Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Sardana R, Parwani A. Basal cell hyperplasia. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/prostatebch.html. Accessed April 1st, 2025.
Definition / general
- Basal cell hyperplasia (BCH) is a benign histologic finding characterized by the proliferation of the basal cells within the prostatic acini (Urology 2019;129:160)
- Benign mimicker of adenocarcinoma and basal cell carcinoma of the prostate gland
- Divided into usual BCH (most common), florid BCH, BCH with prominent nucleoli / atypical hyperplasia, atrophy associated and adenoid cyst / cystic-like hyperplasia (Review Arch Pathol Lab Med 2012;136:721, Urology 2019;129:160)
- Most commonly seen in the transition zone occupying 10 - 60% volume and often associated with nodular, glandular and stromal hyperplasia (Prostate 2017;77:1344)
- Peripheral zone BCH is associated with acinar atrophy and chronic inflammation
Essential features
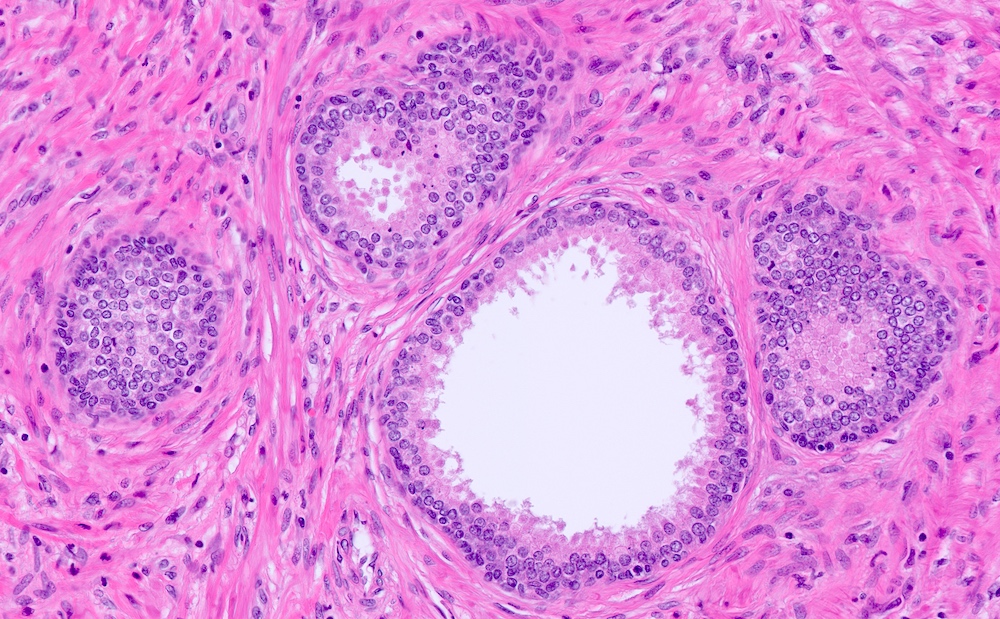
- Solid nests, small crowded acini or pseudocribriform glands; multilayering of round to polygonal uniform cells with scant eosinophilic to clear cytoplasm and conspicuous nucleoli; can show coarse calcifications or eosinophilic cytoplasmic globules (J Clin Pathol 2005;58:290)
- HMWCK / 34 beta E12, p63, GATA3 and CK8 / 18 are positive markers
- AMACR and prostatic markers are typically negative
- No malignant features, including perineural invasion or infiltration into the periprostatic adipose tissue or seminal vesicle(s), are seen
Terminology
- Fetalization or embryonal hyperplasia of the prostate gland as the foci of basal cell hyperplasia has its resemblance with fetal prostatic acini
- Embryonal hyperplasia and atypical basal cell hyperplasia are terms that are no longer used
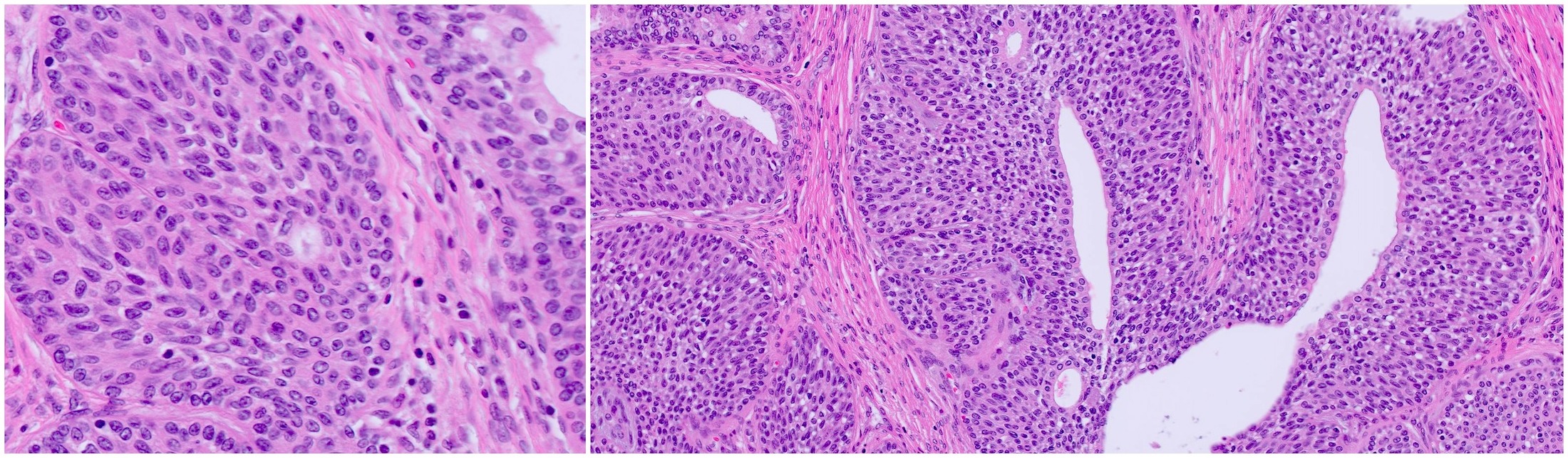
