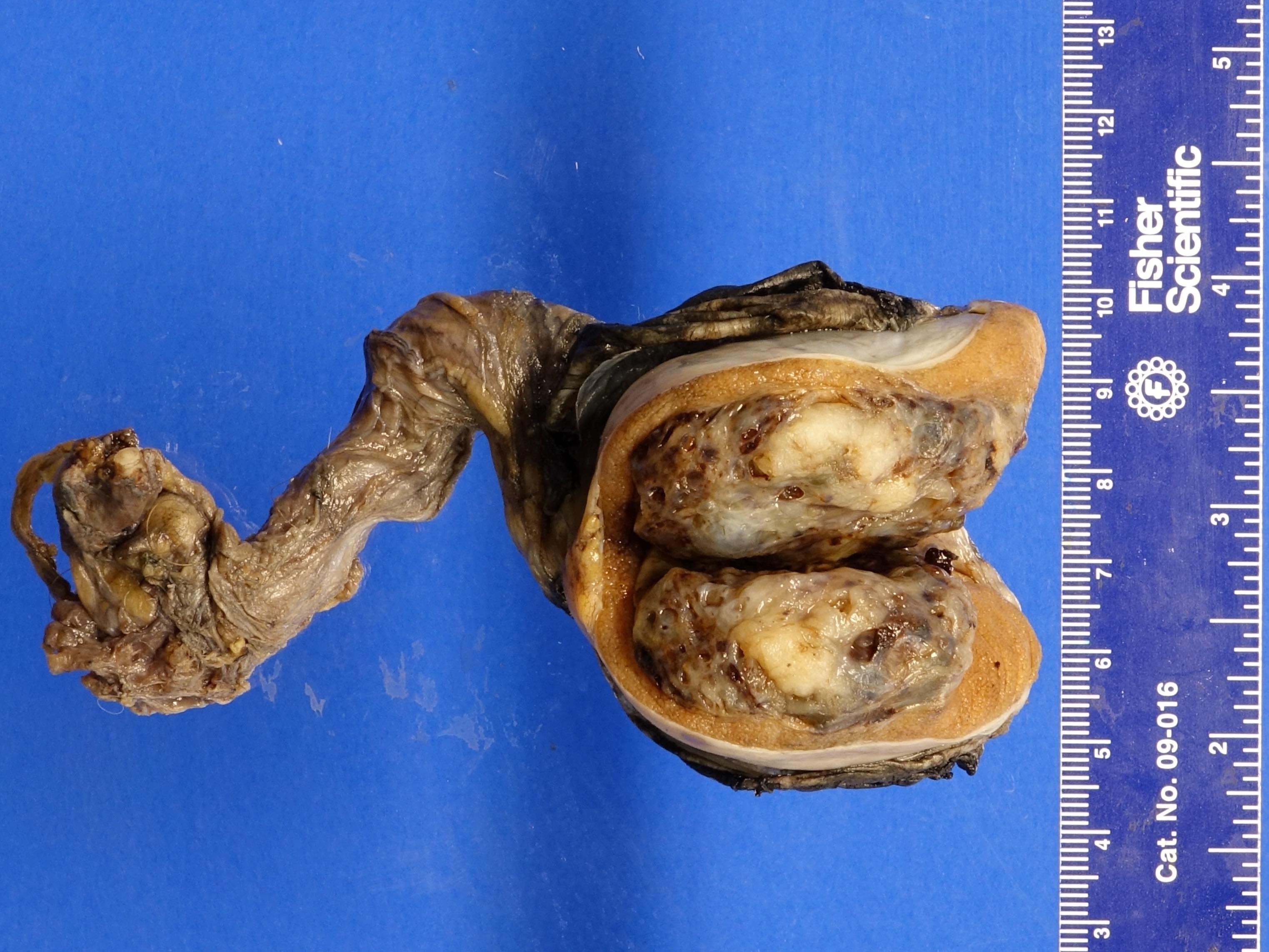
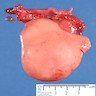
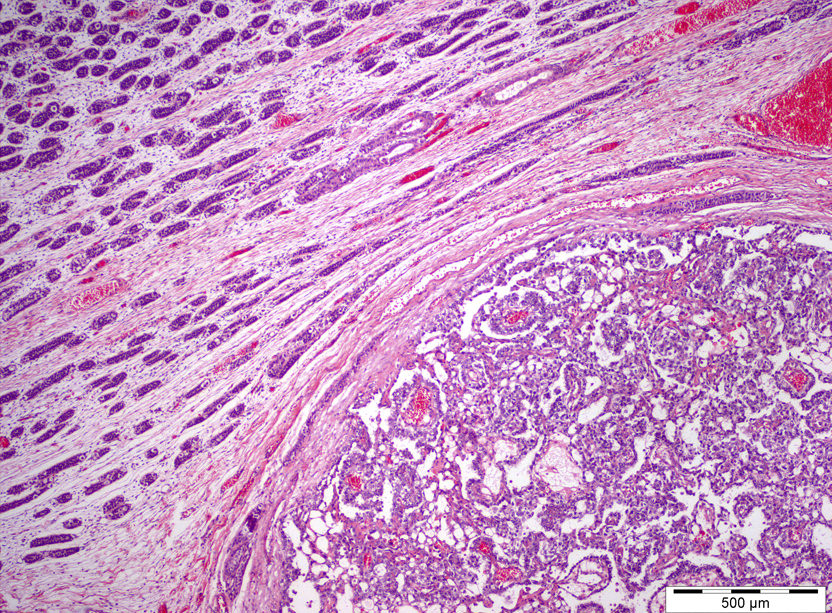
- pTX: cannot be assessed
- pT0: no evidence of primary tumor
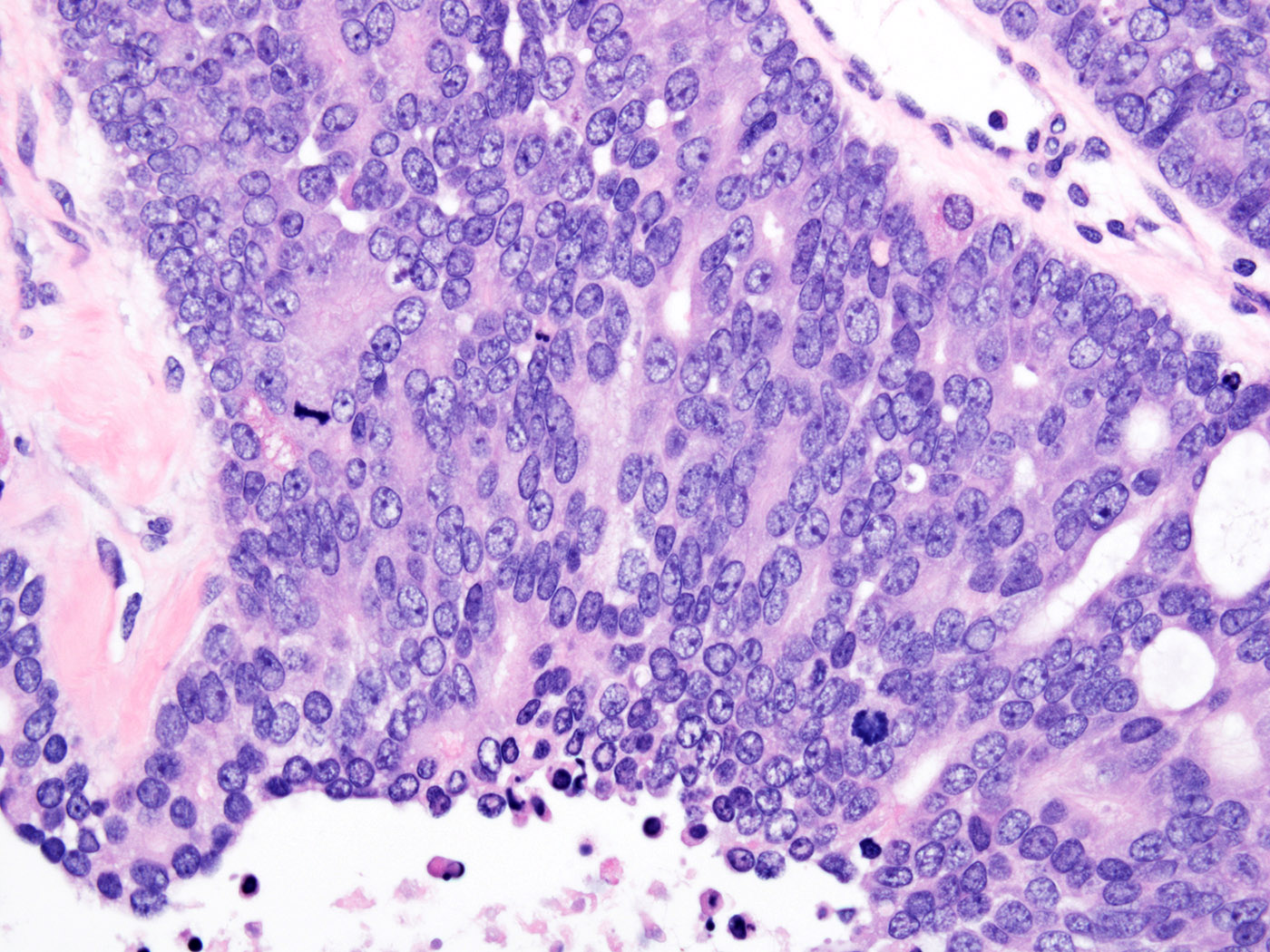
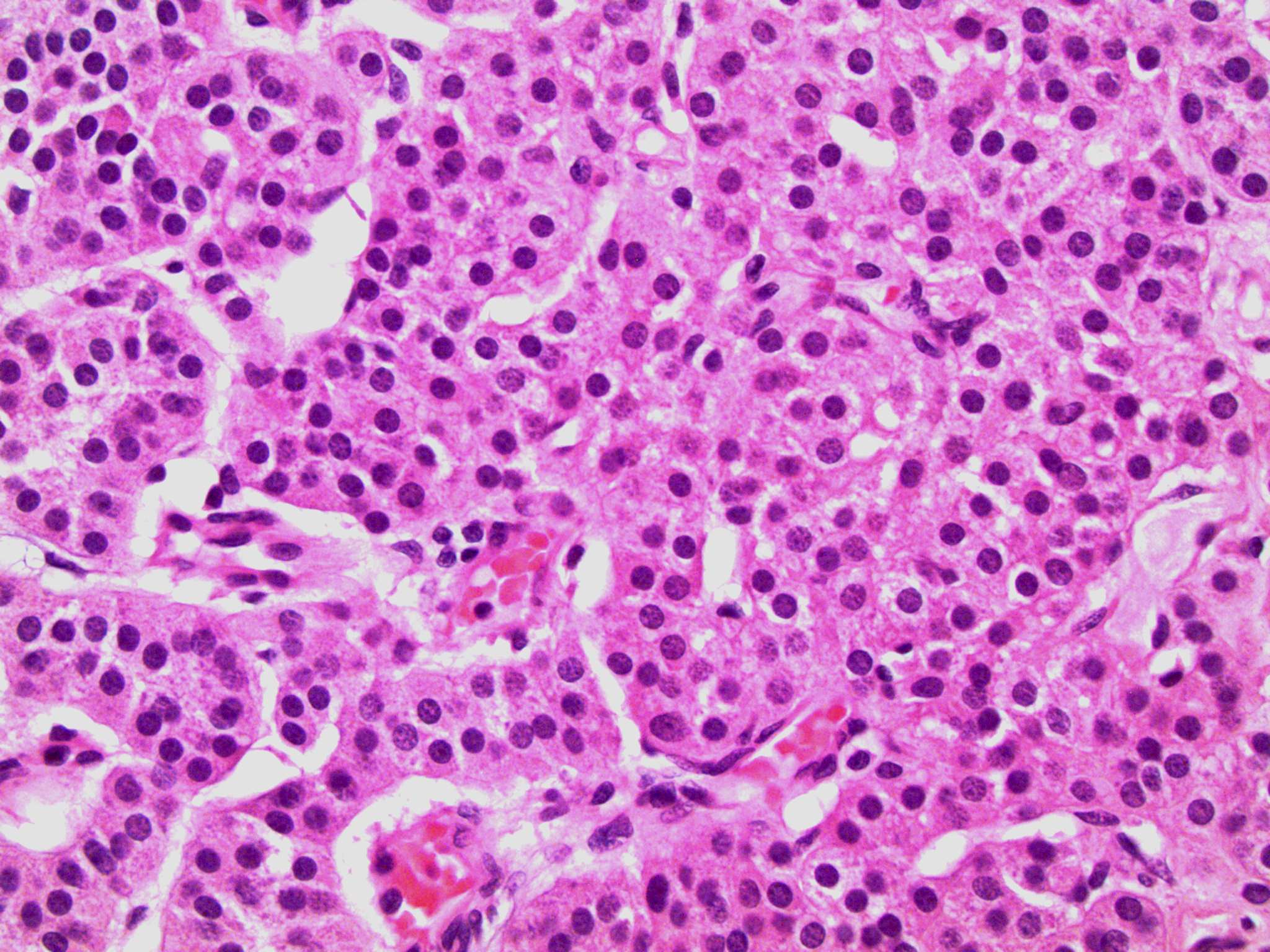
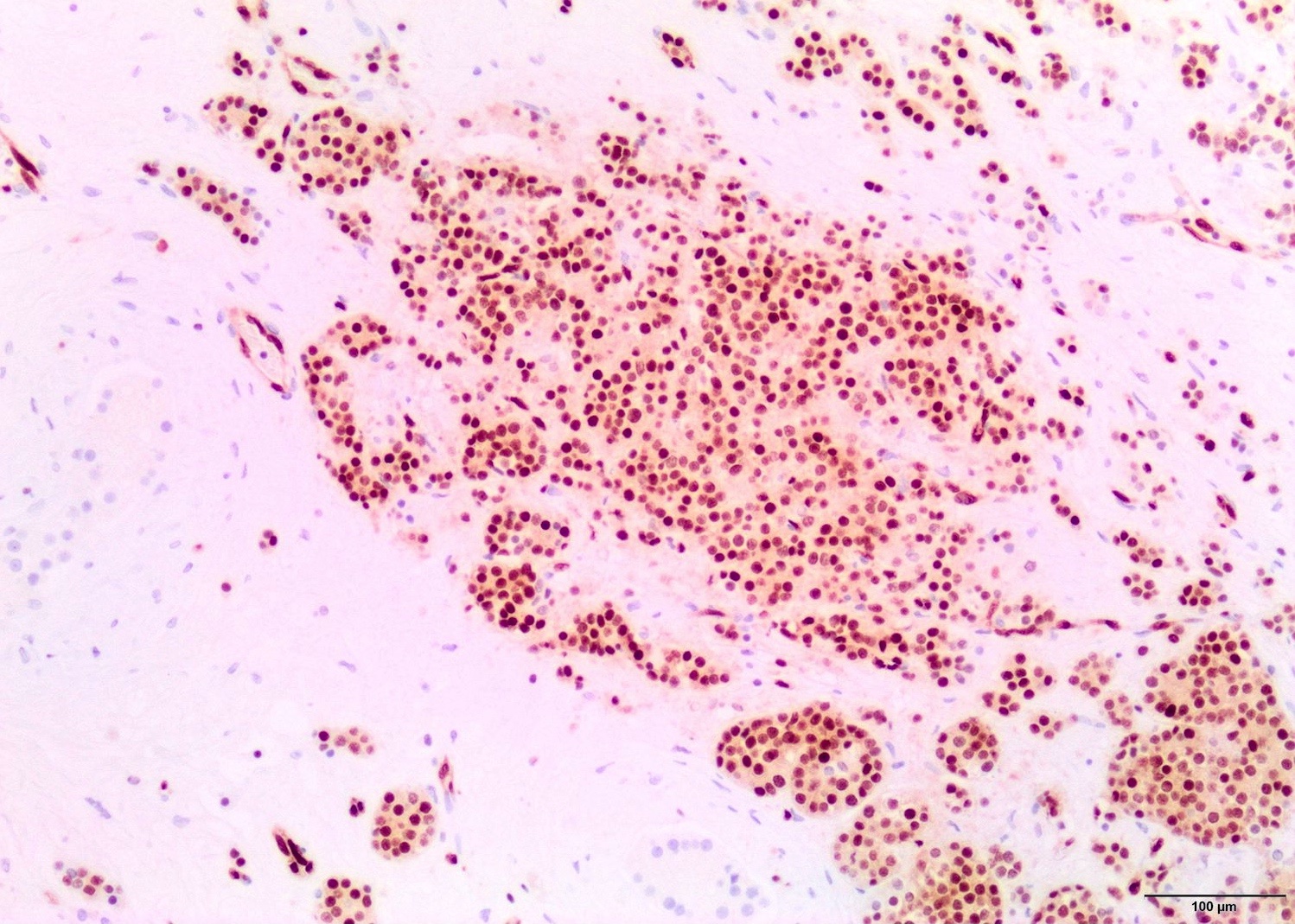
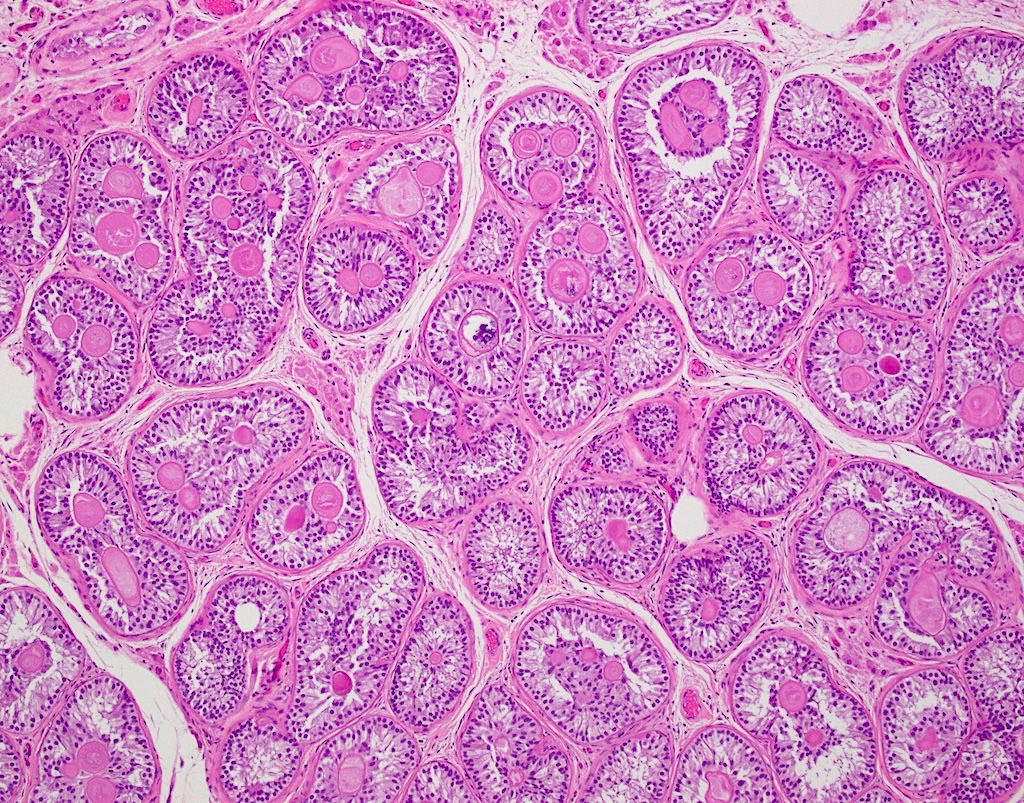
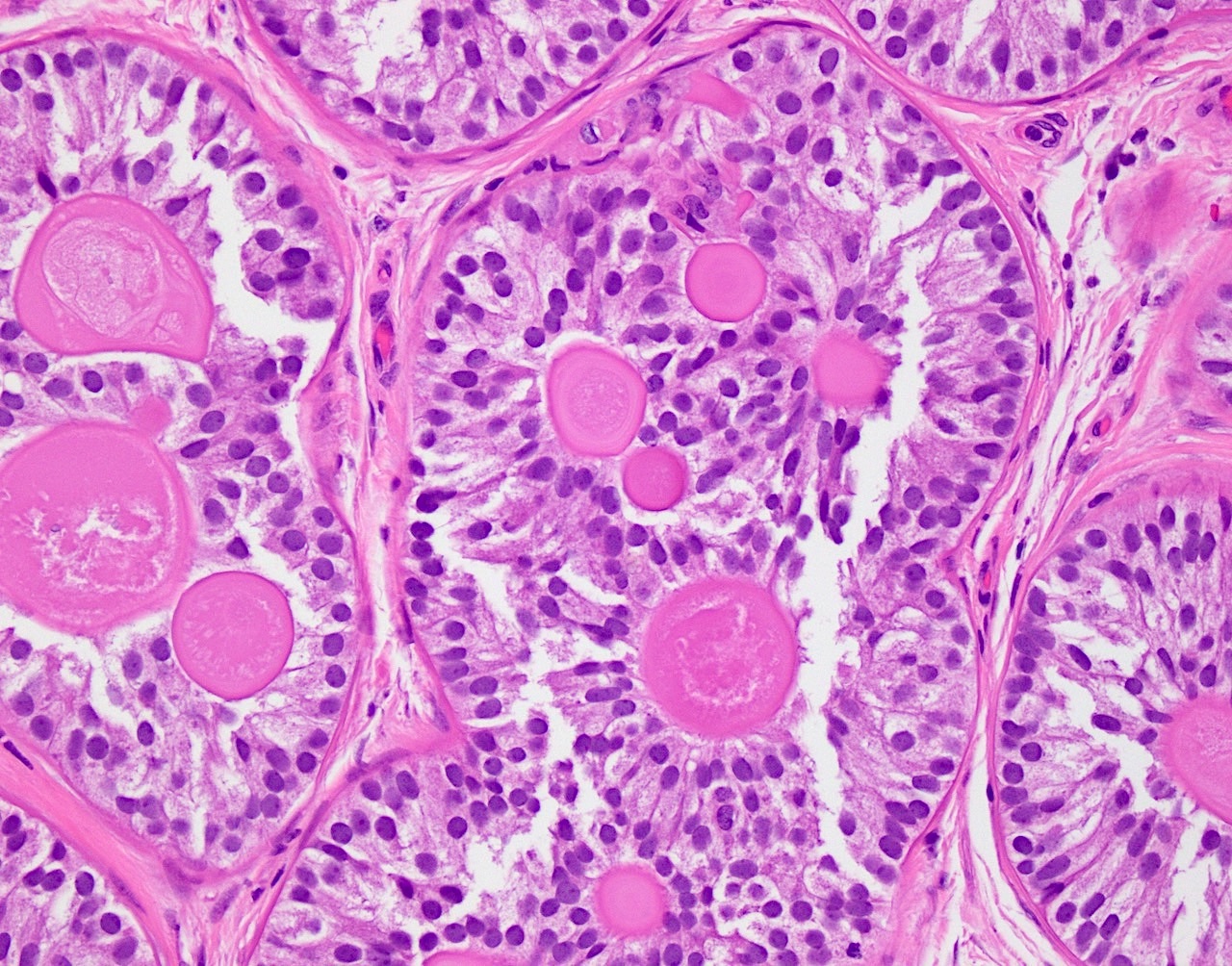
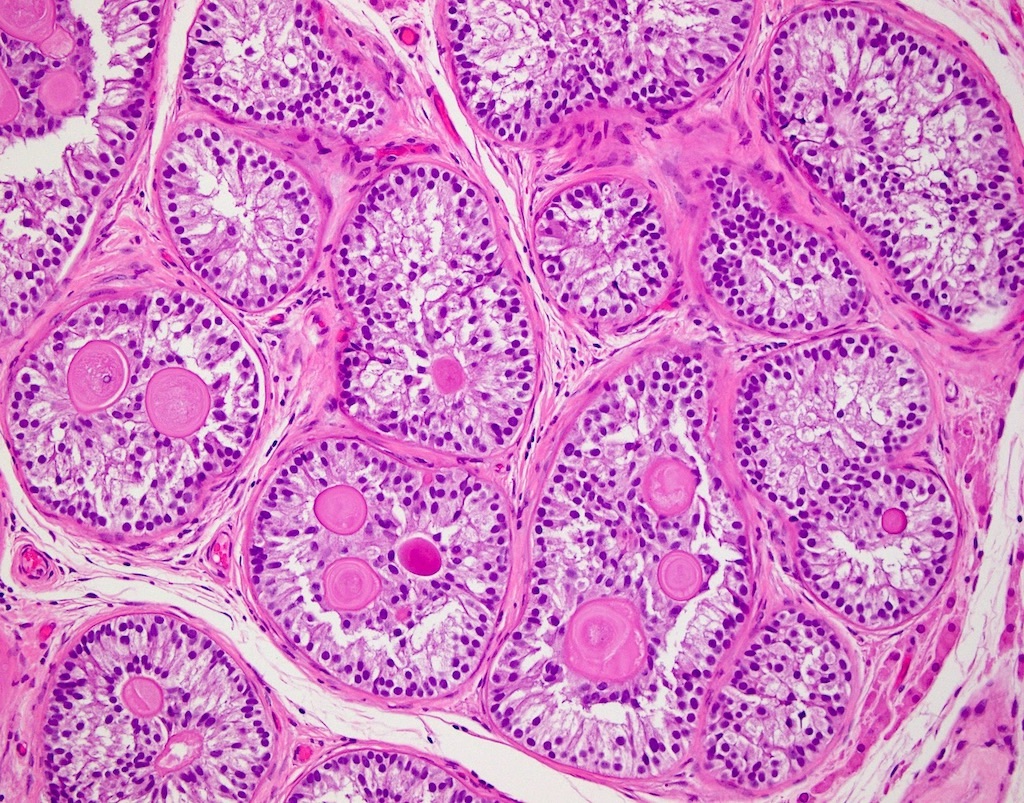
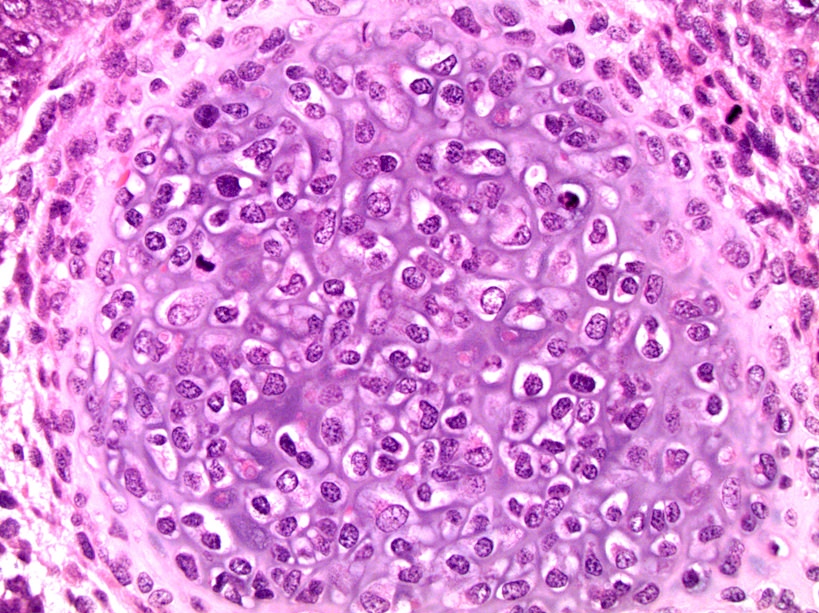
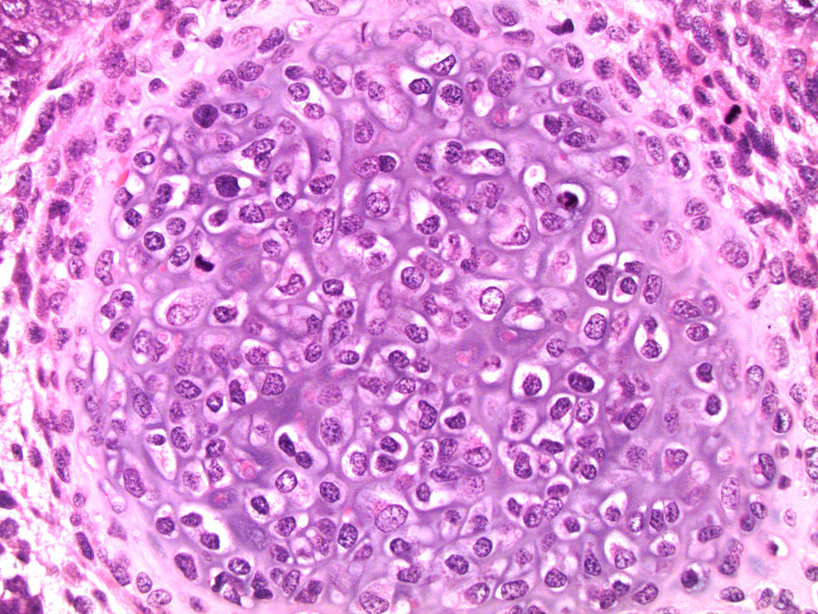
- pTis: germ cell neoplasia in situ
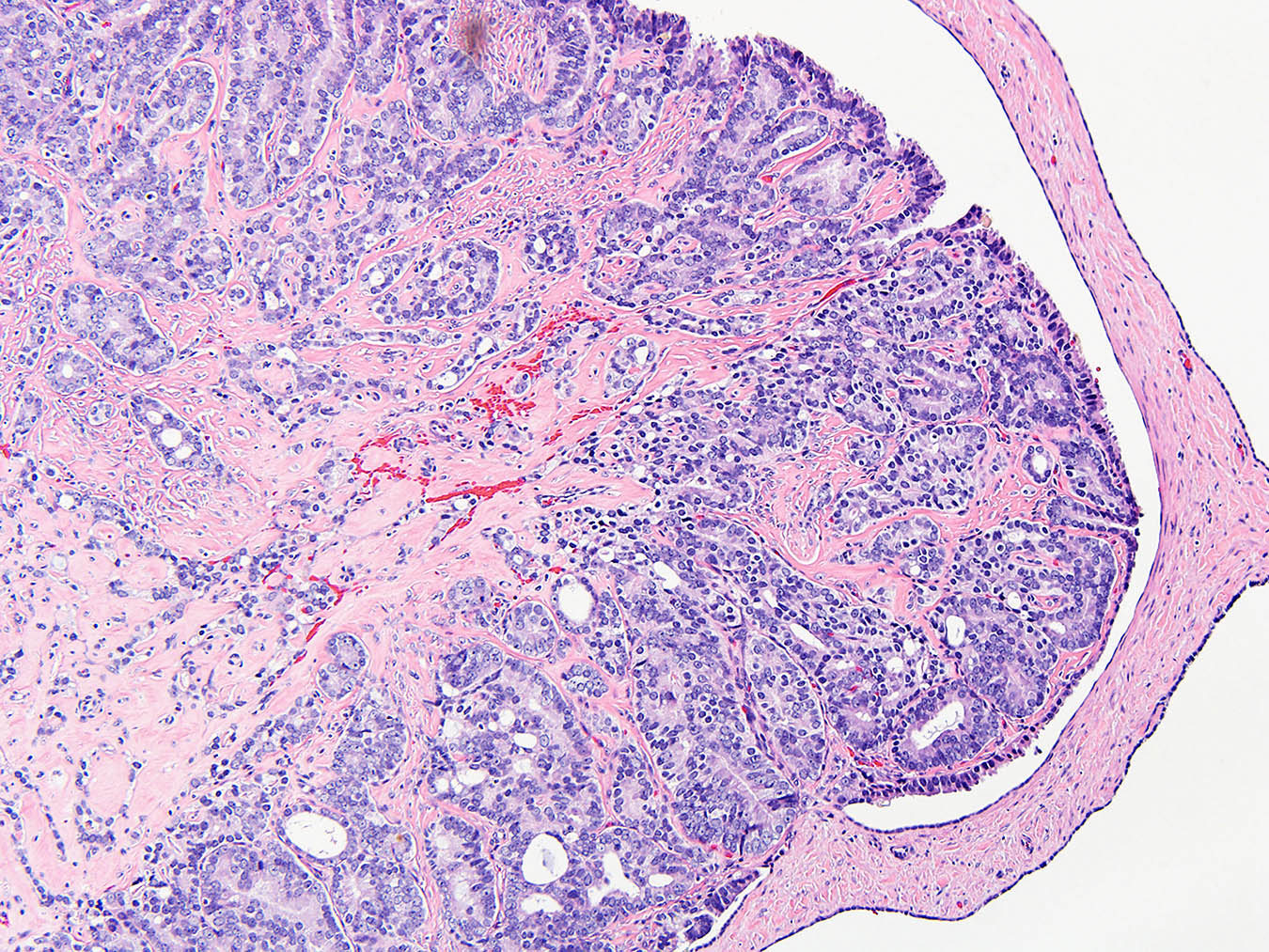
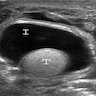
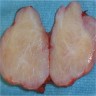
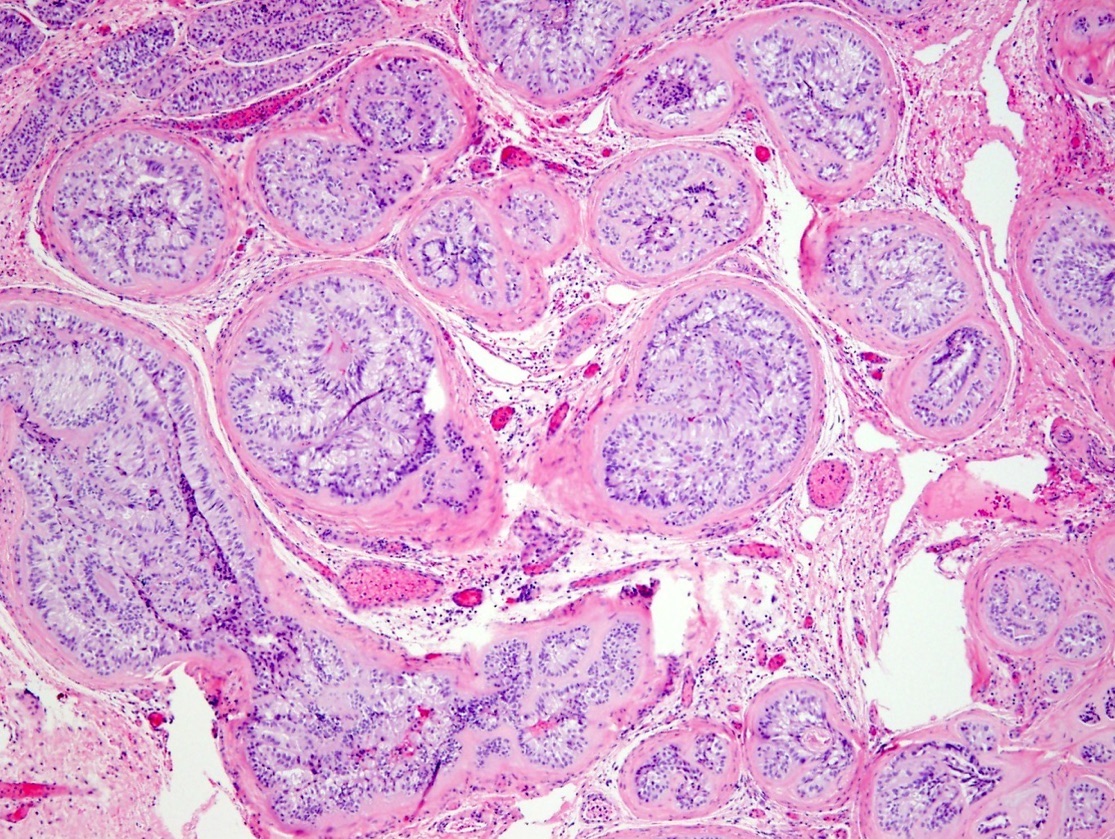
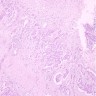
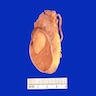
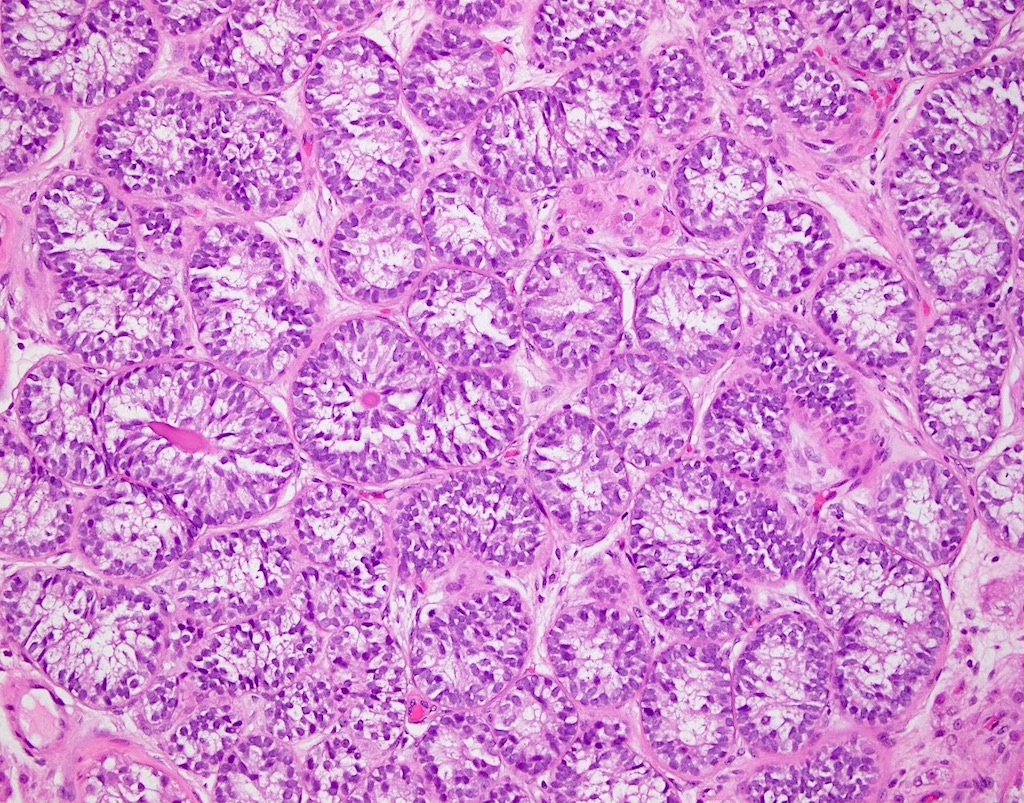
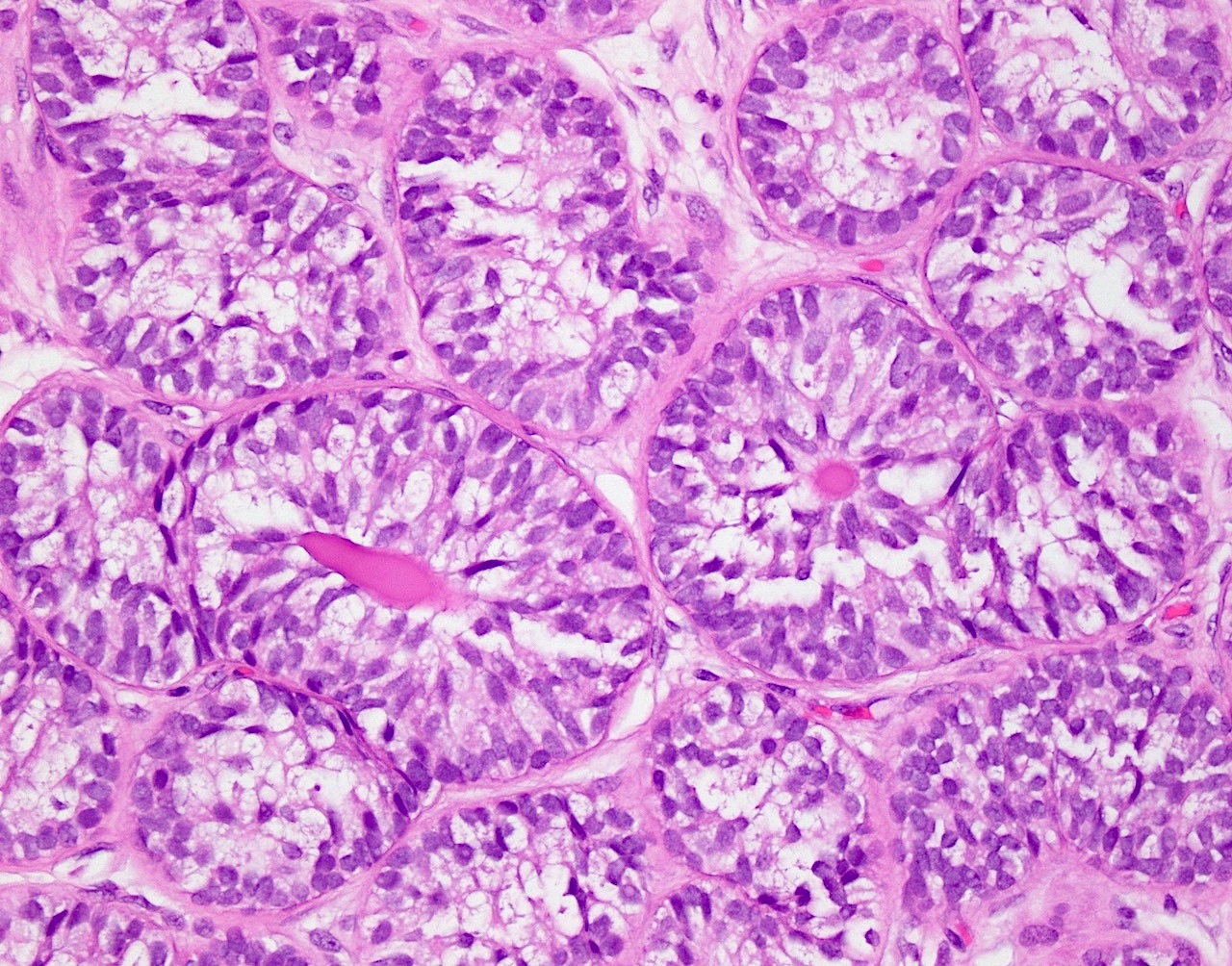
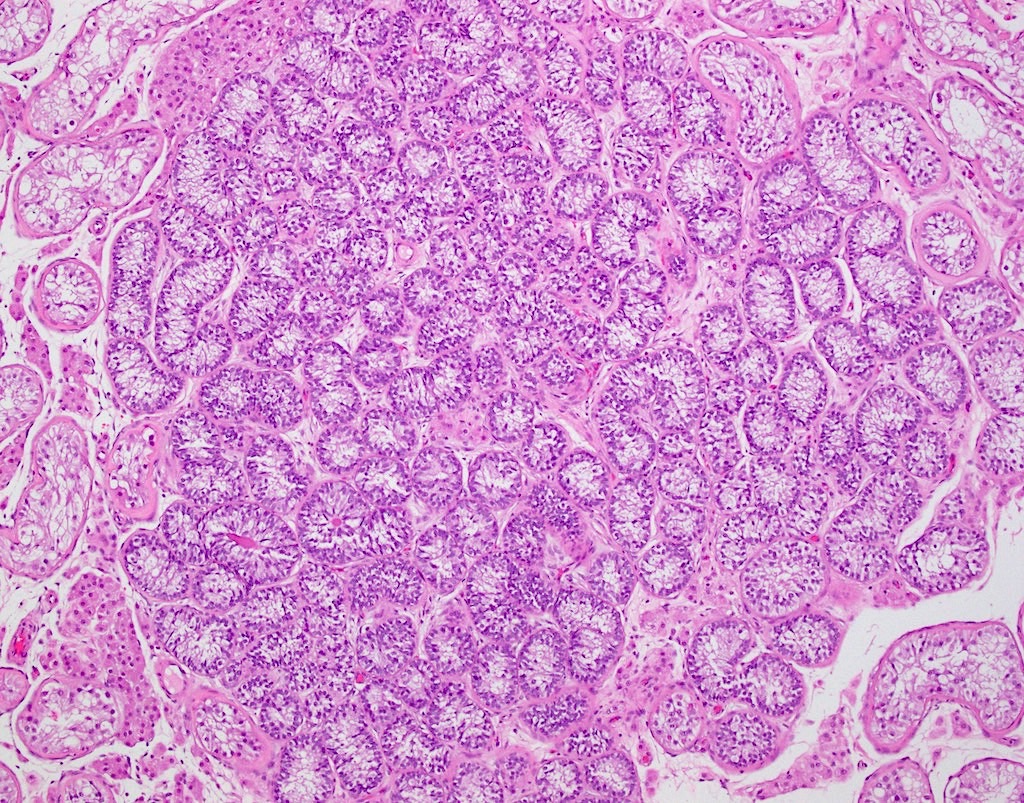
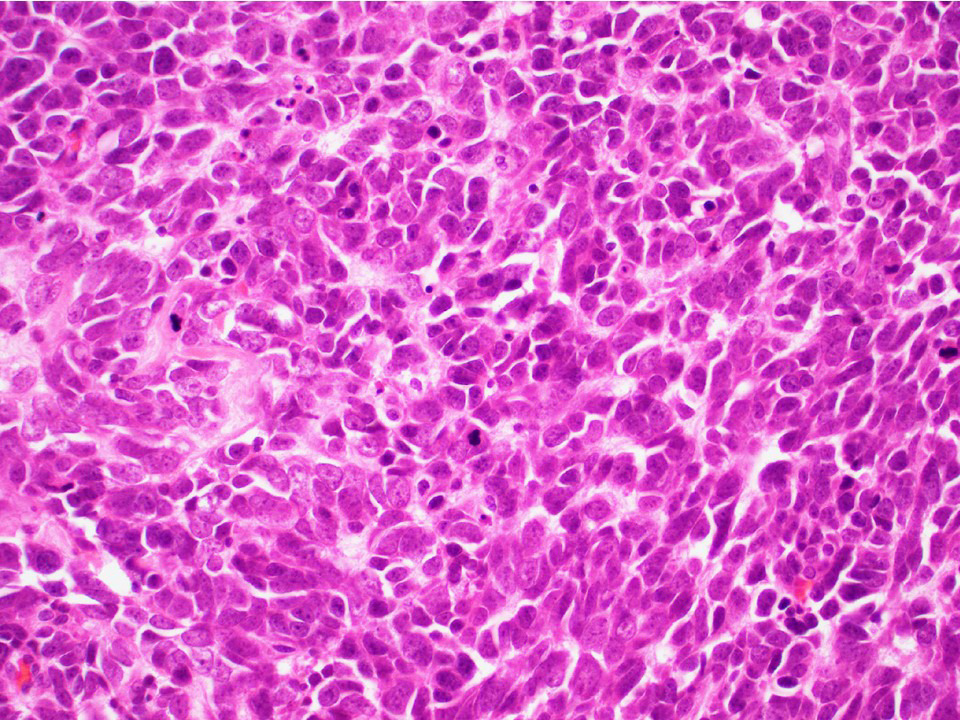
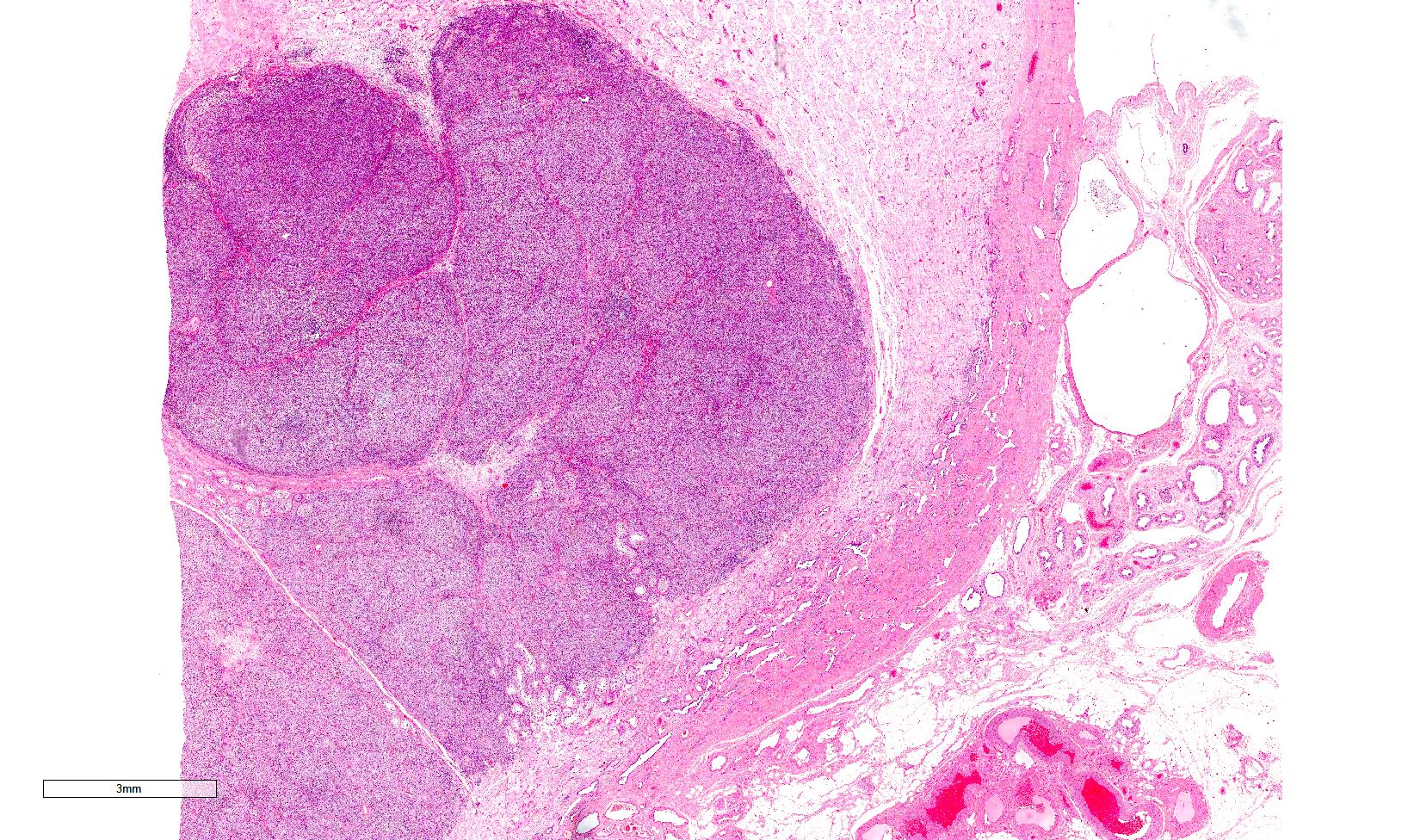
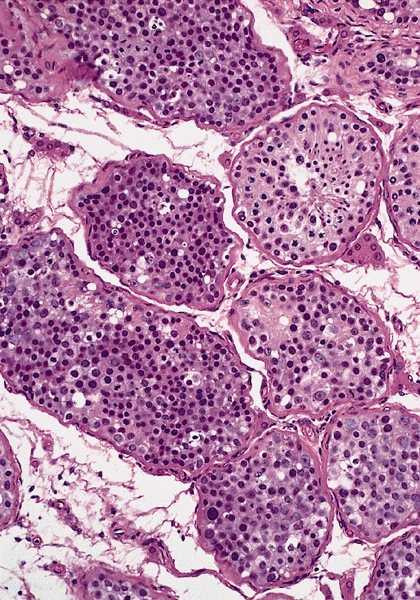
- pT1: nonpure seminoma confined to testis / tunica albuginea / rete and no lymphovascular invasion (LVI)
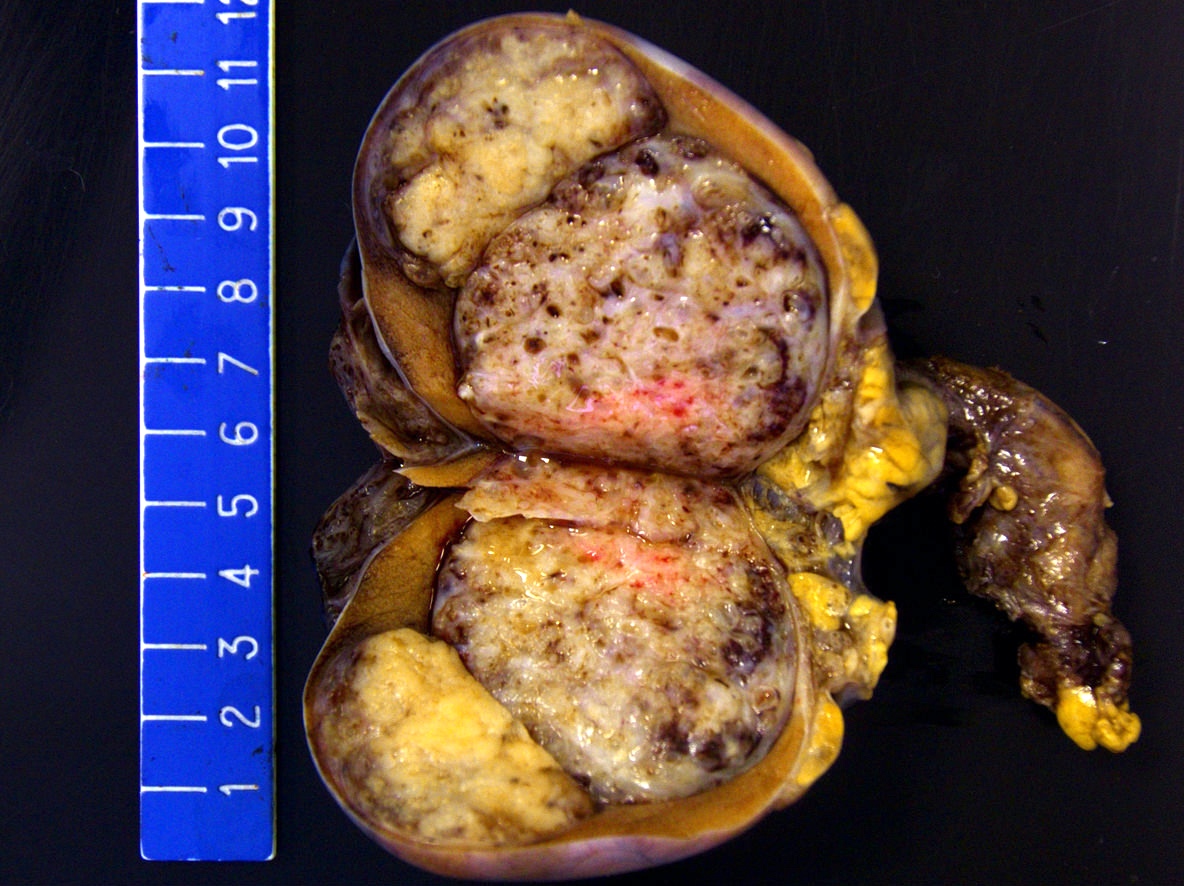
- pT1a: seminoma < 3 cm confined to testis / tunica albuginea / rete and no LVI
- pT1b: seminoma ≥ 3 cm confined to testis / tunica albuginea / rete and no LVI
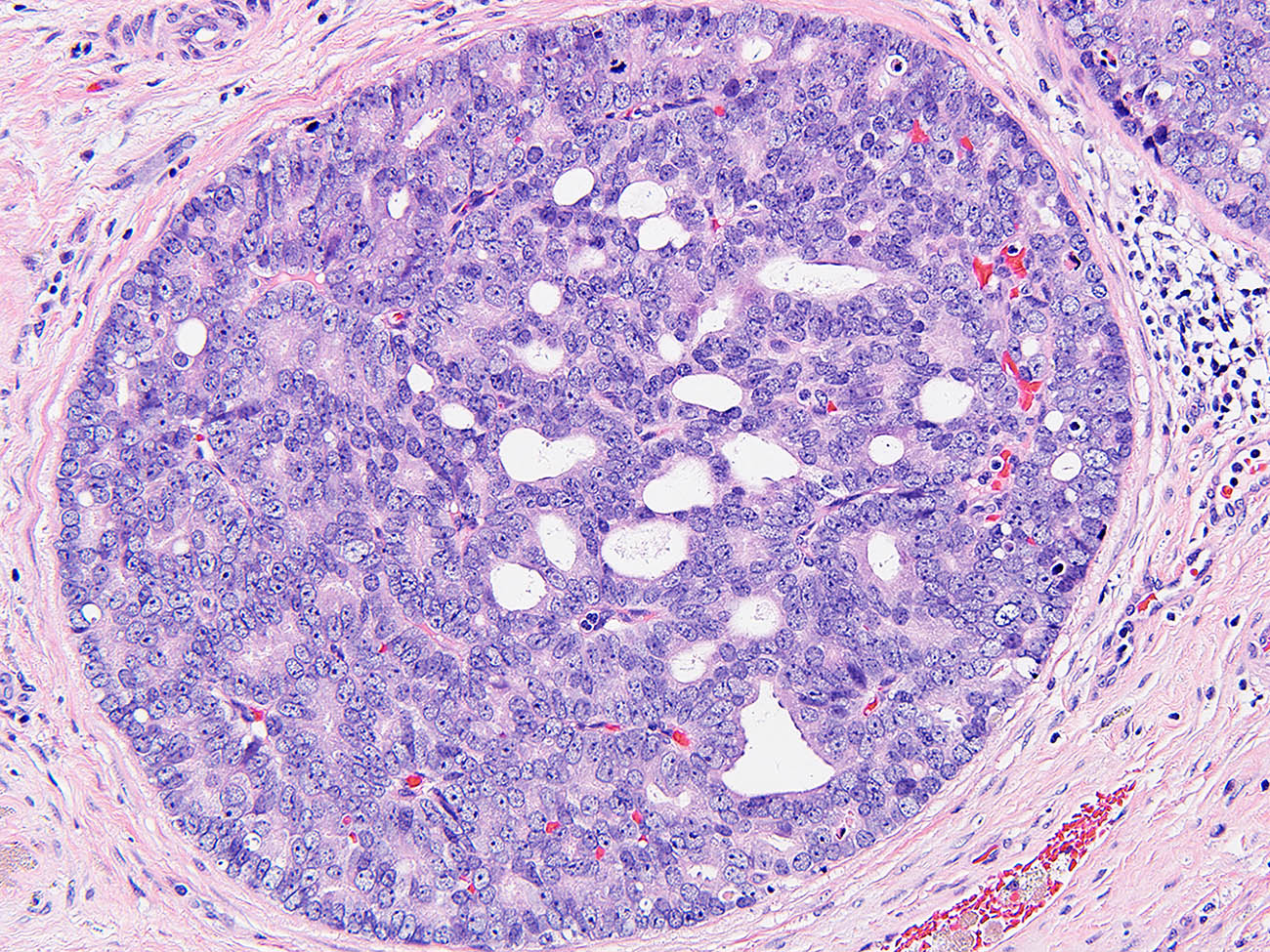
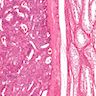
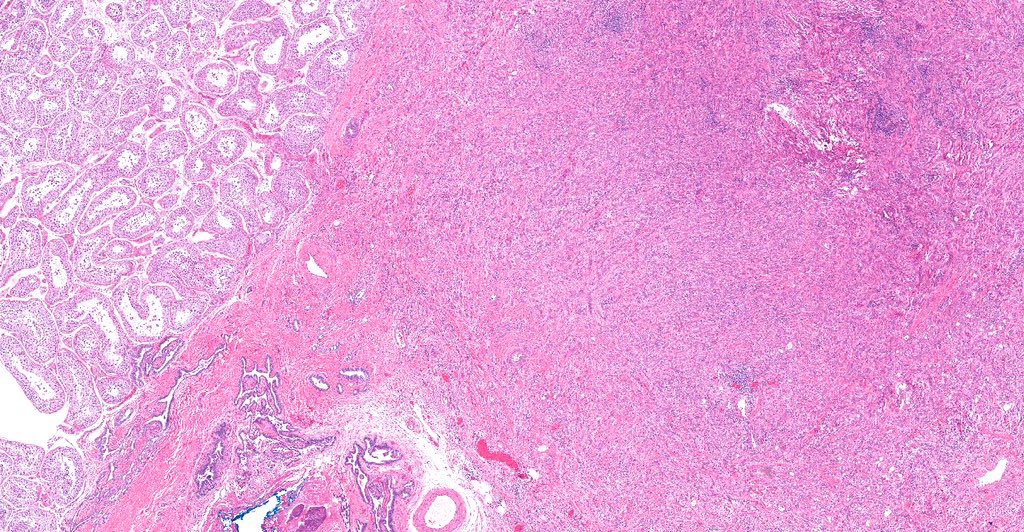
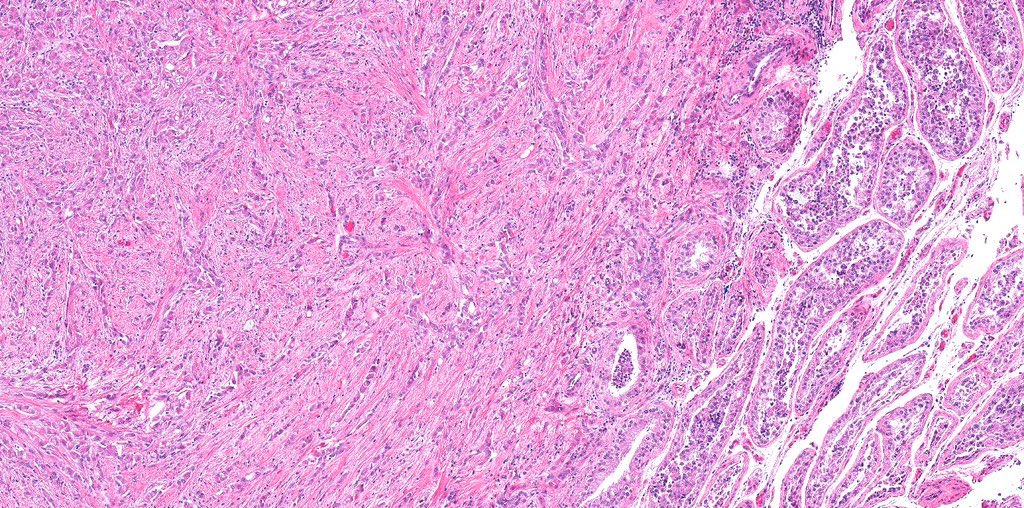
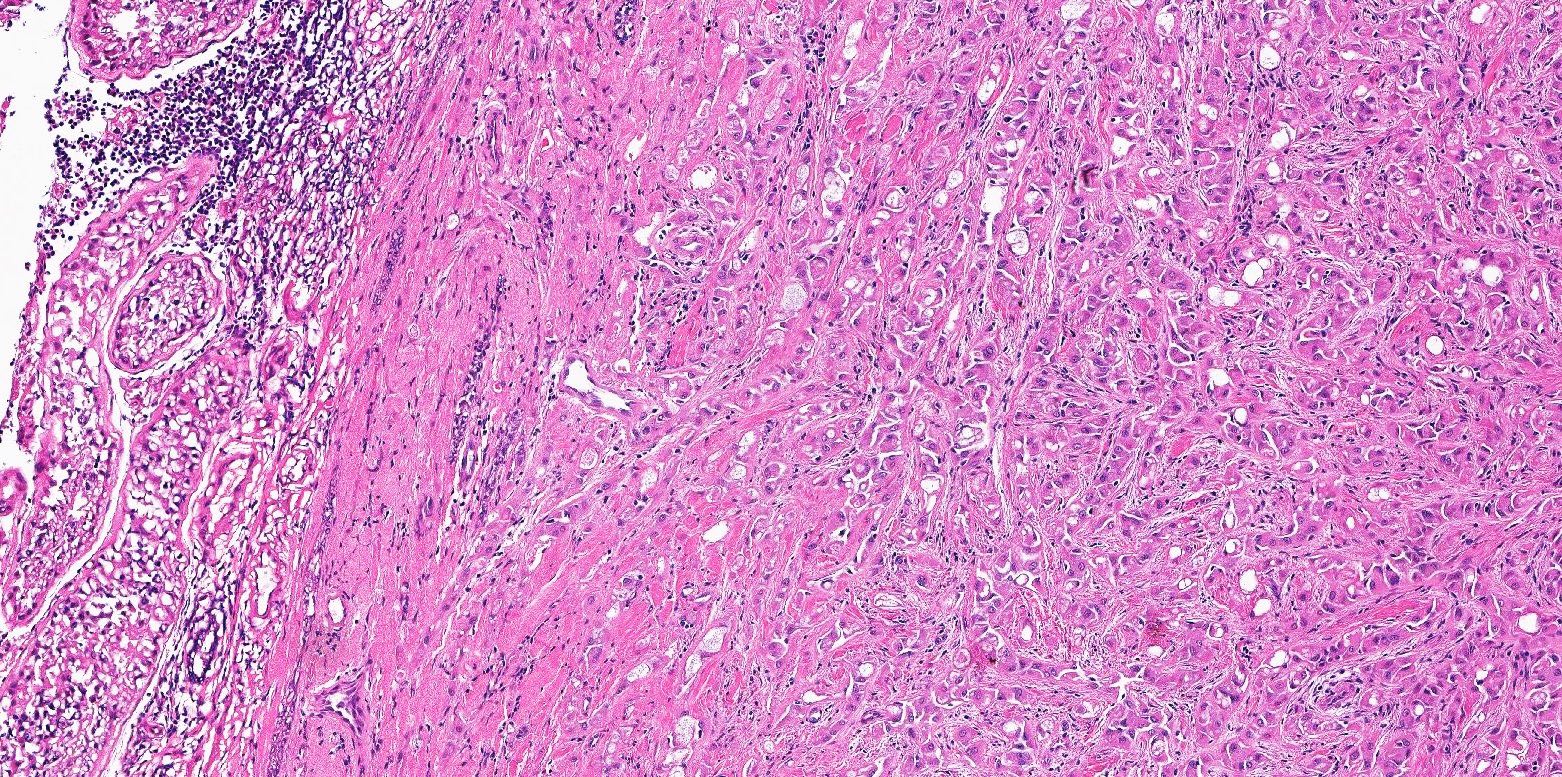
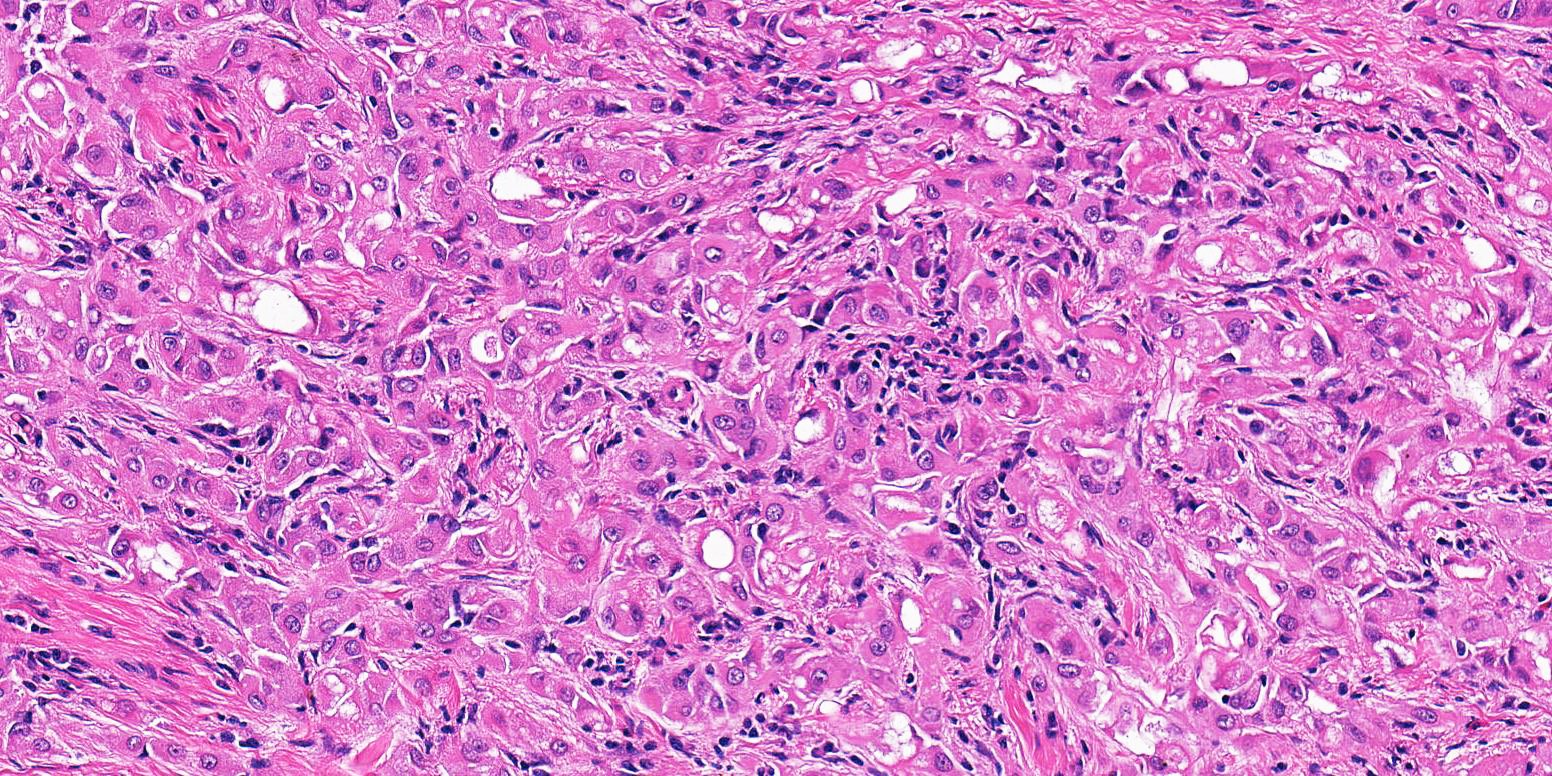
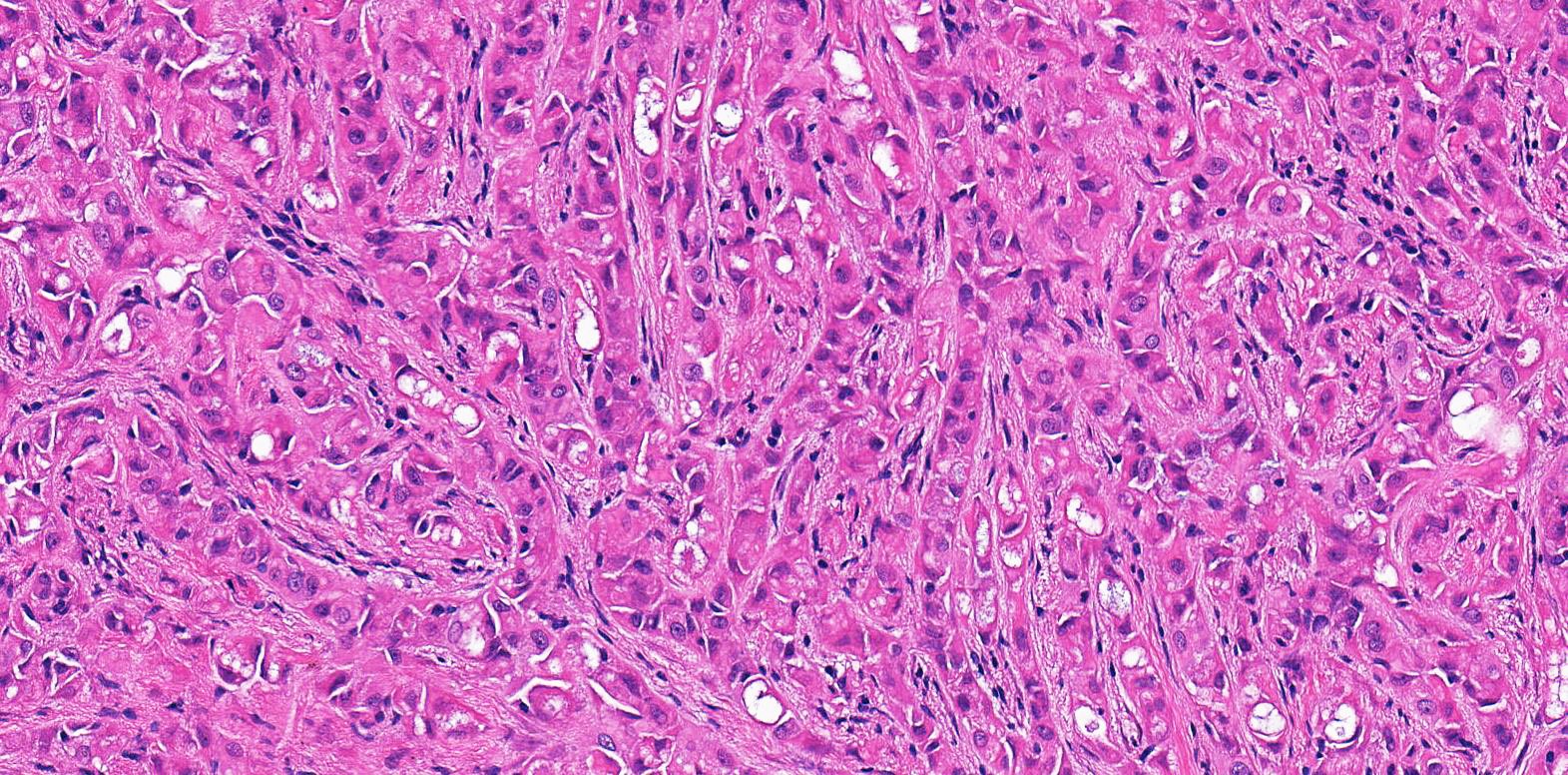
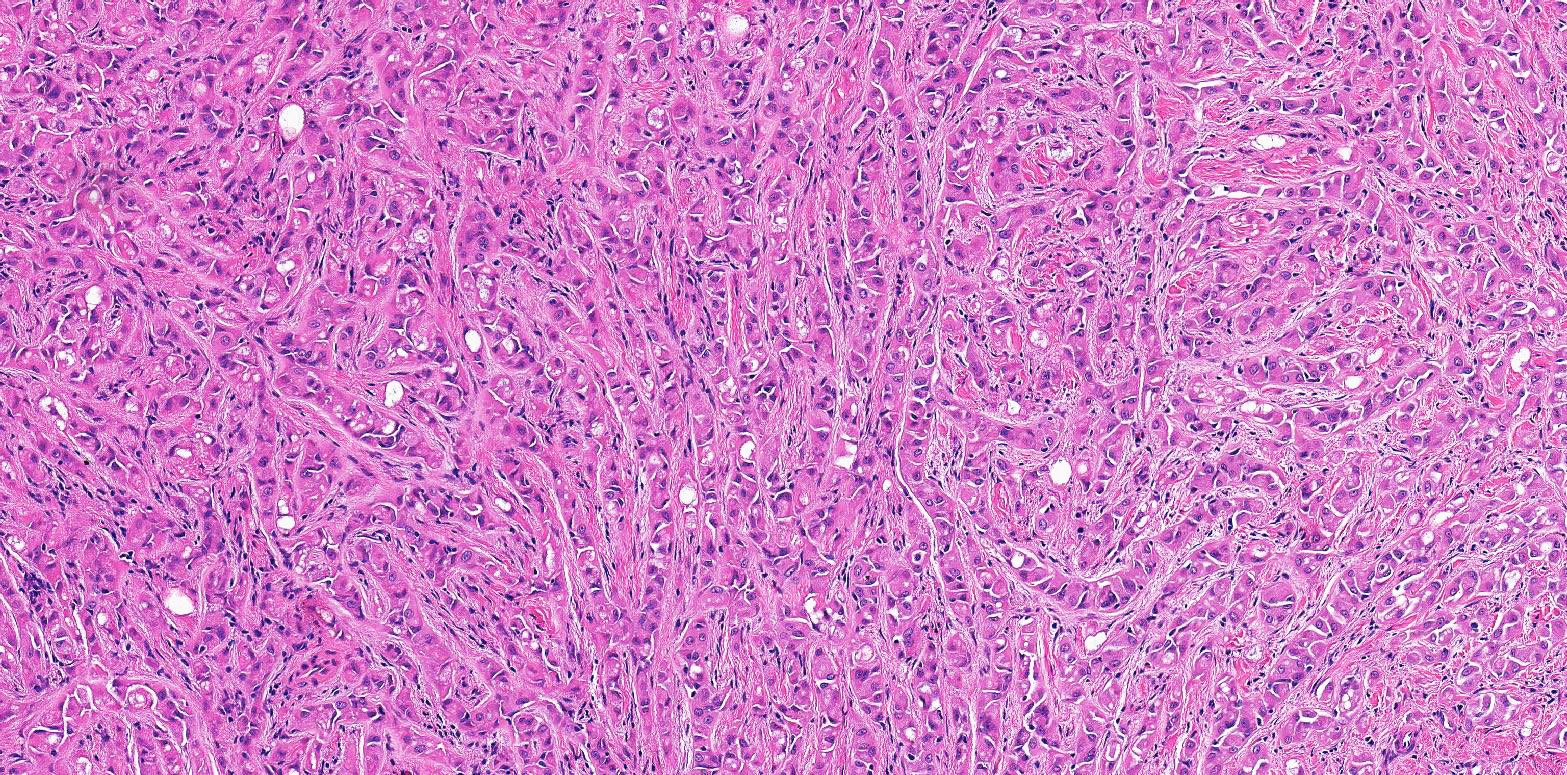
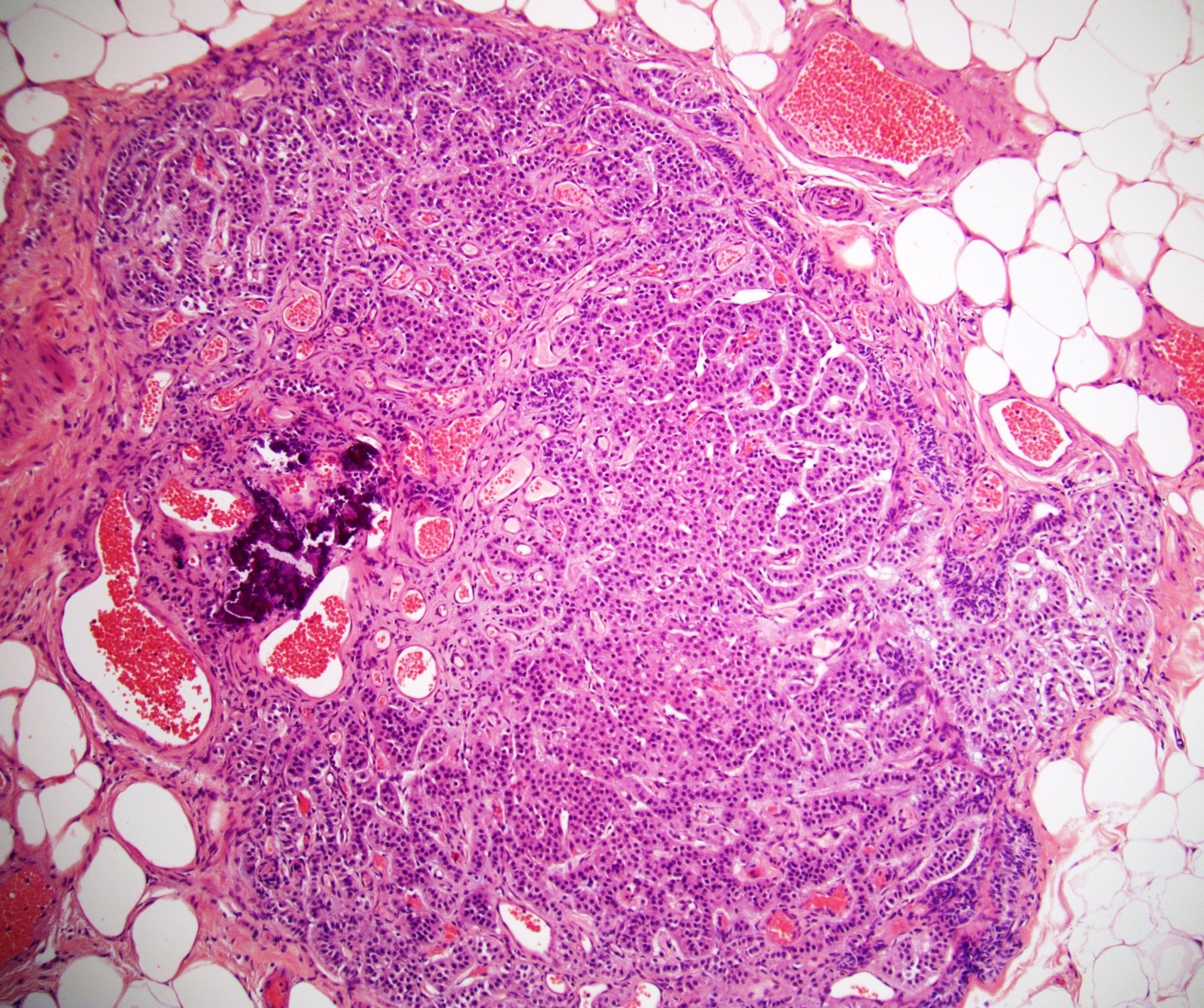
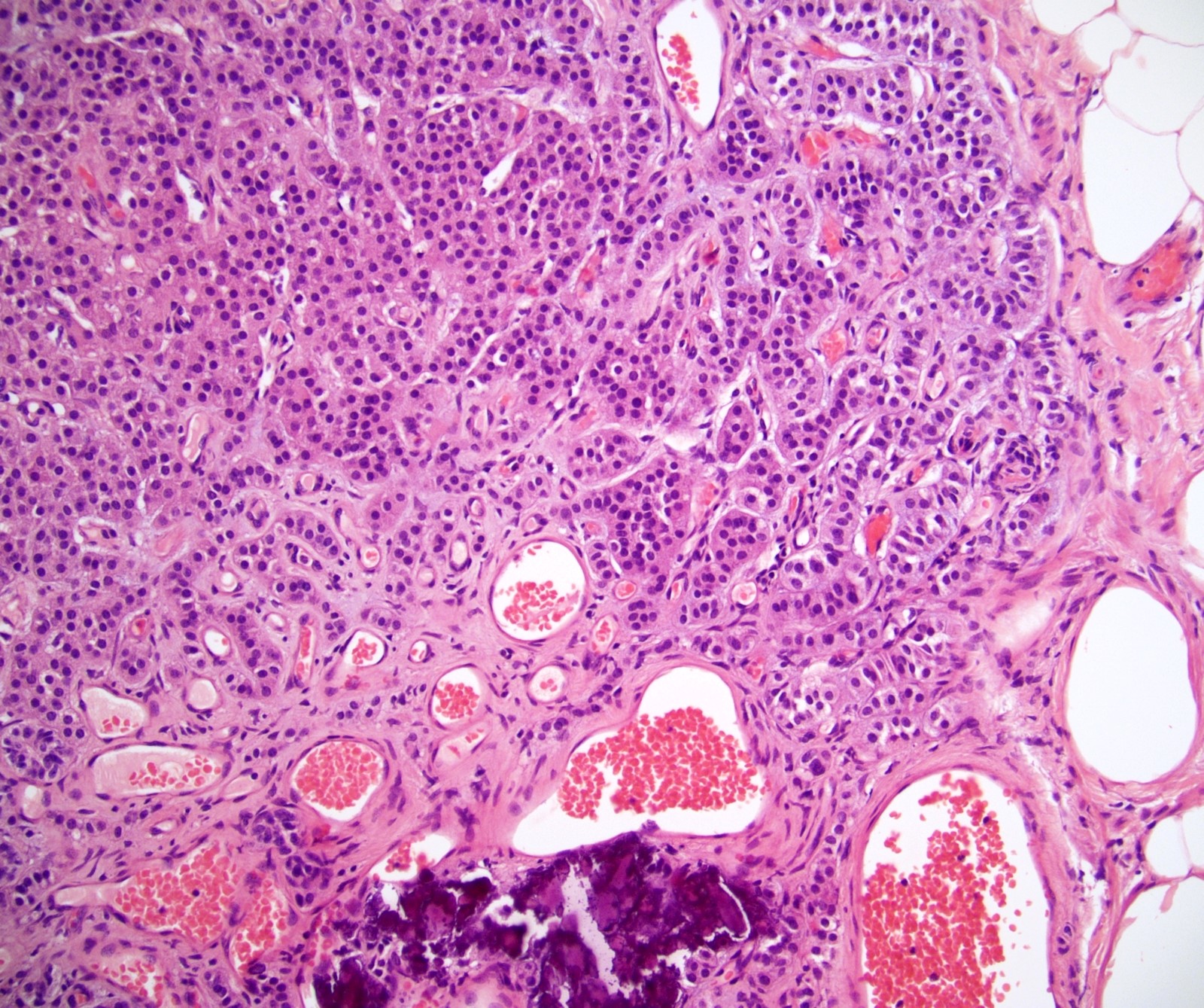
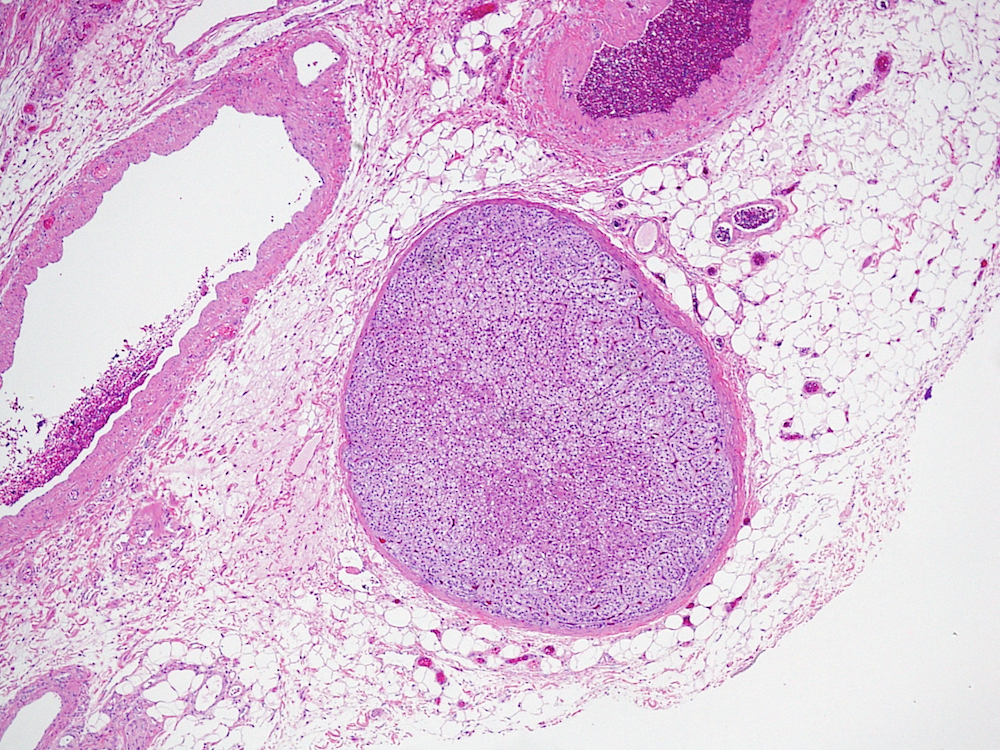
