Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Negative stains | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Ding CC, Wen KW. Neuroendocrine tumor. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/appendixwdneuroendocrine.html. Accessed April 1st, 2025.
Definition / general
- Well differentiated appendiceal epithelial neoplasms that likely arise from neuroendocrine cells, including enterochromaffin (EC) cell neuroendocrine tumors (NETs), L cell NETs and tubular NETs
- Molecular evidence at other anatomic sites supports that poorly differentiated neuroendocrine neoplasms (neuroendocrine carcinomas [NECs]) are biologically different from well differentiated NETs and are recognized as a different entity not discussed here (Mod Pathol 2018;31:1770)
- Appendiceal goblet cell adenocarcinoma (GCA) has a distinct genetic profile and is managed differently; it will not be discussed here (Hum Pathol 2018;77:166, Mod Pathol 2018;31:829)
Essential features
- Appendiceal NETs are well differentiated appendiceal epithelial neoplasms that likely arise from neuroendocrine cells
- Most commonly seen at the tip of the appendix
- Majority (80%) of cases found incidentally, such as after a surgery for acute appendicitis
- Size of the primary tumor is the most reliable indicator of distant metastases
Terminology
- Appendiceal carcinoid tumor, appendiceal carcinoid
ICD coding
- ICD-O: 8240/3 - neuroendocrine tumor, grade 1
- ICD-11:
- 2B81.2 - neuroendocrine neoplasms of appendix