- Florid basal cell hyperplasia refers to extensive proliferation of basal cells involving > 100 small crowded acini (per section) forming a nodule (Hum Pathol 2005;36:480)
Epidemiology
- Incidence ranges from 8 - 10% of needle biopsies to almost 80% in whole prostate mounts (Review Arch Pathol Lab Med 2012;136:721, Mod Pathol 2003;16:598, Urology 2019;129:160)
- More common in transurethral resection (TURP) specimens (due to sampling from the transition zone); can also be seen in needle biopsies and radical prostatectomies
- Prevalence of BCH in biopsies varies according to the sampled area (transitional versus peripheral zone) and gland characteristics such as associated benign prostatic hyperplasia (Urology 2019;129:160)
- No association with ethnicity, BMI, PSA or prostate volume
- 2% of needle core biopsies may show BCH with prominent nucleoli (> 10% of nuclei)
Sites
- More common in the transition zone of the prostate gland; can be present in the peripheral zone
- Although more commonly seen in the TURP specimens, can also be seen in needle biopsies and radical prostatectomies
Pathophysiology
- Hypothesized to be either a primary response to the luminal epithelial apoptosis or a secondary response to the inflammation
- Role of BCL2 antiapoptotic protein and hormones (androgen and estrogen) is attributed (Mod Pathol 2003;16:598)
- Increase in proliferation index with a diminished apoptotic index
- Gene expression study suggests metaplastic BPH nature with conversion of the single layer of basal epithelial to a stratified squamous epithelium (Prostate 2017;77:1344)
- Diffuse BCH along with squamous and urothelial metaplasia are observed in benign prostate glands following androgen deprivation therapy
Etiology
- Increases in response to androgen deprivation and 5 alpha reductase inhibitor therapy (Am J Surg Pathol 2002;26:237, Prostate 2014;74:923, J Urol 1996;156:1194)
- Atrophy associated basal cell hyperplasia is related to post hormonal (androgen deprivation) therapy, radiation or cryosurgery