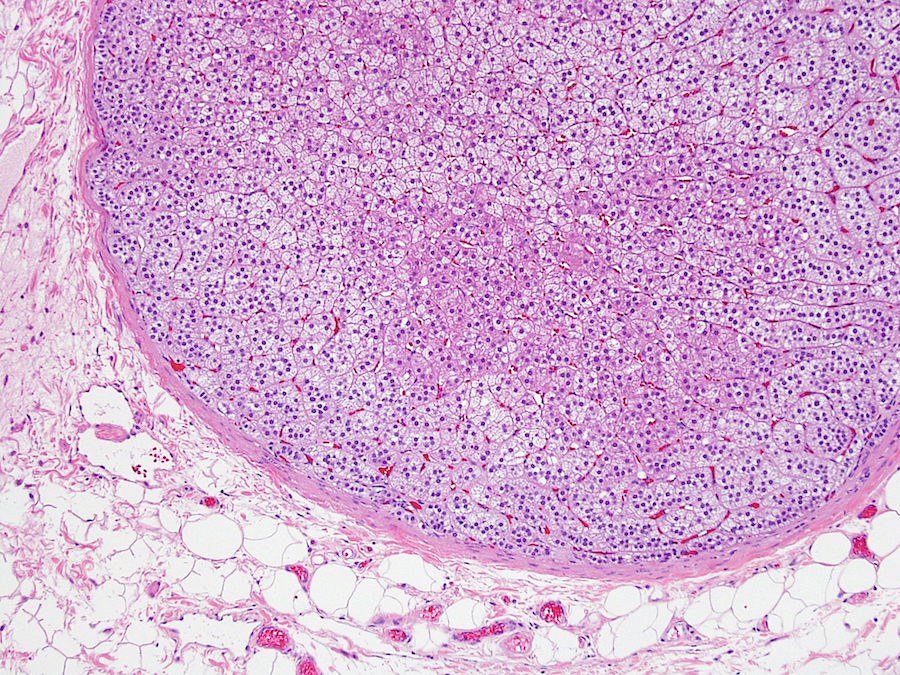
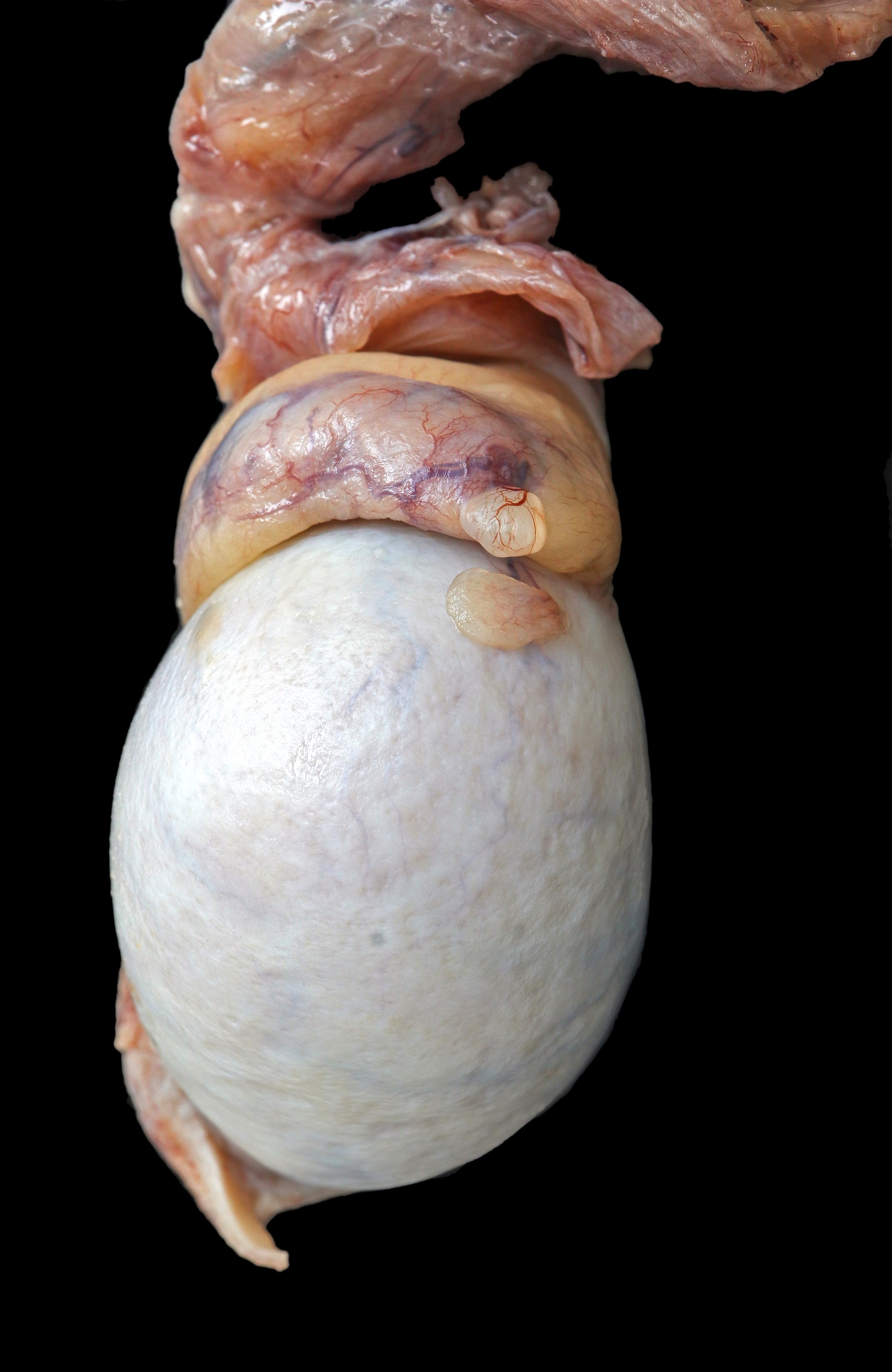
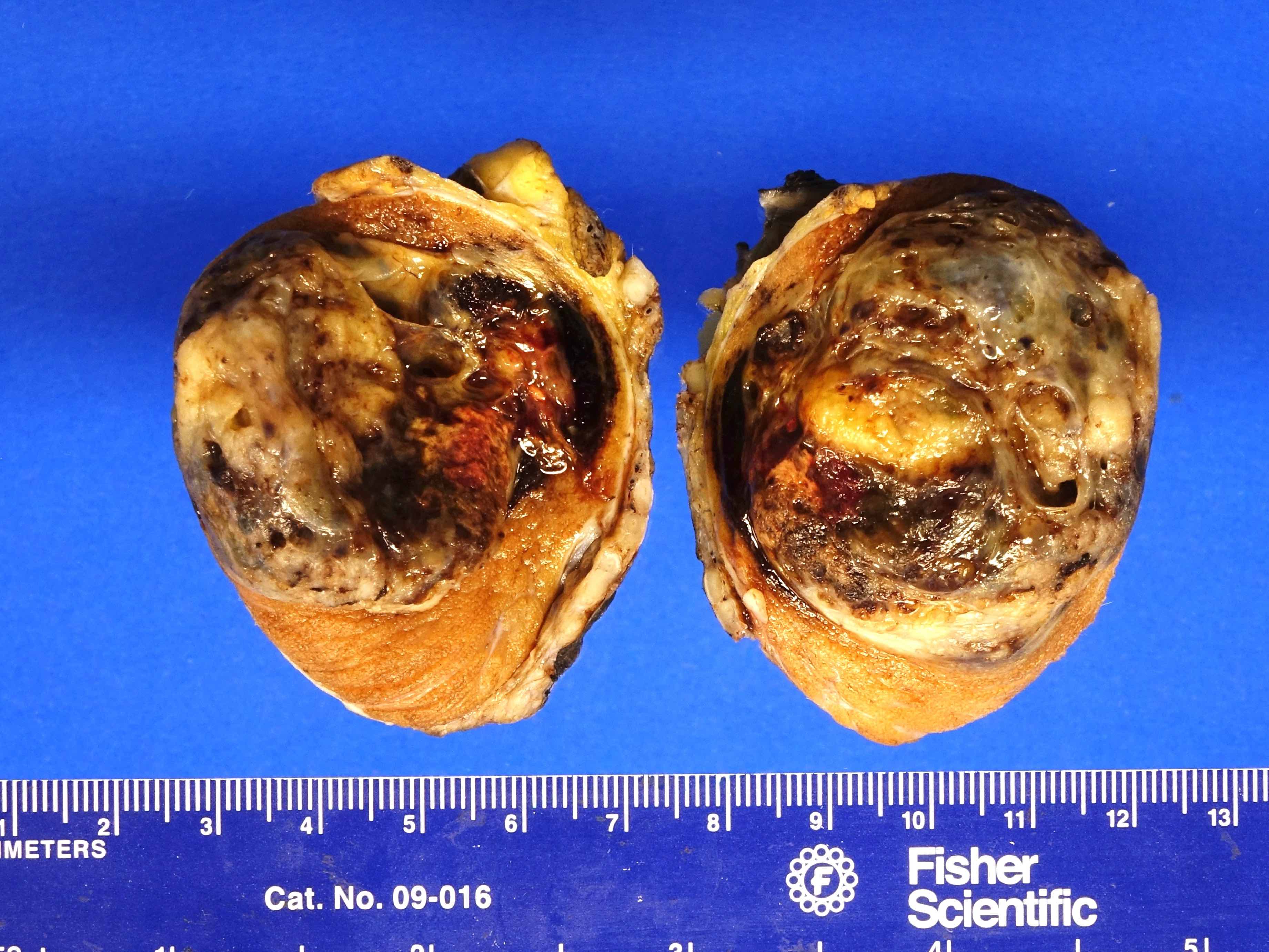
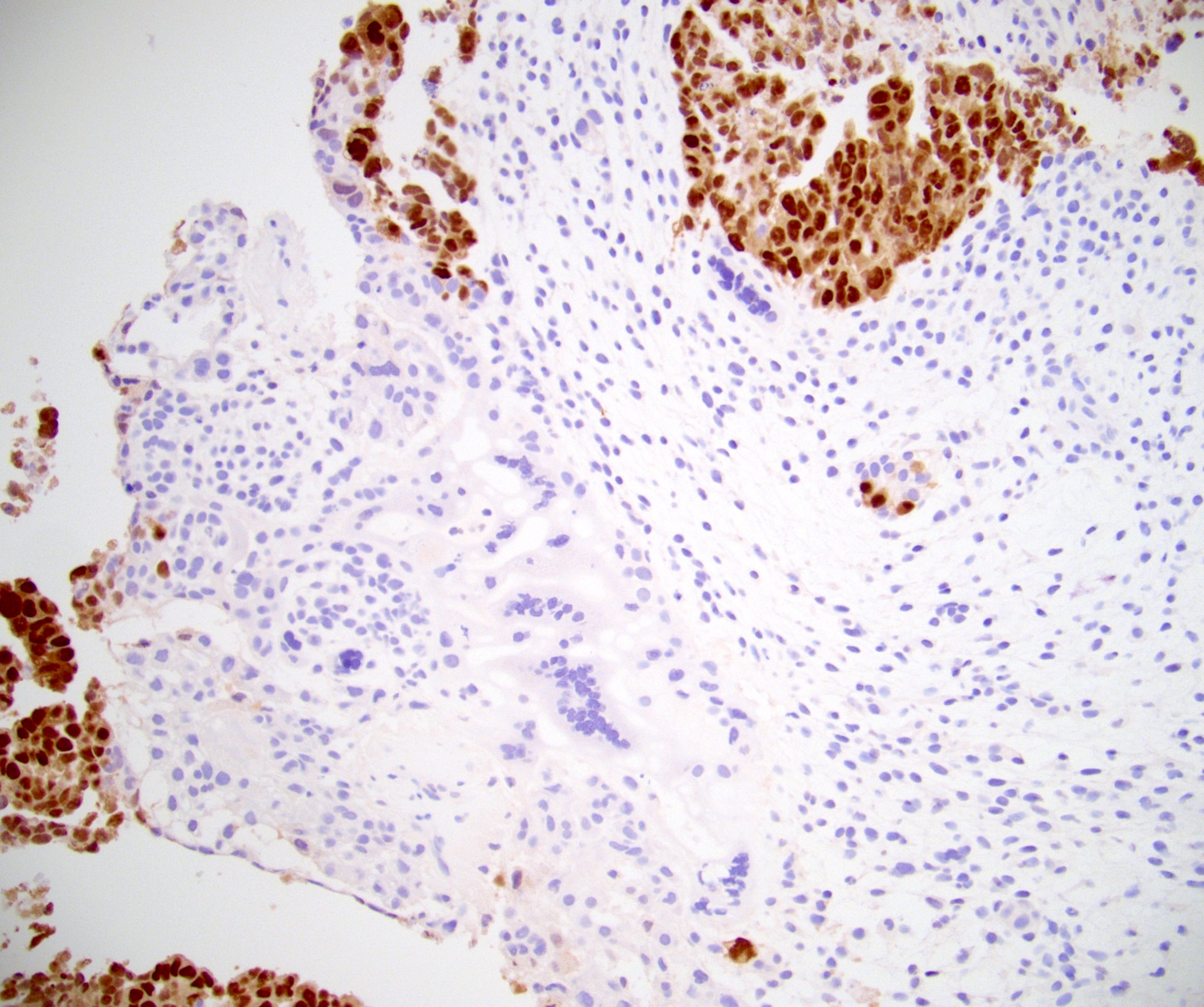
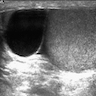
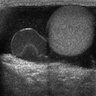
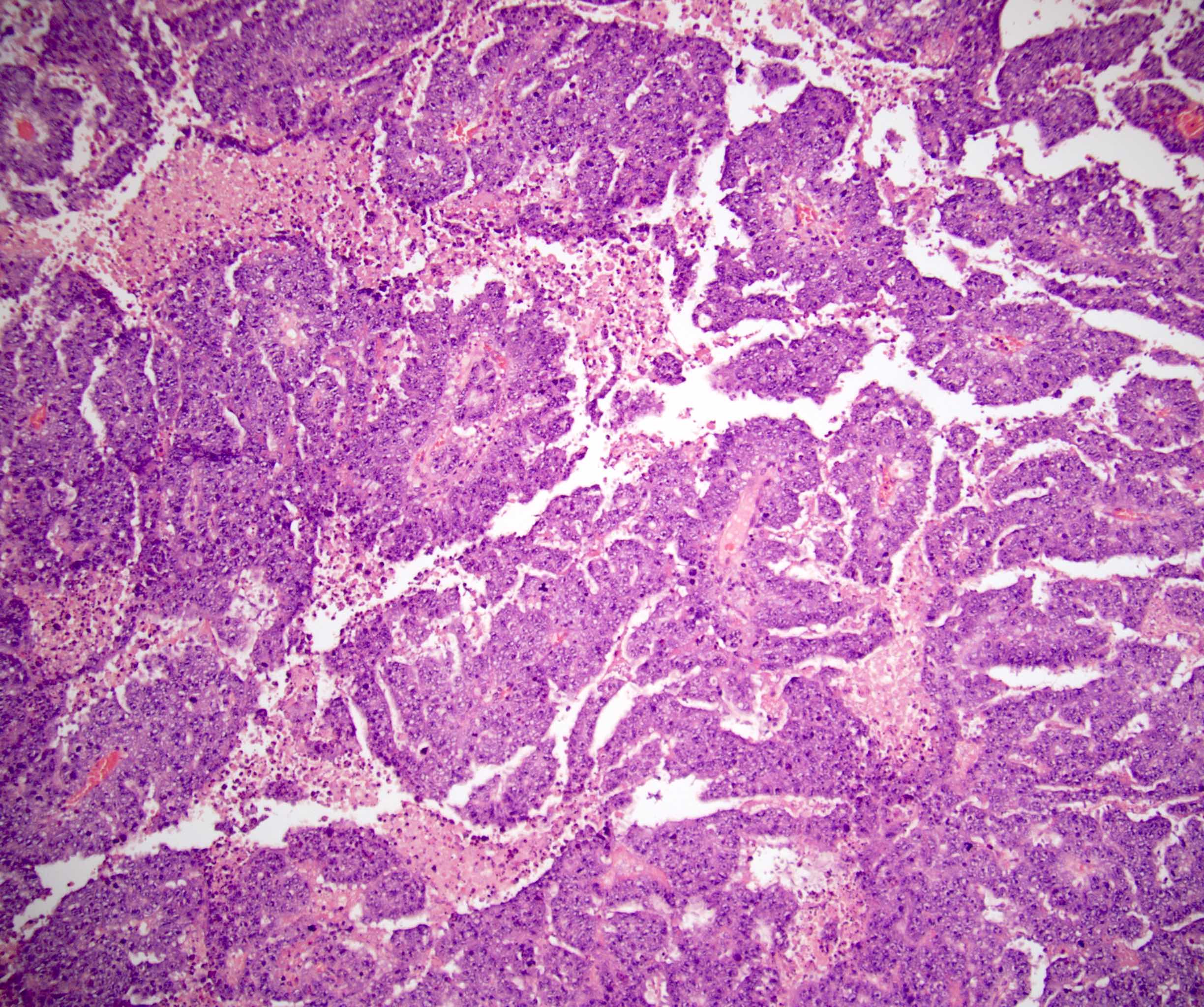
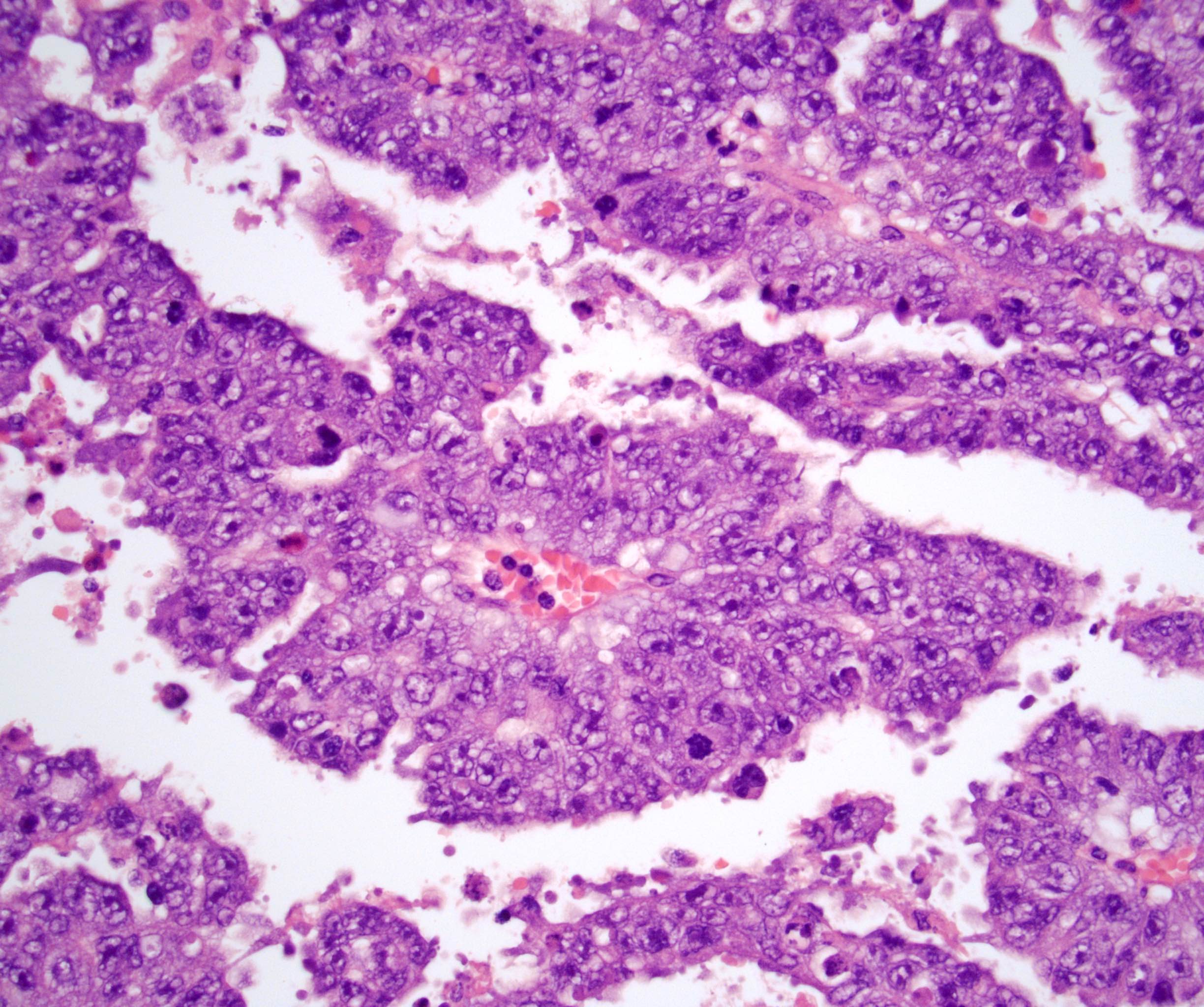
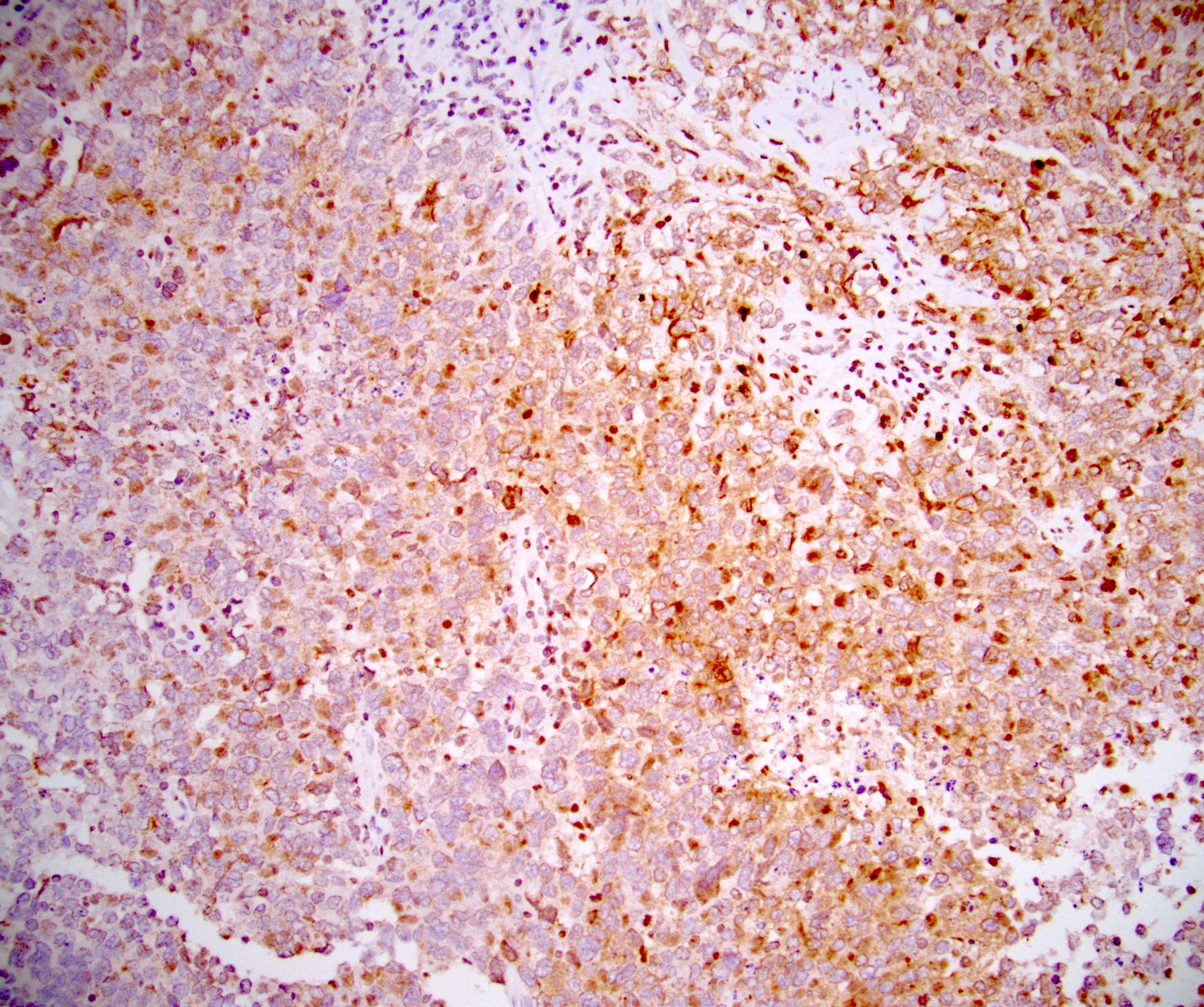
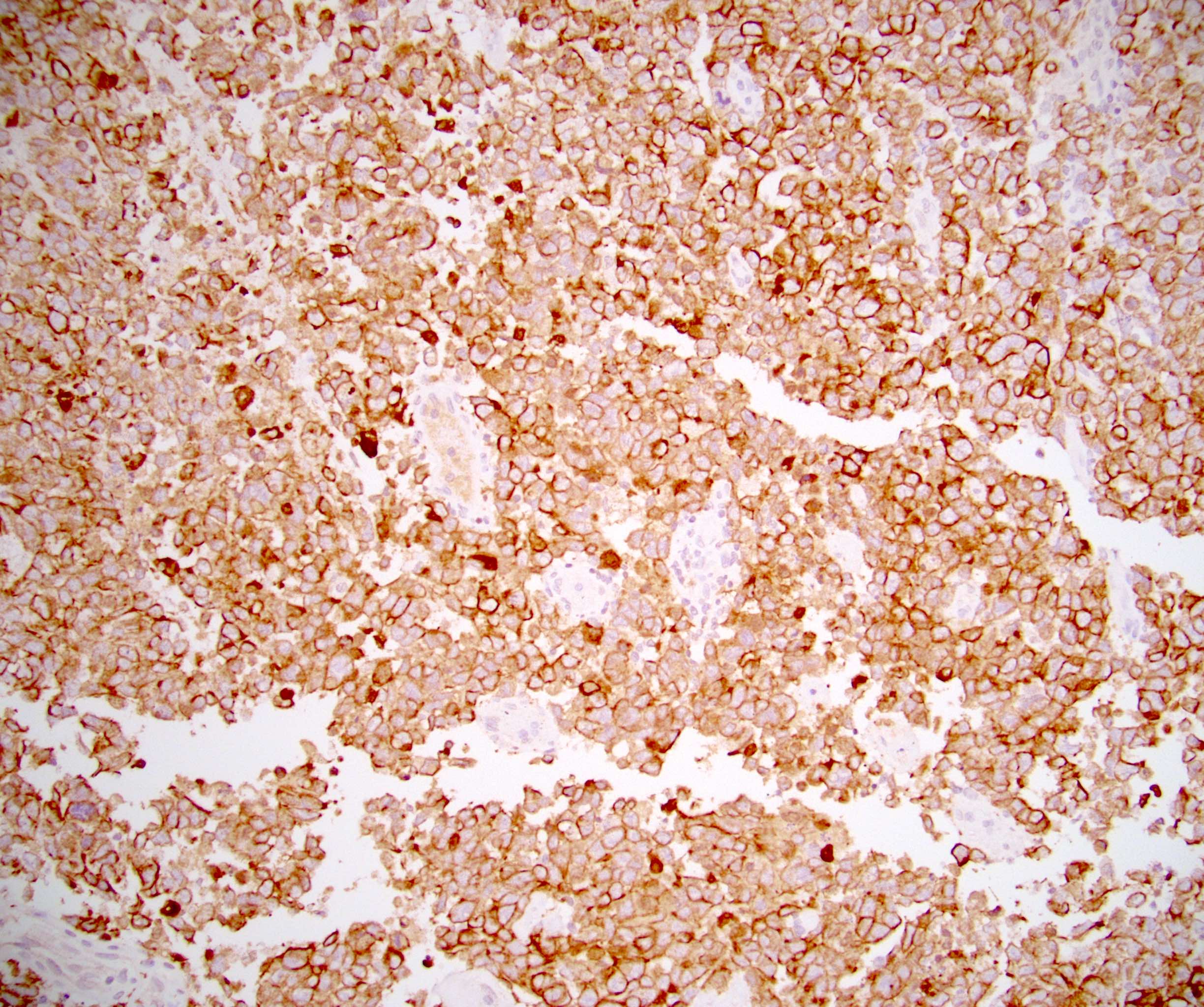
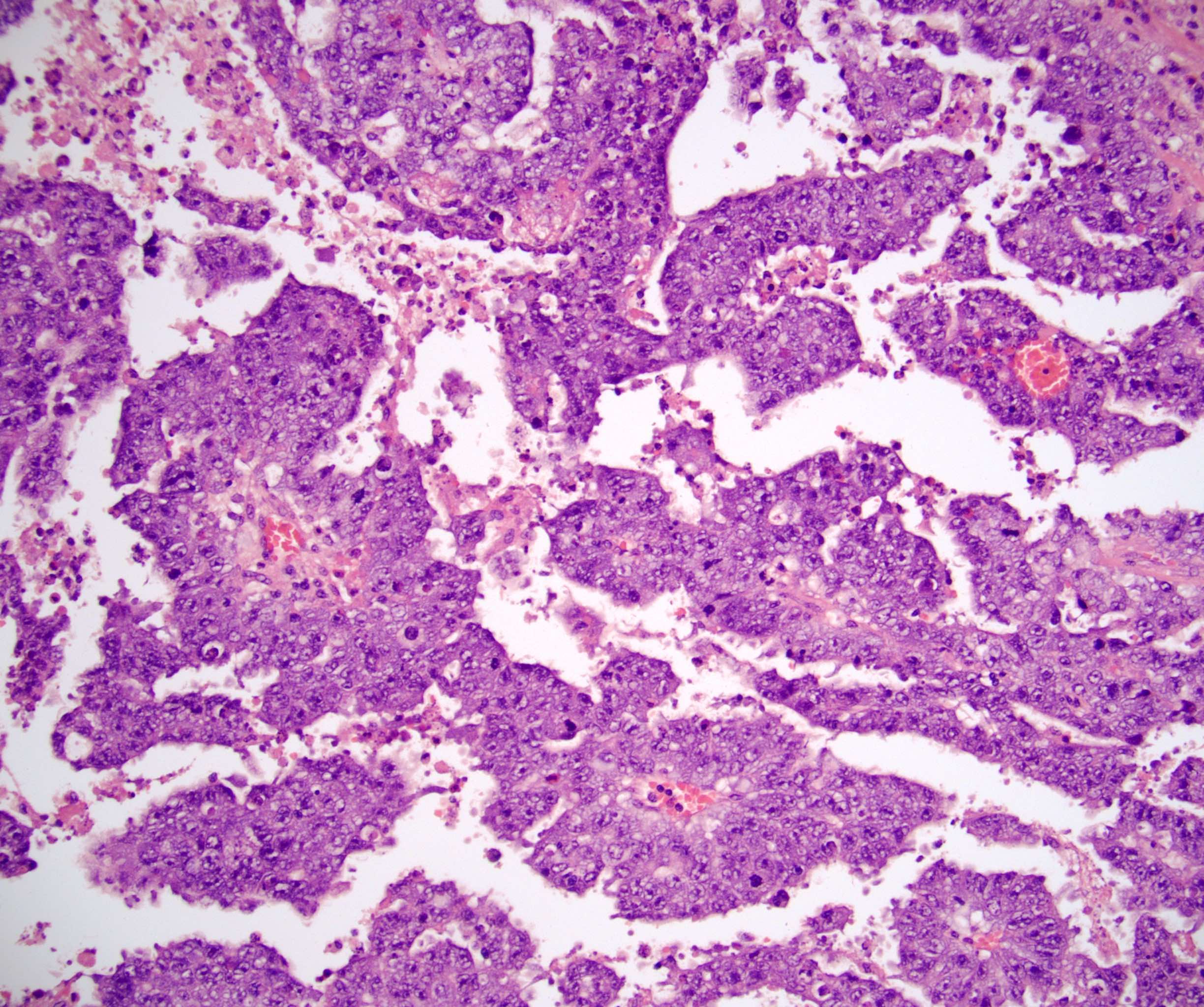
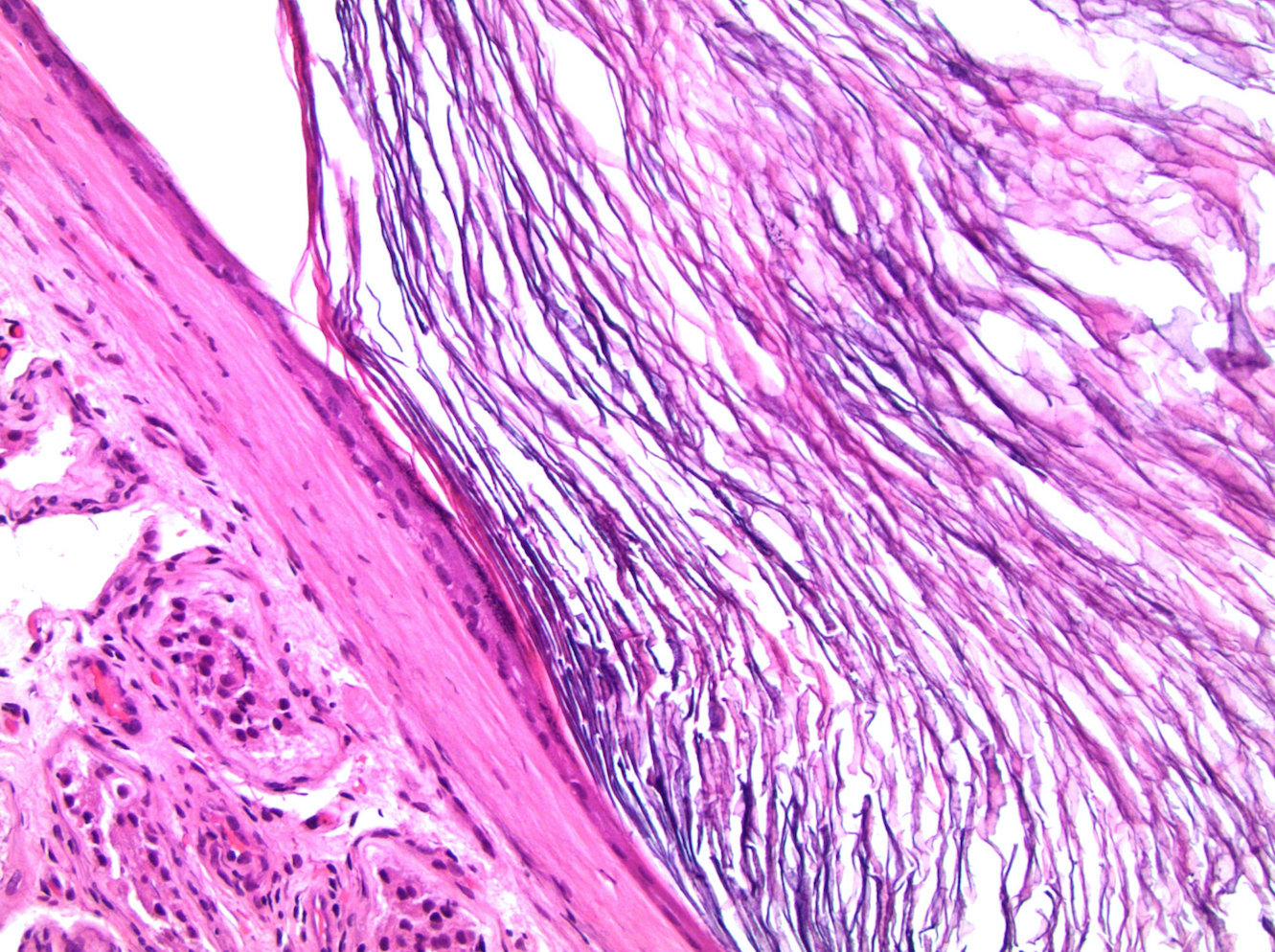
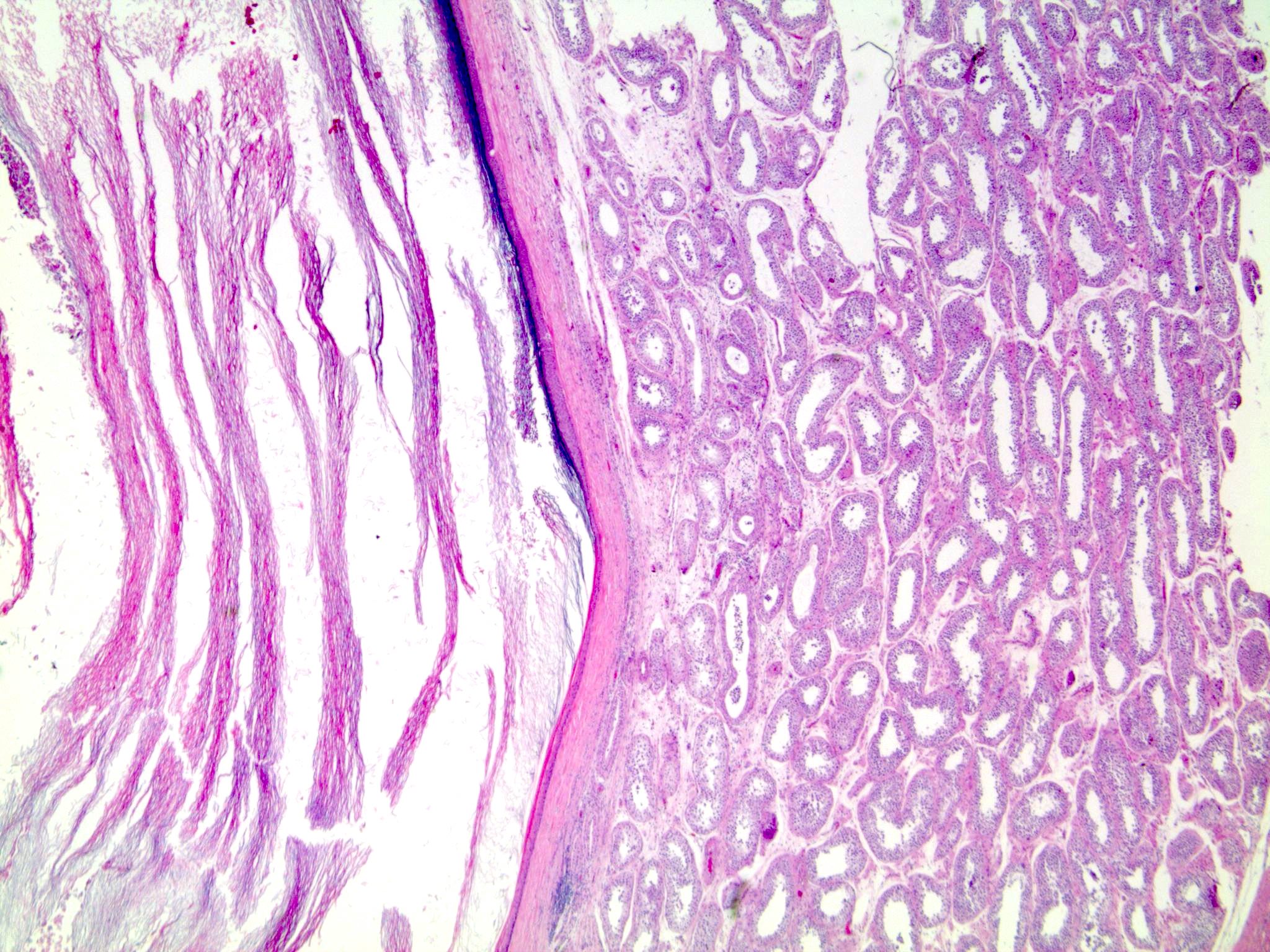
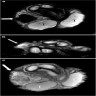
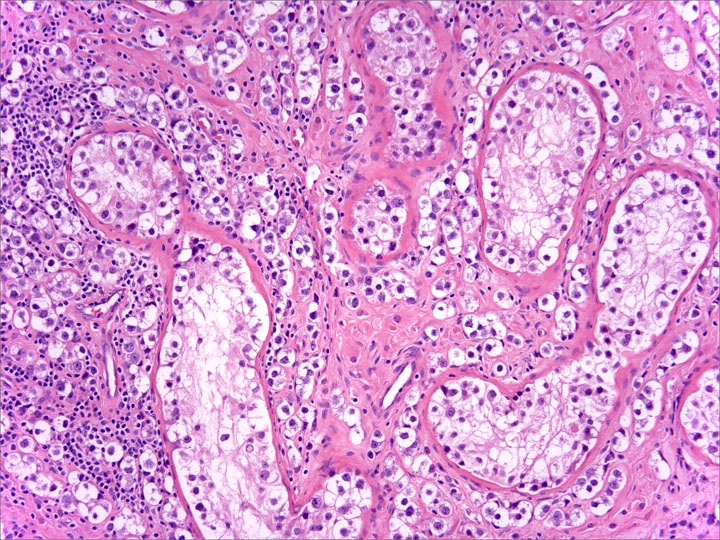
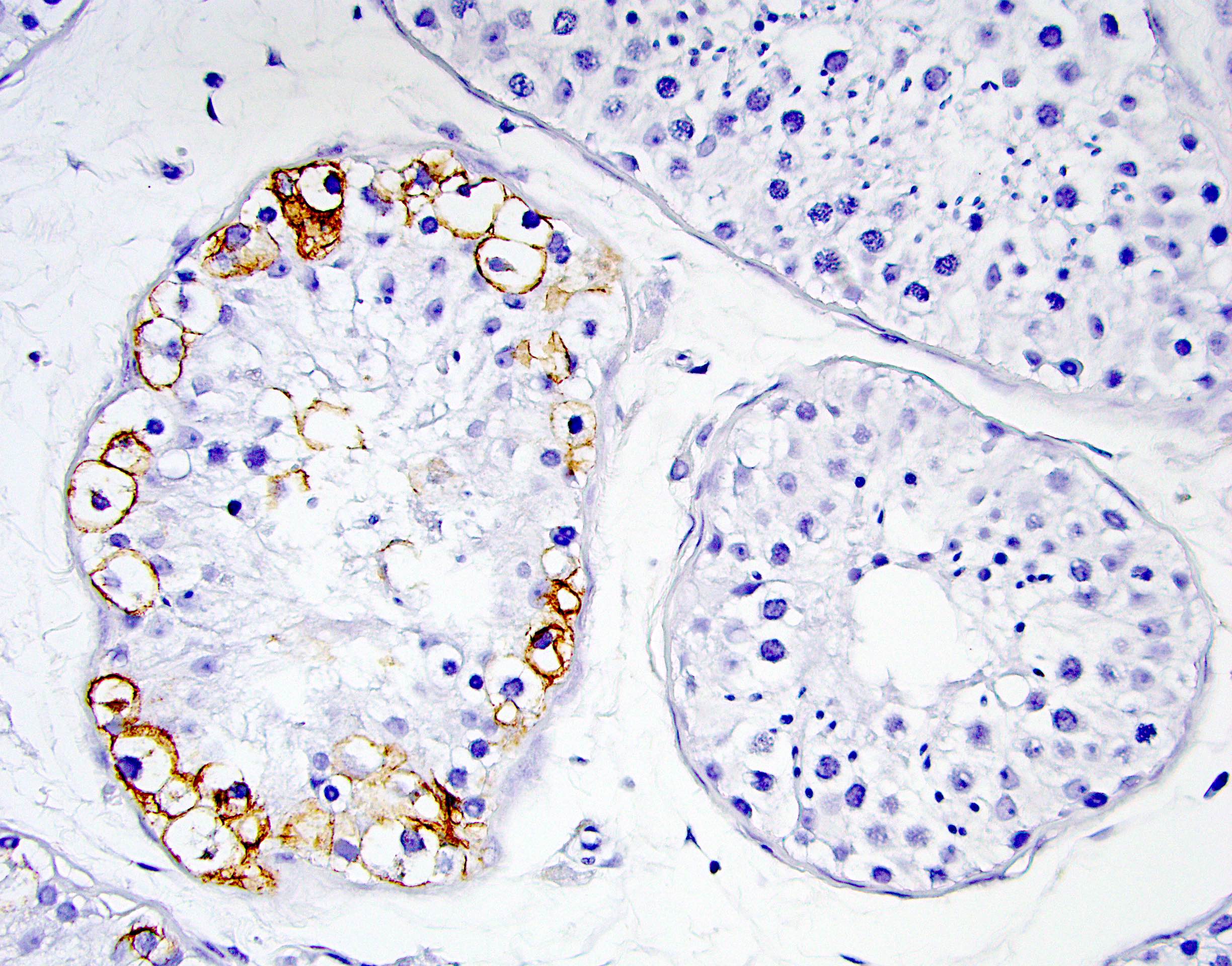
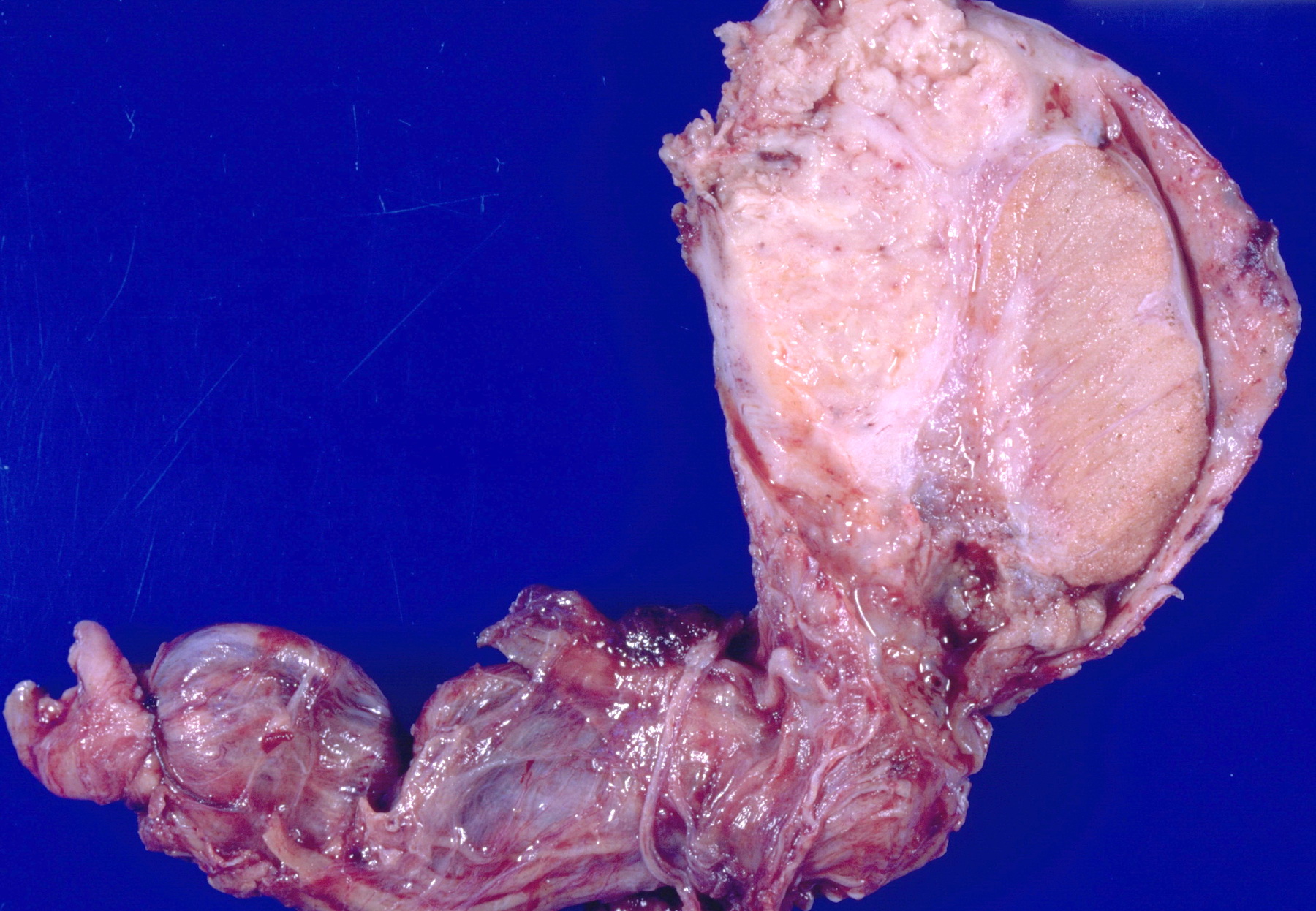
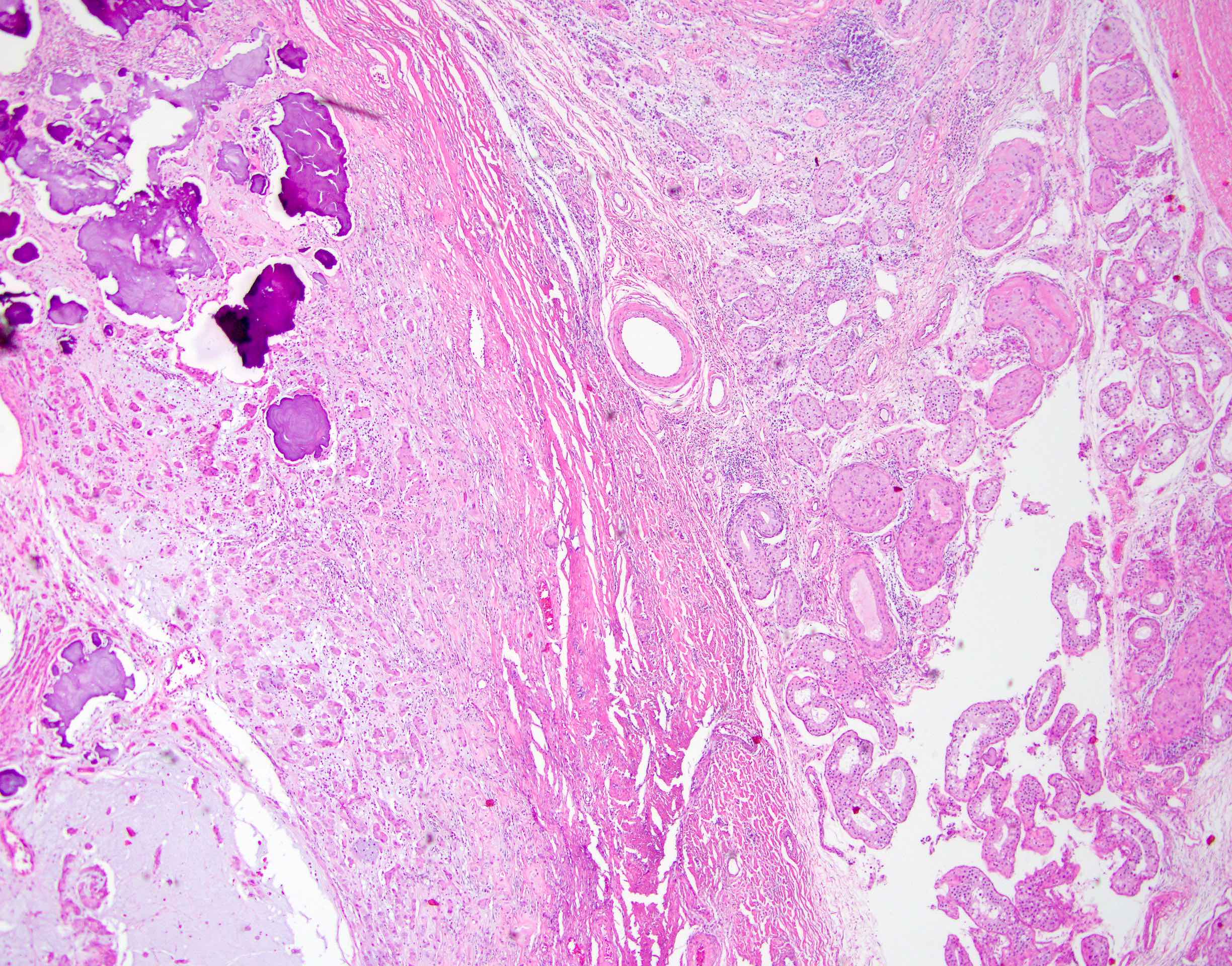
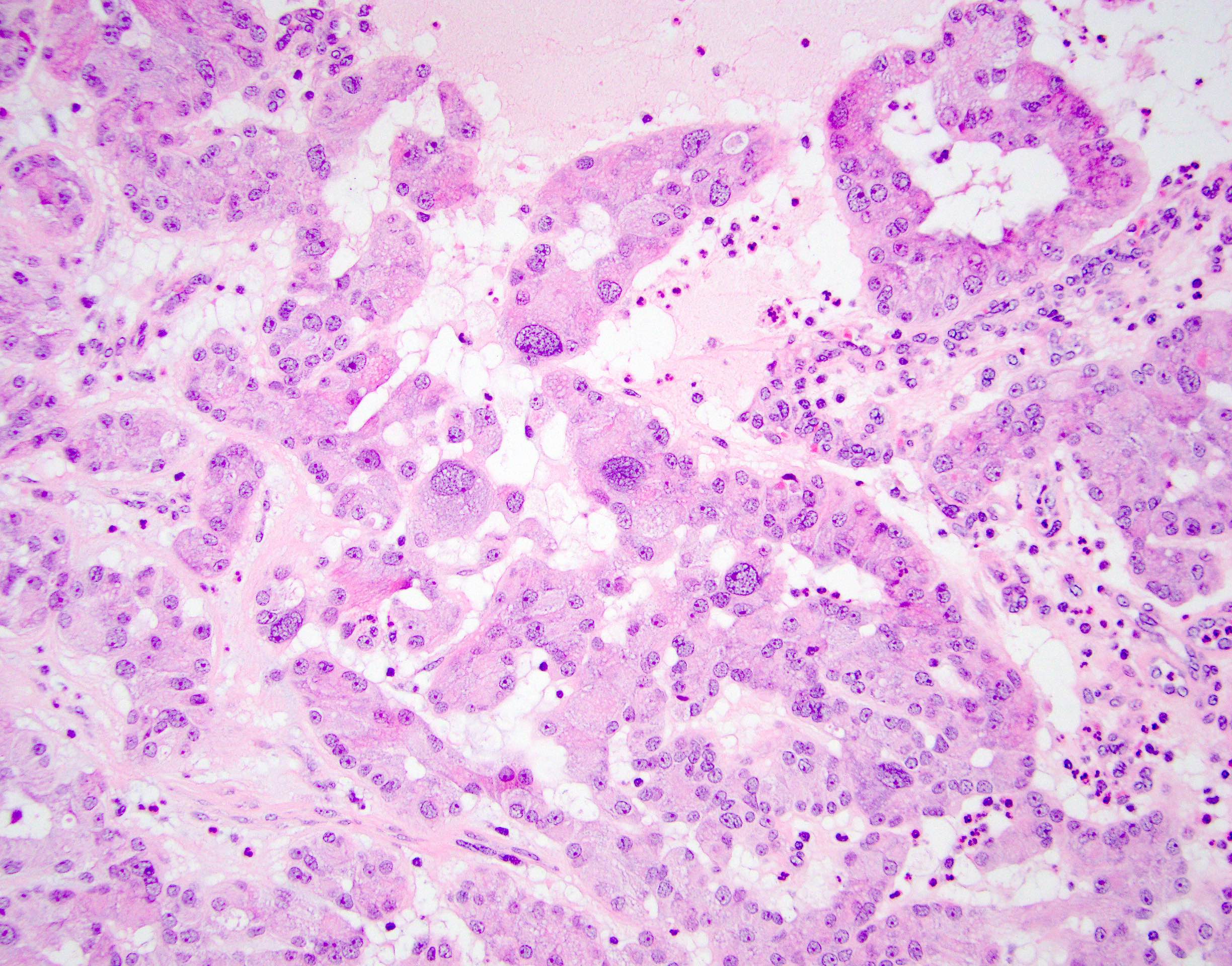
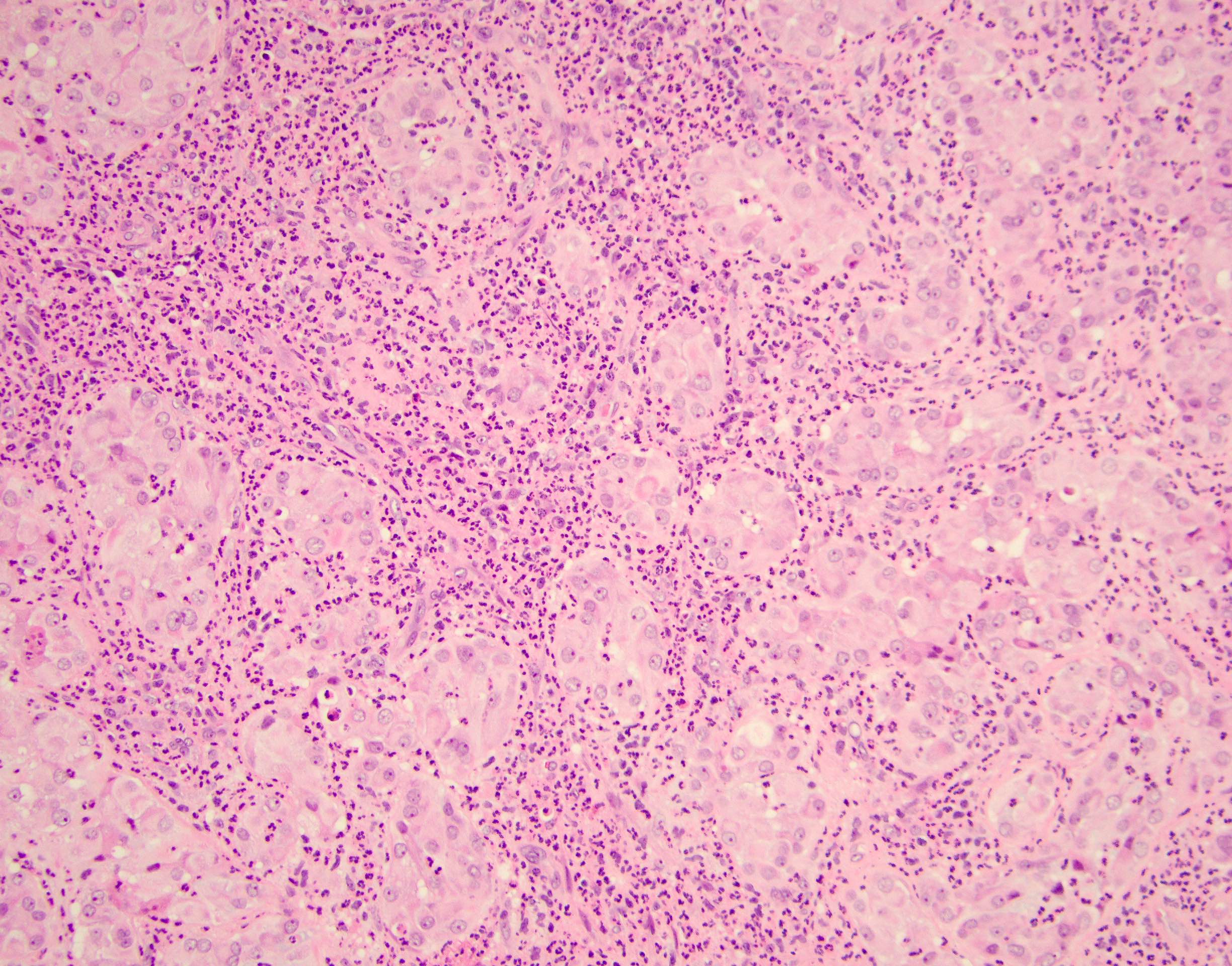
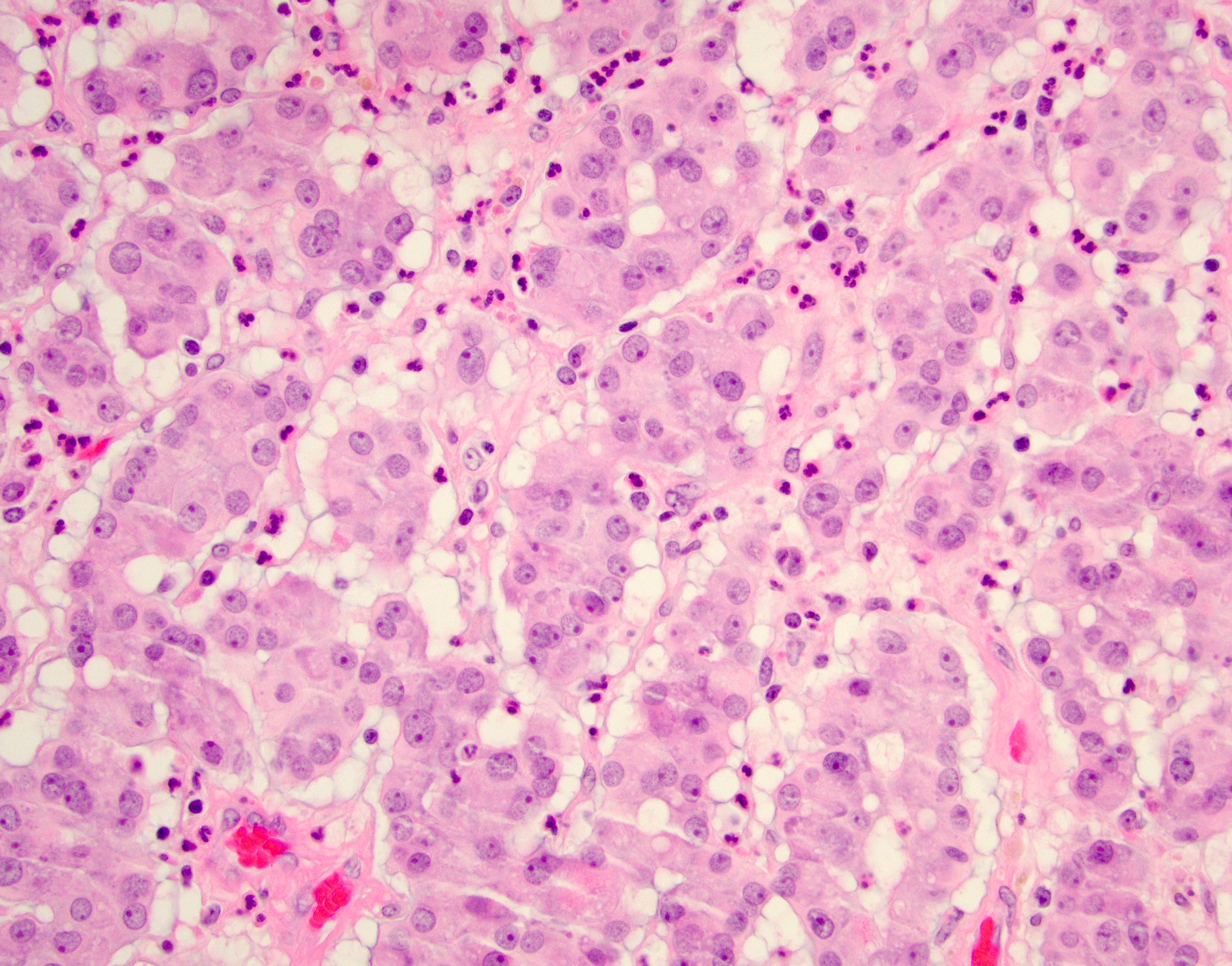
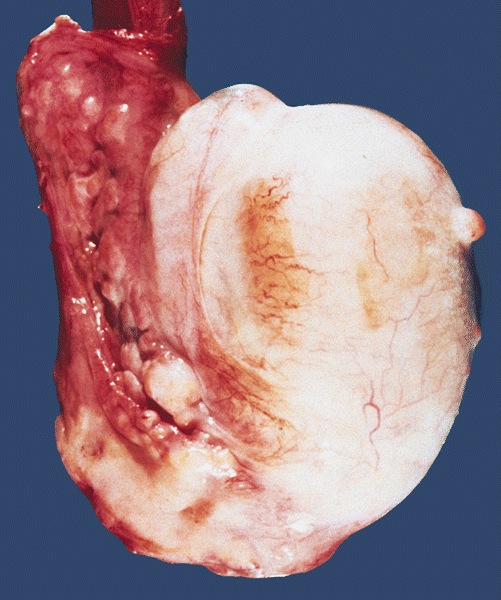
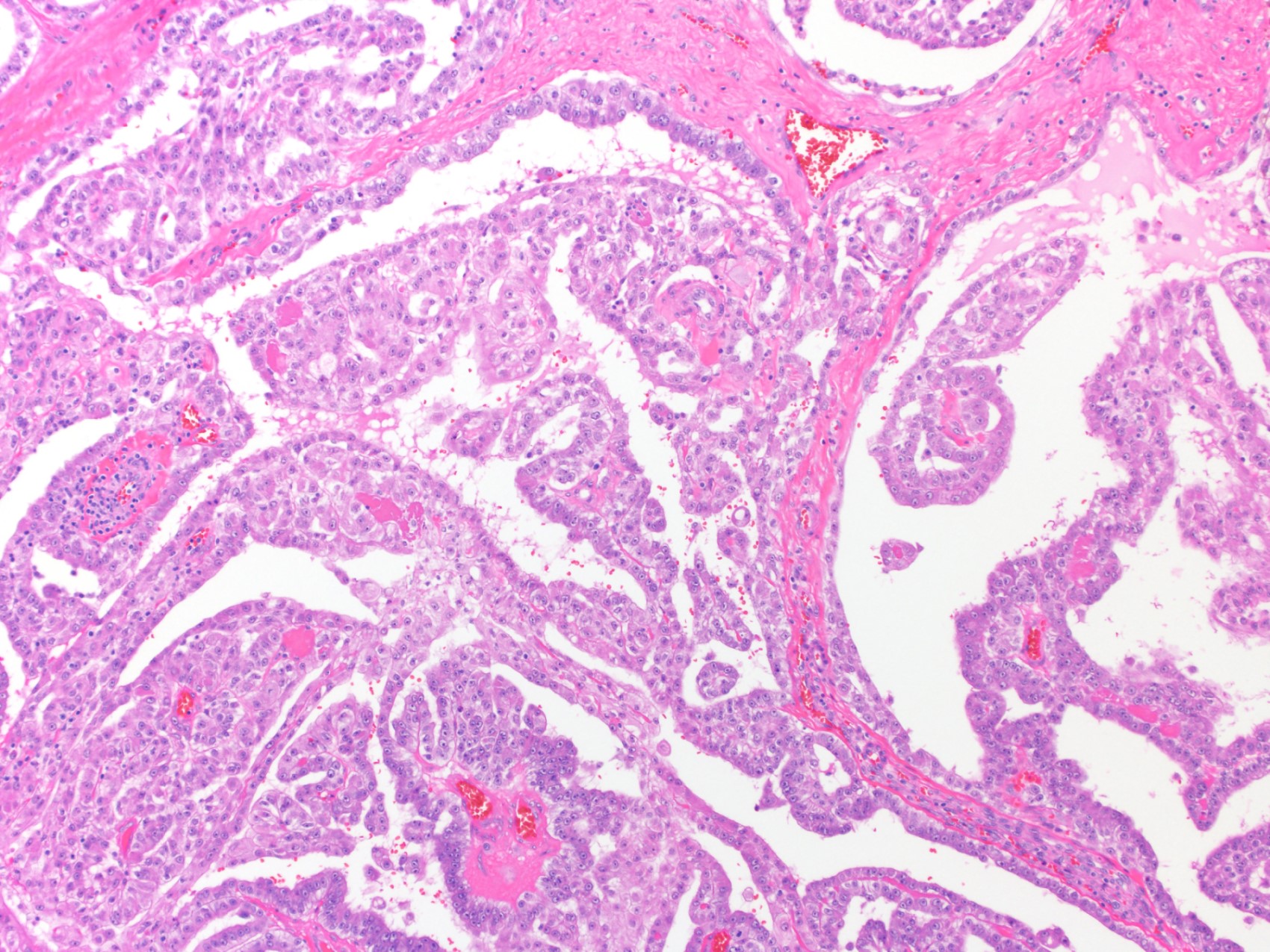
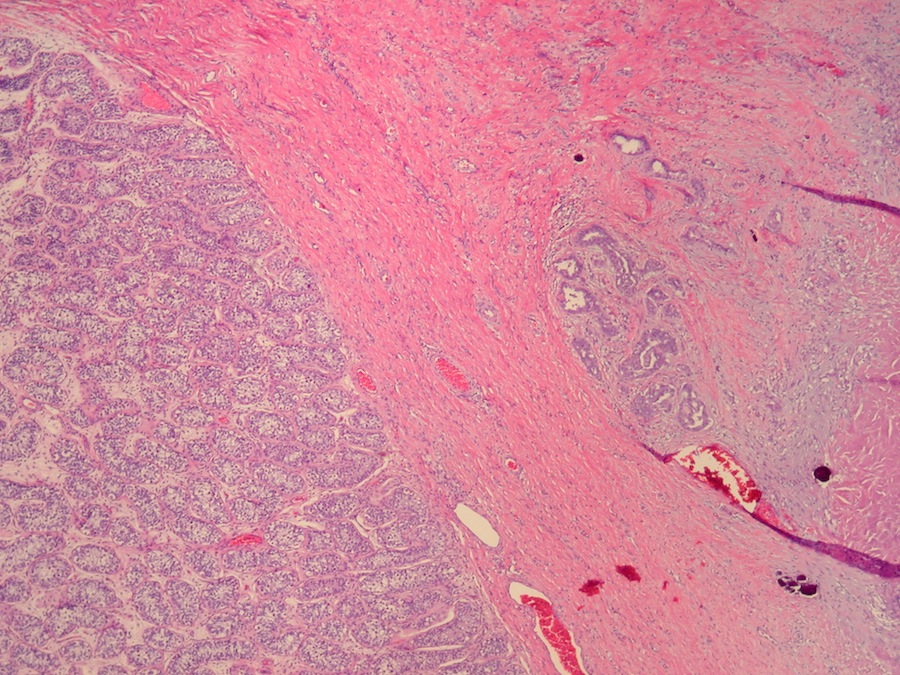
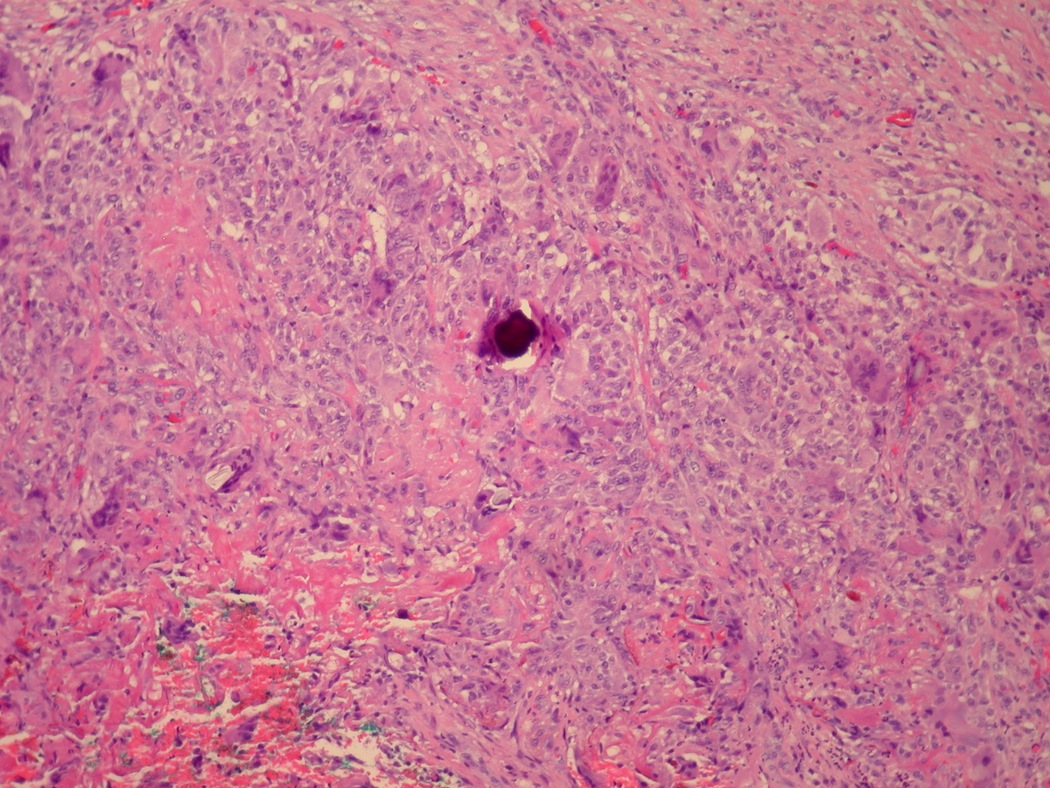
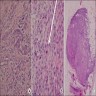
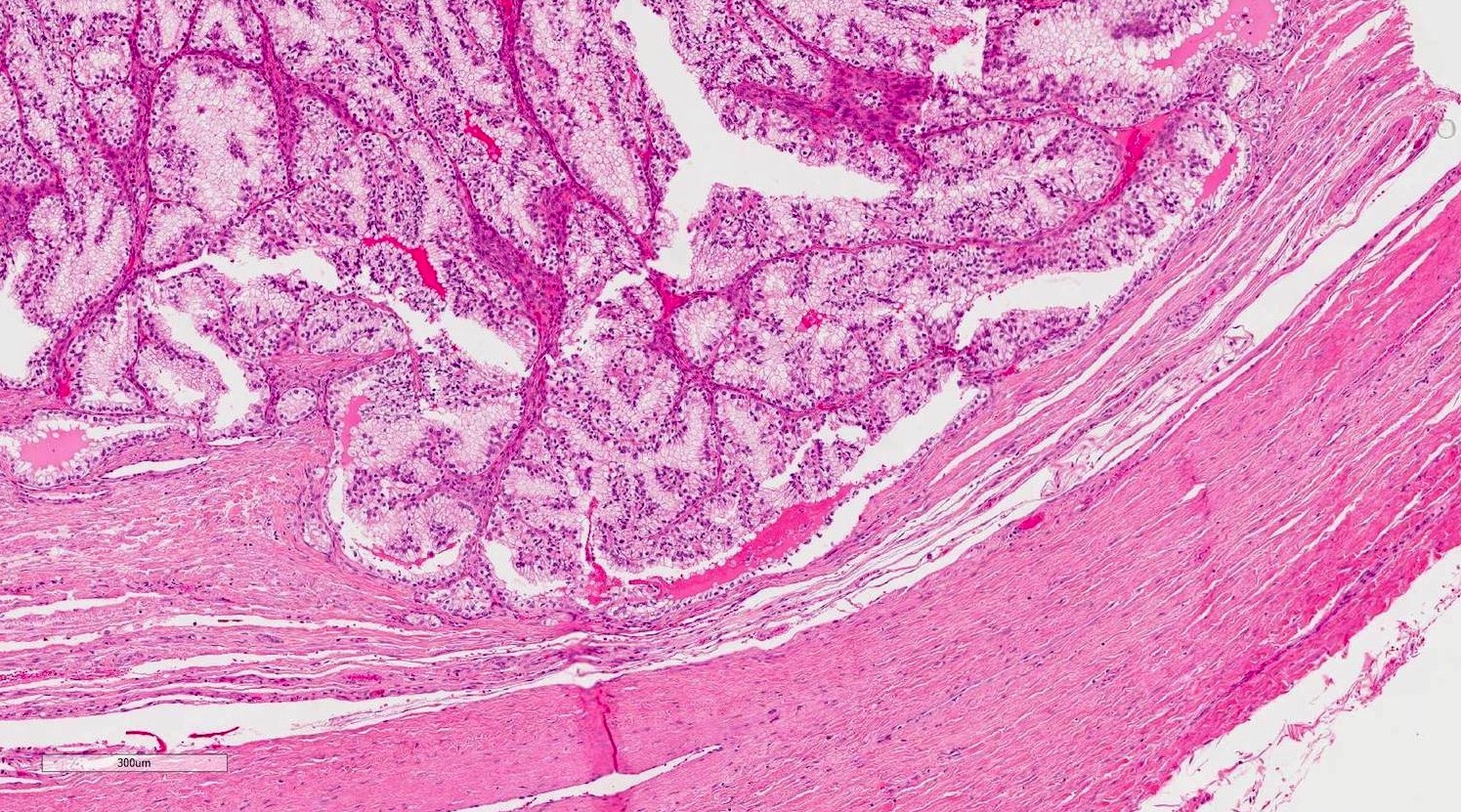
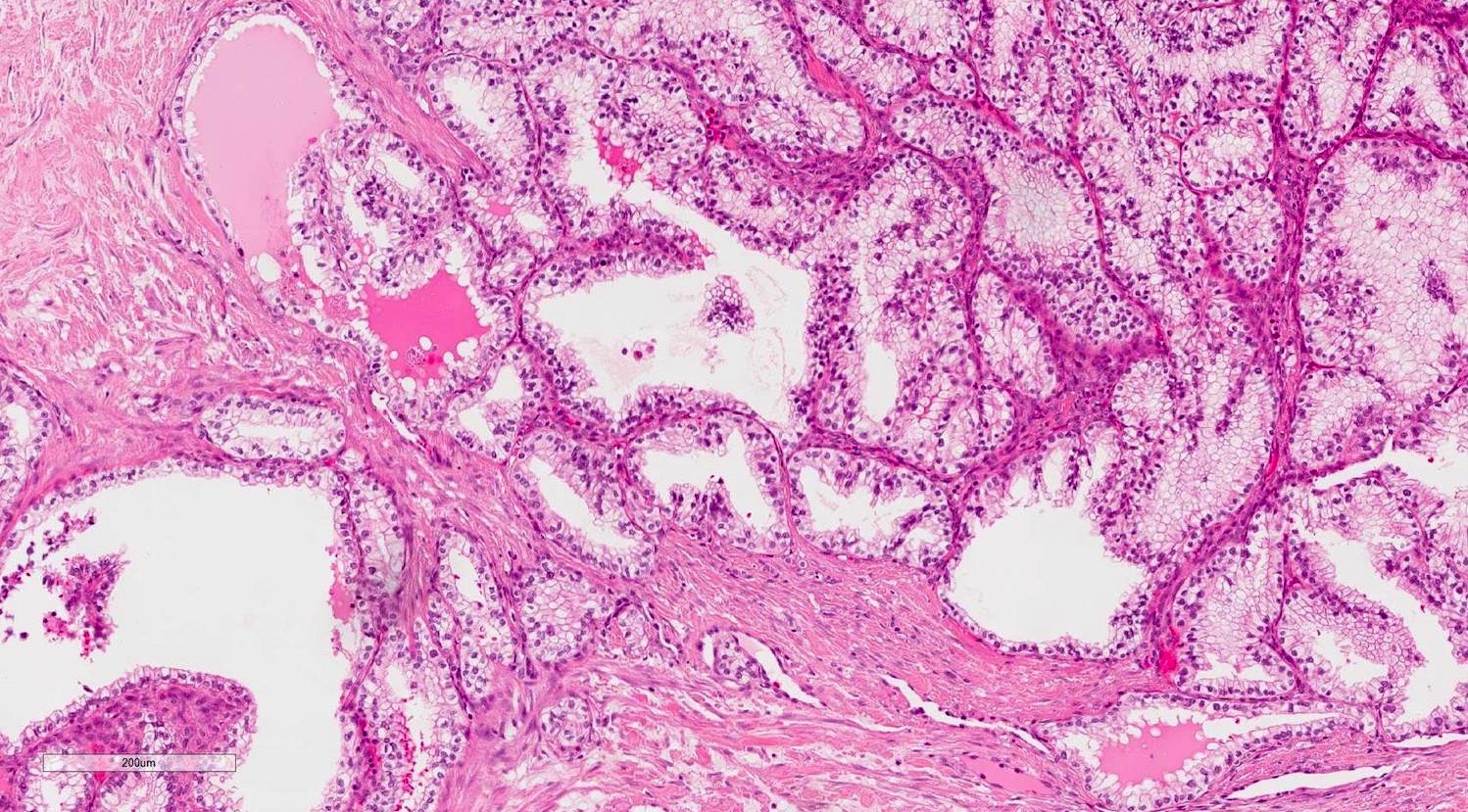
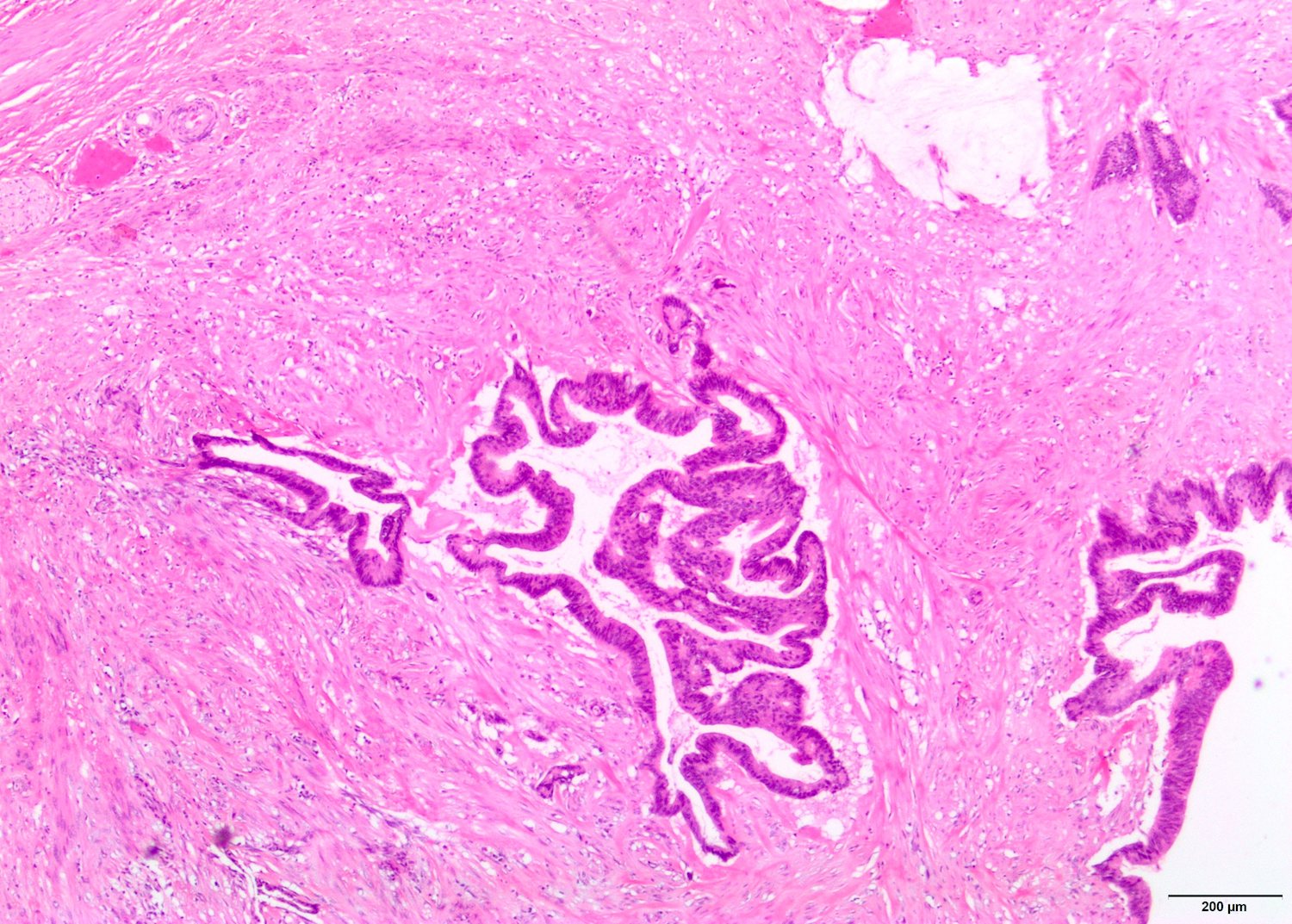
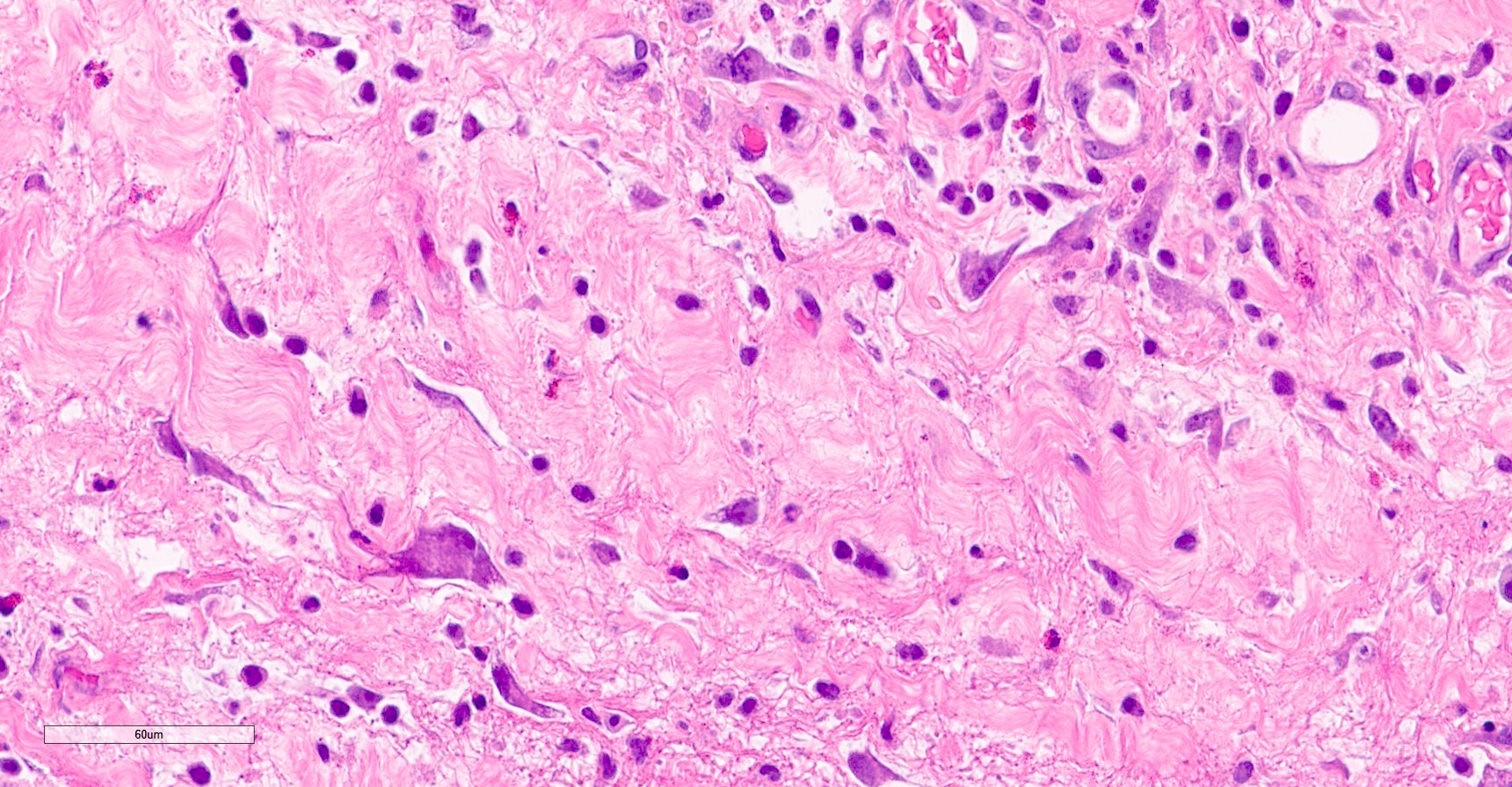
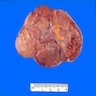
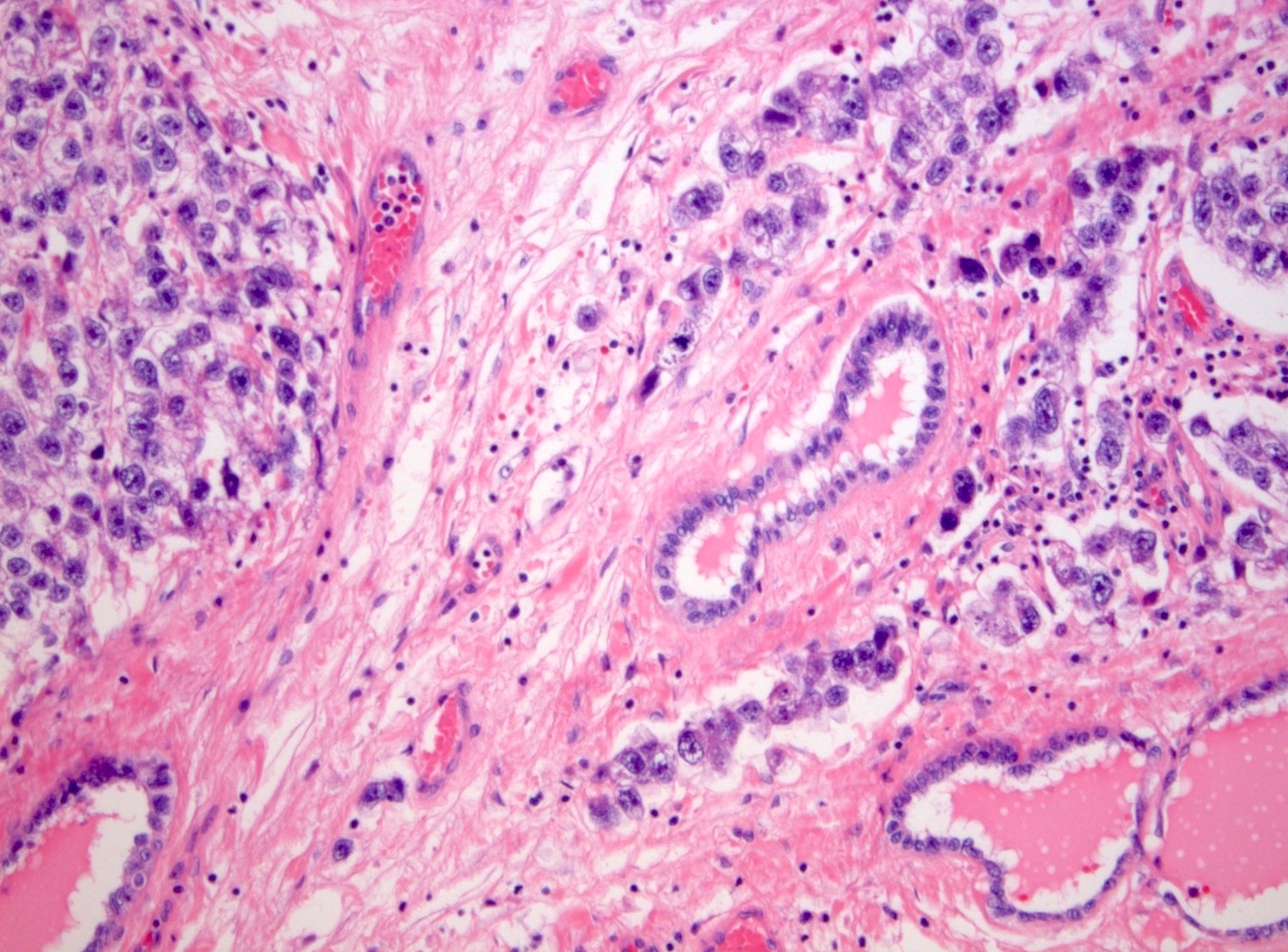
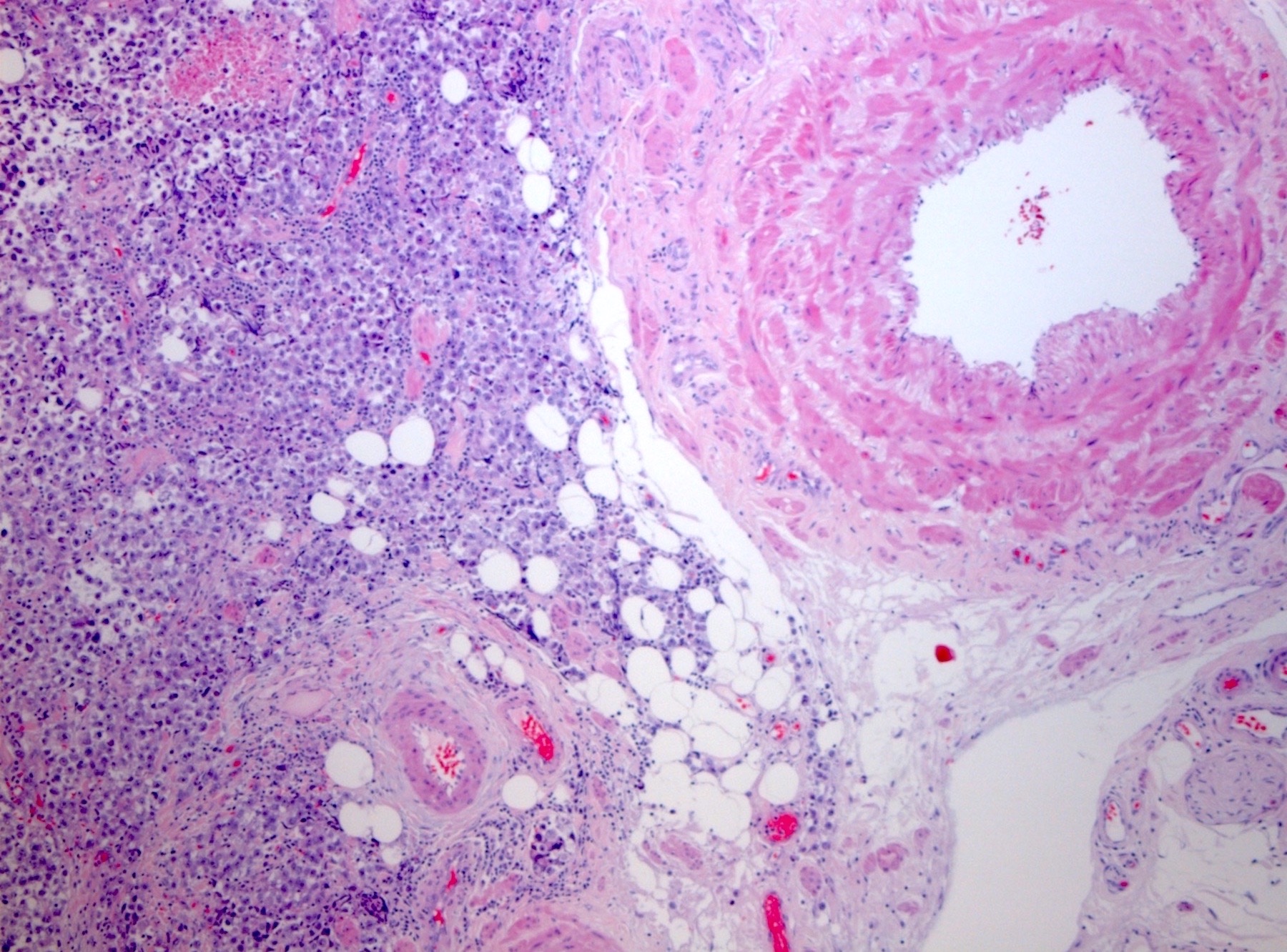
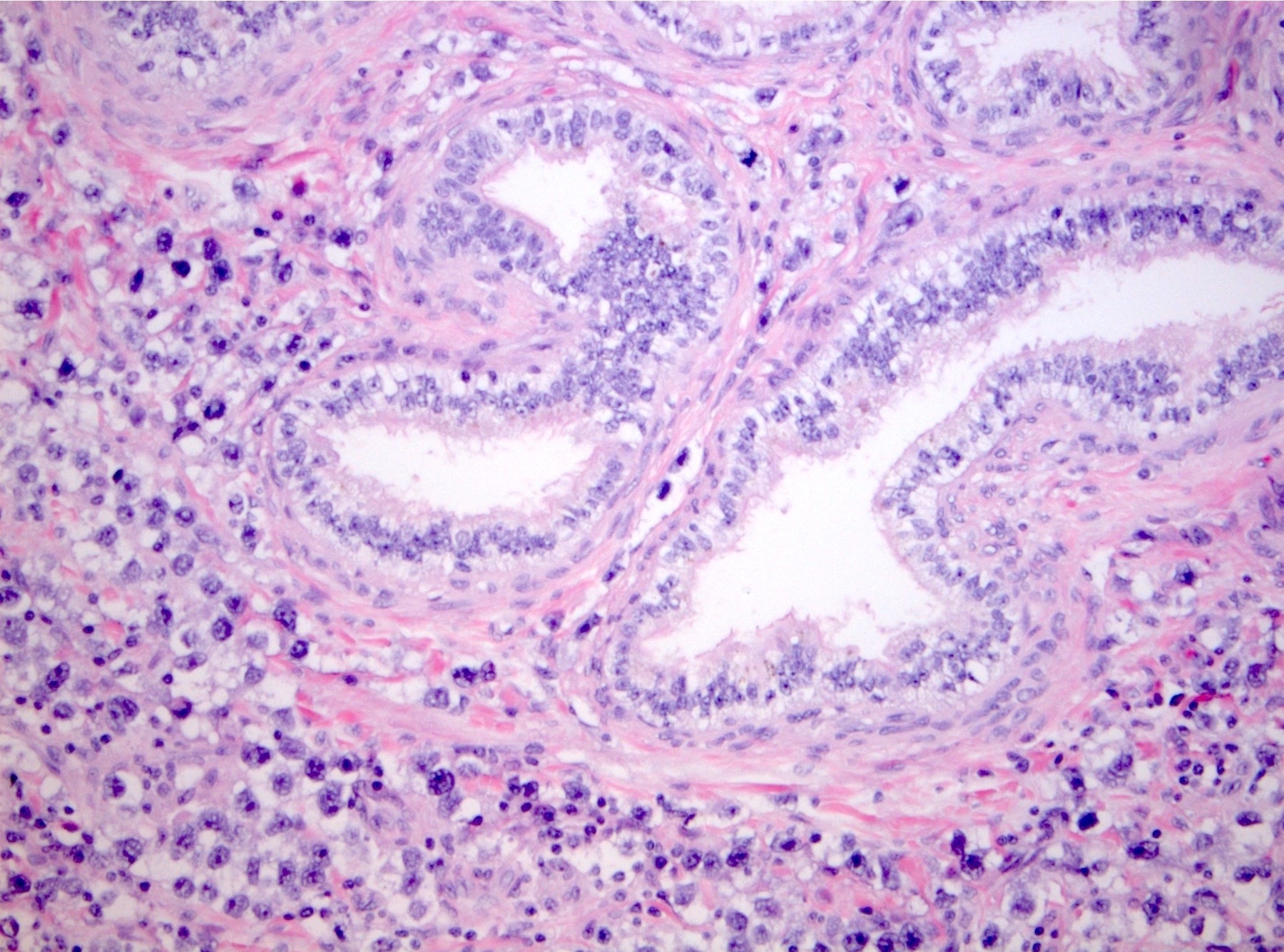
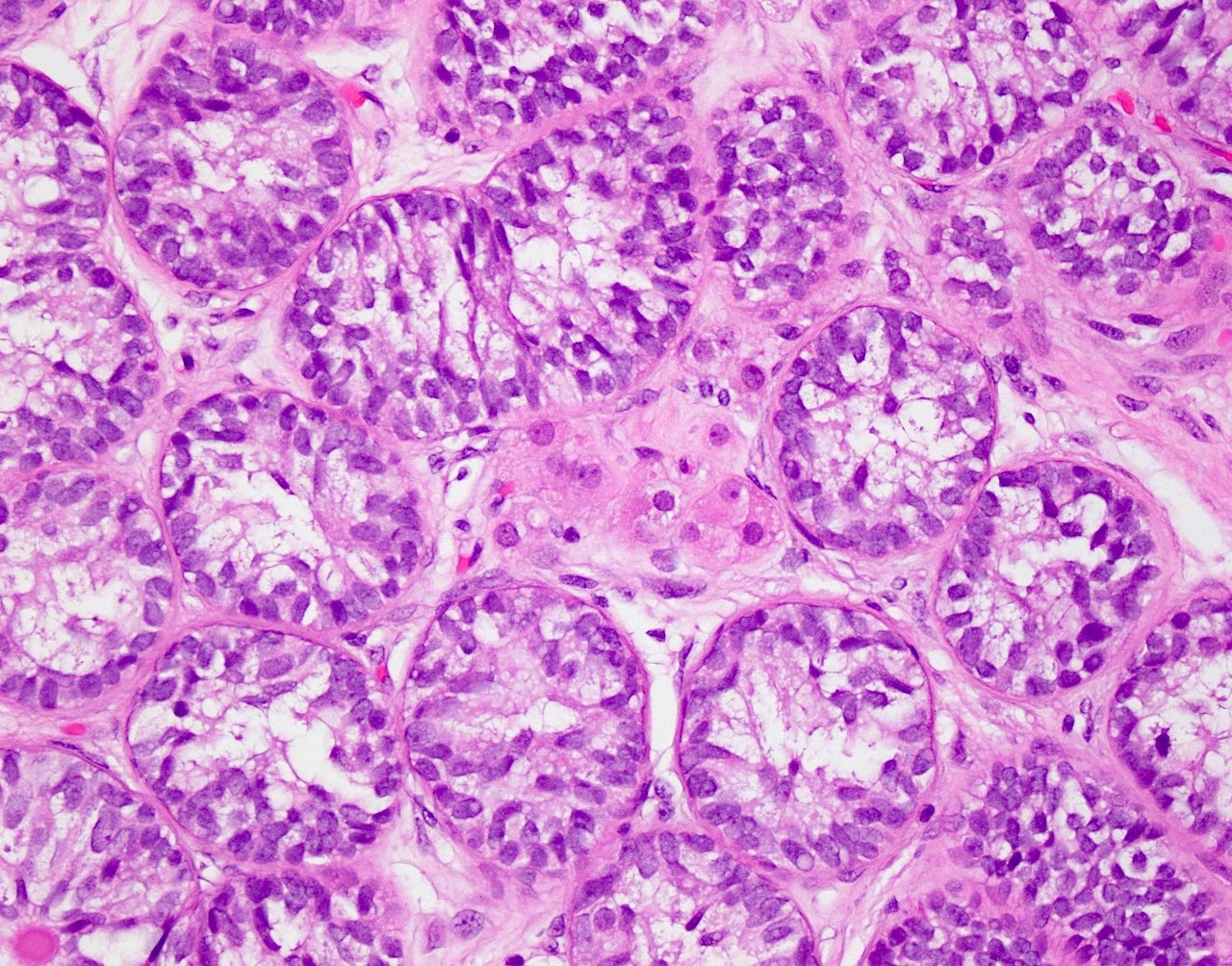
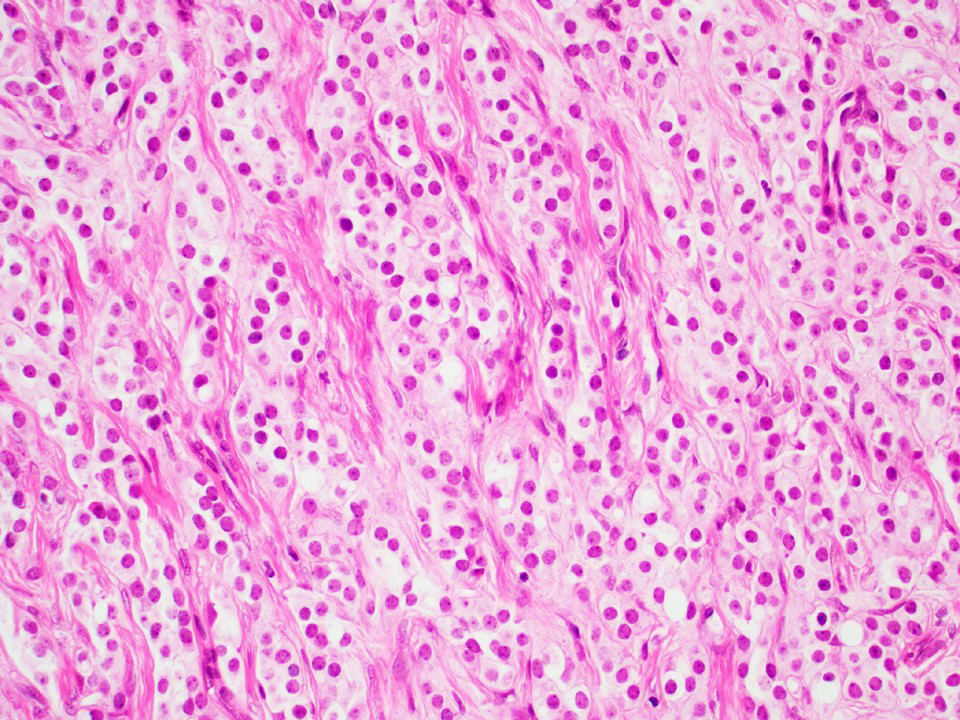
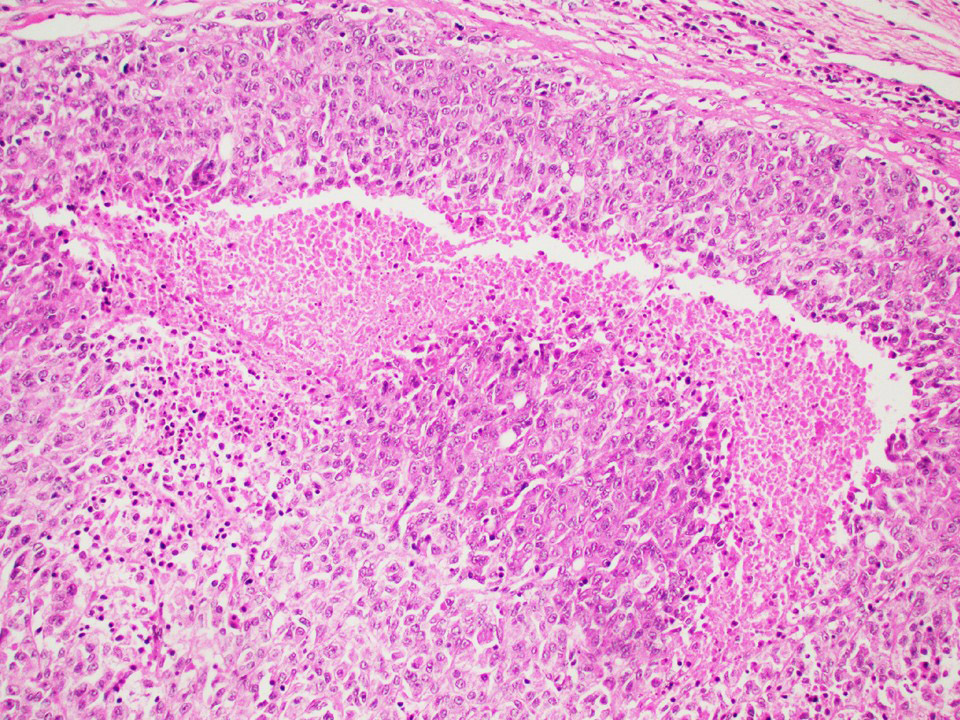
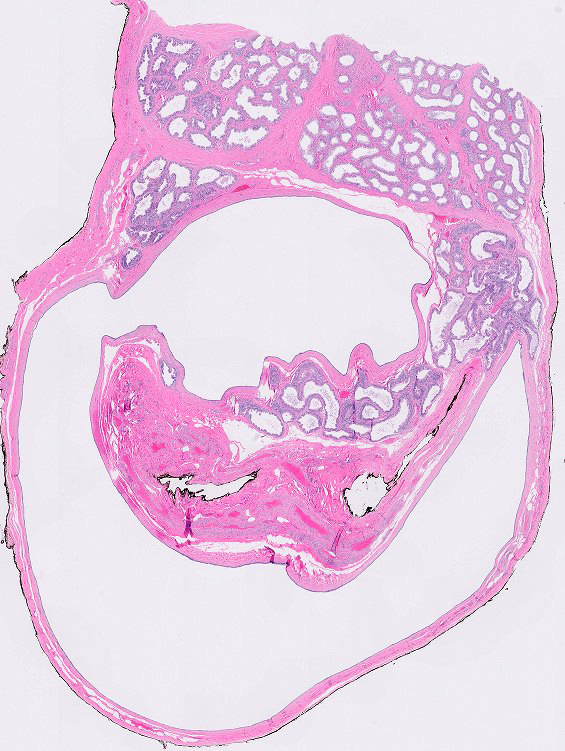
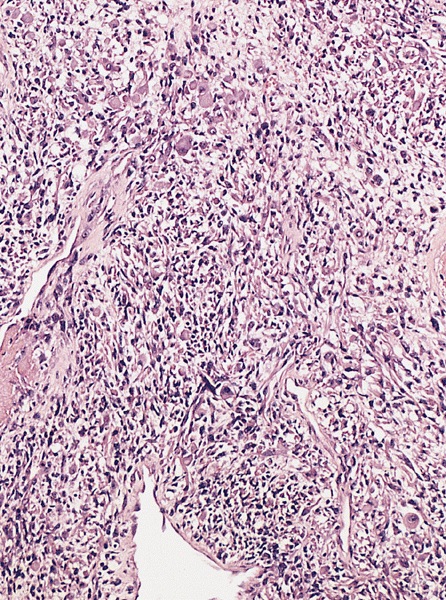
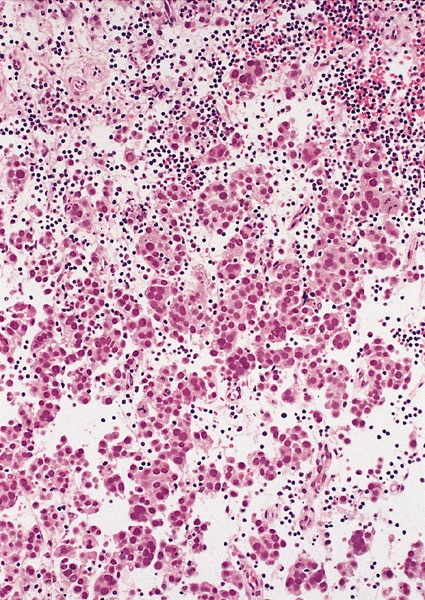
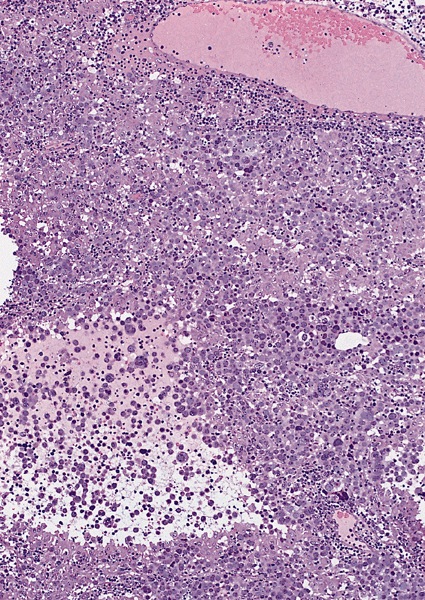
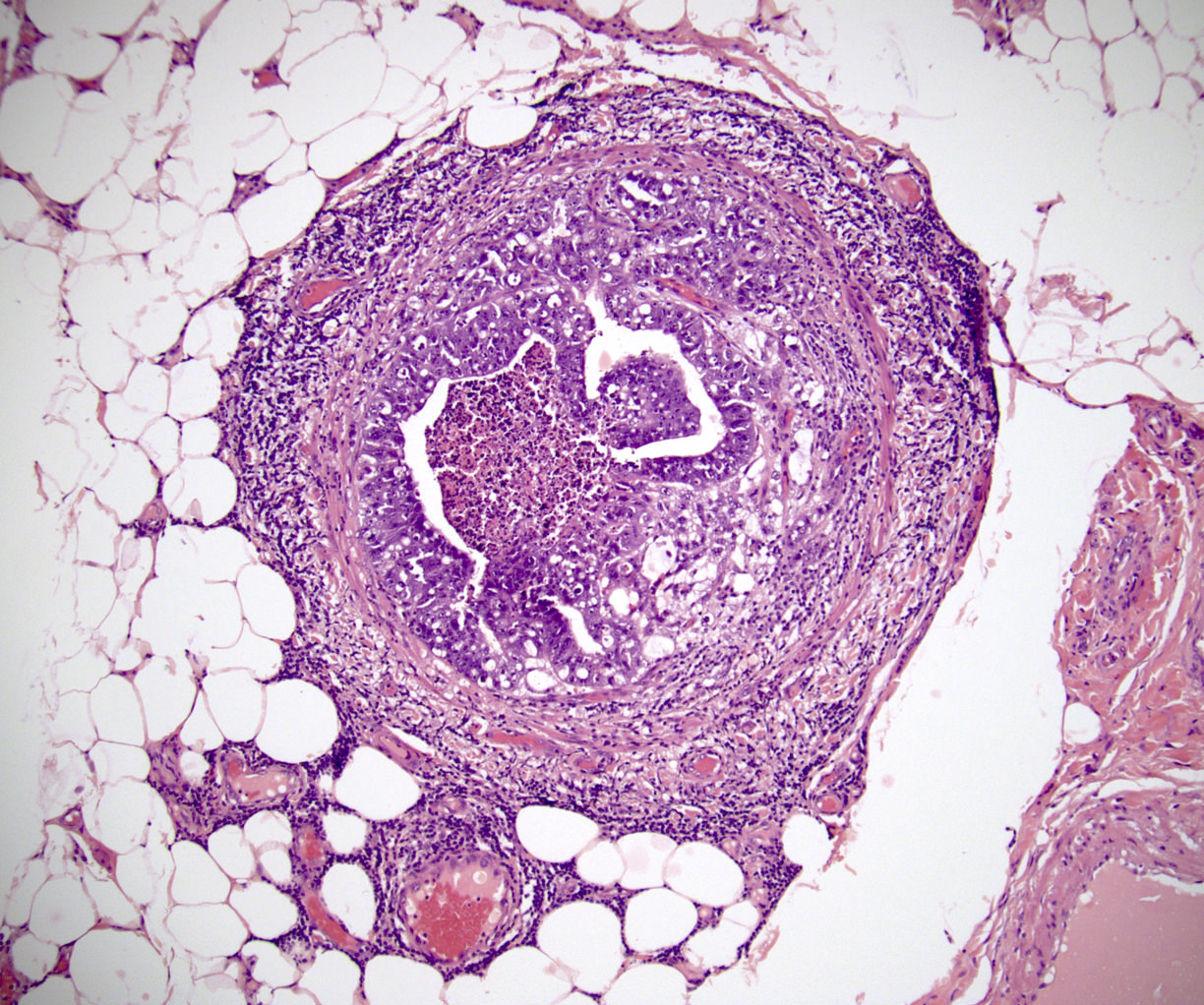
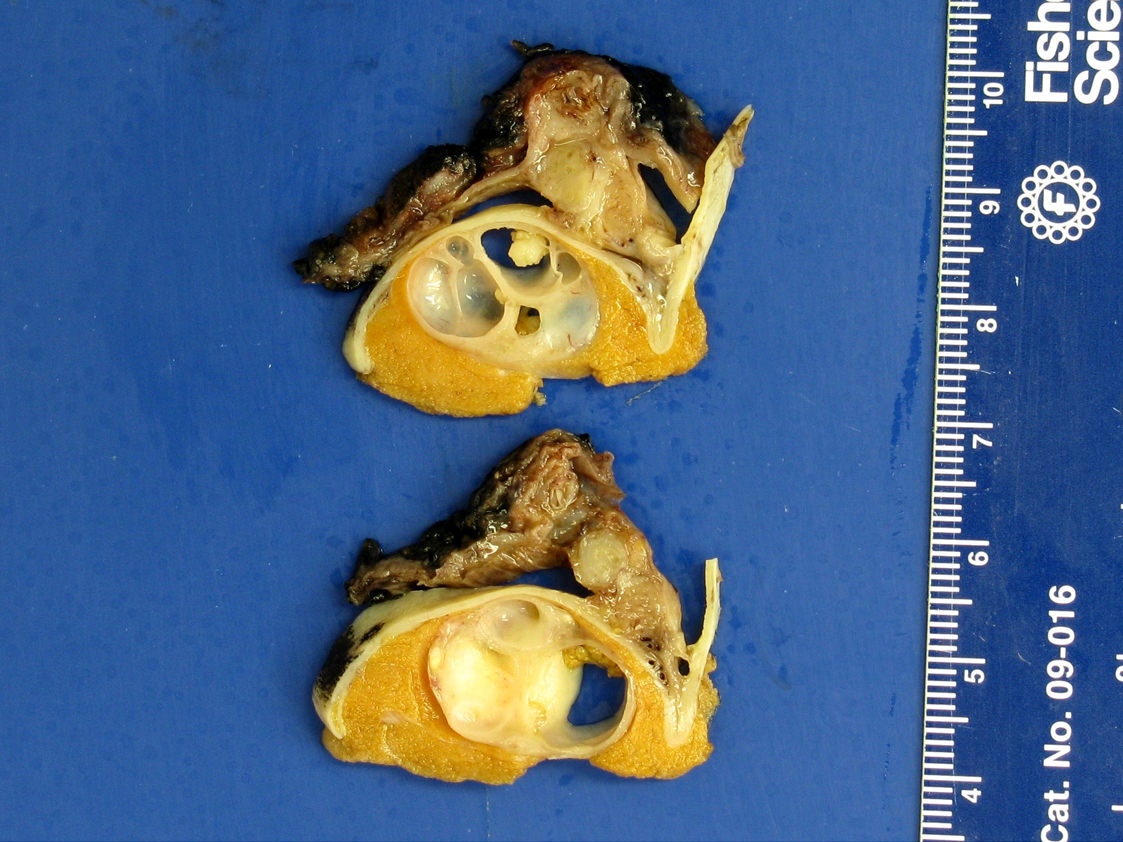
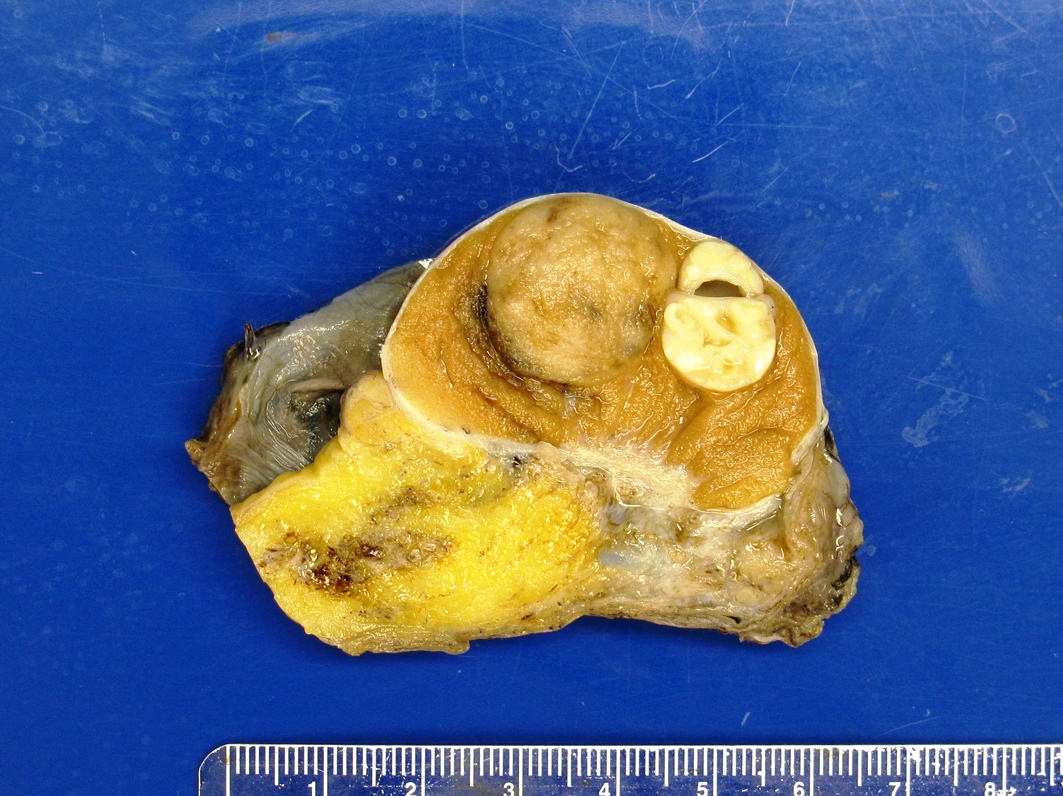
