- pTX: primary tumor cannot be assessed
- pTis: carcinoma in situ (this includes high grade pancreatic intraepithelial neoplasia, intraductal papillary mucinous neoplasm with high grade dysplasia, intraductal tubulopapillary neoplasm with high grade dysplasia and mucinous cystic neoplasm with high grade dysplasia)
- pT1: tumor ≤ 2 cm in greatest dimension
- pT1a: tumor ≤ 0.5 cm in greatest dimension
- pT1b: tumor > 0.5 and < 1 cm in greatest dimension
- pT1c: tumor 1 - 2 cm in greatest dimension
- pT2: tumor > 2 cm and ≤ 4 cm in greatest dimension
- pT3: tumor > 4 cm in greatest dimension
- pT4: tumor involves celiac axis, superior mesenteric artery or common hepatic artery, regardless of size
Superpage
Superpage Topics
Acinar cell carcinoma
Acinar cystic transformation
ACTH secreting tumors
Acute pancreatitis
Adenosquamous carcinoma
Allograft rejection
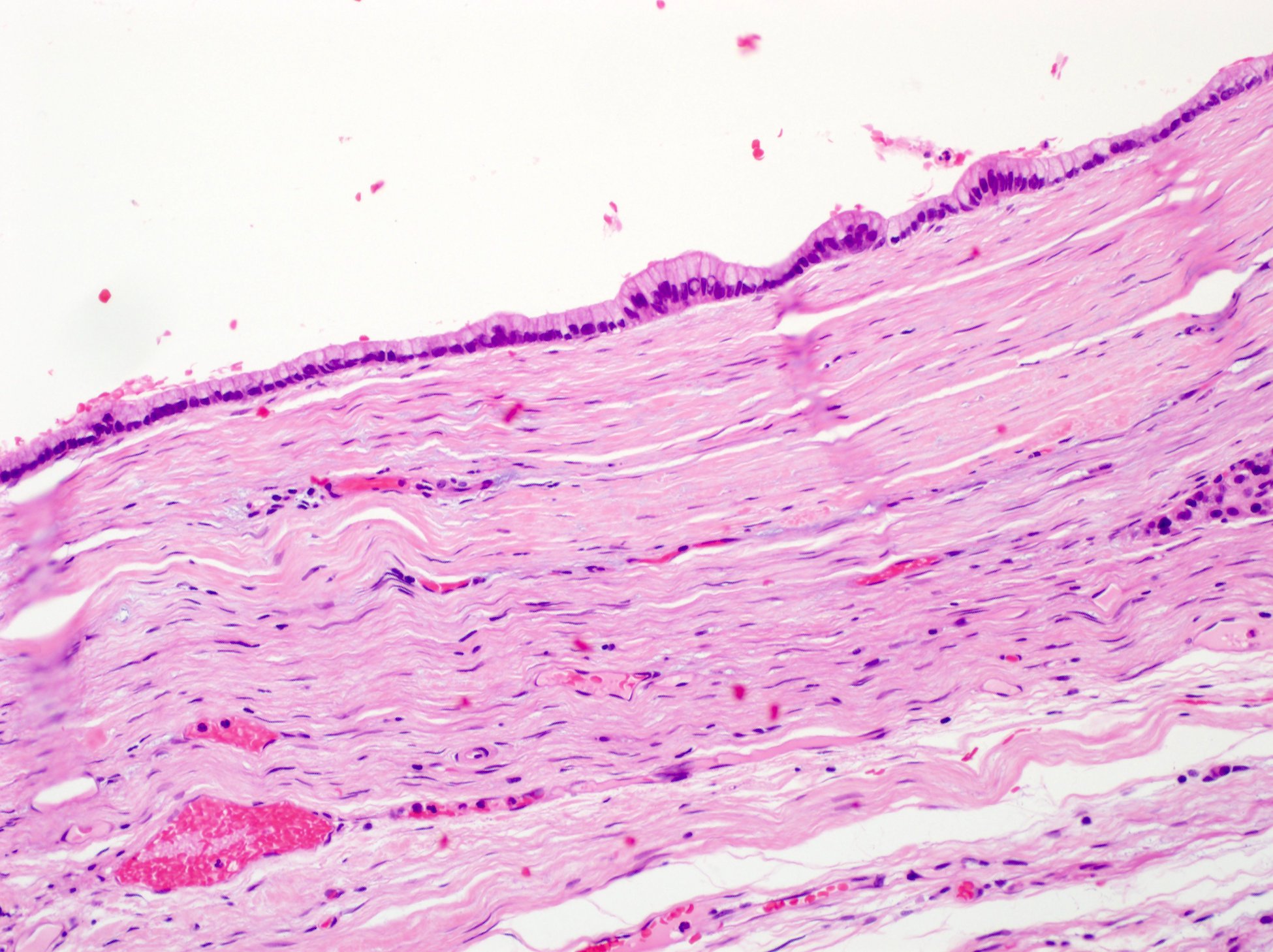
Anatomy & histology
Autoimmune pancreatitis type 1
Autoimmune pancreatitis type 2
Chronic pancreatitis
Clear cell pancreatic endocrine tumor
Colloid carcinoma
Cystic endocrine tumors
Cystic fibrosis
Cytology
Diabetes mellitus
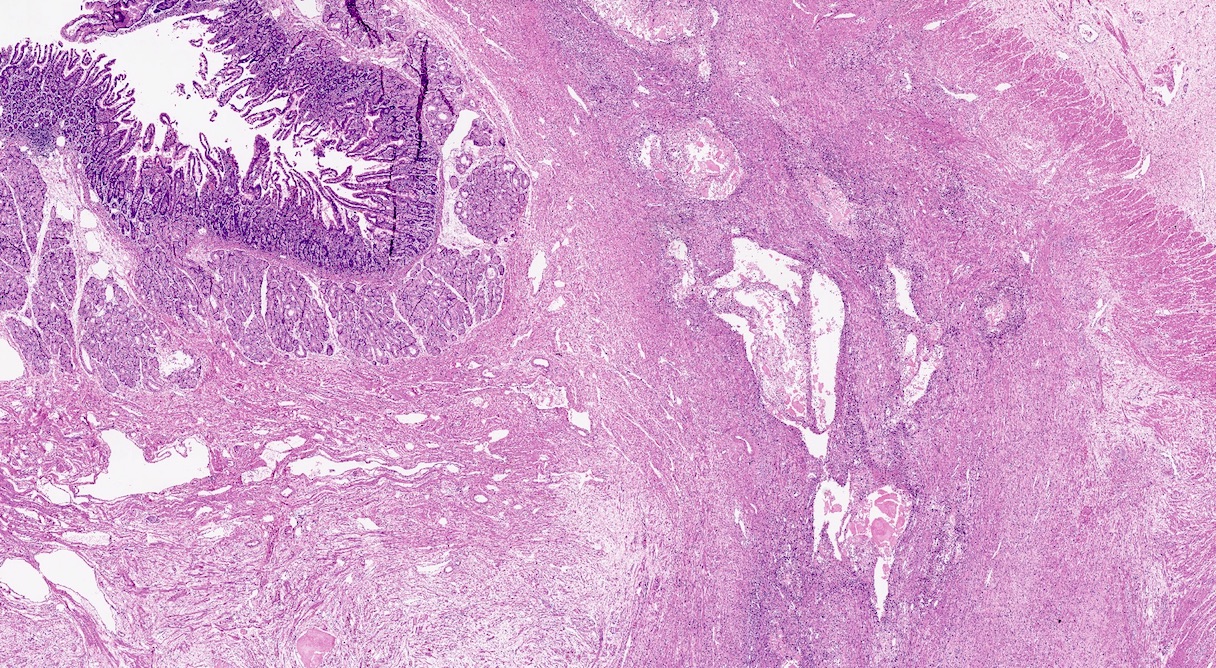
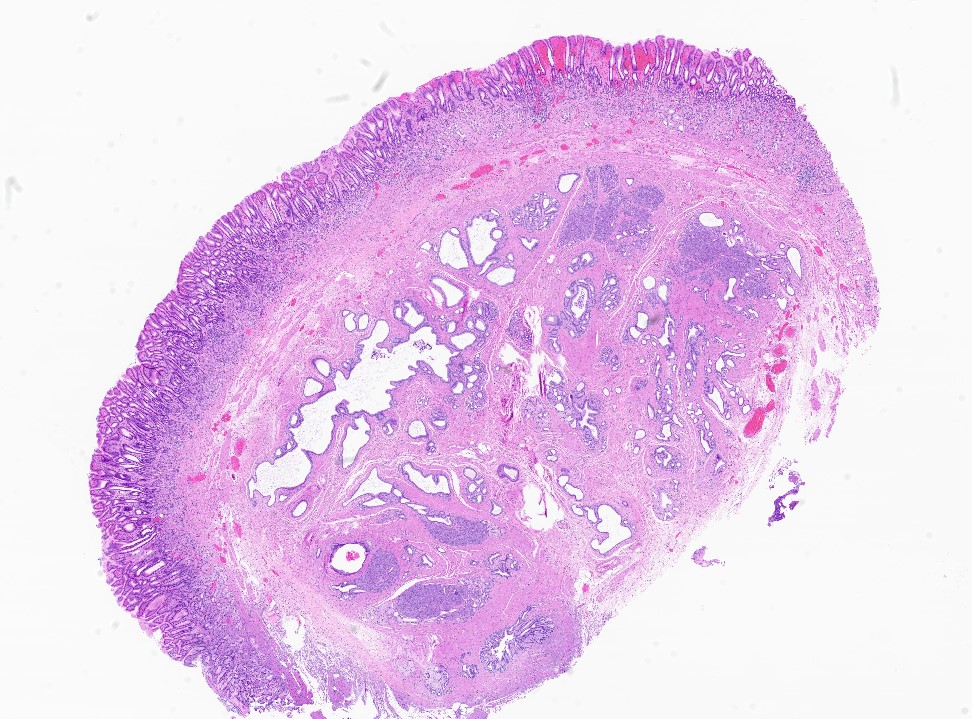
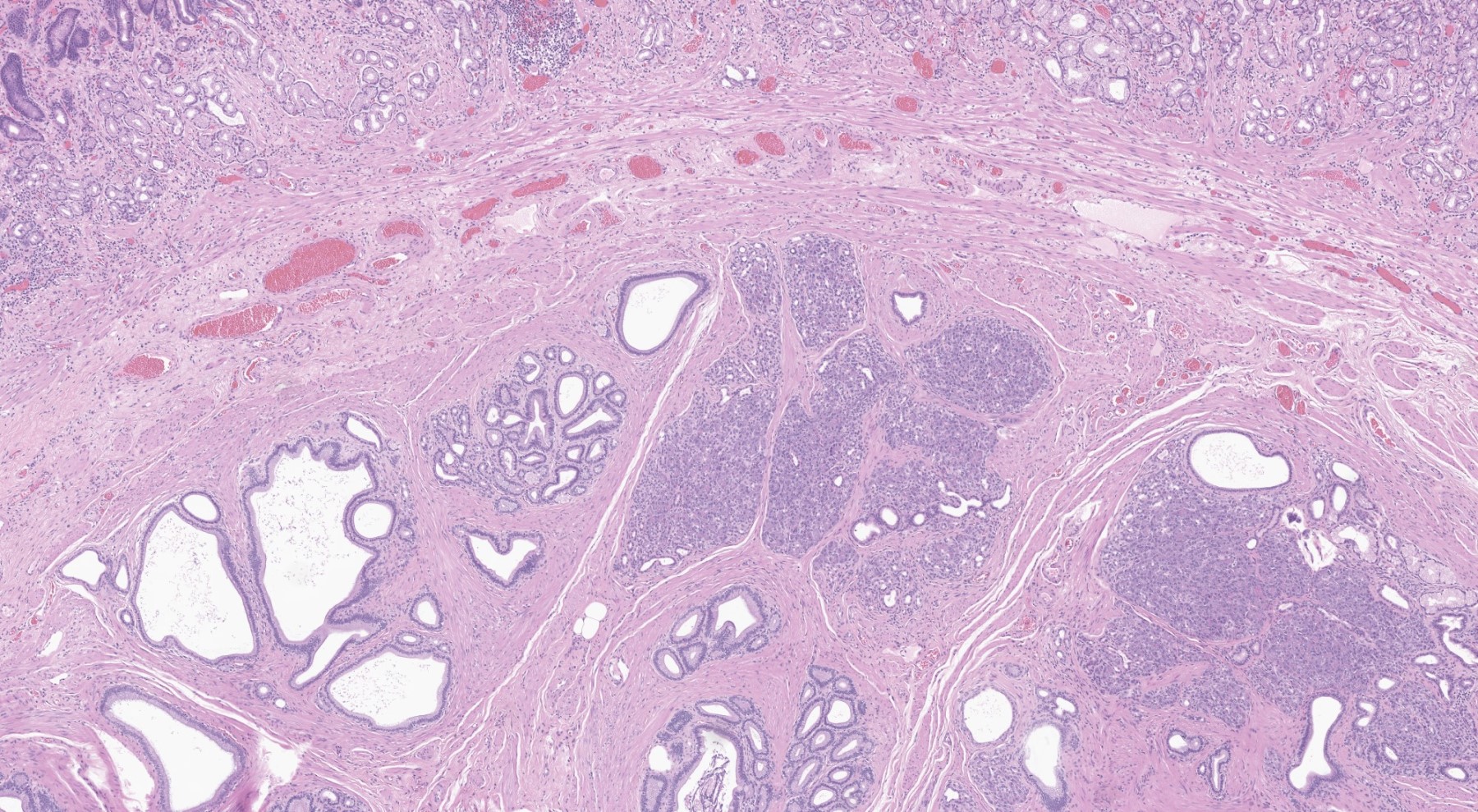
Ductal adenocarcinoma, NOS
Familial pancreatic neoplasms (pending)
Features to report-pancreatectomy
Frozen section
Gastrinoma
Glucagonoma (alpha cell tumors)
Grossing
Heterotopic pancreas
Insulinoma (beta cell tumor)
Intraductal oncocytic papillary neoplasm (IOPN)
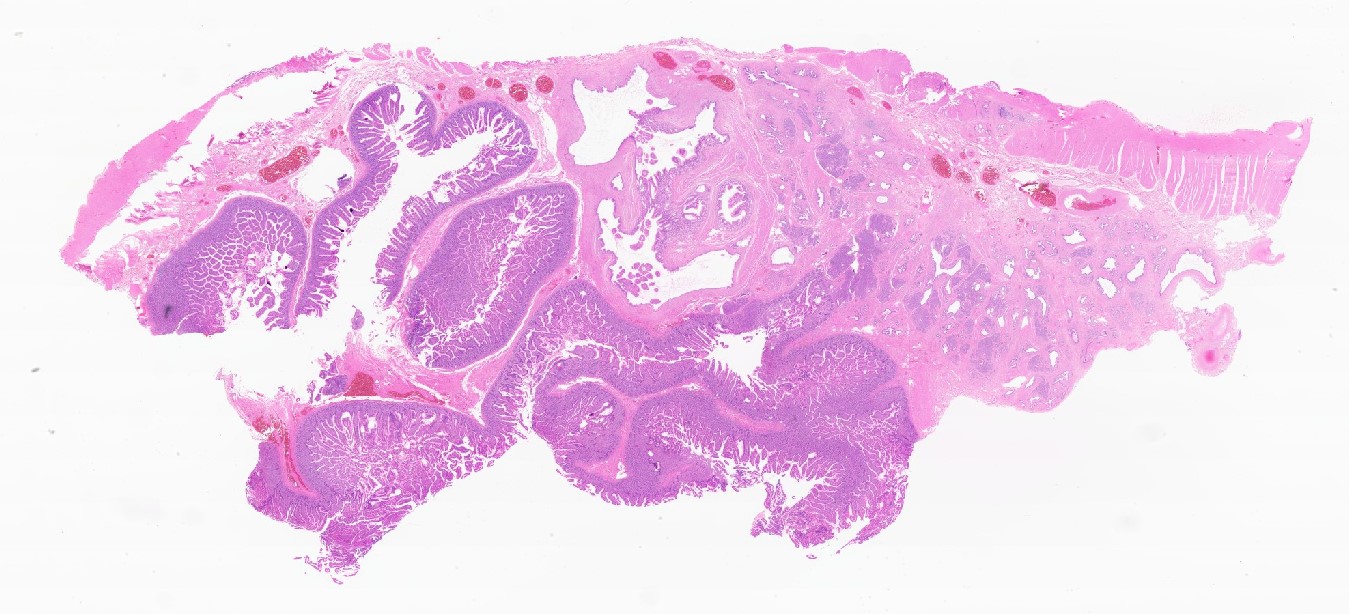
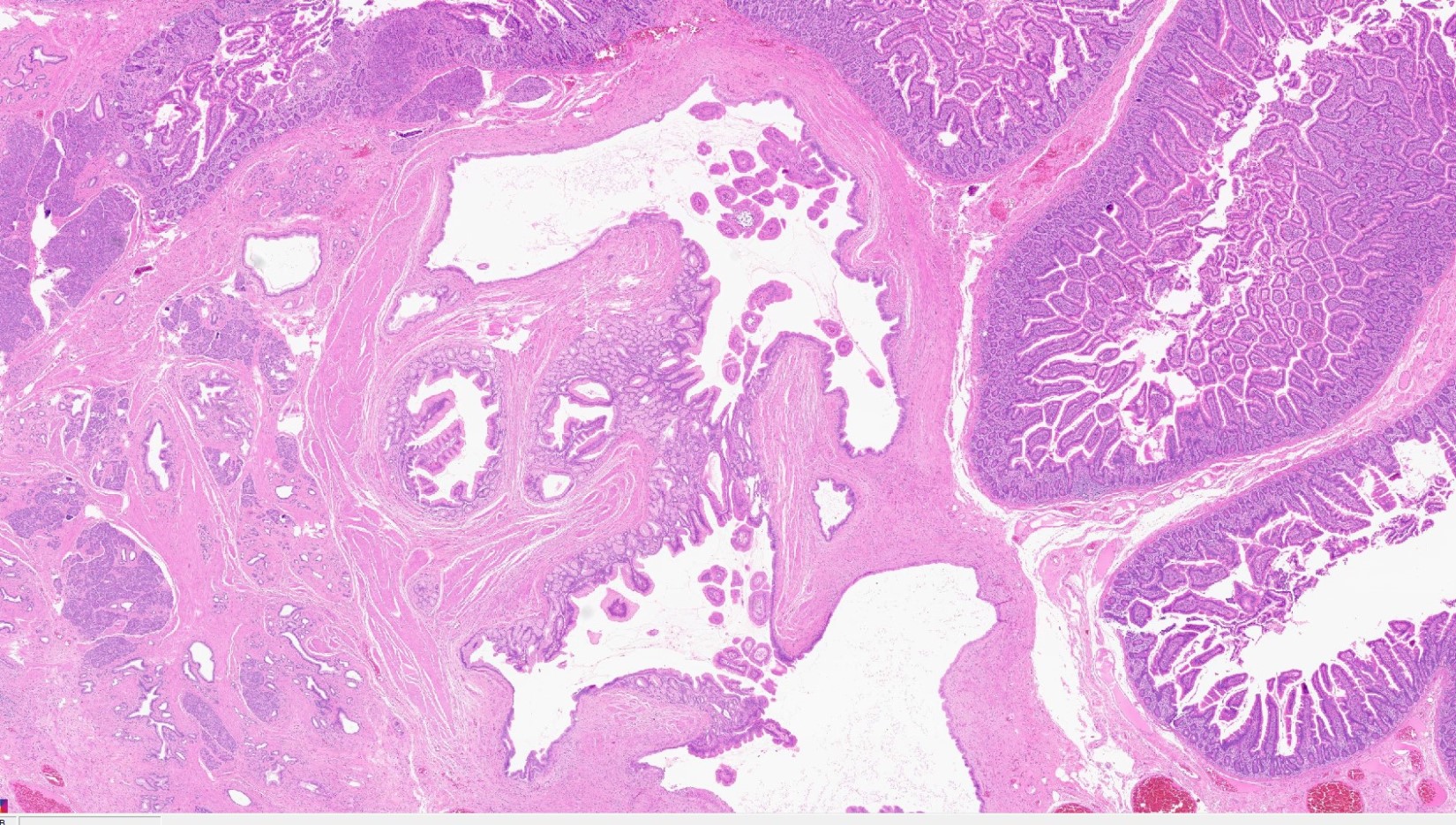
Intraductal papillary mucinous neoplasm (IPMN)
Intraductal tubulopapillary neoplasm (ITPN)
Lymphoepithelial cysts
Medullary carcinoma
MEN1 syndrome
Mixed neuroendocrine nonneuroendocrine neoplasms (MiNENs)
Molecular genetics of pancreatic ductal carcinoma
Mucinous cystic neoplasm (MCN)
Mucinous pancreatic tumor overview
Nesidioblastosis
Neuroendocrine neoplasms-general
Neuroendocrine tumor with sarcomatous differentiation
Pancreatic cystic fluid analysis (pending)
