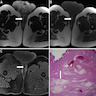
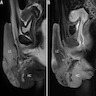
- pTX: primary tumor cannot be assessed
- pT0: no evidence of primary tumor
- pT1 (I): tumor confined to the vagina
- pT1a (I): tumor confined to the vagina, measuring ≤ 2 cm
- pT1b (I): tumor confined to the vagina, measuring > 2 cm
- pT2 (II): tumor invades paravaginal tissues but not to pelvic wall
- pT2a (II): tumor invading paravaginal tissues but not to pelvic side wall, measuring ≤ 2 cm
- pT2b (II): tumor invading paravaginal tissues but not to pelvic side wall, measuring > 2 cm
- pT3 (III): tumor extends to pelvic wall (muscle, fascia, neurovascular structures or skeletal portions of bony pelvis; on rectal examination, there is no cancer free space between the tumor and pelvic wall) or causing hydronephrosis or nonfunctioning kidney
- pT4 (IVA): tumor invades mucosa of bladder or rectum or extends beyond the true pelvis (bullous edema is not sufficient evidence to classify a tumor as pT4)
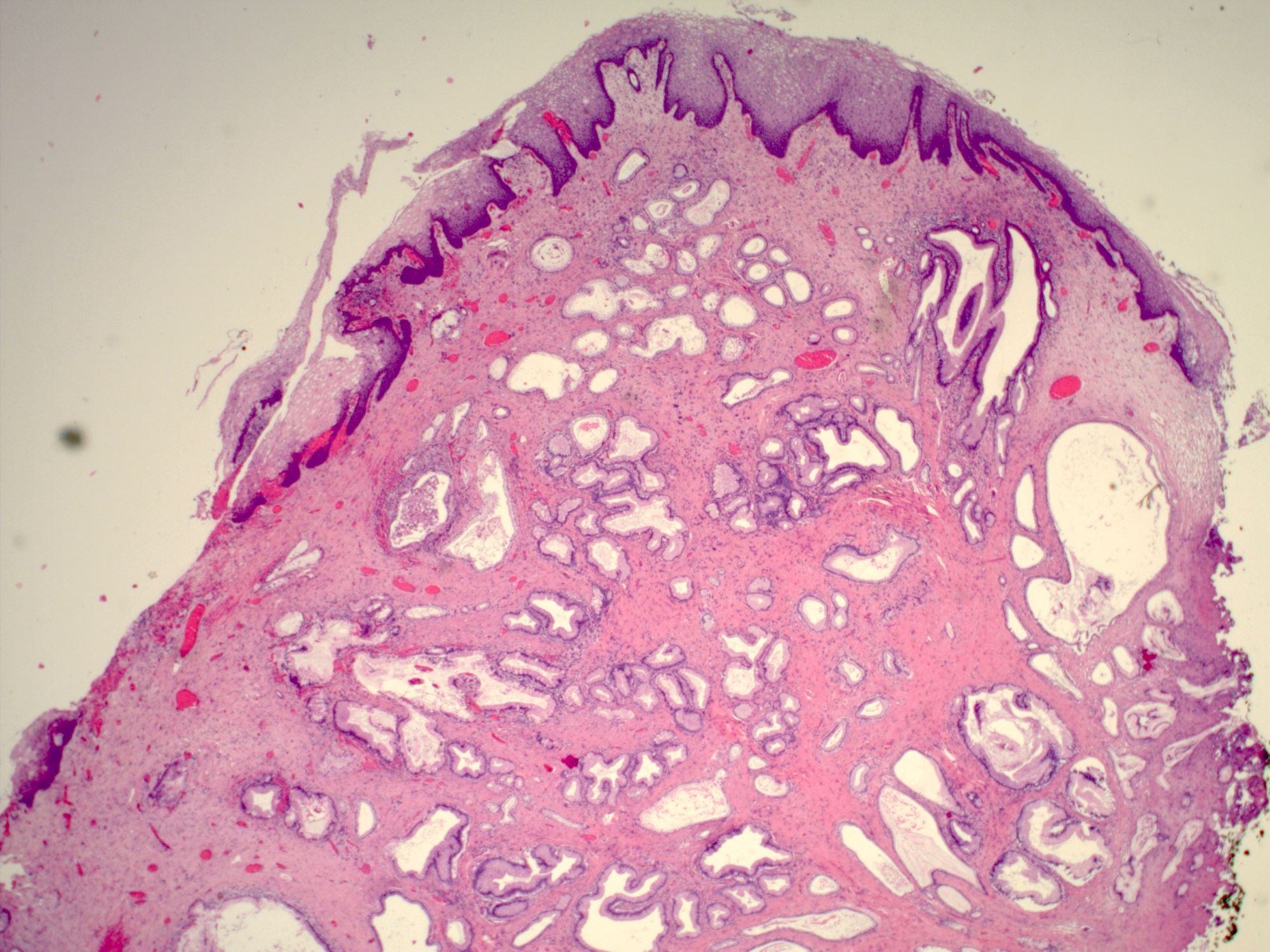
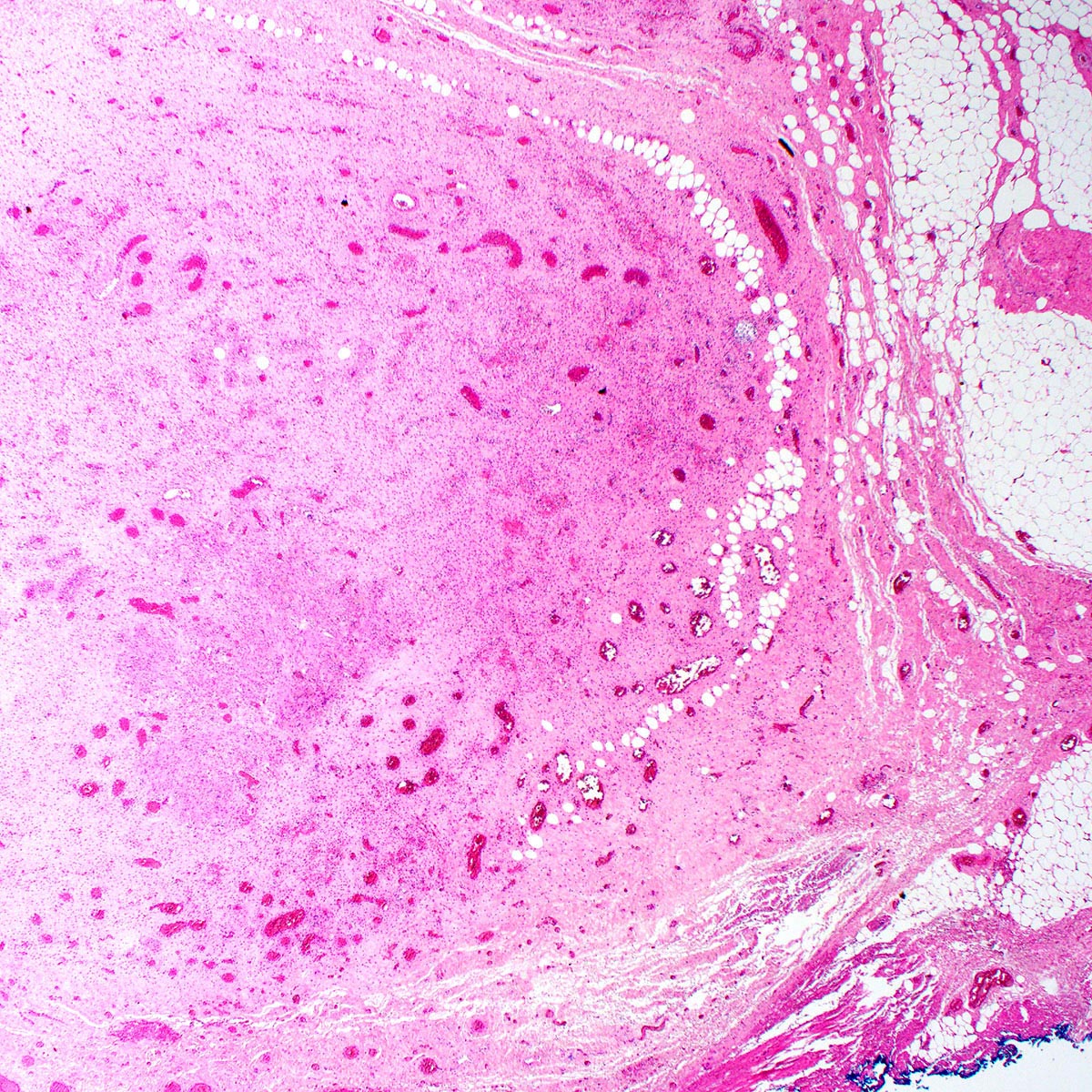
- First described in 1877 by von Preuschen, vaginal adenosis refers to the persistence of Müllerian glandular epithelium in the vagina after birth
- Most noted for its association with diethylstilbestrol (DES) use during pregnancy by mothers of affected young women during the mid 1940s and 1950s; DES use was to prevent a threatened abortion (Reprod Toxicol 2011;31:151)
- Present in 35 - 90% after in utero exposure to diethylstilbestrol (100% have adenosis if diethylstilbestrol started before gestational week 8, 6% if started week 15 or later) (Goldblum: Rosai and Ackerman's Surgical Pathology, 11th Edition, 2017)
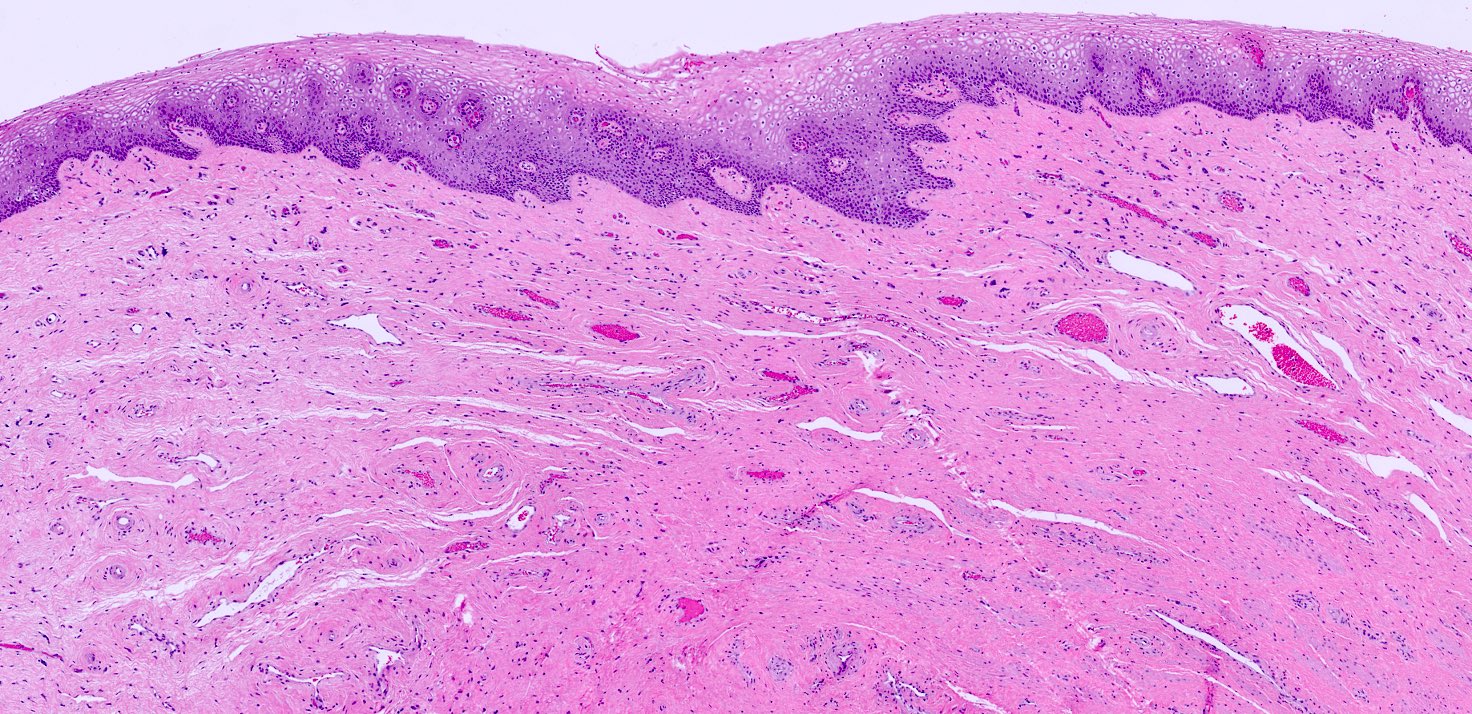
- Dethylstilbestrol adenosis is similar histologically to non-diethylstilbestrol adenosis
- Most common type is mucinous, with endocervical-like glands present; tuboendometrial glands can also be present (Mutter: Pathology of the Female Reproductive Tract, 3rd Edition, 2014)
- Women are at risk of neoplasia, including clear cell adenocarcinoma as well as squamous dysplasia (Am Fam Physician 2004;69:2395)
- An estimated 5 to 10 million women in the United States received diethylstilbestrol during pregnancy (IARC Monogr Eval Carcinog Risks Hum 2012;100:1)
- Approximately 20 - 33% of women who were exposed in utero to diethylstilbestrol demonstrate gross structural changes in the cervix or vagina including vaginal adenosis (Obstet Gynecol Annu 1982;11:187, N Engl J Med 1975;292:334)
- Vaginal adenosis has also been reported in 2 - 10% of nonexposed females (Eur J Gynaecol Oncol 2001;22:260)
- Involves the upper third of the vagina (34% of diethylstilbestrol exposed cases), with the anterior wall more frequently involved than the posterior wall
- Middle third (9% of cases) and lower third (2% of cases) of the vagina may be involved
- Diethylstilbestrol causes irregularities in p63 gene expression, which determines whether Müllerian duct epithelium becomes uterine or vaginal cells and leads to vaginal adenosis
- Mouse model of diethylstilbestrol exposure demonstrates vaginal adenosis and structural changes similar to actual changes observed in women (Hum Pathol 1982;13:190)
- Pathophysiology of non-diethylstilbestrol related adenosis has not been determined conclusively; however, it may result from p63 gene expression changes in squamous epithelium or Müllerian remnants upon epithelial damage or from implantation of Müllerian derived columnar cells into vaginal lesions during menstruation
- Glandular proliferation in adenosis is regulated by estrogen (Rev Obstet Gynecol 2011;4:81)
- Diethylstilbestrol is a synthetic nonsteroidal estrogen that profoundly affects the development of the vagina, uterus and fallopian tubes
- Today, in the post-diethylstilbestrol era, vaginal adenosis usually occurs in women exposed to other hormones, drugs, trauma, inflammation, vulvovaginal involvement by Stevens-Johnson syndrome or toxic epidermal necrolysis (SJS / TEN), laser, radiation or chemotherapy (5-fluorouracil) (Rev Obstet Gynecol 2011;4:81)
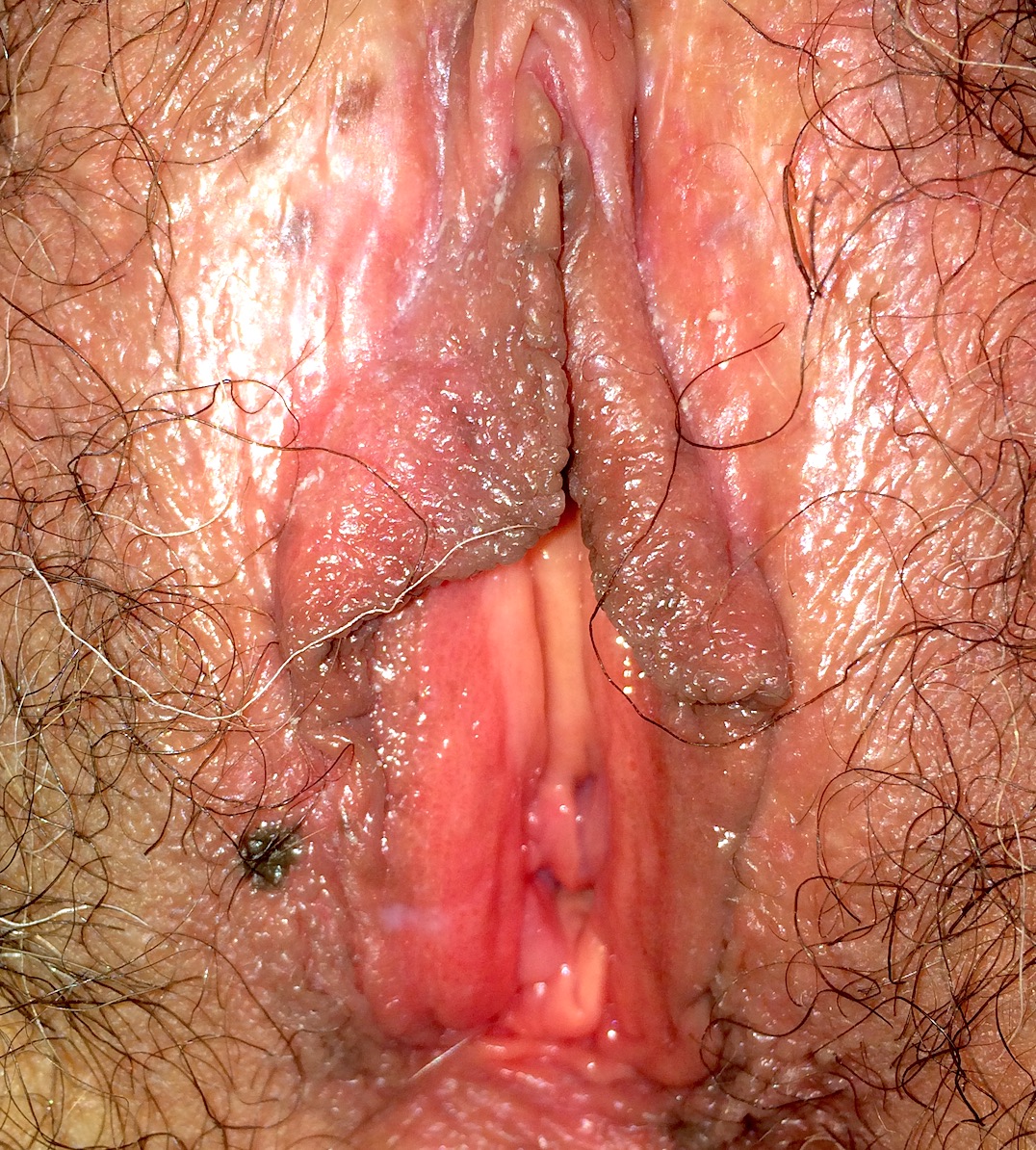
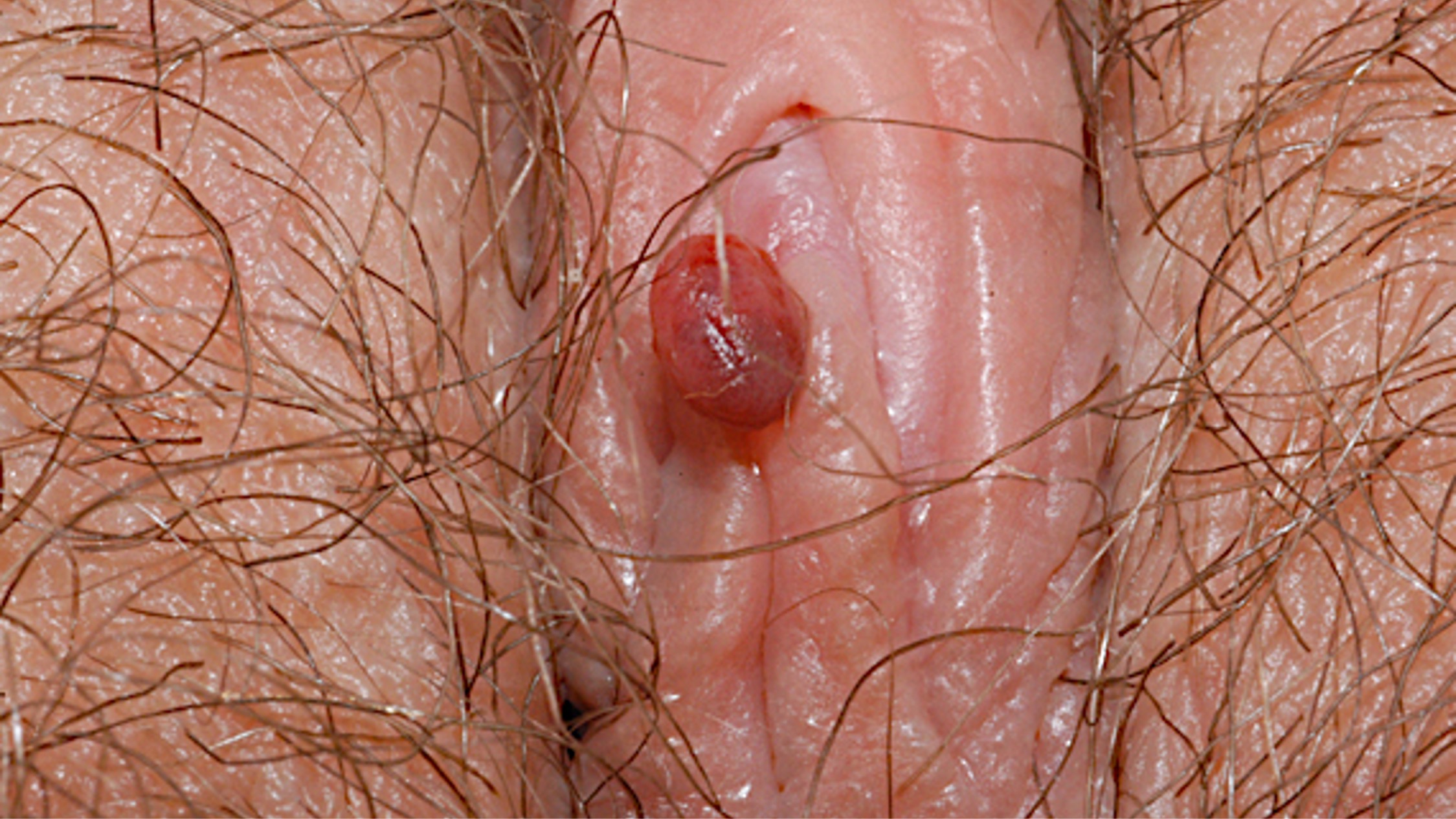
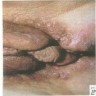
- Usually asymptomatic, although vaginal discharge or postcoital bleeding or dyspareunia have been reported (Mutter: Pathology of the Female Reproductive Tract, 3rd Edition, 2014)
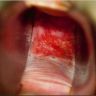
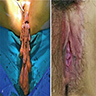
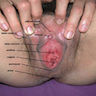
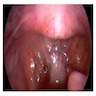
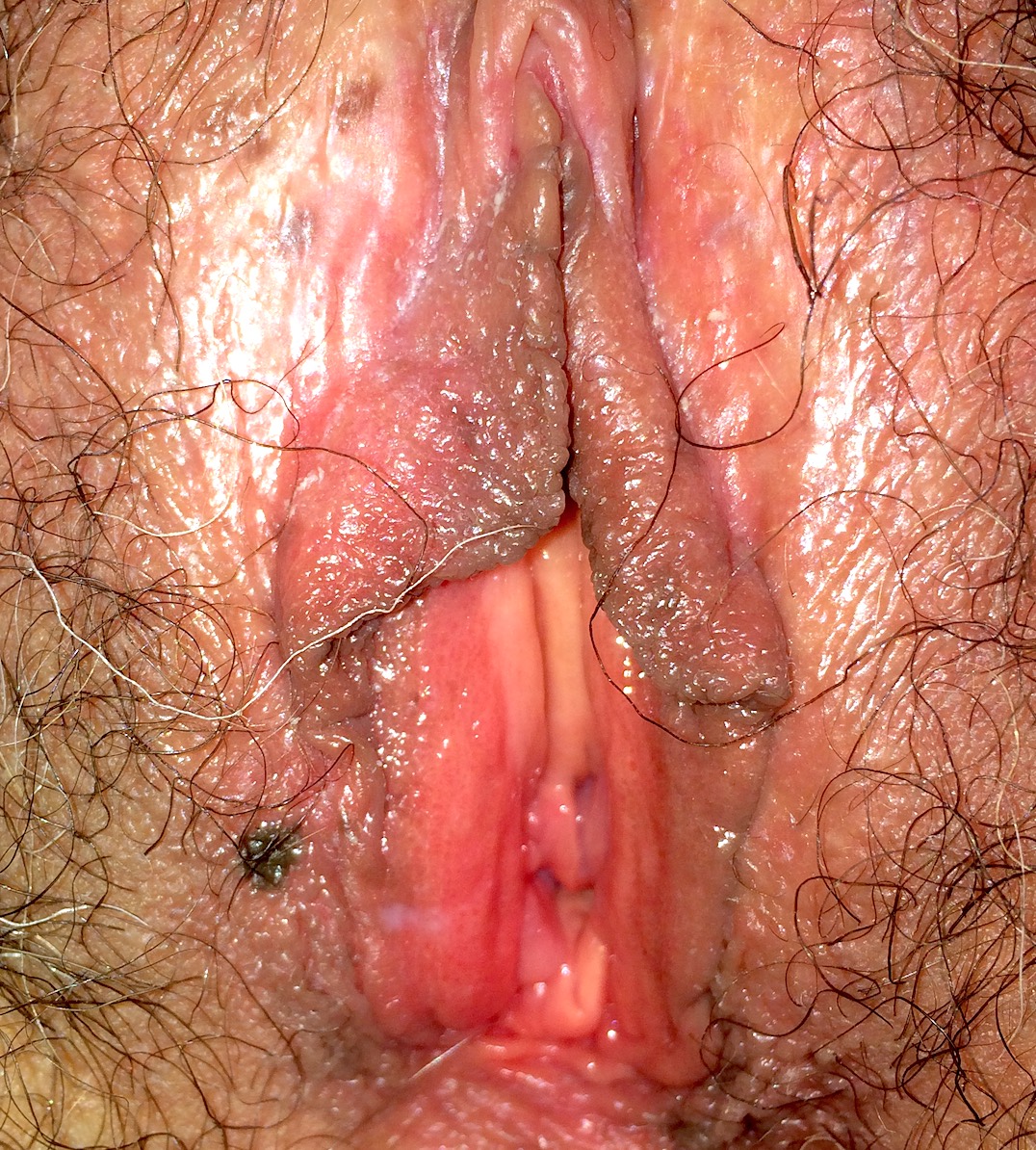
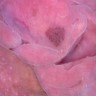
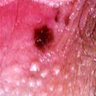
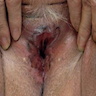
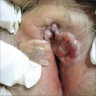
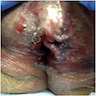
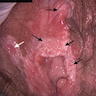
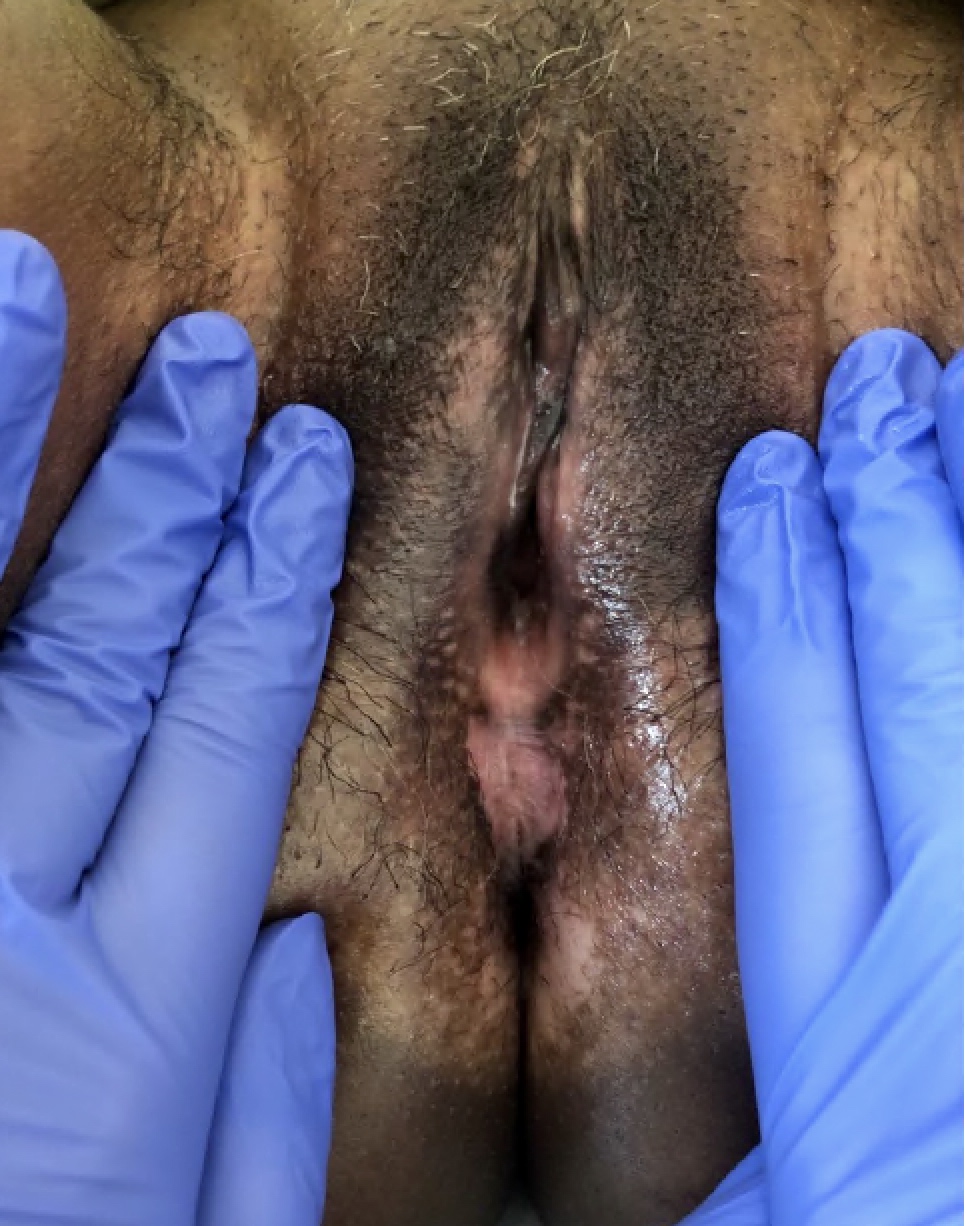
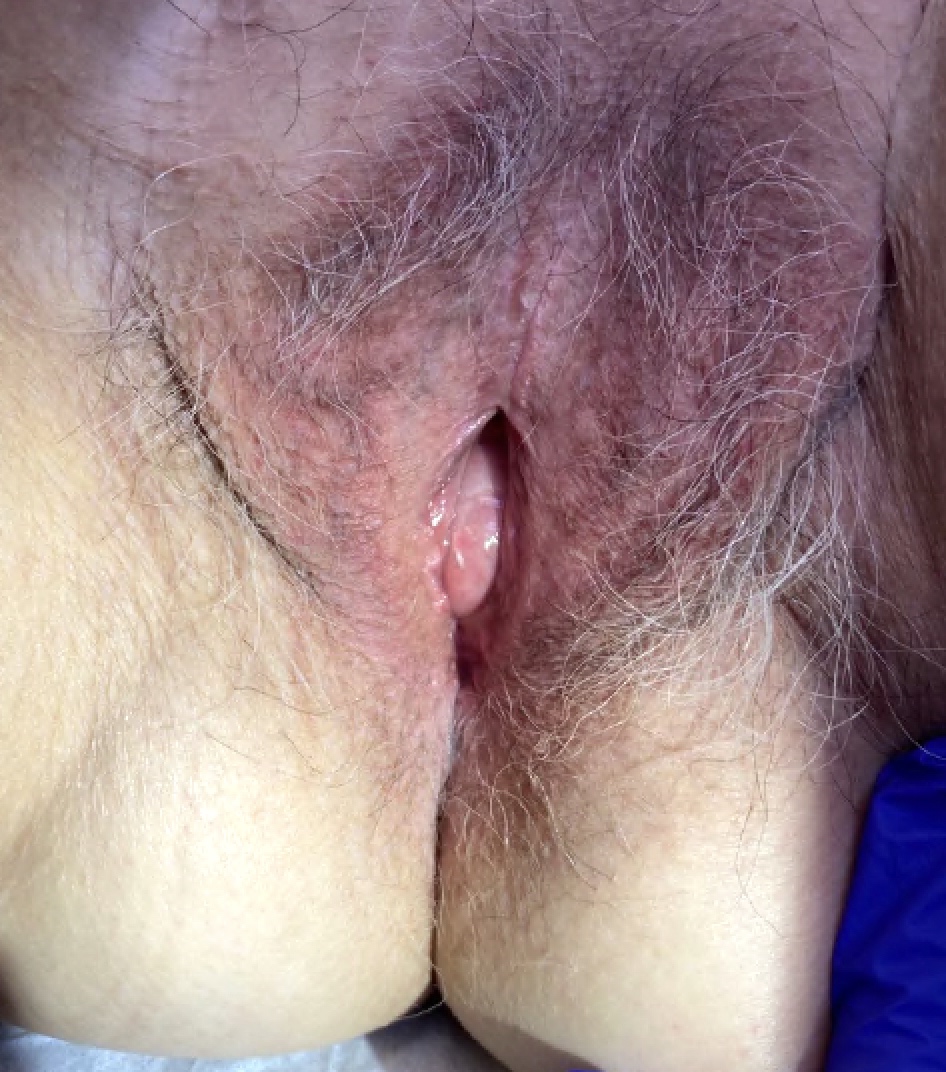
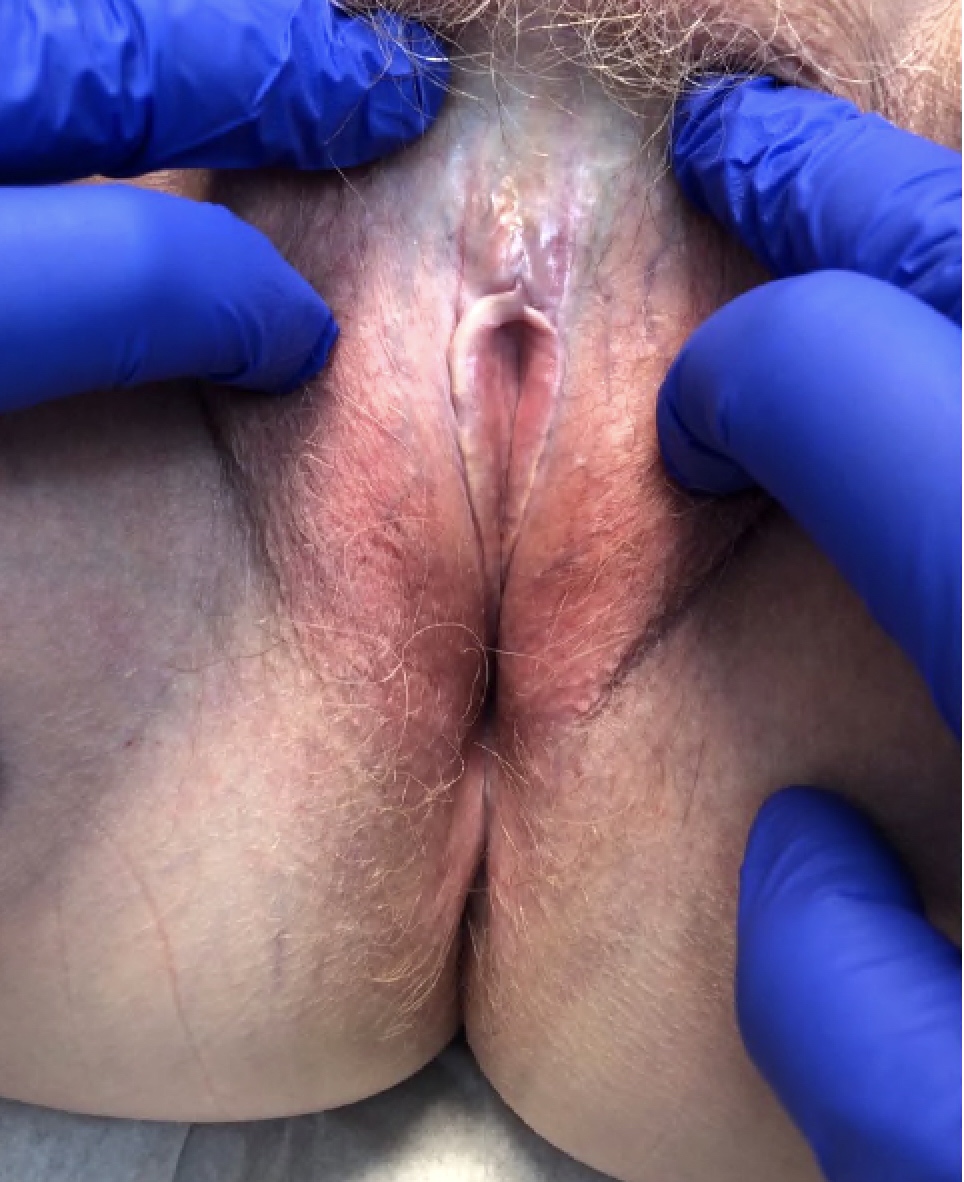
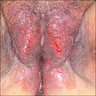
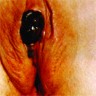
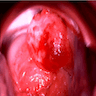
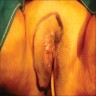
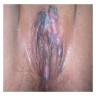
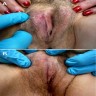
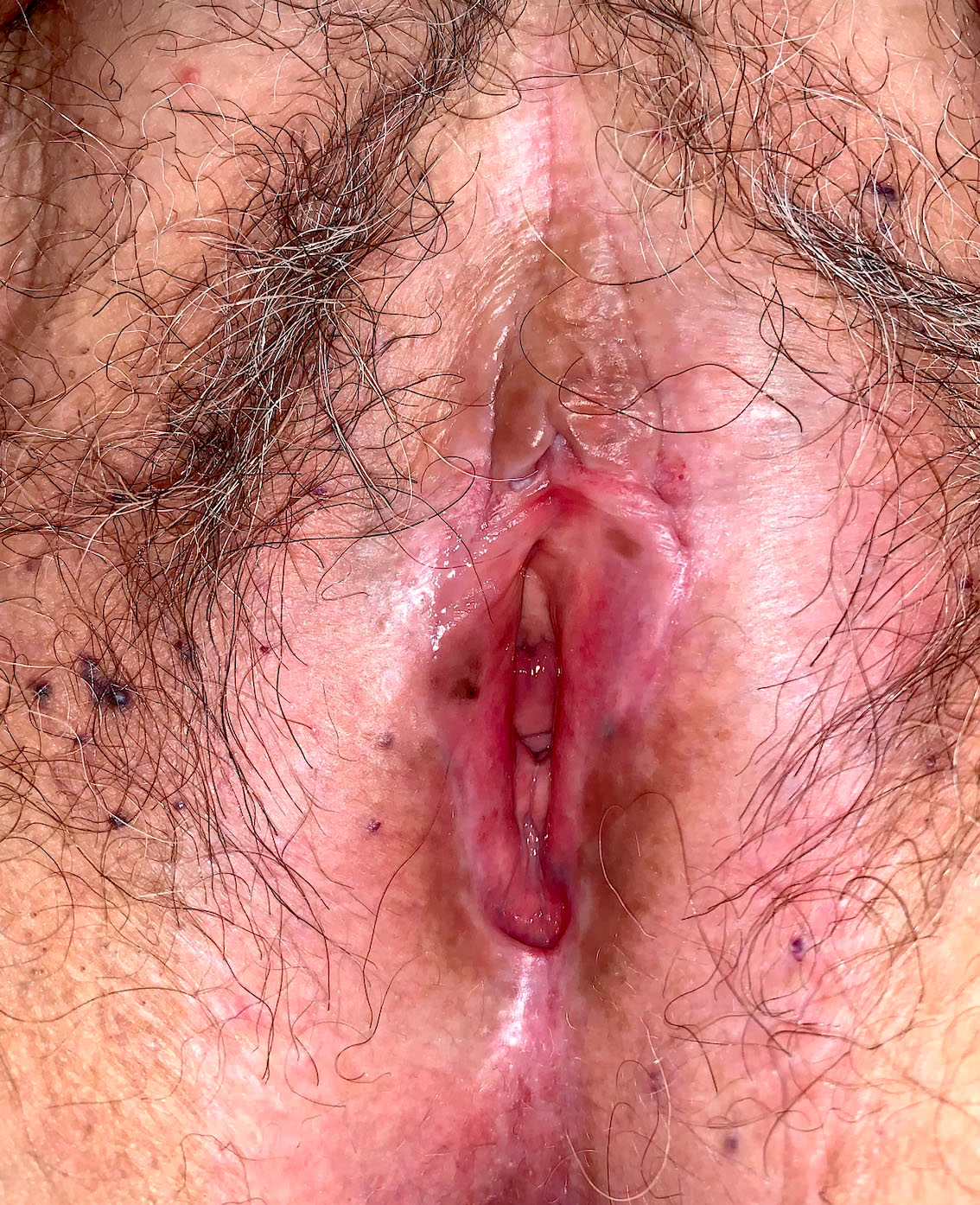
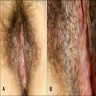
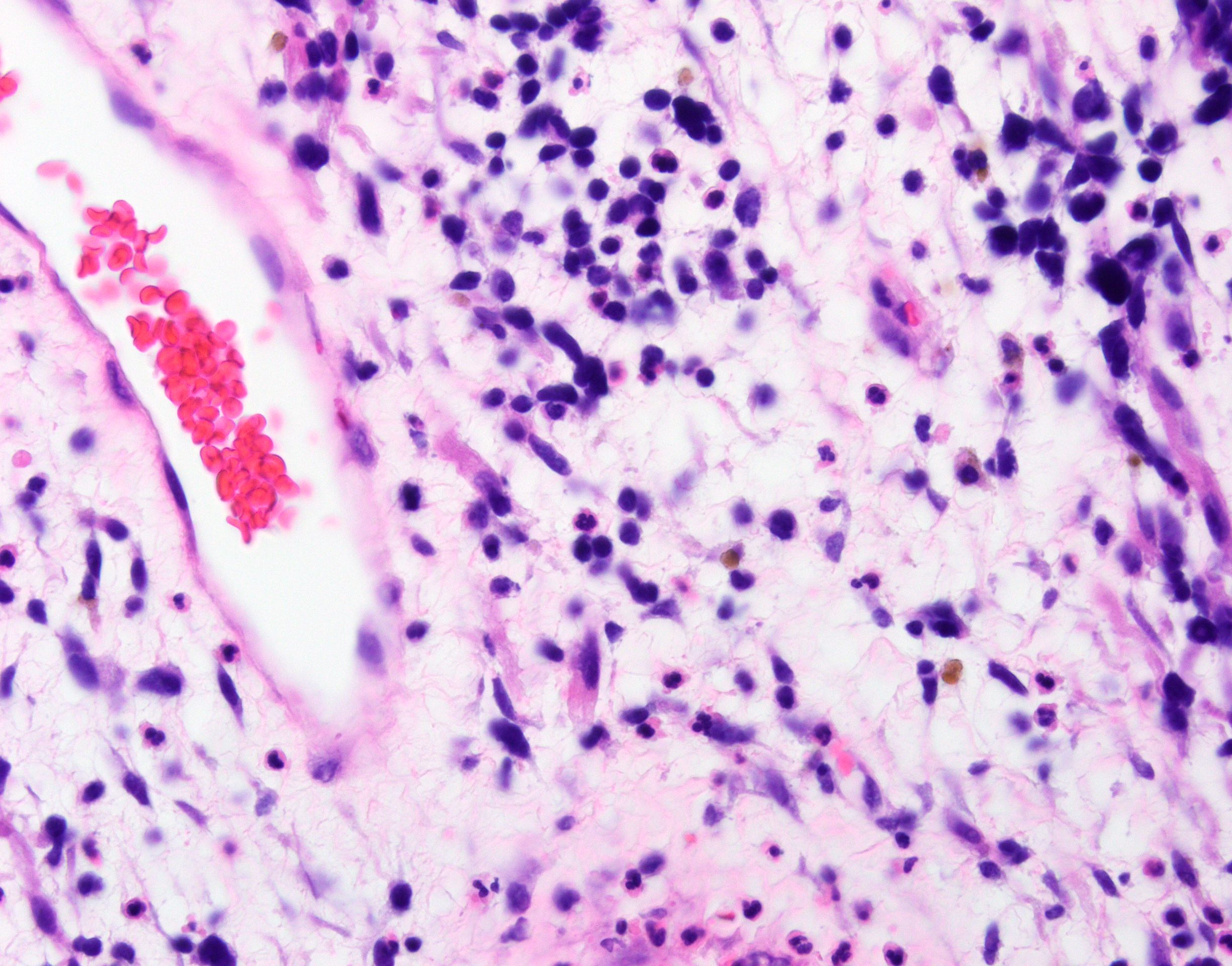
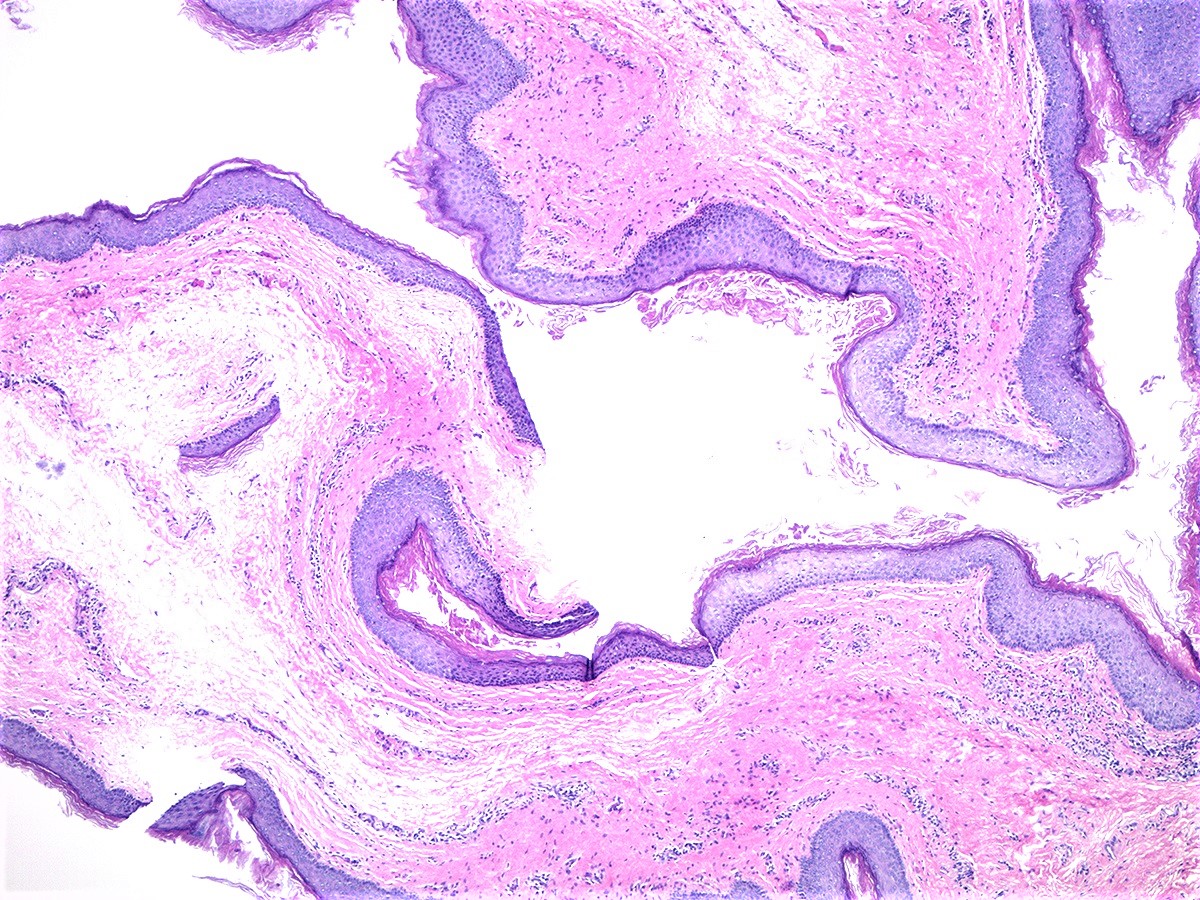
- Vaginal mucosa displays red granular spots or patches and fails to stain with an iodine solution (Mutter: Pathology of the Female Reproductive Tract, 3rd Edition, 2014)
- Mosaicism and punctation are common findings during colposcopy (Obstet Gynecol 1978;52:457)
- Lesions identified on physical examination
- Lesions can be identified on colposcopic examination as mosaicism and punctation (Obstet Gynecol 1978;52:457)
- Diagnosis confirmed on biopsy specimens with or without cytologic brushing of clinically suspicious lesions on colposcopy
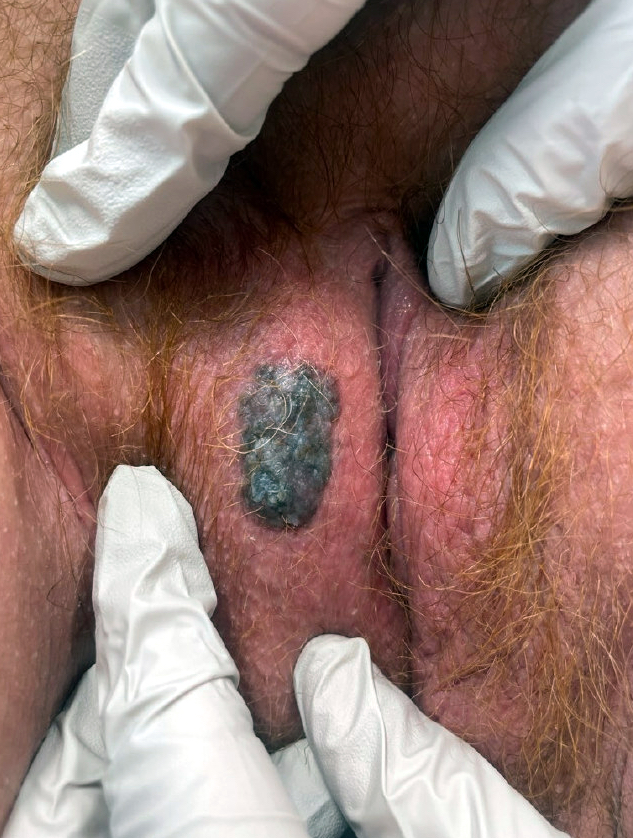
- 39 year old woman with long history of vaginal pain and bleeding as well as dyspareunia (BMC Cancer 2019;19:798)
- 40 year old woman with primary vaginal clear cell adenocarcinoma and Herlyn-Werner-Wunderlich syndrome without DES exposure (Int J Clin Exp Pathol 2020;13:2784)
- 47 year old woman with vaginal cystic appearing mucosa and exophytic mass in mid-vagina (Gynecol Oncol Rep 2020;34:100672)
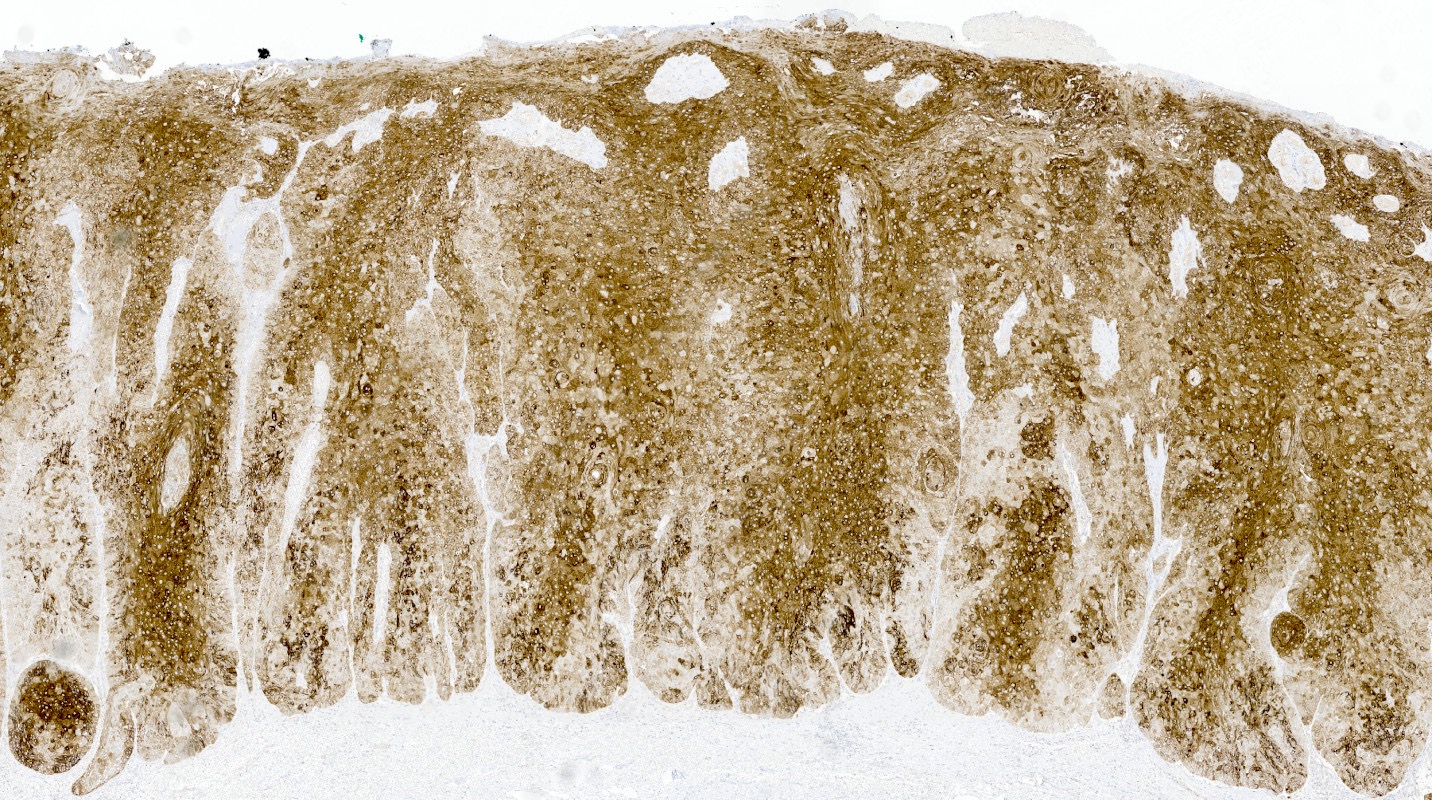
- 11 cases of vaginal glandular lesions exhibiting gastric differentiation (Am J Surg Pathol 2018;42:958)
- In symptomatic patients, treatment with CO2 laser, simple excision or unipolar cautery may be considered (Lasers Surg Med 1983;3:23)
- In patients with vulvovaginal involvement by Stevens-Johnson syndrome and toxic epidermal necrolysis, adenosis prevention includes intravaginal steroids and menstrual suppression ((luteinizing hormone releasing hormone [LHRH] agonists, combined oral contraceptives or progesterone only contraceptives) (Rev Obstet Gynecol 2011;4:81)
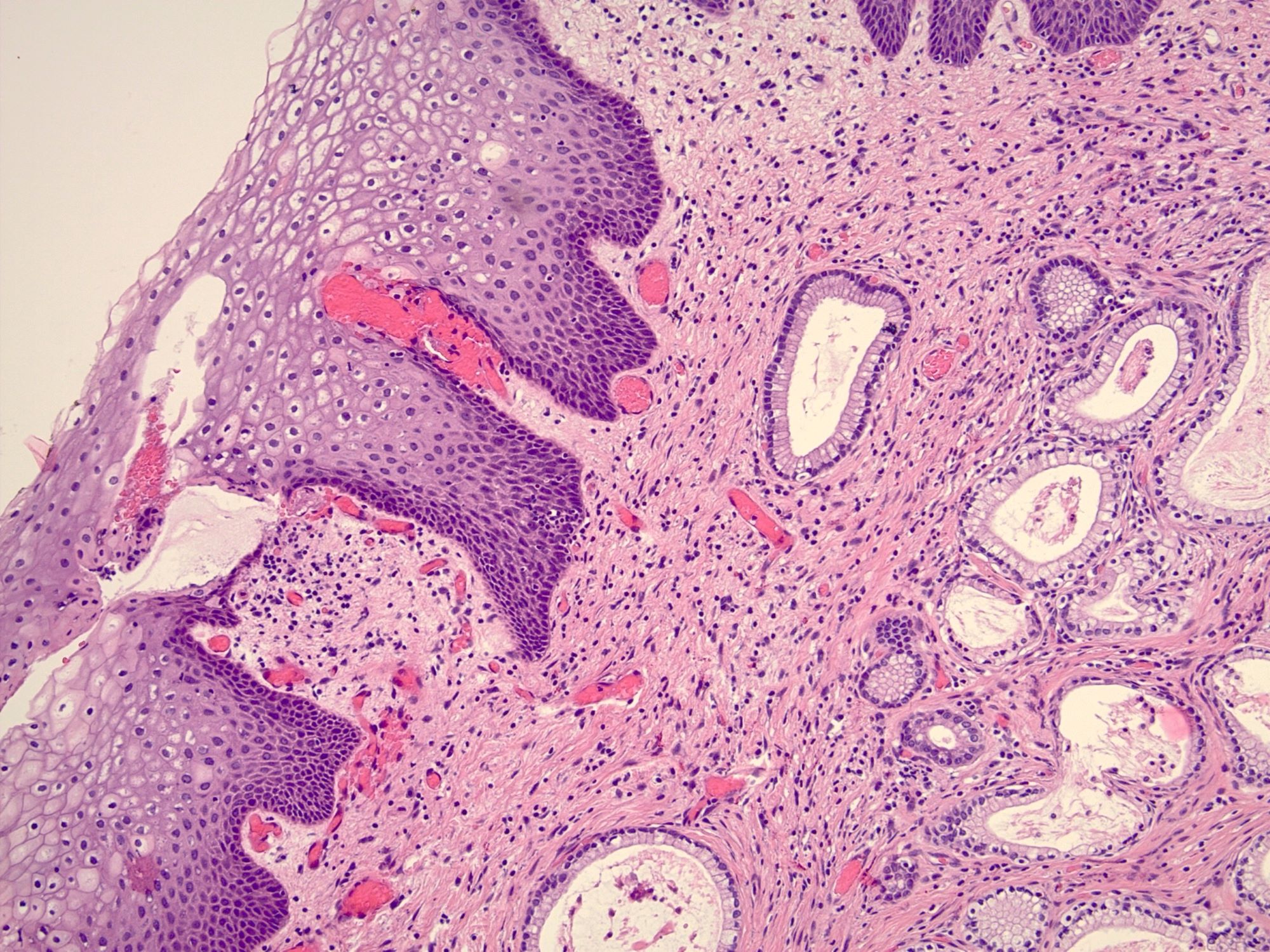
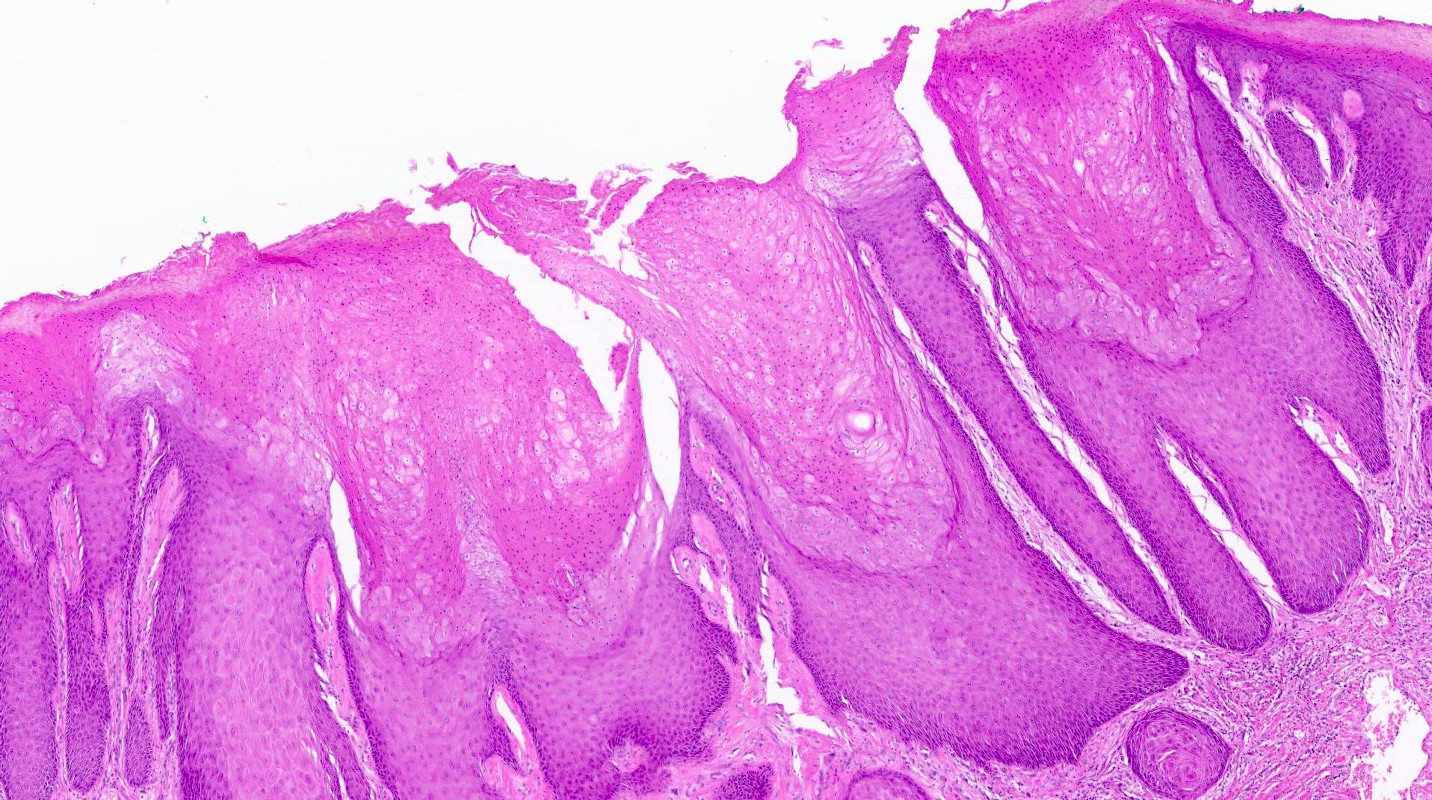
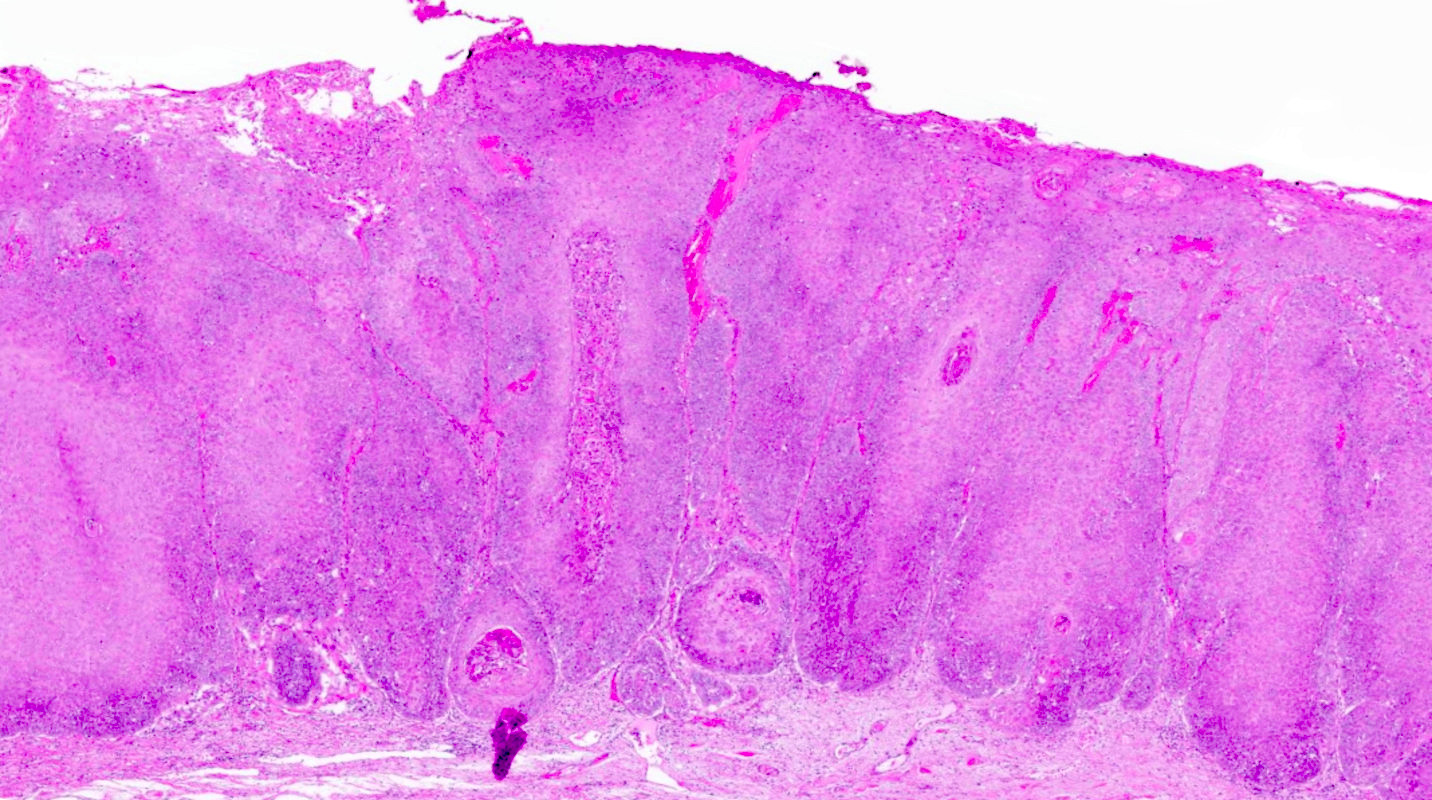
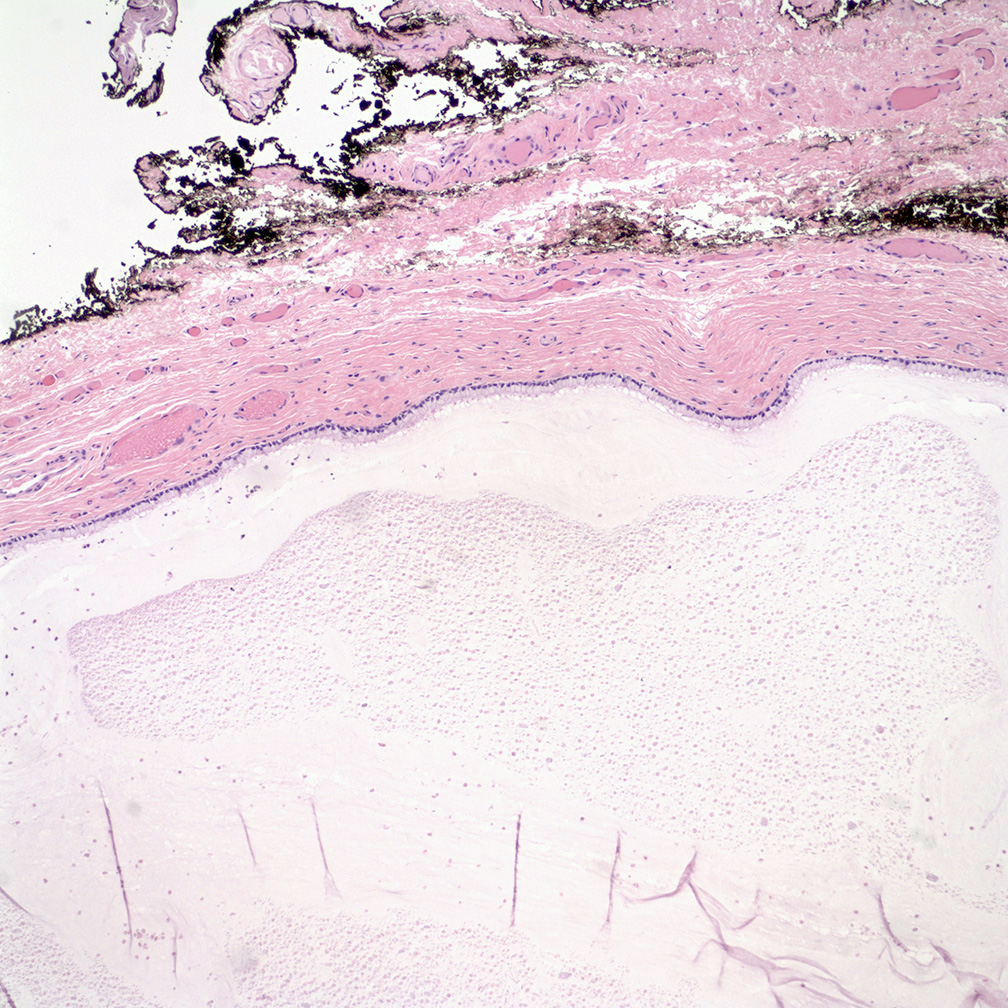
- Vaginal mucosa displays cysts or red granular spots or patches
- In the setting of DES exposure, you may also find a flattened, shallow upper vagina with absent vaginal fornices around a hypoplastic cervix
- Menopause can cause involution of vaginal adenosis into the cervical canal as the upper vagina contracts in these patients (Case Rep Obstet Gynecol 2017;2017:9523853)
- 2 adult (differentiated) forms of adenosis have been described:
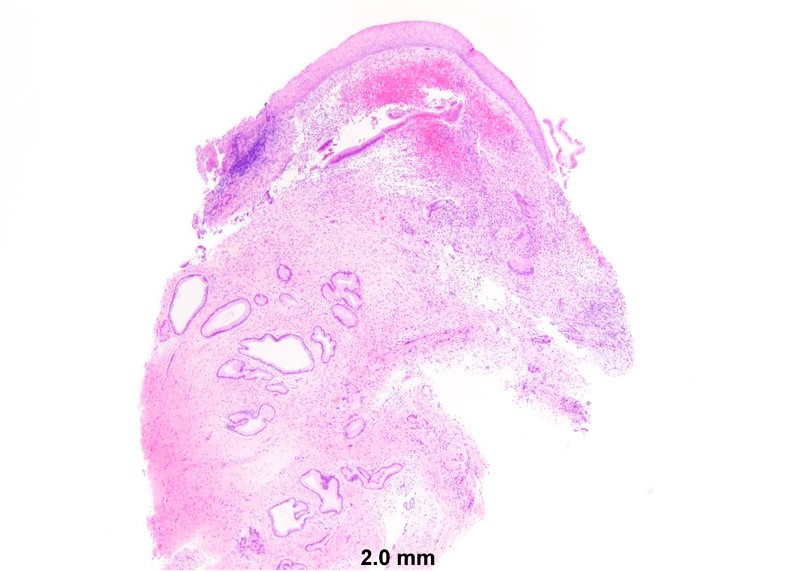
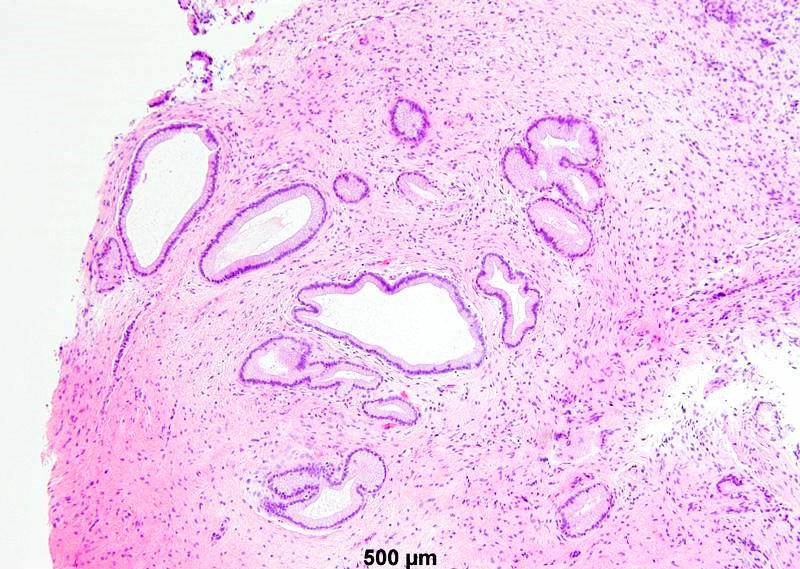
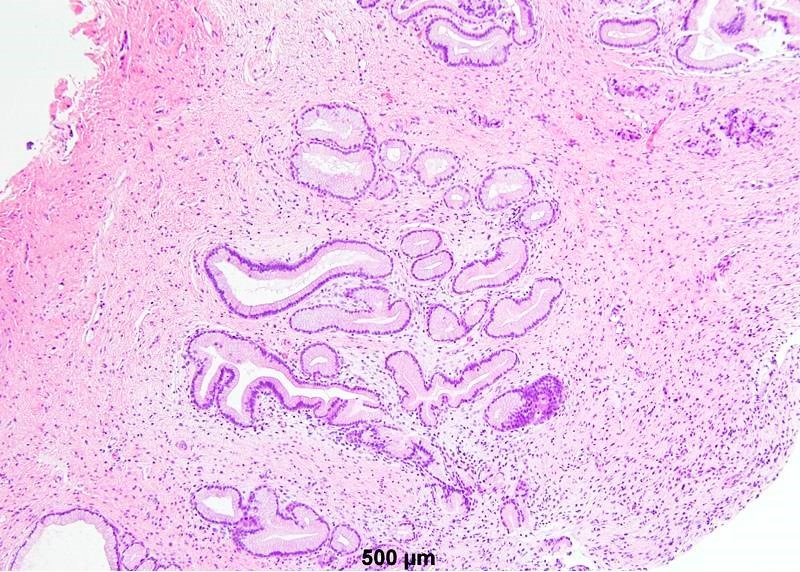
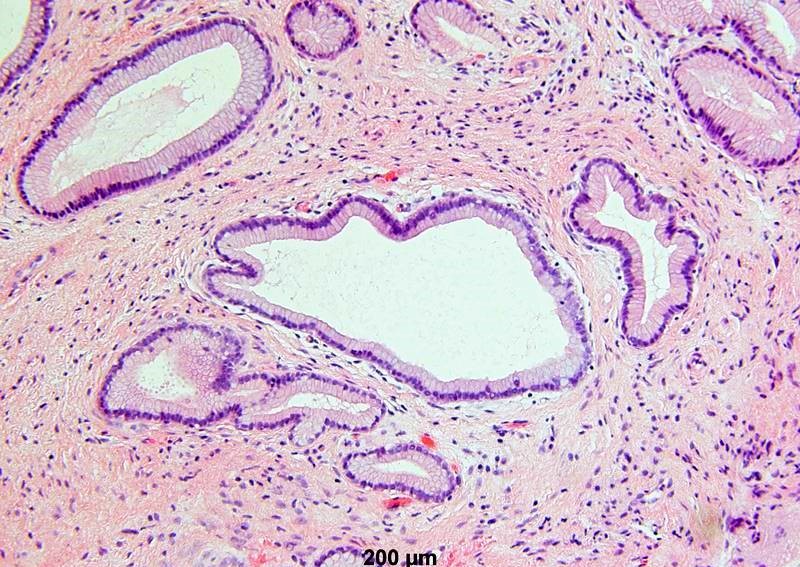
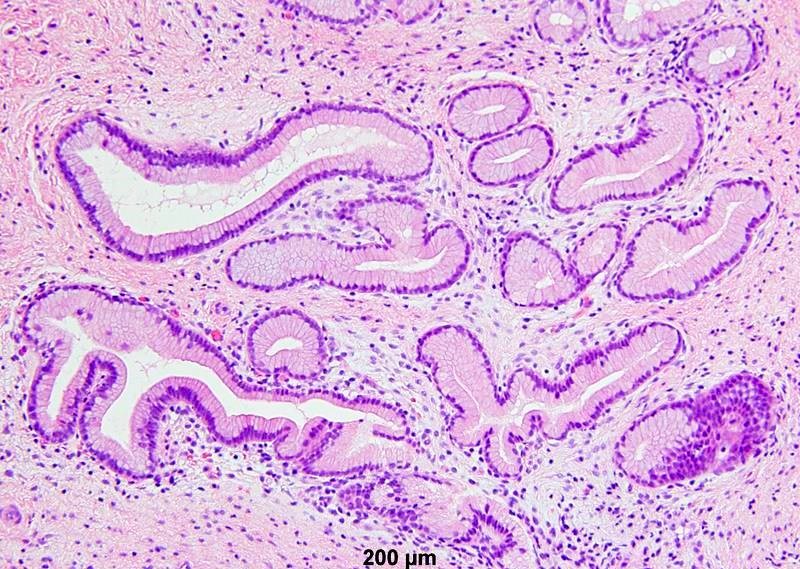
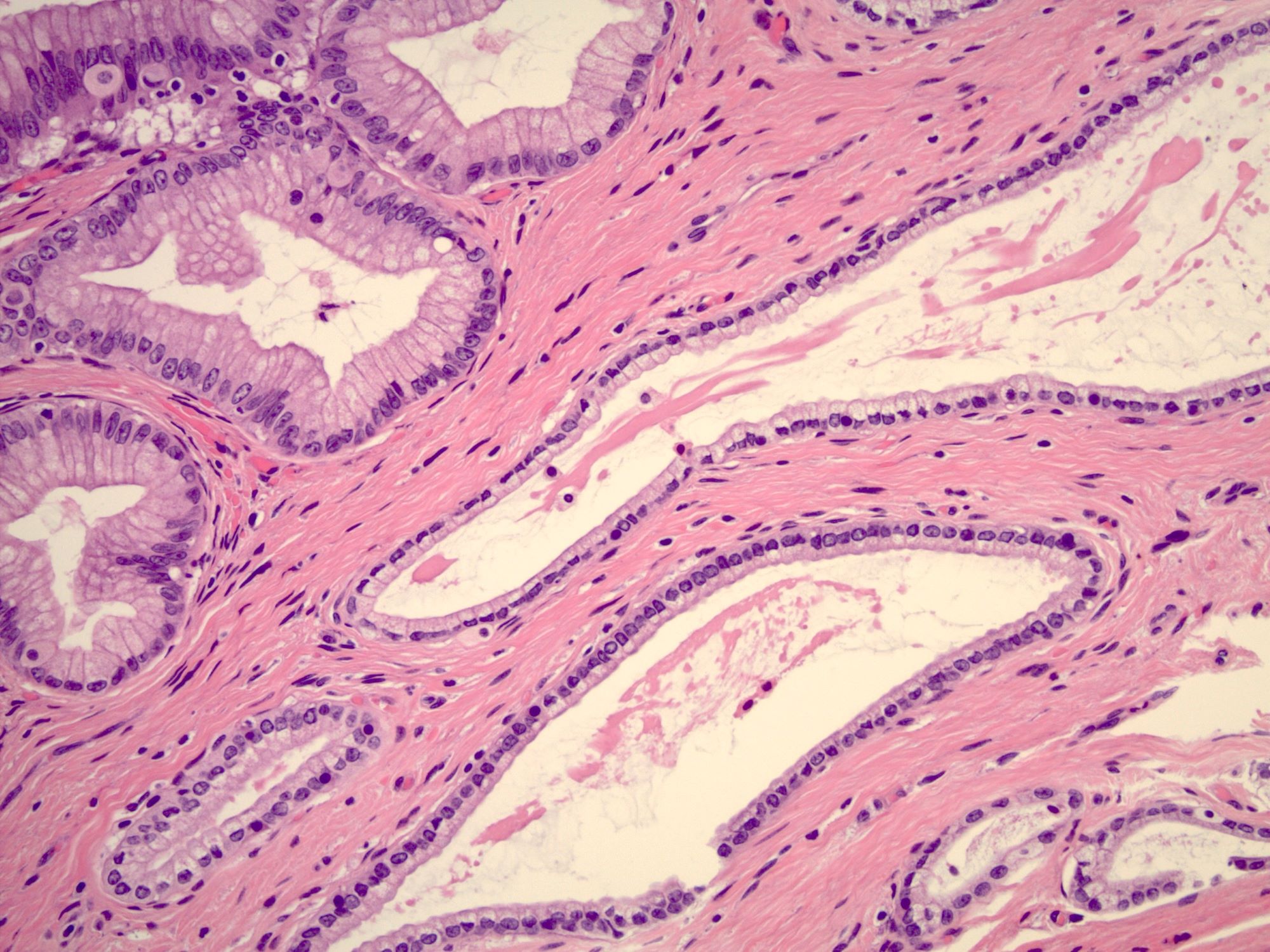
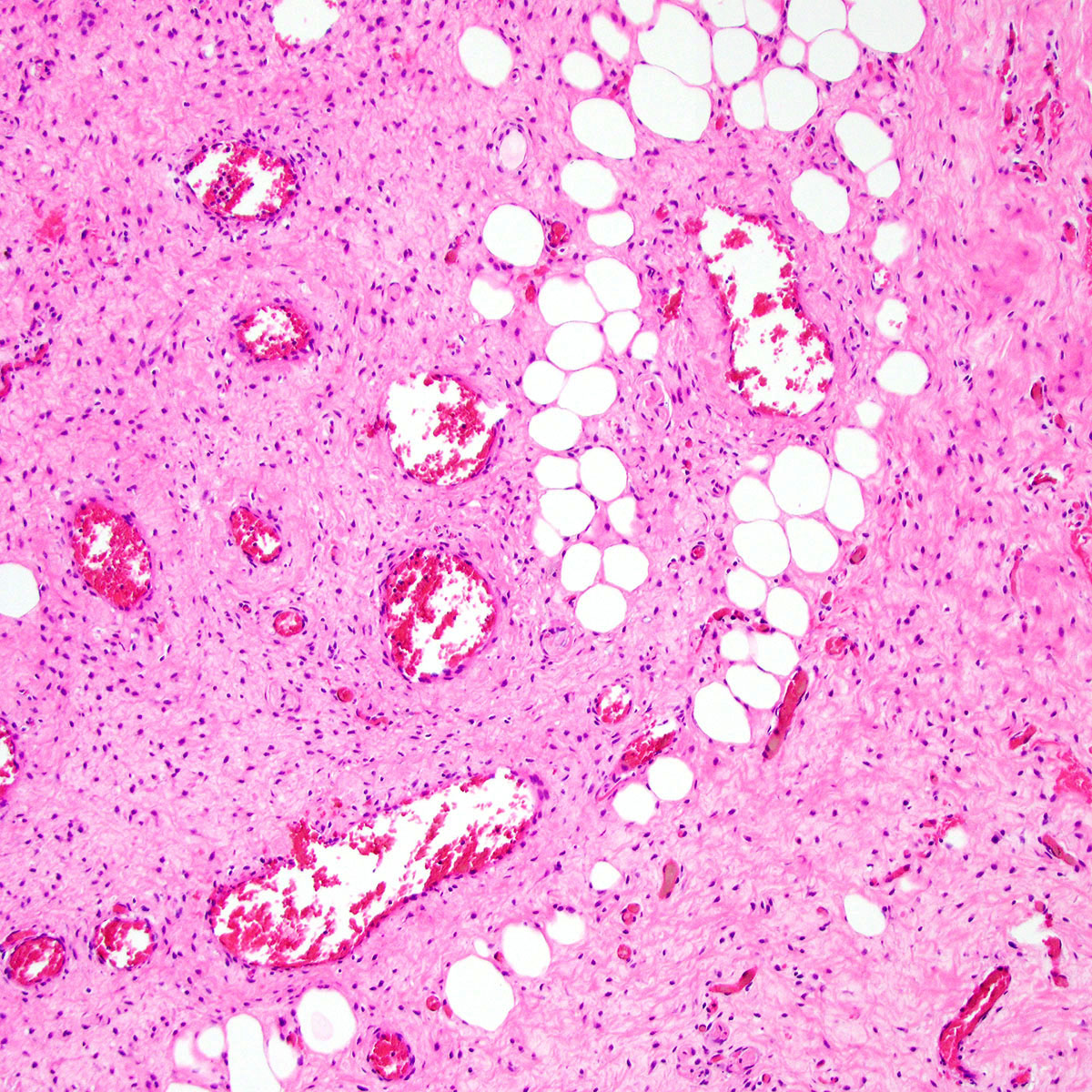
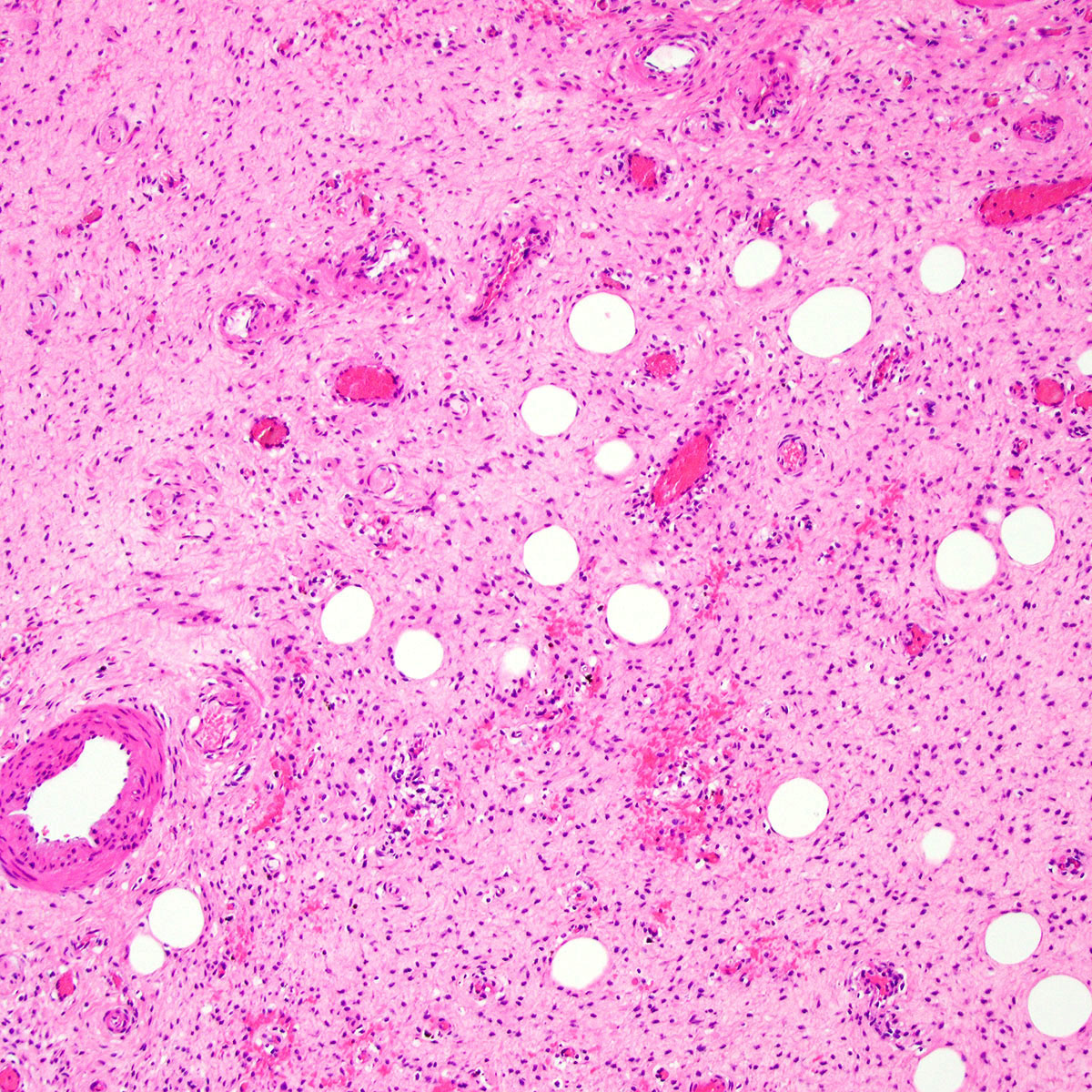
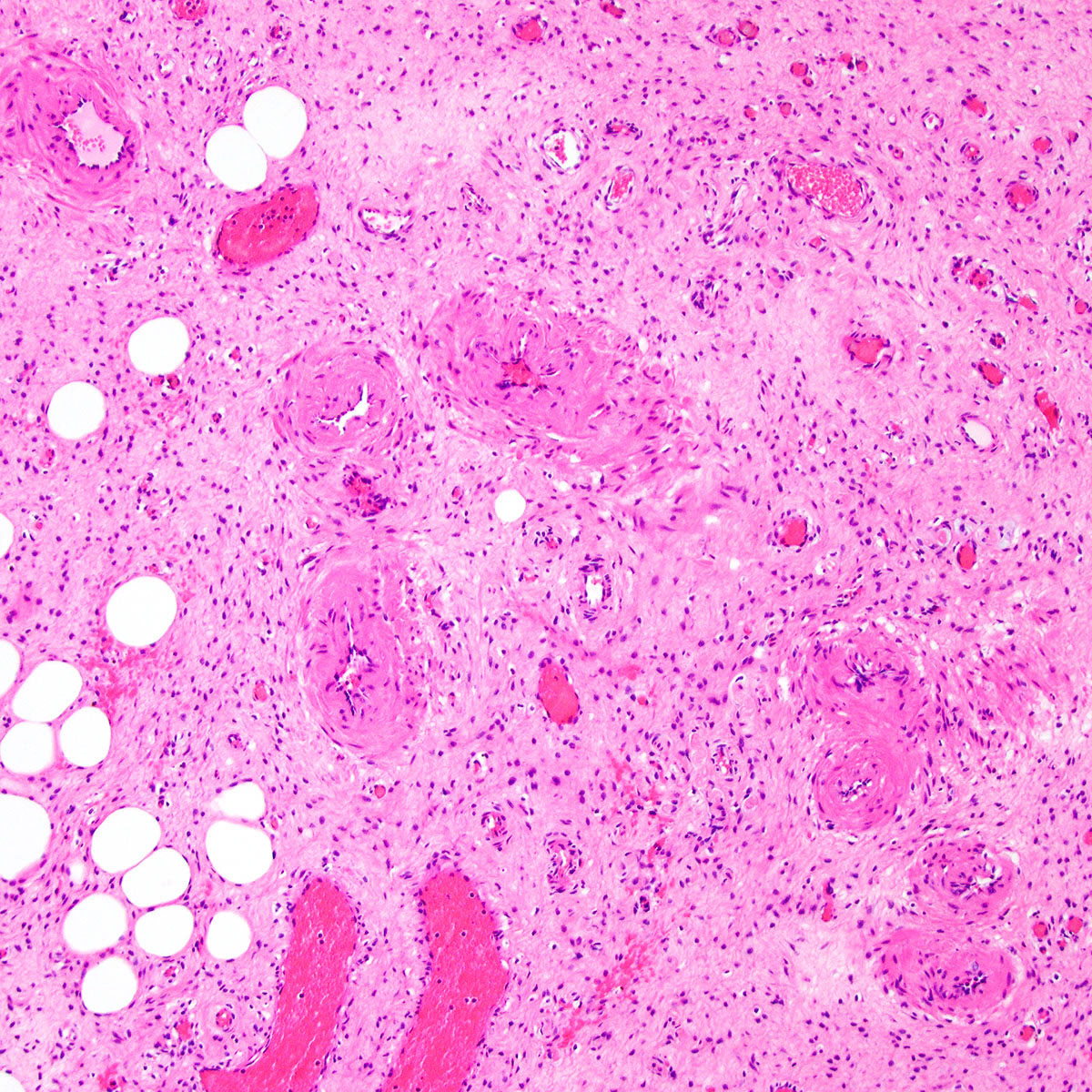
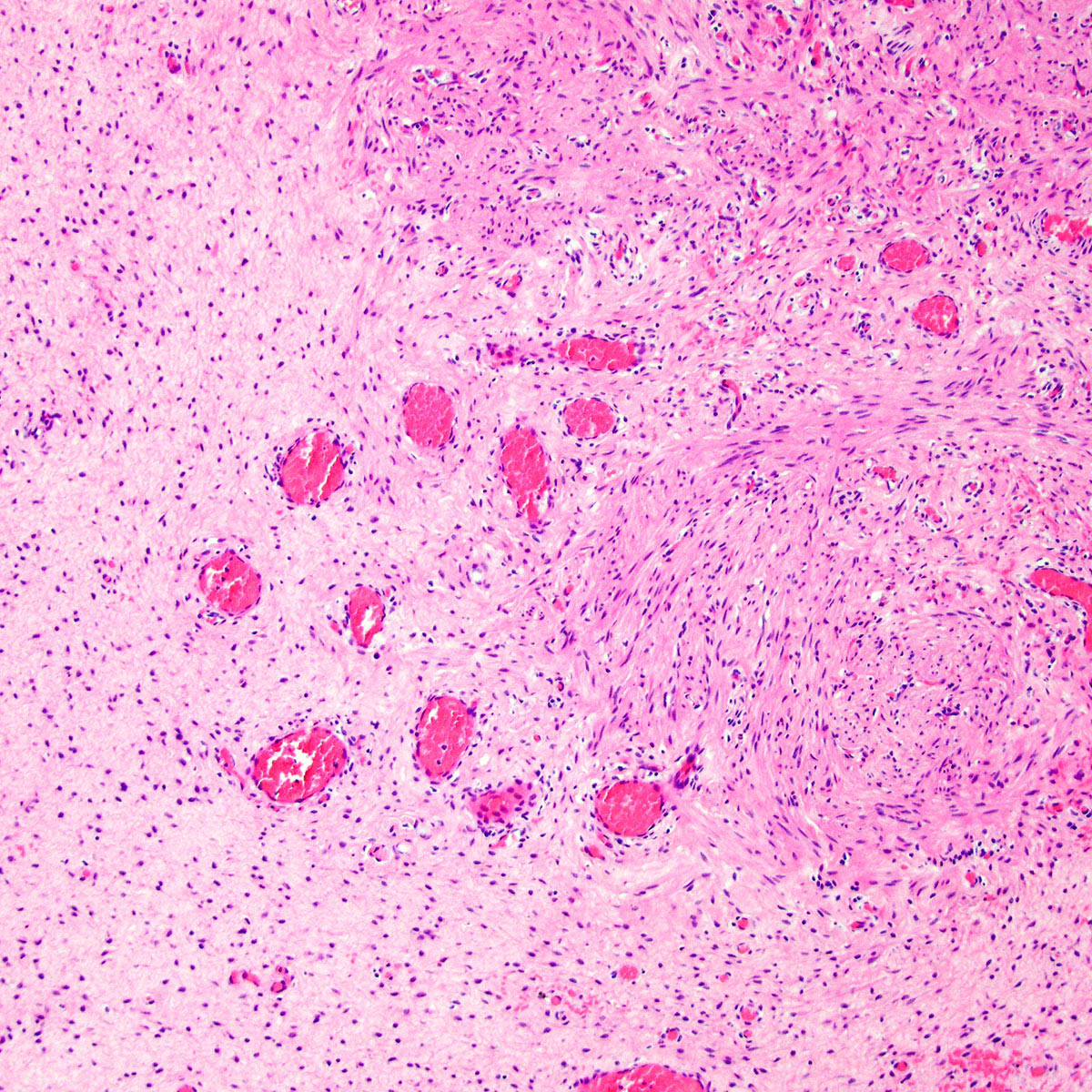
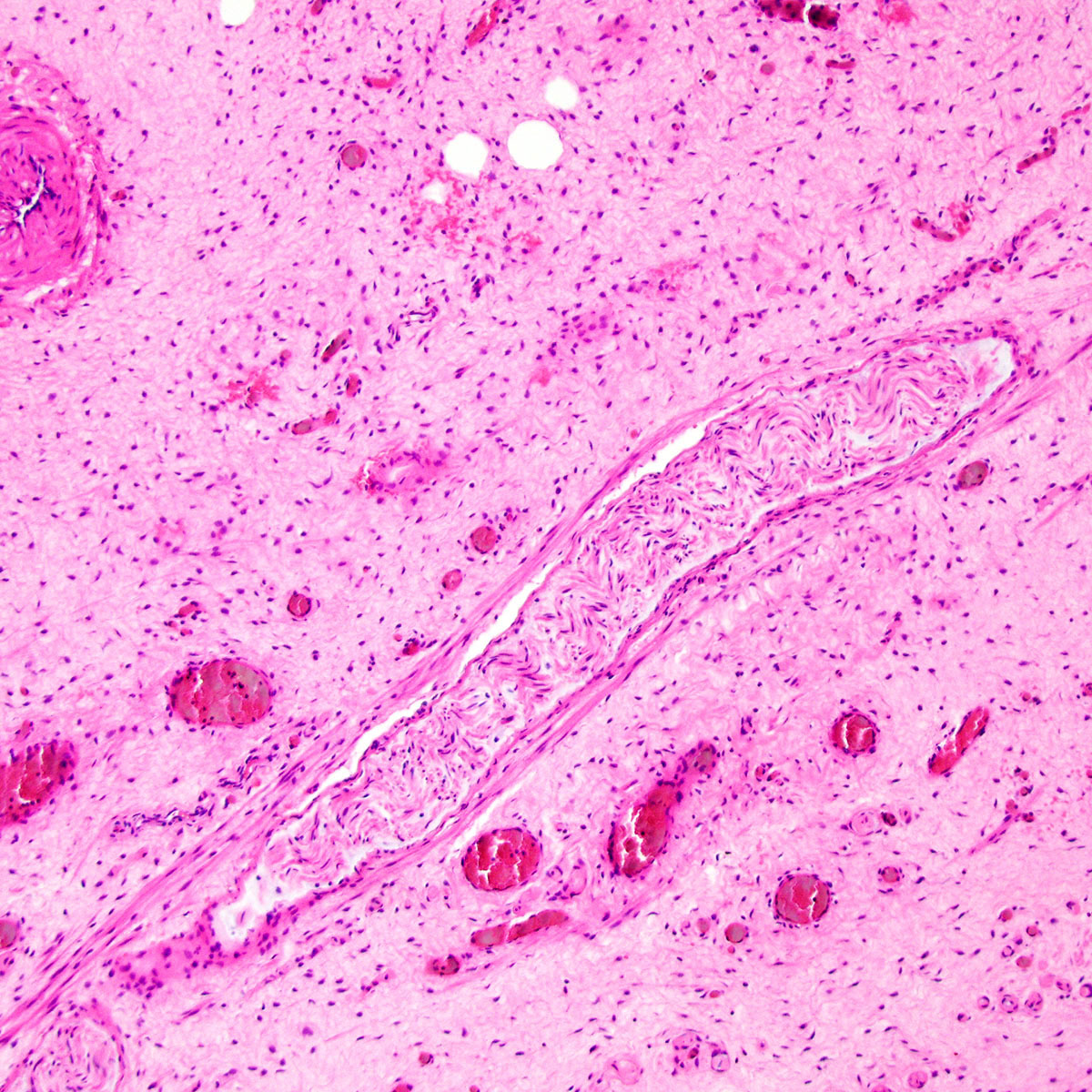
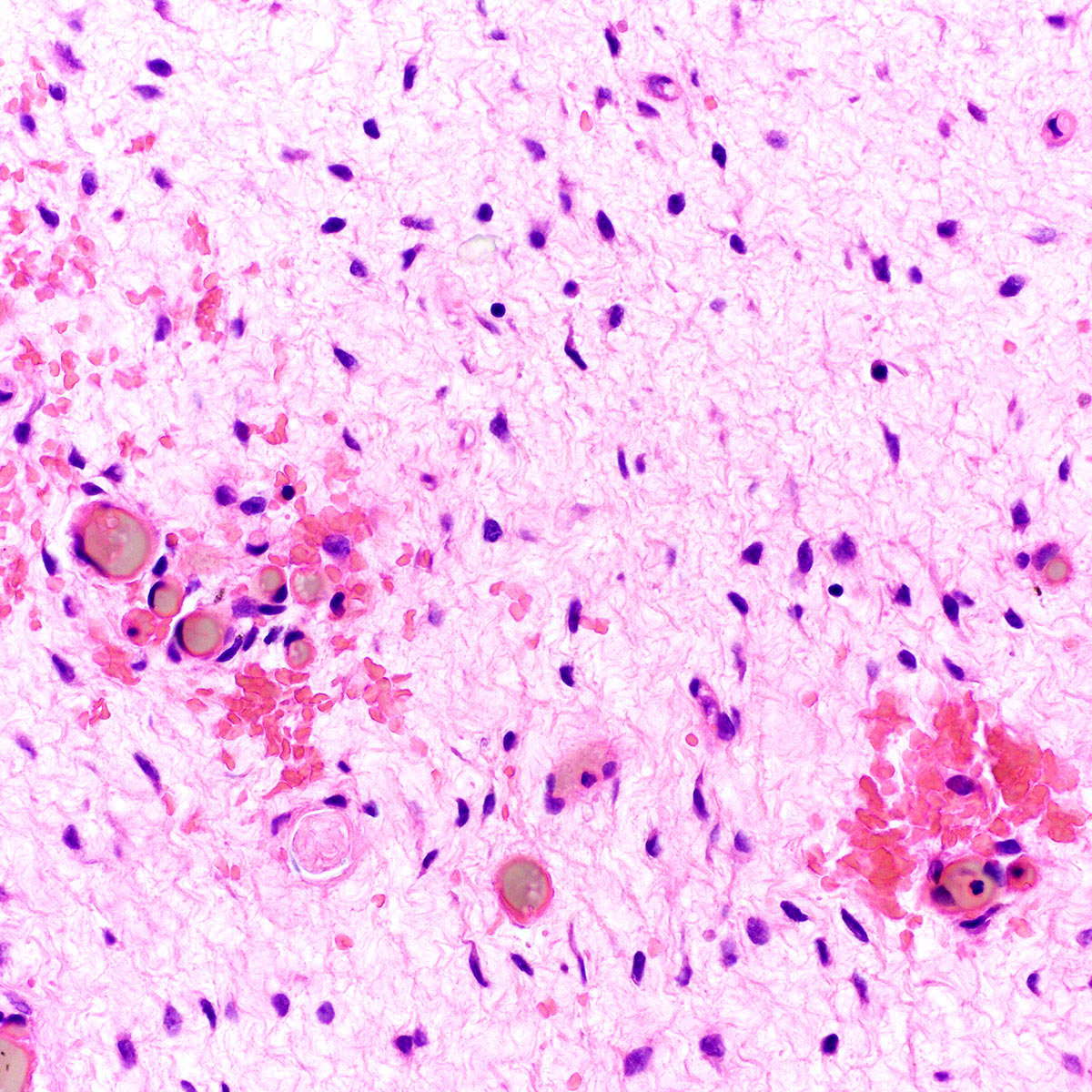
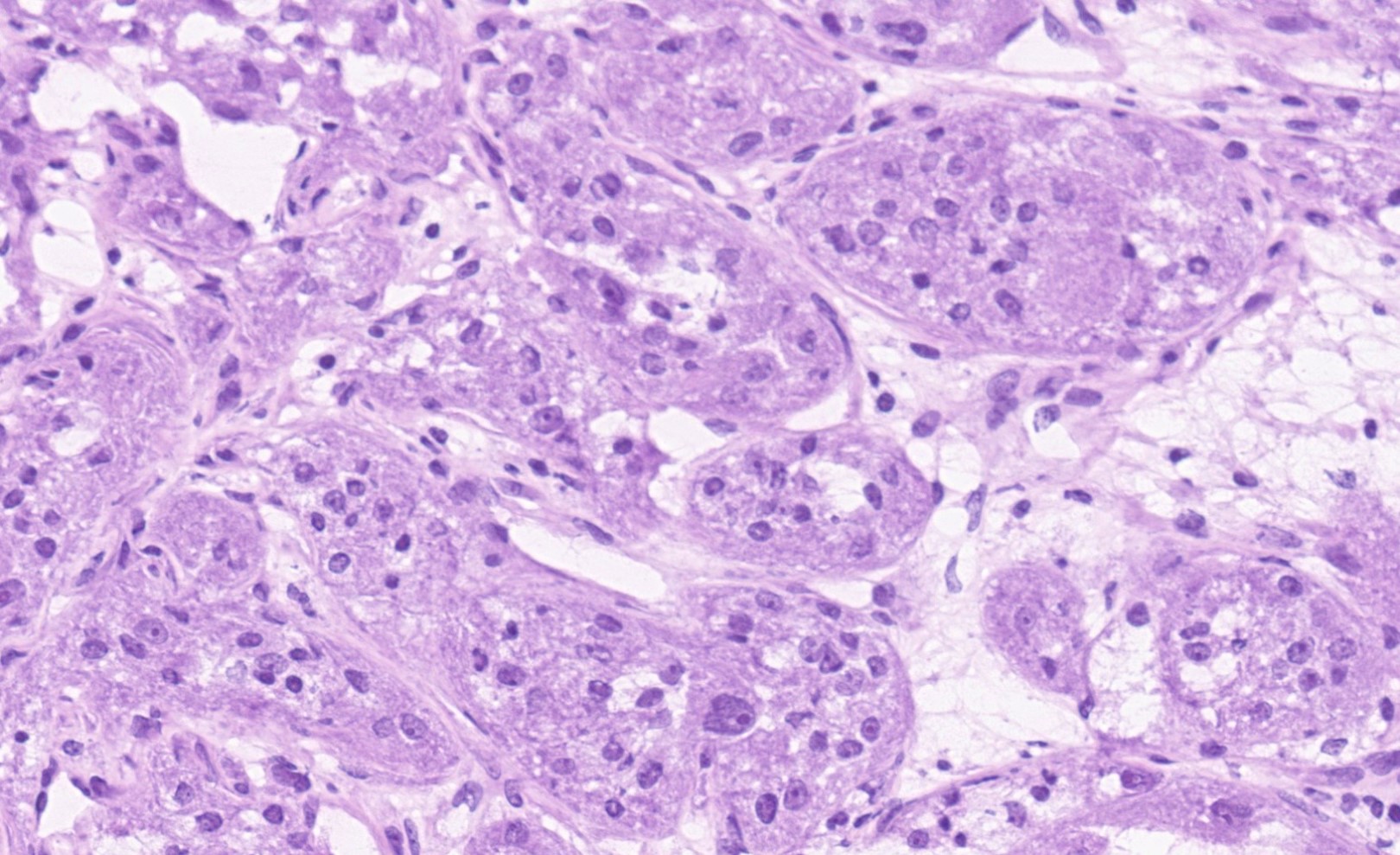
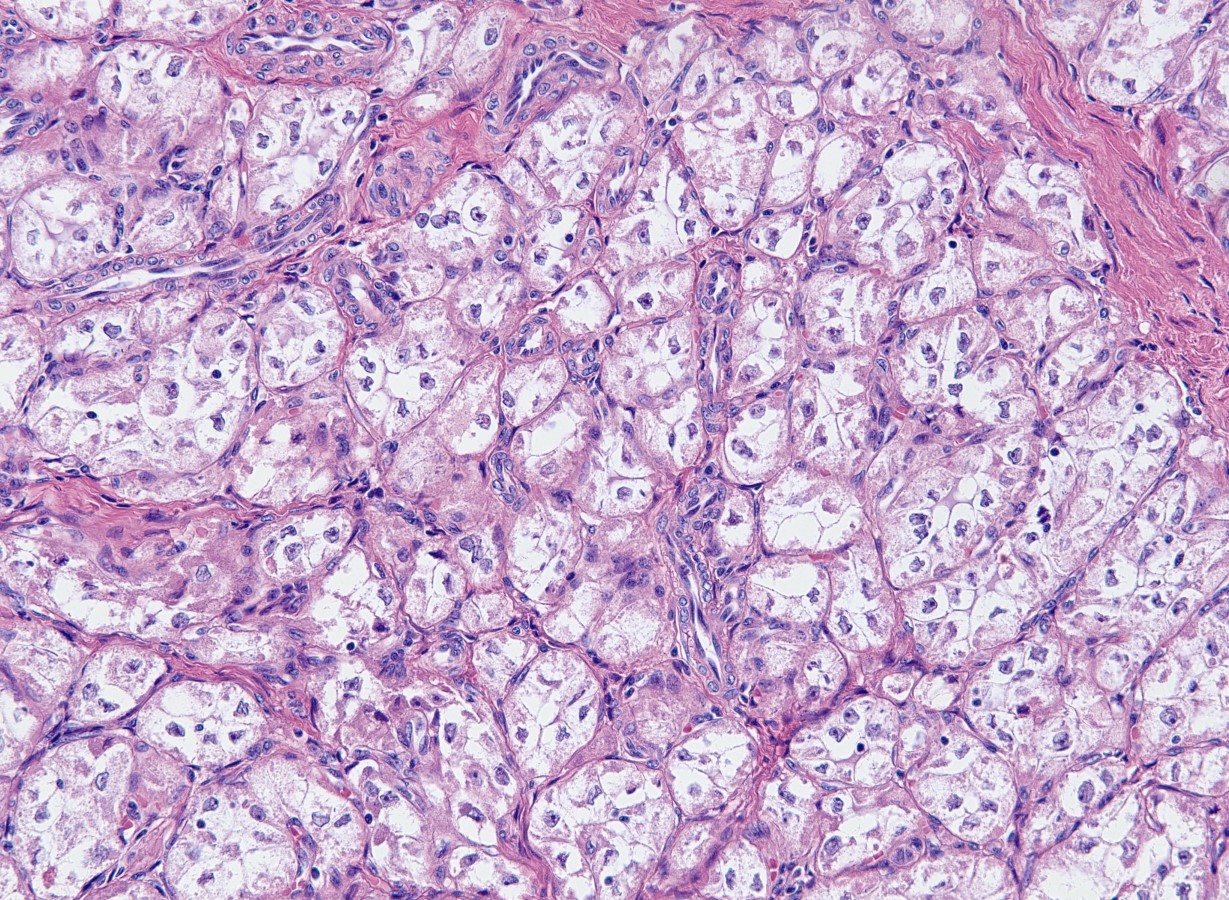
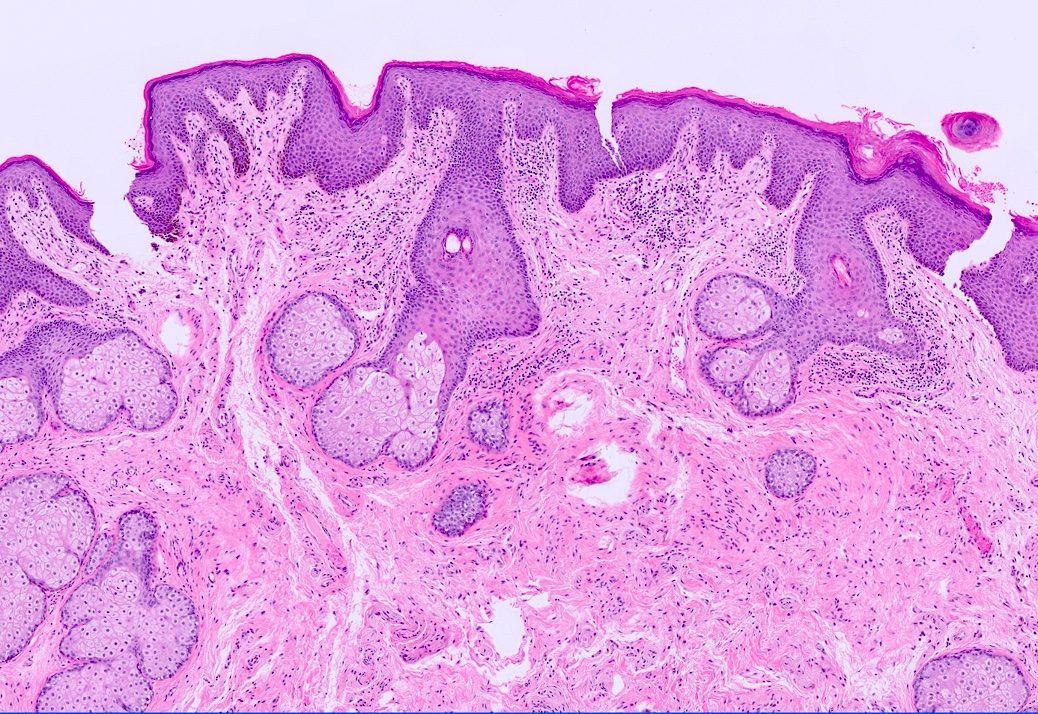
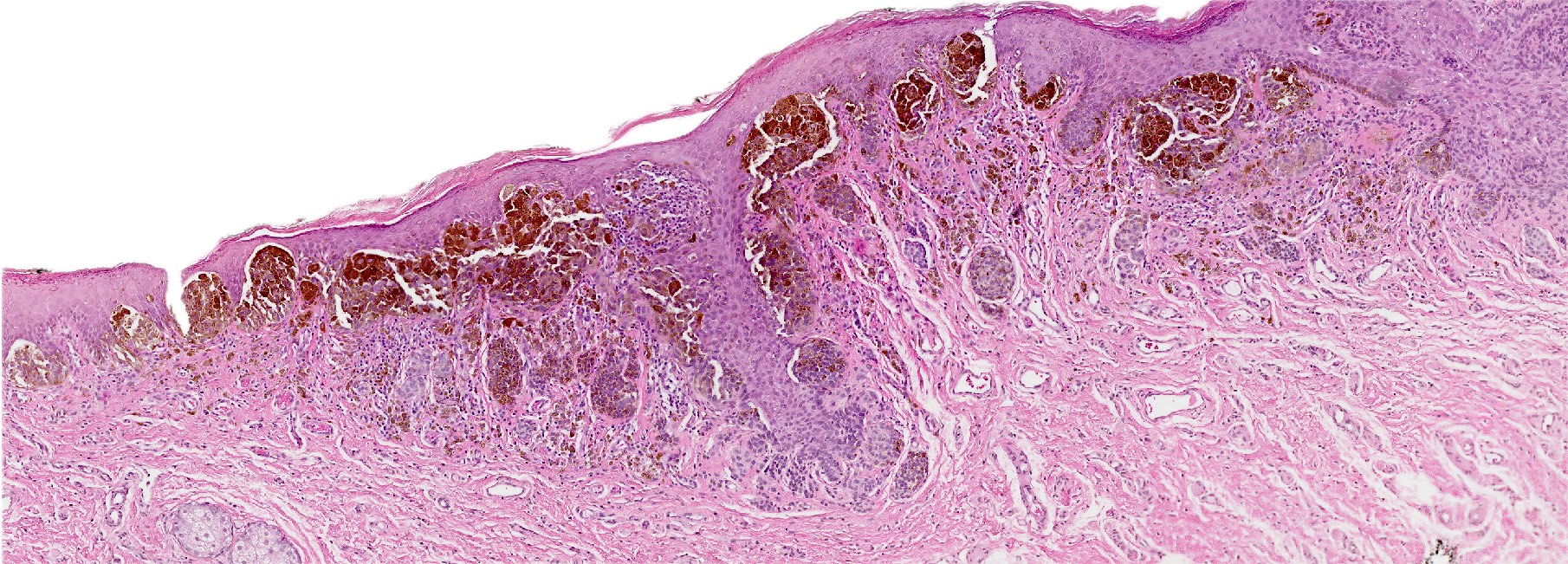
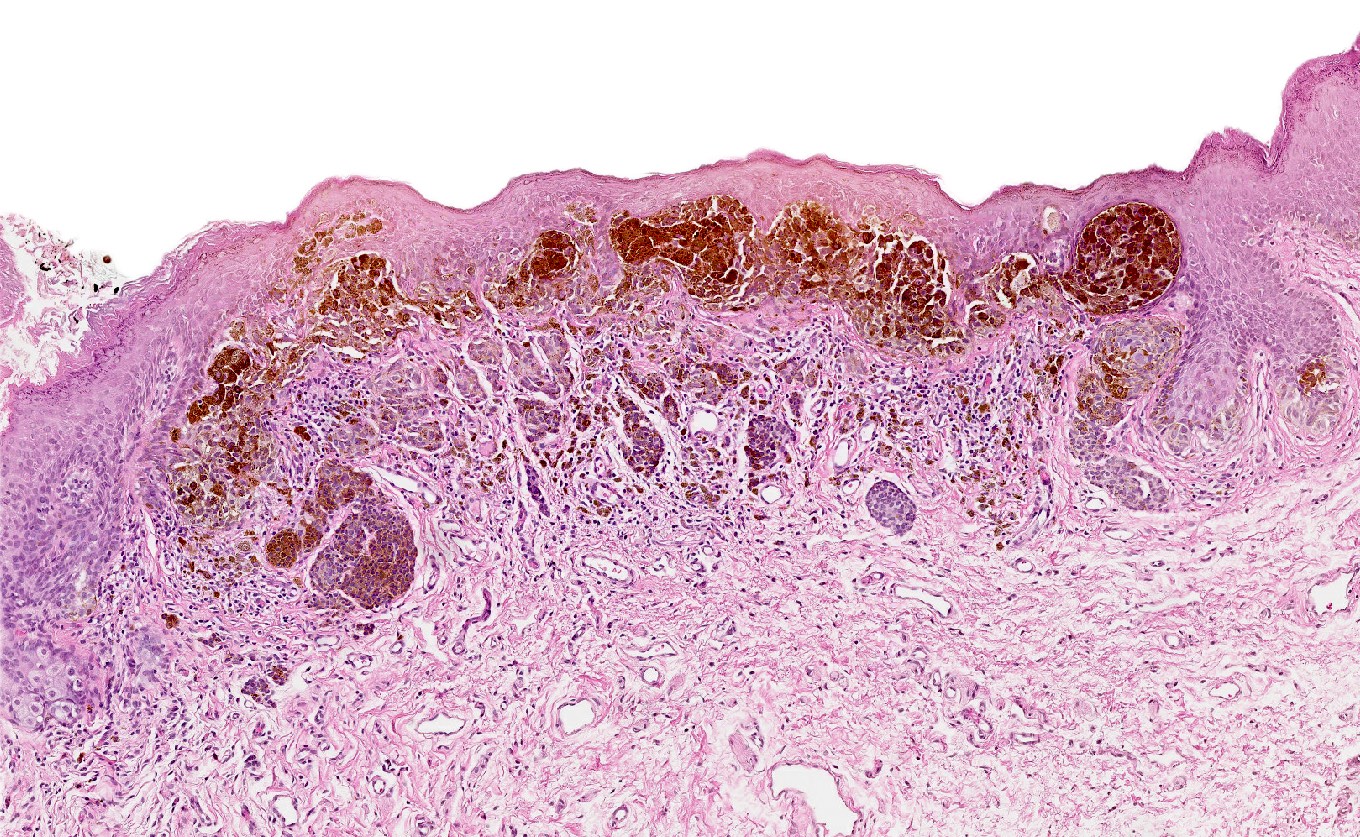
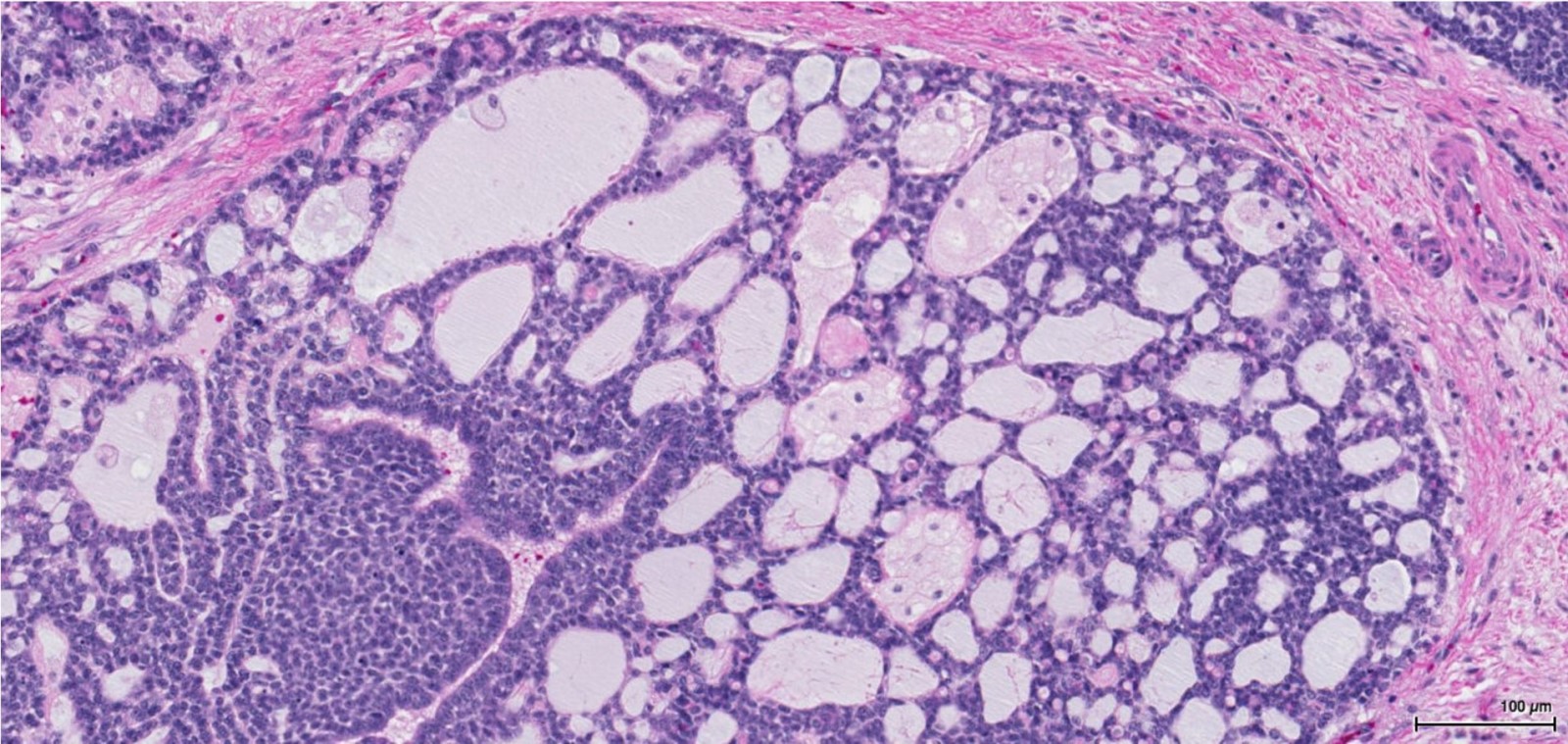
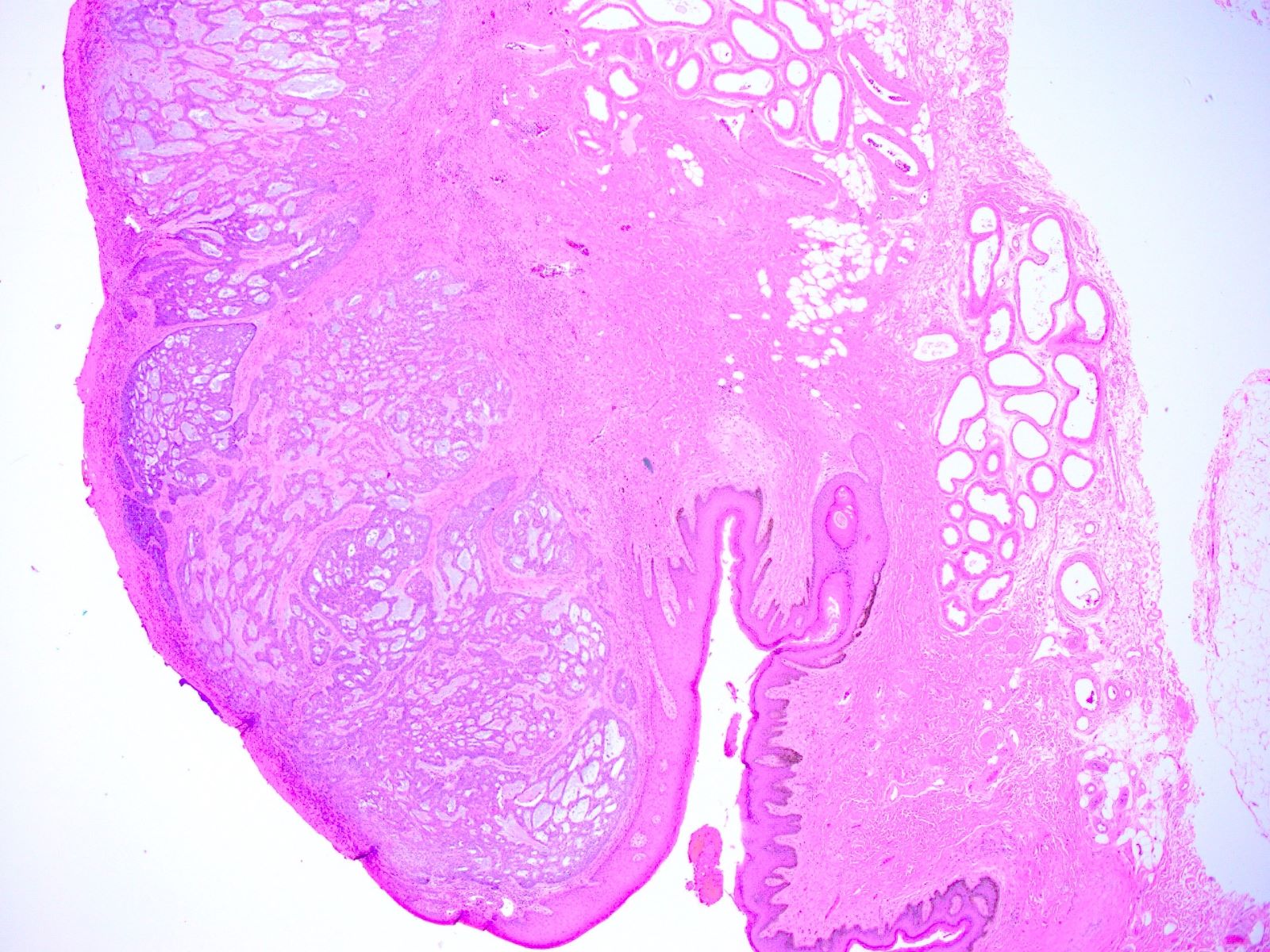
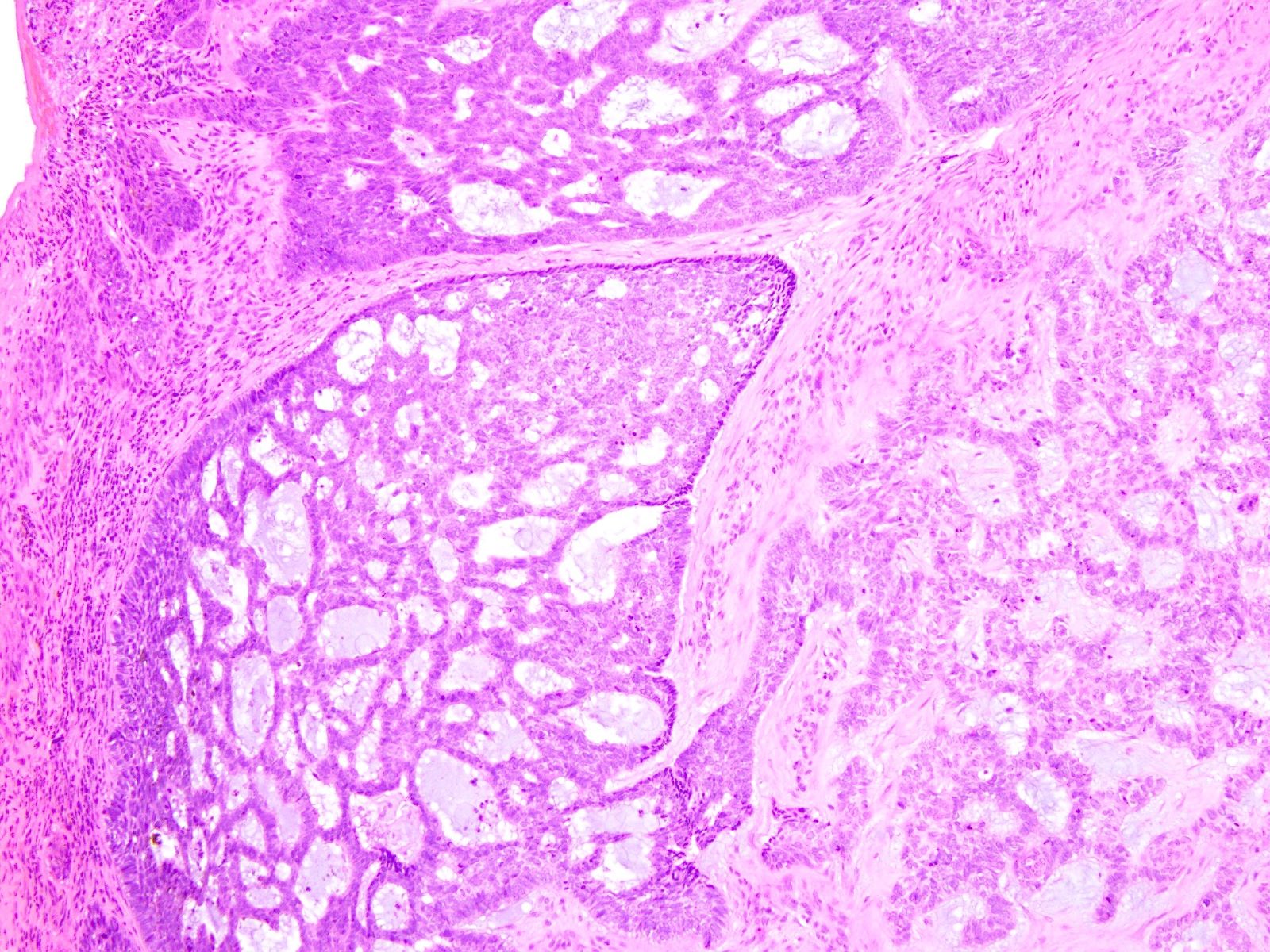
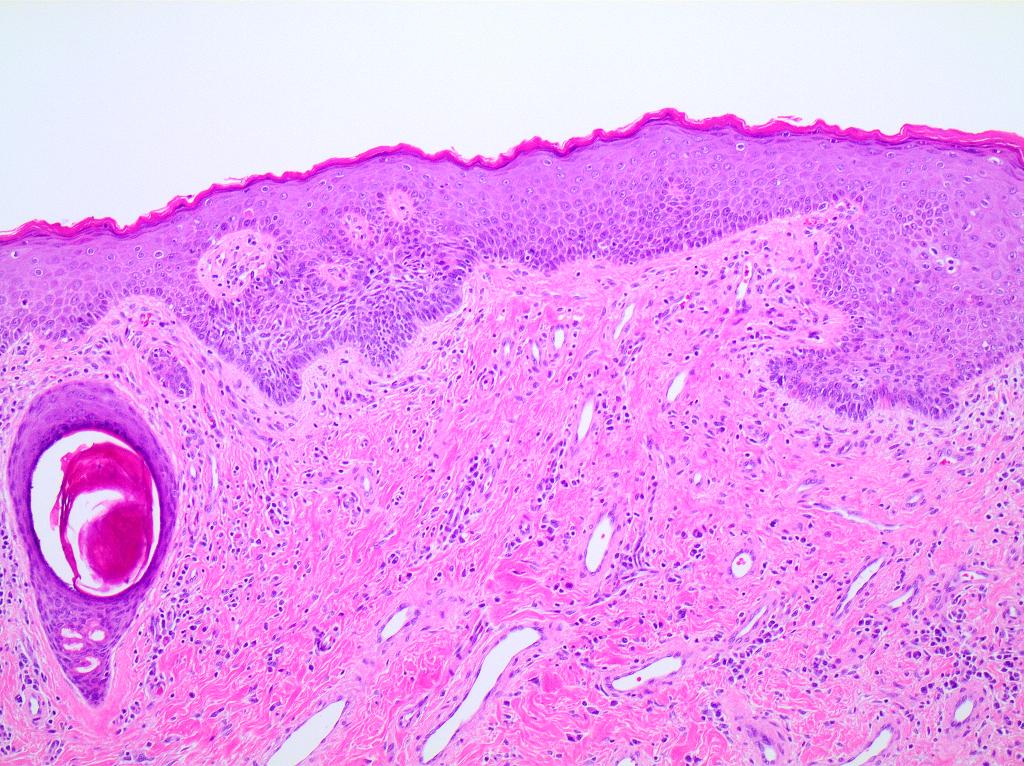
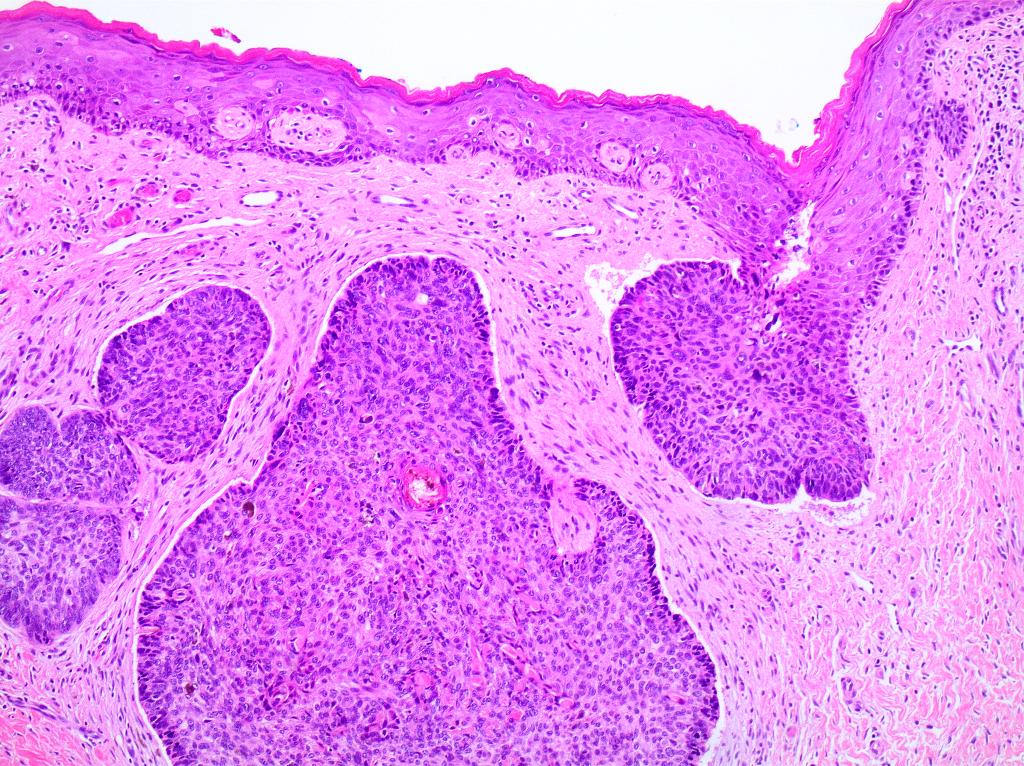
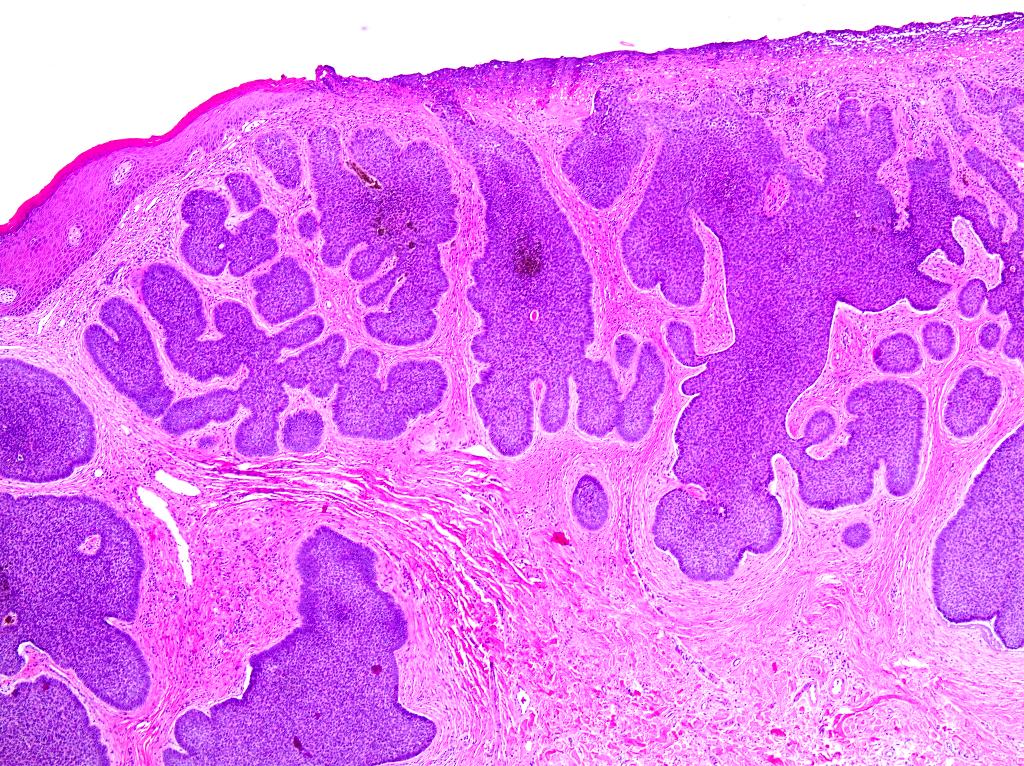
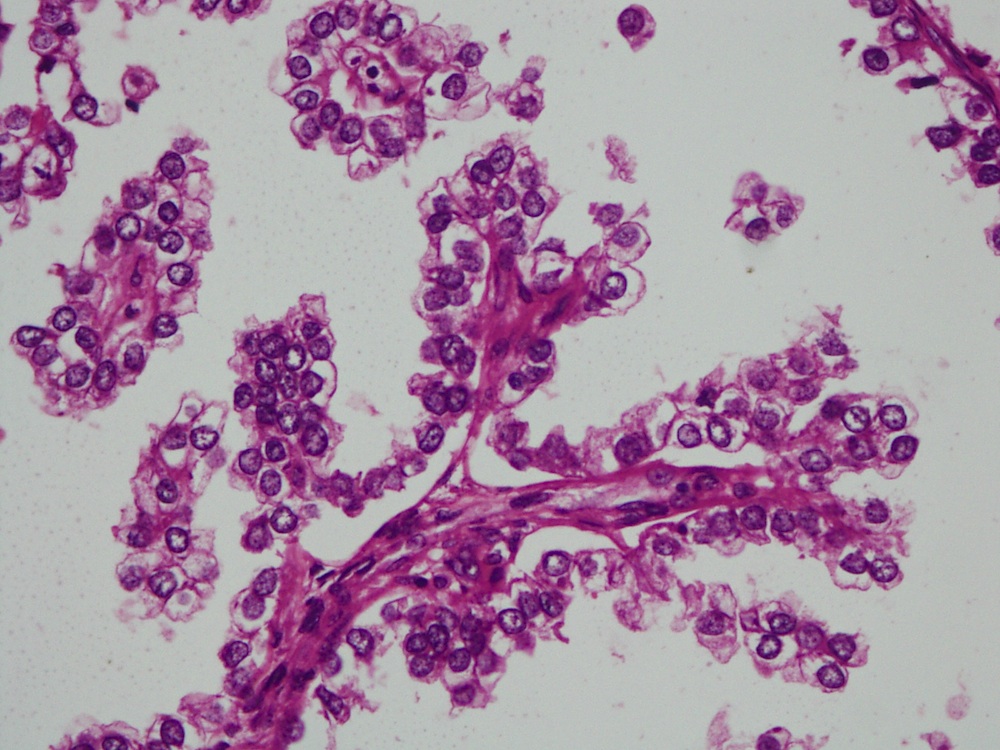
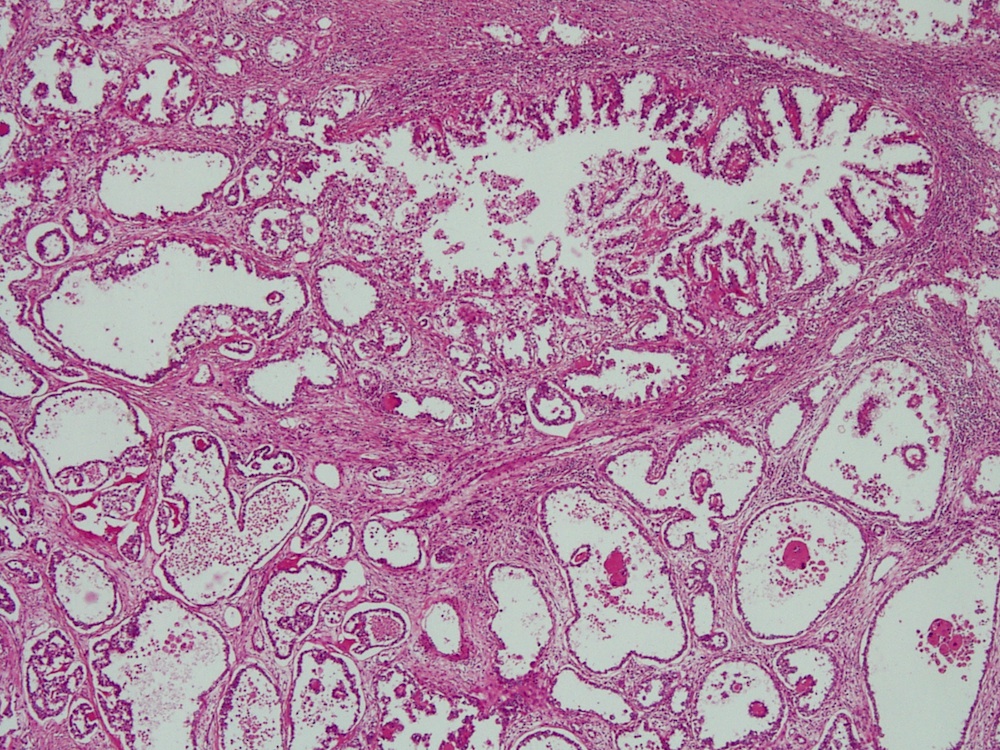
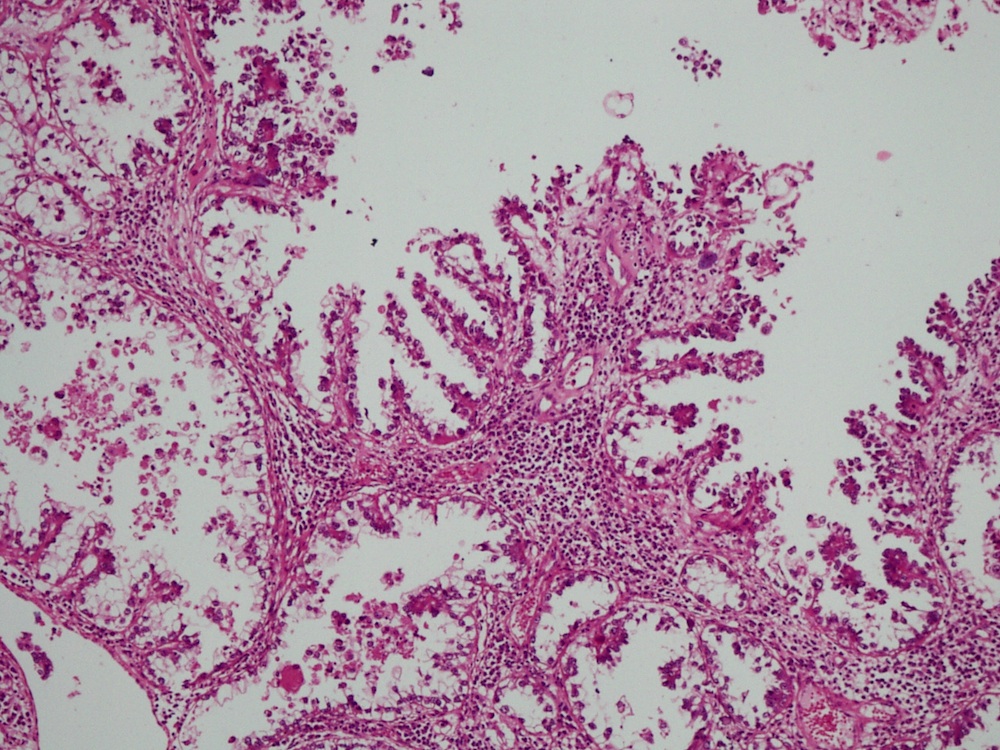
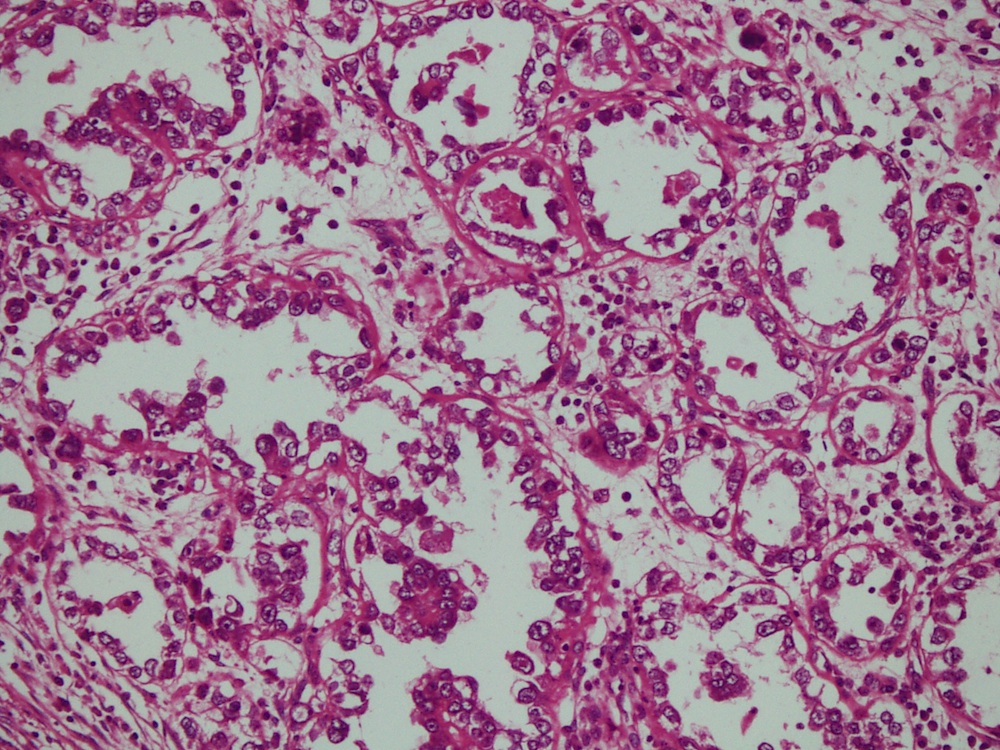
