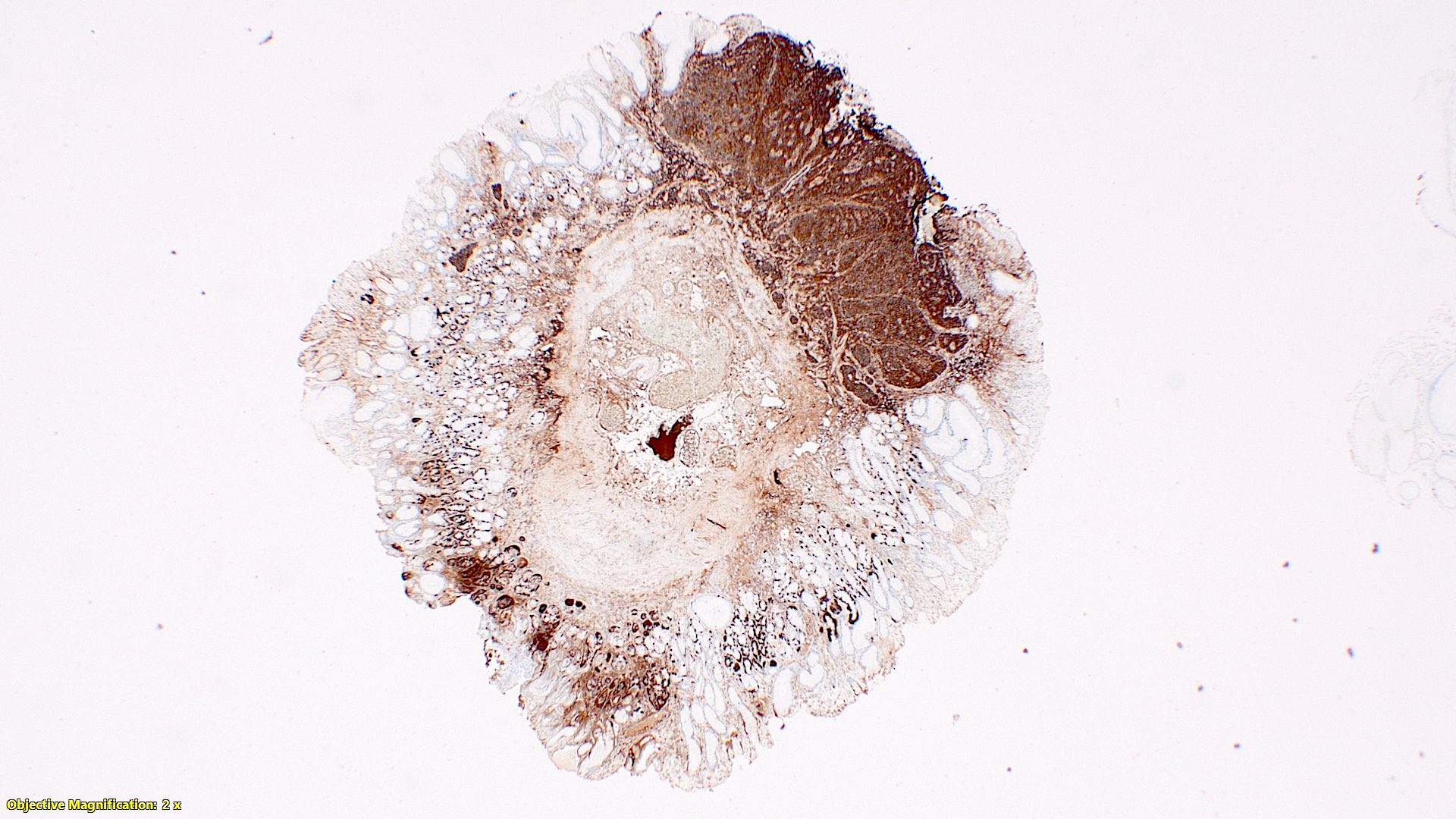
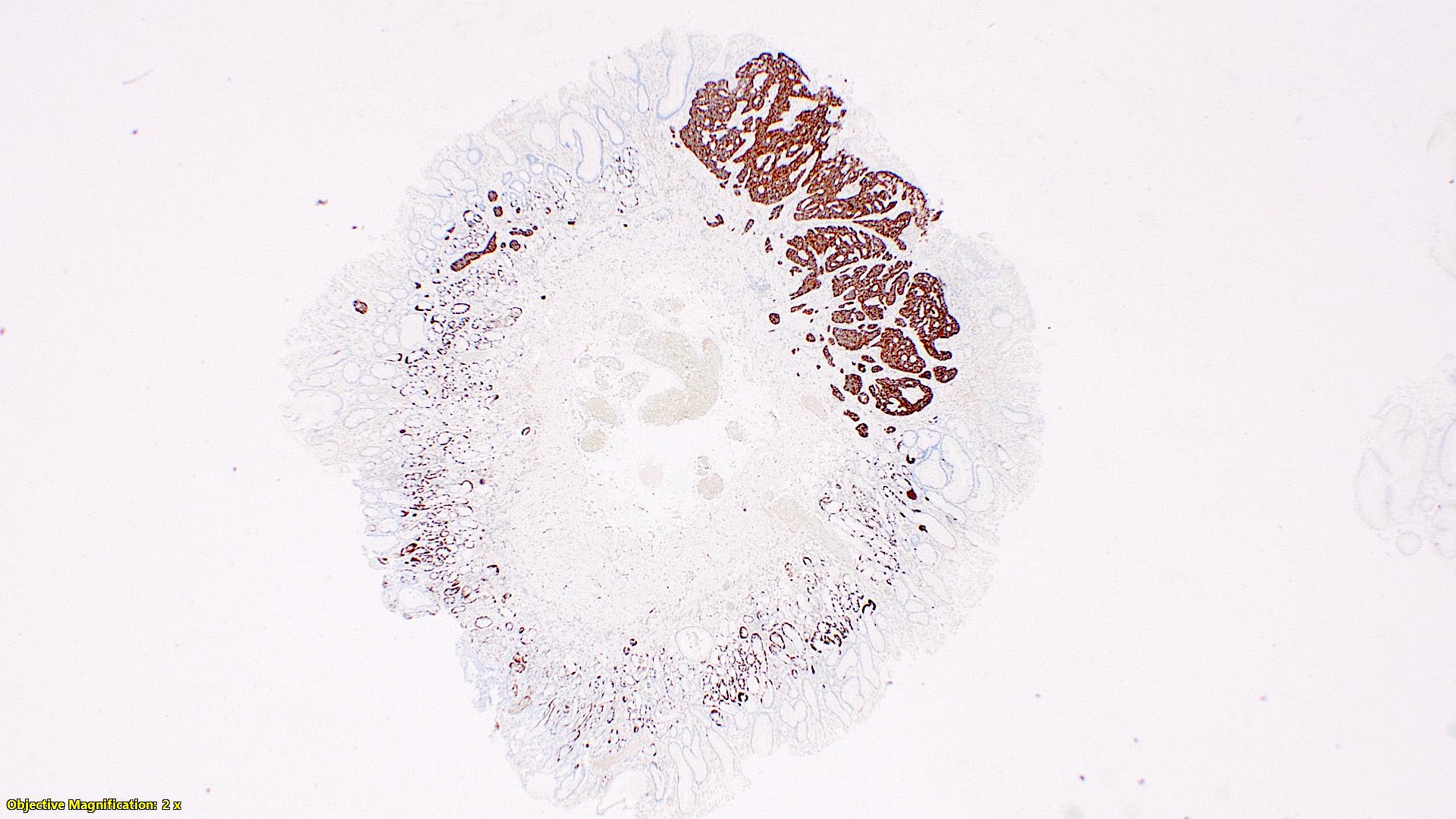
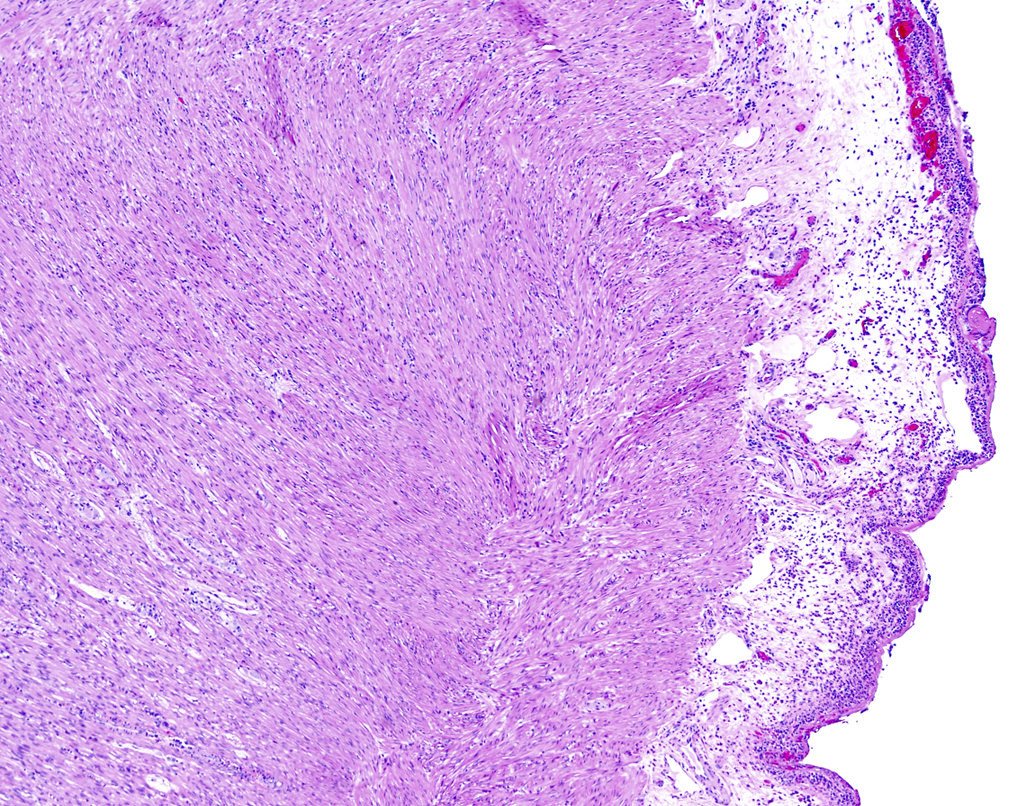
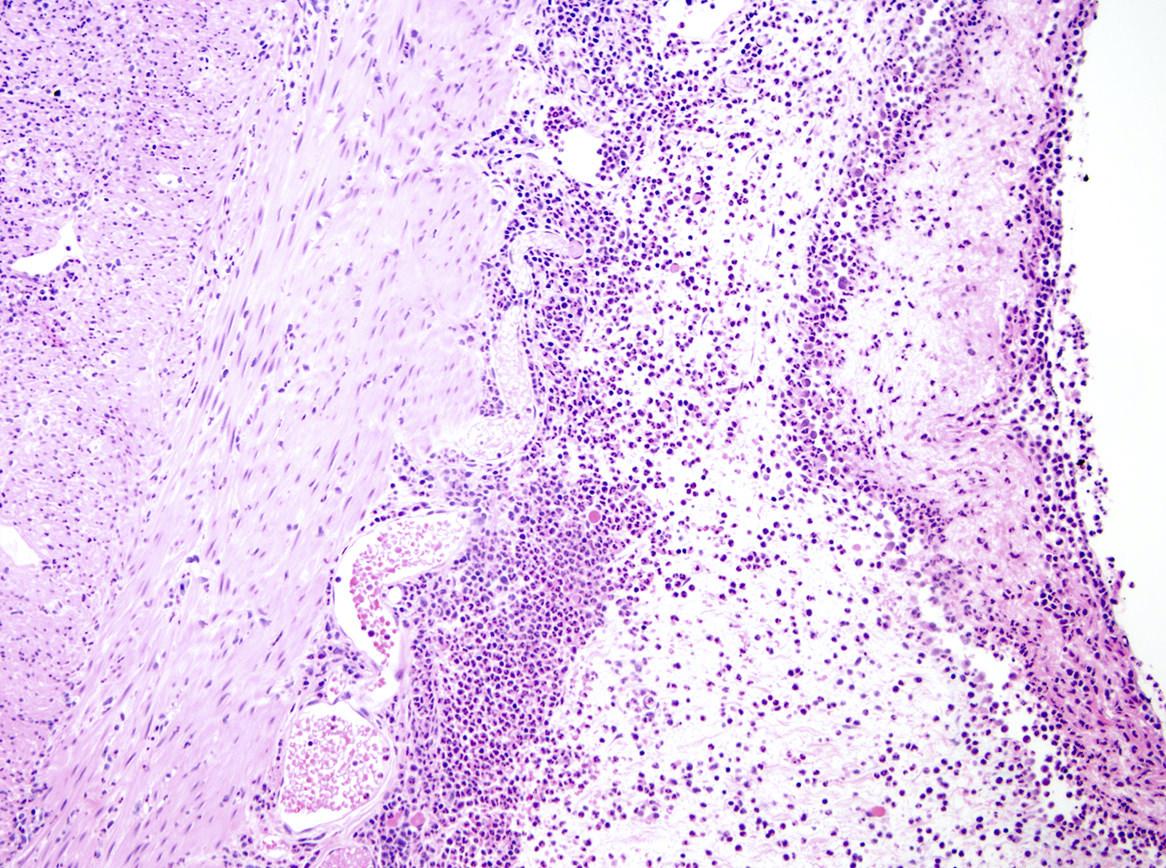
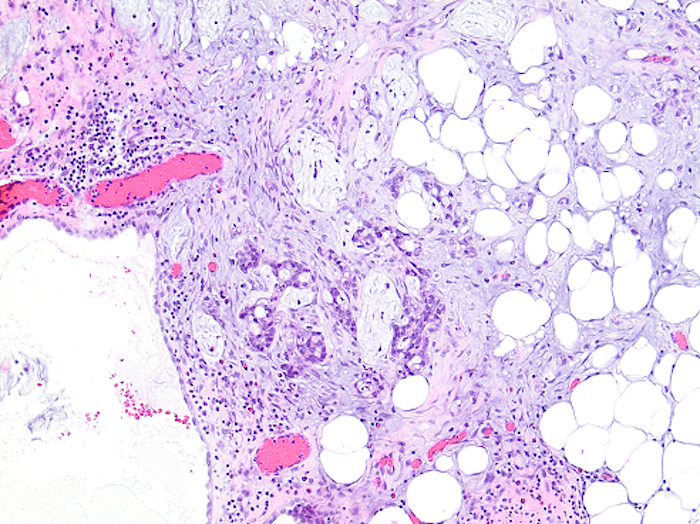
