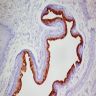
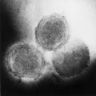
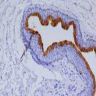
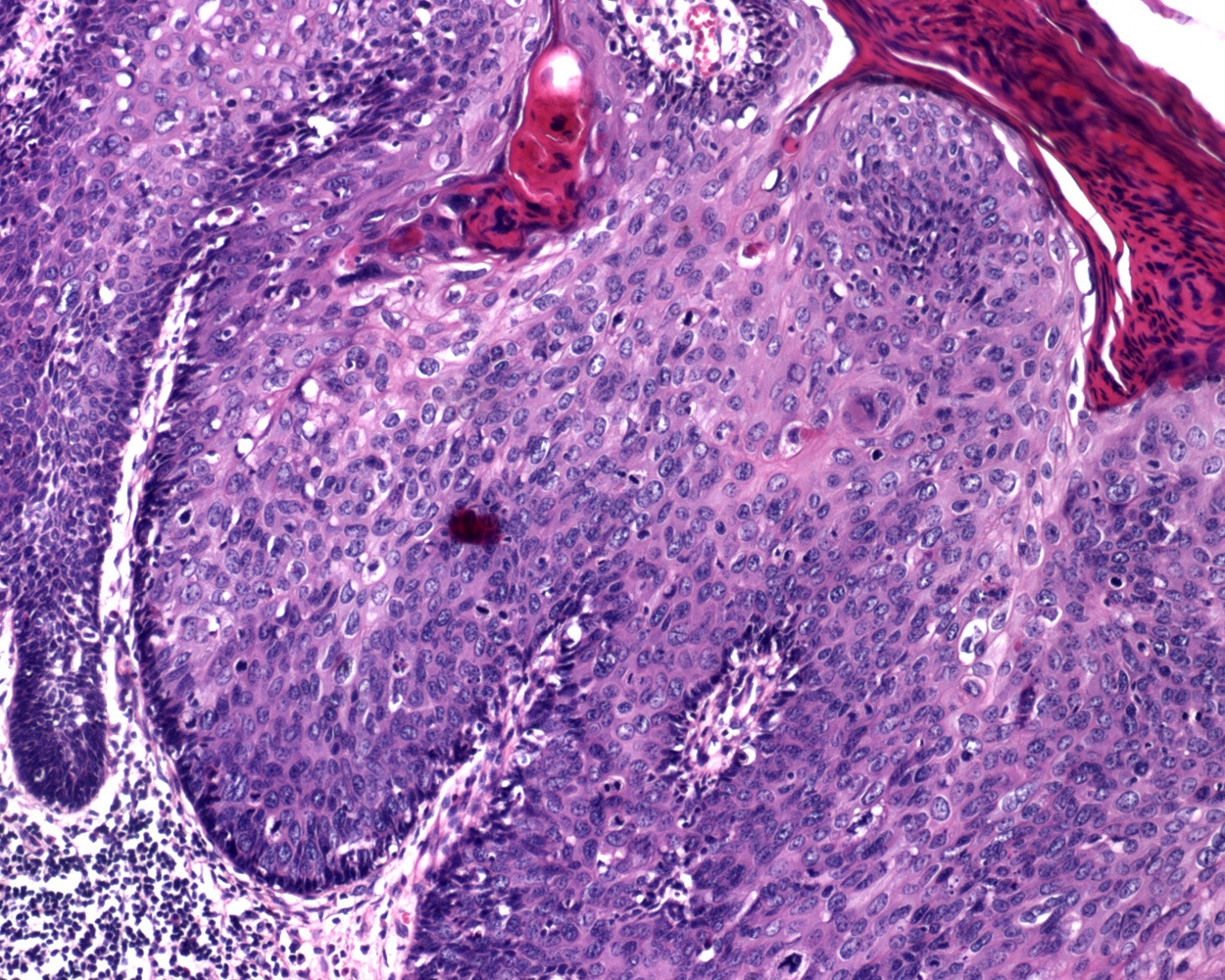
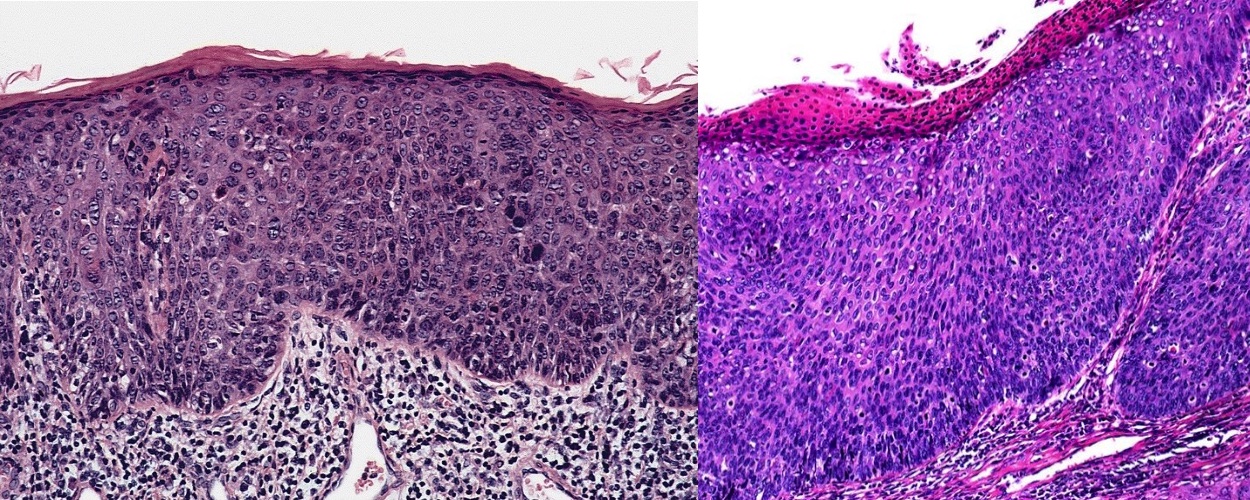
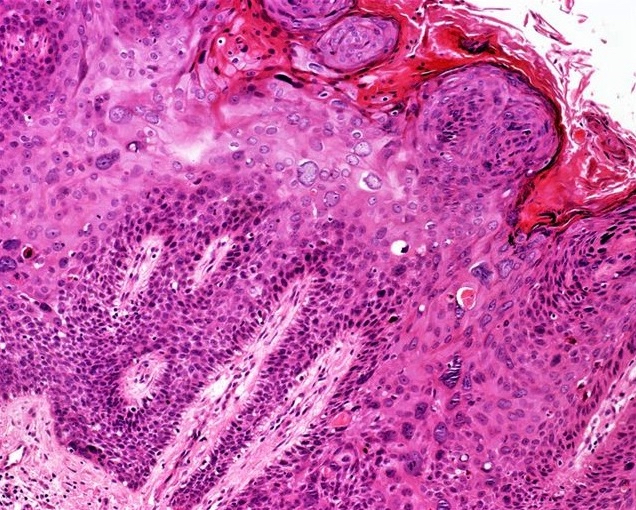
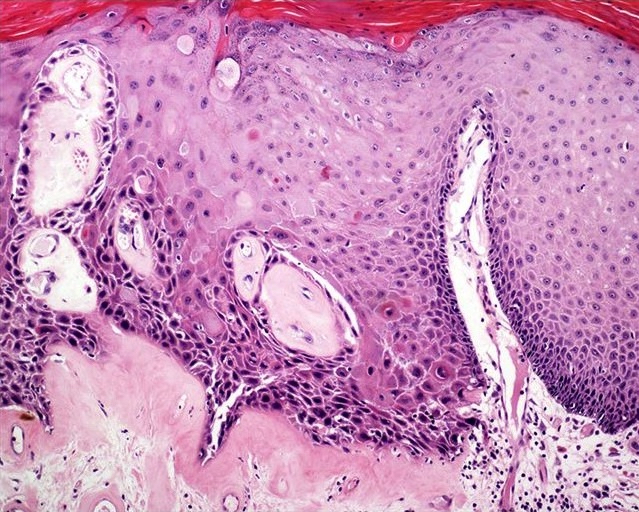
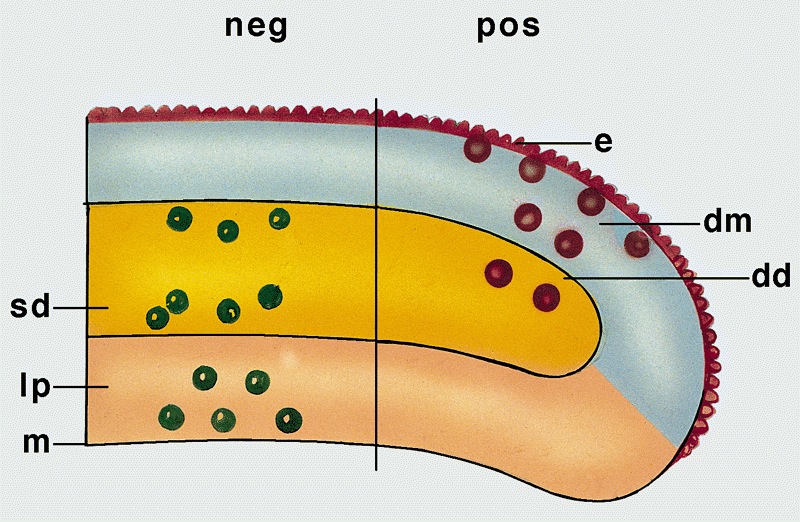
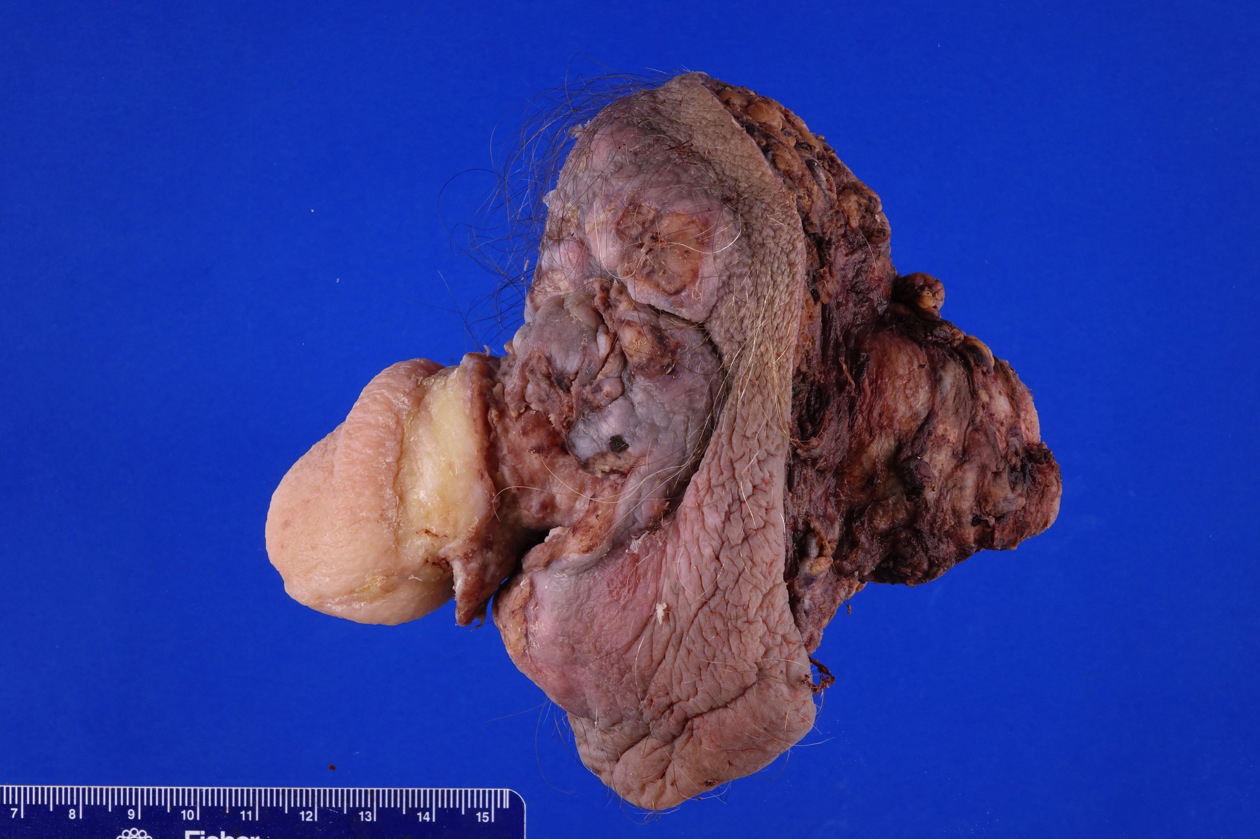
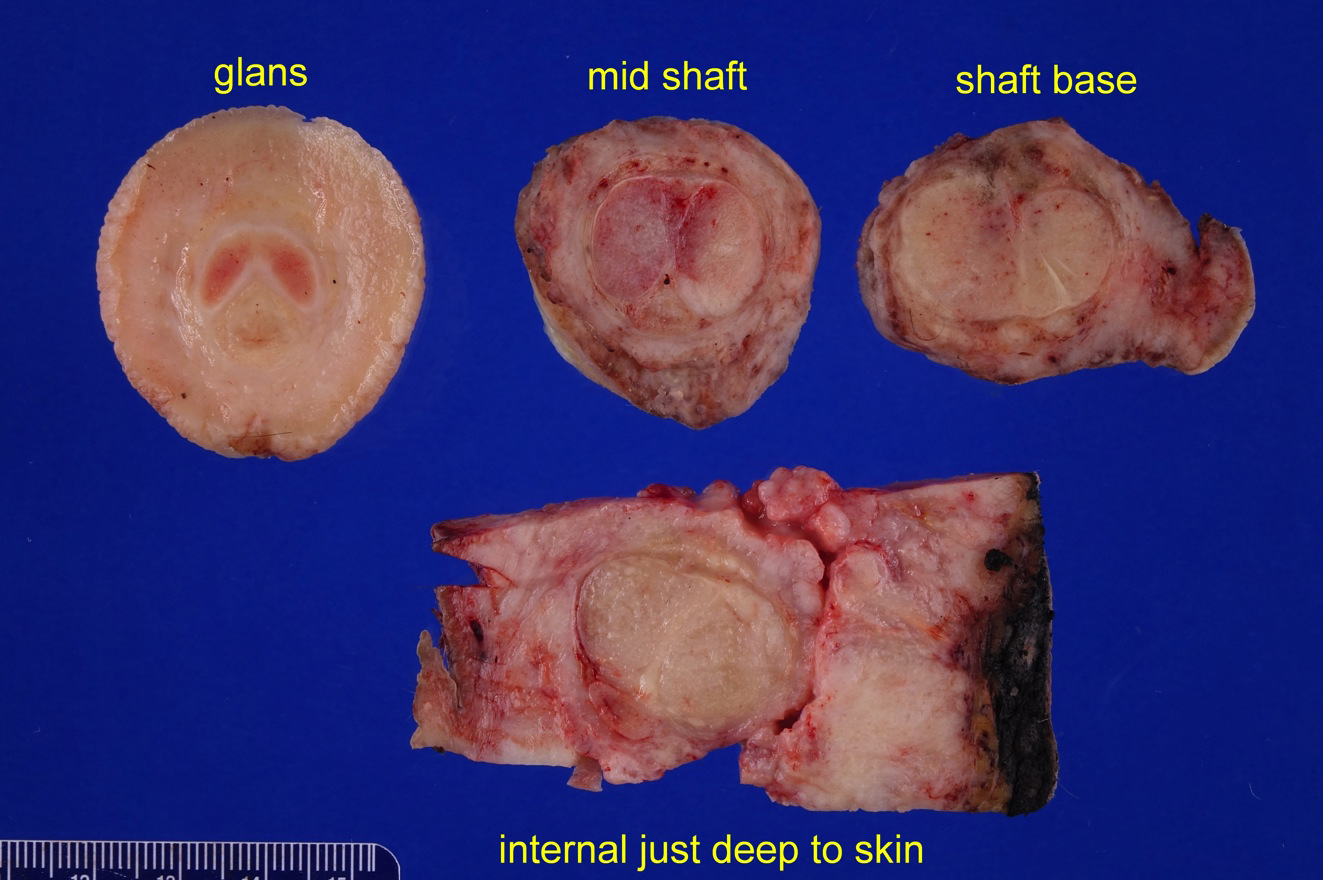
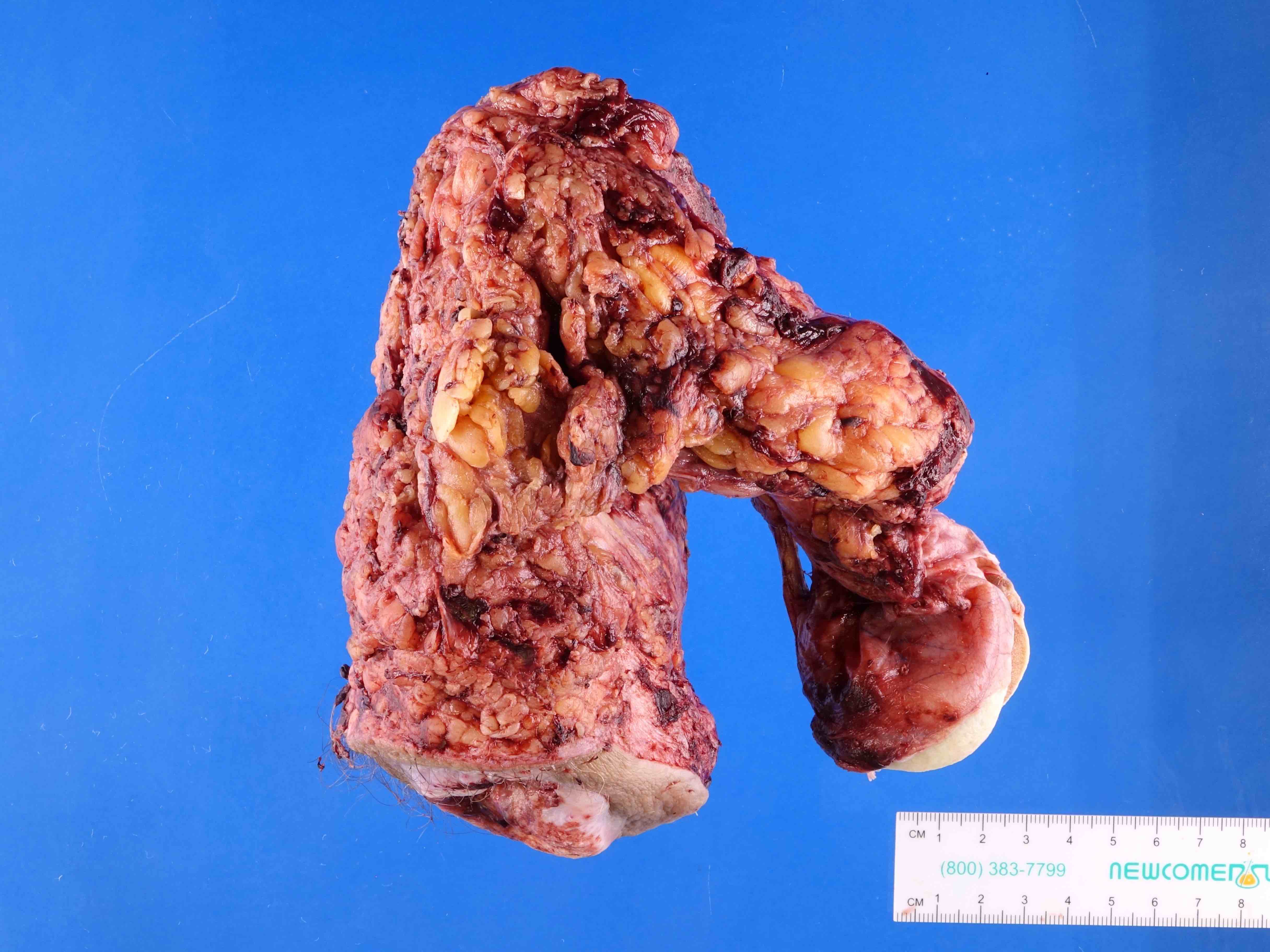
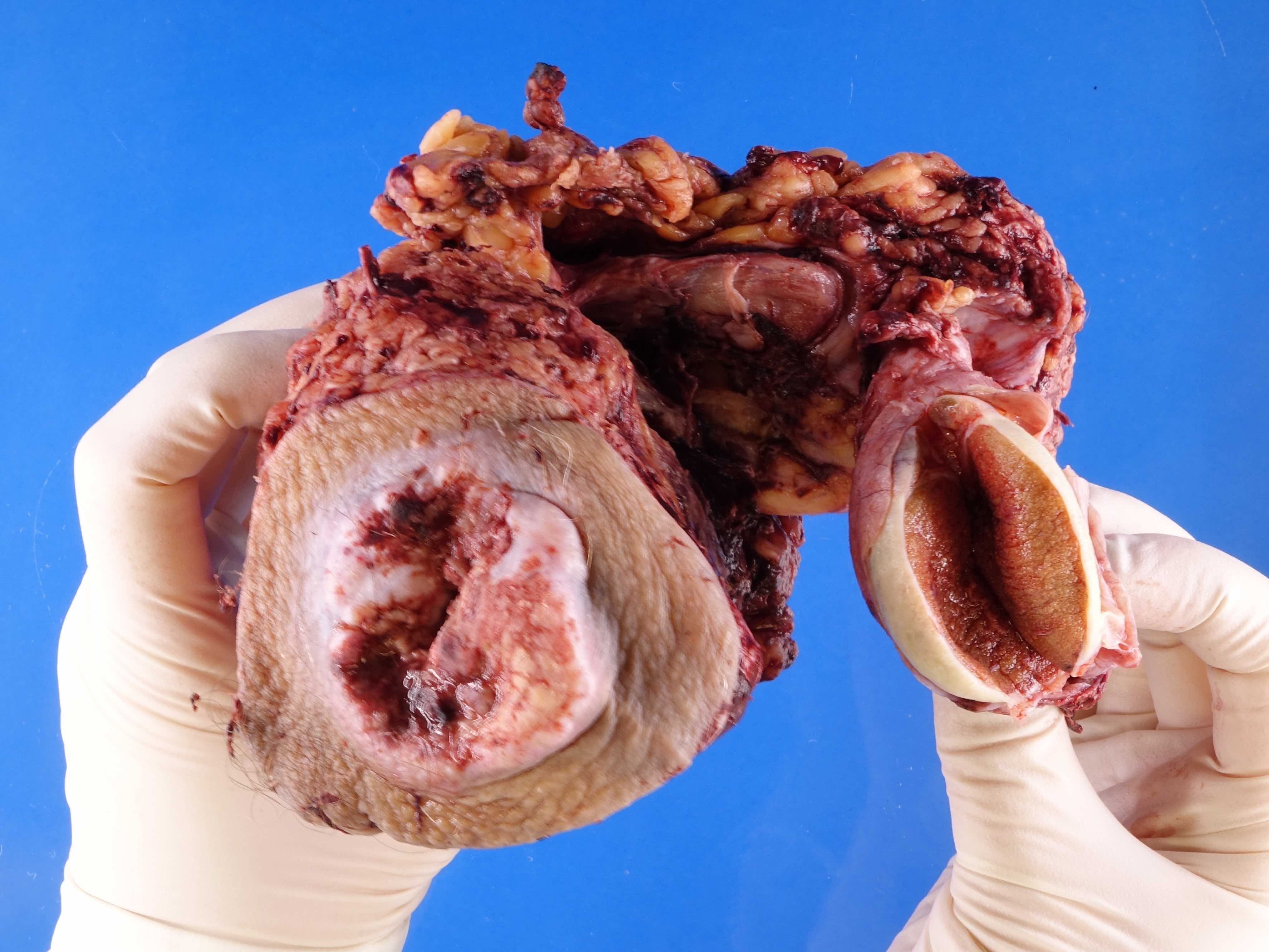
- pTX: cannot be assessed
- pT0: no evidence of primary tumor
- pTa: noninvasive carcinoma (broad pushing penetration is permitted)
- pTis: carcinoma in situ
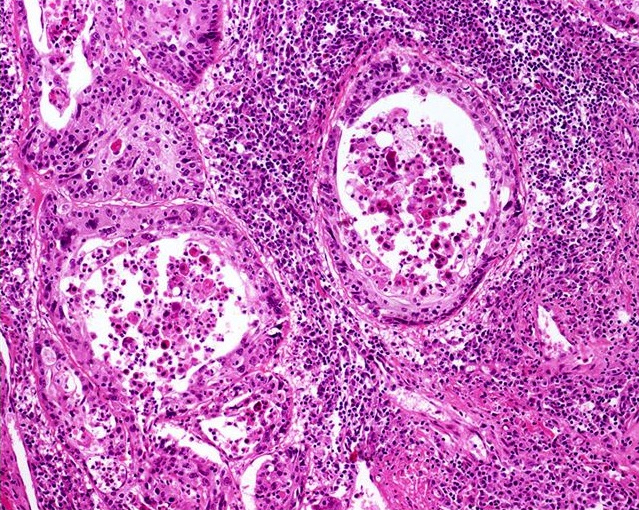
- pT1a: subepithelial invasion without lymphovascular invasion, perineural invasion or grade 3
- pT1b: subepithelial invasion with lymphovascular invasion, perineural invasion or grade 3
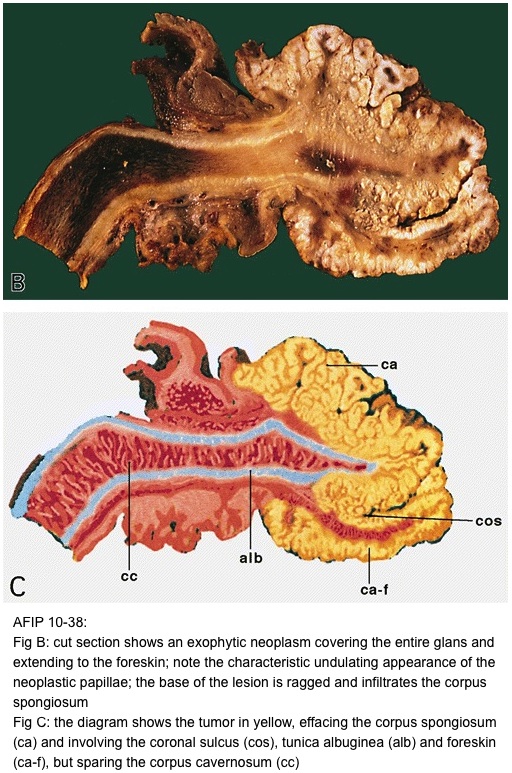
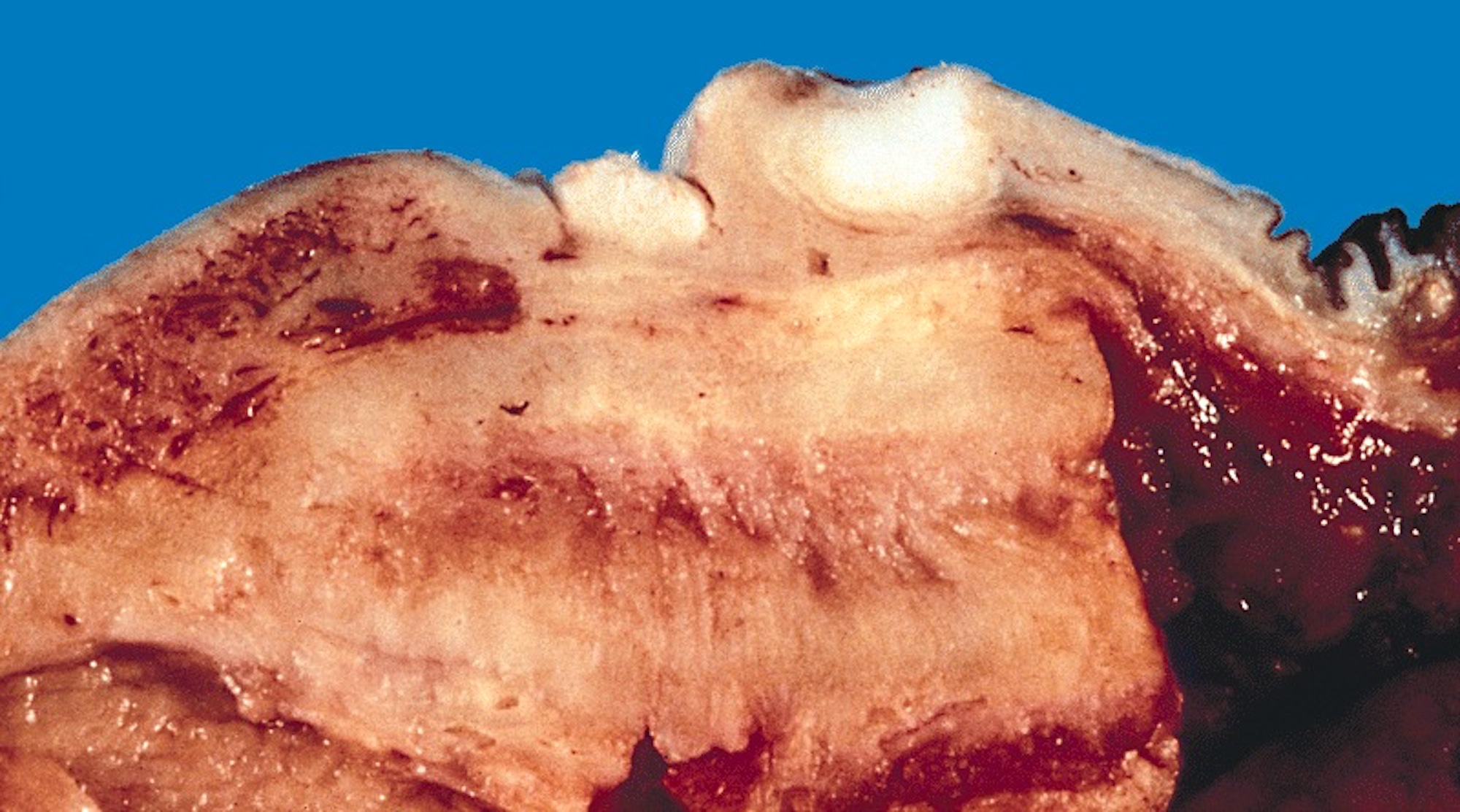
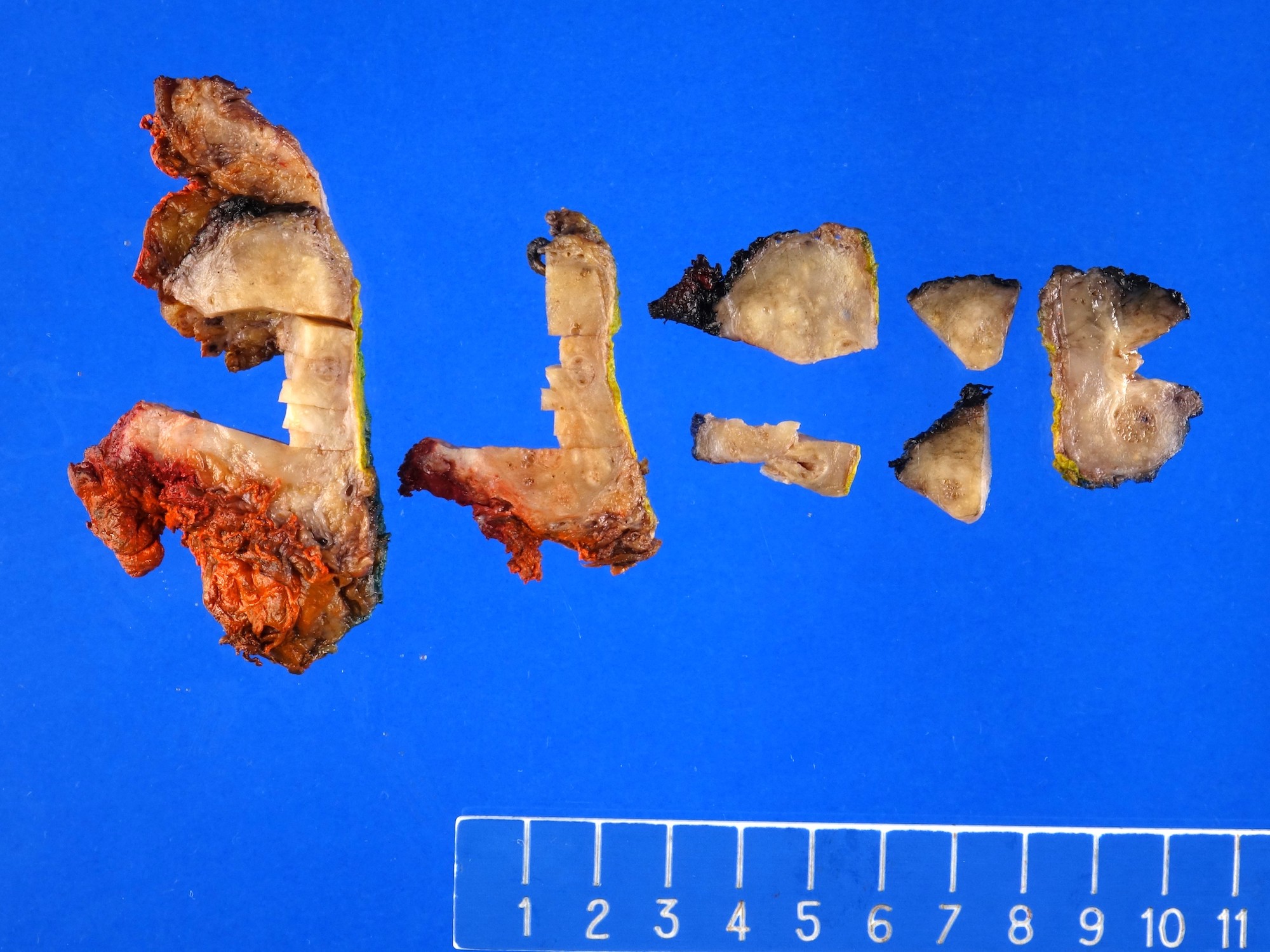
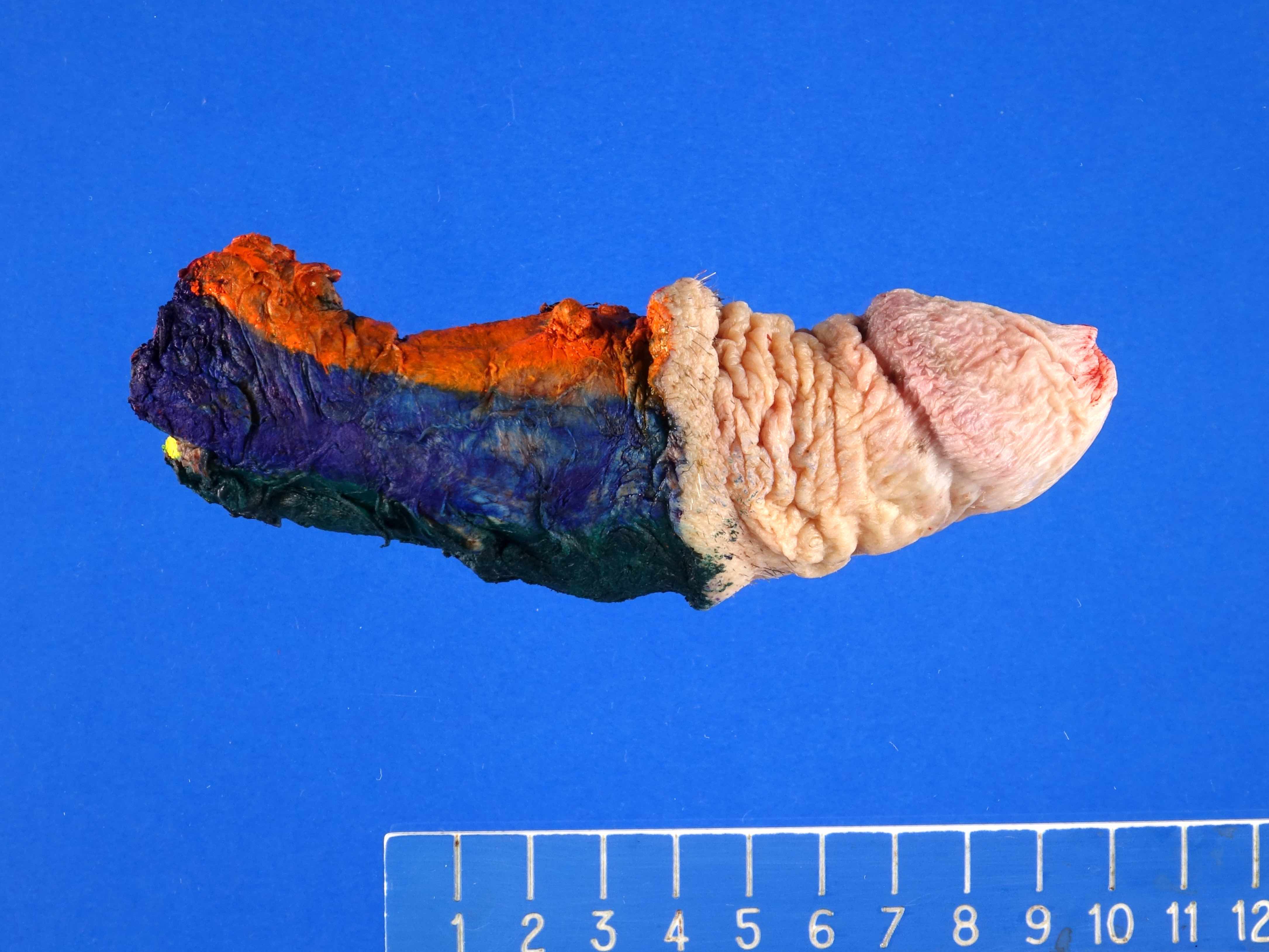
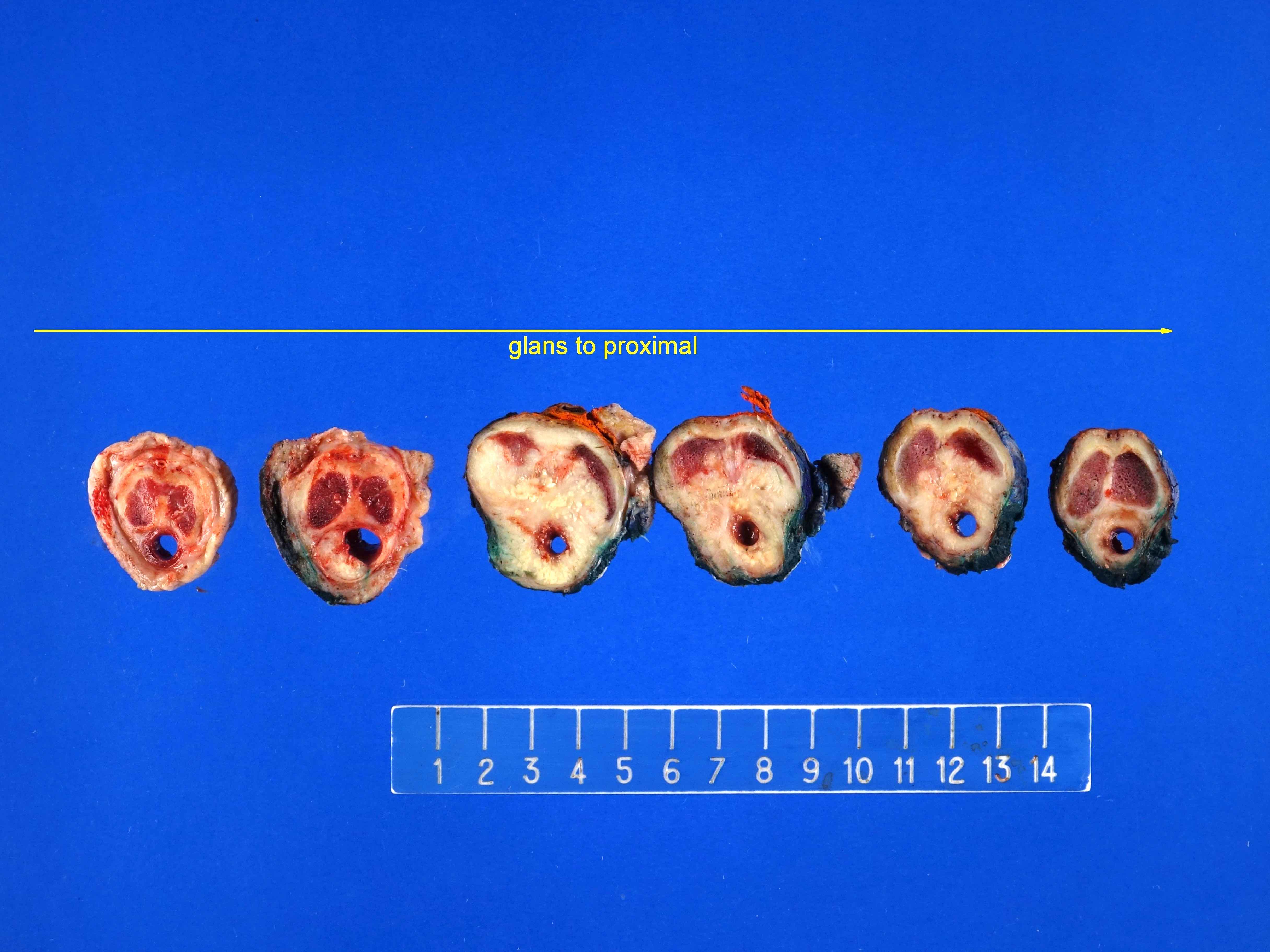
- pT2: invasion of corpus spongiosum
- pT3: invasion of corpus cavernosum
- pT4: invasion of adjacent structures including scrotum, prostate and pubic bone
Superpage
Superpage Topics
Adenosquamous carcinoma
Anatomy & histology-male urethra
Anatomy & histology-penis
Anatomy & histology-scrotum
Balanitis / phimosis
Basal cell carcinoma
Bowenoid papulosis
Cellulitis
Chancroid
Condyloma acuminatum
Congenital anomalies
Extramammary Paget disease
Fournier gangrene
Granuloma inguinale
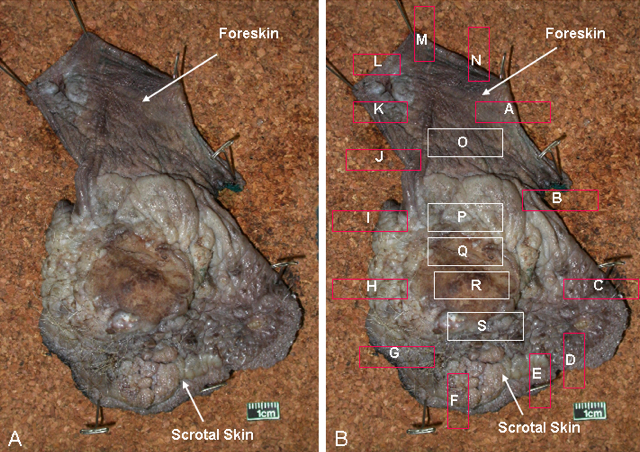
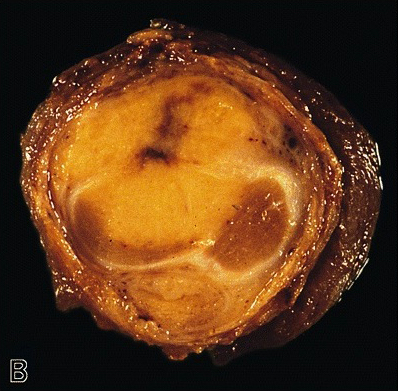
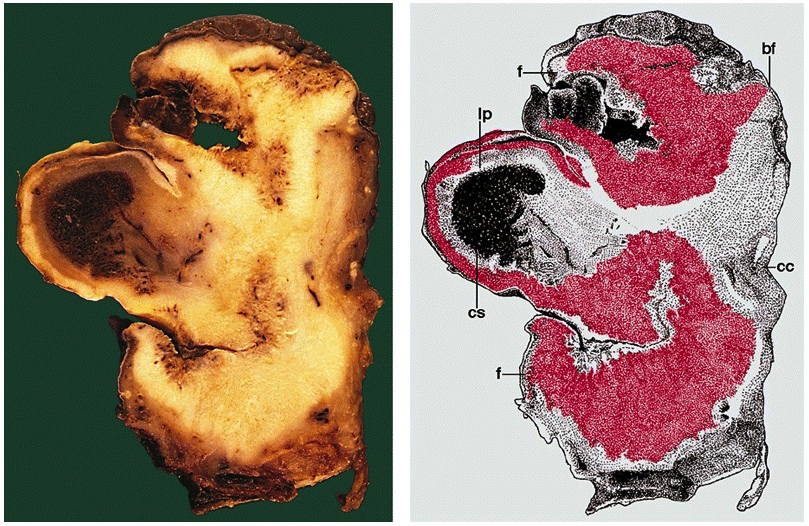
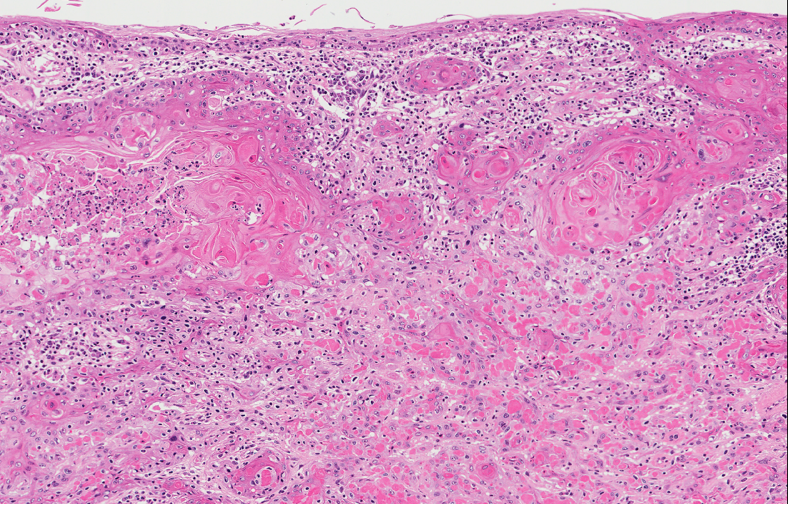
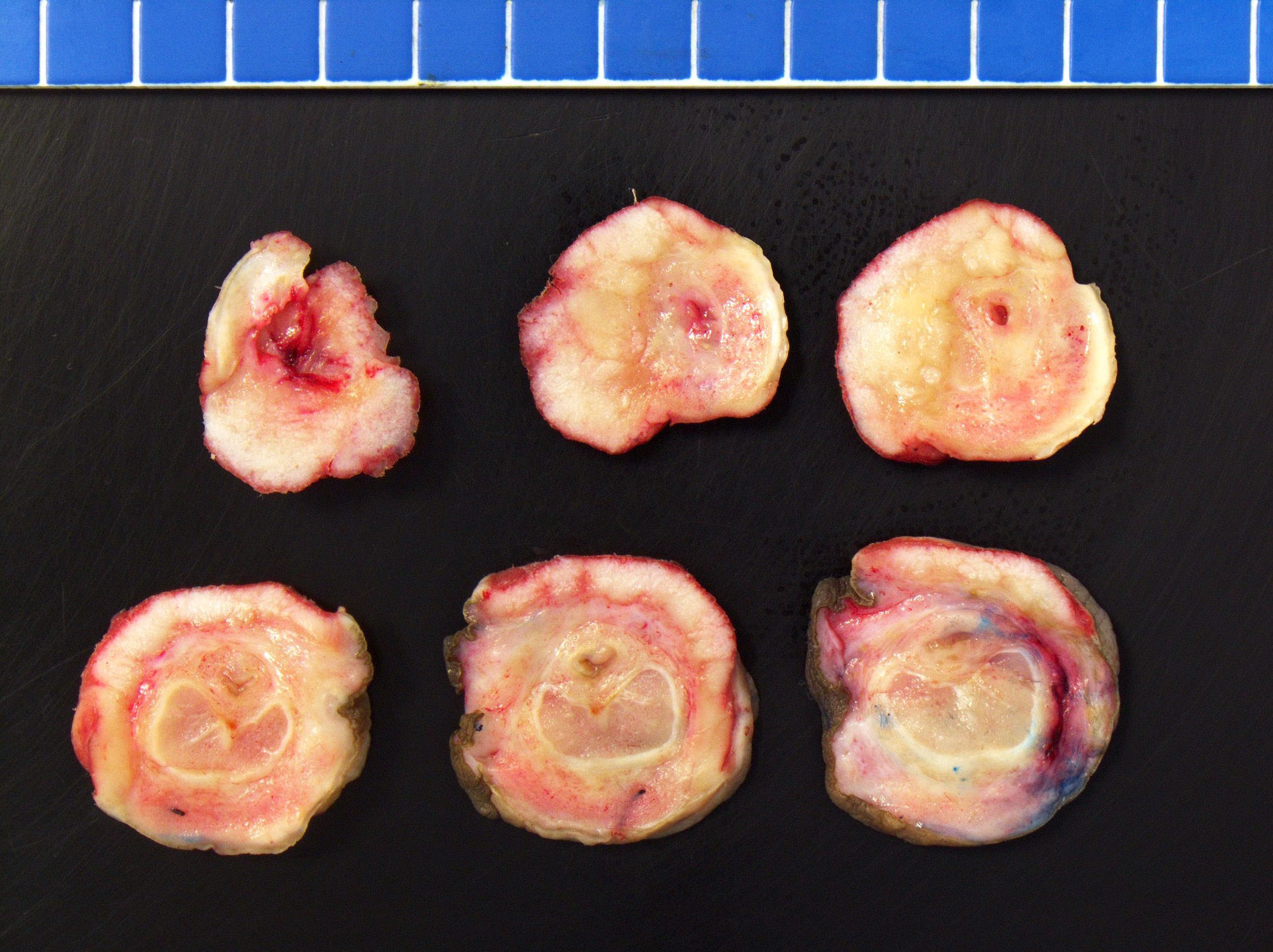
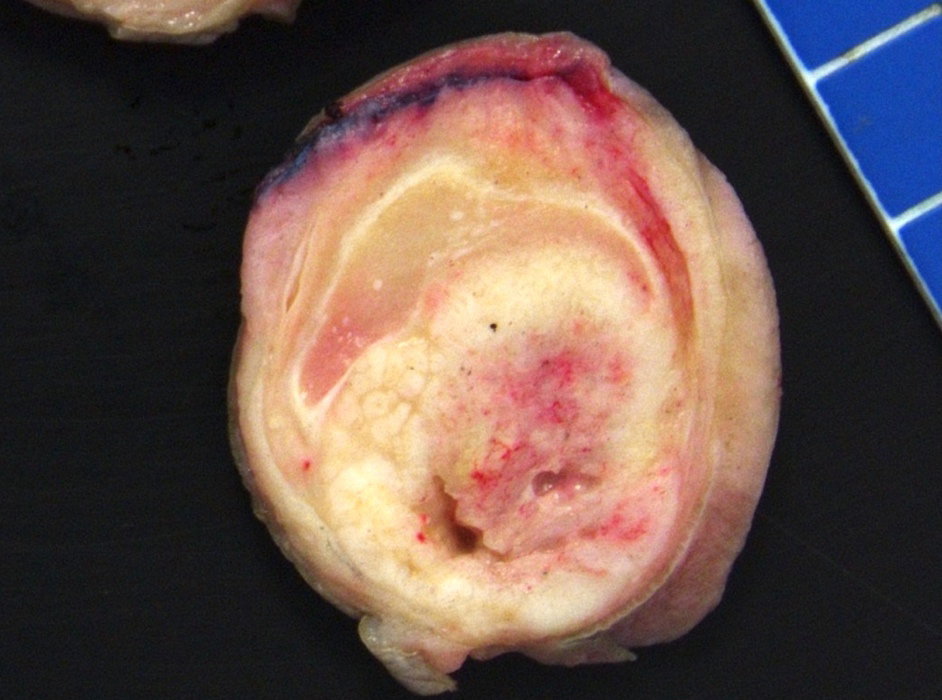
Grossing & features to report
HPV associated squamous cell carcinoma
HPV independent squamous cell carcinoma (usual, verrucous, papillary, sarcomatoid)
Herpes simplex virus
Lichen sclerosus (balanitis xerotica obliterans)
Lymphogranuloma venereum
Melanosis and lentiginosis
Molluscum contagiosum
Mucoid cyst
Myointimoma
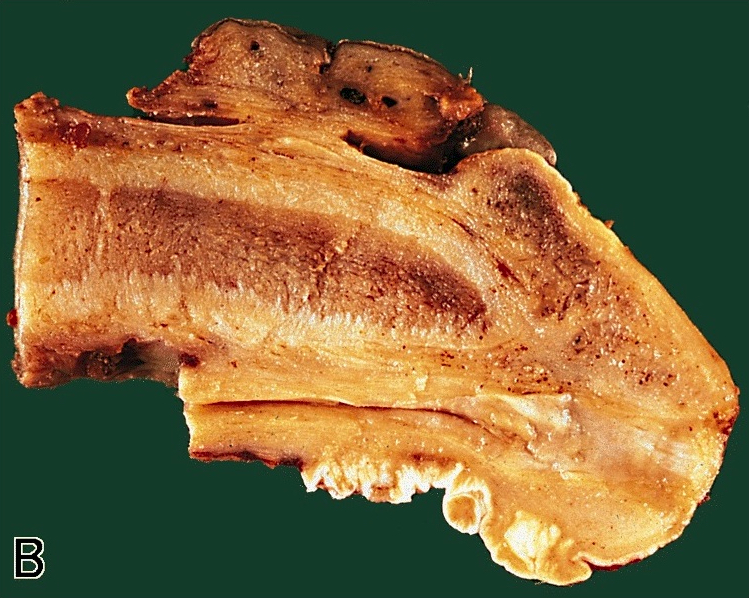
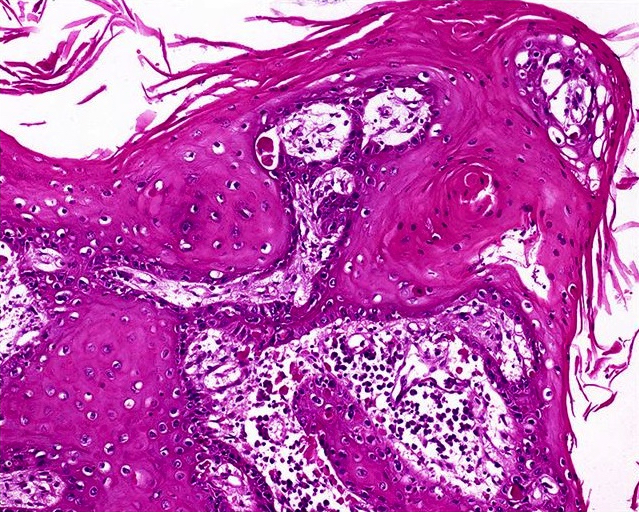
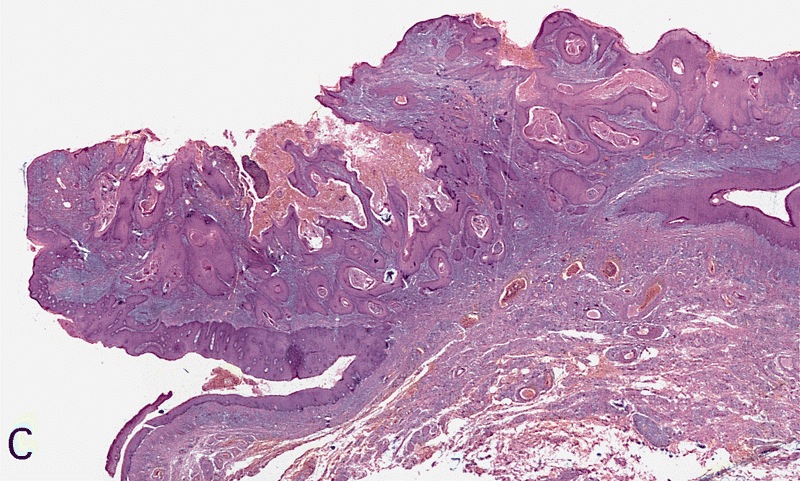
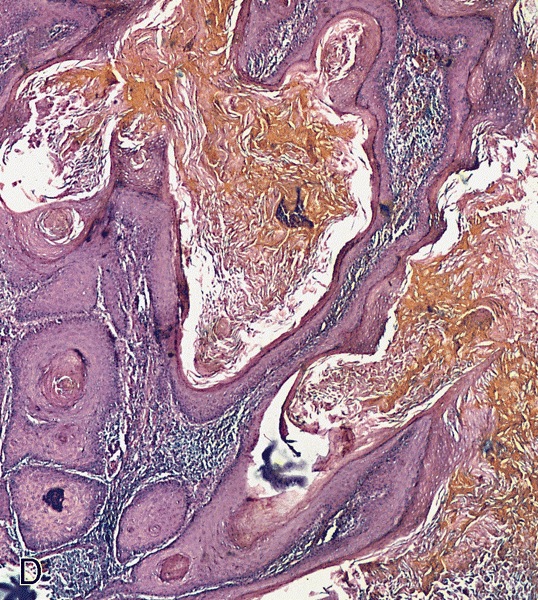
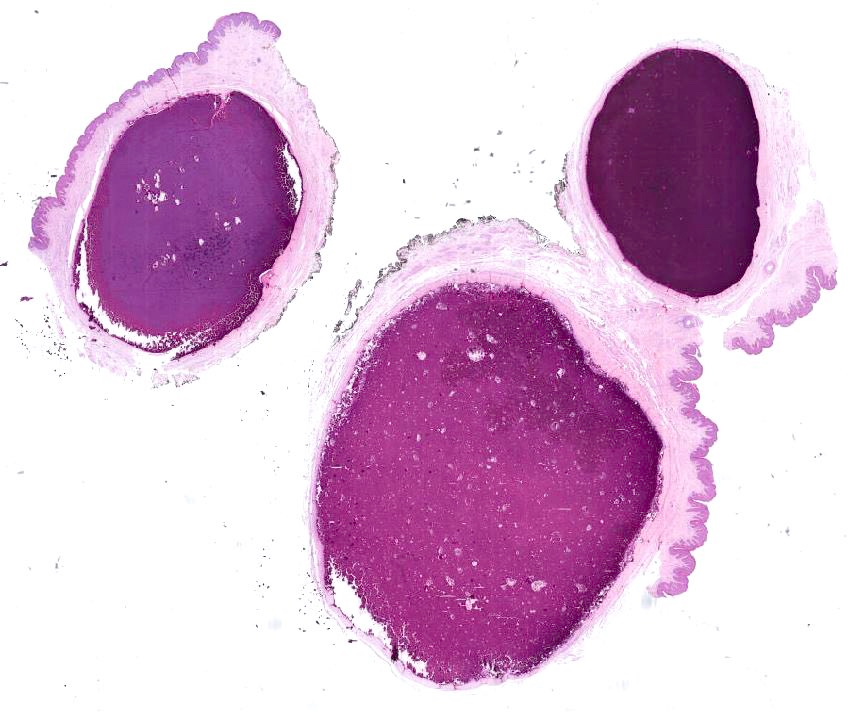
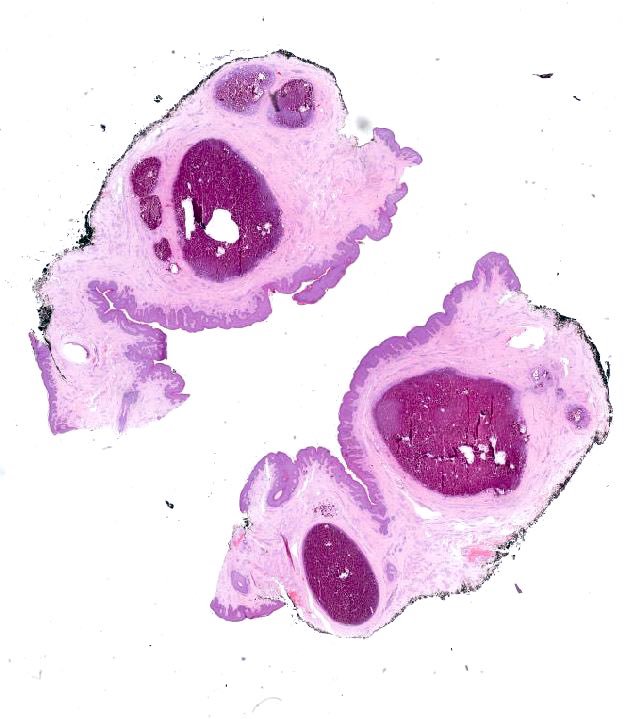
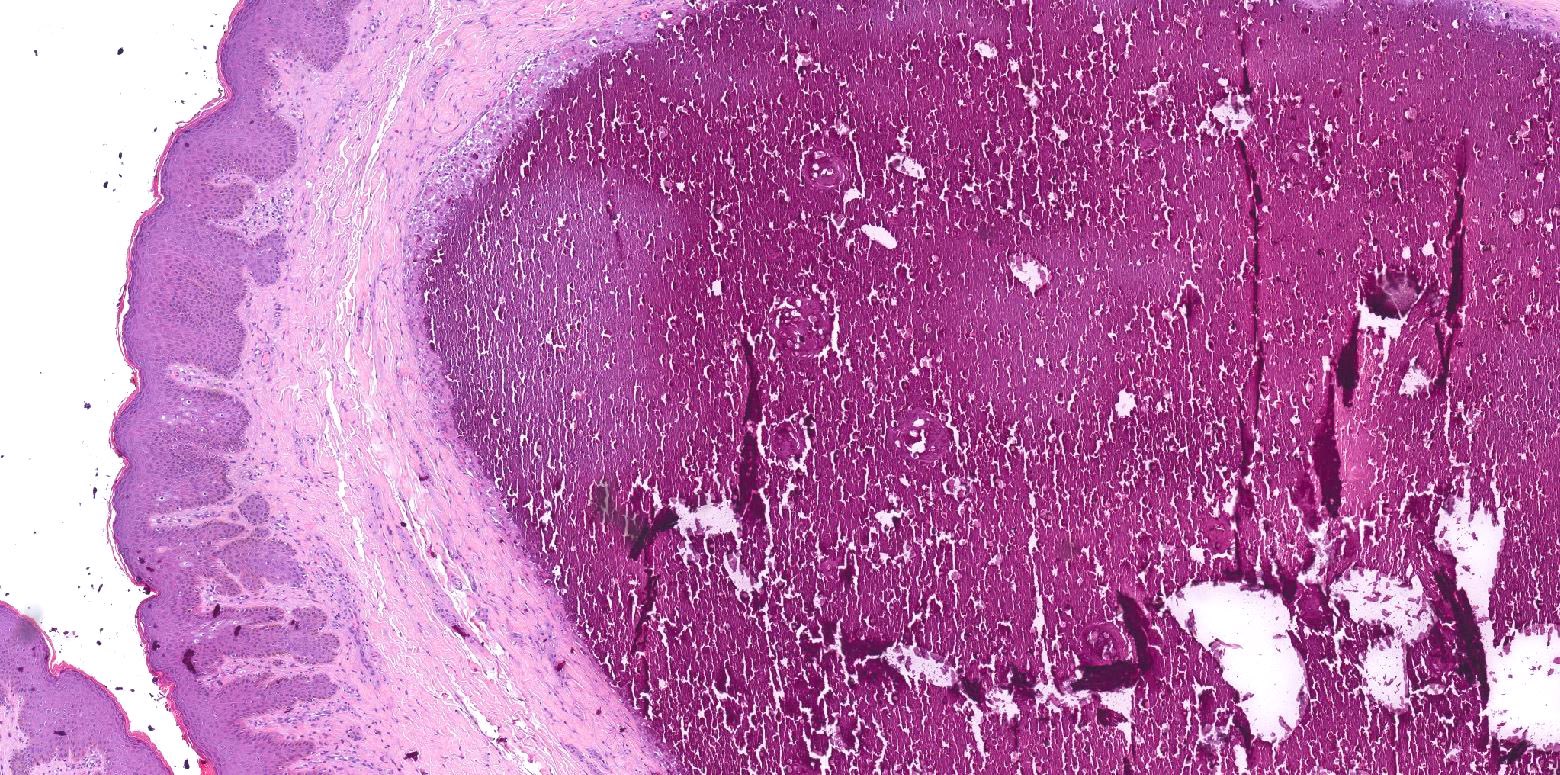
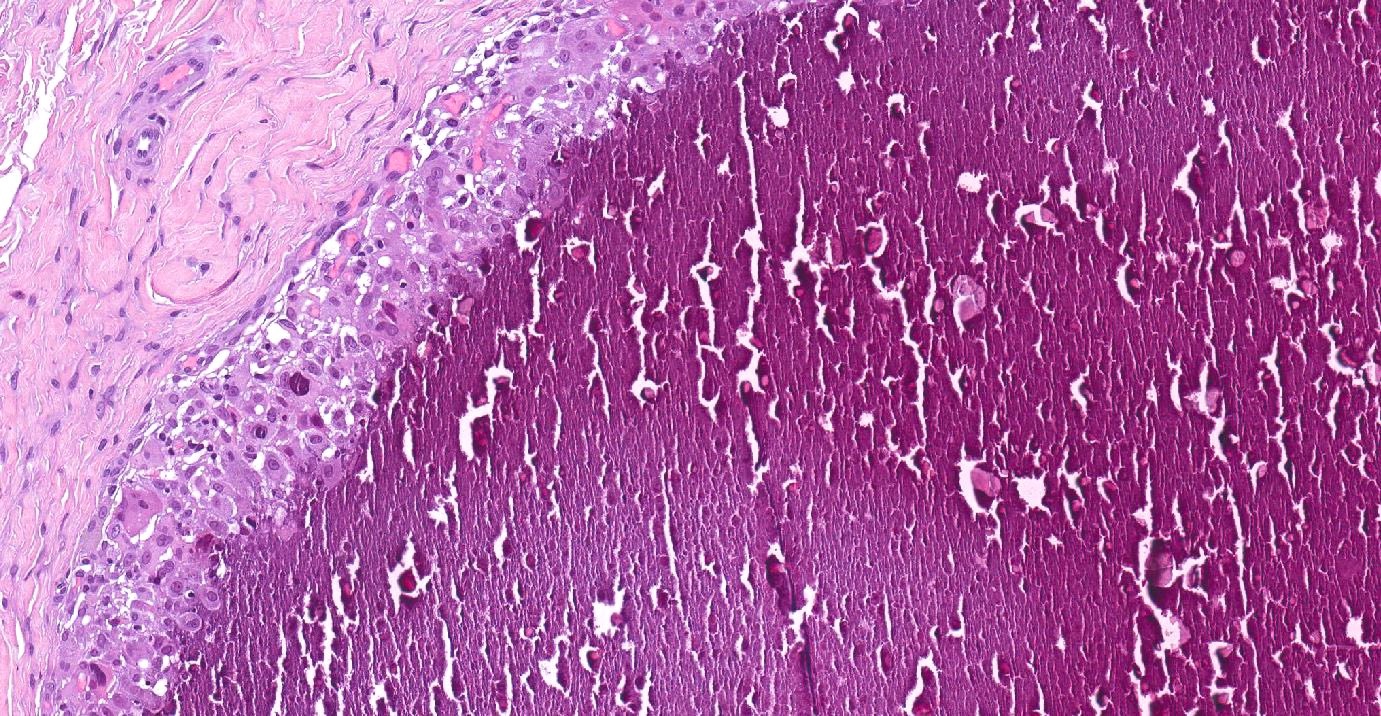
Ossification
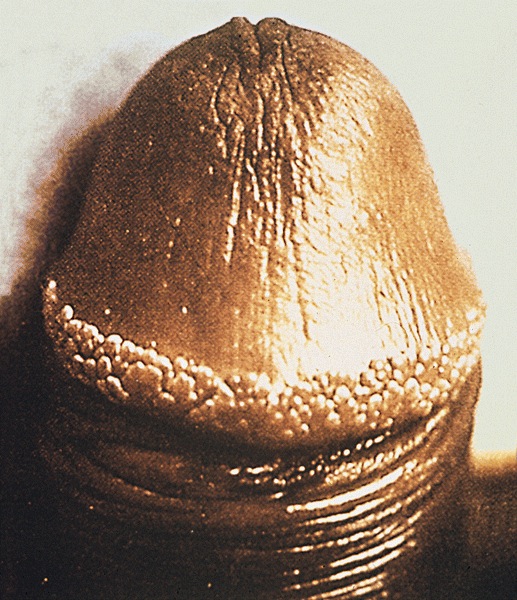
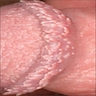
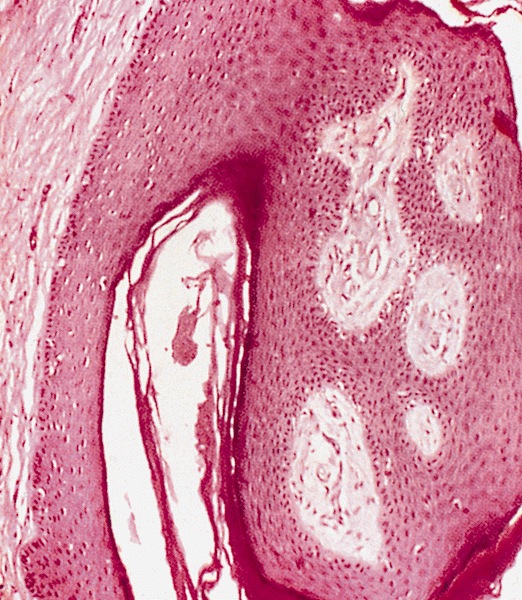
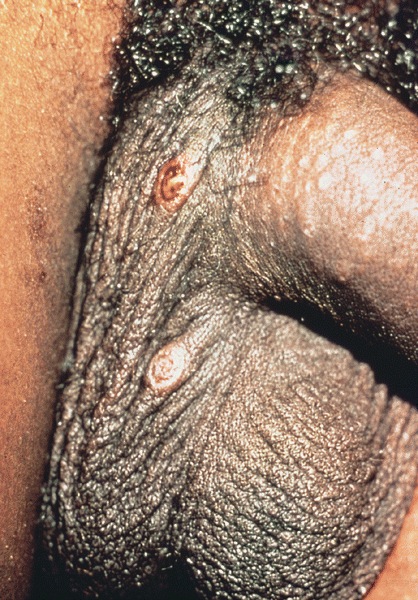
Pearly penile papules
Penile intraepithelial neoplasia (PeIN)
Peyronie disease
Sclerosing lipogranuloma
Scrotal calcinosis
Squamous cell carcinoma-general
Squamous hyperplasia
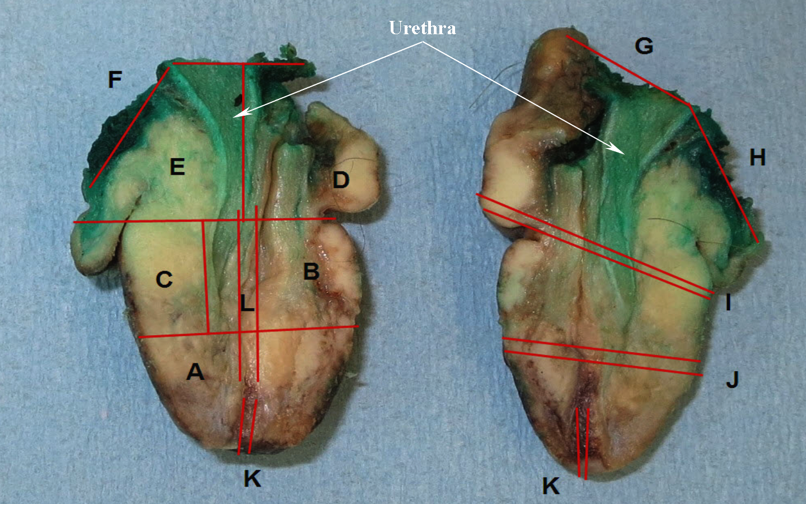
Staging-penis
Syphilis
Tancho nodules / paraffinomas / lipogranulomas
Urethral carcinoma
Verruciform xanthoma
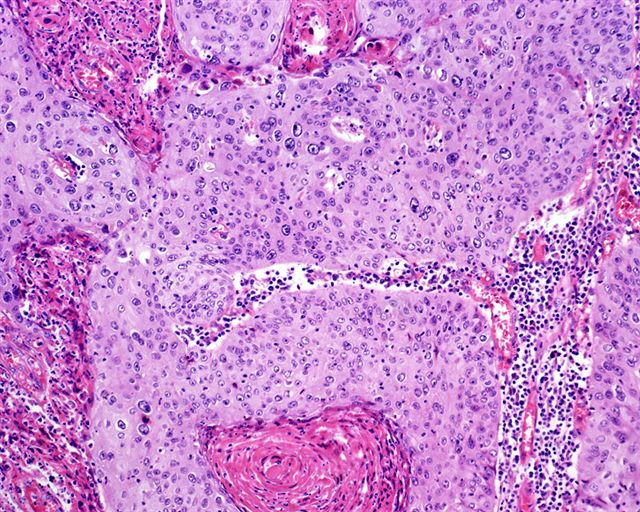
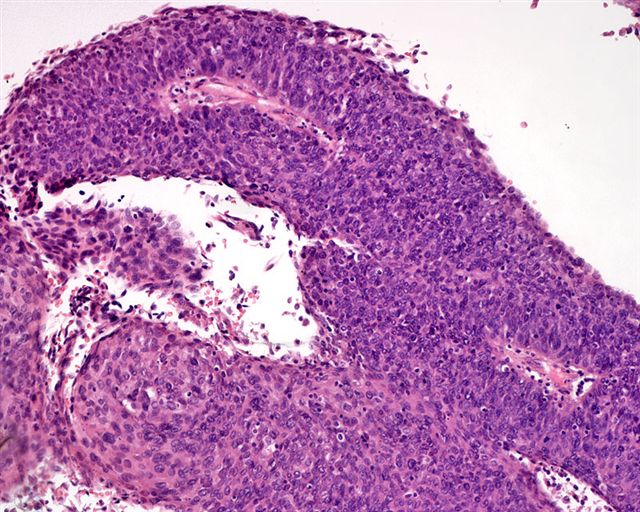
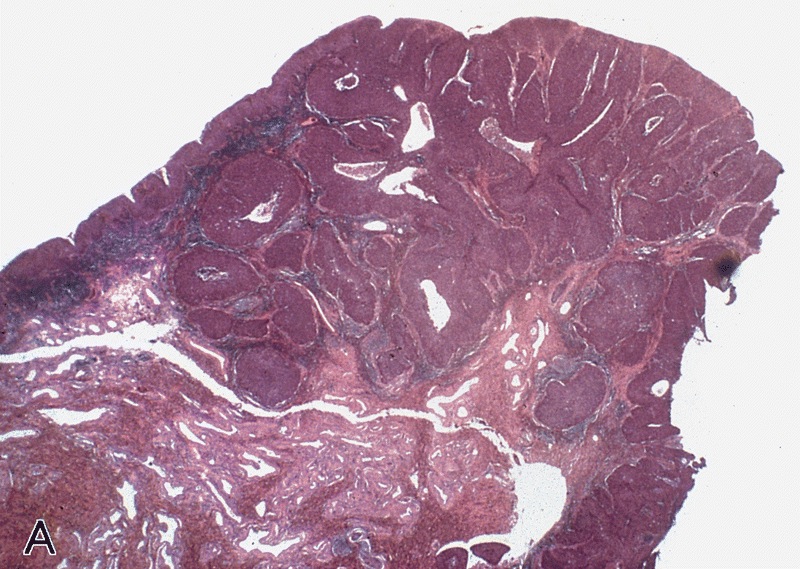
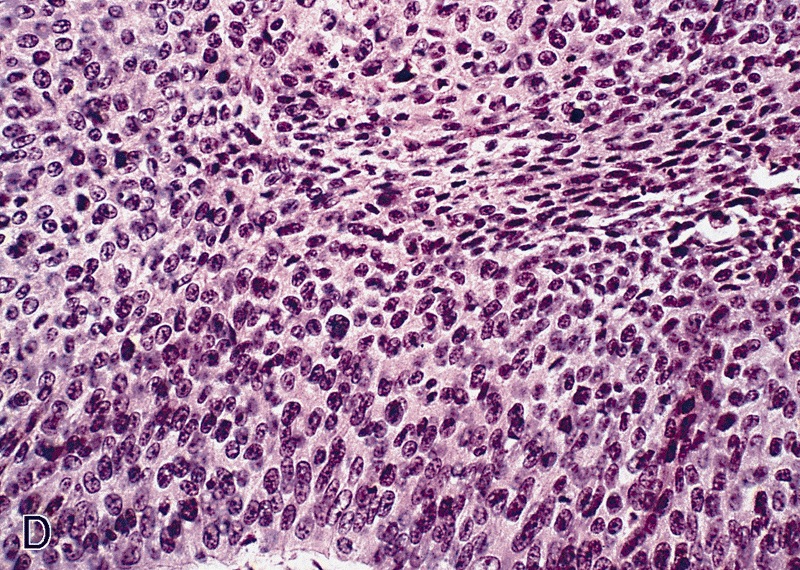
WHO classificationAdenosquamous carcinoma
Table of Contents
Definition / general | Epidemiology | Sites | Etiology | Clinical features | Case reports | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Differential diagnosisDefinition / general
- Mixed tumor composed of neoplastic squamous nests intermingled with areas of glandular differentiation
- ICD-0: 8560 / 3
Epidemiology
- 1 - 2% of all penile carcinomas (Anal Quant Cytol Histol 2007;29:185)
- Mean age of 55 years (range 30 - 74 years)
Sites
- Most common is glans but extension to coronal sulcus and inner foreskin is also common
Etiology
- May originate in misplaced glandular cells in perimeatal region, in metaplastic goblet cells of foreskin mucosa or as aberrant differentiation of squamous epithelium
Clinical features
- Local recurrence in up to 25% and inguinal nodal metastases in 43 - 50% of cases
- Low mortality rate (0 - 14%)
Case reports
- 3 patients (ages 37, 72 and 74 years) with superficial tumors (Am J Surg Pathol 1996;20:156)
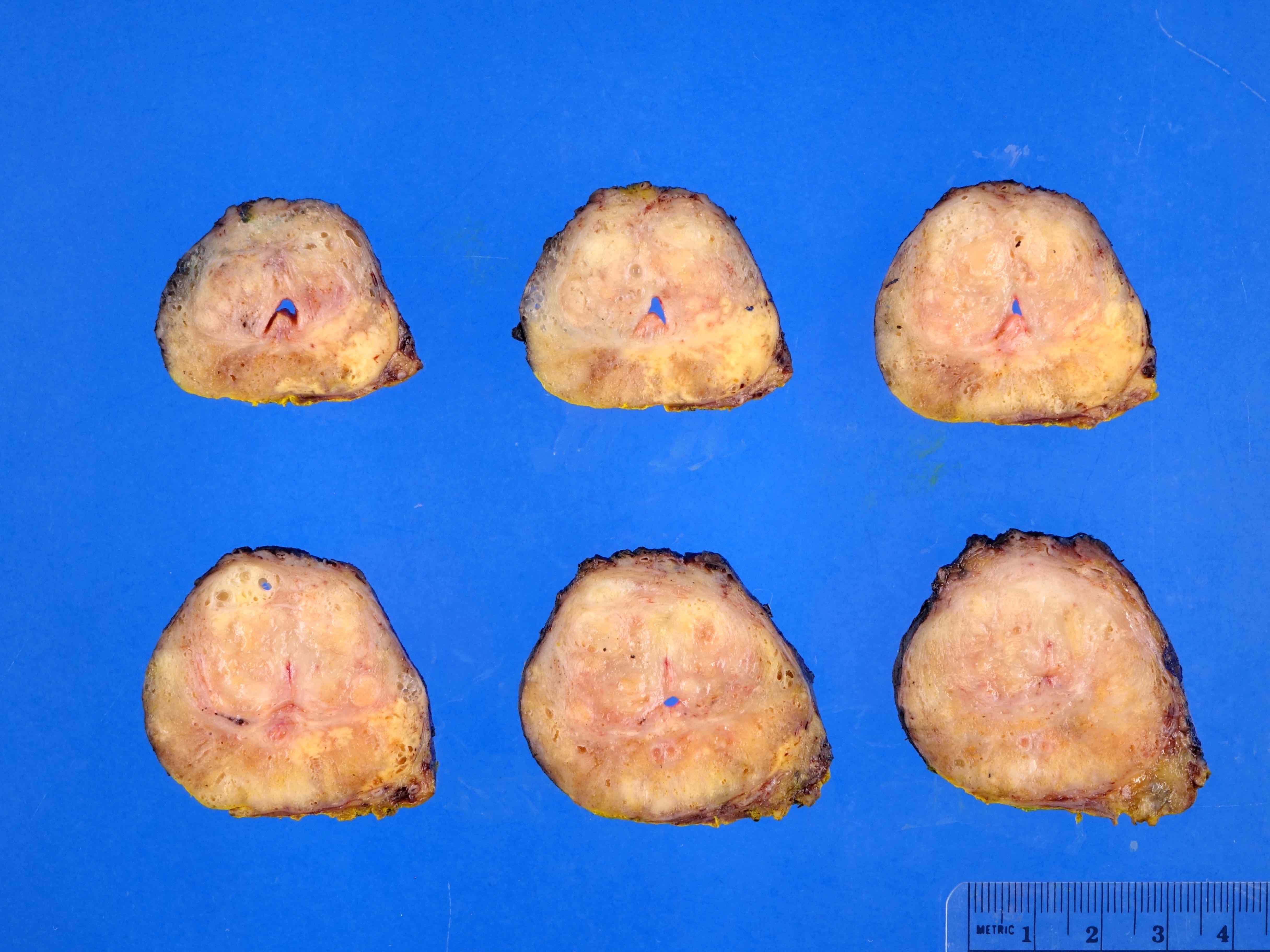
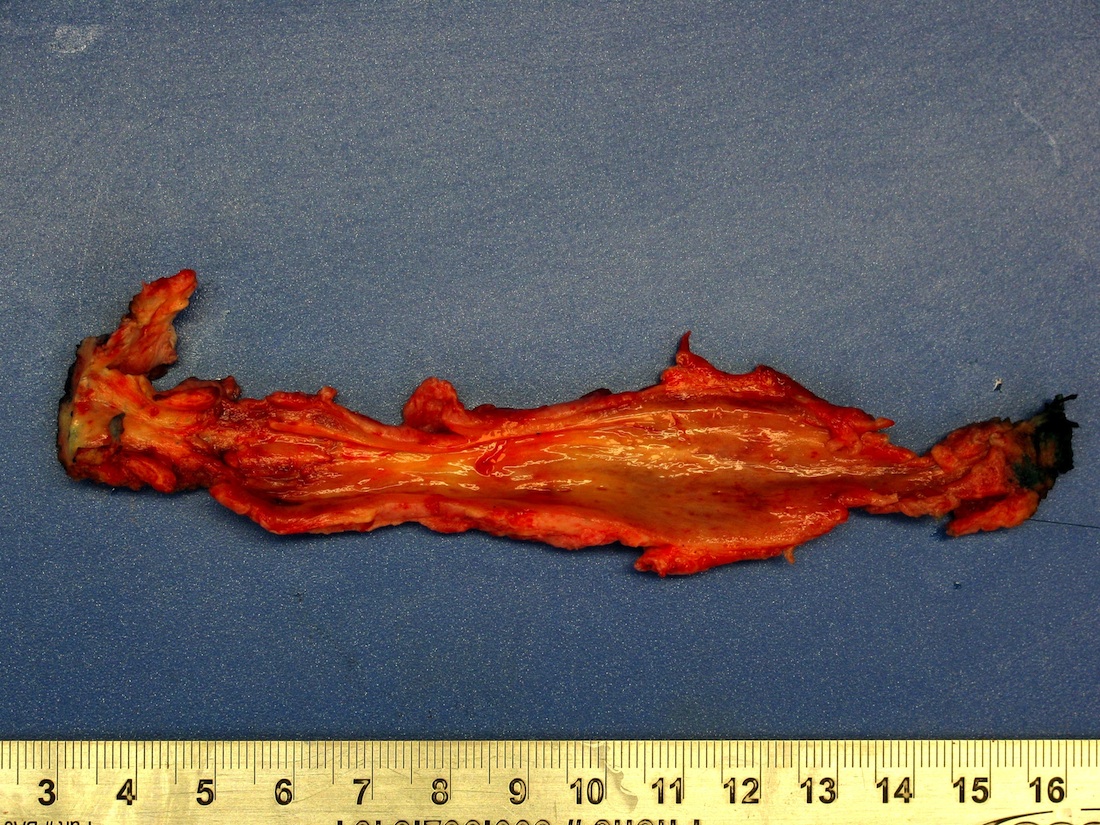
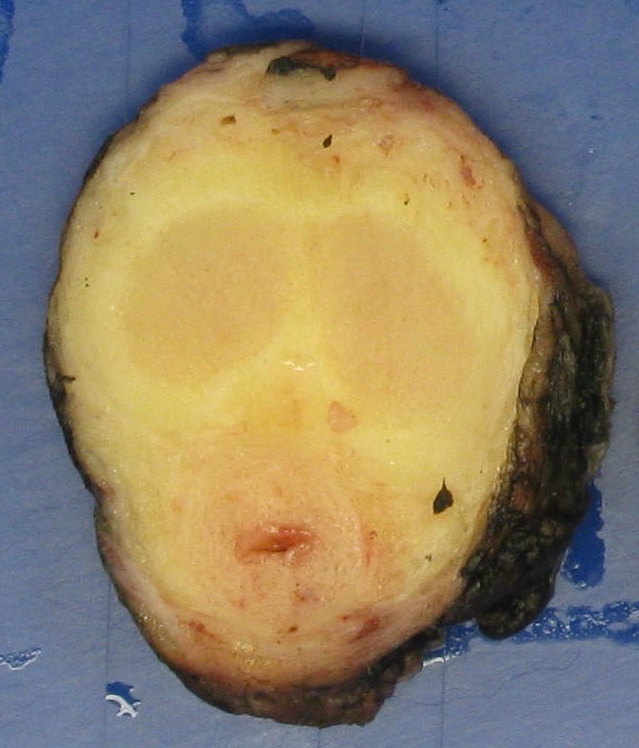
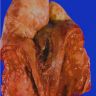
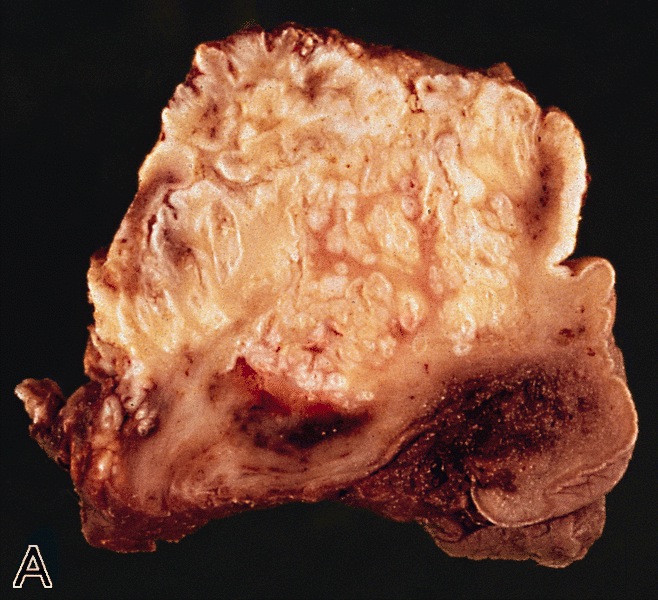
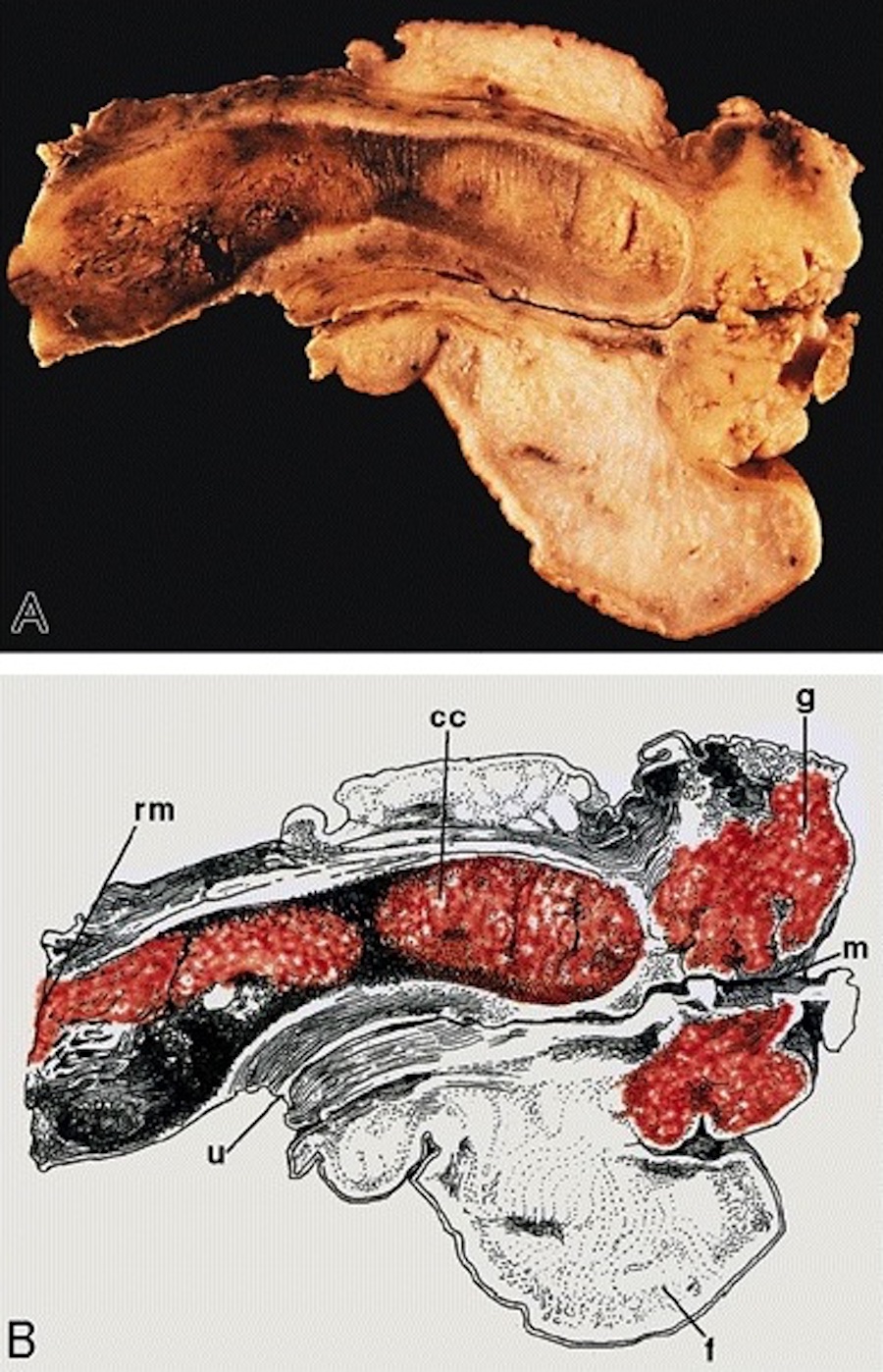
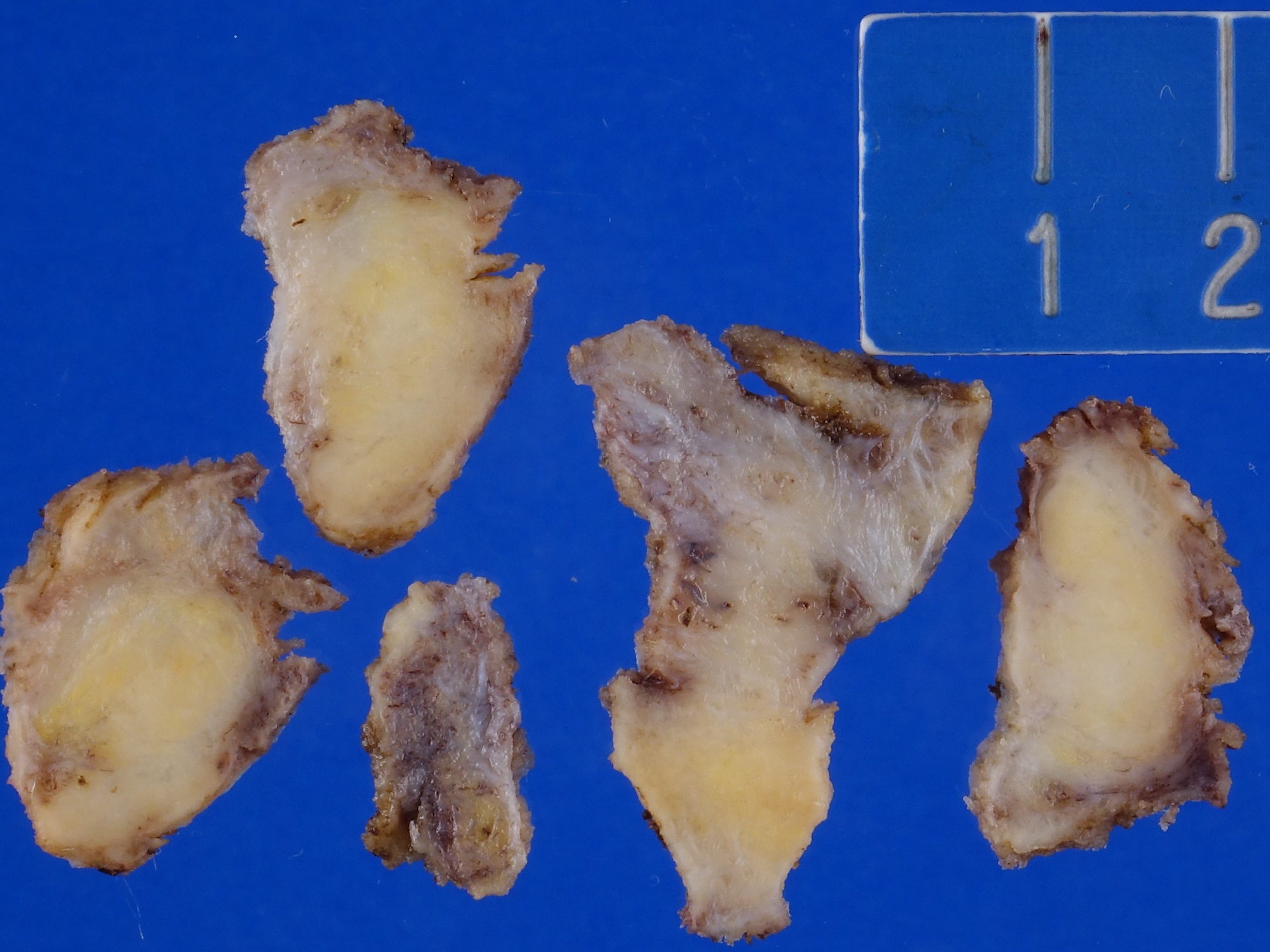
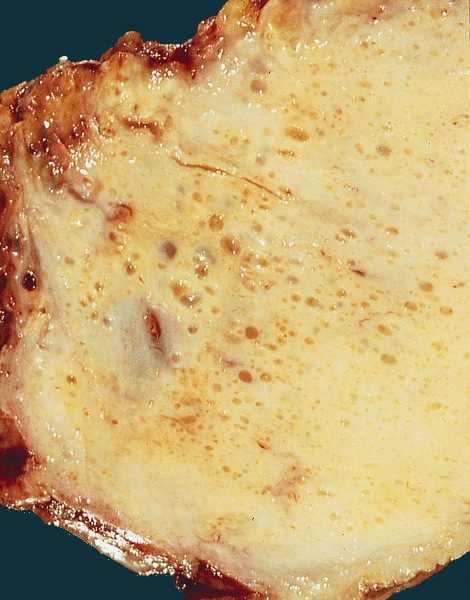
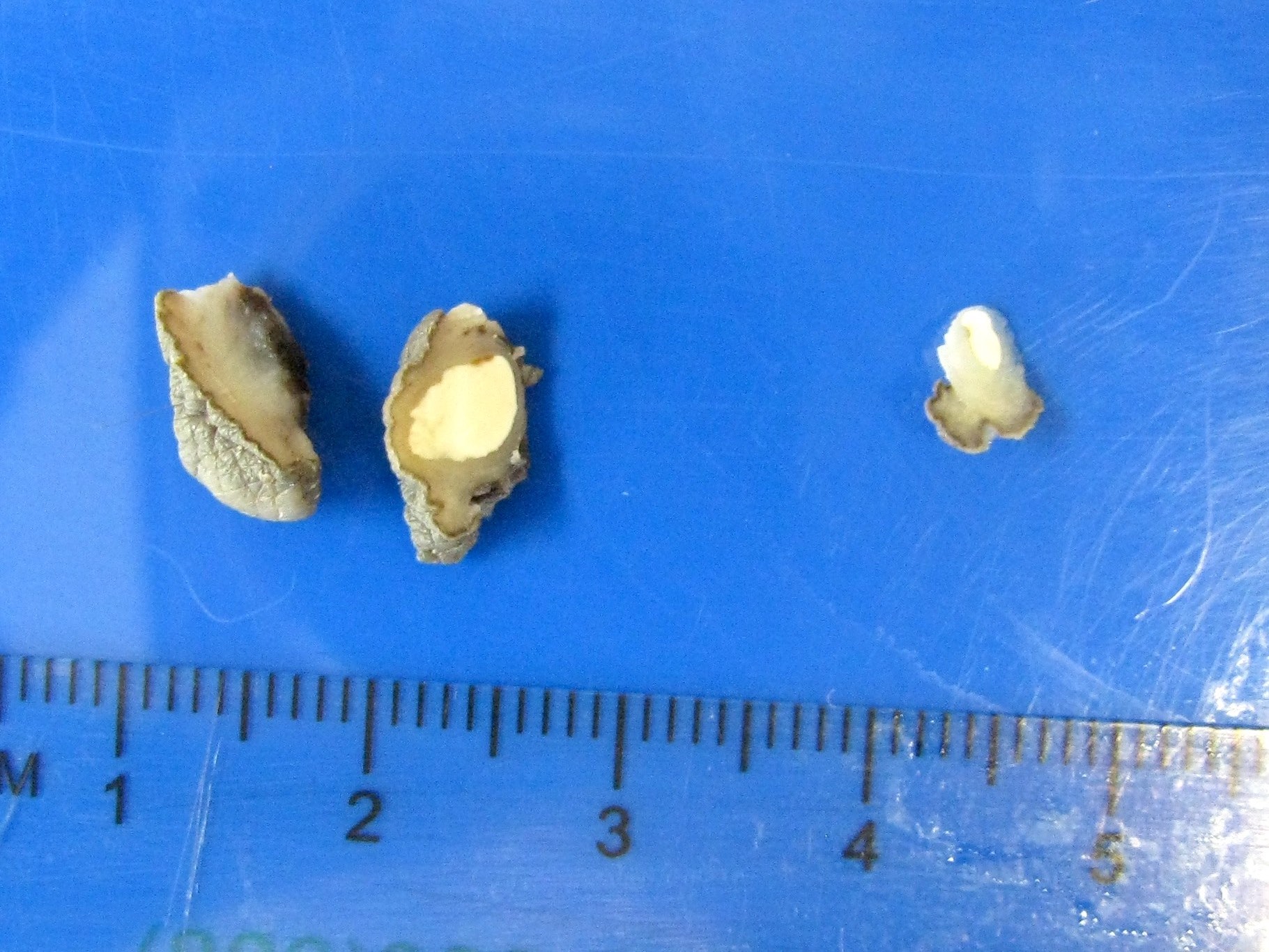
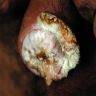
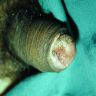
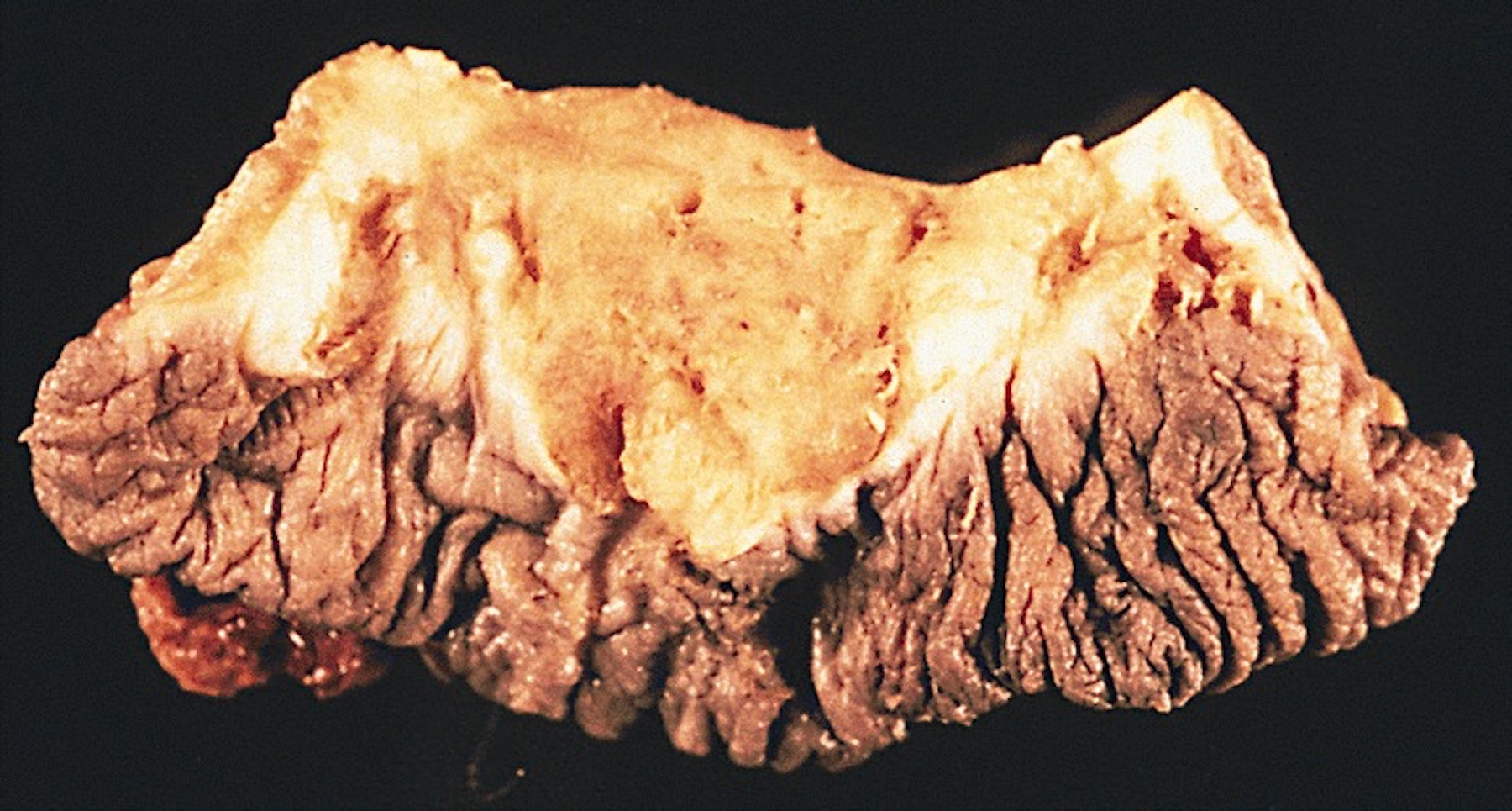
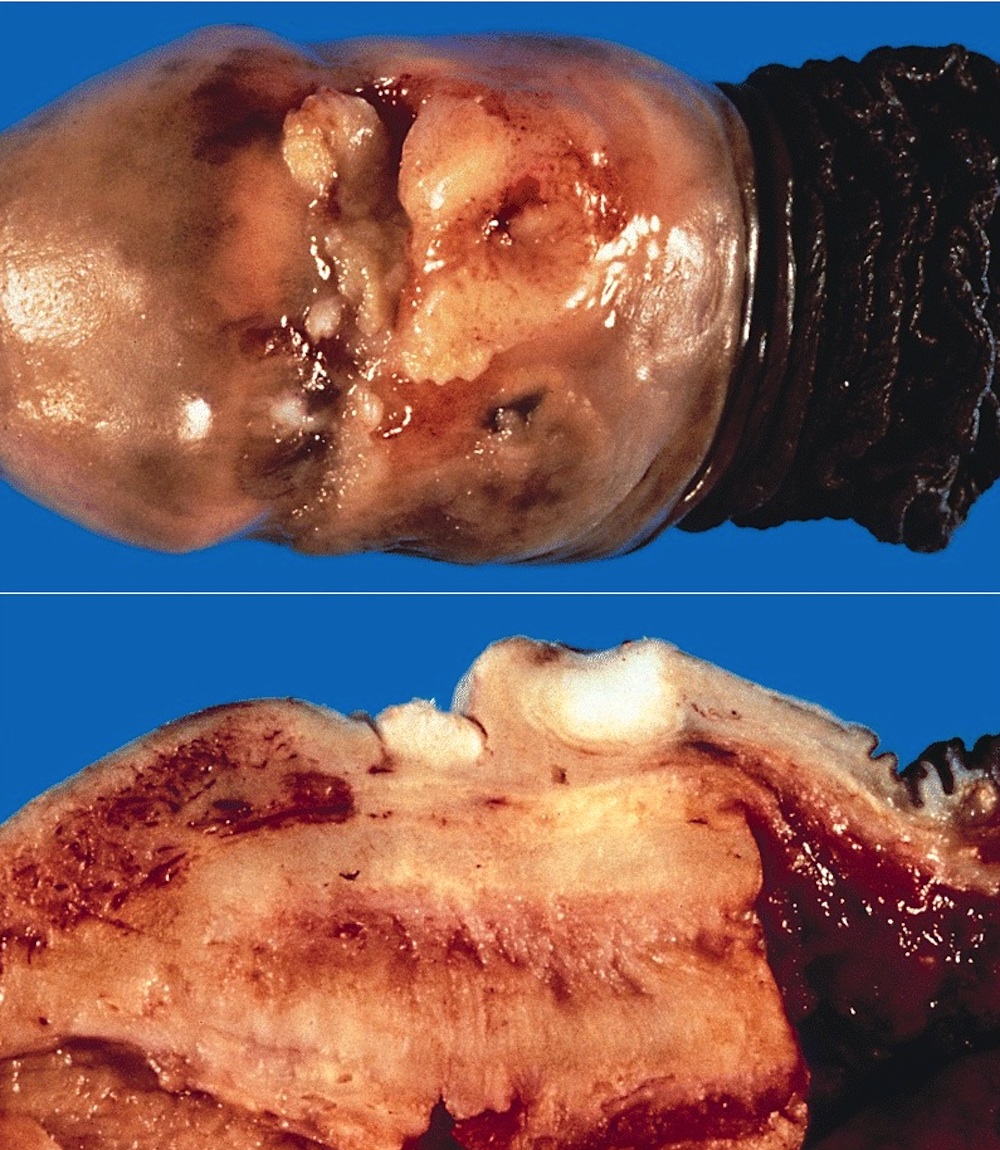
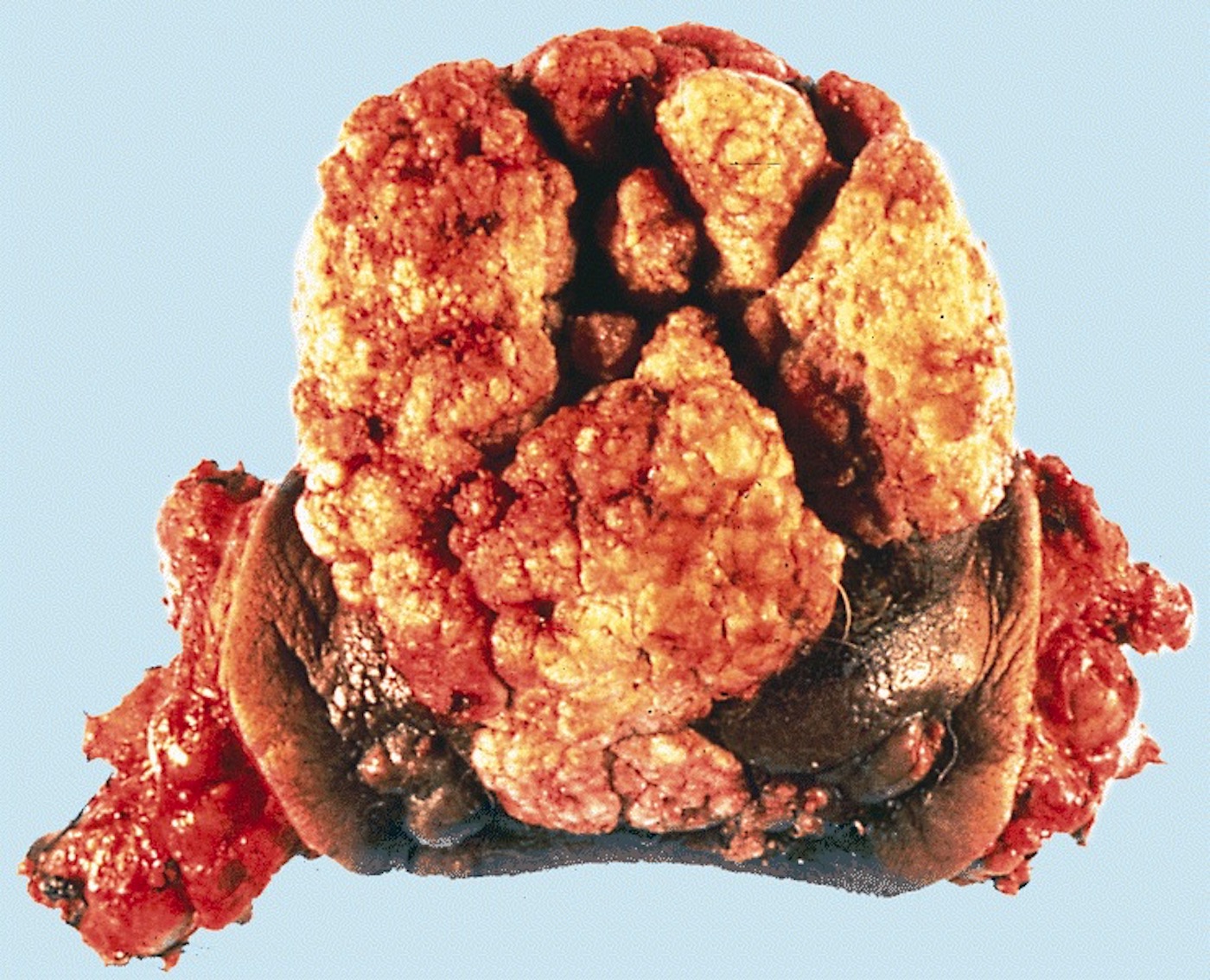
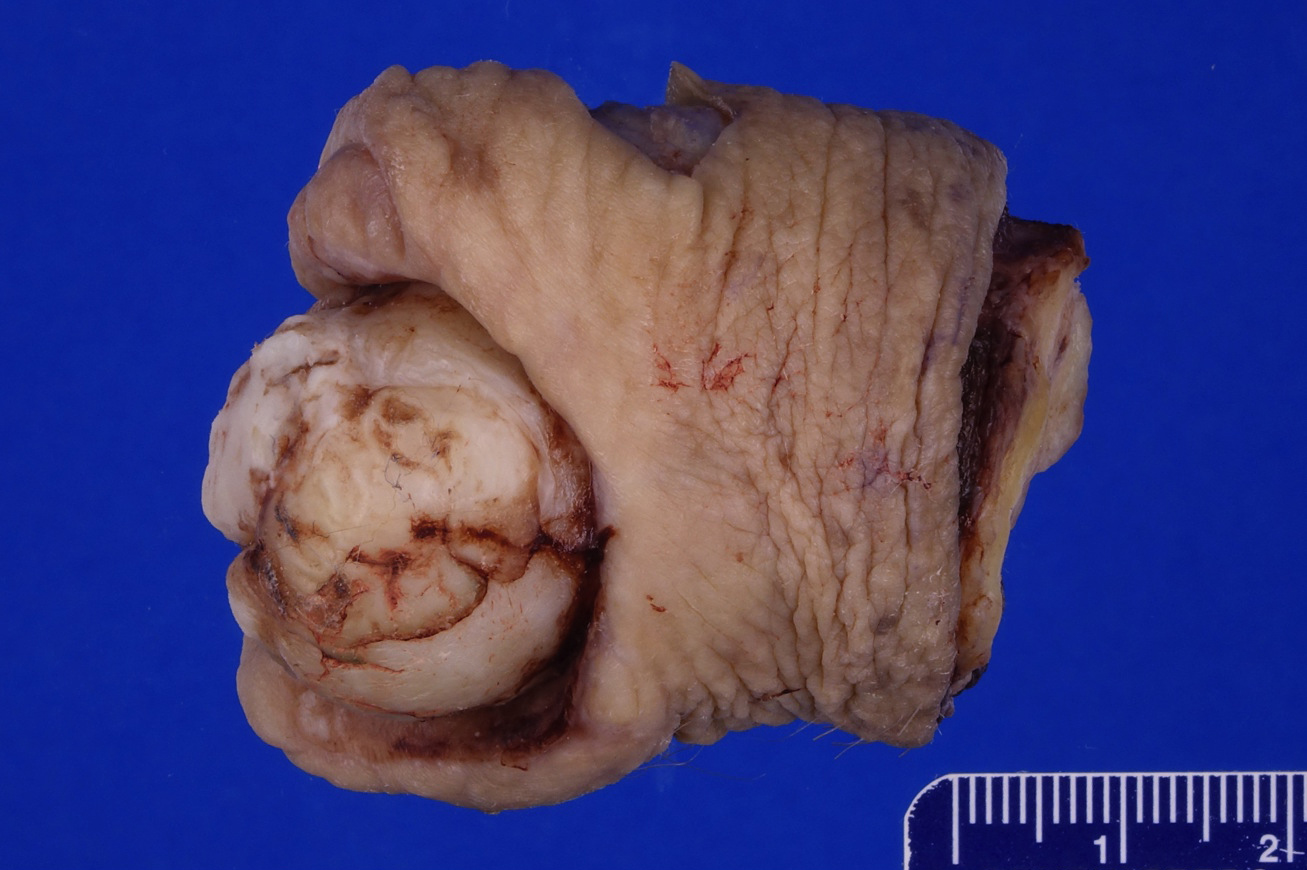
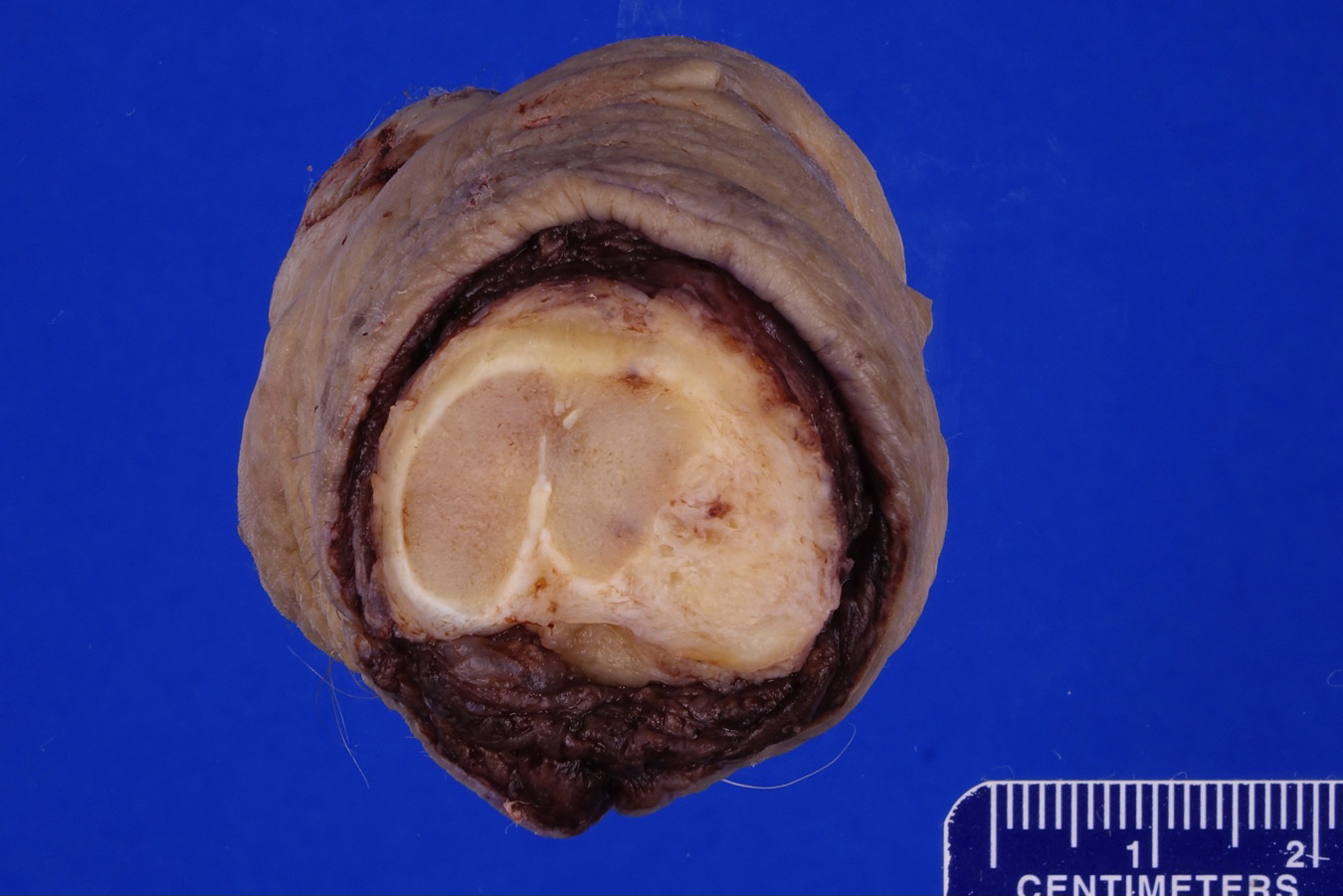
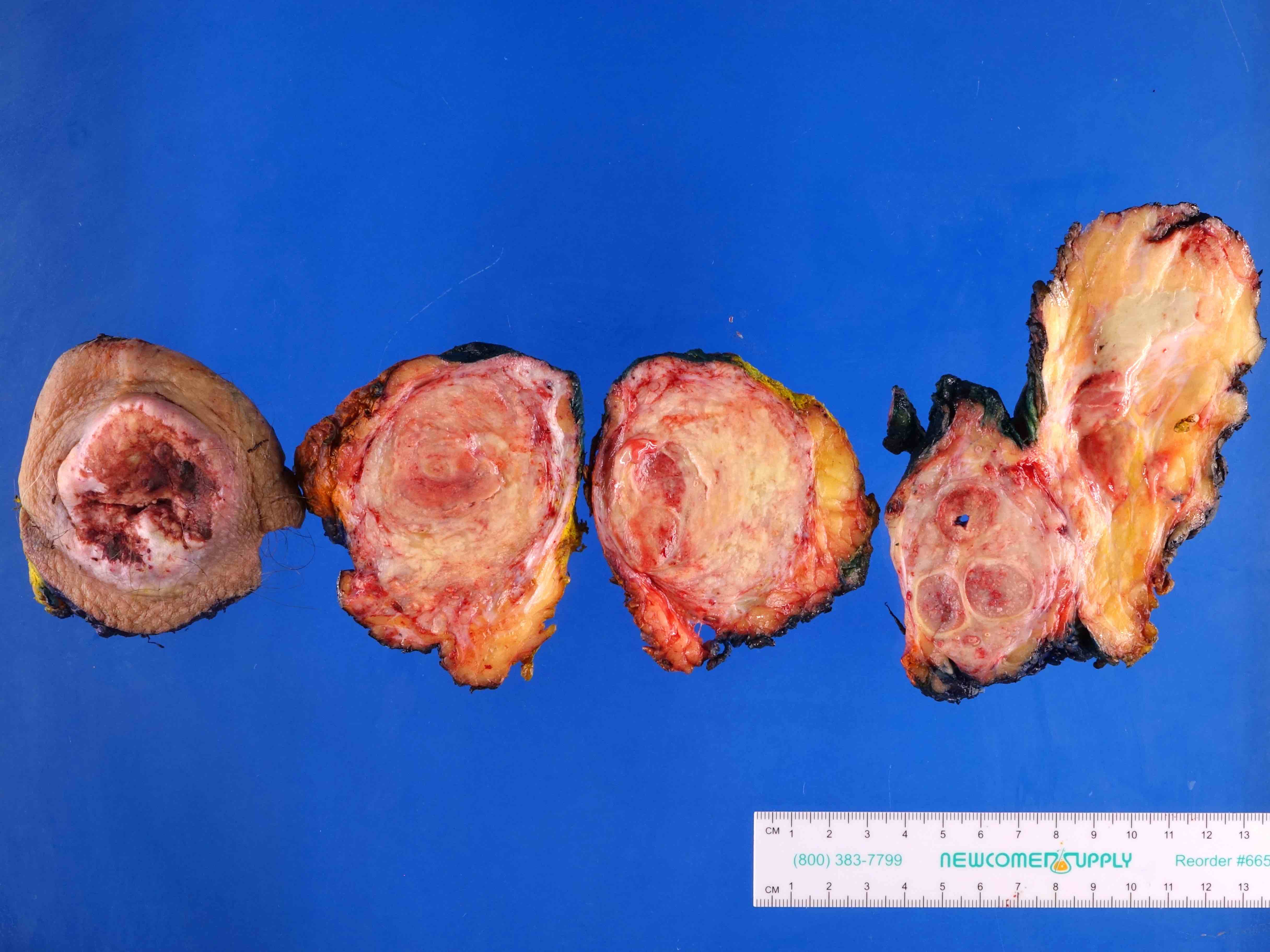
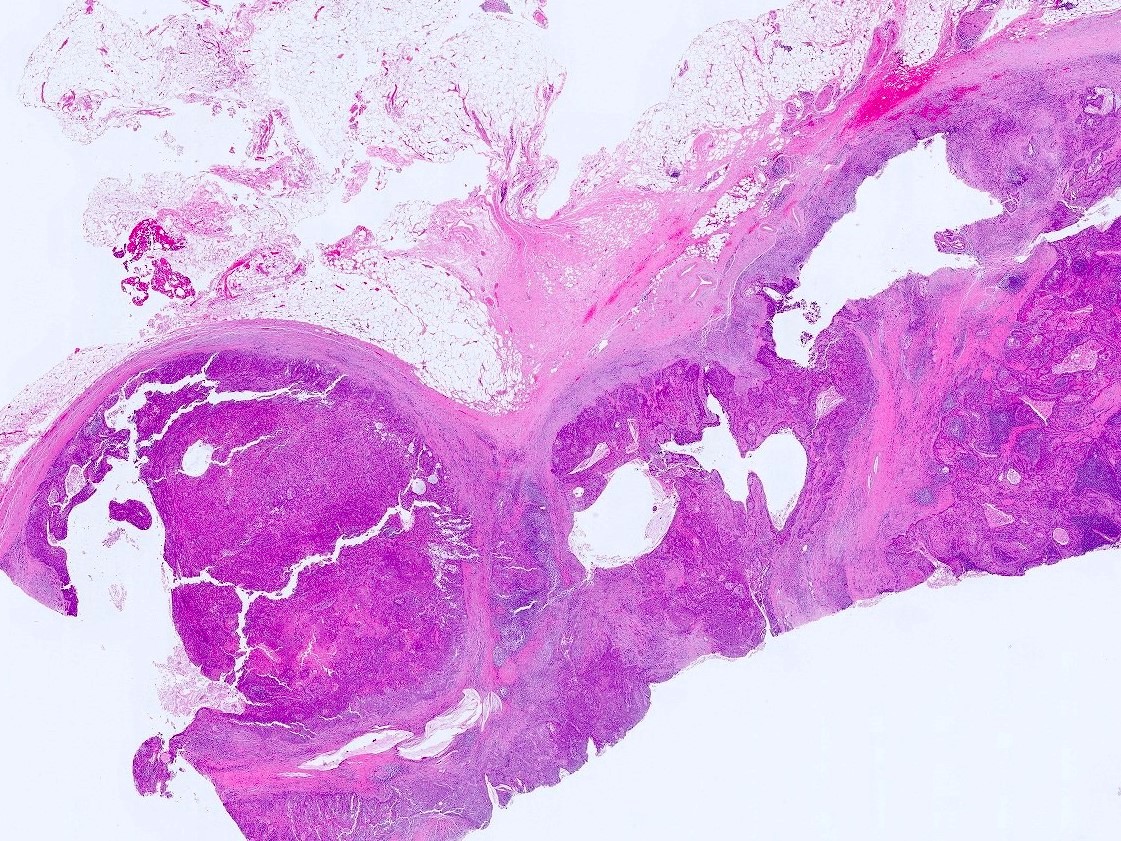
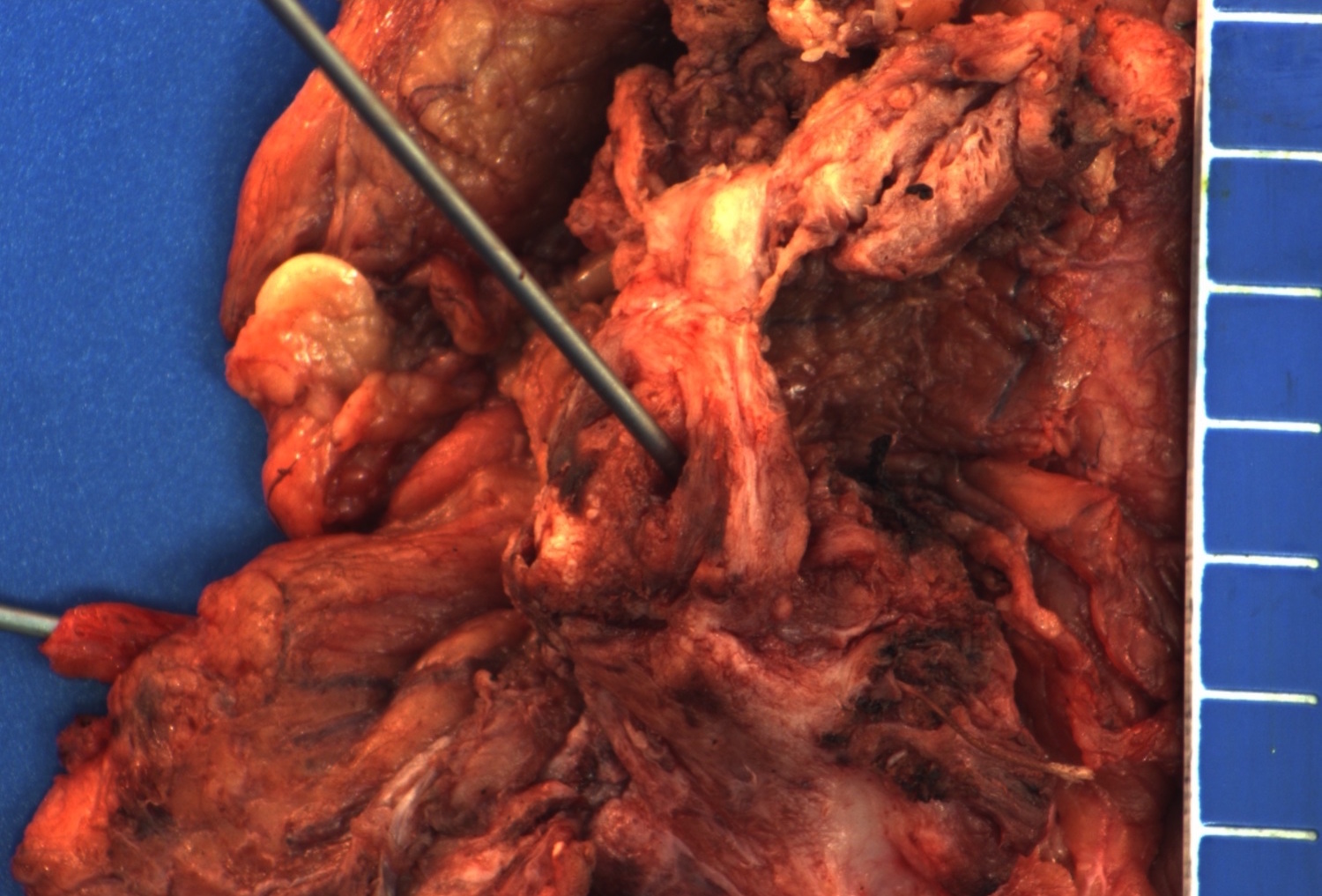
Gross description
- Firm, gray-white and granular tumor
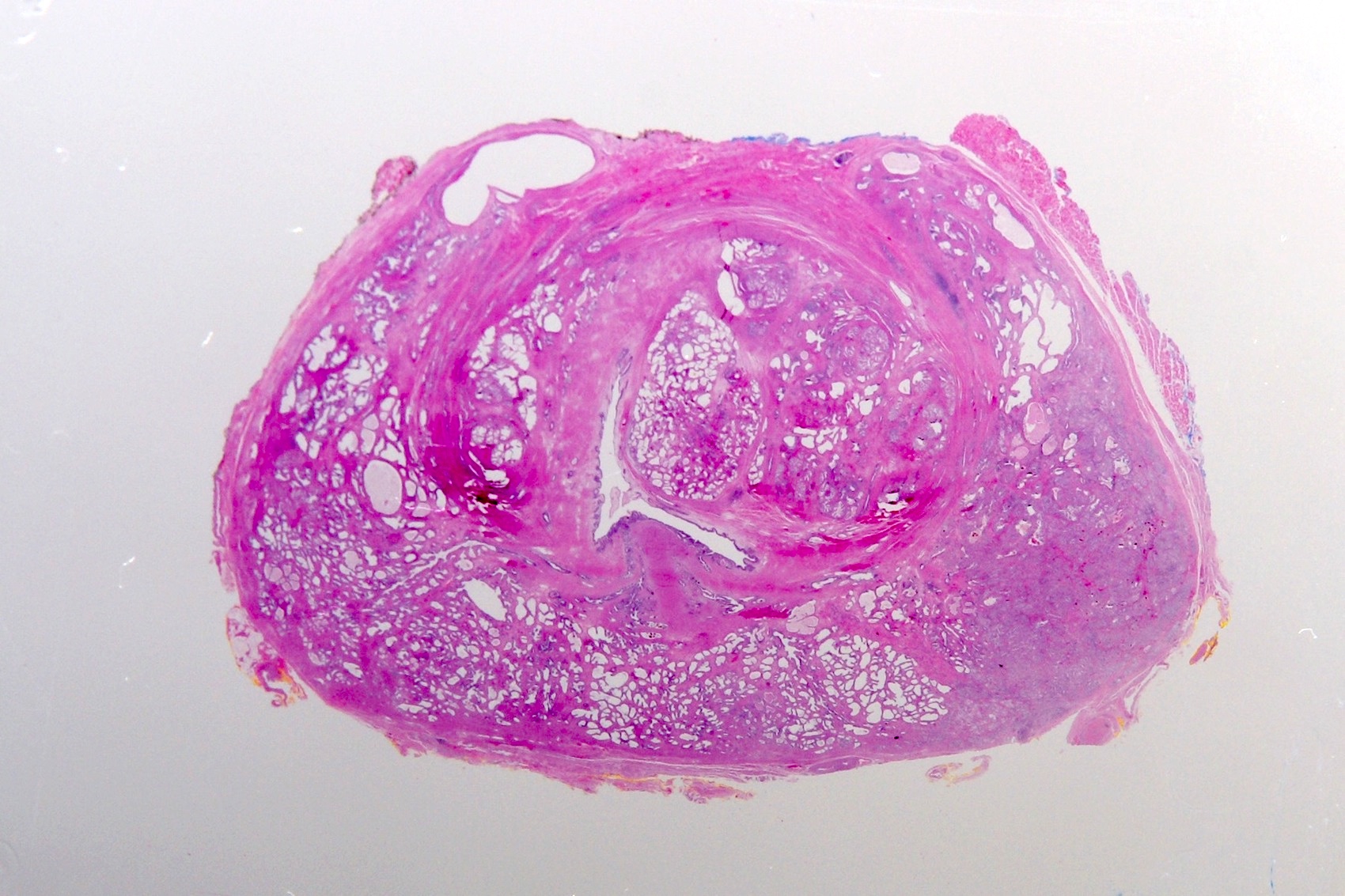
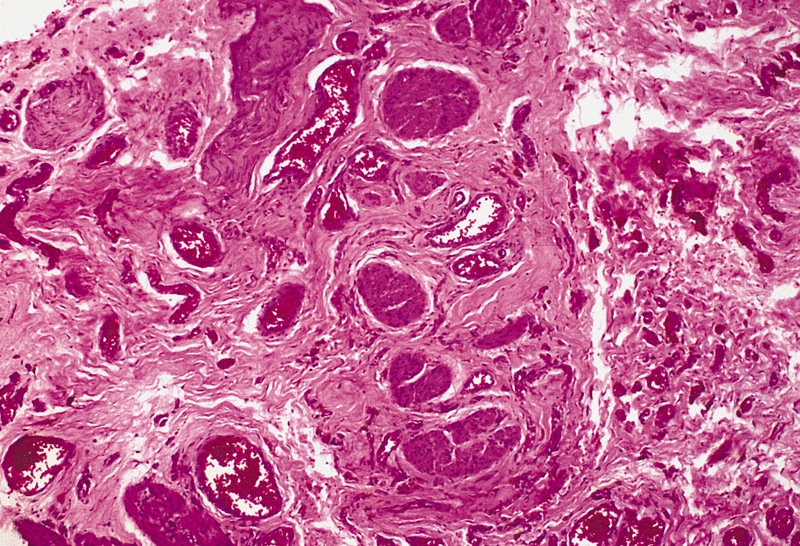
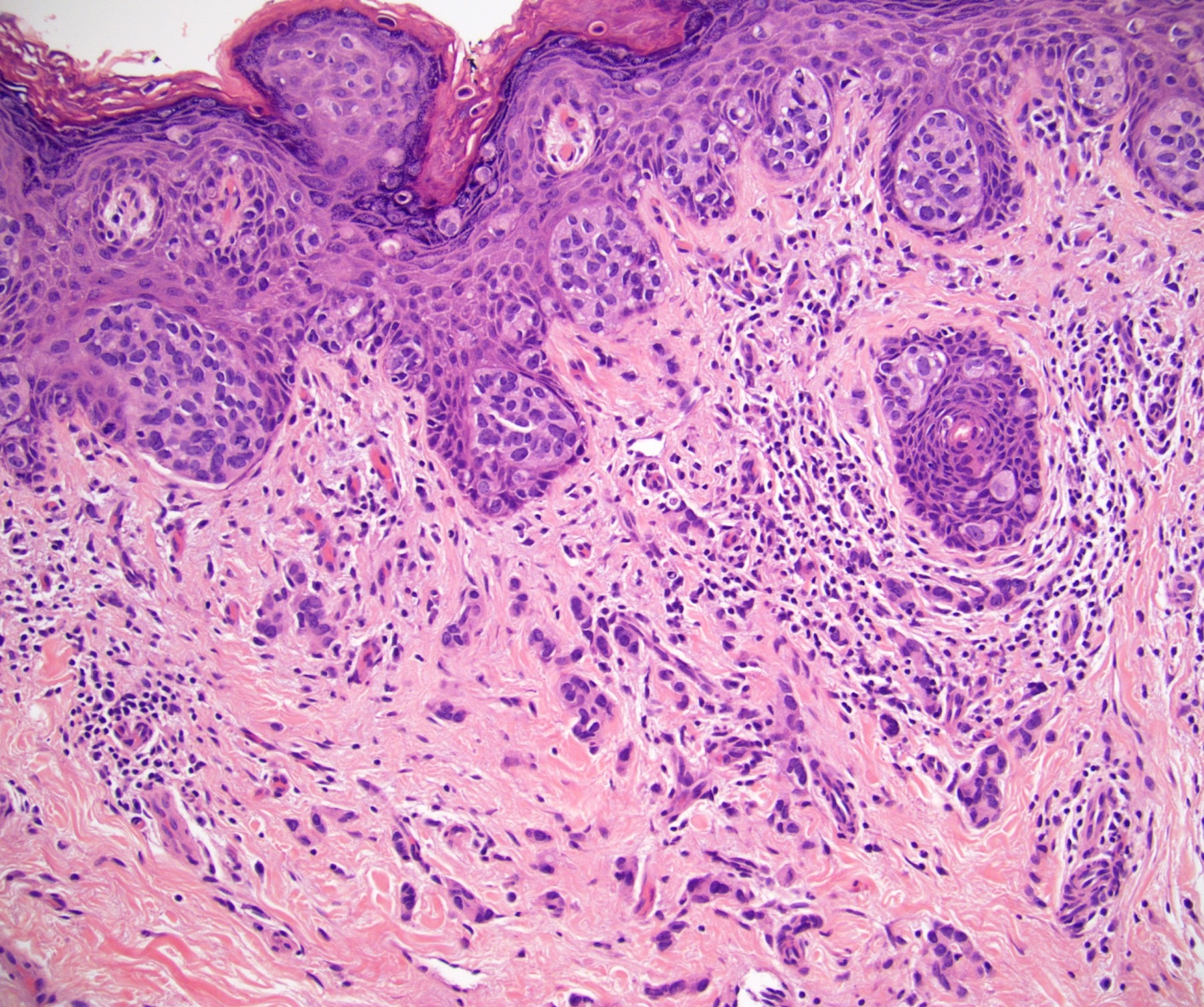
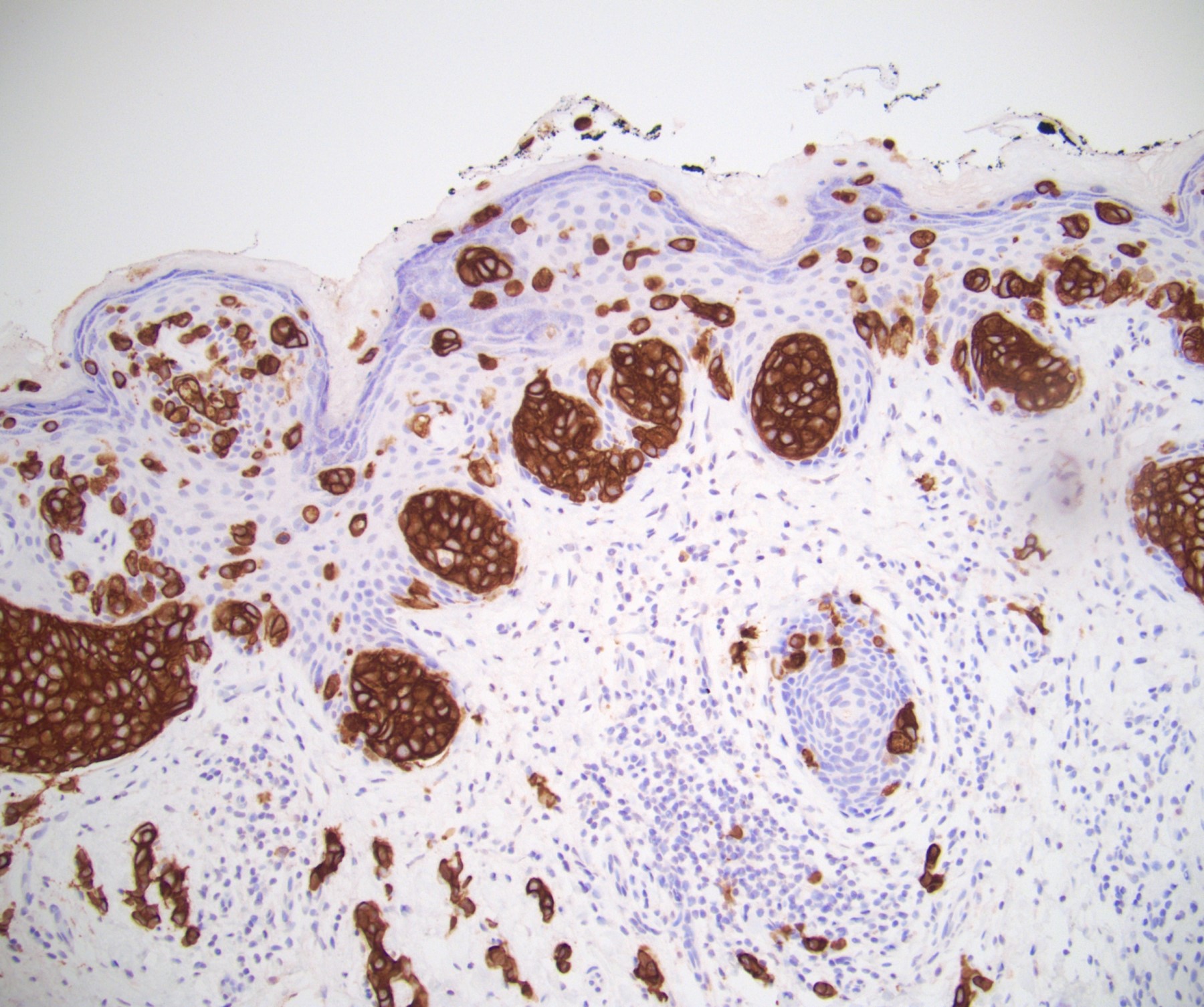
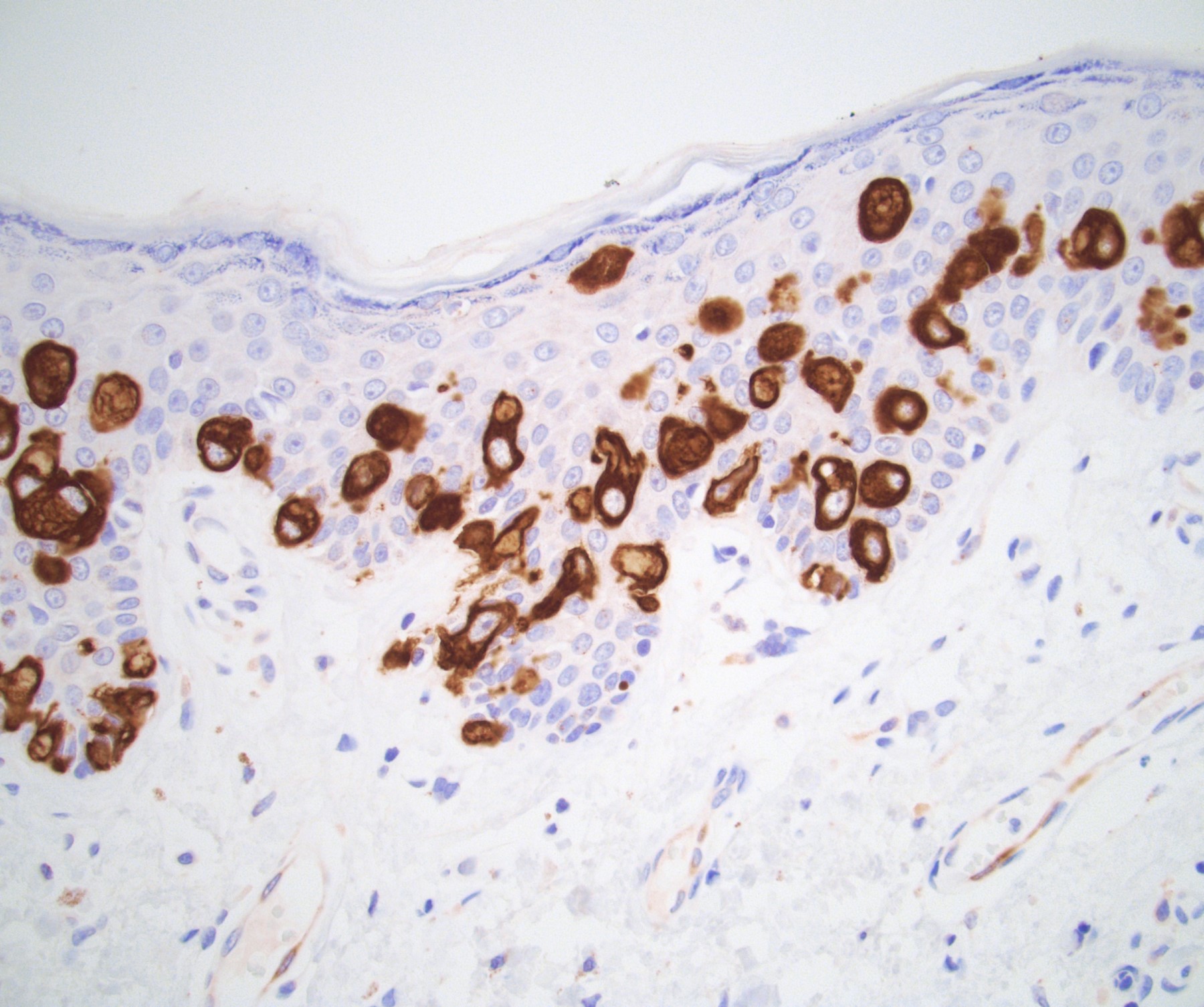
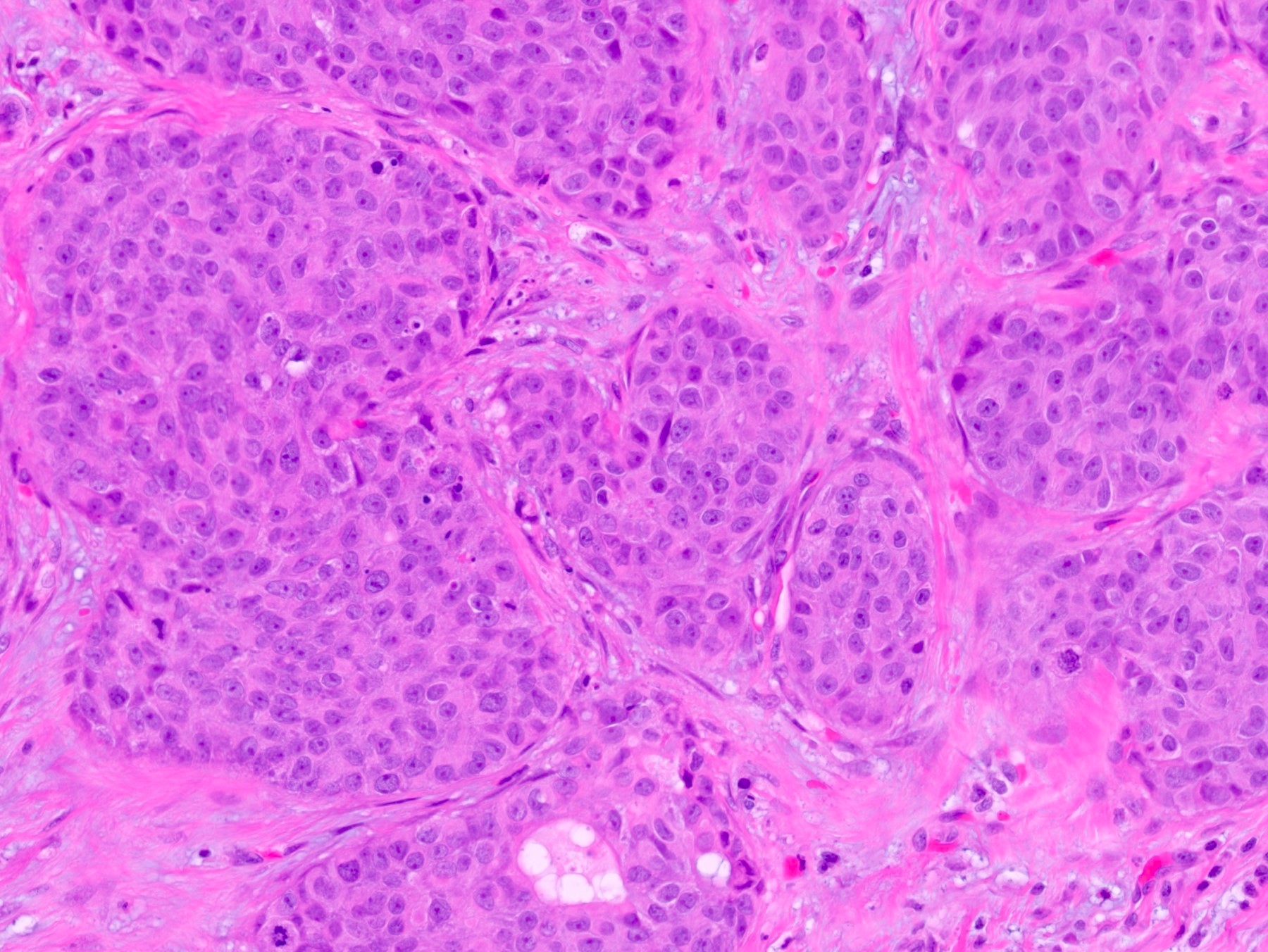
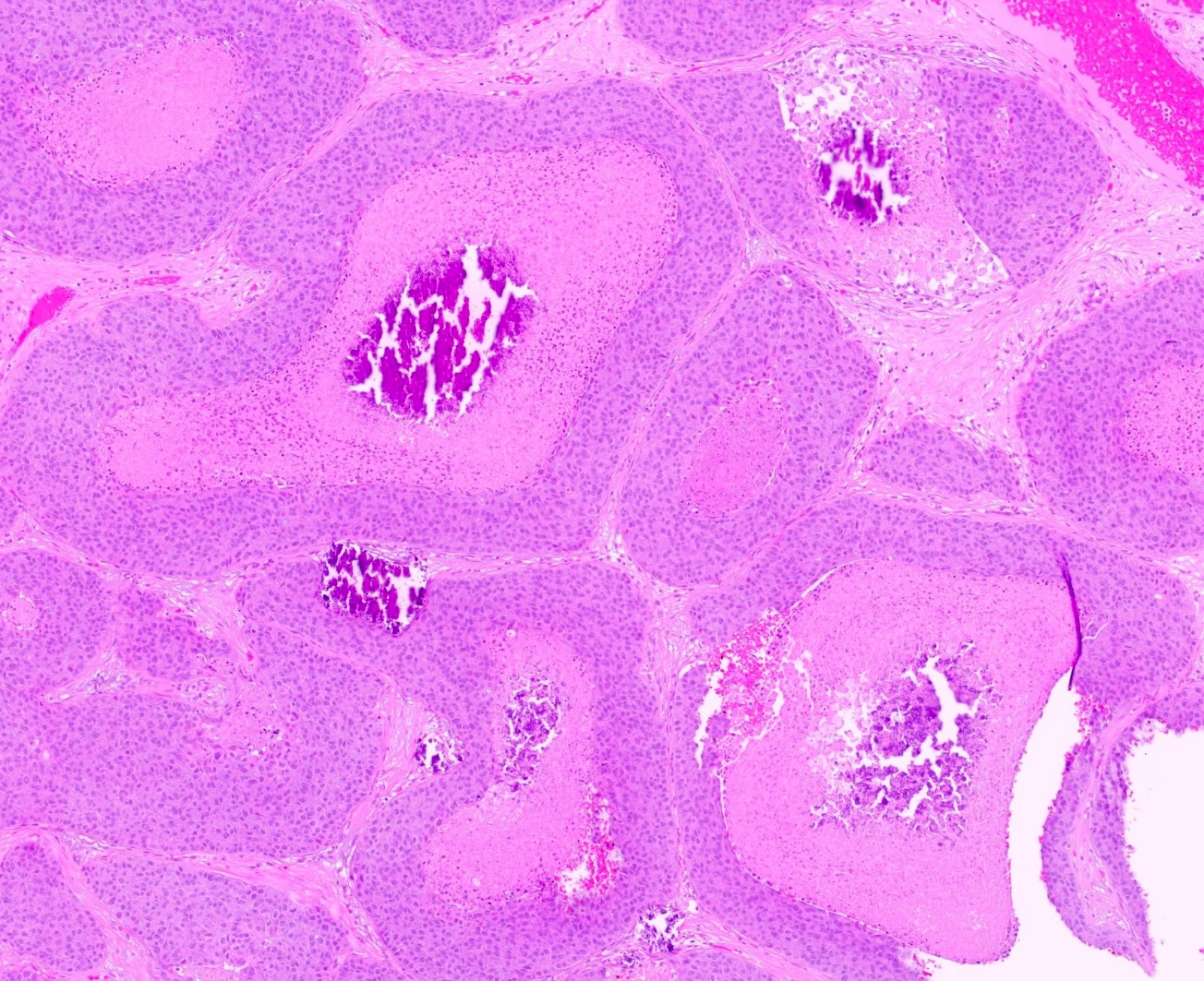
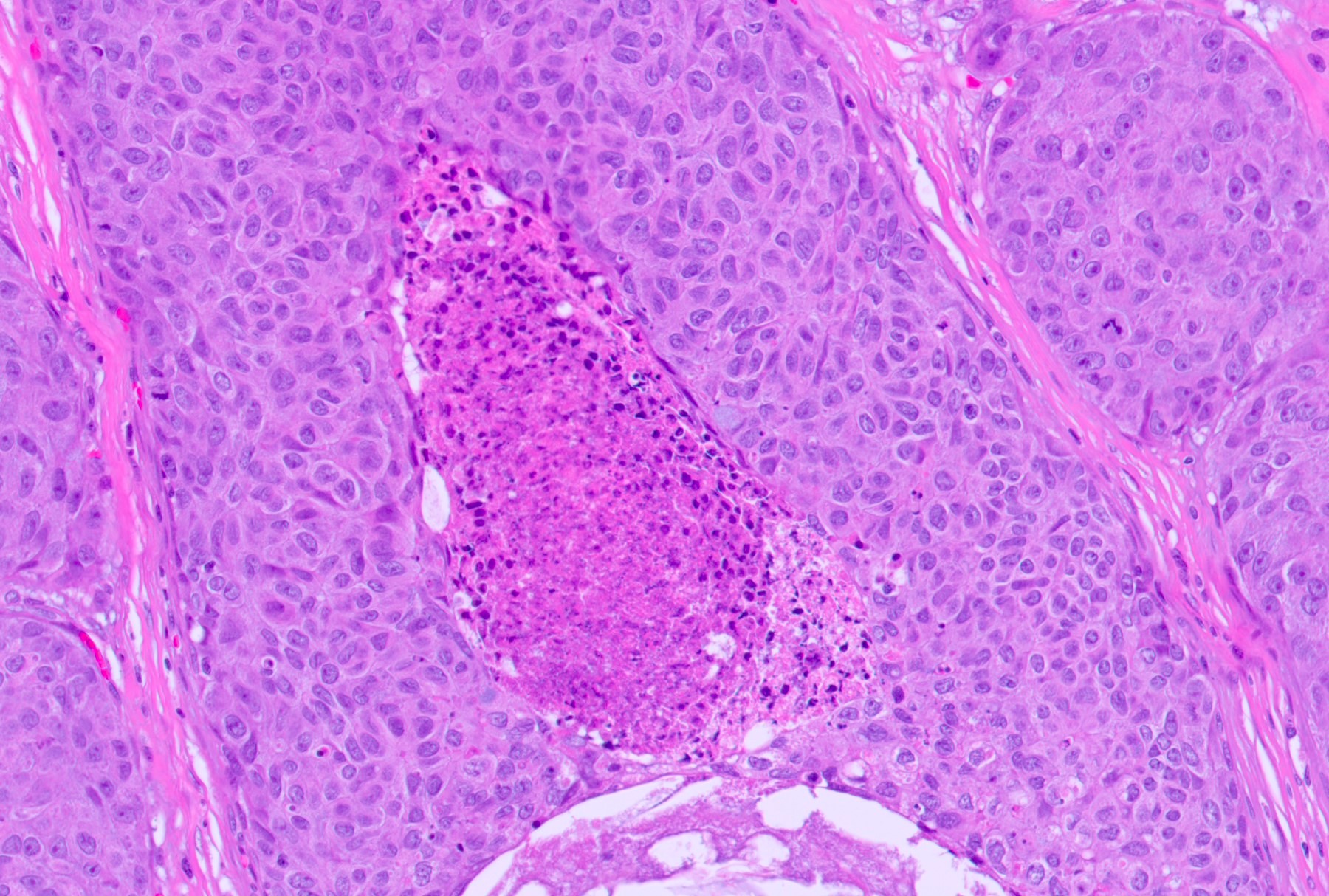