Clinical features
- Seen in adults (Am J Clin Pathol 1983;80:850)
- Usually asymptomatic, unless coexisting BPH or prostatic cancer or any other pathology present
- Symptoms of urinary retention seen in florid BCH (BMJ 1999;318:921)
Diagnosis
- Diagnosis is made on microscopic findings aided by immunohistochemical stains (HMWCK and p63)
- Immunohistochemistry is very valuable in differentiating BCH from high grade prostatic intraepithelial neoplasia, prostatic adenocarcinoma and basal cell carcinoma
Laboratory
- Serum PSA usually within normal limits
- Decrease in PSA may be seen in a post androgen deprivation setting due to ongoing antiandrogen therapy / androgen ablation
Radiology description
- Sometimes calcification may be visible, though very rare
Prognostic factors
- Not associated with adverse prognosis or high risk of prostatic cancer
Case reports
- 50 year old man presented with chronic retention of urine and bilateral hydronephrosis (JCR 2016;6:153)
- 72 year old man with acute urinary retention (Indian J Urol 2000;17:61)
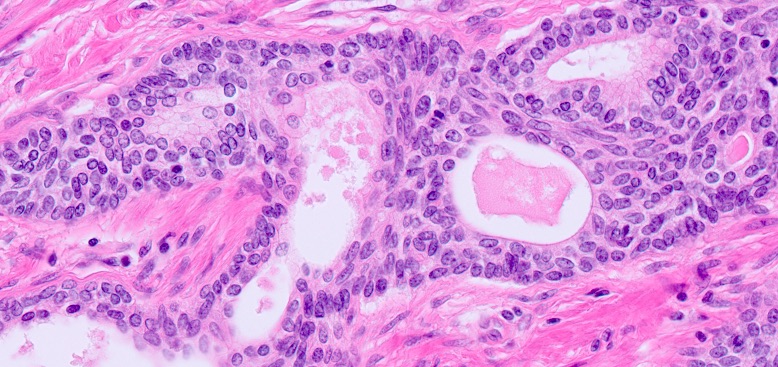
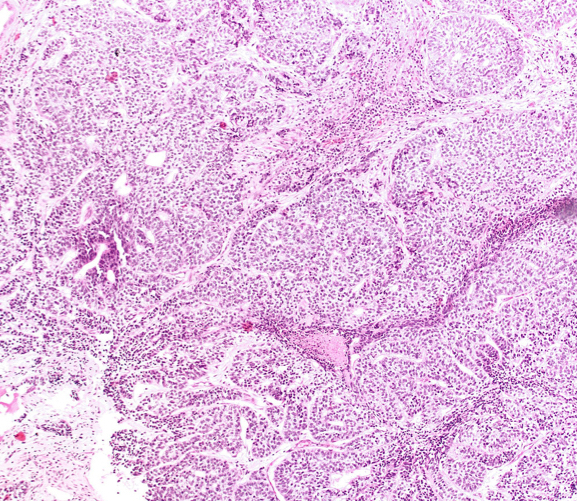
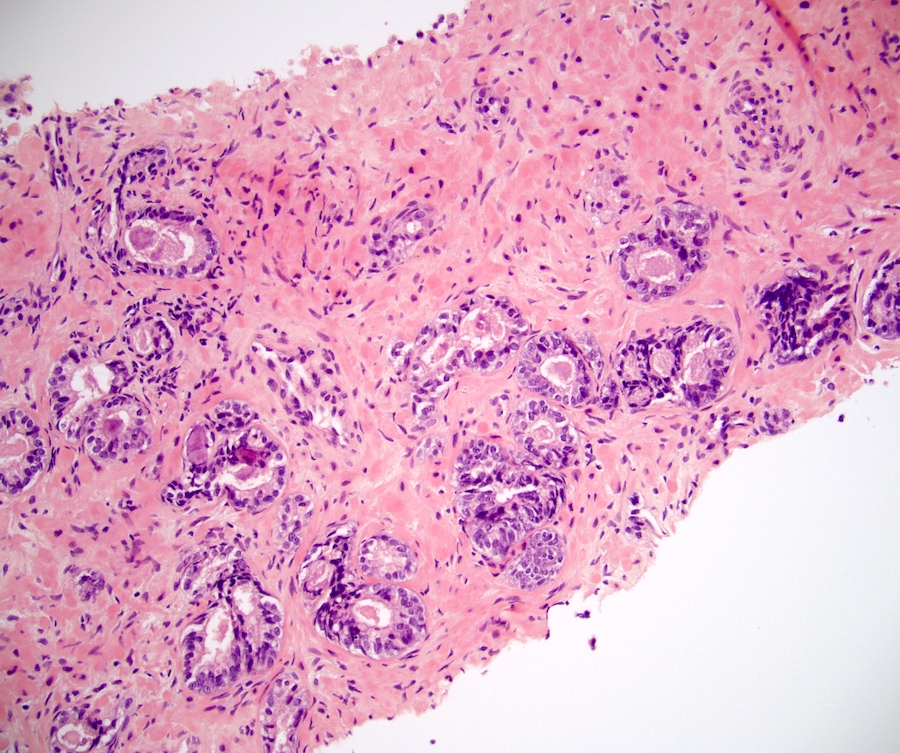
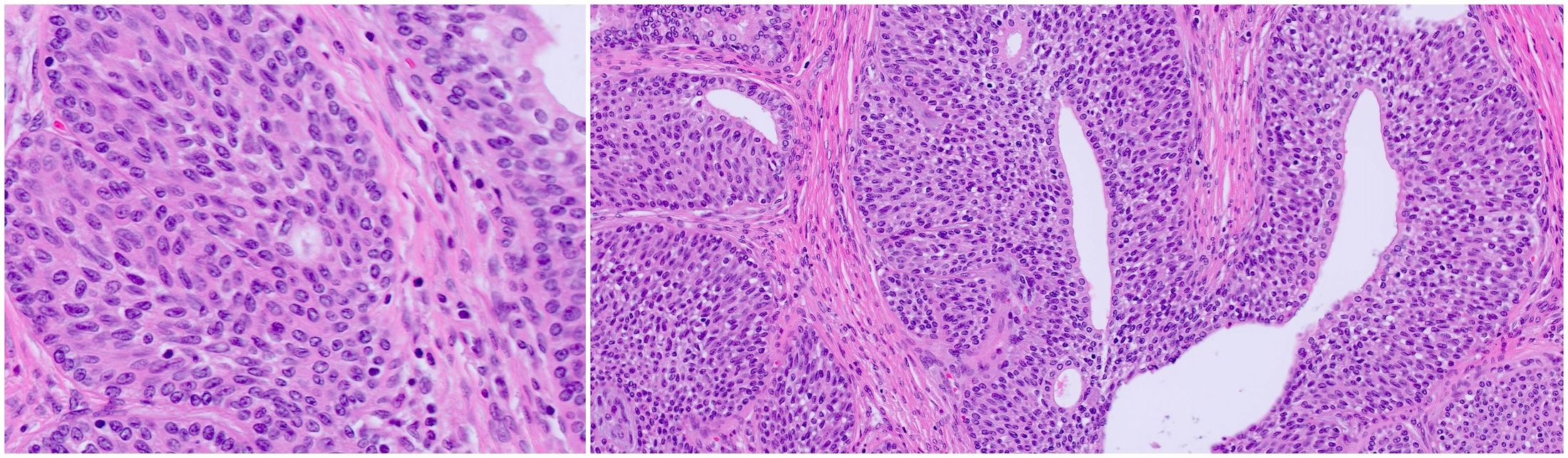
Microscopic (histologic) description
- At low magnification, basal cell hyperplasia has a basophilic appearance of nodular proliferation and back to back uniform glands with stratified crowded nuclei
- Solid nests of benign appearing round to elongated to polygonal acinar epithelial cells, with similar cells having clear to eosinophilic scant cytoplasm
- Piling up of the nuclei within the lumen, ranging from a double cell layer in a few glands to 3 - 4 cells thick in the other glands
- Basal cells show zonal variations: triangular in the peripheral zone, while flattened or low cuboidal with scant cytoplasm in the transition zone (Prostate 2007;67:1686)