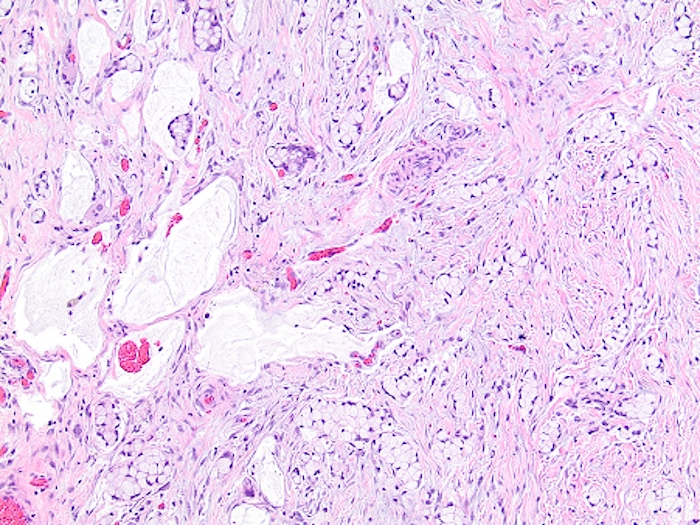
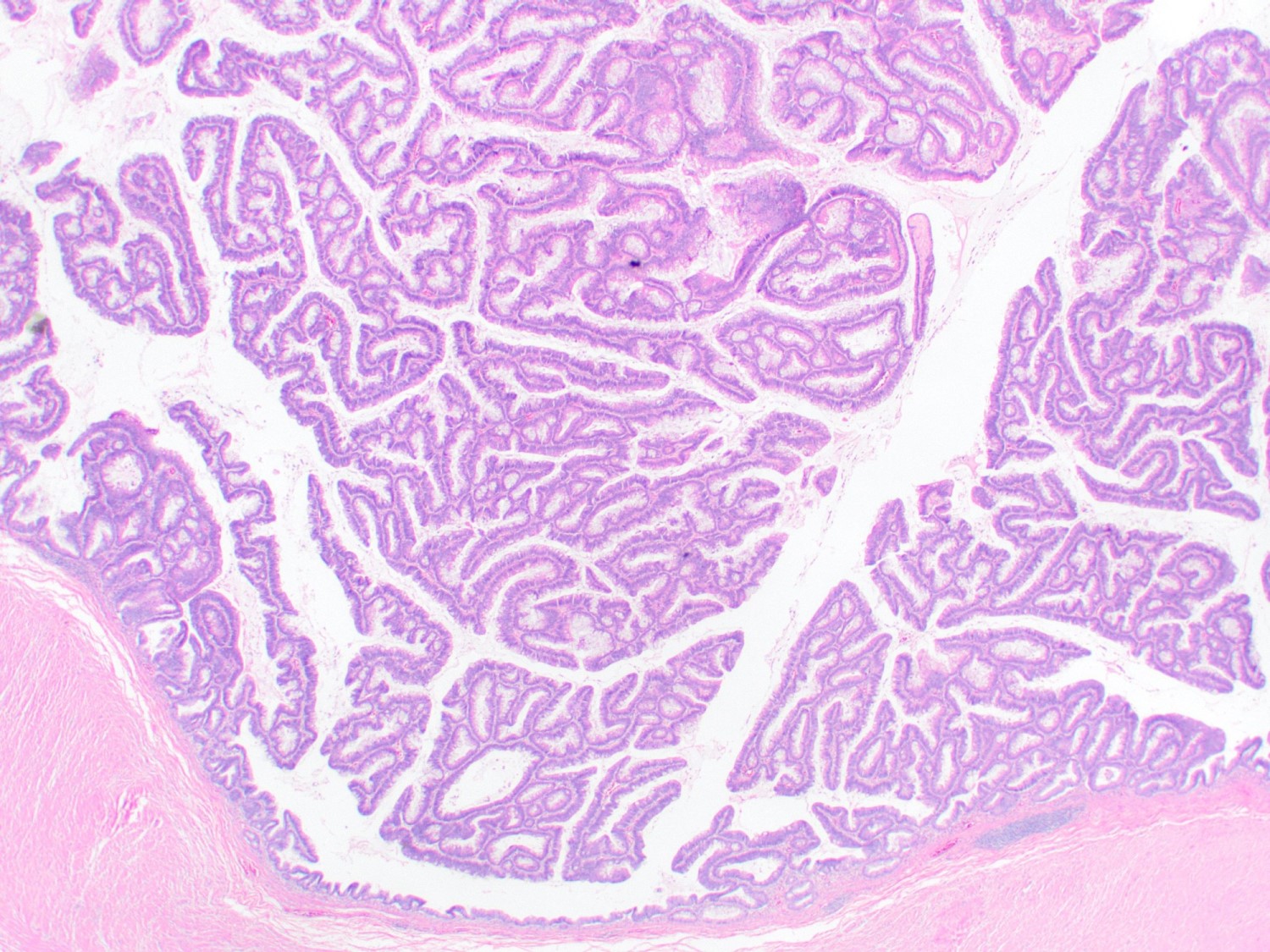
- T3: tumor invades through the muscularis propria into the subserosa or the mesoappendix
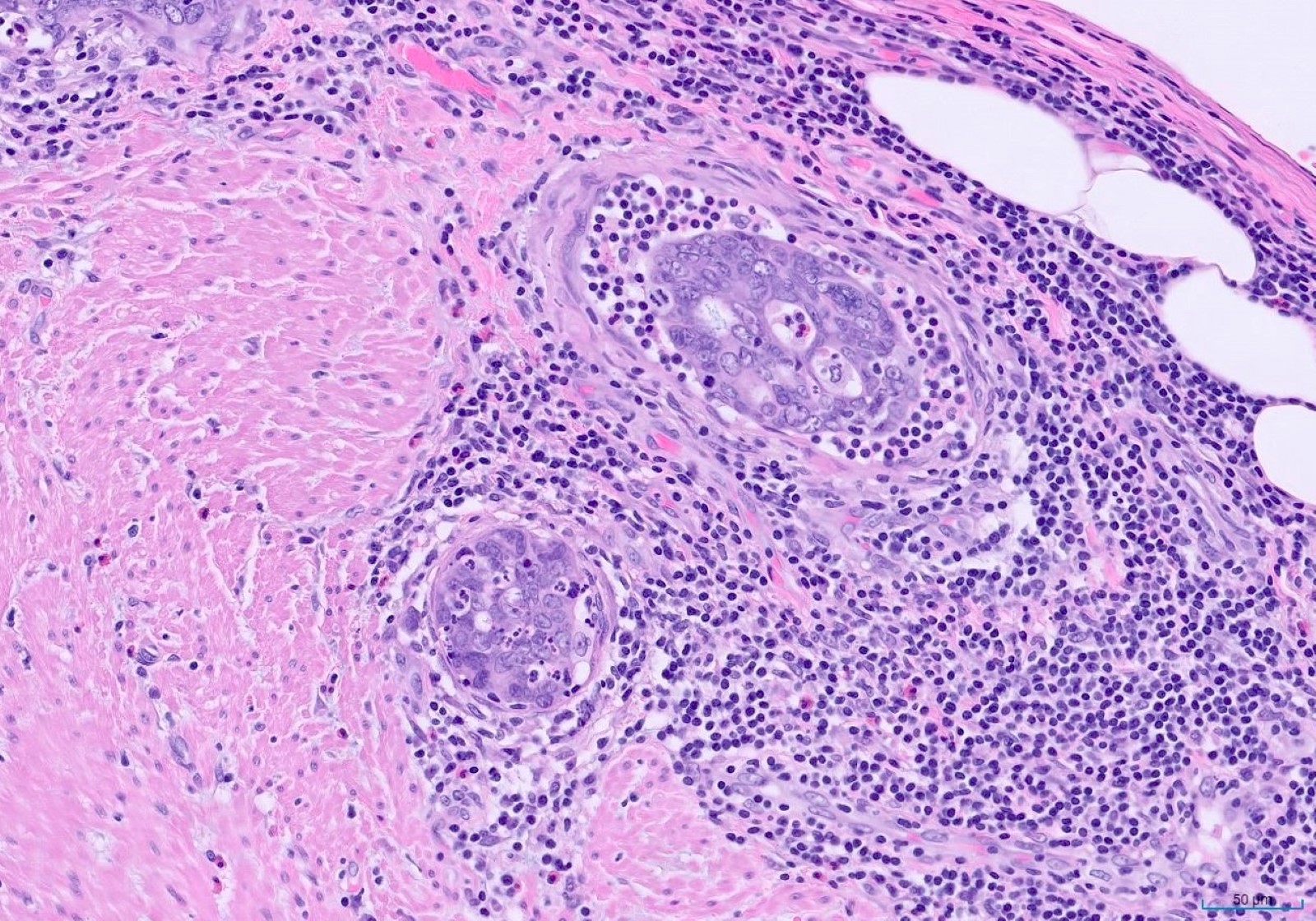