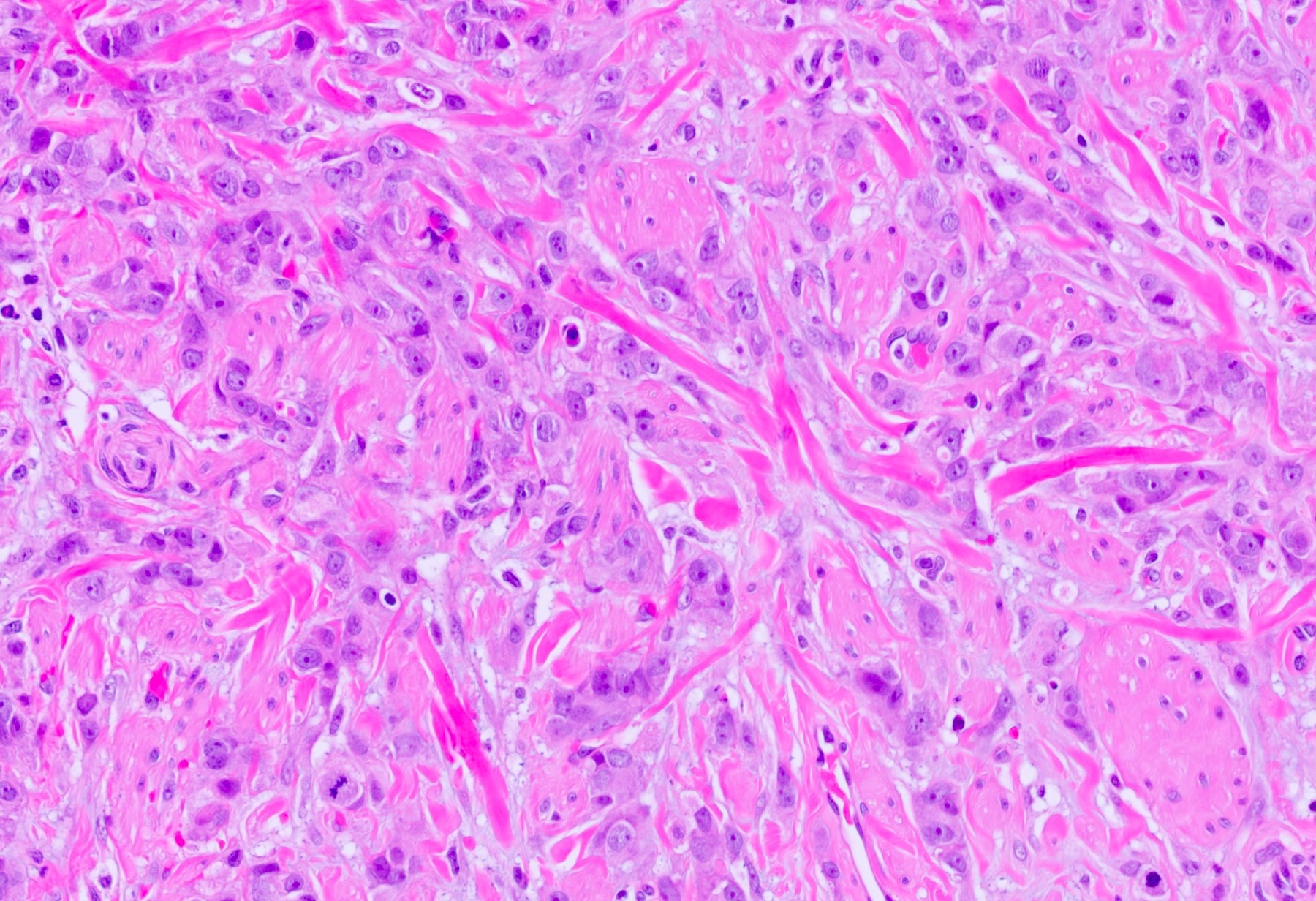
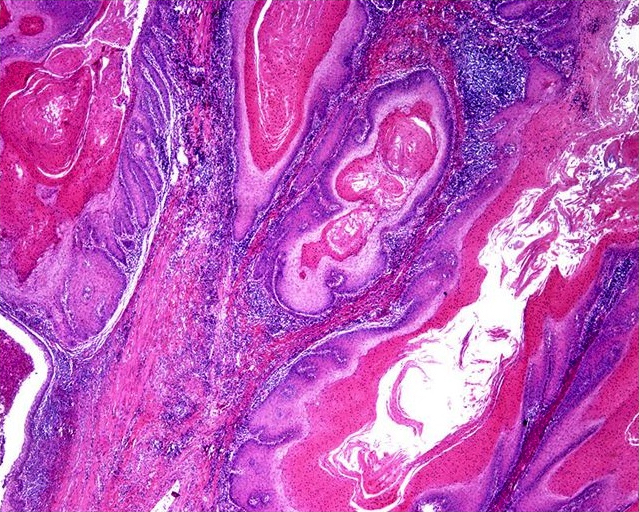
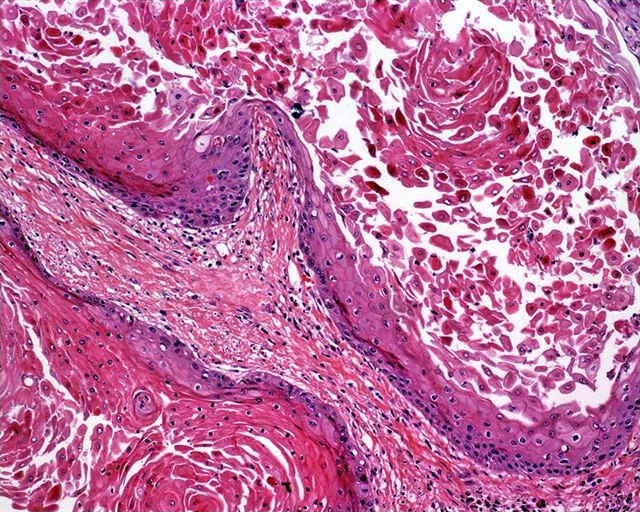
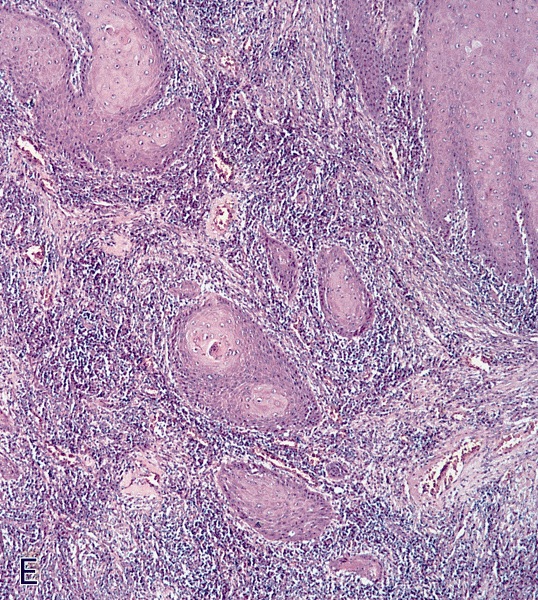
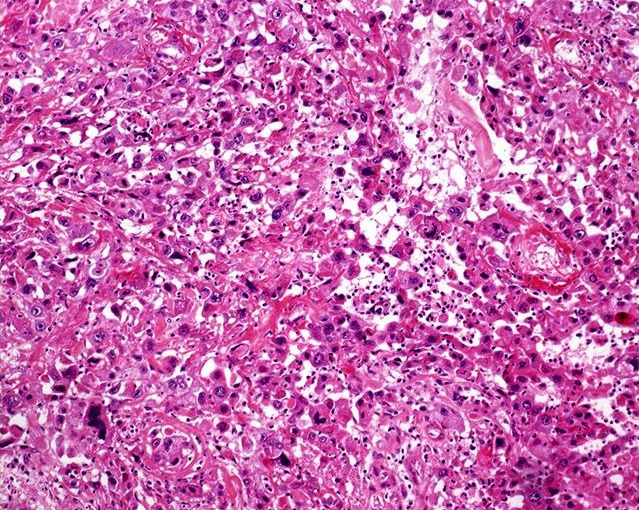
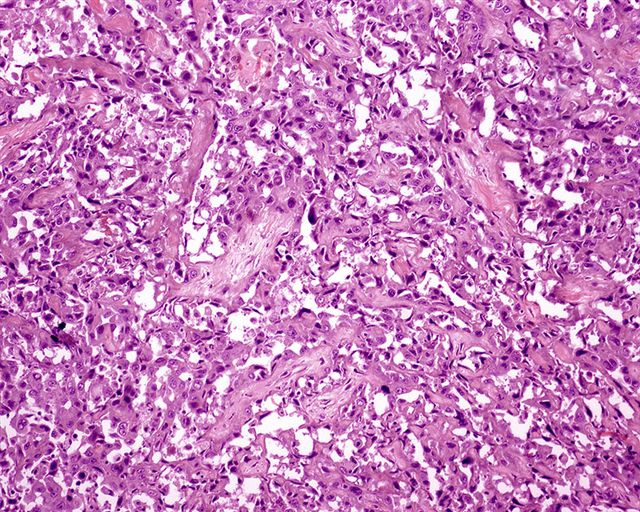
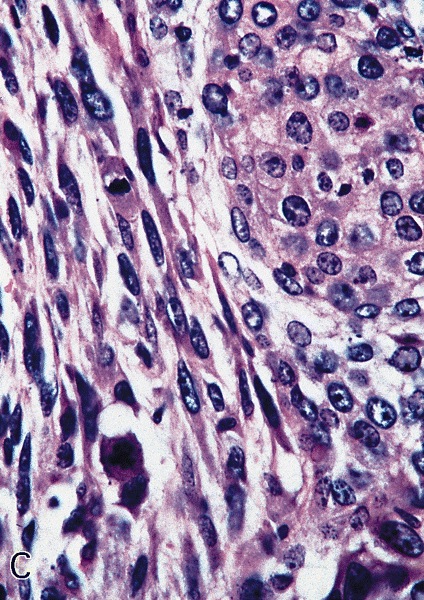
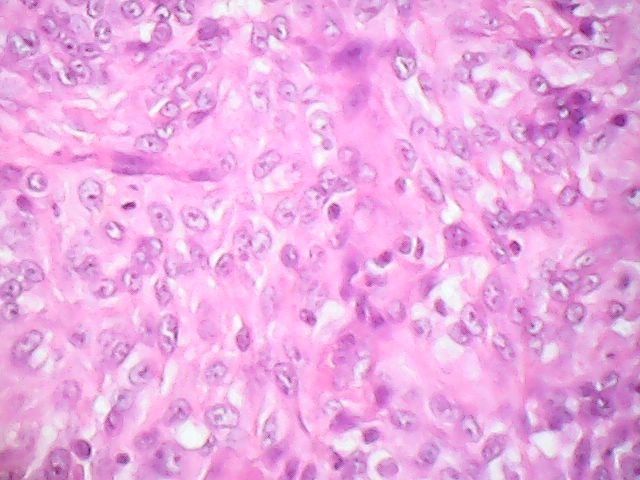
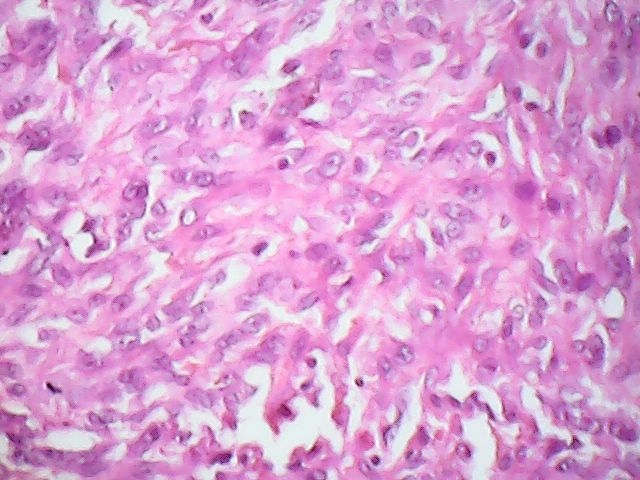
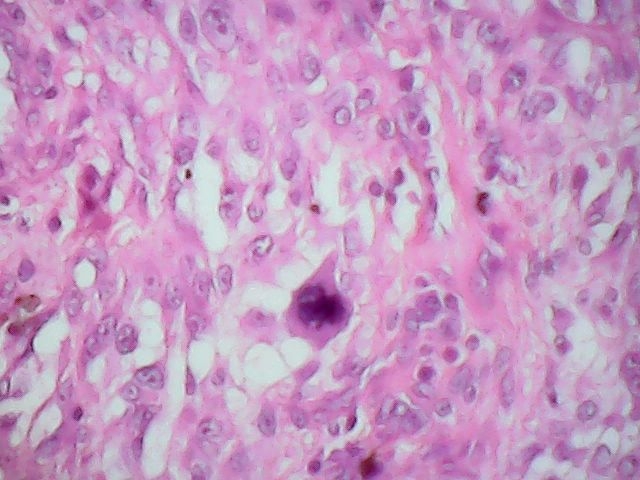
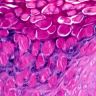
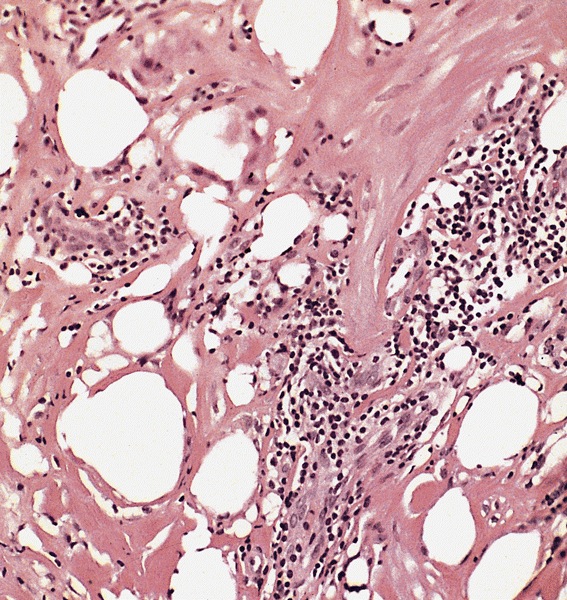
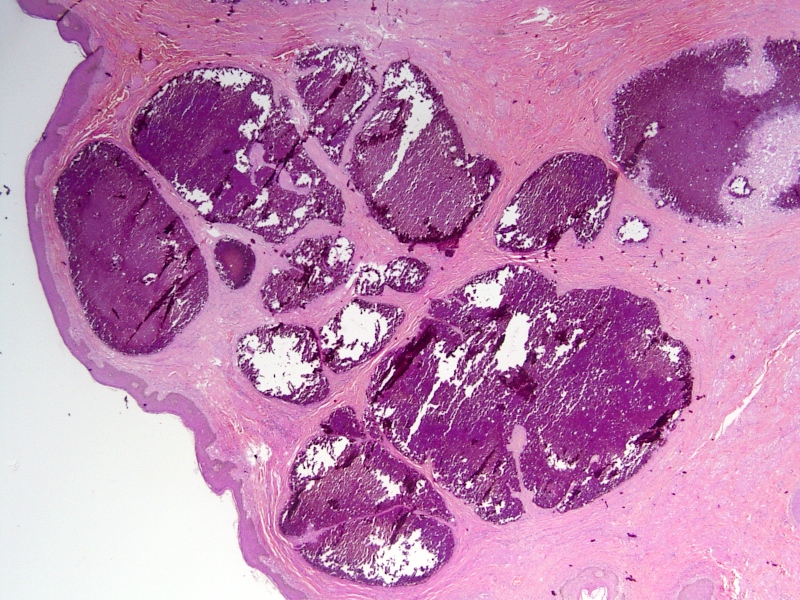
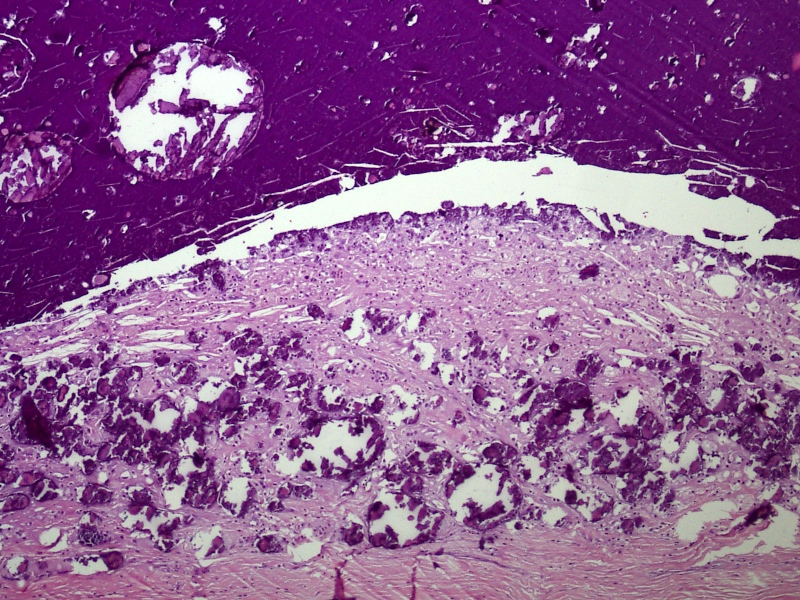
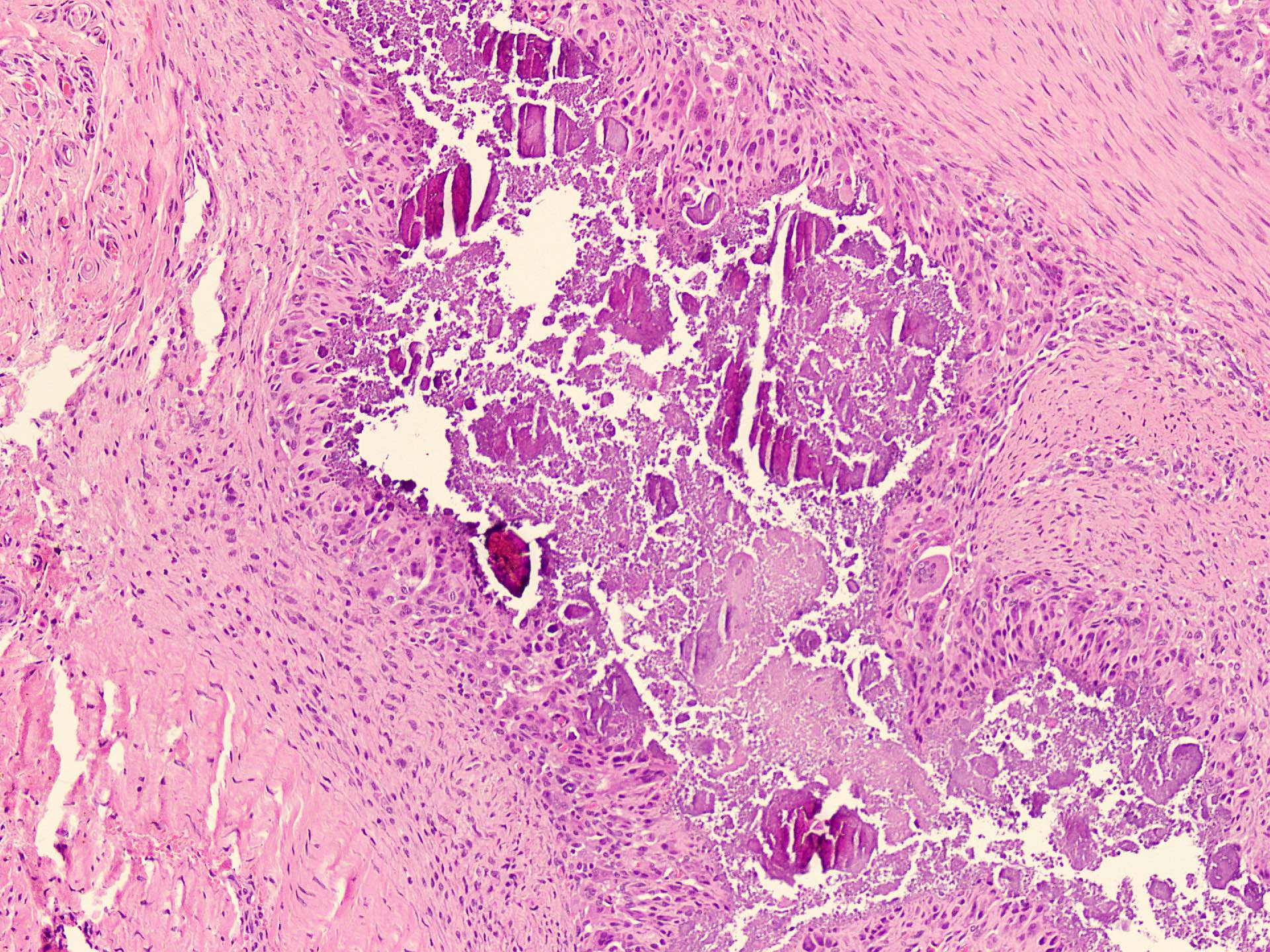
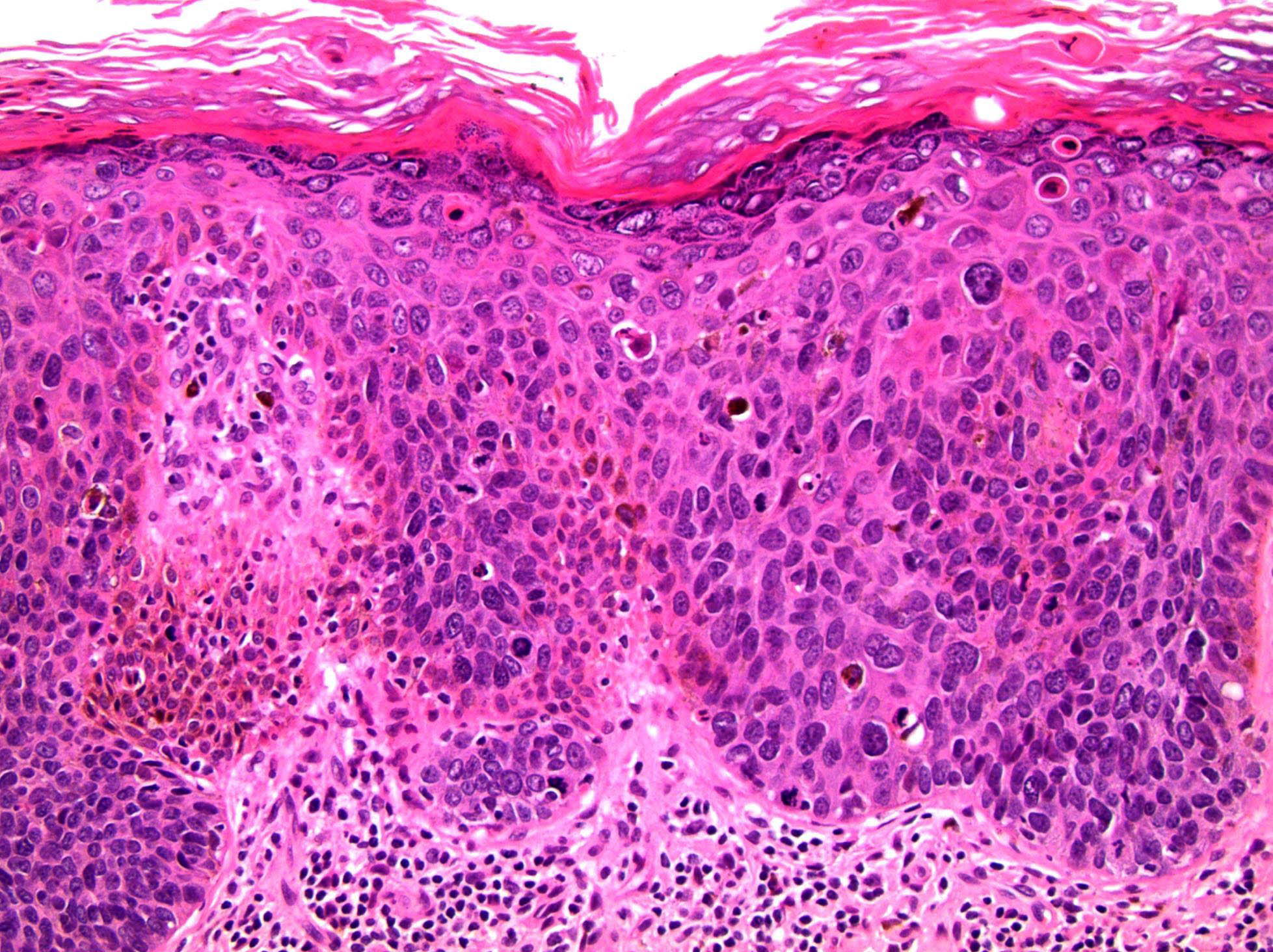
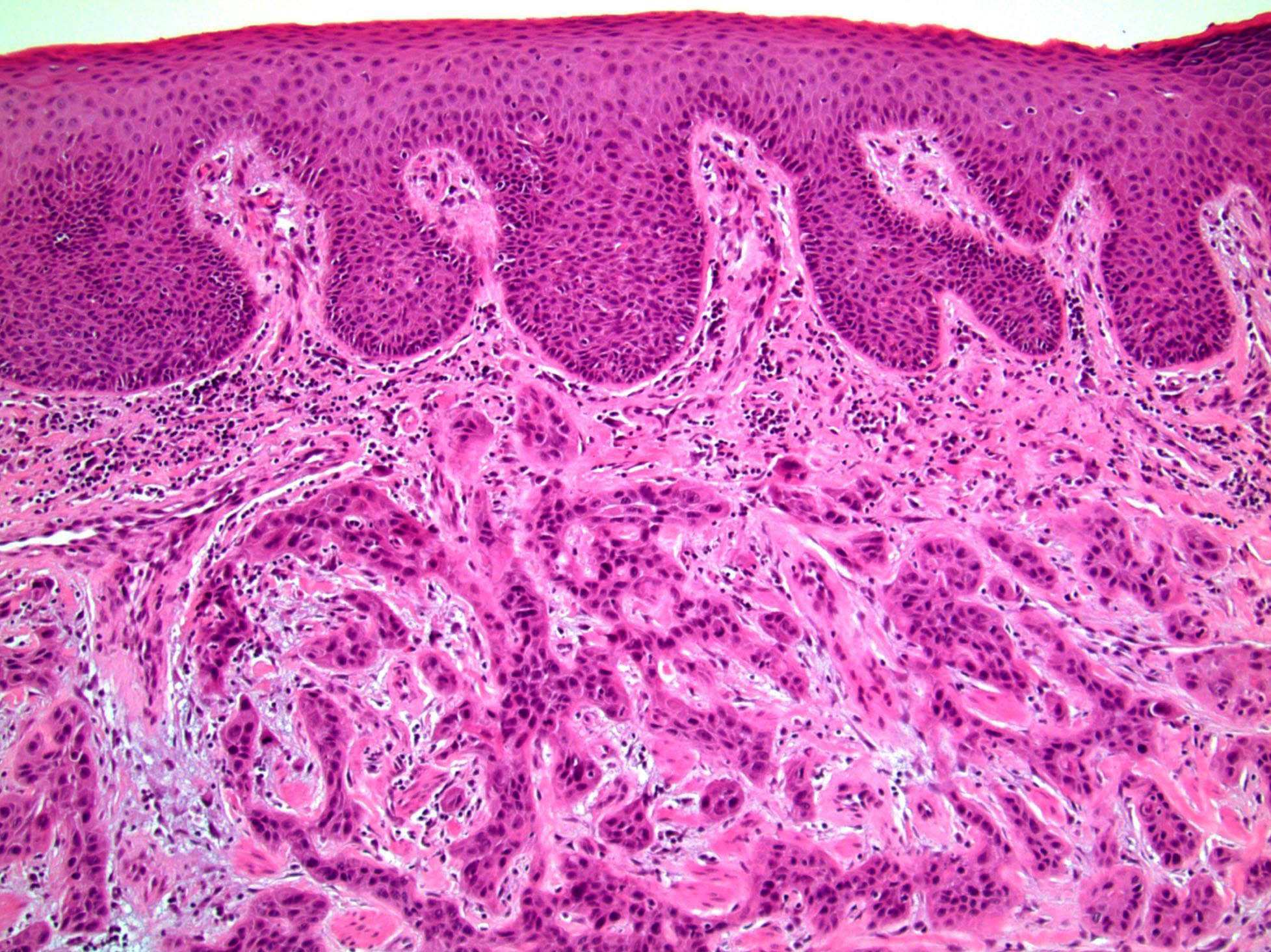
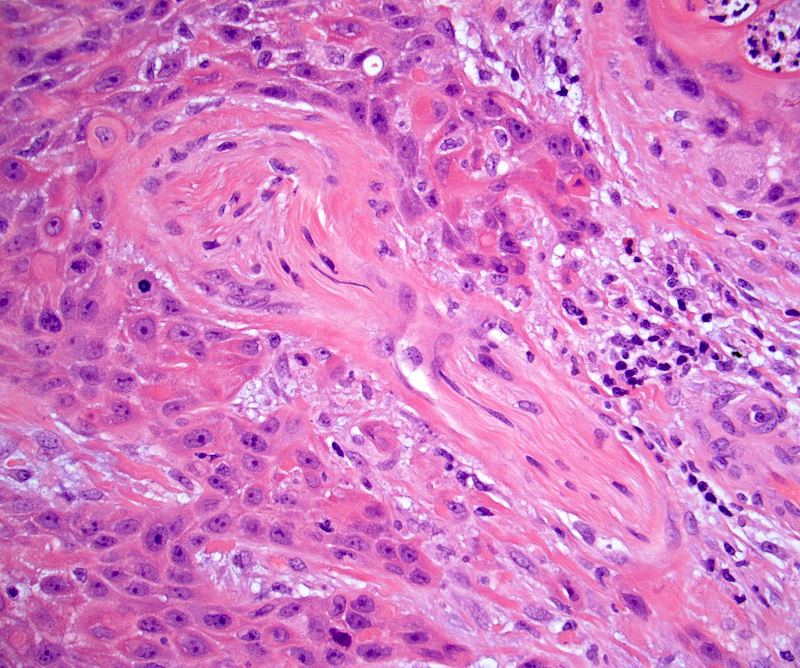
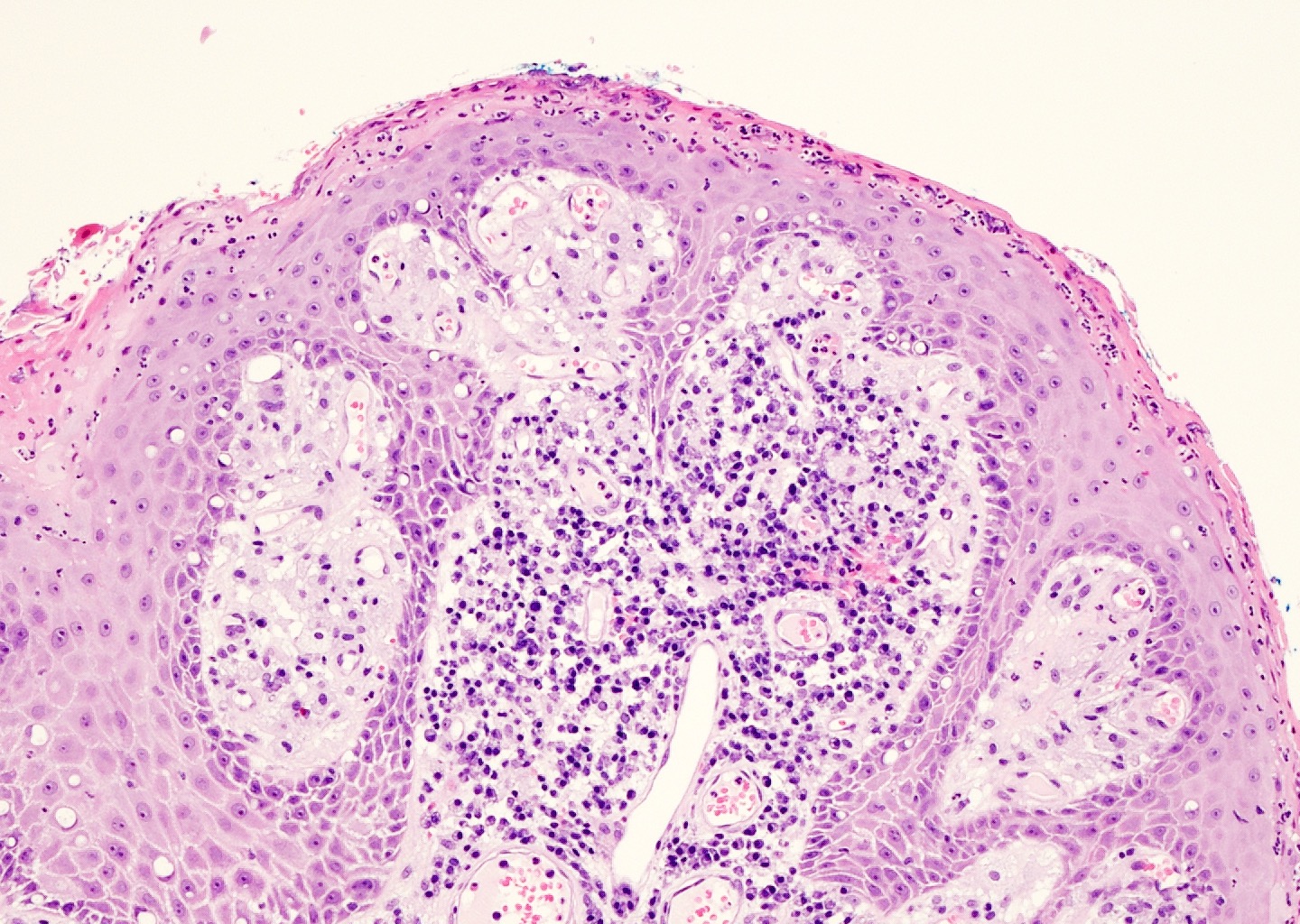
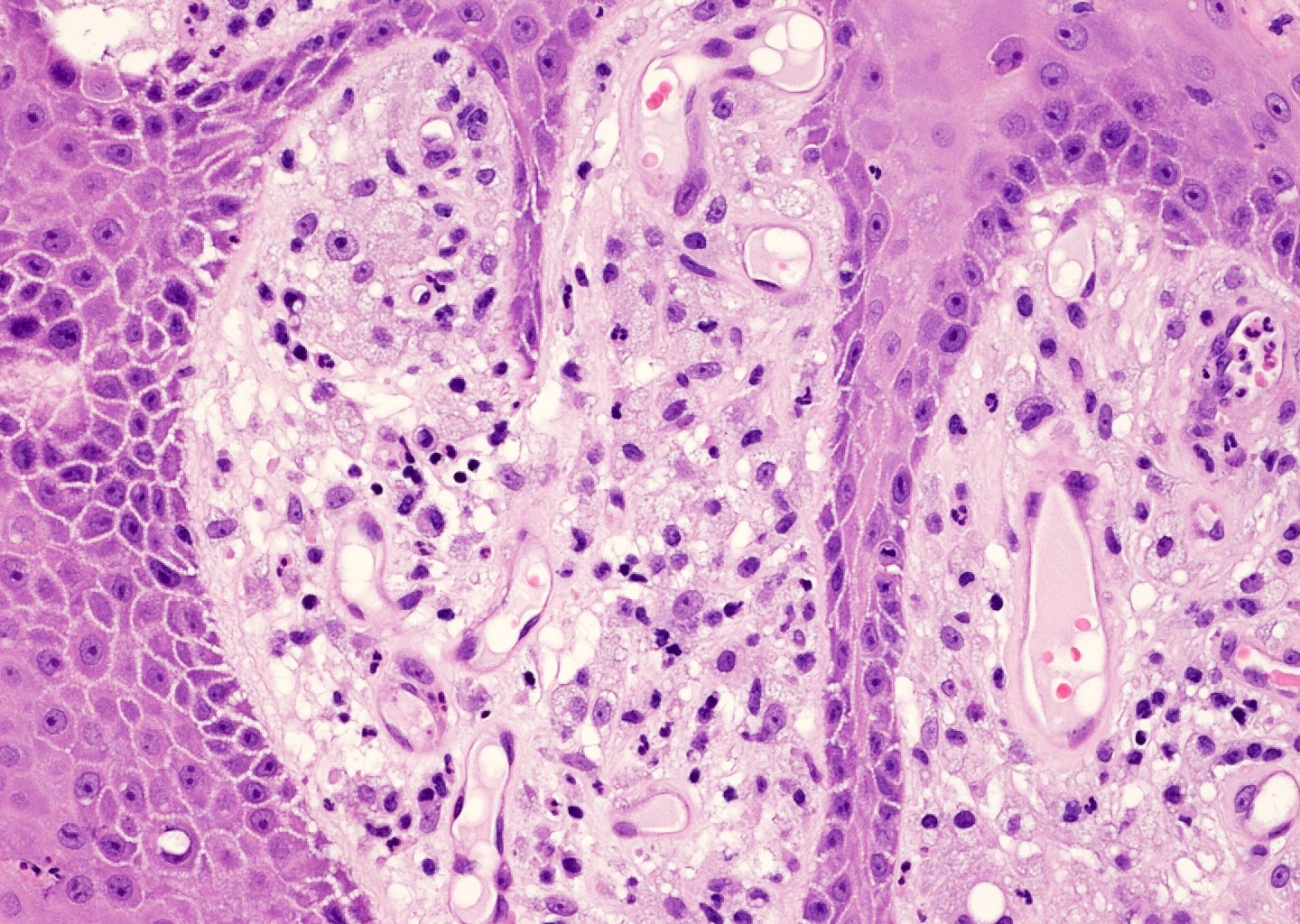
Microscopic (histologic) description
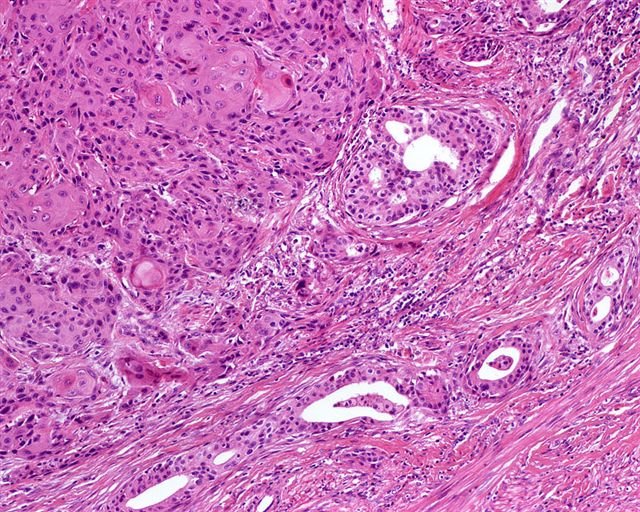
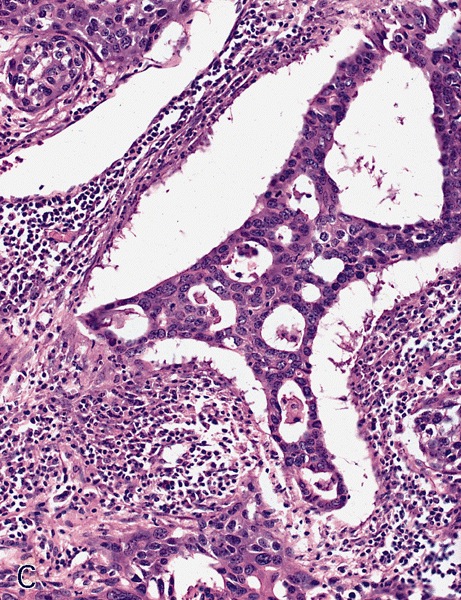
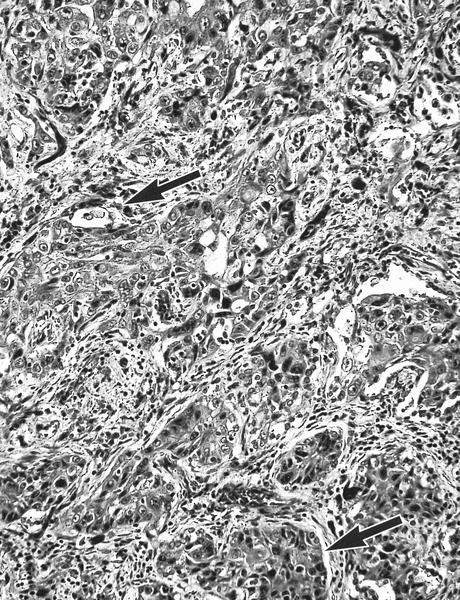
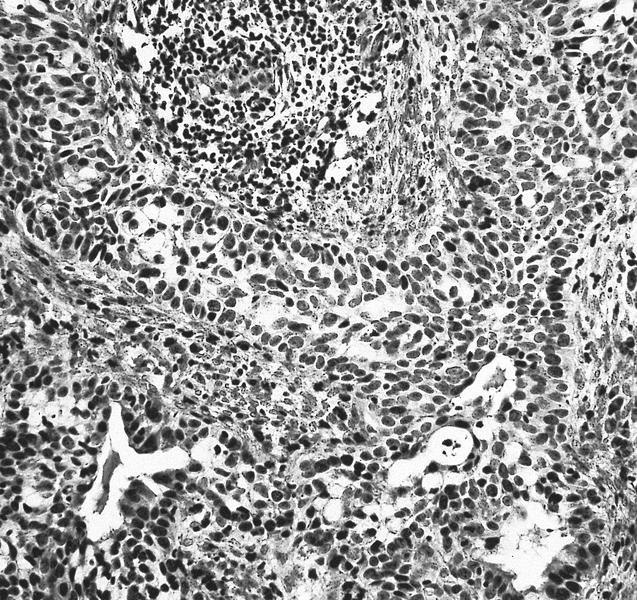
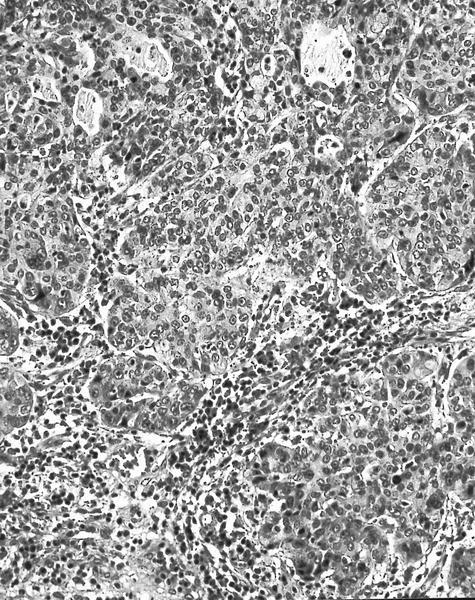
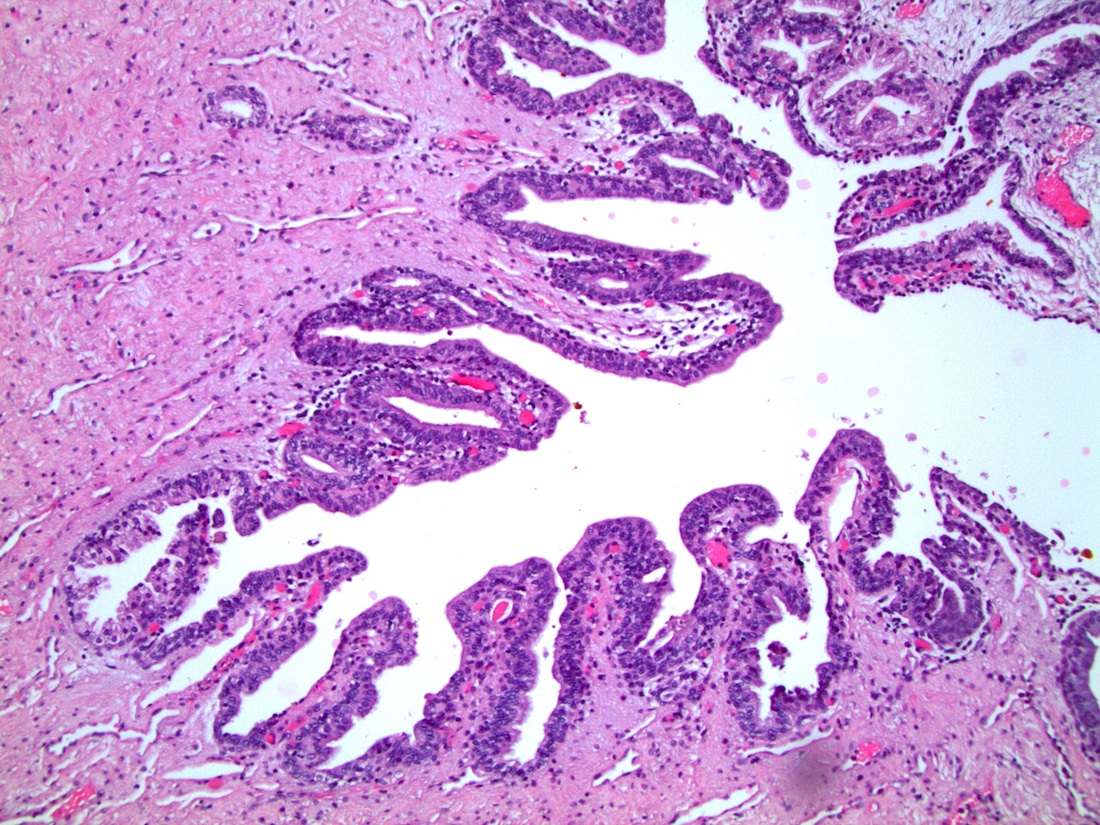
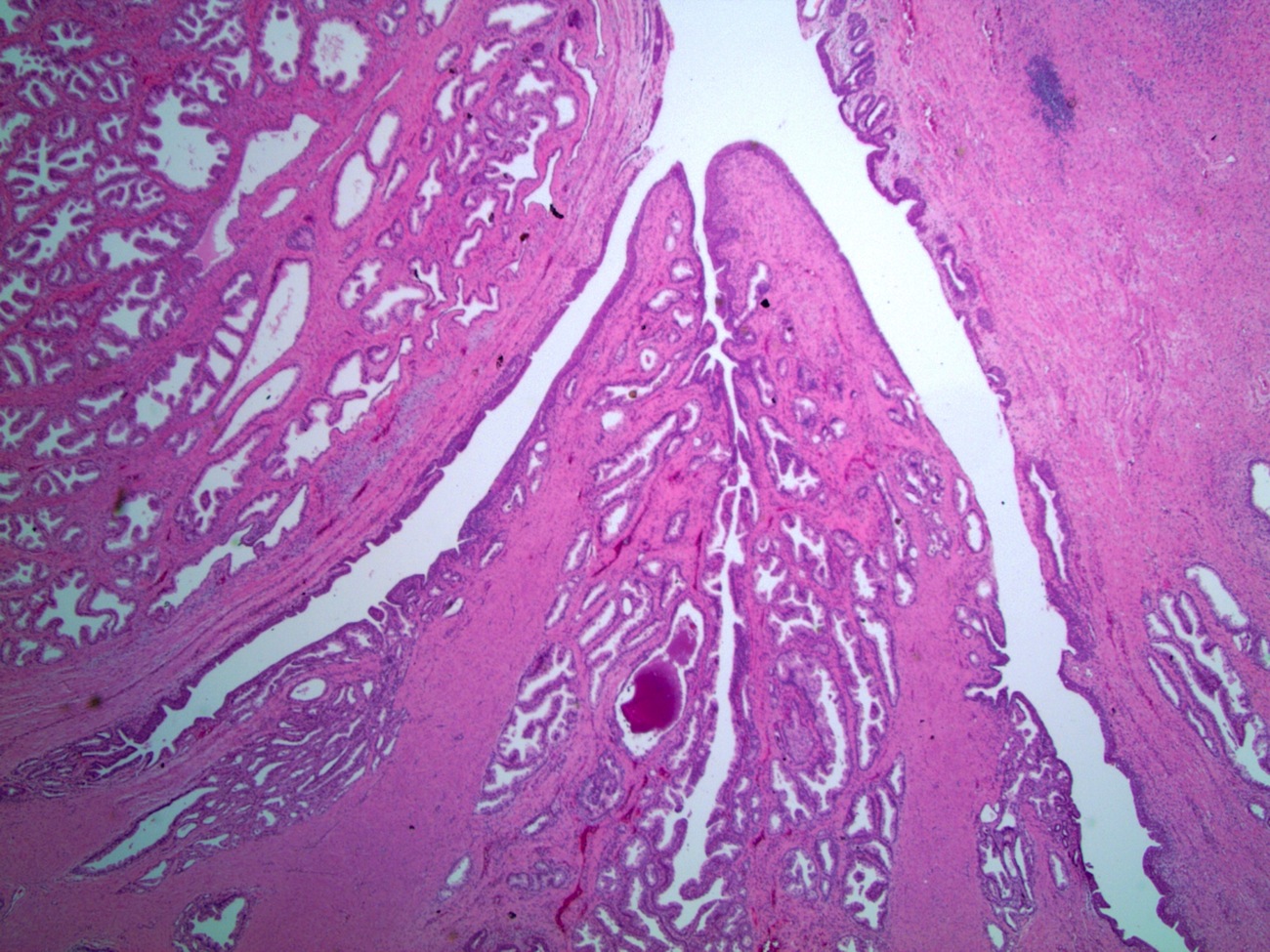
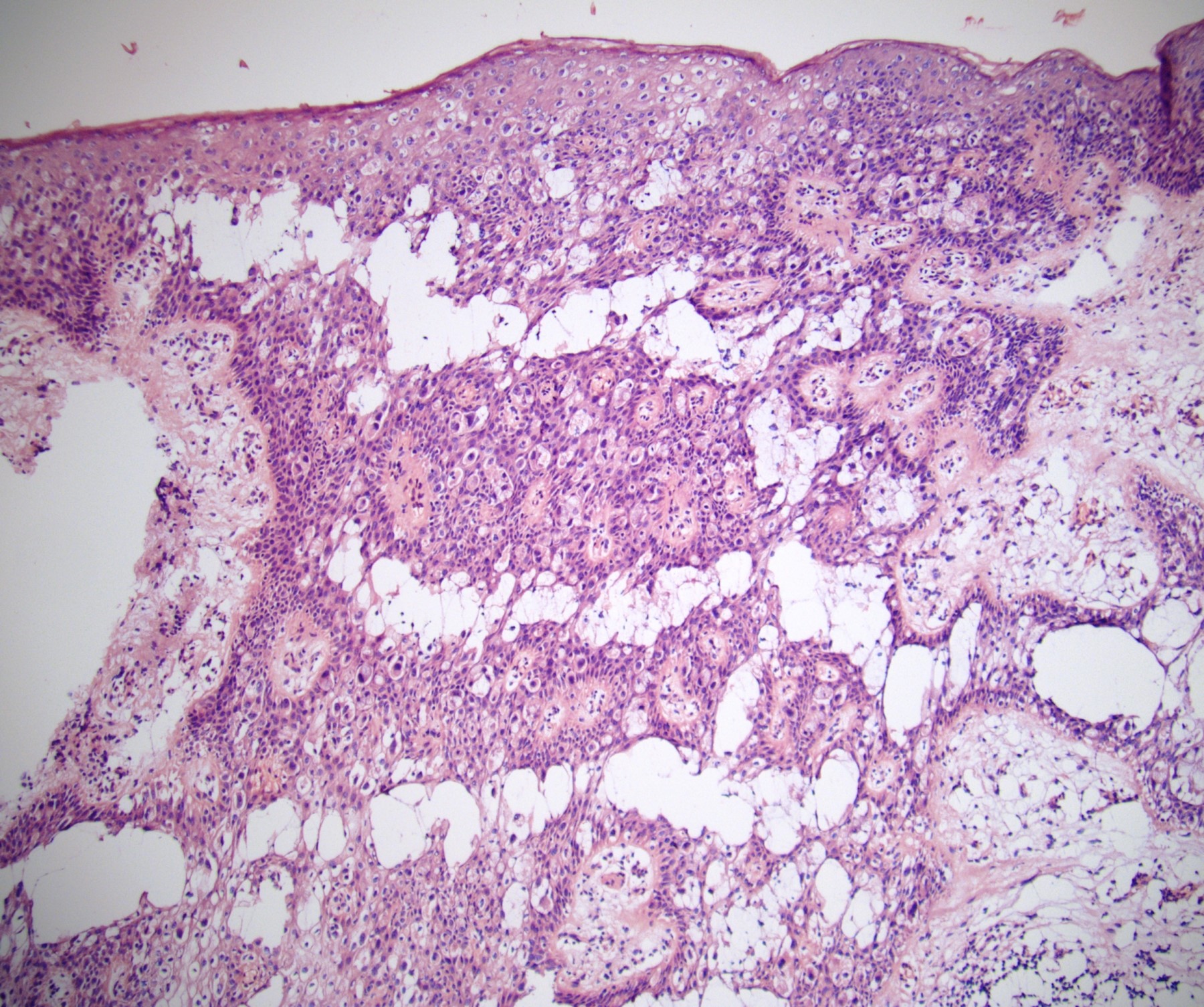
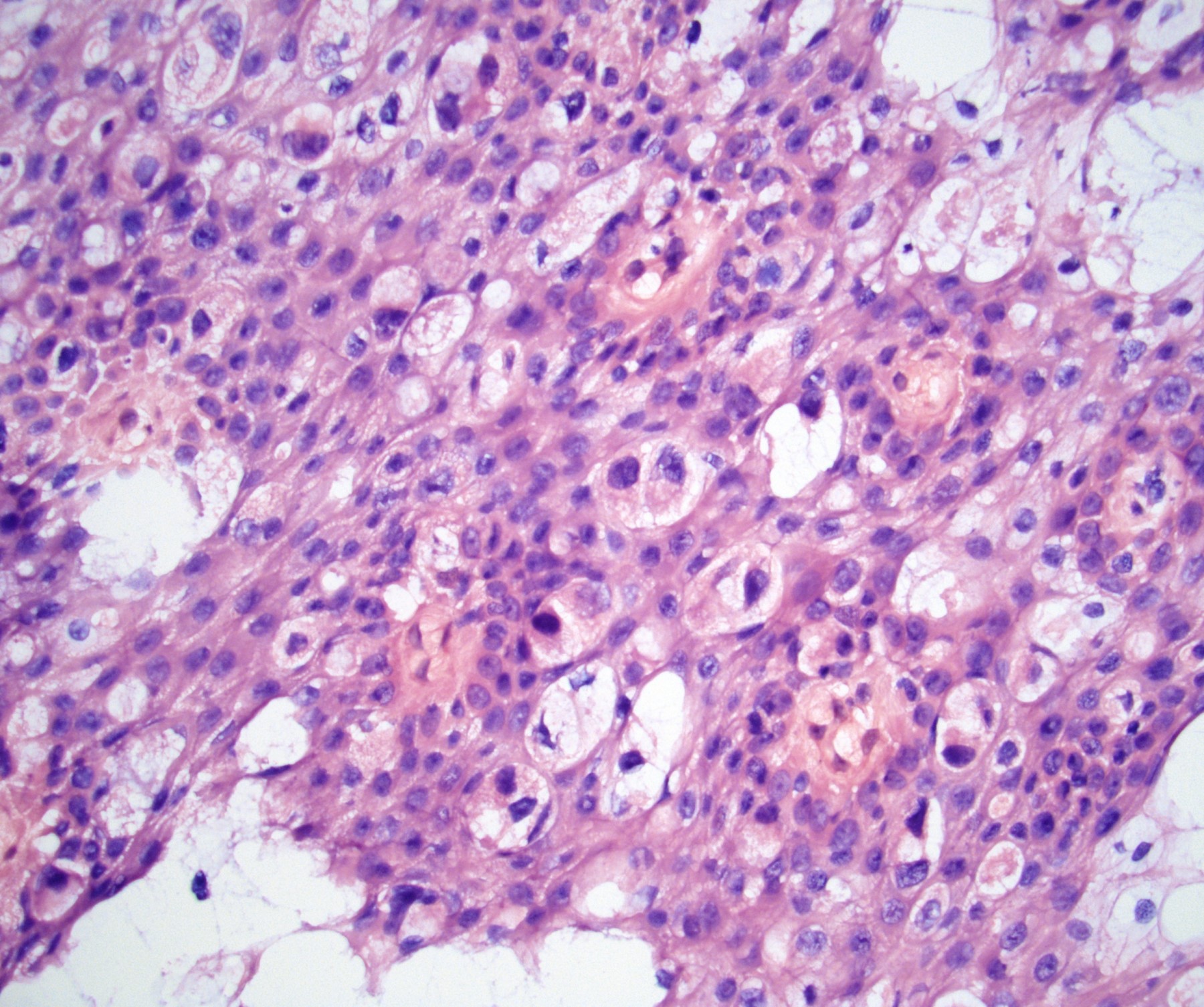
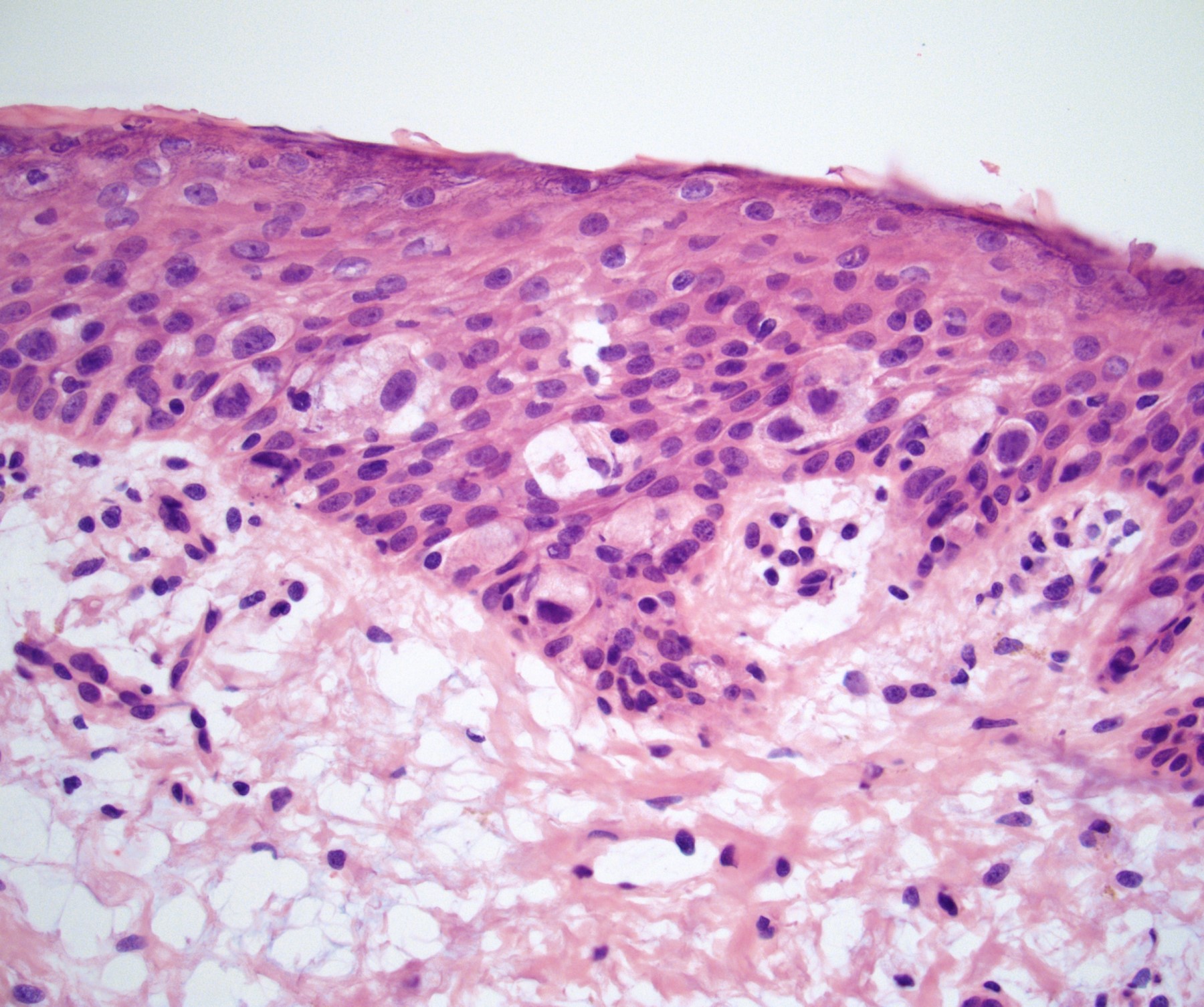
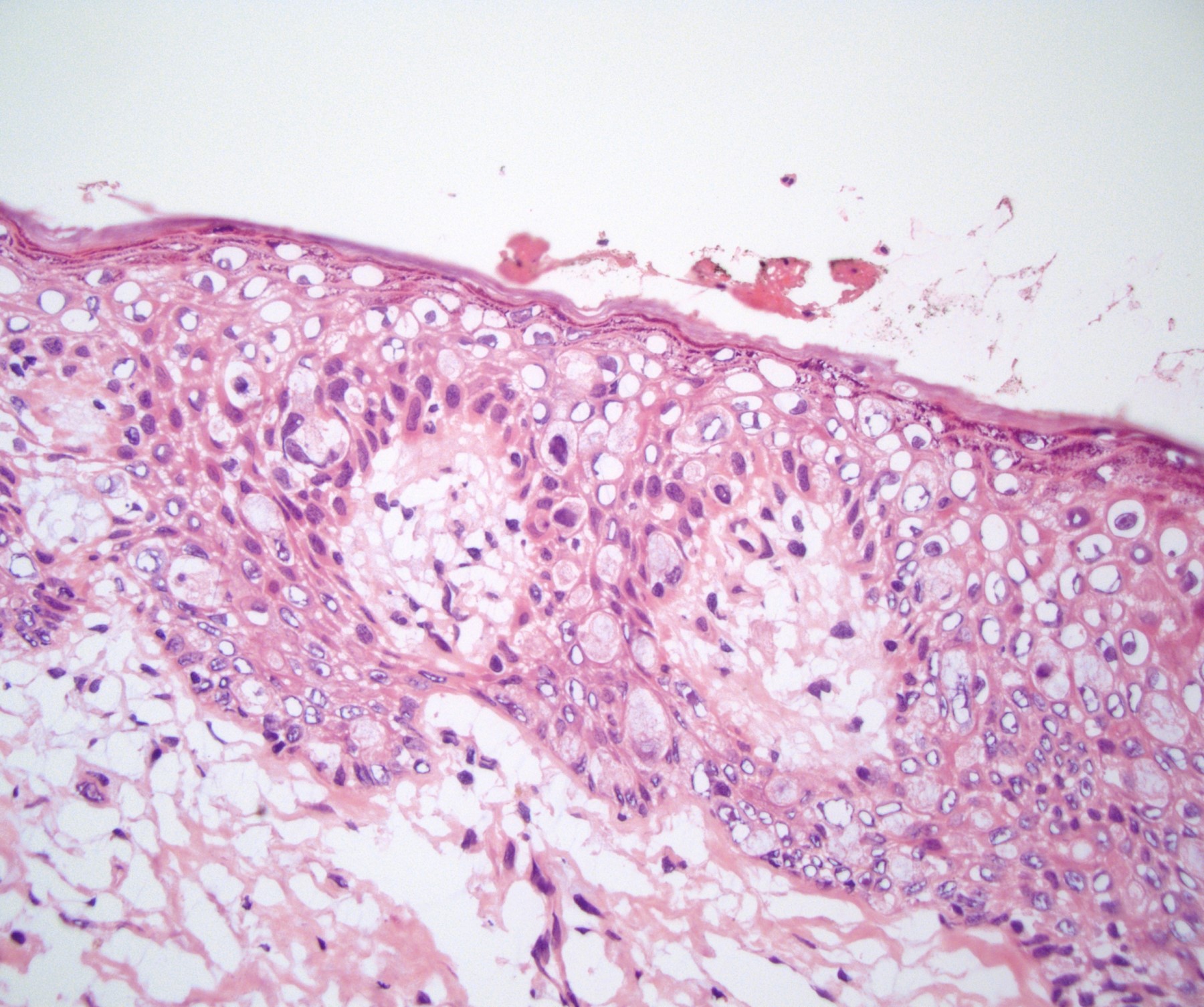
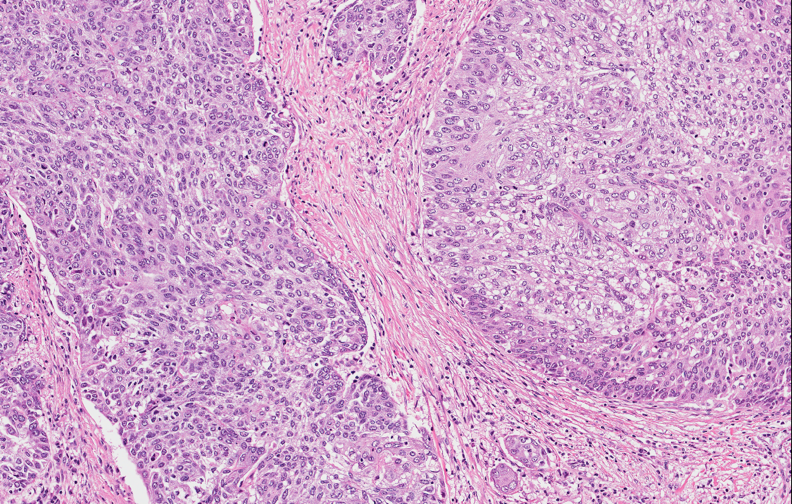
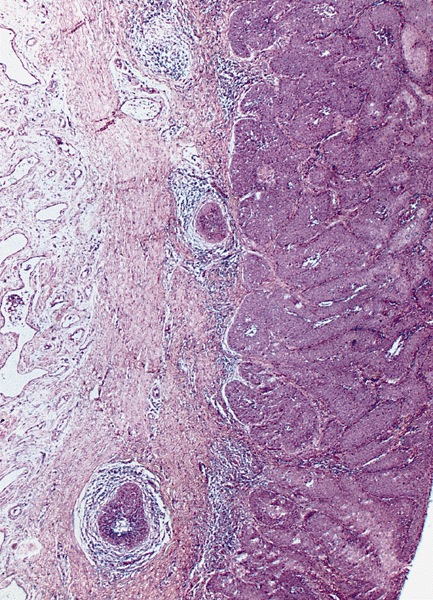
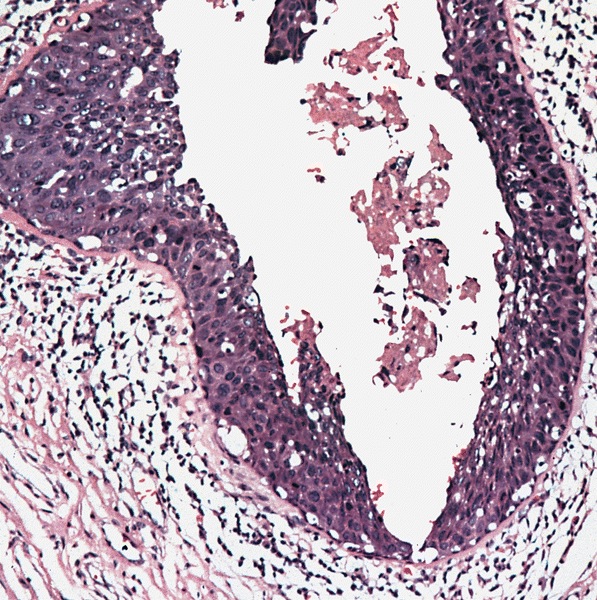
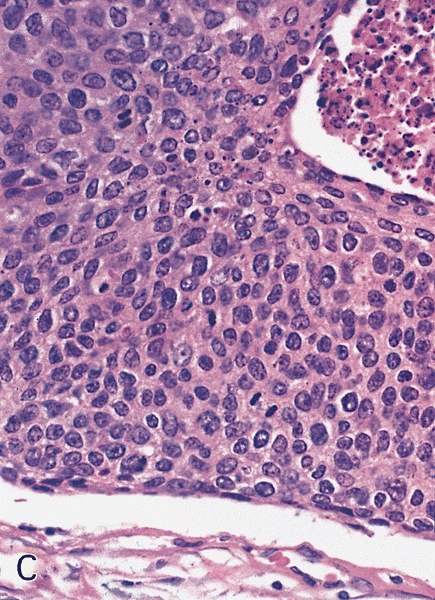
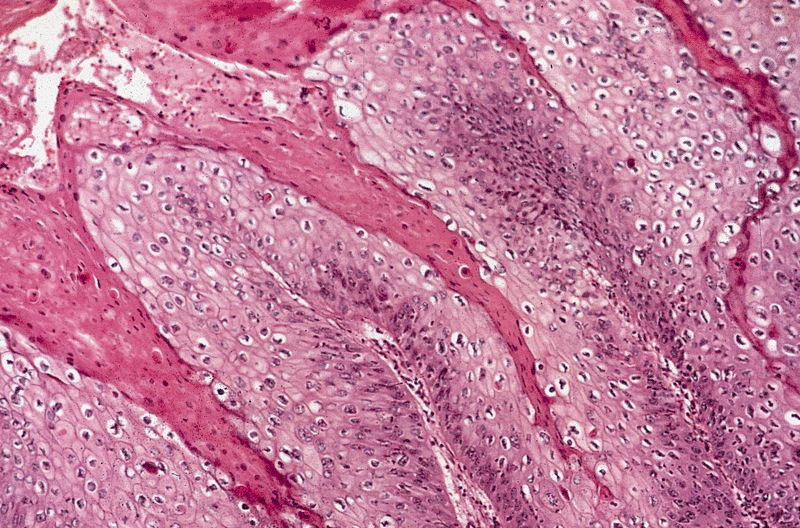
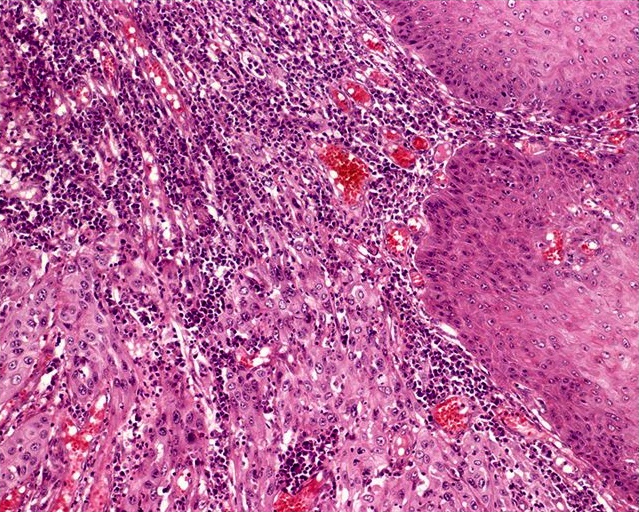
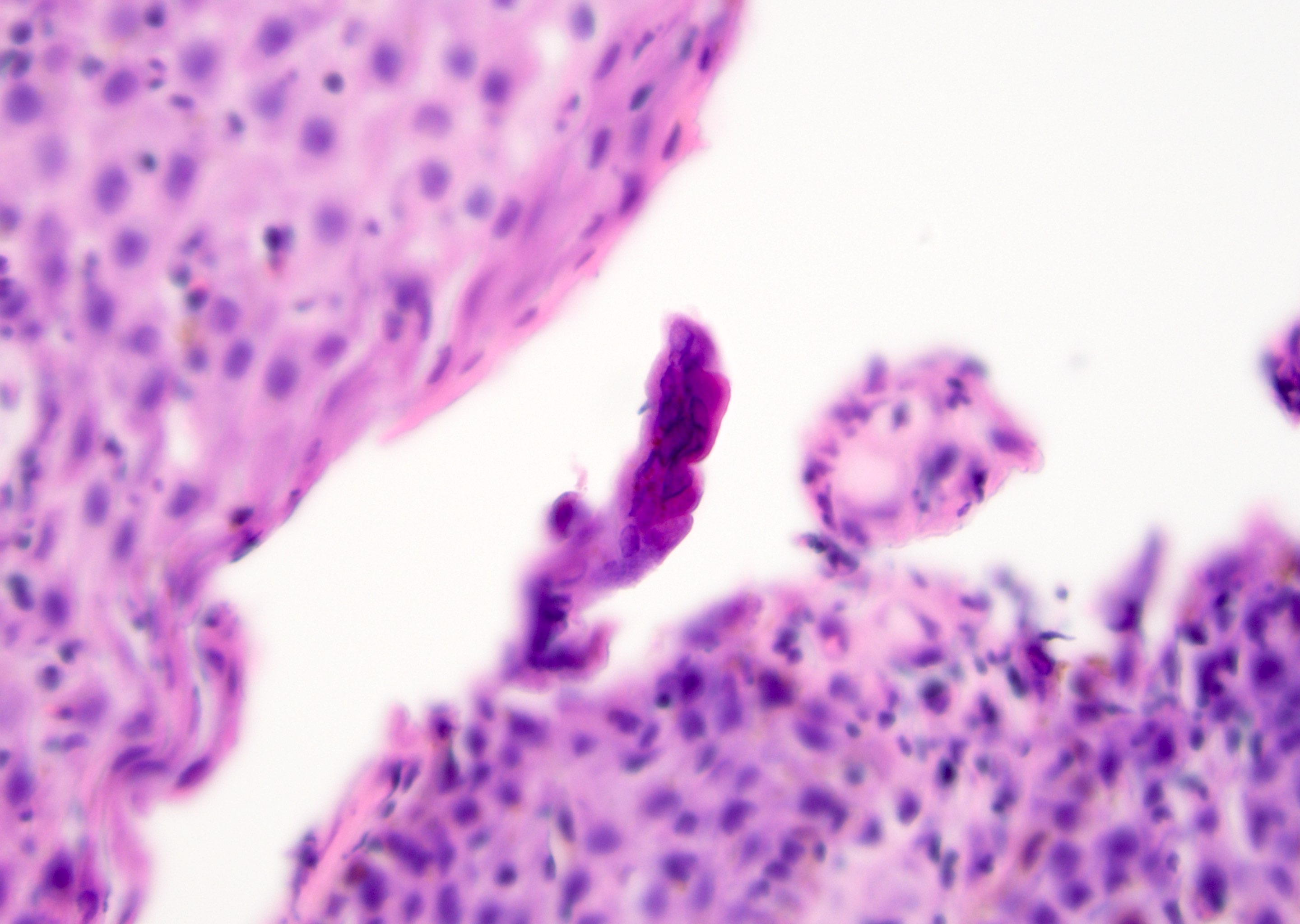
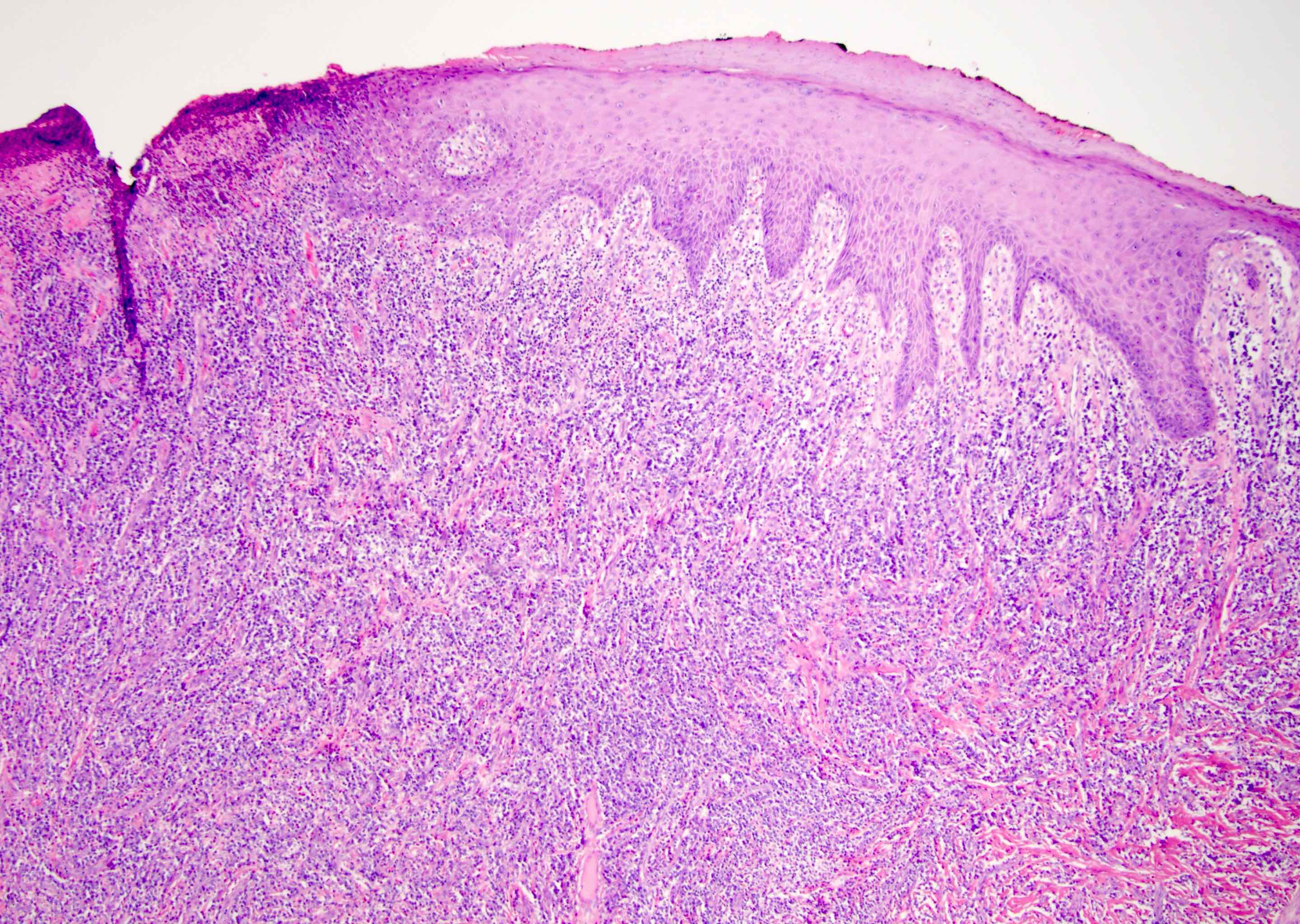
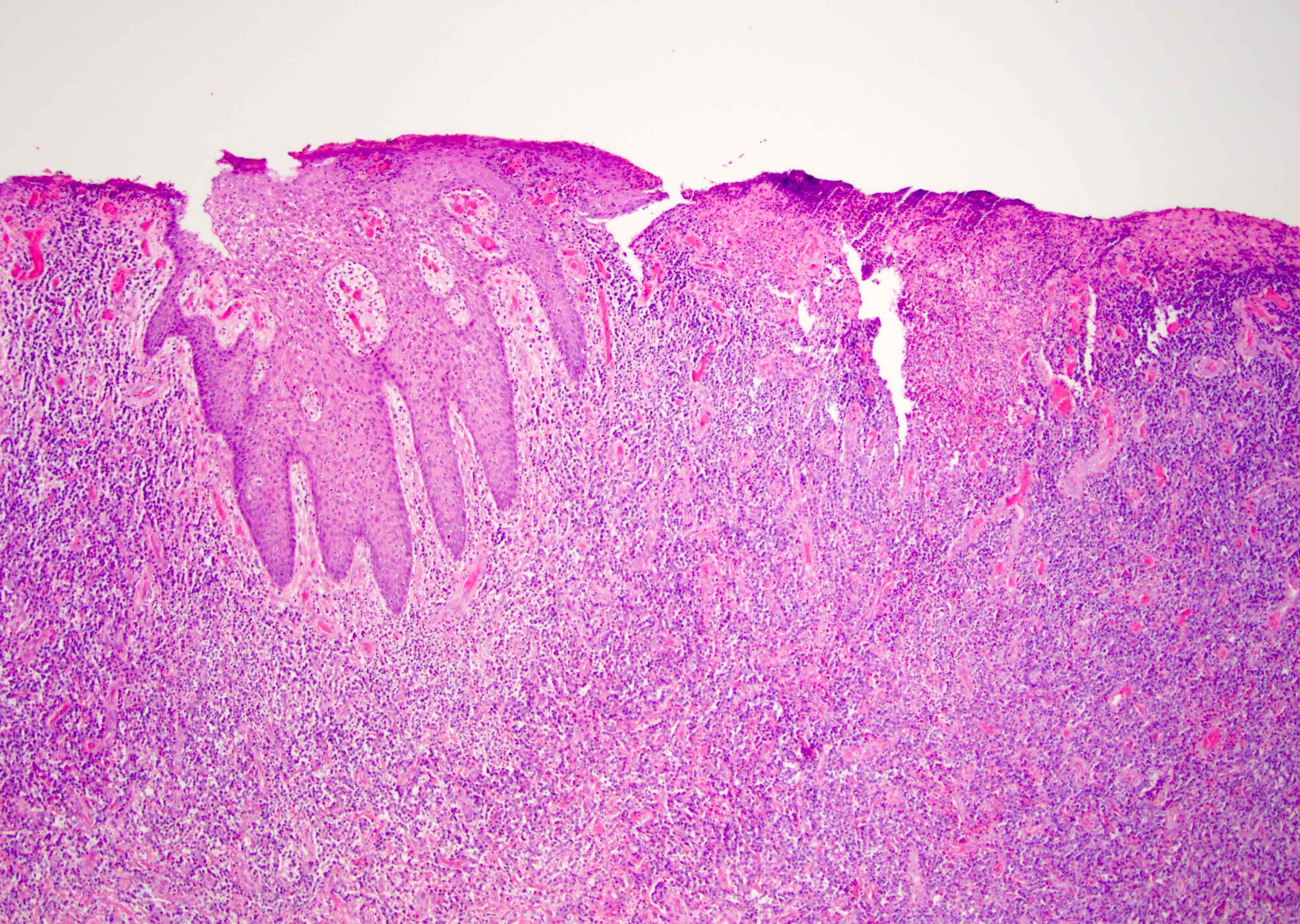
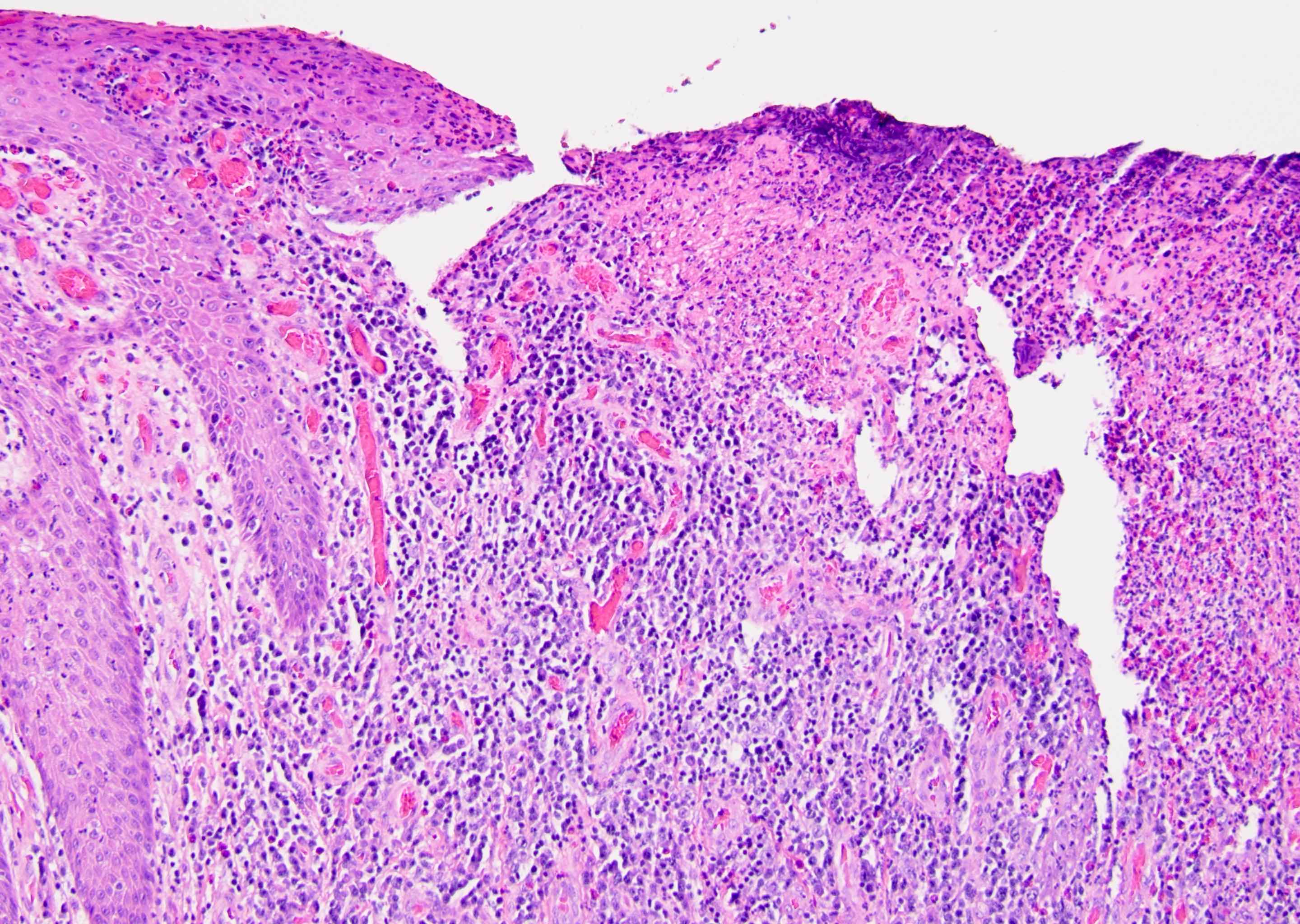
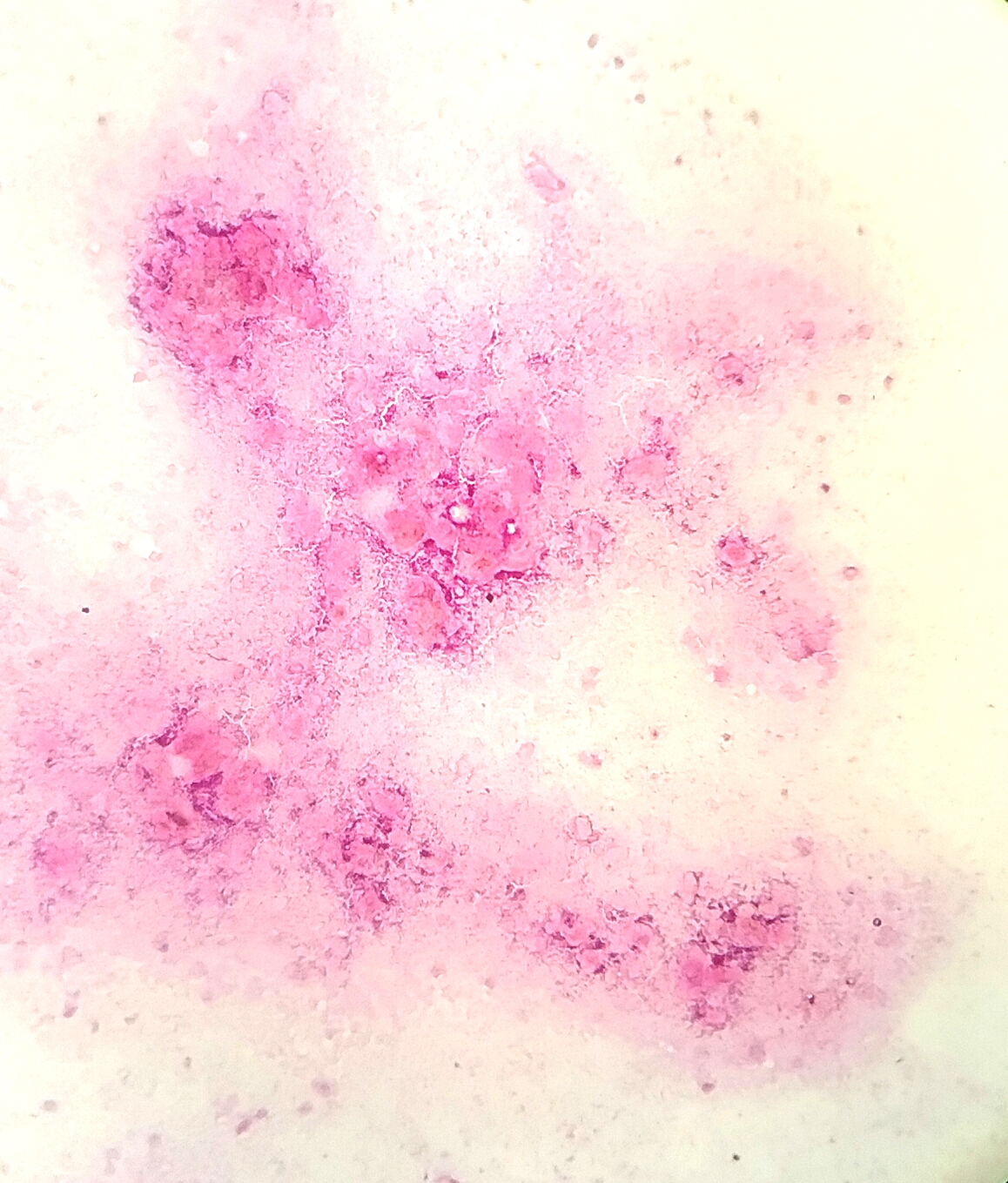
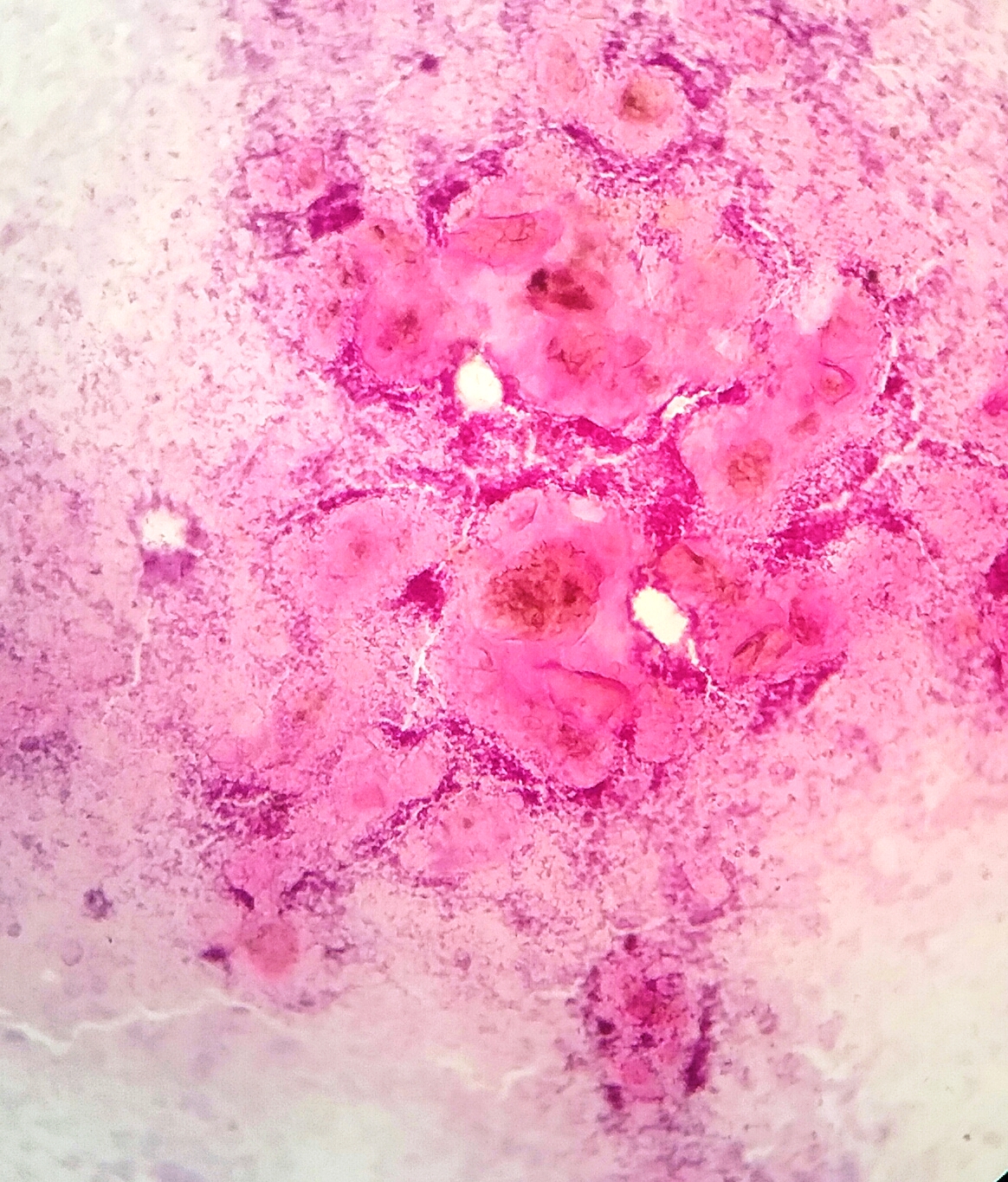
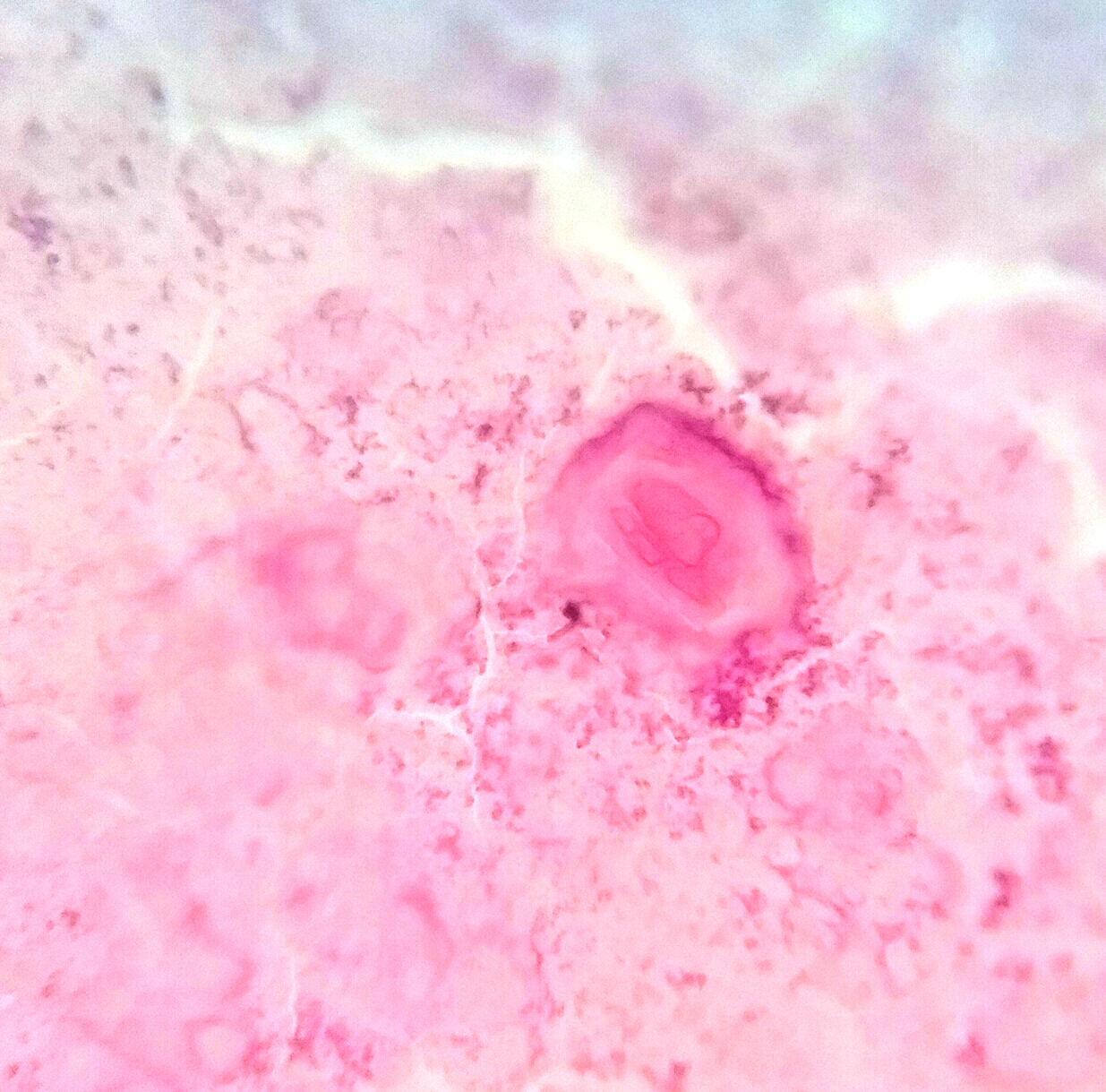
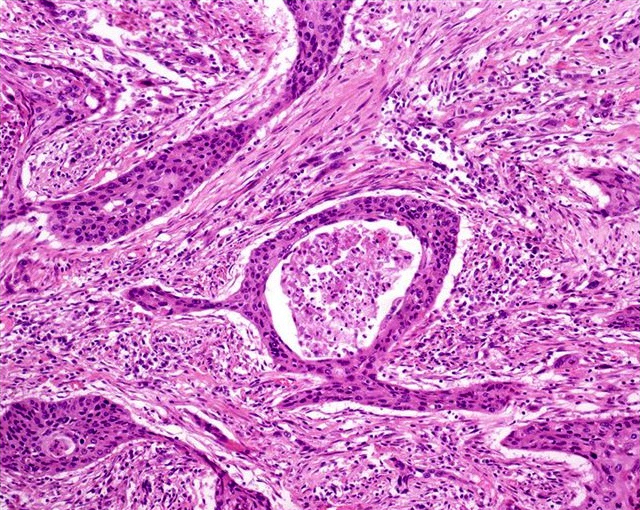
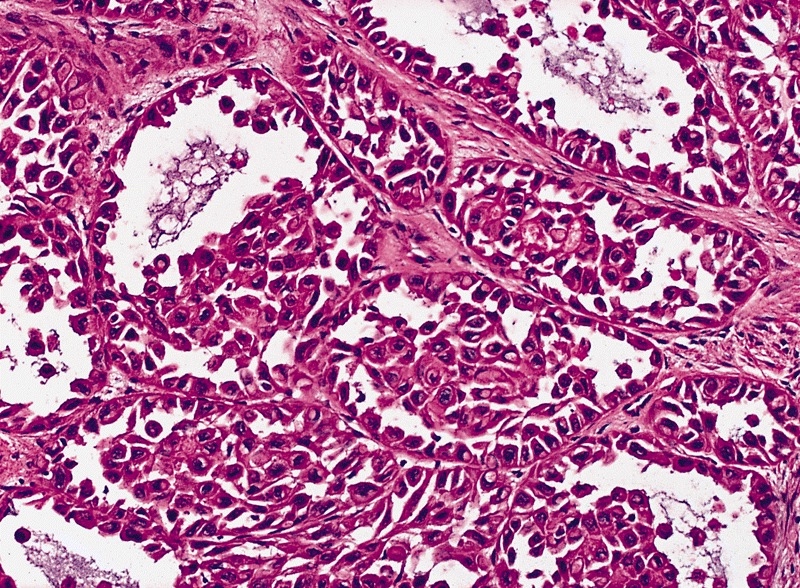
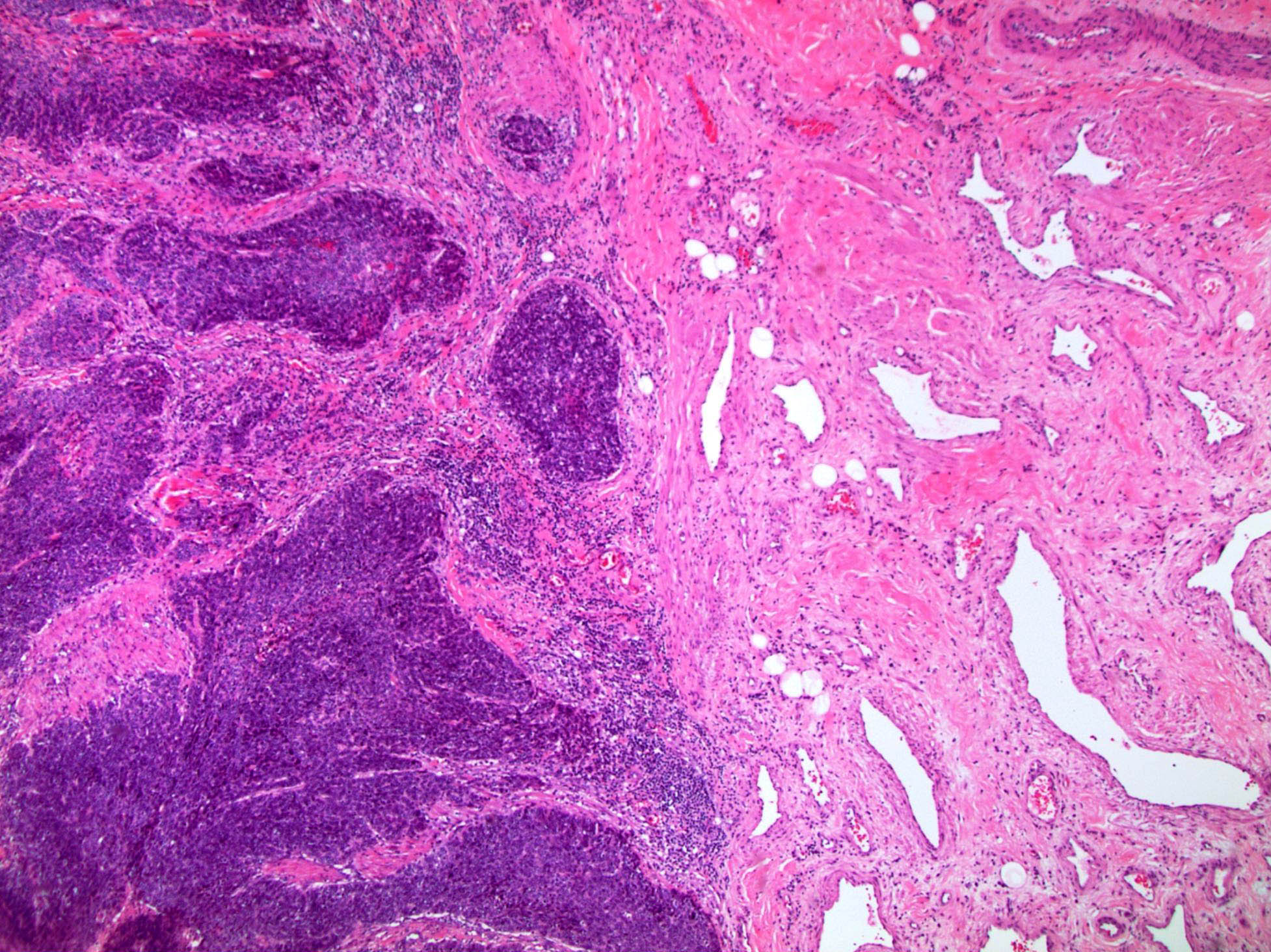
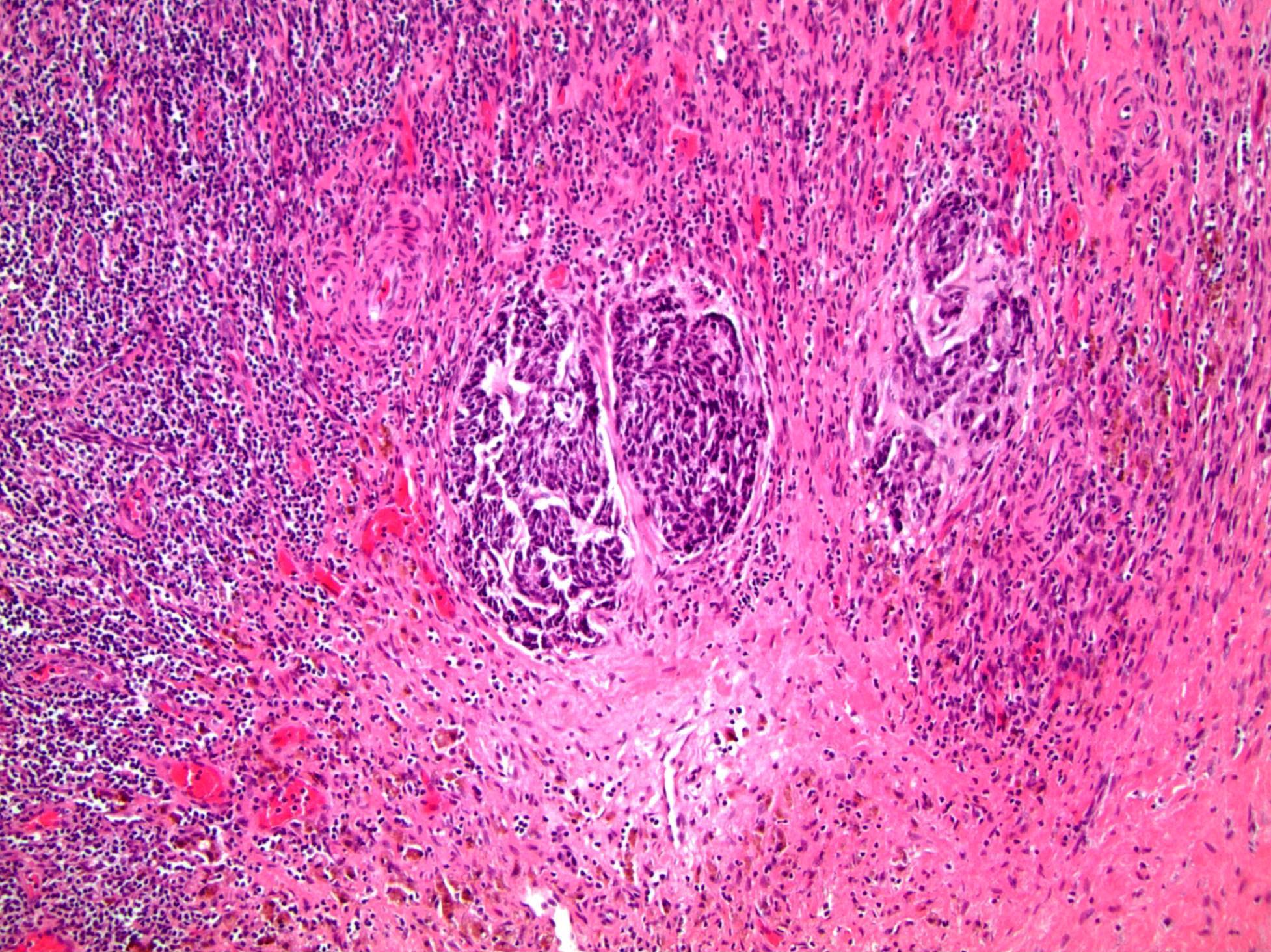
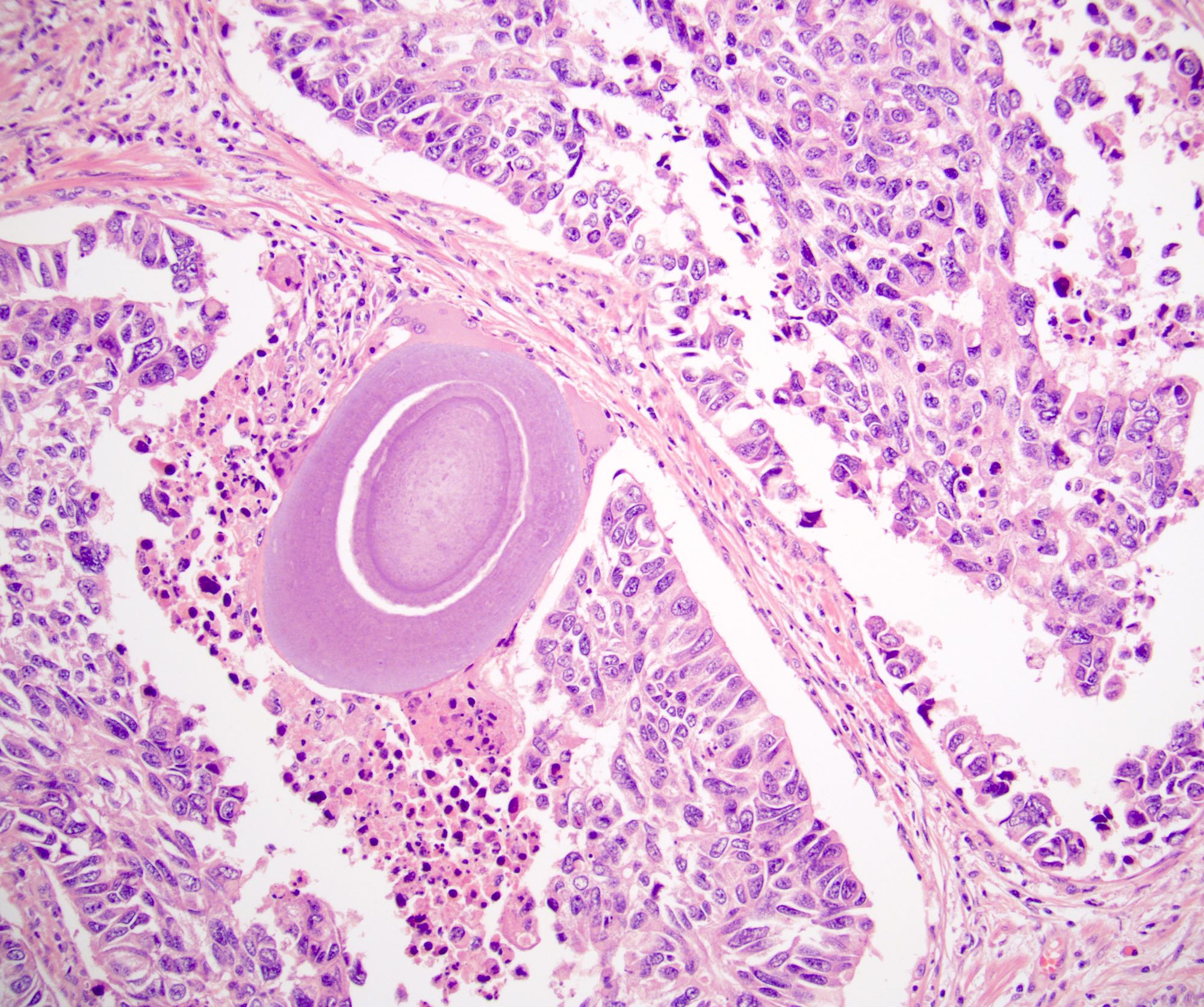
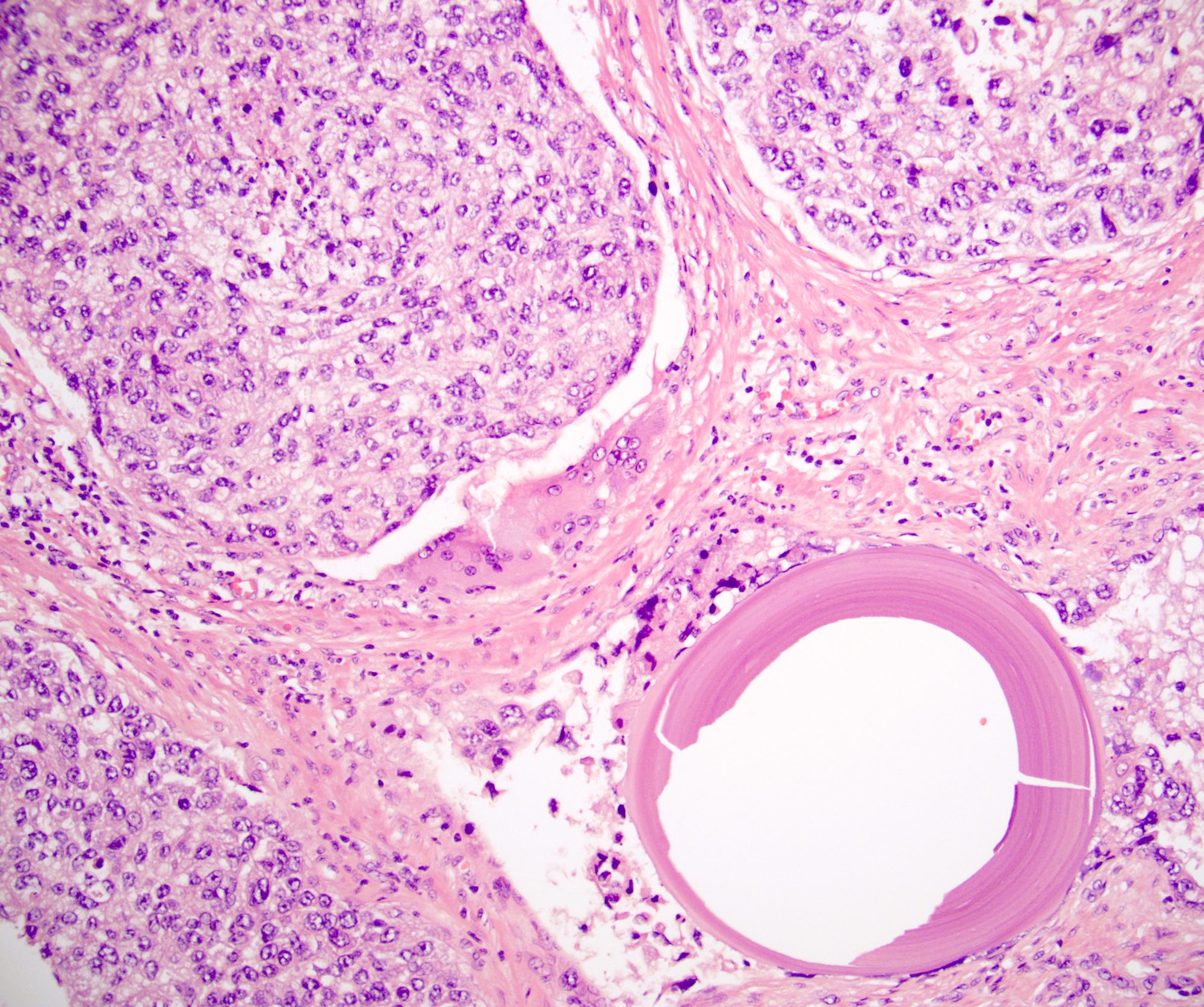
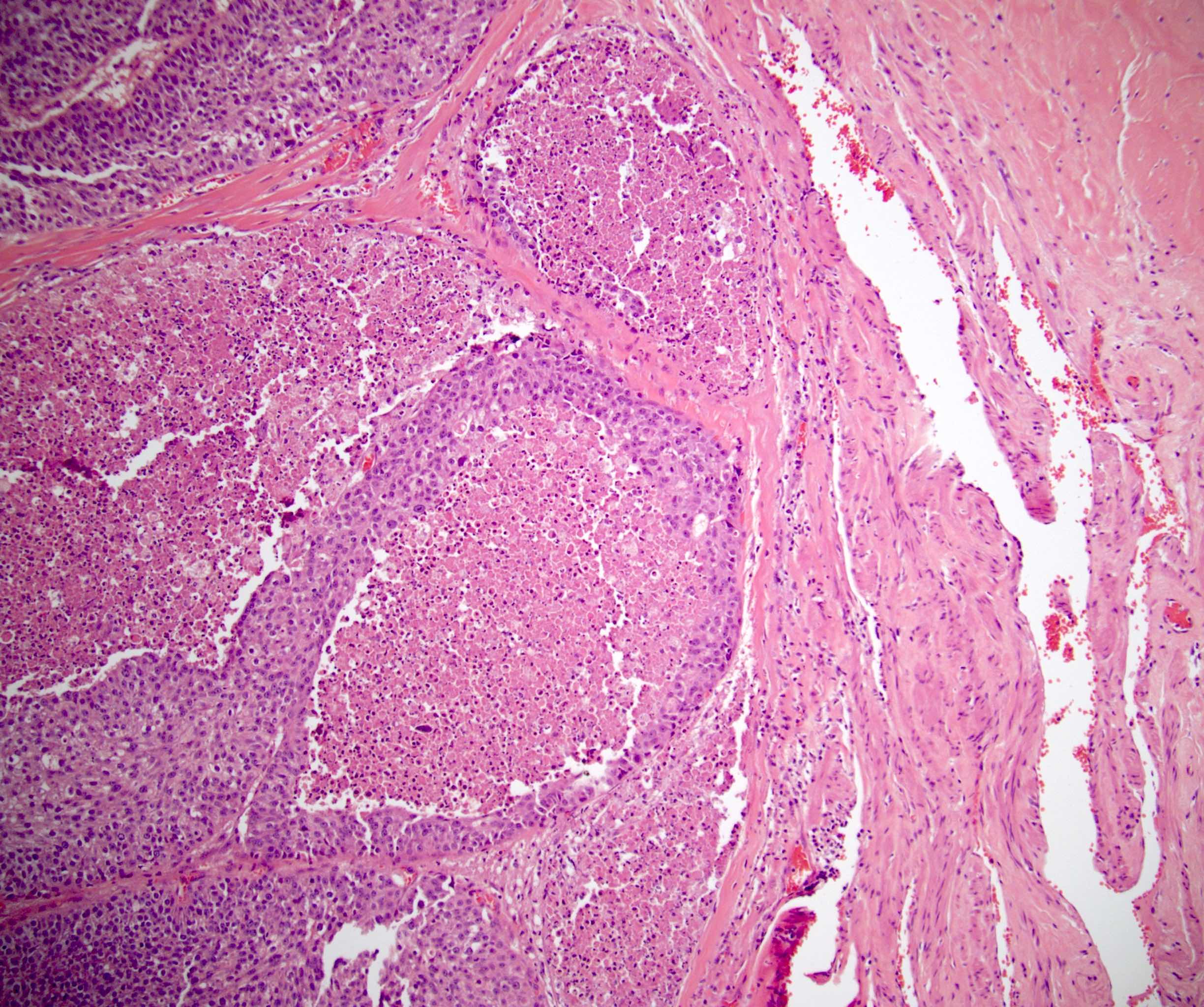
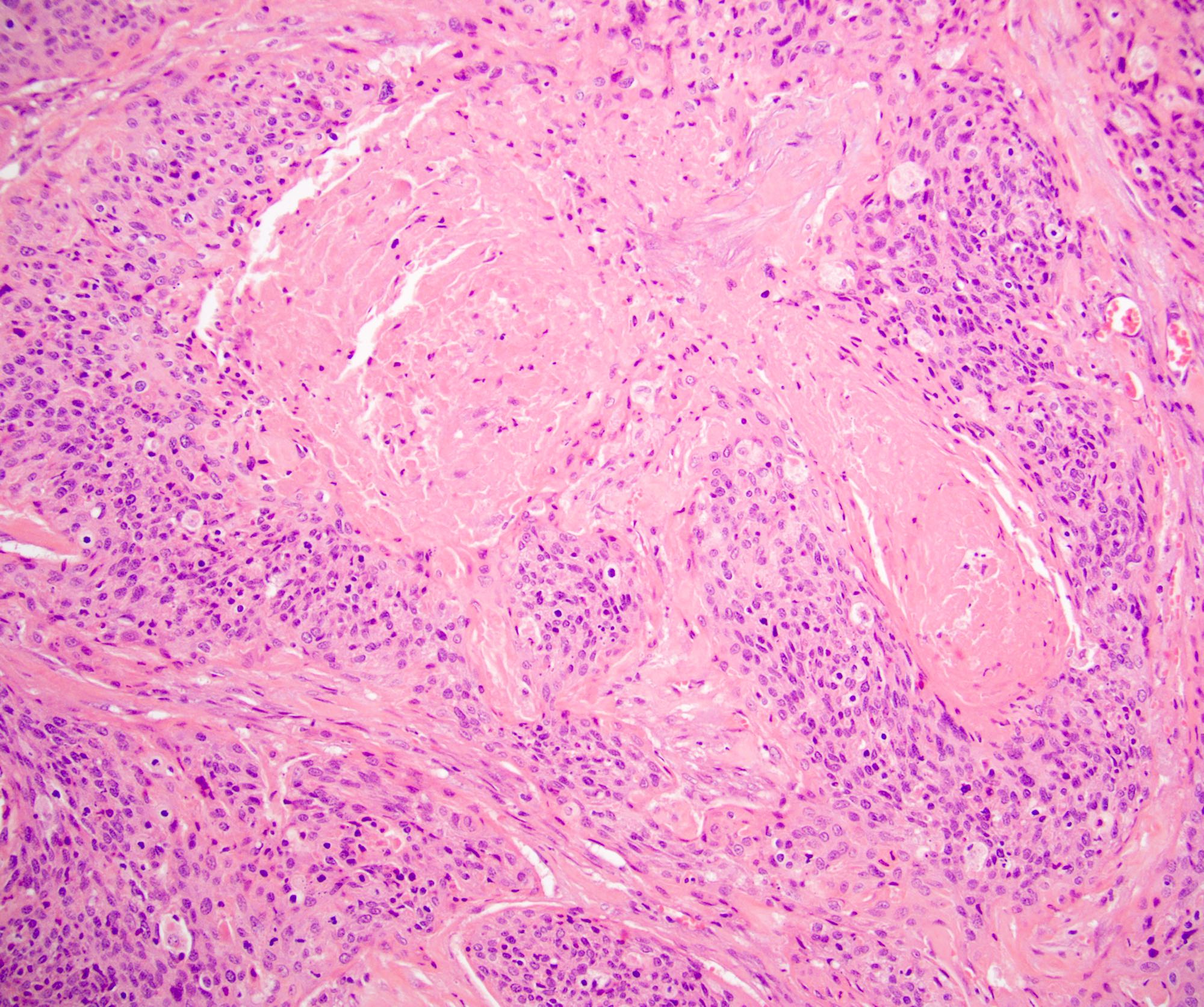
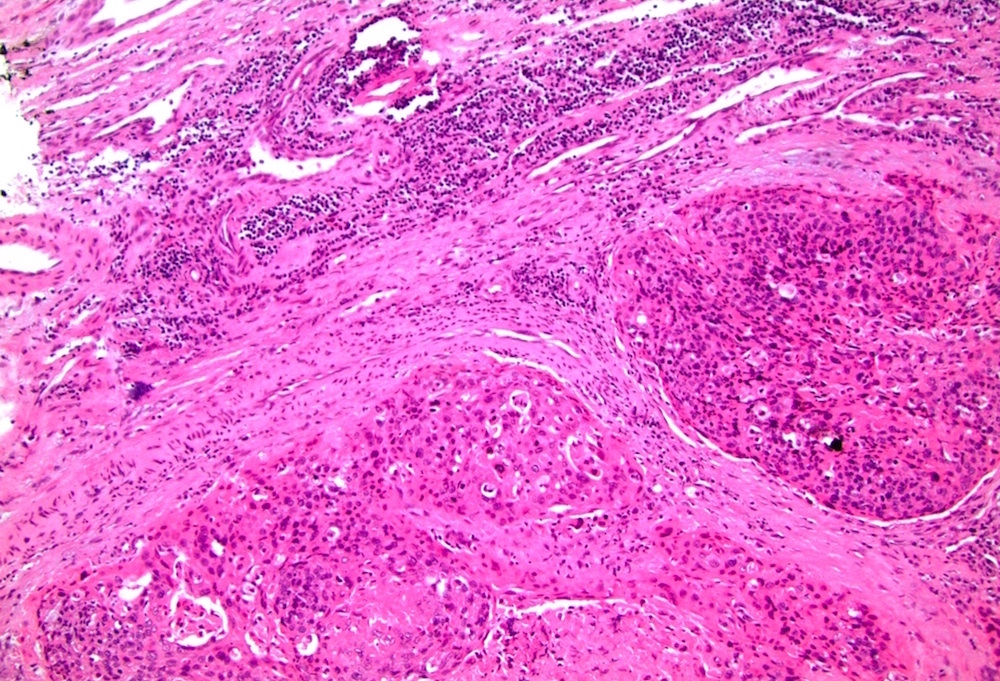
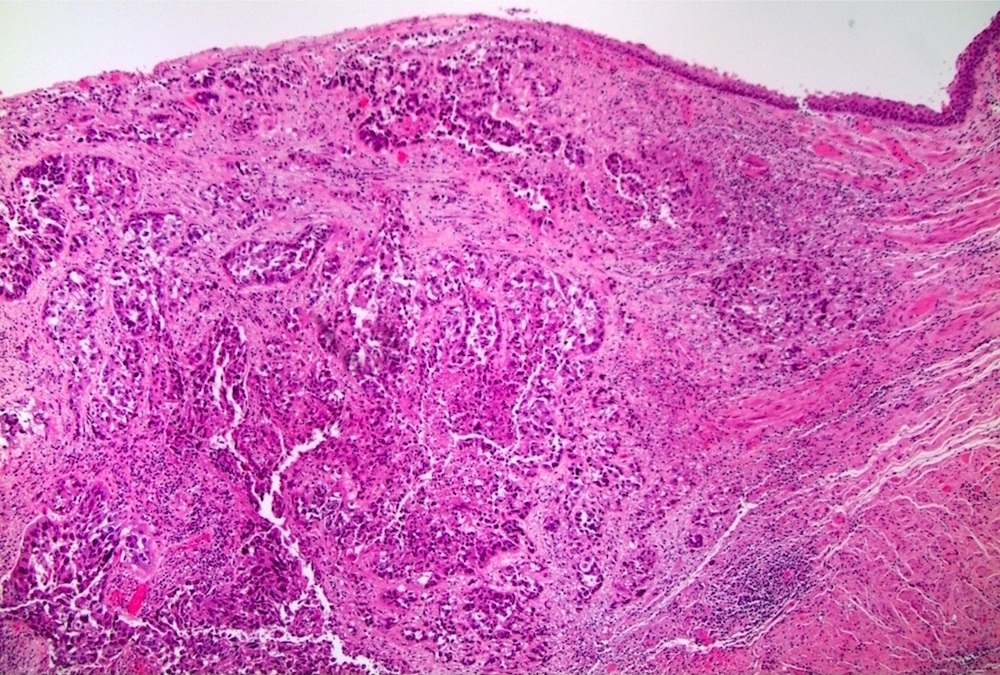
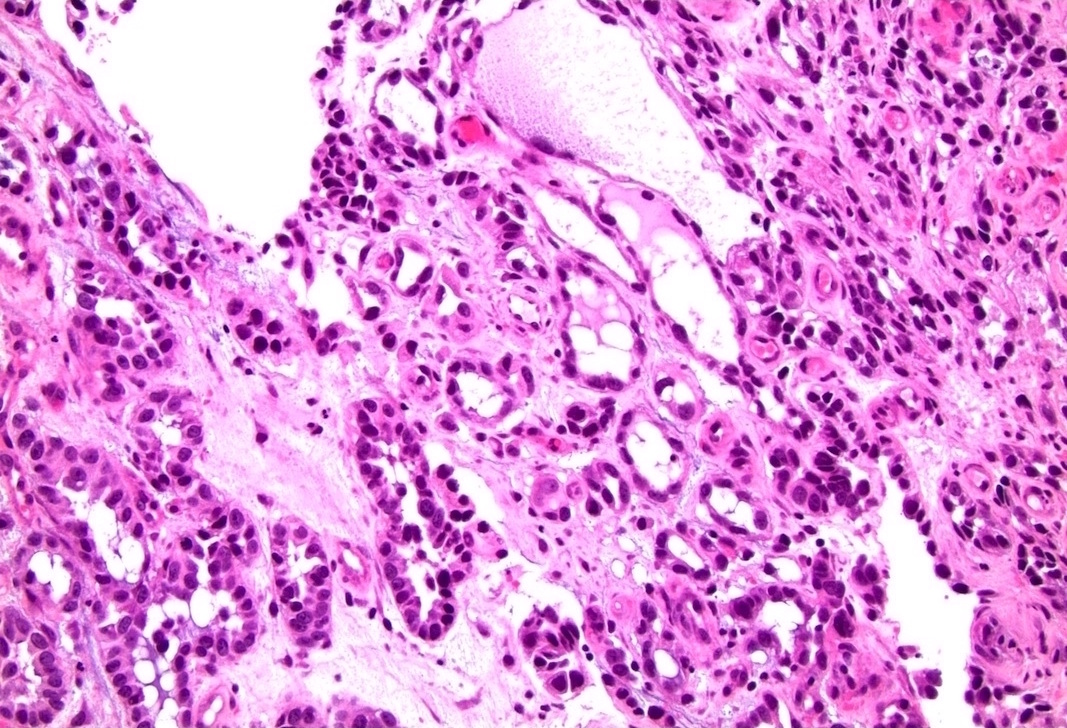
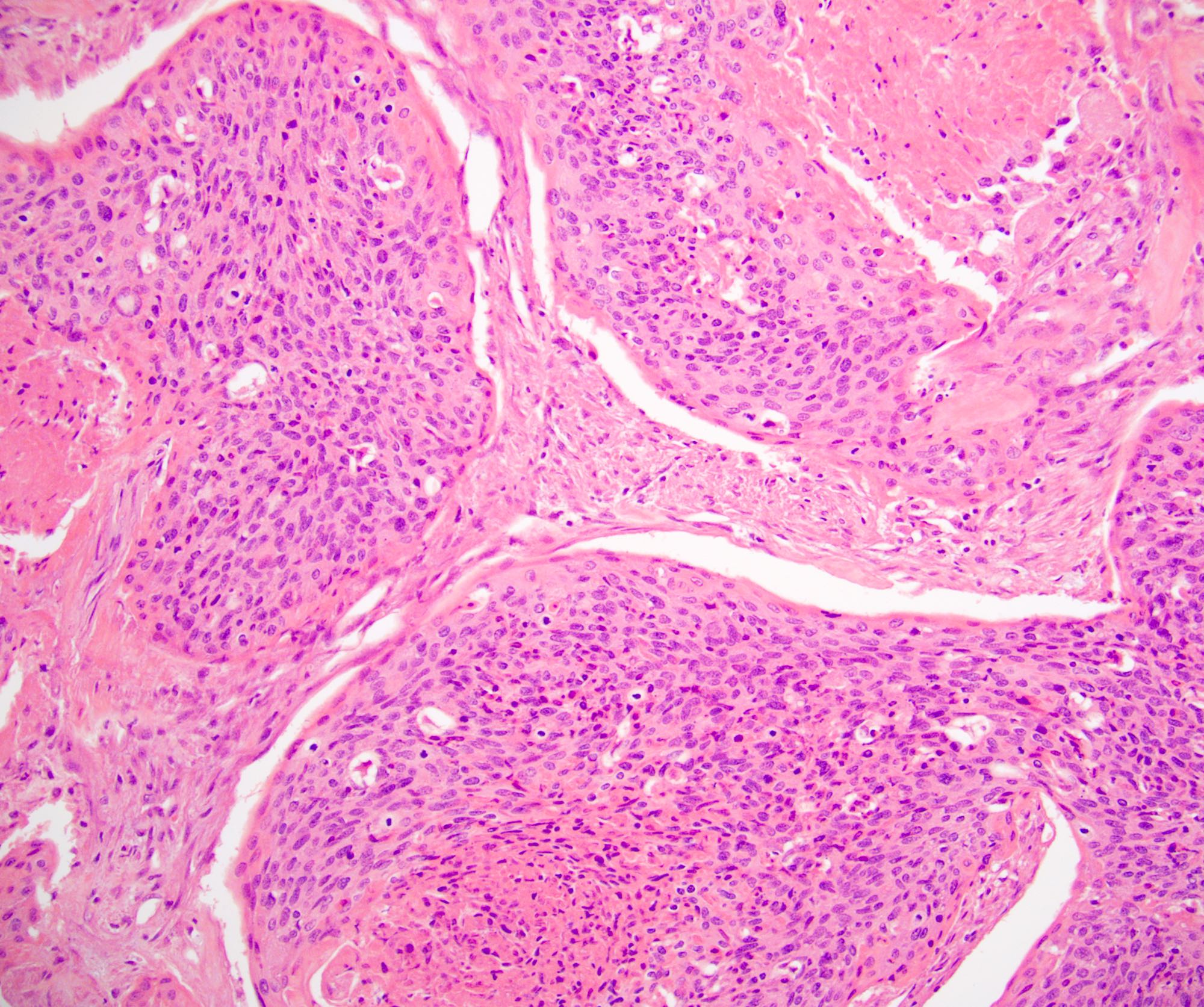
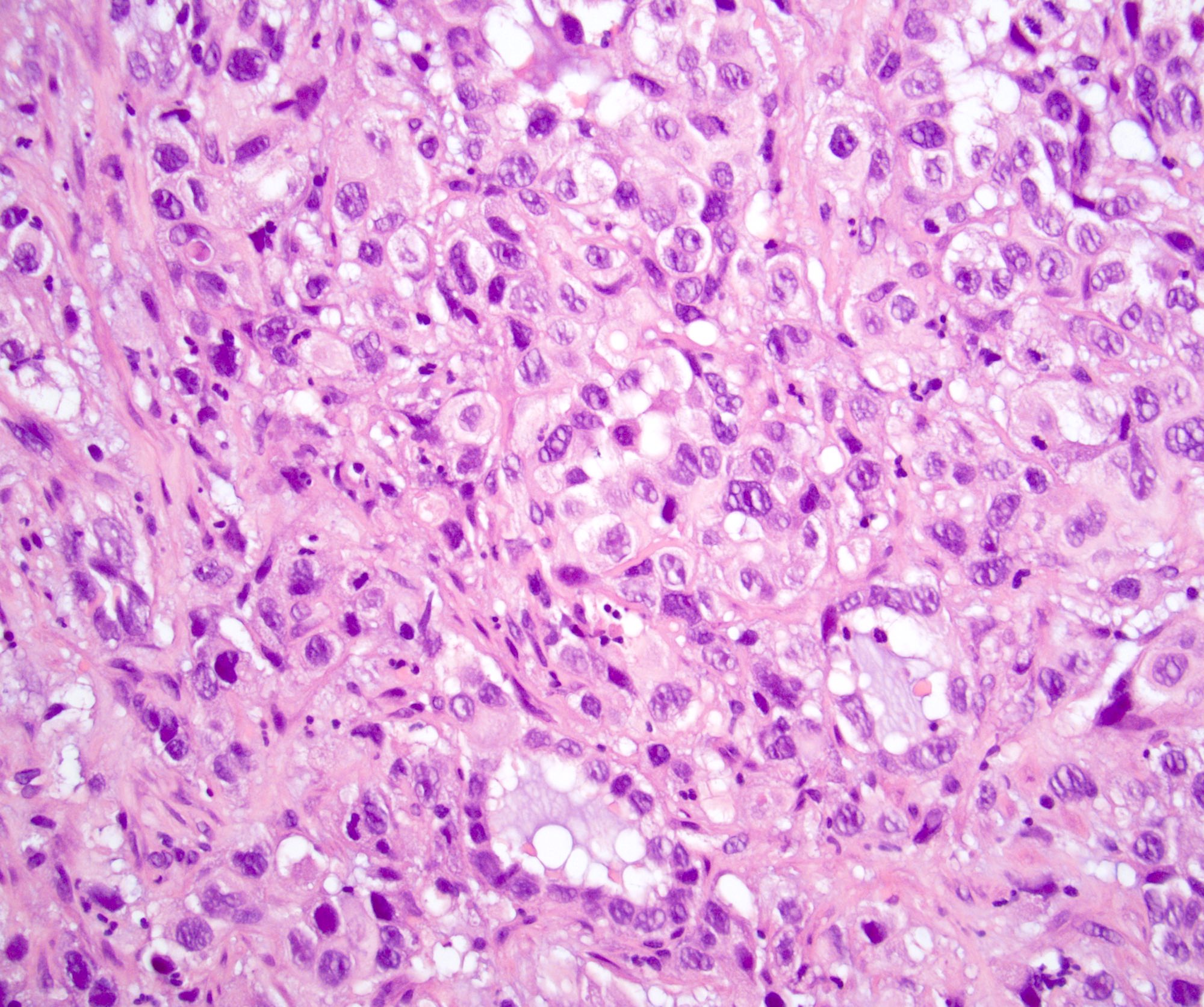
- Squamous cell and glandular patterns, with squamous cell pattern usually predominating
- Both components are usually discrete but mixtures can be found
- Glands produce intraluminal and intracellular mucin
- Frequent presence of penile intraepithelial neoplasia in adjacent mucosa
Microscopic (histologic) images
Contributed by Alcides Chaux, M.D. and Antonio Cubilla, M.D.
AFIP images
Images hosted on other servers:
Differential diagnosis
- Adenosquamous (mucoepidermoid) carcinoma of urethra: ventral in penis, restricted to periurethral tissue and corpus cavernosa
- Littré gland adenocarcinoma: ventral in penis, restricted to periurethral tissue and corpus cavernosa
- Metastatic disease: usually involves shaft, tumor emboli present (Int J Surg Pathol 2011;19:597)
- Mucoepidermoid carcinoma: mixed tumor with mucin but no glandular or ductal structures
- Pseudoglandular (acantholytic, adenoid) carcinoma: prominent acantholysis simulates glandular spaces but lining is composed of squamous epithelium; spaces contain necrotic debris and keratin, not mucin
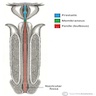
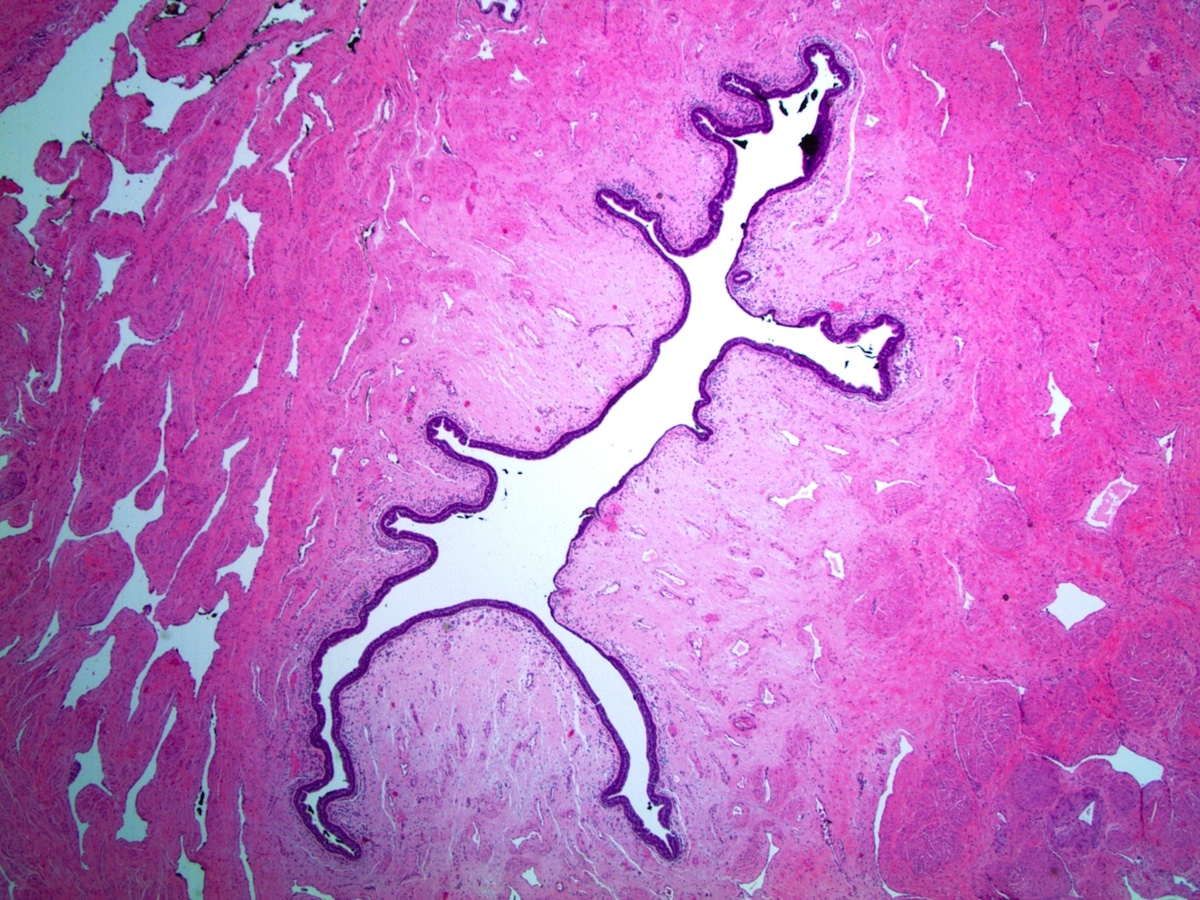
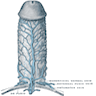
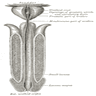
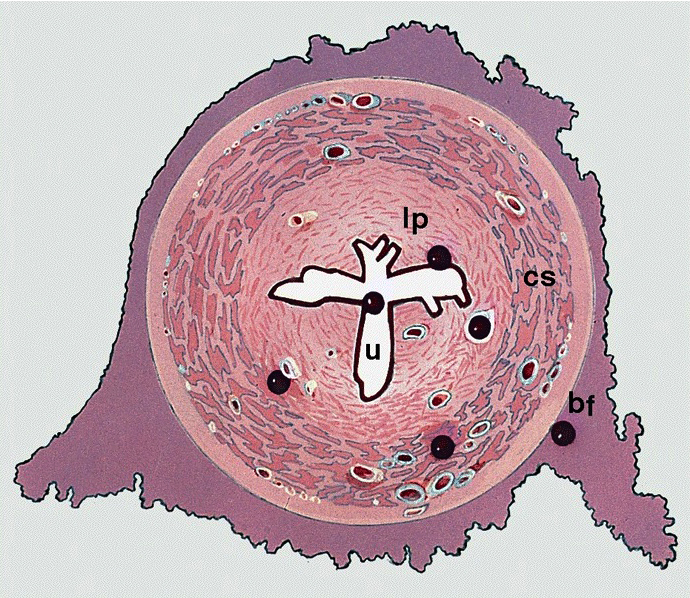
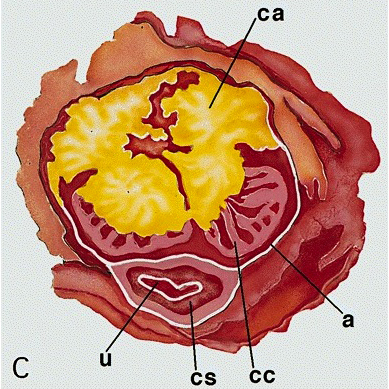
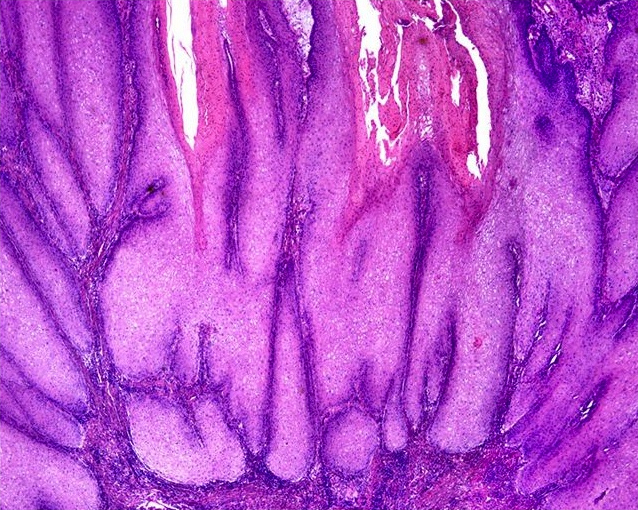
Anatomy & histology-male urethra
Table of Contents
Definition / general | Essential features | Diagrams / tables | Clinical features | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Differential diagnosis | Board review style question #1 | Board review style answer #1Definition / general
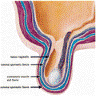
- Fibromuscular tube lined by urothelium, columnar epithelium and nonkeratinized squamous epithelium that takes urine from the urinary bladder to the exterior through the external urethral meatus (Ross: Histology - A Text and Atlas, 7th Edition, 2015, Bostwick: Urologic Surgical Pathology, 3rd Edition, 2014)
Essential features
- Subepithelium composed of loose fibroelastic tissue, glands and abundant vessels
- Muscle layers include smooth muscle and exterior skeletal muscle
- Size, structure and function differ in men and women
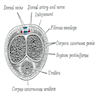
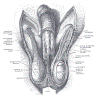
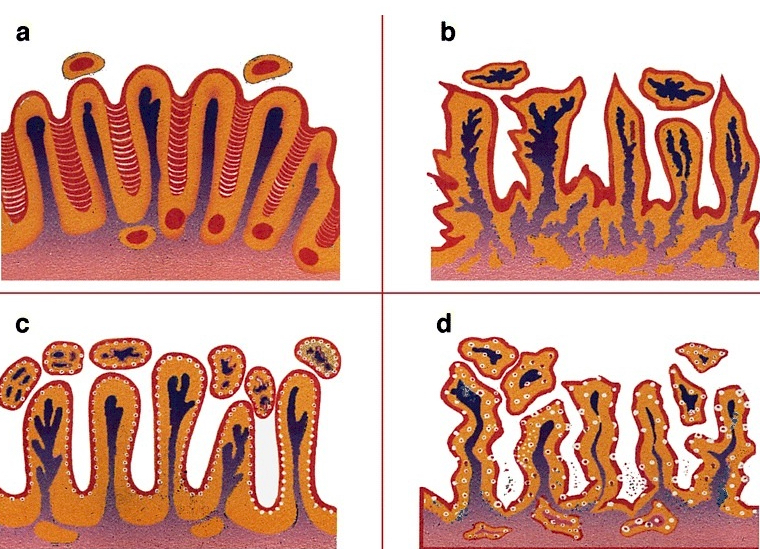
Diagrams / tables
Clinical features
- Functions as the terminal duct for both the genital and urinary system
- Epithelium is derived from the urogenital sinus, which forms from the ventral portion of the endodermal cloaca after its division (Bostwick: Urologic Surgical Pathology, 3rd Edition, 2014)
Gross description
- Measures 15 - 20 cm in length
- Urethral sphincter surrounds the urethra
- Extends from the bladder neck to the inferior fascia of the urogenital diaphragm (Int Urol Nephrol 2014;46:1469)
- Controls urination and discharge of semen into urethra
- Composed of smooth and striated muscle