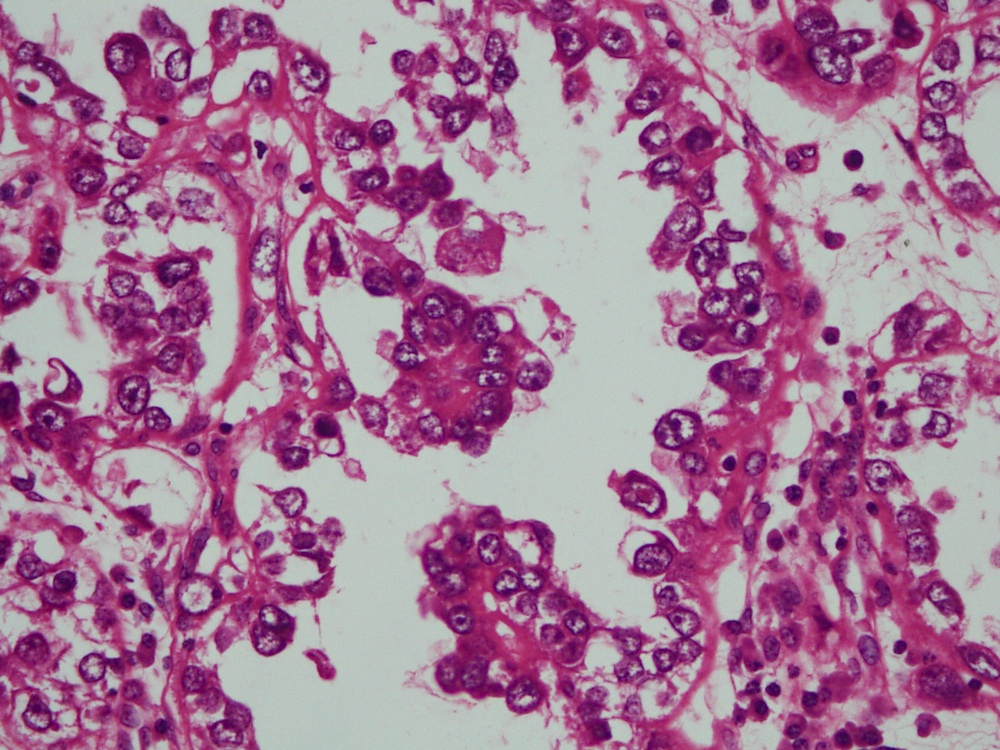
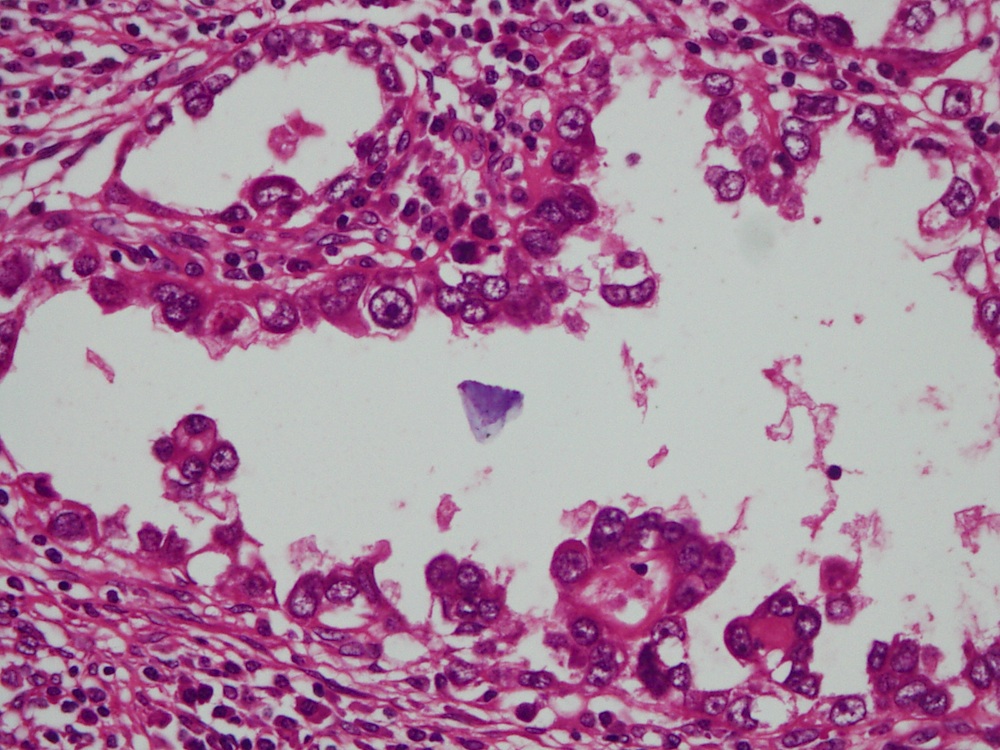
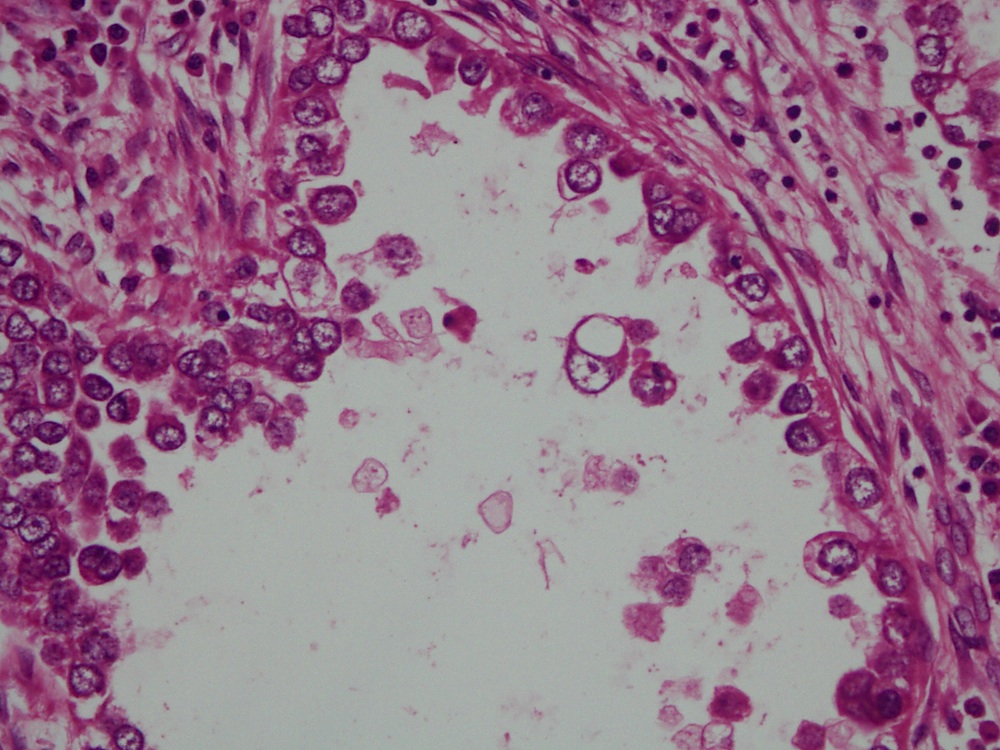
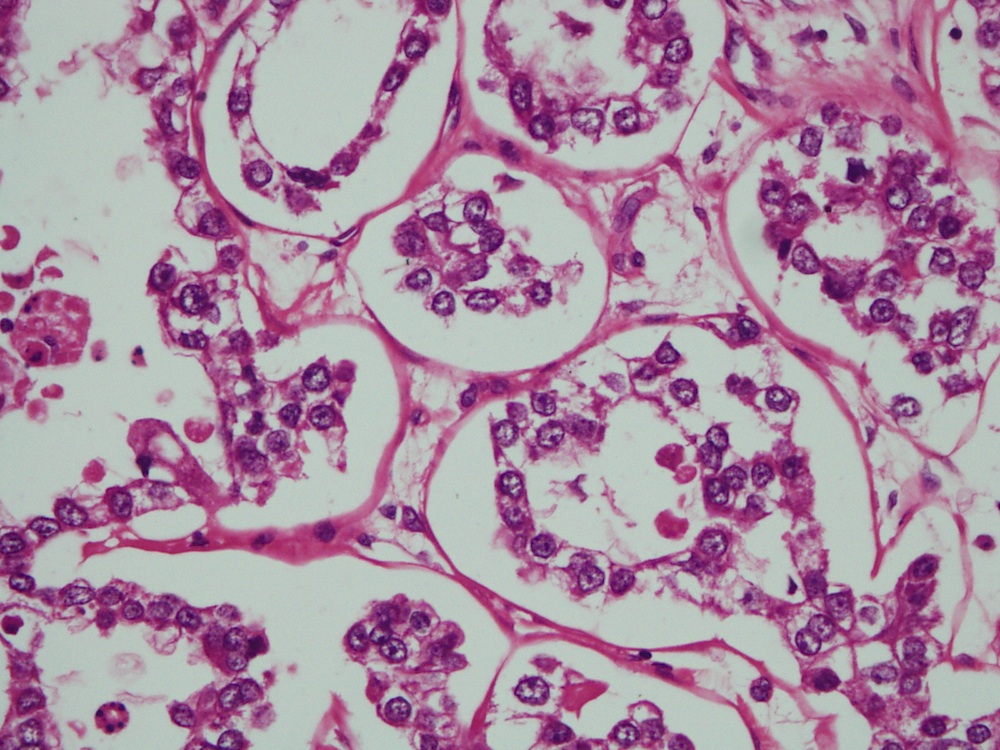
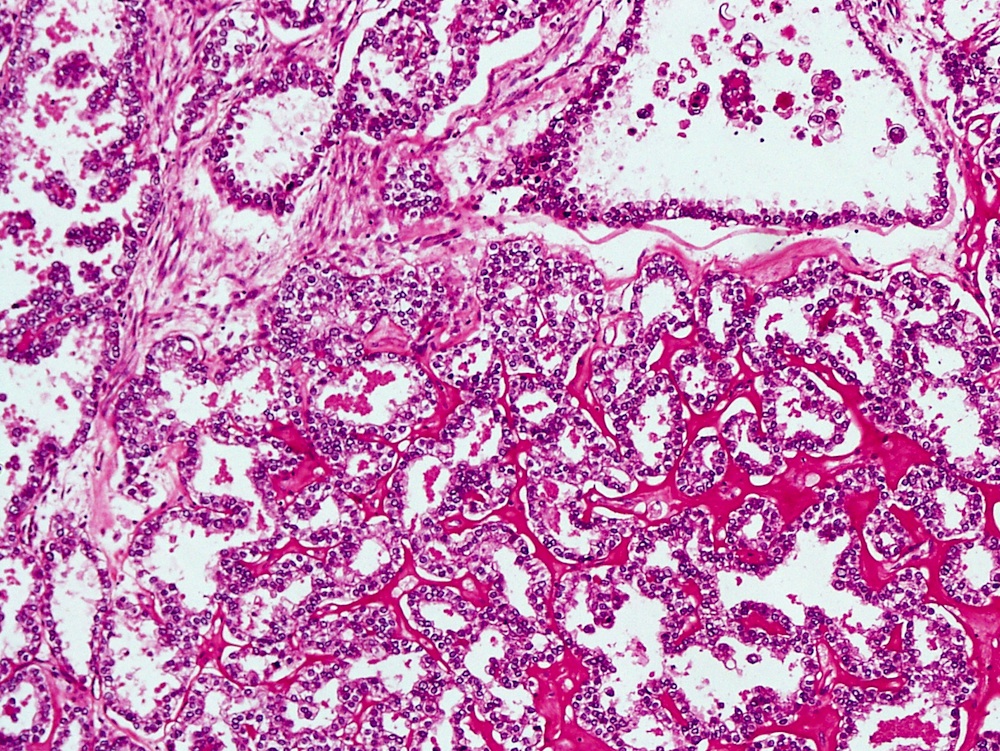
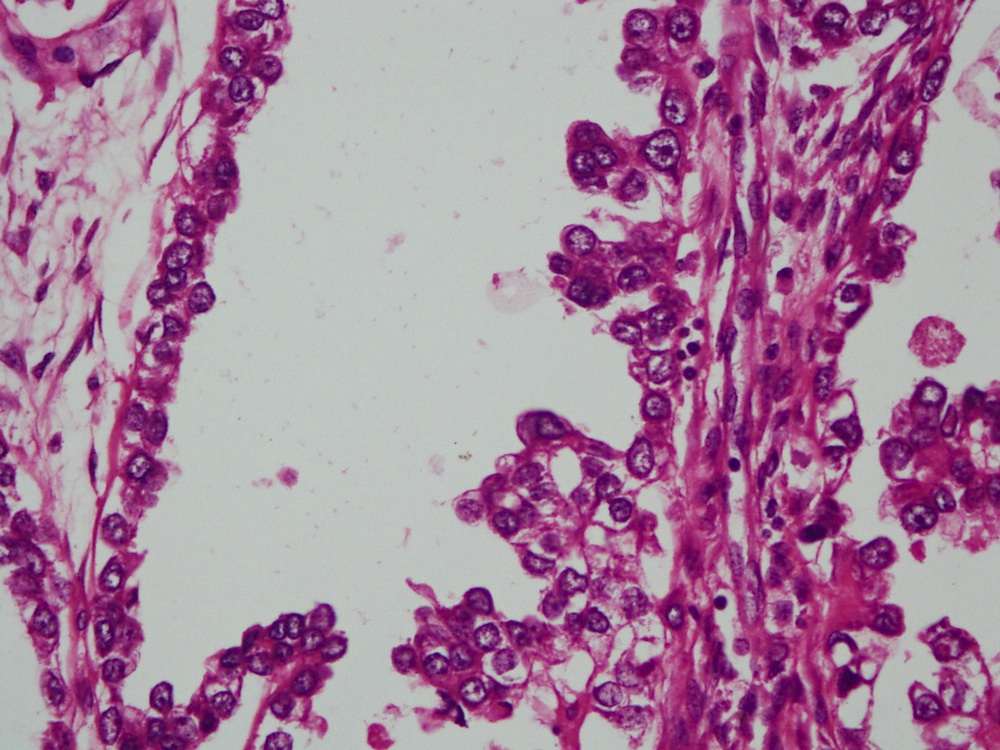
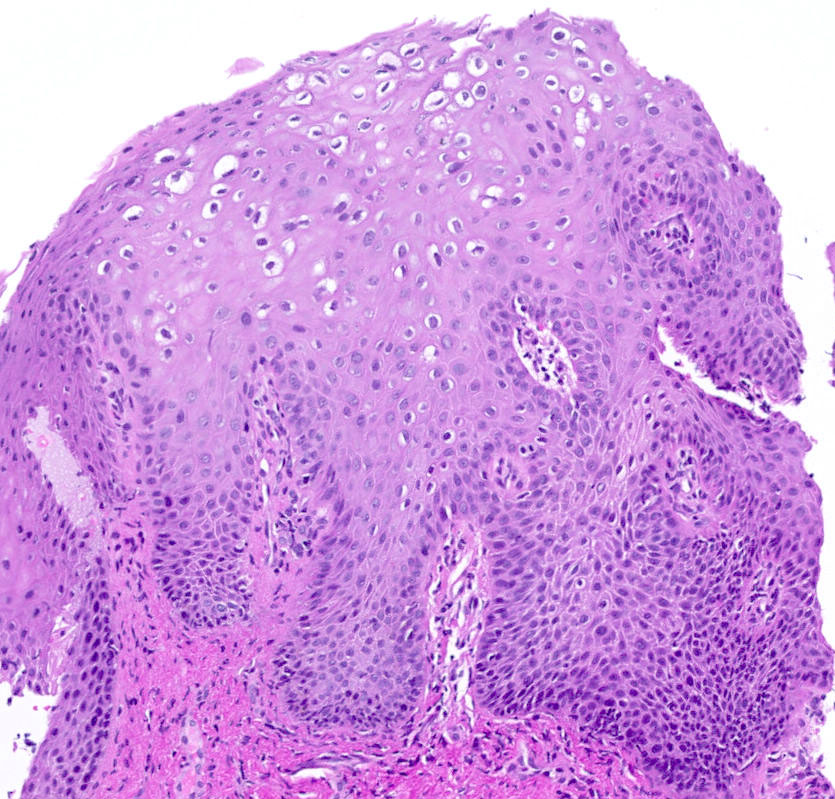
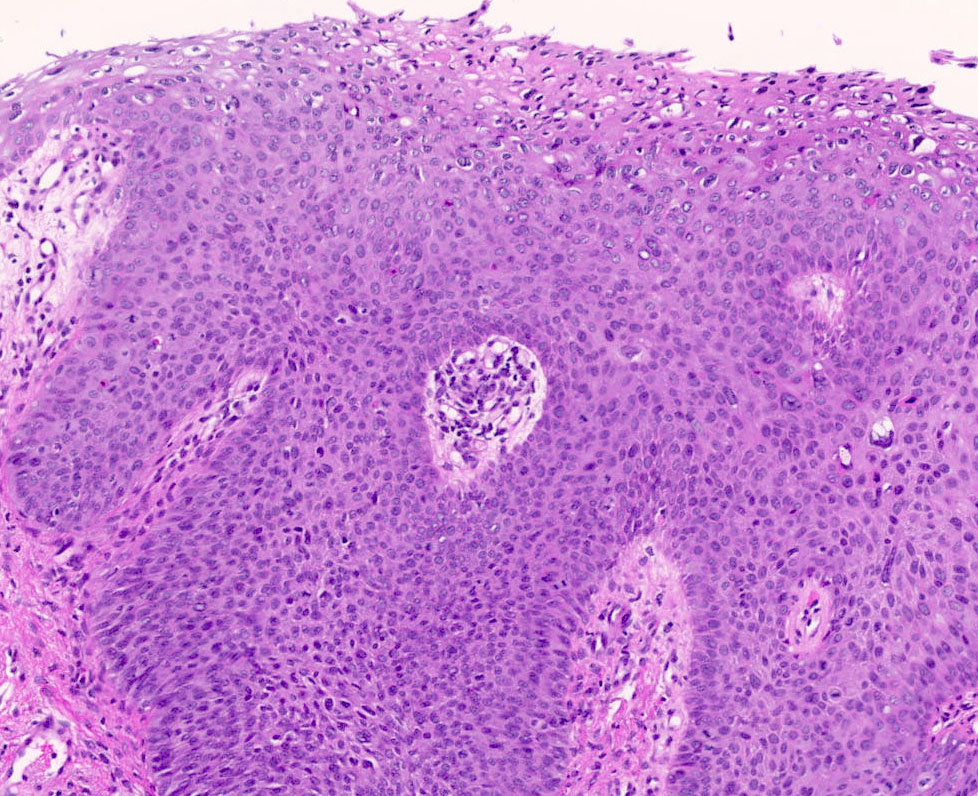
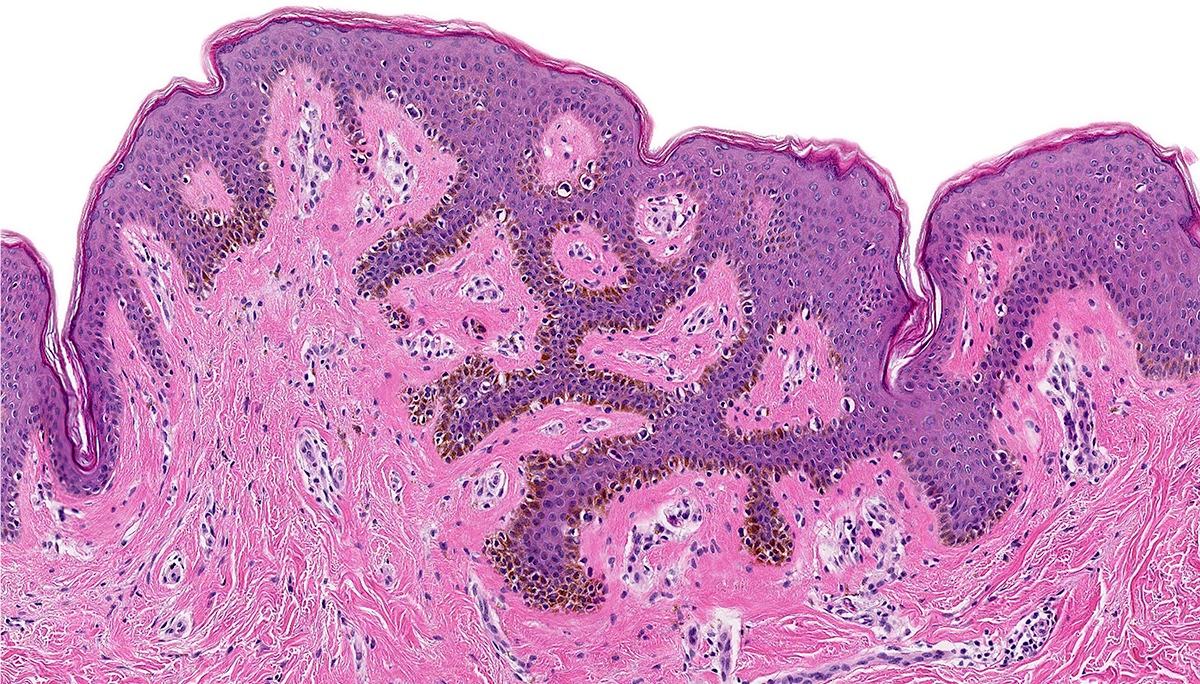
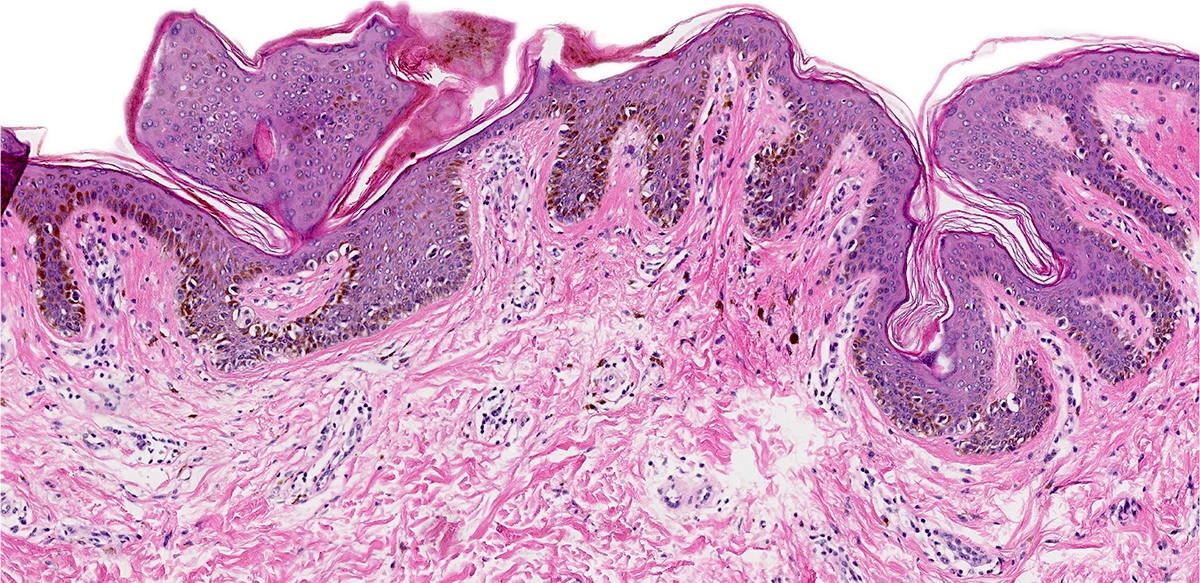
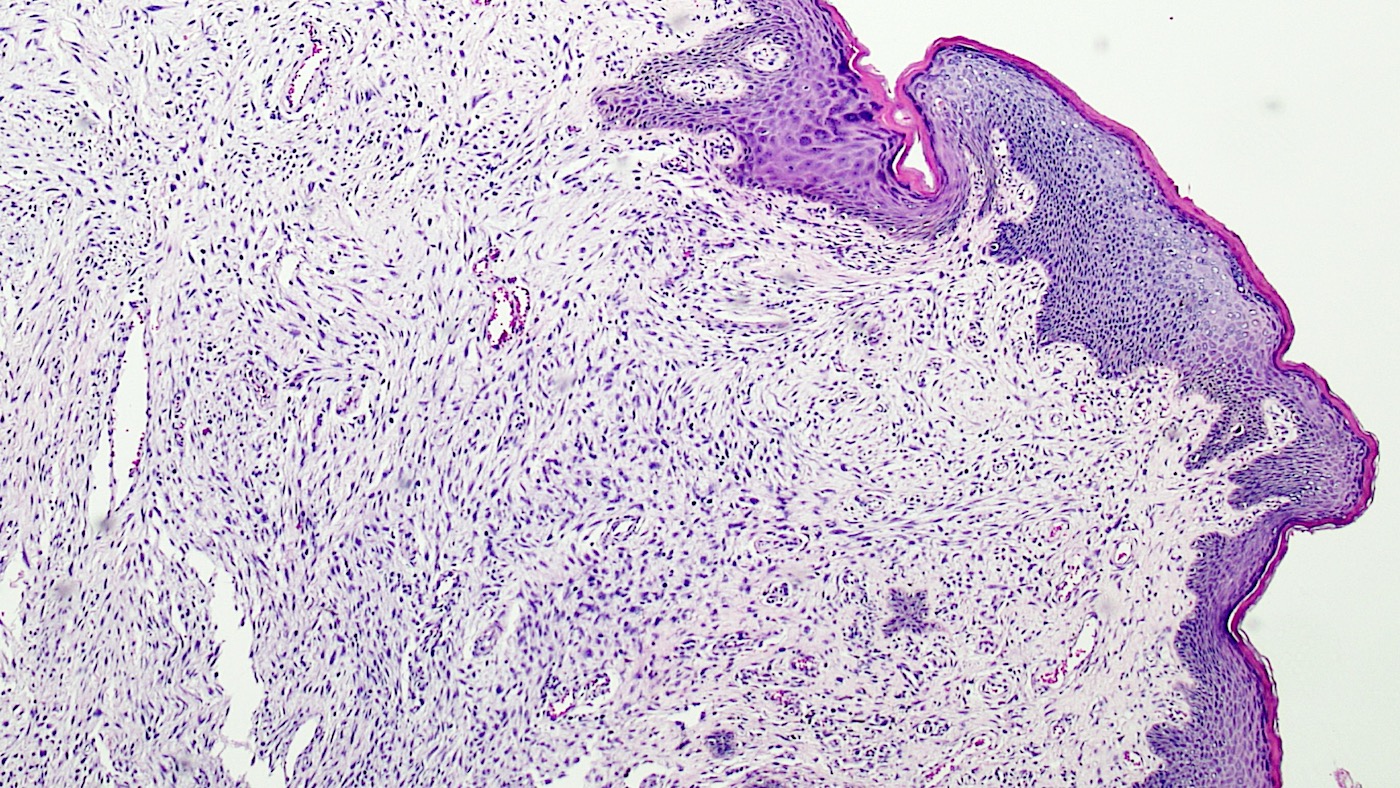
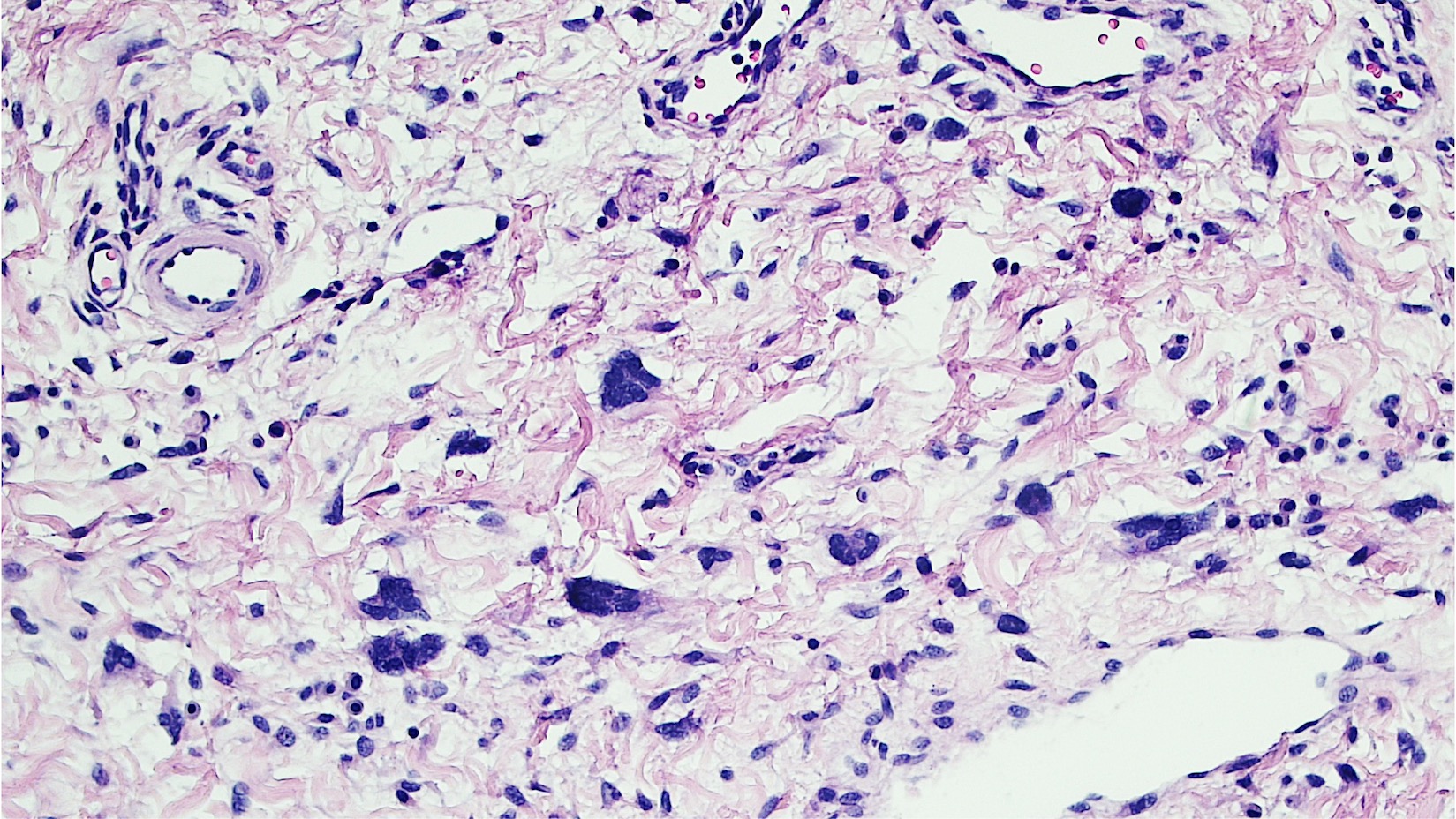
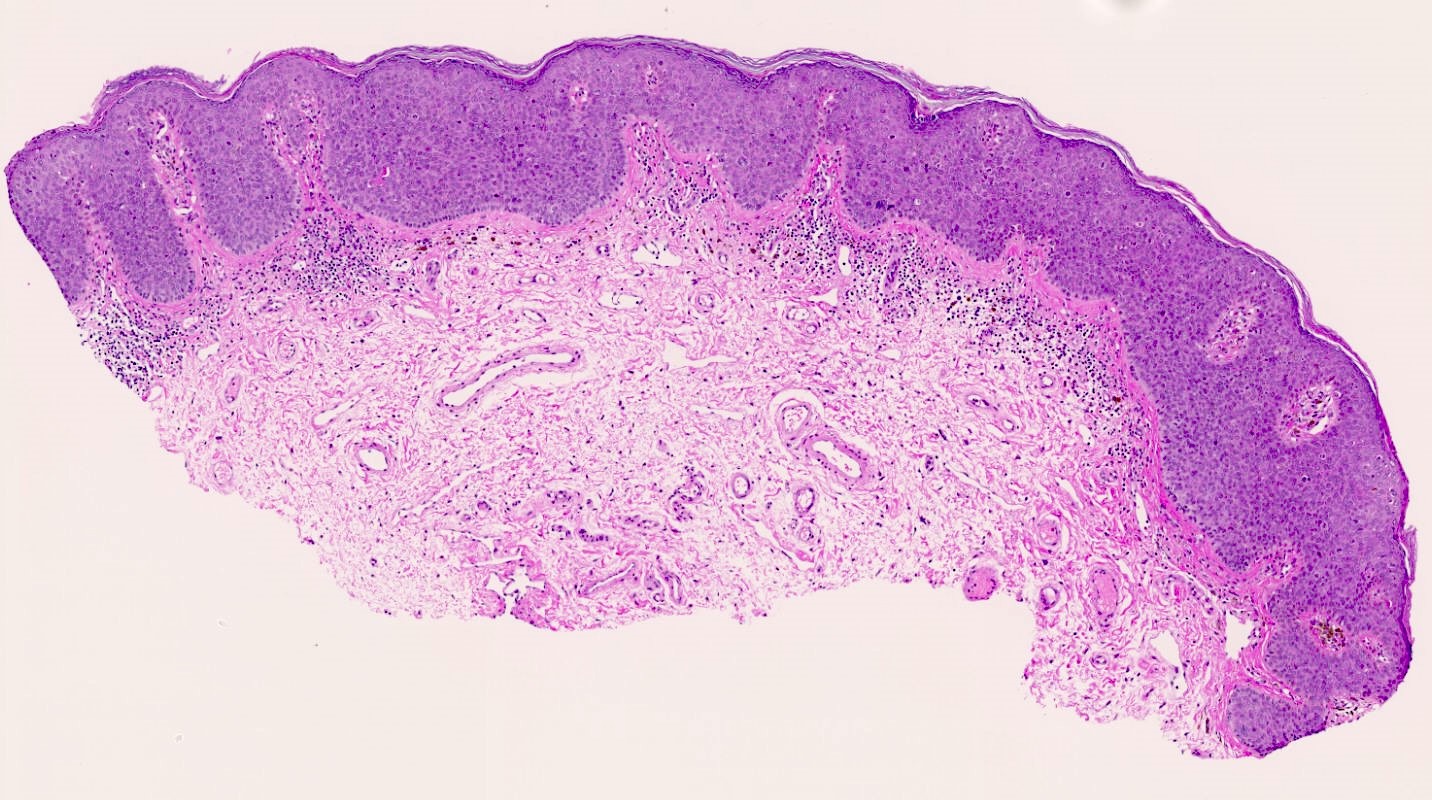
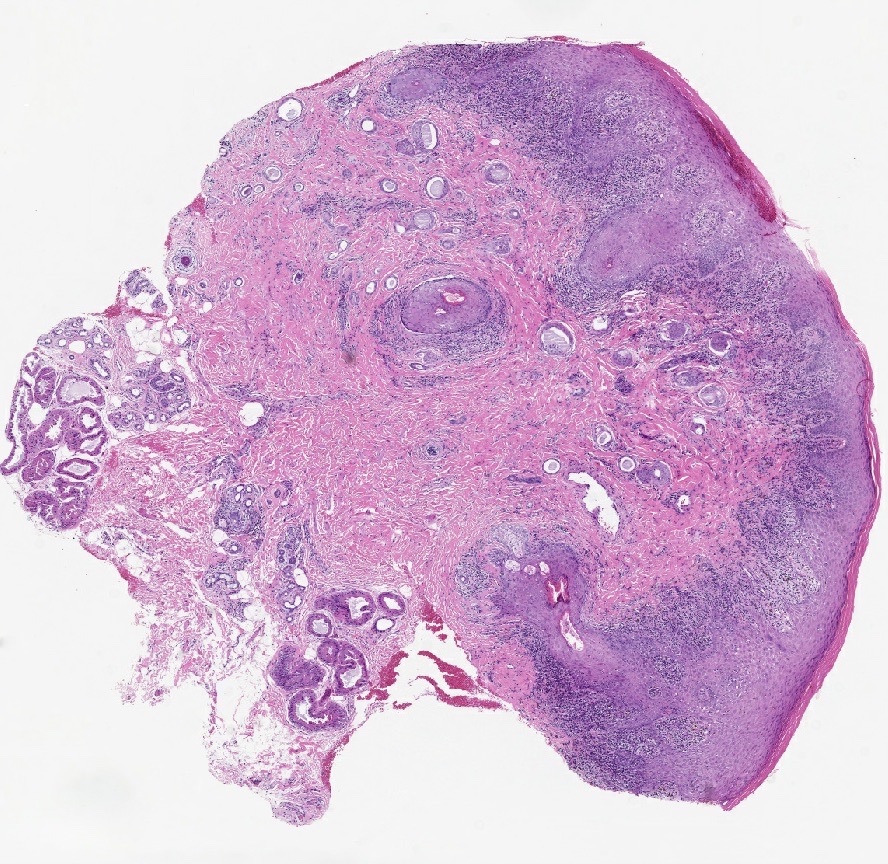
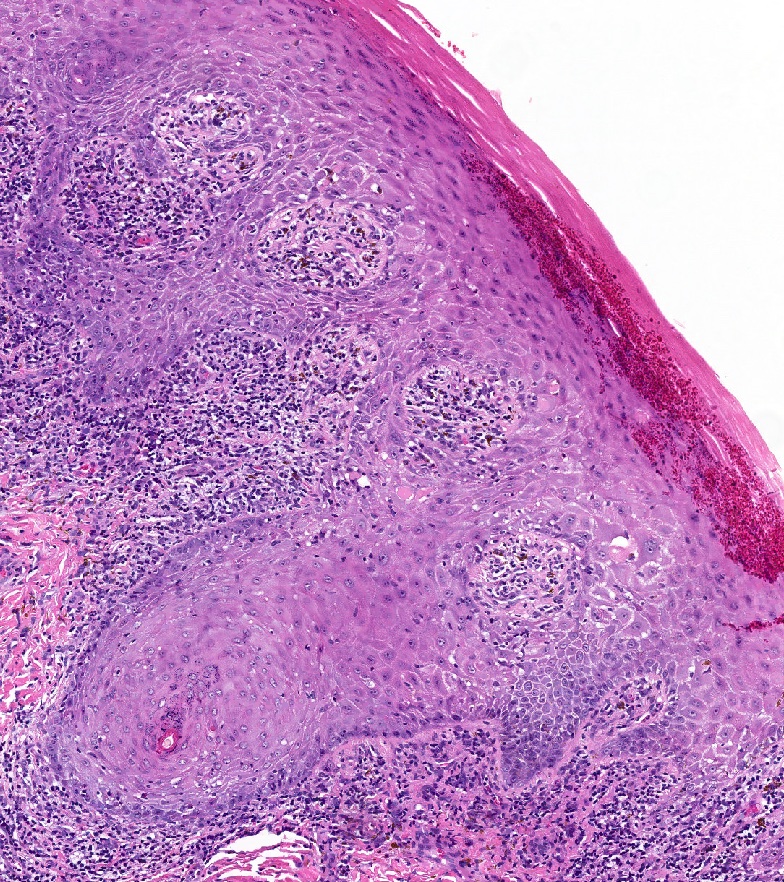
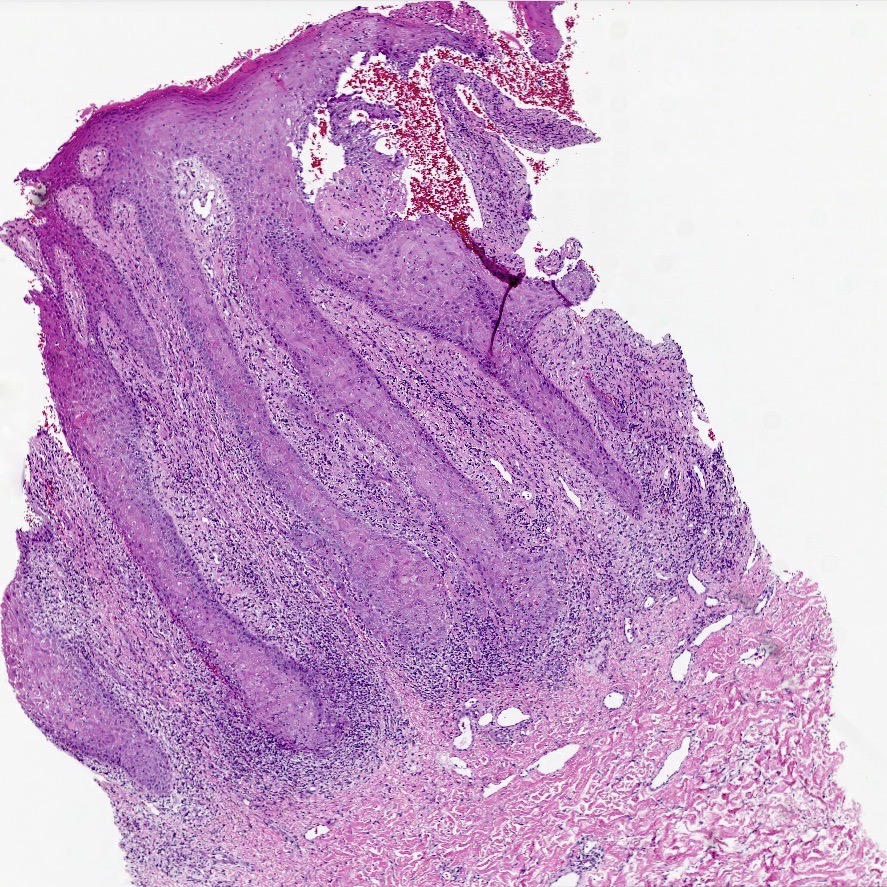
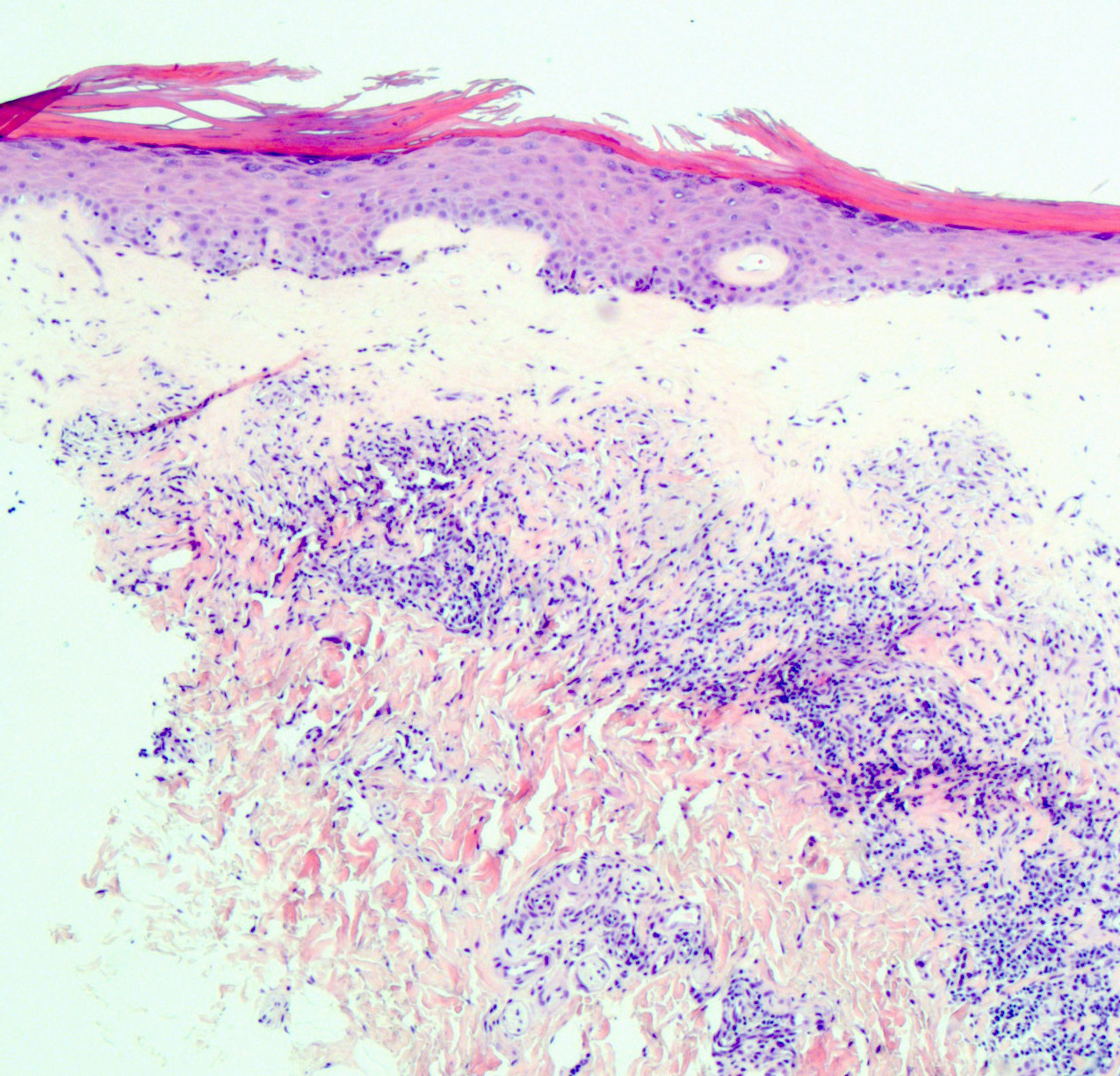
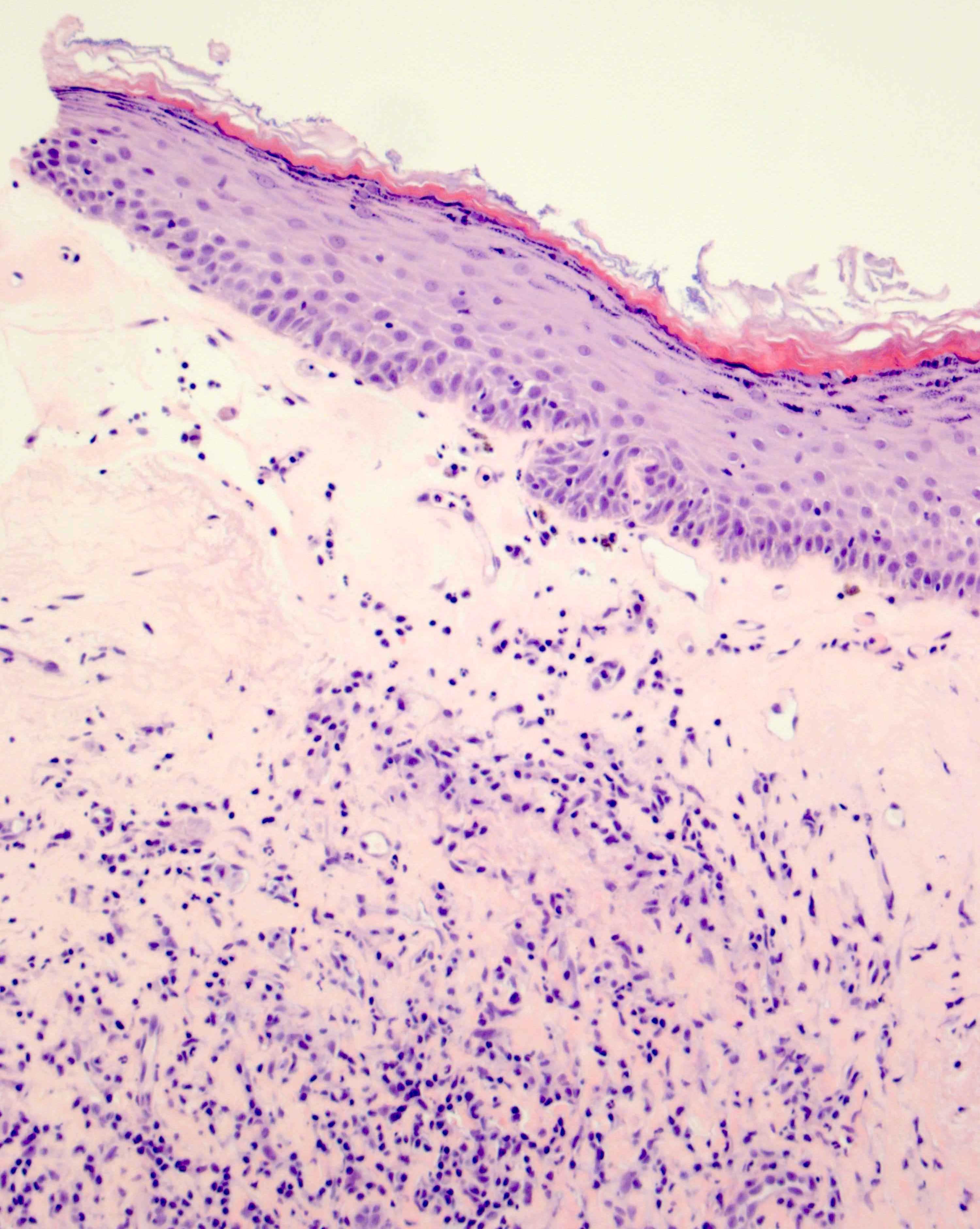
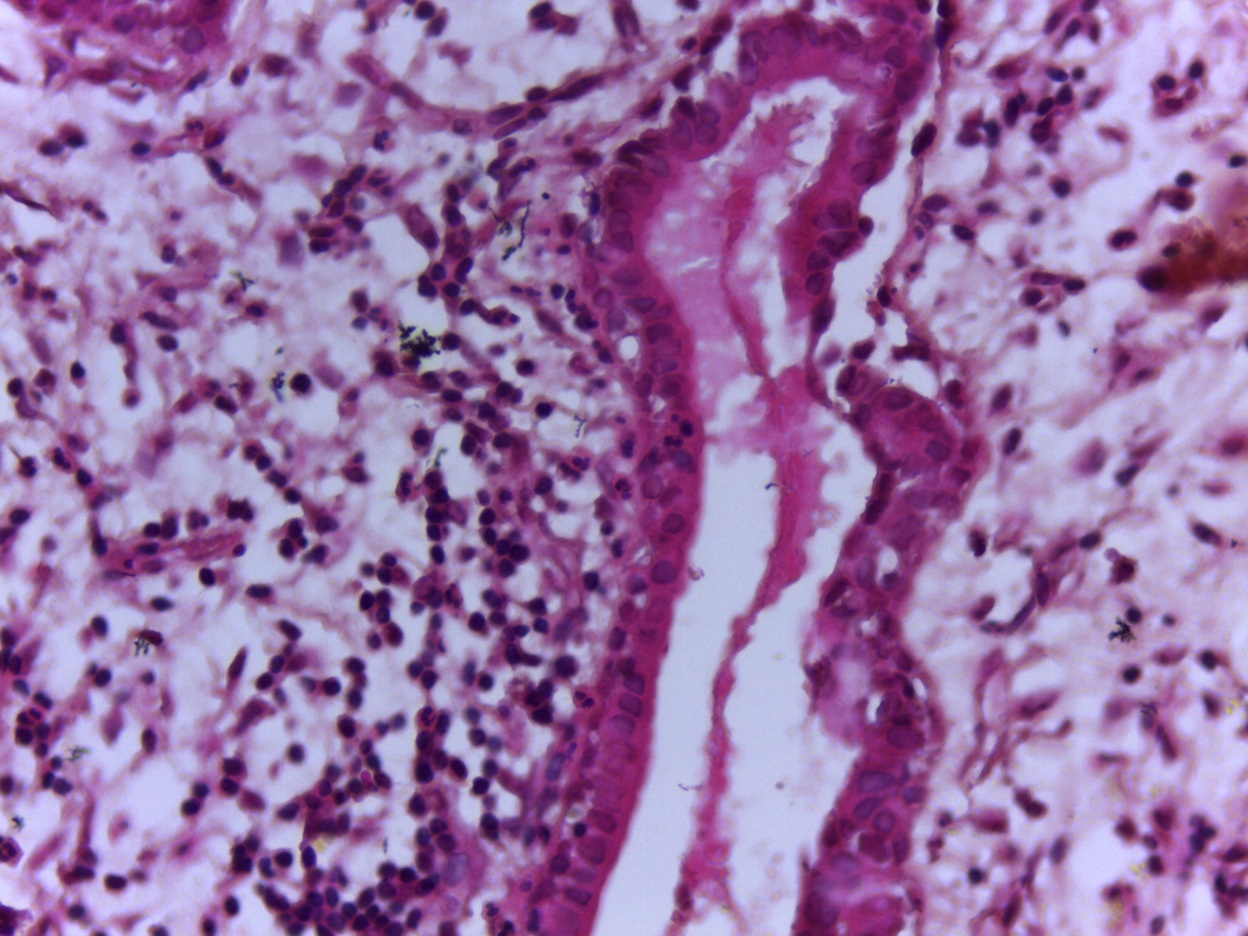
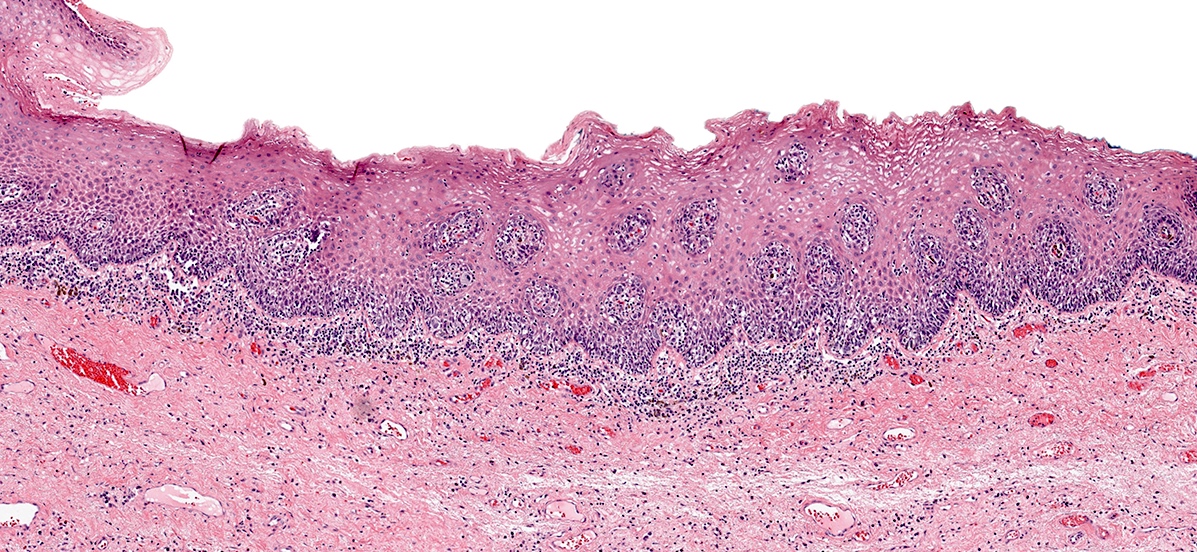
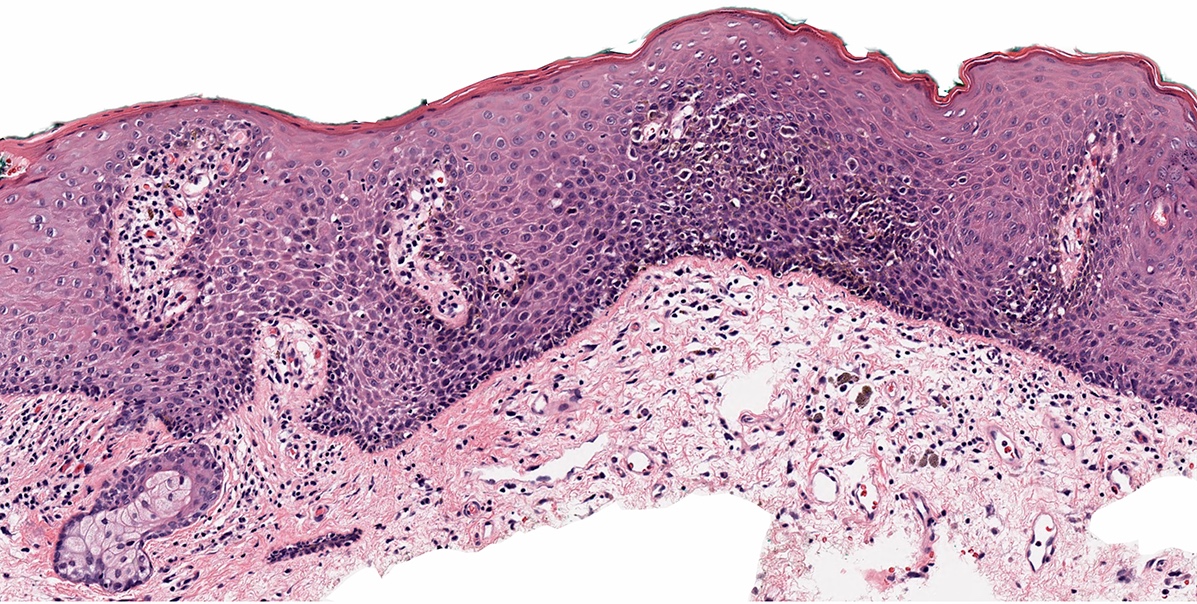
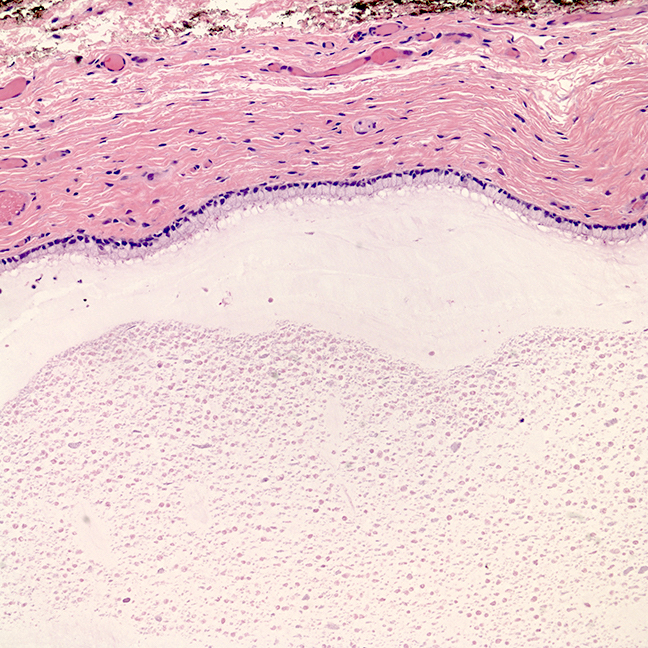
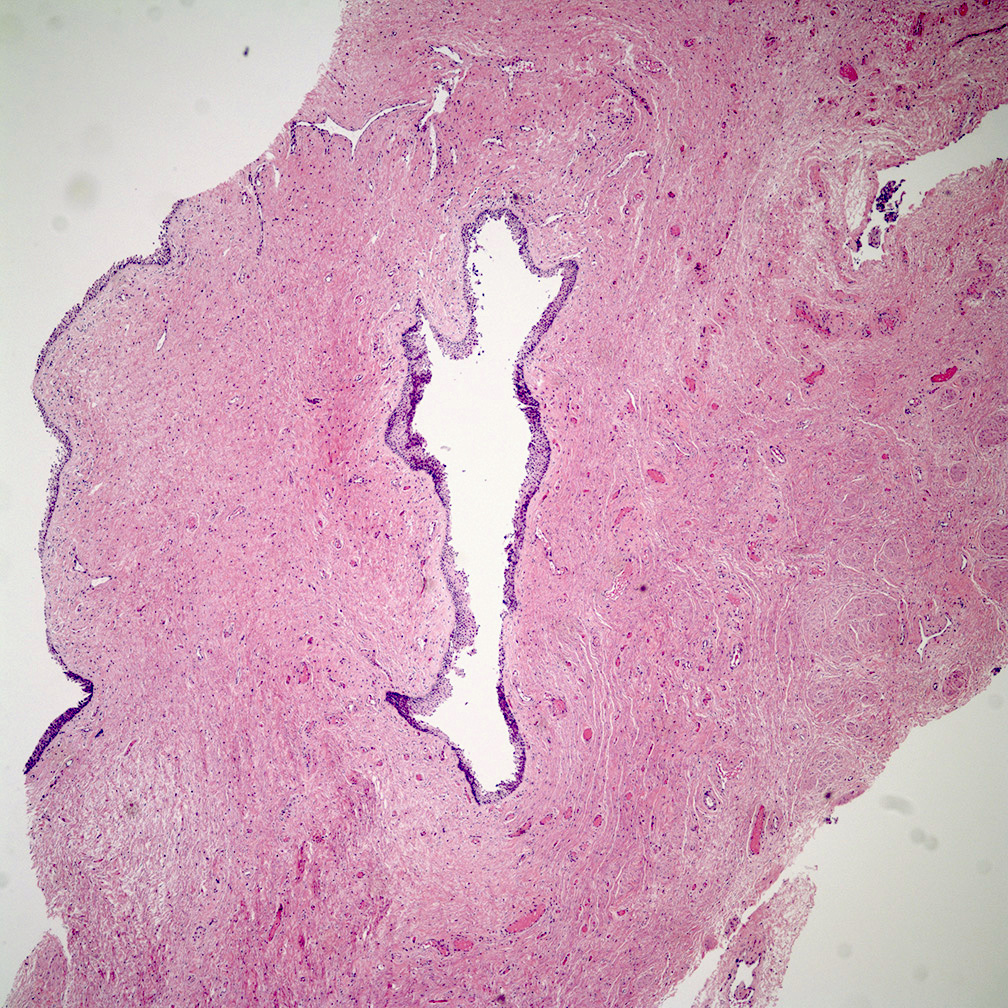
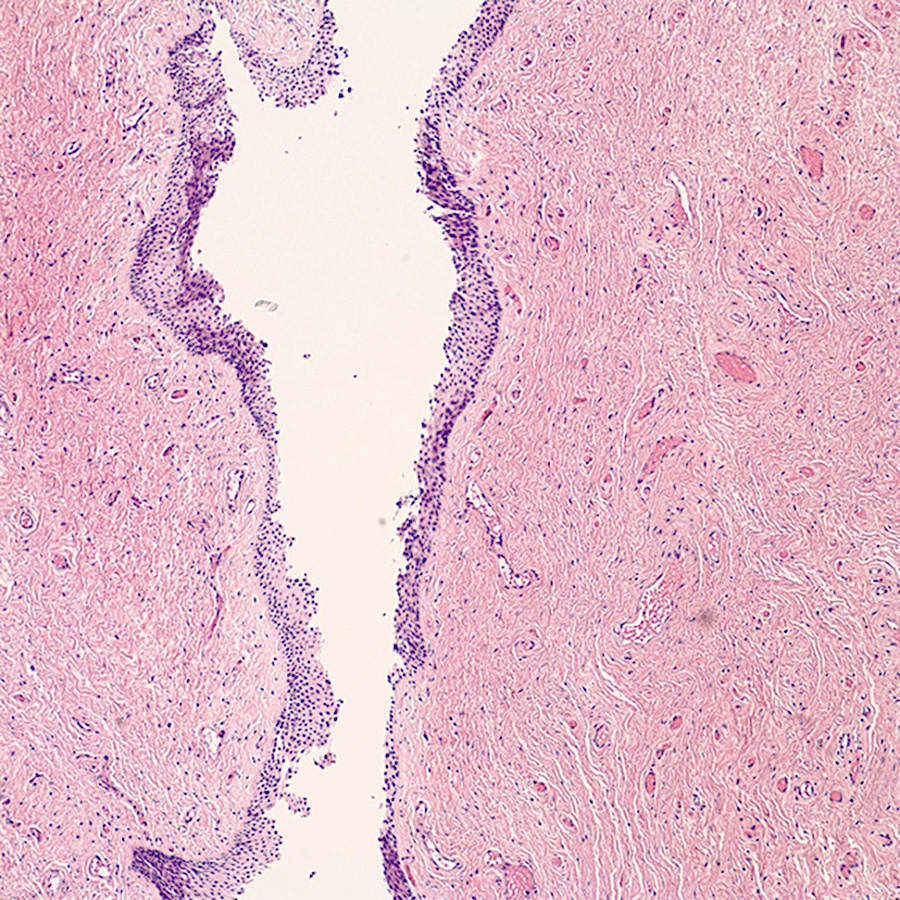
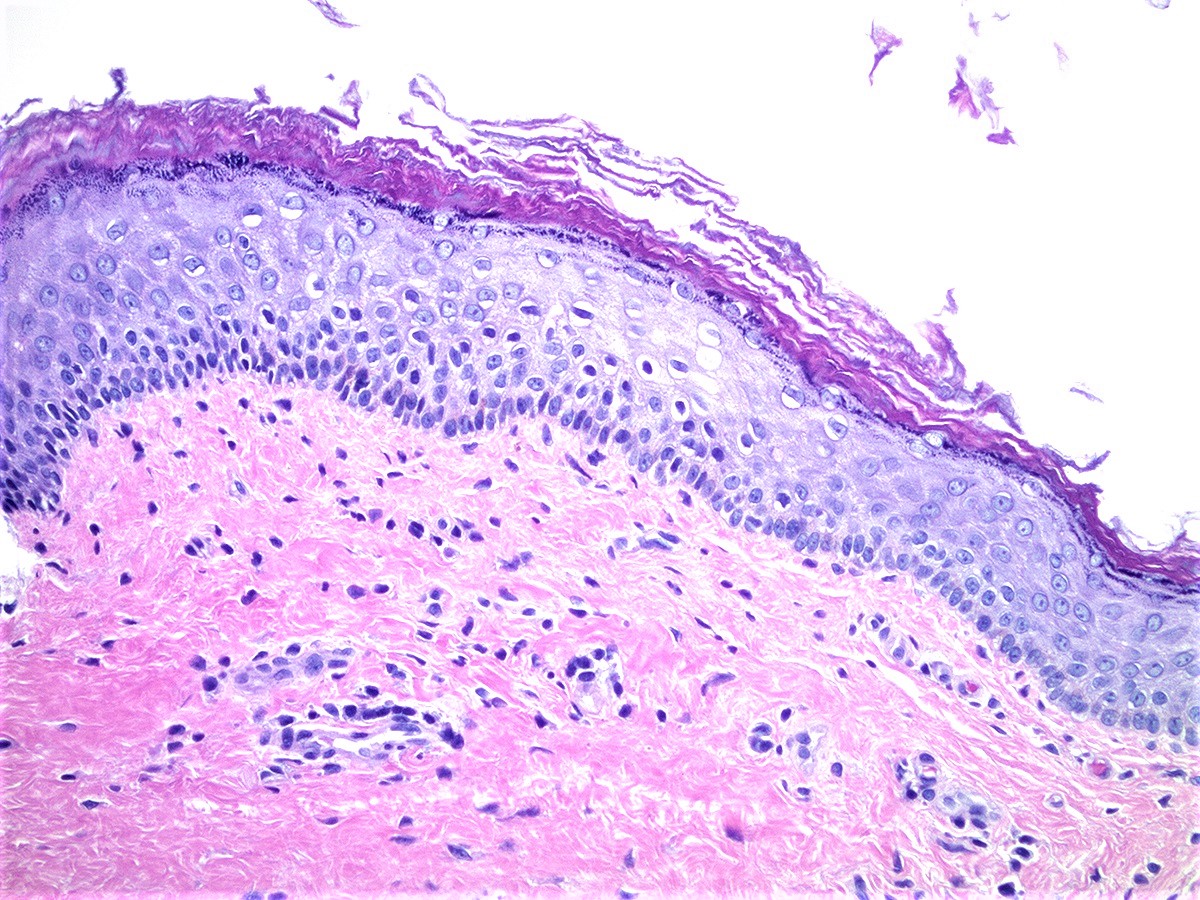
- Mucinous: most common type of adenosis (62% of biopsy specimens); characterized by mucinous columnar cells that resemble those of the normal endocervical mucosa
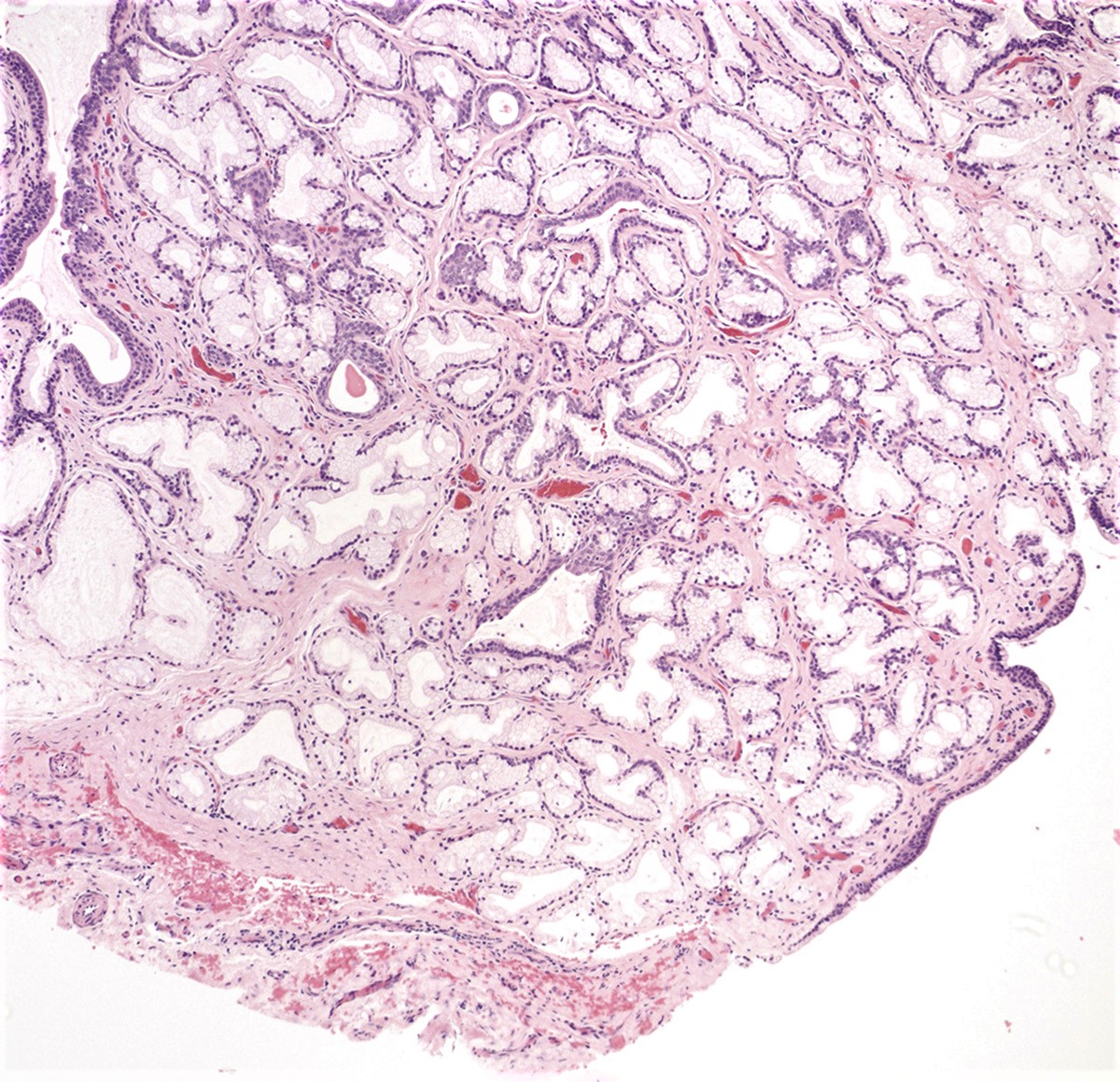
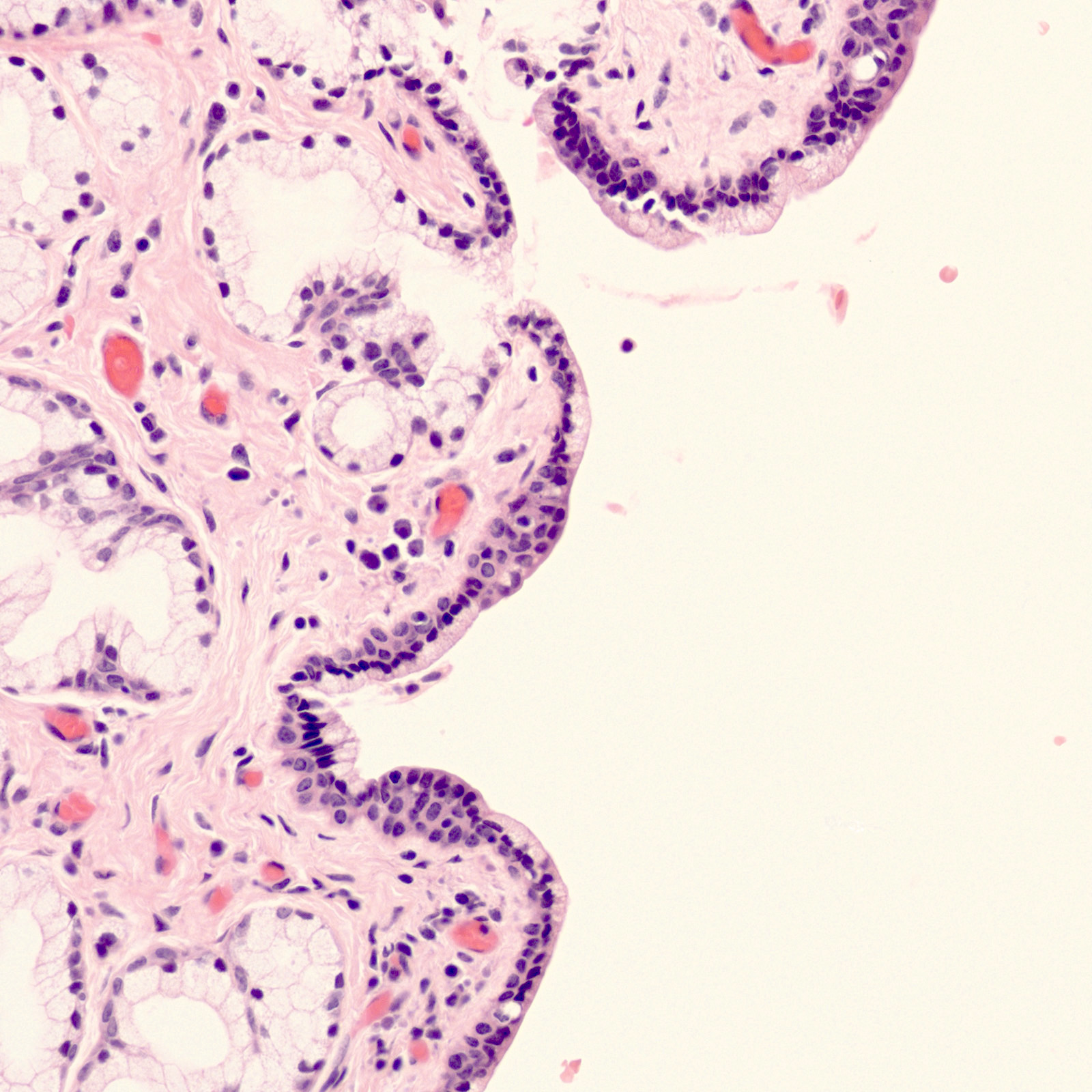
- Tuboendometrial: found in 21% of specimens; glands are lined by light and dark cells, often ciliated and resemble fallopian tube and endometrial gland cells
- Embryonic: glands composed of low columnar or cuboid cells
- Glands may be simple, complex, cystic or papillary and usually found in the lamina propria but also on the mucosal surface
- Cysts lined by a single layer of columnar mucinous cells may resemble cervical nabothian cysts
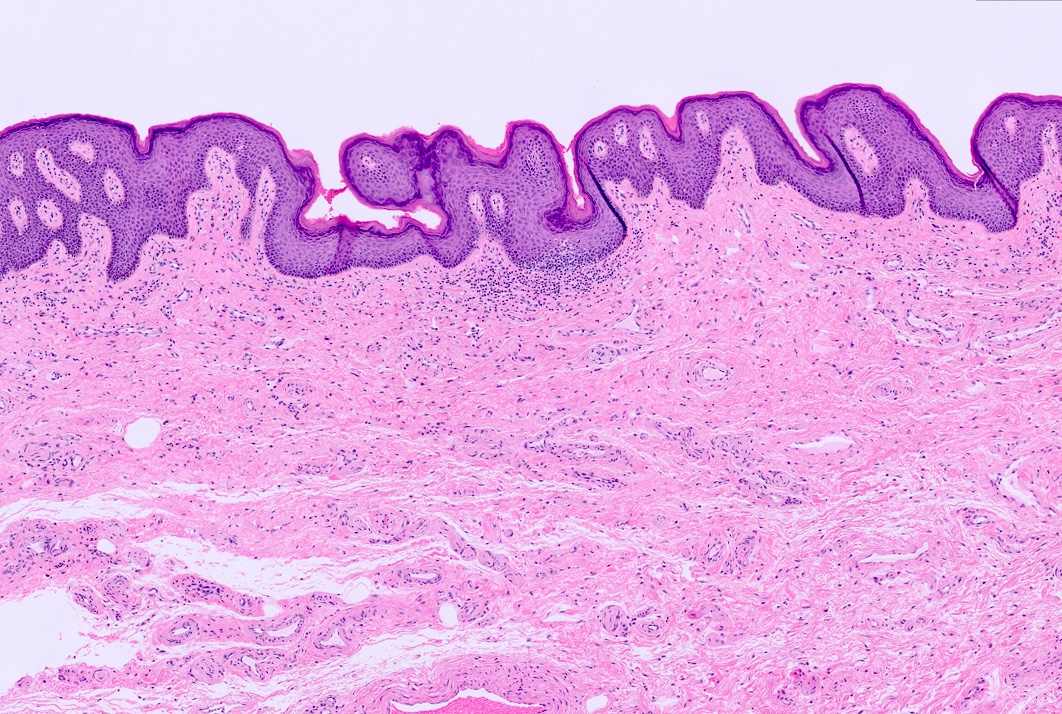
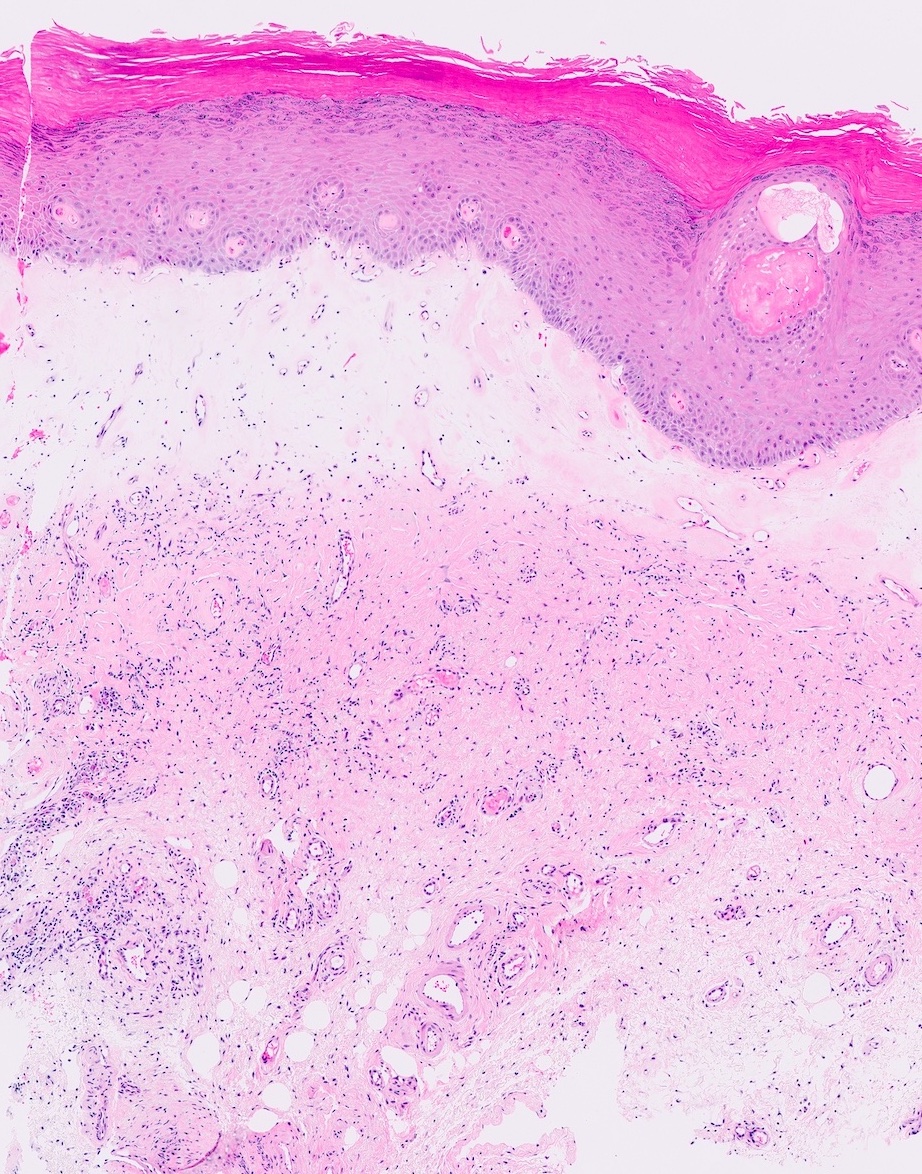
- Squamous metaplasia can be present and represents the process by which adenosis transforms and heals
- Rarely, intestinal metaplasia may be seen
- References: Mutter: Pathology of the Female Reproductive Tract, 3rd Edition, 2014, Kurman: Blaustein's Pathology of the Female Genital Tract, 6th Edition, 2011
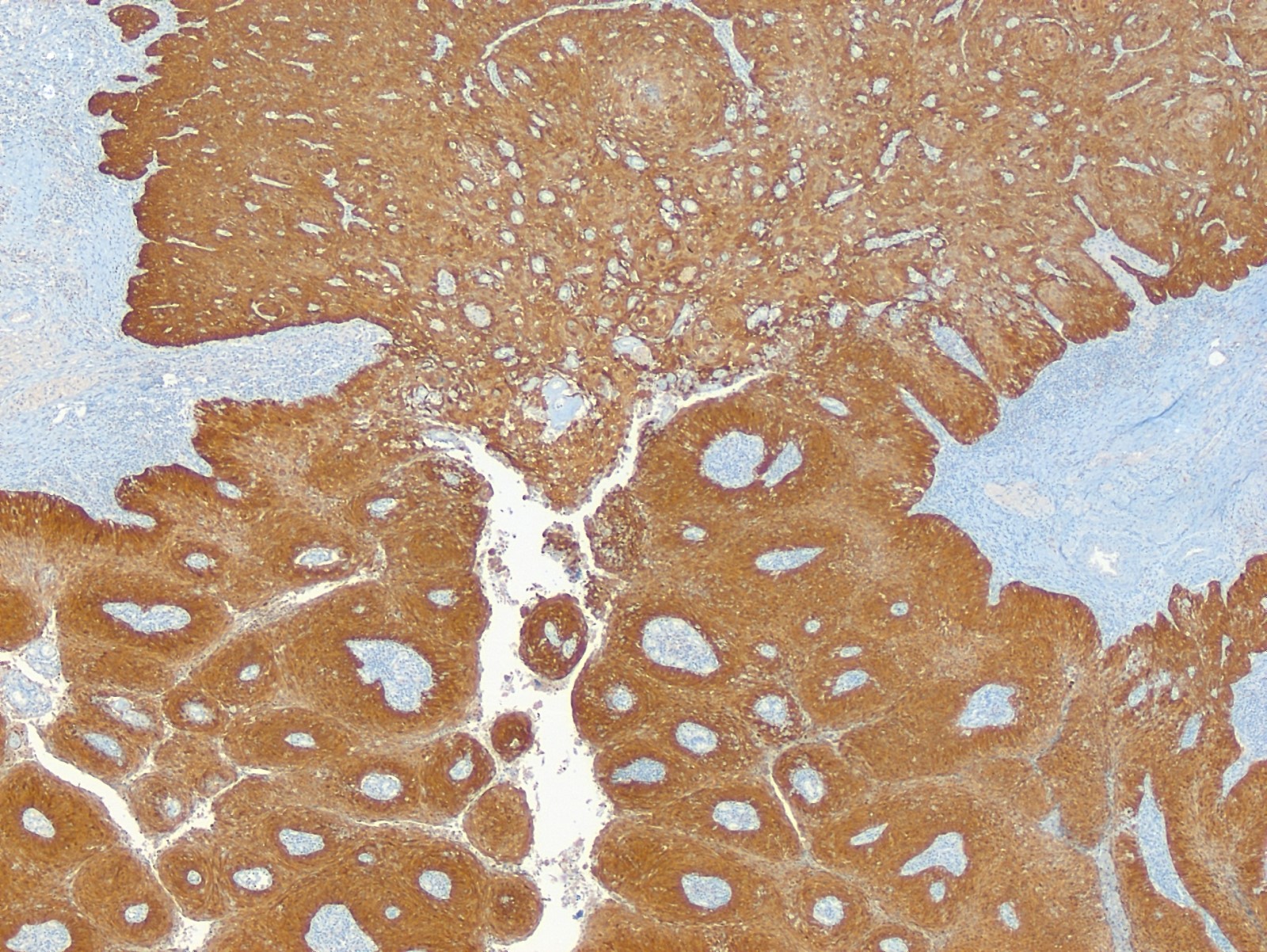
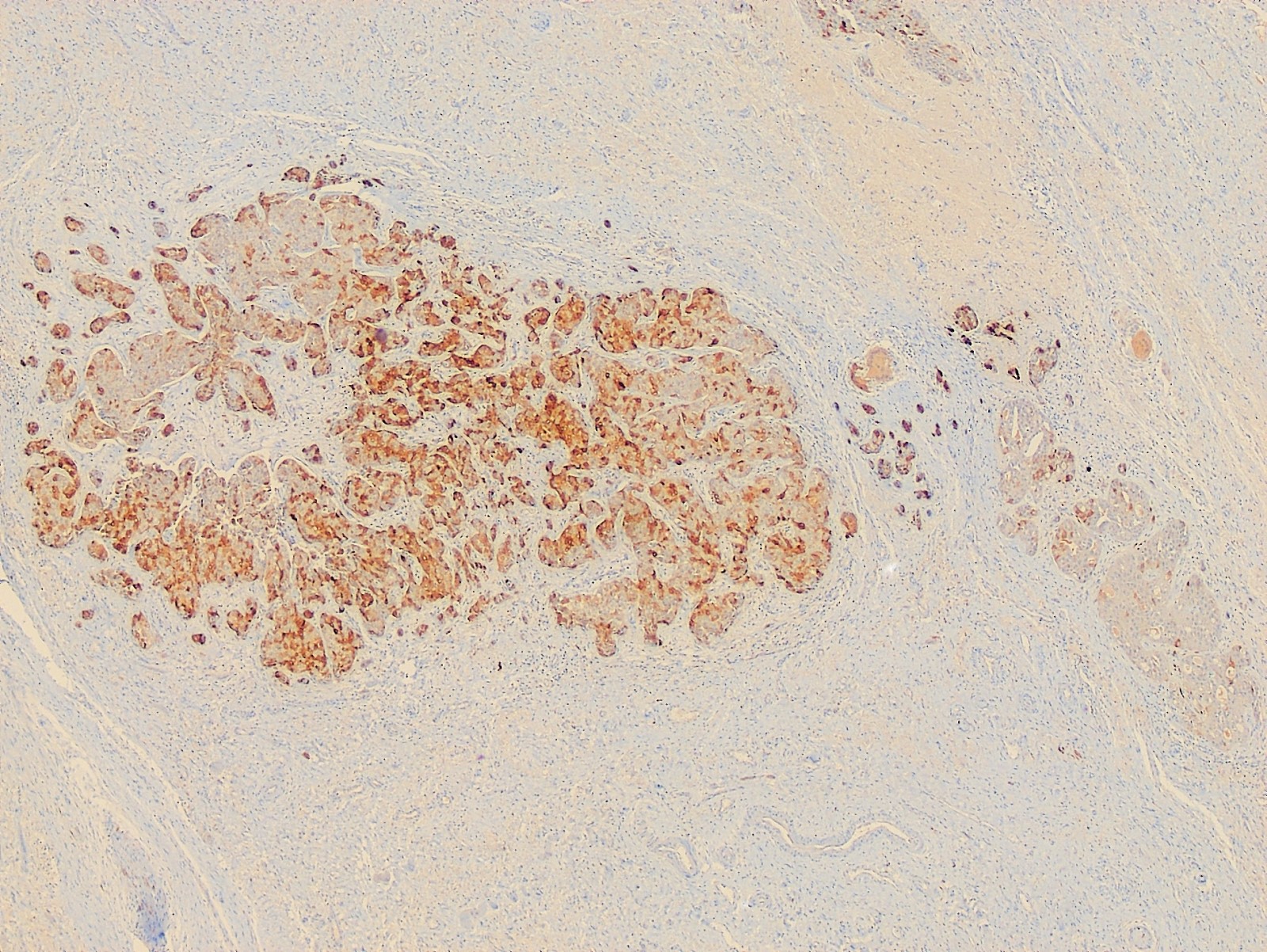
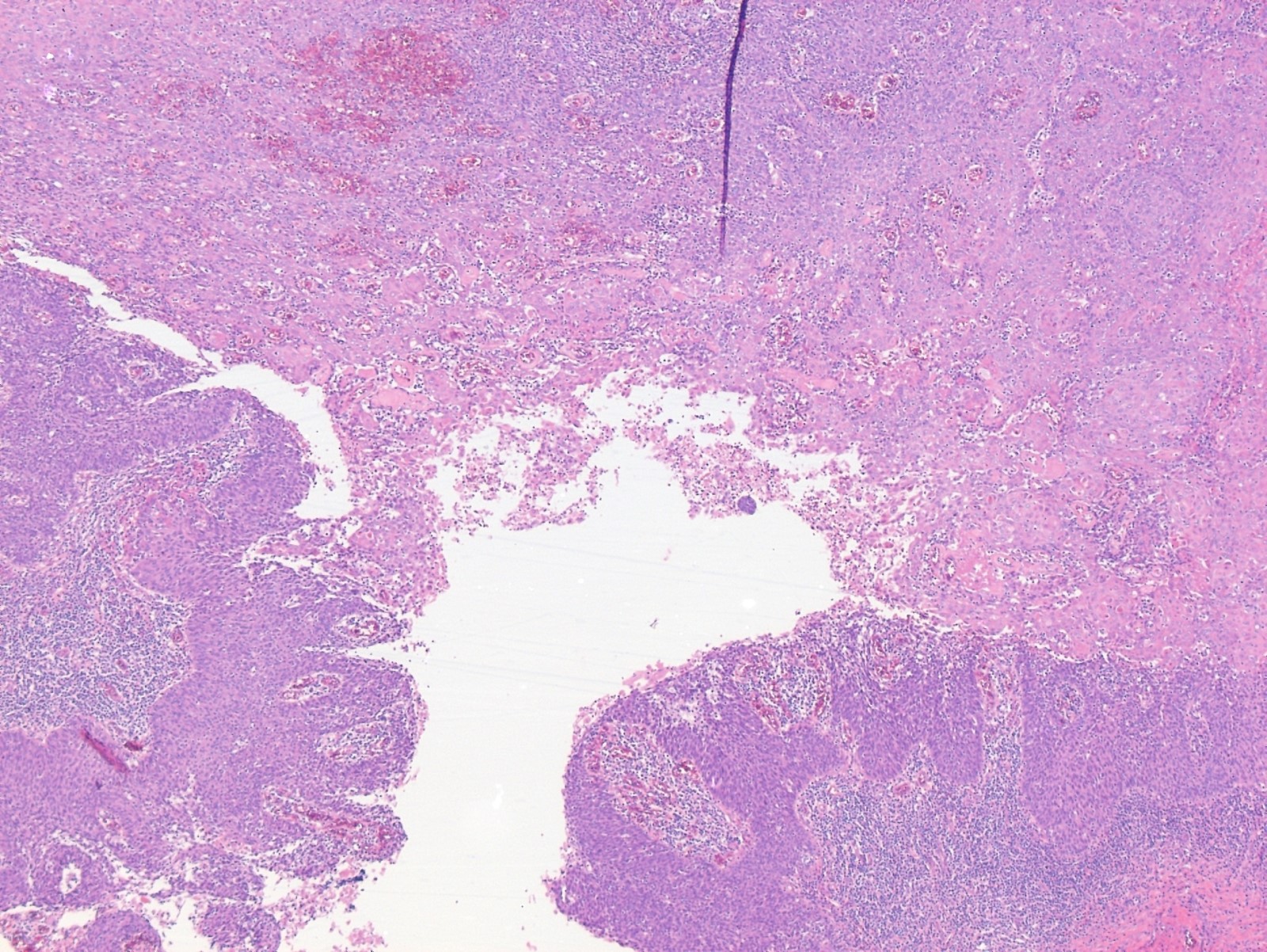
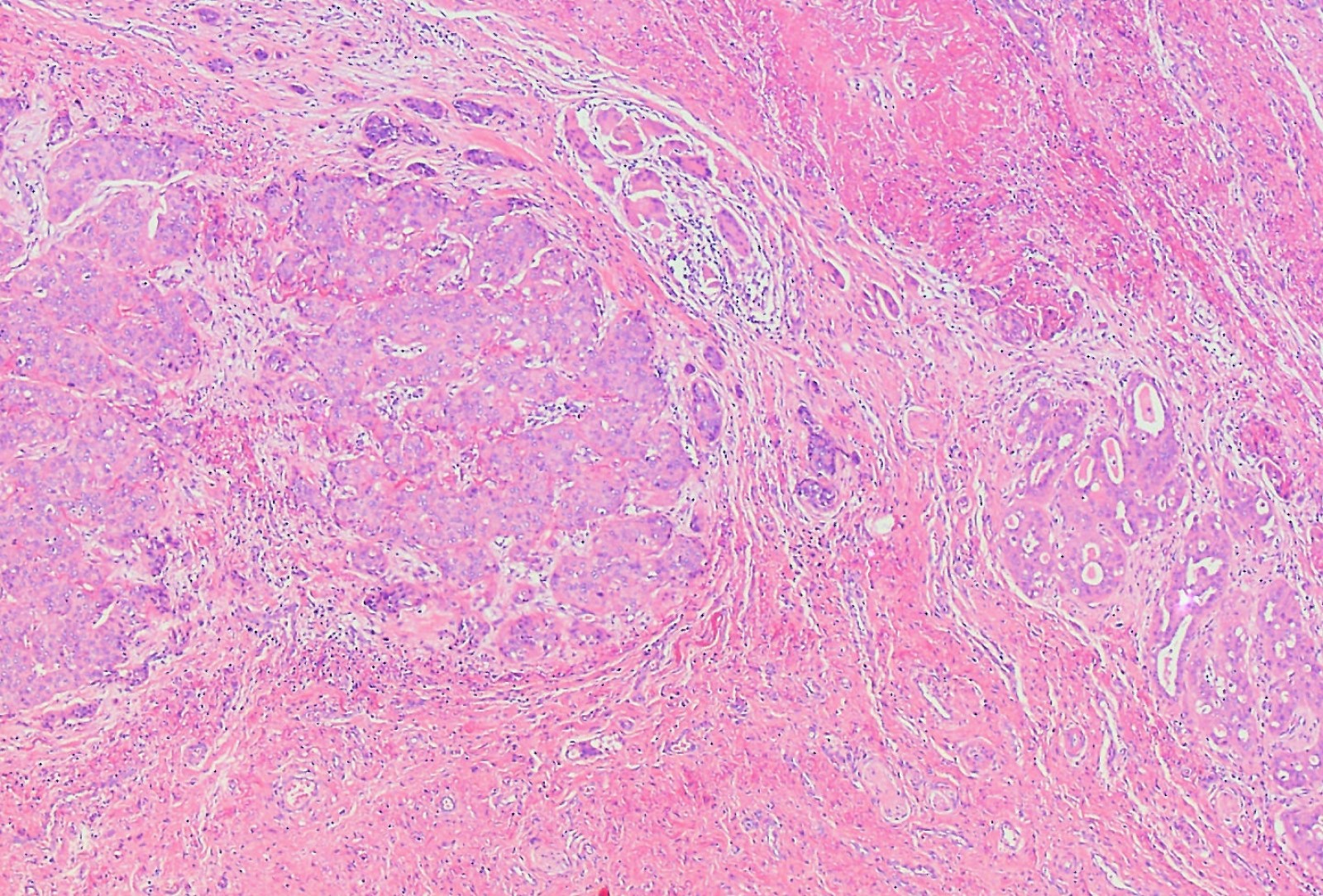
- Vaginal specimens are characterized by endocervical-like glandular cells or metaplastic squamous cells
- Endometrioid glandular cells can also occur
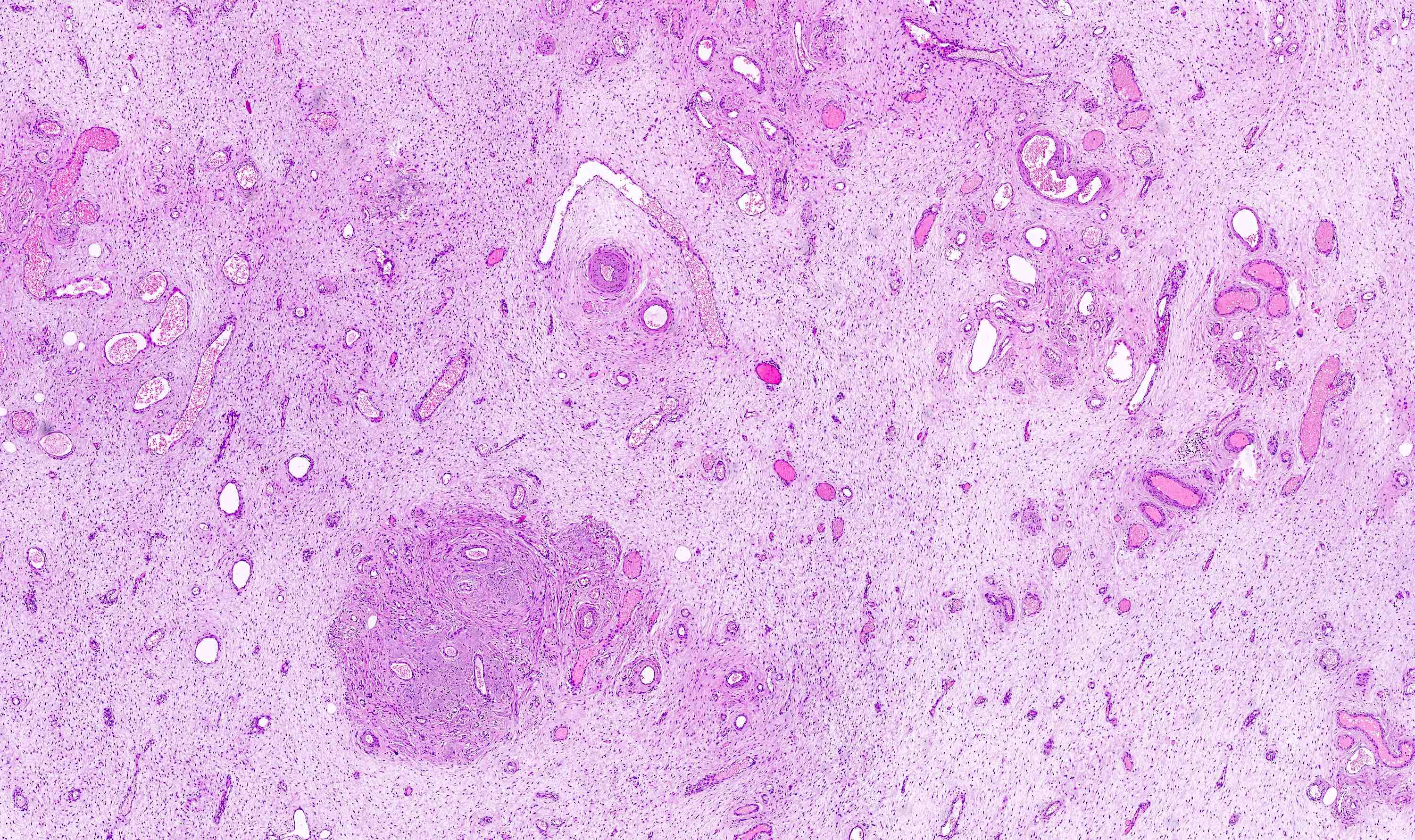
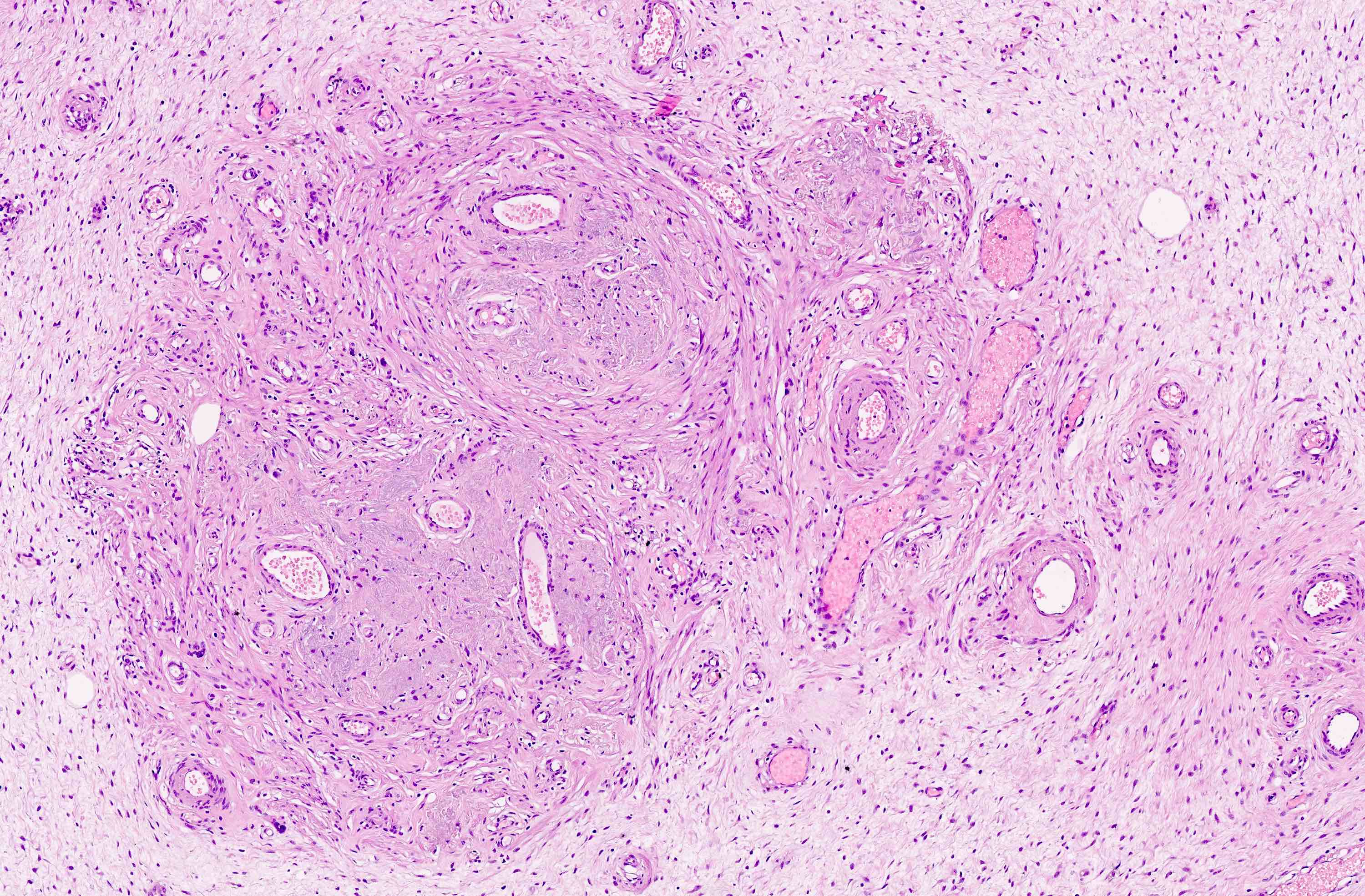
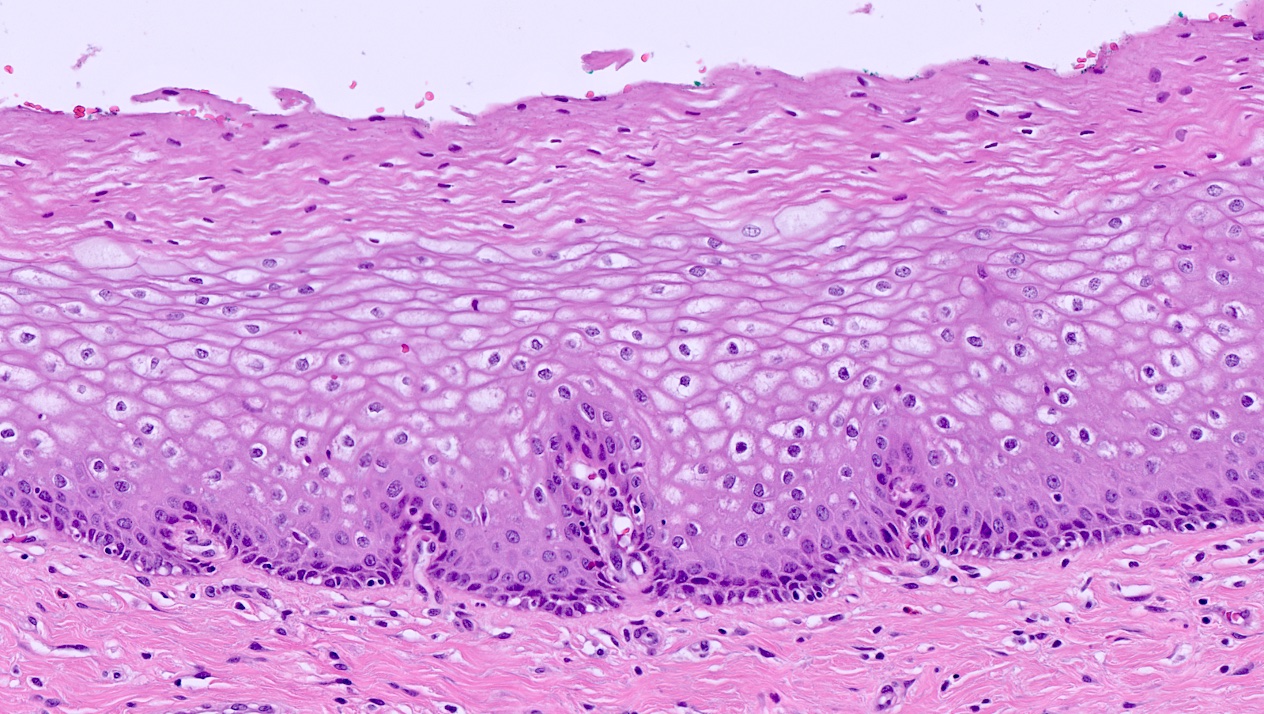
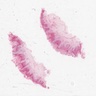
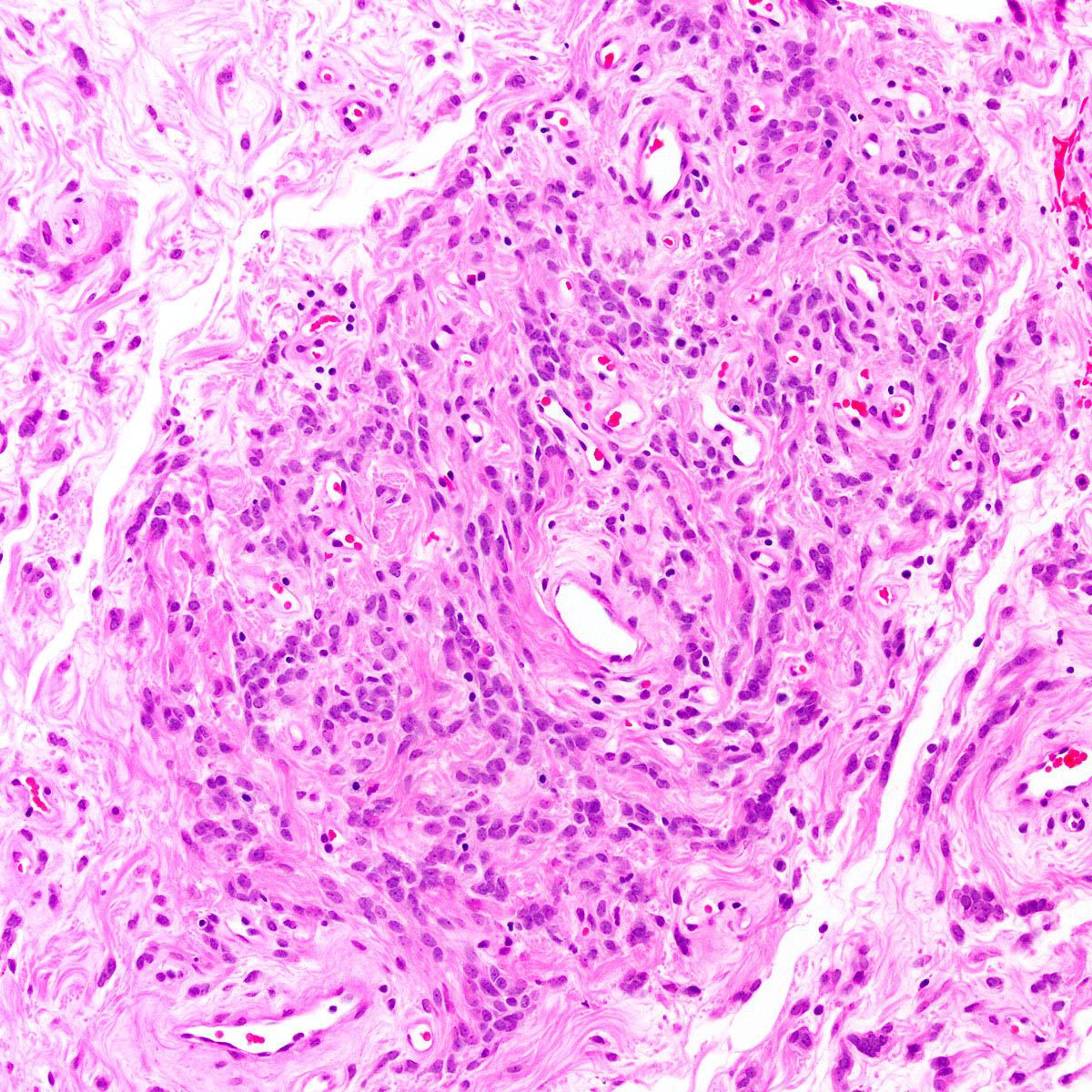
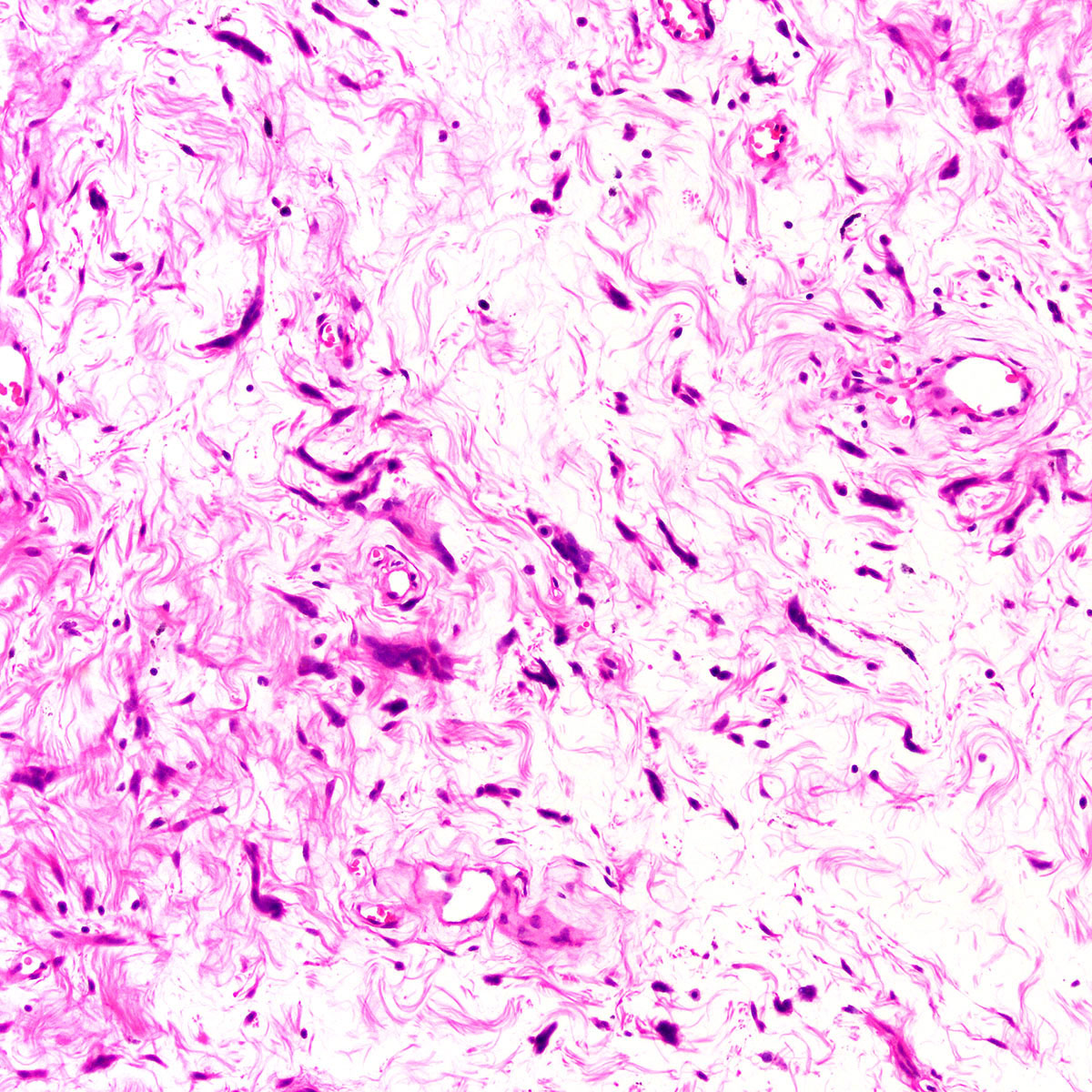
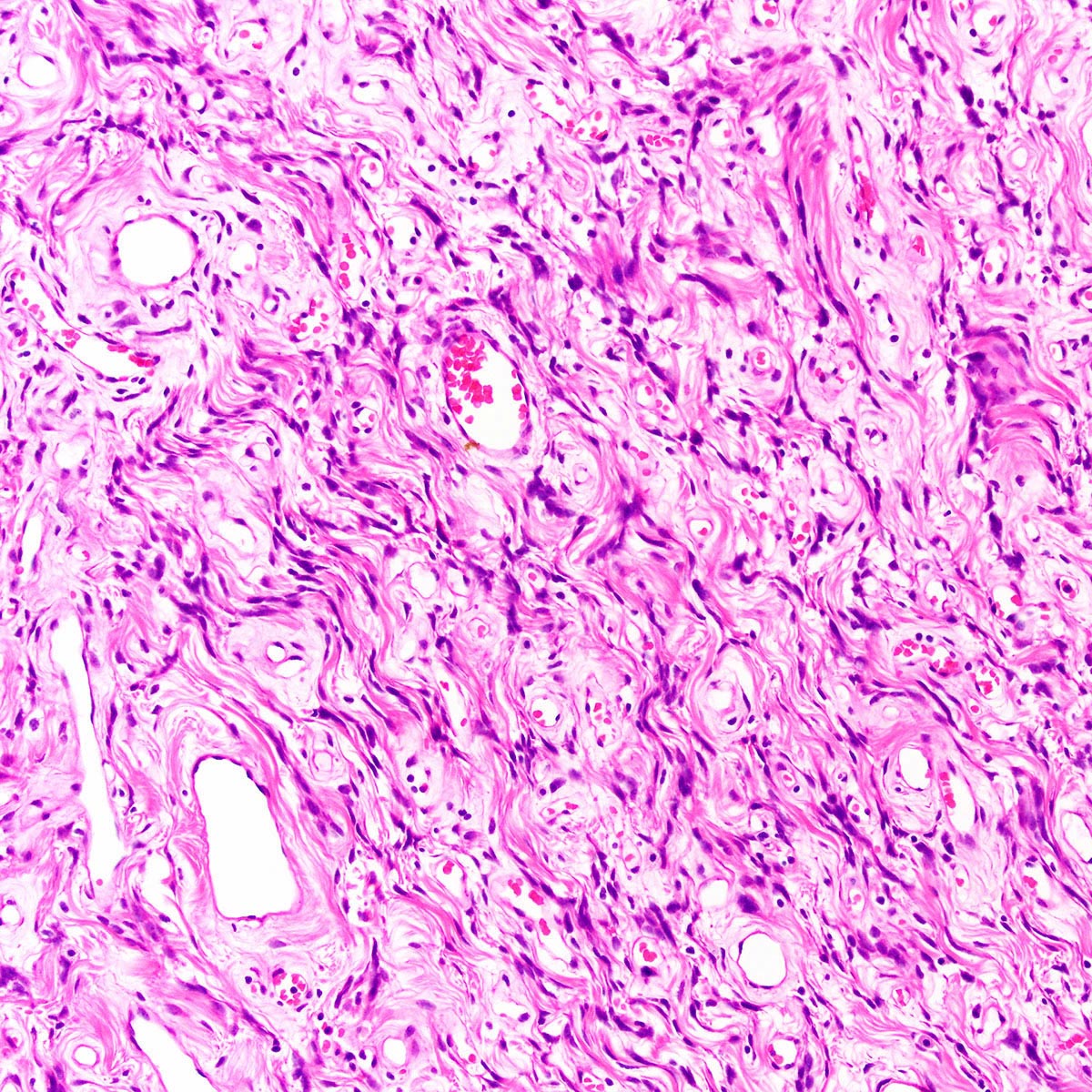
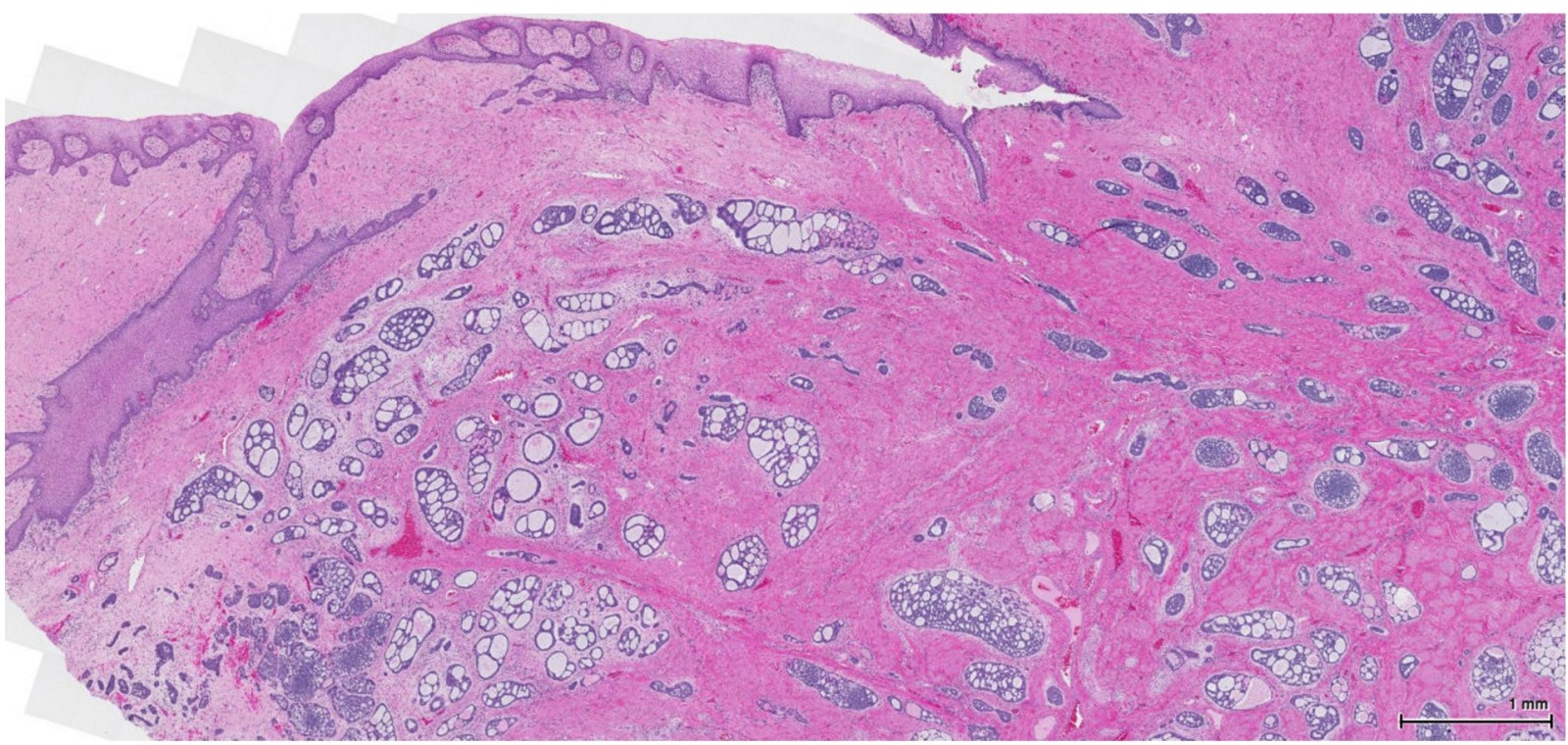
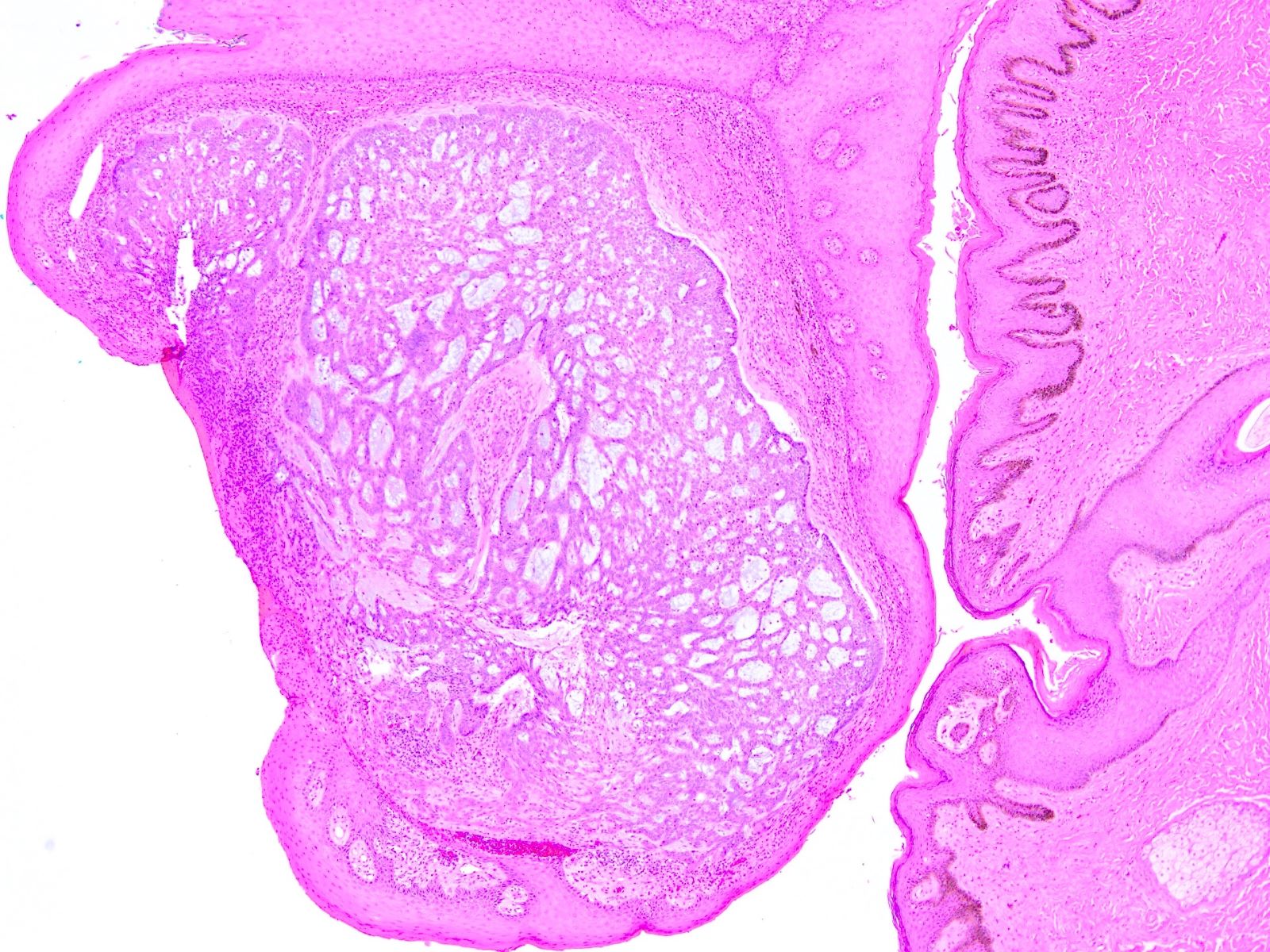
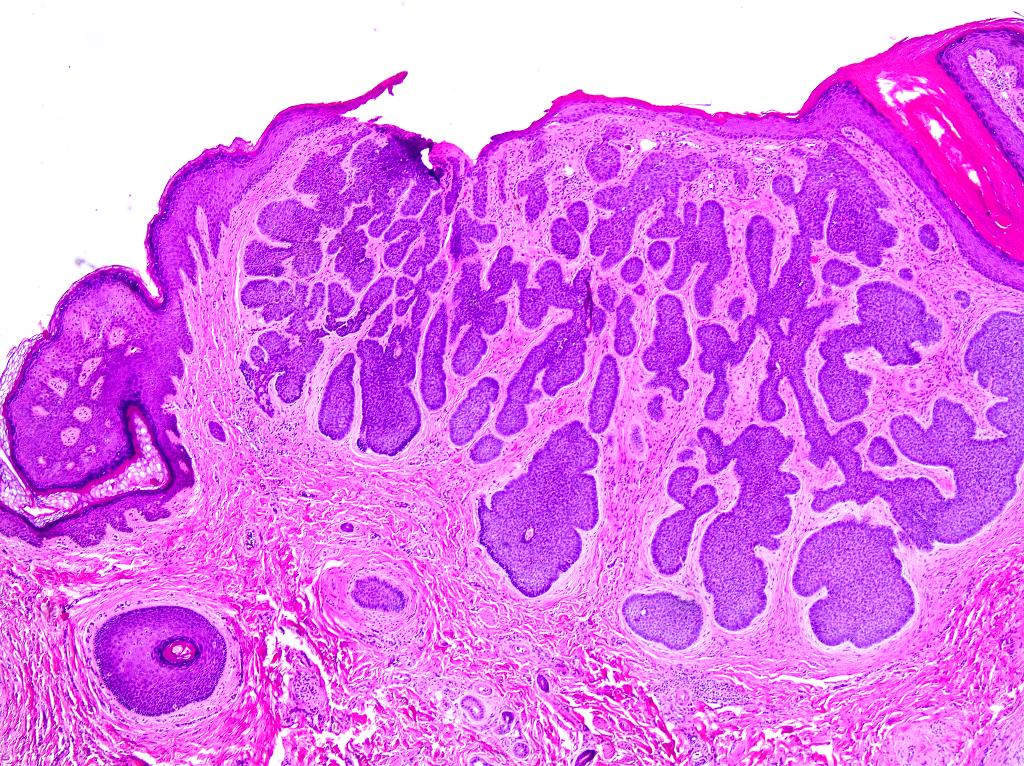
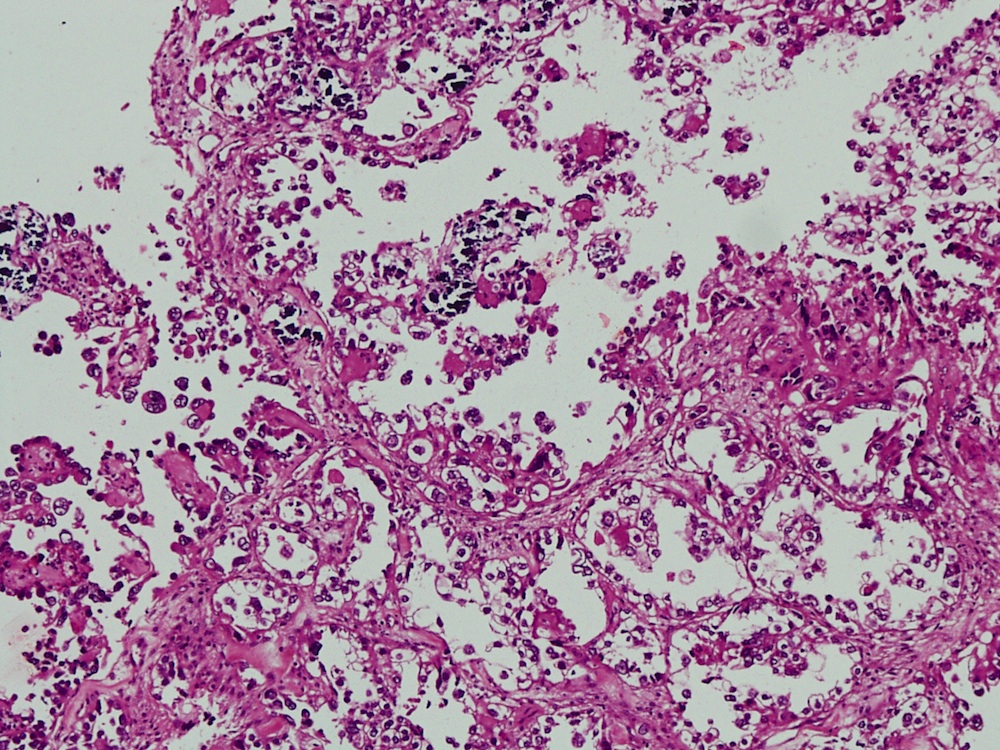
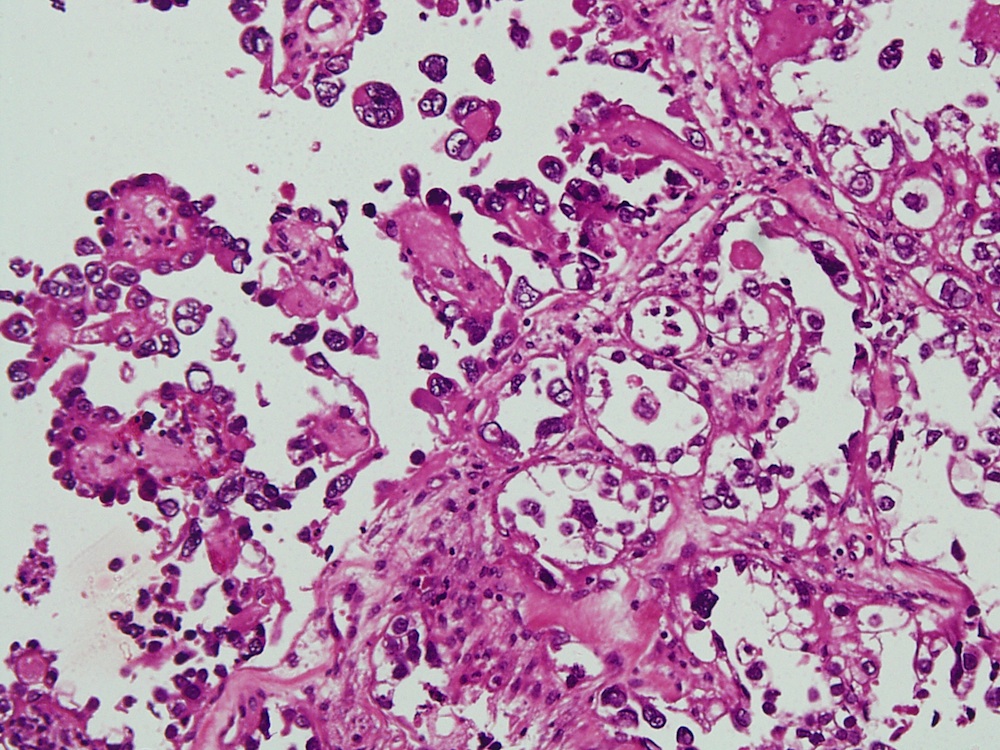
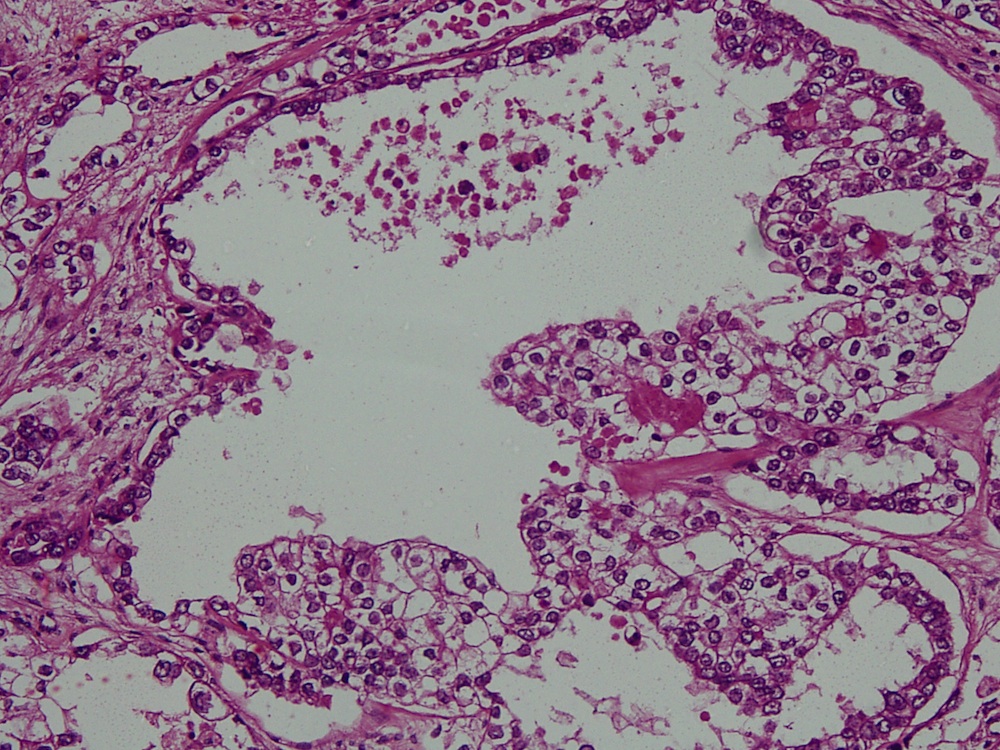
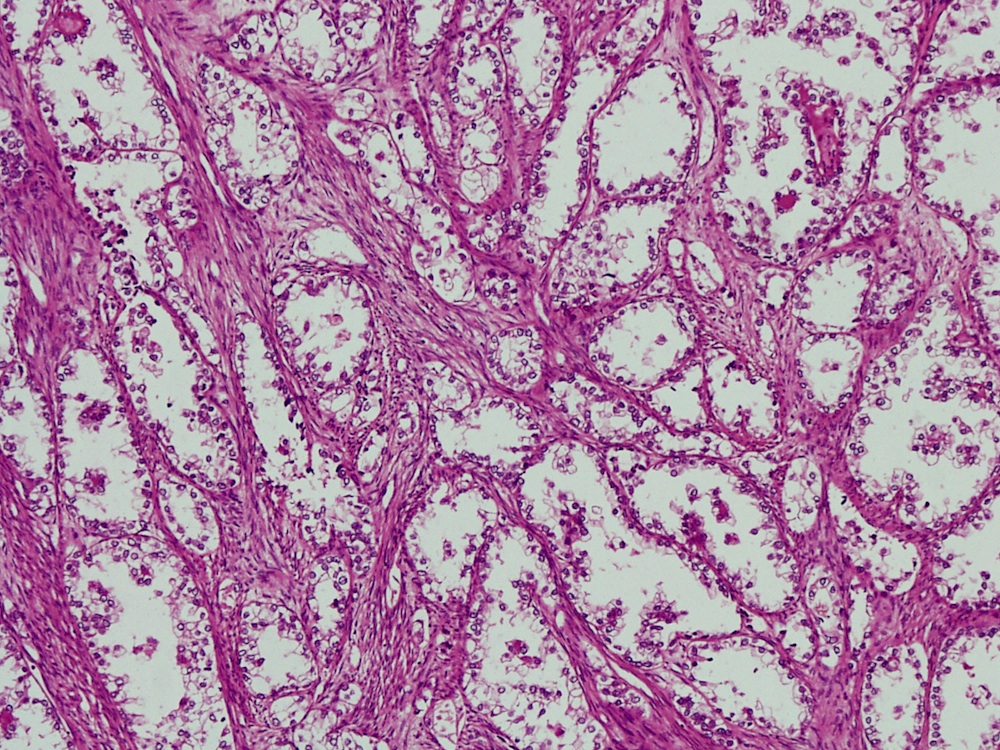
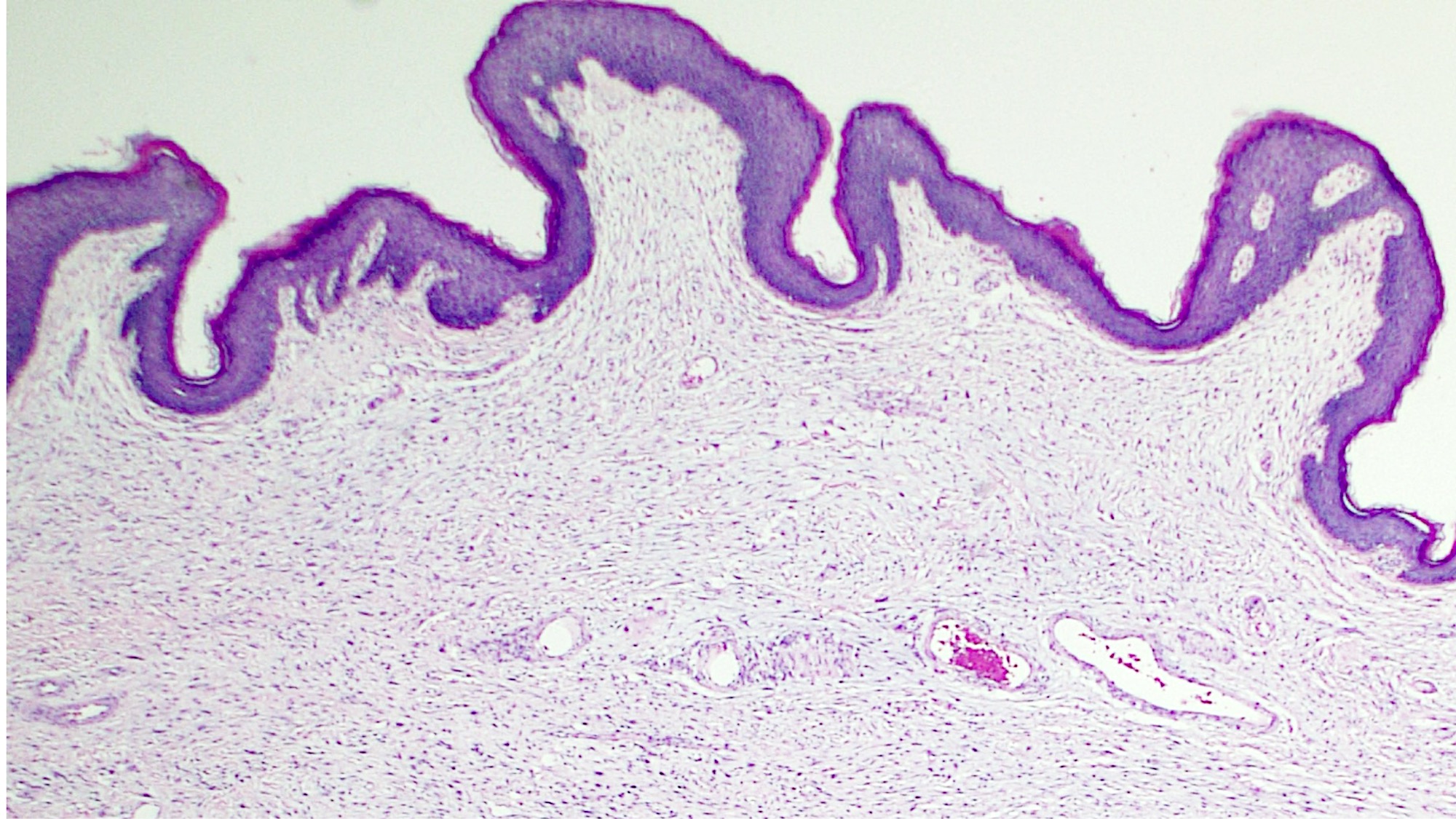
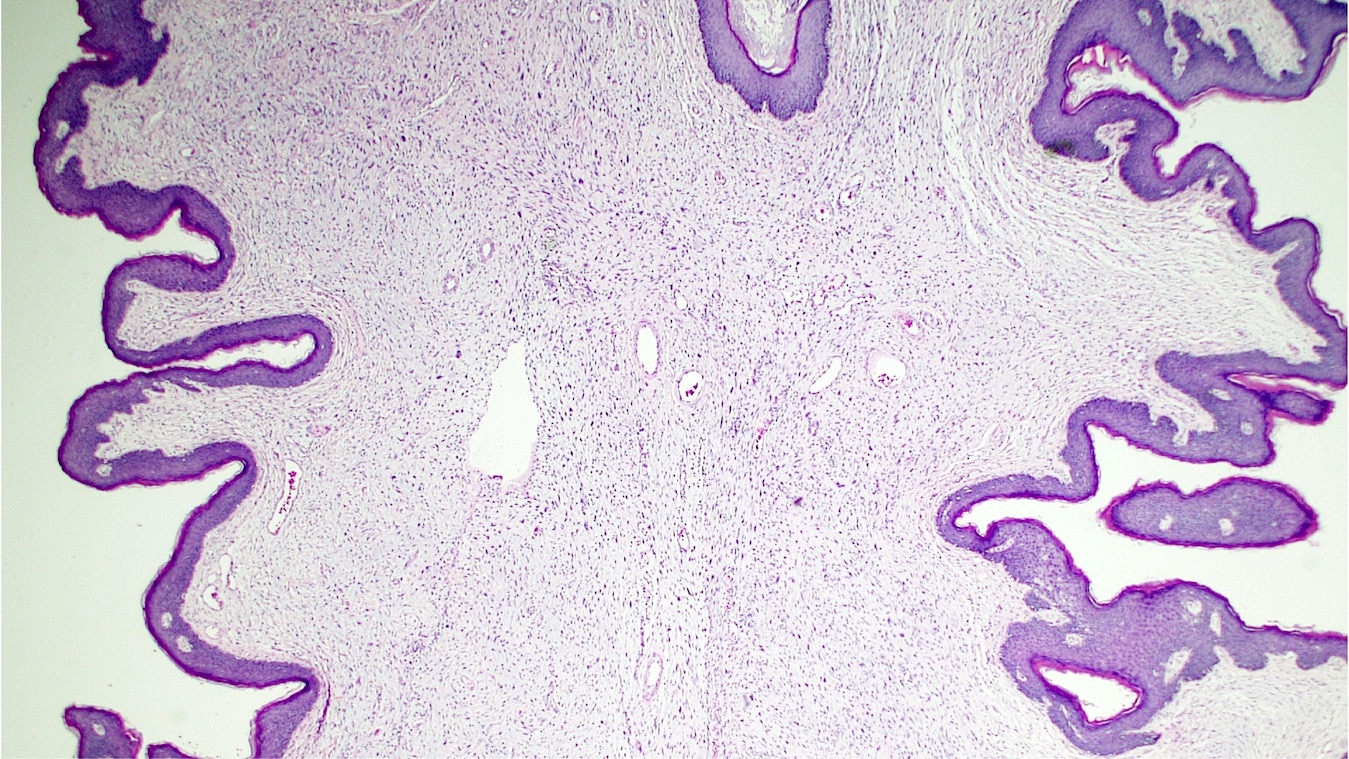
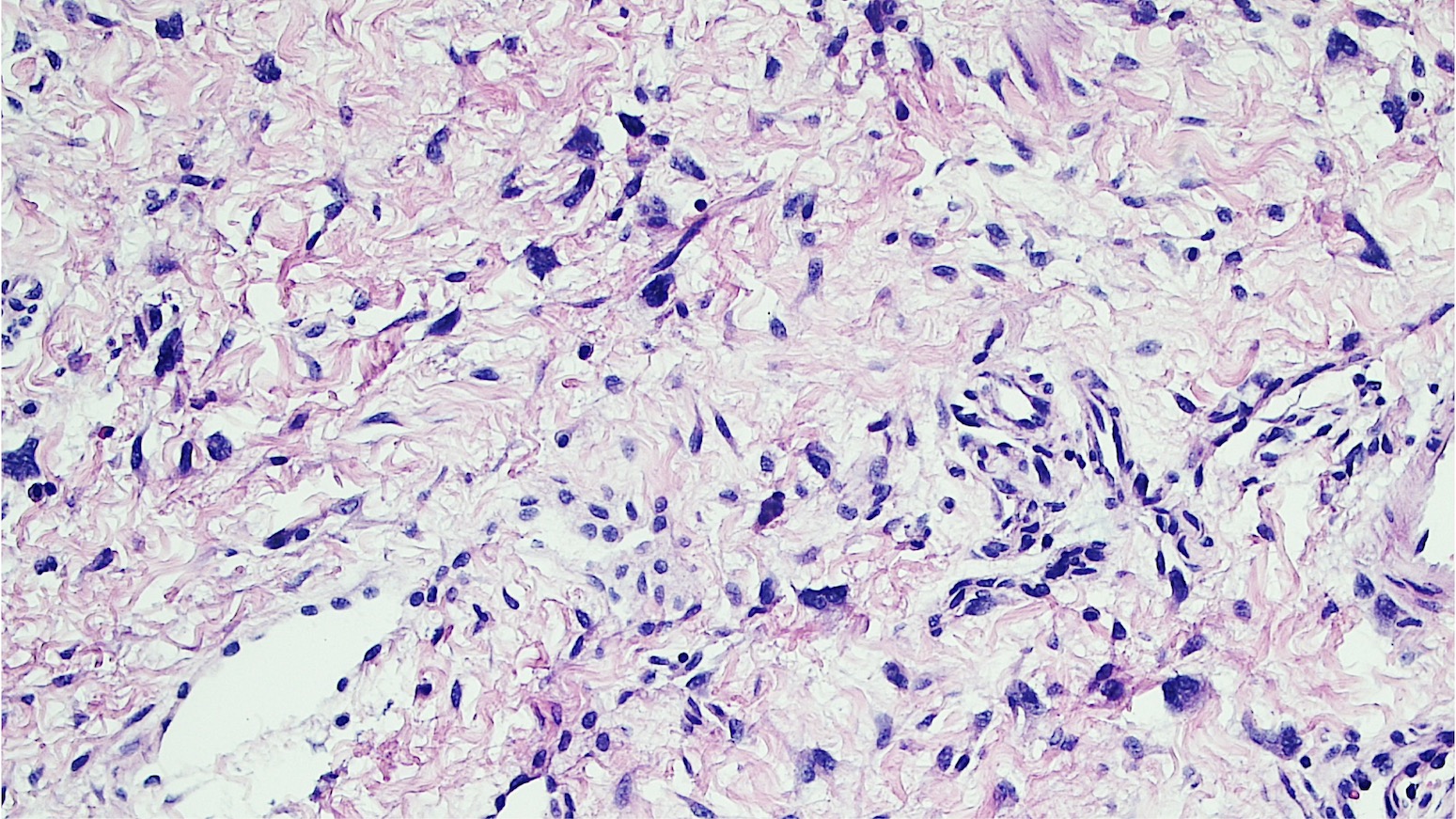
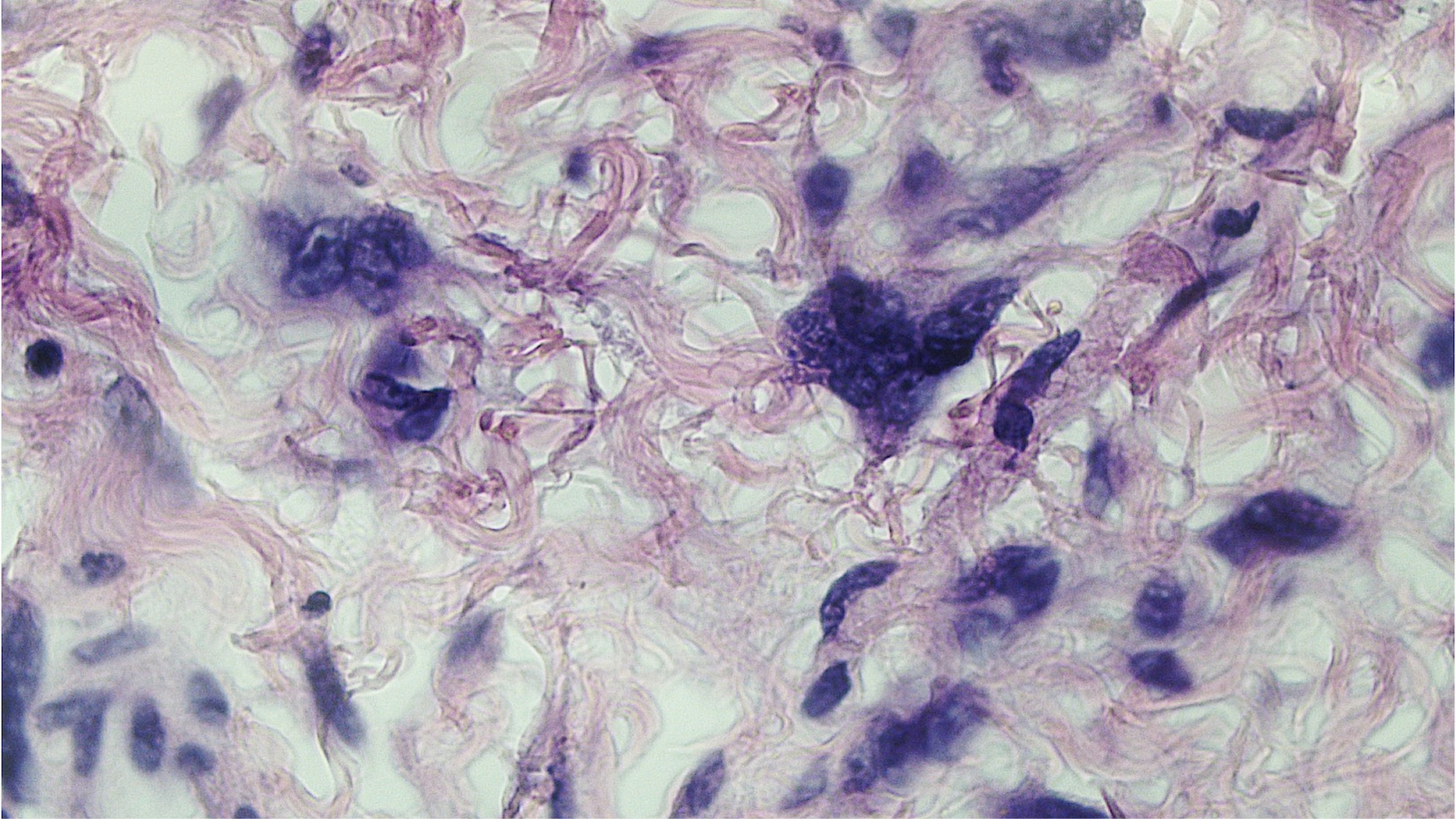
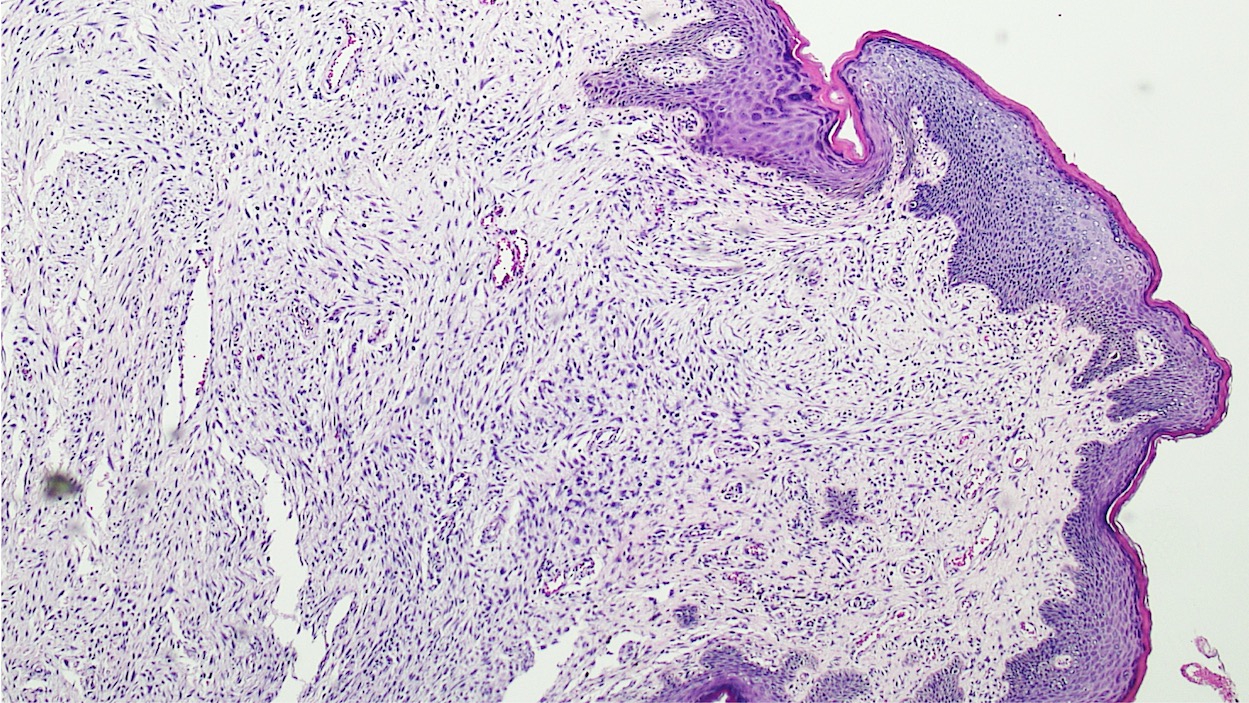
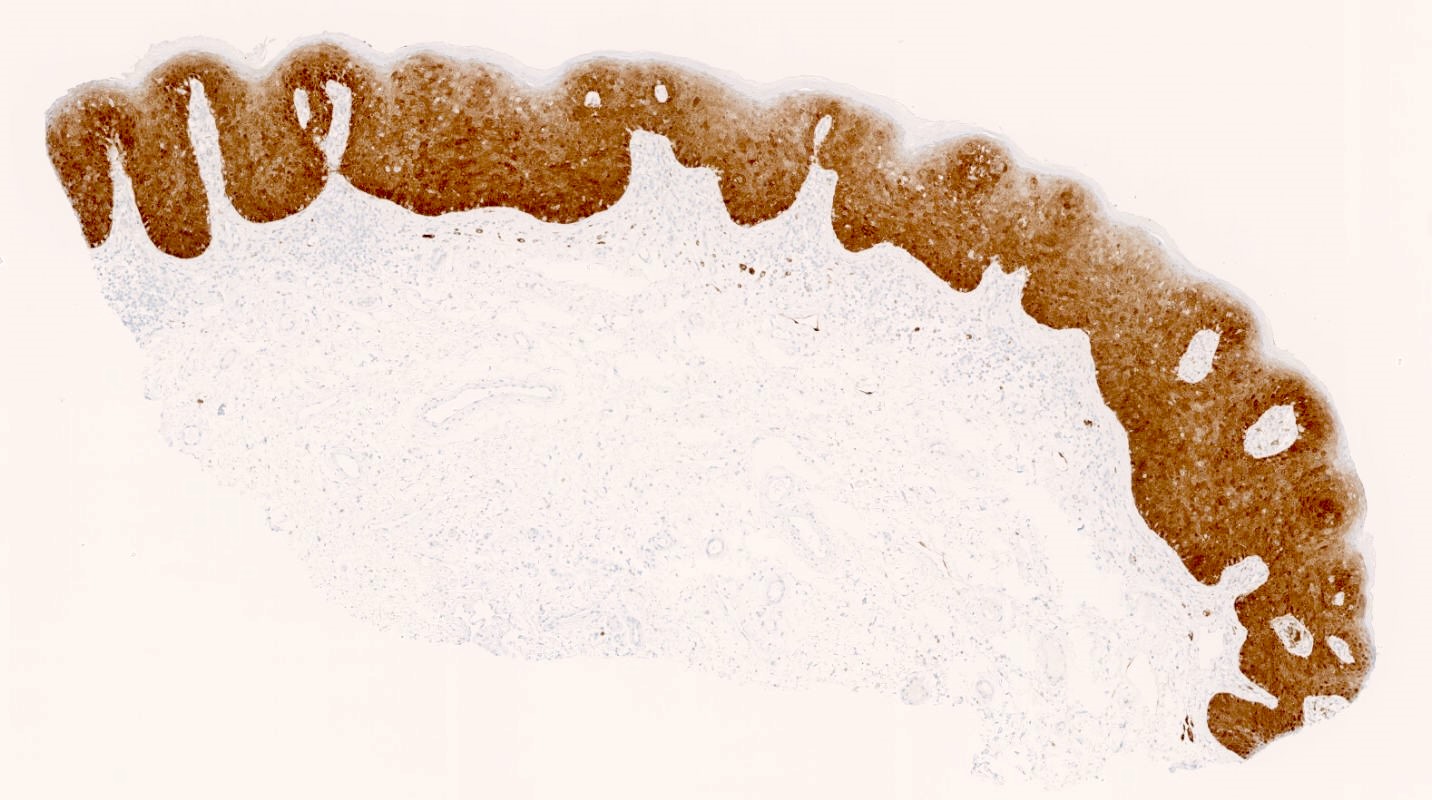
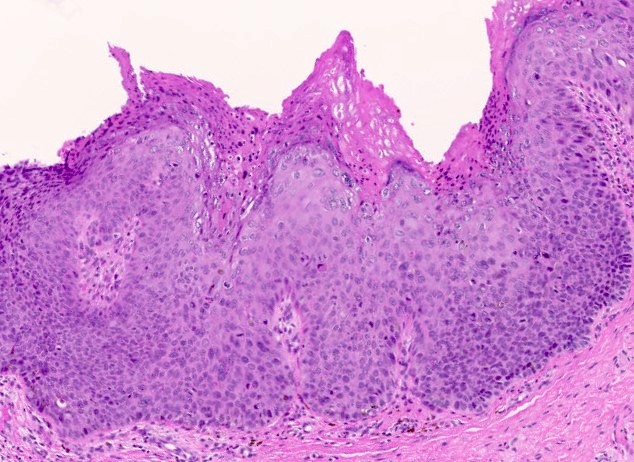
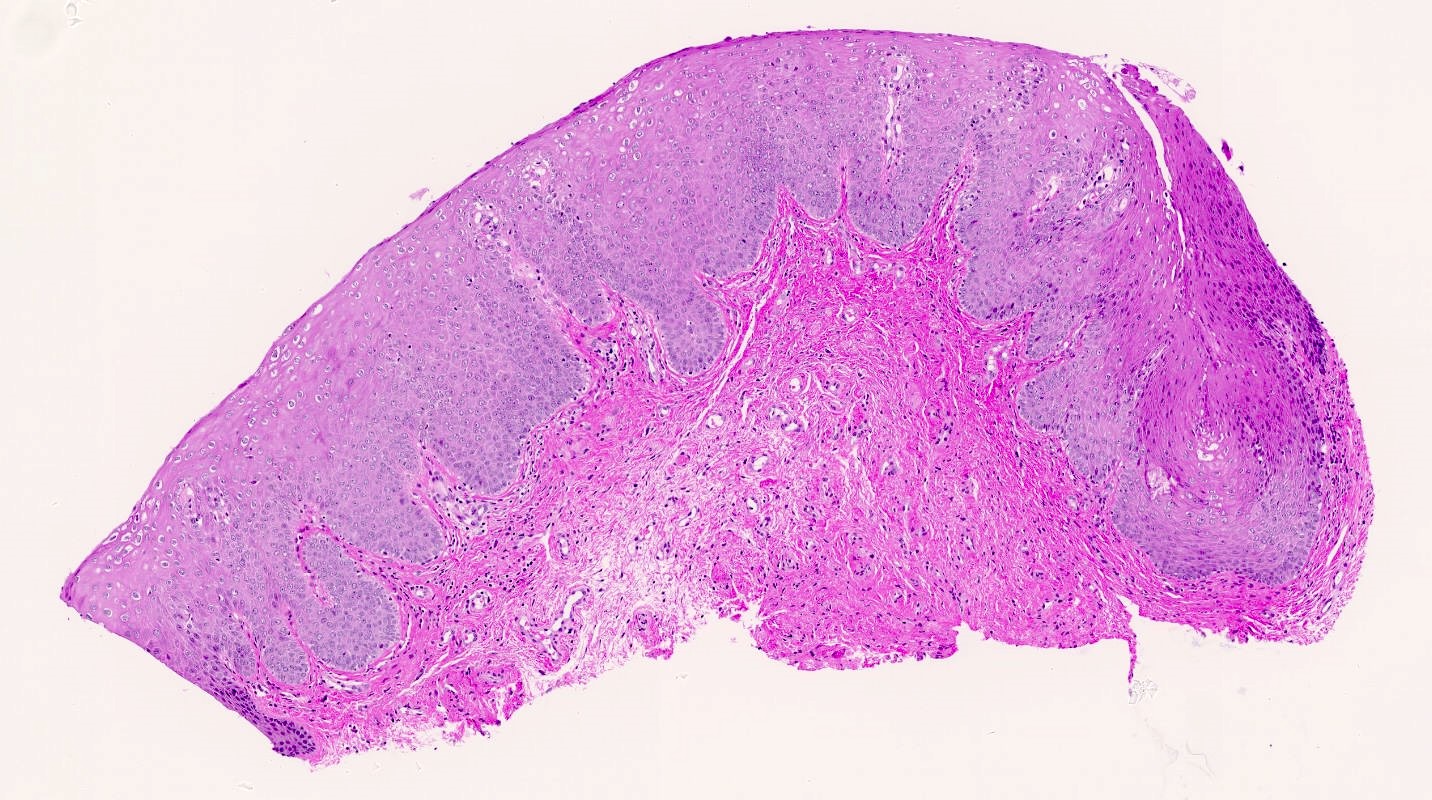
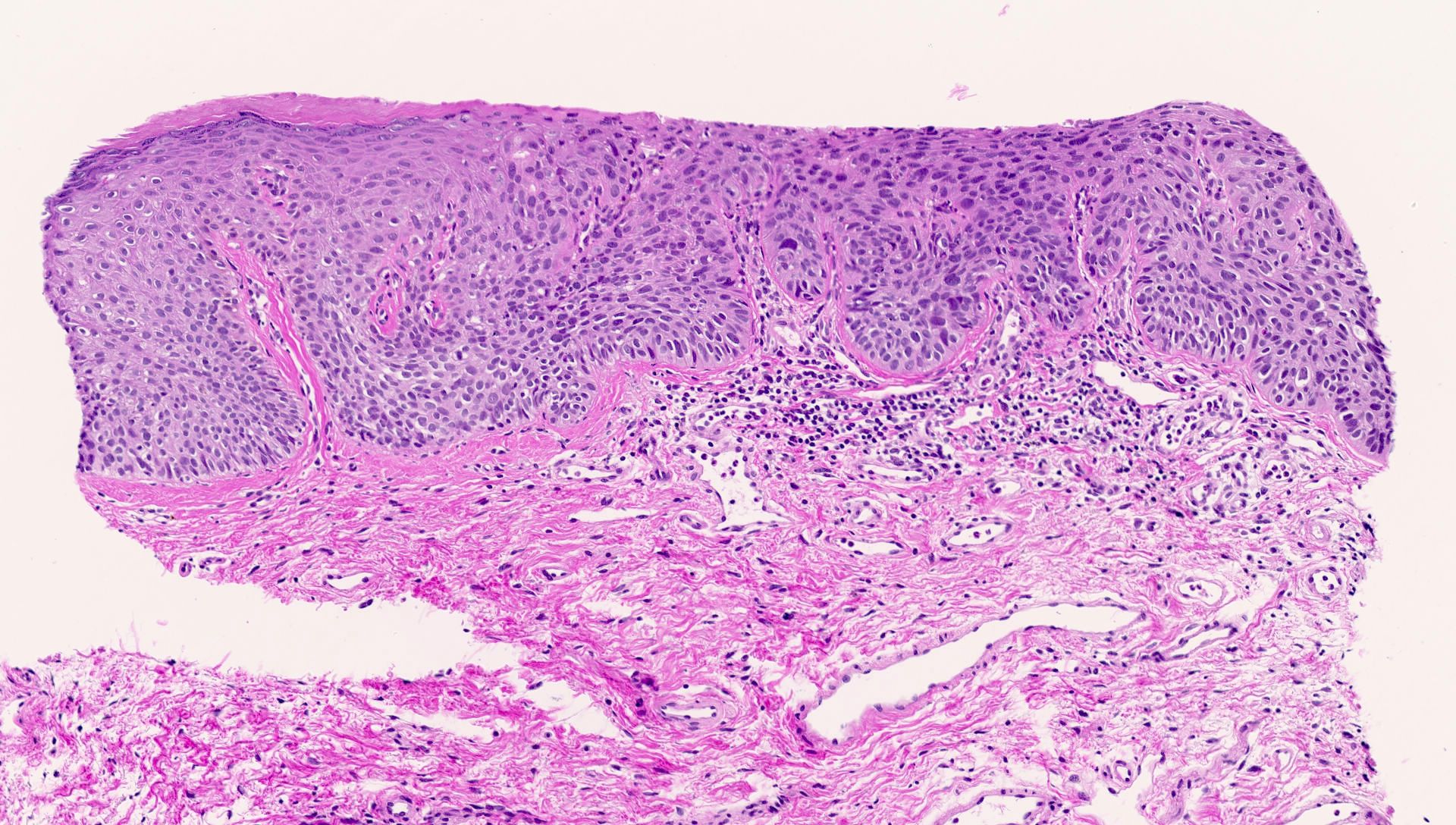
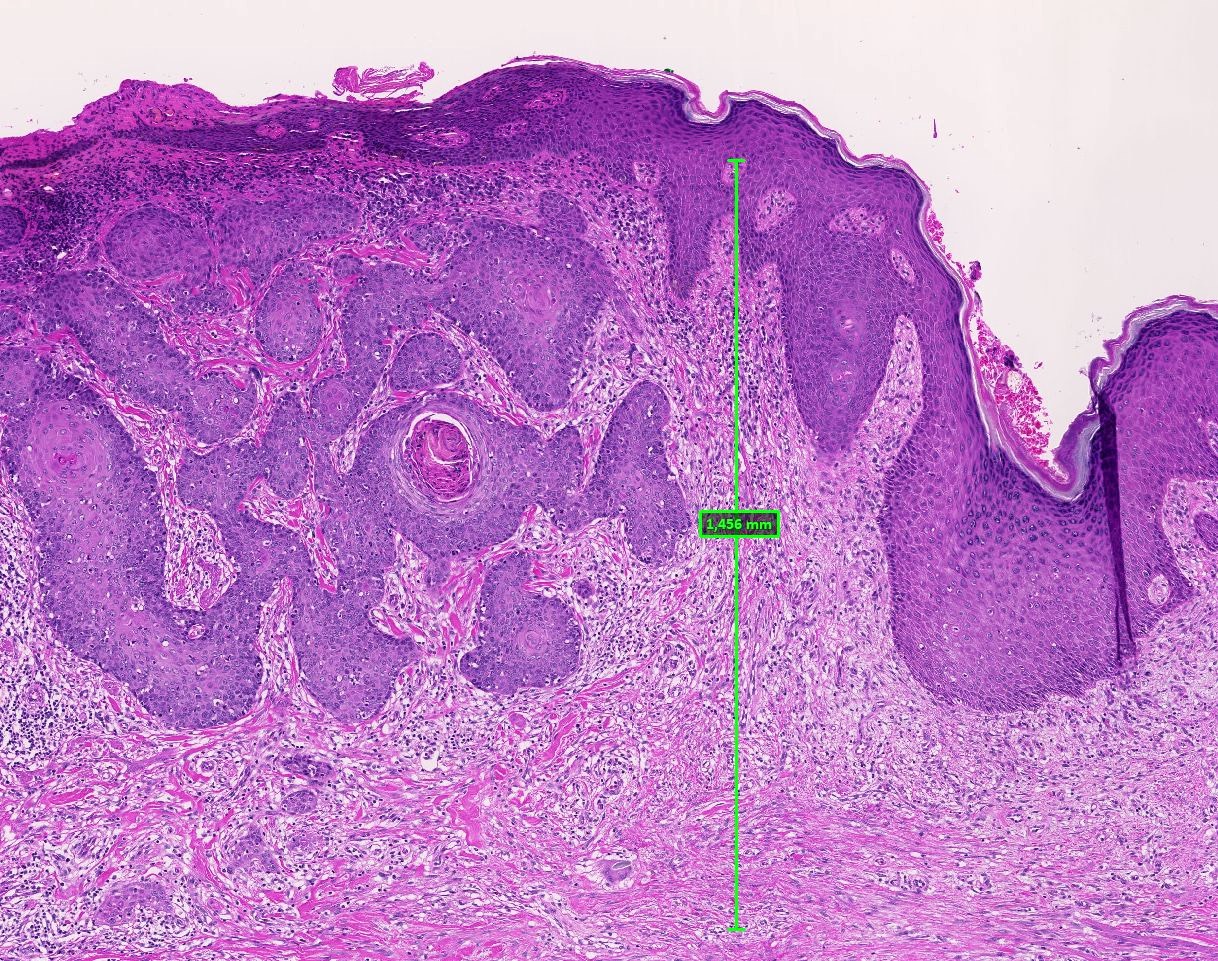
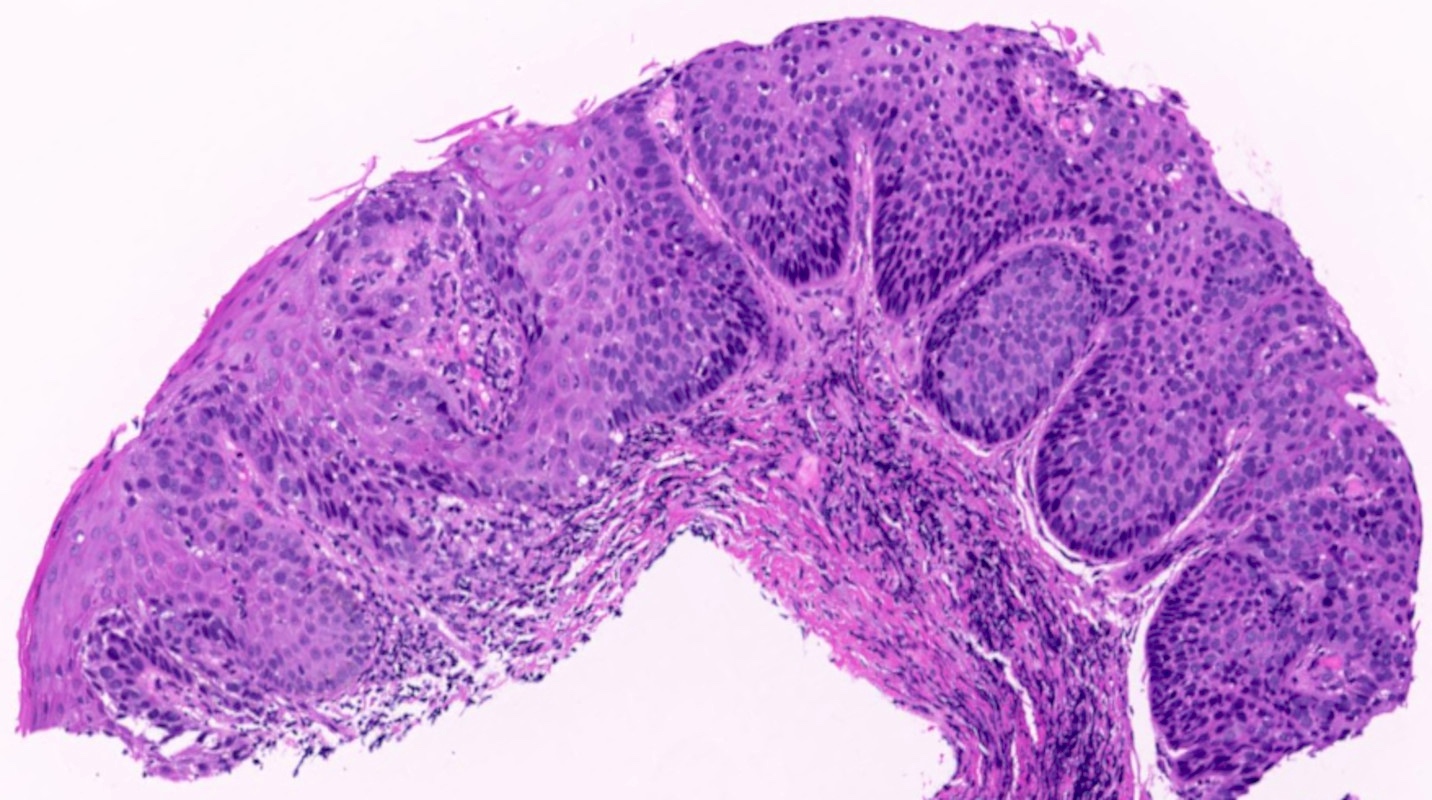
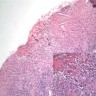
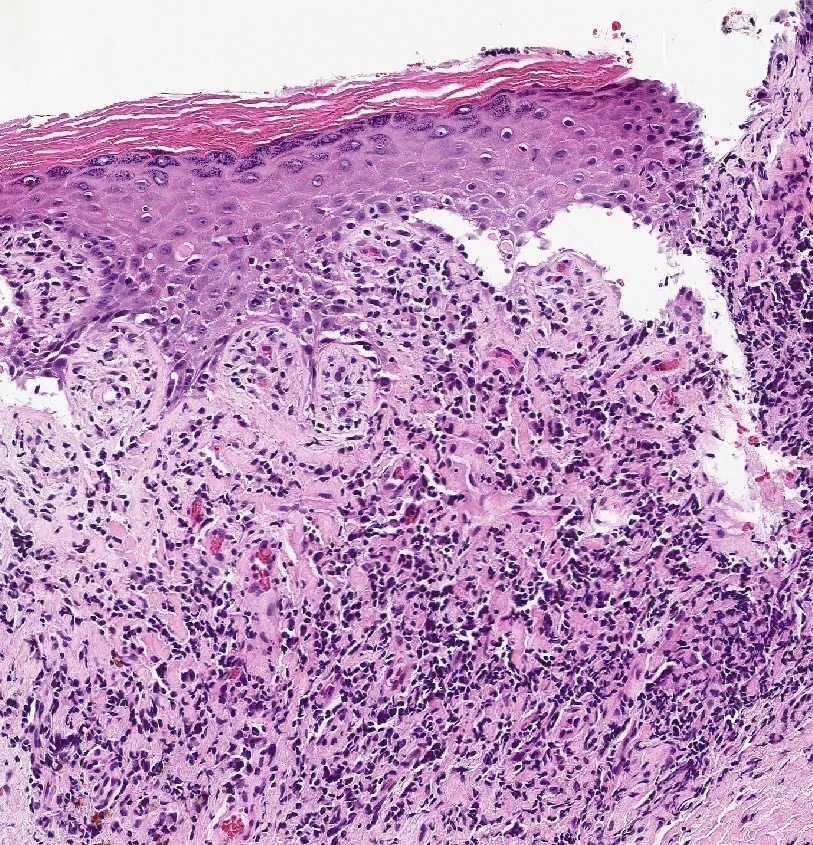
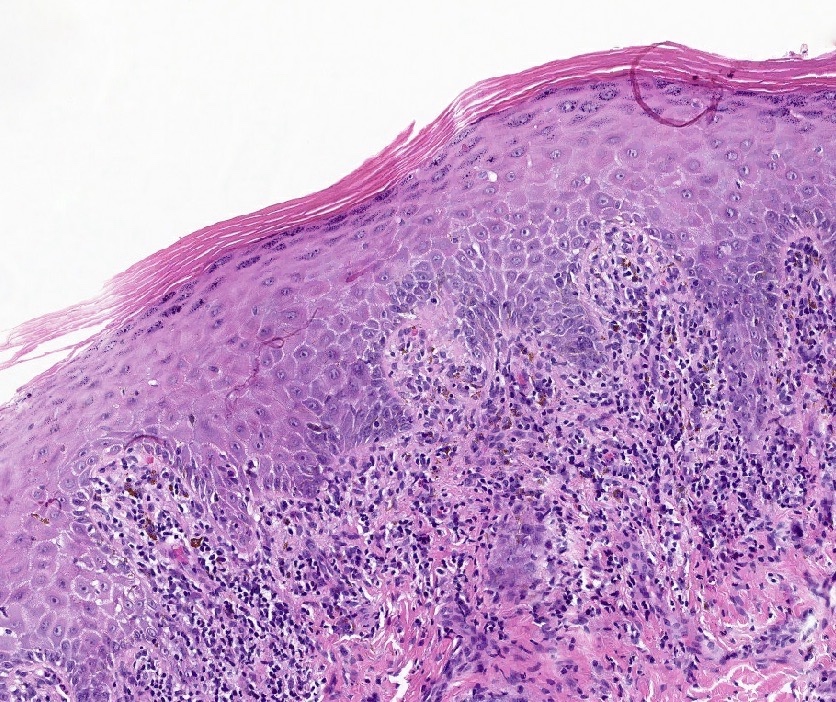
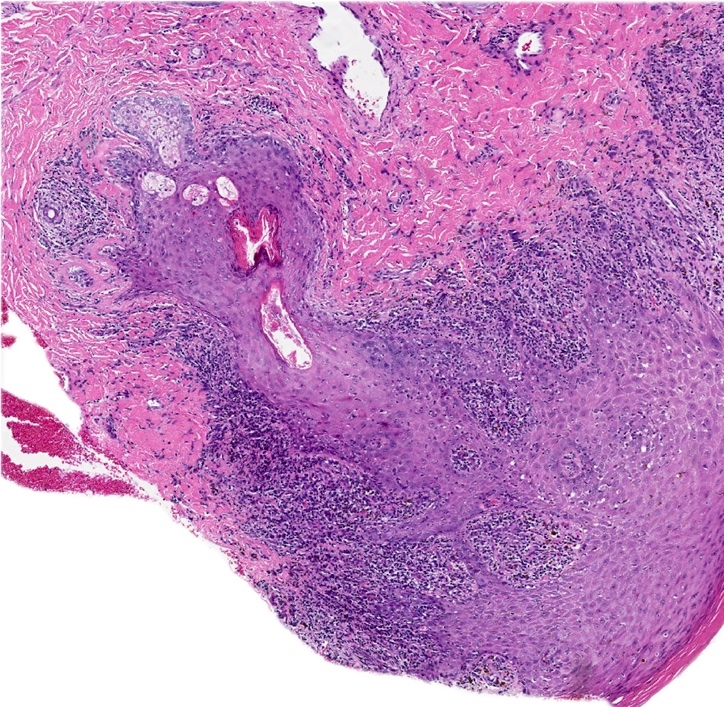
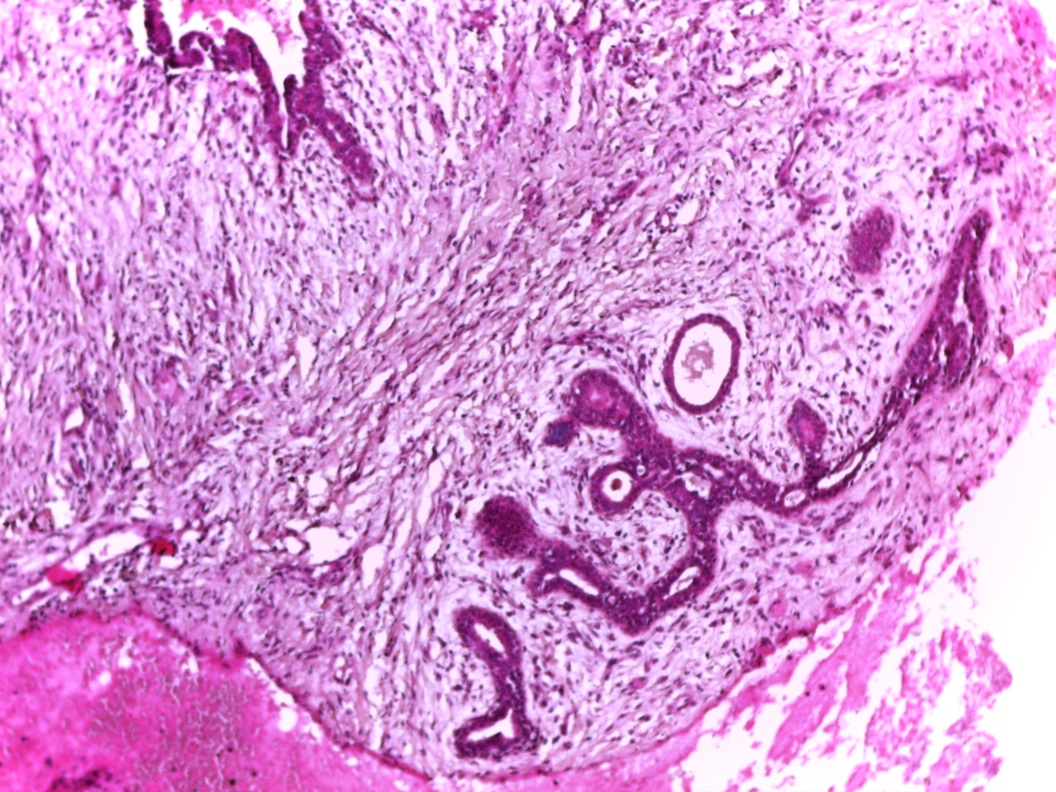
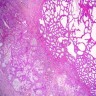
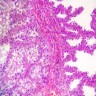
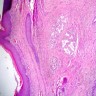
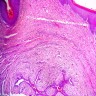
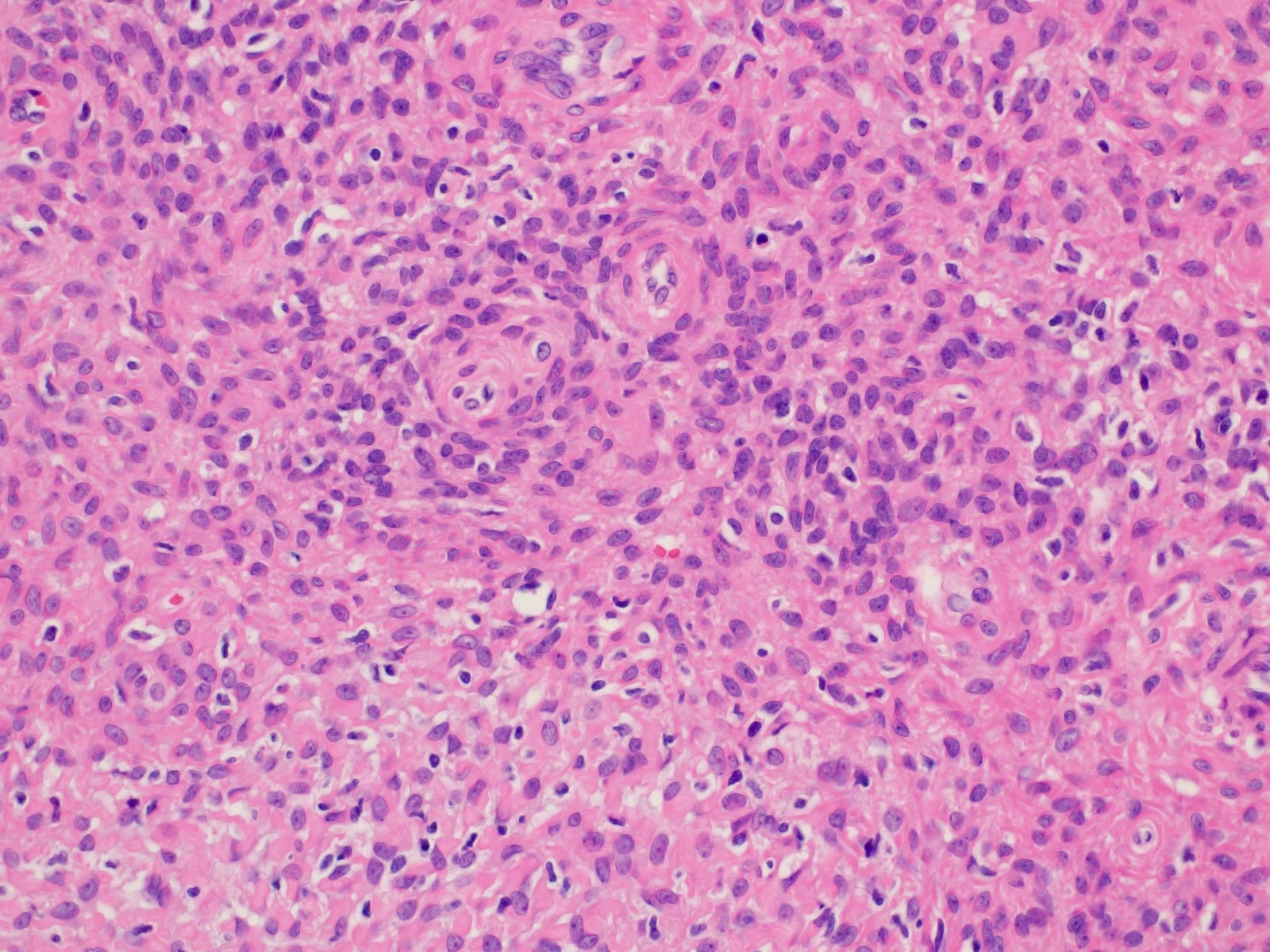
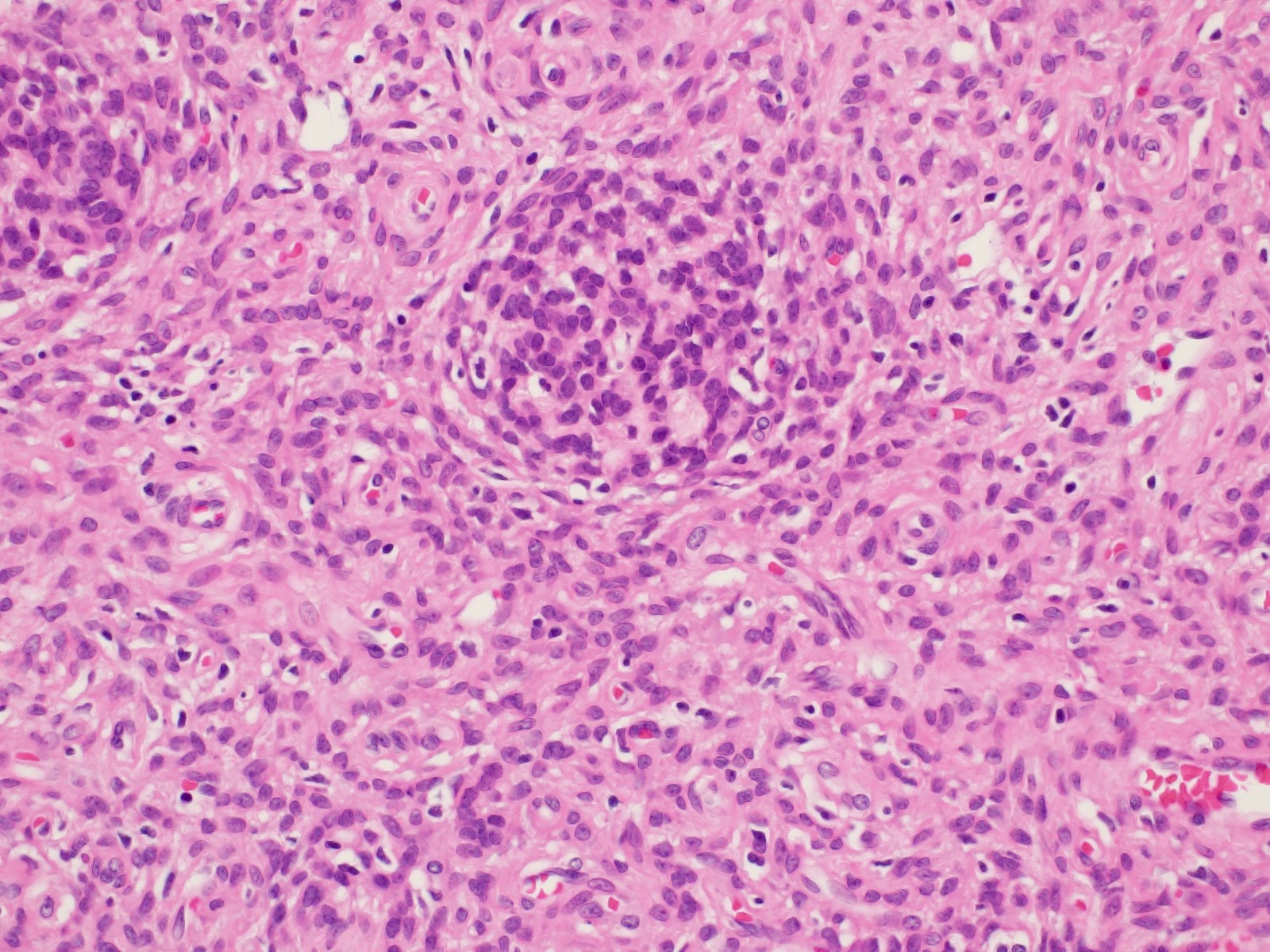
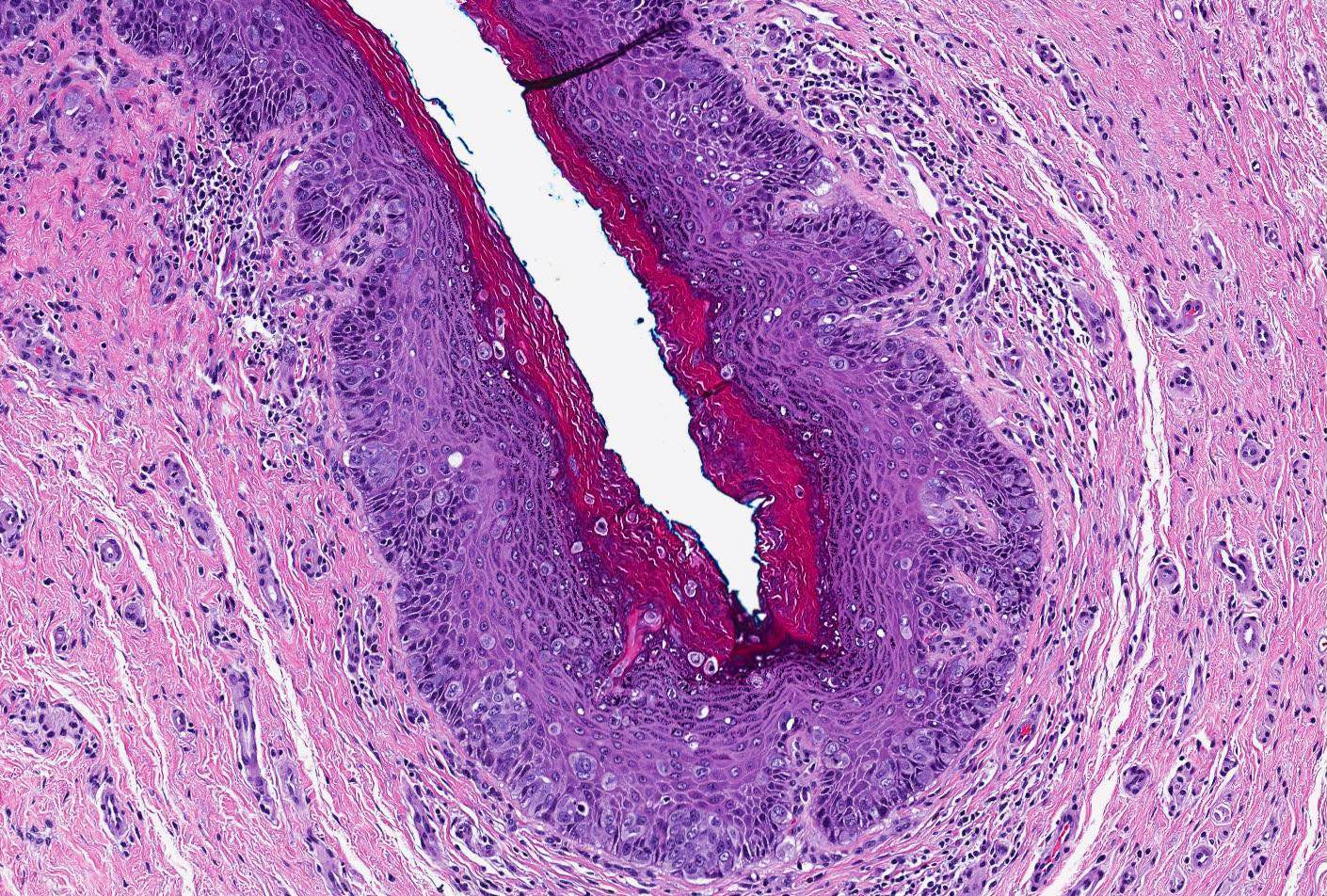
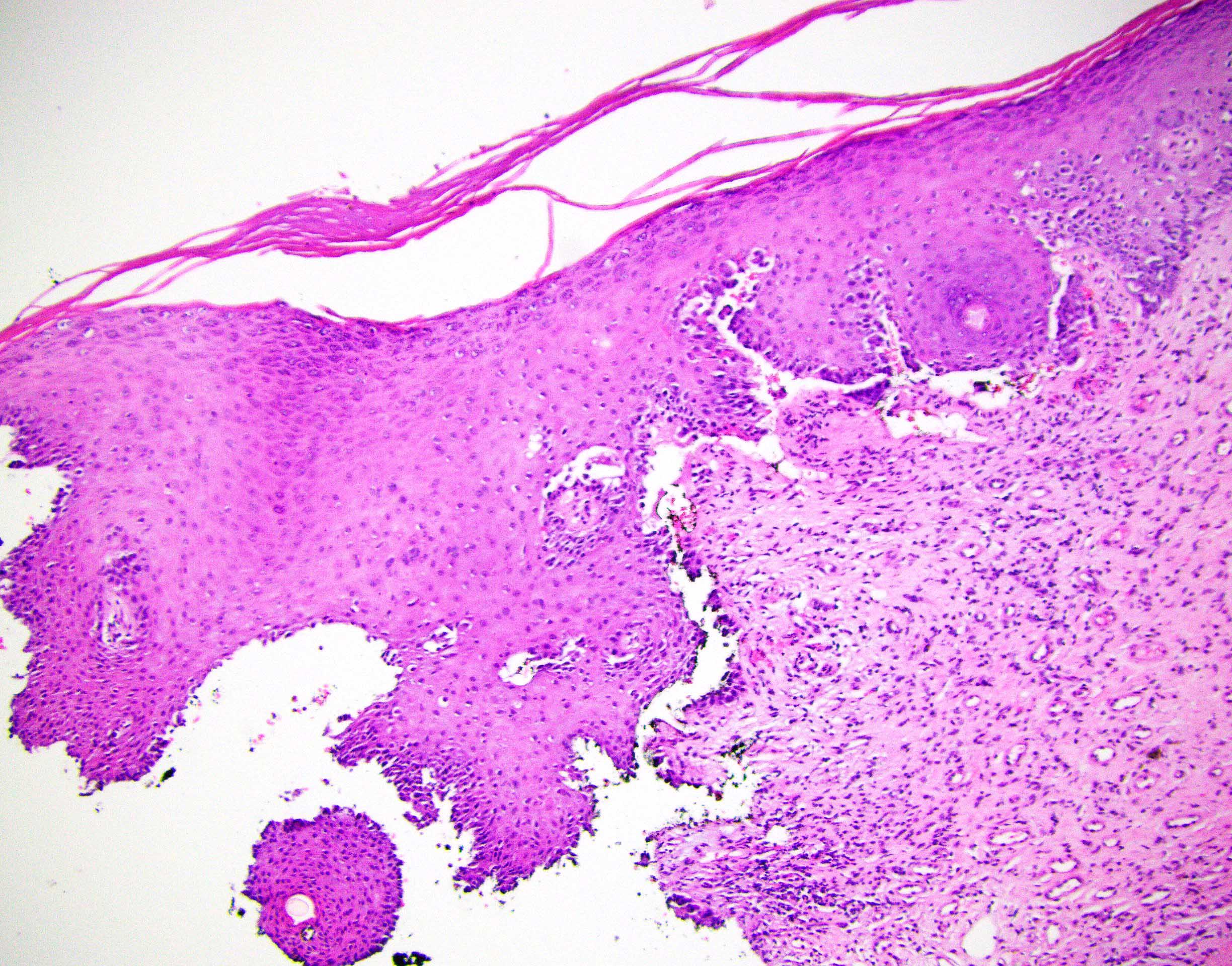
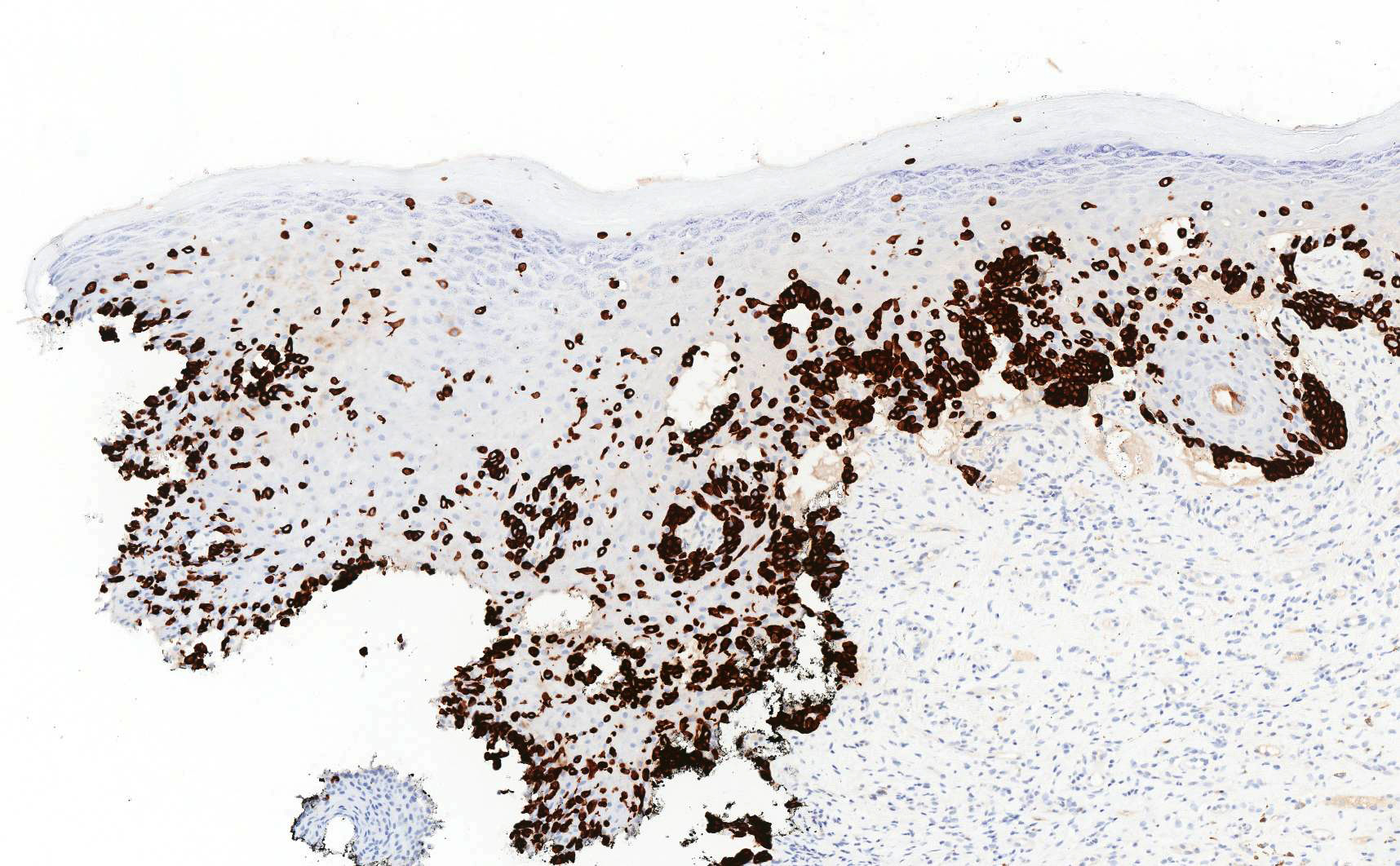
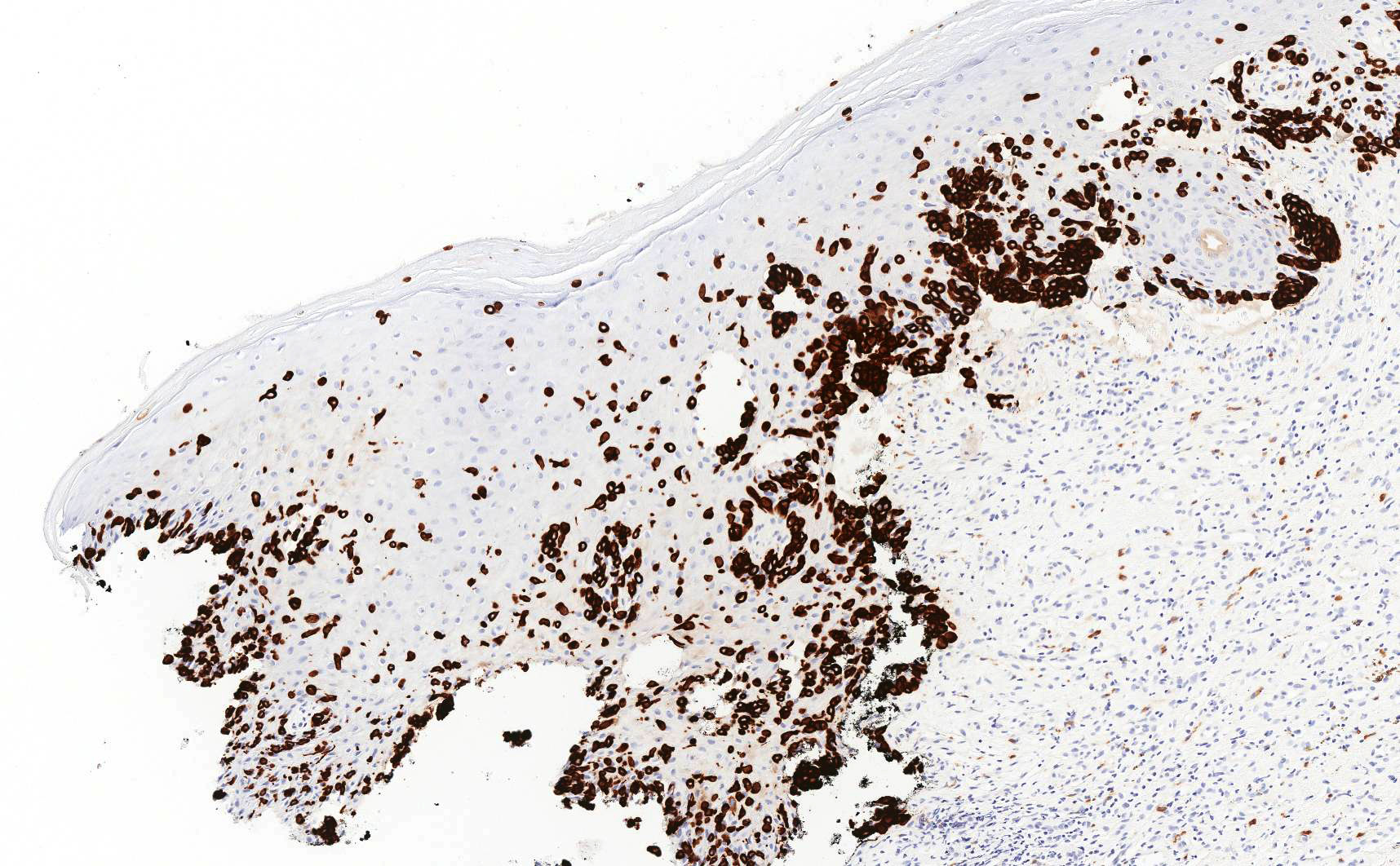
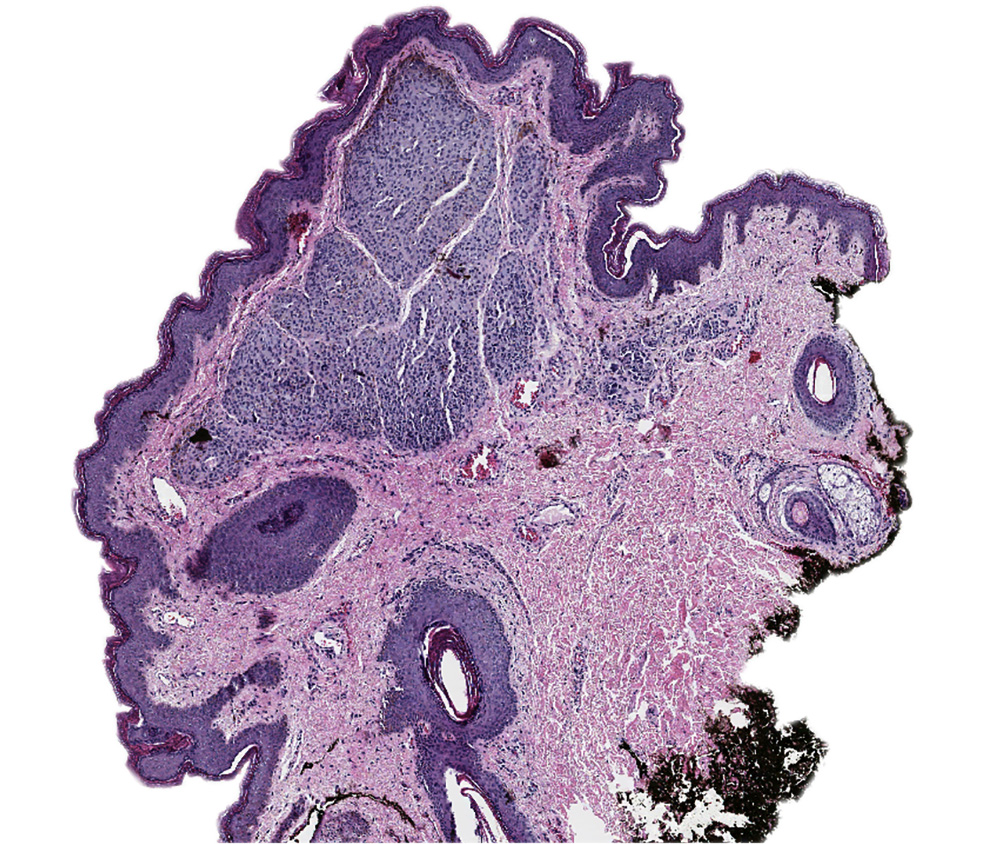
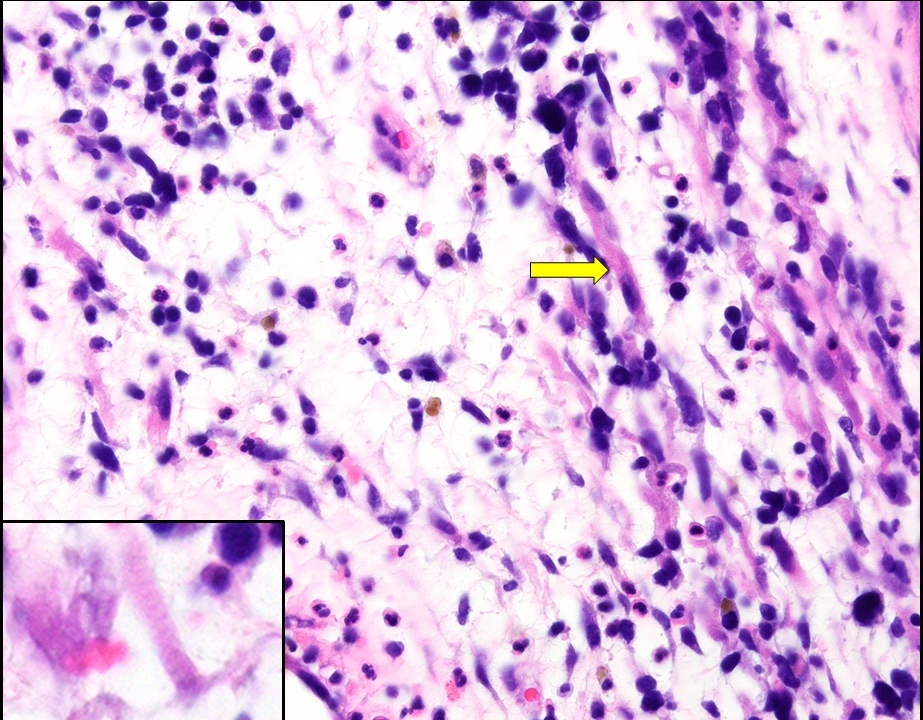
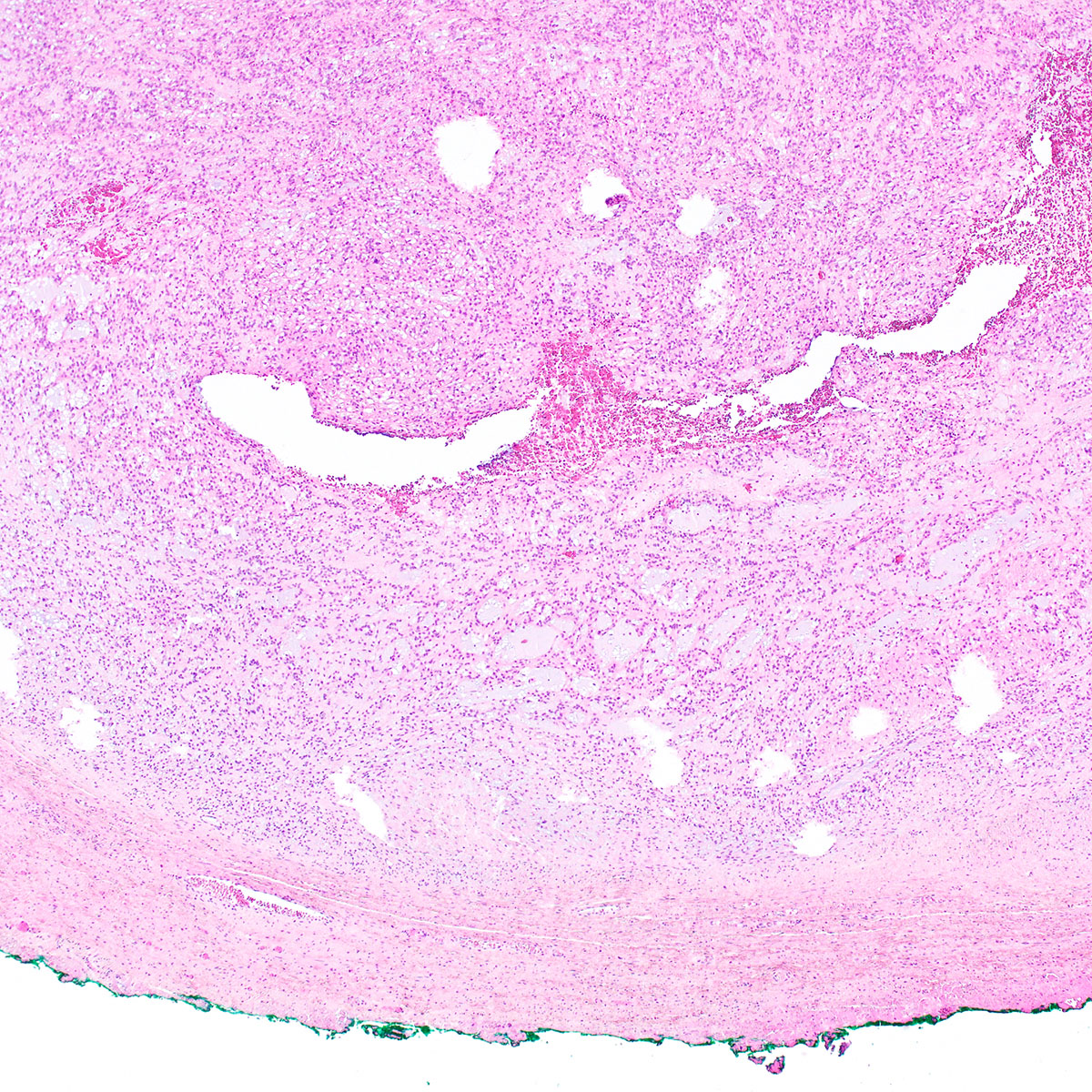
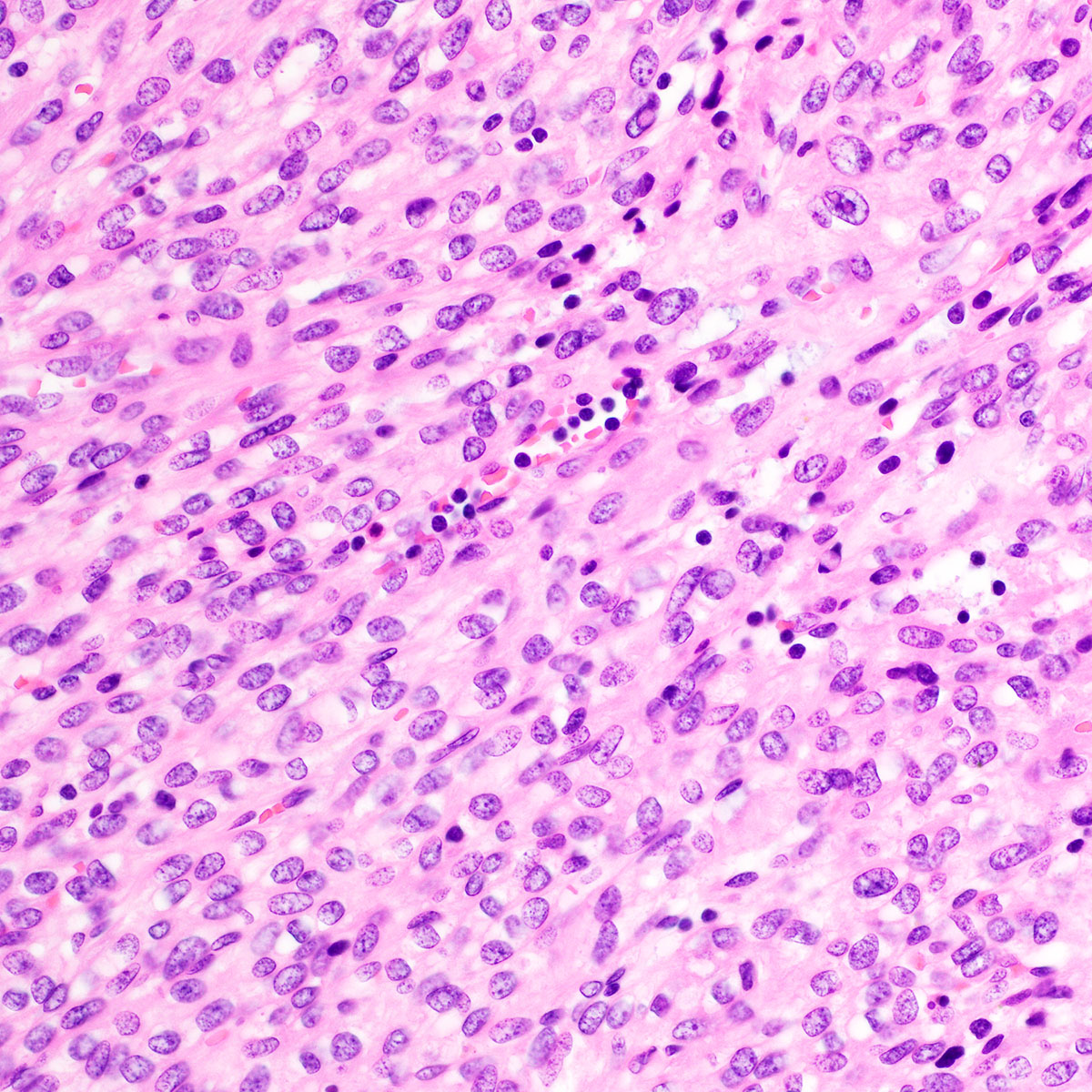
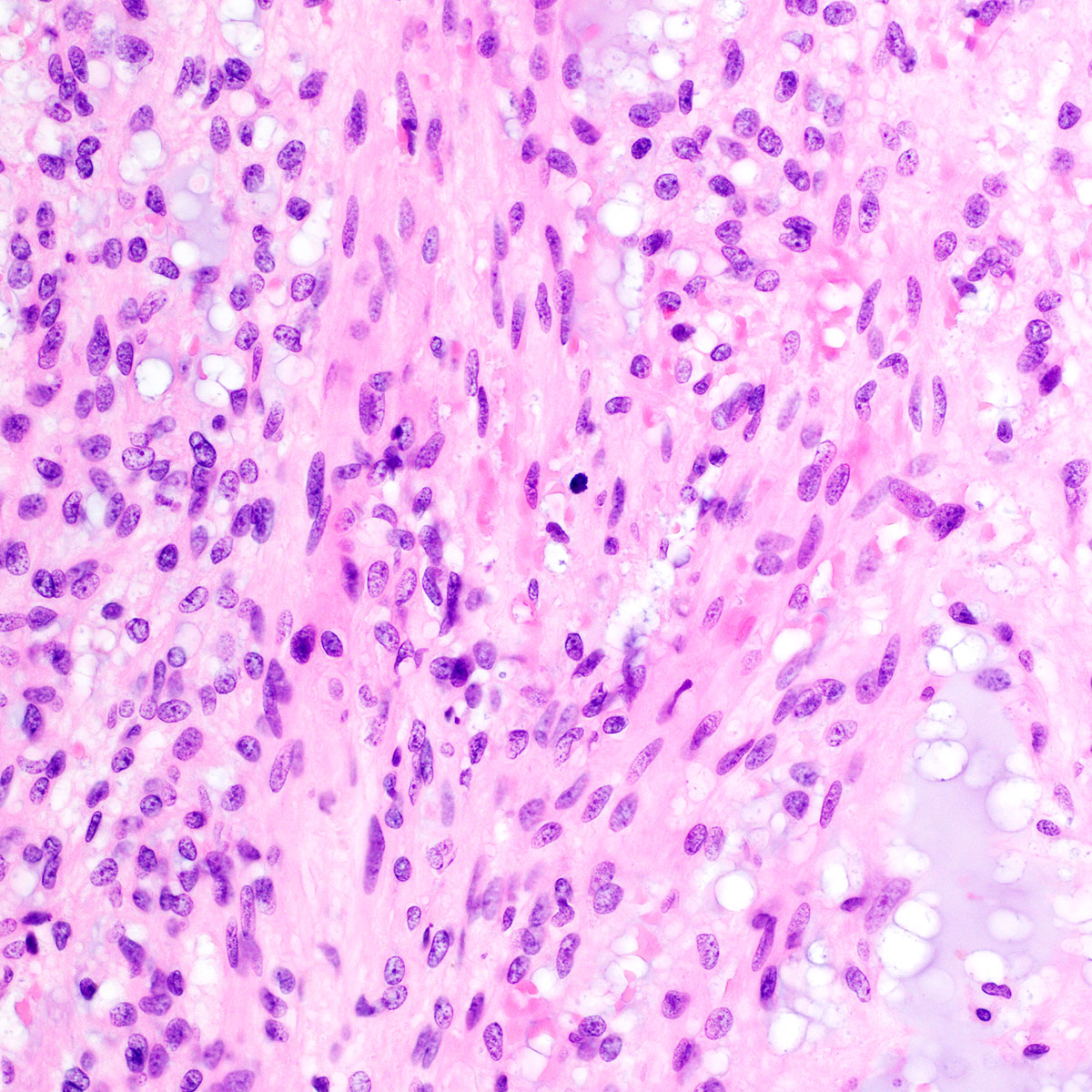
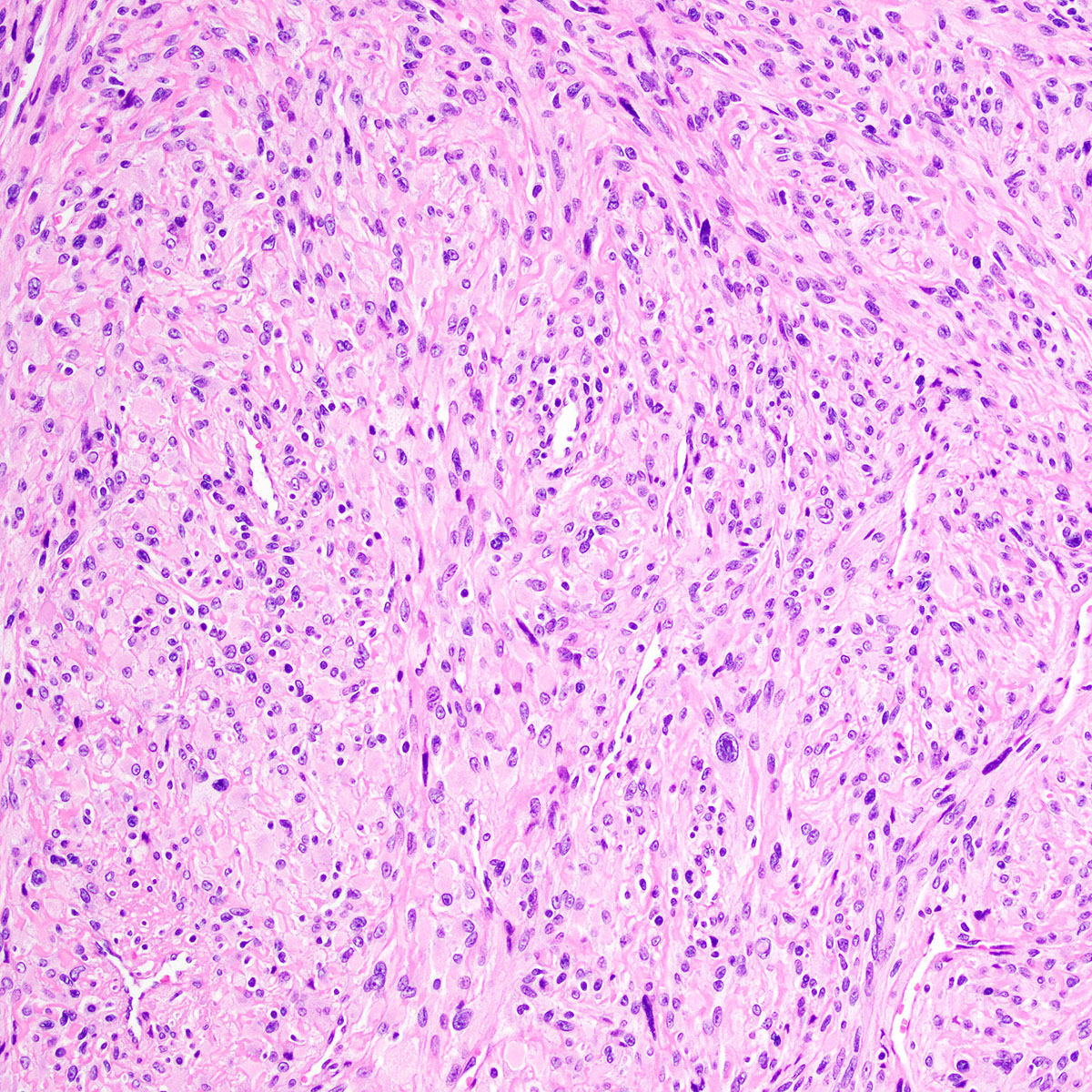
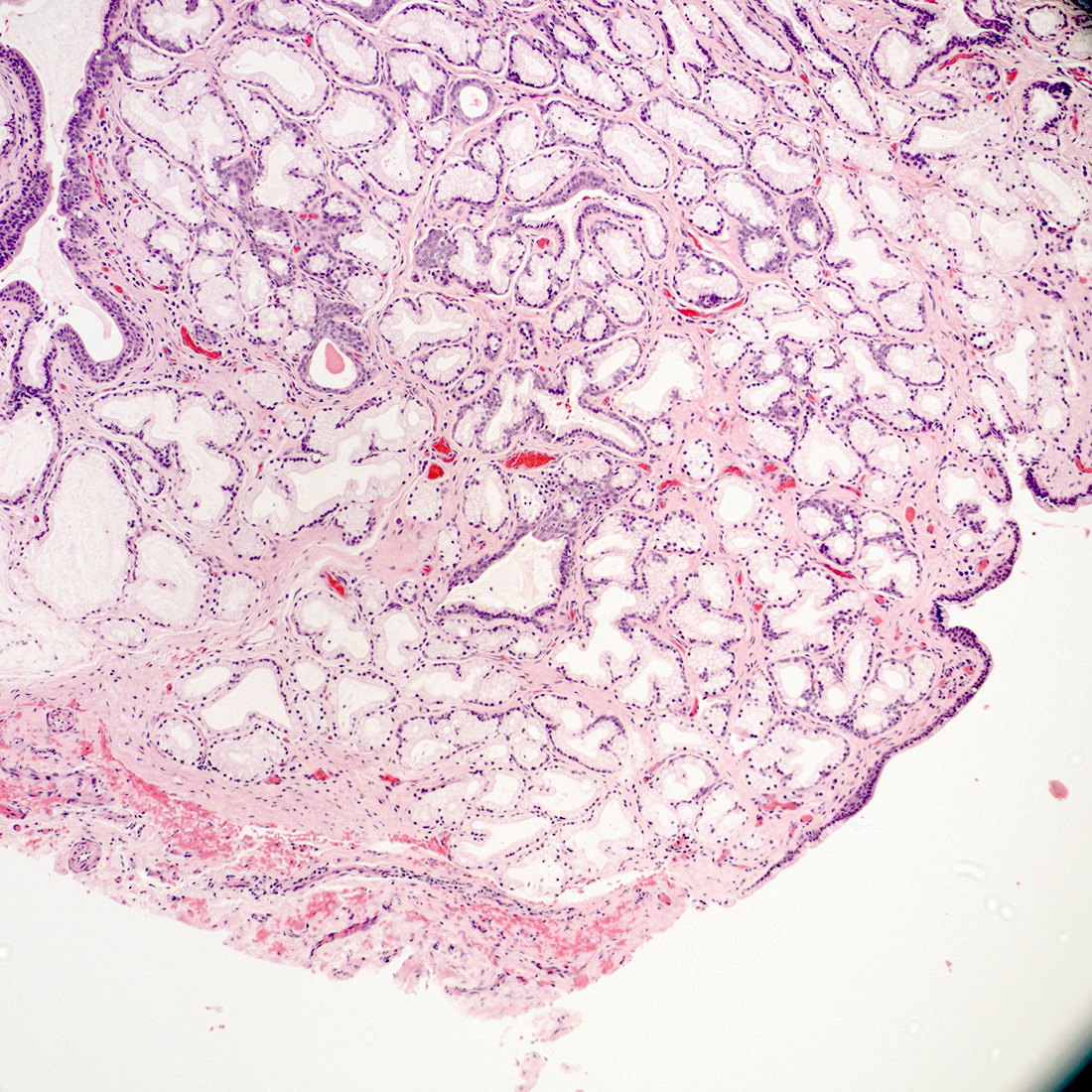
Vaginal adenosis diffuse, rare case
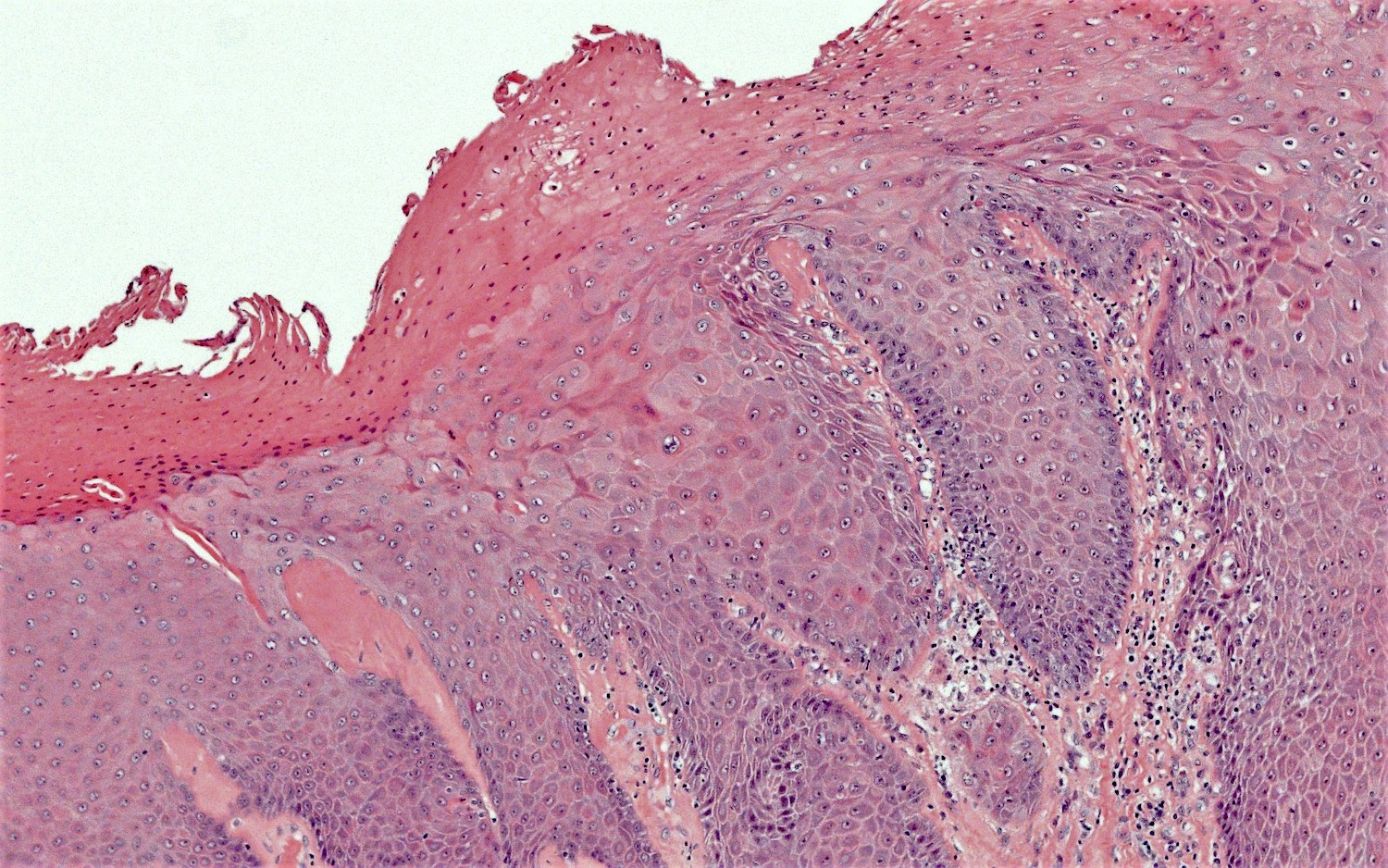
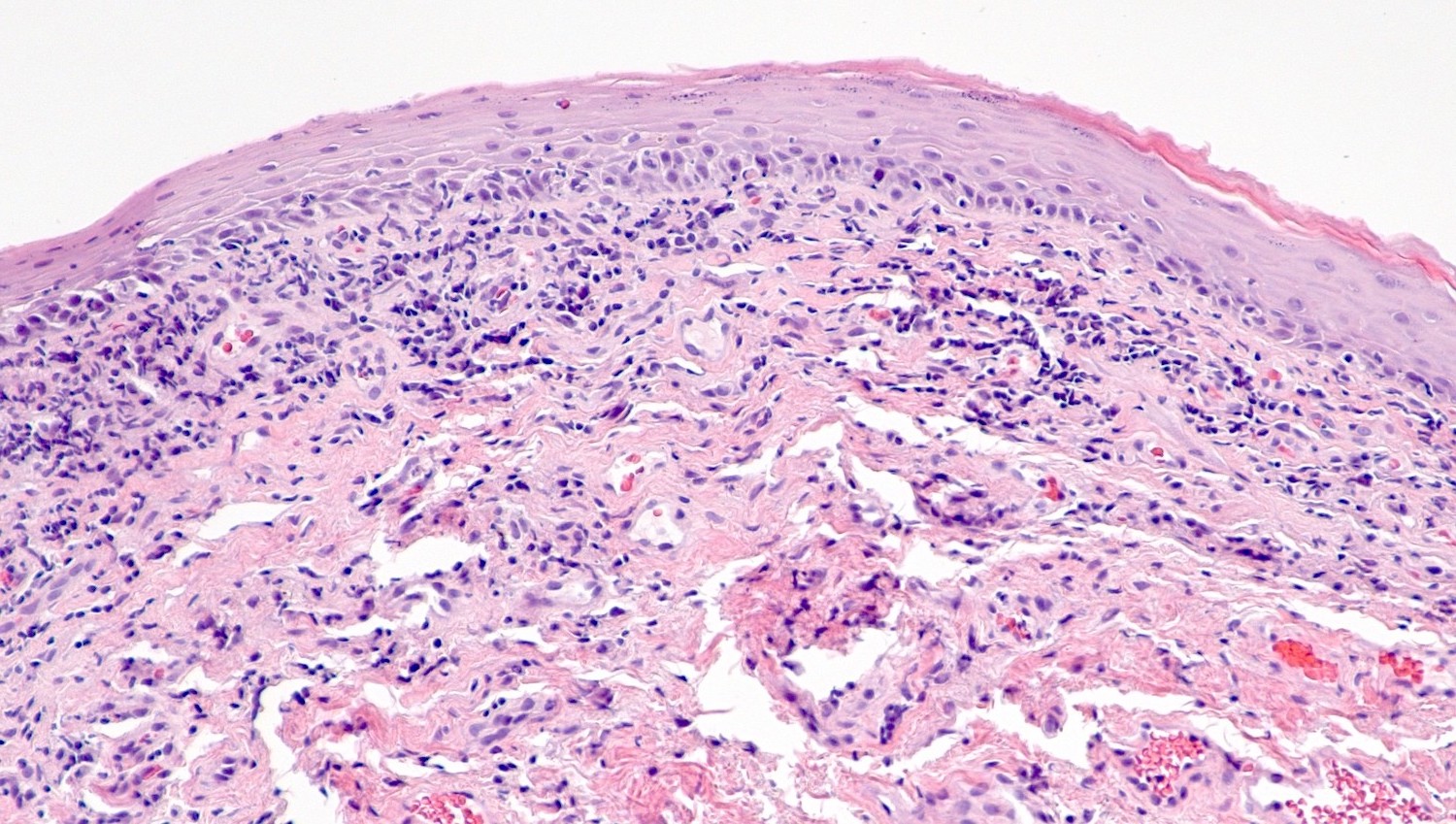
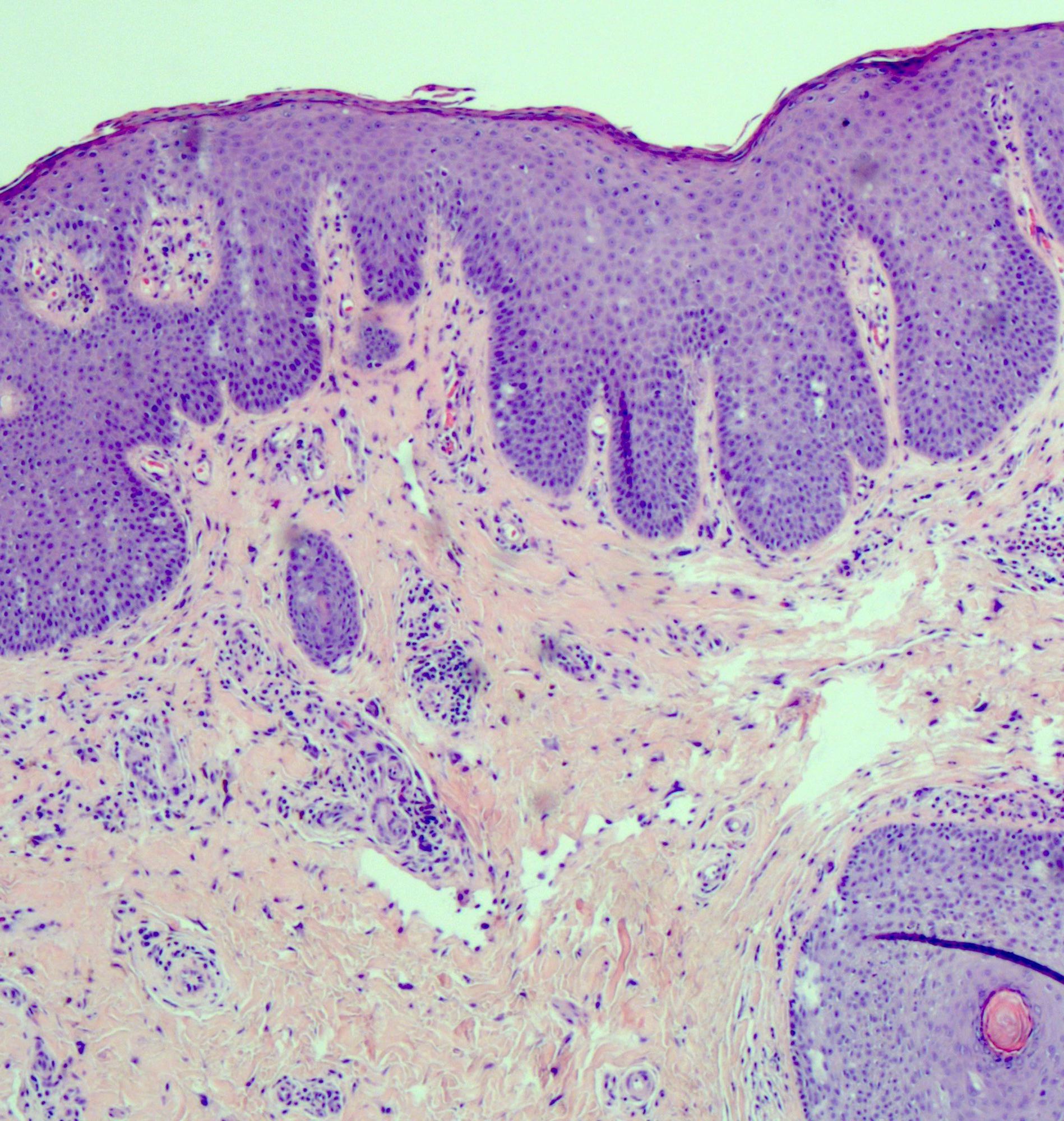
- Vagina, posterior wall, biopsy:
- Squamous mucosa with bland mucinous glands consistent with vaginal adenosis; negative for malignancy
- Clear cell adenocarcinoma (vagina):
- May be confused with the glands of adenosis that undergo microglandular hyperplasia (small, uniform, crowded glands without mucin, nuclear pleomorphism and prominent nucleoli)
- Endometriosis:
- Presence of endometrial stroma with glands that much more closely resemble those of normal endometrium than the glands seen in adenosis
- CD10 is useful to confirm the presence of endometrial stroma
- Mesonephric (Wolffian) remnants:
- Mesonephric tubules are lined by nonciliated, nonmucinous cuboid cells with dense, eosinophilic luminal secretions; these remnants are surrounded by a loose fibrovascular stroma that may contain smooth muscle fibers
- Recurrent endometrial adenocarcinoma:
- Glandular cells in vaginal cytology Papanicolaou tests in patients with hysterectomy for endometrial adenocarcinoma (Diagn Cytopathol 2012;40:138)
A 28 year old woman presents with persistent vaginal discharge and is found to have submucosal mucinous glands on a vaginal biopsy. What is the most likely scenario?
- Maternal grandmother's use of diethylstilbestrol (DES) caused in utero exposure for the patient’s mother
- Maternal use of DES caused in utero exposure for the patient
- The patient had prior treatment for bacterial vaginosis
- The patient had prior treatment for vaginal condyloma acuminata
- Use of DES by the patient
Comment Here
Reference: Vaginal adenosis
- Inapparent on gross examination
- May be associated with microglandular hyperplasia
- Progresses to adenocarcinoma in a majority of the cases
- Usually manifests in prepubertal age
- Usually related to bacterial vaginosis
Comment Here
Reference: Vaginal adenosis
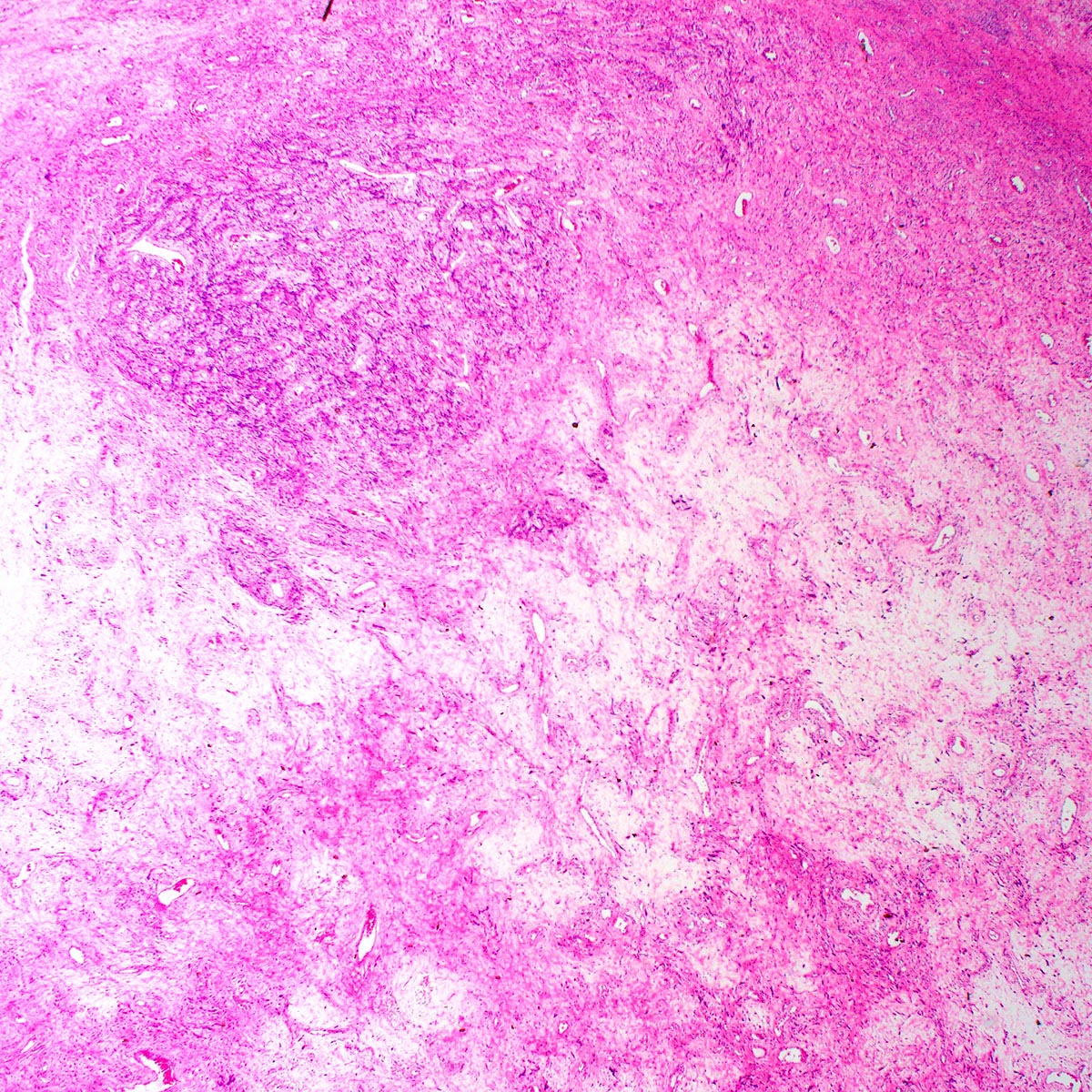
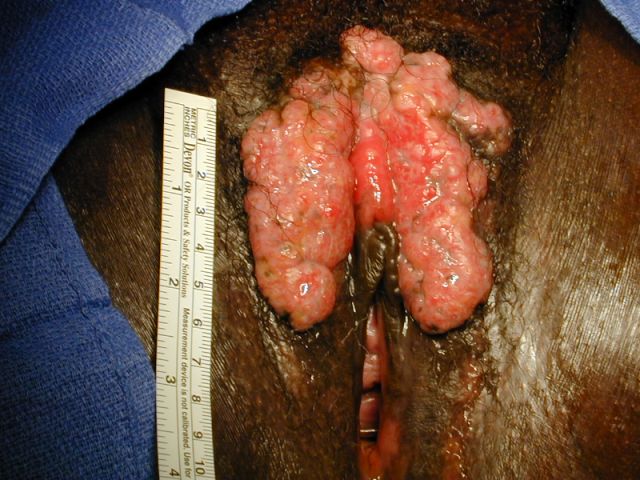
- Aggressive angiomyxoma is an infiltrative spindle cell neoplasm arising in the soft tissues of the lower genital tract, perineum and pelvis; approximately 30% recur locally but distant metastasis is exceptionally rare
- Unique to the soft tissues of the lower genital tract, pelvis and perineum
- Infiltrative hypocellular myxoid lesion with bland spindle cells and prominent variably sized vessels
- HMGA2 overexpressed by immunohistochemistry in > 90%
- Local recurrence in ~ 30% but distant metastasis and death from disease are exceptionally rare
- ICD-10: D48.1 - neoplasm of uncertain behavior of connective and other soft tissue
- F > M (~ 5:1) (Hum Pathol 1985;16:621, Int J Gynecol Cancer 2010;20:303, Arch Gynecol Obstet 2020;302:219)
- Age range: 14 - 77 years (median ~ 40 years) (Cancer 1996;78:79, Int J Gynecol Cancer 2005;15:140, Int J Gynecol Cancer 2010;20:303, Arch Gynecol Obstet 2020;302:219)
- Vulva (Am J Surg Pathol 1983;7:463, Hum Pathol 1985;16:621, Cancer 1996;78:79)
- Perineum
- Deep pelvic (paravaginal, perirectal, ischiorectal) soft tissues
- Gluteal region
- Retroperitoneum
- In men, may affect shaft of penis
- Histogenesis uncertain
- May arise from site specific stromal cells with capacity for fibroblastic, myofibroblastic and smooth muscle differentiation (Cancer 1996;78:79)
- Typically presents as an ill defined, painless, slowly enlarging mass (Am J Surg Pathol 1983;7:463, Hum Pathol 1985;16:621)
- May cause tenderness, pelvic fullness, dyspareunia or frequent urination (Am J Dermatopathol 1993;15:446, Histopathology 1997;30:3)
- Present for months to years prior to presentation (Am J Dermatopathol 1993;15:446, Histopathology 1997;30:3)
- Can clinically resemble a Bartholin cyst, Gartner duct cyst, lipoma or hernia (Am J Surg Pathol 1983;7:463, Am J Dermatopathol 1993;15:446, Histopathology 1997;30:3)
- Physical exam may substantially underestimate size and extent
- May grow rapidly in pregnancy (J Lab Physicians 2018;10:245, Case Rep Obstet Gynecol 2016;2016:8539704)
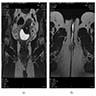
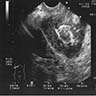
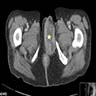
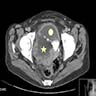
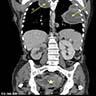
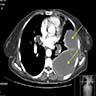