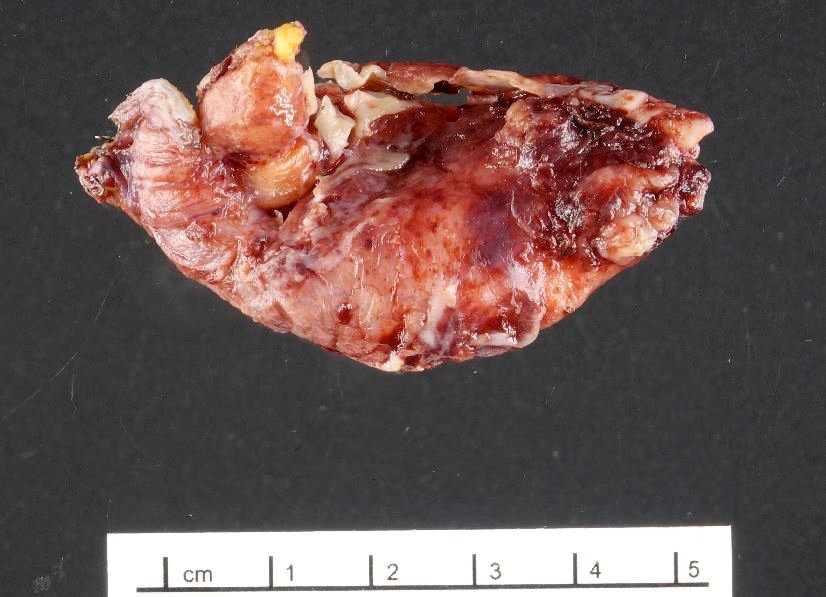
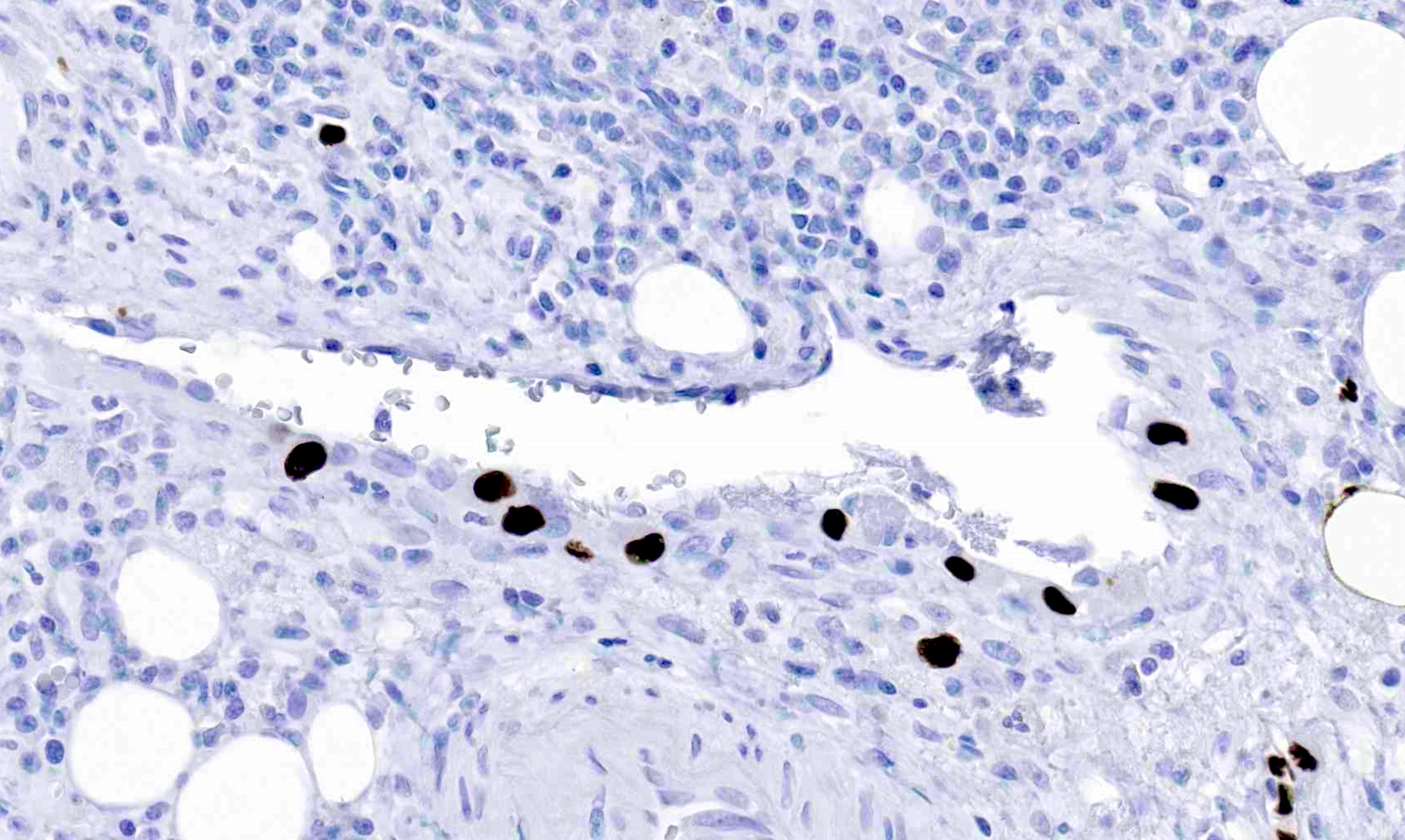
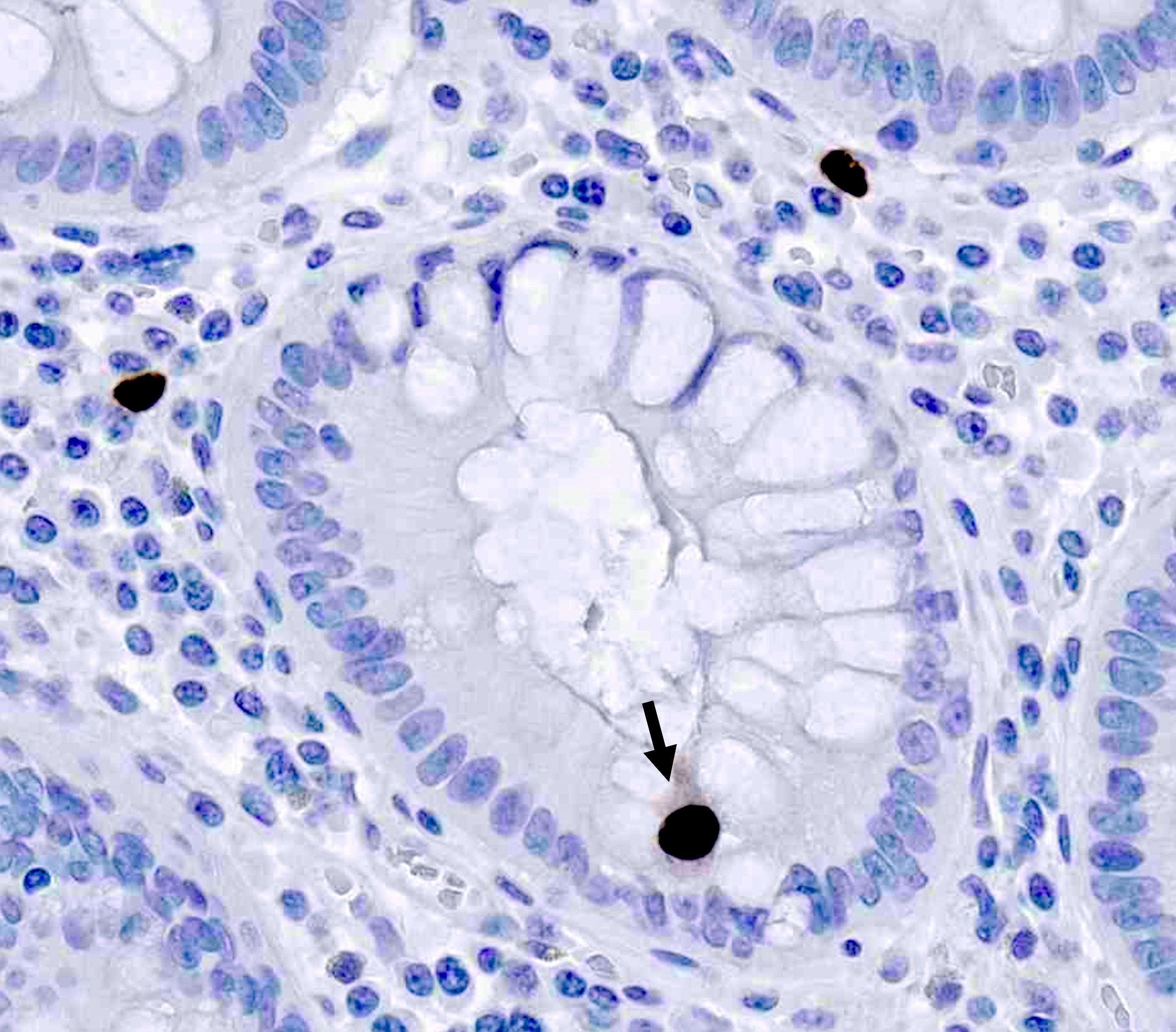
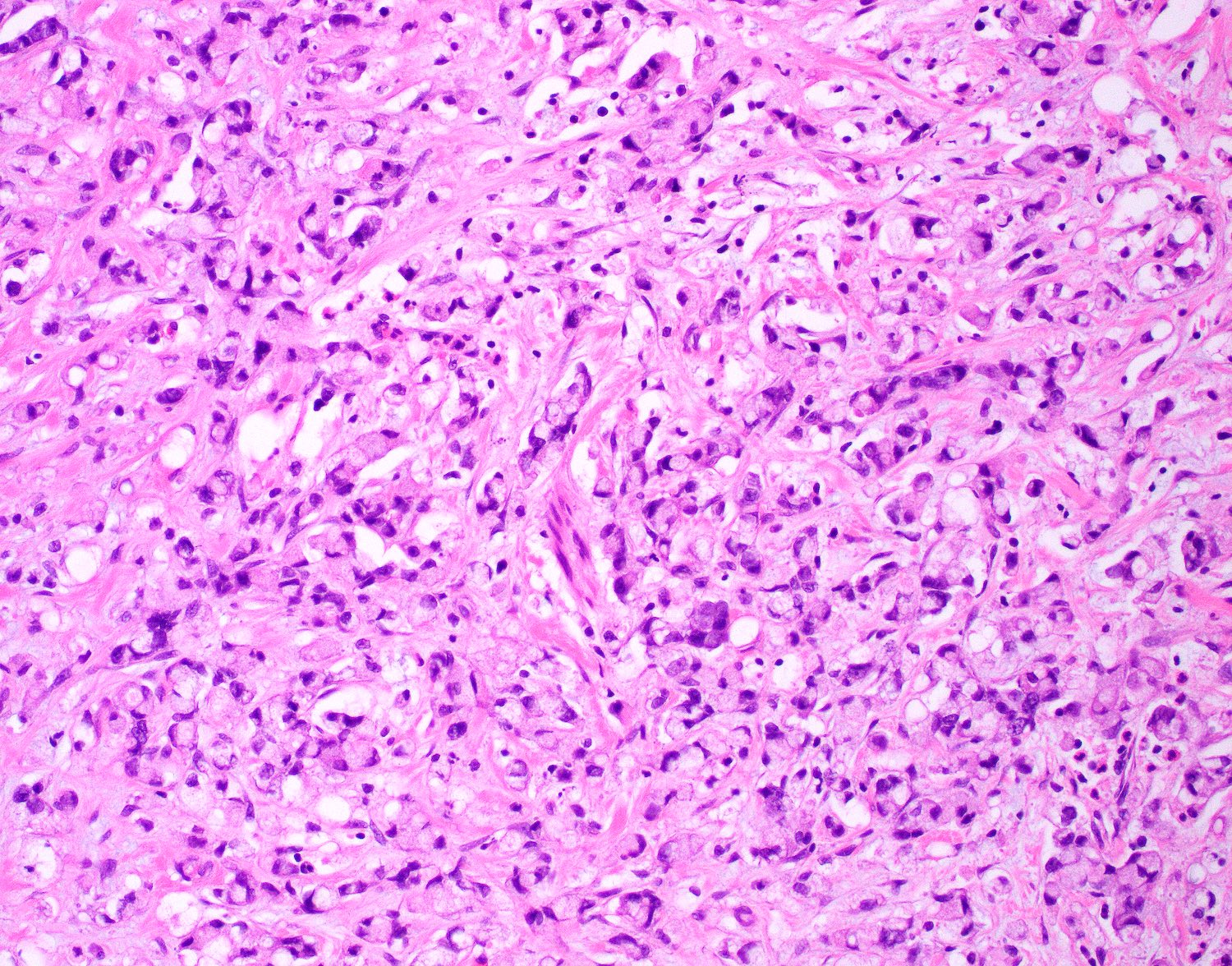