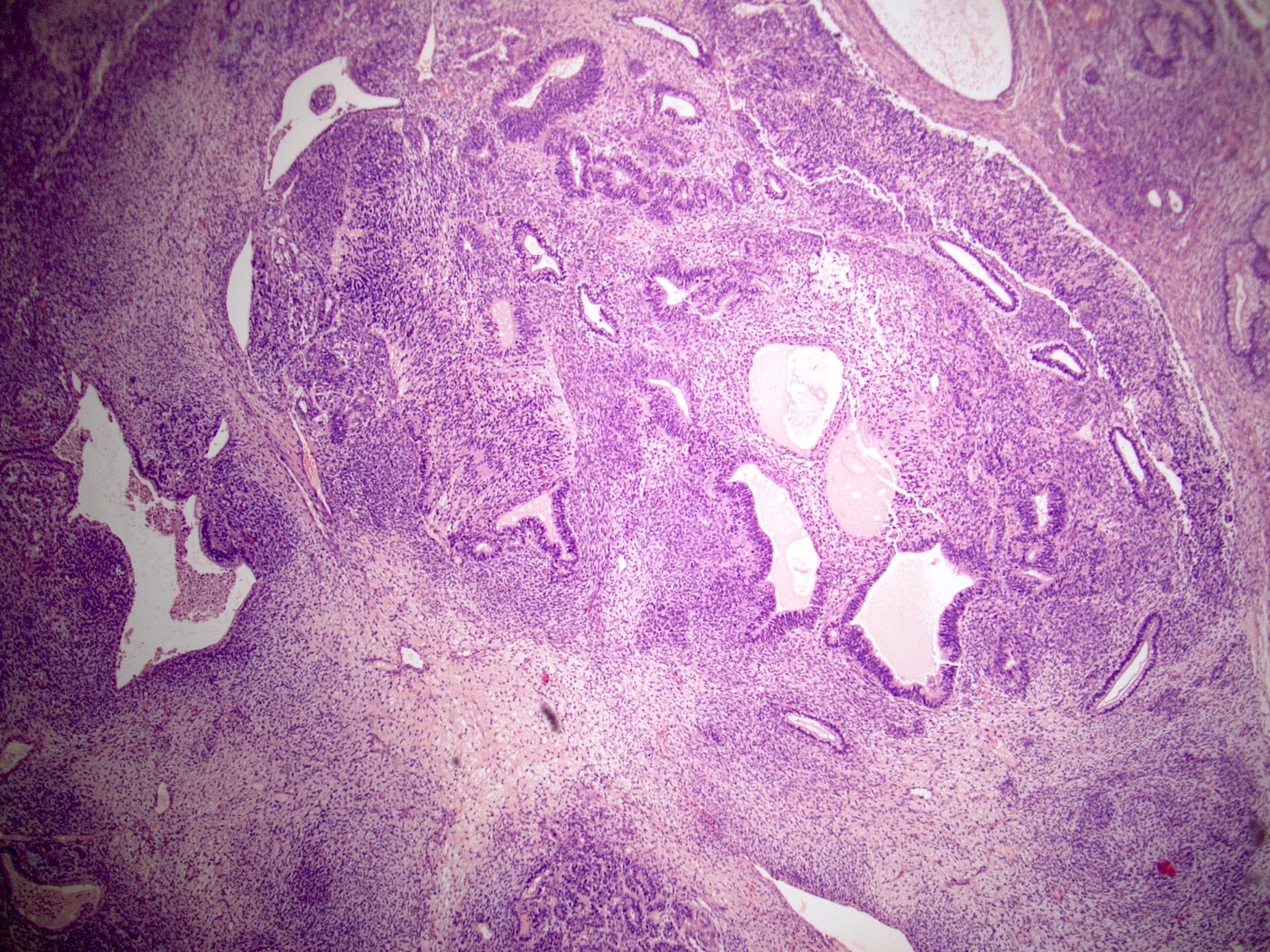
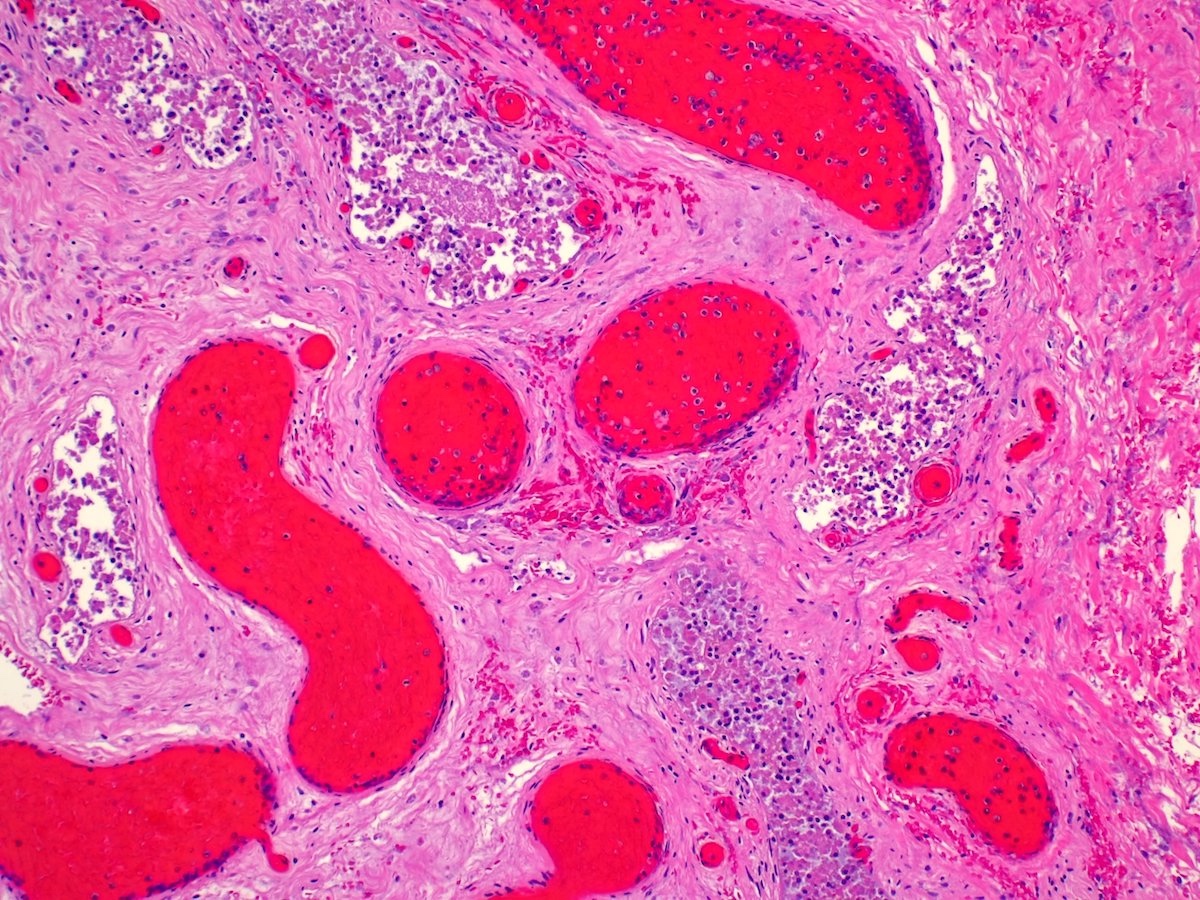
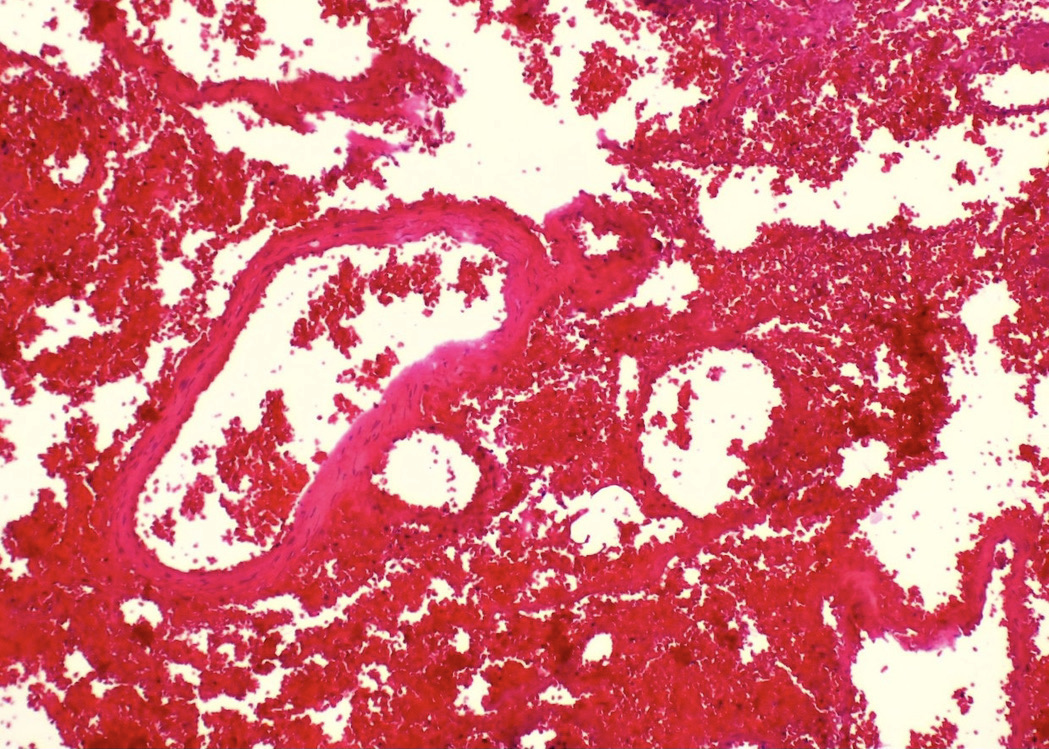
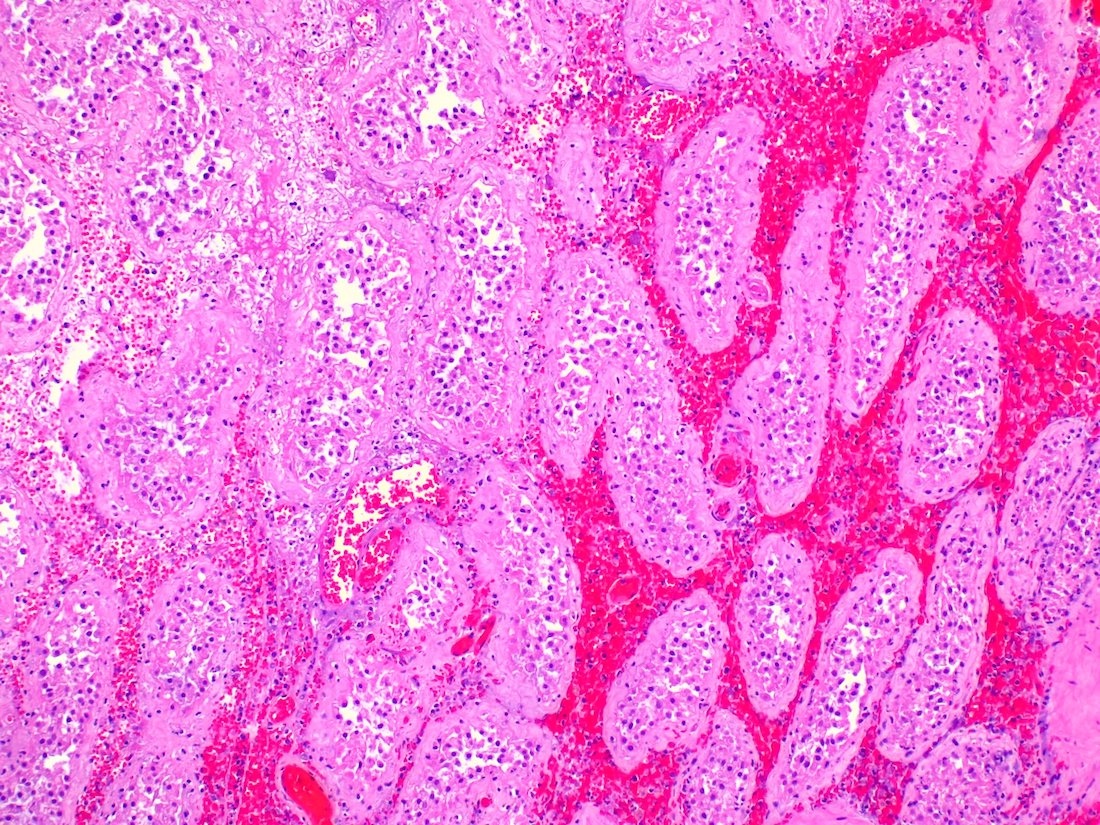
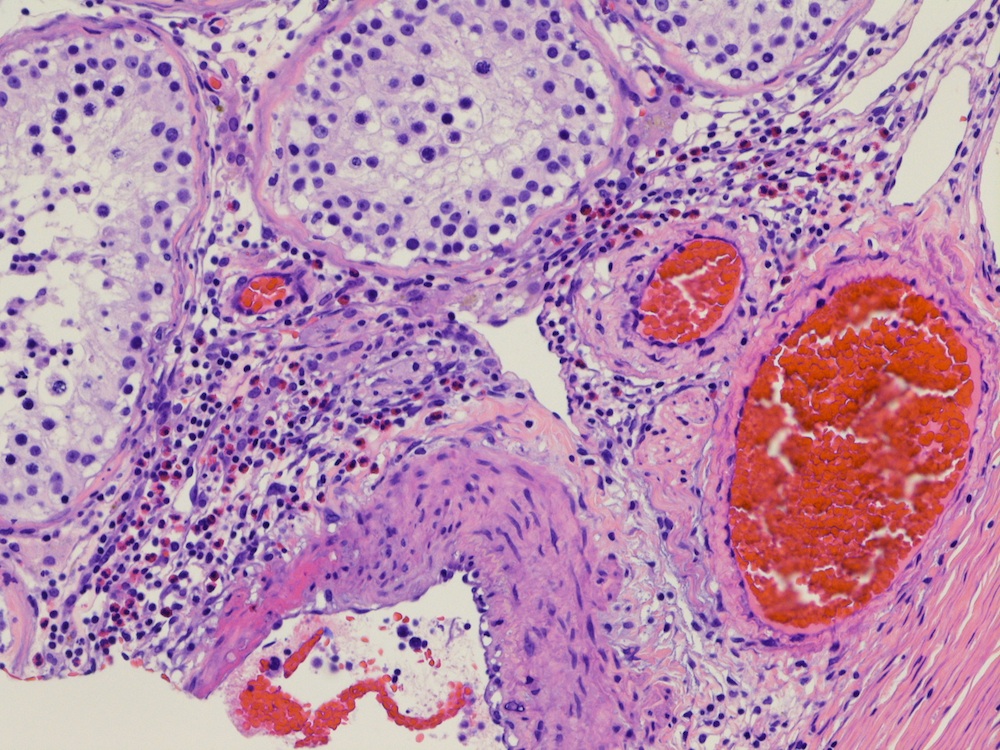
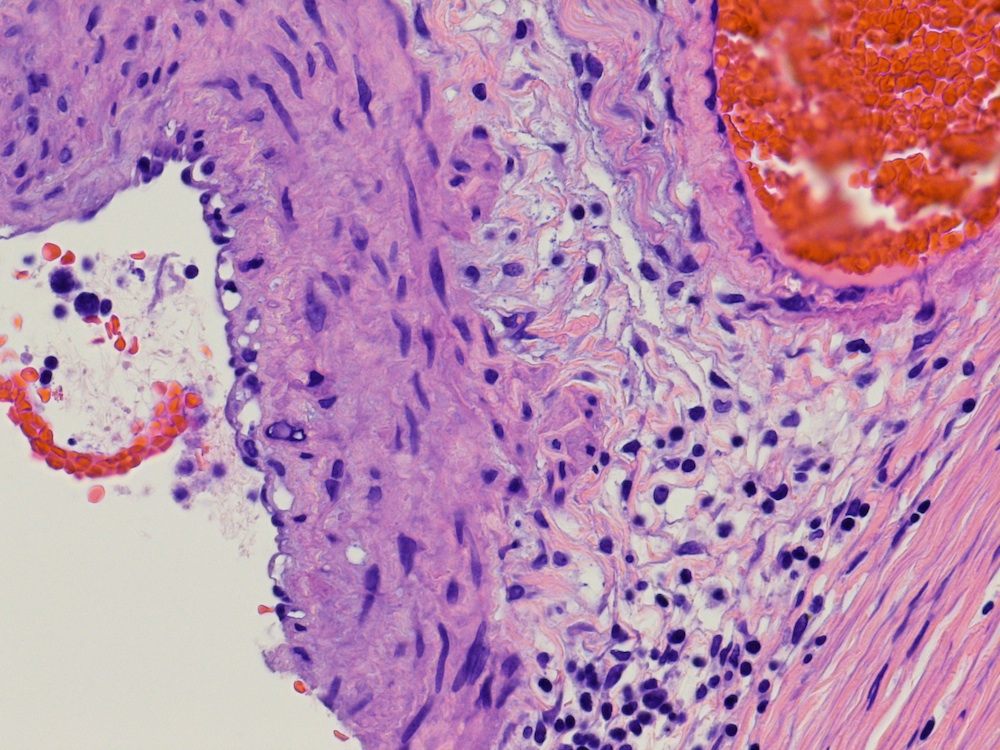
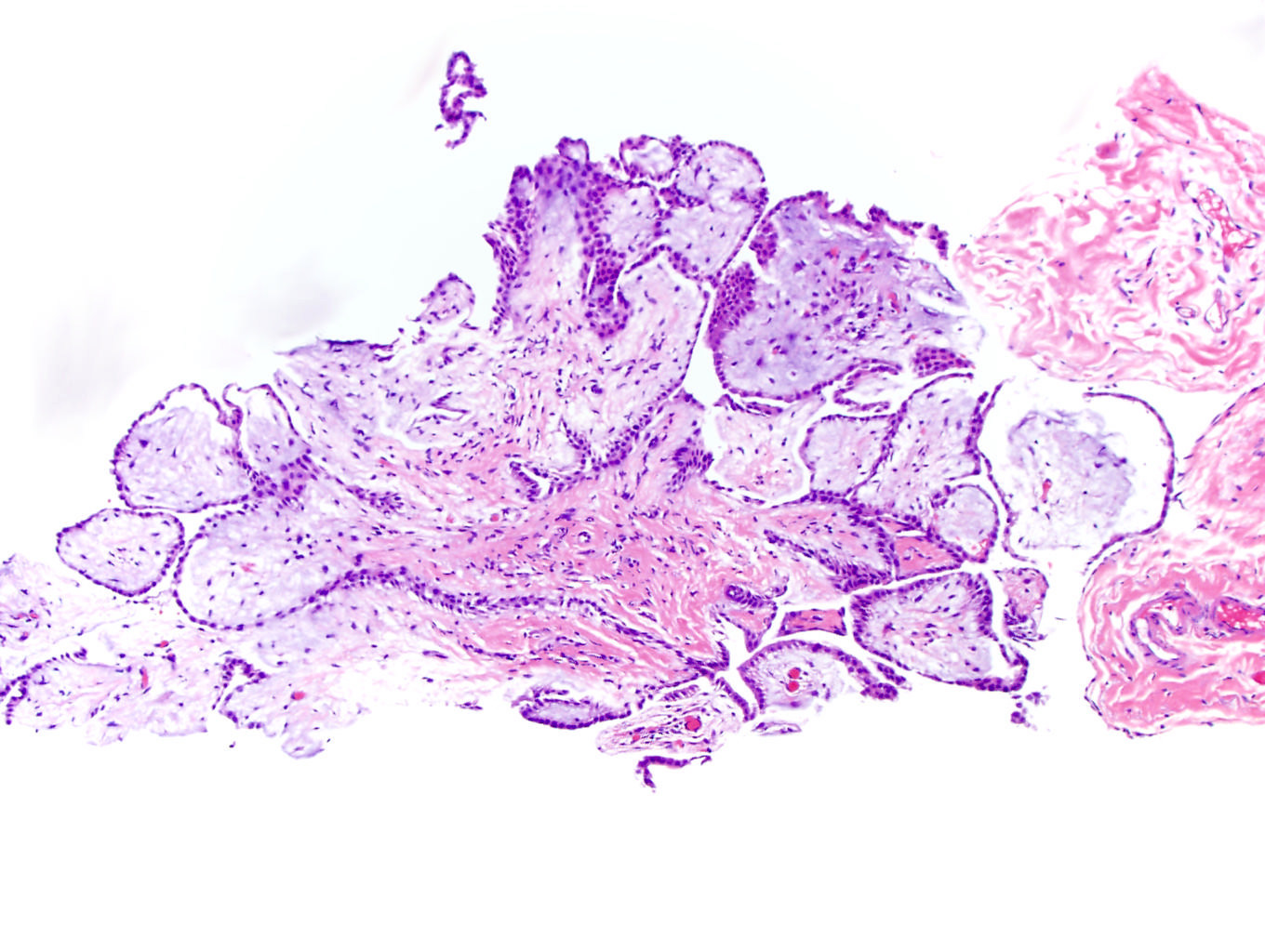
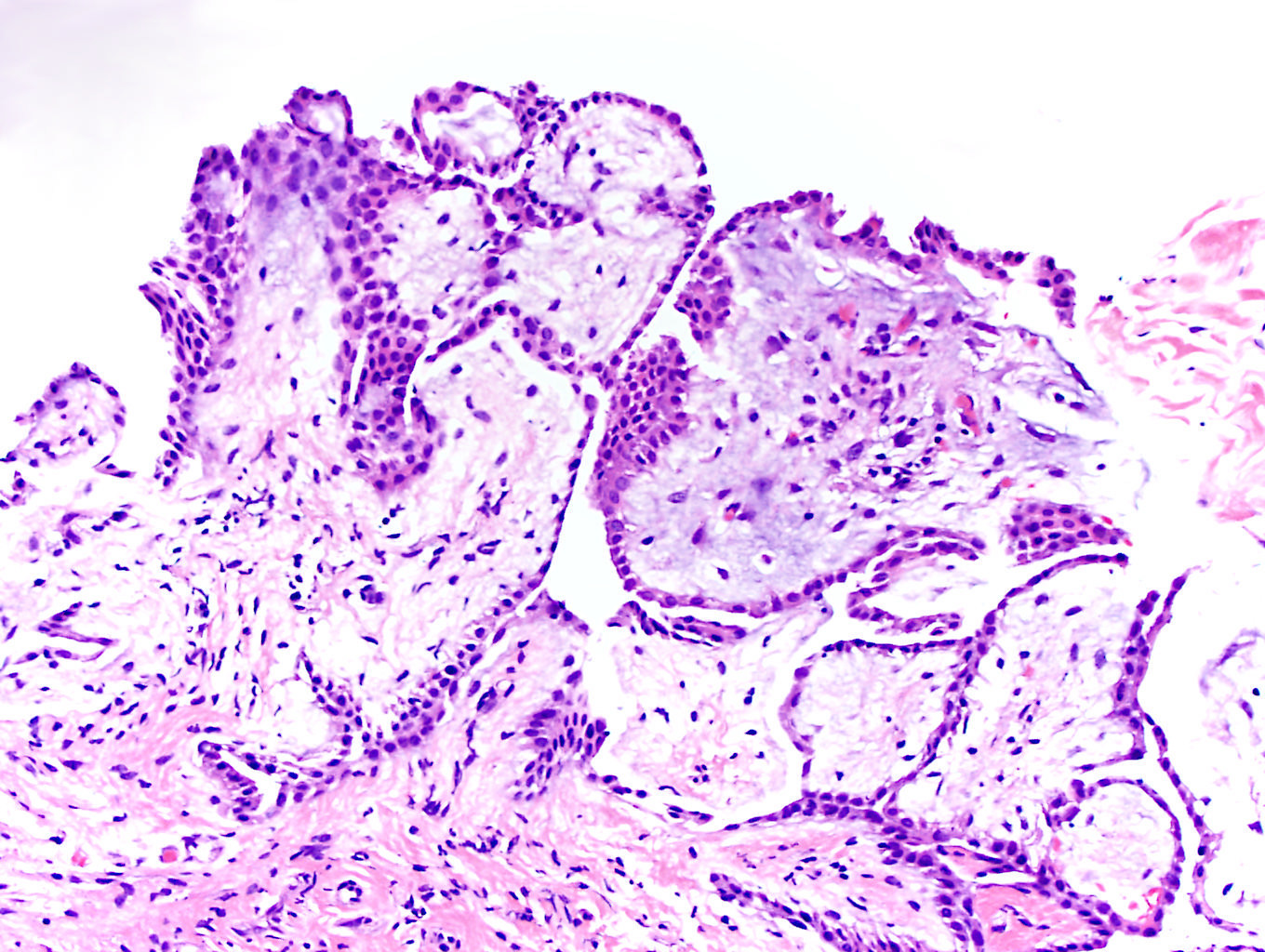
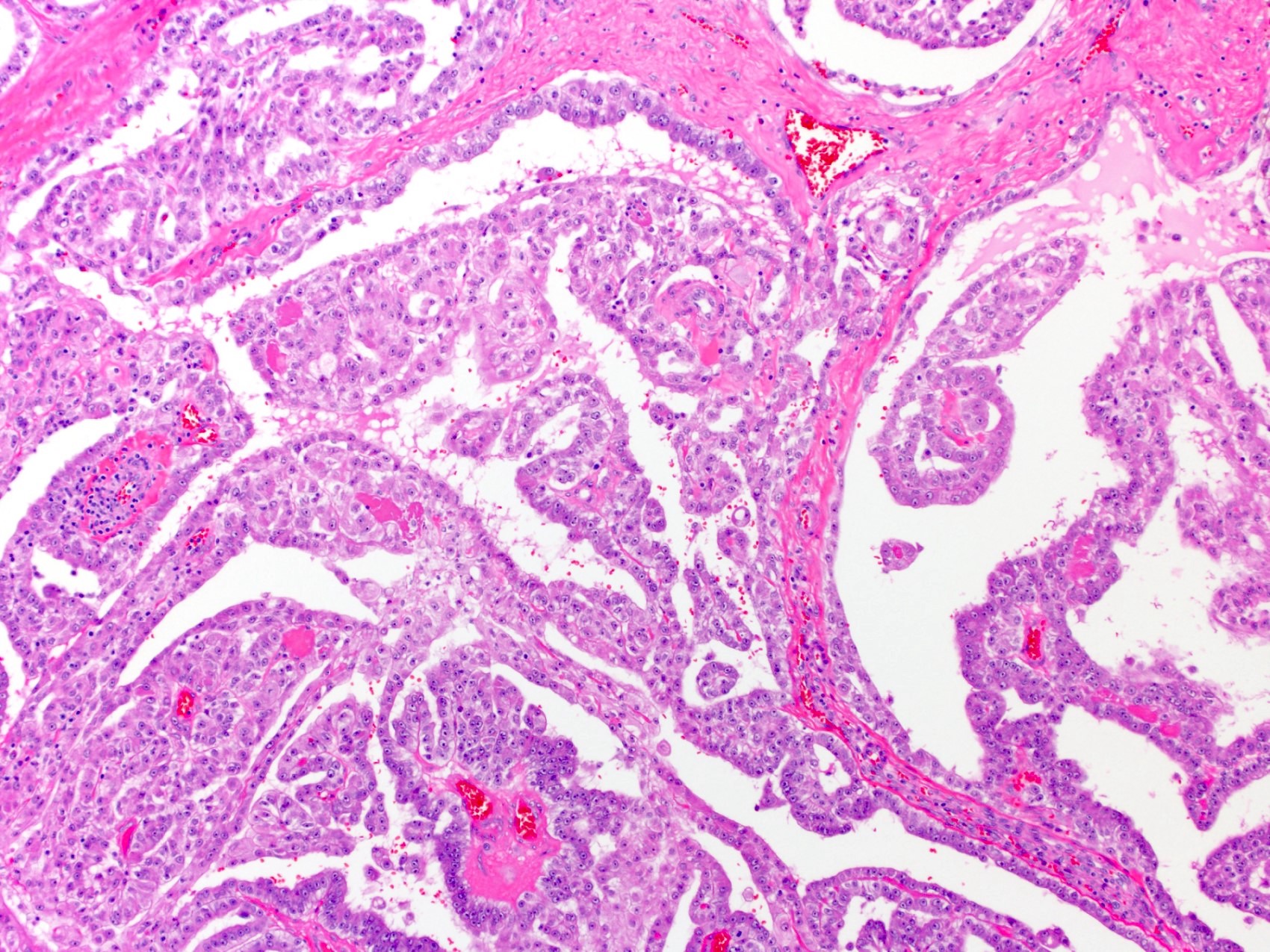
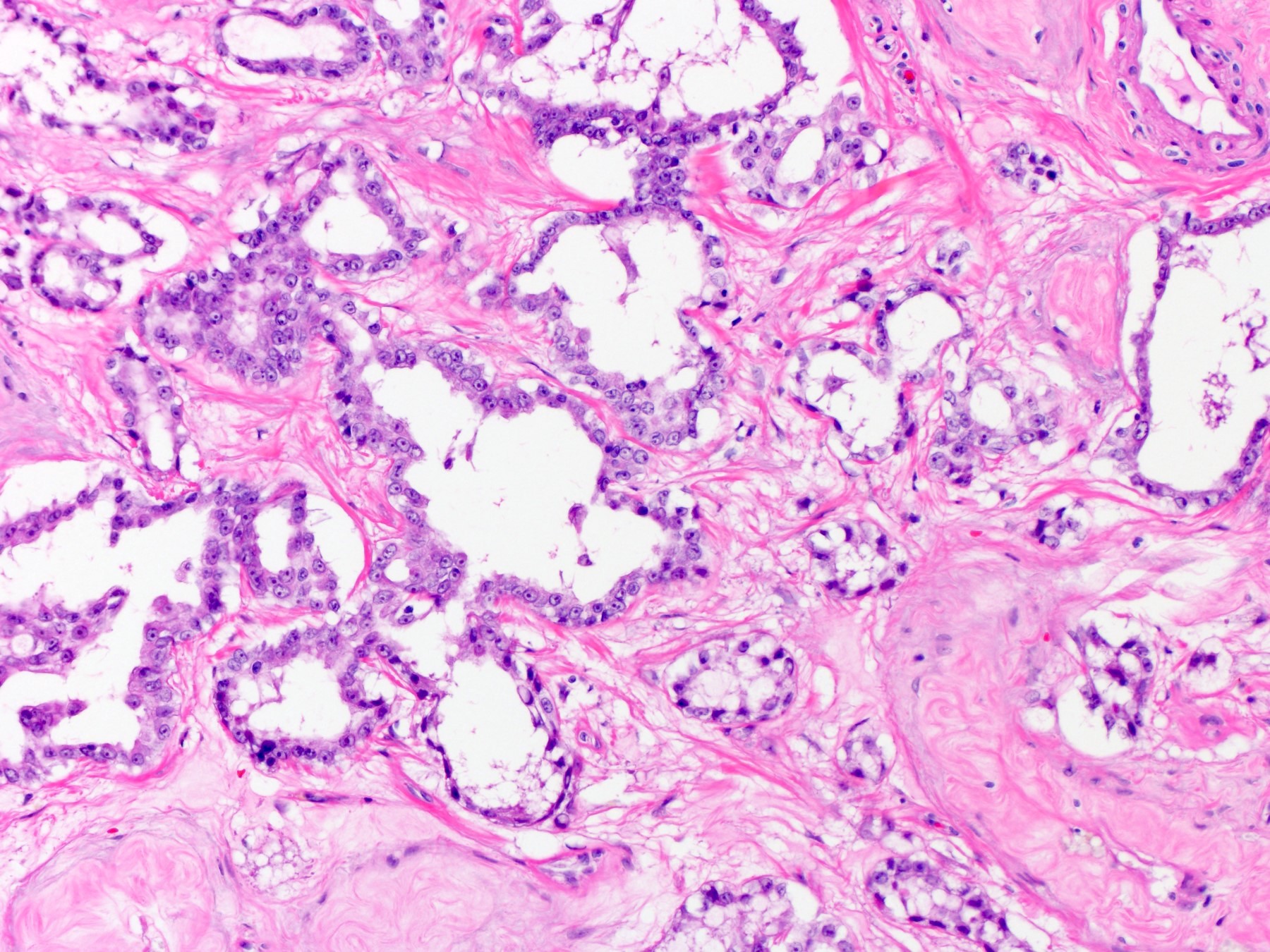
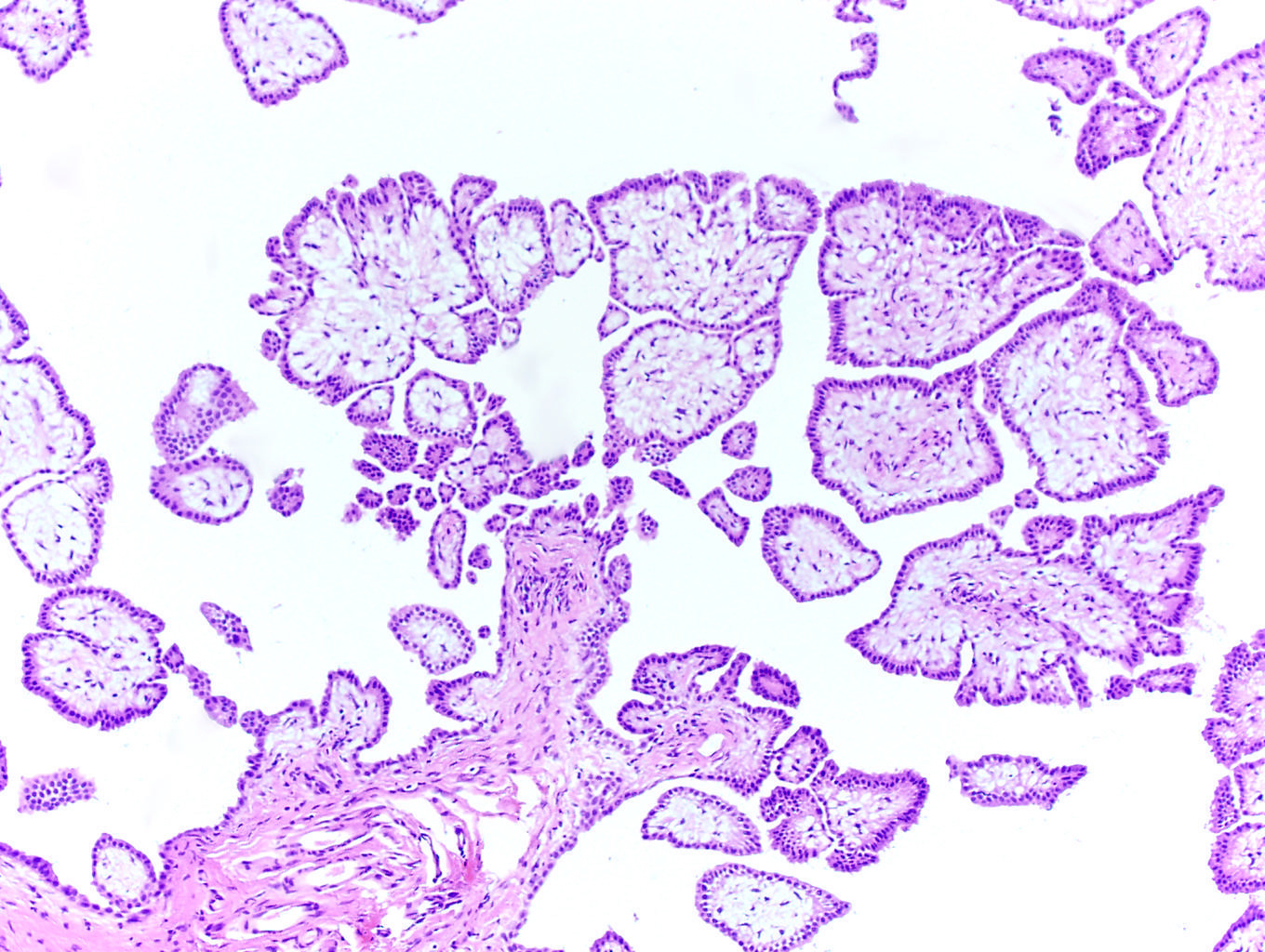
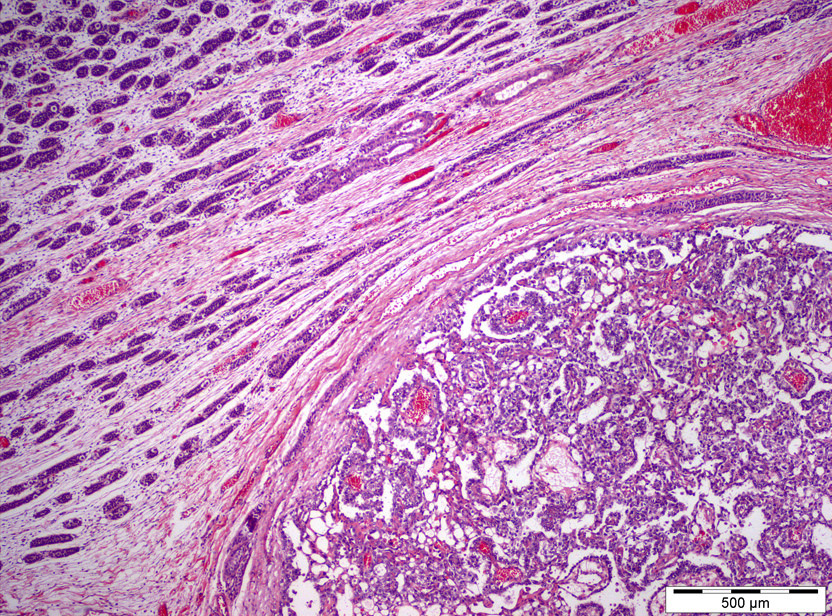
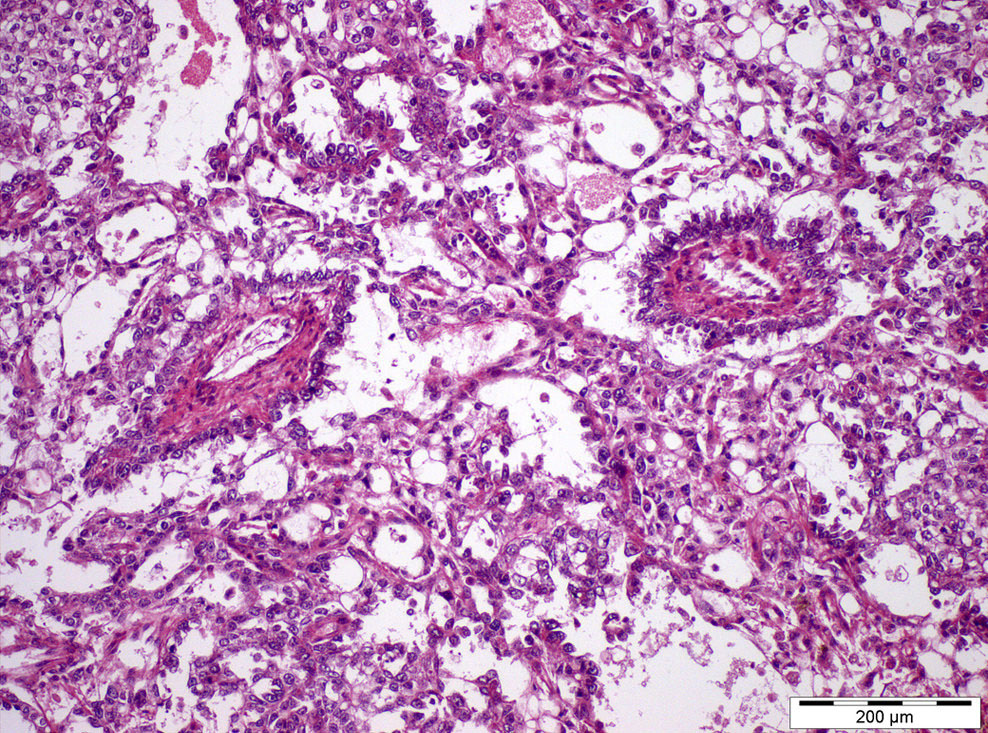
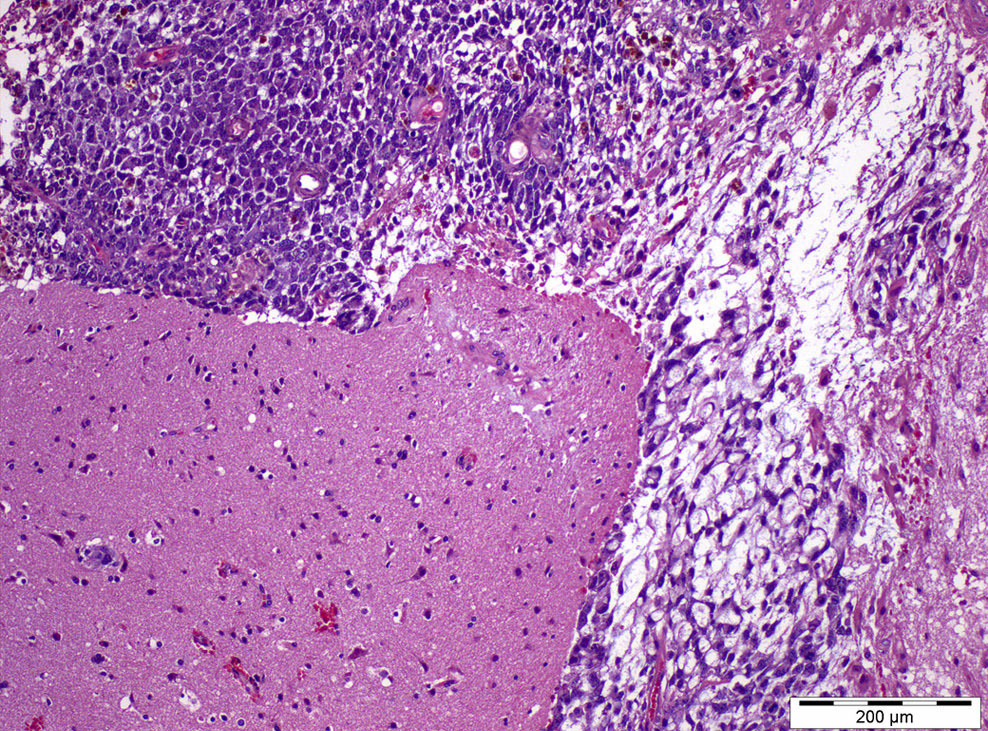
- pT2: lymphovascular, hilar fat, epididymal or tunica vaginalis invasion
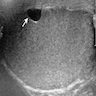
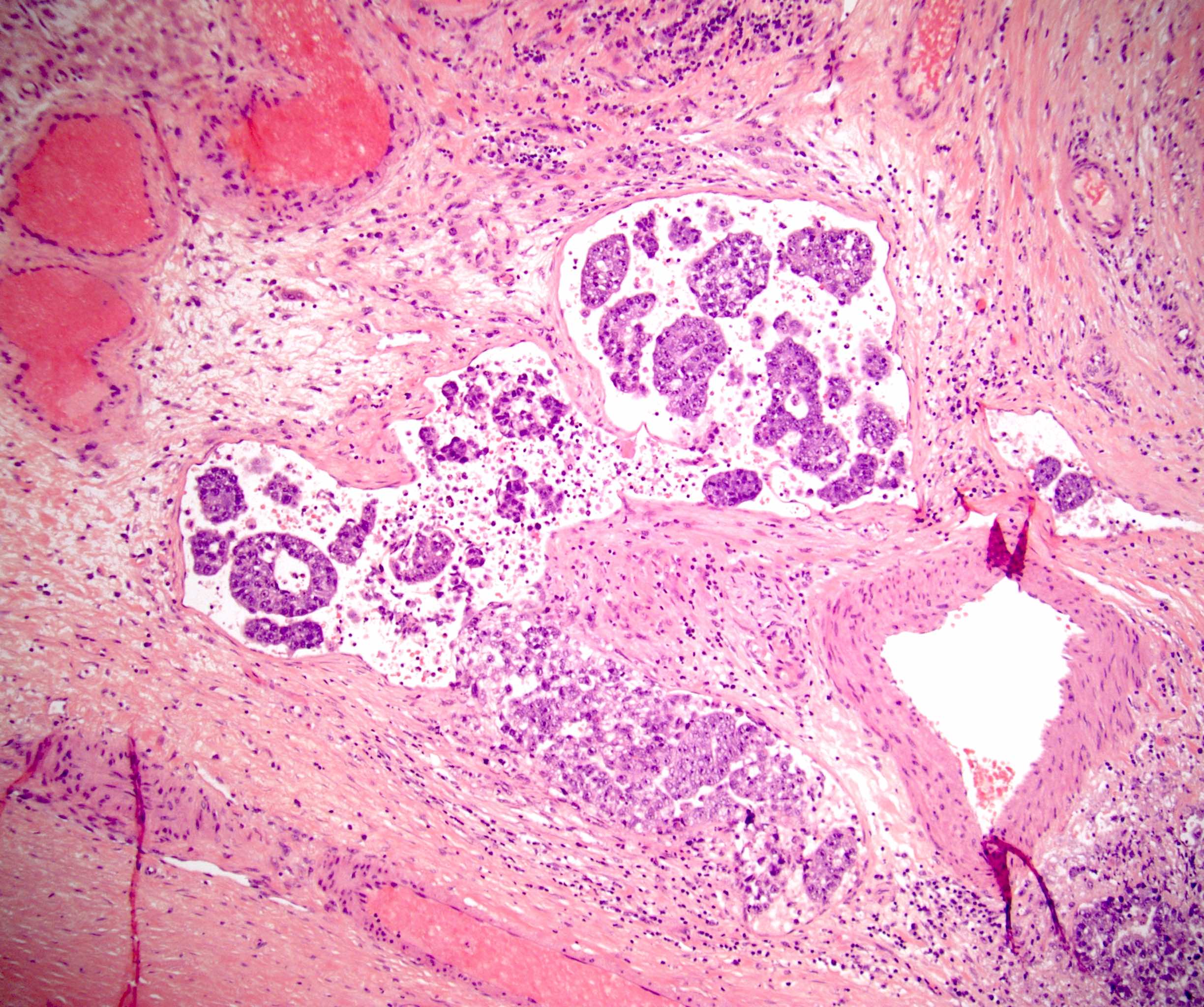
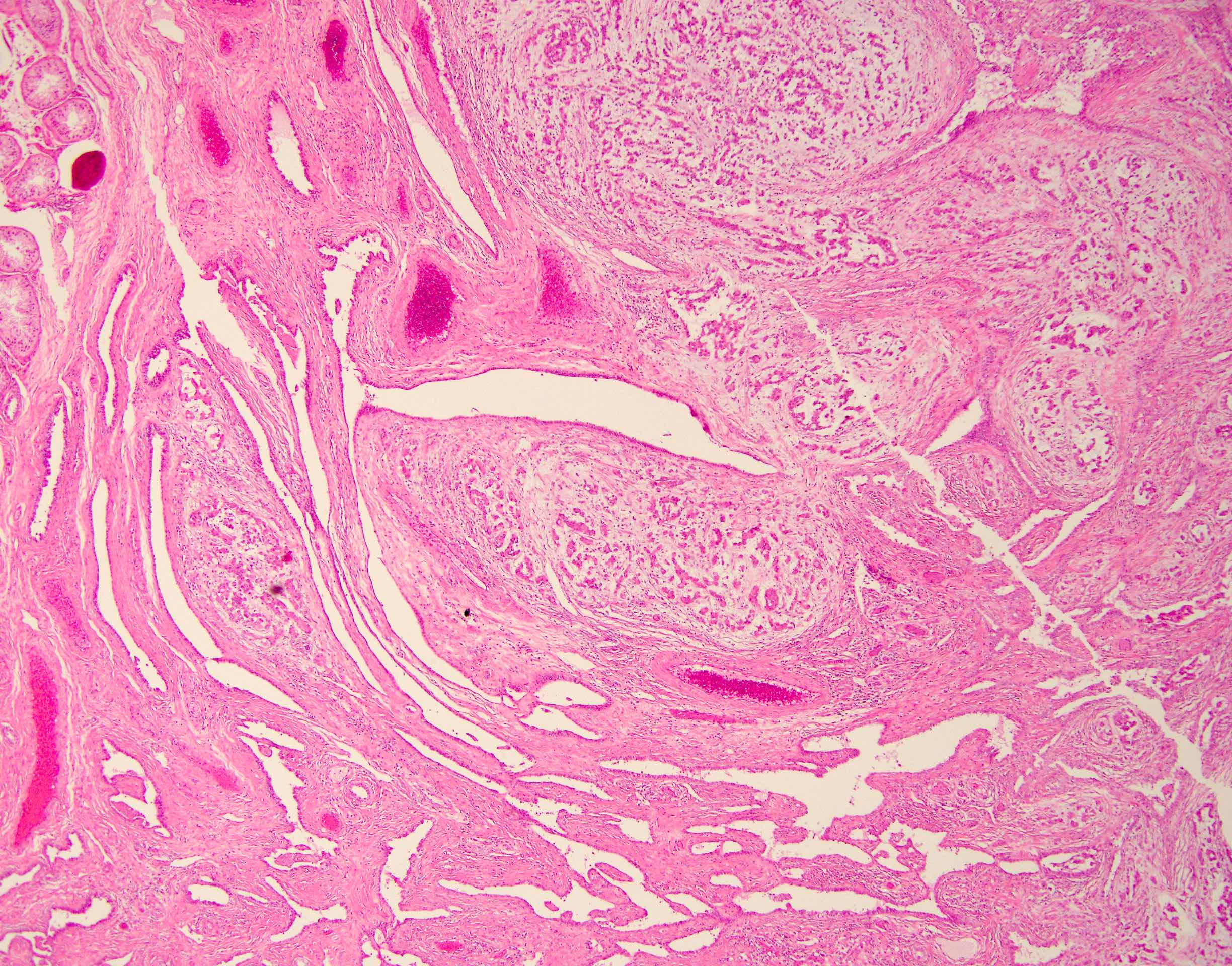
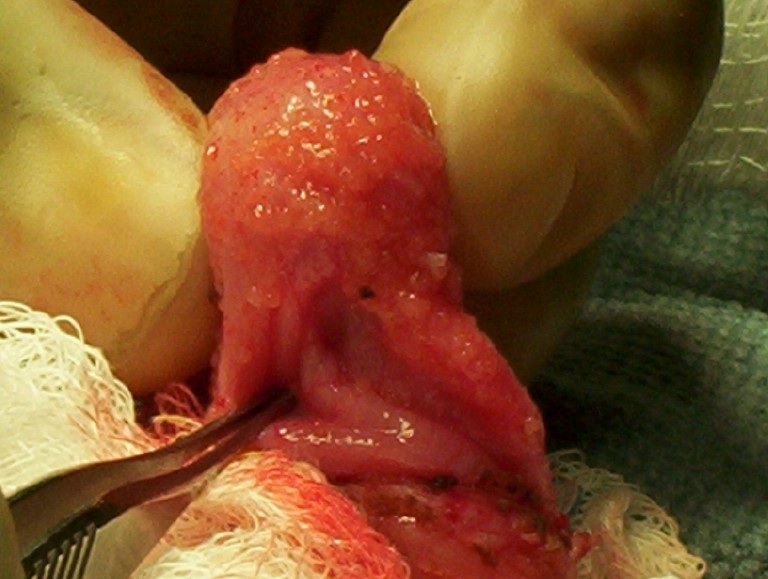
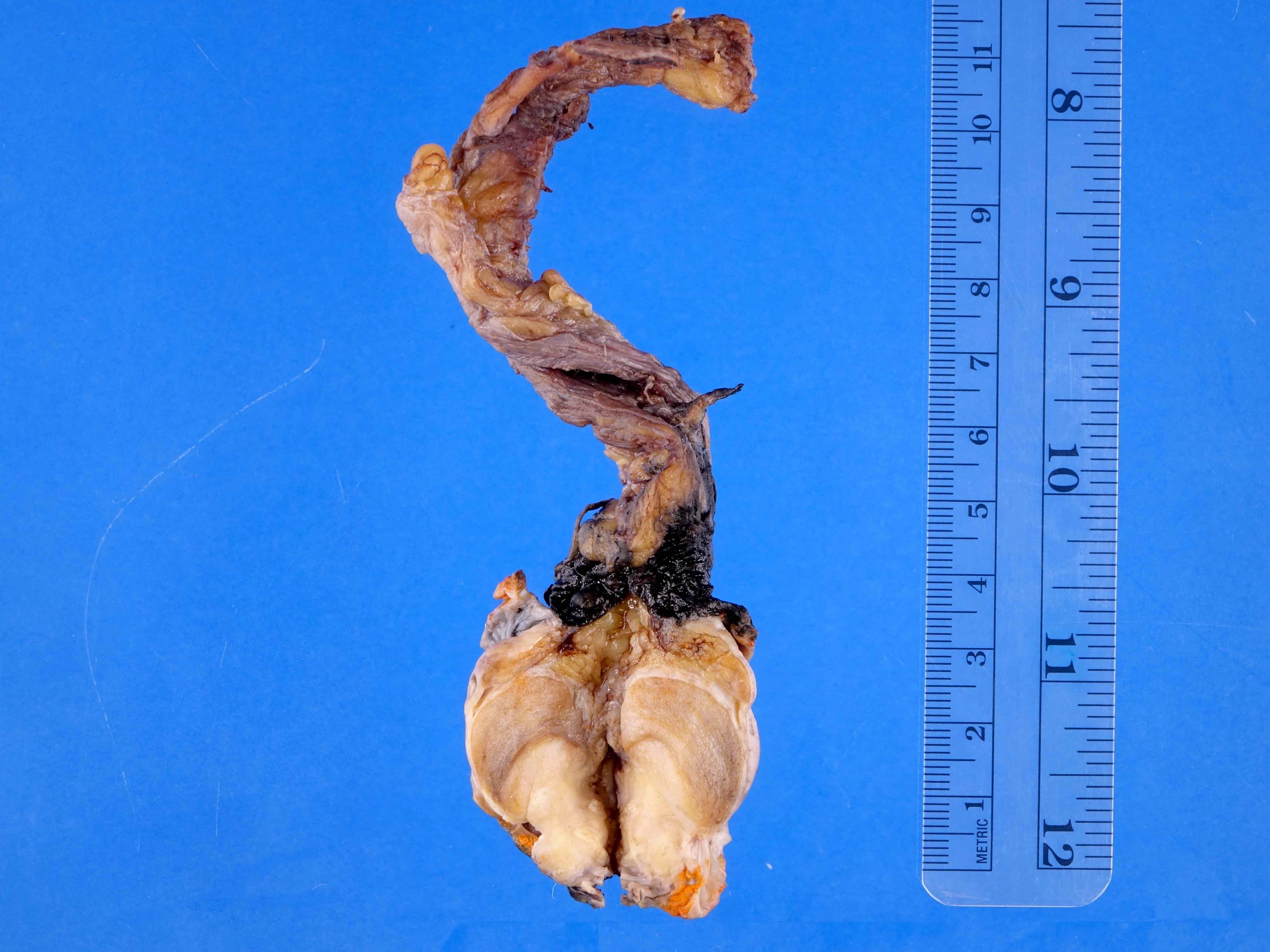
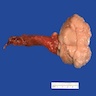
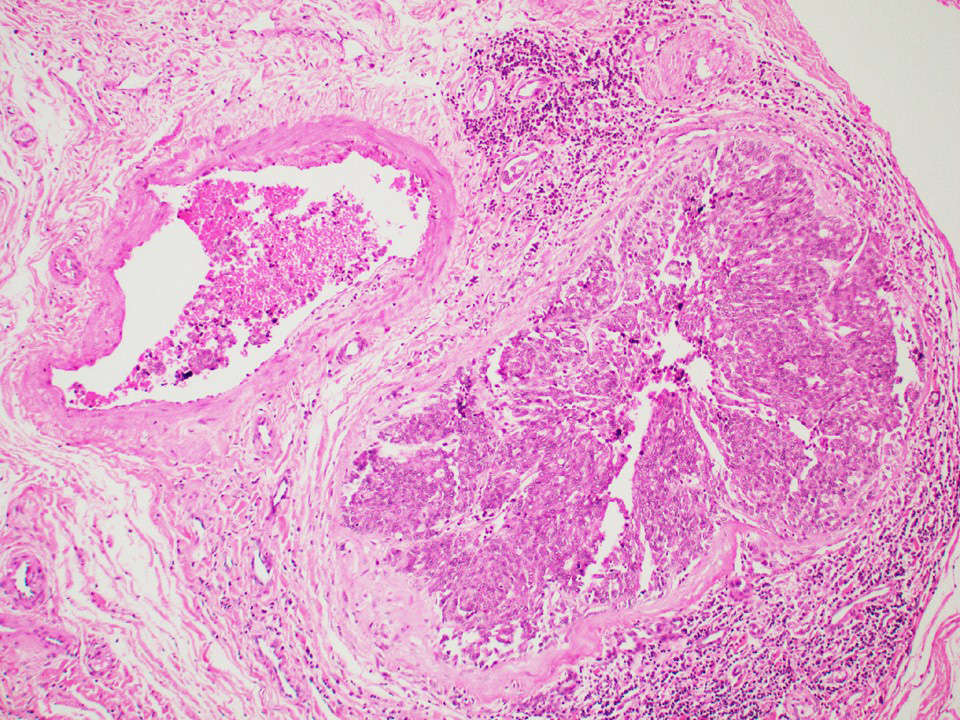
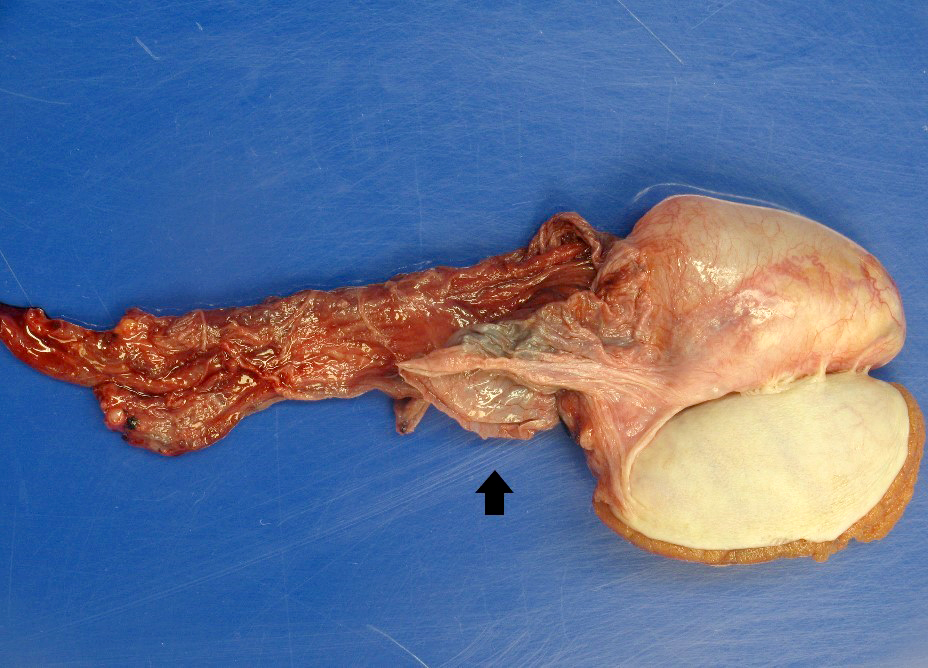
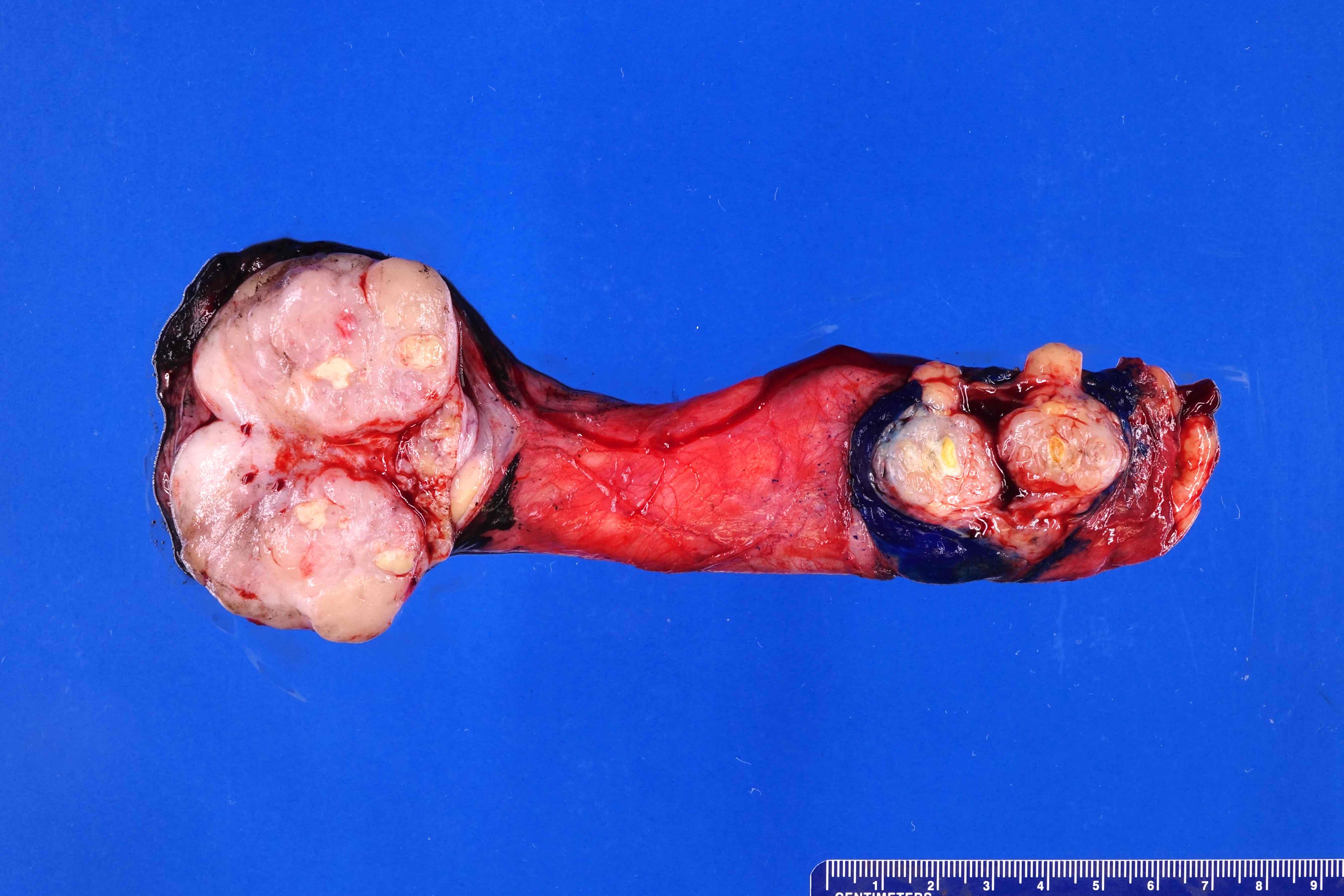
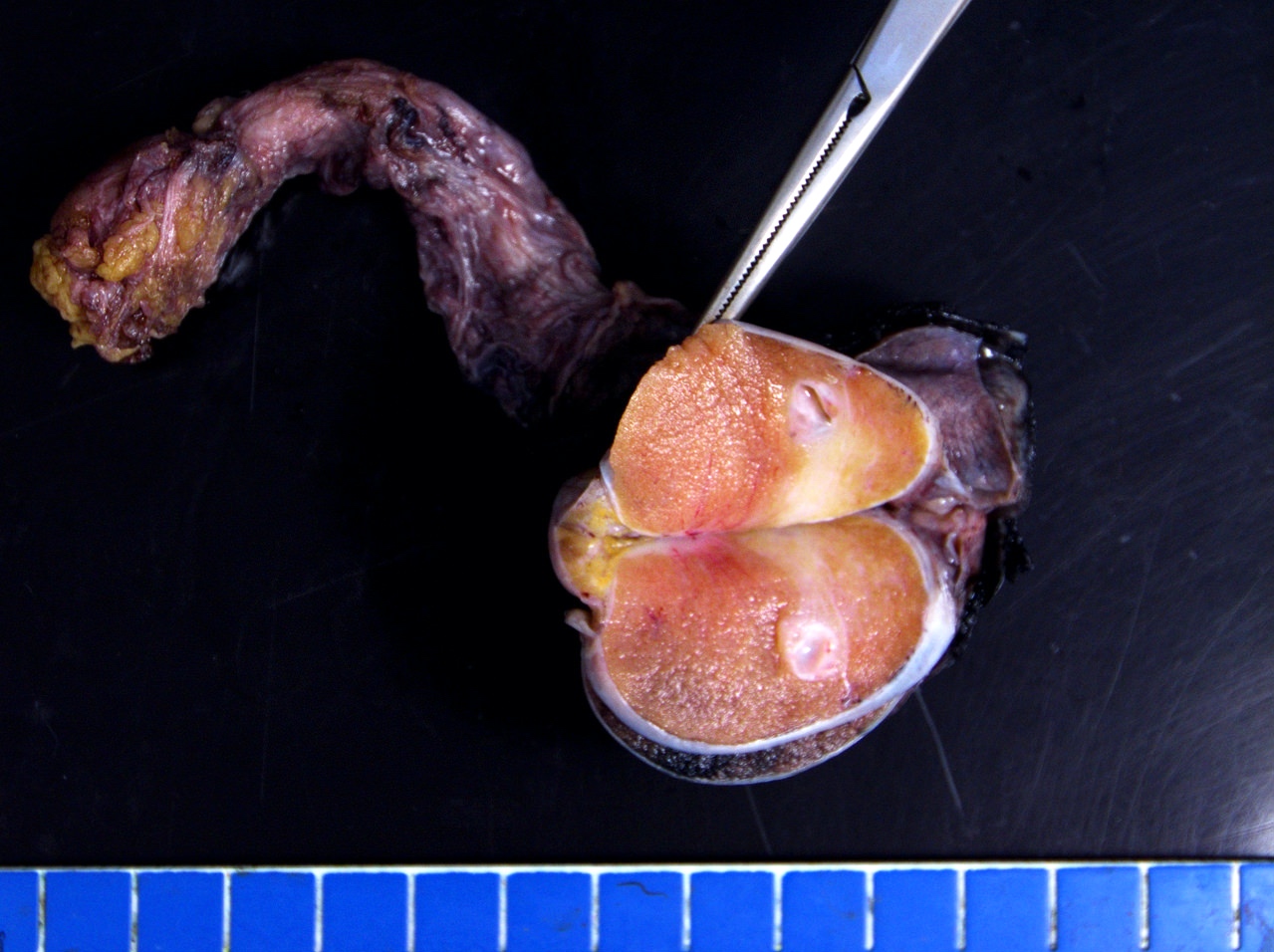
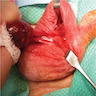
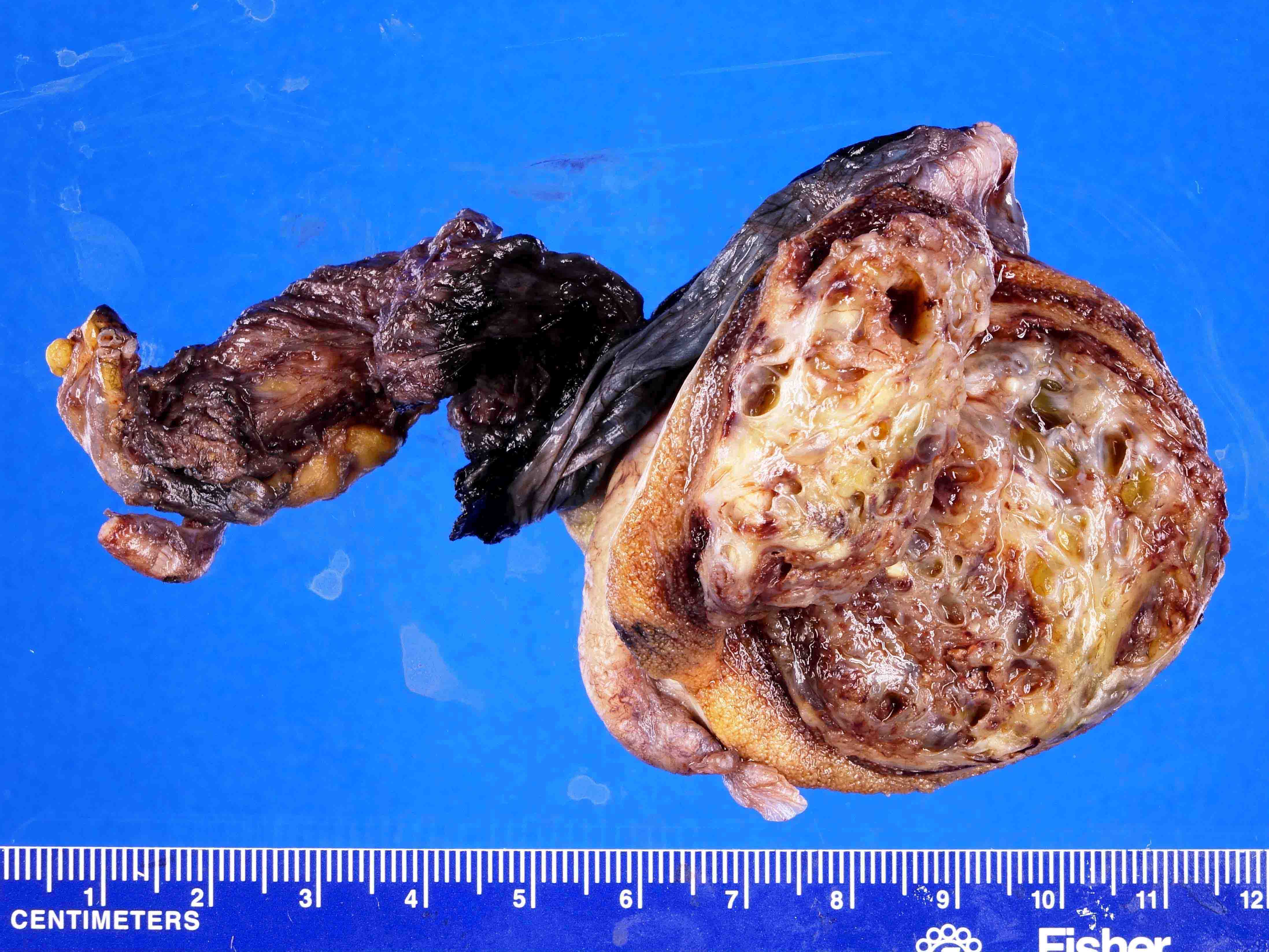
- pT3: direct spermatic cord soft tissue invasion
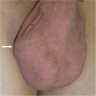
- pT4: direct scrotum invasion
Notes:
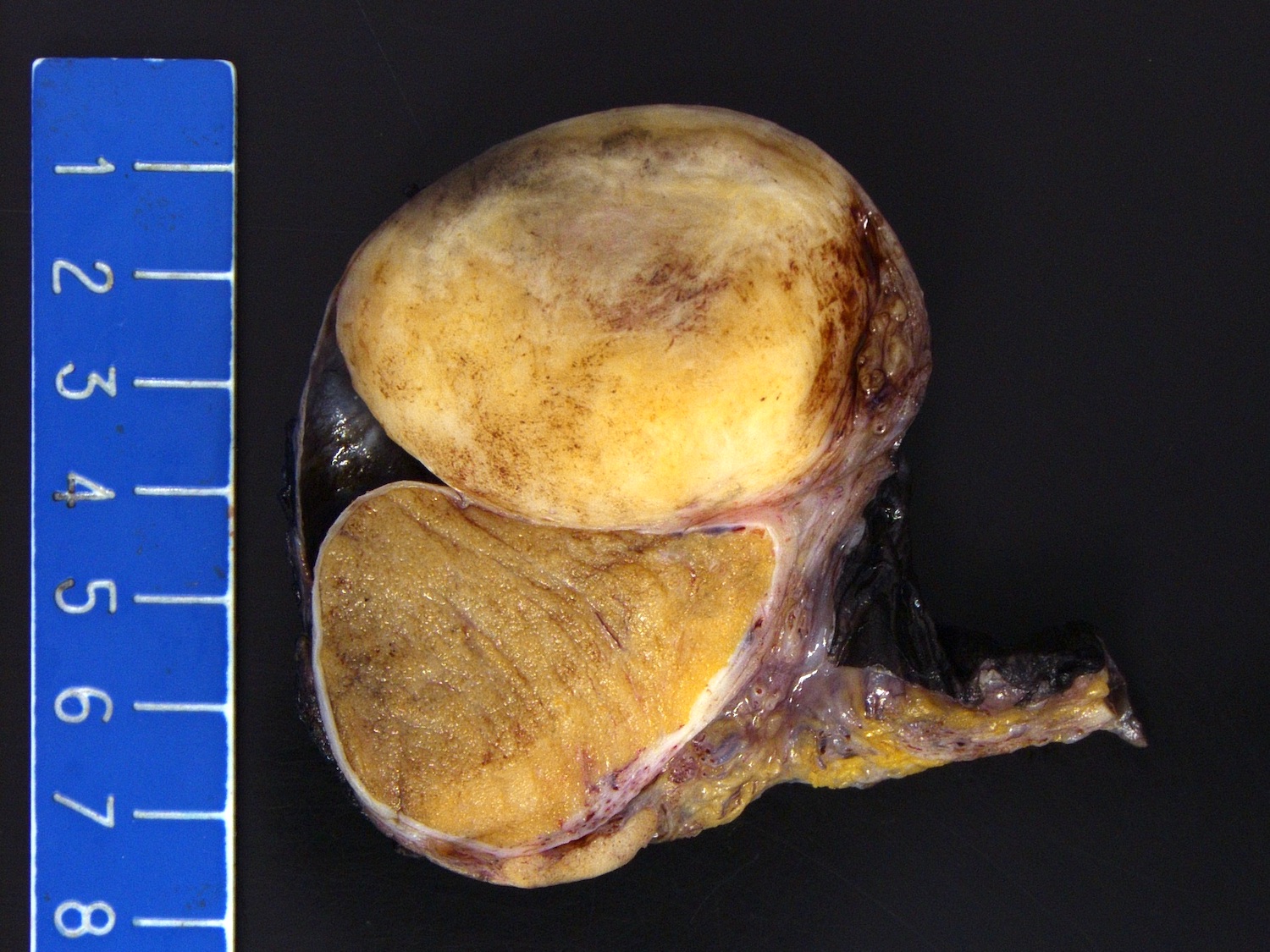
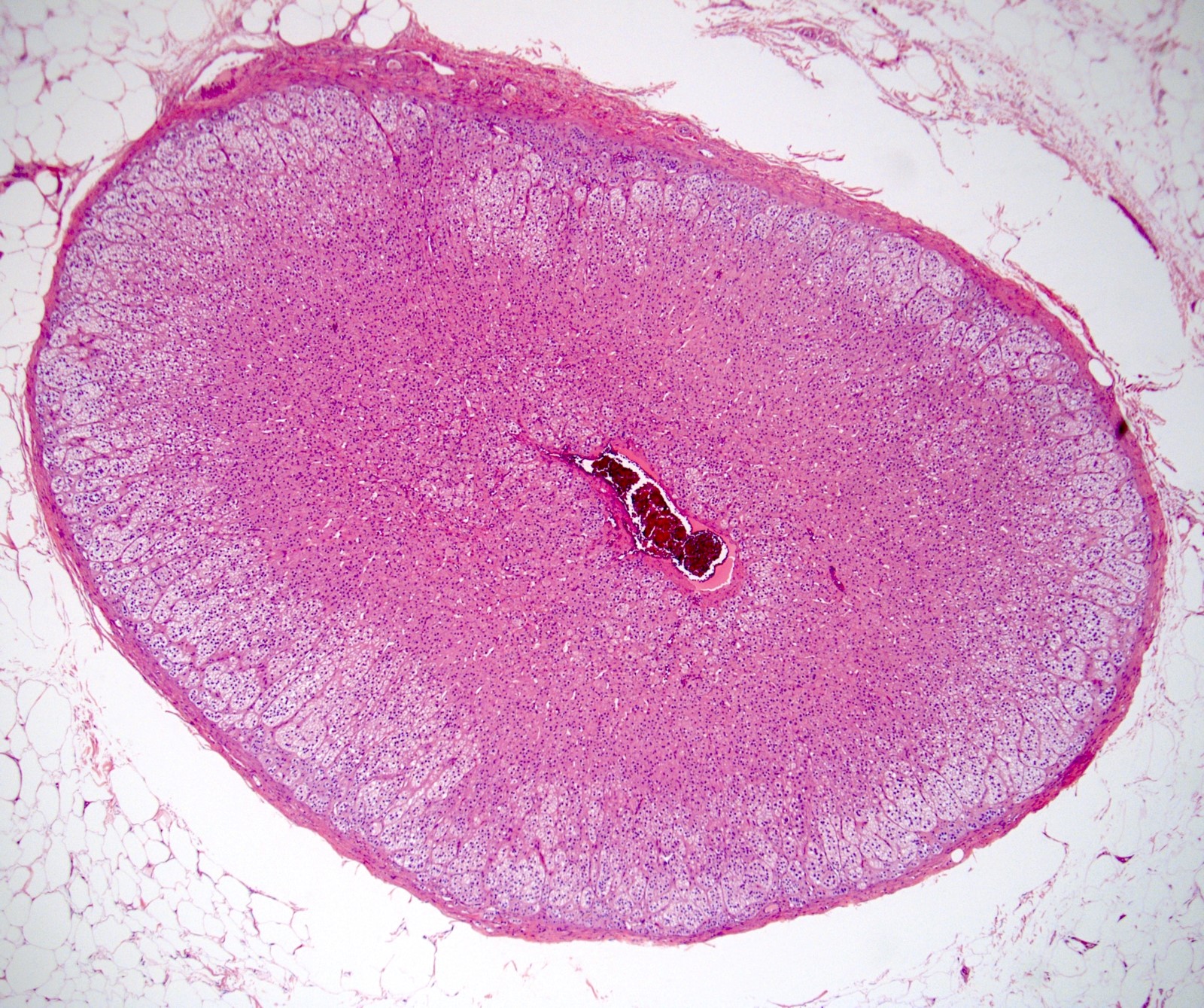
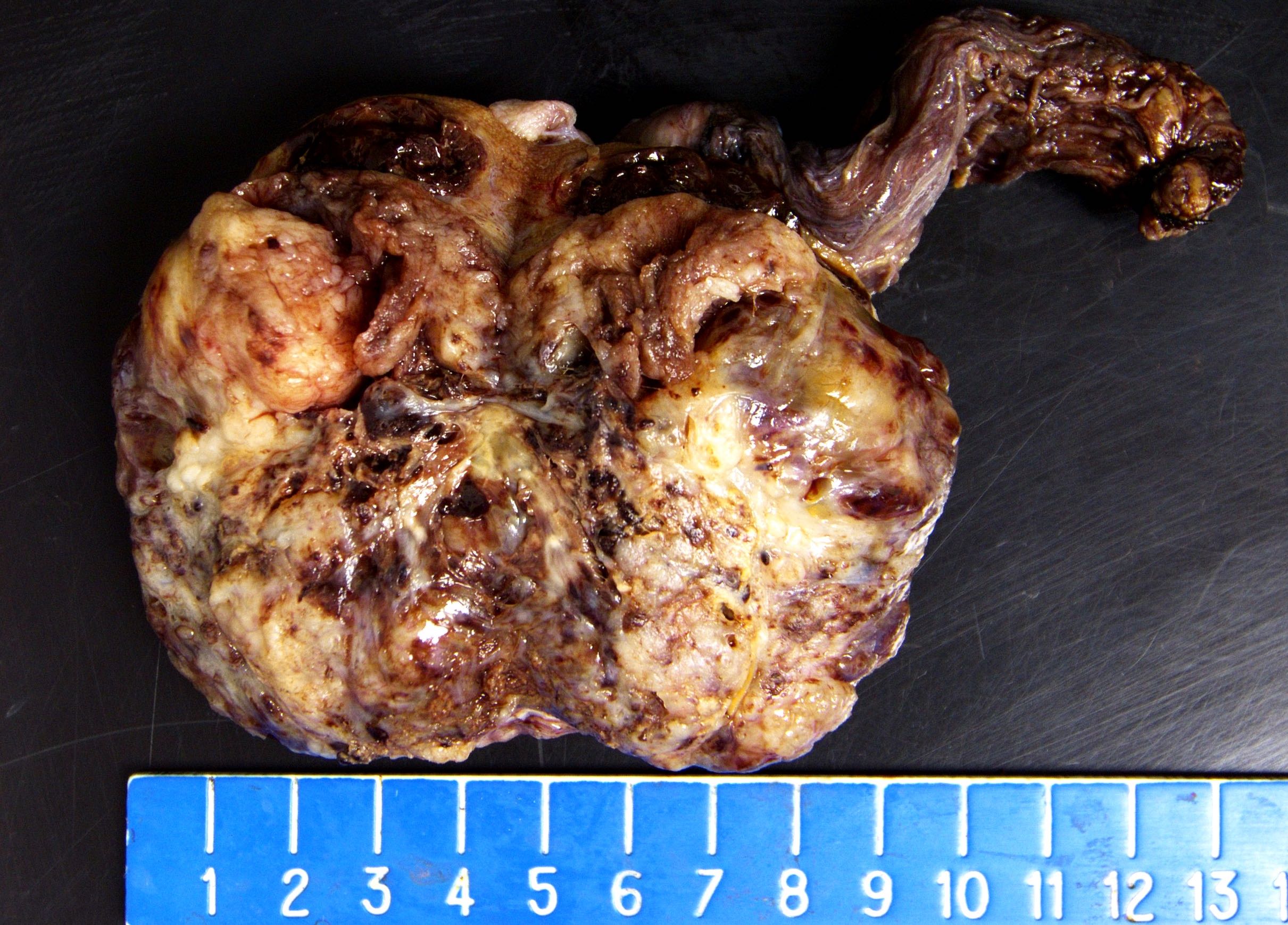
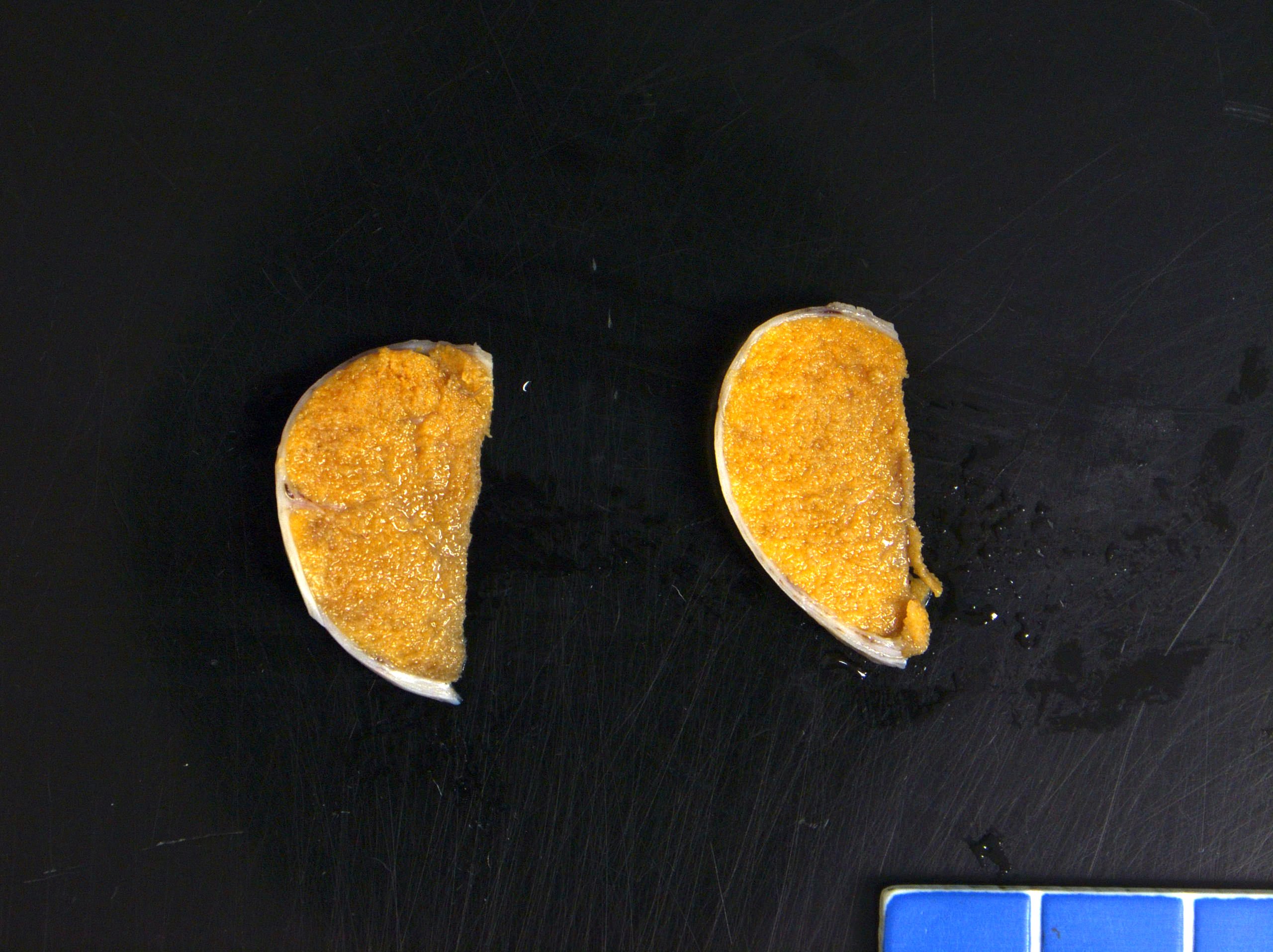
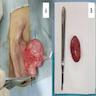
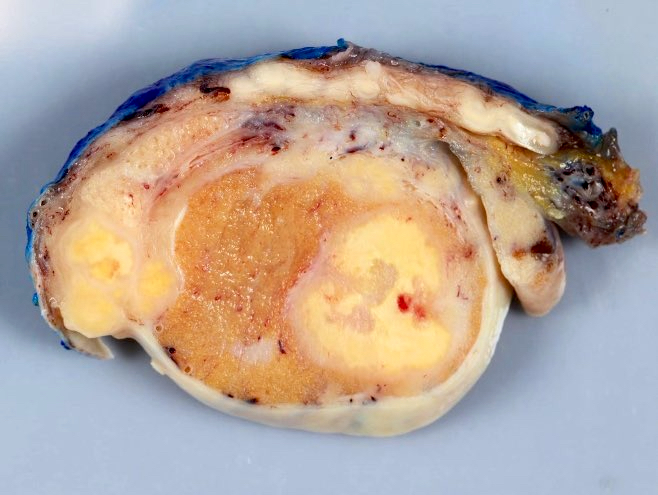
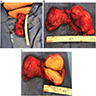
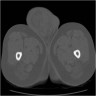
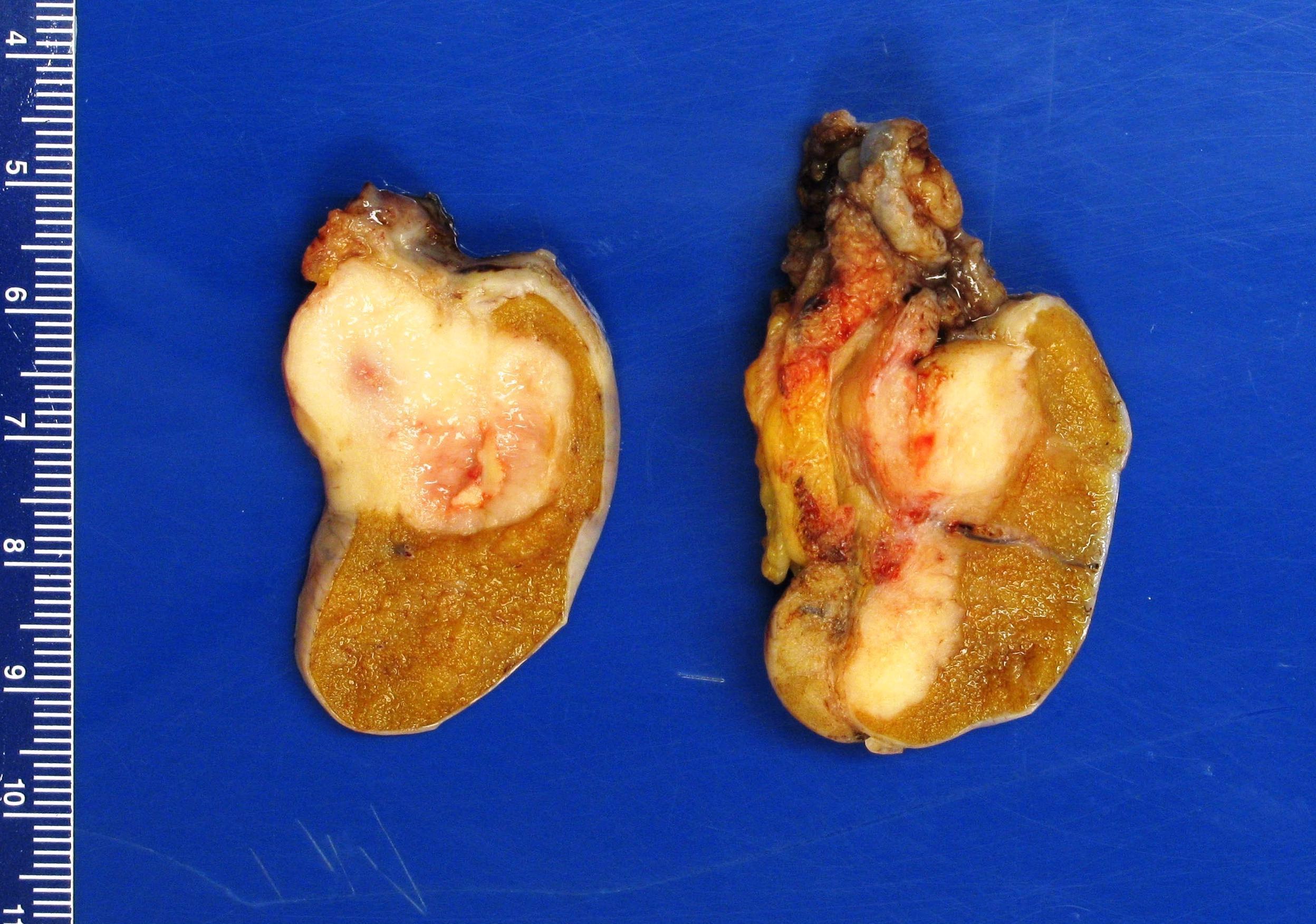
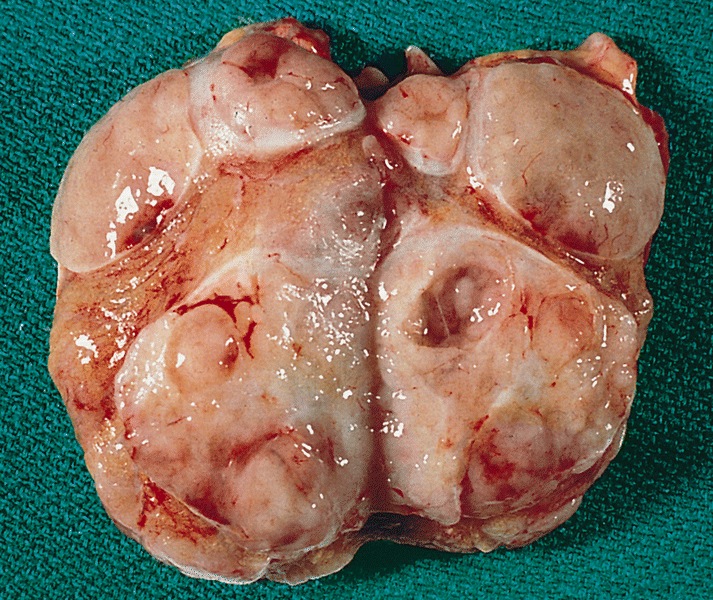
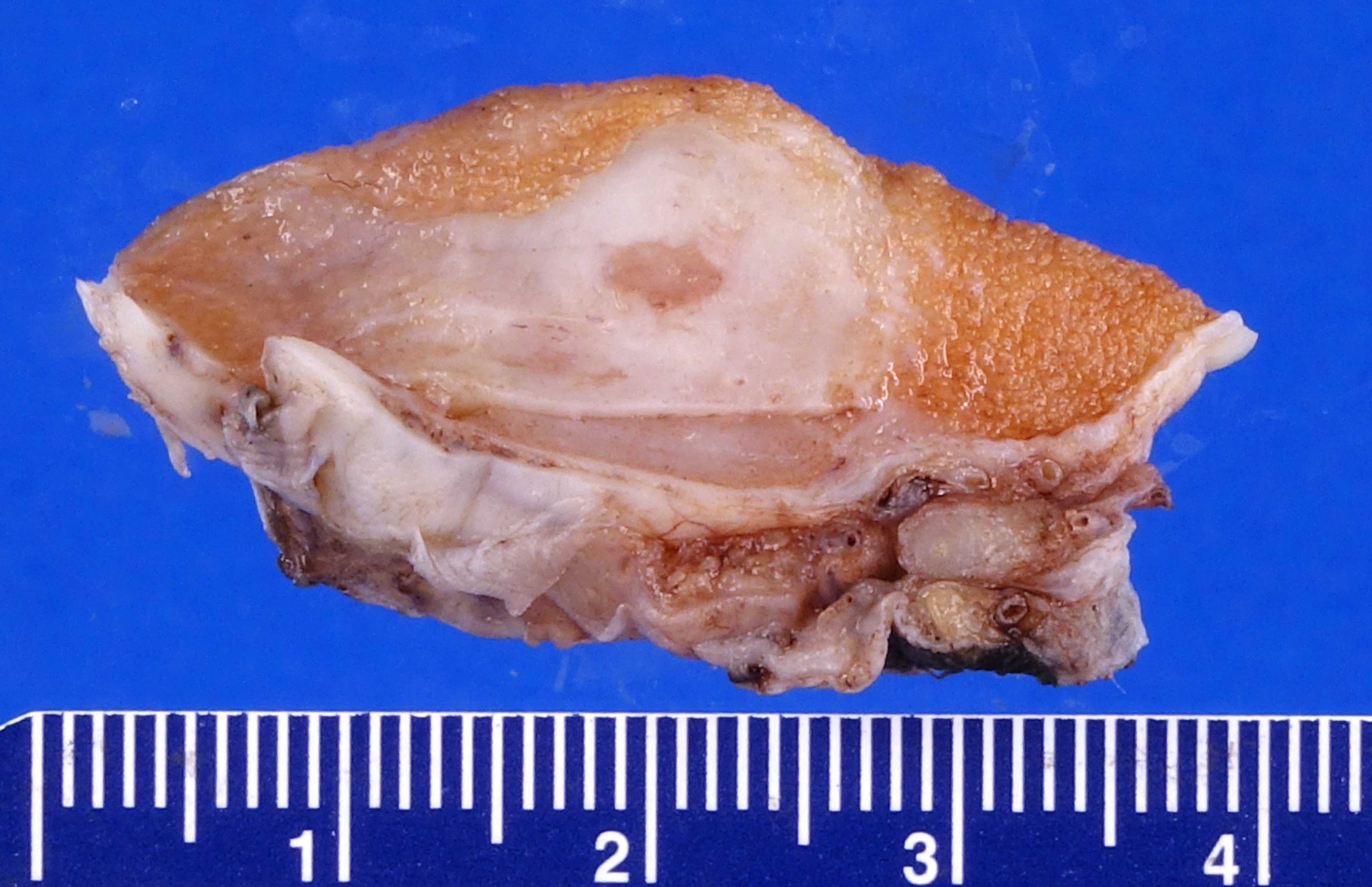
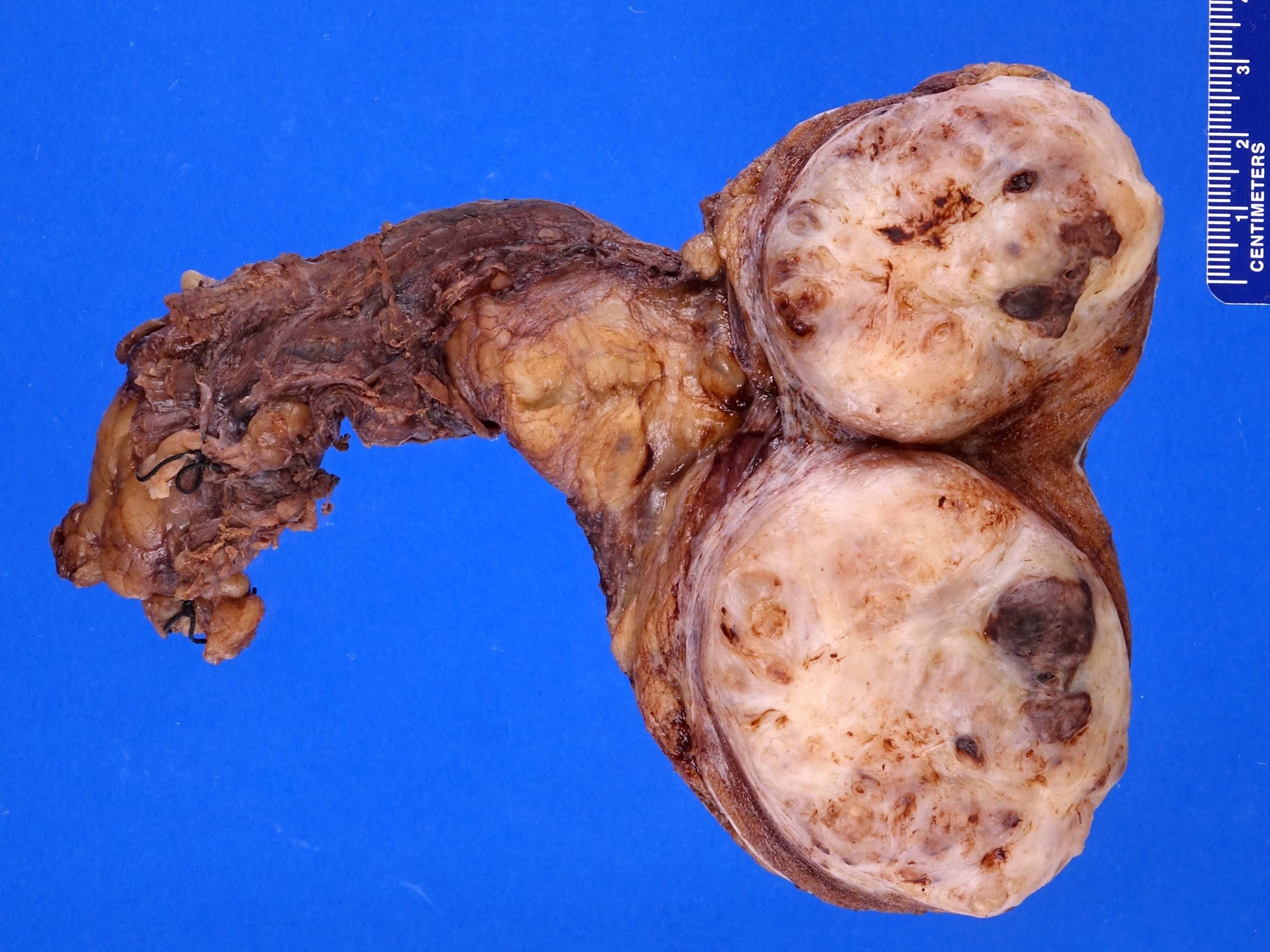
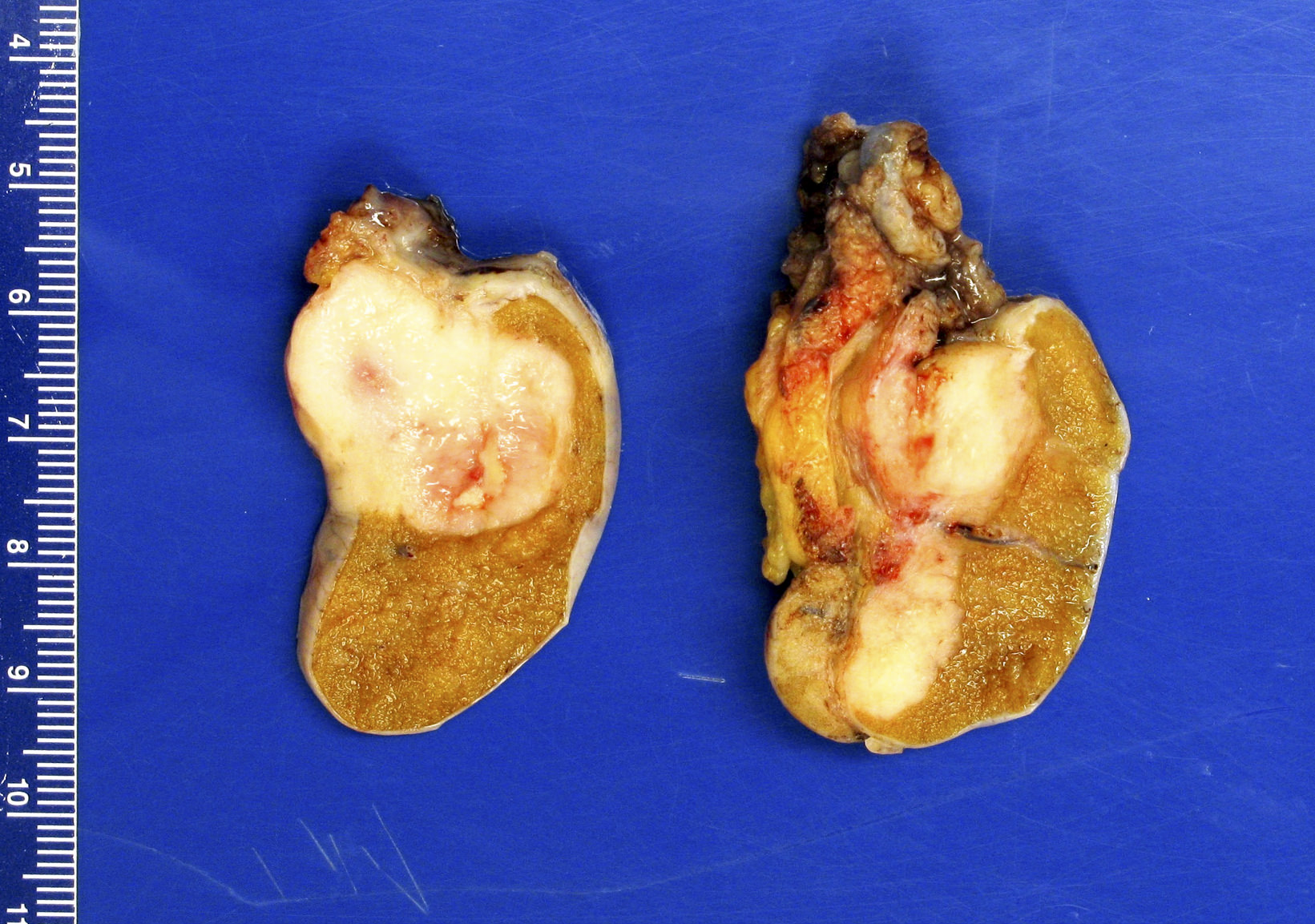