Pancreatic polypeptide secreting tumors
Pancreatoblastoma
PanIN
Poorly differentiated neuroendocrine carcinoma
PRSS1 hereditary pancreatitis
Pseudocysts
Risk stratification of IPMNs (pending)
Sclerosing epithelioid mesenchymal neoplasm
Serous cystadenoma
Simple mucinous cyst
Solid pseudopapillary neoplasm
Somatostatinoma
Staging-exocrine
Staging-neuroendocrine
True cysts
Undifferentiated carcinoma
Undifferentiated carcinoma with osteoclast-like giant cells
VIPoma
Well differentiated neuroendocrine tumor
WHO classification
WHO reporting system for pancreaticobiliary cytopathologyAcinar cell carcinoma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
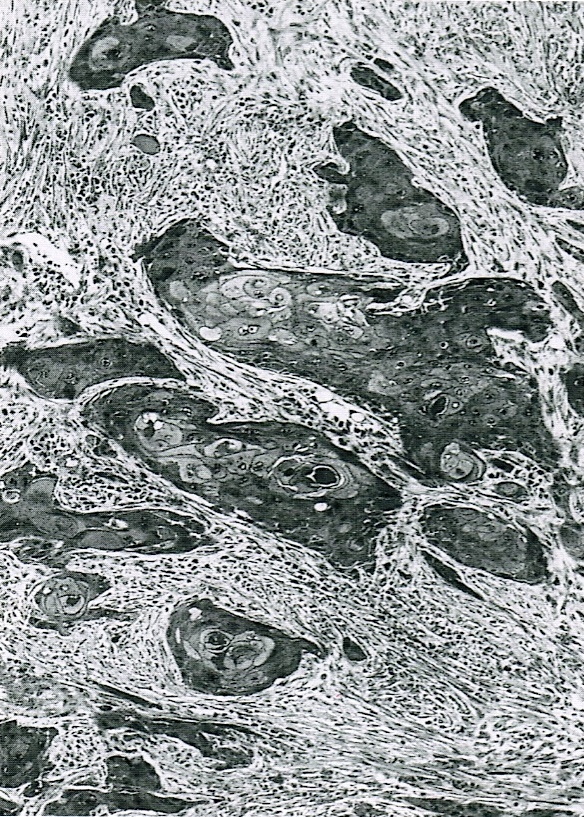
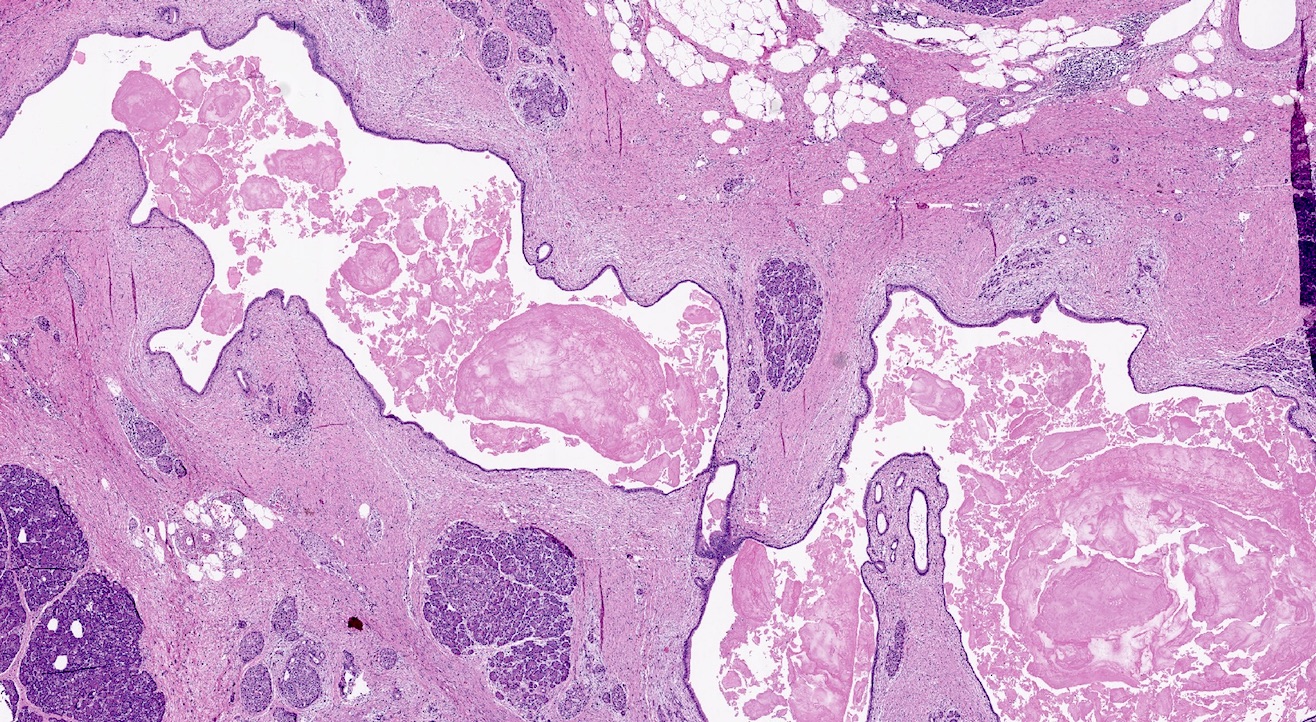
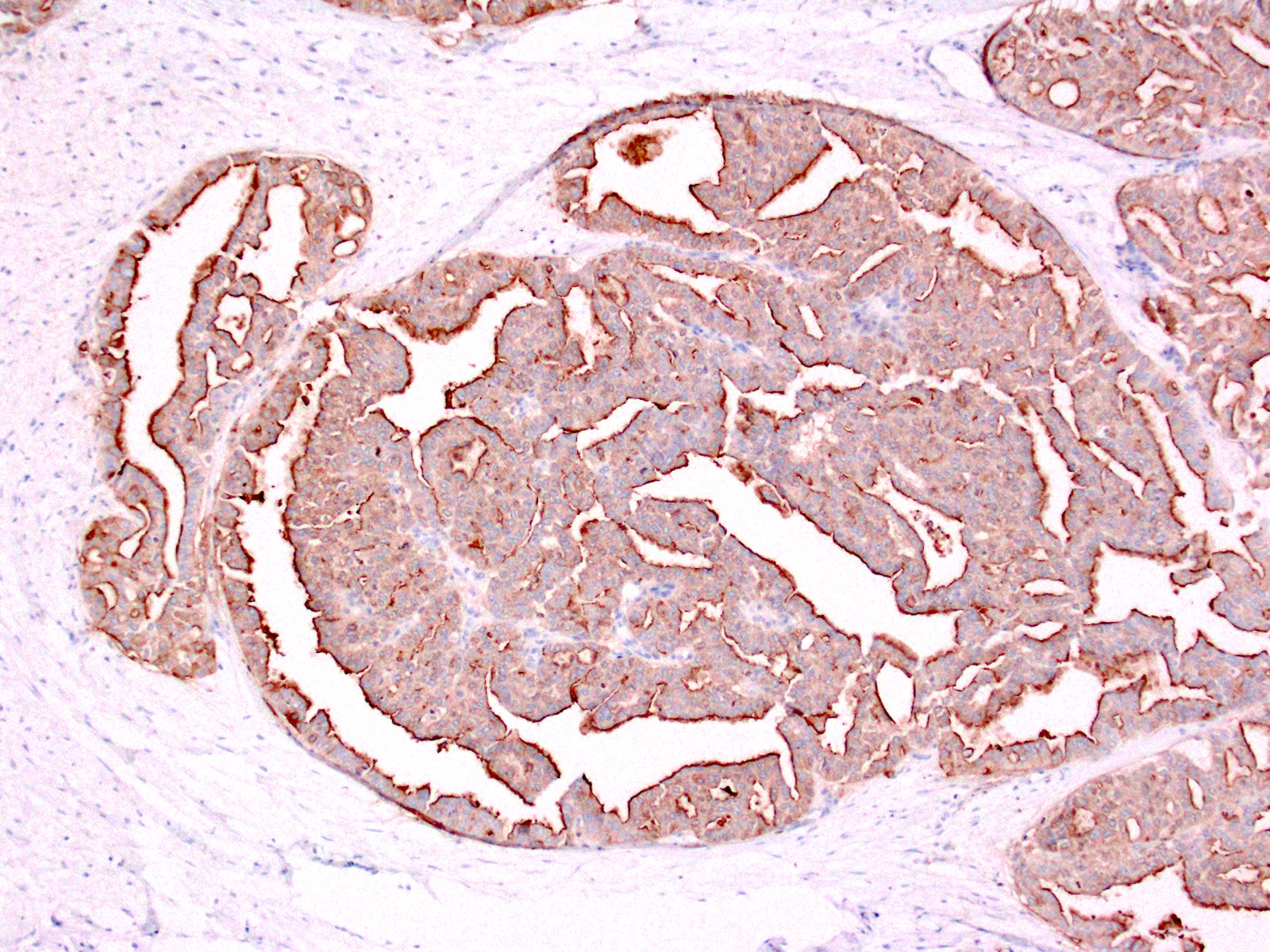
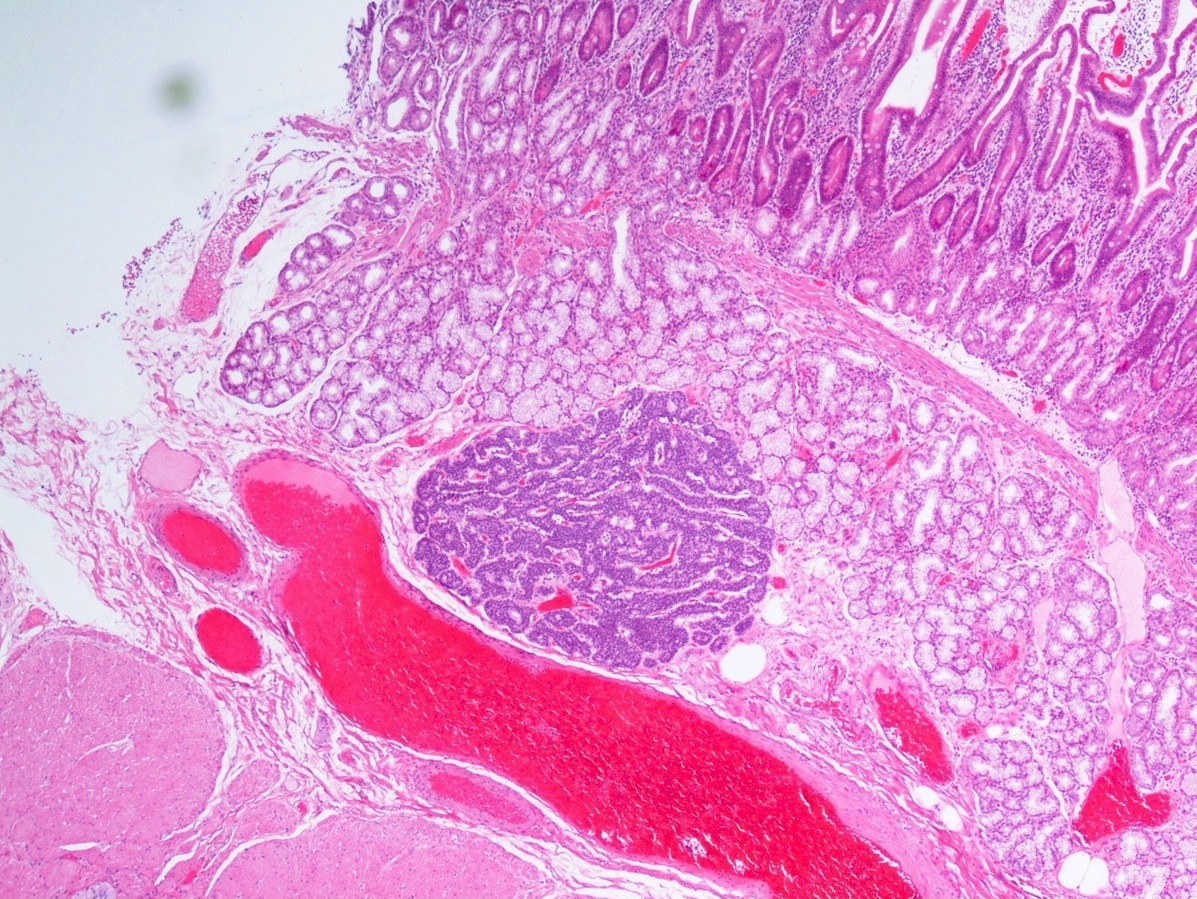
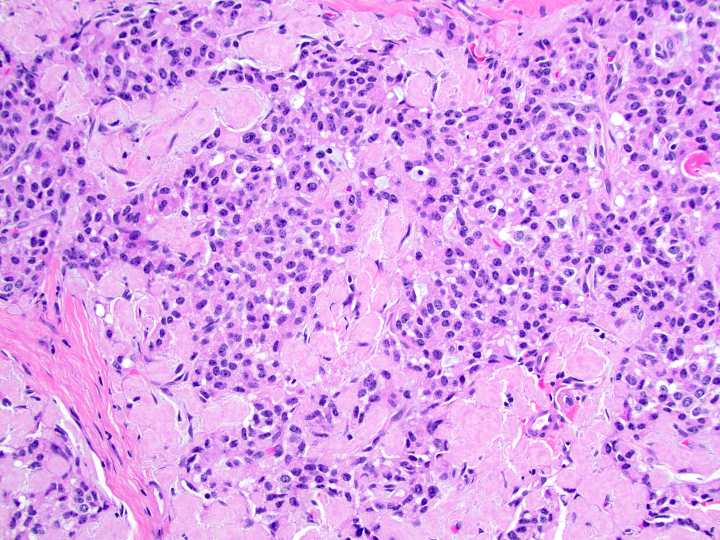
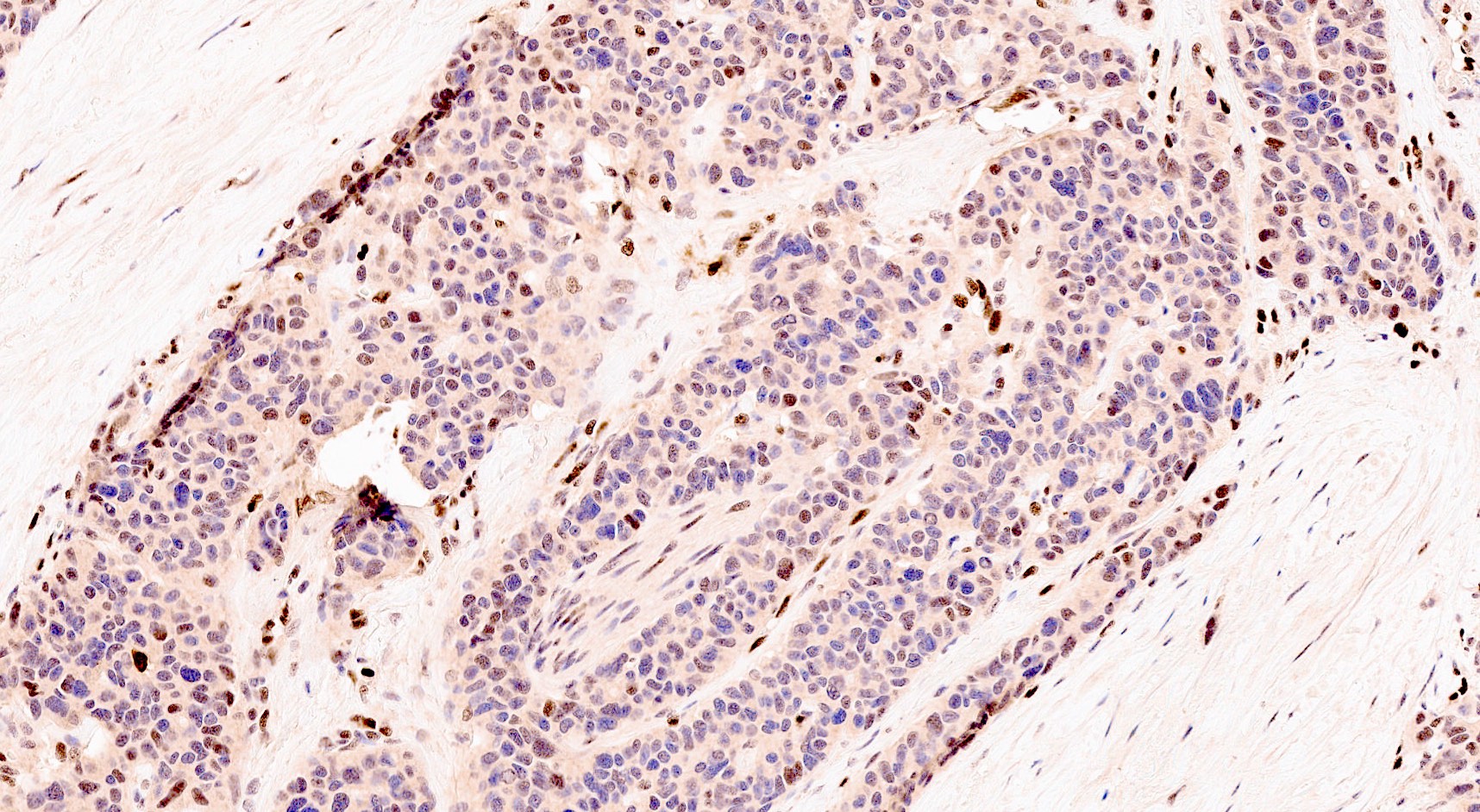
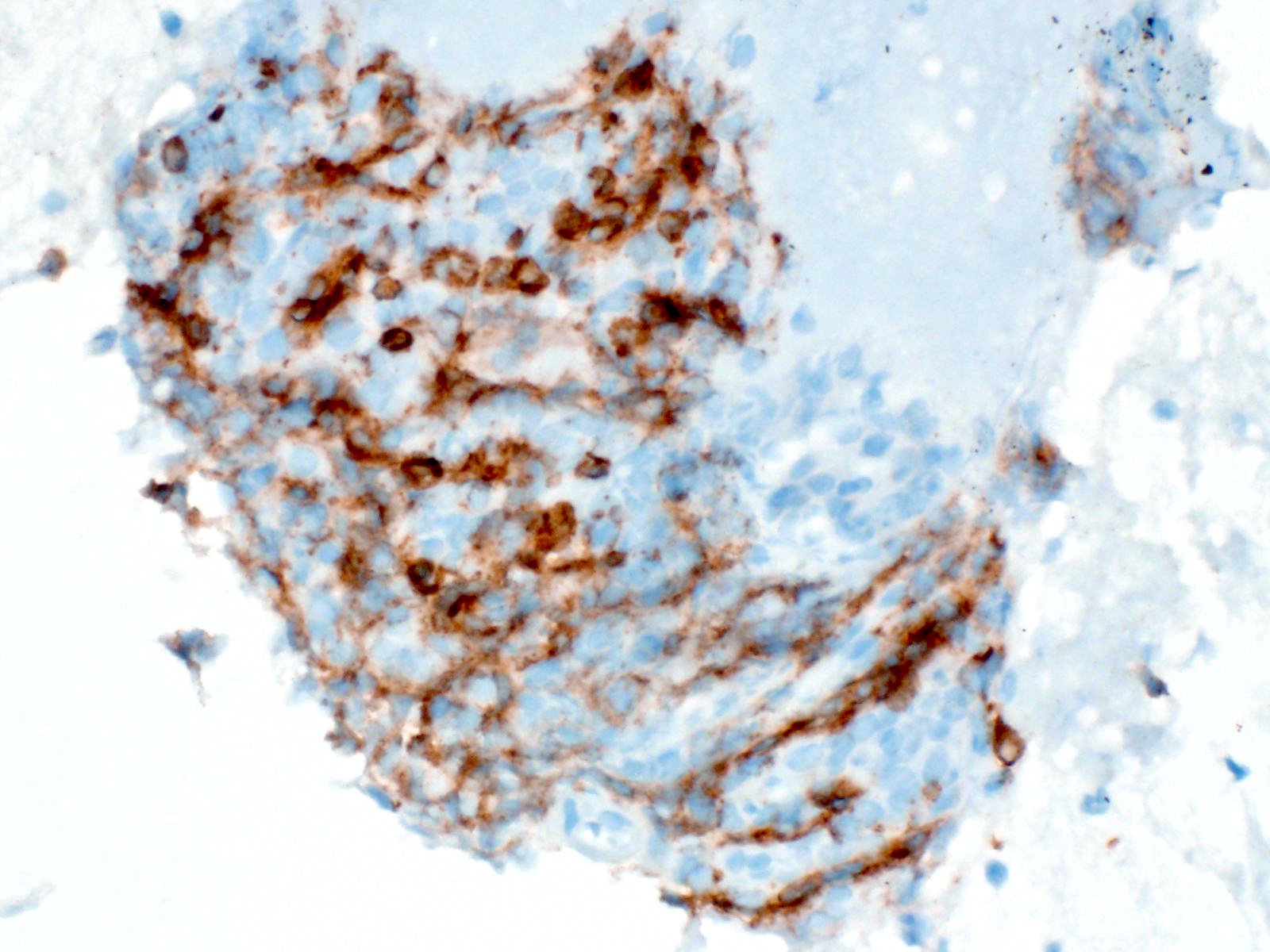
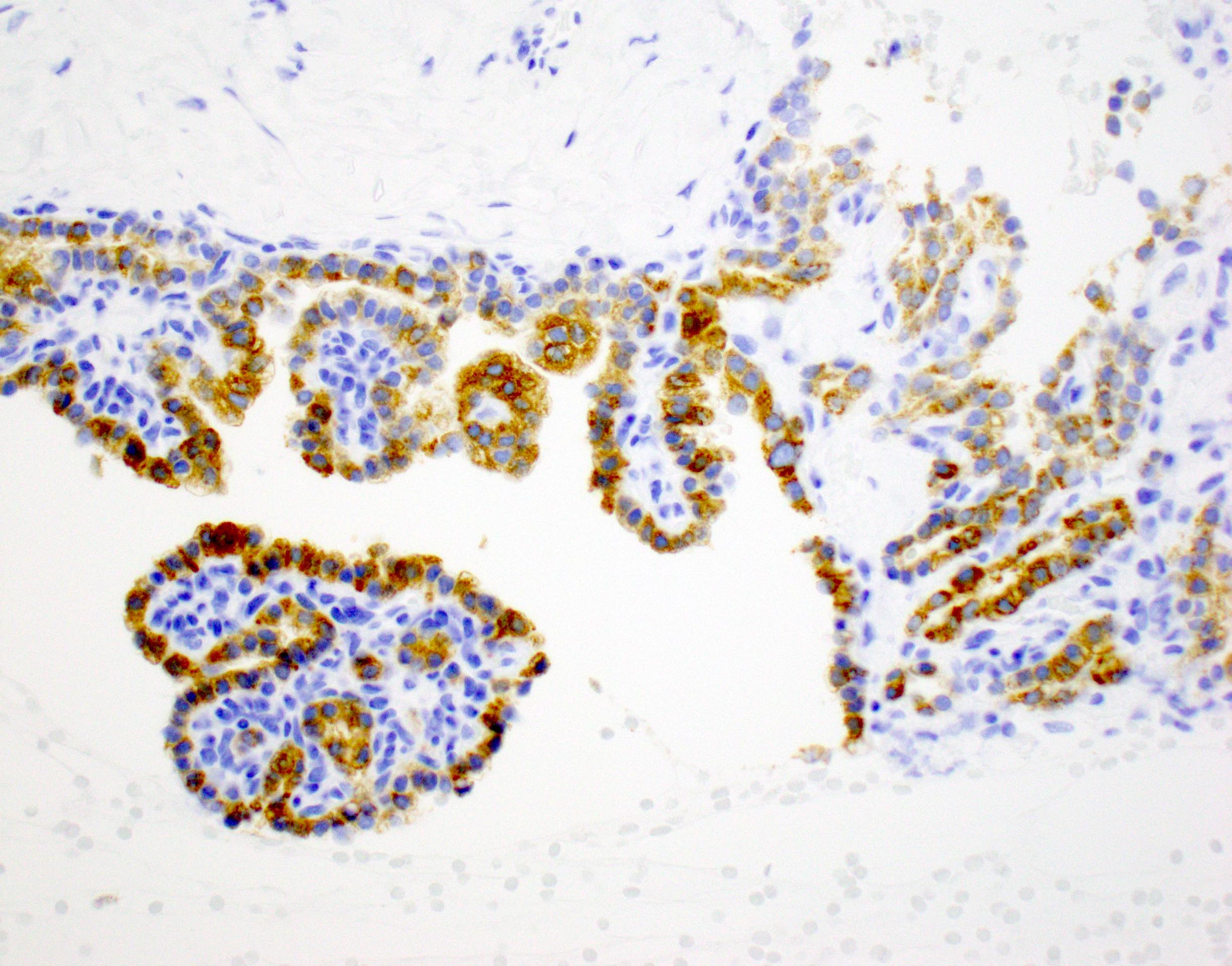
- Malignant exocrine neoplasm of the pancreas composed of cells with morphological resemblance to acinar cells and with immunohistochemistry positive for acinar markers
Essential features
- Epithelial neoplasm showing acinar differentiation
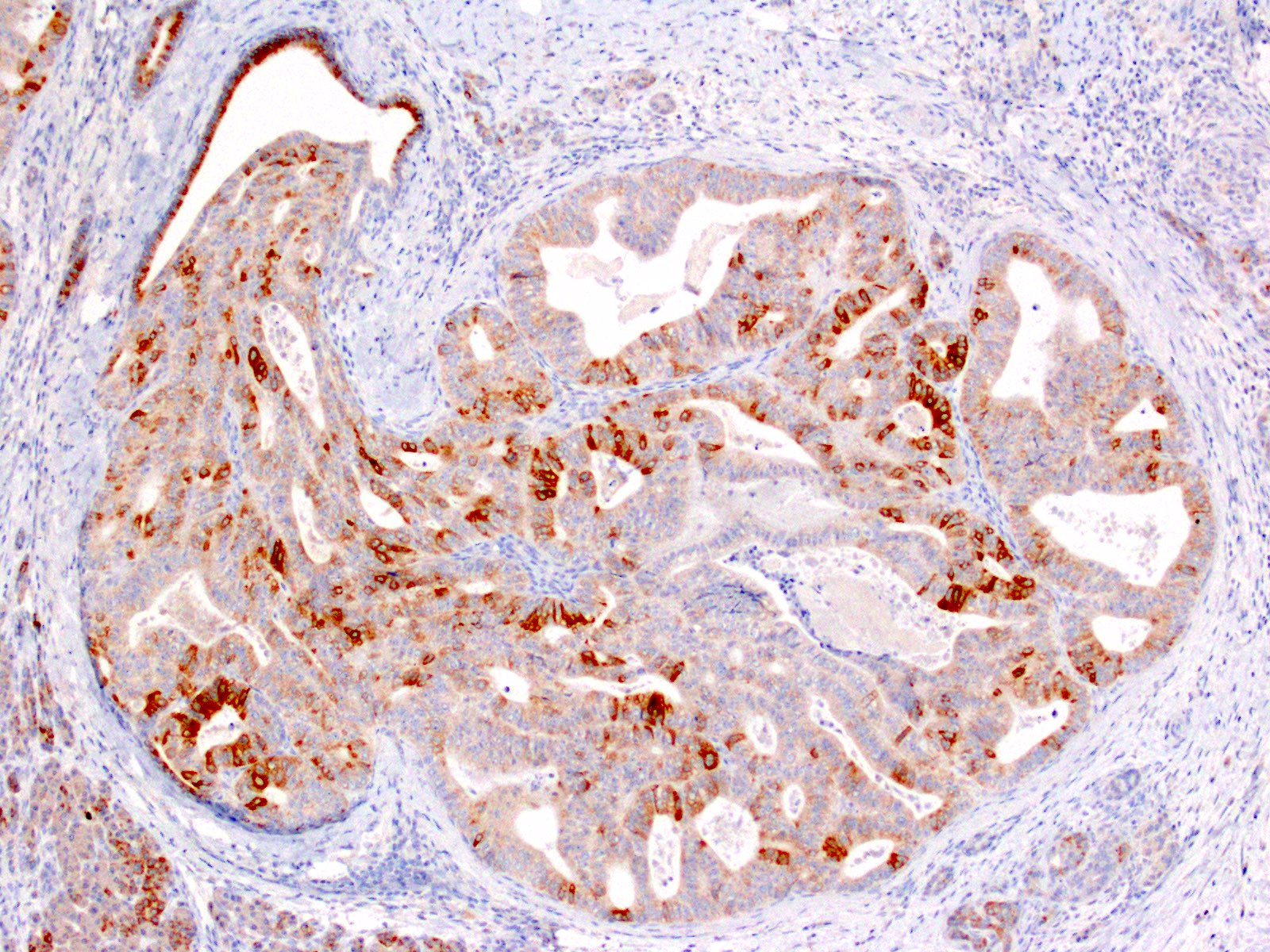
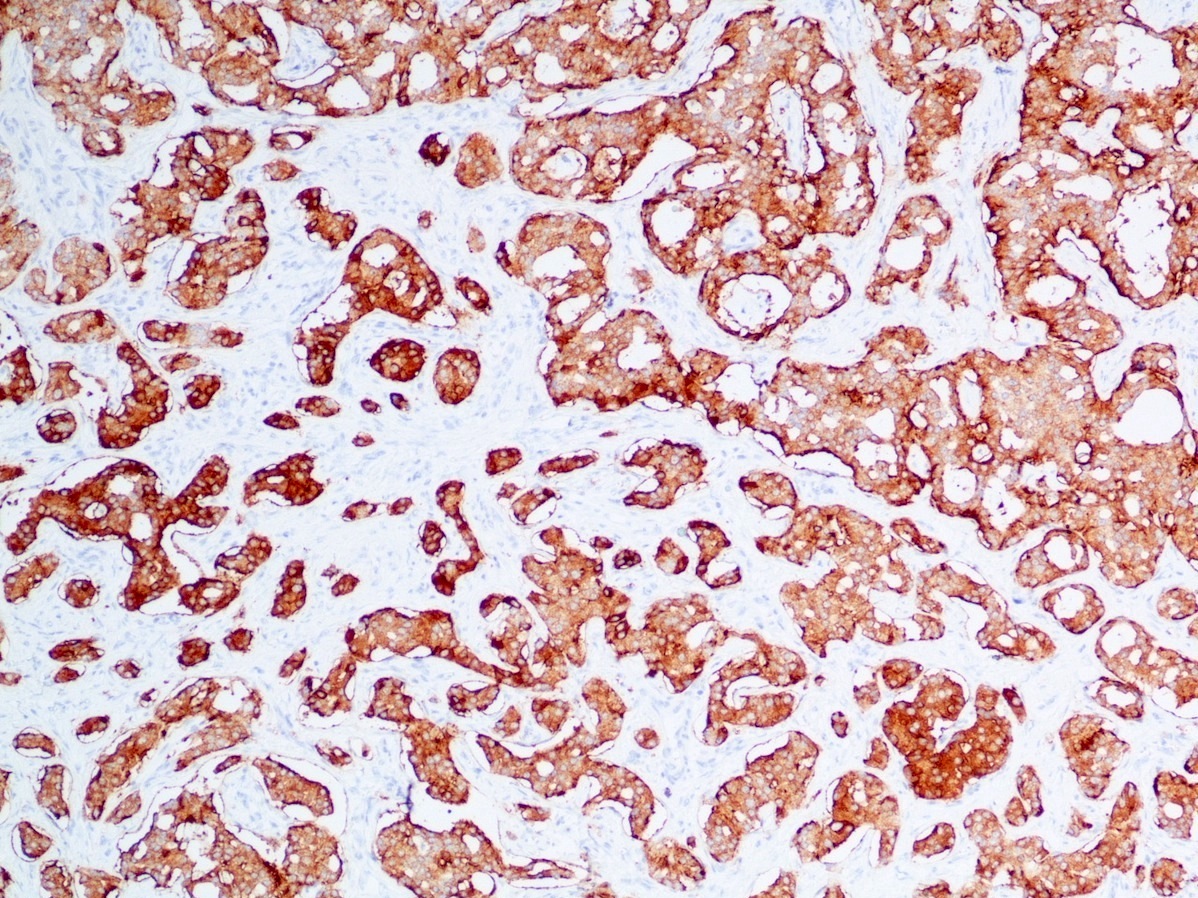
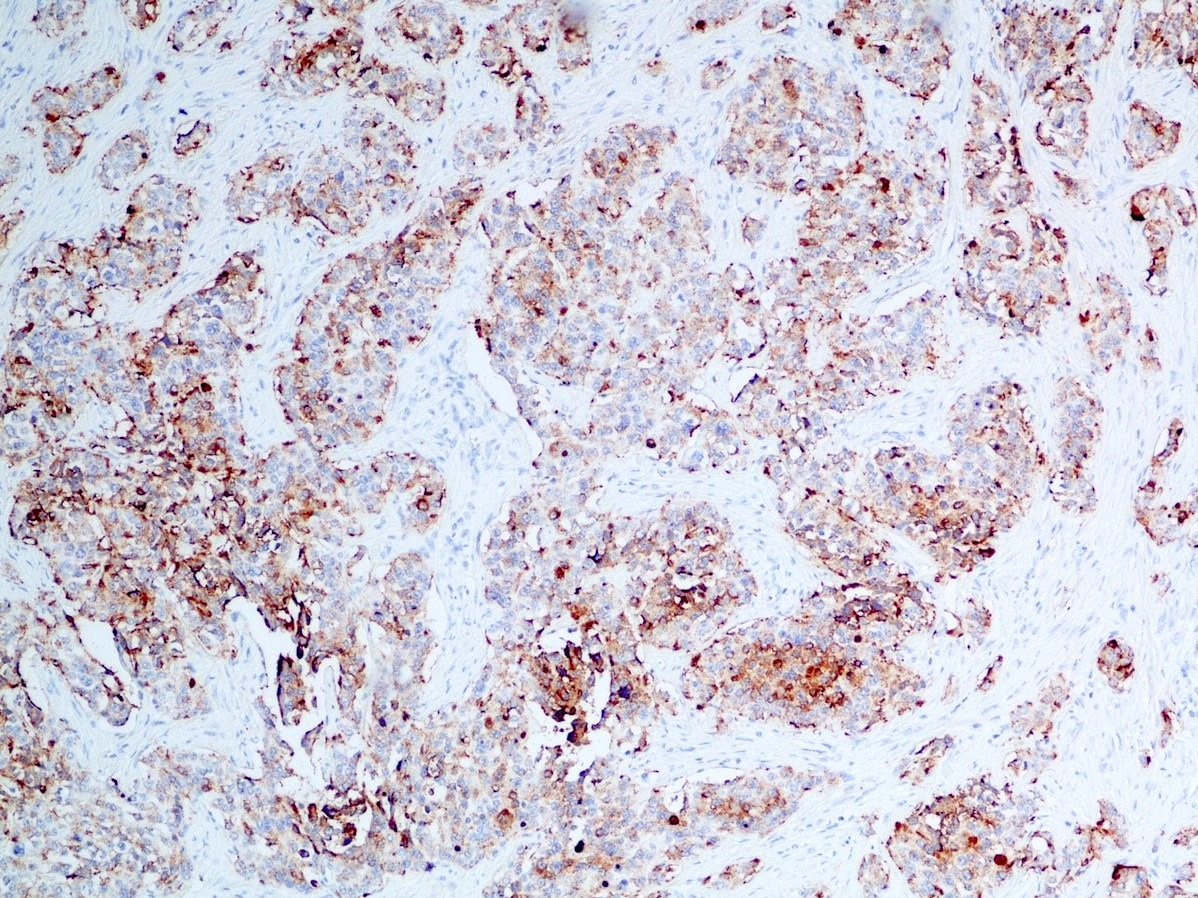
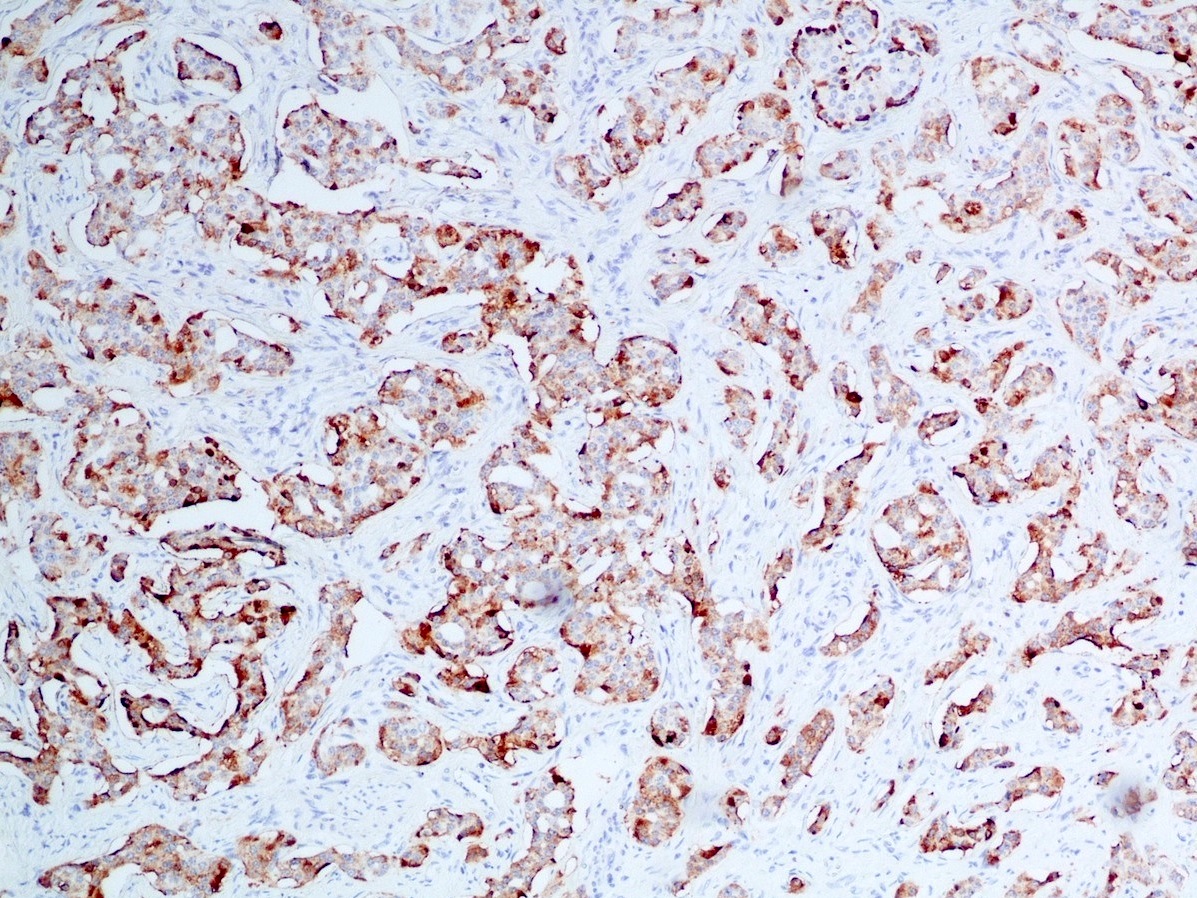
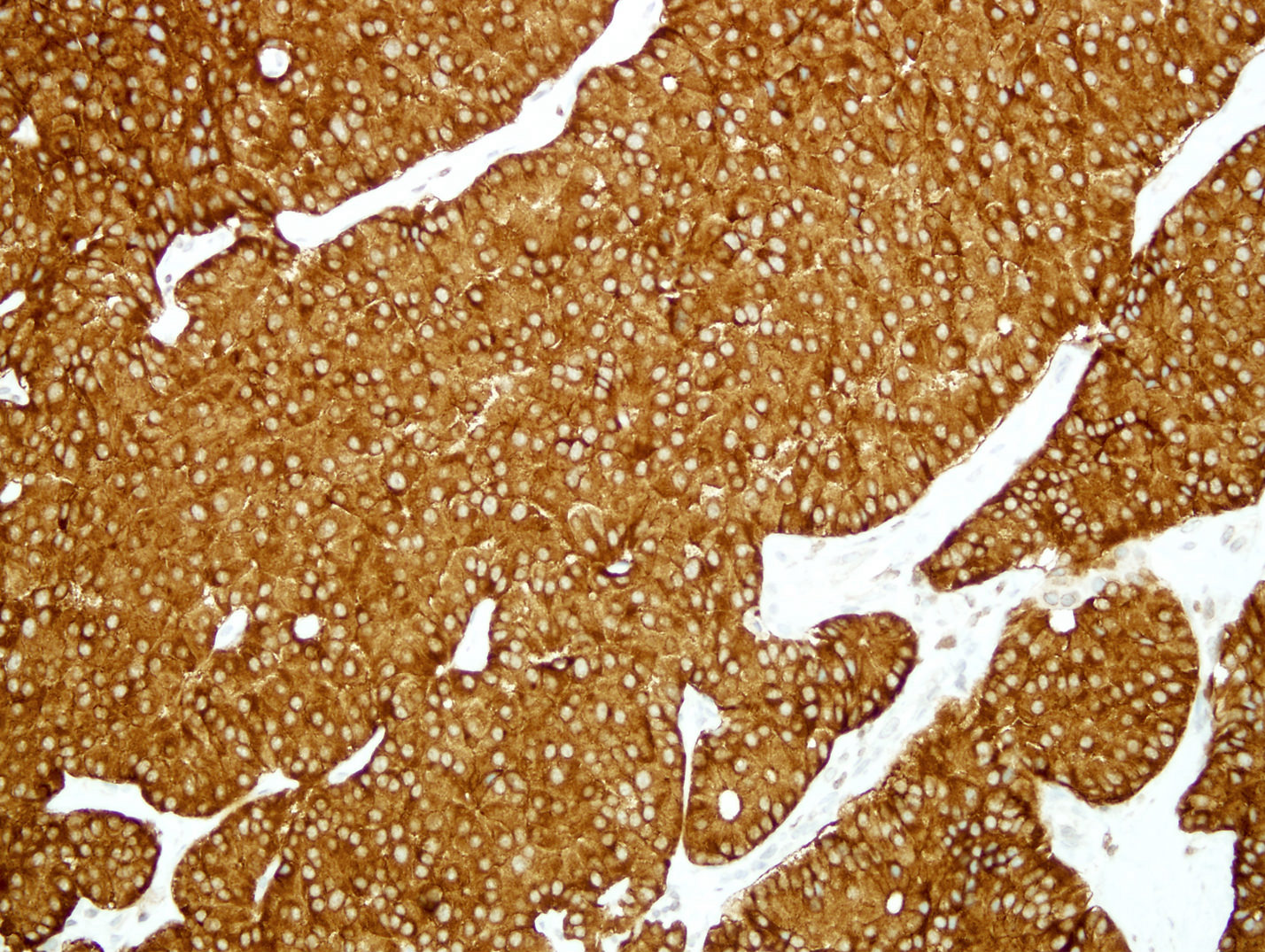
- Immunohistochemical positivity for BCL10 and trypsin (Virchows Arch 2009;454:133, Cancer Cytopathol 2013;121:459, Pathologica 2020;112:210)
Terminology
- Main category: acinar cell carcinoma
- Subtypes: acinar cell cystoadenocarcinoma, mixed acinar neuroendocrine carcinoma, mixed acinar ductal adenocarcinoma
ICD coding
- ICD-O: 8550/3 - acinar cell carcinoma
- ICD-11: 2C10.0 & XH3PG9 - adenocarcinoma of the pancreas & acinar cell carcinoma
Epidemiology
- M:F = 2.1:1 (Semin Diagn Pathol 2016;33:307, Am J Surg Pathol 2012;36:1782)
- Average age: 60 years (Semin Diagn Pathol 2016;33:307)