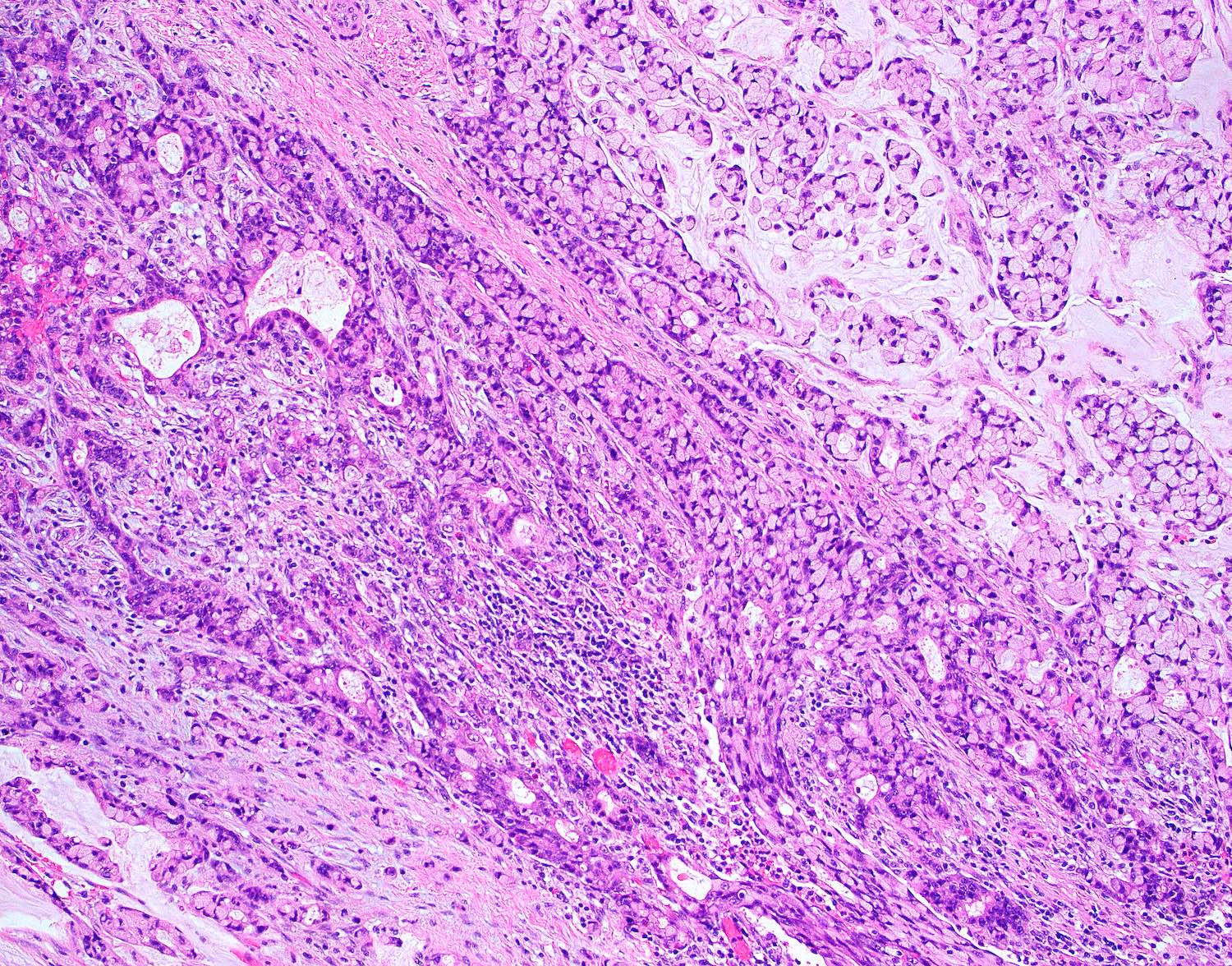
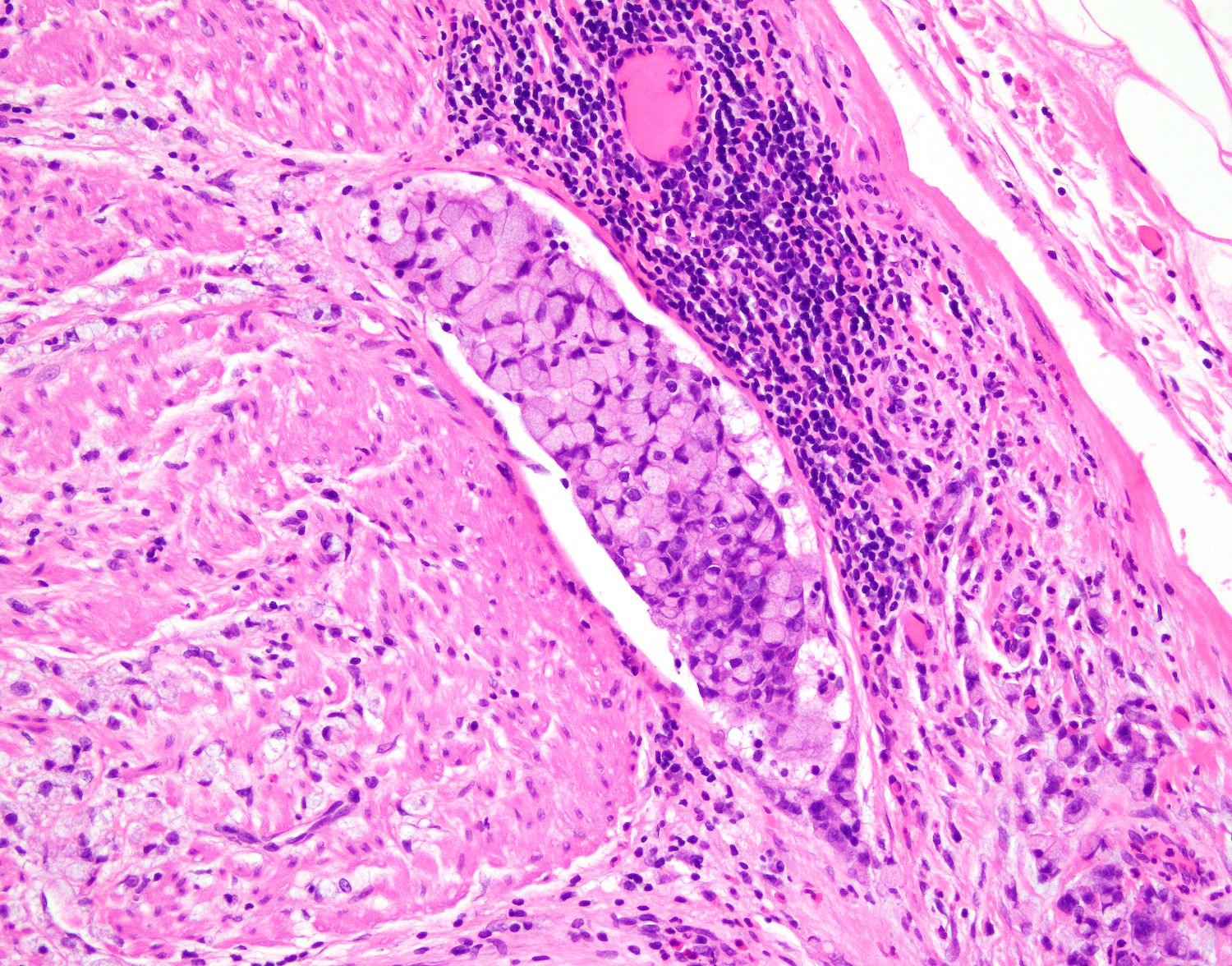
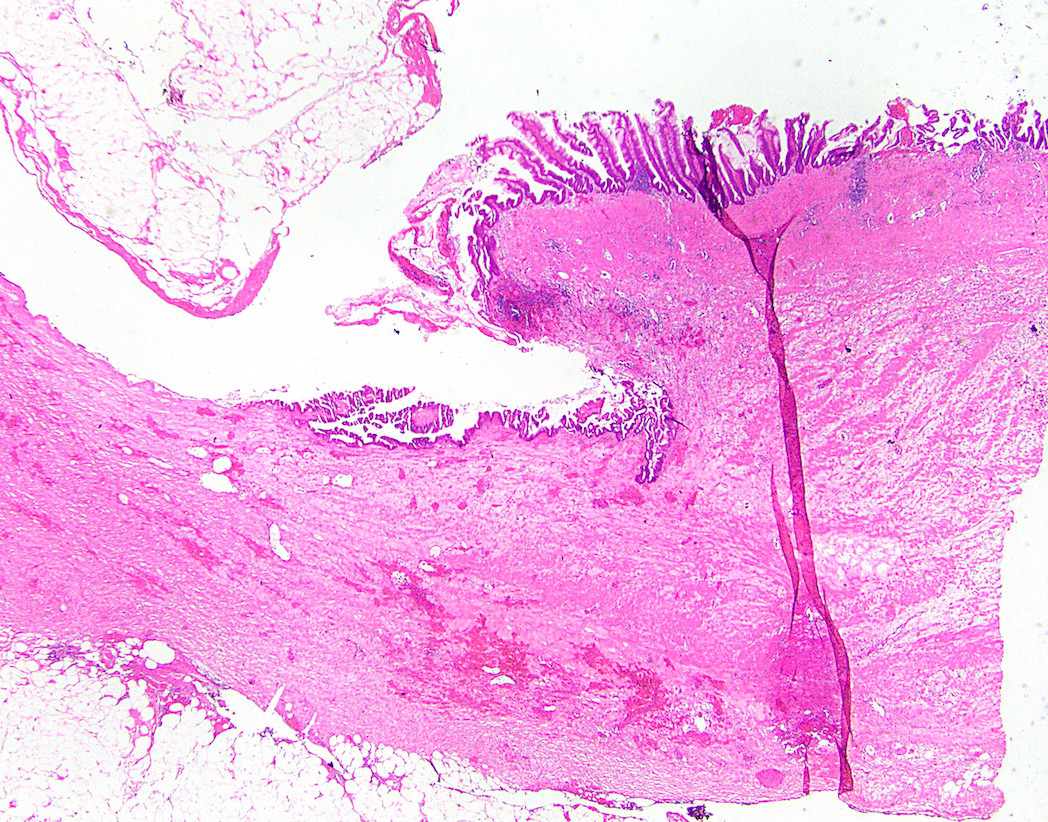
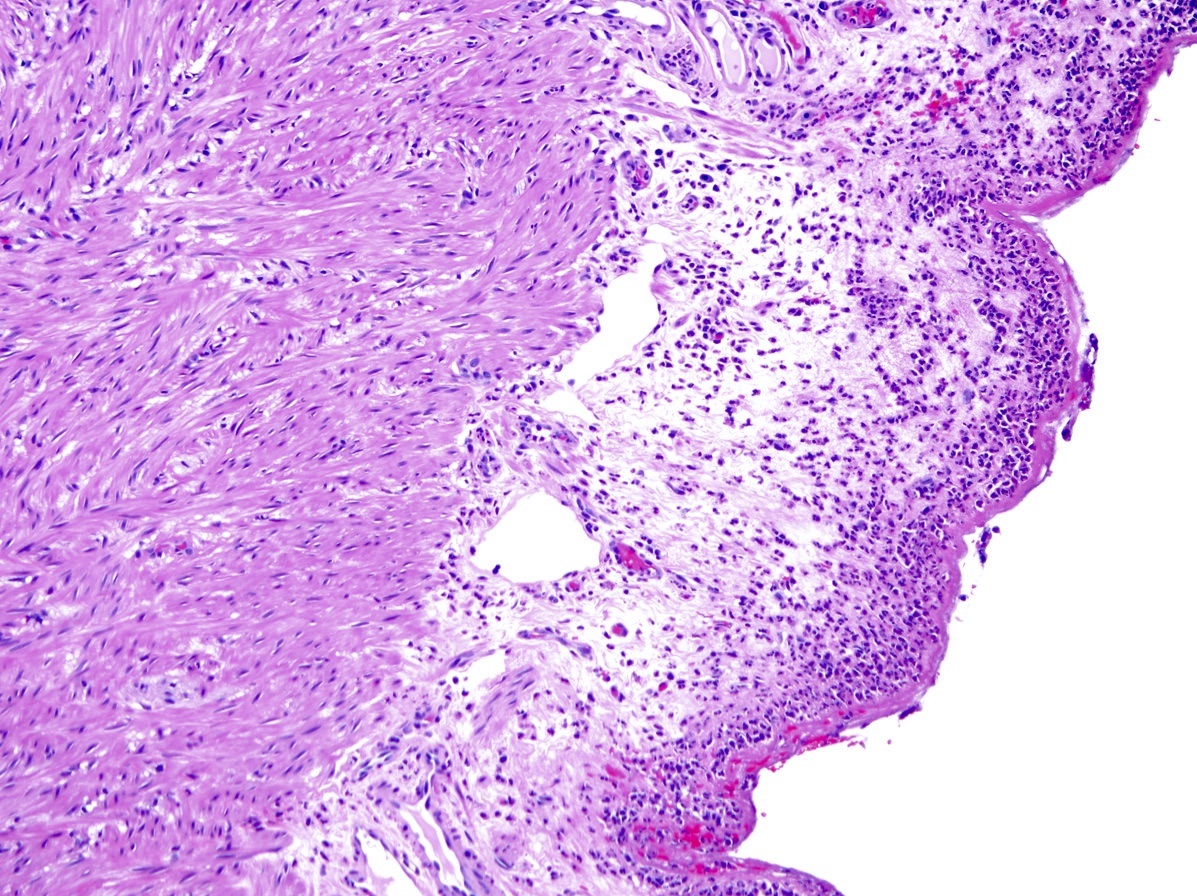
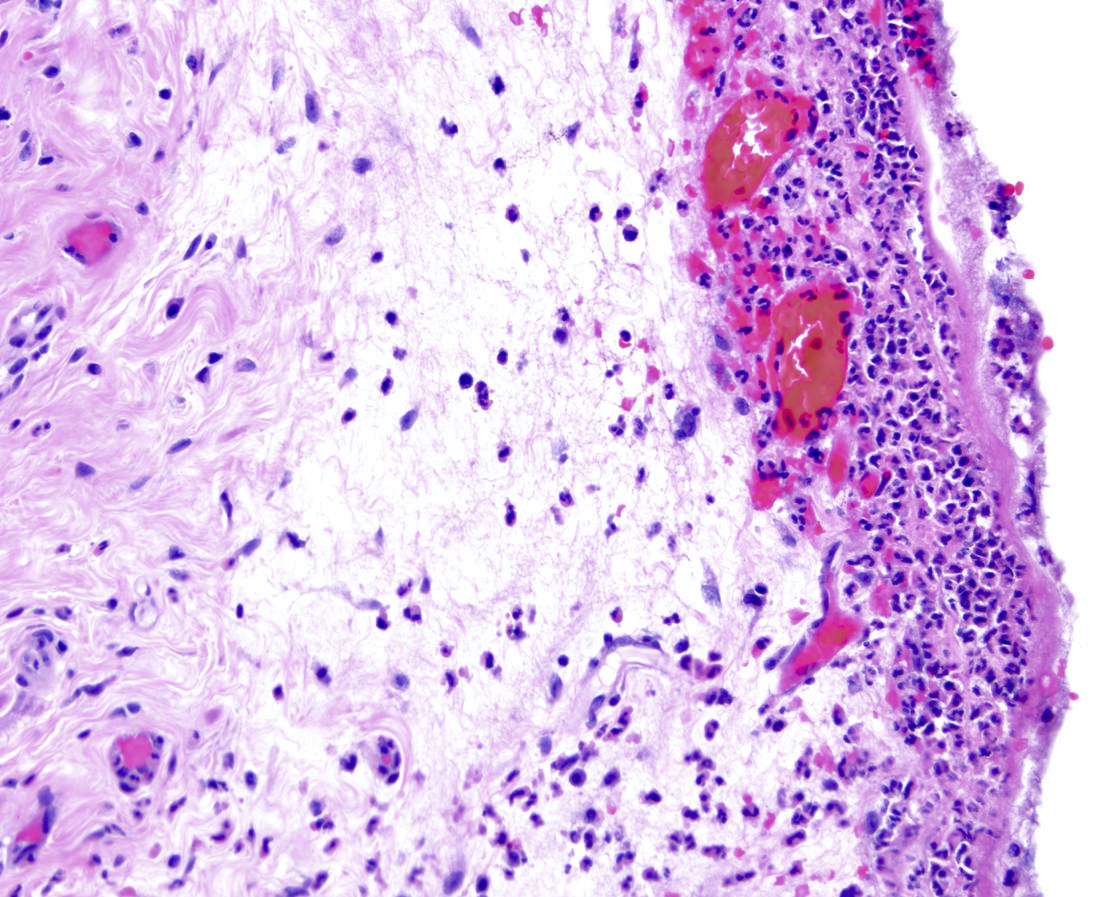