- 2B81.2 & XH9LV8 - neuroendocrine neoplasms of appendix & neuroendocrine tumor, grade 1
- 2B81.2 & XH7NM1 - neuroendocrine neoplasms of appendix & enterochromaffin cell carcinoid
- 2B81.2 & XH7LW9 - neuroendocrine neoplasms of appendix & L cell tumor
- 2B81.2 & XH7152 - neuroendocrine neoplasms of appendix & glucagon-like peptide producing tumour
- 2B81.2 & XH9ZS8 - neuroendocrine neoplasms of appendix & pancreatic peptide and pancreatic peptide-like peptide within terminal tyrosine amide producing tumor
Epidemiology
- Appendiceal NETs are the fifth most frequent gastrointestinal NET
- Incidence of 0.15 - 0.6 cases per 100,000 person years
- Slight female predominance and highest incidence before the age of 40 years
Sites
- Most commonly seen at the tip of the appendix (67% of adult patients, 73% of pediatric patients)
Pathophysiology
- Pathogenesis is largely unknown; possible hypothetical origins include:
- Neuroendocrine cells within the mucosal crypts
- Subepithelial neuroendocrine cells, especially for enterochromaffin cell NETs
Etiology
- Currently unknown
Clinical features
- Majority (80%) of cases are incidental, such as after a surgery for acute appendicitis
- Diagnosed in 1.86% of appendectomies between 2001 and 2015 (Neuroendocrinology 2018;106:242)
- Extremely rare association with carcinoid syndrome
Diagnosis
- Predominantly diagnosed incidentally with other diseases, such as acute appendicitis
- Somatostatin receptor imaging reported to be useful in establishing the diagnosis of neuroendocrine neoplasm in general (World J Gastrointest Surg 2021;13:231)