- 1 - 2% of all pancreatic neoplasms in adults and 15% in children (Semin Diagn Pathol 2016;33:307)
Sites
- Head of the pancreas is the most common site (Semin Diagn Pathol 2016;33:307)
Pathophysiology
- Accumulation of genetic alteration, including chromosomal instability and frequent allelic copy number variation
Etiology
- Tobacco smoking, defective DNA repair; presence of chromosomal instability and frequent allelic copy number variation (Nat Commun 2017;8:1323)
- Most cases are sporadic but a minority (
Clinical features
- Presenting symptoms include abdominal or back pain, weight loss, nausea, vomiting; jaundice is more rarely observed than in ductal adenocarcinoma (Am J Surg Pathol 1992;16:815)
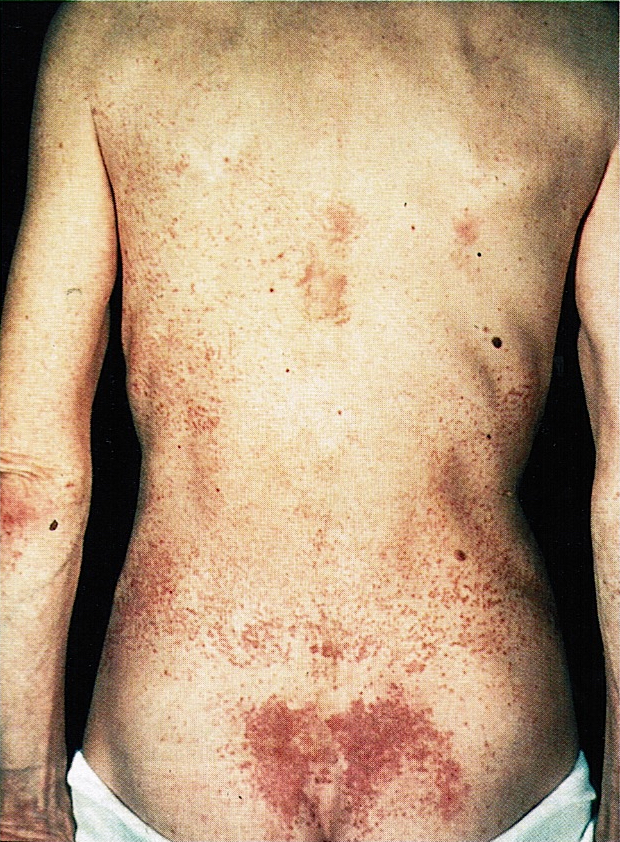
- In the case of extensive metastatic disease, patients may show symptoms related to lipase hypersecretion, including subcutaneous fat necrosis (Front Med (Lausanne) 2015;2:41)
Diagnosis
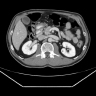
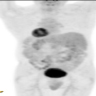
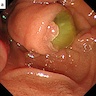
- CT scan and MRI are the preferred imaging modality (J Belg Soc Radiol 2019;103:43)
- Diagnosis is by biopsy or surgical resection
Laboratory
- Rarely, patients can show increased levels of AFP, especially young patients (Hum Pathol 2000;31:938)
- Patients with metastatic disease can show elevated levels of serum lipase (World J Clin Cases 2020;8:5304)
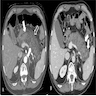
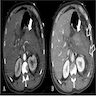
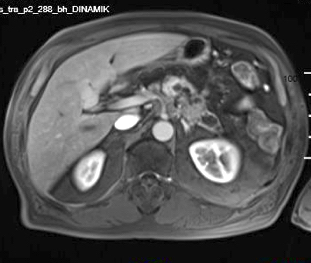
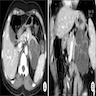
Radiology description
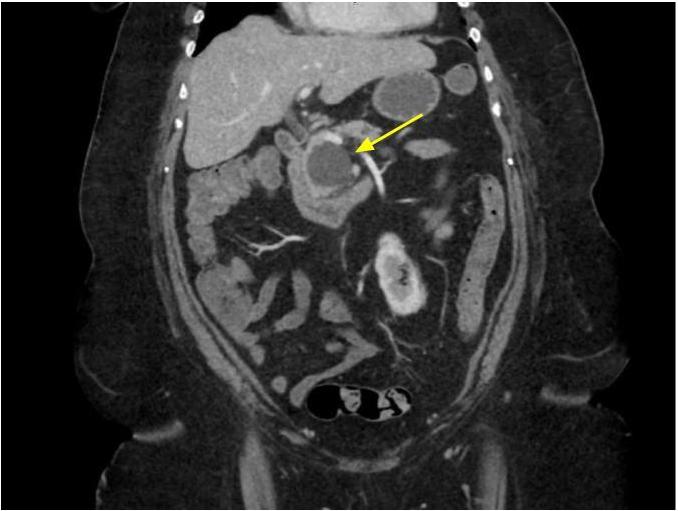
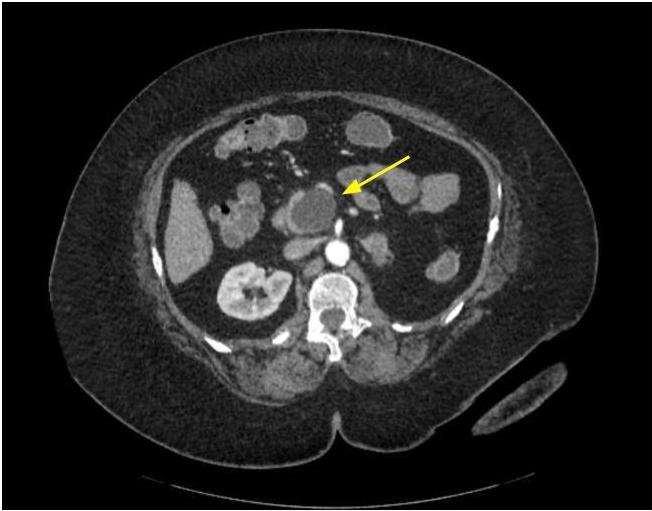
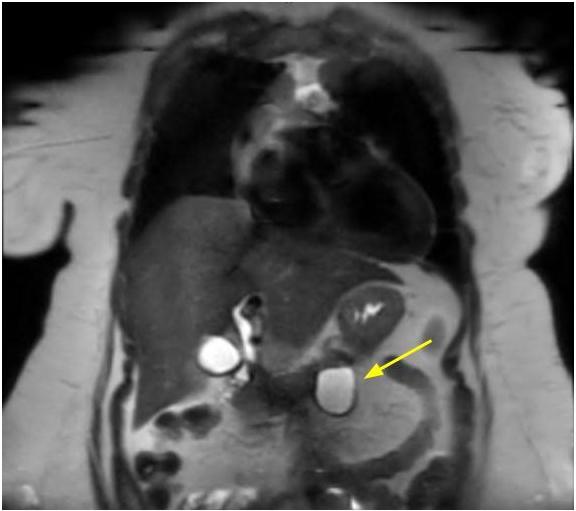
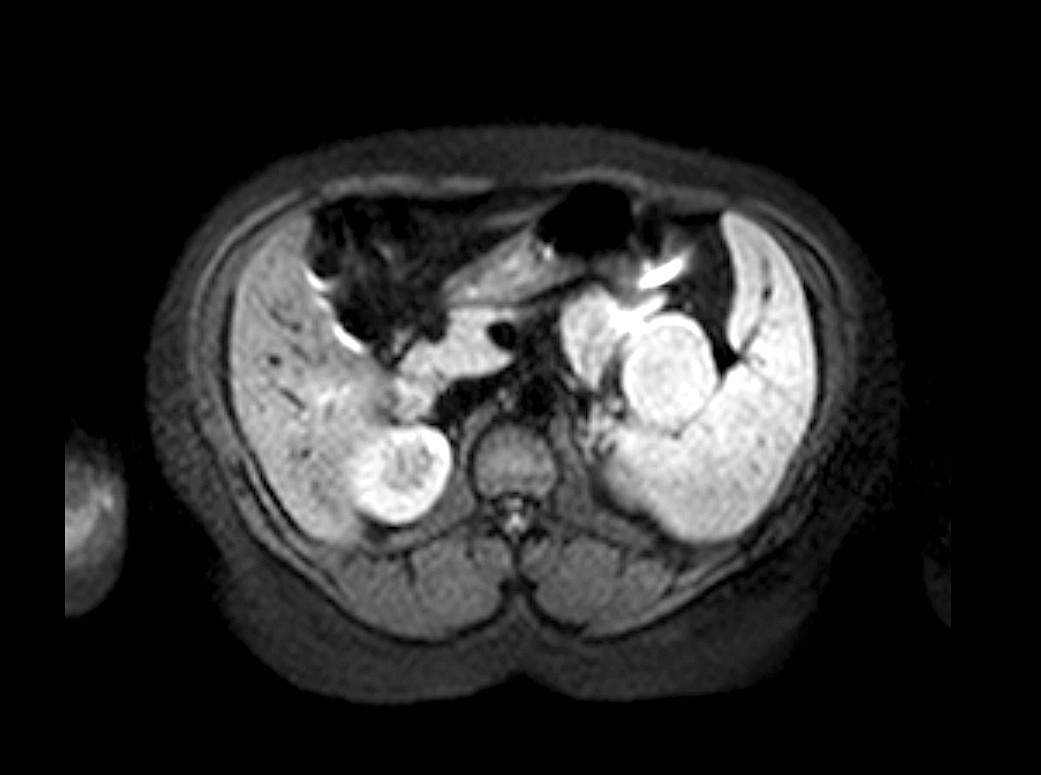
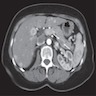
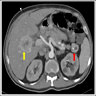
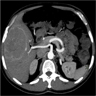
- Typical CT and MRI features of pancreatic acinar cell carcinoma: relatively large mass with a well defined margin, exophytic growth and heterogeneous enhancement (J Belg Soc Radiol 2019;103:43)