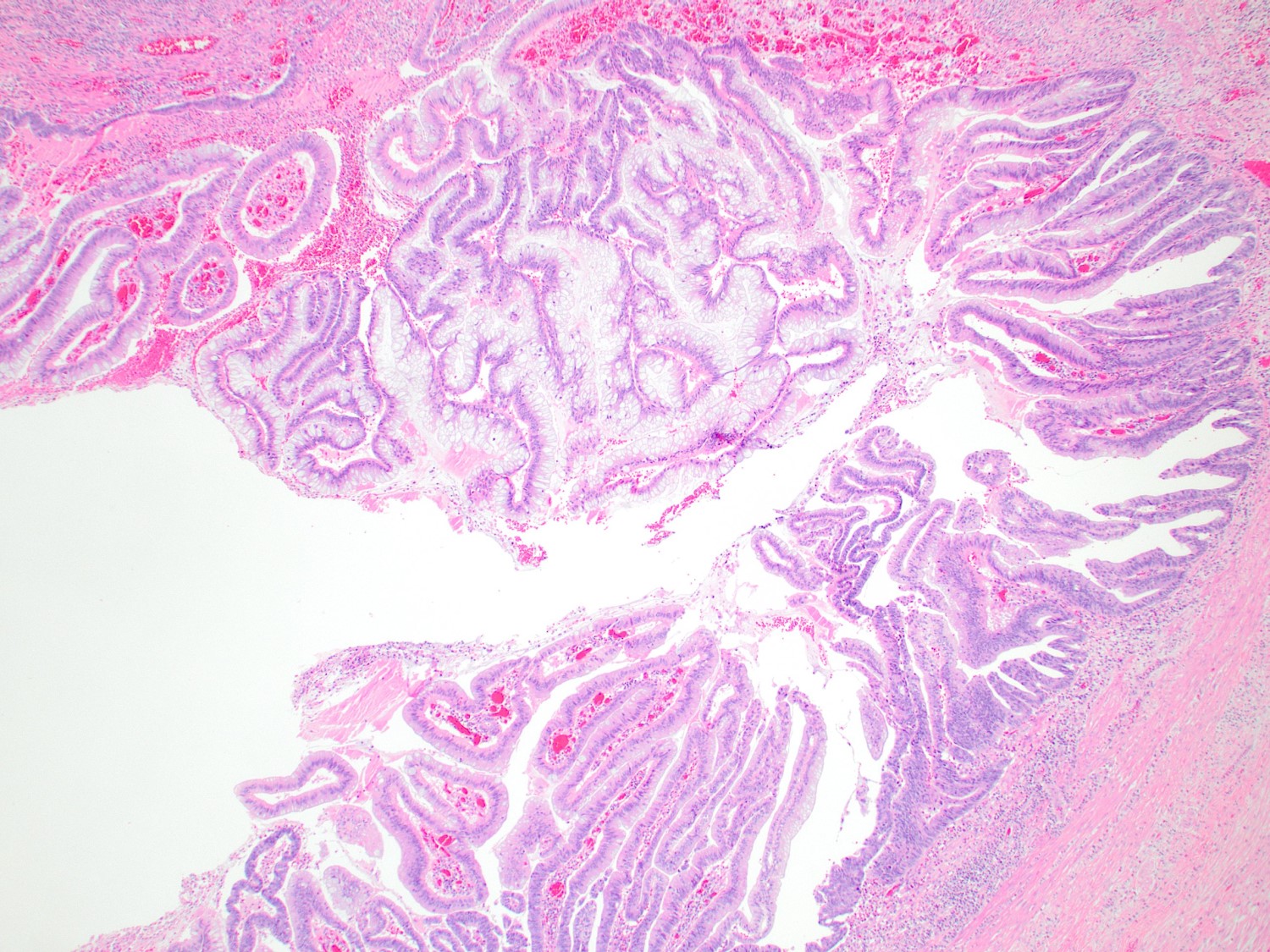
- T4: tumor invades the visceral peritoneum, including the acellular mucin or mucinous epithelium involving the serosa of the appendix or mesoappendix or directly invades