Laboratory
- Chromogranin A, 5-HIAA (serum or urine), neurokinin A and pancreatic polypeptide can be elevated in a minority of cases (World J Gastrointest Surg 2021;13:231)
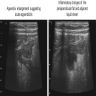
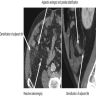
Radiology description
- Usually not identifiable due to small size
- Typical presentation includes ultrasound or CT findings consistent with acute appendicitis (e.g. appendix enlargement, inflammatory changes or densification of the periappendicular fat, adjacent liquid sheet and reactive adenomegaly) (J Surg Case Rep 2019;2019:rjz086)
Prognostic factors
- Size of the primary tumor is the most reliable indicator of distant metastases (30% in tumors ≥ 2 cm) (Arch Pathol Lab Med 1980;104:272)
- Excellent long term outcome
- 10 year survival rate of 92 - 99%
- Metastases in 1.4 - 8.8% of cases, typically only involving regional lymph nodes
- 5 year survival ~85%, even with regional metastases; 34% with distant metastases
- 10 year survival rate of 92 - 99%
- Mesoappendiceal invasion and invasion > 3 mm are associated with lower survival rates (Am J Surg Pathol 2013;37:606)
Case reports
- 10 year old boy with right lower quadrant abdominal pain (World J Surg Oncol 2019;17:197)
- 27 year old woman with rapid onset epigastric tenderness and right lower quadrant pain (Rom J Morphol Embryol 2017;58:1509)
- 30 year old man with abdominal pain and tenderness in right lower quadrant (Ann Med Surg (Lond) 2019;43:44)