Prognostic factors
- The prognosis is poor, with an average survival time of about 19 months (Semin Diagn Pathol 2016;33:307)
- To date, the TNM stage can be considered the only significant prognostic moderator (Am J Surg Pathol 2012;36:1782)
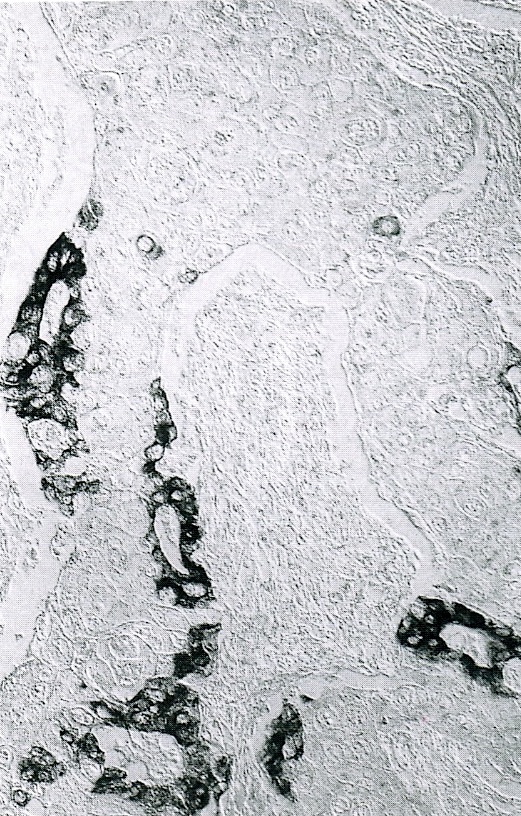
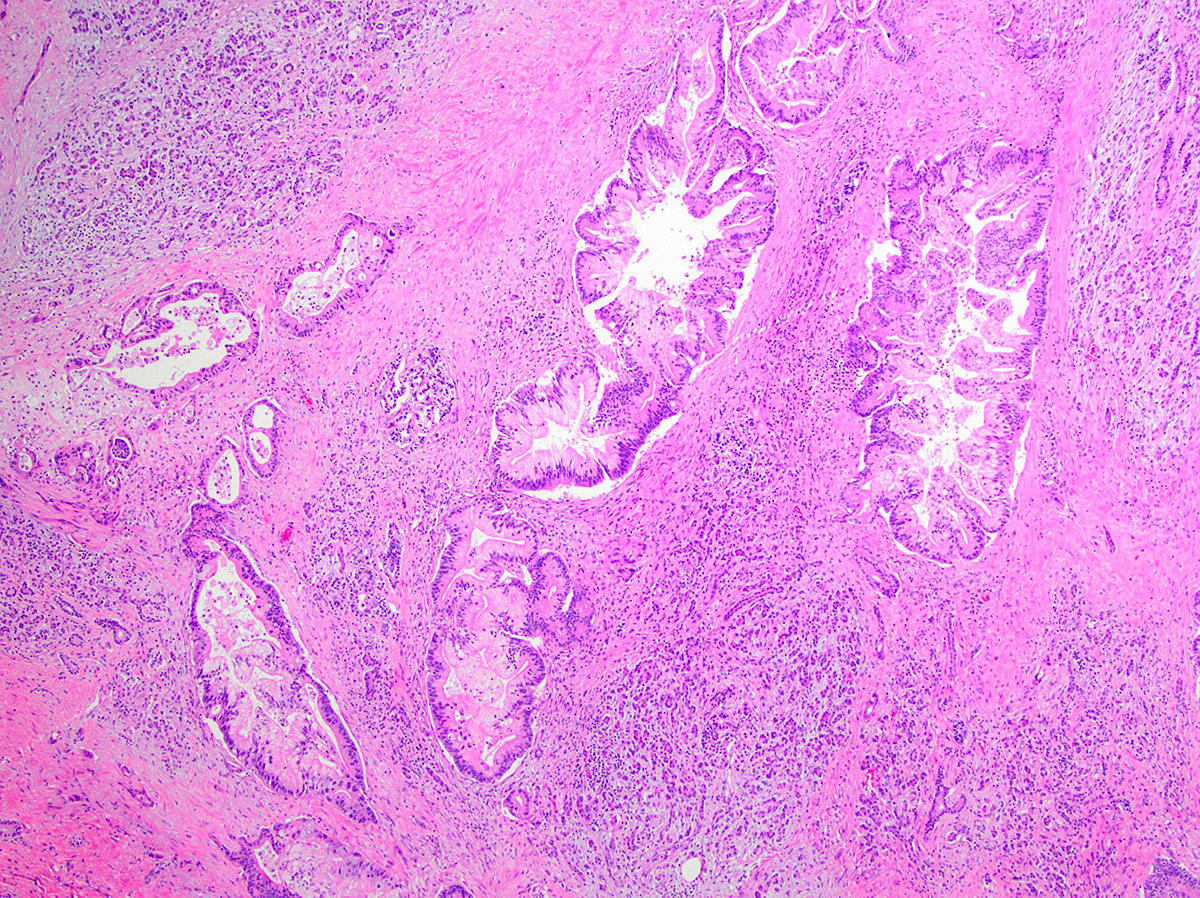
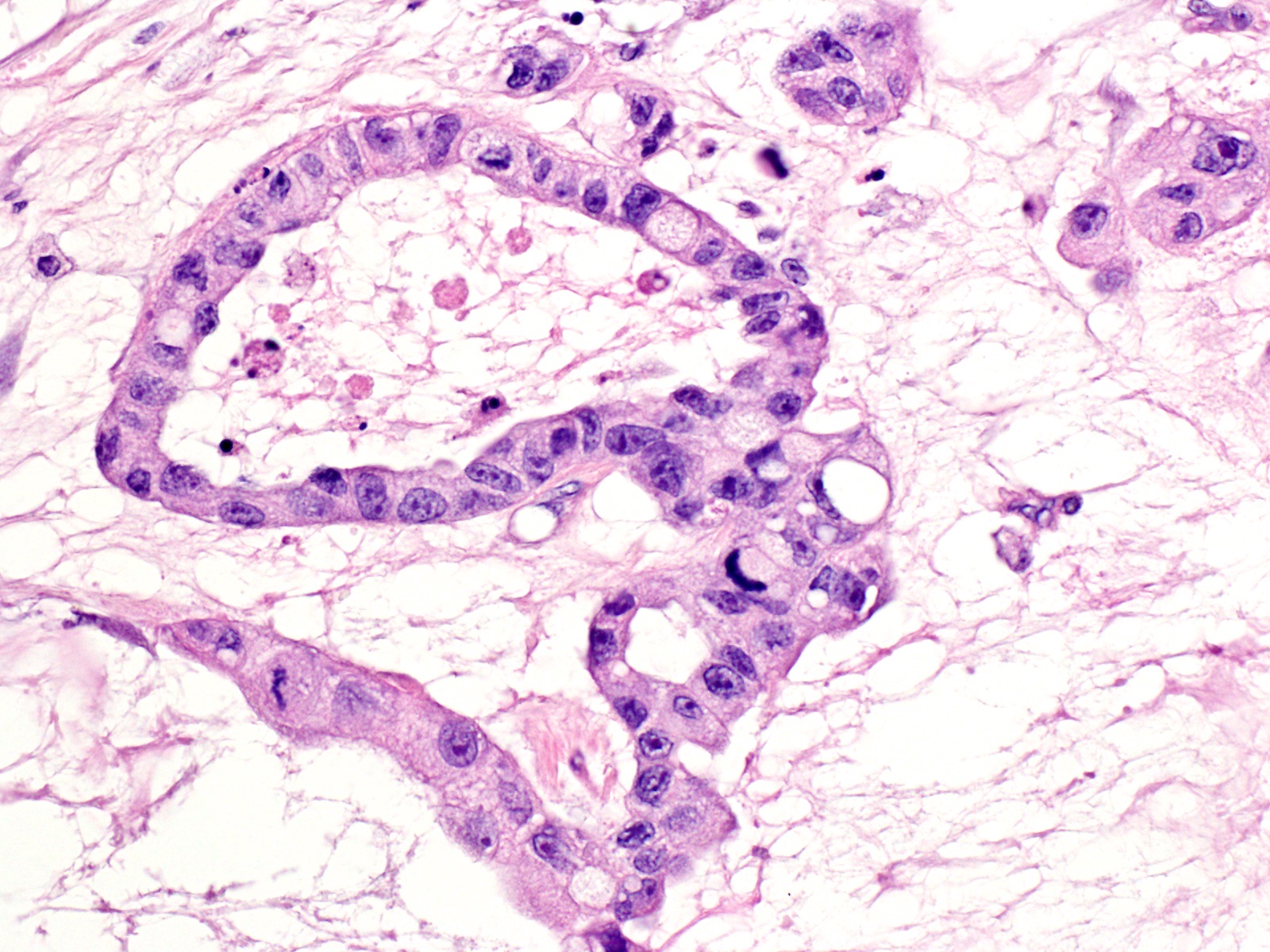
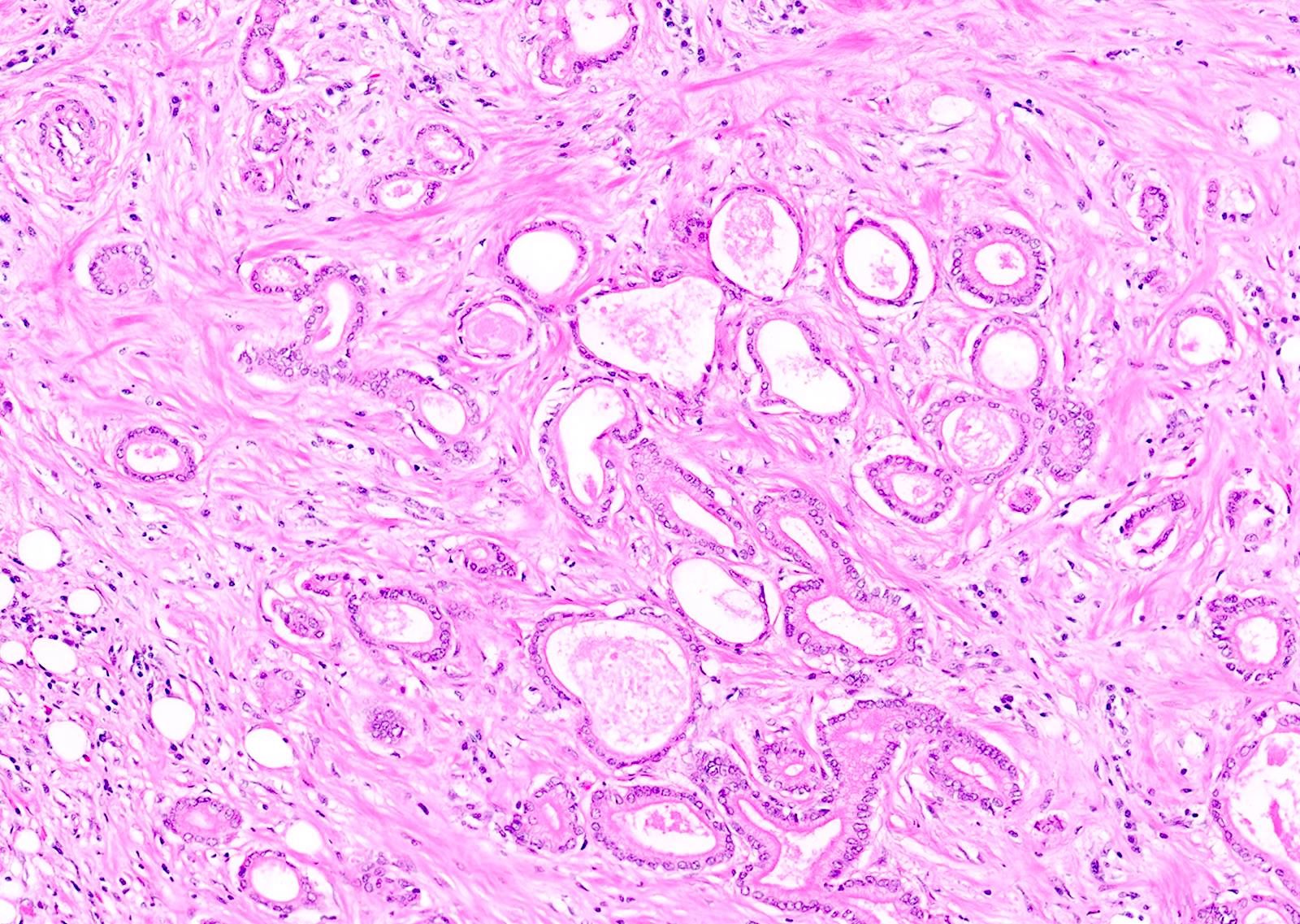
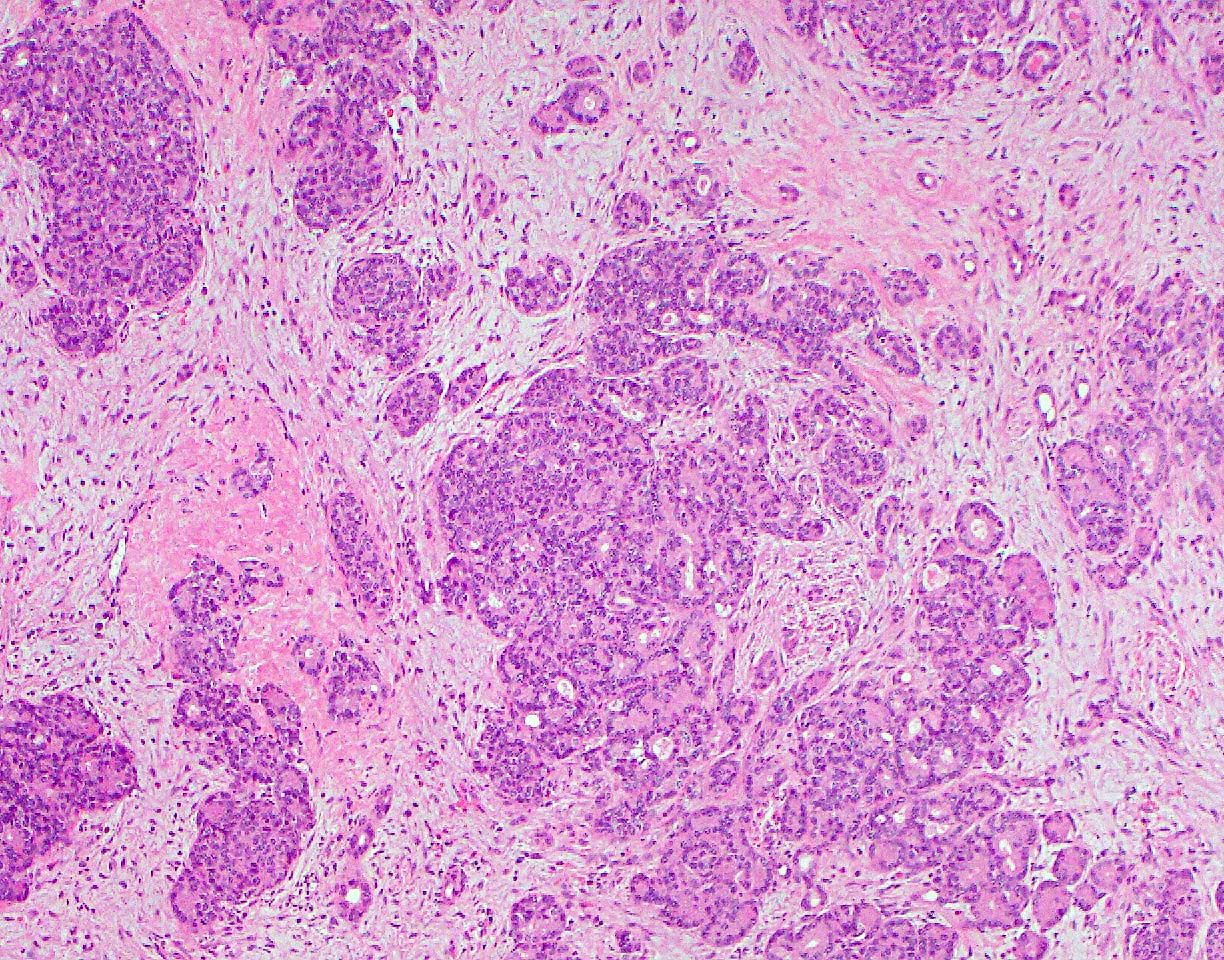
- Tumors with intraductal growth display a better prognosis than conventional acinar cell carcinomas (Am J Surg Pathol 2007;31:363)
Case reports
- 41 and 77 year old men presenting with acinar cell carcinoma extensively involving the pancreas (Medicine (Baltimore) 2017;96:e7904)
- 52 year old man with acinar cell carcinoma of the pancreas harboring BRCA2 germline mutation (Cancer Biol Ther 2019;20:949)
- 69 year old man with acinar cell carcinoma of the pancreas mixed with ductal adenocarcinoma (World J Surg Oncol 2020;18:238)
- 71 year old man with acinar cell carcinoma of the pancreas, with extension into the main pancreatic duct (Surg Case Rep 2021;7:90)
- 81 year old man with acinar cell carcinoma of the pancreas harboring NTRK fusion gene, with exceptional response to molecularly based target therapy (J Natl Compr Canc Netw 2021;19:10)
Treatment
- Surgical resection if possible, gemcitabine based chemotherapy / radiofrequency ablation, molecularly based target therapy in the case of actionable alterations (World J Clin Cases 2020;8:1241)
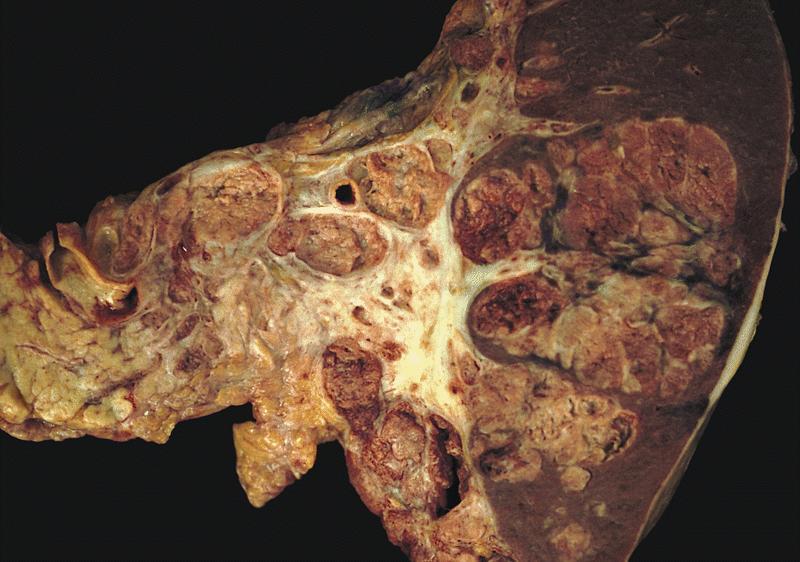
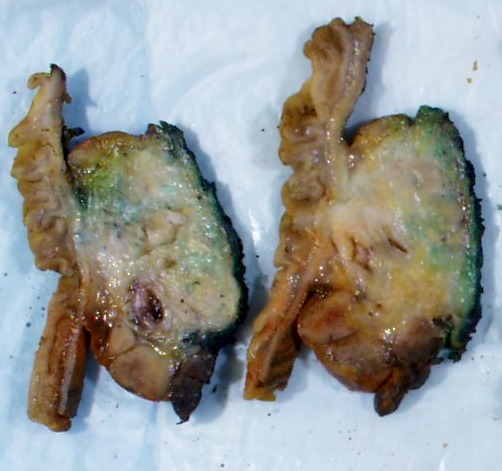
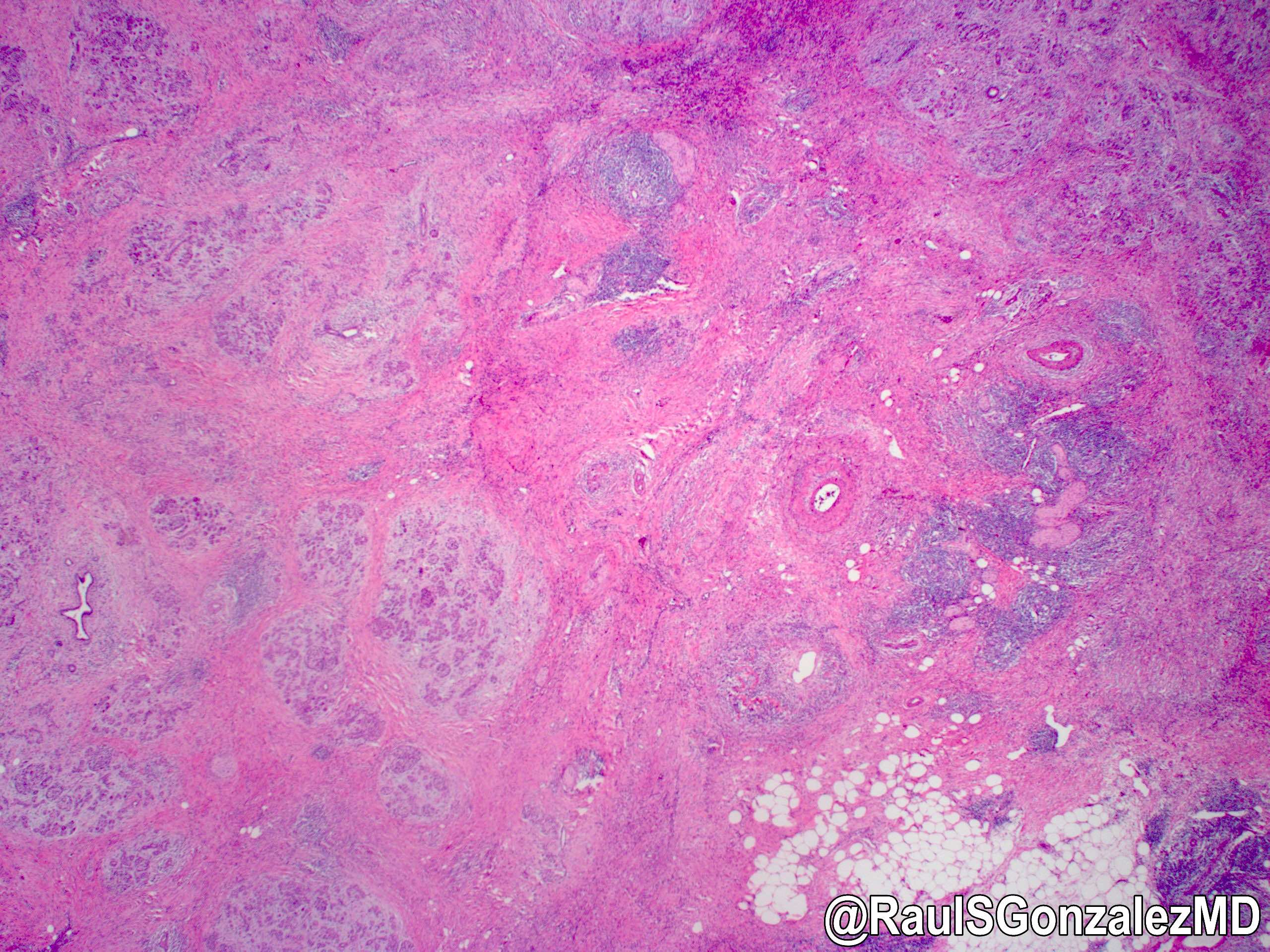
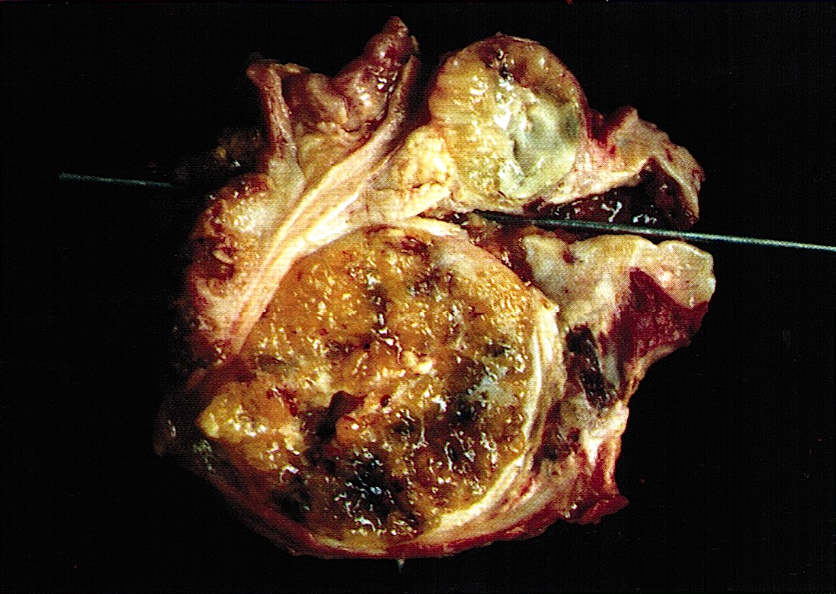
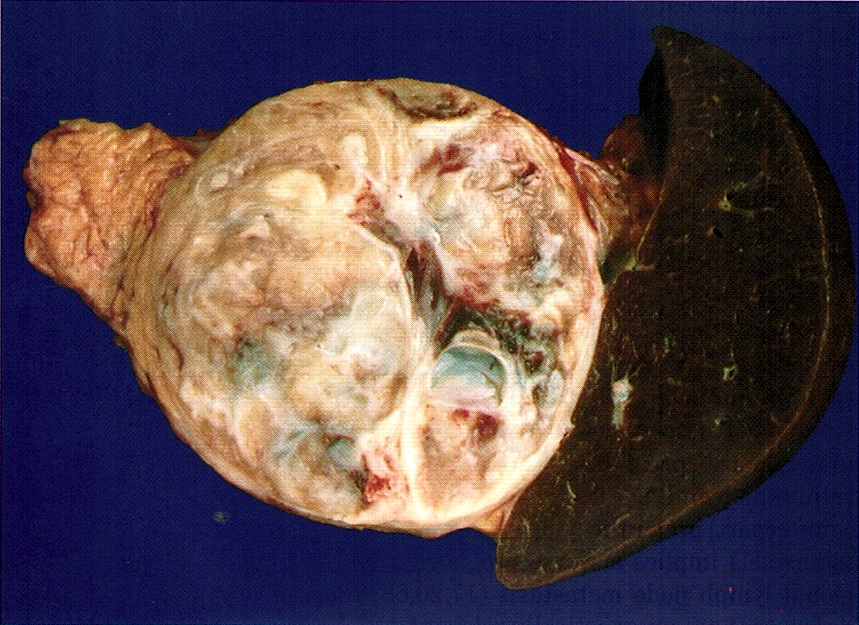
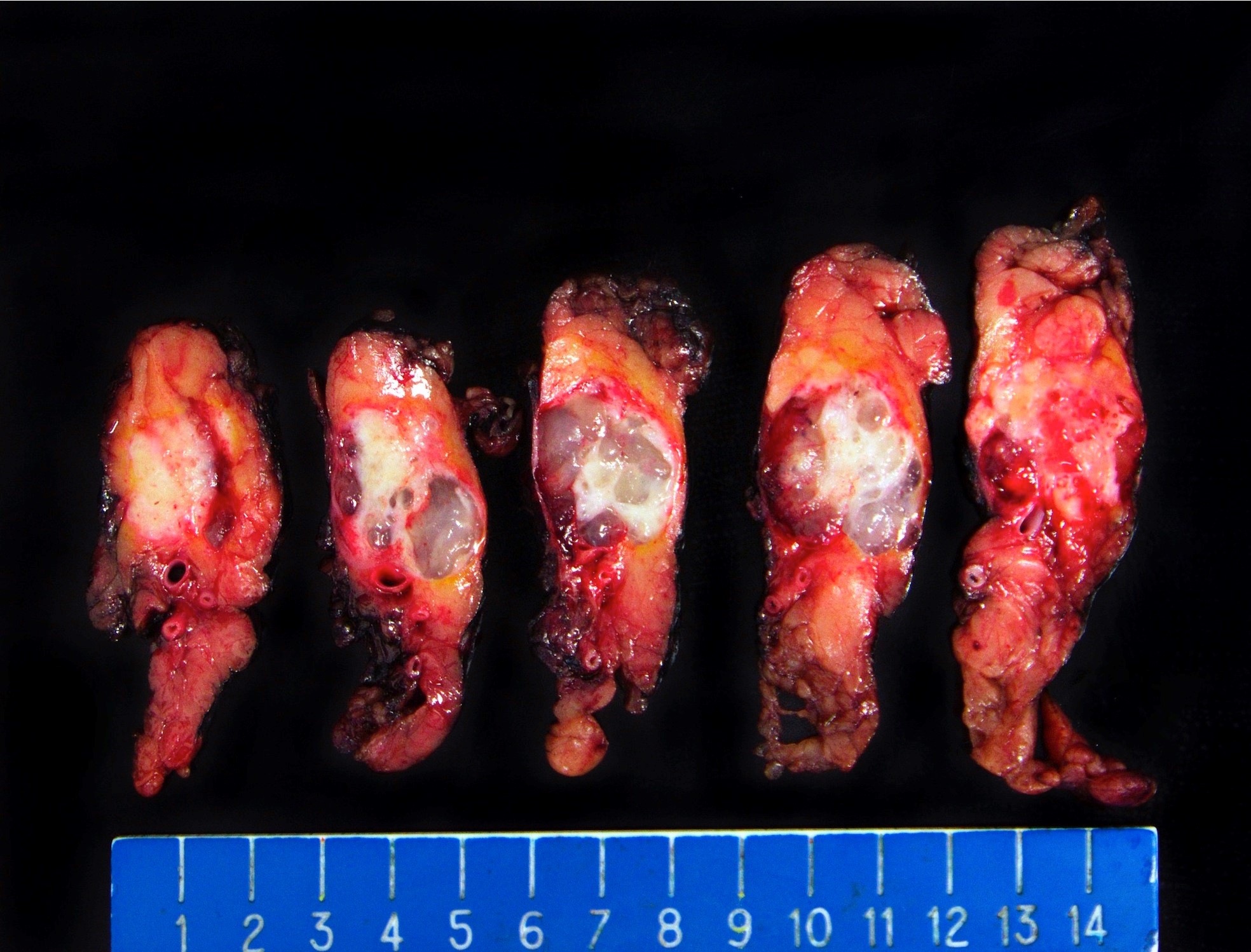
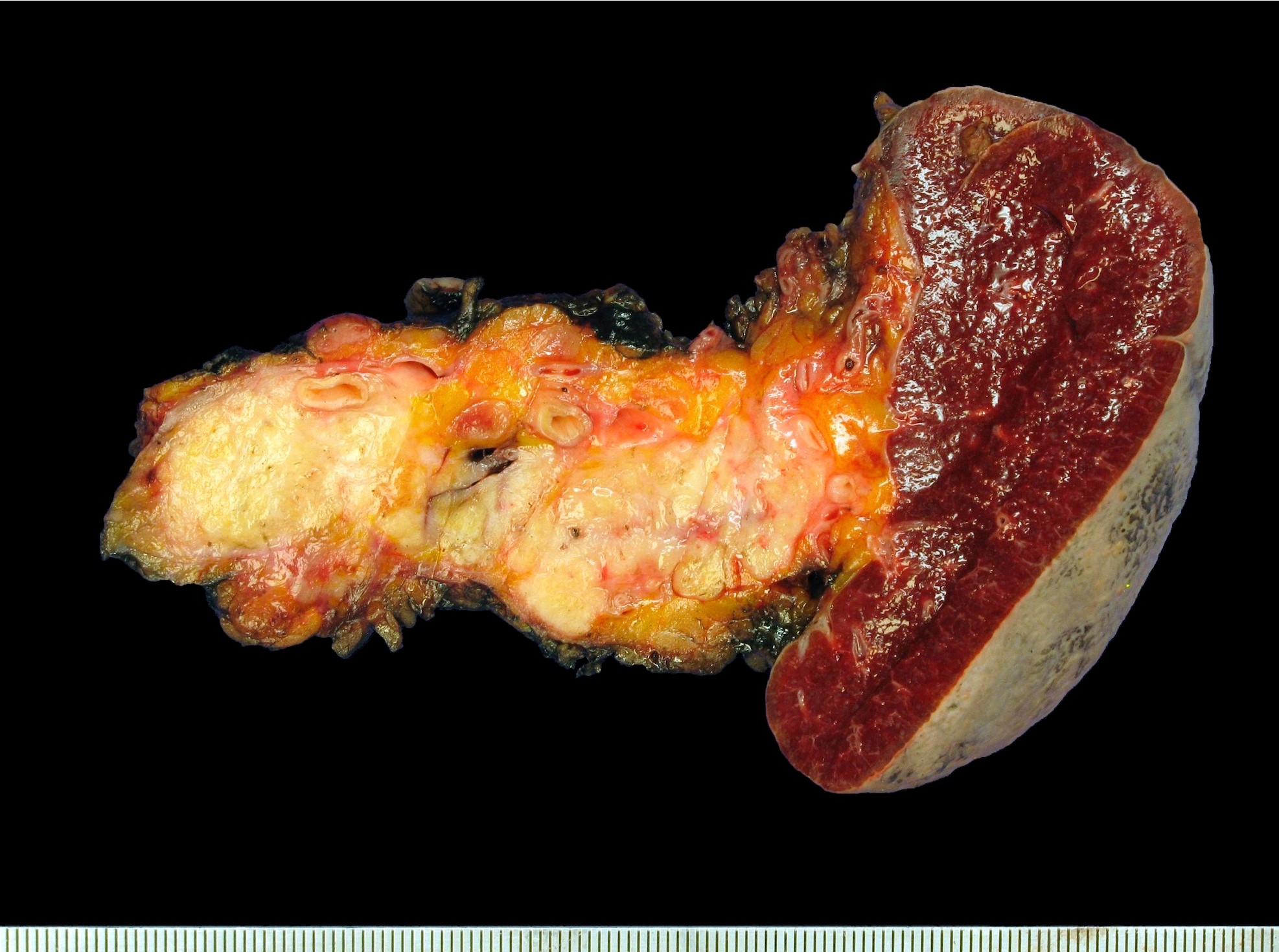
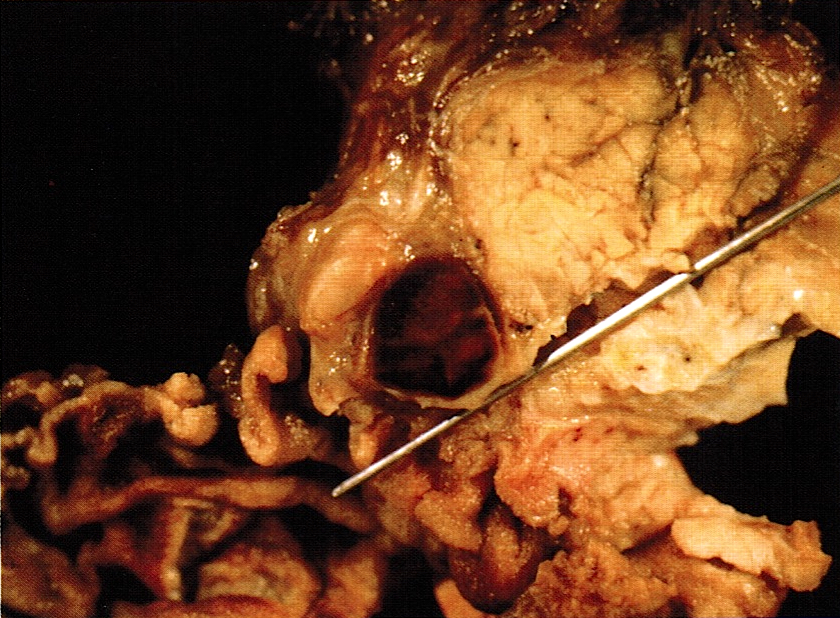
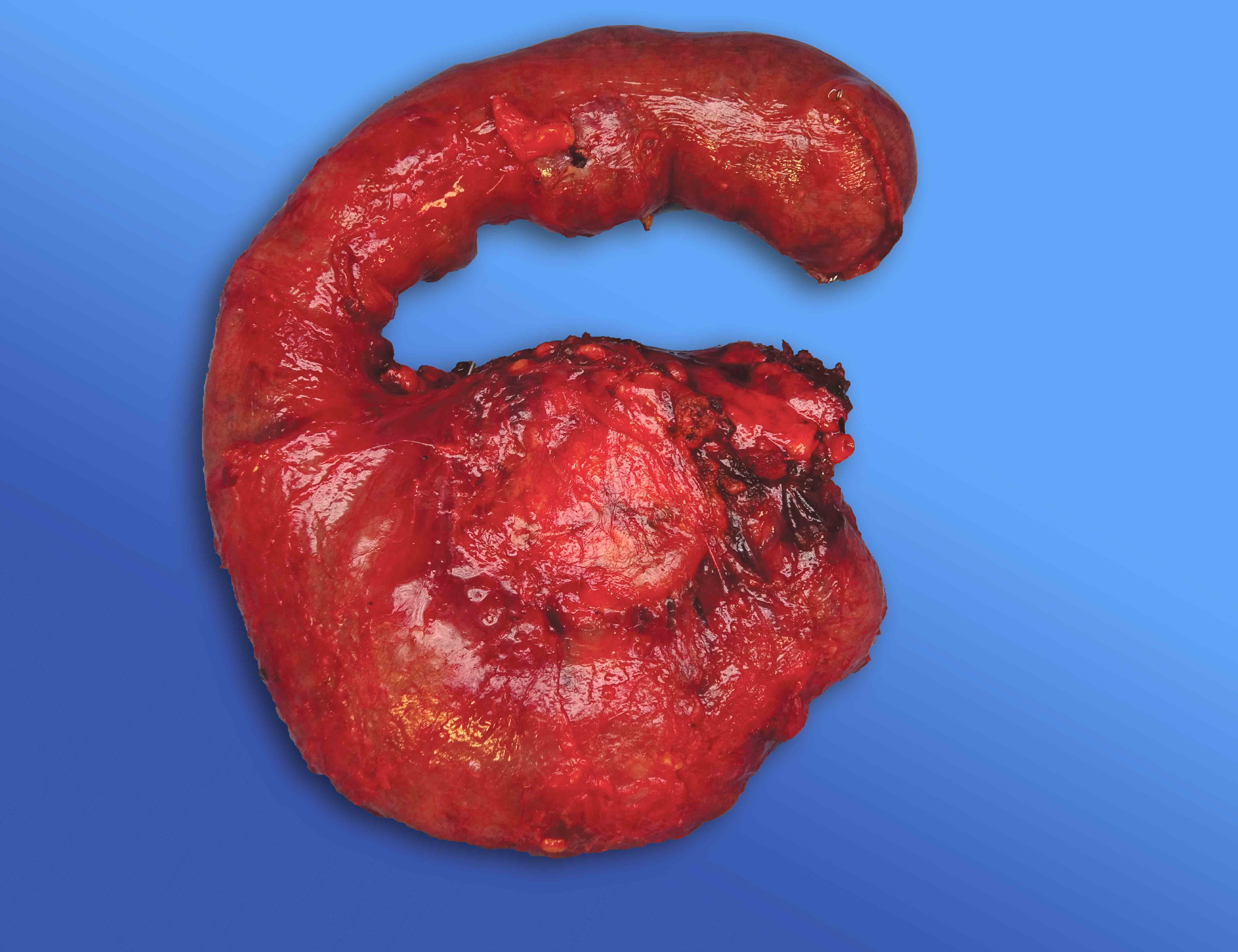
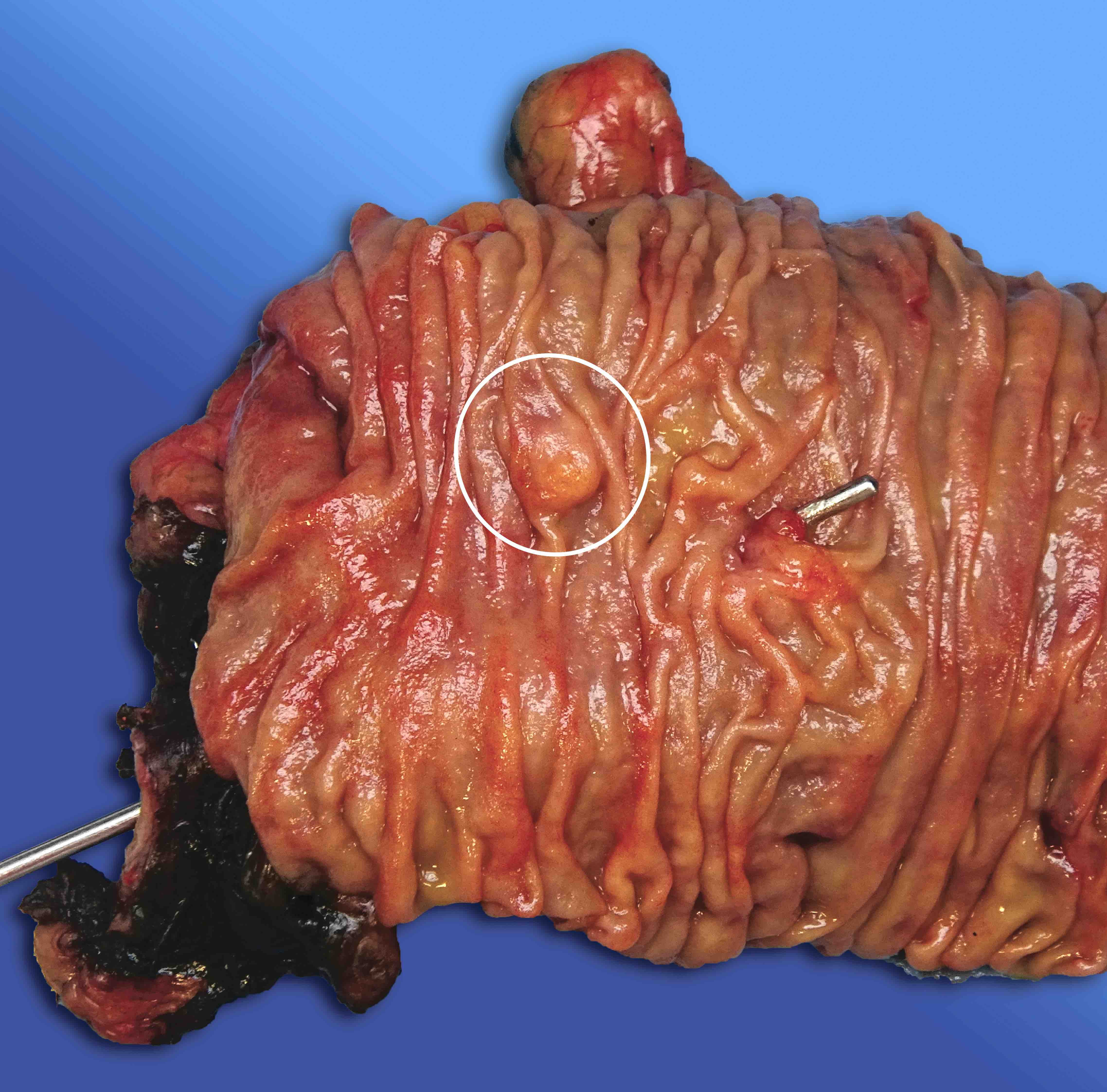
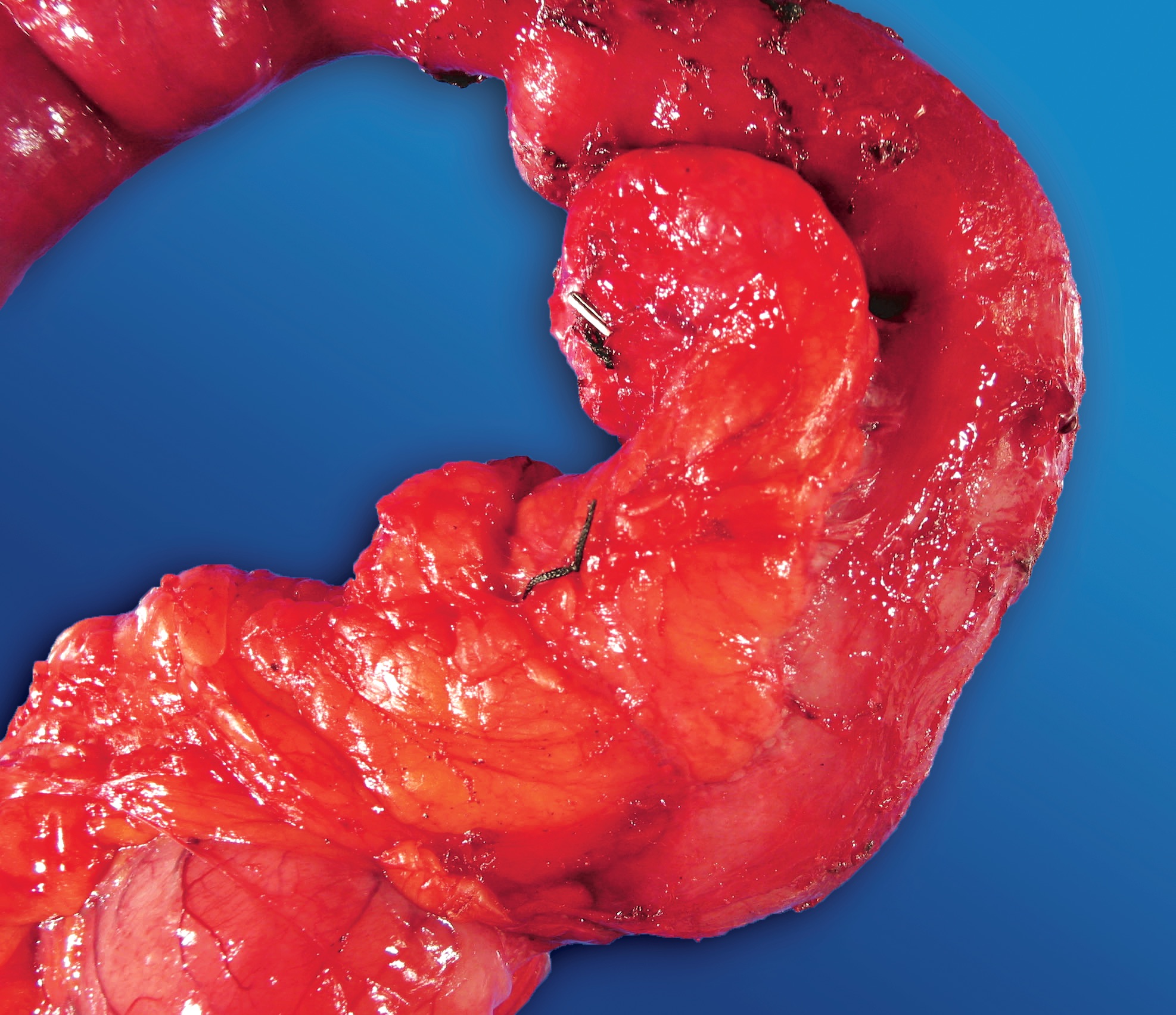
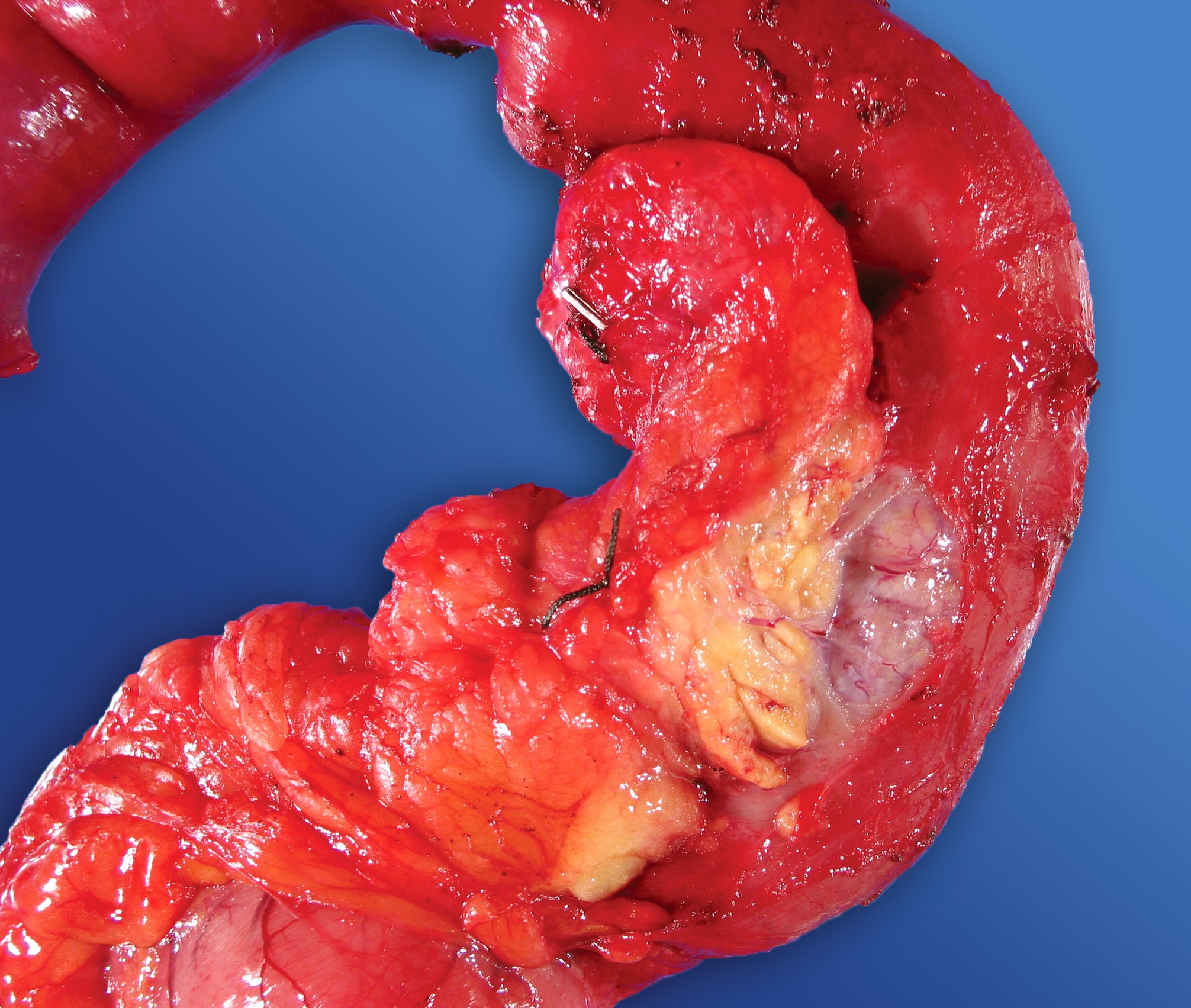
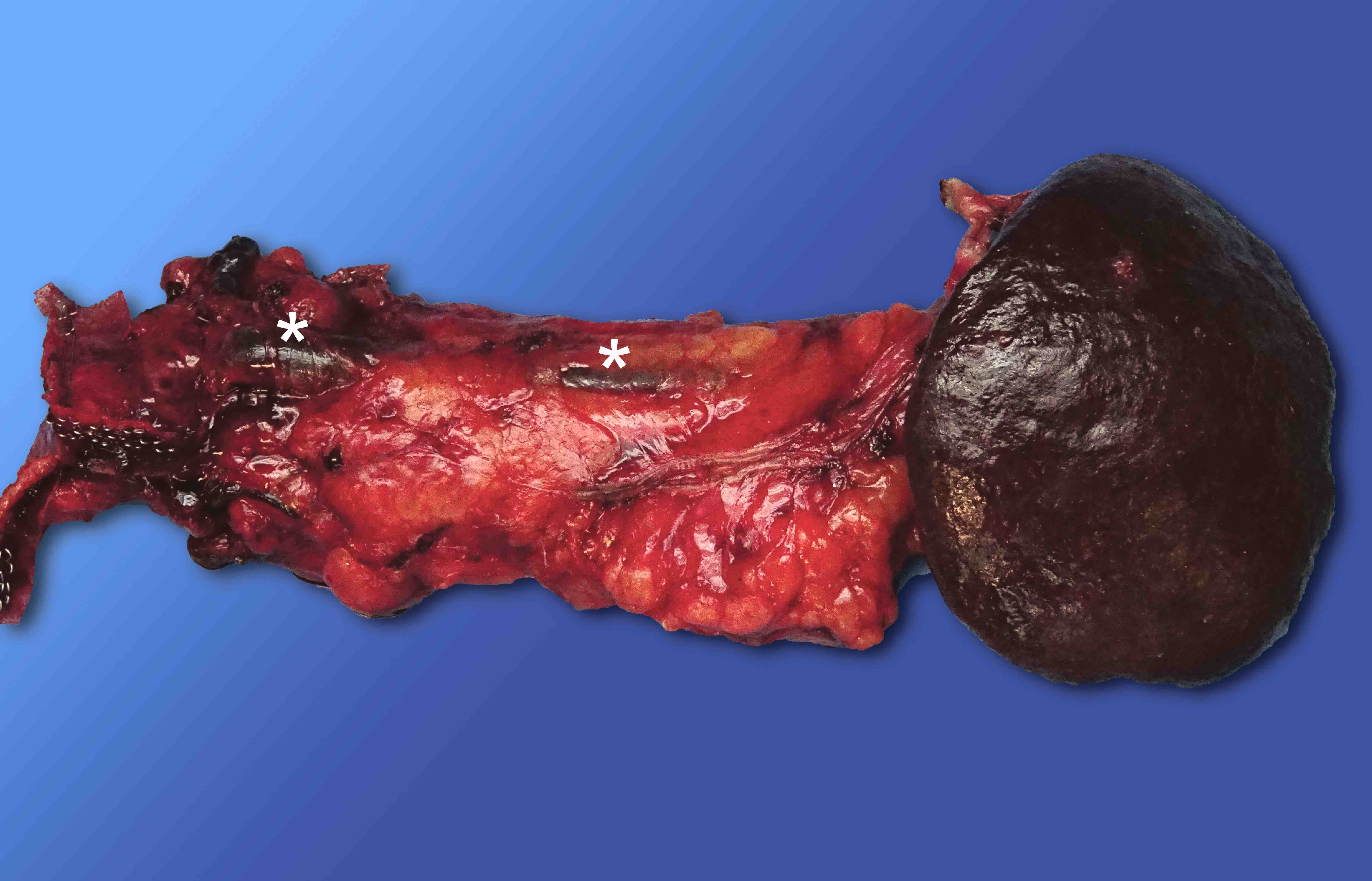