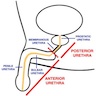
- Divided into prostatic, membranous and penile urethra (Ross: Histology - A Text and Atlas, 7th Edition, 2015, Gartner: Color Textbook of Histology, 3rd Edition, 2006)
- Prostatic urethra
- Extends from the bladder neck through the prostate gland (3 - 4 cm)
- Lined by urothelium
- Ejaculatory ducts enter at the posterior wall
- Most prostatic ducts empty into the posterior and lateral walls (Bostwick: Urologic Surgical Pathology, 3rd Edition, 2014)
- Verumontanum (Müllerian vestige) is posterior to the mid prostatic urethra into which the ejaculatory ducts empty and subsequently join with the prostatic urethra (Ross: Histology - A Text and Atlas, 7th Edition, 2015)
- Membranous urethra
- Extends from the apex of the prostate to the bulb of the penis (1 cm) (Ross: Histology - A Text and Atlas, 2015, 7th Edition, 2015, Bostwick: Urologic Surgical Pathology, 3rd Edition, 2014)
- Lined by stratified / pseudostratified columnar epithelium (similar to urothelium but lacking umbrella cells)
- Passes through the urogenital diaphragm as it enters the perineum
- Surrounded by external voluntary sphincter formed by skeletal muscle of the urogenital diaphragm
- Penile (spongy) urethra
- Extends through the penis to the urethral meatus opening in the glans (15 cm) (Ross: Histology - A Text and Atlas, 7th Edition, 2015, Bostwick: Urologic Surgical Pathology, 3rd Edition, 2014)
- Divided into 3 regions
- Bulbar urethra: spans the bulb of the penis, lined by stratified / pseudostratified columnar epithelium
- Pendulous urethra: spans the pendulous penis (shaft), lined by stratified / pseudostratified columnar epithelium
- Fossa navicularis: dilated portion in the glans, lined by nonkeratinizing squamous epithelium
- Surrounded by the corpus spongiosum
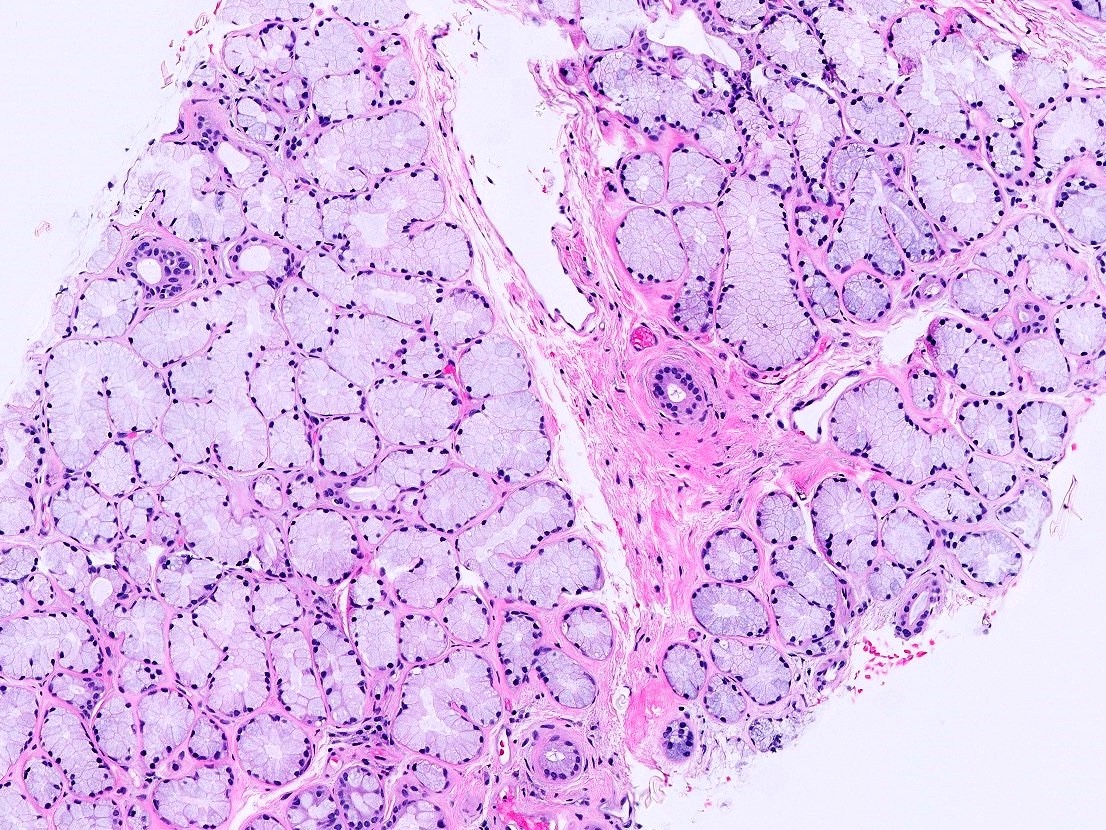
- Bulbourethral (Cowper) glands, glands of Littré (mucous glands) and intraepithelial (juxtaepithelial) glands empty into the penile urethra
- Prostatic urethra
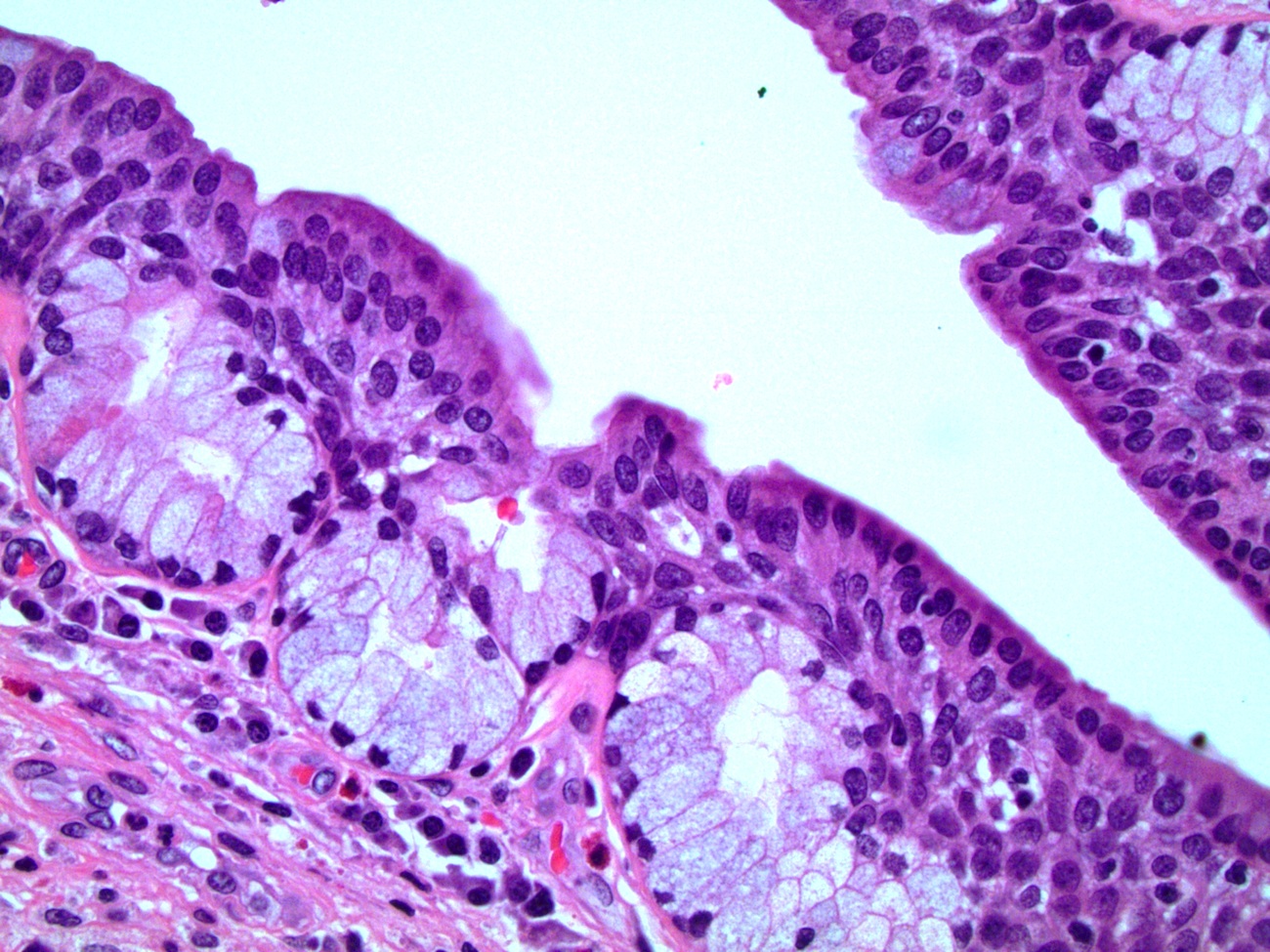
Microscopic (histologic) description
- Urothelium
- Basal layers are composed of either low columnar or cuboidal cells, followed by several layers of polyhedral cells
- Most superficially composed of round, dome shaped umbrella cells that are occasionally multinucleated and flattened according to amount of distention
- Stratified / pseudostratified columnar epithelium
- Multiple cell layers of polyhedral cells; the most superficial are columnar
- Stratified / pseudostratified; in the pseudostratified layer, all cells are in contact with the basal layer
- Nonkeratinizing squamous epithelium
- Composed of multiple layers
- Divided into zones according to cell shapes: cuboidal (deepest), polymorphous (middle), squamous / flattened (superficial)
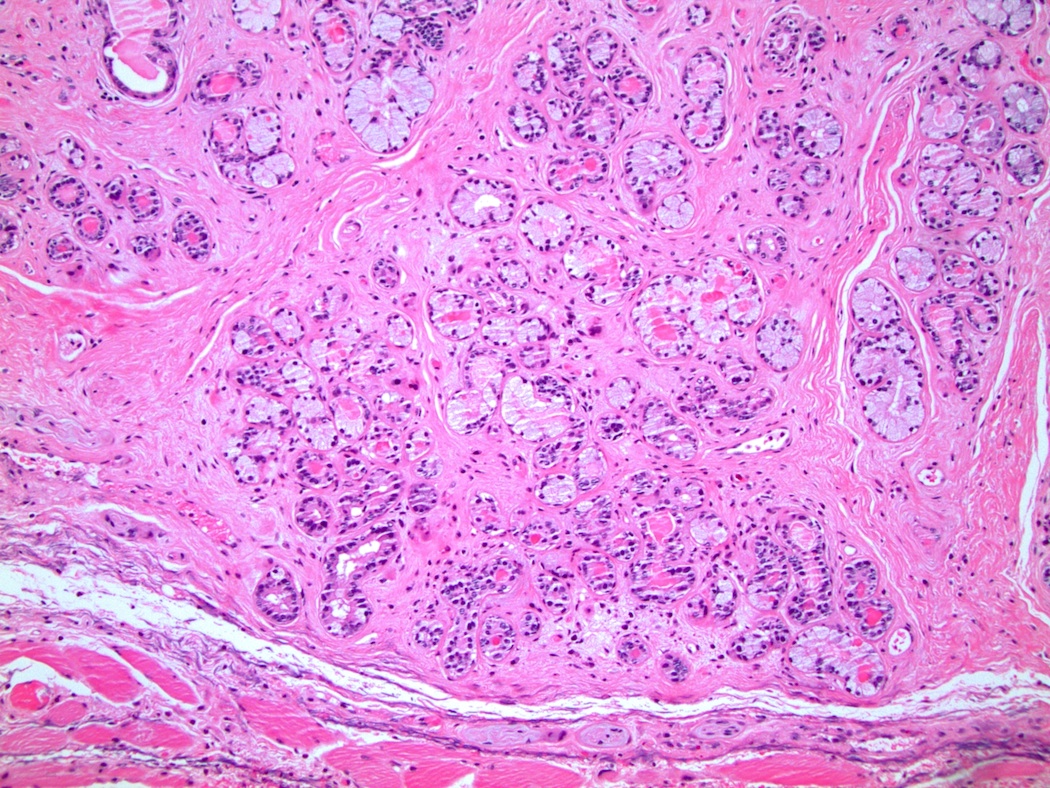
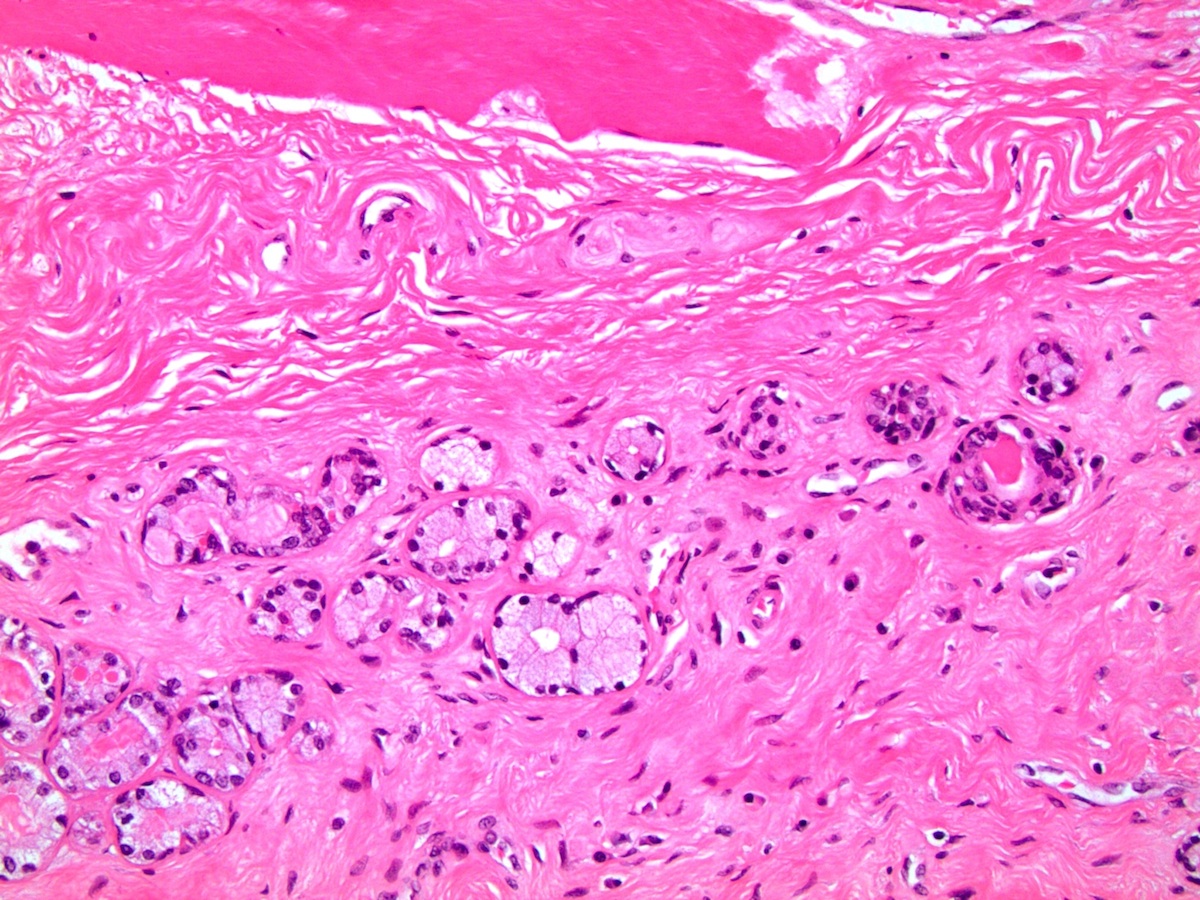
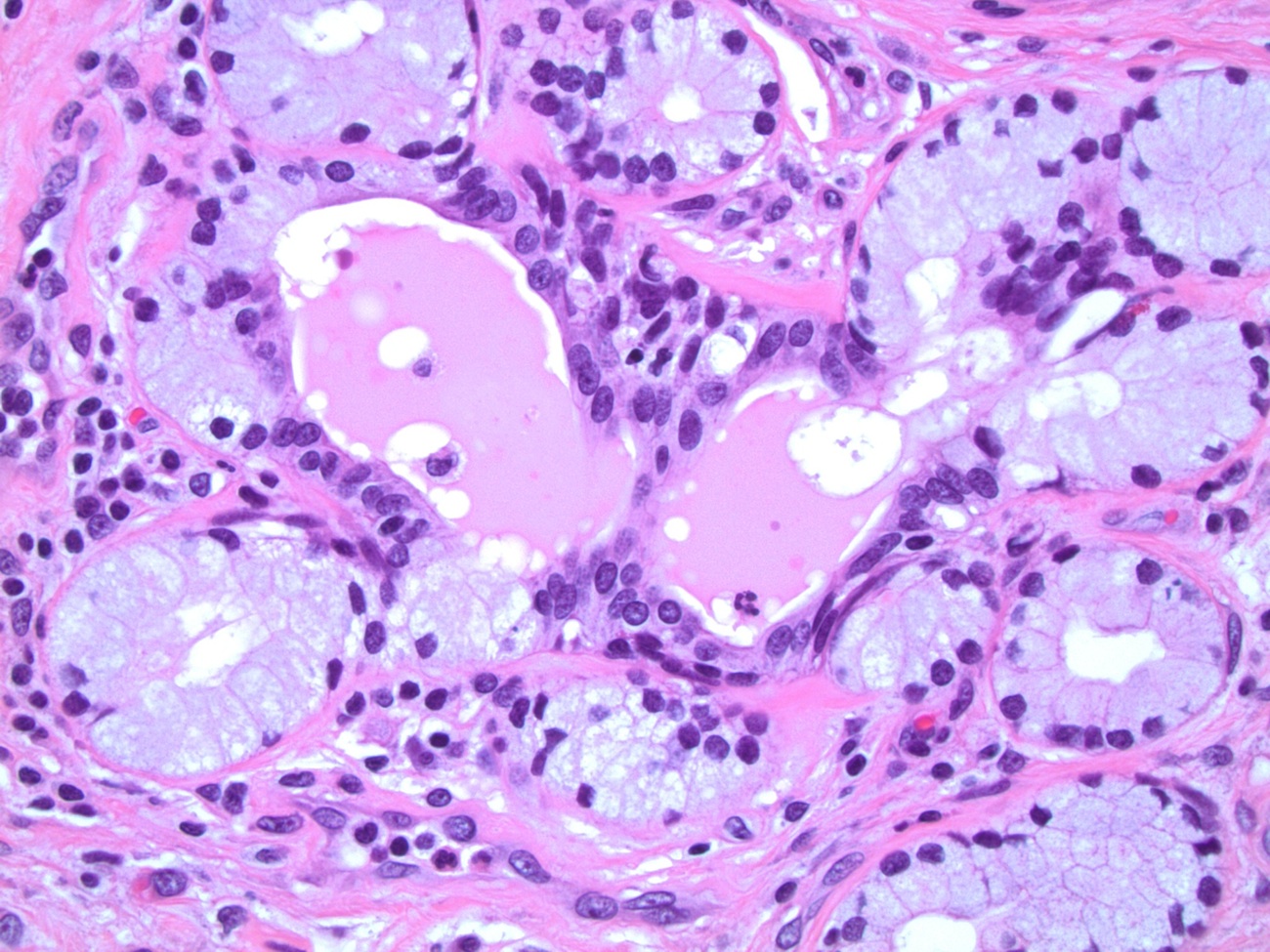
- Urethral / periurethral glands
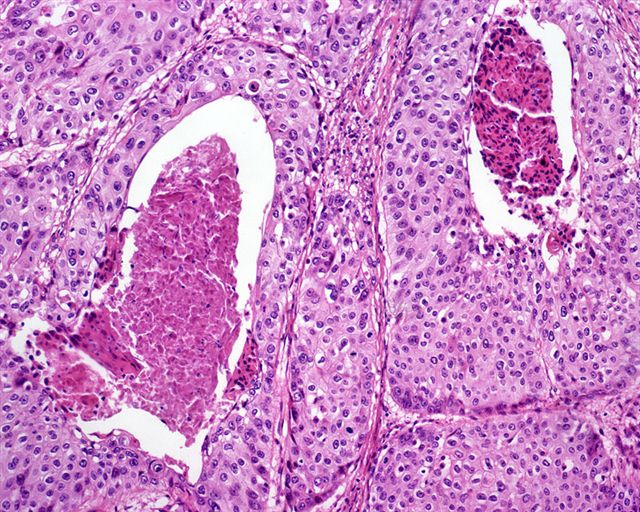
- Bulbourethral (Cowper) glands: mucous - acinous glands in the bulbous and membranous urethra with uniform pale cytoplasm and basal, compressed nuclei
- Littré glands: tubuloacinar mucinous glands with uniform, pale eosinophilic to clear cytoplasm and basally flattened nuclei in penile urethra (Mills: Histology for Pathologists, 4th Edition, 2012)
- Intraepithelial (juxtaepithelial) glands: nests of cells with eosinophilic to flocculent cytoplasm with basally placed nucleus located at the junction of the epithelium and subepithelium
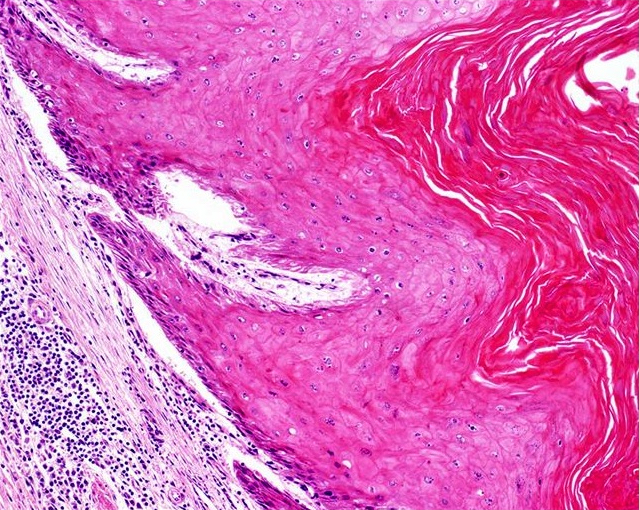
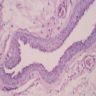
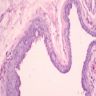
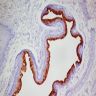
Microscopic (histologic) images
Positive stains
- CK7: upper layers positive (Mills: Histology for Pathologists, 4th Edition, 2012)
- High molecular weight keratin, CK5/6, p63, p40: basal, parabasal cells positive (Mills: Histology for Pathologists, 4th Edition, 2012)
- GATA3
Negative stains
Differential diagnosis
Board review style question #1
Board review style answer #1
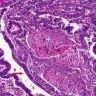
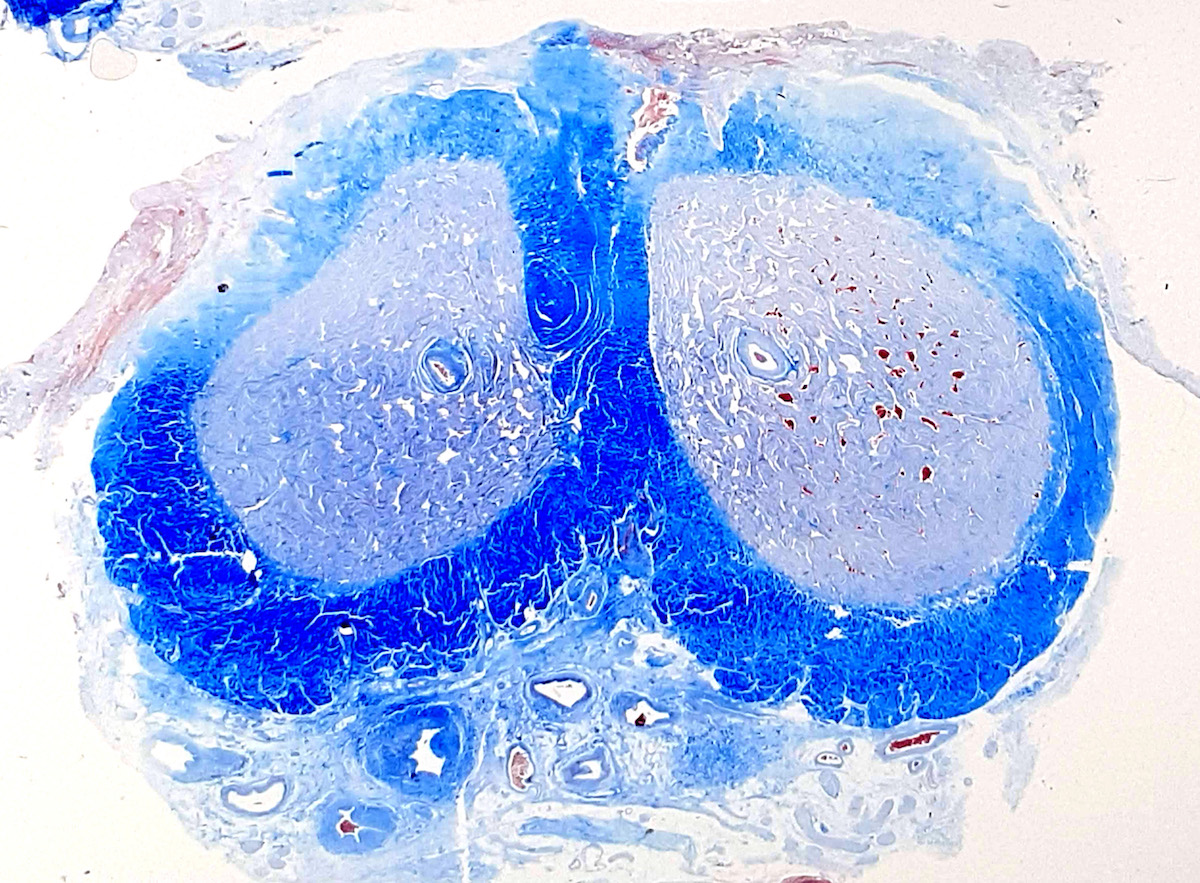
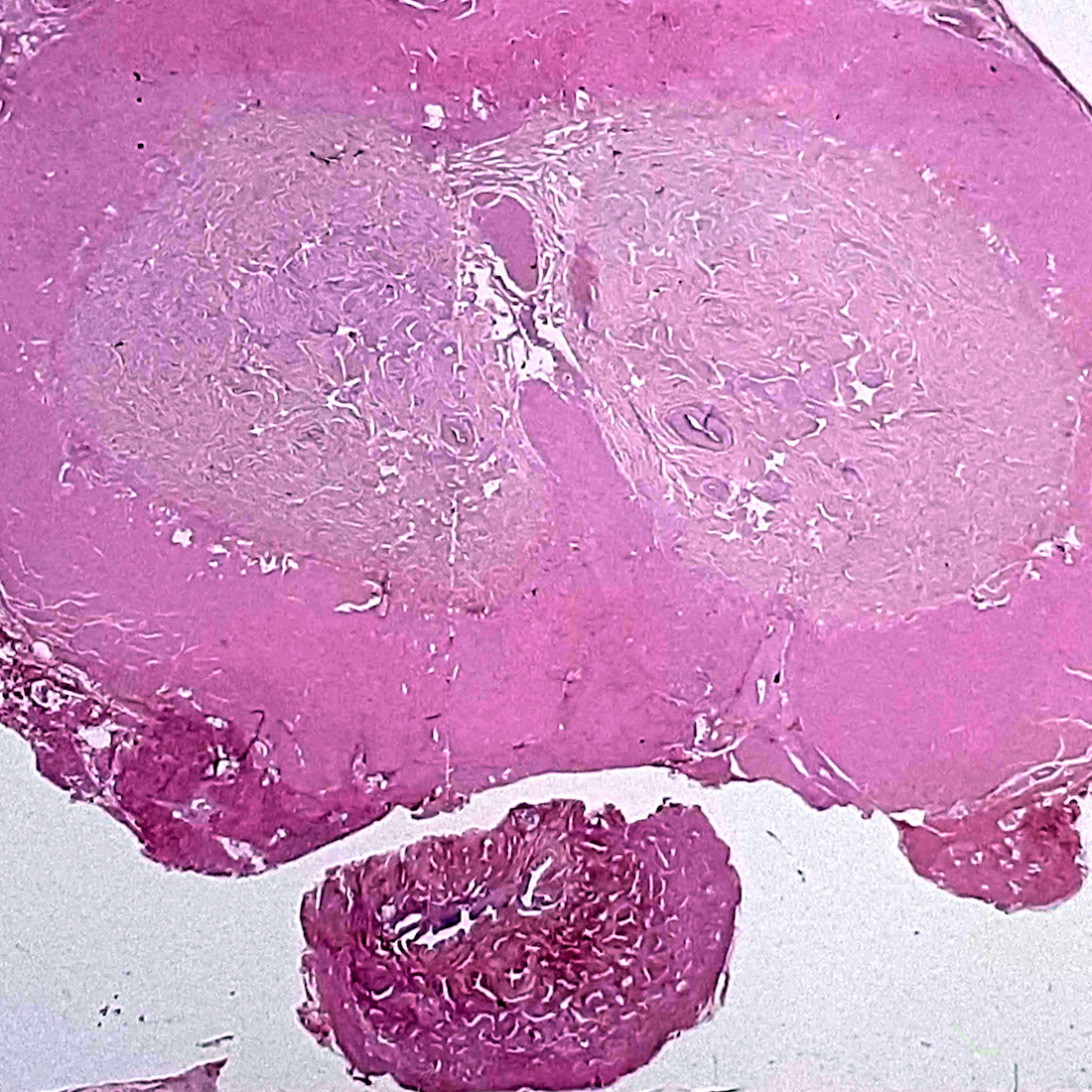
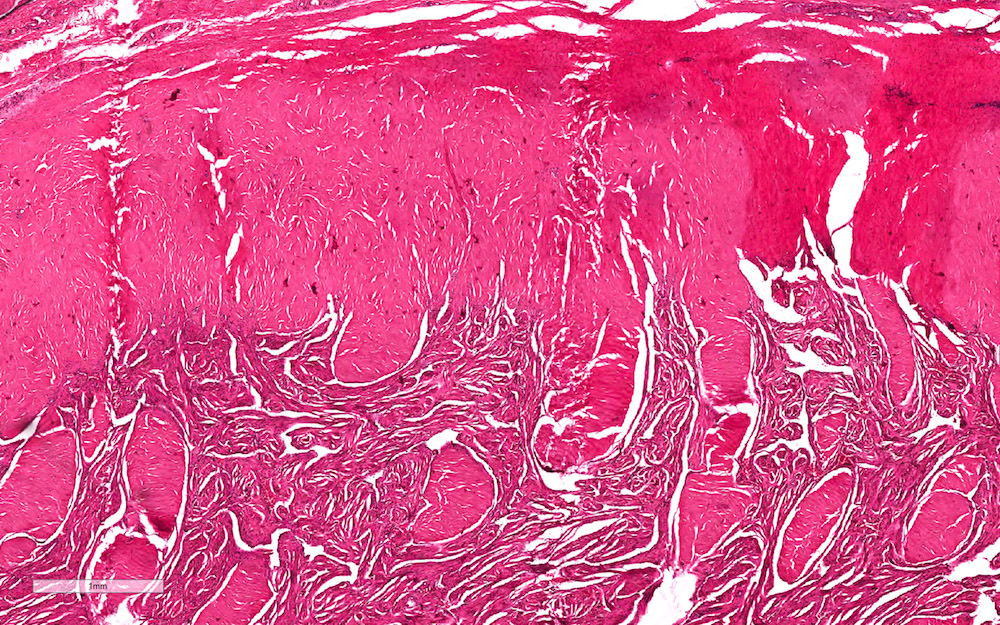
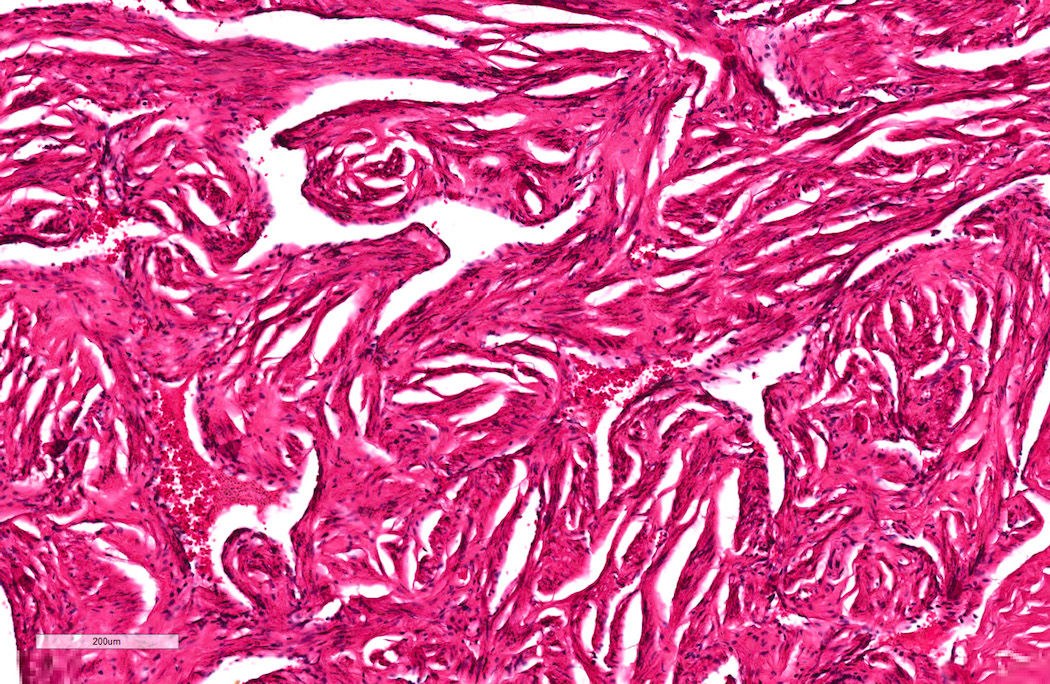
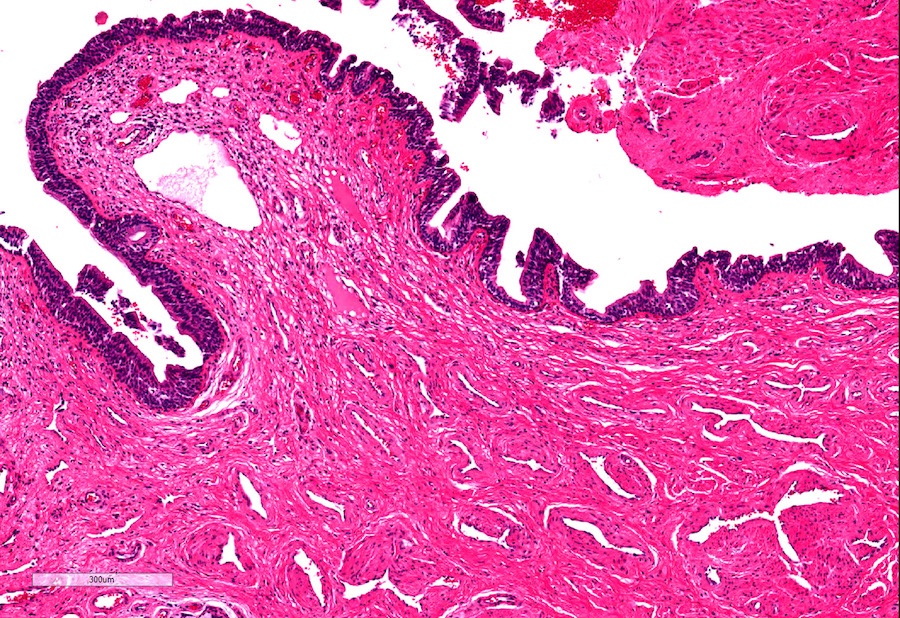
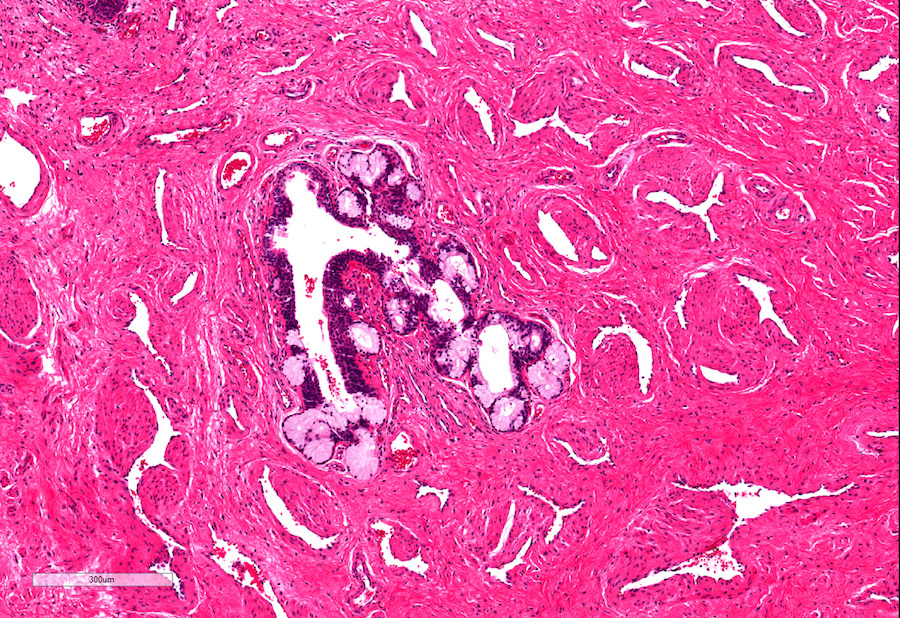
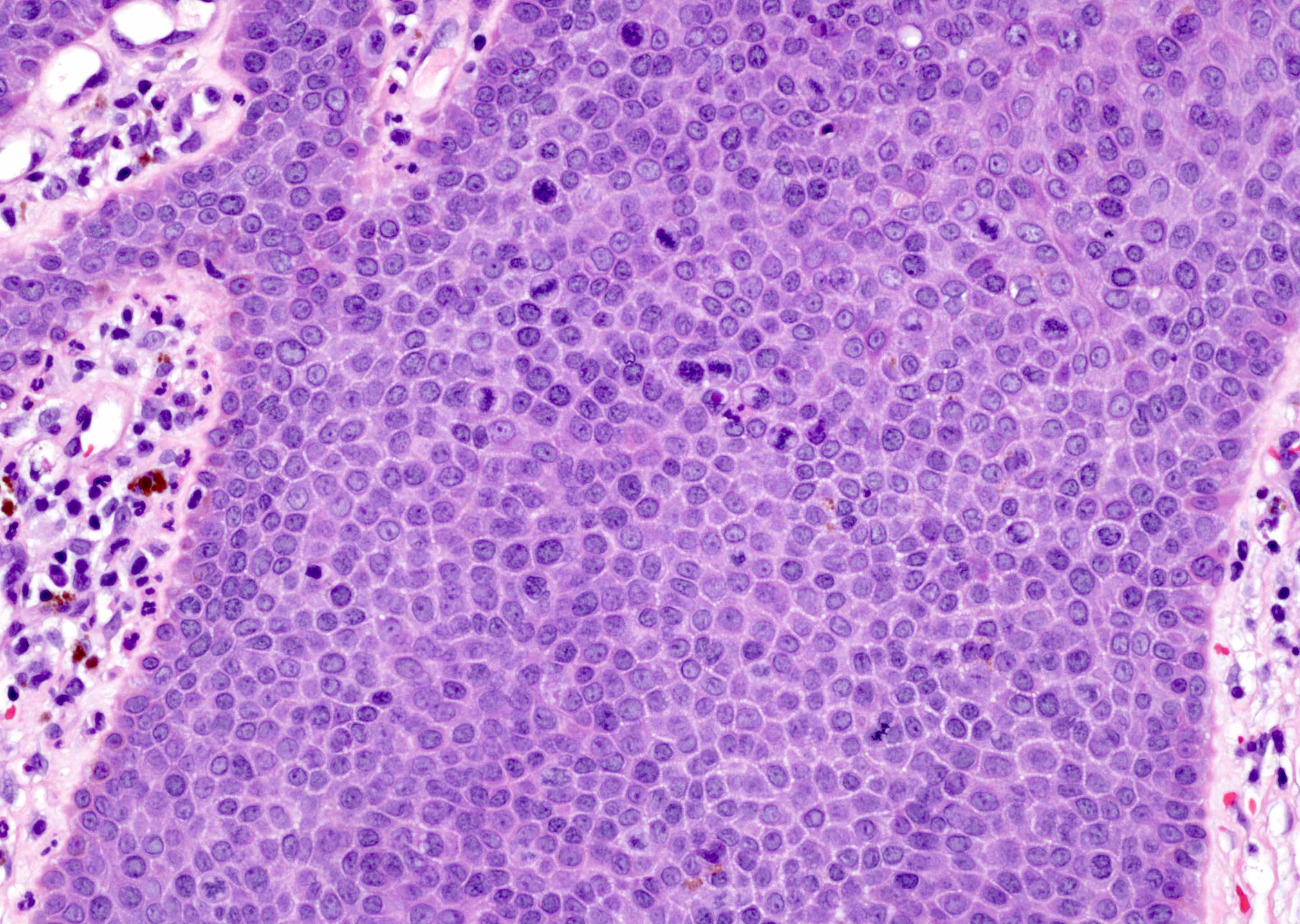
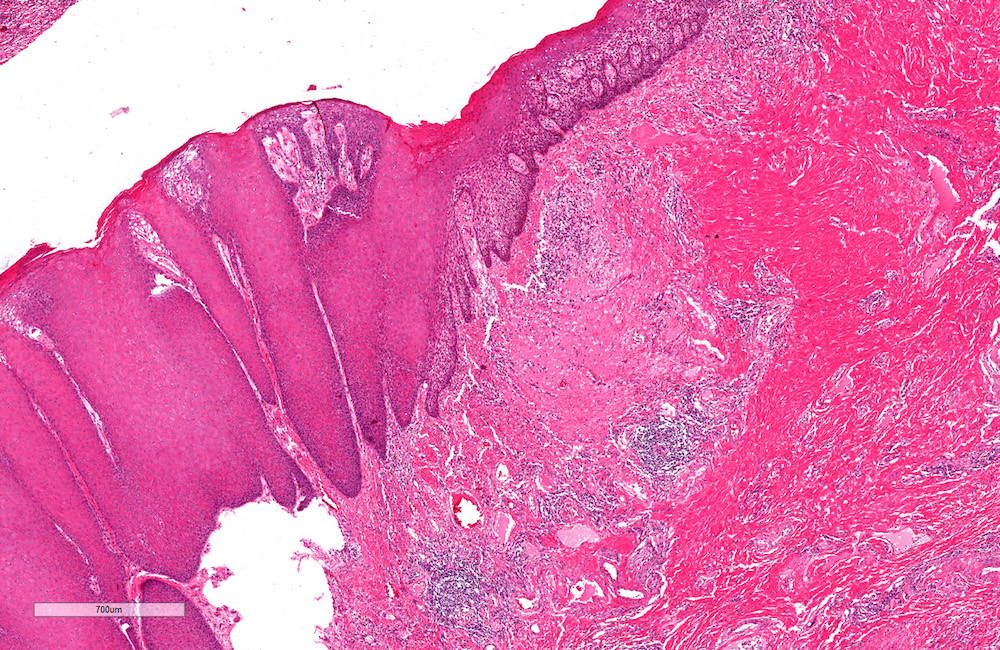
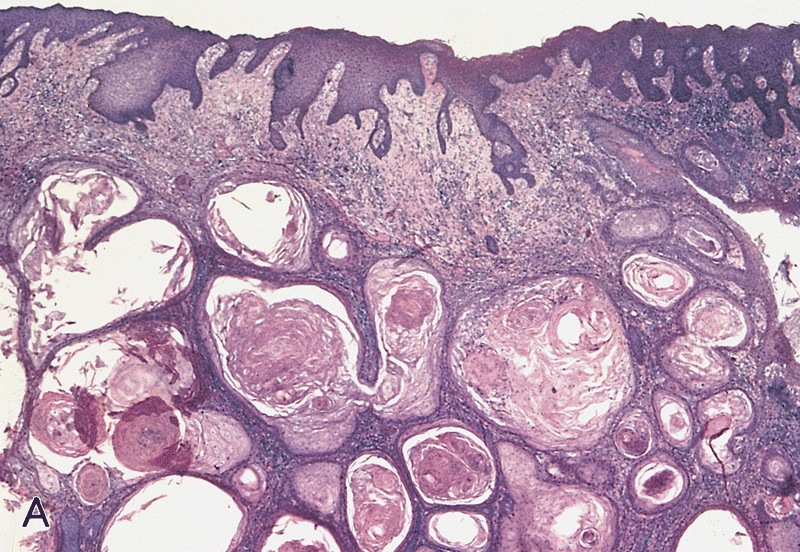
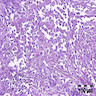
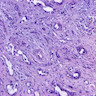
A. Bulbourethral (Cowper) glands. Bulbourethral (Cowper) glands are shown, which are near the prostatic apex and usually in close proximity to skeletal muscle. These are a mixture of mucinous glands with bland flattened nuclei and occasional nonmucinous glands / ducts in which the multilayered nature of the epithelium is easier to appreciate. Bulbourethral (Cowper) glands mimic prostatic adenocarcinoma. In particular, the mucinous glands overlap in morphology with foamy prostatic adenocarcinoma. In difficult cases, immunostains can be performed in which adenocarcinoma will lack basal cells and have expression of AMACR, while bulbourethral (Cowper) glands will have patchy basal cells and have weak to no AMACR staining.