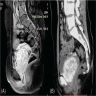
- Radiology may suggest diagnosis and establish extent of local infiltration (Medicine (Baltimore) 2017;96:e6820, World J Clin Cases 2018;6:811)
- Definitive diagnosis on excision specimen
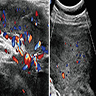
- Ultrasound: shows local infiltration and rich vasculature (Arch Gynecol Obstet 2020;302:219, Medicine (Baltimore) 2017;96:e6820)
- CT: variably dense lesion; highlights local extent of mass (Arch Gynecol Obstet 2020;302:219)
- MRI: hyperintense T2 signal and hypointense T1 signal; highlights local infiltration (J Lab Physicians 2018;10:245, Arch Gynecol Obstet 2020;302:219, Medicine (Baltimore) 2017;96:e6820)
- Local recurrence in ~30% (Am J Surg Pathol 1983;7:463, Hum Pathol 1985;16:621, Cancer 1996;78:79, Pathol Oncol Res 2017;23:131, Histopathology 1997;30:3)
- Positive surgical margins increase recurrence risk (Hum Pathol 1985;16:621, Pathol Oncol Res 2017;23:131, Arch Gynecol Obstet 2020;302:219)
- Recurrences may occur up to 15 years after diagnosis (Am J Surg Pathol 1983;7:463)
- Multiple recurrences not uncommon (Am J Surg Pathol 1983;7:463, Hum Pathol 1985;16:621, Cancer 1996;78:79)
- Distant metastasis and death from disease extremely rare (Hum Pathol 2003;34:1072, Gynecol Oncol Rep 2018;24:15)
- 22 year old woman with vulvar aggressive angiomyxoma (Medicine (Baltimore) 2019;98:e13860)
- 24 year old woman with perineal aggressive angiomyxoma (SAGE Open Med Case Rep 2019;7:2050313X19843391)
- 25 year old pregnant woman with aggressive angiomyxoma (Case Rep Obstet Gynecol 2016;2016:8539704)
- 46 and 61 year old women with pelvic aggressive angiomyxoma (Cureus 2019;11:e4419)
- 47 year old woman with vulvar aggressive angiomyxoma (Gynecol Oncol Rep 2018;24:15)
- Optimal primary management is wide local excision with clear margins (Am J Surg Pathol 1983;7:463, Hum Pathol 1985;16:621, Case Rep Obstet Gynecol 2016;2016:8539704)
- For tumors not amenable to resection, GnRH agonist or radiation therapy may be used at clinical discretion (BMJ Case Rep 2019;12:e227475)
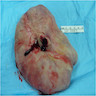
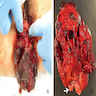
- Large lesions, range 3 - 60 cm (most > 10 cm) (Am J Surg Pathol 1983;7:463, Cancer 1996;78:79, Int J Gynecol Cancer 2005;15:140)
- Poorly defined with gross infiltration of surrounding soft tissue (Histopathology 1997;30:3)
- Lobulated, blue-gray to tan-gray to pink, gelatinous to myxoid to edematous cut surface (Am J Surg Pathol 1983;7:463)
- Punctate hemorrhage in some cases (Hum Pathol 1985;16:621)
- Necrosis absent (Hum Pathol 1985;16:621, Histopathology 1997;30:3)
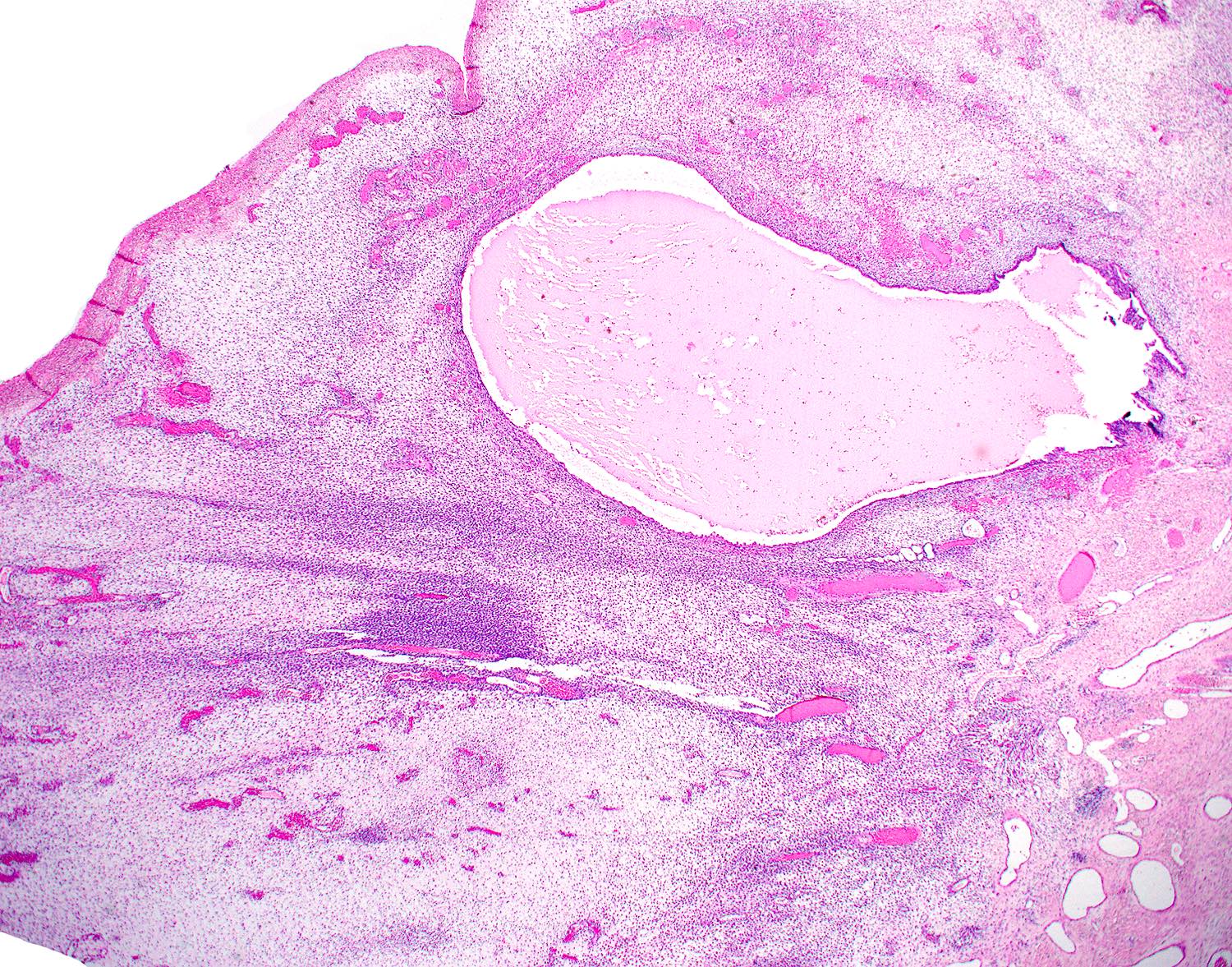
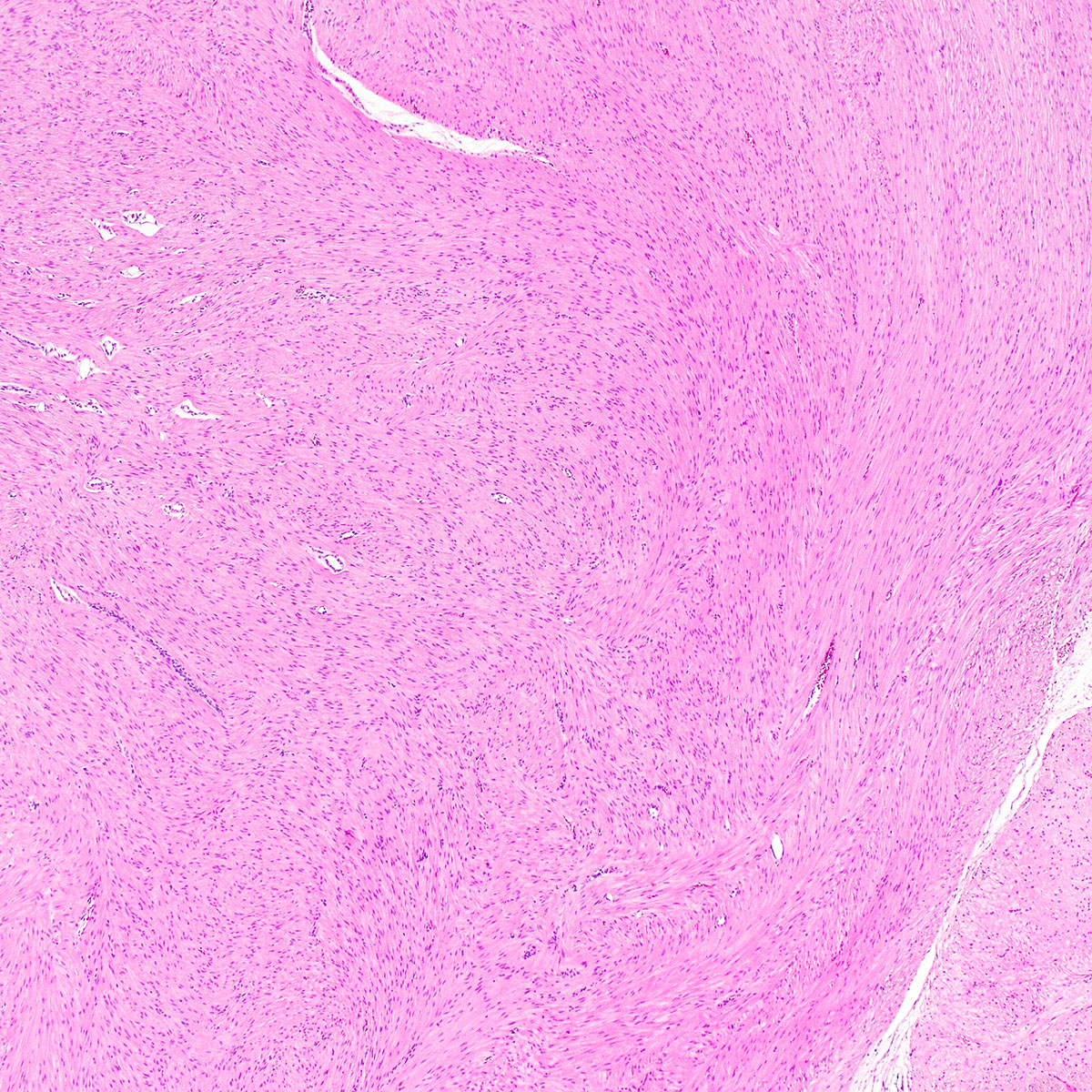
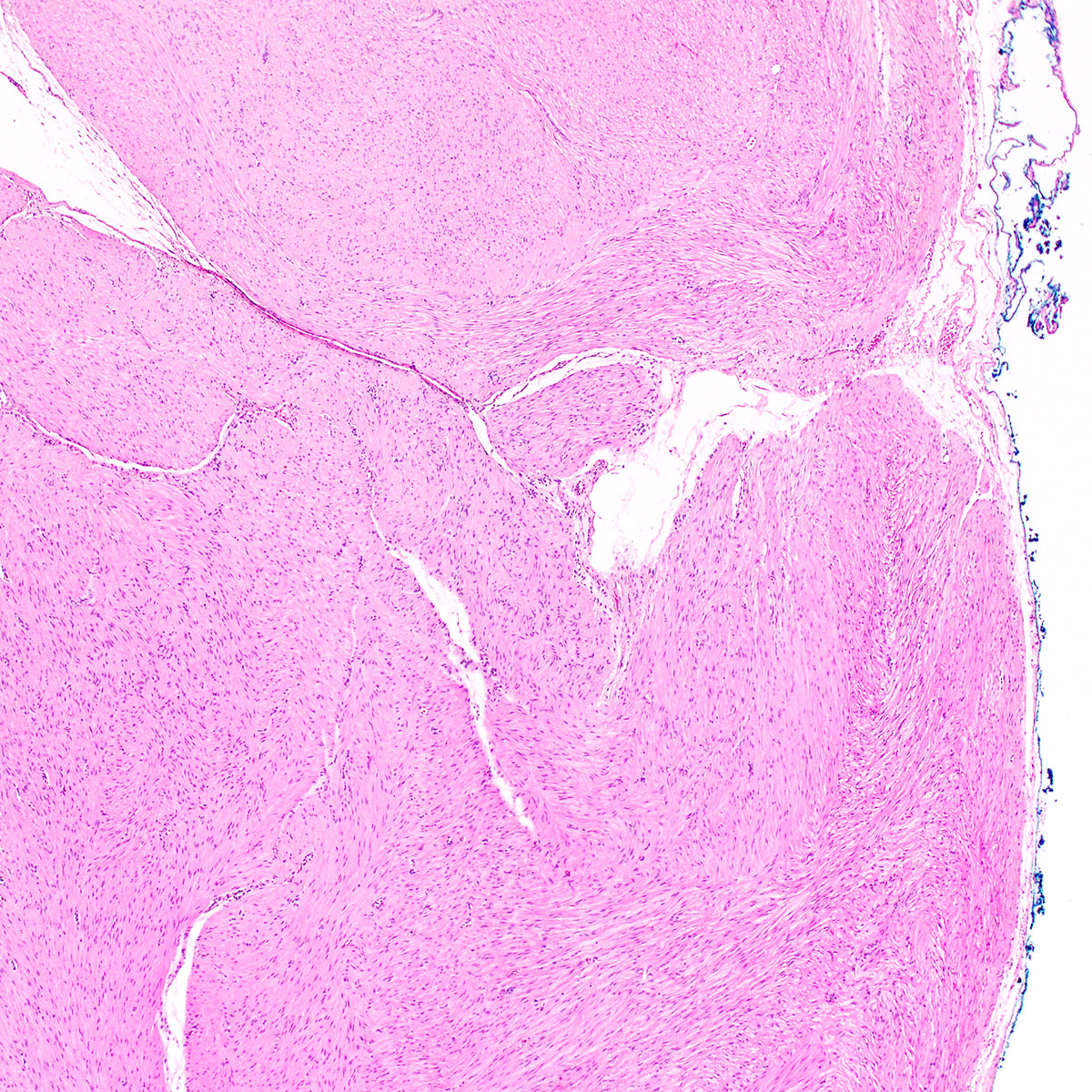
- Unencapsulated and locally infiltrative (Am J Surg Pathol 1983;7:463, Hum Pathol 1985;16:621, Am J Dermatopathol 1993;15:446, Histopathology 1997;30:3)
- Hypocellular (though focally increased cellularity is common) (Histopathology 1997;30:3)
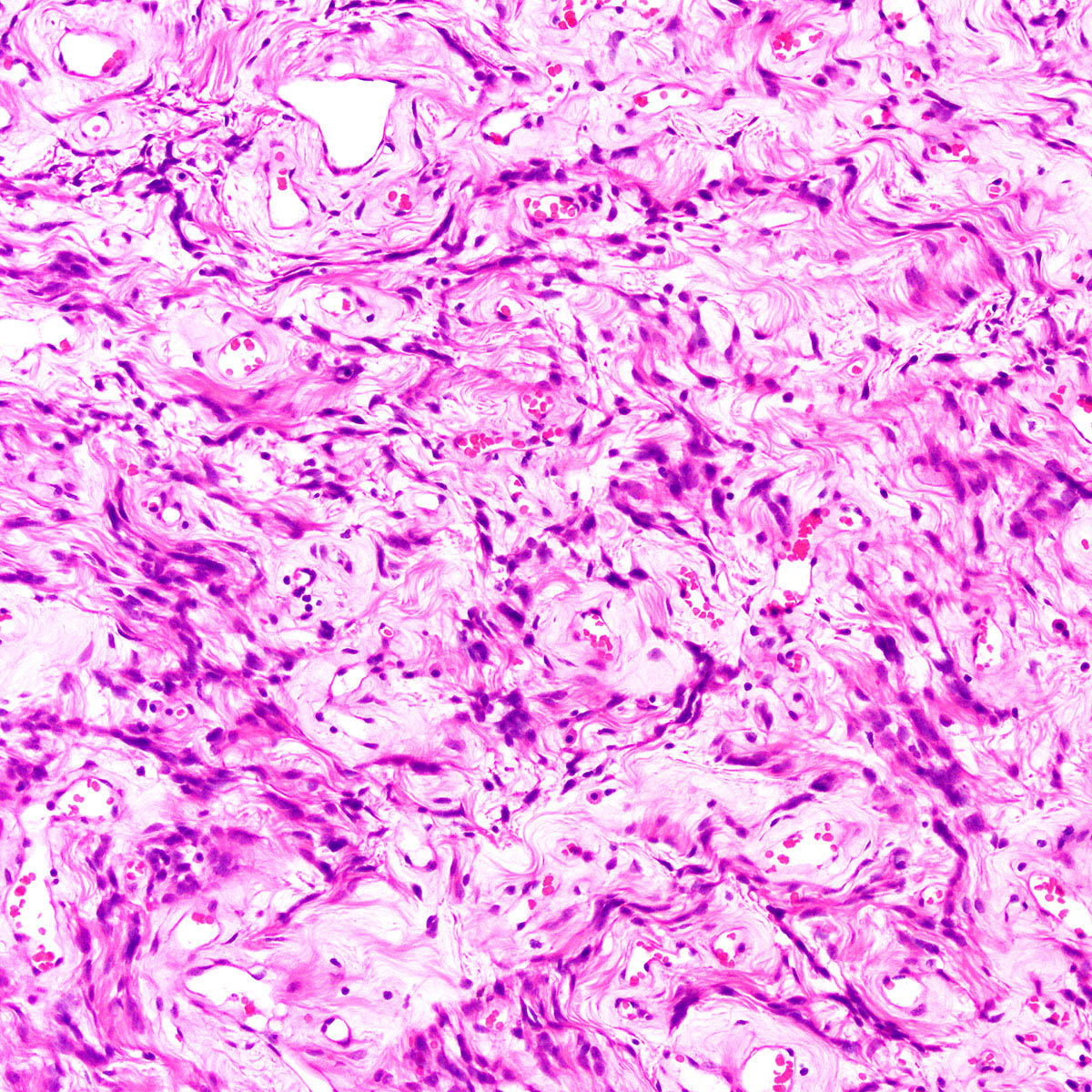
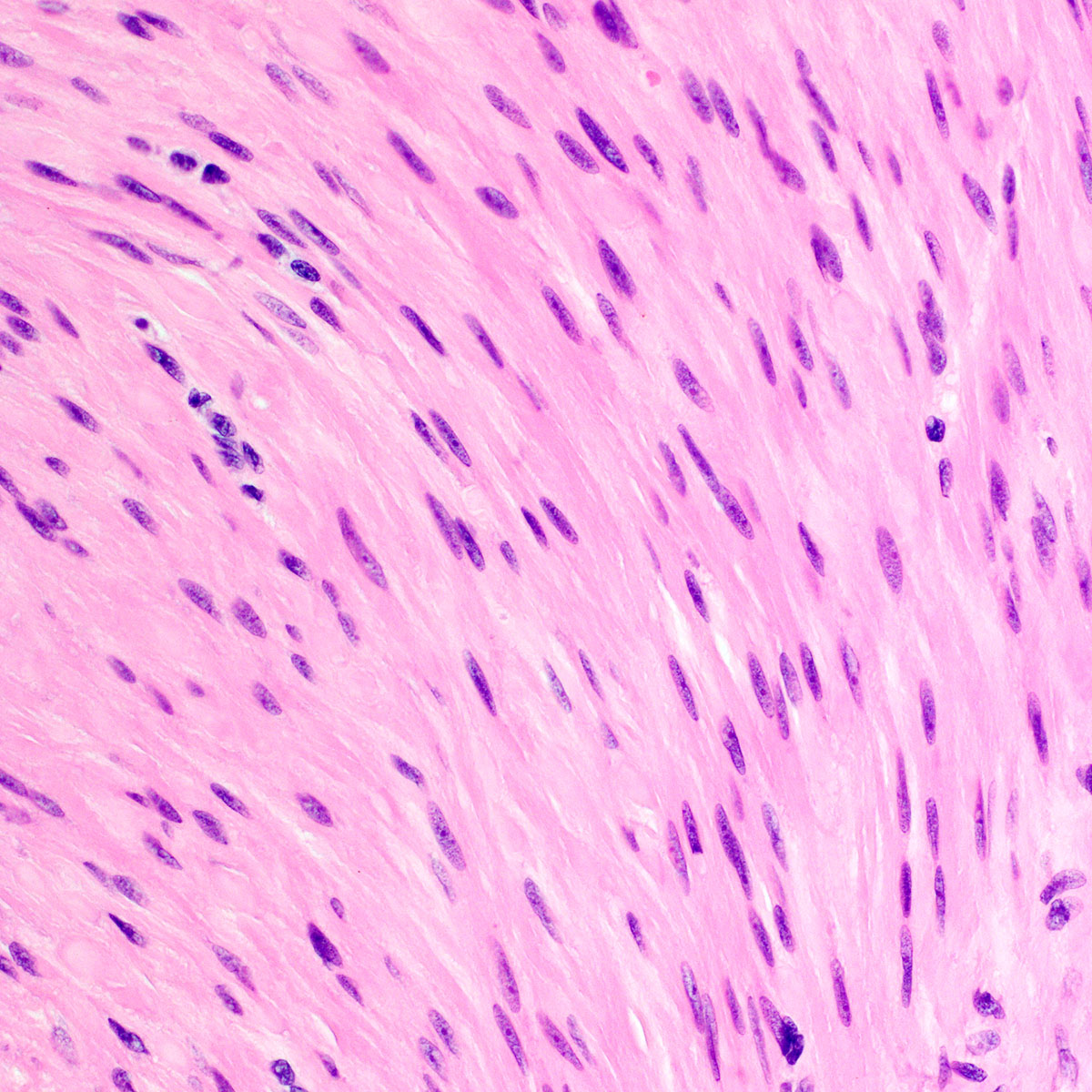
- Tumor cells:
- Spindle to stellate with delicate cytoplasmic processes
- Bland chromatin with small nucleoli
- Mitoses rare or absent
- Tumor stroma:
- Myxoid stroma with scattered delicate collagen fibers
- Stromal mucin positive for Alcian blue
- Stroma peripherally entraps fat, nerves and muscle
- Extravasated red blood cells common (Histopathology 1997;30:3)
- Necrosis absent
- Vasculature:
- Conspicuous haphazard dilated capillaries
- Scattered large, thick walled (medial hypertrophy) or hyalinized vessels
- Vessels are nonanastomosing but may cluster together
- Stromal smooth muscle bundles cluster around tumor vessels (Cancer 1996;78:79, Histopathology 1997;30:3)
- Recurrent lesions may show increased cellularity, increased vasculature and dense stromal collagen
- Rare cases may show minor foci with morphologic features of angiomyofibroblastoma (Histopathology 1997;30:3)
- Myogenic markers: desmin, SMA, calponin, MSA
- May be focal
- Highlight perivascular myoid bundles (Cancer 1996;78:79, Histopathology 1997;30:3)
- Hormone receptors: ER, PR (Cancer 1996;78:79, Int J Gynecol Pathol 2007;26:494, Ultrastruct Pathol 2003;27:227)
- HMGA2 (90%) (Genes Chromosomes Cancer 2001;32:172, Mod Pathol 2010;23:1657)
- CD34 (~ 50%) (Cancer 1996;78:79, Ultrastruct Pathol 2003;27:227)
- CDK4 often positive but without MDM2 co-expression (Virchows Arch 2005;446:157)
- Variable fibroblastic and myofibroblastic differentiation (Am J Surg Pathol 1983;7:463, Hum Pathol 1985;16:621, Am J Dermatopathol 1993;15:446, Ultrastruct Pathol 2003;27:227)
- Balanced HMGA2 rearrangement (chr 12) in approximately 33% (Genes Chromosomes Cancer 2007;46:981)
- HMGA2 rearrangements and overexpression are confined to stromal spindle cells (Genes Chromosomes Cancer 2001;32:172)
- HMGA2 breakpoints and fusion partners highly variable (Genes Chromosomes Cancer 2001;32:172, Virchows Arch 2006;448:838, Int J Gynecol Pathol 2007;26:494, Cancer Genet Cytogenet 2008;181:119)
- Mechanism of HMGA2 overexpression in cases without HMGA2 rearrangement is unclear
Histopathology
- Vulva, mass, wide local excision:
- Aggressive angiomyxoma with involvement of the deep margin (see comment)
- Comment: Microscopic examination reveals a poorly circumscribed hypocellular lesion with myxoid stroma and abundant vasculature, infiltrating fibroadipose tissue. By immunohistochemistry, lesional stromal cells are positive for desmin, ER, PR and HMGA2. The morphologic and immunophenotypic findings are most consistent with aggressive angiomyxoma. Although distant metastasis is exceptionally rare, approximately 30% of such lesions recur locally. Clinical follow up is advised.
- Angiomyofibroblastoma:
- Well circumscribed, noninfiltrative
- Hypo and hypercellular foci
- Epithelioid to plasmacytoid tumor cells
- Tumor cells cluster around small to medium sized, nonhyalinized, thin walled vessels
- HMGA2 negative (rarely positive) (Int J Gynecol Pathol 2021;40:185)
- Lacks HMGA2 rearrangements
- Superficial angiomyxoma:
- Myxoid neurofibroma:
- Characteristic buckled nuclei
- Vasculature less conspicuous
- Contain intrinsic nerve fibers or Meissner (neuroid) bodies
- S100 positive
- Subset occur in context of neurofibromatosis
- Myxoid smooth muscle tumor:
- Cigar shaped nuclei with eosinophilic cytoplasm, at least focally
- Less abundant, large, thick walled vessels
- SMA, desmin and caldesmon positive
- HMGA2 IHC may be positive in a subset (Int J Gynecol Pathol 2021;40:185)
- Myxoid liposarcoma:
- Rarely primary in gynecologic tract (Int J Gynecol Pathol 2015;34:390)
- Lipoblasts present; may be more conspicuous peripherally
- Subset shows cellular, less differentiated round cell component
- Characteristic plexiform (crow's feet) vasculature
- ER and PR negative
- DDIT3 - FUS (t(12;16)) fusion
- Atypical lipomatous tumor / well differentiated liposarcoma:
- Rarely primary in gynecologic tract (Int J Gynecol Pathol 1998;17:17, Int J Gynecol Pathol 2015;34:390)
- Atypical mature fat with scattered hyperchromatic cells
- Vasculature less conspicuous
- CDK4 and MDM2 co-expressed
- ER and PR negative
- Underlying MDM2, CDK4, HMGA2 (chr 12) amplification
- Low grade myxofibrosarcoma:
- Low grade fibromyxoid sarcoma:
- Vulvar soft tissue tumor review (Semin Diagn Pathol 2021;38:85), HMGA2 rearrangement in aggressive angiomyxoma (Int J Gynecol Pathol 2006;25:403), fallopian tube prolapse mimicking aggressive angiomyxoma (Int J Gynecol Pathol 2005;24:292), aggressive angiomyxoma mimicking myxoid liposarcoma (Am J Dermatopathol 2000;22:368)
A 35 year old woman presented to her gynecologist with a complaint of pelvic discomfort and a slowly growing, ill defined vulvar mass. Radiology showed a vascular lesion extending from the vulva into deep pelvic soft tissues. An excision was performed. A representative photomicrograph of the lesion is shown. By immunohistochemistry, the lesional stromal cells were positive for desmin, ER and PR. Which of the following statements about this lesion is true?
- Approximately 50% of patients develop distant metastases
- FISH reveals a characteristic DDIT3 rearrangement
- HMGA2 is positive (overexpressed) by immunohistochemistry
- Immunohistochemistry for S100 is characteristically positive
- Less than 10% of patients experience local recurrence
Comment Here
Reference: Aggressive angiomyxoma-vulva
- Desmin, ER, PR, HMGA2 positive; S100 negative
- S100, EMA, NF positive; ER, PR negative
- SMA, CD34 positive; ER, PR negative
- SMA, desmin, caldesmon positive; S100 negative
- SMA, ER, PR positive; Rb negative (lost)
Comment Here
Reference: Aggressive angiomyxoma-vulva