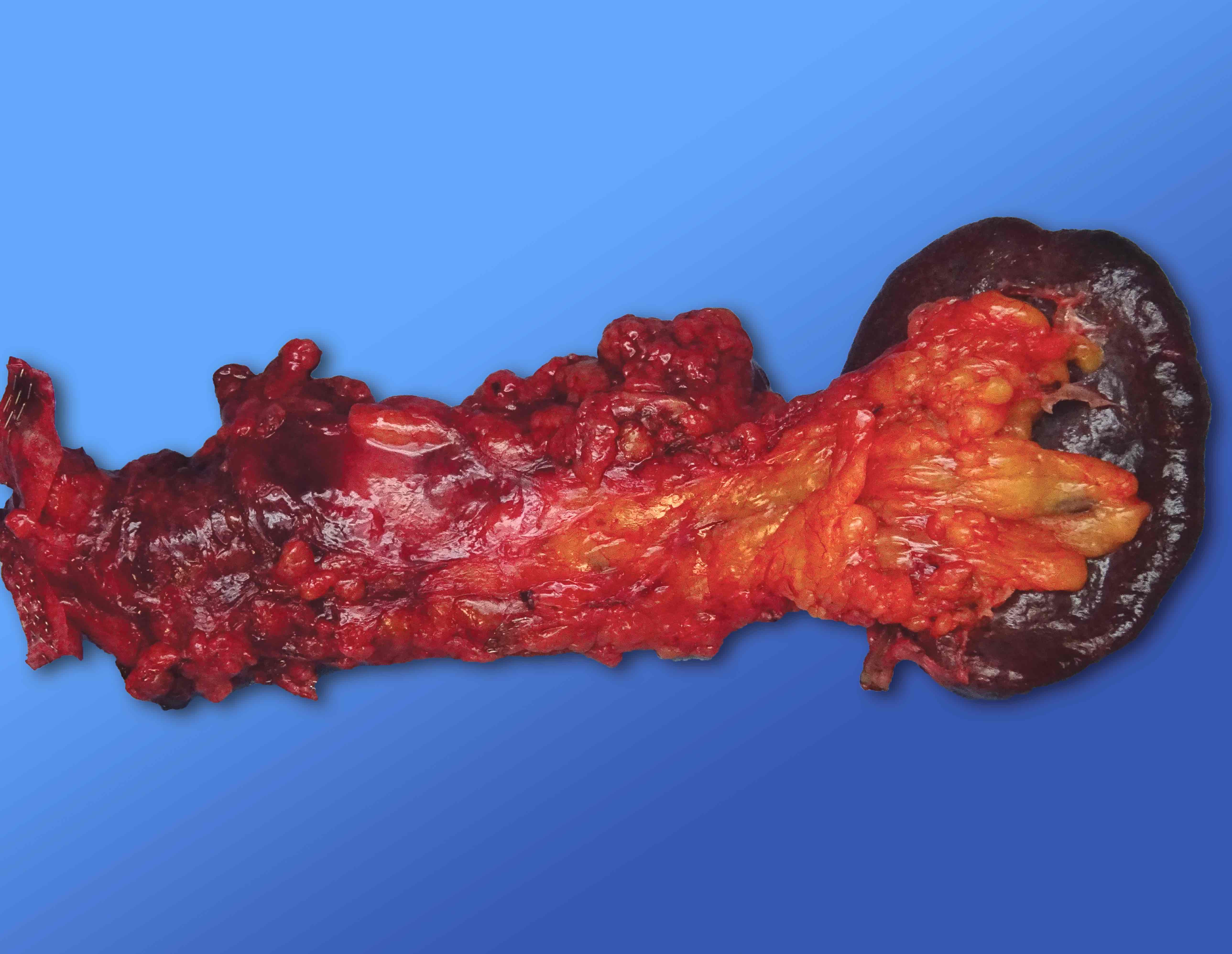
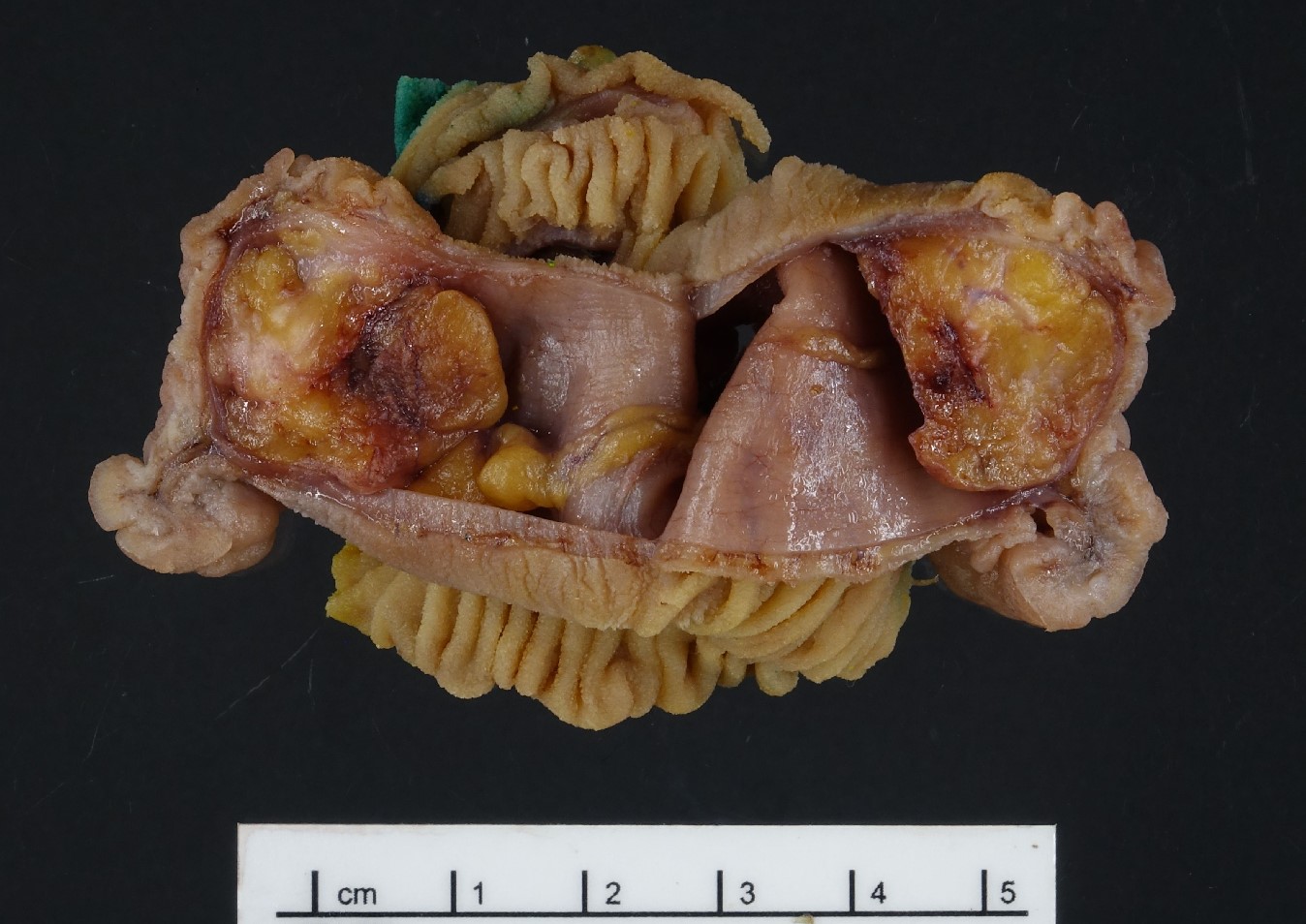
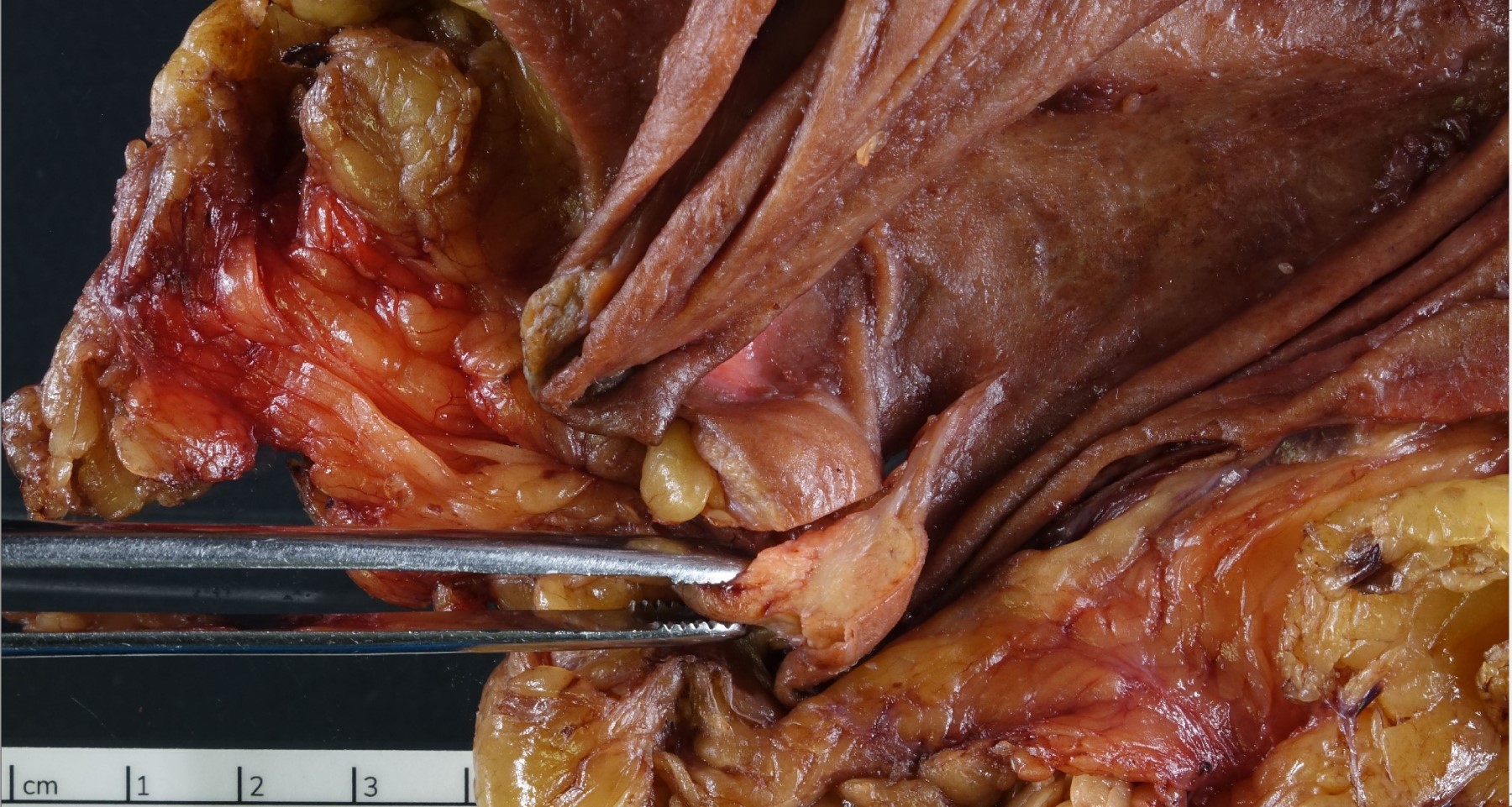
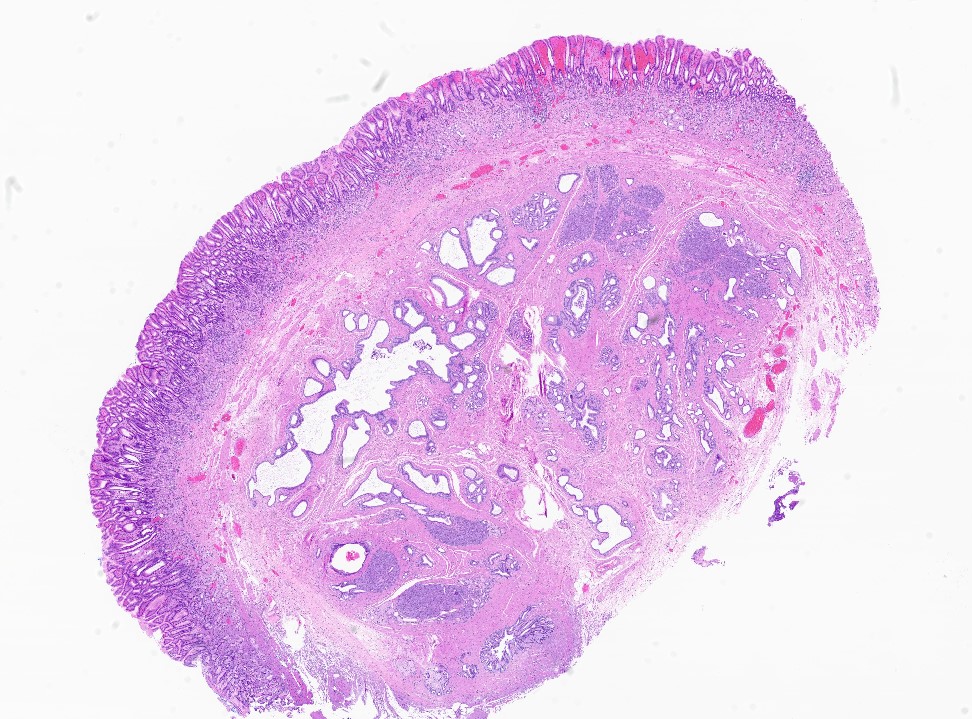
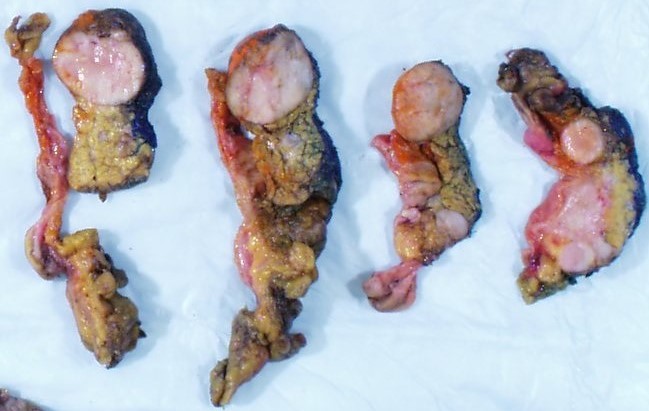
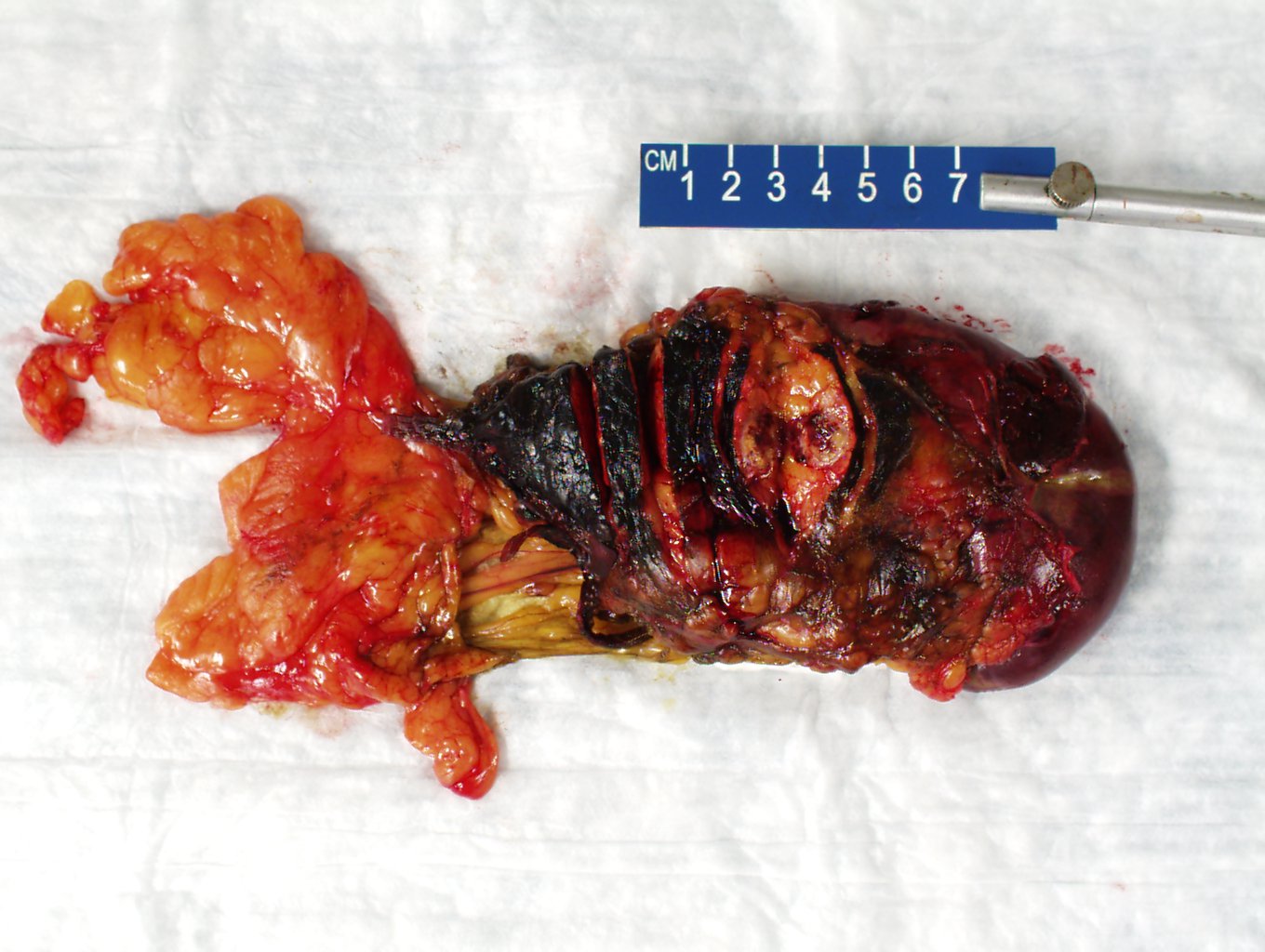
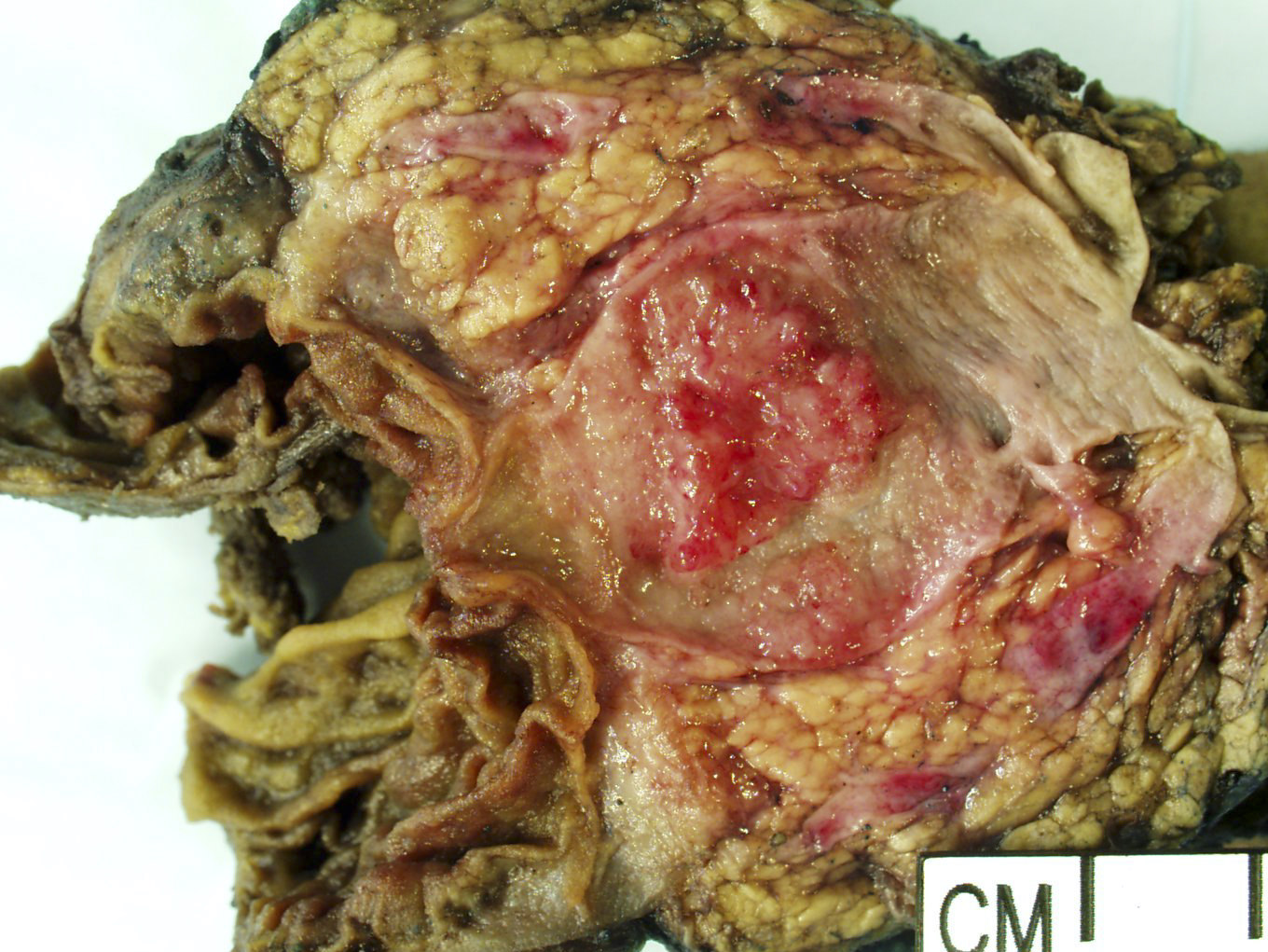
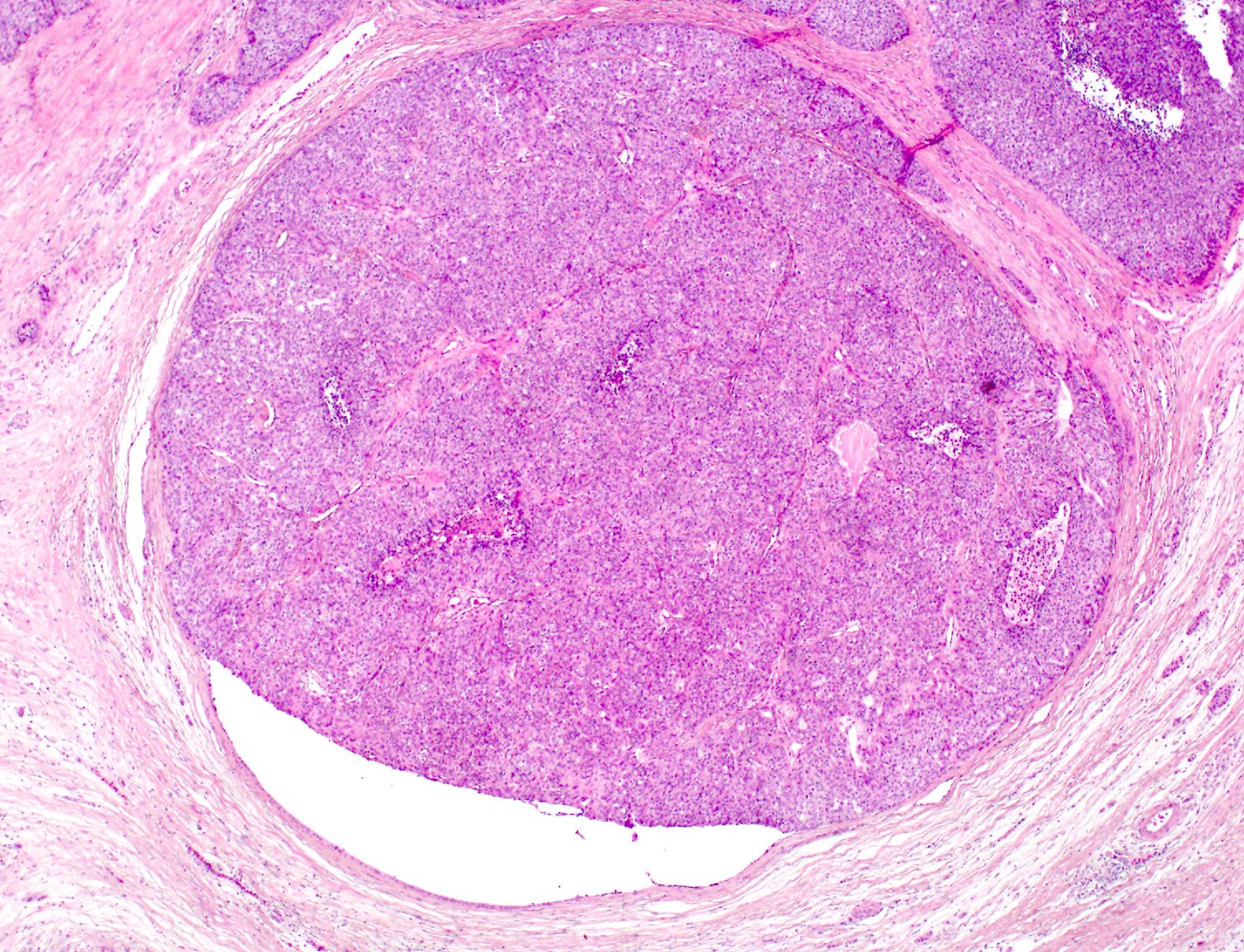
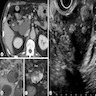
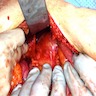
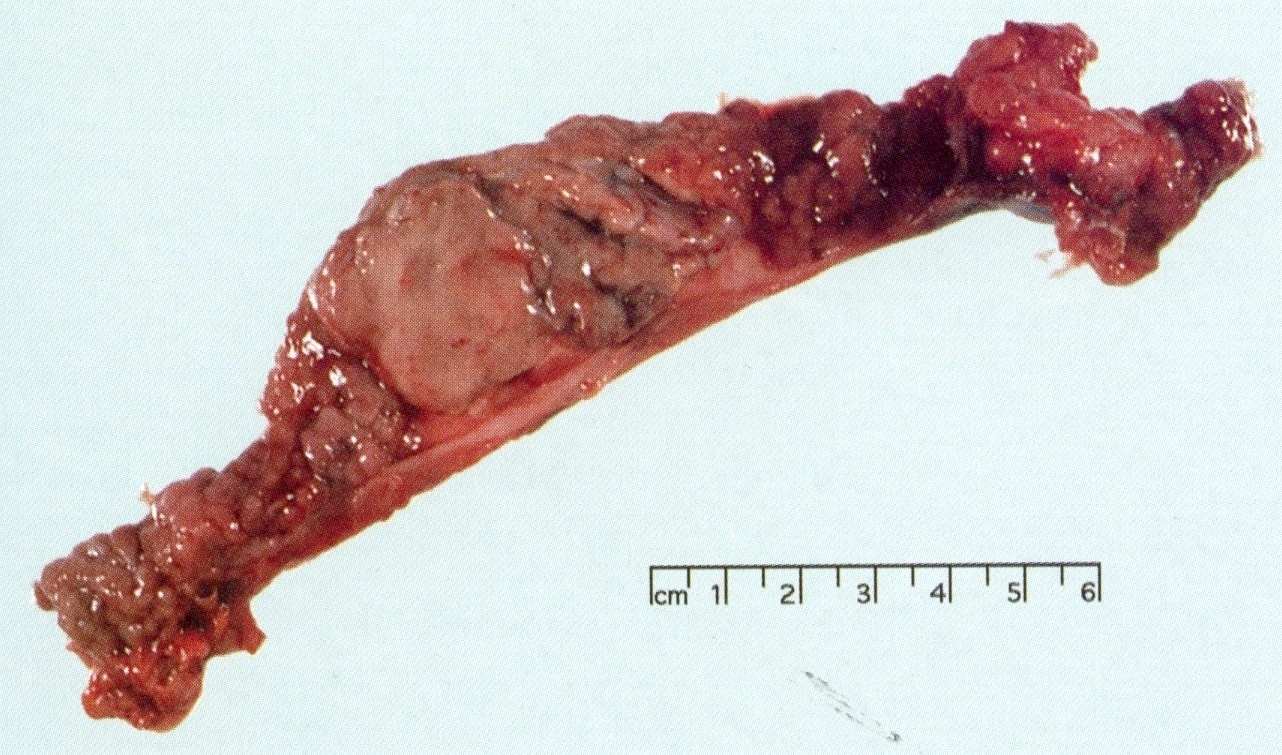
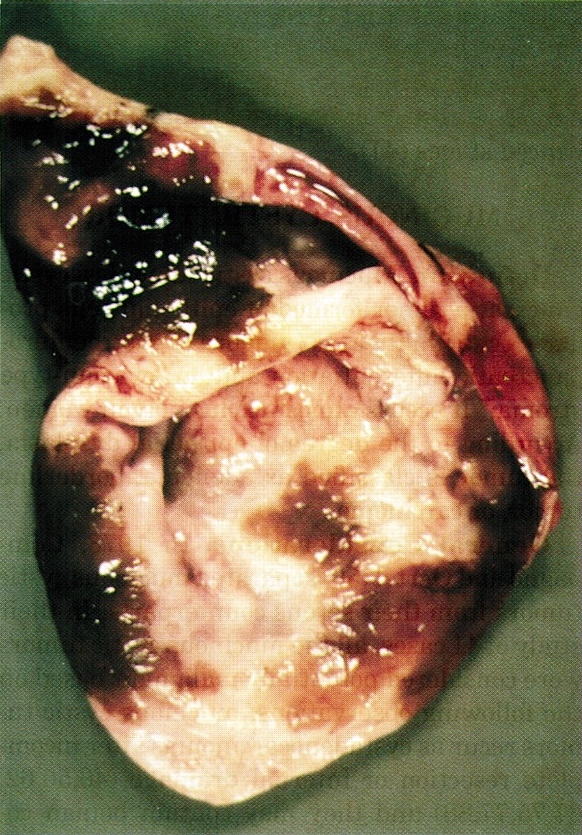
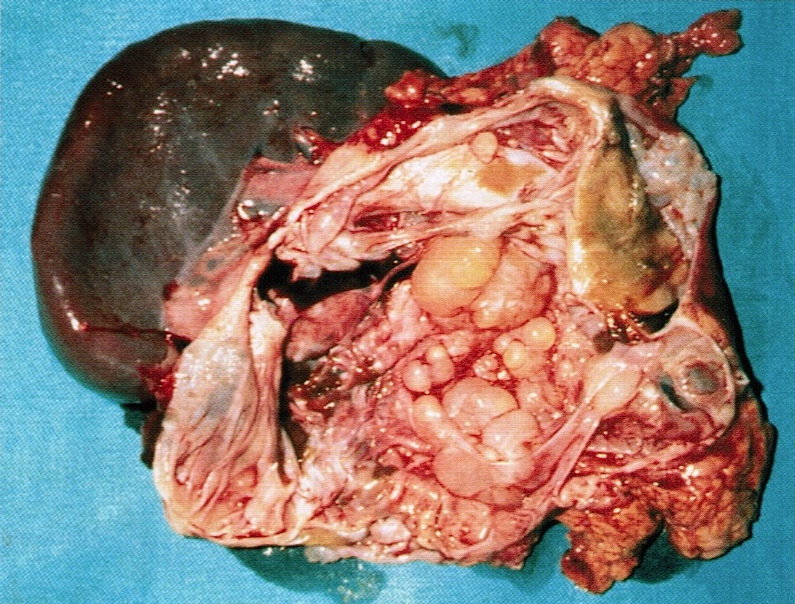
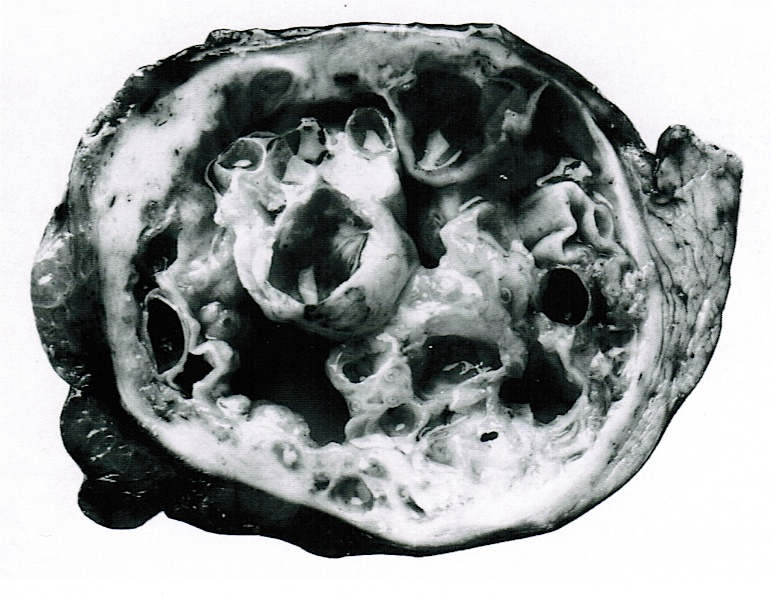
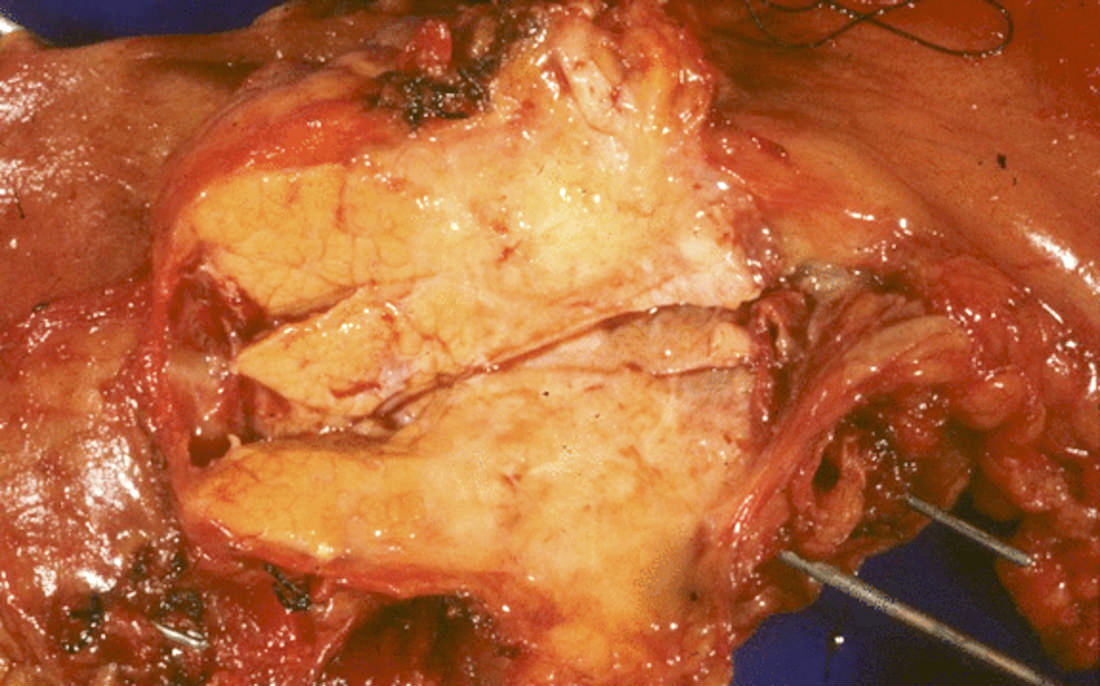
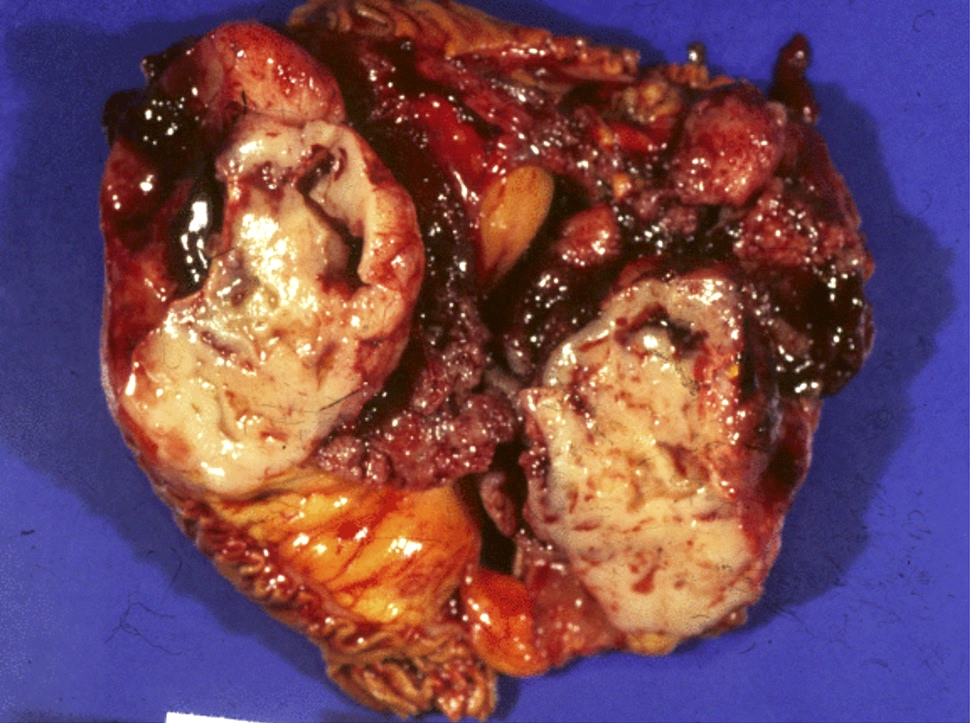
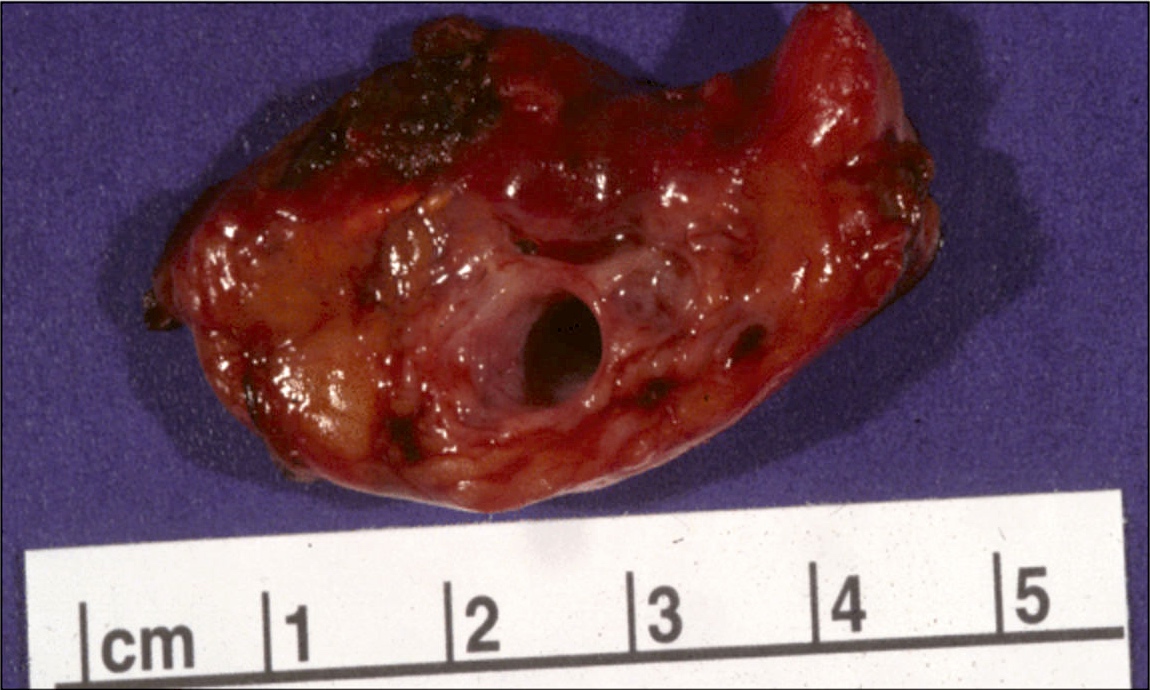
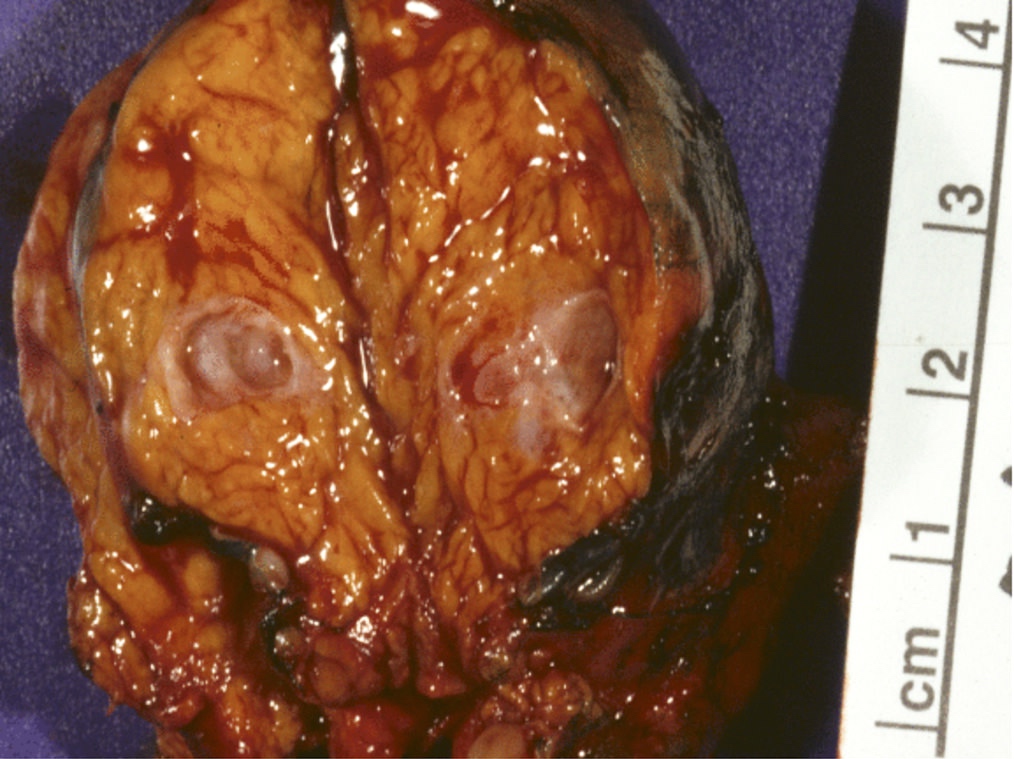
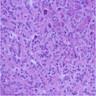
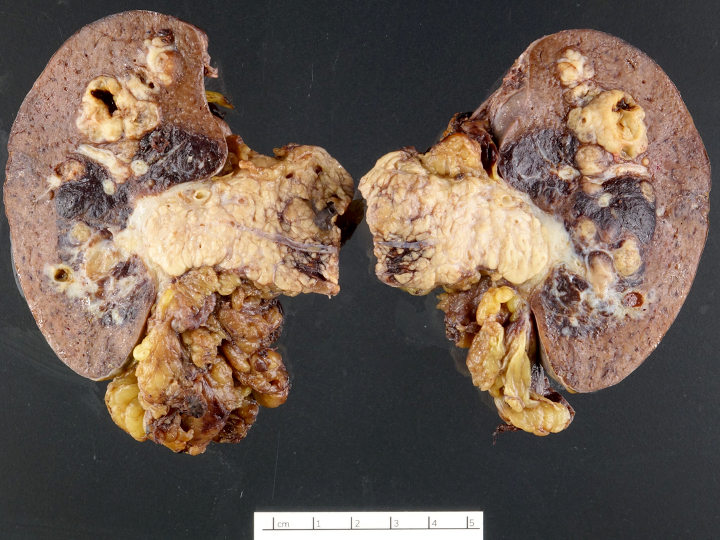
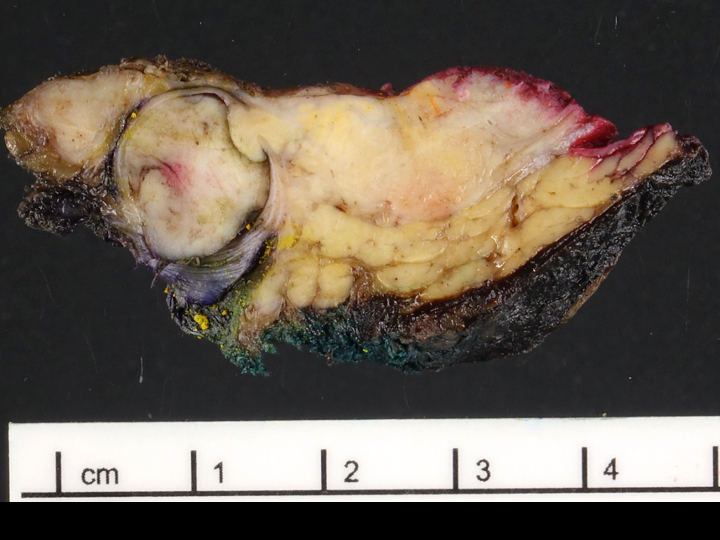
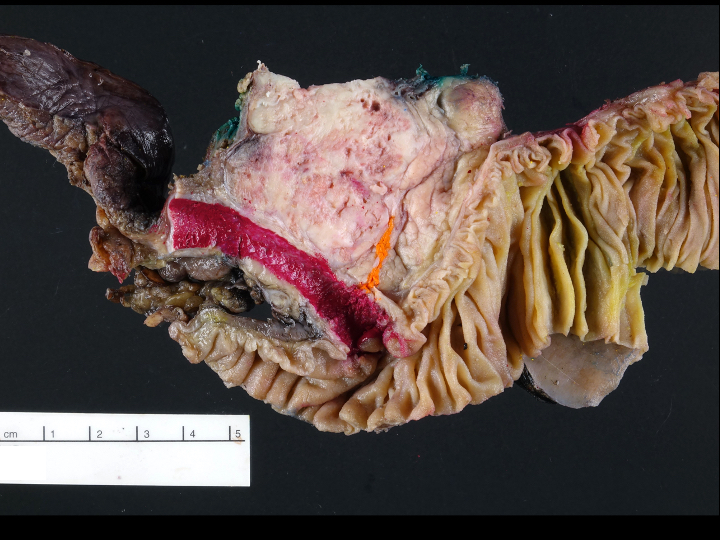
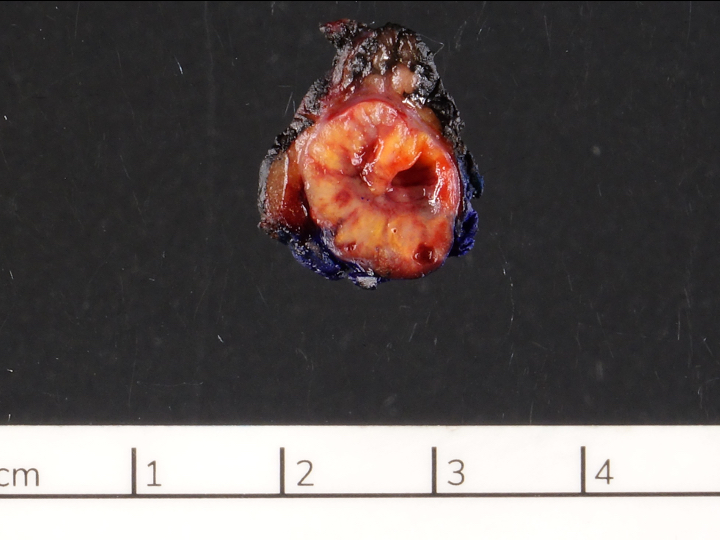
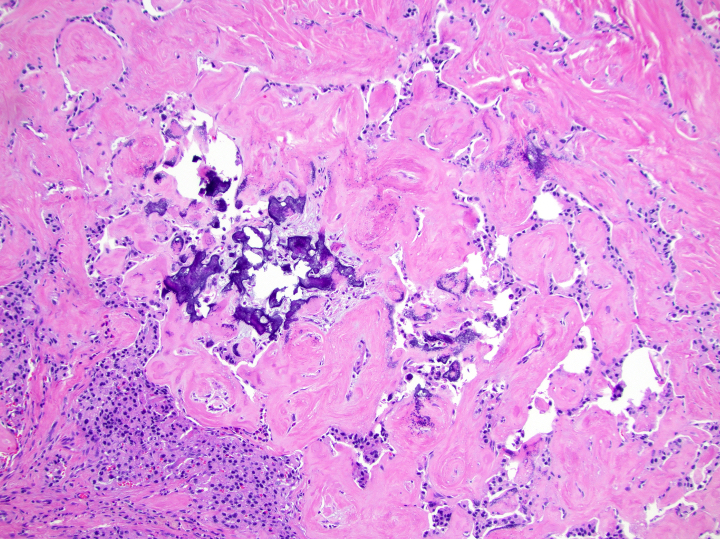
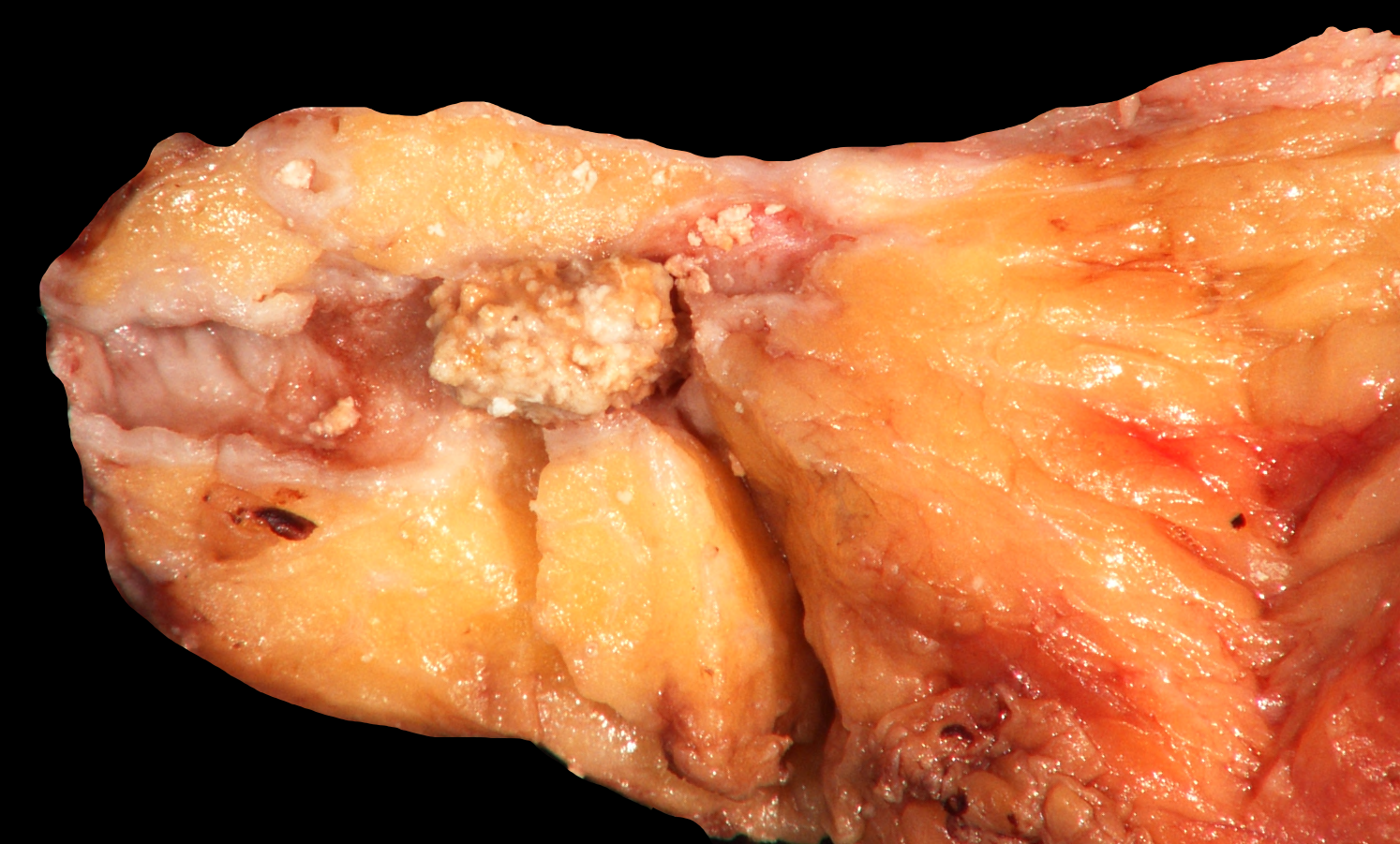
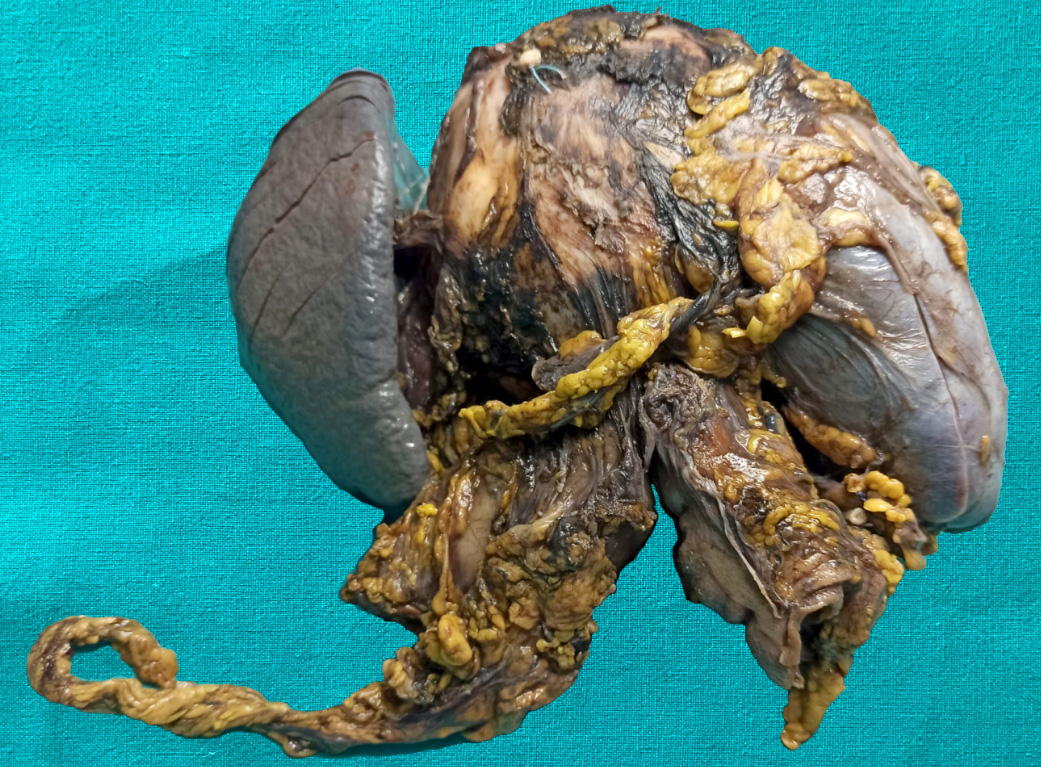
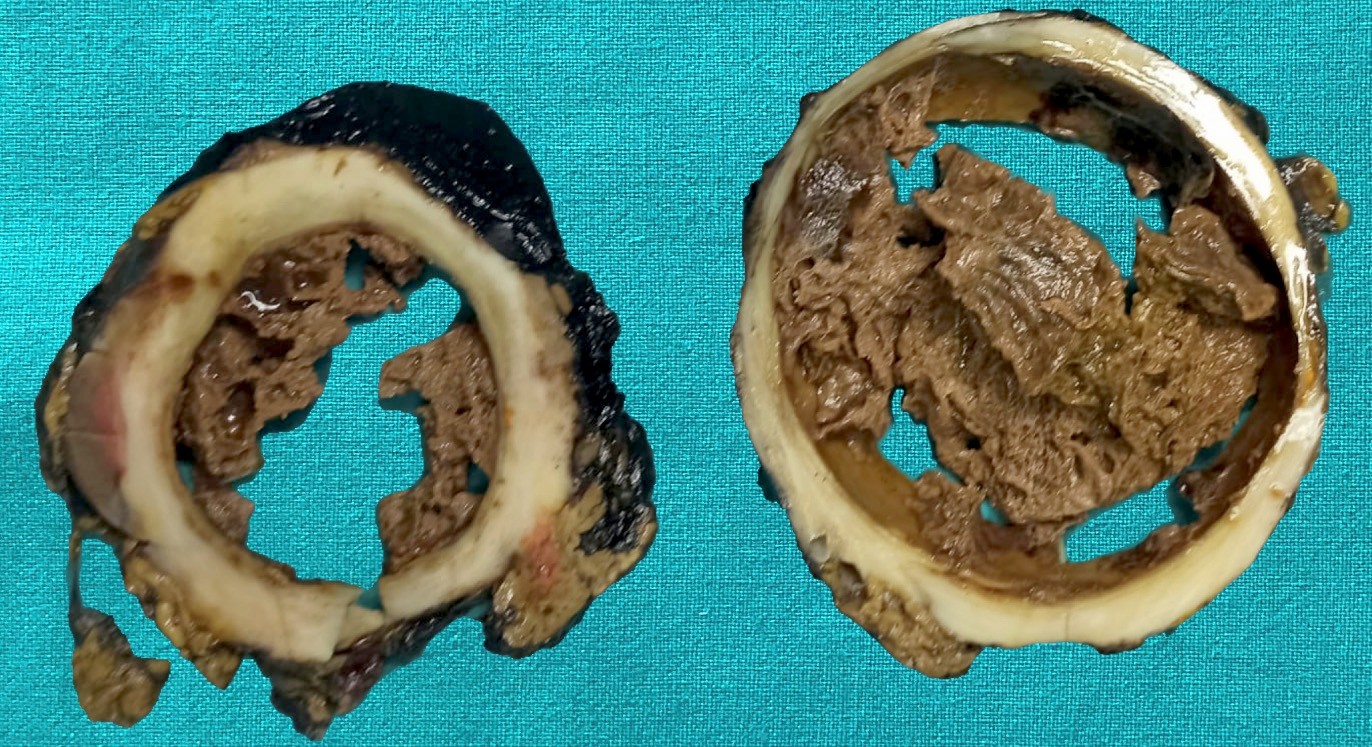
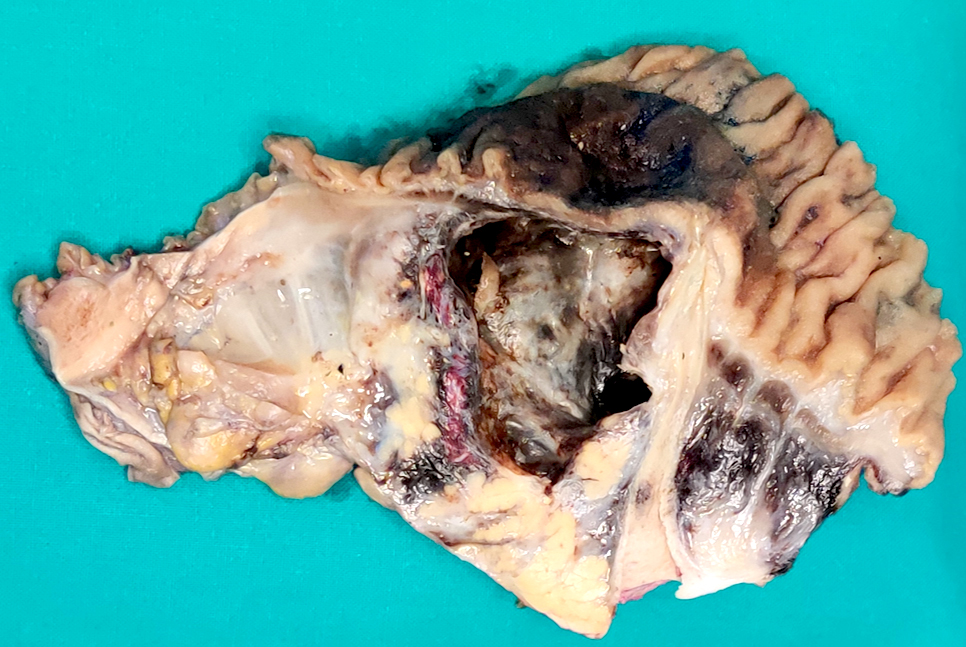
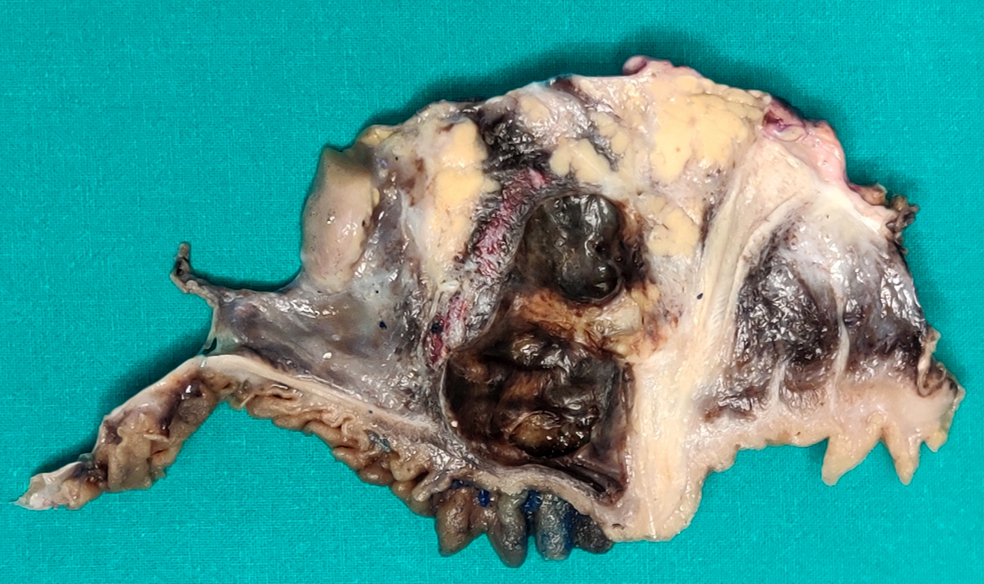
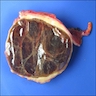
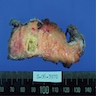
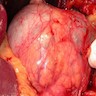
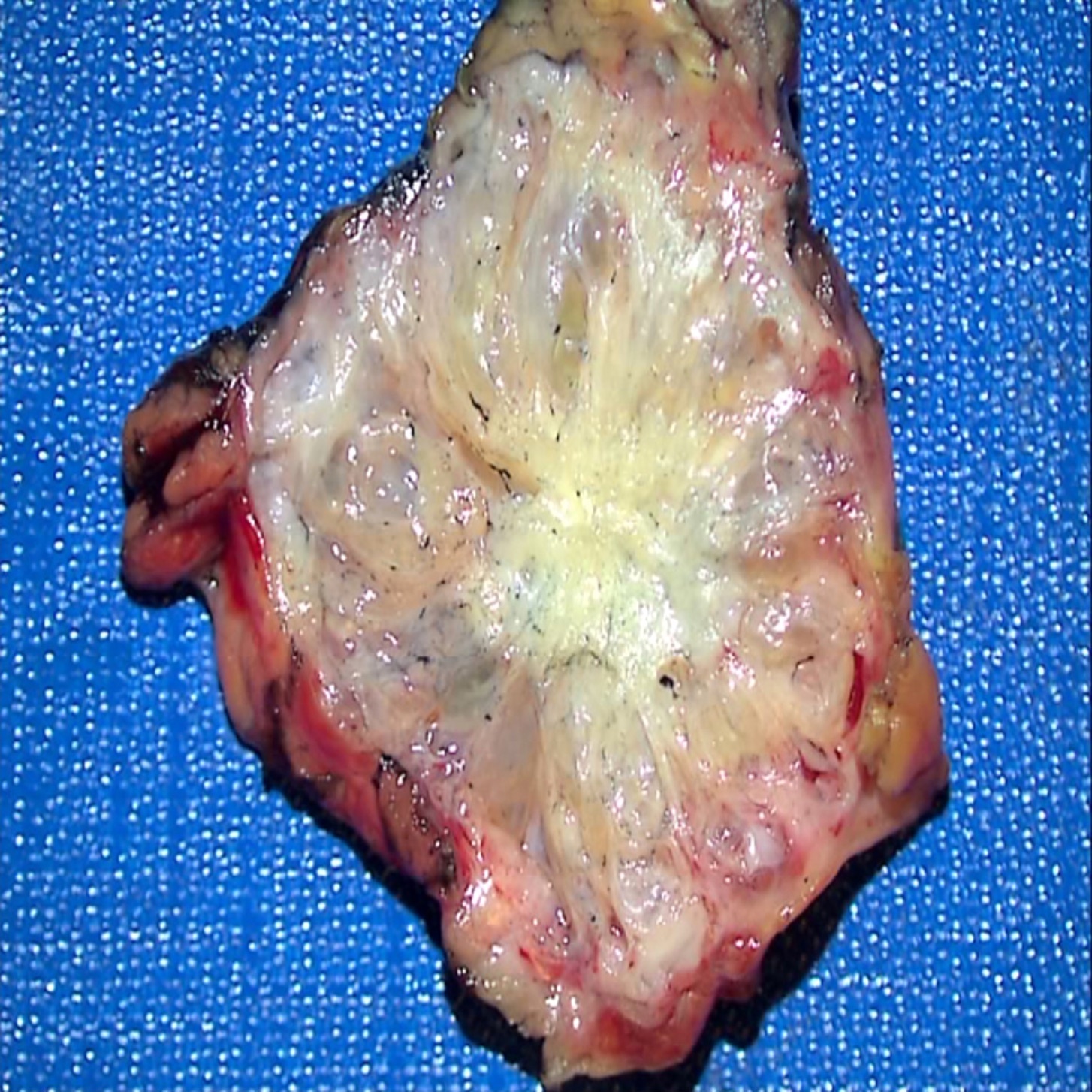
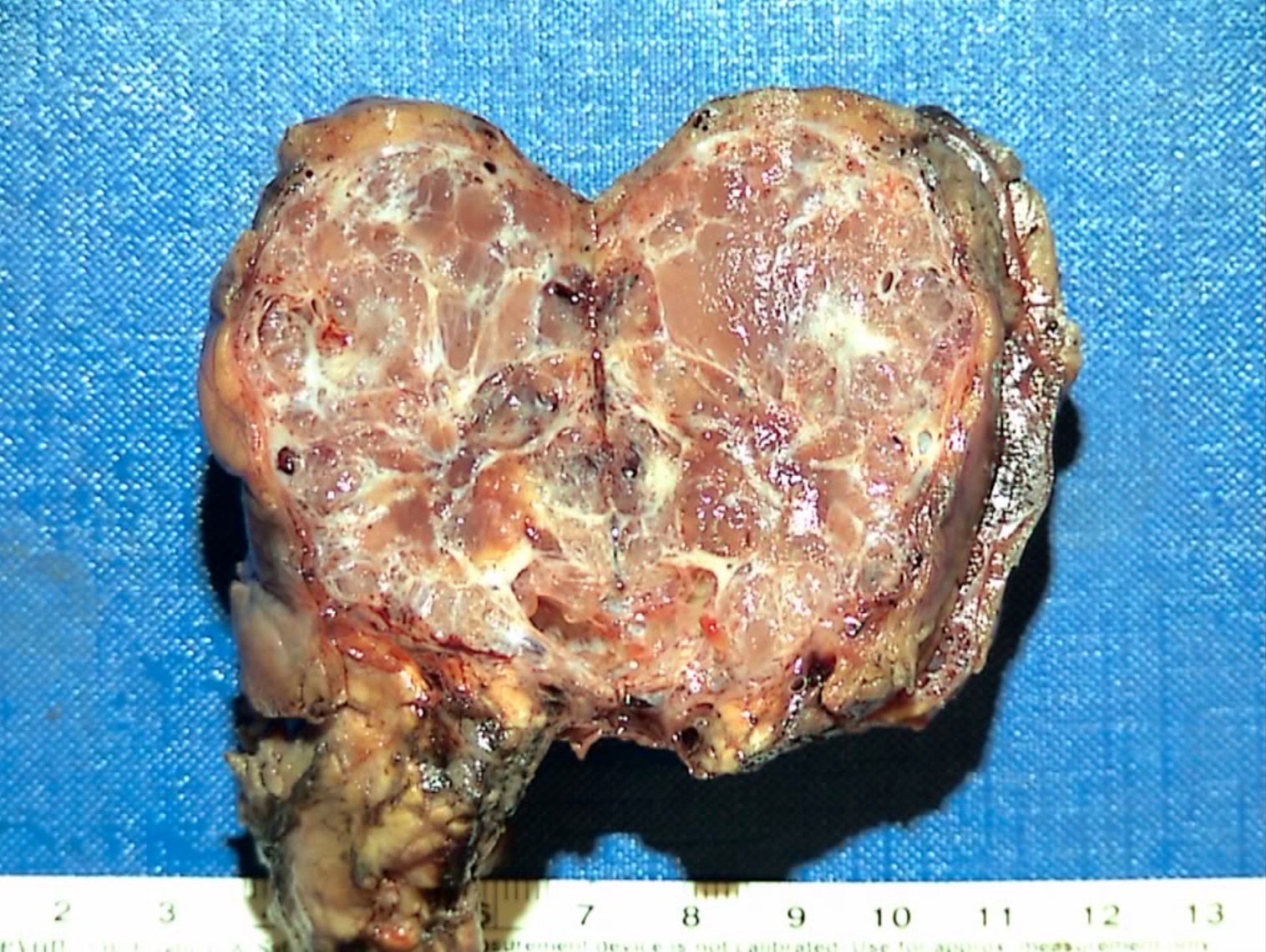
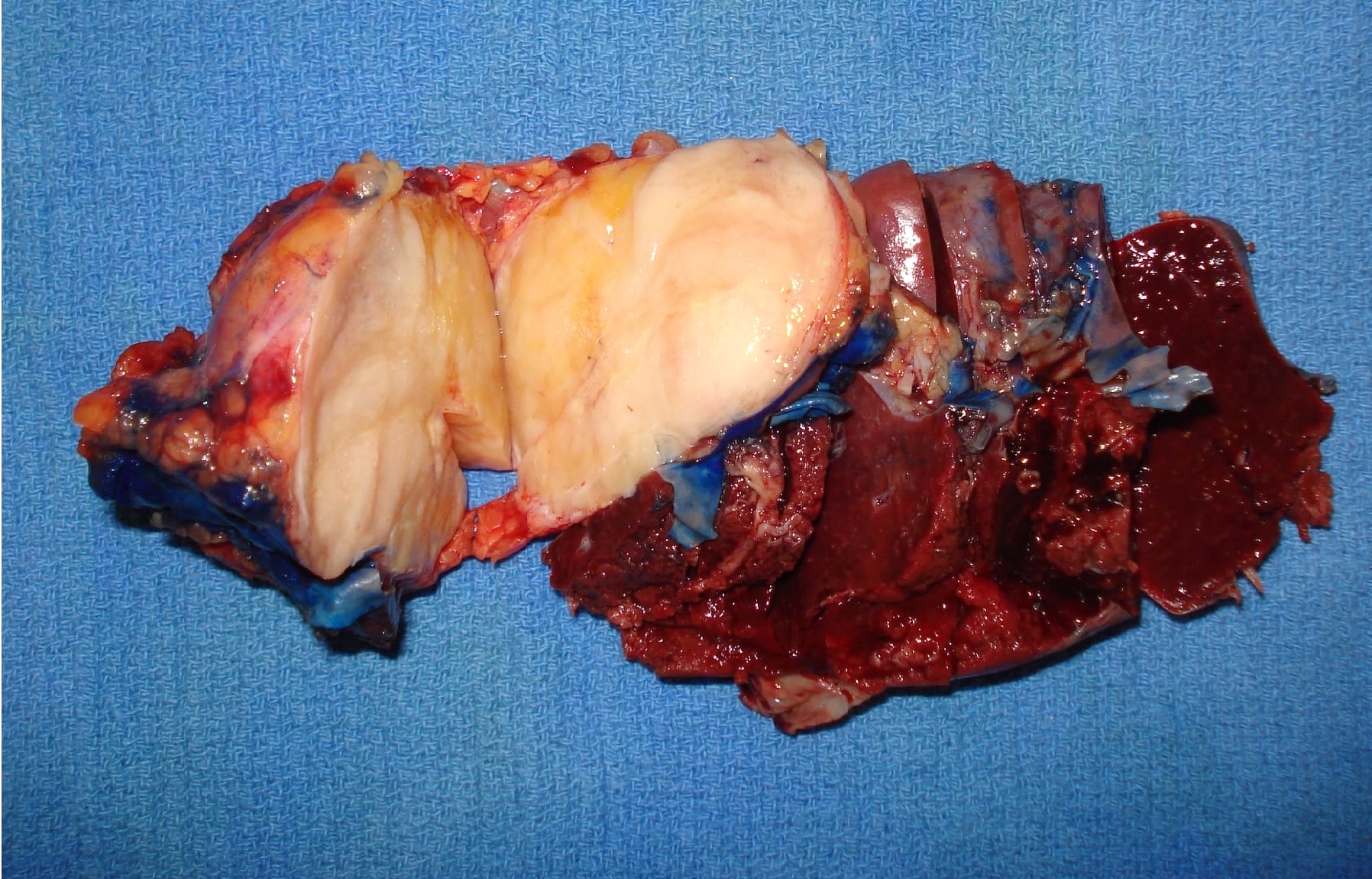
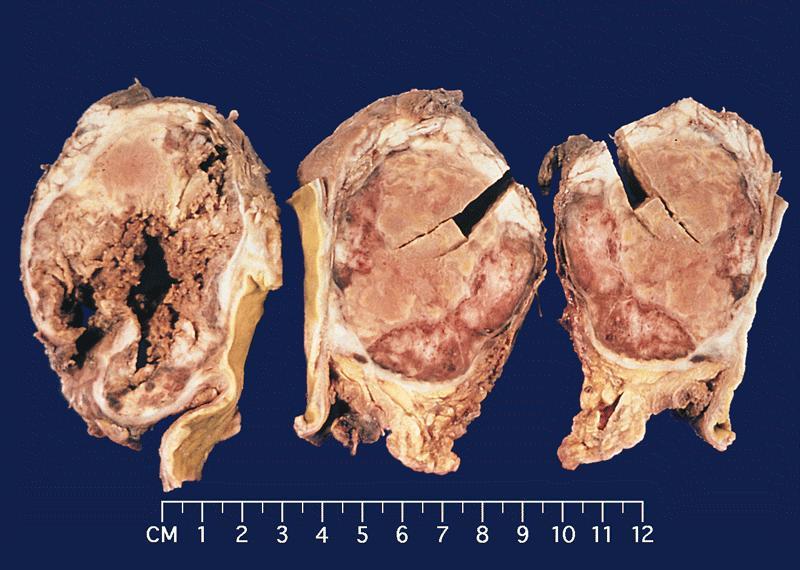
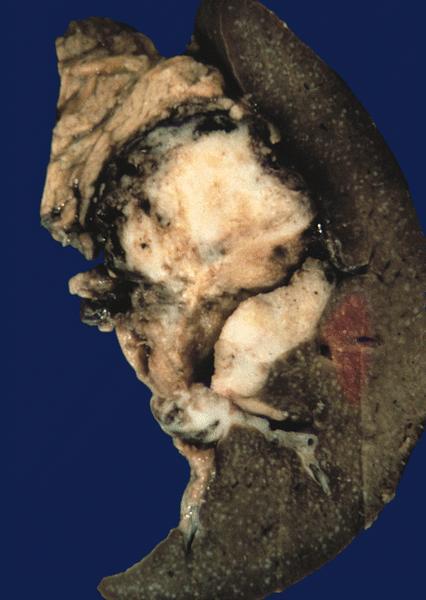
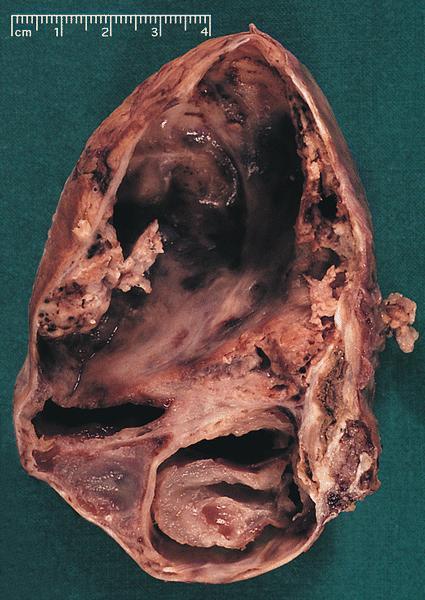
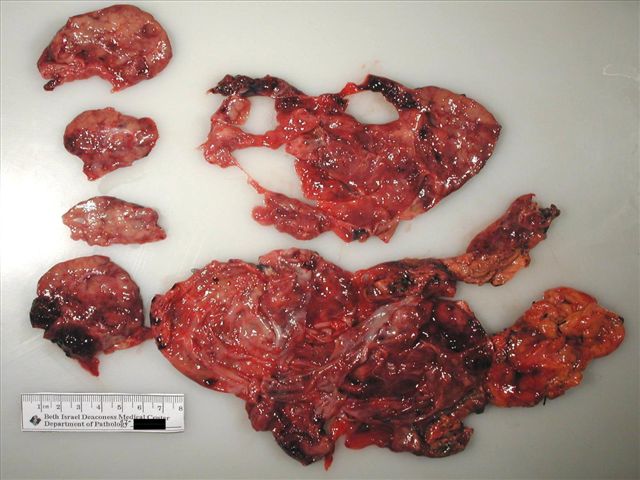
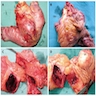
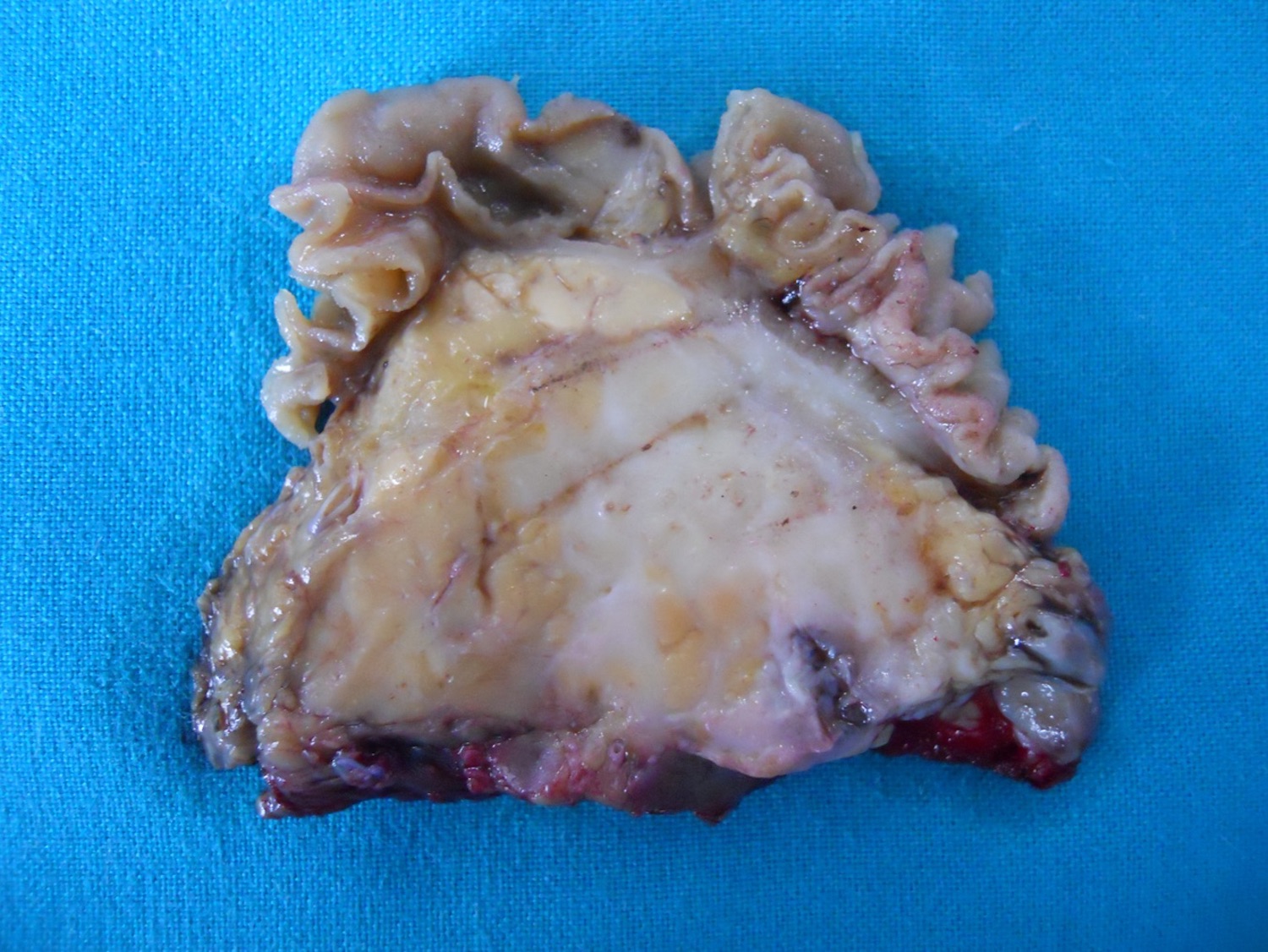
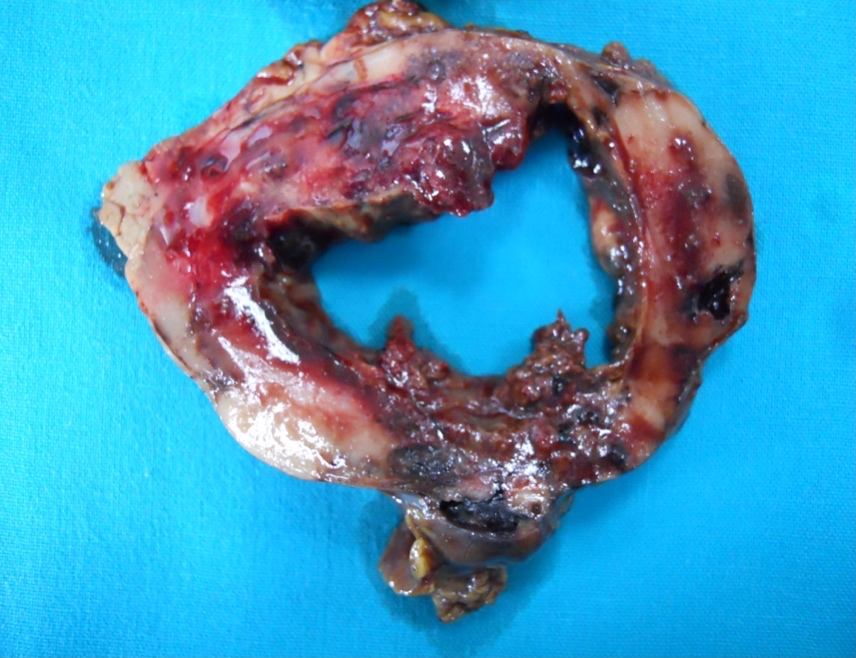
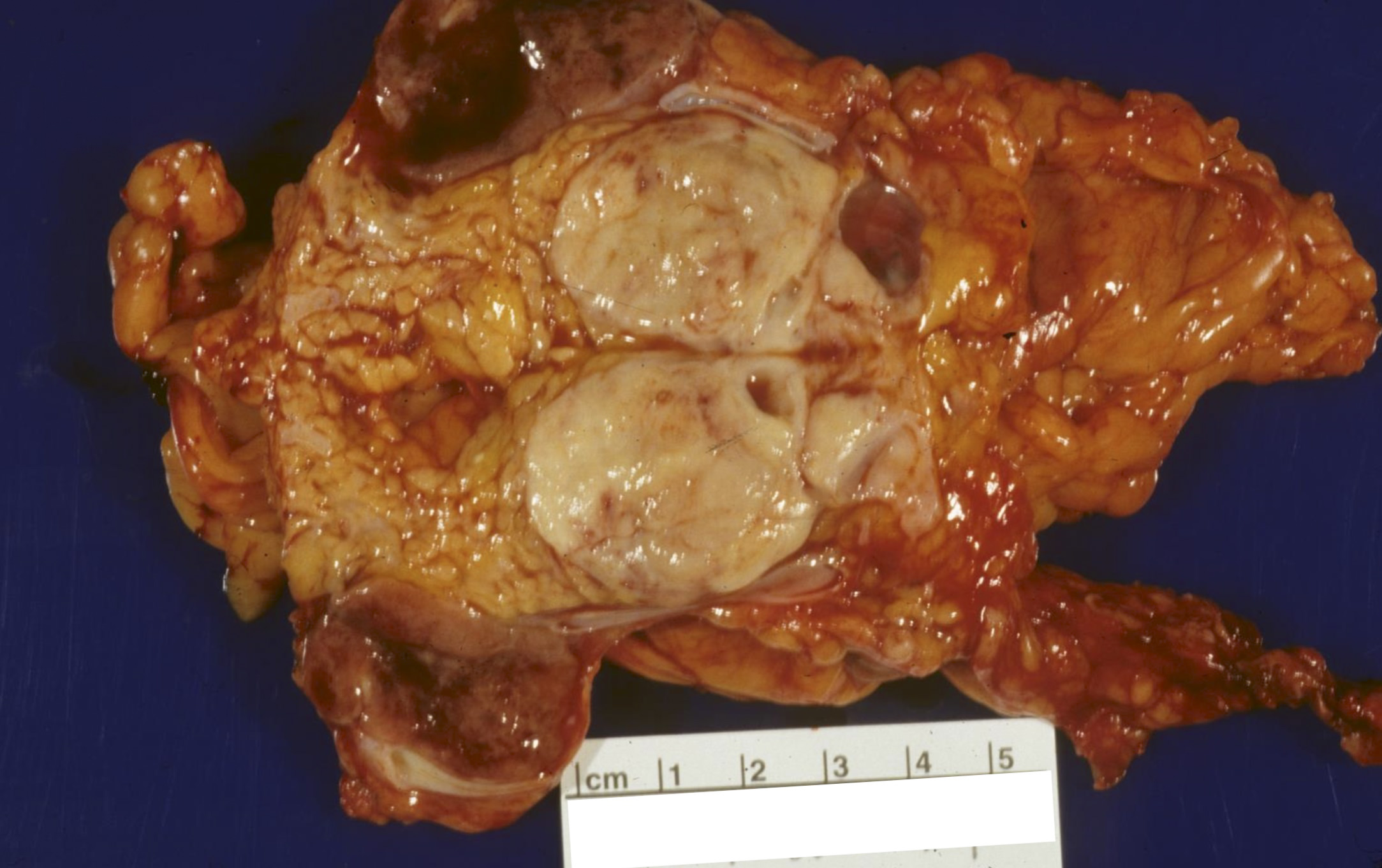
Gross description
- Well circumscribed mass, at least partially encapsulated, solid and large, with fleshy consistency
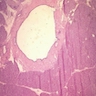
Frozen section description
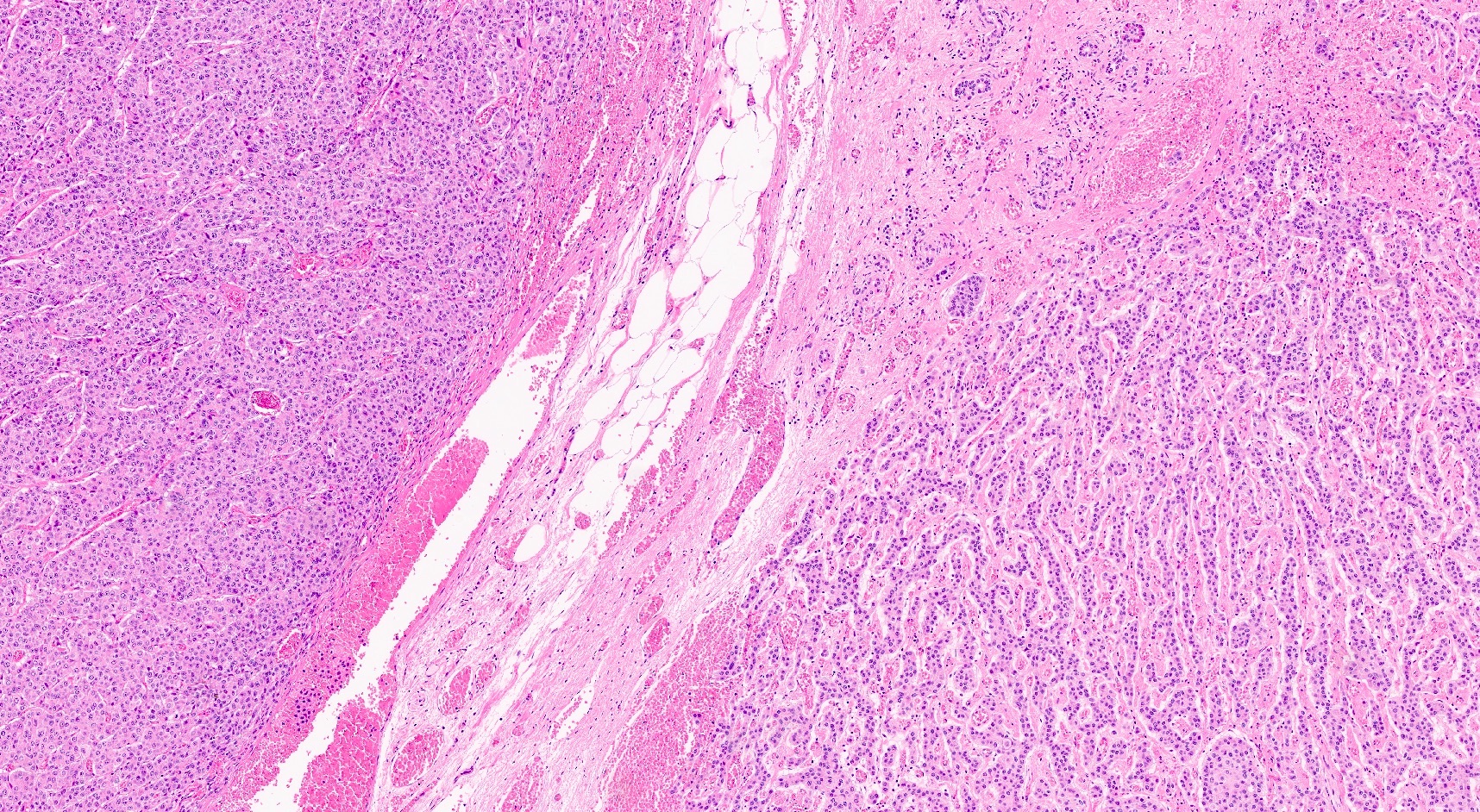
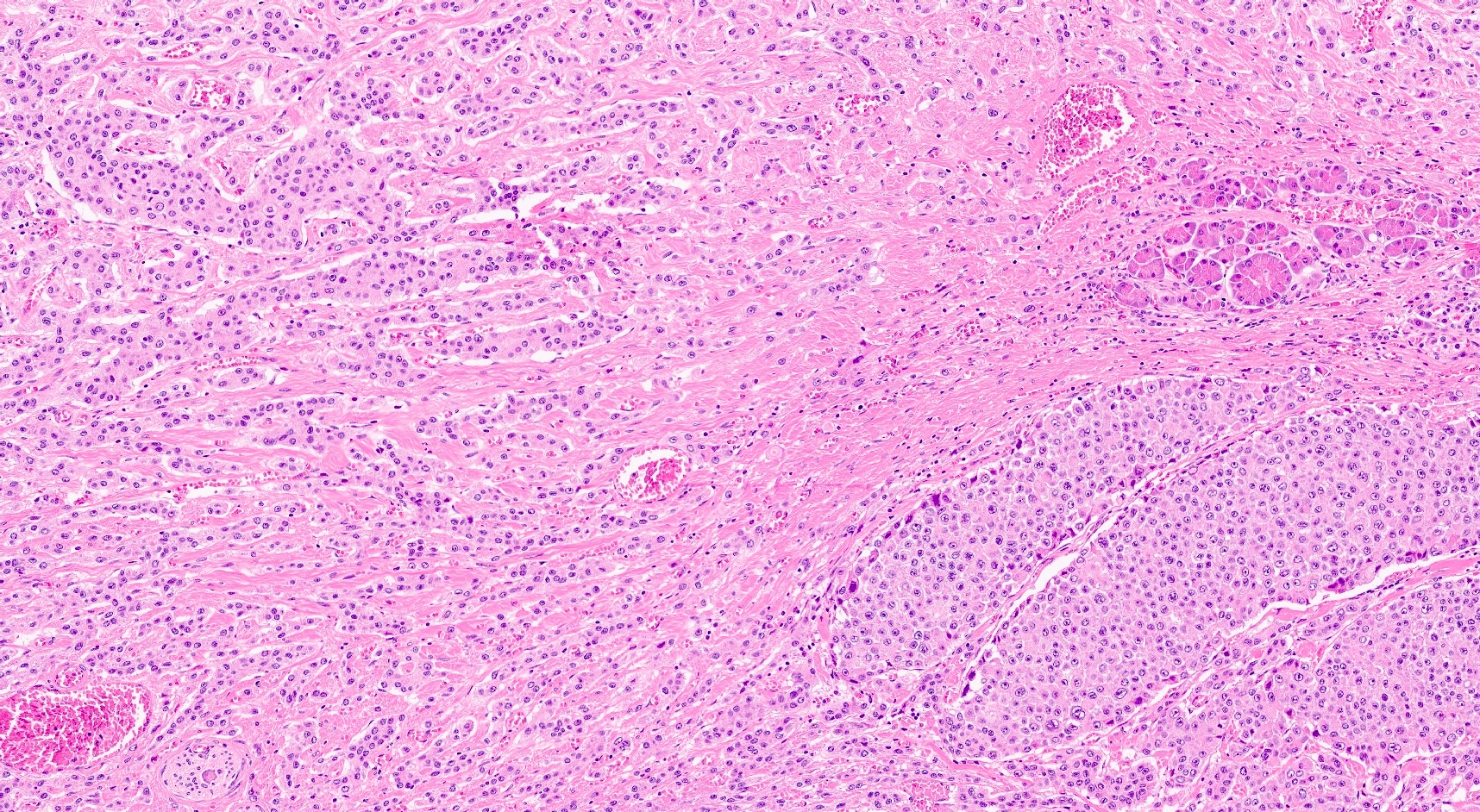