- Pseudocribriform gland pattern with back to back small round glands of BCH rather than a solid nest of cells with punched out lumina that characterize true cribriform glands
- Surrounding stroma either unremarkable or very cellular with abundant spindly fibroblasts or smooth muscle cells; occasionally minimally myxoid stroma; typically lacks desmoplasia
- Lobular configuration is not always obvious, as an entire nodule cannot physically be sampled in a single core; in some instances of florid BCH, the proliferation still retains a lobular configuration, while in other instances, the lobular configuration may either be lost or not appreciated because of the fragmented nature of the transurethral resection specimen
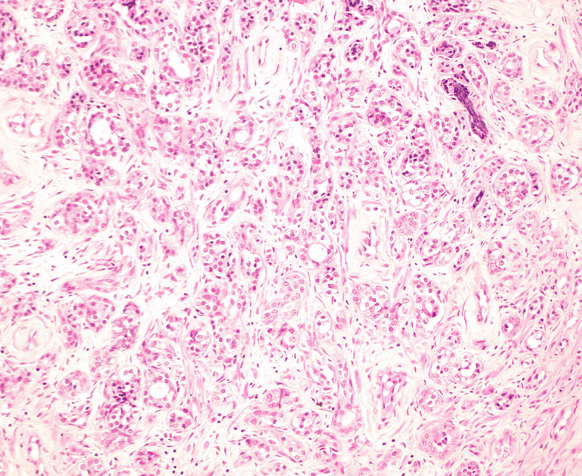
- Intracytoplasmic eosinophilic (hyaline) inclusions (53%)
- Well formed lamellar calcifications (40%)
- Psammomatous calcifications differing from the fine stippled calcifications seen in areas of comedo necrosis within high grade prostatic carcinoma (Am J Surg Pathol 2002;26:237)
- Rare atypical features include perineural involvement, prominent nucleoli, dense intraluminal secretions, luminal blue mucin, intracytoplasmic hyaline globules, crystalloid and individual cell necrosis
- Florid BCH: extensive proliferation of basal cells involving > 100 small crowded acini (per section) forming a nodule (Hum Pathol 2005;36:480)
- BCH with prominent nucleoli: nuclear enlargement, hyperchromasia and nucleolar prominence (earlier known as atypical hyperplasia)
- Adenoid cyst-like hyperplasia: focal glandular anastomosis and cribriform structures with small luminal spaces
- Adenoid basal form of BCH: areas of luminal differentiation similar to the lesions of the salivary gland
Microscopic (histologic) images
Positive stains
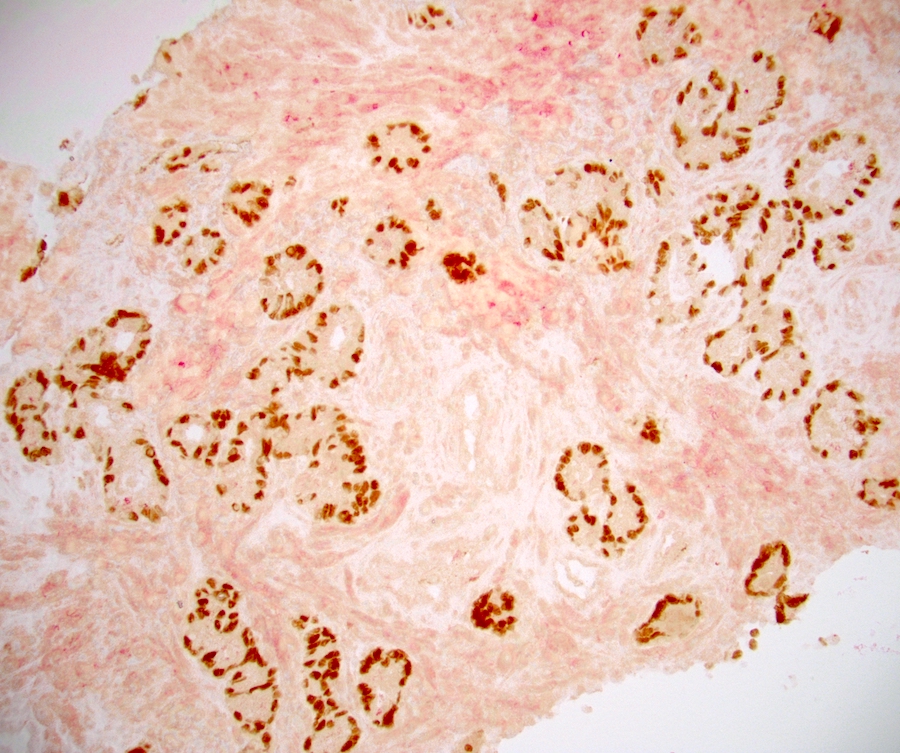
- HMWCK / 34 beta E12, p63, GATA3, AR and CK8 / 18