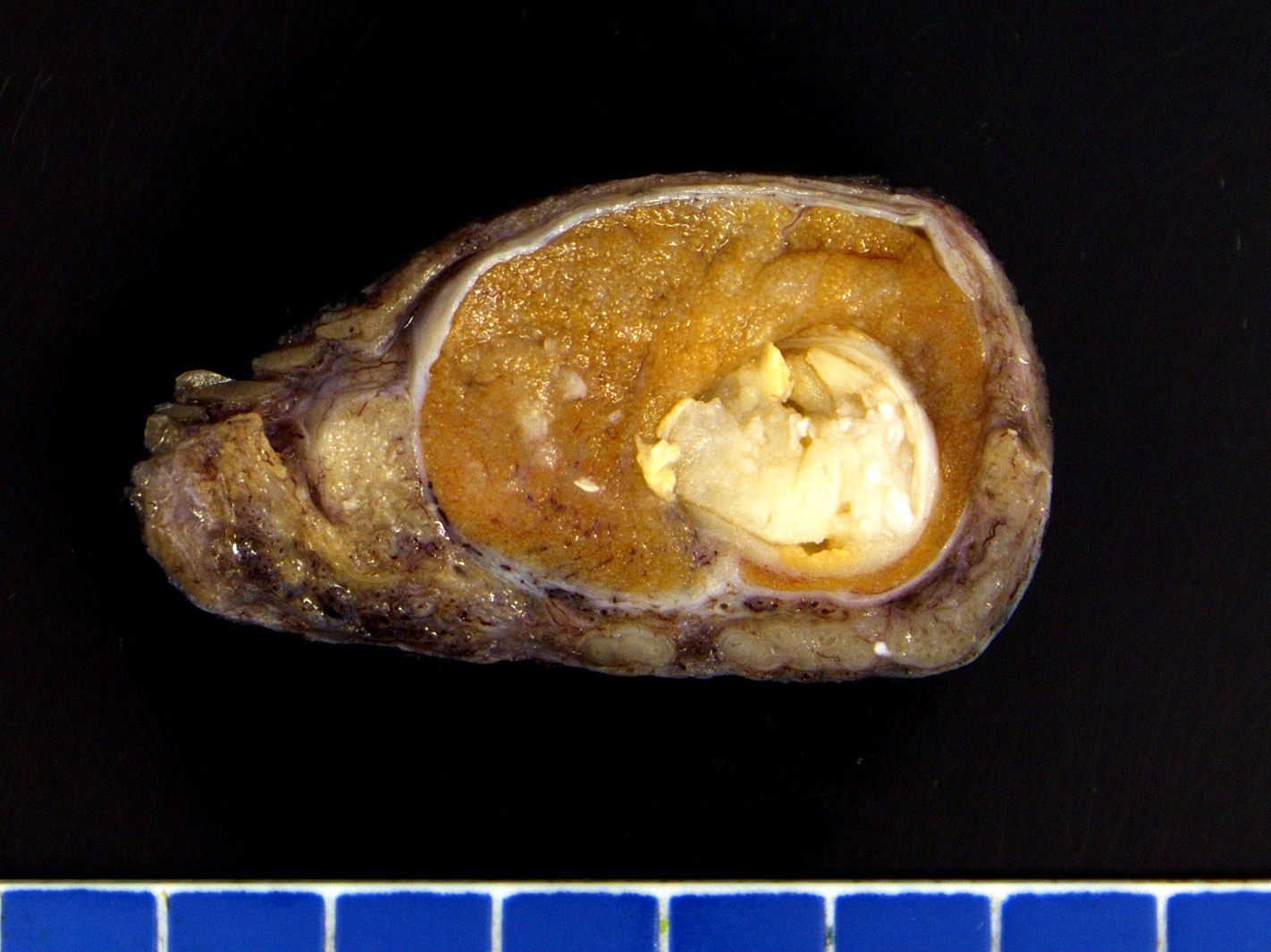
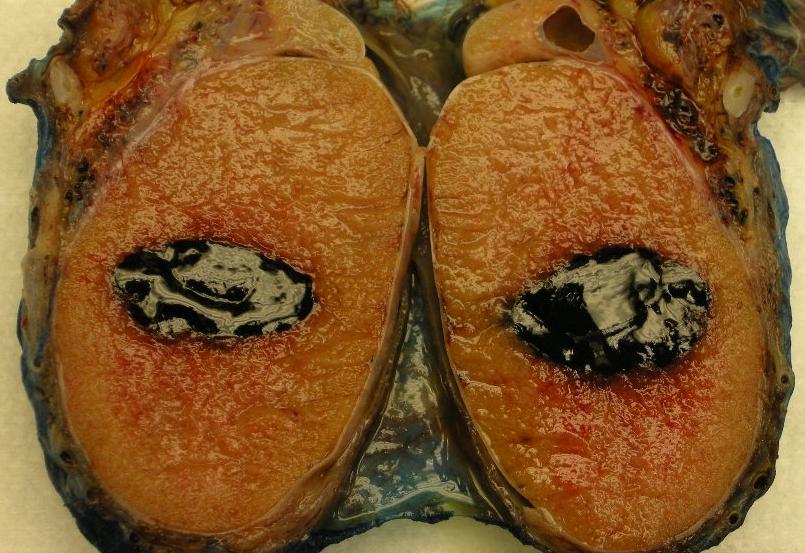
- Size
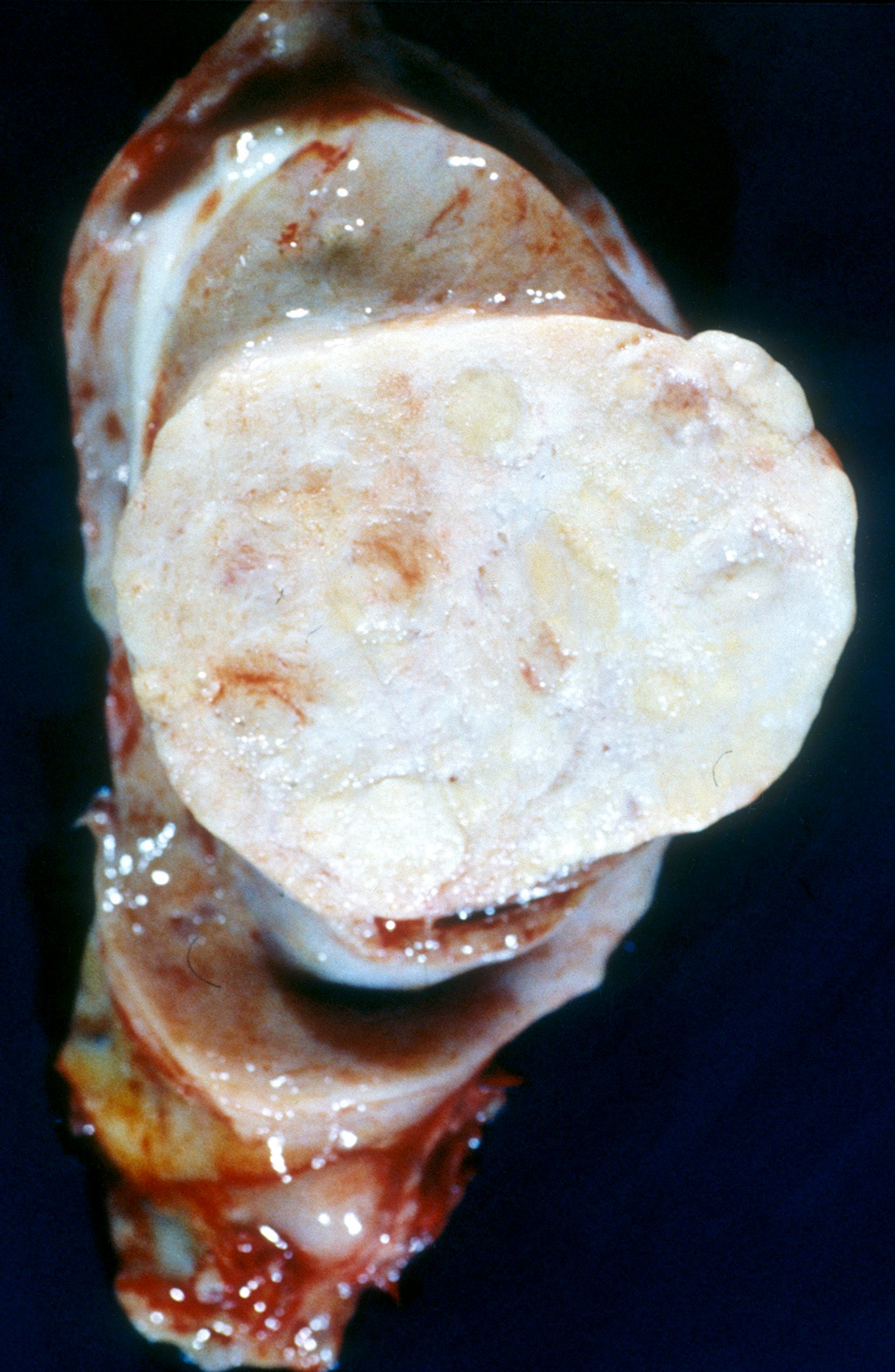
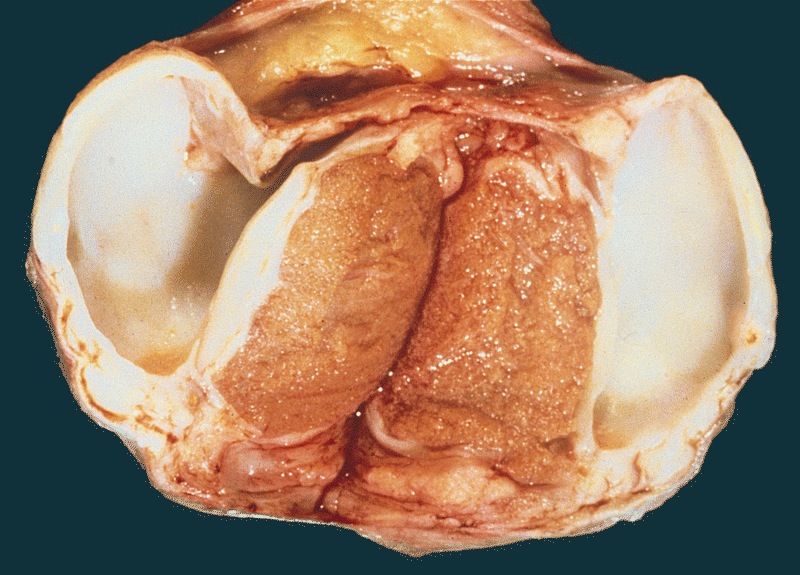
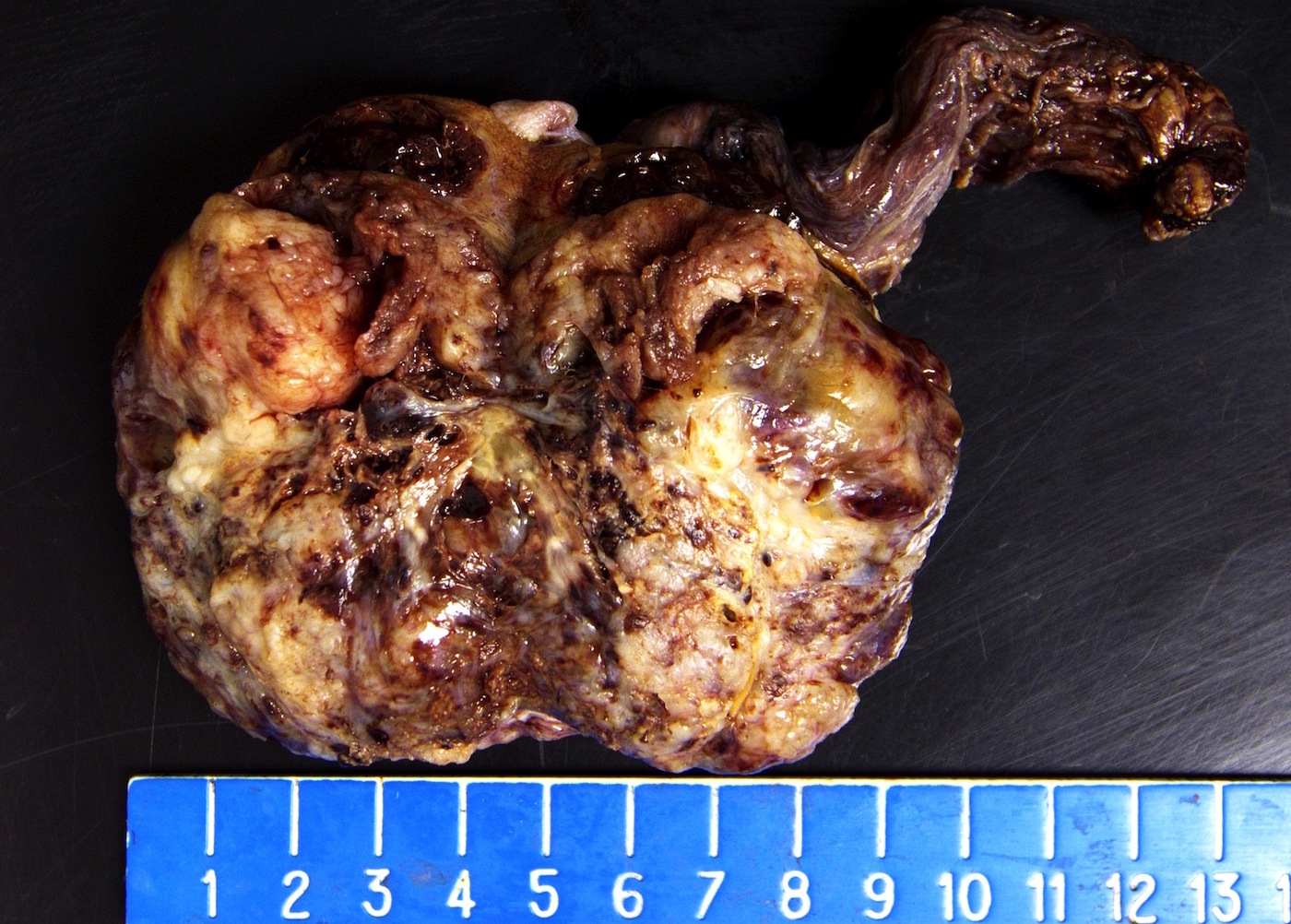
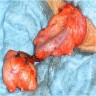
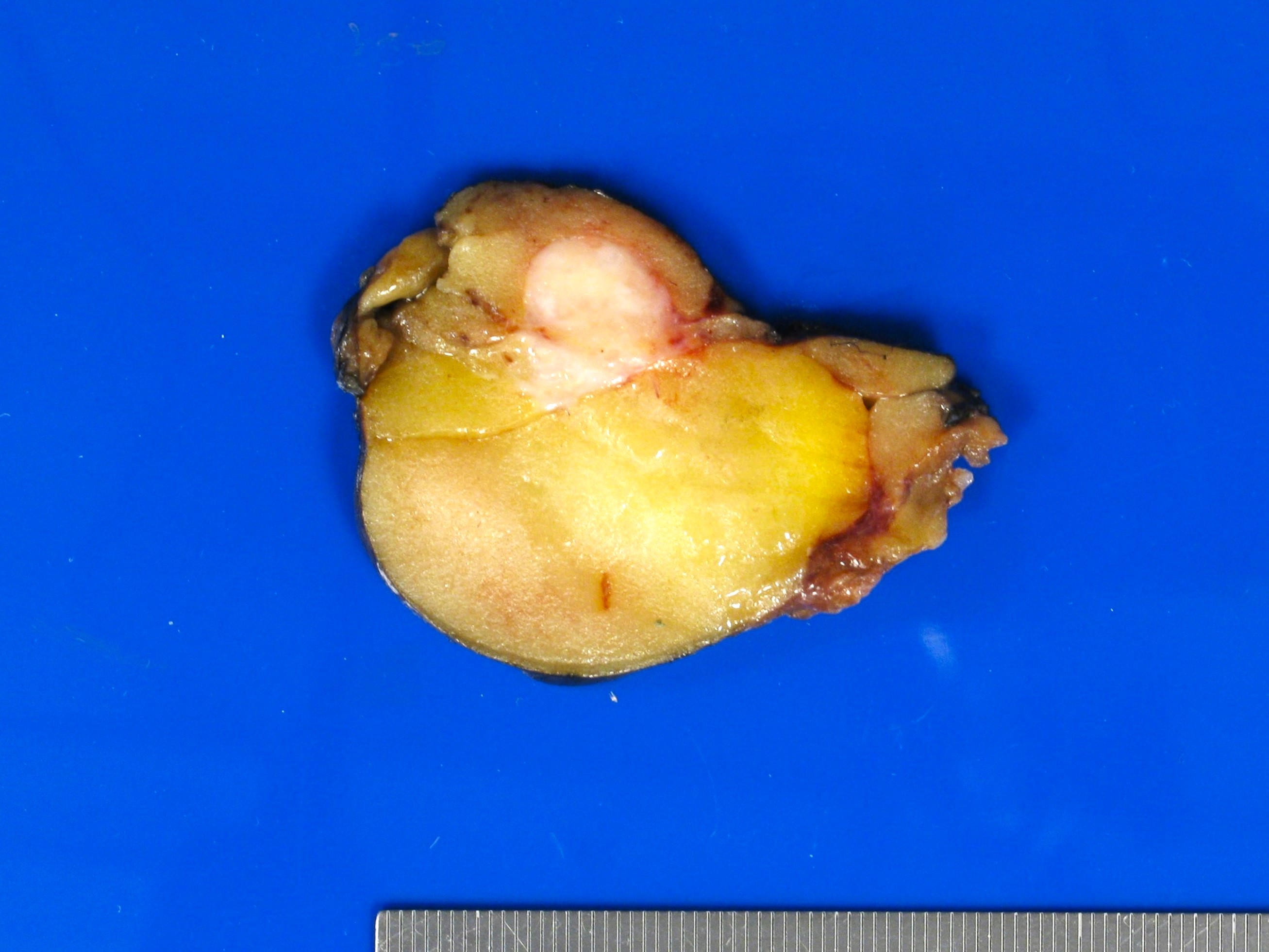
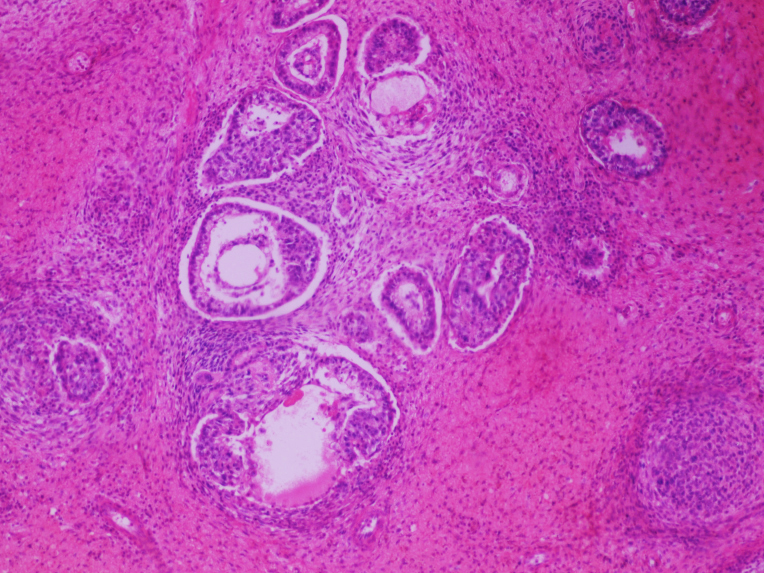
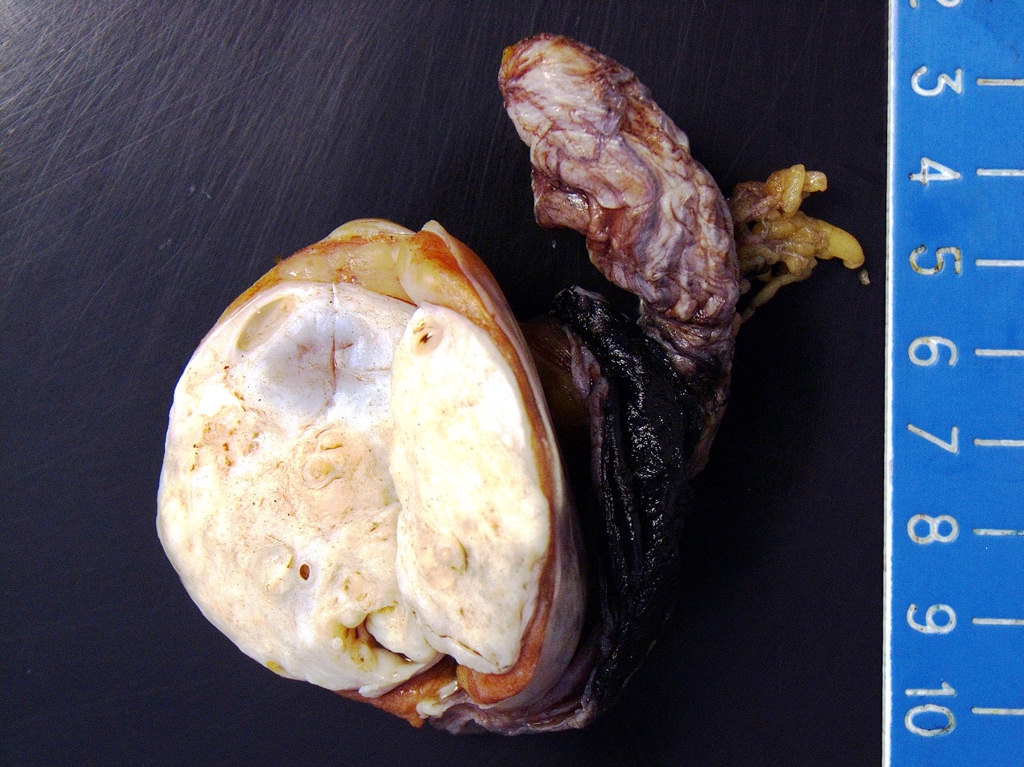
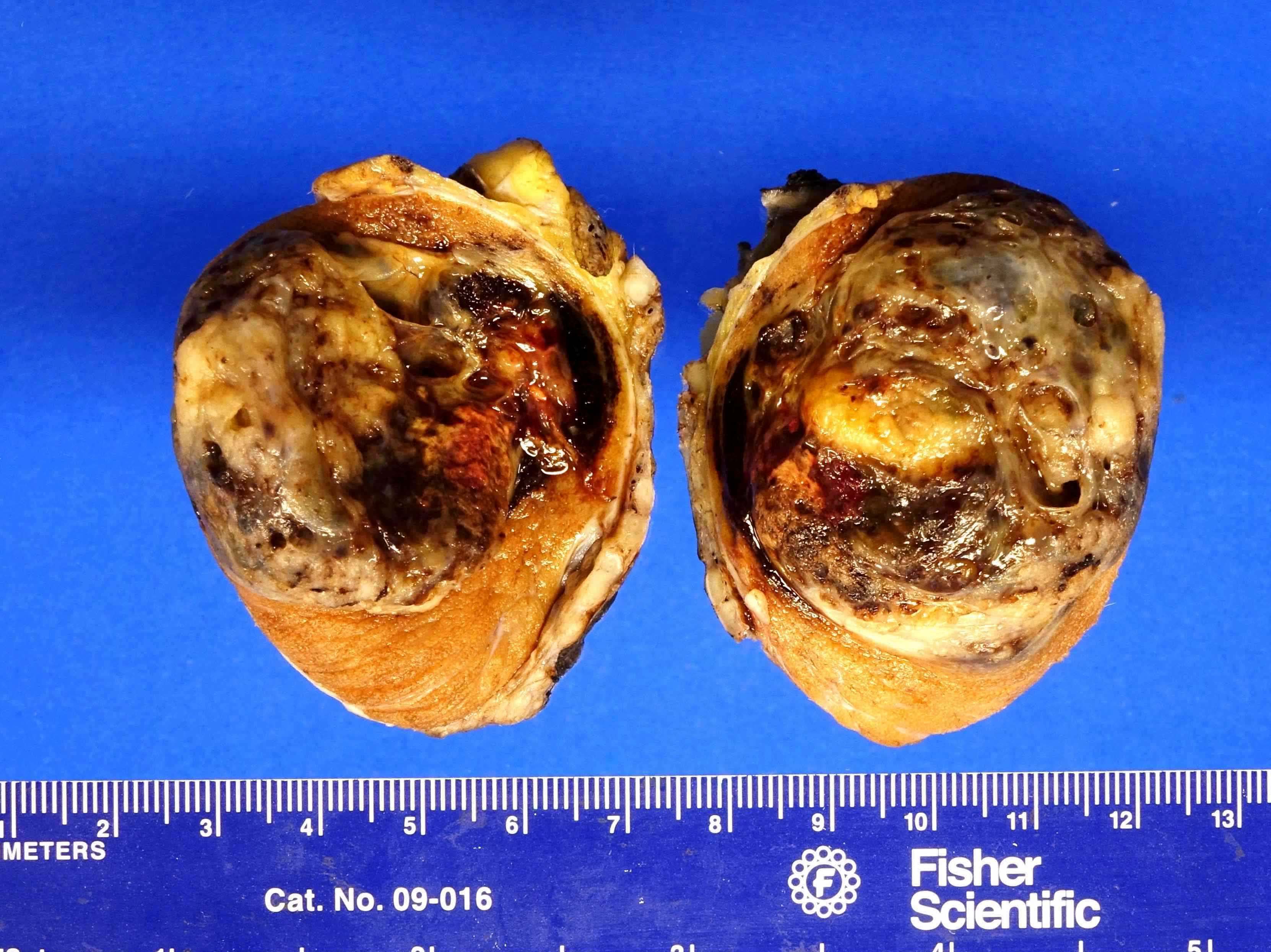
- Largest aggregate tumor size should be obtained, encompassing all adjacent nodules, even with different gross appearances rather than regarding nodules as separate smaller tumors
- Seminoma is subclassified as pT1a / b based on a 3 cm threshold but nonpure seminoma is not
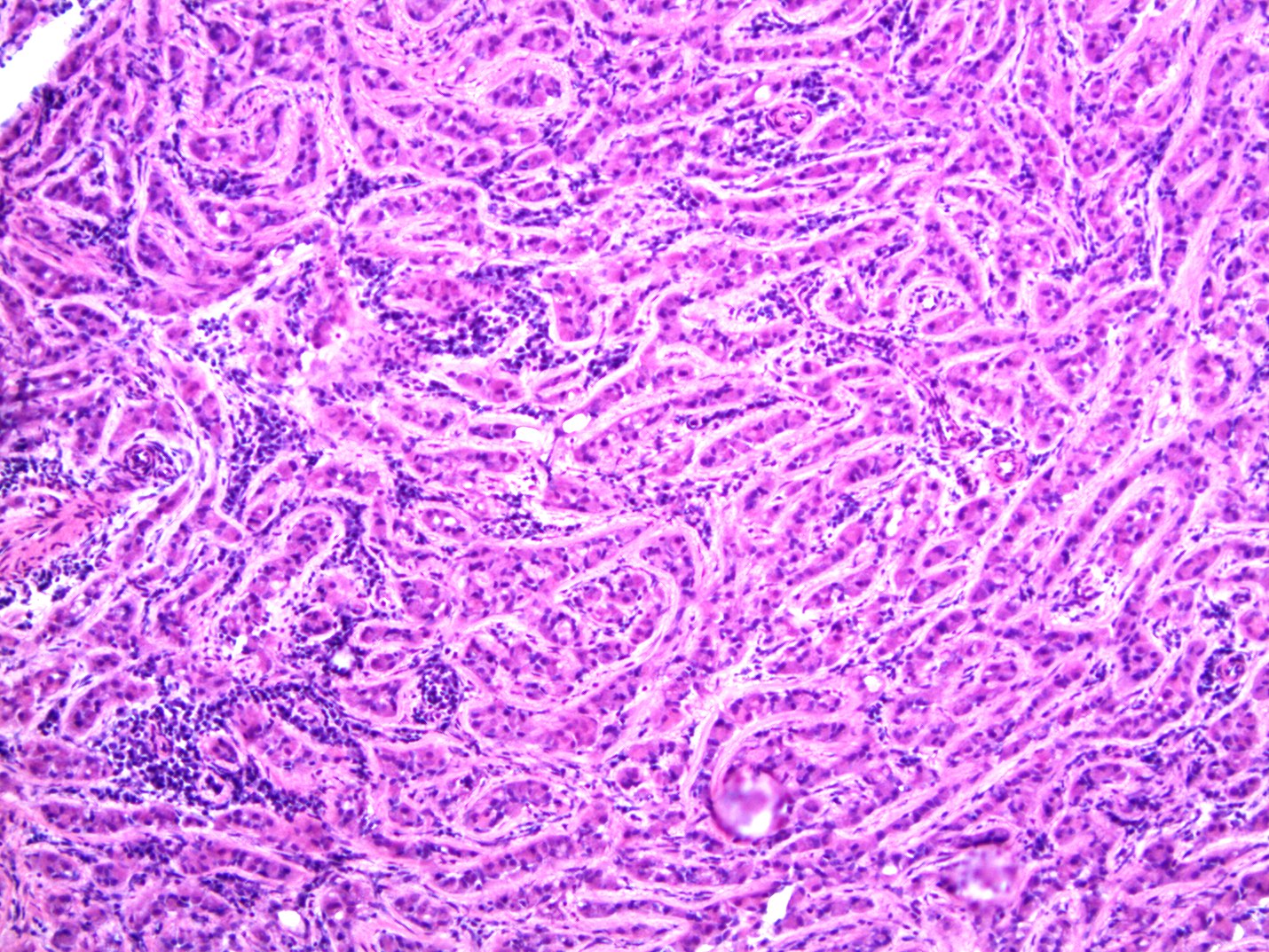
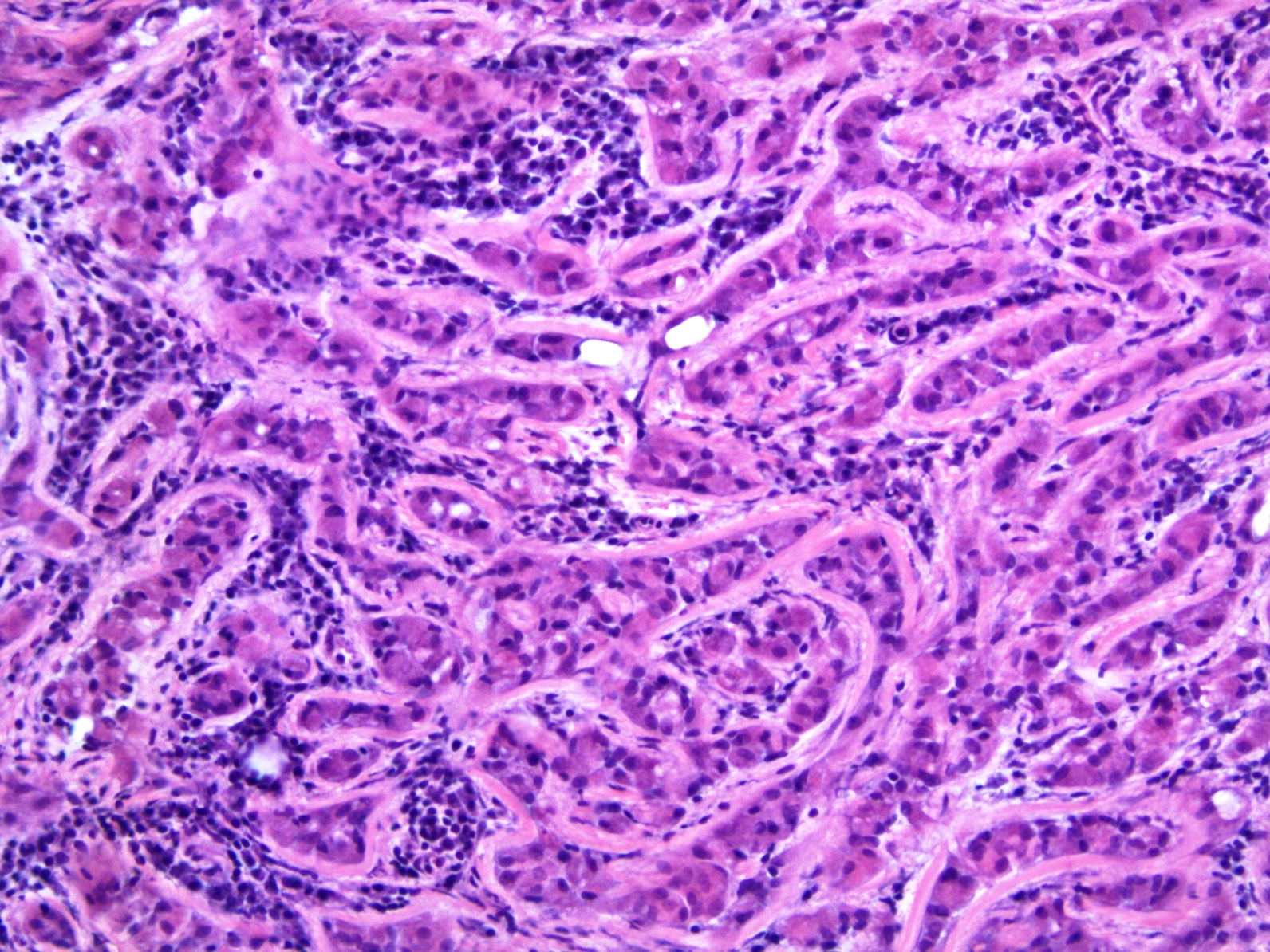
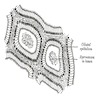
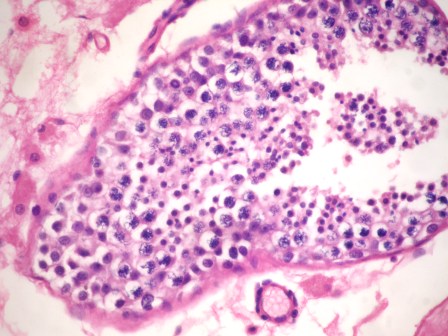
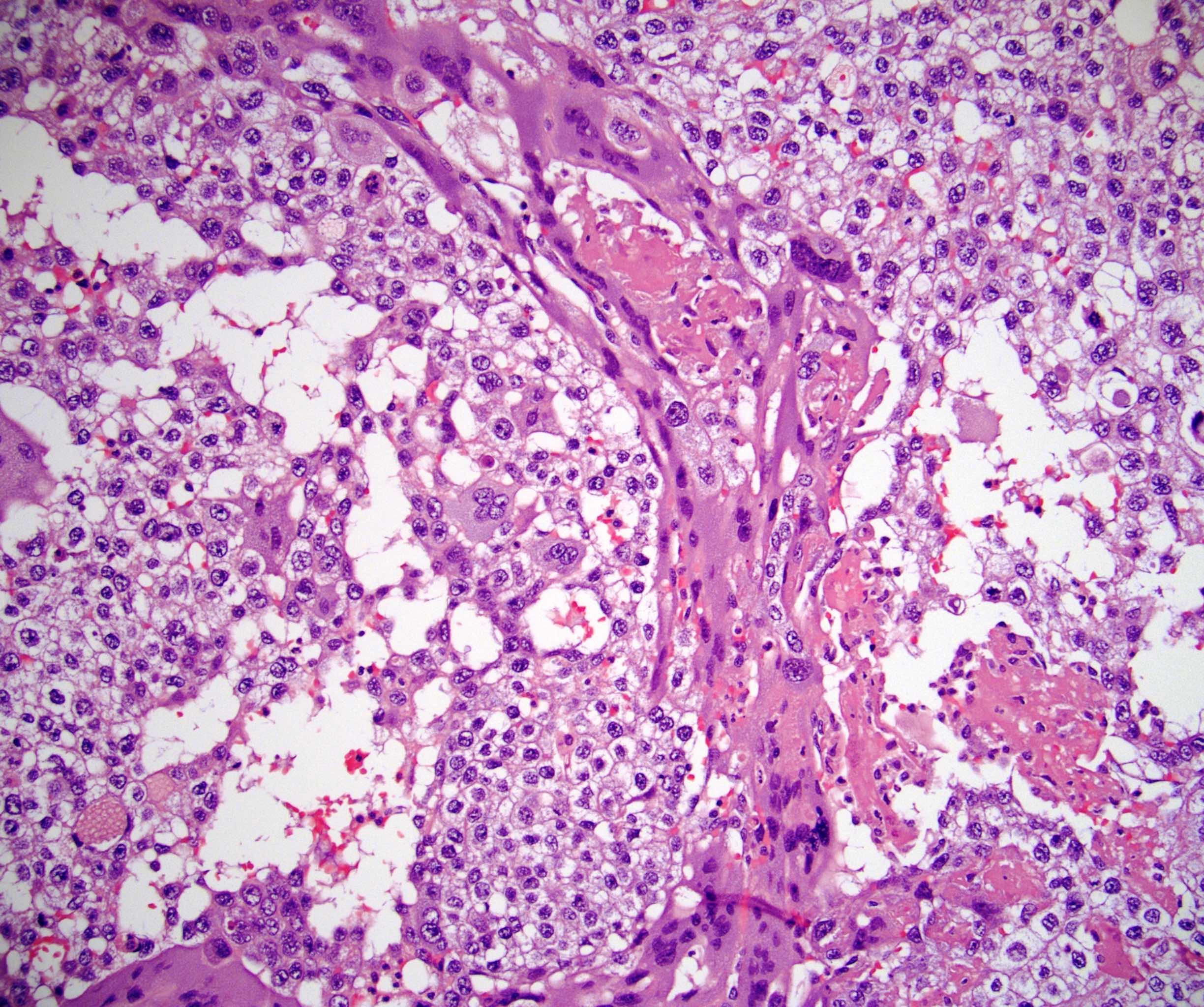
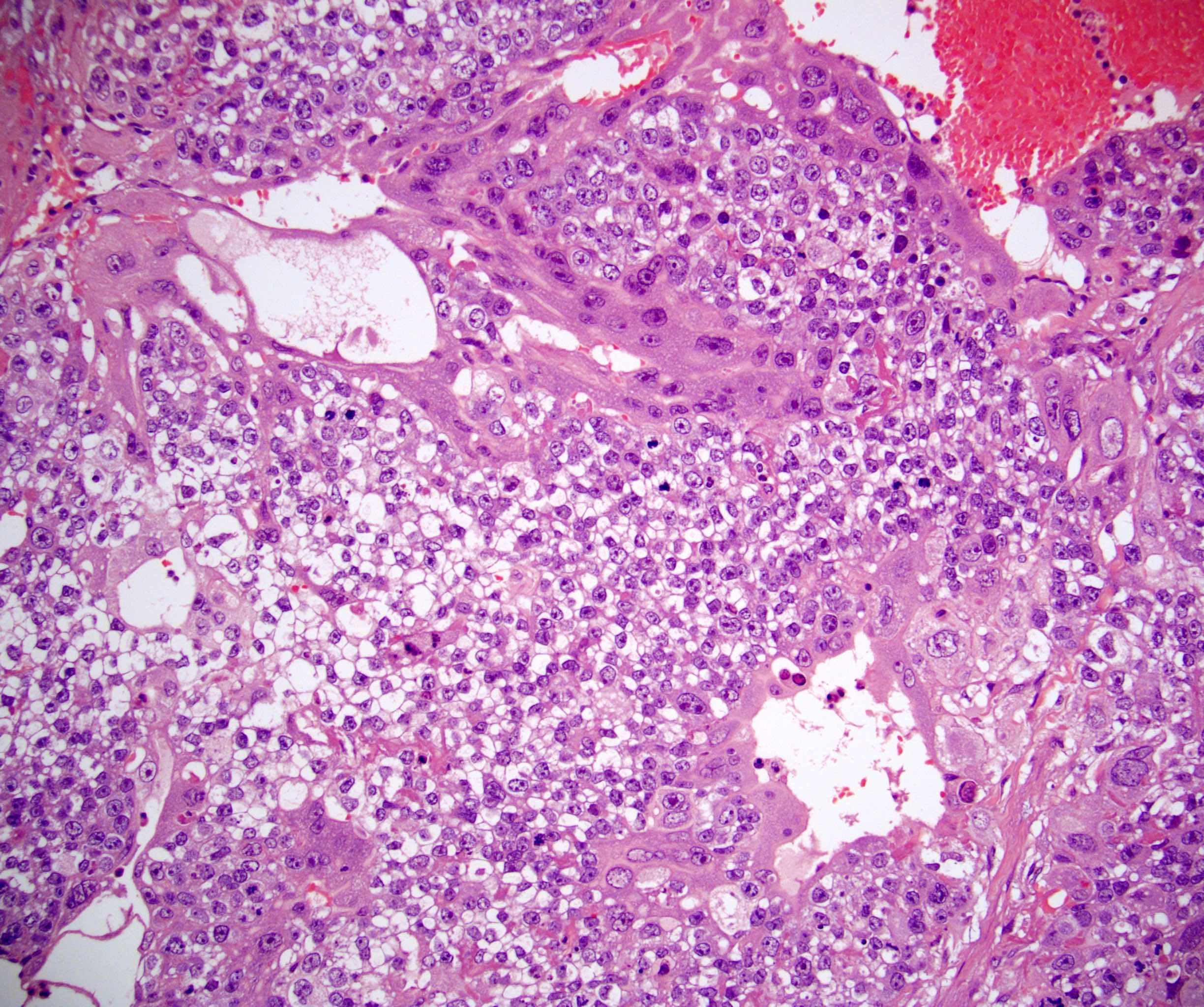
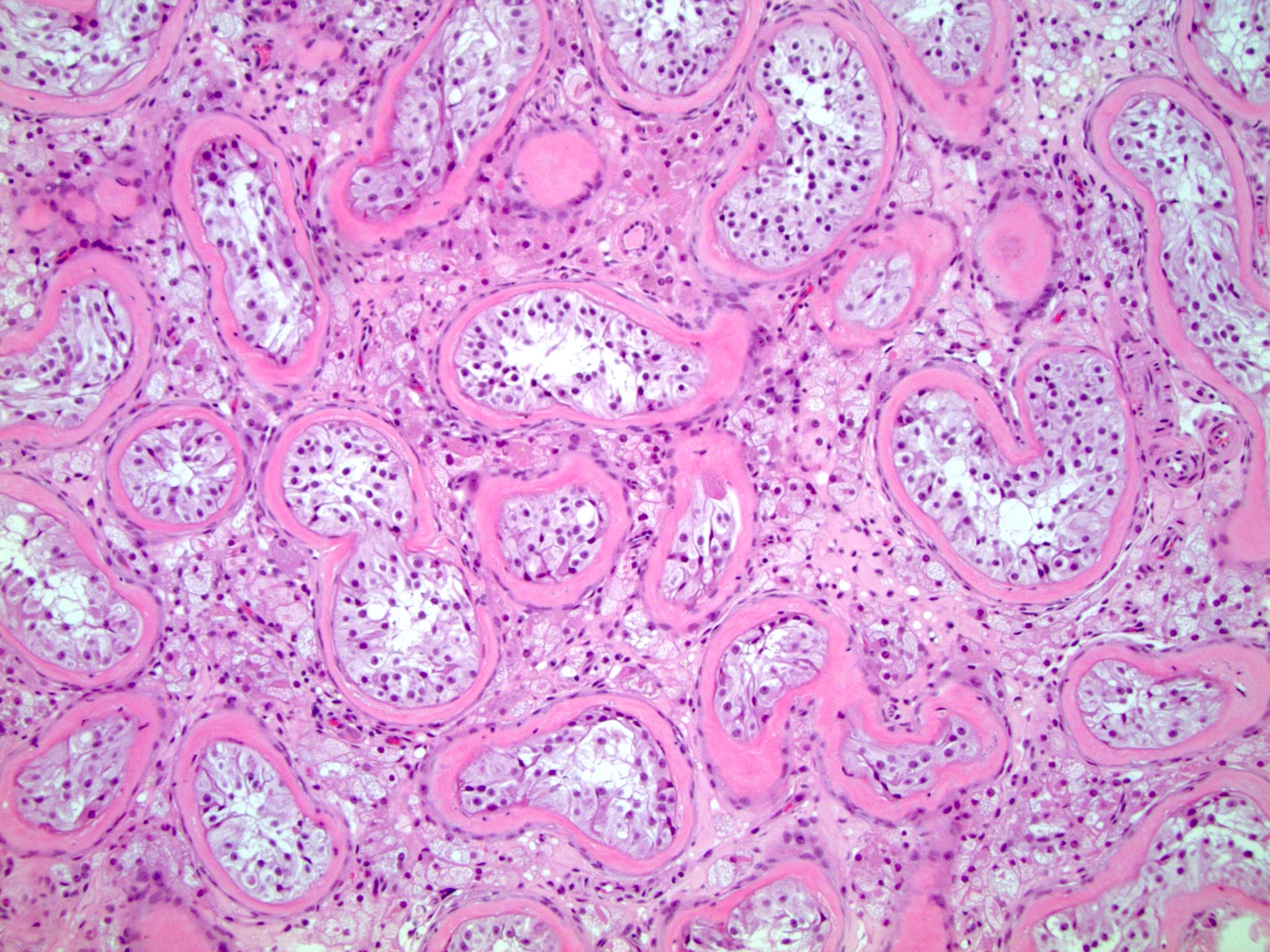
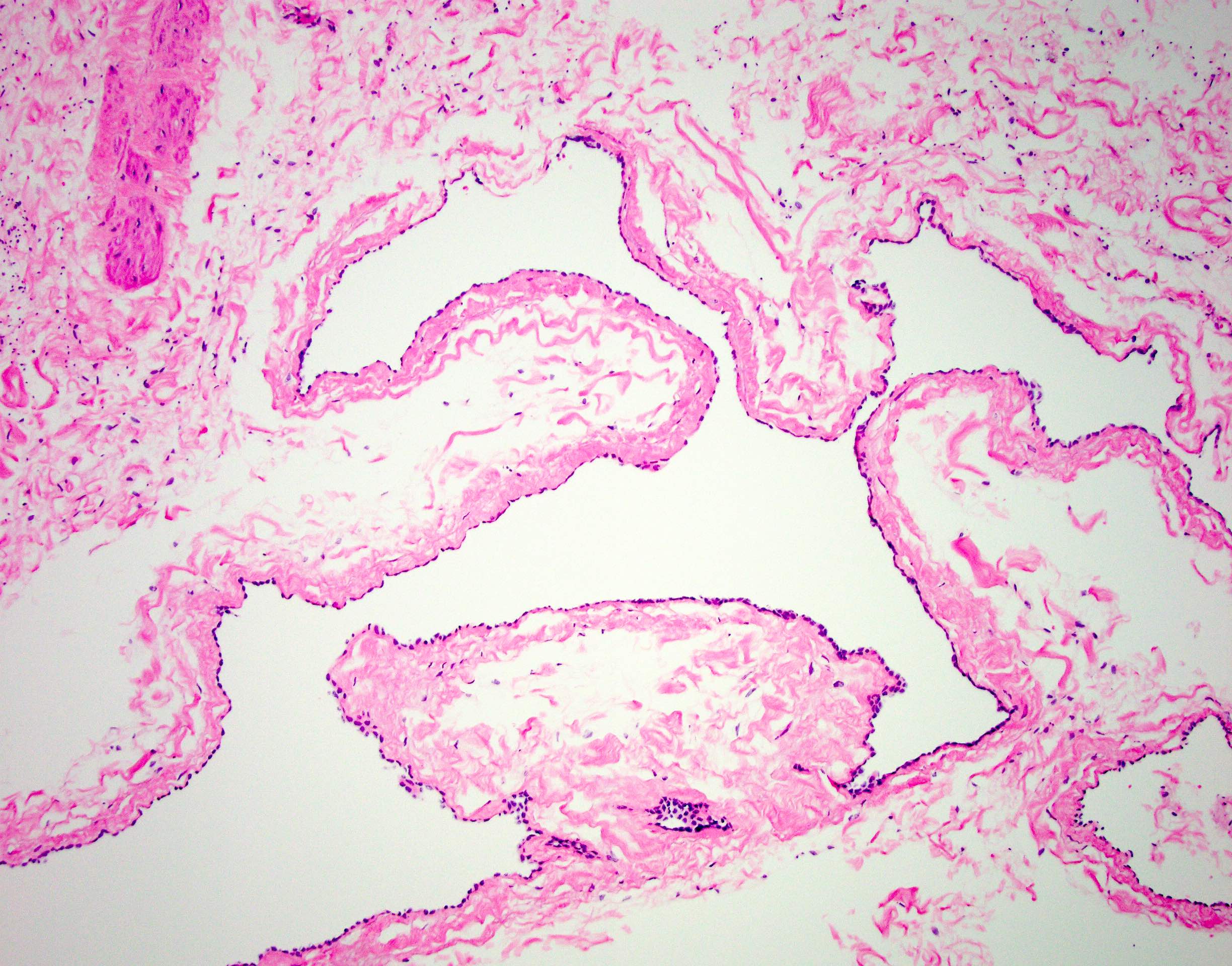
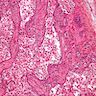
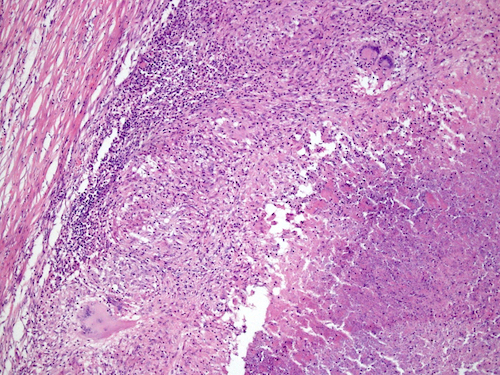
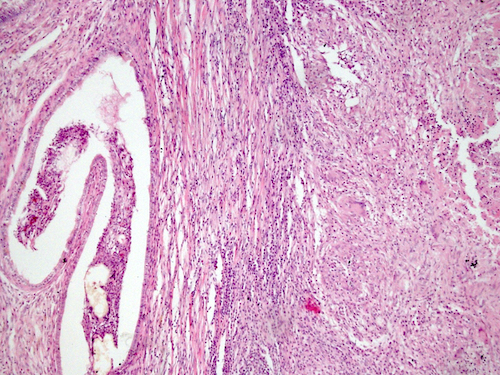
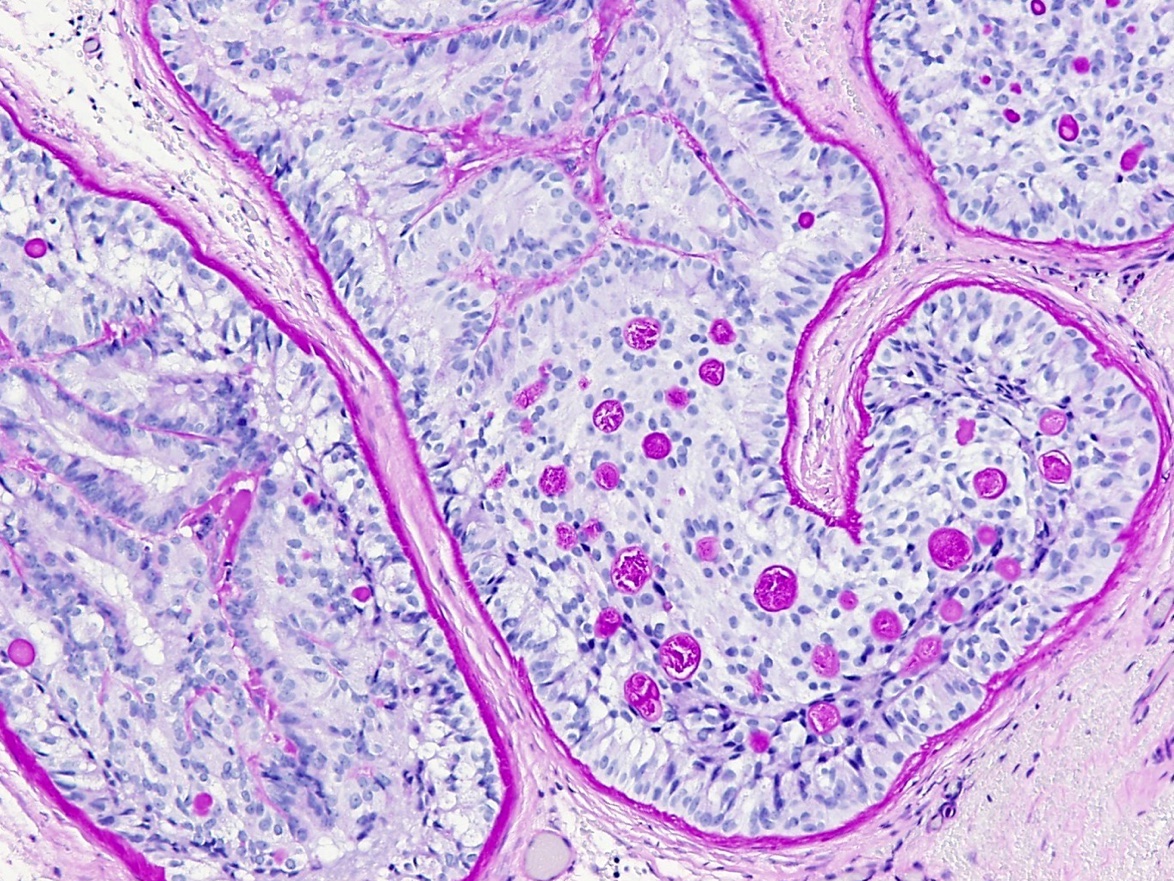
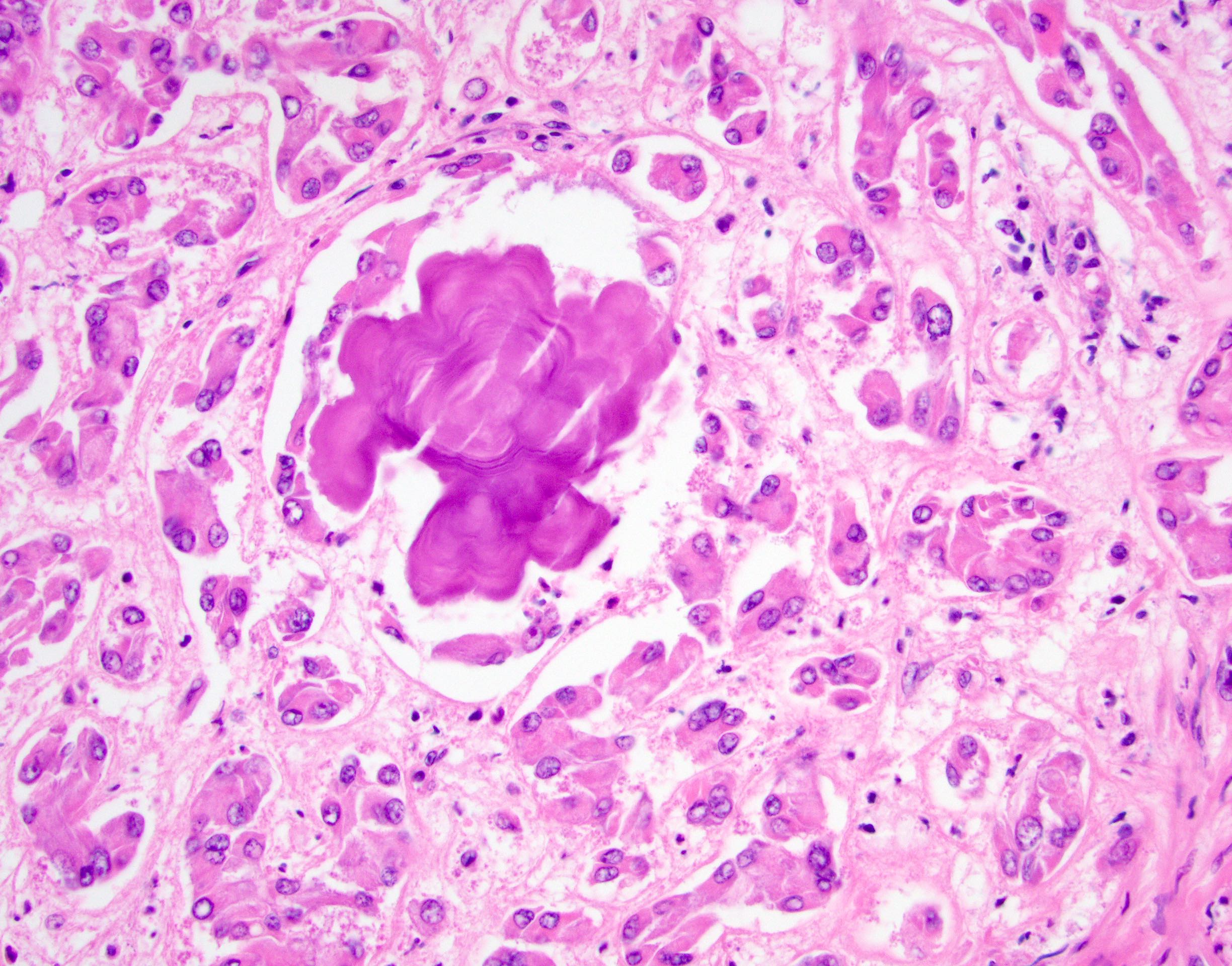
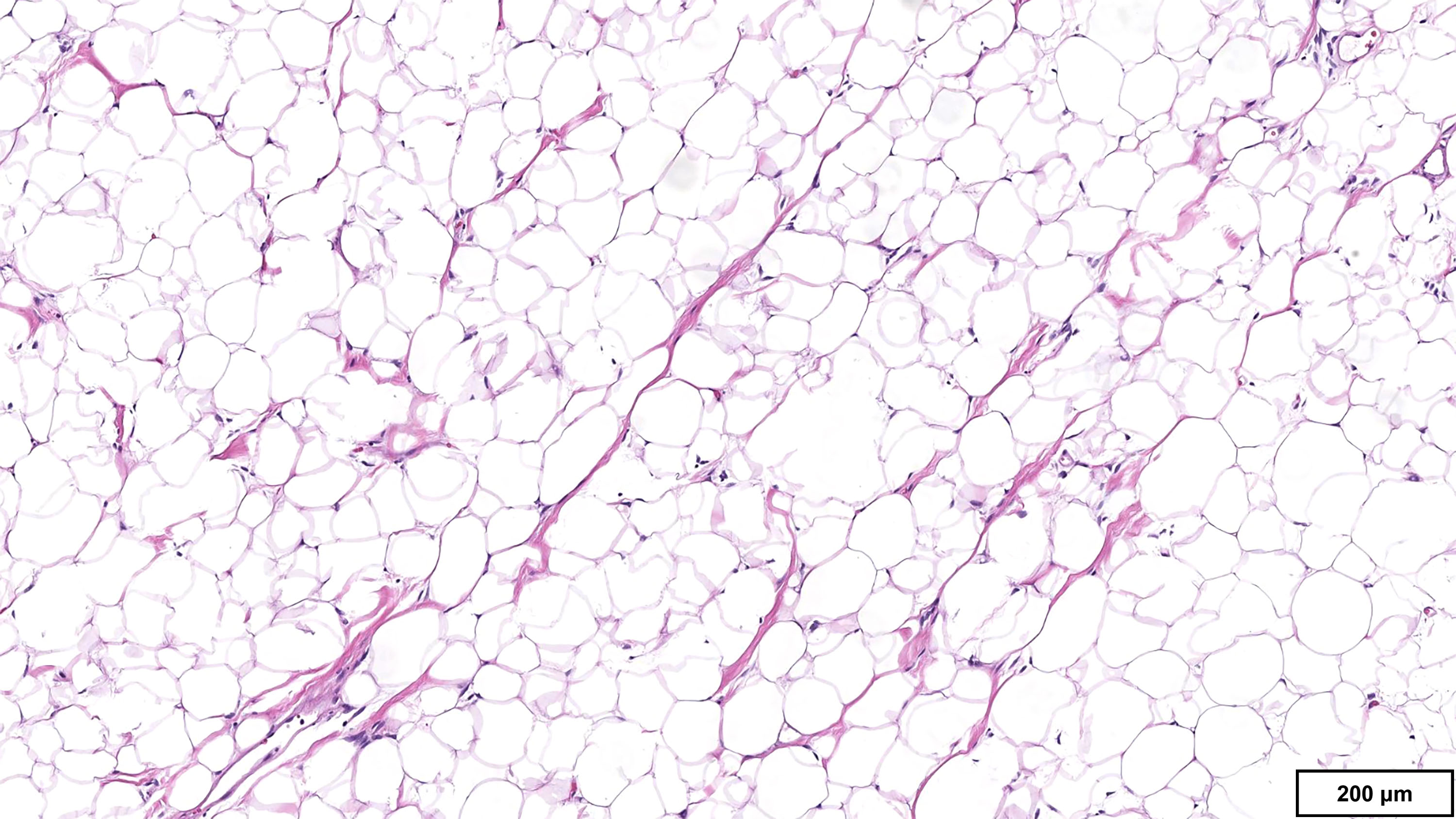
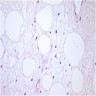
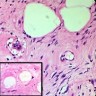
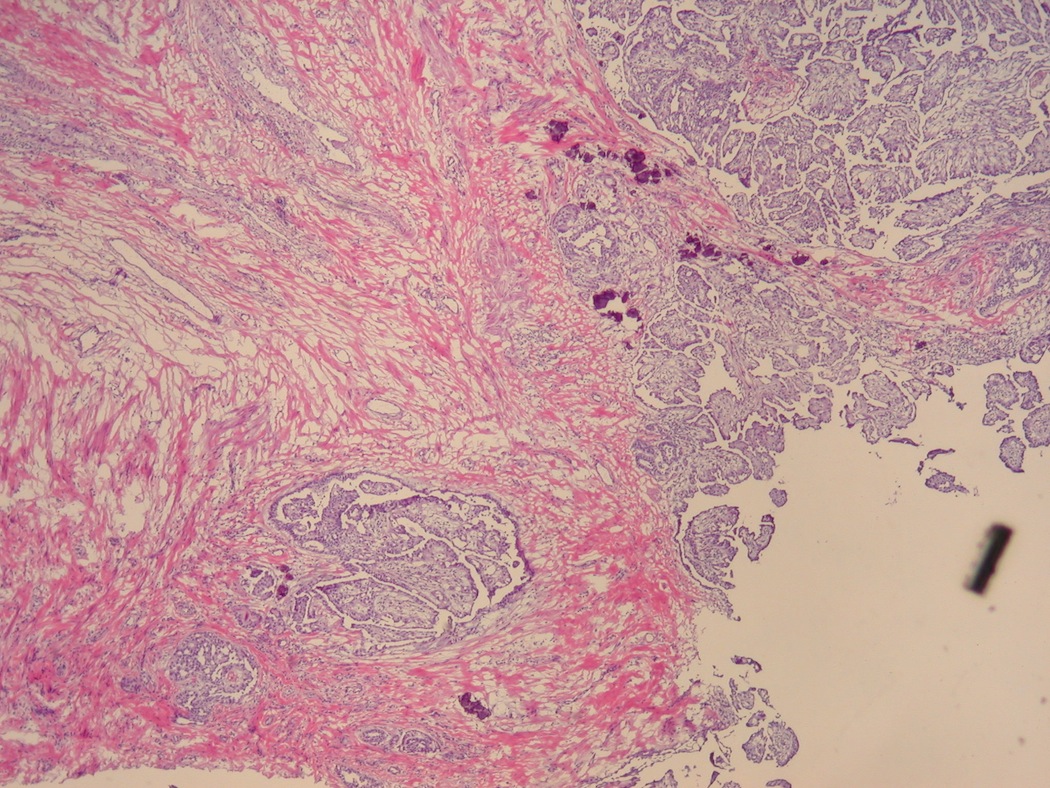
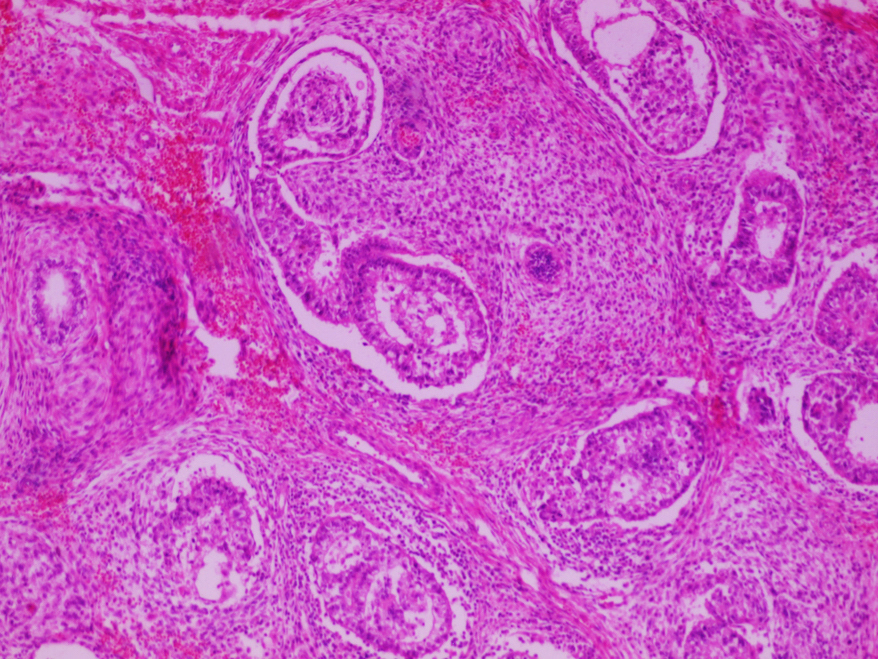
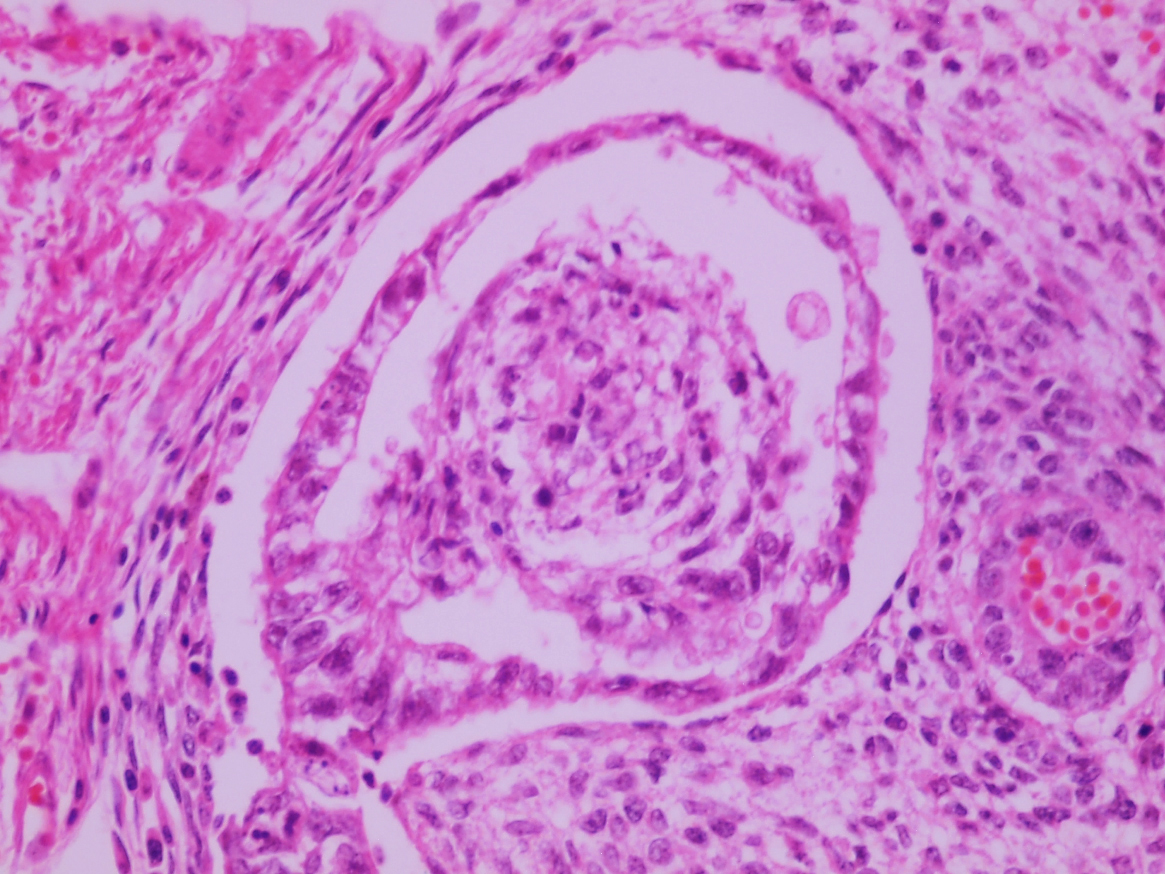
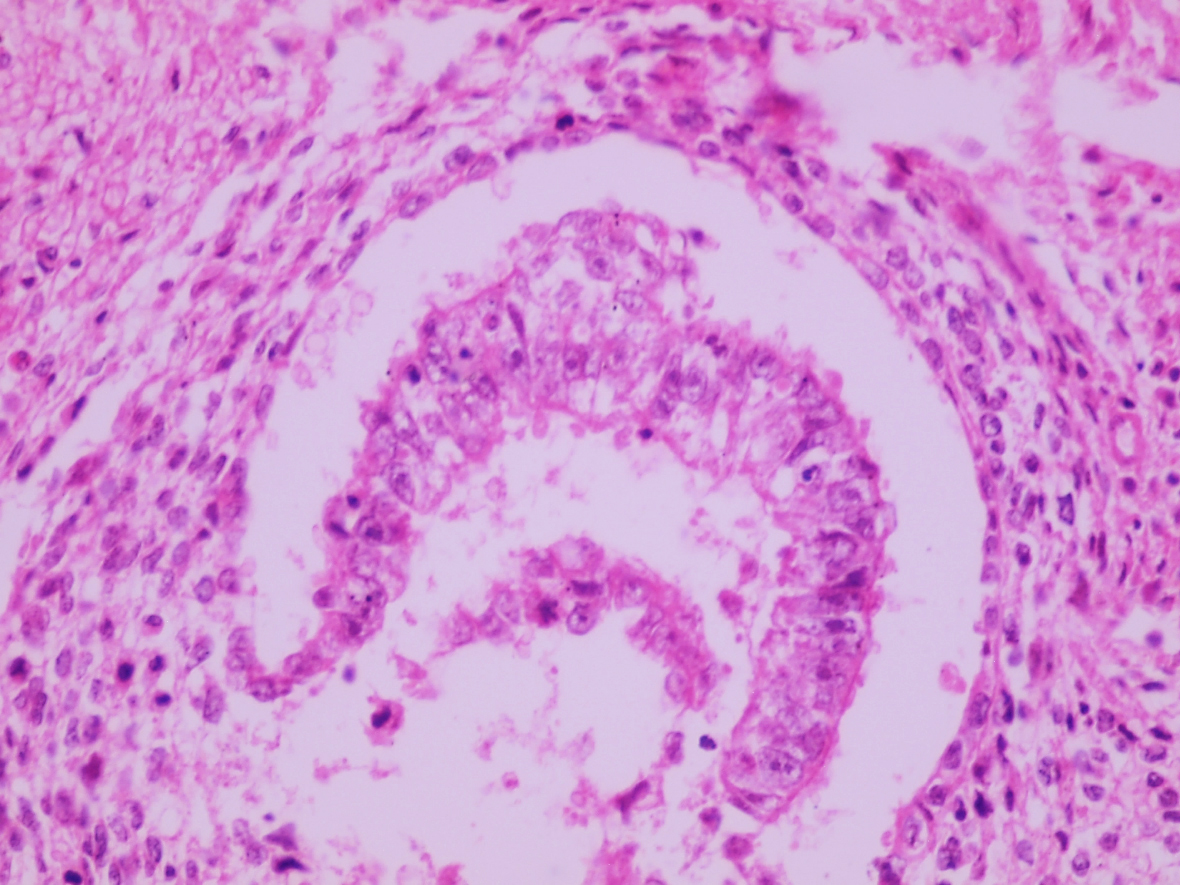
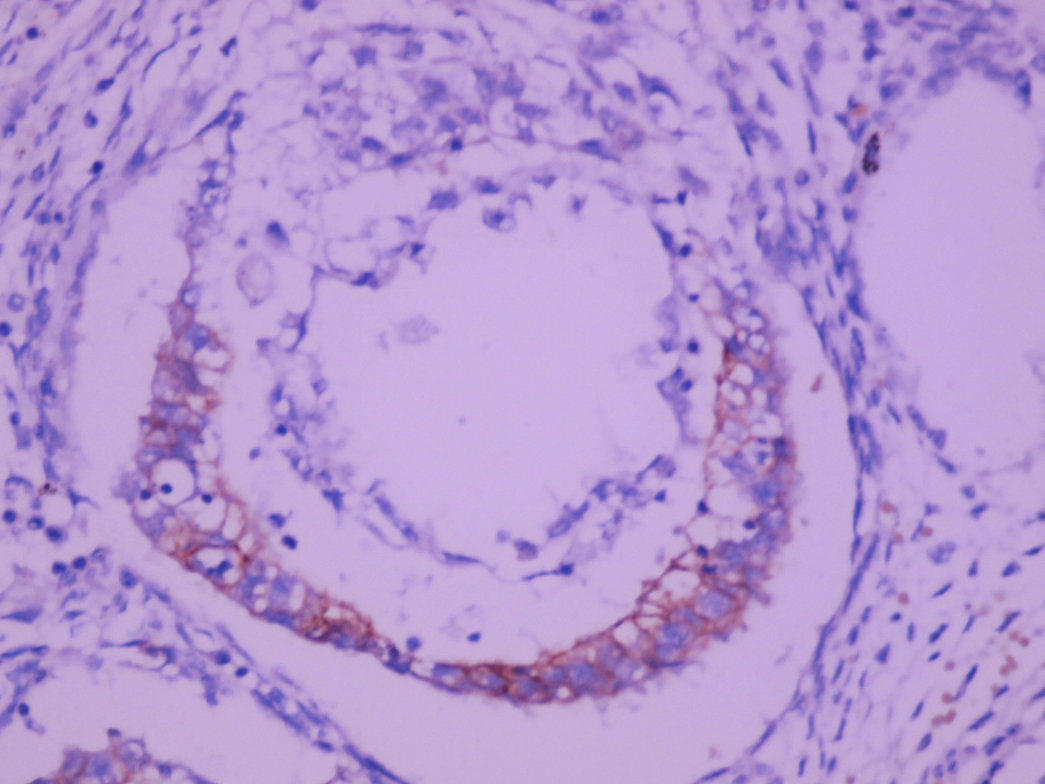
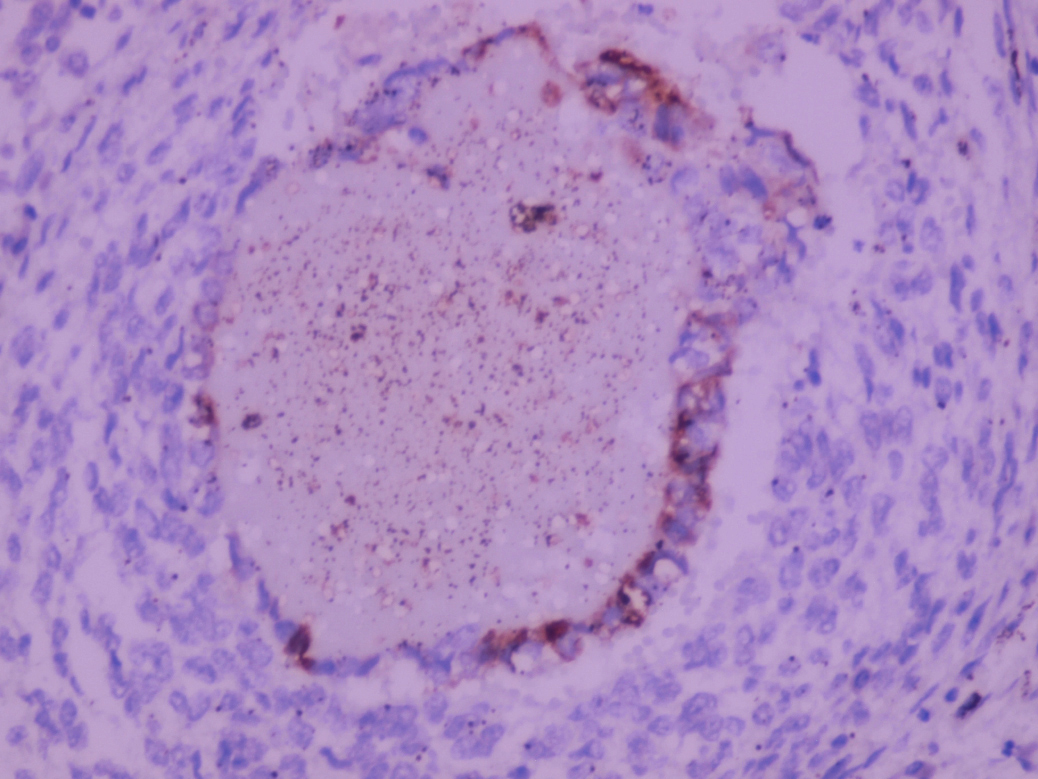
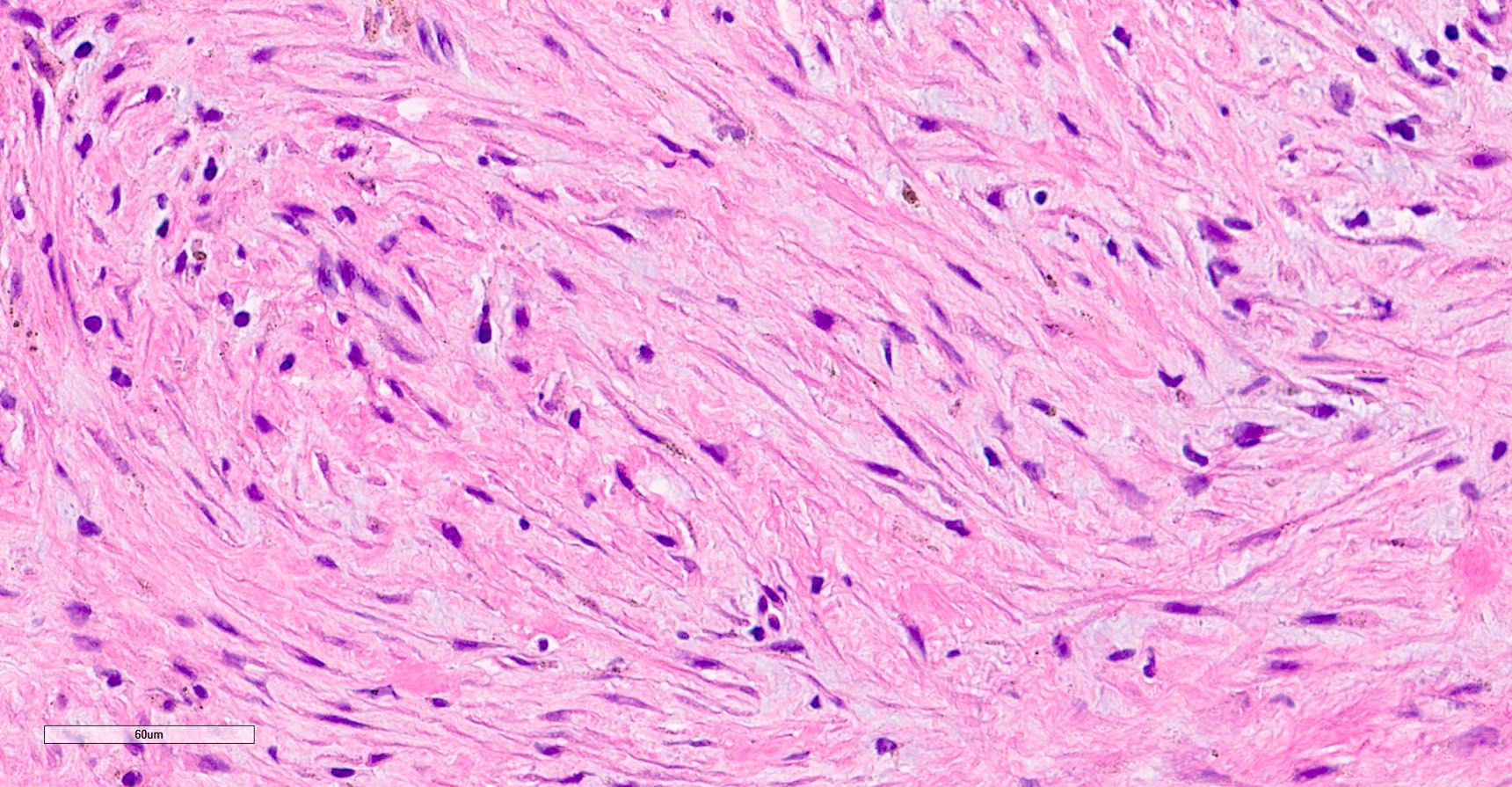
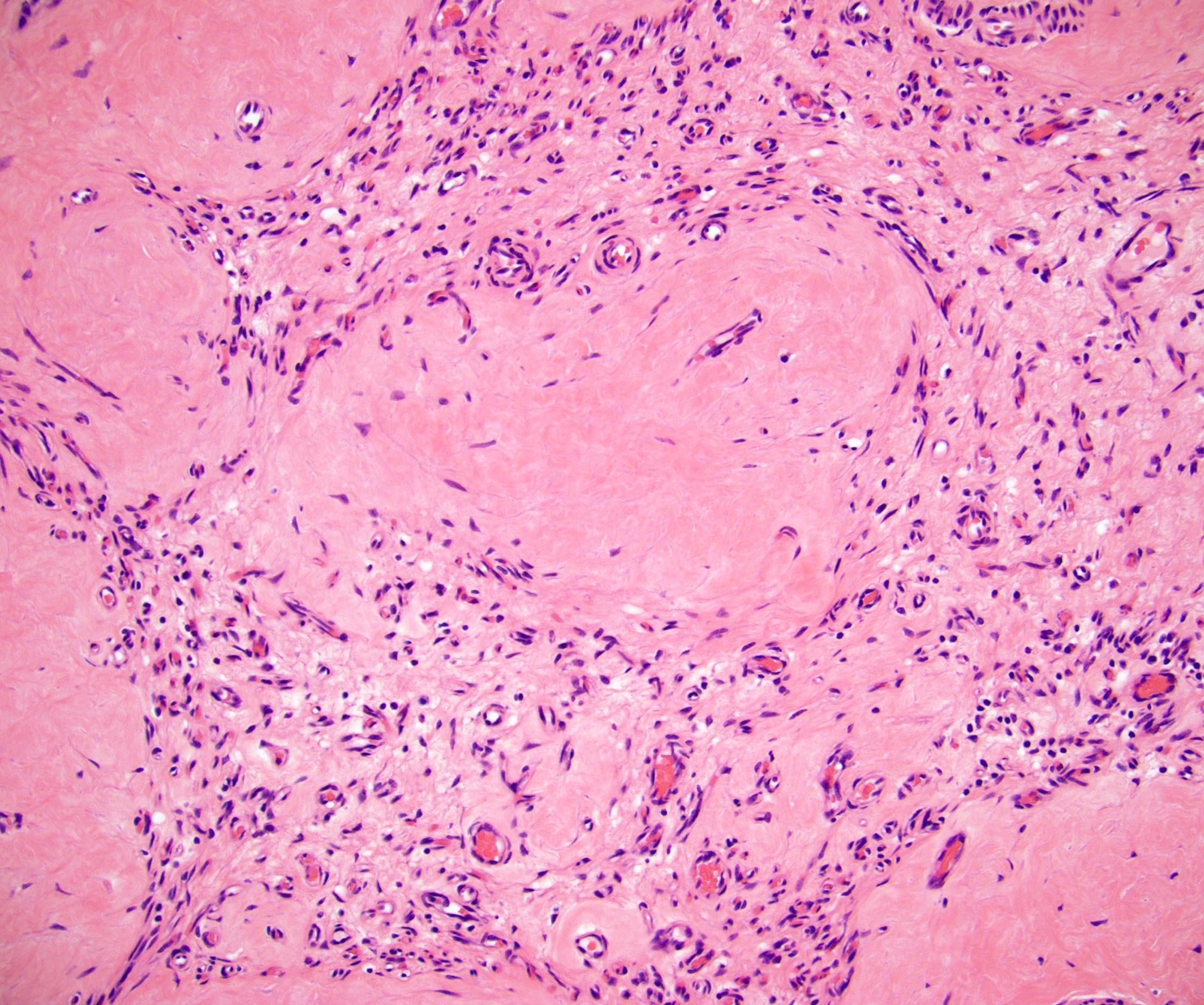
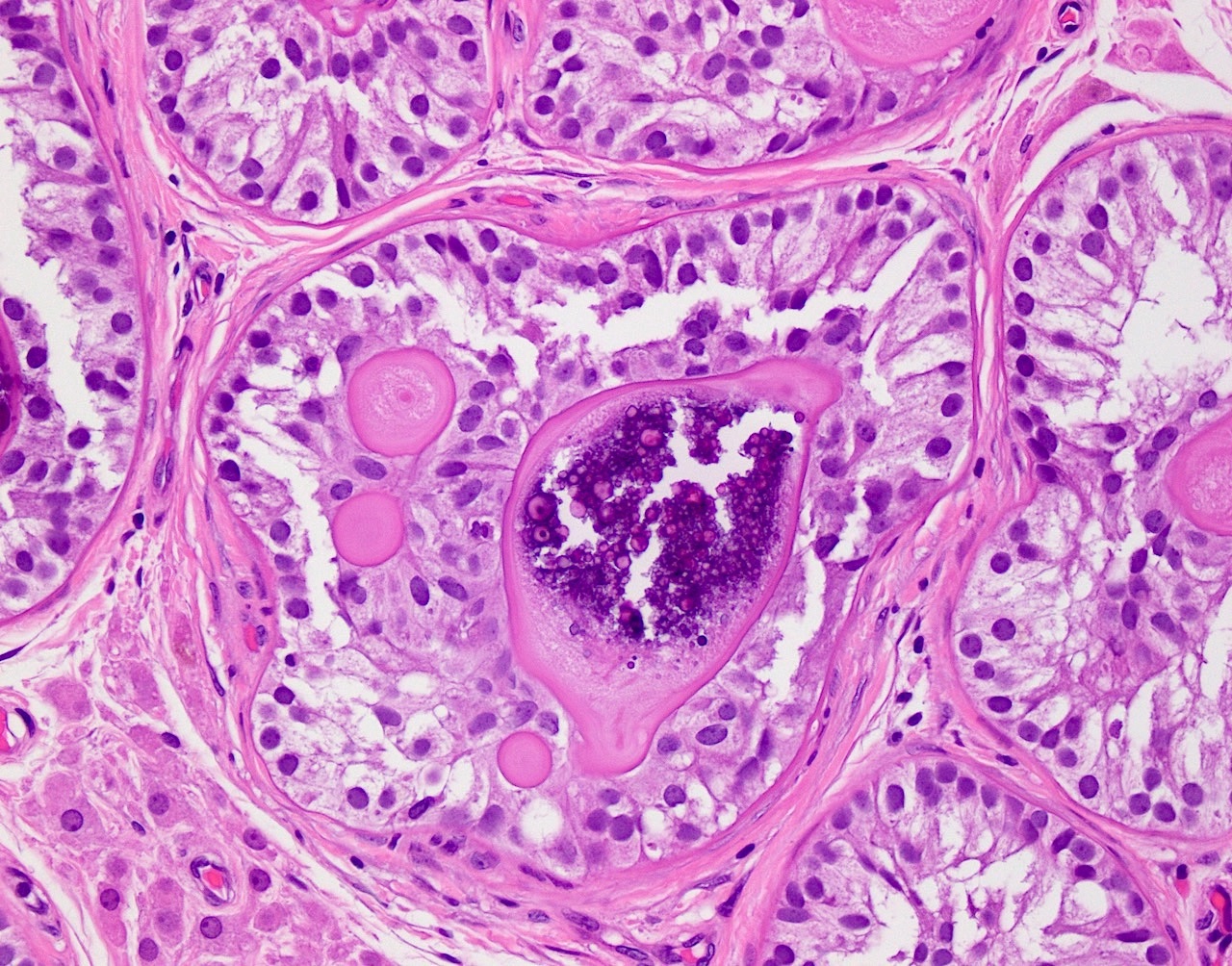
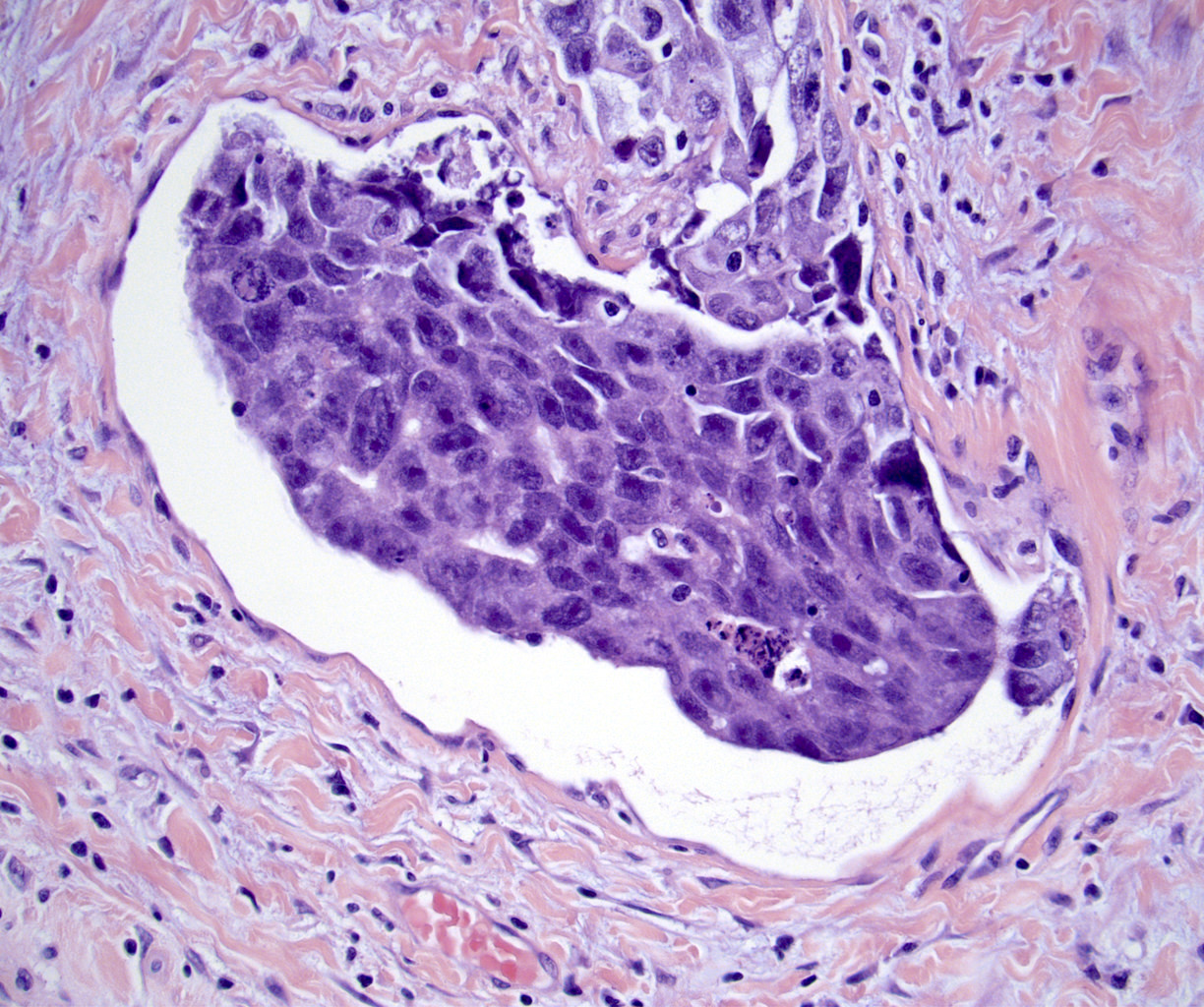
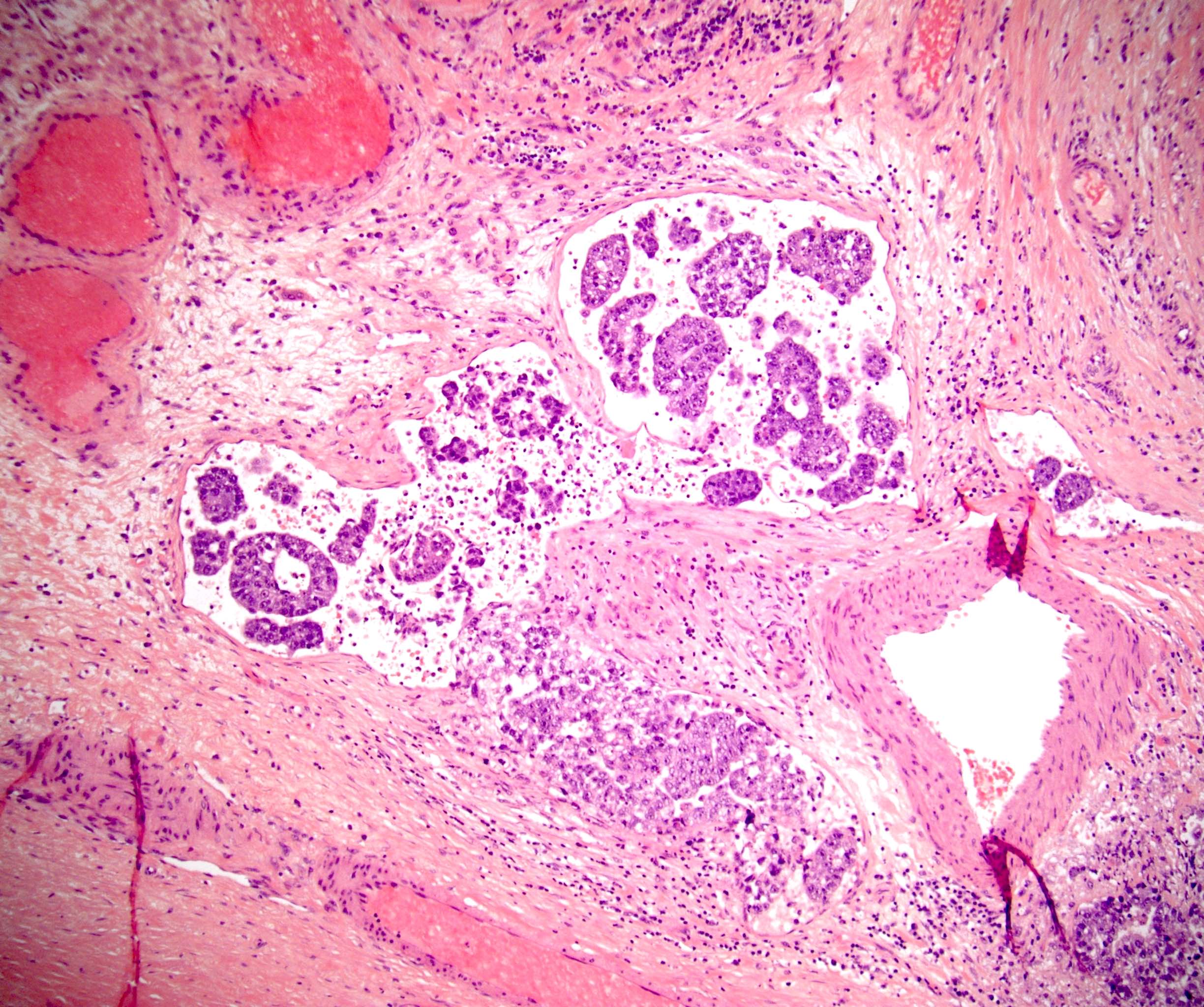