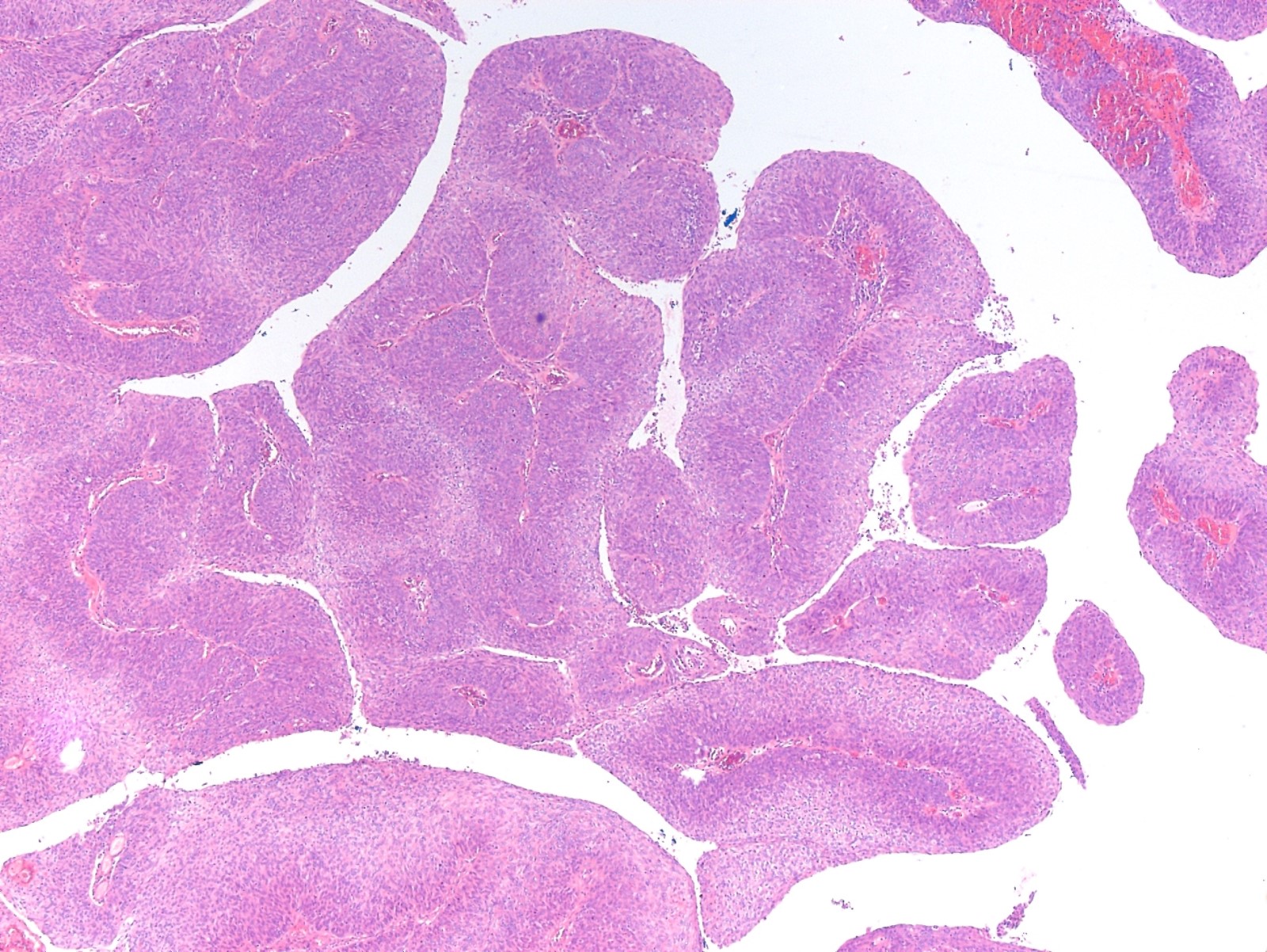
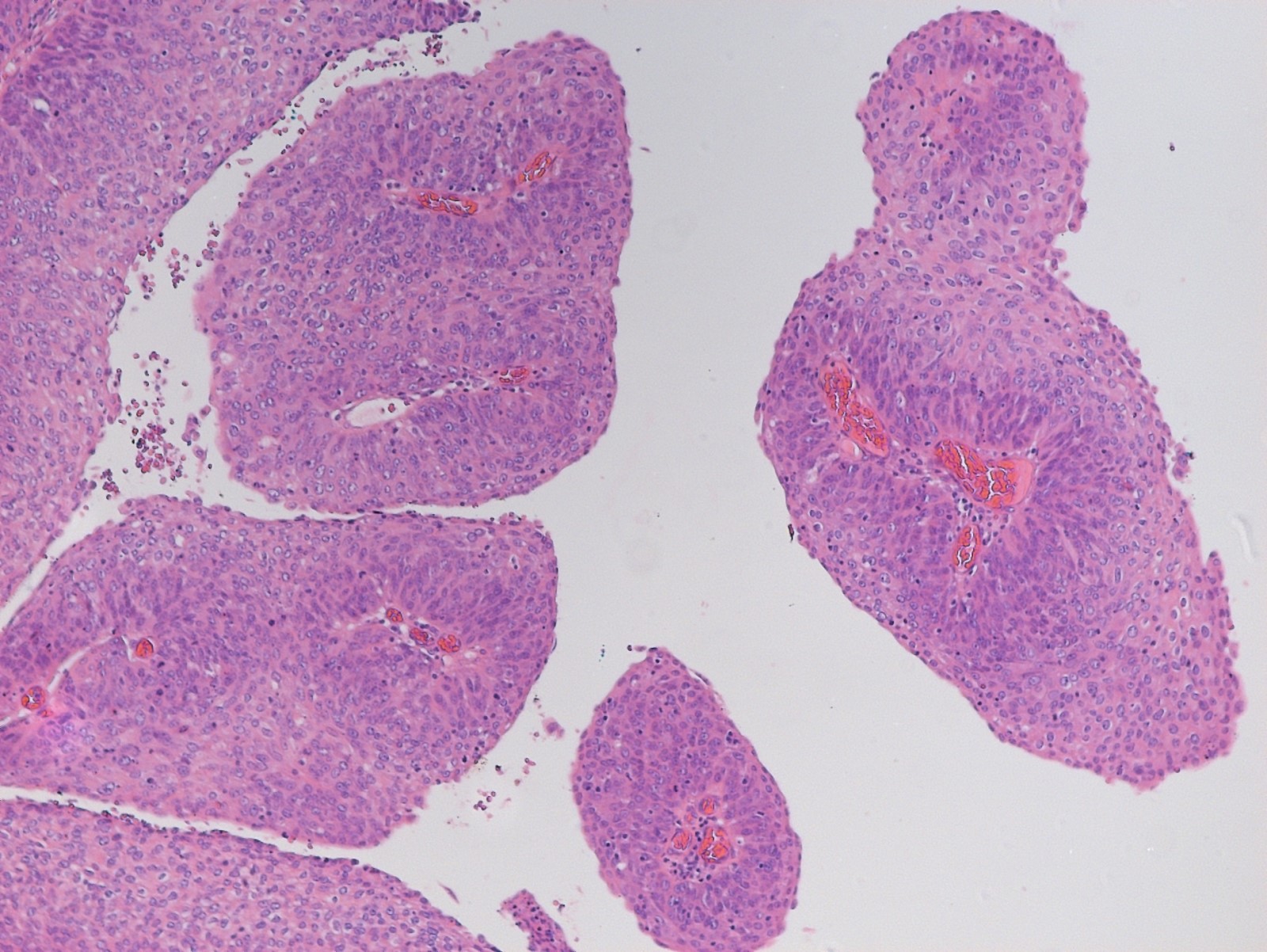
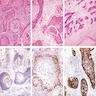
- Alveolar soft part sarcoma (ASPS) is a morphologically distinctive neoplasm of unknown histogenesis / cell lineage, which may rarely occur in the female genital tract
- Alveolar soft part sarcoma is a high grade sarcoma by definition (no formal grading system available)
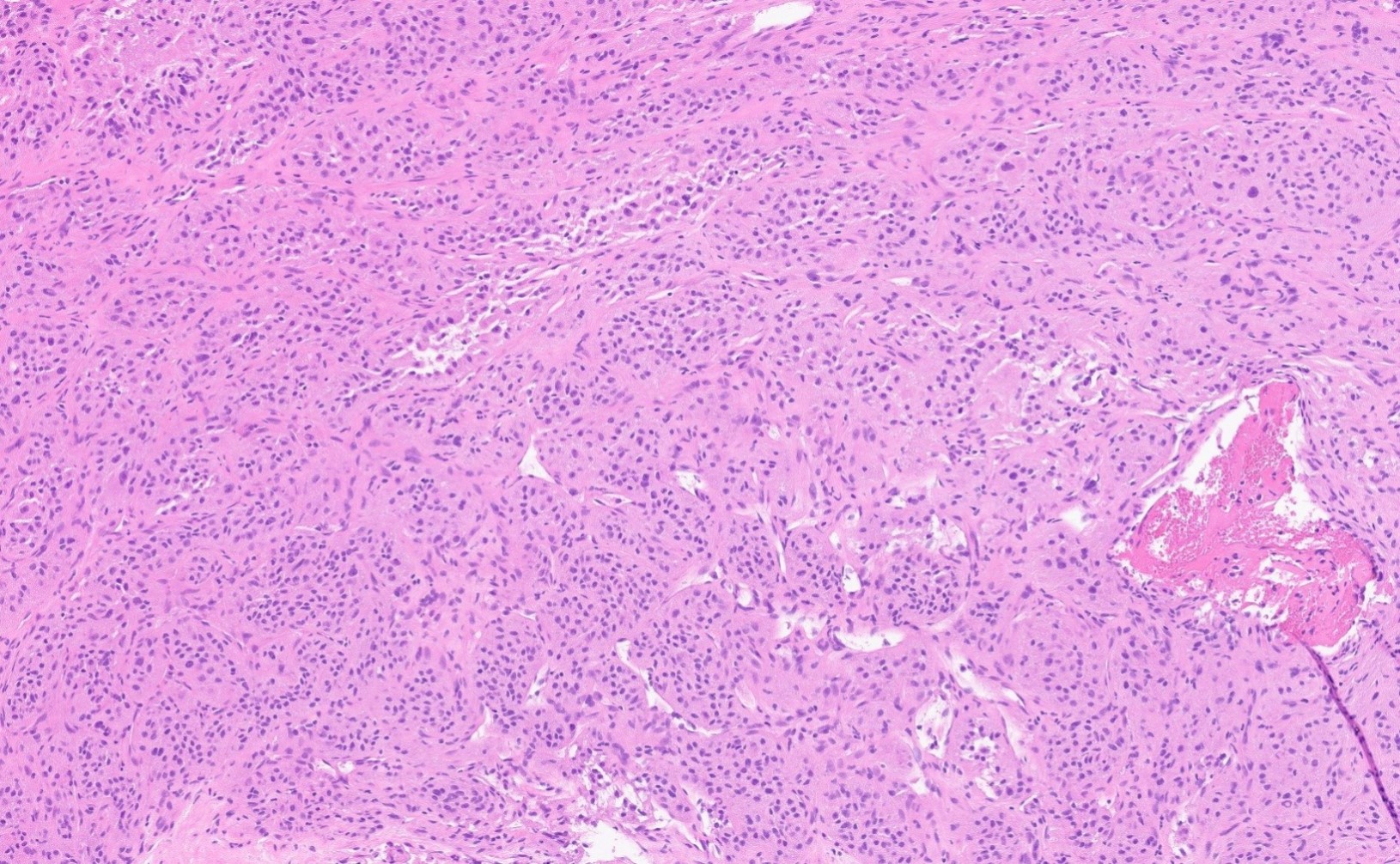
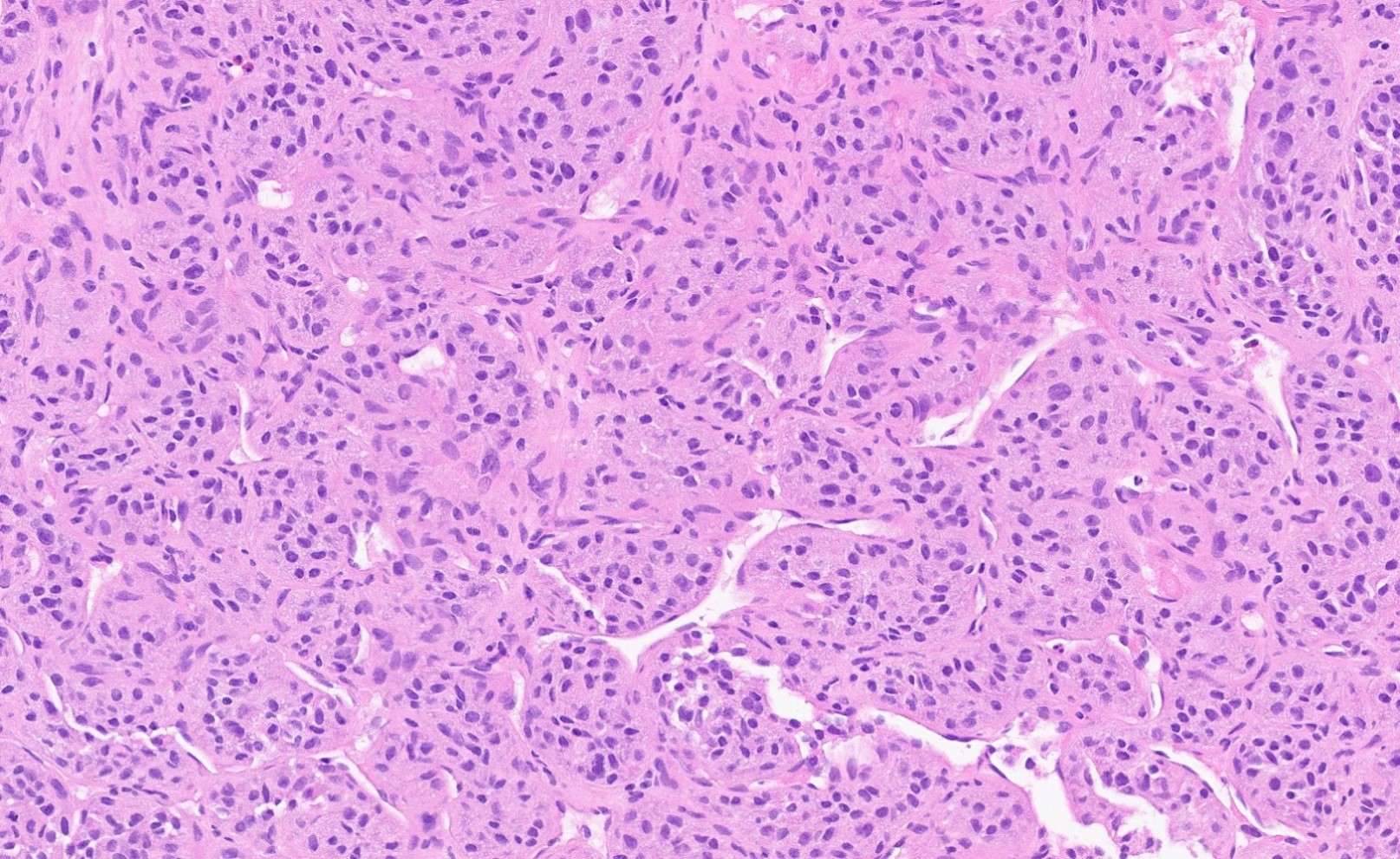
- Rare malignant mesenchymal neoplasm exhibiting nested or solid growth patterns with pseudoalveolar spaces
- Composed of large, polygonal cells with abundant eosinophilic cytoplasm and PASD+ intracytoplasmic granules or crystals
- Harbors characteristic ASPSCR1::TFE3 gene fusion
- Alveolar soft part sarcoma is the preferred WHO terminology
- ICD-O: 9581/3 - alveolar soft part sarcoma
- ICD-10: C49.9 - malignant neoplasm of connective and soft tissue, unspecified
- ICD-11: 2B5F.2 & XH8V95 - sarcoma, not elsewhere classified of other specified sites & alveolar soft part sarcoma
- Alveolar soft part sarcoma of the female genital tract is rare; 50 - 60 cases published to date (Int J Womens Health 2024;16:17)
- Affects women over a wide age range (8 - 68 years), with most cases diagnosed in the third and fourth decades (Am J Surg Pathol 2017;4:622)
- Alveolar soft part sarcoma of nongenital sites also shows a ~2:1 female predilection (J Surg Oncol 2016;113:581)
- ASPS can affect any site in the female genital tract, most commonly cervix (cervix > uterine corpus > vagina) (Int J Gynecol Pathol 1995;14:283, Am J Surg Pathol 2017;4:622, Int J Gynecol Pathol 2014;33:263)
- Vulvar, perineal and adnexal tumors are very rare
- Overall, ASPS is much more common in deep soft tissues of the extremities and head and neck than in the female genital tract
- Unbalanced translocation: der(17)t(X:17)(p11;p25)
- Translocation results in fusion of ASPSCR1 (ASPL) gene on chromosome 17 to the TFE3 gene on X chromosome
- ASPSCR1 gene is joined in frame upstream of either the third or fourth exon of TFE3, yielding 2 fusion variants (type 1 and type 2) (J Clin Pathol 2006;59:1127)
- No risk factors have been identified
- No associated germline mutations
- Uterine, cervical and vaginal tumors typically present with vaginal / abnormal uterine bleeding or menstrual cycle shortening (Int J Clin Exp Pathol 2017;10:9812, Int J Gynecol Pathol 1995;14:283)
- May also be discovered incidentally during pregnancy or at surgery for an unrelated cause
- Rare vulvar or perineal tumors present as a painless mass (Int J Gynecol Pathol 2014;33:263)
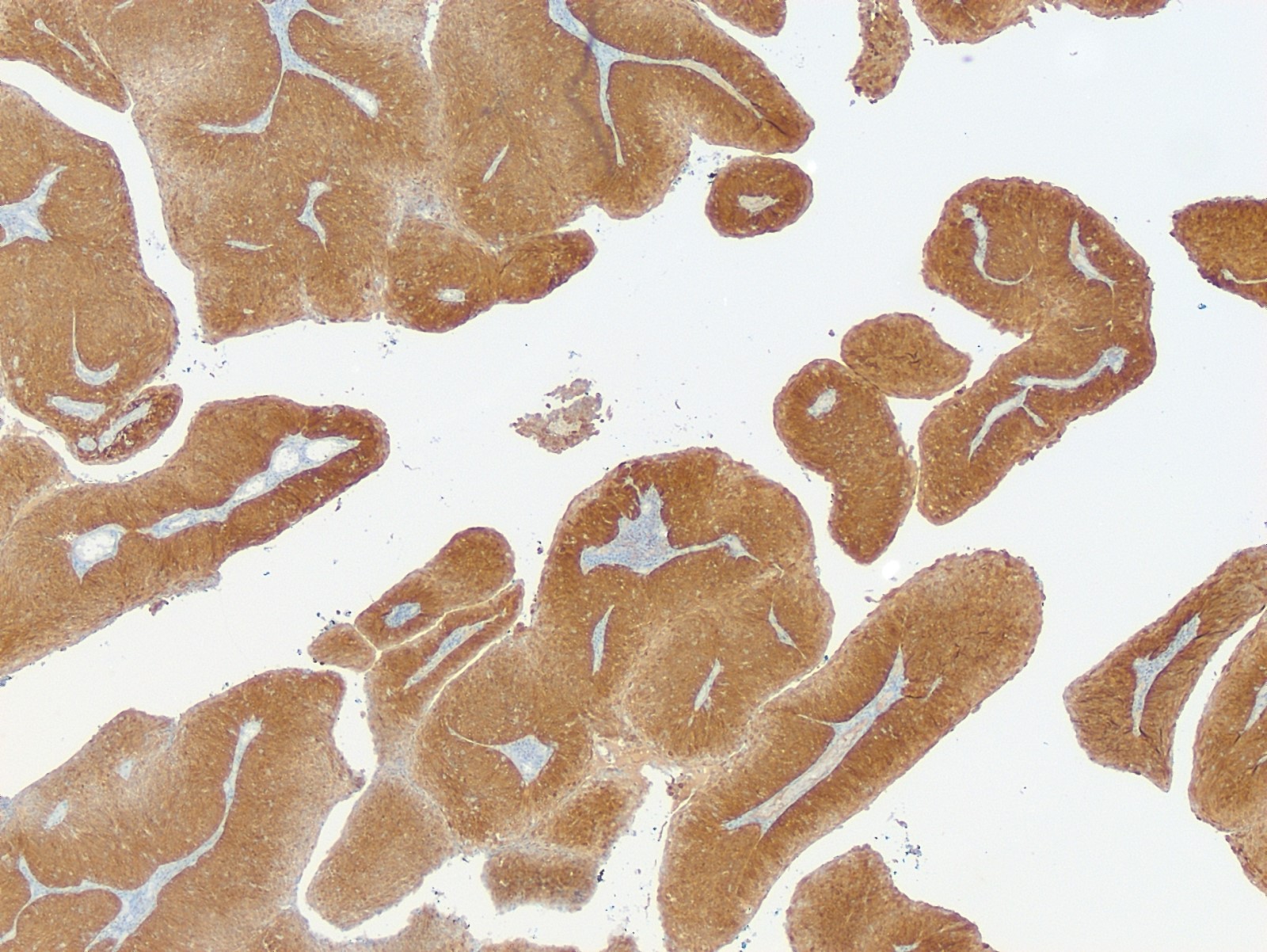
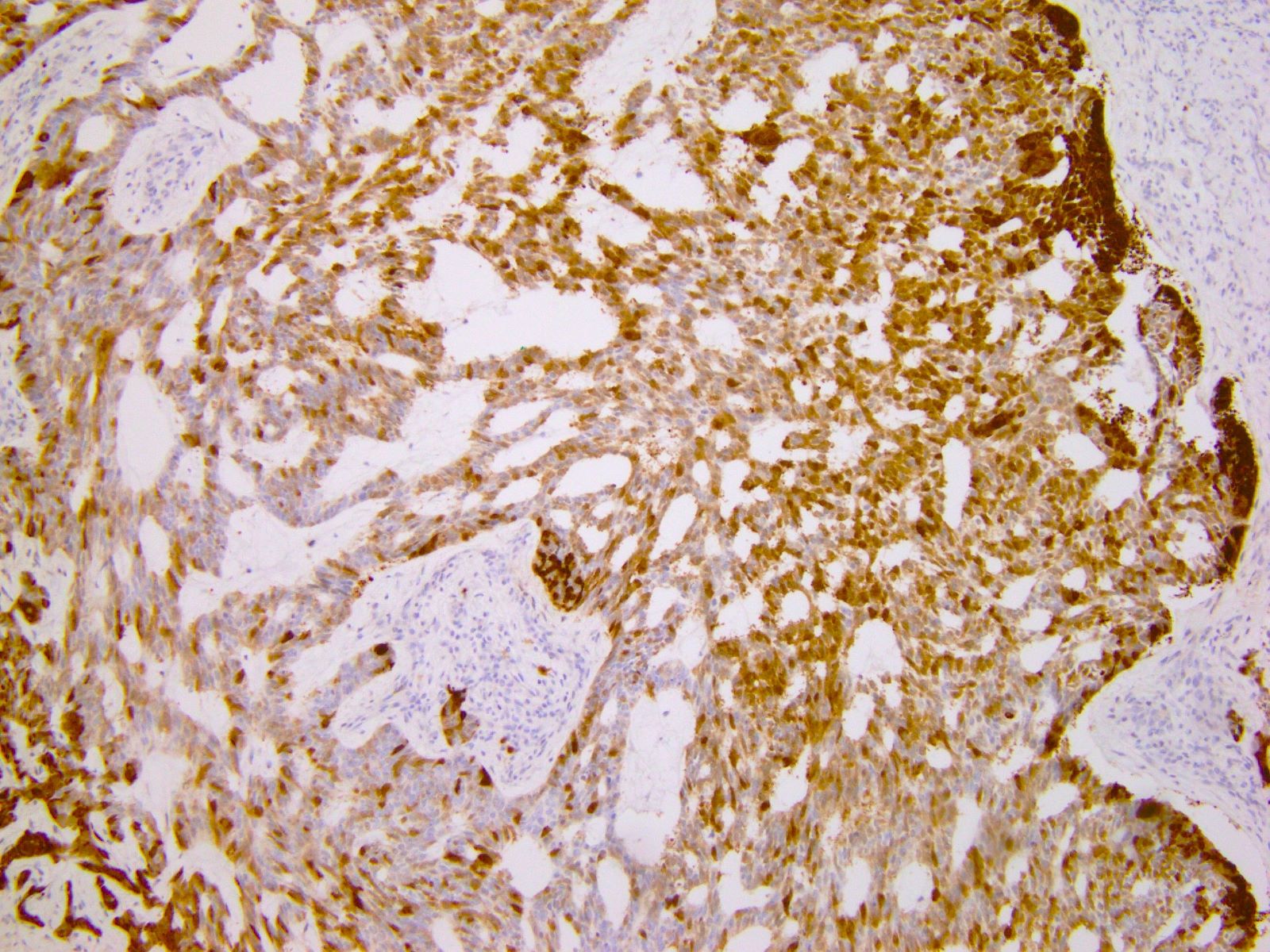
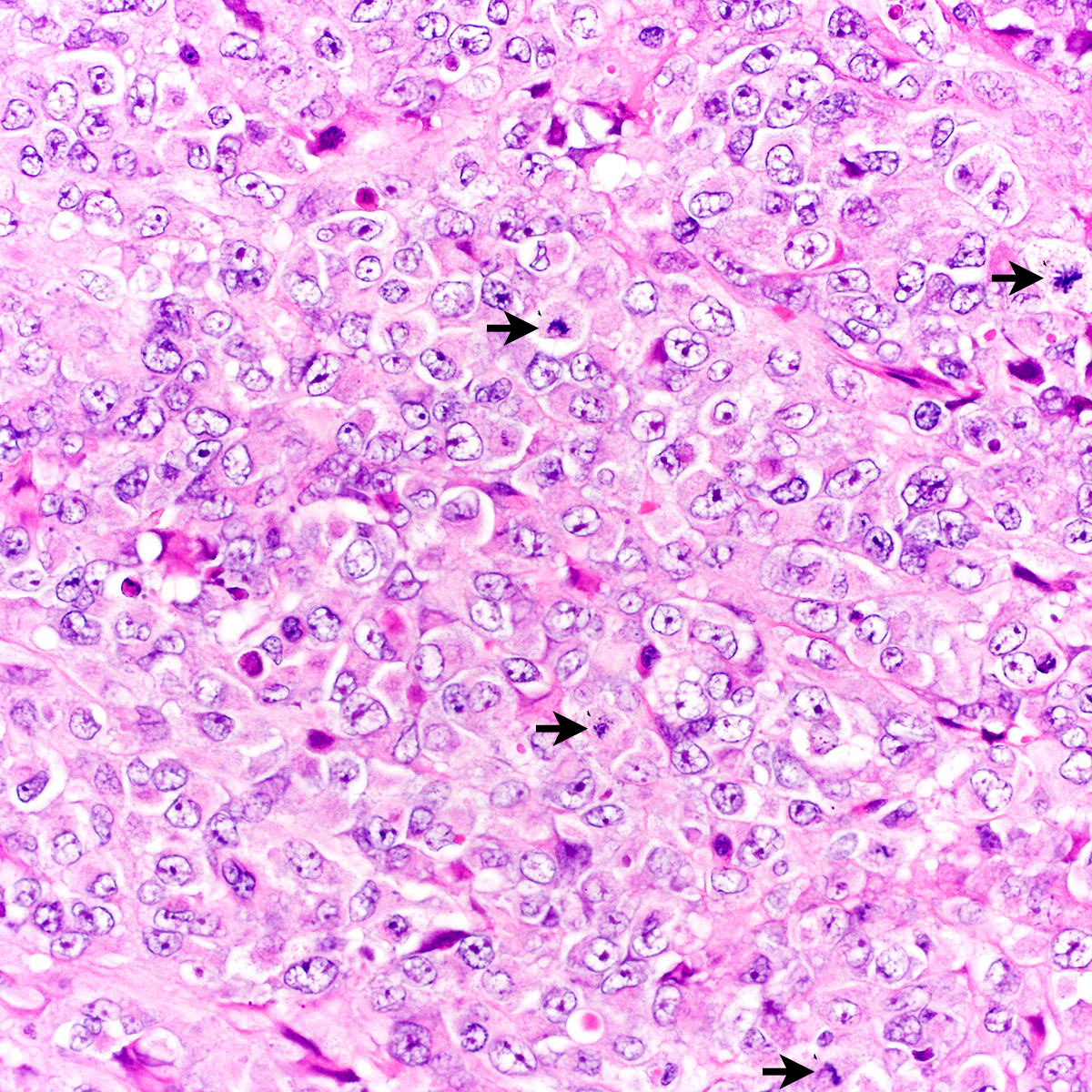
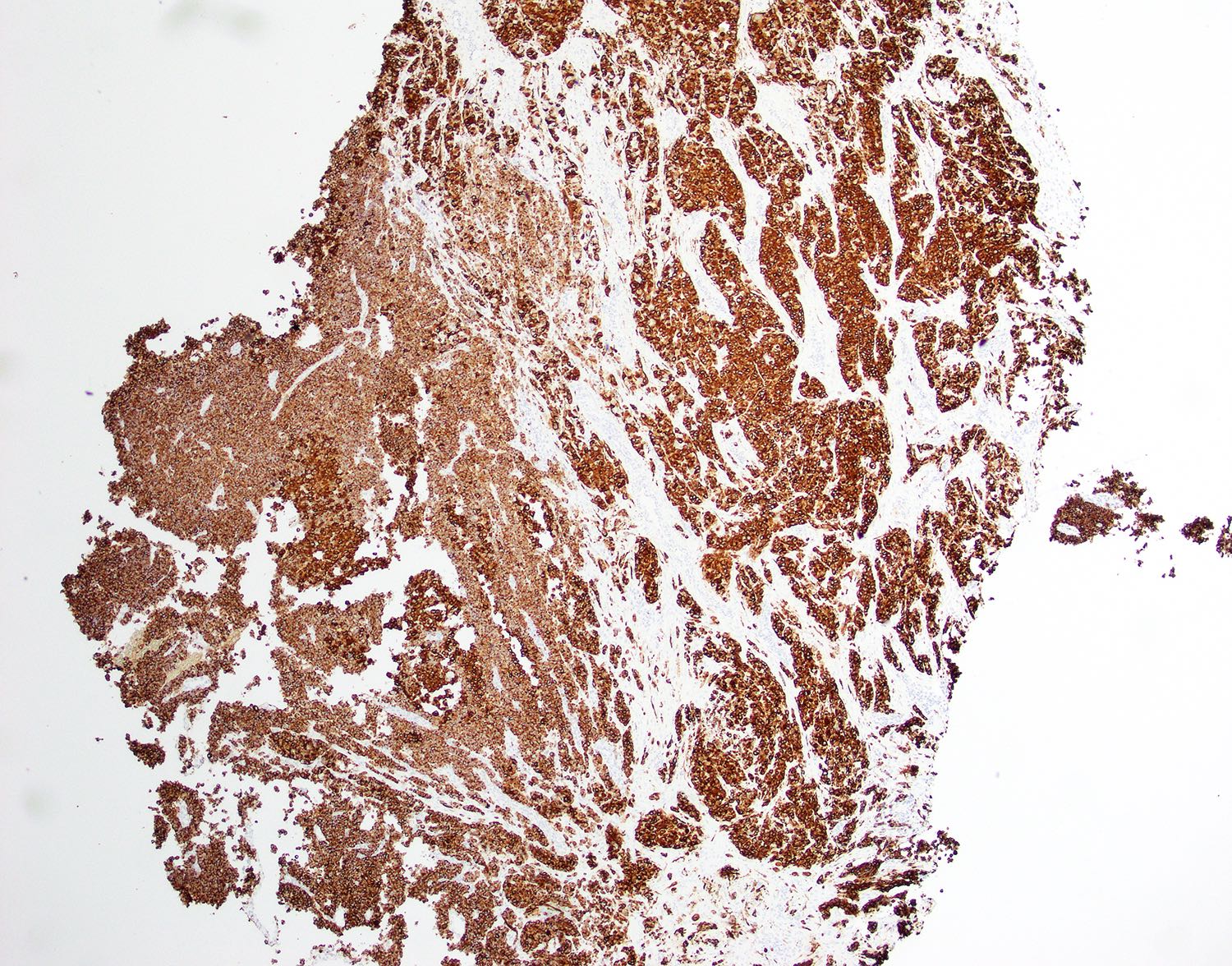
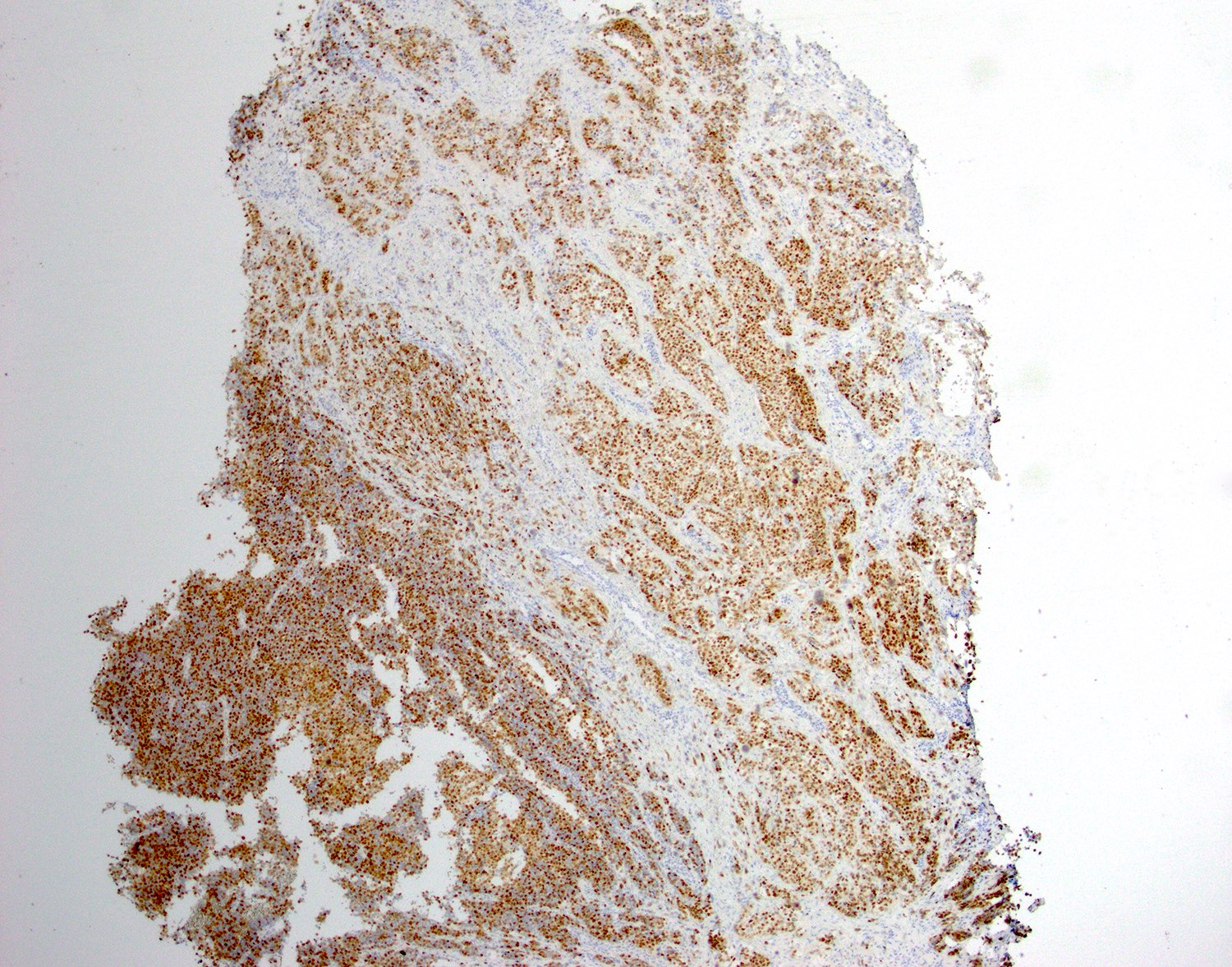
- Characteristic histomorphology: eosinophilic, polygonal cells with a nested or solid growth pattern, delicate vasculature and intracytoplasmic PASD positive material
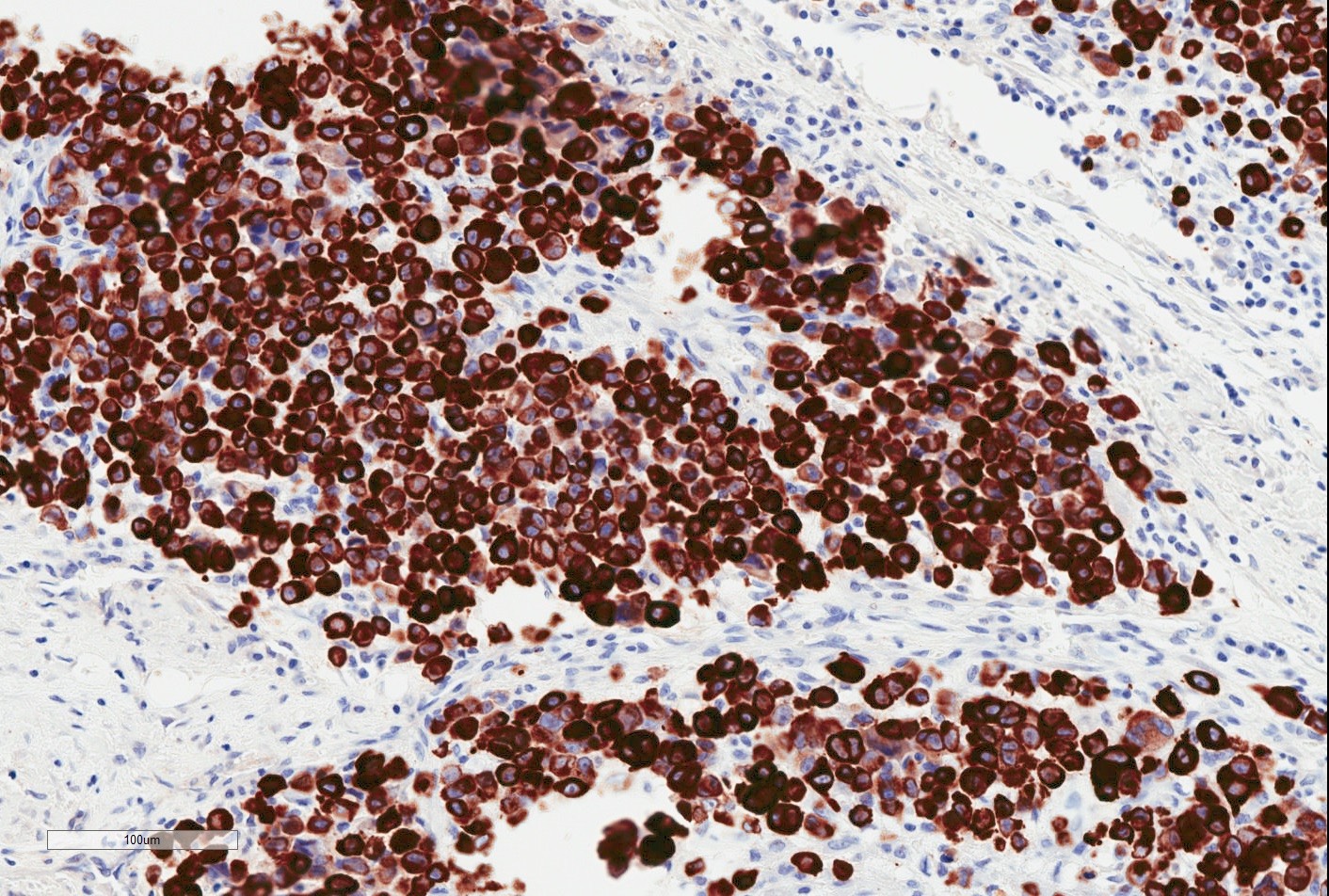
- TFE3 nuclear expression by immunohistochemistry
- Molecular confirmation of TFE3 rearrangement or ASPSCR1::TFE3 fusion
- Reference: JAMA Oncol 2019;5:254
- Hypervascular tumors with high signal intensity on T2 weighted magnetic resonance imaging
- Contrast enhanced masses on computed tomography
- Lobulated contours
- Well defined tumors of the uterine corpus or cervix (compared to more ill defined alveolar soft part sarcoma of other sites) (Korean J Pathol 2014;48:361)
- Prognostic information on ASPS of the female genital tract is limited by short median follow up in published studies
- ASPS of the uterine corpus, cervix or adnexa appear to have a more favorable prognosis than their soft tissue counterparts, likely reflecting early clinical detection, small size or resectability (Korean J Pathol 2014;48:361, Am J Surg Pathol 2017;4:622, Int J Gynecol Pathol 1995;14:283)
- Vulvar and vaginal tumors appear to have a prognosis more comparable to their soft tissue counterparts, with disease related mortality in ~33% of cases
- 10 year old girl with alveolar soft part sarcoma occurring in the uterine cervix (Diagnostics (Basel) 2022;12:1102)
- 33 year old woman with alveolar soft part sarcoma of the uterine corpus (Taiwan J Obstet Gynecol 2023;62:769)
- 57 year old postmenopausal woman with uterine alveolar soft part sarcoma with pelvic lymph node metastasis (Int J Clin Exp Pathol 2012;5:715)
- 68 year old postmenopausal woman with alveolar soft part sarcoma of the uterine cervix (Int J Clin Exp Pathol 2017;10:9812)
- Complete surgical resection is first line therapy
- Most vulvovaginal ASPS reported in the literature have been treated with adjuvant radiation and a subset with adjuvant chemotherapy, though objective data are scarce (Int J Gynecol Pathol 2014;33:263)
- Surgical staging offers little prognostic benefit (Arch Gynecol Obstet 2009;279:263)
- Given the propensity for late recurrence or metastasis, long term follow up is recommended
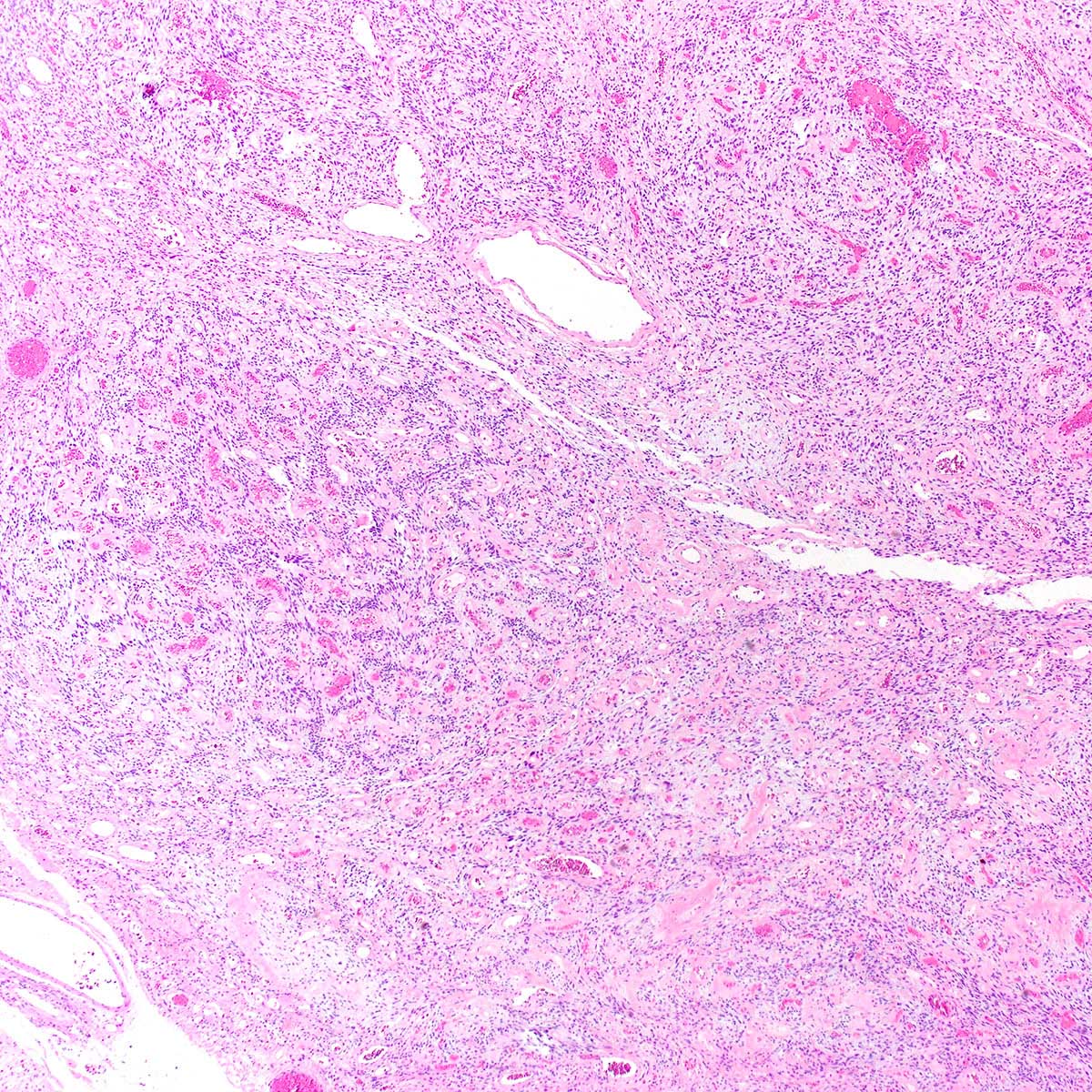
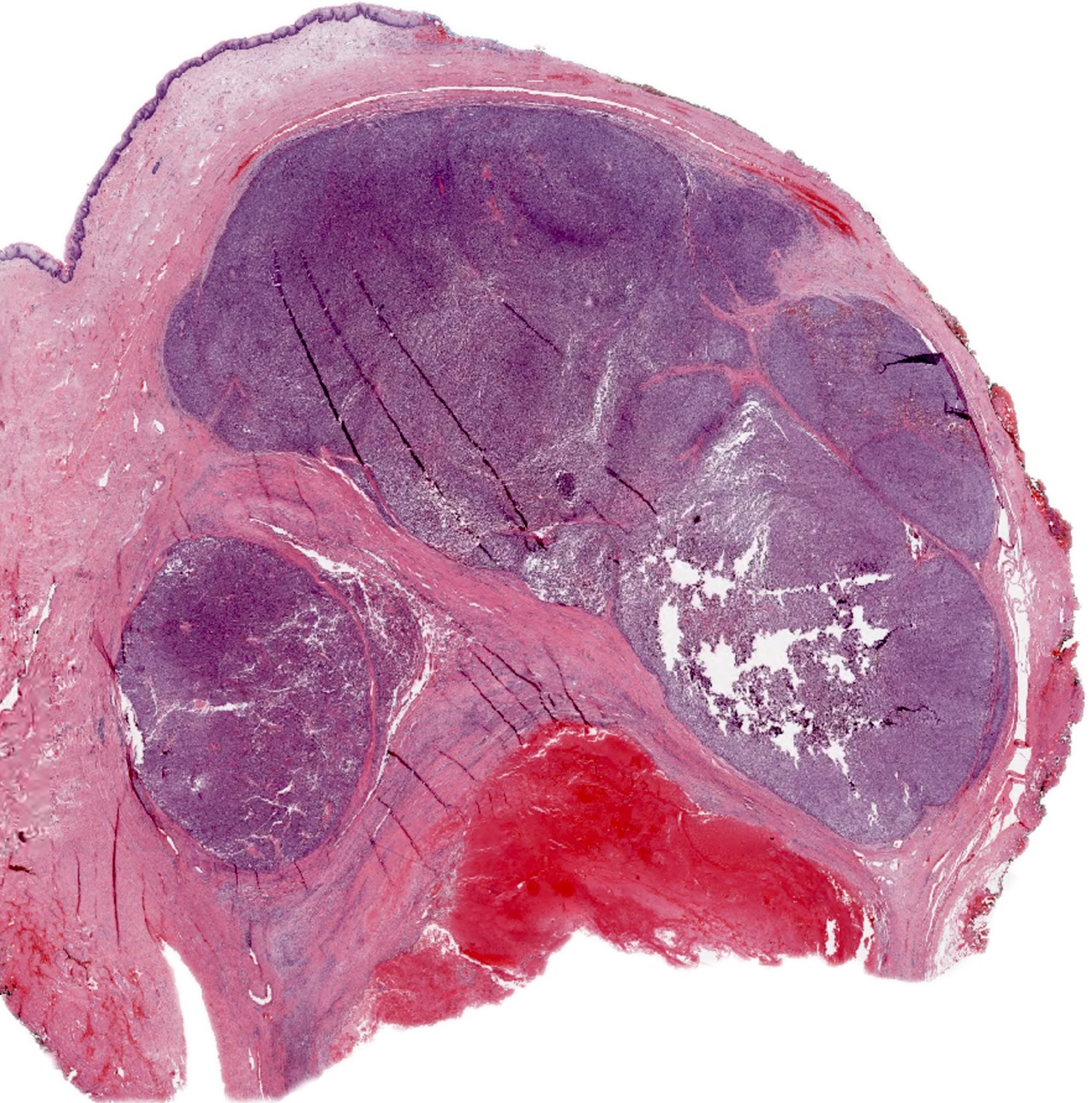
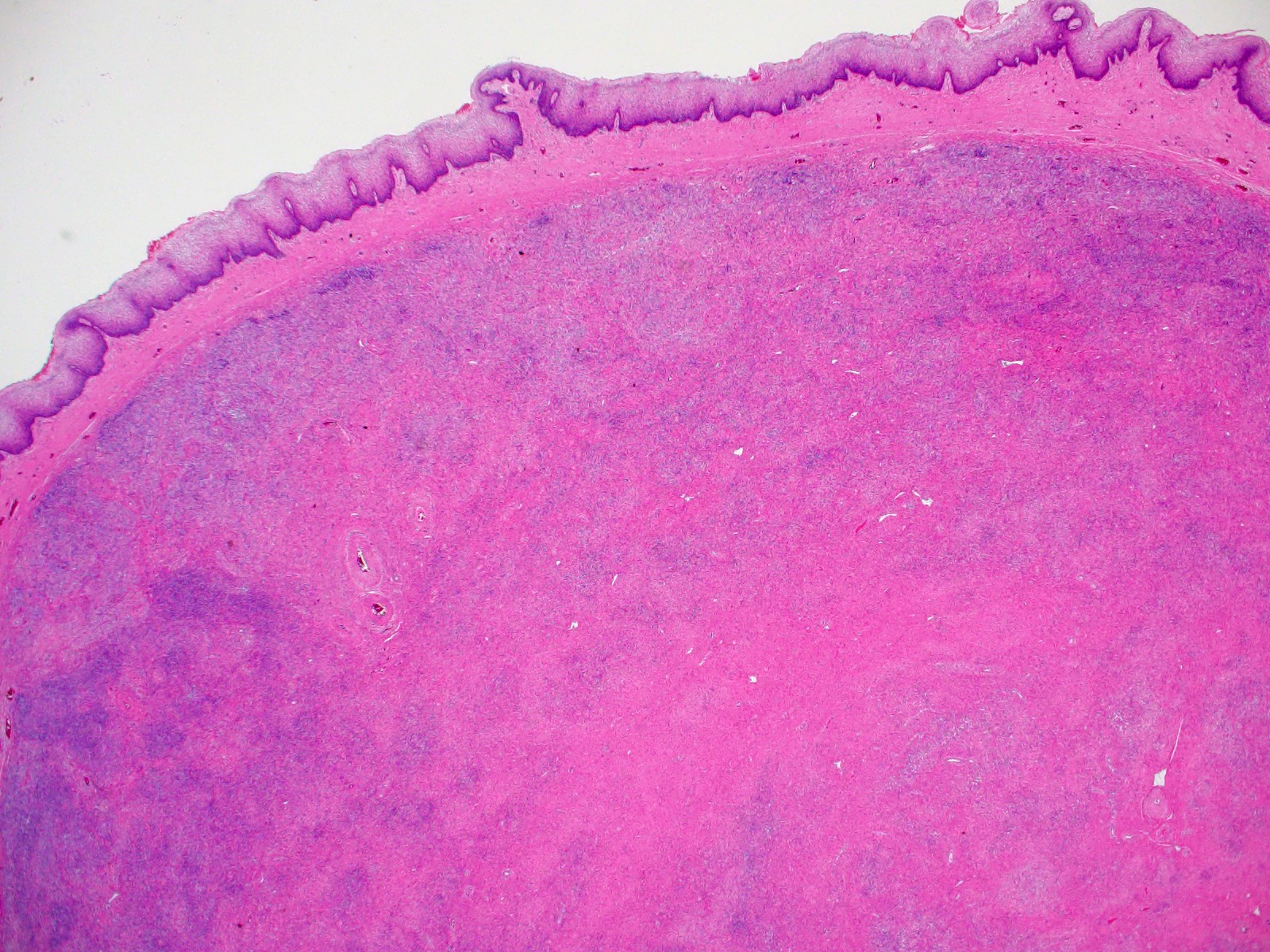
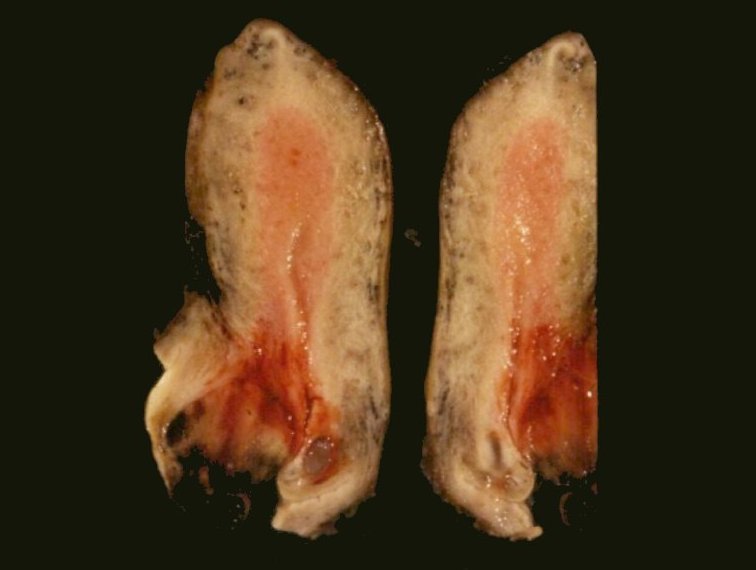
- Circumscribed or infiltrative, solid, lobular mass with white to tan-gray cut surface
- Size variation in female genital tract: 0.5 - 9 cm (median: 5.5) (Int J Gynecol Pathol 2014;33:263)
- Ulceration reported in half of vaginal tumors (Int J Gynecol Pathol 1995;14:283)
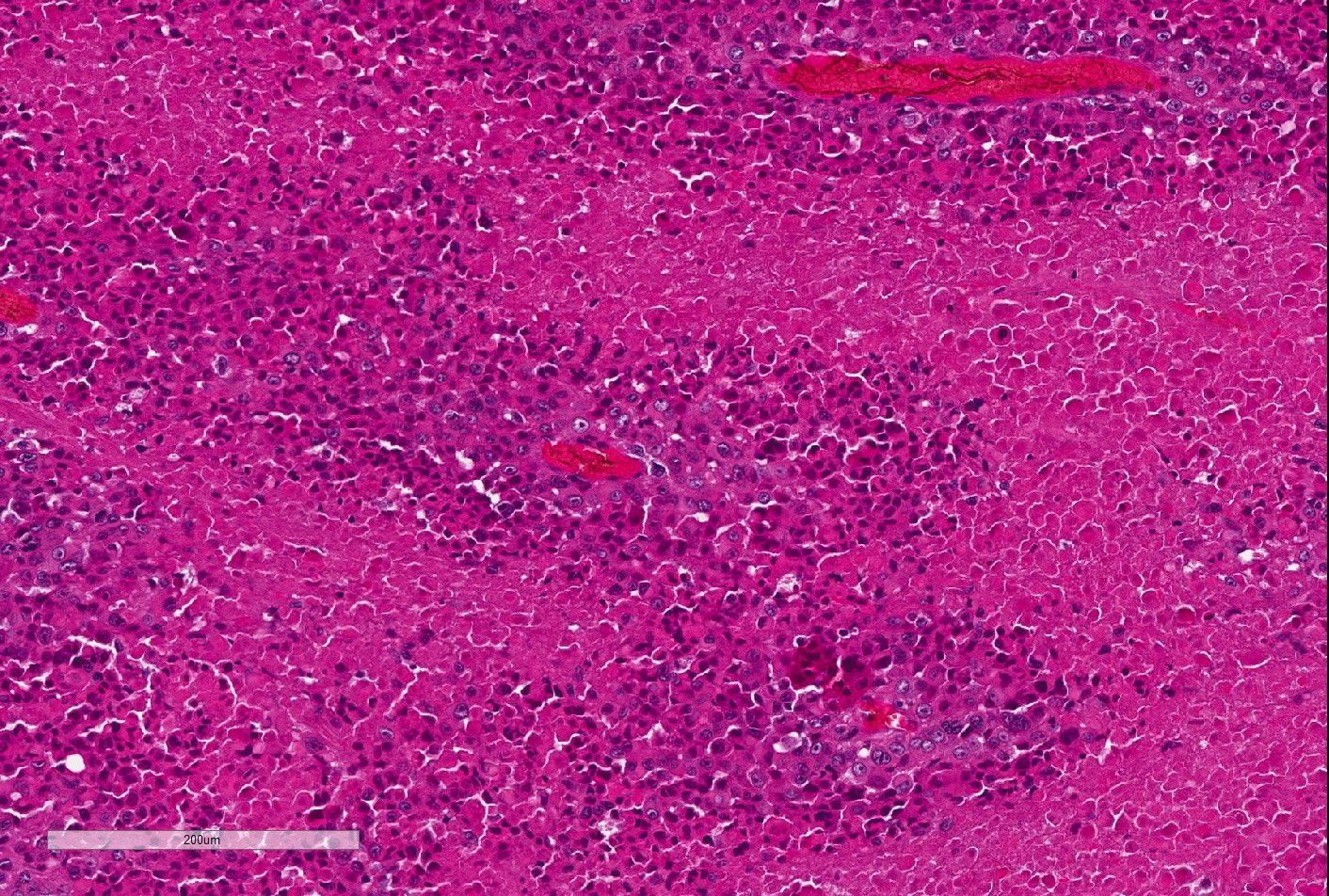
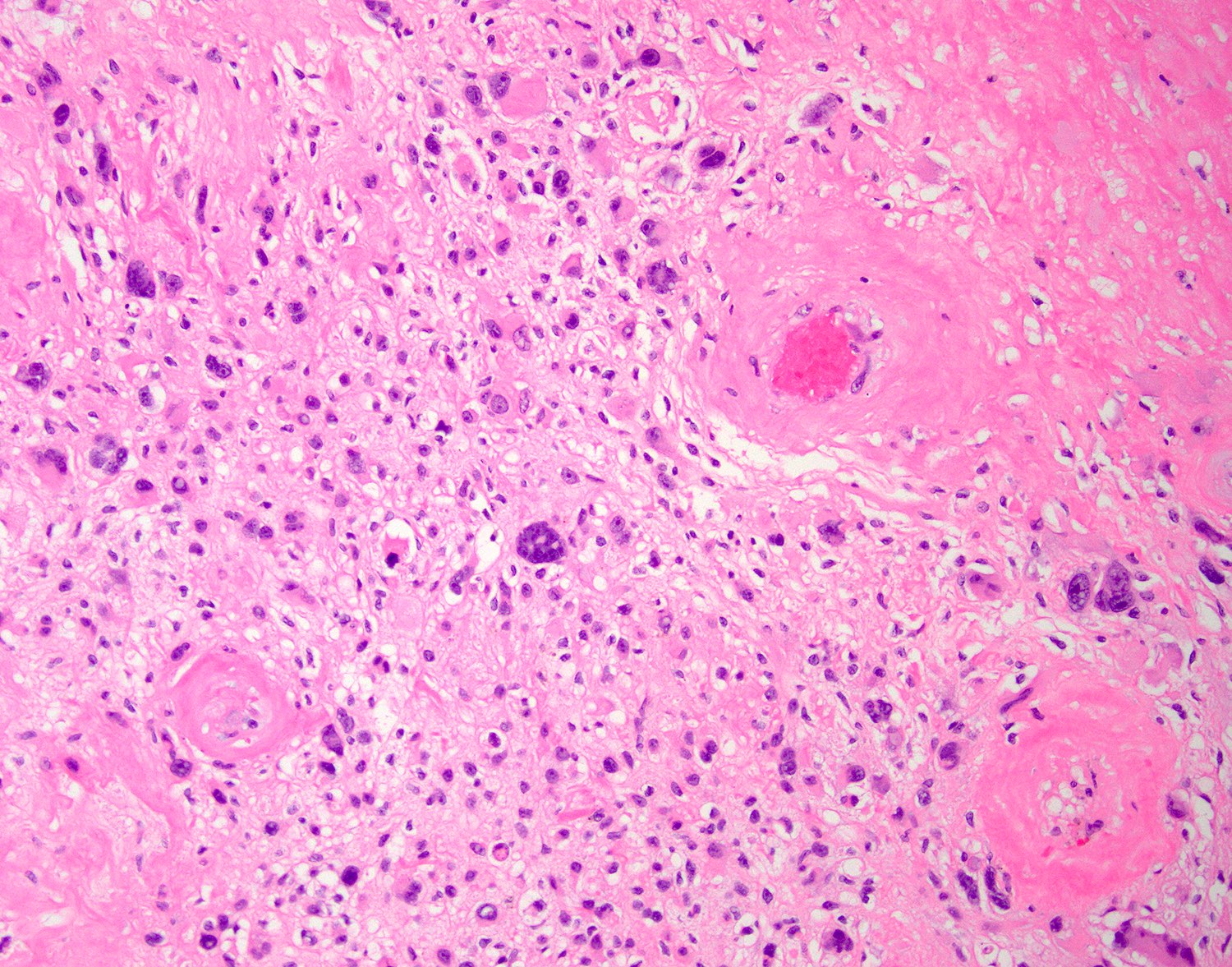
- Large, round to polygonal cells with abundant eosinophilic granular cytoplasm and round, vesicular nucleus with prominent nucleolus
- Organoid / nest-like growth pattern or solid growth pattern
- Central discohesion results in characteristic pseudoalveolar-like structures
- Rich capillary network
- Reference: Cancer 2009;117:500
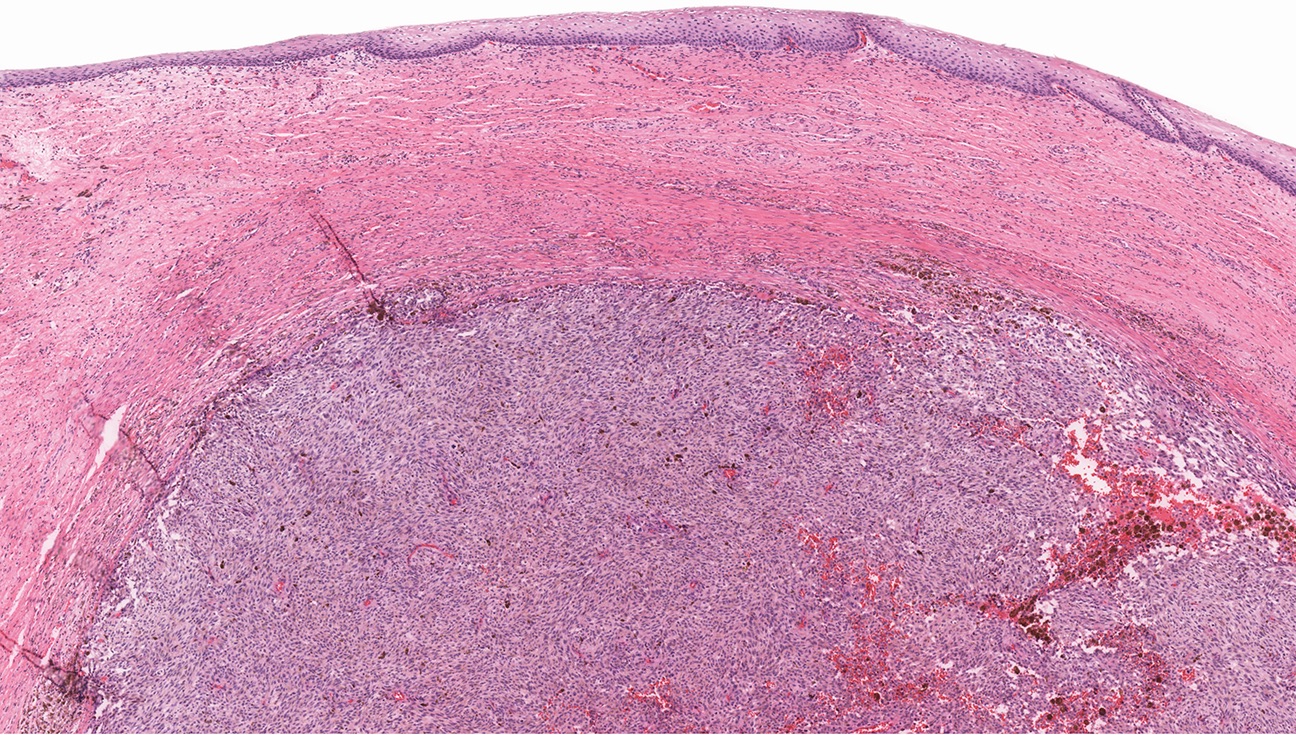
- ASPS of the female genital tract is indistinguishable from its soft tissue counterpart (Int J Gynecol Pathol 1995;14:283, Am J Surg Pathol 2017;4:622)
- Multinodular to lobular architecture with intervening fibrous septa
- Pushing or infiltrative invasion into surrounding tissues
- Well delineated tumor cell nests surrounded by thin walled vessels
- Loss of tumor cell cohesion results in pseudoalveolar pattern
- Subset show areas of solid sheet-like growth
- Tumor cells are large and polygonal with ample granular eosinophilic or clear cytoplasm and monomorphic, round to ovoid nuclei with prominent nucleoli
- Mitoses are rare (typically 0 - 1 per 10 HPF)
- Features seen in a minority of tumors: necrosis, nuclear hobnailing, nuclear pleomorphism (typically focal), bi or multinucleated tumor cells
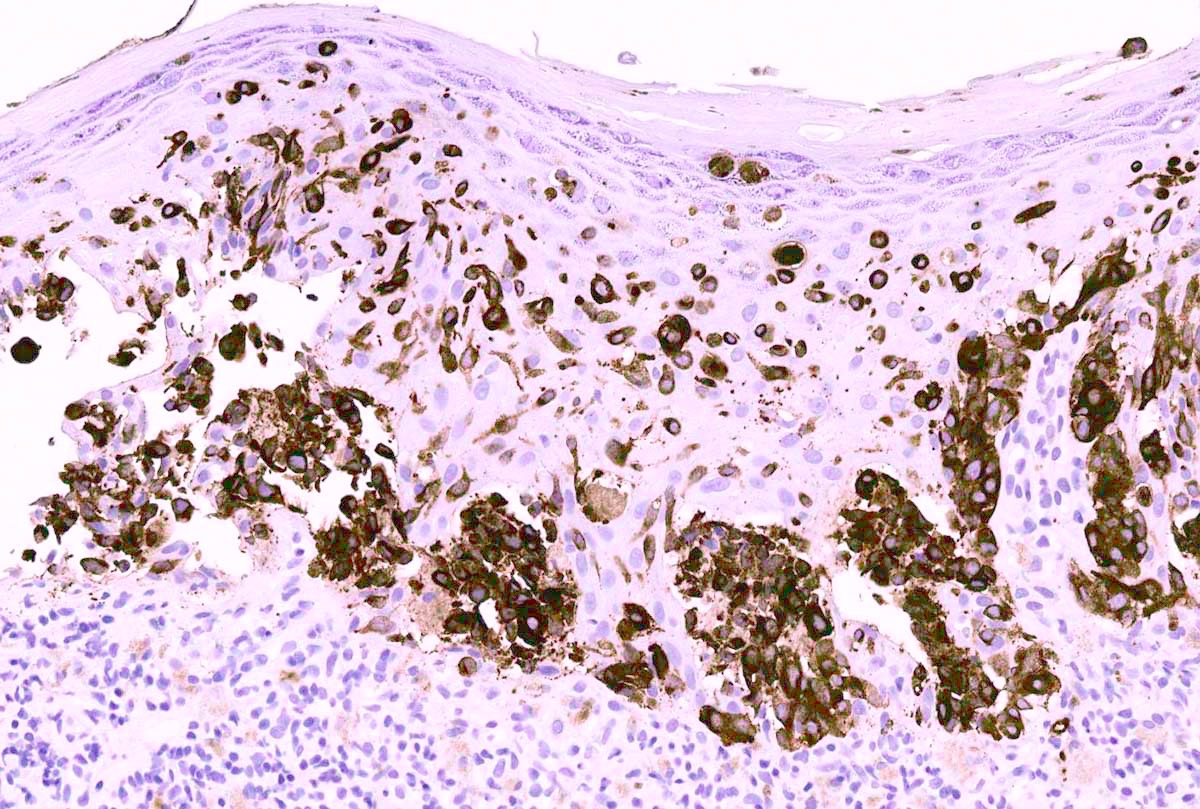
- TFE3 (staining percentage 100%, strong nuclear staining) (Int J Gynecol Pathol 2005;24:131, Am J Surg Pathol 2017;4:622, Int J Gynecol Pathol 2014;33:263)
- Cathepsin K (100%) (Mod Pathol 2011;24:1313)
- PASD highlights intracytoplasmic granules and crystals
- INI1 retained (positive)
- PR positive in alveolar soft part sarcomas of uterus and cervix (Diagnostics (Basel) 2022;12:1102)
- Muscle markers
- SMA (unspecific, weak staining possible)
- Desmin (can be focally positive [50%]) (J Clin Pathol 2006;59:1127)
- h-caldesmon
- Myogenin
- MyoD1
- Melanocytic markers
- HMB45 (rarely focal positivity) (Am J Surg Pathol 2017;4:622)
- MelanA
- MITF
- S100
- Pancytokeratin
- EMA
- PAX8
- CD34
- Inhibin
- Calretinin
- Chromogranin
- Synaptophysin
- Intracytoplasmic rhomboid or rod shaped crystals (Arch Gynecol 1987;240:125)
- FISH confirms TFE3 gene rearrangement
- Reverse transcription polymerase chain reaction (PCR) can detect ASPSCR1::TFE3 fusion transcripts
- ASPSCR1::TFE3 gene fusions are characteristic for ASPS but the same fusion has been reported in a subset of PEComas (tumors with Perivascular and epithelioid cell differentiation) and a small subset of renal cell carcinomas
- References: Am J Surg Pathol 2013;37:1619, Nat Commun 2023;14:1957
- Uterus and cervix, hysterectomy:
- Uterus:
- Proliferative endometrium, negative for hyperplasia and malignancy
- Myometrium and serosa without pathologic findings
- Cervix:
- Alveolar soft part sarcoma (see comment)
- Max tumor size: 4 cm (grossly)
- Lymphovascular invasion present
- Negative for perineural invasion
- All margins negative for tumor, closest margin: proximal (0.7 cm)
- Comment: Alveolar soft part sarcoma rarely affects the cervix or other sites of the female genital tract. It is a neoplasm of unknown differentiation but is defined as a high grade sarcoma. Molecular analysis confirmed the characteristic gene fusion involving ASPSCR1 and TFE3.