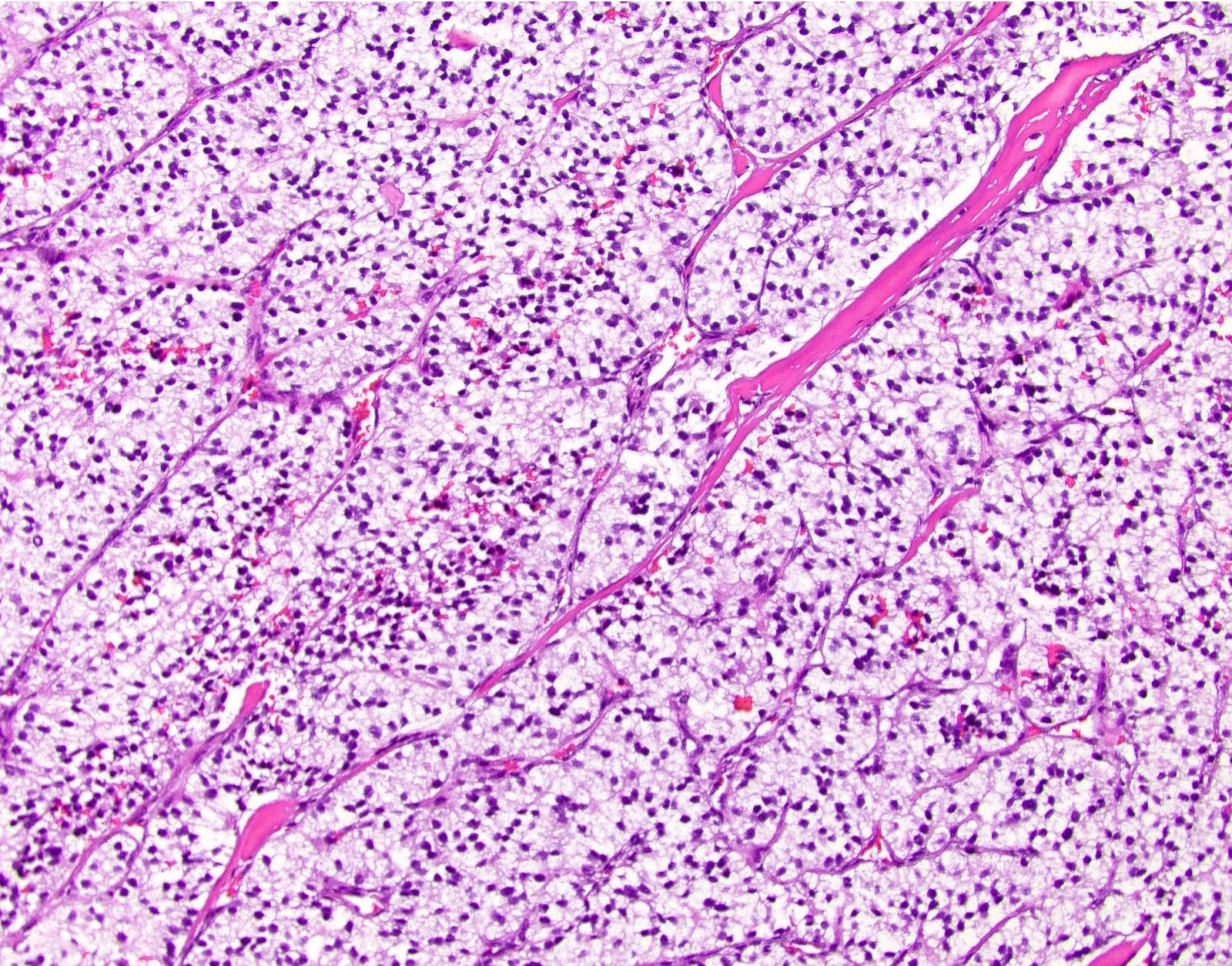
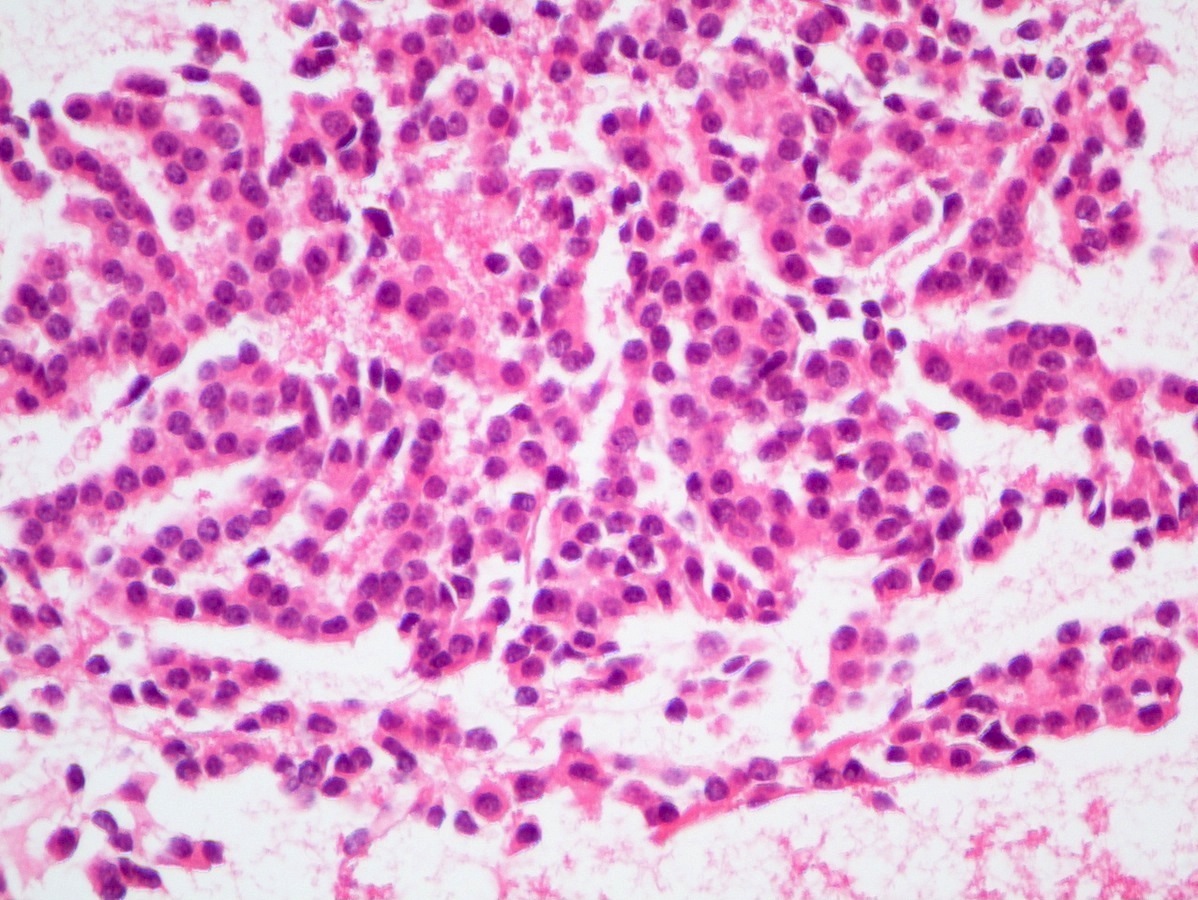
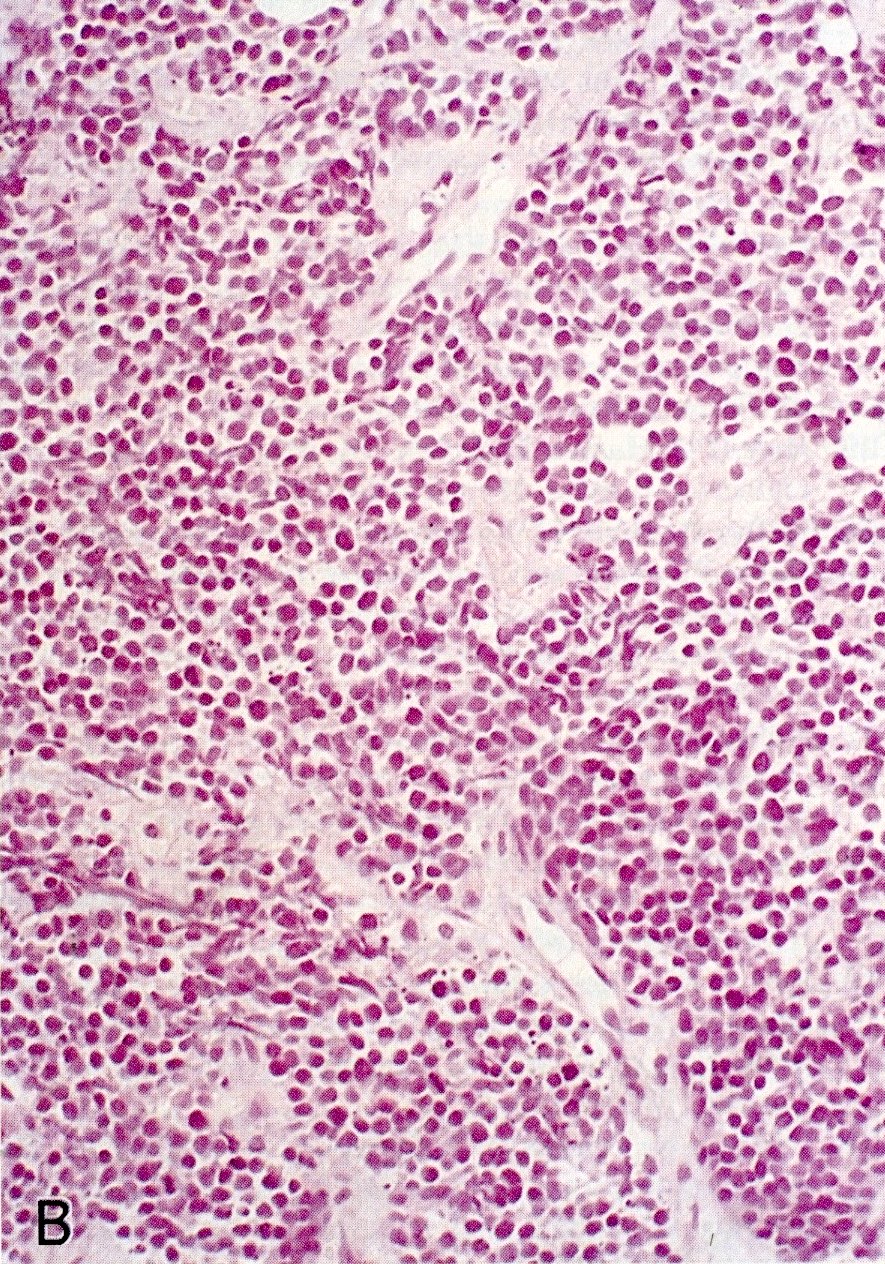
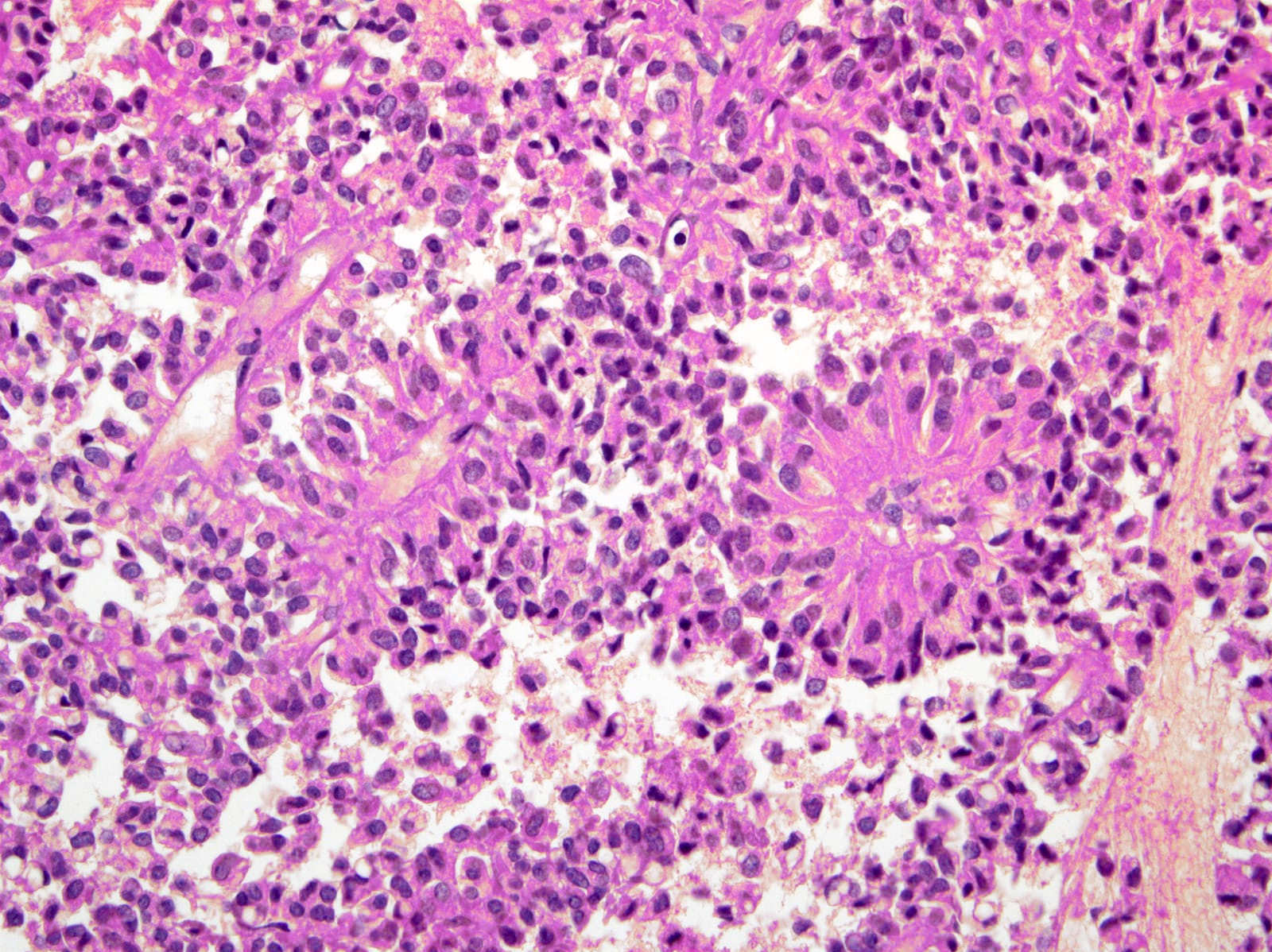
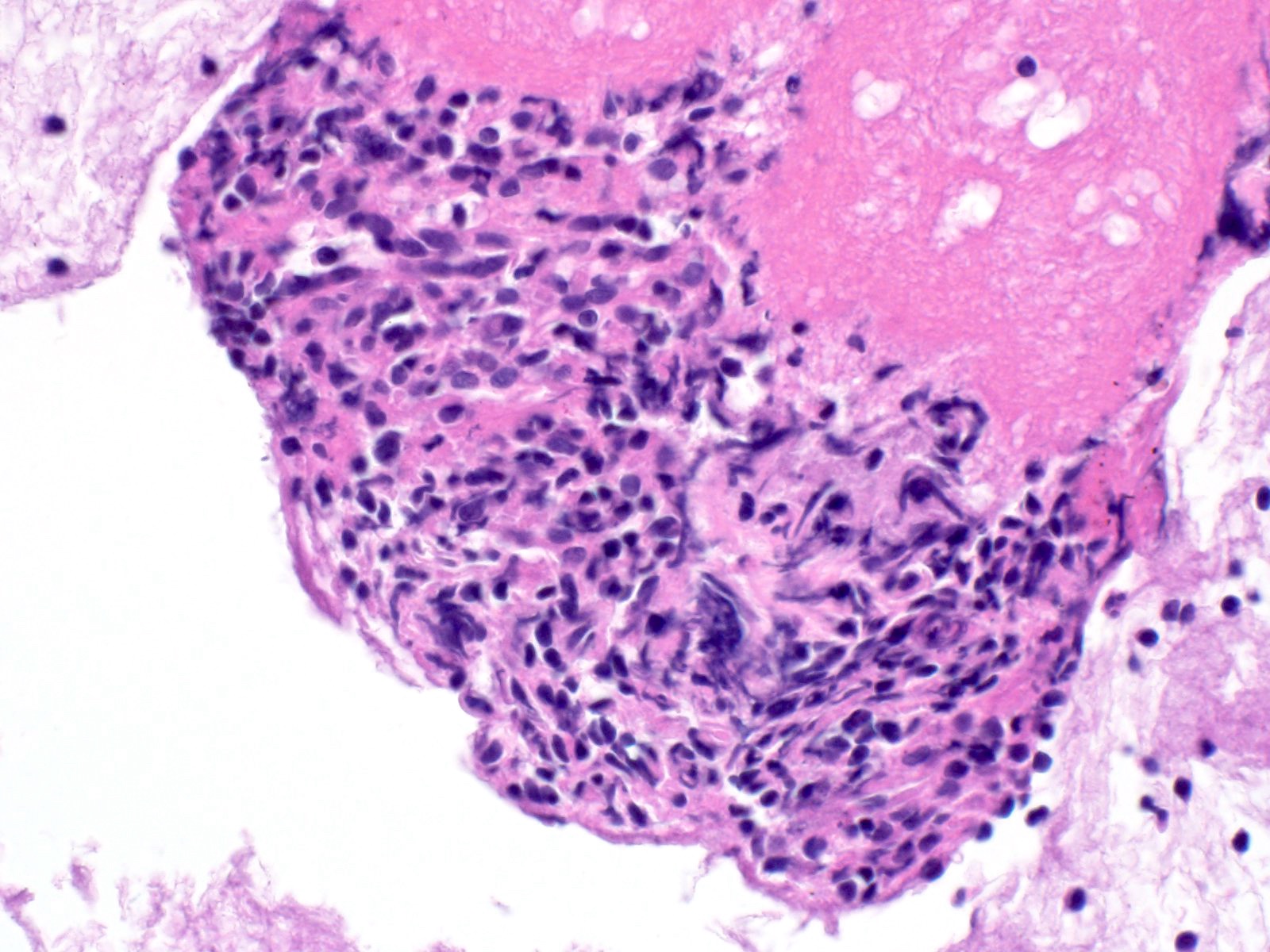
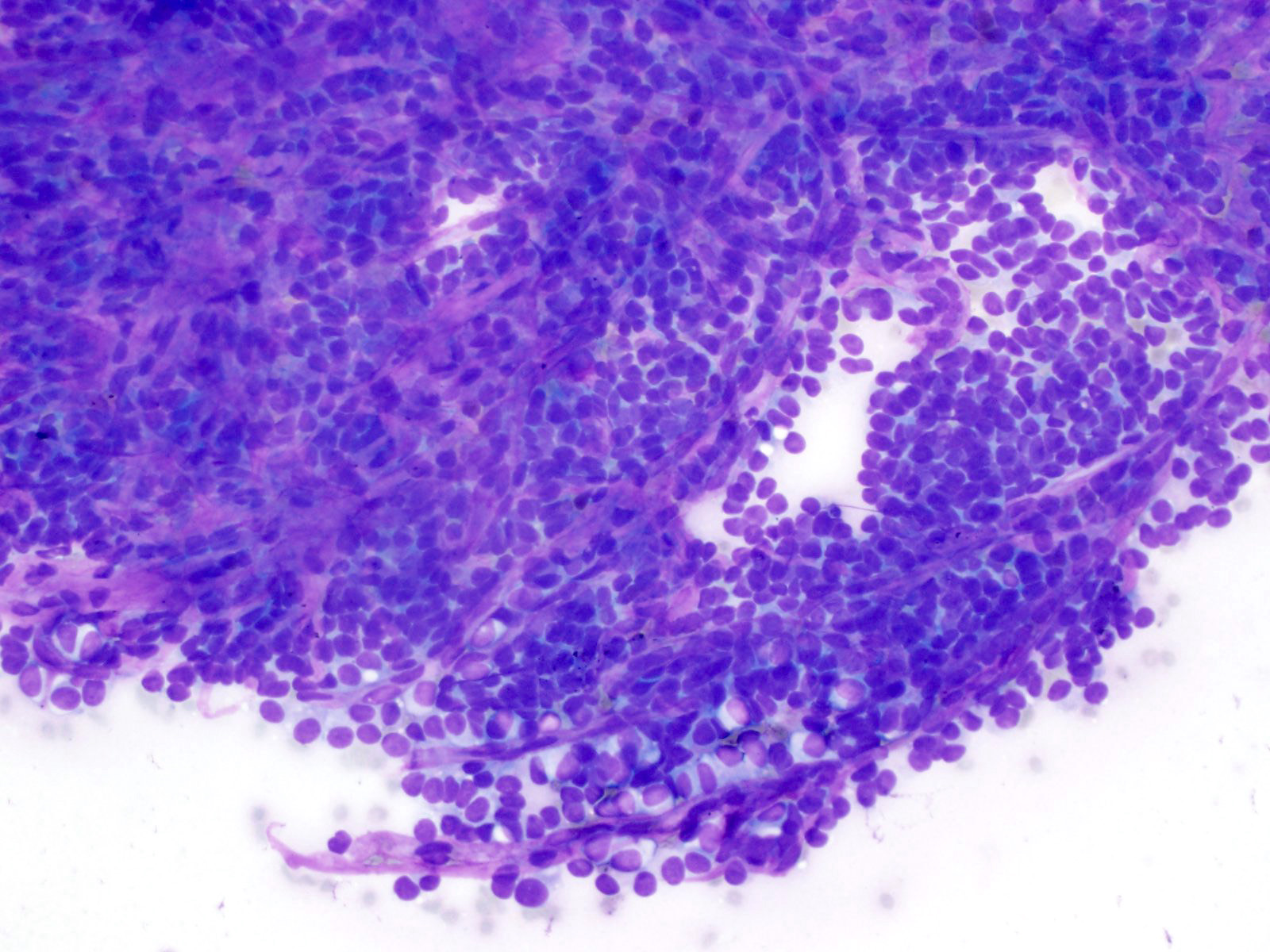
- Hypercellular neoplasm with acinar resembling cells
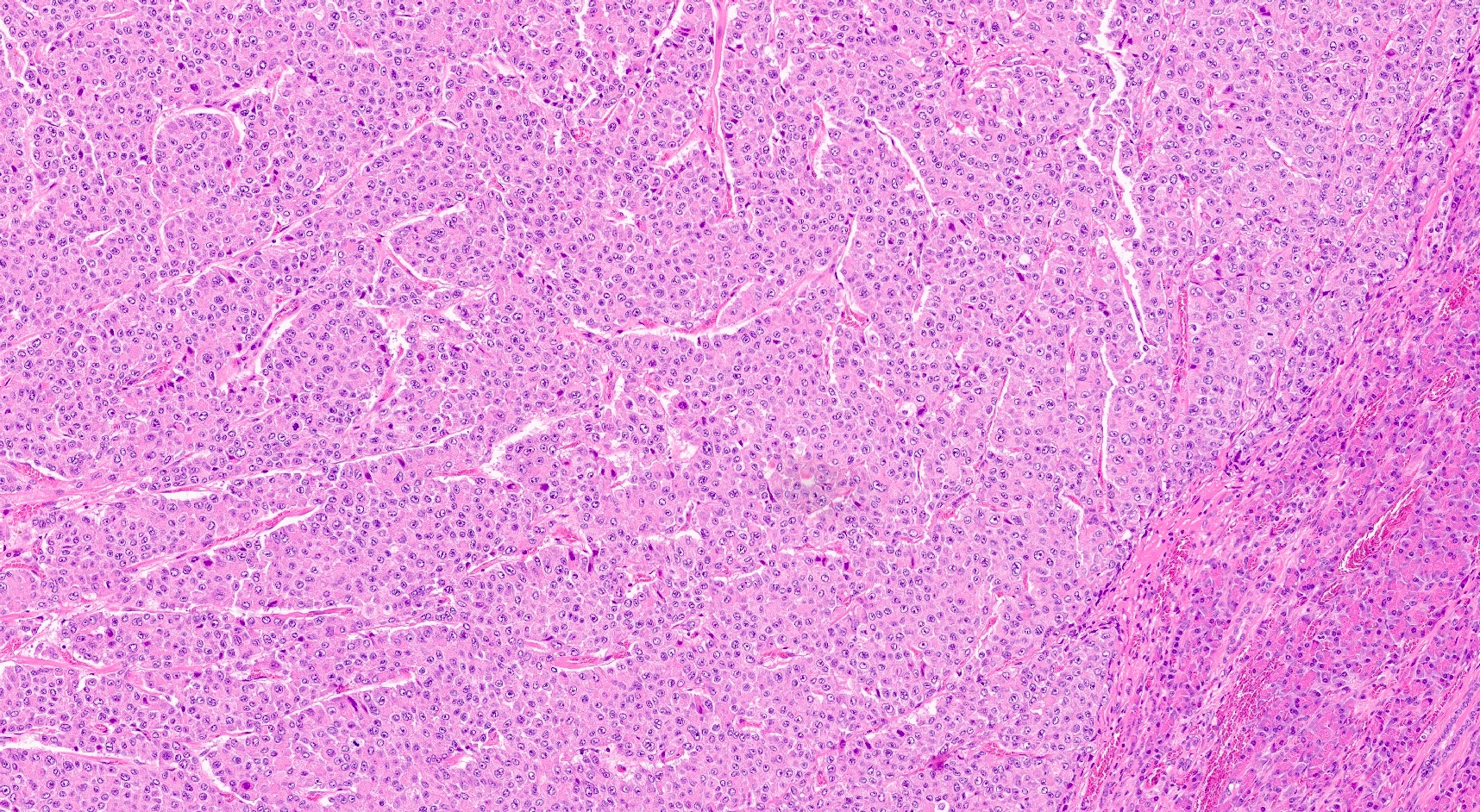
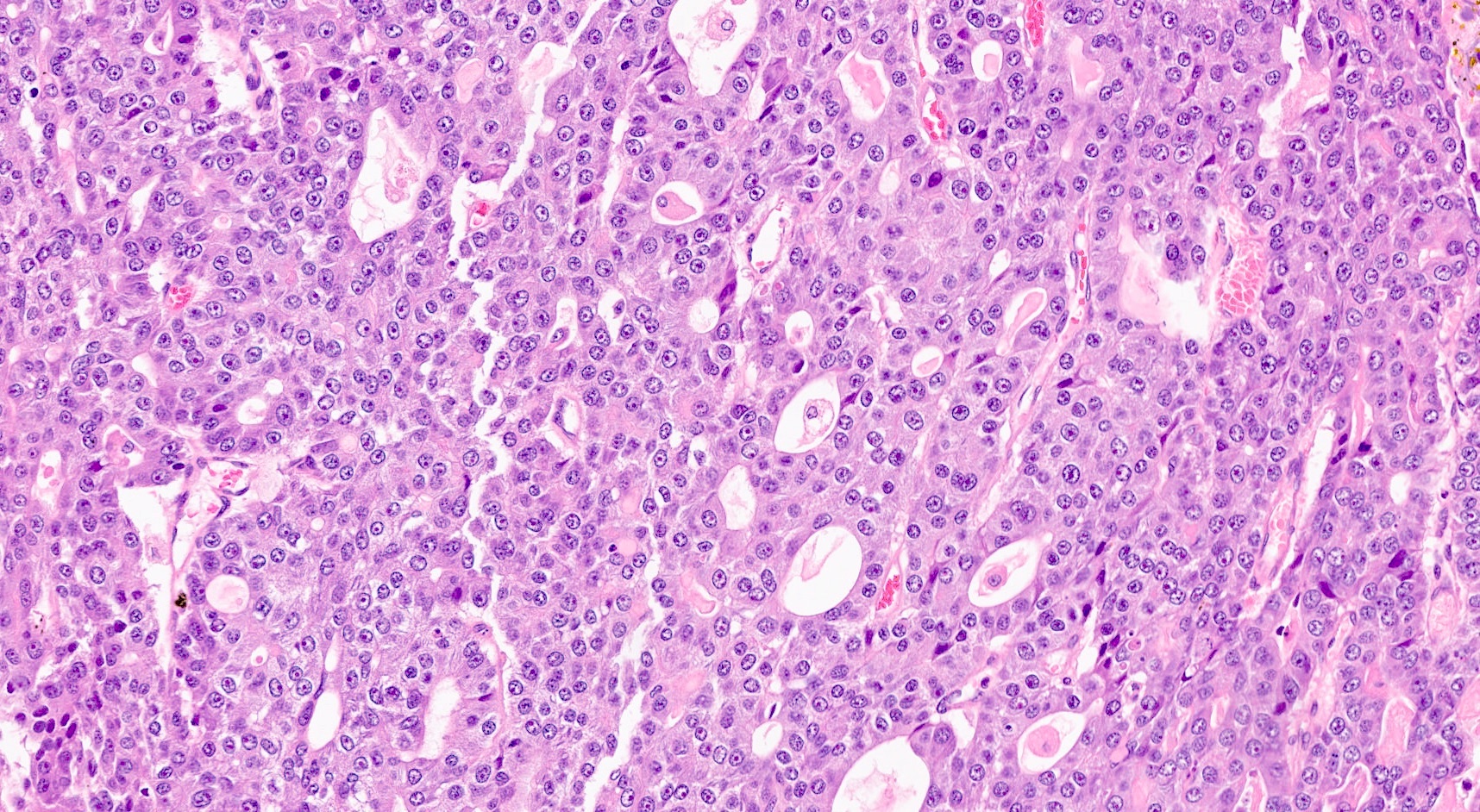
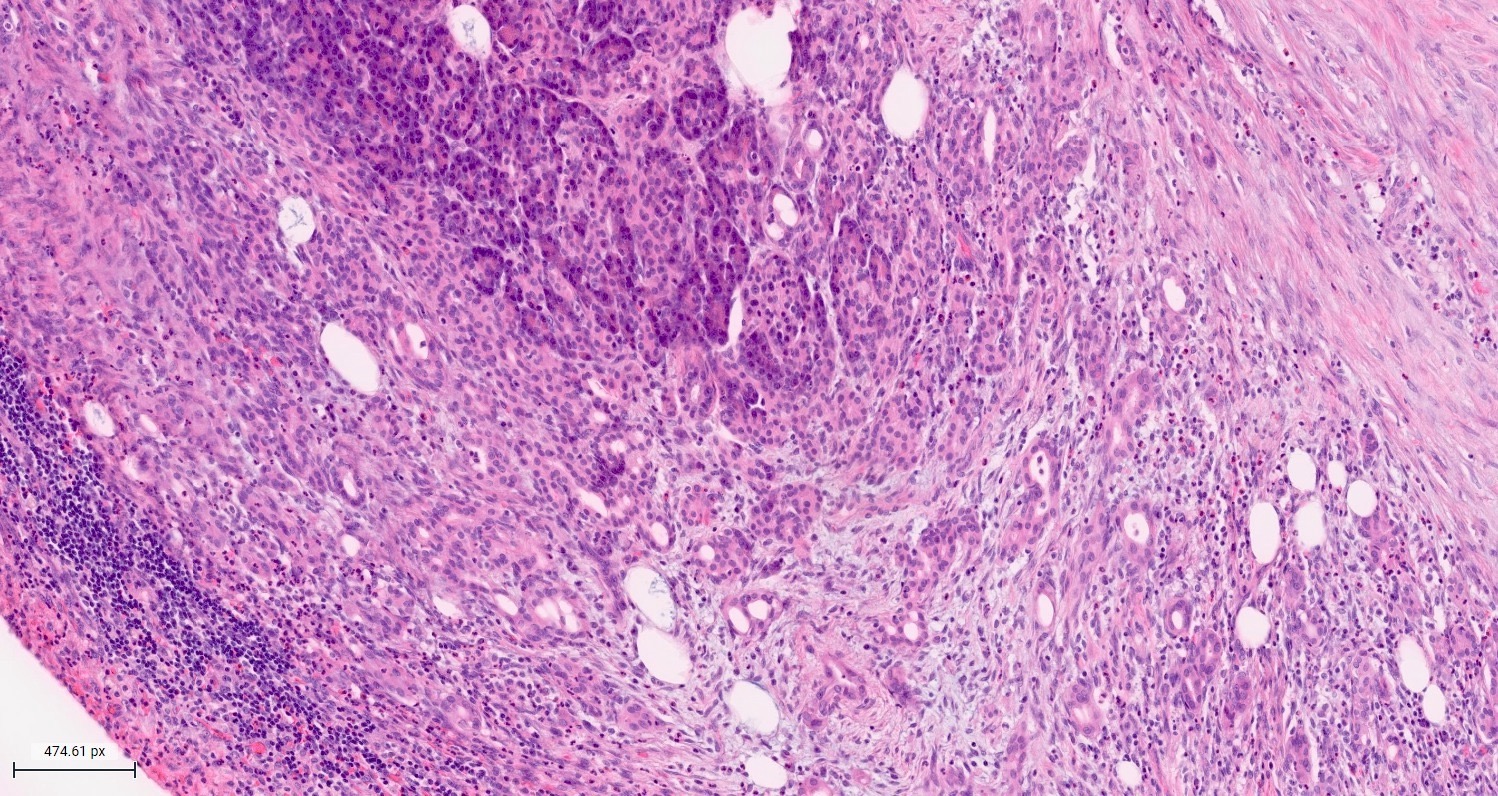
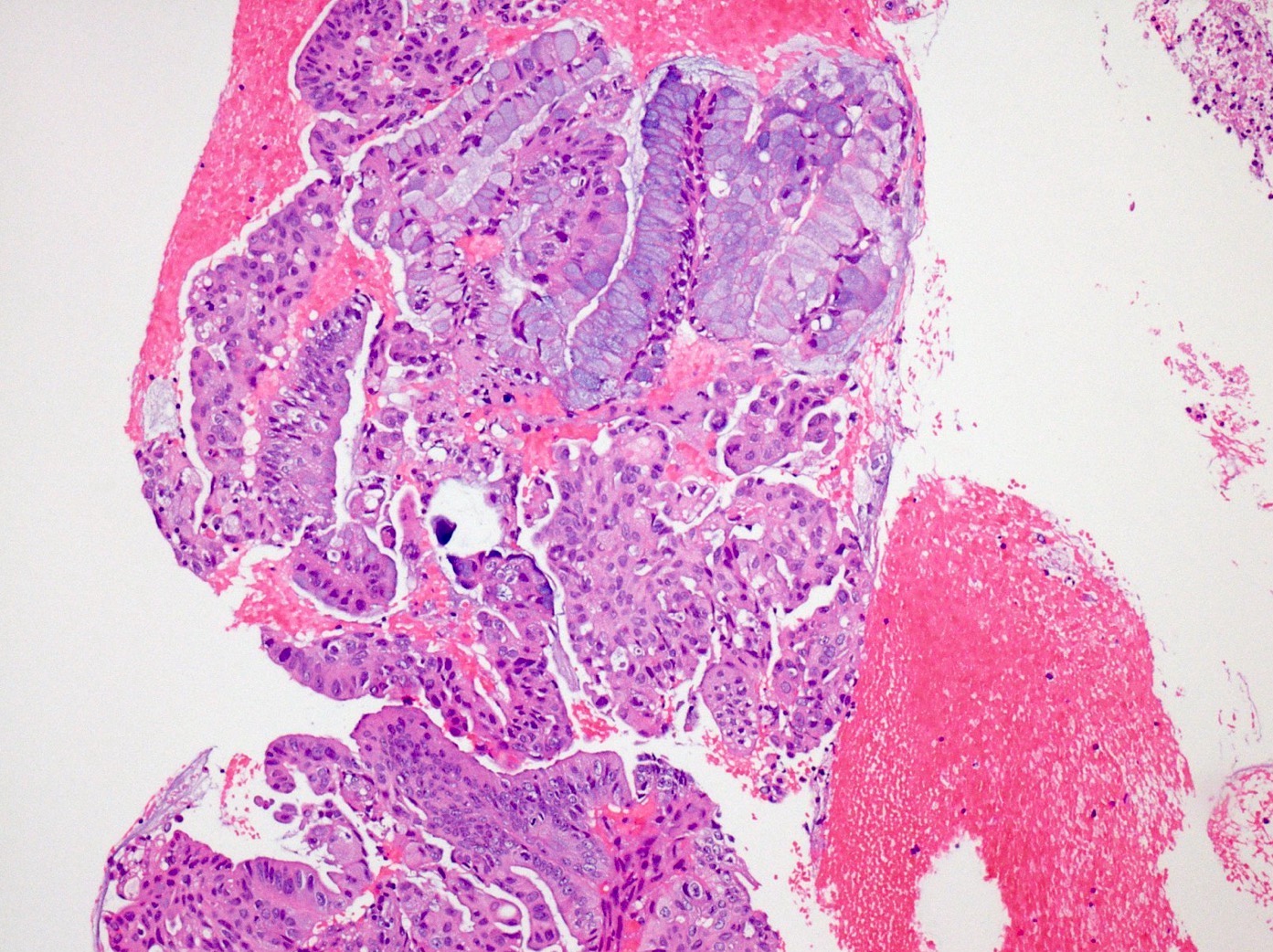
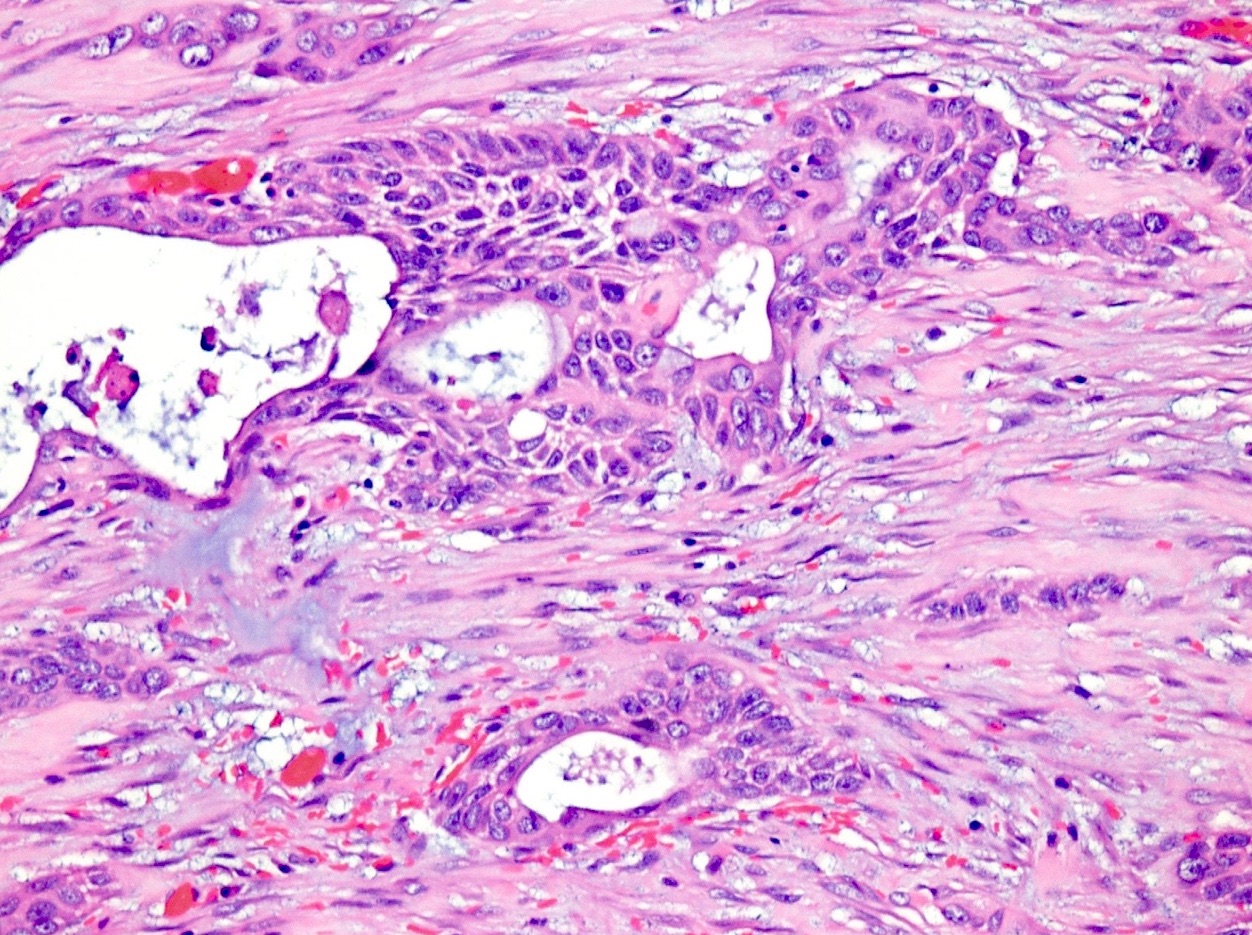
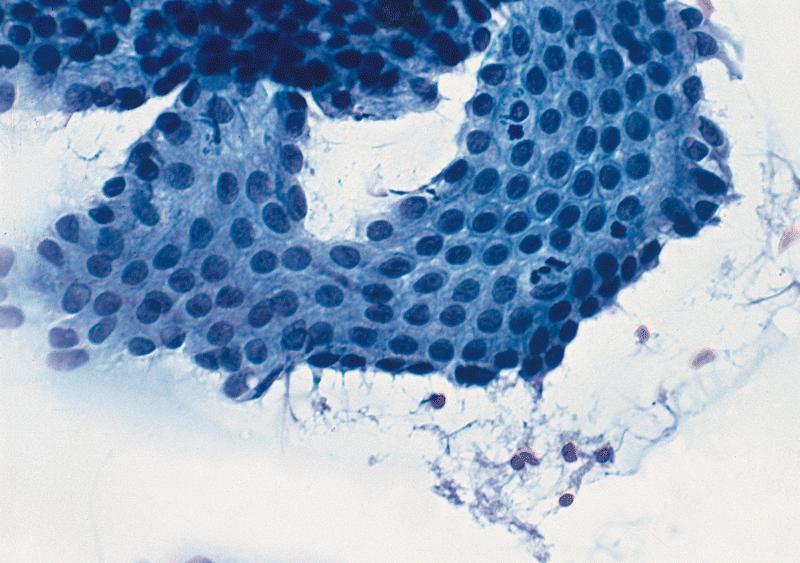
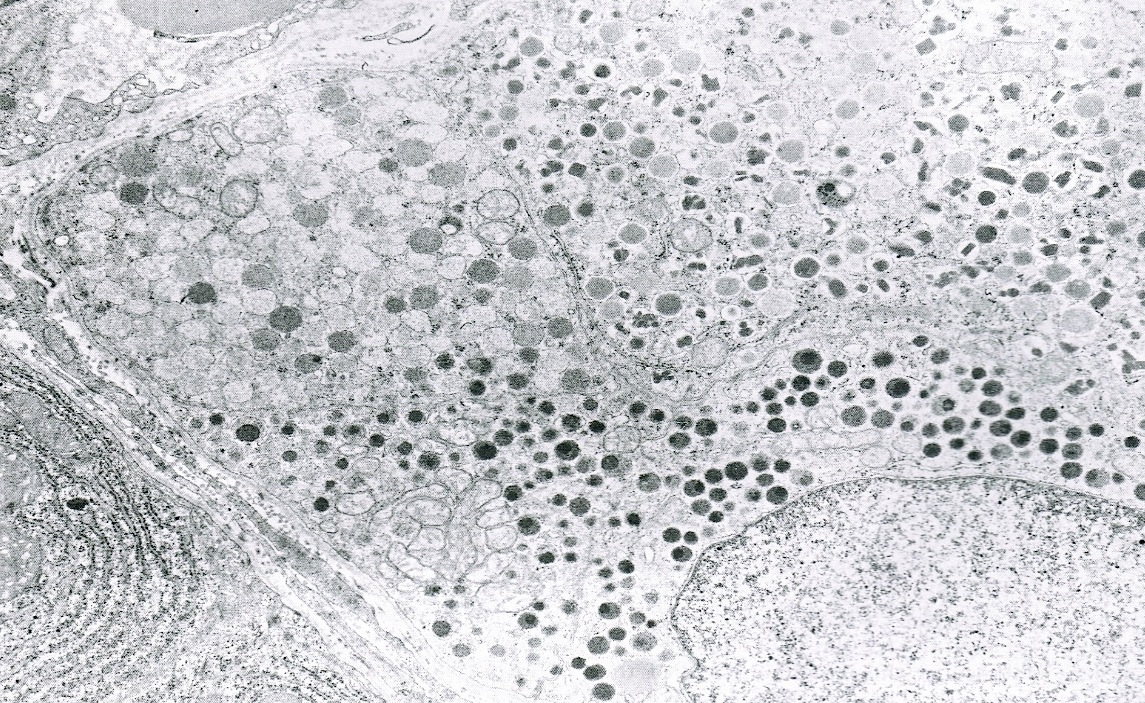
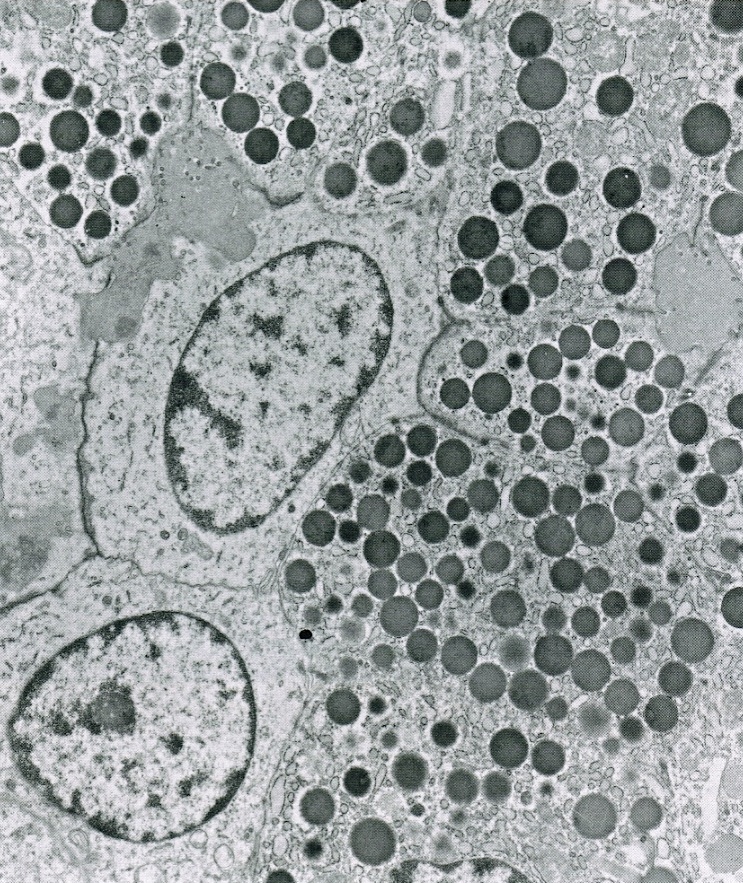
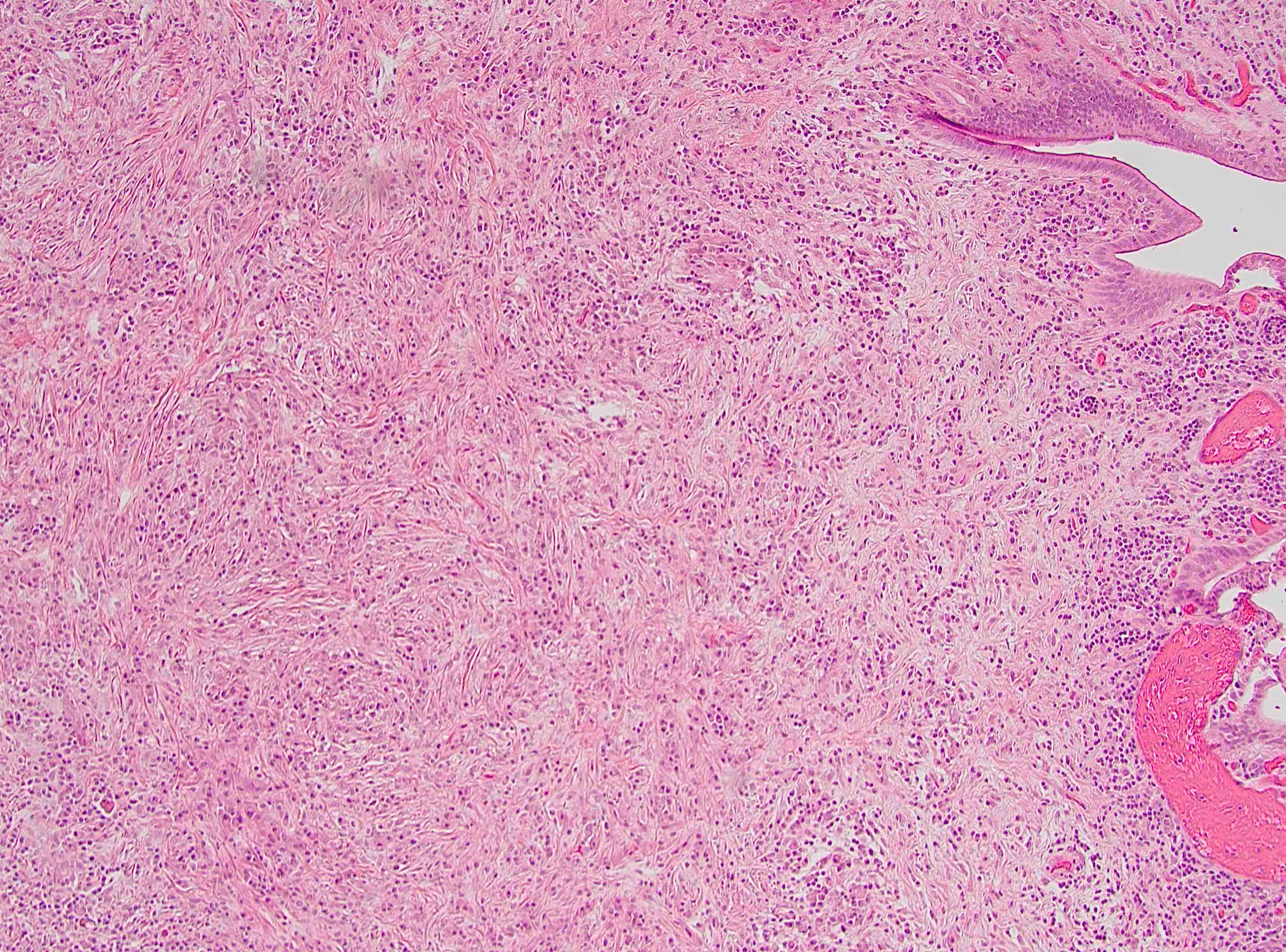
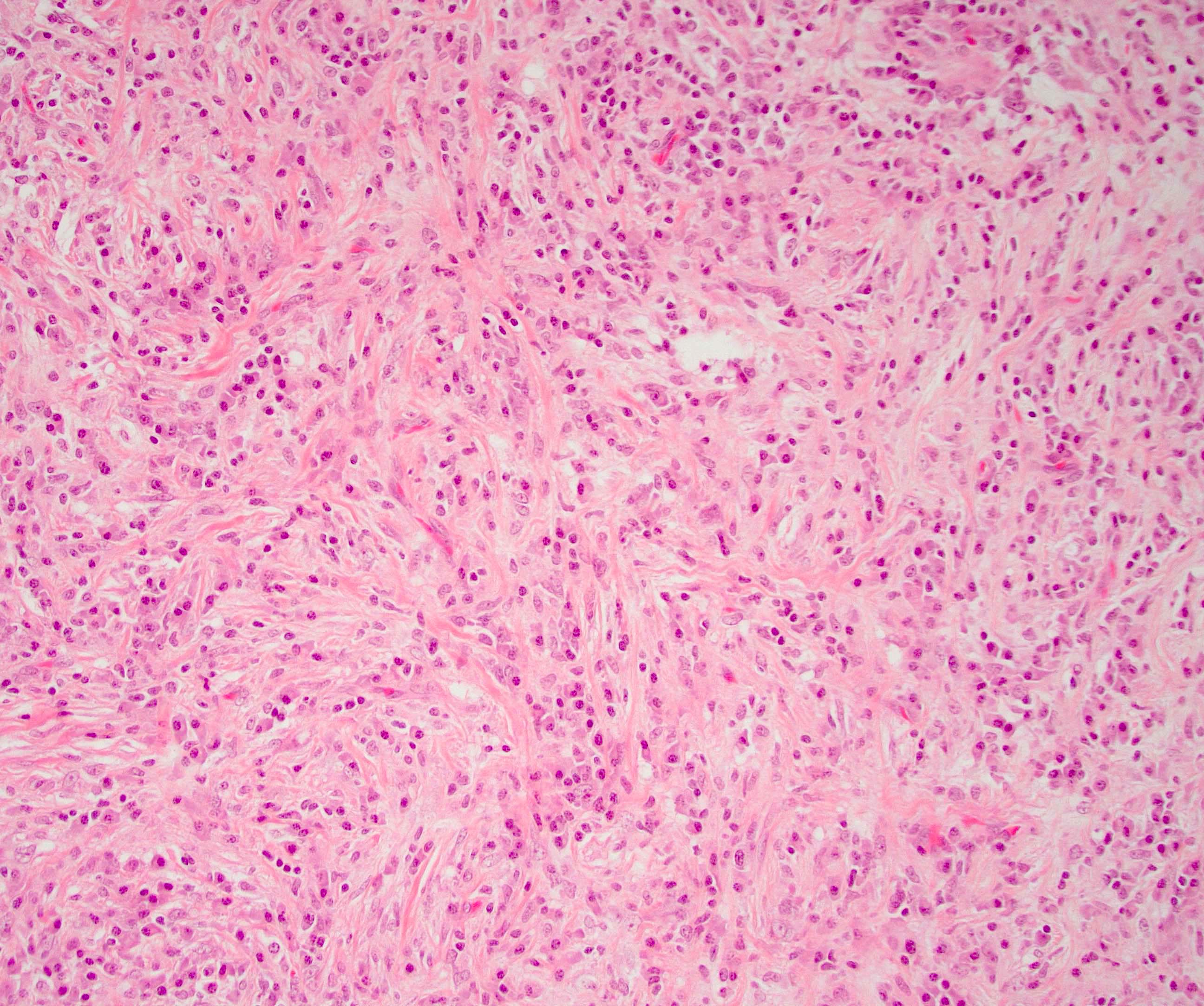
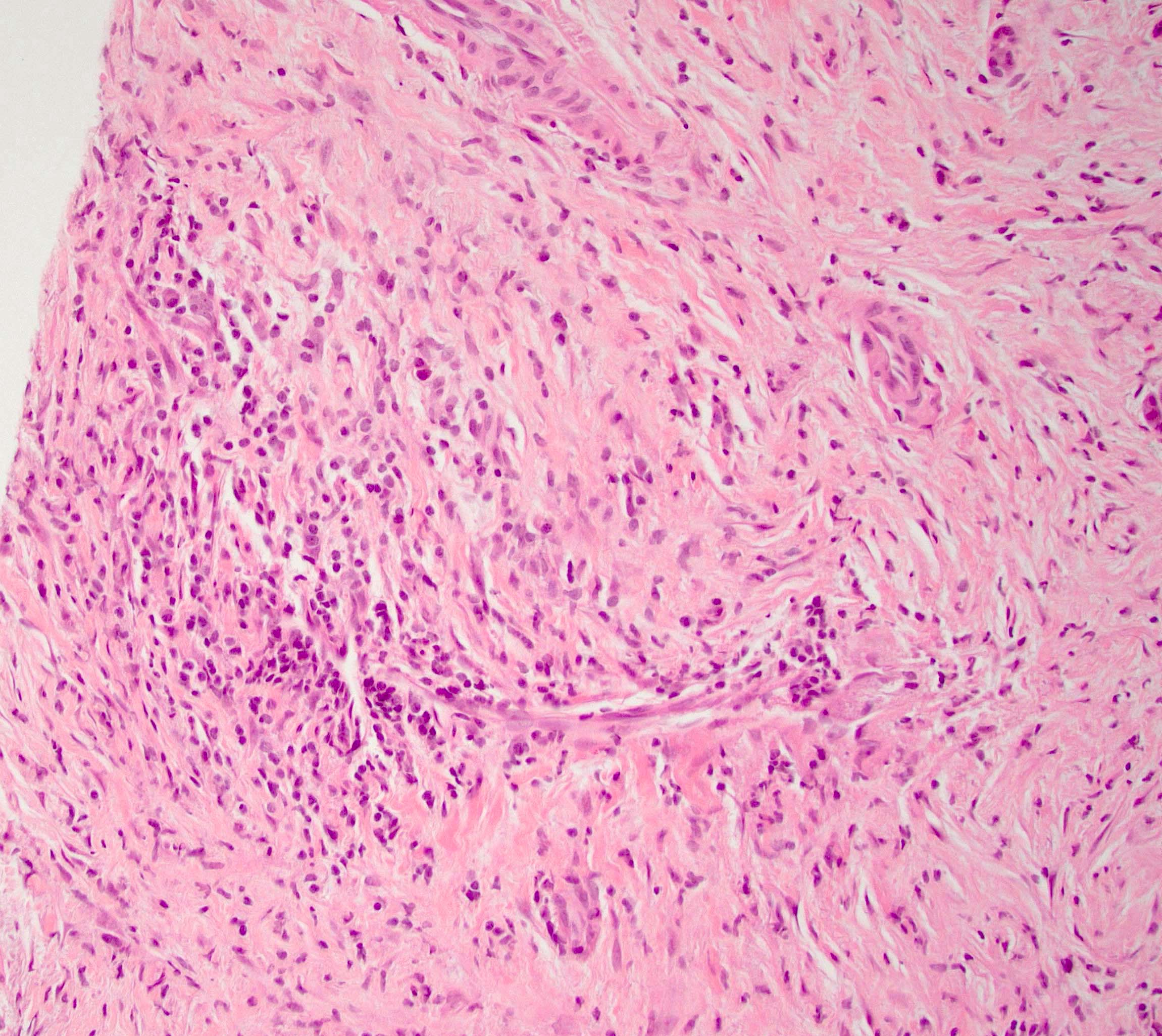
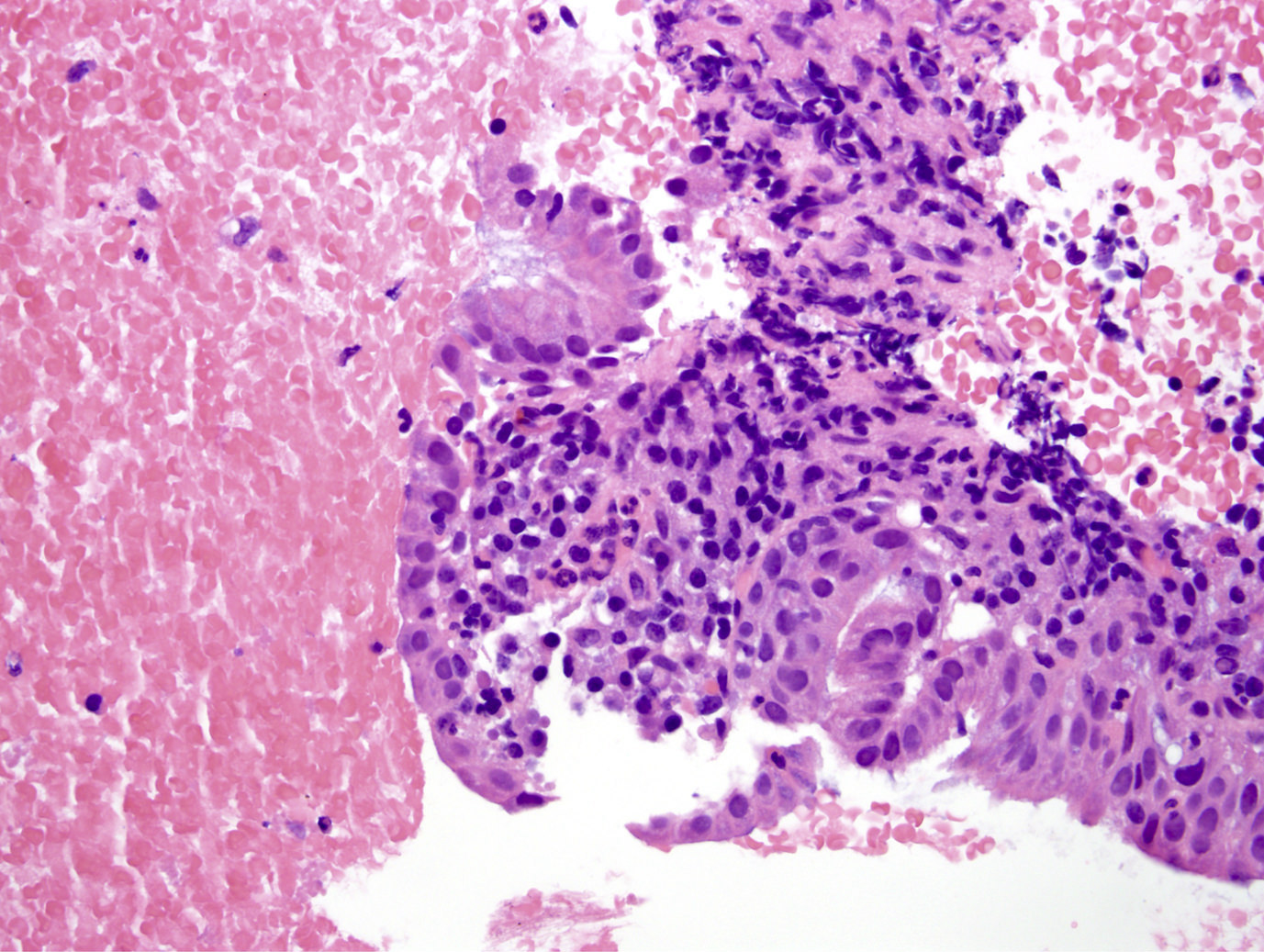
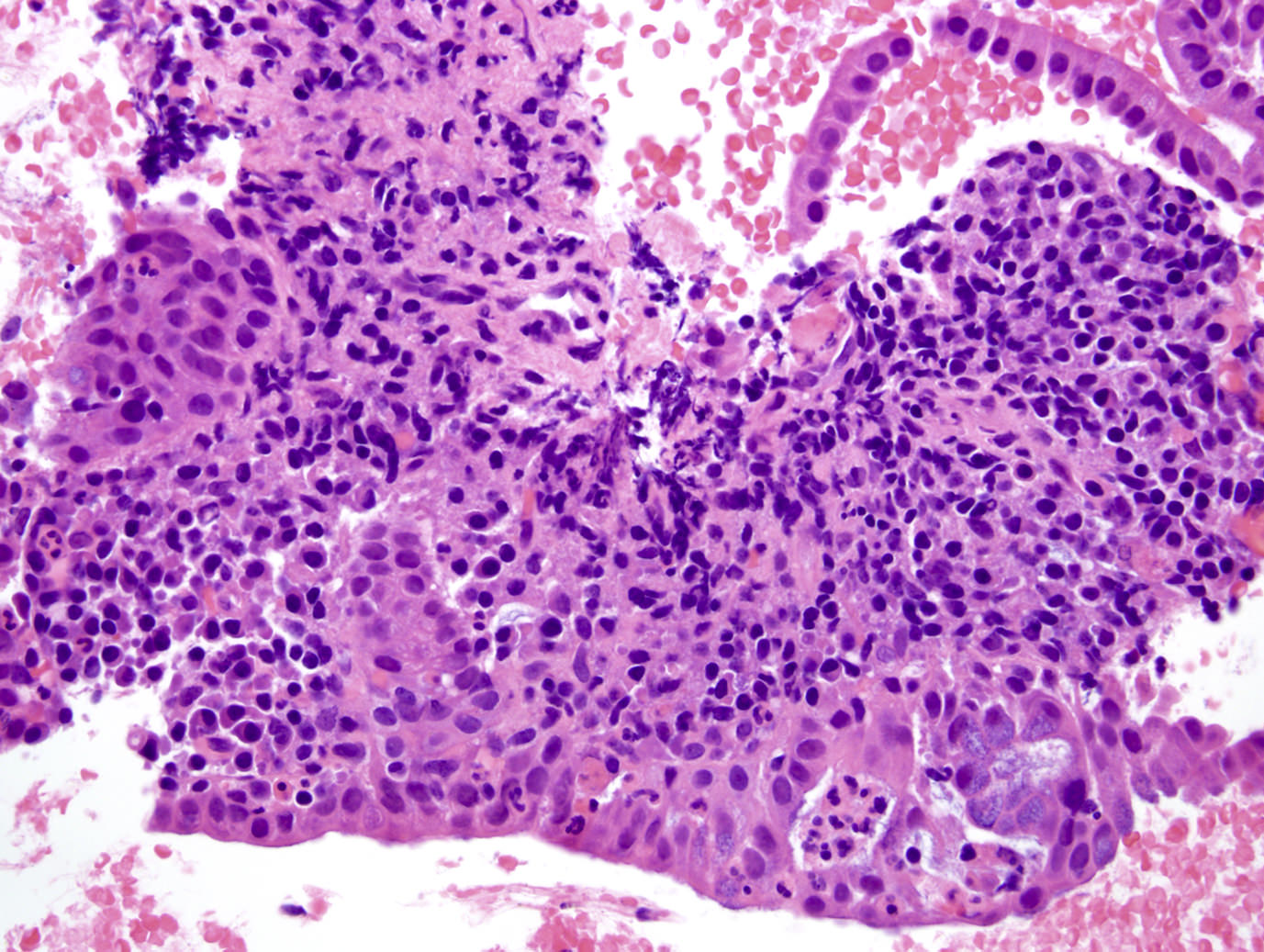
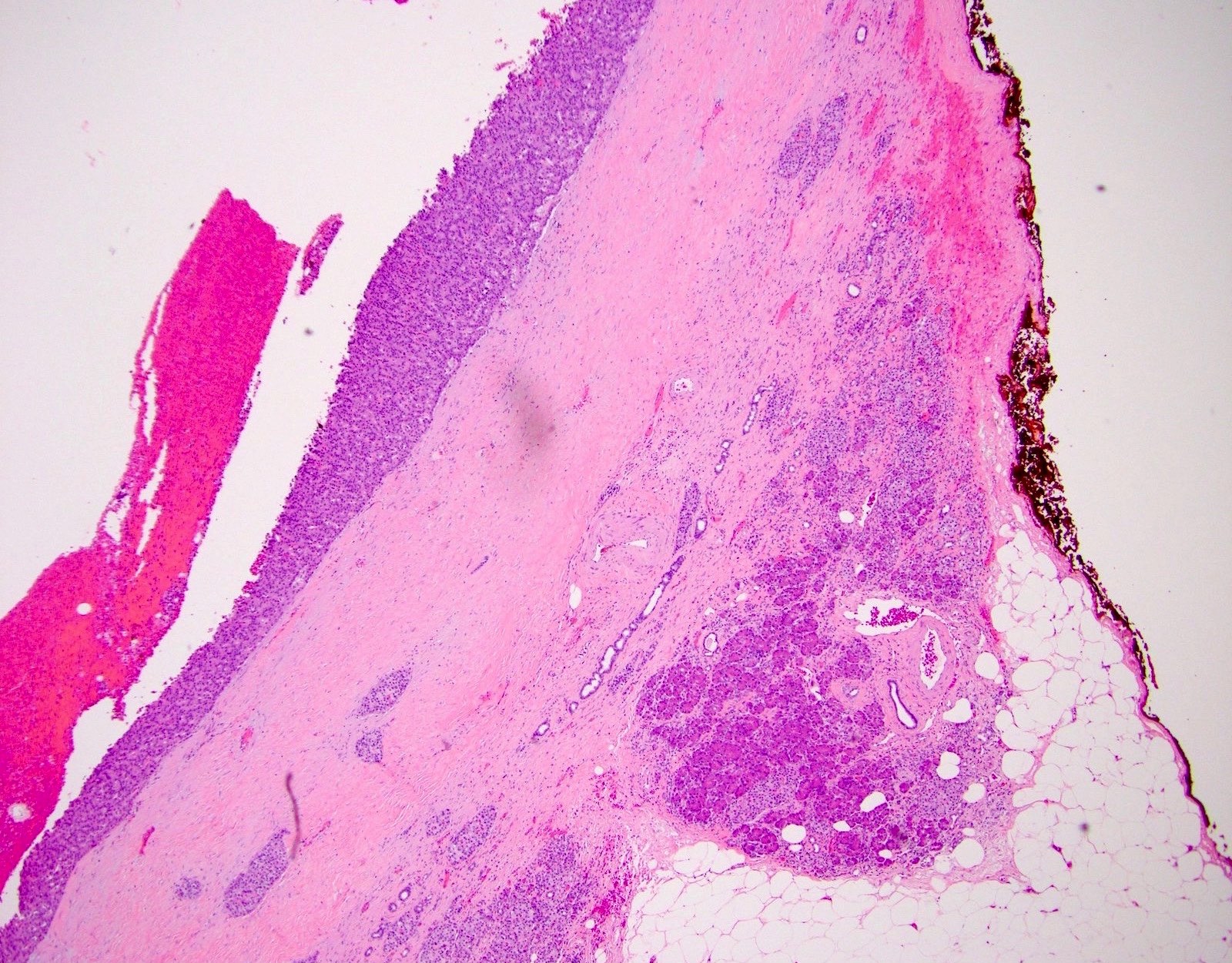
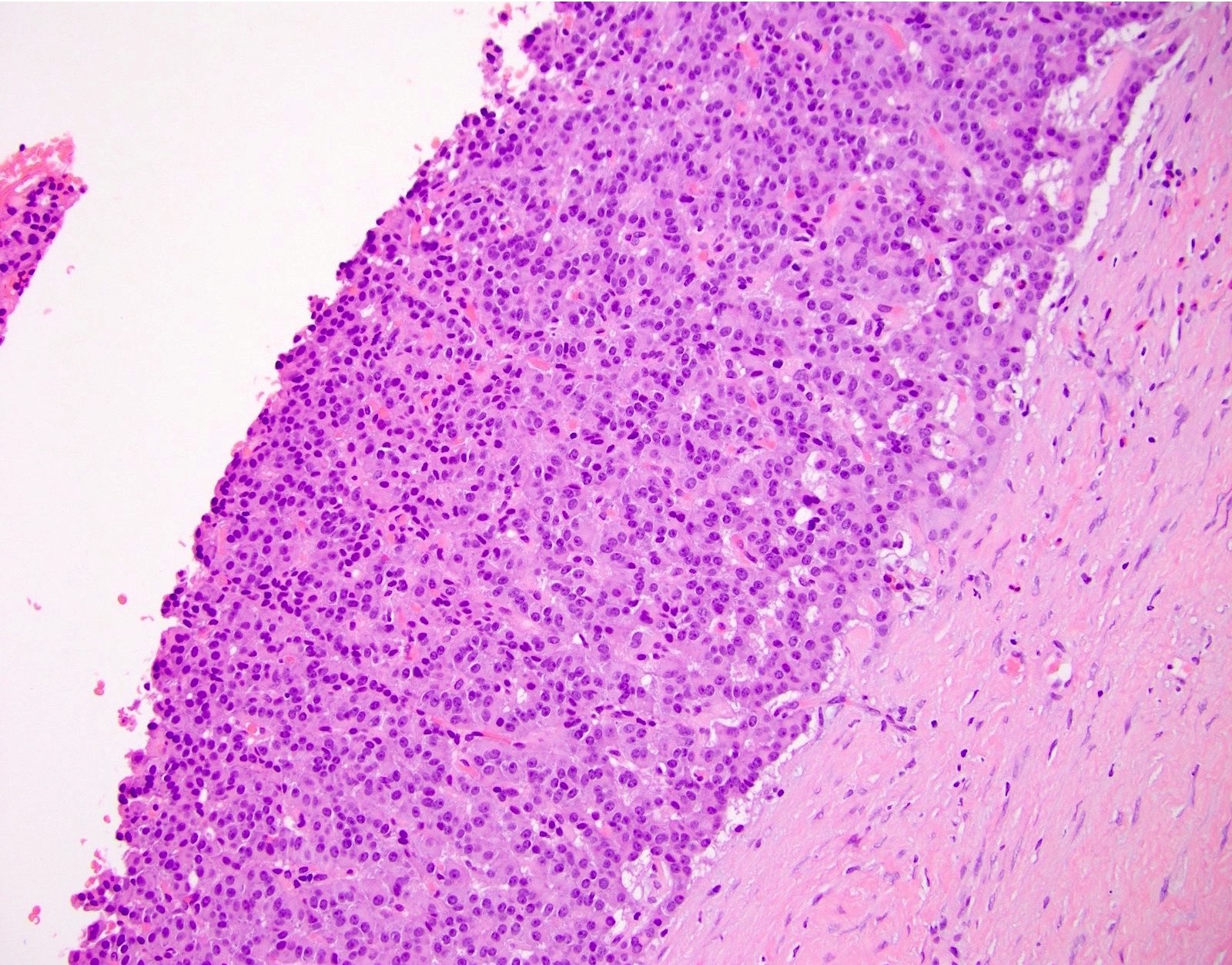
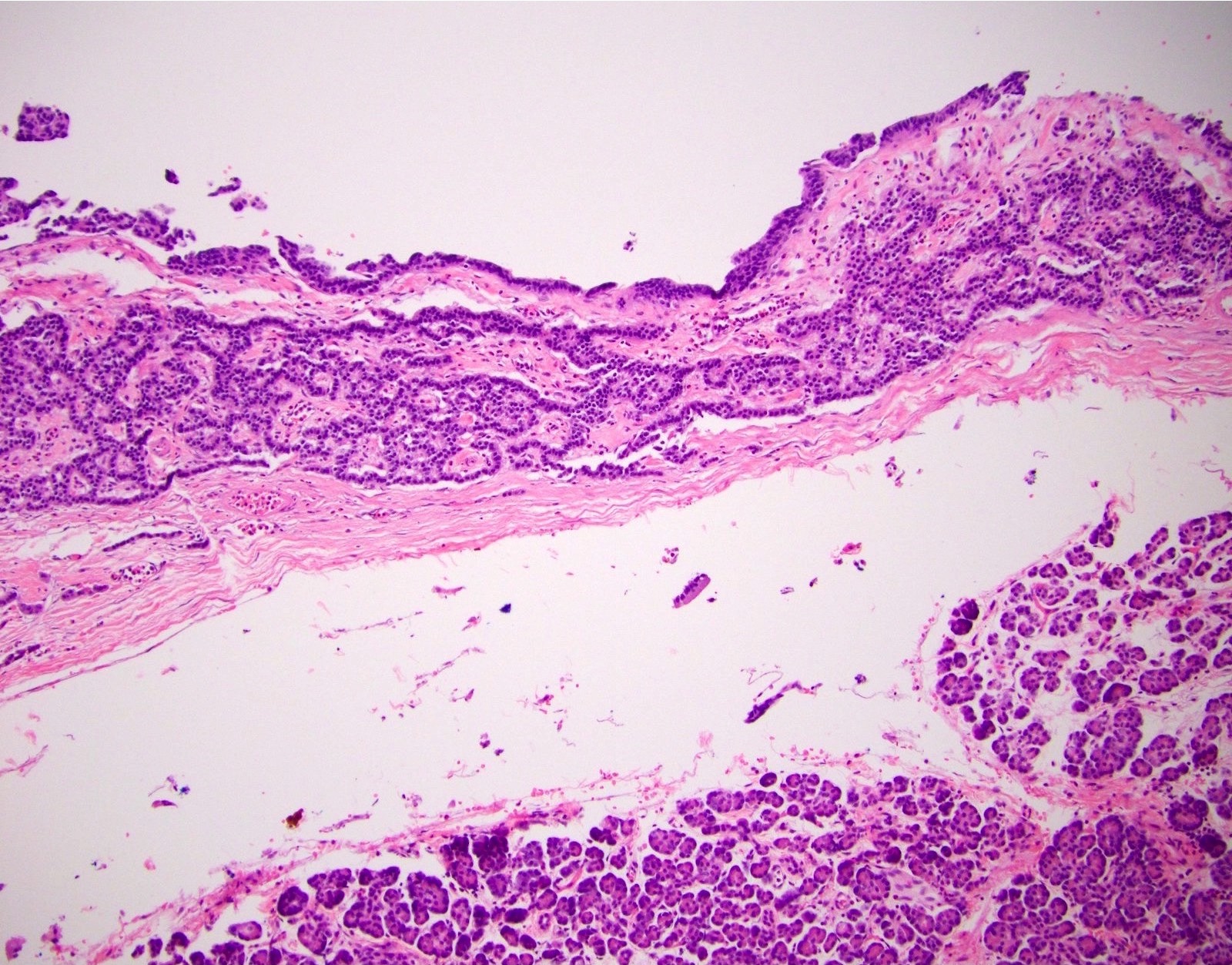
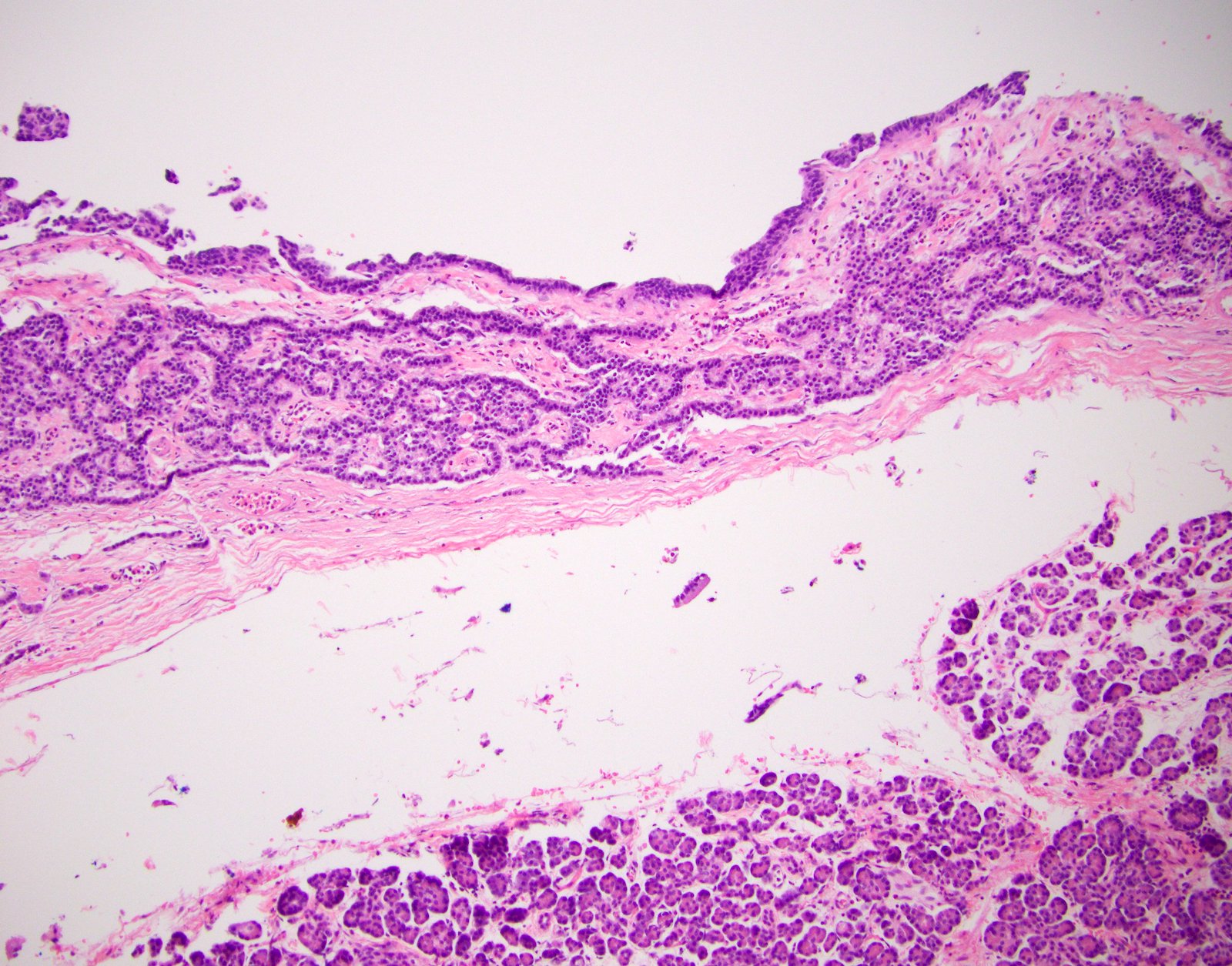
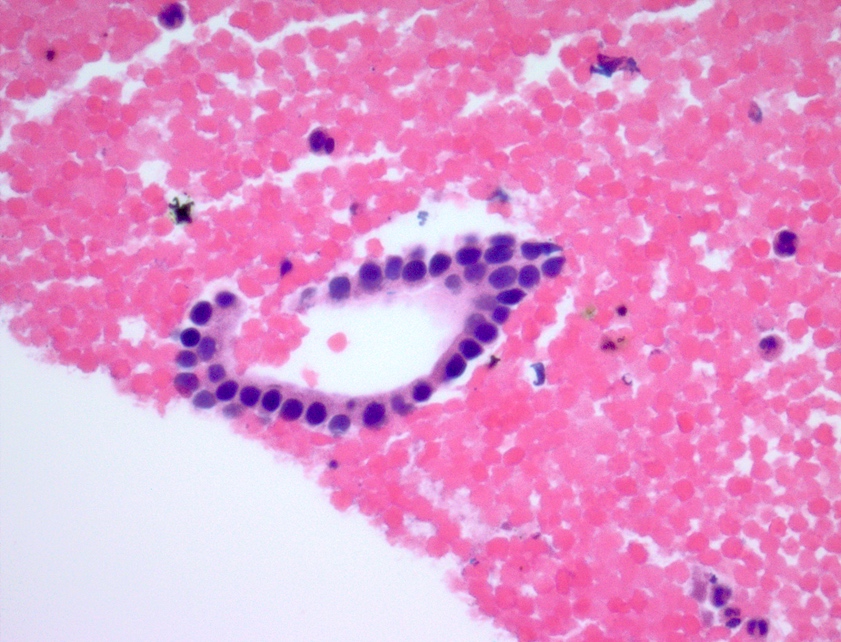
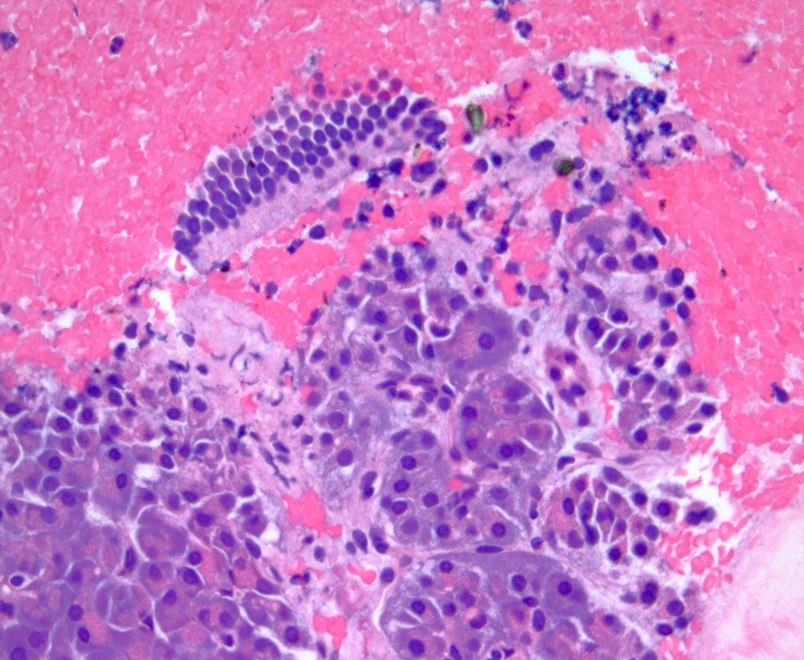
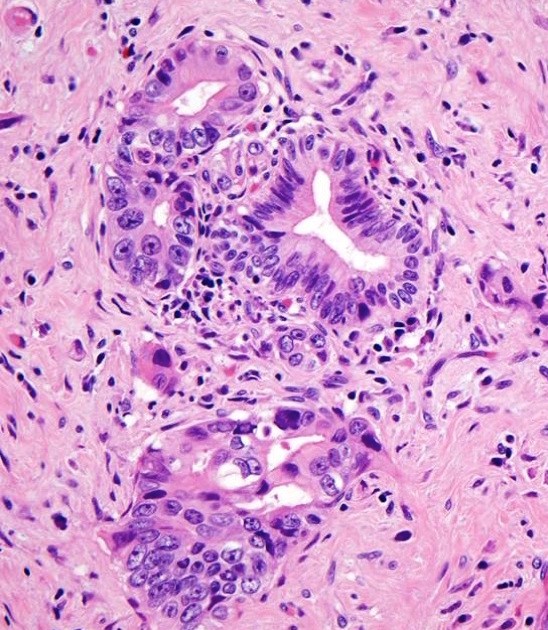
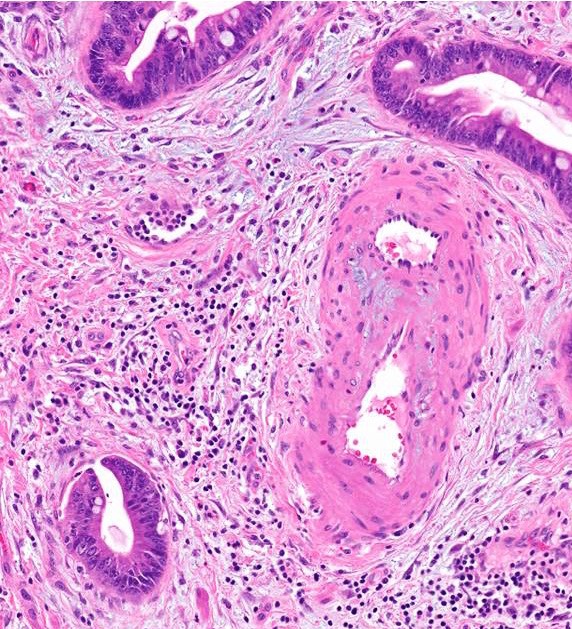
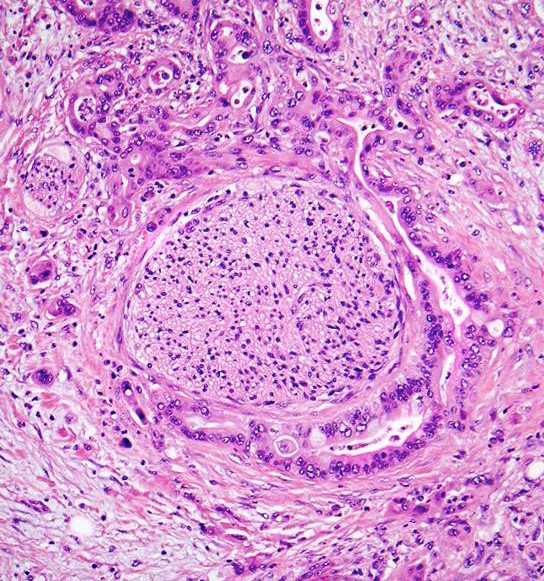
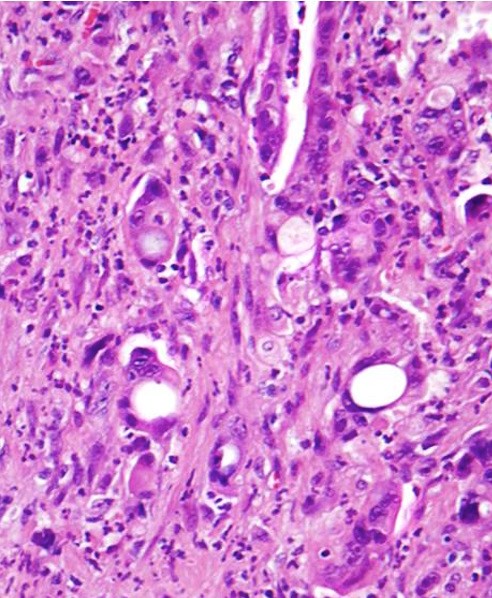
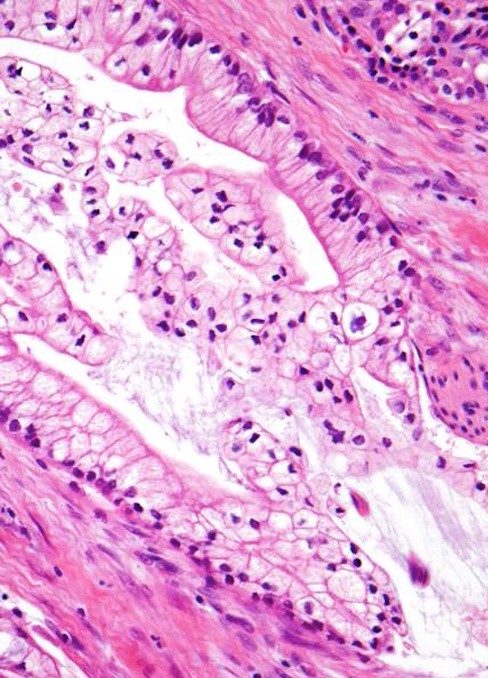
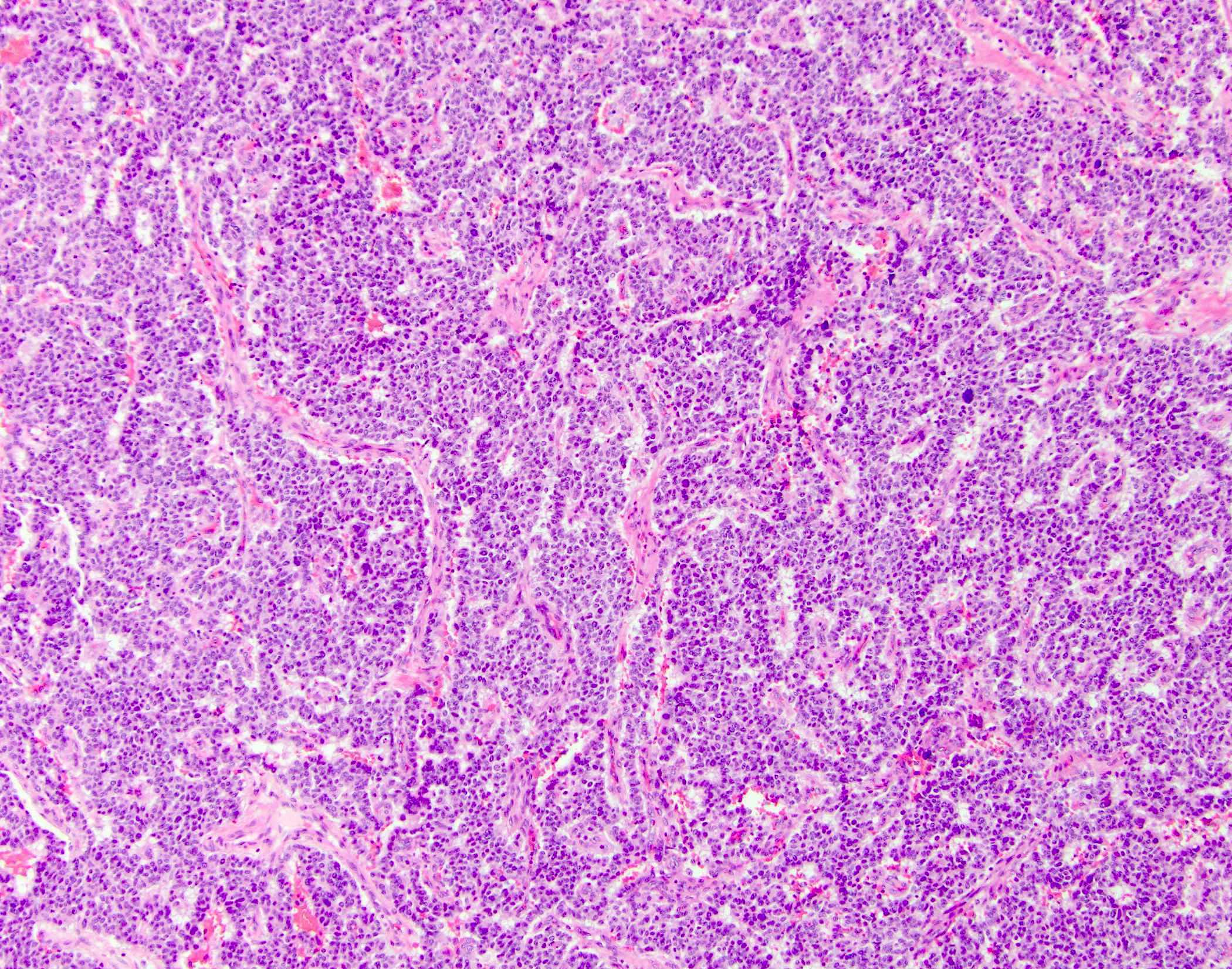
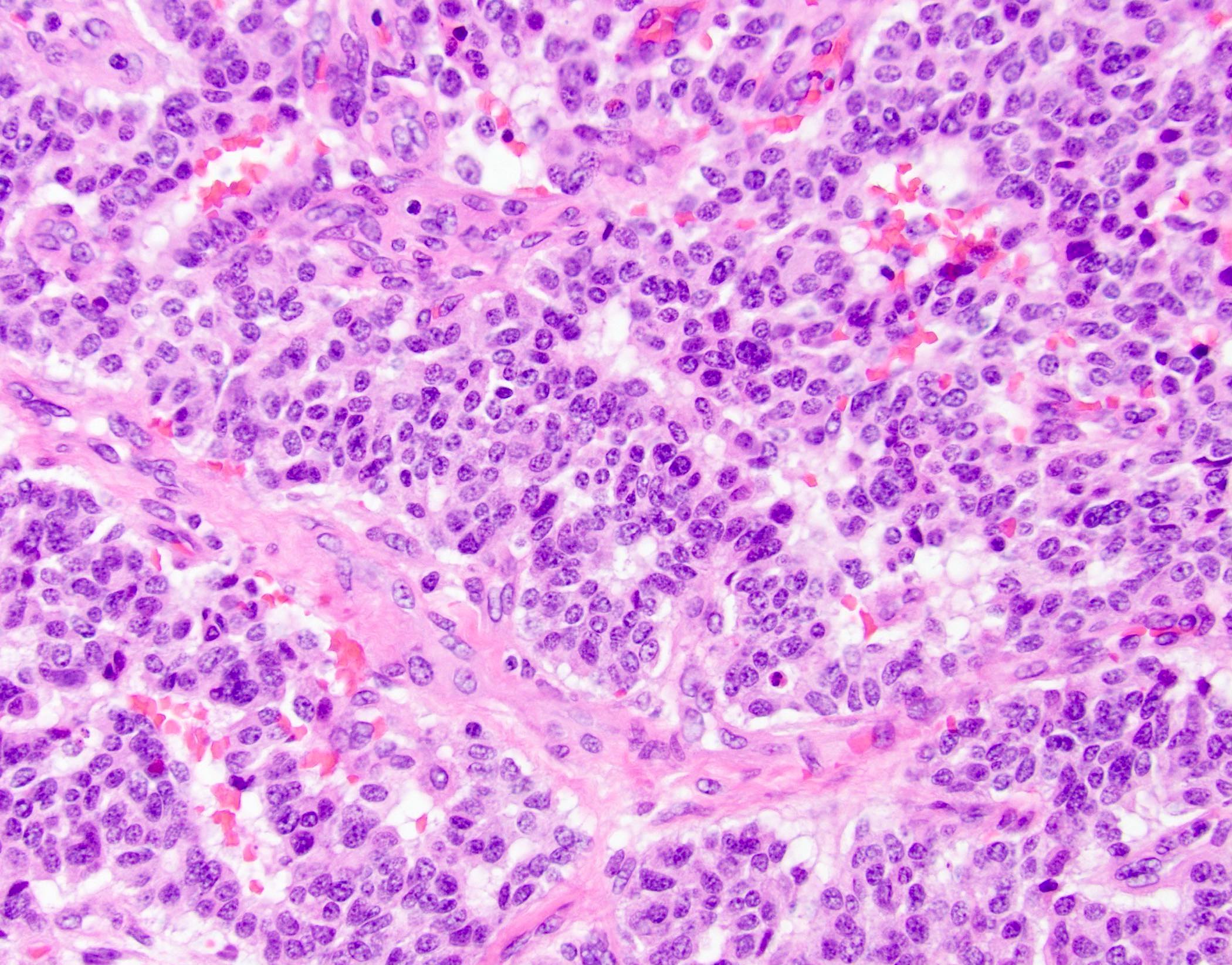
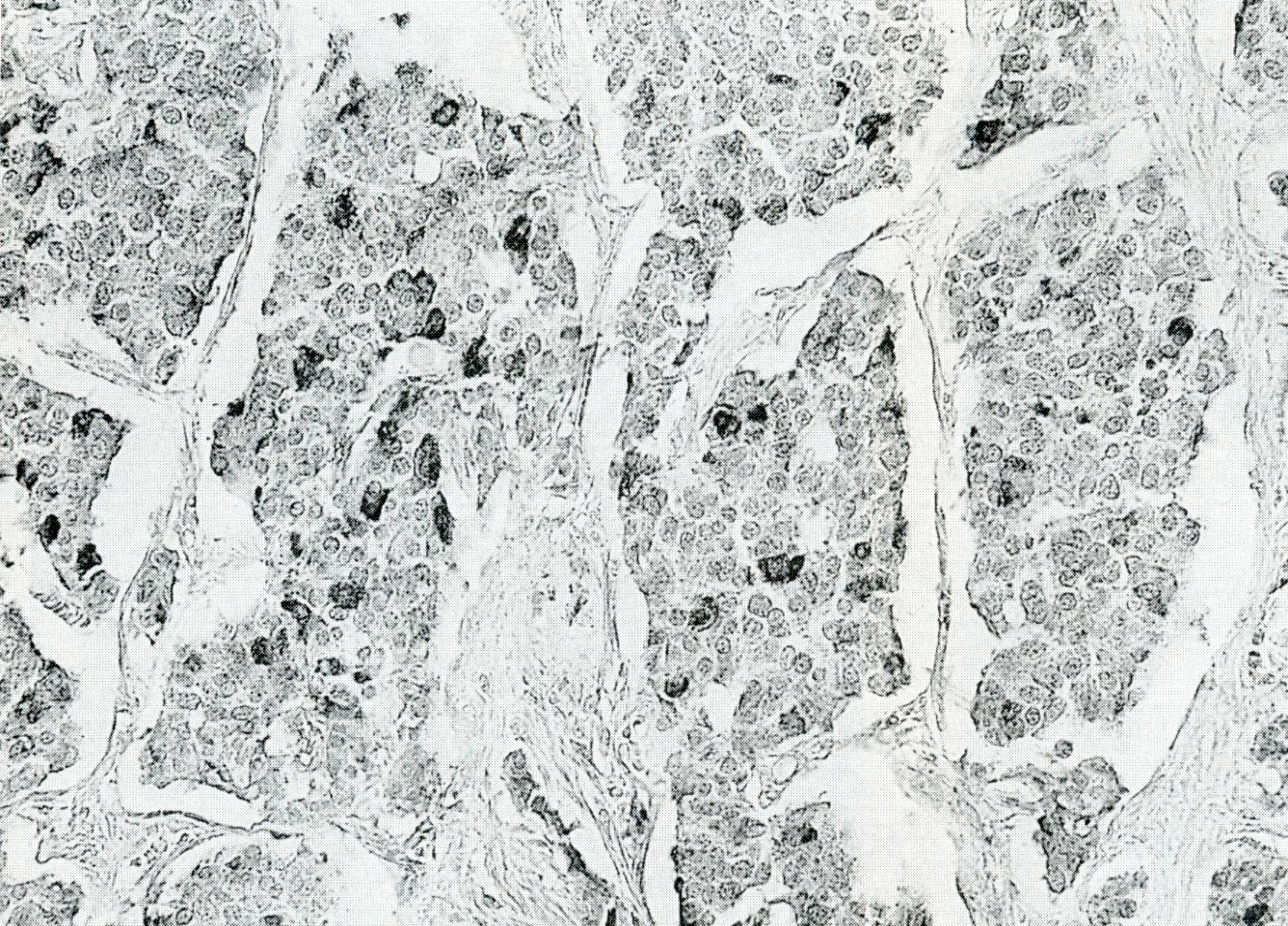
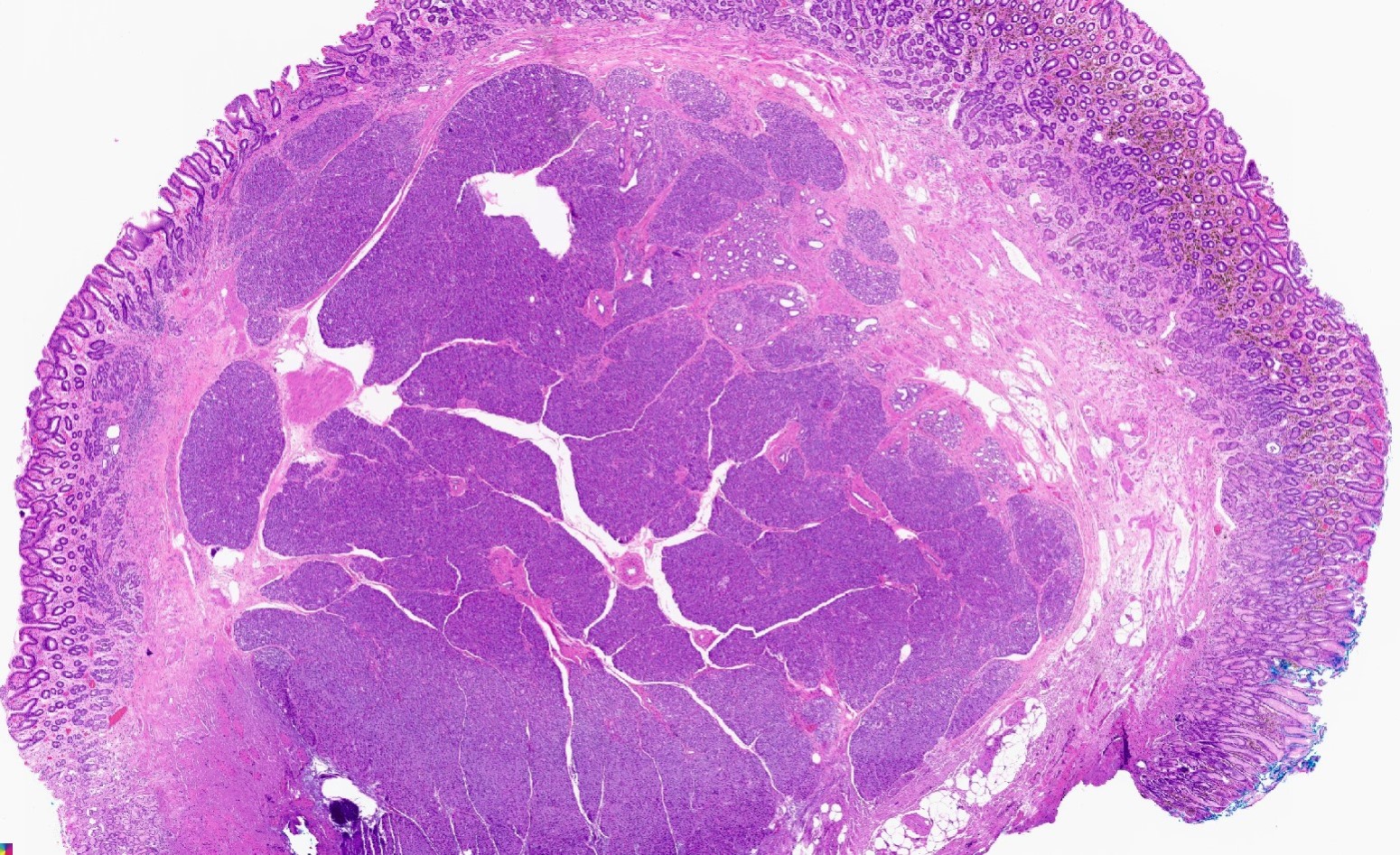
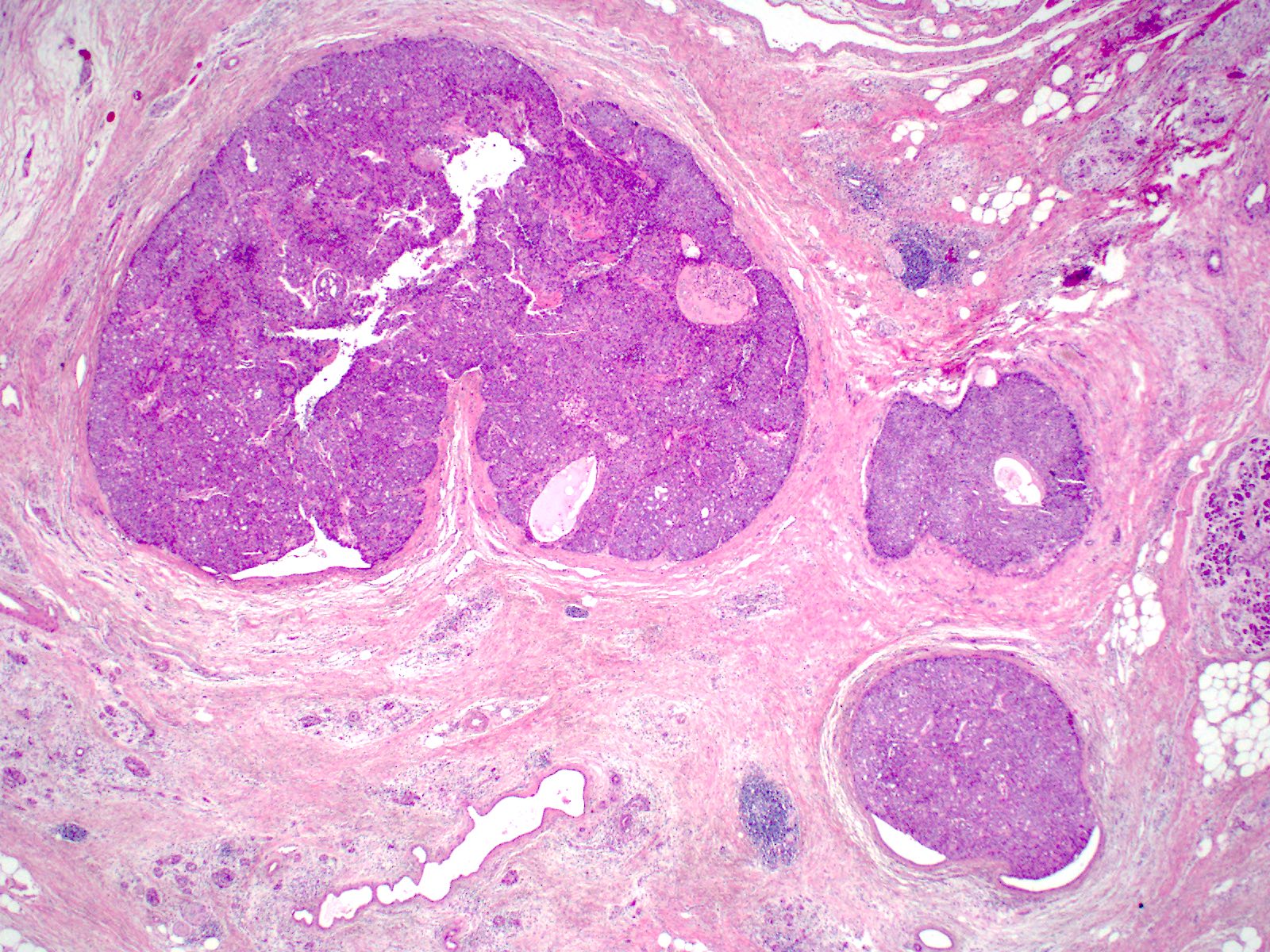
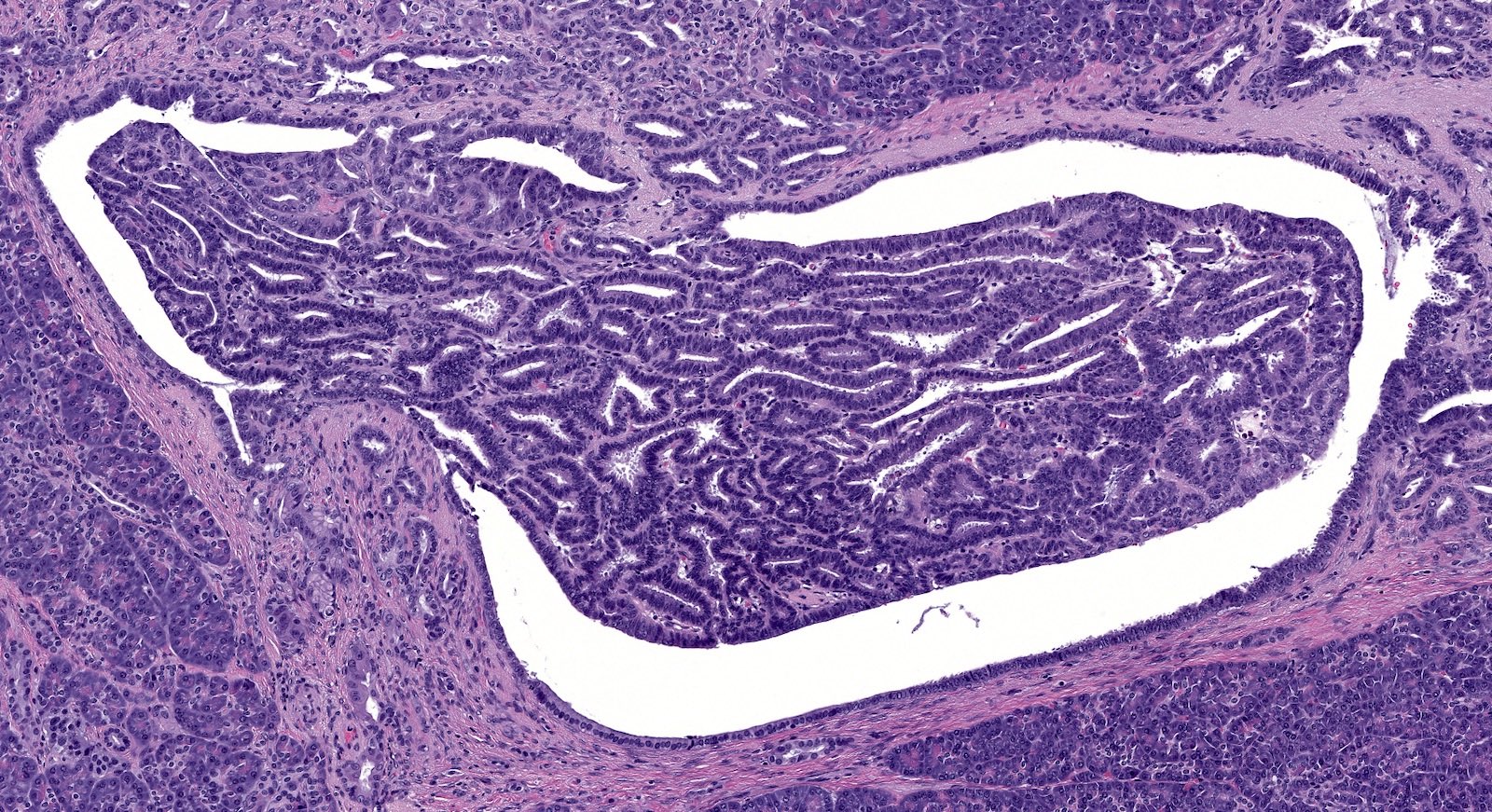
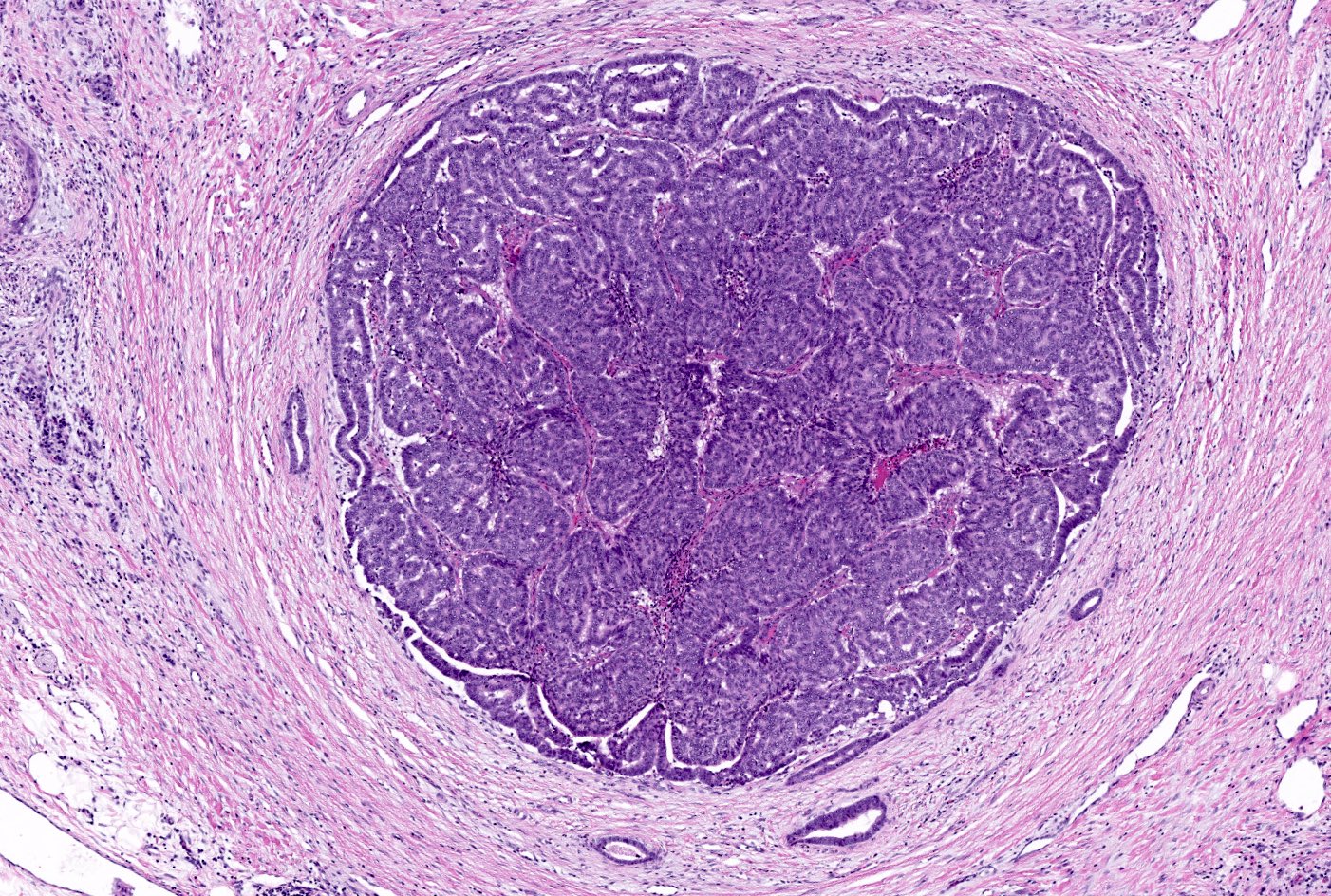
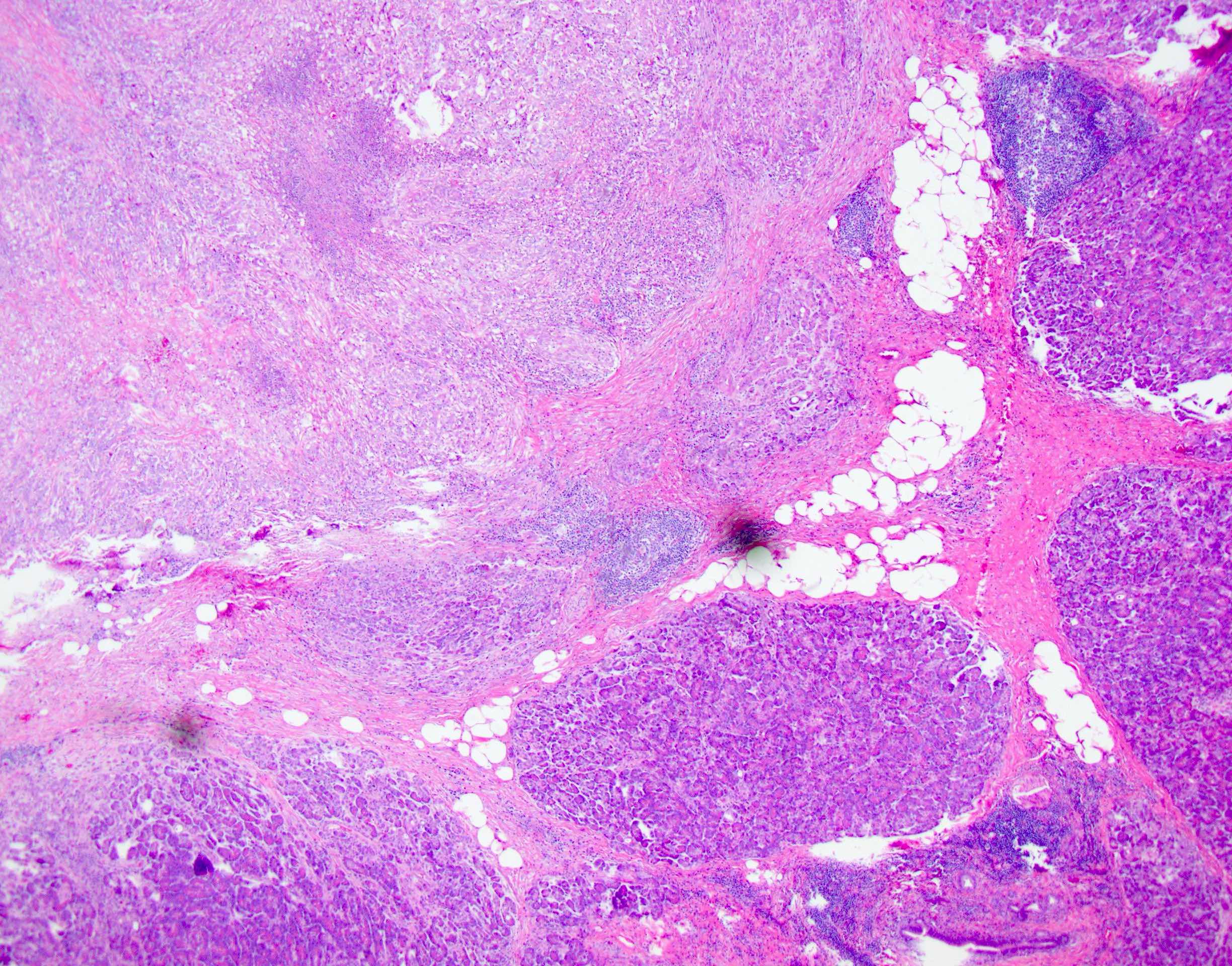
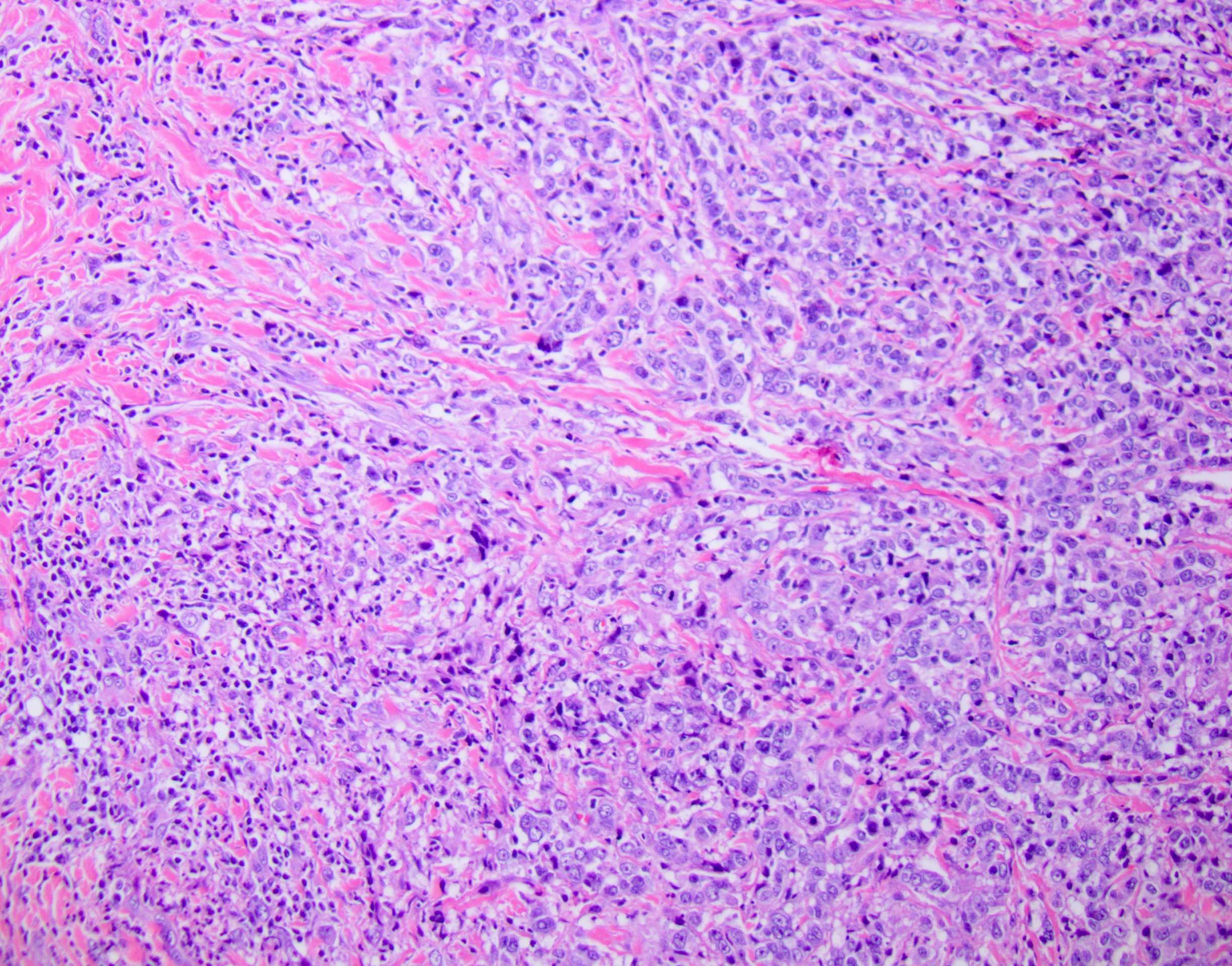
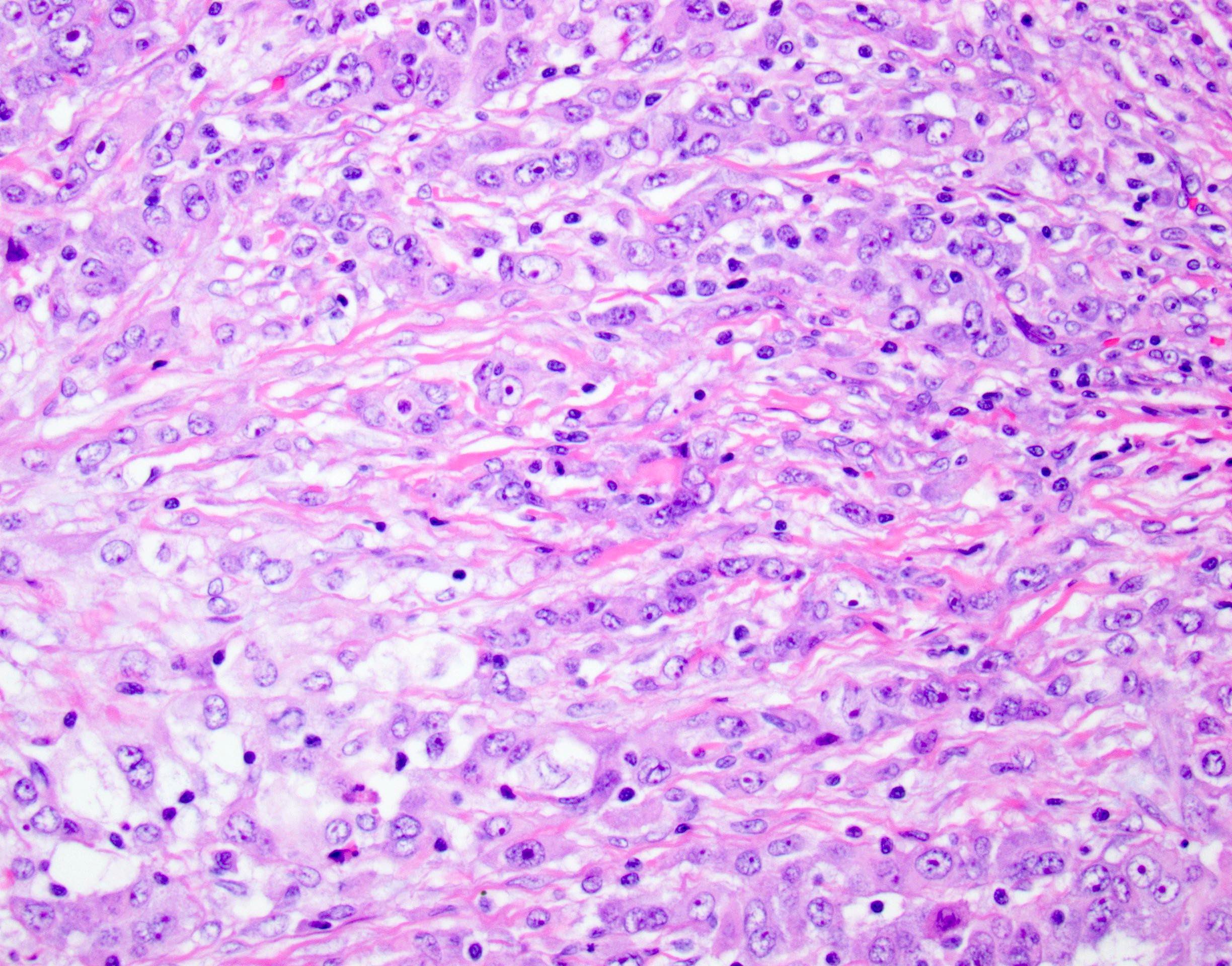
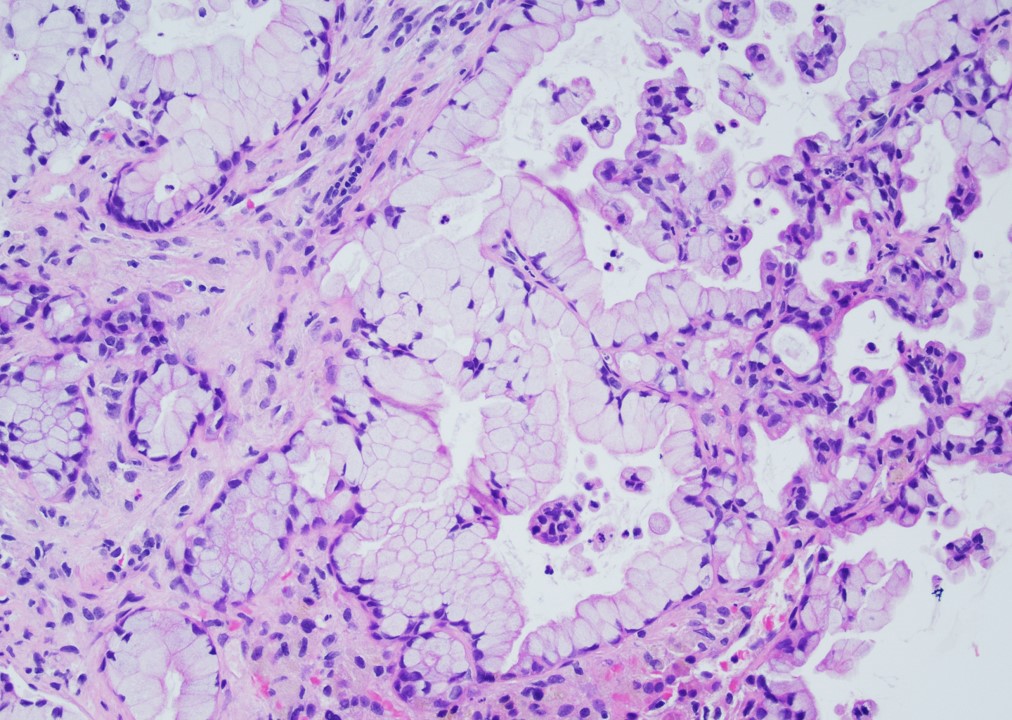
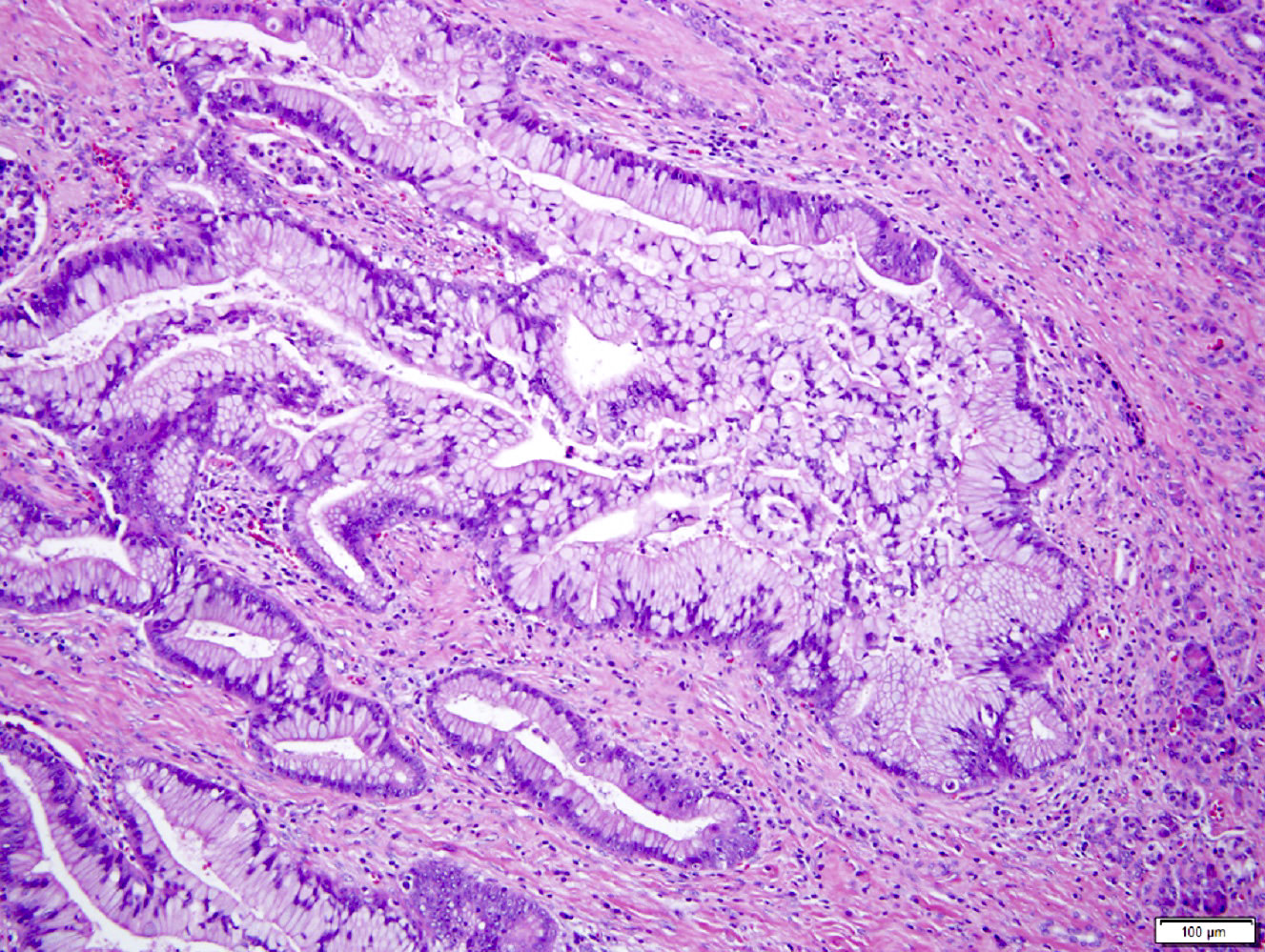
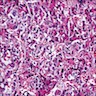
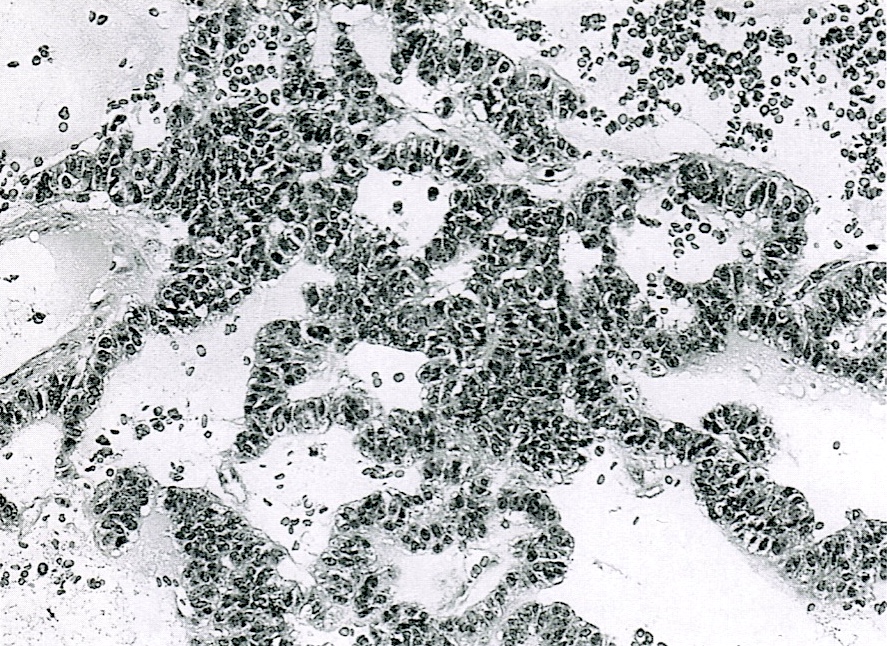
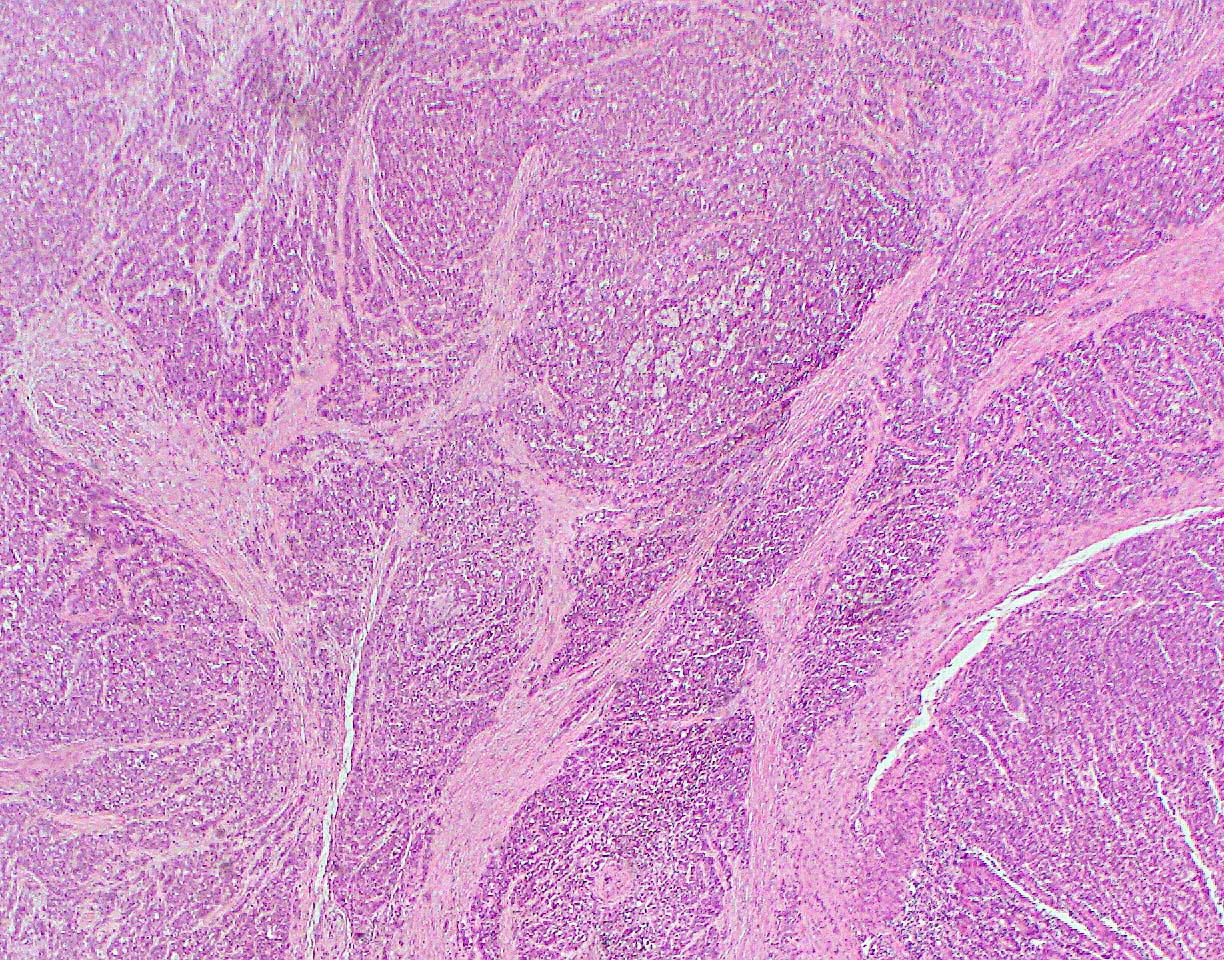
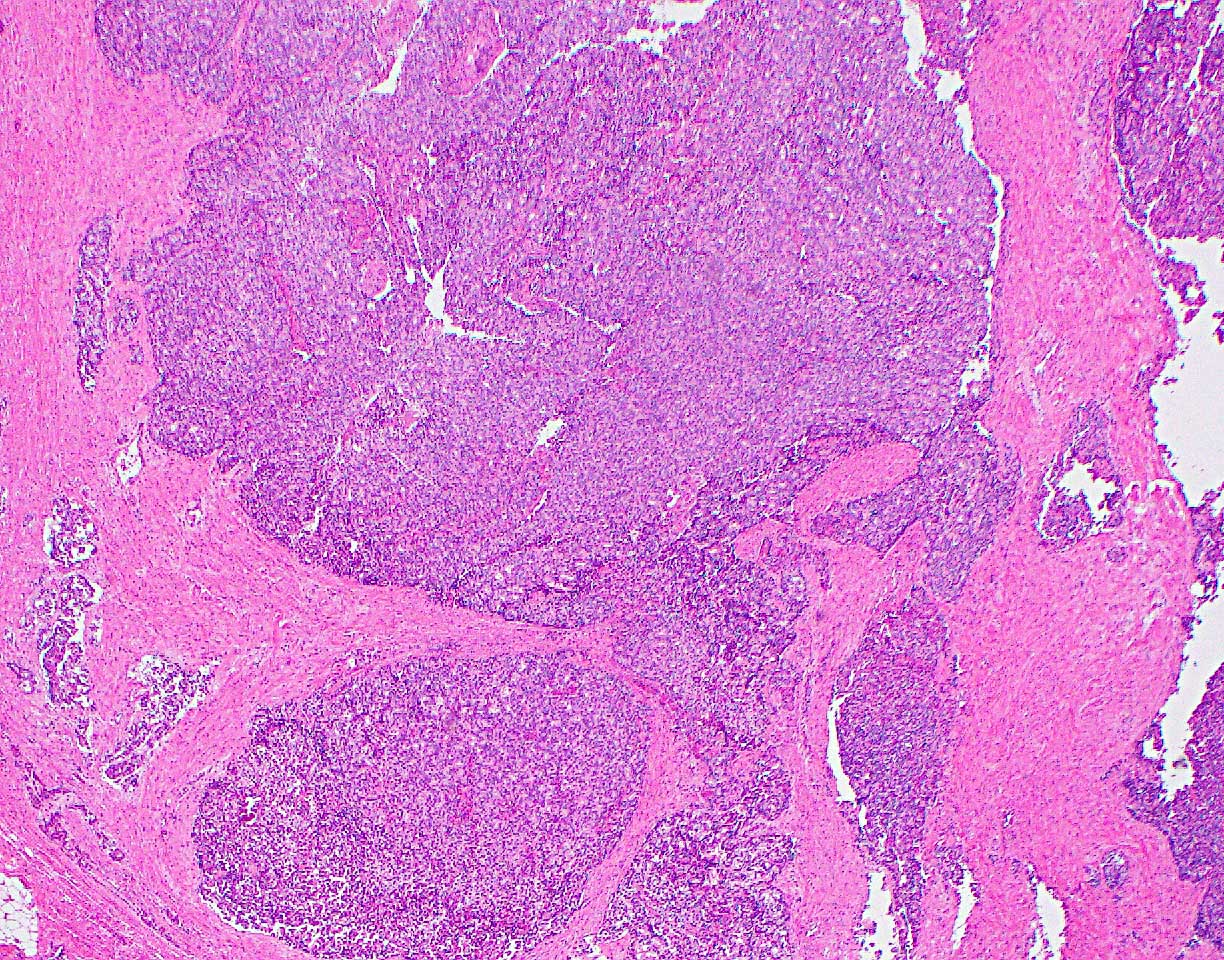
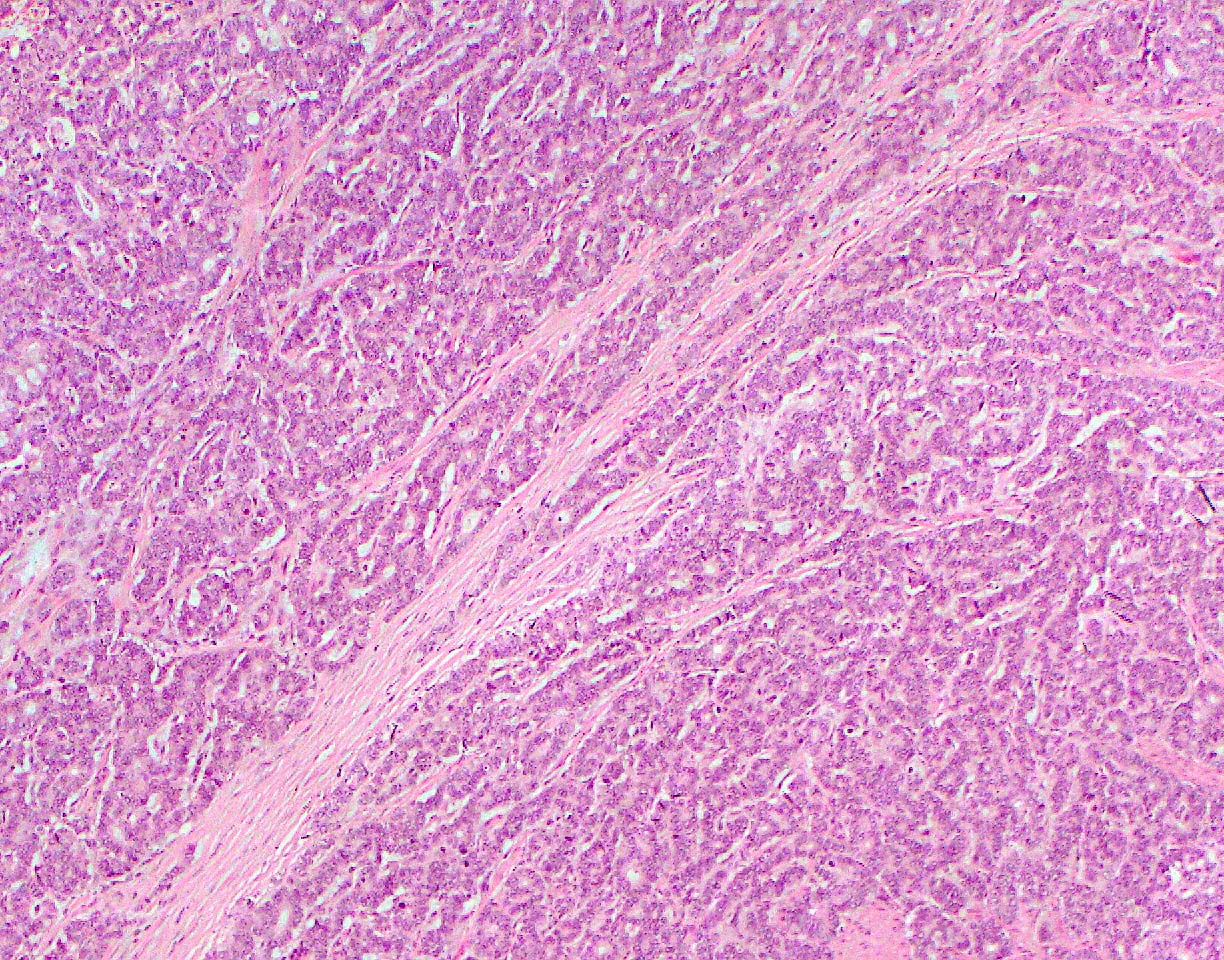
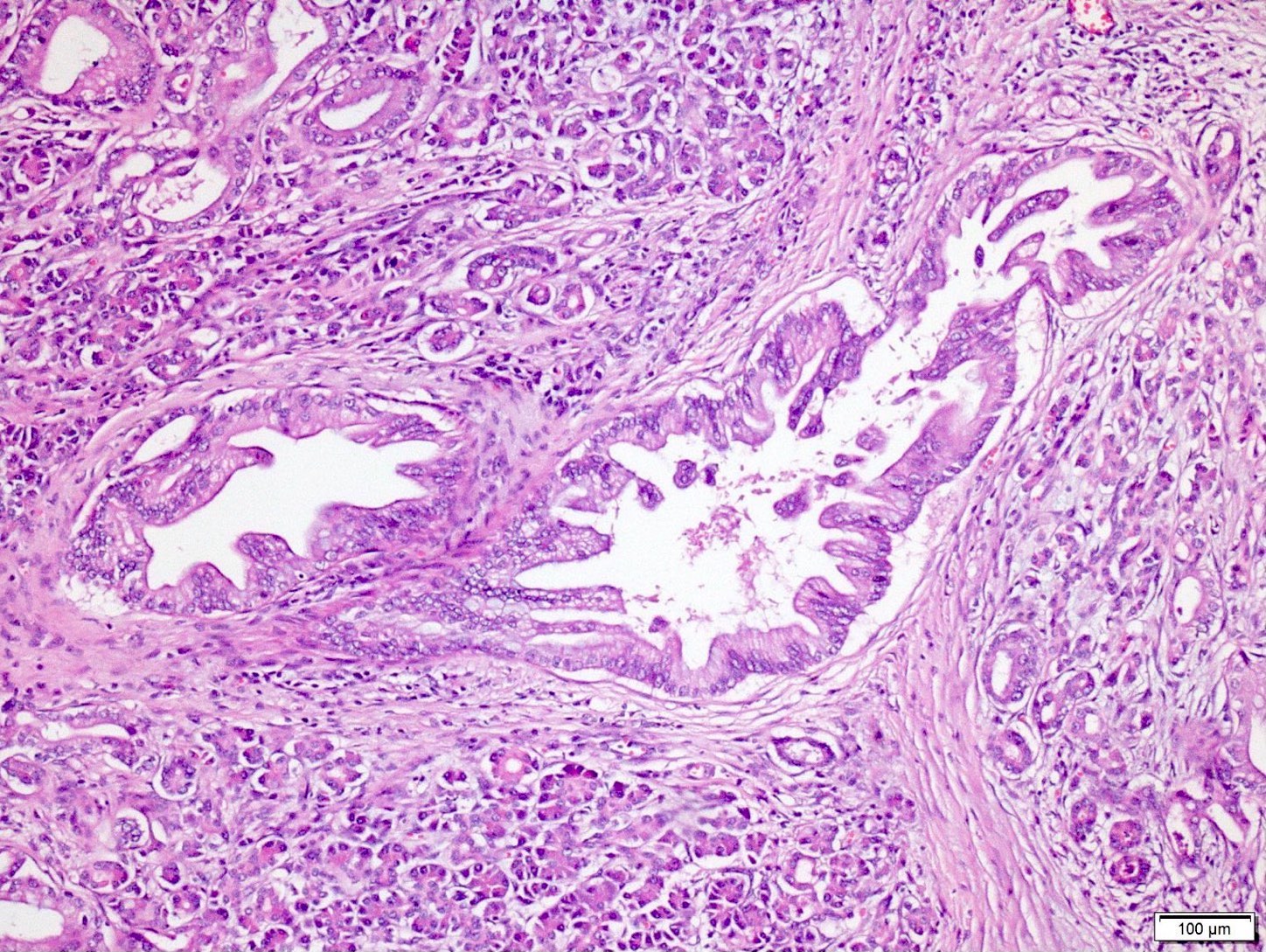
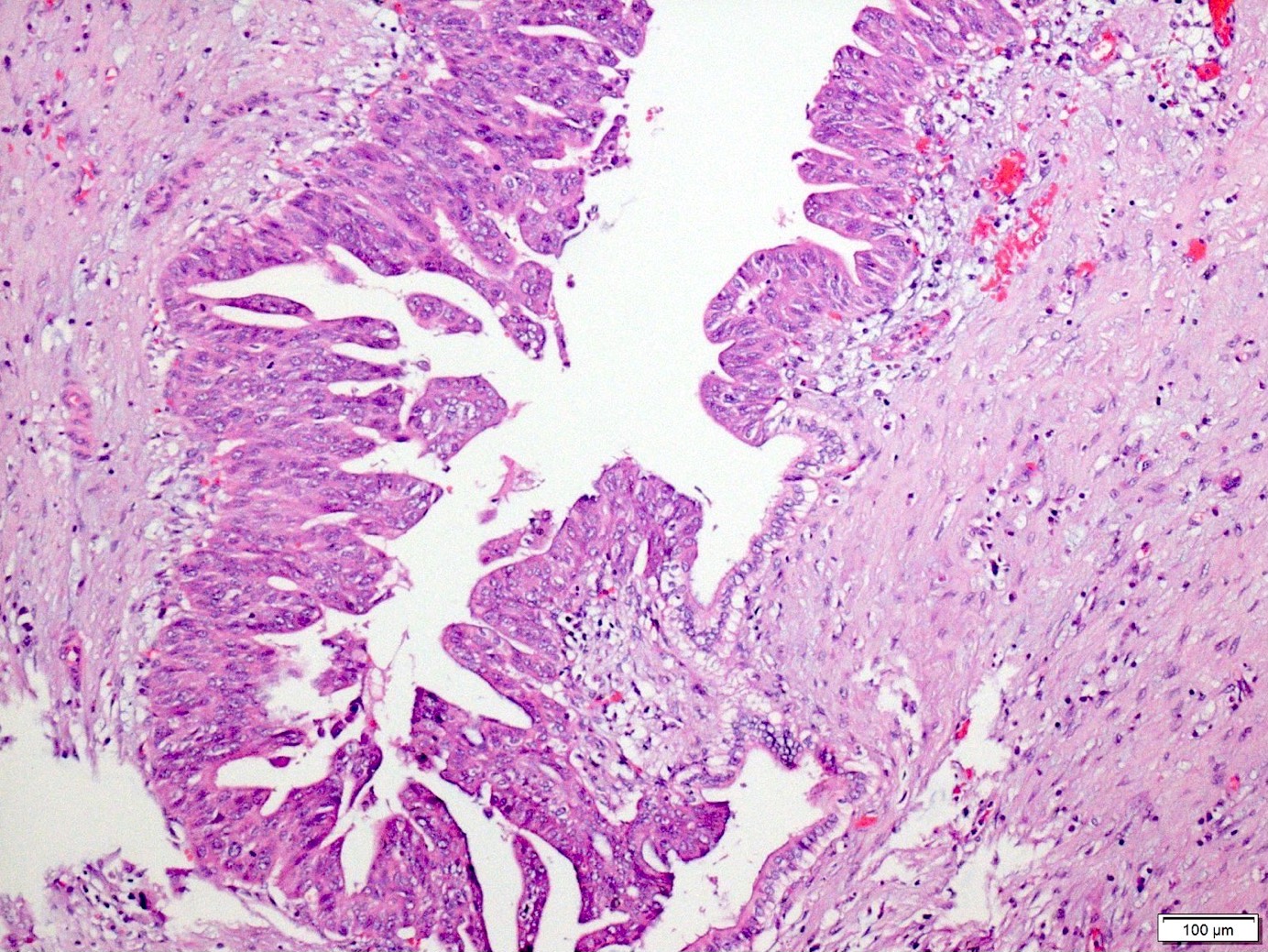