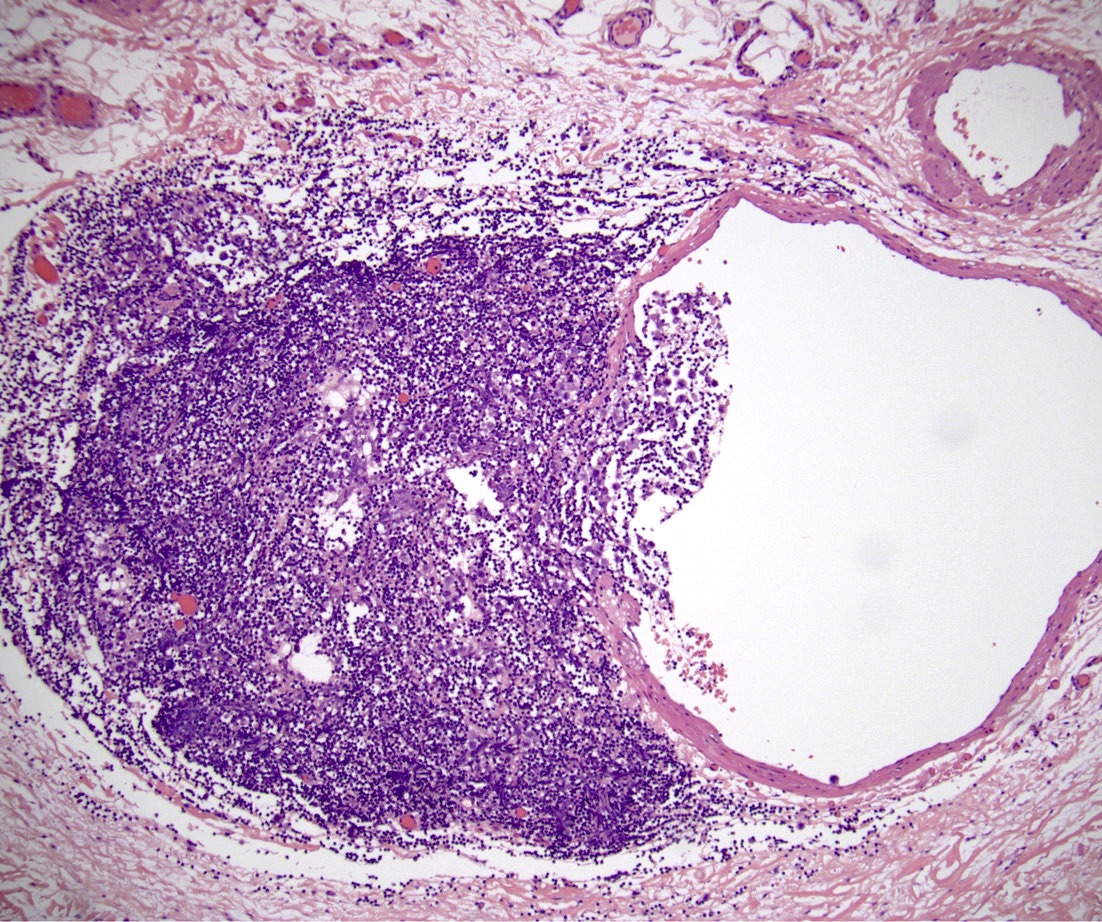
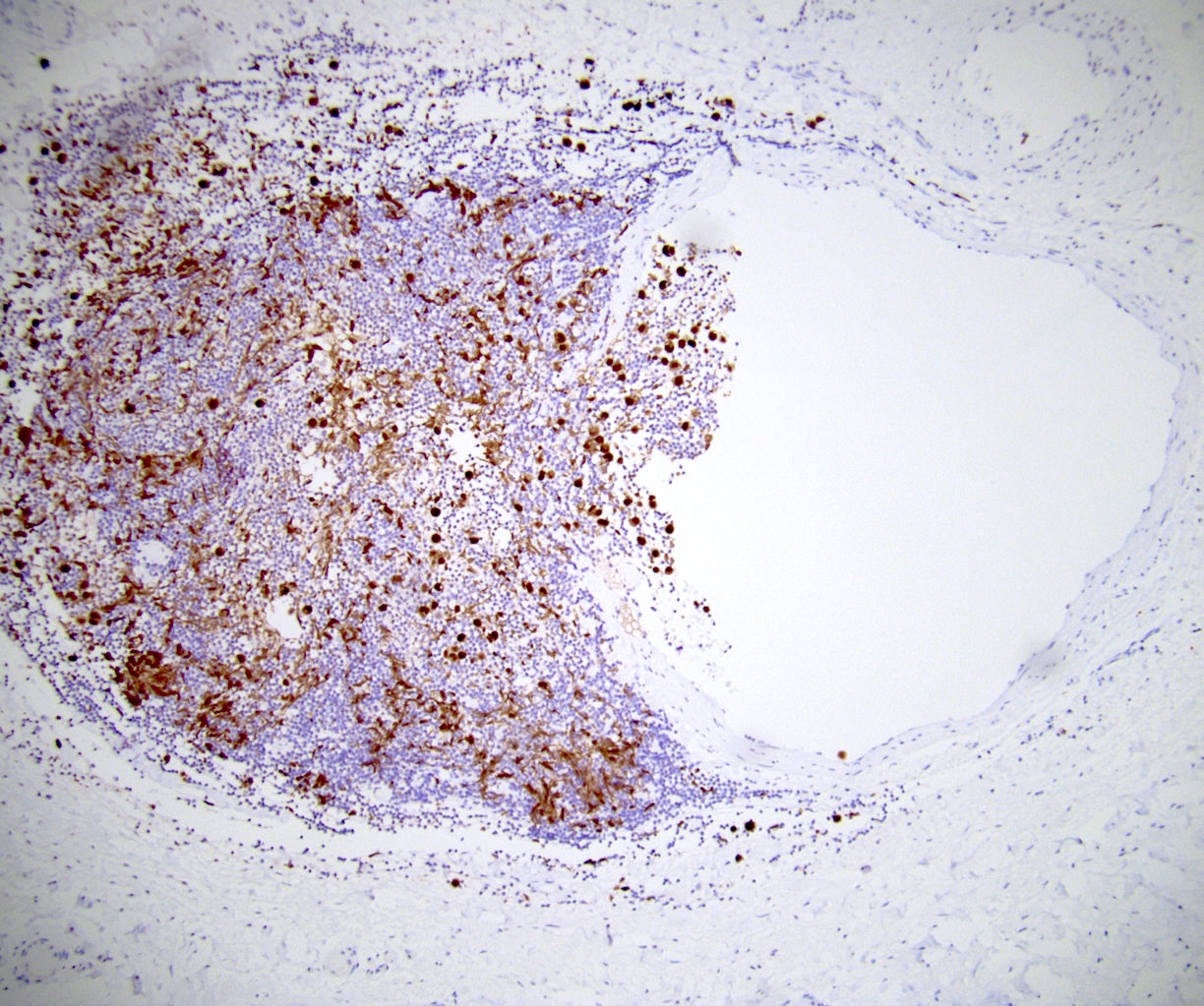
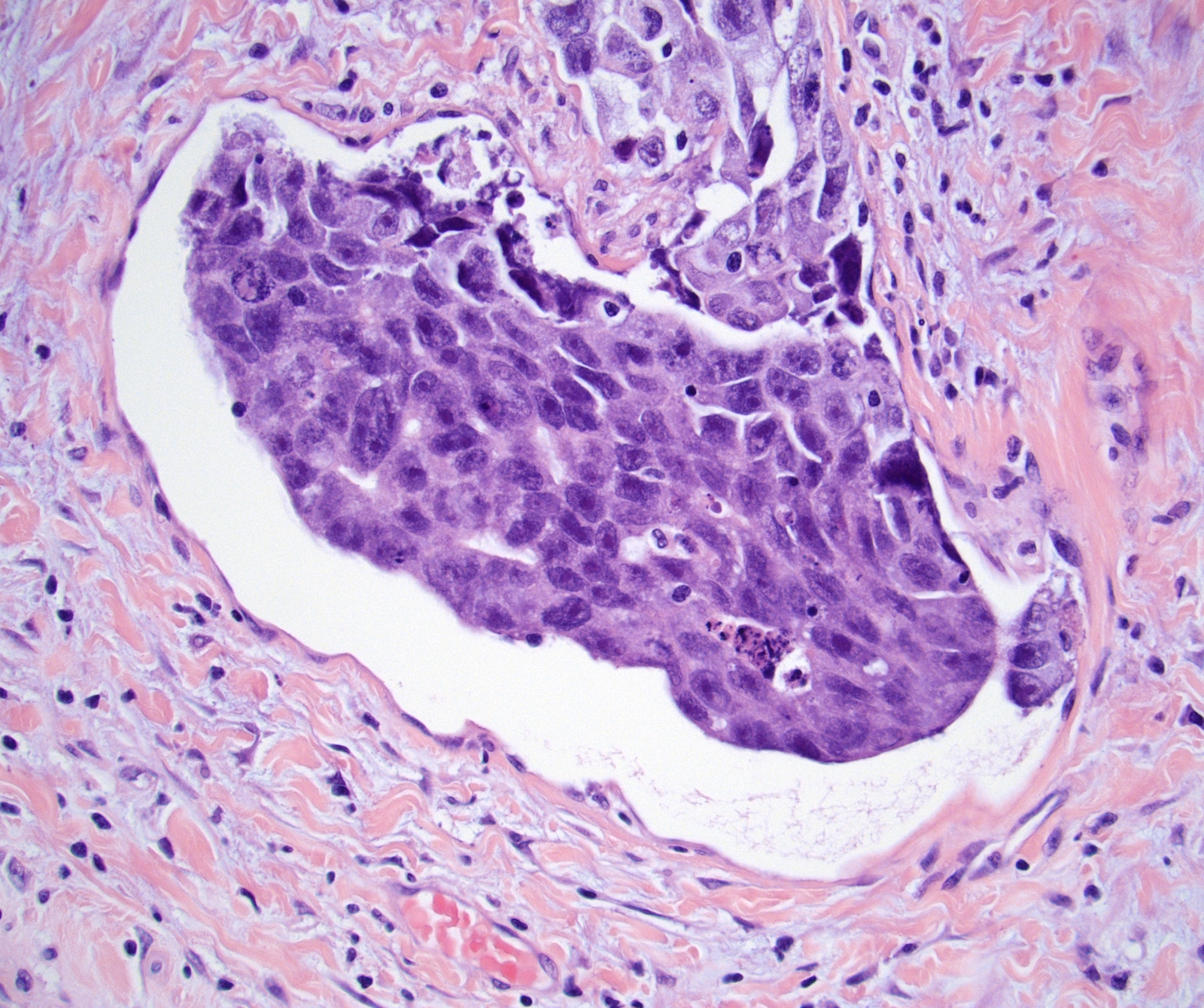
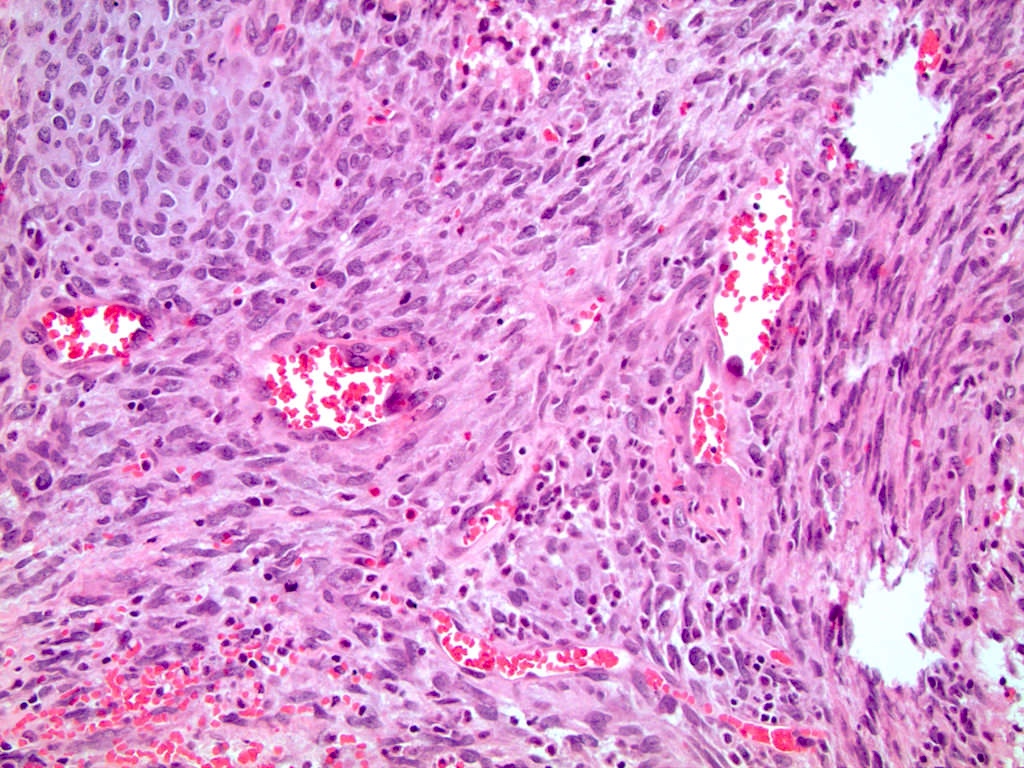
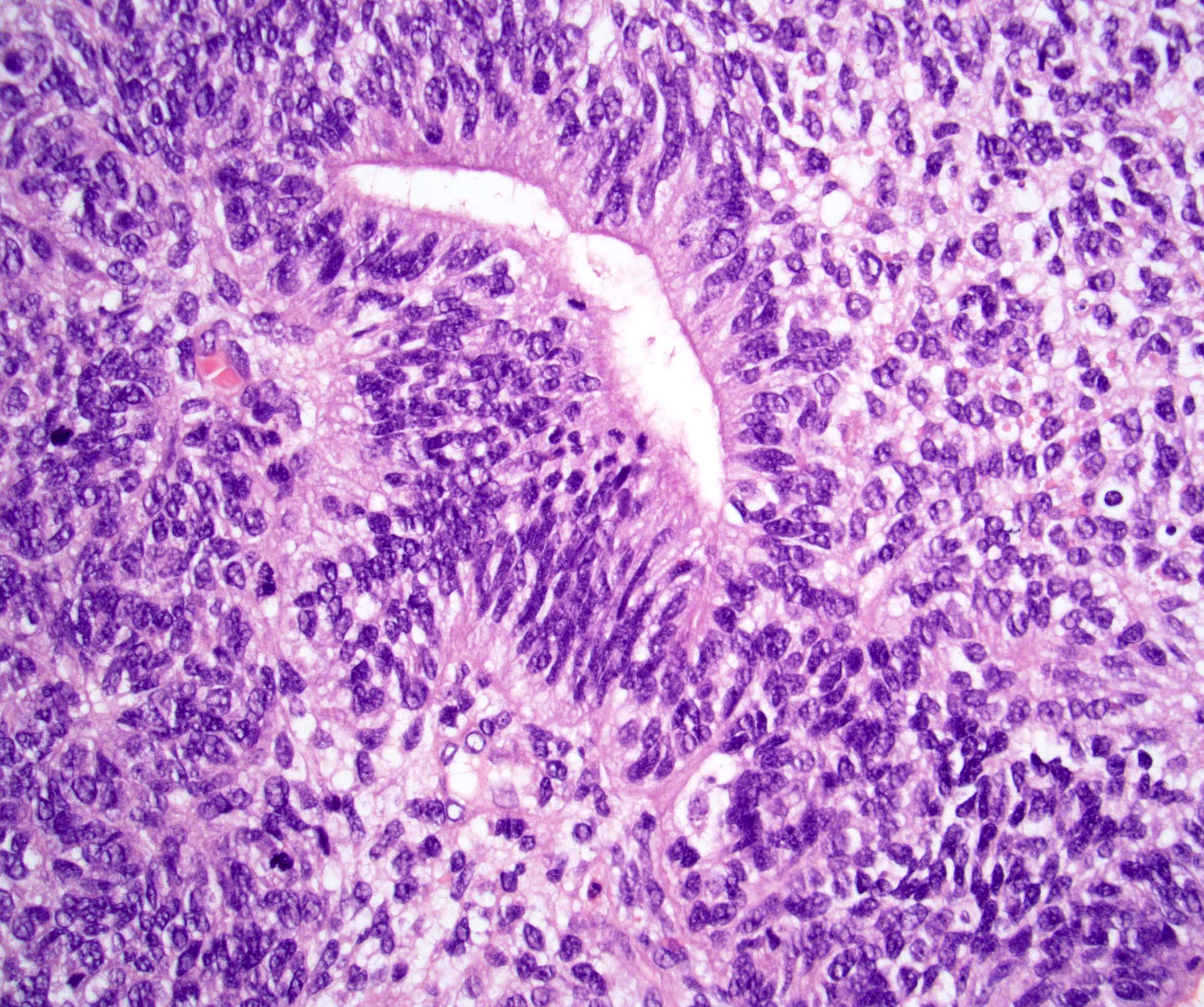
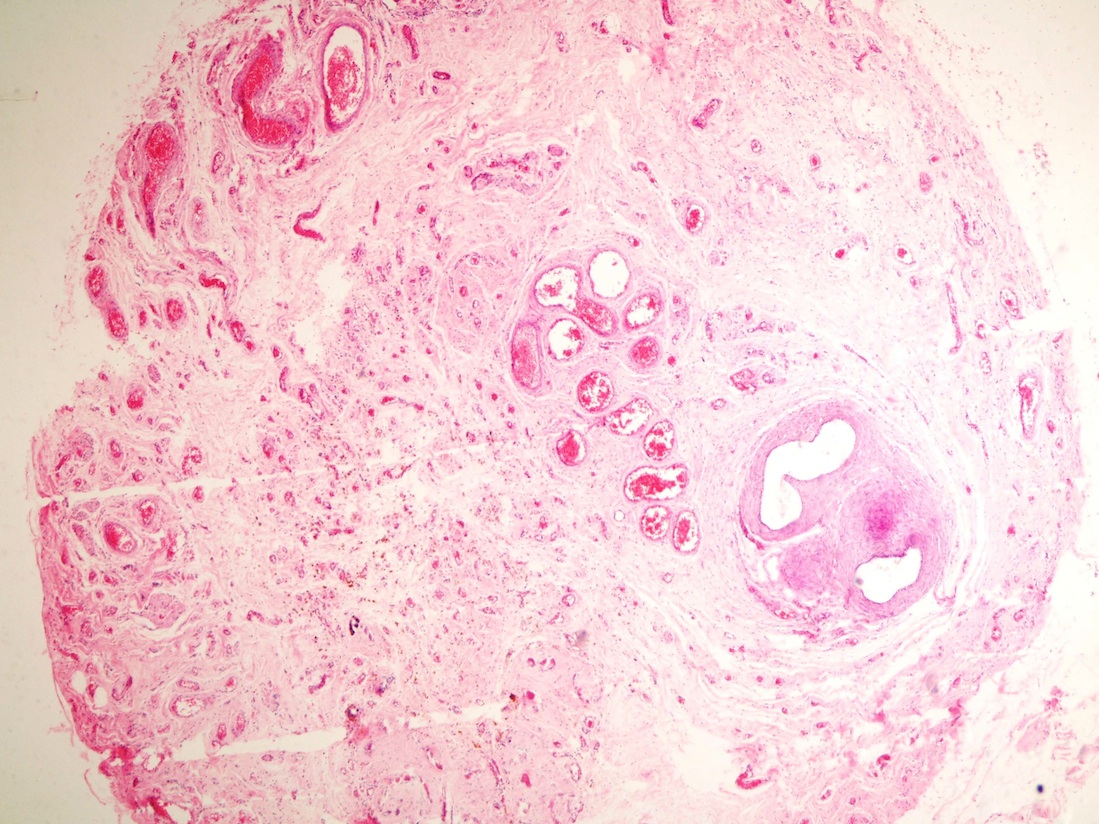
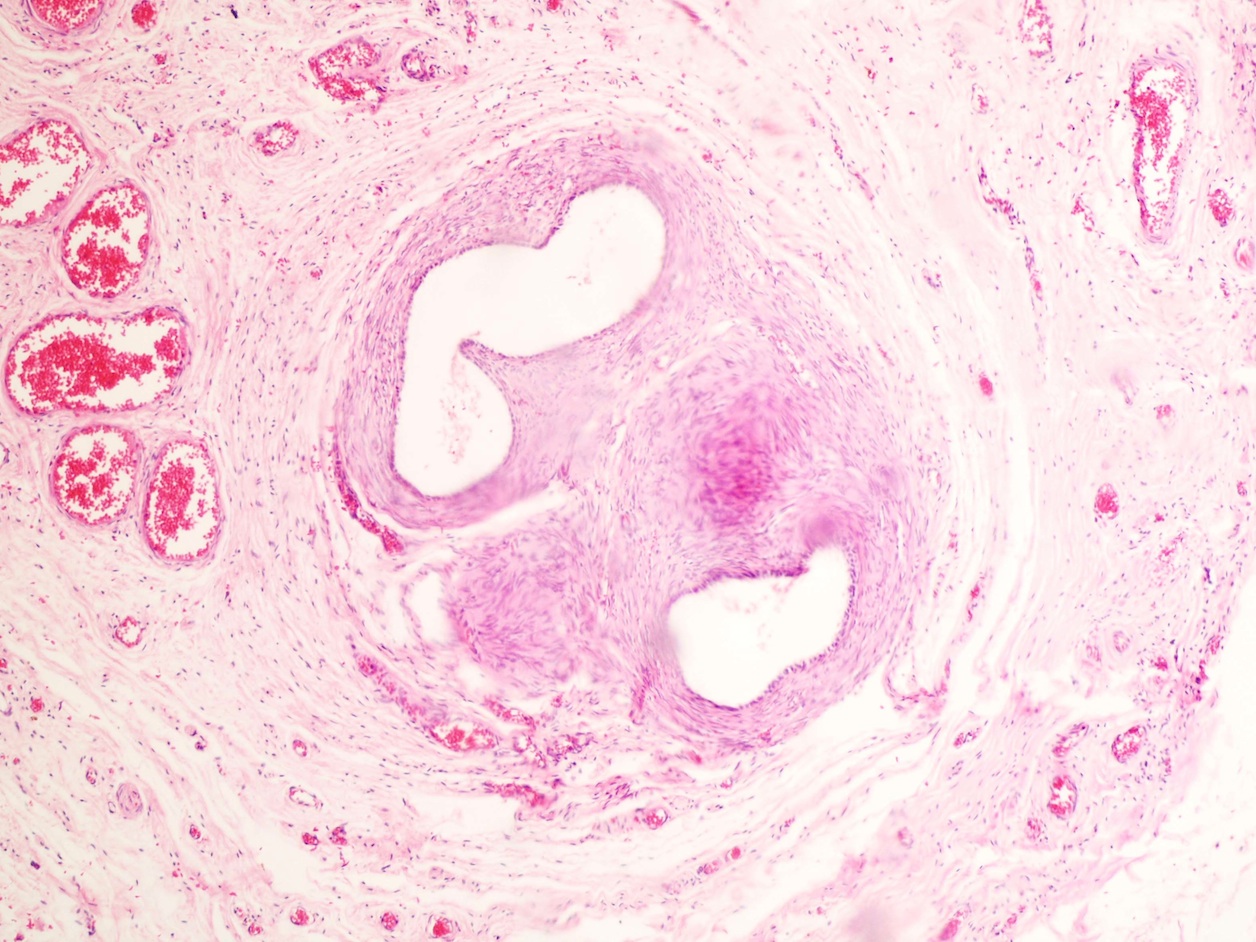
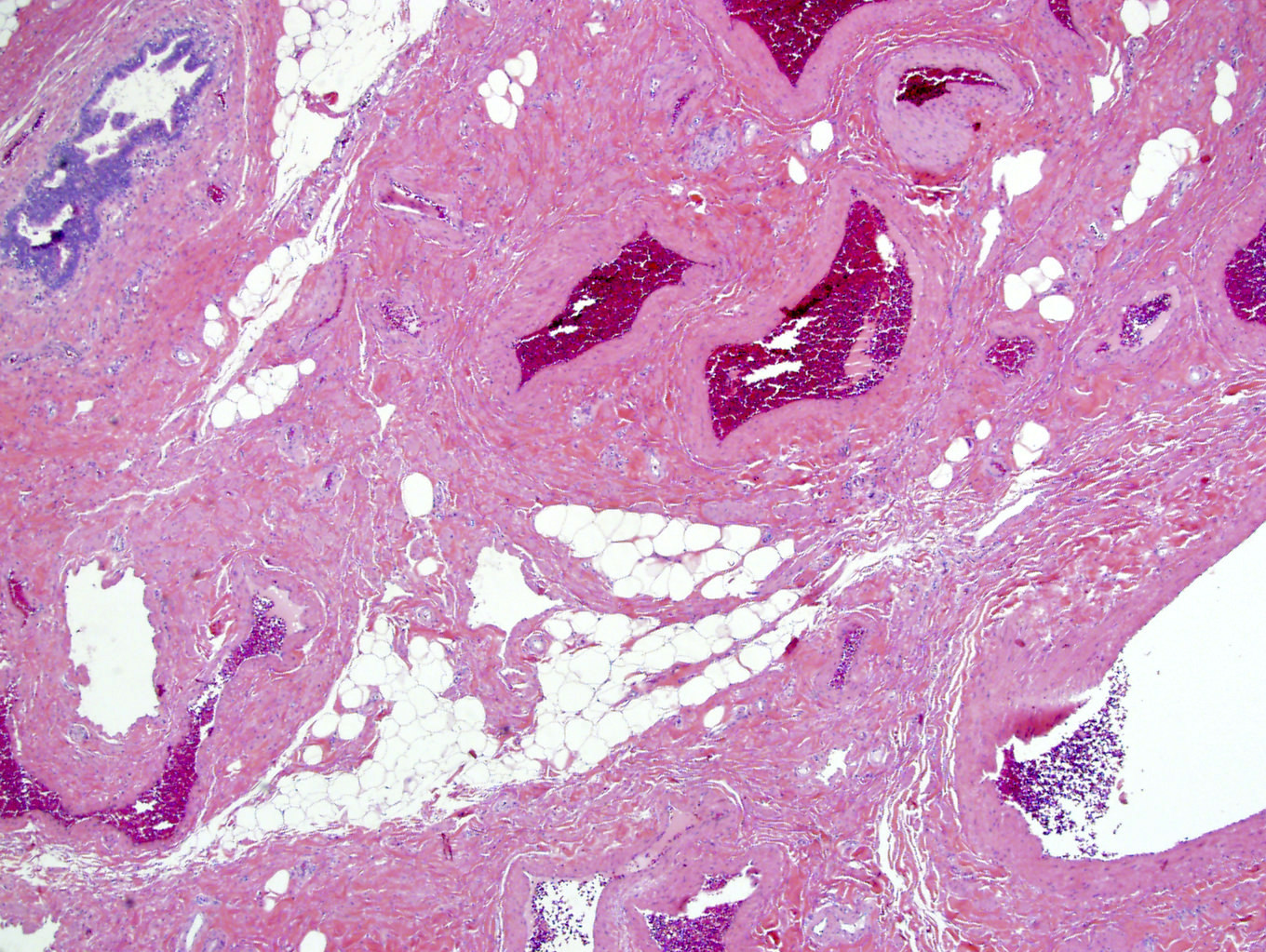
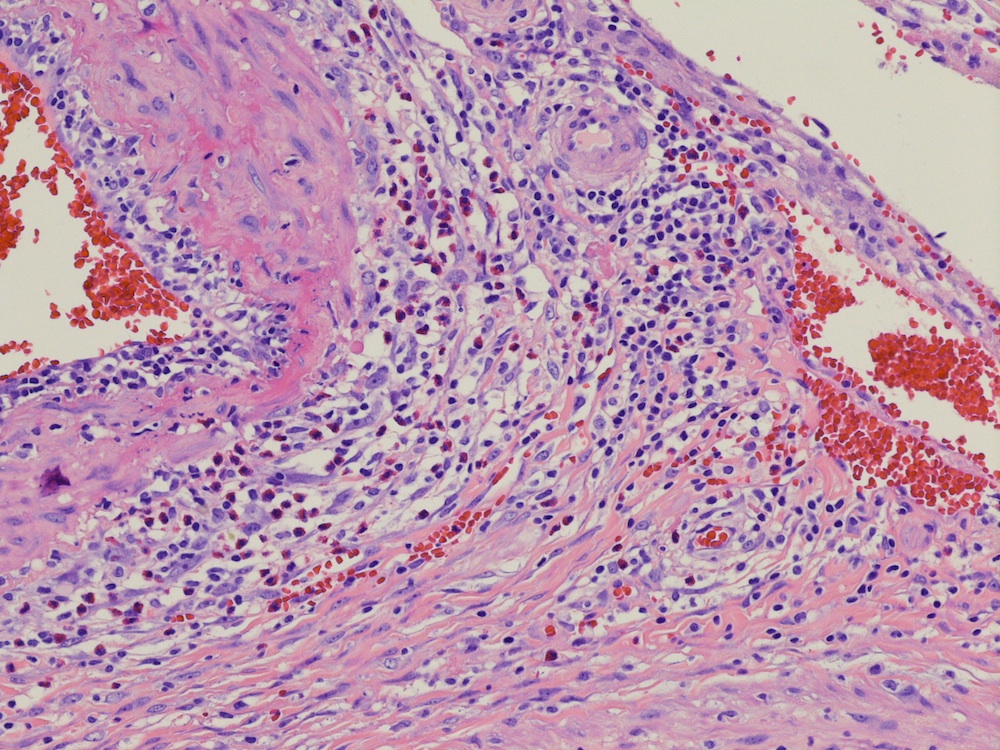
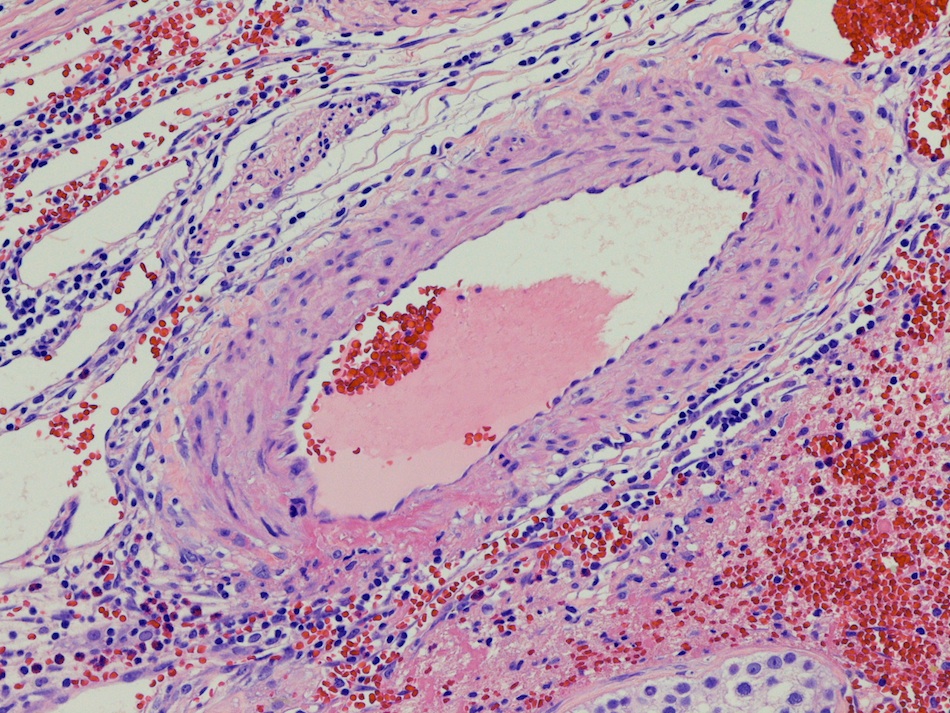
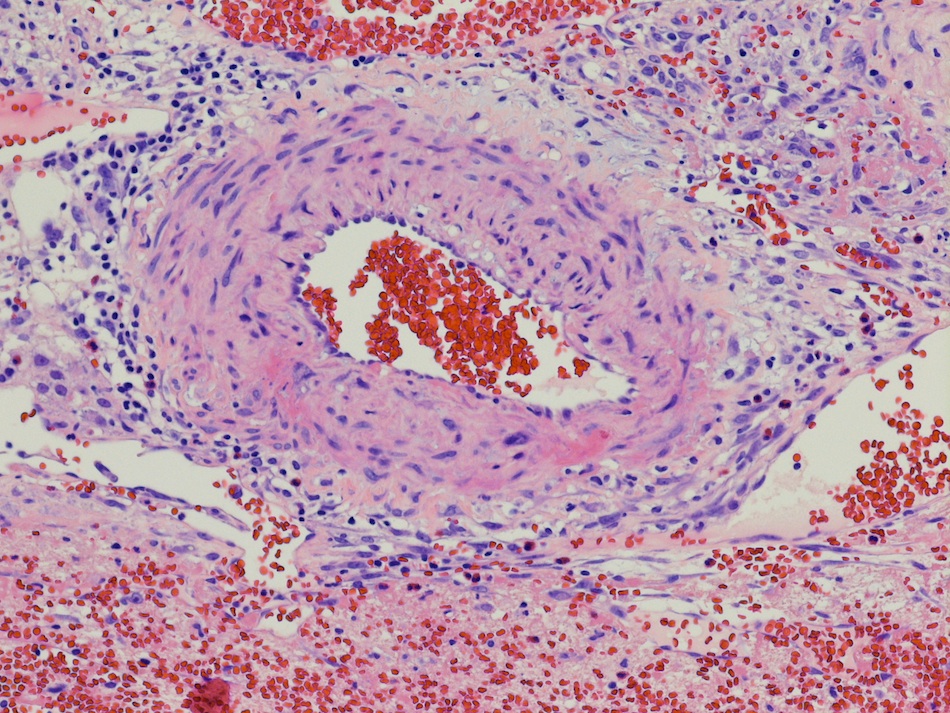
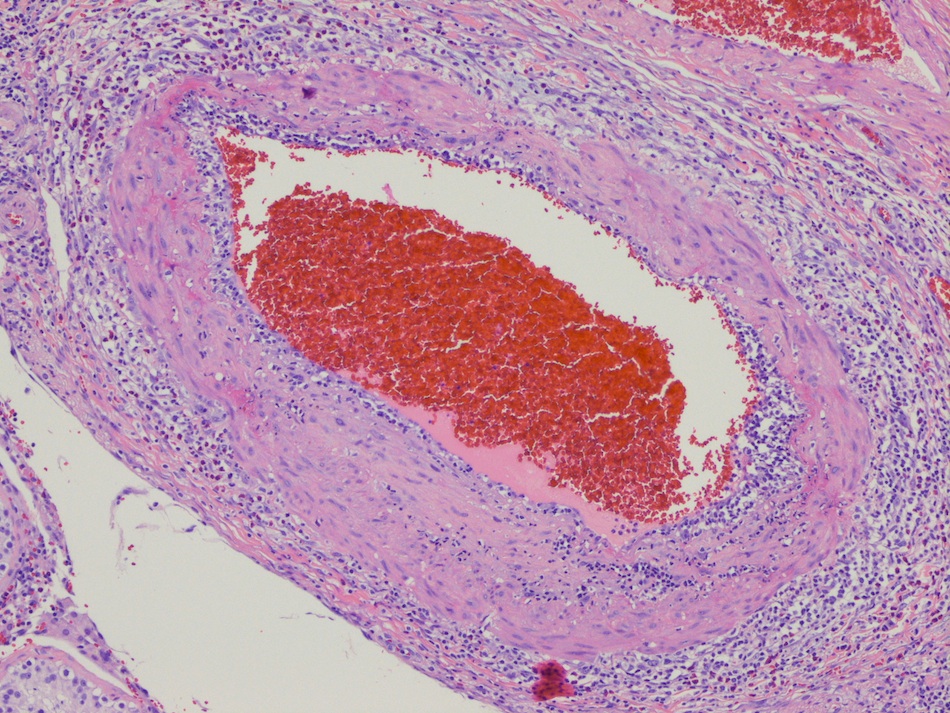
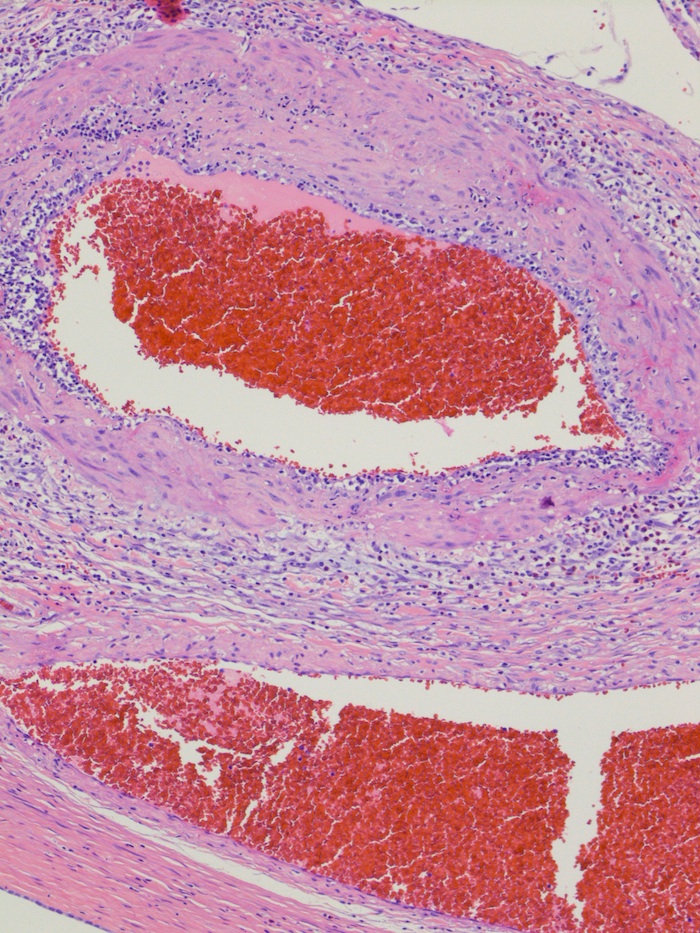
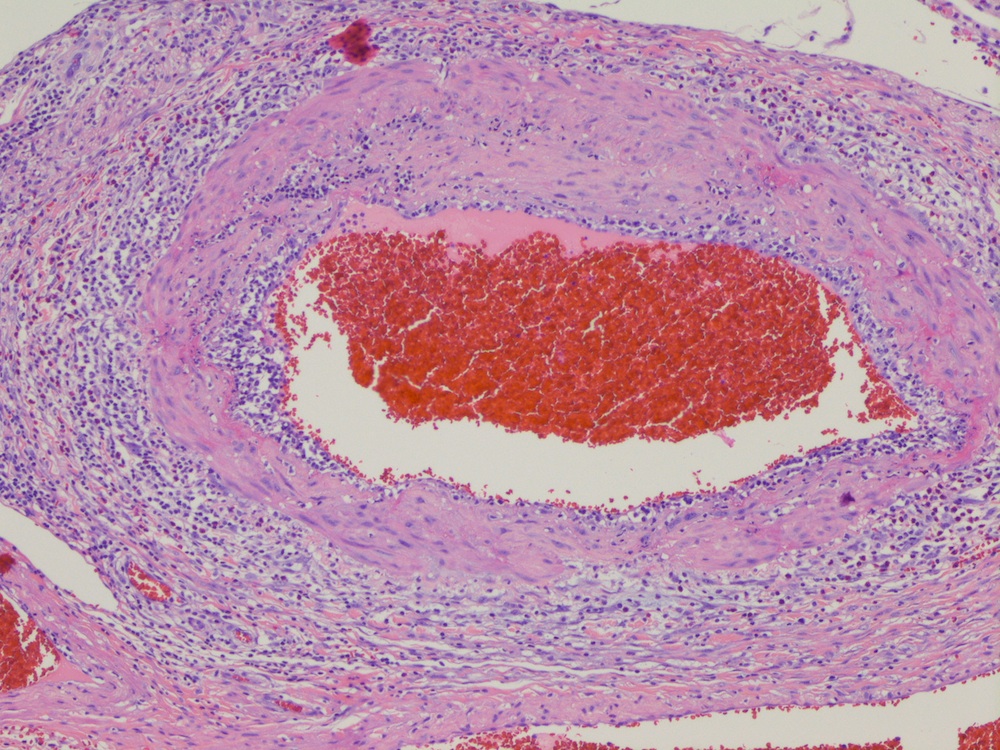
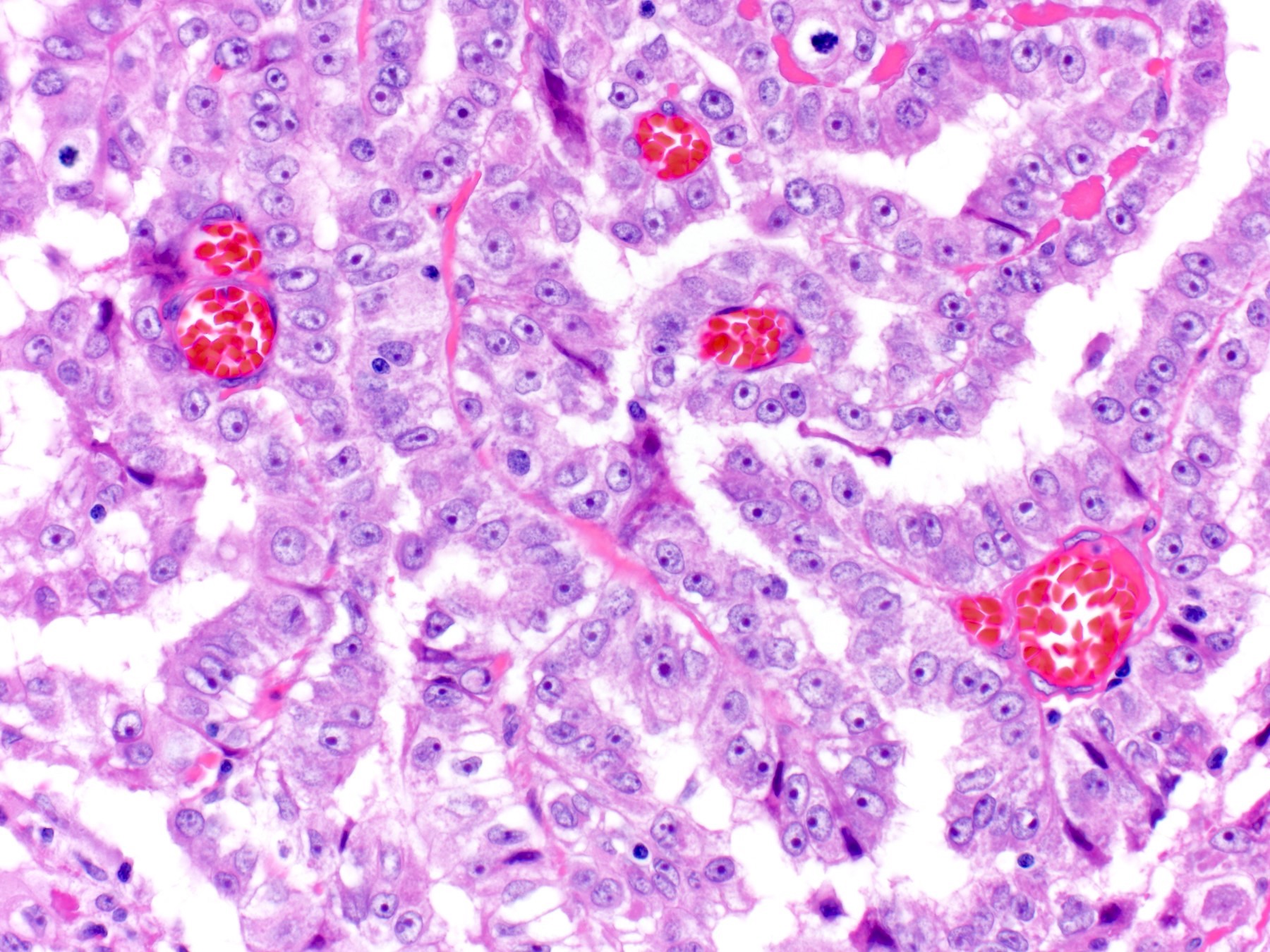
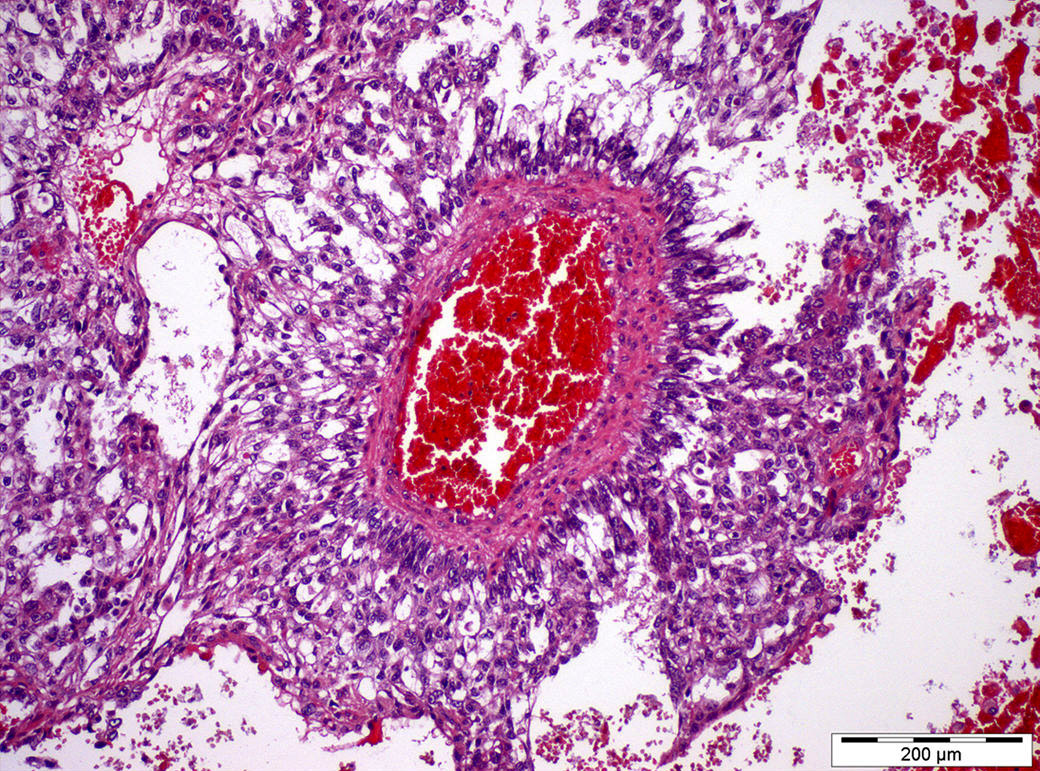
- LVI
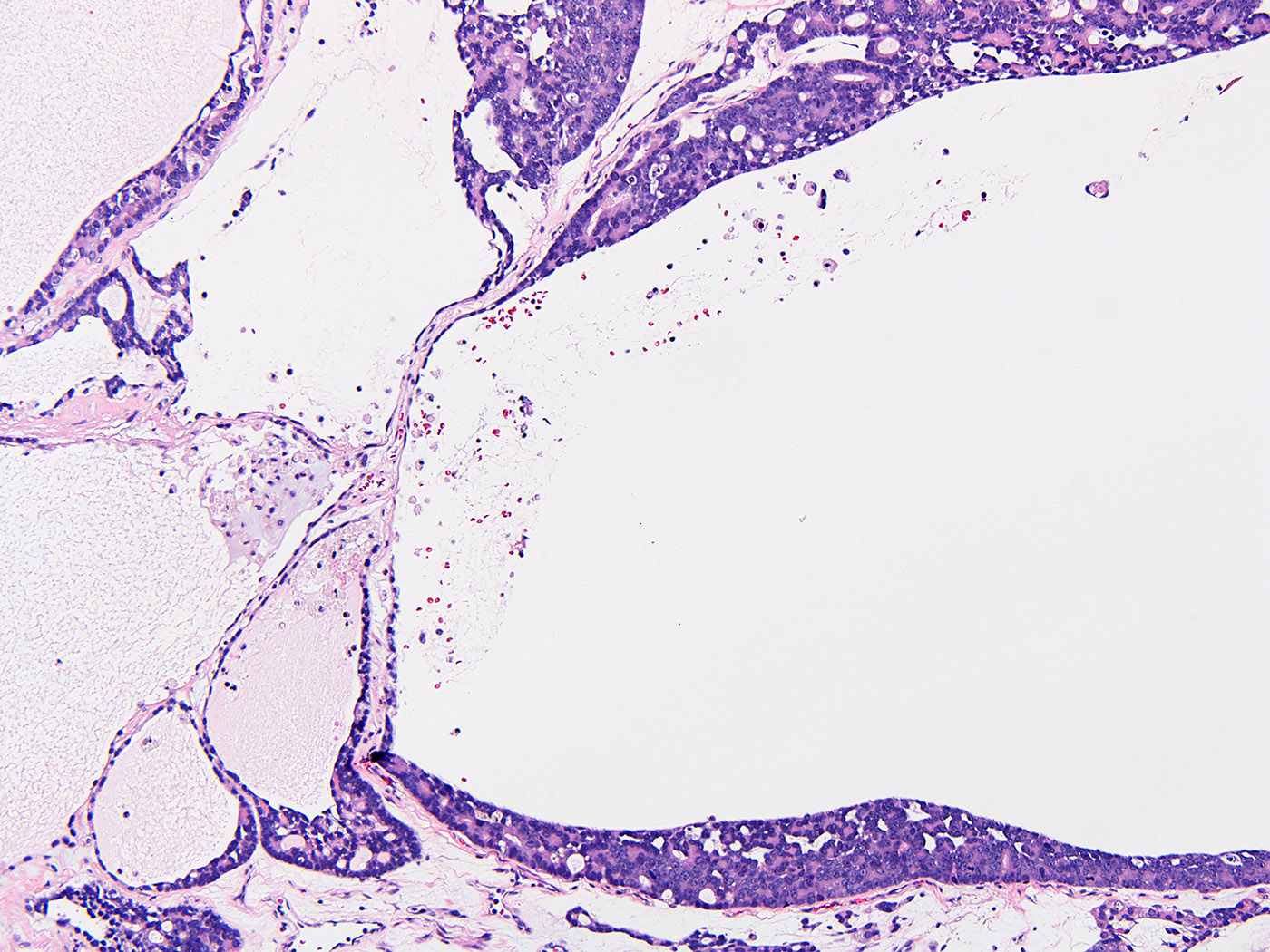
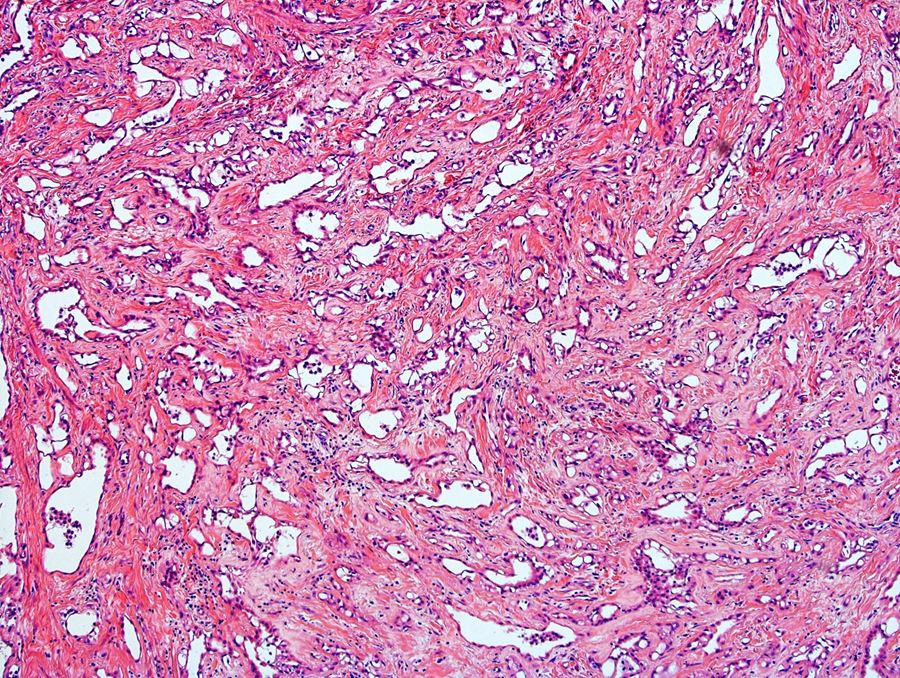
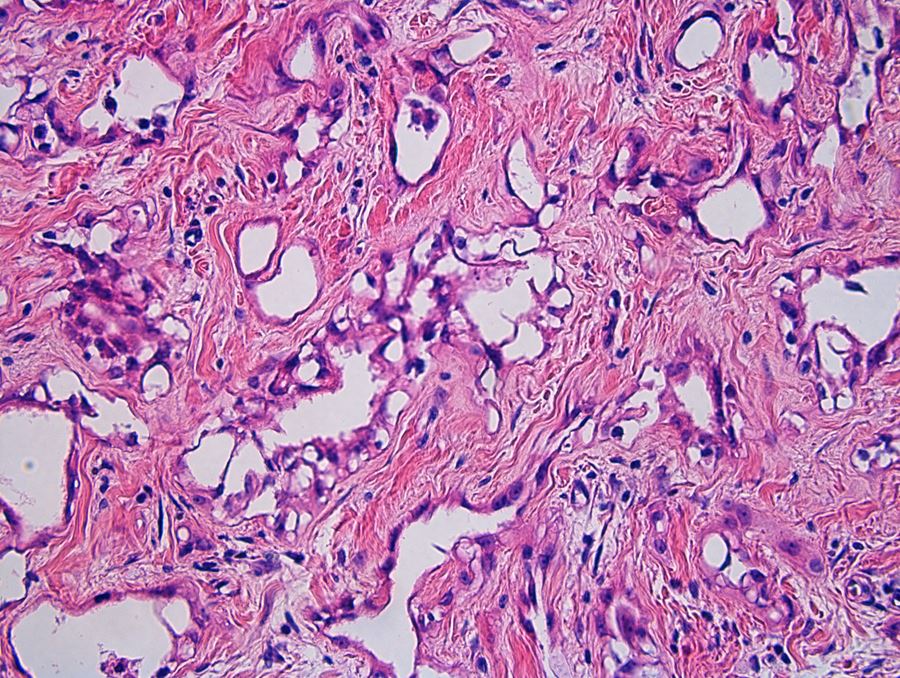
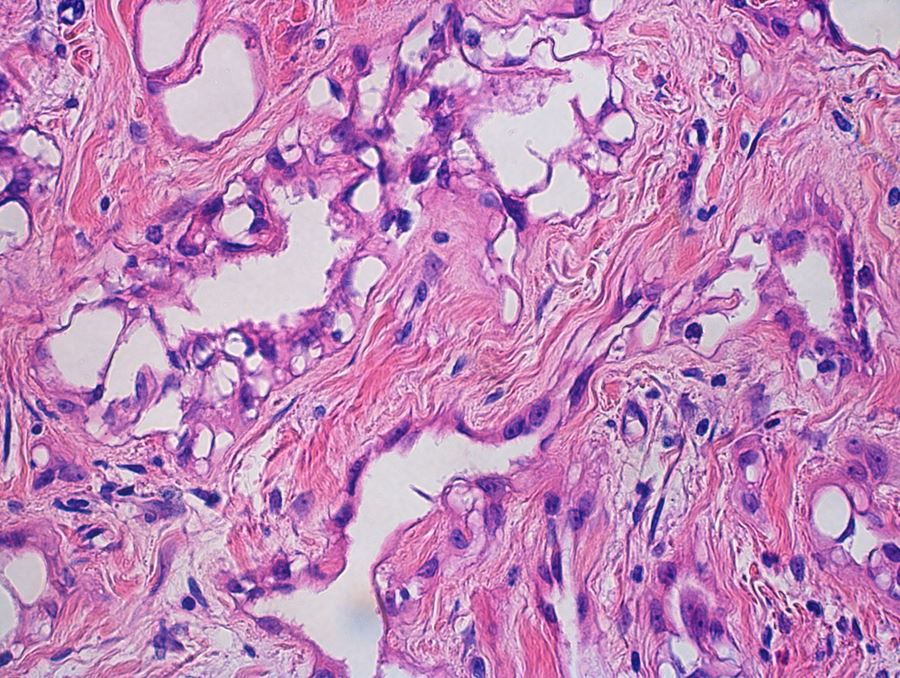
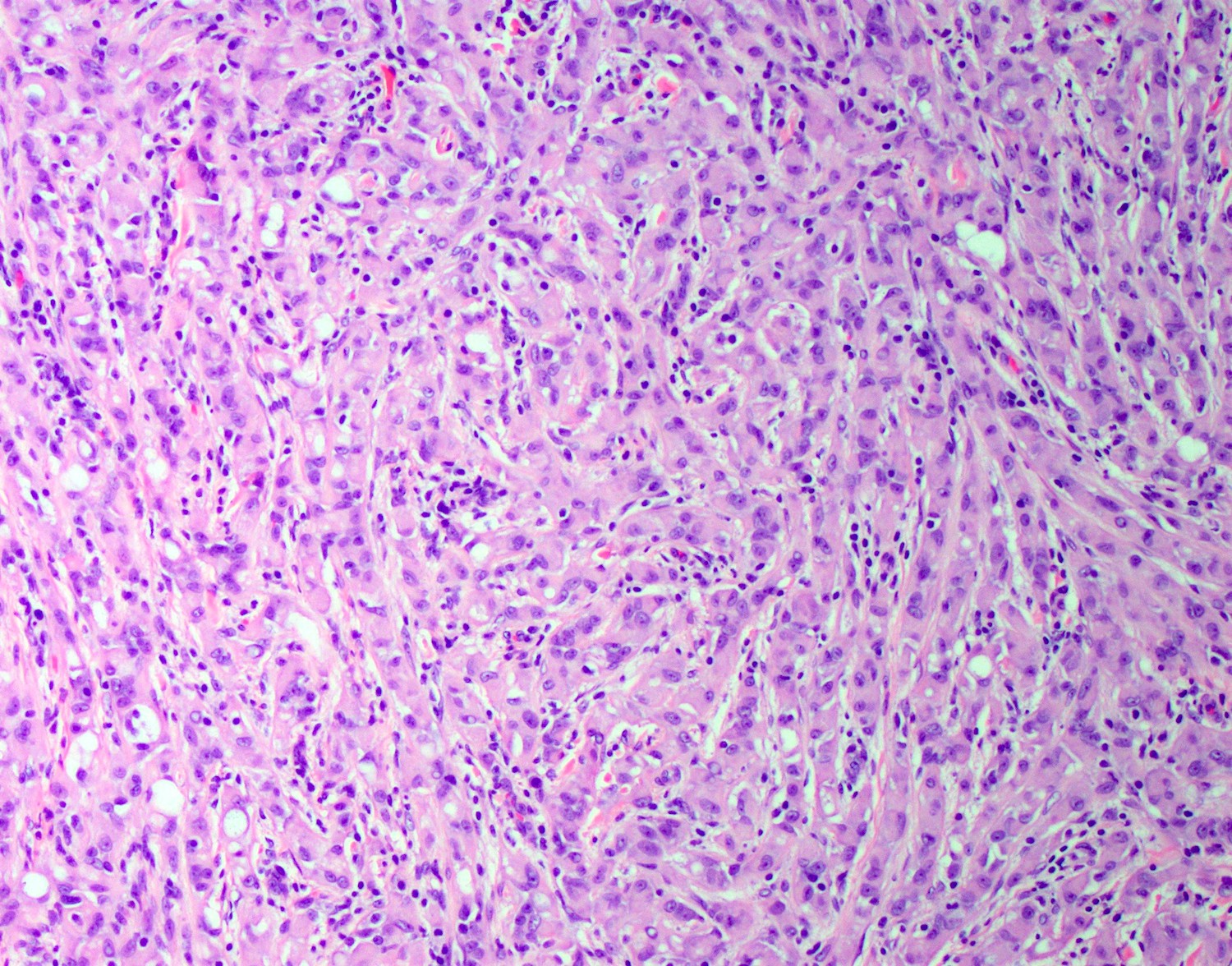
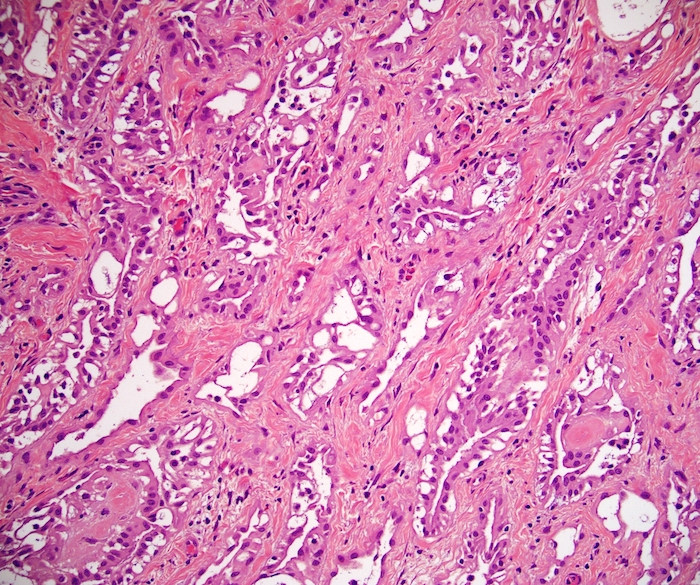
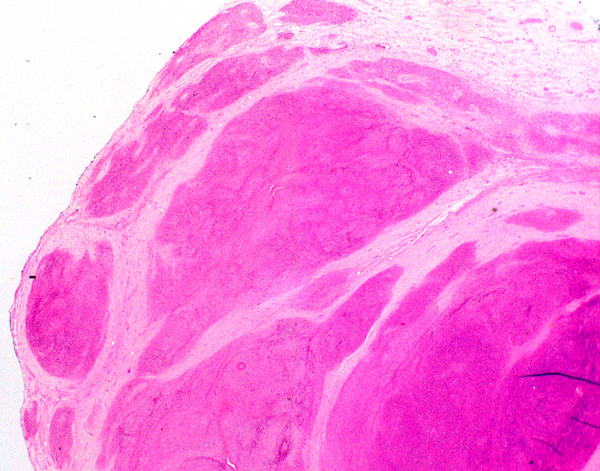
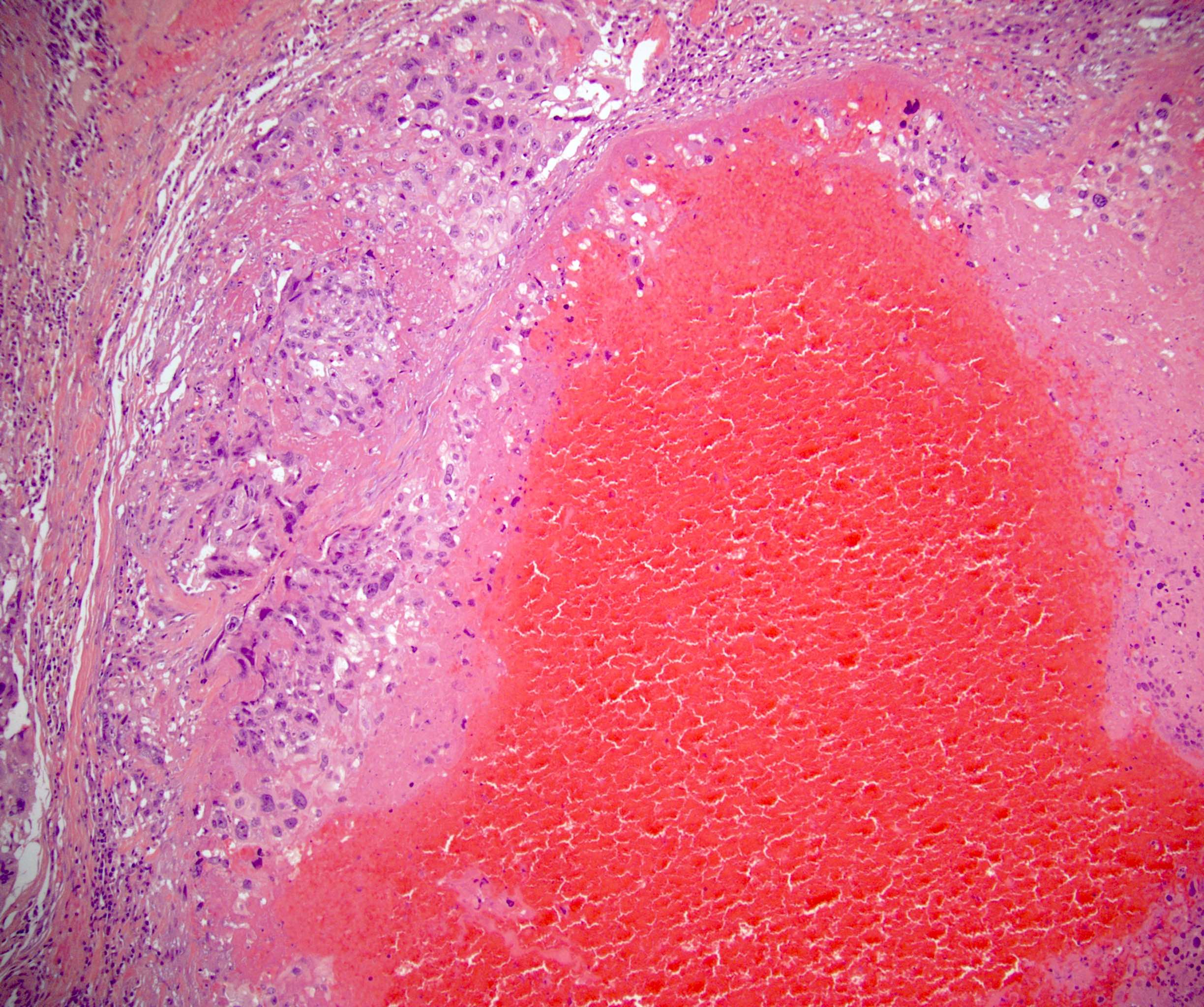
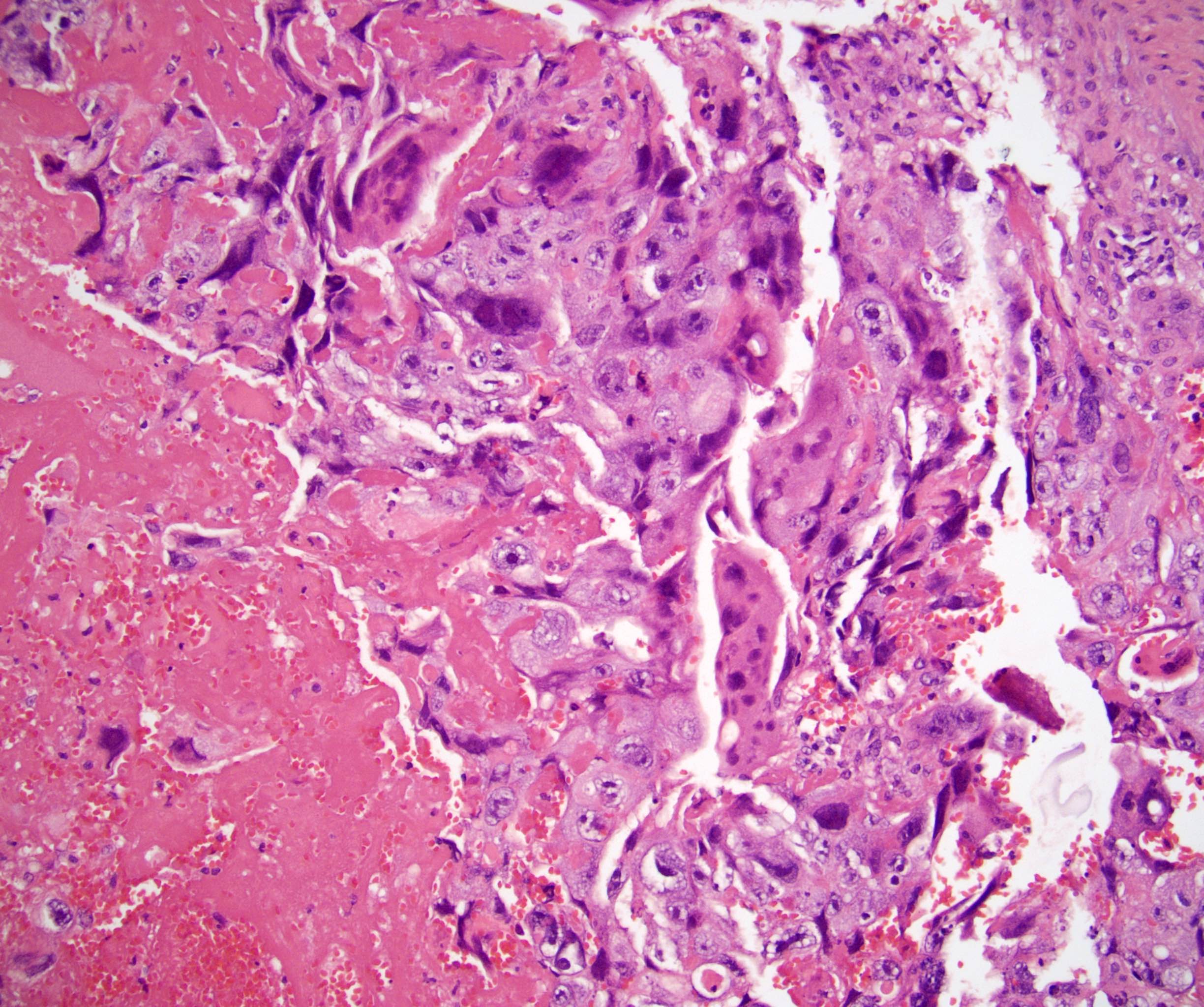
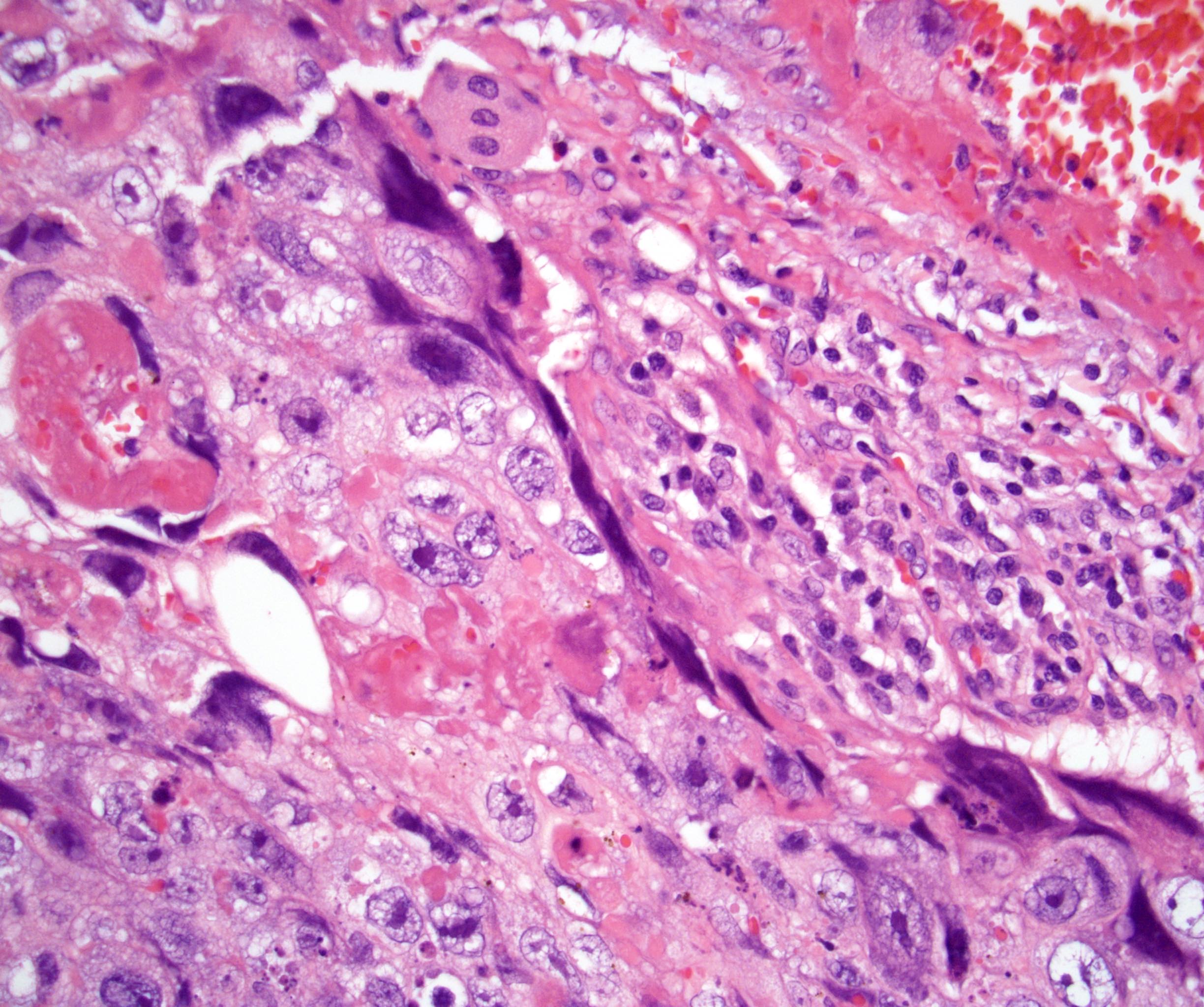
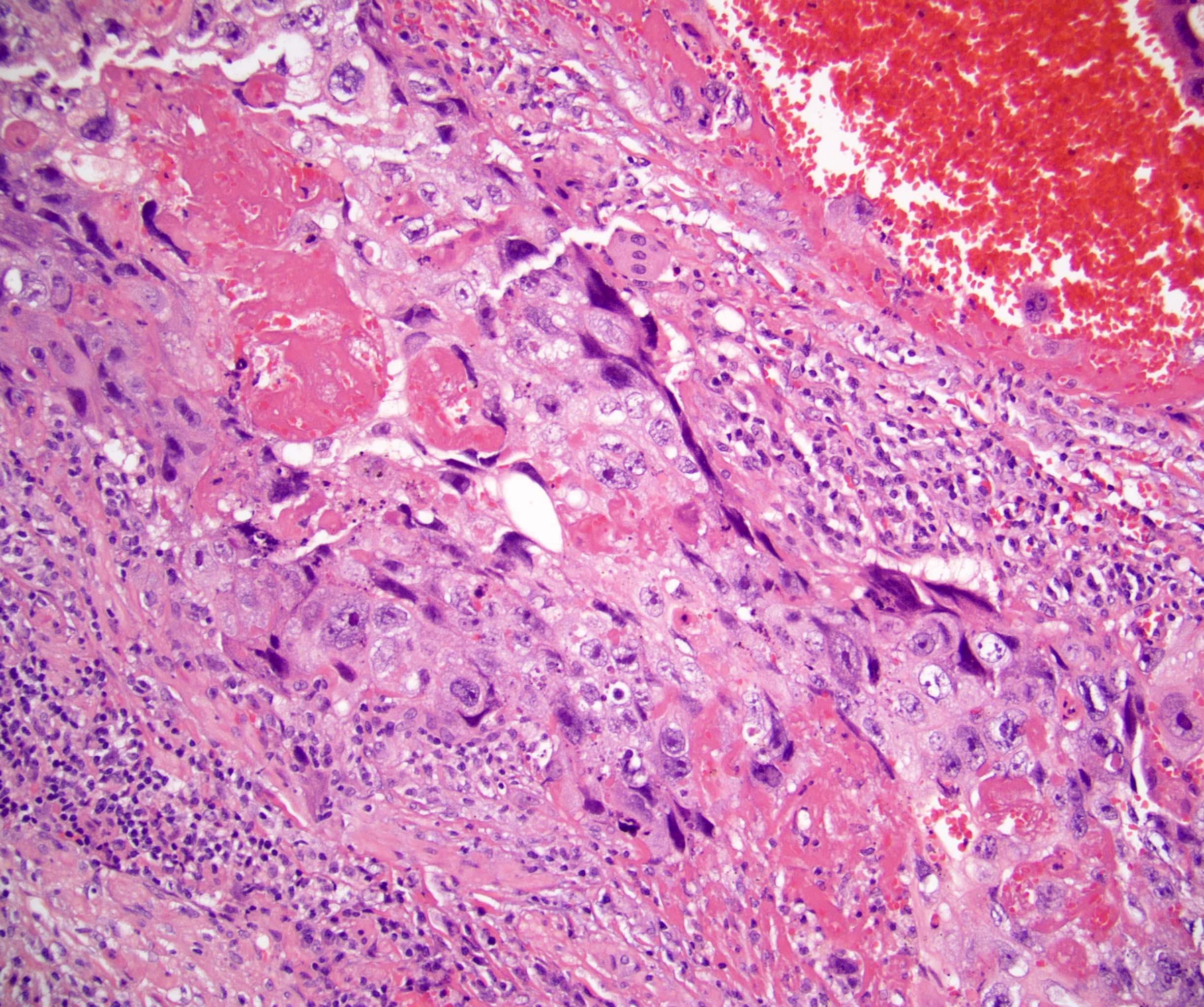
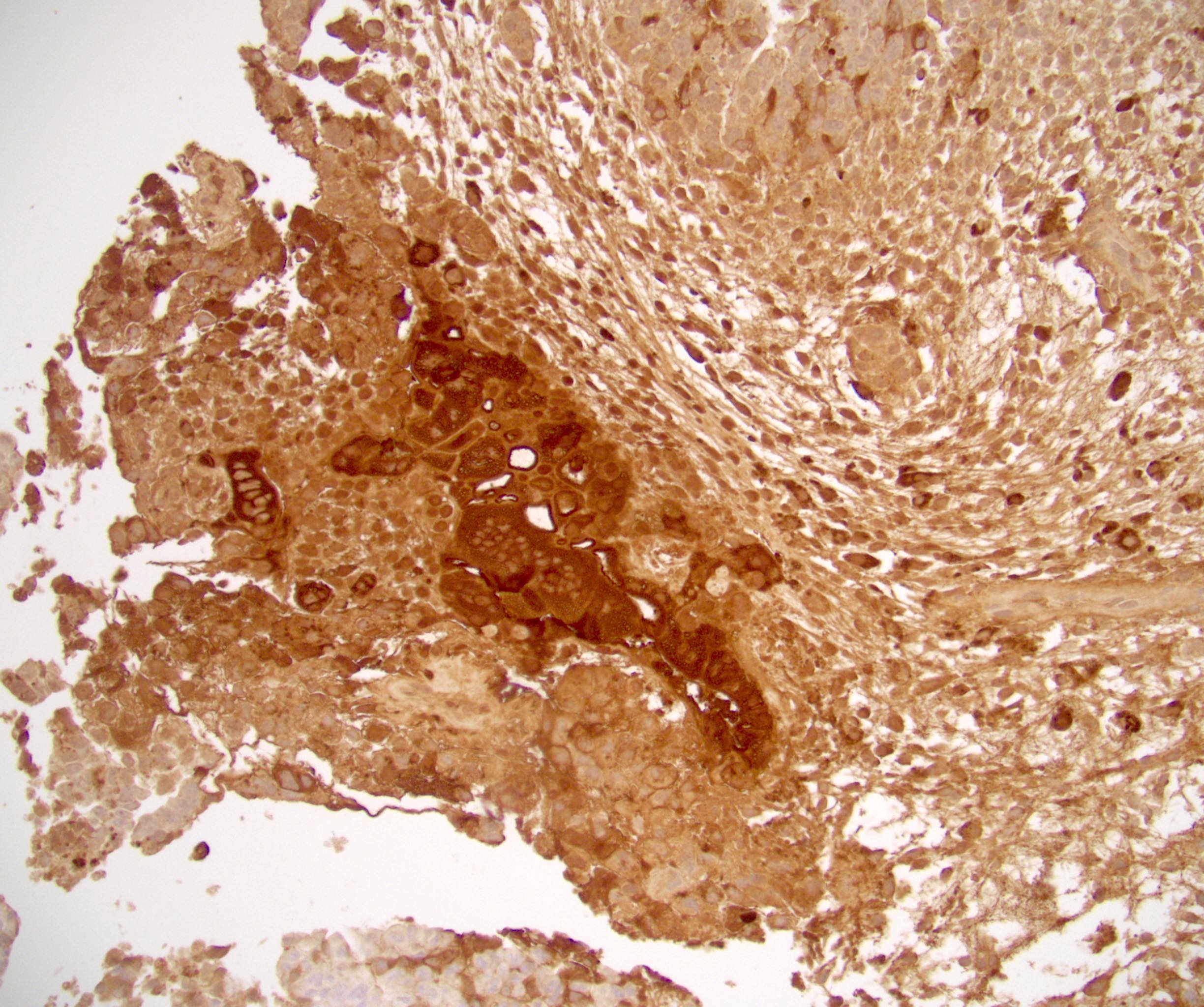
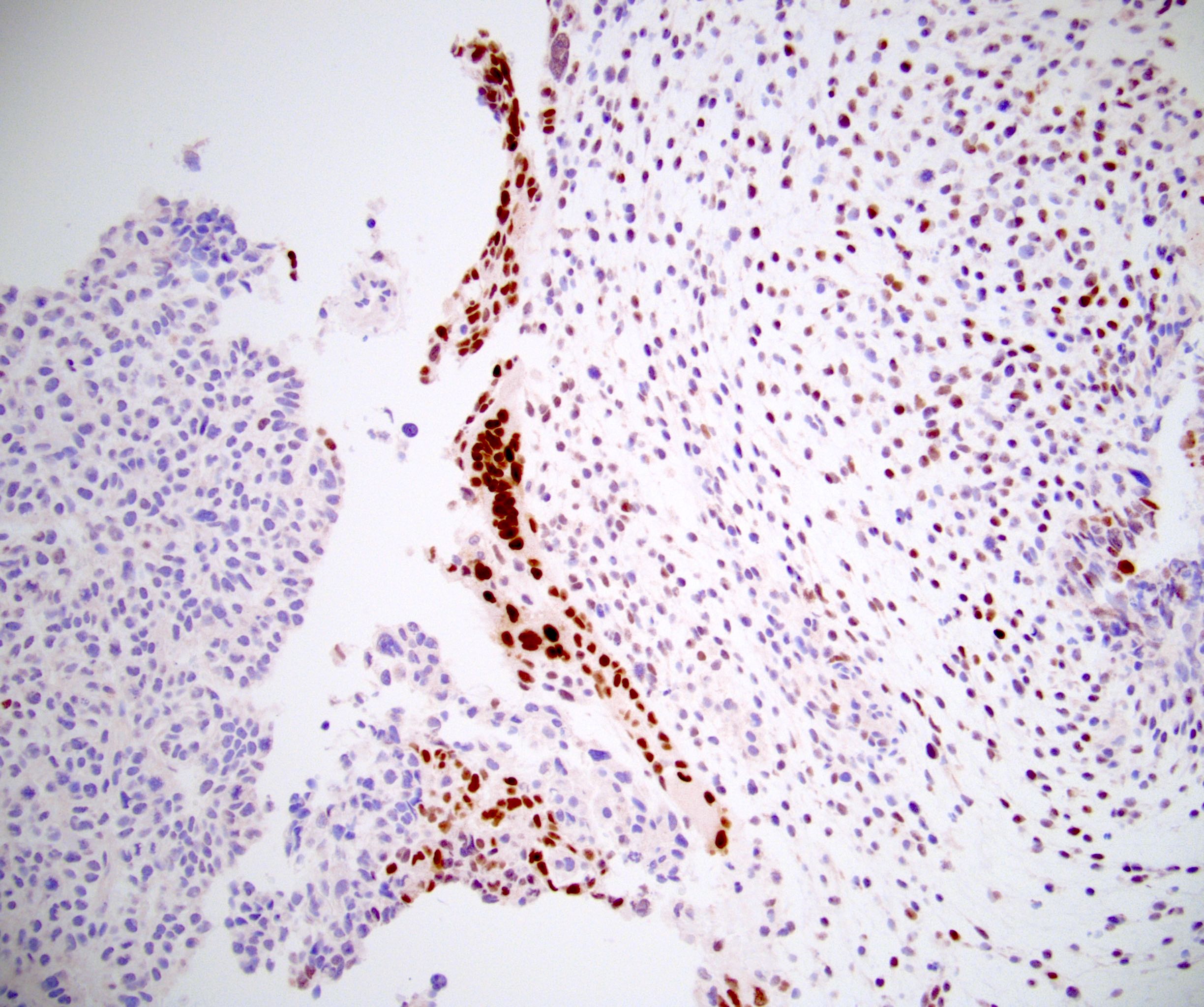
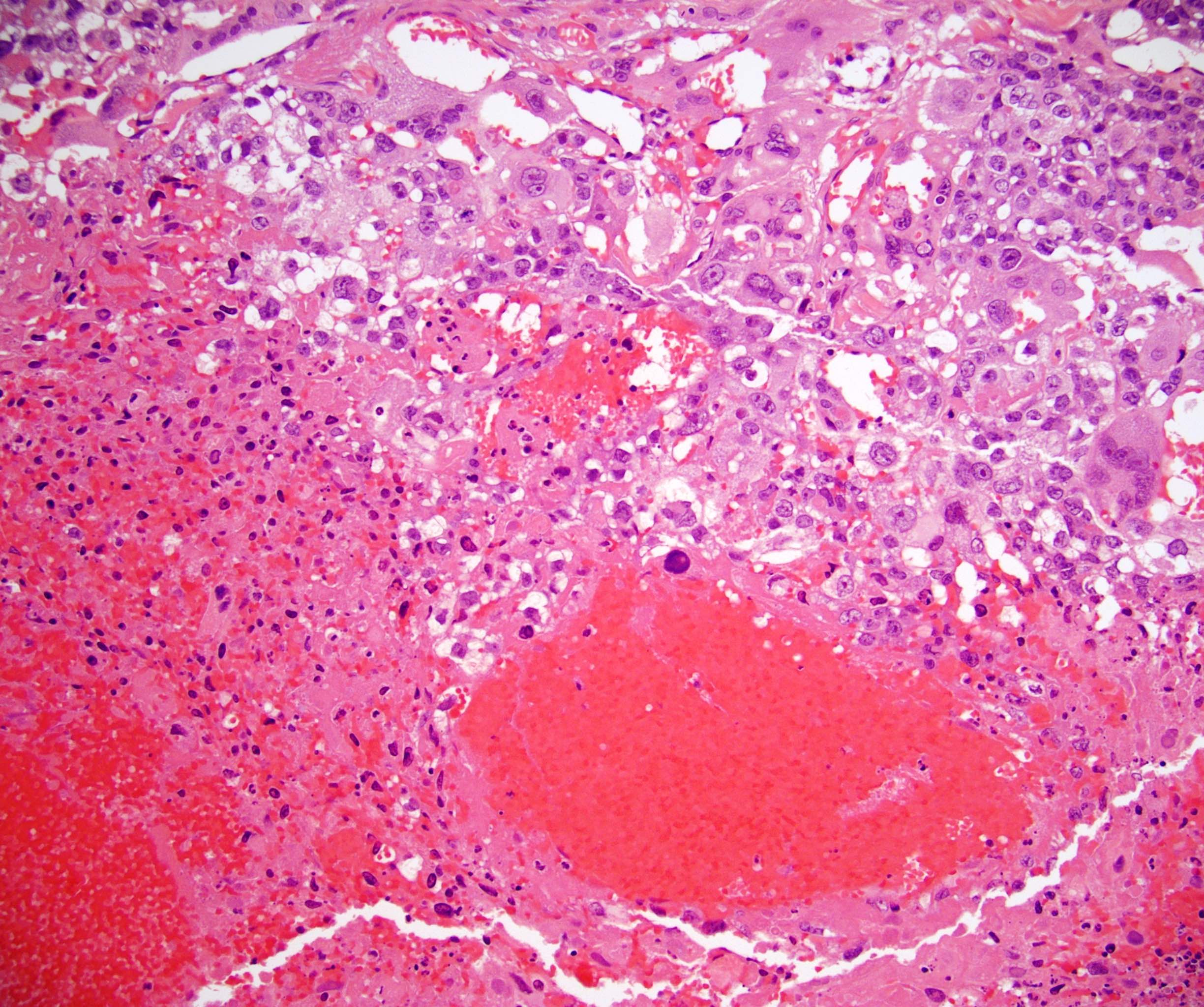
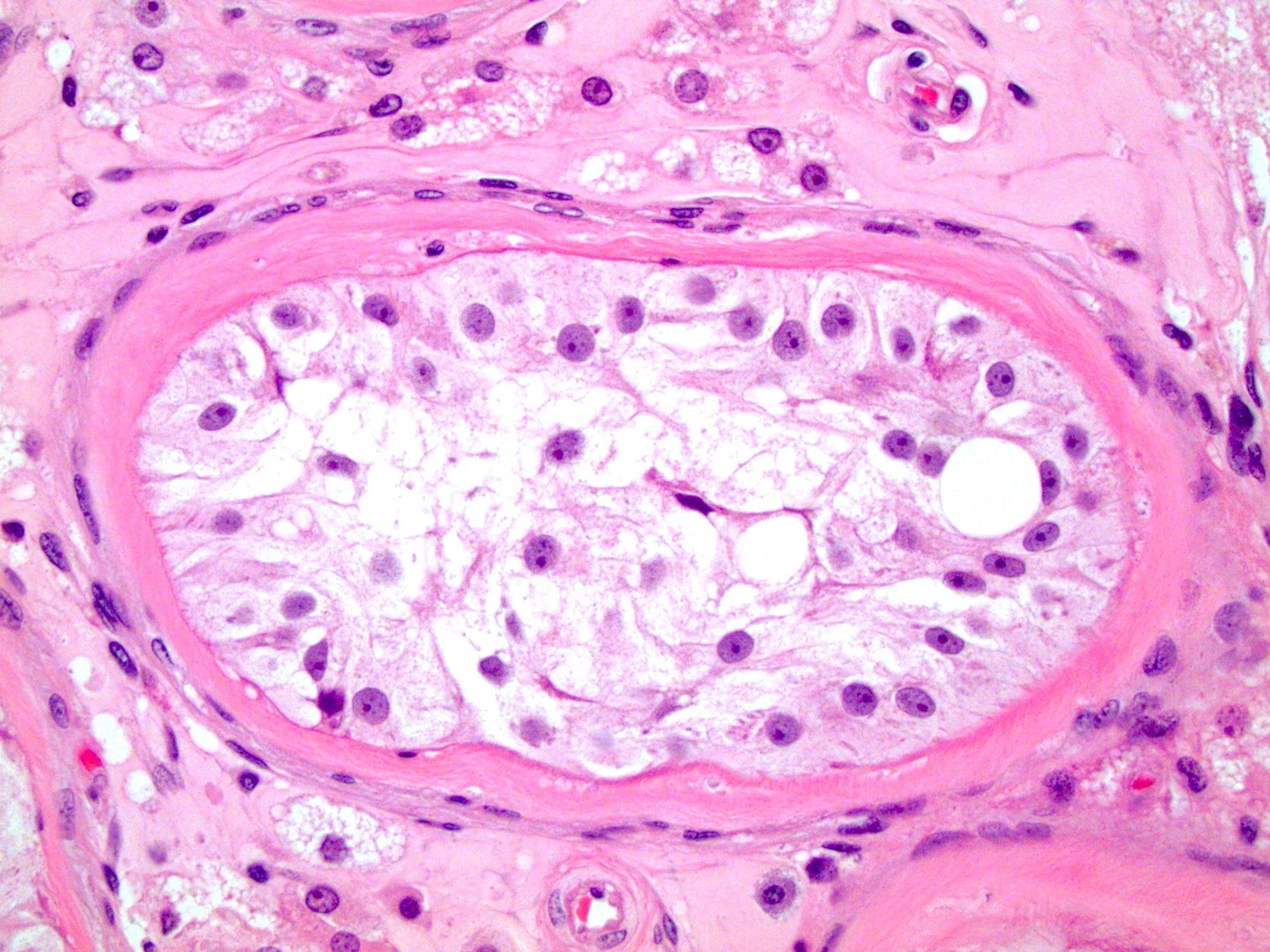
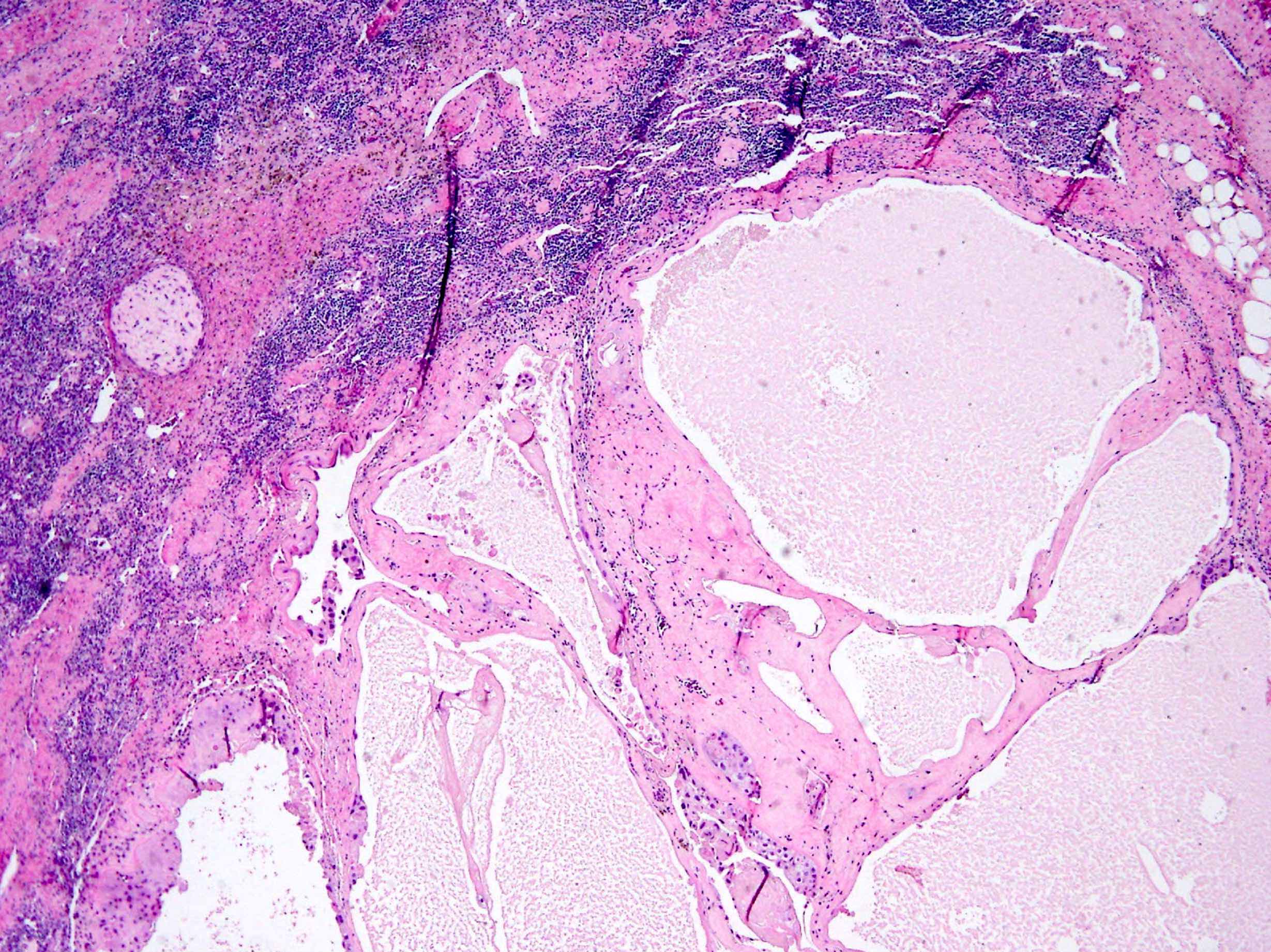
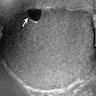
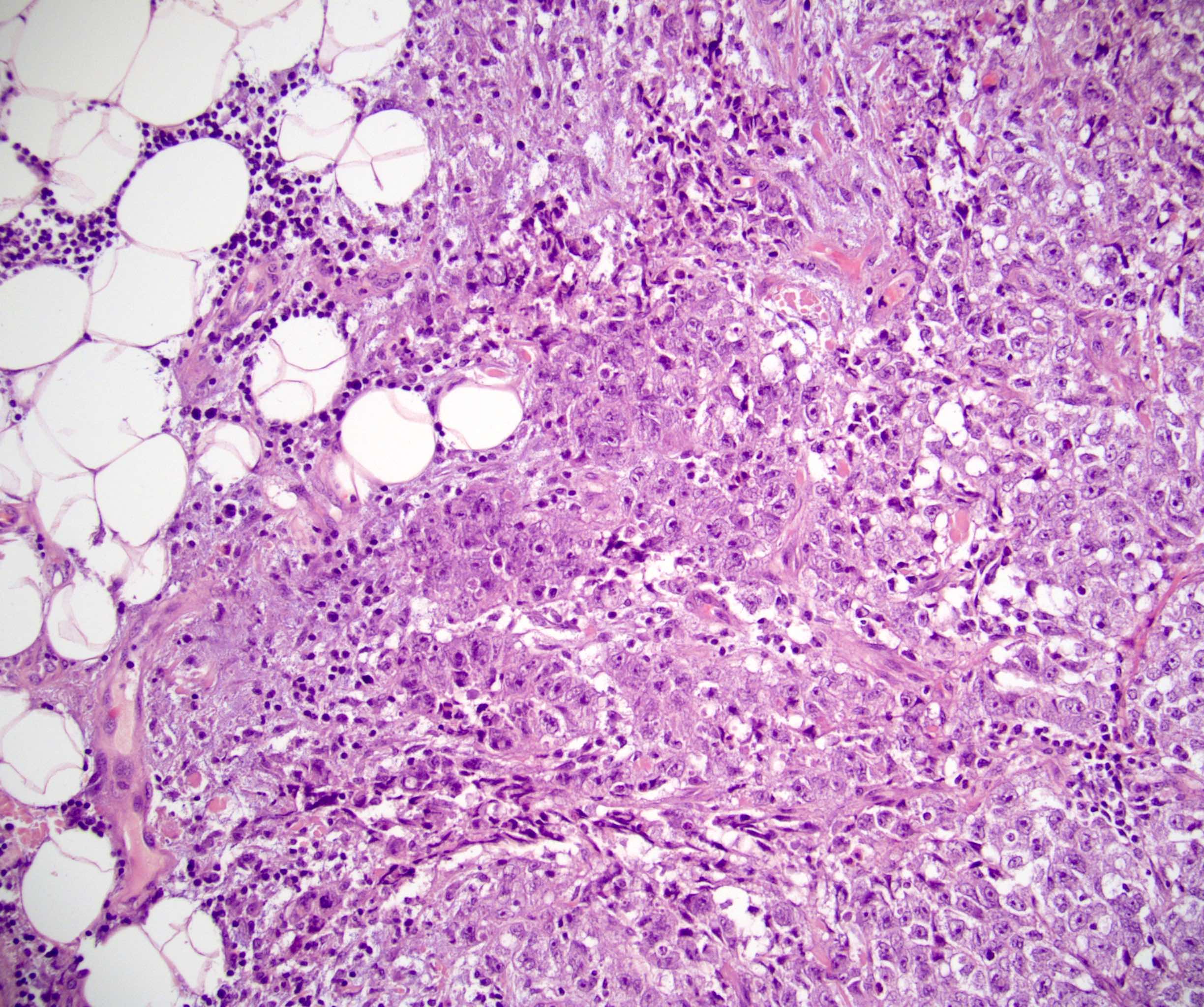
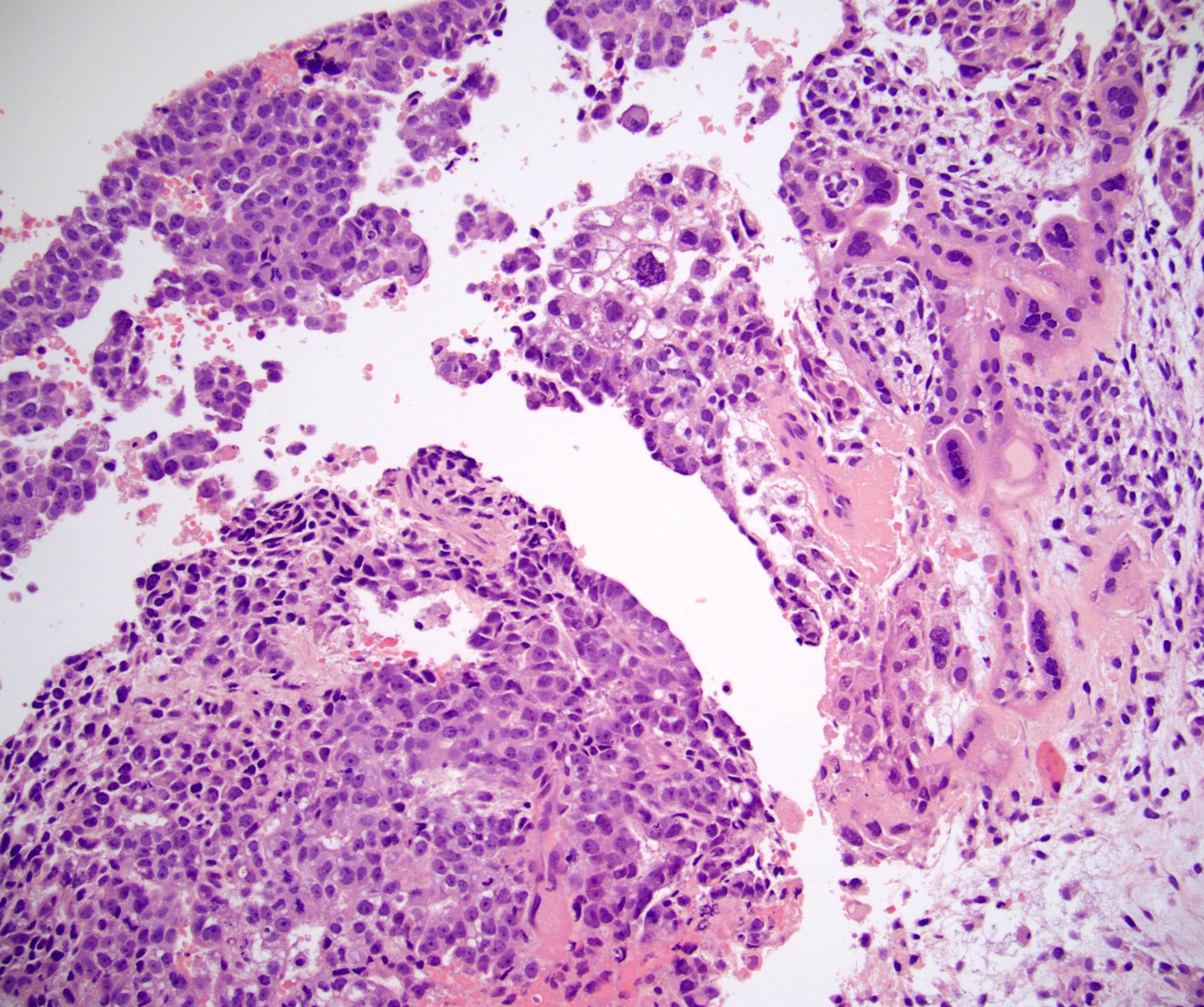
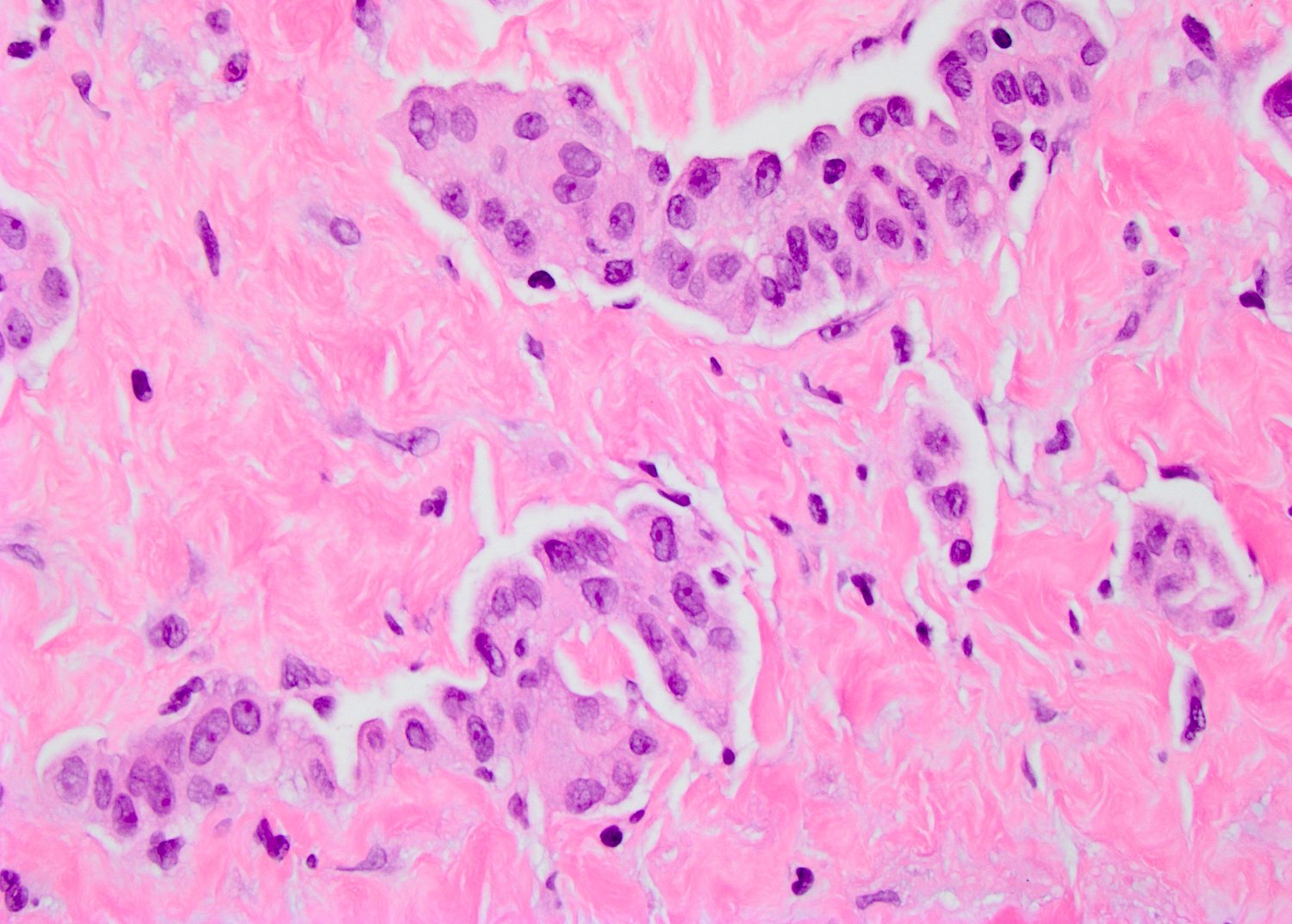
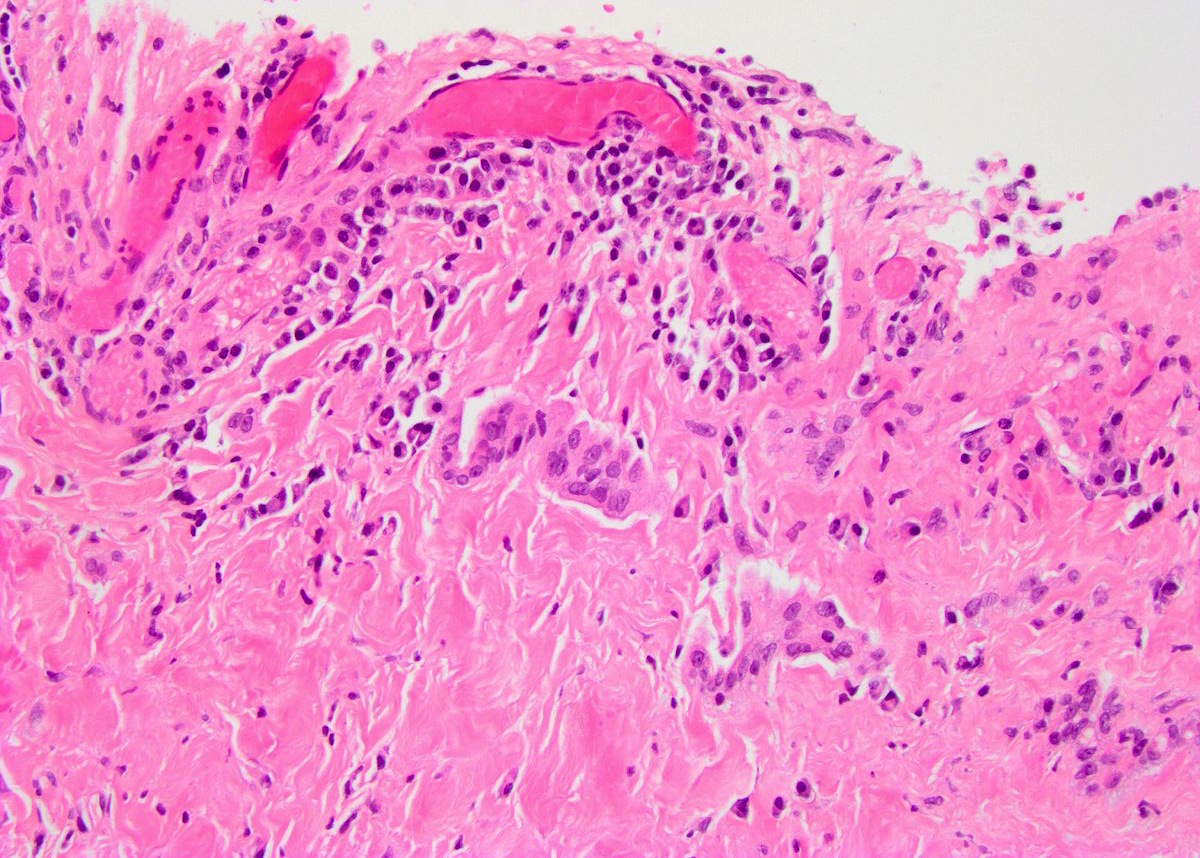
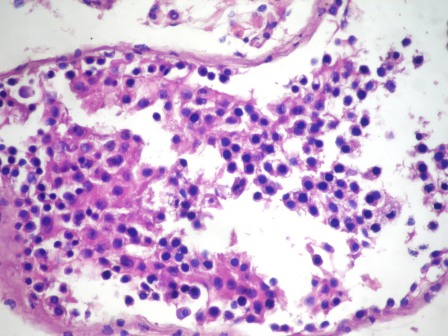
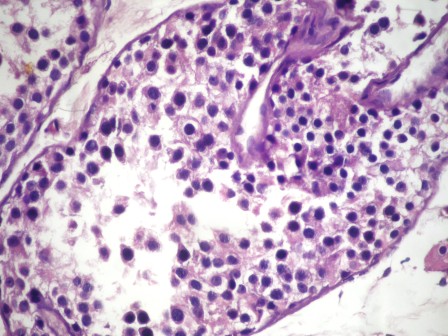
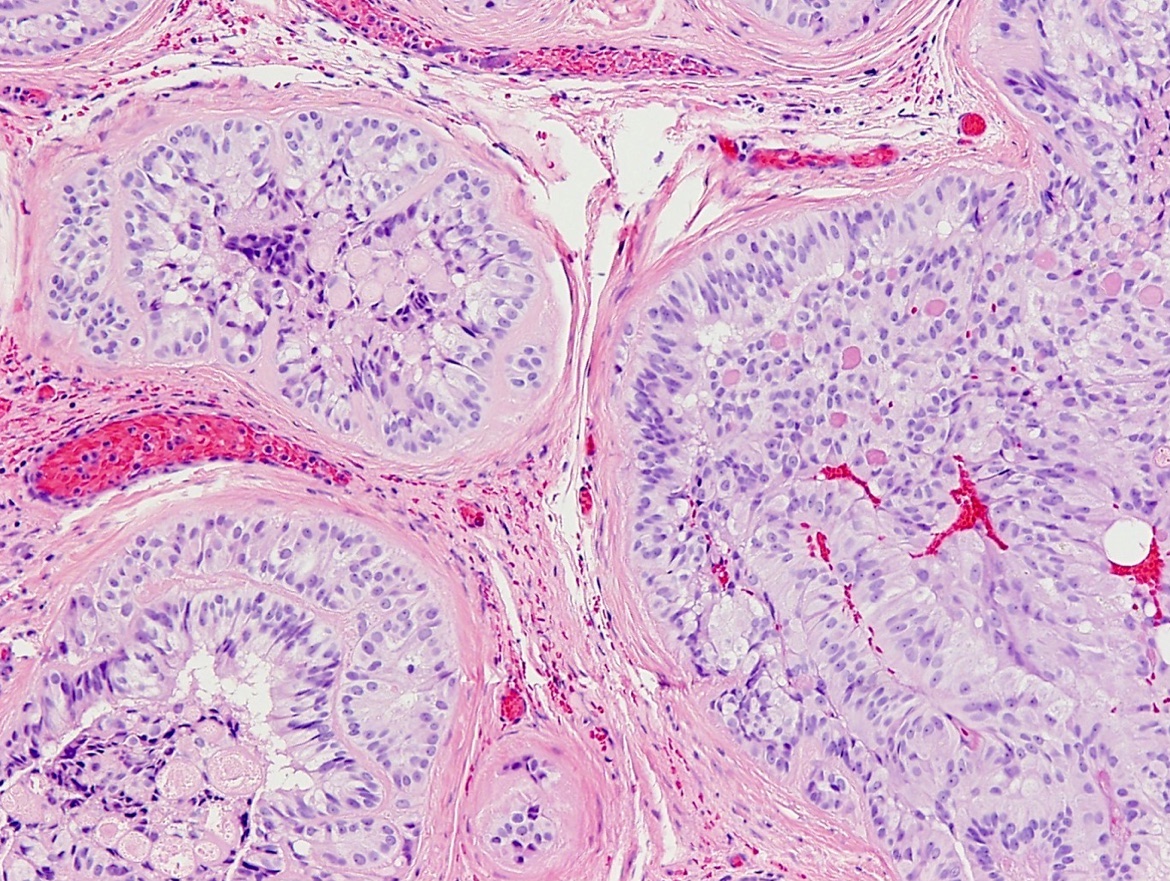
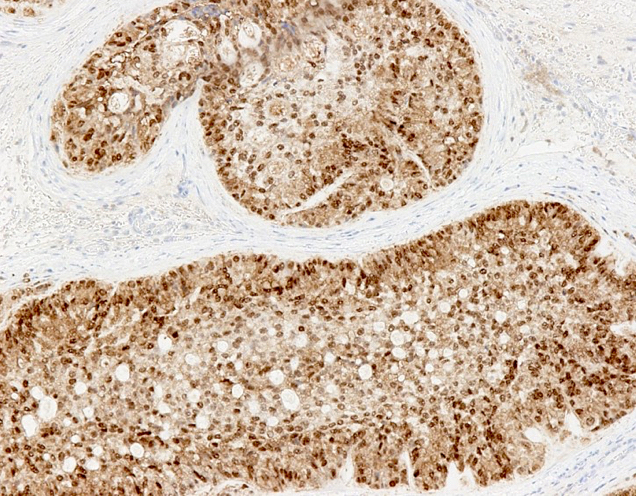
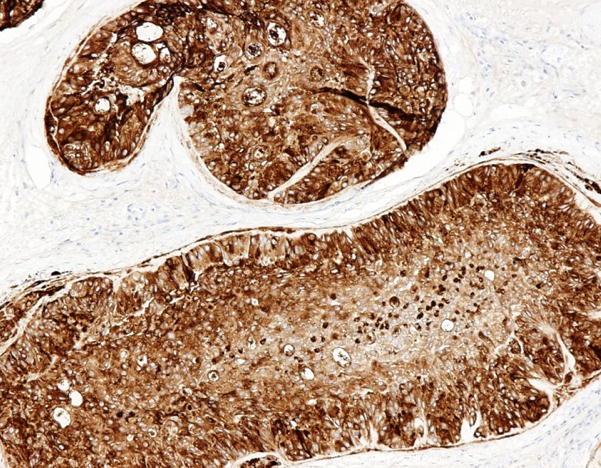
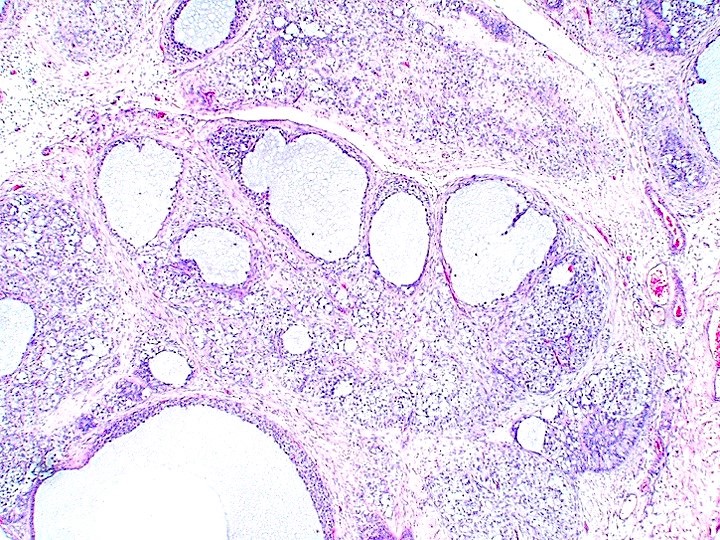
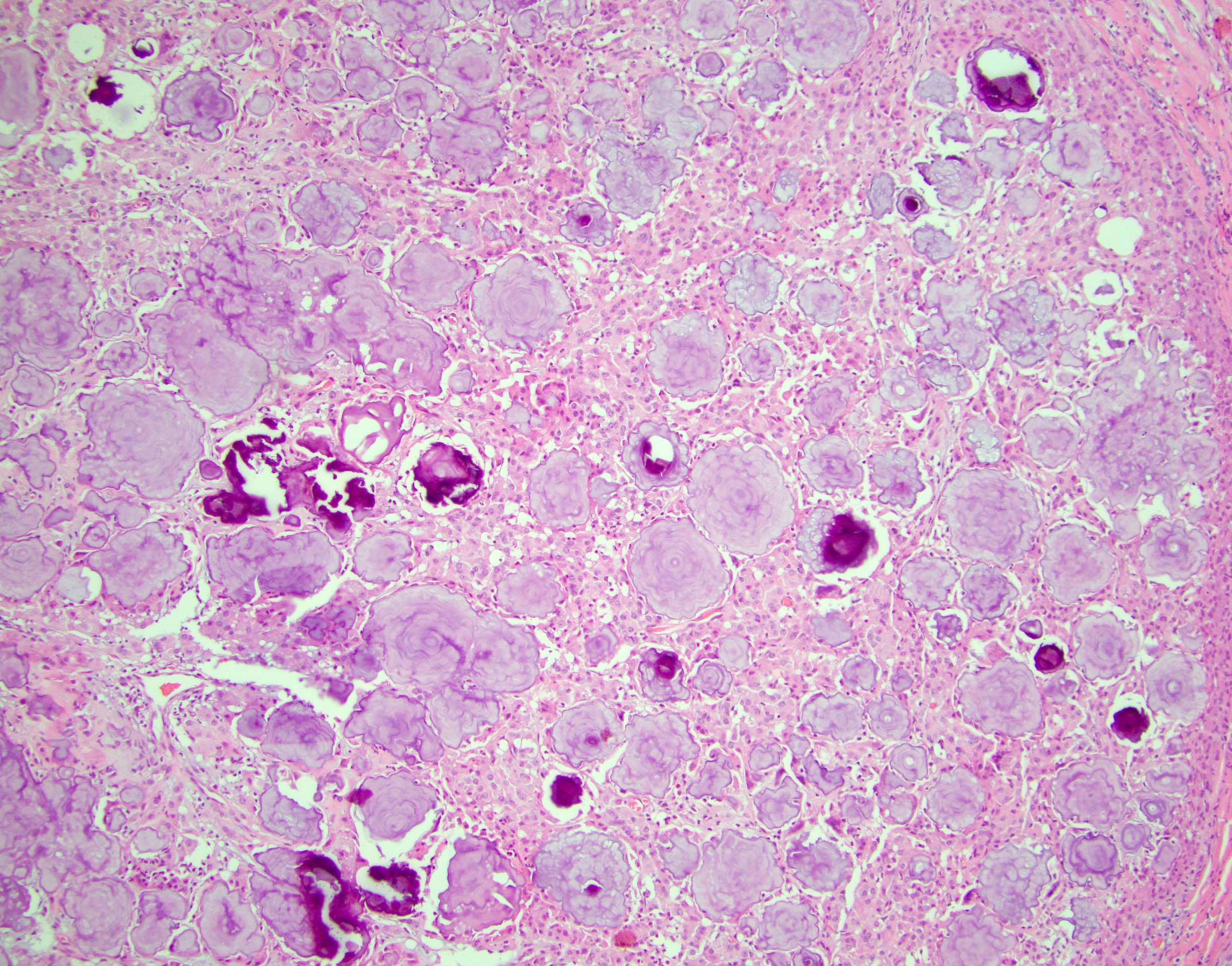