- Uterus:
- PEComa:
- Coexpression of melanocytic (HMB45, MelanA) and myoid markers (SMA, desmin)
- May show nuclear expression of TFE3 as there are TFE3 rearranged subtypes (PSF / SFPQ::TFE3 and other TFE fusions) (Histopathology 2024;84:482, Am J Surg Pathol 2017;4:622)
- Melanoma:
- Clear cell carcinoma:
- Metastatic renal cell carcinoma:
- Paraganglioma:
- Granular cell tumor:
- Rhabdomyoma:
- Alveolar rhabdomyosarcoma:
Comment Here
Reference: Cervix - Alveolar soft part sarcoma
- It is diffusely positive for S100 protein, CD68 and SOX10
- It typically harbors the ASPSCR1::TFE3 fusion protein
- It typically stains negative for PASD
- It typically stains positive for myogenin and MyoD1
Comment Here
Reference: Cervix - Alveolar soft part sarcoma
- Vulva constitutes the portion of female genitalia that is external to the hymen
- Vagina is a fibromuscular tube that extends from the vestibule of vulva to uterine cervix
- Female urethra extends from the bladder to midurethra and exits the body between clitoris and vagina
- Vulva is composed of mons pubis, clitoris, labia minora, labia majora, vulvar vestibule, vestibulovaginal bulbs, urethral meatus, hymen, Bartholin and Skene glands and ducts, vaginal introitus
- Vagina extends from vulva to uterine cervix and is derived from paired Müllerian ducts
- Vulva
- Lies external to hymen and is limited by mons pubis anteriorly, anus posteriorly and inguinal gluteal folds laterally
- Vagina
- Fibromuscular canal that extends from the vestibule of vulva, between labia minora, to the uterine cervix
- Wolffian (mesonephric) duct, also known as Gartner duct, runs deeply along lateral vaginal walls
- Lymphatic drainage: external iliac nodes (upper third of the vagina), the common and internal iliac nodes (middle third) and the superficial inguinal and perirectal nodes (lower third)
- Female urethra
- Fibromuscular tube that takes urine from the urinary bladder to the exterior through the external urethral meatus
- Germ cells from yolk sac migrate to the urogenital ridge, forming the epithelium and stroma of the gonads; genital tubercle becomes the clitoris and the parallel ridges become the labia minora
- Urorectal septum divides the cloaca into the urogenital sinus and anal canal; degeneration of the central portion of the urogenital membrane forms the hymen opening
- Lateral Müllerian ducts (paramesonephric ducts) give rise to upper vagina while the lower vagina is formed by the urogenital sinus
- Vaginal vestibule develops by the joining of the distal vagina and urogenital sinus
- Originates from endoderm, except near the urethra (ectoderm)
- Vestibular line of Hart marks the boundary between these tissues
- Epithelium of female urethra is derived from endoderm of the urogenital sinus while the surrounding connective tissue and smooth muscle tissue is derived from splanchnic mesenchyme (Sadler: Langman's Medical Embryology, 15th Edition, 2023, Am J Obstet Gynecol 1976;126:769)
- Vulva
- Mons pubis
- Anteriormost region of vulva and is anatomically located over the prominence of pubic symphysis
- Hymen
- Corresponds to the distalmost extent of vagina and posterior aspect of vulvar vestibule
- Clitoris
- Erectile tissue similar to corpora cavernosa of penis and is located anterior to frenulum at the junction of labia minora
- Labia majora
- Form the lateral boundaries of the vulva
- Fuse anteriorly into mons pubis
- Posteriorly terminate 3 - 4 cm anterior to the anus where they are united by posterior commissure or fourchette
- Labia minora
- Are medial to labia majora and lateral to vulvar vestibule
- Anteriorly, labia minora divide into 2 parts; one part passes over clitoris to form prepuce and the other joins beneath clitoris and forms frenulum
- Posteriorly, they blend with medial surfaces of labia majora
- Hart line
- Lies at the inferior junction between vulvar vestibule and perineal skin
- Vestibule
- Area between the hymen (anteriorly), Hart line (posterolaterally) and labia minora (anterolaterally)
- Includes vaginal opening and urethral orifice
- Structures found in vestibule include major vestibular (Bartholin) glands, minor vestibular glands, periurethral (Skene) glands, urethra
- Bartholin glands
- Correspond to bulbourethral glands in male
- These are mucin producing glands and are located posterolaterally in the vulva
- Minor vestibular glands
- Correspond to penile glands of Littre
- Concentrically located within the vestibule
- Mons pubis
- Vagina
- Posterior to urinary bladder (from which it is separated by fibroadipose tissue)
- Anterior to rectum (from which is separated by rectouterine space in the upper 25%, rectovaginal septum in the middle portion and sphincter musculature in the distal portion of anal canal)
- Female urethra
- Extends from the bladder to the vestibule of the vagina to its opening posterior to the clitoris
- Measures 4 cm in length
- Striated muscle of the urogenital diaphragm forms the external voluntary sphincter as the urethra penetrates it
- Reference: StatPearls: Anatomy, Abdomen and Pelvis - Female External Genitalia [Accessed 21 August 2023]
- Hymen
- Nonkeratinized stratified squamous epithelium
- Labia majora
- Composed of keratinized stratified squamous epithelium with hair follicles and eccrine, apocrine and sebaceous glands
- Labia minora
- Composed of keratinized stratified squamous epithelium, usually no adnexa
- Stroma
- Composed of stromal cells that can be spindled, stellate, fusiform and may have large multilobated nuclei
- Vestibule
- Lined by nonkeratinized squamous epithelium, may be glycogenated
- Minor vestibular gland
- Superficial glands lined by mucin secreting columnar cells that merge with squamous epithelium of the vestibule
- Open directly onto the surface
- Glands
- Apocrine glands (scent glands)
- Identical to those of axillae, breast and perianal regions
- Height of secretory cells varies
- Lumina of glands are large compared to lumina of eccrine glands
- Eccrine glands (sweat glands)
- Primarily involved in heat regulation
- Lined by layer of epithelial cells that contain eosinophilic cytoplasm
- Sebaceous glands
- Alveolar, holocrine glands that do not contain lumina
- Each gland is composed of several lobules
- Cells in each lobule form a delicate network filled with fat
- Skene glands
- Periurethral glands analogous to prostate
- Mucus secreting columnar epithelium merges with duct urothelium, then stratified squamous epithelium of vestibule
- Apocrine glands (scent glands)
- Vagina
- Lined by nonkeratinized stratified squamous epithelium and is composed of basal, parabasal, intermediate and superficial cell layers
- Basal cell layer is composed of a single layer of columnar cells with high N:C ratio
- Parabasal layer lies above the basal layer and has cells with higher N:C ratio than the more superficial layers
- Intermediate cell layer has more abundant cytoplasm, which can be glycogenated
- Superficial cell layer appears flattened with cells showing pyknotic nuclei (J Mol Med (Berl) 2021;99:531)
- Lamina propria (subepithelial stroma) is composed of loose connective tissue with elastic fibers, rich venous and lymphatic networks, spindle to stellate and some multinucleated stromal cells
- Muscle
- Outer longitudinal and thin inner circular layer of smooth muscle
- Adventitia is composed of inner dense connective tissue layer and outer loose connective tissue layer containing peripheral nerves, blood vessels and lymphatics
- Wolffian (mesonephric) duct, also known as Gartner duct, runs deeply along lateral vaginal walls; single small duct surrounded by a cluster of small glands lined by cuboidal epithelium with eosinophilic secretion in lumen
- Maturation index
- Ratio of parabasal to intermediate to superficial cells of vaginal epithelium (sampled at middle third of lateral vaginal wall)
- Sample is often obtained simultaneous with Pap smear to detect hormonal effects in menopausal and postmenopausal women
- Increased maturation in vaginal epithelium may be due to estrogenic effect of tamoxifen (Clin Exp Obstet Gynecol 1998;25:121)
- Lined by nonkeratinized stratified squamous epithelium and is composed of basal, parabasal, intermediate and superficial cell layers
- Female urethra
- Lined by urothelium (proximal two - thirds), stratified and pseudostratified columnar and squamous epithelium (distal third)
- Basal layers composed of either low columnar or cuboidal cells, followed by several layers of polyhedral cells
- Most superficial layer is composed of round, dome shaped umbrella cells that are occasionally multinucleated and flattened according to amount of distention
- Nonkeratinized squamous epithelium: cuboidal (deepest), polymorphous (middle), squamous / flattened (superficial) (J Urol 1987;138:775)
- Periurethral (Skene) glands (homologous to the prostate gland) open into the distal portion
- Lined by columnar or cuboidal epithelium with surrounding connective tissue and smooth muscle
- Minor vestibular glands (homologous to glands of Littre in males) open along the entire length
- Tubuloacinar mucinous glands with uniform, pale eosinophilic to clear cytoplasm and basally flattened nuclei
- Lined by urothelium (proximal two - thirds), stratified and pseudostratified columnar and squamous epithelium (distal third)
Normal histology of vagina
Anatomy of female urethra
Histology of female urethra
Comment Here
Reference: Anatomy & histology - vulva, vagina & female urethra
- Keratinized stratified squamous epithelium with adnexa
- Keratinized stratified squamous epithelium without adnexa
- Nonkeratinized stratified squamous epithelium with adnexa
- Simple, columnar mucinous epithelial cells
Comment Here
Reference: Anatomy & histology - vulva, vagina & female urethra
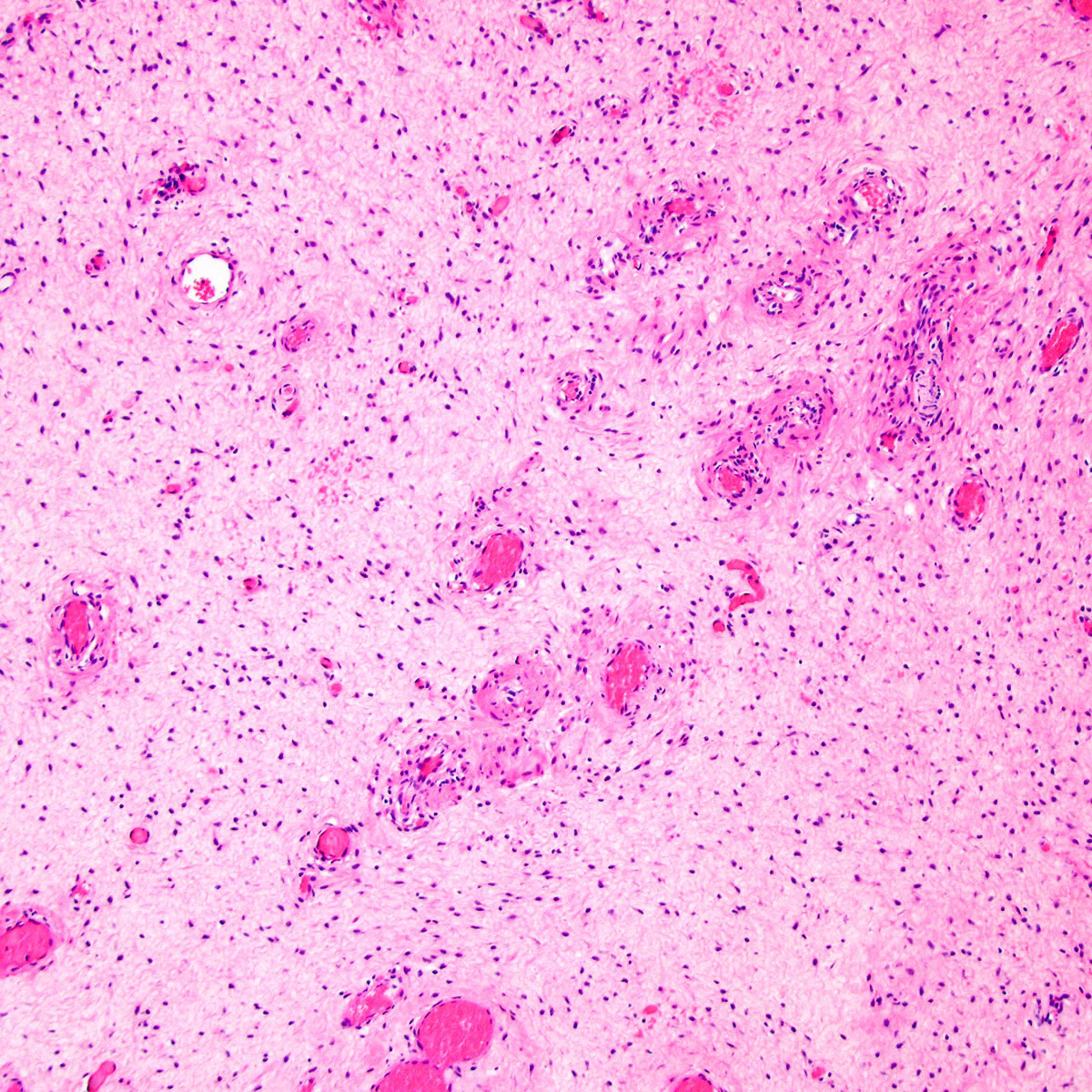
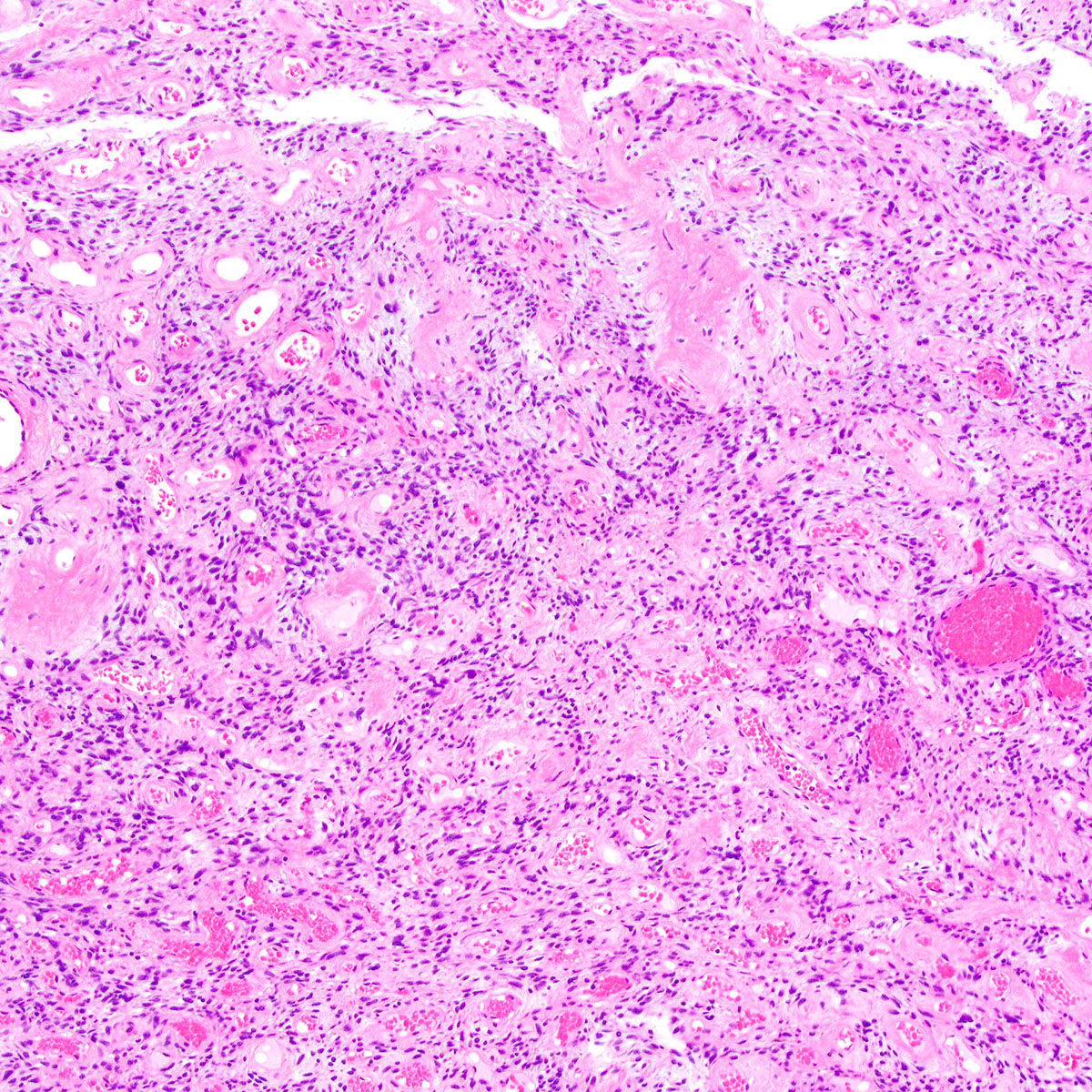
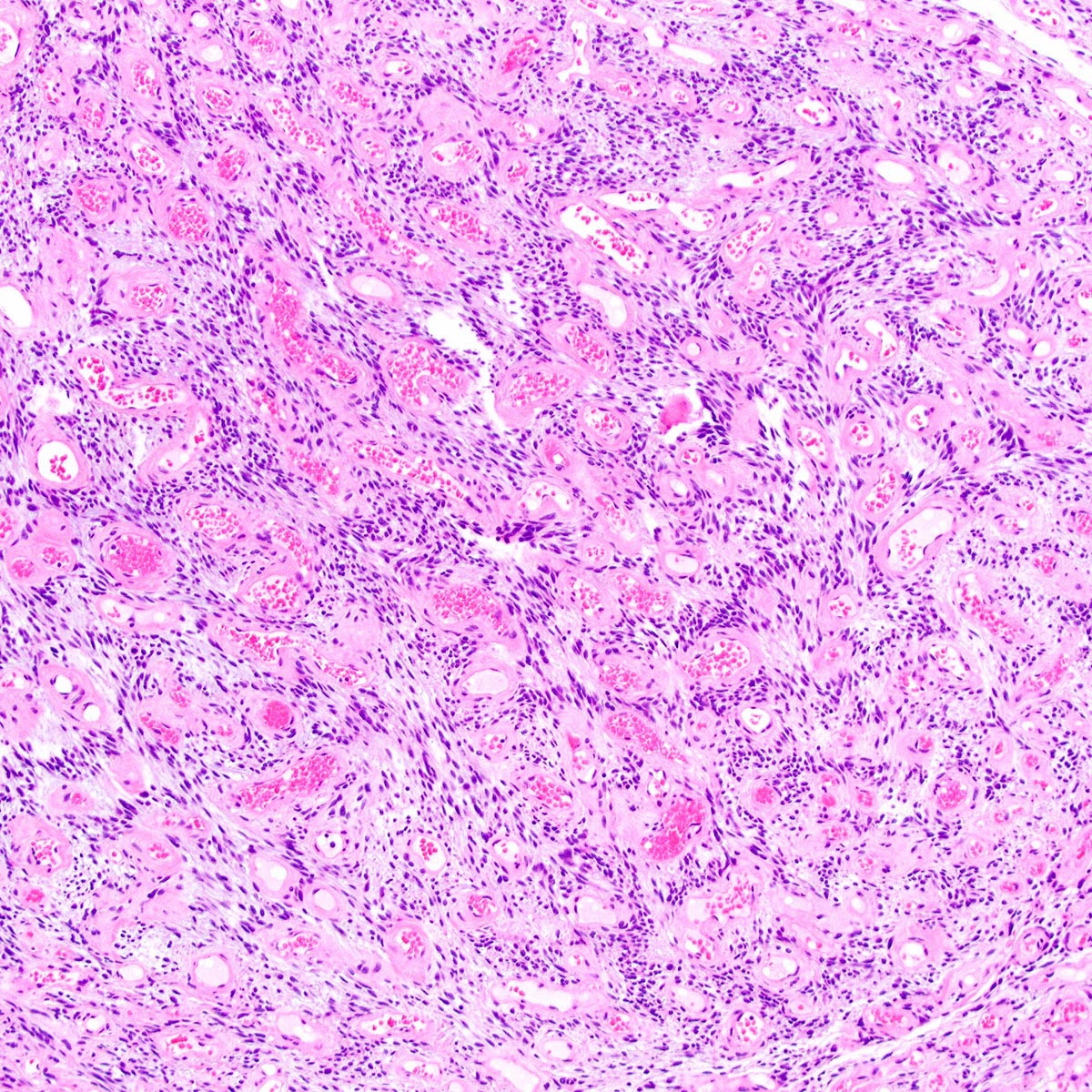
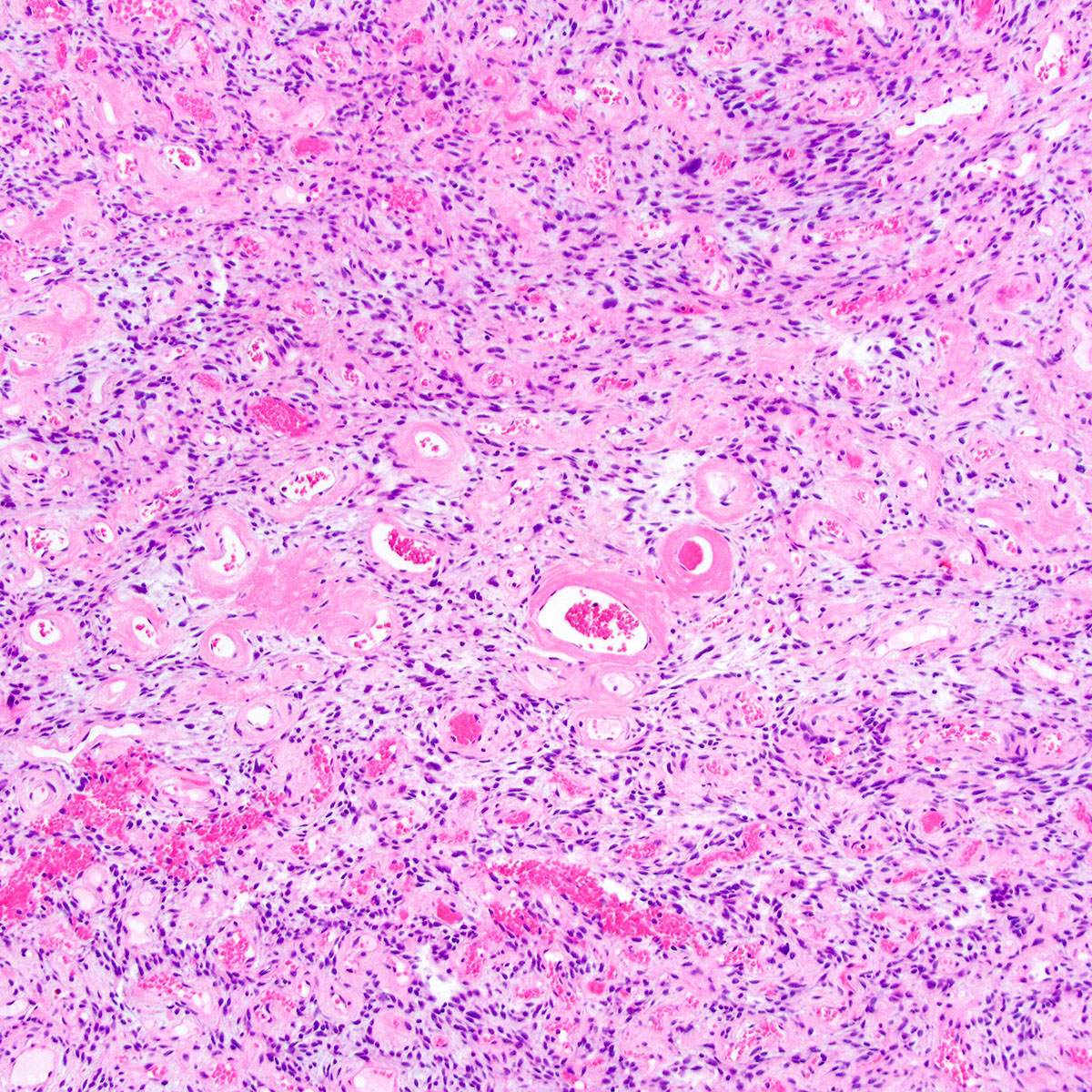
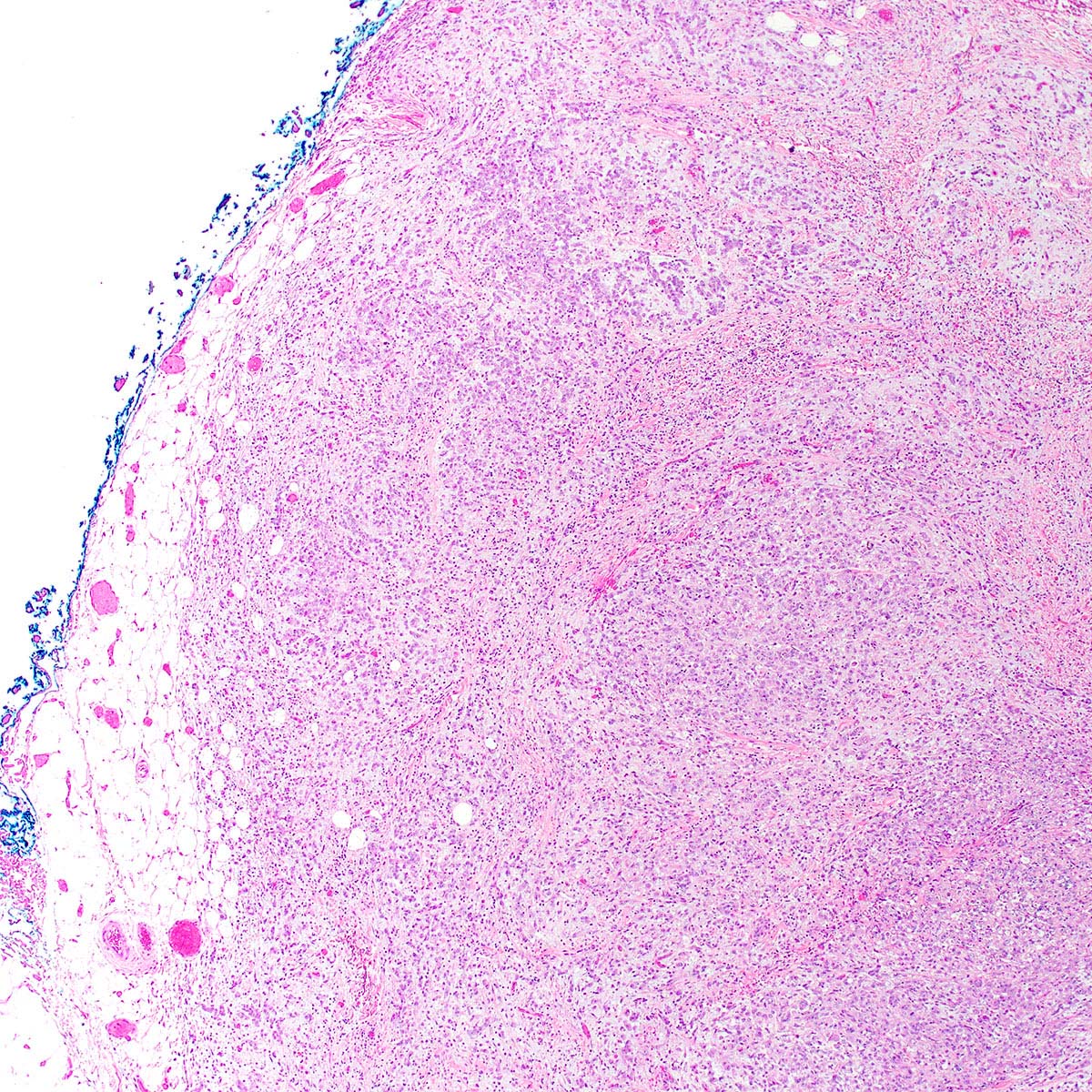
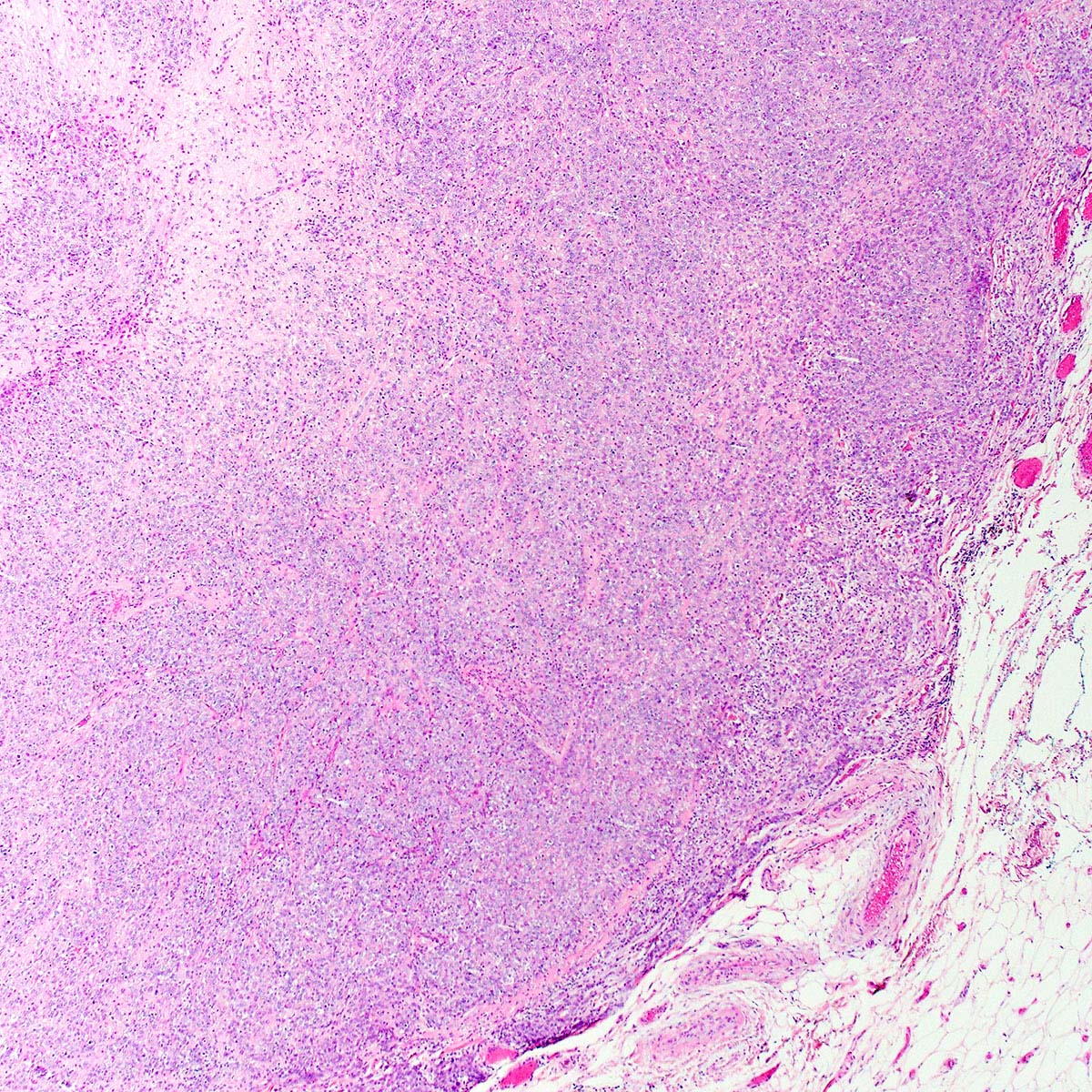
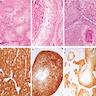
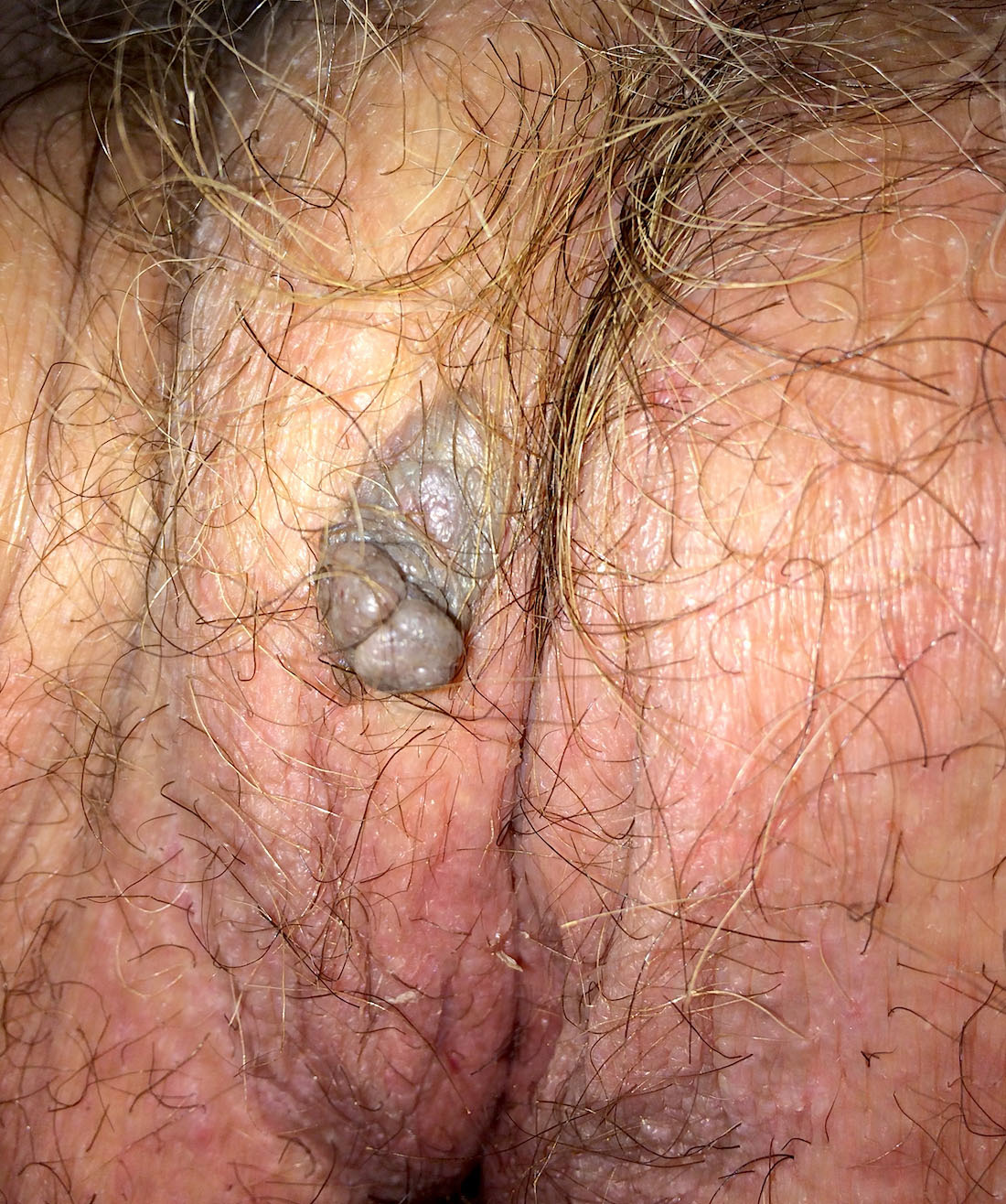
- Angiomyofibroblastoma is a benign, site specific, soft tissue tumor of the lower genital tract with alternating hypo and hypercellular zones, bland spindle to epithelioid cells and abundant vessels
- Benign lower genital tract tumor, seen predominantly in women
- Alternating hypo and hypercellular zones, comprised of bland plump spindle to epithelioid cells clustered around abundant small, thin walled vessels
- No specific immunophenotypic or molecular features reported
- Local excision curative; recurrences exceptionally rare
- F > M
- In women, age range 21 - 71 years (median, fifth decade) (Am J Surg Pathol 1992;16:373, Mod Pathol 1996;9:284, Hum Pathol 1997;28:1046, Hum Pathol 2014;45:1647)
- May occur in pregnancy (J Obstet Gynaecol Res 2011;37:1162)
- Rare examples in men (Am J Clin Pathol 1997;107:36)
- Labia majora most common site; rarely involves vagina or perineum (Am J Surg Pathol 1992;16:373, Mod Pathol 1996;9:284, Hum Pathol 1997;28:1046, J Clin Pathol 2000;53:803, Hum Pathol 2014;45:1647)
- Histogenesis uncertain; may arise from subepithelial stroma or a perivascular stem cell (Am J Surg Pathol 1992;16:373, J Clin Pathol 2000;53:803, Hum Pathol 1997;28:1046)
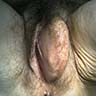
- Painless vulvar mass
- Present for weeks to years before diagnosis (Am J Surg Pathol 1992;16:373)
- Rare cases multifocal
- Clinically resembles a Bartholin cyst or lipoma (Am J Surg Pathol 1992;16:373, Hum Pathol 2014;45:1647)
- Diagnosis typically follows complete local excision of a clinically benign appearing mass
- Ultrasound: well circumscribed mass (Case Rep Obstet Gynecol 2018;2018:8579026, J Med Case Rep 2015;9:248)
- MRI: well circumscribed mass with homogeneous or heterogeneous signal; hypointense on T2 weighted images (Case Rep Obstet Gynecol 2018;2018:8579026, J Med Case Rep 2015;9:248, Int J Clin Exp Pathol 2018;11:5509)
- Benign tumor
- Local recurrence exceptionally rare, even with positive margins (Hum Pathol 2014;45:1647, Am J Surg Pathol 1992;16:373, Mod Pathol 1996;9:284, Hum Pathol 1997;28:1046)
- Single reported case with frank sarcomatous transformation, which recurred locally (Am J Surg Pathol 1997;21:1104)
- 35 year old woman with a 5 cm vaginal angiomyofibroblastoma (J Med Case Rep 2015;9:248)
- 36 year old woman with a 5 cm prolapsing vaginal angiomyofibroblastoma (Case Rep Obstet Gynecol 2018;2018:8579026)
- 42 year old woman with a 4 cm right labial angiomyofibroblastoma (J Midlife Health 2019;10:105)
- 46 year old woman with a 14 cm pedunculated vulvar angiomyofibroblastoma (Int J Clin Exp Pathol 2018;11:5509)
- Conservative local excision considered curative (Am J Surg Pathol 1992;16:373, Hum Pathol 1997;28:1046)
- Positive margins do not necessitate re-excision
- Sole case with sarcomatous transformation managed with radical vulvectomy and adjuvant radiation (Am J Surg Pathol 1997;21:1104)
- Size range: 0.5 - 12 cm (average ~ 3 - 5 cm) (Am J Surg Pathol 1992;16:373, Mod Pathol 1996;9:284, Pathol Int 1995;45:487, Hum Pathol 1997;28:1046)
- Well circumscribed, nodular
- Cut surface homogeneous, white-tan; no necrosis or hemorrhage (Am J Surg Pathol 1992;16:373, Hum Pathol 2014;45:1647)
- Sole case with sarcomatous transformation was large (13 cm) with gross hemorrhage and necrosis (Am J Surg Pathol 1997;21:1104)
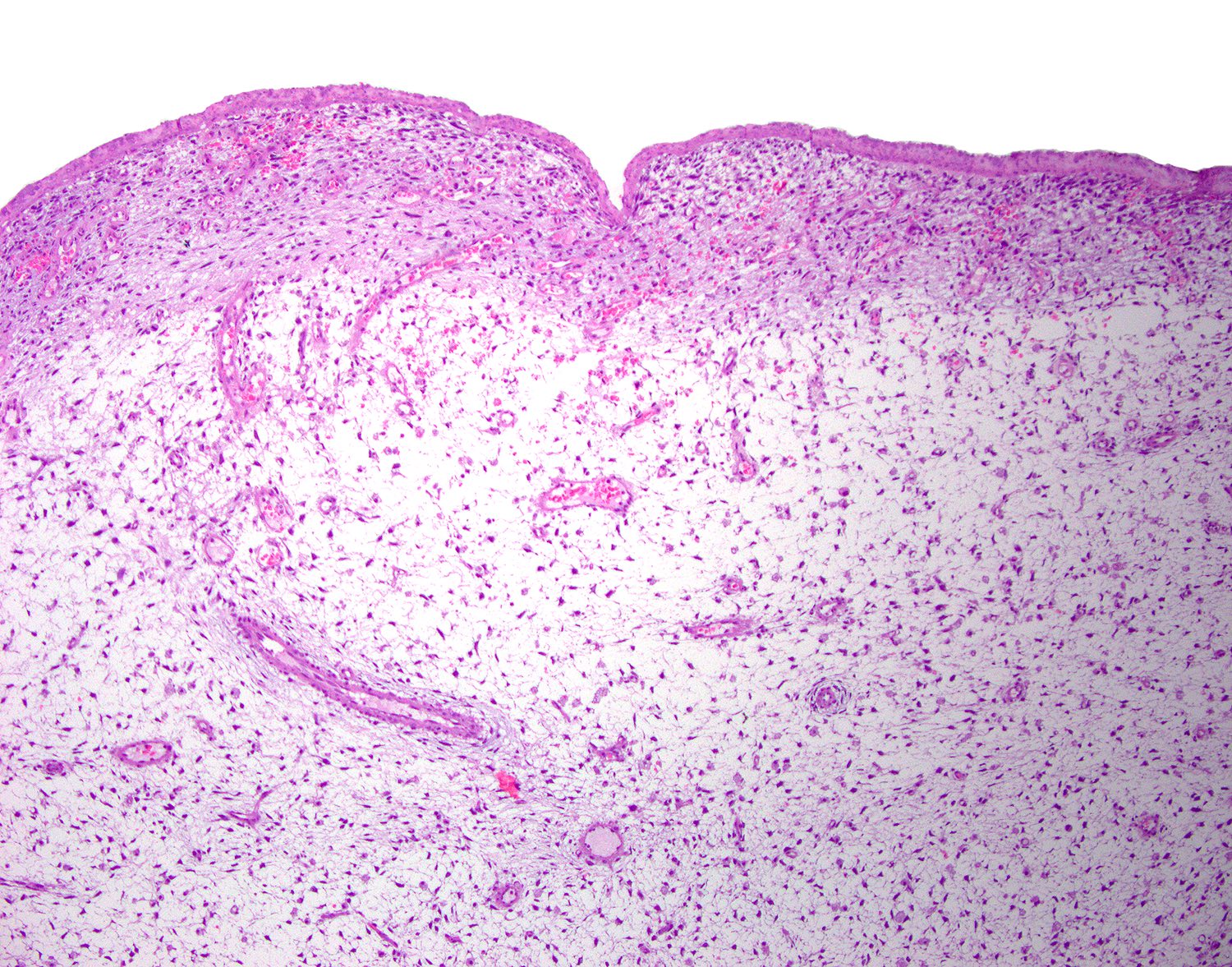
- Well circumscribed, noninfiltrative
- Fibrous pseudocapsule in a subset (Am J Surg Pathol 1992;16:373)
- Characteristic alternating hypo and hypercellular zones (Am J Surg Pathol 1992;16:373)
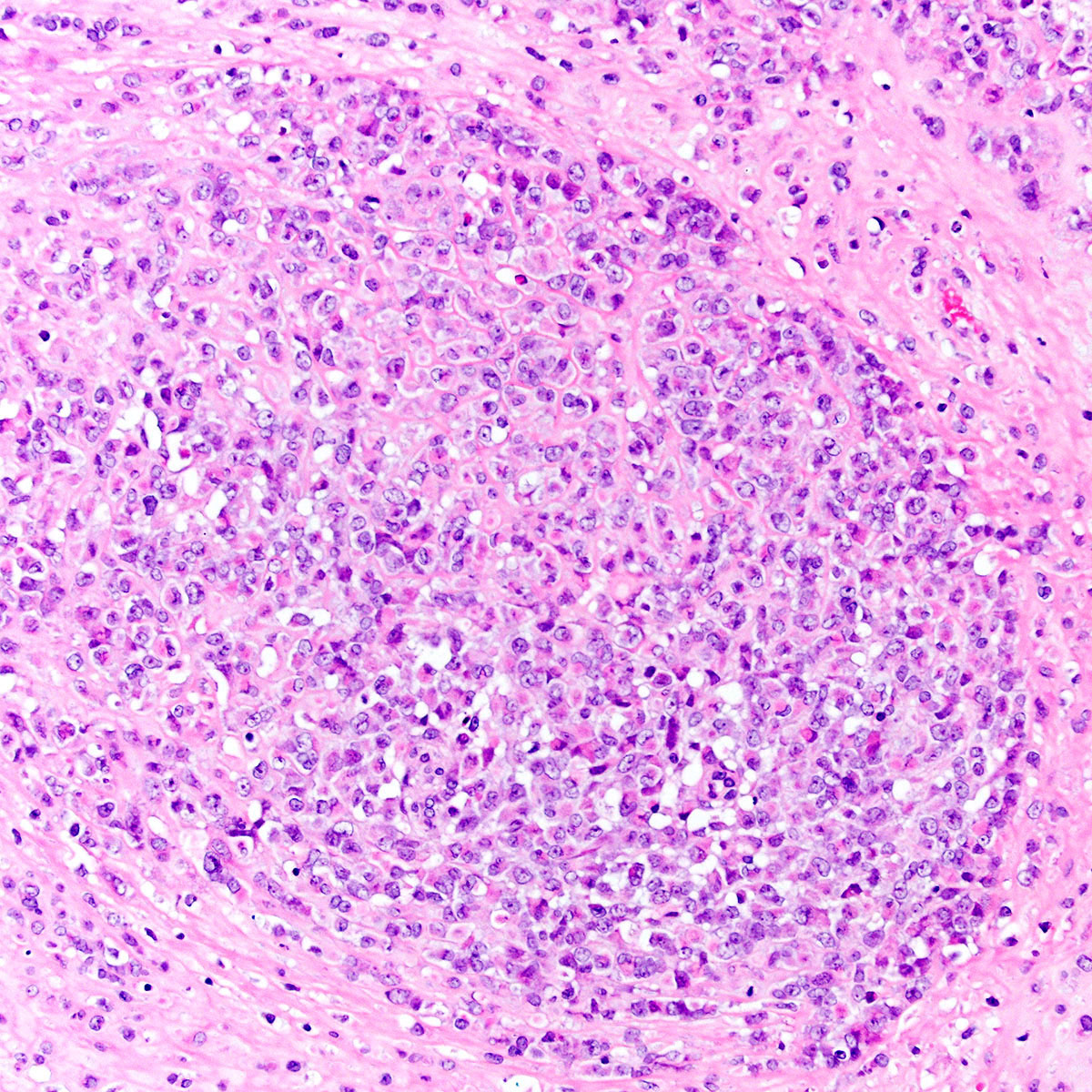
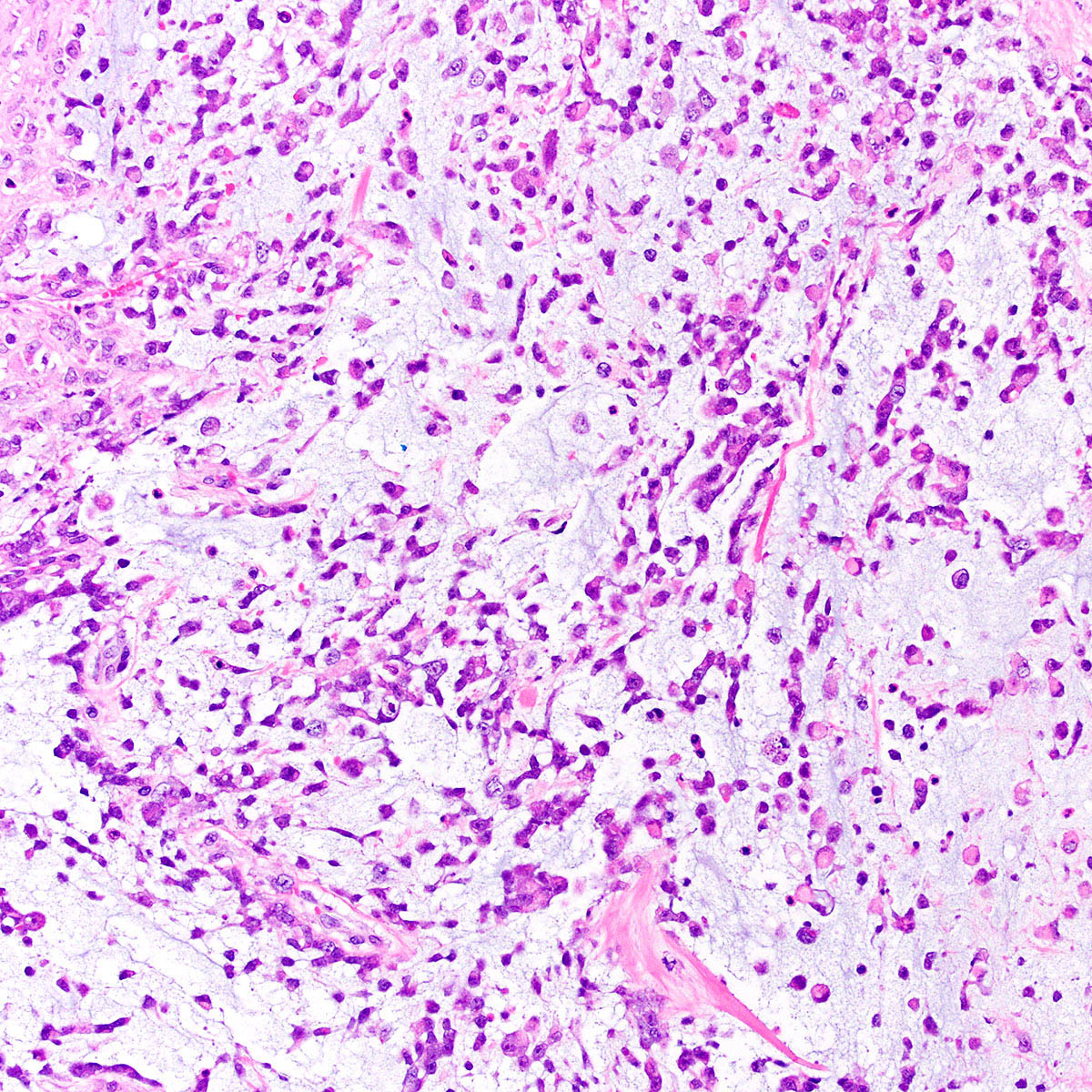
- Tumor cells:
- Spindle to epithelioid to plasmacytoid myofibroblastic cells
- Typically bland chromatin and inconspicuous nucleoli; scattered mildly atypical cells in a minority (Am J Surg Pathol 1992;16:373)
- Bland multinucleated cells common (Am J Surg Pathol 1992;16:373)
- Mitoses rare or absent; no atypical mitoses
- Tumor stroma and vasculature:
- Lacks prominent stromal mucin
- Hypocellular foci show edematous stroma with scattered fine to thick bands of stromal collagen
- In hypocellular foci, tumor cells appear randomly distributed
- In hypercellular foci, tumor cells congregate around small, irregularly distributed, thin walled vessels
- Occasional larger vessels interspersed
- Mast cells typically conspicuous
- Adipocytic differentiation in ~ 25 - 50% (Mod Pathol 1996;9:284, Hum Pathol 1997;28:1046)
- Rare cases with predominant adipocytic differentiation = lipomatous variant of angiomyofibroblastoma (Hum Pathol 1997;28:1046, Hum Pathol 2014;45:1647, Int J Gynecol Pathol 2015;34:204)
- Rare tumors show mixed features of angiomyofibroblastoma and aggressive angiomyxoma; should be managed like aggressive angiomyxoma (Histopathology 1997;30:3)
- Sole case with sarcomatous transformation showed hypercellular foci with cytologic atypia, increased mitoses and lymphovascular invasion (Am J Surg Pathol 1997;21:1104)
- SMA (rarely not negative)
- Muscle specific actin (rarely not negative)
- CD34 (rarely not negative) (Mod Pathol 1996;9:284, Hum Pathol 1997;28:1046, Hum Pathol 2014;45:1647)
- S100 (Am J Surg Pathol 1992;16:373, Mod Pathol 1996;9:284, Pathol Int 1995;45:487, Hum Pathol 2014;45:1647)
- Cytokeratin (AE1 / AE3)
- EMA
- Factor XIIIa
- HMB45
- p63
- Features consistent with myofibroblastic differentiation (Am J Surg Pathol 1992;16:373)
- Stromal cells surrounded by incomplete basal lamina
- Well developed, rough endoplasmic reticulum and Golgi apparatus
- Abundant intermediate filaments and pinocytotic vessels
- Nucleus with delicate heterochromatin
- No specific or recurrent molecular alterations reported
- No deletion of RB1 / FOXO1 (chr 13q14) (Hum Pathol 2014;45:1647)
- No HMGA2 rearrangement (Genes Chromosomes Cancer 2007;46:981)
- Vulva, mass, local excision:
- Angiomyofibroblastoma (3.5 cm) (see comment)
- Comment: Margins are negative.
- Aggressive angiomyxoma:
- Typically large (most > 10 cm)
- Infiltrative
- More uniformly hypocellular
- No perivascular congregation of cells
- Tumor cells more uniformly spindled (versus epithelioid)
- Perivascular myoid bundles characteristic
- Red blood cell extravasation common
- HMGA2 positive
- FISH shows HMGA2 rearrangement in a subset (Genes Chromosomes Cancer 2007;46:981)
- Leiomyoma:
- Solitary fibrous tumor:
- Superficial angiomyxoma:
- More uniformly hypocellular
- No perivascular congregation of cells
- Prominent stromal mucin, often with acellular mucin clefts / pools
- Stromal neutrophils conspicuous
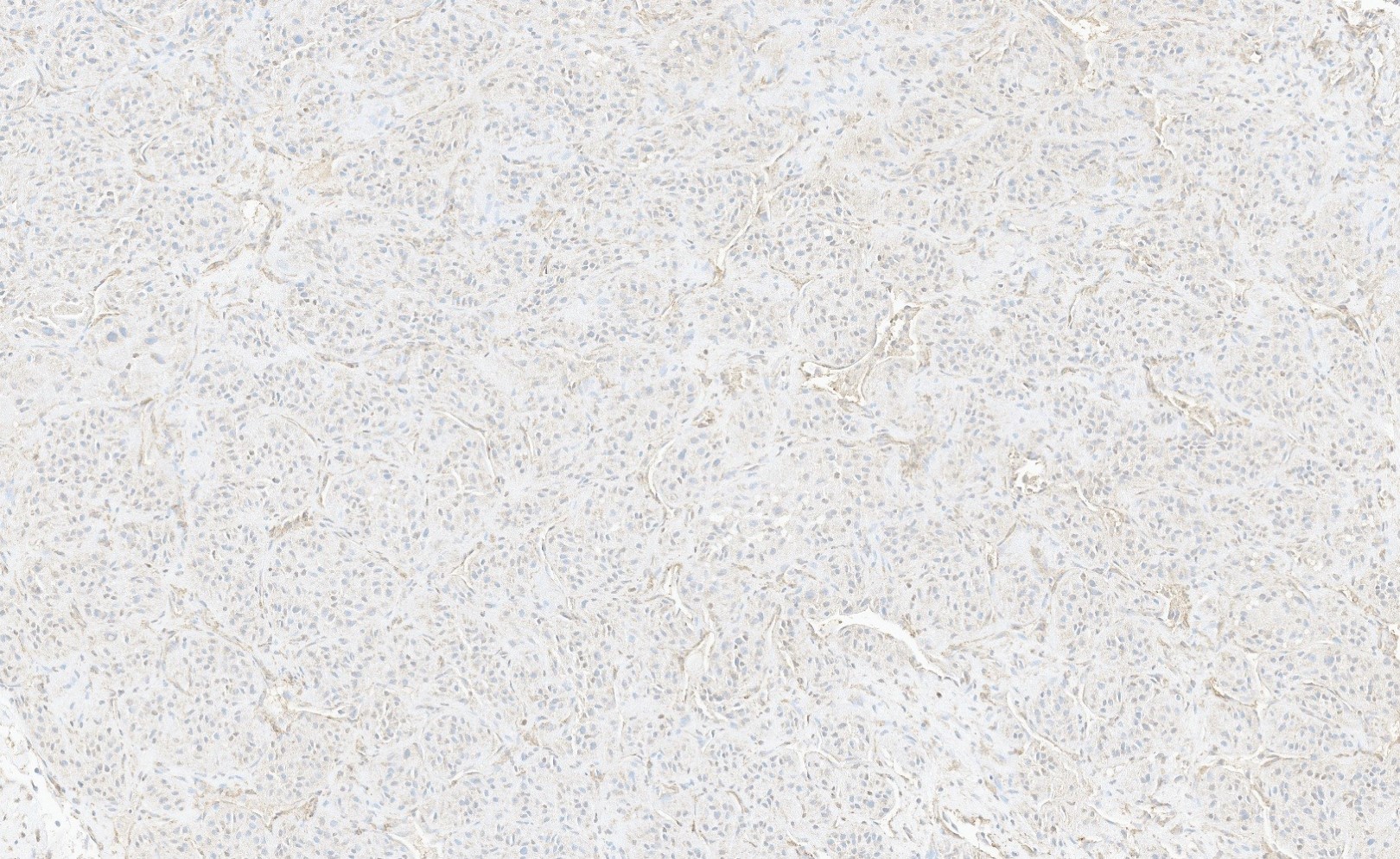
On a routine exam, a 46 year old woman was found to have a 3 cm, painless mass on her labia majora. Her gynecologist suspected a Bartholin cyst and performed a conservative local excision. A representative photomicrograph from the lesion is shown. The lesion was grossly and microscopically well circumscribed but the surgical margin was focally positive. By immunohistochemistry, the tumor cells were positive for desmin, ER and PR. Which of the following statements about this lesion is true?
- Approximately 10% experience distant metastasis
- Conservative local excision is considered curative
- HMGA2 IHC is positive in ~ 90%
- If surgical margins are positive, local recurrence risk exceeds 50%
- Stromal neutrophils are found in virtually all such lesions
Comment Here
Reference: Angiomyofibroblastoma
Which of the following statements about angiomyofibroblastoma is true?
- Abundant, thick walled hyalinized vessels are characteristic
- At low magnification, alternating hypo and hypercellular zones are characteristic
- Men and women are affected in approximately equal numbers
- Rb IHC is negative, reflecting underlying RB1 / FOXO1 (chr 13q14) deletion
- The vagina is affected approximately 3 times as often as the vulva
Comment Here
Reference: Angiomyofibroblastoma
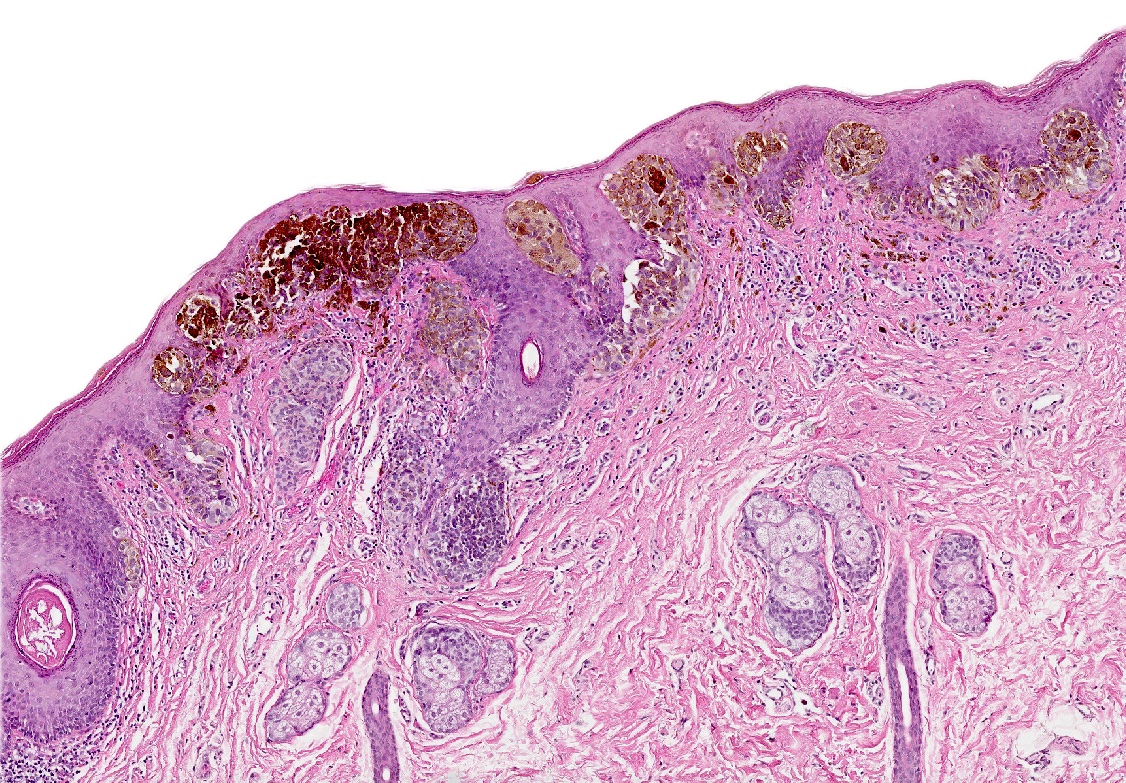
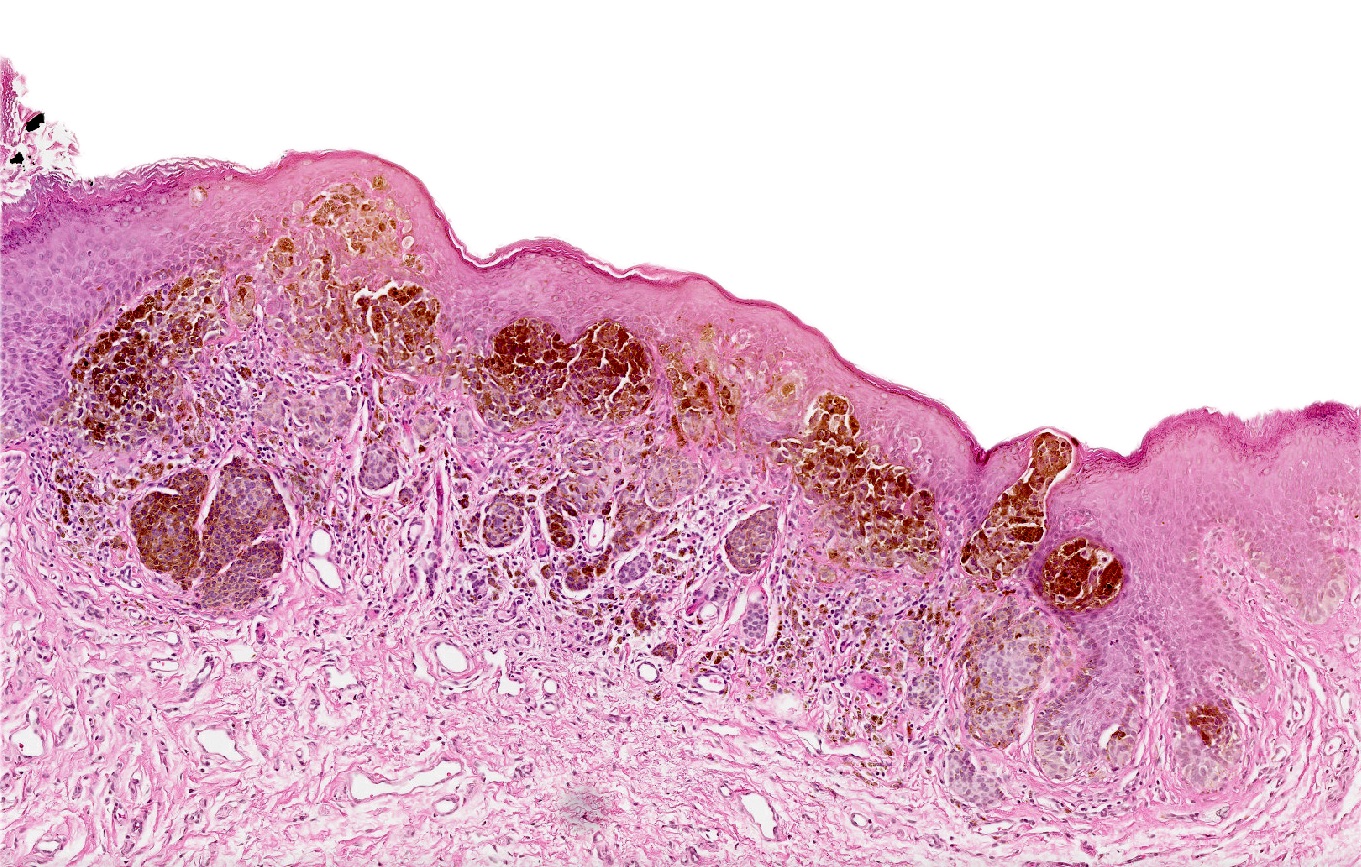
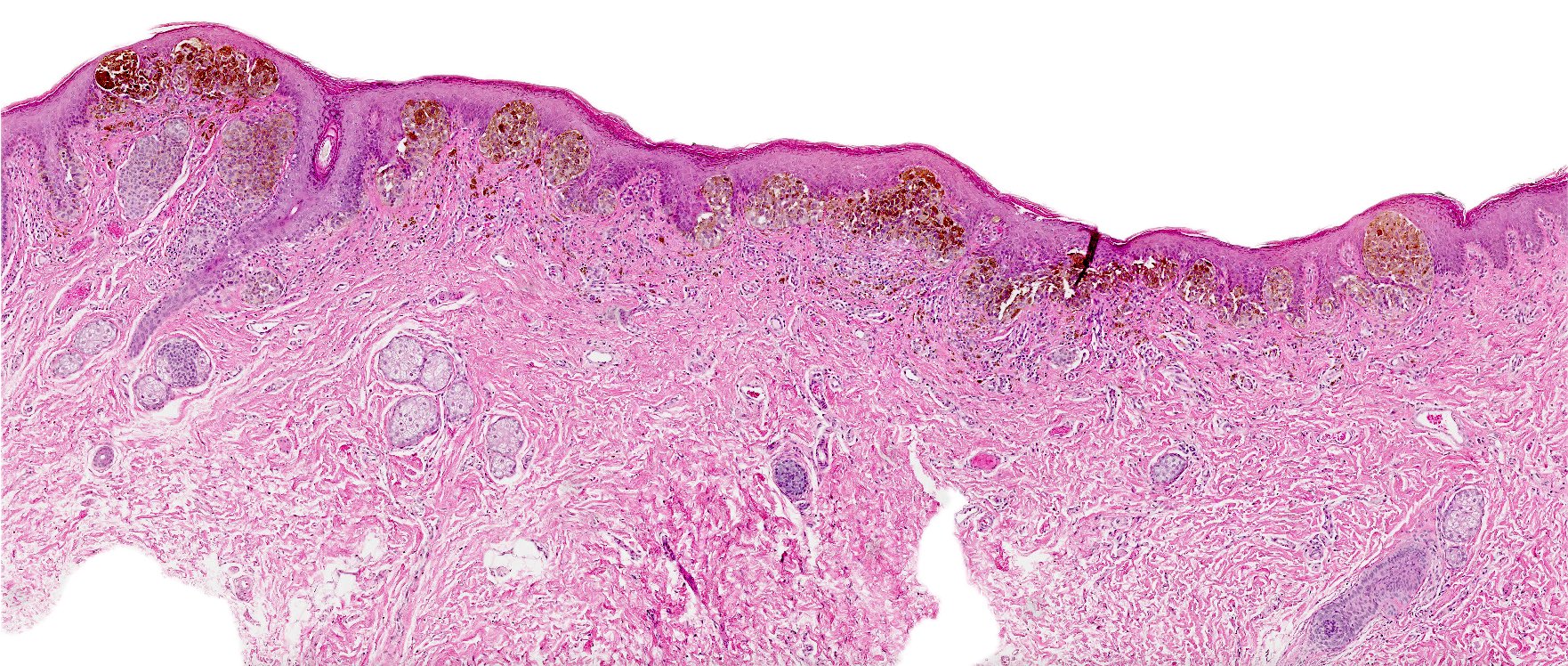
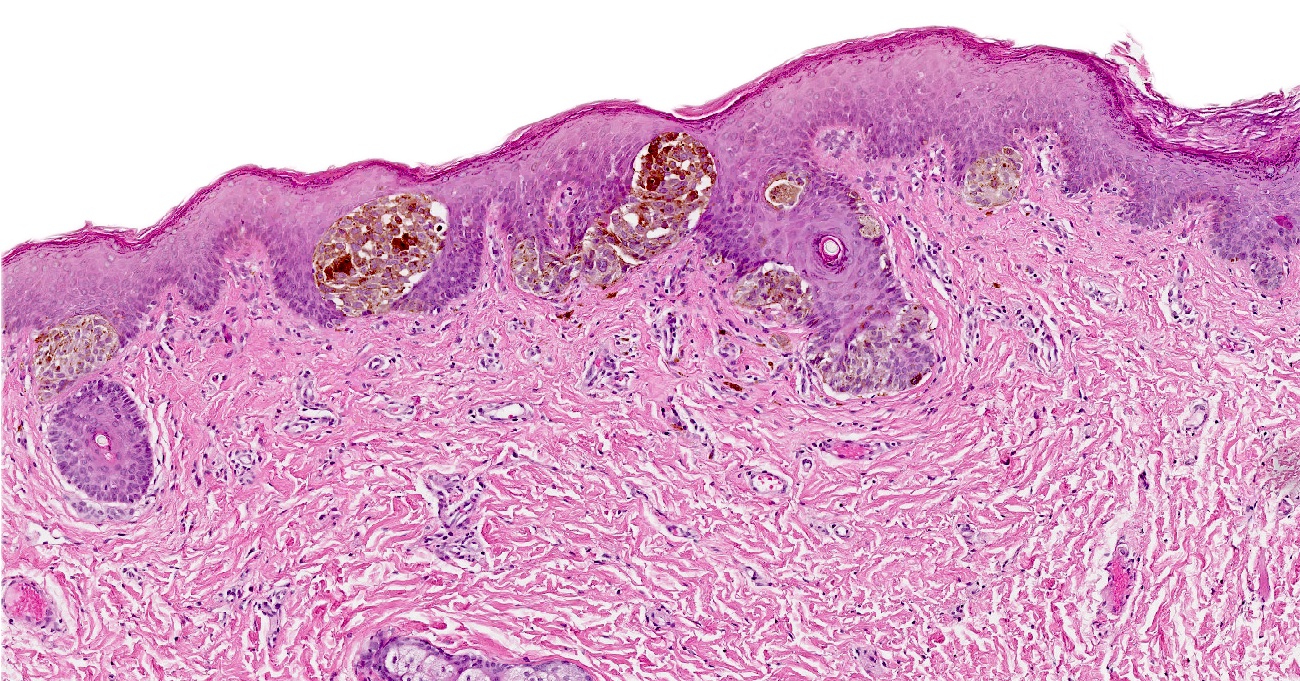
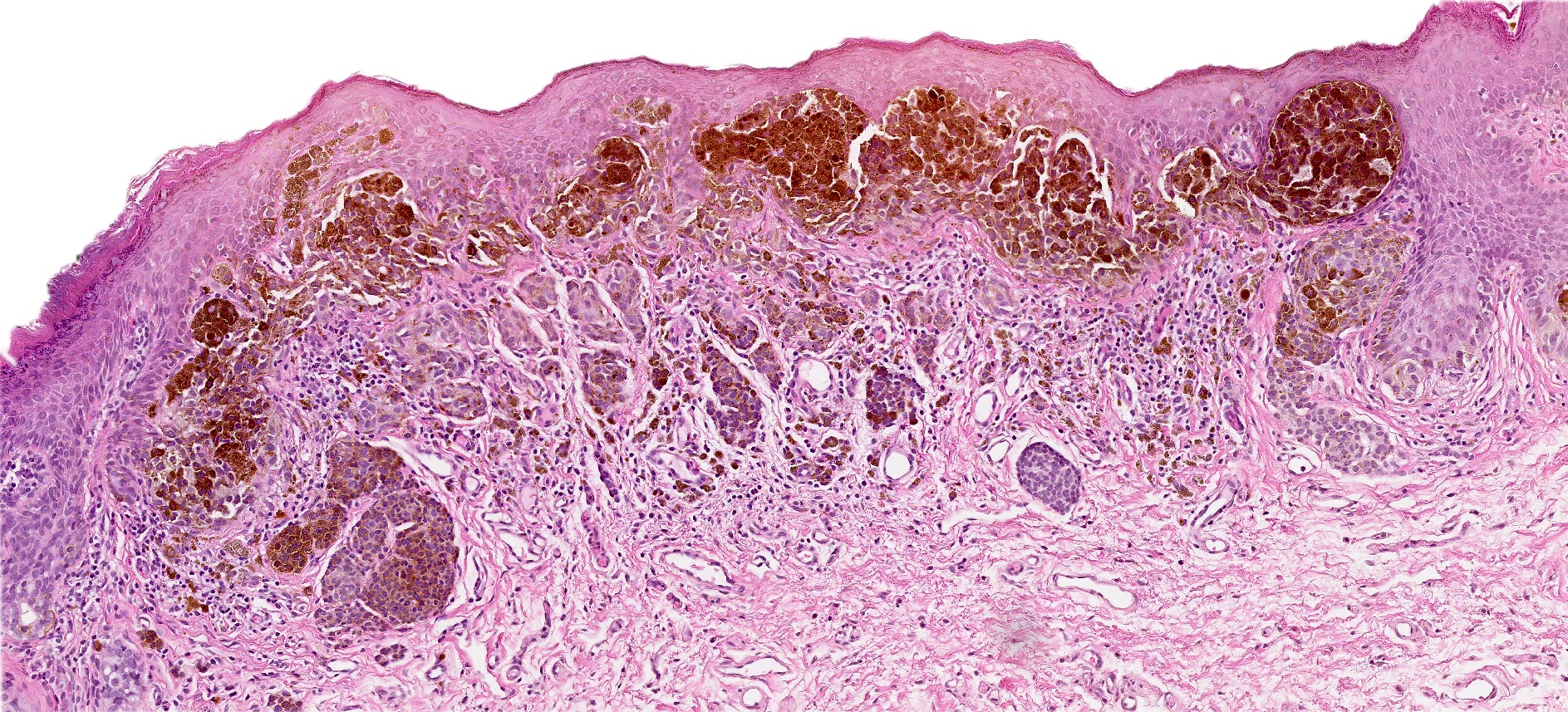
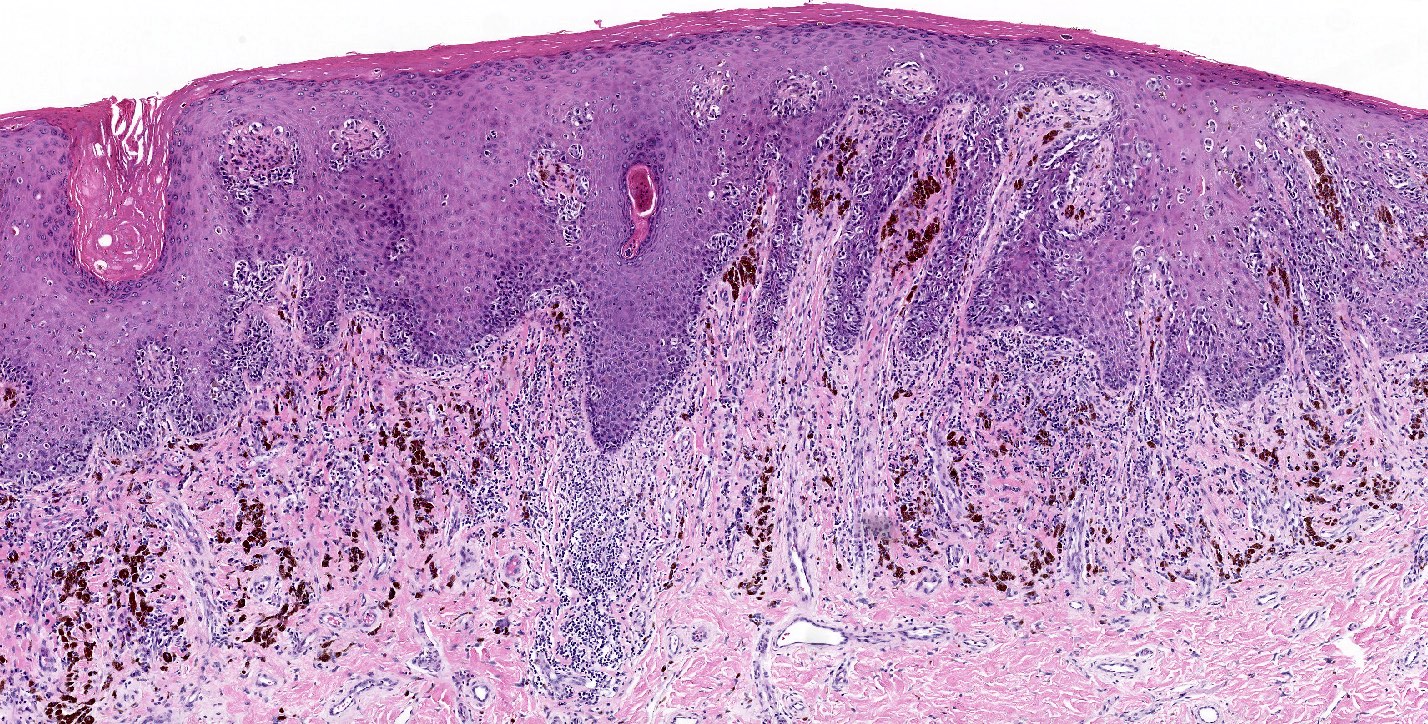
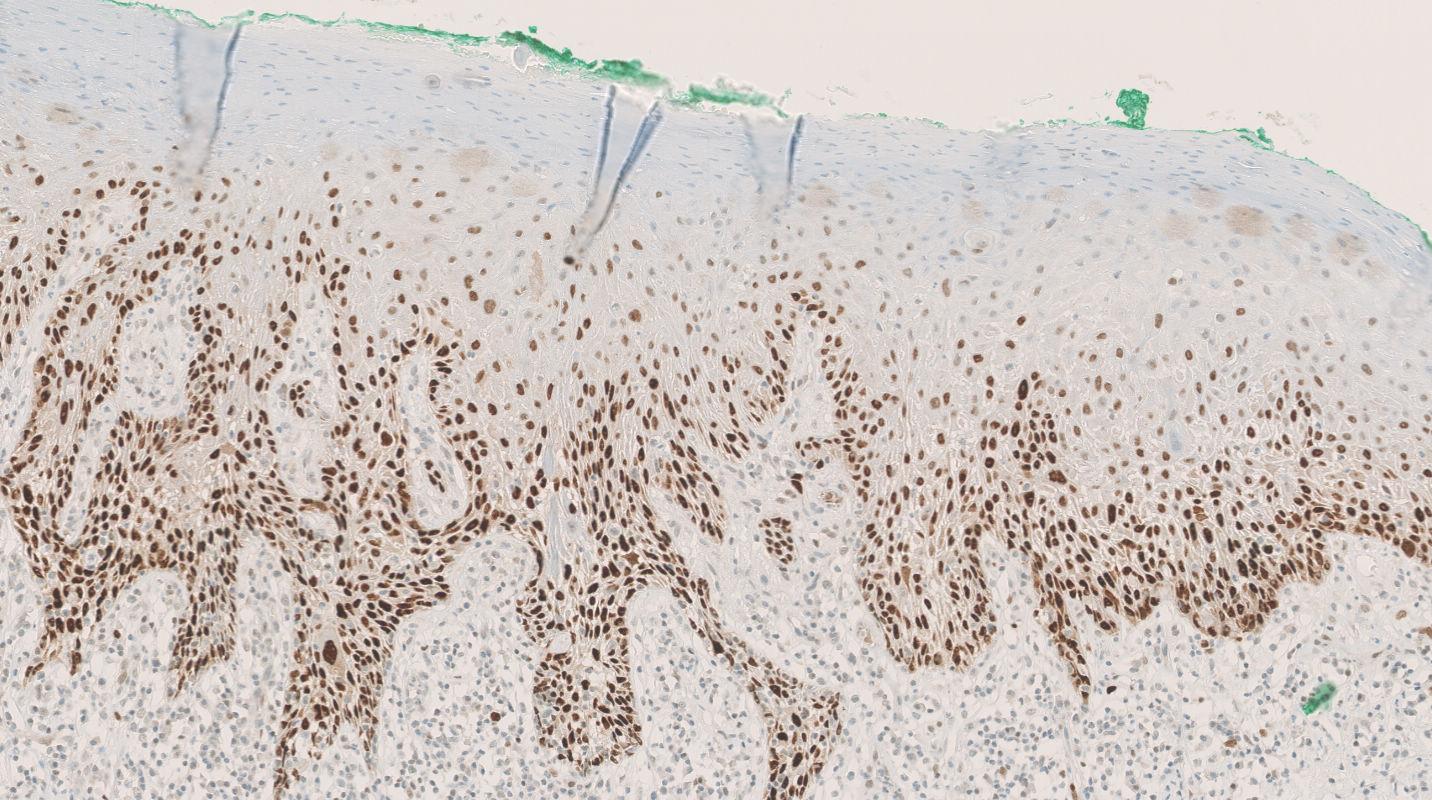
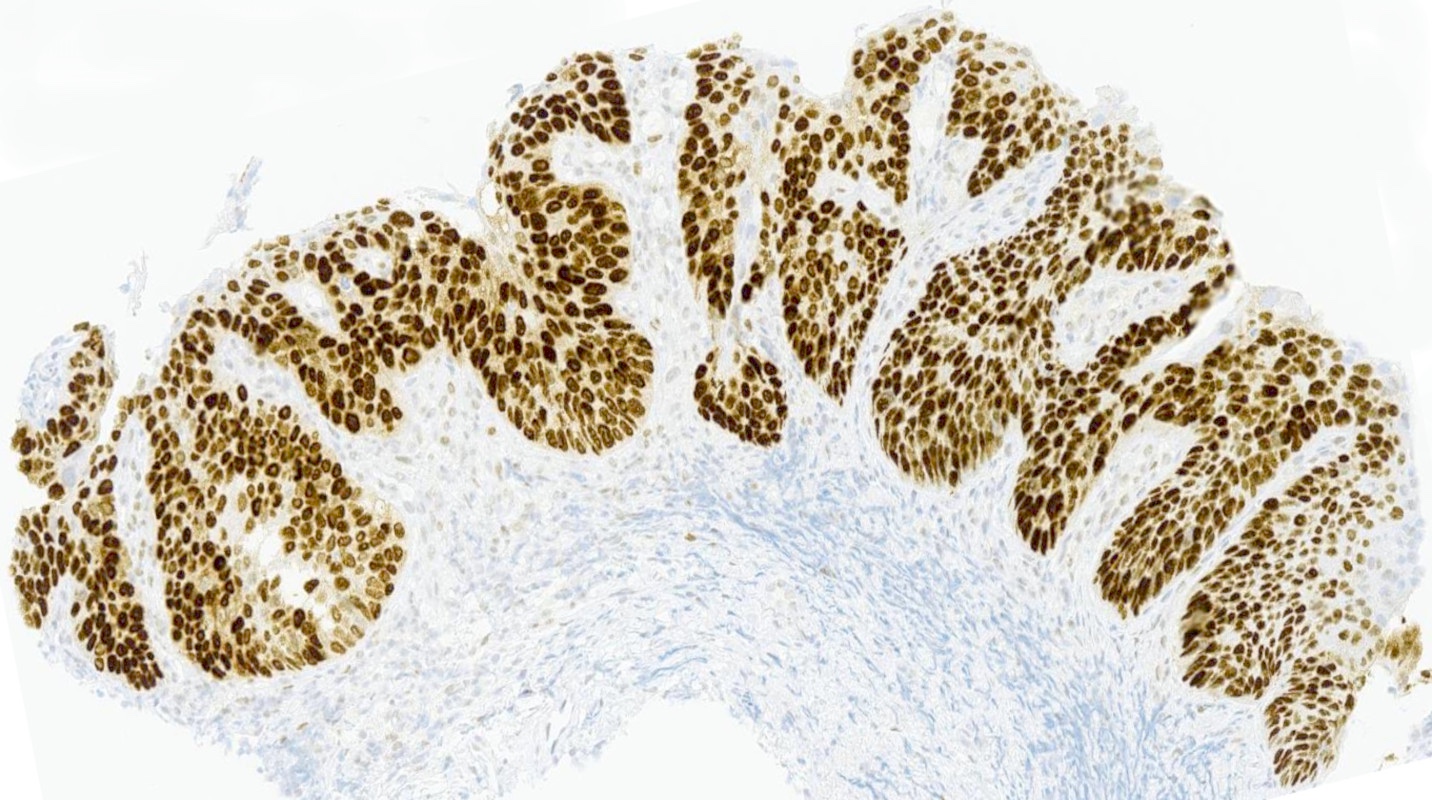
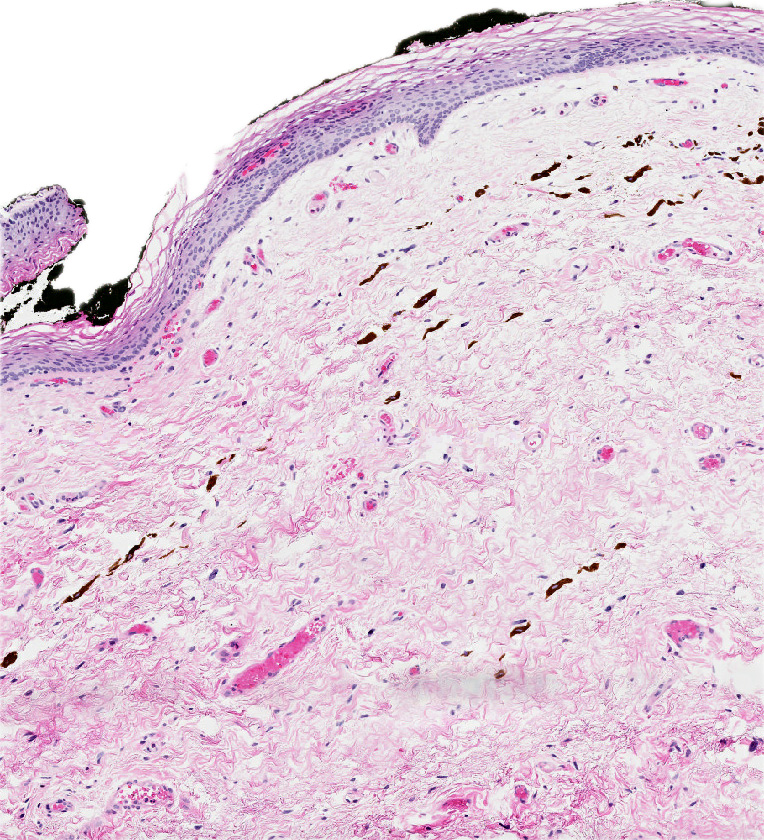
- Rare benign melanocytic lesion, most commonly involving the vulva of young women with concerning histologic features that may overlap with melanoma but are associated with benign behavior (J Cutan Pathol 2008;35:24)
- Belongs to the general group nevi of special sites
- Commonly also part of pigmented lesions of the vulva (J Cutan Pathol 2008;35:24)
- Nonmelanoma histologic diagnosis
- Atypical melanocytic nevi of genital type (AMNGT)
- Atypical genital nevi (AGN)
- Nevi with site related atypia
- Nevi of special sites
- ICD-O
- ICD-10
- ICD-11
- EG9Y - skin disorders involving other specific body regions
- EG9Z - skin disorders involving certain specific body regions, unspecified
- Specific anatomy (use additional code, if desired)
- Generally occurs in young women, with 50% of patients < 20 years old (median: 26 years; range: 6 - 54 years) (Am J Surg Pathol 2008;32:51, J Cutan Pathol 2008;35:24, J Am Acad Dermatol 2014;71:1241, Dermatology 2011;222:157)
- Most common: labia majora > labia minora > clitoris, followed by mons pubis and perineum (Hum Pathol 1998;29:S1, Am J Surg Pathol 2008;32:51, Arch Pathol Lab Med 2011;135:317)
- More frequent on mucosal areas in children < 10 years old (Hum Pathol 1998;29:S1, Am J Surg Pathol 2008;32:51)
- In adults, equal distribution between mucosal and hair bearing surfaces (Am J Surg Pathol 2008;32:51)
- Largely unknown but associated with anatomic milk line, including axillae, breasts, periumbilical region and groin (Am J Surg Pathol 2008;32:51)
- Compared to other nevi of special sites, AMNGT may have alarming clinical features, including darker pigmentation, irregular borders and size up to 2 cm (median: 0.5 cm) (Am J Surg Pathol 2008;32:51, Hum Pathol 1998;29:S1)
- Mucosal sites most commonly present with flat lesions and demonstrate atypical foci of hyperpigmentation (Am J Surg Pathol 2008;32:51)
- See table 1
Table 1: Clinical and histologic differences between atypical genital nevus and vulvar melanoma (adapted from Hoang: Melanocytic Lesions - A Case Based Approach, 1st Edition, 2014)
| Proposed diagnosis | Atypical genital nevus of special anatomic site | Vulvar melanoma |
| Age | Premenopausal, young adult | Postmenopausal |
| Size | < 1 cm | > 1 cm |
| Delineation | Well circumscribed | Infiltrative |
| Symmetry | Present | Absent |
| Lateral extension of junctional component | Focal | Present |
| Lentiginous junctional component | Focal | Present |
| Junctional nests | Discohesive | Confluent |
| Retraction artifact | Present | Absent |
| Ulceration | Absent or due to trauma | Often present |
| Pagetoid upward spread | Focal, central, inconspicuous | Prominent |
| Cytologic atypia | Superficial, mild to moderate | Deep, moderate to severe |
| Dermal mitosis | Rare and superficial | Conspicuous, atypical, deep |
| Dermal maturation | Present | Absent |
| Melanin pigmentation | Coarse, uniform | Fine, irregular |
| Dermal fibrosis | Broad zone of superficial coarse dermal fibrosis | Regression type |
- Dermoscopy for nonmodified mucous membranes is useful (Dermatol Ther 2010;23:449)
- May have any of the dermoscopic patterns seen in benign nevi
- Globular / cobblestone, homogenous, structureless or mixed patterns are most common (Dermatology 2011;222:157, Dermatology 2010;221:55)
- Shiny white streaks (SWS), inverse pigment network and atypical network can be seen in AMNGT in setting of symmetry pattern and absence of melanoma specific dermoscopic features (Dermatology 2011;222:157, Dermatology 2010;221:55)
- See table 2
- Histologic diagnosis is confirmatory
- For optimal diagnosis, lichen sclerosus should be controlled before excision of the nevus (J Am Acad Dermatol 2004;50:690, Arch Dermatol 2002;138:77)
Table 2: Distinguishing vulvar nevi, melanosis and melanoma (adapted from J Am Acad Dermatol 2014;71:1241)
| Reassuring features suggestive of a benign process | Concerning features for possible malignancy | |
| Clinical |
|
|
| Dermoscopy |
|
|
| Reflectance confocal microscopy |
|
|
- Thought to have a benign clinical course (Hum Pathol 1998;29:S1, Am J Surg Pathol 2008;32:51)
- No recurrences noted in cases with negative margins (Hum Pathol 1998;29:S1, Am J Surg Pathol 2008;32:51)
- Limited studies show a 9% recurrence in those that have positive or uncertain margins (Am J Surg Pathol 2008;32:51)
- 7 year old girl with a genital nevus in the background of lichen sclerosus (Am J Dermatopathol 2012;34:838)
- 12 year old girl with a new variant of site related histological atypia (Am J Dermatopathol 2011;33:611)
- 13 year old girl with an atypical melanocytic nevus of genital type (Dermatol Reports 2023;15:9667)
- 30 year old woman with atypical melanocytic nevi of genital tract (Dermatol Online J 2010;16:9)
- Simple excision is usually sufficient
- Symmetric lesion with sharp demarcation and dermal maturation (J Cutan Pathol 2008;35:24)
- 3 histologic patterns described by Clark (Hum Pathol 1998;29:S1)
- Nested: oval, typically large nests, perpendicular or parallel to dermoepidermal junction
- Discohesive nests: nearly contiguous, forming a band that separates the epidermis from the mature dermal melanocytes
- Crowded: closely apposed, ill defined nests and single cells obscuring the dermal epidermal junction
- Usually mild to moderate uniform cytologic atypia (J Cutan Pathol 2008;35:24)
- Single cell growth with focal pagetoid spread may be present but is usually located in the center of the lesion (J Cutan Pathol 2008;35:24)
- Adnexal spread may be present (Hum Pathol 1998;29:S1)
- Dense eosinophilic fibrosis in the superficial dermis (Hum Pathol 1998;29:S1, J Cutan Pathol 2008;35:24)
- Intradermal component with maturation is often present (J Cutan Pathol 2008;35:24)
- Nevi within lichen sclerosus can appear more atypical
- See table 1
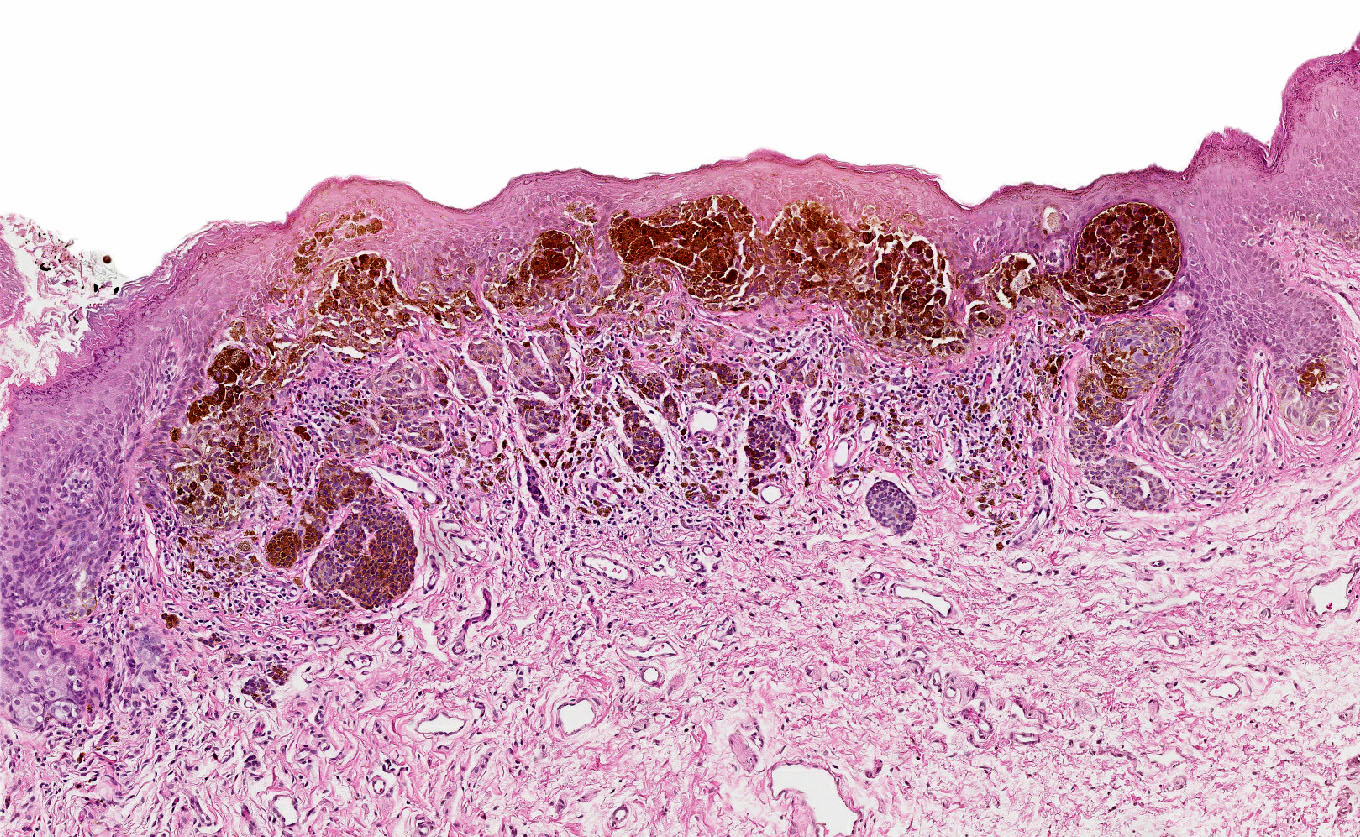
- Melanocytic markers
- MelanA (MART1) may help to highlight fused nests in nevi (J Cutan Pathol 2020;47:446)
- HMB45 (Ann Diagn Pathol 2023;67:152211, Biomed Rep 2016;5:327, J Am Acad Dermatol 2012;67:446)
- In common junctional and compound nevi, expressed in melanocytes at dermoepidermal junction
- Highlights a gradient or maturation (zonation) pattern (diminishing or loss of expression from top to bottom)
- Highly variable in Spitz and congenital nevi
- SOX10 highlights junctional melanocytes to help differentiate melanoma in situ from benign counterparts (Appl Immunohistochem Mol Morphol 2014;22:142)
- Ki67 proliferation rate should be low in the dermal component of genital nevi (J Am Acad Dermatol 2012;67:446)
- HMB45 is commonly negative in intradermal and compound nevi
- PRAME (preferentially expressed antigen in melanoma) is typically lost in nevi (J Cutan Pathol 2020;47:1123)
- BRAF mutation is the most common alteration in melanocytic nevi of genital skin but not in vulvar melanomas (N Engl J Med 2015;373:1926, J Invest Dermatol 2016;136:1858)
- Fluorescence in situ hybridization (FISH) or comparative genomic hybridization (CGH) may be considered in very challenging cases to rule out melanoma
- Sensitivity of both tests may be lower than expected, due to decreased cellularity in vulvar lesions
- For vulvar melanomas, pTERT and CDK4 can be considered but may not be part of each FISH panel (Nat Commun 2019;10:3163, Am J Cancer Res 2020;10:4017)
Atypical nevi and nevi of special sites by Dr. Phillip McKee
Atypical nevi and melanoma by Dr. Steven Wang
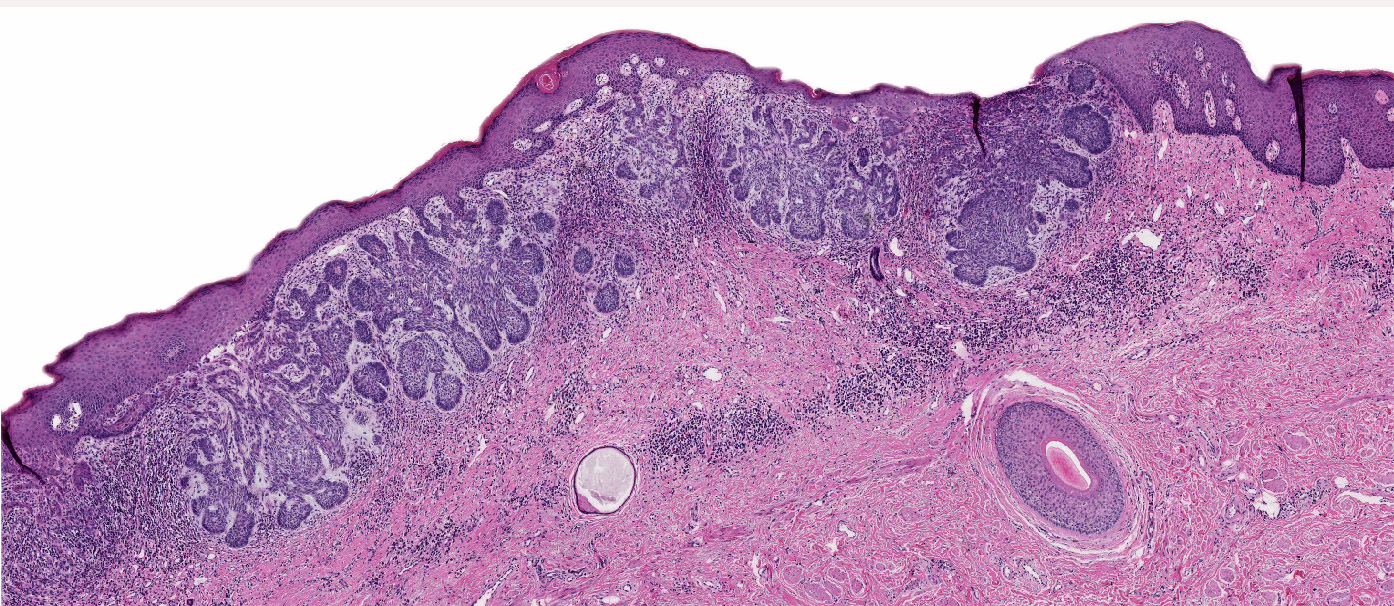
- Vulva, labia minora, excisional biopsy:
- Atypical melanocytic nevus of the genital type, compound pattern, excised entirely
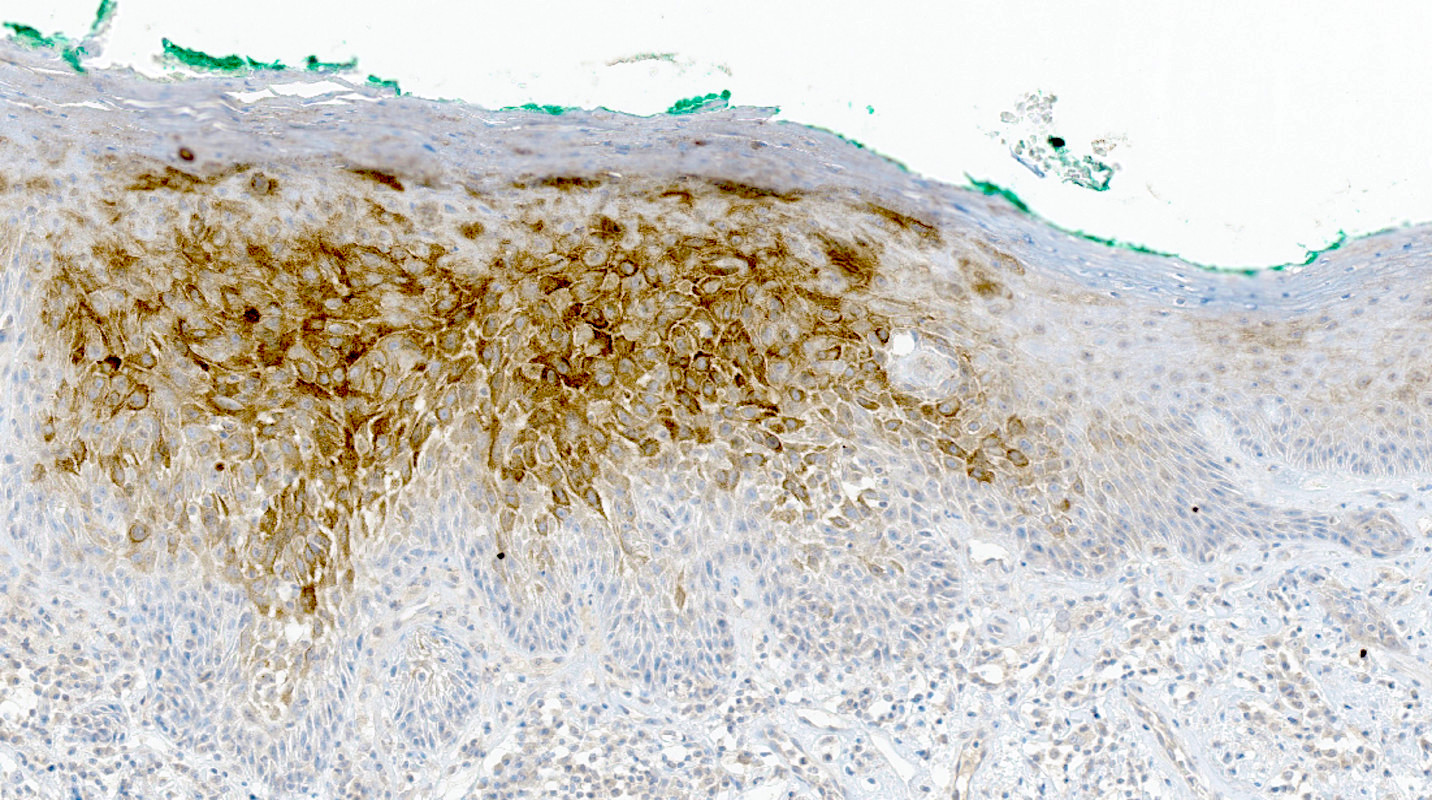
- Melanoma:
- May be most challenging to differentiate from superficial spreading subtype of melanoma (SSM)
- Atypical melanocytes in the epidermis as single cells or forming nests with pagetoid spread
- Dermal component shows no maturation with cells forming sheets, nests, cords, single cells and rarely fascicles
- S100, SOX10 and nerve growth factor receptor (NGFR) are the most sensitive markers in visualization of invasive growth
- PRAME shows diffuse staining
- Melanomas of vulva usually lack BRAF mutation (Br J Dermatol 2010;162:677)
- Pigmented epithelioid melanocytoma (PEM) of the vulva:
- PEM family consists of multiple, usually slow growing, distinct histologic melanocytic entities with potential to metastasize but with a better prognosis than melanoma
- Infiltrative deep dermal tumor that may involve subcutis
- Hypercellular tumor with cells ranging from medium sized epithelioid cells to large epithelioid cells and spindled cells
- Low mitotic activity
- PRKAR1A loss in 67% of PEMs (Am J Surg Pathol 2017;41:1333, Am J Surg Pathol 2019;43:480)
- Dysplastic nevus of vulva:
- Differentiation requires clinical pathologic correlates
- Presence of junctional shoulders; extension of junctional component at least 3 rete ridges beyond the dermal component
- Superficial nests are usually very similar and may show focal bridging or coalescence of the nests
- Elongation and bridging of the rete ridges with nests
- Melanocytes may scatter suprabasally (confined to the lower epidermal layer and centrally)
- 2 tier grading of cytologic atypia is recommended by World Health Organization (WHO) classification and is largely based on nuclear features (Hum Pathol 1999;30:500)
- WHO Classification of Tumours Editorial Board: Skin Tumours, 5th Edition, 2023, J Cutan Pathol 2008;35:889, Am J Surg Pathol 1995;19:792, Ackerman: Pathology of Malignant Melanoma, 1st Edition, 1981, McKee: Pathology of the Skin with Clinical Correlations, 3rd Edition, 2005, Busam: Pathology of Melanocytic Tumors, 1st Edition, 2018
- ALK fusion
- BRAF mutation
- Homozygous deletion of CDKN2A
- PTEN mutation
Comment Here
Reference: Atypical melanocytic nevi of genital type-vulva
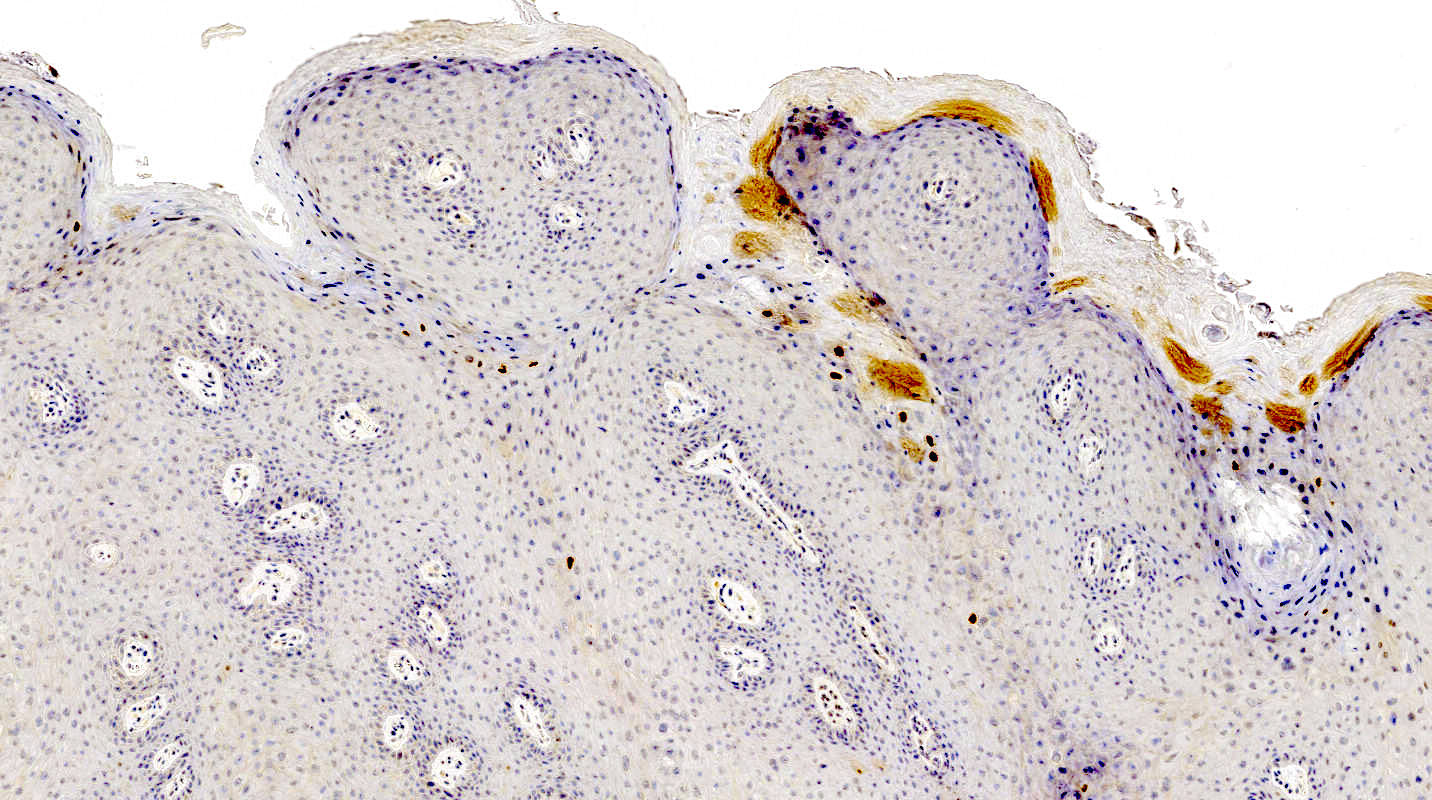
A 16 year old girl presented with her parents to the OBGYN clinic with an irregular, dome shaped, dark pigmented papule on the mons pubis. A biopsy demonstrated large, ill defined nests with focal retraction. What is the most likely interpretation of the immunohistochemical stains for this patient's lesion?