- HMWCK shows multilayered staining of the basal cells in some of the glands and a continuous layer of immunoreactivity
- Basal cells show zonal variations; in the peripheral zone they express HMWCK and p63, whereas in the transition zone they express p63 and variable HMWCK (weak or absent) (Prostate 2007;67:1686, Prostate 2017;77:1344, Hum Pathol 2003;34:462)
Negative stains
- AMACR / P504S (may be focally positive), CD10, AR, NKX3.1, P501s (prostein), PSA, PSMA
Electron microscopy description
- Basal cells oriented parallel or perpendicular to the basement membrane
- 2 types of intracytoplasmic inclusions (Hum Pathol 2003;34:462)
- Type 1: fine, uniform, granular, surrounded by a halo
- Type 2: irregular shape, amorphous content of variable electron density, close to the nucleus
Molecular / cytogenetics description
- BCH glands characterized by K14- / p63+ layers on dual immunostaining and loss of luminal cell AR (Prostate 2017;77:1344)
- RNA sequence analysis confirmed by qPCR shows upregulation of keratinocyte differentiation genes, assuming BCH to be a metaplastic process (Prostate 2017;77:1344)
Sample pathology report
- Prostate, transurethral resection:
- Benign prostatic hyperplasia with florid basal cell hyperplasia
- No evidence of malignancy is seen
Differential diagnosis
- High grade prostatic intraepithelial neoplasia (HGPIN):
- Architecturally benign prostatic acini or ducts lined by cytologically atypical luminal cells that are columnar and pseudostratified perpendicular to the basement membrane
- Basal cells are not readily identifiable (no distinct 2 cell population)
- Prostate adenocarcinoma:
- Unilayered small acini, single cells or true cribriform structures with more abundant amphophilic cytoplasm, enlarged prominent nucleoli, prominent nucleoli and luminal blue mucin or eosinophilic crystalloids
- Negative for HMWCK, p63, GATA3, S100
- Positive for AMACR, P501s (prostein), PSMA, NKX3.1 and PSA