adjacent organs or structures
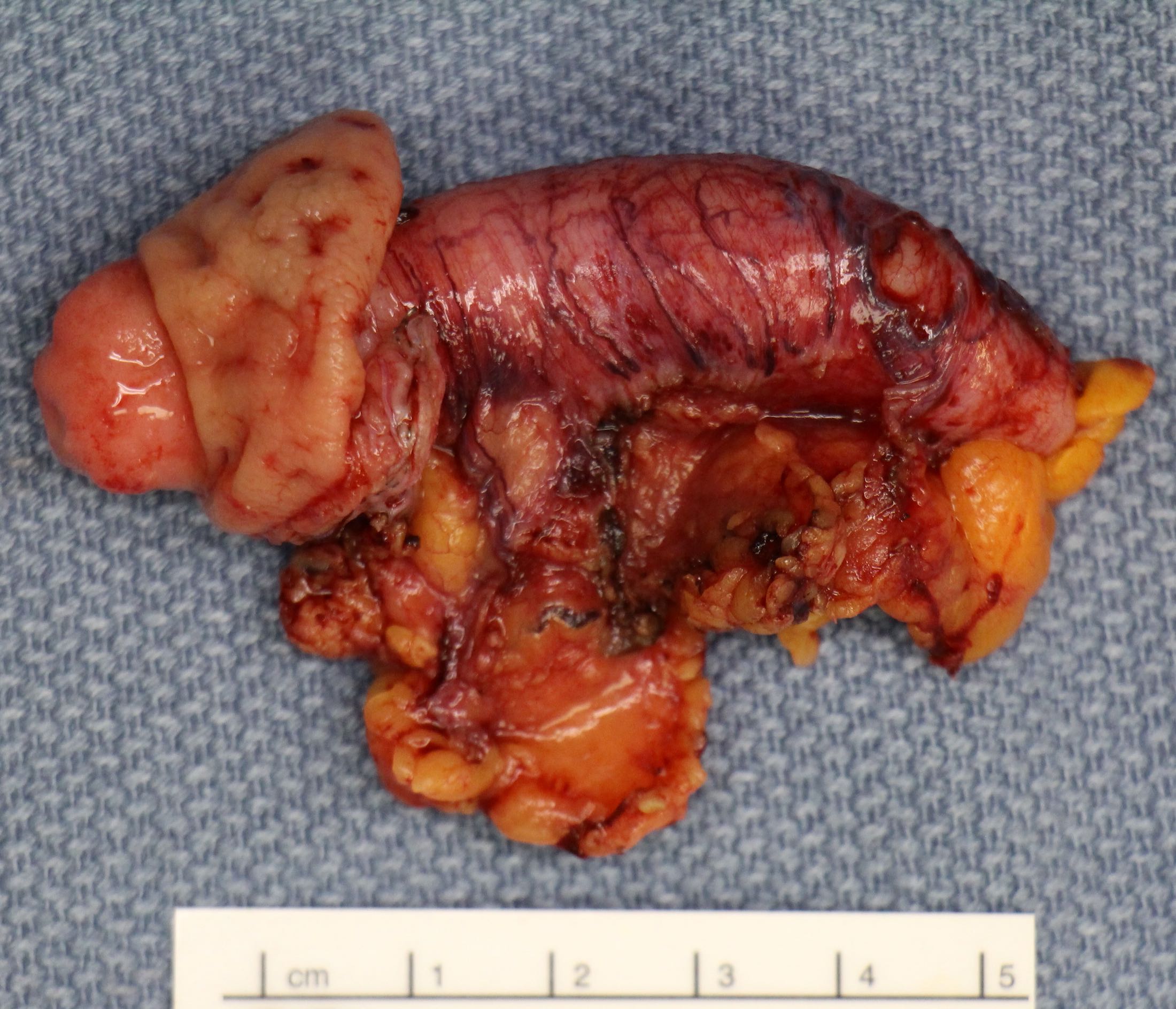
- T4a: tumor invades through the visceral peritoneum, including the acellular mucin or mucinous epithelium involving the serosa