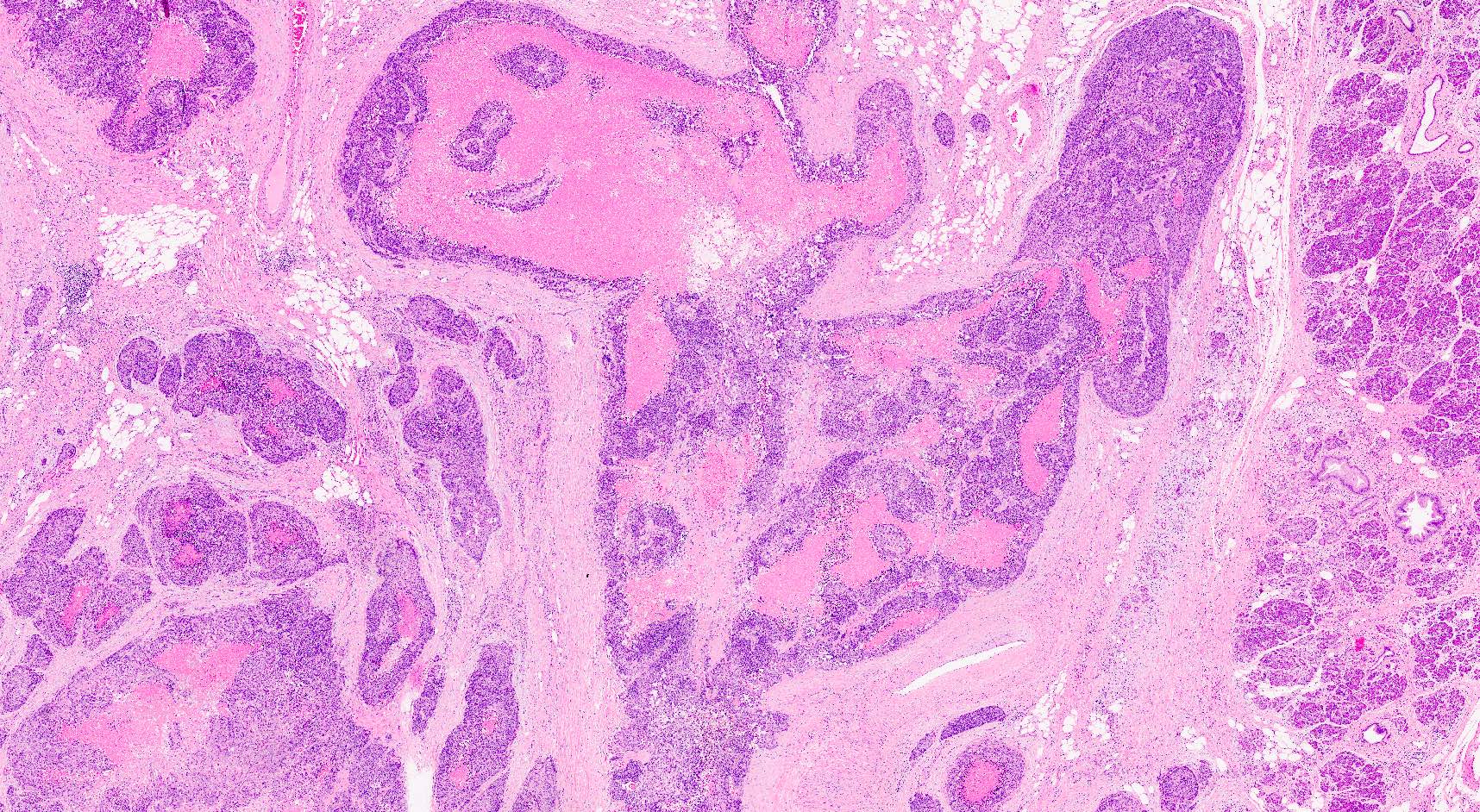
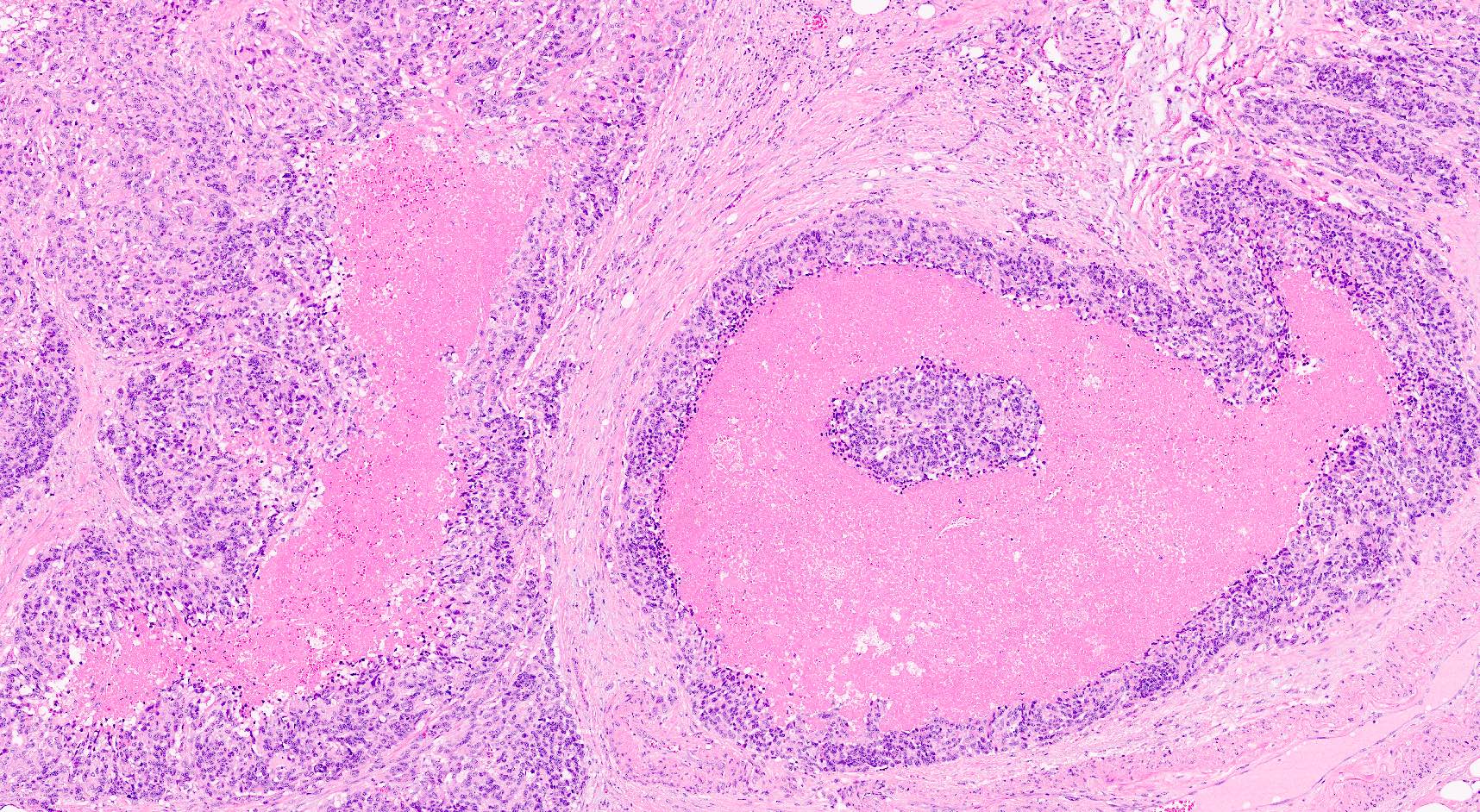
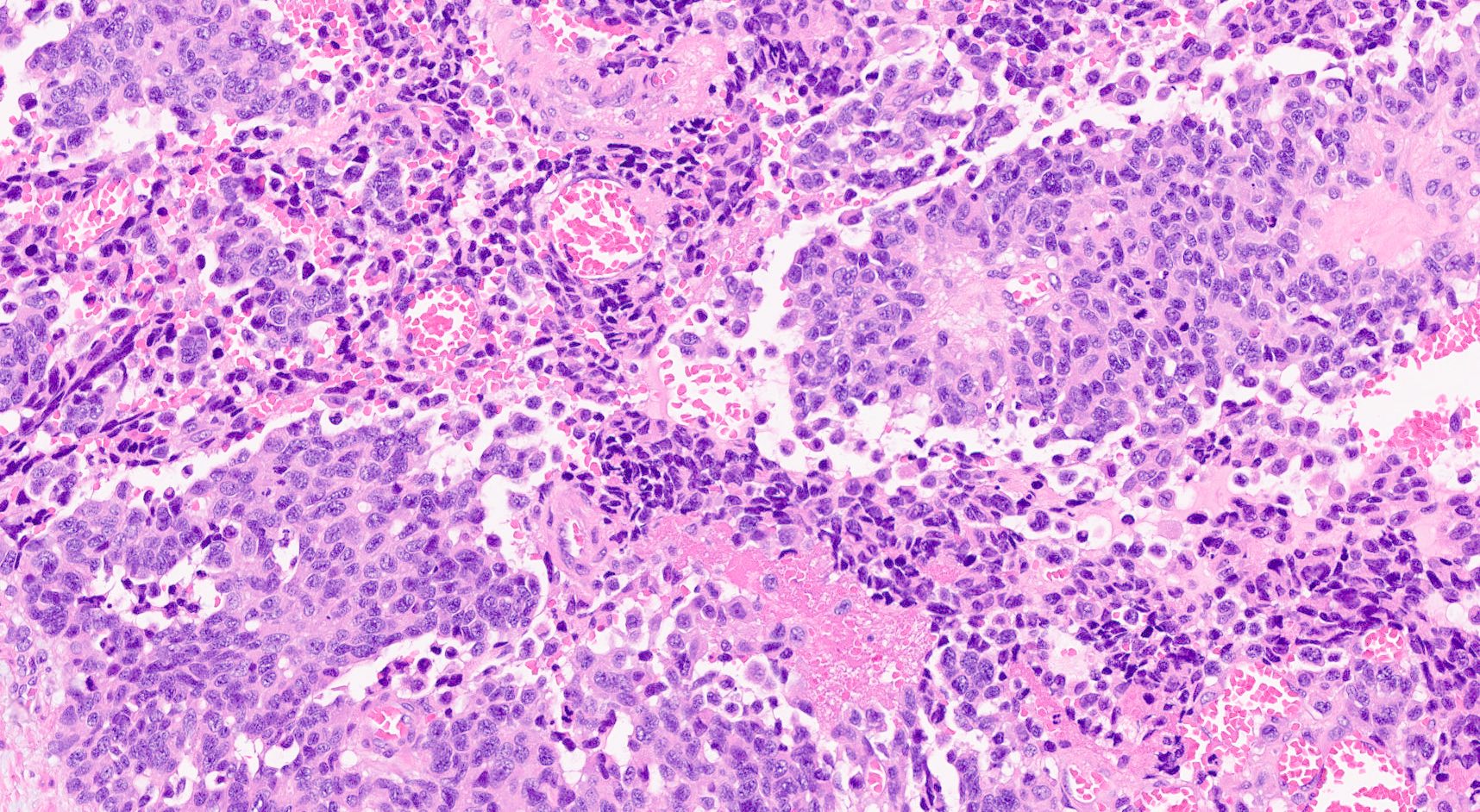
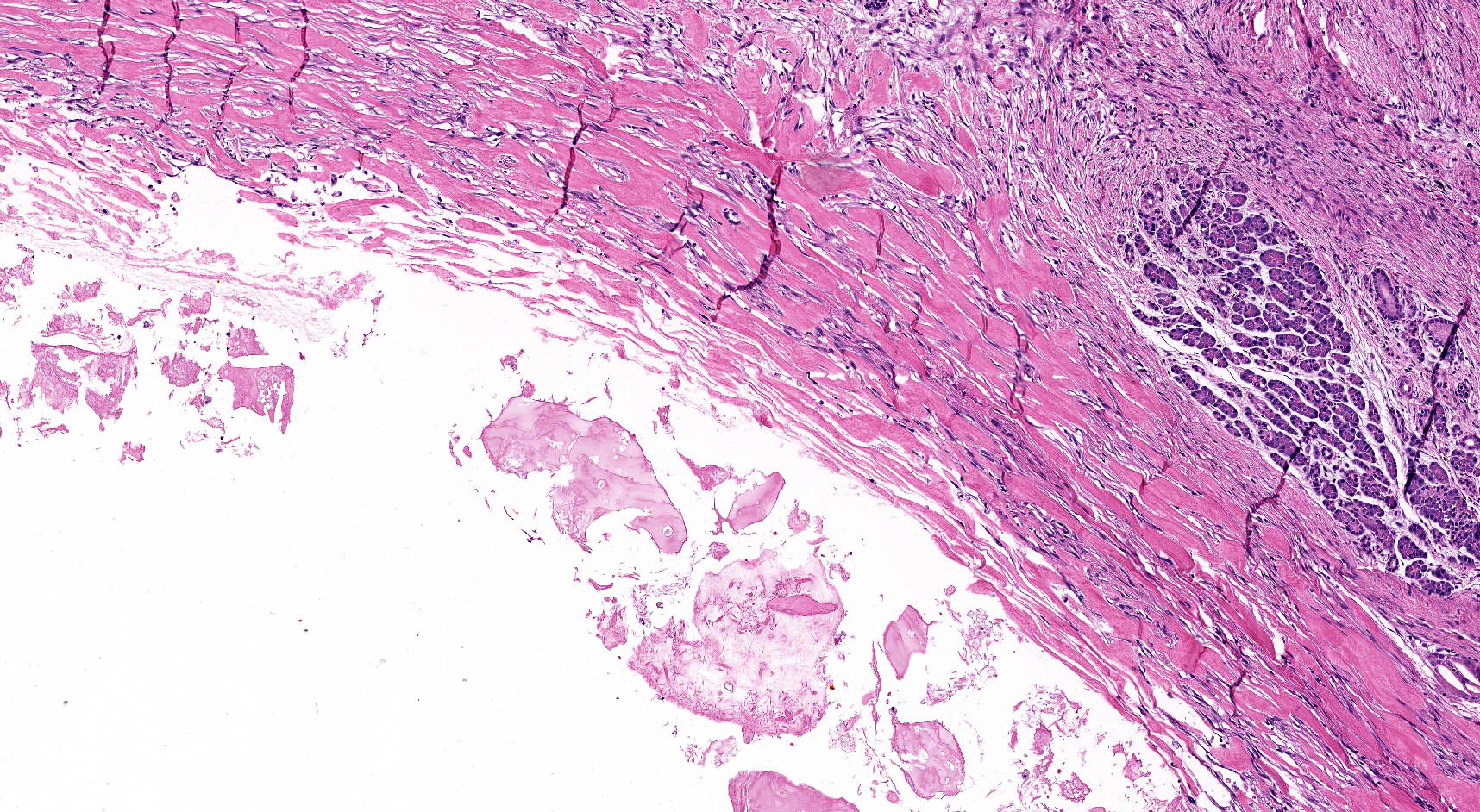
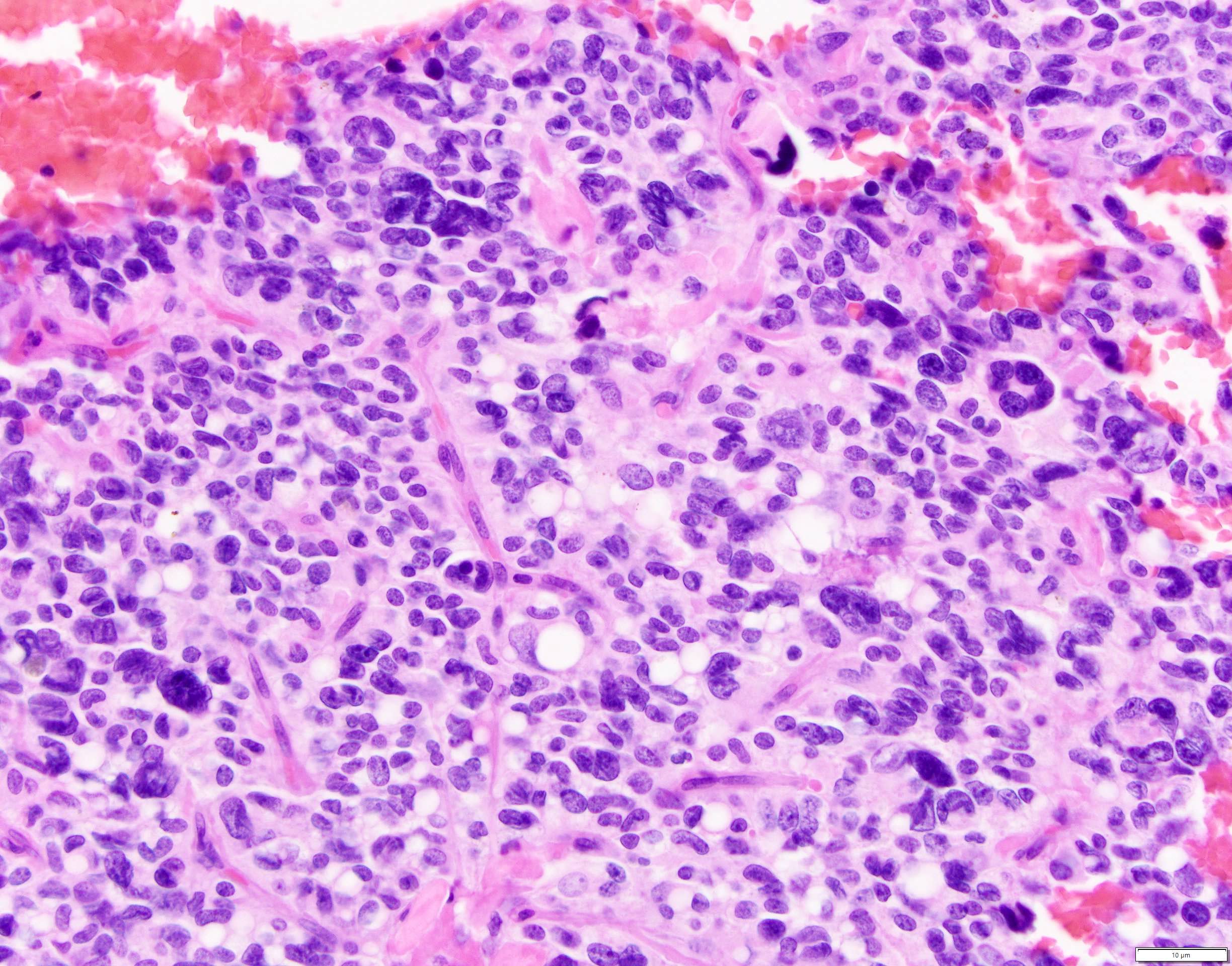
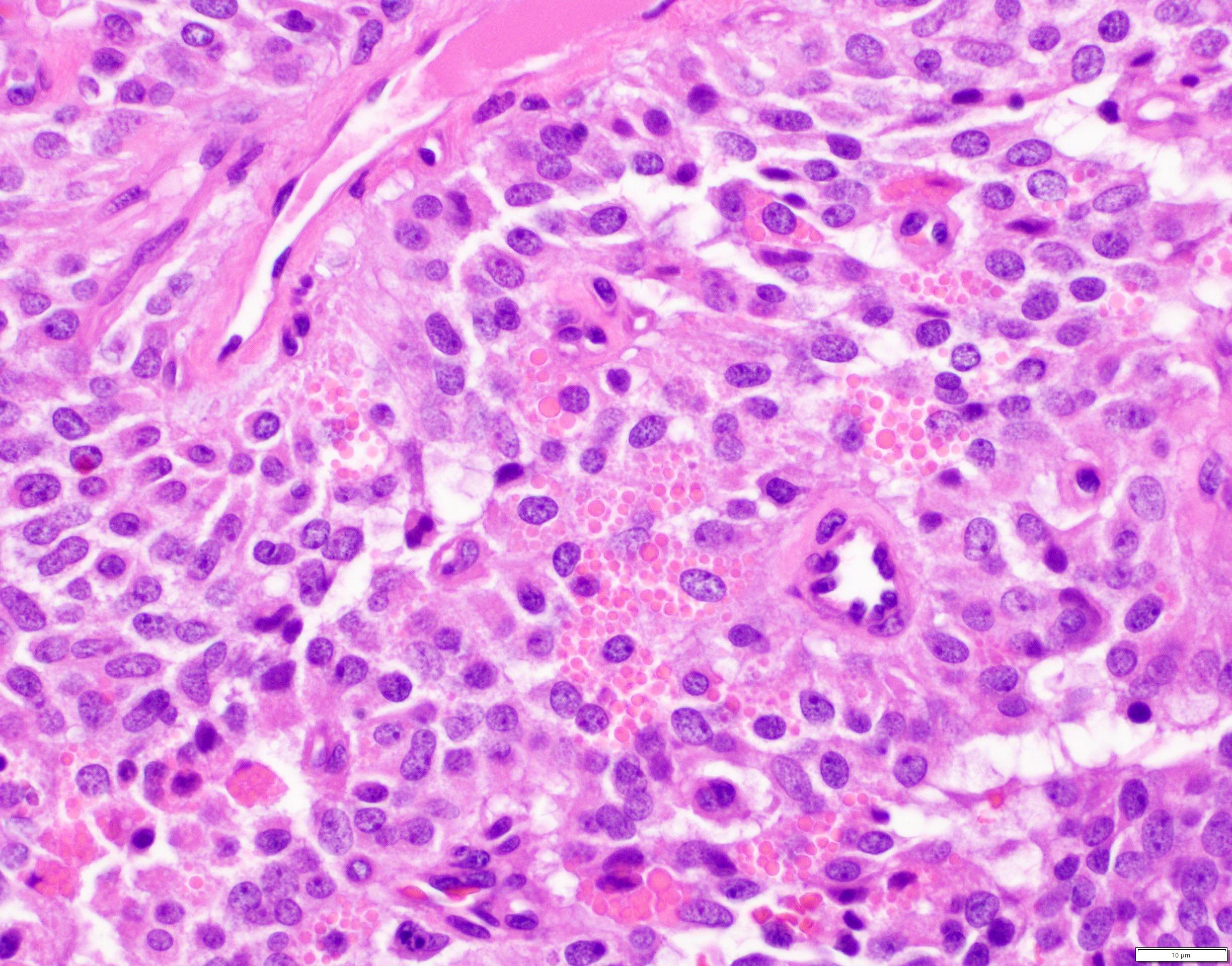
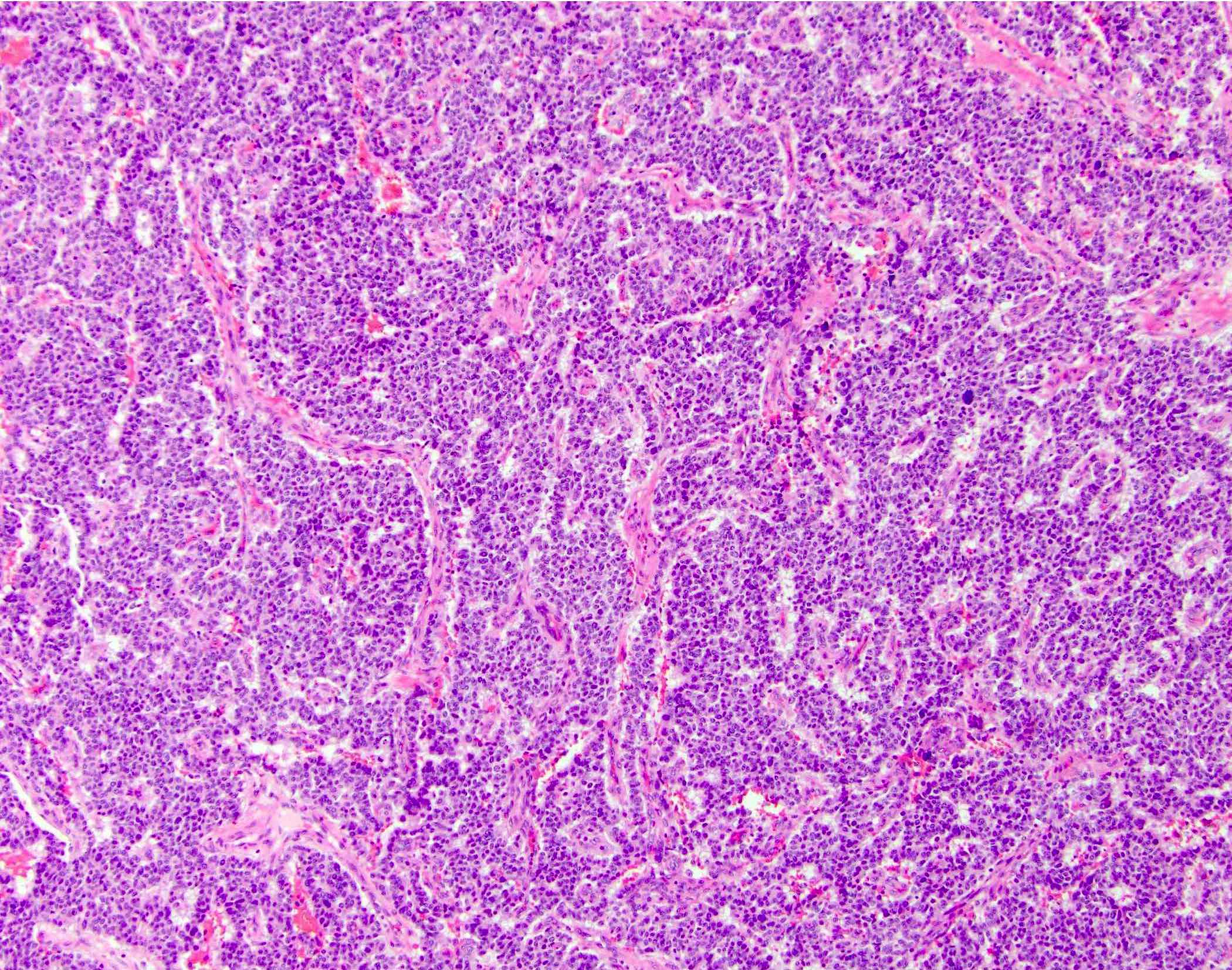
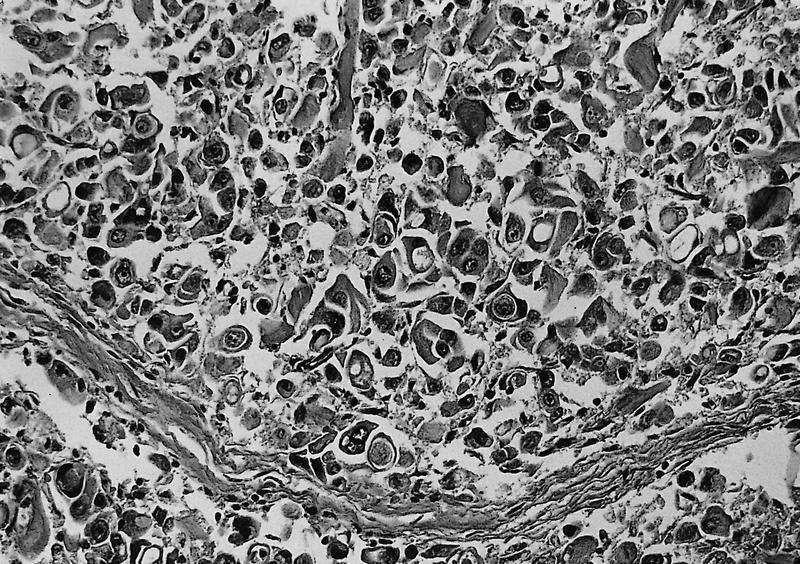
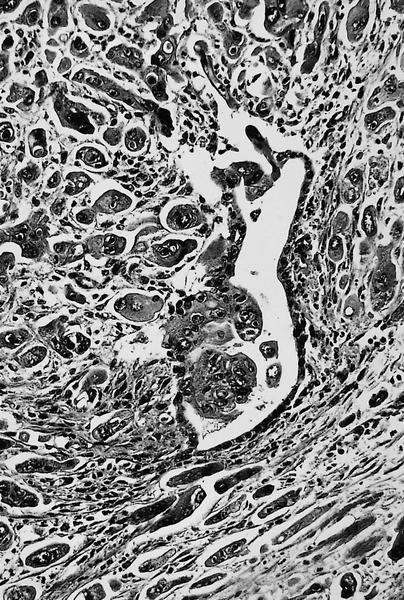
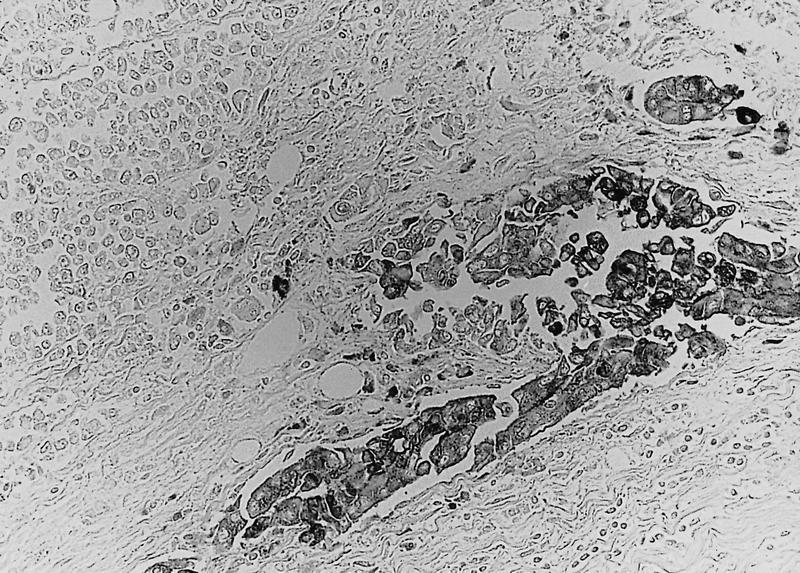
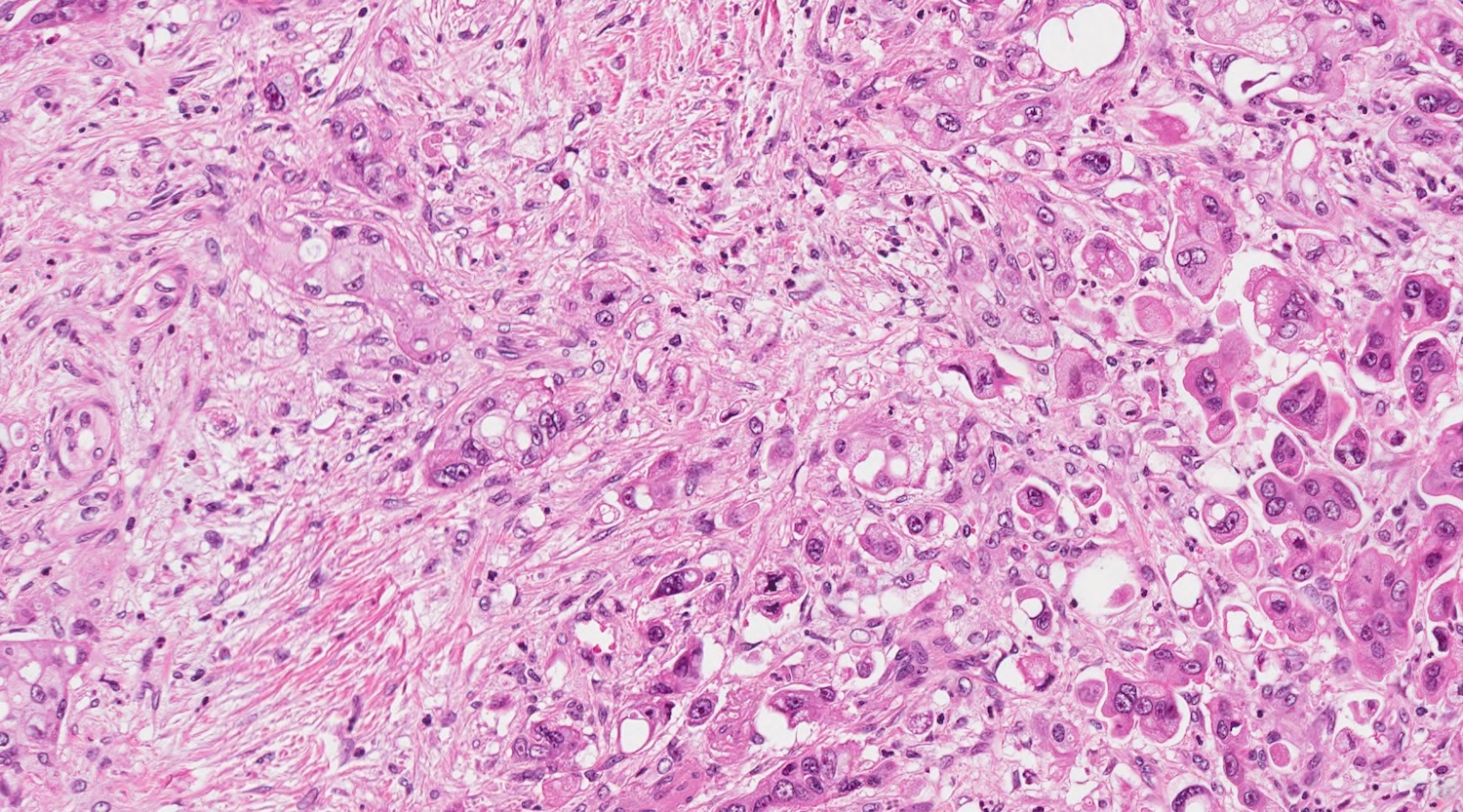
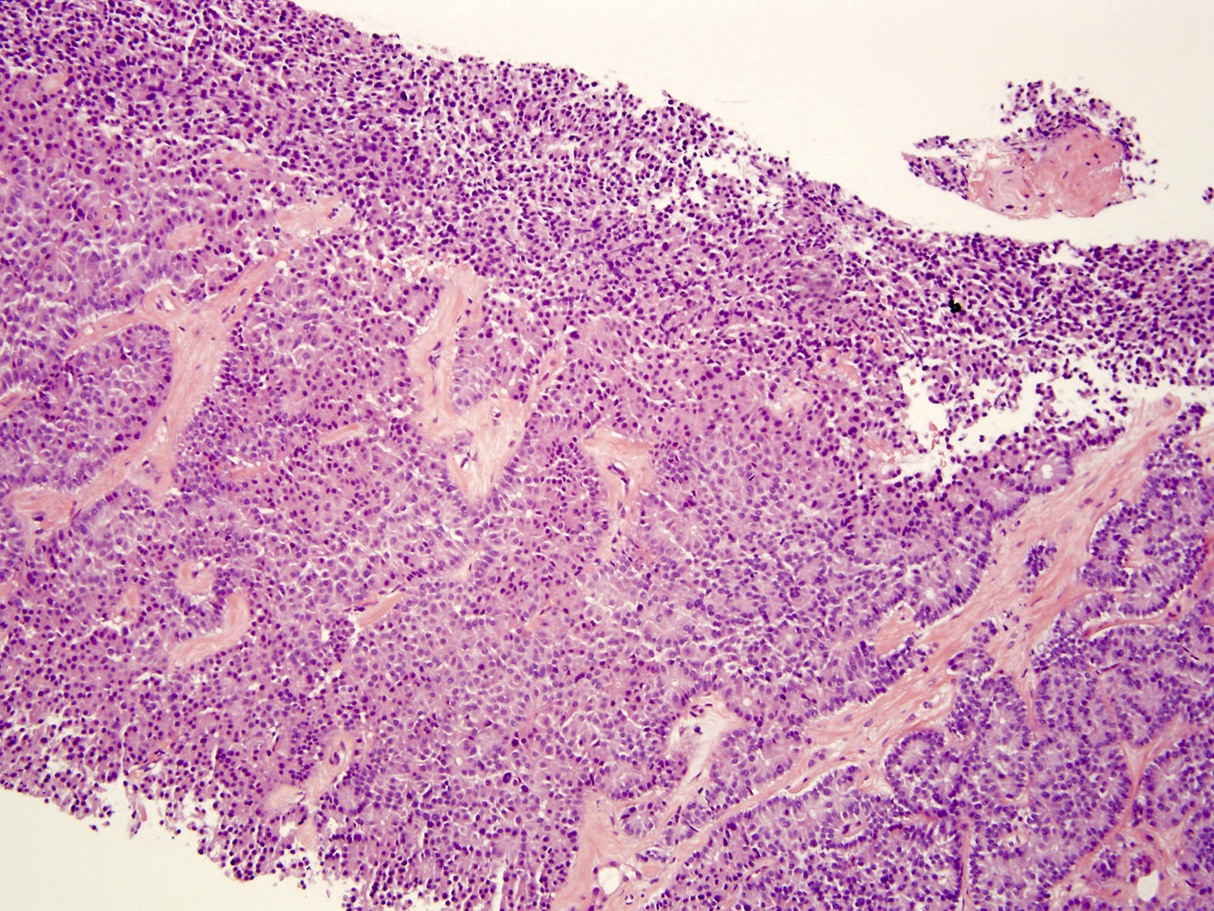
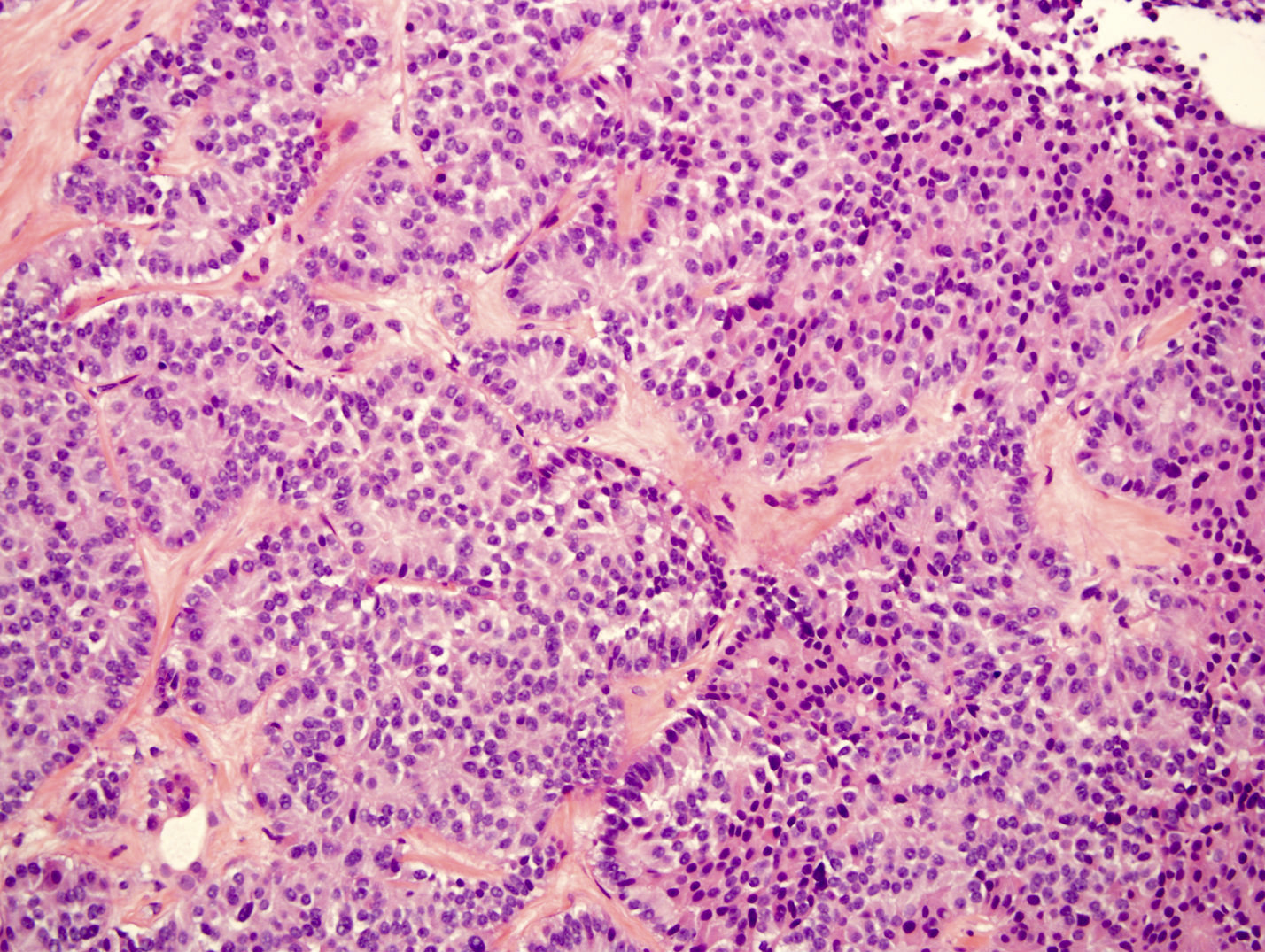
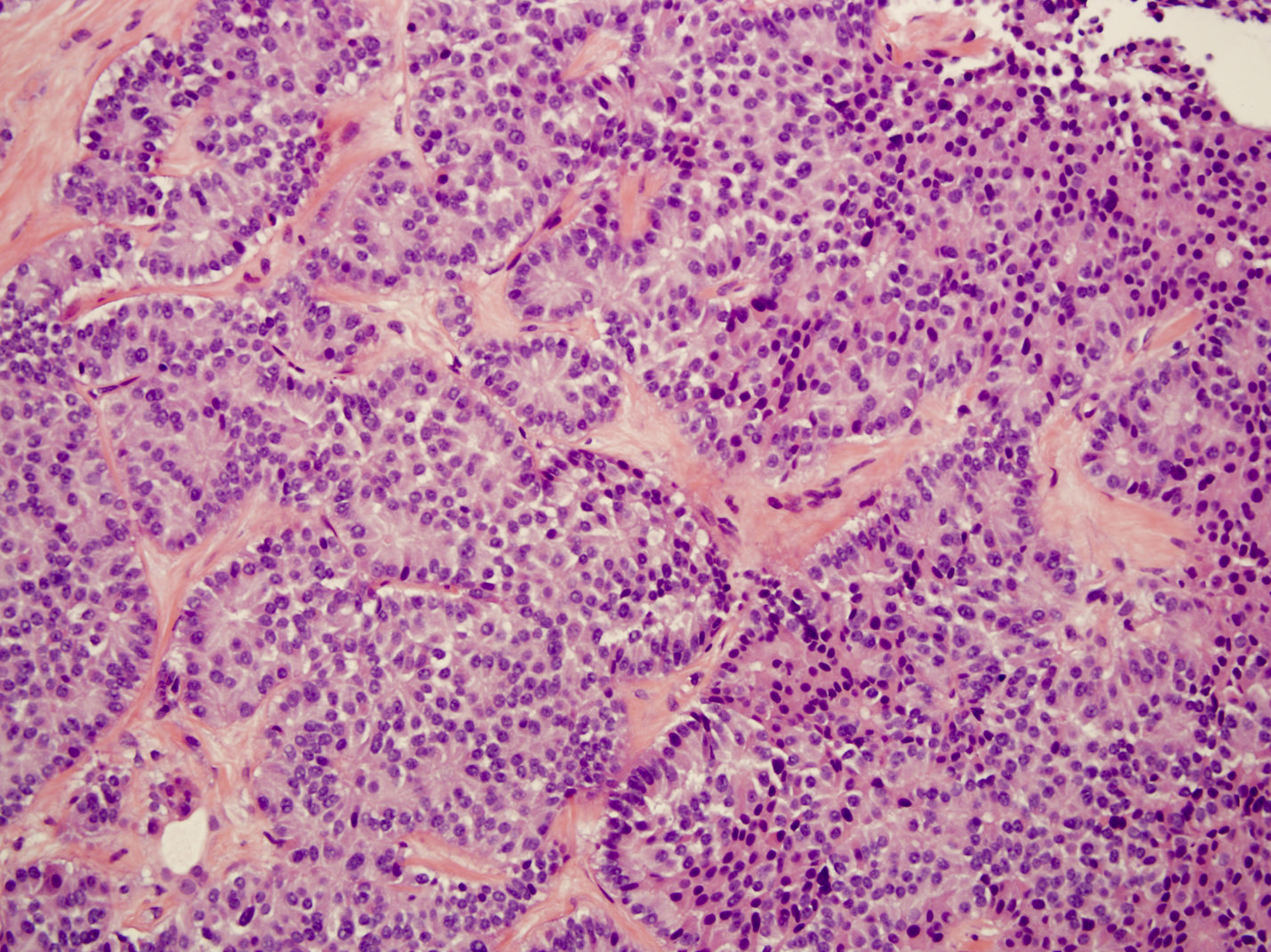
Microscopic (histologic) description
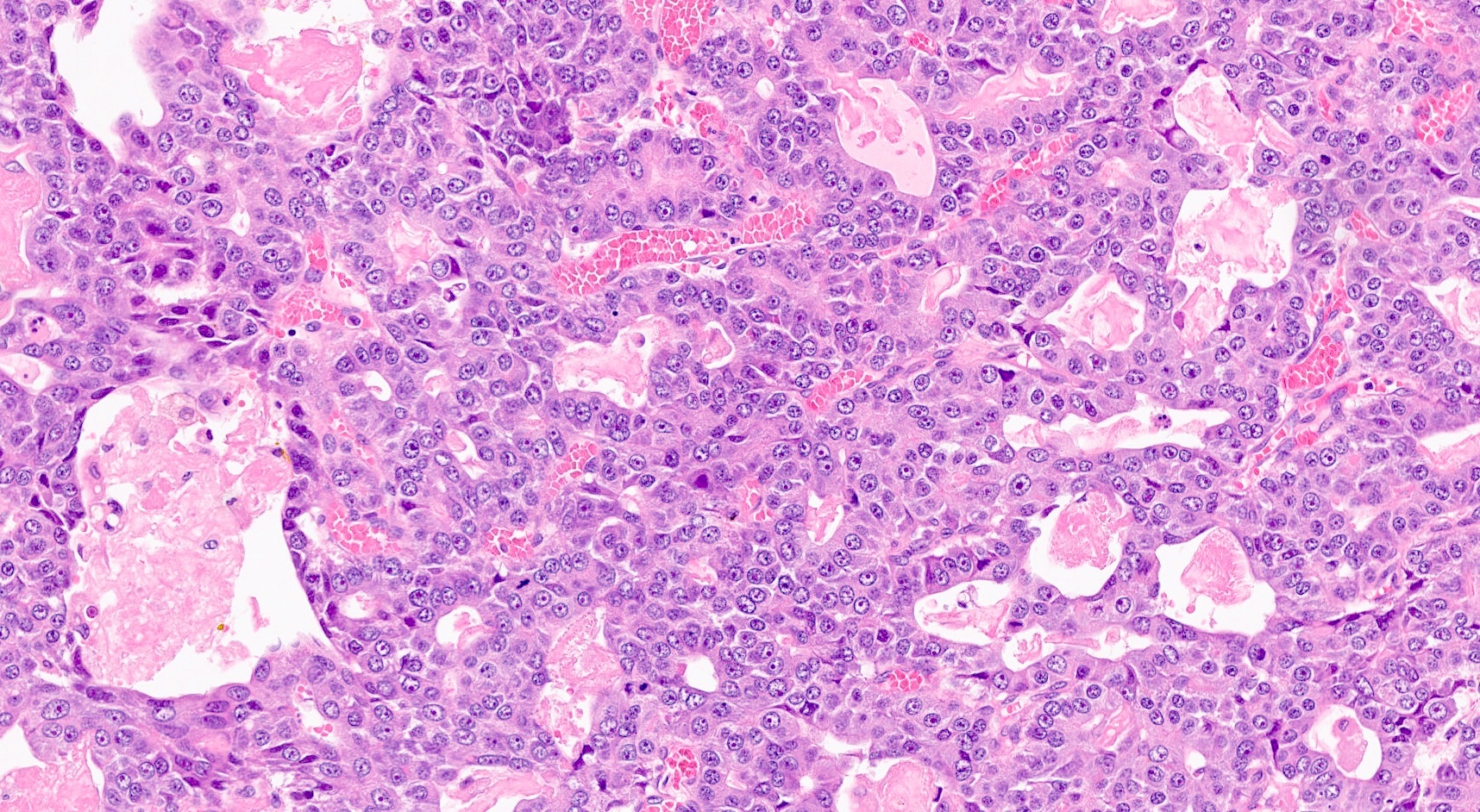
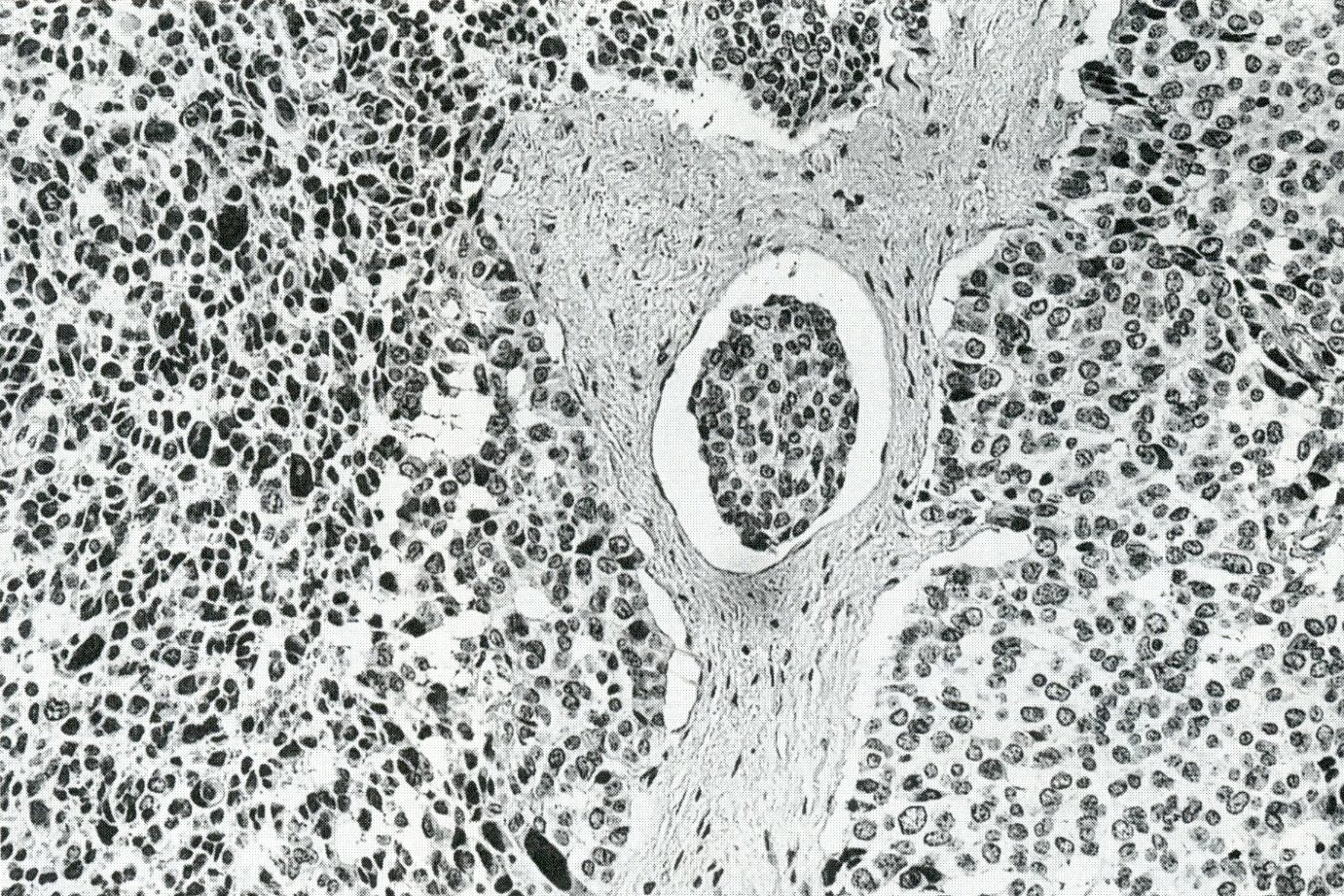
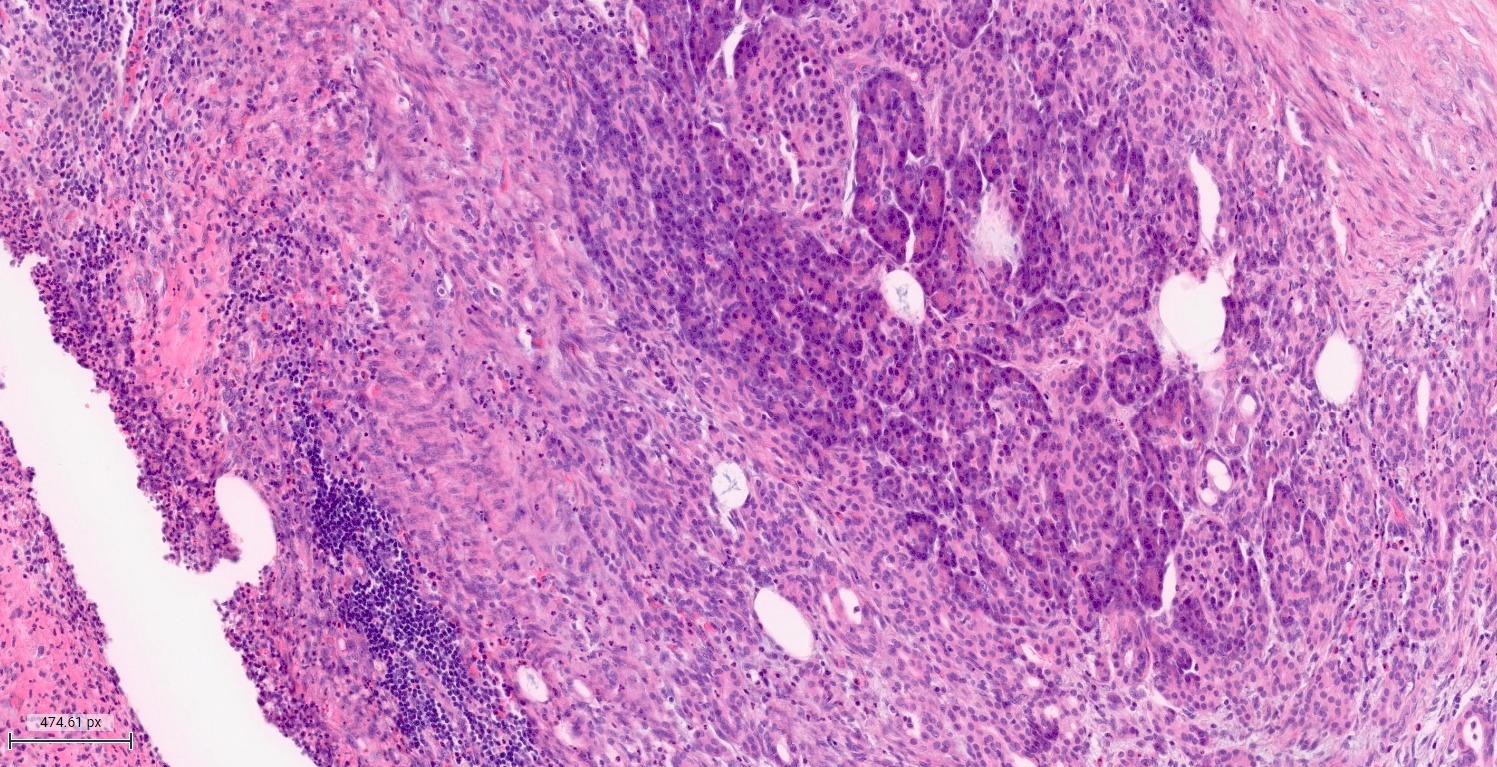
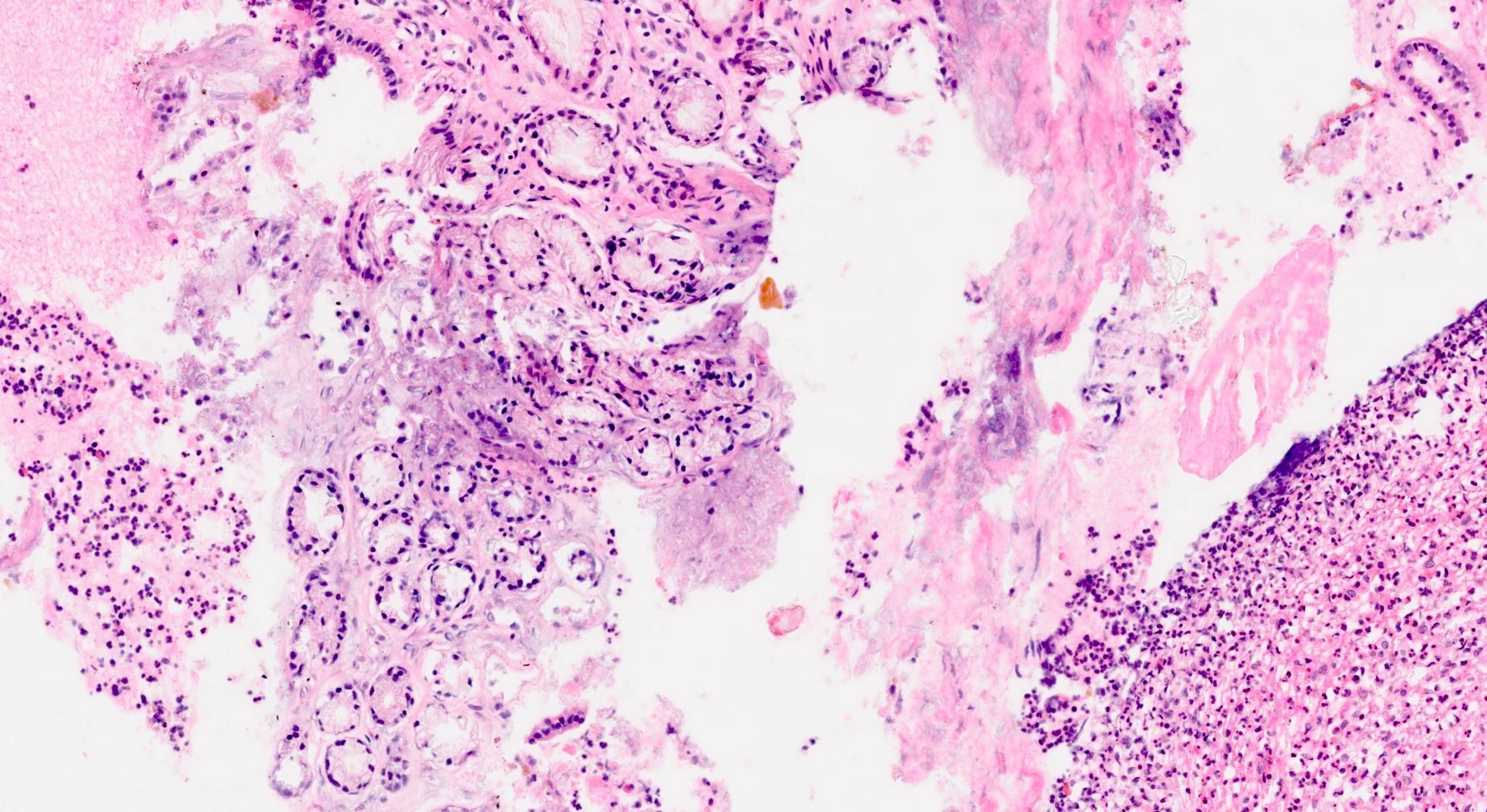
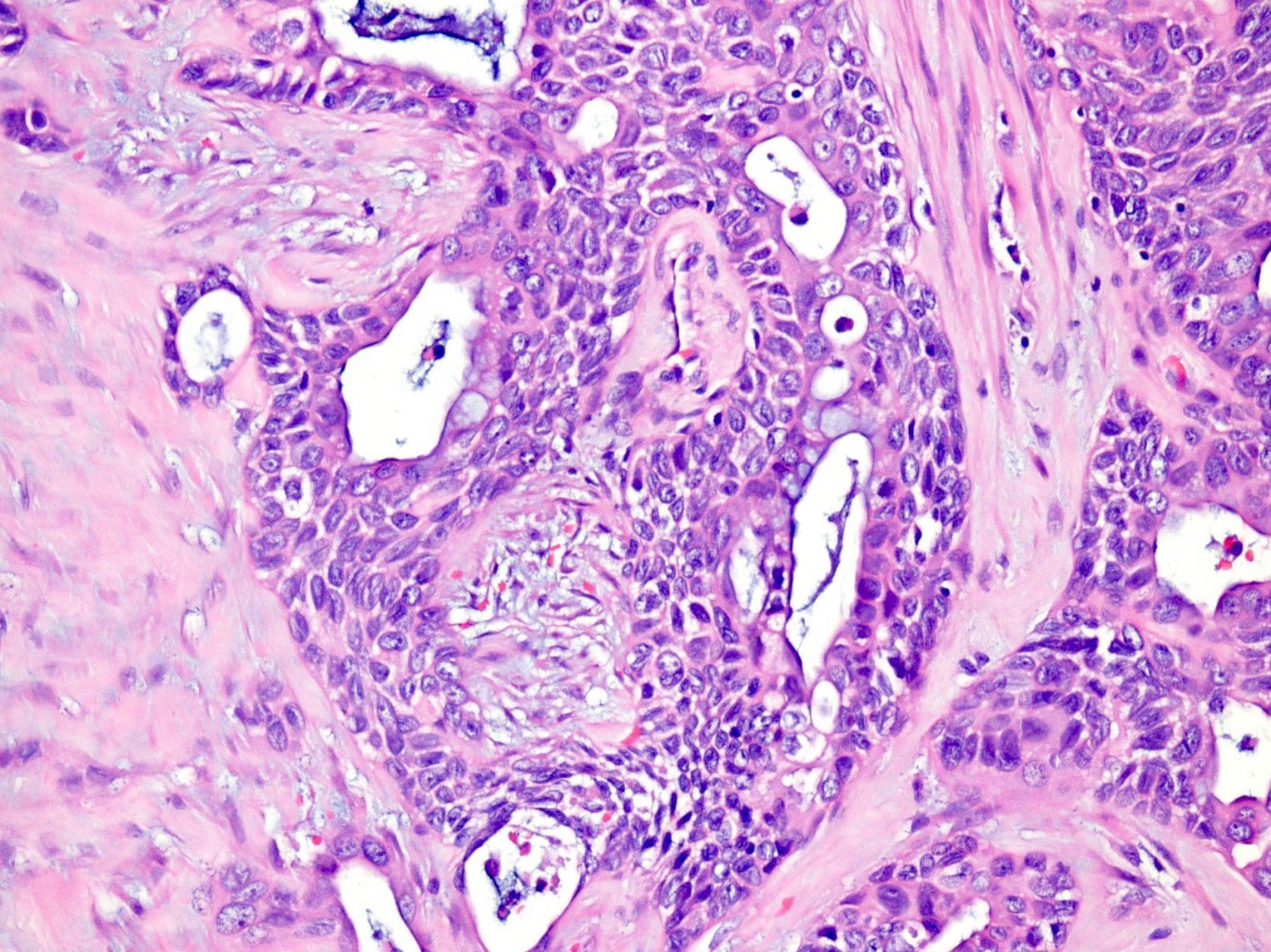
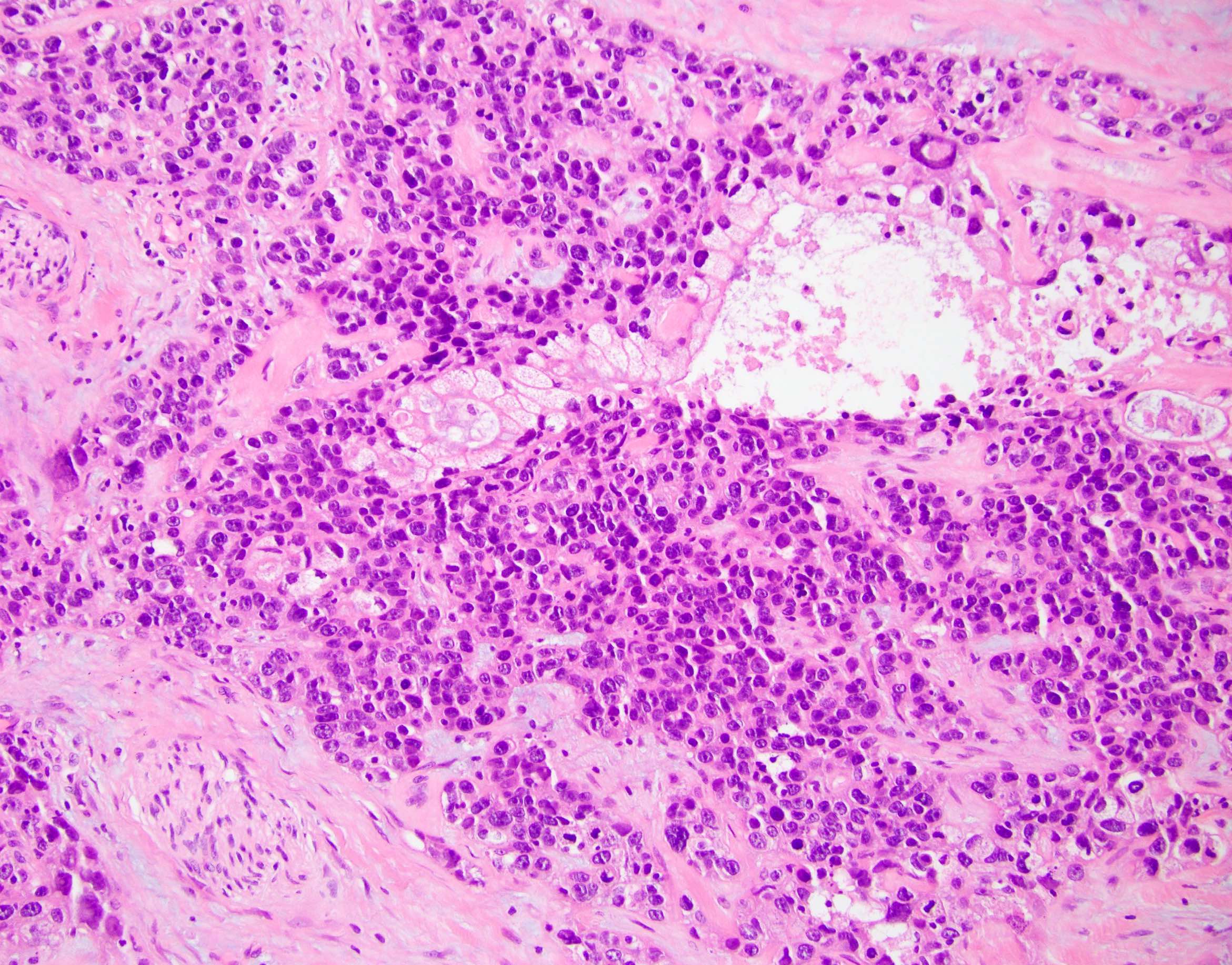
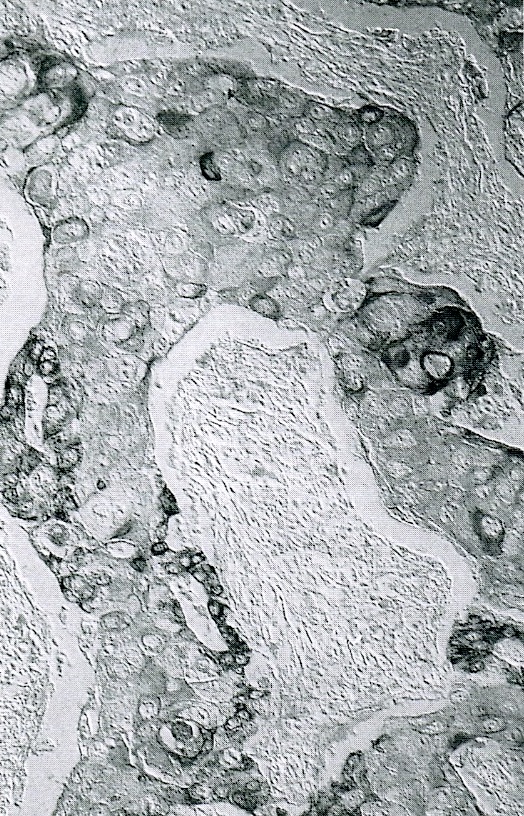
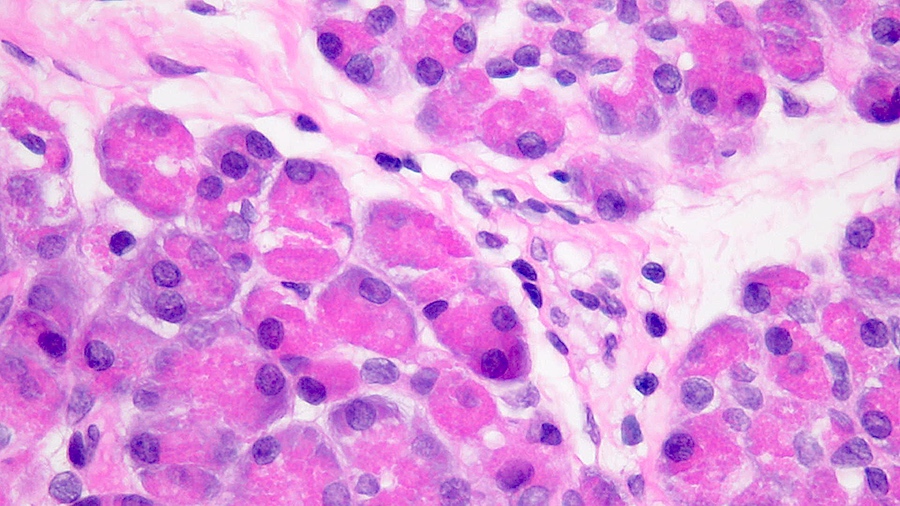
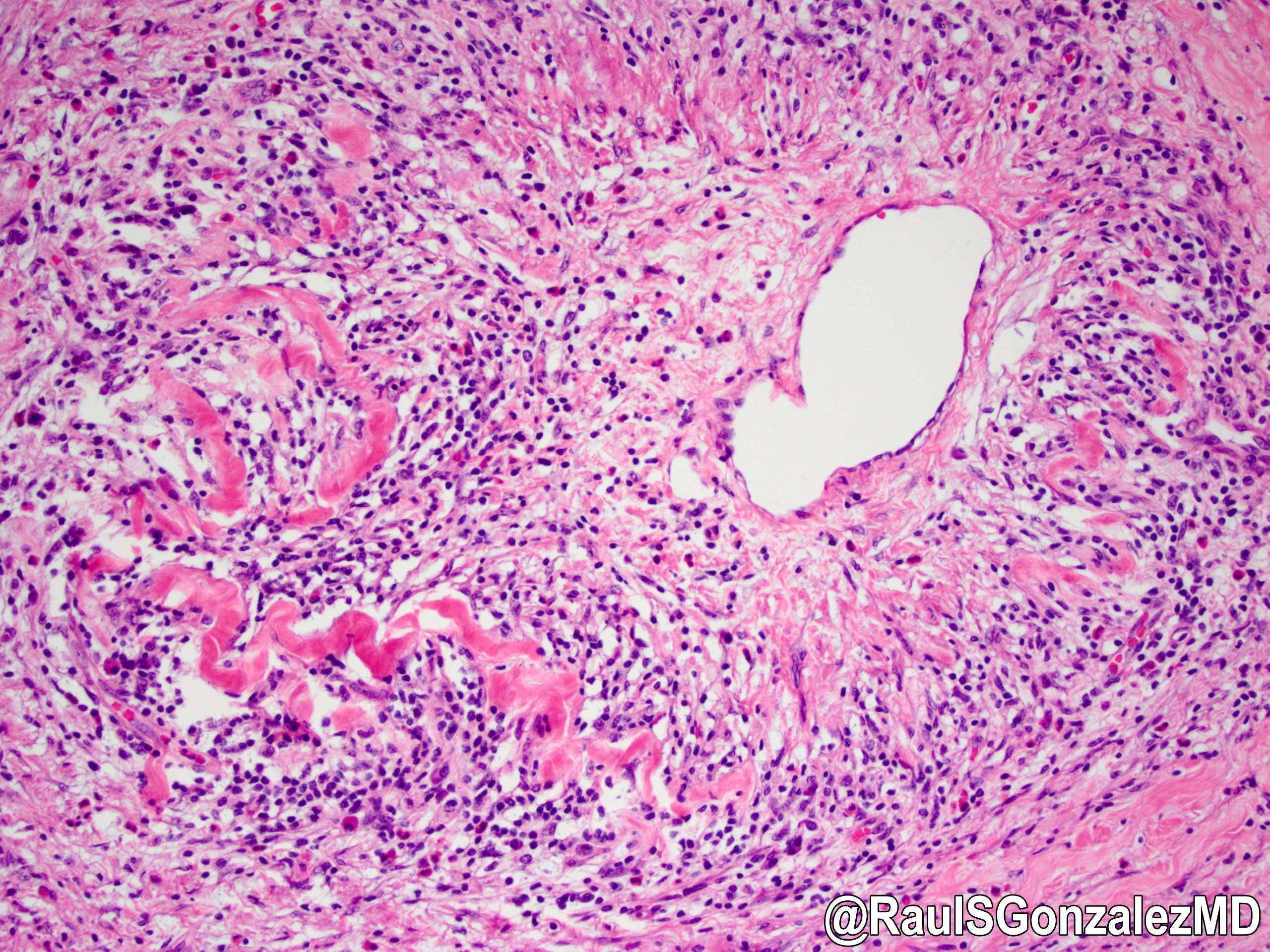
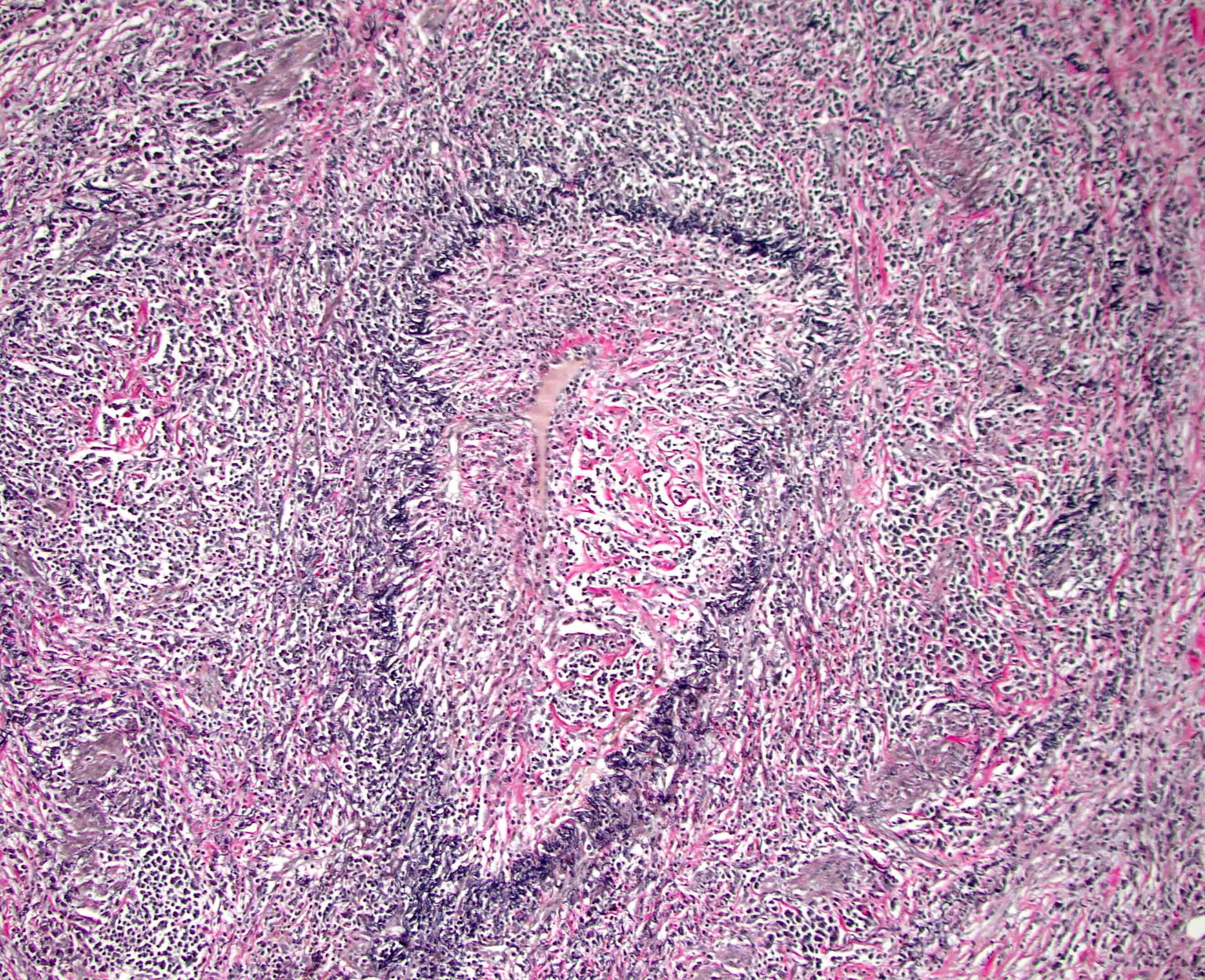
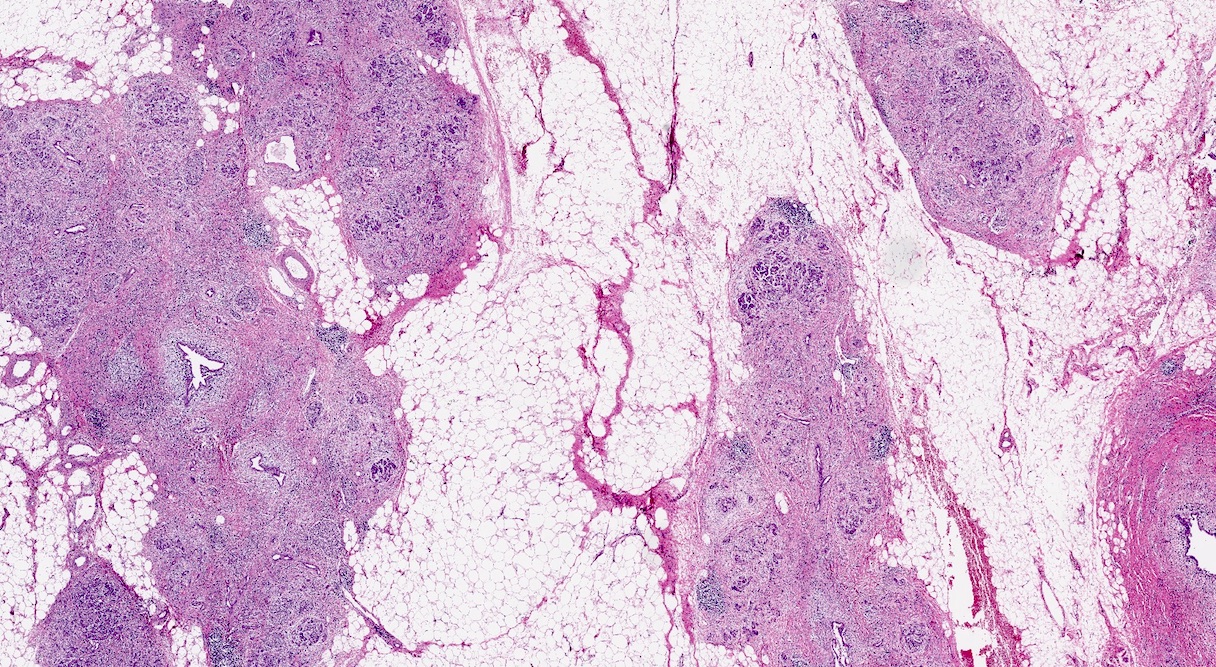
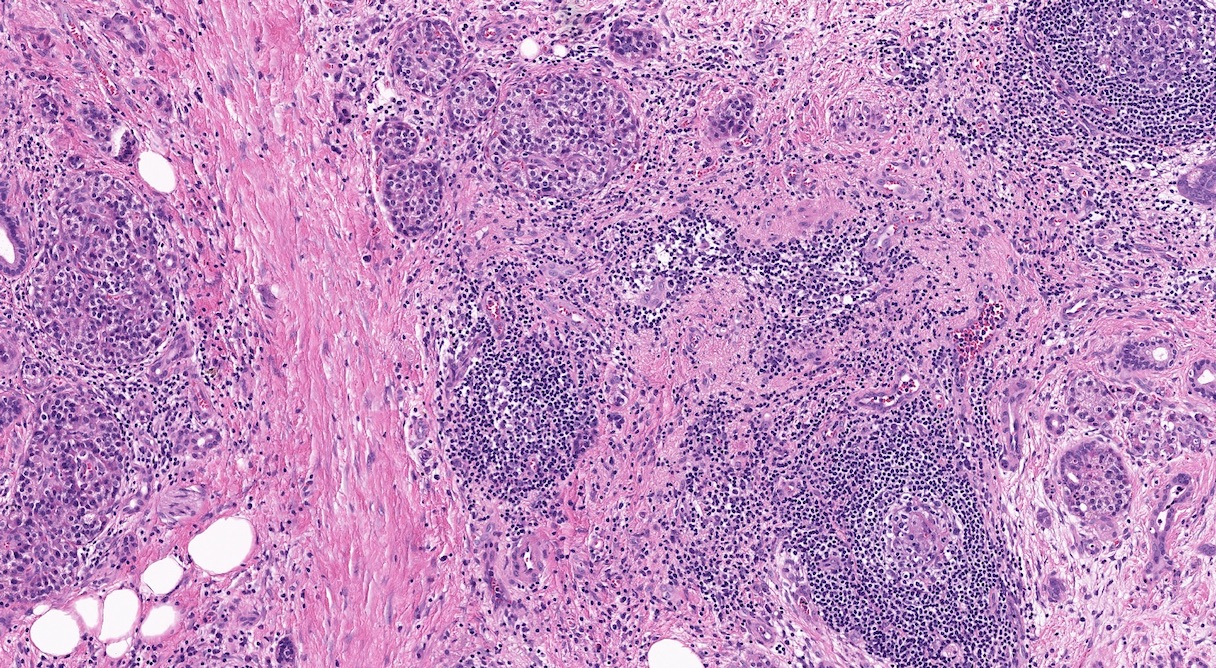
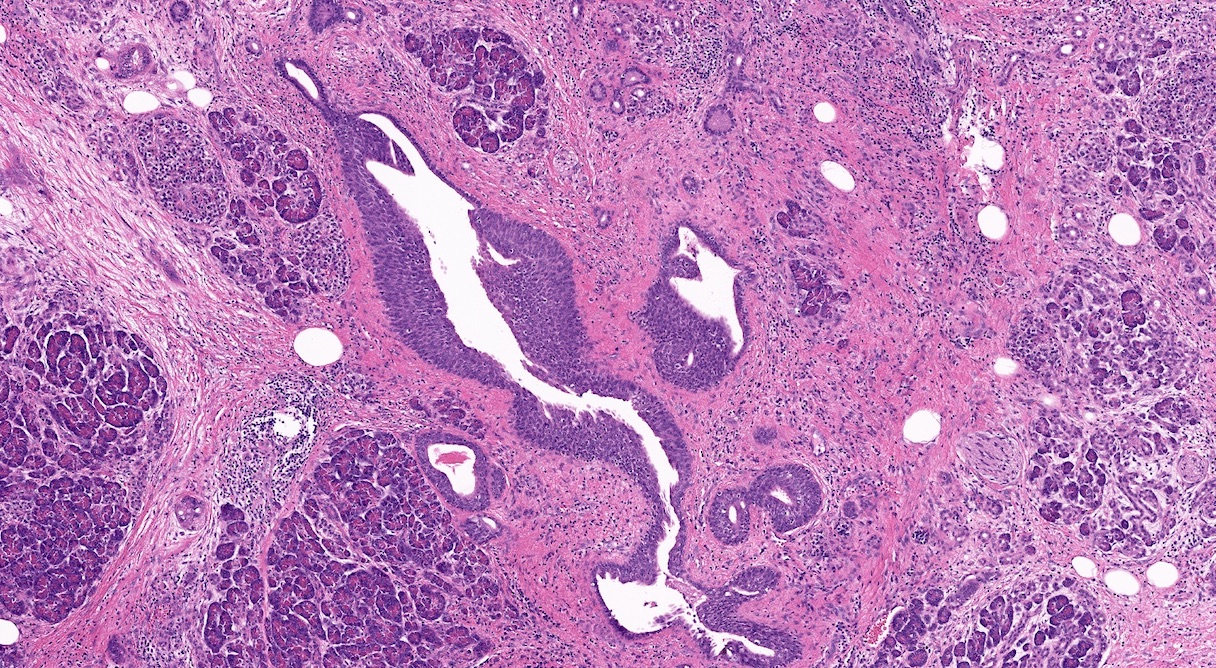
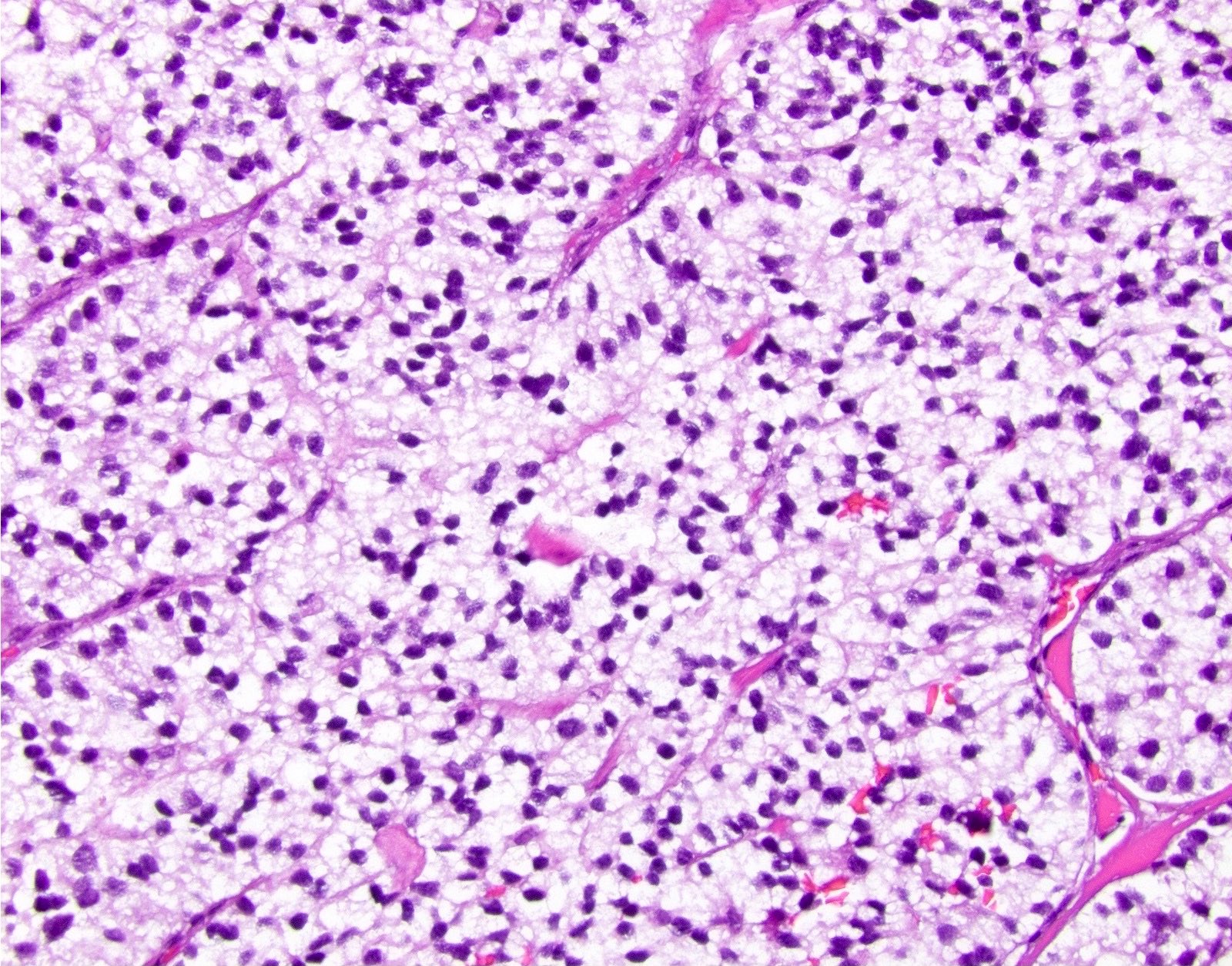
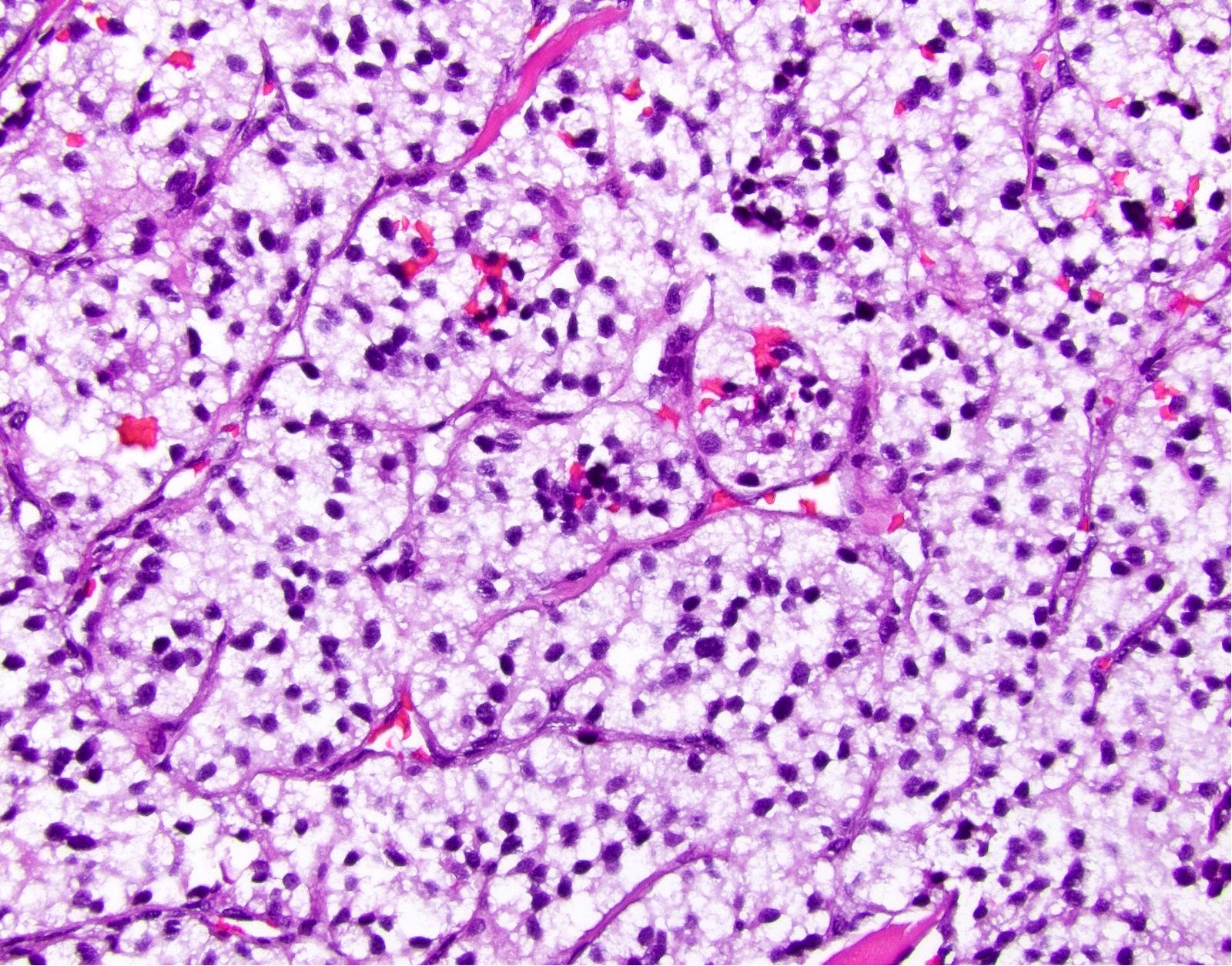
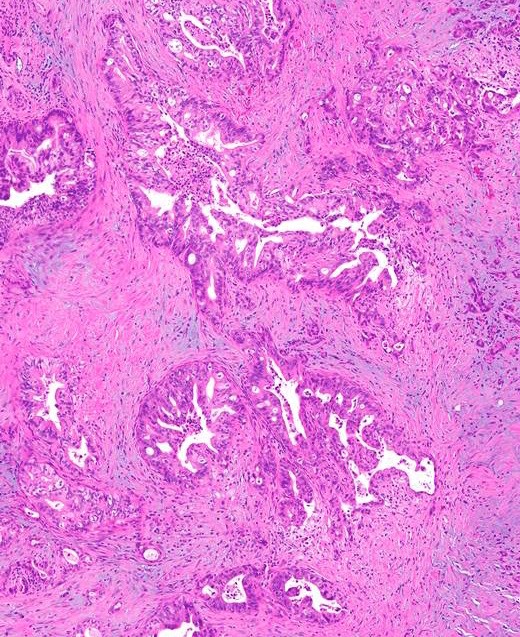
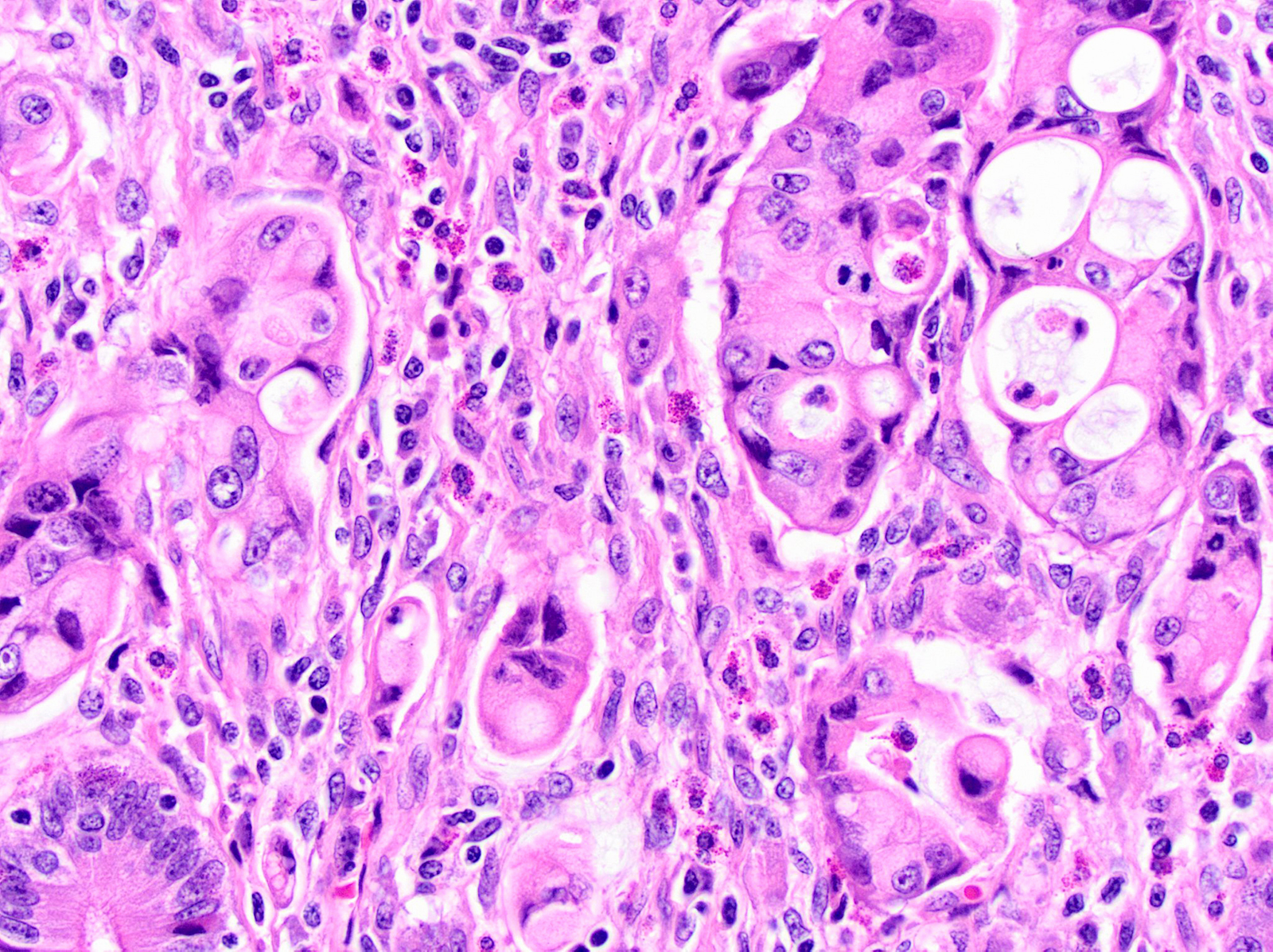
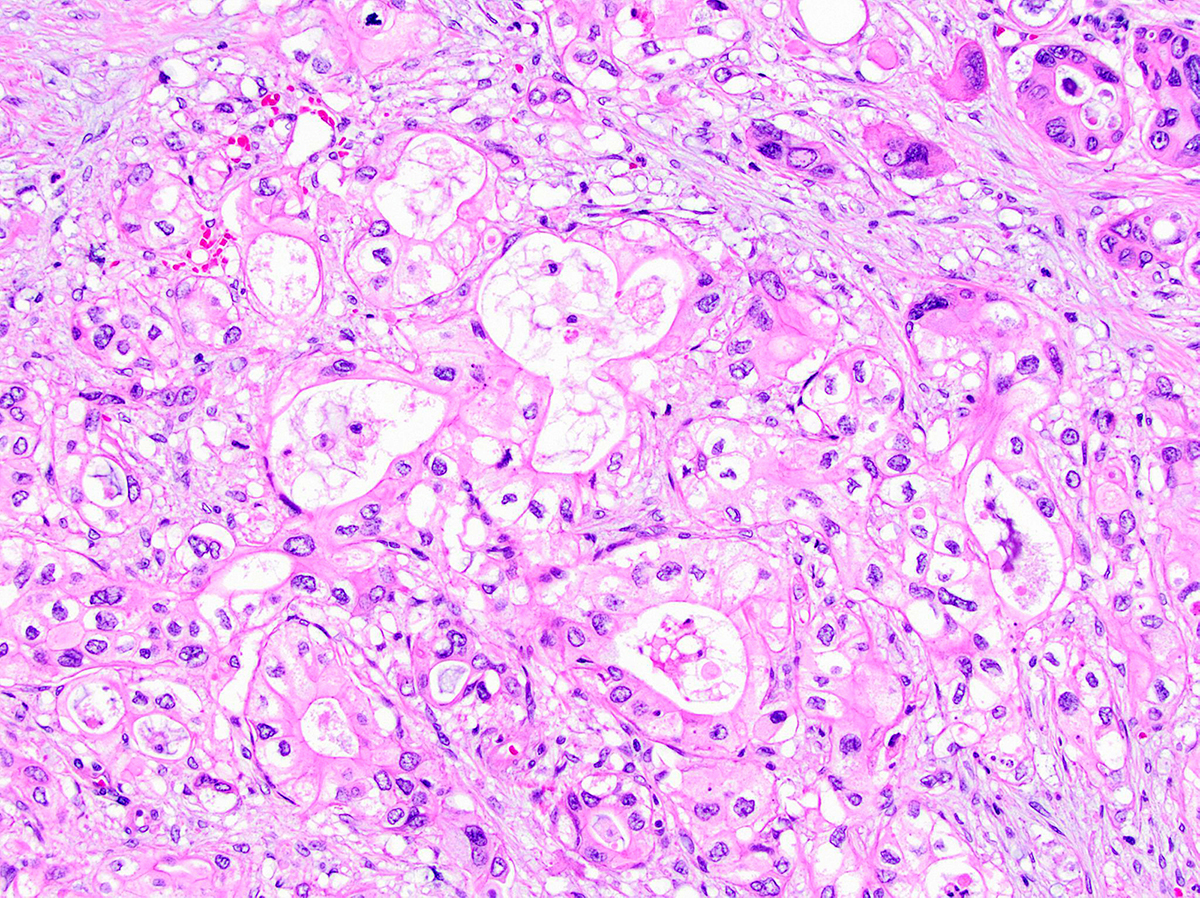
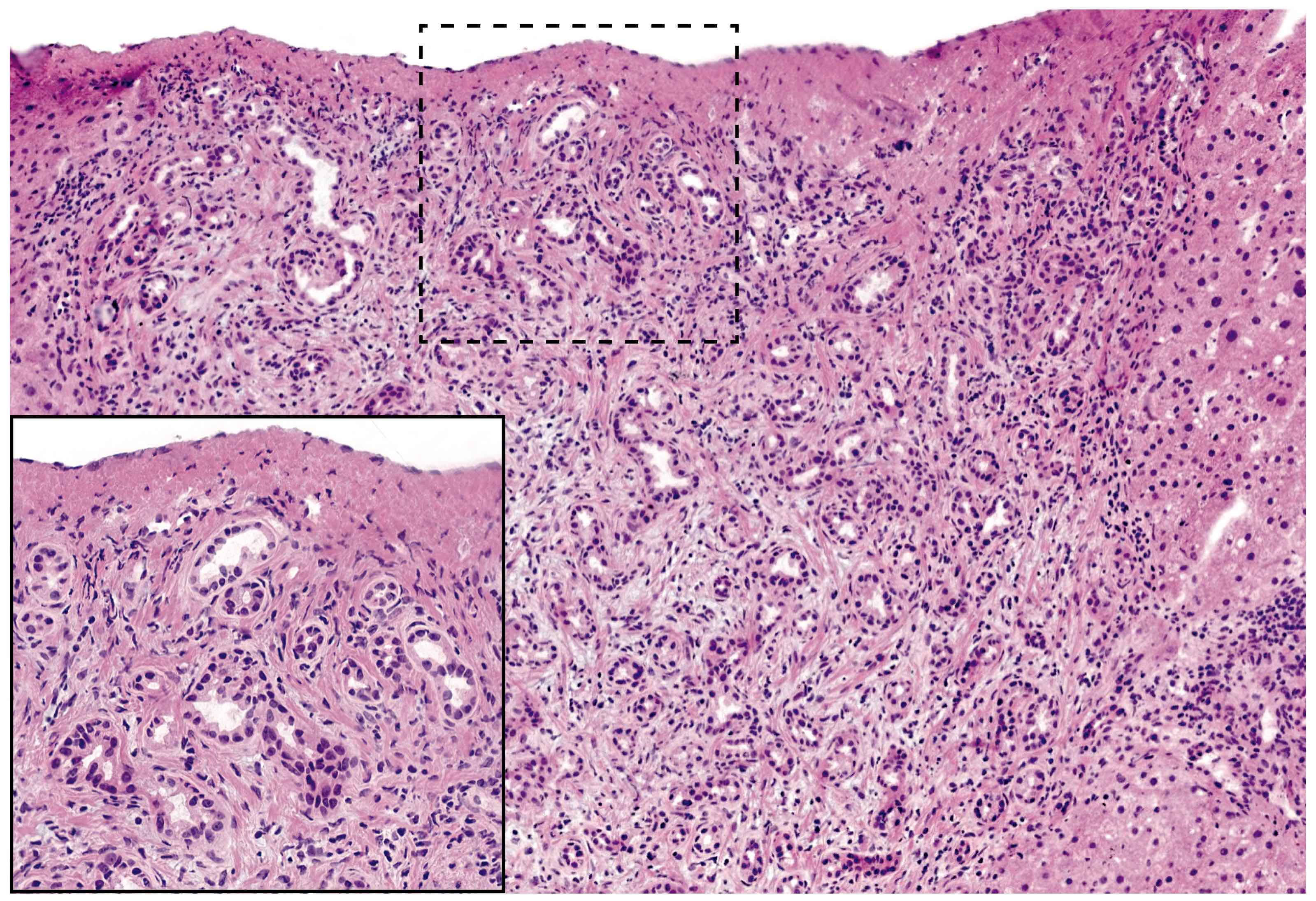
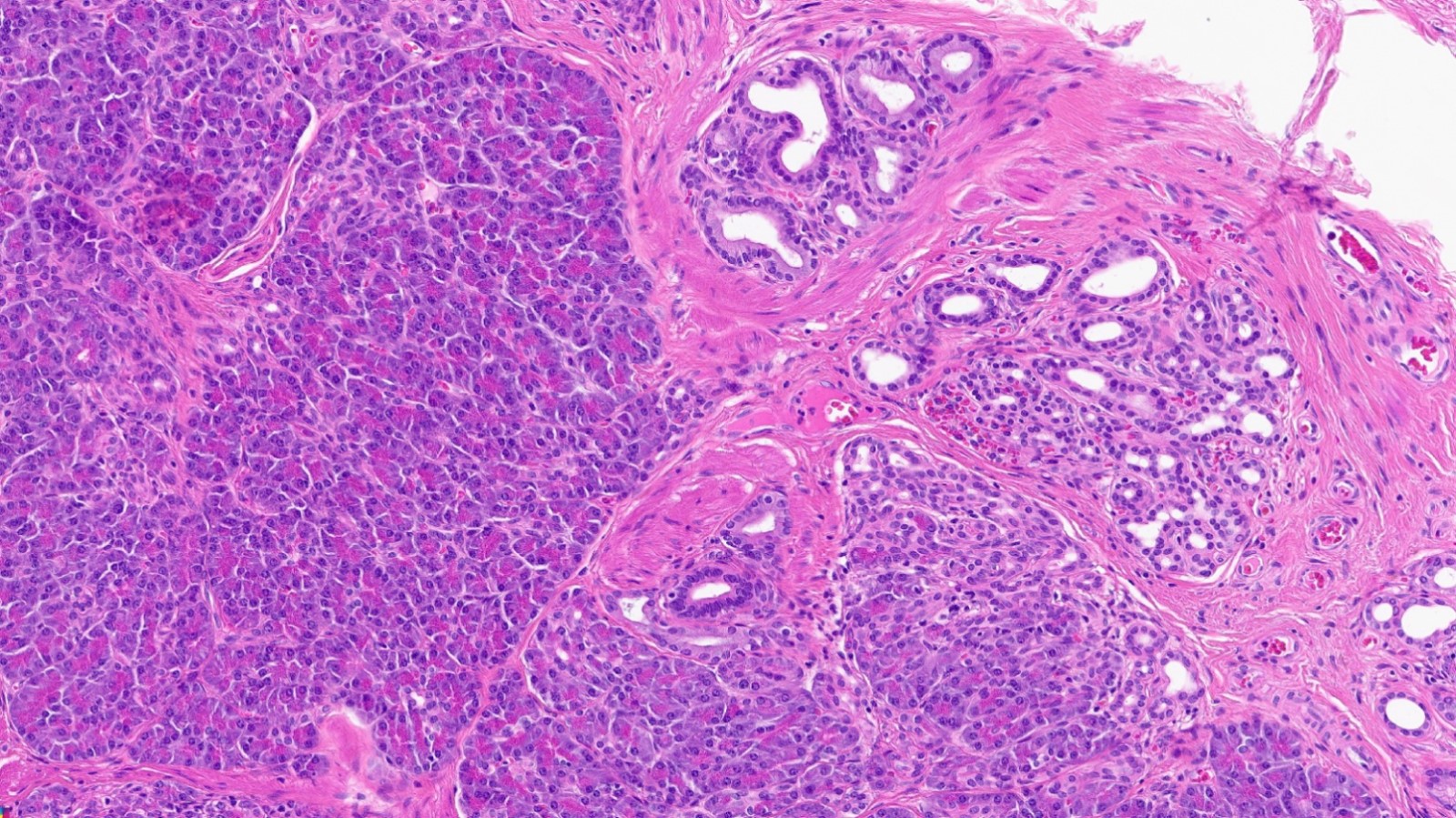
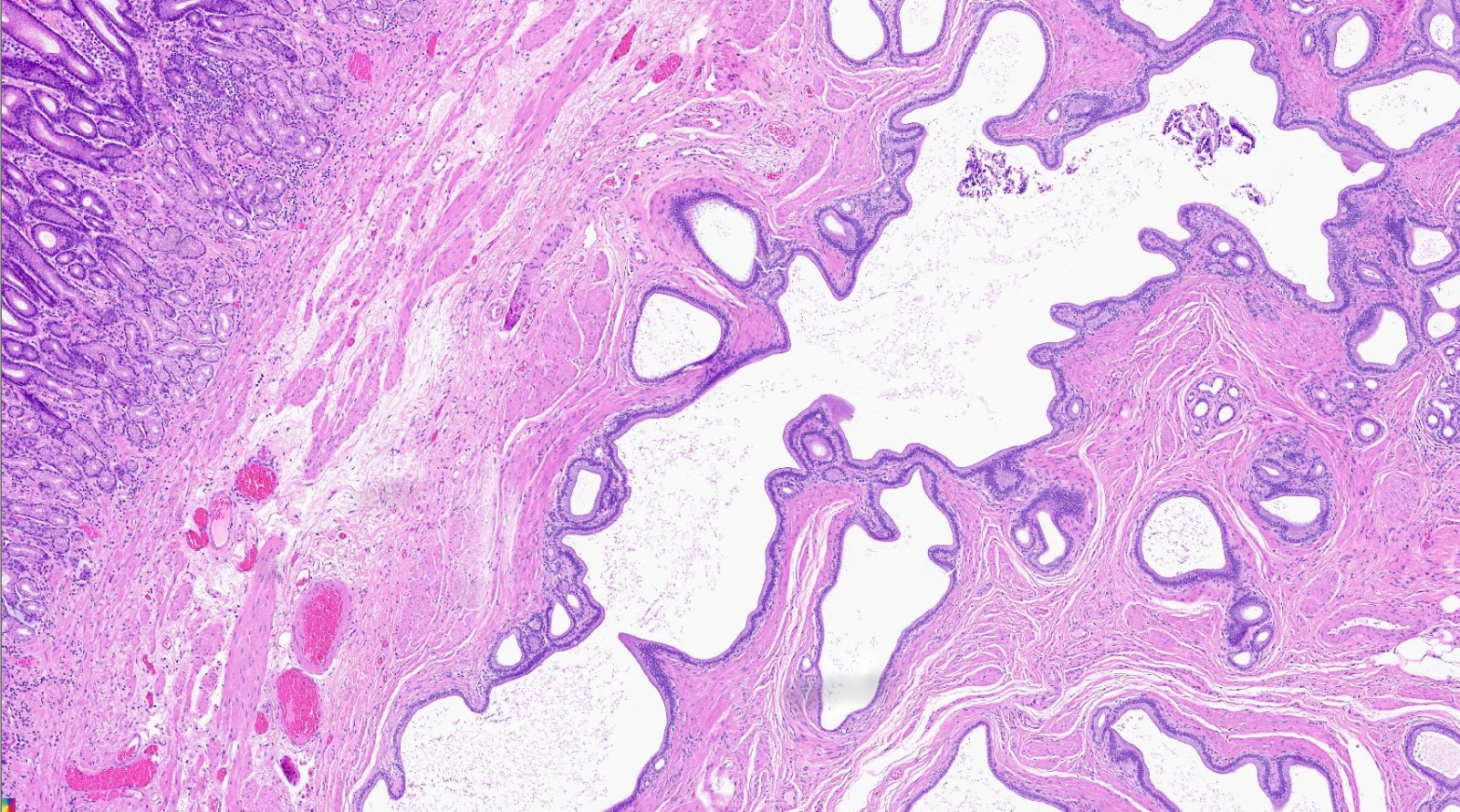
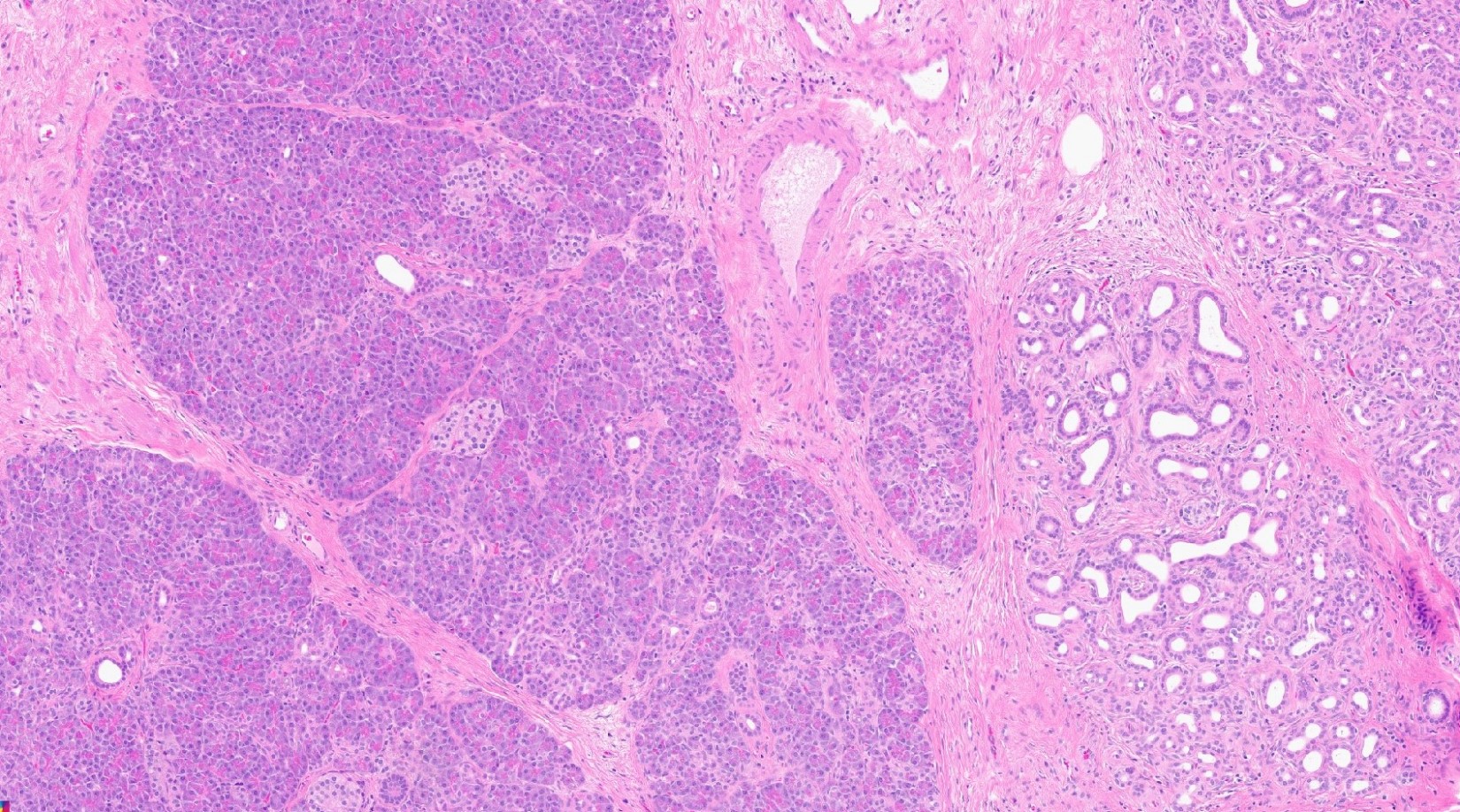
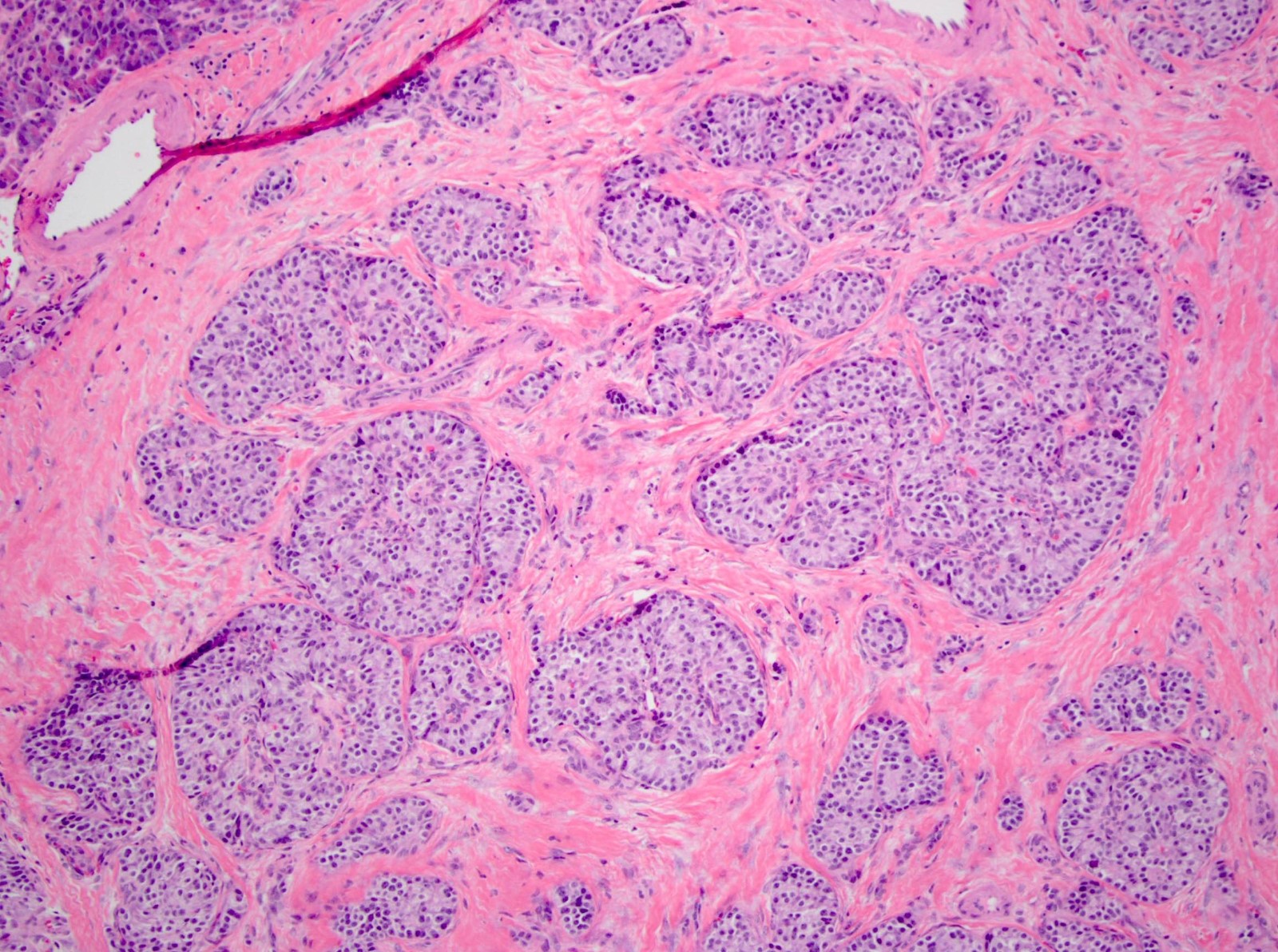
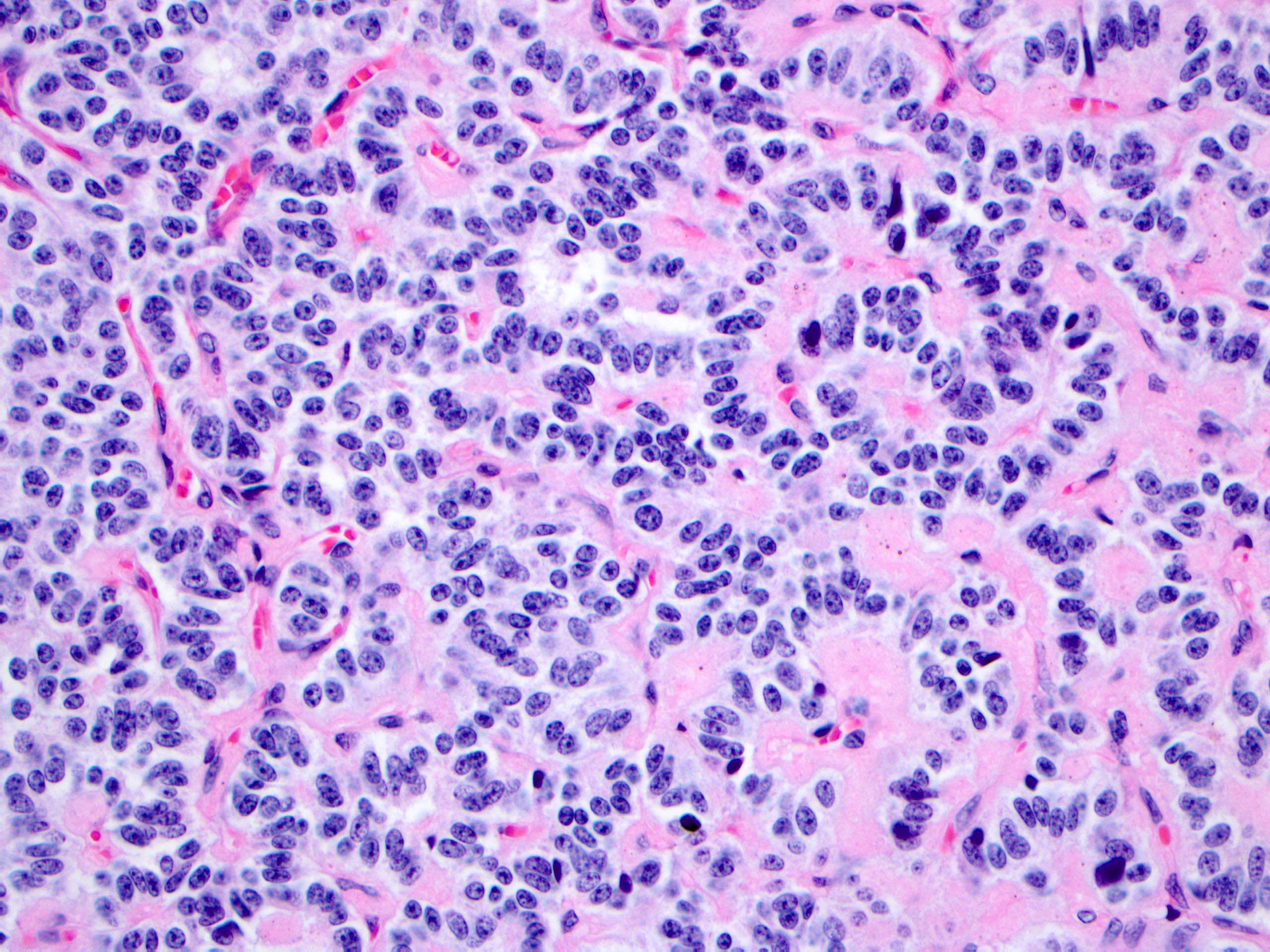
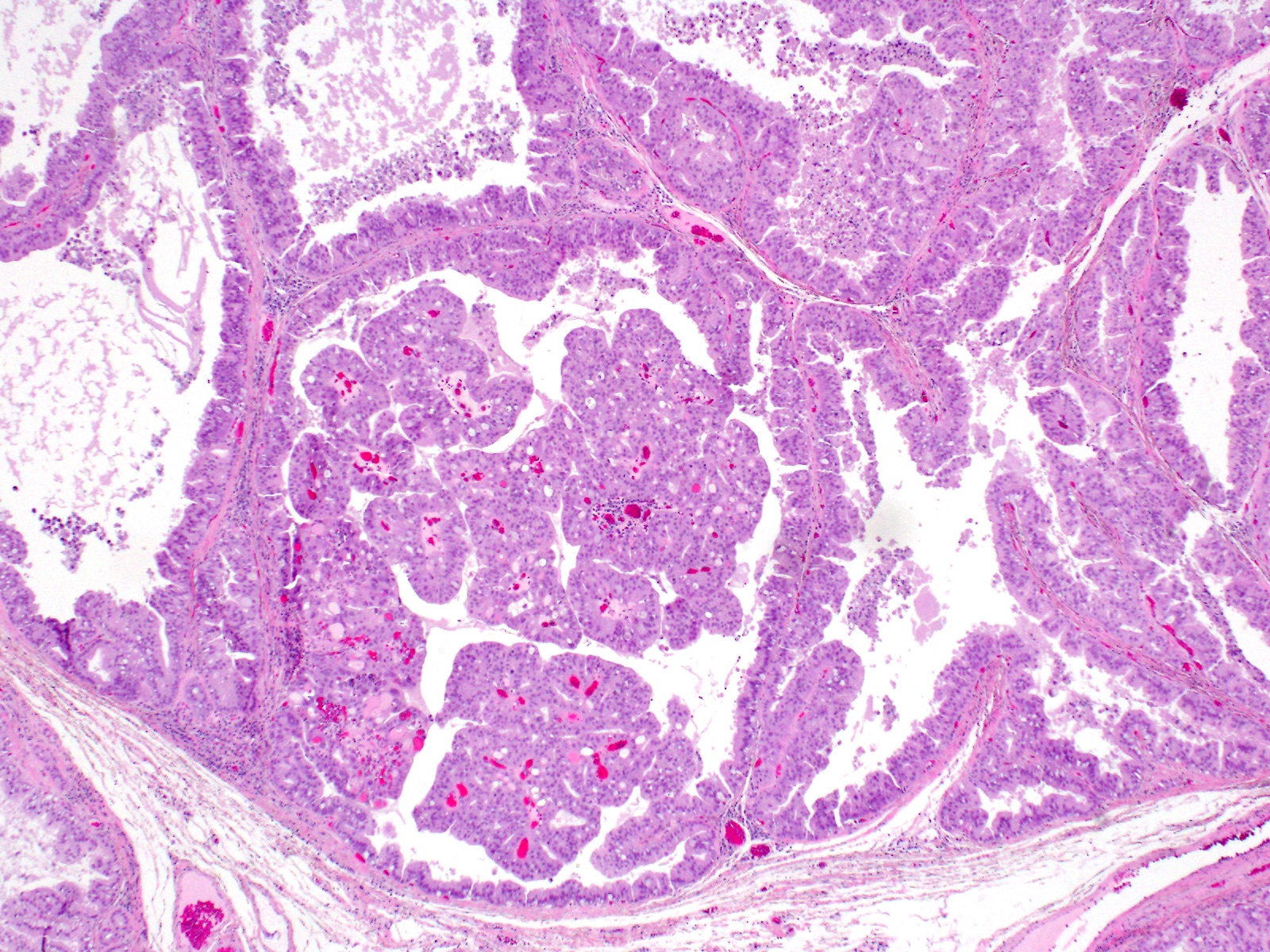
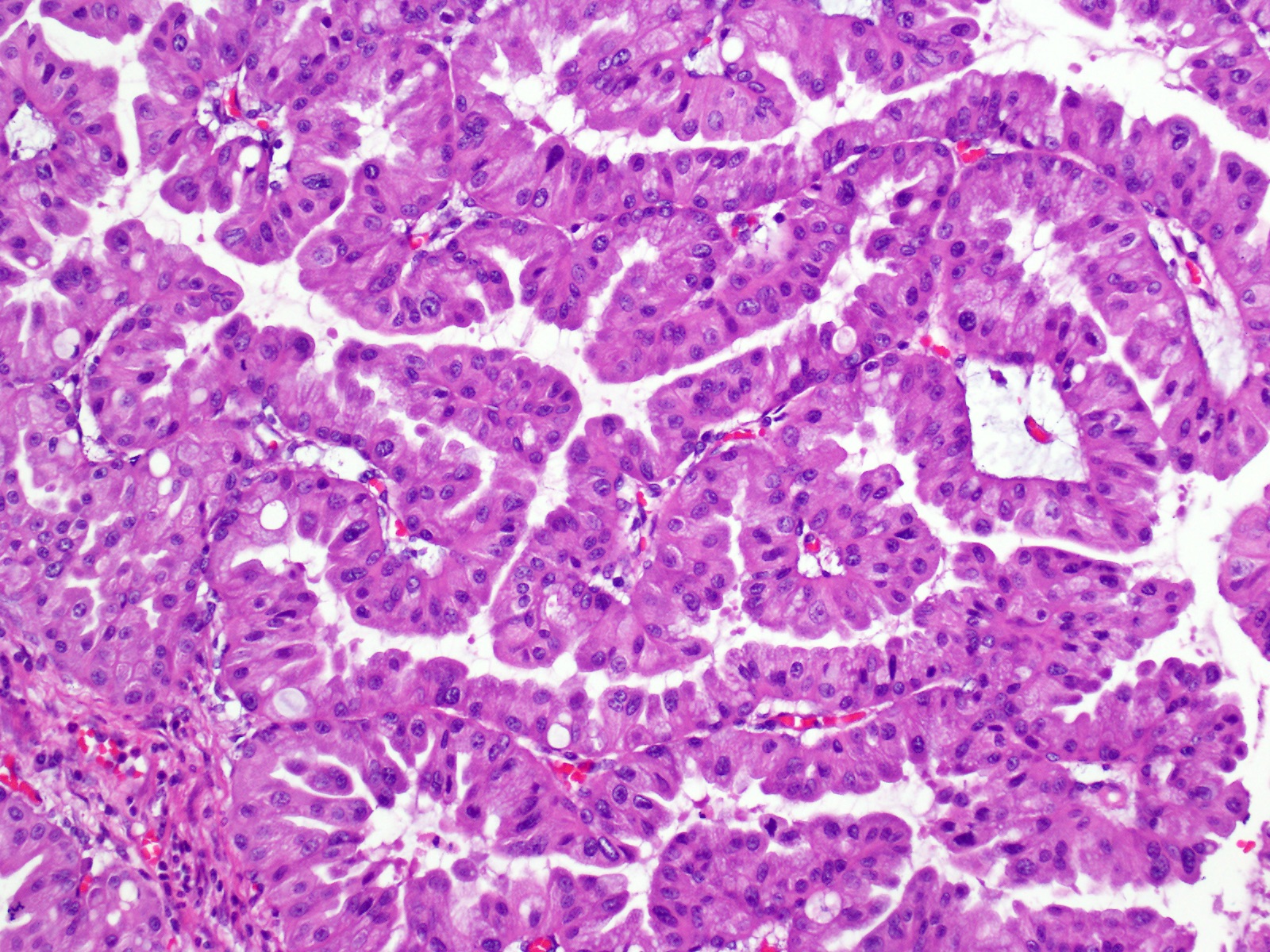
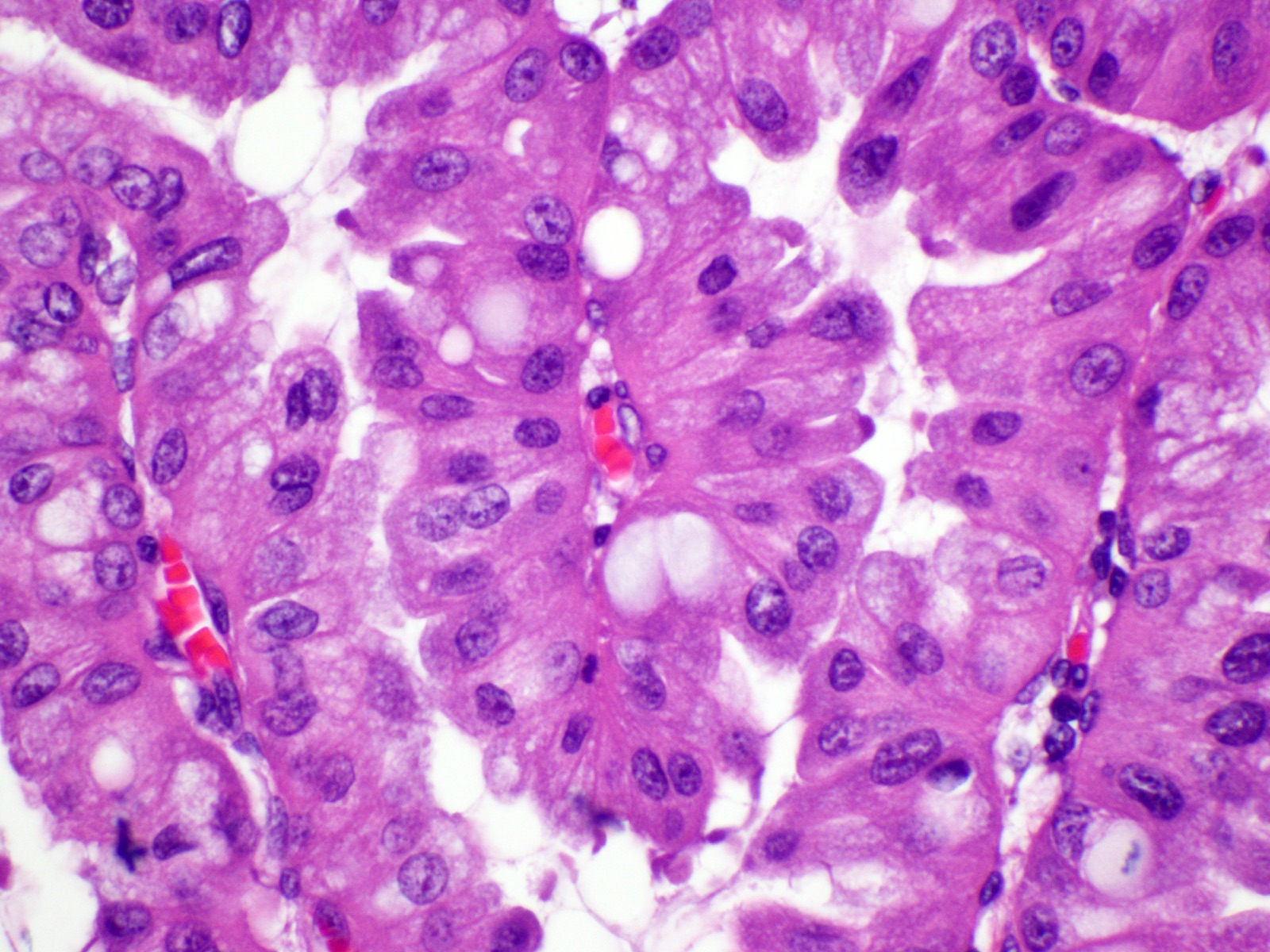
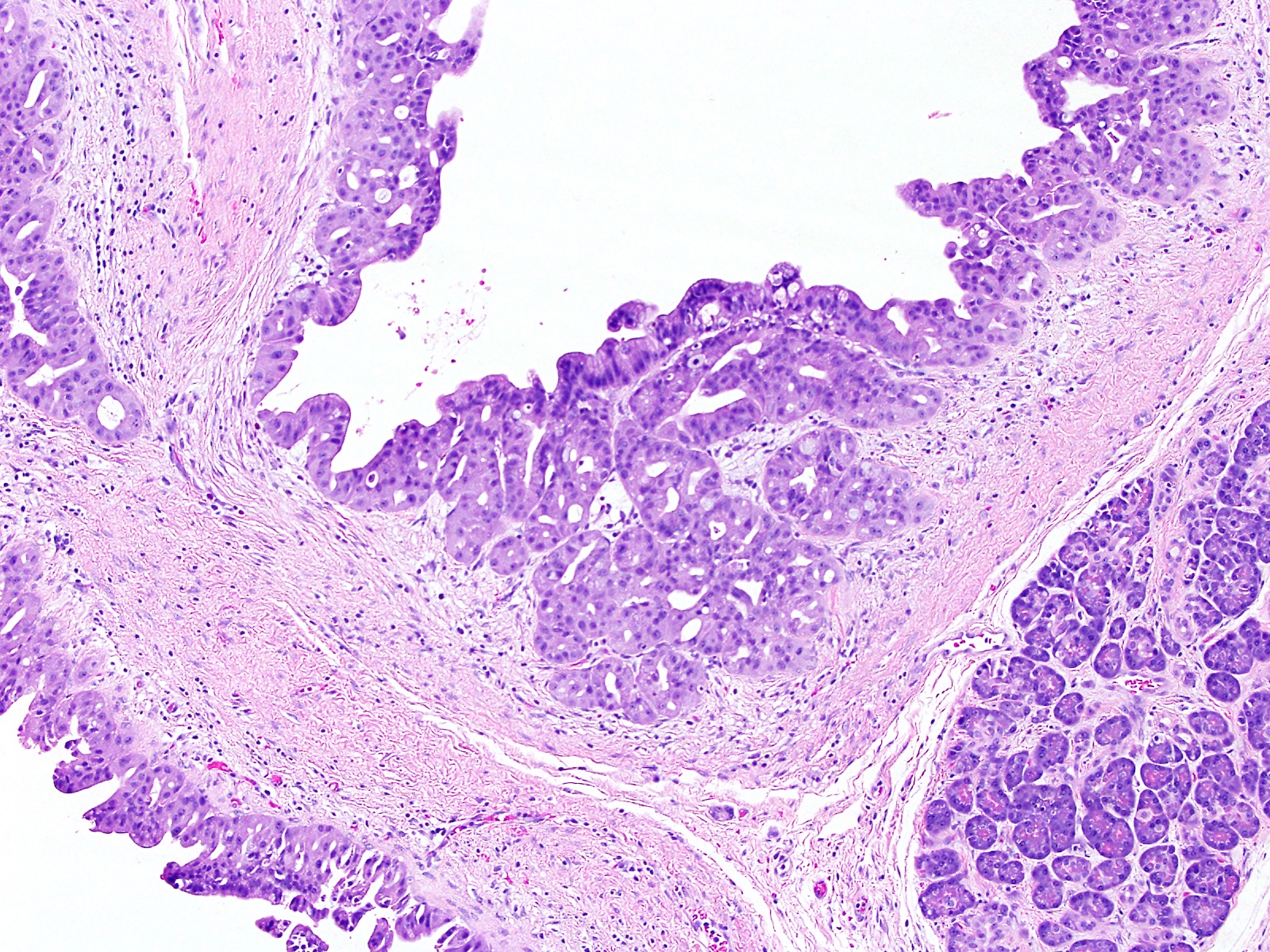
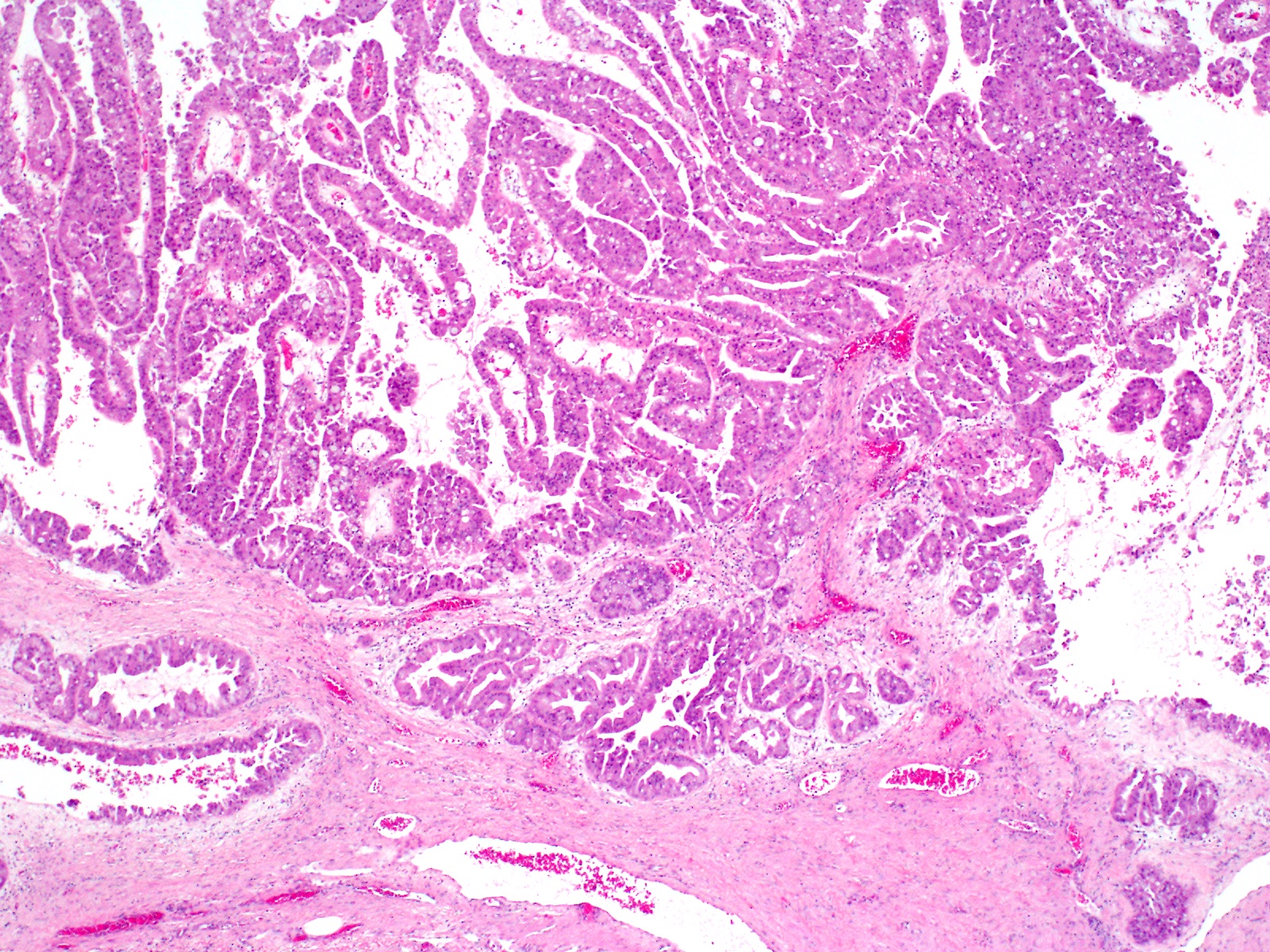
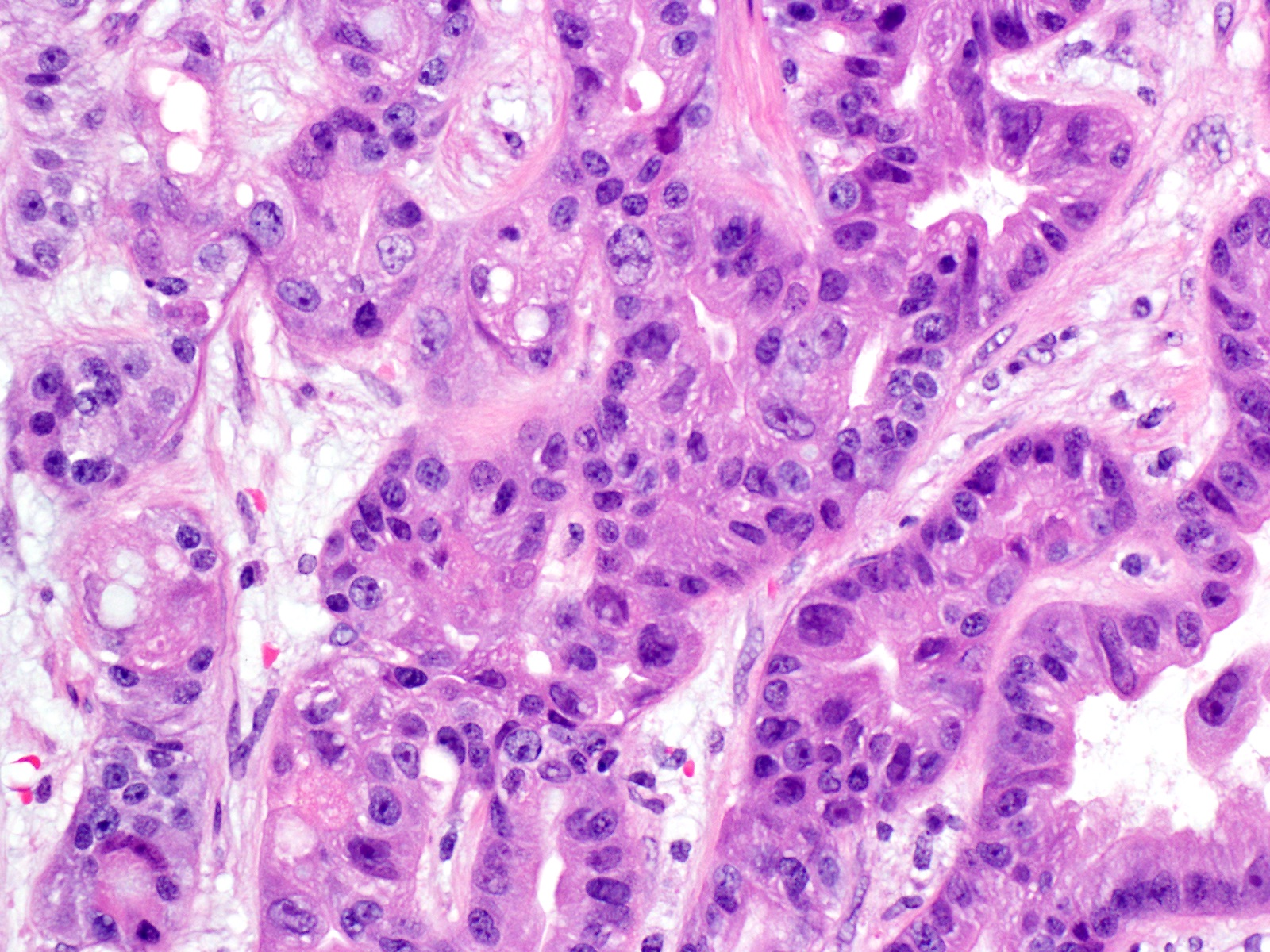
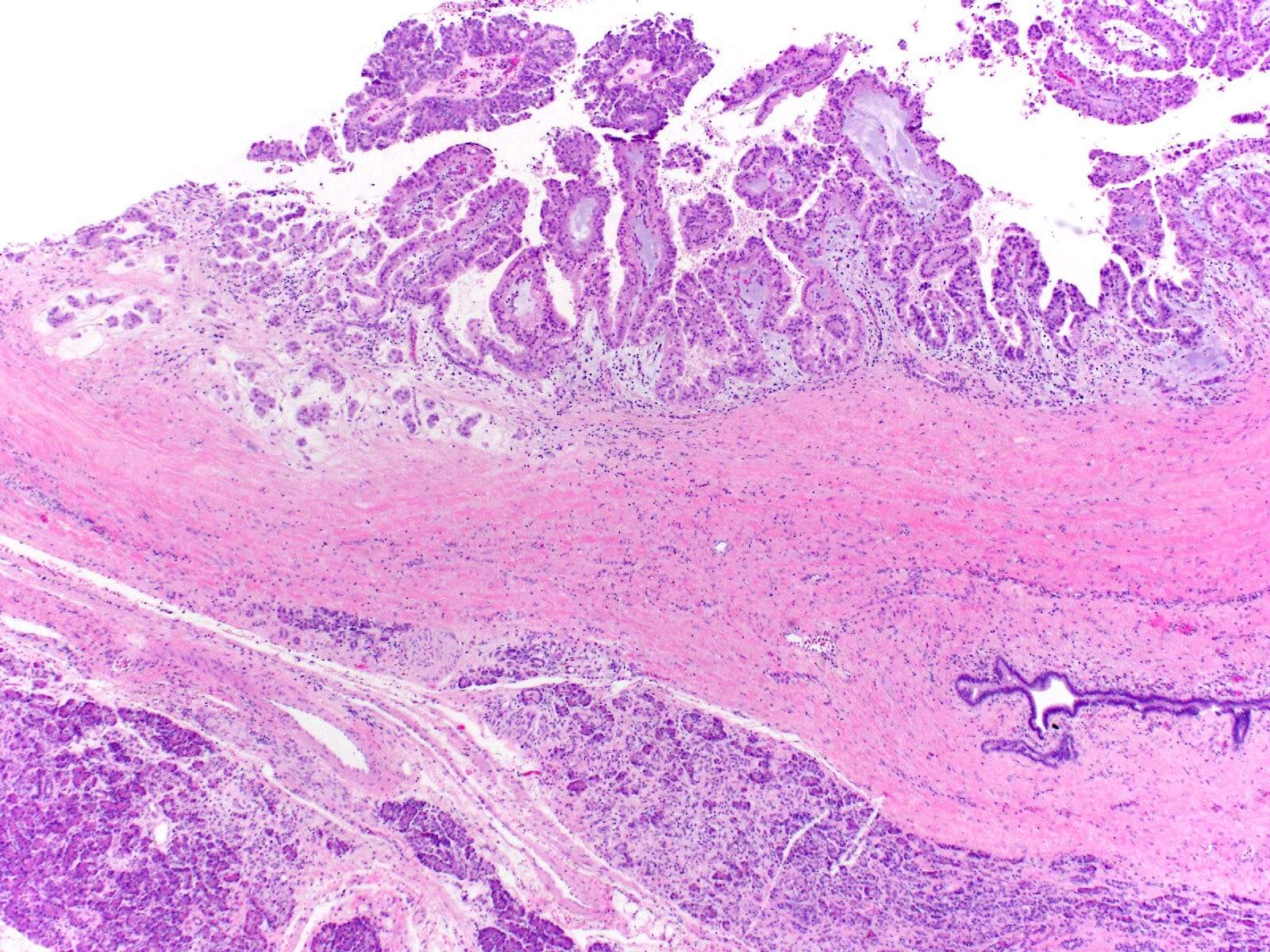
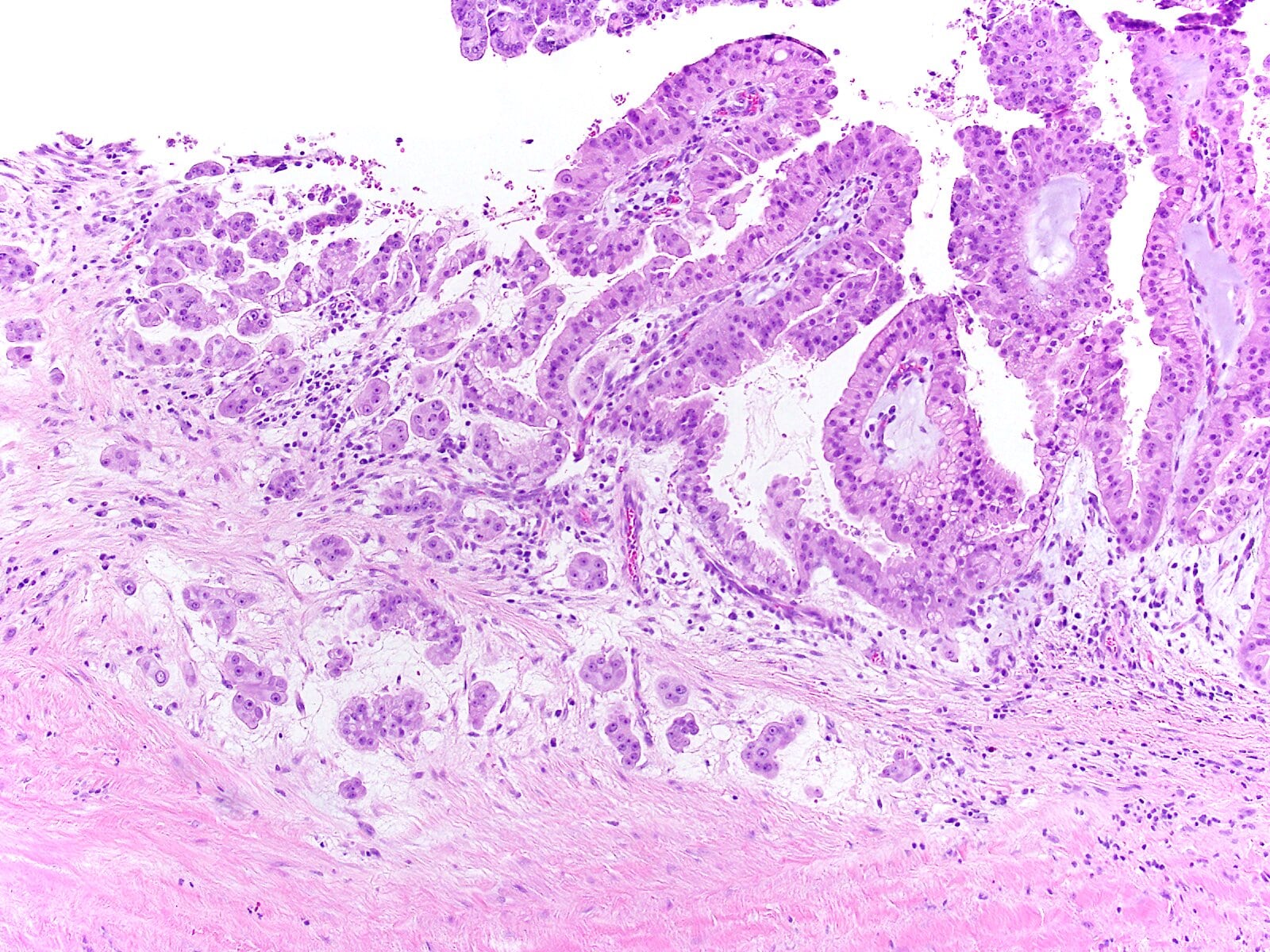
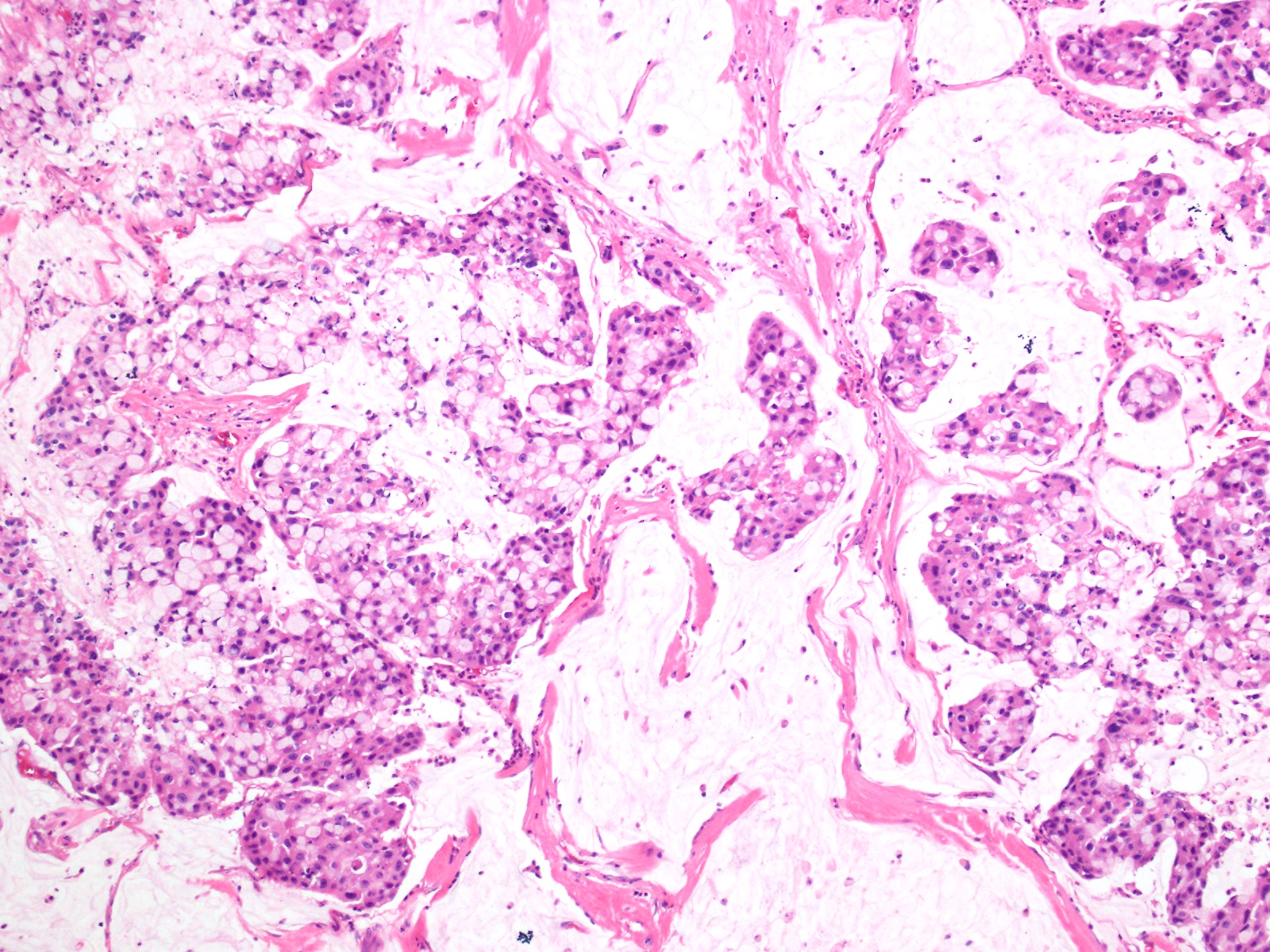
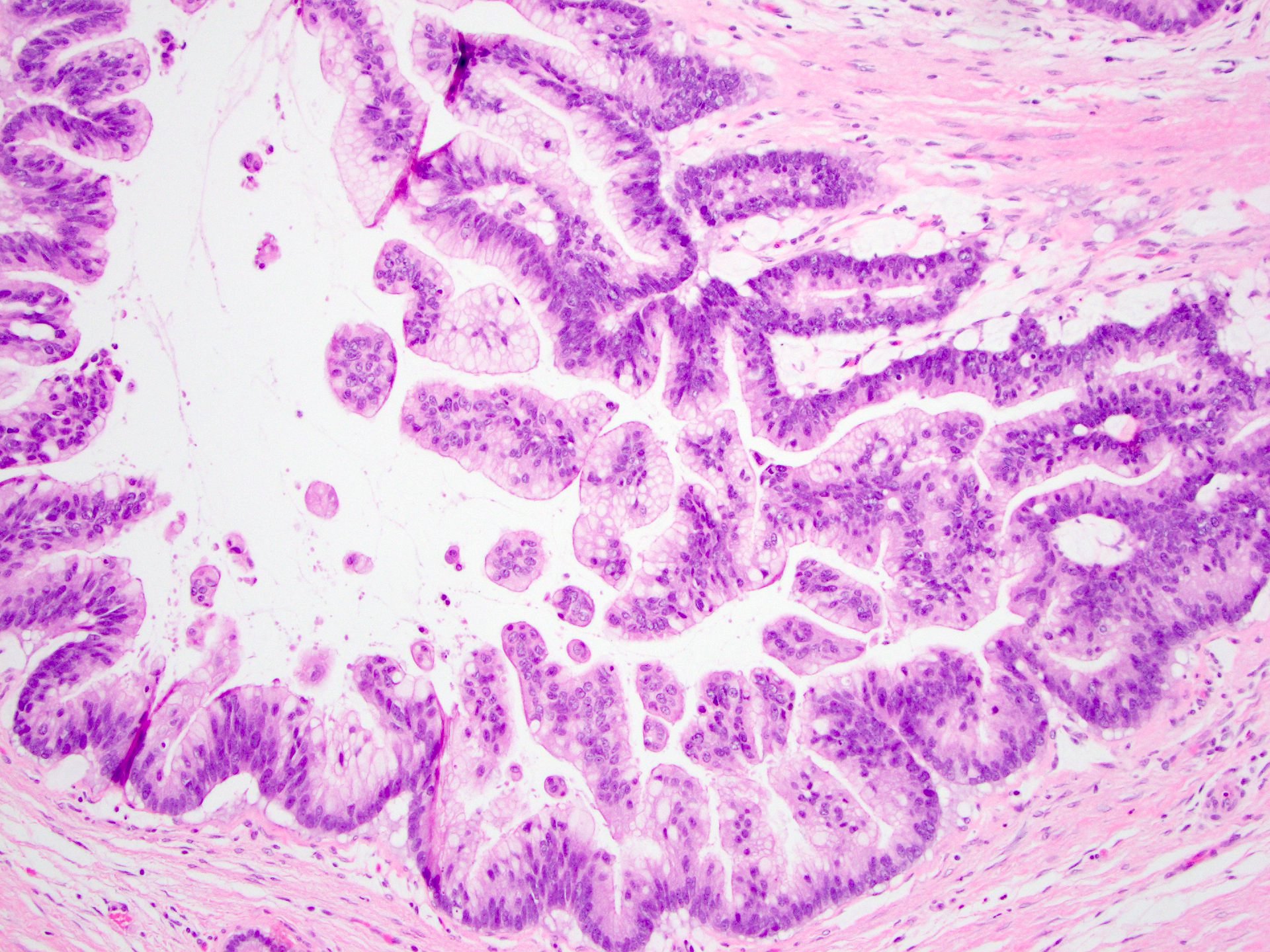
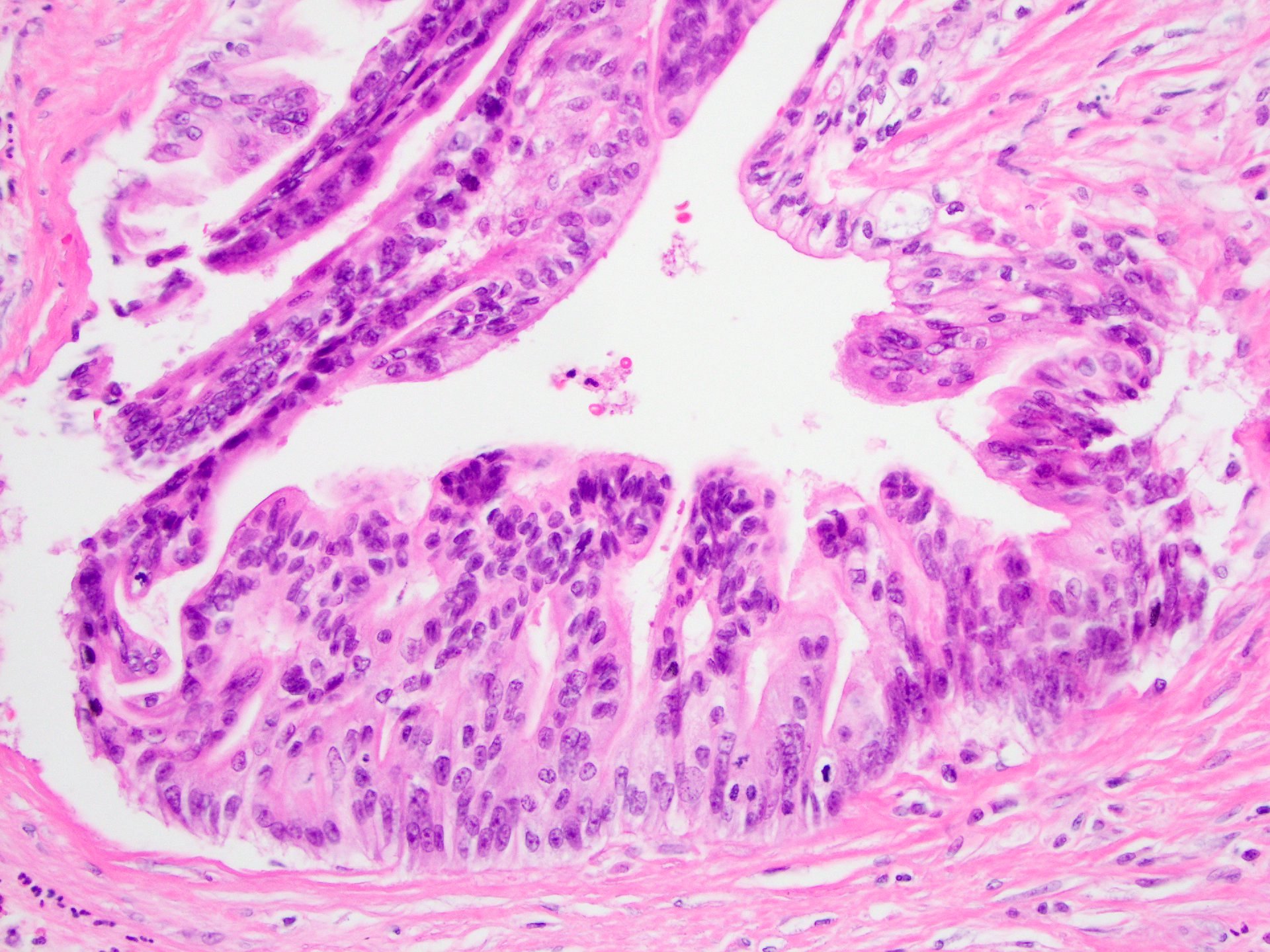