Answer B is incorrect because fossa navicularis is a part of the penile urethra, lined by squamous epithelium. Answer C is incorrect because prostatic adenocarcinoma, atrophic type is a type of carcinoma that mimics atrophy. Answer D is incorrect because prostatic adenocarcinoma, foamy type is a type of carcinoma that mimics bulbourethral glands. Prostatic adenocarcinoma, foamy type will not have the bland duct structures mixed with mucinous glands and will not be exclusively found near skeletal muscle.
Comment Here
Reference: Anatomy & histology-male urethra
Answer B is incorrect because fossa navicularis is a part of the penile urethra, lined by squamous epithelium. Answer C is incorrect because prostatic adenocarcinoma, atrophic type is a type of carcinoma that mimics atrophy. Answer D is incorrect because prostatic adenocarcinoma, foamy type is a type of carcinoma that mimics bulbourethral glands. Prostatic adenocarcinoma, foamy type will not have the bland duct structures mixed with mucinous glands and will not be exclusively found near skeletal muscle.
Comment Here
Reference: Anatomy & histology-male urethra
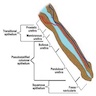
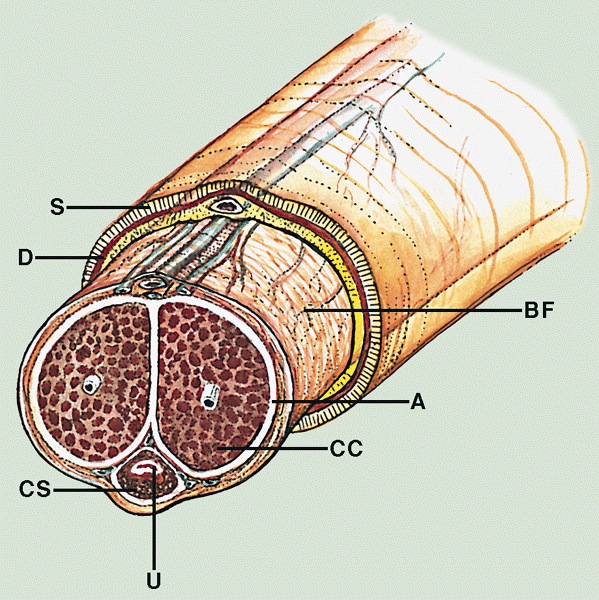
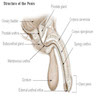
Anatomy & histology-penis
Table of Contents
Definition / general | Essential features | Terminology | Physiology | Diagrams / tables | Clinical features | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Negative stains | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
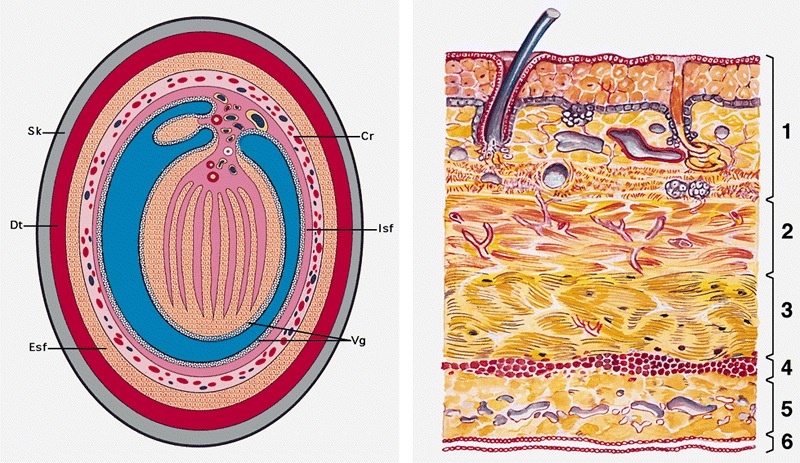
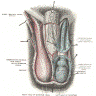
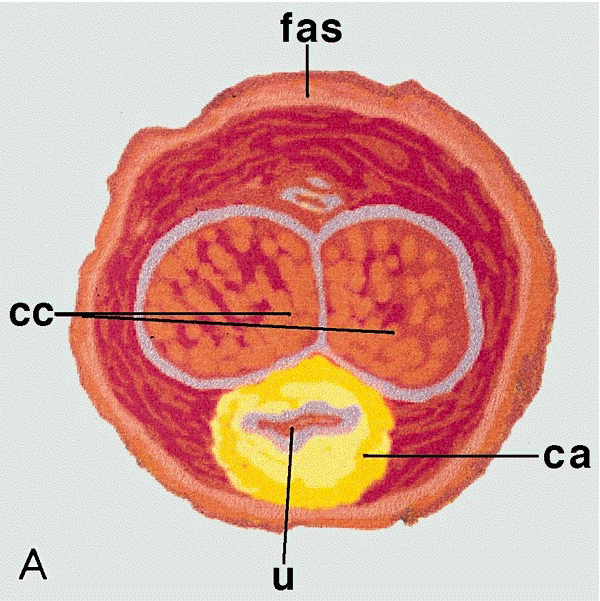
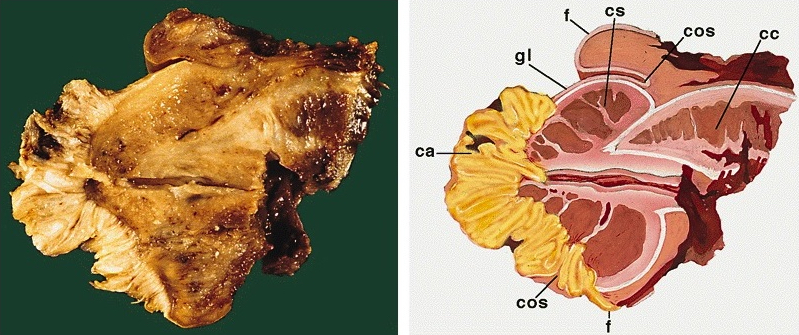
- Cylindric organ suspended from front and sides of pubic arch
- Mainly composed of erectile corpora
- Contains majority of urethra
- Orientation: the upper surface is termed dorsal, the undersurface is termed ventral
Essential features
- Glans and foreskin are the most important anatomic sections of the penis for clinical practice
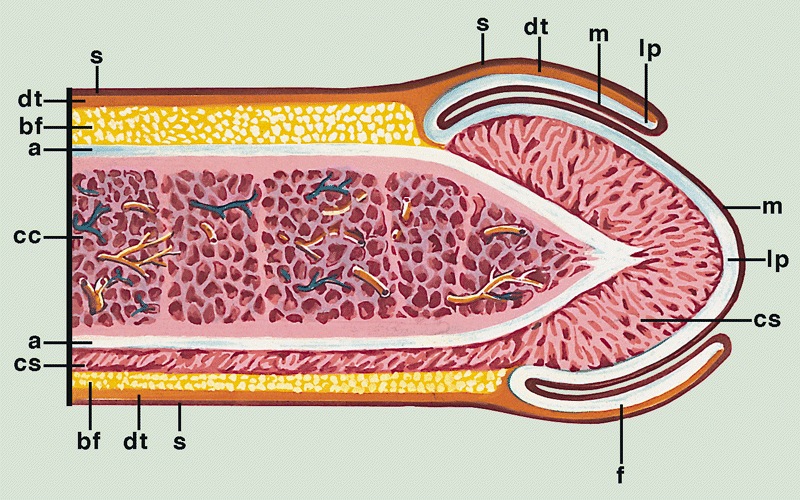
- 3 erectile tissues: 2 corpora cavernosa (dorsal), 1 corpus spongiosum (ventral)
- Foreskin lacks skin adnexa
Terminology
- There are 3 main parts
- Proximal root
- Middle body (corpus or shaft)
- Distal glans (head)
- Root
- Erectile tissues: bulb, crura
- Muscles: ischiocavernosus and bulbospongiosus
- Penile shaft / middle body
- 3 cylindrical masses of erectile tissue
- Specialized venous sinuses of variable diameter and widely interconnected
- Bound together by fibrous tunica albuginea
- Penile (Buck) fascia:
- Loose connective tissue between dartos layer and tunica albuginea
- From penile root to coronal sulcus
- Dartos layer:
- Smooth discontinuous muscle layer from homologous scrotal layer
- Throughout entire shaft between dermis and penile fascia
- Reflects itself over the coronal sulcus before continuing to foreskin (~50%)
- Continues directly to foreskin (~50%)
- Tunica albuginea:
- Dense fibrous membrane
- Encasing and separating corpora cavernosa from corpus spongiosum
- From penile root to tips of corpora cavernosa
- Slits containing small vessels, nerves and adipose tissue
- Considered route for cancer spread (Am J Surg Pathol 2017;41:1542)
- Corpora cavernosa:
- 2 lateral masses of erectile tissue that form the bulk of penis; posterior portions are called crura and connect to pubic arch
- Corpus spongiosum:
- Median ventral mass of erectile tissue
- Contains most of urethra
- Male urethra: see Anatomy & histology-male urethra
- Distal penis
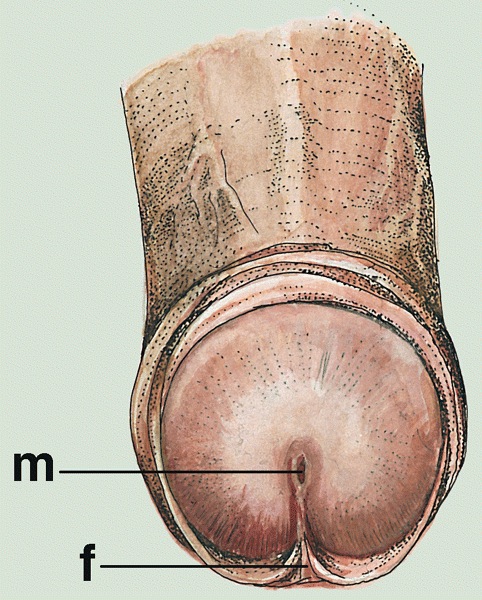
- Glans:
- Conical cup covering distal end of penile shaft
- Portion distal to coronal sulcus
- Glans corona:
- At base of glans
- Slightly elevated circumferential rim
- May contain small papillae over its free border (mistaken for Tyson glands, which are absent in humans), especially in sexually active males
- Meatus urethralis:
- Urethral opening
- Usually at central ventral glans penis
- Vertical cleft, related to frenulum (BJU Int 2007;100:161)
- Fossa navicularis:
- Terminal dilated portion of penile urethra
- Stratified, nonkeratinized squamous epithelium with clear cytoplasm
- Frenulum:
- Fibrous band of tissue attaching foreskin to ventral glans
- Coronal sulcus:
- Narrow and circumferential cul de sac (in noncircumcised) behind glans corona
- Area of insertion of dartos and Buck fascia
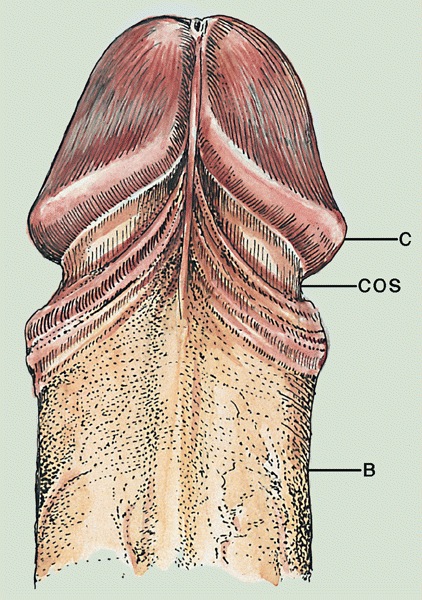
- Foreskin:
- Skin folded on itself covering the glans (clitoris in females)
- 3 types of foreskin (Am J Surg Pathol 2003;27:994)
- Long (orifice covers the glans)
- Medium (orifice is between meatus and glans corona)
- Short (orifice is between corona and sulcus)
- Layers are inner squamous epithelium, lamina propria, dartos layer and preputial skin
- Incidence of completely retractile foreskin increased from 0% at birth to 42% in adolescence
- Phimosis rate decreased with age from 99.7% to 7% (World J Pediatr 2009;5:312)
- Glans:
Physiology
- 2 main functions
- Urination
- Sexual intercourse
- Erection via parasympathetic innervation
- Muscles compression in the penis root prevent veins from draining erectile corpora
- Enlargement is obtained by erectile corpora filled and pressing against tunica albuginea
- Ejaculation via sympathetic innervation
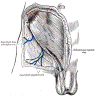
Diagrams / tables
Clinical features
- Regional lymph nodes
- Superficial inguinal nodes (site of 1 - 3 sentinel nodes)
- Deep inguinal
- External iliac
- Internal iliac (pelvic nodes)
- Periurethral glands
- Cowper (bulbourethral) glands: mucinous acinar structures deep at level of membranous urethra
- Intraepithelial glands (Morgagni lacunae): 1 layer cylindrical intraepithelial glands
- Littre glands: tubuloacinar mucinous glands present along entire length of corpus spongiosum
- Miscellaneous
- Penile glycogenated epithelial cells indicate recent vaginal intercourse (Am J Clin Pathol 1985;84:524)
- Penile swabs after recent vaginal intercourse almost always contain female cells identifiable by FISH (Arch Pathol Lab Med 2000;124:1080)
- Skin at root of penis is continuous with skin over scrotum and perineum
- Erectile tissues of corpus spongiosum are composed of straight thin muscle wall whereas those of corpus cavernosum are interanastomosed and composed of thicker muscle walls
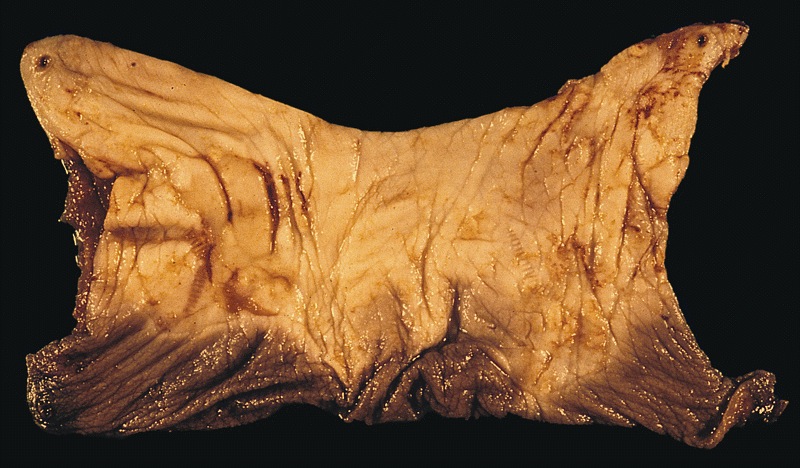
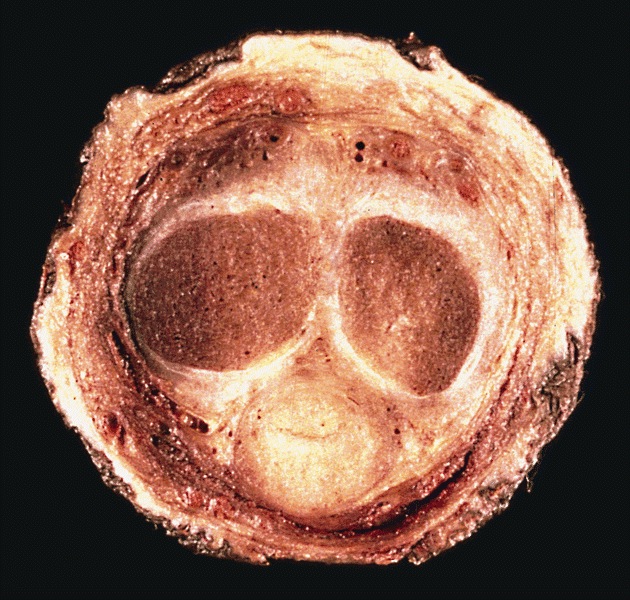
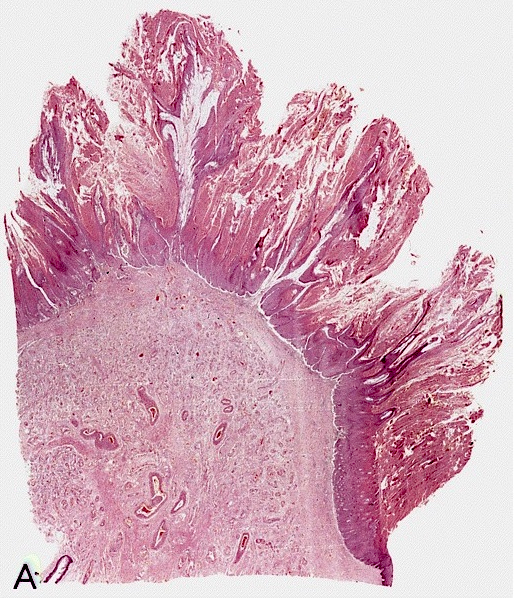
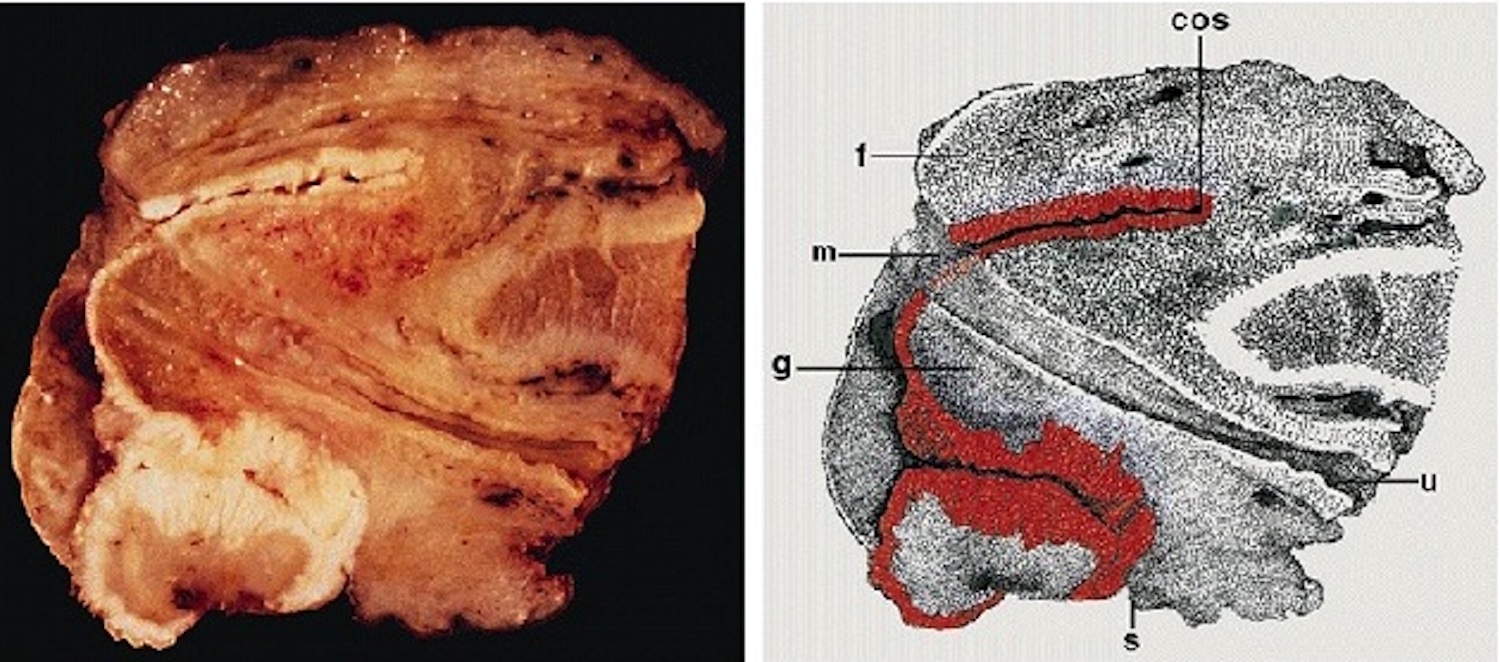
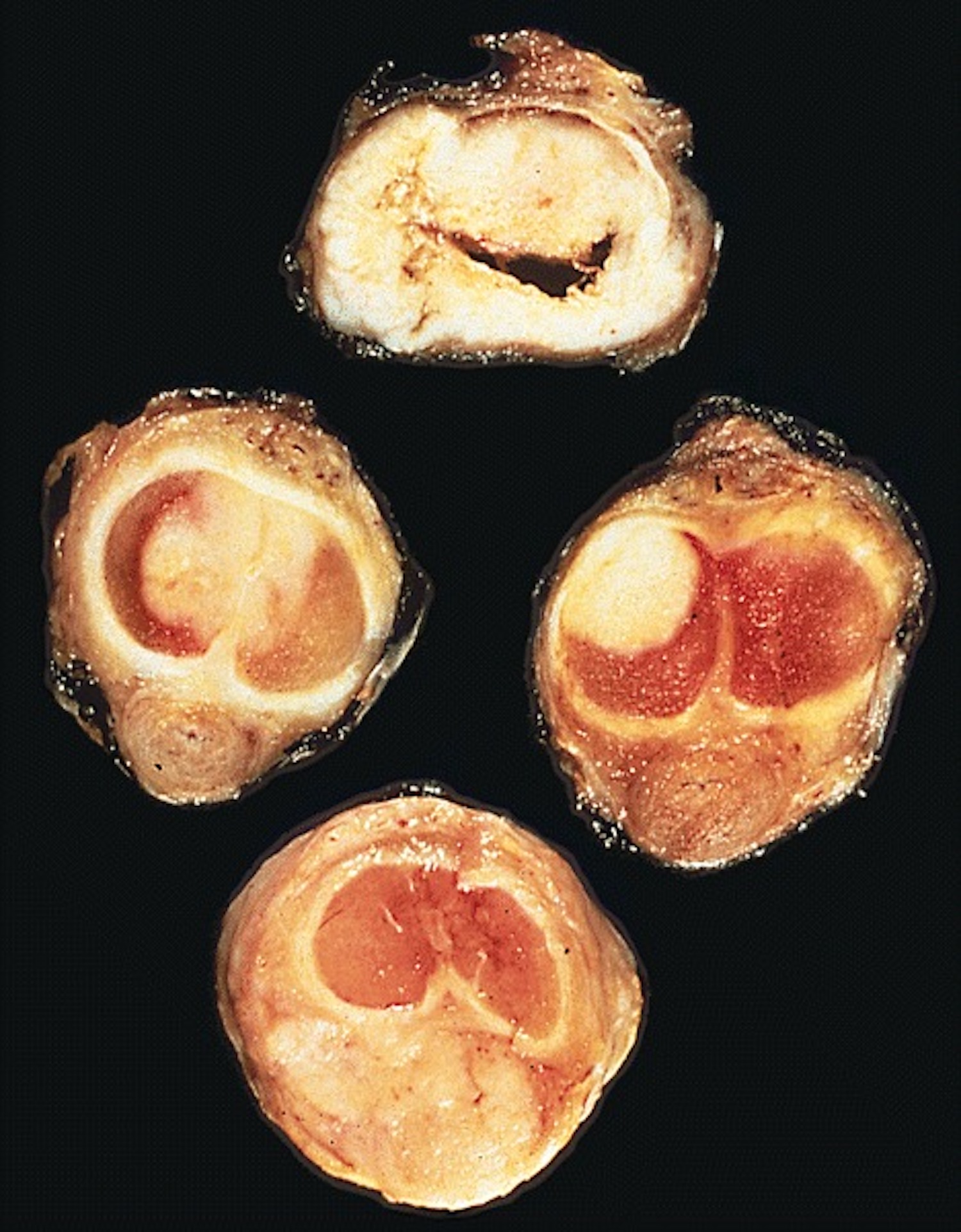
Gross description
- Transversal cut of penile shaft
- Surgical margin
- Must include skin, soft tissues and erectile tissues
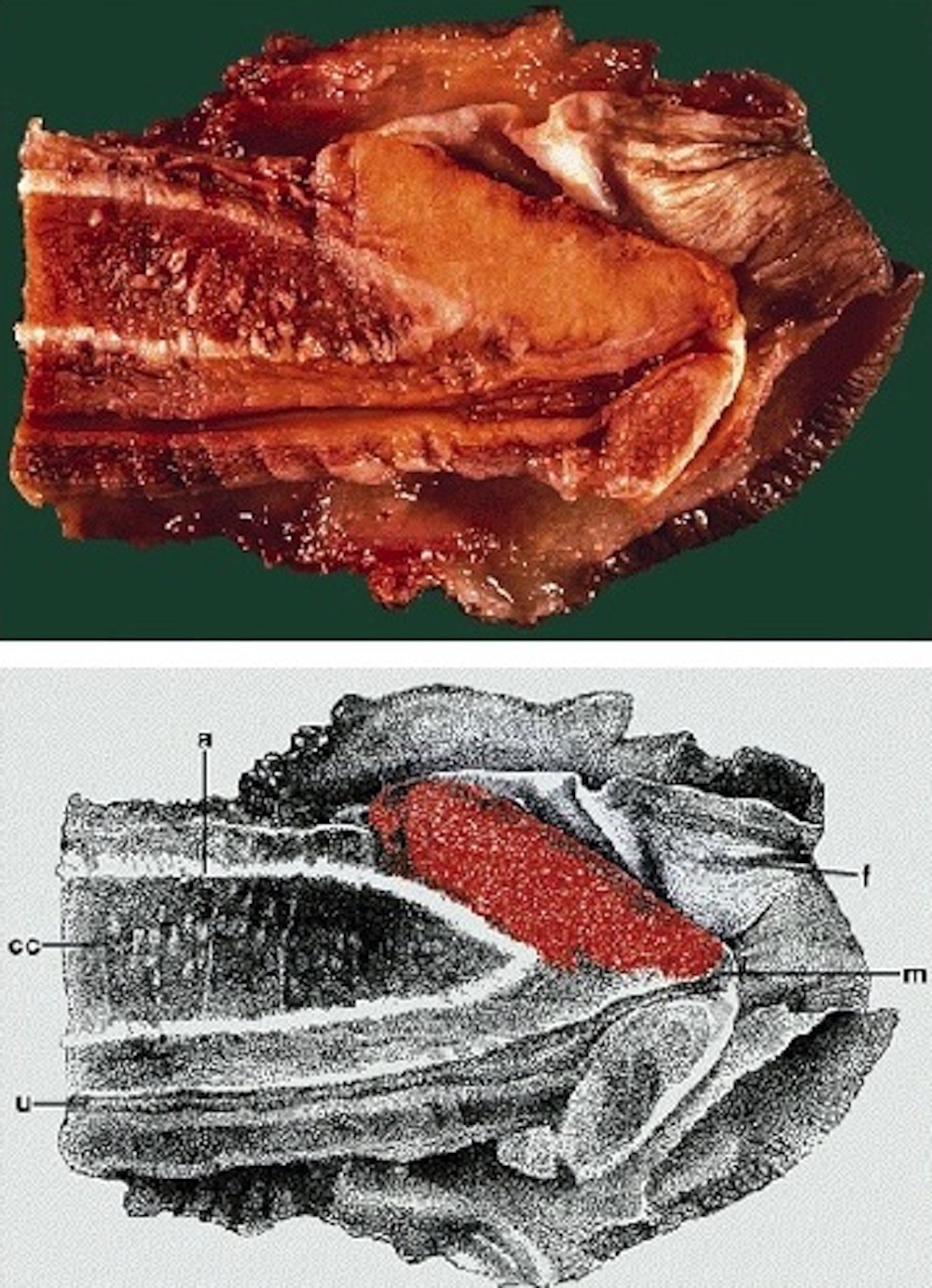
- Shaft, longitudinal cut, from surface to deep structures
- Skin
- Penile fascia and dartos
- Albuginea
- Corpora cavernosa
- Corpus spongiosum
- Urethra
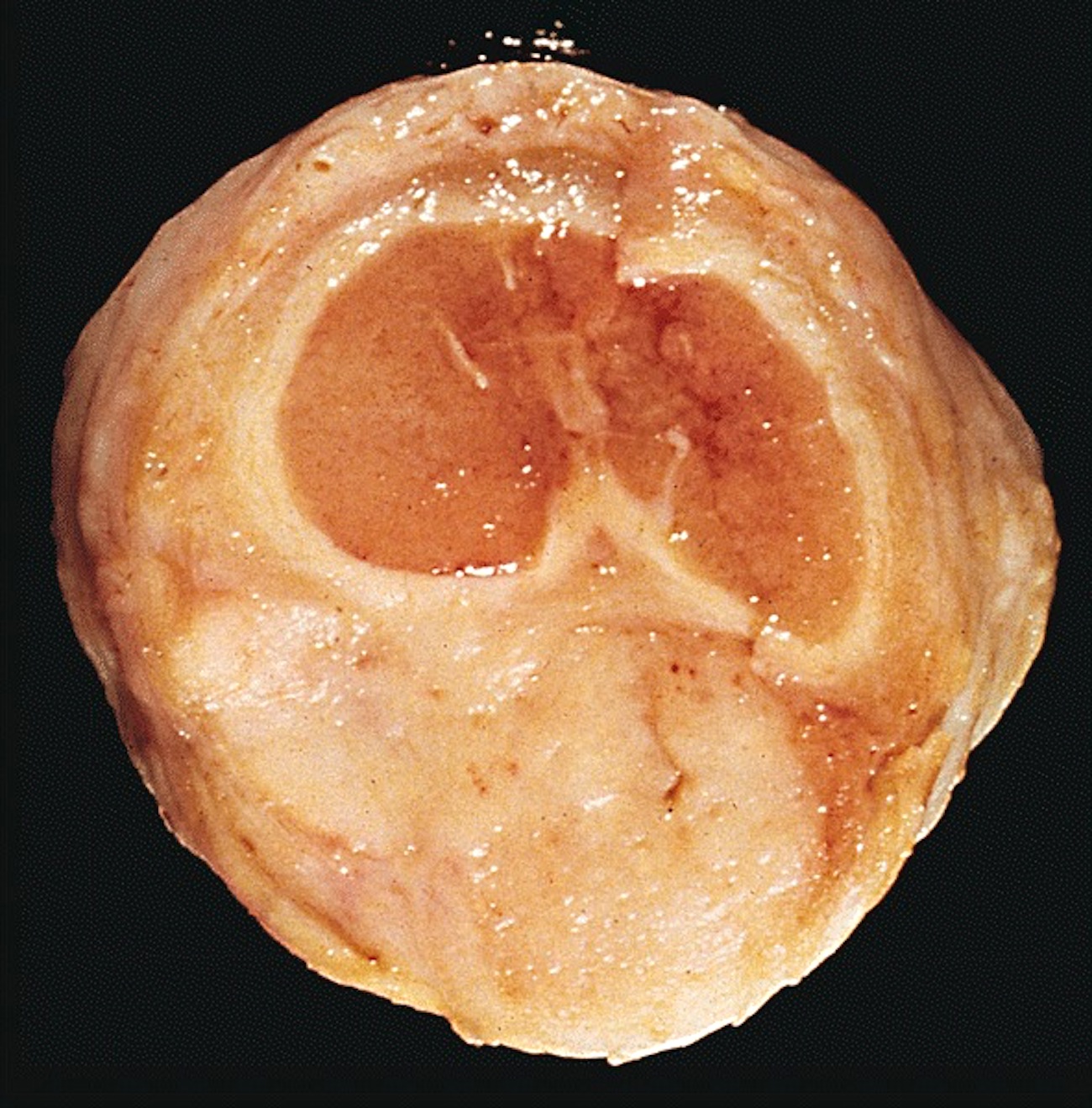
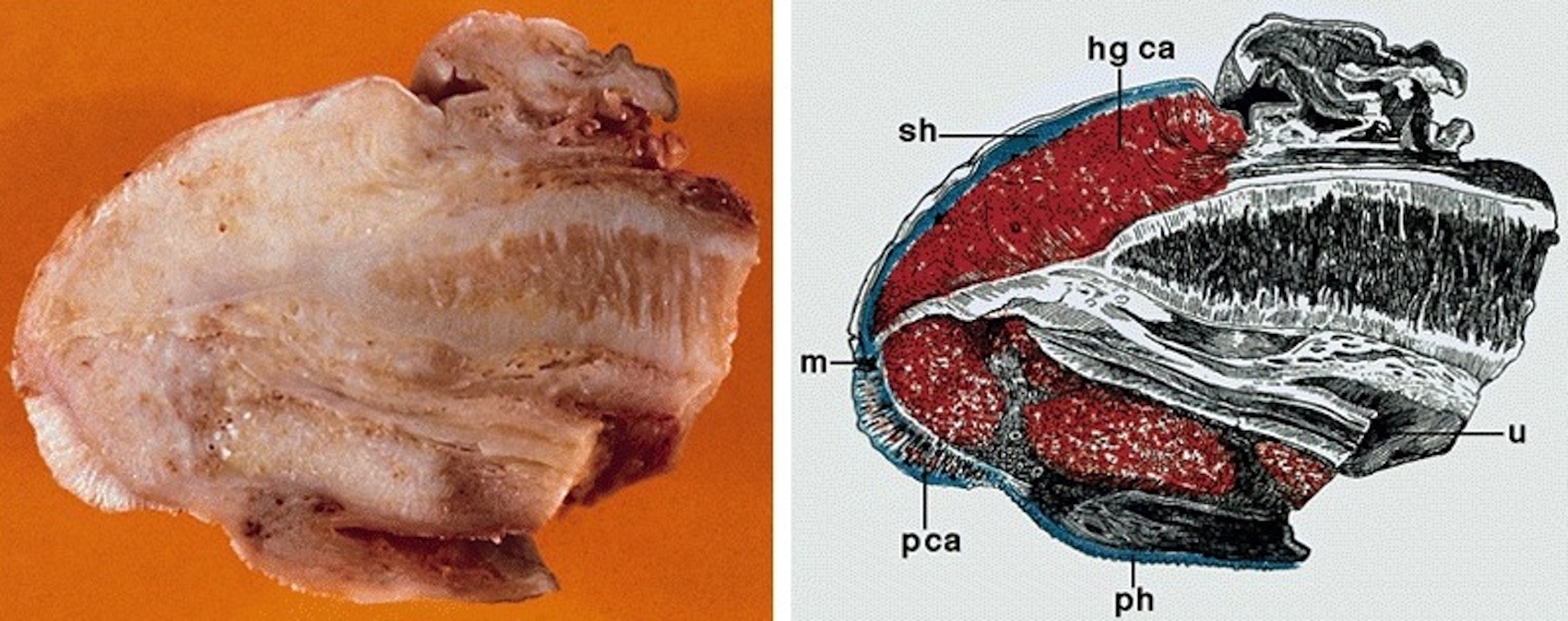
- Glans, longitudinal cut, from surface to deep structures
- Squamous mucosa and lamina propria
- Corpus spongiosum
- Urethra and meatus
- Tip of corpora cavernosa with albuginea
- Foreskin
- Anatomical position: folded with skin on the outer surface and mucosa on the inner surface
- Layers: skin, soft tissues with dartos, mucosa (Mills: Sternberg's Diagnostic Surgical Pathology, 6th Edition, 2015
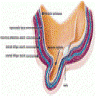
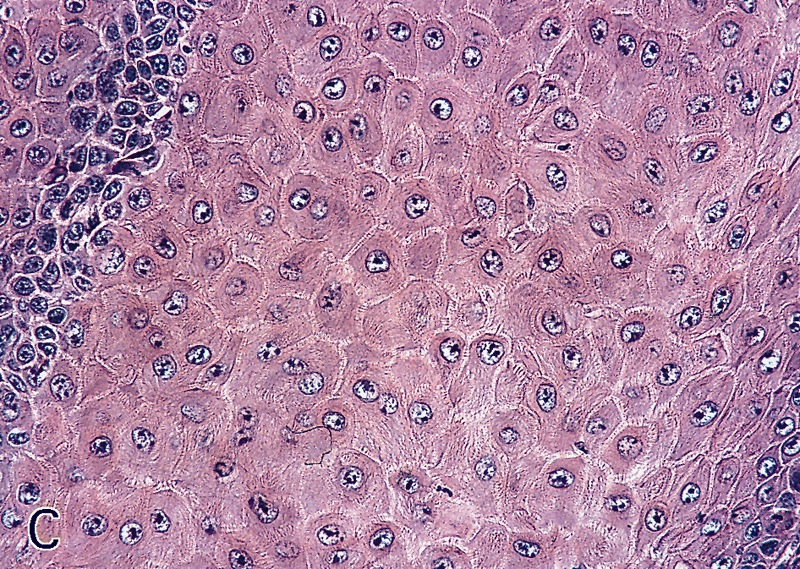
Microscopic (histologic) description
- Skin (Am J Surg Pathol 2017;41:1542, Mills: Histology for Pathologists, 5th Edition, 2019)
- Thin skin covering
- Stratified squamous keratinized epithelium
- Loosely connected to deeper parts of the organ
- Mucosa
- Stratified squamous epithelium
- Up to 10 cell thickness
- Nonkeratinizing at glans penis, keratinized after circumcision
- Lamina propria
- Loose connective tissue
- Small vessels
- Sparse lymphocytic infiltrate
- Penile fascia
- Loose connective tissue
- Small arteries, dorsal veins and nerve bundles
- Adipose tissue
- Corpora cavernosa
- Interanastomosed vascular spaces
- Thicker muscle wall
- Corpus spongiosum
- Vascular spaces
- Straight thin muscle wall
- Abundant elastic fibers
- Urethra
- Prostatic urethra: urothelium
- Membranous urethra: stratified or ciliated pseudostratified columnar epithelium
- Penile urethra: stratified or ciliated pseudostratified columnar epithelium
- Foreskin
- Inner foreskin: continues glans squamous mucosa
- Outer foreskin: squamous keratinized epithelium with no adnexa
- Loose connective tissue with discontinuous smooth muscle bundles (dartos)
Microscopic (histologic) images
Contributed by Diego F. Sanchez, M.D. and Antonio L. Cubilla, M.D.
Positive stains
- CD117: c-kit positive interstitial cells, similar to those in gut (J Sex Med 2007;4:66)
- CK7: urethral upper epithelial layer
- p63 and 34 beta E12: urethral basal layer is positive (Mills: Histology for Pathologists, 5th Edition, 2019)
- GATA3: both urethral layers (Hum Pathol 2013;44:2760)
- PSA: occasional urethral glands (Hum Pathol 2002;33:905)
- CK6: foreskin all layers (Differentiation 2018;103:86)
- CK10: foreskin suprabasal layers (Differentiation 2018;103:86)
Negative stains
- CD20: urethral superficial and basal lining (Hum Pathol 2013;44:2760)
- p63: urethral superficial lining
Additional references
Board review style question #1
Board review style answer #1
D. Smooth muscle (dartos) represented by discontinuous fascicles intermixed with connective tissue
Comment Here
Reference: Anatomy / histology of penis
Comment Here
Reference: Anatomy / histology of penis
Board review style question #2
- What is the epithelium of the penile urethra classified as?
- Pseudostratified columnar
- Simple columnar
- Simple cuboidal
- Simple squamous
- Urothelium
Board review style answer #2
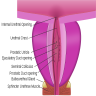
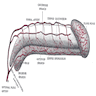
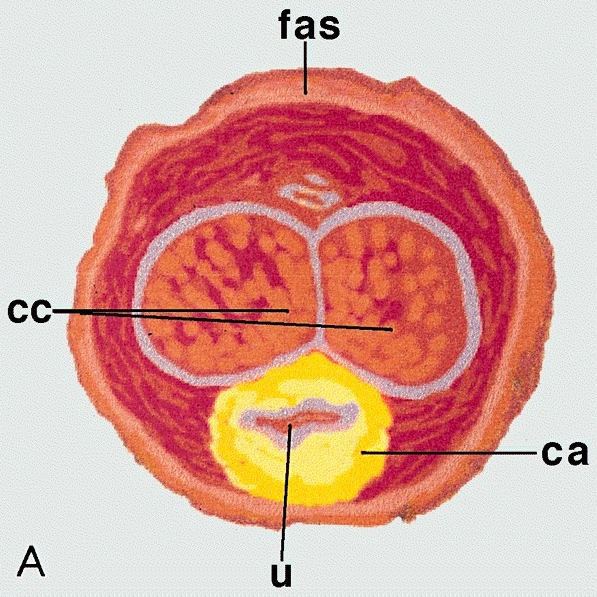
Anatomy & histology-scrotum
Table of Contents
Definition / general | Embryology | Diagrams / tables | Anatomic layers | Drawings | Clinical images | Microscopic (histologic) imagesDefinition / general
- Cutaneous fibromuscular sac containing testes, epididymis and distal spermatic cord
Embryology
- Derives from genital swellings or labioscrotal folds which enlarge and fuse in midline to form scrotal sac
- Formation is mediated by 5 alpha dihydrotestosterone
Diagrams / tables
Anatomic layers
- Skin: thin, corrugated and pigmented; includes keratinized squamous epithelium with skin adnexae, dermis and scattered adipocytes but no subcutaneous tissue; divided in half by a midline cutaneous raphe, which continues to inferior penile surface and along perineum to anus
- Dartos muscular layer: two coherent plexuses of smooth muscle cells; contracts in cold or during sexual stimulation
- External spermatic fascia (intercrural layer of Colles fascia): continuation of external oblique aponeurosis
- Cremasteric muscle (cremasteric layer of Colles fascia): bundles of skeletal muscle, continuation of internal oblique muscle
- Internal spermatic fascia (infundibuliform layer of Colles fascia): partitioned in the midline, continuation of transversalis fascia, attached to tunica vaginalis
- Parietal layer of tunica vaginalis
Clinical images
Microscopic (histologic) images
Balanitis / phimosis
Table of Contents
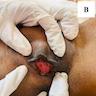
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Phimosis refers to the inability to retract the foreskin, while paraphimosis refers to foreskin trapped in the retracted position; both can be considered urologic emergencies
- Balanoposthitis refers to nonspecific inflammation of the glans (balanitis) and prepuce (posthitis), often occurring secondary to phimosis
- Zoon balanitis refers to inflammatory accumulation of plasma cells at the prepuce / glans penis
Essential features
- Balanoposthitis is inflammation of the mucosa of the glans and prepuce of the penis
- Phimosis and paraphimosis are clinical diagnoses that often occur secondary to balanoposthitis and may warrant urgent surgical attention
Terminology
- Idiopathic lymphoplasmacellular mucositis dermatitis: inflammatory accumulation of plasma cells at mucosal sites; when applied to the penis, it is referred to as Zoon balanitis (Amin: Diagnostic Pathology - Genitourinary, 3rd Edition, 2022)
- Zoon balanitis (first described by Zoon in 1952) is also called plasma cell balanitis or balanitis circumscripta plasmacellularis
ICD coding
Epidemiology
- Phimosis
- Can occur in uncircumcised men of any age (Maclennan: Urologic Surgical Pathology, 4th Edition, 2019)
- Note that in children under the age of 5, the foreskin is not retractable
- Balanoposthitis
- Most common inflammatory disease of penis
- Most affected population of infectious etiology is uncircumcised male patients, including newborns (Kradin: Diagnostic Pathology of Infectious Disease, 2nd Edition, 2017)
- Prevalence of ~35% is seen in uncircumcised male patients with diabetes mellitus (Int J Prev Med 2017;8:32)
- Zoon balanitis
- Third decade onward in uncircumcised men (Amin: Diagnostic Pathology - Genitourinary, 3rd Edition, 2022)
Sites
- Penis: glans penis, prepuce
Pathophysiology
- Phimosis may be the result of recurrent infections (such as balanoposthitis) causing scarring of the preputial ring (Kumar: Robbins & Cotran Pathologic Basis of Disease, 10th Edition, 2020)
- Usually results from an inflammatory reaction to accumulated smegma (Weidner: Modern Surgical Pathology, 2nd Edition, 2009)
- Smegma refers to the accumulation of desquamated epithelial cells, sweat and debris (Kumar: Robbins & Cotran Pathologic Basis of Disease, 10th Edition, 2020)
- Bacteria adhere and colonize the mucosal surface of the prepuce (Kradin: Diagnostic Pathology of Infectious Disease, 2nd Edition, 2017)
Etiology
- Phimosis: may be congenital or acquired
- Congenital
- Anomalous development
- Small preputial orifice
- Abnormally long foreskin (Amin: Diagnostic Pathology - Genitourinary, 3rd Edition, 2022)
- Anomalous development
- Acquired
- Recurrent infections, such as balanitis (Kumar: Robbins & Cotran Pathologic Basis of Disease, 10th Edition, 2020)
- Congenital
- Paraphimosis
- Often iatrogenic, after examination or instrumentation
- Could be caused by malaria (Plasmodium falciparum) or metastatic carcinoma
- Balanoposthitis
- Most commonly in uncircumcised men (Maclennan: Urologic Surgical Pathology, 4th Edition, 2019)
- Risk factors
- Lack of circumcision, diabetes, poor hygiene, buildup of smegma, tight foreskin (Int J Dermatol 2009;48:121)
- Dermatologic conditions
- Contact dermatitis (Weidner: Modern Surgical Pathology, 2nd Edition, 2009)
- Inflammatory dermatoses (Int J Dermatol 2022;61:1467)
- Infection
- Candida balanitis
- Accounts for 30 - 35% of infectious cases (Int J Dermatol 2009;48:121)
- Aerobic bacteria
- Staphylococcus aureus and group A Streptococcus (Int J Dermatol 2009;48:121)
- Gardnerella, uncommonly (Kradin: Diagnostic Pathology of Infectious Disease, 2nd Edition, 2017)
- Anaerobic bacteria
- Viruses
- Parasites
- Trichomonas, uncommonly (Kradin: Diagnostic Pathology of Infectious Disease, 2nd Edition, 2017)
- Trauma (Partin: Campbell-Walsh Urology 12th Edition Review, 3rd Edition, 2020)
- Candida balanitis
- Zoon balanitis
- Lack of circumcision (Maclennan: Urologic Surgical Pathology, 4th Edition, 2019)
- Poor hygiene, warmth, rubbing (Amin: Diagnostic Pathology - Genitourinary, 3rd Edition, 2022)
Clinical features
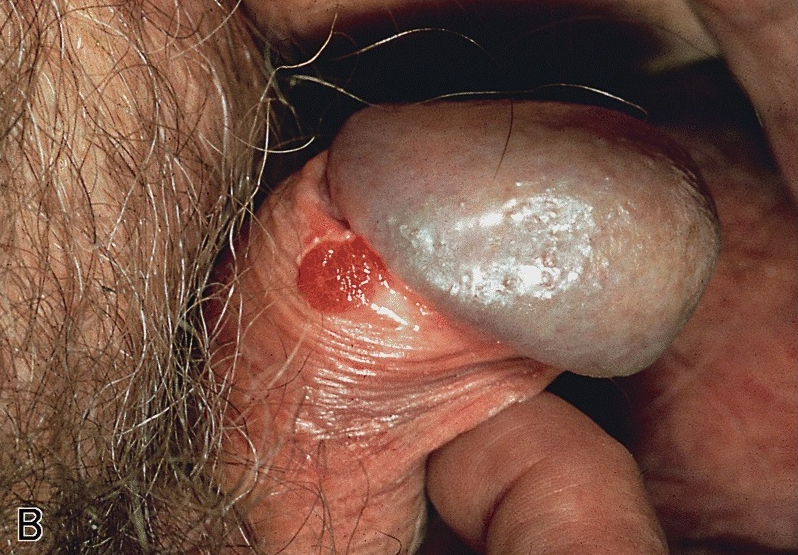
- Phimosis
- Irritation and pain if prolonged
- Paraphimosis
- Substantial pain and penile swelling (Can Fam Physician 2007;53:445)
- Balanoposthitis
- Erythematous rash, pruritus
- Tenderness and pain
- May be associated with ulceration
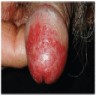
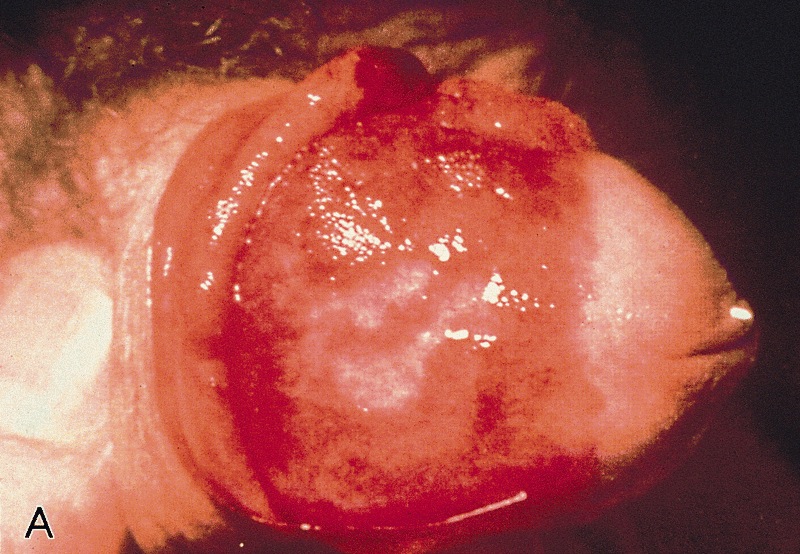
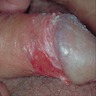
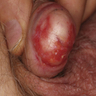
- Zoon balanitis
- Usually single, large, bright red, moist patch on the glans penis or inner prepuce
- May be asymptomatic or associated with dysuria
- Rarely manifests with multiple patches
- Erosions, in severe cases
- References: Maclennan: Urologic Surgical Pathology, 4th Edition, 2019, Amin: Diagnostic Pathology - Genitourinary, 3rd Edition, 2022
Diagnosis
- Phimosis
- Physical examination can be used to differentiate between pathologic and physiologic phimosis (Can Fam Physician 2007;53:445)
- No diagnostic tests required (Emerg Med Clin North Am 2011;29:655)
- Balanoposthitis
- Diagnosis is made based on physical examination with histologic confirmation
- Clinical features are nonspecific and suggestive but should not be considered pathognomonic
- Biopsy can be used to exclude premalignant lesions, which can clinically mimic balanitis
- Sexual history and dermatologic consult may aid in diagnosis
- References: Int J Dermatol 2022;61:1467, J Eur Acad Dermatol Venereol 2023;37:1104
Laboratory
- Phimosis
- Laboratory tests are not indicated or useful in diagnosing (Emerg Med Clin North Am 2011;29:655)
- Balanoposthitis
- Laboratory tests can be useful to determine the causative agent in uncertain cases (J Eur Acad Dermatol Venereol 2023;37:1104)
- Sexually transmitted disease (STD) screening
- Candida swab
- Bacterial cultures
- Urine glucose
- Laboratory tests can be useful to determine the causative agent in uncertain cases (J Eur Acad Dermatol Venereol 2023;37:1104)
Prognostic factors
- Phimosis
- Often coexists with penile carcinoma and is a risk factor for it (Maclennan: Urologic Surgical Pathology, 4th Edition, 2019)
- Balanoposthitis
- Benign, treatable condition
Case reports
- 31 year old man with foreskin pain and swelling (IDCases 2020;21:e00832)
- 44 year old man with discrete polyp on glans penis (Urol Case Rep 2022;45:102262)
- 45 year old circumcised man with recurrent balanoposthitis (Access Microbiol 2023;5:000582)
- 69 year old man with narrowing foreskin after immunotherapy (Urol Case Rep 2020;33:101350)
Treatment
- Phimosis
- Physiologic phimosis
- Reassurance, routine cleaning and close follow up may be sufficient in young children
- Topical corticosteroids can help hasten the process (Can Fam Physician 2007;53:445)
- Pathologic phimosis
- Circumcision, regardless of causative factor (Maclennan: Urologic Surgical Pathology, 4th Edition, 2019)
- Physiologic phimosis
- Paraphimosis
- Often warrants circumcision or emergency dorsal slit surgery (Maclennan: Urologic Surgical Pathology, 4th Edition, 2019)
- Balanoposthitis
- Gentle cleaning of the area, multiple times a day
- Antibiotics or topical antifungals (Indian J Sex Transm Dis AIDS 2014;35:155)
- Zoon balanitis
- Circumcision is the treatment of choice
- Topical calcineurin inhibitors, mupirocin and tacrolimus have been used with variable success (Maclennan: Urologic Surgical Pathology, 4th Edition, 2019)
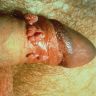
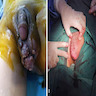
Clinical images
Gross description
- Liberal sampling of foreskin from circumcision to rule out
- Dysplasia
- Carcinoma in situ
- Early invasive carcinoma
- Reference: Amin: Diagnostic Pathology - Genitourinary, 3rd Edition, 2022
Microscopic (histologic) description
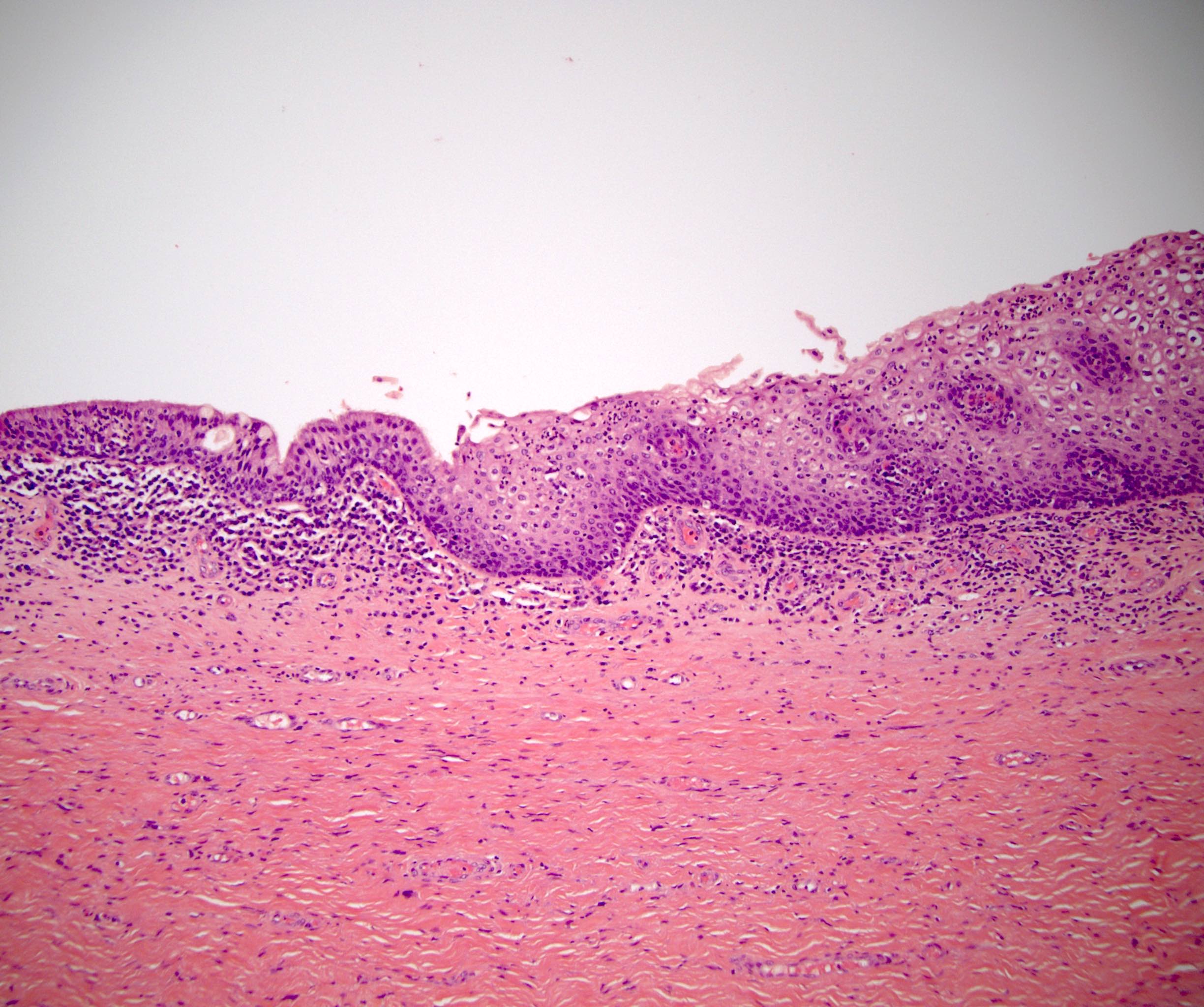
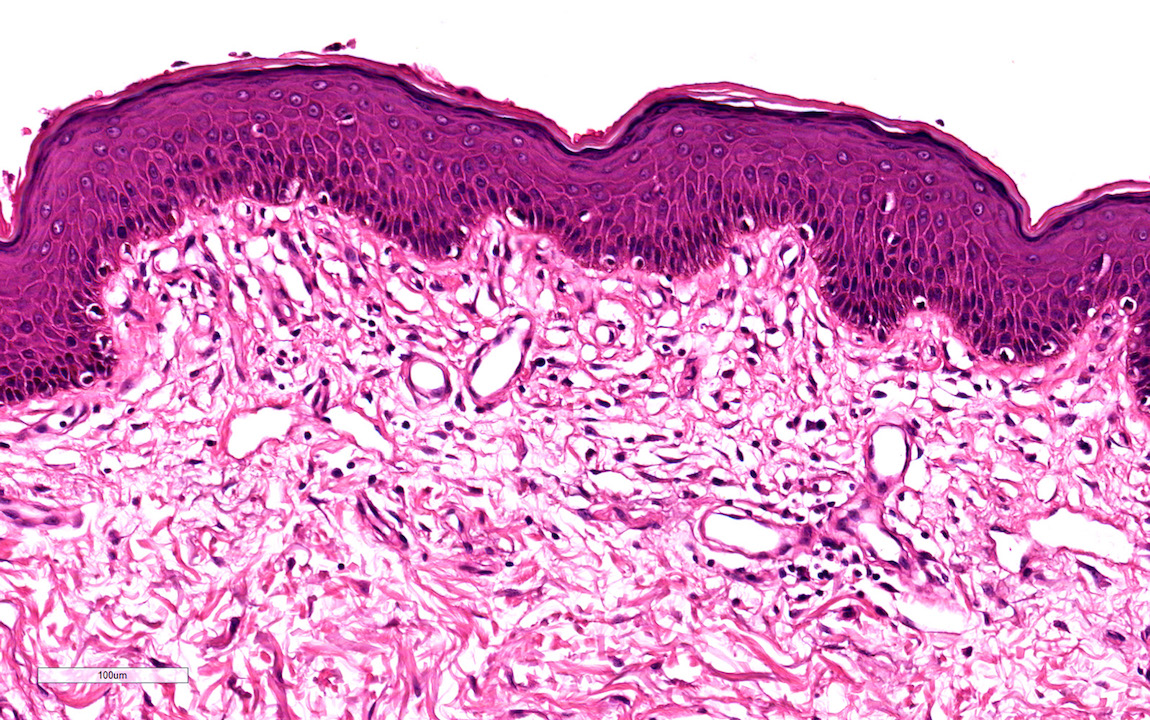
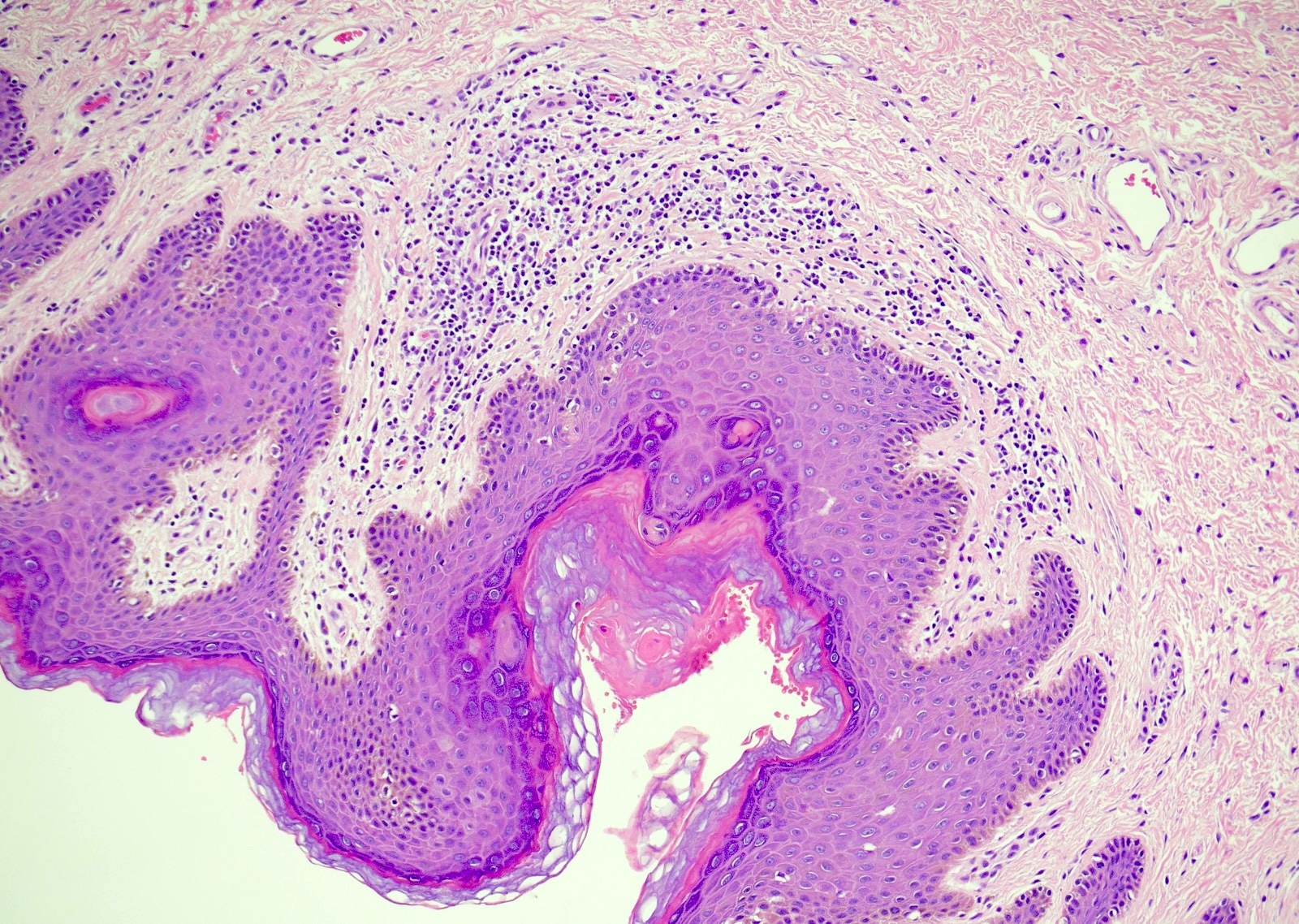
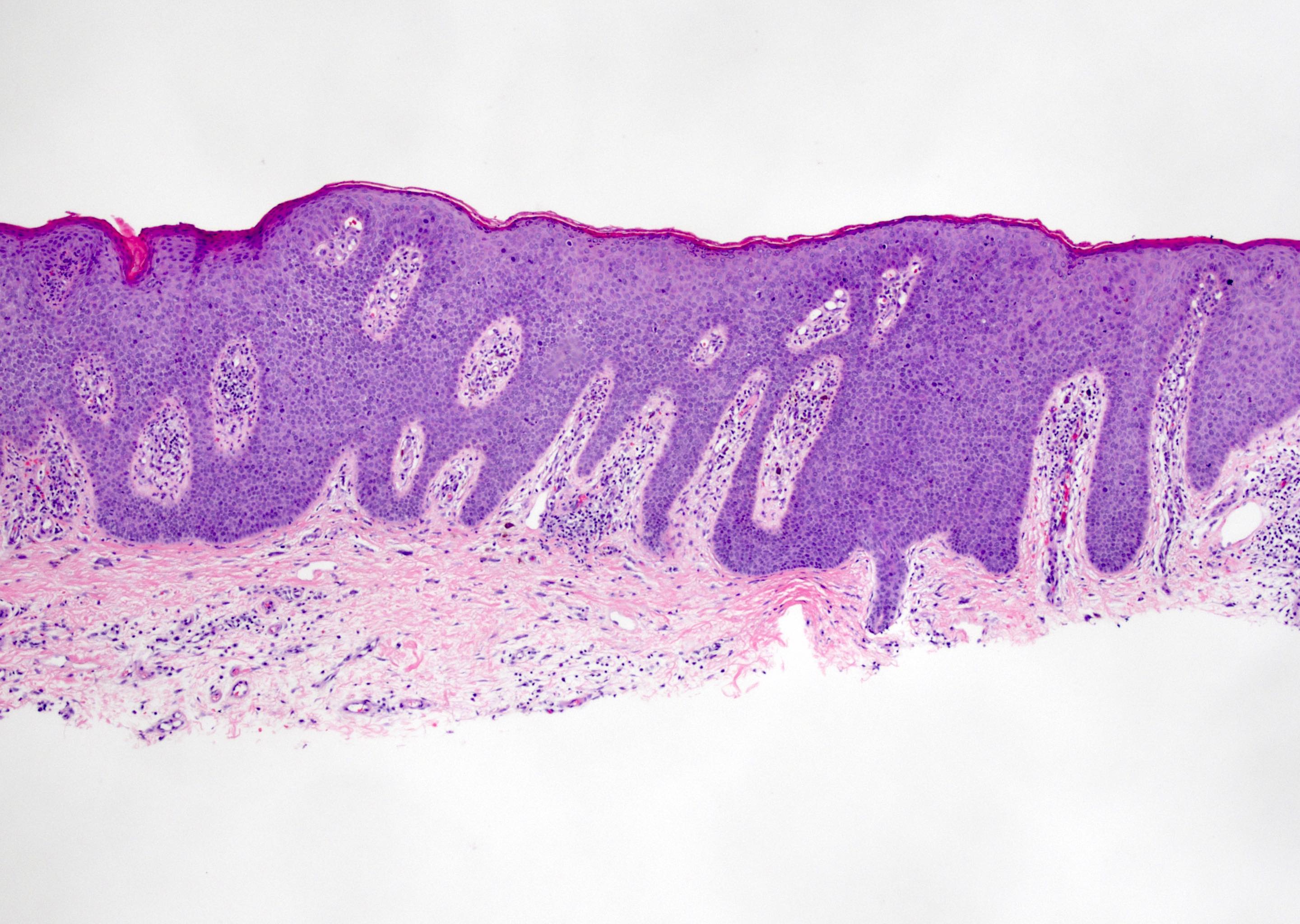
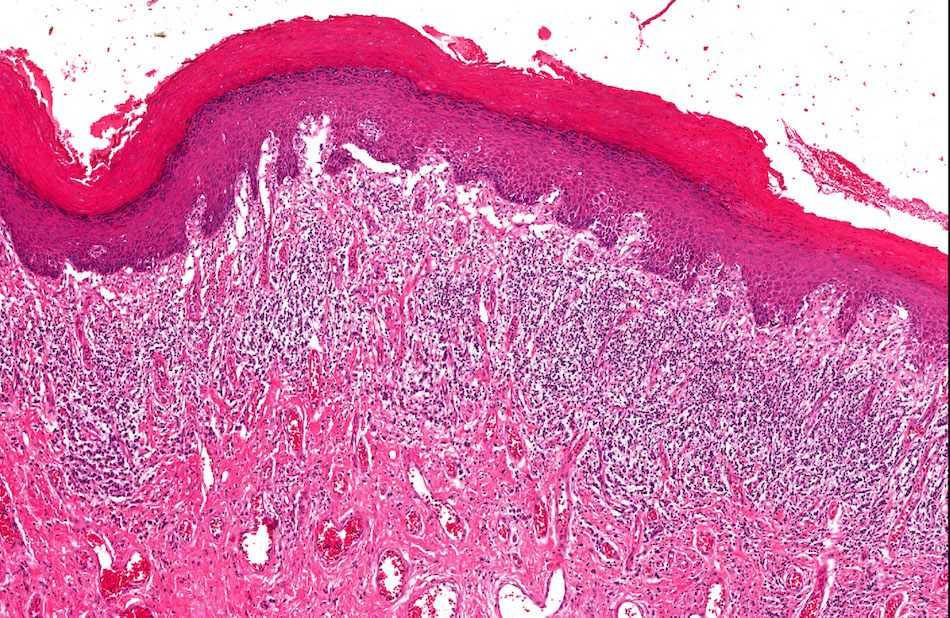
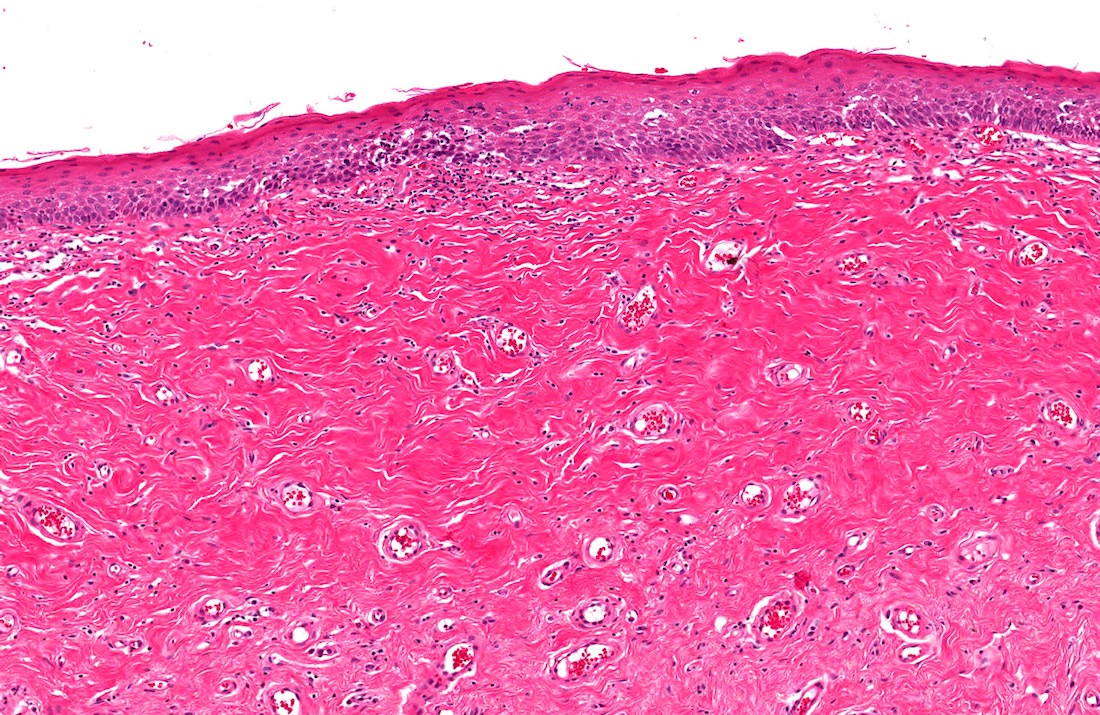
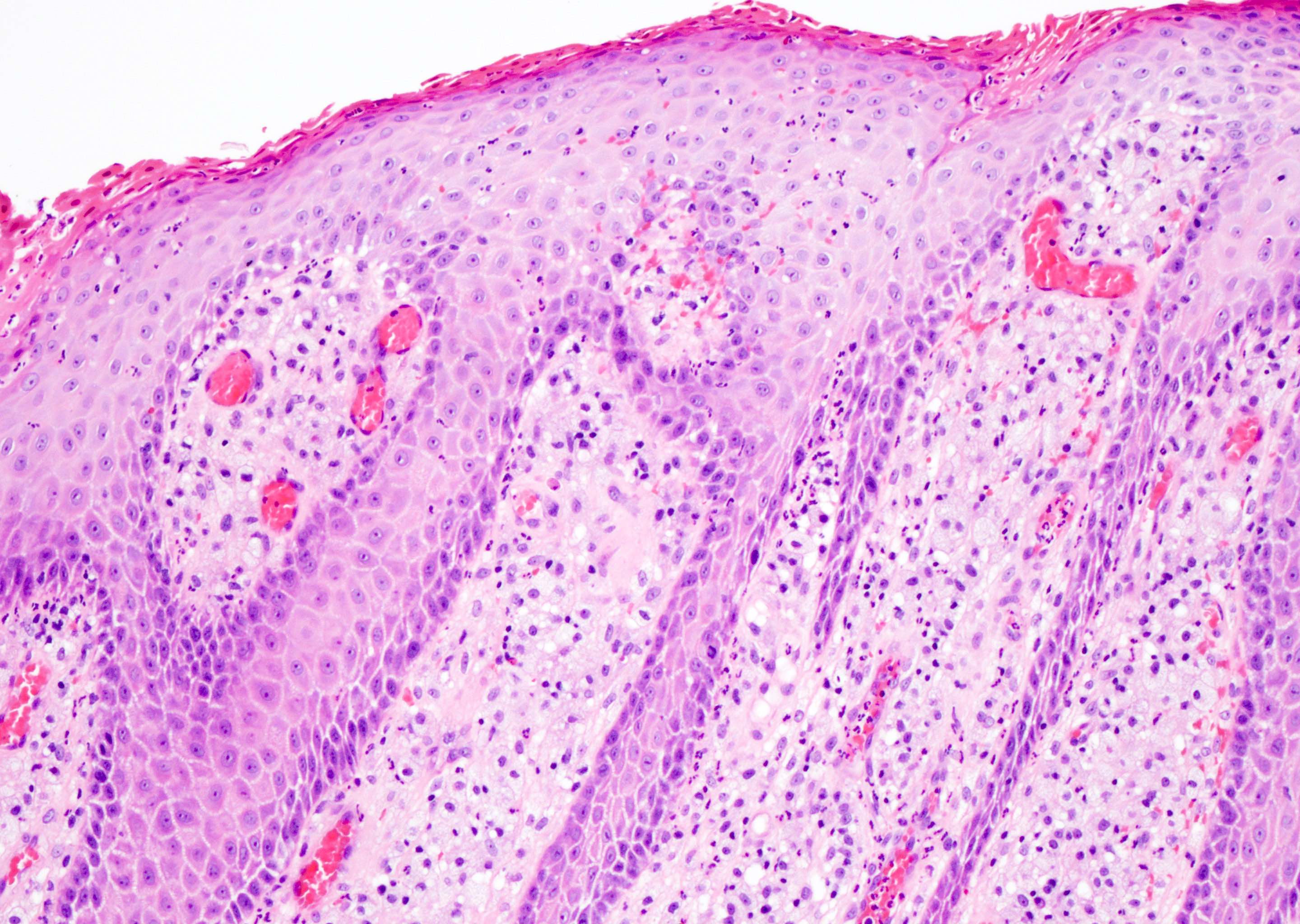
- Phimosis
- Often normal
- May show lymphocytes and plasma cells, fibrosis, edema and vascular congestion
- Balanoposthitis
- Nonspecific inflammatory infiltrate with lymphocytes and plasma cells
- Patterns of inflammation include lichenoid and intraepithelial
- Epithelial changes such as squamous hyperplasia or ulceration may be seen with inflammation
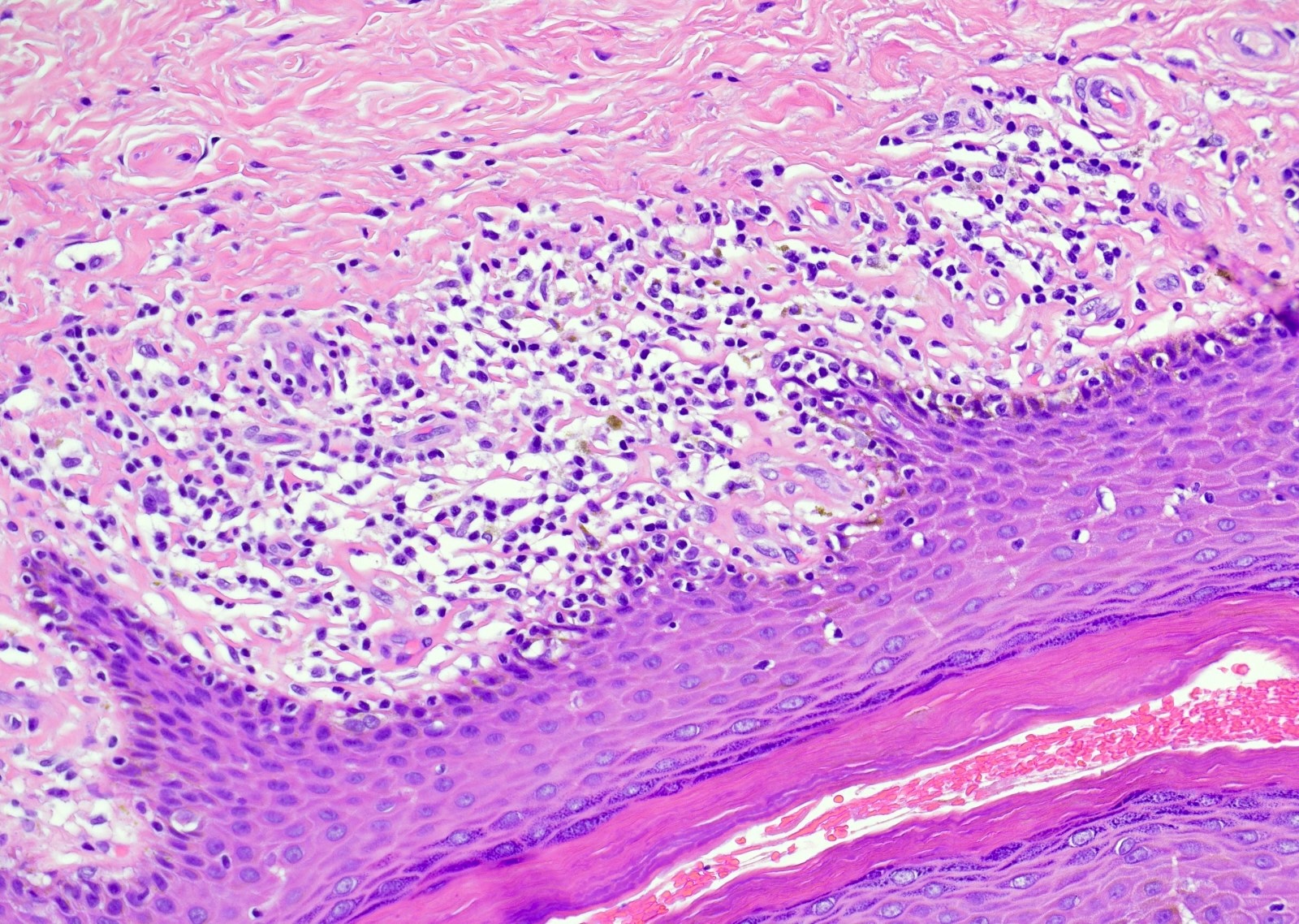
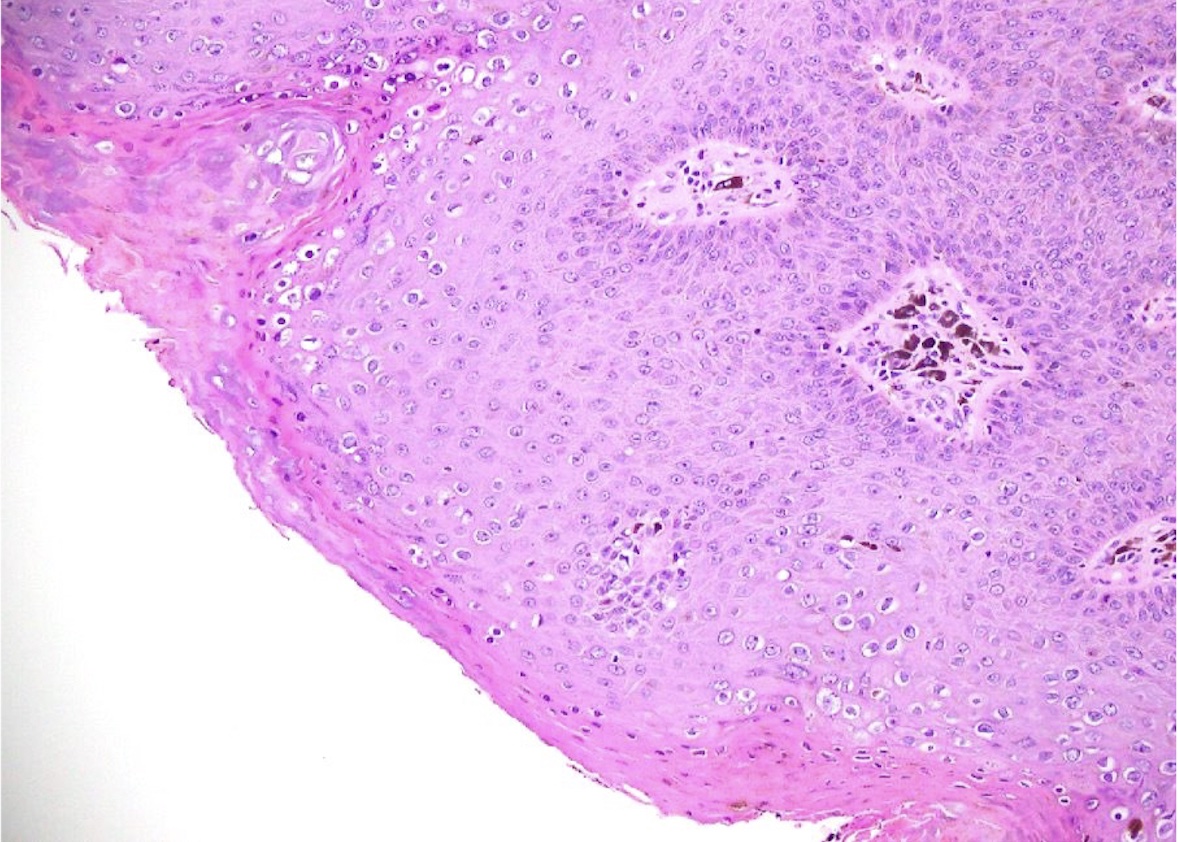
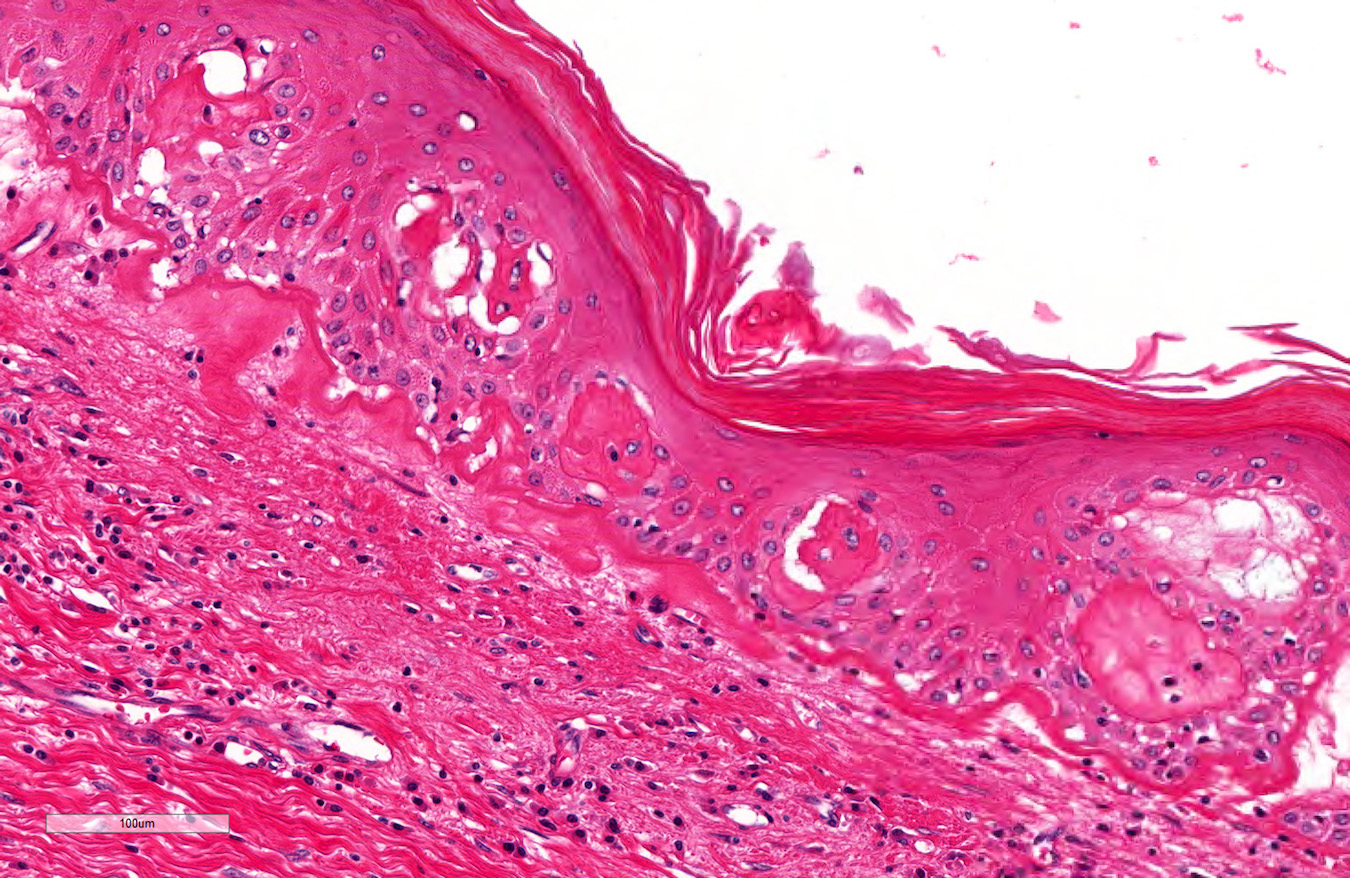
- Zoon balanitis
- Epidermis: thin with possible ulceration and flattened or diamond shaped keratinocytes with intercellular edema
- Upper dermis: band-like infiltrate containing plasma cells (variable amount)
- Dermis: dilated capillaries with adjacent extravasated red blood cells and hemosiderin deposition
- References: Maclennan: Urologic Surgical Pathology, 4th Edition, 2019, Amin: Diagnostic Pathology - Genitourinary, 3rd Edition, 2022
Microscopic (histologic) images
Positive stains
- Silver stains can help to identify spirochetes
- Zoon (plasma cell) balanitis: κ:λ ratio ~ 2:1 (polyclonal)
- Reference: Amin: Diagnostic Pathology - Genitourinary, 3rd Edition, 2022
Sample pathology report
- Foreskin, circumcision:
- Chronic balanoposthitis
- Foreskin, circumcision:
- Keratinized squamous epithelium with minimal, patchy, subepithelial nonspecific chronic inflammation (see comment)
- Comment: Findings are consistent with the clinical diagnosis of phimosis.