- 32 year old man who underwent left inguinal hernia repair but was found to have mucin in the hernia sac and 39 year old woman with right lower quadrant pain (Int J Surg Case Rep 2020;67:76)
- 69 year old man with acute right lower quadrant abdominal pain (J Surg Case Rep 2019;2019:rjz086)
Treatment
- Simple appendectomy is considered curative for tumor < 1 cm
- Right hemicolectomy for tumor > 2 cm or positive surgical margin or extremely pronounced mesoappendiceal spread
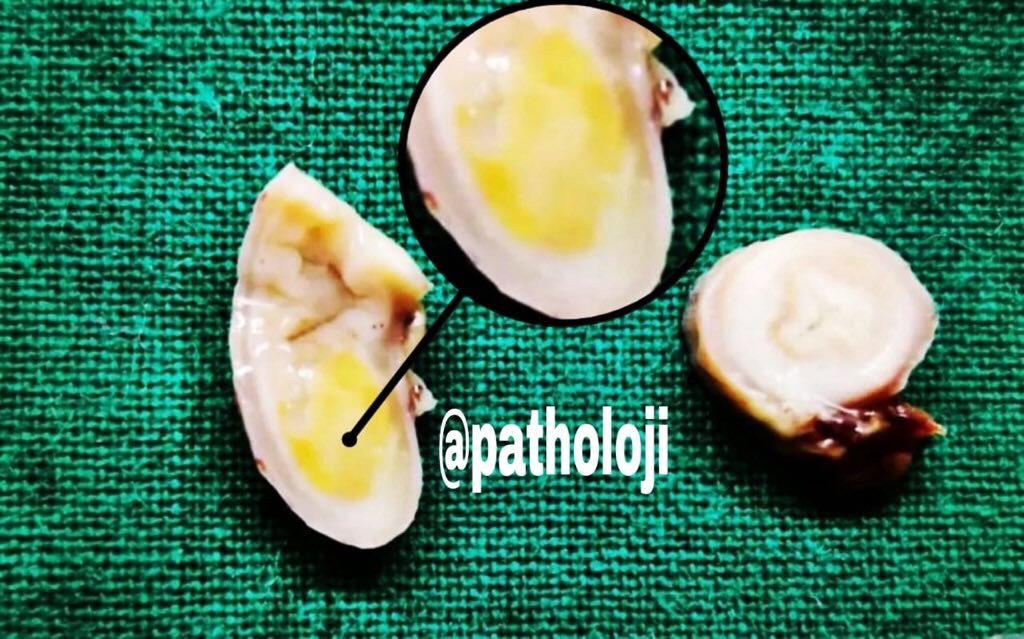
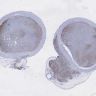
Gross description
- 70% at tip, most appendiceal NETs are < 2 cm (52 - 62% are < 1 cm, 28 - 30% are 1 - 2 cm, 8 - 19% are > 2 cm)
- Well demarcated, yellow (after formalin fixation), firm, intramural nodules that may narrow or obliterate lumen
- Proximal tumors may cause obstruction
- Gross findings are helpful for tumor staging; AJCC and ENETs staging are the 2 most commonly used systems:
- AJCC T staging (8th edition):
- pT0: no evidence of primary tumor
- pT1: tumor 2 cm or less in greatest dimension
- pT2: tumor more than 2 cm but less than or equal to 4 cm
- pT3: tumor more than 4 cm or with subserosal invasion or involvement of the mesoappendix
- pT4: tumor perforates the peritoneum or directly invades other adjacent organs or structures (excluding direct mural extension to adjacent subserosa of adjacent bowel) (e.g. abdominal wall and skeletal muscle)
- ENETS T staging (Neuroendocrinology 2016;103:144):