- Diffuse expression of PRAME
- Gradient pattern of HMB45
- High Ki67 proliferation index in the dermal component
- SOX10 highlighting pagetoid and extensive lentiginous growth
Comment Here
Reference: Atypical melanocytic nevi of genital type-vulva
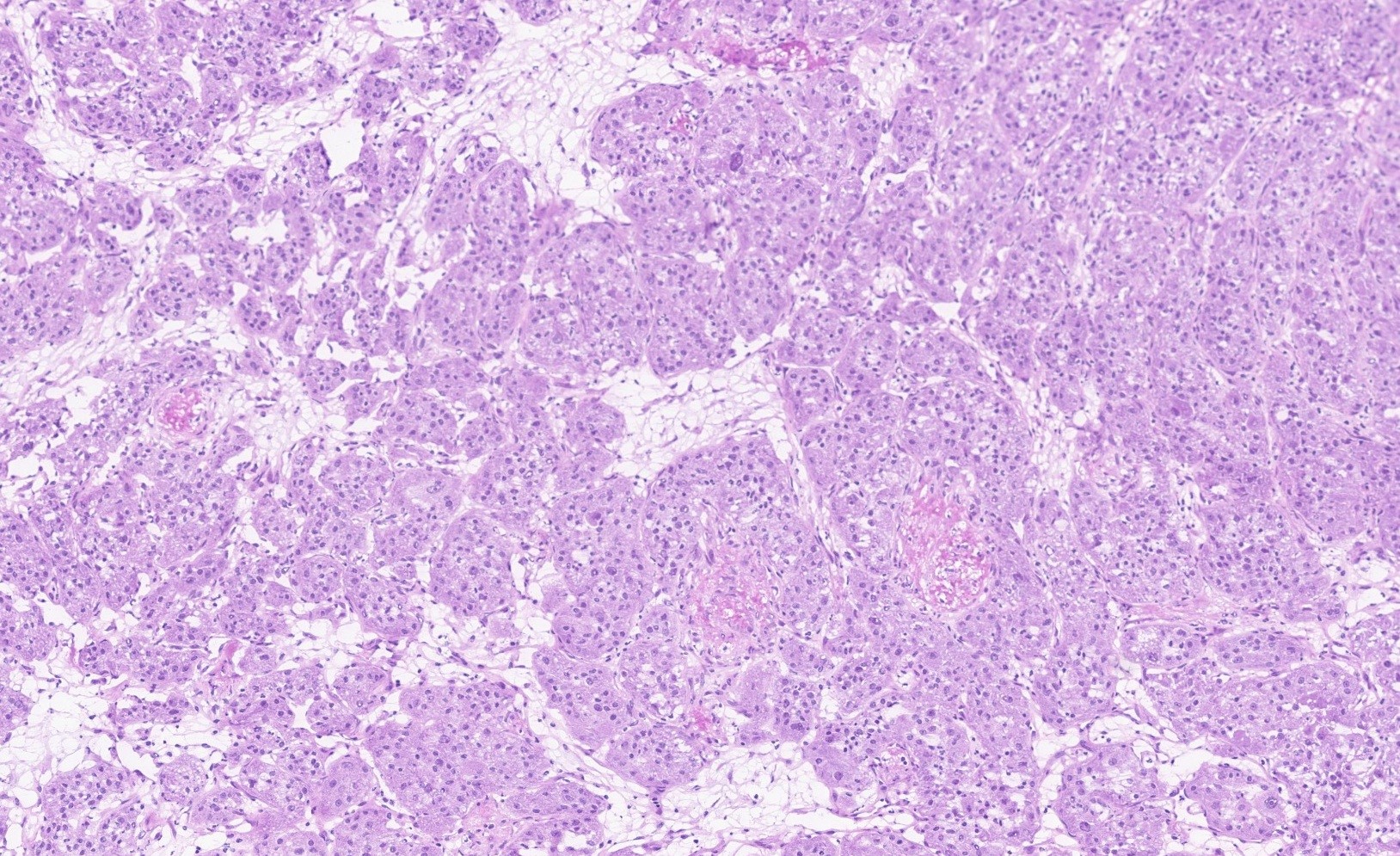
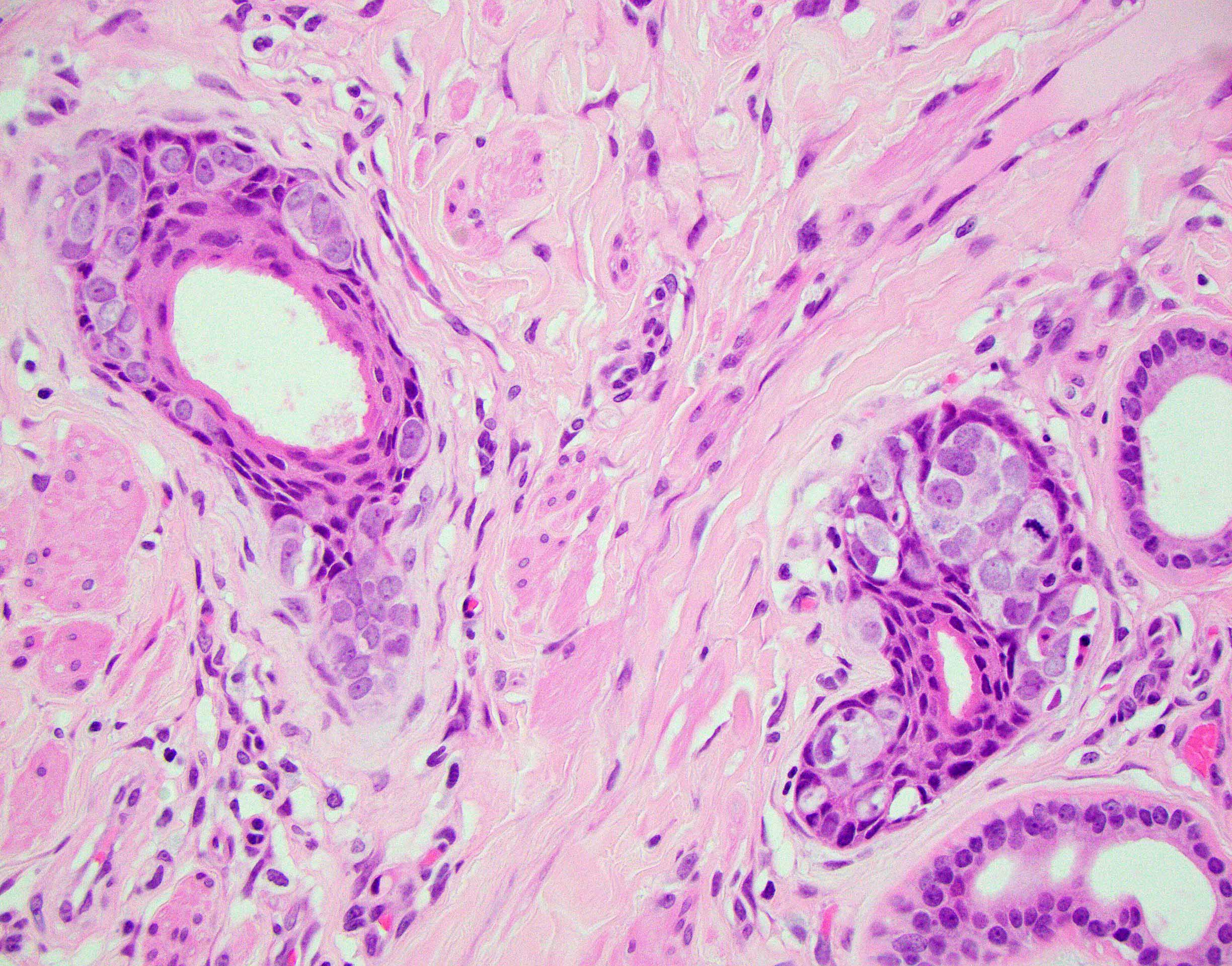
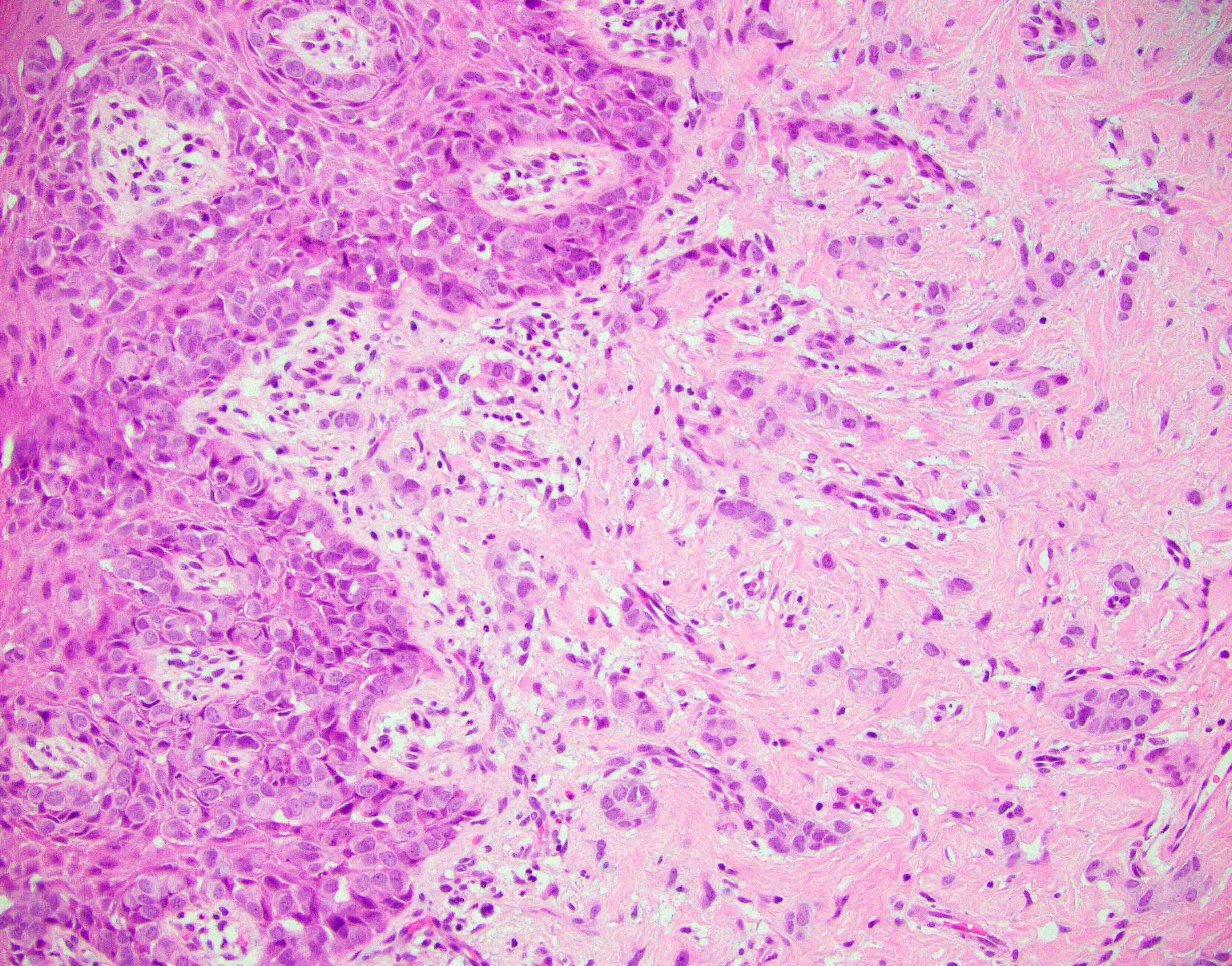
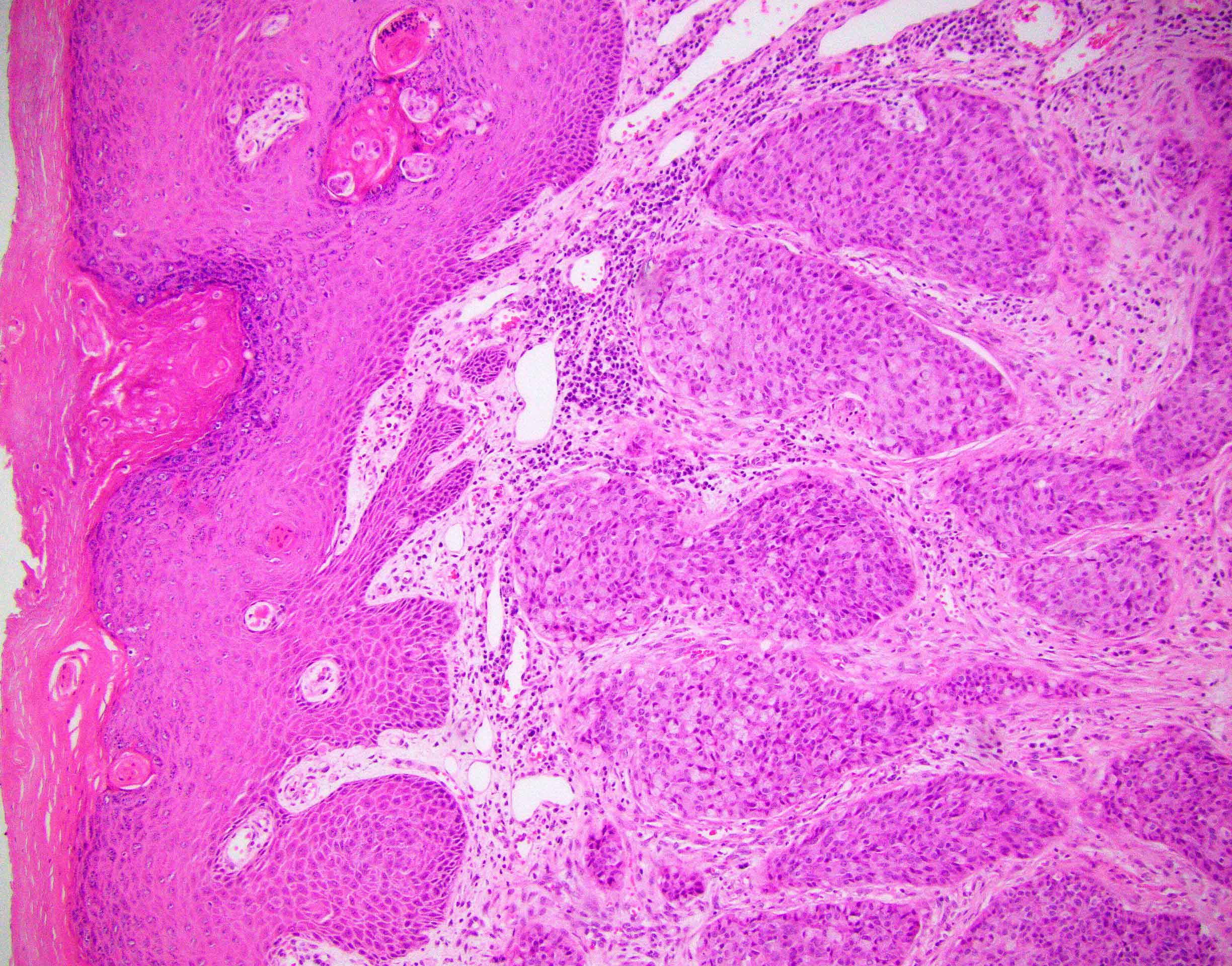
- Rare carcinoma arising from the Bartholin gland
- These can be squamous cell carcinoma, adenocarcinoma, adenoid cystic carcinoma or other rare variants
- Carcinoma arising from Bartholin gland
- Wide range of histologic types can be encountered but most are squamous cell carcinoma, adenocarcinoma or adenoid cystic carcinoma
- Overlying epithelium should be uninvolved, i.e. exclude downgrowth of an overlying vulvar squamous cell carcinoma or Paget disease
- Exclude metastasis to Bartholin gland from a genital or extragenital site
- ICD-11: 2C70.Z & XH74S1 - malignant neoplasms of vulva, unspecified and adenocarcinoma, NOS
- Bartholin gland carcinomas arise in middle aged and elderly women and account for approximately 5% of vulvar malignancies (Surg Oncol 2013;22:117)
- Diagnosis of Bartholin gland carcinoma can only be made if the anatomical location of the tumor is compatible with origin from Bartholin gland, i.e. posterolateral on the vulva (posterior to the labium major), without involvement of overlying squamous epithelium
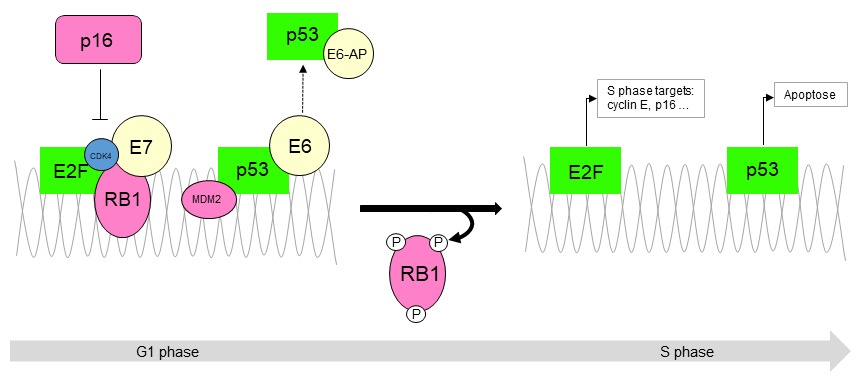
- There is a strong causal association between human papillomavirus (HPV) and squamous cell carcinoma of Bartholin gland (Int J Gynecol Pathol 2019;38:189, Histopathology 2000;37:87, Am J Pathol 1993;142:925)
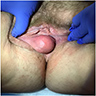
- Painless mass in the region of Bartholin gland in an elderly woman
- May be mistaken for a Bartholin gland cyst or abscess
- Biopsy of a mass in the region of Bartholin gland
- Ultrasound: echogenic mass that may contain calcifications (Crit Rev Oncol Hematol 2017;117:1)
- MRI: solid, isodense to skeletal muscle (Surg Oncol 2013;22:117)
- CT scan: may detect nodal spread (Surg Oncol 2013;22:117)
- Imaging can be used to help exclude a primary tumor elsewhere that has metastasized to the Bartholin gland
- Tumor size and stage are the most important, with prognosis similar to that of vulvar squamous cell carcinoma (Surg Oncol 2013;22:117, J Clin Oncol 2008;26:884)
- Overall prognosis is favorable, especially for tumors localized to the vulva, without nodal metastasis
- 33 year old woman with adenoid cystic carcinoma of the Bartholin gland (Case Rep Obstet Gynecol 2019;2019:1784949)
- 44 and 51 year old women with Bartholin gland epithelial myoepithelial carcinoma (Int J Gynecol Pathol 2009;28:286)
- 49 year old woman with adenoid cystic carcinoma of the Bartholin gland (Oncol Lett 2014;8:849)
- 49 year old woman with Merkel cell carcinoma of the Bartholin gland (Gynecol Oncol 2005;97:928)
- 49 year old woman with primary adenocarcinoma of the Bartholin gland (J Obstet Gynaecol 2015;35:536)
- 51 year old woman with adenoid cystic carcinoma of the Bartholin gland (Eur J Gynaecol Oncol 2013;34:487)
- 61 year old woman with adenoid cystic carcinoma of the Bartholin gland (Pathol Res Pract 2020;216:152968)
- 64 year old woman with adenoid cystic carcinoma of the Bartholin gland (Tokai J Exp Clin Med 2019;44:68)
- 66 year old women with Bartholin gland squamous cell carcinoma (J Obstet Gynaecol 2012;32:318)
- Surgical excision, (vulvectomy with unilateral or bilateral lymphadenectomy) often with adjuvant radiotherapy administered (similar to treatment of vulvar squamous cell carcinoma)
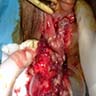
- Firm, irregularly shaped, unencapsulated mass deep to the vulvar skin
- Frozen section findings are not specific and are dependent on the tumor histologic type (e.g. squamous cell carcinoma, adenocarcinoma, adenoid cystic carcinoma)
- Frozen section is typically not required as diagnosis will have been made based on preoperative biopsy
- Approximately 85 - 90% of Bartholin gland carcinomas are squamous cell carcinomas, approximately 10% adenocarcinomas and Gynecol Oncol 2001;82:247, Int J Gynecol Cancer 2016;26:785, Int J Gynecol Pathol 2019;38:189)
- Additionally, several rare to exceedingly rare histological subtypes have been described, such as transitional cell carcinoma, neuroendocrine carcinoma, epithelial myoepithelial carcinoma, Merkel cell carcinoma and lymphoepithelioma-like carcinoma (Crit Rev Oncol Hematol 2017;117:1)
- Histopathologic diagnostic criteria include the following (Obstet Gynecol 1972;39:489):
- Tumor involves the anatomic region of the Bartholin gland and is histologically compatible with origin from the Bartholin gland
- Areas of apparent transition from normal Bartholin gland elements to neoplastic ones are present
- There is no evidence of primary tumor elsewhere
- Histological features
- Squamous cell carcinoma:
- Usually human papillomavirus (HPV) associated and has positive p16 immunostaining (Am J Pathol 1993;142:925, Int J Gynecol Pathol 2019;38:189)
- Morphology is identical to more common HPV associated squamous cell carcinomas of cervix, vagina or vulva
- Adenocarcinomas:
- Nonspecific features (adenocarcinoma NOS) are most common
- May show mucinous differentiation or other growth patterns
- Adenoid cystic carcinoma:
- Morphology is identical to the adenoid cystic carcinoma of salivary gland or lung
- Low grade cells arranged in cribriform pattern; luminal spaces contain mucin or basement membrane material
- Tendency for extensive perineural invasion (Tokai J Exp Clin Med 2019;44:68, Crit Rev Oncol Hematol 2017;117:1)
- Squamous cell carcinoma:
- Cytological examination is typically not used in diagnosis
- It may be used to confirm lymph node metastasis
- Cytological features reflect the histologic type of the carcinoma
- Adenocarcinoma:
- p16- or weak focal positivity (Am J Pathol 1993;142:925, Int J Gynecol Pathol 2019;38:189)
- Adenoid cystic carcinomas show chromosomal rearrangements involving NFIB (also seen in adenoid cystic carcinomas arising at other anatomic sites) in most cases (Int J Gynecol Pathol 2017;36:289)
- Vulva, biopsy:
- Invasive squamous cell carcinoma, consistent with Bartholin gland carcinoma (see comment)
- Comment: This squamous cell carcinoma shows strong diffuse p16 immunoreactivity, consistent with it being associated with high risk human papillomavirus. The clinical setting, i.e. location of the tumor mass and uninvolved overlying vulvar skin, is noted. While the histologic type of this tumor is compatible with it being a primary Bartholin gland carcinoma and there are adjacent benign Bartholin gland acini in this biopsy, clinical correlation is required to exclude a metastatic squamous cell carcinoma from another site.
- Vulvar squamous cell carcinoma:
- Involvement of overlying squamous epithelium
- Invasive Paget disease:
- Epidermocentric with extensive involvement of overlying squamous epithelium
- Mammary-like adenocarcinoma of the vulva:
- Associated benign mammary acini and ducts
- Location away from Bartholin gland
- Expression of breast cancer biomarkers (Breast J 2020;26:1242, Histopathology 2017;71:446)
- Bartholin gland cyst or abscess:
- Simple cyst lined by benign mucinous or squamous epithelium (Case Rep Obstet Gynecol 2018;2018:5256876)
- Which of the following is true about squamous cell carcinoma of the Bartholin gland?
- Has a significantly worse prognosis than other vulvar squamous cell carcinomas
- Is often associated with human papillomavirus infection
- Is the second most common histological subtype of Bartholin gland carcinoma after adenocarcinoma
- Often presents with a painful mass similar to Bartholin gland abscess
Comment Here
Reference: Bartholin gland carcinoma-vulva
- Basal cell carcinoma (BCC) arises from epithelial cells of either epidermis or hair follicle stem cells
- Vulvar BCC is rare
- Histologic features are identical to that of BCC occurring elsewhere on the skin
- Characterized by the presence of nests of basaloid / hyperchromatic nuclei with minimal cytoplasm
- Predominantly seen in older (mean: seventh decade) White women
- Occurs most commonly in the labium majus but can involve any other area of vulva
- Excellent prognosis with up to 21% rate of local recurrence and very rare metastasis
- Not recommended: basalioma of the vulva, basal cell epithelioma of the vulva
- Accounts for J Am Acad Dermatol 2001;45:68, Gynecol Oncol 2005;97:192)
- Mean patient age is ~70 years (Gynecol Oncol 2005;97:192, Dermatol Surg 2023;49:13)
- Predominantly seen in White women
- Cutaneous epithelium of the vulva but may involve mucosal epithelium as well
- Most common location: labium majus followed by clitoris (Int J Dermatol 2019;58:892)
- Arises from skin epithelial cells or hair follicle stem cells (Cell Stem Cell 2015;16:400)
- Inactivating mutations in PTCH1 may contribute to BCC genesis in the absence of ultraviolet irradiation (Cancer Cell 2018;33:229)
- Mutations in TP53
- Activating mutations of SMO
- HPV independent
- Unclear; etiology might differ from that of BCC in sun exposed skin
- Proposed risk factors include
- Chronic vulvar irritation
- History of pelvic radiation therapy (J Am Acad Dermatol 2001;45:68)
- Advanced age
- White race
- Basal cell nevus syndrome (Gorlin syndrome) due to germline PTCH1 mutations
- Xeroderma pigmentosum
- Immunosuppression (Gynecol Oncol 2005;97:192)
- Exposure to arsenic (TJ Obstet Gynaecol Br Emp 1956;63:697)
- Syphilis infection (TJ Obstet Gynaecol Br Emp 1956;63:697)
- Local trauma (Gynecol Oncol 2005;97:192, J Am Acad Dermatol 2001;45:68)
- Frequently asymptomatic
- In symptomatic patients, pruritus is the most frequent symptom (Int J Gynecol Pathol 2023;42:327, Gynecol Oncol 2016;142:440)
- Vulvar lump sensation, palpable vulvar mass, erythema, ulceration, irritation, pain, bleeding (Gynecol Oncol 2005;97:192)
- Clinical appearance ranges from erythematous papules and patches to plaques or nodules with or without ulceration or pigmentation (J Am Acad Dermatol 2001;45:68)
- Dermoscopy findings are identical to BCC in other body areas
- Presence of arborizing vessels and blue globules, shiny white structures (Dermatol Pract Concept 2018;8:68, Arch Dermatol 2007;143:426, J Eur Acad Dermatol Venereol 2017;31:e180)
- Definite diagnosis requires biopsy / excision
- Overall prognosis is excellent
- Local recurrence rates range from 0% to 21% in different case series (Int J Gynecol Pathol 2022;41:86, Cancer 1969;24:460, Int J Dermatol 2019;58:892)
- Lymph node and distant metastasis can occur but is rare
- Features with increased risk for recurrence and metastasis
- Size > 5 - 20 mm, tumor thickness, extension into the subcutis, perineural invasion, aggressive histological subtype (morpheaform, infiltrative, basosquamous) and surgical margins Obstet Gynecol 1997;90:765, Int J Gynecol Pathol 2022;41:86)
- 51 year old woman with a 1.5 cm firm vulvar lesion with shallow red ulcerations at the lower mons pubis (Cureus 2021;13:e20791)
- 51 year old woman with a history of incompletely resected vulvar basal cell carcinoma, now with bilateral lung nodules and inguinal lymphadenopathy (Gynecol Oncol Rep 2016;18:32)
- 70 year old woman with vulvar basal cell carcinoma and bilateral inguinal lymph node metastases (Case Rep Oncol 2019;12:573)
- 80 year old woman with a history of repeated exposure to perineal heat lamps presents with a 1.8 cm pink pearly eroded plaque on the left vulva (JAAD Case Rep 2020;6:103)
- 83 year old woman with a history of lichen sclerosus presents with a tender, 5 mm eroded papule on the right labium majus (Int J Dermatol 2019;58:892)
- Primary treatment is wide local excision or vulvectomy with margin assessment (Gynecol Oncol 2016;142:440)
- Surgical excision by Mohs micrographic surgery or PDEMA (peripheral and deep en face margin assessment) (J Natl Compr Canc Netw 2023;21:1181)
- Radiotherapy for nonsurgical candidates
- If not feasible, systemic therapy with HHIs (hedgehog pathway inhibitors) may be recommended (J Natl Compr Canc Netw 2023;21:1181)
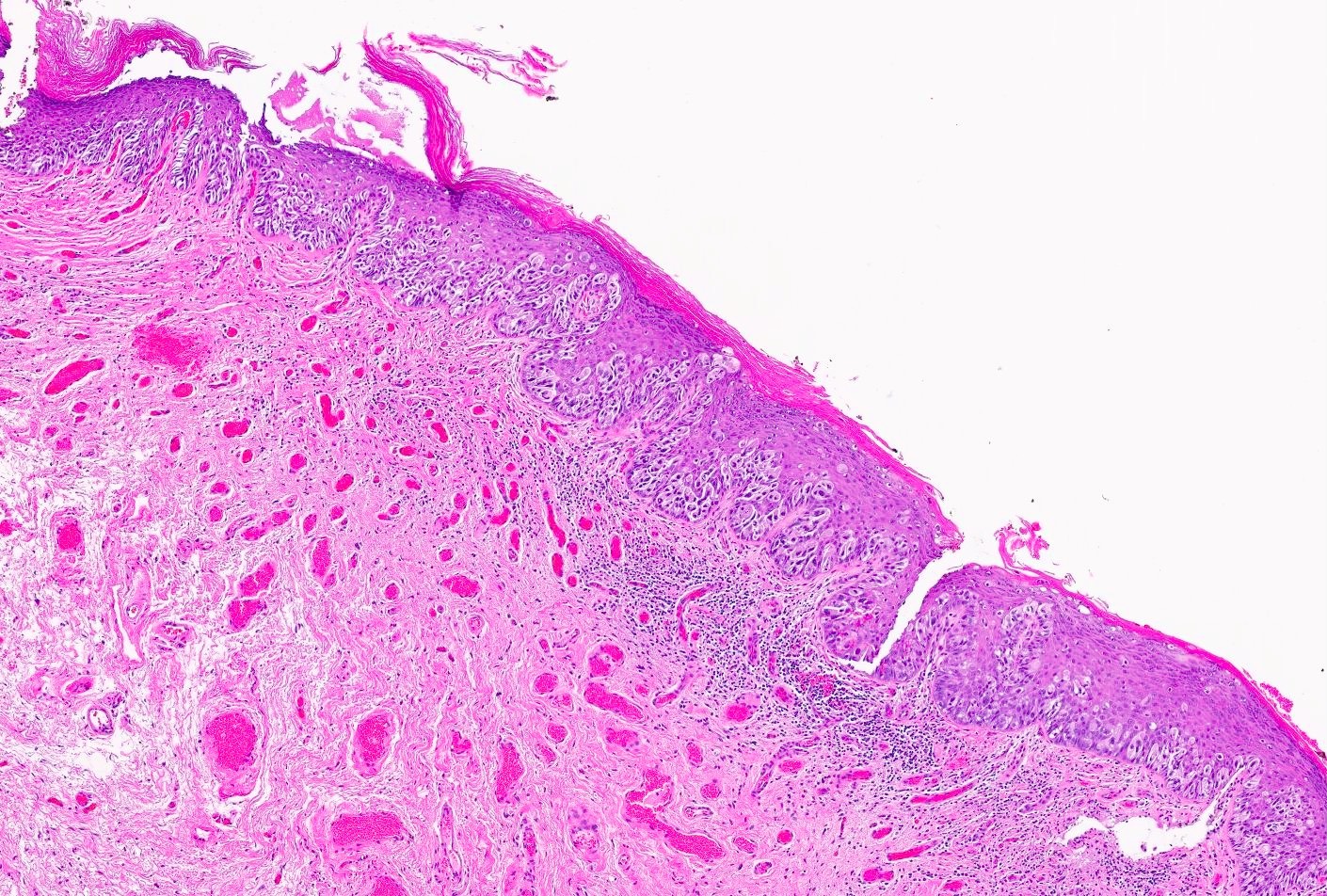
- Pink, pearly nodule, with or without elevated borders and telangiectasias
- Ulceration or dark brown pigment may be present
- Flat, scar-like lesion in superficial BCCs
- Reference: Calonje: McKee’s Pathology of the Skin, 5th Edition, 2019
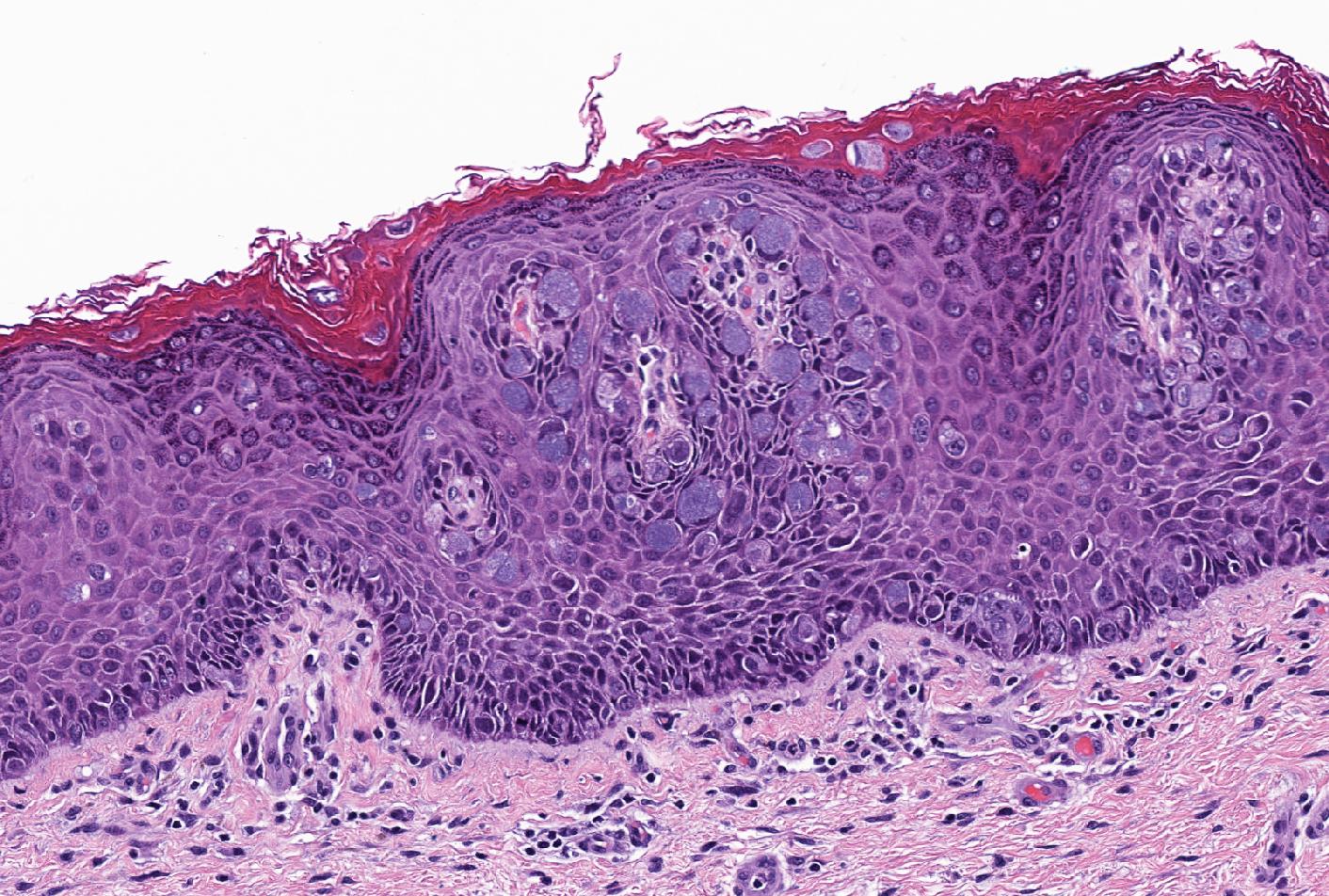
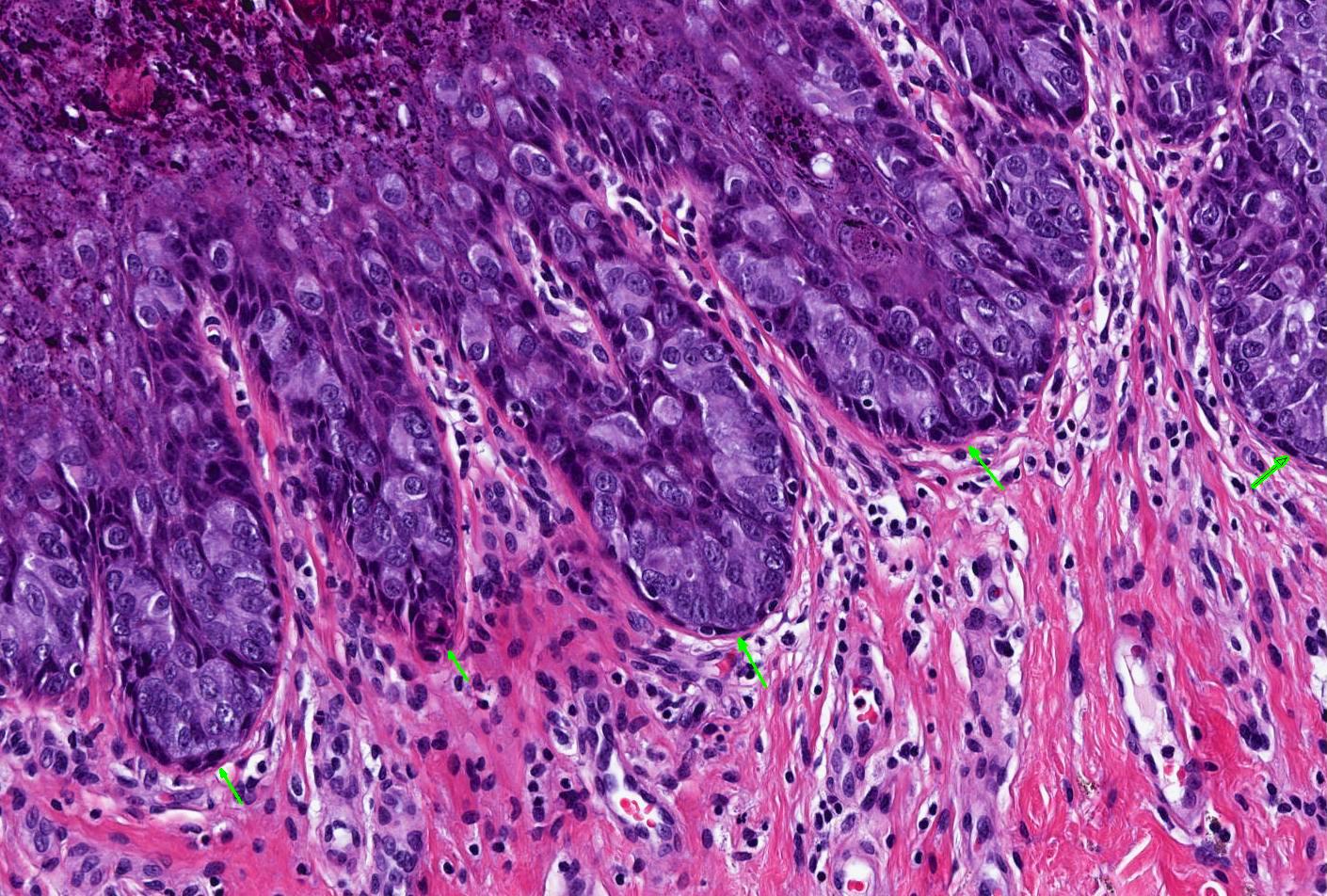
- Identical diagnostic criteria to BCC occurring elsewhere on the skin
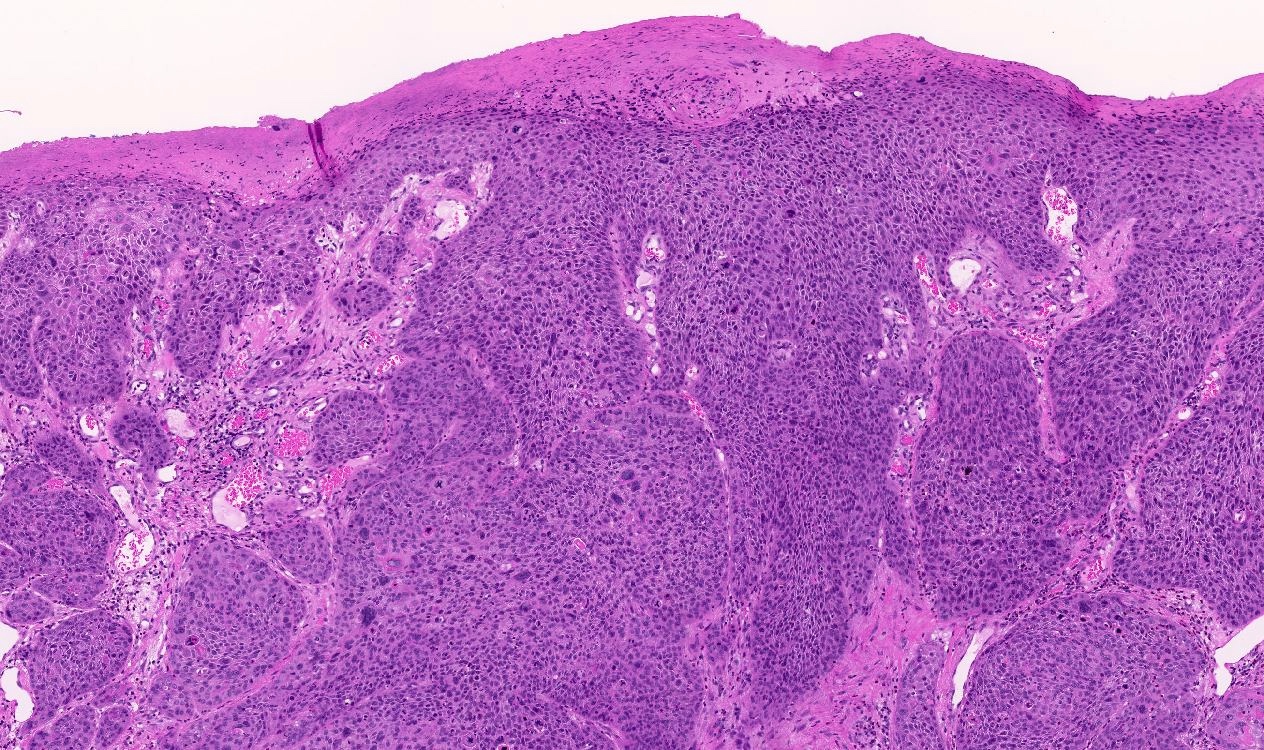
- Basaloid tumor cells with uniform hyperchromatic / basophilic nuclei and scant cytoplasm
- Peripheral palisading of tumor cells with variety of architectural patterns
- Retraction artifact of tumor nests from surrounding stroma (also known as clefting)
- Stromal changes
- Fibromyxoid change, calcification, amyloid deposition
- May be colonized by nonneoplastic melanocytes and may contain melanin pigment
- With or without squamous differentiation
- Most frequent histologic subtype is nodular, followed by superficial and infiltrative
- References: Calonje: McKee’s Pathology of the Skin, 5th Edition, 2019, Kurman: Blaustein's Pathology of the Female Genital Tract, 7th Edition, 2019
Contributed by Lucy Ma, M.D. and Priya Nagarajan, M.D., Ph.D.
- p40, p63, CK5/6
- BerEP4 (typically diffuse), BCL2 (diffuse), CD10 (tumor and stroma), AR (focal)
- CK7, CK19 (J Cutan Med Surg 2017;21:457)
- CD56 (95%), chromogranin A (28%), and synaptophysin (18%) (Med Oncol 2013;30:444, J Immunoassay Immunochem 2017;38:487)
- EMA, CEA, CK20 (may be positive in colonizing Merkel cells)
- PHLDA1 (TDAG51) (J Cutan Pathol 2011;38:542)
- p16 (negative or patchy), high risk HPV in situ hybridization (Am J Surg Pathol 2014;38:542)
- See Pathophysiology
BCC 101 by Dr. Jerad Gardner
Reporting BCC by Dr. Catriona McKenzie, pathCast
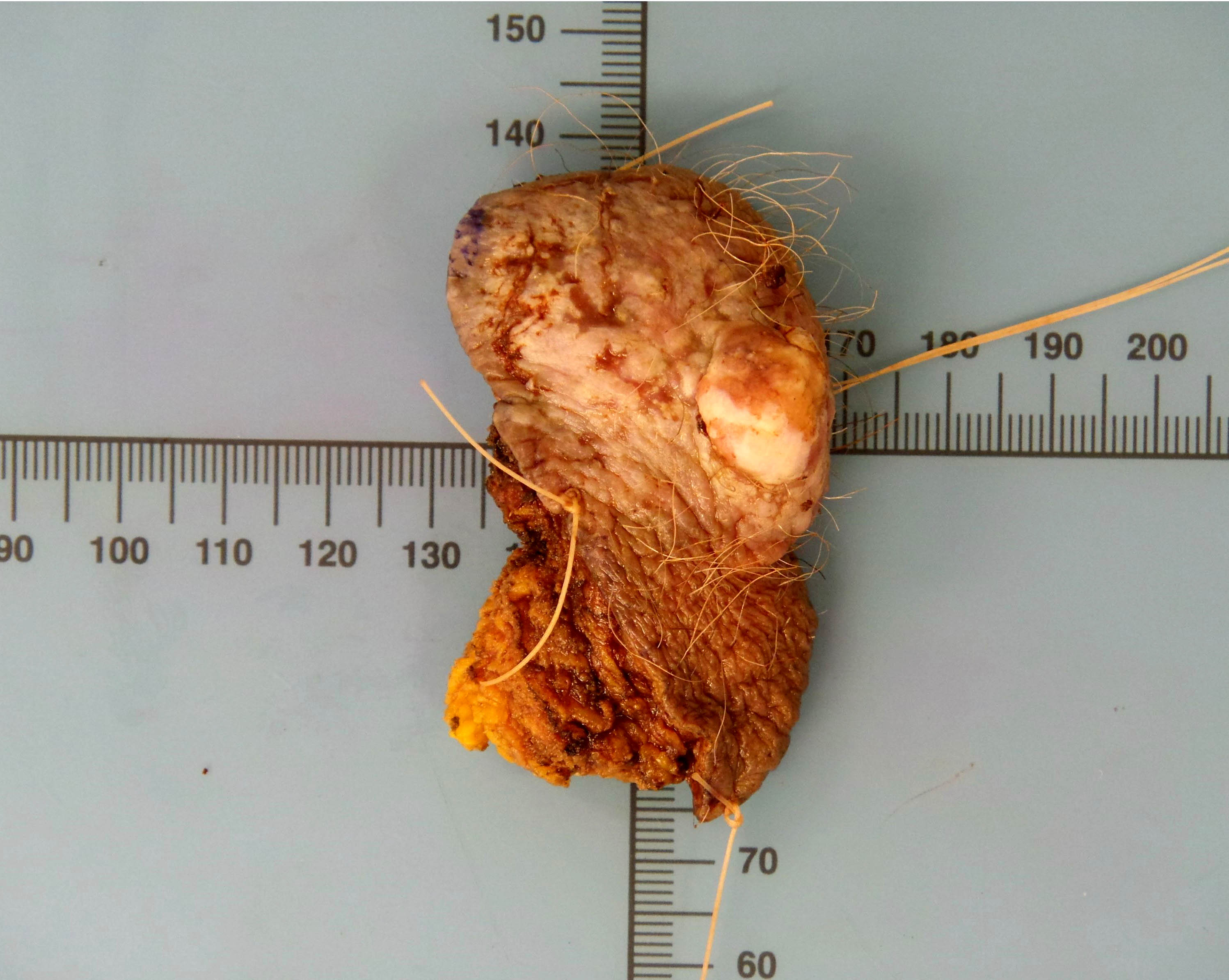
- Vulva, left (partial vulvectomy):
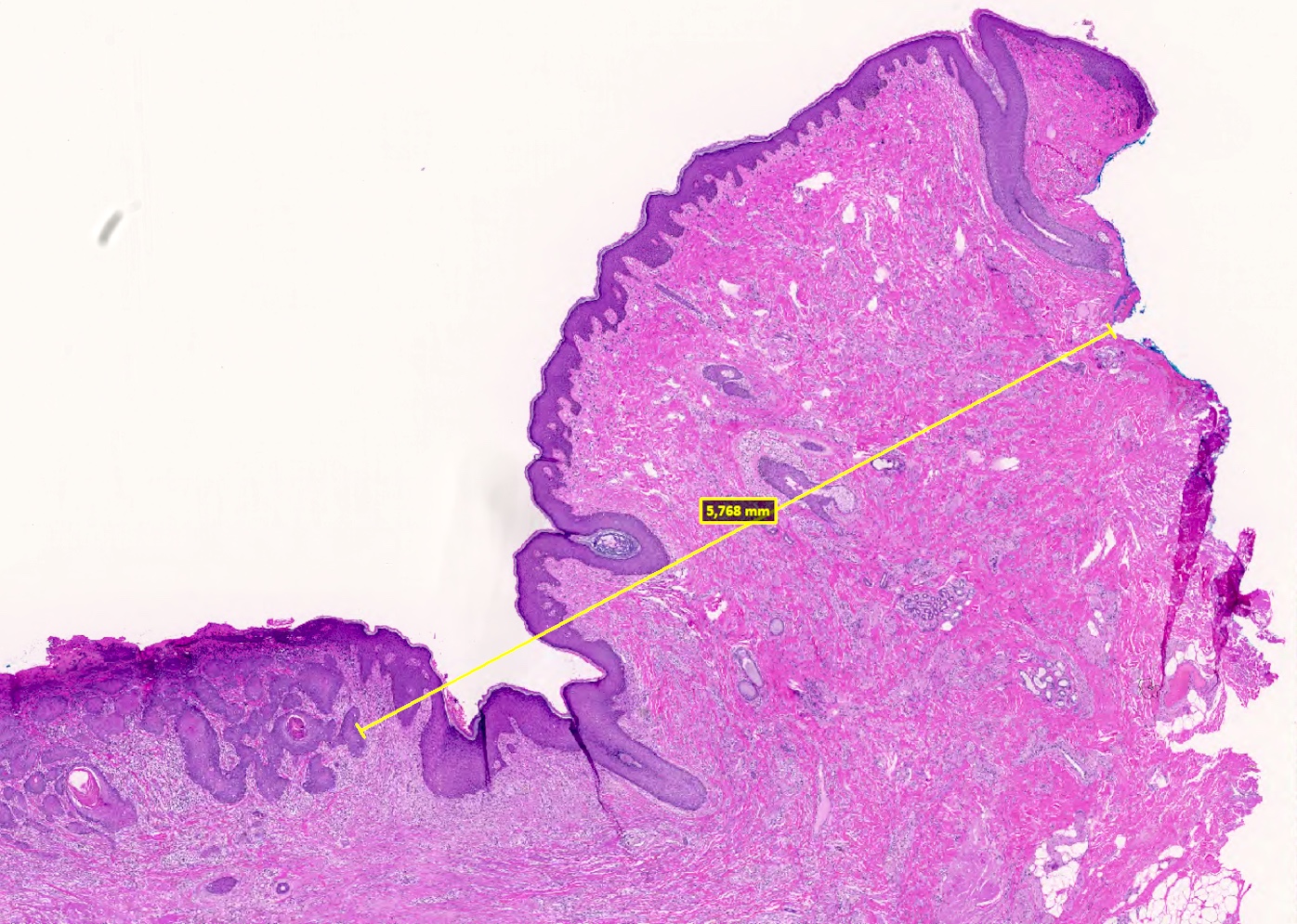
- Basal cell carcinoma, 1.3 cm
- Depth of invasion: 2 mm
- Margins are negative
- Basaloid squamous cell carcinoma:
- Trichoepithelioma / trichoblastoma:
- Basaloid follicular neoplasm
- Presence of horn / keratin cysts and lack of retraction artifact
- CD10 expression limited to stroma
- PHLDA1 (TDAG51)+
- Merkel cell carcinoma:
- Typically no connection to overlying dermis
- Small, round, blue cell tumor with high N:C ratio, round nuclei, salt and pepper chromatin
- Conspicuous mitoses and apoptotic bodies
- CK20+, perinuclear dot-like staining
- Adenoid cystic carcinoma:
- More deeply situated; no connection to overlying epidermis
- Biphasic neoplasm with ductal and myoepithelial differentiation
- Characteristic cribriform, tubular and solid architectural patterns
- Cribriform spaces filled with basement-like material
- MYB:NFIB fusion
- Differentiated vulvar intraepithelial neoplasia:
- Differential diagnosis of superficial subtype of BCC
- Atypical cells confined to basal layer
- Large nuclei with vesicular chromatin, prominent nuclei
- Retained but abnormal maturation of epithelium
- Aberrant p53 expression
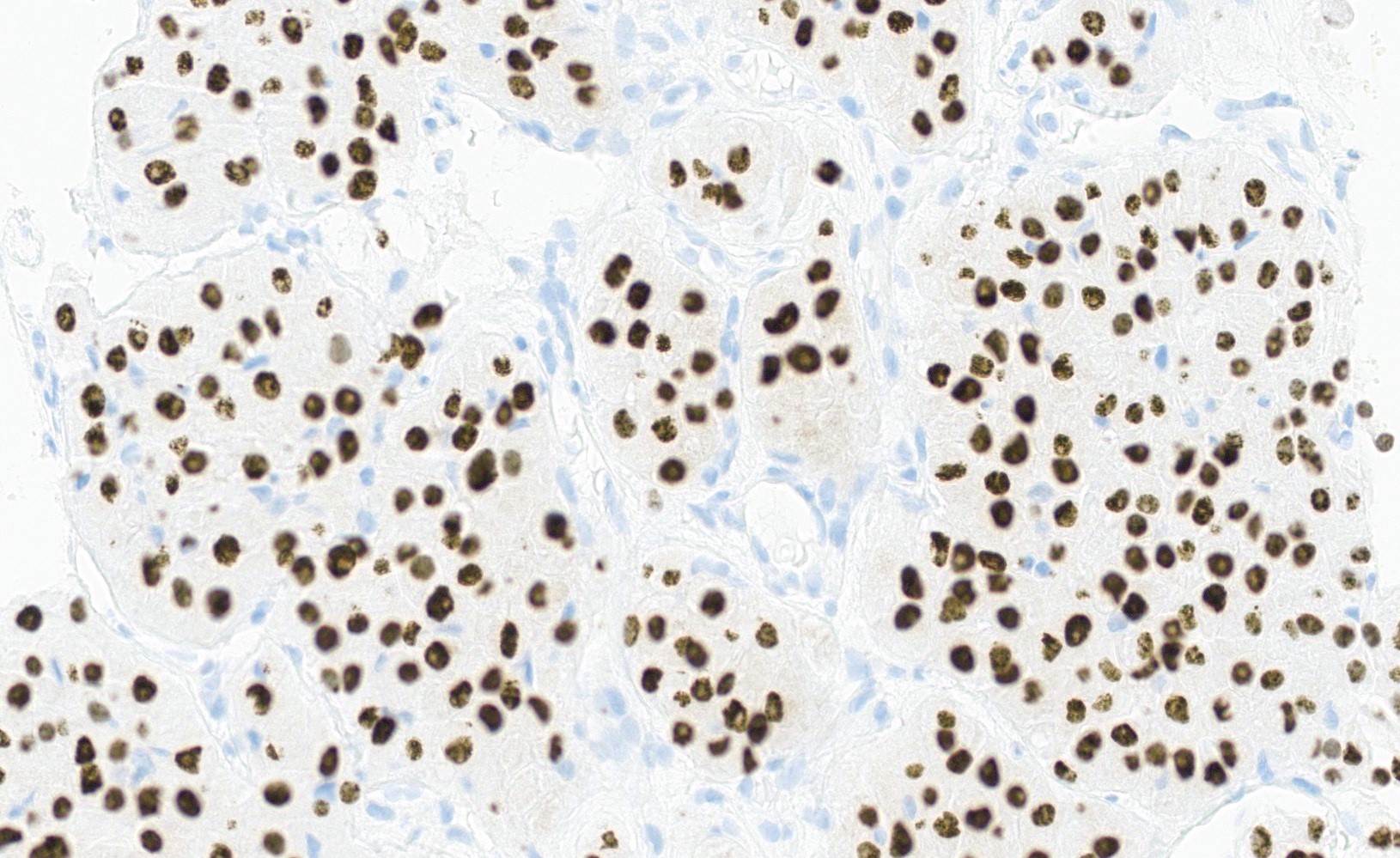
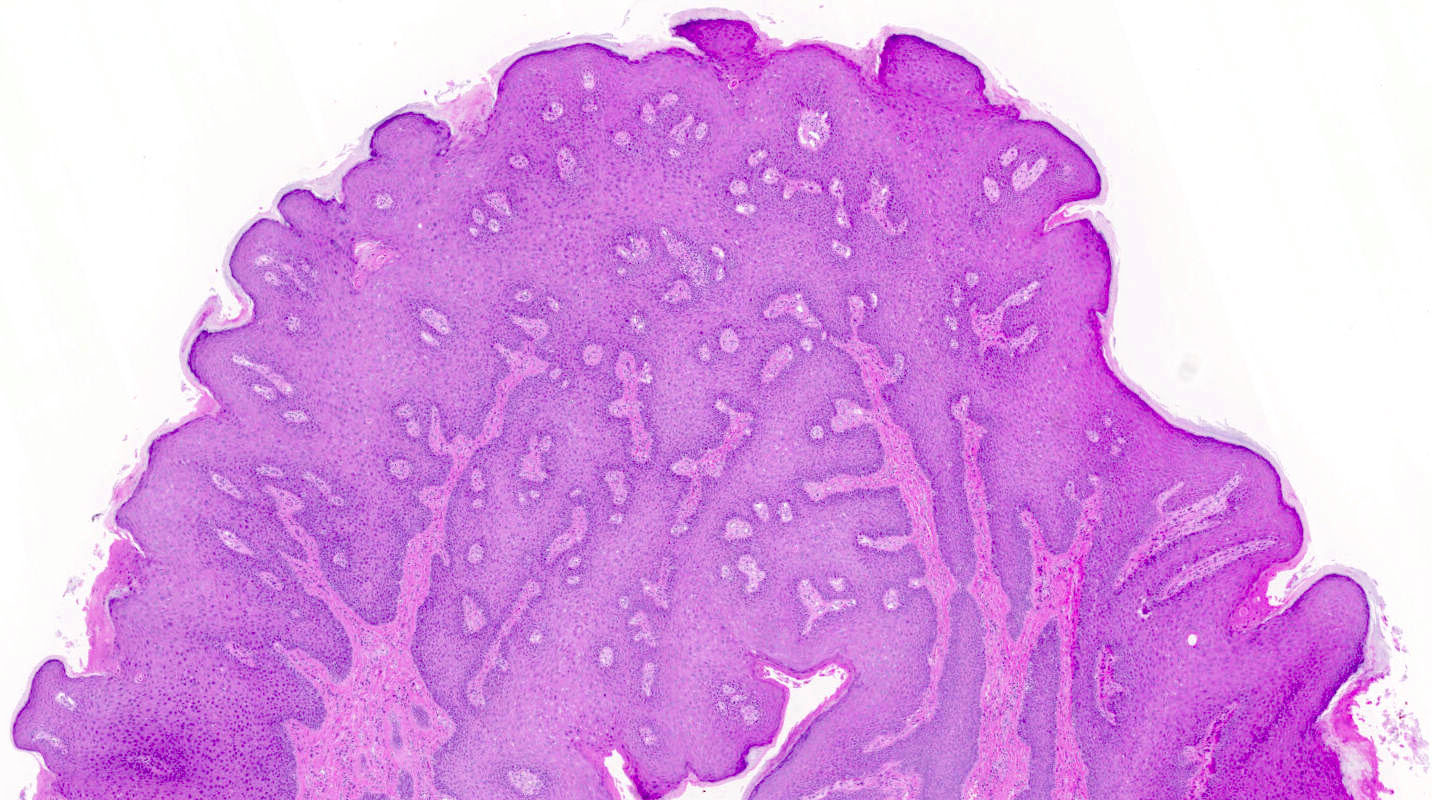
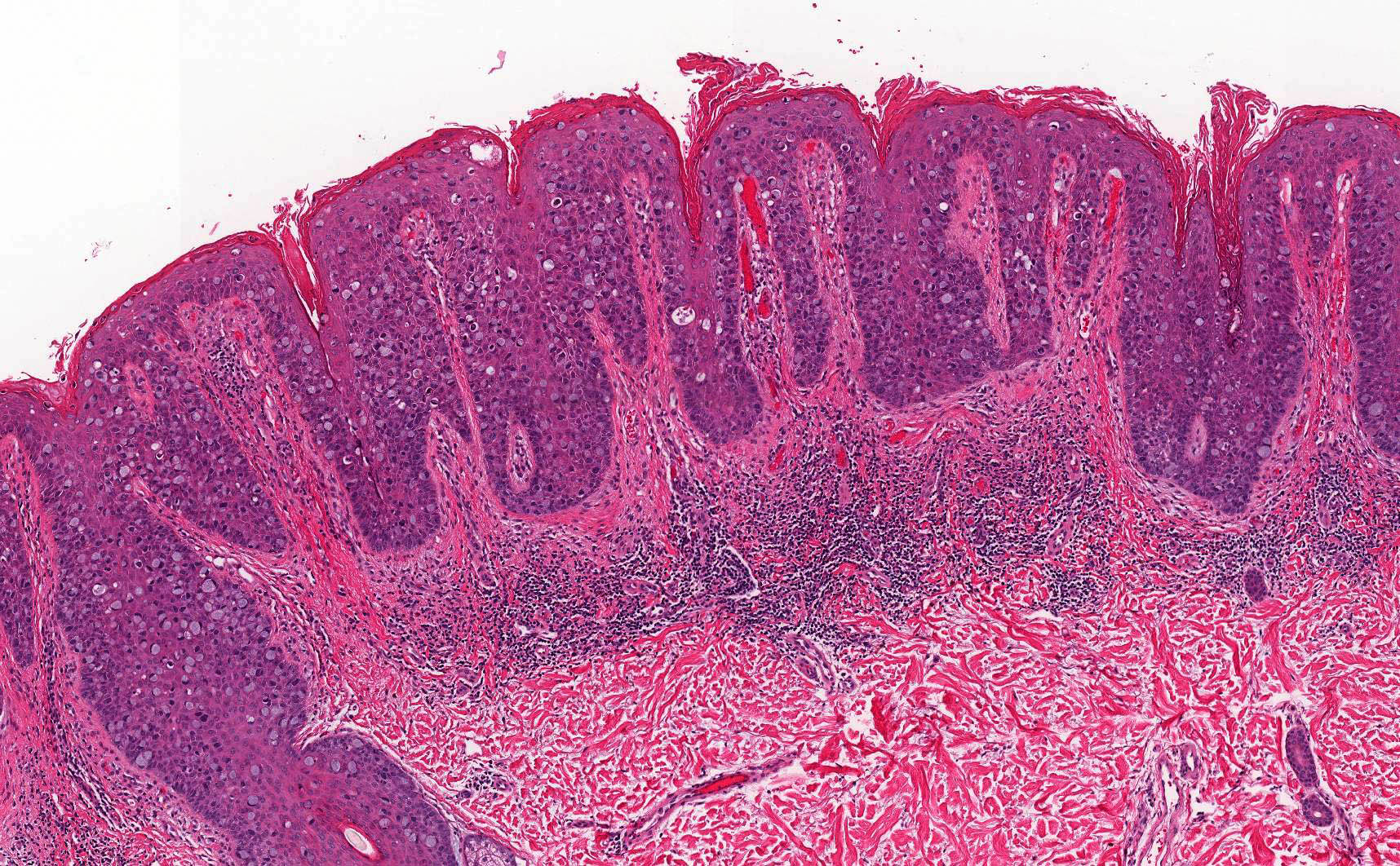
A 78 year old woman presents with a 2 cm pearly pink nodule in the right labium majus. Dermoscopy of the lesion reveals ovoid nests and arborizing fine blood vessels. A biopsy is performed and shows the image above. Which of the following is true?
- Genes usually mutated in this cancer include TP53 and NOTCH1
- Human papillomavirus (HPV) plays a major role in disease pathogenesis
- The tumor is also positive for CK20 perinuclear dot-like staining
- This neoplasm has a high rate of metastatic disease
Comment Here
Reference: Basal cell carcinoma-vulva
- BCL2
- CEA
- CK20
- EMA
Comment Here
Reference: Basal cell carcinoma-vulva
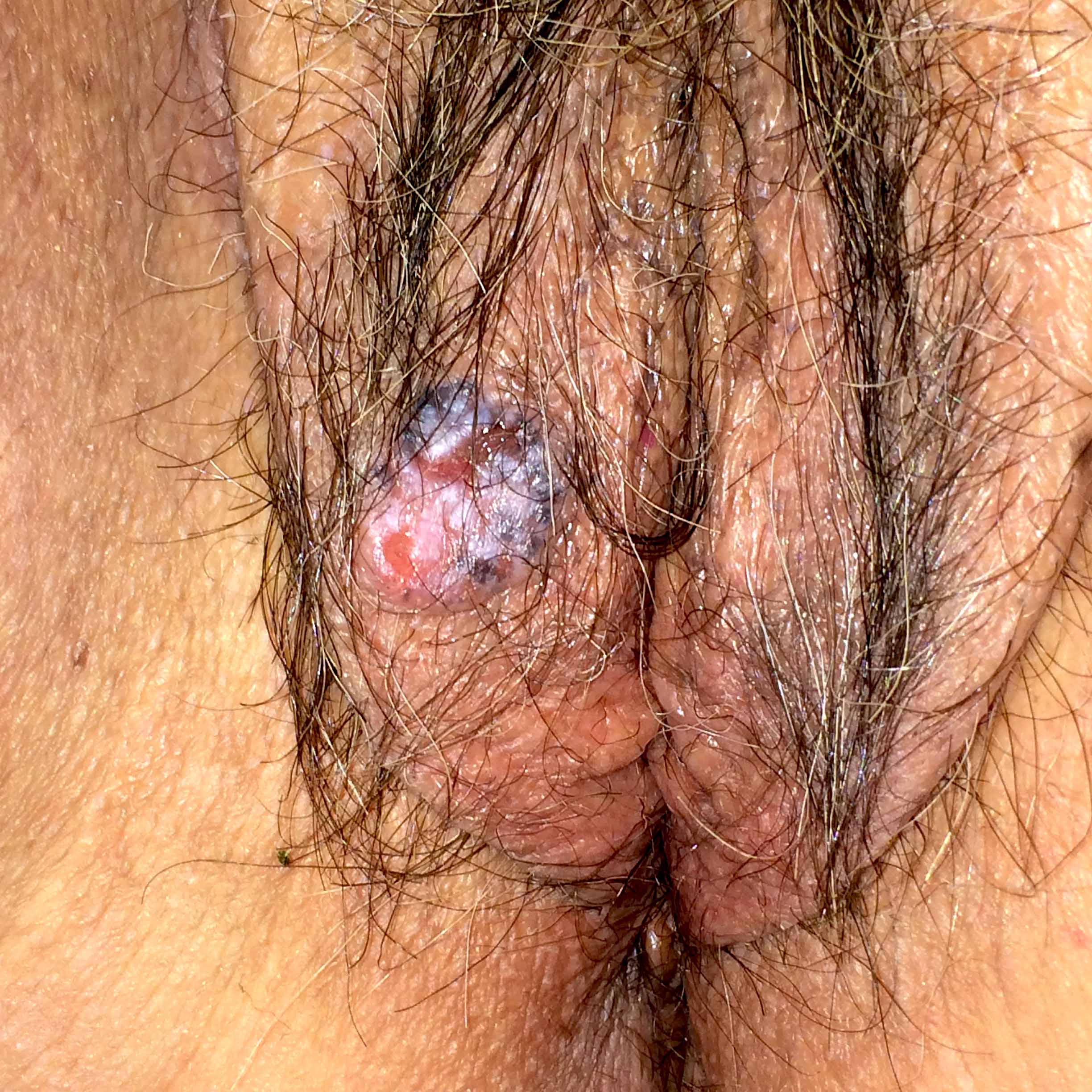
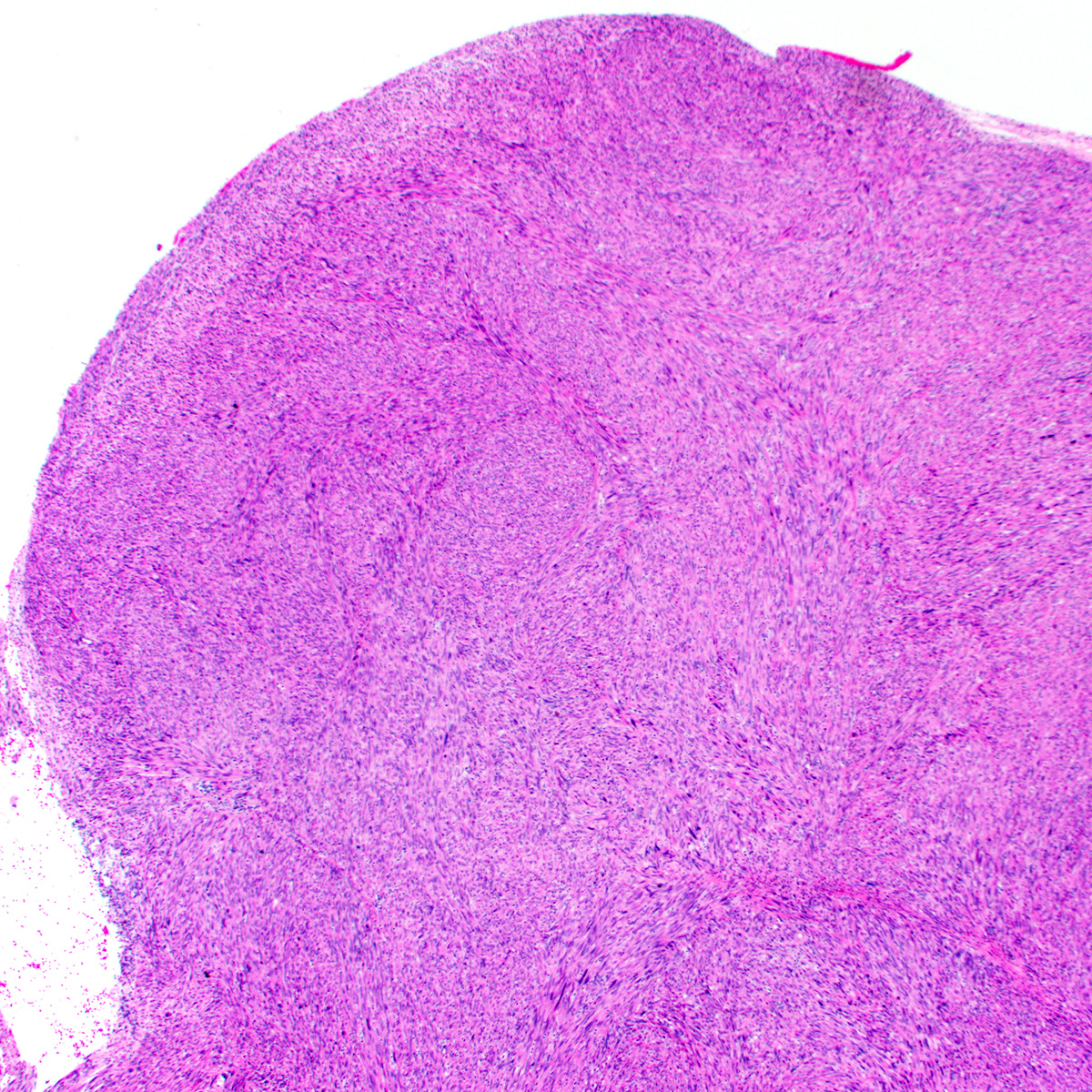
- Cellular angiofibroma is a benign, site specific soft tissue tumor of the lower genital tract
- Benign lower genital tract tumor, occurring equally in women and men
- Bland spindle cell fascicles, abundant medium sized hyalinized vessels and wispy stromal collagen
- Characterized by deletion of RB1 / FOXO1 locus on chr 13q
- Local excision curative; recurrences exceptionally rare
- F ~ M
- In women, age range 22 - 77 years (median ~ 47 years) (Am J Surg Pathol 2004;28:1426, J Cutan Pathol 2003;30:405)
- Men typically older (32 - 88 years; median ~ 65 years) (Mod Pathol 2011;24:82, Am J Surg Pathol 1998;22:6)
- In women, labium majus most common, followed by vagina and perineum (Am J Surg Pathol 2004;28:1426, Mod Pathol 2011;24:82, Am J Surg Pathol 1997;21:636)
- In men, scrotum and groin most common (Am J Surg Pathol 1998;22:6)
- Rare limb, chest wall and retroperitoneal cases (Diagn Pathol 2015;10:114)
- Histogenesis unknown
- Painless, slowly growing mass
- Present for weeks to years before diagnosis (Am J Surg Pathol 1997;21:636, Am J Surg Pathol 2004;28:1426)
- Clinically mimics Bartholin cyst, lipoma or leiomyoma (Am J Surg Pathol 2004;28:1426)
- Diagnosis typically follows complete local excision of a clinically benign appearing mass
- Local recurrence exceptionally rare, even with positive margins (Mod Pathol 2011;24:82, J Clin Pathol 2002;55:477)
- So called sarcomatous transformation does not increase risk of recurrence (Am J Surg Pathol 2010;34:707)
- No reports of distant metastasis
- 37 year old woman with a painless vulvar mass (BMC Clin Pathol 2016;16:8)
- 49 year old woman with a recurrent vulvar cellular angiofibroma (J Clin Pathol 2002;55:477)
- 77 year old man with a left inguinal mass (IJU Case Rep 2020;3:69)
- 79 year old man with a scrotal mass (Diagn Pathol 2017;12:17)
- Simple excision considered curative (Am J Surg Pathol 1997;21:636, Am J Surg Pathol 2004;28:1426)
- Re-excision for positive margins not mandatory
- Well circumscribed, nodular or multilobulated, rubbery mass
- Cut surface white-tan to grey
- Gross hemorrhage and necrosis in < 5% (Am J Surg Pathol 2004;28:1426)
- In women, size 0.6 - 12 cm (mean ~ 3 cm) (Am J Surg Pathol 2004;28:1426, Am J Surg Pathol 1997;21:636, Mod Pathol 2011;24:82)
- In men, size 0.6 - 25 cm (mean ~ 7 cm) (Am J Surg Pathol 2004;28:1426, Am J Surg Pathol 1998;22:6)
- Predominantly based in subcutis; rarely in dermis (Am J Surg Pathol 2004;28:1426)
- Usually well circumscribed; rare cases infiltrative (Am J Surg Pathol 1997;21:636, Am J Surg Pathol 2004;28:1426)
- Fibrous pseudocapsule in a subset (Am J Surg Pathol 1997;21:636, Am J Surg Pathol 2004;28:1426)
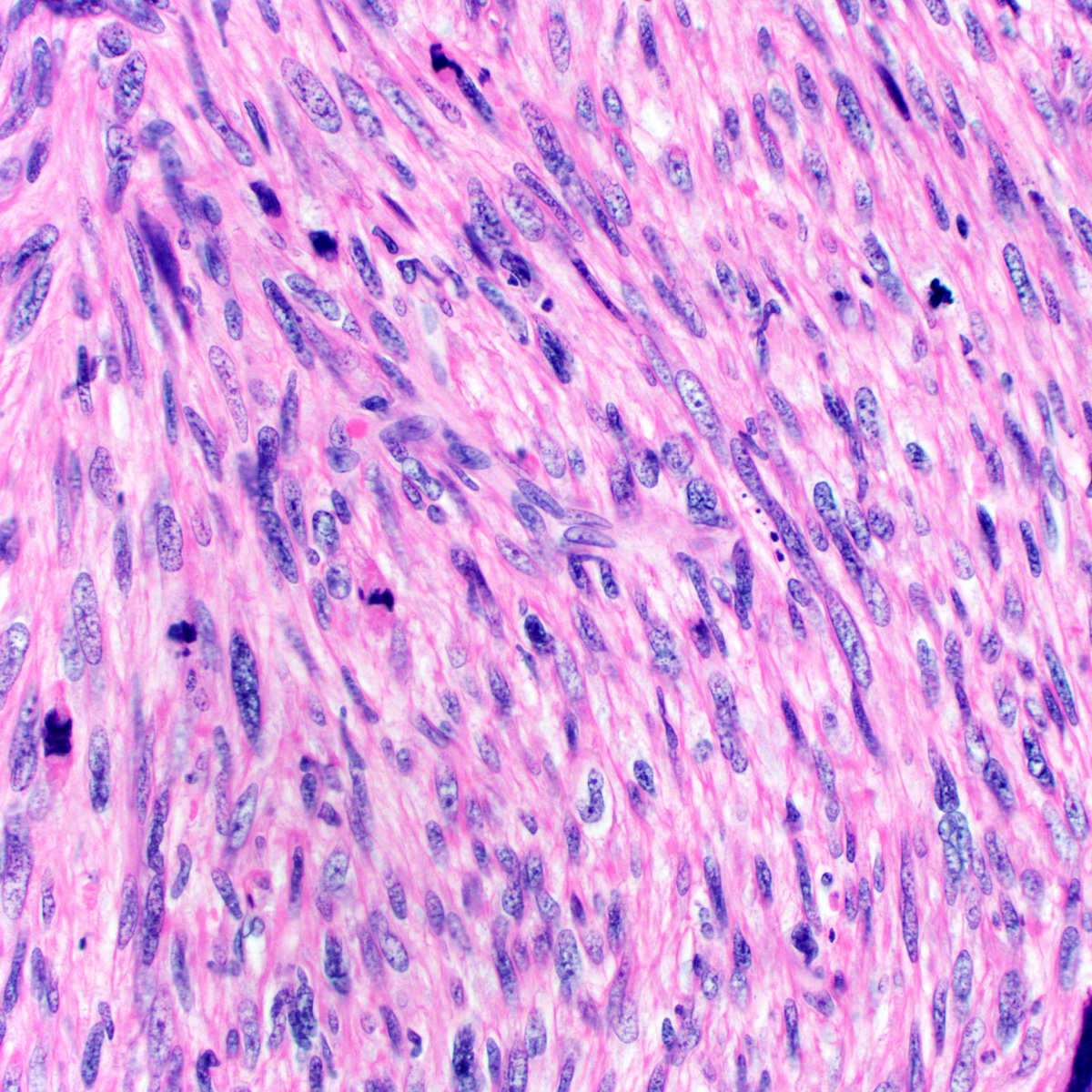
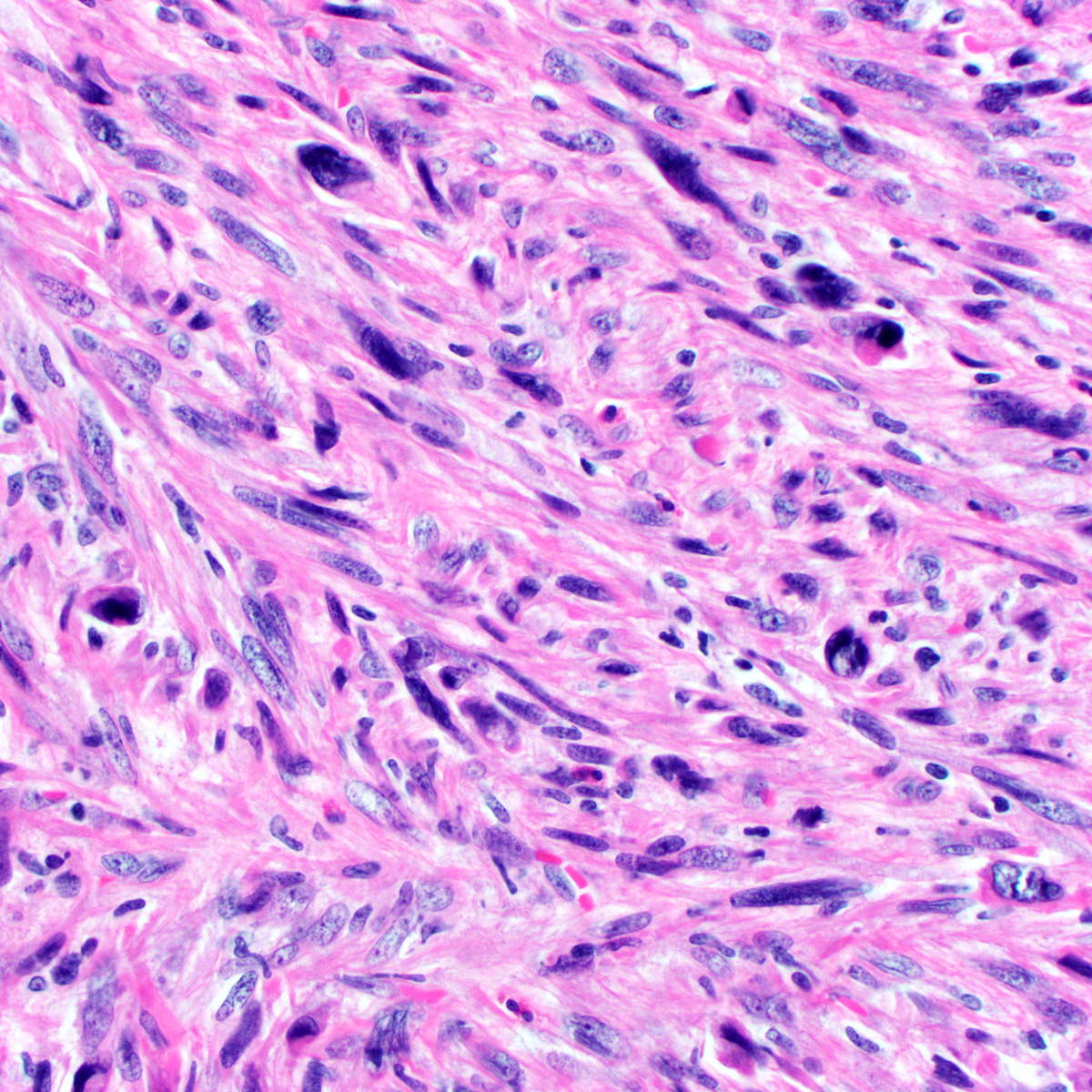
- Tumor cells:
- Small, monotonous spindle cells with bland, ovoid to fusiform nuclei
- Arranged in short intersecting fascicles
- Bland multinucleated cells common (Histopathology 2004;45:360)
- Mitoses typically rare (< 1 per 10 hpf) but occasionally brisk (> 10 per 10 hpf) (Am J Surg Pathol 1997;21:636, Am J Surg Pathol 2004;28:1426)
- Rare cases show focal or diffuse atypia or discrete areas of sarcomatous transformation (Am J Surg Pathol 2010;34:707)
- Sarcomatous transformation may resemble well differentiated liposarcoma, pleomorphic liposarcoma or undifferentiated pleomorphic sarcoma
- Tumor stroma and vasculature:
- Myxoid, edematous, fibrous or hyalinized stroma (Am J Surg Pathol 2004;28:1426)
- Abundant medium sized, thick walled, hyalinized vessels
- Short wispy collagen bundles
- Minor adipocytic component in ~ 50%; rare cases show prominent adipocytic differentiation (Am J Surg Pathol 2004;28:1426)
- Mast cells may be conspicuous
- Necrosis and hemorrhage absent
- Vimentin (Am J Surg Pathol 1997;21:636, Am J Surg Pathol 2004;28:1426, J Cutan Pathol 2003;30:405, Histopathology 2004;45:360, Mod Pathol 2011;24:82)
- ER (most), PR (most)
- CD34 (~50%)
- Tumors with sarcomatous transformation typically show p16 overexpression and rarely mutant p53 (Am J Surg Pathol 2010;34:707)
- Rb (i.e., expression lost) (Am J Surg Pathol 2012;36:1119)
- Myogenic markers: SMA (rarely positive), desmin, caldesmon
- EMA (rarely positive)
- S100 (Am J Surg Pathol 1997;21:636, J Cutan Pathol 2003;30:405, Am J Surg Pathol 2004;28:1426, Histopathology 2004;45:360)
- Cytokeratin (AE1 / AE3, CAM5.2)
- CD117
- MDM2 and CDK4 in those with sarcomatous transformation (Diagnostics (Basel) 2020;10:35)
- Monoallelic RB1 / FOXO1 deletion (chr 13q12-22) in all tested cases (Mod Pathol 2011;24:82, Histopathology 2007;51:410, Diagn Pathol 2017;12:17)
- TP53 mutations present in a subset of cases with sarcomatous transformation (Diagnostics (Basel) 2020;10:35)
- Vulva, mass, excision:
- Cellular angiofibroma (3.2 cm) (see comment)
- Comment: Margins are negative for tumor.
- Angiomyofibroblastoma:
- Alternating hypo and hypercellular foci
- Spindled to epithelioid to plasmacytoid cells, clustering around vessels
- Vascular hyalinization not prominent
- Spindle cell lipoma:
- Typically affects head and neck region in men
- Thick ropy collagen bands
- Hyalinized vessels not prominent
- Mammary type myofibroblastoma:
- Solitary fibrous tumor:
- Characteristic staghorn / hemangiopericytoma-like vessels
- More marked variation in cellularity
- Thick bands of hyalinized collagen
- STAT6 positive
- Leiomyoma:
- Perineurioma:
- Lacks vascular hyalinization
- EMA positive
A 48 year old woman presented with a painless 3.5 cm vulvar mass, which had been slowly growing for 18 months. She underwent complete local excision of a rubbery, well circumscribed mass. A representative photomicrograph is shown. Which of the following is true about this lesion?