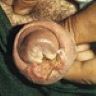
- Glans penis, biopsy:
- Subepithelial plasma cell infiltrate consistent with plasma cell balanitis (Zoon balanitis)
Differential diagnosis
- Sexually transmitted infections (STIs) - primary syphilis:
- Present with papules progressing to ulceration
- Florid lymphocytes and plasma cells
- Often intraepithelial neutrophils
- Pronounced proliferation of endothelial cells
- Psoriasiform hyperplasia as opposed to mucosal thinning
- Organisms (syphilis) may be visualized with special staining
- Cutaneous malignancies (squamous cell carcinoma in situ or extramammary Paget disease):
- Contain intraepithelial malignant cells that are not seen in balanitis
- Cutaneous plasmacytosis:
- Occurs at cutaneous not mucosal sites
- Dense homogenous infiltrate of plasma cells
- Serum electrophoresis shows monoclonal peak
- References: Amin: Diagnostic Pathology - Genitourinary, 3rd Edition, 2022, Hall: Diagnostic Pathology - Nonneoplastic Dermatopathology, 3rd Edition, 2021, Maclennan: Urologic Surgical Pathology, 4th Edition, 2019
Additional references
Board review style question #1
Board review style answer #1
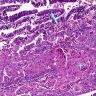
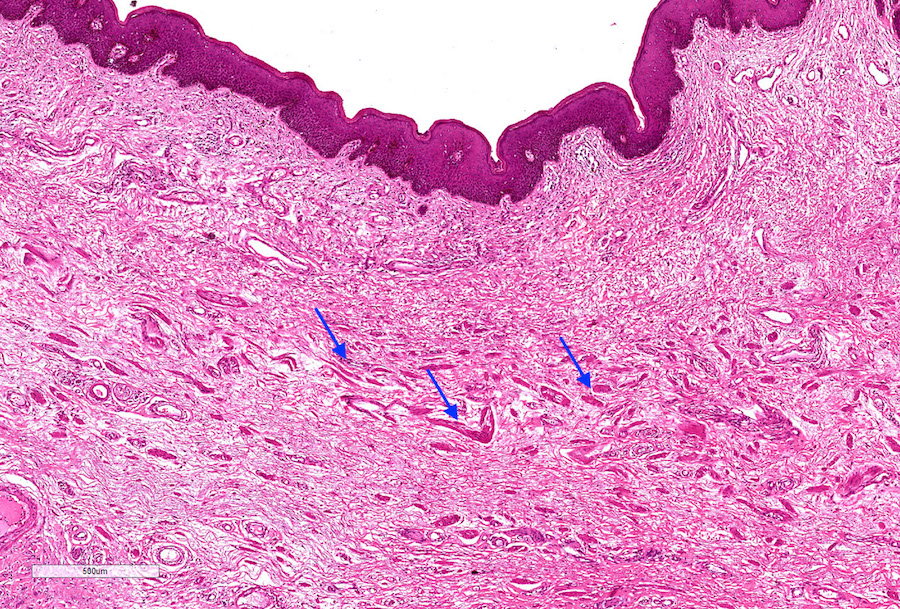
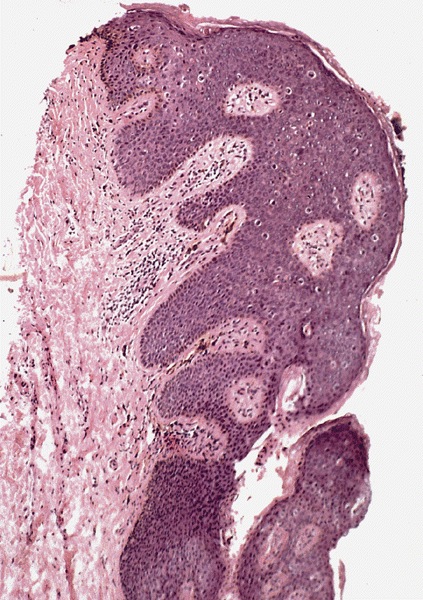
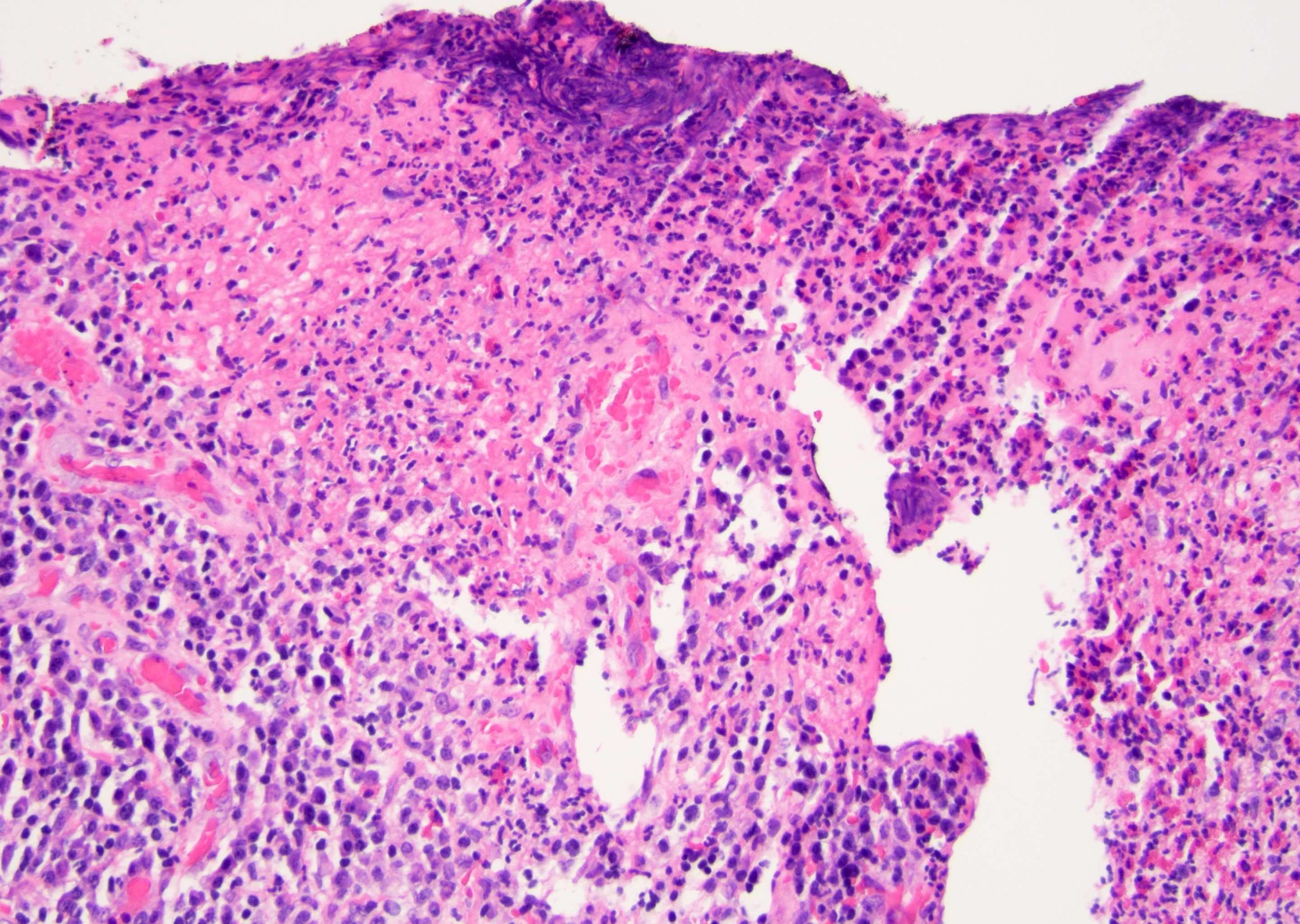
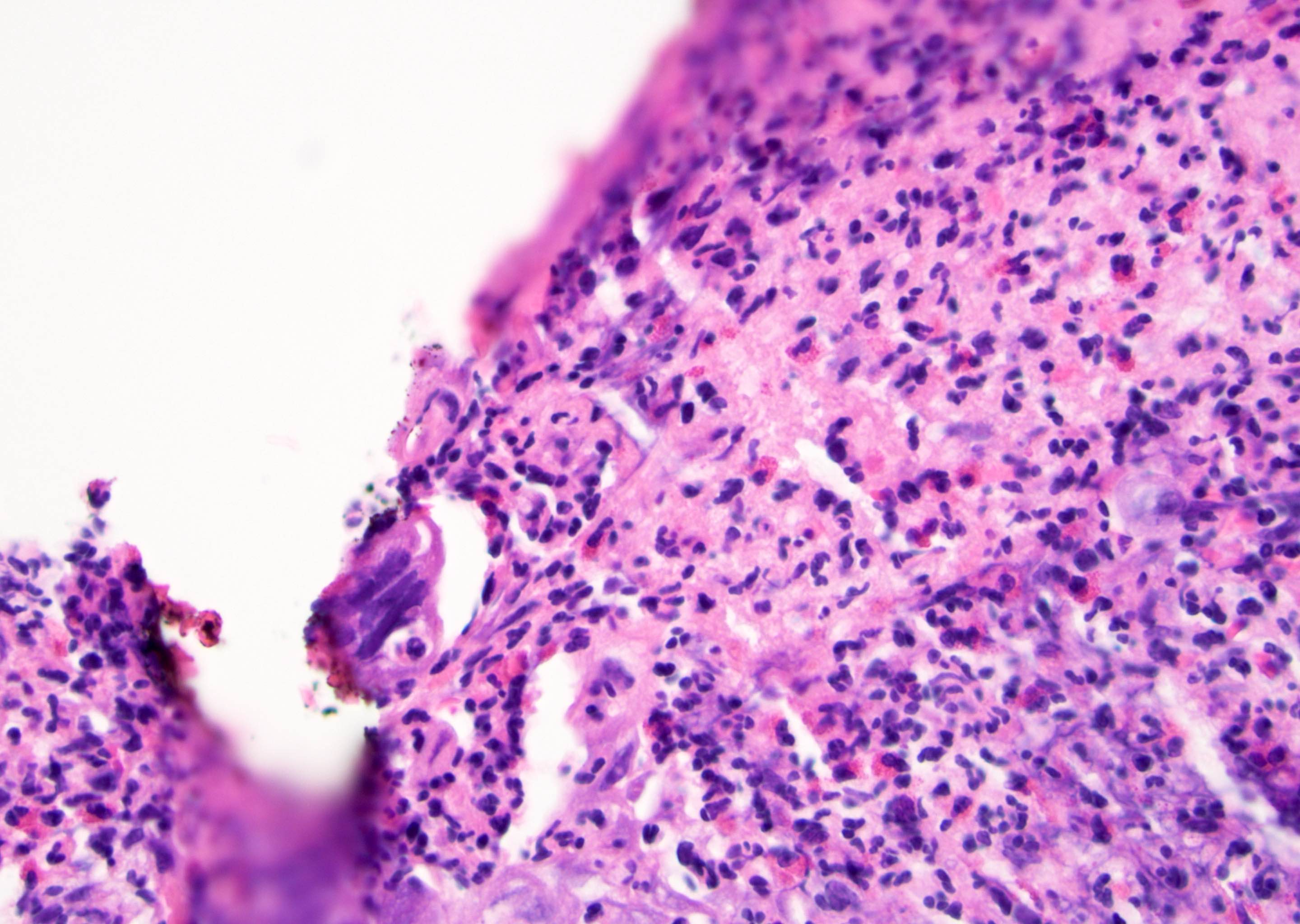
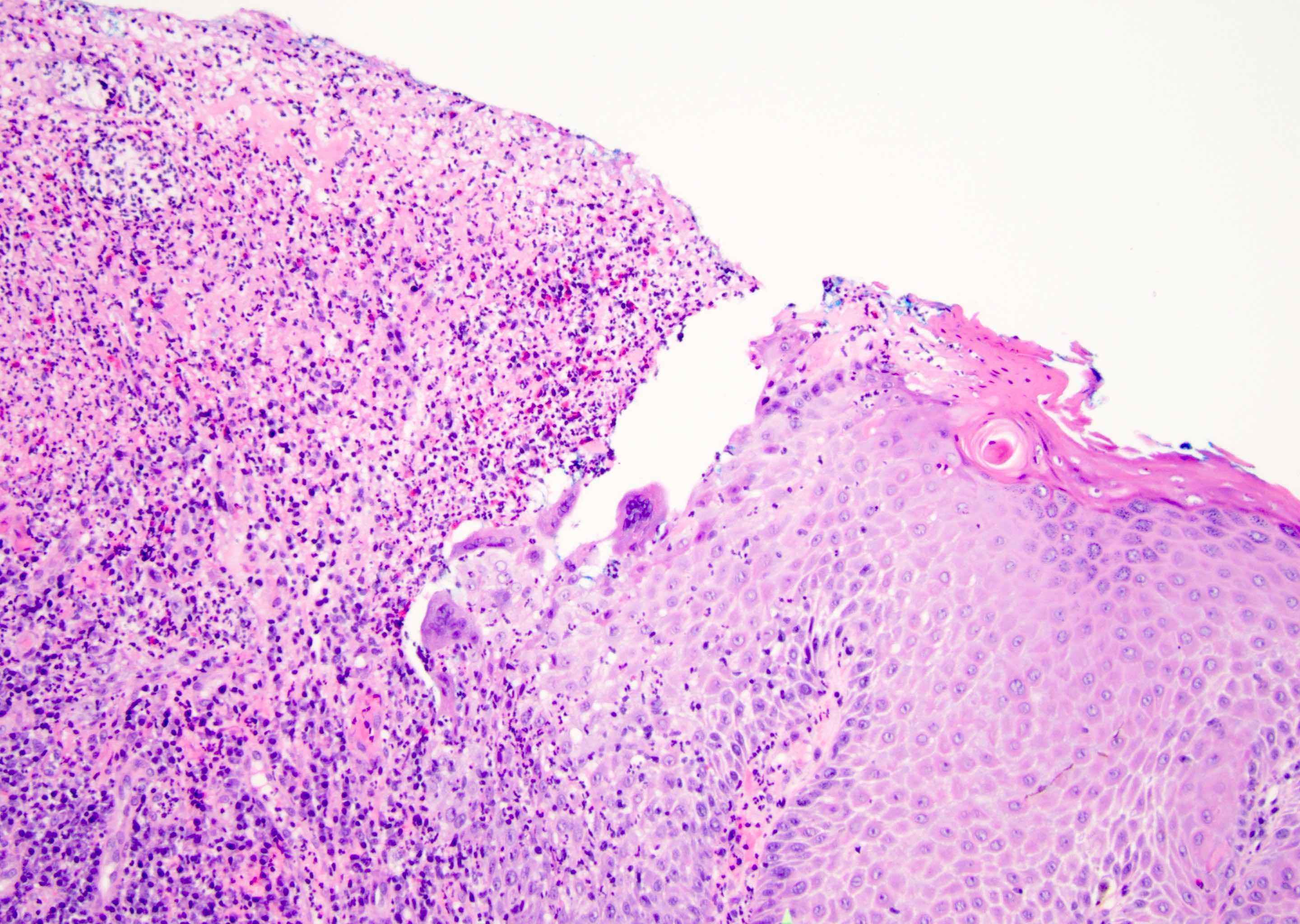
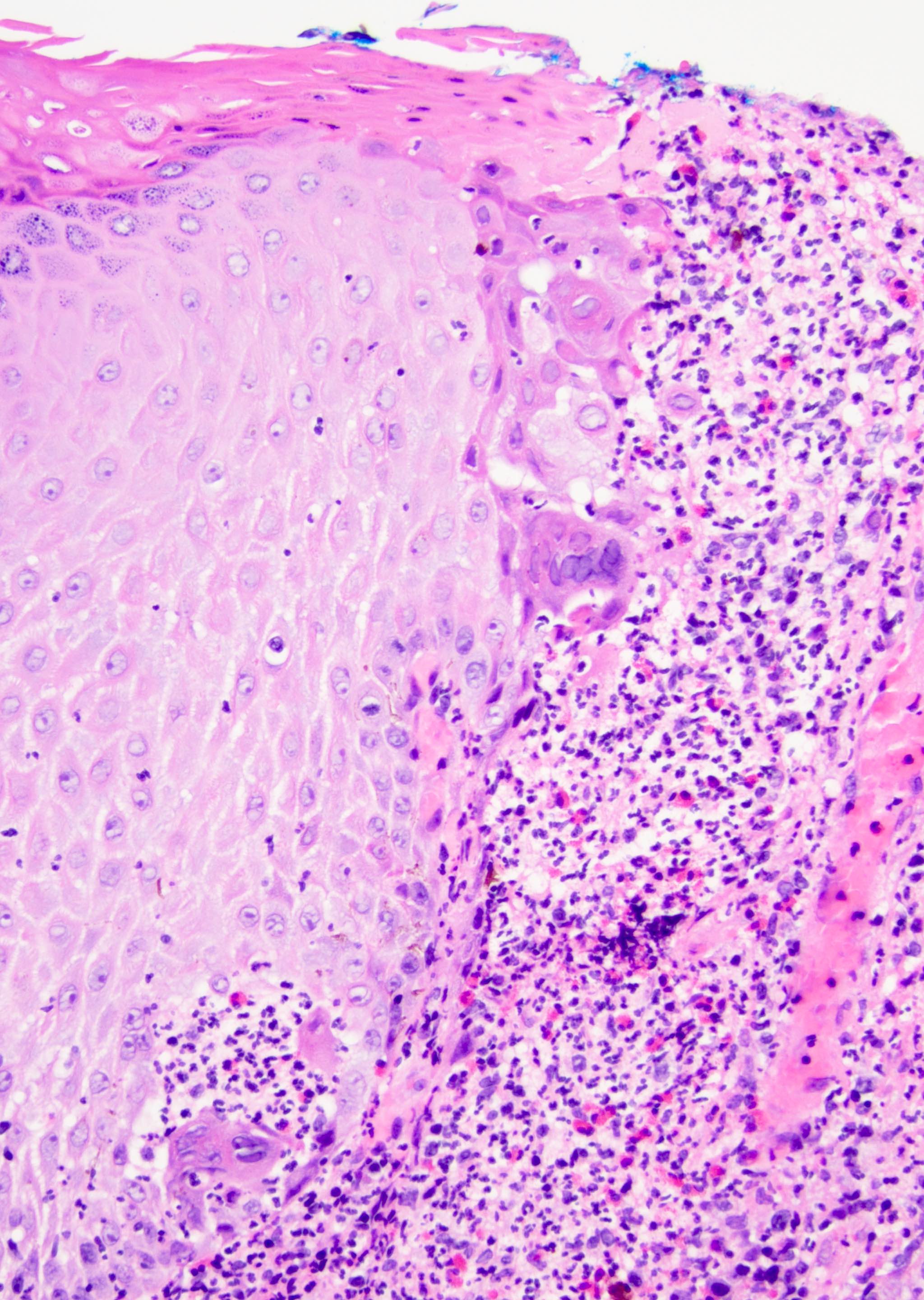
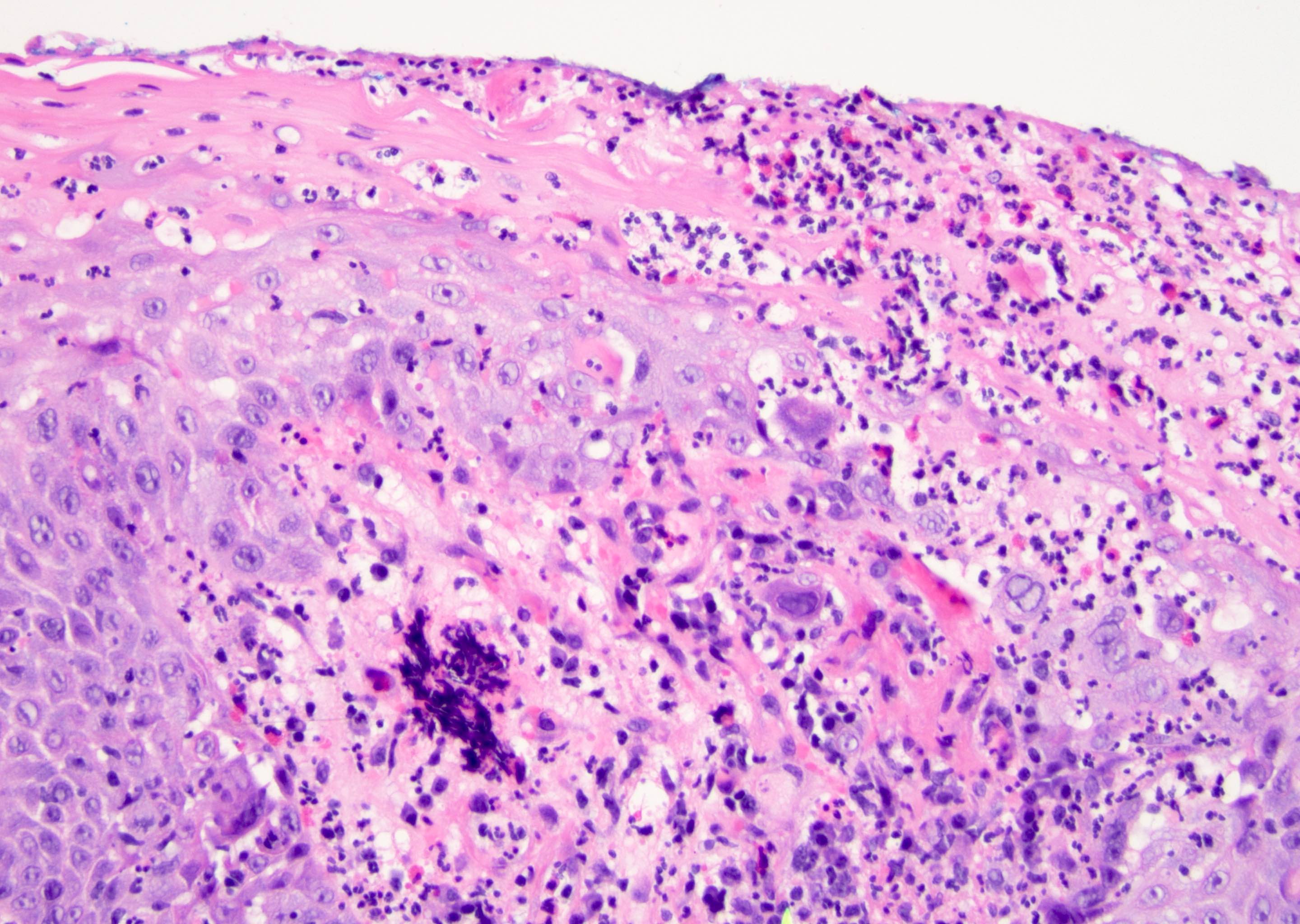
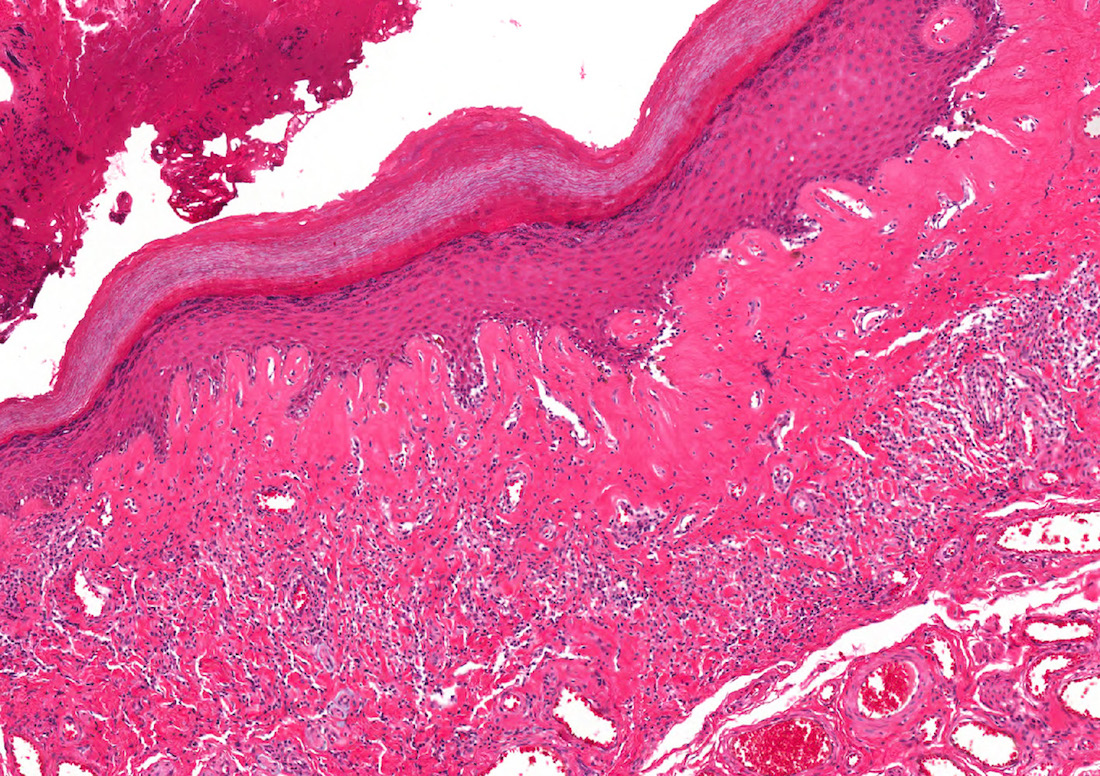
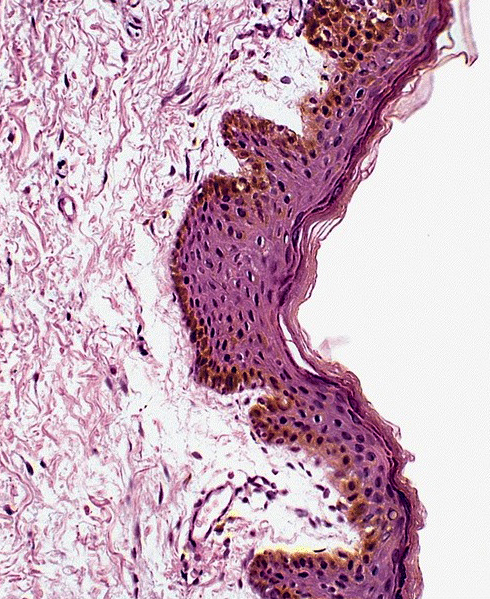
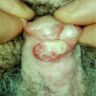
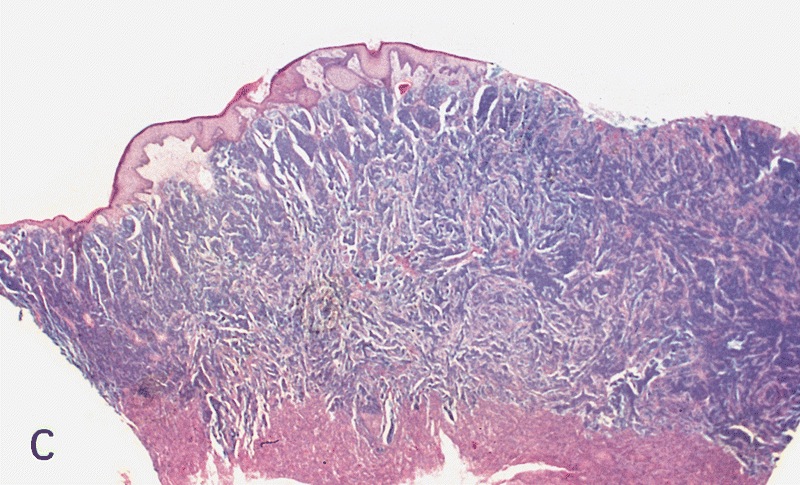
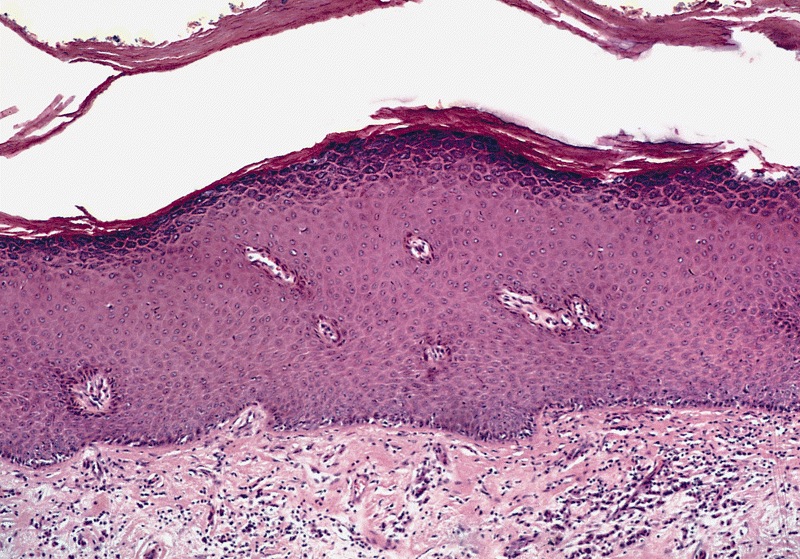
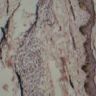
D. Zoon balanitis (plasma cell balanitis). The subepithelial inflammatory infiltrate consists primarily of plasma cells in a lichenoid pattern. The epithelium is thinned with edema and the dermis contains dilated capillaries. Answer A is incorrect because no vacuolar alteration of basal epithelial cells or sclerotic band of the papillary dermis is seen. Answer B is incorrect because the epithelium shown in the image is benign with an absence of malignant cells. Answer C is incorrect because the clinical presentation is inconsistent with the absence of papules or ulceration and there is no psoriasiform hyperplasia or endothelial proliferation.
Comment Here
Reference: Balanitis / phimosis
Comment Here
Reference: Balanitis / phimosis
Board review style question #2
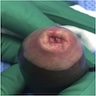
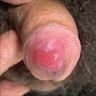
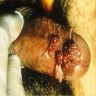
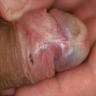
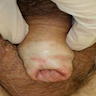
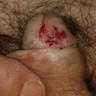
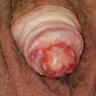
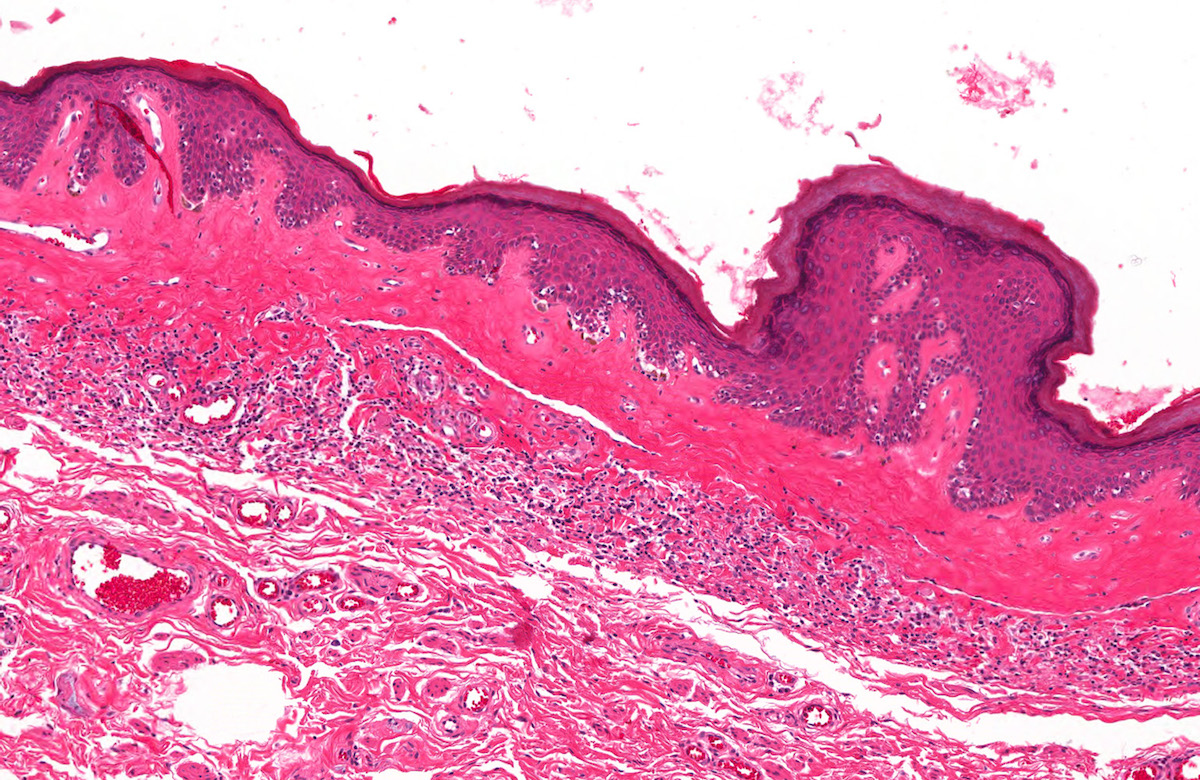
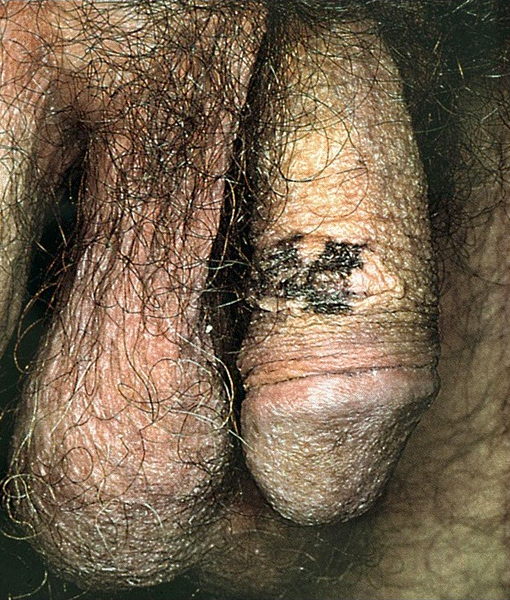
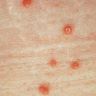
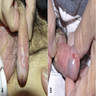
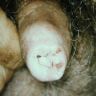
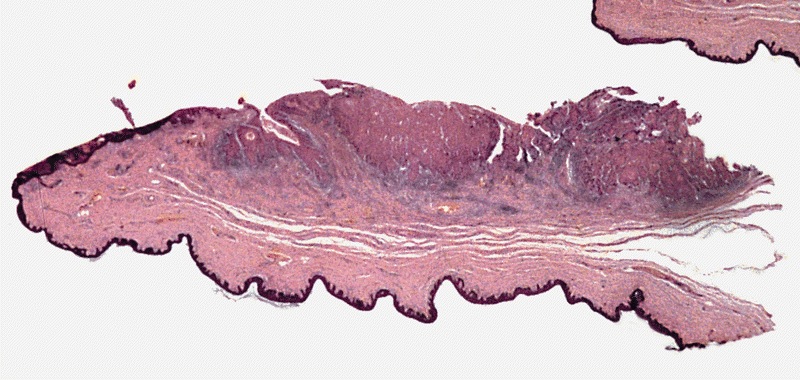
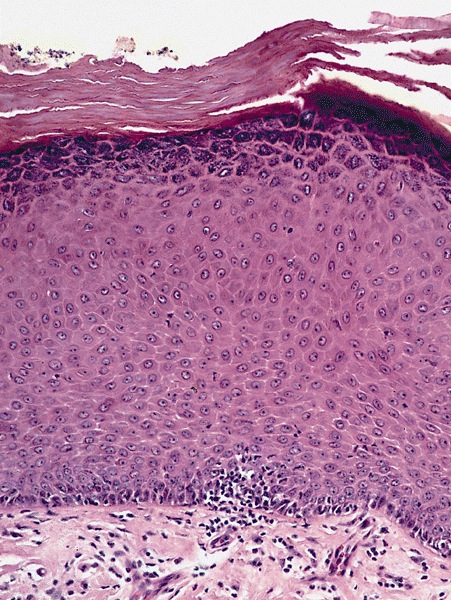
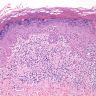
A 34 year old uncircumcised homeless man presents to the emergency department with worsening intense penile pain. Physical examination is remarkable for intractable foreskin and penile swelling. An emergent circumcision is performed and a section of the removed tissue is seen above. If left untreated, this entity is a major risk factor for which of the following entities?
- Condyloma acuminatum
- Kaposi sarcoma
- Melanoma
- Squamous cell carcinoma
Board review style answer #2
D. Squamous cell carcinoma. The clinical presentation, detailing the inability to retract foreskin with penile pain and swelling, is consistent with phimosis. The associated image reveals a subepithelial chronic inflammatory infiltrate of the foreskin, which can commonly be seen with foreskin removed for phimosis. Phimosis is a major risk factor of squamous cell carcinoma. Answer A is incorrect because condyloma acuminatum is associated with human papillomavirus (HPV) infection and phimosis is not a known risk factor. Answer B is incorrect because Kaposi sarcoma is most strongly associated with human herpesvirus 8 (HHV8) and iatrogenic risk factors include immunosuppressed patients. Phimosis is not a known risk factor. Answer C is incorrect because phimosis is not known to be a risk factor of mucosal melanoma.
Comment Here
Reference: Balanitis / phimosis
Comment Here
Reference: Balanitis / phimosis
Basal cell carcinoma
Table of Contents
Definition / general | Epidemiology | Sites | Etiology | Clinical features | Prognostic factors | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosisDefinition / general
- Similar to counterpart in skin (see skin - nonmelanocytic tumor chapter)
- ICD-0: 8090 / 3
Epidemiology
- Very rare
Sites
- Most cases arise in skin of shaft, may be multicentric
Etiology
- No evidence of HPV infection
- Typically develops on sun exposed skin and is closely associated with ultraviolet radiation
Clinical features
- Usually ages 40+ years (J Urol 1994;152:1557)
- Slowly growing hyperpigmented tumor
- Risk factors include ultraviolet and ionizing radiation, arsenic ingestion, immunosuppression and inherited syndromes, such as nevoid basal cell carcinoma syndrome and xeroderma pigmentosum
- Extremely low metastatic potential (J Am Acad Dermatol 2001;45:68)
Prognostic factors
- Multicentric and large tumors may present more aggressive behavior
Case reports
- 75 year old man with multicentric tumor and skin metastases (Scand J Plast Reconstr Surg Hand Surg 2002;36:180)
Treatment
- Excision
Clinical images
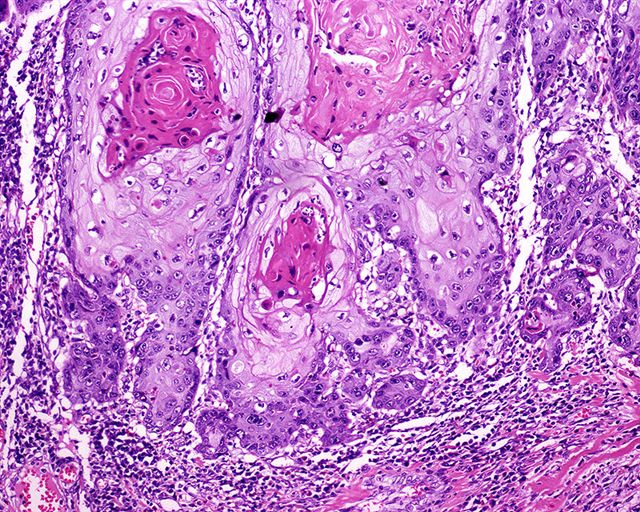
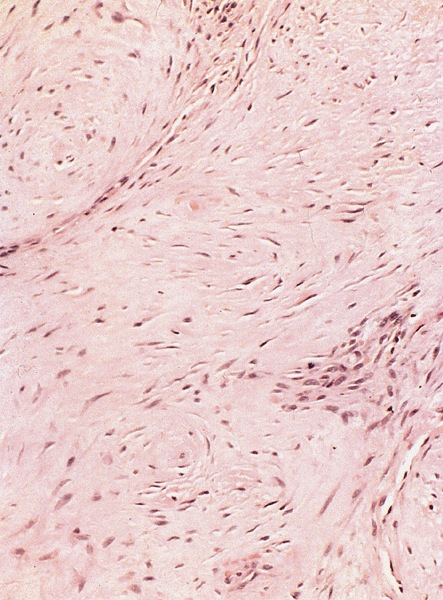
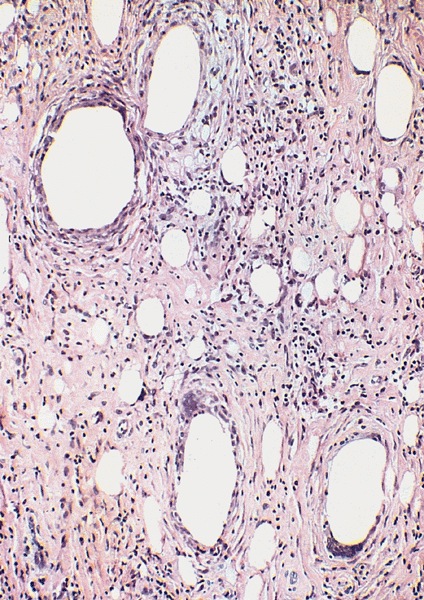
Microscopic (histologic) description
- Regular neoplastic nests with evident peripheral palisading
- No areas of central comedonecrosis
- Frequent myxoid stromal changes
Microscopic (histologic) images
Differential diagnosis
- Basaloid carcinoma: involves glans not shaft, more pleomorphic neoplastic cells, frequent central comedonecrosis and absence of peripheral palisading
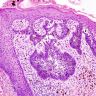
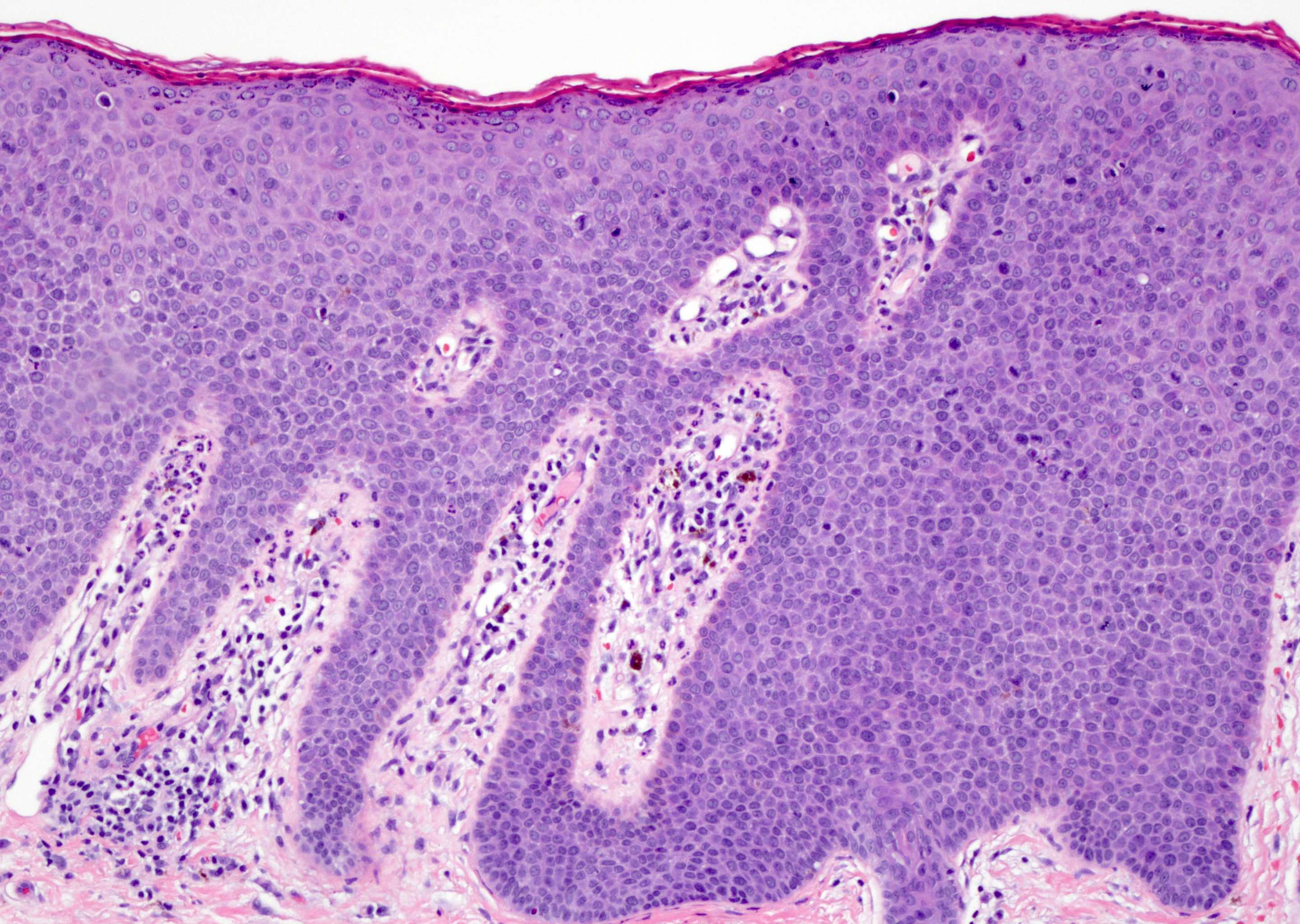
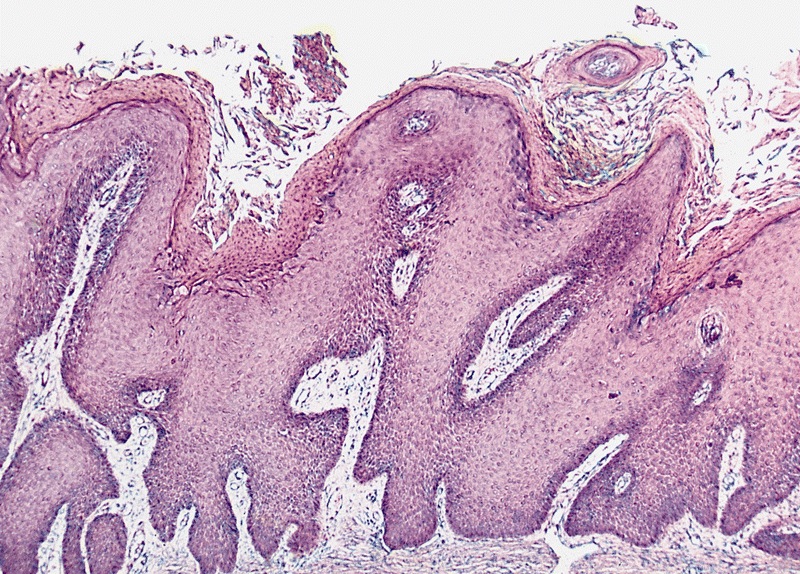
Bowenoid papulosis
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Electron microscopy description | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Human papillomavirus (HPV) related proliferation of atypical basaloid and koilocytic cells; characteristically involves the anogenital skin and mucosa
Essential features
- Characterized by the presence of atypical basaloid and koilocytic cells in squamous epithelium above the basement membrane
- Typically presents as solitary or multiple small pink, brown or violaceous papules or plaques on the penis or other sites
- Diagnosis requires both characteristic clinical and microscopic findings
- Usually caused by HPV 16 infection or (less commonly) other HPV strains
Terminology
- Penile intraepithelial neoplasia (PeIN) is the standardized terminology for pathologic reporting, which may be integrated with clinical presentation for diagnosis of bowenoid papulosis (Eur Urol 2016;70:93)
- Bowenoid papulosis is no longer a term used by pathologists in the World Health Organization (WHO) Classification of Tumors, 5th edition
- First reported as multicentric pigmented Bowen disease (historical, not recommended) (Arch Dermatol 1970;101:48)
ICD coding
Epidemiology
- Young, sexually active males (on average, late 20s to early 30s)
Sites
- Most frequently affects anogenital region but extragenital disease has been reported
- Reports of extragenital lesions include those that involve the mouth, face / neck (within beard distribution) and other areas of skin (Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000;90:466, Br J Dermatol 1999;140:761, Arch Dermatol 1989;125:655, Ann Dermatol 2014;26:381)
Pathophysiology
- Persistent infection with high risk HPV leads to expression of oncoproteins E6 and E7, which inactivate p53 and Rb tumor suppressors, leading to dysregulation of cell division and apoptosis (World J Urol 2009;27:141)
Etiology
- Usually caused by HPV 16 infection or (less commonly) other HPV strains, including 18, 13, 33, 35, 39 and 53 (Patterson: Weedon’s Skin Pathology, 5th Edition, 2020)
- Vaccination against HPV may prevent condition (World J Urol 2009;27:141)
Clinical features
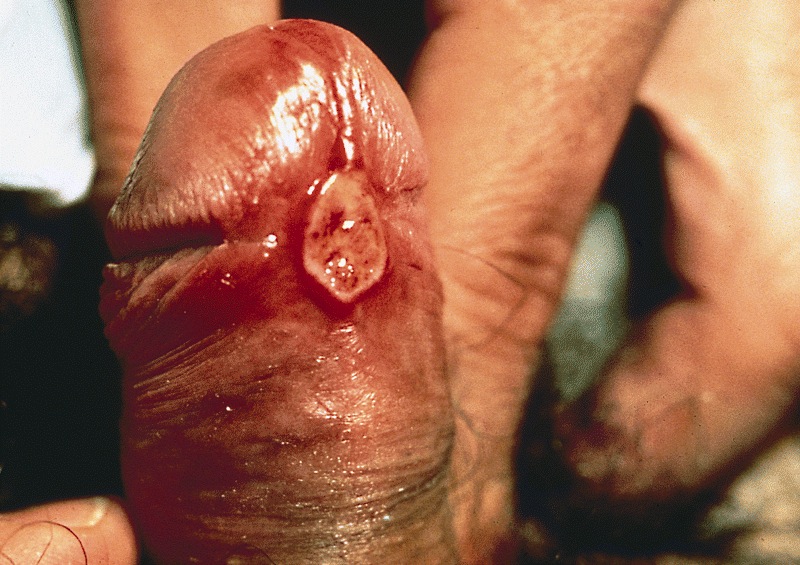
- Typically, multiple well defined skin toned to pigmented papules (< 1 cm)
- May be in linear arrangement (rare) (Postepy Dermatol Alergol 2016;33:146)
- May be a singular lesion or multiple lesions coalescing into a plaque (Cancer 1978;42:1890)
- May be a verrucous / warty lesion (Cancer 1978;42:1890)
- Typically asymptomatic but may be pruritic or painful (Cancer 1986;57:823)
- Reported correlation between recurrence and smoking (StatPearls: Bowenoid Papulosis [Accessed 11 April 2022])
Diagnosis
- Diagnosis requires characteristic clinical findings in conjunction with pathologic features from biopsy
Prognostic factors
- Overall, favorable prognosis, although recurrence is common following conservative treatment (Cancer 1986;57:823)
- Without treatment, may spontaneously regress or persist for years (Arch Dermatol 1978;114:1698, J Am Acad Dermatol 1986;14:433)
- Postulated to rarely progress to Bowen disease or invasive squamous cell carcinoma in older or immunocompromised patients, < 1% of cases (Acta Dermatovenerol Alp Pannonica Adriat 2021;30:117, J Am Acad Dermatol 1980;3:149, J Dermatol 2012;39:646, Dis Colon Rectum 1989;32:1042, Int J Gynecol Pathol 1987;6:1, World J Urol 2009;27:141)
Case reports
- 31 year old man with plaques and papules of glans corona (Postepy Dermatol Alergol 2016;33:146)
- 35 year old man with perianal and genital warty papules and plaques (Br J Dermatol 2000;143:604)
- 46 year old woman with multiple perianal papules (Acta Dermatovenerol Alp Pannonica Adriat 2021;30:39)
Treatment
- Conservative treatment modalities include carbon dioxide laser vaporization, cryotherapy, electrocoagulation, 5-aminolevulinic acid mediated photodynamic therapy, excisional surgery, 5-fluorouracil and topical imiquimod cream 5% (Australas J Dermatol 2017;58:86)
- Female partners are at increased risk for cervical dysplasia and should be monitored (Ther Adv Urol 2011;3:151)
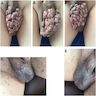
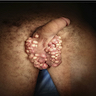
Clinical images
Gross description
- Skin / mucosal punch or shave biopsy with superficial surface demonstrating papule(s), plaque or papillomatous lesion(s) that may be skin toned, violaceous or red-brown
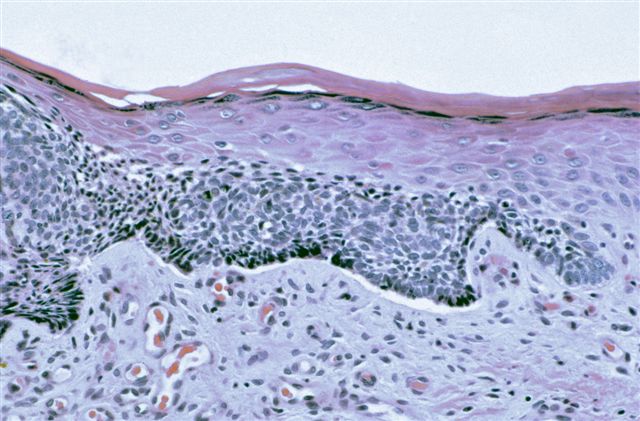
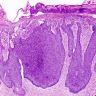
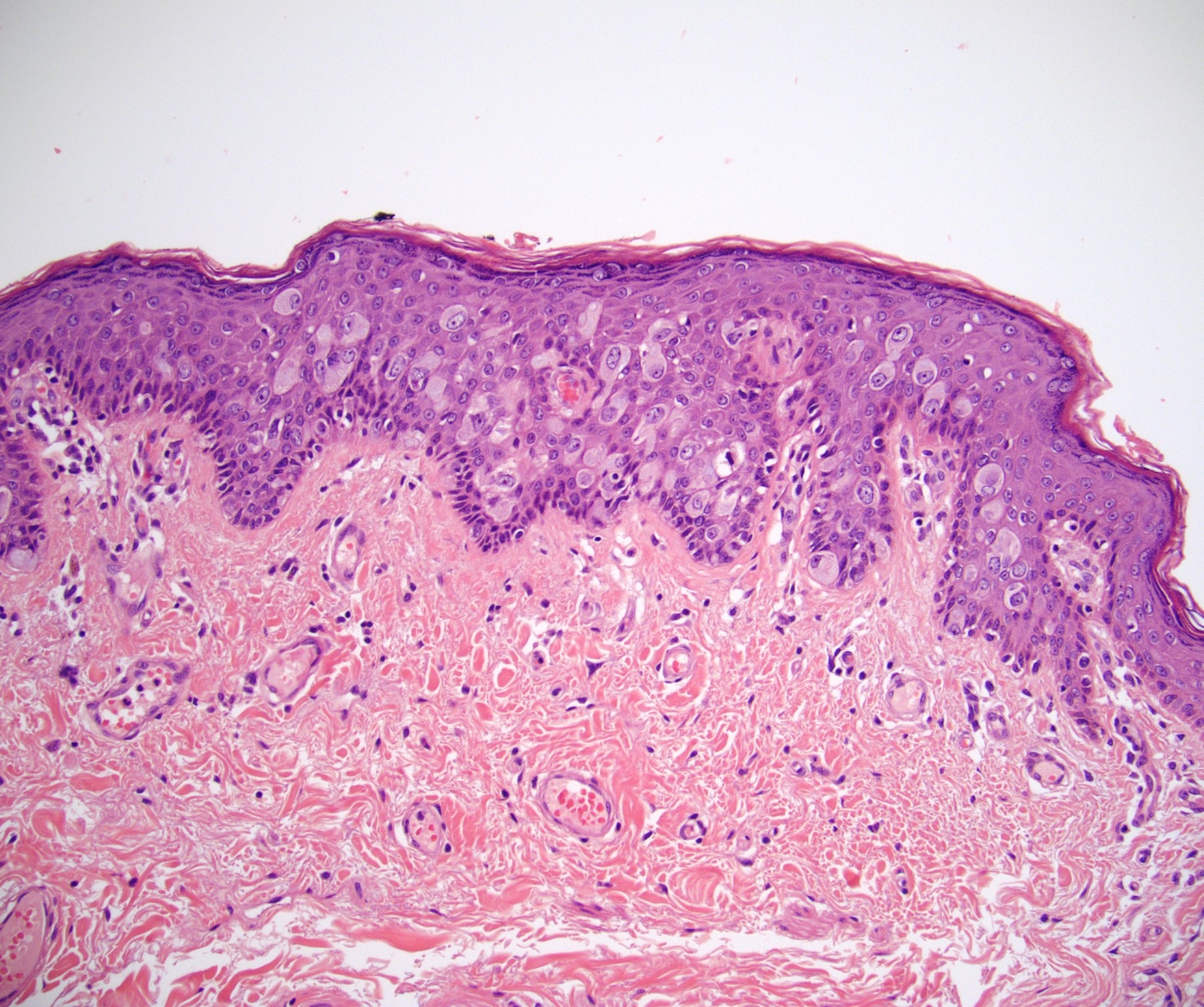
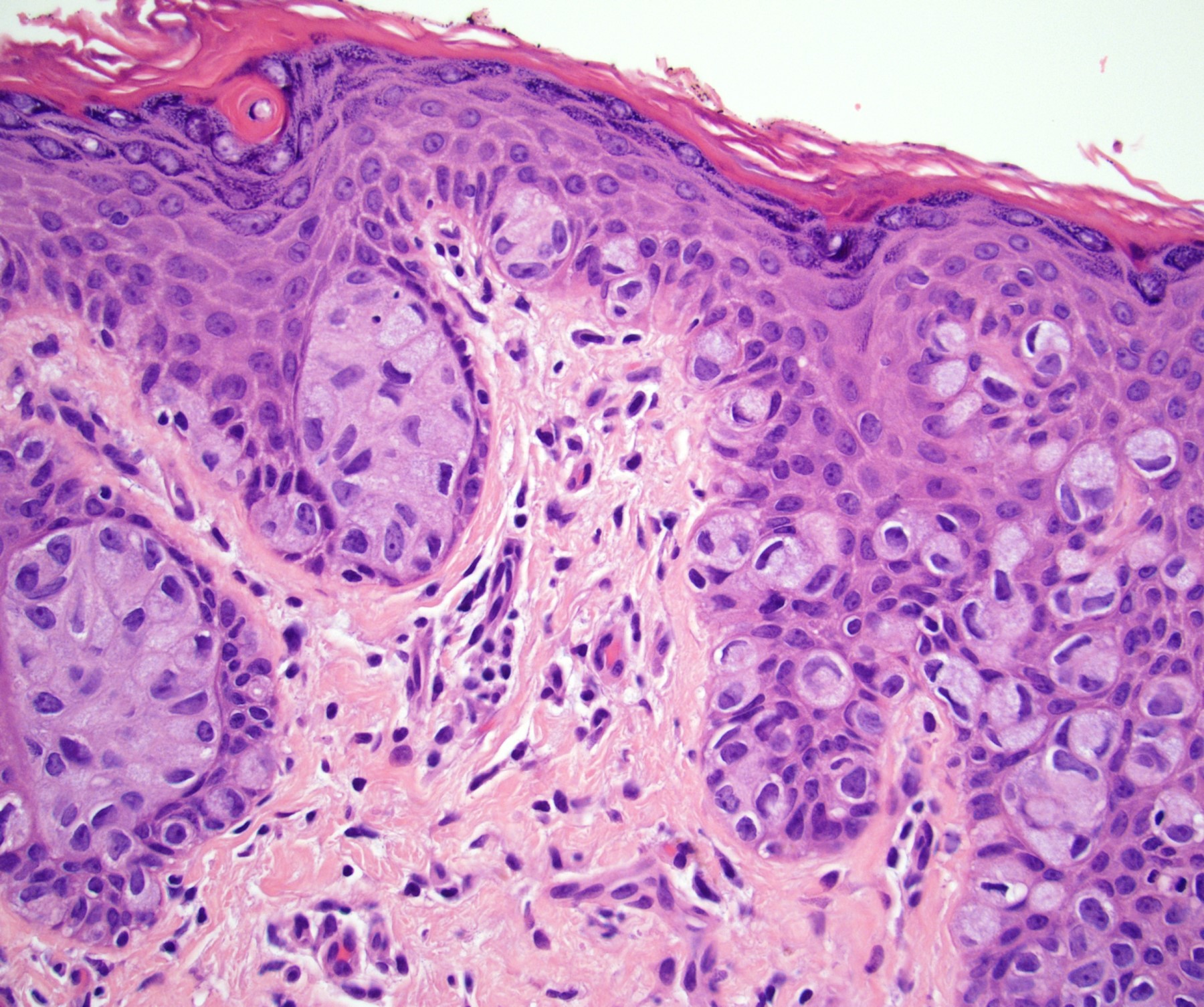
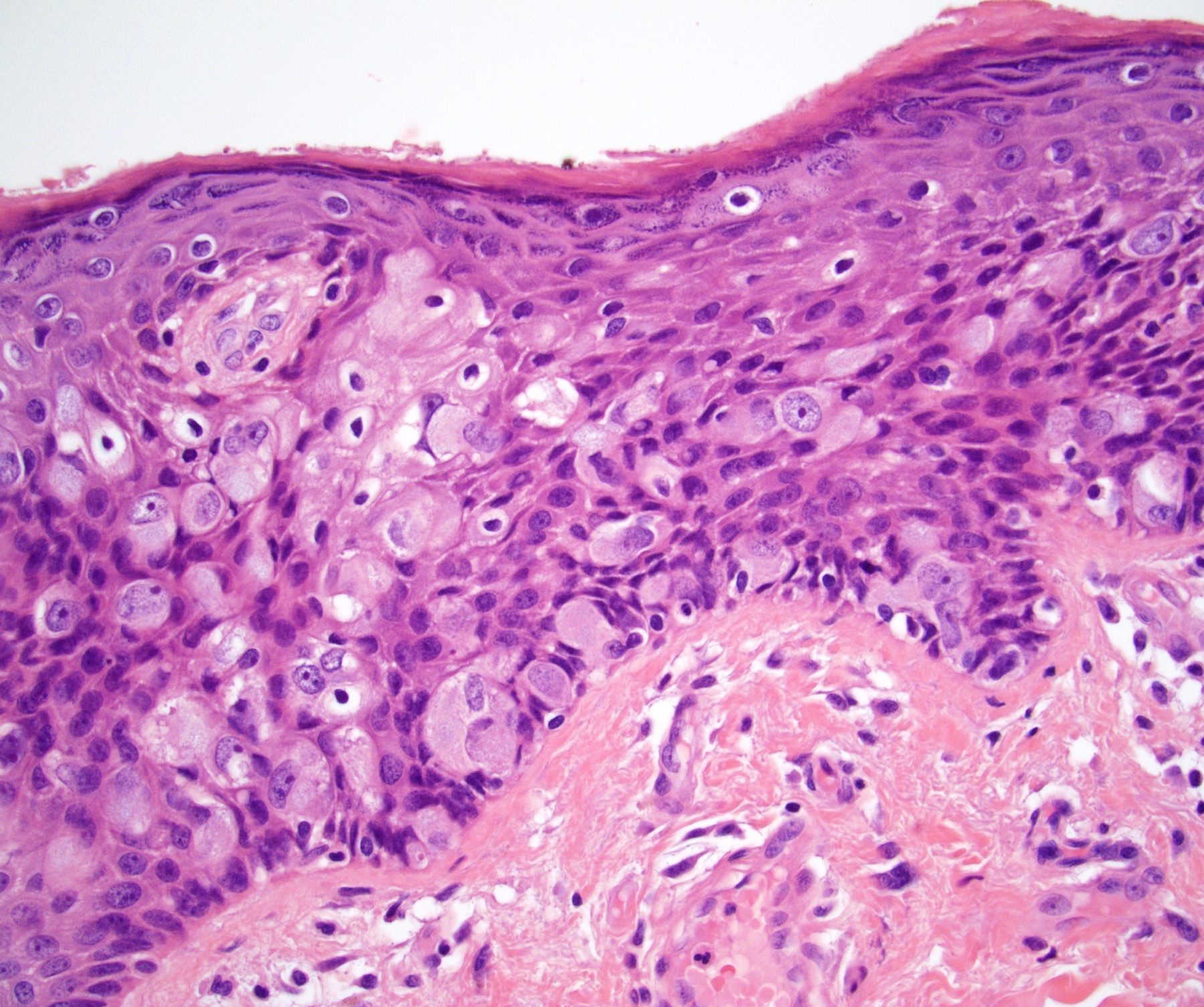
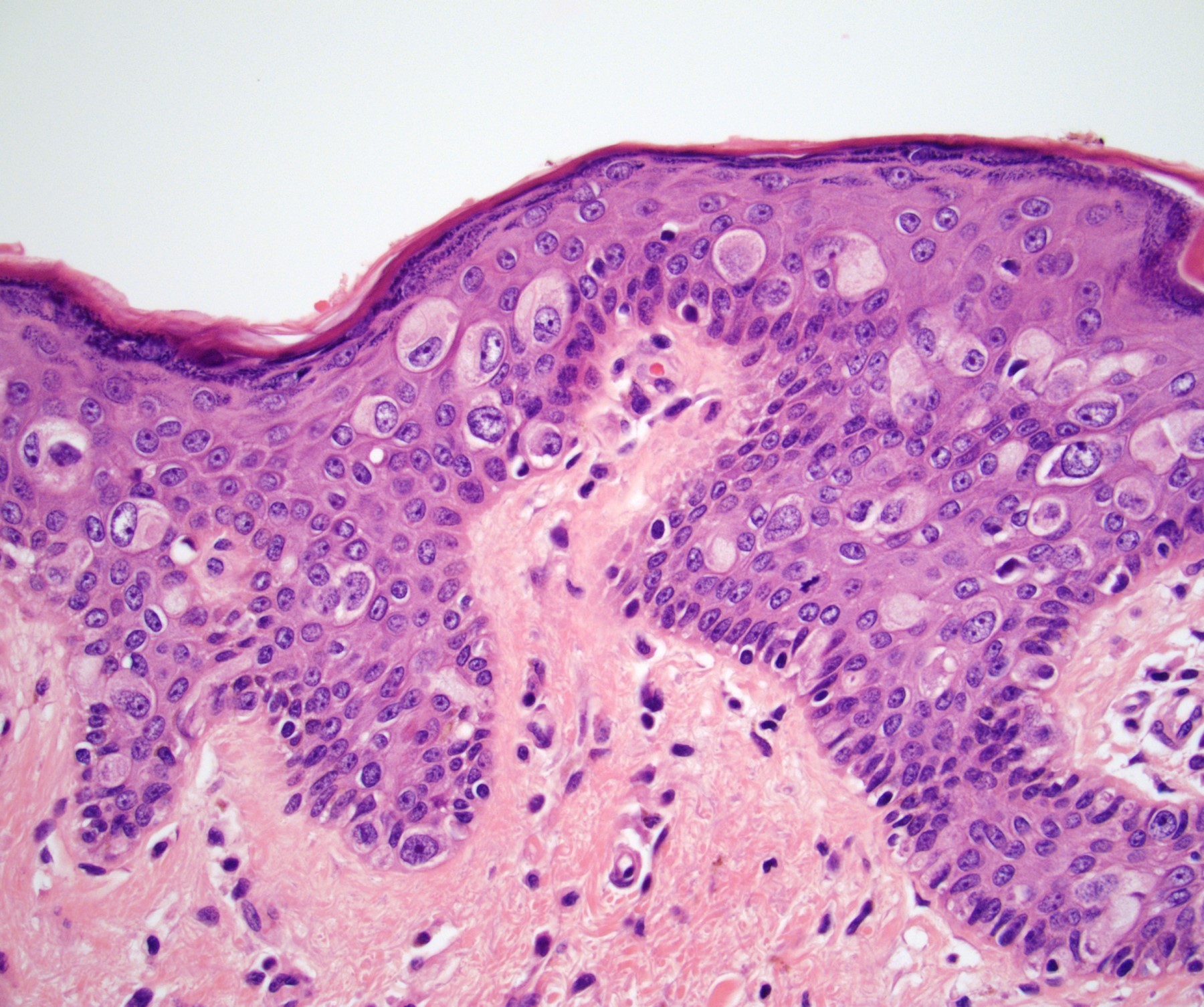
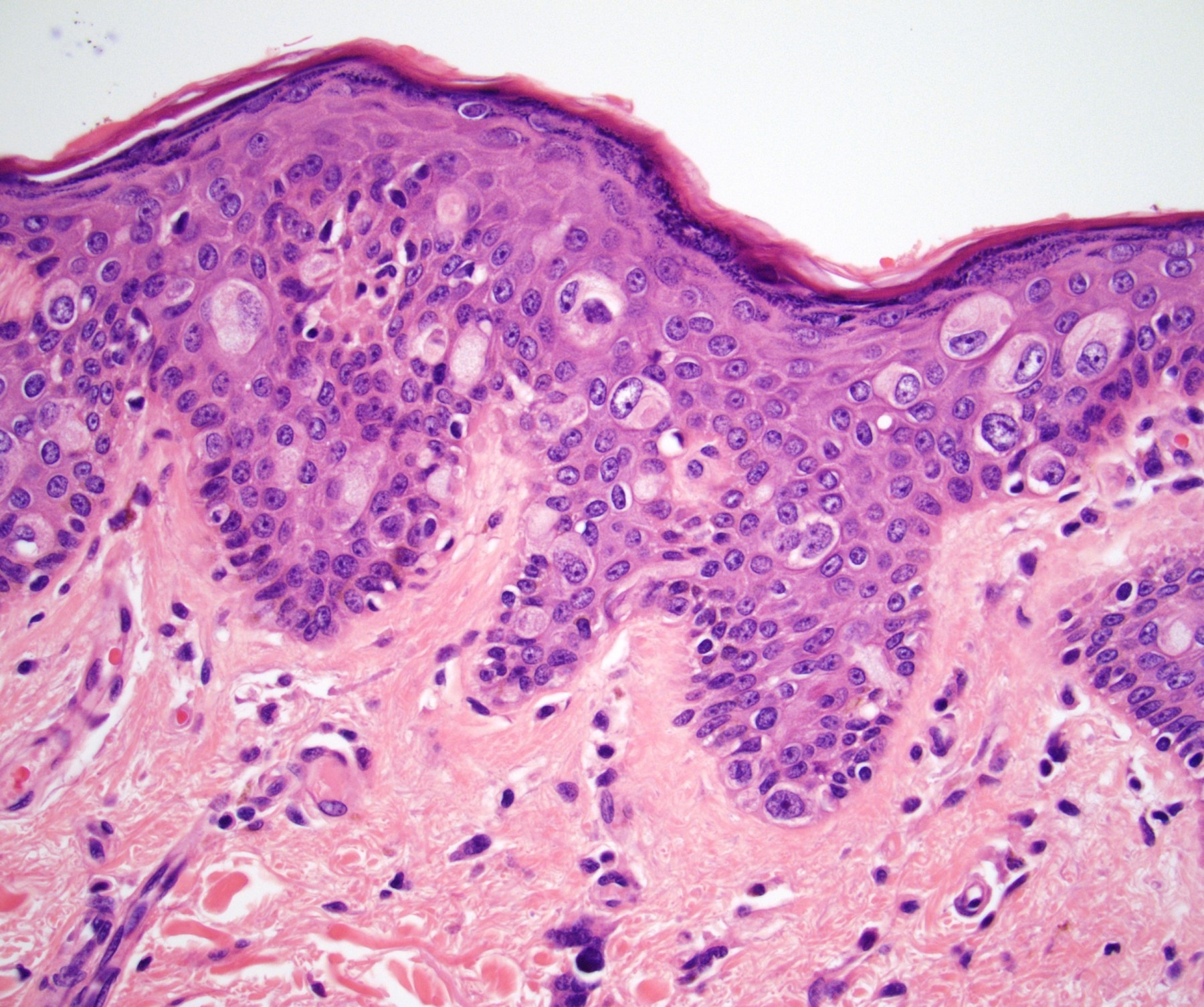
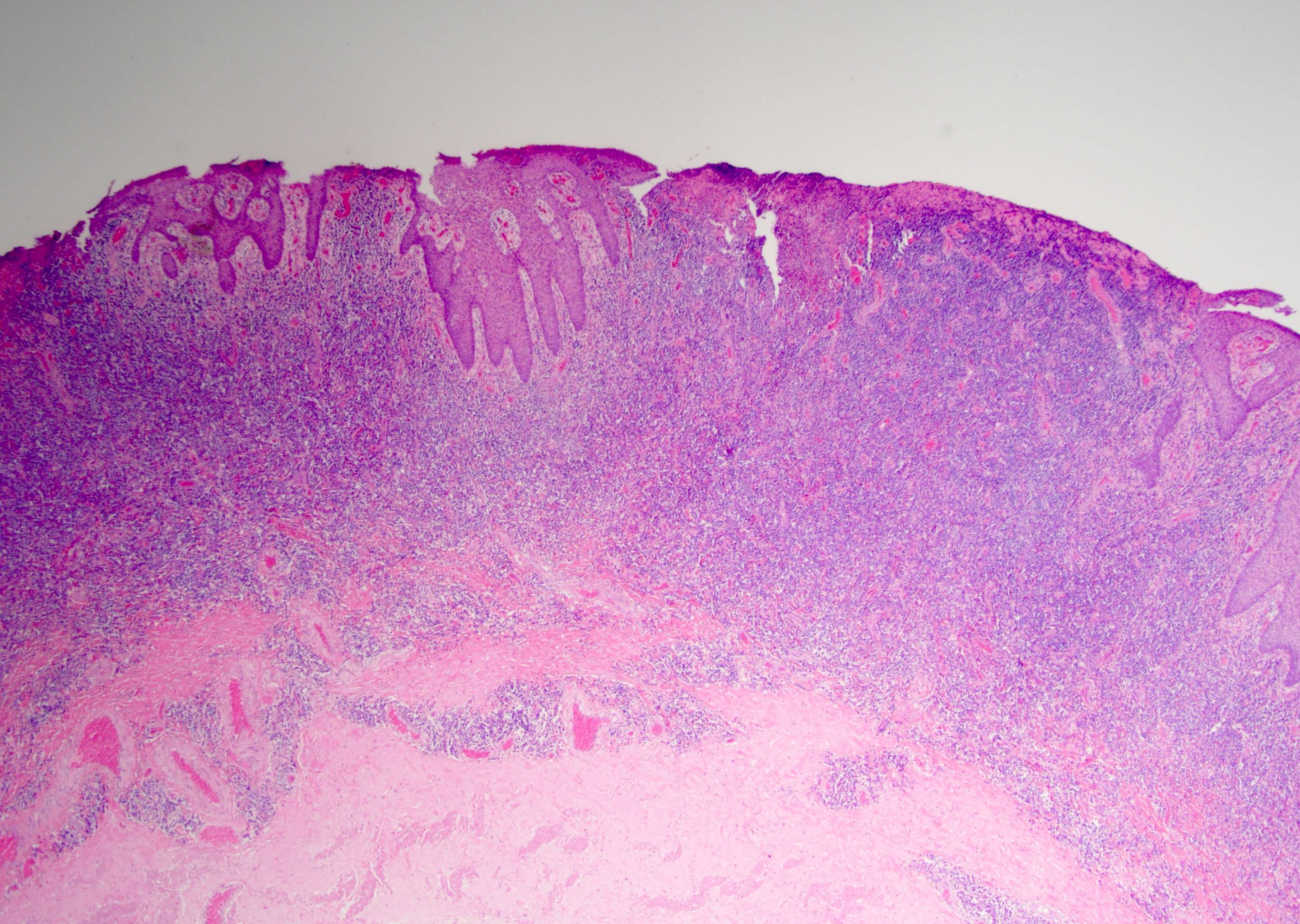
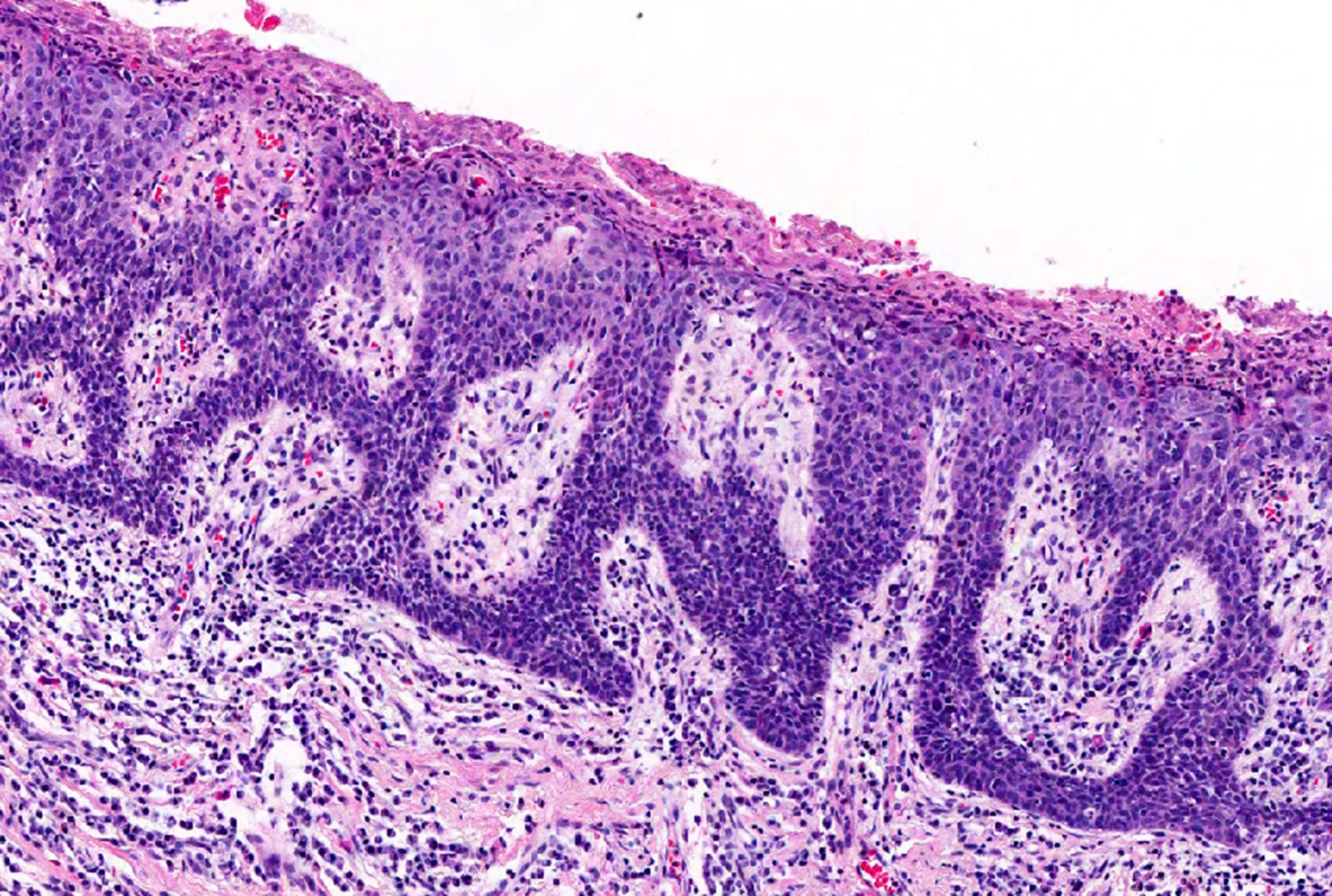
Microscopic (histologic) description
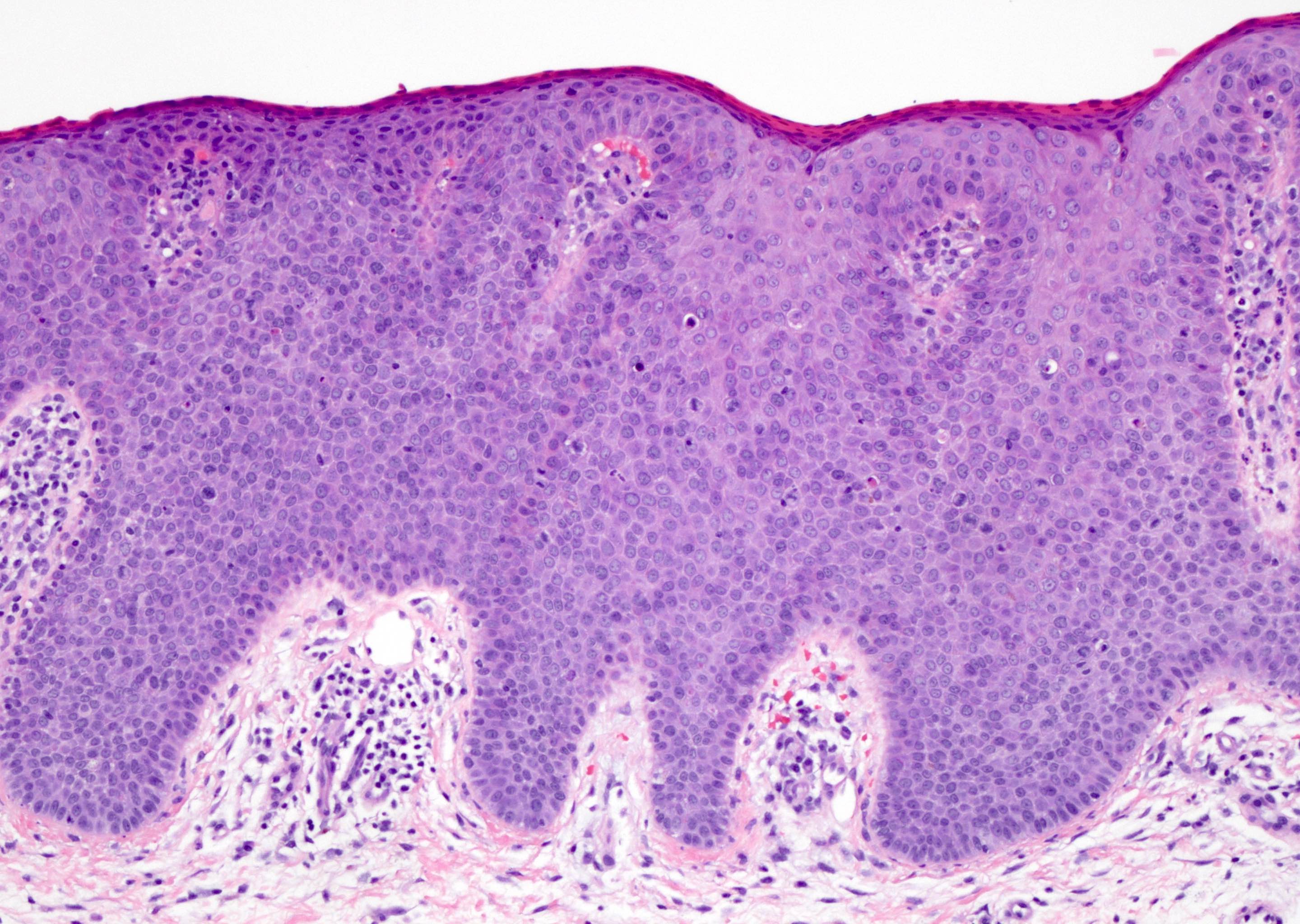
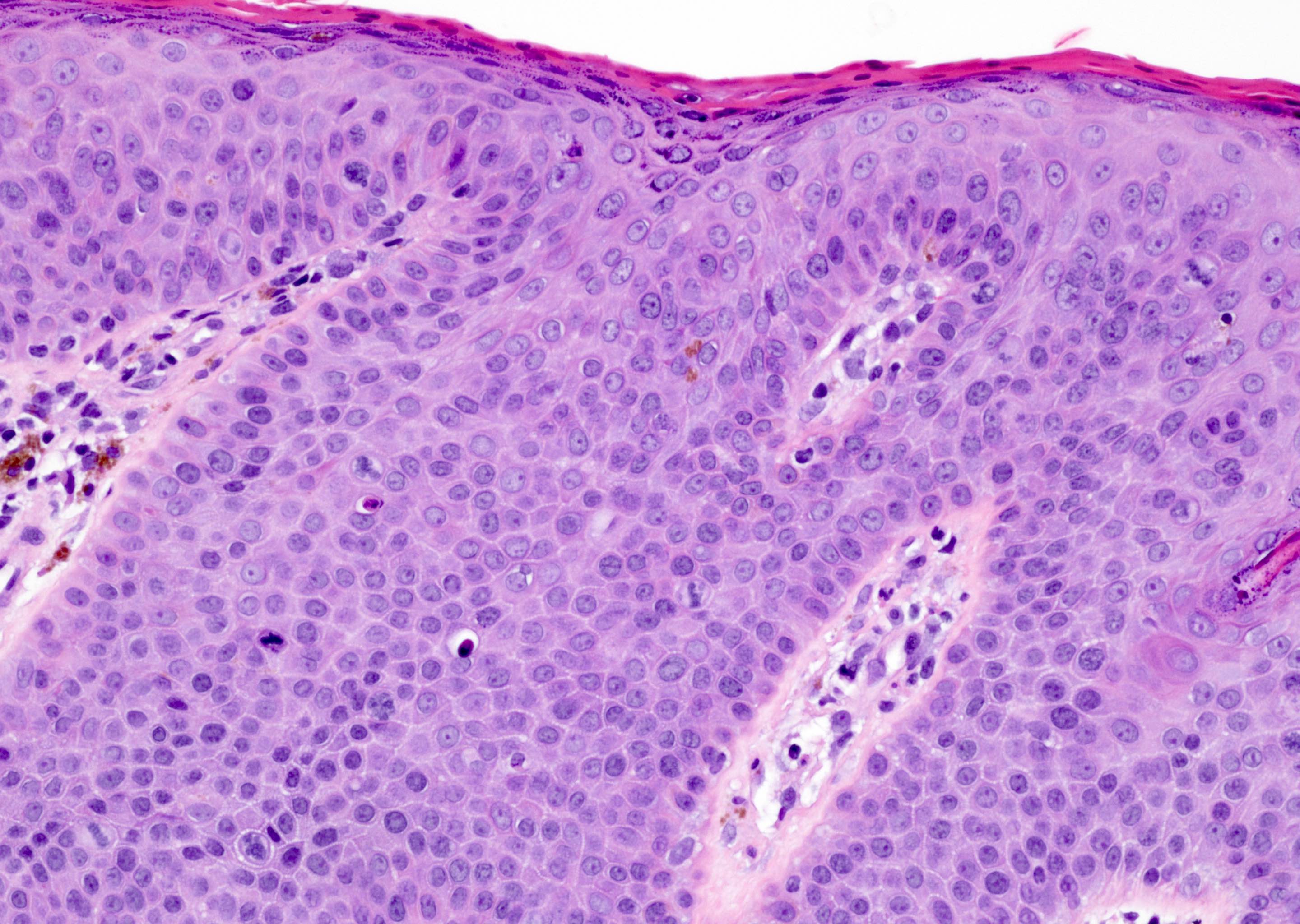
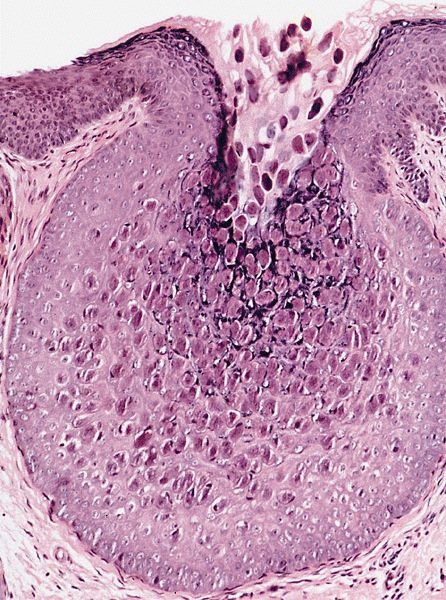
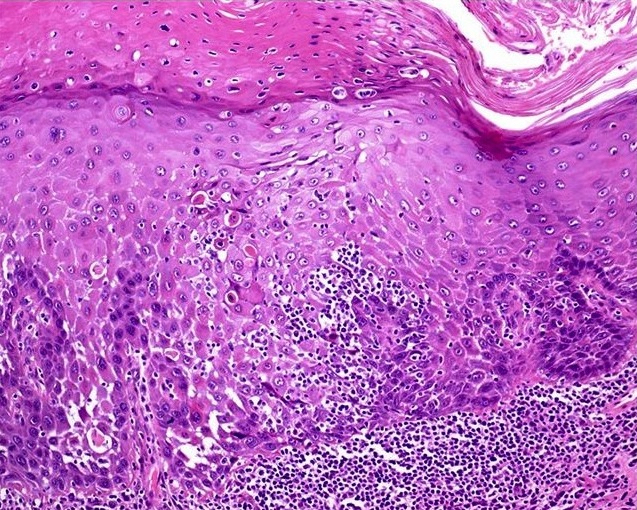
- Dysplastic changes with intact basement membrane, consistent with squamous cell carcinoma in situ (Eur Urol 2016;70:93)
- Proliferation of atypical basaloid and koilocytic cells in squamous epithelium may range from scattered cells to full thickness involvement
- Often accompanied by acanthosis, parakeratosis and mitotic figures above the basal layer
- May demonstrate hyperkeratosis, dyskeratosis, lymphocytic infiltrate, loss of polarity and dilated, tortuous capillaries in dermal papillae (Arch Dermatol 1970;101:48, J Am Acad Dermatol 1986;14:433)
- Cannot definitively be distinguished from other forms of carcinoma in situ, Bowen disease and erythroplasia of Queyrat, based on histology alone (Cancer 1986;57:823, Australas J Dermatol 2019;60:e201)
Microscopic (histologic) images
Positive stains
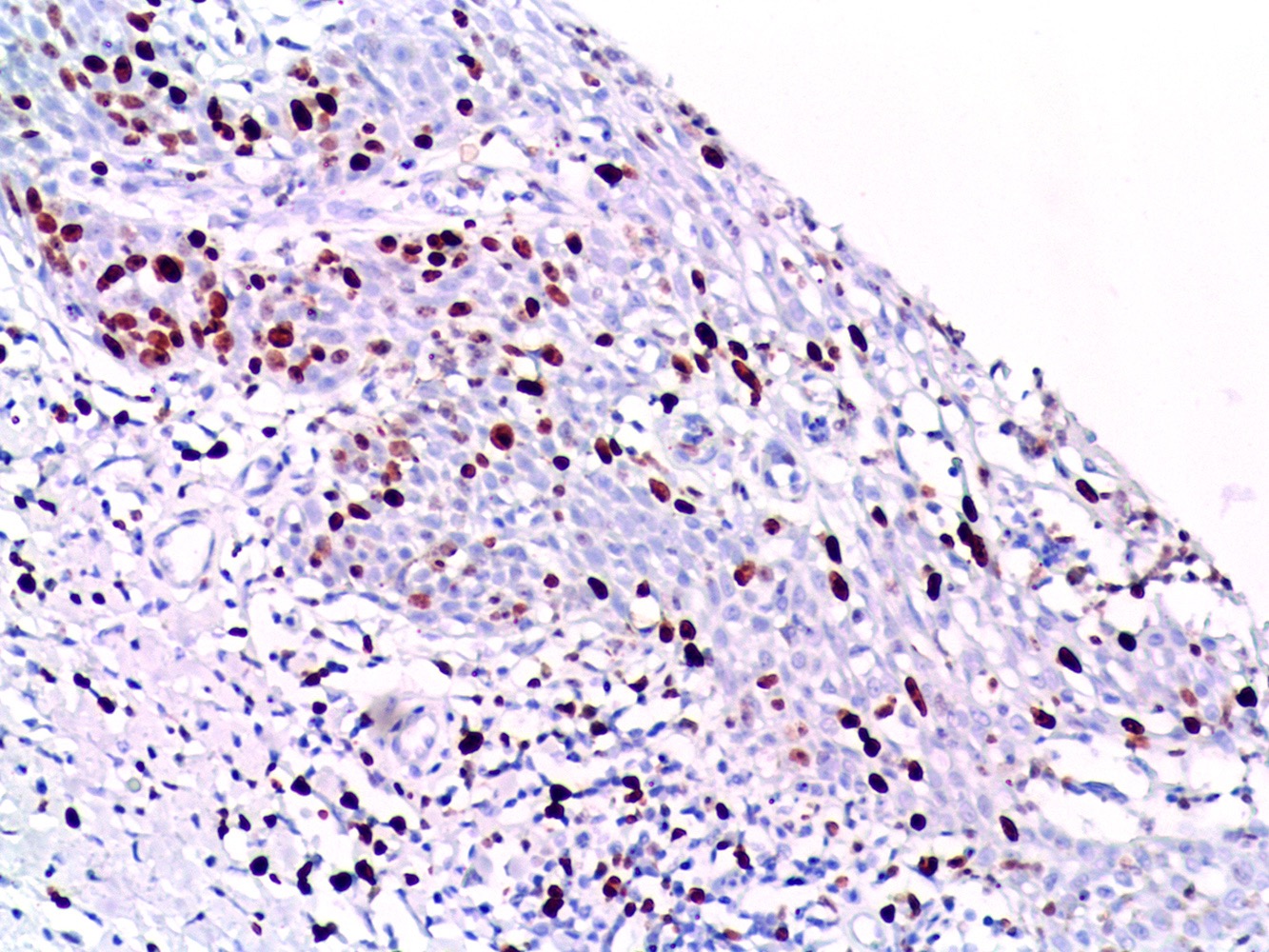
Electron microscopy description
- Viral particles may or may not be visualized (Cancer 1978;42:1890, Arch Dermatol 1979;115:306, Cancer 1986;57:823)
- May demonstrate deformed nucleoli, indented nuclei, widening of spaces between keratinocytes and disruption of tonofilaments (Arch Dermatol 1978;114:1698, Cancer 1986;57:823)
Molecular / cytogenetics description
- DNA PCR and ISH may detect DNA of HPV 16 or other strains (Arch Dermatol 1985;121:858, Br J Dermatol 1994;131:577)
Videos
Microscopic findings of bowenoid papulosis / HSIL
Sample pathology report
- Penis, dorsal glans, punch biopsy:
- Penile intraepithelial neoplasia (PeIN), grade 2 (see comment)
- Comment: Stratified squamous epithelium demonstrates acanthosis with scattered mitoses and atypical koilocytic cells. This moderate dysplasia does not extend below the basement membrane, consistent with PeIN, grade 2. Patient's age and appearance of lesion are noted. Clinical variants of PeIN include bowenoid papulosis, Bowen disease and erythroplasia of Queyrat. Given the presentation of multifocal skin toned, flat topped papules resembling early condyloma acuminata on the glans of a 32 year old man, this may be consistent with bowenoid papulosis. Bowenoid papulosis may spontaneously regress but also may very rarely progress to invasive squamous cell carcinoma.
Differential diagnosis
- Histologically:
- Genital Bowen disease:
- Similar histology to PeIN
- Usually older patients (50s to 70s) with a solitary or multiple large, well demarcated plaque(s) on keratinized genital skin (Open Access Maced J Med Sci 2019;7:696, Acta Derm Venereol 2013;93:228)
- Higher risk of progression to invasive carcinoma, ~5% of cases (World J Urol 2009;27:141)
- Erythroplasia of Queyrat:
- Similar histology to PeIN
- Solitary or multiple erythematous, moist plaques on the mucosal surfaces of the glans, may spread to prepuce (World J Urol 2009;27:141)
- Significant risk of malignant transformation, ~30% of cases (World J Urol 2009;27:141)
- Invasive squamous cell carcinoma:
- Atypical to anaplastic cells infiltrating beyond the basement membrane
- Histology varies depending on subtype (Eur Urol 2016;70:93)
- Most often affects older patients (50s to 70s); typically presents as a slow growing, ulcerated mass (Clin Dermatol 2013;31:362)
- Genital Bowen disease:
- Clinically:
- Condyloma acuminata (StatPearls: Condyloma Acuminata [Accessed 12 April 2022]):
- Koilocytes without atypia in upper layers of dermis
- Associated with low risk HPV; most commonly, strains 6 and 11
- Lichen planus (StatPearls: Lichen Planus [Accessed 12 April 2022]):
- Hyperkeratosis without parakeratosis, irregular thickening of stratum granulosum, liquefactive degeneration of stratum basale, sawtooth appearance of rete ridges and interface dermatitis
- Benign melanocytic nevus:
- Pigment containing melanocytes arranged in regular clusters at the dermal epidermal junction or superficial dermis
- Symmetric, well circumscribed, pigmented macules / papules
- Condyloma acuminata (StatPearls: Condyloma Acuminata [Accessed 12 April 2022]):
Additional references
Board review style question #1
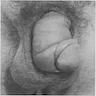
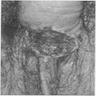
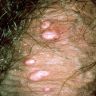
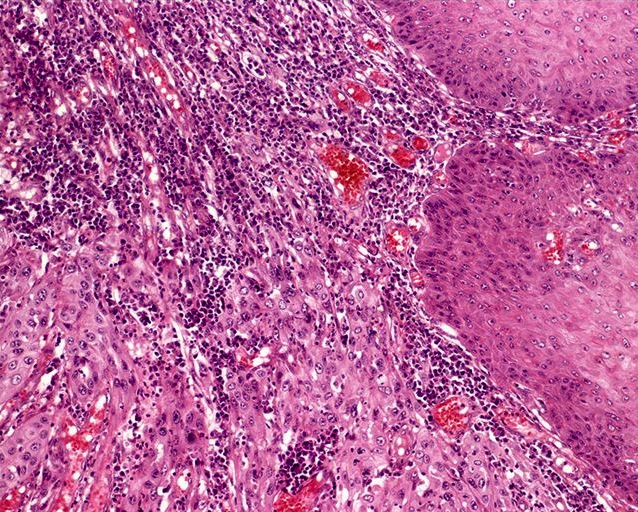
A 25 year old man presents with multiple, violaceous papules (up to 0.5 cm) on the lateral penile shaft. Images of representative microscopic findings are shown above. Immunohistochemical stain for p16 was positive, with a block-like staining pattern. What is the diagnosis?
- Bowen disease
- Bowenoid papulosis
- Invasive squamous cell carcinoma
- Verrucous carcinoma
Board review style answer #1
B. Bowenoid papulosis. Given the patient's young age and multifocality of lesions, this penile intraepithelial lesion (PeIN) is most consistent with bowenoid papulosis. Bowen disease is usually in older patients.
Comment Here
Reference: Bowenoid papulosis
Comment Here
Reference: Bowenoid papulosis
Board review style question #2
What is the current standard of care for bowenoid papulosis lesions?
- Chemotherapy and radiation, as lesions nearly always metastasize
- Conservative management with local resection, ablation or medications
- Observation, as lesions typically spontaneously regress
- Penectomy, as lesions reflect underlying invasive squamous cell carcinoma
Board review style answer #2
B. Conservative management with local resection, ablation or medications. Although bowenoid papulosis may spontaneously regress, it is suspected to be a premalignant lesion that rarely progresses to Bowen disease or even invasive squamous cell carcinoma. Therefore, conservative local resection or ablation is the favored treatment to reduce risk of progression to malignancy.