- pTX: primary tumor cannot be assessed
- pT0: no evidence of primary tumor
- pT1: tumor ≤ 1 cm with infiltration of submucosa and muscularis propria
- pT2: tumor ≤ 2 cm with infiltration of the submucosa, muscularis propria or minimal (≤ 3 mm) infiltration of the subserosa or mesoappendix
- pT3: tumor > 2 cm or extensive (> 3 mm) infiltration of the subserosa or mesoappendix
- pT4: tumor with infiltration of the peritoneum or other neighboring structures
- AJCC T staging (8th edition):
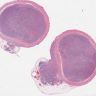
Gross images
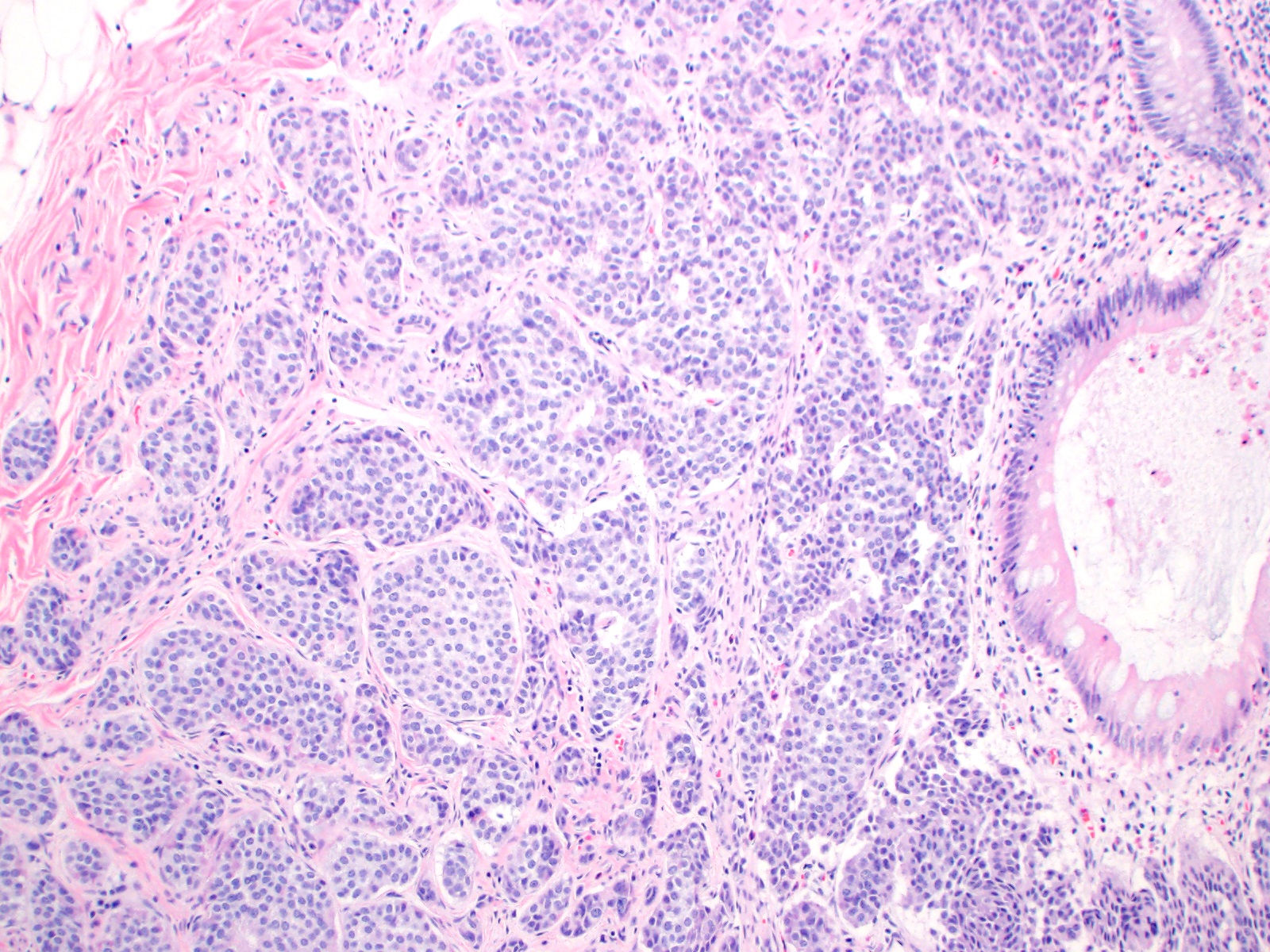
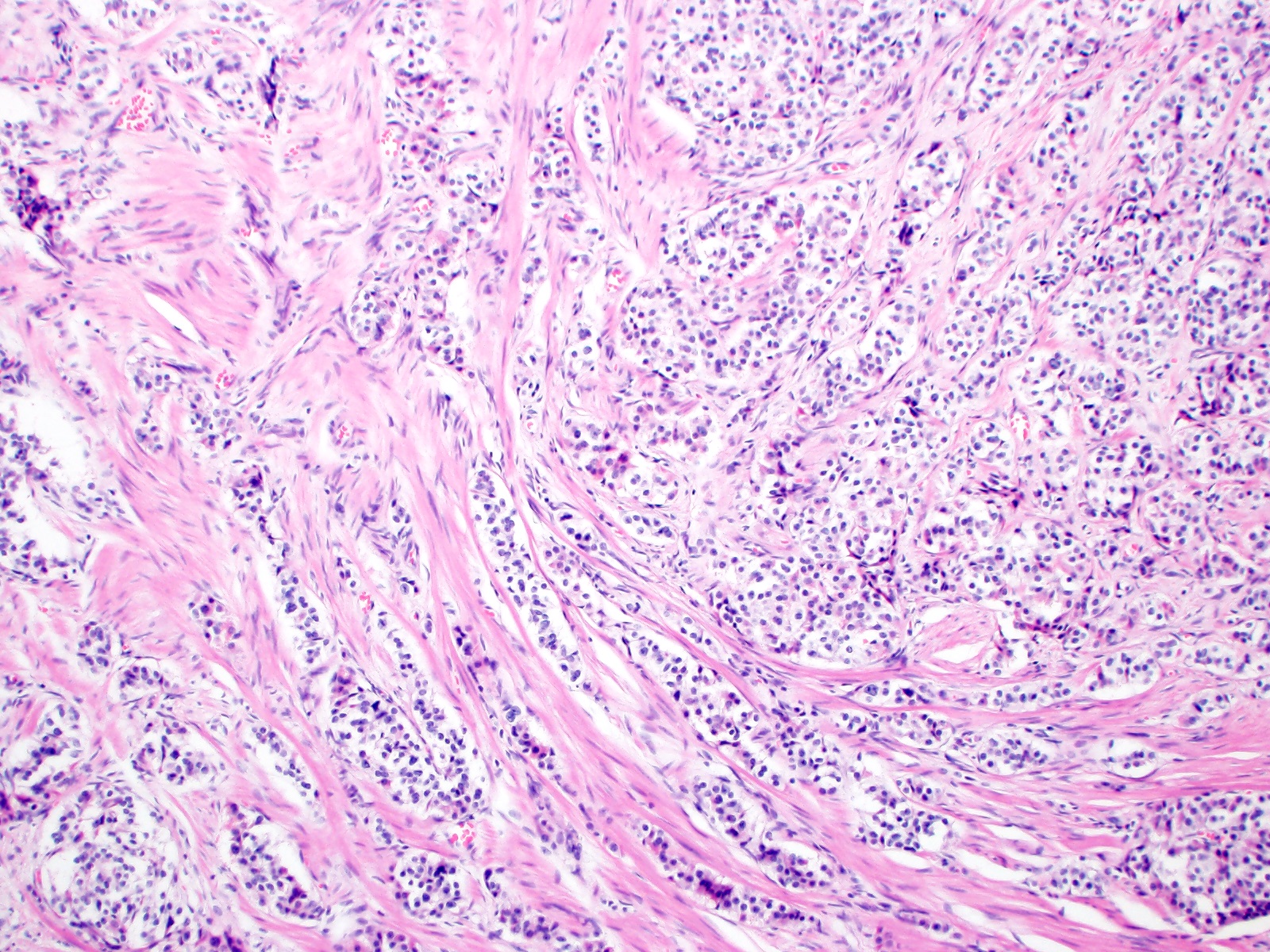
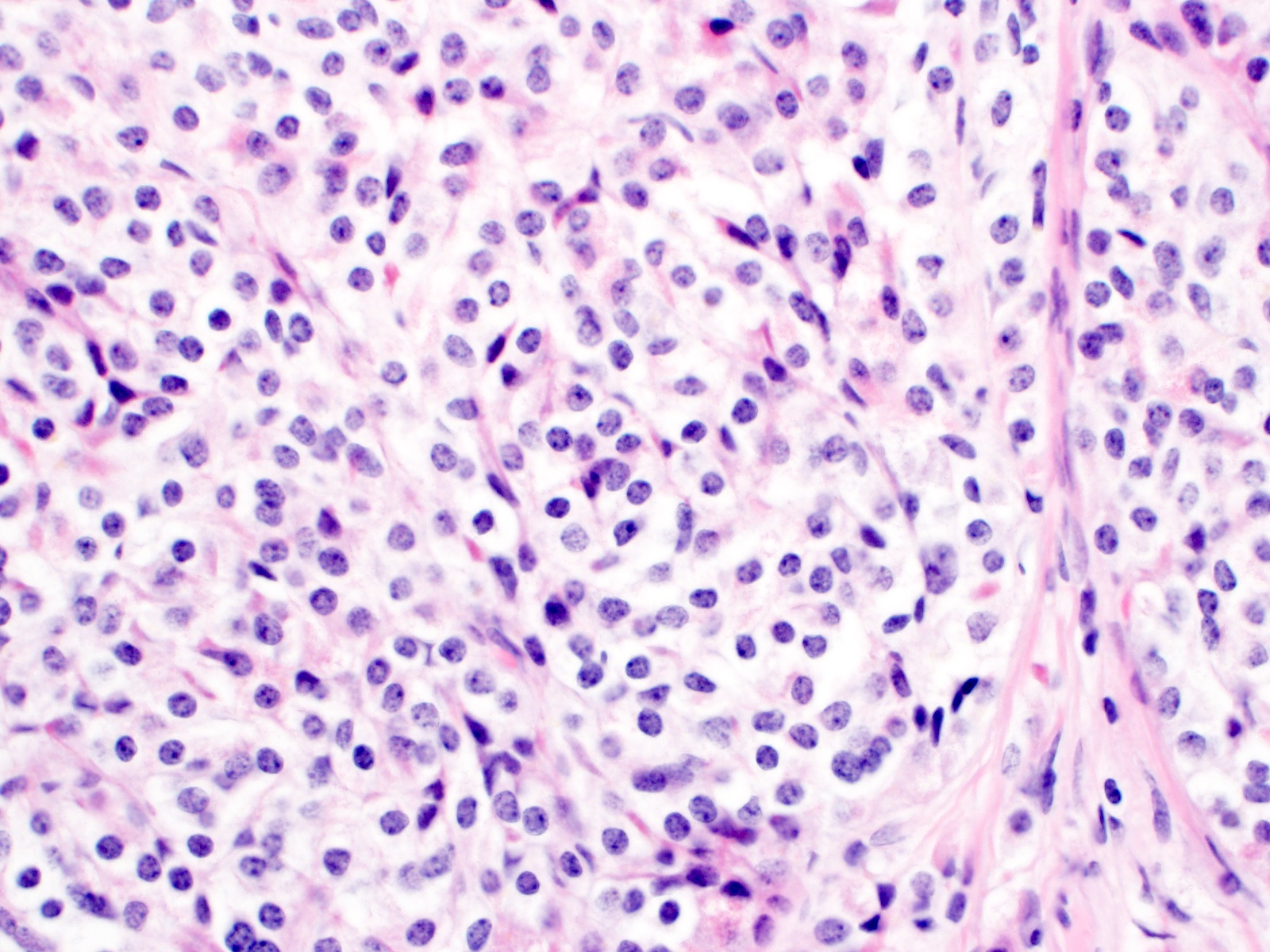
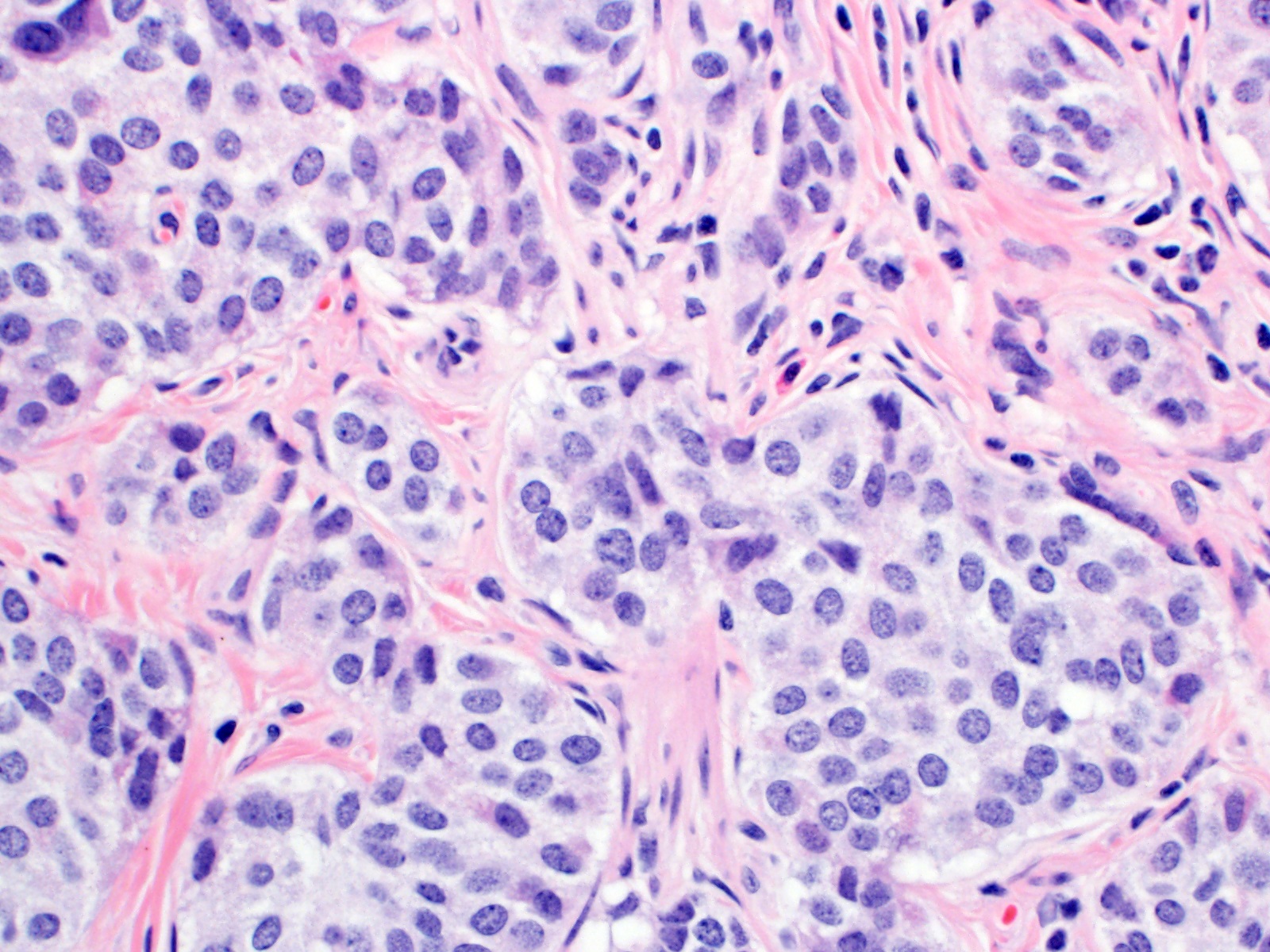
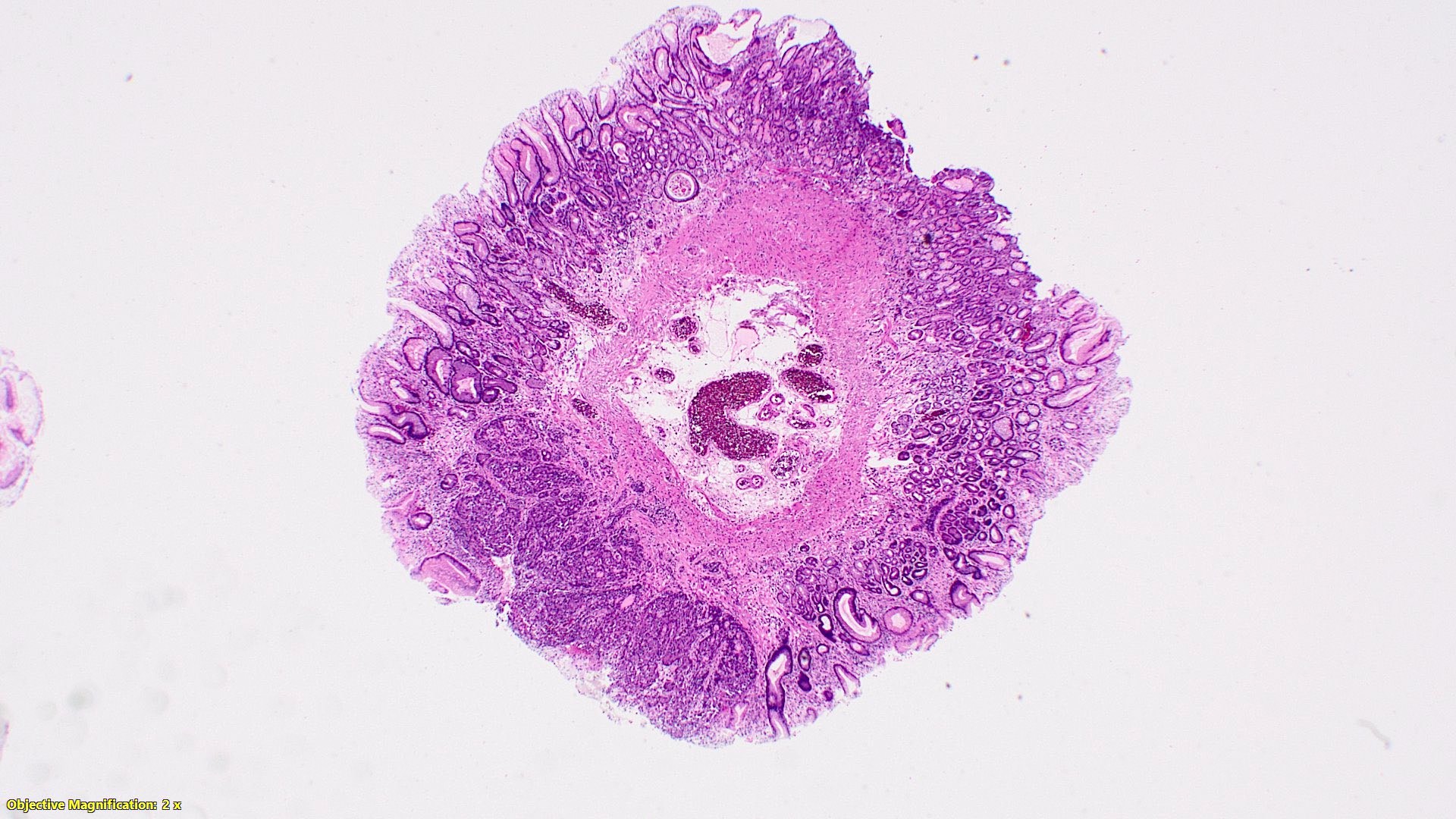
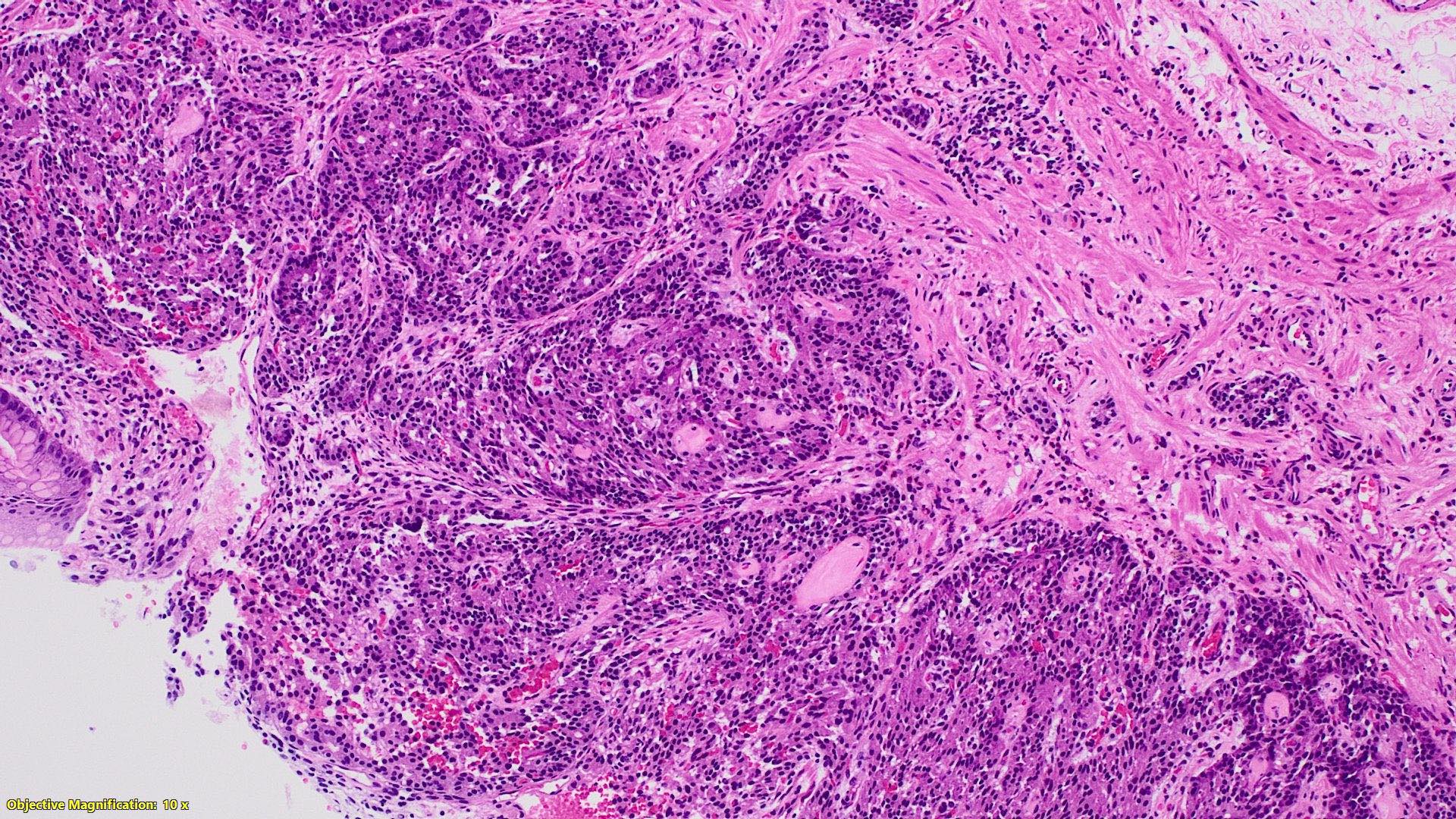
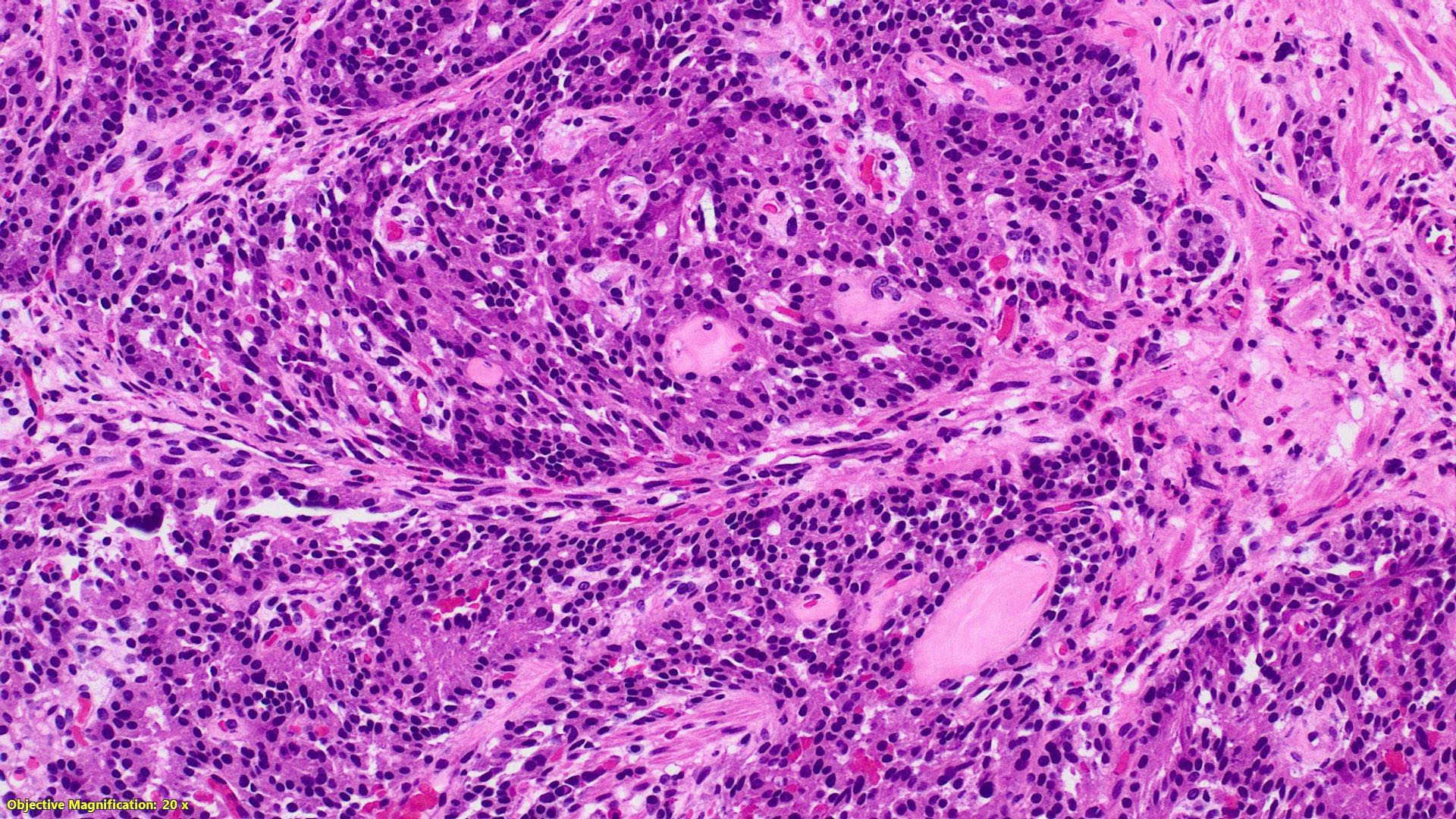
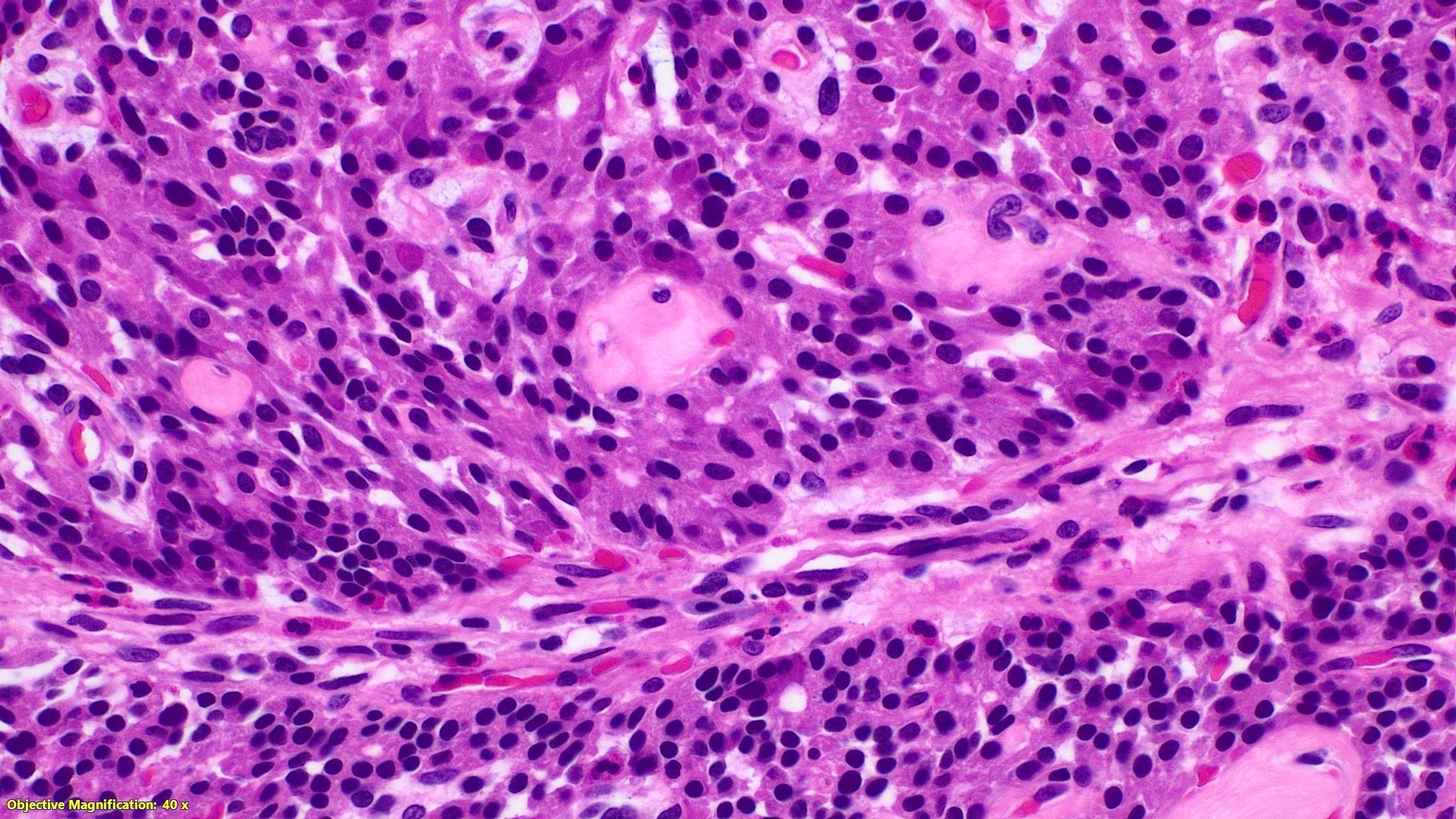
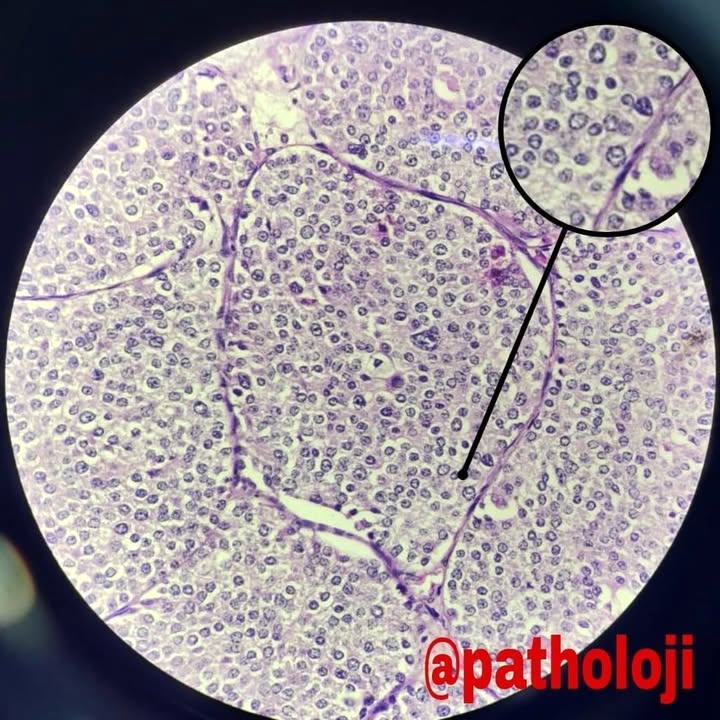
Microscopic (histologic) description
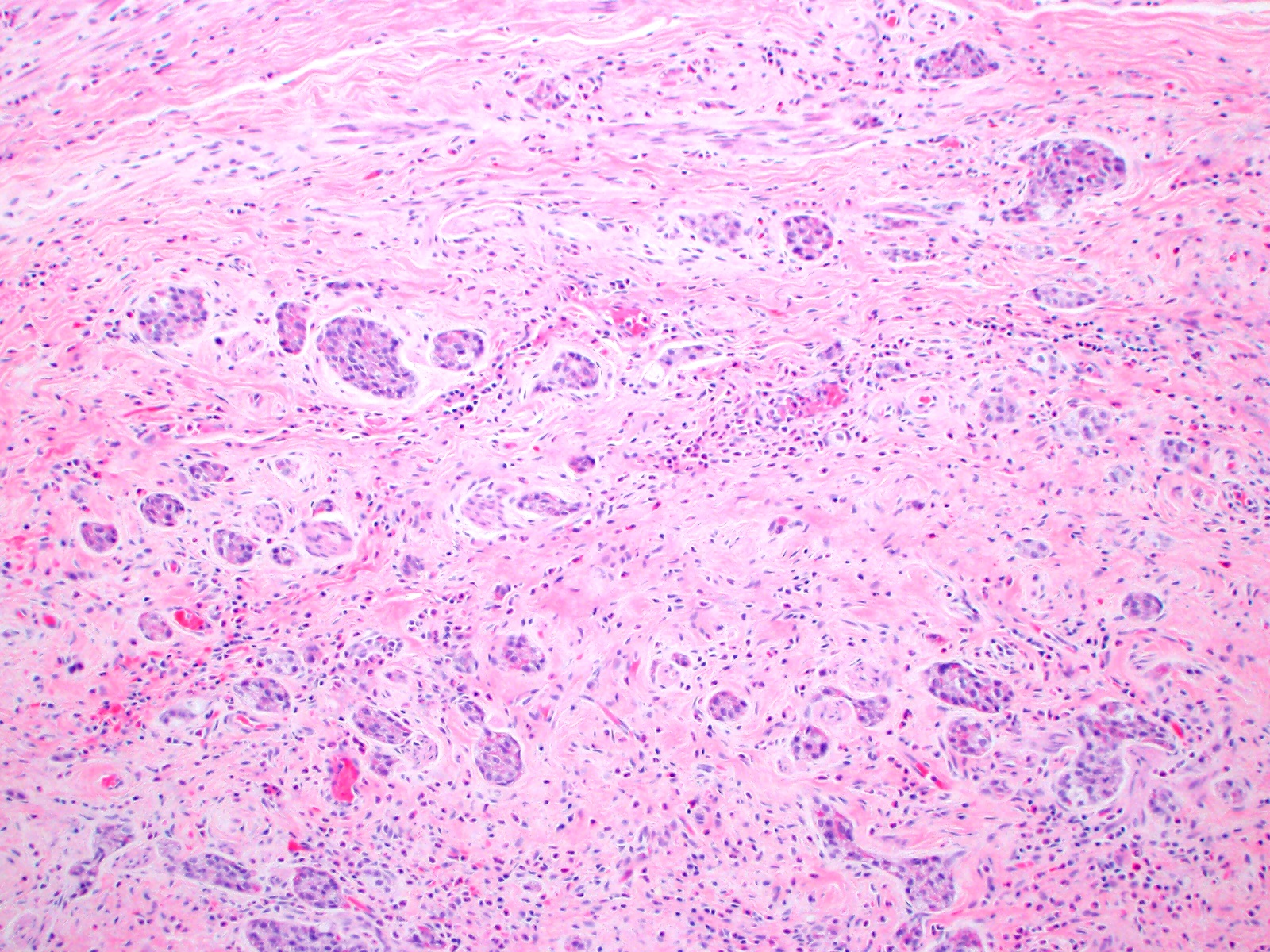
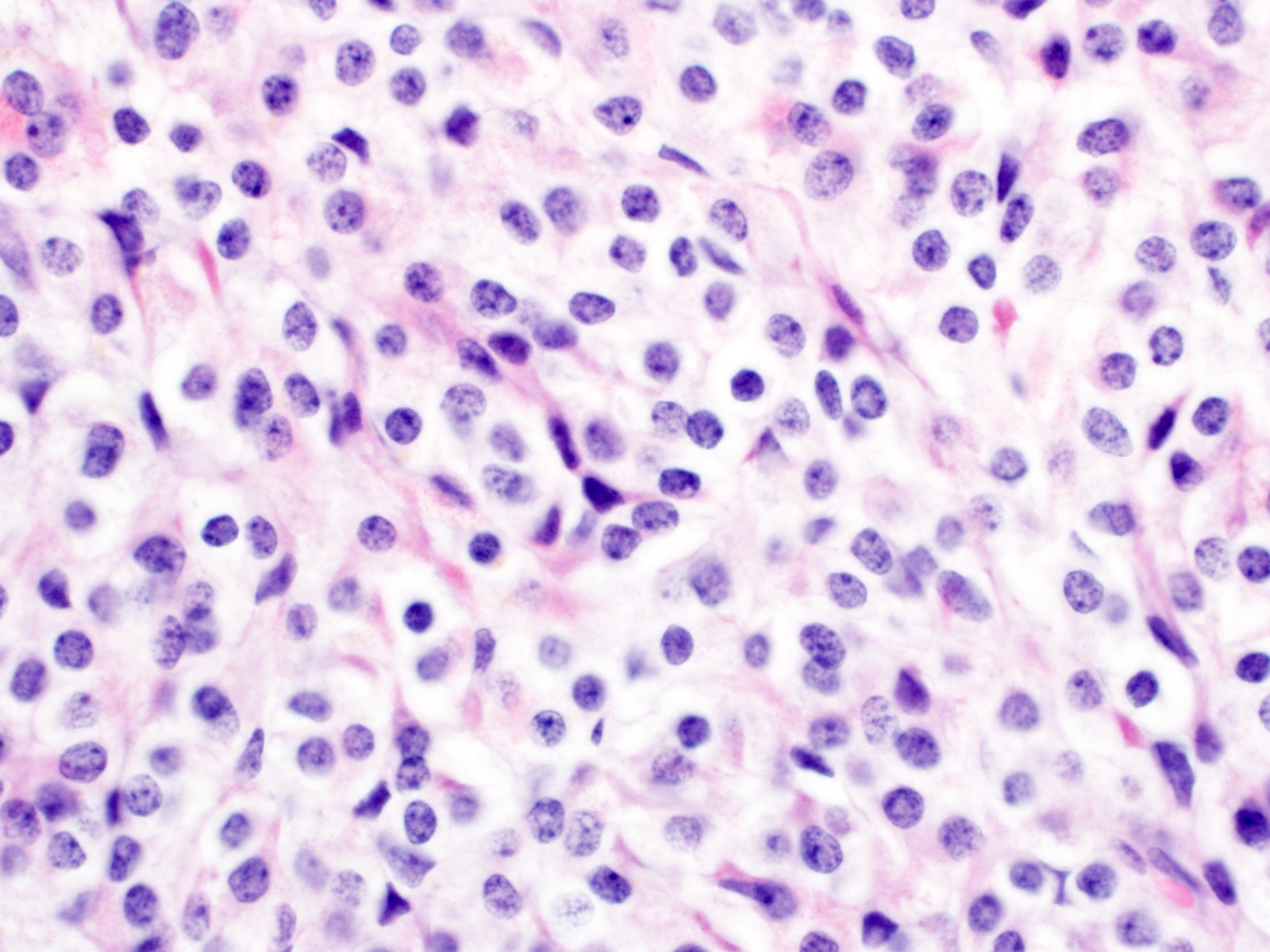
- Monotonous cells with round nuclei and finely stippled chromatin
- Architecture patterns: trabeculae, acini, nests and ribbons
- Grading systems for neuroendocrine tumors of the midgut (jejunum, ileum, appendix and cecum)
- Low grade (G1): < 2 mitoses/2 mm2 and < 3% Ki67 index
- Intermediate grade (G2): 2 - 20 mitoses/2 mm2 or 3 - 20% Ki67 index
- High grade (G3): > 20 mitoses/2 mm2 or > 20% Ki67 index