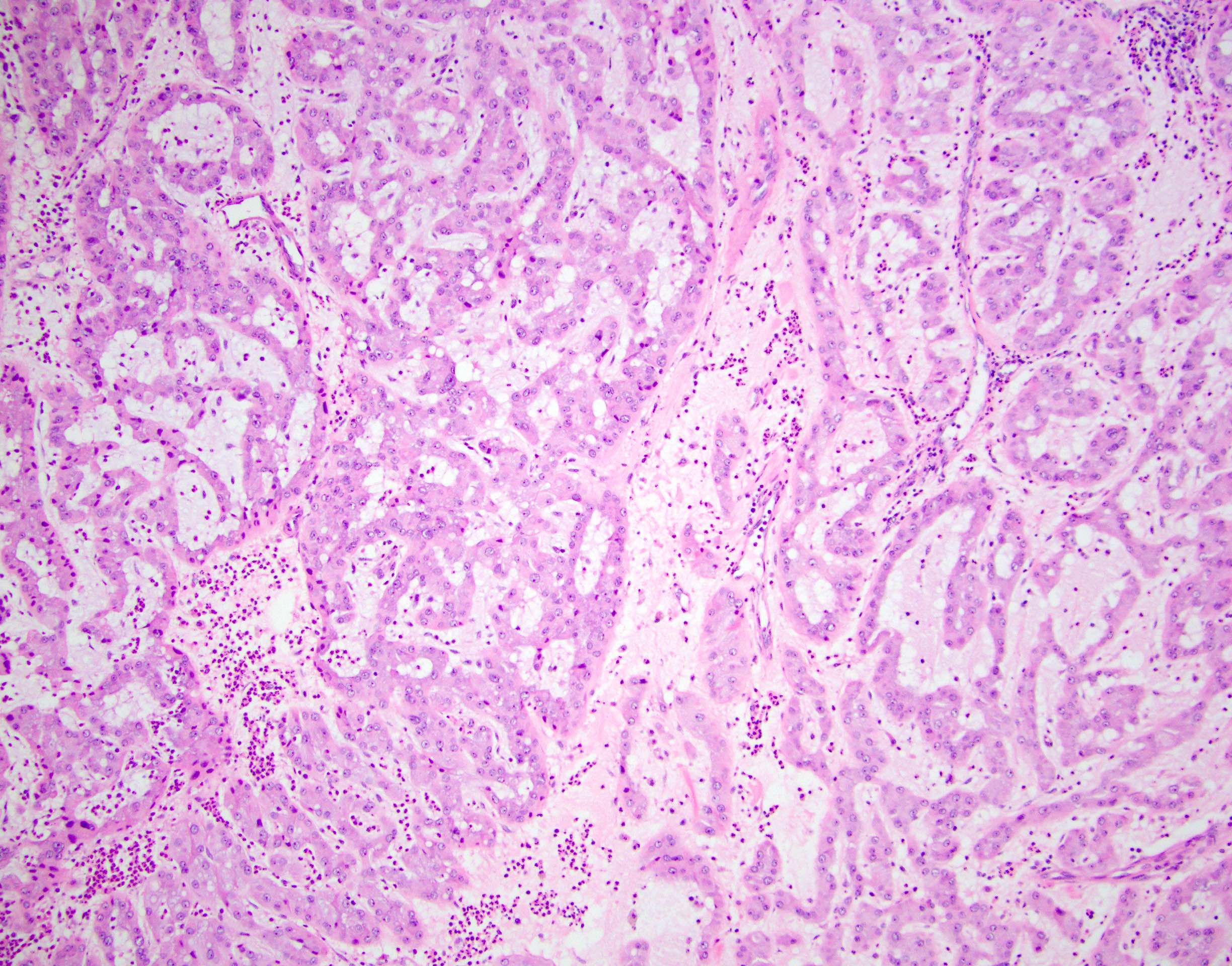
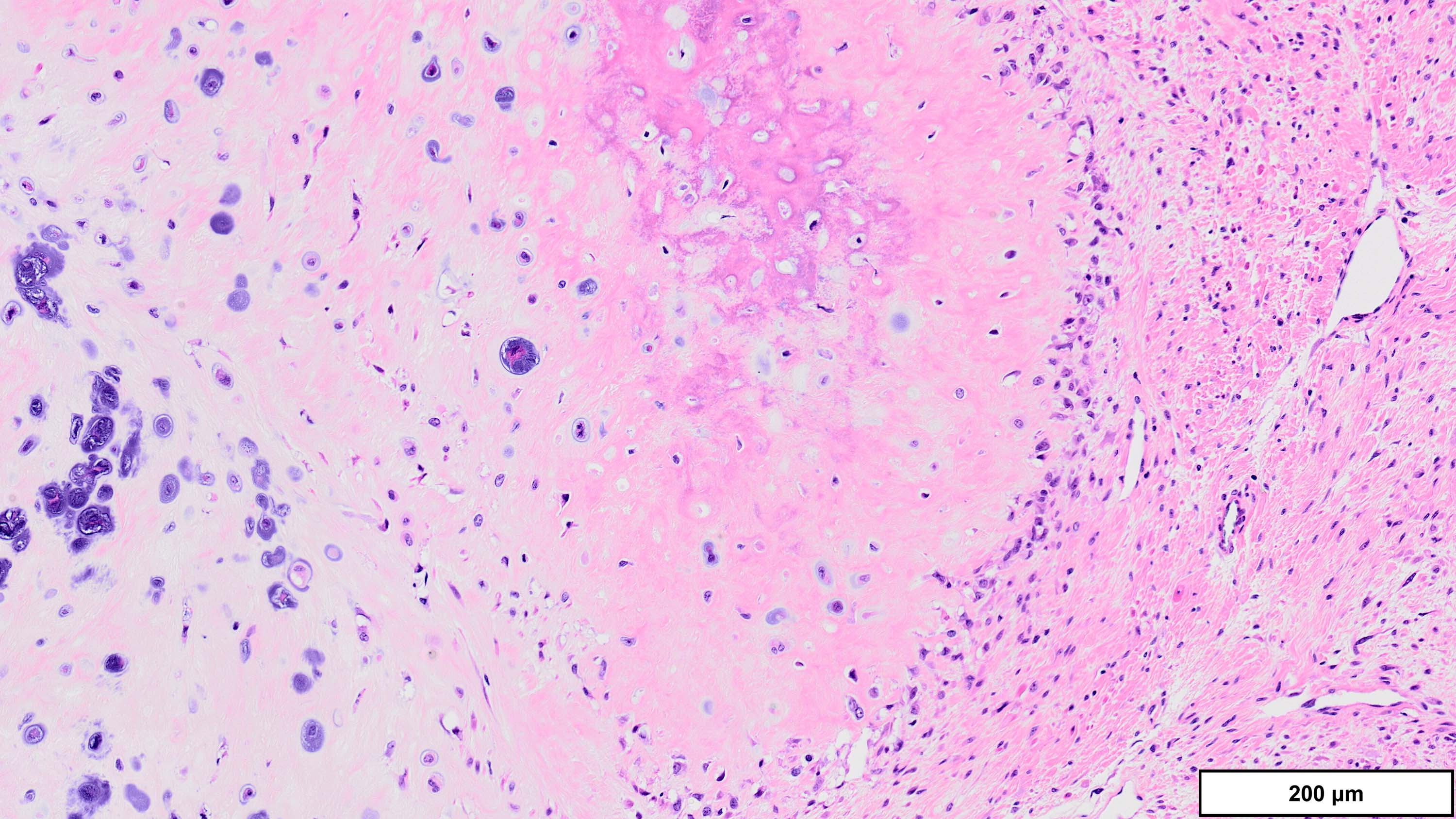
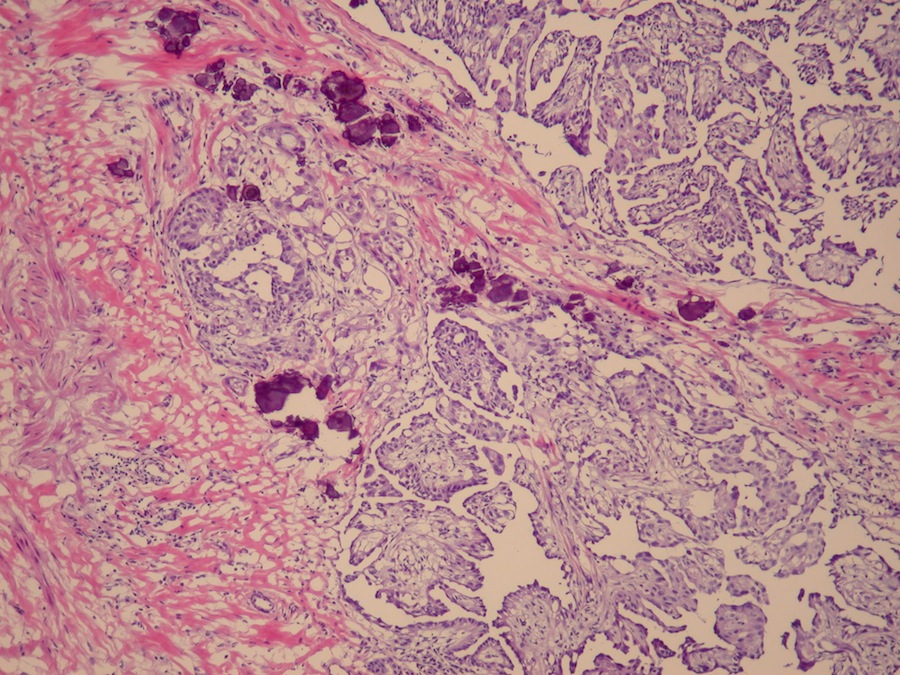
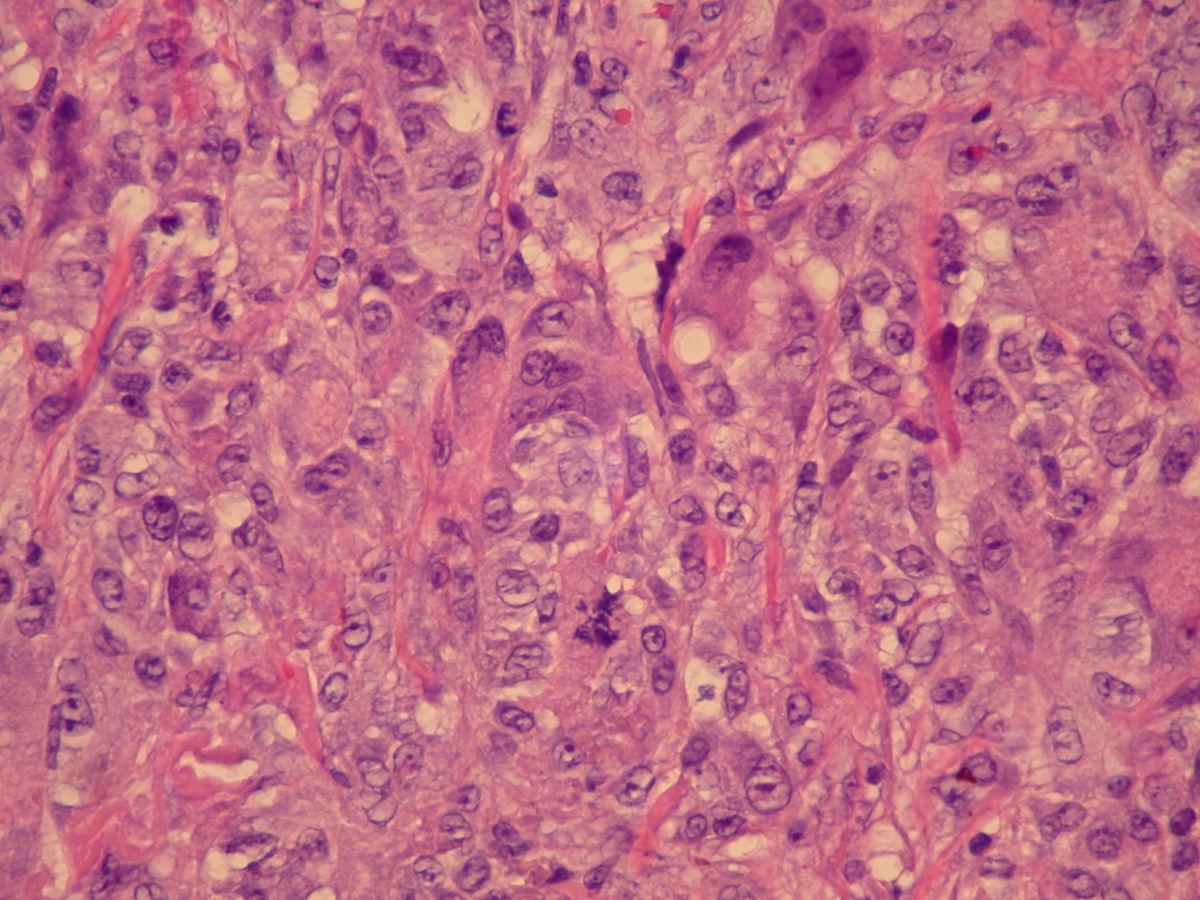
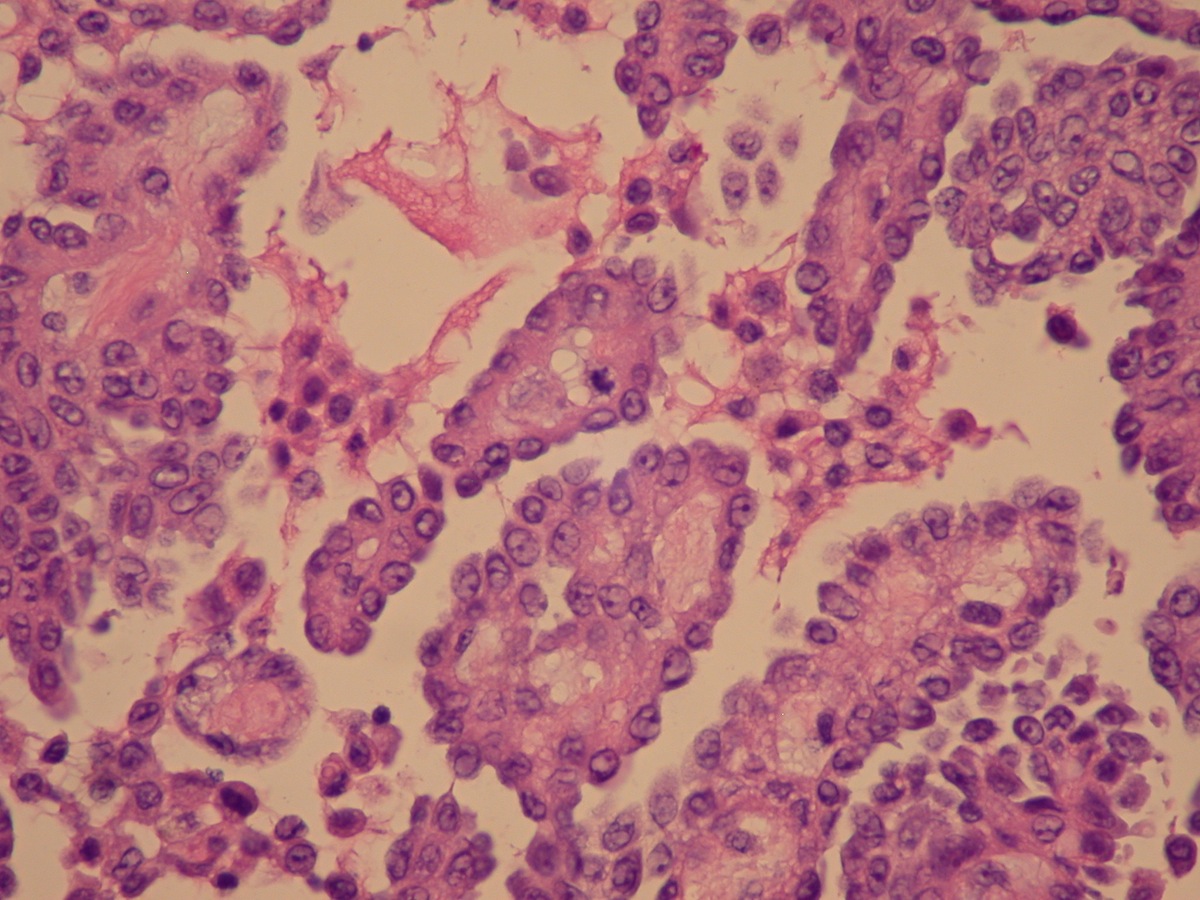
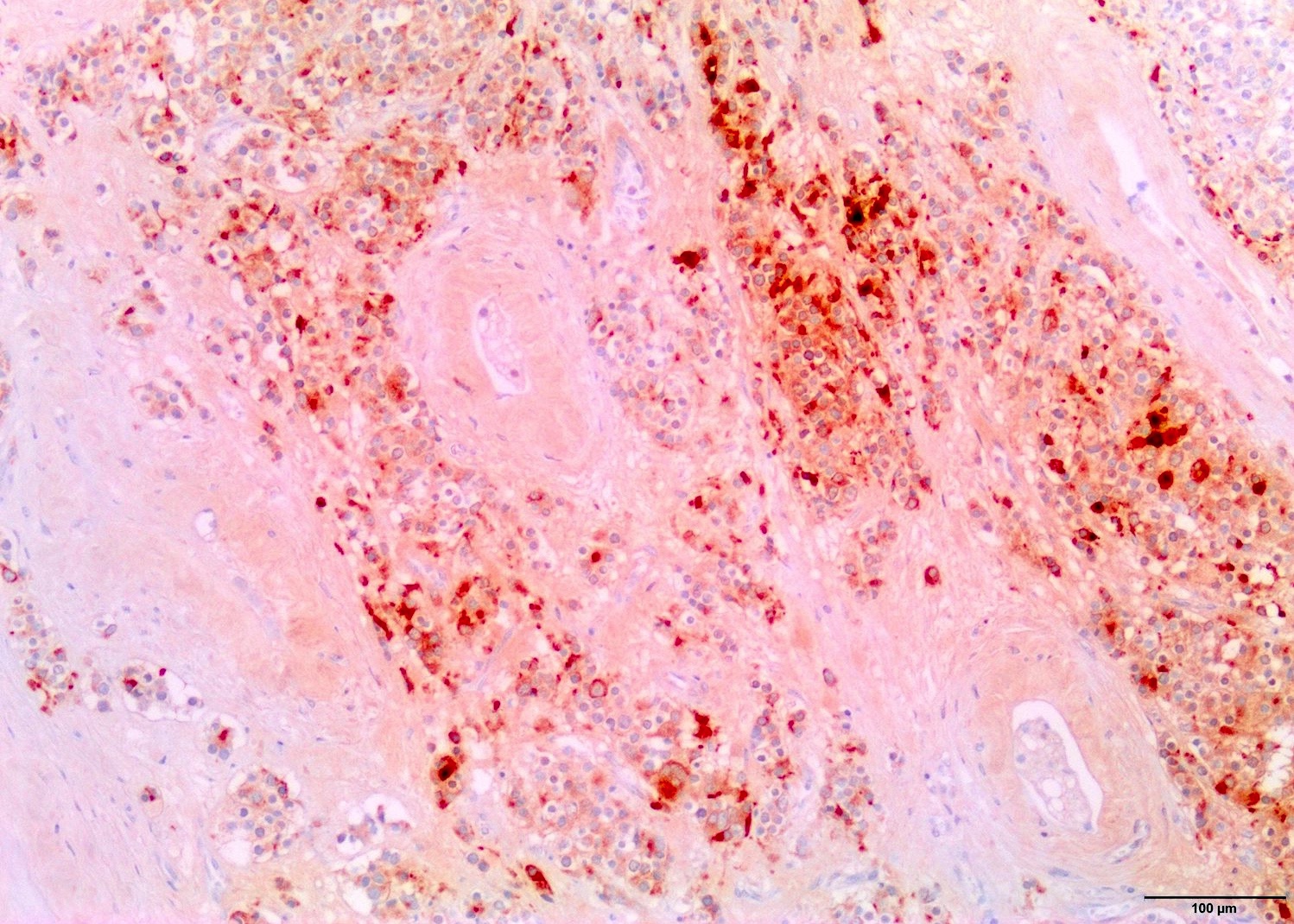
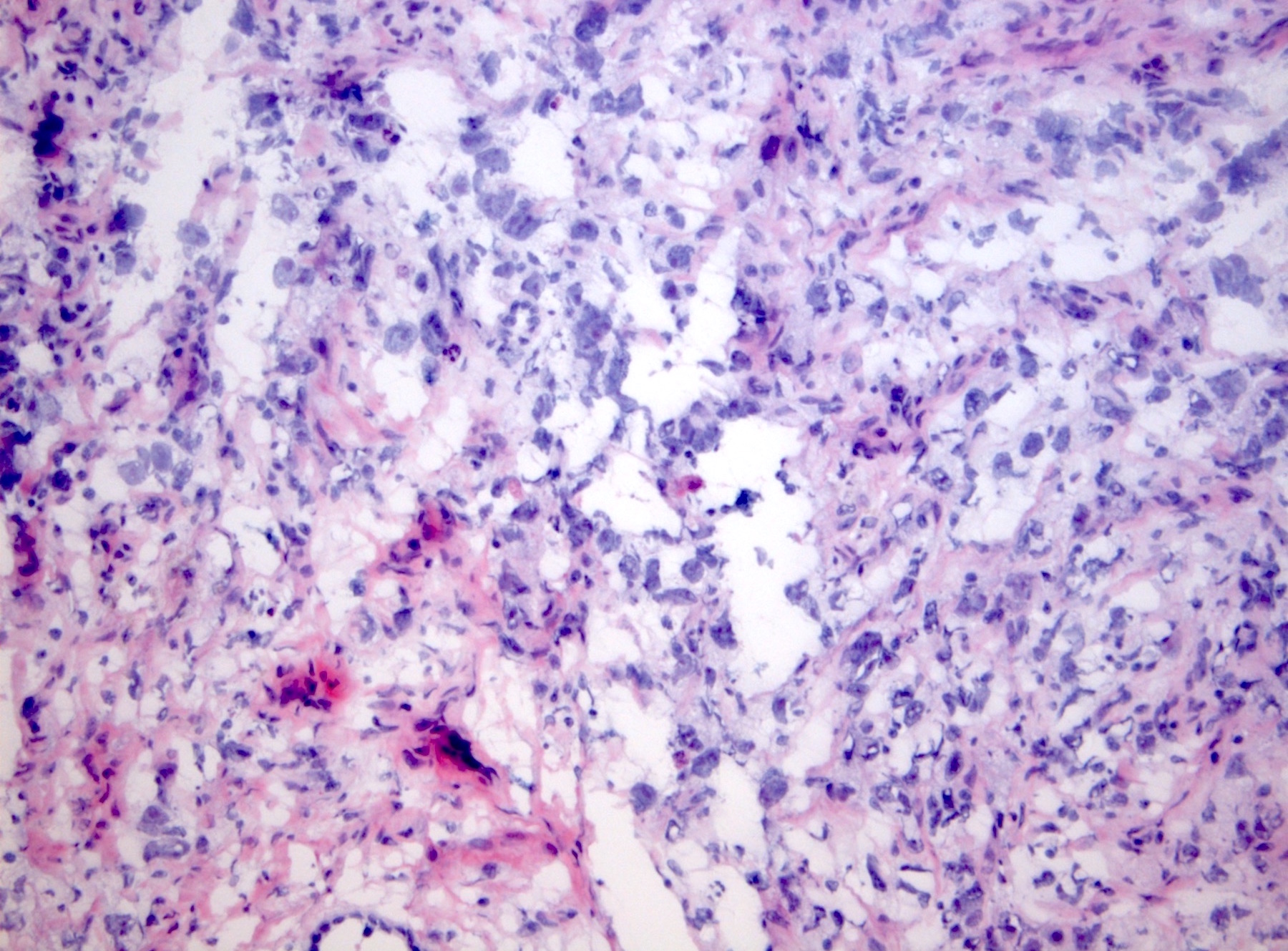
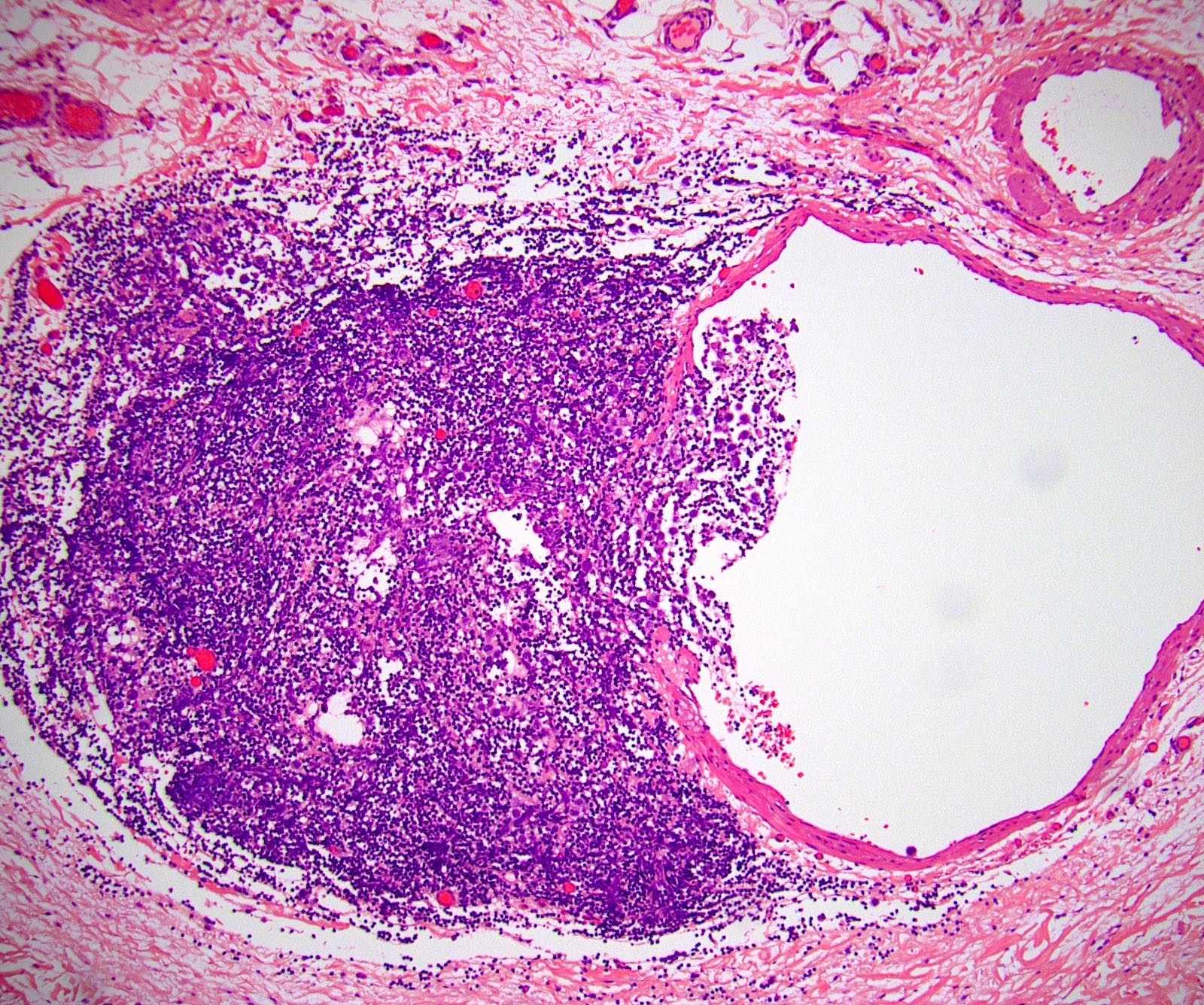
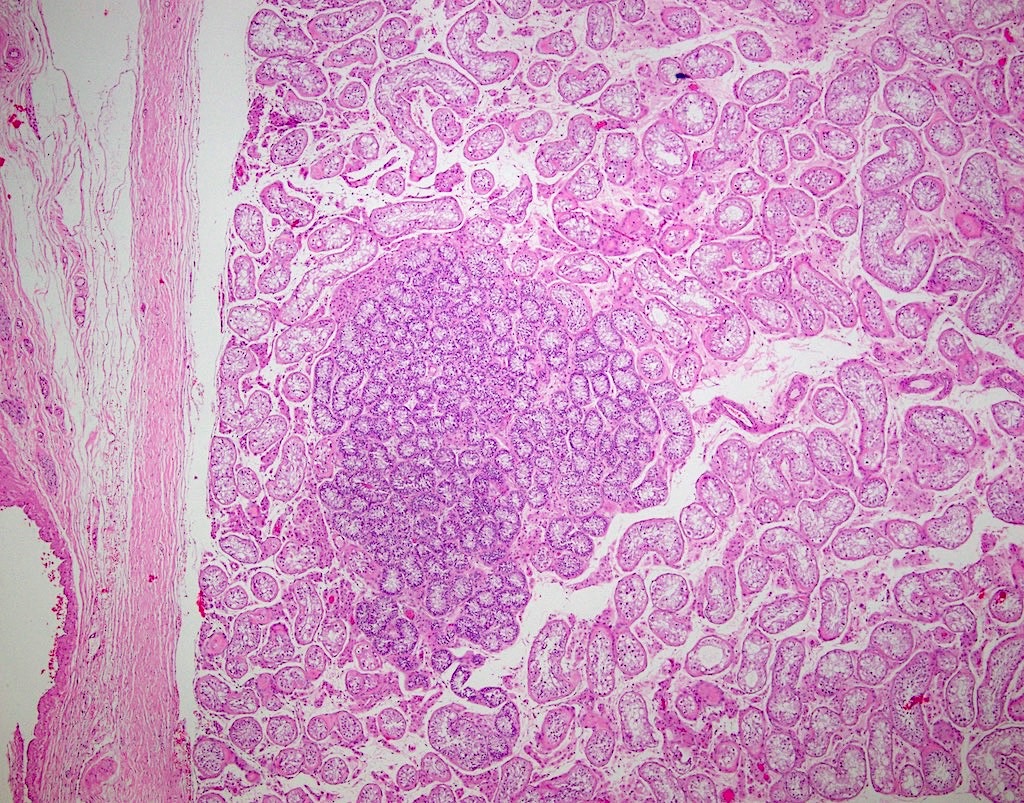
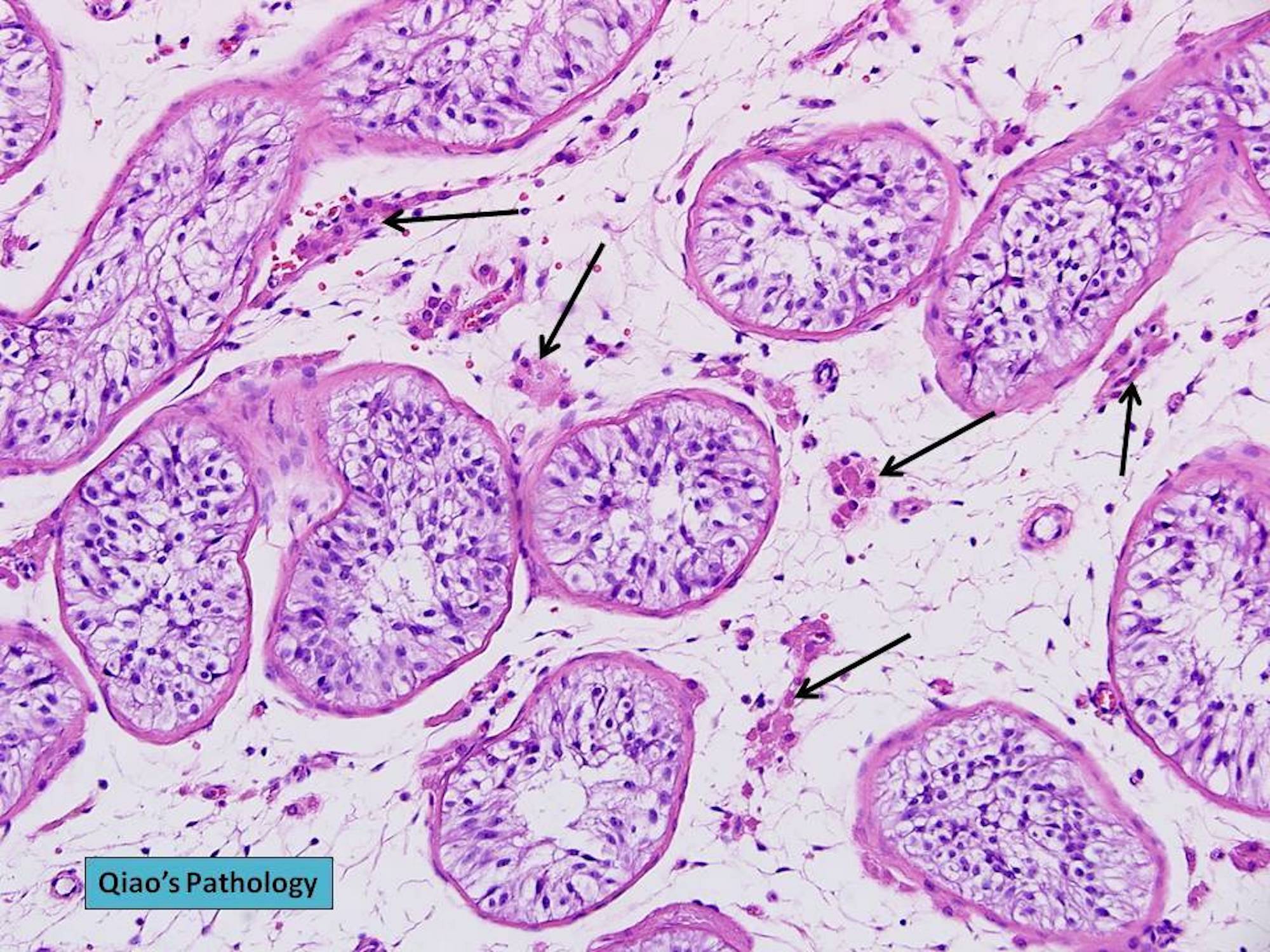
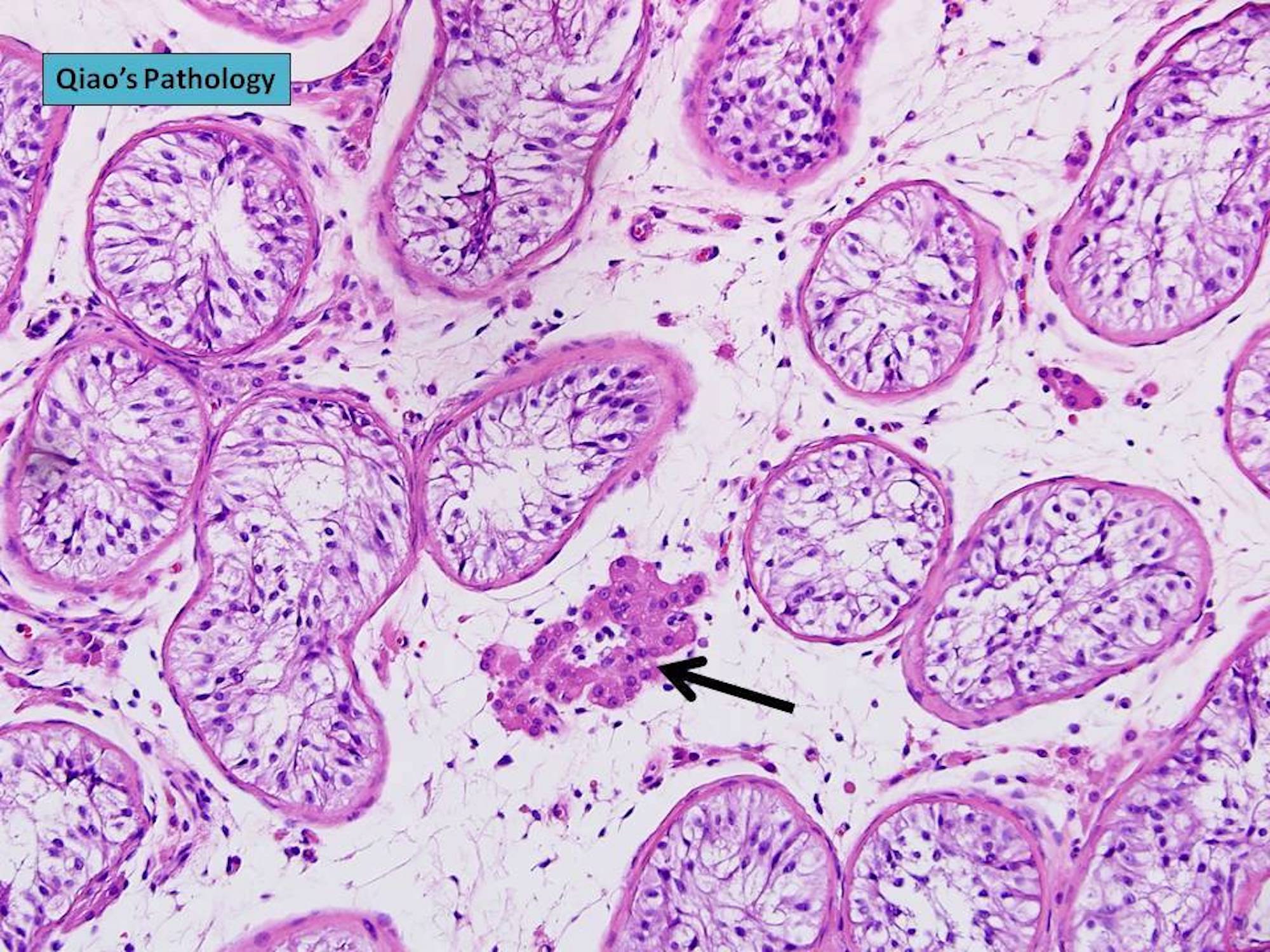
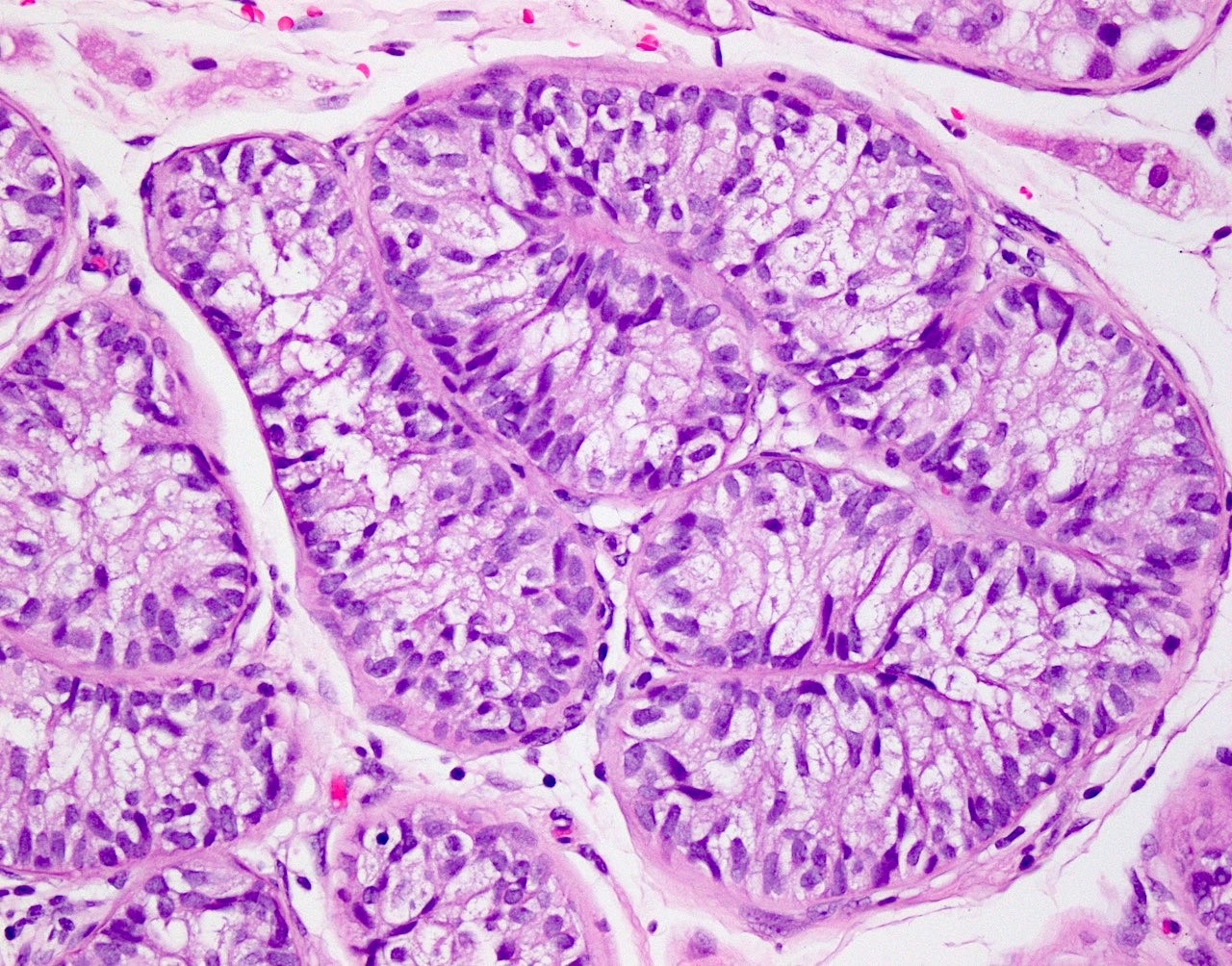
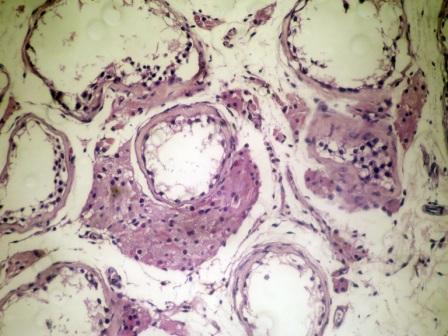
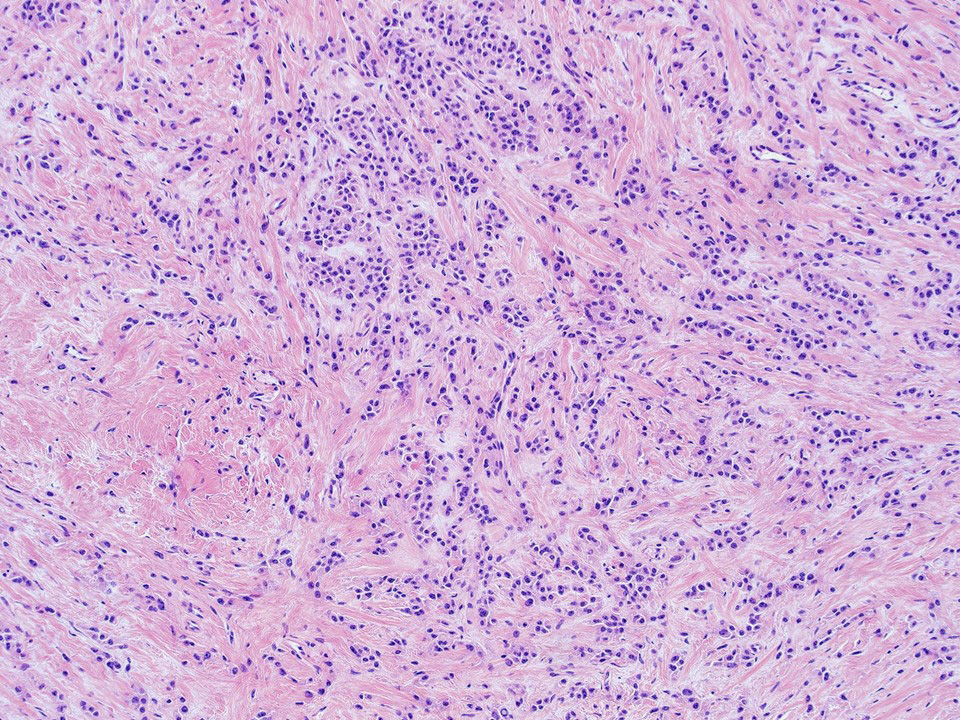
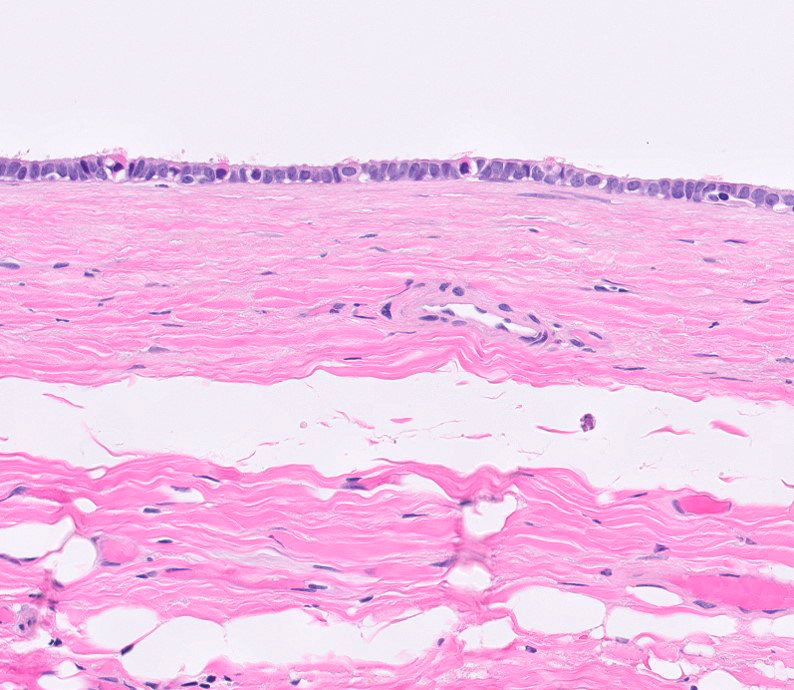
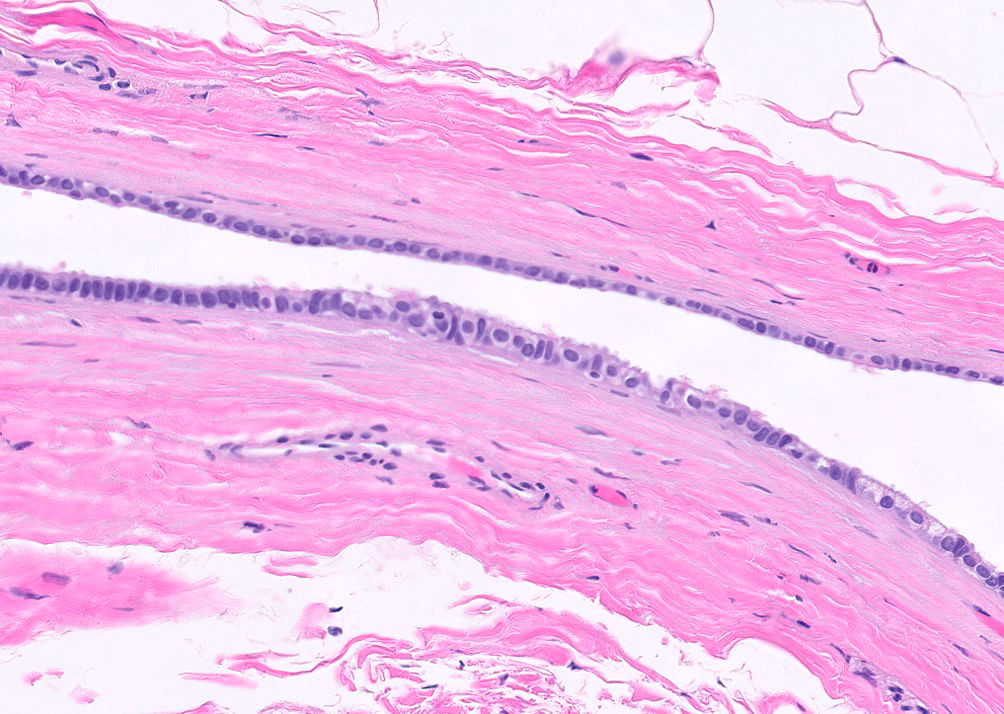
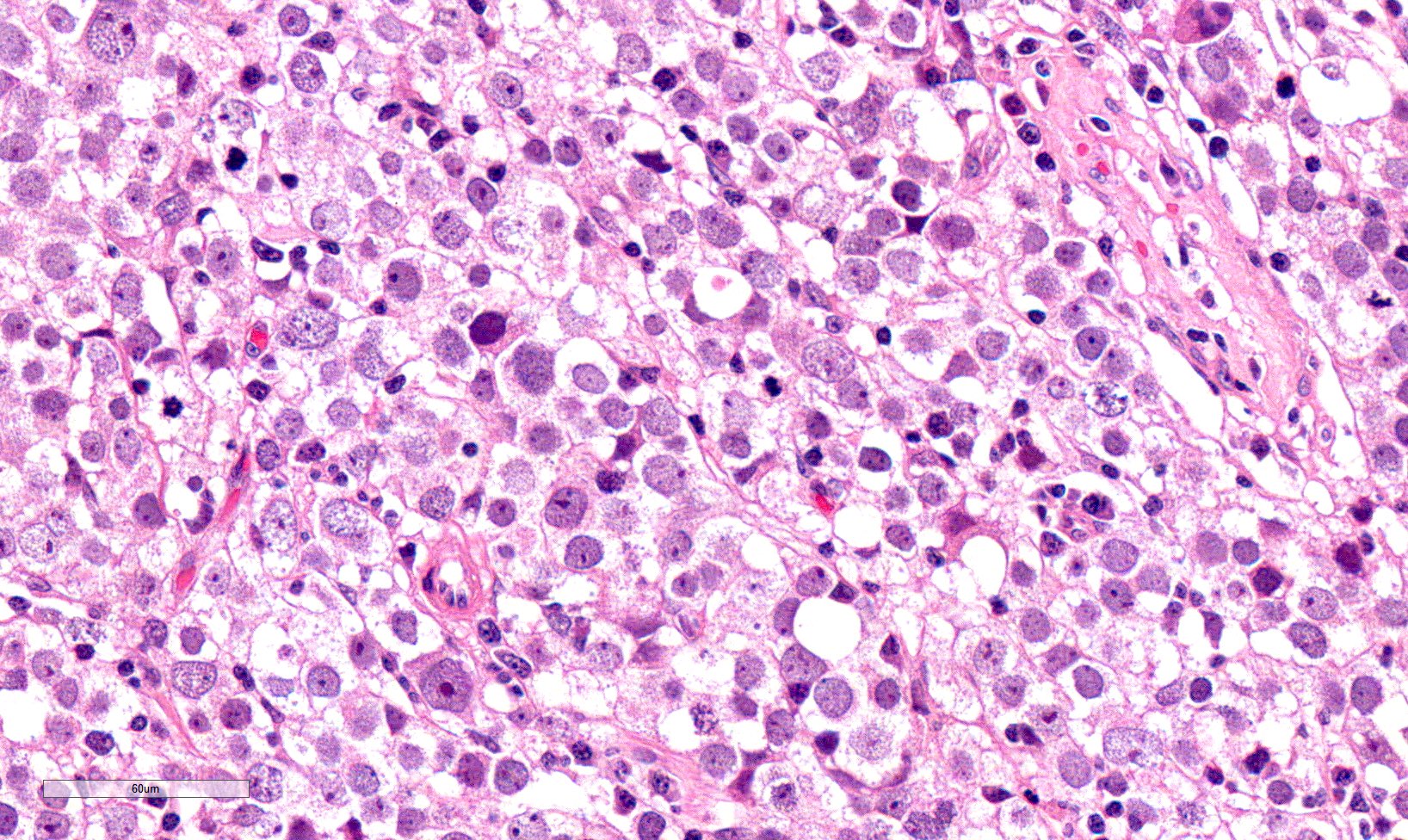
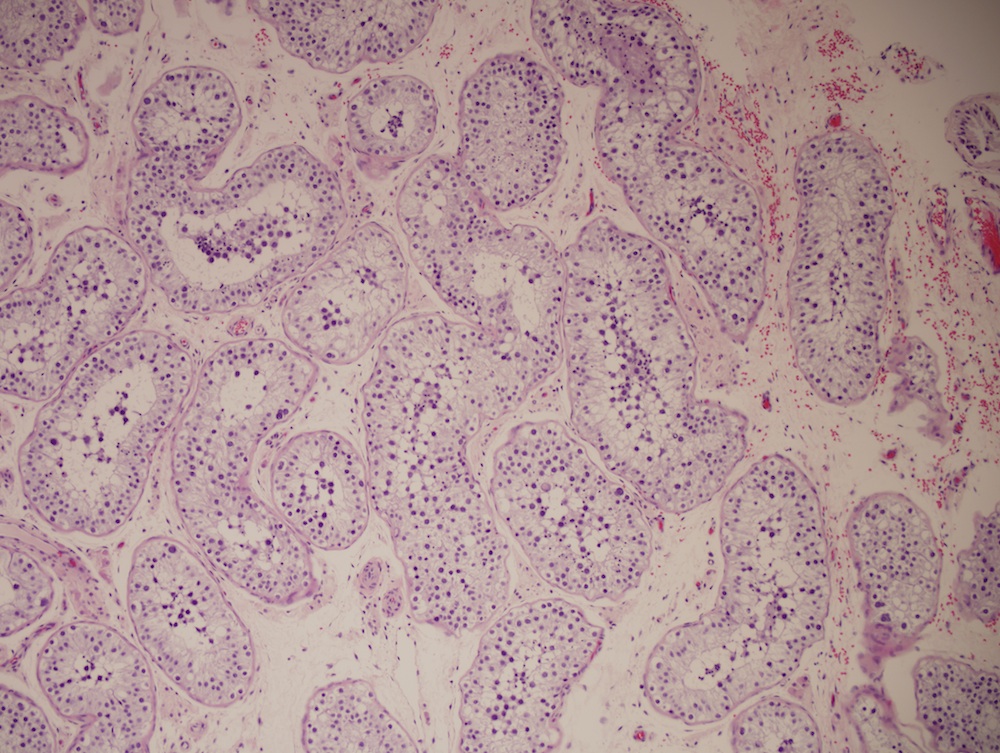
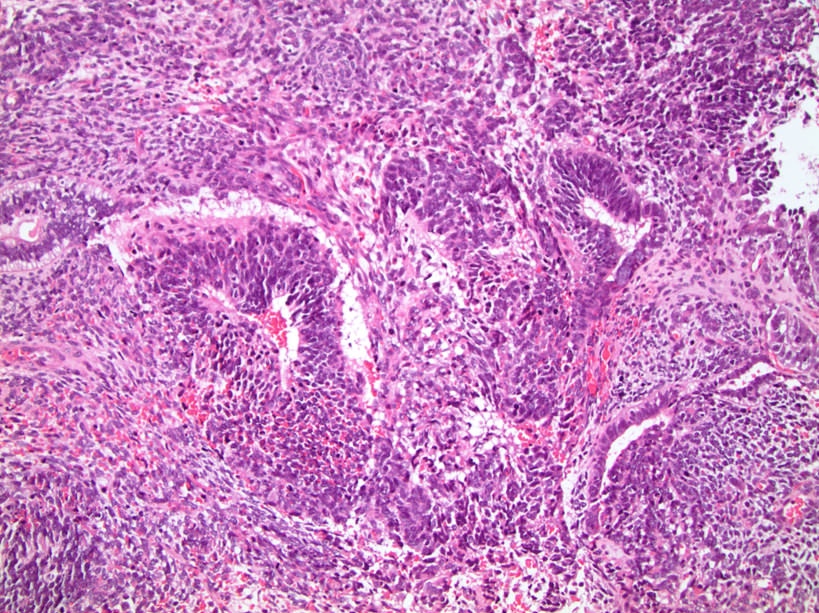
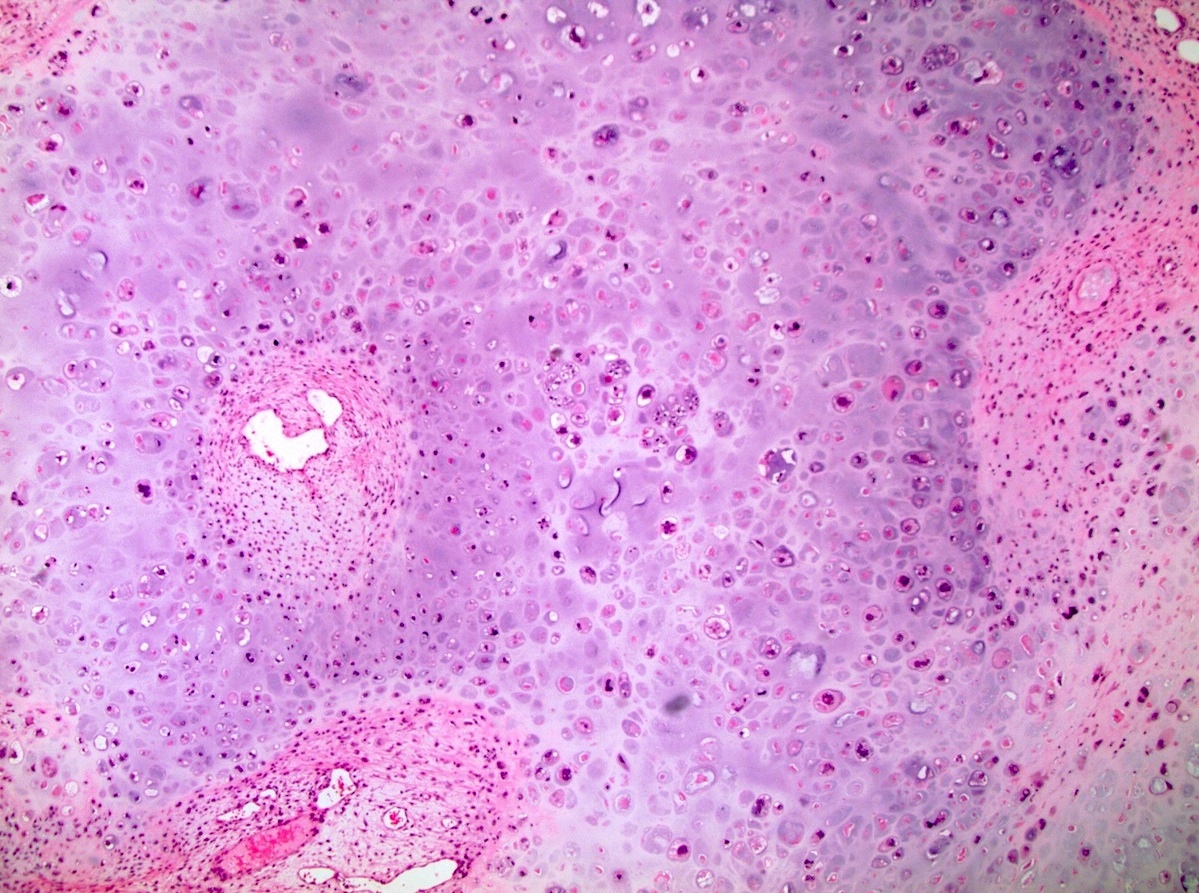
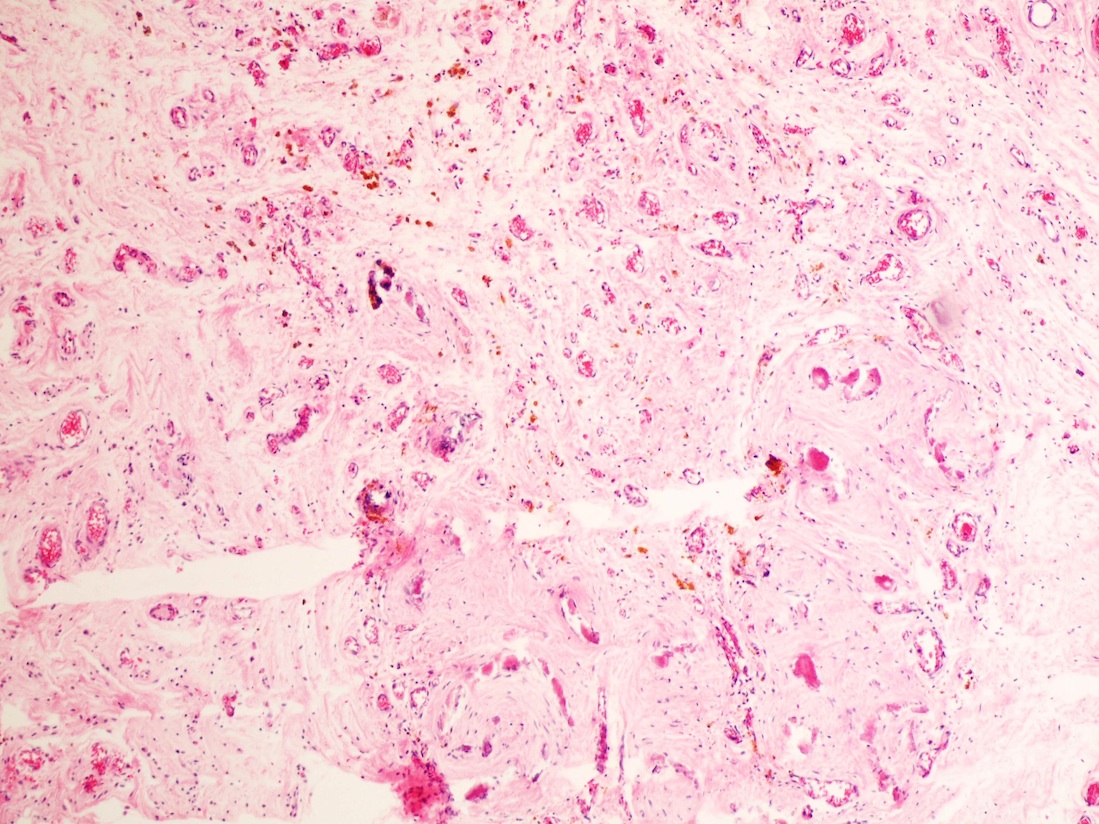
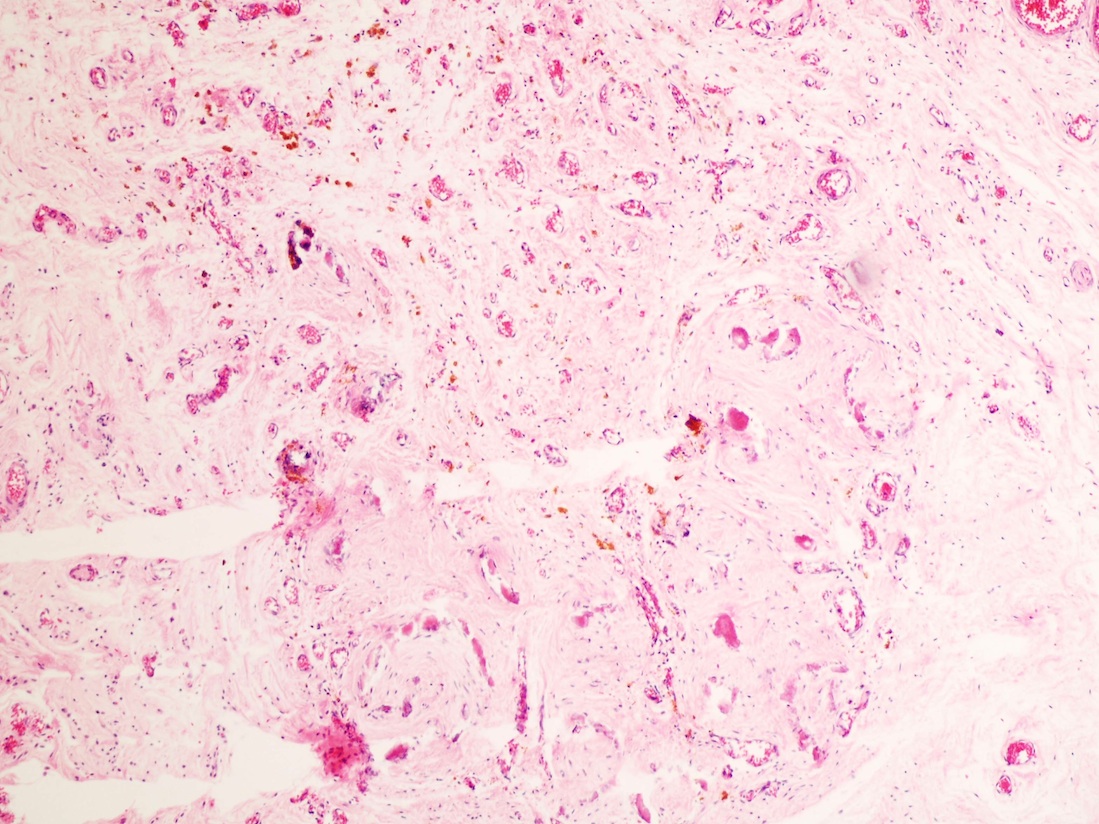
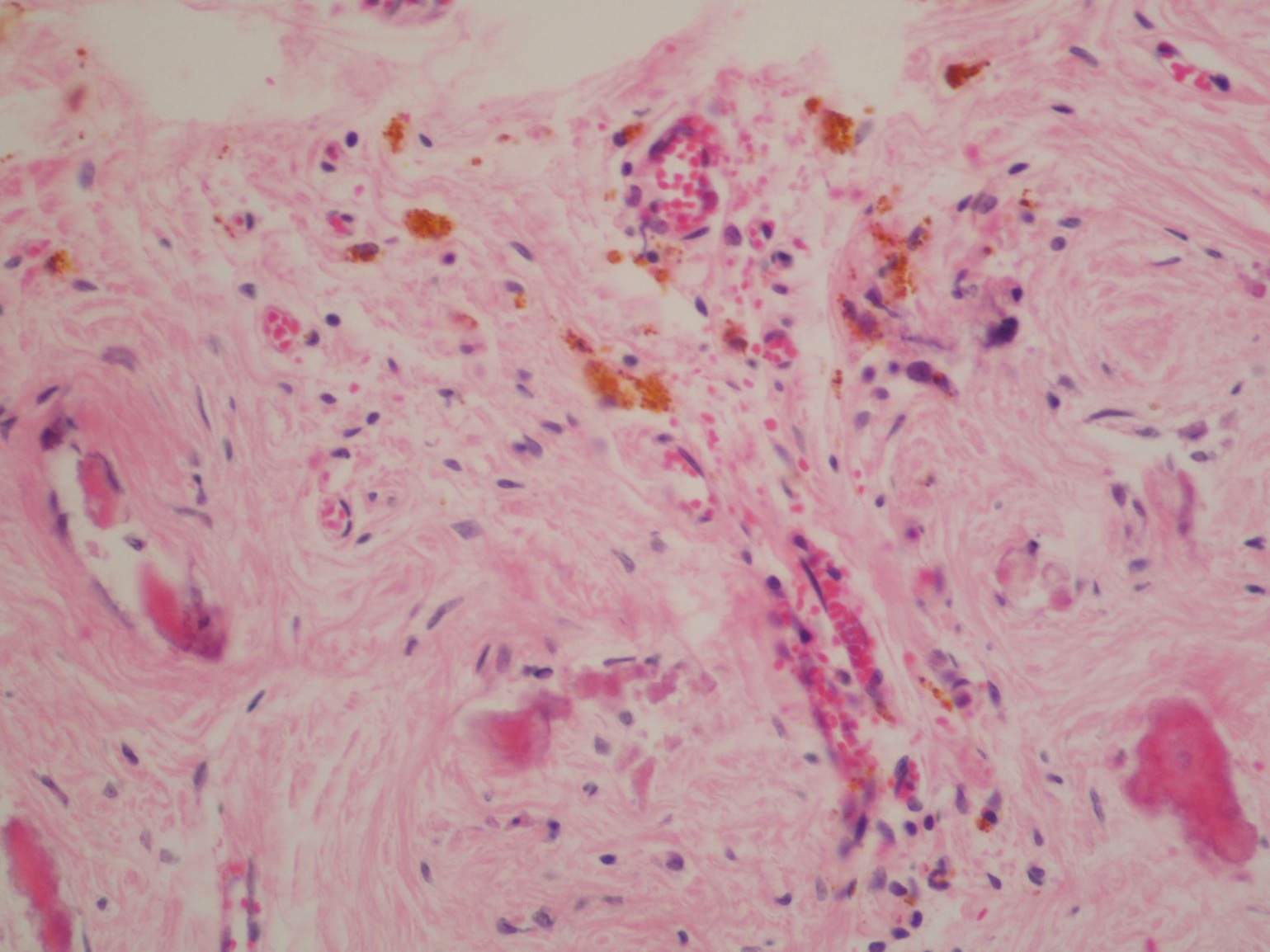
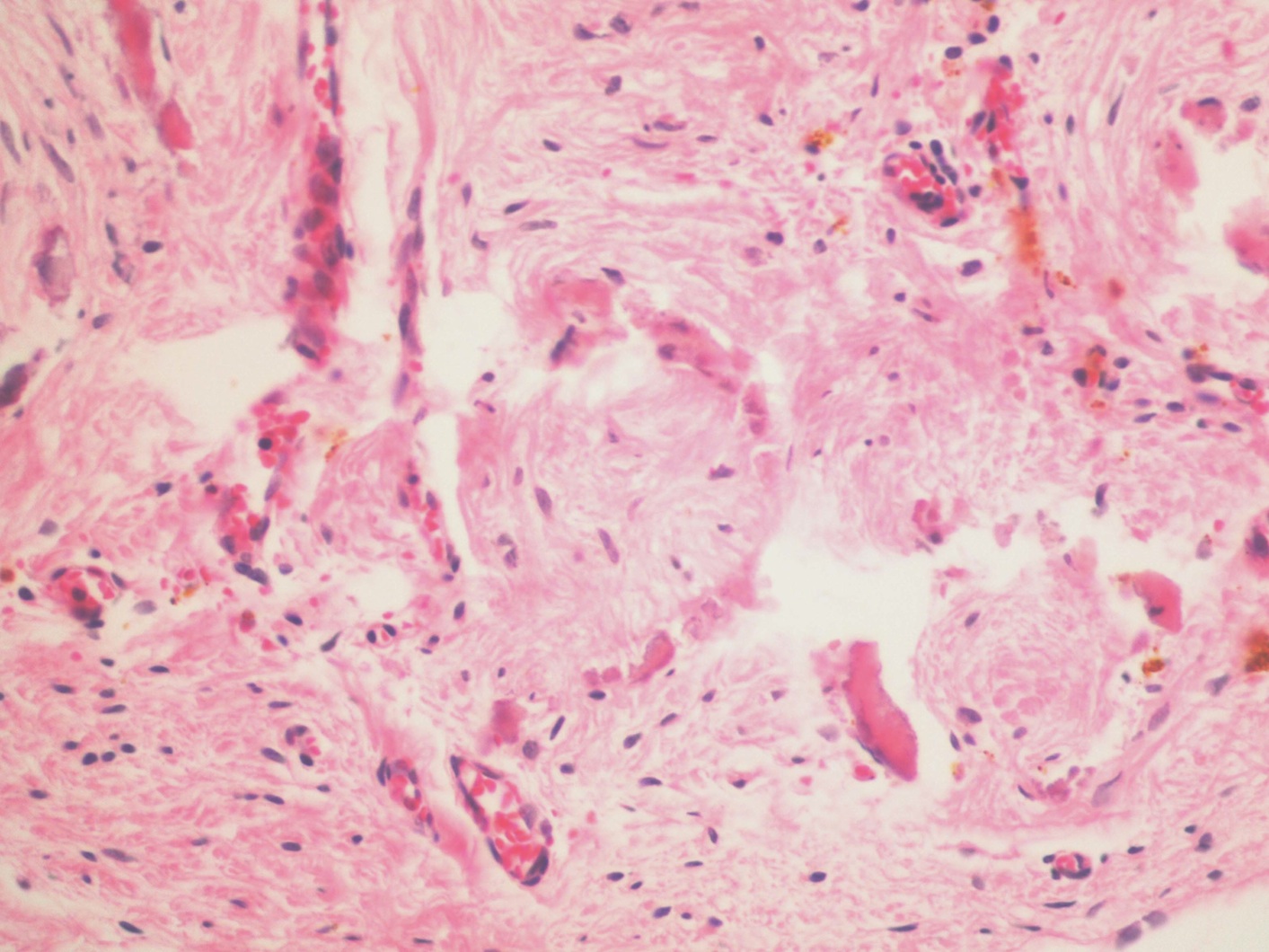
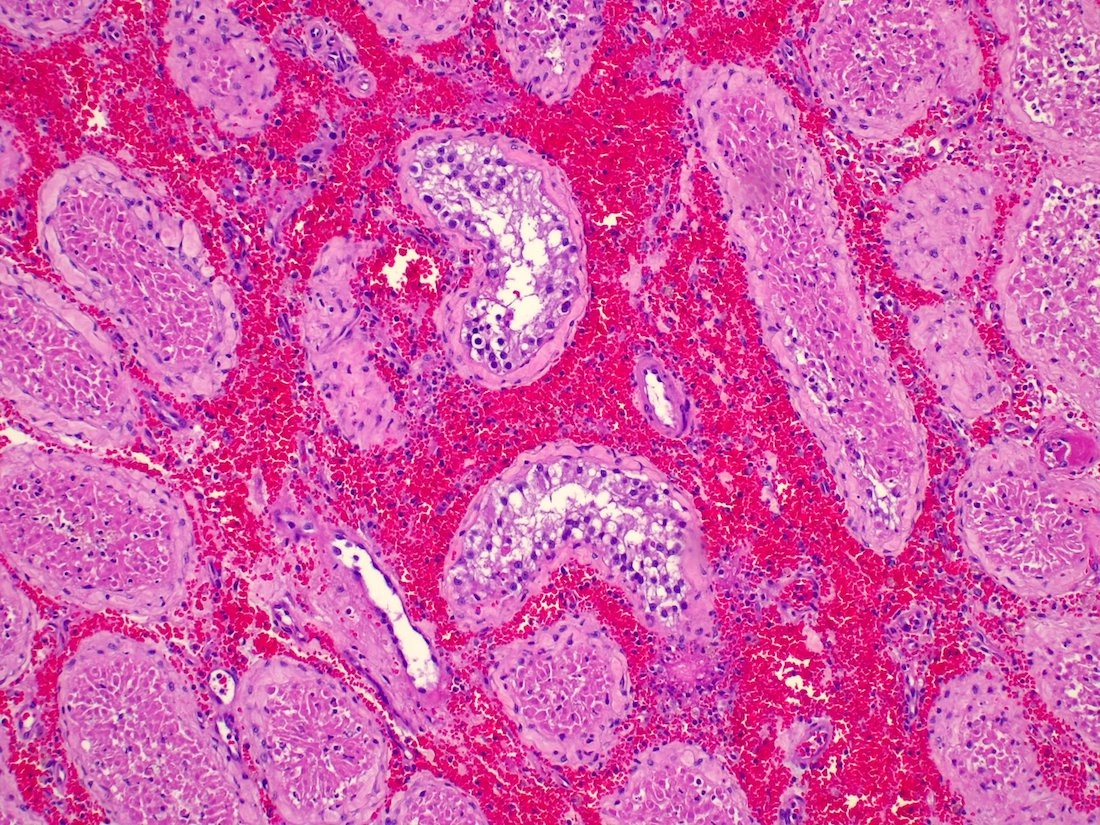
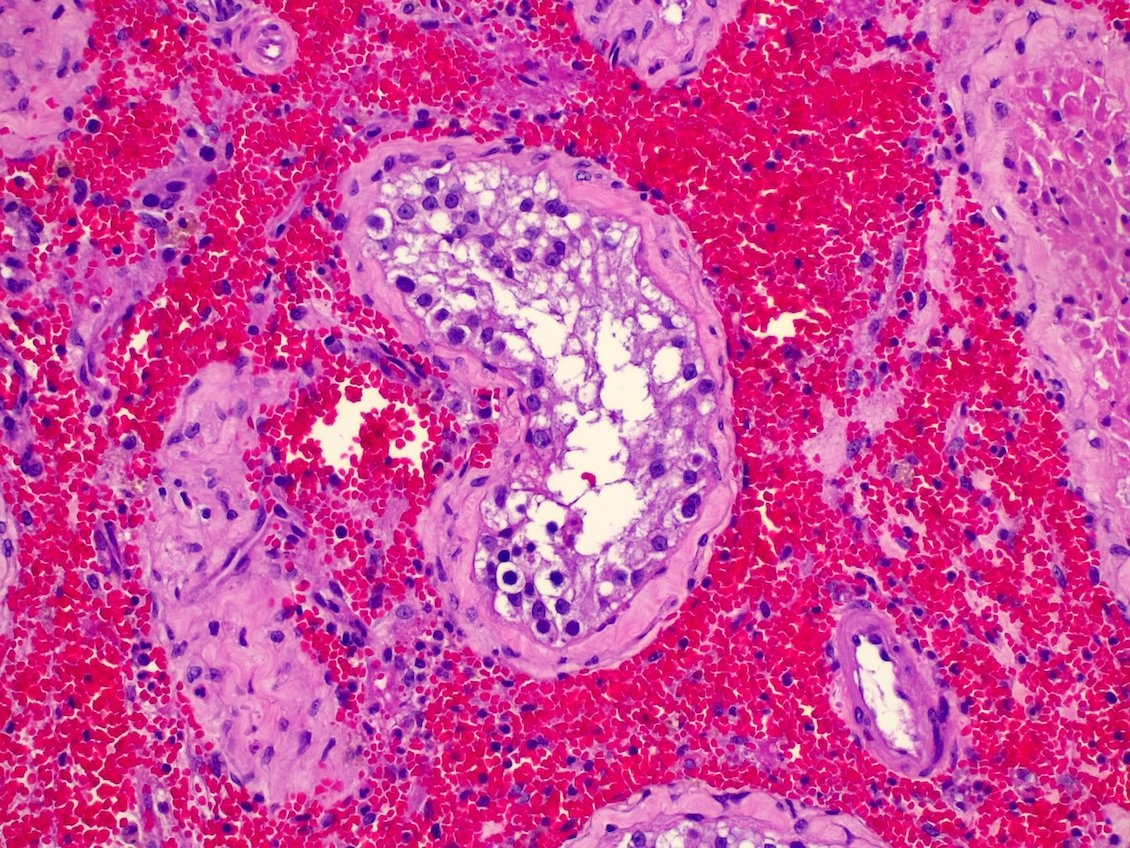
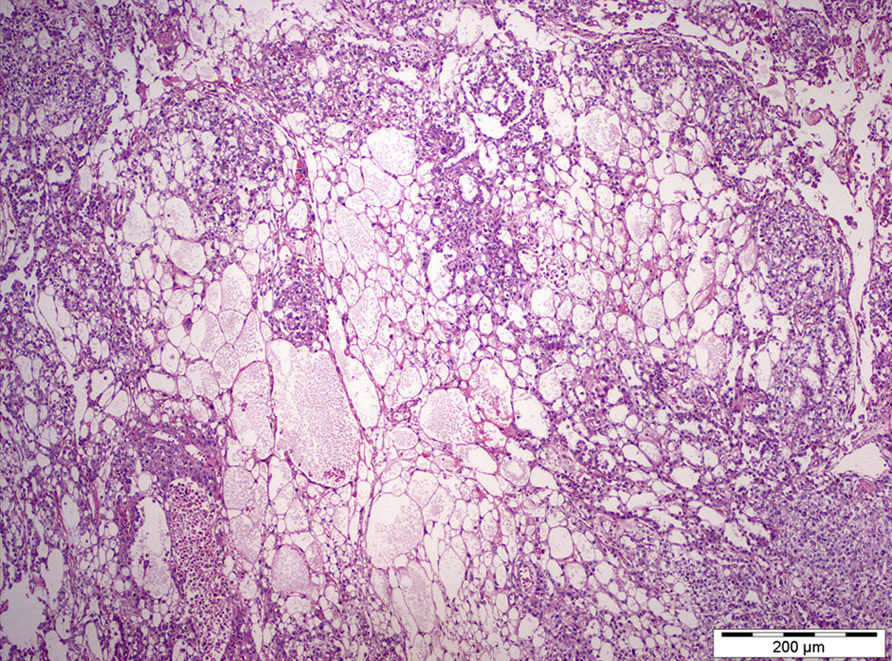
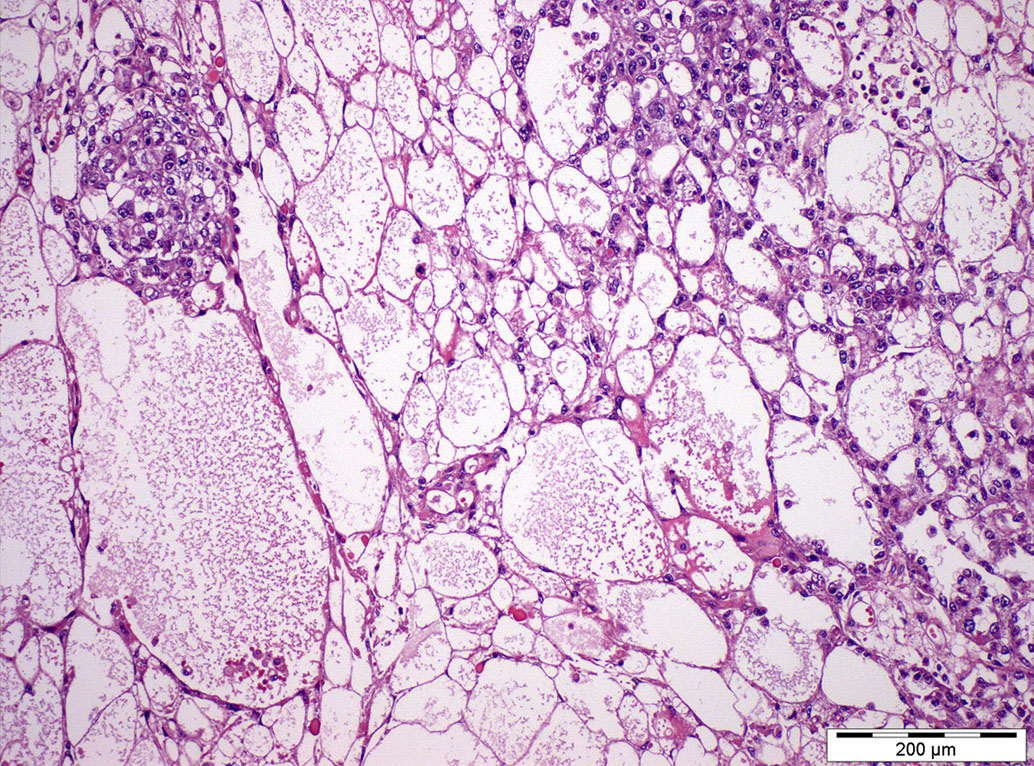
- Freshly cut or minimally fixed testicular germ cell tumors have frequent tissue displacement artifact ("butter"), which confounds the diagnosis of true LVI (classified as pT2) (Am J Clin Pathol 2016;145:341)
- Care must be taken when grossing to decrease the amount of tissue displacement artifact and overnight fixation of a bivalved specimen may be helpful
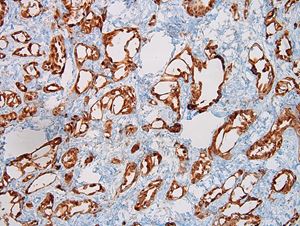
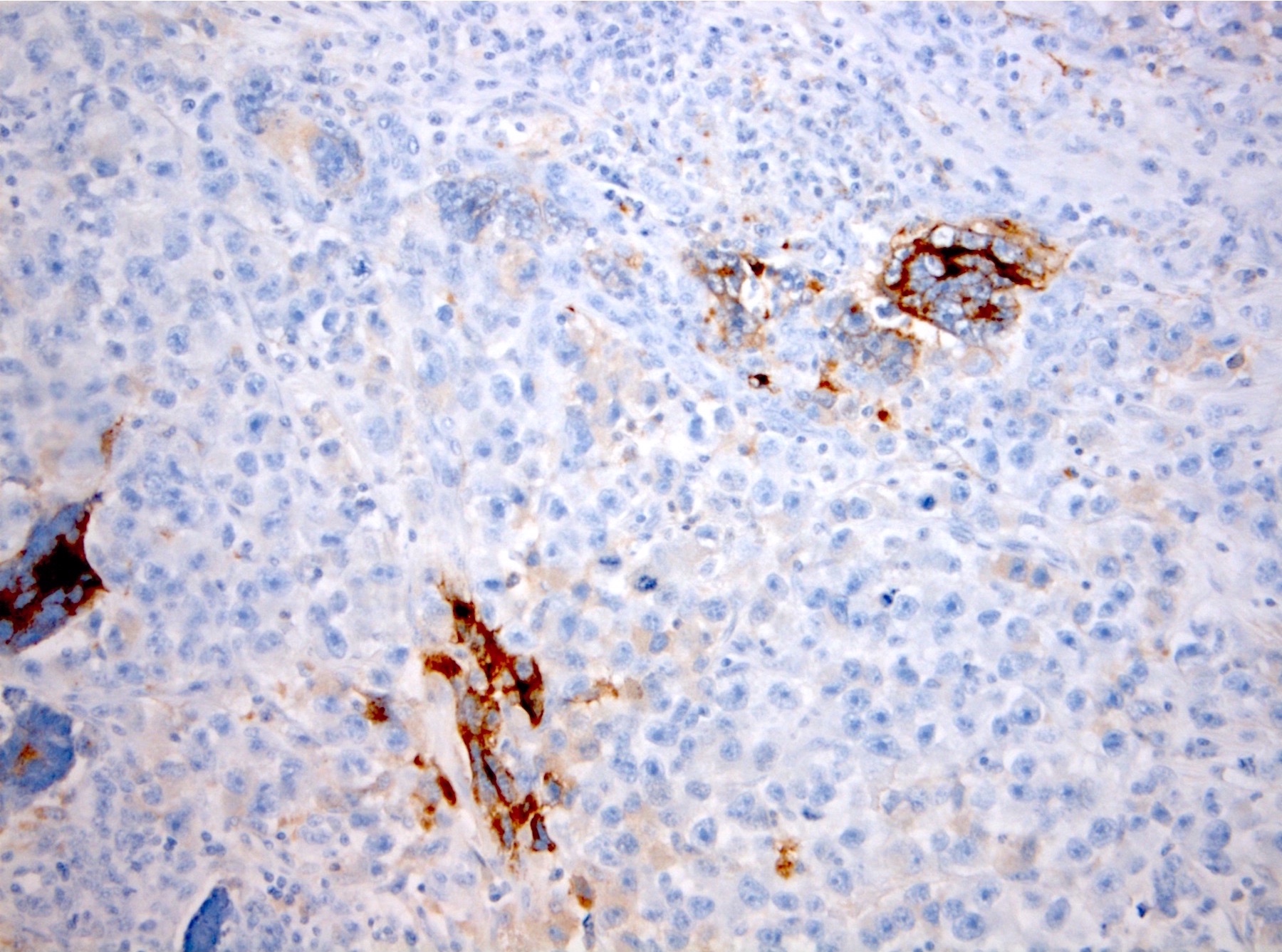
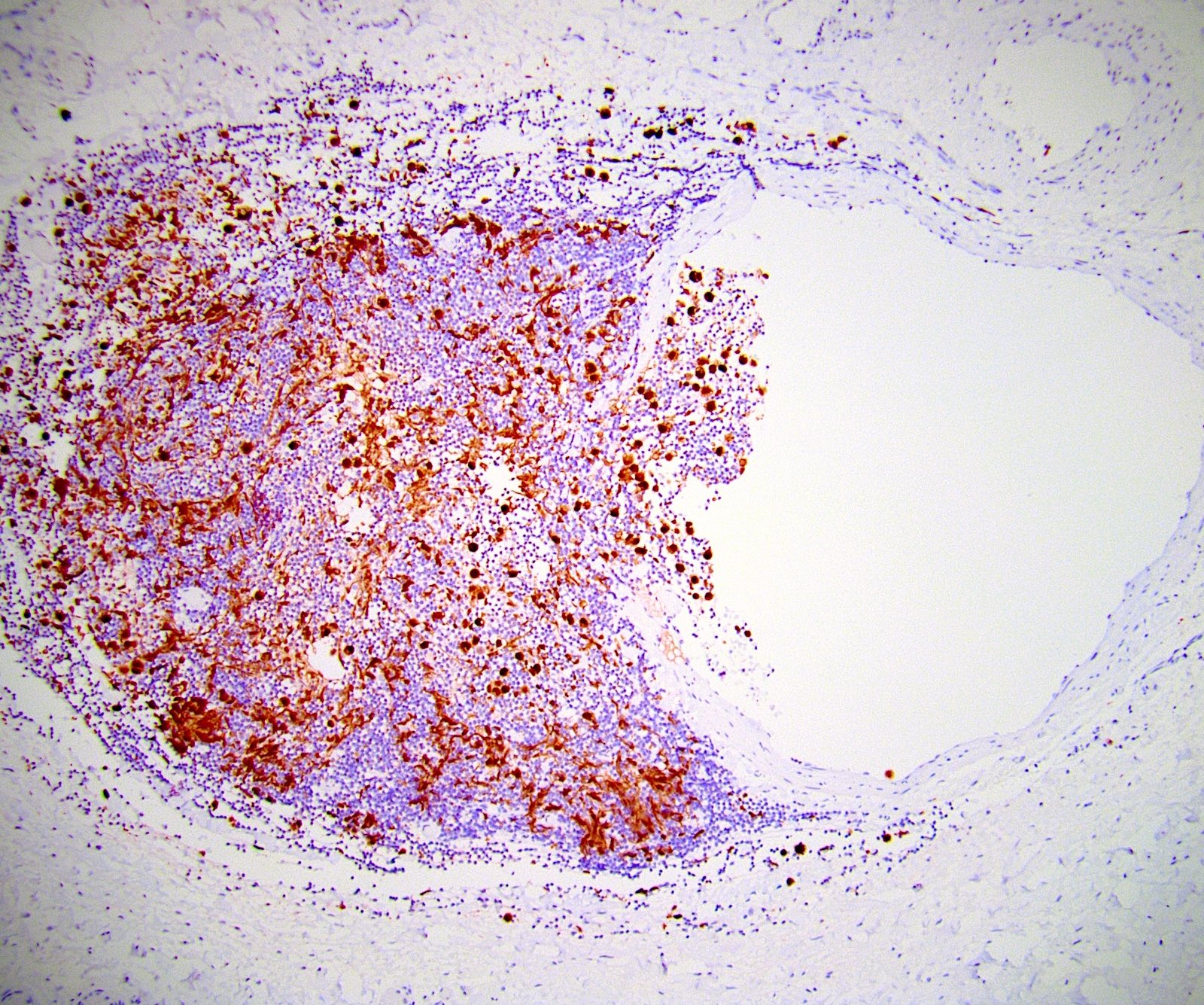
- Histologic features supporting true LVI are tumor emboli that are cohesive, have smooth contours and adhere to the vessel wall (Am J Clin Pathol 2016;145:341)