Comment Here
Reference: Bowenoid papulosis
Comment Here
Reference: Bowenoid papulosis
Cellulitis
Table of Contents
Definition / general | Epidemiology | Etiology | Clinical features | Case reports | Treatment | Clinical images | Microscopic (histologic) descriptionDefinition / general
- Infection of skin of penis
Epidemiology
- More common in newborns and immunosuppressed patients
Etiology
- Usually caused by group A Streptococcus; also Staphylococcus, group B Streptococcus and gram negative organisms
- Deep infections caused by Neisseria gonorrhoeae
- Mixed infections occur as complication of gonorrhea or in debilitated individuals
Clinical features
- Usually involves scrotum
- May have dysuria due to pain and swelling
- Associated with genital lymphangioma (Int J Dermatol 2006;45:800)
Case reports
- Necrotizing cellulitis associated with protein S deficiency (Clin Exp Dermatol 1993;18:305)
Treatment
- Amoxicillin or other oral antibiotics
- Rarely IV antibiotics or surgery (circumcision)
Microscopic (histologic) description
- Severe nonspecific chronic or mixed inflammatory infiltrate
Chancroid
Table of Contents
Definition / general | Terminology | Epidemiology | Etiology | Clinical features | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) imagesDefinition / general
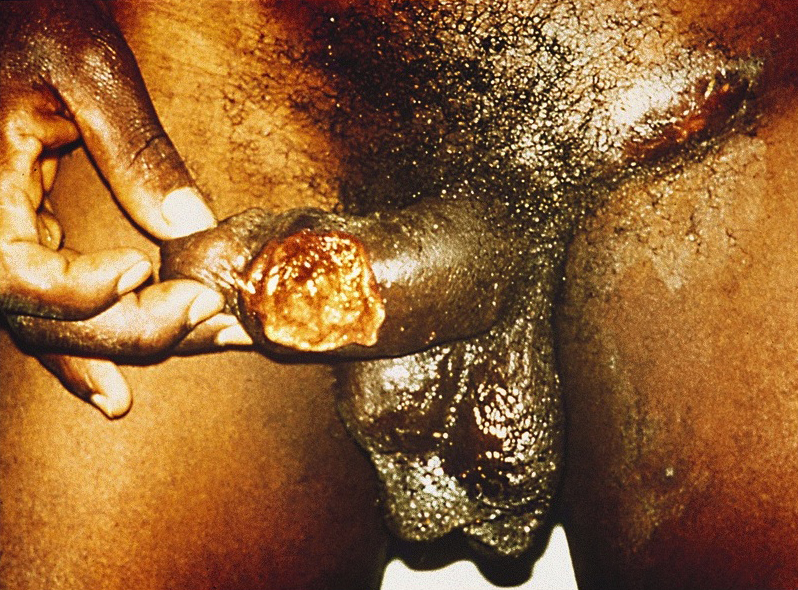
- Sexually transmitted disease caused by Haemophilus ducreyi which produces a painful genital ulcer and inguinal adenopathy
Terminology
- Dwarf chancroid: soft, painful, small ulcer
- Giant chancroid: may extend rapidly and be associated with ruptured inguinal abscess
- Phagedenic chancroid: may destroy external genitalia if superimposed Fusobacterium infection is present
- Do not confuse with chancre, a lesion typical of infection with syphilis
Epidemiology
- Mainly in developing countries, particularly Africa, Asia and Latin America
- Associated with commercial sex workers
Etiology
- Caused by Haemophilus ducreyi, a small gram negative rod
Clinical features
- Painful genital ulcer associated with tender suppurative inguinal adenopathy is suggestive
- Cofactor for HIV transmission (CDC: Sexually Transmitted Diseases Treatment Guidelines, 2006 [Accessed 28 March 2018])
- Often culture negative because Haemophilus ducreyi is very fragile in transport
- Molecular techniques are useful for diagnosis
- Must rule out Treponema pallidum (serology or darkfield examination) and HSV, which may coexist
Treatment
- Single oral dose of azithromycin or a single IM dose of ceftriaxone or oral erythromycin for seven days
Clinical images
Microscopic (histologic) description
- Zonation phenomenon at ulcer base
- Upper layer is ulcer base with fibrin, neutrophils and necrosis
- Middle layer has granulation tissue, palisading blood vessels and thrombosis
- Deep layer has marked lymphoplasmacytic infiltrate
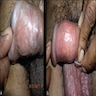
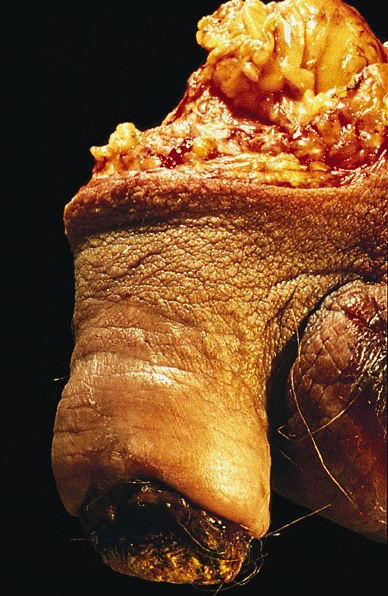
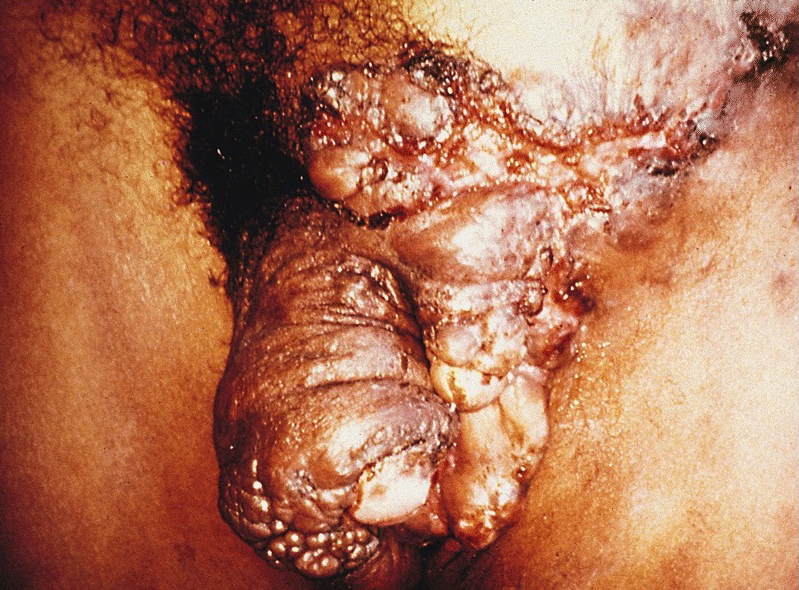
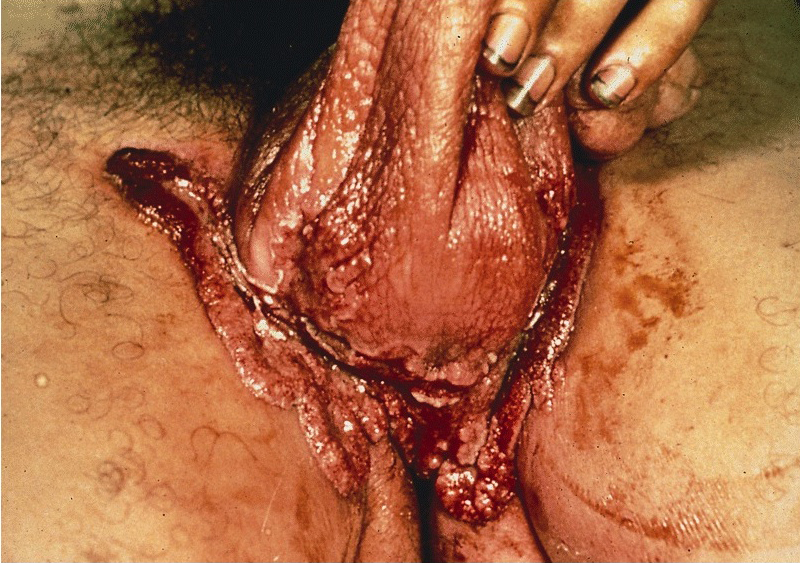
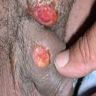
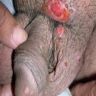
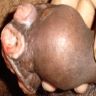
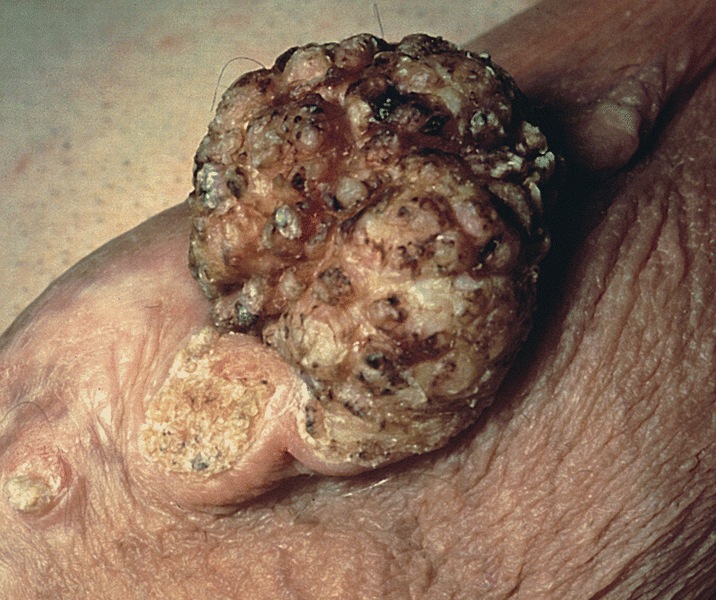
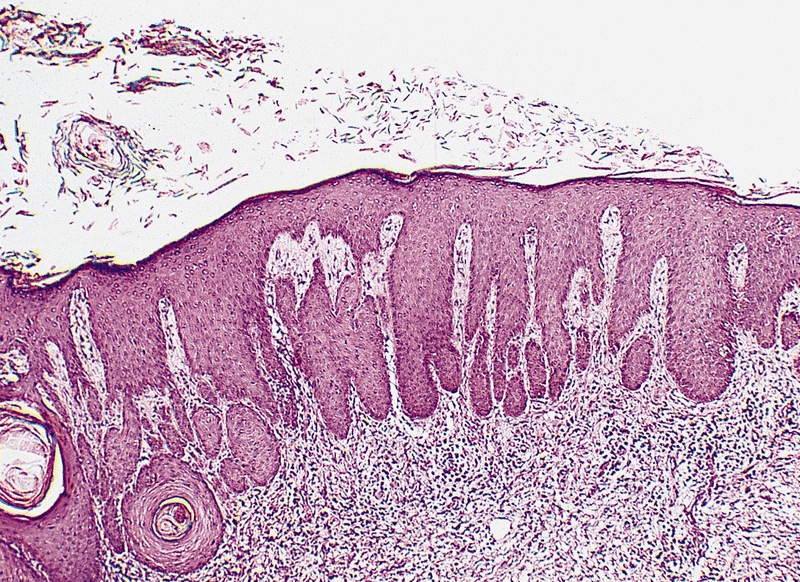
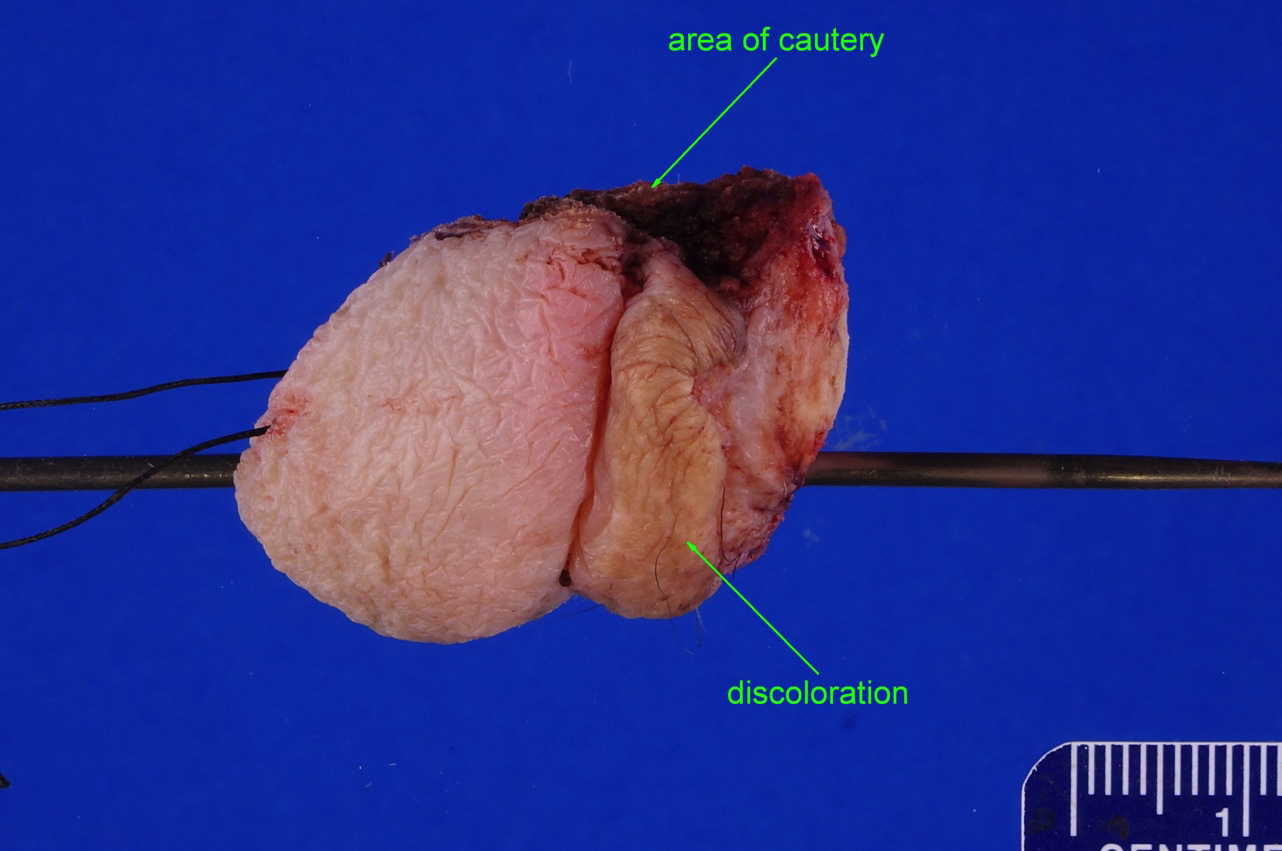
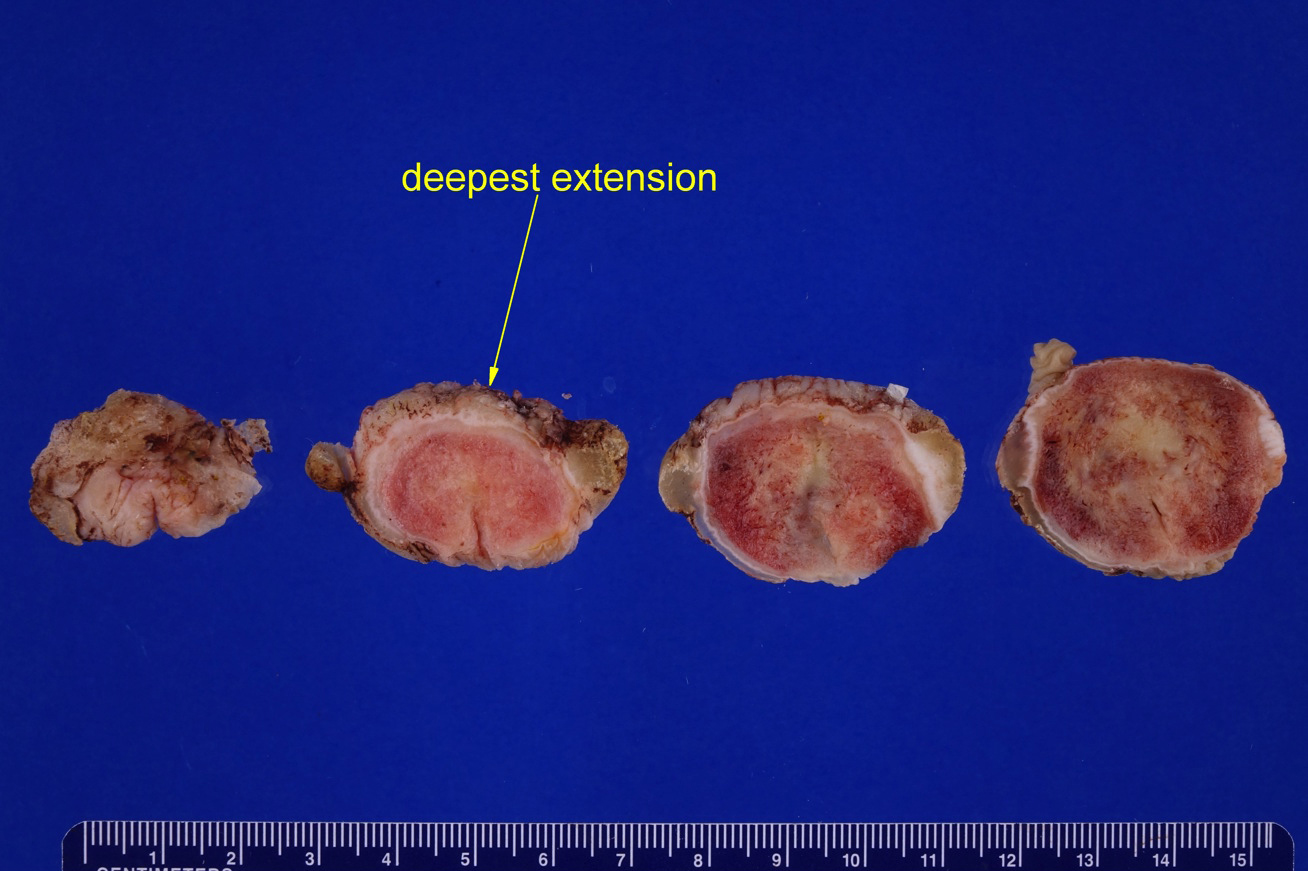
Condyloma acuminatum
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Definition / general
- Human papillomavirus (HPV) associated, nonneoplastic tumor-like growths
- Typically considered benign
Essential features
- HPV (6 and 11) associated lesions
- Frequently occurs in young, sexually active men
- Soft, flesh colored, cauliflower-like, raised or flat lesions
- Hallmark of the lesion is koilocytic atypia
- Benign course with high recurrence rate
Terminology
- Genital wart
- Anogenital wart
- Usual condyloma
- Flat condyloma
- Venereal wart
- References: Amin: Diagnostic Pathology - Genitourinary, 3rd Edition, 2022, Partin: Campbell-Walsh Urology 12th Edition Review, 3rd Edition, 2020
Epidemiology
- Most frequently occurs in men between 25 and 29 years of age
- Spread by direct skin to skin contact
- Transmitted sexually
- If found in children, sexual abuse should be suspected
- Autoinfection is common after initial infection
- References: Cheng: Urologic Surgical Pathology, 4th Edition, 2019, Medicine (Baltimore) 2019;98:e15109, Drugs Context 2018;7:212563
Sites
- Occurs most commonly on the penis, scrotum, perineum and anus
- Usual site of occurrence on the penis is the glans, followed by foreskin and penile shaft
- 5% of patients demonstrate urethral involvement, which may extend to the prostatic urethra
- Urinary bladder is rarely involved
- Giant condyloma acuminatum (Buschke-Löwenstein tumor) occurs in the urethra, coronal sulcus, frenulum or shaft
- Reference: Partin: Campbell-Walsh Urology 12th Edition Review, 3rd Edition, 2020, Drugs Context 2018;7:212563, J Clin Aesthet Dermatol 2016;9:S2
Pathophysiology
- Microabrasions cause HPV virus inoculation into the epithelial structures
- Virus replicates in the epithelial basal layer
- Warty plaques or papules form due to viral replication
- Viral genome of HPV has 6 early open reading frames (E1, E2, E4, E5, E6, E7) as well as 2 late open reading frames (L1, L2)
- Early open reading frames play a role in regulating and coding of proteins involved in viral replication and cell transformation
- High risk HPV strains directly integrate their genetic material into the host cell, resulting in uncontrolled activation of E6 and E7 genes, transcription of oncoproteins and inactivation of p53 and retinoblastoma tumor suppressor genes
- HPV integration into host cells causes formation of atypical and altered morphological cells called koilocytes
- Viral gene amplification occurs with migration of infected basal layer cells to adjacent layers
- Site of assembly of virions is in the superficial layer of the epithelium, from which they are released and infect their own or foreign adjacent tissues
- Viral effects on epithelium causes condyloma's exophytic phenotype
- Reference: Rom J Morphol Embryol 2021;62:369
Etiology
- Contagious disease
- Transmission by oral, genital and anal sex, through skin contact
- Nononcogenic mucosal HPV types 6 and 11 are detected in 75 - 100% of condylomas
- 15% are coinfected with high risk HPV (16 and 18) types
- HPV 16 and 18 can lead to squamous cell carcinoma
- Factors associated with higher rates of infection with HPV include
- Absence of circumcision
- Multiple sexual partners
- Lack of condom use
- Smoking
- References: Partin: Campbell-Walsh Urology 12th Edition Review, 3rd Edition, 2020, Amin: Diagnostic Pathology - Genitourinary, 3rd Edition, 2022, Heliyon 2020;6:e03547
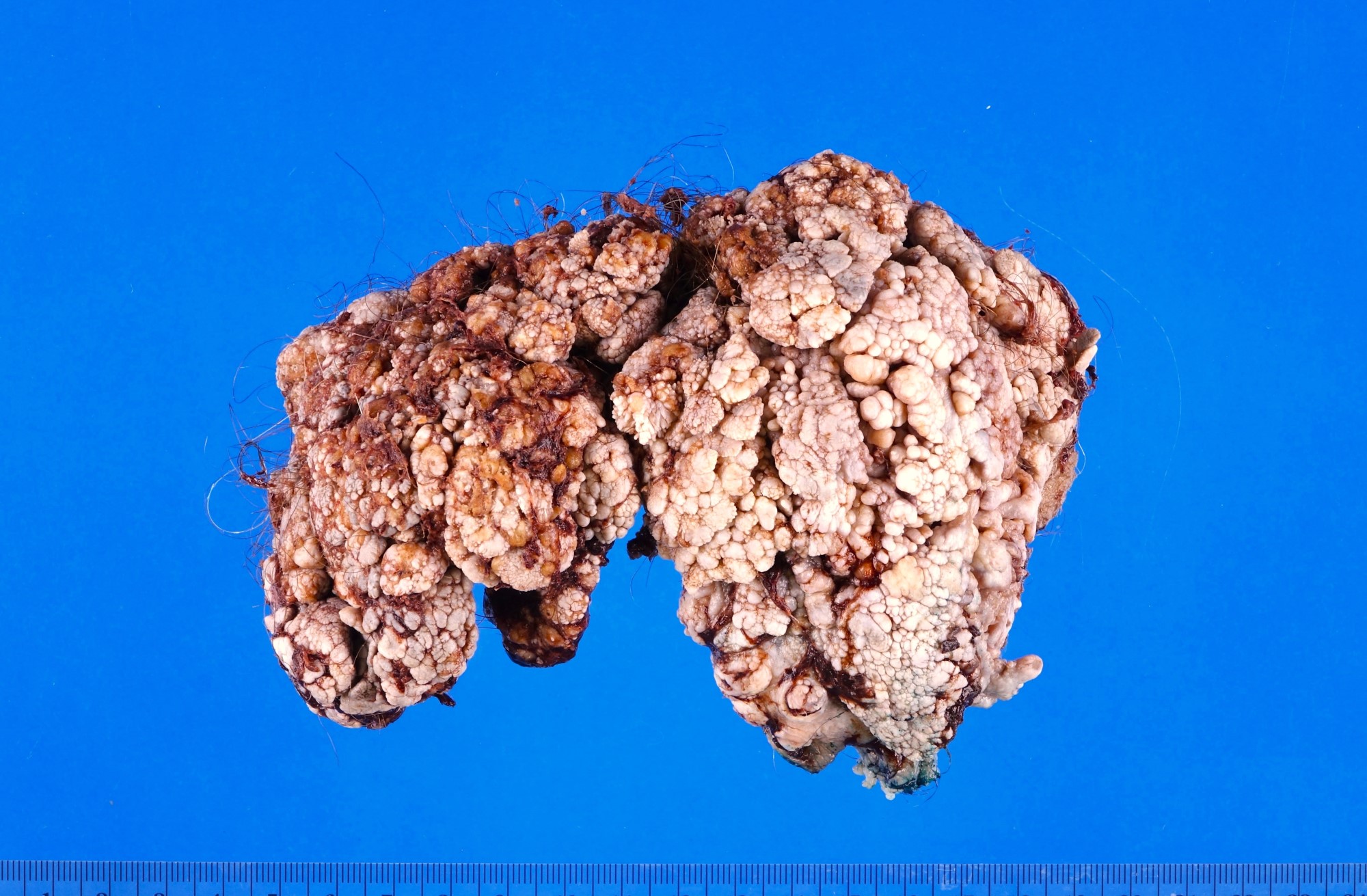
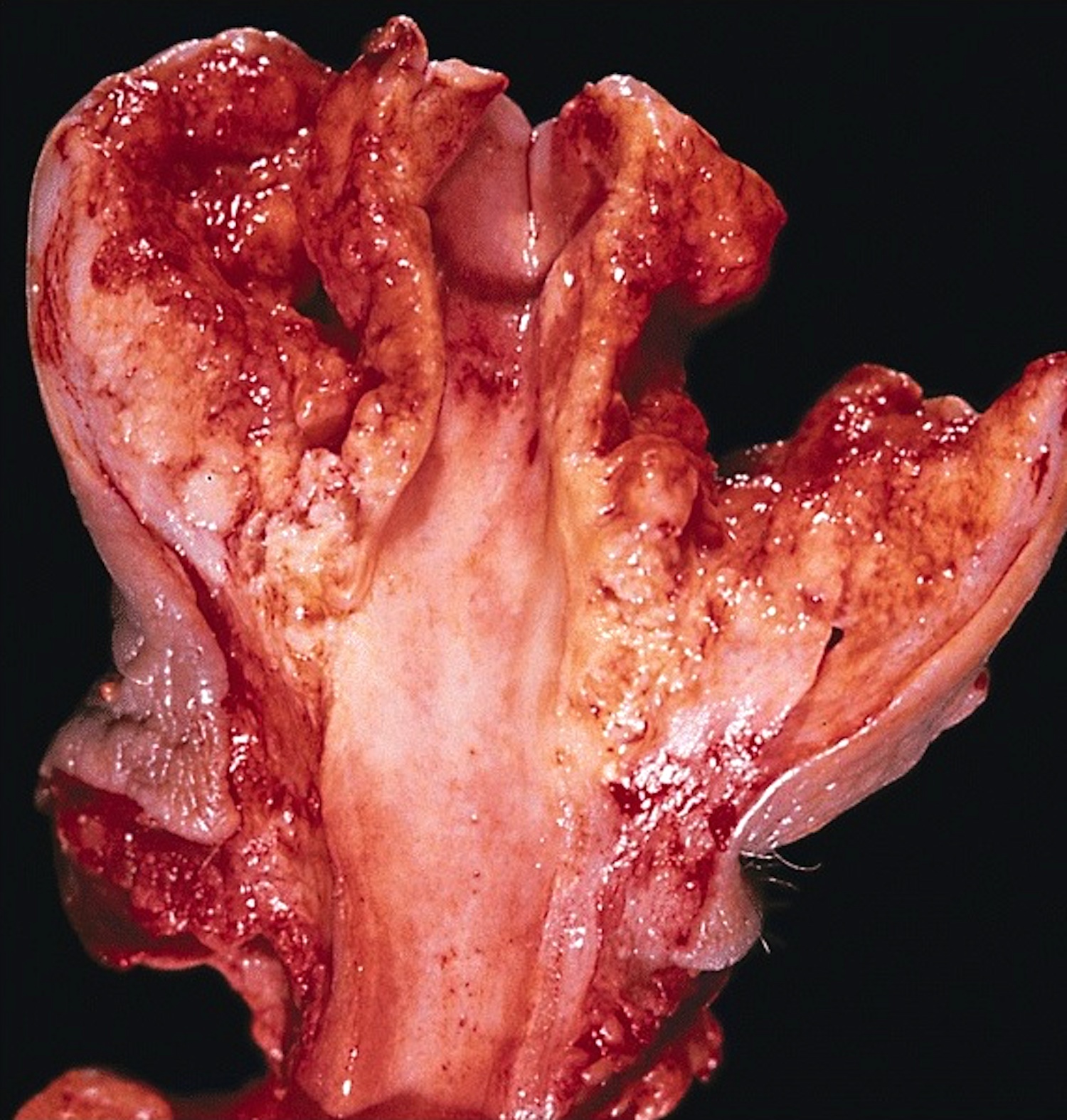
Clinical features
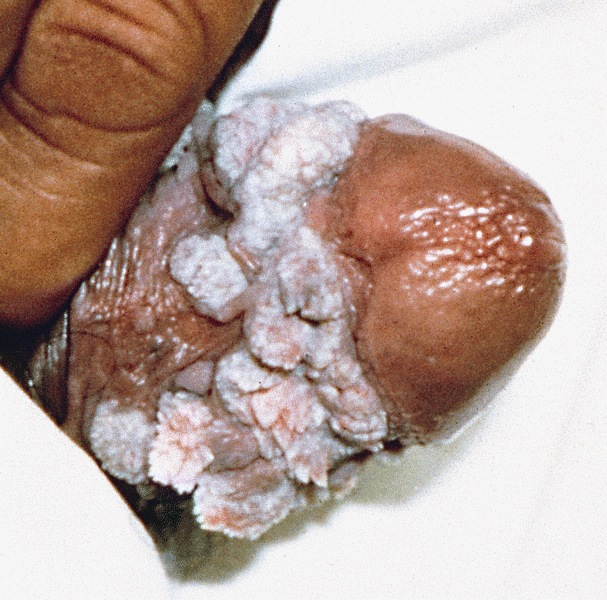
- Soft and friable papillomatous growth
- May occur singly or in moruloid clusters
- Giant condyloma acuminatum can become as large as 15 cm, destroying normal tissue and imparting a cancerous appearance
- Reference: Partin: Campbell-Walsh Urology 12th Edition Review, 3rd Edition, 2020
Diagnosis
- Detection is through thorough clinical history, physical examination and biopsy
- Subclinical disease and flat lesions, which may not be obvious on inspection, may be detected by application of 5% acetic acid solution to the penis, followed by inspection with a magnifying glass
- Lesions will turn white
- These acetowhite lesions should be confirmed by biopsy, since not all these lesions are caused by HPV
- Virus can be identified by immunohistochemistry, in situ hybridization and PCR
- Reference: Partin: Campbell-Walsh Urology 12th Edition Review, 3rd Edition, 2020, Drugs Context 2018;7:212563
Prognostic factors
- Benign course with high recurrence rate
- Posttreatment recurrence is seen in 38 - 73% patients with usual benign lesions
- Spontaneous regression is common
- Malignant transformation is rare; more frequently seen in longstanding giant condylomas
- Reference: Amin: Diagnostic Pathology - Genitourinary, 3rd Edition, 2022, Drugs Context 2018;7:212563
Case reports
- 26 year old White man with 6 months history of papillomatous lesions on the glans penis and orificium urethrae (Open Access Maced J Med Sci 2018;6:110)
- 26 year old Lebanese man with hypopigmented lesions on the penis (Indian J Dermatol Venereol Leprol 2016;82:572)
- 29 year old man with 1 month history of papillary lesions on the coronary sulcus of the penis (Medicine (Baltimore) 2019;98:e15109)
- 62 year old man with 6 year history of warty lesions on the genital areas (Acta Dermatovenerol Alp Pannonica Adriat 2021;30:117)
- 62 year old man with urinary obstruction (Am J Case Rep 2018;19:1522)
Treatment
- Commonly used treatment modalities include
- Podophyllotoxin 0.5% solution or gel (used historically)
- Trichloroacetic acid 35 - 85%
- Cryotherapy with liquid nitrogen
- Electrofulguration
- CO2 laser therapy
- Imiquimod cream (5%) - topical treatment of choice
- Surgical approaches include electrofulguration, cryosurgery, laser ablation for smaller lesions, local excision for medium sized lesions and wide local excision or penectomy for giant condylomas
- Significant rates of recurrence have been noted despite using laser, electrocautery or cryotherapy treatment options
- References: Amin: Diagnostic Pathology - Genitourinary, 3rd Edition, 2022, Partin: Campbell-Walsh Urology 12th Edition Review, 3rd Edition, 2020, J Clin Aesthet Dermatol 2016;9:S2, J Eur Acad Dermatol Venereol 2019;33:1006
Clinical images
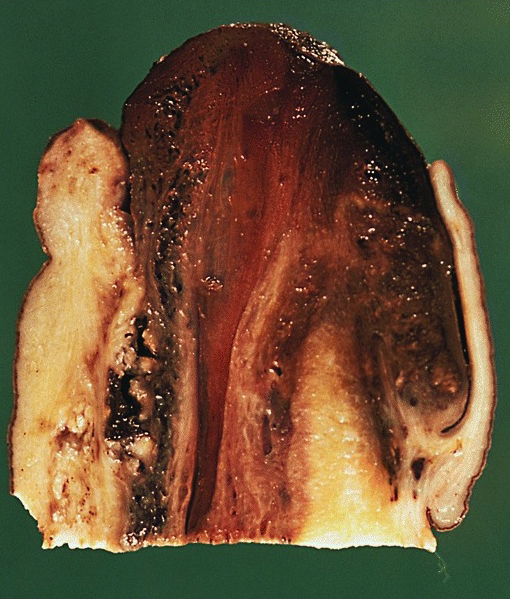
Gross description
- Soft, flesh colored lesions
- Can be flat, delicately papillary or warty and cauliflower-like
- References: Cheng: Urologic Surgical Pathology, 4th Edition, 2019, Heliyon 2020;6:e03547
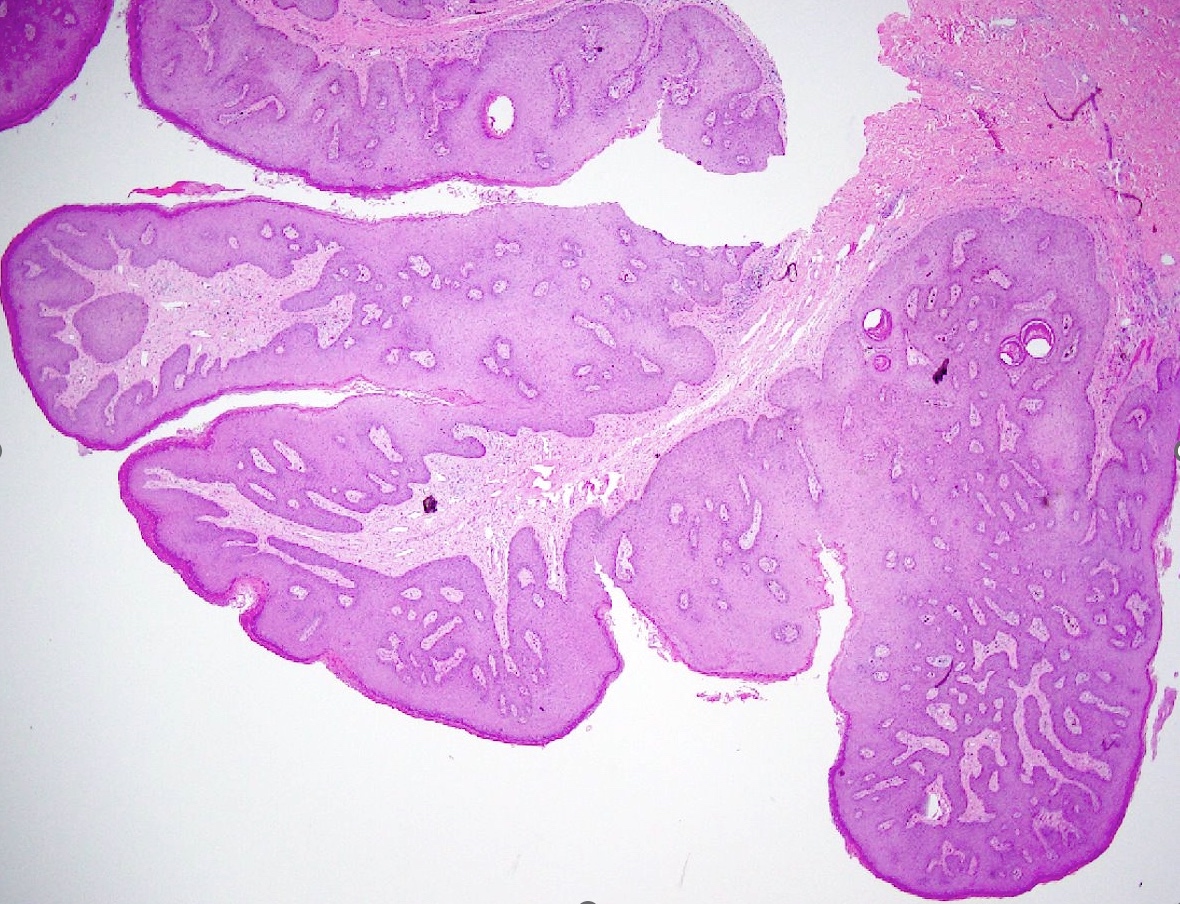
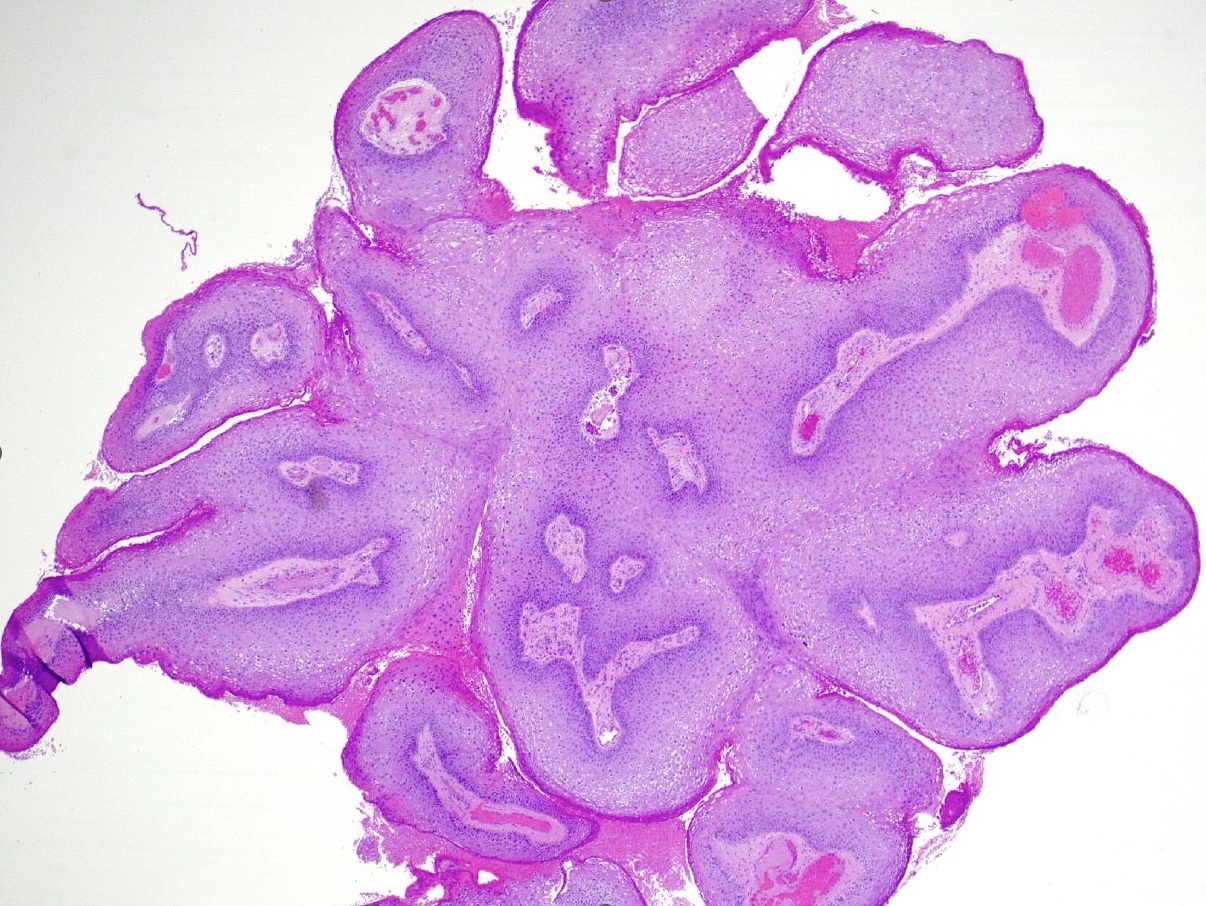
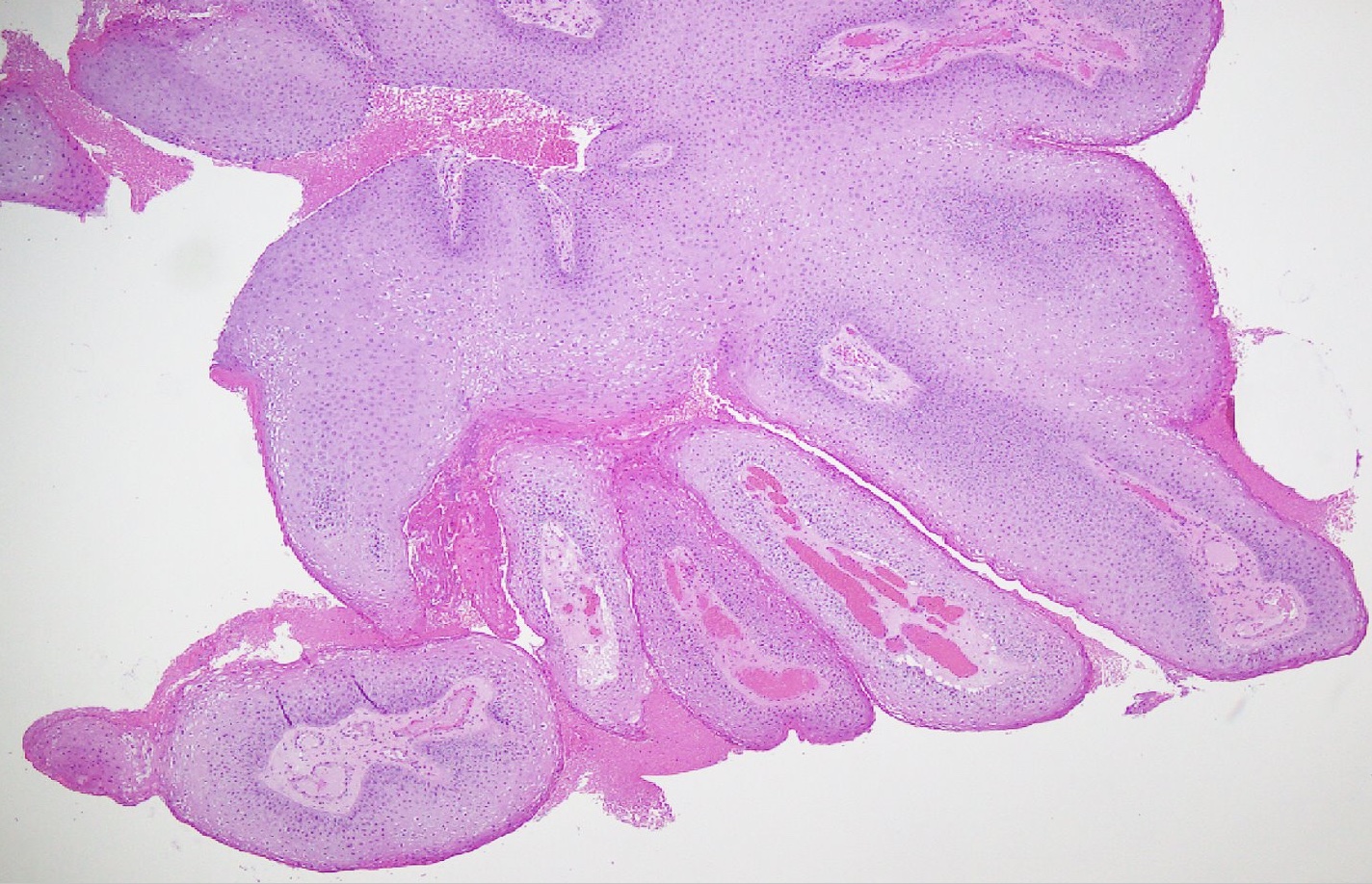
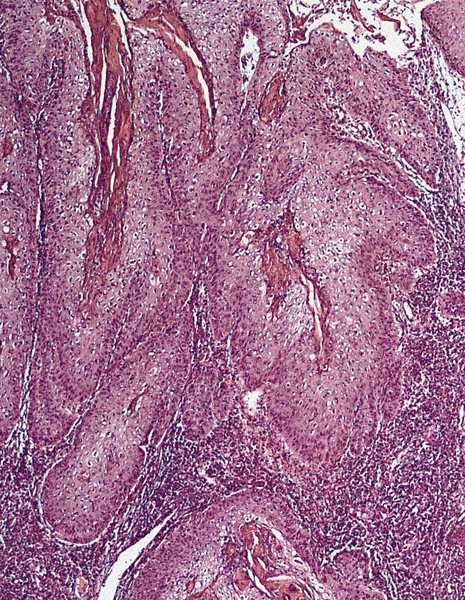
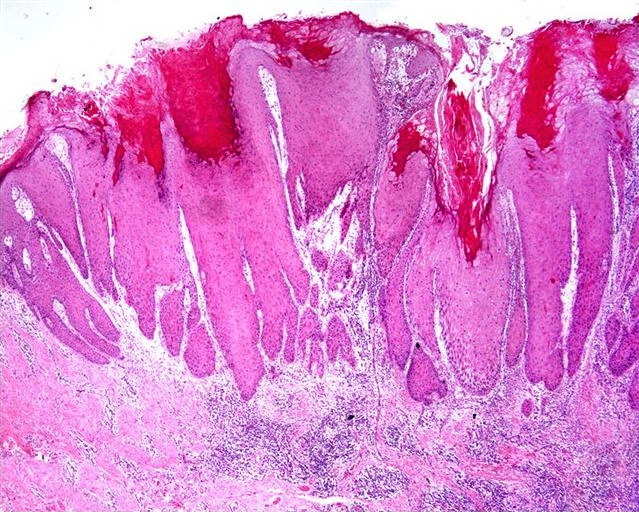
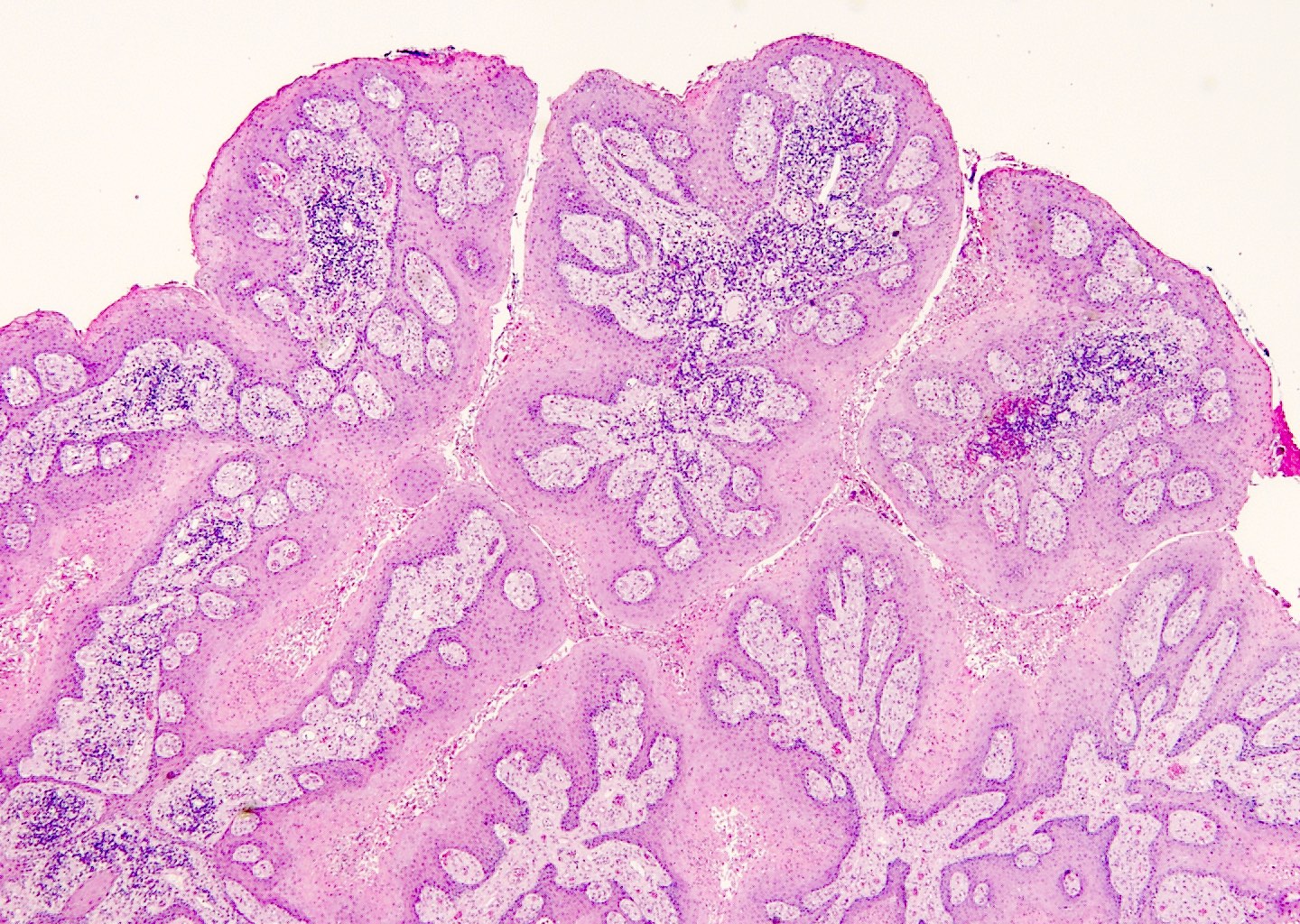
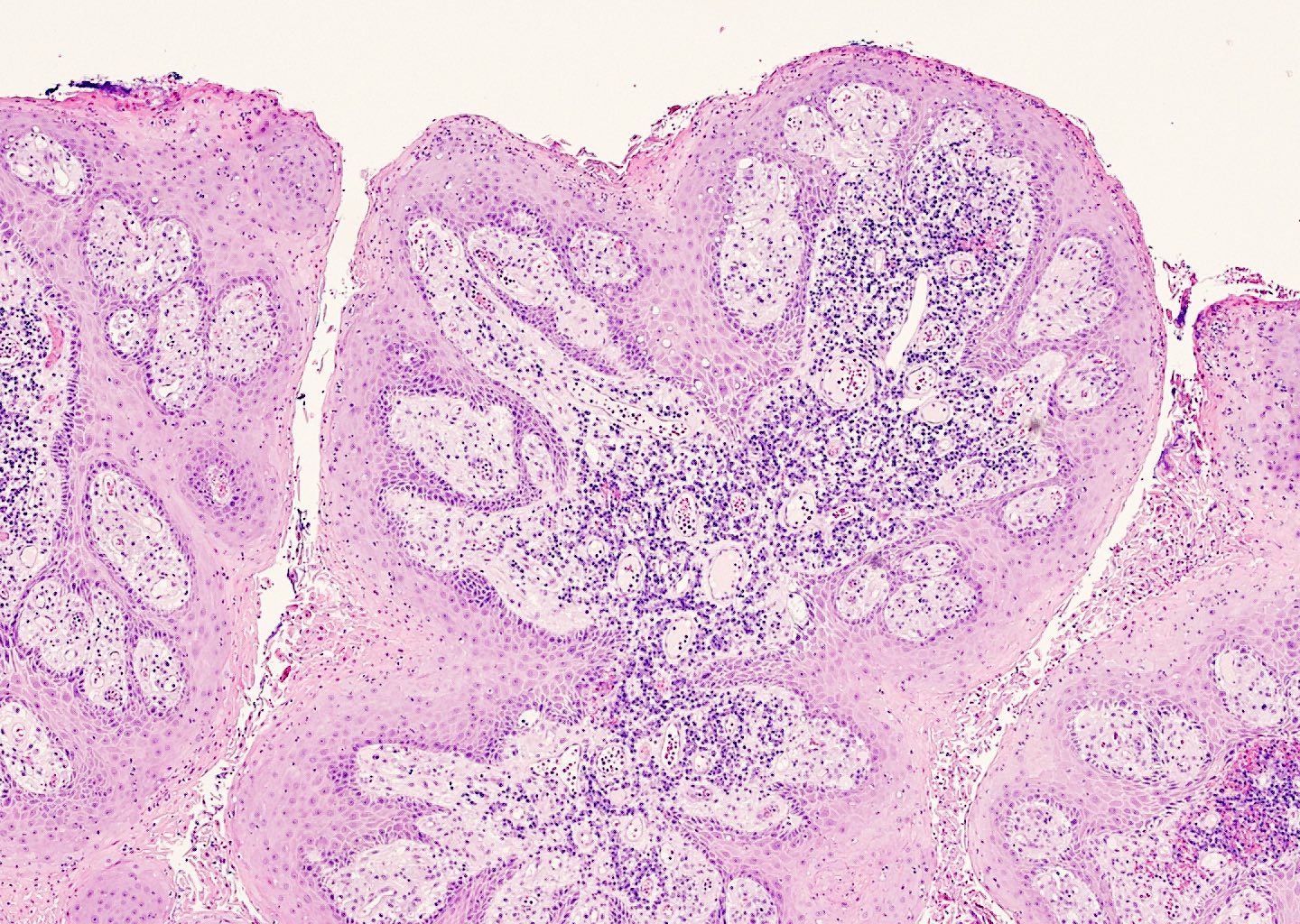
Microscopic (histologic) description
- Lesions show papillomatosis, acanthosis with a well demarcated bulbous base
- Prominent central fibrovascular cores with branching patterns
- Surface hyperkeratosis and parakeratosis are frequently seen in the papillae
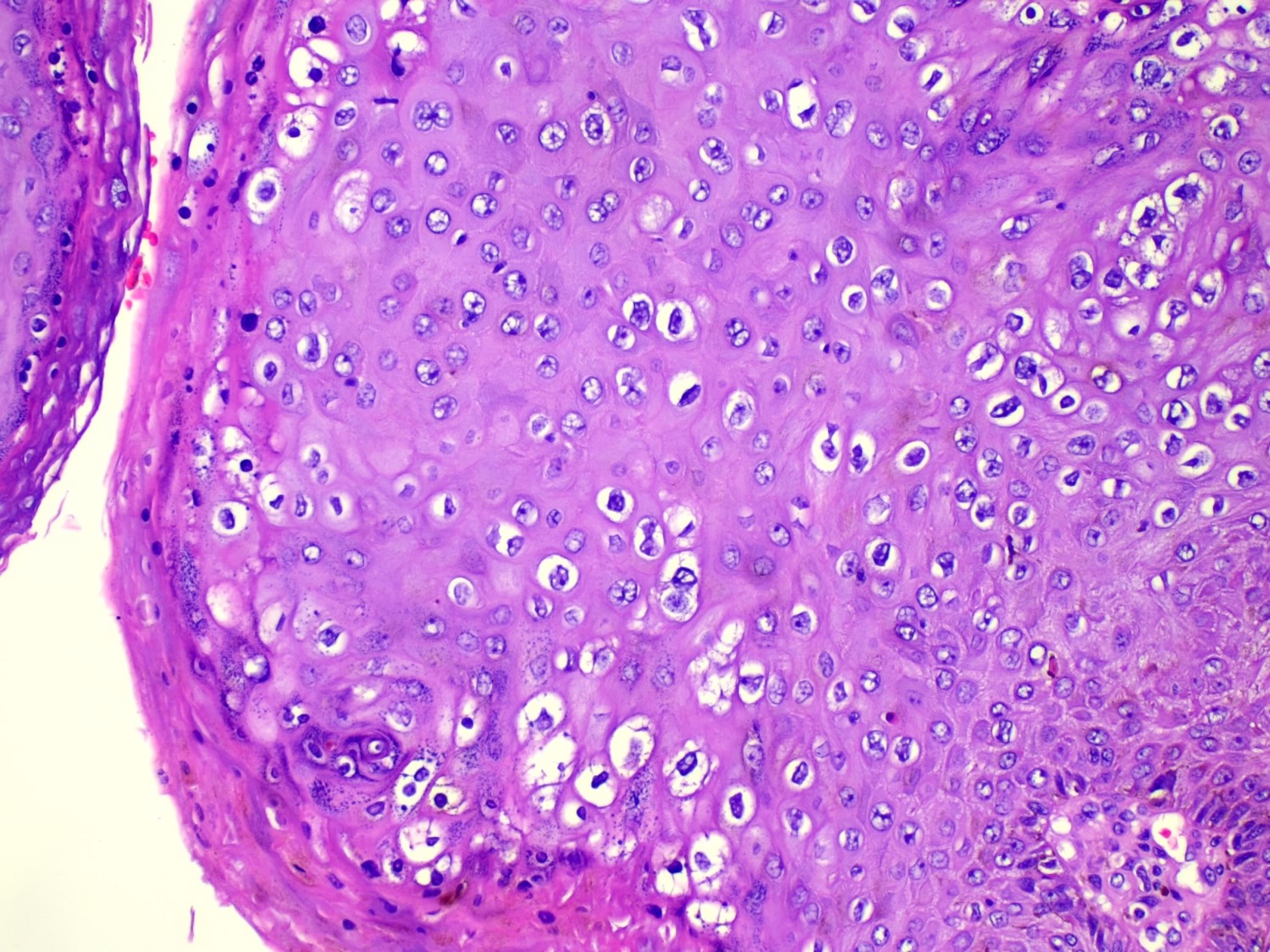
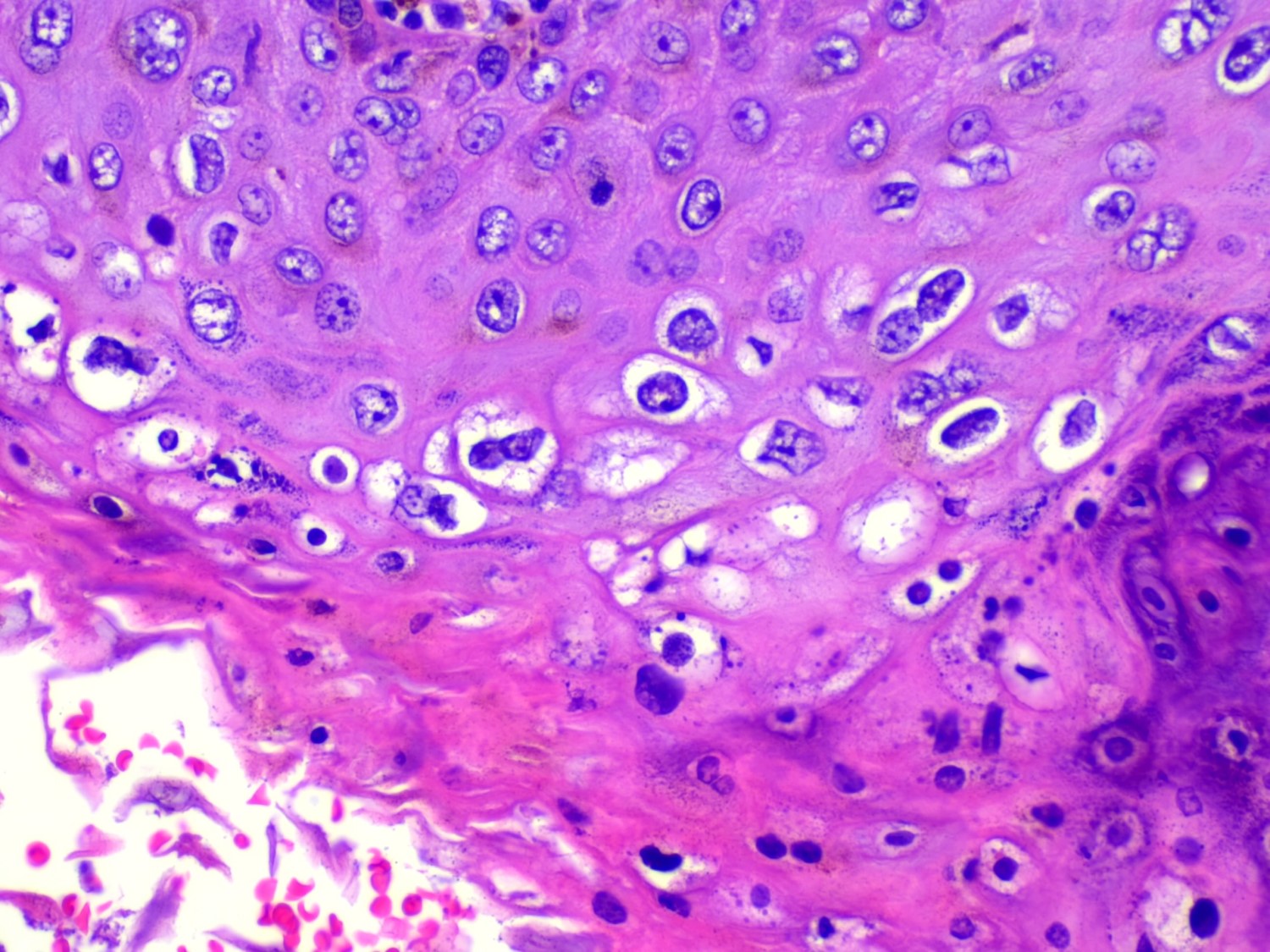
- Koilocytic atypia is the hallmark of the lesions
- Koilocytes have enlarged, wrinkled nuclei surrounded by a perinuclear halo
- Dyskeratotic cells with binucleation and multinucleated forms may be seen
- Koilocytic changes are more prominent on the upper levels of the epithelium
- Despite being the most classic and best recognized feature, koilocytes are not always prominent
- Sessile (flat) and inverted patterns are rare
- Prominent degenerative changes such as vacuolization, nuclear enlargement, numerous necrotic keratinocytes in the lower half of the epidermis and an increased number of mitotic figures are seen in condyloma acuminata that has been previously treated with topicals (e.g., podophyllum resin, also known as podophyllin)
- References: StatPearls: Condyloma Acuminata [Accessed 29 February 2024], Rom J Morphol Embryol 2021;62:369
Microscopic (histologic) images
Positive stains
- Immunochemistry is not routinely used, although positivity for p16 in condylomas is associated with high risk HPV (Asian Pac J Cancer Prev 2021;22:3219)
Molecular / cytogenetics description
- HPV, which are usually low risk types, can be detected by in situ hybridization and PCR
Videos
Condyloma histopathology
Genital warts under microscope
Sample pathology report
- Penis, lesion, biopsy:
- Condyloma acuminata (see comment)
- Comment: Koilocytic cells are seen within papillomatous lesion. There is no high grade dysplasia or malignancy identified.
Differential diagnosis
- Early penile plaques:
- Hyperkeratosis
- No koilocytosis
- HPV negative
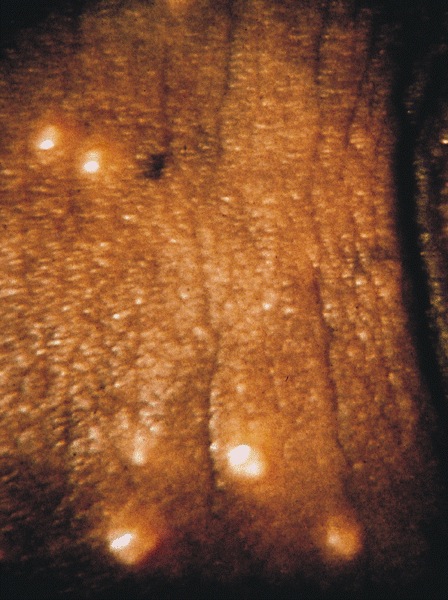
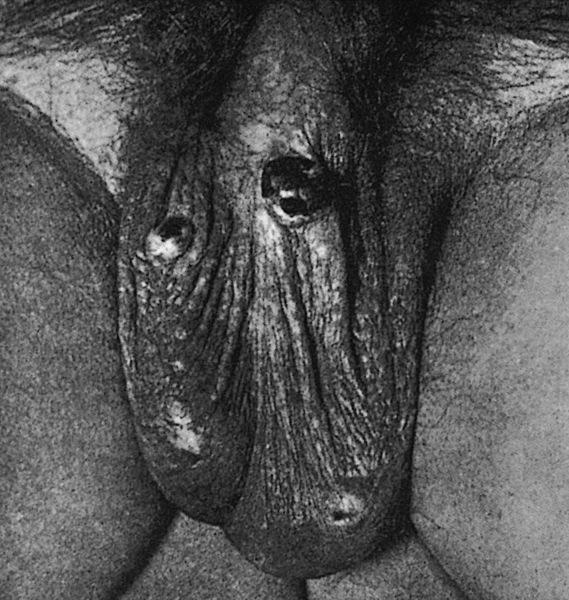
- Pearly penile papules:
- Also known as papillomatosis of glans corona
- Monomorphic yellow-white papules on the coronal sulcus and penile corona in uncircumcised men
- Thick epithelial covering over fibrovascular cores, resembling angiofibromas
- HPV negative
- References: Cheng: Urologic Surgical Pathology, 4th Edition, 2019, Rom J Morphol Embryol 2021;62:369
- Acrochordons:
- Also known as skin tags
- Soft, flesh colored, sessile or pedunculated lesions
- Occasionally may be hyperkeratotic or warty appearing (Drugs Context 2018;7:212563)
- HPV negative
- Seborrheic keratosis:
- Acanthosis, papillomatosis and occasional hyperpigmentation
- HPV negative
- Warty carcinoma (Amin: Diagnostic Pathology - Genitourinary, 3rd Edition, 2022):
- Papillary squamous cell carcinoma:
- Exophytic invasive squamous cell carcinoma with complex irregular papillae possessing jagged interface with the underlying stroma
- Irregular wide areas of keratinization are present between adjacent papillae
- Condylomas with malignant transformation (Amin: Diagnostic Pathology - Genitourinary, 3rd Edition, 2022):
- Rare event for common condylomas
- Usually associated with longstanding giant condylomas
- Malignant foci are composed of conventional squamous cell carcinoma and tend to occur at tumor base or front
- Molluscum contagiosum (Cheng: Urologic Surgical Pathology, 4th Edition, 2019, Drugs Context 2018;7:212563, Rom J Morphol Embryol 2021;62:369):
- Diagnosis is usually made clinically
- Caused by molluscum contagiosum virus (MCV), a pox virus
- Smooth, dome shaped, 1 - 5 mm papules with central umbilication
- Cytoplasmic inclusions (molluscum bodies) are seen in cells of the stratum malpighii and progressively enlarge as they reach the surface
- Verrucous carcinoma (Amin: Diagnostic Pathology - Genitourinary, 3rd Edition, 2022):
- Condyloma latum (Cheng: Urologic Surgical Pathology, 4th Edition, 2019, Rom J Morphol Embryol 2021;62:369):
- Cutaneous lesion of secondary syphilis caused by Treponema pallidum
- Appear as wet, sometimes weepy plaques
- Dark field microscopy can be used to visualize spirochetes
- Prominent epithelial hyperplasia that may become pseudoepitheliomatous with ulceration and neutrophilic exocytosis
- Plasma cell infiltrate and capillary endothelial proliferation
Additional references
Board review style question #1
Board review style answer #1
A. Condyloma acuminatum. The clinical presentation along with the microscopic findings of koilocytosis, acanthosis and hyperkeratosis are classic for condyloma acuminatum caused by HPV infection. Answer B is incorrect because condyloma latum is a cutaneous lesion of secondary syphilis caused by treponema pallidum, which would show prominent epithelial hyperplasia with plasma cell infiltrate and capillary endothelial proliferation. Answer C is incorrect because molluscum contagiosum would have molluscum bodies. Answer D is incorrect because verrucous carcinoma would show endophytic growth with a broad based pushing pattern of invasion and absent koilocytic atypia.
Comment Here
Reference: Condyloma acuminatum
Comment Here
Reference: Condyloma acuminatum
Board review style question #2
Board review style answer #2
C. Smoking. Smoking is one of the risk factors associated with higher rates of infection with HPV.
Answers A, B and D are incorrect because lack of multiple sexual partners, safe sex practices (use of condoms) and circumcision decrease risk of acquiring HPV infection.
Comment Here
Reference: Condyloma acuminatum
Comment Here
Reference: Condyloma acuminatum
Board review style question #3
Which of the following is true regarding the pathogenesis of condyloma acuminatum?
- E6 and E7 viral gene activation causes inactivation of p53 and retinoblastoma tumor suppressor genes
- Viral effects on the epithelium causes condyloma's endophytic phenotype
- Virus does not integrate into host cells
- Virus remains confined to the basal cell layer
Board review style answer #3
A. E6 and E7 viral gene activation causes inactivation of p53 and retinoblastoma tumor suppressor genes.
Answer B is incorrect because viral effects on the epithelium cause exophytic growth. Answer C is incorrect because viral integration into host cells does occur, causing koilocyte formation. Answer D is incorrect because although the viral replication happens in the basal layer, the infected basal layer cells migrate to adjacent layers causing amplification and the assembly of the virions takes place in the superficial layer of the epithelium.
Comment Here
Reference: Condyloma acuminatum
Comment Here
Reference: Condyloma acuminatum
Congenital anomalies
Table of Contents
Aphallia | Chordae (chordee) | Concealed penis | Diphallia | Epispadias | Hypospadias | Clinical images | Lateral curvature | Median raphe cysts | Microscopic (histologic) images | Micropenis | Torsion | Webbed penisAphallia
- Agenesis of penis caused by failure in embryologic development of genital tubercle
- Very rare, incidence of 1 per 10 million male births; < 100 cases reported
- Associated with other GU abnormalities and with musculoskeletal and cardiopulmonary defects
- Can classify based on site of urethral meatus (J Urol 1989;141:589)
- Case reports: associated with urethrorectal fistula (Saudi J Kidney Dis Transpl 2008;19:435), various anomalies (J Pediatr Surg 2010;45:E13)
Chordae (chordee)
- Fibrous band associated with hypospadias or epispadias that causes bending of penis
Concealed penis
- Also called hidden or buried penis
- Penis is normally developed but hidden under fat in suprapubic region, scrotum, perineum and thigh
- May be complication of circumcision
- In adults, surgical repair may be complicated (J Sex Med 2009;6:876)
Diphallia
- Duplication of penis
- Occurs in 1 per 5 million male births
- Associated with hypospadias, bifid scrotum, bladder duplication (Cir Pediatr 2008;21:235) and renal agenesis
Epispadias
- Urethra opens onto dorsal surface of penis
- Very rare, incidence of 1/300,000 male births
- Part of exstrophy-epispadias complex (Orphanet J Rare Dis 2009;4:23)
- Not related to hypospadias (has a different embryologic defect)
- Penopubic epispadias (opening in penopubic junction) is most common, associated with urinary incontinence
- Treatment is surgical
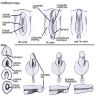
Hypospadias
- Most common congenital abnormality of male external genitalia other than cryptorchidism
- Urethra opens onto ventral surface of penis or scrotum
- 3 - 5/1000 live male births
- Due to failure of fusion of urethral folds; may be due to mutations in MAMLD1 (CXorf6) gene (Horm Res 2009;71:245)
- Urethral opening is usually near glans
- Hypospadias and epispadias are associated with abnormal descent of testes, urinary tract malformations, obstruction, urinary tract infections and possibly infertility if orifices are near base of penis
- Treatment is usually surgical unless hypospadias is minor (eMedicine: Hypospadias [Accessed 28 March 2018])
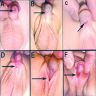
Classified by location of opening of meatus (see first clinical image below):
- A: anterior (inferior surface of glans)
- B: coronal (in balanopenile furrow)
- C: distal third of shaft
- D: penoscrotal (at base of shaft in front of scrotum)
- E: scrotal (on scrotum or between the genital swellings)
- F: perineal (behind scrotum or genital swellings)
Clinical images
Lateral curvature
- Due to hypo / hyperplasia of one corpora cavernosa
- Surgical treatment is often effective (J Urol 2008;179:1495)
Median raphe cysts
- Relatively common
- Due to anomalies in development of urethral groove, trapped epithelial cells or migration of epithelial cells after closure of genital folds
- Usually in foreskin or glans; may also be present in frenulum
- Lined by squamous, columnar, mucus producing, apocrine-like or distal urethra type epithelium
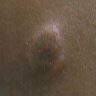
- Case report: 43 year old man with an asymptomatic nodule on glans (Dermatol Online J 2005;11:37)
Microscopic (histologic) images
Micropenis
- Penis small but normal ratio of shaft length to circumference
- Defined as stretched length < 2.5 standard deviations below mean for age (Wikipedia: Micropenis [Accessed 28 March 2018], eMedicine: Microphallus [Accessed 28 March 2018])
- Usually associated with endocrine abnormalities (insufficient androgen stimulation during embryologic growth of external genitalia, Arch Dis Child 1991;66:1033)
Torsion
- Fibrous tissue surrounding corpus spongiosum or short urethra causes rotational defect of penile shaft
- Isolated neonatal torsion occurs in 27%, usually to left (J Pediatr Urol 2007;3:495)
- Can be surgically corrected in adults, although patients often tolerate it without complaint (J Sex Med 2008;5:735)
Webbed penis
- Scrotal skin extends to ventral portion of penis and hides it
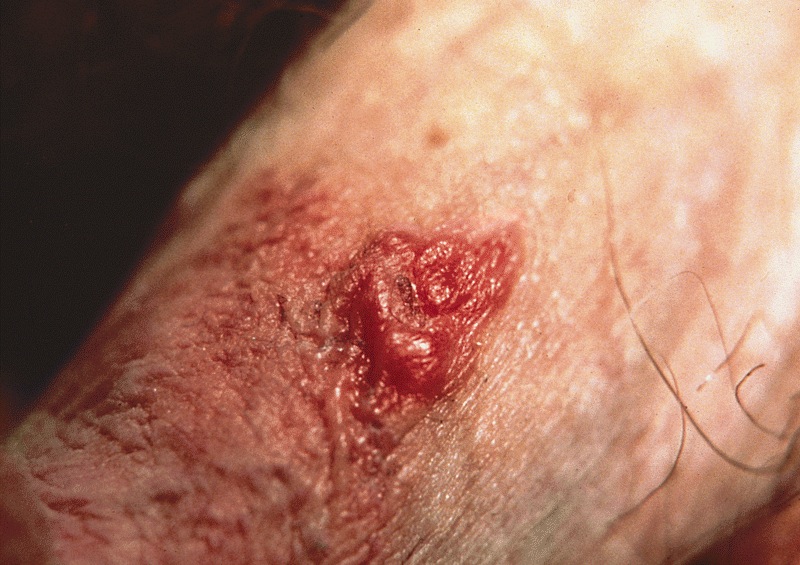
Extramammary Paget disease
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Paget disease of the penis and scrotum is a rare, intraepidermal adenocarcinoma; it arises as a primary tumor or from secondary involvement of a nonpenoscrotal neoplasm
Essential features
- Single or clusters of atypical intraepithelial cells involving the penis or scrotum
- Primary tumor expresses CK7 and is negative for CK20 and melanocytic markers
- Correlation with clinical history to evaluate for an secondary malignancy etiology
Terminology
- Paget's disease, extramammary Paget disease, extramammary Paget's disease
ICD coding
- ICD-O: 8542/3 - Paget disease, extramammary (except Paget disease of bone)
Epidemiology
- Limited data on population incidence
- Surveillance, Epidemiology and End Results (SEER) registry reported 61% prevalence in White populations and 36% in Asian or Native American populations (Dis Colon Rectum 2019;62:1283)
Sites
- Penis or scrotum epidermis or dermis
Pathophysiology
- Primary Paget disease: unknown precursor cell
- May be Toker cell, as seen in other regions but not yet identified on penile or scrotal sites (Dermatol Online J 2019;25:13030)
- May also originate from apocrine glands, eccrine glands or epidermal basal cells (Dermatol Online J 2019;25:13030)
- Secondary Paget disease: direct extension or epidermotropic spread of underlying tumor from urogenital, colorectal or cutaneous origin sites (Dis Colon Rectum 2019;62:1283)
- Usually metachronous, with presentation at a median of 4 years after diagnosis of Paget disease (Dis Colon Rectum 2019;62:1283)
Etiology
- No known risk factors
- Not human papillomavirus (HPV) related (Int J Surg Pathol 2018;26:617)
- 10% also have a genitourinary malignancy, most commonly prostatic adenocarcinoma, followed by urothelial carcinoma of the upper urinary tract / bladder (Dis Colon Rectum 2019;62:1283)
Clinical features
- Older men; median age: 65 - 72 years (range: 42 - 91) (Indian J Dermatol Venereol Leprol 2020;86:134, Dis Colon Rectum 2019;62:1283, Sci Rep 2017;7:44933, Hum Pathol 2016;47:70, BJU Int 2008;102:485, BJU Int 2015;115:153)
- Presents as lesions with pruritus, erythema, pain, rash, erosion and exudation (Indian J Dermatol Venereol Leprol 2020;86:134, Hum Pathol 2016;47:70)
- Often involves a large area (median size: 20.5 cm²) (Indian J Dermatol Venereol Leprol 2020;86:134)
- Often misdiagnosed and initially given a nonneoplastic clinical diagnosis
- Median delay of 3 - 4 years between initial symptoms to correct diagnosis (Indian J Dermatol Venereol Leprol 2020;86:134, Sci Rep 2017;7:44933, Hum Pathol 2016;47:70, Urol Oncol 2010;28:28, BJU Int 2008;102:485, BJU Int 2015;115:153)
Diagnosis
- Clinical history and evaluation with a punch biopsy (Dermatol Online J 2019;25:13030)
- Microscopic examination of tissue
- Clinical and radiologic evaluation in order to evaluate for secondary involvement from a tumor of nonpenoscrotal origin
- Sentinel lymph node biopsy may be considered to assist in disease staging (Dermatol Online J 2019;25:13030)
Laboratory
- Evaluate tumor serum markers such as carcinoembryonic antigen (CEA) and prostate specific antigen (PSA)
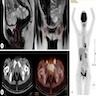
Radiology description
- Ultrasound may show skin lesion with irregular contours, heterogenous echogenicity, increased vascularity and dermal invasion (Ultrasound Q 2020;36:84)
- Imaging may be used to assess for nonscrotal primary tumors and to rule out distant metastases
Prognostic factors
- Unfavorable prognostic factors
- Younger age, shorter symptom duration, delay in diagnosis, exudation, elevated serum CEA, adnexal involvement, greater depth of invasion, wide horizontal invasion, nodule formation, marked inflammation, lymphovascular invasion, HER2 / neu and p53 expression (Indian J Dermatol Venereol Leprol 2020;86:134, Sci Rep 2017;7:44933, Hum Pathol 2016;47:70, Br J Dermatol 2009;161:577)
- Complete resection is associated with improved survival (Indian J Dermatol Venereol Leprol 2020;86:134, Dermatol Online J 2019;25:13030, Dermatol Surg 2017;43:708, Dis Colon Rectum 2019;62:1283, BMC Cancer 2018;18:403)
- Recurrence in 10 - 40% of patients (Sci Rep 2017;7:44933, Hum Pathol 2016;47:70, BJU Int 2008;102:485)
- Metastatic sites
- Regional lymph nodes (most common), nonregional lymph nodes, bone (Br J Dermatol 2009;161:577, Urol Oncol 2010;28:28, BJU Int 2015;115:153)
- Lymph node metastasis rate: 5 - 34% (Sci Rep 2017;7:44933, Hum Pathol 2016;47:70, Urol Oncol 2010;28:28)
- 3 - 5 year survival: ~70% (Dis Colon Rectum 2019;62:1283, Hum Pathol 2016;47:70, Sci Rep 2017;7:44933, BJU Int 2015;115:153)
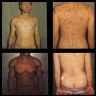
Case reports
- 60 year old man with erythematous plaque on inner prepuce of penis (J Cancer Res Ther 2020;16:1535)
- 61 year old man with flaky rash in the penoscrotal region for 9 months (Case #520)
- 77 year old man with erythematous lesions on his scrotum extending to the inguinal area (Cureus 2022;14:e29486)
- 79 year old man with scaling, erythematous plaques on his scrotum (JAAD Case Rep 2022;23:87)
- 85 year old man with recurrent erythematous plaque lesions on the penis secondary to urothelial carcinoma (J Cutan Pathol 2022;49:663)
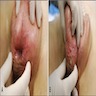
Treatment
- Wide local excision or Mohs micrographic surgery (Asian J Surg 2023;46:4261)
- If surgery is not possible, alternative therapies (with limited data supporting their use) include imiquimod cream, photodynamic therapy, radiotherapy and chemotherapy (Dermatol Online J 2019;25:13030, Am J Cancer Res 2023;13:4492)
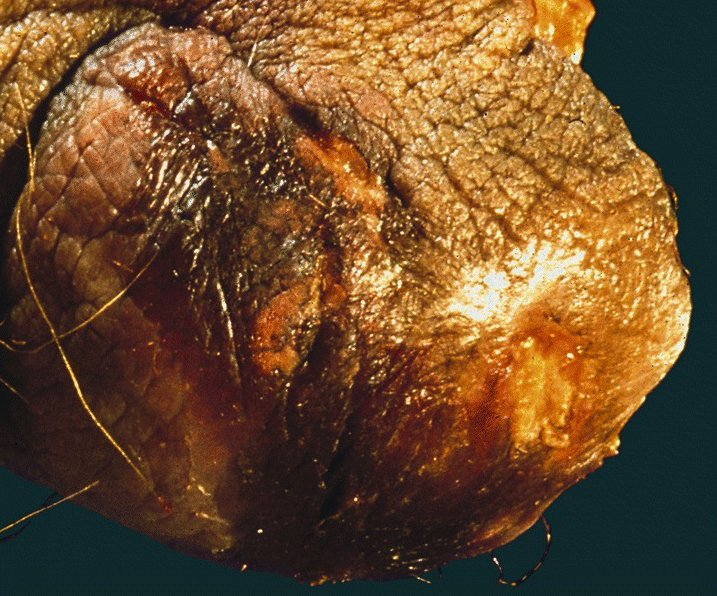
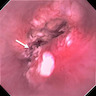
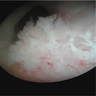
Clinical images
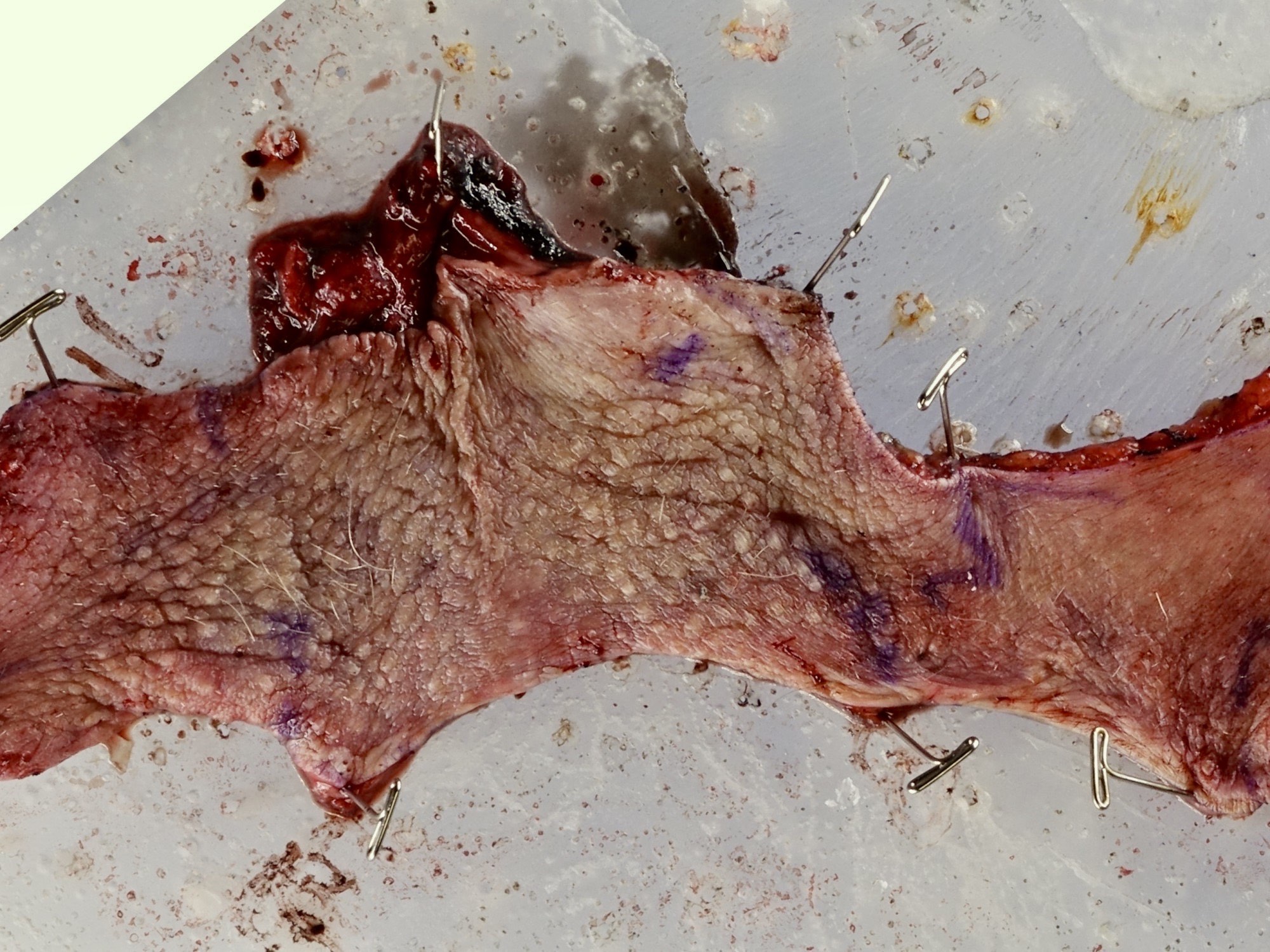
Gross description
- Erythematous, scaly epidermal lesions with undulating edge
- Often with ulceration and exudation
- Median size: 20.5 cm² (Indian J Dermatol Venereol Leprol 2020;86:134)
Frozen section description
- Intraoperative frozen section can be used to assess margins for local wide excision (J Med Case Rep 2009;3:4, J Plast Reconstr Aesthet Surg 2020;73:1700, BJU Int 2007;100:1282, Urol Oncol 2009;27:483)
- Difficult to diagnose via frozen section; false negative rate up to 40% (Ann Surg Oncol 2017;24:3229, Asian J Androl 2013;15:508)
- Clues to help identify tumoral cells include retraction artifact, basal location, paler cytoplasm, nucleomegaly and prominent nucleoli
- Frozen artifact to the squamous epithelium can mask and retracted tumor cells
Frozen section images
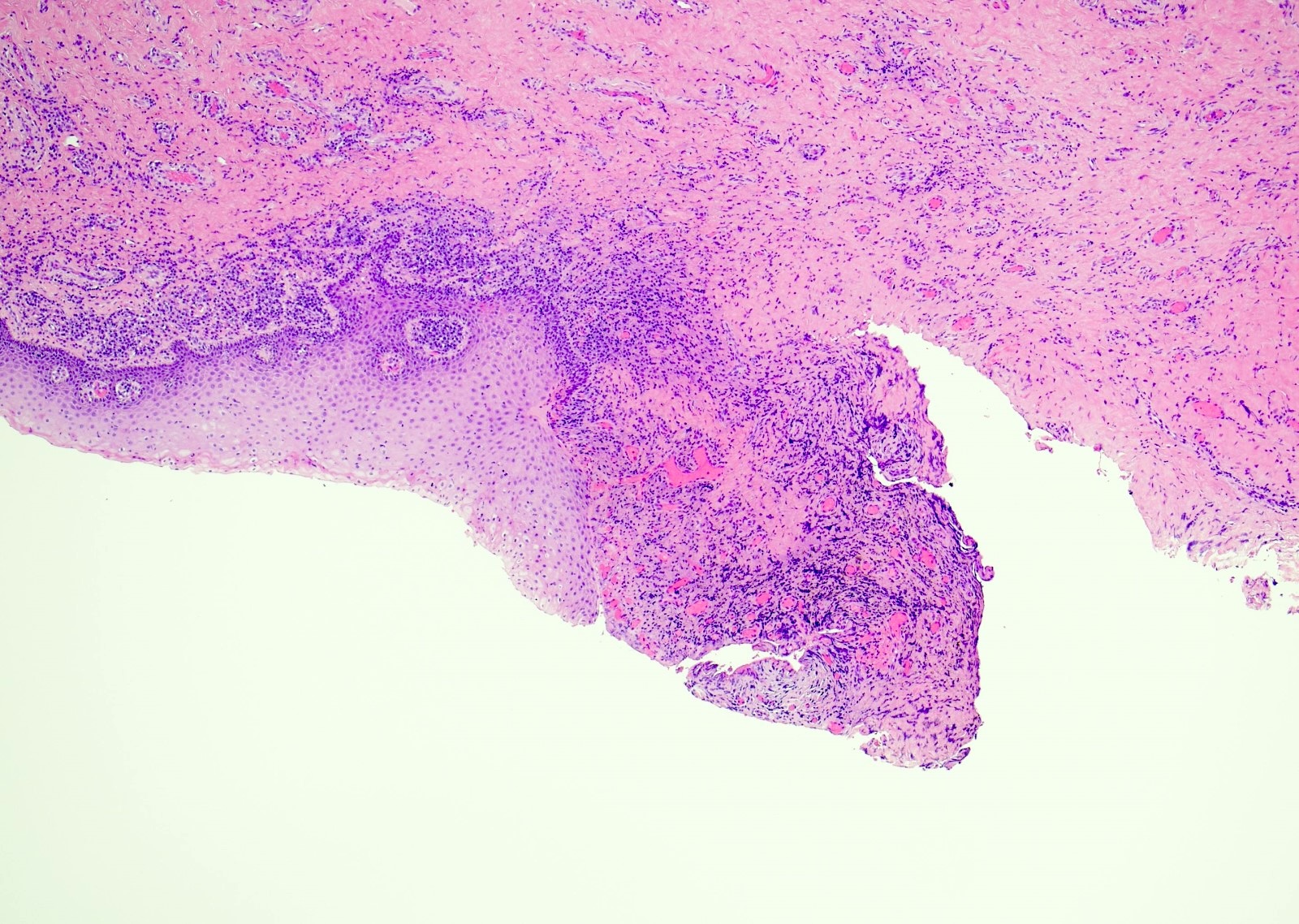
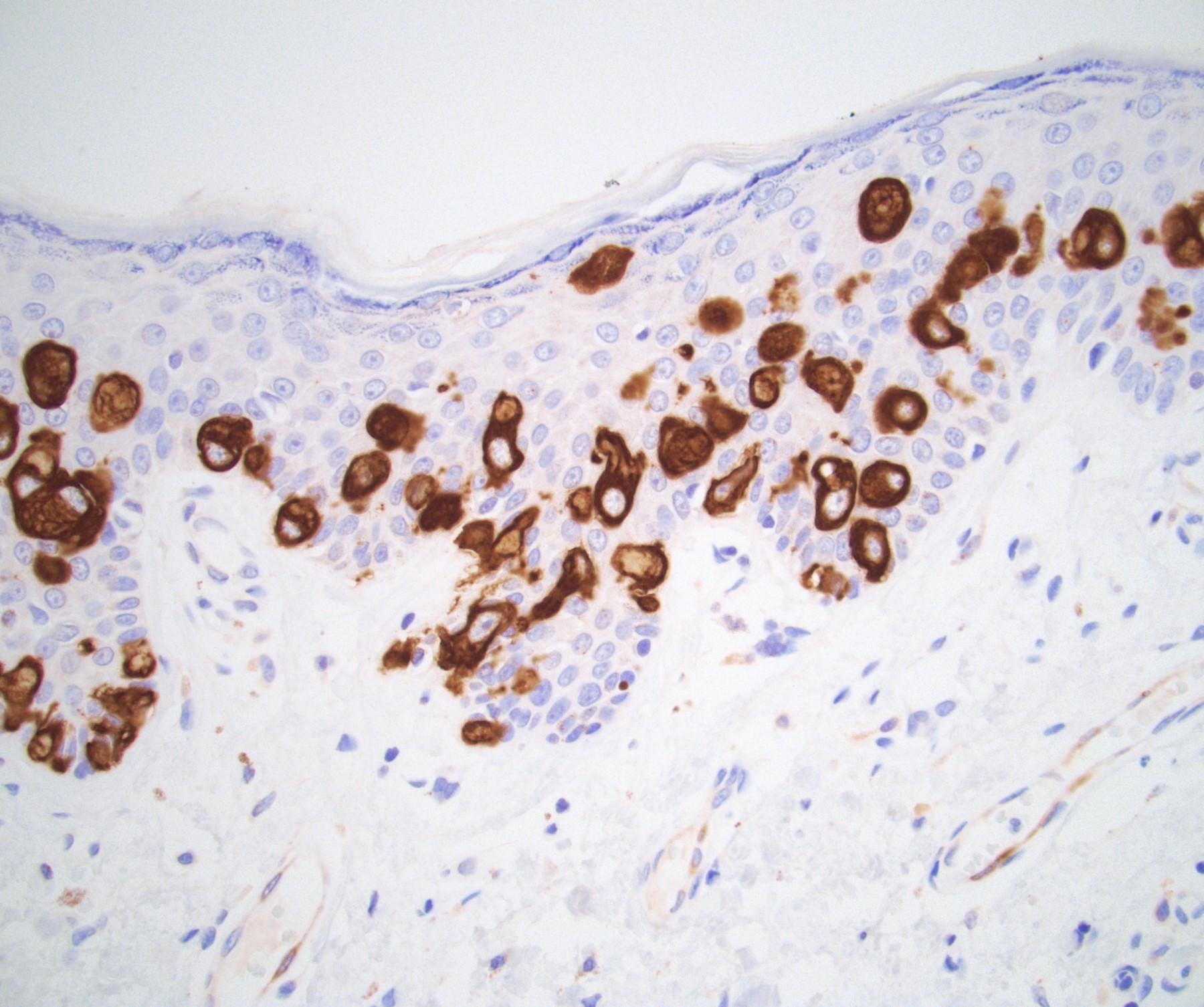
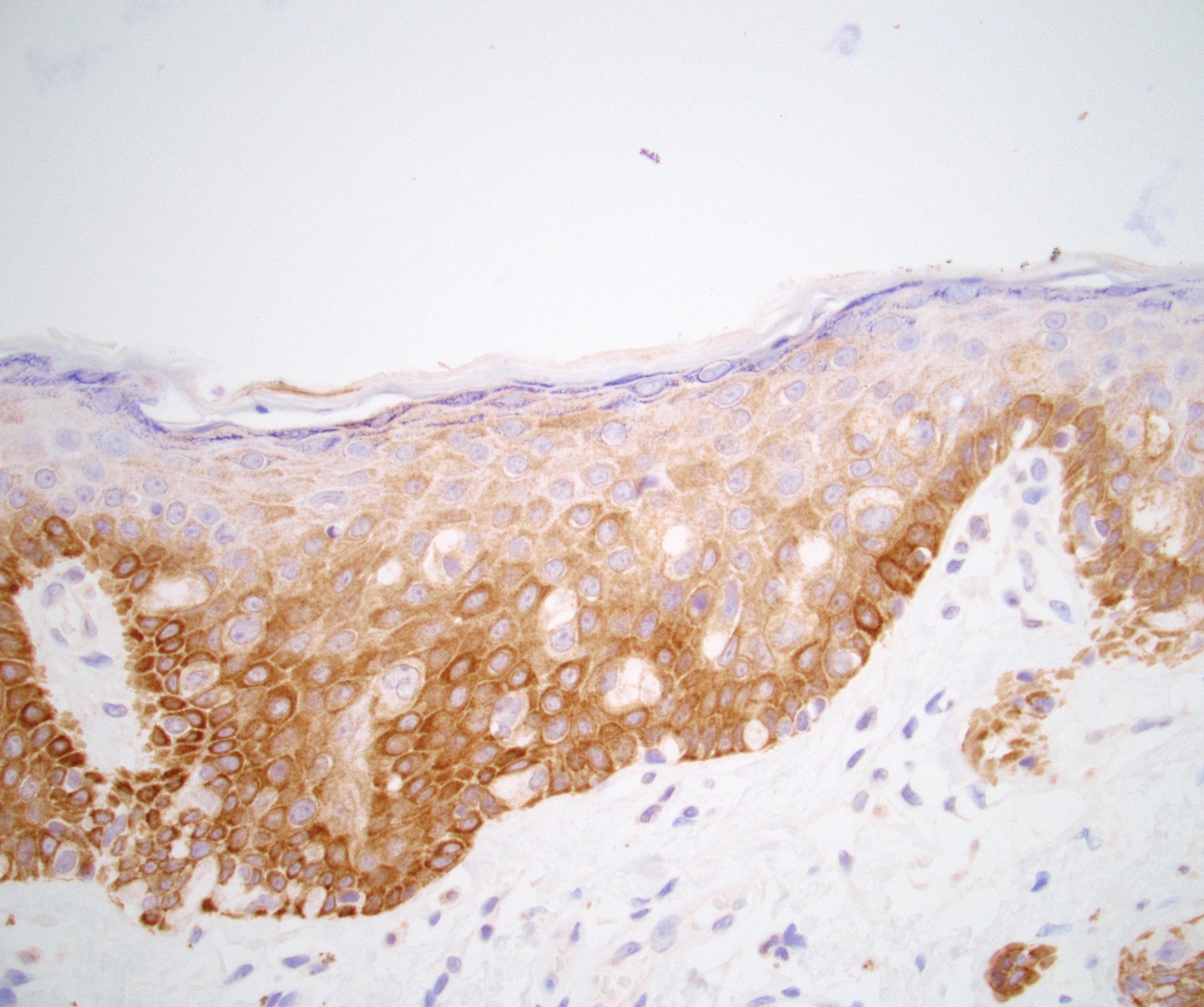
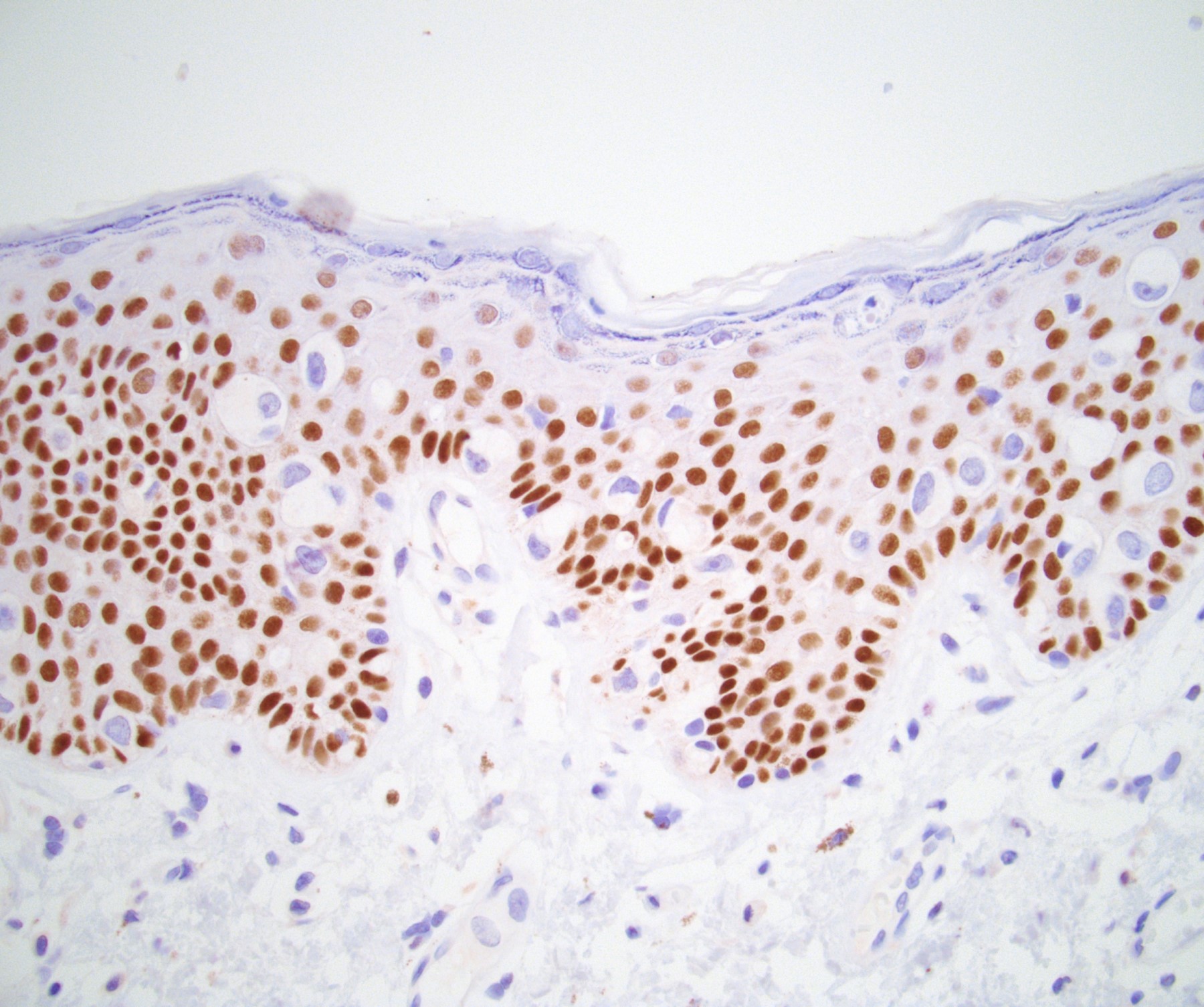
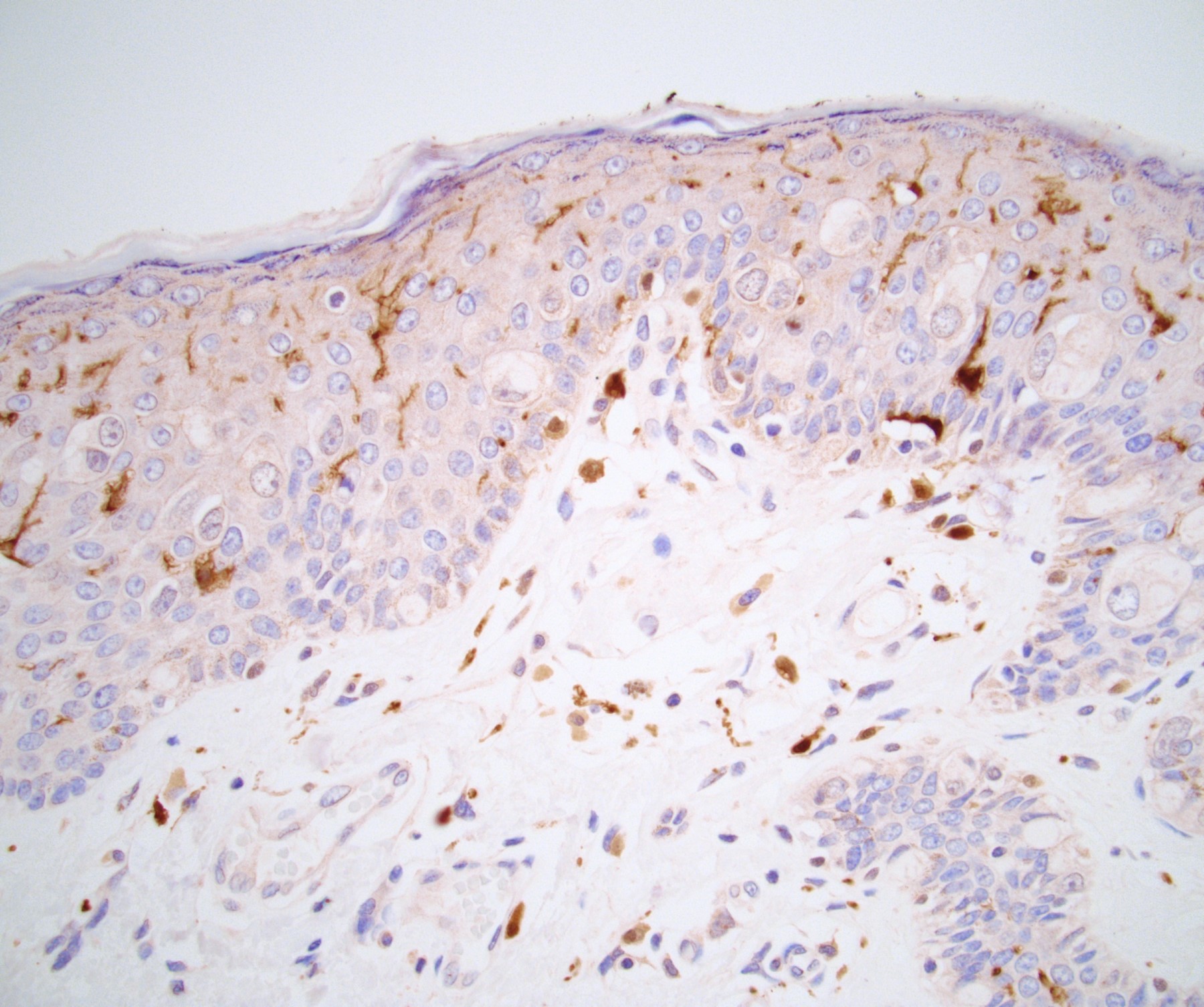
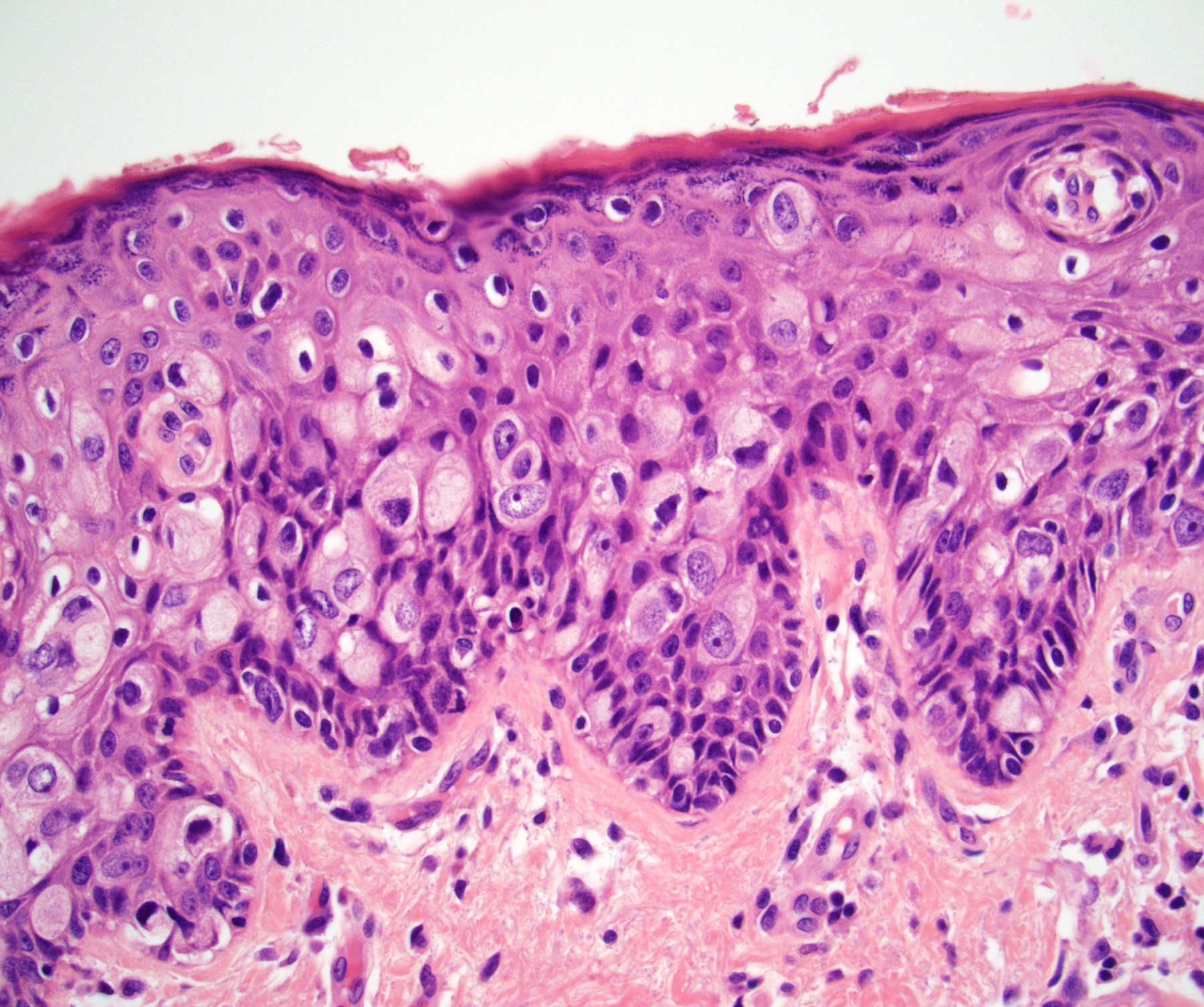
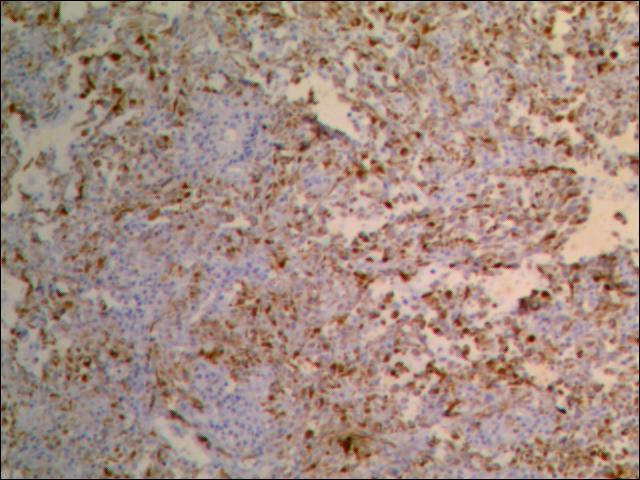
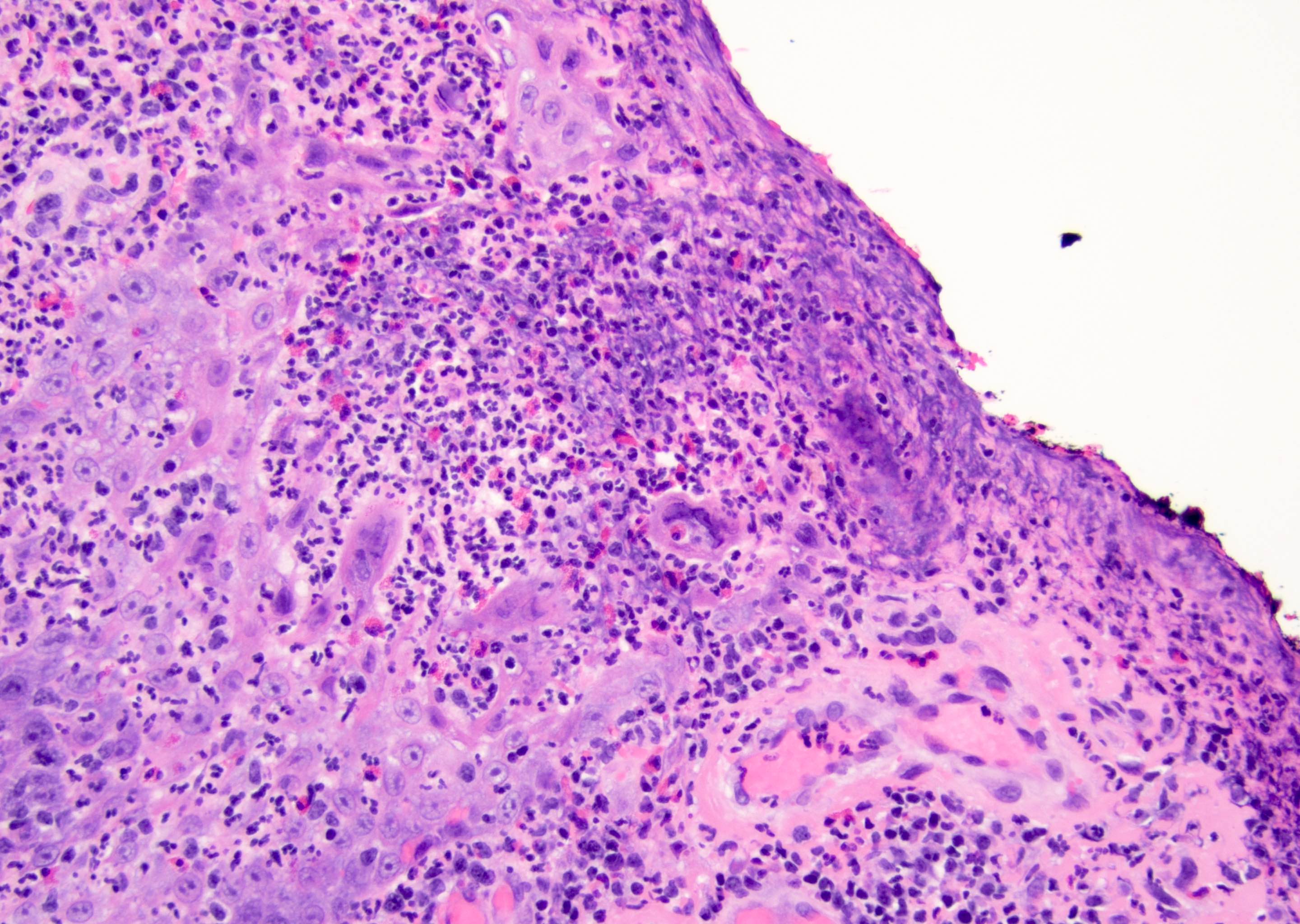
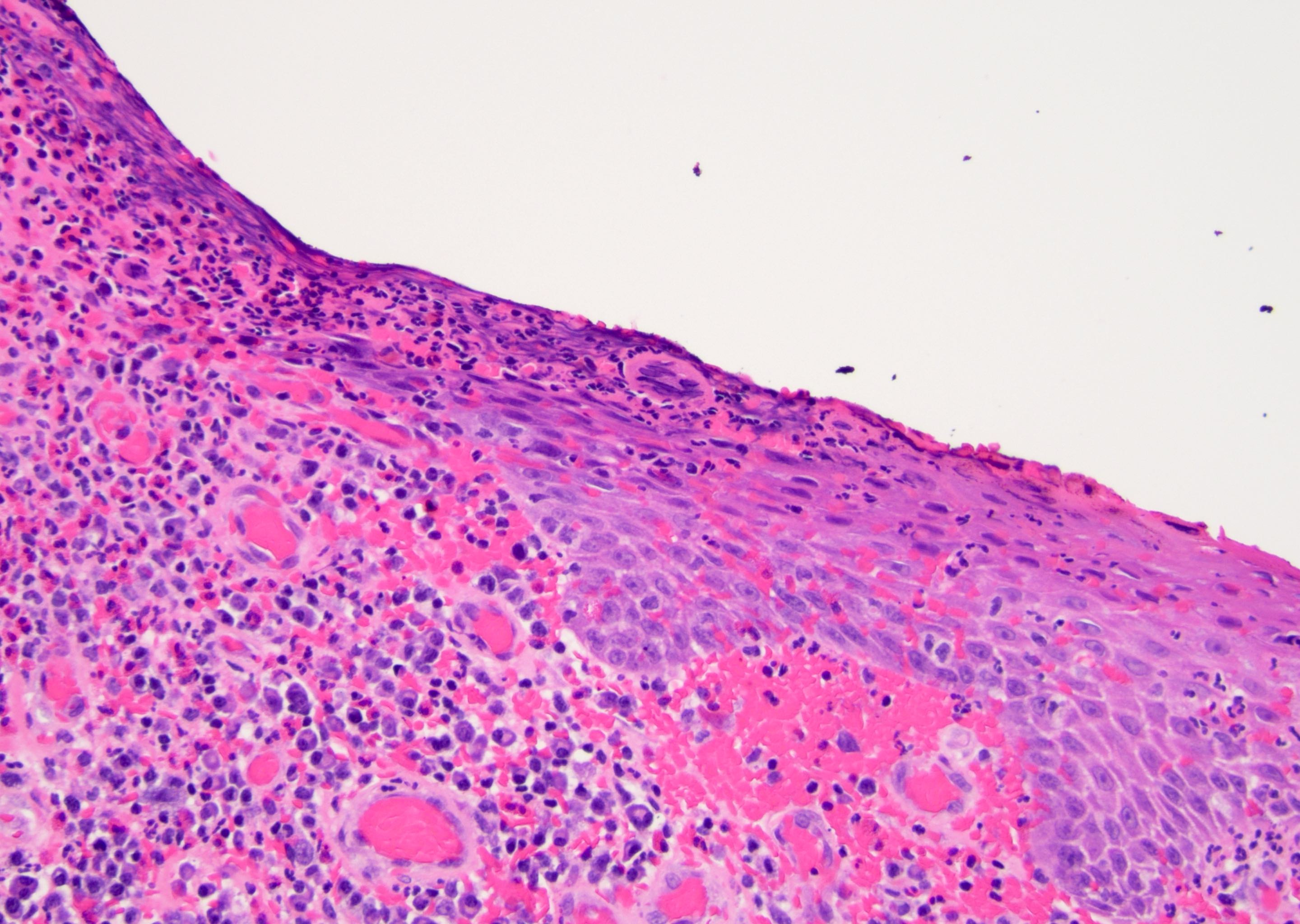
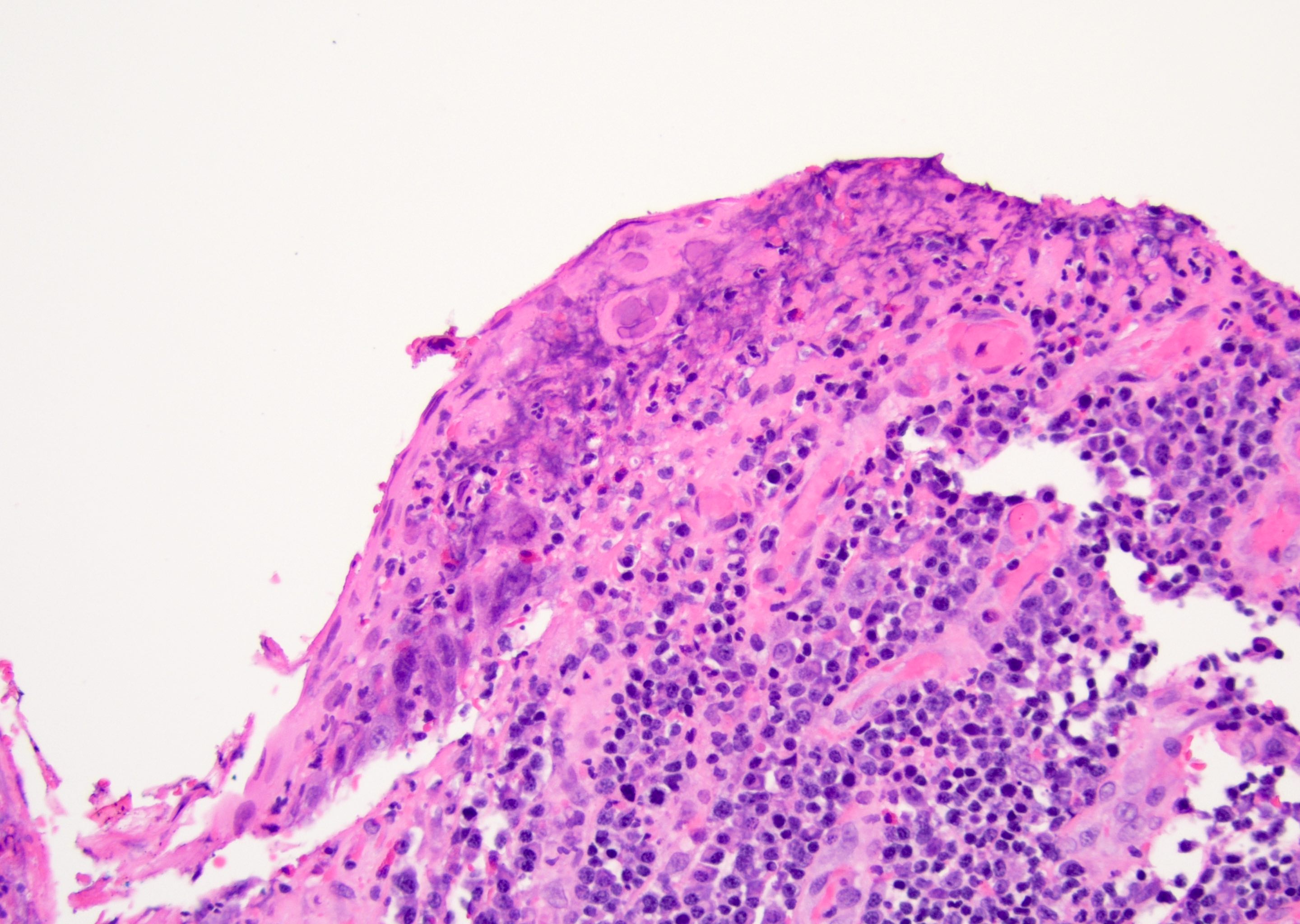
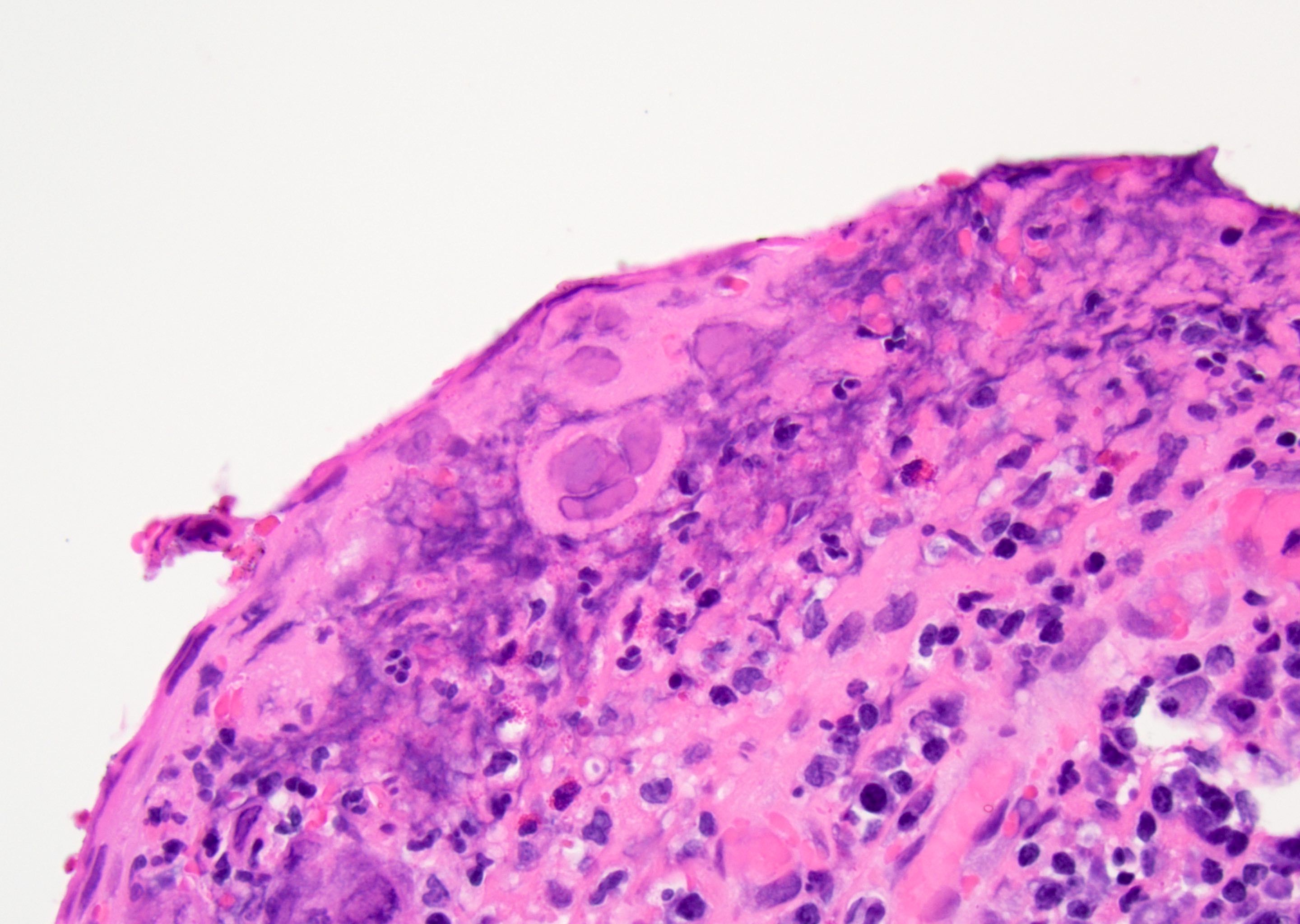
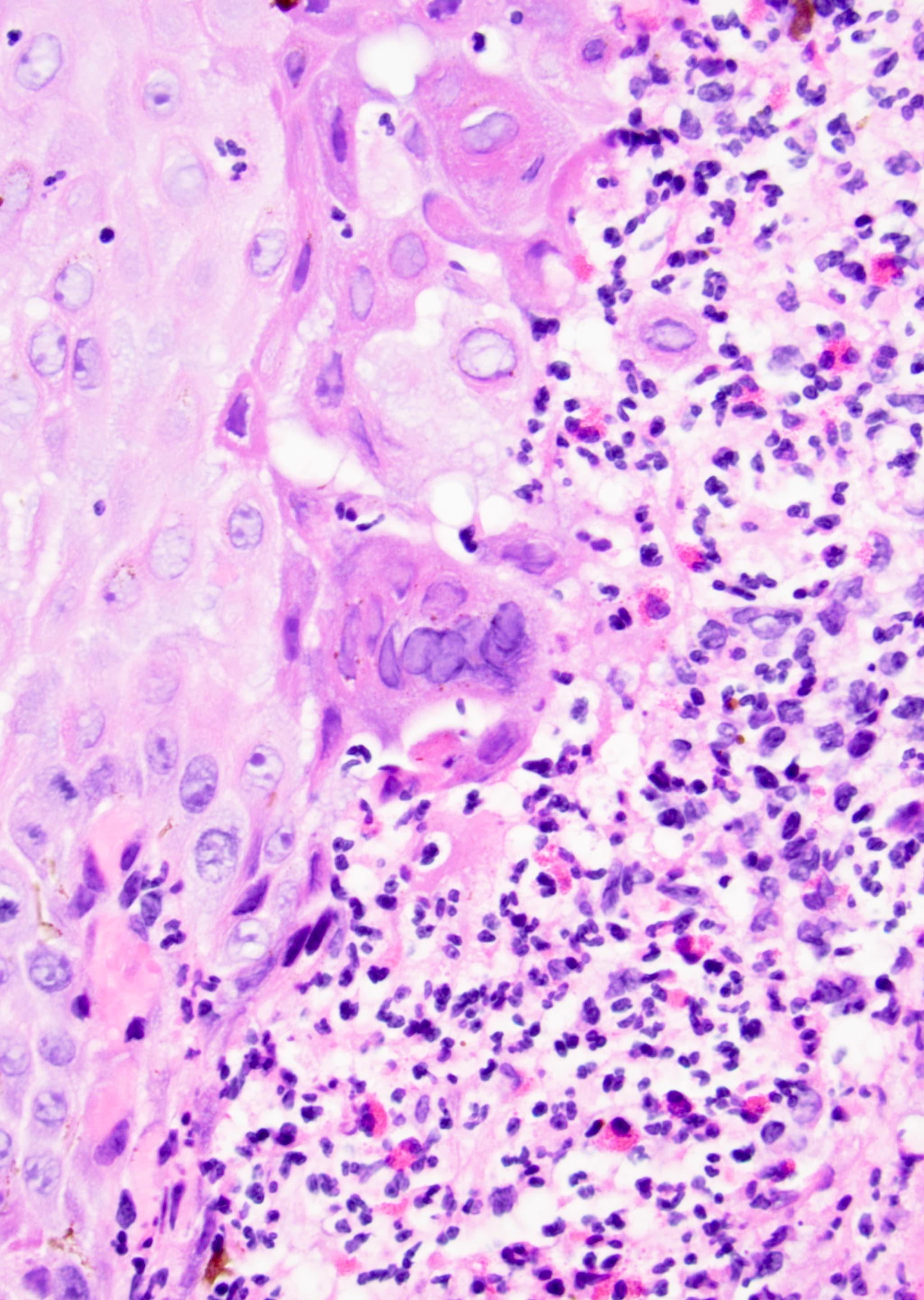
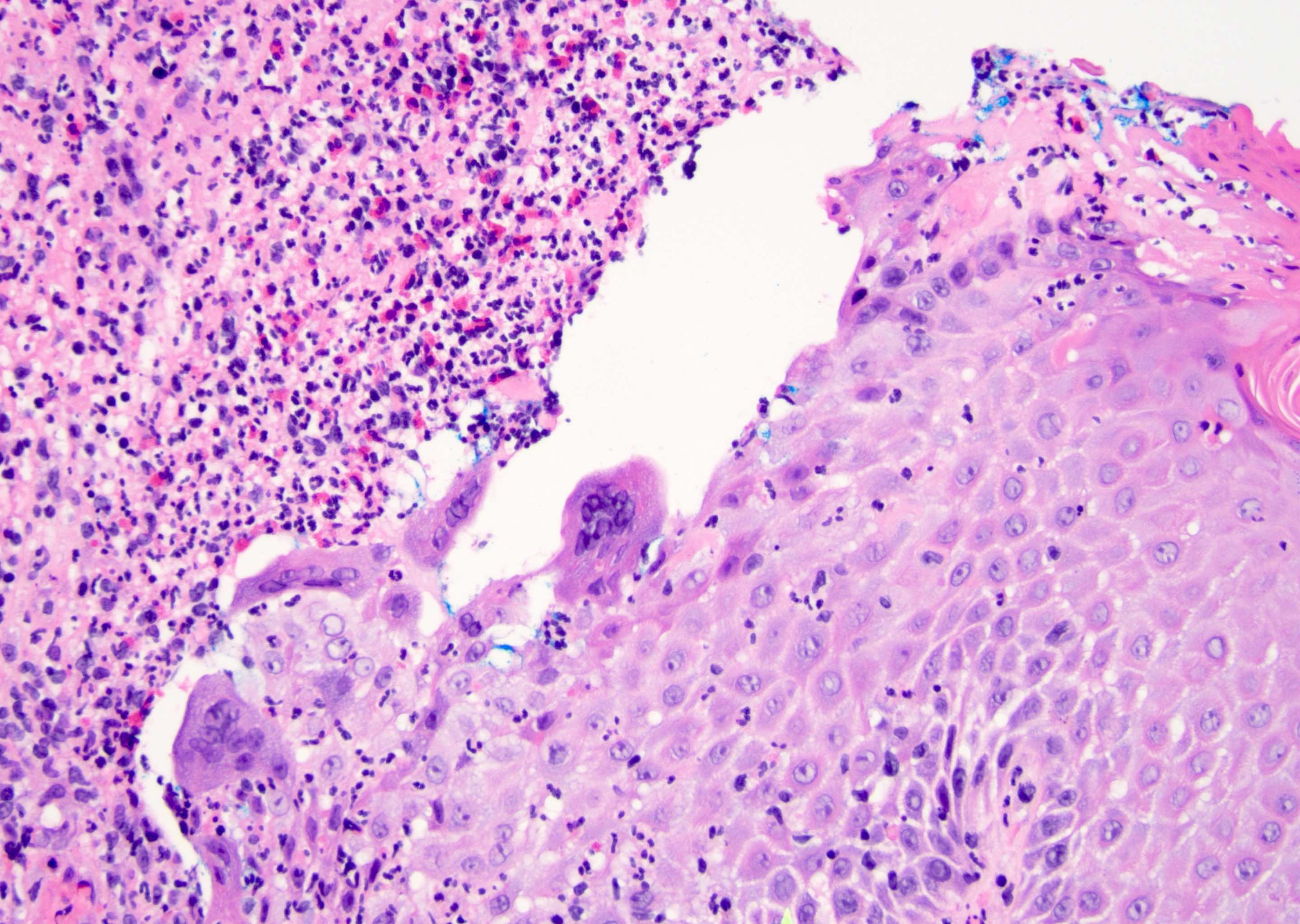
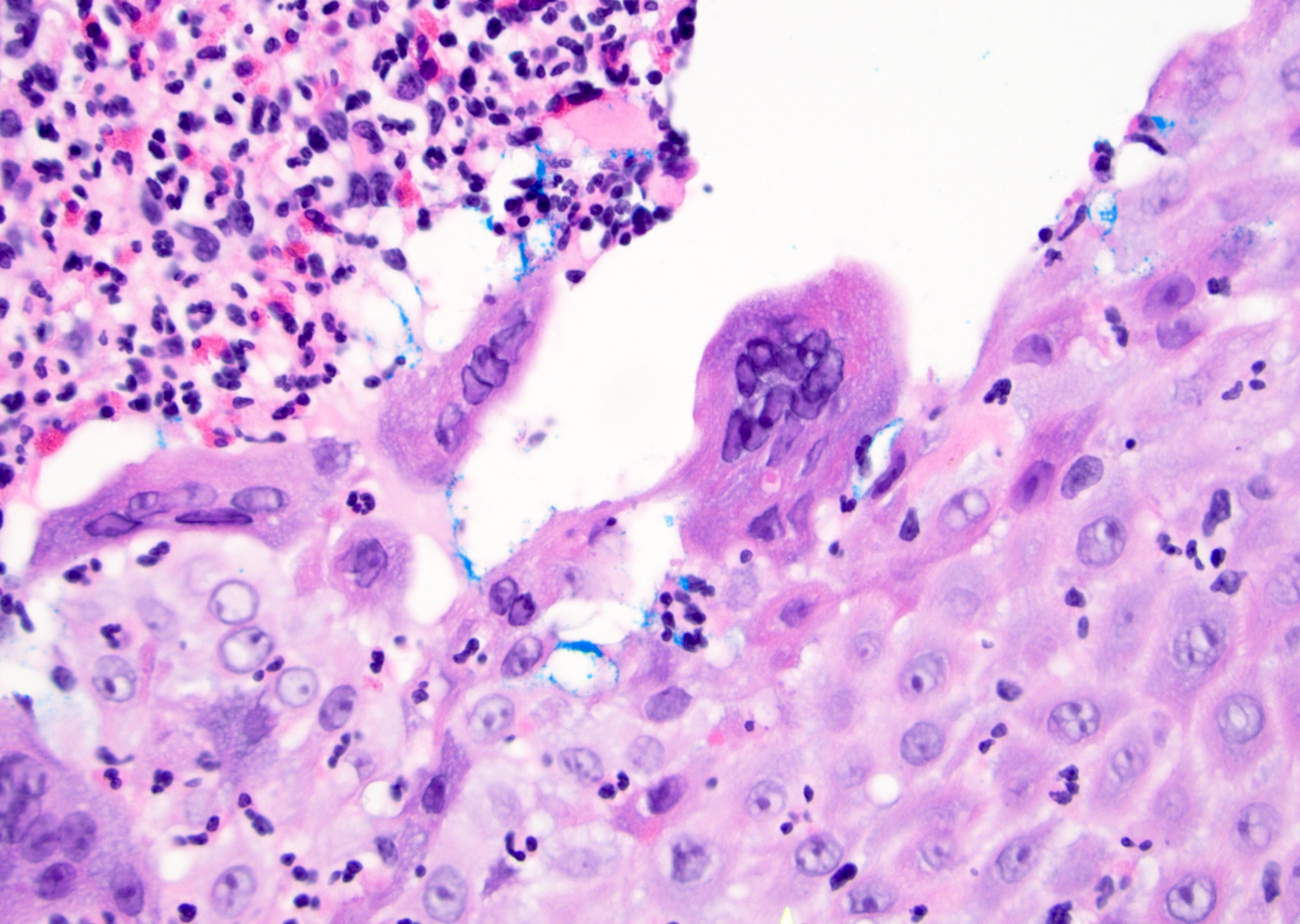
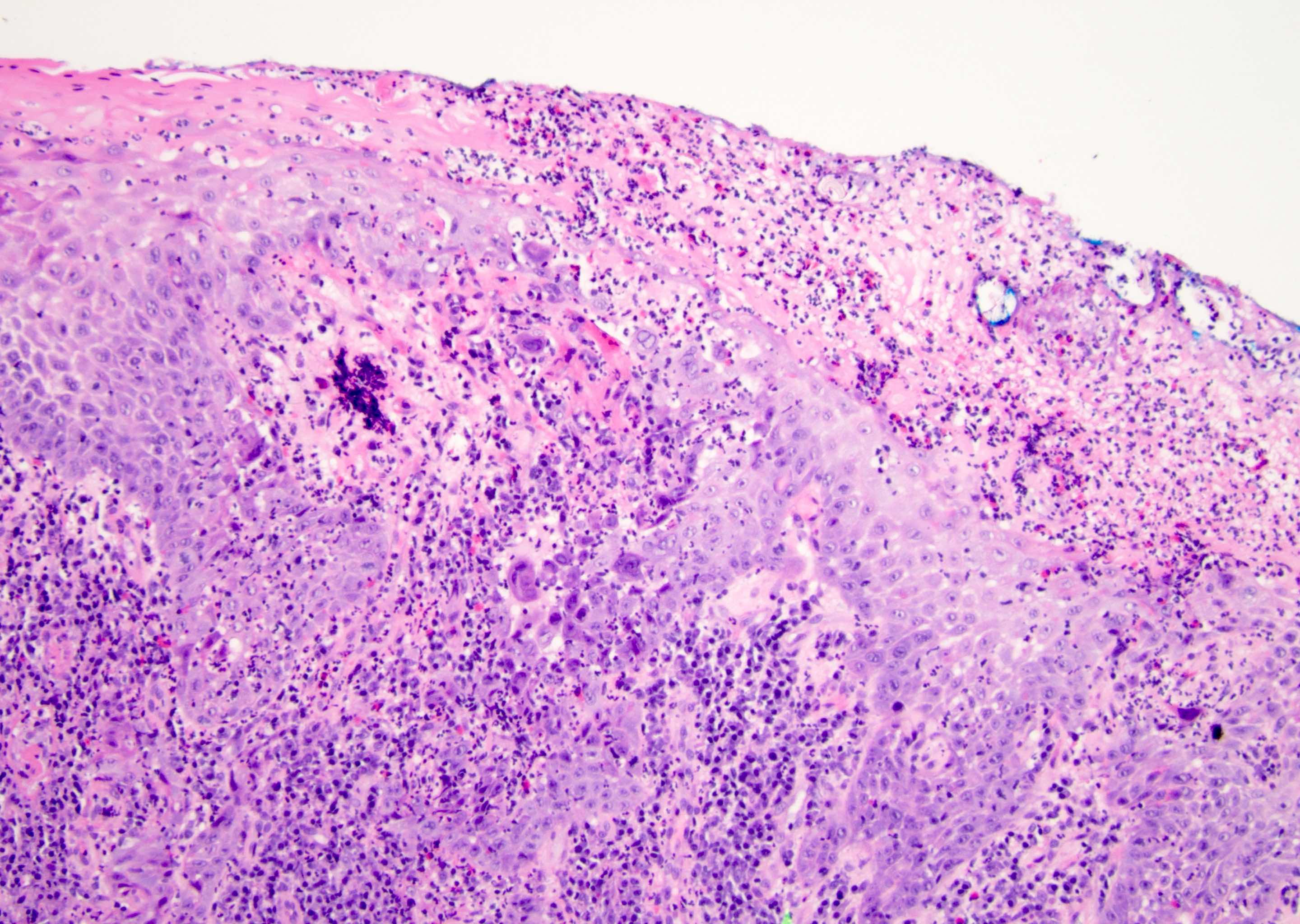
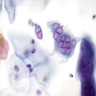
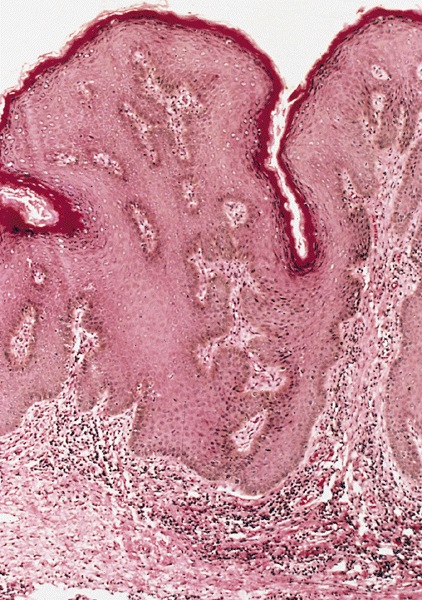
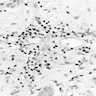
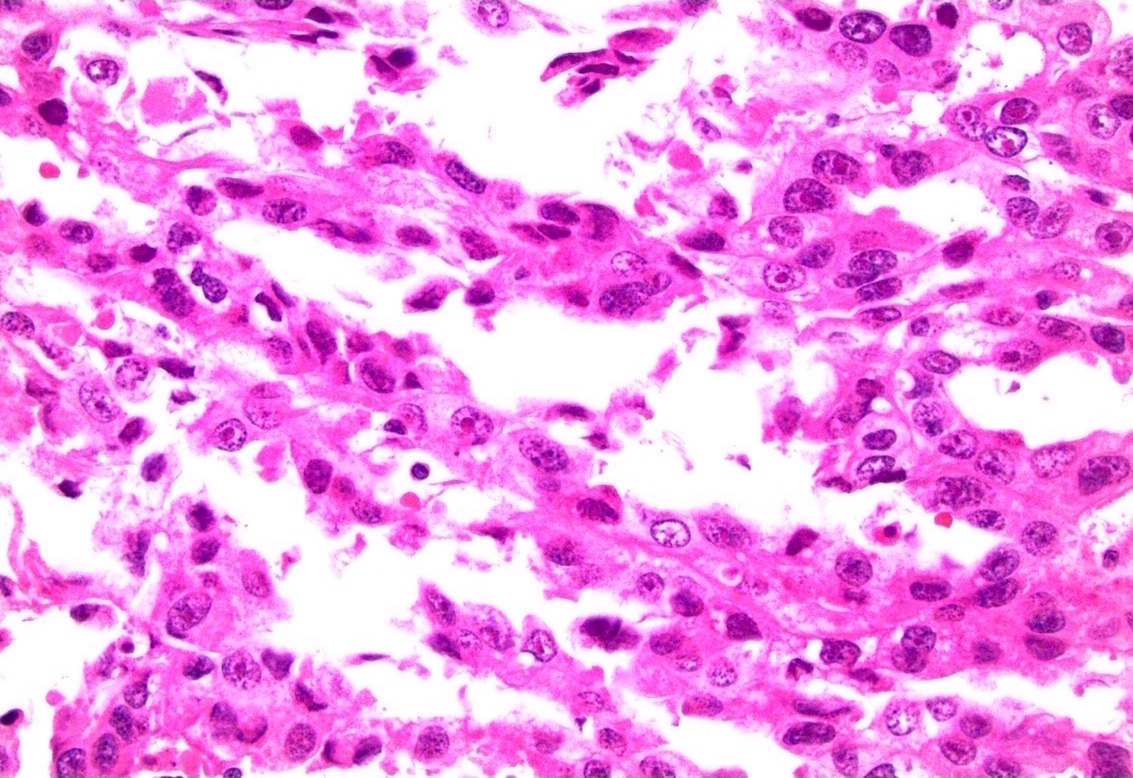
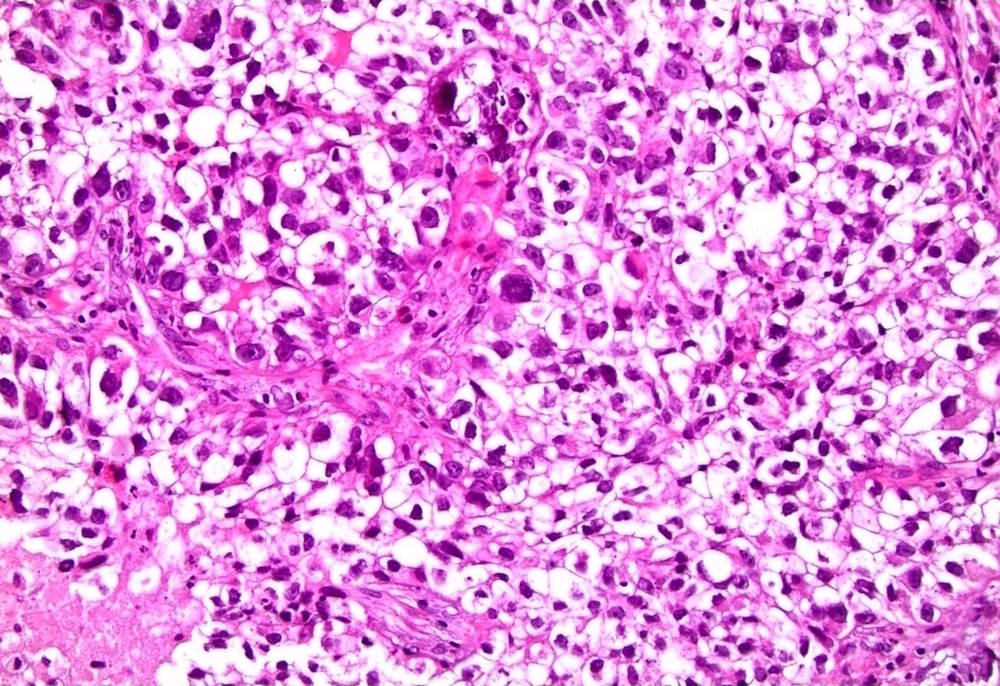
Microscopic (histologic) description
- Atypical intraepidermal epithelial cells present individually and as clusters
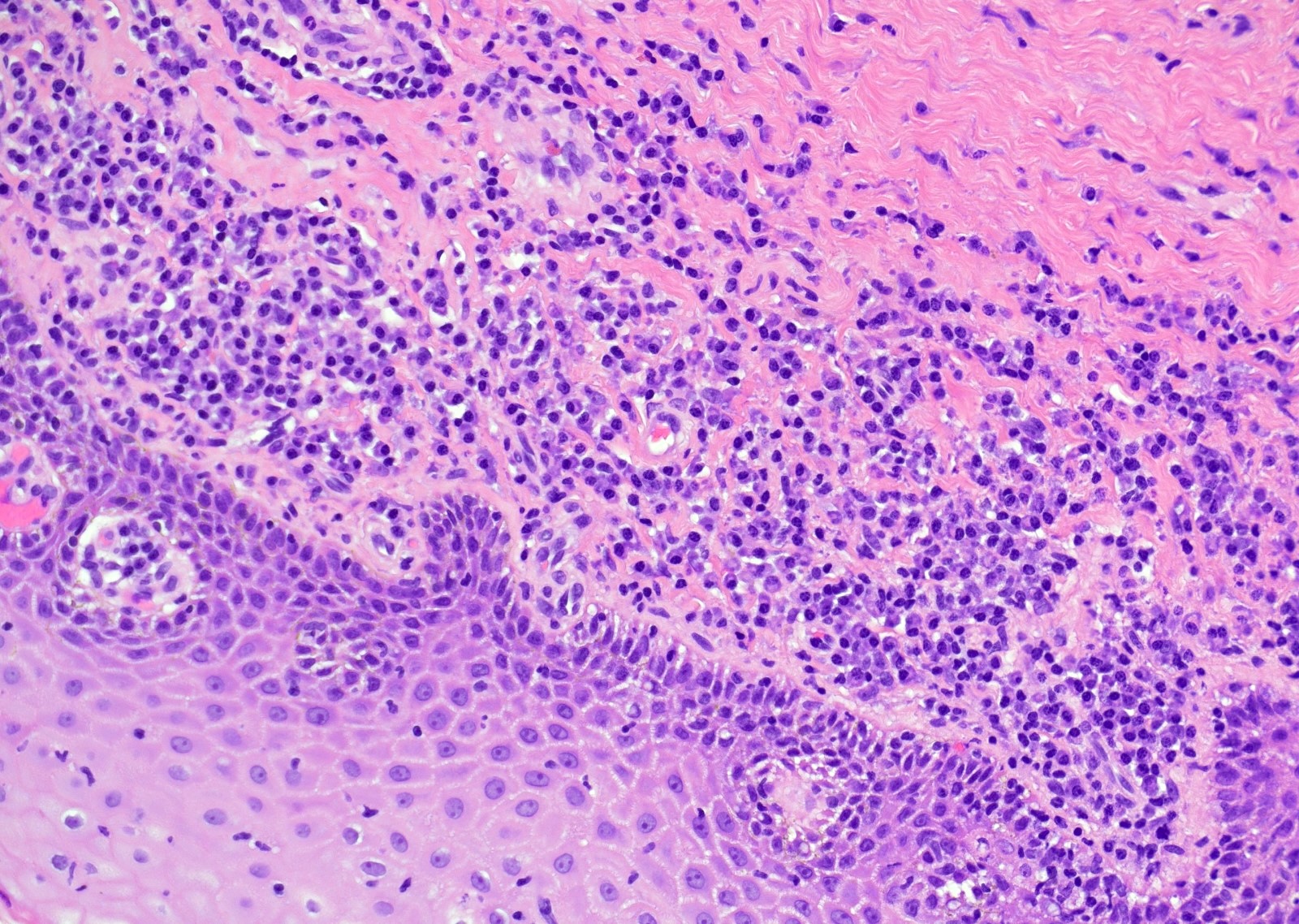
- Aggregates form in basal layers of epidermis with underlying flattened basal keratinocytes (unless invasive)
- Can form glandular structures
- Cells are large and round
- Abundant, pale eosinophilic cytoplasm
- Large nuclei and prominent nucleoli
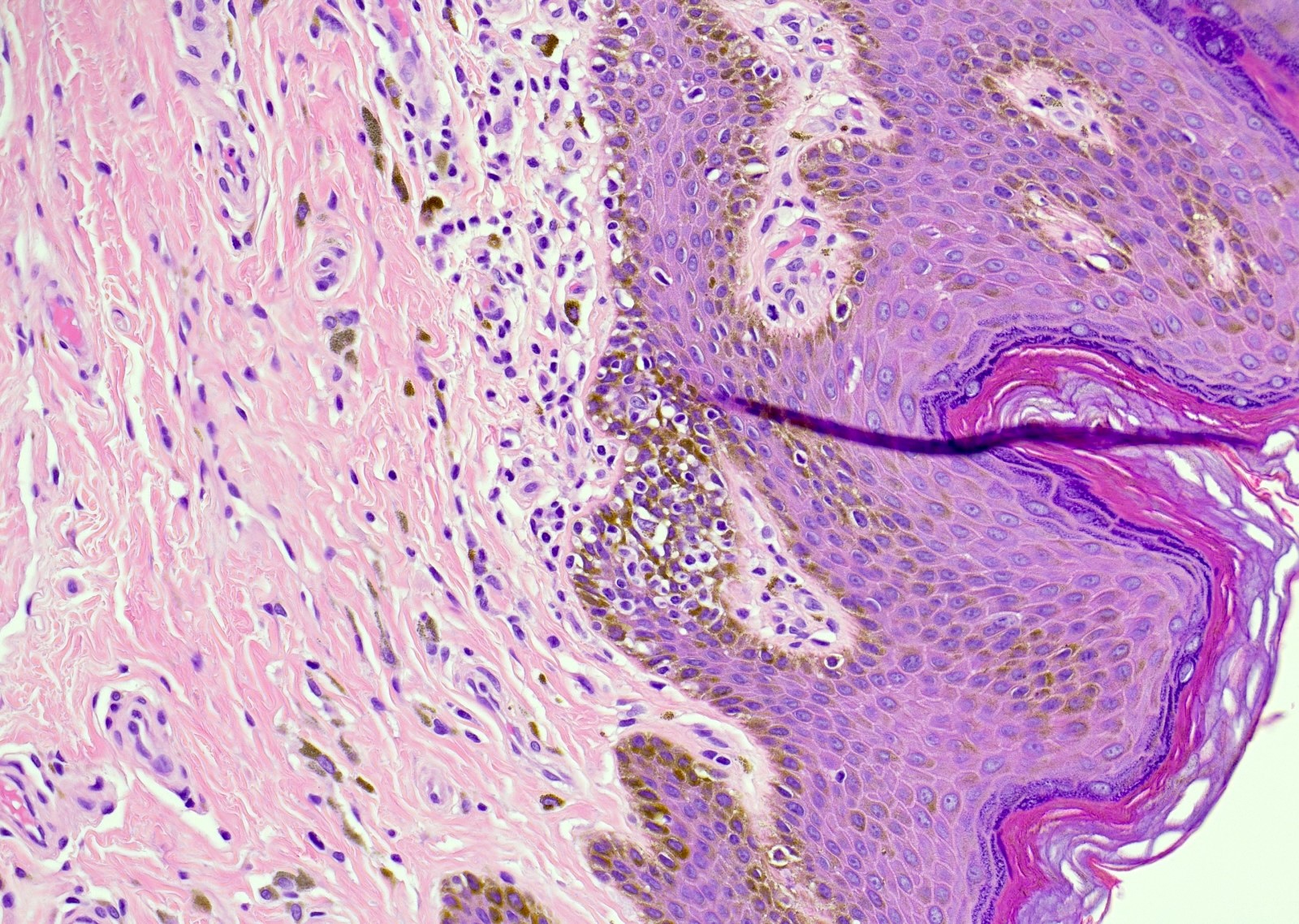
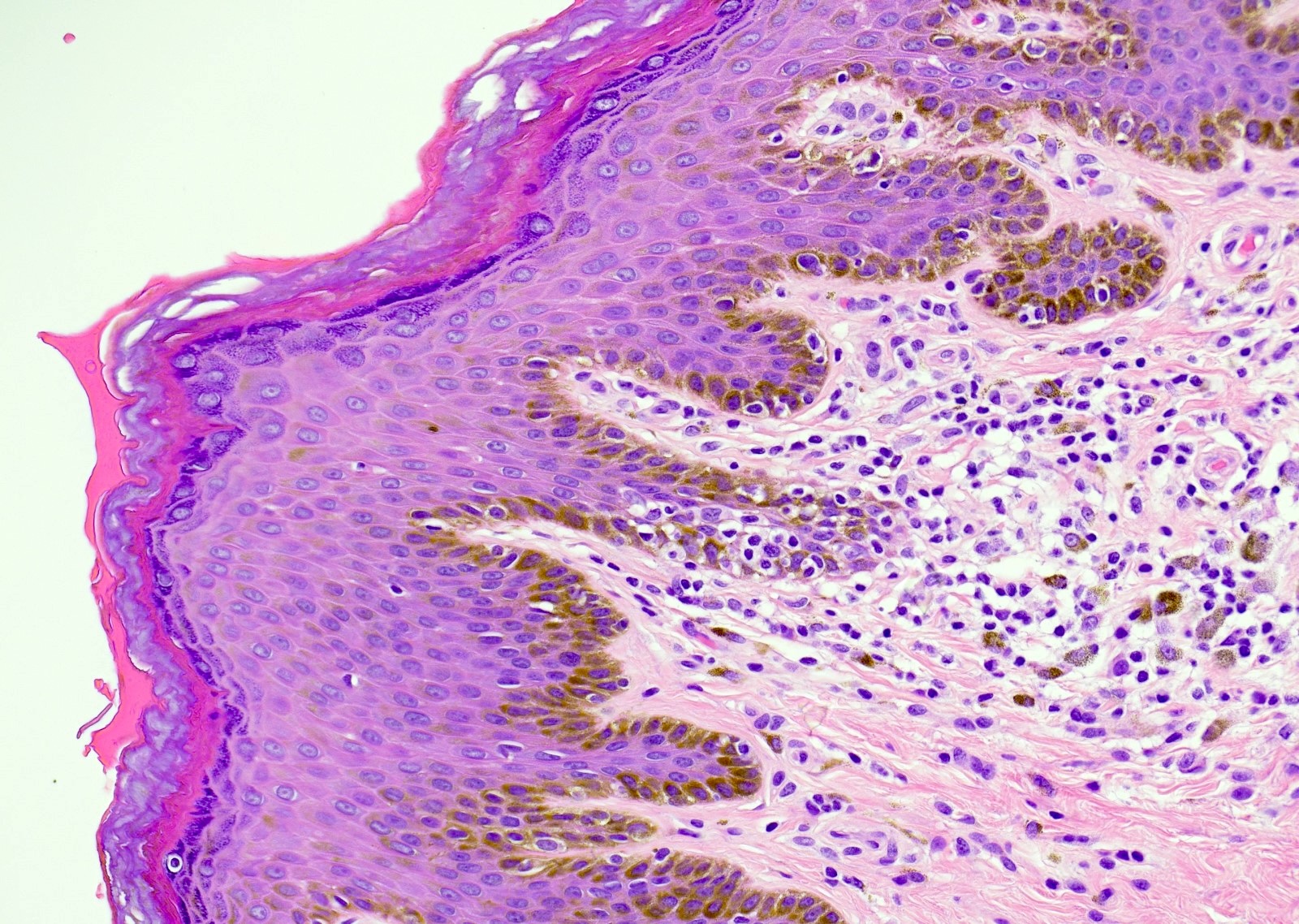
- 10 - 20% have dermal invasion with tumor as individual cells or forming nodules, glands or sheets (Indian J Dermatol Venereol Leprol 2020;86:134, Sci Rep 2017;7:44933, Hum Pathol 2016;47:70)
- May involve hair follicles and sweat glands
- Rarely infiltrates the dartos muscle of the scrotum (Indian J Dermatol Venereol Leprol 2020;86:134, Sci Rep 2017;7:44933, Hum Pathol 2016;47:70)
- Hyperkeratosis, parakeratosis and dense chronic inflammation often present
- Can have intracytoplasmic melanin pigment
- Lymphovascular invasion is rare but possible
- Perineural invasion is uncommon (Hum Pathol 2016;47:70)
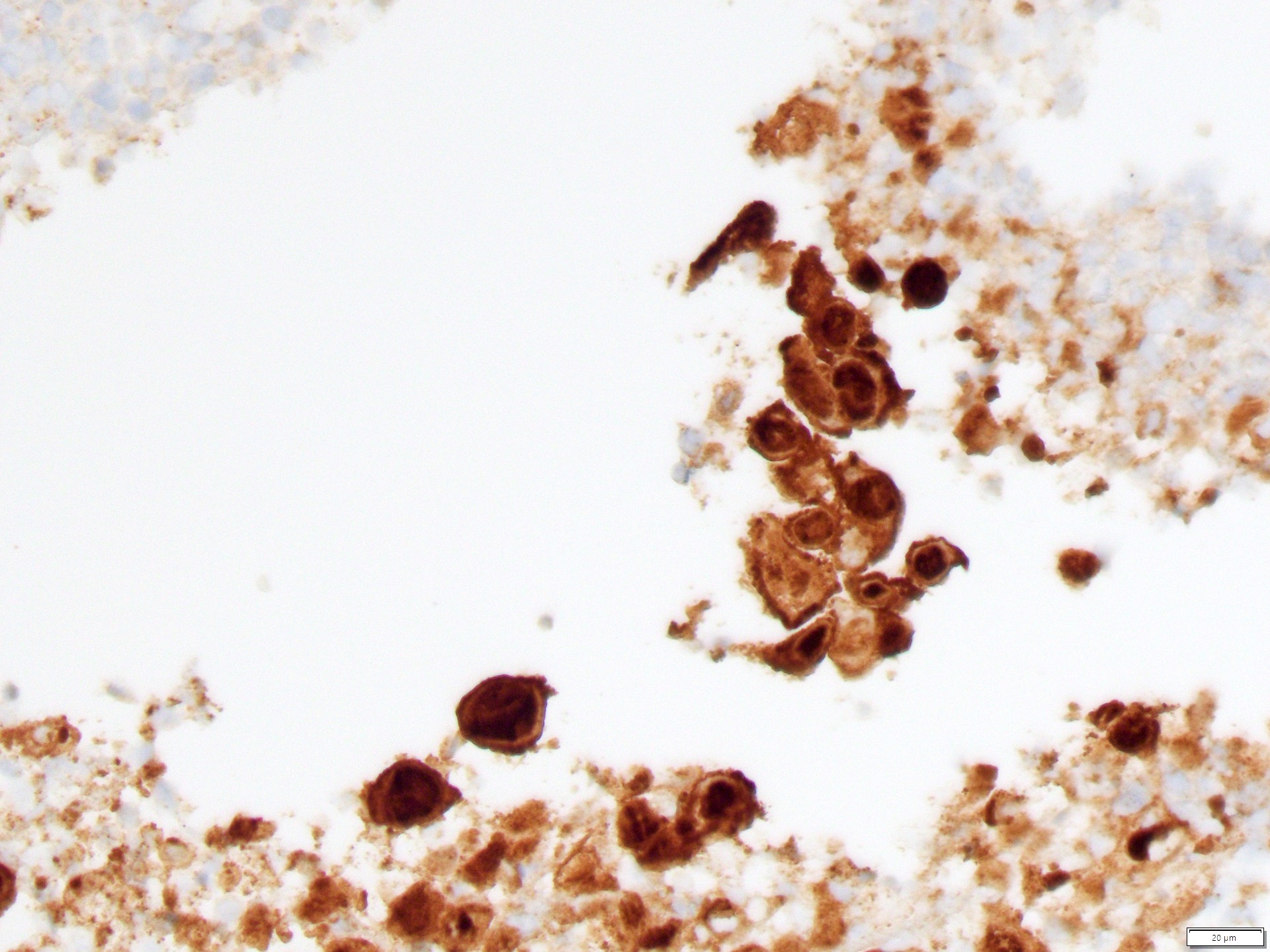
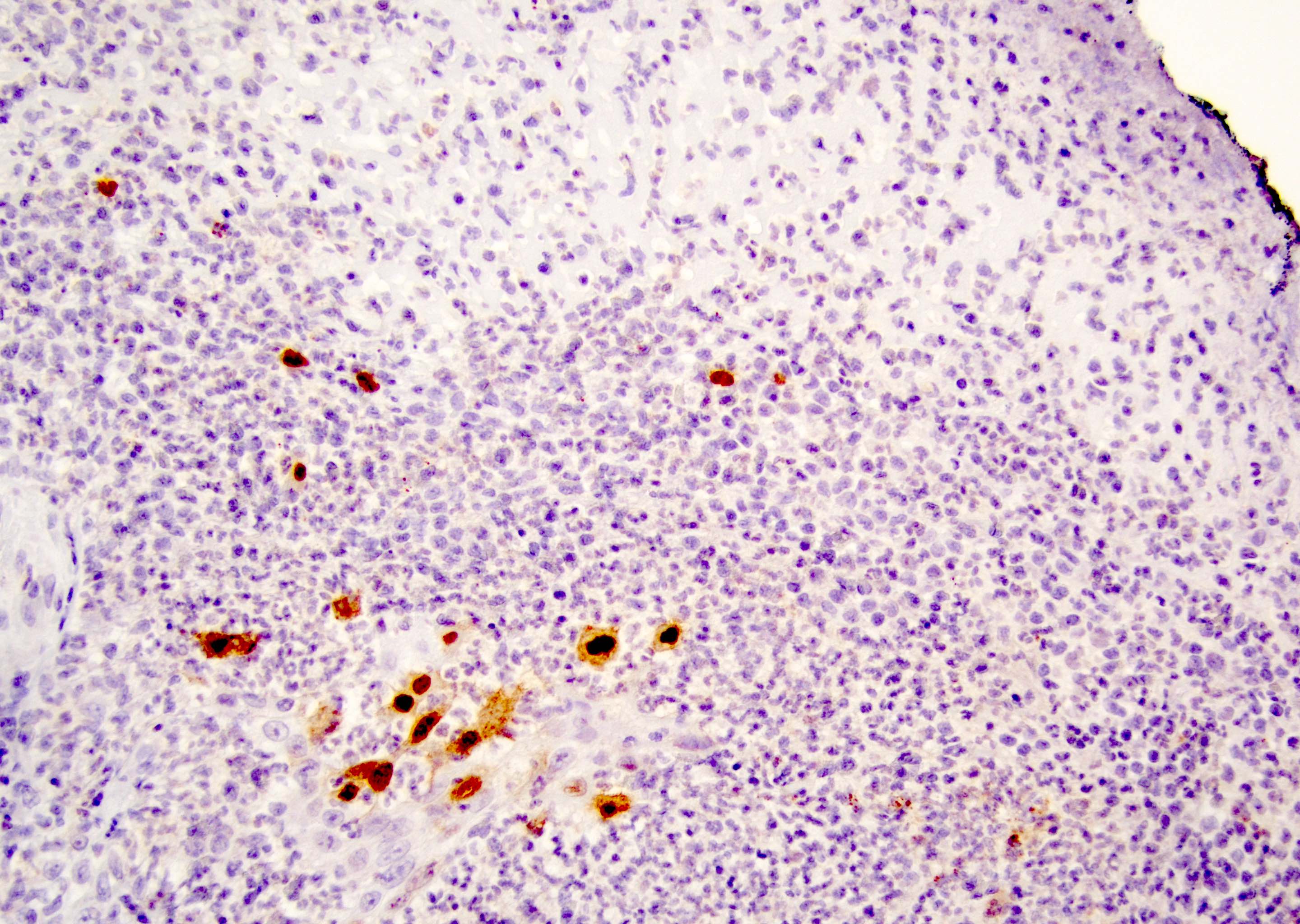
Microscopic (histologic) images
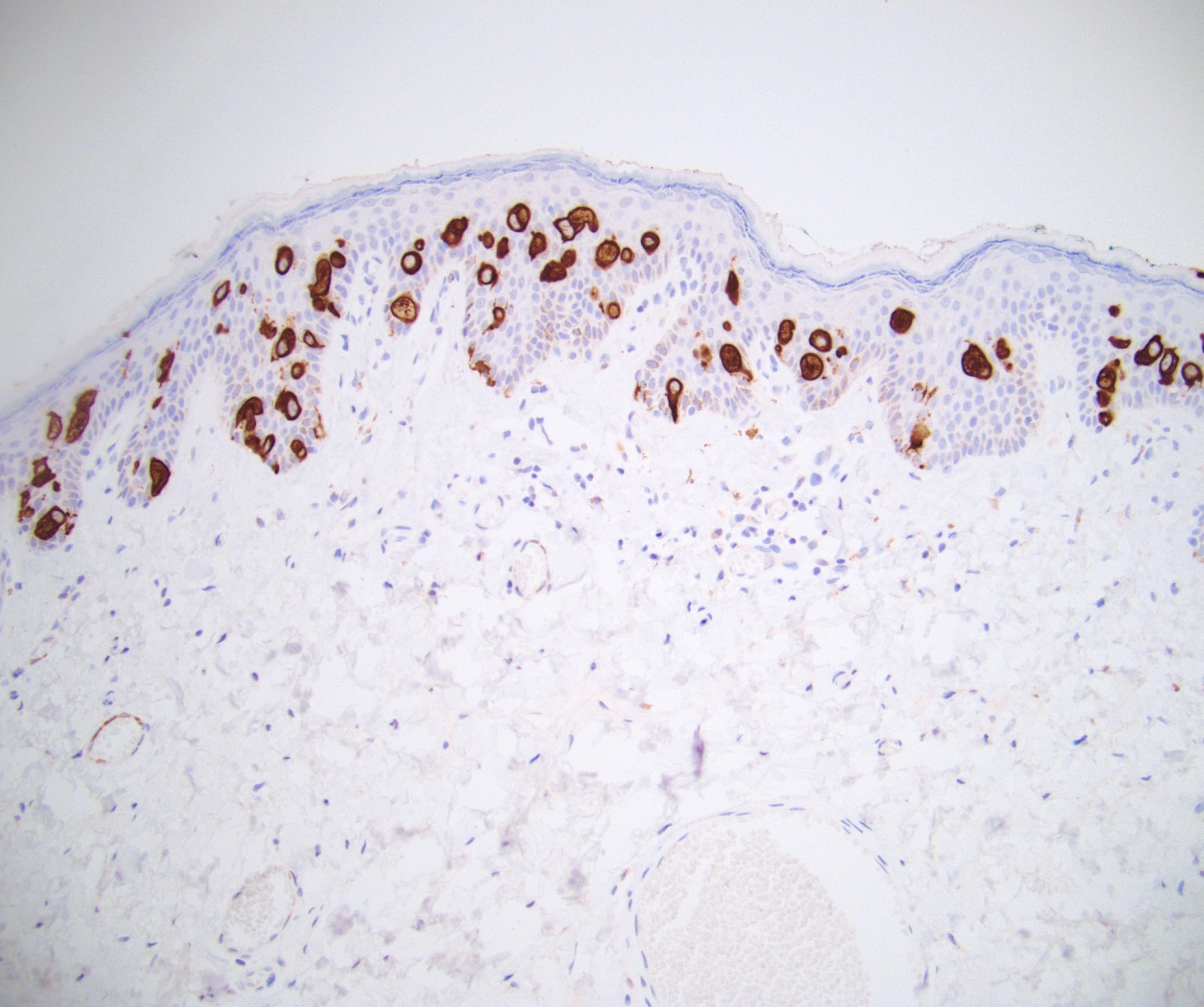
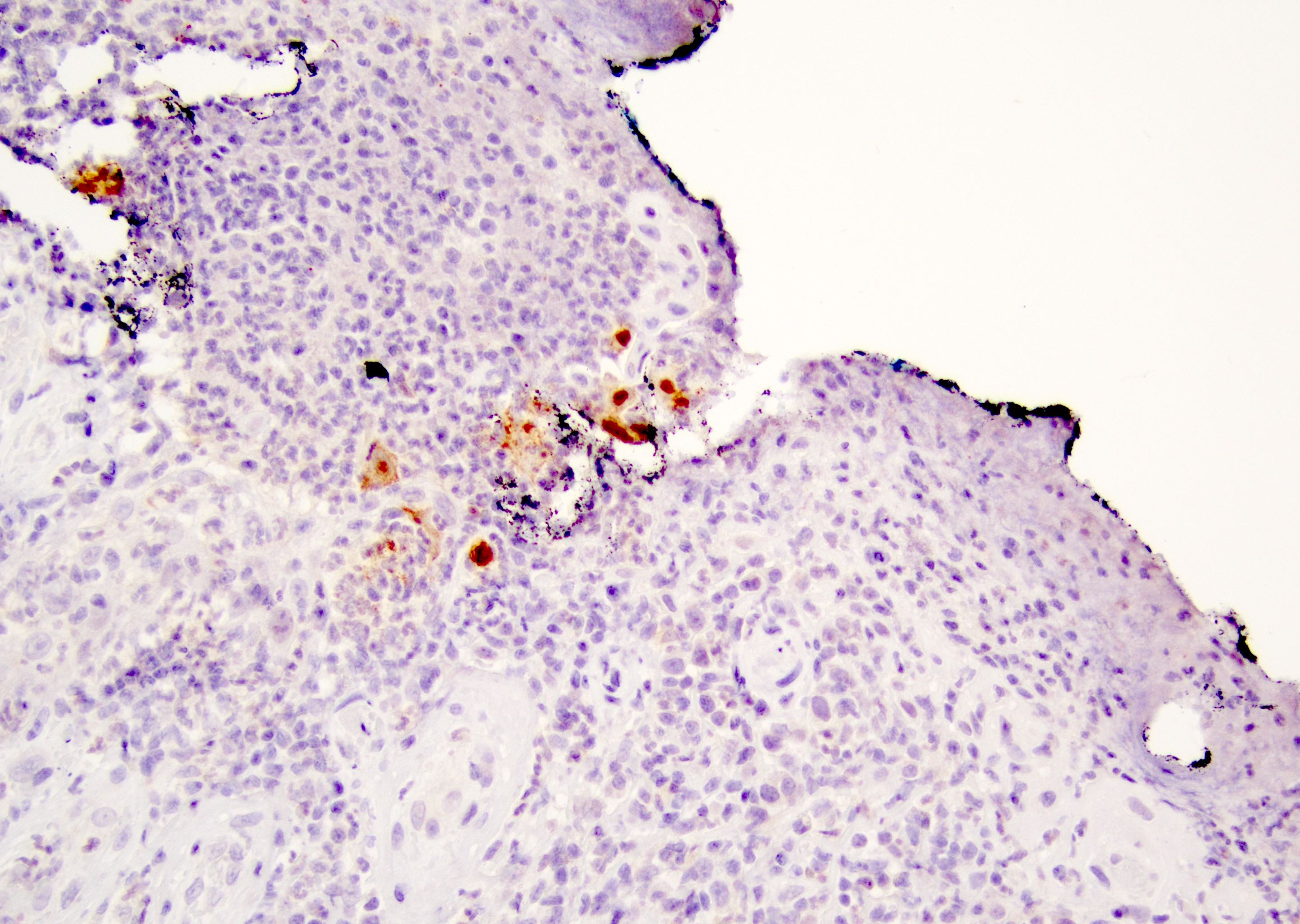
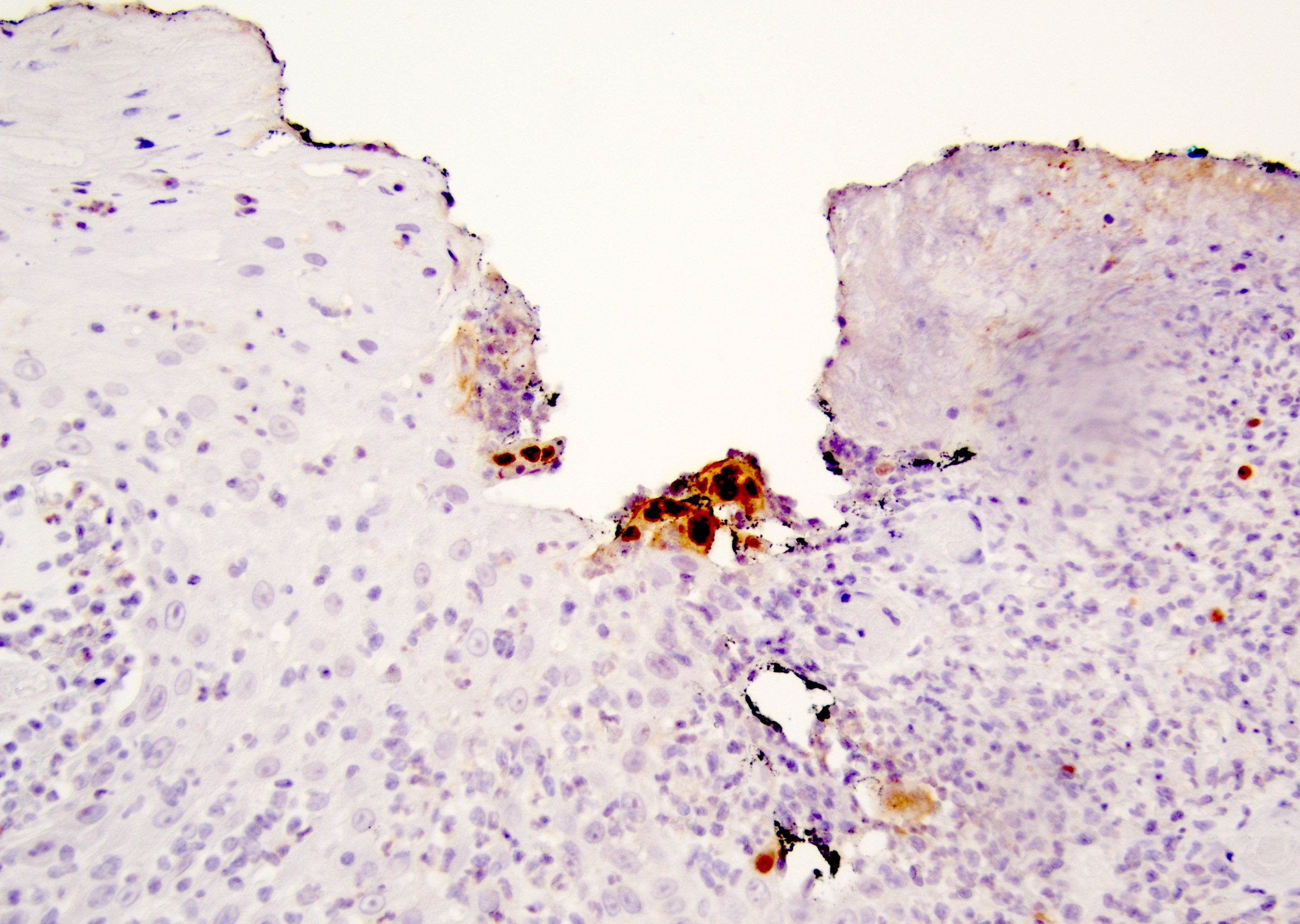
Positive stains
- Primary (Dermatol Online J 2019;25:13030, Indian J Dermatol Venereol Leprol 2020;86:134, Hum Pathol 2016;47:70, Int J Surg Pathol 2018;26:617, Hum Pathol 2018;77:152, Appl Immunohistochem Mol Morphol 2020;28:524, Histopathology 2023;83:104)
- AE1 / AE3, CAM 5.2, EMA, LMWK
- CK7
- CEA
- GATA3
- AR
- TRPS1 (Histopathology 2023;83:104)
- PAS, Alcian blue, colloidal iron, mucicarmine
- GCDFP-15: variable expression in ~60 - 100% of cases
- HER2: ~50% positive
- Secondary
- Urothelial carcinoma: p63, CK5/6 (note: both express GATA3)
- Prostatic adenocarcinoma: PSA, NKX3.1 (note: both express AR)
- Colorectal adenocarcinoma: CK20 (note: both express CEA)
Negative stains
- Primary
- Secondary
- Reference: Appl Immunohistochem Mol Morphol 2020;28:524
Electron microscopy description
- Mucin vesicles, well developed Golgi apparatus and a small number of glycogen particles (Br J Dermatol 1993;128:189)
Molecular / cytogenetics description
- Low risk and high risk HPV in situ hybridization (ISH) negative (Int J Surg Pathol 2018;26:617, Cancer Med 2020;9:1441)
Sample pathology report
- Penis and scrotum, wide local excision:
- Extramammary Paget disease with focal microinvasion (see comment)
- Tumor involves margins in blocks A1 (12 - 3:00 penile margin shave), A3 (3 - 6:00 penile margin shave) and A4 (9 - 12:00 penile margin shave); all other resection margins are negative for tumor
- Comment: Tumor cells are positive for CK7 and GATA3 and are negative for CK5/6, p63, CK20, CDX2 and S100.
Differential diagnosis
- Melanoma in situ:
- Squamous cell carcinoma in situ / penile intraepithelial neoplasia (PeIN):
- Mucinous metaplasia:
- Bland cells intraepidermal cells containing mucin
- Pagetoid dyskeratosis:
- Most common in head and neck
- Usually incidental finding
- Has been reported in the prepuce
- Also has enlarged cells with pale cytoplasm
- Found in the upper layers of the squamous epithelium rather than basal predominant
- Has nuclear halo / clearing
- Pyknotic, bland nuclei
- Clear cell papulosis:
- Usually occurs in Asian children
- Numerous small lesions
- Cells are large with pale cytoplasm but lack aggressive nuclear features like nuclear pleomorphism or prominent nucleoli
- Similar staining profile
- Secondary versus primary extramammary Paget disease:
Board review style question #1
A 68 year old man presented with a whitish, itchy plaque-like lesion that was located at the base of his penis and had grown larger over the past 2 years. The lesion was resected and a microscopic photo is shown above. Immunostains reveal that the lesional cells are positive for AE1 / AE3, CK7 and CEA and are negative for S100, CK20, CK5/6, p63 and NKX3.1. What is the diagnosis?
- Malignant melanoma
- Primary extramammary Paget disease
- Prostatic adenocarcinoma
- Squamous cell carcinoma
- Urothelial carcinoma
Board review style answer #1
B. Primary extramammary Paget disease. Penoscrotal extramammary Paget disease presents in elderly men as a whitish plaque. The lesion is often misdiagnosed for several years as a nonneoplastic lesion. Primary penoscrotal extramammary Paget disease tumoral cells express AE1 / AE3, CK7 and CEA and are negative for S100, CK20, CK5/6 and NKX3.1. Answer A is incorrect because melanoma is positive for S100 and negative for AE1 / AE3. Answer C is incorrect because prostatic adenocarcinoma is negative for CK7 and positive for NKX3.1. Answer D is incorrect because squamous cell carcinoma is negative for CEA and positive for p63 and CK5/6. Answer E is incorrect because urothelial carcinoma is positive for p63. Of note, primary penoscrotal extramammary Paget disease expresses GATA3 and AR, overlapping with urothelial carcinoma and prostatic adenocarcinoma.
Comment Here
Reference: Extramammary Paget disease
Comment Here
Reference: Extramammary Paget disease
Board review style question #2
Board review style answer #2
B. CK7 (shown in the image). Most cases of primary extramammary Paget disease express AE1 / AE3, EMA, CK7, CEA, GATA3 and AR. There is variable expression of GCDFP and HER2. Answers A, C, D and E are incorrect because most cases are negative for CK5/6, CK20, p63 and S100 and are also negative for CDX2, SOX10, HMB45, NKX3.1, PSA and prostein. Lack of expression for these makers helps exclude malignant melanoma, prostatic adenocarcinoma, squamous cell carcinoma and urothelial carcinoma.
Comment Here
Reference: Extramammary Paget disease
Comment Here
Reference: Extramammary Paget disease
Fournier gangrene
Table of Contents
Definition / general | Epidemiology | Sites | Etiology | Diagrams / tables | Clinical features | Prognostic factors | Case reports | Treatment | Clinical images | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosisDefinition / general
- First documented in 1883 by Professor Jean Alfred Fournier (Whonamedit: Fournier Gangrene [Accessed 28 March 2018], eMedicine: Fournier Gangrene [Accessed 28 March 2018])
- Serious life threatening condition characterized by necrotizing fasciitis of genitalia and perineum
- Risk factors: trauma, burns, anorectal disease, diabetes, leukemia and alcoholic cirrhosis
Epidemiology
- Elderly adults (male and female), immunocompromised (particularly diabetes) or those with depressed mental status
Sites
- Dartos and penile fascia are preferred sites
Etiology
- 50 - 60% of infections have GI or GU source of infection
- Usually a polymicrobial infection
- Streptococcus and Staphylococcus most common in children
- Gram negative bacilli and anaerobic bacteria most common in adults
- Source of infection may be colorectal, urologic or cutaneous
- Infection spreads from skin down fascial plane, causing inflammation, ischemia and necrosis; low oxygen tension and necrosis promote anaerobes and cause rapid dissemination
- Obliterative endarteritis plays a key role in pathogenesis
Clinical features
- Patients present with genital induration, pain, erythema and crepitus
- Xrays may show air in perineal tissue
- To diagnose, MUST examine genitals, particularly in elderly or patients with diminished mental status
- Finding nidus of infection is important - may be periurethral or perirectal
- History of perineal trauma is important
- Affects Buck fascia and foreskin, sparing glans
- Mortality rate of 7 - 22%, even with timely and aggressive therapy (J Urol 2009;181:2120)
Prognostic factors
- Fournier gangrene severity index (FGSI) scores > 9 predicts severity and mortality (J Urol 1995;154:89, J Postgrad Med 2008;54:102)
- FGSI: nine variables are assigned scores of 0 - 4, which are added together
- Variables are body temperature, heart rate, respiratory rate, serum sodium, serum potassium, serum creatinine, hematocrit and white blood count and serum bicarbonate (see table above)
- Cirrhosis, not a FGSI factor, is also a poor prognostic factor (J Microbiol Immunol Infect 2007;40:500)
Case reports
- 50 year old man with blackened skin on penis and scrotum (Internet Journal of Emergency Medicine 2008;5(1))
- 65 year old man with third degree burns (J Med Case Rep 2009;3:7264)
Treatment
- Aggressive medical treatment (fluids, broad spectrum antibiotics) plus aggressive surgical debridement with aggressive wound care
- Skin grafts are usually not required due to elasticity of genital skin (West J Emerg Med 2009;10:281)
- Hyperbaric oxygen does not appear to be useful
Clinical images
Gross images
Microscopic (histologic) description
- Penile fascia with severe inflammation (neutrophils), bacteria and necrotic tissue
- Thrombosis of small vessels (obliterative endarteritis)
- Deep erectile tissue usually remains unaffected
Microscopic (histologic) images
Differential diagnosis
- Corbus disease (gangrenous balanitis):
- Similar to Fournier gangrene, affects exclusively glans penis and foreskin is usually spared
Granuloma inguinale
Table of Contents
Definition / general | Terminology | Epidemiology | Sites | Etiology | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Electron microscopy description | Differential diagnosis | Additional referencesDefinition / general
- Initially described in India by McLeod (1882) and Donovan (1905)
- Sexually transmitted disease caused by Klebsiella granulomatis, formerly Calymmatobacterium granulomatis, a gram negative rod
- Initially a small painful nodule at infection site that ulcerates; may have satellite lesions
Terminology
- Also called donovanosis
Epidemiology
- Rare in U.S. (100 cases/year)
- More common in African Americans, in individuals with a lower socioeconomic status and among those untrained in hygiene