- G3 NETs are less commonly seen in GI tract compared with pancreas and do not indicate a diagnosis of NEC
- Enterochromaffin cell NETs
- Most common
- Uniform polygonal tumor cells, frequently arranged in large nests or ribbons
- Common histologic features include
- Production of serotonin
- Peripheral palisading and pseudogland formation
- Associated with a fibrotic stromal response
- Mitoses infrequent to absent
- Necrosis uncommon
- Often located in the deep muscular wall and subserosa
- L cell NETs: distinct trabecular or pseudoglandular growth pattern, producing GLP1 and other proglucagon derived peptides
- Tubular NETs (formally called tubular carcinoid)
- Predominantly tubular pattern
- Produce serotonin and substance P
- Usually no desmoplastic response
- Morphologically mimic adenocarcinoma and goblet cell adenocarcinoma but no nuclear atypia
- Stromal retraction artifact must be distinguished from vascular invasion
- Subtyping of NETs is not required and provides no prognostic significance
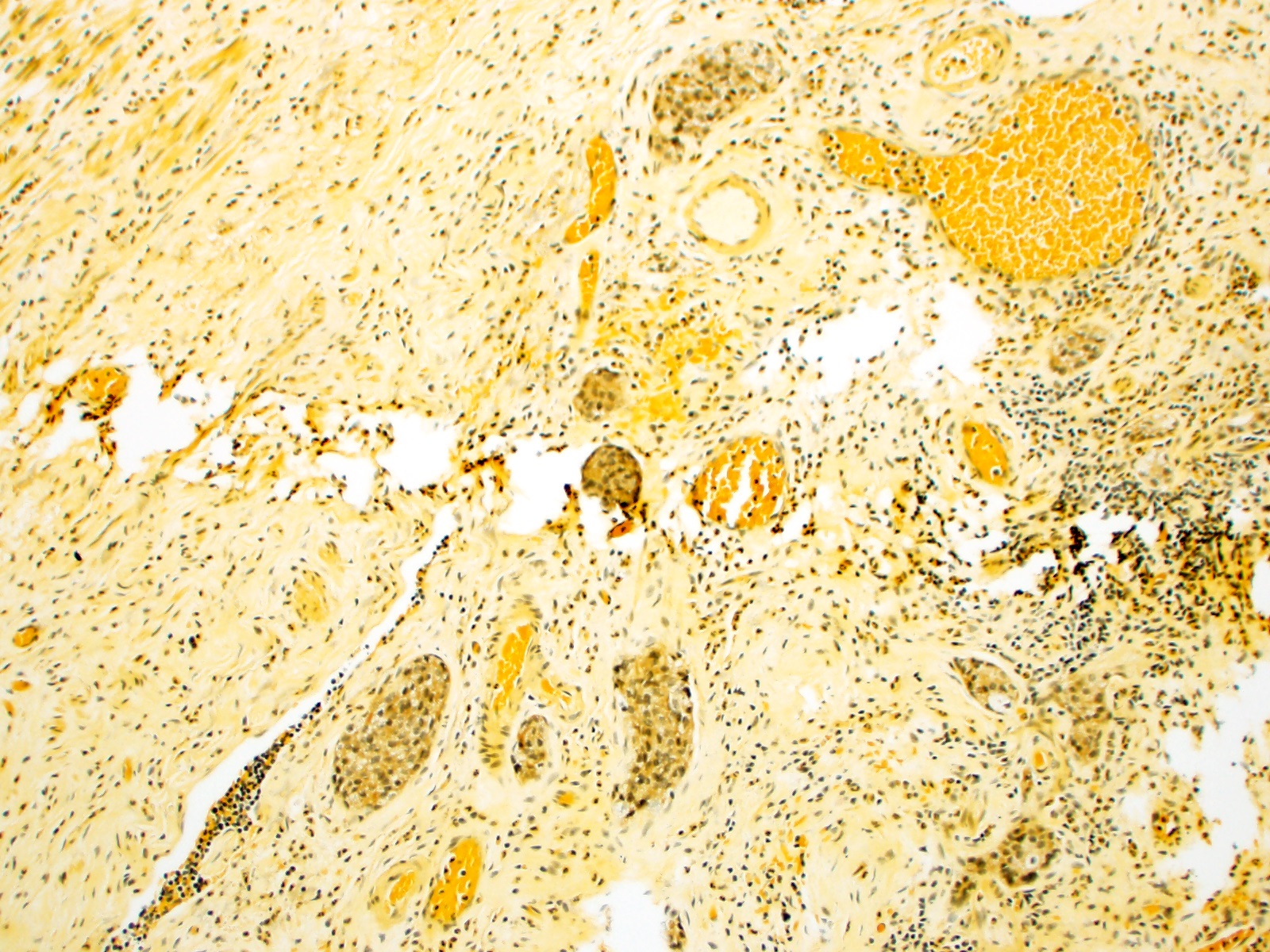
- ~33% of appendiceal NETs infiltrate the mesoappendix, of which 50 - 82% are < 3 mm in thickness
Microscopic (histologic) images
Positive stains
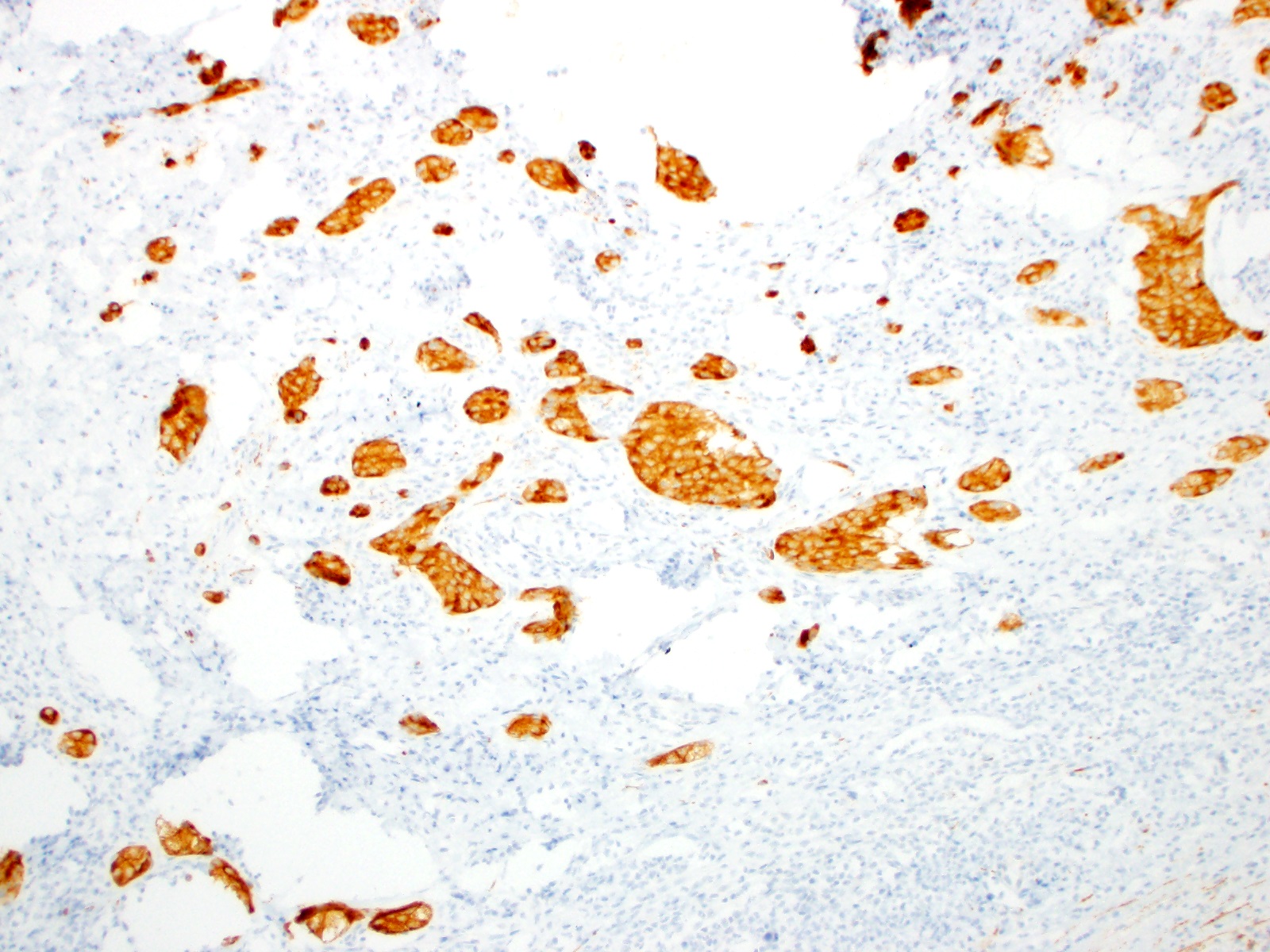
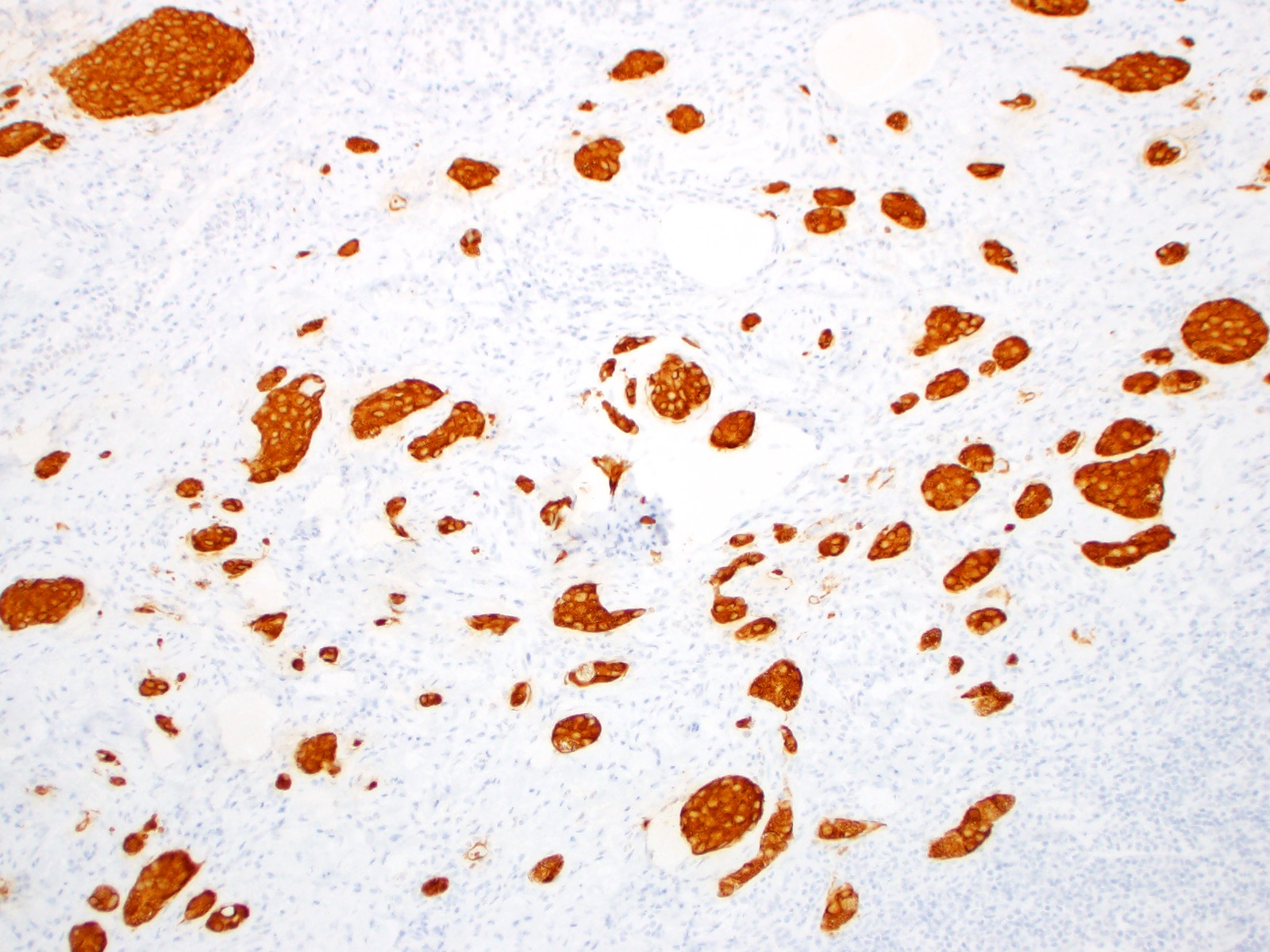
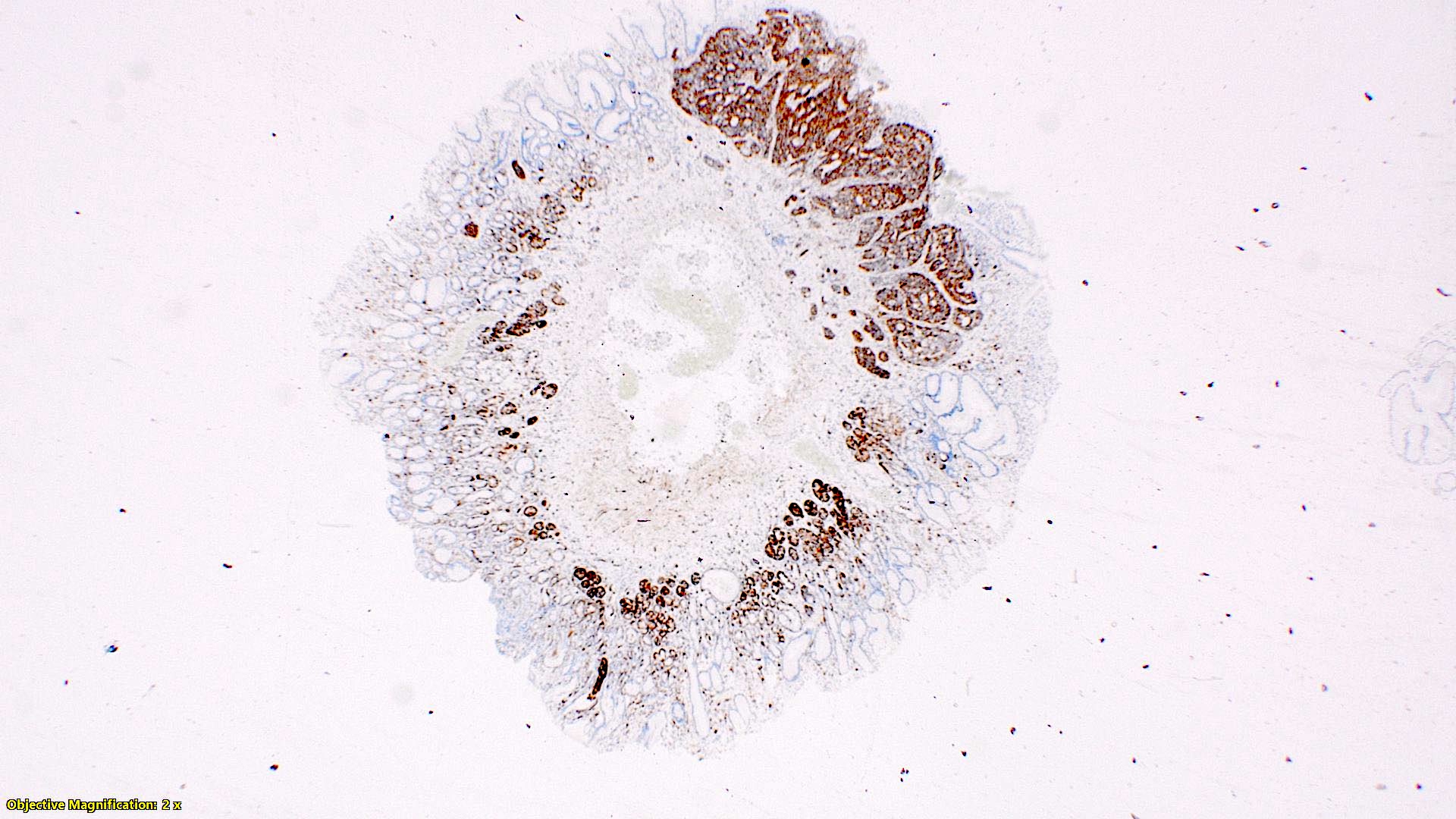
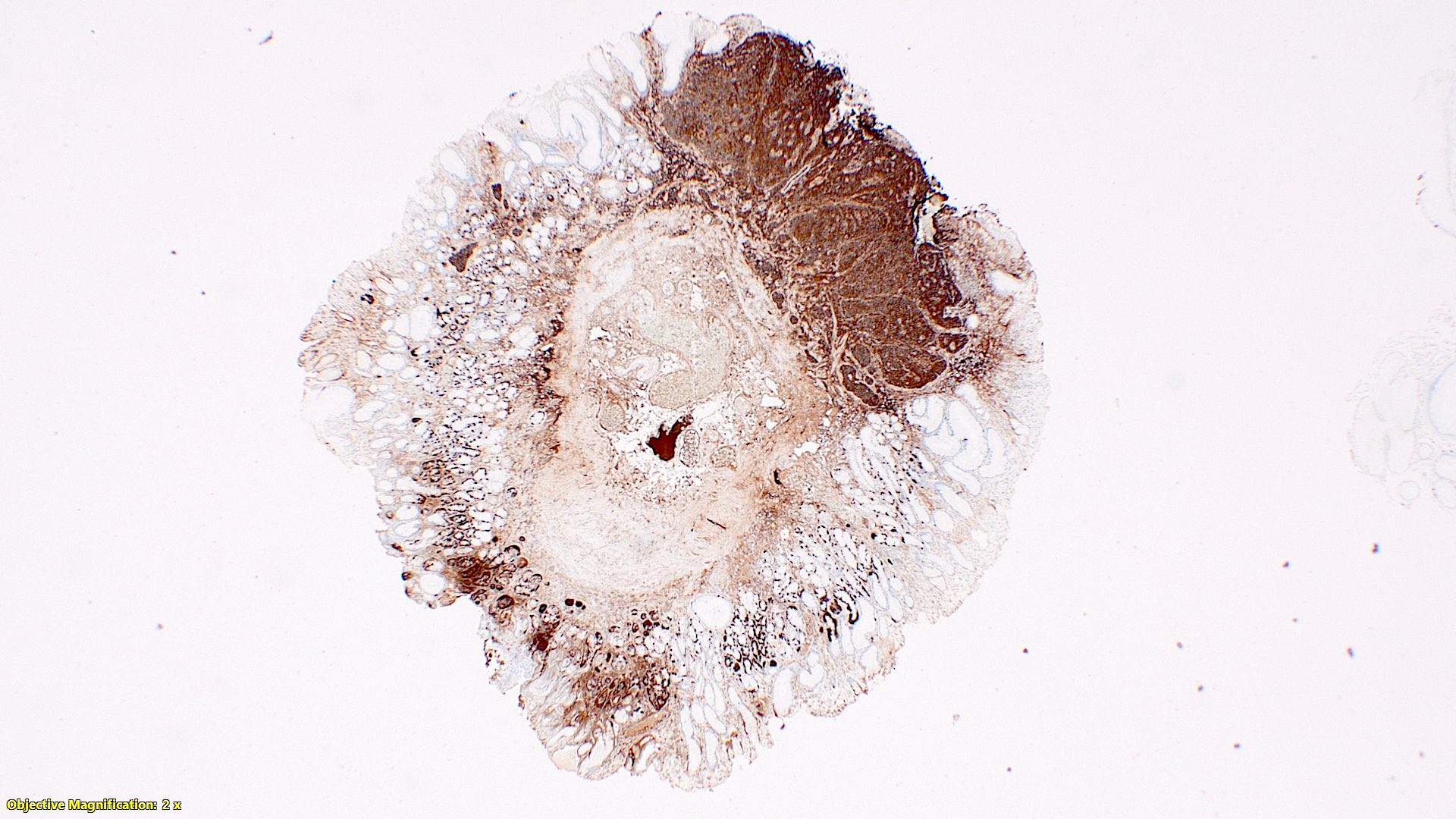
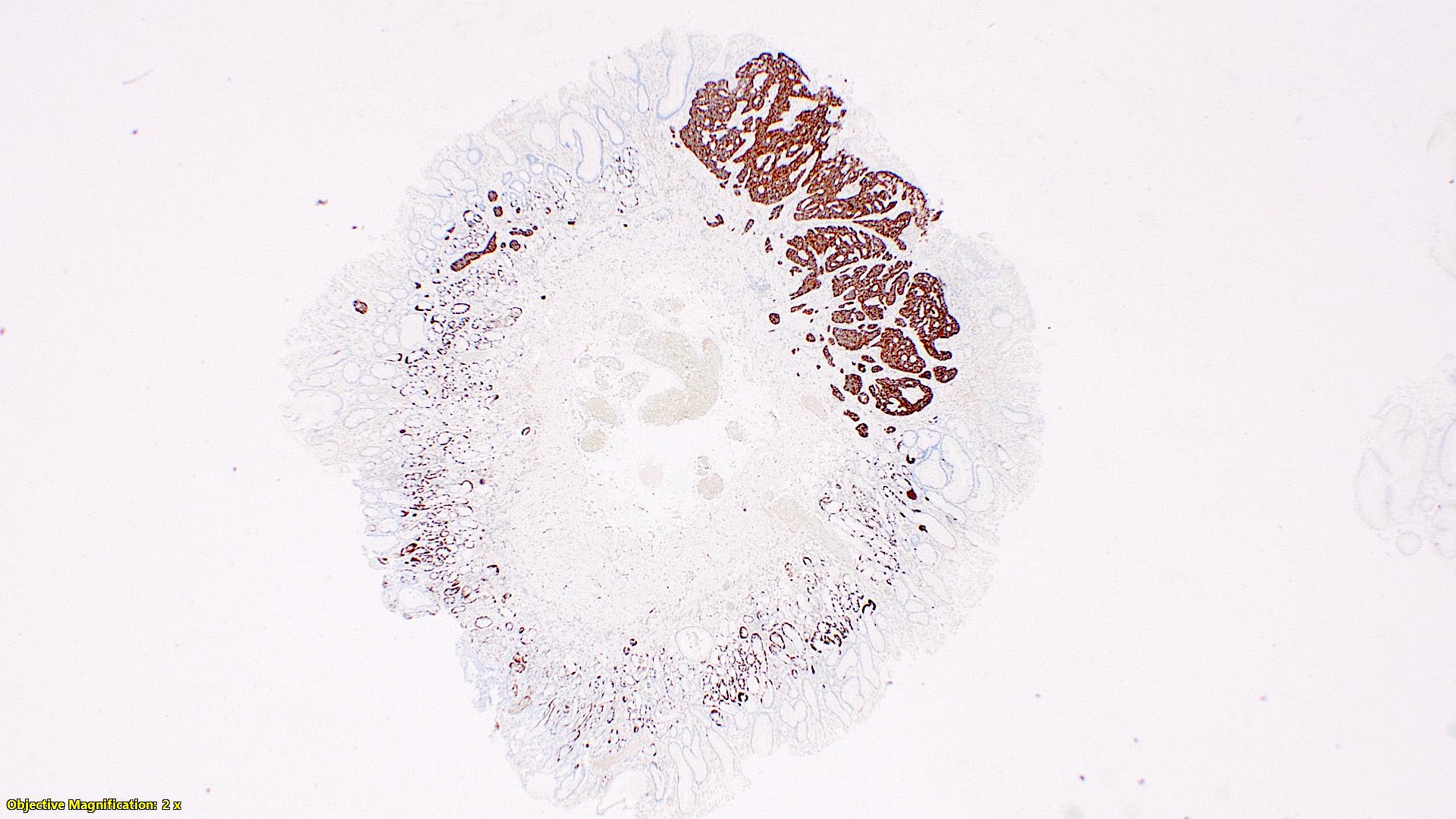
- Enterochromaffin cell NETs: serotonin, chromogranin A, synaptophysin
- L cell NETs: chromogranin B, synaptophysin, GLP1, peptide YY, pancreatic polypeptide, CEA