- Complete local excision is considered curative
- Immunohistochemistry for estrogen receptor and progesterone receptor is typically negative
- Nuclear pleomorphism is associated with an increased risk of distant metastasis
- Rb protein is typically overexpressed
- This lesion occurs exclusively in women
Comment Here
Reference: Cellular angiofibroma
- Most common subtype of vaginal adenocarcinoma associated with DES exposure in young females; can also occur in postmenopausal women without exposure to DES
- Mesonephroid carcinoma, mesonephric carcinoma (Cancer 1970;25:745)
- Rare vaginal cancer, accounting for 5% to 10% of primary vaginal malignancies (J Minim Invasive Gynecol 2006;13:237)
- Bimodal age distribution, mean age 22 years (Gynecol Oncol 1996;60:339) in women with exposure to DES and 55 years in postmenopausal women with no history of DES, but who may have pelvic endometriosis (J Minim Invasive Gynecol 2006;13:237, Gynecol Oncol 2006;103:1130)
- Most common - anterior vaginal wall (Gynecol Oncol 1993;51:266, Gynecol Oncol 2007;105:273); can also occur in elsewhere in vagina
- DES causes persistence of Müllerian epithelium while inducing contact between epithelium and the vaginal mesenchyme
- Unopposed estrogen and obesity causes increase in the peripheral conversion of steroid hormones to estrone by the enzyme aromatase leading to a hyperestrogenic environment (Gynecol Oncol 2006;103:1130)
- Most cases occur among women born 1947 through 1971, when pregnant women were most frequently prescribed DES in the United States (Cancer Causes Control 2012;23:207, Gynecol Oncol 1996;60:339)
- 70% occur in women having intrauterine exposure to DES - also known as DES daughters (Gynecol Oncol 1996;60:339, Gynecol Oncol 2007;105:273, Gynecol Oncol 1993;51:266, Cancer Causes Control 2010;21:999)
- Endometriosis of vagina and perivaginal area may be a precursor in non-DES exposed females; the frequency of vaginal tumors arising in endometriosis ranges from 4% - 11% (Gynecol Oncol 2006;103:1130, J Minim Invasive Gynecol 2006;13:237)
- Vaginal adenosis (especially tuboendometrial adenosis) is associated with CCA (clear cell adenocarcinoma) in 90% of cases, suggesting adenosis is a precursor lesion (J Obstet Gynaecol Res 2010;36:681, Adv Anat Pathol 2012;19:296, Gynecol Oncol 1993;51:266)
- Abnormal vaginal bleeding or discharge, although 16 - 25% are asymptomatic (Gynecol Oncol 2007;105:273)
- Postmenopausal bleeding (Gynecol Oncol 2006;103:1130, J Minim Invasive Gynecol 2006;13:237)
- Congenital anomalies of GU tract without DES exposure: associated with metanephric and mesonephric remnants and Mü¸llerian duct anomalies (J Obstet Gynaecol Res 2010;36:681, J Pak Med Assoc 2009;59:568)
- Lymphatic and vascular spread can occur (J Minim Invasive Gynecol 2006;13:237)
- Can metastasize to regional lymph nodes (J Minim Invasive Gynecol 2006;13:237), lungs (Gynecol Oncol 1993;51:266, Gynecol Oncol 2007;105:273, J Minim Invasive Gynecol 2006;13:237), kidney (Gynecol Oncol 1993;51:266), peritoneum, omentum, ovary, liver and brain (Gynecol Oncol 2007;105:273)
- Can present with malignant pericardial effusion and cardiac tamponade (Int J Gynecol Cancer 2006;16:1458)
- May recur in distant sites even in absence of pelvic disease (Gynecol Oncol 1993;51:266)
- Women exposed to DES in utero (DES daughters) also have increased risk of clear cell adenocarcinoma persisting at older ages and an increased risk of melanoma at young ages, no increased risk of other cancers (Cancer Causes Control 2010;21:999)
- Biologic behavior and prognosis differ from squamous cell carcinoma:
- Better 5 year survival of localized vaginal CCA but greater risk of developing late recurrences after disease free interval; recurrences can occur 2 years to 8 years later (Gynecol Oncol 1993;51:266, Gynecol Oncol 2007;105:273)
- Non-DES exposed patients may have poorer prognosis compared with DES exposed individuals (J Minim Invasive Gynecol 2006;13:237)
- 5 year old girl with clear cell adenocarcinoma of vagina (Clin Radiol 1998;53:69)
- 14 year old girl with vaginal bleeding had a mass involving the vagina and cervix (Case of the Week #363)
- 22 year old woman with fertility sparing radical abdominal trachelectomy for clear cell adenocarcinoma of the upper vagina (Gynecol Oncol 2007;105:820)
- 43 year old woman with diethylstilbestrol (DES) induced clear cell adenocarcinoma of the vagina metastasizing to the brain (Gynecol Oncol 2007;105:273)
- 55 year old woman with clear cell adenocarcinoma of the vagina and vaginal endometriosis (Gynecol Oncol 2006;103:1130)
- Late recurrence of clear cell adenocarcinoma of the cervix (Obstet Gynecol 1990;76:525)
- Clear cell adenocarcinoma of the vagina: MR features (Br J Radiol 1993;66:168)
- Clear cell adenocarcinoma of the cervix and vagina in a woman with mixed gonadal dysgenesis (J Reprod Med 1989;34:981)
- Primary clear cell adenocarcinoma of the vagina (Hiroshima J Med Sci 1976;25:141)
- Clear cell (mesonephric) adenocarcinoma of the vagina (Acta Cytol 1973;17:493)
- Surgery is primary therapy for low stage vaginal CCA tumors
- Small tumors: local excision, evaluation of retroperitoneal lymph nodes followed by local irradiation to the bed of tumor (J Minim Invasive Gynecol 2006;13:237)
- Large tumors: neoadjuvant chemoradiation therapy (J Minim Invasive Gynecol 2006;13:237)
- Stage I or early stage II CCA:
- Surgical treatment includes radical hysterectomy with partial or complete vaginectomy, pelvic lymphadenectomy, vaginal reconstruction if needed
- Ovarian function is usually preserved (J Minim Invasive Gynecol 2006;13:237)
- Following surgery, combination chemotherapy paclitaxel and carboplatin may be given (J Minim Invasive Gynecol 2006;13:237)
- To preserve fertility, selective trachelocolpectomy may be chosen (J Obstet Gynaecol 2010;30:420)
- Constant close longterm followup required (Gynecol Oncol 1993;51:266, Gynecol Oncol 2007;105:273) including continued surveillance in postmenopausal women with cytological smears (both cervical and vaginal) and biopsies (Gynecol Oncol 1999;75:338)
- Superficially located polypoid, exophytic mass that typically originates from the anterior wall of the upper two thirds of the vagina (Gynecol Oncol 2007;105:273)
- Tumor has cystic, papillary, tubular / glandular and solid architectural patterns with focal necrosis
- Cells have distinct cell membranes, are large with moderate to abundant clear cytoplasm, occasionally may be oxyphilic
- Cells are usually cuboidal and sometimes hobnail type with nuclei protruding into the lumen
- Nuclei are round to irregular, hyperchromatic with conspicuous nucleoli (Int J Gynecol Pathol 2001;20:252)
- Provides diagnostic information in 41% of cases (Gynecol Oncol 2007;105:273)
- Cells are arranged in sheets, clusters or papillae; cells have delicate vacuolated glycogen rich cytoplasm
- May have naked nuclei and a tigroid background, similar to other glycogen containing tumor cells such as seminoma and Ewing sarcoma
- Nuclei are large, pale and round with prominent nucleoli (Cytojournal 2013;10:17)
- CK7, CAM 5.2, 34 beta E12, CEA, LeuM1, vimentin, bcl2, p53 and CA125
- Variable positivity for ER and HER2 / neu (Int J Gynecol Pathol 2001;20:252)
- Similar ultrastructural features as CCA of ovary, cervix or endometrium
- Glands have short, thick microvilli in the lumina; cells are attached by desmosomes and interdigitating cytoplasmic processes
- Cells contain abundant glycogen granules, also many small, uniform mitochondria and "stacked" parallel rows of granular endoplasmic reticulum (Cancer 1972;29:1680, Cancer 1977;40:3019)
- p53 nuclear staining patterns is heterogeneous in both proportion and intensity of tumor cells stained (Gynecol Oncol 1996;60:339)
- Overexpression of p53 protein is not due to mutational inactivation of the p53 gene but probably due to persistent DNA damage or genetic instability
- Intrauterine exposure to DES, therefore, is unlikely to directly alter the p53 gene as has been suggested for other mutagens
- Persistence of wild type p53 in these rare tumors may correlate with their typically favorable prognosis and radiosensitivity (Gynecol Oncol 1996;60:339)
Histopathology vagina - clear cell carcinoma
- Arias-Stella reaction: cells are either not mitotically active or have only rare mitotic figures and the nuclei show degenerative features with pseudoinclusions and no mass lesion present (Adv Anat Pathol 2012;19:296)
- Mesonephric remnants: may also mimic clear cell carcinoma at low power (Adv Anat Pathol 2012;19:296)
- Metastatic tumor: rare; rule out kidney primary (Indian J Urol 2004;20:169)
- History, imaging, immunohistochemical stains and EM may be useful
- Microglandular hyperplasia: occasionally occurs in vagina (Adv Anat Pathol 2012;19:296)
- Serous carcinoma, yolk sac tumor and alveolar soft part sarcoma (Adv Anat Pathol 2012;19:296)
- Condyloma acuminatum is an HPV associated wart-like lesion of squamous epithelium
- Benign HPV related lesion of the anogenital region with warty appearance and histologically bland papillomatous hyperplasia of squamous epithelium
- Giant condyloma (Buschke-Löwenstein tumor) develops over confluence of longstanding condyloma acuminata with the propensity to transform into invasive carcinoma
- Acceptable: condylomatous low grade squamous intraepithelial lesion (LSIL), genital wart
- ICD-11: 1A95.1 - genital warts
- Sexually active adults (1%), peak incidence between 20 - 29, HIV predisposing
- Buschke-Löwenstein tumor is observed mostly in the fifth decade after median condyloma diagnosis for 5 years
- Labia majora, vestibule, perineum, vagina, perianal, rare extragenital
- Low risk HPV types 6 and 11 most common (Sex Transm Dis 2013;40:123, Acta Derm Venereol 2013;93:223)
- High risk HPV types in up to 42% (Am J Surg Pathol 2006;30:1513)
- Range from only colposcopically visible lesions to small papules to large masses with cauliflower-like appearance, often multiple (Best Pract Res Clin Obstet Gynaecol 2014;28:1063)
- Buschke-Löwenstein tumor shows clinically expansive and destructive growth; there is no sharp criterion for size to differentiate it from large condyloma
- Clinical appearance, punch biopsy
- Benign process, frequent recurrence, spontaneous regression possible (Sex Transm Dis 2011;38:949)
- Buschke-Löwenstein tumor shows focal development of invasive carcinoma in up to 50%, rarely metastasizes; mortality 20%; relapse in up to 50% after therapy (Dis Colon Rectum 1994;37:950)
- 25 year old pregnant woman with large condyloma acuminatum in the vagina (Int J STD AIDS 2011;22:534)
- 29 year old woman with giant condyloma during pregnancy (Acta Obstet Gynecol Scand 2007;86:762)
- 32 year old pregnant woman with disseminated condyloma acuminata (Indian J Pathol Microbiol 2019;62:310)
- Local excision or laser ablation, imiquimod
- Overall verrucous or warty architecture (Adv Anat Pathol 2005;12:20)
- Acanthosis, papillomatosis, hyperkeratosis and hypergranulosis of squamous epithelium
- Broad papillae with rounded ends which are fused at the base
- Viral cytopathic effects: variable koilocytosis (may be absent or inconspicuous), binuclear cells
- Usually no stromal inflammation
- Giant condyloma Buschke-Löwenstein shows the same histological changes with more endophytic growth but without invasion
- HPV detection by RNA in situ hybridization or immunohistochemical demonstration of L1 capsid protein
- p16 stains in patchy / nonblock type pattern or is negative
- Labium minora, biopsy:
- Condyloma acuminatum
- Seborrheic keratosis:
- Horn pearls, squamous eddies, minimal hypergranulosis
- HPV negative
- Vestibular papillomatosis:
- Branching delicate papillae without keratinization, no cytopathic viral effects
- HPV ISH negative
- Squamous papilloma:
- Minimal branching, fibrovascular core, no cytopathic viral effects
- HPV ISH negative
- Condyloma with HSIL:
- Verrucous carcinoma:
- Unequivocal pushing border invasion, stromal inflammation, no koilocytosis / no HPV association (as seen in giant condyloma Buschke Löwnstein)
HPV6 is a low risk HPV and is most often found in condyloma as shown in several studies. The frequency of HPV16 is lower than that of HPV6. HIV is only predisposing, not found in the epithelium. There is no association with HHV8 and HSV. (Am J Surg Pathol 2006;30:1513)
Comment Here
Reference: Condyloma
- HPV IHC
- HPV ISH
- Ki67
- p16
- p53 IHC
HPV ISH has high sensitivity and specificity while HPV IHC has low sensitivity. Both entities are p53 wildtype, p16 patchy / negative and have elevated Ki67 indices.
Comment Here
Reference: Condyloma
- Acquired nevus in the genital area with architectural and cytologic atypia
- Clinical and epidemiologic features are similar to atypical genital nevi
- Most commonly occurs in women of reproductive age
- Morphologically characterized by a significant lentiginous component, well developed shoulder, randomly distributed melanocytic atypia and marked inflammatory infiltrate
- Nevus with architectural and cytologic atypia (acceptable by the 5th edition of World Health Organization [WHO] Classification of Female Genital Tumors)
- Dysplastic melanocytic nevi account for about 5% of genital melanocytic lesions (J Cutan Pathol 2008;35:24)
- Most often diagnosed in reproductive age women (mean: 32 years; median: 28 years) (Obstet Gynecol Clin North Am 2017;44:339, J Am Acad Dermatol 2014;71:1241)
- Usually affects the cutaneous part of labia majora > > perineum (Hum Pathol 1998;29:S1, J Cutan Pathol 2008;35:24, Arch Pathol Lab Med 2011;135:317)
- Mucosa is typically uninvolved (J Cutan Pathol 2008;35:24)
- Dysplastic melanocytic nevi are usually associated with activating mutations in genes such as BRAF (typically BRAF V600E) and NRAS (N Engl J Med 2015;373:1926)
- Largely unknown
- May be associated with dysplastic nevus syndrome (Am J Surg Pathol 2008;32:51)
- During obstetrics - gynecologic visit
- Women rarely examine their vulva closely; consequently, duration and change of vulvar melanocytic lesions are often unclear (Dermatol Clin 2010;28:795)
- Decision to biopsy is based on atypical appearance of pigmented lesion (Dermatol Clin 2010;28:795)
- Genital pigmented lesions usually appear more atypical than nongenital lesions (Dermatol Clin 2010;28:795)
- Punch or excisional biopsy is preferred over shave biopsy (Dermatol Clin 2010;28:795)
- Dysplastic melanocytic nevus
- Commonly presents as macules or papules with irregular borders, pink in the center and tan to brown at the periphery (Dermatol Ther 2010;23:449)
- Usually measures > 0.6 cm in size contrasting with common acquired nevi, which are typically Difficult to distinguish clinically from atypical melanocytic nevus of genital type (Am J Surg Pathol 2008;32:51, Hum Pathol 1998;29:S1)
Table 1: Clinical - pathologic features of vulvar melanocytic lesions (adapted from Diagn Histopathol 2024;30:P15)
| Vulvar melanocytic nevus | Vulvar melanocytic nevus in association with lichen sclerosus | Atypical genital nevus of special anatomic site | Dysplastic melanocytic nevus | Vulvar melanoma | |
| Age | Premenopausal | Premenopausal | Premenopausal, young adult | Premenopausal | Postmenopausal |
| Size | 0.5 - 1 cm* | > 1 cm | |||
| Delineation | Well circumscribed | Well circumscribed | Well circumscribed | Well circumscribed | Infiltrative |
| Symmetry | Present | Present | Present | Present | Absent |
| Lateral extension of junctional component | Absent | Absent | Focal | Present | Present |
| Pigmented junctional nests | Absent | Present | Absent | Absent | Absent |
| Junctional nests | Discrete | Coalescent | Mostly discrete | Discrete | Coalescent |
| Retraction artefact | Absent | Absent | Present | Usually absent | Absent |
| Ulceration | Absent | Absent | Absent or traumatic | Absent or traumatic | Often present |
| Pagetoid spread | Absent | Focal Central | Focal Central | Focal Central | Prominent |
| Cytologic atypia | None to mild | Moderate to severe | Mild to moderate Superficial | Mild to severe** | Severe Uniform Deep |
| Dermal mitosis | Rare in pregnancy | Rare | Rare Superficial | Rare | Conspicuous Atypical Deep |
| Dermal maturation | Present | Present | Present | Present | Absent |
| Dermal fibrosis | Absent | Dermal sclerosis | Broad zone of superficial coarse dermal fibrosis | Concentric or fibrolamellar fibrosis | Regression type |
**See Table 3 for dysplasia grading per WHO 5th edition
- Dermoscopy for nonmodified mucous membranes is useful (Dermatol Ther 2010;23:449)
- May show any of the dermoscopic patterns seen in benign nevi
- Globular / cobblestone, homogenous, structureless or mixed patterns are most common (Dermatology 2011;222:157, Dermatology 2010;221:55)
- Histologic diagnosis is confirmatory
- For optimal diagnosis, lichen sclerosus should be controlled before excision of the nevus (J Am Acad Dermatol 2004;50:690, Arch Dermatol 2002;138:77)
Table 2: Differential diagnosis of vulvar melanocytic nevi, melanosis and melanoma (adapted from J Am Acad Dermatol 2014;71:1241)
| Reassuring features suggestive of a benign process | Concerning features for possible malignancy | |
| Clinical |
|
|
| Dermoscopy |
|
|
| Reflectance confocal microscopy |
|
|
- Thought to have a benign clinical course (Obstet Gynecol Clin North Am 2017;44:339)
- Grade 2 cytologic atypia (former severe dysplasia) may be a precursor to melanoma (Obstet Gynecol Clin North Am 2017;44:339)
- No recurrences noted in cases with negative margins (Hum Pathol 1998;29:S1, Am J Surg Pathol 2008;32:51)
- 21, 27 and 30 year old women with dysplastic melanocytic nevi of the vulva (Obstet Gynecol 1991;78:968)
- Simple excision is usually sufficient
- Dysplastic melanocytic nevus is characterized by architectural and cytologic atypia
- Architectural atypia
- Bridging / fusion of junctional nests between adjacent rete ridges
- Variation in the size, shape or position of junctional nests
- Scattered mild to moderate cytologic atypia in the melanocytes
- Pagetoid spread of melanocytes may be seen, which is typically central and confined to lower epidermal layer
- Bland melanocytes may extend into the papillary dermis
- Concentric, lamellar eosinophilic fibroplasia in the papillary dermis near the dermoepidermal junction
- Presence of shouldering (junctional shoulders): a lentiginous junctional component (basilar proliferation of atypical melanocytes) at the periphery of the lesion, extending beyond the dermal component (> 3 rete ridges)
- Inflammatory infiltrate is usually confined to the superficial dermis
- Usually papillomatous appearance of the epidermis
- Cytologic atypia
- See Table 3 for grading
- Nuclear features are evaluated based on nuclear size, shape and presence of prominent nucleoli
- To estimate the nuclear size, the adjacent keratinocytes are typically referenced
- Chromatin evaluation includes hyperchromasia, coarse versus clumping of chromatin and its condensation within the nucleus
- Typically, absent or low mitotic activity (2)
- Architectural atypia
- Stereotypical features of junctional nevi are usually seen, including a well circumscribed, symmetrical lesion with intradermal component demonstrating maturation (J Cutan Pathol 2008:35:24)
- Nevi in the background of lichen sclerosus may appear more atypical (Arch Dermatol 2002;138:77)
- See Table 1 for clinicopathologic correlations
Table 3: Grading of cytologic atypia (adapted from WHO Classification of Skin Tumors, 5th edition)
| Grade | Previously | WHO grade, 4th edition | Nucleus | Presence of nucleoli | Chromatin | |
| Size | Shape variation | |||||
| 0 | Mild | Not dysplastic nevus | 1x | Minimal | Absent or small | Can be hyperchromatic |
| 1 | Moderate | Low grade dysplasia | 1 - 1.5x | Random atypia* | Hyperchromatic or dispersed | Hyperchromatic or dispersed |
| 2 | Severe | High grade dysplasia | 1.5x or more | More than random atypia but still in minority of cells** | Prominent, often lavender | Hyperchromatic Coarse granular Peripheral condensation |
*Random atypia is referred to prominent shape variation in the minority of cells
**Shape variation would be more than random atypia or in a larger minority of cells, hence, not diagnostic for melanoma
- Melanocytic markers
- MelanA (MART1) may help to highlight fused nests (J Cutan Pathol 2020;47:446)
- HMB45 (Ann Diagn Pathol 2023;67:152211, Biomed Rep 2016;5:327, J Am Acad Dermatol 2012;67:446)
- In common junctional and compound nevi, expressed in melanocytes at dermoepidermal junction
- Highlights a gradient or maturation (zonation) pattern (diminishing or loss of expression from top to bottom)
- Highly variable in Spitz and congenital nevi
- SOX10 highlights junctional melanocytes to help differentiate melanoma in situ from benign counterparts (Appl Immunohistochem Mol Morphol 2014;22:142)
- Ki67 proliferation rate should be low in the dermal component of genital nevi (J Am Acad Dermatol 2012;67:446)
- HMB45 is often negative in intradermal and compound nevi
- PRAME (preferentially expressed antigen in melanoma) differentiates dysplastic melanocytic nevi with grade 2 cytologic atypia (see Table 3) from melanoma with 85% specificity and 80% sensitivity (Virchows Arch 2023 Dec 19 [Epub ahead of print])
- Dysplastic melanocytic nevi typically have a higher mutational burden than benign lesions with broader spectrum of initiating oncogenes, including BRAF V600K or BRAF K601E and NRAS (N Engl J Med 2015;373:1926)
Dysplastic nevus: 5 minute pathology pearls by Dr. Jerad Gardner
Dysplastic nevi by Prof. Naseem Ahmed
Melanoma arising in a dysplastic nevus by Drs. Philip H. Mckee and Antonina Kalmykova
Histopathology and dermoscopy of severely dysplastic nevus / in situ melanoma by Dr. Sasi Kiran Attili
Dysplastic nevus: fact or fiction by Prof. Cliff Rosendahl
Junctional dysplastic lentiginous nevus by Dr. Ian McColl
Dysplastic nevus by Dr. Sonam Kumar Pruthi
Dysplastic nevus by Audiopedia
- Vulva, labium majus, biopsy:
- Dysplastic melanocytic nevus with moderate to focally severe cytologic atypia and moderate architectural atypia; the lesion extends to 1 peripheral section edge (see comment)
- Comment: Multiple levels have been examined. Given the presence of the focally severe atypia present in this melanocytic proliferation and extension to a peripheral section edge, re-excision to ensure complete removal and minimize the risk of recurrence is recommended.
- Atypical melanocytic nevus of genital type:
- Symmetrical lesion with dermal component maturation
- A few atypical melanocytes in the epidermis as single cells with rare pagetoid spread, predominantly in the center of the lesion
- S100, SOX10 and nerve growth factor receptor (NGFR) fail to demonstrate invasive growth
- HMB45 highlights a gradient pattern (diminishing or loss of expression from top to bottom)
- PRAME is lost or weakly expressed (J Cutan Pathol 2020;47:1123)
- BRAF is the most common alteration (N Engl J Med 2015;373:1926, J Invest Dermatol 2016;136:1858)
- Melanoma:
- Atypical melanocytes in the epidermis as single cells or forming nests with pagetoid spread
- Dermal component shows no maturation with cells forming sheets, nests, cords, single cells and rarely fascicles
- S100, SOX10 and NGFR are the most sensitive markers in visualization of invasive growth
- PRAME shows diffuse staining
- Usually lacks BRAF mutation (Br J Dermatol 2010;162:677)
- Pigmented epithelioid melanocytoma of the vulva:
- Consists of multiple, usually slow growing, distinct melanocytic entities with the potential to metastasize
- Infiltrative deep dermal tumor that may involve subcutis
- Hypercellular tumor with cells ranging from medium sized epithelioid cells to large epithelioid and spindled cells with low mitotic activity
- PRKAR1A loss in ~66% of cases (Am J Surg Pathol 2017;41:1333, Am J Surg Pathol 2019;43:480)
- Am J Surg Pathol 1995;19:792, J Am Acad Dermatol 2023;88:1, J Am Acad Dermatol 2023;88:13, J Cutan Pathol 2008;35:889, Ackerman: Pathology of Malignant Melanoma, 1981, McKee: Pathology of the Skin - With Clinical Correlations, 3rd Edition, 2005, Busam: Pathology of Melanocytic Tumors, 1st Edition, 2018, WHO Classification of Tumours Editorial Board: Female Genital Tumours, 5th Edition, 2020, WHO Classification of Tumours Editorial Board: Skin Tumours, 5th Edition, 2024
- Cytologic atypia, grade 1
- Dermal mitotic activity (2 mitoses/2 mm2)
- Focal fused junctional nests
- Focal pagetoid spread
Comment Here
Reference: Dysplastic nevi
A 38 year old woman presented to the OBGYN clinic with a 0.9 cm, irregular, dome shaped, dark pigmented papule on the right labium majus. A biopsy demonstrated symmetric melanocytic proliferation, with maturation, adjacent bridges of rete ridges and focal pagetoid spread mostly confined to the lower level of the epidermis. What is the most likely molecular alteration in this lesion?
- ALK fusion
- BRAF V600E mutation
- Homozygous deletion of CDKN2A
- NRAS mutation
Comment Here
Reference: Dysplastic nevi
- This topic lists new entities described but not part of the WHO classification for this site
- Email possible additions or changes to Authors@PathologyOutlines.com
- Myoepithelioma-like tumors of the vulvar region (J Obstet Gynaecol Res 2022;48:2015)
- Second most common subtype of primary vaginal adenocarcinoma with majority of cases associated and likely arising from endometriosis
- Metastatic endometrioid adenocarcinoma to vagina and local spread from a neoplasm arising in an adjacent organ needs to be excluded (Am J Surg Pathol 2007;31:1490)
- Age range 45 to 81 years (mean age 60 years) (Am J Surg Pathol 2007;31:1490)
- Most common site is vaginal apex
- Can also occur in posterior wall, lateral wall and anterior wall (Am J Surg Pathol 2007;31:1490)
- Prior hysterectomy and trauma due to surgery might predispose to development of endometriosis at vaginal apex and further development of carcinoma at the same site (Am J Surg Pathol 2007;31:1490)
- Vaginal endometriosis (Am J Surg Pathol 2007;31:1490, Pathol Int 2010;60:636, Gynecol Oncol 1989;34:232, Am Fam Physician 2000;62:734, Obstet Gynecol 1984;64:592, Gynecol Oncol 1982;14:271)
- Patient can have history of prior hysterectomy for endometriosis
- Unrelated to exposure to DES that is usually seen in association with clear cell carcinoma (Am J Surg Pathol 2007;31:1490 but see Cancer 1970;25:745)
- Most common symptoms are vaginal bleeding or vaginal discharge (Am J Surg Pathol 2007;31:1490)
- Also pelvic pain and constipation
- Can be discovered incidentally as pelvic mass on routine vaginal examination
- May have history of prior hysterectomy due to endometriosis or other benign disease (Pathol Int 2010;60:636)
- Based on histologic examination of biopsy or resection specimen which shows pure or predominant component of typical endometrioid adenocarcinoma and excluding local spread or metastatic carcinoma to vagina
- May recur and can metastasize to distant sites including lungs, bowel
- Stage I and II do well without distant metastasis and have better 5 year survival (Am J Surg Pathol 2007;31:1490)
- 34 year old woman with primary invasive vaginal cancer in setting of Mayer-Rokitansky-Küster-Hauser syndrome (Gynecol Oncol 2002;85:384)
- 42 year old woman (Proc R Soc Med 1967;60:999)
- 57 year old woman with endometrioid adenocarcinoma of the vagina with a microglandular pattern arising from endometriosis after hysterectomy (Pathol Int 2010;60:636)
- 68 year old woman with adenocarcinoma arising in endometriosis (Am Fam Physician 2000;62:734)
- Arising in vaginal endometriosis (Gynecol Oncol 1989;34:232)
- Primary vaginal endometrioid carcinoma following unopposed estrogen administration (J Obstet Gynaecol 2003;23:316)
- Malignant transformation of vaginal endometriosis (Obstet Gynecol 1984;64:592)
- Adenocarcinoma of vagina arising in endometriosis (Gynecol Oncol 1982;14:271)
- Vaginal adenocarcinoma developing in residual pelvic endometriosis (Gynecol Oncol 1989;33:96)
- Adenocarcinoma of vaginal vault following prolonged unopposed estrogen hormone replacement therapy (J Obstet Gynaecol 2005;25:220)
- Radical resection of tumor; if tumor is small, conservative local resection can be attempted
- Post surgical radiotherapy, chemotherapy, hormonal therapy or a combination (Am J Surg Pathol 2007;31:1490)
- Polypoid, papillary, rough, granular, fungating, exophytic or flat
- Can also be partially cystic
- Size ranges from 1.4 cm to 7.0 cm (Am J Surg Pathol 2007;31:1490)
- Atypical glandular proliferation composed of tubular glands lined by columnar cells with moderate amount of eosinophilic cytoplasm and occasional intracytoplasmic mucin
- Nuclei are oval to elongated, large and stratified or pseudostratified
- Glands can show microcysts and numerous neutrophils within and around cysts, microglandular pattern (Pathol Int 2010;60:636, Am J Surg Pathol 2007;31:1490)
- Nuclear features are bland in microglandular pattern so careful histological examination for classic endometrioid adenocarcinoma and architectural complexity is required (Pathol Int 2010;60:636)
- Squamous metaplasia can also be seen with cytoplasmic clearing due to glycogen accumulation
- Rare cases have nonvillous papillary budding pattern (Am J Surg Pathol 2007;31:1490)
- Grades: vary from well differentiated to moderately to poorly differentiated (Am J Surg Pathol 2007;31:1490, Am Fam Physician 2000;62:734)
- Atypical glandular cells with hyperchromatic nuclei and high N:C ratio with prominent nucleolus
- Microglandular pattern has clusters of epithelial cells in papillary arrangement and microglandular structures; neutrophils are seen within and around cystic glands
- Cells have lacy and pale cytoplasm, round to oval small nuclei with fine chromatin and small but distinct nucleoli (Pathol Int 2010;60:636)
- Metastasis from adjacent organs including: uterus, cervix, ovary, vulva, urinary tract and from distant sites such as lower GI tract
- Other subtypes of primary vaginal adenocarcinoma: serous adenocarcinoma, adenosarcoma, polypoid endometriosis (Am J Surg Pathol 2007;31:1490)
- Epithelioid sarcoma of the vulva and deep pelvic soft tissue is a rare malignant neoplasm characterized by SMARCB1 / INI1 deletion
- Most vulvar and pelvic epithelioid sarcomas are of the proximal type and show more aggressive clinical behavior than distal type (classical) epithelioid sarcoma
- In vulva and pelvic soft tissues, proximal type epithelioid sarcoma > distal type
- Proximal type epithelioid sarcoma: sheets or nests of epithelioid to rhabdoid cells with nuclear atypia
- Positive immunostains: CK, EMA, CD34 (~ 50%)
- SMARCB1 / INI1 deletion in > 90%, resulting in SMARCB1 / INI1 loss by IHC
- Frequent local recurrence, lymph node metastasis, distant metastasis (especially to lung) and death
- Women, 17 - 80 years (most often in fourth and fifth decades) (Am J Surg Pathol 2015;39:836, Int J Gynecol Pathol 2010;29:600, Mod Pathol 2001;14:655)
- Rarely diagnosed in pregnancy (Gynecol Oncol 2002;85:218, Appl Immunohistochem Mol Morphol 2009;17:270)
- Vulvar epithelioid sarcoma most often involves the labia majora and mons pubis (Cancer 1983;52:1462)
- Superficial inguinal region and deep pelvic soft tissues also affected (Am J Surg Pathol 1997;21:130, Mod Pathol 2001;14:655)
- Histogenesis uncertain
- Typically presents with painless, slow growing vulvar mass (Am J Surg Pathol 1997;21:130, Mod Pathol 2001;14:655)
- Occasional lesions painful or pruritic (Am J Surg Pathol 1989;13:848, Int J Gynecol Pathol 2010;29:600)
- Present for weeks to years before diagnosis (Am J Surg Pathol 1997;21:130)
- Rare cases ulcerated (Mod Pathol 2001;14:655)
- Clinically resemble lipoma or Bartholin cyst (Am J Surg Pathol 1989;13:848, Cancer 1983;52:1462)
- Clinical evaluation may underestimate lesion size (Cancer 1983;52:1462)
- Diagnosis typically established on examination of excision specimen
- Occasionally diagnosed on preoperative biopsy (Mod Pathol 2001;14:655, BMJ Case Rep 2015;2015:bcr2014208488)
- Ultrasound: may show local tumor infiltration or lymph node metastases (BMJ Case Rep 2015;2015:bcr2014208488)
- CT: may show local tumor infiltration or lymph node or distant metastases (BMJ Case Rep 2015;2015:bcr2014208488)
- T2 weighted MRI: signal intensity similar to fat (Int J Clin Exp Pathol 2015;8:7526)
- Diffusion weighted MRI: high signal intensity
- Proximal type epithelioid sarcoma (more common in vulva) has poorer prognosis than distal type (classical) epithelioid sarcoma (rare in vulva) (Cancer 1983;52:1462, Mod Pathol 2001;14:655)
- Local recurrence in approximately 67% (Mod Pathol 2001;14:655)
- Metastases (most often to lymph nodes, lung, liver, bone and skin) in approximately 70%, often after one or more local recurrences (Cancer 1983;52:1462, Mod Pathol 2001;14:655, Gynecol Oncol 1980;9:237)
- Approximately 50% die of disease, often within 2 years of diagnosis (Cancer 1983;52:1462, Am J Surg Pathol 1997;21:130)
- Large tumor size (> 7.8 cm) and early metastasis (< 28 months after diagnosis) associated with increased mortality (Mod Pathol 2001;14:655)
- 32 year old woman with proximal type epithelioid sarcoma of the vulva (Gynecol Oncol Rep 2016;15:31)
- 34 year old woman with proximal type epithelioid sarcoma of the vulva (Case Rep Obstet Gynecol 2015;2015:971217)
- 42 year old woman with proximal type epithelioid sarcoma of the vulva (Int J Clin Exp Pathol 2015;8:7526)
- 55 year old woman with proximal type epithelioid sarcoma of the vulva (BMJ Case Rep 2015;2015:bcr2014208488)
- Early wide en bloc excision or radical vulvectomy with node dissection; local excision is inadequate (Gynecol Oncol 1980;9:237, Obstet Gynecol 1976;48:14S, Obstet Gynecol 1972;40:839)
- Long term followup for late recurrence (Mod Pathol 2001;14:655)
- Chemotherapy and radiation administered at clinical discretion; robust trials lacking (Gynecol Oncol 1980;9:237, Gynecol Oncol 2007;107:130, Appl Immunohistochem Mol Morphol 2009;17:270)
- EZH2 inhibitor tazemetostat FDA approved for epithelioid sarcoma (Drugs 2020;80:513)
- Size range, 1 - 8 cm (average, ~ 3 - 5 cm) (Am J Surg Pathol 1997;21:130, Mod Pathol 2001;14:655, Gynecol Oncol Rep 2016;15:31)
- Poorly circumscribed, multinodular, firm gray to fleshy white mass (Cancer 1983;52:1462)
- Most often based in subcutis or deep pelvic soft tissues; occasionally dermal (Cancer 1983;52:1462, Am J Surg Pathol 1997;21:130, Mod Pathol 2001;14:655)
- Gross necrosis may be present (Cancer 1983;52:1462)
- In vulva: proximal type > distal type
- Proximal type epithelioid sarcoma (Cancer 1983;52:1462, Am J Surg Pathol 1989;13:848, Am J Surg Pathol 1997;21:130, Mod Pathol 2001;14:655, Am J Surg Pathol 2015;39:836):
- Extensively infiltrates surrounding tissues
- Multinodular or diffuse growth pattern in collagenous or myxoid stroma
- Atypical polygonal epithelioid cells
- Minor spindle cell population may be present peripherally or admixed
- Coarse vesicular chromatin and 1 - 2 prominent nucleoli
- Abundant eosinophilic cytoplasm with distinct borders
- Cytoplasm may contain an inclusion displacing nucleus peripherally (rhabdoid morphology)
- Subset show exclusively rhabdoid morphology
- Mitoses highly variable (2 - 57 mitoses per 10 high power fields) (Cancer 1983;52:1462)
- Atypical mitoses, necrosis, hemorrhage and lymphovascular invasion common
- Distal type epithelioid sarcoma of the vulva (Am J Surg Pathol 1989;13:848, Am J Surg Pathol 1997;21:130):
- Pseudo granulomatous growth pattern: scattered small tumor nodules with central necrosis in a collagenous stroma with prominent lymphocytic inflammation
- Monomorphic eosinophilic epithelioid or histiocytoid cells
- Bland nuclei with inconspicuous nucleoli
- Rhabdoid morphology not common
- 8 mitoses per 10 high power fields in 1 vulvar case (Am J Surg Pathol 1989;13:848)
- Cellular smear or touch prep with dispersed large epithelioid cells (Cytopathology 2018;29:471, Am J Surg Pathol 1989;13:848)
- Rhabdoid morphology may be seen
- Mitoses brisk
- Perivascular arrangements may be seen (Cancer Cytopathol 2018;126:934)
- Cytokeratin (AE1 / AE3, CAM5.2; virtually 100%) (Am J Surg Pathol 1989;13:848, Am J Surg Pathol 1997;21:130, Mod Pathol 2001;14:655, Am J Surg Pathol 2015;39:836)
- EMA (~ 90%)
- CD34 (~ 50%)
- ERG, N terminus (~70%) (Mod Pathol 2014;27:496)
- FLI1 (Mod Pathol 2014;27:496)
- SMARCB1 / INI1 (loss of expression in ~ 95%) (Int J Gynecol Pathol 2010;29:600, Am J Surg Pathol 2009;33:542, Am J Surg Pathol 2018;42:312, Mod Pathol 2013;26:385)
- S100 (Am J Surg Pathol 1997;21:130)
- CD31 (Mod Pathol 2014;27:496)
- ER, PR (Gynecol Oncol 2002;85:218)
- ERG, C terminus (Mod Pathol 2014;27:496)
- Mononuclear cells
- Conspicuous intermediate filaments, sometimes as perinuclear whorls (Cancer 1983;52:1462, Am J Surg Pathol 1997;21:130, Am J Surg Pathol 1989;13:848)
- Epithelial type cell - cell adhesions (tonofilaments or desmosomes) (Am J Surg Pathol 1997;21:130)
- Well developed rough endoplasmic reticulum, ribosomes and lysosomes
- Few mitochondria (Gynecol Oncol 1980;9:237)
- Homozygous deletions of SMARCB1 / INI1 in 80 - 90% of proximal type epithelioid sarcoma (Am J Surg Pathol 2009;33:542, Cancer Res 2005;65:4012, Genes Chromosomes Cancer 2014;53:475, Am J Surg Pathol 2018;42:312, Mod Pathol 2013;26:385)
- SMARCB1 / INI1 deletion correlates strongly with SMARCB1 / INI1 loss by IHC (Eur J Cancer 2011;47:287)
- SMARCA4, SMARCC2 or SMARCC1 alterations in rare cases (Am J Surg Pathol 2018;42:312)
Proximal type epithelioid sarcoma of the groin
- Vulva, mass, wide local excision:
- Epithelioid sarcoma, proximal type (6.0 cm) (see comment)
- Margins are negative for tumor
- Comment: Microscopic examination reveals an infiltrative tumor composed of atypical epithelioid cells, some of which show prominent rhabdoid cytoplasmic inclusions. By immunohistochemistry, tumor cells are positive for cytokeratin AE1 / AE3, EMA and CD34 and show loss of SMARCB1 / INI1 expression. S100, CD31, ER and PR are negative. The findings are most consistent with proximal type epithelioid sarcoma. These are aggressive tumors with a high rate of local recurrence, lymph node metastasis and distant metastasis. Clinical and radiographic correlation are advised.
- Squamous cell carcinoma:
- Keratinization or intercellular bridging frequently present
- Associated with usual or differentiated type vulvar intraepithelial neoplasia
- p63 / p40 positive
- HPV in situ hybridization positive in HPV associated tumors
- Mutant pattern p53 immunostaining in a subset of HPV independent tumors
- SMARCB1 / INI1 positive (retained)
- CD34 negative
- Myoepithelial carcinoma (Am J Surg Pathol 2015;39:836):
- Less common than epithelioid sarcoma in vulva
- More varied cytology and more prominent spindle cells
- Reticulated architecture in myxoid stroma
- S100 or SMA positive
- SMARCB1 / INI1 IHC negative (lost) in ~ 50% (Am J Surg Pathol 2009;33:542)
- May harbor EWSR rearrangements (not yet detected in vulvar tumors)
- Extrarenal malignant rhabdoid tumor:
- In adults, most often represents carcinoma, sarcoma or melanoma with rhabdoid morphology (composite rhabdoid tumor)
- Debated whether malignant rhabdoid tumor is an entity distinct from proximal type epithelioid sarcoma (Cancer Res 2005;65:4012, Int J Gynecol Pathol 2010;29:600, Hum Pathol 2015;46:225, Hum Pathol 2009;40:349)
- Malignant melanoma:
- S100, SOX10, HMB45, MelanA positive
- SMARCB1 / INI1 positive (retained)
- Cytokeratin and EMA negative
- Epithelioid angiosarcoma:
- True vascular spaces lined by multiple layers of tumor cells
- Vacuolated tumor cells may contain red blood cells
- CD31 and ERG positive
- Cytokeratin may be positive but EMA is negative
- Epithelioid malignant peripheral nerve sheath tumor:
- S100 positive
- SMARCB1 / INI1 IHC negative (lost) in ~ 50% (Am J Surg Pathol 2009;33:542)
- CD34 positive in ~ 50%
- Cytokeratin and EMA negative
- Epithelioid leiomyosarcoma:
- Rhabdomyosarcoma:
- Desmin, myogenin, myoD1 positive
- SMARCB1 / INI1 positive (retained)
- Epithelioid monophasic synovial sarcoma:
- SMARCB1 / INI1 positive (retained)
- Characteristic t(X;18) fusion, with positive fusion specific immunostain (Am J Surg Pathol 2020;44:922)
- Necrotizing granulomas (in differential diagnosis with distal type epithelioid sarcoma):
- CD68 and PU.1 positive
- SMARCB1 / INI1 positive (retained)
- Cytokeratin and EMA negative
- Infectious organisms, foreign material or autoimmune etiology may be present
- Vulvar soft tissue tumor review (Semin Diagn Pathol 2020 Sep 6 [Epub ahead of print]), additional review of distal type (classical) epithelioid sarcoma (Adv Anat Pathol 2006;13:114), prognostic factors in epithelioid sarcoma (Eur J Surg Oncol 2020;46:1320, Ann Surg Oncol 2015;22:2624)
A 44 year old woman presented with a 3 cm painless vulvar mass. Her gynecologist diagnosed a Bartholin cyst and performed a conservative local excision. A representative photomicrograph of the lesion is shown. On immunohistochemical studies, tumor cells were positive for CK AE1 / AE3 and showed loss of INI1 staining. Which of the following is true about this tumor?
- CD34 is expressed in ~ 50%
- Exposure to ultraviolet radiation is the most common risk factor
- Fewer than 10% of patients experience local recurrence
- Hormone receptors (ER, PR) are usually positive
- Lymph node metastases are exceptionally rare
Comment Here
Reference: Epithelioid sarcoma-vulva
- Anatomic site and location
- Size
- Depth of invasion
- Histologic type
- Histologic grade
- Pagetoid spread
- Type of invasion (infiltrating, pushing, mixed)
- Angiolymphatic invasion
- Adjacent organs
- Margins
- Presence of VAIN, condyloma acuminatum, adenosis
- Descriptive features (ulcers, etc.)
- Lymph nodes: total, number positive, location, tumor size
- If adjacent tissue is involved
- References: Arch Pathol Lab Med 1999;123:62
- Specimen type
- Procedure
- Tumor site / location
- Tumor focality
- Tumor size
- Histologic type and grade
- Thickness of tumor: measured in millimeters from the surface of the tumor or, if keratinization, from the bottom of the granular layer to the deepest point of invasion
- Depth of invasion: measured in millimeters from the epithelial-stromal junction of the adjacent, most superficial dermal papilla to the deepest point of invasion
- Tumor growth pattern (infiltrating, pushing, mixed)
- Margins
- Lymphovascular invasion
- Lymph nodes (total, location, number positive, tumor size, extranodal extension)
- Distant metastasis
- Pathologic staging
- Presence of vulvar intraepithelial neoplasia
- Benign mesenchymal mass characterized by a polypoid proliferation of the stroma with overlying squamous epithelium
- Originally described by Norris and Taylor in 1966 (Cancer 1966;19:227)
- Common benign polypoid proliferation of the stroma with overlying squamous epithelium
- Hormonally related and affects women, mostly of reproductive age
- The key to diagnosis is the characteristic stellate and multinucleated stromal cells
- May raise concern of a sarcoma due to the stromal cellularity
- Complete surgical resection is curative or otherwise it can recur
- Formerly known as pseudosarcoma botryoides (not recommended)
- ICD-10: N84.3 - polyp of vulva
- Common
- Reproductive age women, often during pregnancy or in postmenopausal women taking hormone replacement therapy
- Most common in vagina
- Can also occur in the vulva or cervix (Gynecol Oncol 2005;98:168)
- Rarely extragenital sites (BMC Res Notes 2013;6:345)
- Reactive hyperplastic process arising from the distinctive specialized subepithelial stromal cells of the lower female genital tract (Histopathology 2000;36:97)
- Some of these stromal cells exhibit hormonal responsiveness and immunoreactivity to estrogen and progesterone receptors (Am J Perinatol 1991;8:236)
- Due to hormone induced hyperplasia of loose subepithelial connective tissue or end stage of granulation tissue (J Clin Pathol 1992;45:235)
- Usually asymptomatic
- Occasionally can cause bleeding, discharge and general discomfort
- Morphology is the key to diagnosis
- Imaging is rarely done but the typical findings are (Jpn J Radiol 2010;28:609):
- Polypoid stromal proliferation
- Hyper and hypointense areas
- Covering surface epithelium
- Central fibrovascular core
- No focal or diffusion restriction
- No pelvic lymph node enlargement
- Benign, may recur during pregnancy, often regresses following delivery
- 7 month old with a labial papule (Am J Dermatopathol 2009;31:465)
- 16 year old girl with large pedunculated mass (Pril (Makedon Akad Nauk Umet Odd Med Nauki) 2018;39:127)
- 16 year old girl with vulvar mass (Gynecol Oncol 2005;98:168)
- 21 year old woman with a giant mass (Ann Surg Innov Res 2013;7:8)
- 31 year old woman with labial mass (BMJ Case Rep 2018;2018:bcr2017222789)
- Surgical excision is curative
- Recurrence is possible if incompletely excised (Int J Gynecol Pathol 1988;7:351)
- Polypoid, multilobulated or pedunculated mass usually < 5 cm
- Giant polyps (15 - 20 cm) are rare; likely arise from proliferation of mesenchymal cells within the hormonally sensitive subepithelial stromal layer and have been reported in association with chronic lymphedema (Gynecol Oncol 2005;98:168)
- Polypoid and noncircumscribed, extending to the epithelial / subepithelial interface (J Low Genit Tract Dis 2011;15:69, Am J Surg Pathol 2000;24:231)
- 2 stromal cellularity variants: hypocellular form (spindle cells set within a loose collagenous myxoid-like stroma) or hypercellular variant (exhibits marked nuclear pleomorphism and frequent mitoses, including atypical forms), especially during pregnancy, therefore mimicking leiomyosarcoma or rhabdomyosarcoma (Am J Surg Pathol 2000;24:231)
- Overlying squamous epithelium may display reactive changes but without papillomatous architecture or koilocytosis, which distinguishes it from condyloma acuminatum (caused by human papillomavirus) (Am J Surg Pathol 2005;29:460)
- Stellate cells should not be interpreted as sarcoma (J Low Genit Tract Dis 2011;15:134)
- Commonly found around blood vessels or near the epidermal stromal interface
- Acrochordon of vulva (skin tag) may be either predominantly epithelial (with papillomatosis, hyperkeratosis or attenuation of the epithelium) or primarily stromal (hypo or hypercellular with atypical cells) (Pril (Makedon Akad Nauk Umet Odd Med Nauki) 2018;39:127)
- Usually associated with systemic conditions: type 2 diabetes mellitus, genital psoriasis, congenital lymphedema, Crohn's disease or obesity
- Desmin, ER, PR, vimentin variable, actin (occasional), MyoD1 (focal rarely) (Gynecol Oncol 2005;98:168, Hum Pathol 2020;99:75)
- SMA, HMGA2, S100, cytokeratin, myogenin (commonly) (Int J Gynecol Pathol 1988;7:351)
- Vulva, lesion, excision:
- Fibroepithelial stromal polyp
- Aggressive angiomyxoma:
- Deep location
- Large size (> 10 cm)
- Infiltrative borders
- Lack of hypercellular areas
- Presence of uniformly distributed blood vessels throughout the tumor
- Rearrangements of the high mobility group AT-hook 2 (HMGA2) gene (chromosome 12q15)
- Immunohistochemistry: strong nuclear reactivity for HMGA2
- Angiomyofibroblastoma:
- Subcutaneous rather than a polypoid mass
- Stromal cells are:
- Epithelioid with abundant eosinophilic cytoplasm
- Cluster around blood vessels
- Abundant thin walled blood vessels
- Mean age: fifth decade of life
- Mast cells within the stroma
- Epithelioid and spindle cells are myofibroblastic in origin
- Immunohistochemistry: positive for desmin with coexpression of BCL2 and CD99
- Cellular angiofibroma:
- Well circumscribed
- Moderately cellular stroma
- Intersecting short fascicles of bland spindle cells
- Numerous medium sized blood vessels often with thick hyalinized walls
- Immunohistochemistry: weak to absent staining with desmin and SMA and variably positive for CD34
- Loss of 13q14 (FOX1A1) (similar to spindle cell lipoma and extramammary myofibroblastoma)
- Superficial angiomyxoma:
- Superficial location
- Multinodular growth pattern in a myxoid stroma
- Thin blood vessels
- Neutrophils in the stroma
- Lack of stellate stromal cells
- Mostly found in the head and neck and rarely in the vulva
- Multiple extragenital superficial angiomyxomas are strongly associated with Carney complex
- Prepubertal vulvar fibroma:
- Botryoid rhabdomyosarcoma:
- Usually under 5 years of age
- Cambium layer
- Invasion
- Rapid growth
- Cross striations
- A 25 year old woman presented with a vulvar mass, measuring 3.0 cm in diameter. The lesion was excised and the H&E image is shown. Which of the following is the most likely diagnosis?
- Superficial angiomyxoma
- Cellular angiofibroma
- Fibroepithelial stromal polyp
- Angiomyofibroblastoma
- Acrochordon of vulva
- Which of the following morphologic features is characteristic of fibroepithelial stromal polyps?
- Uniformly distributed blood vessels
- Thin blood vessels
- Thick walled blood vessels
- Stellate cells around the blood vessels
- Abundant thin walled blood vessels
- Squamous intraepithelial lesion (SIL) is the morphological manifestation of HPV infection, both low and high risk types
- Precursor lesion of HPV associated vulvar carcinoma
- p16 positivity in block type pattern (strong diffuse nuclear or nuclear / cytoplasmic staining)
- Harbors clinically undetected carcinomas in up to 20% of cases (J Gynecol Oncol 2017;28:e27)
- Low grade squamous intraepithelial lesion (LSIL): vulvar intraepithelial neoplasia 1, low grade vulvar intraepithelial neoplasia, condyloma accuminatum, mild dysplasia, condyloma/VaIN 1 (Pathology 2016;48:291)
- High grade squamous intraepithelial lesion (HSIL): vulvar intraepithelial neoplasia of usual type (u-VIN), high grade vulvar intraepithelial neoplasia, vulvar intraepithelial neoplasia 2, vulvar intraepithelial neoplasia 3, moderate, severe dysplasia, VaIN 2, VaIN 3
- Reproductive age women
- High grade squamous intraepithelial lesion is the most frequent (> 90%) vulvar intraepithelial neoplasia type
- More common in patients with HIV
- Risk factors: smoking, oral contraception, herpes infection, HPV infection elsewhere
- Often component of multitopic lower genital tract neoplasia
- References: Ecancermedicalscience 2015;9:531, Gynecol Oncol 2017;145:298
- Frequently multifocal (70%)
- Introitus, labia minora, labia majora, posterior fourchette, periclitoral
- Fornix and upper portions of the vagina, multifocal
- HPV integrates in the host genome or resides episomally
- Virus proteins E6 and E7 promote cell transformation and proliferation due to suppression of p53, upregulation of telomerase and stabilization of retinoblastoma protein (Nat Rev Cancer 2002;2:342)
- High grade squamous intraepithelial lesion: HPV high risk types, most common HPV type 16, others 18, 31, 33, 45
- Low grade squamous intraepithelial lesion: HPV low risk 6 / 11 and high risk types (~40%) (Int J Gynecol Pathol 2017;36:486)
- White, pink or red macules or elevated plaques
- Papules or verrucae with pruritus or asymptomatic
- Some lesions are pigmented
- Acetic acid positive
- Reference: Best Pract Res Clin Obstet Gynaecol 2014;28:1051
- Punch biopsy
- Most low grade squamous intraepithelial lesions regress spontaneously, low risk for progression to high grade squamous intraepithelial lesion or invasive carcinoma
- Up to 9% of high grade squamous intraepithelial lesion progress to squamous cell carcinoma if untreated, 3 - 4% if treated; spontaneous regression possible; recurrence rate ~15% after treatment (Gynecol Oncol 2005;97:645)
- 6 young women aged 20 to 36 with regression of vulvar intraepithelial neoplasia 3 (J Low Genit Tract Dis 2012;16:56)
- 36 year old pregnant woman with vulvar intraepithelial neoplasia 3 (Ann Agric Environ Med 2017;24:459)
- 44 year old woman with vulvar intraepithelial neoplasia 3 and Behçet’s disease (BMC Res Notes 2016;9:172)
- 49 year old woman with multinucleated atypia (Cutis 2005;75:118)
- 51 year old woman with vulvar intraepithelial neoplasia 3 (Int J Clin Oncol 2011;16:610)
- 75 year old woman with vulvar intraepithelial neoplasia 3 (Int J Womens Dermatol 2016;2:35)
- Wide local excision if cancer is suspected; otherwise local excision, laser ablation, local topical agent imiquimod (Obstet Gynecol 2016;128:937)
- No treatment for low grade squamous intraepithelial lesion if asymptomatic
- High grade squamous intraepithelial lesion
- Acanthosis, papillomatosis, enlarged atypical nuclei in all cell layers including middle and upper third of the epithelium (full thickness atypia), suprabasal mitoses, atypical mitosis, extension in hair follicles and skin appendages (Pathology 2013;45:214)
- Warty type vulvar intraepithelial neoplasia: extensive hyper and parakeratosis at the surface, some koilocytosis possible, condylomatous appearance with wide rete pegs which extend into the subepithelial stroma
- Basaloid type vulvar intraepithelial neoplasia: atypical immature parabasal type cells in the entire epithelium, numerous mitotic figures
- Rarely pagetoid pattern of vulvar intraepithelial neoplasia is observed simulating extramammary Paget disease
- Various types often admixed, classification of the predominant type, distinction of types has no known clinical relevance
- Can be a component of a condyloma
- Foci of unsuspected invasion in up to 20% cases (J Gynecol Oncol 2017;28:e27)
- Low grade squamous intraepithelial lesion
- Acanthosis / papillomatosis, atypical koilocytosis in upper layers, usually mild atypia of immature cells and mitoses limited to the lower third, binucleated epithelial cells
- Squamous cell markers: p63, CK5/6
- High grade squamous intraepithelial lesion
- Nuclear or nuclear and cytoplasmic p16 positivity involving at least the lower one third of epithelium with frequent extension upward (J Low Genit Tract Dis 2012;16:205)
- Numerous Ki67 positive nuclei in middle and higher portions
- Heterogeneous p53 staining with varying intensities especially in lower epithelial layers (wild type pattern)
- CK17 shows only patchy, weak to moderate staining in superficial cell layers (Virchows Arch 2018;473:739)
- Low grade squamous intraepithelial lesion
- p16 usually negative but may be rarely positive in block type pattern in the lower third of the epithelium
- Ki67 is elevated, mostly in the lower third of the epithelium, preserved compartments
- Immunohistochemically positive HPV detection in koilocytic cells in a subset of cases depending on the antibody used
- HPV DNA/mRNA positive
- Labium minor, left side, biopsy:
- High grade squamous intraepithelial lesion (vulvar intraepithelial neoplasia 2-3) (see comment)
- Comment: p16 is positive (block type).
- High grade squamous intraepithelial lesion
- Invasive squamous cell carcinoma (versus involvement of skin adnexa):
- Desmoplasia, irregular contours, inflammatory response, distorted architecture are features indicative of invasion
- Paget disease:
- Positive for CK7, CEA and mucin
- p16 may be positive (Int J Gynecol Pathol 2013;32:221)
- Melanoma:
- Positive for melanoma markers (S100, HMB45, melanA, tyrosinase, SOX10)
- Basal cell carcinoma:
- Peripherial palisading
- BerEP4 positive
- Seborrheic keratosis:
- Keratin pearls, uniform cells without atypia
- Differentiated vulvar intraepithelial neoplasia:
- Invasive squamous cell carcinoma (versus involvement of skin adnexa):
- Low grade squamous intraepithelial lesion
- Squamous cell hyperplasia, lichen simplex:
- Ki67 limited to basal cell layers
- Squamous cell hyperplasia, lichen simplex:
- Which immunohistochemical stain shows a block type pattern in high grade squamous intraepithelial lesion?
- CK17
- Ki67
- p16
- p53
- SOX2
- Stromal invasive squamous cell carcinoma with immunohistochemically or molecularly verified association with human papillomavirus (HPV)
- Stromal invasive squamous cell carcinoma
- p16 block type positivity or positive molecular testing (PCR or ISH) for HPV
- Coincident HPV associated vulvar intraepithelial neoplasia (VIN) (desirable)
- ICD-O: 8085/3 - squamous cell carcinoma, HPV positive
- ICD-10: C51 - malignant neoplasm of vulva
- ICD-11: 2C70.2 & XH0EJ7 - squamous cell carcinoma of vulva & squamous cell carcinoma, HPV positive
- ~25 - 33% of all vulvar squamous cell carcinomas (Lancet Glob Health 2020;8:e180)
- Highest incidence in seventh decade but also younger patient ages
- HPV 16 is the most commonly associated virus subtype (Int J Cancer 2017;141:1161)
- > 90% of vulvar squamous cell carcinomas in sub-Saharan Africa are HPV associated (Int J Cancer 2023;152:496)
- All parts of the vulva including labia majora and minora, clitoris (midline carcinomas), vestibule, introitus
- Human papillomaviruses: mostly high risk HPV 16 followed by HPV 33; very rare low risk HPV 6 and 11 (Lancet Oncol 2023;24:403)
- Risk factors: smoking, immunodeficiency
- Punch biopsy, vulvectomy specimen
- Outcome of HPV associated vulvar squamous cell carcinoma (VSCC) is favorable compared to HPV independent VSCC (Int J Cancer 2018;142:1158, Histopathology 2017;71:238)
- 5 year overall survival: 59 - 89%
- Stage (tumor size and invasion depth) and lymph node status are the main prognostic factors (Semin Diagn Pathol 2021;38:37)
- 22 year old woman with HPV positive squamous cell carcinoma of the vulva (Gynecol Oncol Rep 2021:36:100760)
- 58 year old woman with warty squamous cell carcinoma and condylomata acuminata (Med Arch 2017;71:72)
- 63 year old woman with combined squamous cell carcinoma and Merkel cell carcinoma (JAAD Case Rep 2015;1:196)
- Wide local excision, partial or total vulvectomy depending on stage and patient condition (J Natl Compr Canc Netw 2017;15:121)
- Exophytic mass or ulcerated lesion, frequently multifocal
- Tumor diameter should be measured in millimeters parallel to the skin surface
- Morphologic variants: keratinizing (~33%, most common), nonkeratinizing, basaloid, warty / condylomatous type (Int J Cancer 2017;141:2517)
- Basaloid and warty type are more frequent in HPV associated squamous cell carcinomas than in HPV independent tumors (blue appearance)
- Rare verrucous and spindle cell differentiation
- Frequent necrosis, koilocytic changes and nuclear pleomorphism
- There is no established grading system
- Measurement of tumor invasion depth is as follows
- Union for International Cancer Control (UICC): from the tip of the nearest most superficial dermal papilla to the deepest tumor deposit
- International Federation of Gynecology and Obstetrics (FIGO): from the deepest adjacent dysplastic rete ridge to the deepest point of tumor invasion
- FIGO staging may downstage some tumors from IB to IA
- Surgical margin is measured from the peripheral edge of tumor straight to the nearest epithelial or stromal margin through tissue (Int J Gynecol Pathol 2020;39:420)
- p53 wild type pattern
- Vulva, wide local excision:
- Poorly differentiated basaloid squamous cell carcinoma (HPV associated) (see synoptic report)
- Comment: p16 IHC block-like positive
- HPV independent squamous cell carcinoma:
- Frequently, keratinizing squamous cell carcinomas but significant overlap with morphologically identical HPV associated carcinomas
- HPV independent carcinomas are usually p16 negative with mutation type p53 expression in most cases
- VIN with tangential section or extension in adnexal structures:
- Regular tumor cell infiltrates with smooth outline, evidence of cross sectioning, adnexal structures with VIN, no single cells, no desmoplastic stroma, no paradoxical maturation
- Loose inflammatory infiltrates are common
Comment Here
Reference: HPV associated squamous cell carcinoma-vulva
- Basal cell layer
- Dermal papilla
- Rete ridge
- Surface epidermis
- Tumor surface
Comment Here
Reference: HPV associated squamous cell carcinoma-vulva
- Human papillomavirus (HPV) independent vulvar intraepithelial neoplasia is the putative precursor lesion of HPV independent vulvar squamous cell carcinoma
- Subtypes include p53 mutant differentiated vulvar intraepithelial neoplasia (dVIN) and p53 wild type lesions such as differentiated exophytic vulvar intraepithelial lesion (DEVIL) and vulvar acanthosis with altered differentiation (VAAD), recently also designated as vulvar aberrant maturation (VAM) or HPV independent, p53 wild type verruciform acanthotic vulvar intraepithelial neoplasia (Mod Pathol 2022;35:1317)
- Highly differentiated with overlapping histological features to benign vulvar lesions
- Currently, there are no reliable immunohistochemical or molecular markers to establish the diagnosis
- HPV independent VIN can be p53 abnormal (dVIN) or p53 wild type (DEVIL, VAAD, VAM, vaVIN)
- Differentiated vulvar intraepithelial neoplasia is an aggressive lesion with higher potential to become invasive than HPV associated vulvar intraepithelial neoplasia (usual type)
- Old: simplex vulvar intraepithelial neoplasia
- Postmenopausal women, sixth to eighth decade; 2 - 29% of all vulvar intraepithelial neoplasia (Pathology 2016;48:291)
- Labia minora; commonly found adjacent to invasive squamous cell carcinoma (70%)
- Background of chronic inflammatory dermatoses, lichen sclerosus, lichen simplex chronicus (J Low Genit Tract Dis 2016;20:180)
- Unifocal, gray-white papules or plaques
- Difficult to diagnose in punch biopsies alone; overlapping histological features with other common vulvar lesions, especially lichen simplex
- More likely to progress to invasive carcinoma (32%) than usual vulvar intraepithelial neoplasia; shorter latency to become invasive (Eur J Cancer 2009;45:851, Int J Gynecol Cancer 2009;19:741)
- VAAD may be a possible precursor of verrucous carcinoma
- 52 year old woman with progression to carcinoma (Int J Gynecol Pathol 2007;26:248)
- 6 55 - 82 year old women with dVIN in association with squamous cell carcinoma (Int J Gynecol Pathol 2008;27:125)
- 61 and 78 year old women with DEVIL, which was initially misinterpreted (J Low Genit Tract Dis 2022;26:283)
- 70 year old woman with background of lichen sclerosus (Am J Dermatopathol 2011;33:e27)
- 73 year old woman with longstanding illness and diagnosis of dVIN, VAAD and vulval carcinoma (JAAD Case Rep 2020;8:53)
- 82 year old woman with dVIN (JAAD Case Rep 2019;5:448)
- 85 year old woman with VAAD and progression to carcinoma (Int J Gynecol Pathol 2015;34:385)
- Conservative excision, careful follow up (Best Pract Res Clin Obstet Gynaecol 2014;28:1051)
- dVIN
- Acanthosis, parakeratosis, elongation and anastomosis of rete ridges (Histopathology 2020;76:128, Semin Diagn Pathol 2021;38:27)
- Mild to moderate atypical cells in basal and parabasal layers with hyperchromatic nuclei; however, full thickness atypia is lacking
- Enlarged squamous cells with eosinophilic cytoplasm, vesicular nuclei and prominent nucleoli; prominent intercellular bridges
- Rare basaloid differentiation of dVIN with atypical cells in upper cell layers (Am J Surg Pathol 2009;33:1659, Am J Surg Pathol 2020;44:1506, Histopathology 2023;82:731)
- DEVIL: exophytic verruciform lesion with acanthosis; no basal atypia, no koilocytosis (Mod Pathol 2017;30:448)
- VAAD: verruciform acanthosis, parakeratosis, absence of nuclear atypia, cytoplasmic pallor in superficial cells (Am J Surg Pathol 2004;28:638)
- p53 and p16 immunohistochemistry is recommended for classification (see below)
- p53
- Mutation type pattern in dVIN: basal overexpression, diffuse overexpression, absent (null) or cytoplasmic (Mod Pathol 2020;33:1595, Mod Pathol 2023;36:100145)
- Wild type in DEVIL and VAAD
- Interpretation may be difficult in small biopsies
- Ki67 confined to basal cell layers
- Retained CK17 expression (Int J Gynecol Pathol 2017;36:273)
- Loss of GATA3 expression (Mod Pathol 2018;31:1131)
- p16 negative or patchy (Histopathology 2005;46:718)
- HPV
- HPV ISH negative
- Mutations in TP53 (Mod Pathol 2010;23:404)
- Labium majus, biopsy:
- Differentiated vulvar intraepithelial neoplasia (see comment)
- Comment: p16 is negative and p53 is overexpressed (aberrant pattern).
- Condyloma:
- Mild nuclear atypia, most commonly superficially
- HPV related
- Lichen sclerosus:
- Lacks basal atypia
- May be concurrent
- Lichen simplex chronicus:
- Lacks basal atypia
- p53 wild type
- Hypertrophic lichen planus:
- Band-like chronic inflammation
- Lacks basal atypia
Comment Here
Reference: HPV independent VIN-vulva
- CK18
- Ki67
- p16
- p53
- SOX10
Comment Here
Reference: HPV independent VIN-vulva
- Stromal invasive squamous cell carcinoma with no evidence for association with human papillomavirus (HPV) infection
- Stromal invasive squamous cell carcinoma
- p16 nonblock type positivity or aberrant p53 mutation type expression pattern
- Coincident HPV independent vulvar intraepithelial neoplasia (dVIN, vaVIN) (desirable)
- No synonyms
- ~67% of all vulvar squamous cell carcinomas (VSCC) independent of HPV prevalence in the population (Lancet Oncol 2023;24:403)
- Highest incidence in the eighth decade (Int J Cancer 2017;141:2517)
- All parts of the vulva including labia majora et minora, clitoris (midline carcinomas), vestibule, introitus
- 30 - 80% of cases present with TP53 mutations (Semin Diagn Pathol 2021;38:50)
- Risk factors: lichen simplex chronicus, lichen sclerosus (5% with subsequent vulvar carcinoma), lichen planus (Surg Pathol Clin 2019;12:249)
- Punch biopsy, vulvectomy specimen
- Prognosis of HPV independent VSCC is worse than HPV associated VSCC (Histopathology 2017;71:238, Int J Gynecol Pathol 2020;39:391)
- Higher local recurrence rate in HPV negative VSCC (Gynecol Oncol 2016;142:420)
- Stage (tumor size and invasion depth) and lymph node status are main prognostic factors (Semin Diagn Pathol 2021;38:37)
- Tumor margin plays a role in local recurrence (Eur J Cancer 2016;65:139)
- 28 year old woman with VSCC and simplex VIN (Int J Gynecol Pathol 2008;27:591)
- 74 year old woman with HPV negative spindle cell carcinoma of the vulva (Am J Dermatopathol 2002;24:135)
- 79 year old woman with verrucous carcinoma and lichen planus (World J Surg Oncol 2017;15:7)
- 82 year old woman with acantholytic squamous cell carcinoma (Int J Gynecol Pathol 2022;41:122)
- 4 cases with spindle cell carcinoma of the vulva (Int J Gynecol Pathol 2014;33:203)
- Wide local excision, partial or total vulvectomy, sentinel or inguinofemoral lymph node dissection depending on stage and patient condition (J Obstet Gynaecol Can 2019;41:89)
- Diminished response to radiotherapy (Int J Gynecol Cancer 2020;30:100)
- Exophytic mass or ulcerated lesion, frequently multifocal
- Tumor diameter should be measured parallel to the skin surface in millimeters
- Frequently (up to 80%) keratinizing squamous cell carcinomas; other histological patterns: nonkeratinizing, basaloid, warty differentiation (Int J Cancer 2017;141:2517)
- Keratin pearl formation and high percentage of differentiated tumor cells are characteristic features
- Verrucous carcinomas and carcinomas with spindle cell differentiation are mostly HPV negative (Surg Pathol Clin 2019;12:249, Int J Gynecol Pathol 2023;42:207)
- Rare acantholytic morphology has been described (Int J Gynecol Pathol 2022;41:122)
- No established grading system
- Combined use of p16 and p53 immunohistochemistry (IHC) is recommended for demonstration of HPV independence (Mod Pathol 2023;36:100145)
- p53 mutation type pattern in the majority of HPV independent VSCC (Mod Pathol 2020;33:1595)
- p16 nonblock-like staining or negative in most cases (Int J Gynecol Pathol 2016;35:385)
- Vulva, biopsy:
- Keratinizing squamous cell carcinoma (HPV independent) (see comment)
- Comment: p16 IHC nonblock-like (negative)
- HPV associated squamous cell carcinoma:
- Histomorphological overlapping with HPV associated carcinomas, which also show keratinizing morphology in up to 36% of cases (Int J Cancer 2017;141:2517)
- p16 and p53 immunohistochemistry for differential
- Condyloma acuminatum:
- Morphological similarities to verrucous carcinoma: postmenopausal woman, pushing border infiltration, mostly HPV negative but sometimes also positive for low risk HPV
- HPV immunohistochemistry
- HPV RNA in situ hybridization
- p16 immunohistochemistry
- p16 and p53 immunohistochemistry
- TP53 next generation sequencing
Comment Here
Reference: HPV independent squamous cell carcinoma-vulva
Comment Here
Reference: HPV independent squamous cell carcinoma-vulva
- Causes vulvovaginitis; most frequent mycoses of women; 10% of women are carriers
- Risk factors: diabetes, oral contraceptives, pregnancy
- Transmission: male genitalia are not a relevant reservoir for recurrent vulvovaginal candidosis, thus decreasing the possibility of sexual heterosexual transmission (J Eur Acad Dermatol Venereol 2011;25:145)
- Recurrent vulvovaginal candidosis: mostly caused by identical Candida strains suggesting C. albicans persistence in female anogenital area, particularly in external vulva (Mycoses 2011;54:e807)
Etiology
- Acute vulvovaginitis: most commonly C. albicans, followed by C. glabrata
- Chronic recurrent vulvovaginitis: C. albicans and C. glabrata are often equally distributed (Med Monatsschr Pharm 2010;33:324)
Clinical features
- Small white surface patches with leukorrhea and itching
Diagnosis
- Fungal hyphae on wet mount
Treatment
- Local and systemic antimycotic agents based on the severity of disease and etiological agent
- Small gram negative rods, implicated in vaginitis when other causes can't be found
- Presence of 3 of 4 criteria indicate BV (bacterial vaginosis, Medscape Womens Health 1997;2:2):
- Homogenous noninflammatory discharge (not many WBCs)
- pH > 4.5
- Clue cells (bacteria attached to border of epithelial cells, > 20% of epithelial cells)
- Positive "whiff" test
- Caused by Calymmatobacterium granulomatis, an encapsulated, nonmotile gram negative rod
- Soft granulomatous area enlarges by peripheral extension and ulcerates
Clinical features
- Genital ulceration, genital tract bleeding
Microscopic (histologic) description
- Donovan bodies (small round encapsulated bodies within histiocytes), seen best with silver or Giemsa stains
- Also pseudoepitheliomatous hyperplasia, plasma cells, histiocytes, small abscesses
Differential diagnosis
- Soft tissue neoplasm if it spreads to retroperitoneum
- Squamous cell carcinoma
- Sexually transmitted disease characterized by labial ulcers with punched out centers
- Usually HSV2 in young women
- 1/3 are symptomatic (lesions 3 - 7 days after sex); lesions heal in 1 - 3 weeks but virus is latent in regional nerve ganglia
- 2/3 suffer recurrences (less painful)
- High risk of transmission to neonate during vaginal birth, especially if active primary infection
- Rarely, in HIV positive patients, presents as chronic hyperproliferative plaque or mass in vulva or perianal region that clinically resembles malignancy (J Drugs Dermatol 2003;2:198)
Clinical features
- Extremely painful ("heartbreak of herpes"), papules in vulva, progress to vesicles, later coalescent ulcers
- Also affects vagina and cervix
Case reports
- 23 year old woman with associated necrotizing lymphadenitis (Arch Pathol Lab Med 1985;109:1043)
- 38 year old HIV+ woman with chronic herpes infection (Case Rep Dermatol 2012;4:192)
- 44 year old HIV+ woman with hypertrophic herpes simplex genitalis with concomitant early invasive squamous cell carcinoma (Int J Gynecol Pathol 2012;31:286)
- Patient with large fungated vulvar mass (Case of the Week #53)
Clinical images
Case #53
Images hosted on other servers:
Microscopic (histologic) description
- Multinucleated giant cells with molding, ground glass nuclei
- Hypertrophic masses show epithelial hyperplasia, brisk infiltrate of lymphocytes and plasma cells (Dis Colon Rectum 2005;48:2289)
Microscopic (histologic) images
Case #53
Images hosted on other servers:
- HPV subtypes 6, 8,11,13 associated with papillary lesions
- HPV subtypes 16, 18, 31, 33 associated with flat lesions
- HPV 16 produces E6 protein that binds to p53 and E7 protein that binds to Rb protein
- Koilocytotic atypia is a viral cytopathic effect, often NOT present in vulvar condylomas
- Verrucopapillary lesions, even in children / young adults, are likely to be HPV associated (Am J Surg Pathol 1994;18:728)
- HPV and vulvar neoplasia
- HPV leads to pathogenetic pathways for vulvar squamous cell carcinoma and vulvar intraepithelial neoplasia (VSCC and VIN)
Microscopic (histologic) images
Images hosted on other servers:
- Sexually transmitted disease caused by Chlamydia trachomatis, L1 - L3 serotypes, treated with tetracycline
- Initially small ulcer at contact site, then inguinal adenopathy with stellate abscesses surrounded by epithelioid histiocytes, then scarring, fistulas and strictures of urethra, vagina, rectum (Prim Care 1990;17:153)
- Squamous cell carcinoma or adenocarcinoma may be engrafted on lymphogranulomatous structures
Diagnosis
- Frei test (intradermal skin test), complement fixation, immunofluorescence
Case reports
- 22 year old woman (Br J Vener Dis 1984;60:390)
Clinical images
Images hosted on other servers:
Microscopic (histologic) images
Images hosted on other servers:
- Sexually transmitted disease that affects vulva only
Microscopic (histologic) description
- Molluscum bodies
- Causes spontaneous abortions and chorioamnionitis
- Affects entire gynecologic tract in adults except vagina; only children get vaginitis
- Causes infertility
- Begins in Bartholin glands or other vestibular or periurethral glands, then spreads to cervix, tubes, ovaries
Microscopic (histologic) description
- Acute suppurative reaction, inflammation within mucosa and submucosa only
- Clinical syndrome due to various bacteria
- Compared to N. gonorrhea, exudates are less with staphylococcal or streptococcal infections or coliforms but infection extends throughout wall to serosa and may cause bacteremia
- Complications: bacteremia, infertility, intestinal obstruction due to adhesions, peritonitis
Additional references
- First symptom is small, round, firm ulcer known as chancre, which is infectious
- Occurs where bacteria first enters body - usually vulva in women ~3 weeks after infection
- Usually disappears in 3 - 6 weeks even without treatment
- Composed of plasma cells, lymphocytes, histiocytes, covered by zone of ulceration with neutrophils and necrosis; also endarteritis
- Adjacent lymph nodes may be enlarged with plasma cells, endarteritis within or outside capsule, fibrosis (capsular, pericapsular), follicular hyperplasia
- See also Penis & scrotum - Syphilis
Secondary syphilis
- Usually rash on palms and soles that doesn't itch, which appears 2 - 10 weeks after chancre
- Other symptoms include headache, sore throat, swollen lymph glands, tiredness
- Lesions contain bacteria and are infectious
- This stage may disappear without treatment but will recur and progress if not treated appropriately
Latent syphilis
- Begins when symptoms of secondary syphilis are over
- Early latent syphilis is infectious
- Late latent syphilis has low to no risk of infecting the partner
- If not treated appropriately, latent syphilis may progress to tertiary syphilis
Tertiary syphilis
- Affects very small number of syphilis patients even if never treated
- Can affect heart, eyes, brain, joints, nervous system, bones
Additional references
- Large, flagellated, ovoid protozoan, causes up to 25% of vaginitis cases
- Diagnose with wet mount
- 15% of women in sexually transmitted disease clinic are infected
- Purulent discharge, local discomfort, "strawberry" cervix (fiery red with thin epidermis)
- Infection limited to epithelium and lamina propria
Diagnosis
- Foul frothy discharge, pH > 4.5 (in 70% of cases), punctuate cervical microhemorrhages (25% of cases) and motile trichomonads on wet mount (25 - 75% of cases, Medscape Womens Health 1997;2:2)
Treatment
- Oral metronidazole; treatment failure is usually due to nontreatment of male partner
- Chronic, mucocutaneous, inflammatory disease, likely T cell mediated, characterized by band-like lymphocytic inflammation (Am J Surg Pathol 1998;22:473)
- Papule or plaque located on hair bearing or hairless vulvar or perianal area
- Band-like lymphocytic infiltrate at the dermoepidermal junction
- Features of destruction of basal keratinocytes
- Vulvovaginal lichen planus (VLP) with concomitant oral involvement described as vulvovaginal gingival syndrome (Ann Dermatol Venereol 1982;109:797)
- Although it was previously believed that desquamative inflammatory vaginitis (DIV) can occur as erosive VLP, it is now a distinct entity (Obstet Gynecol 2011;117:850, J Reprod Med 2008;53:124)
- ICD-O:
- ICD-10:
- ICD-11:
- EA91 - lichen planus
- EA91.0 - acute eruptive lichen planus
- EA91.1 - hypertrophic lichen planus
- EA91.2 - follicular lichen planus
- EA91.3 - lichen planus of genital skin and mucous membranes
- EA91.4 - lichen planus and lichenoid reactions of oral mucosa
- EA91.5 - lichen planus of the nails
- EA91.6 - subacute lichen planus
- EA91.Y - other specified lichen planus
- EA91.Z - lichen planus of unspecified type
- Most commonly involving the introitus (nonhair bearing mucosa) (J Low Genit Tract Dis 2020;24:317, Australas J Dermatol 2016;57:284, Br J Dermatol 2013;169:226)
- Women in fifth to sixth decade
- Incidence of VLP has not been studied; ~1 - 2% worldwide at any site (Int J Womens Dermatol 2017;3:58, N Engl J Med 2012;366:723)
- In a study of 3,350 women over 13 years, 3.7% showed biopsy proven VLP, including 17.6% with erosive VLP (Br J Dermatol 2000;143:1349)
- Erosive VLP
- Up to 68% with concomitant oral lichen planus (OLP) (J Reprod Med 2007;52:43)
- 20% with concomitant cutaneous lichen planus (CLP) (J Eur Acad Dermatol Venereol 2005;19:301)
- 29% present with other autoimmune disorders, most commonly involving the thyroid gland, followed by alopecia areata and celiac disease (Arch Dermatol 2008;144:1432)
- 25 - 34% present with concomitant vulvar disease, most commonly contact dermatitis and atrophic vaginitis (J Reprod Med 2007;52:43)
- Well documented association between VLP and hepatitis C virus (HCV) in Japan but not in European studies (Eur J Clin Invest 1995;25:910, J Eur Acad Dermatol Venereol 2005;19:301, BJOG 2004;111:271)
- Skin: flexors of wrists, ankles, hands, scalp and genital skin
- Mucosa: genital and oral
- Although exact pathophysiology remains unknown, lichen planus is believed to be a T cell mediated autoimmune disease (Clin Exp Dermatol 2005;30:551)
- Epitopic alteration of basal keratinocytes in the epidermis may lead to lymphocytic attack, followed by their destruction and repair (Adv Anat Pathol 2017;24:278)
- Variety of cytokines may play a role in upregulation of cytotoxic T cells (Adv Anat Pathol 2017;24:278)
- Interferon γ, tumor necrosis factor α, interleukins and CXCL10, a chemokine selectively expressed by basal keratinocytes in the presence of cytotoxic T cells in lichenoid dermatoses
- ICAM1, an intercellular adhesion molecule, is crucial in the migration of the T cells to a particular skin site and is overexpressed in lichen planus
- Associated with viruses (most commonly HCV)
- HCV may alter the cytokine expression that predisposes to lichen planus development (Adv Anat Pathol 2017;24:278)
- HCV and damaged keratinocytes may share epitopes that cytotoxic T cells target (Med Oral Patol Oral Cir Bucal 2005;10:E40)
- Contact allergens (metal amalgam in oral lichen planus) (Adv Anat Pathol 2017;24:278)
Table 1: Diagnostic criteria of vulvar erosive lichen planus by the International Society of the Study of Vulvovaginal Disease (ISSVD) (adapted from Br J Dermatol 2013;169:337)
| 3 out of 9 criteria needed for diagnosis |
| Well demarcated erosions or glazed erythema of introitus |
| Wickham striae on surrounding skin |
| Pain or burning |
| Scarring or loss of architecture |
| Vaginal inflammation |
| Involvement of other mucosal surfaces |
| Well defined inflammatory band in superficial connective tissue involving dermoepidermal junction |
| Inflammatory band consists of predominantly lymphocytes |
| Evidence of basal cell layer degeneration (e.g., Civatte bodies, abnormal keratinocytes or basal apoptosis) |
Table 2: Summary of minimum clinicopathologic criteria to diagnose vulvar erosive lichen planus, more specific than those proposed by ISSVD (adapted from J Low Genit Tract Dis 2020;24:317)
| All 5 criteria must be present | |
| Clinical presentation (signs) | Well demarcated glazed red macule or patch located on vestibule, labia minora or vagina* |
| Histopathological correlate | Nonkeratinized squamous epithelium, mucocutaneous junction or adjacent hairless skin |
| Band-like lymphocytic infiltrate abuts the epithelial layer | |
Basal layer with one of the following patterns
| |
| Sclerosis is absent | |
- *Supportive features
- Architectural changes include midline fusion or labia minora, adhesions between affected opposed structures
- Common comorbidities: lichen sclerosis (LS), other types of locations of lichen planus, systemic autoimmune diseases
Table 3: Clinicopathologic features of classic hypertrophic lichen planus (adapted from J Low Genit Tract Dis 2020;24:317)
| Classic lichen planus | Hypertrophic lichen planus | |
| Clinical features | Red, gray-white or purple-brown plaque or papules on hairless or hair bearing skin | Circumferential red plaque over hairless and hair bearing skin of vulva or perianal area |
| Histopathological correlate | ||
| Stratum corneum* | Hyperkeratosis | Marked hyperkeratosis |
| Granular cell layer* | Hypergranulosis | Diffuse and marked hypergranulosis |
| Rete ridge morphology | Mild to moderate acanthosis with irregular rete ridges, often sawtoothed or spiky | Marked acanthosis with deep and irregular rete ridges |
| Basal layer | Apoptotic bodies, squamatization, vacuolar change Lymphocytosis | Apoptotic bodies, squamatization, vacuolar change may be confined to rete tips / basal layer above papillary processes
Lymphocytosis |
| Dermis | Band-like applied lymphocytic infiltrate | |
| Papillary dermal fibrosis | ||
- *May be altered by excoriation, especially in hypertrophic lichen planus
- Cutaneous
- Pleuritis, planar, polygonal, purple papules
- Wickham striae represent a fine reticulate network of white lines and are considered pathognomonic
- Mucosal
- Erosive lichen planus (ELP) is most common on vulvovaginal surface
- Reticular pattern and white lacy stria
- Less commonly, atrophic, hypertrophic, papular and bullous lesions
- Reference: J Low Genit Tract Dis 2020;24:317
- Most reliable diagnosis is based on clinicopathologic correlations
- Vulvar ELP
- Clinical diagnosis is sufficient per the international electronic Delphi consensus exercise conducted by the International Society of the Study of Vulvovaginal Disease (ISSVD) (see Table 1) (Br J Dermatol 2013;169:337)
- To achieve the most reliable diagnosis of vulvar ELP, the recommended clinicopathologic criteria include clinical appearance, site and 3 histopathologic features: lymphocytic infiltrate, basal layer damage (degenerative / regenerative) and absence of sclerosis (see Table 2) (J Low Genit Tract Dis 2020;24:317)
- Biopsy should be taken at the edge of vulvar erosion (Br J Dermatol 2013;169:337)
- Classic and hypertrophic lichen planus diagnosis require their characteristic clinical appearance, accompanied by specific changes at the 5 epidermal layers (see Table 3) (J Low Genit Tract Dis 2020;24:317)
- Concurrent histopathologic evidence should be considered in the diagnosis of complex cases (J Low Genit Tract Dis 2020;24:317)
- Overall favorable for lichen planus with cutaneous involvement (self limiting within a year)
- Mucosal involvement is persistent and commonly refractory to the treatment
- Association of lichen planus with human papillomavirus (HPV) independent squamous cell carcinoma is unclear (J Low Genit Tract Dis 2018;22:159)
- 30 year old woman with multiple vulvar hyperpigmented papulonodules (Int J STD AIDS 2017;28:1048)
- 32 year old woman with orovaginalvulvar lichen planus (J Med Case Rep 2021;15:97)
- 46 year old woman with severe oral and vulvovaginal erosive lichen planus (Int J Gynaecol Obstet 2022;156:172)
- 55 year old woman with erosive lichen planus vaginitis (Obstet Gynecol 2003;101:1121)
- 79 year old woman with a 3 cm verrucous carcinoma in an area of lichen planus (World J Surg Oncol 2017;15:7)
- Topical
- First line: ultrapotent corticosteroids
- Second line: calcineurin inhibitors (can be used in combination with steroids, if there is no initial response to first line)
- Promising initial results in a limited study with aloe vera gel used as an adjunct anti-inflammatory treatment (82% of 17 patients showed significant clinical improvement compared with 1 in the placebo group)
- Topical photodynamic therapy (PDT): 1 session showed a similar reduction in clinical symptom scores compared with months long steroid therapy
- Intralesional injections: triamcinolone acetonide (Kenalog) 3 - 10 mg/mL used where topical steroids failed (Obstet Gynecol Surv 2020;75:624)
- Systemic
- Methotrexate as an additional treatment regimen in recalcitrant vulvovaginal disease
- Prednisone to treat flares of widespread desquamative disease
- The International Society of the Study of Vulvovaginal Disease (ISSVD) tasked the Difficult Pathologic Diagnoses Committee with development of a consensus document for the clinicopathologic diagnosis of vulvar ELP, distributed in 2013 based on the Delphi consensus exercise
- Well defined inflammatory band in superficial connective tissue involving dermoepidermal junction
- Inflammatory band consists of predominantly lymphocytes
- Evidence of basal cell layer degeneration (e.g., Civatte bodies, abnormal keratinocytes or basal apoptosis)
- Summary of microscopic findings only to diagnose vulvar ELP, more specific than proposed by ISSVD (see Diagrams / tables for the entire clinicopathologic criteria) (J Low Genit Tract Dis 2020;24:317)
- Nonkeratinized squamous epithelium, mucocutaneous junction or adjacent hairless skin
- Band-like lymphocytic infiltrate abuts the epithelial layer
- Basal layer with one of the following patterns
- Degenerative pattern: apoptotic bodies, vacuolar change or squamatization
- Specific features: Civatte bodies (dyskeratosis) within epidermis and colloid bodies in papillary dermis secondary to basal keratinocyte damage
- Regenerative patterns: high N:C ratio, mitoses, diminished epithelial maturation
- Degenerative pattern: apoptotic bodies, vacuolar change or squamatization
- Sclerosis is absent
- Classic lichen planus (see Diagrams / tables for the entire clinicopathologic criteria) (J Low Genit Tract Dis 2020;24:317)
- Hyperkeratosis and hypergranulosis
- Mild to moderate acanthosis with irregular rete ridges, often sawtoothed or spiky
- Apoptotic bodies squamatization, vacuolar change and lymphocytosis
- Dermis: band-like applied lymphocytic infiltrate
- Hypertrophic lichen planus (see Diagrams / tables for the entire clinicopathologic criteria) (J Low Genit Tract Dis 2020;24:317)
- Marked hyperkeratosis
- Diffuse and marked hypergranulosis
- Rete ridges morphology: marked acanthosis with deep and irregular rete ridges
- Basal layer: apoptotic bodies squamatization, vacuolar change may be confined to rete tips / basal layer above papillary processes and lymphocytosis
- Dermis: band-like applied lymphocytic infiltrate
- Papillary dermal fibrosis
- Although not specific, immunofluorescence can support diagnosis
- IgM > IgA, IgG, C3 deposits
- Fibrin stains colloid bodies
- p53: aberrant expression of basal cells in degenerative and regenerative areas
- p16: negative in regenerative areas, focally positive in degenerative areas of vulvar ELP
Lichen planus & hypertrophic lichen planus
Hypertrophic lichen planus: 5 minute pathology pearls
Lichen planus
- Vulva, labium majus, left, punch biopsy:
- Findings consistent with lichen planus (see comment)
- Comment: Sections show epidermal acanthosis, hypergranulosis and a lichenoid chronic inflammatory infiltrate with Civatte bodies. No basal atypia is seen. A special stain for PASF is negative for fungal organisms. A p53 immunohistochemical stain shows a wild type staining pattern. The morphologic features are consistent with lichen planus. Clinicopathologic correlation is recommended.
- Lichenoid drug eruption:
- Parakeratosis and dyskeratosis
- Perivascular and periadnexal inflammatory infiltrate composed of plasma cells and eosinophils
- Early lichen sclerosus:
- Psoriasiform epidermal hyperplasia
- Superficial band-like chronic inflammation with lymphocytes extravasation into lower epidermis
- Papillary dermal fibrosis
- Vacuolization of basal epidermal layer
- Syphilis:
- Minimal dyskeratosis
- Early infection: neutrophilic infiltrate in early infection
- Granulomatous inflammation later in the disease
- Deep perivascular plasmacytic infiltrate
- Endothelial cell swelling (endarteritis)
- Positive T. pallidum immunohistochemistry or Warthin-Starry silver stain
- Plasmacytosis mucosae (Zoon vulvitis):
- 1 - 3 cm isolated brown-red patch or plaque
- ≥ 50% polyclonal plasmacytic infiltrate
- Loss of stratum granulosum with mild spongiotic change
- Red cell extravasation with hemosiderin deposition
- High grade squamous intraepithelial lesion (HSIL):
- Full thickness epithelial atypia with acanthosis, papillomatosis, suprabasal mitoses, extension in hair follicles and skin appendages
- Diffuse, block-like p16 expression
- Differentiated vulvar intraepithelial neoplasia (dVIN):
- Basal atypia with enlarged, hyperchromatic nuclei, prominent nucleoli, prominent intercellular bridges, eosinophilic cytoplasm
- Acanthosis, parakeratosis, elongation and anastomosis of rete ridges
- Aberrant p53 expression
- Desquamative inflammatory vaginitis:
- Purulent vaginal discharge with abnormal vaginal flora
- Mixed predominantly polymorphonuclear leukocytes in parabasal epithelial cells
- Thinning of adjacent epithelium
- Complete loss of p53
- Diffuse, block-like expression of p16
- Prior history of HPV independent squamous cell carcinoma
- Wild type p53 expression
Comment Here
Reference: Lichen planus
A 45 year old woman with a past medical history of hypothyroidism presents to the clinic with pain and burning discomfort in the vulvar area. On physical examination, a well delineated red plaque in the vaginal introitus is observed extending onto the labia majora. There is no scarring or obvious vaginal inflammation. A punch biopsy was obtained. PASF shows no fungal elements. Based on the microphotograph above and clinical information provided, what is the most likely diagnosis?
- Fixed drug reaction
- High grade squamous intraepithelial lesion (HSIL)
- Syphilis
- Vulvar hypertrophic lichen planus
Comment Here
Reference: Lichen planus
- Immune mediated chronic fibroinflammatory condition of vulvar skin
- Most commonly postmenopausal at onset; rarely can occur in children
- Lichenoid interface inflammatory reaction
- Hyalinization and homogenization of the superficial dermal collagen with displacement of inflammatory cells downward, below the abnormal collagen layer
- Epidermis is usually thin (atrophic)
- Lichen sclerosus et atrophicus
- Alternative spelling: lichen sclerosis
- Typically seen in postmenopausal women (Climacteric 2017;20:339)
- Associated with other autoimmune disorders (J Eur Acad Dermatol Venereol 2010;24:1031)
- Vulvar skin
- Can involve perianal skin
- Typically does not involve vaginal mucosa but focal extension from vulvar skin onto the adjacent mucosa may be seen
- Cell mediated immune response with associated degenerative changes of the basal keratinocytes
- Secondary fibrosis of the superficial dermis, leading to a subepithelial hypocellular band of homogenous appearing collagen
- Autoimmune (Int J Biol Sci 2019;15:1429)
- May be familial (J Eur Acad Dermatol Venereol 2010;24:1031)
- Intensely pruritic
- May become excoriated
- Associated with increased risk of developing human papillomavirus (HPV) independent vulvar intraepithelial neoplasia (VIN) and squamous cell carcinoma (Int J Cancer 2017;140:1998)
- Vulvar skin becomes thinned (cigarette paper appearance), with destruction of normal anatomic landmarks as the disease progresses
- Characteristic clinical findings are highly suggestive but a biopsy can provide a definitive diagnosis if there is diagnostic uncertainty and also rule out neoplasia (e.g., VIN or Paget disease)
- Early diagnosis and treatment may prevent disease progression
- 44 year old woman with bullous lichen sclerosus (Dermatol Online J 2018;24:13030)
- 50 year old woman with lichen sclerosus undergoing treatment with nivolumab (Dermatol Online J 2020;26:13030)
- 60 and 61 year old women with lichen sclerosus with vaginal involvement (Facts Views Vis Obgyn 2017;9:171)
- 66 year old woman with lichen sclerosus after treatment for vulvar squamous cell carcinoma (Gynecol Oncol Rep 2017;20:73)
- Topical corticosteroids (first line), with topical calcineurin inhibitor therapy as second line (Am J Clin Dermatol 2013;14:27)
- Diagnosis is made based on small biopsy specimens
- May be an incidental finding in a resection specimen (e.g., for vulvar squamous cell carcinoma)
- Vacuolar interface reaction pattern in conjunction with dermal sclerosis (homogenized and hyalinized eosinophilic collagen bundles) of any thickness intervening between inflammatory infiltrate and epithelium or vessel walls (Mod Pathol 1998;11:844)
- Early lesions show only the inflammation and no or minimal fibrosis (inflammatory phase); the histopathological findings at this stage of disease development are not diagnostic
- Severe hyperkeratosis; thin epidermis, loss of rete pegs, basal cell degeneration, homogenized band of dense fibrosis at papillary dermis, upper dermal edema, band-like chronic inflammation
- In early stages, findings are subtle and often more prominent in adnexal structures than in interfollicular skin; adnexal structures show acanthosis, luminal hyperkeratosis and hypergranulosis
- Early dermal changes are homogenized collagen and wide ectatic capillaries in dermal papillae immediately beneath basement membrane
- Superficial dermal collagen may be wire-like with lymphocyte entrapment (J Cutan Pathol 2015;42:510)
- Lymphocytic infiltrate can be sparse or dense, lichenoid or interstitial with epidermal lymphocyte exocytosis
- Erosions or ulceration can occur (J Low Genit Tract Dis 2021;25:255)
- Wild type pattern staining for p53
Introduction to lichen sclerosus
- Right labium majus, biopsy:
- Lichen sclerosus (see comment)
- Comment: This vulvar biopsy shows established lichen sclerosus. Negative for dysplasia or malignancy.
- HPV independent vulvar intraepithelial neoplasia (VIN):
- Other terminology: differentiated VIN (dVIN), differentiated exophytic vulvar intraepithelial lesion (DEVIL), vulvar acanthosis with altered differentiation (VAAD), vulvar altered maturation (VAM)
- Shows epithelial hyperplasia and loss of normal maturation; often shows significant basal atypia and may show mutant pattern p53 immunostaining (Mod Pathol 2011;24:297)
- Lichen planus:
- Clinically, the presence of erosions, oral involvement, a burning sensation or a hyperkeratotic lesional margin favor a diagnosis of lichen planus over lichen sclerosus (Australas J Dermatol 2020;61:324)
- Typically involves mucosa or nonhair bearing skin
- Subepithelial band-like inflammatory infiltrate is directly under the squamous epithelium, without a separating area of fibrosis / sclerosis
- Pointed rete ridges are more common in lichen planus, while the presence of epidermal atrophy or basal lamina thickening favor lichen sclerosus (Am J Surg Pathol 1998;22:473)
- Early lesions of lichen sclerosus, before the fibrosis becomes established, can be difficult or impossible to distinguish from lichen planus; such cases can be signed out descriptively, indicating that follow up, with or without rebiopsy, should allow for definitive diagnosis
- Lichen planus can coexist with lichen sclerosus (J Low Genit Tract Dis 2017;21:204)
- Lichen simplex chronicus:
- Common; spares the vaginal mucosa (Dermatol Clin 2010;28:669)
- Epidermal hyperplasia is present, rather than atrophic changes, with no degenerative changes of the basal epithelial layer and no superficial subepidermal sclerosis
- Excoriation is common and may lead to subepidermal scarring but with variably sized collagen bundles and not the homogenized sclerotic band of lichen sclerosis
- Spongiosis / spongiotic dermatitis may be present but is not necessary for diagnosis (Int J Womens Dermatol 2017;3:58)
- Hypergranulosis is common
- High grade squamous intraepithelial lesion (HSIL / VIN3)
- Human papillomavirus (HPV) independent vulvar intraepithelial neoplasia (VIN)
- Lichen planus
- Psoriasis
Comment Here
Reference: Lichen sclerosus
- Recently described entity that occurs in the vulva and shares remarkable histologic overlap with lipoblastoma and myxoid liposarcoma
- These lesions have been shown to have Rb loss like the spindle cell lipoma family of tumors
- Does not have PLAG1 or FUS rearrangements (Int J Gynecol Pathol 2019;38:204, Am J Surg Pathol 2015;39:1290)
- Has only a limited ability to locally recur and these lesions should be distinguished from myxoid liposarcoma
- Occurs along primitive milk line, from axilla to groin; may be source of papillary hidradenoma
- Mammary-like anogenital glands are now considered a normal constituent of the perineum rather than ectopic tissue (Adv Anat Pathol 2011;18:1)
- Histology consistent with breast carcinoma
- Nonneoplastic breast tissue or DCIS present in vulva
- Supportive immunohistochemistry (ER+, PR+, GCDFP-15+, HER2+)
- No carcinoma in ordinary breast tissue or other organs
- Inability to locate other primary carcinoma of vulvar glands or skin appendages
- 28 year old woman with vulvar lactating adenoma and fibroadenoma (Pan Afr Med J 2012;13:47)
- 42 year old pregnant woman with ulcerated nodule on labia (Case of the Week #273)
- 43 and 50 year old women with phyllodes tumors (Turk Patoloji Derg 2013;29:73)
- 47 year old woman with mucinous (colloid) adenocarcinoma (Arch Pathol Lab Med 2002;126:1216)
- 58 year old woman with synchronous Paget disease of breast and vulva (Eur J Gynaecol Oncol 2012;33:534)
- Lactational ectopic breast tissue of vulva (Breastfeed Med 2013;8:223)
- Multiple lactating vulvar adenomas (Obstet Gynecol 2011;118:478)
- Primary malignant melanocytic tumor
- Malignant melanocytic tumor arising from melanocytes in mucosal or cutaneous areas of the lower genital tract
- Tumor thickness is the most important prognostic factor
- Malignant melanoma (MM)
- Primary malignant melanoma of vulva
- Primary malignant melanoma of vagina
- Primary malignant melanoma of lower genital tract
- ICD-O
- ICD-10
- ICD-11
- 2C70 - malignant neoplasm of the vulva
- Specific anatomy (use additional code, if desired)
- 2C71 - malignant neoplasms of vagina
- Specific anatomy (use additional code, if desired)
- 2C7Y - other specified malignant neoplasms of female genital organs
- 2C7Z - malignant neoplasms of female genital organs, unspecified
- Accounts for Lancet Oncol 2008;9:973)
- 200 of 100,000 (0.2%) women per year will be diagnosed with vulvar melanoma (Obstet Gynecol 2007;110:296, Gynecol Oncol 2011;122:612)
- Difference in incidence of vulvar and vaginal melanomas among ethnic groups is significantly smaller than in cutaneous melanomas of other sites (Melanoma Res 2010;20:153)
- Vulvar melanoma
- Although vulvar melanomas are rare, accounting for 1 - 2% of all melanomas, they represent the most common primary genitourinary (GU) melanomas and primary gynecologic melanomas (J Natl Compr Canc Netw 2016;14:450, Case Rep Oncol 2021;14:1144, J Am Acad Dermatol 2014;71:1241, J Am Acad Dermatol 2016;75:144, Urol Oncol 2016;34:166.e7)
- Review of the Survival Epidemiology and End Results (SEER) database between 1973 - 2010 indicates that 75% of primary GU melanomas are vulvar and 25% are vaginal (Urol Oncol 2016;34:166.e7)
- Melanoma is the second most common vulvar malignancy following squamous cell carcinoma (Am J Surg Pathol 2002;26:1450, Lancet Oncol 2008;9:973, Obstet Gynecol 2007;110:296)
- Occurs predominantly in postmenopausal White patients in the fifth to eighth decades (Obstet Gynecol 2007;110:296, J Am Acad Dermatol 2014;71:1241)
- Association with lichen sclerosus has been reported in the pediatric population (Cancers (Basel) 2022;14:5217, Pediatr Dermatol 2016;33:e190, Pediatr Dermatol 2004;21:473, Am J Dermatopathol 1984:6:253, Arch Dermatol 1997;133:345)
- Although vulvar melanomas are rare, accounting for 1 - 2% of all melanomas, they represent the most common primary genitourinary (GU) melanomas and primary gynecologic melanomas (J Natl Compr Canc Netw 2016;14:450, Case Rep Oncol 2021;14:1144, J Am Acad Dermatol 2014;71:1241, J Am Acad Dermatol 2016;75:144, Urol Oncol 2016;34:166.e7)
- Vaginal melanoma: occurs in postmenopausal women in their fifth to seventh decades (J Gynecol Oncol 2013;24:330, Int J Gynecol Cancer 2014;24:149)
- Vulvar melanoma
- Labia majora and labia minora > periclitoral / clitoris > midline structures (e.g., periurethral, introitus and posterior fourchette) (Ann Surg Oncol 2015;22:1959)
- Most common: glabrous (hairless, mucosal) site in about 50% of cases, followed by hairy - glabrous skin junction (38%) and hairy skin (13%) (Acta Oncol 2004;43:421)
- Vaginal melanoma
- Most common: lower third of vagina, in the anterior wall (J Cancer Res Ther 2019;15:1392)
- Pathogenesis of all subtypes is poorly understood (Biomedicines 2021;9:758)
- Vulvar melanoma (sun protected cutaneous melanomas, majority originating in mucosal epithelium)
- Similar pathway to cutaneous melanomas arising in sun protected areas (e.g., acral and subungual melanomas)
- It has been hypothesized that a single melanocyte can act as a spontaneous promoter
- Vitamin D receptor (VDR) expression has been implicated (Am J Cancer Res 2020;10:4017)
- Molecular profile differs from cutaneous melanomas (J Low Genit Tract Dis 2023;27:40)
- Rate of KIT and NRAS mutations is higher than in cutaneous melanomas
- Both KIT and NRAS are upstream to BRAF and part of Ras / Raf / MEK / ERK pathway
- BRAF is relatively rare (J Low Genit Tract Dis 2015;19:350)
- Vaginal melanomas (J Low Genit Tract Dis 2023;27:40)
- Although the data on strictly vaginal melanomas are limited and typically grouped with vulvar melanomas, KIT mutations appear to play a role in pathogenesis (Melanoma Res 2014;24:360, Int J Gynecol Pathol 2020;39:587, Br J Dermatol 2017;177:1376)
- Unlike cutaneous melanomas, ultraviolet (UV) radiation exposure does not appear to impact disease onset (J Am Acad Dermatol 2014;71:366, J Cutan Pathol 2005;32:191)
- HPV infection may play a role (Oncologist 2021;26:e473, Am J Dermatopathol 2007;29:13)
- Clinical presentation is often delayed due to a variety of symptoms and rare self inspection (Gynecol Oncol 2021;161:202)
- Symptoms are nonspecific (Cancers (Basel) 2022;14:5217, Asian J Urol 2022;9:407, Gynecol Oncol 2021;161:202, Obstet Gynecol 2007;110:296)
- Early symptoms: pruritis, spontaneous bleeding or atypical discharge
- May present as a distinct mass, swelling or lump sensation
- Lymphadenopathy in advanced cases
- May be asymptomatic (J Low Genit Tract Dis 2016;20:e24, Melanoma Res 2008;18:127)
- On physical examination (Cancers (Basel) 2022;14:5217, Asian J Urol 2022;9:407, Gynecol Oncol 2021;161:202, Obstet Gynecol 2007;110:296)
- ABCD: asymmetry, border irregularity, color variation / discoloration, diameter (concerning, > 6 mm)
- 25 - 30% of cases are amelanotic, particularly on mucosal surfaces where melanoma may appear as a nonpigmented red polyp (J Am Acad Dermatol 2004;50:554, Dermatol Ther 2010;23:449, Melanoma Res 2008;18:127)
- May be multifocal (Dermatol Ther 2010;23:449)
- See table 1 and table 2
Table 1: Clinical and histologic differences between atypical genital nevus and vulvar melanoma (adapted from Hoang: Melanocytic Lesions - A Case Based Approach, 2014)
| Proposed diagnosis | Atypical genital nevus of special anatomic site | Vulvar melanoma |
| Age | Premenopausal, young adult | Postmenopausal |
| Size | > 1 cm | |
| Delineation | Well circumscribed | Infiltrative |
| Symmetry | Present | Absent |
| Lateral extension of junctional component | Focal | Present |
| Lentiginous junctional component | Focal | Present |
| Junctional nests | Discohesive | Confluent |
| Retraction artefact | Present | Absent |
| Ulceration | Absent or due to trauma | Often present |
| Pagetoid upward spread | Focal, central, inconspicuous | Prominent |
| Cytologic atypia | Superficial, mild to moderate | Deep, moderate to severe |
| Dermal mitoses | Rare and superficial | Conspicuous, atypical, deep |
| Dermal maturation | Present | Absent |
| Melanin pigmentation | Coarse, uniform | Fine, irregular |
| Dermal fibrosis | Broad zone of superficial coarse dermal fibrosis | Regression type |
Table 2: Clinical differences in mucosal melanoma of the vulva and vagina (adapted from Ann Surg Oncol 2015;22:1959 and Am J Cancer Res 2020;10:4017)
| Proposed diagnosis | Vulvar melanoma | Vaginal melanoma | |||
| Frequency | 3 - 10% of vulvar tumors | 4 - 5% of vaginal tumors | |||
| Age | Postmenopausal (50 - 70 years) | Postmenopausal (50 - 70 years) | |||
| Location | Labia majora and labia minora > periclitoral / clitoris > midline structures (e.g., periurethral, introitus and posterior fourchette)* | Lower vagina | |||
| Presentation | Bleeding, abnormal discharge, pruritis or visible exophytic mass to the patient | Similar to vulvar melanoma; polypoid mass may be only seen on closer gynecologic examination | |||
| Prognosis | Poor; 5 year survival of 25 - 60% | Very poor; 5 year survival of | Histologic growth patterns | Lentiginous (most common) Superficial spreading (less common) Nodular and pagetoid (rare) | Nodular (most common) |
| Differential diagnosis | Poorly differentiated carcinoma Metastatic melanoma Melanocytic nevus Lymphoma Soft tissue neoplasm | Poorly differentiated carcinoma Metastatic melanoma Lymphoma Soft tissue neoplasm |
- Dermoscopy for nonmodified mucous membranes is useful
- Dermoscopic evaluation aids in avoiding unnecessary biopsies (Arch Dermatol 2011;147:1181, Dermatology 2011;222:157)
- Combination of blue, gray or white color with structureless zones and atypical vascularization is highly predictive of melanoma (Arch Dermatol 2011;147:1181, Dermatology 2011;222:157)
- In the absence of melanoma specific dermoscopic features, shiny white streaks (SWS), inverse pigment network and vascular network in a symmetrical pattern can be seen in atypical melanocytic nevi of genital type (AMNGT) (Dermatology 2011;222:157, Dermatology 2010;221:55)
- Experienced dermatologist will take a biopsy of clinically or dermoscopically concerning lesions
- See table 3
- Histologic diagnosis is confirmatory
Table 3: Differential diagnosis of vulvar melanocytic nevi, melanosis and melanoma (adapted from J Am Acad Dermatol 2014;71:1241)
| Reassuring features suggestive of a benign process | Concerning features for possible malignancy | |
| Clinical |
|
|
| Dermoscopy |
|
|
| Reflectance confocal microscopy |
|
|
- Lactate dehydrogenase (LDH) has been suggested as a potential predictive and prognostic biomarker (Cell Mol Biol Lett 2020:25:35, Cancer Immunol Immunother 2014;63:449)
- This has not been widely adopted and may lack sensitivity and specificity depending on LDH isoform utilized
- Recurrence rate
- Overall recurrence rate of 60% (Gynecol Oncol 2021;161:202, J Low Genit Tract Dis 2016;20:e24, Int J Gynecol Cancer 2016;26:1307)
- Independent prognostic factor for local recurrence: tumor size (Int J Gynecol Cancer 2016;26:1307)
- Independent prognostic factor for distant recurrence: American Joint Committee on Cancer (AJCC) stage and lymph node involvement (Int J Gynecol Cancer 2016;26:1307, N Engl J Med 2017;376:2211, Ann Surg Oncol 2000;7:738)
- Survival (Cancers (Basel) 2022;14:5217)
- Clinical presentation
- Central vulvar involvement is associated with worse overall survival (OS) and increased lymph node involvement (Clin Cancer Res 2017;23:2093, Urol Oncol 2016;34:166.e7)
- Multifocal involvement is associated with worse overall survival (Urol Oncol 2016;34:166.e7)
- Histopathologic factors
- 5 year overall survival (5Y OS) is associated with number of mitoses and lymph node status (J Low Genit Tract Dis 2016;20:e24, Ann Surg Oncol 2000;7:738)
- Factors independently associated with overall survival and disease specific survival (DSS): tumor thickness (≤ 2 mm2 or > 2 mm2) and dermal mitotic rate (2 or ≥ 2 mm2) (Clin Cancer Res 2017;23:2093)
- Independent prognostic factor: tumor infiltrating lymphocytes (Cancer Med 2018;7:583, J Am Acad Dermatol 2017;76:264)
- Ulceration is associated with worse survival (Cancer Med 2018;7:583, J Am Acad Dermatol 2017;76:264)
- Lymphovascular invasion (LVI) is associated with positive sentinel lymph nodes (Cancer Med 2018;7:583, J Am Acad Dermatol 2017;76:264)
- Stage by AJCC 2017 (Am J Clin Dermatol 2021;22:639)
- 5 year disease specific survival (5Y DSS) decreased from 32.3% at stage I to 4.9% at stage IV
- 5 year overall survival decreased from 73.6% to 3.9%, at stage I to stage IV, respectively
- Other prognosticators
- Age is a widely accepted independent prognostic factor for 5 year overall survival, disease free survival (DFS) and overall survival (Obstet Gynecol 2007;110:296, Int J Gynecol Cancer 2013;23:1118, Lancet Oncol 2016;17:757, Clin Cancer Res 2017;23:2093, Urol Oncol 2016;34:166.e7)
- See table 4 and table 5
- Clinical presentation
Table 4: Revised 2017 AJCC TNM staging for melanoma
| Primary tumor (T) | ||
| TX | Primary tumor thickness cannot be assessed (i.e., diagnosis by curettage) | |
| T0 | No evidence of primary tumor (i.e., axillary metastases without known primary tumor) | |
| Tis | Intraepithelial (i.e., melanoma in situ) | |
| T1 | Tumor ≤ 1.0 mm thick, without or with ulceration | |
| T1a | ≤ 0.8 mm thick and Clark level II or III, without ulceration | |
| T1b | 0.8 - 1.0 mm thick and Clark level IV or V, with or without ulceration | |
| T2 | Tumor 1.01 - 2.0 mm thick, without (T2a) or with ulceration (T2b) | |
| T3 | Tumor 2.01 - 4.0 mm thick, without (T3a) or with ulceration (T3b) | |
| T4 | Tumor > 4.0 mm thick without (T4a) or with (T4b) ulceration | |
| Regional lymph node (N) | ||
| Nx | Regional lymph nodes cannot be assessed | |
| N0 | No regional lymph node metastasis | |
| N1 | Metastasis to 1 lymph node or in transit, satellite or microsatellite metastases with no tumor involved nodes | |
| N1a | Clinically occult (i.e., detected by SLNB) | |
| N1b | Clinically apparent (i.e., macroscopic) | |
| N1c | In transit, satellite or microsatellite metastases with no tumor involved nodes | |
| N2 | Metastasis to 2 or 3 regional lymph nodes or in transit, satellite or microsatellite metastases with no tumor involved nodes | |
| N2a | Clinically occult (i.e., detected by SLNB) | |
| N2b | Clinically apparent (i.e., macroscopic) | |
| N2c | In transit, satellite or microsatellite metastases combine with 1 clinically occult or apparent | |
| N3 | Metastasis to 4 or more regional lymph nodes, matted lymph nodes or combination of in transit metastasis or satellite(s) and metastatic regional lymph node(s) | |
| N3a | Clinically occult (i.e., detected by SLNB) | |
| N3b | Clinically apparent (i.e., macroscopic) or presence of matted nodes | |
| N3c | In transit, satellite or microsatellite metastases combine with 2 or more clinically occult or clinically detected or presence of matted nodes | |
| Distant metastasis (M) | ||
| Mx | Distant metastasis cannot be assessed | |
| M0 | No distant metastasis | |
| M1 | Distant metastasis, LDH status (designated as 0 for not elevated and I for elevated level; no suffix is used if LDH is not recorded or is unspecified) | |
| M1a | Distant skin, subcutaneous or lymph node | |
| M1b | Lung | |
| M1c | All other non-central nervous system (CNS) visceral sites | |
| M1d | CNS | |
Table 5: 2017 eighth AJCC prognostic stage group for cutaneous melanoma
| Stage | T | N | M |
| 0 | Tis | N0 | M0 |
| IA | T1a | N0 | M0 |
| IB | T1b | N0 | M0 |
| T2a | |||
| IIA | T2b | N0 | M0 |
| T3a | |||
| IIB | T3b | N0 | M0 |
| T4a | |||
| IIC | T4b | N0 | M0 |
| IIIA | T1a, T1b | N1, N2a | M0 |
| T2a | |||
| IIIB | T0 | N1b, N1c | M0 |
| T1a / b - T2a | N1b / c, N2b | ||
| T2b / T3a | N1a - N2b | ||
| IIIC | T0 | N2b / c, N3b / c | M0 |
| T1a - T3a | N2c, N3 | ||
| T3b / T4a | Any N ≥ N1 | ||
| T4b | N1a - N2c | ||
| IIID | T4b | N3 | M0 |
| IV | Any T, Tis | Any N | M1 |
- 18 year old Black woman with widely metastatic melanoma originating from a primary vulvar lesion (Pediatr Dermatol 2023;40:749)
- 47 year old woman with metastatic vulvar melanoma, harboring an exon 17 KIT mutation (Ther Adv Med Oncol 2020:12:1758835920946158)
- 47 year old woman with vulvar melanoma and a family history of cutaneous melanoma (Cancer Genet 2022:262:102)
- 51 year old woman with recurrent vulvar melanoma with neurofibromatosis and postmenopausal bleeding (BMJ Case Rep 2019;12:e224744)
- 54 year old gravida 2 para 0 woman with recurrent metastatic vaginal melanoma (Gynecol Oncol Rep 2019:30:100508)
- 69 year old woman with melanoma of the vulva treated with topical imiquimod (Gynecol Oncol Rep 2021:38:100875)
- 73 year old woman with metastatic vaginal mucosal melanoma (Gynecol Oncol 2021;161:645)
- 84 year old woman with primary vulvar melanoma and melanocytic metastatic tissue (Curr Oncol 2022;29:3130)
- 89 year old woman with primary amelanotic vaginal melanoma (Diagn Cytopathol 2019;47:230)
- Vulvar melanoma
- Clinical management is mainly based on cutaneous melanomas (Eur J Cancer 2017:81:36, Int J Gynecol Cancer 2014;24:S117, Cancers (Basel) 2022;14:5217)
- For localized disease: wide local excision (WLE) with adequate surgical margins
- Sentinel lymph node biopsy (SLNB) use is recommended (Cancers (Basel) 2022;14:5217)
- If there is clinical or radiologic evidence of positive lymph nodes
- If Breslow thickness is > 4 mm (J Obstet Gynaecol Can 2019;41:762, Eur J Cancer 2020:135:22, Int J Gynecol Cancer 2014;24:S117)
- Benefit of SLNB in intermediate thickness (1 - 4 mm) is unclear (Int J Gynecol Cancer 2019;29:1077)
- Bilateral SLNB is appropriate if the tumor is 2 cm from the midline (Int J Gynecol Cancer 2019;29:1077, Cancers (Basel) 2022;14:5217)
- If SLNB is positive, the benefit of complete groin dissection is currently unclear; it will be based on a multidisciplinary treatment plan and individual circumstances (Cancers (Basel) 2022;14:5217)
- DeCOG-SLT study suggested a complete groin dissection only for SLN metastases > 1 mm (Lancet Oncol 2016;17:757)
- In case of inguinofemoral metastases, an elective lymph node dissection (ELND) should be considered (Eur J Cancer 2020:135:22, Int J Gynecol Cancer 2014;24:S117)
- Data on adjuvant and neoadjuvant therapy in mucosal melanoma is based on retrospective case series, which makes the development of clinical guidelines challenging (Cancers (Basel) 2022;14:5217, Am J Cancer Res 2020;10:4017)
- Personalized therapy is based on the success of cutaneous melanoma treatment and is currently under investigation (Cancers (Basel) 2022;14:5217, Biomedicines 2021;9:758, Am J Cancer Res 2020;10:4017)
- Immunotherapy: programmed death 1 (PD-1), cytotoxic T lymphocyte antigen (CTLA4) checkpoint inhibitors show some success in locally advanced tumors and tumors with distant metastases; however, the clinical data is limited and the trials are ongoing (Immunotargets Ther 2018:7:35)
- Potential targeted therapy: BRAF, c-KIT, and MEK inhibitors with c-KIT show limited success in comparison to cutaneous melanoma (Ther Adv Med Oncol 2020:12:1758835920946158, N Engl J Med 2010;363:711, Br J Cancer 2010;102:1219)
- Chemotherapy: currently considered second line treatment (Cancers (Basel) 2022;14:5217, Am J Cancer Res 2020;10:4017)
- Radiation therapy plays a limited role and its utility remains to be investigated (Cancers (Basel) 2022;14:5217, Am J Cancer Res 2020;10:4017)
- See diagram 1 for a summary of vulvar melanoma management (Am J Cancer Res 2020;10:4017)
- Vaginal melanoma
- Complete local resection with adequate surgical margins (1 - 2 cm based on Breslow thickness) (Ann Surg Oncol 2004;11:34, Ann Surg Oncol 2002;9:840)
- Radical surgery alone or in combination with adjuvant radiotherapy can be considered in cases where complete resection is not feasible, the tumor is closely approaching resection margin, the tumor is large (> 3 cm) or the lymph nodes are positive (Ann Surg Oncol 2004;11:34, J Gynecol Oncol 2013;24:330)
- Groin or pelvic lymphadenectomy is recommended with evidence of positive lymph nodes on clinical or radiologic evaluation (Am J Cancer Res 2020;10:4017)
- SLNB utility is currently unclear (Am J Cancer Res 2020;10:4017)
- Role of chemotherapy remains to be determined (Am J Cancer Res 2020;10:4017)
- Personalized therapy may be considered with targetable mutations (Biomedicines 2021;9:758)
- Potential targeted therapy to be investigated: c-KIT, SF3B1, NRAS, BRAF
- PD-1 and CTLA4 checkpoint inhibitors have been utilized with mixed success and their utility in specifically vaginal melanoma remains to be determined (J Gynecol Oncol 2019;30:e94, J Low Genit Tract Dis 2021;25:146, Cancers (Basel) 2022;14:5217, J Eur Acad Dermatol Venereol 2018;32:e39)
- See diagram 2 for treatment strategies for vaginal melanoma (Am J Cancer Res 2020;10:4017)
- See table 6 for treatment strategies based on melanoma subtype
Table 6: Clinicopathologic correlates of melanoma in the lower genital tract (adapted from Am J Cancer Res 2020;10:4017)
| Subtype | Sun protected cutaneous vulvar melanoma | Mucosal melanoma (vulvar and vaginal) |
| Molecular alterations | BRAF, NRAS, TERT, CDKN2A, PTEN | KIT, NRAS, BRAF, NF1, CDKN2A, TERT, PTEN, SF3B1 |
| Metastatic pattern | Initially involving regional lymph nodes, distant metastases (lung, brain) at later stage | More likely to develop distant metastases (lung, liver, brain) |
| Surgical modality | Wide local excision with adequate margins | Complete excision |
| Lymph node assessment | Sentinel lymph node biopsy, regional lymphadenectomy if needed | Sentinel lymph node biopsy or routine regional lymphadenectomy not recommended |
| Systemic therapy | ||
| Chemotherapy | Interferon, dacarbazine, paclitaxel | Cisplatin, vinblastine, dacarbazine, interferon |
| Radiotherapy | Neoadjuvant or adjuvant | Palliative treatment of local or metastatic disease |
| Targeted therapy | BRAF and MEK inhibitors | BRAF and c-KIT inhibitors |
| Immunotherapy | Programmed cell death protein 1 (PD-1) and cytotoxic T lymphocyte antigen 4 (CTLA4) checkpoint inhibitors | |
| Palliative therapy | Palliative surgery or radiotherapy | |
| Prognosis factors | AJCC staging, Breslow thickness, lymph node status, distant metastases | Breslow thickness, depth of invasion, lymph node status, distant metastases |
- Vulvar melanoma
- Usually presents as a pigmented plaque or amelanotic ulcerated nodule (Cancers (Basel) 2022;14:5217, Dermatol Ther 2010;23:449)
- Vaginal melanoma (Asian J Urol 2022;9:407)
- Usually presents as a large, ulcerated, nodular mass, often extending to the cervix
- Rarely, an associated nevus may be present
- Vulvar melanoma
- Per AJCC and College of American Pathologists (CAP), vulvar melanoma are staged as primary cutaneous melanomas
- 25 - 30% of tumors are amelanotic (Cancer 1999;86:1273, J Am Acad Dermatol 2004;50:554, Dermatol Ther 2010;23:449, Melanoma Res 2008;18:127)
- Radial growth pattern (lentiginous / mucosal and superficial spreading) >> vertical growth pattern (nodular) > unclassified (Cancer 1999;86:1273, J Am Acad Dermatol 2012;67:598)
- Most tumors are asymmetric or ill defined lesions (Ann Diagn Pathol 2018:37:91)
- Lentiginous pattern (Cancer 1999;86:1273, J Am Acad Dermatol 2012;67:598, Ann Diagn Pathol 2018:37:91)
- Solitary units of melanocytes with nuclear atypia irregularly distributed along the dermoepidermal junction (DEJ)
- Occasional nests with skip areas and consumption of the rete ridges
- Adnexal involvement by isolated melanocytes or small nests is common
- Follicular extension along the epithelial stromal junction may be evident
- Overlying epidermis may resemble lichen planus with
- Hyperkeratosis and hypergranulosis
- Focal epidermal atrophy
- Dense lichenoid infiltrate
- Superficial spreading type (Cancer 1999;86:1273, J Am Acad Dermatol 2012;67:598, Ann Diagn Pathol 2018:37:91)
- In situ component shows isolated cells and nests of atypical epithelioid melanocytes spreading into spinus layer in a pagetoid pattern
- Variable pagetoid patterns (J Am Acad Dermatol 2012;67:598)
- Amelanotic pagetoid melanoma in situ may closely resemble extramammary Paget disease and Bowen disease
- Immunohistochemical studies will support a definitive diagnosis
- Epithelioid morphology is most common, followed by mixture of epithelioid and spindle cells, although pure spindle cell melanomas have been described (Am J Surg Pathol 2004;28:1518)
- Effacement of dermal rete with moth eaten appearance
- Dermal component shows no maturation with severely atypical epithelioid or spindle cells with brisk mitoses forming sheets, nests, cords, single cells and rarely fascicles (Cancer 1999;86:1273, J Am Acad Dermatol 2012;67:598, Ann Diagn Pathol 2018:37:91)
- Nodular subtype (J Am Acad Dermatol 2013;68:568)
- In situ component should not extend beyond the periphery of the invasive tumor nodule; therefore, caution should be exercised in small biopsies
- Excisional specimens are the most helpful to examine the entire epidermis adjacent the nodular component
- May display a wide variation of architectural and cytologic features
- Usually polypoid / exophytic but can present with endophytic / infiltrative growth
- Nests with a mixture of fascicles and high cell density with solid growth pattern
- Ulceration, necrosis and LVI are common
- Desmoplastic (very rare but can be a diagnostic pitfall) (Pathology 1997;29:241, Gynecol Oncol 1997;64:180, Pathologica 2011;103:337)
- May induce an extensive desmoplastic reaction (Am J Surg Pathol 2004;28:1518, Ann Diagn Pathol 2018:37:91)
- Typically paucicellular with mild cytologic atypia and low mitotic activity
- Pure desmoplastic melanoma contains single spindle cells arranged in small nests or short fascicles embedded in the desmoplastic stroma
- Mixed / combined desmoplastic melanoma contains an additional epithelioid or spindled invasive dermal component (Ann Diagn Pathol 2018:37:91, Pathologica 2011;103:337)
- Peripheral lymphoid aggregates on low power are a soft sign of desmoplastic melanoma (Ann Diagn Pathol 2018:37:91)
- Perineural invasion is more common (Am J Surg Pathol 2004;28:1518, Ann Diagn Pathol 2018:37:91)
- Unless it is paucicellular (early onset) of lentiginous pattern, vulvar melanoma is usually associated with variable lymphocytic (typically lymphoplasmacytic) infiltrate
- In the absence of malignant cells, dermal fibrosis, increased vascularity and lymphocytic infiltrate are indicative of regression
- See table 7
Table 7: AJCC versus CAP features recommended for histopathologic assessment of primary vulvar melanomas (adapted from CAP: Protocol for the Examination of Specimens From Patients With Melanoma of the Skin [Accessed 30 November 2023])
| Histopathologic feature | AJCC recommended | CAP recommended |
| Histologic type | Yes | |
| Clarka / anatomicb level | ||
| Breslowa / tumorb thickness | Yes | Yes |
| Radial (nontumorigenic) growth phase | ||
| Vertical (tumorigenic) growth phase | ||
| Mitotic rate (number/mm2) | Yes | Yes |
| Ulceration | Yes | Yes |
| Regression | ||
| Lymphovascular invasion | Yes | |
| Perineural invasion | ||
| Microscopic satellitosis | Yes | Yes |
| Tumor infiltrating lymphocytes | ||
| Associated precursor melanocytic nevus | ||
| Predominant cytology | ||
| Margins | Yes |
b Used in evaluation of primary melanomas of mucosal origin
Table 8: Clark and Chung classifications comparative aspects (adapted from Biomedicines 2021;9:758, Ann Surg 1970;172:902, Cancer Res 1969;29:705, Obstet Gynecol 1975;45:638)
| Level of invasion | Clark classification Level of invasion of cutaneous melanoma | Chung classification Level of invasion of mucosal melanoma |
| I | Lesions involving only the epidermis (in situ melanoma) | Tumor confined to the epithelium (in situ melanoma) |
| II | Invasion of the papillary dermis, does not reach the papillary - reticular dermal interface | Invasion into the basement membrane to a depth of ≤ 1 mm |
| III | Invasion fills and expands the papillary dermis, does not extend to reticular dermis | Invasion to a depth of 1 - 2 mm |
| IV | Invasion into the reticular dermis, does not extend to the subcutaneous tissue | Invasion to a depth of > 2 mm, does not extend to the subcutaneous fat |
| V | Invasion through the reticular dermis into the subcutaneous tissue | Tumor penetrates the subcutaneous fat |
Contributed by Anna Sarah Erem, M.D., Gulisa Turashvili, M.D., Ph.D. and Priya Nagarajan, M.D., Ph.D.