- Endemic in tropical and subtropical climates such as Papua New Guinea, parts of South Africa, parts of India, Indonesia and Australian aborigines (Braz J Infect Dis 2008;12:521)
Sites
- Can affect foreskin, glans, penile shaft or scrotum
Etiology
- Caused by Klebsiella granulomatis, formerly Calymmatobacterium granulomatis, a gram negative rod (Int J Syst Bacteriol 1999;49:1695)
Case reports
- 21 year old HIV+ man with coexisting squamous cell carcinoma (Dermatol Online J 2008;14:8)
- 48 year old man (Dermatol Online J 2006;12:14, free full text)
Treatment
- Three weeks of treatment with erythromycin, streptomycin or tetracycline or 12 weeks of treatment with ampicillin
- Usually clinical improvement within 1 week
Clinical images
Microscopic (histologic) description
- Massive plasma cell infiltrate without lymphocytes in granulation tissue
- Diffuse infiltration by neutrophils forming microabscesses
- Large mononuclear cells (also called Pund cells) with Donovan bodies (large intracytoplasmic encapsulated bipolar bodies, highlighted with Warthin-Starry or Wright-Giemsa stain)
Microscopic (histologic) images
Positive stains
- Wright-Giemsa or Warthin-Starry stains show Donovan bodies in tissue sample
Electron microscopy description
- Bacteria residing inside phagosomes of macrophages
Differential diagnosis
Additional references
Grossing & features to report
Table of Contents
Definition / general | Grossing - penectomy specimens | Grossing - circumcision | Features to report | Features to report by organization | Gross images | Microscopic (histologic) images | Sample gross description report | Diagrams / tables | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- This topic describes how to gross specimens obtained from penectomy and partial penectomy procedures
- Essential clinical history: clinical diagnosis, procedure performed, prior biopsies
Grossing - penectomy specimens
- In the fresh state, cut the proximal resection margin en face
- 3 important areas of the resection margin need to sampled:
- Proximal urethra and surrounding periurethral cylinder composed of epithelium, subepithelial connective tissue (lamina propria), corpus spongiosum and penile fascia
- Urethra may be retracted but it is important to locate it and submit its circumference entirely
- Corpora cavernosa separated and surrounded by tunica albuginea and Buck fascia
- Skin of shaft with underlying corporal dartos
- Proximal urethra and surrounding periurethral cylinder composed of epithelium, subepithelial connective tissue (lamina propria), corpus spongiosum and penile fascia
- Fix the remaining specimen in 10% buffered formalin overnight
- After fixation, section the glans and shaft longitudinally (sagittally) in 2 halves, using the meatus and anterior urethra as a guide
- Do not probe the urethra as doing so can result in distortion of the urethral mucosa
- If foreskin is present:
- Measure its length and identify the presence / absence of phimosis
- If not affected by tumor, separate the foreskin, leaving a 3 mm margin from the coronal sulcus and include it as a circumcision specimen
- Do not remove foreskin if it is affected by tumor
- Document the tumor size, location, color, growth pattern and distance from resection margin
- Take a photograph of the specimen showing the maximum tumor depth of invasion
- Map the photograph according to sections submitted
- Section each half longitudinally along the specimen's longest axis, at 3 - 5 mm intervals
- Submit entirely the section which depicts the deepest anatomical level infiltrated by tumor
- If tumor affects multiple anatomical compartments, submit at least 3 sections of each compartment affected
- Sections should always attempt to include adjacent nontumoral mucosa
Grossing - circumcision
- Benign circumcision specimens:
- Sample with routine sections (1 - 2), including any grossly identified lesions / abnormalities
- Circumcision specimens containing tumor / suspicious for tumor:
- Lightly stretch and pin the specimen to cardboard / sheet of cork
- Fix in 10% buffered formalin overnight
- Measure and describe the specimen:
- Identify color and consistency
- Identify areas of flattening / thickening / induration
- Describe focal lesions, hemorrhage, exudates and edema
- Describe the tumor and its relation / distance to surgical resection margins
- Ink the mucosal and cutaneous margins of resection with different colors
- Take a photograph of the specimen
- Section the specimen transversally
- Map the photograph according to sections submitted, labeling each section in a clockwise fashion
- Submit the entire tumor and sample each surgical resection margin
- References: Eur Urol 2004;46:434, Am J Surg Pathol 2001;25:1091, Am J Surg Pathol 2003;27:994
Features to report
- Features to report are according to the College of American Pathologists Cancer Protocols (CAP: Cancer Protocol Templates [Accessed 24 January 2023])
- Foreskin: presence and type (Am J Surg Pathol 2003;27:994)
- Uncircumcised
- Phimosis
- Circumcised
- Uncircumcised
- Number of lymph nodes examined and involved
- Specimen size
- Tumor site
- Glans
- Foreskin (mucosal surface or skin surface)
- Coronal sulcus
- Skin of shaft
- Penile urethra
- Tumor size (greatest dimension + additional dimensions)
- Tumor focality
- Unicentric
- Multicentric
- Macroscopic features
- Flat
- Ulcerated
- Polypoid
- Verruciform
- Necrosis
- Hemorrhage
- Tumor deep borders
- Pushing
- Infiltrative
- Anatomic level of involvement: macroscopic and microscopic
- Glans
- Involving subepithelial connective tissue (lamina propria)
- Involving corpus spongiosum
- Involving tunica albuginea
- Involving corpus cavernosum
- Involving distal (penile) urethra
- Foreskin
- Involving subepithelial connective tissue (lamina propria)
- Involving tunica albuginea
- Involving corpus cavernosum
- Involving distal (penile) urethra
- Shaft
- Involves skin
- Involves dartos
- Involves Buck fascia
- Involves corpus spongiosum
- Involves corpus cavernosum
- Involves proximal urethra
- Glans
- Gross assessment of surgical resection margins
- Tumor type (invasive, noninvasive, in situ)
- Histological type
- Benign and precursor squamous lesions
- Condyloma acuminatum
- Squamous cell carcinoma precursors, HPV associated
- Penile intraepithelial neoplasia (PeIN), HPV associated
- Squamous cell tumors and precursors, HPV independent
- Differentiated penile intraepithelial neoplasia (PeIN), HPV independent
- Invasive epithelial tumors of the penis and scrotum
- Invasive squamous epithelial tumors
- HPV associated squamous cell carcinoma
- Basaloid squamous cell carcinoma
- Warty carcinoma
- Clear cell squamous cell carcinoma
- Lymphoepithelioma-like carcinoma
- Non-HPV associated squamous cell carcinoma
- Squamous cell carcinoma, usual type
- Verrucous (including carcinoma cuniculatum)
- Papillary squamous cell carcinoma
- Sarcomatoid squamous cell carcinoma
- Squamous cell carcinoma, NOS
- HPV associated squamous cell carcinoma
- Other epithelial tumors
- Adenosquamous carcinoma
- Mucoepidermoid carcinoma
- Paget disease, extramammary
- Other scrotal tumors
- Basal cell carcinoma of the scrotum
- Invasive squamous epithelial tumors
- Benign and precursor squamous lesions
- Histological grade
- Well differentiated (G1)
- Moderately differentiated (G2)
- Poorly differentiated (G3); % present (J Urol 2001;165:1138)
- Tumor thickness
- Lymphovascular invasion
- Perineural invasion
- Presence of associated lesions
- Squamous hyperplasia
- PeIN (differentiated, basaloid, warty, warty basaloid)
- Lichen sclerosus
- Depth of invasion (Mod Pathol 2001;14:963)
- From deepest malignant cell to highest overlying dermal papilla
- Note: if tumor replaces most of penis, measure tumor thickness from nonkeratinized tumor surface to the deepest point of invasion
- Prognostic index optional (Am J Surg Pathol 2009;33:1049)
Features to report by organization
Gross images
Microscopic (histologic) images
Sample gross description report
- Specimen labeled as the requisition and with the matching Co-Path tag as penis consists of a portion of penis and measures 5.5 cm in length by up to 4.3 x 3.5 cm in diameter.
- On the skin surface, a white ulcerated / granular mass in distal portion measuring up to 3.8 cm in length and involves up to 70% of luminal circumference, which is 1.5 cm from the proximal resection margin.
- The margin is inked with silver nitrate, skin margin inked green. On longitudinal sectioning, the tumor partially involves the distal 2 cm of the corpus cavernosum and spongiosum.
- The proximal shaft / urethral is free of tumor.
- Macroscopic digital photos taken and representative sections are submitted as follows:
- B1 - B4: the entire longitudinal section of specimen including proximal resection margin, into 4 quadrants, in toto
- B5 - B6: the remainder of proximal resection margin bisected in toto, en face
Diagrams / tables
Additional references
Board review style question #1
You are grossing a partial penectomy and note that the tumor affects multiple anatomical compartments. How would you approach gross evaluation of the specimen?
- Submit 2 - 3 representative sections per centimeter of specimen
- Submit at least 3 sections of each affected compartment
- Submit the section of the highest stage compartment
- Submit the specimen in toto
Board review style answer #1
B. Submit at least 3 sections of each affected compartment
Comment Here
Reference: Penis & scrotum - Grossing & features to report
Comment Here
Reference: Penis & scrotum - Grossing & features to report
HPV associated squamous cell carcinoma
Basaloid carcinoma
Definition / general
ICD coding
Epidemiology
Sites
Etiology
Diagrams / tables
AFIP images
Clinical features
Prognostic factors
Treatment
Gross description
Gross images
AFIP images
Microscopic (histologic) description
Microscopic (histologic) images
Contributed by Alcides Chaux, M.D., Antonio Cubilla, M.D. and AFIP
Positive stains
Differential diagnosis
- Squamous cell carcinoma composed of uniform, small to intermediate cells in solid sheets or nests and often with central comedo-like necrosis
ICD coding
- ICD-O: 8083/3 - basaloid squamous cell carcinoma
Epidemiology
- 4 - 10% of all penile carcinomas (Anal Quant Cytol Histol 2007;29:185)
- Median age 52 years (range 33 - 84 years)
Sites
- Glans is the preferred site but extension to coronal sulcus and inner foreskin is common
Etiology
- HPV detected in 71 - 81% of all cases (HPV 16 in 81% of all positive tumors) (Am J Pathol 2001;159:1211)
Diagrams / tables
AFIP images
Clinical features
- Aggressive, high grade and deeply invasive penile tumor
- Inguinal nodal metastases in 50 - 100% and local recurrence in 36% (J Urol 2006;176:1431)
- High mortality rate (21 - 67%)
Prognostic factors
- Regional metastasis and mortality associated with tumor thickness > 10 mm and infiltration of corpus cavernosum (Am J Surg Pathol 1998;22:755)
Treatment
- May require total penectomy, bilateral groin dissection and adjuvant chemotherapy from the onset (Am J Surg Pathol 2009;33:1299)
Gross description
- Flat, ulcerated, irregular mass with solid tan tissue replacing corpus spongiosum and invading tunica albuginea and corpus cavernosa
- Mean tumor size of 3 - 4.5 cm
Gross images
AFIP images
Microscopic (histologic) description
- Closely attached nests of tumor cells, often with central comedo-like necrosis
- Vertical growth pattern is typical
- Composed of small to intermediate basophilic cells with scant cytoplasm, indistinctive cell borders and high mitotic / apoptotic rate
- Occasionally peripheral palisading and focal central abrupt keratinization
- May have peripheral clefts due to retraction artifact
- Frequently associated with basaloid or warty penile intraepithelial neoplasia (Int J Surg Pathol 2004;12:351)
- Prominent perineural and vascular invasion
Microscopic (histologic) images
Contributed by Alcides Chaux, M.D., Antonio Cubilla, M.D. and AFIP
Positive stains
Differential diagnosis
- Basal cell carcinoma:
- Neoplastic cells have less atypia, tumor occurs usually in skin of shaft, nests have prominent peripheral palisading and characteristic myxoid stromal changes
- Neuroendocrine carcinoma:
- Similar morphology
- Immunohistochemistry for neuroendocrine differentiation may be useful
- Poorly differentiated usual squamous cell carcinoma:
- More irregular nests
- Neoplastic cells with eosinophilic cytoplasm and distinct cellular boundaries
- Gradual (not abrupt) keratinization and clear tendency towards squamous differentiation
- Urothelial carcinoma:
- More pleomorphism, features of urothelial differentiation usually evident in the invasive or in situ component
- Positive for uroplakin III and thrombomodulin
Clear cell carcinoma
Definition / general
Epidemiology
Sites
Etiology
Clinical features
Case reports
Treatment
Gross description
Microscopic (histologic) description
Positive stains
Differential diagnosis
- Recently described aggressive variant of penile carcinoma
Epidemiology
- Middle aged men
Sites
- Foreskin inner mucosa
Etiology
- Most likely of sweat gland origin
- HPV 16 DNA found in all reported cases
Clinical features
- All reported patients presented with inguinal nodal metastases with clear cells and sclerotic basement membrane material
Case reports
- Series of 5 cases of middle aged men is only report to date (Am J Surg Pathol 2004;28:1513)
Treatment
- Excision with wide surgical margin
Gross description
- Large, exophytic and partially ulcerated
- Size 2.5 - 5.5 cm
Microscopic (histologic) description
- Solid proliferation of large neoplastic clear cells
- Marked nuclear atypia
- Extensive geographical necrosis
- Angiolymphatic invasion is common
- Warty PeIN may be found at surface
Positive stains
Differential diagnosis
- Clear cell urothelial carcinoma:
- Previous / concurrent history of urothelial carcinoma elsewhere (Int J Urol 2003;10:348)
- Preferential central location, concurrent areas of urothelial carcinoma in situ
- Positive for urothelial markers (uroplakin III, thrombomodulin)
- Metastatic renal cell carcinoma:
- Sebaceous carcinoma:
- Foreskin outer skin, smaller nuclei
- HPV-, PAS- (Arch Pathol Lab Med 2007;131:1655)
- Warty carcinoma:
- Less aggressive behavior, prominent papillae with condylomatous feature, conspicuous koilocytosis, necrosis uncommon
- MUC1-
- Well differentiated squamous cell carcinoma with glycogenated (clear) cells:
- Clear glycogenated cells are usually focal and not prominent, retained squamous maturation, pyknotic nuclei, superficially located
- MUC1-
Warty carcinoma
Definition / general
Terminology
Epidemiology
Sites
Clinical features
Prognostic factors
Treatment
Gross description
Gross images
AFIP images
Microscopic (histologic) description
Microscopic (histologic) images
Contributed by Alcides Chaux, M.D., Antonio Cubilla, M.D. and AFIP
Positive stains
Molecular / cytogenetics description
Differential diagnosis
- HPV related low grade verruciform tumor, identical to vulvar, cervical or anal counterparts (J Urol 2009;182:528)
Terminology
- Also called condylomatous carcinoma
Epidemiology
- 7 - 10% of penile carcinomas, 34 - 36% of verruciform carcinomas (Anal Quant Cytol Histol 2007;29:185)
- Mean age 48 - 55 years
Sites
- Affected sites include glans, foreskin and coronal sulcus
- Usually affects multiple anatomical compartments
- Tends to multicentricity
Clinical features
- Slow growing
- Lymph node metastasis in 17 - 18% of cases; associated with deep invasion
- Intermediate behavior between low grade verrucous or papillary carcinomas and usual squamous cell carcinomas of penis
- May recur due to inadequate excision or multicentric disease not identified at time of surgery
- Low mortality rate (0 - 9%) (Am J Surg Pathol 2001;25:673, Am J Surg Pathol 2000;24:505)
Prognostic factors
- Poor: invasion of corpora cavernosa; high grade areas; presence of vascular / perineural invasion
Treatment
- Partial or total penectomy; circumcision
- Groin dissection according to risk group stratification
Gross description
- Typical lesion is exophytic mass arising from glans; also coronal sulcus or foreskin
- Verruciform, white-tan, cauliflower-like and up to 5 cm
- May have cobblestone surface
- Endophytic cut surface
- May penetrate deep into corpus spongiosum or corpora cavernosa with broad or irregular contours
Gross images
AFIP images
Microscopic (histologic) description
- Low grade verruciform tumor with acanthosis, hyperkeratosis and parakeratosis
- Identical to warty carcinomas of vulva, uterine cervix or anus
- Arborescent papillary pattern with long, rounded or spiky papillae with prominent fibrovascular cores
- Conspicuous koilocytosis (increased nuclear size with hyperchromasia, wrinkling and bi or multinucleation, perinuclear halos and individual cell necrosis) throughout entire tumor (not just surface)
- May have intraepithelial abscesses
- Early: sharply delineated interface between tumor and stroma with no invasion (noninvasive warty carcinoma)
- Later: jagged boundary between tumor and stroma (invasive warty carcinoma)
Microscopic (histologic) images
Contributed by Alcides Chaux, M.D., Antonio Cubilla, M.D. and AFIP
Positive stains
Molecular / cytogenetics description
- 22 - 100% are associated with HPV, usually HPV 16 (Am J Pathol 2001;159:1211)
Differential diagnosis
- Carcinoma cuniculatum:
- Deep tumoral invaginations forming irregular, narrow and elongated neoplastic sinus tracts connecting surface to deep anatomic structures
- Giant condyloma:
- Benign, HPV changes only in superficial layers, no pleomorphism and no invasion
- Papillary carcinoma:
- No HPV changes, irregular fibrovascular cores with complex papillae and invasive jagged border
- More likely to have inguinal metastases
- Verrucous carcinoma:
- No HPV changes
- Inconspicuous fibrovascular cores
- Broad based invasive front
- No regional or distant metastases
- Warty basaloid carcinoma:
- Warty carcinoma mixed with basaloid squamous cell carcinoma
- Basaloid cells present in bottom layers of papillae or in deeply infiltrative nests
- More aggressive than pure warty carcinoma
HPV independent squamous cell carcinoma (usual, verrucous, papillary, sarcomatoid)
Table of Contents
Usual | Usual - pseudohyperplastic carcinoma | Verrucous | Verrucous - carcinoma cuniculatum | Papillary | SarcomatoidUsual - pseudohyperplastic carcinoma
Definition / general
Epidemiology
Sites
Clinical features
Treatment
Gross description
Microscopic (histologic) description
Microscopic (histologic) images
Contributed by Alcides Chaux, M.D. and Antonio Cubilla, M.D.
Molecular / cytogenetics description
Differential diagnosis
- Nonverruciform, highly differentiated invasive tumor resembling pseudoepitheliomatous hyperplasia
Epidemiology
- Affects elderly patients (mean age 69 years)
- ~12 cases have been reported
Sites
- Preferentially involves inner mucosa of foreskin
- Tends to be multicentric
Clinical features
- Good prognosis
- Usually no recurrence after excision
- Negative inguinal nodes
Treatment
- Circumcision or partial penectomy
- Groin dissection is not needed in absence of clinically evident metastatic disease
Gross description
- Often multicentric
- Typically flat or slightly elevated, white and granular, 2 cm
Microscopic (histologic) description
- Nonverruciform, highly differentiated and downward proliferation of keratinizing nests of squamous cells with minimal atypia
- Squamous pearls in almost all cases
- Most nests are orderly and surrounded by reactive fibrous stroma with variable inflammatory cells
- Tumor cells have eosinophilic cytoplasm with distinct intercellular bridges
- Definite invasion with irregular stromal interface is present but may need additional sections to identify
- Frequent extension beyond lamina propria
- Adjacent squamous epithelium exhibits squamous hyperplasia and differentiated penile intraepithelial lesion
- Well developed lichen sclerosus is almost always present (Am J Surg Pathol 2004;28:895)
- Peripheral palisading is not evident
Microscopic (histologic) images
Contributed by Alcides Chaux, M.D. and Antonio Cubilla, M.D.
Molecular / cytogenetics description
- HPV- in analyzed cases
Differential diagnosis
- Pseudoepitheliomatous hyperplasia:
- Regular nests, conspicuous peripheral palisading, scant stromal reaction, no extension beyond lamina propria and no cytological atypia
- Squamous cell carcinoma, usual type:
- Glans penis is preferential location, has irregular nests with moderately differentiated neoplastic cells
- The presence of broad areas of highly differentiated tumor is uncommon
- Verrucous carcinoma:
- Exophytic pattern of growth, broad pushing tumor base
Verrucous
Definition / general
Terminology
ICD coding
Epidemiology
Sites
Etiology
Clinical features
Treatment
Gross description
Gross images
AFIP images
Microscopic (histologic) description
Microscopic (histologic) images
Contributed by Alcides Chaux, M.D., Antonio Cubilla, M.D. and AFIP
Negative stains
Differential diagnosis
- Verruciform, slow growing, extremely well differentiated variant of squamous cell carcinoma with low malignant potential
Terminology
- Also called Buschke-Löwenstein tumor
ICD coding
- ICD-O: 8051/3 - verrucous carcinoma, NOS
Epidemiology
- Represents 3 - 8% of all penile carcinomas and 12 - 38% of all verruciform tumors (Anal Quant Cytol Histol 2007;29:185)
- Median age 57 years
Sites
- Glans is the preferred site but there is occasionally extension to other compartments
- Tends to be multicentric in foreskin
Etiology
- Consistently HPV- or only rarely HPV+ when applying strict diagnostic criteria (Mod Pathol 1992;5:48, Am J Surg Pathol 2010;34:104)
Clinical features
- Many cases classified as verrucous carcinoma could be reclassified as other verruciform neoplasms
- Slow growing but may recur locally
- No inguinal nodal metastases and no death due to disease in pure verrucous carcinoma
Treatment
- Partial or total penectomy
- Possibly intra-arterial chemotherapy (Urology 2003;61:1216)
Gross description
- Broad based white to gray exophytic neoplasm with a verruciform pattern of growth
- Invasion is usually limited to lamina propria or superficial corpus spongiosum
Gross images
AFIP images
Microscopic (histologic) description
- Very well differentiated with prominent intercellular bridges, minimal atypia and rare mitotic figures
- Penetrates through lamina propria with broad base and pushing borders
- Hyperkeratotic and acanthotic papillae with keratin cysts
- Orthokeratosis more prominent than parakeratosis
- Tumor cells are polygonal squamous cells with glassy cytoplasm, central vesicular nuclei and intercellular edema; may have superficial vacuolated clear cells but no koilocytosis
- Dense inflammatory infiltrate may obscure tumor stroma boundary
- Intraepithelial abscess and crust formation is common
- Frequently associated with squamous hyperplasia and differentiated penile intraepithelial neoplasia
- Central fibrovascular cores are uncommon
Microscopic (histologic) images
Contributed by Alcides Chaux, M.D., Antonio Cubilla, M.D. and AFIP
Negative stains
- Low / negative p16 INK4a and Ki67 (Mod Pathol 2009;22:1160)
Differential diagnosis
- Giant condyloma:
- Conspicuous koilocytosis, prominent fibrovascular cores
- Hybrid verrucous carcinoma:
- Foci of usual squamous cell carcinoma intermingled with a typical verrucous carcinoma
- Papillary carcinoma:
- Invasive and jagged border, more atypia, irregular but usually evident fibrovascular cores
- Squamous hyperplasia:
- No atypia, no stromal reaction and no extension beyond lamina propria (in some cases distinction is not possible)
- Warty carcinoma:
- Koilocytotic change present, jagged tumor front, neoplastic cells with more pleomorphism, prominent fibrovascular cores and usually deeper invasion
Verrucous - carcinoma cuniculatum
Definition / general
Terminology
Epidemiology
Sites
Etiology
Clinical features
Case reports
Gross description
Gross images
Images hosted on other servers:
Microscopic (histologic) description
Microscopic (histologic) images
Contributed by Alcides Chaux, M.D. and Antonio Cubilla, M.D.
Differential diagnosis
- Rare, low grade variant of verruciform penile carcinoma with deeply penetrating and burrowing pattern of growth
- More common in plantar surface of foot, first described in 1954 (Br J Surg 1954;42:245)
Terminology
- Cuniculatum: from cuniculus, rabbit's burrow (hole)
Epidemiology
- Elderly men
- Mean age 77 years (range 73 - 83 years)
Sites
- Glans and coronal sulcus frequently involved
- Multiple anatomical compartments usually affected
Etiology
- No evidence of HPV infection
Clinical features
- Slow growing
- Tumors may begin as small warts and slowly progress to larger tumors
- No reported cases with regional nodal metastasis or cancer related death
Case reports
- Series of seven cases (Am J Surg Pathol 2007;31:71)
Gross description
- Verruciform pattern of growth
- Average size 6.3 cm (range 5 - 9 cm)
- Tumor invade deep erectile tissues with a burrowing pattern of growth on cut section
- Stroma tumor interface sharply delimited
- Presence of cyst-like and sinus structures
- Fistulae to preputial or shaft skin are common
Gross images
Images hosted on other servers:
Microscopic (histologic) description
- Low grade keratinizing neoplastic nests resembling verrucous carcinoma
- Hyperkeratosis, papillomatosis and marked acanthosis
- Bulbous front of invasion
- Cyst-like and sinus lumina filled with hyperkeratotic material
- No / rare vascular and perineural invasion; no fibrovascular cores; no koilocytes
Microscopic (histologic) images
Contributed by Alcides Chaux, M.D. and Antonio Cubilla, M.D.
Differential diagnosis
- Mixed (hybrid) usual verrucous carcinoma:
- Verrucous carcinoma with foci of usual squamous cell carcinoma typically located at tumor front, absence of a burrowing pattern of growth
- Verrucous carcinoma:
- Similar in the exophytic component, usually limited to lamina propria or superficial corpus spongiosum and absence of a burrowing pattern of growth
- Warty carcinoma:
- Condylomatous papillae with prominent fibrovascular cores, conspicuous koilocytosis and jagged tumor front
Papillary
Definition / general
ICD coding
Epidemiology
Sites
Etiology
Clinical features
Gross description
Gross images
AFIP images
Microscopic (histologic) description
Microscopic (histologic) images
Contributed by Alcides Chaux, M.D., Antonio Cubilla, M.D. and AFIP
Negative stains
Differential diagnosis
- Slow growing, low grade verruciform variant of squamous cell carcinoma representing 5 - 15% of all penile carcinomas and 27 - 53% of all verruciform tumors (Anal Quant Cytol Histol 2007;29:185)
- Diagnosis is made after exclusion of other verruciform tumors
ICD coding
- ICD-O: 8050/3 - papillary carcinoma, NOS
Epidemiology
- Mean age 57 years (range 26 - 84 years)
Sites
- Usually involves glans, possibly with foreskin and coronal sulcus
Etiology
- Low HPV detection rate (Am J Surg Pathol 2010;34:104)
Clinical features
- Inguinal nodal metastases in 0 - 25% and local recurrence in 12% of all cases
- Low mortality rate (0 - 6%) (Am J Surg Pathol 2010;34:223)
Gross description
- Usually in glans but extension to coronal sulcus and inner foreskin is common
- Large gray-white exophytic destructive lesion
- Mean tumor size 5.5 cm (range 1 - 9 cm)
- Cut surface shows pearly white papillomatous tissue, poor demarcation between tumor and stroma
Gross images
AFIP images
Microscopic (histologic) description
- Well differentiated papillary squamous neoplasm
- Prominent hyperkeratosis and acanthosis
- Complex papillae with irregular fibrovascular cores
- Irregular / infiltrative tumor base
- Frequent association with squamous hyperplasia (74%), differentiated penile intraepithelial neoplasia (46%) and lichen sclerosus (34%)
- May have keratin cysts and intraepithelial abscesses
- High grade foci are unusual
- No koilocytotic changes
Microscopic (histologic) images
Contributed by Alcides Chaux, M.D., Antonio Cubilla, M.D. and AFIP
Negative stains
Differential diagnosis
- Squamous cell carcinoma, NOS:
- No prominent papillary features, most cases are moderately differentiated
- Squamous hyperplasia:
- No atypia, no stromal reaction and no extension beyond lamina propria
- Verrucous carcinoma:
- Inconspicuous fibrovascular cores, broad / bulbous boundary of tumor and stroma
- Warty carcinoma:
- Prominent fibrovascular cores, pleomorphic cells with koilocytotic changes
Sarcomatoid
Definition / general
Terminology
ICD coding
Epidemiology
Sites
Etiology
Clinical features
Case reports
Gross description
Gross images
AFIP images
Microscopic (histologic) description
Microscopic (histologic) images
Contributed by Alcides Chaux, M.D., Antonio Cubilla, M.D., Ajeeth Kumar, M.D., Eliz Thomas, M.D., Mythreye Karthikeyan, Ph.D. and AFIP
Positive stains
Negative stains
Differential diagnosis
- Aggressive variant of penile squamous cell carcinomas composed mostly of anaplastic spindle cells (Am J Surg Pathol 2005;29:1152)
Terminology
- Also called spindle cell carcinoma
- Rare tumors with distinct sarcoma and carcinoma components are called carcinosarcoma
ICD coding
- ICD-O: 8074/3 - squamous cell carcinoma, sarcomatoid
Epidemiology
- Median age 59 years (range 28 - 81 years)
Sites
- Preferred site is glans but extension to coronal sulcus and foreskin is not unusual
Etiology
- May occur after radiation therapy; low HPV detection rate
Clinical features
- Represents 1 - 3% of all penile carcinomas (J Urol 2004;172:932)
- High mortality rate (40 - 75%)
- Inguinal nodal metastases in 75 - 89% and local recurrence in 67% of all cases
Case reports
- 50 year old man with 3 cm tumor of glans (Indian J Urol 2008;24:267)
Gross description
- Large gray-white or red polypoid or fungating mass with frequent ulceration and hemorrhage
- Mean tumor size 3 - 5 cm (up to 7 cm)
- Cut surface shows deep invasion of corpus spongiosum or corpora cavernosa
- Superficial or deep tumor satellite nodules
Gross images
AFIP images
Microscopic (histologic) description
- Predominantly anaplastic spindle cells resembling fibrosarcoma or leiomyosarcoma
- Occasional giant or multinucleated malignant fibrohistocytoma-like cells
- Foci of usual squamous cell carcinoma present in most cases
- Prominent necrosis and mitotic activity
- Areas of myxoid, chondroid, osteosarcomatous or angiosarcomatous-like changes may be observed
- Penile intraepithelial neoplasia in adjacent mucosa is not uncommon
Microscopic (histologic) images
Contributed by Alcides Chaux, M.D., Antonio Cubilla, M.D., Ajeeth Kumar, M.D., Eliz Thomas, M.D., Mythreye Karthikeyan, Ph.D. and AFIP
Positive stains
Negative stains
Differential diagnosis
Herpes simplex virus
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Positive stains | Videos | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Herpes simplex virus 1 (HSV1) and herpes simplex virus 2 (HSV2) are double stranded DNA viruses that cause painful vesicles or ulcers
- Most common cause of genital ulcers (McAninch: Smith and Tanagho's General Urology, 19th Edition, 2020)
Essential features
- Sexually transmitted infection causing intraepidermal vesicle formation, ulceration, acantholysis and characteristic nuclear features, including viral nuclear inclusions, chromatin margination, nuclear molding and multinucleation
Terminology
- Herpes comes from the Greek word for creeping, ἕρπης (Online Etymology Dictionary: Herpes [Accessed 7 December 2023])
- Herpesviridae family has at least 9 viruses known to have humans as their primary host: herpes simplex virus 1 (HSV1), herpes simplex virus 2 (HSV2), Epstein-Barr virus (EBV), human cytomegalovirus (HCMV), varicella zoster virus (VZV), human herpesvirus 6A, 6B and 7 (HHV6A, HHV6B, HHV7) and Kaposi sarcoma associated herpesvirus (also known as human herpesvirus 8 [HHV8])
- Herpesviridae is currently organized into 3 subfamilies, including Alphaherpesvirinae (includes HSV1, HSV2 and VZV), Betaherpesvirinae, Gammaherpesvirinae and a single unassigned genus
ICD coding
Epidemiology
- Sexually transmitted through direct contact with lesions or by asymptomatic shedding
Sites
- Genital region, mouth, other areas
Pathophysiology
- In primary infection, herpes virus infects epithelial cells, nerve endings and sacral ganglia via axonal transport
- During reactivation, the virus travels down the axon to the skin or mucosa, where ulceration or asymptomatic viral shedding occurs
- Viral replication occurs within the nucleus resulting in the cytopathic effect
- Reference: Whitley: Pathogenesis and Disease, 2007
Etiology
- Most cases of genital herpes are caused by HSV2
- However, HSV1 is a growing cause of first episode genital herpes particularly in high income countries, though it causes less frequent genital recurrences than HSV2 (JAMA 2022;328:1730)
Clinical features
- HSV infection is the most common cause of genital ulceration (BJU Int 2002;90:498)
- Classic primary presentation of painful papular lesions progressing to vesicles and ulcers, usually with local adenitis
- Typically arises 4 - 7 days after exposure and may persist 4 - 15 days until crusting and re-epithelization
- Median of 5 recurrences in the first year after primary infection with genital HSV2 and a median of 1 recurrence after infection with genital HSV1 (Ann Intern Med 1994;121:847)
- Recurrences are typically milder than initial outbreak and duration of recurrent episodes are typically shorter
- Atypical presentations include fissures, furuncles, linear excoriations and ulcerations
- In immunocompromised patients, especially those with human immunodeficiency virus / acquired immunodeficiency syndrome (HIV / AIDS), ulcerations can be deep and persistent
- Atypical manifestations can include tumor-like nodules and condylomatous / hypertrophic lesions (herpes vegetans or hypertrophic herpes simplex genitalis) in immunocompromised (e.g., HIV positive) patients, raising the risk of misdiagnosis (HCA Healthc J Med 2022;3:247, J Hematol 2017;6:68, Int J Infect Dis 2015:33:165, Ann Clin Lab Sci 2014;44:208)
- Typical involvement of the glands, prepuce or shaft; however, the scrotum may be involved and blisters can occur around the anus in men who have sex with men
Diagnosis
- Usually diagnosed clinically but may require microbiology or serologic work up
- Biopsy may be required in cases that are unusual or do not respond to initial therapy (McAninch: Smith and Tanagho's General Urology, 19th Edition, 2020)
Laboratory
- Confirmation by cell culture or PCR for viral DNA collected from the base of the genital ulcers
- Testing for syphilis, chancroid (in locations where it is prevalent) and HIV could also be considered
- Serologic testing may be useful in some cases
Case reports
- 39 year old man with HIV and exophytic plaques with central ulceration (N Engl J Med 2023;388:2466)
- 44 year old man with HIV / AIDS and growing groin nodules (J Gen Intern Med 2018;33:393)
- 48 year old man with HIV / AIDS and enlarging nodular lesion on penis (Urol Case Rep 2021:36:101594)
- Man with ulcers on scrotum (Johns Hopkins Medicine: Week 503 - Case 1 [Accessed 14 November 2023])
Treatment
- Systemic (oral) antiviral therapy results in partial control of signs and symptoms
- Antiviral agents include acyclovir, valacyclovir and famciclovir
Clinical images
Microscopic (histologic) description
- Classic findings are an intraepidermal vesicle or ulcer with dirty necrosis, acantholysis and characteristic nuclear findings
- Look for viral changes in ulcerated and nonulcerated areas, hair follicles and interface between nonulcerated and ulcerated areas
- Nuclear changes
- Pale intranuclear viral inclusion bodies surrounded by peripheral condensation of the native chromatin (chromatin margination)
- Balloon degeneration
- Nuclear molding
- Fusion of infected cells (multinucleation also known as Tzanck cells)
- Cowdry bodies (dark nuclear inclusions composed of nucleic acid) may also be seen
- Reticular degeneration, where epidermal cells undergo progressive hydropic swelling and cytoplasmic clearing with peripheral cytoplasmic strands remaining, can occur
- Cytoplasmic vacuolization may be present
- Older lesions may appear more necrotic with ghosts of epithelial cells remaining
- Superficial and deep perivascular and periadnexal lymphocytic predominant infiltrate, lichenoid inflammatory response, intraepithelial lymphocytes, vasculitis and perineural inflammation may be seen
- Neutrophils may be seen including within vesicles
- Other less common patterns
- In some resolving lesions, no viral inclusions are evident and the histologic features are a nonspecific granulomatous dermatitis (Billings: Inflammatory Dermatopathology - A Pathologist's Survival Guide, 1st Edition, 2010)
- Herpes incognito (Am J Dermatopathol 2006;28:181)
- Vasculitis pattern (Am J Dermatopathol 1984;6:561)
- Folliculitis pattern (Br J Dermatol 2006;154:743)
- Herpes vegetans with pseudoepitheliomatous epidermal hyperplasia (N Engl J Med 2023;388:2466)
Microscopic (histologic) images
Contributed by Daniel Anderson, M.D., M.B.A. and Garrison Pease, M.D.
Virtual slides
Cytology description
- Characteristic nuclear changes in epithelial cells, including multinucleation, molding of nuclei, chromatin margination leading to thick appearing nuclear membranes, ground glass nuclei and eosinophilic intranuclear inclusions
Videos
Herpes under the microscope
by Dr. Jared Gardner
Sample pathology report
- Penis, shave biopsy:
- Ulcer and cellular changes consistent with herpes infection
Differential diagnosis
- Inflammatory dermatitis, including erythema multiforme, pemphigus and even acute spongiotic dermatitis:
- VZV (herpes zoster) infection:
- Will infect the anogenital area in a dermatomal distribution through the third and fourth sacral sensory nerves
- HSV1 / HSV2 negative (Sex Transm Infect 2022;98:71, Sex Transm Dis 2022;49:e34)
- Squamoproliferative neoplasms, including squamous cell carcinoma:
Board review style question #1
A 36 year old man presents with a painful penile lesion, which is biopsied. An ulcer with the above cellular changes is identified. What is the cause of the lesion?