- Ki67 is essential for grading
Negative stains
- TTF1, CK7 (usually) (Hum Pathol 2001;32:1087)
- Enterochromaffin cell NETs: CEA
- L cell NETs: mucin, chromogranin A (usually)
Molecular / cytogenetics description
- Appear to lack mutational changes in common cancer associated genes
- May have 18q- (rare, compared to ileal NETs)
- May show nonrecurrent large copy number changes in several chromosomes
Videos
Neuroendocrine tumor, appendix - histopathology
Sample pathology report
- Appendix, appendectomy:
- Well differentiated neuroendocrine tumor, WHO grade 1, 0.6 cm, negative margins (see comment)
- Acute appendicitis with periappendicitis
- Comment: The following immunohistochemical stains were performed on block XX:
- Synaptophysin: Diffusely positive
- Chromogranin: Diffusely positive
- Ki67: < 1% proliferation index
- Neuroendocrine tumor synoptic
- Location of tumor: Appendix, tip
- Procedure: Appendectomy
- Tumor size: 0.6 cm in greatest dimension
- Histologic type (WHO 2017 classification update): Well differentiated neuroendocrine tumor (NET)
- Histologic grade (WHO 2017 classification): G1 - low grade
- Mitotic count: < 1 mitotic figure / 2 mm2
- Ki67 index: < 1%
- Tumor focality: Unifocal
- Microscopic tumor extension: Tumor invades through muscularis propria and infiltrates < 1 mm into the subserosa or mesoappendix but does not extend to the serosal surface
- Tumor necrosis: None
- Lymphovascular invasion: Not identified
- Perineural invasion: Not identified