- S100, SOX10 and nerve growth factor receptor (NGFR) are the most sensitive markers to visualize invasive melanoma
- Melanocytic markers
- MelanA (MART1) helps to highlight invasion and intraepidermal melanocytes (J Cutan Pathol 2020;47:446)
- Caution: staining of dendritic processes from melanocytes wrapped around keratinocytes may lead to overestimation and false positive interpretation
- HMB45 (Ann Diagn Pathol 2023:67:152211, Biomed Rep 2016;5:327, J Am Acad Dermatol 2012;67:446)
- Homogenous expression in primary conventional melanomas, especially epithelioid
- Absence of gradient or maturation pattern (does not diminish from top to bottom)
- May be weak or completely lost in spindle cell and desmoplastic melanomas
- MelanA (MART1) helps to highlight invasion and intraepidermal melanocytes (J Cutan Pathol 2020;47:446)
- SOX10 highlights junctional melanocytes to help differentiate melanoma in situ from benign counterparts (Appl Immunohistochem Mol Morphol 2014;22:142)
- PRAME (preferentially expressed antigen in melanoma) is typically diffusely and strongly expressed in melanomas (J Cutan Pathol 2020;47:1123)
- Melanomas of vulva usually lack BRAF mutation (Br J Dermatol 2010;162:677)
- p16: complete loss of expression is usually seen in melanomas
- Its utility in differentiation between benign and malignant melanocytic lesions has been questioned
- Current studies have not consistently separated melanomas arising from glabrous / mucosal from hair bearing vulva; hence, their molecular distinction is challenging (J Low Genit Tract Dis 2023;27:40)
- KIT mutations are the most common alterations in mucosal melanomas (J Clin Oncol 2006;24:4340, Melanoma Res 2014;24:360, J Low Genit Tract Dis 2023;27:40)
- Vulvar melanoma > vaginal melanomas >> cutaneous melanomas
- NRAS, NF1, TP53, TERT, CDKN2A, PTEN have also been identified
- BRAF mutation in vulvovaginal melanomas (J Low Genit Tract Dis 2023;27:40)
- Rare and identified in
- Fluorescence in situ hybridization (FISH) or comparative genomic hybridization to confirm or rule out melanoma
- Sensitivity of both tests may be lower than expected due to decreased cellularity in vulvar lesions
- For vulvar melanomas, pTERT and CDK4 can be considered but may not be part of each FISH panel (Nat Commun 2019;10:3163, Am J Cancer Res 2020;10:4017)
- See table 6 for clinicopathologic correlates
Vulvovaginal melanoma
by Dr. Lewis Hassell
Vaginal melanoma radiology
by Dr. Ayushi Gupta
Vaginal melanoma
- Vulva, right labium majus, excision:
- Melanoma, superficial spreading growth pattern, with features of regression (Breslow thickness = 1.7 mm, Clark level = IV; pT2a)
- Compound nevus
- Dermal scar
- All margins are free of melanoma (see comment and synoptic report)
- Comment: Immunohistochemical stains for MelanA performed on blocks A3 - A7 highlight the invasive melanoma. A BRAF V600E stain performed on block A6 is positive in the tumor cells.
- Vagina, radical hysterectomy and bilateral salpingo-oophorectomy:
- Vagina:
- Multifocal melanoma, nodular type (largest focus: 4.7 x 1.7 x 1.0 cm)
- Vaginal cuff margin involved by melanoma
- Cervix:
- Involved by melanoma (direct extension)
- Uterus:
- Endometrium: secretory
- Myometrium: adenomyosis
- Uterine serosa and cervix: benign
- Fallopian tubes: benign, fimbriated
- Ovaries: benign
- Vagina:
- Atypical melanocytic nevus of genital type (AMNGT):
- May be most challenging to differentiate from superficial spreading subtype of melanoma
- Symmetrical lesion with dermal component maturation
- Rare or very few atypical melanocytes in the epidermis as single cells with rare pagetoid spread, predominantly in the center of the lesion
- S100, SOX10 and nerve growth factor receptor (NGFR) fail to demonstrate invasive growth
- HMB45 highlights a gradient pattern (diminishing or loss of expression from top to bottom)
- PRAME is lost or very weakly expressed (J Cutan Pathol 2020;47:1123)
- BRAF is the most common alteration in AMNGT (N Engl J Med 2015;373:1926, J Invest Dermatol 2016;136:1858)
- Pigmented epithelioid melanocytoma (PEM) of the vulva:
- PEM family consists of multiple, usually slow growing, distinct histologic melanocytic entities with potential to metastasize but with a better prognosis than melanoma (WHO 5th edition)
- Infiltrative deep dermal tumor that may involve subcutis
- Hypercellular tumor with cells ranging from medium sized epithelioid cells to large epithelioid cells and spindled cells
- Low mitotic activity
- PRKAR1A loss in 67% of PEMs (Am J Surg Pathol 2017;41:1333, Am J Surg Pathol 2019;43:480)
- Dysplastic nevus of vulva:
- Differentiation requires clinical - pathologic correlates
- Relative symmetry
- Presence of junctional shoulders: extension of junctional component at least 3 rete ridges beyond the dermal component
- Superficial nests are usually very similar and may show focal bridging or coalescence of the nests
- Elongation and bridging of the rete ridges with nests
- Melanocytes may scatter suprabasally (confined to the lower epidermal layer and centrally)
- 2 tier grading of cytologic atypia is recommended by World Health Organization (WHO) classification and is largely based on nuclear features (WHO 5th edition) (Hum Pathol 1999;30:500)
- Am J Surg Pathol 1995;19:792, J Cutan Pathol 2008;35:889, J Cutan Pathol 2008:35:24, McKee: Pathology of the Skin - With Clinical Correlations, 3rd Edition, 2005, Busam: Pathology of Melanocytic Tumors, 1st Edition, 2018, Amin: AJCC Cancer Staging Manual, 8th Edition, 2017, WHO Classification of Tumours Editorial Board: Skin Tumours, 5th Edition, 2023, American Cancer Society: Key Statistics for Melanoma Skin Cancer [Accessed 5 December 2023], Nucci: Gynecologic Pathology - A Volume in Foundations in Diagnostic Pathology Series, 2nd Edition, 2020, Frumovitz: Diagnosis and Treatment of Rare Gynecologic Cancers, 1st Edition, 2022
- ALK fusion
- BRAF mutation
- KIT mutation
- NTRK 1/3
- PRKAR1A loss
Comment Here
Reference: Melanoma
A 69 year old woman presented to the OBGYN clinic with severe pruritis. Clinical exam showed a 12 x 15 mm asymmetric, dark brown patch on the right labia minora extending into the clitoral hood. Biopsy demonstrated a broad, asymmetric, junctional melanocytic proliferation with dense lichenoid infiltrate. What is most likely to be demonstrated by the immunohistochemical stains for this patient’s lesion?
- HMB45 showing diffuse nuclear expression
- Loss of PRAME expression
- MelanA highlighting invasive melanoma cells
- p16 demonstrating strong nuclear expression
- SOX10 highlighting increased number of melanocytes with focal pagetoid spread
Comment Here
Reference: Melanoma
- First described by Brown in 1953 (Am J Clin Pathol 1953;23:237)
- Rare; only ~60 reported cases
- Of unclear histogenesis
- Rare, benign tumor commonly of the lower posterior vaginal wall (near hymenal ring)
- Well circumscribed, unencapsulated, detached from overlying epithelium
- Predominantly bland spindle cells (cytokeratin+, SMA+, S100-) with sparsely admixed glands / squamous islands
- Recurrence following complete excision is rare
- Mixed tumor of vagina is the term currently endorsed by the World Health Organization classification of female genital tract tumors
- The term spindle cell epithelioma (SCE) is not recommended
- Middle aged women (mean 40; range 20 - 80) (Am J Surg Pathol 1993;17:509, Pathol Res Pract 2012;208:424, Eur J Obstet Gynecol Reprod Biol 1996;69:143, Am J Surg Pathol 1981;5:413)
- 1 familial case (2 sisters) has been reported (Gynecol Oncol 1979;8:21)
- Most commonly within the posterior vaginal wall near the hymenal ring (Am J Surg Pathol 1993;17:509)
- Unlike mixed tumors of other anatomic sites (e.g., pleomorphic adenoma), the spindle cell component of SCE shows ultrastructural and immunohistochemical evidence of epithelial differentiation (Am J Surg Pathol 1993;17:509, Pathol Res Pract 2012;208:424, Mod Pathol 2004;17:1243)
- Müllerian origin versus urogenital sinus origin is debated
- High frequency of CD10, BCL2, ER, PR positivity favors Müllerian (Pathol Res Pract 2012;208:424)
- Propensity for hymenal ring favors urogenital sinus (Int J Gynecol Cancer 2003;13:543)
- CD34 and WT1 expression argue for a common progenitor cell with the capacity for multiple pathways of differentiation (Arch Pathol Lab Med 2001;125:547, Int J Surg Pathol 2015;23:677)
- Often discovered incidentally
- Well defined, may be pedunculated / polypoid or intramural (Int J Gynecol Cancer 2003;13:543, Clin Imaging 2018;50:181)
- May be perceived as a cyst (Int J Surg Pathol 2015;23:677)
- Diagnosis is made by histopathologic examination, either via biopsy or excision of the lesion
- Ovoid, well circumscribed
- T1 isointense, T2 hyperintense relative to adjacent vaginal wall; homogenous enhancement and restricted diffusion (Clin Imaging 2018;50:181)
- Benign
- May recur with incomplete excision (Gynecol Oncol 1991;40:84)
- 20 year old woman with a perineal itching sensation and posterior vaginal wall mass on MRI (J Korean Med Sci 2002;17:845)
- 28 year old woman with a mass protruding from the vagina (Ann Saudi Med 2002;22:202)
- 45 year old woman with a clinical diagnosis of vaginal wall leiomyoma (J Clin Diagn Res 2013;7:1743)
- Local excision
- 1 - 6 cm in size (Am J Surg Pathol 1993;17:509)
- Intramural without connection to mucosa
- Well circumscribed, unencapsulated (Am J Surg Pathol 1993;17:509)
- Soft to rubbery consistency
- Tan-white, homogenous cut surface with or without grossly visible cysts (Int J Gynecol Cancer 2003;13:543)
- Well circumscribed but unencapsulated with a pushing border
- Located just deep (Biphasic population of predominantly spindle cells with a minor squamous or glandular component and numerous small vessels (Am J Surg Pathol 1993;17:509)
- Spindle cell (major) component:
- Variably cellular spindle cell component
- Hypocellular areas of fibroblastic type cells
- Bland cytology, minimal mitotic activity
- Cytoplasm is usually indistinct but can appear eosinophilic
- Condensed extracellular matrix forms eosinophilic hyaline globules
- Glandular / squamoid (minor) component:
- More often seen at the periphery of the lesion
- Simple glands lined by cuboidal to columnar epithelium
- Glandular epithelium may resemble endocervical epithelium with intracytoplasmic mucin
- Squamous metaplasia is common, resulting in nests / morules and cords of plump squamous cells with glycogenated cytoplasm (Am J Surg Pathol 1993;17:509, Pathol Res Pract 2012;208:424)
- Spindle and epithelial components show unique staining (Mod Pathol 2004;17:1243):
- Spindle cells: cytokeratin, vimentin, SMA, desmin, CD10, CD34, BCL2, ER, PR
- Epithelial cells: cytokeratin, EMA, ER, PR, PAS (diastase sensitive) (Am J Surg Pathol 1993;17:509)
- Spindle cells show variable staining for caldesmon
- Both epithelial and spindle cells are frequently CK7+ / CK20-
- WT1 and calretinin expression has been reported (Int J Surg Pathol 2015;23:677)
- Spindle cells show prominent tonofilaments with associated desmosomes (Am J Surg Pathol 1993;17:509)
- Myoepithelial features (dense myofibrils, pinocytotic vesicles, basal laminar investitures) are not seen
- Vagina, local excision:
- Benign mixed tumor of vagina, 1.5 cm, margins are uninvolved by tumor (see comment)
- Comment: Immunostaining shows AE1 / AE3, CD10, SMA positivity in lesional cells. S100 and SOX10 are negative. The cytomorphology and immunohistochemical profile are consistent with the above diagnosis.
- Carcinosarcoma of vagina:
- Infiltrative border
- Overtly sarcomatous mesenchymal component
- Malignant glandular component (usually high grade morphologically)
- Müllerian adenosarcoma:
- Cambium layer
- Mitotic activity (≥ 2 per 10 high power fields)
- Leaf-like (phyllodes-like) architecture
- Periglandular stromal condensation
- Atypical / frankly malignant spindle cell component
- Tubulosquamous polyp:
- Anatomically more superior
- Thought to arise from mesonephric rests
- Epithelial elements with squamoid and glandular appearance are discrete but are not associated with a surrounding spindle cell population
- GATA3+ and NKX3.1+ epithelium, CD10- and cytokeratin- stroma (Am J Surg Pathol 2007;31:1013)
- Metastatic endometrial stromal sarcoma:
- Infiltrative border
- Usually epithelial elements are absent
- Previous history of the disease
- Metastatic / primary spindle cell carcinoma:
- Infiltrative border and marked atypia
- Overlying mucosal dysplasia or better differentiated squamous cell carcinoma components (if primary)
- Synovial sarcoma:
- Infiltrative border
- SS18::SSX+ (Am J Surg Pathol 2020;44:922)
A 31 year old woman presents with a 2 cm solid mass protruding from the vagina. A local resection is performed and shows a submucosal, well circumscribed but unencapsulated lesion composed of predominantly bland spindle cells with sparse glands and squamous metaplasia (see image shown above). Electron microscopy of the spindle cells shows abundant tonofilaments and desmosomes but no pinocytotic vesicles. Which of the following is true regarding these spindle cells?
- They are derived from mesonephric rests
- They are derived from normal myoepithelial cells found in the vagina
- They are likely keratin+, CD10+ and SMA+
- They are the malignant component of this biphasic tumor
Comment Here
Reference: Mixed tumor of vagina
- Intraepithelial adenocarcinoma primarily involving the epidermis of vulvar skin, with or without an underlying invasive adenocarcinoma
- May be either primary or secondary
- Large, round epithelial cells with abundant pale cytoplasm and large nuclei present within the epidermis
- In primary Paget disease, neoplastic cells are consistently strongly positive for CK7
- Recurrence of disease is common despite seemingly adequate excision
- Extramammary Paget disease (EMPD)
- Primary cutaneous Paget disease
- Secondary Paget disease
- Accounts for 1 - 2% of vulvar neoplasms (Cancers (Basel) 2023;15:1803)
- Typically affects postmenopausal White women > 60 years of age; mean age is 65 years (Australas J Dermatol 2013;54:9)
- Most cases are primary Paget disease
- ~15 - 20% of vulvar extramammary Paget disease cases are associated with invasive disease
- Labia majora is the most common site
- Labia minora, clitoris; can extend to the vaginal or (rarely) cervical mucosa (Diagn Cytopathol 2016;44:931)
- Can extend onto extravulvar skin
- Primary Paget disease (cutaneous)
- Presumably originates in the appendages
- Pluripotent stem cells of the adnexa, cells from sweat ducts or cells from anogenital mammary-like glands including the clear cells of Toker (Am J Dermatopathol 2005;27:185)
- Paget cells can thus display apocrine, eccrine and keratinocyte differentiation
- Presumably originates in the appendages
- Secondary Paget disease (extracutaneous)
- Associated primary anal, rectal, bladder, cervical or other noncutaneous adenocarcinoma secondarily involving vulvar skin
- Symptoms include pruritus, burning and vulvar pain; few cases are asymptomatic
- Generally appears as erythematous and eczematoid plaques; may also demonstrate crusting, focal erosion or discharge
- Frequently multifocal
- As most cases present similarly to inflammatory processes, delay in diagnosis is common and often occurs months to years after symptom onset (Gynecol Oncol 2011;122:42)
- Vulvar biopsy
- Once diagnosis is confirmed, may be prudent to exclude secondary involvement by other underlying malignancy (e.g., rectal, bladder or cervical origin)
- May require physical, endoscopic or radiologic examination
- No validated tumor markers for extramammary Paget disease
- Tumor cells express carcinoembryonic antigen (CEA)
- Serum CEA may be elevated in patients with metastatic or invasive extramammary Paget disease (Br J Dermatol 2008;158:313)
- Elevated CEA levels have been associated with poor prognosis
- Generally good prognosis; 5 year overall survival rate is 75 - 95% (Dermatol Surg 2020;46:151)
- Recurrence is common, occurring in up to 44% of patients who undergo wide local excision
- Recurrence is common regardless of surgical margin status (Gynecol Oncol 2007;104:547)
- Negative prognostic factors include the presence of a nodule within the primary lesion, palpable lymphadenopathy, deep invasion into the dermis and lymph node metastases (Dermatol Surg 2012;38:1938)
- Increased p53 expression may be associated with invasion and thus poor prognosis (Hum Pathol 2003;34:880)
- 59 year old woman with a history of low grade papillary urothelial carcinoma of the bladder status postresection and postimmunotherapy who later presented with severe vulvar pruritus (Diagn Pathol 2019;14:125)
- 65 year old woman presented with a 10 cm left vulvar slow growing pruritic erythematous plaque (Indian J Sex Transm Dis AIDS 2020;41:210)
- 72 year old woman presented with an erythematous and pruritic vulvar plaque (Rev Bras Ginecol Obstet 2019;41:412)
- 73 year old White woman presented with a 3 month history of left vulvar erythema and pruritus unresponsive to topical creams (Gynecol Obstet Invest 2017;82:1)
- Primary treatment is wide local excision or vulvectomy (Gynecol Oncol 2020;157:146)
- Other treatment options include Mohs micrographic surgery, radiation and topical agents such as 5-fluorouracil, imiquimod, rapamycin and bleomycin
- Targeted therapy may be used to treat metastatic extramammary Paget disease when HER2 amplification is present
- Trastuzumab has been used alone and in combination with cytotoxic chemotherapy (JCO Precis Oncol 2018;2:1)
- In secondary Paget disease, treatment of the underlying malignancy is necessary
- May require managements tailored to the primary malignancy
- Often ill defined, raised, red scaly plaque(s)
- Identifying Paget cells on frozen section is difficult
- Reported false negative rates of ~37% (Diagn Cytopathol 2016;44:931)
- Routine frozen section evaluation is not advised
- Paget cells are enlarged polygonal epithelial cells with abundant pale cytoplasm, large nuclei and small to prominent nucleoli
- Intracytoplasmic mucin is frequently present
- Intracytoplasmic melanin pigment may be present (rare)
- Rarely, gland formation may be seen
- Predominantly located in the basal and parabasal portions of the squamous epithelium
- Extends upward as single cells or in small clusters
- Commonly involves the epithelium of skin adnexa
- Basal layer keratinocytes are preserved but may appear compressed by the tumor cells (Histopathology 2006;48:723)
- Prominent host inflammatory response in the superficial dermis is frequently seen
- Dermal invasion
- Single cells or small groups of cells infiltrating the dermis with an associated desmoplastic reaction
- Groups of cells may form glandular structures
- May be associated with dense dermal inflammatory response
Contributed by Priya Nagarajan, M.D., Ph.D. and Lucy Ma, M.D.
- Round to columnar, moderately enlarged atypical cells, dispersed or in loose groups, with abundant clear cytoplasm, vesicular nuclei and prominent nucleoli (Acta Cytol 2010;54:1007, Acta Cytol 2010;54:898, Diagn Cytopathol 2010;38:127)
- Nuclei can appear compressed due to vacuoles in the cytoplasm
- Primary and secondary Paget disease: CAM 5.2, EMA, CEA, mucicarmine, periodic acid-Schiff (PAS)
- Primary Paget disease: CK7, GATA3, GCDFP-15, androgen receptor, p16 (weak to moderate) (Am J Clin Pathol 2000;113:572)
- Secondary Paget disease, anorectal primary: CK20, CDX2, SATB2
- Secondary Paget disease, urothelial primary: CK7, CK20, GATA3, uroplakin III, p63
- Secondary Paget disease, cervical primary: CK7, PAX8, p16 (block positive), high risk HPV ISH
- Primary and secondary Paget disease: HMB45, MelanA, SOX10, MITF, p63, p40, CK5/6, ER and PR (Am J Clin Pathol 2000;113:572)
- Primary Paget disease: CK20
- Secondary Paget disease, anorectal primary: CK7, GATA3, GCDFP-15, uroplakin III
- Secondary Paget disease, urothelial primary: GCDFP-15
- Secondary Paget disease, cervical primary: CK20, GATA3, GCDFP-15
- Mutations RAS and RAF genes (19%) and in PIK3CA and AKT1 genes (35%) (Int J Cancer 2013;132:824, Am J Dermatopathol 2017;39:358)
- Hypermethylation of CDH1 gene, which encodes for protein E-cadherin (Int J Cancer 2013;132:824)
- ERBB2 amplification (although rate is less frequent than Paget disease of the breast) (J Low Genit Tract Dis 2023;27:40)
- Amplification at chromosome Xcent-q21 and 19 and loss at 10q24-qter, indicating that androgen receptor may play a role in the pathogenesis (Br J Dermatol 2005;153:290)
Extramammary Paget disease: 5 minute pathology pearls
Paget disease versus melanoma versus squamous cell carcinoma in situ
- Vulva, left, wide local excision:
- Invasive extramammary Paget disease
- Invasive tumor size: 13 mm
- Depth of invasion: 6 mm
- Lymphovascular invasion: present
- Margins are negative for invasive tumor; extramammary (in situ) Paget disease extensively involves the medial peripheral margin
- Vulva, right, biopsy:
- Extramammary Paget disease (see comment)
- Comment: On immunostains, the neoplastic cells are strongly positive for CK7 and CAM 5.2 and negative for SOX10, supporting the diagnosis.
- Melanoma in situ:
- Vulvar intraepithelial neoplasia (VIN), HPV associated and HPV independent:
- Sebaceous carcinoma:
- Tumor cells show multiple cytoplasmic vacuoles, which characteristically indent the nuclei
- CEA and GCDFP-15 negative
- Adipophilin and perilipin positive (due to the lipid content)
- Pagetoid dyskeratosis:
- Clear cells of Toker:
- CK7 positive but lacks cytologic atypia
- Not associated with clinical findings
- Clear cell papulosis:
- Generally seen in children (rarely in adults)
- Lacks cytologic atypia and mitoses
- Primary extramammary Paget disease
- Secondary Paget disease, likely associated with breast carcinoma
- Secondary Paget disease, likely associated with rectal carcinoma
- Secondary Paget disease, likely associated with urothelial carcinoma
Comment Here
Reference: Vulva & vagina - Paget disease
A 66 year old woman presents with a pruritic plaque on the right labia majora of 2 years duration. She has no significant oncologic history. As her symptoms have not improved, a punch biopsy is performed (image shown above). A mucicarmine stain is positive within the tumor cells. Which of the following ancillary stains would also likely be positive in this tumor?
- CK5/6
- CK7
- High risk HPV in situ hybridization
- HMB45
Comment Here
Reference: Vulva & vagina - Paget disease
- Vulvar lesions that appear pigmented either grossly or microscopically
- Pigmented vulvar lesions are a poorly characterized group of entities with variable gross and histologic appearances
- Lesions containing melanin and those that appear pigmented but lack melanin microscopically
- Postinflammatory reactive and reparative changes, purpura, vascular lesions, debris filled comedones and normal genital skin variations may be included in this category (Dermatol Clin 2010;28:795)
- ICD-O
- 8720/0 - nevus, NOS
- 8761/0 - congenital melanocytic nevus, NOS
- 8720/0 - atypical melanocytic nevus of genital type
- 8727/0 - dysplastic nevus
- 8720/3 - malignant melanoma, NOS
- 8077/0 - low grade squamous intraepithelial lesion
- 8077/2 - high grade squamous intraepithelial lesion
- 8071/2 - differentiated vulvar intraepithelial neoplasia (VIN)
- 8085/3 - squamous cell carcinoma, HPV associated
- 8086/3 - squamous cell carcinoma, HPV independent
- 8070/3 - squamous cell carcinoma, NOS
- 8090/3 - basal cell carcinoma, NOS
- 8542/3 - Paget disease, extramammary
- C51.0 - labia majus / labia majora, NOS; Bartholin gland; skin of the labia majora
- C51.1 - labia minus / labia minora
- C51.2 - clitoris
- C51.8 - overlapping lesions of the vulva
- C51.9 - vulva, NOS
- ICD-10
- D22.9 - melanocytic nevi, unspecified
- N90.89 - vulvar lesion(s), nontraumatic
- N76 - inflammation of the vulva
- D28.0 - benign neoplasm of the vulva
- D39.0 - neoplasm of uncertain behavior of female genital organs
- N90.3 - intraepithelial neoplasia (VIN)
- D07 - carcinoma in situ of other and unspecified genital organs
- C51 - malignant neoplasm of the vulva
- ICD-11
- 2C70 - malignant neoplasm of the vulva
- 2C70.0 - basal cell carcinoma of the vulva
- 2C70.2 - squamous cell carcinoma of the vulva
- 2E67.1 - carcinoma in situ of the vulva
- 2E67.11 - vulvar Paget disease
- 2E67.12 - vulvar intraepithelial neoplasia, HPV independent
- 2E67.13 - high grade squamous intraepithelial lesions of the vulva
- EA83.00 - lichen simplex of the vulva
- EB60.0 - lichen sclerosus of the vulva
- ED61.11 - vulvar melanotic macule
- GA13.1 - low grade squamous intraepithelial lesions of the vulva
- XH5FT2 - vulvar intraepithelial neoplasia, grade III
- Different types of vulvar pigmented lesions are found in 8 - 12% of females (Ann Pathol 2022;42:79)
- In reproductive aged women (median age: 40 - 44 years), 68% of vulvar pigmented lesions are lentigines (Dermatol Clin 2010;28:795, J Am Acad Dermatol 1990;22:104)
- In children with vulvar melanosis, multisystem genodermatoses should be considered (see Table 3)
- Prevalence of vulvar melanocytic nevi is 2.3% (J Am Acad Dermatol 1990;22:104)
- Vulvar nevus (nevus of special site; pigmented nevi, nevocellular nevi, common nevi)
- Premenopausal women (median age: 28 - 33 years) (J Am Acad Dermatol 2014;71:1241)
- Prevalence is 3.3% in girls younger than 18 years (Am Acad Dermatol 2014;70:429)
- Majority of nevi are acquired, while congenital nevi are uncommon
- Atypical melanocytic nevi of the genital type (also known as nevi with site related atypia)
- Generally occur in young women (median age: 17 - 26 years) (see Table 4) (J Cutan Pathol 2008;35:24, J Am Acad Dermatol 2014;71:1241, Dermatology 2011;222:157, Am J Surg Pathol 2008;32:51)
- Seborrheic keratoses mostly occur in Caucasian patients aged > 40 years but can be diagnosed in all age groups and races (Dermatol Clin 2010;28:795)
- Angiokeratoma of Fordyce (genital angiokeratoma)
- Usually occurs in young women and may be associated with pregnancy or vulvar varicosity (Arch Dermatol 1967;95:166)
- Pigmented condylomata acuminata (anogenital warts)
- More common in patients with darker skin tones in association with high risk human papillomavirus (HPV) types (Dermatol Ther 2010;23:449)
- Anogenital warts affect 1% of adults
- Pigmented vulvar intraepithelial neoplasia (previously known as Bowen disease or Bowenoid papulosis) (Dermatol Clin 2010;28:795, J Low Genit Tract Dis 2013;17:320)
- HPV associated high grade squamous intraepithelial lesion (linked to high risk HPV types, most commonly 16, 18, 31)
- Risk factors: smoking, multiple sex partners, immunosuppression
- Younger women
- Typically multifocal, hyperpigmented flat topped papules or plaques with distinct margins
- HPV independent, differentiated vulvar intraepithelial neoplasia
- Older women
- Arises in a background of lichen sclerosus
- HPV associated high grade squamous intraepithelial lesion (linked to high risk HPV types, most commonly 16, 18, 31)
- Pigmented basal cell carcinoma
- Accounts for 5% of primary vulvar cancers (Dermatol Clin 2010;28:795)
- Prevalence of genital basal cell carcinoma ranges from 0.5 to 1.7% and affects older women (J Cutan Pathol 2018 Jun 19 [Epub ahead of print], Int J Gynecol Pathol 2022;41:86)
- Vulvar pigmented lesions generally show predilection for labia majora, labia minora and clitoris, less commonly mons pubis and perineum (Arch Pathol Lab Med 2011;135:317)
- Vulvar melanosis
- Most common in mucosal surfaces followed by hair bearing skin of external genitalia (Dermatol Clin 2010;28:795)
- Vulvar nevus
- Most commonly located on labia majora, labia minora or clitoral hood
- Atypical melanocytic nevi of the genital type
- Labia majora (most common)
- More frequent on mucosal areas in children younger than 10 years (Hum Pathol 1998;29:S1, Am J Surg Pathol 2008;32:51)
- In adults, equal distribution between mucosal and hair bearing surfaces (Am J Surg Pathol 2008;32:51)
- Angiokeratoma of Fordyce
- Labia majora
- Physiologic hyperpigmentation
- Dark skin > light skin complexion
- Hormonal variation
- Postinflammatory hyperpigmentation (Dermatol Clin 2010;28:795)
- Most commonly occurs in areas of previous injury or dermatosis
- Associated with known inflammatory conditions
- Lichen sclerosus
- Lichen planus
- Erythema multiforme
- Fixed drug eruptions
- Trauma and dermatologic treatments
- Acanthosis nigricans
- Strong association with insulin resistance (diabetes mellitus, obesity, hyperandrogenism) (Am J Clin Dermatol 2004;5:199)
- Malignancy (adenocarcinoma most common) related acanthosis nigricans
- Production of hormones and peptides by malignancy have been implicated
- Secretion of transforming growth factor alpha (TGFα) in large quantities is associated with increased keratinocyte proliferation (J Gastroenterol 1997;32:71)
- Medication: nicotinic acid, glucocorticoids, oral contraceptives and diethylstilbestrol, protease inhibitors in human immunodeficiency virus infected patients (Clin Infect Dis 2002;34:716)
- Seborrheic keratosis
- Proliferation of keratinocytes of unknown cause (Dermatol Clin 2010;28:795)
- Subset of genital seborrheic keratosis may be related to low risk HPV, though this is controversial (Hum Pathol 2003;34:559, APMIS 2012;120:477, J Low Genit Tract Dis 2014;18:190)
- Genital / vulvar melanosis / lentiginosis
- Largely unknown
- Variation in hormonal levels may play a role (onset of melanosis after oral contraceptive use and the immediate postpartum period) (J Am Acad Dermatol 2014;71:1241)
- Vulvar nevus
- May relate to hormonal milieu (J Low Genit Tract Dis 2013;17:320)
- Atypical melanocytic nevi of the genital type
- Largely unknown but associated with anatomic milk line (Am J Surg Pathol 2008;32:51)
- Angiokeratoma of Fordyce
- Associated with pregnancy or vulvar varicosity (Arch Dermatol 1967;95:166)
- Speculated pathogenesis is localized hypertension (Arch Dermatol 2005;141:1325)
- Pigmented condylomata acuminata
- HPV infection (90% accounted for HPV 6 and 11)
- Pigmented basal cell carcinoma
- Sun exposure, ultraviolet (UV) specific p53 mutations in sun exposed areas
- Previous radiation
- Limited studies in Chinese population proposed p53 overexpression independent to UV specific pathway (J Cutan Pathol 2018 Jun 19 [Epub ahead of print])
Table 1: Features distinguishing vulvar nevi, melanosis and melanoma (adapted from J Am Acad Dermatol 2014;71:1241)
| Reassuring features suggestive of a benign process | Concerning features for possible malignancy | |
| Clinical |
|
|
| Dermoscopy |
|
|
| Reflectance confocal microscopy |
|
|
Table 2: Clinical morphology for pigmented vulvar lesions (adapted from Dermatol Ther 2010;23:449)
| Papules and macules | Patches and plaques |
| Pigmented nevi (nevocellular nevi) | Physiologic hyperpigmentation |
| Dysplastic nevi | Postinflammatory hyperpigmentation |
| Malignant melanoma | Vulvar melanosis (vulvar lentiginosis) |
| Anogenital warts | Acanthosis nigricans |
| Vulvar intraepithelial neoplasia | |
| Seborrheic keratosis | |
| Basal cell carcinoma | |
| Angiokeratoma |
Table 3: Genodermatosis syndromes with vulvar melanosis (adapted from J Am Acad Dermatol 2014;71:1241)
| Genodermatosis | Areas affected | Key features | Genetic mutation |
| Peutz-Jeghers syndrome | Oral, perianal and genital area, lips, nostrils, hands, feet | Hamartomatous polyps in the gastrointestinal tract (GIT), breast, ovarian, pancreatic and GIT cancers | STK11 |
| Carney complex | Oral and genital area, lips, eyelids, conjunctiva | Blue nevus, psammomatous melanotic schwannoma, myxomas (usually cardiac), endocrine neoplasms | PRKAR1A |
| Multiple lentigines syndrome (LEOPARD syndrome) | Face, neck, trunk, genital area | Delayed growth, sensorineural deafness, ECG abnormalities, ocular hypertelorism, pulmonic stenosis, abnormal genitalia | PTPN11, RAF1, BRAF |
| Bannayan-Riley-Ruvalcaba syndrome | Face, genital area | Macrocephaly, intellectual and motor deficiencies, joint hyperextensibility, pectus excavatum, scoliosis, intestinal hamartomatous polyposis, lipomas, hemangiomas | PTEN |
| Dowling-Degos syndrome | Axillae, neck, flexural folds, inguinal and genital area | Follicular papules, comedone-like lesions, perioral scar, reticulated hyperpigmentation, hypopigmented or erythematous macules | KRT5 |
Table 4: Clinical and histologic differences between atypical genital nevus and vulvar melanoma
(adapted from Hoang: Melanocytic Lesions - A Case Based Approach, 1st Edition, 2014)
| Proposed diagnosis | Atypical genital nevus of special anatomic site | Vulvar melanoma |
| Age | Premenopausal, young adult | Postmenopausal |
| Size | > 1 cm | |
| Delineation | Well circumscribed | Infiltrative |
| Symmetry | Present | Absent |
| Lateral extension of junctional component | Focal | Present |
| Lentiginous junctional component | Focal | Present |
| Junctional nests | Dyscohesive | Confluent |
| Retraction artefact | Present | Absent |
| Ulceration | Absent or due to trauma | Often present |
| Pagetoid upward spread | Focal, central, inconspicuous | Prominent |
| Cytologic atypia | Superficial, mild - moderate | Deep, moderate to severe |
| Dermal mitosis | Rare and superficial | Conspicuous, atypical, deep |
| Dermal maturation | Present | Absent |
| Melanin pigmentation | Coarse, uniform | Fine, irregular |
| Dermal fibrosis | Broad zone of superficial coarse dermal fibrosis | Regression type |
- During obstetrics / gynecologic visit
- Women rarely examine their vulva closely; consequently, duration and change are of minimum relevance (Dermatol Clin 2010;28:795)
- Consider endocrine disorders (e.g., congenital adrenal hyperplasia, Addison disease and Cushing disease) (Dermatol Clin 2010;28:795)
- Decision to biopsy is based on atypical appearance of pigmented lesion (Dermatol Clin 2010;28:795)
- Pigmented lesions on genital skin appear more atypical than on extragenital skin (Dermatol Clin 2010;28:795)
- Punch or excisional biopsy is preferred over shave biopsy (Dermatol Clin 2010;28:795)
- Physiologic hyperpigmentation (Dermatol Clin 2010;28:795)
- Most common in patients with dark complexion
- May vary with different hormonal stages (e.g., puberty, menopause, pregnancy, contraception use)
- Distribution
- Macular, symmetric, nonscaly or change in texture; usually darker at the posterior introitus, labia minora and perianal skin
- Proximal medial thighs may show uniform hyperpigmentation with fading into the color of nonmodified mucous membranes
- Postinflammatory hyperpigmentation
- May occur in all skin types
- Most notable in patients with dark skin complexion
- Most commonly in areas of previous injury or dermatosis
- Distribution
- Usually less symmetric than physiologic hyperpigmentation
- Macules and patches with different shades of brown
- Macular, symmetric, nonscaly or change in texture; usually darker at the posterior introitus, labia minora and perianal skin
- Proximal medial thighs may show uniform hyperpigmentation with fading into the color of nonmodified mucous membrane
- Acanthosis nigricans
- Velvety, leathery, wrinkly texture with a darker appearance versus unaffected sites of the body
- Skin tags within skin folds are common
- Spares mucous and modified mucous membranes (MMB)
- Seborrheic keratosis
- Typically single, keratotic, flat topped, sharply demarcated, brown lesions with a stuck on appearance, showing keratotic and follicular plugging; uniform color and shape
- Because of irritation, moisture and hair in genital area, the lesions may appear less keratotic due to loss of their coloration and uniformity (Dermatol Clin 2010;28:795)
- May have slightly verrucous surface
- Absence of seborrheic keratosis on other body parts coupled with a younger age and Ki67 immunohistochemical stain extending into the superficial part of the lesion would favor condyloma
- Detection of low risk HPV test would confirm diagnosis
- Unlikely to occur in the vulva if other skin sites appear unaffected (Dermatol Clin 2010;28:795)
- Clinically seborrheic keratosis may mimic nevi, pigmented condylomata acuminata and vulvar intraepithelial neoplasia and less often dysplastic nevi or melanoma (Dermatol Clin 2010;28:795)
- Only seborrheic keratosis that appear atypical, clinically and on dermoscopy, should be biopsied
- Genital / vulvar melanosis / lentiginosis
- Incidental finding on inspection
- Single or multiple asymptomatic, asymmetric, macules or patches with variation of size and color ranging from tan to black within the lesion, irregular and poorly demarcated borders (Dermatol Clin 2010;28:795, J Low Genit Tract Dis 2013;17:320)
- Concerning features: papular component, erosions, reported pruritis or pain
- Can be associated with a postinflammatory hyperpigmentation, such as in patient with lichen sclerosus and lichen planus (Dermatol Clin 2010;28:795)
- Biopsy is recommended to definitely exclude the presence of melanoma or pigmented vulvar intraepithelial neoplasia (Dermatol Clin 2010;28:795)
- Vulvar nevus
- Nevi can be flat (junctional) or domed (compound or intradermal)
- Common nevi range from tan to dark brown in color; bluish color is indicative of pigment in the dermis (Dermatol Clin 2010;28:795)
- Generally symmetrical, with sharp and regular border and even color throughout the lesion
- Common acquired nevi are usually < 0.6 cm, dysplastic are usually > 0.6 cm
- Atypical lesions should be biopsied to rule out dysplastic nevi versus melanoma
- Atypical melanocytic nevi of the genital type (Hum Pathol 1998;29:S1, Am J Surg Pathol 2008;32:51)
- Compared to nevi of special site, they may have alarming clinical features, darker pigmentation, irregular border and large size
- Angiokeratoma of Fordyce
- Typically multiple, small, shiny red to purple; some can appear very dark blue to black
- Early lesions are soft and blanchable; later lesions may become darker and keratotic
- Pigmented condylomata acuminata
- Usually multiple with variety of morphology (papillomatous, verrucous, fleshy papules and flat topped) (Dermatol Ther 2010;23:449)
- In patients with dark complexion, pigmented warts can be flat topped, lobular or papular; only the filiform variant is less likely to be hyperpigmented (Dermatol Ther 2010;23:449)
- Flat topped, brown, anogenital warts in individuals of light skin should be biopsied with concern of vulvar intraepithelial neoplasia (Dermatol Clin 2010;28:795)
- Clinically, anogenital warts may be indistinguishable with seborrheic keratosis
- Pigmented vulvar intraepithelial neoplasia
- HPV associated high grade squamous intraepithelial lesion
- Typically multifocal, hyperpigmented, flat topped papules or plaques with distinct margins
- Biopsies should be avoided if lesions were recently treated (e.g., podophyllin); treatment effect may be interpreted as vulvar intraepithelial neoplasia histologically
- HPV independent differentiated vulvar intraepithelial neoplasia
- Unifocal, often erythematous, well demarcated patches and plaques, with occasional hyperpigmentation
- Clinical differentials include nevi, seborrheic keratosis and anogenital warts
- Keratotic scale may be lacking on mucous and modified mucous membranes
- Clinical differentials include psoriasis, lichen simplex chronicus and tinea
- May be associated with lichen sclerosus or lichen planus
- HPV associated high grade squamous intraepithelial lesion
- Pigmented basal cell carcinoma
- Usually pink and flat or nodular with flesh colored, pearly appearing, translucent sheen but may be irregularly pigmented (brown-black)
- Can appear as nodules, polyps, ulcers or flat areas of hyperpigmentation and hypopigmentation
- Basal cell nevus syndrome may affect perineum
- Regular anogenital examinations are recommended
- See Table 2
- Dermoscopy for nonmodified mucous membranes is useful (Dermatol Ther 2010;23:449)
- Pigmented condylomata acuminata are characterized by exophytic papillary structures with variation in pigment that includes jet black color, red dots and whitish halo (keratinization)
- Histologic diagnosis is confirmatory for other vulvar pigmented lesions (Dermatol Clin 2010;28:795, JAMA Dermatol 2020;156:1185)
- See Table 1
- Additional laboratory workup may be considered if syndromic association is suspected
- Vulvar nevus
- Atypical nevi formed along the milk line have no increased risk for malignant transformation (Dermatol Ther 2010;23:449)
- Risk for vulvar melanoma arising from vulvar nevi remains unclear
- Out of 219 Swedish patients, 98% of de novo melanomas occur on mucosal surfaces or the hairy - mucosal junction (Acta Oncol 2004;43:421, Cancer 1999;86:1273)
- 35% of these cases involve pre-existing nevi at the melanoma sites on the hair bearing skin
- Dysplastic nevi indicate increased risk for melanoma (Dermatol Ther 2010;23:449)
- Atypical melanocytic nevi of the genital type
- Thought to have a benign clinical course (Hum Pathol 1998;29:S1, Am J Surg Pathol 2008;32:51)
- Pigmented vulvar intraepithelial neoplasia
- Risk of progression to invasive vulvar carcinoma can be as high as 87.5% in women with untreated disease
- HPV independent differentiated vulvar intraepithelial neoplasia may coexist with lichen sclerosus
- Multifocal warty / basaloid HPV associated vulvar intraepithelial neoplasia may regress spontaneously in young women (Eur J Obstet Gynecol Reprod Biol 2008;137:97)
- Pigmented basal cell carcinoma
- Metastasis is very rare (Arch Dermatol 2003;139:643)
- 10% recurrence rate for vulvar basal cell carcinomas (Arch Dermatol 2003;139:643)
- Basal cell carcinomas can be locally disfiguring and destructive, unless treated with excision and radiation
- 18, 57 and 68 year old women with vulvar pigmented cystic lesions (Int J Gynecol Pathol 2022;41:93)
- 20 year old woman with irregular, darkly pigmented, vulvar lesion (J Cutan Pathol 2022;49:172)
- 24 year old woman with vulvar pigmented epithelioid melanocytoma (PEM) (Am J Dermatopathol 2020;42:544)
- 27 year old pregnant woman with massive vulvar Kaposi sarcoma (Int Med Case Rep J 2016;9:227)
- 33 year old woman with HIV and syphilis coinfection and genital Kaposi sarcoma (BMC Infect Dis 2019;19:1095)
- 34 year old woman with a hyperpigmented vulvar nodule (Am J Dermatopathol 2020;42:46)
- 39 year old woman with dark brown vulvar discoloration (Acta Dermatovenerol Croat 2022;30:261)
- 50 year old woman with vulvar mucosal PEM (Dermatol Pract Concept 2020;10:e2020070)
- 80 and 92 year old women with hypopigmented and heavily pigmented vulvar nodules (Ceska Gynekol 2021;86:325)
- 86 year old White woman with hyperpigmentation on upper vulva (Medicina (Kaunas) 2023;59:206)
- Vulvar nevus
- Atypical lesions should be biopsied to rule out dysplastic nevi and melanoma
- Simple excision is usually sufficient
- Angiokeratoma of Fordyce (Dermatol Ther 2010;23:449)
- Therapy is usually not needed
- If irritated or for cosmetic purposes, the individual lesions can be lightly hyfrecated or removed by shave
- Multiple lesions can be treated with cryotherapy, electrocautery, radiofrequency and ablative laser
- Depending on the exact location, simple excision may be preferred for some lesions
- Pigmented condylomata acuminata (Dermatol Ther 2010;23:449)
- Cryotherapy, laser, shave / snip excision
- Chemodestruction with podofilox, trichloroacetic / bichloroacetic acid
- Alternatively, immunotherapy with imiquimod can be used
- Pigmented vulvar intraepithelial neoplasia:
- Surgical excision (wide local excision), cryotherapy, laser, vaporization, shave / snip excision (Obstet Gynecol 2016;128:e178)
- For larger lesions, imiquimod is commonly used
- Less often, 5 fluorouracil (5FU) cream may be considered
- Topical 5 aminolevulinic, acid based, photodynamic therapy is a new modality
Contributed by José Alberto Fonseca Moutinho, M.D.
Images hosted on other servers:
- Physiologic hyperpigmentation
- Number of melanocytes and keratinocytes in the basal layer is usually higher compared with adjacent normal epithelium
- Genital / vulvar melanosis / lentiginosis
- Basilar hyperpigmentation with mild melanocytic hyperplasia at the dermoepidermal junction arranged as solitary units more commonly than in nests, epithelial hyperplasia and stromal melanophages without cytologic atypia (Dermatol Clin 2010;28:795, J Low Genit Tract Dis 2013;17:320)
- Postinflammatory hyperpigmentation
- Basement membrane damage with pigment incontinence followed by melanin release into the superficial dermis (Dermatol Clin 2010;28:795, J Low Genit Tract Dis 2013;17:320)
- Acanthosis nigricans
- Mild hyperkeratosis, acanthosis and papillomatosis
- Can alternate with atrophic areas
- Occasional keratin filled cysts
- Nonspecific perivascular chronic inflammatory infiltrate may be present in superficial dermis
- Seborrheic keratosis
- Hyperkeratosis, acanthosis or pseudohorned cysts
- Occasional dendritic melanocytes in epidermis
- Vulvar nevus
- Pigmented nevi (nevocellular nevi) of the special site
- Proliferation of nonpigmented and pigmented melanocytes with no to mild cytologic atypia
- Involving the dermal epidermal junction in junctional nevus
- Involving the dermal epidermal junction and dermis in compound nevus
- Involving the dermis in intradermal nevus
- Typically, symmetrical silhouette with very minor irregularity or asymmetry
- Zonation (also known as maturation)
- Decrease in size of nests
- Decrease of individual melanocytes from the large epithelioid (type A melanocytes) appearance superficially to small epithelioid lymphocyte-like (type B) and fusiform schwannian (neural, type C) at the deep part of the lesion
- If melanin is present, it tends to be most prominent in epidermal and superficial dermal melanocytes with loss of pigmentation in the deeper part of the lesion
- Lack of mitoses
- No evidence of regression
- Proliferation of nonpigmented and pigmented melanocytes with no to mild cytologic atypia
- Dysplastic nevi diagnosis has undergone multiple revisions and the new World Health Organization (WHO) classification is still pending final diagnostic criteria
- Practically considered an entity that entails a deviation from stereotypical junctional nevi comprising architectural disorder and cytologic atypia (WHO 5th edition)
- Histologic features supporting dysplastic nevus
- Presence of junctional shoulders - basilar proliferation of atypical melanocytes that must extend beyond the dermal component
- Superficial nests are usually very similar and may show focal bridging or coalescence of the nests
- Elongation and bridging of the rete ridges with nests
- Melanocytes may scatter suprabasally (confined to the lower epidermal layer)
- Lamellar and concentric fibroplasia
- Patchy lymphocytic infiltrate
- Cytologic atypia is based on nuclear size comparing with neighboring basal keratinocytes, presence of nucleoli, chromatin hyperchromasia and clumping
- Pigmented nevi (nevocellular nevi) of the special site
- Atypical melanocytic nevi of the genital type
- Symmetrical lesion with sharp demarcation and dermal maturation (J Cutan Pathol 2008;35:24)
- 3 histological patterns described by Clark (Hum Pathol 1998;29:S1)
- Nested: oval, typically large nests, perpendicular or parallel to dermoepidermal junction
- Dyshesive nests: nearly contiguous forming a band that separates the epidermis from the mature dermal melanocytes
- Crowded: closely apposed, ill defined nests and single cells obscuring the dermal epidermal junction
- Usually mild to moderate uniform cytologic atypia (J Cutan Pathol 2008;35:24)
- Single cell growth with focal pagetoid spread may be present but is usually located in the center of lesion (J Cutan Pathol 2008;35:24)
- Adnexal spread may be present (Hum Pathol 1998;29:S1)
- Dense eosinophilic fibrosis in the superficial dermis (Hum Pathol 1998;29:S1, J Cutan Pathol 2008;35:24)
- Intradermal component with maturation is often present (J Cutan Pathol 2008;35:24)
- Nevi within lichen sclerosus can appear more atypical
- For best diagnosis, lichen sclerosus should be controlled before excision of the nevus (J Am Acad Dermatol 2004;50:690, Arch Dermatol 2002;138:77)
- See Table 4
- Angiokeratoma of Fordyce (J Cutan Med Surg 2020;24:188)
- Dilated capillaries in superficial dermis only with overlaying acanthosis and hyperkeratosis
- Vessels may appear to herniate into epidermis (J Cutan Med Surg 2020;24:188)
- Lack of dilated vasculature in deep dermal and subcutaneous component
- Pigmented condylomata acuminata
- HPV related histologic changes
- Exophytic growth with marked acanthosis and trabecular or solid pattern
- Superficial koilocytes are characteristic
- Coarse keratohyalin granules may be seen
- Condylomas treated with podophyllin demonstrate marked epidermal pallor and increased mitosis as well as necrosis of keratinocytes in the lower half of epidermis
- Pigmented vulvar intraepithelial neoplasia
- Both types show hyperkeratosis with parakeratosis
- HPV associated high grade squamous intraepithelial lesion
- Parakeratosis with koilocytosis and full thickness epithelial involvement
- Enlarged hyperchromatic nuclei
- Easily identifiable mitoses
- Apoptotic bodies may be present
- HPV independent differentiated vulvar intraepithelial neoplasia
- Parakeratosis with acanthosis
- Elongation and anastomosis of rete ridges
- Enlarged hypereosinophilic keratinocytes with prominent intercellular bridges
- Basal layer with prominent cytologic atypia
- Pigmented basal cell carcinoma
- Islands or nests of basaloid cells with peripheral palisading
- HPV associated vulvar intraepithelial neoplasia: diffuse, block-like p16 expression as opposed to patchy expression in pigmented condylomata acuminata; wild type p53 expression (Adv Anat Pathol 2006;13:8)
- HPV independent differentiated vulvar intraepithelial neoplasia: aberrant p53 expression, negative or focal to patchy p16 expression (Int J Gynecol Pathol 2008;27:591)
- Basal cell carcinoma: BCL2 and BerEP4 expression in tumor cells (Mod Pathol 2013;26:1382, Clin Cancer Res 2015;21:1289, Arch Dermatol 2003;139:643)
- Melanocytic markers (Arch Med Res 2020;51:827)
- MelanA / MART1 may help highlighting fused nests in nevi
- HMB45 (Semin Diagn Pathol 2022;39:239)
- In common junctional and compound nevi, expressed in melanocytes at dermal epidermal junction
- Highlights maturation (zonation) pattern (diminished or loss of expression from top to bottom)
- Highly variable in Spitz and congenital nevi
- SOX10 highlights junctional melanocytes to help differentiate melanoma in situ from benign counterparts
- Ki67 rate should be low in the dermal component of genital nevi
- HMB45 is commonly negative in intradermal and compound nevi
- PRAME (preferentially expressed antigen in melanoma) is typically lost in nevi (J Cutan Pathol 2020;47:1123)
Angiokeratoma by Filip Sokol
Angiokeratoma by Pathology mini tutorials
Atypical nevi and nevi of special sites by Dr. Phillip McKee
- Vulva, labium majus, biopsy:
- Angiokeratoma
- Vulva, biopsy:
- Melanocytic macule
- Vulva, left, biopsy:
- Intradermal nevus
- Vulva, labium minus, biopsy:
- Blue nevus
- Vulva, labia minora, biopsy:
- Atypical melanocytic nevus of the genital type, compound pattern
- Melanoma:
- Atypical melanocytes in epidermis as single cells or forming nests with pagetoid spread
- Dermal component shows no maturation with cells forming sheets, nests, cords, single cells and rarely fascicles
- S100, SOX10 and NGFR (nerve growth factor receptor) are the most sensitive markers in visualization of invasive growth
- PRAME shows diffuse staining
- Pigmented epithelioid melanocytoma (PEM) of vulva:
- PEM family consists of multiple, usually slow growing, distinct histologic melanocytic entities with potential to metastasize but with a better prognosis than melanoma (WHO 5th edition)
- Infiltrative deep dermal tumor that may involve subcutis
- Hypercellular tumor with cells ranging from medium sized epithelioid cells to large epithelioid cells and spindled cells
- Low mitotic activity
- PRKAR1A loss in 67% of PEMs (Am J Surg Pathol 2017;41:1333, Am J Surg Pathol 2019;43:480)
- Kaposi sarcoma:
- Associated with HIV / AIDS
- Increased number of anastomosing vascular channels in reticular dermis
- Promontory sign is rarely present: protrusion of native vessels into the lumen of dilated vascular neoplastic channels
- Infiltrative spindle cells with eccrine gland destruction
- Hemorrhage, extravasated erythrocytes and plasma cells are commonly present
- Nuclear HHV8 / LANA1 is positive in all cases of Kaposi sarcoma
- Pigmented fibroadenoma:
- Well circumscribed
- Uniformly paucicellular stroma surrounding epithelial component
- Focal intraluminal polypoid projections and cystic changes may be seen
- Pancytokeratin AE1 / AE3, CK7, estrogen and progesterone receptors (epithelial component)
- Purpura:
- Dilated superficial capillaries with extravasation of blood into the dermis
- Chronic forms may show hemosiderin laden macrophages and pigment incontinence in adjacent superficial dermis
- Am J Surg Pathol 1995;19:792, Dermatol Surg 2004;30:1118, J Cutan Pathol 2005;32:40, J Cutan Pathol 2008;35:889, Ackerman: Pathology of Malignant Melanoma, 1st Edition, 1981, McKee: Pathology of the Skin - With Clinical Correlations, 3rd Edition, 2005, WHO Classification of Tumours Editorial Board: Skin Tumours, 5th Edition, 2023
- Adnexal spread
- Dense fibrosis in papillary dermis
- Lack of a mature dermal component
- Pagetoid spread to granular layer in the center of the lesion
Comment Here
Reference: Pigmented lesions-vulva
A 65 year old woman presented with a dark brown macule with irregular borders measuring 0.7 cm in the largest dimension, located at the mucosal junction of right labia majora (left image). Shave biopsy is shown (right image). Which of the following is true for this patient's condition?
- HPV test will help to diagnose the lesion
- p63 will highlight neoplastic cells
- PRAME will highlight an in situ component
- SOX10 may demonstrate slight increase in density of the isolated melanocytes at the dermal - epidermal junction
Comment Here
Reference: Pigmented lesions-vulva
- Rare sarcoma showing evidence of skeletal muscle differentiation
- Multiple subtypes: embryonal, alveolar and pleomorphic
- Most common variant in the gynecologic tract is embryonal, which typically presents as a polypoid vaginal or cervical mass in children and adolescents
- Rhabdomyosarcoma originating in the uterine corpus, fallopian tube or ovary is less common and occurs over a wider age range
- Presence of rhabdomyoblasts, typically expressing MyoD1 or myogenin, is essential for diagnosis
- Sarcoma botryoides: refers to the macroscopic appearance of embryonal rhabdomyosarcoma (i.e. polypoid mass resembling a cluster of grapes)
- Dependent upon subtype and location:
- Embryonal (most common)
- Vagina: most common vaginal cancer in children < 5 years of age
- Uterine cervix: peak incidence 10 - 19 years but may occur in older women
- Uterine corpus: middle aged and older women
- Alveolar, pleomorphic (rare)
- Wide age range, usually older women
- Embryonal (most common)
- References: Int J Gynecol Cancer 2008;18:190, Gynecol Oncol 1988;29:290, Hum Pathol 2018;74:122
- Most common: cervix and vagina
- Rarely occurs in the uterine corpus, fallopian tube and ovary
- DICER1 alterations are identified in 65 - 95% of embryonal rhabdomyosarcomas of the uterine cervix, with ~50% of these patients harboring germline alterations; conversely, most DICER1 alterations in uterine corpus tumors are somatic (Mod Pathol 2020;33:1207, Mod Pathol 2021 May 20 [Epub ahead of print], Am J Surg Pathol 2020;44:738)
- ~80% of alveolar rhabdomyosarcomas demonstrate recurrent fusions involving FOXO1, most commonly t(2;13)(PAX3-FOXO1) or t(1;13)(PAX7-FOXO1) (Cancer Lett 2008;270:10)
- Subset of embryonal rhabdomyosarcomas, particularly of the uterine cervix, occur as a component of DICER1 syndrome with germline DICER1 mutations
- Dependent on tumor location and subtype
- Tumors of the vagina, uterine cervix and corpus may present with vaginal bleeding
- Embryonal rhabdomyosarcomas form a polypoid, grape-like cluster in the vagina and uterus which may protrude from the vagina (Am J Obstet Gynecol 1970;107:484, Gynecol Oncol 1988;29:290)
- Pleomorphic and alveolar rhabdomyosarcomas are more likely to form a solitary mass centered in the myometrium, cervical stroma or ovary (Hum Pathol 2018;74:122, Int J Gynecol Pathol 1998;17:113)
- Based on morphologic features and the identification of rhabdomyoblasts (by morphology or immunohistochemistry)
- Confirmatory genetic testing can be helpful in select cases
- References: Int J Gynecol Cancer 2008;18:190, Gynecol Oncol 1988;29:290, Hum Pathol 2018;74:122
- Embryonal rhabdomyosarcoma appears as a heterogenous mass, with cystic and solid components on ultrasound; CT demonstrates a similar appearance to that seen on ultrasound (Radiographics 1997;17:919)
- Overall 5 year survival for all subtypes: 68.4% (Arch Gynecol Obstet 2017;296:327)
- Favorable prognostic factors:
- Embryonal histology (Arch Gynecol Obstet 2017;296:327)
- Young age (Arch Gynecol Obstet 2017;296:327)
- Poor prognostic factors:
- Adult age (Am J Surg Pathol 2007;31:382)
- Alveolar rhabdomyosarcomas with proven FOXO1 fusions
- Alveolar rhabdomyosarcomas without FOXO1 fusions have a prognosis similar to embryonal rhabdomyosarcoma (Pediatr Blood Cancer 2016;63:634)
- Pleomorphic subtype (Cancer Med 2018;7:4023)
- Anaplasia in cases of embryonal rhabdomyosarcoma (Am J Surg Pathol 1993;17:443)
- 18 month old girl with an abdominal pelvic mass, vaginal bleeding and a protruding mass in the introitus (J Surg Case Rep 2017;2017:rjx080)
- 10 year old girl with a history of cystic nephroma and 3 week history of vaginal bleeding (J Pediatr Adolesc Gynecol 2020;33:173)
- 24 year old woman with a history of cervical embryonal rhabdomyosarcoma and a synchronous ovarian Sertoli-Leydig cell tumor (Gynecol Oncol Rep 2018;25:94)
- 54 year old postmenopausal woman with abdominal pain and vaginal bleeding (Cureus 2020;12:e9841)
- Embryonal rhabdomyosarcoma may be treated with a combination of surgery, chemotherapy and radiation therapy (Int J Gynecol Cancer 2008;18:190)
- Limited data in treating adults, nonembryonal subtypes and noncervicovaginal sites in the female genital tract
- Protocols are typically extrapolated from nongynecologic sites and involve a combination of resection (total hysterectomy and bilateral salpingo-oophorectomy) and chemotherapy with or without radiation (Int J Radiat Oncol Biol Phys 2013;86:58)
- Botryoid embryonal rhabdomyosarcoma: grape-like polypoid cluster of tan to gray, semitranslucent tissue protruding from the vagina or cervical os
- Uterine rhabdomyosarcoma: myometrially centered tumors with tan-white, fleshy cut surface with hemorrhage and necrosis
- References: Am J Obstet Gynecol 1970;107:484, Gynecol Oncol 1988;29:290
- Embryonal rhabdomyosarcoma (Gynecol Oncol 1988;29:290, Mod Pathol 2012;25:602, Mod Pathol 2021 May 20 [Epub ahead of print])
- Alternating hypercellular areas and hypocellular areas with myxoid / edematous (more common) or collagenous (less common) stroma
- Densely cellular subepithelial zone (cambium layer) composed of primitive, small cells with hyperchromatic nuclei and mitotic activity
- Rhabdomyoblasts typically seen in hypocellular foci
- Eccentric, dense eosinophilic cytoplasm
- Elongated strap cells (bipolar) or tadpole cells (unipolar) with eosinophilic cytoplasm and cross striations
- Hyaline or fetal type cartilage in ~50% of cases
- Alveolar rhabdomyosarcoma (Hum Pathol 2018;74:122)
- Nests and sheets of primitive small round cells with abundant eosinophilic cytoplasm
- Noncohesive cells floating in empty spaces create characteristic alveolar pattern
- Pleomorphic rhabdomyosarcoma (Hum Pathol 2018;74:122, Int J Gynecol Pathol 2010;29:122)
- Sheet-like growth of pleomorphic round to spindled cells
- Scattered large rhabdomyoblasts
- Clusters of atypical hyperchromatic round to ovoid cells (Diagn Pathol 2016;11:3)
- Occasional characteristic unipolar tadpole cells
- Desmin: diffusely positive in all subtypes
- Myogenin and MyoD1
- High sensitivity and specificity
- Diffuse nuclear staining in alveolar and pleomorphic subtypes
- Typically focal in embryonal
- Myoglobin: less sensitive than myogenin and MyoD1
- Also positive in mature tumors (rhabdomyoma)
- Reference: Am J Surg Pathol 2001;25:1150
- Keratins (rarely may be positive)
- S100
- CD34
- Reference: Am J Surg Pathol 2001;25:1150
- Embryonal rhabdomyosarcoma
- DICER1 alterations are identified in 65 - 95% of embryonal rhabdomyosarcomas of the uterine cervix, with ~50% of these patients harboring germline alterations; conversely, most DICER1 alterations in uterine corpus tumors are somatic (Mod Pathol 2020;33:1207, Mod Pathol 2021 May 20 [Epub ahead of print], Am J Surg Pathol 2020;44:738)
- Alveolar rhabdomyosarcoma
- Recurrent fusions of FOXO1 gene in ~80%, most commonly t(2;13)(PAX3-FOXO1) or t(1;13)(PAX7-FOXO1) (Cancer Lett 2008;270:10)
- Pleomorphic rhabdomyosarcoma
- Often complex karyotype without consistent genetic abnormality (Cancer Genet Cytogenet 2003;140:73)
- Cervix, polypectomy:
- Embryonal rhabdomyosarcoma (see comment)
- Comment: The tumor demonstrates polypoid fragments with myxoid / edematous stroma and scattered aggregates of primitive cells. Clusters of differentiating rhabdomyoblasts with focal cross striations are seen, which are highlighted by an immunohistochemical stain for desmin. There is also focal positivity for myogenin and myoD1, supporting the above diagnosis.
- Müllerian adenosarcoma:
- Most important differential consideration for botryoid embryonal rhabdomyosarcoma due to polypoid architecture and consolidation of stroma underneath epithelium
- Typically shows more abundant epithelial elements with phyllodes-like architecture
- Stromal overgrowth may make epithelial component difficult to identify but extensive sampling often reveals areas of classic adenosarcoma
- Fibroepithelial polyp, especially those with atypical cells:
- Malignant mixed Müllerian tumor (MMMT) with rhabdomyoblastic differentiation:
- Extensive sampling typically reveals the malignant epithelial component
- Undifferentiated endometrial carcinoma / undifferentiated uterine sarcoma:
- Uterine tumor resembling ovarian sex cord tumor:
- ESR1-NCOA2 fusions can show rhabdoid morphology (Am J Surg Pathol 2020;44:1563)
- Sex cord-like morphology may provide clue to diagnosis (corded, retiform, trabeculated)
- SMARCA4 deficient uterine sarcoma:
- Rhabdomyoma:
- Lacks cambium layer and primitive small cells
Which of the following is true about the pictured tumor?
- Benign entity with minimal chance of recurrence
- Most common presentation is a polypoid mass in the cervix or vagina of children and adolescents
- Most often occurs in the uterus of adult women
- Immunohistochemistry is unhelpful in establishing the correct diagnosis
Comment Here
Reference: Rhabdomyosarcoma
- Age < 10 years at diagnosis
- Embryonal histology
- FOXO1 gene fusion
- Somatic DICER1 mutation
- Leiomyoma and leiomyosarcoma are, respectively, the most common benign and malignant mesenchymal neoplasms of the vulva and vagina
- Subset of tumors with equivocal features is diagnosed as smooth muscle tumor of uncertain malignant potential (STUMP)
- Leiomyoma and leiomyosarcoma are, respectively, the most common benign and malignant vulvovaginal mesenchymal tumors
- The morphologic spectrum of vulvovaginal smooth muscle neoplasia mirrors that seen in the uterus, including leiomyoma variants
- Although myxoid stroma is more common in vulvovaginal smooth muscle tumors than in their uterine counterparts, conventional spindled morphology still predominates
- The diagnostic criteria for vulvovaginal leiomyosarcoma have evolved over the last 5 decades, with recent evidence suggesting that criteria for diagnosing malignancy in uterine smooth muscle tumors are also accurate in vulvovaginal tumors
- The diagnosis smooth muscle tumor of uncertain malignant potential (STUMP) is applied to tumors with worrisome morphologic features that fall short of the diagnostic threshold for leiomyosarcoma
- For uniformity of diagnosis, smooth muscle tumor of uncertain malignant potential should be used, when applicable, in place of atypical leiomyoma or atypical smooth muscle tumor
- Most common benign (leiomyoma) and malignant (leiomyosarcoma) vulvovaginal mesenchymal tumors (Obstet Gynecol 1979;53:689, Obstet Gynecol 1979;53:213, Obstet Gynecol 1995;86:269, Am J Surg Pathol 2018;42:84)
- Age range: 15 - 87 years (Obstet Gynecol 1979;53:689, Obstet Gynecol 1979;53:213, Am J Surg Pathol 2018;42:84, Hum Pathol 2020;103:83, Am J Surg Pathol 1996;20:779, Histopathology 1991;18:523)
- Most common in fourth and fifth decades
- Vagina > vulva (approximately 3:1) (Obstet Gynecol 1979;53:689, Obstet Gynecol 1979;53:213, Obstet Gynecol 1995;86:269, Am J Surg Pathol 2018;42:84)
- Vagina: upper, middle and lower thirds equally affected (Obstet Gynecol 1979;53:689)
- Vulva: mons pubis and labia majora > labia minora (Obstet Gynecol 1979;53:213, Histopathology 1991;18:523)
- May present incidentally or as a painless or tender prolapsing mass (Obstet Gynecol 1979;53:689, Obstet Gynecol 1979;53:213, Am J Surg Pathol 2018;42:84, Hum Pathol 2020;103:83, Histopathology 1991;18:523)
- Present for weeks to years before diagnosis
- Vulvar tumors often diagnosed clinically as Bartholin cyst
- Rarely, vaginal wall tumors grow posteriorly, impinging on the rectum or pouch of Douglas (Case Rep Obstet Gynecol 2017;2017:2190135)
- Fumarate hydratase (FH) deficient vulvovaginal smooth muscle tumors may be associated with hereditary leiomyomatosis renal cell carcinoma (HLRCC) syndrome (Mod Pathol 2021;34:767 - see abstract 614, page 735)
- Multiple vulvar leiomyomas (leiomyomatosis) may co-occur with multiple esophageal leiomyomas (retained FH expression, distinct from HLRCC) (Gynecol Oncol 1991;41:92)
- Definitive diagnosis best made on an excision specimen
- Diagnostic core biopsy obtained in a subset (particularly in vaginal tumors) (Case Rep Obstet Gynecol 2017;2017:2190135)
- MRI:
- Circumscription favors benign diagnosis (Indian J Radiol Imaging 2009;19:238)
- Infiltration highly worrisome for malignancy (Taiwan J Obstet Gynecol 2020;59:314)
- May show necrosis in leiomyosarcoma (Clin Imaging 2009;33:482)
- Ultrasound principally used to characterize vaginal tumors (J Midlife Health 2019;10:204, Indian J Radiol Imaging 2009;19:238, Medicine (Baltimore) 2021;100:e24911)
- CT:
- Examination of chest, abdomen and pelvis to exclude tumor metastasis
- PET avidity may highlight primary or recurrent leiomyosarcoma (Gynecol Oncol Rep 2017;21:28, Cureus 2016;8:e550)
- Leiomyoma: excellent prognosis
- Negligible recurrence risk, even with positive margins (Am J Surg Pathol 2018;42:84)
- Leiomyoma variants (plexiform, cellular, with bizarre nuclei) follow benign course (Hum Pathol 2020;103:83, Histopathology 1991;18:523)
- Smooth muscle tumor of uncertain malignant potential (STUMP): intermediate prognosis
- 1 of 5 recently reported cases recurred (Hum Pathol 2020;103:83)
- Few cases and limited follow up available
- Leiomyosarcoma: guarded prognosis
- In a recent study with median follow up of 64 months (Am J Surg Pathol 2018;42:84):
- Local or distant recurrence in 12 of 15 (80%) vulvovaginal leiomyosarcomas
- Distant metastasis in 10 of 15
- Death from disease in 8 of 15
- Recurrences reported up to 10 years after diagnosis (Am J Surg Pathol 1996;20:779)
- In a recent study with median follow up of 64 months (Am J Surg Pathol 2018;42:84):
- Leiomyoma
- 24 year old woman with a vaginal leiomyoma (Medicine (Baltimore) 2021;100:e24911)
- 36 year old woman with a vaginal leiomyoma (BMC Womens Health 2020;20:12)
- 39 year old woman with a vulvar leiomyoma BMC Womens Health 2020;20:89)
- 45 year old woman with a vulvar epithelioid leiomyoma (Medicine (Baltimore) 2019;98:e17423)
- 45 year old woman with a vaginal leiomyoma (J Midlife Health 2019;10:204)
- STUMP
- 34 year old woman with a vulvar STUMP (Gynecol Oncol Rep 2017;20:1)
- Leiomyosarcoma
- 37 year old woman with vulvar leiomyosarcoma (Cureus 2016;8:e550)
- 58 year old woman with vaginal leiomyosarcoma (Taiwan J Obstet Gynecol 2020;59:314)
- 63 year old woman with vulvar leiomyosarcoma (Oman Med J 2020;35:e153)
- 66 year old woman with vaginal leiomyosarcoma (J Gynecol Oncol 2008;19:261)
- 73 year old woman with vulvar leiomyosarcoma (Prz Menopauzalny 2020;19:184)
- Leiomyoma
- Conservative simple excision curative
- STUMP (based on limited data)
- Complete excision with negative margins
- At least 10 mm margin has been advocated (Histopathology 2000;36:97)
- Close follow up (Int J Gynecol Pathol 2001;20:105)
- Leiomyosarcoma
- Wide local excision
- (Neo)adjuvant chemotherapy or radiation per clinical indication (Cureus 2016;8:e550, J Gynecol Oncol 2008;19:261)
- Hormonal therapy may be effective in some cases (Gynecol Oncol Rep 2017;21:28)
- Effective use of neoadjuvant GnRH analog therapy reported (Medicine (Baltimore) 2021;100:e24911)
- Long term follow up required
- Genetic counseling advised for patients with morphologic or immunohistochemical features of FH deficiency
- Wide size range: 0.5 - 16 cm, with most Obstet Gynecol 1979;53:689, Obstet Gynecol 1979;53:213, Am J Surg Pathol 2018;42:84, Am J Surg Pathol 1996;20:779, Histopathology 1991;18:523)
- Broad size overlap between leiomyoma and leiomyosarcoma (Am J Surg Pathol 2018;42:84)
- Leiomyoma:
- Well circumscribed
- Rubbery, white-tan, whorled surface (Obstet Gynecol 1979;53:689, Obstet Gynecol 1979;53:213, Hum Pathol 2020;103:83)
- Leiomyosarcoma:
- May be well or poorly circumscribed
- Soft, fleshy to variegated cut surface
- Hemorrhage and necrosis may be apparent (Am J Surg Pathol 2018;42:84)
- Leiomyoma: morphologic spectrum similar to uterine smooth muscle tumors
- Spindle cell morphology most common (Am J Surg Pathol 1996;20:779, Histopathology 1991;18:523)
- Intersecting fascicles of spindle cells
- Cigar shaped nuclei
- Eosinophilic cytoplasm
- Epithelioid and myxoid morphologies well documented (Am J Surg Pathol 1996;20:779, Histopathology 1991;18:523, Medicine (Baltimore) 2019;98:e17423, Gynecol Oncol 2005;96:548)
- Myxoid change more common in vulvovaginal smooth muscle tumors than in uterine smooth muscle tumors
- Myxoid change may predominate in pregnancy (Obstet Gynecol 1979;53:213)
- Uncommon morphologic variants reported (Obstet Gynecol 1979;53:689, Obstet Gynecol 1979;53:213, Am J Surg Pathol 2018;42:84, Hum Pathol 2020;103:83, Am J Surg Pathol 1996;20:779, Histopathology 1991;18:523, Medicine (Baltimore) 2019;98:e17423, Gynecol Oncol 2005;96:548):
- Plexiform leiomyoma
- Cellular leiomyoma
- Leiomyoma with bizarre nuclei
- Fumarate hydratase (FH) deficient leiomyomas show characteristic morphologic features
- Nuclear atypia
- Orangeophilic macronucleoli with perinucleolar clearing
- Staghorn (hemangiopericytoma-like) vessels
- Alveolar pattern edema
- Chain-like nuclear arrangement
- Vaginal leiomyomas: degenerative hyalinization, calcification, hemorrhage and edema common (probably secondary traumatic changes) (Obstet Gynecol 1979;53:689)
- Spindle cell morphology most common (Am J Surg Pathol 1996;20:779, Histopathology 1991;18:523)
- Leiomyosarcoma: criteria for malignancy have evolved
- Original criteria for vaginal leiomyosarcoma (Obstet Gynecol 1979;53:689)
- ≥ 5 mitoses per 10 high power fields AND moderate or marked atypia OR
- Infiltrative border
- Original criteria for vulvar leiomyosarcoma (Obstet Gynecol 1979;53:213)
- ≥ 5 cm AND ≥ 5 mitoses per 10 high power fields AND infiltrative border
- Modified criteria for vulvar leiomyosarcoma (Am J Surg Pathol 1996;20:779)
- At least three of the following:
- Moderate to severe nuclear atypia
- ≥ 5 cm
- ≥ 5 mitoses per 10 high power fields
- Infiltrative border
- At least three of the following:
- Recent data suggest uterine criteria for leiomyosarcoma are applicable to vulvovaginal tumors (Am J Surg Pathol 2018;42:84, Hum Pathol 2020;103:83, Gynecol Oncol Rep 2017;20:1)
- At least two of the following:
- Moderate to severe nuclear atypia
- Mitoses ≥ 10 per 10 high power fields
- Coagulative tumor necrosis
- Uterine criteria for epithelioid and myxoid leiomyosarcoma not yet validated in vulvovaginal tumors (Medicine (Baltimore) 2019;98:e17423)
- At least two of the following:
- Original criteria for vaginal leiomyosarcoma (Obstet Gynecol 1979;53:689)
- STUMP: criteria difficult to establish, given rarity and lack of long term follow up
- Nielsen, et al., defined atypical leiomyoma by presence of two atypical features (Am J Surg Pathol 1996;20:779):
- Moderate to severe nuclear atypia
- ≥ 5 cm
- ≥ 5 mitoses per 10 high power fields
- Infiltrative border
- Nucci and Fletcher defined STUMP by presence of any of the following (Histopathology 2000;36:97):
- Infiltrative margins
- Nuclear atypia
- Any mitotic activity
- More recent data suggest that uterine criteria for STUMP may apply to vulvovaginal tumors (Hum Pathol 2020;103:83, Gynecol Oncol Rep 2017;20:1)
- Nielsen, et al., defined atypical leiomyoma by presence of two atypical features (Am J Surg Pathol 1996;20:779):
- CD34
- S100
- Cytokeratins
- Note: CAM5.2 may show cross reactivity with smooth muscle
- Myogenin
- MyoD1
- FH deficiency reported in a subset (Mod Pathol 2021;34:767 - see abstract 614, page 735)
- Pleomorphic cells with complex cytoplasmic projections (Taiwan J Obstet Gynecol 2020;59:314)
- Well developed rough endoplasmic reticulum
- Large indented nuclei with prominent nucleoli
- Myofilaments may be rare
- Intercellular junctions absent
- Leiomyoma:
- Right labial mass, excision:
- Leiomyoma (3 cm) (see comment)
- Margins negative
- Comment: Microscopic examination reveals a well circumscribed tumor composed of fascicles of bland spindle cells with abundant eosinophilic cytoplasm. The tumor is entirely submitted for histologic examination. No necrosis or mitoses are identified. The morphology is consistent with a leiomyoma.
- Right labial mass, excision:
- STUMP:
- Right labial mass, excision:
- Smooth muscle tumor of uncertain malignant potential (4 cm) (see comment)
- Margins negative
- Comment: Microscopic examination reveals a smooth muscle tumor, comprised of fascicles of spindle cells with moderate to focally marked nuclear atypia. Mitoses number up to 6 per 10 high power fields. There is no necrosis and the tumor is well circumscribed, without infiltration of surrounding vulvar tissues. Margins are negative (tumor approaches to within 0.3 cm of one unoriented inked margin, block A3). The morphologic findings, including nuclear atypia and increased mitoses, are worrisome but do not reach the threshold for diagnosis of leiomyosarcoma. Accordingly, this tumor is best classified as a smooth muscle tumor of uncertain malignant potential (STUMP). Recent reports indicate that vulvovaginal STUMPs have the potential for local recurrence (see reference) but specific prognostic factors are not well defined. Clinical correlation is advised and close clinical follow up is recommended (Hum Pathol 2020;103:83).
- Right labial mass, excision:
- Leiomyosarcoma:
- Right labial mass, wide local excision:
- Leiomyosarcoma (6.5 cm) (see comment)
- Comment: Microscopic examination reveals a high grade spindle cell neoplasm, composed of fascicles of atypical to focally pleomorphic cells, with conspicuous mitoses (up to 12 per 10 high power fields), including atypical forms. Tumor type coagulative necrosis is identified and there is multifocal tumor infiltration of surrounding vulvar tissues. Margins are negative (tumor approaches to within 0.4 cm of the inked lateral margin). Immunostains for SMA, desmin and caldesmon are positive, whereas CD34, S100 and CK AE1 / AE3 immunostains are negative. The findings are diagnostic of leiomyosarcoma. Correlation with clinical and radiographic findings is advised.
- Right labial mass, wide local excision:
- Cellular angiofibroma (Am J Surg Pathol 2018;42:84):
- Angiomyofibroblastoma:
- Alternating hypo and hypercellular foci
- Short spindle to epithelioid or plasmacytoid cells, clustered around abundant small, thin walled vessels
- Aggressive angiomyxoma:
- Inflammatory myofibroblastic tumor:
- Conspicuous inflammatory infiltrate, often with plasma cells
- ALK IHC positive and rearranged by molecular testing
- Solitary fibrous tumor:
- Variable (patternless) architecture
- Alternating hypocellular and hypercellular zones
- Staghorn (HPC-like) vasculature
- Adipocytic component in a subset
- STAT6 IHC positive
- NAB2-STAT6 gene fusion
- PEComa:
- Epithelioid cells with clear to eosinophilic and granular cytoplasm
- HMB45, MelanA, PNL2, cathepsin K positive
- Melanoma:
- Myoepithelial neoplasm:
- Prominent myxoid stroma
- Coexpression of EMA, cytokeratin and S100 typical
- GFAP and p63 expressed in ~50%
- SMARCB1 / INI1 lost in ~50%
- Dermatofibrosarcoma protuberans:
- Spindle cells arranged in short storiform fascicles
- Sarcomatous transformation: increased mitoses, increased atypia, more sweeping fascicles in a herringbone pattern
- Dermal based tumor with subcutaneous infiltration (characteristic honeycomb fat trapping)
- CD34 positive
- Desmin and SMA negative
- COL1A1-PDGFRB t(17;22) fusion
A 36 year old woman undergoes excision of a 3 cm, slow growing, painless vulvar mass. The lesion is well circumscribed and microscopic examination reveals no nuclear atypia, necrosis or mitotic activity. A representative photomicrograph is shown above. Immunostains for smooth muscle actin, desmin and caldesmon are positive. Which of the following statements is true regarding the depicted tumor?
- Approximately 50% recur after simple local excision
- CD34 immunohistochemistry is positive in > 90% of cases
- Estrogen and progesterone receptor are expressed in Plexiform architecture merits a malignant diagnosis
- This is the most common vulvar mesenchymal neoplasm
Comment Here
Reference: Smooth muscle tumors
A 60 year old woman presents with vaginal bleeding and is found to have a 7 cm prolapsing mass. A preoperative MRI shows infiltration of the rectovaginal septum. A core biopsy is obtained, showing atypical spindle cells with brisk mitoses (up to 12 per 10 high power fields) and coagulative necrosis. A representative photomicrograph is shown above. Immunostains for desmin and caldesmon are positive. Which of the following statements is true regarding the depicted tumor?
- Local recurrence or distant metastasis occur in > 50%
- Most are grossly and microscopically well circumscribed
- Most cases show predominantly epithelioid morphology
- MyoD1 immunohistochemistry is strongly and diffusely positive
- Positive surgical margins are not associated with increased risk of local recurrence
Comment Here
Reference: Smooth muscle tumors
- Primary squamous cell carcinoma arising in vagina without involvement of surrounding structures, such as cervix or vulva
- Comprises 1 - 3% of all gynecological cancers (Int J Gynecol Cancer 1994;4:389, Gynecol Oncol 2013;131:380, Crit Rev Oncol Hematol 2015;93:211)
- 80 - 90% of primary vaginal cancers are squamous cell carcinomas (Crit Rev Oncol Hematol 2015;93:211)
- Usually in 6th decade of life (median age 58 to 68) but can also be seen in younger patients (Int J Gynecol Cancer 1994;4:389, Gynecol Oncol 2013;131:380)
- Most common in upper half of vagina (Int J Gynecol Cancer 1994;4:389, Gynecol Oncol 2013;131:380, Crit Rev Oncol Hematol 2015;93:211)
- Tumor may be missed on initial examination if small and involves lower 2/3 of vagina (Int J Gynecol Cancer 1994;4:389)
- In some cases, vaginal intraepithelial neoplasia (VAIN) can be found prior to invasive squamous cell carcinoma
- History of prior hysterectomy in up to 50% of cases
- Also associated with vaginal or uterovaginal prolapse (Crit Rev Oncol Hematol 2015;93:211)
- Strong relationship with high risk human papilloma virus (HPV), especially HPV 16 (seen in up to 80% of cases), HPV 18 and HPV 31
- More common in smokers because smoking increases the risk of high grade VAIN in women with oncogenic HPV (Crit Rev Oncol Hematol 2015;93:211)
- Most common symptom is vaginal bleeding or discharge (Int J Gynecol Cancer 1994;4:389, Gynecol Oncol 2013;131:380, Crit Rev Oncol Hematol 2015;93:211)
- Other symptoms include urinary symptoms and lower abdominal pain (Int J Gynecol Cancer 1994;4:389)
- May remain asymptomatic, especially if small
- Clinical history along with histological features on biopsy / resection specimen
- Tumor involving both the vagina and the cervix should be classified as a cervical carcinoma; similarly a tumor involving both the vagina and the vulva should be considered a vulvar carcinoma
- Imaging required to determine extent of disease and to look for distant metastasis
- FIGO stage is most important predictor of overall survival
- Tumor size > 4 cm associated with decreased local control and lower overall survival, while total radiation dose in excess of 70 Gy is associated with improved local control of disease and improved overall survival (Gynecol Oncol 2013;131:380, Crit Rev Oncol Hematol 2015;93:211)
- Vaginal squamous cell carcinoma can spread to vulva, cervix, bladder, rectum and through lymphatics can metastasize to obturator, hypogastric, external iliac and groin nodes
- Rarely distant metastasis to liver, lungs, bones and brain (Crit Rev Oncol Hematol 2015;93:211)
- 28 year old woman with invasive squamous cell carcinoma of vagina during pregnancy (Obstet Gynecol 2002;100:1105)
- 39 year old woman with primary vaginal squamous cell carcinoma arising in a squamous inclusion cyst (Cesk Patol 2012;48:153)
- 57 year old woman with synchronous papillary cystadenocarcinoma of ovary and squamous cell carcinoma of lower vagina (BMJ Case Rep 2013 Jan 11;2013)
- 59 year old woman with vaginal cancer and complicated prolapse history (Female Pelvic Med Reconstr Surg 2014;20:295)
- 68 year old woman with vaginal cancer and multiple liver and pulmonary metastases (Obstet Gynecol Sci 2013;56:416)
- 84 year old woman with carcinoma of vagina in uterovaginal prolapse (JNMA J Nepal Med Assoc 2012;52:82)
- Aggressive primary invasive vaginal carcinoma associated with HPV 61 (Tumori 2012;98:57e)
- Various treatment modalities are used including external beam radiation therapy / EBRT, interstitial brachytherapy, intracavitary brachytherapy, chemotherapy and surgical resection
- Mainstay of treatment is typically definitive radiation therapy with external beam radiation or brachytherapy (Gynecol Oncol 2013;131:380, Int J Clin Oncol 2013;18:314, Crit Rev Oncol Hematol 2015;93:211)
- Surgical resection is recommended for early stage cancer involving upper posterior vagina (Gynecol Oncol 2013;131:380)
- For stage I disease, surgery consists of radical hysterectomy, upper vaginectomy and pelvic lymphadenectomy (Crit Rev Oncol Hematol 2015;93:211)
- Cisplatin based chemotherapy can be administered with radiation therapy (Gynecol Oncol 2013;131:380)
- Exophytic or ulcerative with necrosis
- Histologically graded as well differentiated (G1), moderately differentiated (G2), poorly differentiated or undifferentiated (G3) (Crit Rev Oncol Hematol 2015;93:211)
- Well differentiated tumors have polygonal squamous cells with ample eosinophilic cytoplasm, abundant keratin pearls and intercellular bridges
- Poorly differentiated tumors have small cells with scant cytoplasm and hyperchromatic nuclei
- Nuclear pleomorphism and mitotic activity increases from well to poorly differentiated
- Moderately differentiated tumors have histological features intermediate between well and poorly differentiated
- HPV+ tumors are more frequently of nonkeratinizing, basaloid or warty type than HPV- tumors (84% versus 14.3%; p < 0.001) and more often showed diffuse p16 immunoreactivity (96% versus 14.3%, p < 0.001)
- Keratinizing squamous cell carcinomas have polygonal cells with bizarre shapes including spindle shaped and tadpole cells, with dense orangeophilic / eosinophilic cytoplasm
- Cells can present singly or in small groups in a dirty necrotic background
- Nonkeratinizing SCC needs to be differentiated from repair and adenocarcinoma
Pathologic TNM staging of carcinoma of the vagina, AJCC 8th edition and FIGO 2018 update
- Based on the American Joint Committee on Cancer (AJCC) Staging Manual (8th edition) and the International Federation of Gynecology and Obstetrics (Fédération Internationale de Gynécologie et d'Obstétrique, FIGO) 2018 update
- Includes tumors with primary site of growth in vagina only; tumors with secondary spread to the vagina from other genital (vulva, cervix, endometrium) or extragenital sites should not be included
- Staging is mostly clinical; however, information available from pathologic evaluation of resection specimens needs to be used
- Based on AJCC Cancer Staging Manual (8th edition) and the FIGO 2018 update
- pNX: regional lymph nodes cannot be assessed
- pN0: no regional lymph node metastasis
- pN0(i+): isolated tumor cells in regional lymph node(s) ≤ 0.2 mm
- pN1 (III): pelvic or inguinal lymph node metastasis
- pM0: no distant metastasis
- pM1 (IVB): distant metastasis
| Stage 0: | Tis | N0 | M0 |
| Stage I: | T1 | N0 | M0 |
| Stage II: | T2 | N0 | M0 |
| Stage III: | T1 - 3 | N1 | M0 |
| T3 | N0 | M0 | |
| Stage IVA: | T4 | any N | M0 |
| Stage IVB: | any T | any N | M1 |
- Depth of invasion
- Involvement of paravaginal adipose tissue
- Lymphovascular space invasion
- Multifocality
- Tumor size
Comment Here
Reference: Staging-vagina carcinoma
Pathologic TNM staging of carcinoma of the vulva, AJCC 8th edition and FIGO 2018 update
- Based on the American Joint Committee on Cancer (AJCC) Staging Manual (8th edition) and the International Federation of Gynecology and Obstetrics (Fédération Internationale de Gynécologie et d'Obstétrique, FIGO) 2018 update
- Includes tumors with primary site of growth in vulva only; excludes mucosal melanoma
- Depth of invasion is defined as the measurement of the tumor from the epithelial stromal junction of the adjacent most superficial dermal papilla to the deepest point of invasion
- AJCC / FIGO no longer uses Tis
- Site and laterality of lymph node metastases should be described
- Multifocality does not affect staging, although it is a prognostic factor; thus, multifocal lesions should be designated as such
- Largest lesion or lesion with the greatest depth of invasion will determine the pT stage
- Based on AJCC Cancer Staging Manual (8th edition) and the FIGO 2018 update
- pTX: primary tumor cannot be assessed
- pT0: no evidence of primary tumor
- pT1 (I): tumor confined to the vulva or perineum
- pT1a (IA): lesions ≤ 2 cm in size, confined to the vulva or perineum and with stromal invasion ≤ 1.0 mm
- pT1b (IB): lesions > 2 cm in size or any size with stromal invasion > 1.0 mm, confined to the vulva or perineum
- pT2 (II): tumor of any size with extension to adjacent perineal structures (lower / distal 1/3 of urethra, lower / distal 1/3 of vagina, anal involvement)
- pT3 (IVA): tumor of any size with extension to any of the following: upper / proximal 2/3 of urethra, upper / proximal 2/3 of vagina, bladder mucosa, rectal mucosa or fixed to pelvic bone
- pNX: regional lymph nodes cannot be assessed
- pN0: no regional lymph node metastasis
- pN0(i+): isolated tumor cells in regional lymph node(s) ≤ 0.2 mm
- pN1 (IIIA): 1 or 2 regional lymph nodes with the following features:
- pN1a (IIIA): 1 or 2 lymph node metastases < 5 mm **
- pN1b (IIIA): 1 lymph node metastasis ≥ 5 mm
- pN2 (IIIB): regional lymph node metastasis with the following features:
- pN2a (IIIB): 3 or more lymph node metastases each < 5 mm **
- pN2b (IIIB): 2 or more lymph node metastases ≥ 5 mm
- pN2c (IIIC): lymph node metastasis with extracapsular spread
- pN3 (IVA): fixed or ulcerated regional lymph node metastasis
** Includes micrometastases (N1mi and N2mi)
- pM0: no distant metastasis
- pM1 (IVB): distant metastasis (including pelvic lymph node metastasis)
| Stage 0: | Tis | N0 | M0 |
| Stage I: | T1 | N0 | M0 |
| Stage IA: | T1a | N0 | M0 |
| Stage IB: | T1b | N0 | M0 |
| Stage II: | T2 | N0 | M0 |
| Stage IIIA: | T1 - 2 | N1a - b | M0 |
| Stage IIIB: | T1 - 2 | N2a - b | M0 |
| Stage IIIC: | T1 - 2 | N2c | M0 |
| Stage IVA: | T1 - 2 | N3 | M0 |
| T3 | any N | M0 | |
| Stage IVB: | any T | any N | M1 |
- Depth of invasion is defined as the measurement of the tumor from the tumor surface to the deepest point of invasion
- Depth of invasion of > 1 mm, in a tumor confined to the vulva, qualifies as stage IB disease
- Multifocal lesions are by definition stage IB
- Size of metastatic tumor to lymph nodes and extranodal extent are not required for staging
Comment Here
Reference: Staging-vulva carcinoma
- Vaginal intraepithelial neoplasia (VAIN) is defined as squamous cell dysplasia including maturation abnormalities and cytopathic changes, which do not extend beyond the basement membrane
- VAIN lesions are divided into a 2 tier system, low grade (VAIN 1) and high grade (VAIN 2 - 3) (Arch Pathol Lab Med 2012;136:1266)
- Squamous dysplasia, which does not extend beyond the basement membrane
- Most commonly associated with human papillomavirus (HPV) but may also be associated with tobacco use, postmenopause and history of cervical intraepithelial neoplasia (CIN) (Chin Med J (Engl) 2012;125:1219)
- This disease is rare, representing PLoS One 2016;11:e0167386)
- Related terminology includes low grade squamous intraepithelial lesion (LSIL) (VAIN 1 / mild squamous dysplasia) and high grade squamous intraepithelial lesion (HSIL) (VAIN 2 / moderate squamous dysplasia and VAIN 3 / severe squamous dysplasia)
- True incidence of VAIN is unknown but is estimated to be 0.1 cases per 100,000 women in the U.S. (J Womens Health (Larchmt) 2009;18:1731)
- Current reported values may underestimate the incidence
- Risk factors include advanced age, HPV infection, concomitant cervical intraepithelial neoplasia, cervical cancer and hysterectomy (J Obstet Gynaecol Res 2021;47:1624)
- Mean age is ~50 - 60 years although can occur over a wide age range (22 - 80 years) (Acta Obstet Gynecol Scand 1999;78:648, J Obstet Gynaecol Res 2010;36:94)
- Recurrence rate is high and malignant potential is well established
- More common in upper third of the vagina and fornices (J Obstet Gynaecol Res 2010;36:94)
- Exact pathophysiological mechanisms are still under investigation
- HPV E6 and E7 protein expression induce high grade changes of squamous epithelium (Nat Rev Dis Primers 2016;2:16086)
- HPV has been implicated in pathogenesis of VAIN
- HPV associated lesions are often multifocal and multicentric and foci of squamous intraepithelial neoplasia in the cervix are commonly found (Eur J Gynaecol Oncol 2017;38:10)
- Prevalence of HPV in VAIN 2 - 3 and VAIN 1 is 92.6% and 98.5%, respectively, higher than in vulvar lesions (Obstet Gynecol 2009;113:917)
- VAIN 1 / LSIL may be associated with either low risk or high risk HPV types, while VAIN 2 - 3 / HSIL is associated with high risk HPV
- HPV 16 is the most common HPV type in vaginal (55.4%) cancers and VAIN 2 - 3 (65.8%) (Obstet Gynecol 2009;113:917, Int J Cancer 2019;145:78)
- On the basis of their association with premalignant and malignant lesions, 13 HPV types (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68) have been defined as high risk (HR) (J Pathol 2014;234:431)
- History of prior pelvic radiation (7.4%), associated neoplasia of the lower genital tract (67.6%) and history of prior hysterectomy (54.4%) (J Obstet Gynaecol Res 2010;36:94)
- Arises from native squamous epithelium, not metaplastic epithelium as in cervix
- Lesions may be raised, flat, white, gray or have irregular pigmentation
- VAIN is usually asymptomatic but may present with postcoital spotting or vaginal discharge
- Most common presentation is abnormal cytology (J Obstet Gynaecol Res 2010;36:94)
- Colposcopy directed biopsy has a sensitivity of > 80% (Arch Gynecol Obstet 2020;301:769)
- Must be excluded in all women with an abnormal Pap smear who had a hysterectomy or who do not have identifiable cervical lesions that could account for the abnormality
- VAIN is a histologic diagnosis, typically based on colposcopic assessment and directed biopsy of the vagina (J Obstet Gynaecol Res 2010;36:94)
- Lugol iodine solution can be used to detect lesions and confirm boundaries prior to excision
- HSIL / VAIN 2 - 3 is a precursor to invasive squamous cell carcinoma of the vagina
- HPV serotypes 16 and 18 are associated more with VAIN 2 - 3 (HSIL)
- HPV serotypes 6 and 11 are associated more with VAIN 1 (LSIL)
- Reference: Obstet Gynecol 2009;113:917
- 32 year old woman with concurrent nonavalent HPV vaccination and immune stimulation with imiquimod to treat recalcitrant HPV associated high grade vaginal intraepithelial neoplasia (Gynecol Oncol Rep 2024;52:101350)
- 39 year old woman with vaginal intraepithelial neoplasia 3, without simultaneous cervical intraepithelial neoplasm (J Med Cases 2020;11:246)
- 53 year old woman with recurrent vaginal intraepithelial neoplasia successfully treated with topical imiquimod (Mol Clin Oncol 2020;13:19)
- 54 year old woman with inherited chromosomally integrated human herpesvirus type 6A and vaginal neoplasia induced by an unusual papillomavirus subtype (Gynecol Obstet Invest 2017;82:307)
- 10 cases of vaginal intraepithelial neoplasia after radiation therapy for gynecologic malignancies (Gynecol Oncol 2011;120:108)
- Case series of 657 women with intraepithelial neoplasia (Eur J Gynaecol Oncol 2022;43:42)
- Surgical excision is the mainstay of VAIN treatment (J Low Genit Tract Dis 2012;16:306)
- Surgical approaches include local excision, partial vaginectomy; rarely total vaginectomy for extensive and persistent disease
- Partial or total vaginectomy appears to be the safest method of treating multifocal high grade VAIN
- Complications include shortening or stenosis of the vagina following wide local excision and significant postoperative morbidity following abdominal procedures
- CO2 laser therapy is also used for local tissue ablation, with pain and bleeding being the most frequent complications (J Reprod Med 1990;35:941)
- Ablative therapy should not be performed if the entire area of abnormal epithelium cannot be visualized or if there is any suspicion of invasion through colposcopy
- Medical therapy
- Topical application of therapeutic agents has the advantage of treating the entire vaginal mucosa with good coverage of multifocal disease and disease in folds and recesses of the vagina
- Imiquimod 5% cream is commonly used (Int J Gynaecol Obstet 2008;101:3, Int J Gynecol Cancer 2005;15:898, J Low Genit Tract Dis 2003;7:290, J Low Genit Tract Dis 2012;16:306)
- Complications of topical fluorouracil (5FU) include vaginal irritation or burning and ulcerations (Obstet Gynecol 1981;58:580)
- Radiation therapy includes high dose brachytherapy (Gynecol Oncol 1997;65:74)
- Conservative options in the form of laser ablation and topical agents are useful as first line treatment methods, especially in young women and for multifocal disease
- Radical options like brachytherapy and vaginectomy should be reserved for highly selected cases (J Low Genit Tract Dis 2012;16:306)
- On colposcopy with application of 3 - 5% acetic acid, lesions appear as raised or flat white, granular epithelium with sharply demarcated borders with punctation and mosaic pattern more prevalent in VAIN 2 - 3 (J Obstet Gynaecol Res 2010;36:94)
- By definition, lesions must not extend beyond the basement membrane
- Basal cells may display large, dark nuclei with basophilic cytoplasm
- Affected cells may display koilocytic / viral cytopathic changes and eosinophilic cytoplasm as they approach the surface
- VAIN is classified in a similar manner to cervical dysplasia / cervical intraepithelial neoplasia
- VAIN 1: mild dysplasia
- VAIN 2: moderate dysplasia
- VAIN 3: severe dysplasia / carcinoma in situ
- VAIN is classified according to the depth of epithelial involvement
- VAIN 1 involves the lower one - third of epithelium
- VAIN 2 involves lower two - thirds of epithelium
- VAIN 3 involves more than two - thirds of epithelium
- Carcinoma in situ, which encompasses the full thickness of the epithelium, is included under VAIN 3
- Low grade VAIN corresponds to VAIN 1 and is analogous to LSIL
- High grade VAIN corresponds to VAIN 2 - 3 and is analogous to HSIL
- Reference: Am J Obstet Gynecol 2013;208:410.e1
- Cytological features are similar to cervical Pap smear
- Low grade: mild squamous dysplasia, including koilocytic atypia and nuclei that are enlarged at least 3 - 4 times that of the normal intermediate cell nucleus; HPV cytopathic changes including distinct perinuclear cytoplasmic halo and binucleation or multinucleation may be noted
- High grade: moderate to severe squamous dysplasia comprising high N:C ratio, immature cytoplasm and greater nuclear pleomorphism
- Reference: Am J Obstet Gynecol 2013;208:410.e1
- p16 strong diffuse block positivity involving basal keratinocytes and extending beyond the lower third of the epithelium is found in nearly all high grade lesions
- Low risk HPV E7 protein expression may produce focal or weak p16 staining in lower grade lesions
- p53 immunostaining pattern is wild type
- Ki67 proliferation index may be elevated in both low grade and high grade lesions
- Reference: J Obstet Gynaecol Res 2018;44:2077
- Vagina, 3 o’clock, biopsy:
- Low grade squamous intraepithelial lesion (VAIN 1)
- Atrophy / transitional metaplasia (StatPearls: Genitourinary Syndrome of Menopause [Accessed 24 October 2024]):
- Squamous cell carcinoma:
- Evaluation of the basement membrane is necessary to rule out an invasive process
- HPV 16
- HPV 18
- HPV 31
- HPV 33
- HPV 35
Comment Here
Reference: Vaginal intraepithelial neoplasia (VAIN)
A raised vaginal lesion in a 52 year old woman was biopsied and showed the features in the image above. What is your diagnosis?
- High grade squamous intraepithelial lesion (HSIL) / VAIN 2
- HSIL / VAIN 3
- Low grade squamous intraepithelial lesion (LSIL) / VAIN 1
- Reactive changes, no dysplasia is identified
Comment Here
Reference: Vaginal intraepithelial neoplasia (VAIN)
- Benign cysts located within the vulva or vagina
- Vulvovaginal cysts may be congenital or acquired
- Congenitally derived vulvovaginal cysts may represent an embryological derivative, a result of an urological abnormality or ectopic tissue
- Cysts of the vagina are relatively common and represent generally benign conditions
- Bartholin gland cyst: often a product of chronic bacterial inflammation; nonsulfated sialomucin secretion product
- Epidermal / epithelial inclusion cyst: lined by squamous epithelium; may occur post surgery or trauma
- Gartner duct cyst: mesonephric origin (rare), non mucin secreting
- Müllerian cyst: lined by mucin secreting columnar cell
- Urothelial cyst: derived from periurethral and Skene glands
- Mammary-like gland cyst
- Cyst of canal of Nuck: mesothelial cyst
- Endometriotic cyst / cystic endometriosis
- Vaginitis emphysematosa: variably sized vaginal nodules that produce a characteristic popping sound
- Müllerian cyst
- Comprises 30 - 44% of all vaginal cysts (Obstet Gynecol Surv 2021;76:101)
- Gartner duct cyst
- Most commonly identified vaginal cyst in children (Obstet Gynecol Surv 2021;76:101)
- Accounts for 4 - 21% of all vaginal cysts in adult females (Obstet Gynecol Surv 2021;76:101)
- Bartholin gland cyst
- Accounts for 7 - 21% of all vaginal cysts in reproductive age females (Obstet Gynecol Surv 2021;76:101)
- Vaginitis emphysematosa
- Rarely reported (Arch Pathol Lab Med 1987;111:746)
- Average age ranges from 42 to 65 years
- Gartner duct cyst
- Most commonly located along the anterolateral vaginal wall at 11 o'clock and 1 o'clock regions
- May extend deep into the vaginal wall
- Bartholin gland cyst
- Found in the posterolateral inferior third of the vagina
- Urothelial cyst
- Usually situated low in the vagina, close to the urethra due to its urogenital sinus derivation
- Müllerian cyst
- May be found anywhere in the vagina but most commonly found in the vulvar vestibule
- Gartner duct cyst
- Mesonephric duct (Wolffian duct) normally forms genital organs in males and regresses in females; a remnant of mesonephric duct sometimes fails to regress and results in the formation of a Gartner duct cyst (JNMA J Nepal Med Assoc 2020;58:505)
- Bartholin gland cyst
- Often results from a blockage of the Bartholin gland due to inflammation, trauma, childbirth or infection (StatPearls: Bartholin Gland Cyst [Accessed 8 December 2021])
- Müllerian cyst
- Formation occurs when a portion of Müllerian epithelium fails to involute during the normal replacement of Müllerian epithelium with squamous epithelium of the urogenital sinus (Differentiation 2018;103:46)
- May show focal squamous metaplasia (Differentiation 2018;103:46)
- Epidermal / epithelial inclusion cyst
- May occur post surgery or trauma
- May present as a painful swollen cystic lesion
- Often asymptomatic
- Most diagnosed clinically by position and appearance
- If clinical diagnosis remains unclear, further evaluation with transvaginal ultrasound (TVUS) may be employed (Aust N Z J Obstet Gynaecol 2018;58:388)
- Histopathological confirmation in surgically excised specimens
- Ultrasound findings: well defined, unilocular cyst
- 29 year old woman with a recurrent cystic mass and an enlarged left labia majora (Case Rep Womens Health 2019;24:e00141)
- 32 year old woman with a chief complaint of pelvic organ prolapse (JNMA J Nepal Med Assoc 2020;58:505)
- 57 year old woman with a bulky appearing left labial mass (Case Rep Womens Health 2019;22:e00115)
- Bartholin duct cyst: complete excision or marsupialization with antibiotic administration if necessary
- Bartholin duct cyst
- Lined by residual mucinous epithelium, low cuboidal or transitional epithelium
- May exhibit focal squamous metaplasia or denudation
- Müllerian cyst
- Lined by mucin secreting or ciliated (tubal type) columnar cells
- May show focal squamous metaplasia
- Gartner duct cyst
- Lined by a single layer of cuboidal to low columnar nonmucinous epithelium
- Urothelial cyst
- Lined by transitional or squamous epithelium
- Epidermal / epithelial inclusion cyst
- Lined by squamous epithelium (keratinizing or nonkeratinizing)
- Mammary-like gland cyst
- Ectopic mammary tissue may present as a persistent vulvar cyst (World J Surg Oncol 2009;7:70)
- Cyst of canal of Nuck
- Lined by mesothelial cells
- Endometriotic cyst / cystic endometriosis
- Cystic endometrial type glands surrounded by variable amount of endometrial stroma and hemosiderin laden macrophages
- Vaginitis emphysematosa
- Variably sized cysts with pink, hyaline-like material, foreign body giant cells and chronic inflammatory infiltrate (Arch Pathol Lab Med 1987;111:746)
Bartholin gland cyst of the vulva - histopathology
- Vulva, right posterior, cystectomy:
- Bartholin gland cyst (2 cm)
- Urethral diverticulum:
- Communication with urethra detected by MRI (AJR Am J Roentgenol 1993;161:809)
- Diagnostic confirmation at the time of surgery
A 42 year old woman presents with a painful cystic swelling along the right posterolateral vulva, near the vaginal introitus. The cyst is excised and sent to pathology, where a benign appearing cystic lesion lined by simple cuboidal epithelium with areas of denudation and surrounding inflammation are seen. These findings, along with the image shown above, best describe which of the following vulvovaginal cysts?
- Bartholin cyst
- Epidermal inclusion cyst
- Gartner duct cyst
- Urothelial cyst
- Bartholin cyst
- Epidermal inclusion cyst
- Gartner duct cyst
- Urothelial cyst
Find related Pathology books: gynecologic