- Basal cell carcinoma:
Additional references
Board review style question #1
Board review style answer #1
C. Most commonly seen in the transition zone of prostate. The picture shows basal cell hyperplasia of prostate, which is commonly seen in the transition zone of prostate as nodular, glandular or stromal hyperplasia. It is frequently encountered during TURP due to its preference to transition zone. Although rare, BCH can also be seen in the peripheral zone.
Comment Here
Reference: Basal cell hyperplasia
Comment Here
Reference: Basal cell hyperplasia
Board review style question #2
Basal cell hyperplasia of the prostate, a benign mimicker, can be diagnosed by which set of findings?
- Anastomosing basaloid nests and tubules centrally lined by eosinophilic cells with strong BCL2 reactivity
- Architecturally benign prostatic acini or ducts lined by luminal cells that are columnar and pseudostratified perpendicular to the basement membrane
- Solid nests of epithelial cells with piling up of the nuclei within the lumen and well formed lamellar calcifications, GATA3+ and AMACR-
- Unilayered small acini, single cells with enlarged prominent nucleoli and nuclei, showing HMWCK negativity
Board review style answer #2
C. Solid nests of epithelial cells with piling up of the nuclei within the lumen and well formed lamellar calcifications, GATA3+ and AMACR-. BCH is the benign proliferation of basal cells within the prostatic acini. Histologically, solid nests of benign appearing round to polygonal acinar epithelial cells and back to back glands with similar cells having clear to eosinophilic cytoplasm (usually atrophic cytoplasm) are seen. There is piling up of the nuclei within the lumen ranging from a double cell layer in a few glands, to 3 - 4 cells thick in the other glands.
Comment Here
Reference: Basal cell hyperplasia
Comment Here
Reference: Basal cell hyperplasia