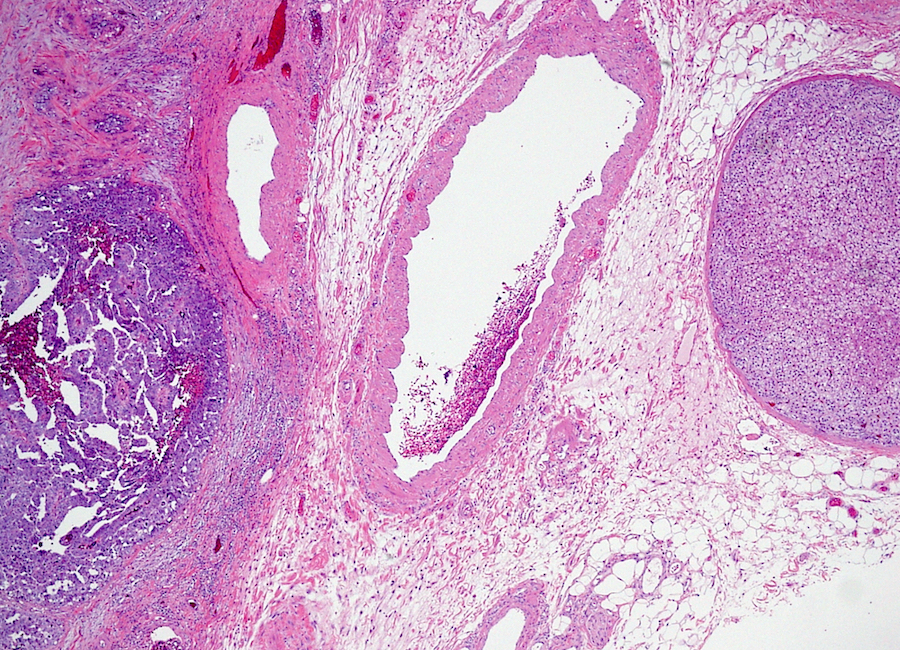
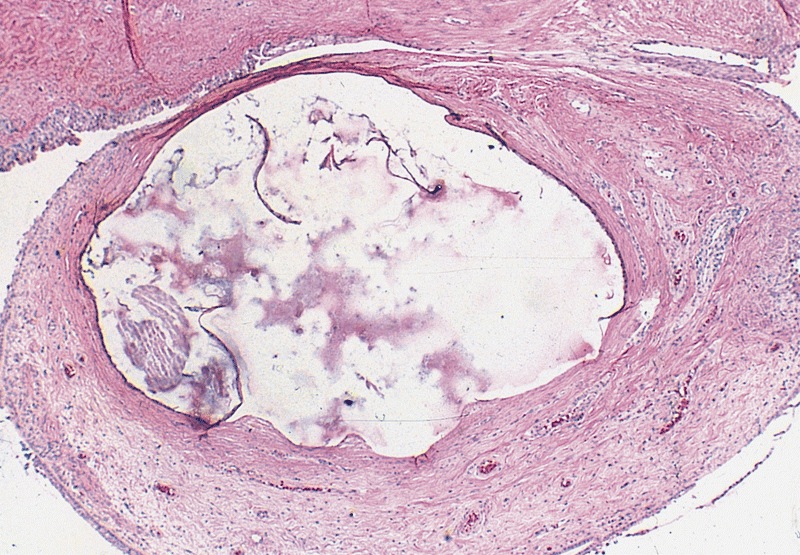
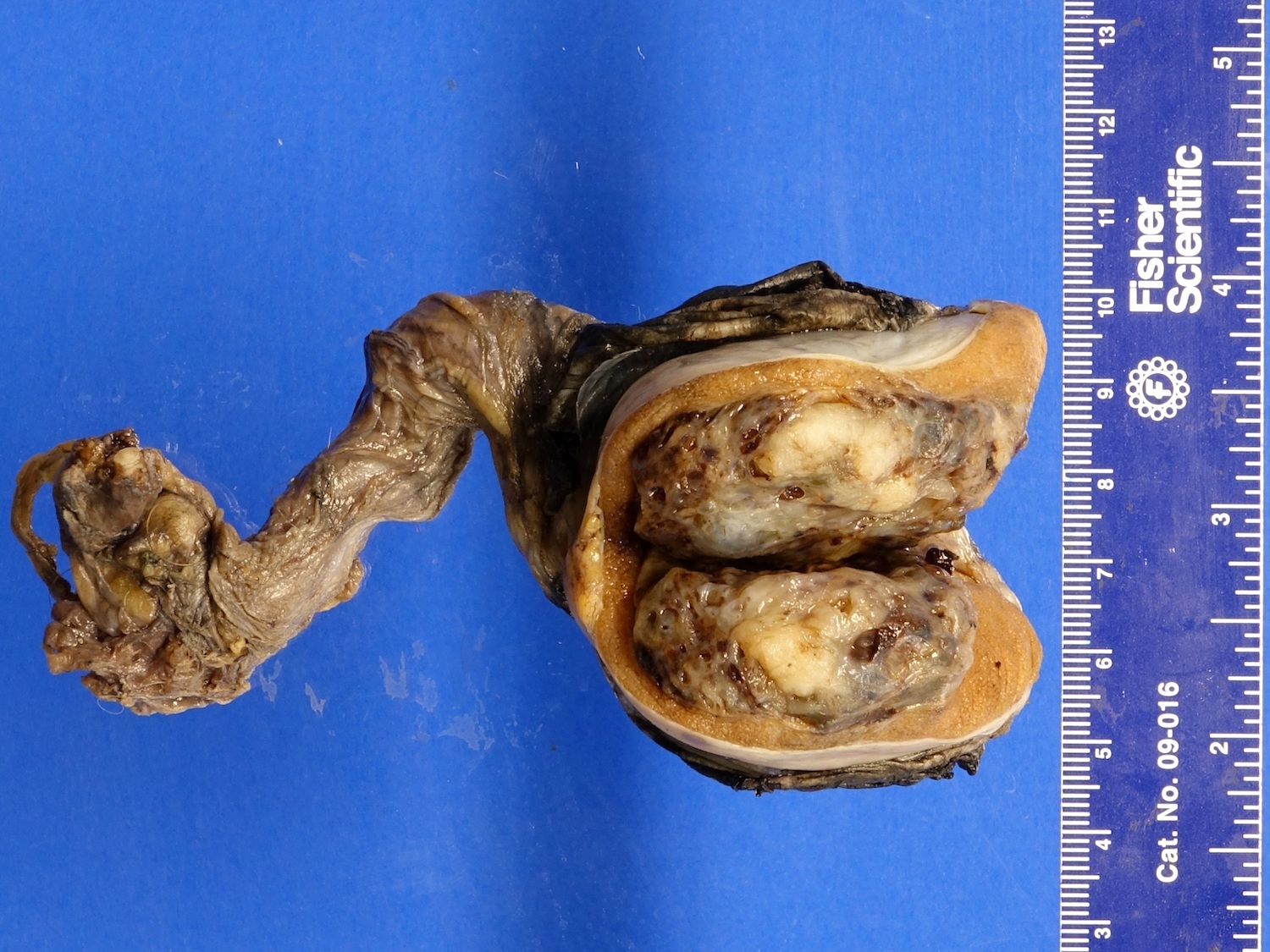
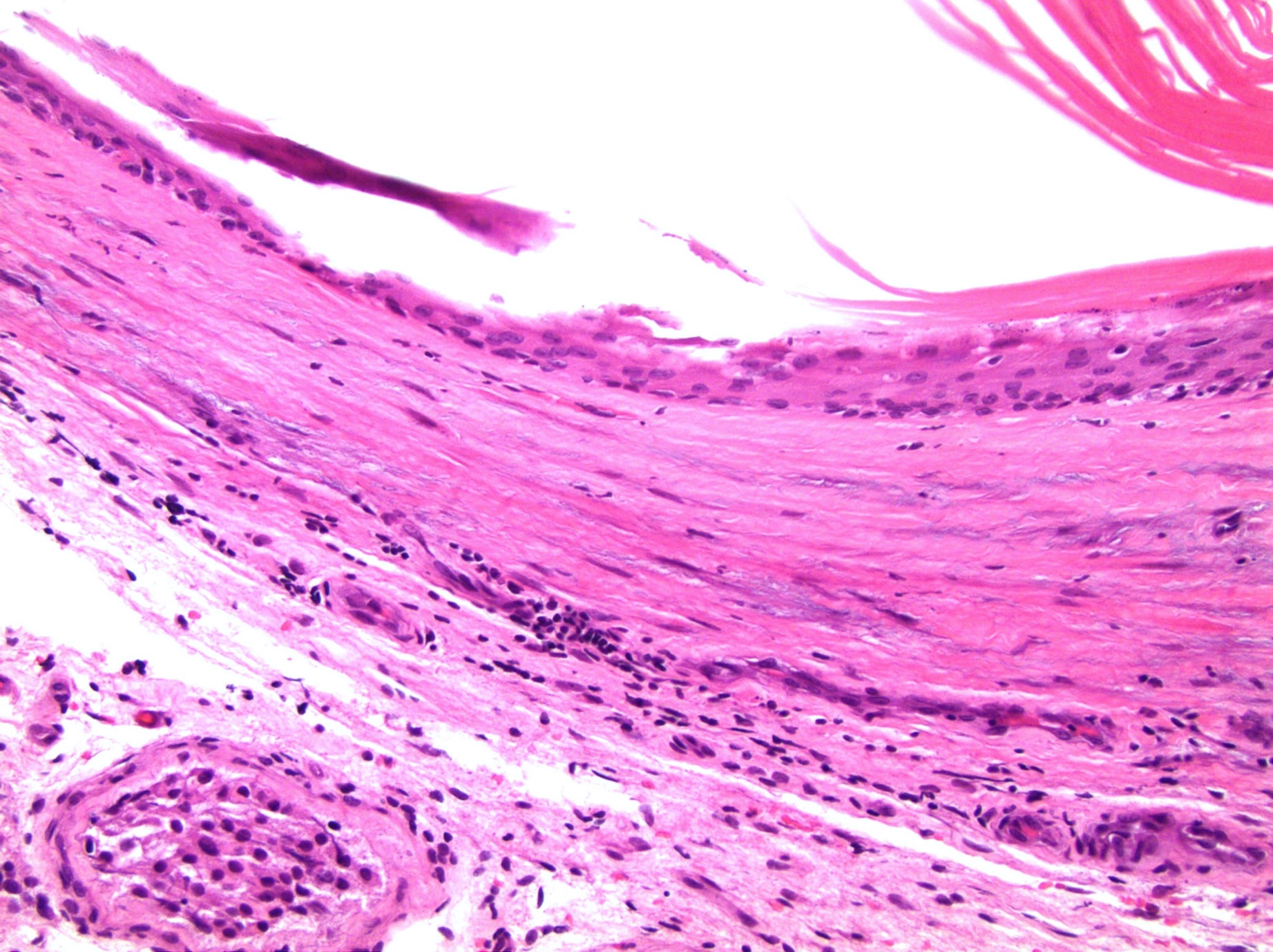
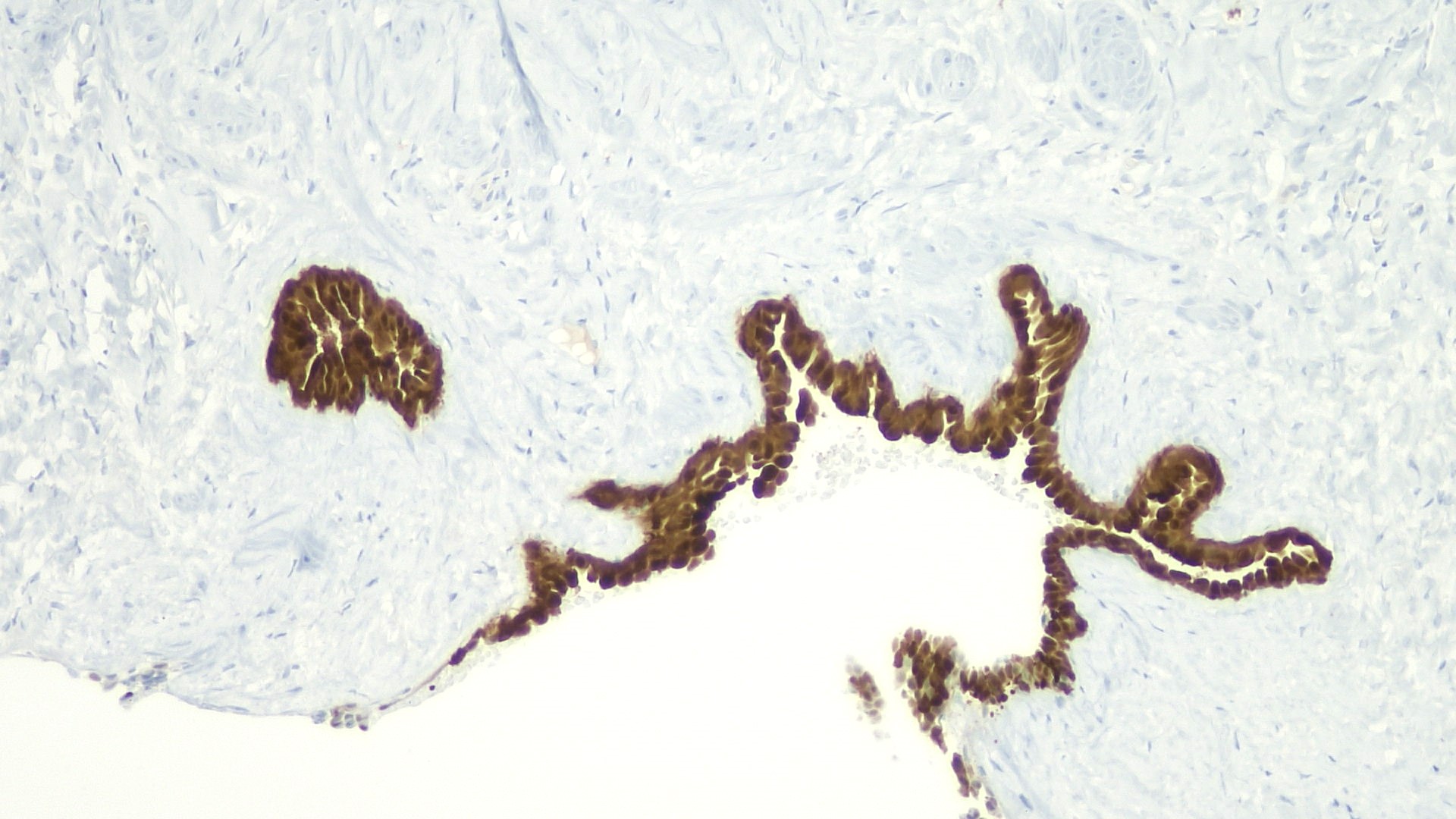
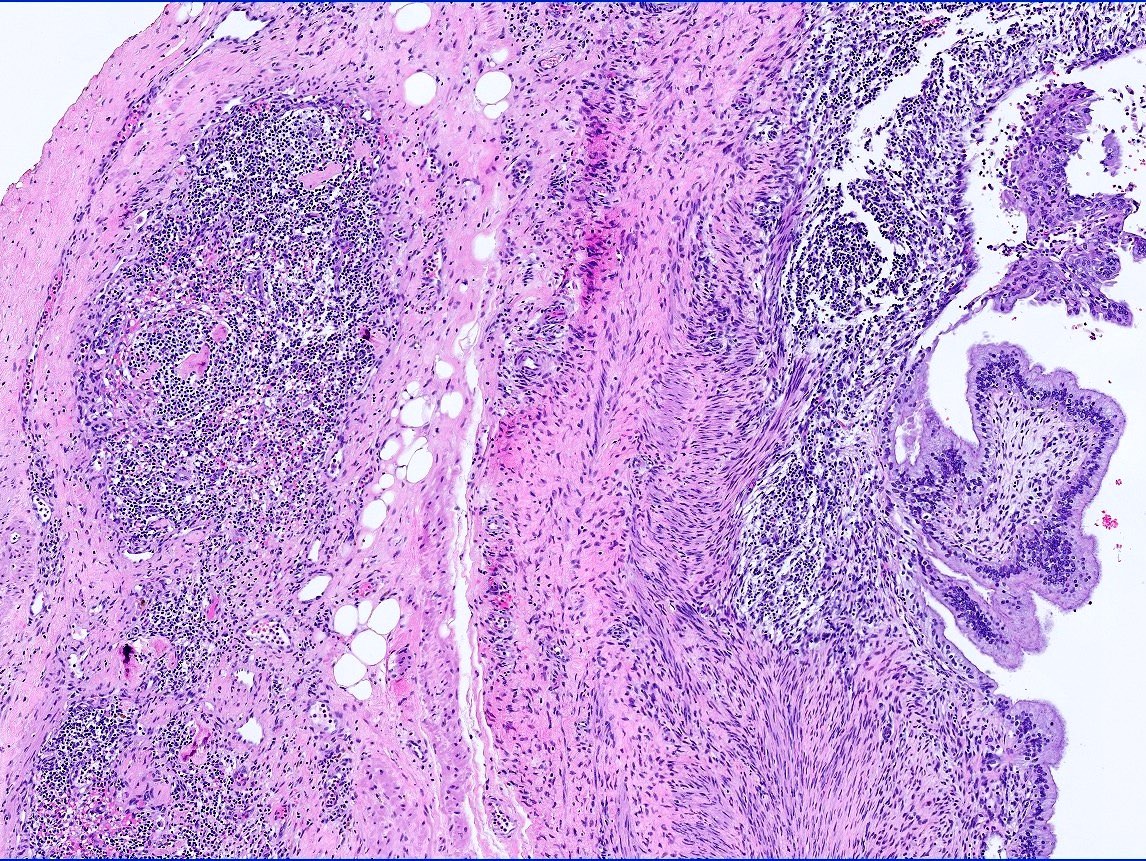
- LVI of the spermatic cord is pT2 not pT3
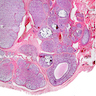
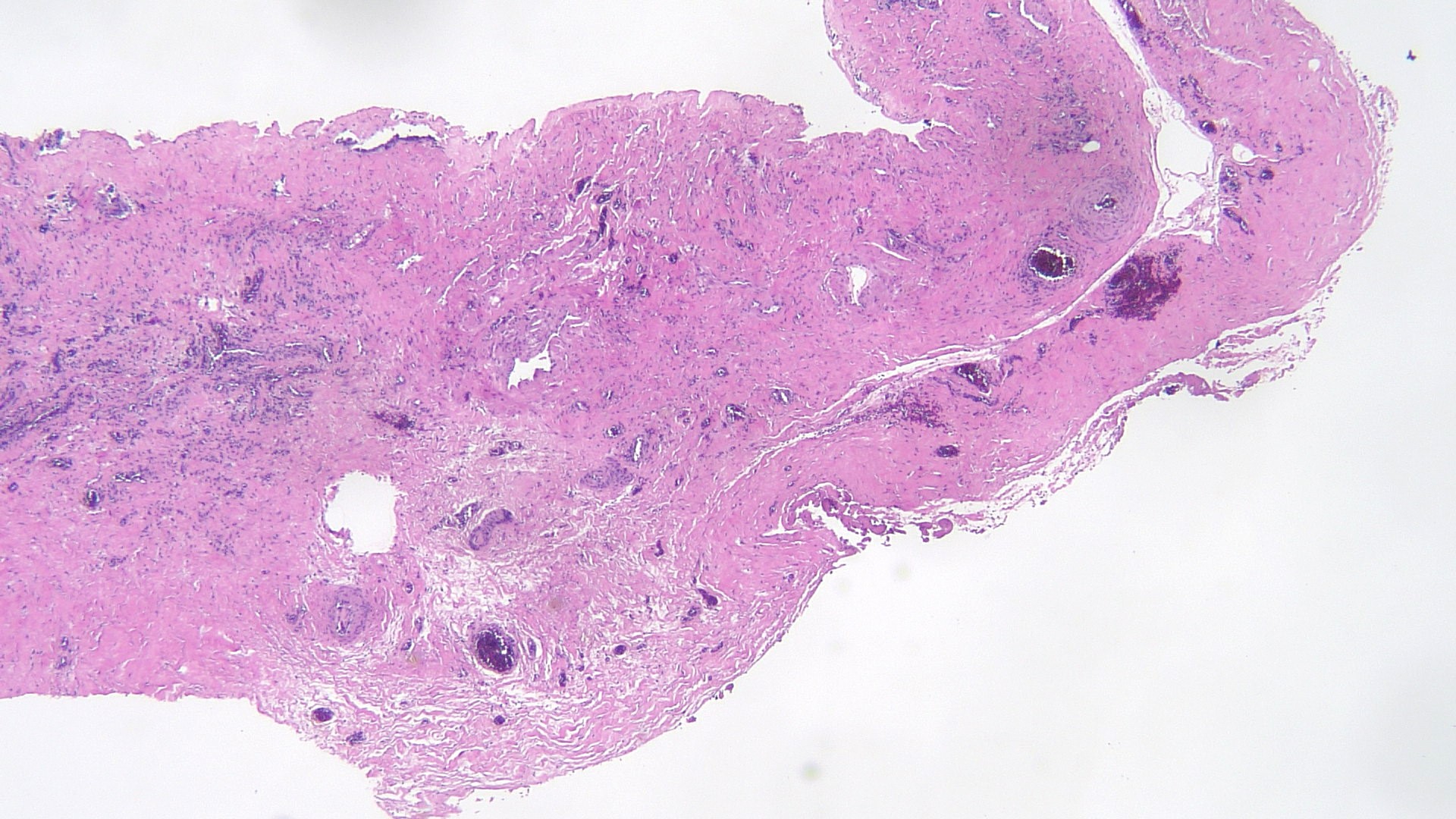
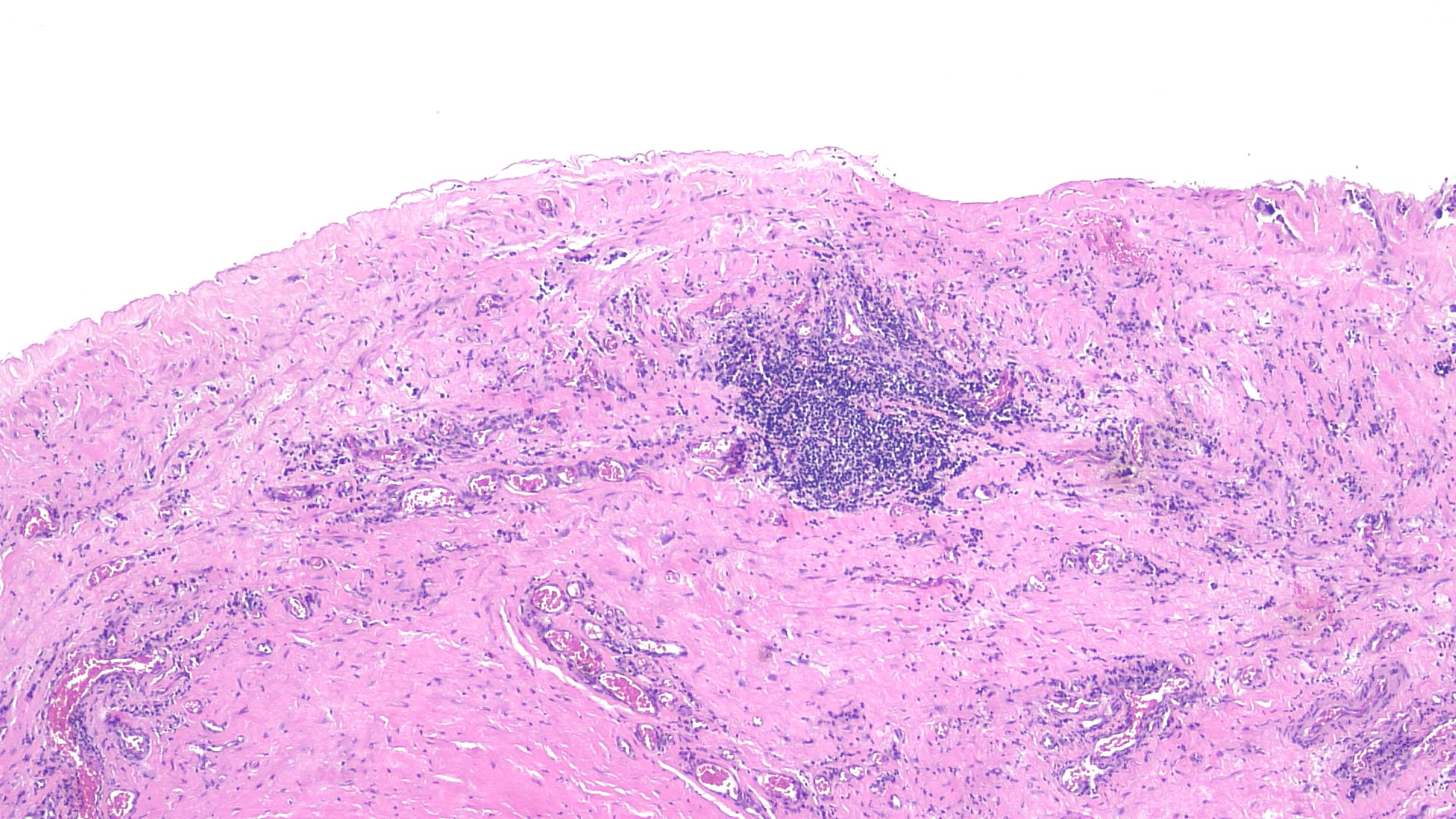
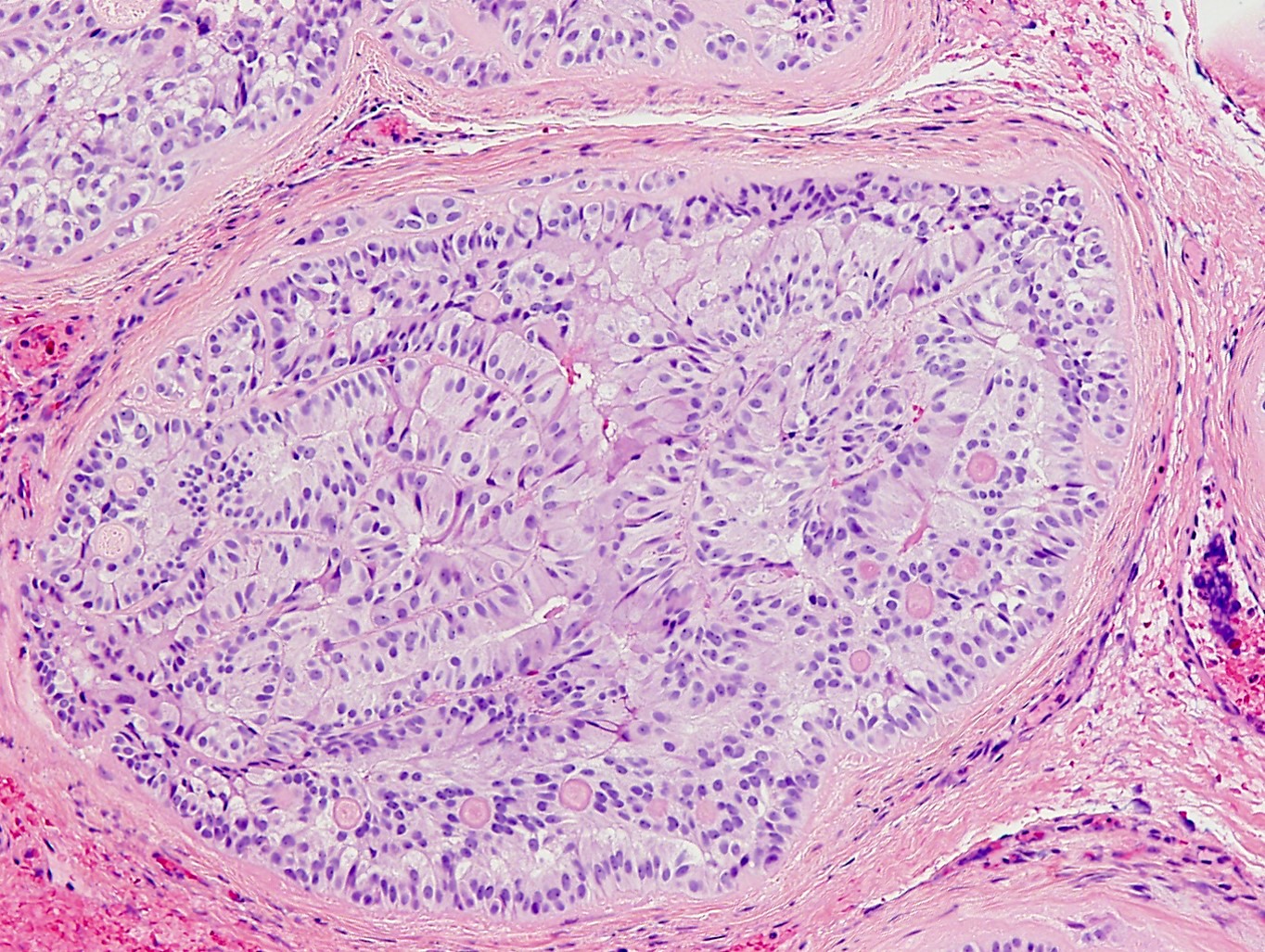
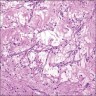
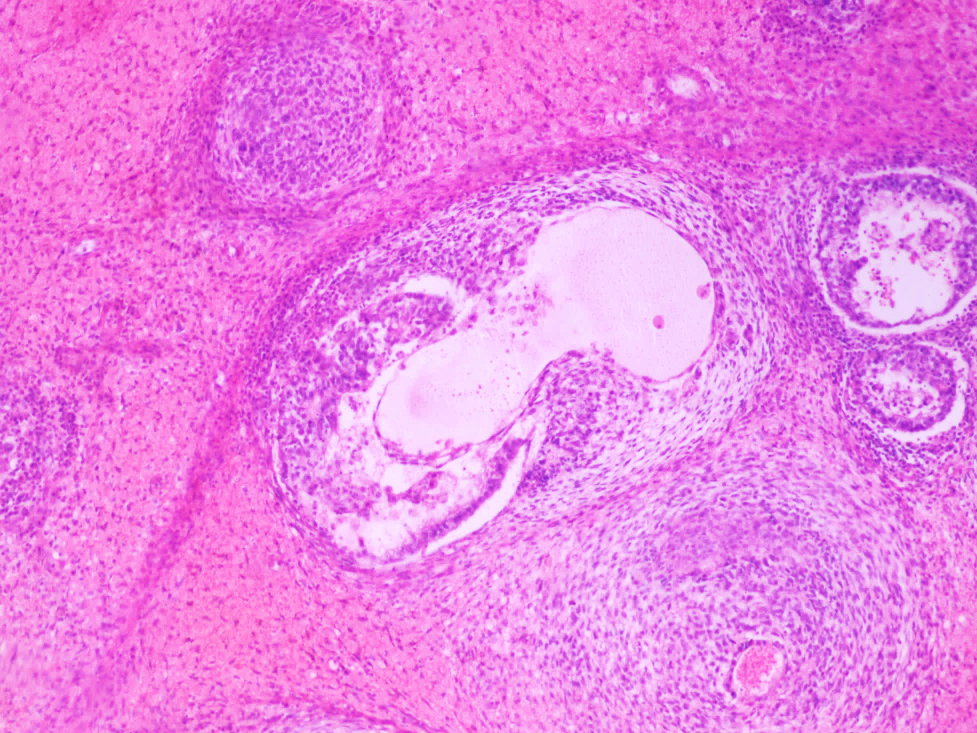
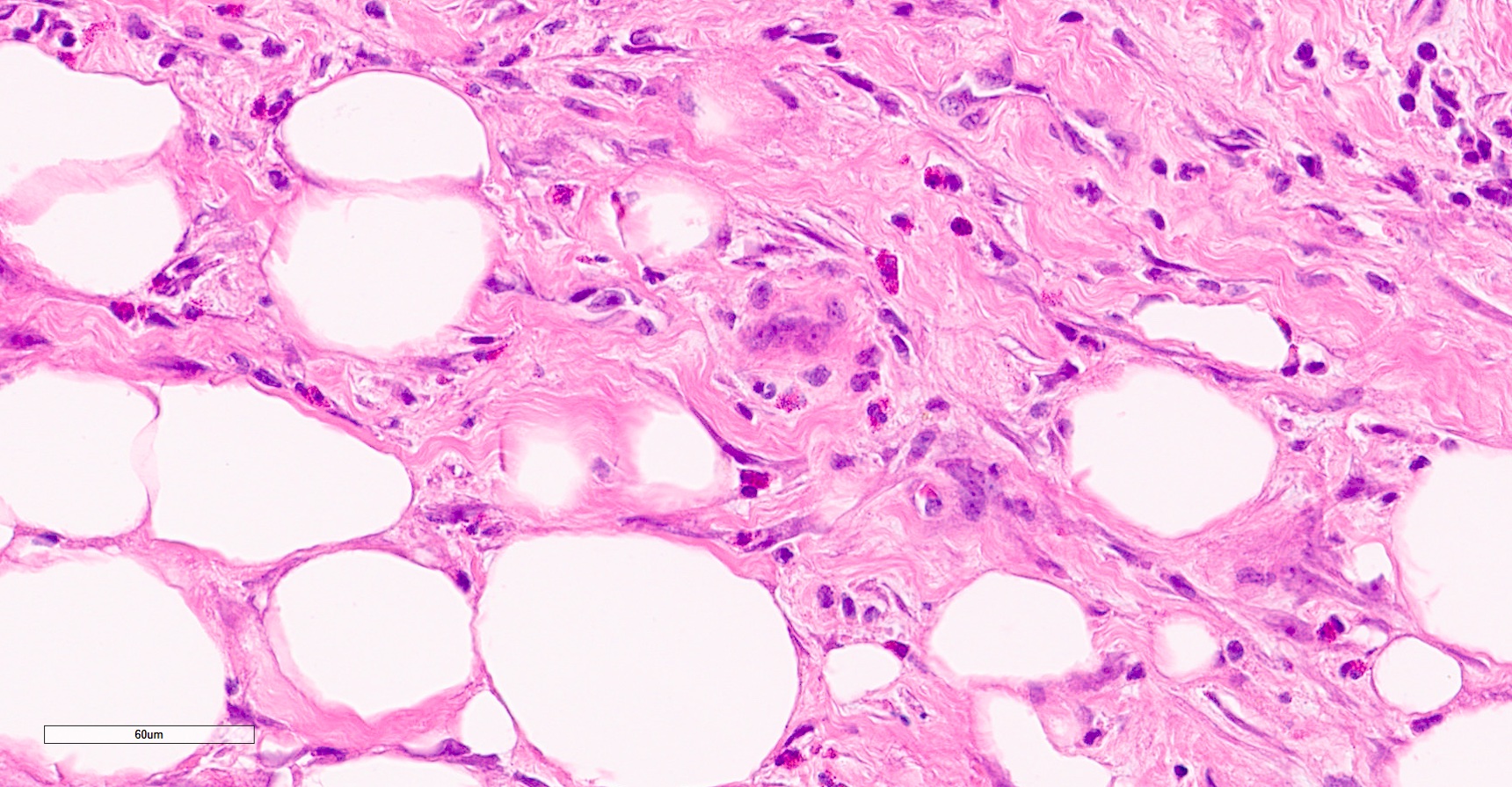
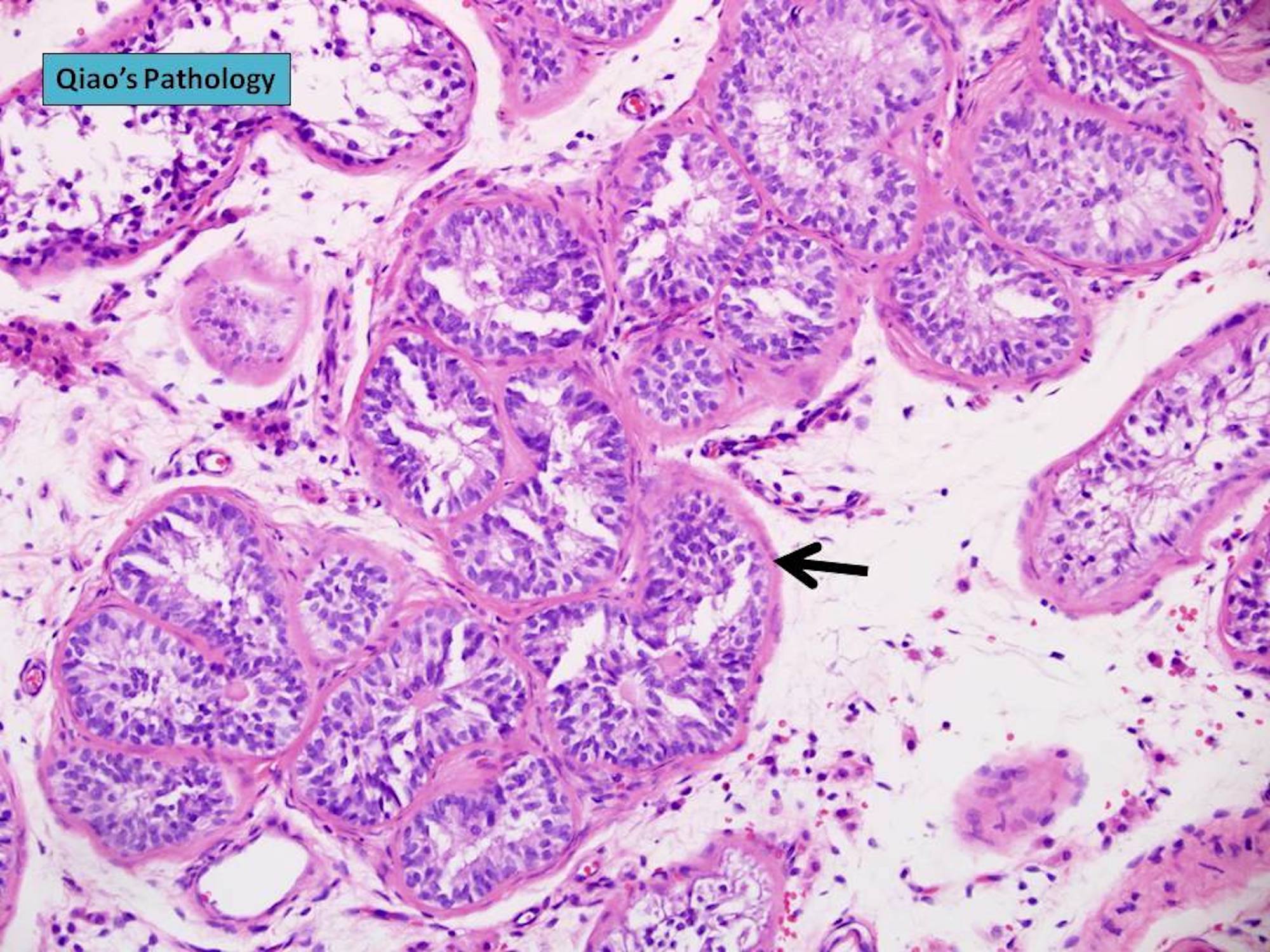
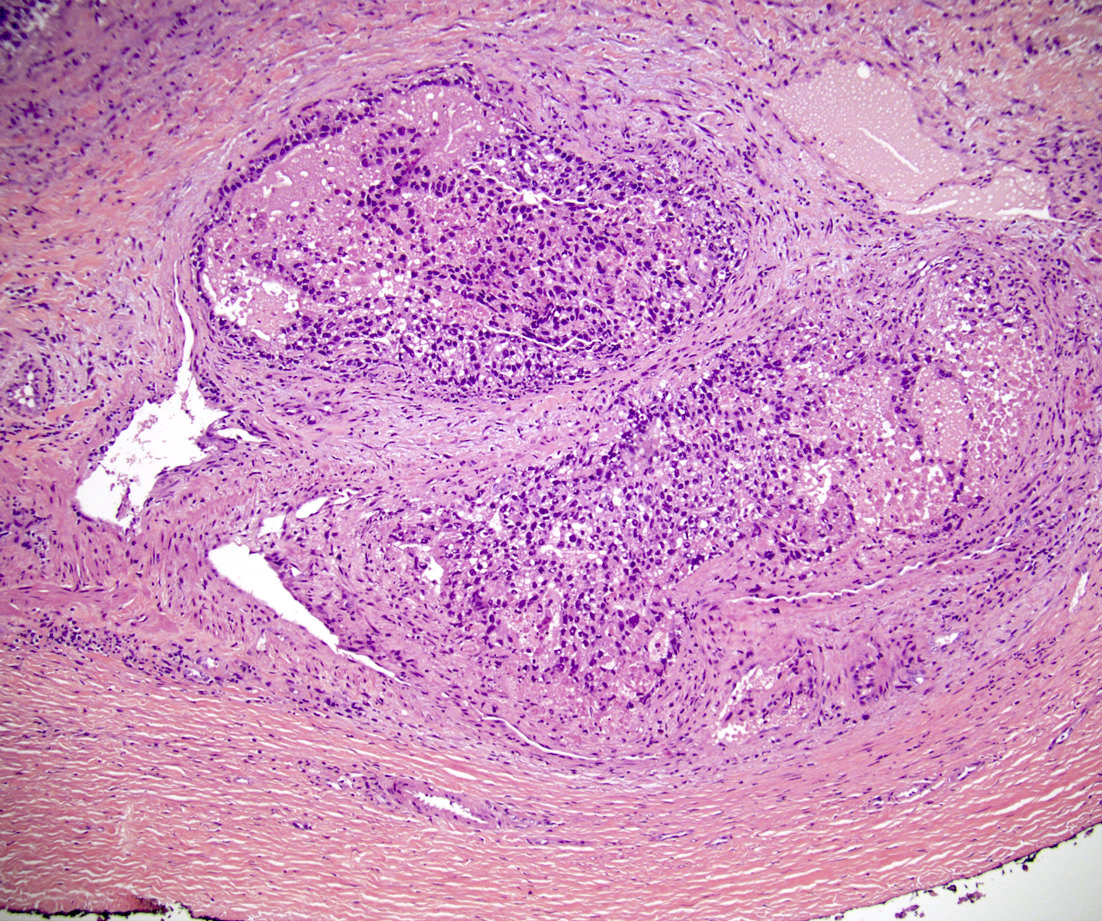
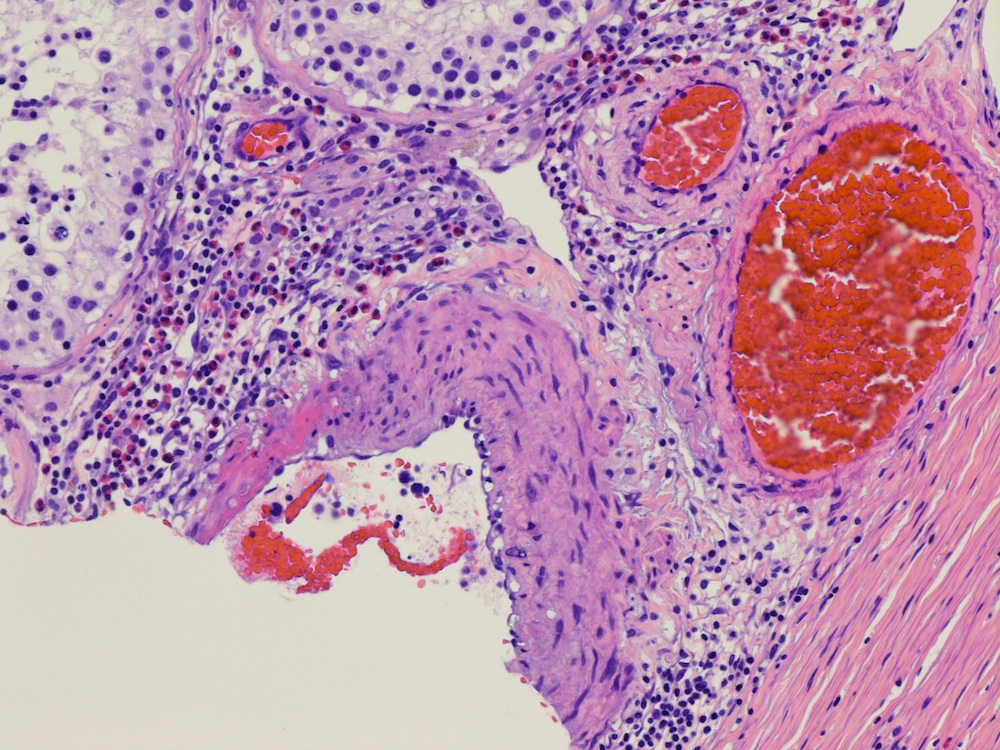
- pT2
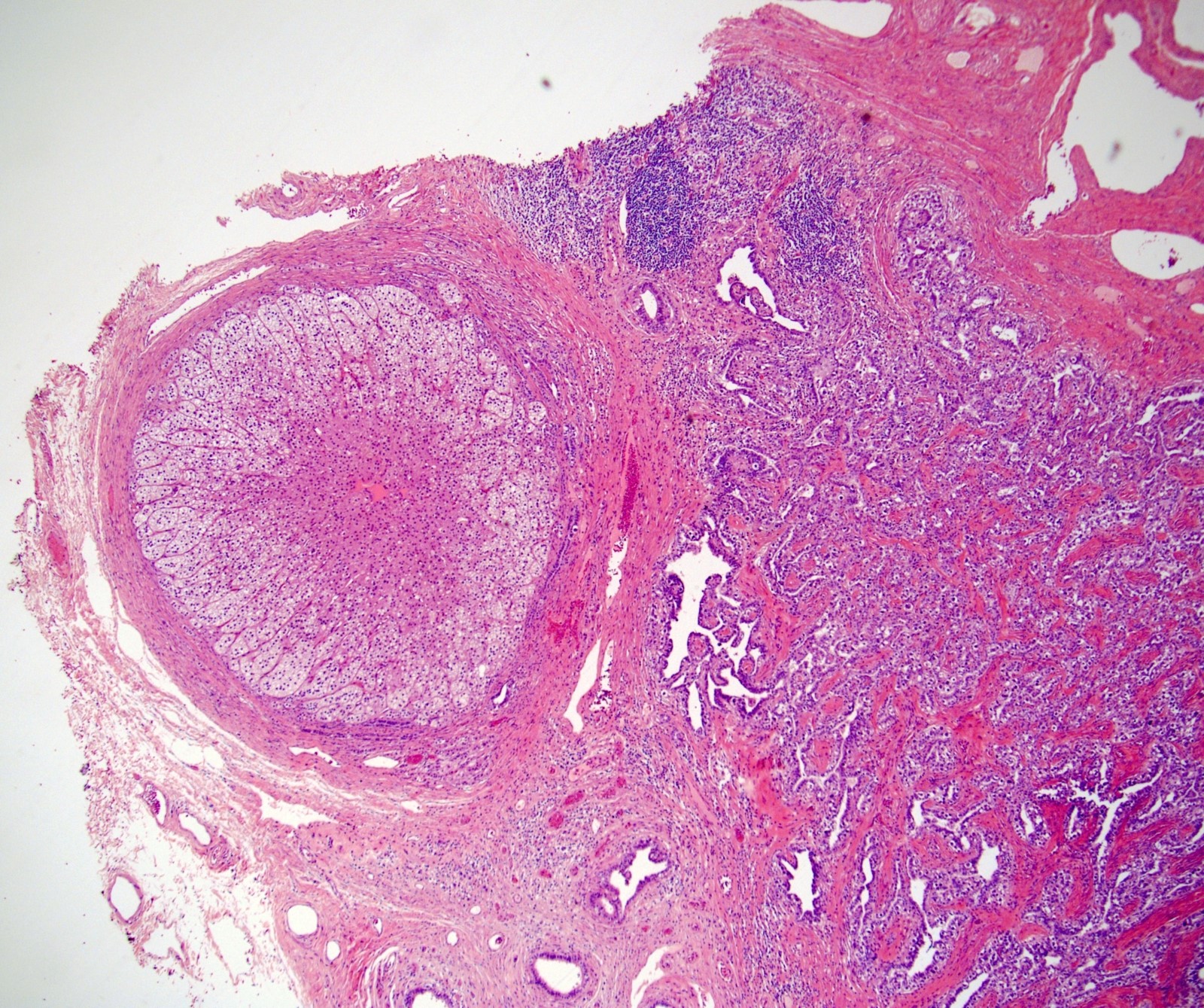
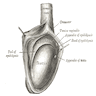
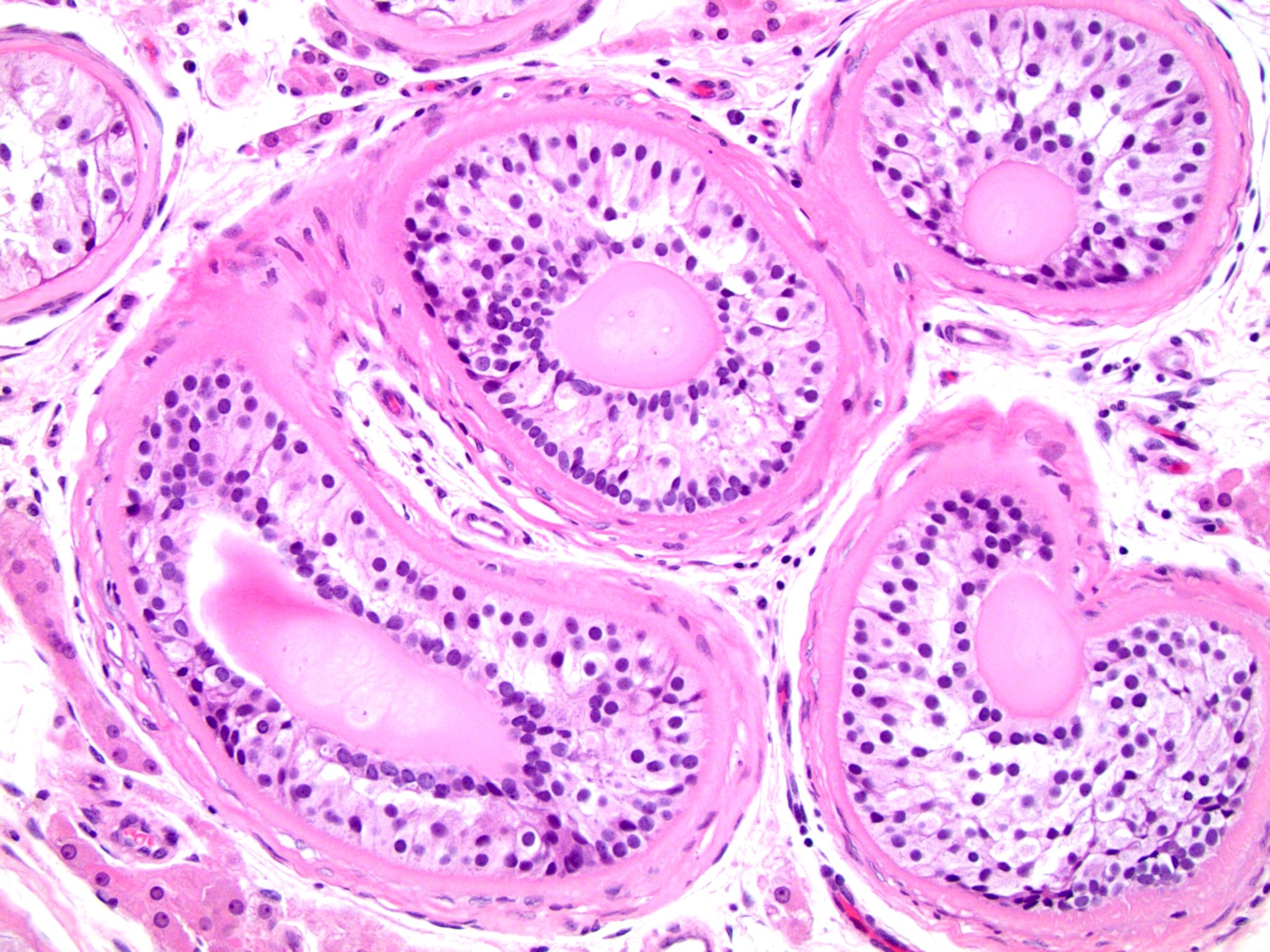
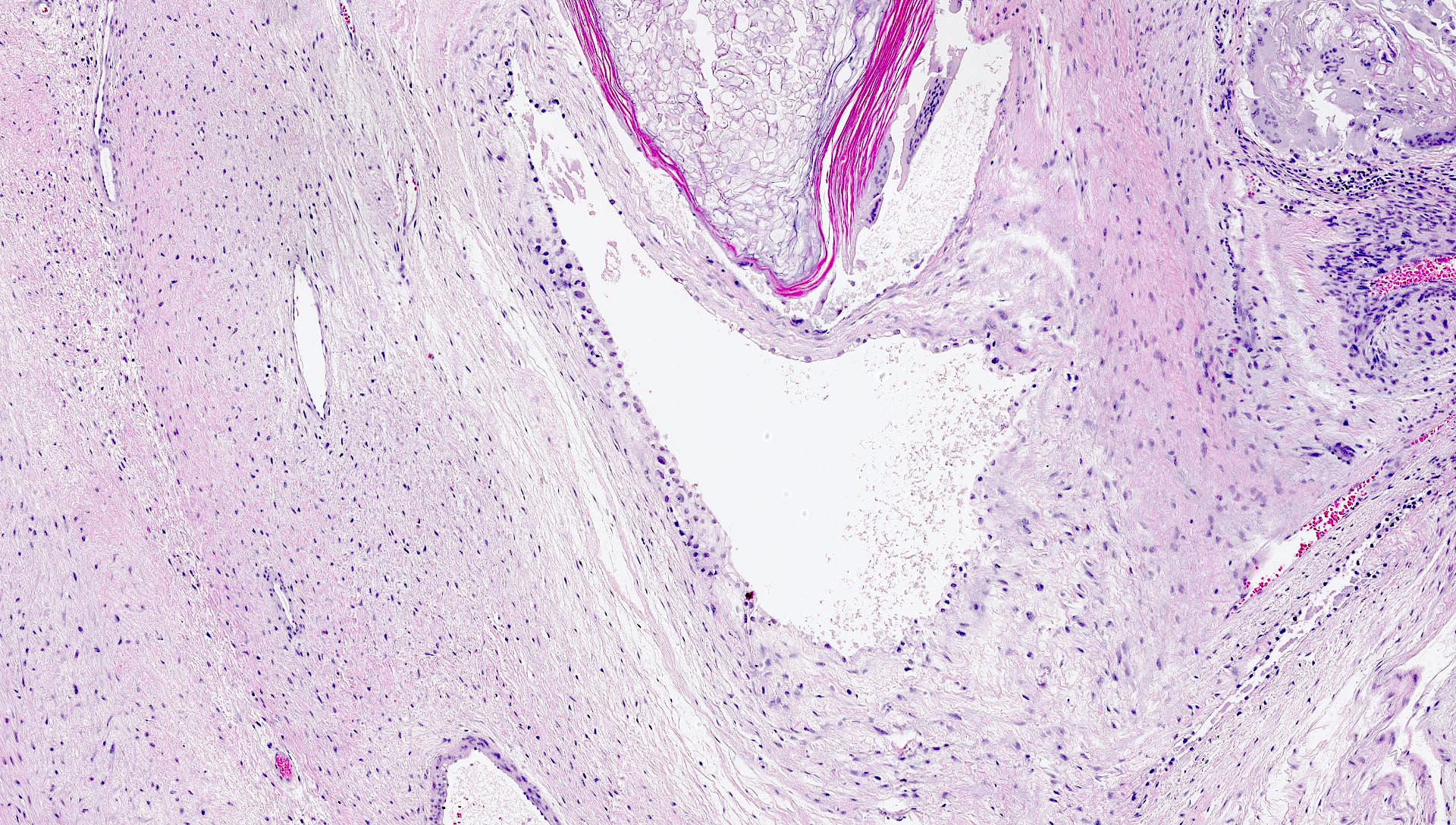
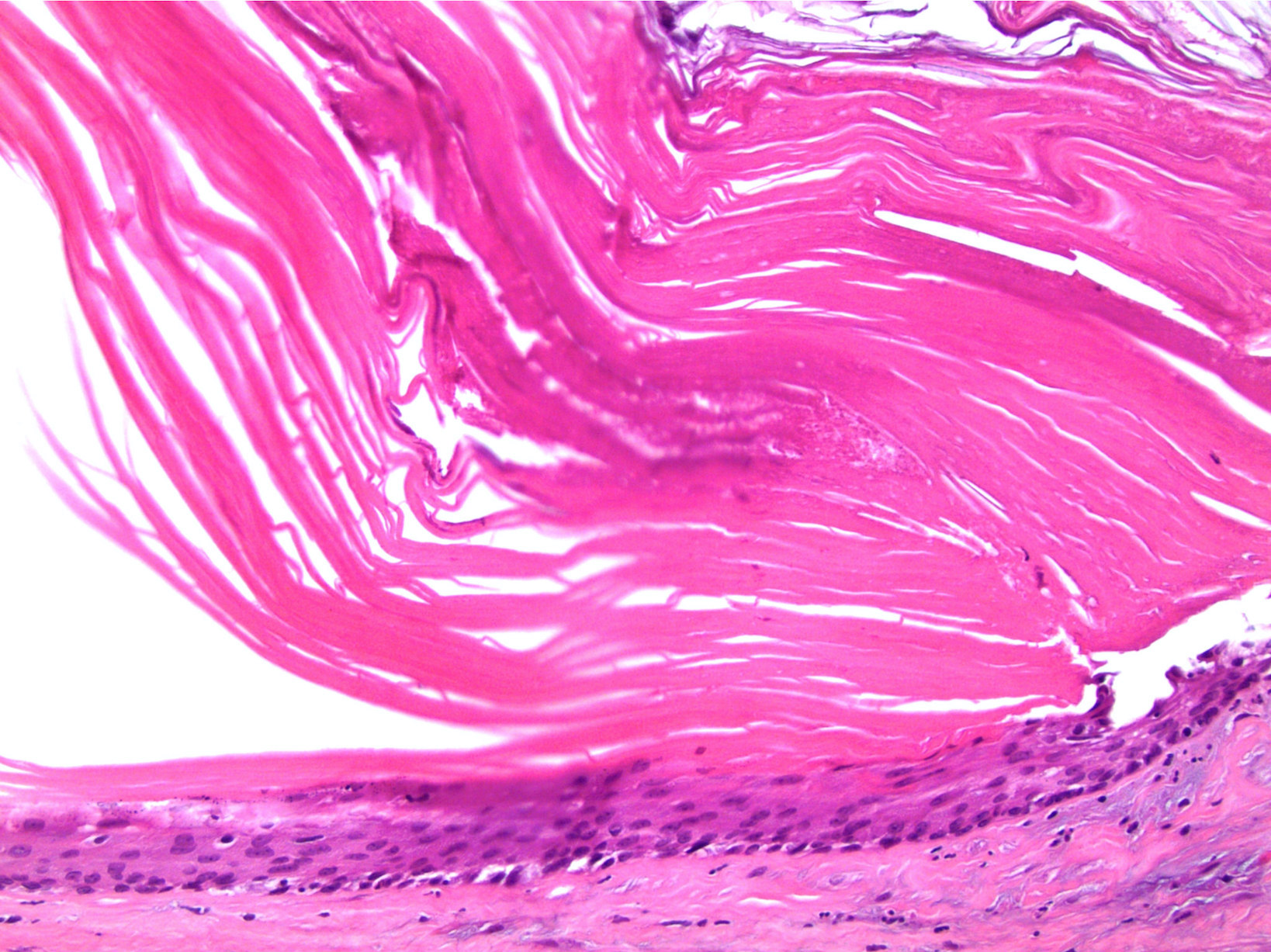
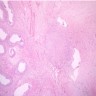
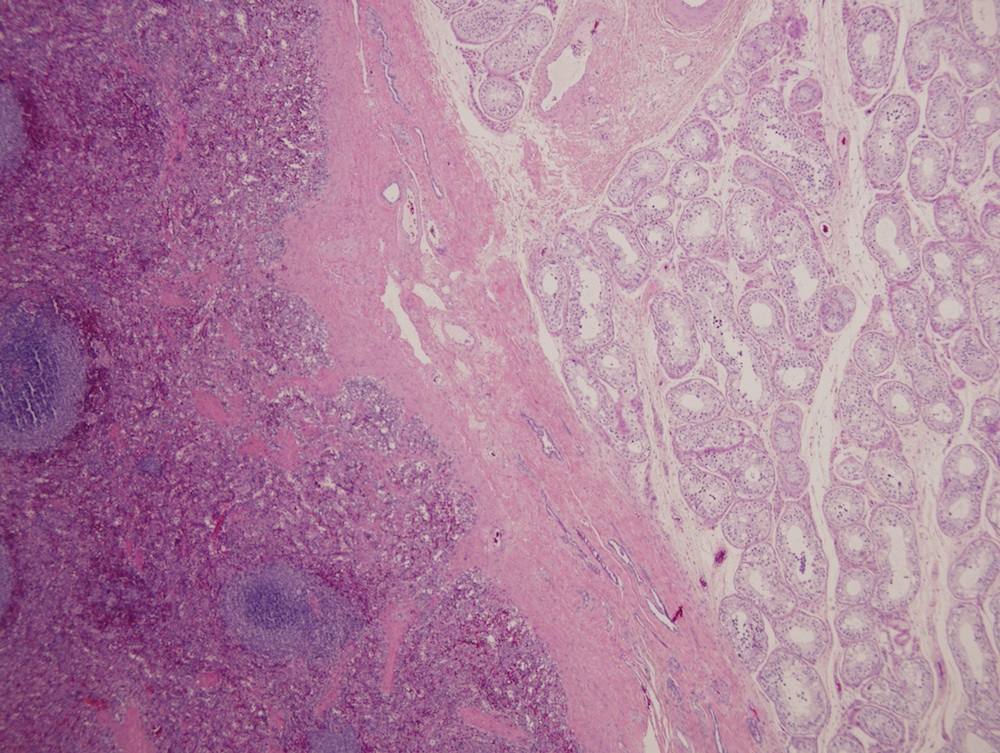
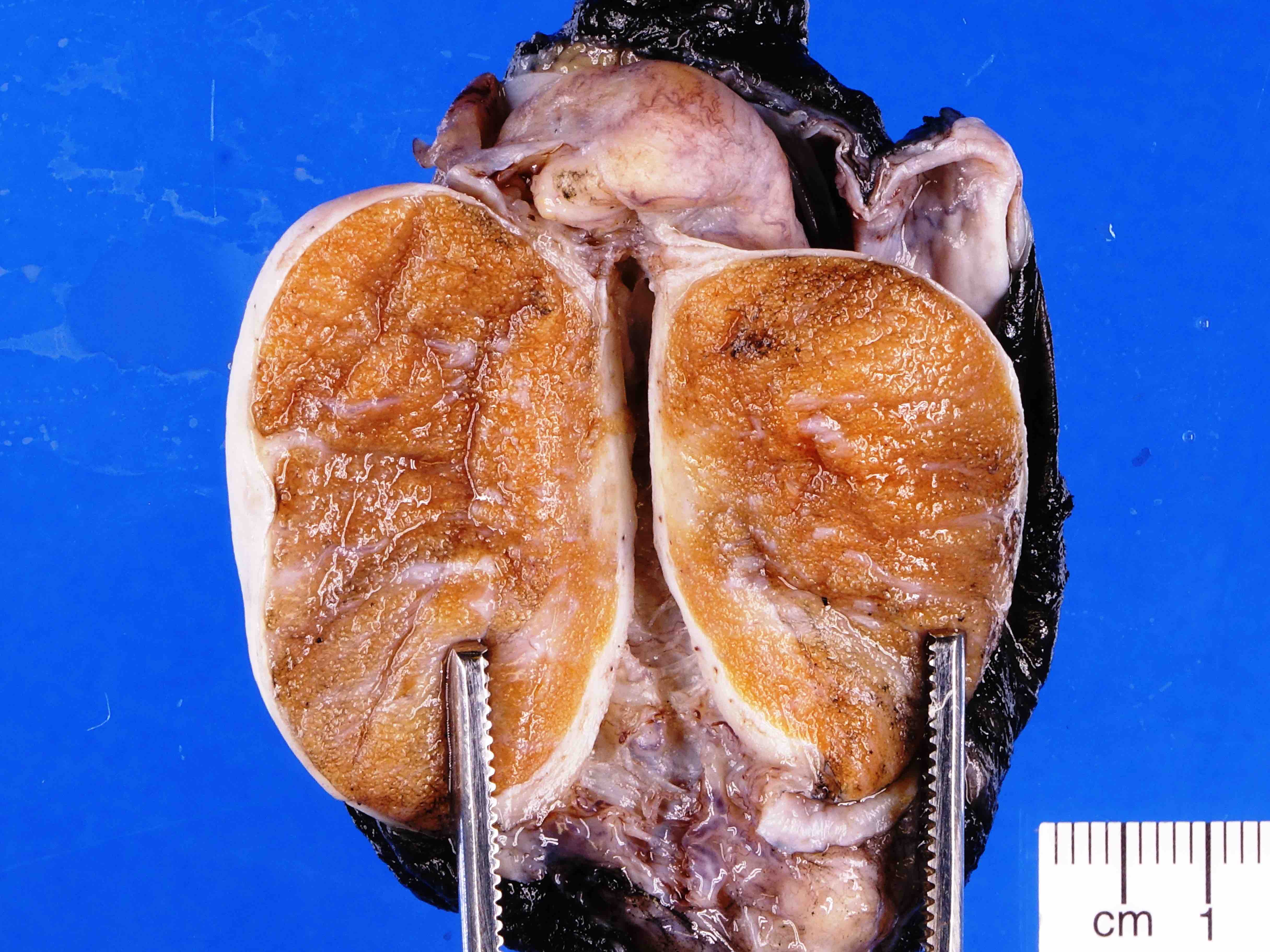
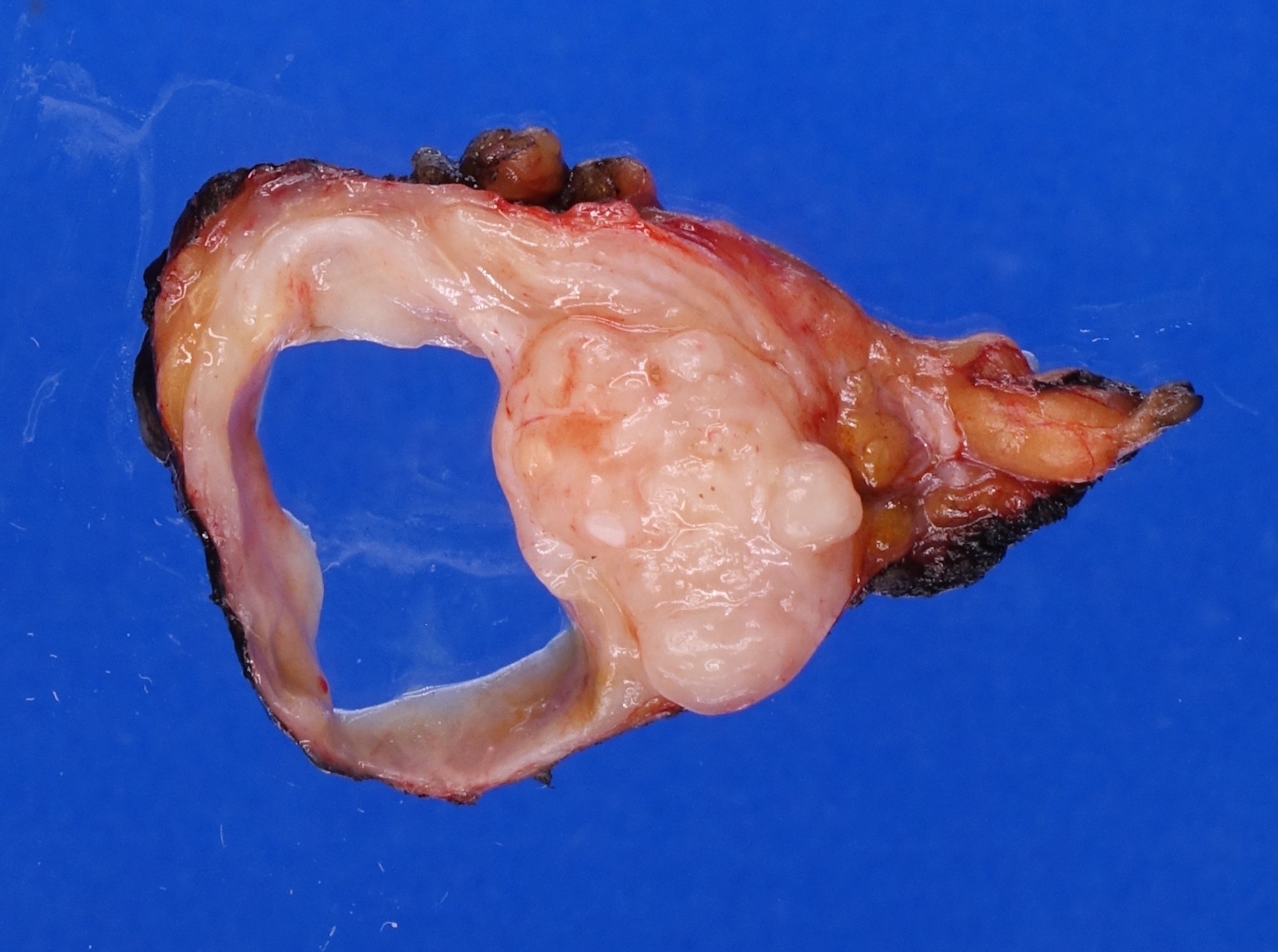
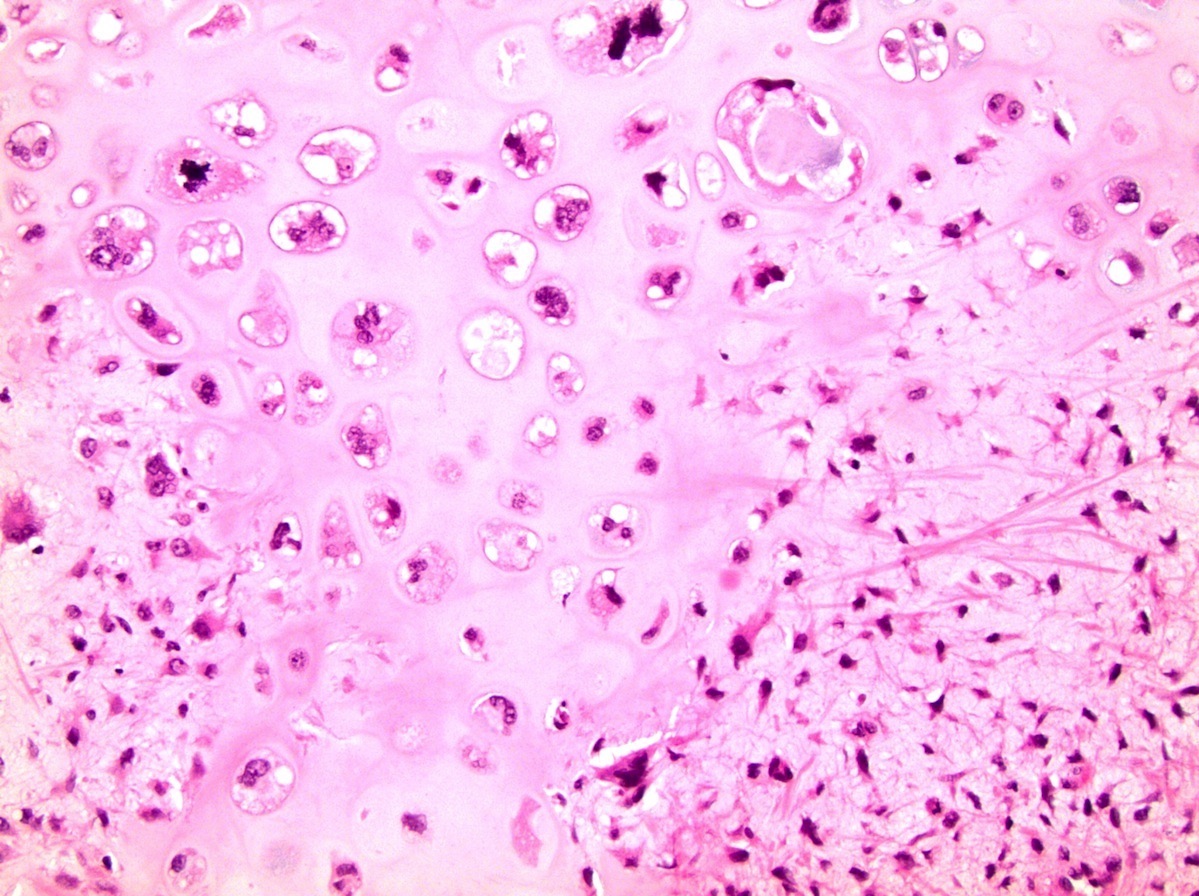
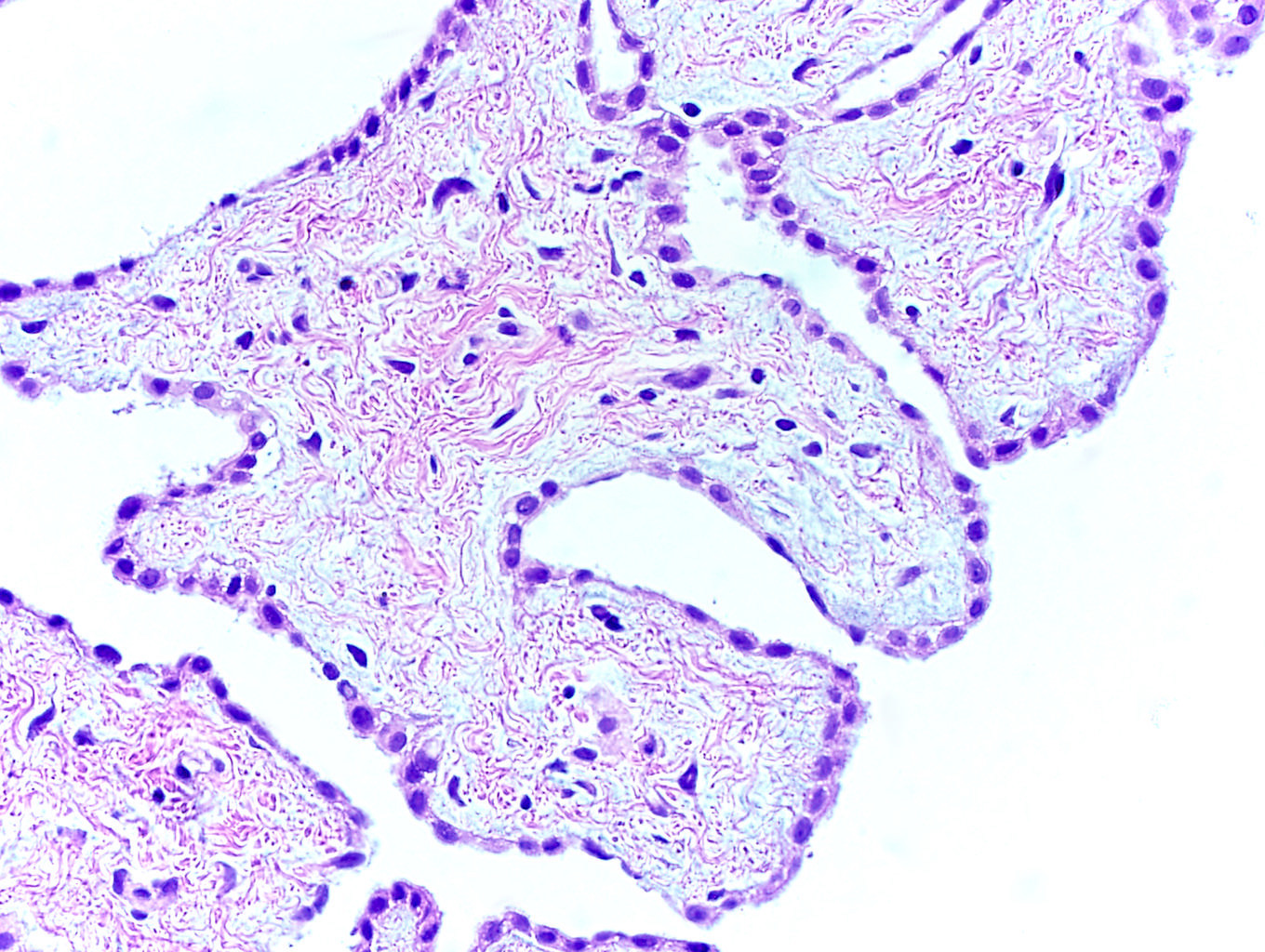
- Involvement of the tunica albuginea does not impact pT classification
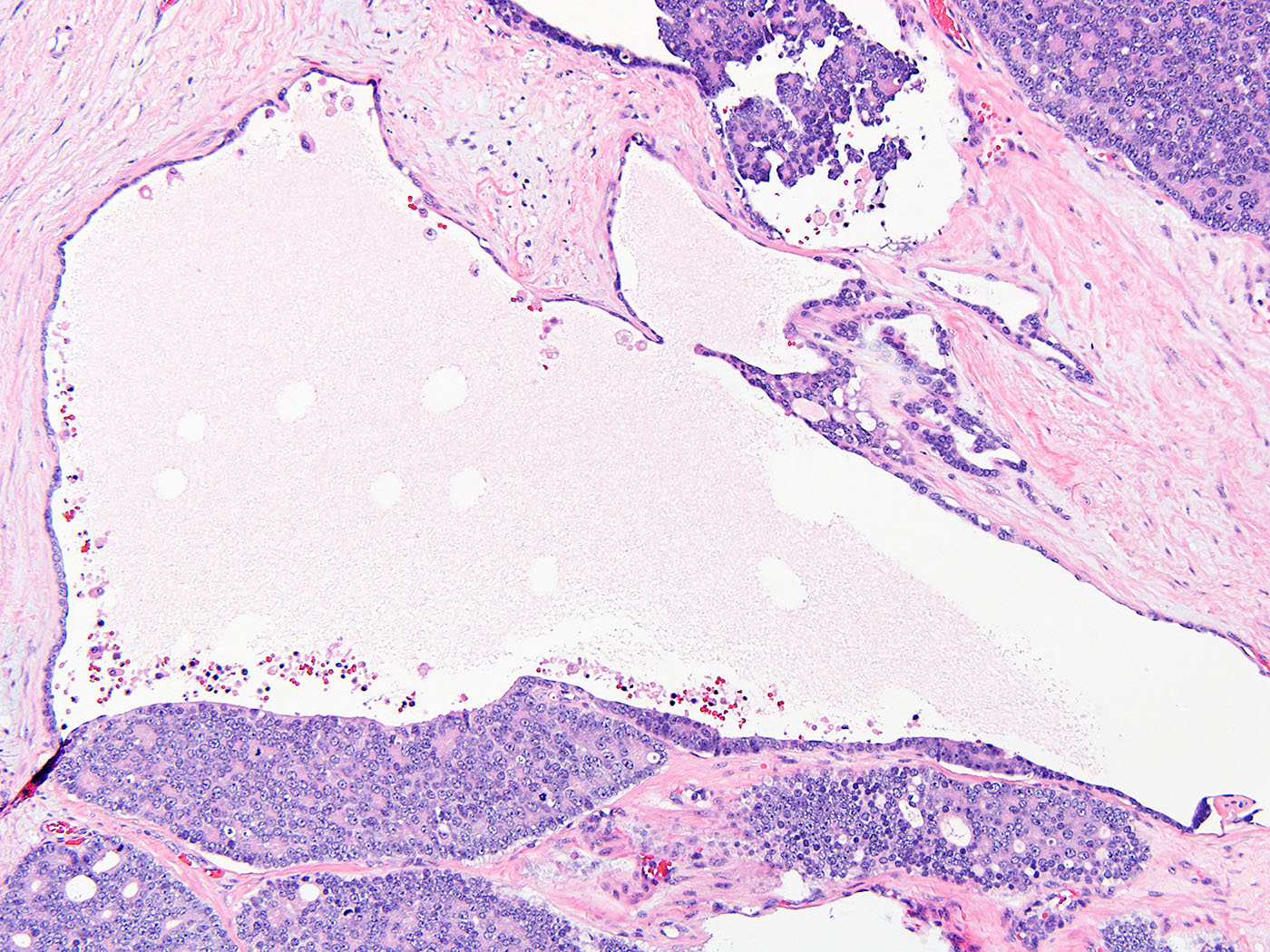
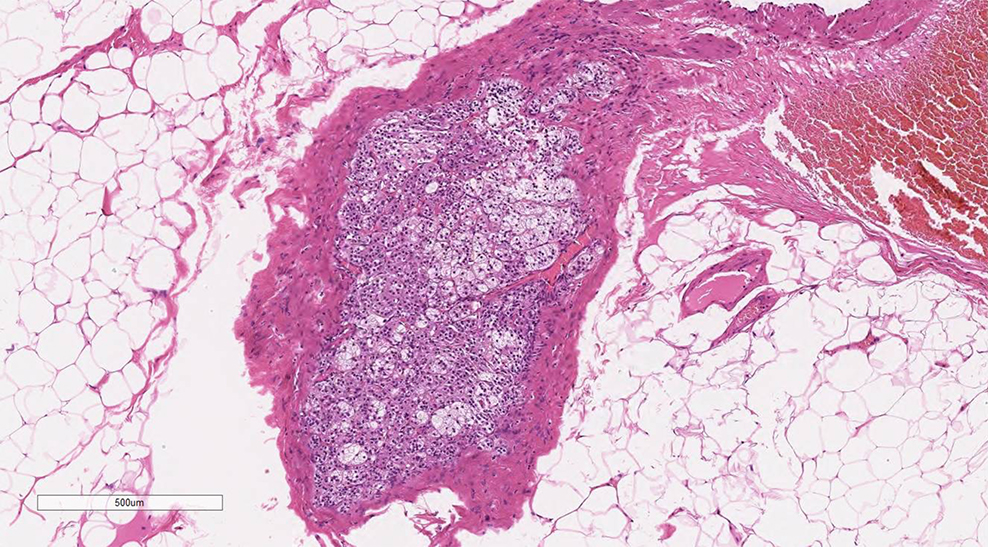
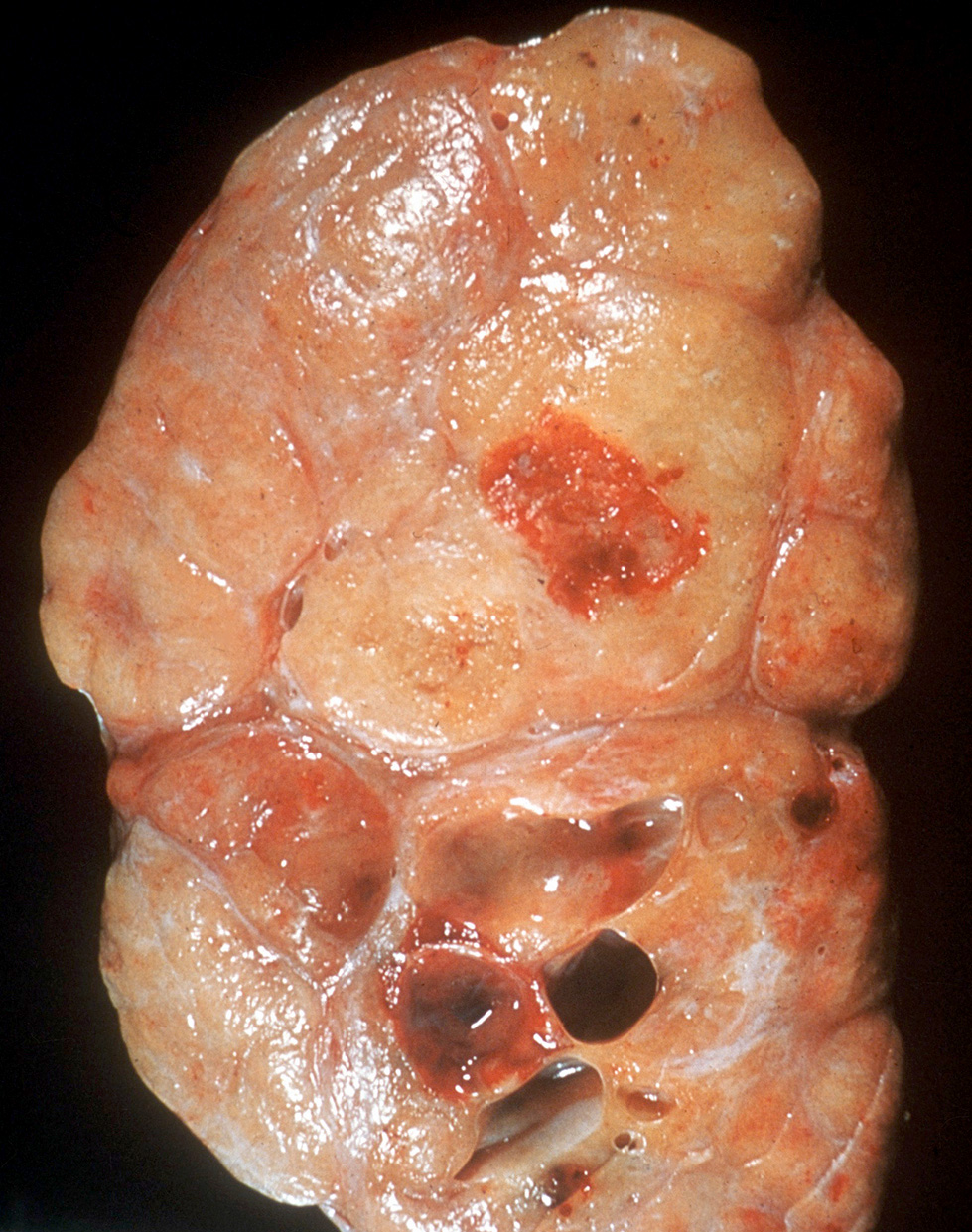
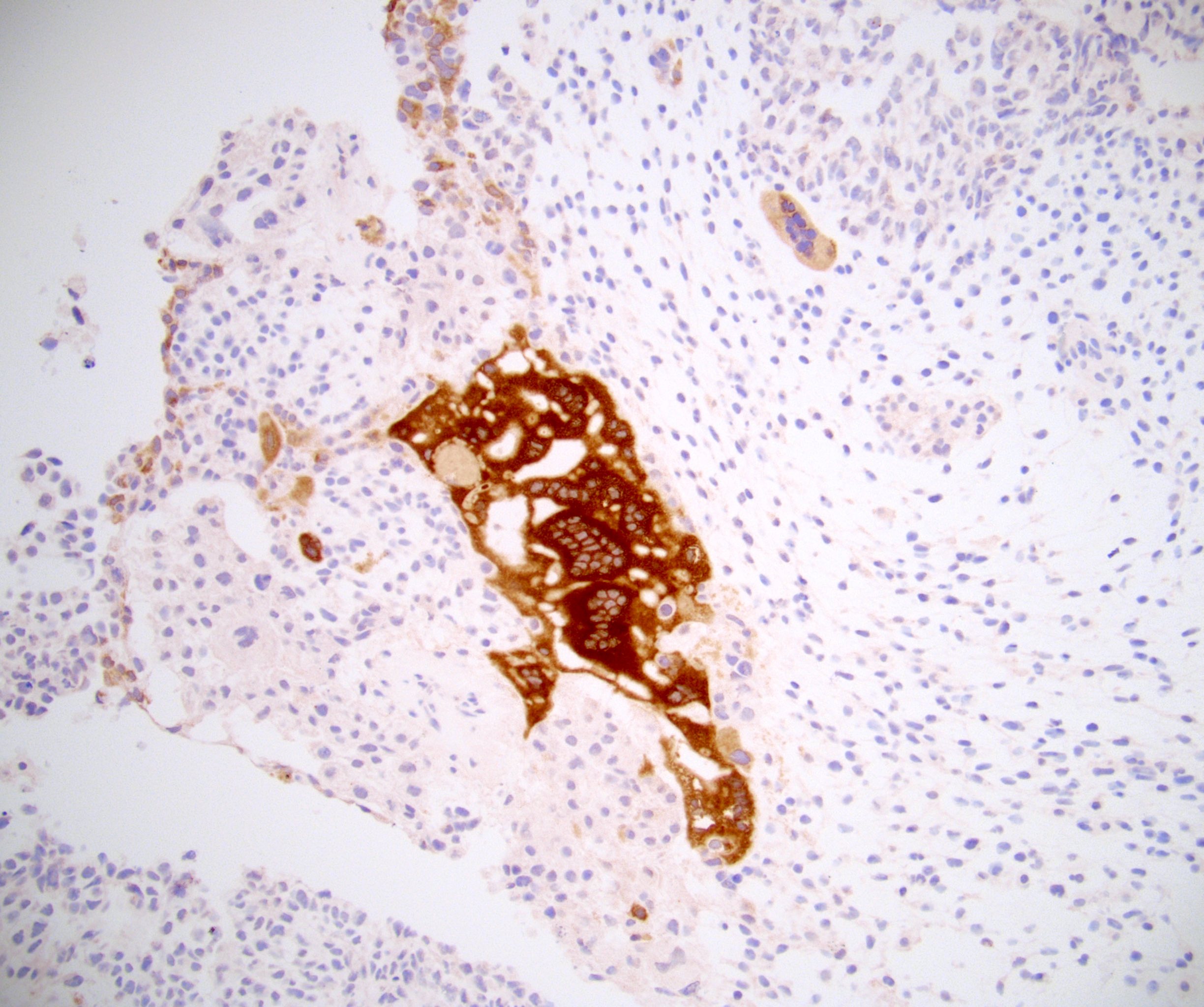
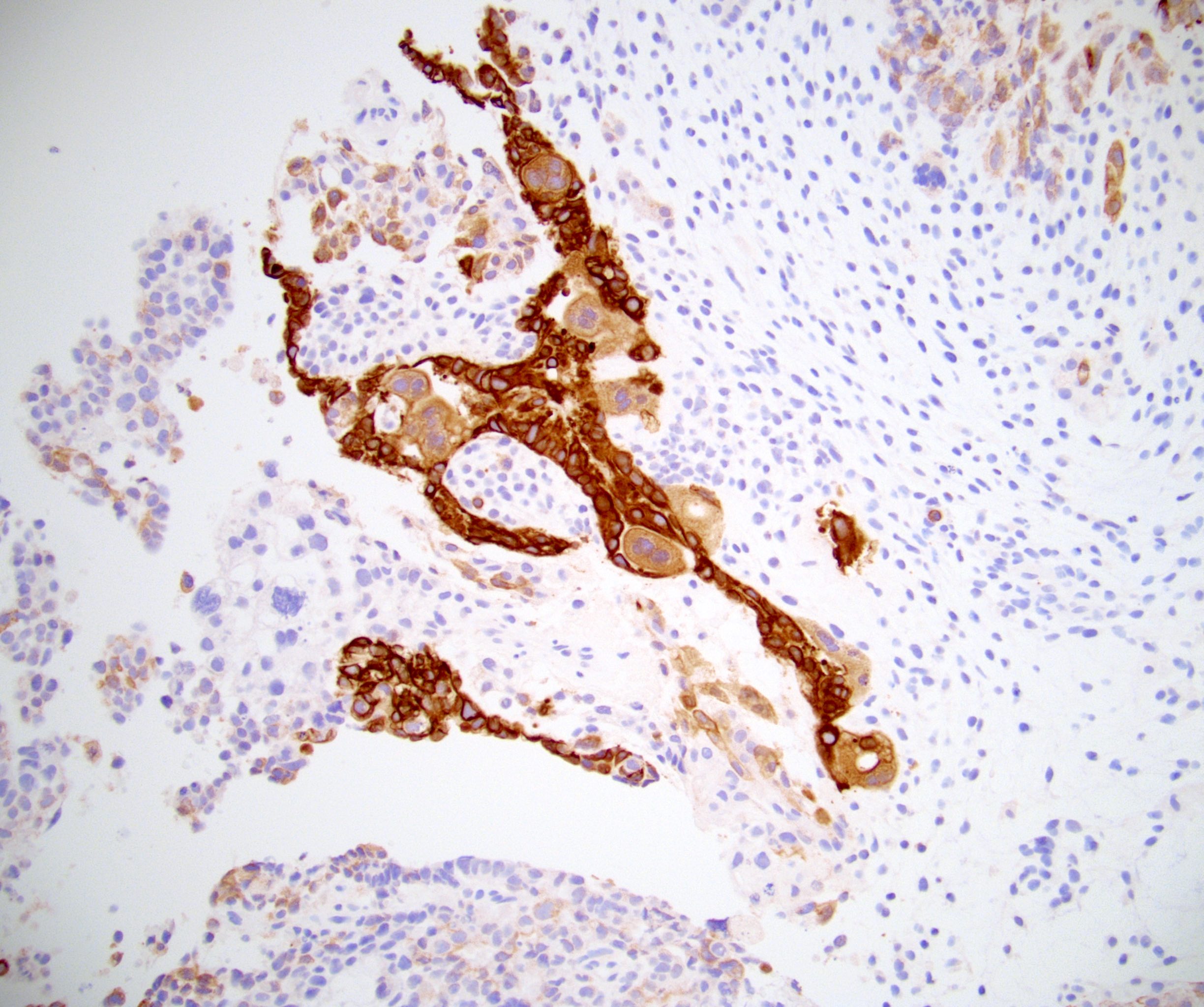
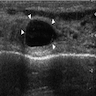
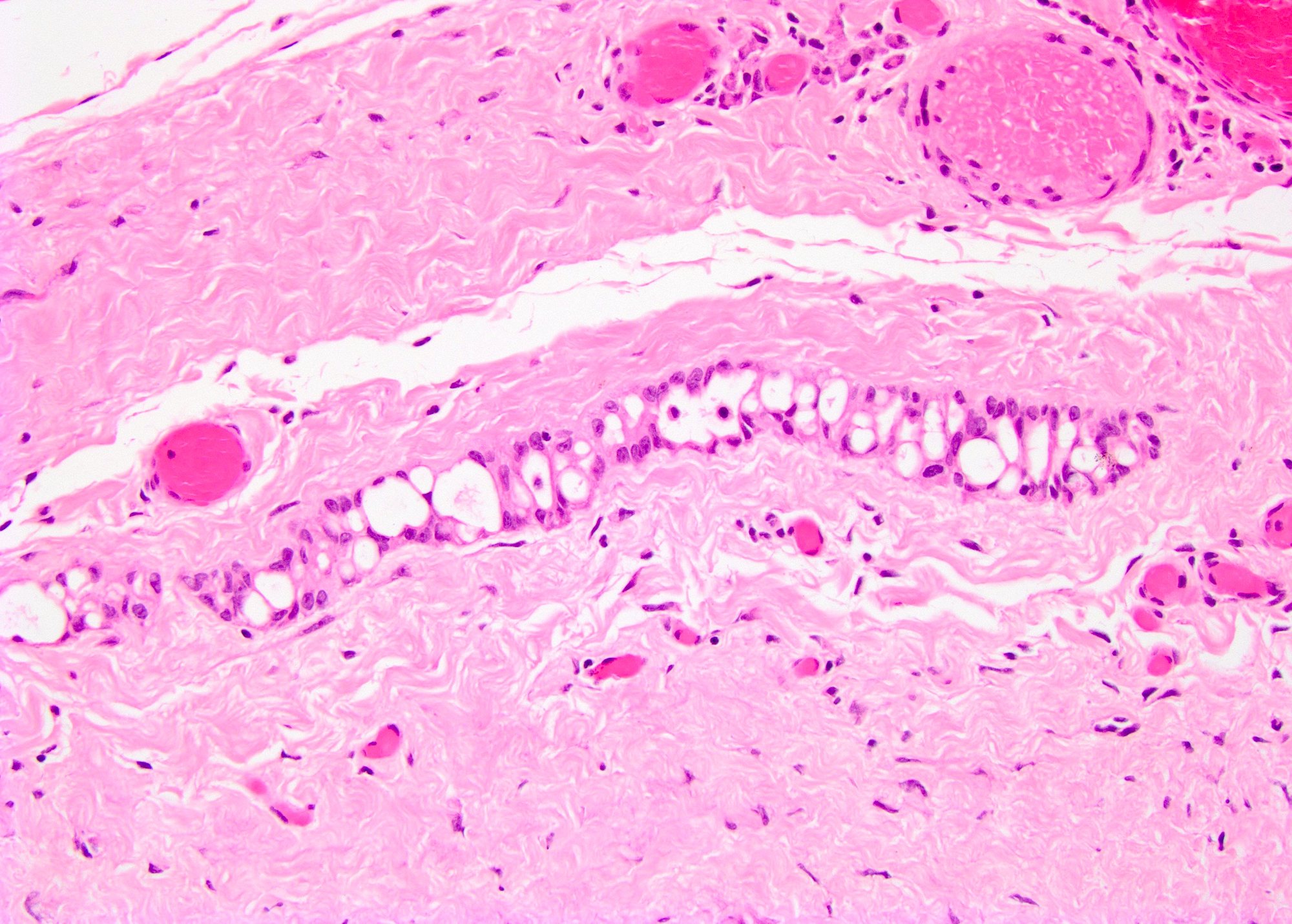
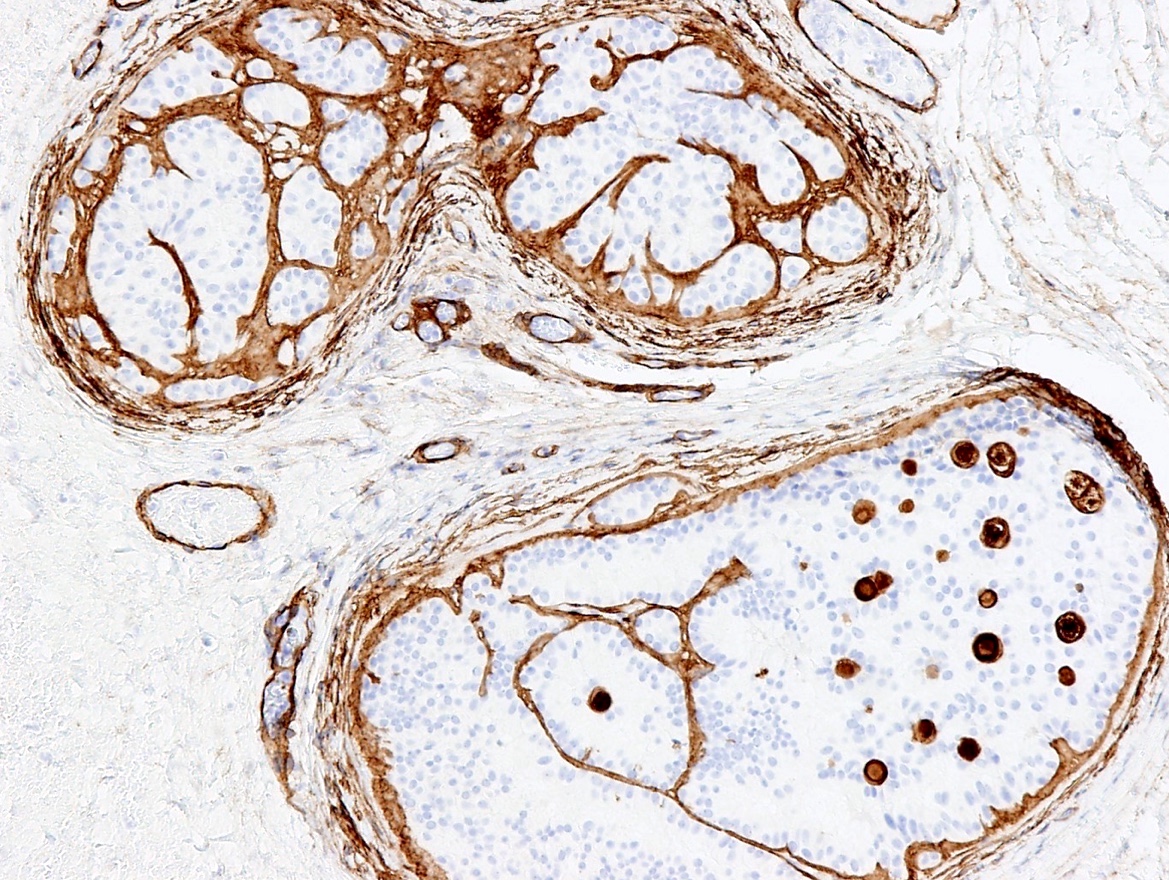
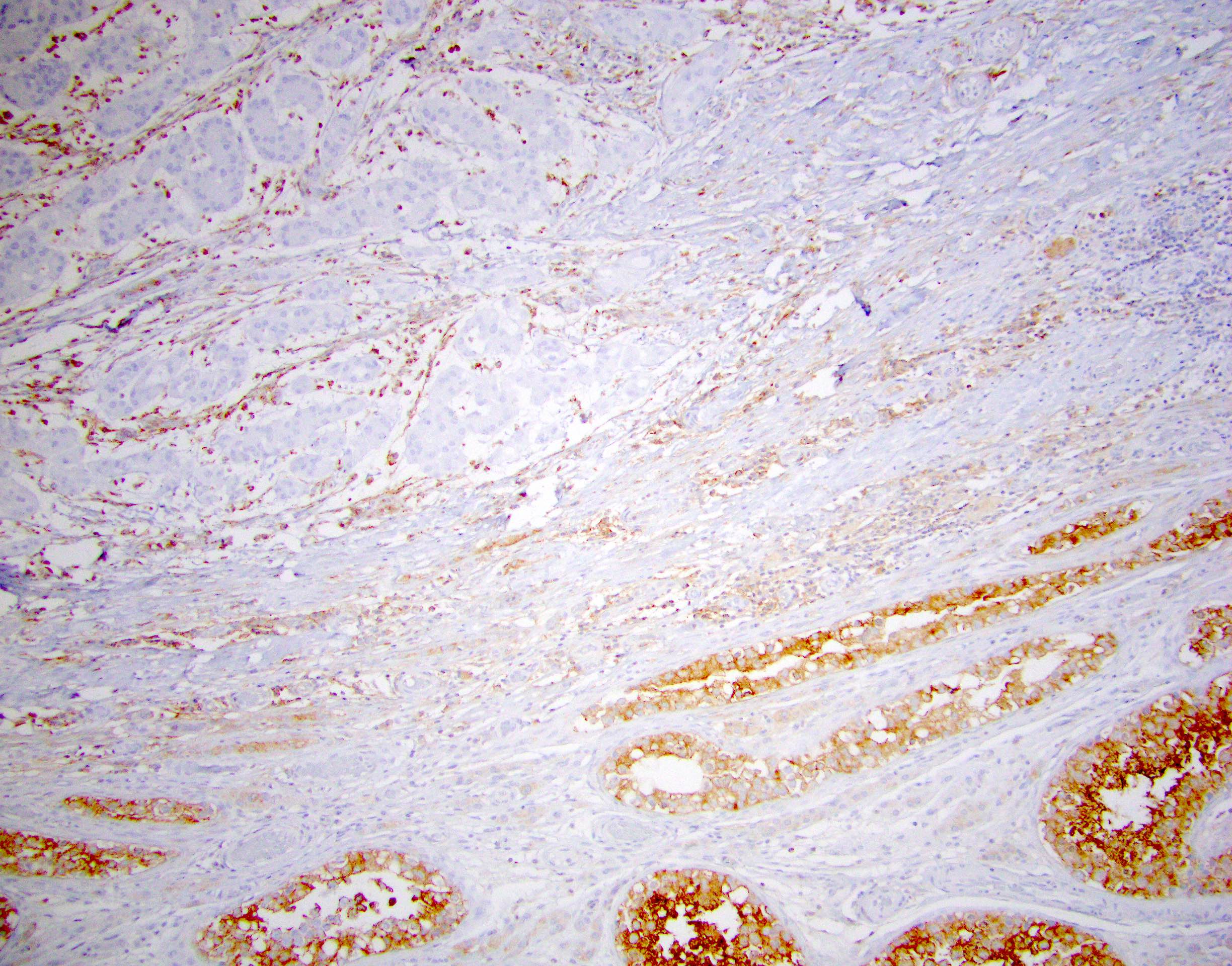
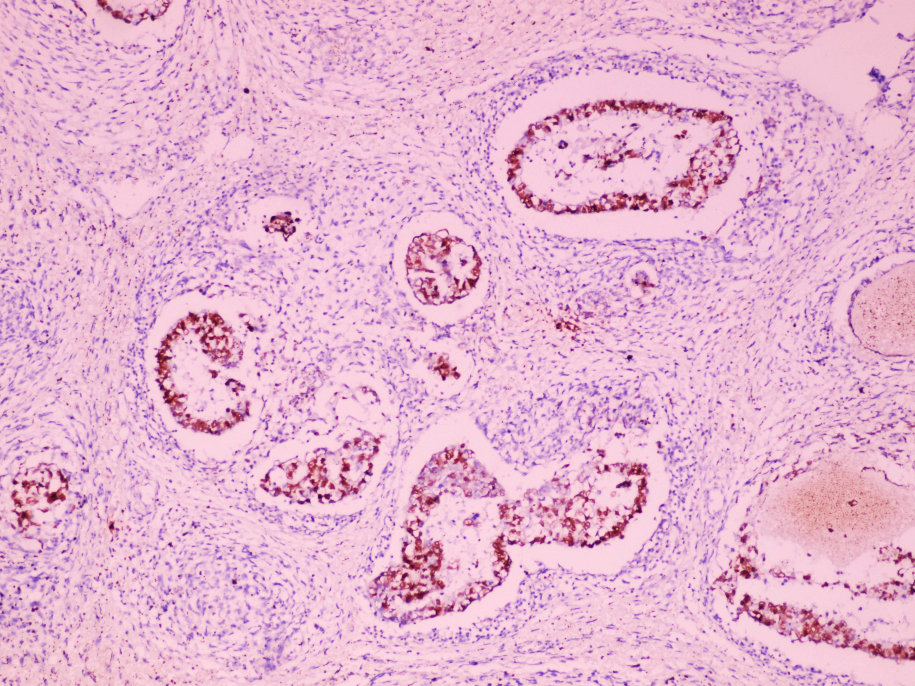
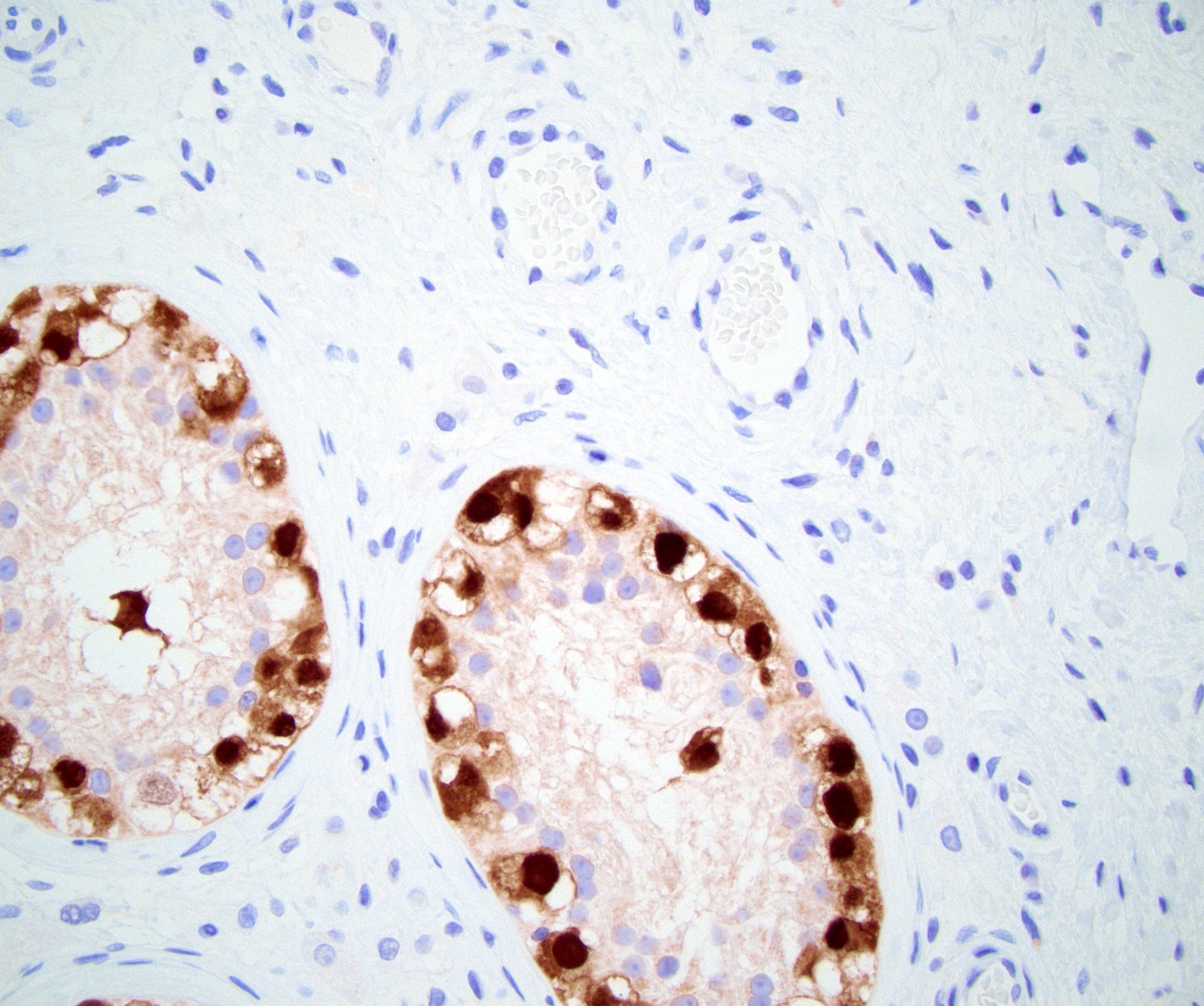
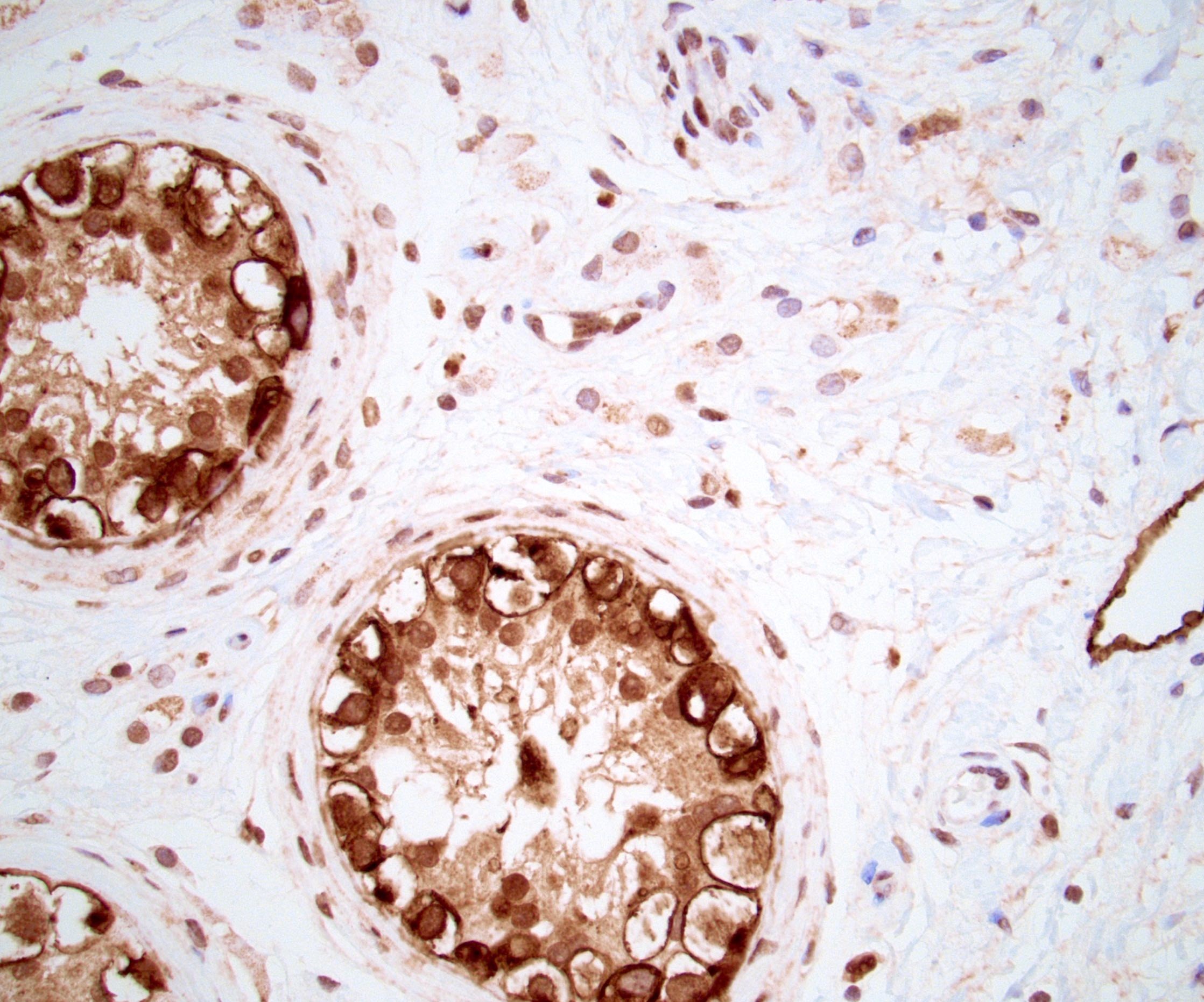
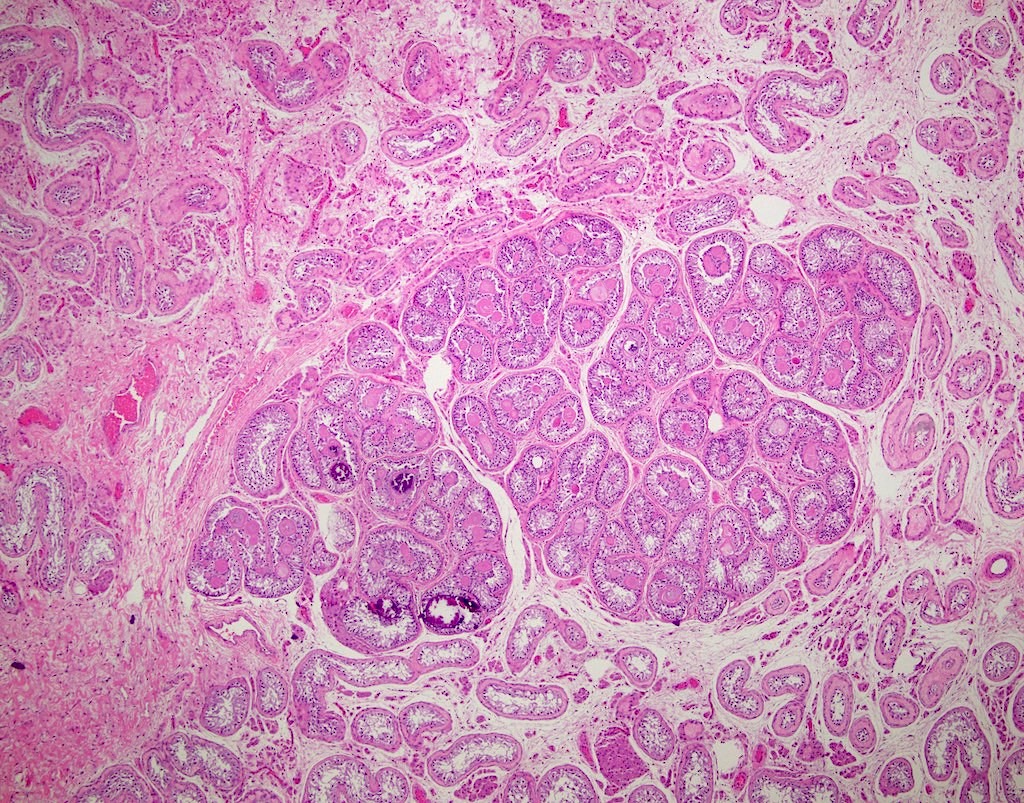
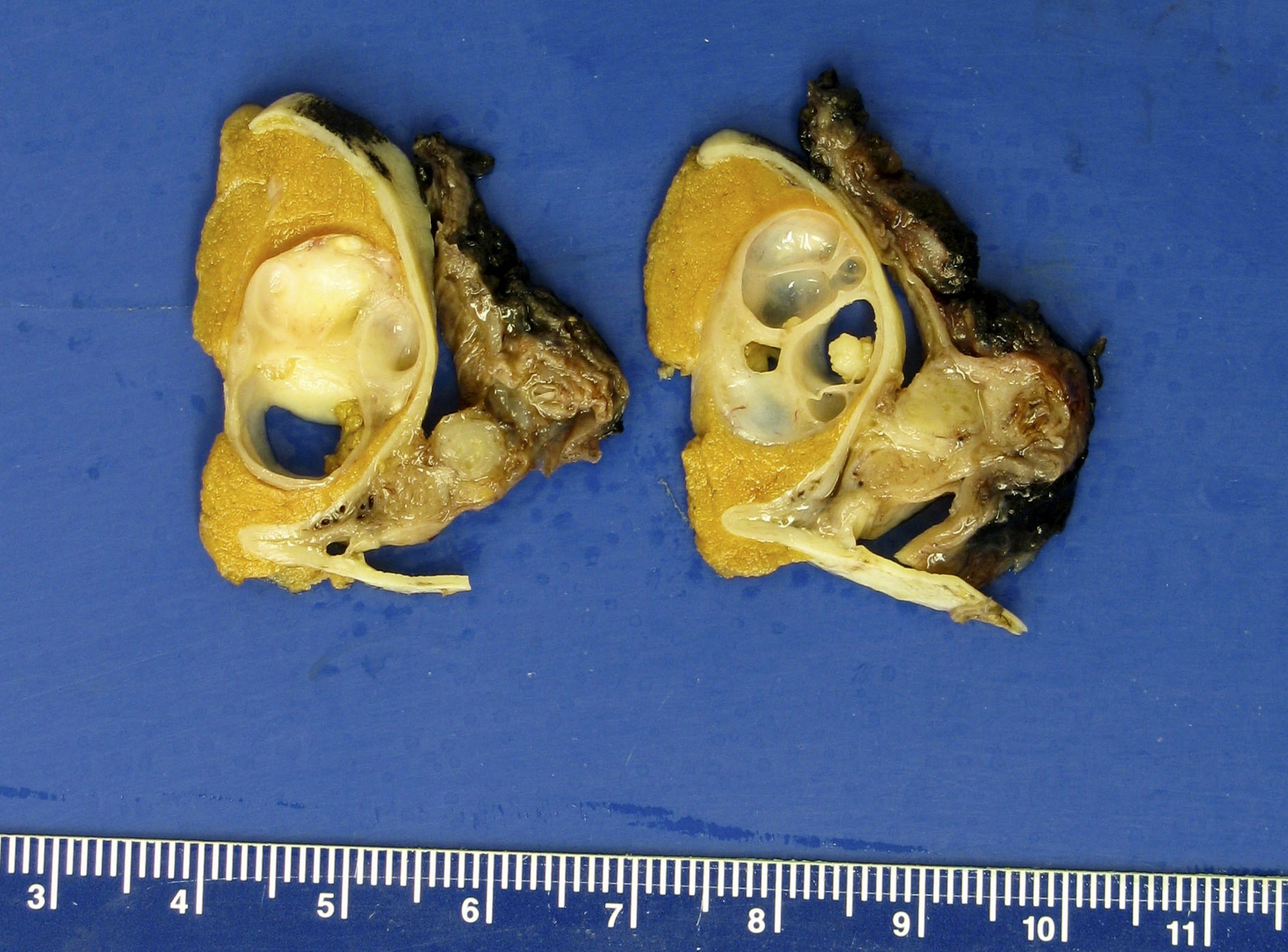