of the appendix or serosa of the mesoappendix
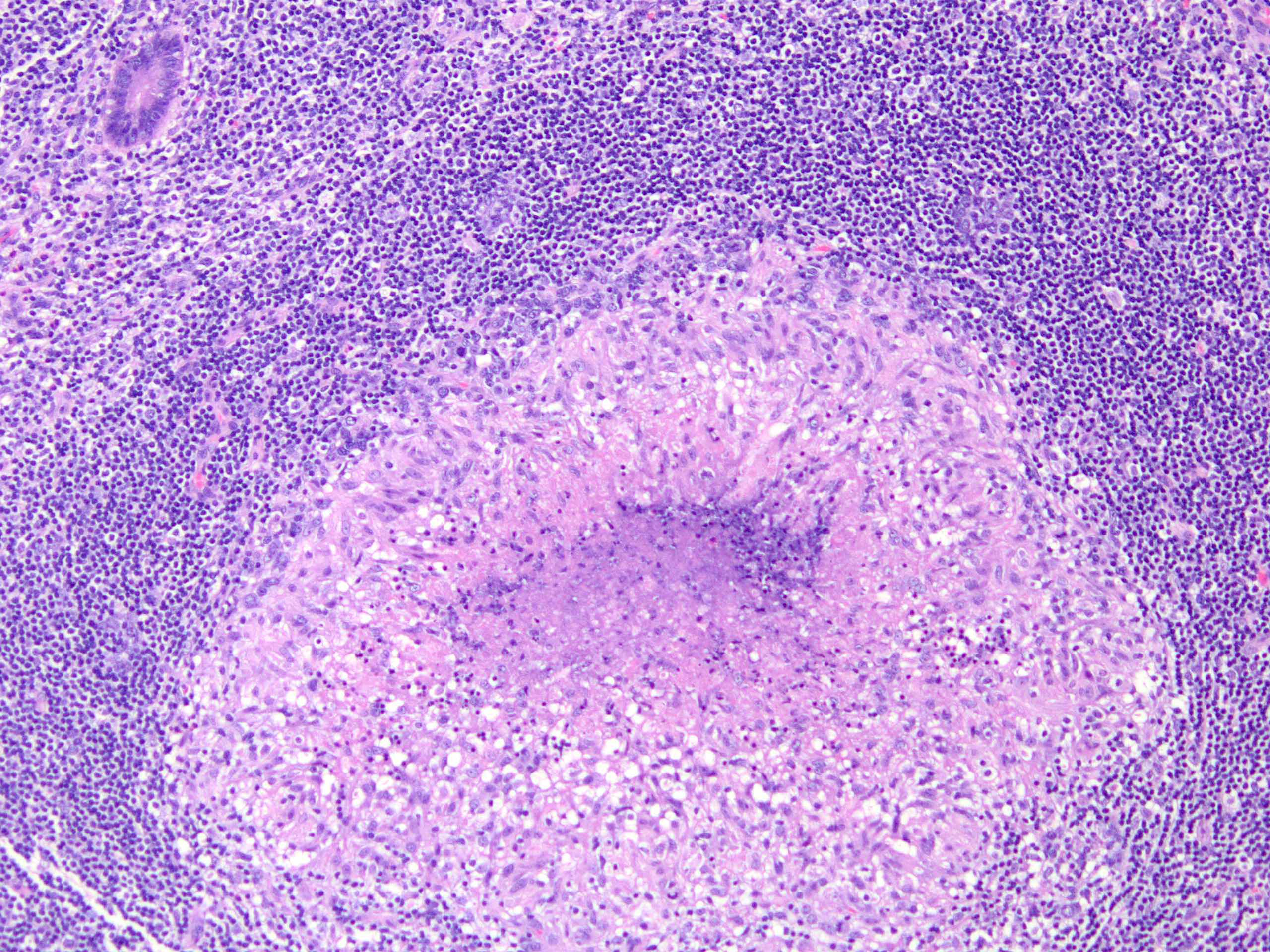
- T4b: tumor directly invades or adheres to adjacent organs or structures
Notes:
- T1 and T2 are not applicable to LAMN; acellular mucin or mucinous epithelium that extends into the subserosa or serosa should be classified as T3 or T4a, respectively (Hum Pathol 2017;69:81)
- T1 and T2 are applicable to HAMN