- Autoantibodies for epidermal cell junction proteins
- Circulatory compromise from tight underwear / skinny jeans
- Double stranded DNA virus
- Single stranded RNA virus
- Syncytiotrophoblastic change in metastatic choriocarcinoma
Board review style answer #1
C. Double stranded DNA virus. The histologic findings are of viral cytopathic effect from herpes, a double stranded DNA virus. Answer A, B, D and E are incorrect because the cytopathic effects are not those of a bullous disease (answer A), an ischemic injury (answer B) or neoplasm (answer E). HSV is a double stranded DNA virus, not a single stranded RNA virus (answer D).
Comment Here
Reference: Herpes simplex virus
Comment Here
Reference: Herpes simplex virus
Board review style question #2
A 54 year old HIV positive man has an exophytic mass on the penis. The lesion is clinically worrisome for squamous cell carcinoma and was excised by the urologist. Histologic sections are shown above. What is the correct diagnosis?
- Corrosive chemical injury
- HSV viral infection causing ulcer and reactive / hyperplastic epidermal changes
- Treponemal infection
- Ulcerative squamous cell carcinoma
Board review style answer #2
B. HSV viral infection causing ulcer and reactive / hyperplastic epidermal changes. The findings are of an atypical HSV infection showing tumor-like condylomatous / hypertrophic epidermal changes as can sometimes be seen in immunocompromised patients. Though biopsies of HSV of the penis are rarely seen, unusual presentations should sometimes be considered in one's differential diagnosis. Answer A is incorrect because while corrosive chemical injury is a mechanism of cell injury, it will not lead to viral changes. The immunostain shown is HSV1 / HSV2 with positive cells. The viral cytopathic changes and positive HSV immunohistochemical stain makes answer C (syphilis infection) incorrect. Answer D is incorrect as the hypertrophic changes are due to HSV infection, not neoplasm.
Comment Here
Reference: Herpes simplex virus
Comment Here
Reference: Herpes simplex virus
Lichen sclerosus (balanitis xerotica obliterans)
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Chronic inflammatory mucocutaneous condition (J Eur Acad Dermatol Venereol 2018;32:91)
- Male equivalent of lichen sclerosus et atrophicus of vulva
Essential features
- Chronic inflammatory and sclerotic benign condition
- Clinical diagnosis is usually straightforward
- Middle aged men
- Unknown etiology
- Associated with penile intraepithelial neoplasia (PeIN) and penile carcinomas
Terminology
- Also called lichen sclerosus et atrophicus
- Balanitis: inflammation of glans, from Greek ("acorn")
- Xerotica: unable to determine origin of term but used by Stuhmer in 1928 (see also Arch Dermatol Syph 1941;44:547)
ICD coding
Epidemiology
- Middle aged men
- Frequency (J Urol 2011;185:522):
- Overall: 1.4/100,000
- Asian: 0.9/100,000
- African American: 1.4/100,000
- Other: 1.7/100,000
- White: 2.1/100,000
- Reported in young boys (Ann R Coll Surg Engl 2007;89:62, Pediatr Surg Int 2018;34:1245)
- Common cause of phimosis (Histopathology 2011;58:925)
Sites
- Inner foreskin, coronal sulcus and glans mucosae
- Urethra may be affected
- Rarely extends to the shaft
Pathophysiology
- Unknown at this time
Etiology
- Chronic inflammation (BJU Int 2011;108:14)
- Autoimmune association remains elusive (Acta Derm Venereol 2013;93:238)
- HPV, Borrelia burgdorferi, EBV and were reported as concurrent (Int J Dermatol 2018;57:139, Arch Dermatol 2008;144:591)
Clinical features
- Most common cause of pathological phimosis in boys
- Narrowing of urethral meatus and paraphimosis
- Associated with low grade keratinizing squamous cell carcinoma (non-HPV variants: squamous cell, NOS, pseudohyperplastic, verrucous and papillary, in glans and foreskin) (Am J Surg Pathol 2003;27:1448, Int J Surg Pathol 2004;12:351)
- Rarely found in basaloid or warty invasive carcinoma
- The majority are associated with differentiated PeIN versus HPV related PeIN
- Atypical lichen sclerosus, with epithelial dysplastic changes (low grade or high grade, are currently classified as differentiated PeIN) (Moch: WHO Classification of Tumours of the Urinary System and Male Genital Organs, 4th Edition, 2016)
- There is a sequential spatial distribution of lichen sclerosus with precancerous and invasive carcinoma suggesting it is a precancerous condition (Int J Surg Pathol 2019;27:477)
Diagnosis
- Peniscopy: grayish white plaques in foreskin, coronal sulcus or glans
- Biopsy all suspected cases (J Urol 2007;178:2268)
Prognostic factors
- Local anatomy alterations if untreated (Br J Dermatol 2018;178:839)
- Meatal stenosis
- Urethral strictures
- Concealed buried penis
- Sexual dysfunction
- More than half of patients responds to local therapy (J Eur Acad Dermatol Venereol 2012;26:730)
- Up to 25% of surgical treated require further interventions (J Eur Acad Dermatol Venereol 2012;26:730)
- p53, CDKN2A alterations suggest progression to neoplasia (Int J Biol Sci 2019;15:1429)
Case reports
- 45 year old man with lichen sclerosus and granuloma annulare in his foreskin (Rom J Morphol Embryol 2015;56:1179)
- 56 year old man with urethral squamous cell carcinoma and long history of genital lichen sclerosus (Am J Mens Health 2018;12:493)
- 70 year old man with lichen sclerosus, nevus elasticus and neoplastic progression (JAAD Case Rep 2015;1:163)
- 76 year old man with lichen sclerosus associated nevus on glans mimicking melanoma (JAAD Case Rep 2020;6:323)
Treatment
- Local corticosteroids
- Imiquimod
- PUVA (Psoralen and UltraViolet A) therapy
- Biopsy all cases of possible BXO (J Urol 2007;178:2268)
- Circumcision is indicated for preputial lesions (J Eur Acad Dermatol Venereol 2012;26:730)
Clinical images
Gross description
- Grayish, bluish to white irregular geographic foci of atrophy
- Erosion, ulceration and raised pearly white areas
- In advanced cases, inner preputial folds may disappear due to replacement of elastic fibers by fibrous tissue (Am J Clin Dermatol 2013;14:27)
Microscopic (histologic) description
- Topographical evaluation from surface epithelia to deep penile layers (dartos in foreskin and corpus spongiosum in glans) is recommended as follows (Int J Surg Pathol 2020;28:468)
- Squamous epithelium:
- Normal, atrophic or hyperplastic, the latter most common, with more than 10 cell epithelial layers
- Hyperkeratosis associated with hyperplasia
- Vacuolar degeneration of basal layer, landmark lesions in all types of lichen
- Tissue separation at the basal layer, which indices late epithelial ulceration
- Lamina propria:
- Thickening and loss of structures
- Edema, hypervascularity and typically sclerosis
- Distinctive sclerotic patterns
- At the surface: perivascular, globular or linear sclerosis
- Diffuse sclerosis in deeper lamina propria tissues
- Presence of a sclerotic globule is sufficient for diagnosis
- Sclerotic changes spares corpus spongiosum of glans and foreskin dartos
- Lymphocytic infiltration:
- Landmark lesion with variable presentation
- At the interface of epithelium and lamina propria
- Distant from basal layer deep in lamina propria
- Lymphocytes are the basis for subtyping
- Lichenoid: lymphocytes at the basal layer
- Band-like or classic: lymphocyte in deep lamina propria
- Lymphocytic depletion: only few lymphocytes present
- Depletion of lymphocytes is typical of cancer associated lichen sclerosis
- During progression sclerosis increases; fully developed lesions are characterized by:
- Epithelial thinning and ulceration
- Wide hyalinized band in the upper dermis
- Lymphocytic infiltrate below the hyalinized band
- Penile intraepidermal neoplasia and/or carcinoma can be associated
Microscopic (histologic) images
Contributed by Diego F. Sanchez, M.D. and Antonio L. Cubilla, M.D.
Positive stains
- Immunohistochemistry is not needed for diagnosis but can suggest progression to neoplastic lesions
- p53: 40% of cases adjacent to cancer showed positive parabasal cells in other organs (Br J Cancer 2003;89:2249)
- Ki67: positive in basal epithelial cells (J Urol 2013;190:399)
- MCM3 (mini chromosome maintenance 3 protein): positivity in long standing lichen sclerosus and correlates with Ki67 expression (J Urol 2013;190:399)
- CD3, CD4, CD8: T cells marker are positive in the lymphocytic infiltrate (Histopathology 2006;48:730)
Molecular / cytogenetics description
- Some reports regarding RNA expression are (Int J Biol Sci 2019;15:1429):
- Upregulation of miR-155, TNF, IL6, galectin 7, collagen (type I, III, V), p53
- Downregulation of FOXO3, CDKN1B, IL10 and endothelial ECM1
- CDKN2A and p53 epigenomic modifications
Videos
Lichen sclerosus et atrophicus
Sample pathology report
- Foreskin, circumcision:
- Lichen sclerosus (see comment)
- Comment: Epithelial hyperplasia and hyperkeratosis was noted. Scarce lymphocytic infiltrate was seen in the lamina propria and between sclerotic tissue suggesting lymphocytic depleted variant. This subtype has been reported associated with preneoplastic lesions and carcinoma which were not observed in this specimen.
Differential diagnosis
- Lichen planus:
- Dense lymphoid infiltrate is the hallmark
- Absence of hyalinization / sclerosis
- This later can be difficult to differentiate with early lichen sclerosus
- Zoon balanitis:
- Plasma cell balanitis
- Usually absence of granular and cornified layers
- Very rare
Additional references
Board review style question #1
- A 30 year old man went to the urologist complaining about difficulty with coitus. He has a history of untreated phimosis from childhood. At the clinical examination, a buried penis is found. Circumcision is performed and the following image shows the findings. What is your diagnosis?
- Acute postitis
- Amyloidosis
- Lichen planus
- Lichen sclerosus
- Zoon balanitis
Board review style answer #1
D. Lichen sclerosus. Atrophic epithelium, dense hyalinized fibrotic tissue replacing lamina propria and subjacent lymphocytic inflammatory infiltrate.
Comment Here
Reference: Lichen sclerosus (balanitis xerotica obliterans)
Comment Here
Reference: Lichen sclerosus (balanitis xerotica obliterans)
Board review style question #2
- Regarding lichen sclerosus, which of the following is correct?
- Circumcision is always curative
- HPV is the most common etiology
- Immunohistochemistry is needed for accurate diagnosis
- It is associated with neoplastic lesions
- Only seen in anogenital area
Board review style answer #2
D. It is associated with neoplastic lesions. Although it is a benign chronic inflammatory condition, this lesion is associated with non-HPV related PeIN and carcinomas.
Comment Here
Reference: Lichen sclerosus (balanitis xerotica obliterans)
Comment Here
Reference: Lichen sclerosus (balanitis xerotica obliterans)
Lymphogranuloma venereum
Table of Contents
Definition / general | Epidemiology | Etiology | Clinical features | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Electron microscopy descriptionDefinition / general
- Sexually transmitted disease caused by Chlamydia trachomatis, an obligate intracellular parasite (Wikipedia: Lymphogranuloma Venereum [Accessed 29 March 2018], eMedicine: Lymphogranuloma Venereum (LGV) in Emergency Medicine [Accessed 29 March 2018], eMedicine: Dermatologic Manifestations of Lymphogranuloma Venereum [Accessed 29 March 2018])
Epidemiology
- Incidence highest in the tropics and subtropics, although outbreaks have occurred in West among men who have sex with men (MMWR Morb Mortal Wkly Rep 2004;53:985)
Etiology
- Sexually transmitted disease caused by Chlamydia trachomatis, an intracellular bacteria
- Different genovars produce specific clinical manifestations; i.e. types A, B, Ba and C cause trachoma, types LGV I, II and III (serovars L1, L2 and L3) cause LGV and types D to K cause oculogenital diseases
Clinical features
- Causes either inguinal, rectal or rarely pharyngeal syndrome
- Inguinal syndrome: painless papule or ulcer at inoculation site appears and rapidly disappears; followed 1 - 2 weeks later by enlarged inguinal lymph nodes with suppurative inflammation; followed by lymphocytic hyperplasia and massive plasma cell infiltration; then get stellate abscess; then suppurative granuloma with sinuses and tracts
- Rectal syndrome: most patients have proctitis, many with severe symptoms (Clin Infect Dis 2007;44:26)
- LGV infection may facilitate transmission of HIV
- Diagnosis: based primarily on clinical findings, can be supported by culture (30 - 50% sensitive), complement fixation (80% sensitive but does not distinguish different serovars) or PCR (limited availability)
Treatment
- Tetracycline (except during pregnancy) or erythromycin
Microscopic (histologic) description
- Nonspecific features of ulceration and granulation tissue in dermis
- Suppurative inflammation of inguinal lymph nodes (neutrophils in necrotic foci); followed by lymphocytic hyperplasia and massive plasma cell infiltration; then stellate abscess from merging of microfoci of suppuration; then suppurative nonnecrotizing granuloma with epithelioid and multinucleated giant cells
- Sinuses and tracts can develop and fibrosis may ultimately replace most of lymph node architecture
- In lymph nodes, appear as intravacuolar organisms, associated with necrosis and suppuration
- Etiological agent is not apparent with routine stains
- Organisms are 0.2 - 2.0 micrometers in diameter, stain Gram negative with the Brown-Hopps tissue Gram stain, faintly blue with hematoxylin and eosin stain and black with Warthin-Starry stain (Mod Pathol 1995;8:924)
Electron microscopy description
- Elementary and reticulate bodies and intermediate forms characteristic of the genus Chlamydia
Melanosis and lentiginosis
Table of Contents
Definition / general | Clinical features | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosisDefinition / general
- Penile melanosis and penile lentiginosis are benign pigmented lesions frequently found in glans and foreskin
- Penile melanosis shares clinicopathological features with Laugier-Hunziker syndrome of oral mucosa (eMedicine: Laugier-Hunziker Syndrome [Accessed 30 March 2018]) and vulvovaginal melanosis (J Am Acad Dermatol 1989;20:567)
Clinical features
- Benign, although associated with melanoma
Penile melanosis:
- Large, often single, flat and pigmented macule with irregular borders
- Pigmentation may be associated with Laugier-Hunziker syndrome (Int J Dermatol 2004;43:571)
Penile lentiginosis:
- Penile lentigines are 0.2 - 2 cm, oval to irregular lesions with uniform or variegated pigmentation
- Areas of depigmentation are characteristic
- Lesions are scattered on shaft or glans
- Clinically may resemble an atypical melanocytic lesion
- May be associated with Cowden disease (J Cutan Med Surg 2001;5:228), Bannayan-Riley-Ruvalcaba syndrome (J Am Acad Dermatol 2005;53:639)
Microscopic (histologic) description
Penile melanosis:
Penile lentiginosis:
- Melanocytic hyperplasia, hyperpigmentation of basal epithelium and otherwise normal epithelium
Penile lentiginosis:
- Elongation of rete ridges with basal layer hyperpigmentation, slight melanocytic hyperplasia, epithelial hyperplasia and stromal melanophages, no atypia (J Am Acad Dermatol 1990;22:453)
- In hyperpigmented areas, there are increased number of melanocytes along the basal layer
- Lymphocytes, which are found in close apposition, destroy melanocytes and surrounding keratinocytes lack pigmentation (Pigment Cell Res 1992;5:404)
Differential diagnosis
- Congenital melanocytic nevus
- Melanoma: difficult to distinguish clinically, may need to biopsy (Urology 1976;7:323)
Molluscum contagiosum
Table of Contents
Definition / general | Sites | Etiology | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology images | Electron microscopy imagesDefinition / general
- Dome shaped papule with central umbilication, caused by DNA poxvirus called molluscum contagiosum virus (MCV) (eMedicine: Molluscum Contagiosum [Accessed 29 March 2018])
Sites
- In children ages 1 - 5 years, occurs commonly on almost all body sites, including trunk, arms and legs
- In adults, is considered a sexually transmitted disease, often due to MCV2 virus
Etiology
- Caused by DNA poxvirus, which only infects humans
- Spreads by skin to skin contact, autoinnoculation (spread to neighboring areas by touch), sexual transmission or by handling objects with the virus on them
Case reports
- 65 year old HIV+ man with genital lesions appearing after institution of HAART therapy, as part of the immune reconstitution syndrome (Dermatol Online J 2007;13:6)
Treatment
- Usually resolves within months in people with a normal immune system
- Virus lives only in lesions - once they are gone, patient is cured, unless reinfected
- Treatment is similar to that for warts - cryotherapy, acid, electrocautery, curetting or laser therapy; also topical trichloroacetic acid, cantharidin, retinoic acid or imiquimod
Clinical images
Gross description
- 3 - 6 mm dome shaped pearly painless papule with central umbilication
Microscopic (histologic) description
- Prominent Henderson-Patterson (molluscum) bodies (intracytoplasmic eosinophilic inclusions containing virus particles) in keratinocytes of stratum spinosum and granulosum
- Epidermal lobular acanthosis with inverted epidermal hyperplasia
Microscopic (histologic) images
Mucoid cyst
Table of Contents
Definition / general | Terminology | Clinical features | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stainsDefinition / general
- Inflammatory lesion due to accessory urethral canals (entrapped urothelium during development) on foreskin or glans, with intraepithelial mucous cells or glands
Terminology
- Also includes some median raphe cysts (uncommon; found from distal penis and scrotum toward the perineum in a midline position; considered a congenital alteration in embryologic development, Urology 2008;71:830)
Clinical features
- Slow growing painless cystic mass
- Often has an incorrect clinical and pathologic diagnosis (J Urol 1976;115:397)
Case reports
- 43 year old man with mucinous median raphe cyst of glans penis (Dermatol Online J 2005;11:37)
Treatment
- Excision is curative
Microscopic (histologic) description
- Epithelium is stratified columnar with intraepithelial mucous cells or associated mucous glands
Microscopic (histologic) images
Negative stains
Myointimoma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1Definition / general
- Benign myointimal proliferation with predilection to the corpus spongiosum of the glans penis
Essential features
- Benign mesenchymal neoplasm in the corpus spongiosum of the glans penis
- Intravascular proliferation of the vascular intimal cells
- Lesional cells stain with α smooth muscle actin but not desmin
Terminology
- Penile myointimoma
- Myointimoma of the penis
ICD coding
- ICD-O: 9137/0 - myointimoma
Epidemiology
- Rare tumor with ~28 cases reported in the literature (Am J Surg Pathol 2000;24:1524, Am J Surg Pathol 2007;31:1622, Int J Surg Pathol 2022 Aug 9 [Epub ahead of print])
- Previously described as intravascular leiomyoma, leiomyomatosis, late stage intravascular fasciitis, leiomyoma of the glans penis, solitary cutaneous myofibroma of the glans penis (Cancer 1970;25:1431, J Urol 2000;164:791, Am J Dermatopathol 1996;18:317)
- Wide age range (2 - 74 years old) (Am J Surg Pathol 2000;24:1524, Actas Dermosifiliogr 2009;100:511)
Sites
- Corpus spongiosum of the glans penis
Pathophysiology
- Intravascular proliferation from the intimal cells of the inner layer of the corpus spongiosum vasculature in the glans penis (Am J Surg Pathol 2000;24:1524)
Etiology
- Mesenchymal tumor unrelated to the status of circumcision, history of trauma or the presence of disease (Am J Surg Pathol 2000;24:1524, Actas Dermosifiliogr 2009;100:511)
Diagrams / tables
Clinical features
- Small, distinct, nonmobile, firm, painless nodule on the glans penis (J Am Acad Dermatol 2005;53:1084)
- Benign, rapidly growing lesions that remain stable for years, even after incomplete excision (Am J Surg Pathol 2000;24:1524)
Diagnosis
- Laboratory tests, including urine analysis and ultrasonic evaluation of the abdomen and the scrotum to exclude other potential causes (Pathol Int 2007;57:158, J Pediatr Surg Case Rep 2019;44:101189)
- Punch, incisional or excisional biopsy (Am J Surg Pathol 2000;24:1524)
Prognostic factors
- Benign outcome with no recurrence; spontaneous regression may occur (Am J Surg Pathol 2000;24:1524, Am J Surg Pathol 2007;31:1622, Int J Surg Pathol 2022 Aug 9 [Epub ahead of print)
Case reports
- 14 year old boy with a 1 cm nodule on the right side of his glans penis (J Cutan Pathol 2009;36:817)
- 49 year old man with a 12 month history of a palpable lesion involving the glans penis (Int J Impot Res 2021;33:583)
- 50 year old man with a nodule located in the glans penis (Pathol Int 2007;57:158)
Treatment
- Simple excision
Gross description
- Firm white mass of 0.4 - 1.9 cm (Am J Surg Pathol 2007;31:1622, Am J Surg Pathol 2000;24:1524, J Am Acad Dermatol 2005;53:1084)
Frozen section description
- Lesion may be present at margin of excision (Am J Surg Pathol 2007;31:1622)
Microscopic (histologic) description
- Multinodular or plexiform pattern; composed of occlusive intravascular myointimal proliferation (Am J Surg Pathol 2000;24:1524)
- Nodules contain spindle or stellate shaped cells embedded in abundant fibromyxoid matrix or sometimes chondroid matrix (Am J Surg Pathol 2007;31:1622)
- Cells have long eosinophilic cytoplasmic processes, blunt ended nuclei, fine chromatin and juxtanuclear vacuoles
- Foci of degenerative changes appear as ghost cells (Am J Surg Pathol 2000;24:1524)
- No cytologic atypia, nuclear pleomorphism, prominent nucleoli or mitoses
- Residual smooth muscle bundles surrounding the tumor (Actas Dermosifiliogr 2009;100:511)
- Overlying skin may show slight hyperkeratosis (J Cutan Pathol 2009;36:817)
- No necrosis or significant inflammation
Microscopic (histologic) images
Positive stains
Negative stains
- Desmin (shows the residual native smooth muscle of the vessel walls)
- CD34 / CD31 / factor VIII related antigen (highlight the penetrating capillaries and the residual endothelial cells)
- S100 protein
- AE1 / AE3 (Am J Surg Pathol 2000;24:1524)
- Verhoeff-van Gieson elastic histochemical stain (demonstrates the meshwork of the elastic fibers surrounding the nodules) (Am J Surg Pathol 2000;24:1524)
Sample pathology report
- Glans penis, excisional biopsy:
- Penile myointimoma (see comment)
- Comment: Sections show a plexiform vascular proliferation composed of spindle cells in a fibromyxoid background with occluded lumens. The neoplastic cells are α smooth muscle actin positive and desmin negative.
Differential diagnosis
- Plexiform fibrohistiocytic tumor:
- Not intravascular
- Common in extremities, trunk, head and neck
- Dimorphic population of cells:
- Epithelioid hemangioendothelioma:
- Myofibroma:
- Biphasic pattern of myoid nodules similar to myointimoma; however, there are hemangiopericytoma-like areas
- Can involve vessels but is not limited to intravascular lumina
- Intravascular fasciitis:
- May overlap
- Intralesional inflammatory cells, mucoid pools
- Nerve sheath tumor:
- Diffuse nuclear S100
- Leiomyoma:
Board review style question #1
Board review style answer #1
Ossification
Table of Contents
Definition / general | Terminology | Etiology | Clinical images | Microscopic (histologic) images | Differential diagnosisDefinition / general
- Heterotopic bone in penis (Eur Urol 1984;10:420), most commonly in elderly and also children
- Note: male mammals except chimpanzees and humans have an intrapenile bone (bacula); humans have an equivalent strong distal ligament composed of type I collagen in the glans (J Androl 2005;26:624)
Terminology
- Also called baculum, penis / penile bone
Etiology
- Penile ossification may occur after trauma (Urology 1990;35:349)
- Also associated with diabetes, gout, venereal disease, Peyronie disease and neoplasia
- Rarely congenital (J Urol 1964;91:663)
Clinical images
Differential diagnosis
- Ossification in the corpora cavernosa (Eur Urol 1995;27:252)
Pearly penile papules
Table of Contents
Definition / general | Terminology | Epidemiology | Etiology | Clinical features | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosisDefinition / general
- Benign condition of 20 - 30% of normal young and middle aged males, asymptomatic
Terminology
- Also called hirsutoid papillomas, papillomatosis of glans corona
Epidemiology
- Prevalence of 38% in men < 25 years old and 11% in men > 50 years old (Int J STD AIDS 2009;20:768)
Etiology
- Hyperplastic, reactive
- Not related to HPV (J Am Acad Dermatol 2003;49:50)
Clinical features
- Multiple pearly gray-white fibroepithelial papillomas, 1 - 2 mm and in dorsal glans corona
- Usually arranged in 2 - 3 rows
- Rarely covers most of glans
- Reduced prevalence in circumcised men
- Has been confused with Tyson glands, which don't exist in humans (Wikipedia: Preputial Gland [Accessed 2 April 2018])
Treatment
- None required; disappears with age; CO2 laser if patients request removal (Dermatol Surg 2002;28:617)
- Also cryotherapy, electrodesiccation, podophyllin or curettage
Clinical images
Microscopic (histologic) description
- Hyperkeratosis associated with a fibrovascular stroma, simulating an angiofibroma
- No koilocytosis, no significant inflammation
Microscopic (histologic) images
Differential diagnosis
- Condyloma acuminatum: nonuniform, not arranged in rows and has HPV related changes
- Syphilis: ulcer with indurated and punched out base, marked plasmacytic inflammation and serologic evidence of syphilis
Penile intraepithelial neoplasia (PeIN)
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Negative stains | Molecular / cytogenetics description | Molecular / cytogenetics images | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Intraepithelial neoplastic proliferation with variable degree of dysplasia, keratinization and nuclear atypia
- Penile intraepithelial neoplasia (PeIN) is classified as HPV related / dependent or HPV unrelated / independent, similar to invasive carcinomas (Am J Pathol 2001;159:1211, Semin Diagn Pathol 2015;32:198)
- Considered a precursor of penile invasive carcinoma
Essential features
- Classified as HPV related / dependent or HPV unrelated / independent
- May occur in any of the penile mucosal epithelial areas, most commonly in the glans and foreskin
- Differentiated PeIN is related to lichen sclerosus
- HPV16 is the most frequent genotype associated with PeIN
Terminology
- Synonyms are as follows: erythroplasia of Queyrat, Bowen disease, carcinoma in situ (CIS), squamous intraepithelial lesion, dysplasia (mild, moderate and severe) and bowenoid papulosis
- PeIN is the recommended nomenclature for penile precancerous lesions (Epstein: Tumors of the Prostate Gland, Seminal Vesicles, and Scrotum, 2020, Eur Urol 2016;70:93)
- PeIN refers to squamous lesions and excludes Paget disease, urothelial carcinoma in situ and malignant melanoma in situ
- Some authors stratify PeIN into grades I, II and III (Int J Urol 2019;26:353)
ICD coding
Epidemiology
- There is geographic variation in the presentation of PeIN (Hum Pathol 2012;43:190)
- Differentiated PeIN is more commonly diagnosed in countries with a high frequency of penile cancer
- HPV related PeIN is more common in countries with a low frequency of penile cancer
- In countries with a high frequency of invasive penile carcinomas (2 - 5 cases/100,000), PeIN is rarely diagnosed as a solitary lesion (Hum Pathol 2012;43:190)
- In countries in which invasive penile carcinomas are rare (≤ 1 case/100,000), PeIN is the most common penile neoplasia at clinical diagnosis
- PeIN affects patients younger than those with invasive cancers (Hum Pathol 2012;43:1020)
- Mean age is ~58 years old
- HPV related PeIN preferentially affects younger patients
- Predisposing factors: HIV / immunosuppression, lack of or delayed circumcision, inflammatory / irritative conditions (balanitis, buried penis, phimosis) (Mod Pathol 2022;35:1101)
Sites
- PeIN is most commonly found in the glans and foreskin (Histopathology 2011;58:925, Int J Urol 2019;26:353)
- Differentiated PeIN, non-HPV related, preferentially involves the foreskin inner mucosal epithelium
- ~33% of cases with PeIN are multicentric, especially the HPV related types
- PeIN may occur in the skin of the shaft of the penis
Etiology
- Undifferentiated: high risk HPV
- Differentiated: lichen sclerosus
Clinical features
- Lesions can be subclassified according to morphological features and HPV genotypes present (Am J Surg Pathol 2017;41:820)
- Non-HPV related PeIN / differentiated
- Represented by differentiated PeIN
- Squamous or simplex PeIN are synonyms
- Putative precursors of non-HPV related keratinizing squamous cell invasive carcinomas, the majority of penile cancers
- Most cases in association with invasive carcinoma and rarely as a solitary lesion
- Difficult to diagnose and underrecognized by pathologists due to only subtle histologic changes
- Foreskin is a preferential site but the glans is also involved
- Frequently associated with lichen sclerosus
- HPV related PeIN / undifferentiated
- Subclassified in basaloid, warty and warty basaloid subtypes (Epstein: Tumors of the Prostate Gland, Seminal Vesicles, and Scrotum, 2020, Eur Urol 2016;70:93)
- Bowen disease, CIS, high grade PeIN are synonyms
- In young males with multicentric lesions, it has been referred to as bowenoid papulosis (Cancer 1978;42:1890)
- More frequently multicentric than differentiated PeIN
- Putative precursors of HPV related basaloid and condylomatous (warty) invasive carcinomas or mixtures, ~33% of penile cancers (Am J Surg Pathol 2017;41:820)
- Straightforward diagnosis and well recognized by pathologists
- Glans is a preferential site but the foreskin, coronal sulcus and shaft may also be involved
- HPV16, 6 and 11 are the most commonly found genotypes (Lancet Oncol 2019;20:145, Int J Surg Pathol 2020;28:265)
- HIV positive patients are more susceptible to develop HPV related PeIN (AIDS 2006;20:1201)
- Mixed PeIN
- Coexisting non-HPV and HPV related PeIN in the same specimen
- Rarely found as solitary lesions
- Multicentric lesions may be in collision, next to each other or in separate foci
- Pathological features and HPV composition are similar in PeIN and invasive carcinomas (Int J Surg Pathol 2020;28:265)
- HPV is usually negative in differentiated PeIN and the corresponding invasive non-HPV related squamous cell carcinoma
- HPV is usually positive in PeIN and the corresponding invasive basaloid or warty invasive carcinoma
- Morphological similarity and HPV genotype composition indicate a causal relation of PeIN and corresponding invasive carcinoma
Diagnosis
- Dermatoscopy
- Biopsy
- Excision
Prognostic factors
- Frequent recurrence (48%) (Mod Pathol 2022;35:1101)
- Low rate of progression to invasive carcinoma (2%) (Mod Pathol 2022;35:1101)
Case reports
- 51 year old man with pruritic lesions on the penis, scrotum, pubis and gluteal region (JAAD Case Rep 2017;3:542)
- 68 year old man presented with phimosis (Open Access Maced J Med Sci 2017;6:61)
- 79 year old man with penile lesion involving the distal urethra (SAGE Open Med Case Rep 2020;8:2050313X20918985)
Treatment
- Local excision (Mohs surgery, glans resurfacing, glansectomy) is the most frequent approach (BJU Int 2022;129:752, Semin Diagn Pathol 2015;32:232)
- Laser therapy is associated with higher complication and recurrence rate (BJU Int 2022;129:752, Eur Urol Focus 2021 May 11 [Epub ahead of print])
- Topical agents (5-fluorouracil, imiquimod 5%)
- Local destructive methods (photodynamic therapy, cryotherapy, curettage and electrocautery)
Clinical images
Gross description
- Non-HPV related PeIN / differentiated
- Solitary white or pink macule, plaque or slightly elevated geographical lesion
- Affects the foreskin and glans and rarely the shaft
- HPV related PeIN
- Lesions are flat or slightly elevated or papular, velvety, erythematous, dark brown or black
- Warty PeINs are granular or villous
- Borders are irregular or sharply delineated
- References: Epstein: Tumors of the Prostate Gland, Seminal Vesicles, Penis, and Scrotum, 2020, Eur Urol 2016;70:93
Microscopic (histologic) description
- Non-HPV related PeIN / differentiated
- Common features are hyperkeratosis, parakeratosis, hypergranulosis, acanthosis, elongation of rete ridges, abnormal squamous maturation and squamous cell atypia
- Prominent intercellular edematous bridges and intraepithelial keratinization
- Some histological heterogeneity: hyperplasia-like, classic and pleomorphic features
- Most common or classic feature is a keratinized maturing lesion with obvious atypical cells involving 2 or 3 basal epithelial layers
- Less common are the hyperplasia-like features, with acanthotic thickening of the epithelium and subtle basal cell atypia
- In the pleomorphic variant, there are anaplastic cells involving most of the epithelial thickness but with evident maturation or cellular keratinization
- Grading system if used can classify as follows: hyperplasia-like as grade 1, classic as grade 2, pleomorphic as grade 3
- Basaloid PeIN
- Most frequent subtype
- Uni or multicentric
- Usually, flat lesions with a broad or undulating base
- Occasionally, papillary lesions simulating urothelial tumors
- Monotonous uniform small anaplastic basaloid cell population
- Replace the full epithelial thickness
- Superficial hyper and parakeratosis often with some koilocytes is typical
- Rarely, cells are larger, spindly or pleomorphic
- High nuclear cytoplasmic ratio
- Numerous mitoses may be noted
- Starry sky pattern is not uncommon
- Warty PeIN
- Presentation as a solitary lesion is unusual (Am J Surg Pathol 2017;41:820)
- Most commonly, it is part of a multicentric lesion
- Associated with invasive warty or basaloid carcinomas
- Squamous maturing lesion
- Striking micropapillary spiking features
- Surface shows hyper and parakeratosis
- Hallmark is atypical superficial or deep pleomorphic koilocytosis
- Multinucleation, nuclei with irregular contours, perinuclear halo and dyskeratosis are common
- Warty basaloid PeIN
- Warty cells and basaloid cells in about equal proportions
- Unifocal or multicentric lesions
- May be associated with invasive basaloid or warty carcinomas
- Hyper and parakeratosis, papillary or spiking features at the upper half
- Upper half is composed of clear warty-like cells
- Lower half is composed of small, anaplastic basaloid type cells
Microscopic (histologic) images
Contributed by Alcides Chaux, M.D. and Antonio Cubilla, M.D.
Positive stains
- Non-HPV related PeIN / differentiated
- HPV related PeIN / undifferentiated
- Ki67: positive in most cells, full thickness
- p16: 99% positive, en bloc for basaloid PeIN and ≤ 50% of epithelial thickness in warty basaloid and warty PeIN (Am J Surg Pathol 2010;34:385, Mod Pathol 2022;35:1101)
Negative stains
- Non-HPV related PeIN / differentiated
Molecular / cytogenetics description
- HPV detection by PCR or ISH is negative in differentiated PeIN
- High risk HPV is detected in most HPV related PeIN
- HPV16 found in 67% of basaloid PeIN (Am J Surg Pathol 2017;41:820)
- Low risk HPV is present in 16% of warty PeIN (Am J Surg Pathol 2017;41:820)
- More variable HPV genotypic composition in warty PeIN
Sample pathology report
- Penis, glans, biopsy:
- HPV related basaloid penile intraepithelial neoplasia (PeIN) (see comment)
- Comment: Lateral margins are involved by PeIN. High risk HPV ISH positive. p16 immunostaining is positive en bloc.
Differential diagnosis
- Non-HPV related / differentiated PeIN:
- Squamous hyperplasia:
- Condyloma acuminatum, flat type:
- HPV related PeIN (versus pleomorphic differentiated PeIN):
- HPV related PeIN:
- Urothelial carcinoma in situ (versus basaloid PeIN):
- p16 negative
- Condyloma acuminatum with atypical features (versus warty PeIN) (Int J Surg Pathol 2020;28:265):
- p16 negative
- May harbor low or high risk HPV
- Squamous cell carcinoma warty and basaloid types (versus warty and basaloid PeIN)
- Urothelial carcinoma in situ (versus basaloid PeIN):
Board review style question #1
A 55 year old man undergoes excision of a 1 cm, slow growing, erythematous plaque on the glans penis. At microscopy, the epithelium showed full thickness dysplasia, pleomorphic cells and mitoses. A representative photomicrograph is shown above. p16 immunostaining was strongly positive and Ki67 was positive in all cell layers. Which of the following statements is true regarding the depicted tumor?
- Differentiated PeIN hyperplasia-like is the diagnosis
- Distinguishing this lesion from squamous hyperplasia is difficult
- HPV16 is the most frequent genotype identified in the lesion
- It is the precursor of the most frequent invasive penile carcinoma subtype
- This neoplasia is usually related to lichen sclerosus
Board review style answer #1
C. HPV16 is the most frequent genotype identified in the lesion. This is HPV related PeIN. 67% of basaloid PeIN are positive for HPV16 genotype.
Comment Here
Reference: Penile intraepithelial neoplasia
Comment Here
Reference: Penile intraepithelial neoplasia
Board review style question #2
A 78 year old man undergoes excision of a 1 cm whitish plaque on the foreskin. At microscopy, the epithelium showed full thickness dysplasia, pleomorphic cells with ample cytoplasm and mitoses. Which of the following steps is needed for a final diagnosis?
- CD4 / CD8 for evaluating lichen sclerosus
- HMB45 immunostaining
- Ki67 for differentiating warty from basaloid PeIN
- In situ hybridization for high risk HPV
- p53 for ruling out squamous hyperplasia
Board review style answer #2
D. In situ hybridization for high risk HPV. In situ hybridization for high risk HPV is needed to confirm HPV related basaloid PeIN versus non-HPV related differentiated PeIN with pleomorphic features.
Comment Here
Reference: Penile intraepithelial neoplasia
Comment Here
Reference: Penile intraepithelial neoplasia
Peyronie disease
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosisDefinition / general
- Fibrous thickening of dermis and Buck’s fascia between corpora cavernosa and tunica albuginea, causing curvature towards side of lesion and restricting movement of these structures during erection
Essential features
- Proliferation of fibroblasts and myofibroblasts on or around the tunica albuginea of the penis, most often the dorsal aspect, that may lead to plaques with curvature of the penis towards the plaque
Terminology
- Induratio penis plastica, penile fibromatosis
Epidemiology
- Usually 40+ years, rare in younger population (J Androl 2003;24:27)
- Prevalence 3 - 9% (Int J Impot Res 2002;14:379)
Sites
- Tunica albuginea, most commonly the dorsal aspect
Etiology
- May be related to microtrauma (coital, urethral instrumentation), which releases fibroblast related cytokines
- May arise secondary to urethritis as a sclerosing inflammatory process
- Etiology may be related to Parc protein (BJU Int 2010;106:1706) or Wnt2 (J Sex Med 2012;9:1430)
Clinical features
- May be associated with carcinoid syndrome, Dupuytren contracture
- May be associated with beta blockers, thiazides (J Sex Med 2010;7:1529), hypertension, diabetes and immune reactions
- Fibrosis produces an abnormal curvature of the penis, which may cause pain during erection and intercourse
- In some cases, nodules adherent to tunica albuginea are noted over the mid dorsal line
- May be clinically mimicked by epithelioid sarcoma (Int J Impot Res 2003;15:378)
Diagnosis
- Usually clinical, however, occasionally may be a histologic confirmation
Radiology description
- Ultrasound
- Echogenic plaques, frequently calcifications
- MRI
- Thickened and hypointense plaques on T1 and T2 in and around tunica albuginea
- T2 is superior (Radiographics 2009;29:477)
Case reports
- 59 year old man with penile ossification (Sao Paulo Med J 2007;125:124)
Treatment
- May need surgical treatment (Adv Urol 2008;2008:263450, Eur Urol 2009;55:1469, Curr Urol Rep 2011;12:444)
- Some nonsurgical options may stabilize disease (Asian J Androl 2008;10:79)
- Can respond to laser therapy and small amounts of irradiation and injected steroids (J Androl 2009;30:397, J Androl 2010;31:445) and interferon
- Prosthesis may be useful (Adv Urol 2008;2008:646052)
- May spontaneously regress (J Androl 2009;30:397)
Gross description
- Irregular areas of fibrosis in tunica albuginea with or without nodules in penile shaft
- Occasionally nodular areas of fibrosis may predominate
Microscopic (histologic) description
- Dense fibrous nodules, similar to those in Dupuytren contracture, fibromatosis and other desmoplastic conditions involving myofibroblasts
- More dense and less cellular than other types of superficial fibromatosis
- Disorganization of collagen of tunica albuginea with formation of nodules, often hyalinizing fibrosis
- Perivascular lymphoid infiltrate in early stages of disease in 1/3
- Fibrotic tunica albuginea with extension of fibrosis to corpus cavernosum
- Abnormal vessels with venous leakages
- Calcification or ossification may occur, linear band of calcification in 1/4 (J Urol 1997;157:282)
Positive stains
- Vimentin, variable actin - muscle specific and smooth muscle actin, less frequently desmin
Electron microscopy description
- Penile plaques are composed of collagen fibrils, amorphous particulate material and fibroblasts (Int J Urol 1997;4:274)
Molecular / cytogenetics description
- No somatic mutations of beta catenin genes unlike desmoid fibromatosis (Mod Pathol 2001;14:695)
Videos
Penile fibromatosis
Sample pathology report
- Penis, plaque, excision:
- Features consistent with Peyronie disease
Differential diagnosis
- Spindle cell sarcomas (i.e. synovial sarcoma, malignant peripheral nerve sheath tumor, etc.)
- Epithelioid hemangioma (J Med Case Rep 2011;5:260)
- Epithelioid sarcoma: may clinically appear similar (Int J Impot Res 2003;15:378)
Sclerosing lipogranuloma
Table of Contents
Definition / general | Epidemiology | Sites | Etiology | Clinical features | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Differential diagnosisDefinition / general
- See also Tancho nodules / paraffinomas
Epidemiology
- Rare; most patients are young adults
Sites
- Usually affects penis, scrotum, spermatic cord and perineum
Etiology
- Usually due to injection or topical application of oil based substances (paraffin, silicone, oil or wax) for cosmetic or therapeutic use (Arch Pathol Lab Med 1977;101:321)
- Foreign body reaction is response to degenerated or damaged fatty tissue or lipids (Med Mol Morphol 2007;40:108)
- May also be due to trauma and cold weather
- Idiopathic cases with peripheral eosinophilia
Clinical features
- Localized painless or slightly tender, indurated plaque / mass
- Up to several centimeters
Gross description
- Firm, yellow to grayish white areas
- Solid or solid and cystic
- Often fragmented
Gross images
Microscopic (histologic) description
- Fat necrosis, histiocytes, giant cells with extensive fibrosis and hyalinization
- Lipid vacuoles with marked variation in size
- Cysts, if present, lack epithelial lining but may contain giant cells
- Also T lymphocyte infiltrate (Pathol Int 2003;53:121)
Microscopic (histologic) images
Positive stains
- Lipid stains: Oil Red O for frozen tissue
Differential diagnosis
- Adenomatoid tumor: epitheliod and spindle cells, cystic spaces lined by flat, cuboidal or low columnar cells, no fat necrosis and no giant cells
- Lymphangioma: cystic spaces lined by endothelium; no fat necrosis, no giant cells
- Sclerosing liposarcoma: irregular adipocytes of variable sizes, presence of lipoblasts and usually no giant cells
Scrotal calcinosis
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Videos | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Nonneoplastic condition characterized by multiple calcific nodular deposits in scrotal skin, caused by dystrophic calcification (G Ital Dermatol Venereol 2015;150:495)
Essential features
- Clinically, multiple subcutaneous painless nodules in scrotum
- Multiple varying sized calcified deposits in the dermis and subcutis
- Rarely shows extensive involvement of the scrotum
Terminology
- Previously described as idiopathic scrotal calcinosis
- First described by Lewinski in 1883 and was established as a distinct entity by Shapiro et al. in 1970 (Ann Dermatol 2018;30:236)
Epidemiology
- Lesions begin in childhood or early adulthood
- Mean age of 31.5 years, 70% in 30s to 40s, range of 15 - 77 years (Dermatol Online J 2010;16:5)
Sites
- Scrotum
- Exceedingly rare vulvar calcinosis may represent the female counterpart of scrotal calcinosis (J Low Genit Tract Dis 2019;23:75)
Etiology
- Pathogenesis of scrotal calcinosis remains unknown and has been the subject of longterm controversy (Urol Case Rep 2020;32:101225)
- Originally termed idiopathic scrotal calcinosis (ISC); however, many authors suggest that at least some cases are not genuinely idiopathic
- Dubey et al. evaluated 100 cases, out of which 53% had associated cysts containing calcific material (Dermatol Online J 2010;16:5)
- Shah et al. found cyst walls in 14 out of 20 cases and suggested that scrotal calcinosis results from calcification of hair follicle or epidermal cysts but as reported in most of the cases, this epithelium disappears and may not be seen (Am J Dermatopathol 2007;29:172)
- The following are various hypotheses for the origin of scrotal calcinosis:
- Truly idiopathic (Heliyon 2022;8:e10762)
- Secondary to calcification of epidermal inclusion cysts, pilar cysts or eccrine gland duct milia (G Ital Dermatol Venereol 2015;150:495, Am J Clin Exp Urol 2022;10:194)
- Result from dystrophic calcification in the degenerated and necrotic dartos smooth muscle of scrotum, which may be due to trauma (Acta Dermatovenerol Alp Pannonica Adriat 2022;31:123)
- Secondary to localized skin infection / inflammation causing a sudden increase in collagen synthesis around blood vessels and fat, with subsequent calcium deposition (Am J Clin Exp Urol 2022;10:194)
- Some suggested that it could be the end stage of a single disease that started from multiple etiologies (Clin Cosmet Investig Dermatol 2018;11:333)
Clinical features
- Typically presents as multiple nodular, bosselated scrotal masses of many years duration before the patients seek medical advice (Am J Clin Exp Urol 2022;10:194)
- Lesions increase in size and number over time (Dermatol Online J 2010;16:5)
- They are pearly / yellowish white nodular masses of different sizes in the scrotum with no obvious cause, rock hard, partly smooth surface and an individually uneven surface (Am J Clin Exp Urol 2022;10:194)
- Number of lesions varies from 1 to 100 nodules, 45% of patients have 11 to 20 lesions (Dermatol Online J 2010;16:5)
- Size ranging from a few millimeters to several centimeters
- Some of the nodules get fused with the surrounding ones to form a large mass (Heliyon 2022;8:e10762)
- Generally asymptomatic or feeling of heavy sensation when extensive; in some rare cases it can cause itching or pelvic and perineal pain or chalky white exudate or secondary infection of the skin (Heliyon 2022;8:e10762, Am J Clin Exp Urol 2022;10:194, Zhonghua Nan Ke Xue 2019;25:544, Int J Surg Case Rep 2018;44:51)
- Main discomfort to the patient is psychological and aesthetic with impairment of quality of life, self esteem and fear of sexual dysfunction
- In most cases, there is no impairment of calcium and phosphorus metabolism (Am J Clin Exp Urol 2022;10:194)
- There is no history of endocrine disorder, trauma or a family member with similar diseases (Zhonghua Nan Ke Xue 2019;25:544)
Diagnosis
- Diagnosis is usually straight forward and is solely based on physical exam (G Ital Dermatol Venereol 2015;150:495, Dermatol Online J 2010;16:5)
Radiology description
- Ultrasound: typically appear as round or oval echogenic foci with or without acoustic shadowing in cases of large and small calculi, respectively (J Ultrasound Med 2007;26:1775)
Prognostic factors
- Nonneoplastic lesions with little chance of recurrence following localized excision
Case reports
- 22 and 23 year old brothers with familial example of scrotal calcinosis (Ann Dermatol 2018;30:236)
- 23 year old man presenting with extensive scrotal calcinosis associated with vitiligo (BMJ Case Rep 2020;13:e238695)
- 24 year old man with scrotal calcinosis treated by surgical excision (Urol Case Rep 2020;32:101225)
- 28 year old man with hypercalcemia caused by isotretinoin taken for the treatment of severe acne presented with scrotal calcinosis (Acta Dermatovenerol Alp Pannonica Adriat 2022;31:123)
- 46 year old man on maintenance hemodialysis and severe calcium and phosphorus metabolism disorder presented with scrotal calcinosis (Urol Case Rep 2020;33:101270)
Treatment
- Surgical treatment remains the mainstay in the management of bothersome scrotal calcinosis (BMJ Case Rep 2020;13:e238695, Urol Case Rep 2020;32:101225, Am J Clin Exp Urol 2022;10:194, Zhonghua Nan Ke Xue 2019;25:544)
- Occasionally scrotal reconstruction may be required in case of extensive involvement of the scrotal skin
- Newer options like CO2 laser therapy have been used with promising results (J Lasers Med Sci 2020;11:500)
Clinical images
Gross description
- Multinodular and skin covered masses with rock hard and pearly white surface (Diagn Cytopathol 2017;45:922, Dermatol Online J 2010;16:5)
- Surface erosion / ulceration may be present
- Cut surface is chalky to cheesy white solid
- Size of the nodules ranges from 1 to 50 mm; 50% of patients have lesions that are 6 to 10 mm (Dermatol Online J 2010;16:5)
Microscopic (histologic) description
- Multiple varying sized, granular, fractured calcium deposits within the dermis and or subcutis (Arch Ital Urol Androl 2020;92:64)
- May be surrounded with fibroblast proliferation chronic inflammation and foreign body giant cell reaction (G Ital Dermatol Venereol 2015;150:495)
- Some cases may have associated remnants of epidermal inclusion cyst (G Ital Dermatol Venereol 2015;150:495, Am J Clin Exp Urol 2022;10:194)
- Overlying epidermis may show atrophy or ulceration
- Decalcification of the sections may be required to properly visualize the deposits on the slides
Microscopic (histologic) images
Cytology description
- Cytological evaluation of scrotal calcinosis is usually not done due to its typical clinical appearance
- Aspirate is difficult to obtain as it is thick and shows sticky, chalky white material
- Smears reveal calcified, basophilic, amorphous, granular material along with refractile crystals
- These features are nonspecific and raise a differential of calcified epidermal inclusion cysts, dystrophic calcification following any injury or inflammation and scrotal calcinosis (Diagn Cytopathol 2017;45:922)
Cytology images
Positive stains
- Immunohistochemistry is not done in most cases since it is a straightforward histologic diagnosis
- CK AE1 / AE3 may be utilized to highlight the presence of any component of epidermal inclusion cyst, seen in some cases (Dermatol Online J 2010;16:5)
Videos
Scrotal calcinosis: pathology mini tutorial
Sample pathology report
- Scrotal mass, excision biopsy:
- Scrotal calcinosis (see comment)
- Comment: Scrotal calcinosis is a nonneoplastic condition. Localized excision is considered adequate treatment.
Differential diagnosis
- Multiple epidermal inclusion cysts or pilar cysts in scrotum:
- Some cases of scrotal calcinosis may have remnants of an epithelial cyst
- True cysts are lined by stratified squamous epithelium
- Lumen showing keratinous material
- Idiopathic cutaneous calcinosis:
- Usually in the extremities
- Isolated scrotal involvement is rare
- Dystrophic calcification due to Onchocerca volvulus:
- Presence of parasite in tissue sections
Board review style question #1
Which of the following is true regarding scrotal calcinosis?
- Idiopathic in most cases
- More common in elderly
- Only affects Caucasians
- Related to hypercalcemia
- Usually a solitary lesion
Board review style answer #1
Board review style question #2
Board review style answer #2
Squamous cell carcinoma-general
Table of Contents
Definition / general | Terminology | Epidemiology | Risk factors | HPV related squamous cell carcinoma | Sites | Etiology | Diagrams / tables | Clinical features and outcome | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Differential diagnosisDefinition / general
- Malignant epithelial tumor composed of squamous cells; diagnosis often delayed (eMedicine: Urogenital Squamous Cell Carcinoma [Accessed 4 April 2018])
- Squamous cell carcinoma, NOS: not otherwise specified (i.e., usual histologic pattern); represents 48 - 65% of all penile squamous cell carcinomas (Anal Quant Cytol Histol 2007;29:185)
- Pseudoglandular growth: unusual and aggressive variant of penile carcinoma with pseudoglands, often filled with amorphous material
Terminology
- Squamous cell carcinoma, NOS is also called usual, typical, conventional, classical or epidermoid squamous cell carcinoma
- Pseudoglandular growth: also called adenoid or acantholytic carcinoma
Epidemiology
- Most penile neoplasms are squamous cell carcinoma
- Rare in U.S.; < 1% of carcinomas in men versus 10 - 20% in Asia (excluding Japan), Africa and South America (Cancer 2008;113:2883)
- Usually age 40 - 70 years, median age 58 years
- Incidence is 0.29 per 100,000 in U.S. whites versus 4.2 per 100,000 in Paraguay versus 4.4 per 100,000 in Uganda
- Rare if circumcision at birth, more common if late circumcision (after age 10)
- More prevalent in populations with lower education and higher poverty (Cancer 2008;113:2910)
- More common in Hispanic and black men
- Familial cases have occasionally been reported
- Patients from high risk areas tend to be younger and present with a higher stage disease
- Rarely associated with genital piercing (J Sex Med 2010;7:2280)
- In squamous cell carcinoma, NOS, mean age is 58 years
- In pseudoglandular growth, average age is 54 years
Risk factors
- Phimosis and long foreskin, paraphimosis (Am J Surg Pathol 2003;27:994)
- Genital warts (6 times increased risk) (J Natl Cancer Inst 1993;85:19)
- Also HPV infection in general, particularly HPV 16 and related risk factors (Cancer Epidemiol Biomarkers Prev 2008;17:2683)
- ~33% of non-HPV cases are associated with lichen sclerosus (balanitis xerotica obliterans) (Am J Surg Pathol 2003;27:1448)
- Penile injury, tears and chronic balanitis
- Smoking, psoriasis patients treated with UVB radiation, penile rash > 1 month, immunosuppression and radiation therapy
Sites
- Most tumors arise from glans or inner foreskin near coronal sulcus as a slow growing, irregular mass
- In high incidence areas, tumors involve multiple anatomical compartments in up to 50% of cases
- In squamous cell carcinoma, NOS, glans is the preferred site but extension to coronal sulcus and inner foreskin is common
- In pseudoglandular growth, glans, coronal sulcus and inner foreskin are usually involved
Etiology
- In squamous cell carcinoma, NOS, 25% of cases are HPV+ (Am J Surg Pathol 2010;34:104)
Diagrams / tables
Clinical features and outcome
- Patients occasionally present with inguinal nodal metastases with occult penile cancer due to severe phimosis or very small primary tumor
- Local recurrence in 33% is due to insufficient surgery or positive margins, which also increases risk of regional inguinal and pelvic nodal metastases
- 10 year survival rate of 82% (J Urol 2009;182:528)
- Histologic subtypes have similar frequency in Paraguay and U.S. (Int J Surg Pathol 2010;18:268)
- Metastases:
- 5% have metastases at diagnosis
- Common sites are inguinal and pelvic lymph nodes, liver, lung, heart or bone (Int J Surg Pathol 2011;19:164)
- Nodes are often enlarged at clinical presentation due to infection, not metastases
- 5 year survival is related to nodal involvement: 66% (not involved) versus 27% (involved)
- Low grade:
- Usually no regional metastases if only superficial invasion of ≤ 6 mm (Mod Pathol 2001;14:963)
- High grade:
- Deep invasion (8 - 10 mm) into corpus spongiosum, dartos or corpora cavernosa is associated with 80% rate of metastases
- Intermediate / high grade with invasion of 5 - 10 mm have 15% risk of metastases
- Squamous cell carcinoma, NOS:
- Inguinal nodal metastases in 28 - 39% and recurrences in 28% of all cases
- Intermediate mortality rate (20 - 38%)
- Pseudoglandular growth has a higher metastatic rate and cancer related deaths when compared with usual SCC
Prognostic factors
- Poor prognostic factors:
- High stage
- High histologic grade (Am J Surg Pathol 2008;32:974, J Surg Oncol 2008;97:487)
- Deeper invasion (anatomic levels are epithelium, lamina propria, corpus spongiosum and corpus cavernosum) but anatomic variations exist (corpus cavernosum may not be located in glans in 25% of cases) (Am J Surg Pathol 2001;25:1091)
- Angiolymphatic invasion (J Urol 2008;180:1354)
- Perineural invasion (World J Urol 2009;27:169)
- Anaplastic, basaloid, pseudoglandular, sarcomatoid or solid subtypes
- Lymph node density in one study (J Urol 2009;182:2721)
- Prognostic index score:
- Combines histologic grade, anatomical level of tumor infiltration and perineural invasion to predict the likelihood of inguinal nodal involvement (Am J Surg Pathol 2009;33:1049)
- Useful for risk group stratification and clinical management
- Appropriate for surgical specimens, not for biopsies
- Score is sum of points for histologic grade (grade 1: 1, grade 2: 2, grade 3: 3), anatomical level of maximum tumor infiltration (lamina propria: 1, corpus spongiosum / dartos: 2, corpus cavernosum / preputial skin: 3) and perineural invasion (absent: 0, present: 1)
- Low risk: score of 2 - 3, intermediate risk: score of 4, high risk: score of 5 - 7
Case reports
- 66 year old man with metastatic disease in pleural effusion (Arch Pathol Lab Med 1992;116:198)
- Series of 7 cases where patients presented with predominant pseudoglandular or adenoid features (Am J Surg Pathol 2009;33:551)
Treatment
- Local resection, partial / total penectomy (NIH: Skin Cancer Treatment [Accessed 4 April 2018])
- Local excision and partial penectomy are inadequate for sarcomatoid and basaloid carcinomas (Am J Surg Pathol 2009;33:1299); poor outcomes with metastatic disease (Ann Surg Oncol 2007;14:3614)
- Higher risk for recurrence if node positive or partial penectomy (Eur Urol 2008;54:161)
- Possibly brachytherapy for tumors confined to glans (Int J Radiat Oncol Biol Phys 2009;74:1150)
- Criteria for inguinal lymphadenopathy are controversial, as palpable nodes may be reactive (Can Urol Assoc J 2008;2:525)
- Patients with prognostic index scores of 2 - 3 may not need inguinal nodal dissection
- Patients with prognostic index scores of 5 - 7 may benefit from prophylactic groin dissection
Clinical images
Gross description
- Grossly noted growth patterns may have prognostic implications (Am J Surg Pathol 1993;17:753, World J Urol 2009;27:169)
- Superficial spreading: tumors are limited to lamina propria or superficial corpus spongiosum and usually extend horizontally through multiple anatomical compartments
- Vertical growth: tumors invade deep anatomical levels, surface is nonverruciform and frequently ulcerated
- Verruciform: tumors are exophytic and papillomatous with a cauliflower-like aspect, may be limited to surface (verrucous) or invade deep anatomical levels (cuniculatum)
- Mixed patterns: observed in 10 - 15% of all cases
- In some cases, multicentric tumors (2 or more independent foci of carcinomas) are identified
- Squamous cell carcinoma, NOS:
- Predominant growth patterns are vertical and superficial spreading
- Gross aspect is nondistinctive and variable
- Mean tumor size varies from 2 cm in low incidence areas to 4 - 5 cm in high incidence areas
- Cut surface shows tan-white solid irregular tumor with superficial or deep penetration
- Pseudoglandular growth:
- Large, irregular white-gray ulcerative or exophytic masses
- Frequent invasion of deep erectile tissues
- Average size is 4.6 cm
Gross images
AFIP images
Glans:
Foreskin:
Coronal sulcus:
Multiple compartments:
Superficial spreading (SCC):
Assessment of depth of invasion:
Images hosted on other servers:
Microscopic (histologic) description
- Most histologic subtypes resemble those in vulva, anus or buccal mucosa
- 48 - 65% are usual squamous cell carcinoma
- Verruciform tumors are verrucous, warty, papillary or cuniculatum carcinomas
- Basaloid and sarcomatoid carcinomas usually have a vertical growth pattern
- Often undifferentiated (bowenoid) penile intraepithelial neoplasia and lichen sclerosis (J Am Acad Dermatol 2010;62:284)
- Features to report: depth of invasion measured from deepest malignant cells to highest overlying dermal papilla; resection margins
- Grading:
- Grade 1: well differentiated cells, almost undistinguishable from normal squamous cells except for the presence of minimal basal / parabasal cell atypia
- Grade 2: all tumors not fitting into criteria for grade 1 or 3 (Am J Surg Pathol 2009;33:1042)
- Grade 3: any anaplastic cells
- Squamous cell carcinoma, NOS:
- Usually keratinized with moderate differentiation
- Up to 50% of cases are heterogeneous (> 1 histological grade)
- Most cases have differentiated penile intraepithelial neoplasia and squamous hyperplasia
- Tumors composed exclusively of extremely well differentiated or poorly differentiated areas are uncommon
- In some cases, clear glycogenated cells may predominate (but must differentiate from koilocytes)
- Stroma has variable lymphoplasmacytic infiltrate
- Foreign body type giant cells are often seen in highly keratinized tumors
- Pseudoglandular growth:
- Hallmark is the pseudogland: open space lined by atypical, cubical or cylindrical uni or multistratified cells
- Pseudoglands frequently filled with amorphous material containing debris, keratin and desquamated cells
- Intraluminal neutrophilic microabscesses are not uncommon
- Frequent presence of intracytoplasmic clear vacuoles, sometimes with a collaret arrangement
- Variegated tumor aspect showing solid areas intermingled with pseudoglandular spaces
- Frequent vascular and perineural invasion
Microscopic (histologic) images
Contributed by Antonio L. Cubilla, M.D., Alcides Chaux, M.D. and AFIP images
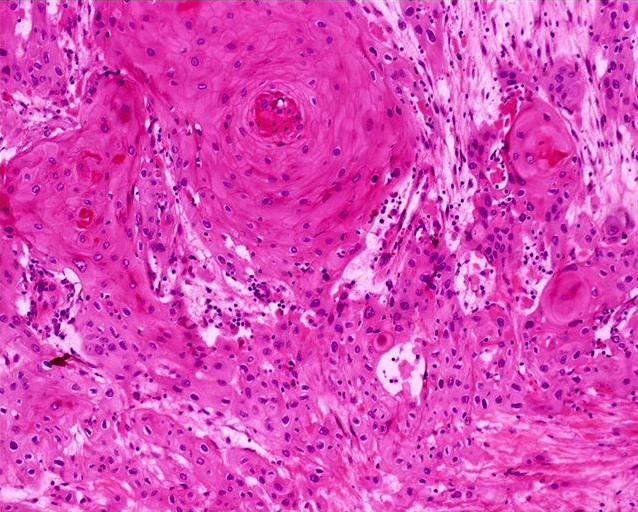
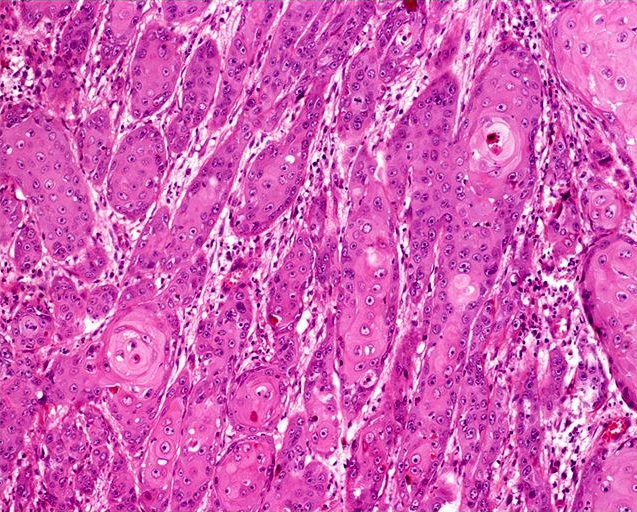
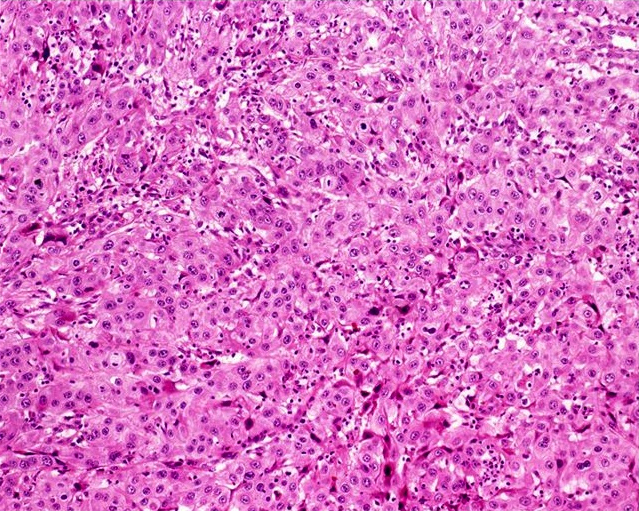
Usual type: well differentiated (left, grade 1); moderately differentiated (middle, grade 2); poorly differentiated (right, grade 3)
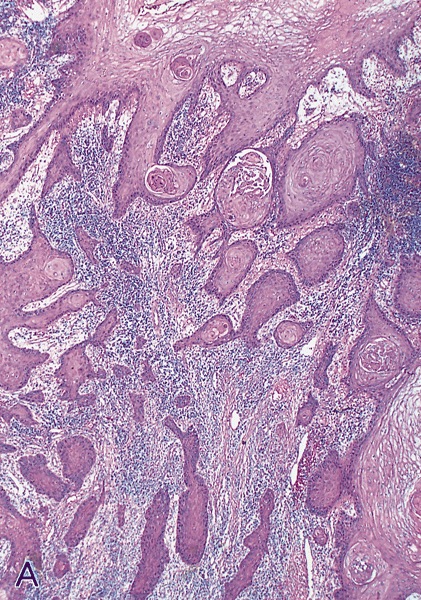
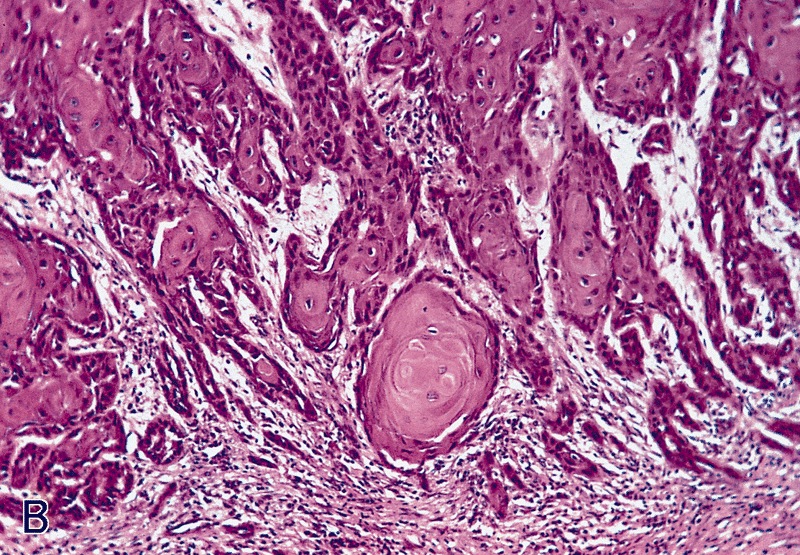
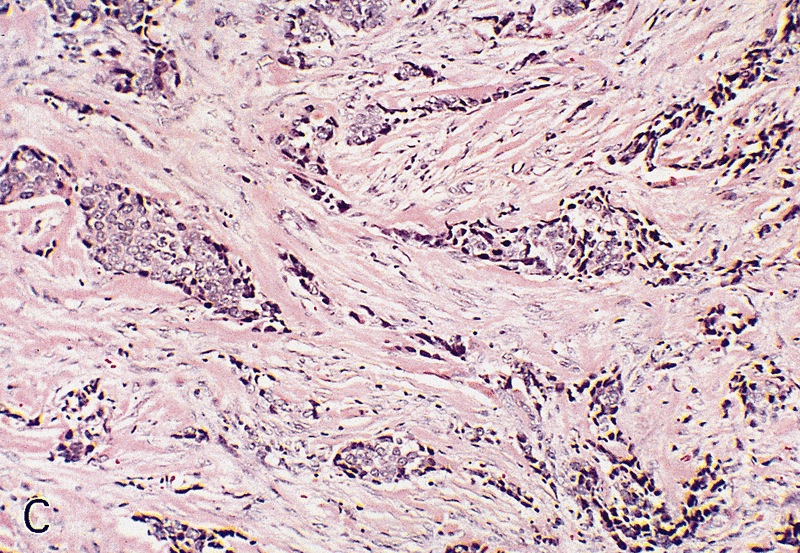
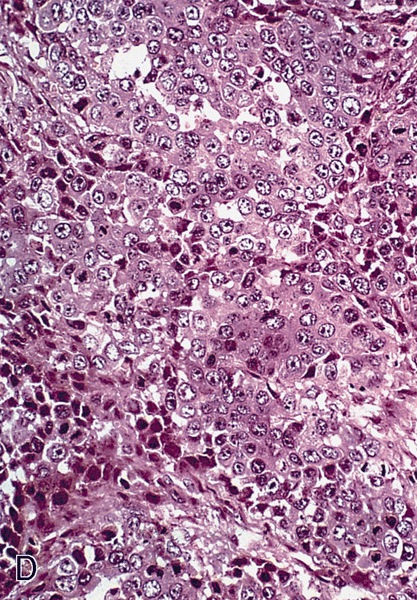
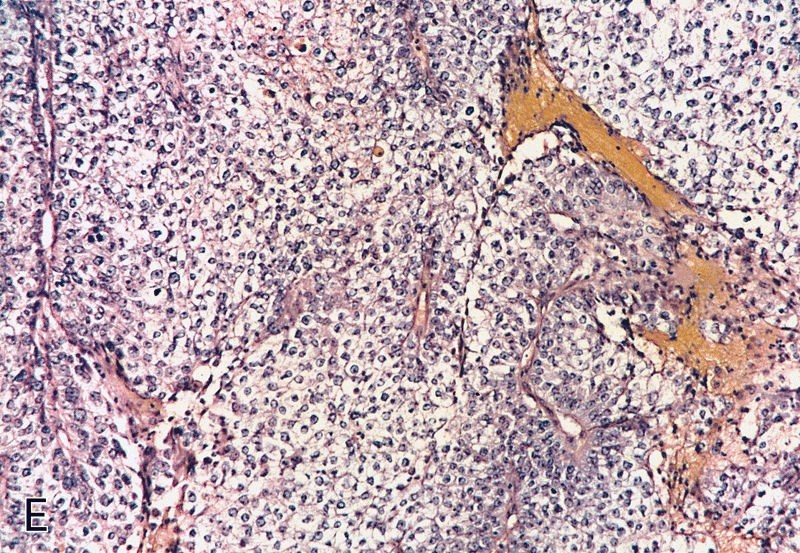
Low grade keratinizing (A, B); moderate to high grade nonkeratinizing (C); high grade nonkeratinizing tumor (D); and carcinoma with prominent glycogenated clear cells (E)
Images hosted on other servers:
Pseudoglandular growth:
Positive stains
Negative stains
- Negative staining in pseudoglandular variant for factor VIII, PAS and Alcian blue
Molecular / cytogenetics description
- Mutations in PIK3CA, HRAS or KRAS genes in 39% (J Urol 2008;179:2030)
- Epigenetic silencing (by methylation) of FHIT gene in 92% (Virchows Arch 2008;452:377)
Differential diagnosis
- Squamous cell carcinoma, NOS:
- Basaloid carcinoma:
- Basophilic cytoplasm, indistinctive cellular borders and mostly HPV+
- Clear cell carcinoma:
- Pseudoepitheliomatous hyperplasia:
- Elongated rete ridges, no nuclear atypia, regular epithelial nests with evident peripheral palisading and no stromal reaction
- Urothelial carcinoma:
- Ventral surface of penis, absence of squamous metaplasia, microglandular hyperplasia, lichen sclerosus or penile intraepithelial neoplasia, presence of urothelial carcinoma in situ or history of urothelial CIS or bladder tumor
- Basaloid carcinoma:
- Pseudoglandular growth:
- Adenocarcinoma of Littré glands:
- True glandular differentiation, ventrally located with secondary extension to perimeatal area
- Adenosquamous carcinoma:
- Pseudoangiosarcomatoid variant of sarcomatoid carcinoma:
- Urothelial carcinoma of distal urethra with glandular features:
- Previous / concurrent history of urothelial carcinoma elsewhere and presence of urothelial carcinoma in situ
- Positive for urothelial markers (uroplakin III, thrombomodulin)
- Adenocarcinoma of Littré glands:
Squamous hyperplasia
Table of Contents
Definition / general | Sites | Clinical features | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosisDefinition / general
- Benign thickening of squamous epithelium (more than 15 cell layers) without atypia
Sites
- May affect any penile anatomical compartment
Clinical features
- Most common epithelial change associated with keratinizing penile carcinoma
- Usually found adjacent to neoplastic changes (in situ or invasive carcinoma)
- Uncertain if reactive or precancerous (Anal Quant Cytol Histol 2007;29:185)
- Benign but associated with squamous cell carcinoma, particularly verrucous and low grade papillary subtypes (Int J Surg Pathol 2004;12:351)
Gross description
- Flat, smooth and slightly raised pearly white areas
Microscopic (histologic) description
- Hyperkeratosis, acanthosis and hypergranulosis but normal maturation of squamous epithelium
- Minimal to no parakeratosis
- No cytological atypia, no koilocytosis
- May be adjacent to carcinoma or merge with adjacent low grade carcinoma
Morphological patterns:
- Flat: most common type, linear interface between epithelium and lamina propria
- Papillary: serrated appearance at low power view, jagged interface with stroma
- Pseudoepitheliomatous: downward florid but superficial proliferation of regular squamous cell nests with peripheral palisading, often appearing detached but with no keratinization, no stromal reaction, no desmoplasia and no extension beyond lamina propria
- Verrucous: marked acanthosis with hyperkeratosis, slight papillomatosis and linear interface with stroma
Microscopic (histologic) images
Differential diagnosis
- Penile intraepithelial neoplasia, differentiated type:
- Cytological atypia, more frequent parakeratosis
- Pseudohyperplastic carcinoma:
- Irregular nests, no peripheral palisading, evident stromal reaction and extension beyond lamina propria
- Squamous cell carcinoma with pseudohyperplastic features
- Verruciform carcinomas:
- Cytological atypia, evidence of stromal invasion
- Verruciform xanthoma:
- Lipid laden histiocytes (foamy cells) in lamina propria
Staging-penis
Table of Contents
Definition / general | Essential features | Primary tumor (pT) | Regional lymph nodes (pN) | Distant metastasis (pM) | AJCC prognostic stage groups | Registry data collection variables | Histologic grade (G) | Histopathologic type | Gross images | Microscopic (histologic) images | Board review style question #1 | Board review style answer #1Definition / general
- All carcinomas of the penis are covered by this staging system
- These topics are not covered: penile urethral carcinoma, sarcoma and melanoma
Essential features
- AJCC 7th edition staging was sunset on December 31, 2017; as of January 1, 2018, use of the 8th edition is mandatory
Primary tumor (pT)
Regional lymph nodes (pN)
- pNX: cannot be assessed
- pN0: no regional lymph node metastasis
- pN1: metastasis in 1 or 2 unilateral inguinal node(s)
- pN2: metastasis in 3 or more unilateral inguinal nodes or bilateral inguinal nodes
- pN3: extranodal extension or metastasis to pelvic lymph node
Notes:
- Regional lymph nodes include superficial / deep inguinal and pelvic (external iliac, internal iliac / hypogastric, obturator, pelvic NOS)
Distant metastasis (pM)
- pM1: distant metastasis
Notes:
- Distant metastasis includes common iliac and aortic / caval lymph nodes
AJCC prognostic stage groups
| Stage group 0is: | Tis | N0 | M0 |
| Stage group 0a: | Ta | N0 | M0 |
| Stage group I: | T1a | N0 | M0 |
| Stage group IIA: | T1b - 2 | N0 | M0 |
| Stage group IIB: | T3 | N0 | M0 |
| Stage group IIIA: | T1 - 3 | N1 | M0 |
| Stage group IIIB: | T1 - 3 | N2 | M0 |
| Stage group IV: | T4 | N1 - 3, X | M0 |
| T1 - 4, X | N3 | M0 | |
| T1 - 4, X | N1 - 3, X | M1 |
Registry data collection variables
- Histologic subtype
- Size of largest node metastasis
- Total number of nodes removed
- Presence of high risk HPV
- p16 immunohistochemical expression
- Urethral mucosal invasion
Histologic grade (G)
- GX: cannot be assessed
- G1: well differentiated
- G2: moderately differentiated
- G3: poorly differentiated
Histopathologic type
- Non-HPV related squamous cell carcinoma
- Usual type
- Pseudoglandular
- Verrucous
- Cuniculatum
- Papillary, NOS
- Adenosquamous
- Sarcomatoid
- Mixed
- HPV related squamous cell carcinoma
- Basaloid
- Papillary basaloid
- Warty
- Warty basaloid
- Clear cell
- Rare other types of squamous cell carcinoma
Gross images
Microscopic (histologic) images
Board review style question #1
Board review style answer #1
D. pT3. Corpus cavernosum invasion is classified as pT3.
Comment Here
Reference: Pathologic TNM staging of penis carcinoma (AJCC 8th edition)
Comment Here
Reference: Pathologic TNM staging of penis carcinoma (AJCC 8th edition)
Syphilis
Table of Contents
Definition / general | Terminology | Epidemiology | Etiology | Clinical features | Laboratory | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Electron microscopy images | Differential diagnosisDefinition / general
- Caused by Treponema pallidum; humans are the only natural host (Wikipedia: Syphilis [Accessed 30 March 2018])
- Sexually transmitted disease with primary, secondary and tertiary phases (not very infectious in tertiary stage) (eMedicine: Syphilis [Accessed 30 March 2018])
Terminology
- Historically called lues
Epidemiology
- U.S. incidence dropped through 2000 but has risen since with more cases in men having sex with men
Etiology
- Caused by Treponema pallidum, subspecies pallidum, a microaerophilic spirochete that is pathogenic only to humans
Clinical features
- Called "the great imitator" because it may mimic other disorders if presentation is unusual
- 30% transmission rate from sexual intercourse
Primary syphilis:
- Painless hard chancre (ulcer with indurated and punched out base) at site of inoculation, often in glans
- Most common affected sites are inner foreskin, coronal sulcus, penile shaft and penile base
- Chancres are usually solitary
Secondary syphilis:
- Bacteremic stage with greatest number of organisms in the body
- Classically has widespread rash (small red macular lesions), including on palms and soles and mucous membranes
- Condyloma lata, formed by soft, flat topped, moist, red / rose / gray to pale maculopapules, nodules or plaques, is the characteristic anogenital lesion and may become confluent; are common in scrotum
- May have varied clinical presentation
Tertiary syphilis:
- Gummatous form is characteristic: granulomas with epithelioid and giant cells, obliterative endarteritis and necrosis
- Also cardiovascular form and neurosyphilis
- Accelerated time course occurs with HIV infection (1 year to neurosyphilis)
Laboratory
- Screening tests: rapid plasma reagin (RPR, Wikipedia: Rapid Plasma Reagin [Accessed 30 March 2018]) and VDRL (Wikipedia: Venereal Disease Research Laboratory Test [Accessed 30 March 2018])
- Confirmatory tests: fluorescent treponemal antibody absorption (FTA-ABS) or Treponema pallidum hemagglutination assay
- Note: patients receiving IV immunoglobulin may passively acquire treponemal antibodies (Arch Pathol Lab Med 2002;126:1237)
Case reports
- 22 year old man with nodular and annular skin lesions over face, back and limbs (Dermatol Online J 2003;9:9)
- 26 year old homosexual man with secondary syphilis localized to penis / scrotum (G Ital Dermatol Venereol 2009;144:725)
Treatment
- Penicillin G
Clinical images
Microscopic (histologic) description
- Presence of obliterative endarteritis surrounded by a predominantly plasmocytic infiltrate is characteristic of all stages
- Spirochetes can be identified in primary and secondary lesions but are difficult to demonstrate in gummas
- Primary syphilis: ulceration, granulation tissue and obliterative endarteritis at ulcer base; plasma cells and lymphocytes underlying ulcer, endothelial cell proliferation and capillaritis
- Secondary syphilis: psoriasiform epidermal hyperplasia or spongiform pustular lesions with superficial or deep obliterative endarteritis and lymphoplasmacytic infiltrate at the dermal epidermal junction; perivascular infiltrate and possible granulomas; also nodal involvement with florid follicular hyperplasia, unusually shaped follicles, endothelial swelling and perivascular cuffs of plasma cells and lymphocytes
- Tertiary syphilis: gummas formed by granulomas with epithelioid and multinucleated giant cells, obliterative endarteritis and necrotic foci
Microscopic (histologic) images
Positive stains
- Spirochete identified by darkfield microscopy, Warthin-Starry stain or Steiner stain
- Treponema pallidum immunostain is more sensitive than Warthin-Starry stain (Hum Pathol 2009;40:624)
Differential diagnosis
- Lymphoma: monoclonal lymphoplasmacytic infiltrate; no clinical or laboratory evidence of syphilis
- Plasma cell myeloma: monoclonal plasma cells, often binucleated; monoclonal gammopathy; no clinical or laboratory evidence of syphilis
Tancho nodules / paraffinomas / lipogranulomas
Table of Contents
Definition / general | Clinical features | Case reports | Treatment | Clinical images | Microscopic (histologic) descriptionDefinition / general
-
Tancho nodules:
- Custom among some Asian populations to implant, inject or insert paraffin or other foreign material (such as glass spheres) under the skin of the penis to improve sexual pleasure
- Tancho is a brand of hair pomade widely used in the Far East and Southeast Asia
- Custom among some Asian populations to enlarge penis by injecting mineral oil, paraffin (paraffinoma), silicone or vaseline (Urology 2008;71:1132)
- Inflammatory / foreign body reaction may occur years after the injection
Lipogranuloma:
Clinical features
- Associated with genital tattoo
- May cause fistulas or ulcers (J Eur Acad Dermatol Venereol 2008;22:845)
- May be important to recognize for forensic cases (Med Sci Law 2006;46:349)
Tancho nodules:
- Palpable subcutaneous firm nodules
Lipogranuloma:
- Distortion of organ with evident gross abnormalities
- May extend to adjacent structures such as scrotum
Case reports
- 71 year old man with paraffinoma almost 40 years after injections (Int Urol Nephrol 2007;39:553)
Treatment
- May require local surgical resection
Microscopic (histologic) description
Tancho nodules:
Lipogranuloma:
- Inflammatory foreign body reaction with variable fibrosis surrounding inserted material
Lipogranuloma:
- Numerous vacuoles ranging from tiny to large and cystically dilated (pseudocysts)
- Stroma may be sclerotic (sclerosing lipogranuloma) and contain inflammatory cells and foreign body giant cells
Urethral carcinoma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Primary urethral carcinoma is a rare but aggressive genitourinary malignancy that leads to urethral obstruction (Res Rep Urol 2021;13:325)
Essential features
- Primary urethral carcinoma is rare with diverse tumor histology
- Nonspecific clinical findings and lack of screening techniques often lead to late detection and poor prognosis
Terminology
- Urothelial carcinoma
- Squamous cell carcinoma
- Adenocarcinoma of the urethra
ICD coding
- ICD-O: 8140/3 - carcinoma of Littré glands
- ICD-10: C68.0 - malignant neoplasm of urethra
- ICD-11
- 2C93 - malignant neoplasms of urethra or paraurethral gland
- 2C93.0 & XH22Z8 - adenocarcinoma of urethra or paraurethral gland & carcinoma of Skene, Cowper and Littré glands
Epidemiology
- From 2004 to 2016, 436 - 622 new cases of primary urethral carcinoma were diagnosed each year (Cancer Causes Control 2021;32:627)
- Men (68 - 71%) > women (29 - 32%) (Hum Pathol 2018;72:35, World J Urol 2016;34:97, Cancer Causes Control 2021;32:627)
- Median age of diagnosis among men and women is 61 - 71 years old (Hum Pathol 2018;72:35, World J Urol 2016;34:97, Clin Genitourin Cancer 2018;16:e1003, Int J Surg Pathol 2022;30:15, J Surg Oncol 2022;125:907)
- 46% of all patients (male and female) were age 75 or older, 42% were 55 - 74 and 12% were 54 or younger (Cancer Causes Control 2021;32:627)
- Incidence rate highest in Black populations (3.33), followed by White populations (1.72), other race groups (1.57) and Hispanic populations (1.57) (Cancer Causes Control 2021;32:627)
- Race reported as 71% for White population, 16% Black population, 7% Hispanic population and 5% other (Cancer Causes Control 2021;32:627)
- Higher proportion in Black women (12% male, 26% female) (Cancer Causes Control 2021;32:627)
- Overall, urothelial carcinoma is the most common histological subtype (53%), followed by squamous cell carcinoma (24%), adenocarcinoma (15%) and other (9%) (Cancer Causes Control 2021;32:627)
- Men: urothelial (39 - 64%), squamous cell carcinoma (24 - 54%), adenocarcinoma (9%) and other (4%) (Actas Urol Esp (Engl Ed) 2022;46:70, Cancer Causes Control 2021;32:627)
- Women: urothelial (29 - 45%), squamous cell carcinoma (19 - 25%), adenocarcinoma (27 - 40%) and other (7 - 19%) (Cancer Causes Control 2021;32:627, World J Urol 2013;31:147, J Surg Oncol 2022;125:907)
- One study proposed classification of a new hybrid tumor with both urothelial and squamous features, a basaloid appearance and expression and frequent identification of p16 or HPV18 as the overwhelming histology (82%) in the urethra for both men and women (Hum Pathol 2018;72:35)
Sites
- Men (among all histologic subtypes) (World J Urol 2016;34:97)
- Proximal (prostatic + membranous) urethra only: 54%
- Distal (penile) urethra only: 36%
- Proximal + distal urethra: 10%
- Women (among all histologic subtypes) (Clin Genitourin Cancer 2018;16:e1003, World J Urol 2016;34:97)
- Proximal urethra only: 29 - 44%
- Distal urethra only: 21 - 69%
- Proximal + distal urethra: 2 - 33%
Etiology
- Chronic inflammation and irritation of the urinary tract are risk factors for the development of primary urethral carcinoma
- Men: history of sexually transmitted infections (HPV16), urethral stricture, intermittent catheterization and history of radiation therapy (Hum Pathol 2022;129:71)
- Women: recurrent urinary tract infections and urethral diverticula (Clin Genitourin Cancer 2018;16:e1003)
- 77% of patients with urothelial carcinoma of the urethra had a prior history of urothelial carcinoma of the bladder (Int J Surg Pathol 2022;30:15)
Clinical features
- Common presenting features include mass lesion (75 - 79%), urethral narrowing (20%) obstructive urinary tract symptoms (82%) and hematuria (67%) (Hum Pathol 2018;72:35, Clin Genitourin Cancer 2018;16:e1003)
- Among men with bulbar and penile urethral carcinoma, 46% presented with urethral abscess (Actas Urol Esp (Engl Ed) 2022;46:70)
- Inguinal lymph nodes are the most common regional metastatic site (Hum Pathol 2018;72:35)
- Liver and lung are the most frequent distant metastatic sites (Hum Pathol 2018;72:35)
Diagnosis
- Physical examination for suspicious masses or indurations through palpation of external genitalia (Eur Urol 2013;64:823)
- Bilateral inguinal lymph node palpation to assess for the presence of enlarged lymph nodes (Eur Urol 2013;64:823)
- Role of urinary cytology limited due to varying sensitivity (Eur Urol 2013;64:823)
- Urethrocytoscopy with biopsy is diagnostic (Eur Urol 2013;64:823)
Radiology description
- Magnetic resonance imaging (MRI) visualized all tumors in one study with appearance as intermediate signal intensity on T2 imaging (Hum Pathol 2018;72:35)
Radiology images
Prognostic factors
- Staging is based on TNM, refer to links for the current system for women and men
- Recurrence rate of 53% for urethral carcinoma (World J Urol 2016;34:97)
- Pathologically advanced (pT3 or greater), pathologically or clinically node positive disease and proximal tumor location associated with recurrence (World J Urol 2016;34:97)
- Most common sites of recurrence were lymph nodes (12%) and urethra (18%) (World J Urol 2016;34:97)
- Clinical nodal assessment 93% accurate in predicting pathological lymph node involvement (World J Urol 2016;34:97)
- Median survival of 21 months (mean of 39 months) and 10 year survival of 25% for surgical patients (Hum Pathol 2018;72:35)
- Among patients without metastatic disease that underwent radical urethrectomy, 5 year cancer specific mortality free survival was 62% (87% for those with 36 month disease free interval) (J Surg Oncol 2024;129:1348)
- 5 year survival for T3 - 4 N0 - 2 patients was 54% (84% for those with 36 month disease free interval)
- Urothelial carcinoma
- 1 year overall survival rate of 73%; 3 year overall survival rate of 33% (Int J Surg Pathol 2022;30:15)
- Previous history of urothelial carcinoma of the bladder confers no effect on overall survival (Int J Surg Pathol 2022;30:15)
- Men
- Bulbar or penile urethral carcinoma (all histologic subtypes) yielded a mean 5 year overall survival of 50%, cancer specific survival 66% and relapse free survival of 58%; 62% with recurrence after surgery after a median of 6.3 months (Actas Urol Esp (Engl Ed) 2022;46:70)
- Proximal squamous cell carcinoma confers poor survival outcomes with a mean survival of 14 months and 90% develop distant metastatic disease (Eur Urol Focus 2021;7:163)
- Women
- Median overall survival (all histologic subtypes): 70 months (J Surg Oncol 2022;125:907)
- 5 year survival: 67% for stage 0 - II, 53% for stage III and 17% for stage IV (World J Urol 2013;31:147)
- Node positive and tumor size of ≥ 3 cm confer worse overall survival (J Surg Oncol 2022;125:907)
- 5 year survival: adenocarcinoma (31%), urothelial carcinoma (61%) and squamous cell carcinoma (64%) (World J Urol 2013;31:147)
- Advanced disease present: adenocarcinoma (65%), urothelial carcinoma (32%) and squamous cell carcinoma (53%) (World J Urol 2013;31:147)
Case reports
- 54 year old man with low grade papillary tumor of the anterior urethra (Ann Med Surg (Lond) 2022;76:103561)
- 67 year old woman with primary clear cell adenocarcinoma of the urethra (BMC Womens Health 2022;22:251)
- 68 year old man with urethral bulbar carcinoma with mixed urothelial and squamous differentiation (Mol Clin Oncol 2022;17:142)
- 76 year old woman with urothelial carcinoma with squamous metaplasia (Int J Surg Case Rep 2023;109:108505)
Treatment
- Among all patients with primary urethral carcinoma, rates of treatment with adjuvant radiotherapy and chemotherapy have increased over time (World J Urol 2013;31:147)
- Adjuvant radiotherapy: 28% of patients from 1989 to 1998; 43% from 1999 to 2008
- Adjuvant chemotherapy: 4% from 1989 to 1998; 12% from 1999 to 2008
- Men
- Locally advanced disease or metastasis: neoadjuvant therapy often recommended before surgical resection (Eur Urol Focus 2021;7:163)
- Distal (penile and bulbar carcinoma): partial penectomy (8 - 75%), total penectomy (17 - 54%) and transurethral resection (8 - 38%) (Int J Urol 2006;13:716, Actas Urol Esp (Engl Ed) 2022;46:70)
- Proximal: total penectomy (41%) and radical cystectomy / total penectomy with ileal conduit (59%) (Int J Urol 2006;13:716)
- Proximal squamous cell carcinoma: panurethrectomy (80%) and radical prostatectomy (20%) (Eur Urol Focus 2021;7:163)
- Women
- Among all histologic subtypes: 43% surgery alone, 16% radiotherapy alone and 22% surgery and radiotherapy (World J Urol 2013;31:147)
- T1 or Tis: 30% were treated with cystourethrectomy, 40% underwent local excision and 30% received chemotherapy and radiation (Clin Genitourin Cancer 2018;16:e1003)
- Locally advanced, nonmetastatic disease: 52% received multimodal therapy (radical cystourethrectomy, lymph node dissection and chemotherapy or radiation) and 48% received nonmultimodal therapy (radical cystourethrectomy alone, local excision with or without additional therapy or chemotherapy with or without radiation therapy), with no difference in survival (Clin Genitourin Cancer 2018;16:e1003)
Clinical images
Gross description
- Poorly circumscribed mass with white to yellow-tan appearance (Hum Pathol 2018;72:35)
- Mean size: 3.7 cm (range: 0.5 - 6 cm) (Hum Pathol 2018;72:35)
- Range of appearances
- Fungated mass with protrusion to urethral lumen
- Flat lesions with invasion of surrounding tissue (Hum Pathol 2018;72:35)
Gross images
Microscopic (histologic) description
- Urothelial carcinoma
- Cytologically malignant urothelial cells with visible cell membranes, marked nucleomegaly, irregular nuclei, prominent nucleoli, dark chromatin and identifiable mitoses
- Squamous cell carcinoma
- Sheets of large, pleomorphic tumor cells with focal or abundant keratinization (depending on the degree of differentiation), ample cytoplasm, intercellular bridges, prominent nuclear atypia and high mitotic activity
- Adenocarcinoma
- Composed of simple or pseudostratified columnar epithelium, apical cytoplasm and basally located hyperchromatic nuclei
- Occasional vacuolated cytoplasm with mucin or can be a true mucinous tumor with mucin pools
- Clear cell adenocarcinoma
- May have glandular, tubulocystic, solid / diffuse, papillary or micropapillary growth patterns
- Cuboidal, variably sized cells with abundant clear or eosinophilic cytoplasm and cytoplasmic vacuoles
- Nuclei that are hyperchromatic, pleomorphic and have prominent nucleoli
- Hobnail changes and extracellular mucoid material may be present
- Mitoses and necrosis are often seen
- One study proposed a hybrid tumor with urothelial and squamous features, similar to basaloid squamous cell carcinoma, as the histology for most urethral tumors (Hum Pathol 2018;72:35)
Microscopic (histologic) images
Cytology description
- Positive cytology seen in 59% with primary urethral carcinoma (Urology 2004;63:33)
- Sensitivity greatest for urothelial cell carcinoma (80%) and lowest for squamous cell carcinoma (50%) (Urology 2004;63:33)
Positive stains
- Urothelial carcinoma (Hum Pathol 2013;44:2760)
- Squamous cell carcinoma
- Adenocarcinoma
- CK20 (variable), CDX2 (variable), cytoplasmic beta catenin
- Clear cell adenocarcinoma (Virchows Arch 2013;462:193, Arch Pathol Lab Med 2008;132:1417, Diagn Pathol 2022;17:87)
Negative stains
- Urothelial carcinoma (Hum Pathol 2013;44:2760)
- E-cadherin, CD44s, PAX8, PSA, PSAP
- Squamous cell carcinoma
- Adenocarcinoma
- Clear cell adenocarcinoma
Molecular / cytogenetics description
- PIK3CA found to be the most frequently targeted genomic alterations of advanced urethral carcinoma (Urol Oncol 2021;39:731.e1)
- Adenocarcinoma: PTEN was the top potentially targetable genomic alteration (Urol Oncol 2021;39:731.e1, Int J Surg Pathol 2021;29:447)
- Clear cell adenocarcinoma: focal copy number loss at SMAD4 and ARID2 loci, truncating mutation in ATM, ALK mutation (Am J Pathol 2014;184:584)
Sample pathology report
- Prostate, radical prostatectomy:
- Invasive urothelial carcinoma, high grade (see synoptic report)
- Surgical margins, negative for carcinoma
- Comment: The tumor expresses GATA3, CK7, CK20 and p63 and has focal, patchy expression of CK5/6.
- Penile urethra, biopsy:
- Carcinoma consistent with invasive urothelial carcinoma, high grade (see comment)
- Carcinoma invades corpus spongiosum
- Comment: The tumor expresses GATA3, CK7, CK20 and p63 and has focal, patchy expression of CK5/6.
- Female urethra, biopsy:
- Clear cell adenocarcinoma of the urothelial tract
- Comment: The tumor expresses PAX8, CK7 and AMACR and is negative for p16, CK20 and p63.
Differential diagnosis
- Mimickers of primary urethral urothelial carcinoma
- Urothelial carcinoma arising from the bladder:
- Clinical history, imaging and gross description are critical for differentiation
- Inverted urothelial papilloma:
- Typically develops at trigone of bladder but can develop anywhere that urothelium is present (Ann Diagn Pathol 2019;38:11)
- Absent mitotic activity without cellular atypia (Ann Diagn Pathol 2019;38:11)
- Urothelial carcinoma arising from the bladder:
- Mimickers of primary urethral squamous cell carcinoma
- Mimickers of primary urethral adenocarcinoma
- Prostatic adenocarcinoma:
- Colonic adenocarcinoma:
- Colonic adenocarcinoma expresses nuclear beta catenin but not CK7, p63 and GATA3
- Mimickers of clear cell adenocarcinoma
- Nephrogenic metaplasia:
- Minimal atypia or mitotic activity
- Overlapping immunoprofile
- Nephrogenic metaplasia:
Board review style question #1
A female urethra is resected with the primary tumor shown in the above image. The tumor strongly and diffusely expresses CK5/6 and p63 but is negative for PAX8 and only has weak expression of GATA3. What is the best diagnosis?
- Adenocarcinoma, intestinal type
- Clear cell adenocarcinoma
- Squamous cell carcinoma
- Urothelial carcinoma
Board review style answer #1
C. Squamous cell carcinoma. The most common 2 tumors primary to the female urethra are urothelial carcinoma and squamous cell carcinoma. Squamous cell carcinoma characteristically expresses CK5/6 and p63 and does not express PAX8. GATA3 can be positive but is often weaker in intensity compared to urothelial carcinoma. Answer A is incorrect because adenocarcinoma, intestinal type would show gland formation and be negative for CK5/6 and p63. Answer B is incorrect because clear cell adenocarcinoma would show tubules, hobnailed cells and clear cells and would express PAX8 but not CK5/6 and p63. Answer D is incorrect because urothelial carcinoma usually has a strong expression of GATA3 and does not have a diffuse and strong expression of CK5/6. Both urothelial carcinoma and squamous cell carcinoma express p63.
Comment Here
Reference: Urethral carcinoma
Comment Here
Reference: Urethral carcinoma
Board review style question #2
Board review style answer #2
B. Clear cell adenocarcinoma. Clear cell adenocarcinoma can have many growth patterns including glandular, tubulocystic, solid / diffuse, papillary or micropapillary. The cells can be cuboidal or hobnailed and have clear or eosinophilic cytoplasm. The tumor typically expresses PAX8. Answers A, C and D are incorrect because adenocarcinoma, intestinal type, squamous cell carcinoma and urothelial carcinoma do not have the described morphology and they do not express PAX8.
Comment Here
Reference: Urethral carcinoma
Comment Here
Reference: Urethral carcinoma
Verruciform xanthoma
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Electron microscopy description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Rare, nonneoplastic lesion of verrucous epidermal acanthosis, foamy histiocytes aggregates in papillary dermis and neutrophilic inflammation
Essential features
- Nonneoplastic verrucous lesion characterized by aggregates of lipid laden macrophages in papillary dermis
- Closely resembles other verrucous lesions clinically and microscopically
ICD coding
- ICD-10: E75.5 - other lipid storage disorders
Epidemiology
- Mean age: 54.5 - 62 years; range: 8 - 85 years (Dermatol Ther (Heidelb) 2017;7:65, Histopathology 2020;77:841)
- M > F (Histopathology 2020;77:841)
- Majority reported in Caucasians (Dermatol Ther (Heidelb) 2017;7:65)
- May be present several years before diagnosis (Dermatol Ther (Heidelb) 2017;7:65)
Sites
- Usually in oral cavity; majority of nonoral cases involve anogenital skin (penis, scrotum and vulva) (Dermatol Ther (Heidelb) 2017;7:65, Australas J Dermatol 2015;56:e99)
Etiology
- Uncertain etiology; likely local irritation, inflammation or trauma inciting an immune reaction with consequent keratinocyte degradation (Am J Surg Pathol 1998;22:479, J Oral Maxillofac Pathol 2013;17:392, J Cutan Pathol 2010;37:895)
- Keratinocyte degradation resulting in release of chemotactic cytokines, attracting neutrophils as well as plasma cells and lymphocytes
- Macrophages (CD68+ dermal dendritic cells) phagocytose neutrophilic and keratinocyte debris with eventual formation of foamy cells (Am J Surg Pathol 1998;22:479, Oral Oncol 2001;37:326)
- HPV association variable (Australas J Dermatol 2015;56:e99, J Cutan Pathol 2010;37:895)
- In several reports coexisting with inflammatory cutaneous disorders (graft versus host disease, pemphigus vulgaris, discoid lupus erythematosus, lichen planus) or associated with immunocompromise
- Not associated with underlying disorders of lipid metabolism (J Cutan Pathol 2010;37:895)
Clinical features
- Asymptomatic, slow growing, persistent and usually solitary papule(s) or plaque resembling viral warts, particularly on the scrotum in males (Dermatol Ther (Heidelb) 2017;7:65, Histopathology 2020;77:841)
- Difficult to distinguish clinically; may resemble fibroepithelial polyp, seborrheic keratosis, condyloma acuminatum, squamous cell carcinoma or verrucous carcinoma (Histopathology 2020;77:841)
Diagnosis
- Punch biopsy with subsequent histological examination
Case reports
- 38 year old man with multiple, coexisting verruciform xanthomas of anogenital region associated with cutaneous trauma (J Cutan Pathol 2010;37:895)
- 50 year old man with scrotal verruciform xanthoma (Dermatol Online J 2014;20:21253)
- 64 year old man with penile verruciform xanthoma (Arch Ital Urol Androl 2016;88:284)
- 75 year old man with scrotal verruciform xanthoma (J Med Case Rep 2012;6:260)
- 78 year old man with penile verruciform xanthoma (Indian J Dermatol Venereol Leprol 2018;84:600)
Treatment
- Majority cured by complete excision; typically do not recur (Dermatol Ther (Heidelb) 2017;7:65)
Gross description
- Yellow, pink, brown or flesh colored pedunculated papules or plaques, 0.5 - 2.5 cm, with a verrucous or pebbly surface (Australas J Dermatol 2015;56:e99, Dermatol Ther (Heidelb) 2017;7:65, Histopathology 2020;77:841)
Microscopic (histologic) description
- Verrucous epidermal acanthosis, papillomatosis and hyperkeratosis
- Uniform depth of rete ridges
- Parakeratosis, often wedge shaped, filling clefts between epidermal projections (Histopathology 2020;77:841)
- Large foamy histiocytes (lipid laden macrophages) in aggregates in elongated dermal papillae, may be scattered or scarce (J Oral Maxillofac Pathol 2013;17:392, Dermatol Ther (Heidelb) 2017;7:65, Histopathology 2020;77:841)
- Neutrophils, particularly in the stratum corneum, as well as dermis and occasionally prominent neutrophilic exocytosis (Australas J Dermatol 2015;56:e99, Dermatol Ther (Heidelb) 2017;7:65, Histopathology 2020;77:841)
- Mixed inflammatory infiltrate in the submucosa (J Oral Maxillofac Pathol 2013;17:392)
- Vascularized papillary dermis (J Am Acad Dermatol 2015; 72:e147)
- Absence of keratinocyte atypia
Microscopic (histologic) images
Positive stains
- Foamy cells: CD68+, PAS+ and diastase resistant (Virchows Arch A Pathol Anat Histopathol 1993;423:243, J Periodontol 2001;78:504)
- Adipophilin (Clin Exp Dermatol 2015;40:156)
- Factor VIIIa
- Vimentin
Negative stains
Electron microscopy description
- Xanthoma cells contain membrane bound lysosomes, myelin figures and fragmented desmosomes (Oral Surg Oral Med Oral Pathol 1975;40:246)
Sample pathology report
- Penis, dorsal, biopsy:
- Verruciform xanthoma
Differential diagnosis
- Verruca vulgaris:
- Koilocytes, coarse hypergranulosis, prominent papillomatosis, rete ridges curve inward
- Condyloma acuminatum:
- Prominent koilocytotic atypia in upper epidermis
- No prominent foamy macrophages
- Xanthoma:
- Foamy cells are located in the mid dermis
- Clinical hyperlipidemia
- Squamous cell carcinoma:
- Marked atypia, no prominent foamy histiocytes
- Verrucous carcinoma:
- Ulcerating or fungating lobules of mature squamous epithelium
- Minimal atypia but no prominent foamy histiocytes
- Bowenoid papulosis:
- Resembling basaloid PeIN
- Spotty cytological atypia
Board review style question #1
A 58 year old Caucasian man presents with a 1.0 cm pink, warty lesion on the glans penis. The biopsy shows verruciform hyperplasia of the epidermis with hyperkeratosis, parakeratosis and acanthosis (figure 1). No keratinocyte atypia or dyskeratotic cells are seen. There is a dense infiltrate of lipid laden macrophages (foamy cells) in the papillary dermis (figure 2) which are positive for CD68 and negative for S100. Which statement is true of cutaneous verruciform xanthoma?
- It is frequently caused by HPV genotypes 6 and 11
- Approximately 2 - 5% will either harbor or progress to a squamous cell carcinoma
- It is usually associated with underlying immunosuppression
- It is a nonneoplastic lesion that typically does not recur after excision
Board review style answer #1
D. It is a nonneoplastic lesion that typically does not recur after excision. The diagnosis of the lesion is cutaneous verruciform xanthoma.
Comment Here
Reference: Cutaneous verruciform xanthoma
Comment Here
Reference: Cutaneous verruciform xanthoma
Board review style question #2
Which best summarizes the immunohistochemical profile of the characteristic cells infiltrating the dermal papillae in cutaneous verruciform xanthoma?
- CD68 positive; PAS positive diastase resistant; positive granular cytoplasmic staining of adipophilin; S100 negative
- CD68 positive; S100 positive; CD1a positive; Langerin positive
- CK5/6 positive; p16 positive; p63 positive
- CD10 positive; CK5/6 negative; S100 negative
Board review style answer #2
A. CD68 positive; PAS positive diastase resistant; positive granular cytoplasmic staining of adipophilin; S100 negative
Comment Here
Reference: Cutaneous verruciform xanthoma
Comment Here
Reference: Cutaneous verruciform xanthoma
WHO classification
Table of Contents
Definition / general | Major updates | WHO classification 2022 (ICD-O morphology terms if different) | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- WHO classification of tumors of the urinary and male genital tumors
- Currently on 5th edition, published in 2022
Major updates
- Terminology for squamous cell carcinoma groupings has been changed from non-HPV related to HPV independent and from HPV related to HPV associated
- Papillary basaloid carcinoma, warty basaloid carcinoma, pseudohyperplastic carcinoma, pseudoglandular carcinoma, carcinoma cuniculatum and mixed squamous cell carcinoma have been removed as distinct subtypes
- Pseudohyperplastic and pseudoglandular patterns are now a part of squamous cell carcinoma, usual type
- Carcinoma cuniculatum pattern is now a part of verrucous carcinoma
WHO classification 2022 (ICD-O morphology terms if different)
-
Benign and precursor squamous lesions
- Condyloma acuminatum
- Squamous cell carcinoma precursors, HPV associated
- Squamous cell carcinoma precursors, HPV independent
- Invasive squamous epithelial tumors
- HPV associated squamous cell carcinoma (squamous cell carcinoma, HPV associated)
- Basaloid squamous cell carcinoma
- Warty carcinoma
- Clear cell squamous cell carcinoma
- Lymphoepithelioma-like carcinoma (lymphoepithelial carcinoma)
- HPV independent squamous cell carcinoma (squamous cell carcinoma, HPV independent)
- Squamous cell carcinoma NOS (squamous cell carcinoma, NOS)
- HPV associated squamous cell carcinoma (squamous cell carcinoma, HPV associated)
- Other epithelial tumors
- Penile adenosquamous carcinoma (adenosquamous carcinoma)
- Penile mucoepidermoid carcinoma (mucoepidermoid carcinoma)
- Extramammary Paget disease (Paget disease, extramammary)
-
Invasive epithelial tumors of the penis and scrotum
-
Other scrotal tumors
Additional references
Board review style question #1
Which marker is utilized in the classification scheme of penile squamous cell carcinoma?
- ALK
- HPV
- PTEN
- TFEB
- TFE3
Board review style answer #1
Recent Penis & scrotum Pathology books
Find related Pathology books: GU/adrenal