- Margins:
- Proximal margin: Negative (tumor is > 5 cm from margin)
- Mesenteric margin: Negative (tumor is 0.2 cm from margin)
- Other margin(s): Not applicable
- Regional lymph node status: None present
- Additional pathologic findings: Acute appendicitis
- AJCC pathologic stage: pT3NX
- ENETS pathologic stage: pT2NX
Differential diagnosis
- Neuroendocrine carcinoma (NEC):
- Typically with a poorly differentiated morphology (i.e. small cell NEC or large cell NEC)
- Usually with a very high Ki67 labeling index
- May or may not be positive for synaptophysin or chromogranin A
- Goblet cell adenocarcinoma (GCA):
- Typically with circumferential growth pattern of goblet cells with glandular differentiation (reminiscent of normal crypt)
- Mucicarmine positivity in neoplastic cells and mucin pools
- May have additional signet ring cell or poorly differentiated components
Additional references
Board review style question #1
What feature(s) determine(s) the difference between appendiceal neuroendocrine tumor (NET) and neuroendocrine carcinoma?
- Invasive versus noninvasive
- Ki67 labeling index and counts of mitotic figures
- Morphologically well differentiated or poorly differentiated
- Presence of TP53 mutation
Board review style answer #1
C. Morphologically well differentiated or poorly differentiated
Comment Here
Reference: Well differentiated neuroendocrine tumor
Comment Here
Reference: Well differentiated neuroendocrine tumor
Board review style question #2
Board review style answer #2