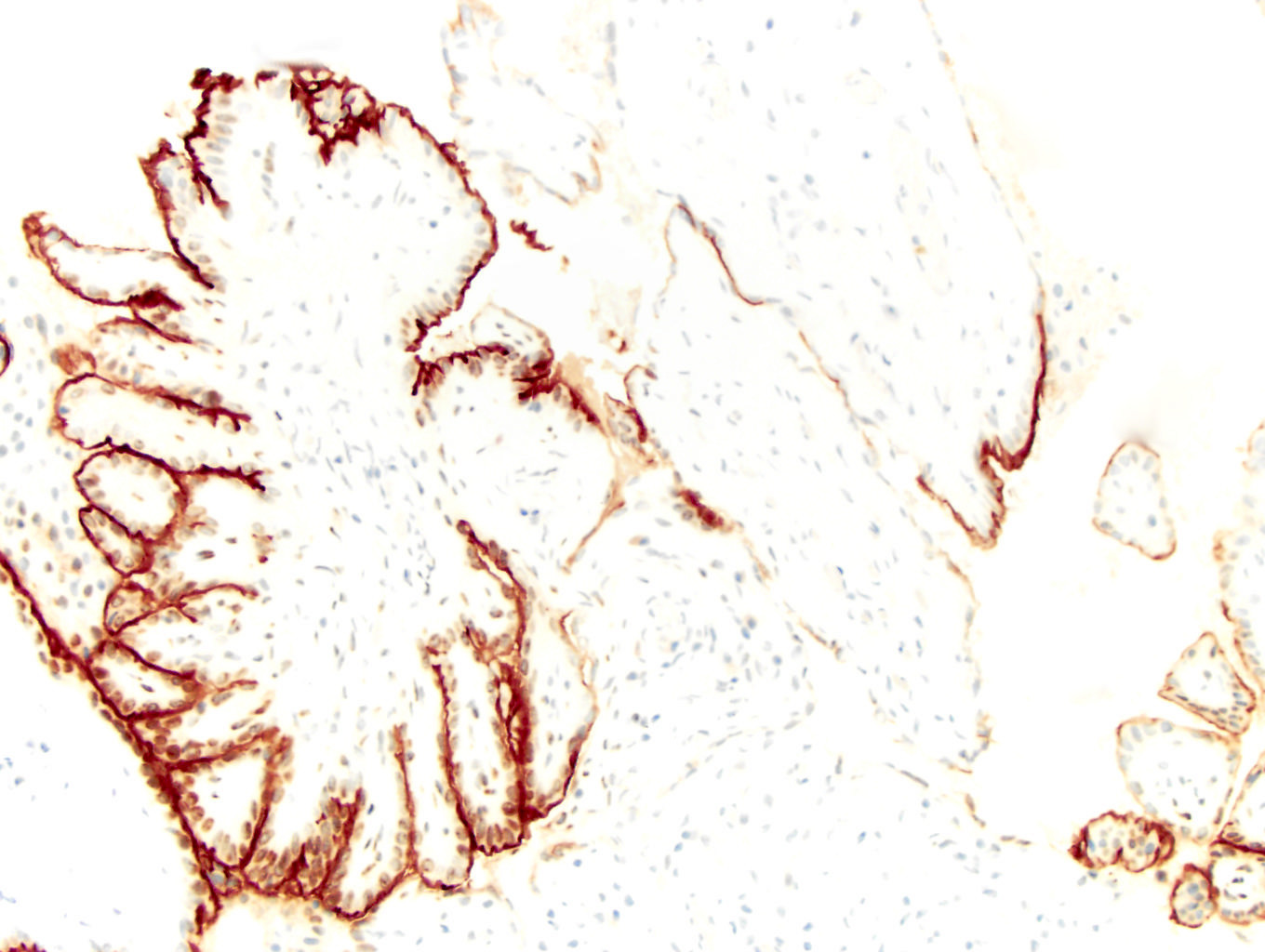
- Tumor involvement of the tunica vaginalis is classified as pT2 and is rare (4/170, 2% seminoma; 0/148, 0% nonseminoma) (Histopathology 2018;73:741, Mod Pathol 2013;26:579)
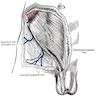
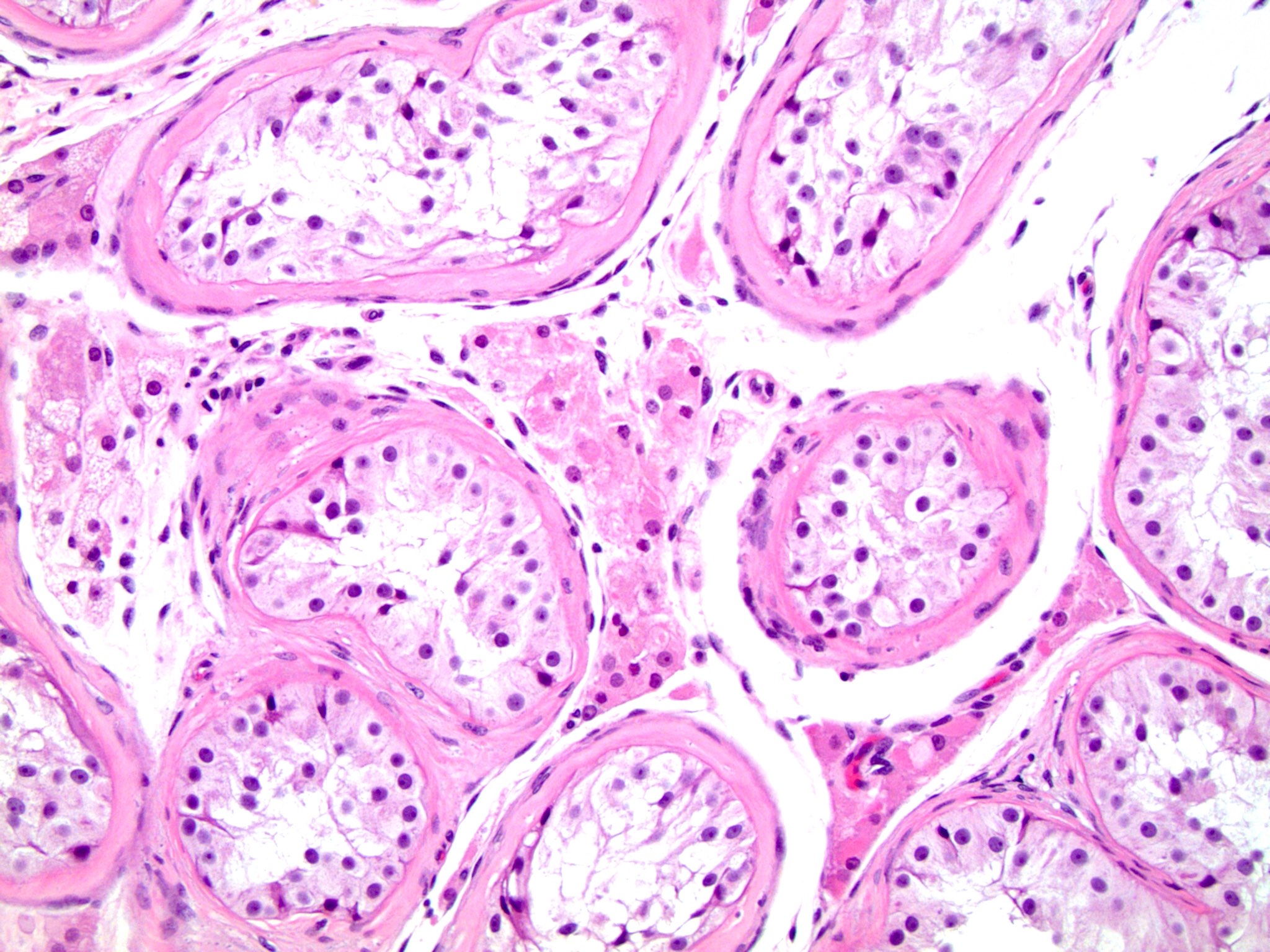
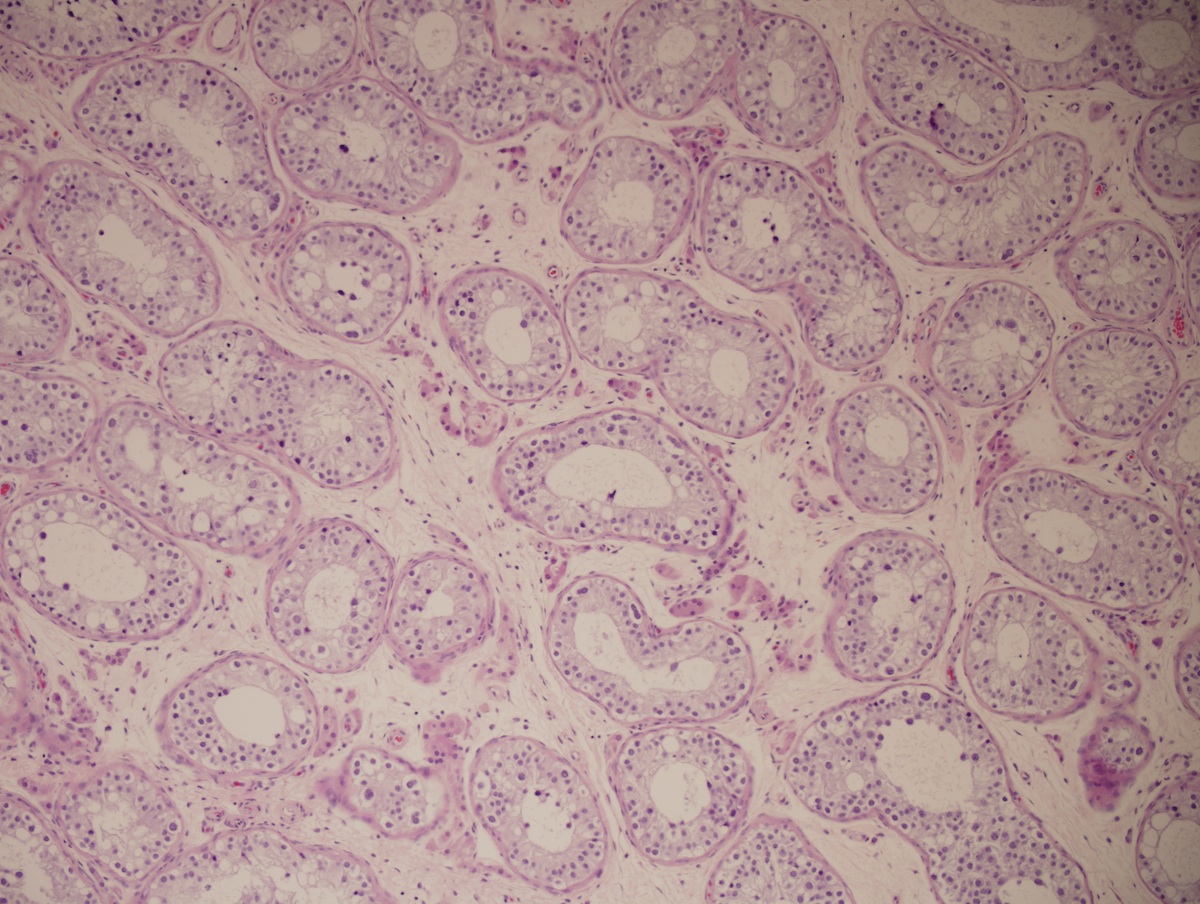
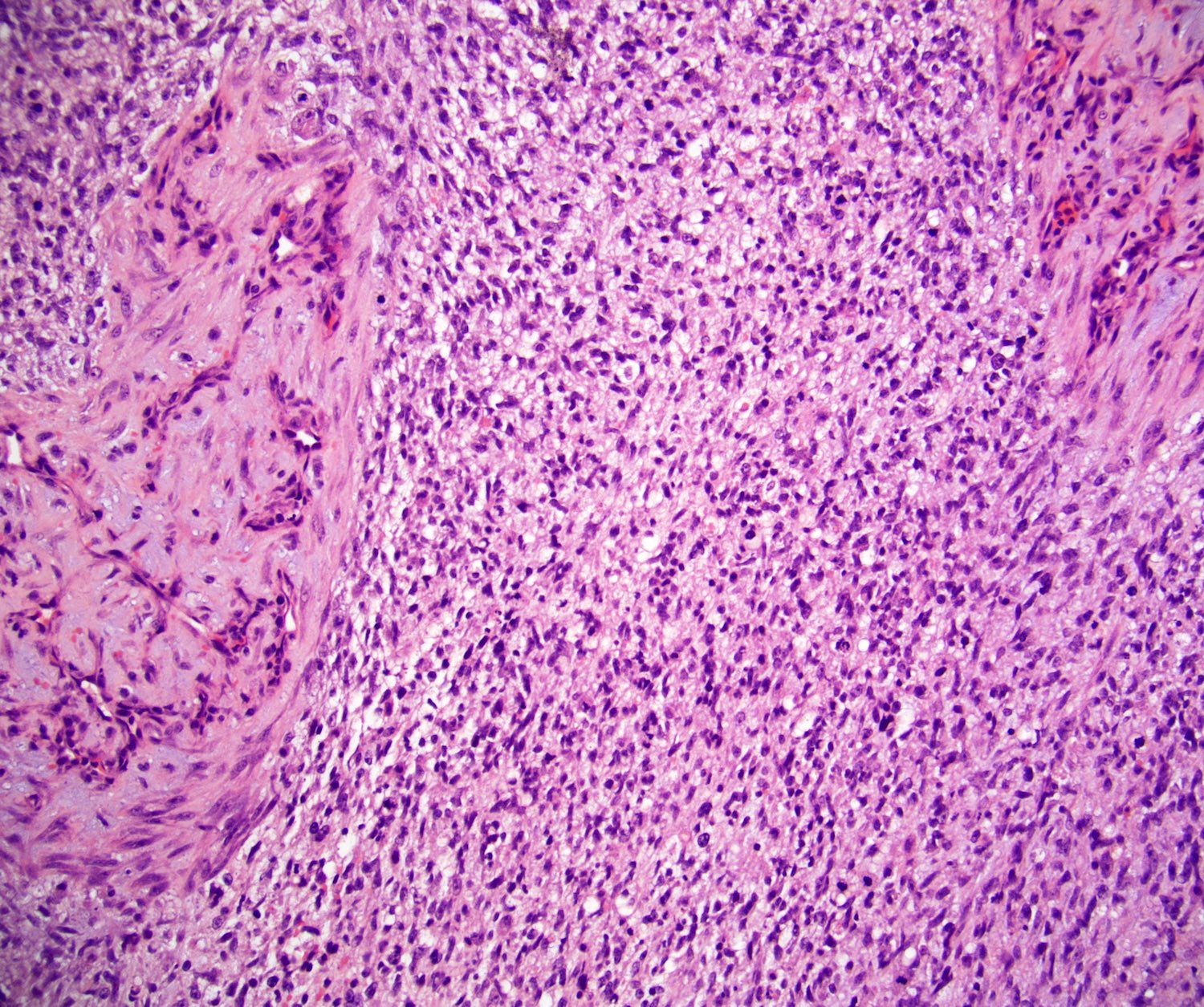
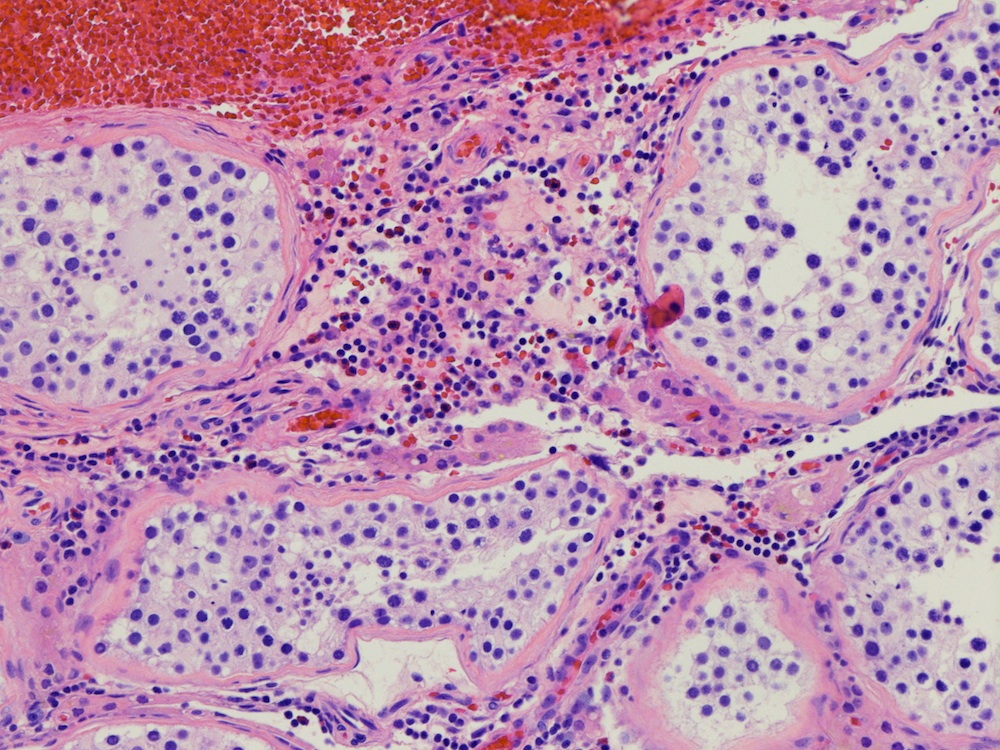
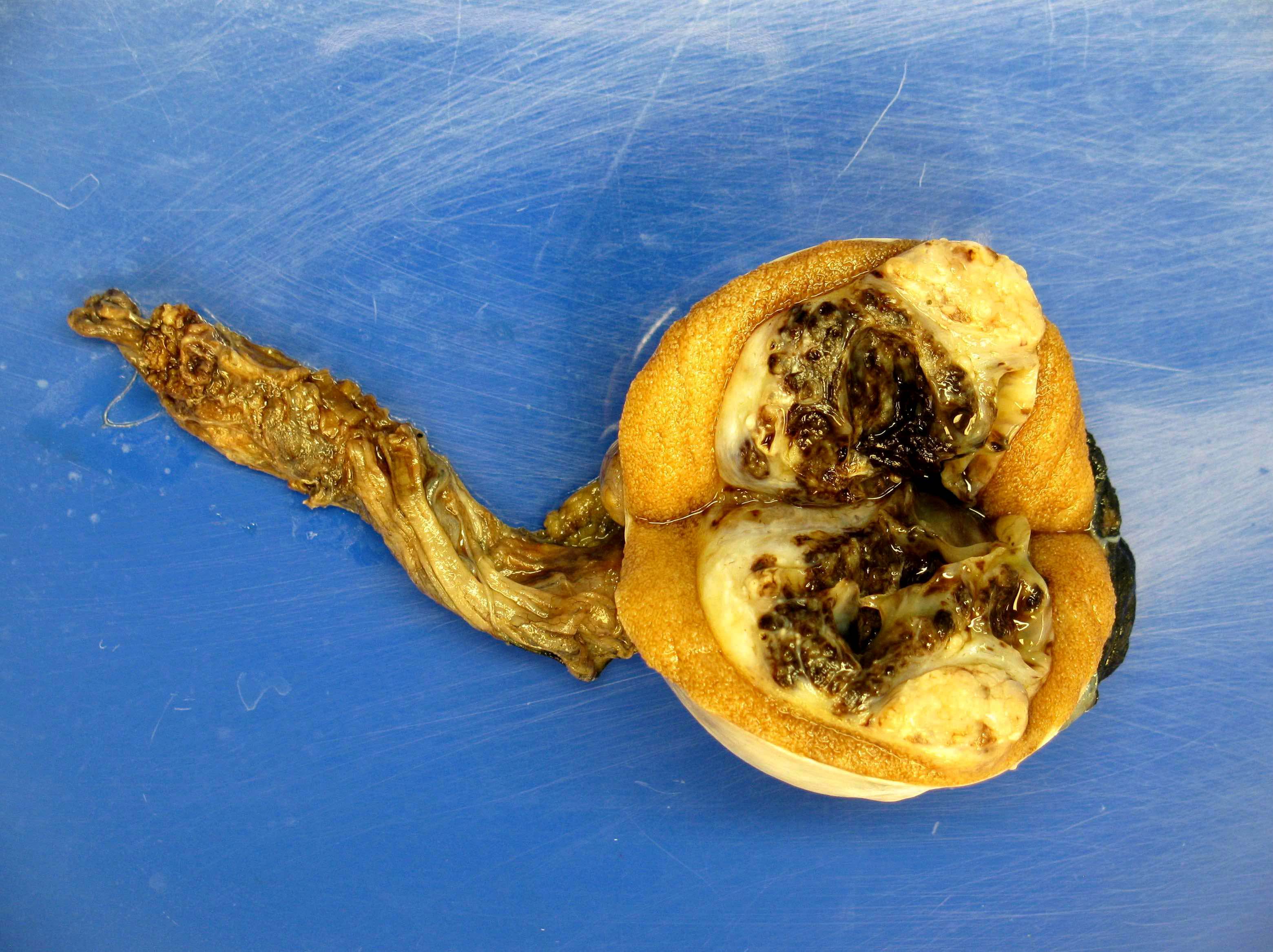
- Epididymal invasion is uncommon (6% seminoma, 8% nonseminoma) and is classified as pT2; however, it is not associated with a higher clinical stage (Histopathology 2018;73:741, Mod Pathol 2013;26:579)
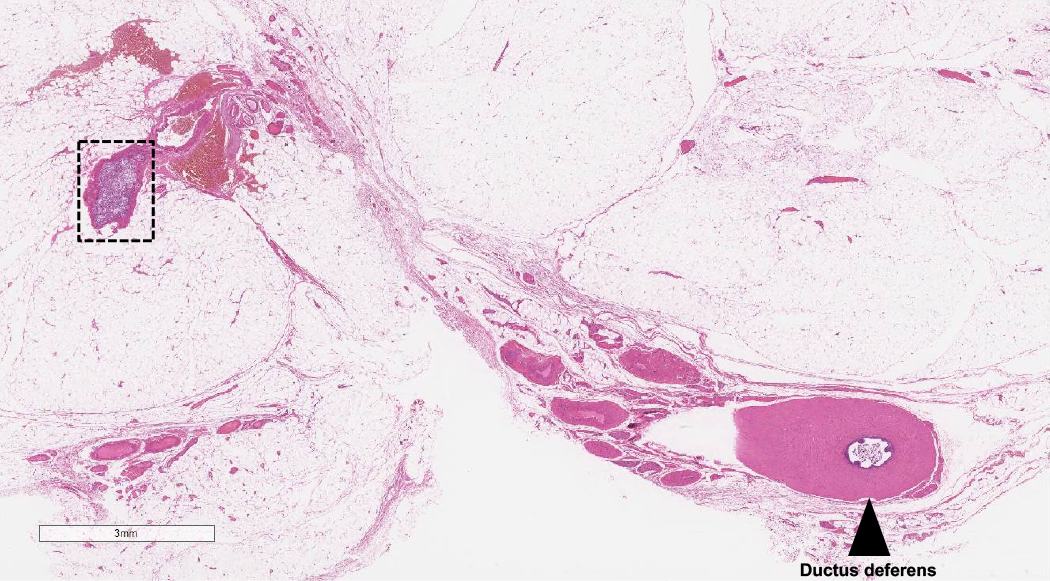
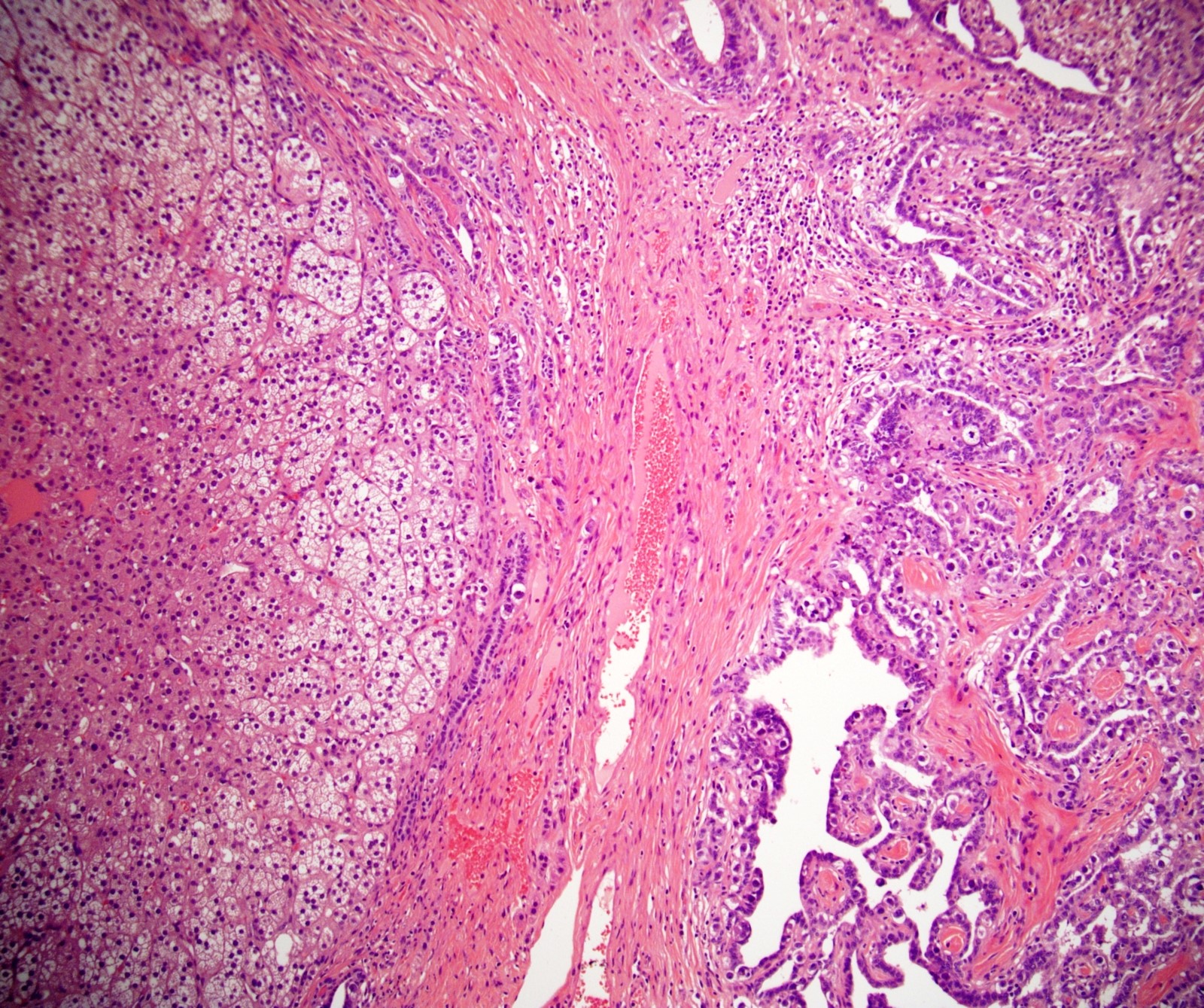
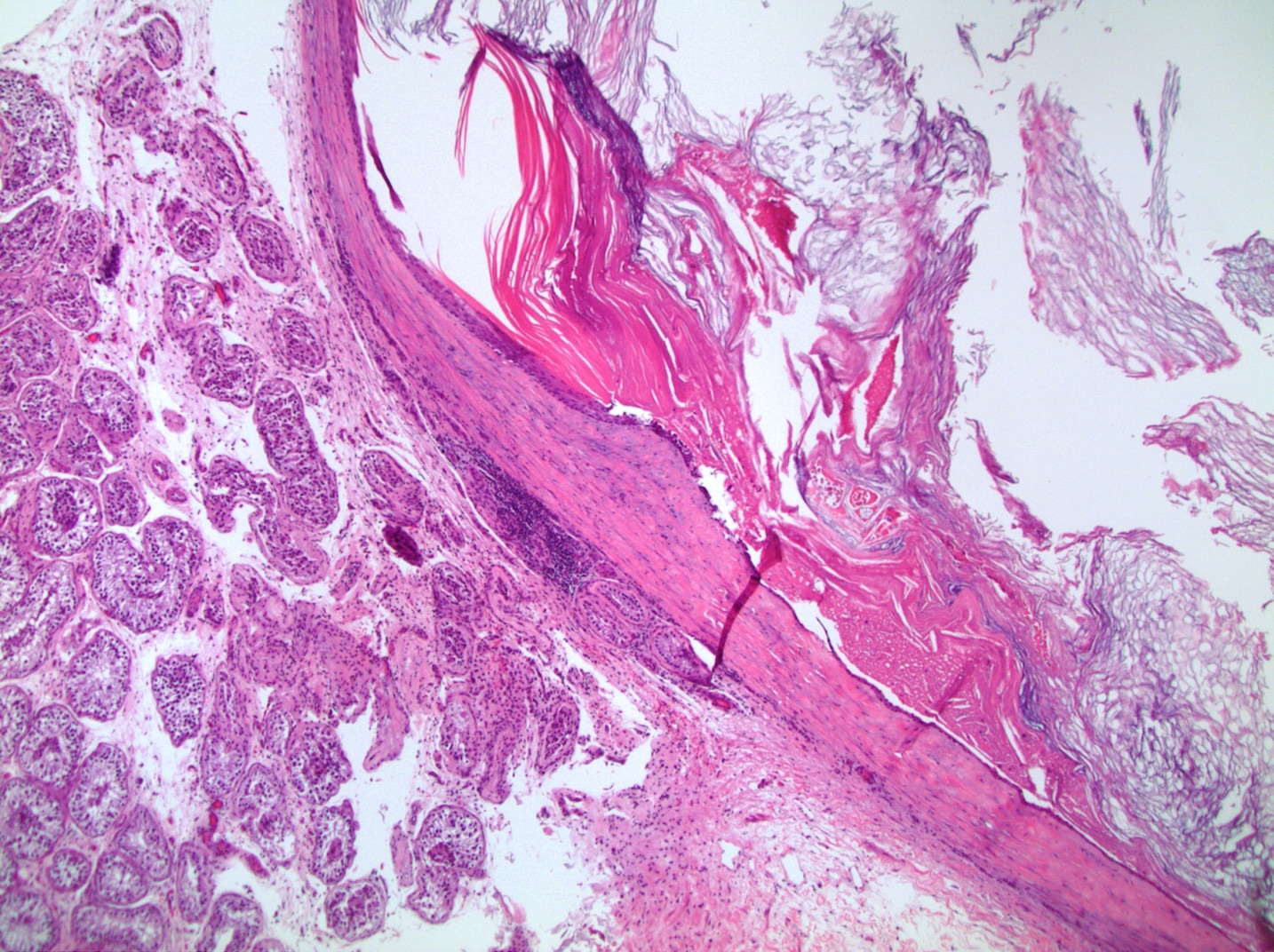
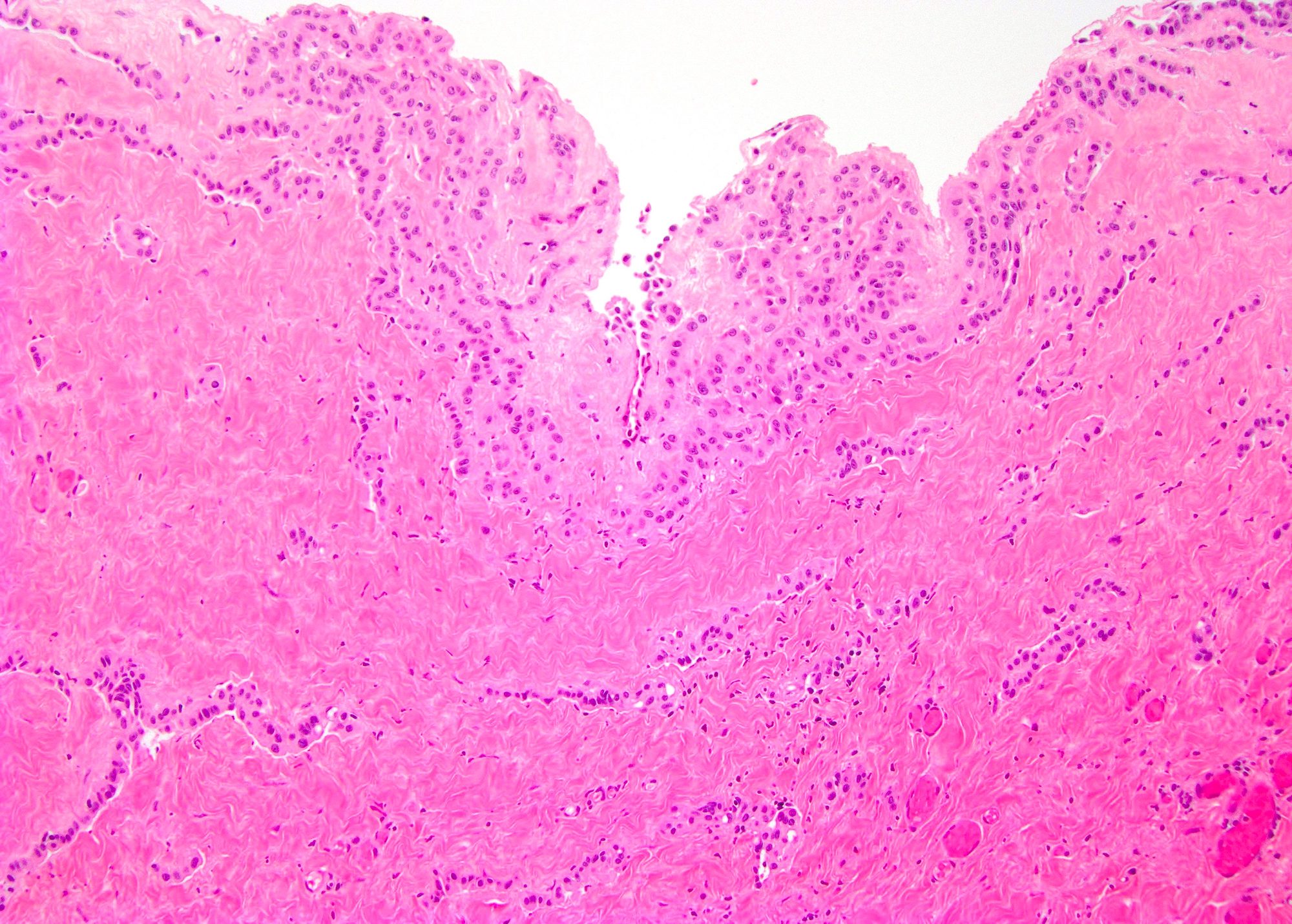
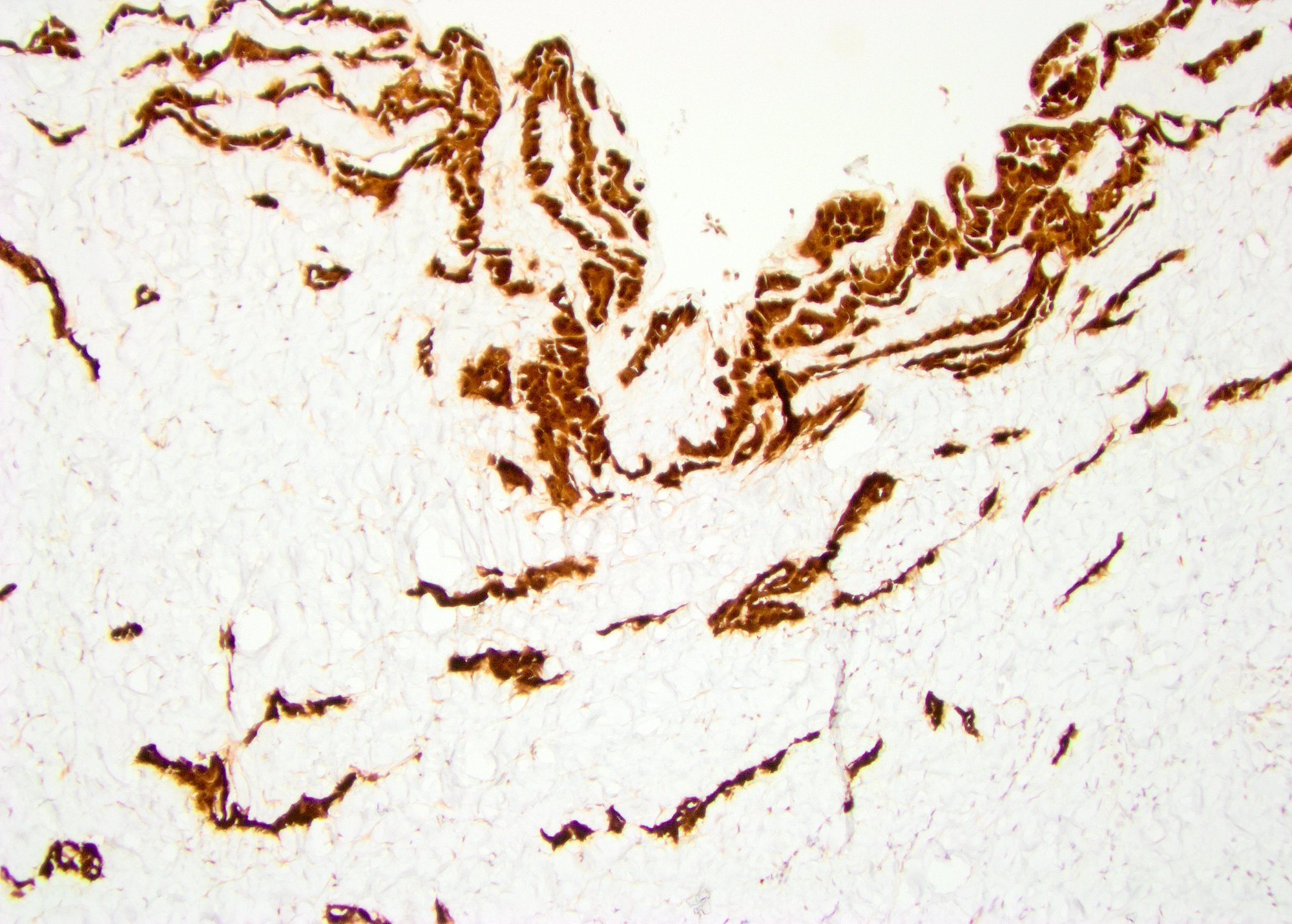
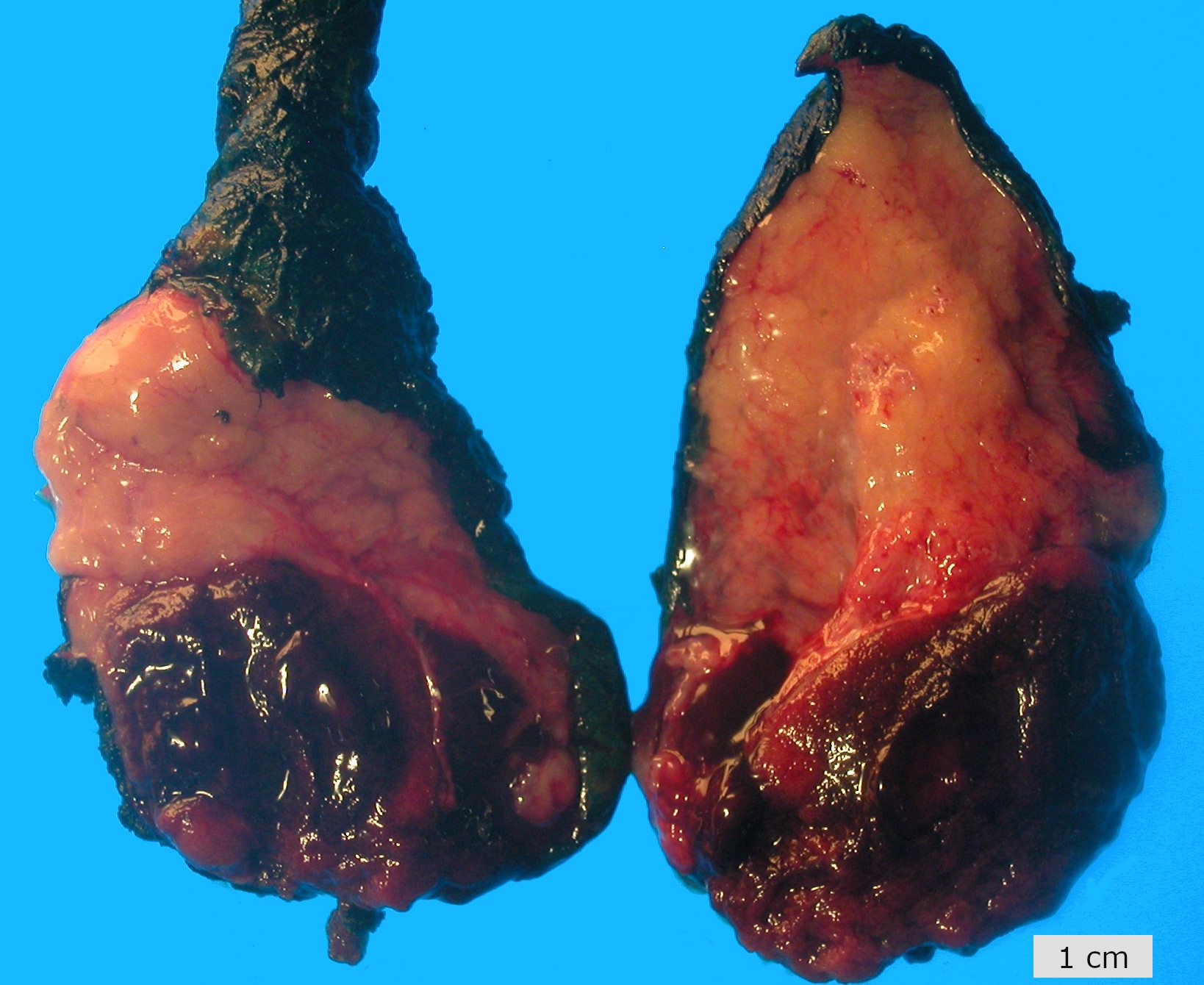
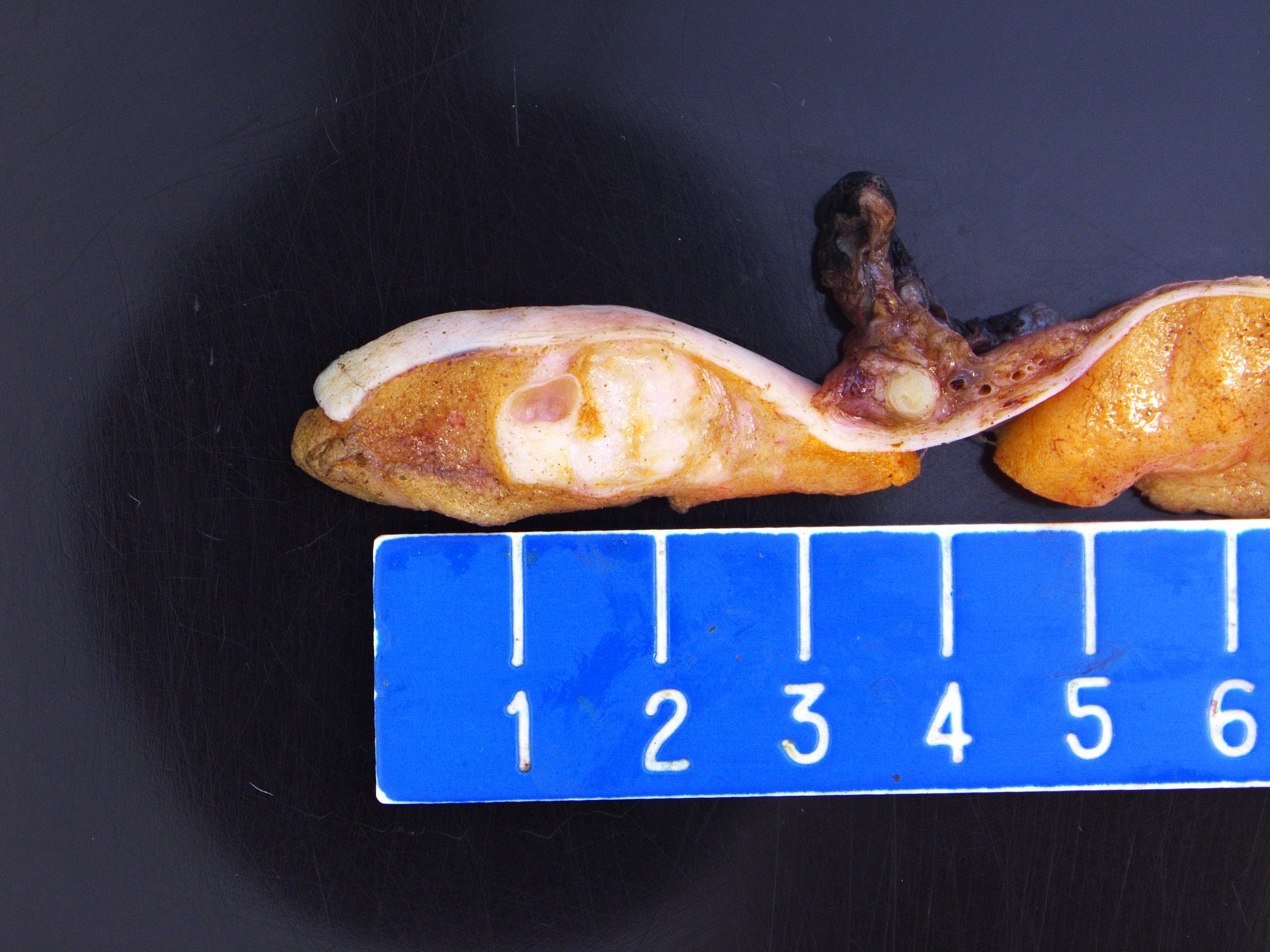
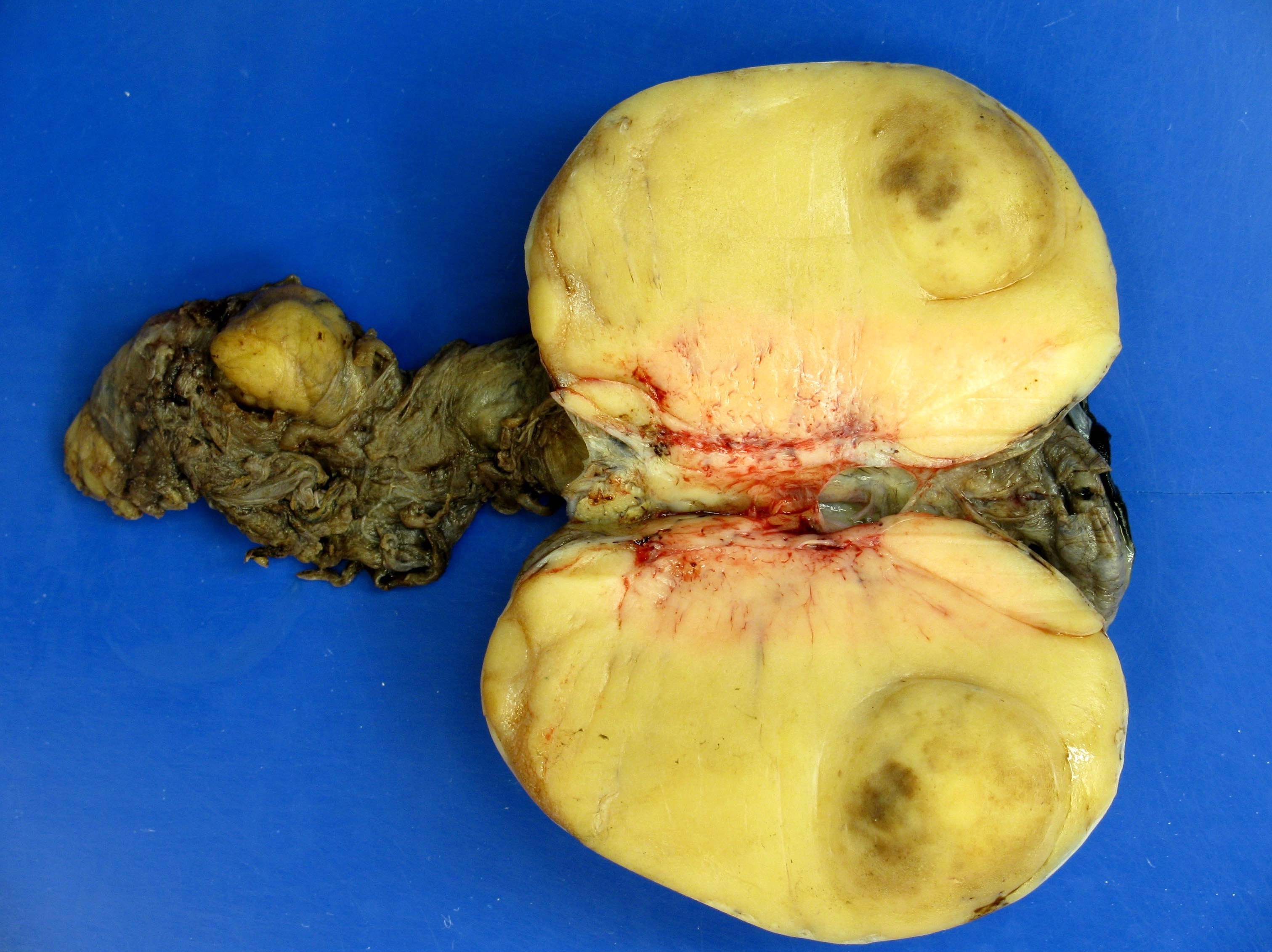
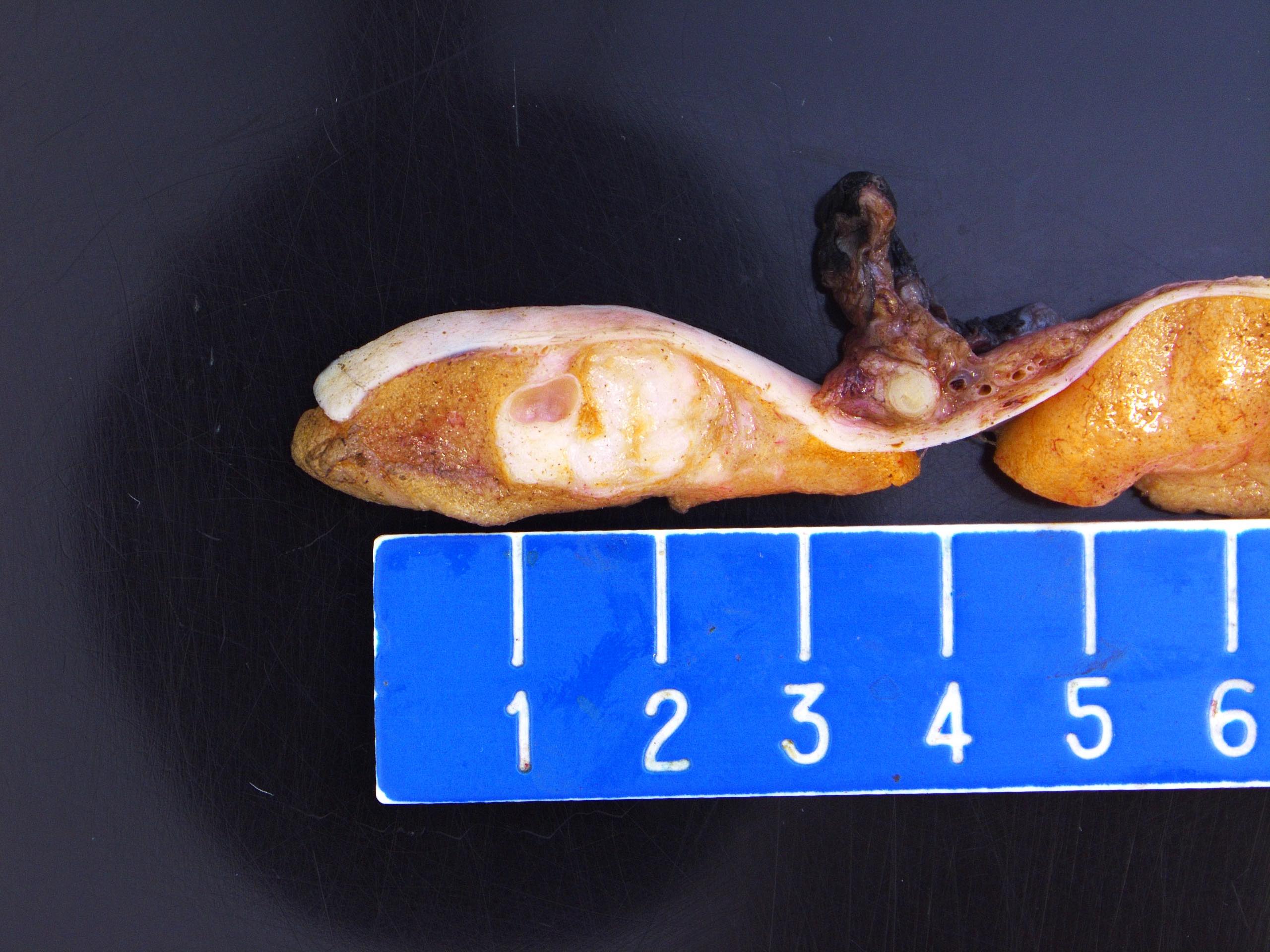
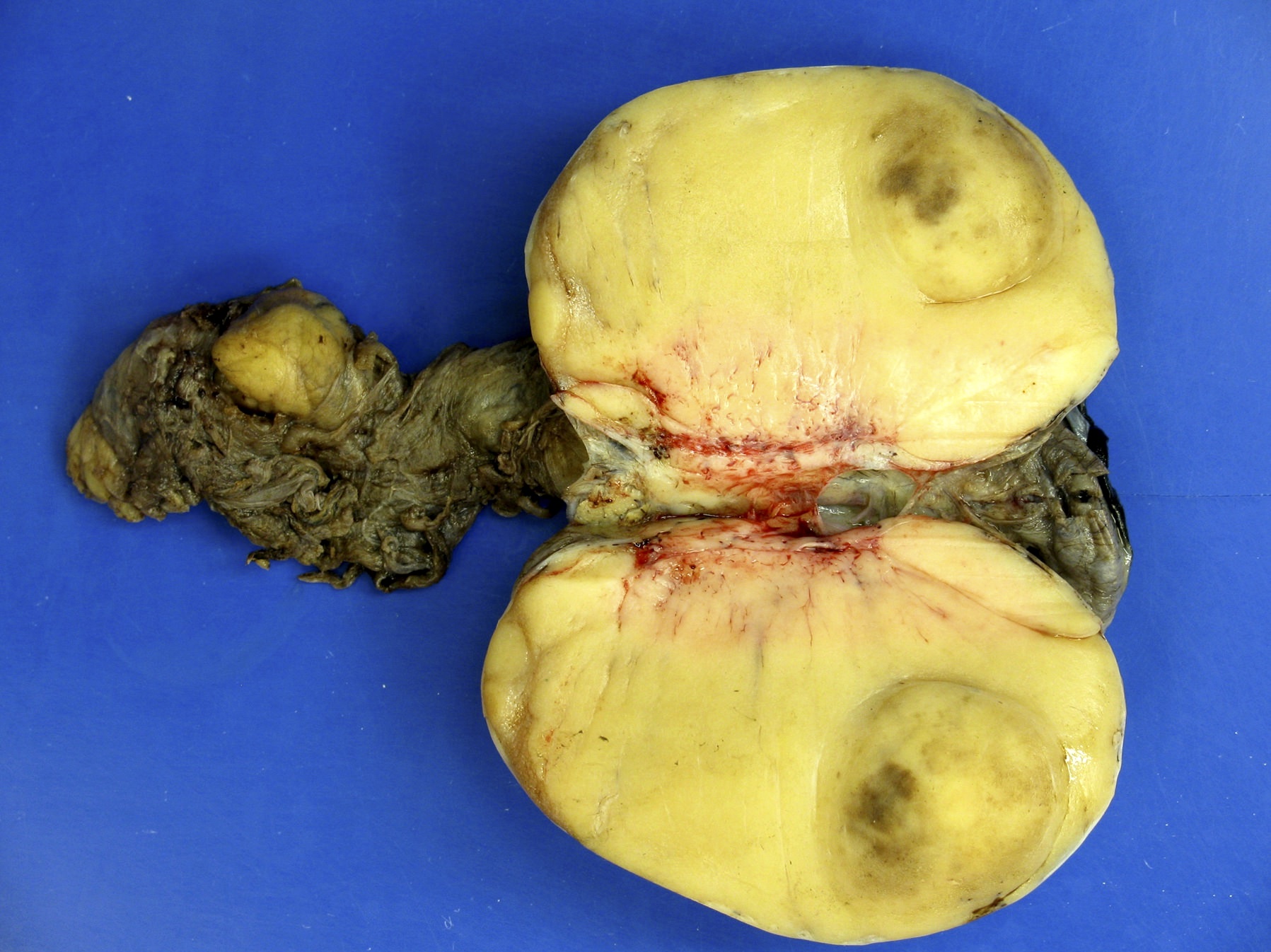
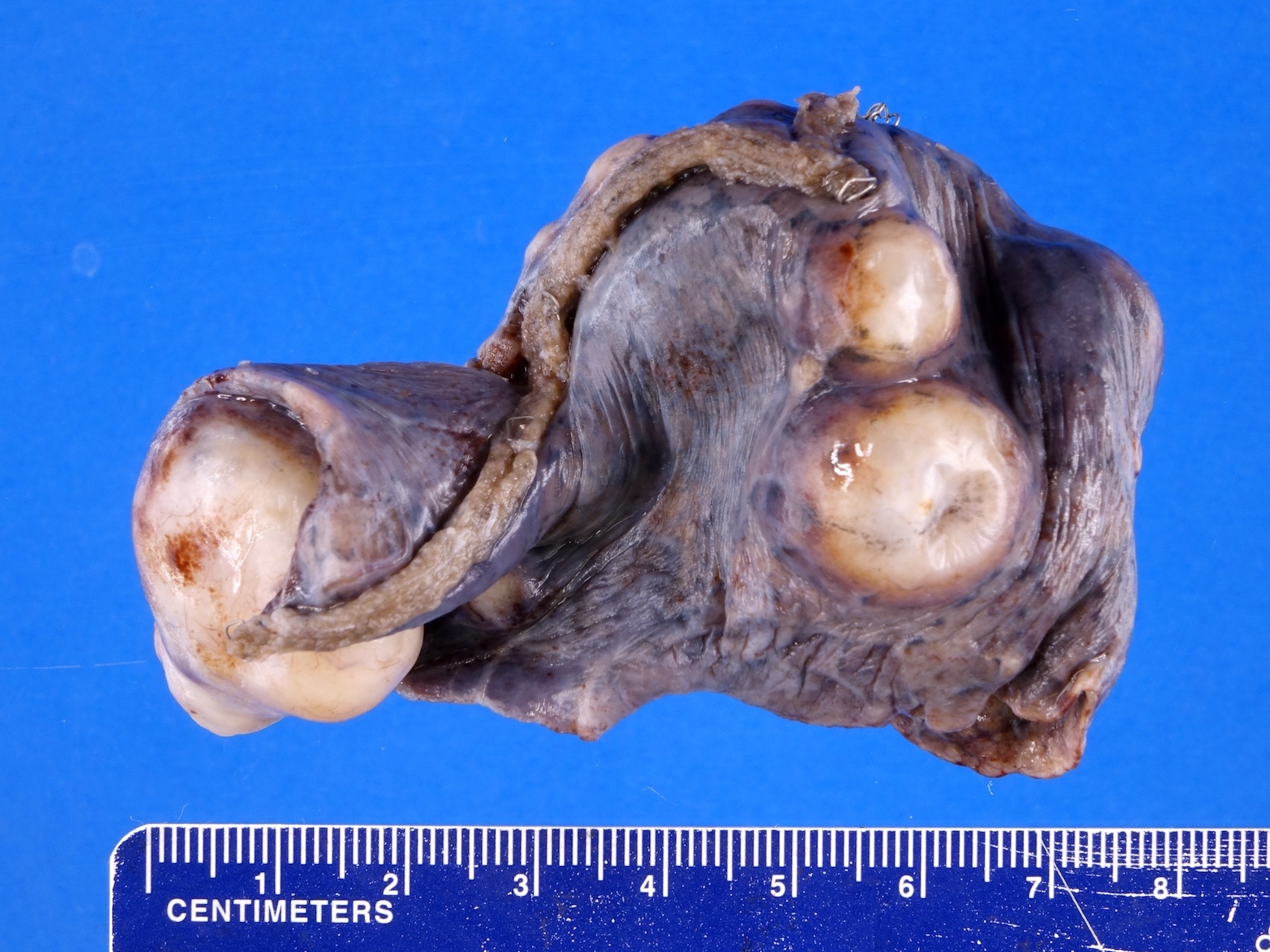
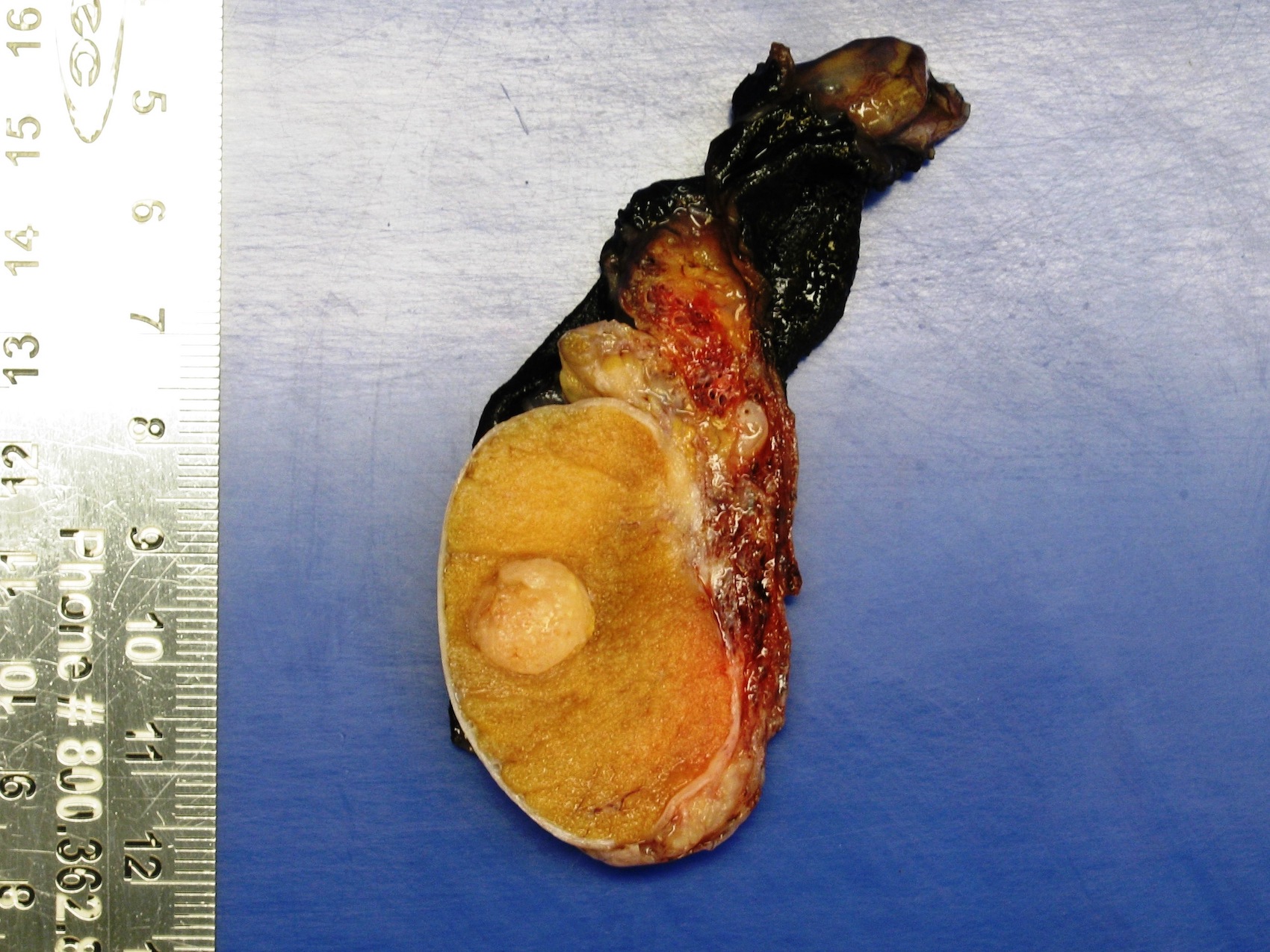
- Tumor within the soft tissue below the level of the epididymal head is considered hilar fat invasion and is classified as pT2
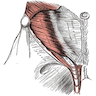
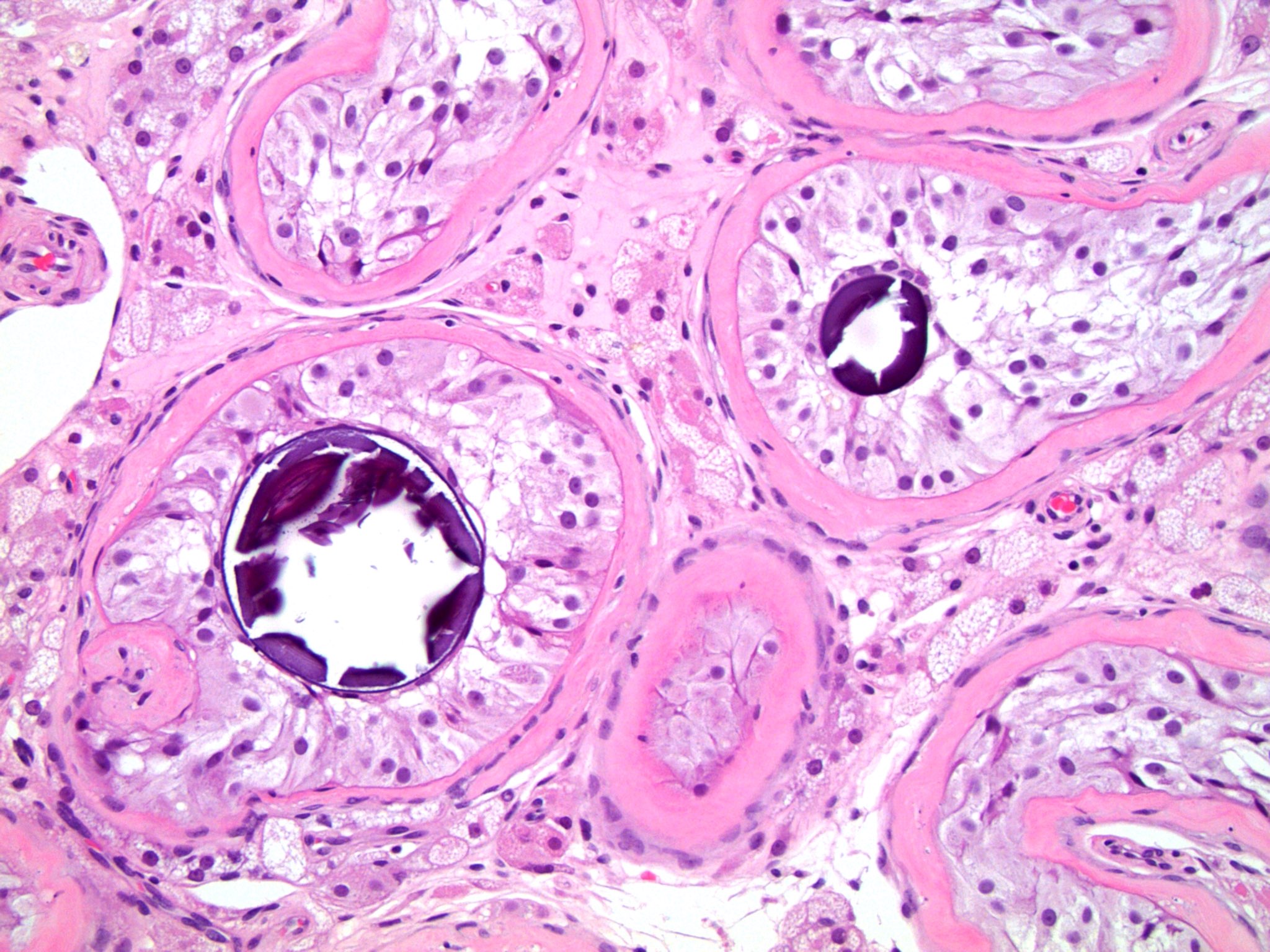
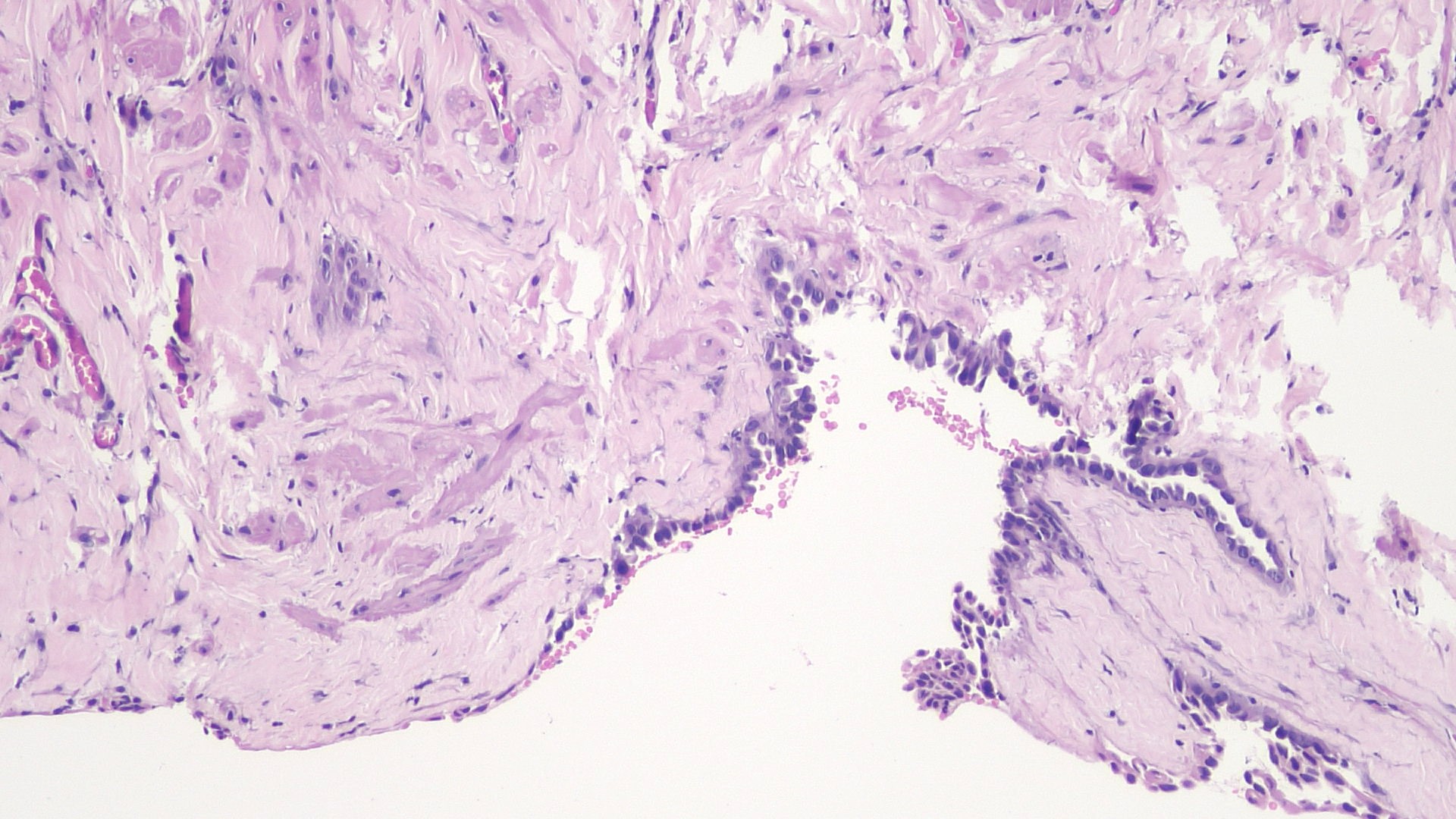
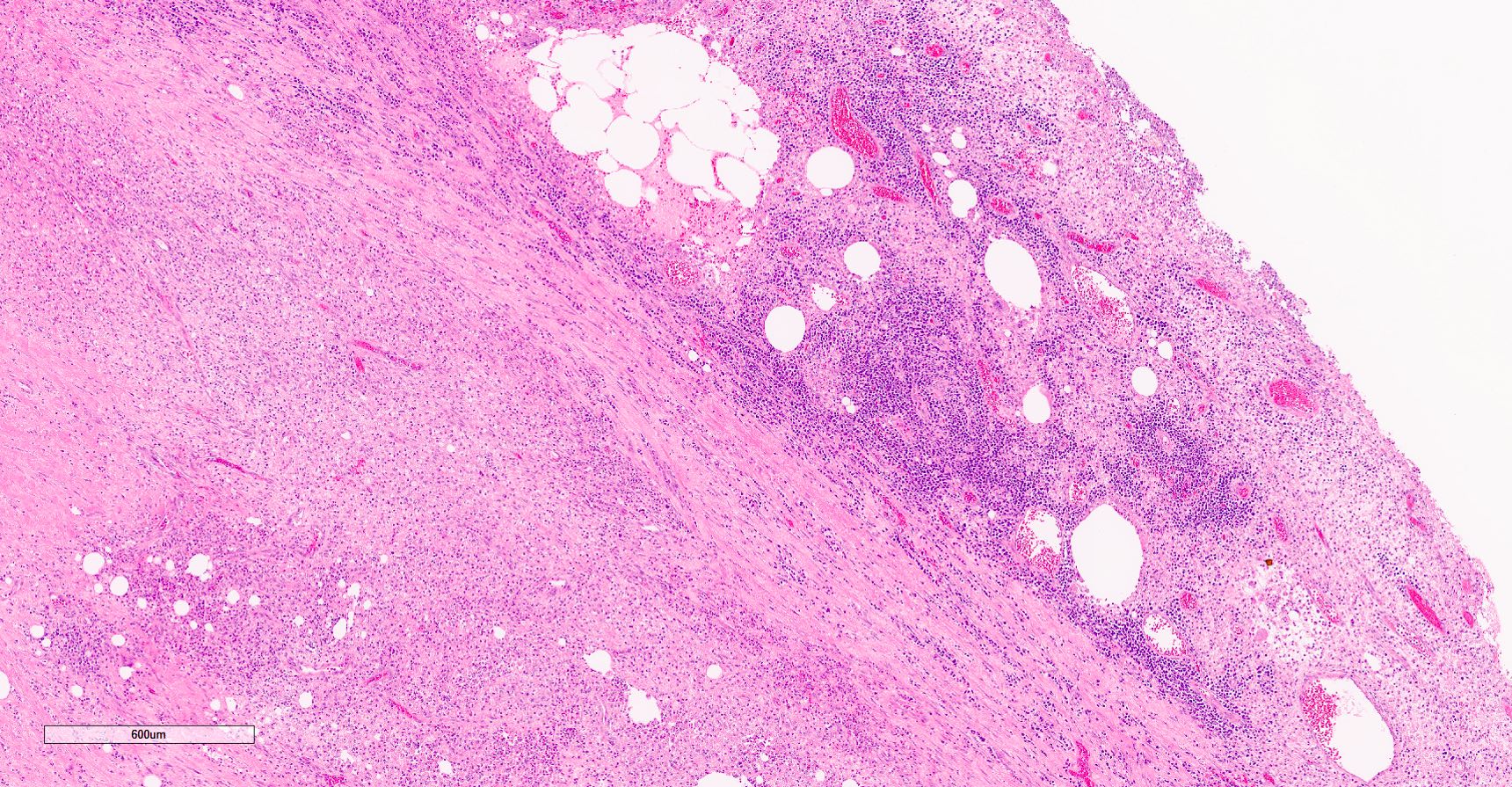
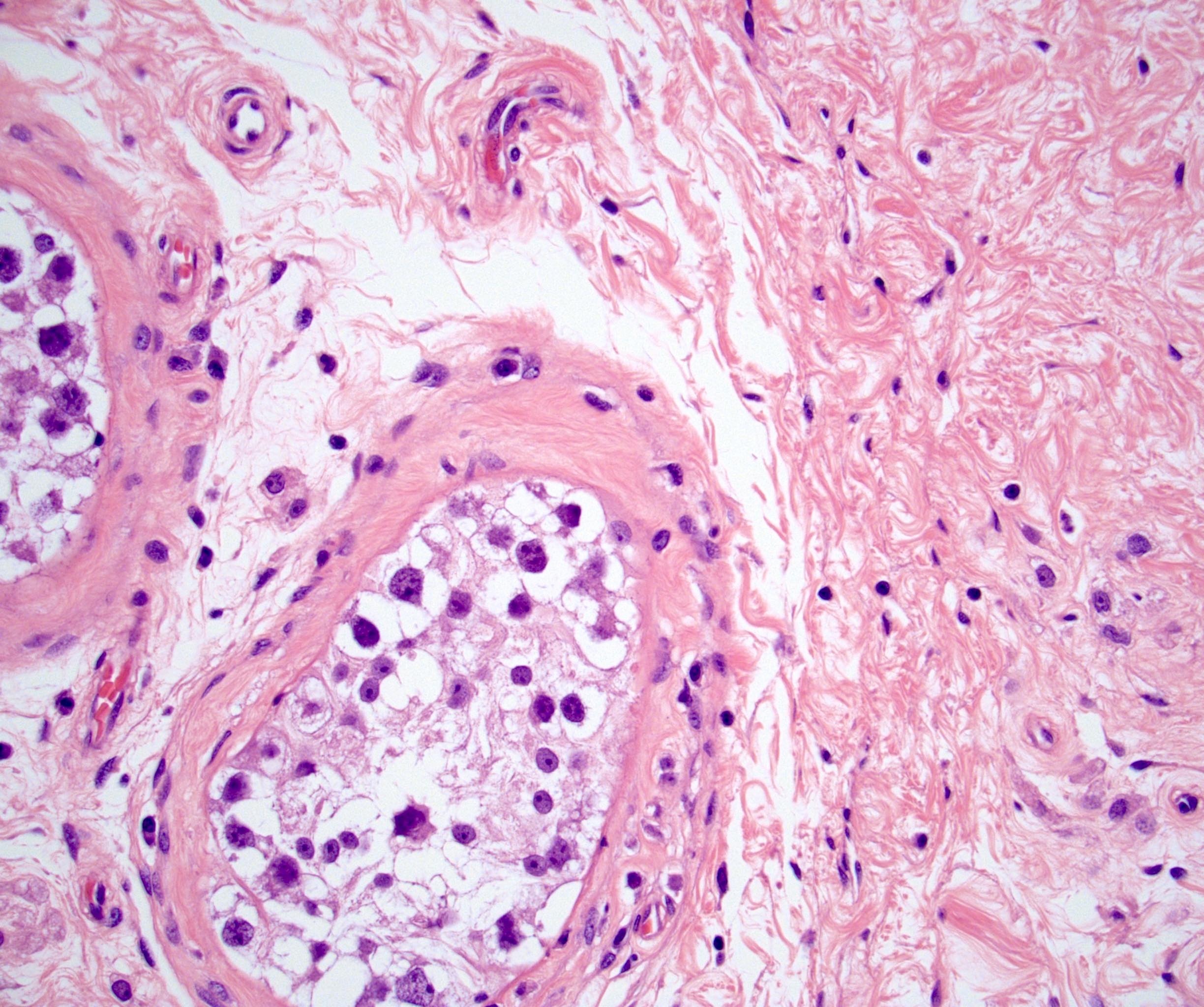
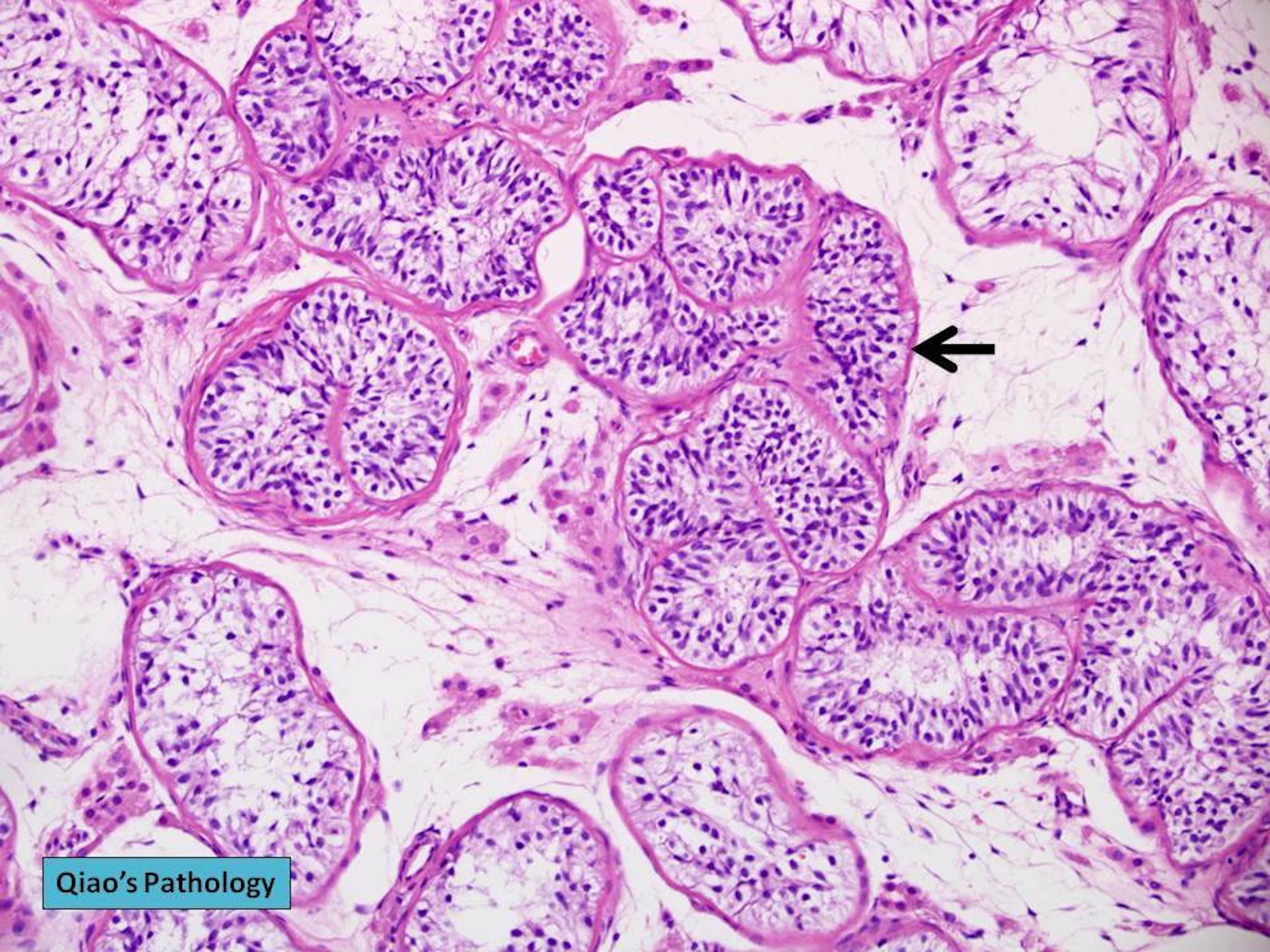
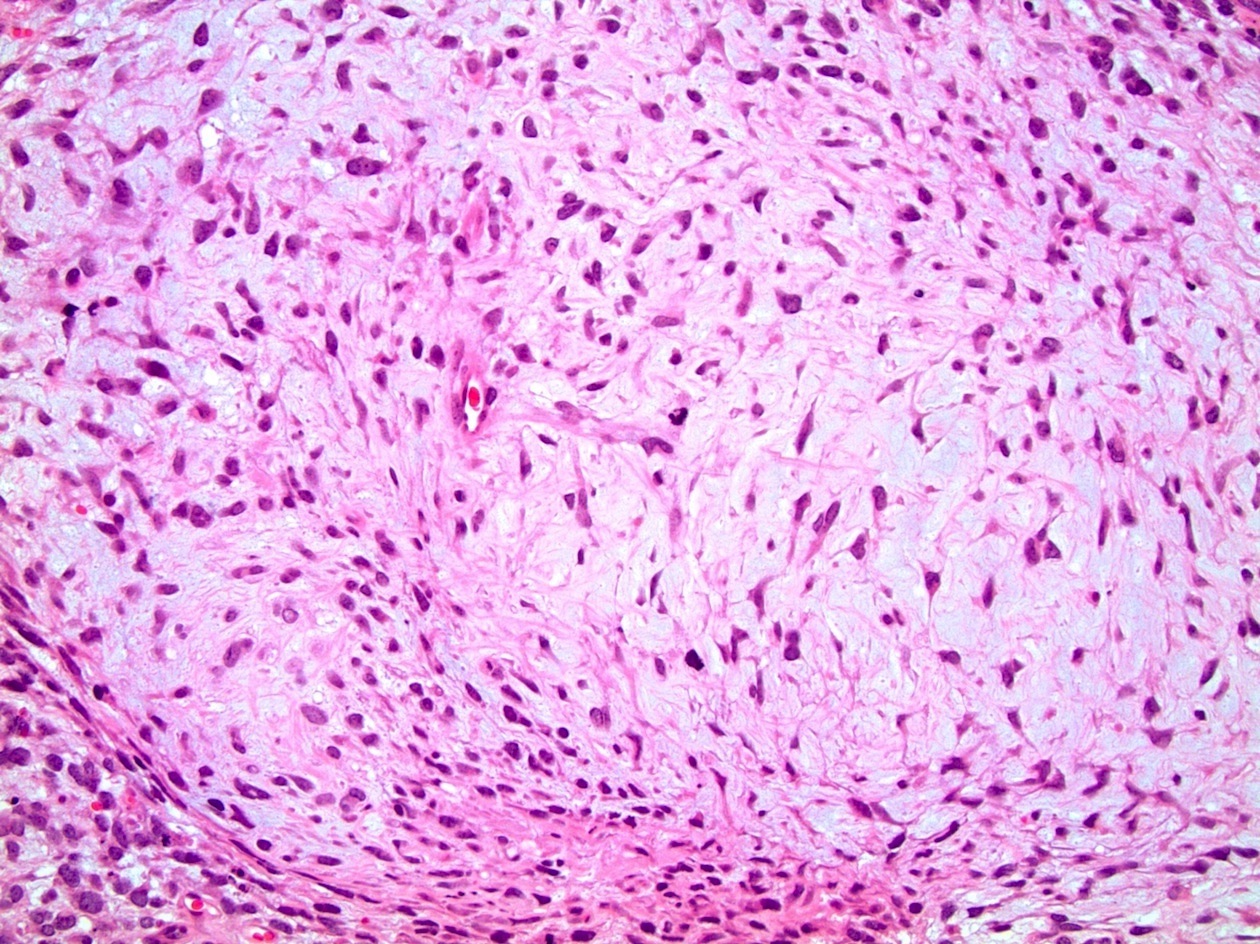
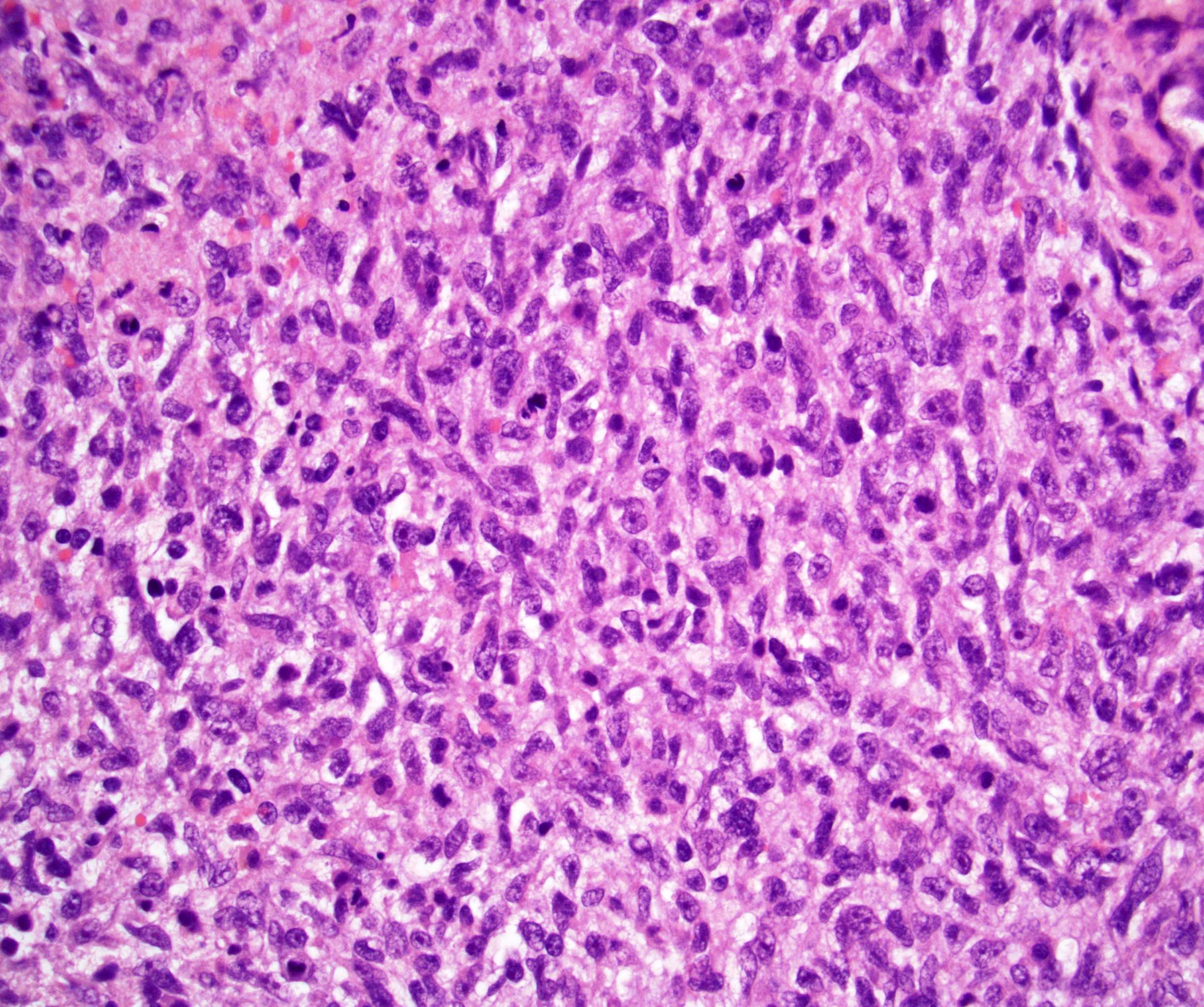
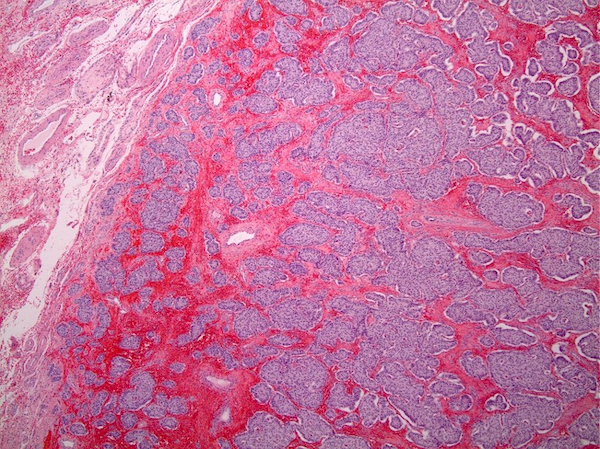
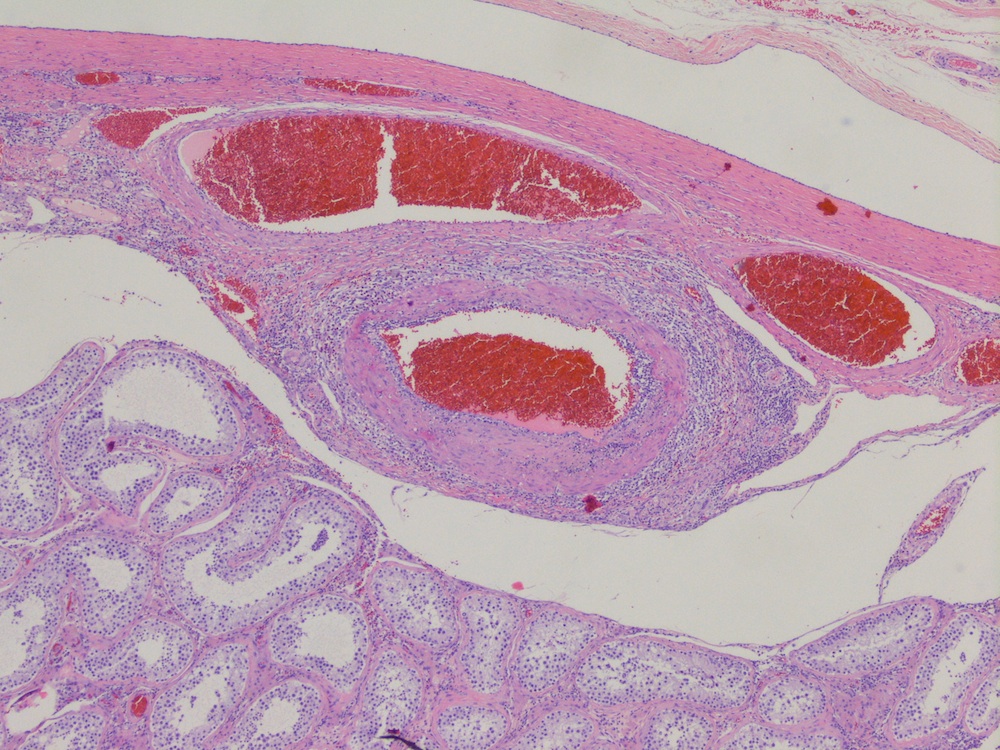
- pT3
- Tumor within the soft tissue beyond the angle between the epididymis and spermatic cord is classified as pT3