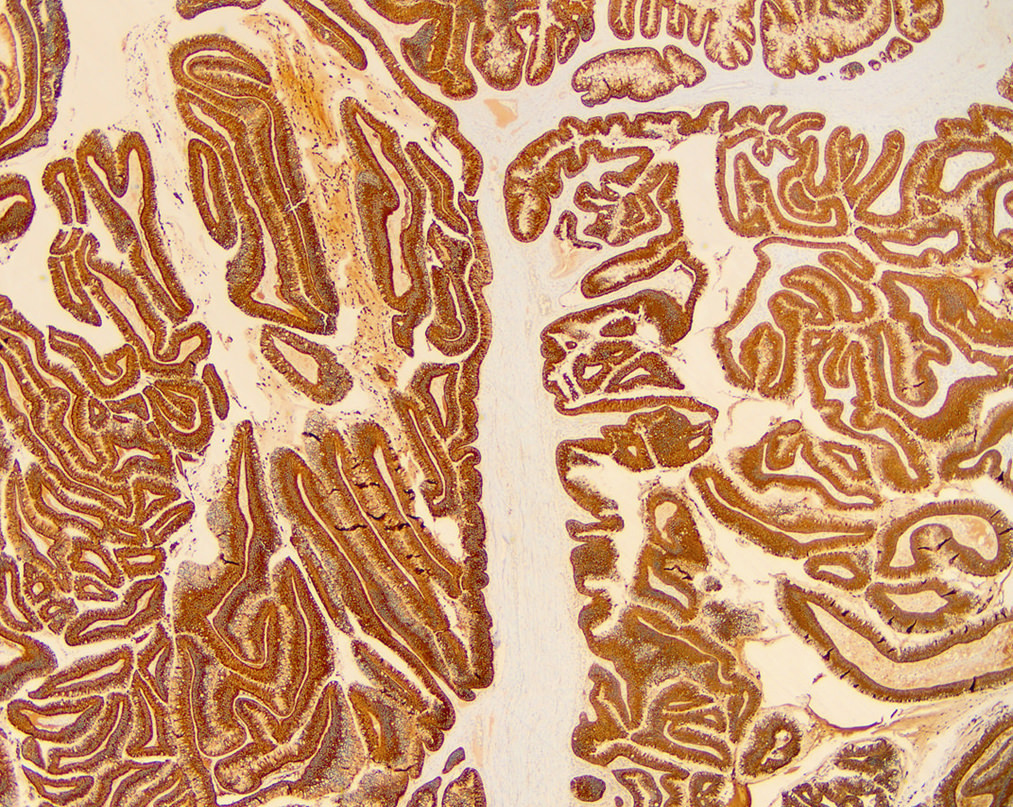
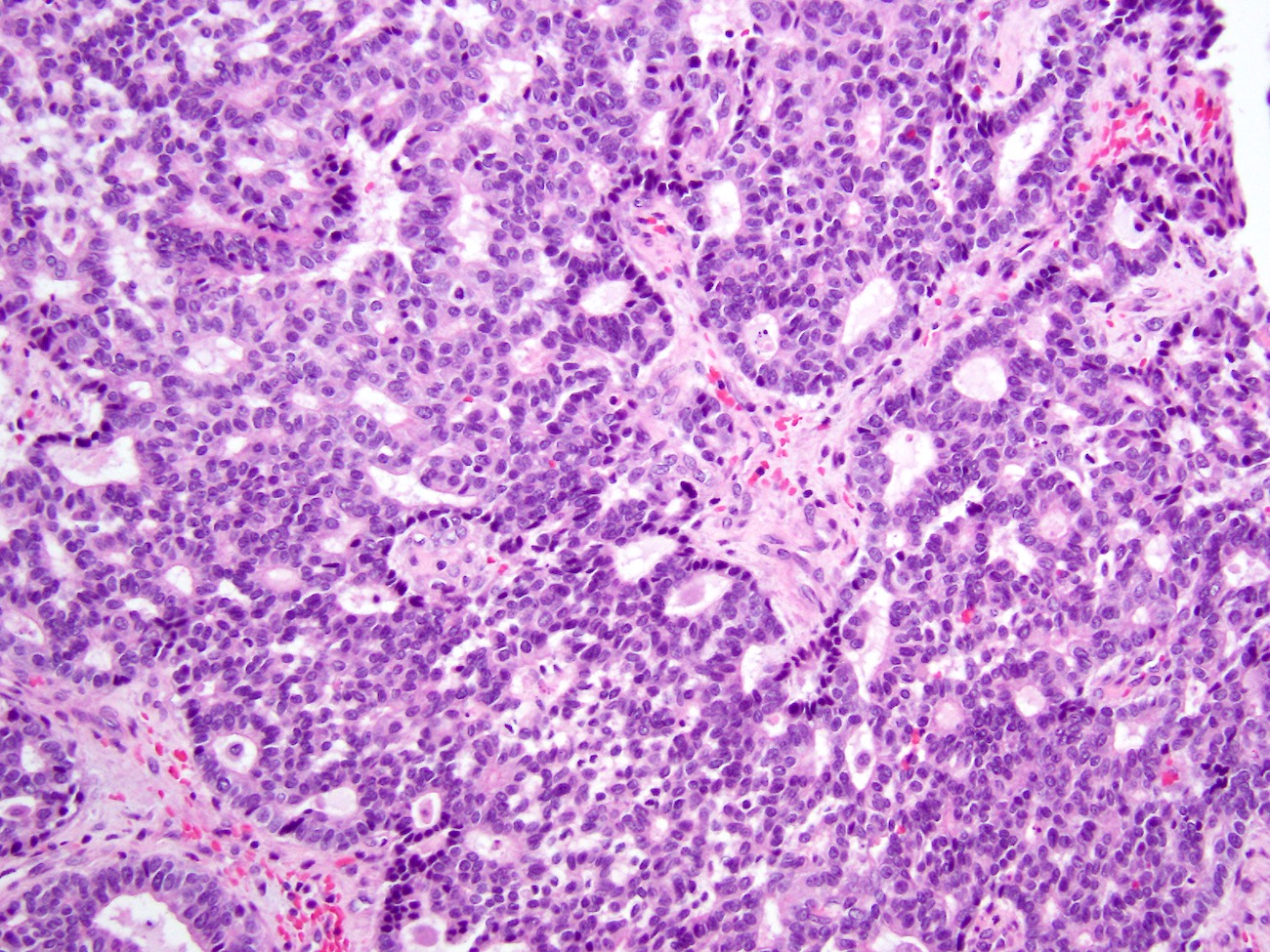
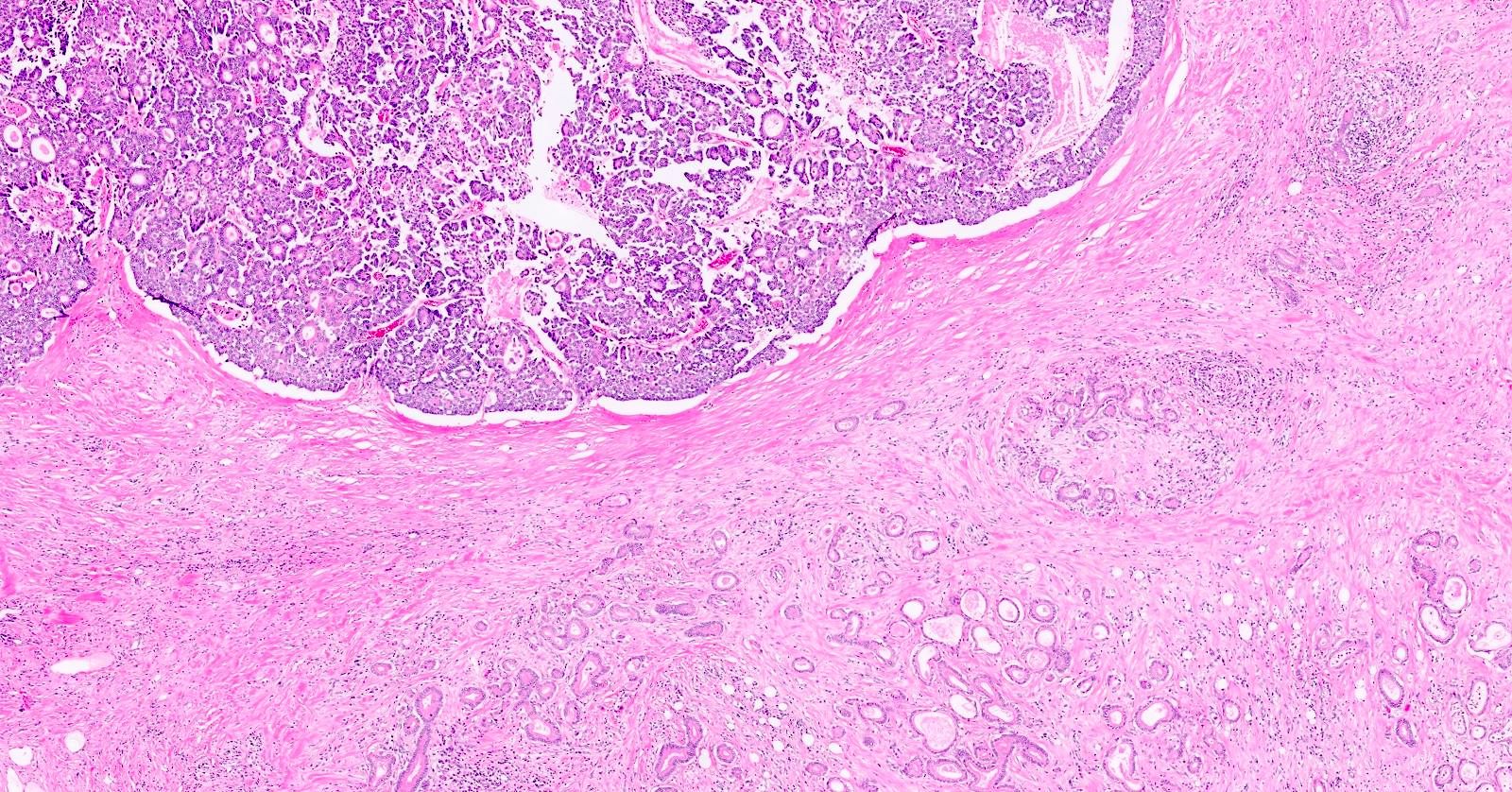
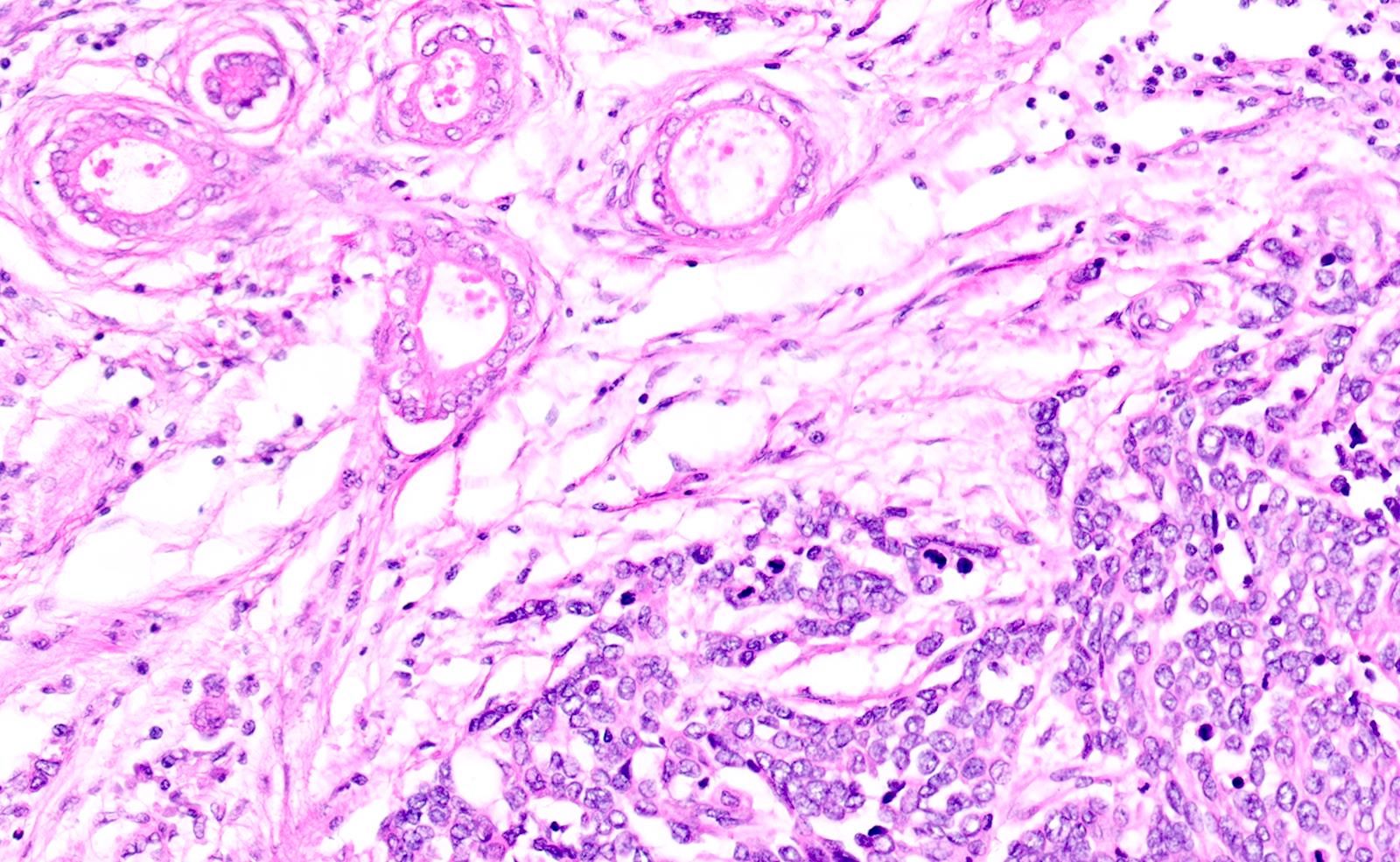
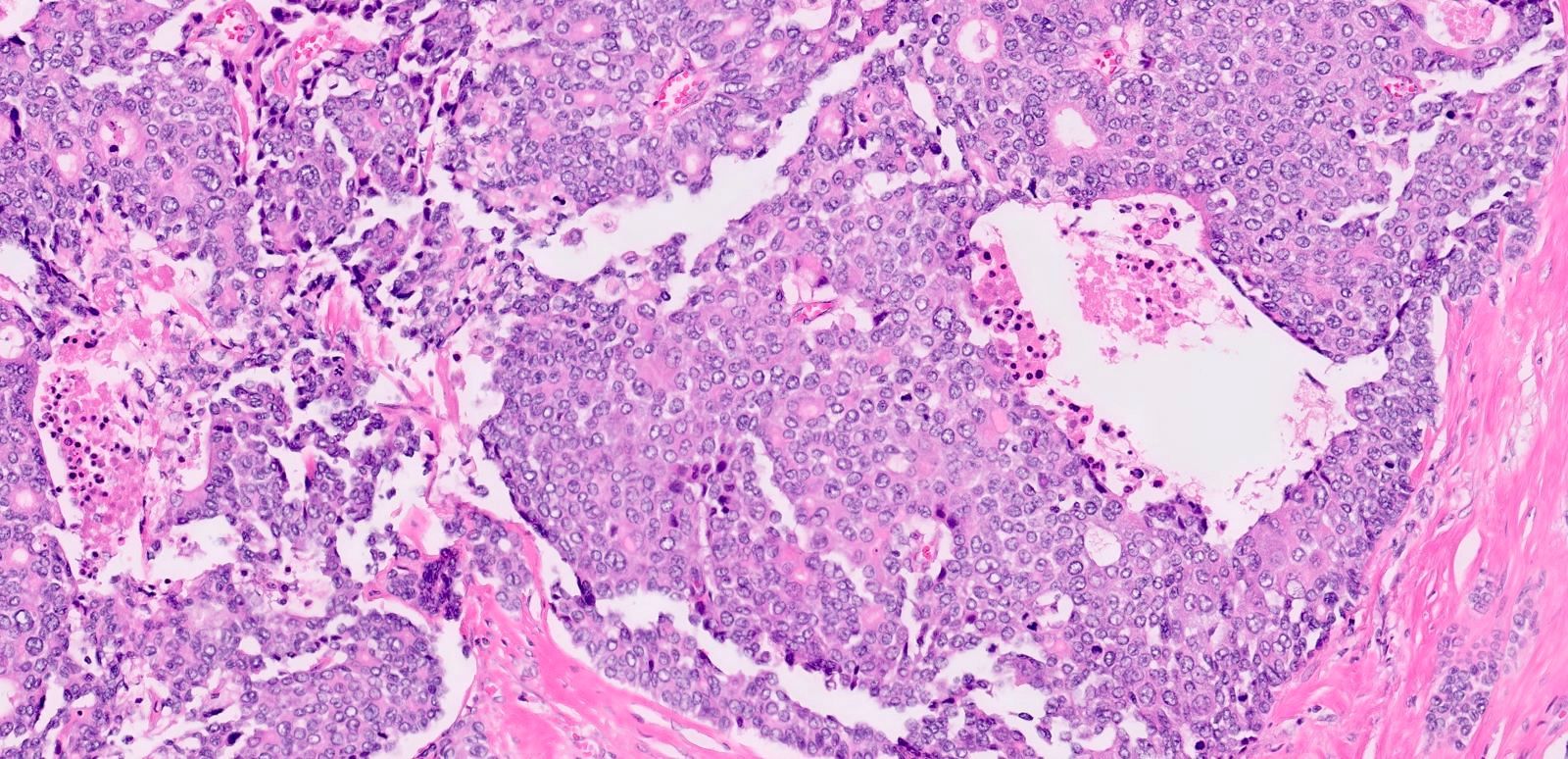
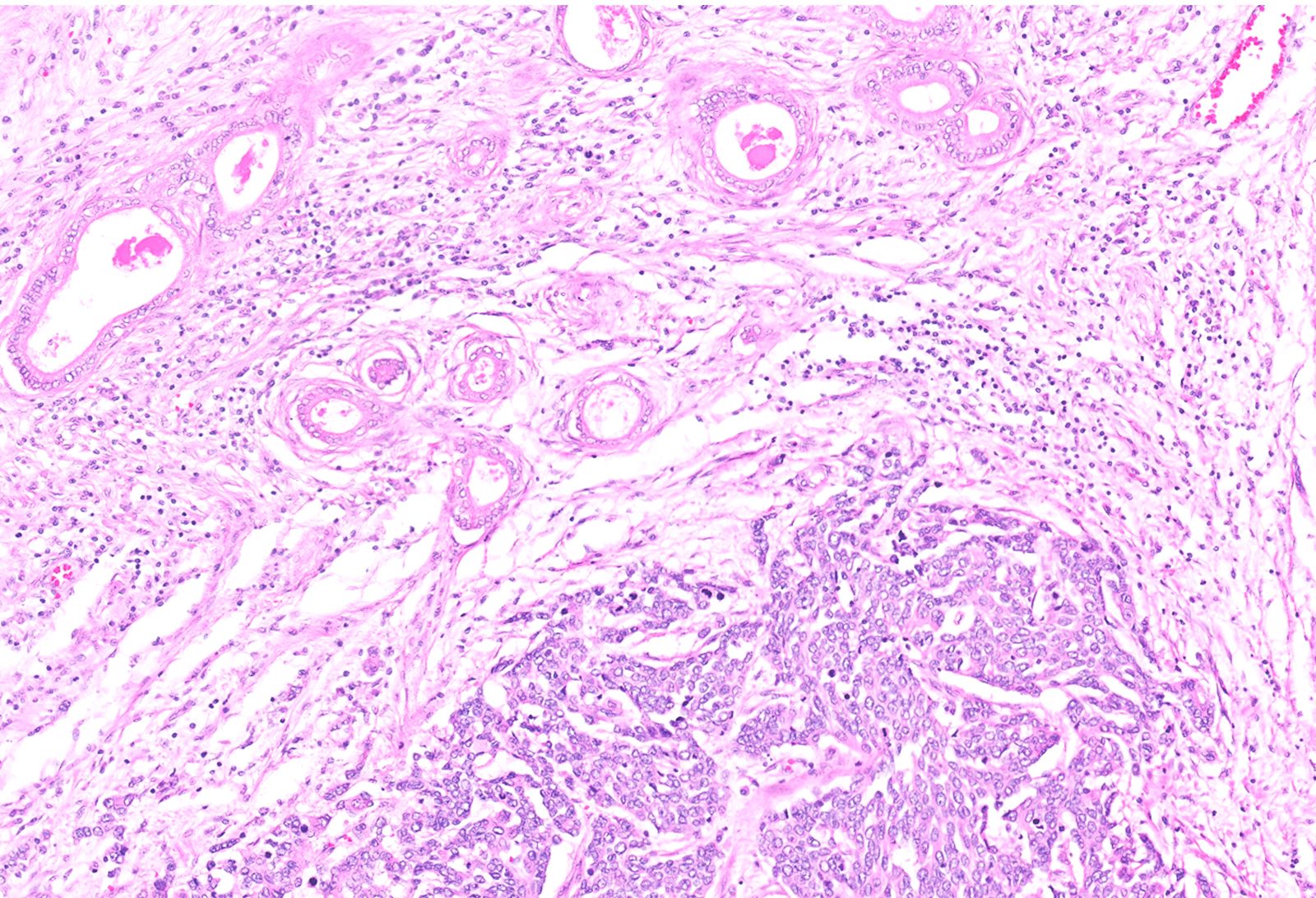
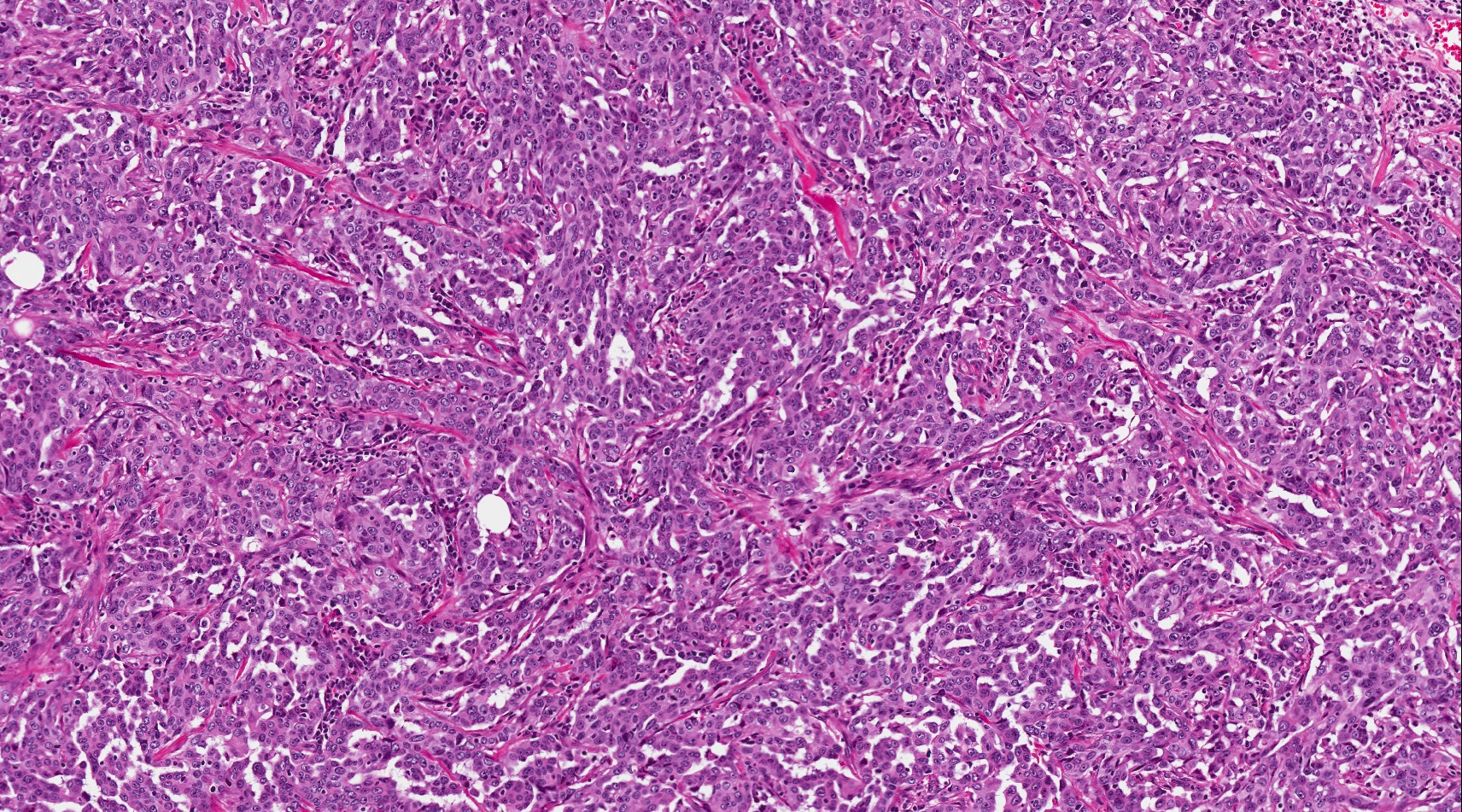
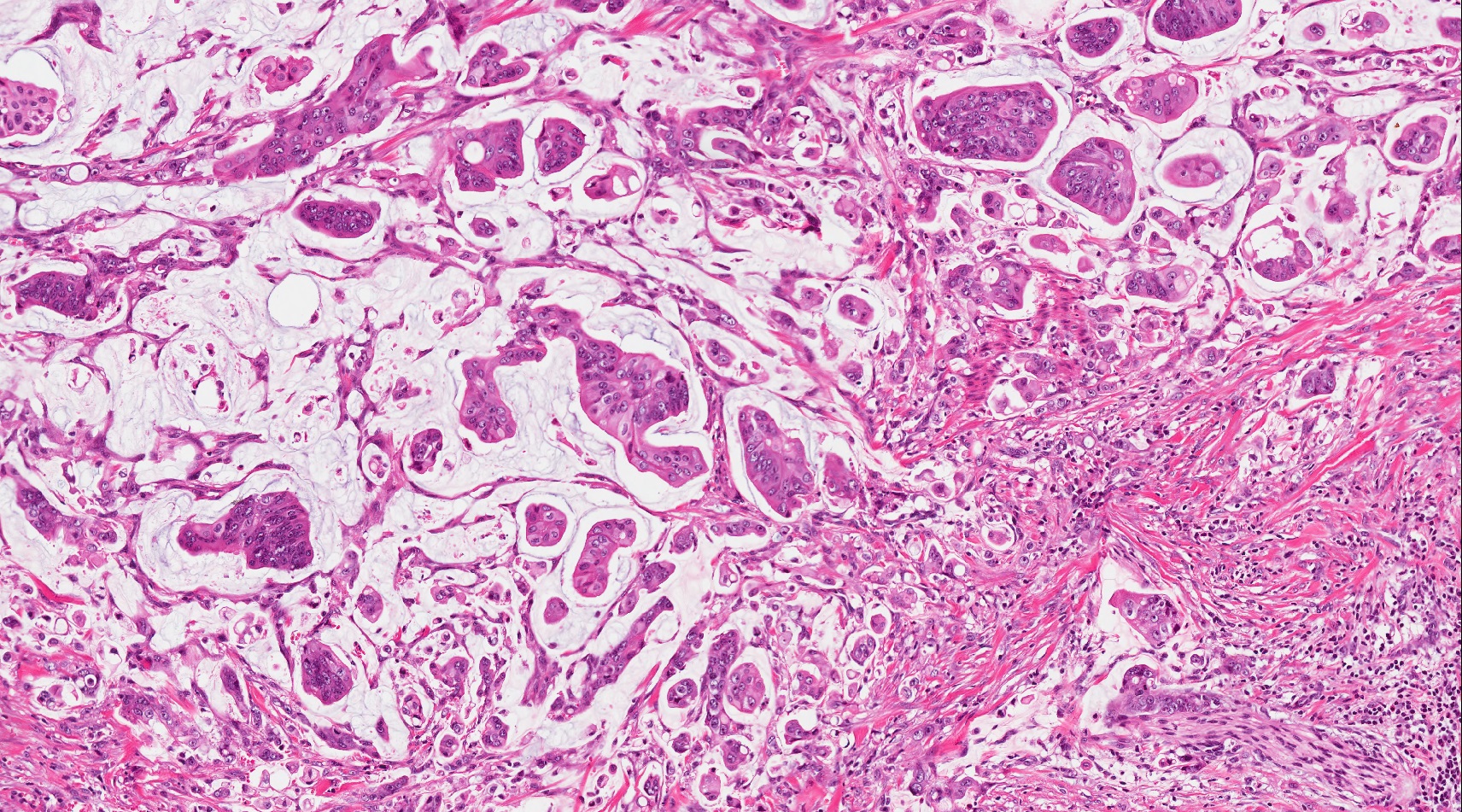
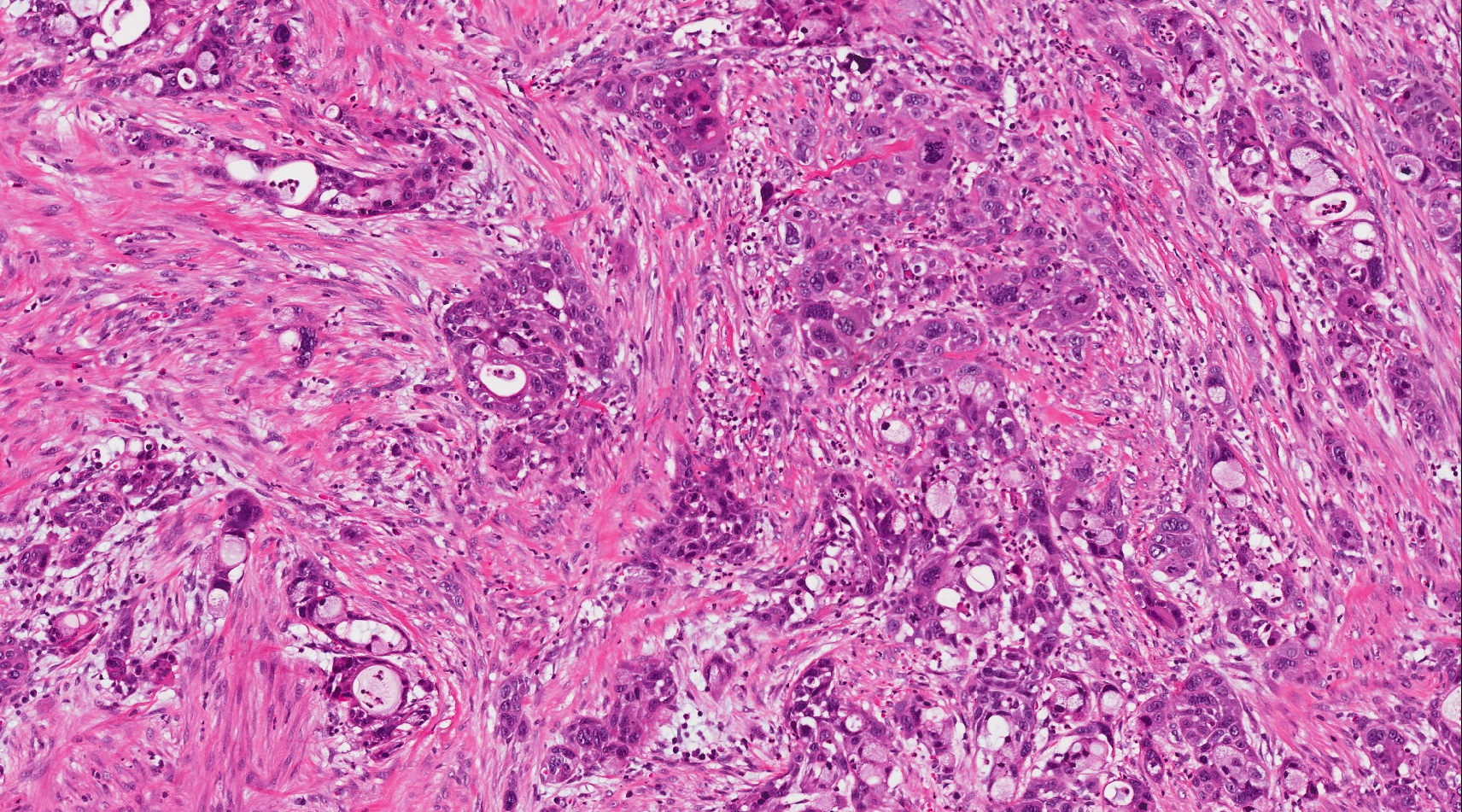
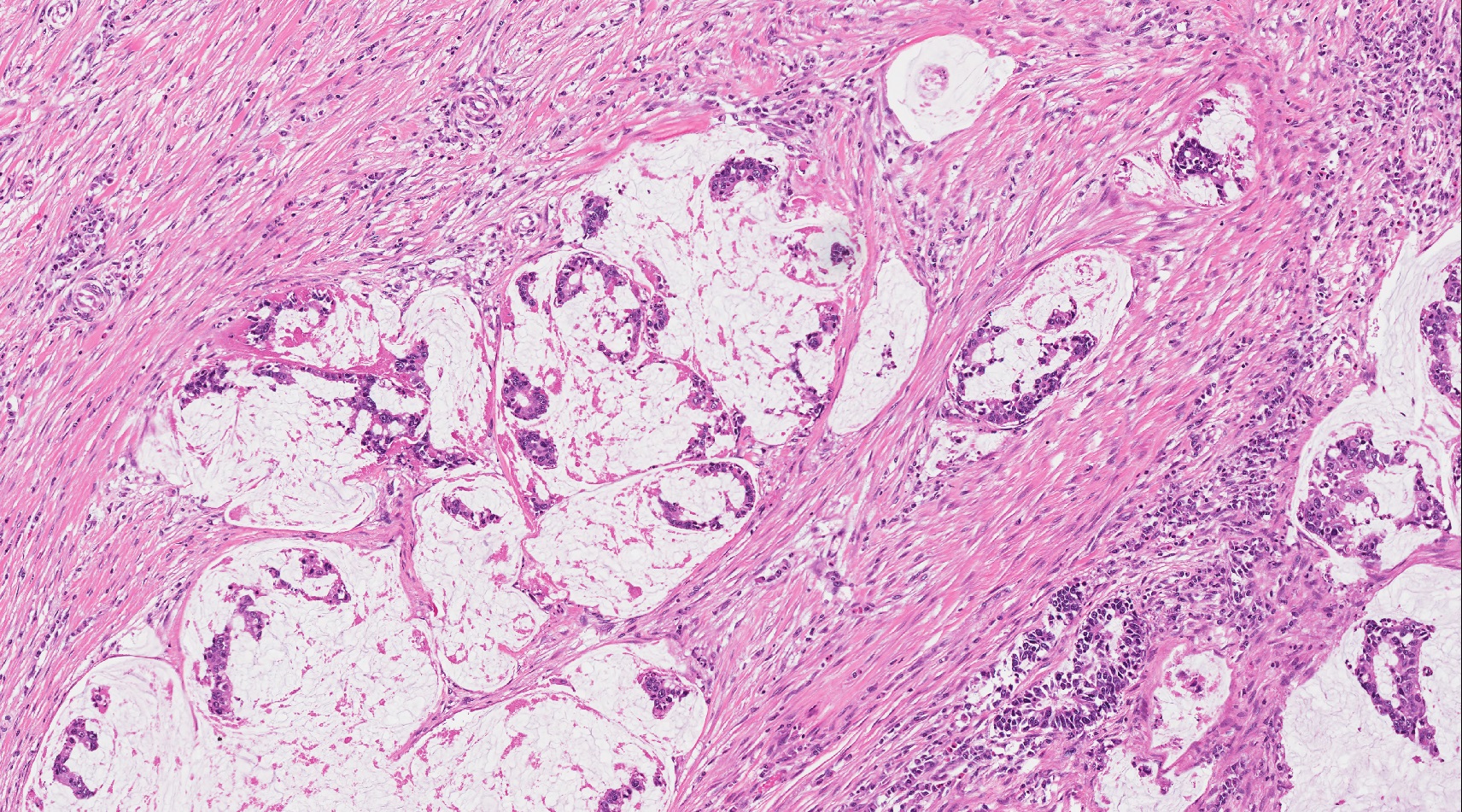
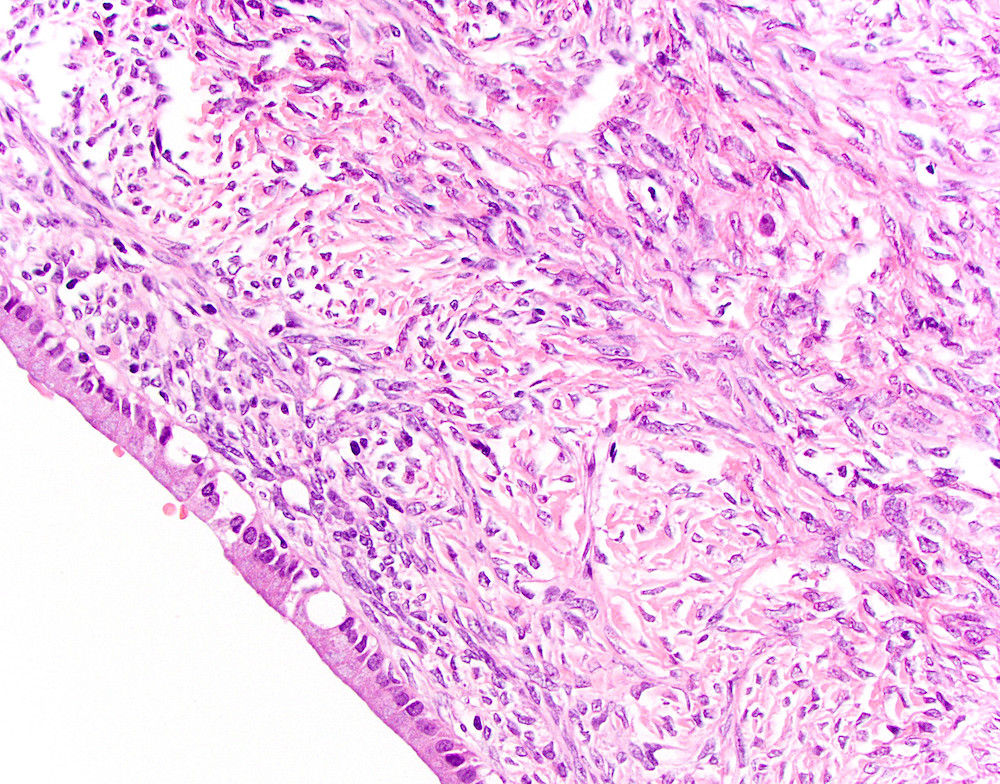
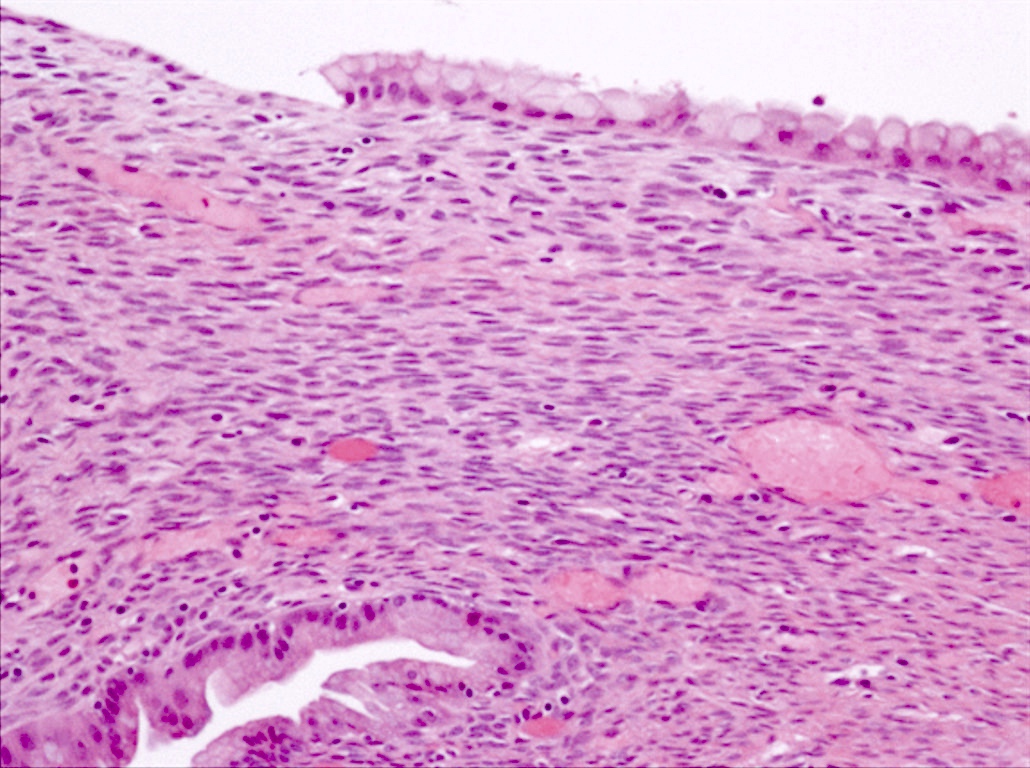
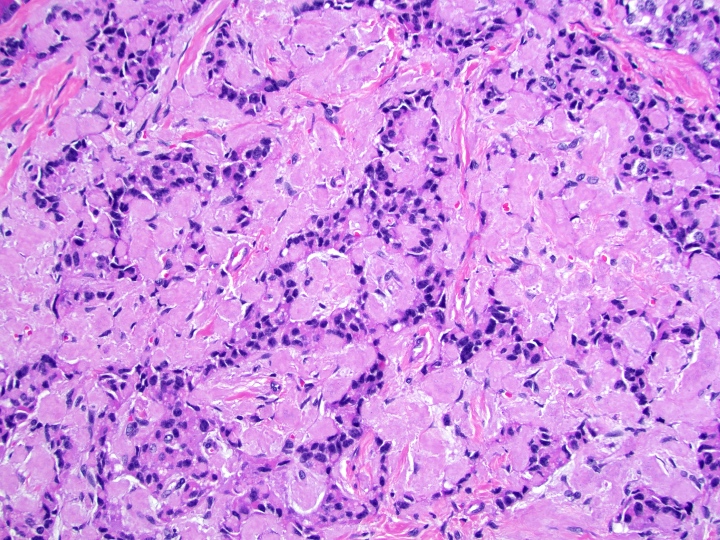
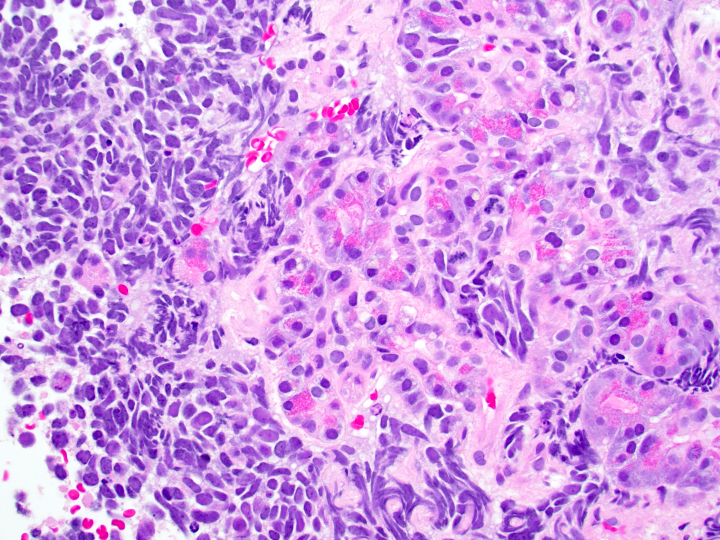
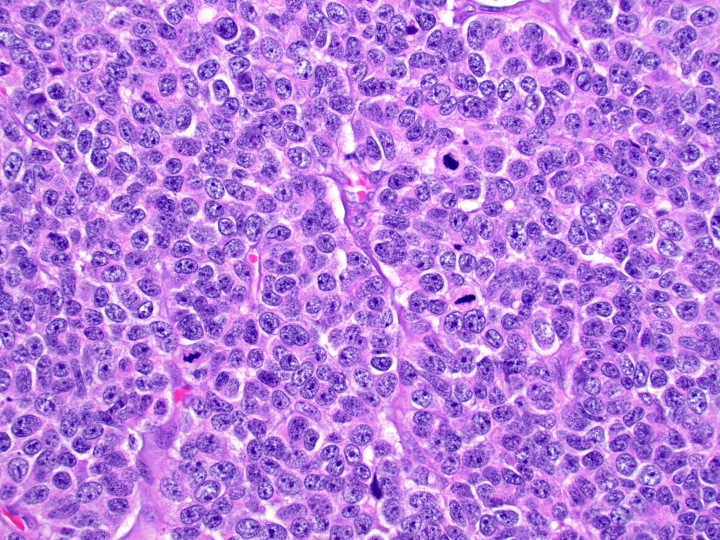
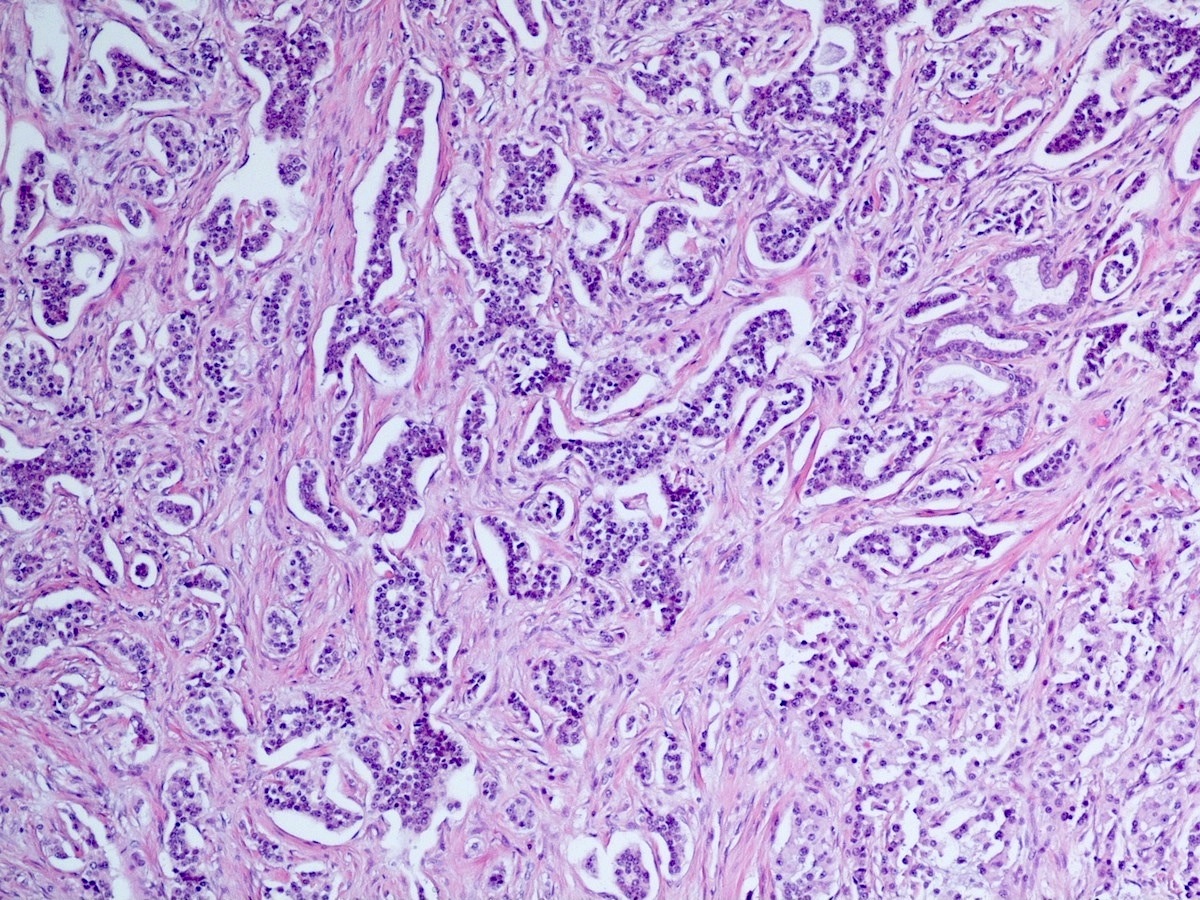
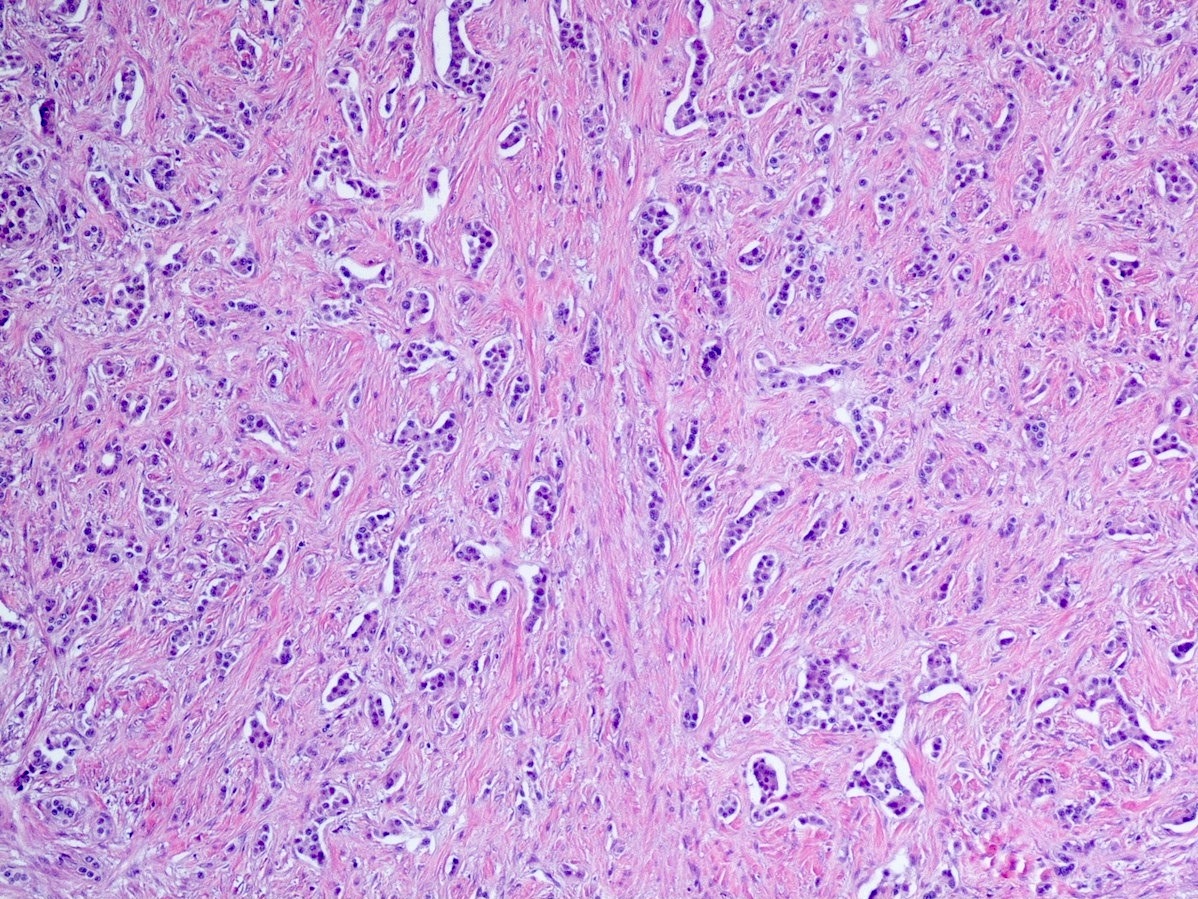
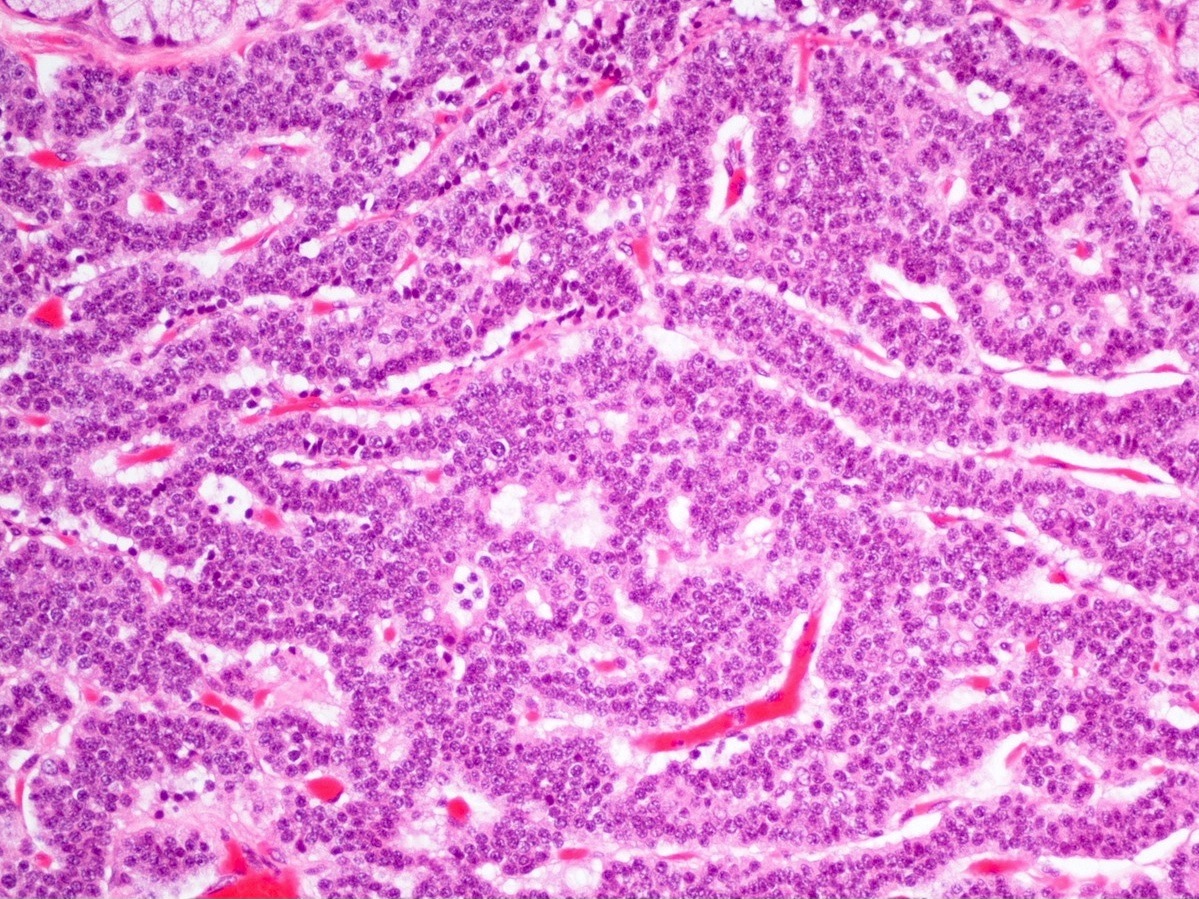
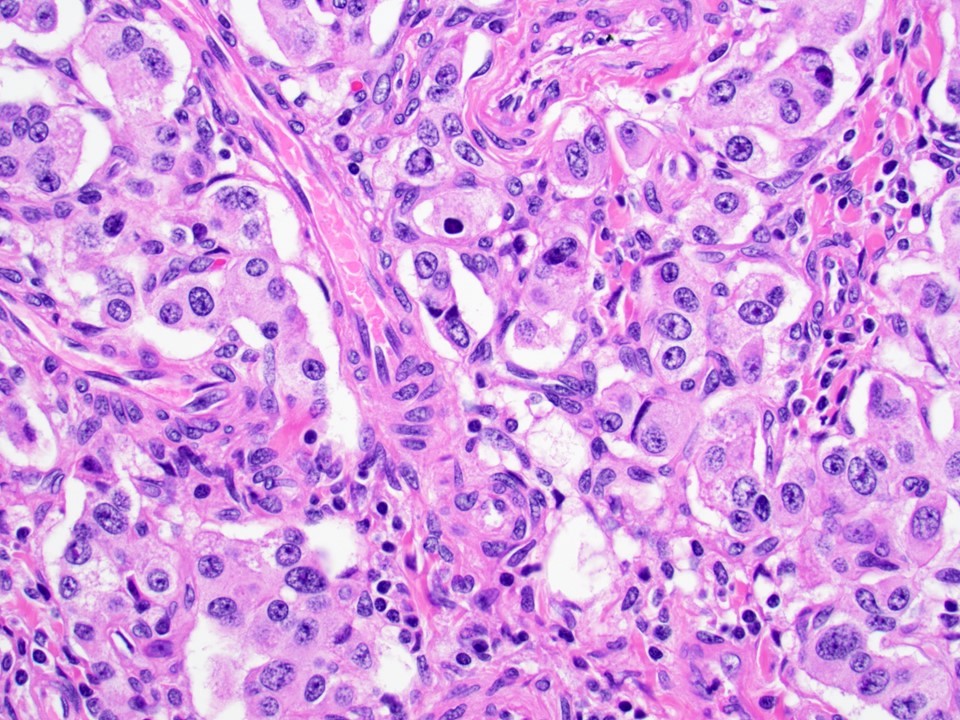
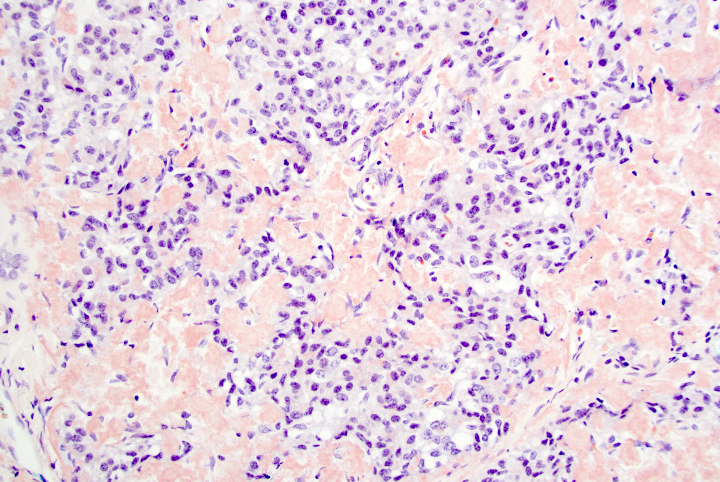
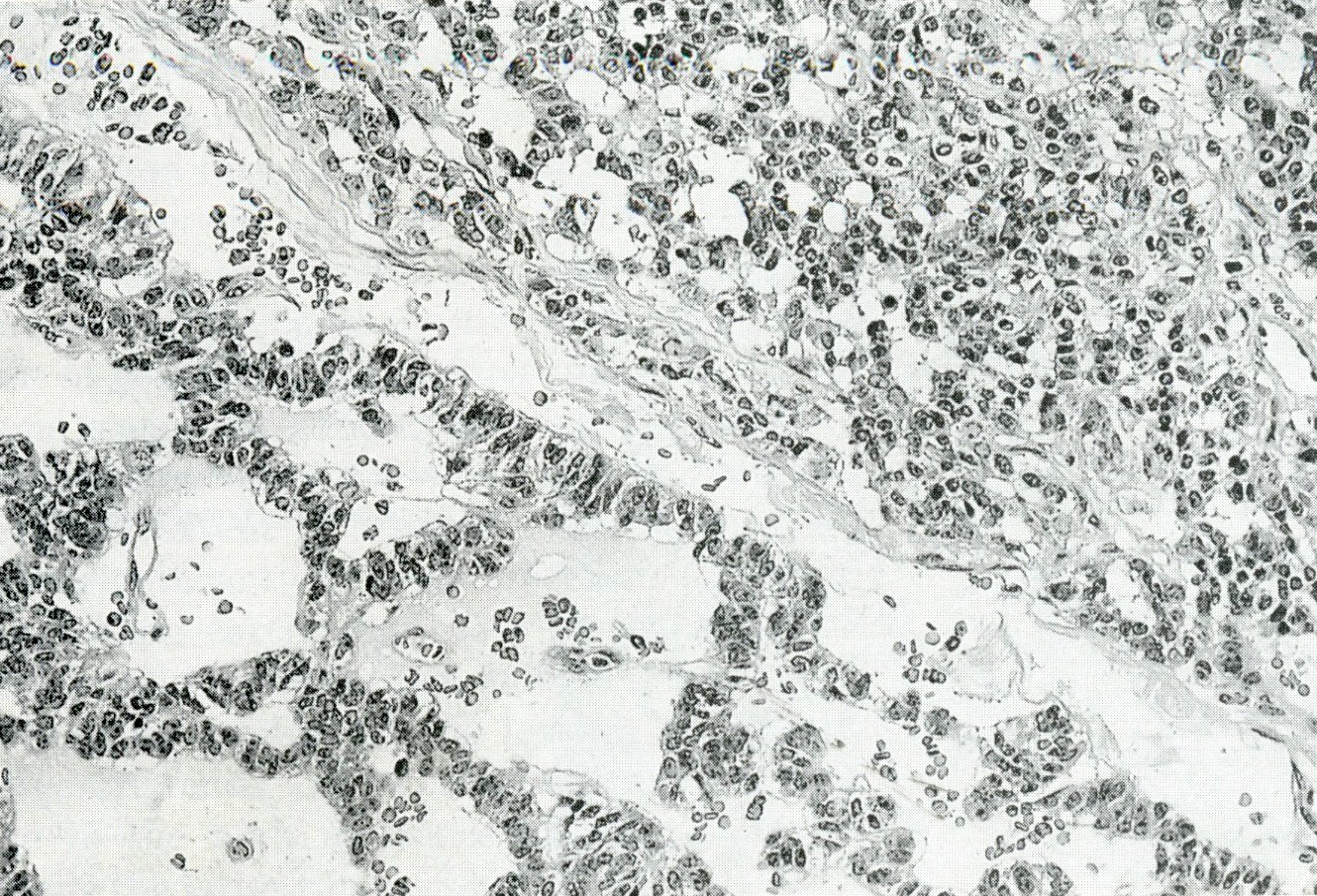
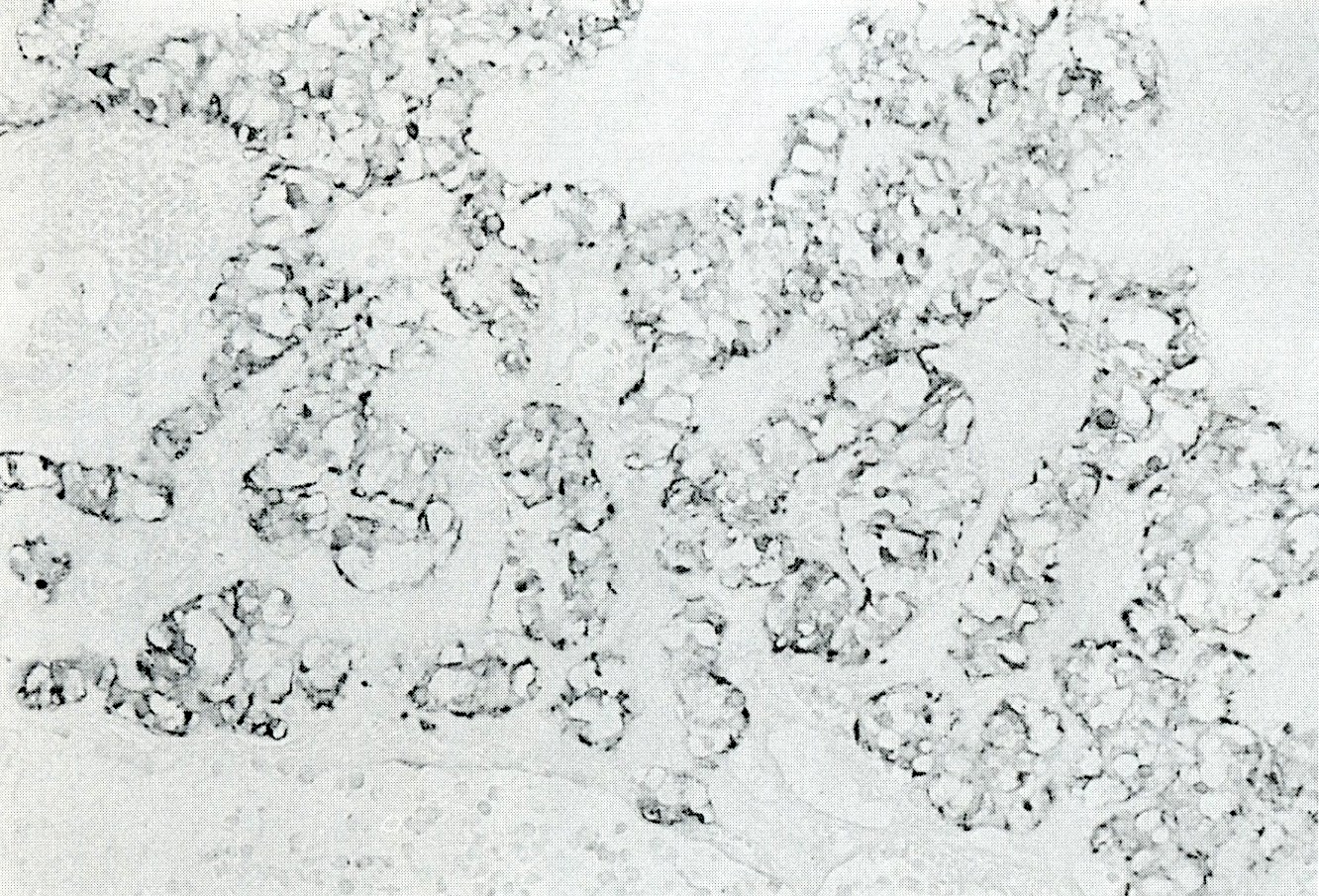
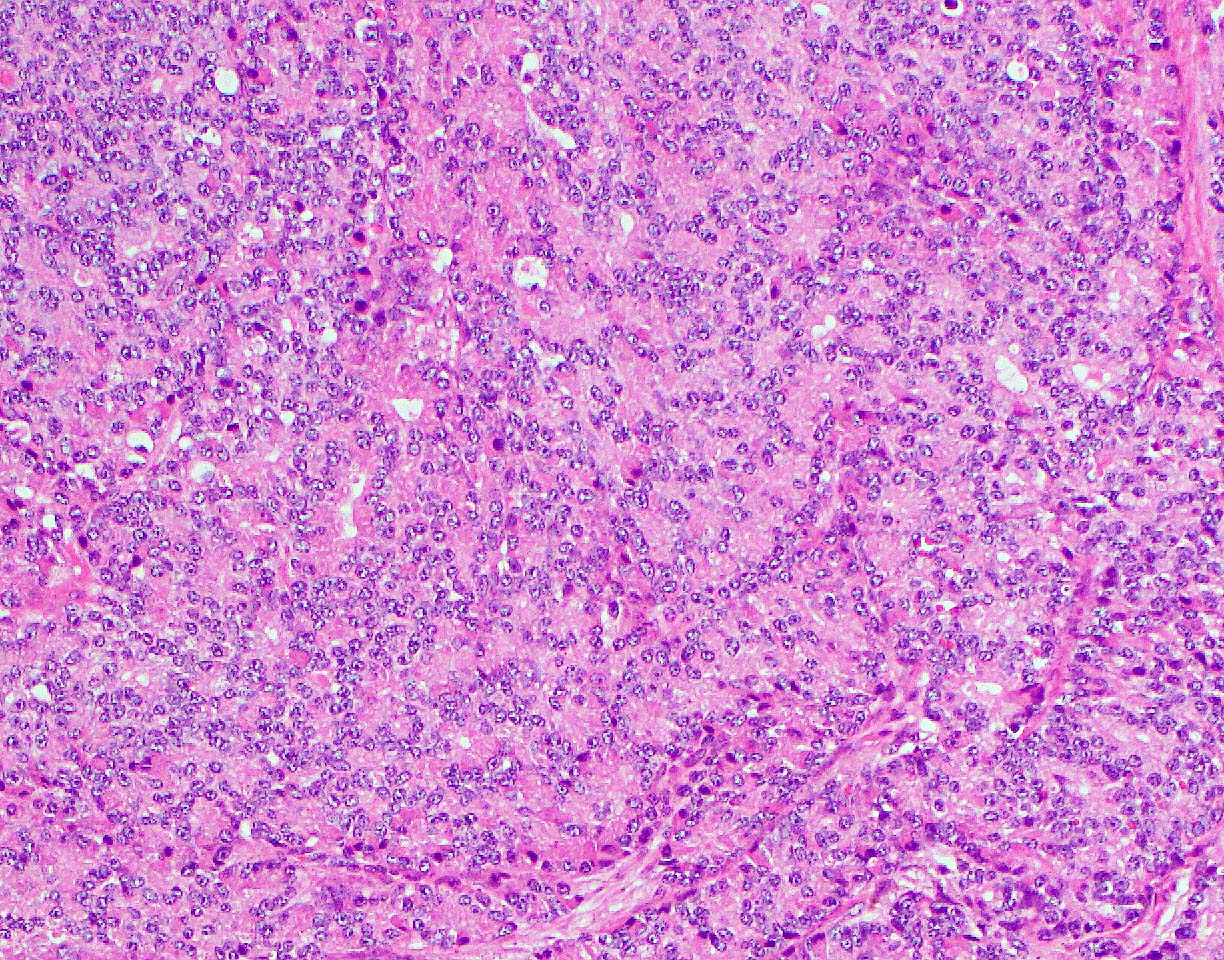
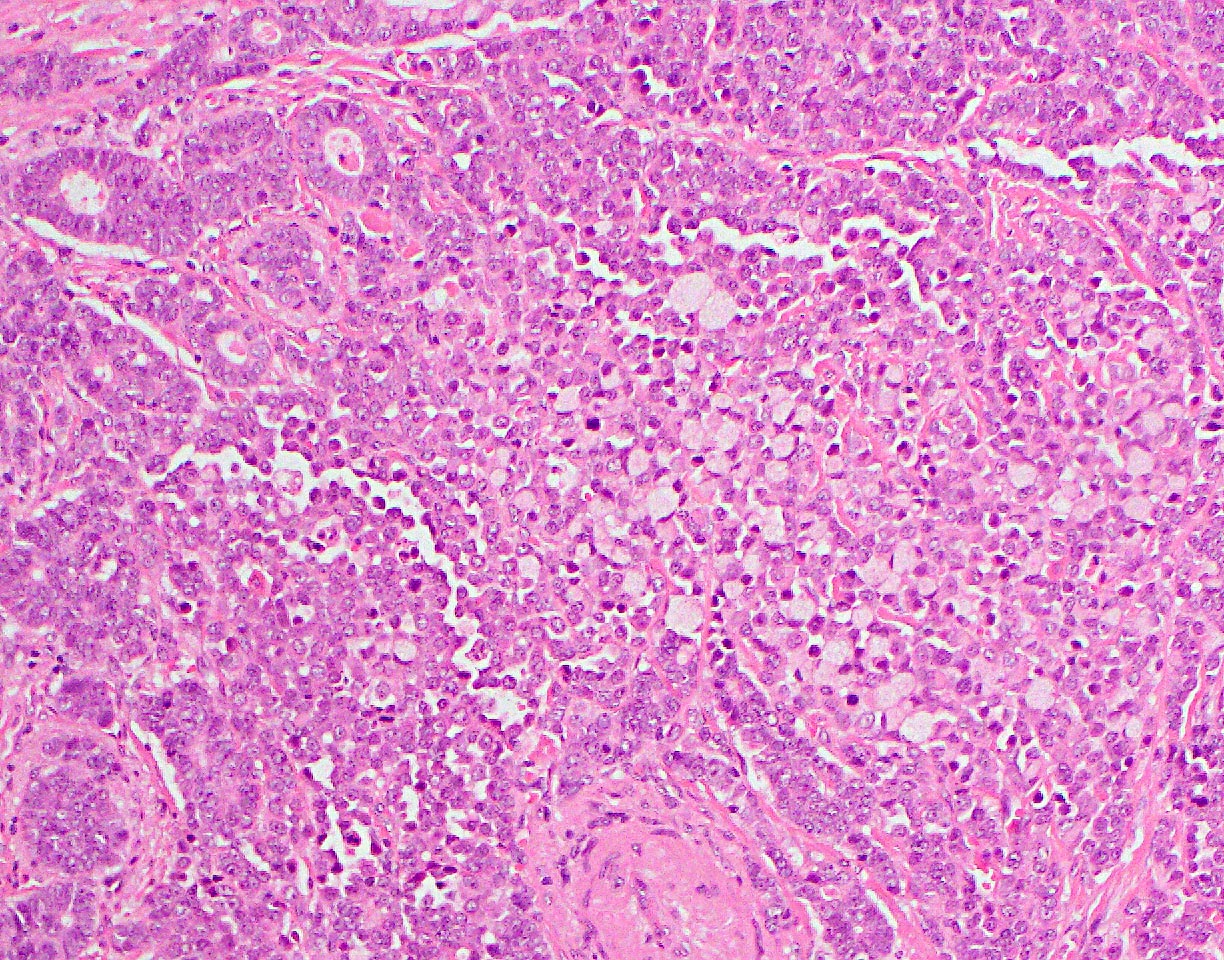
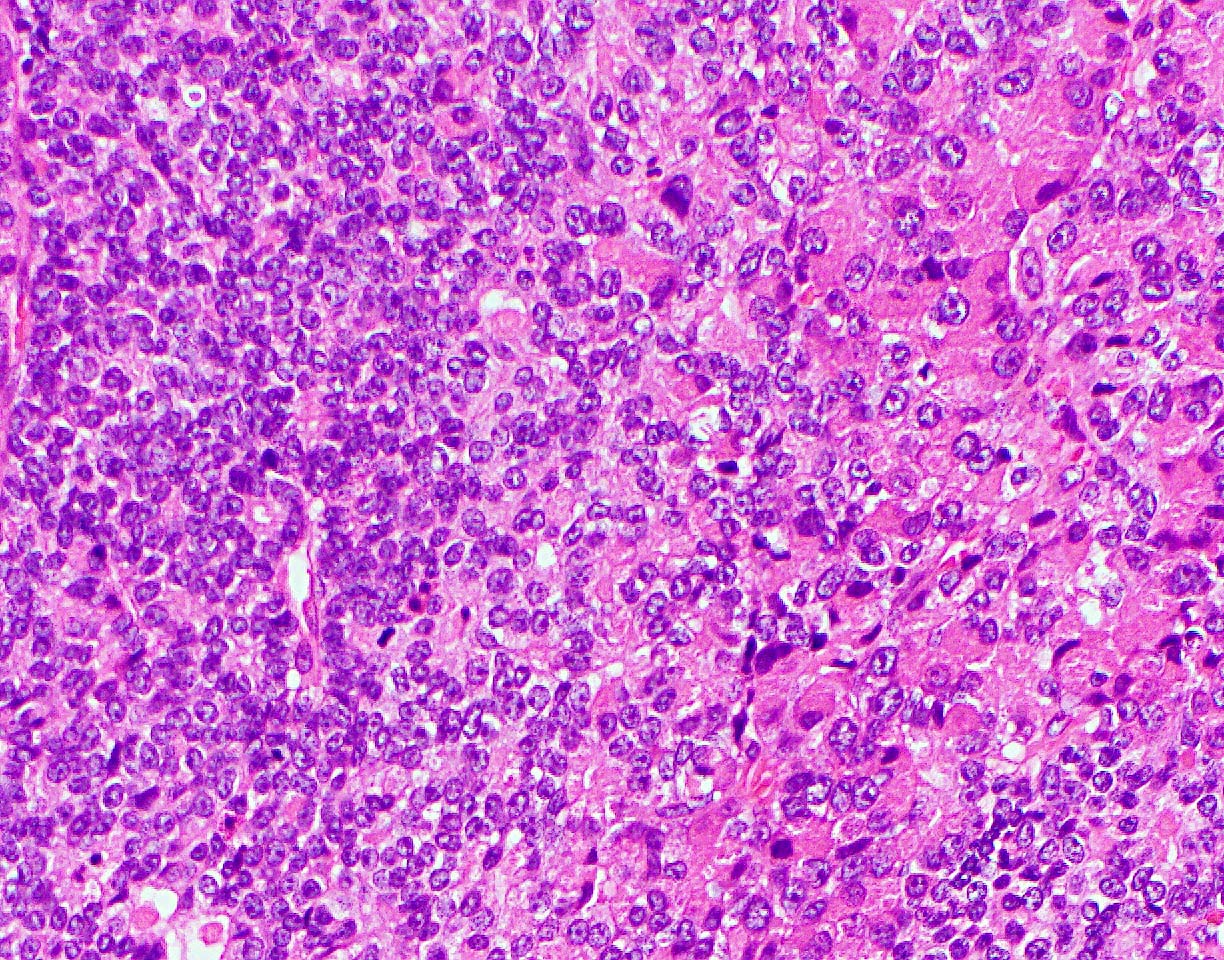
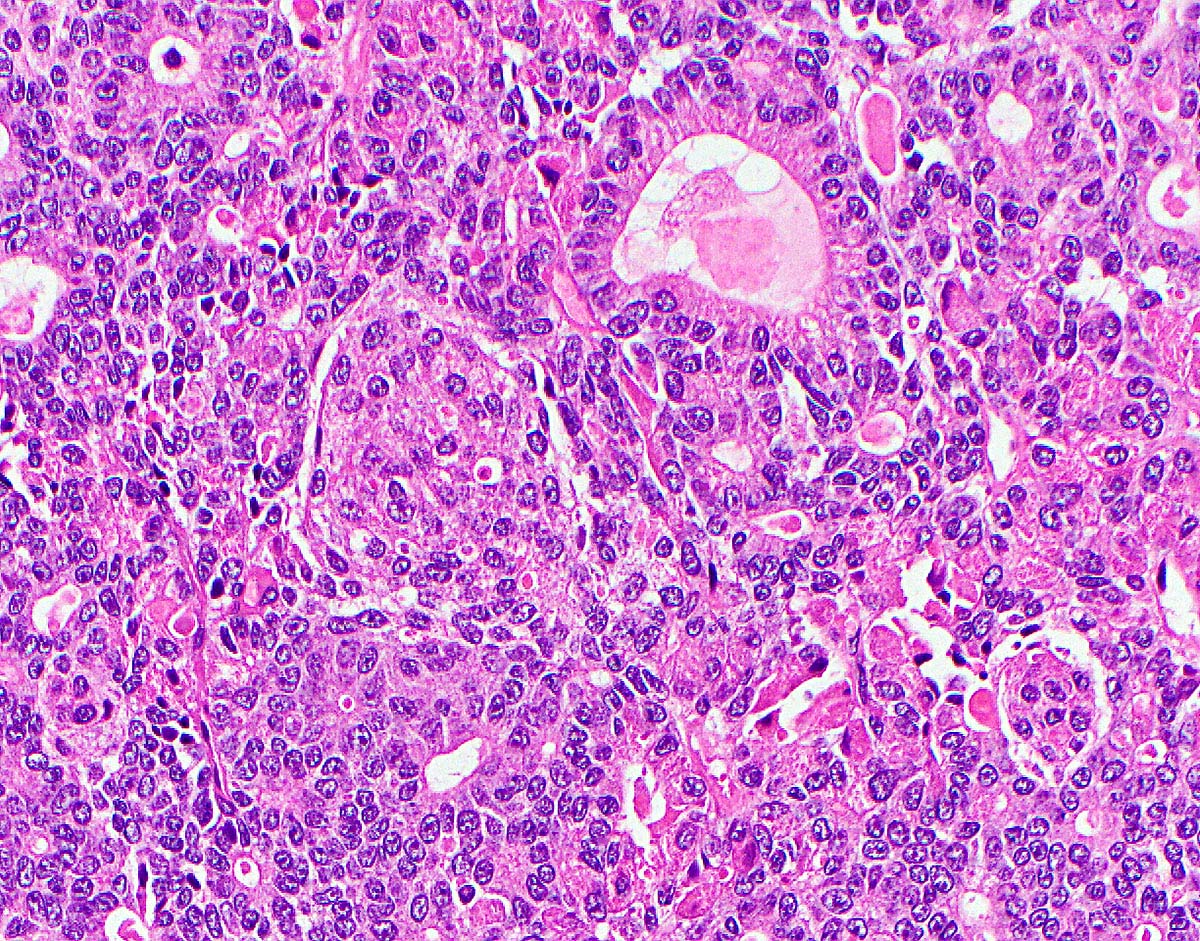
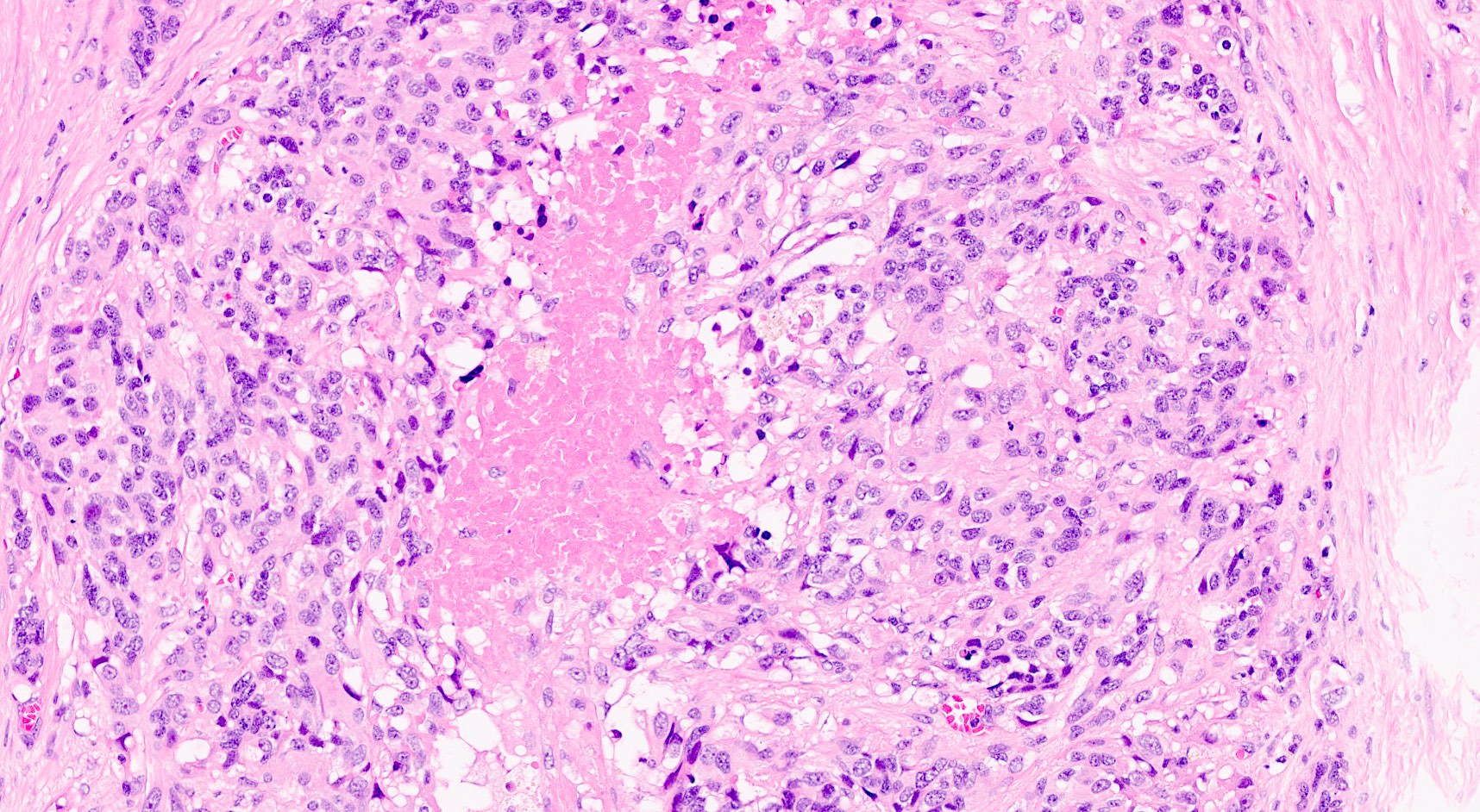
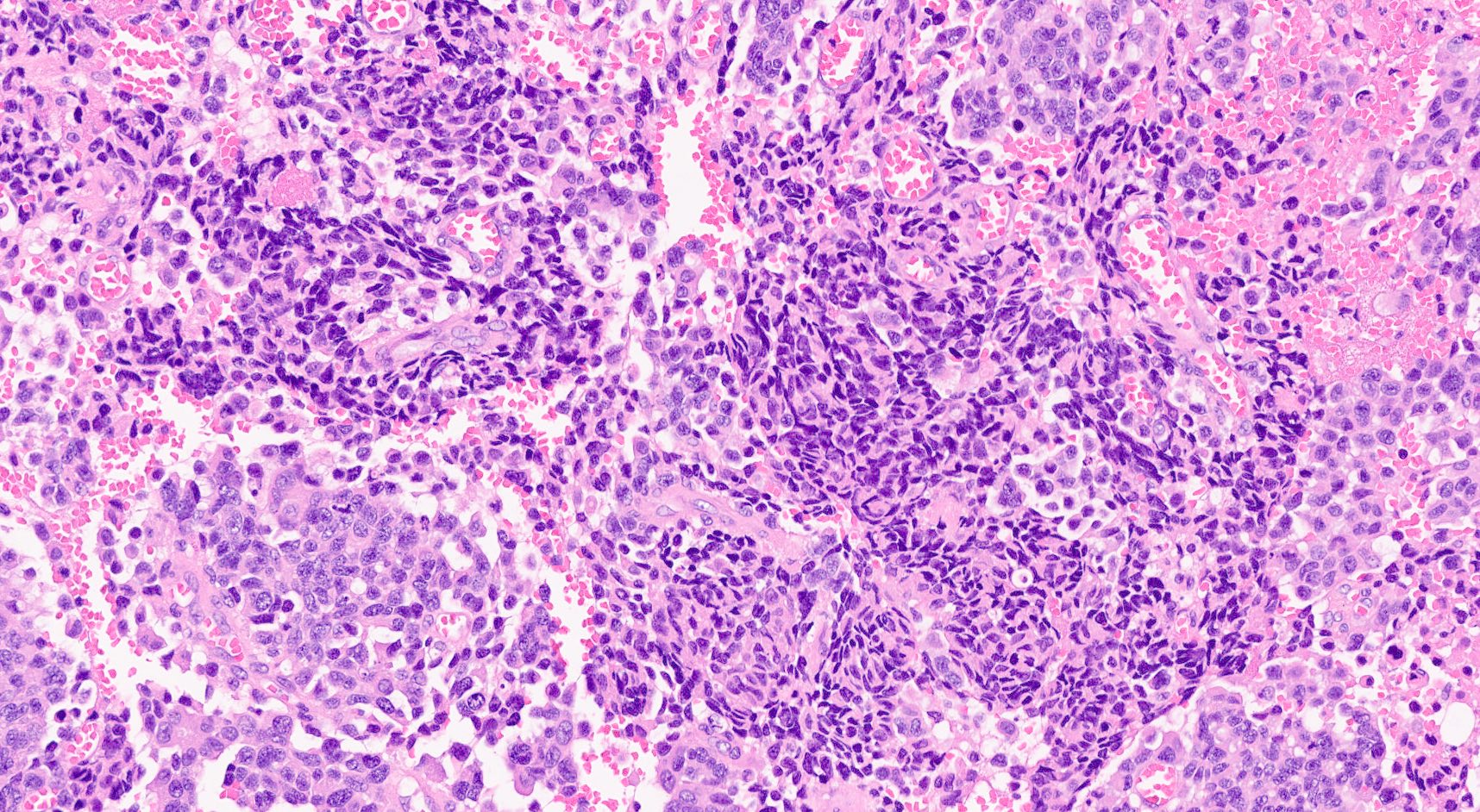
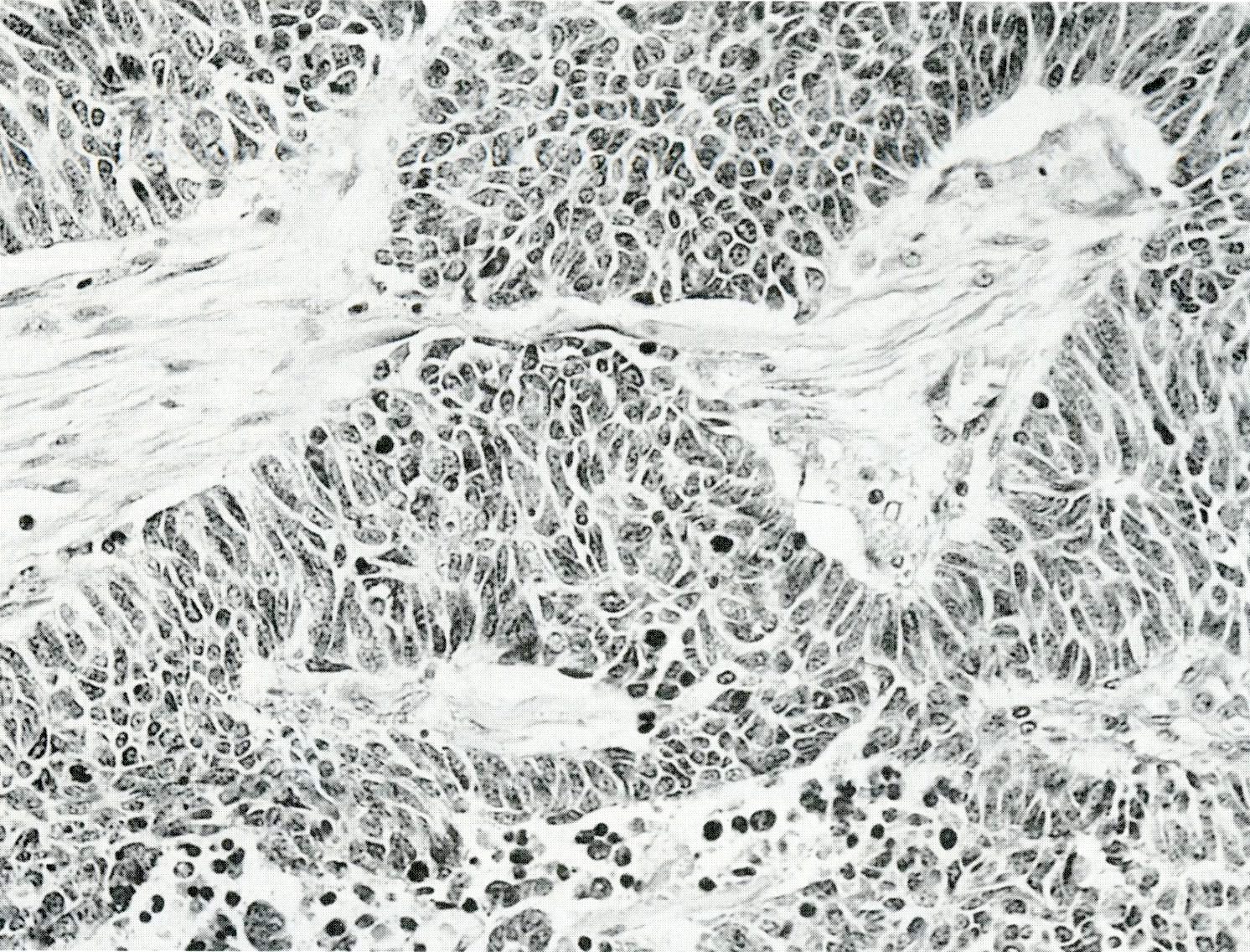
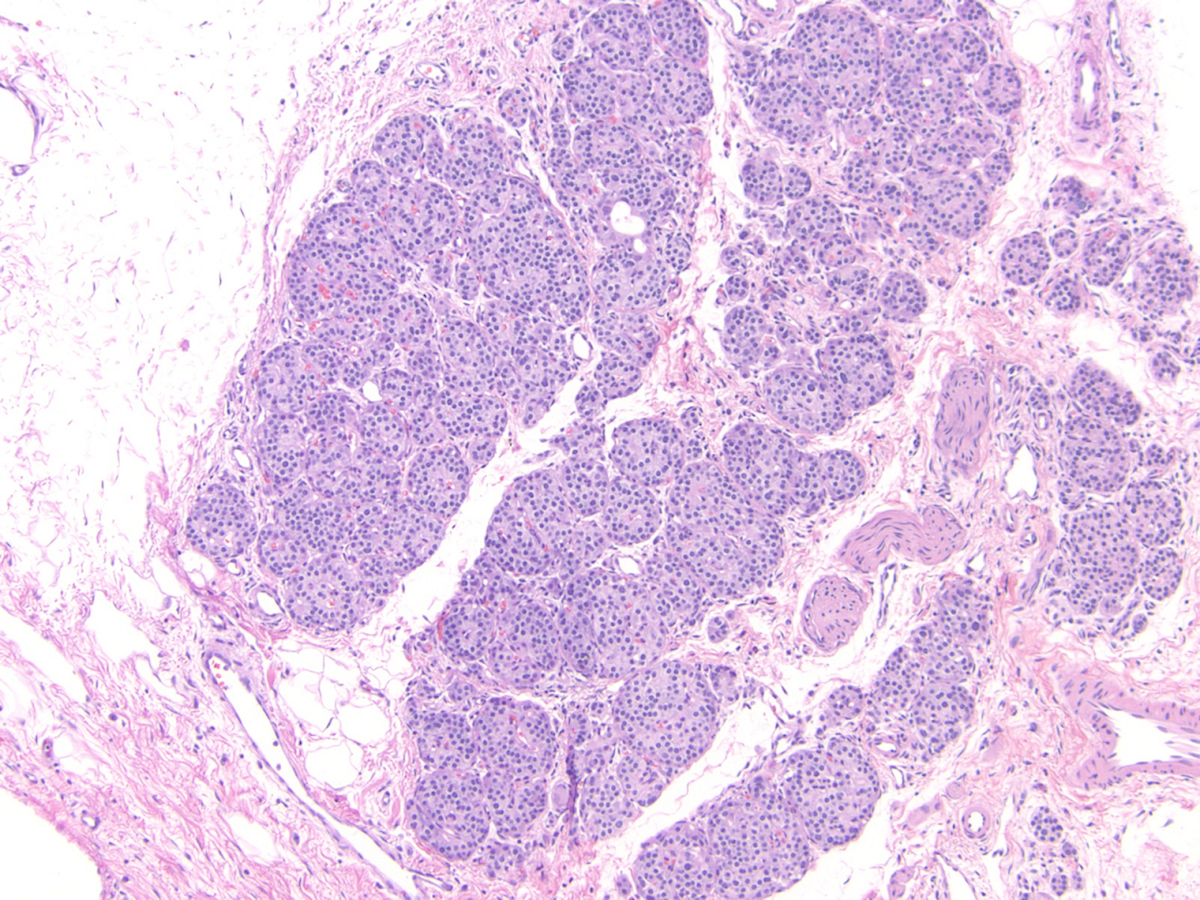
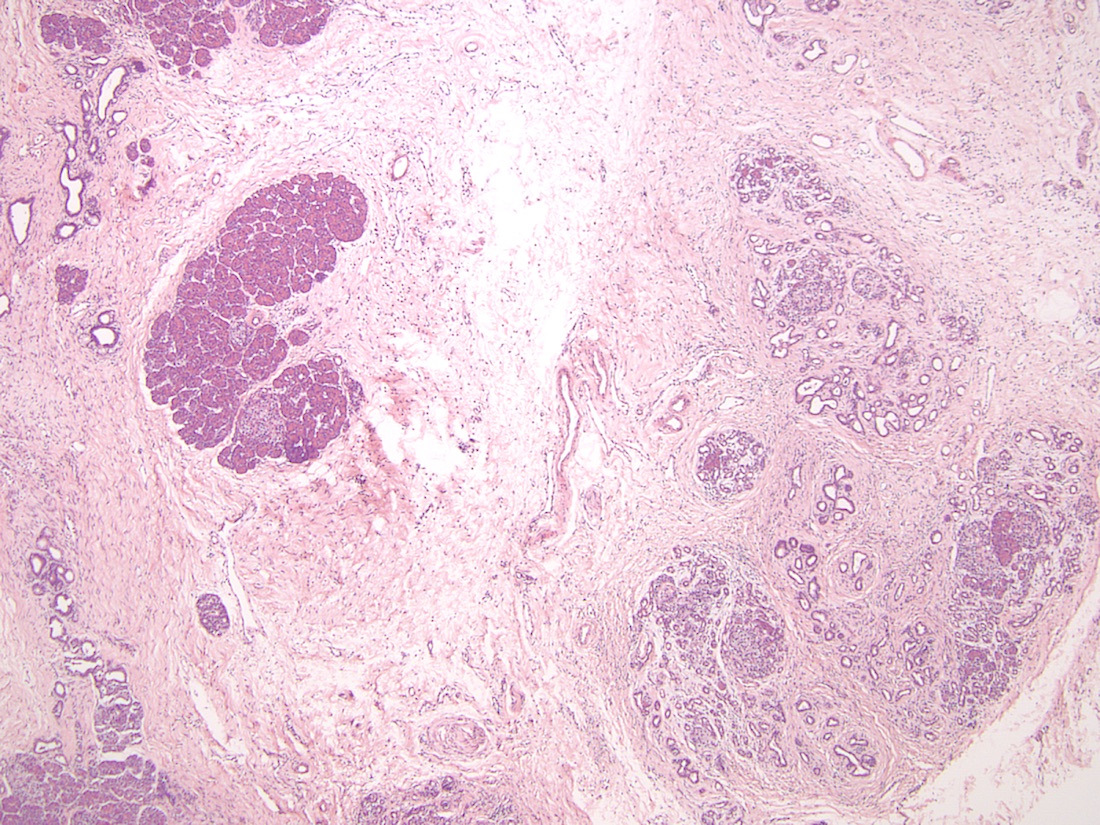
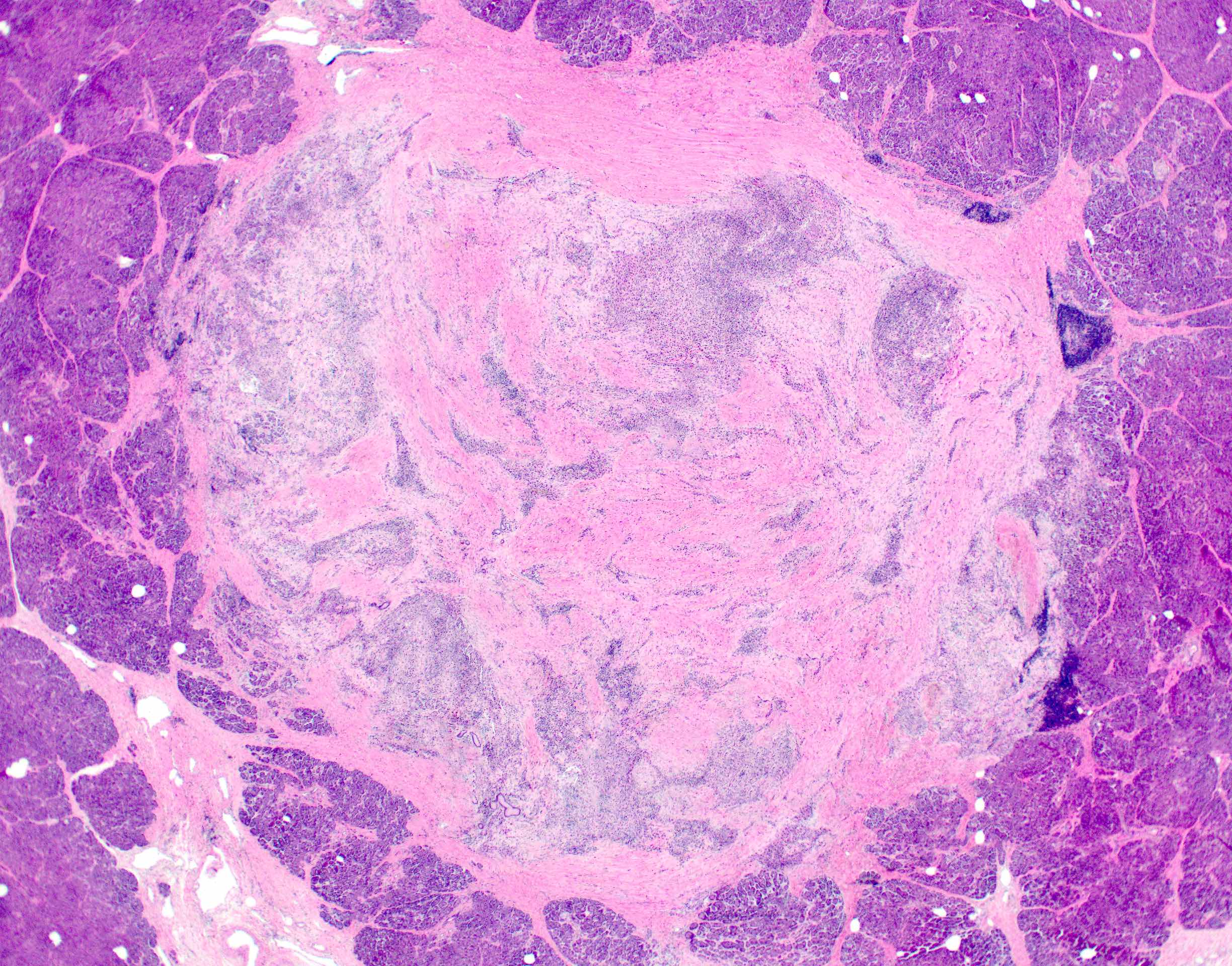
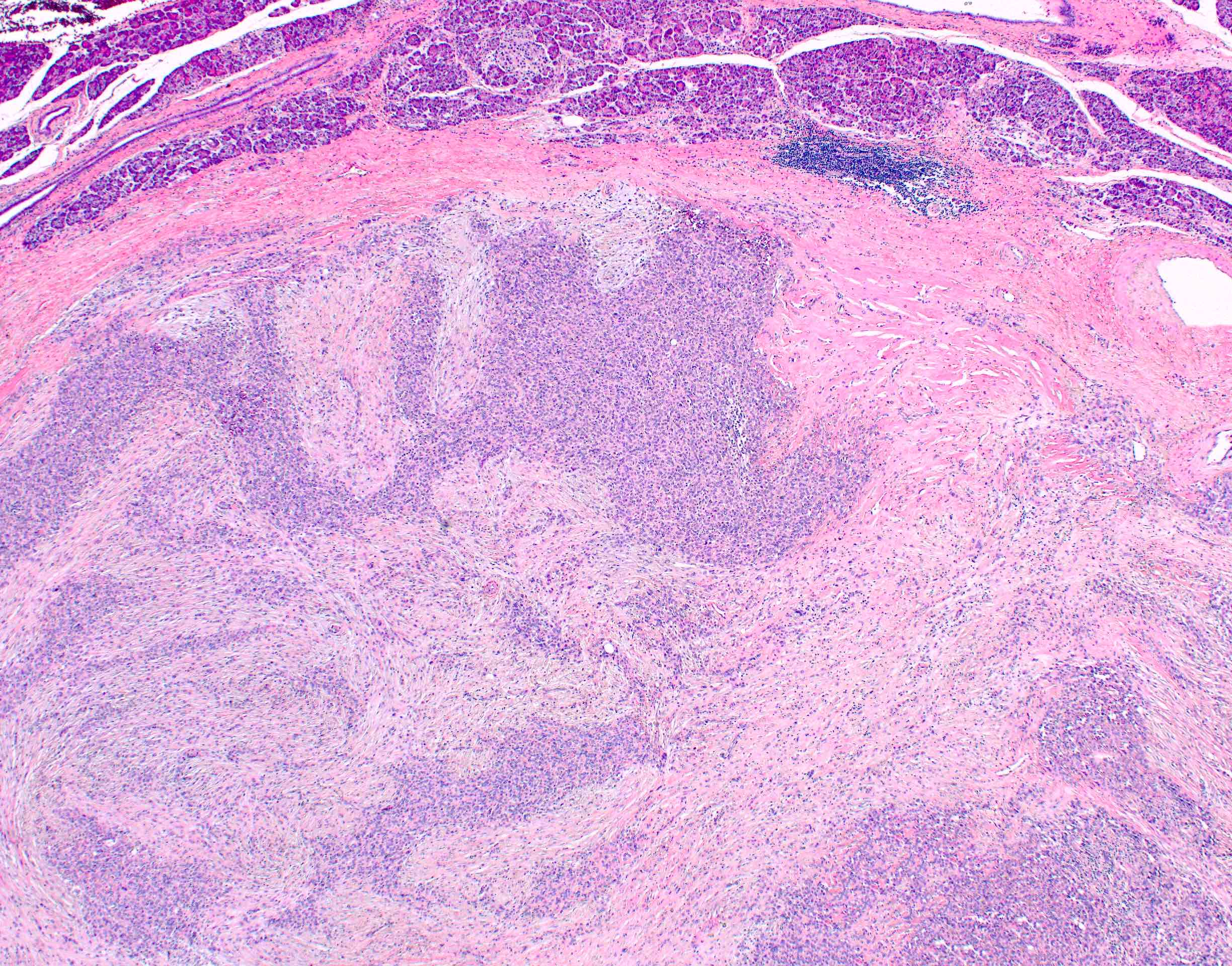
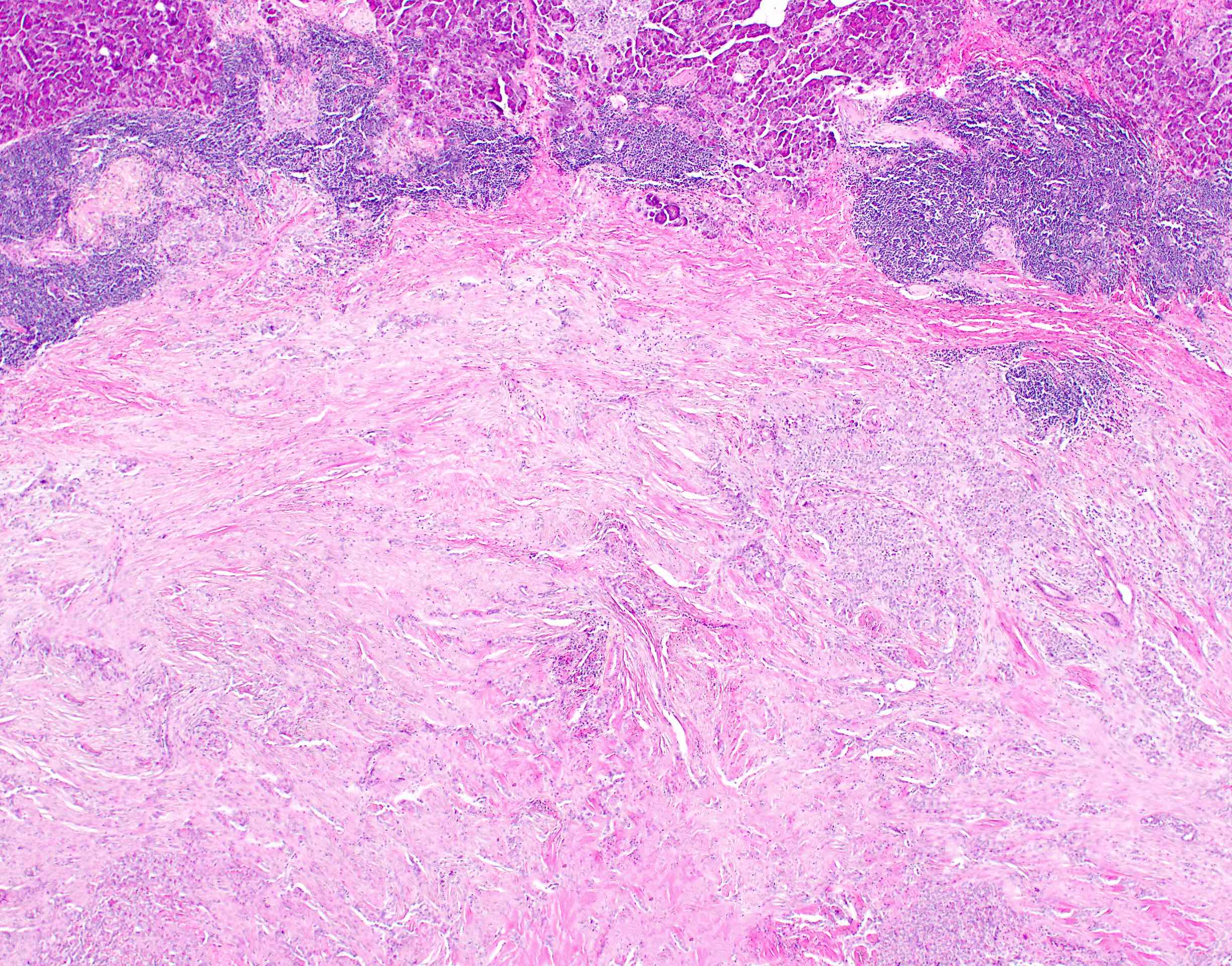
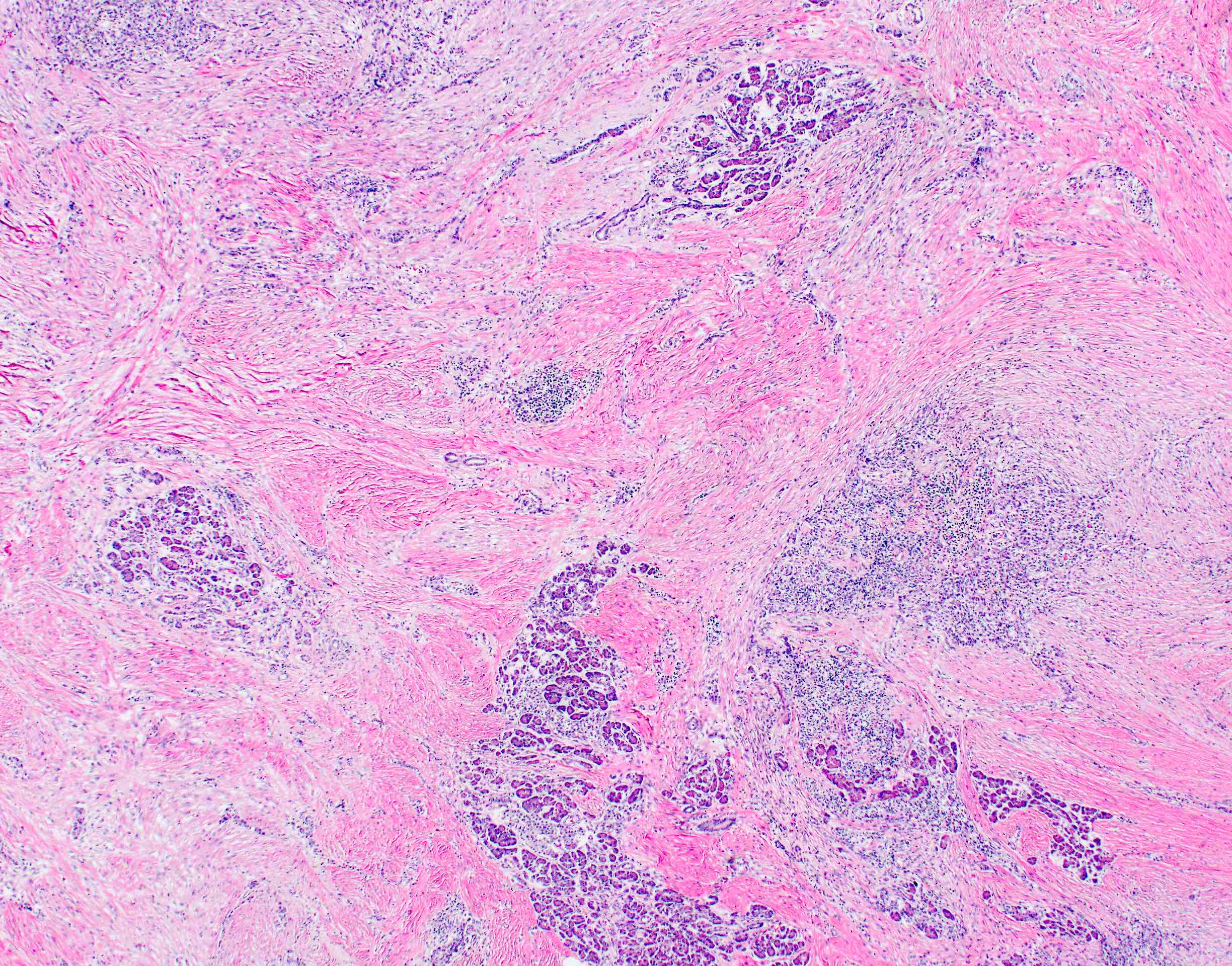
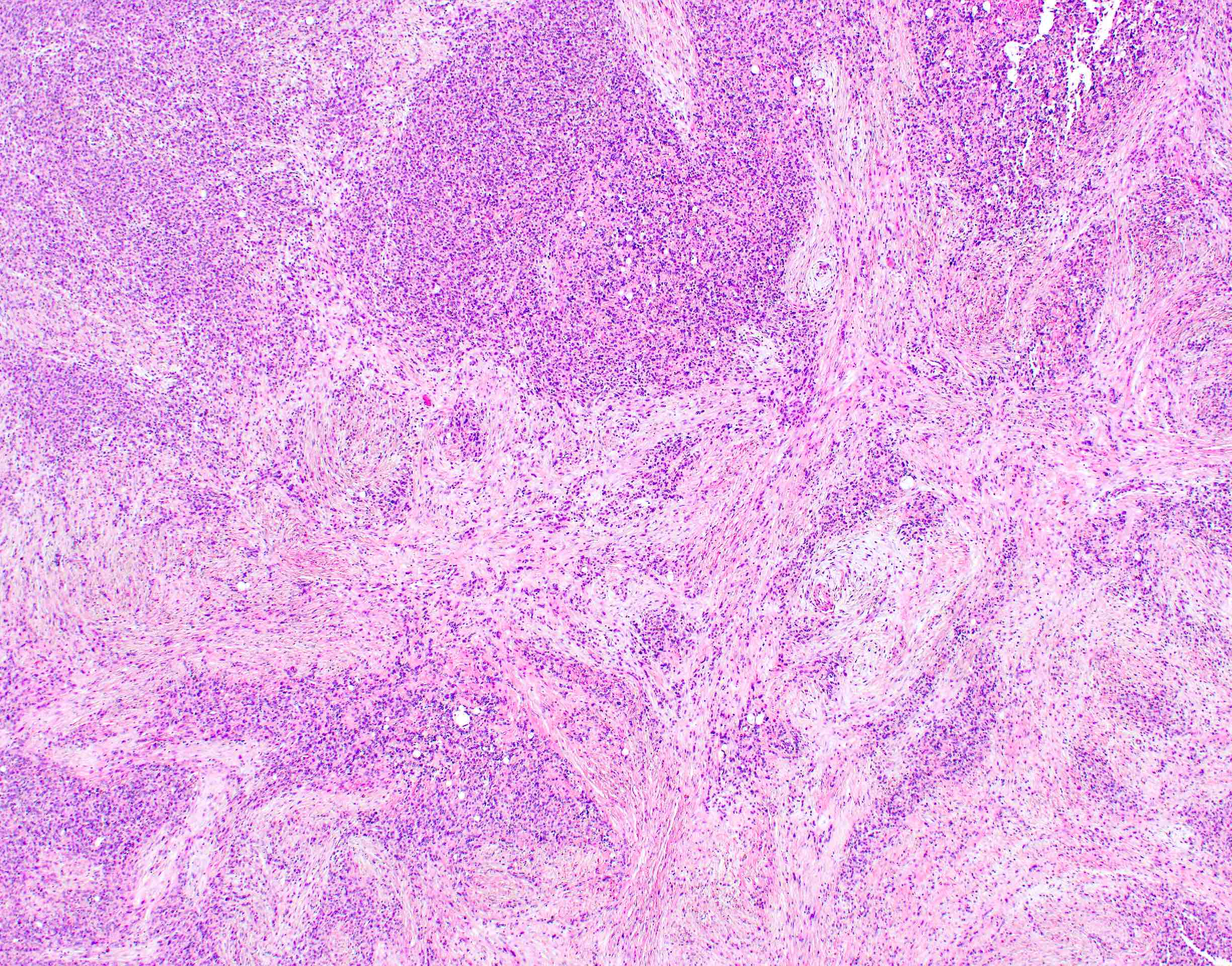
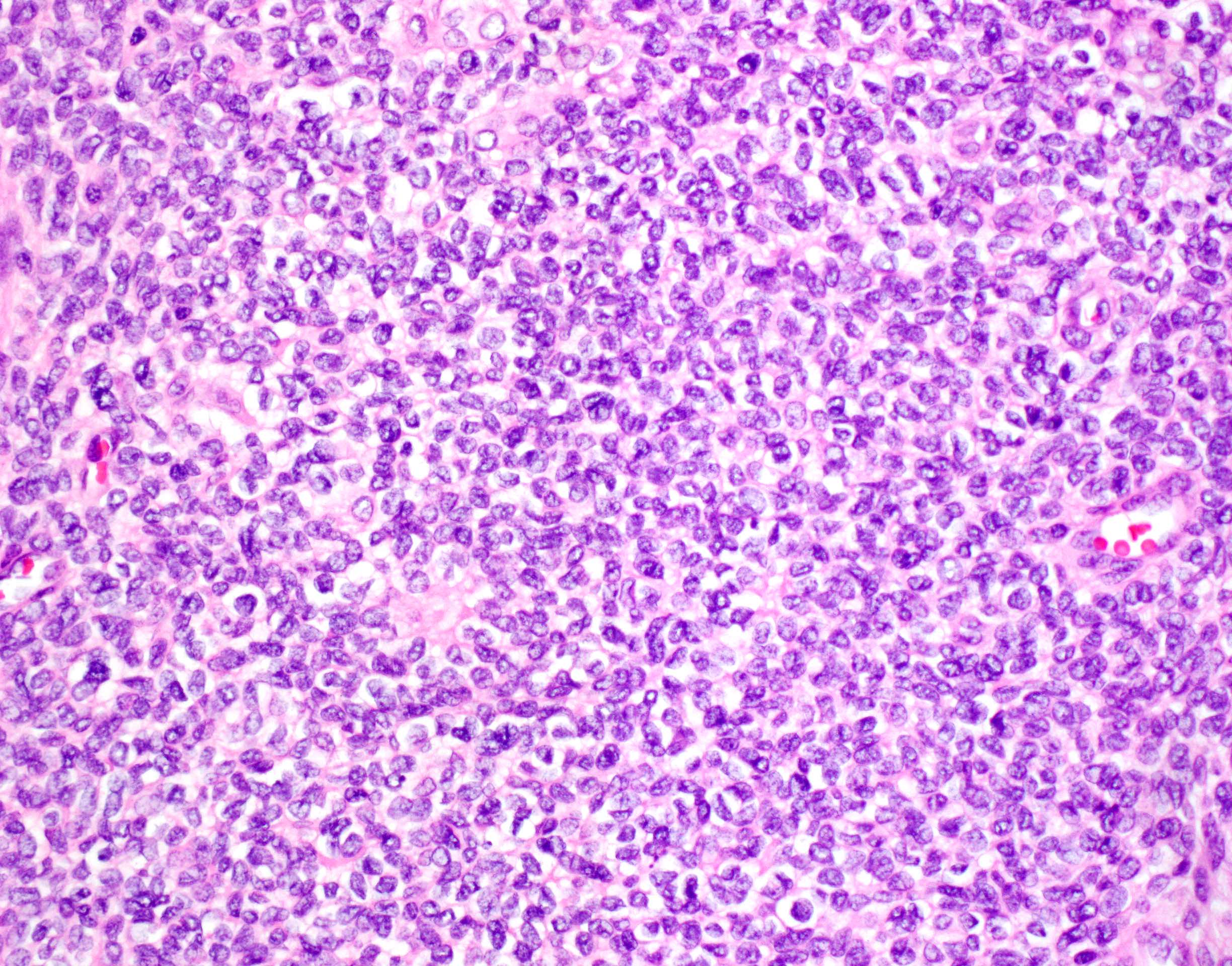
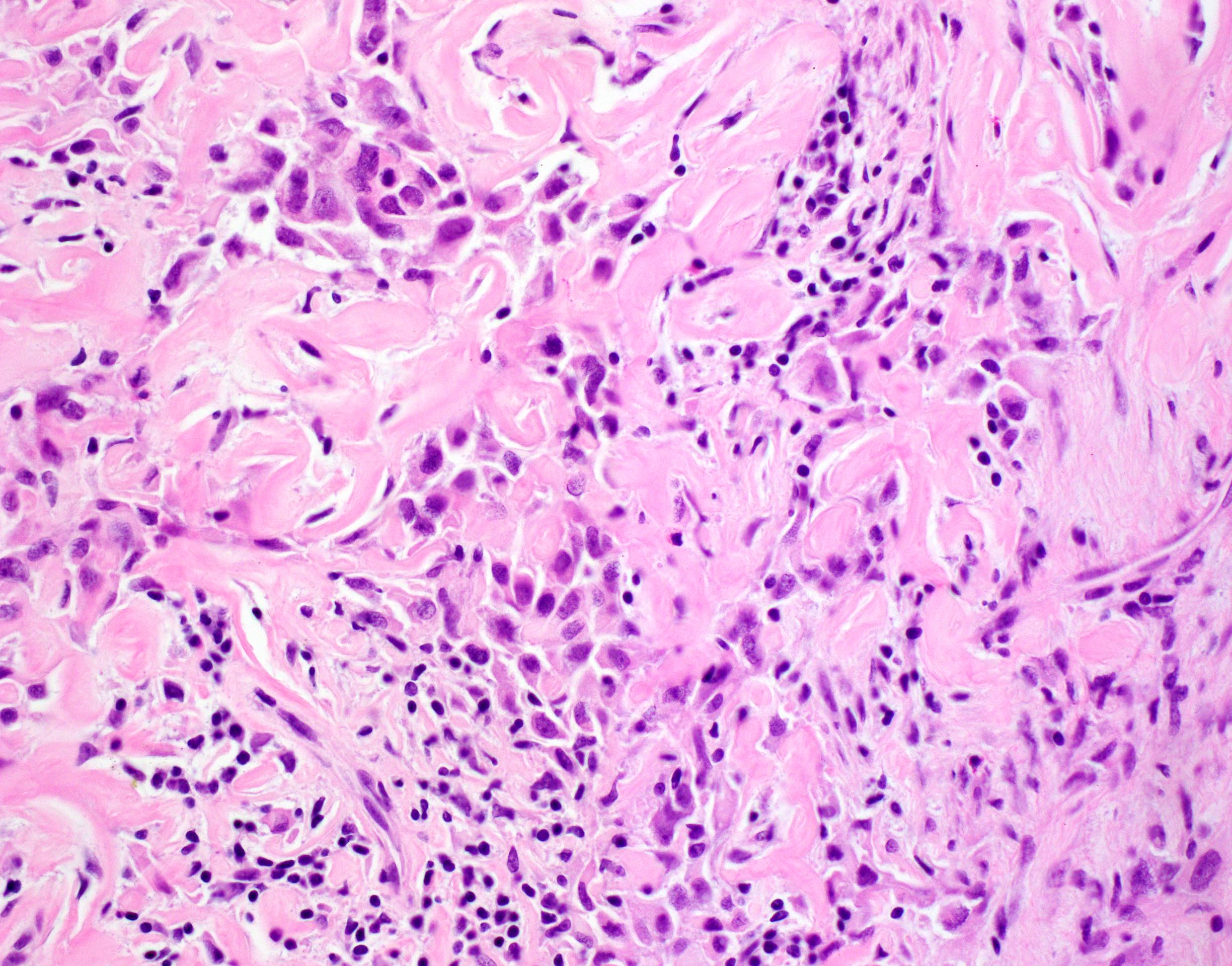
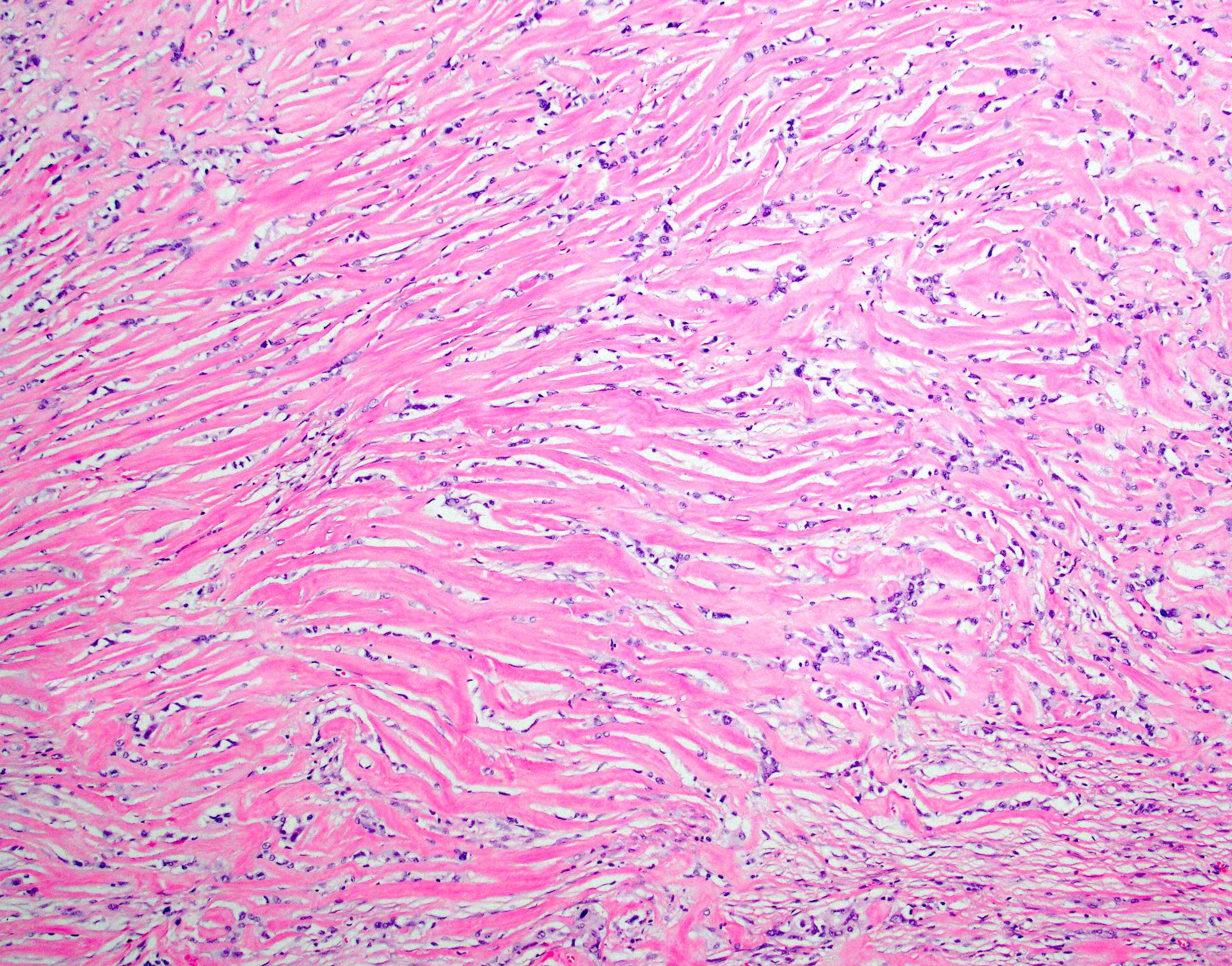
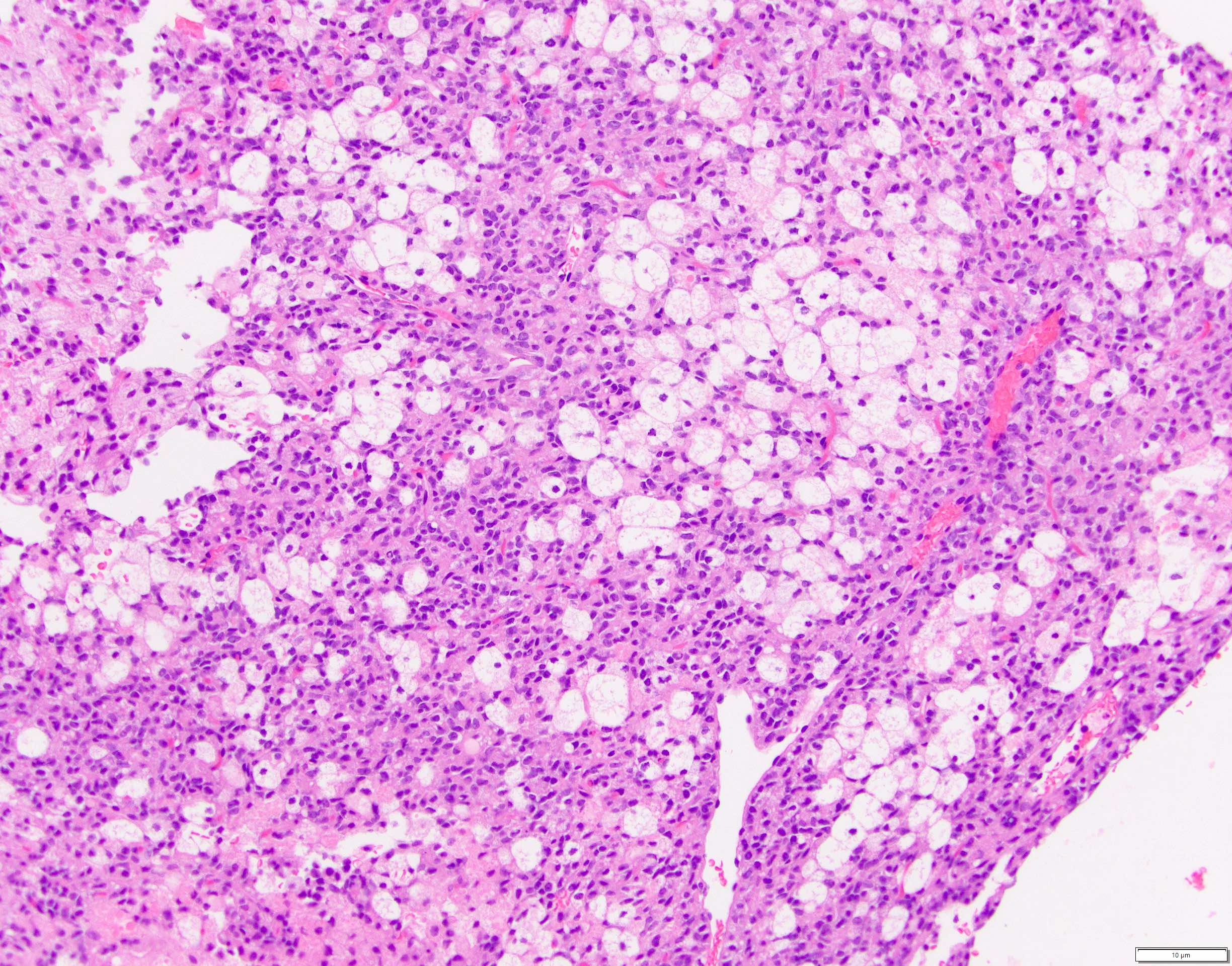
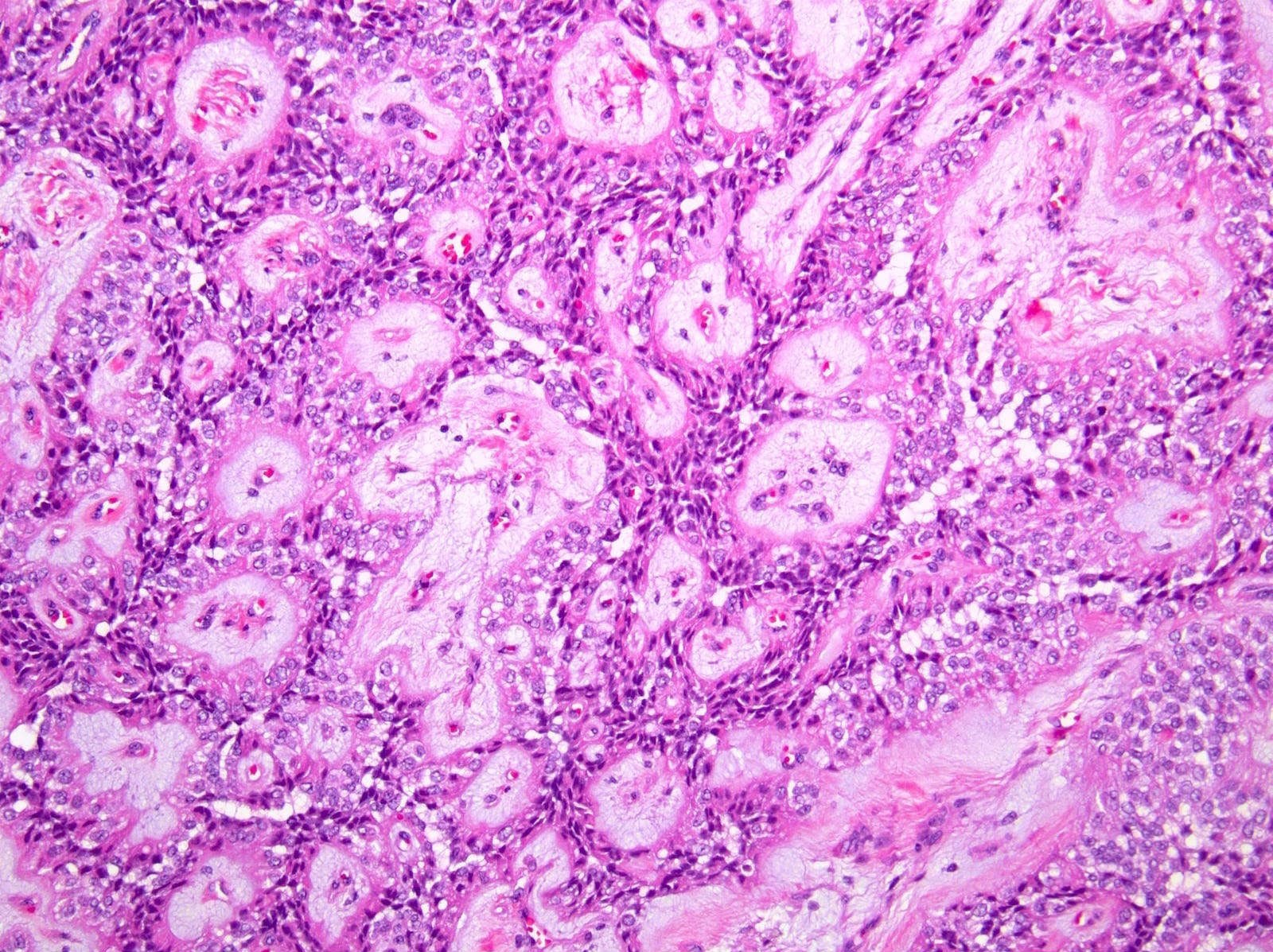
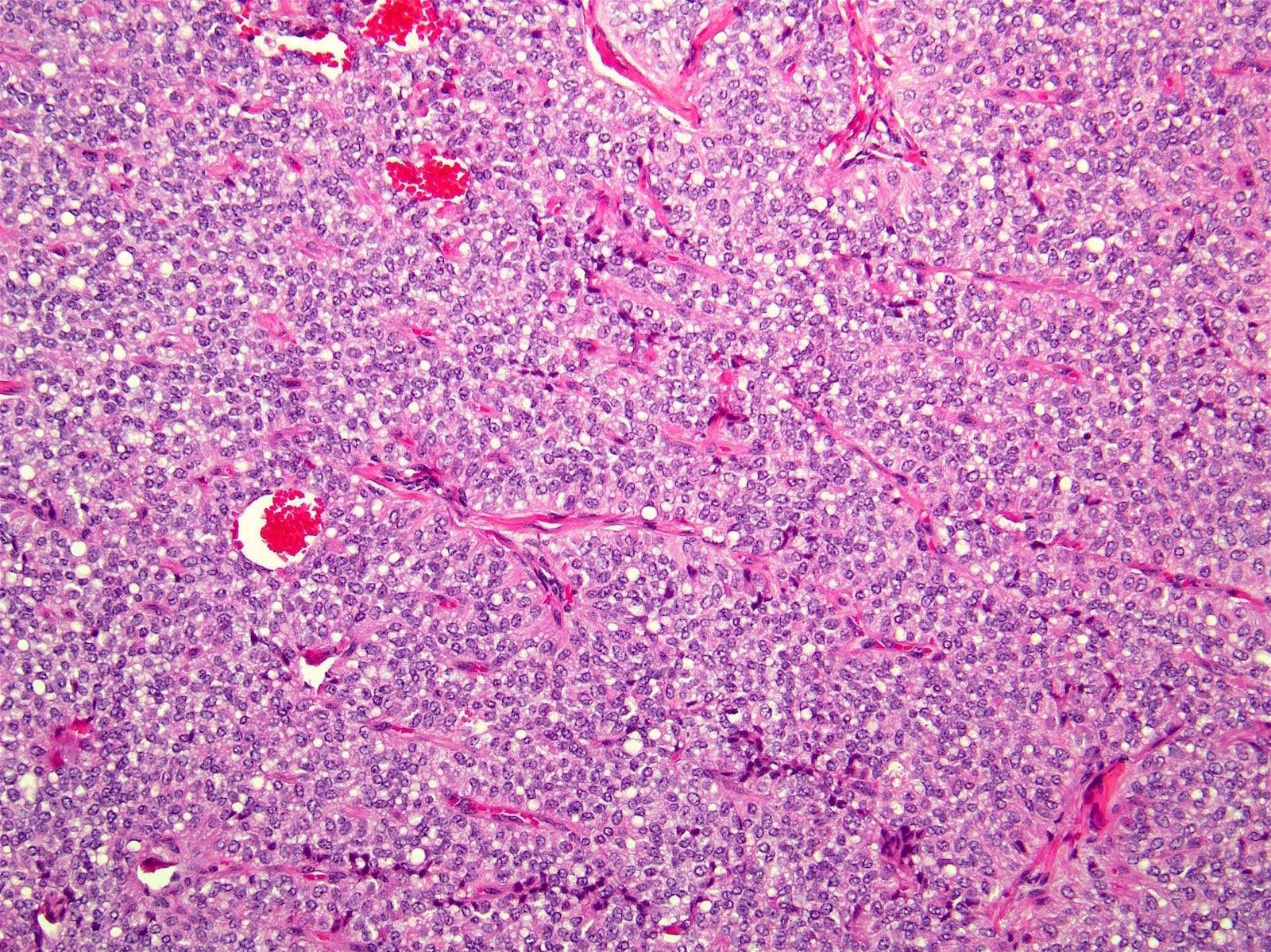
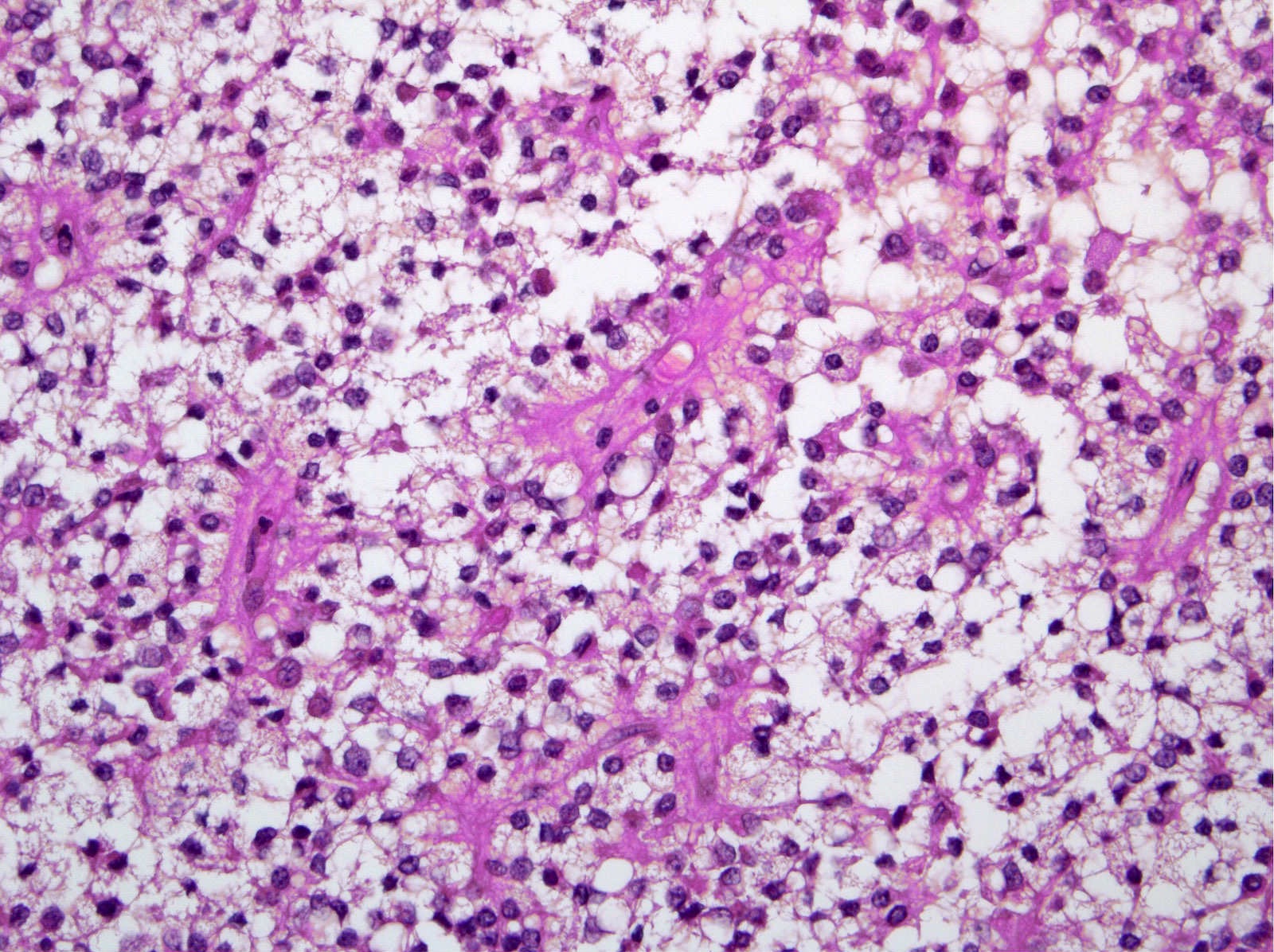
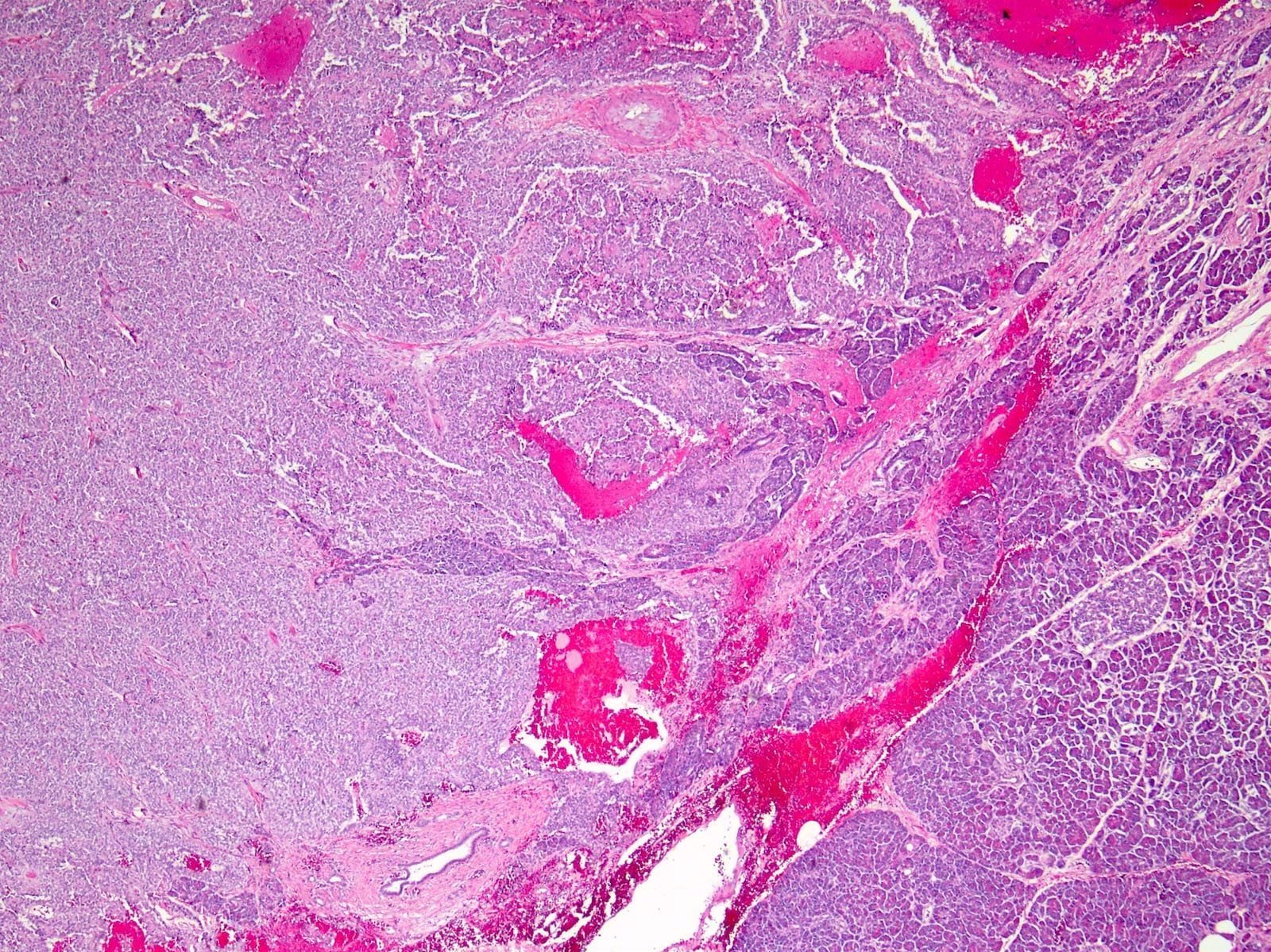
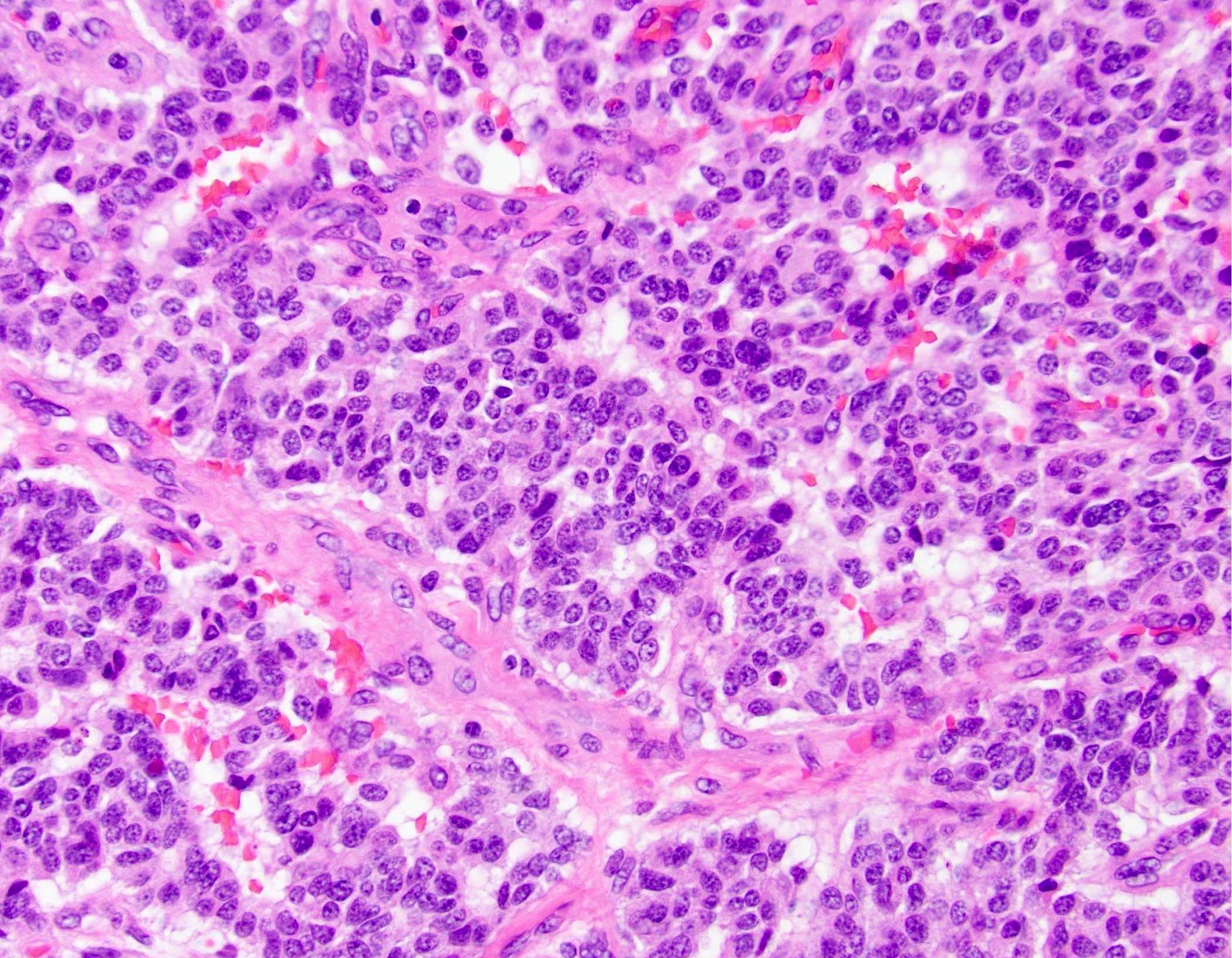
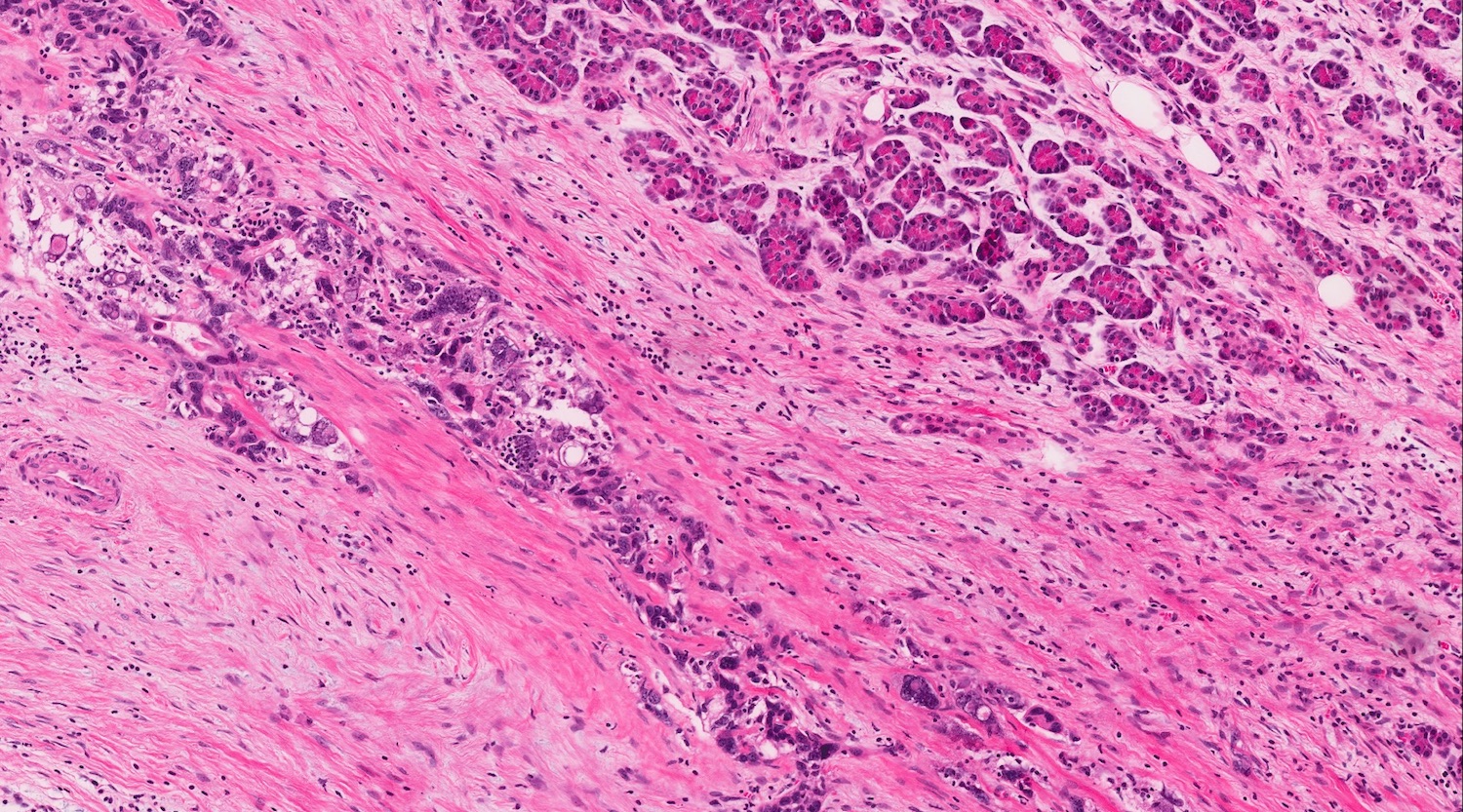
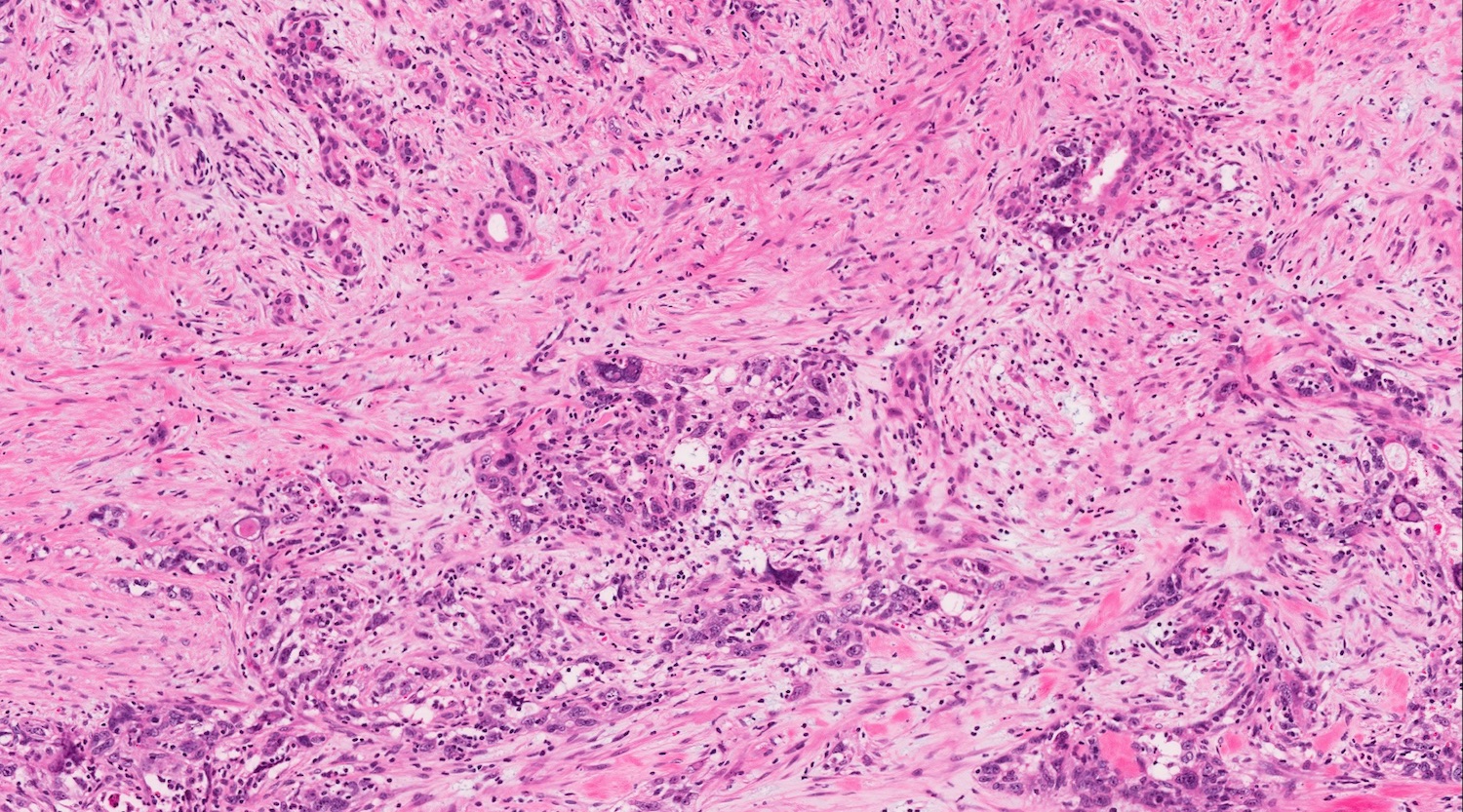
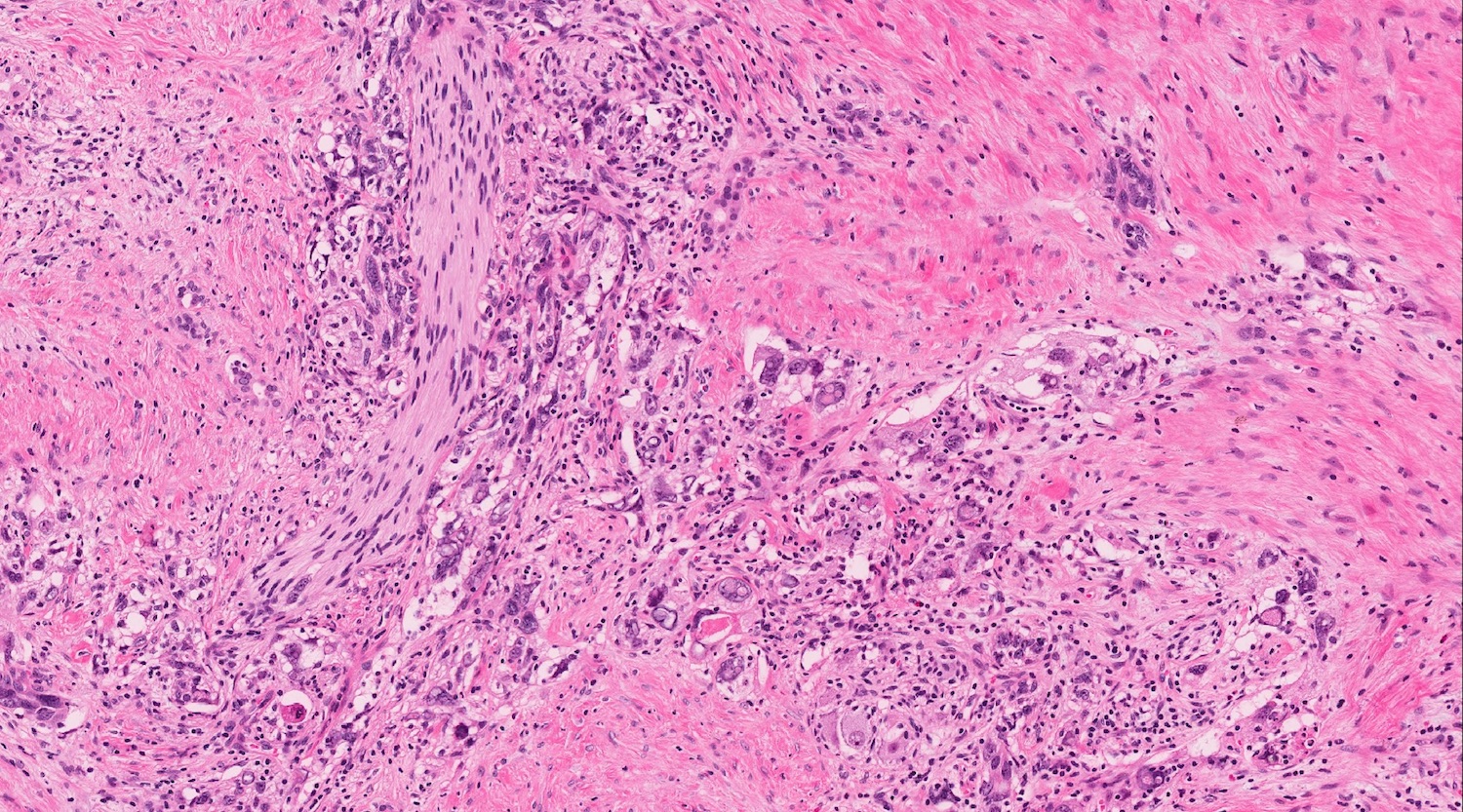
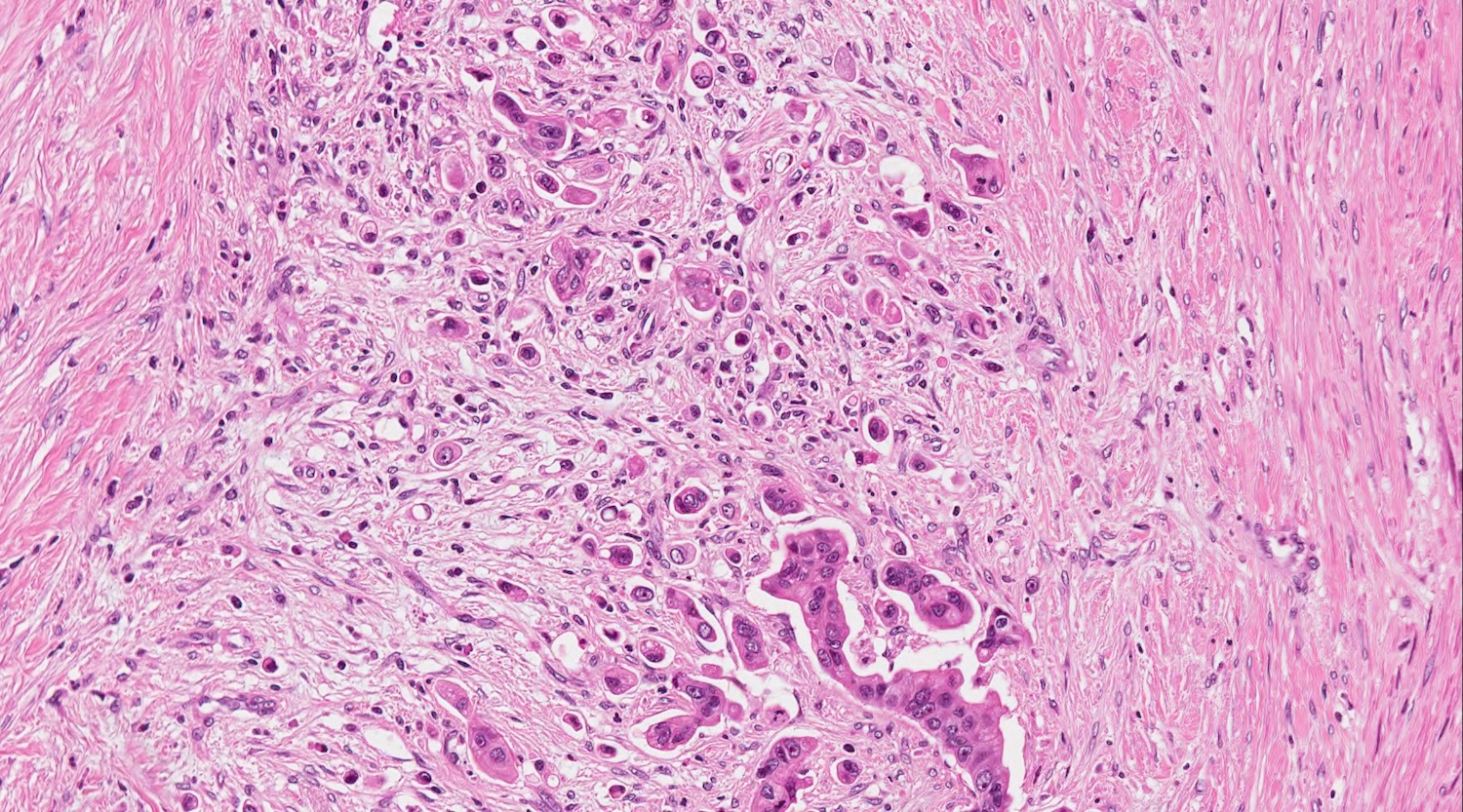
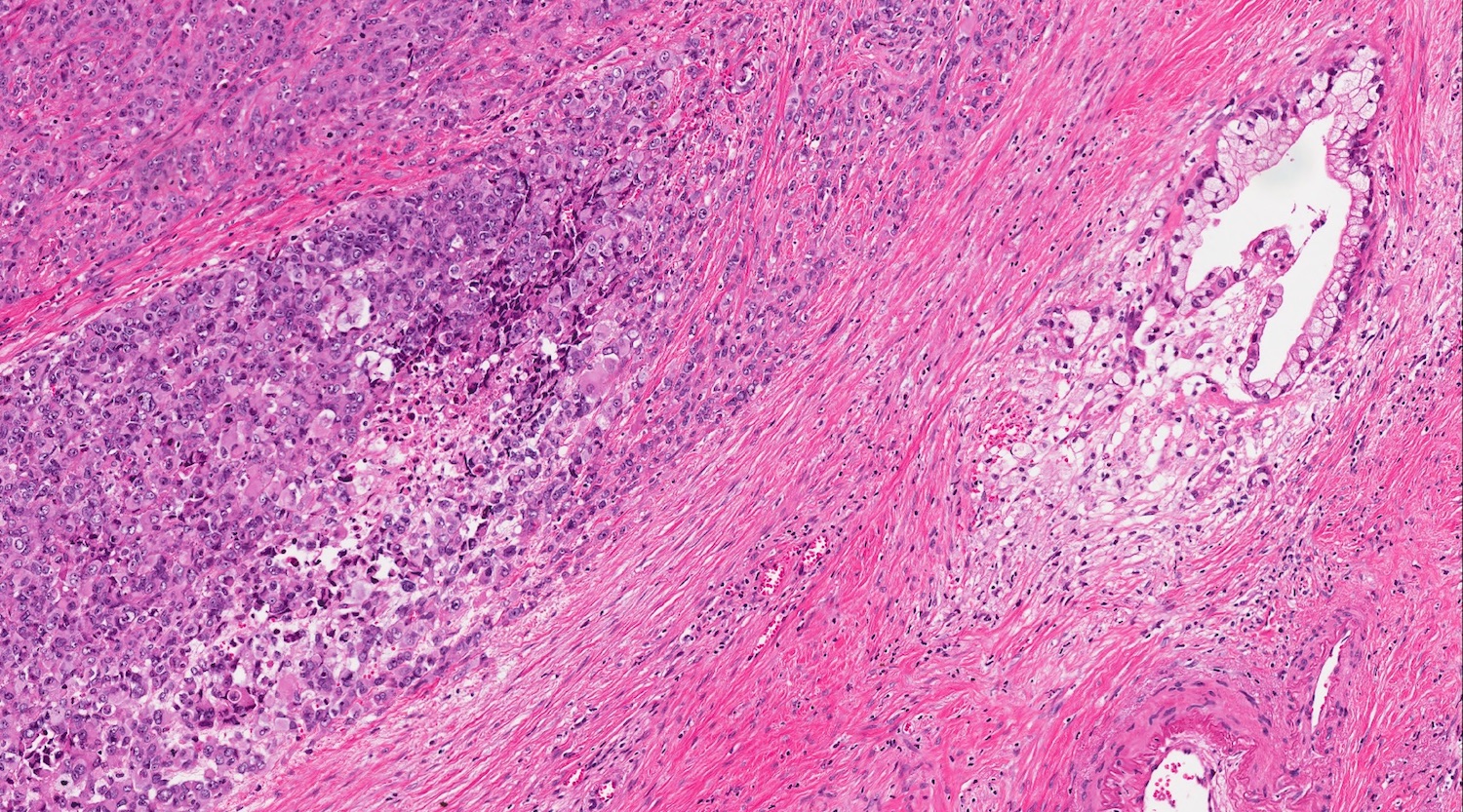
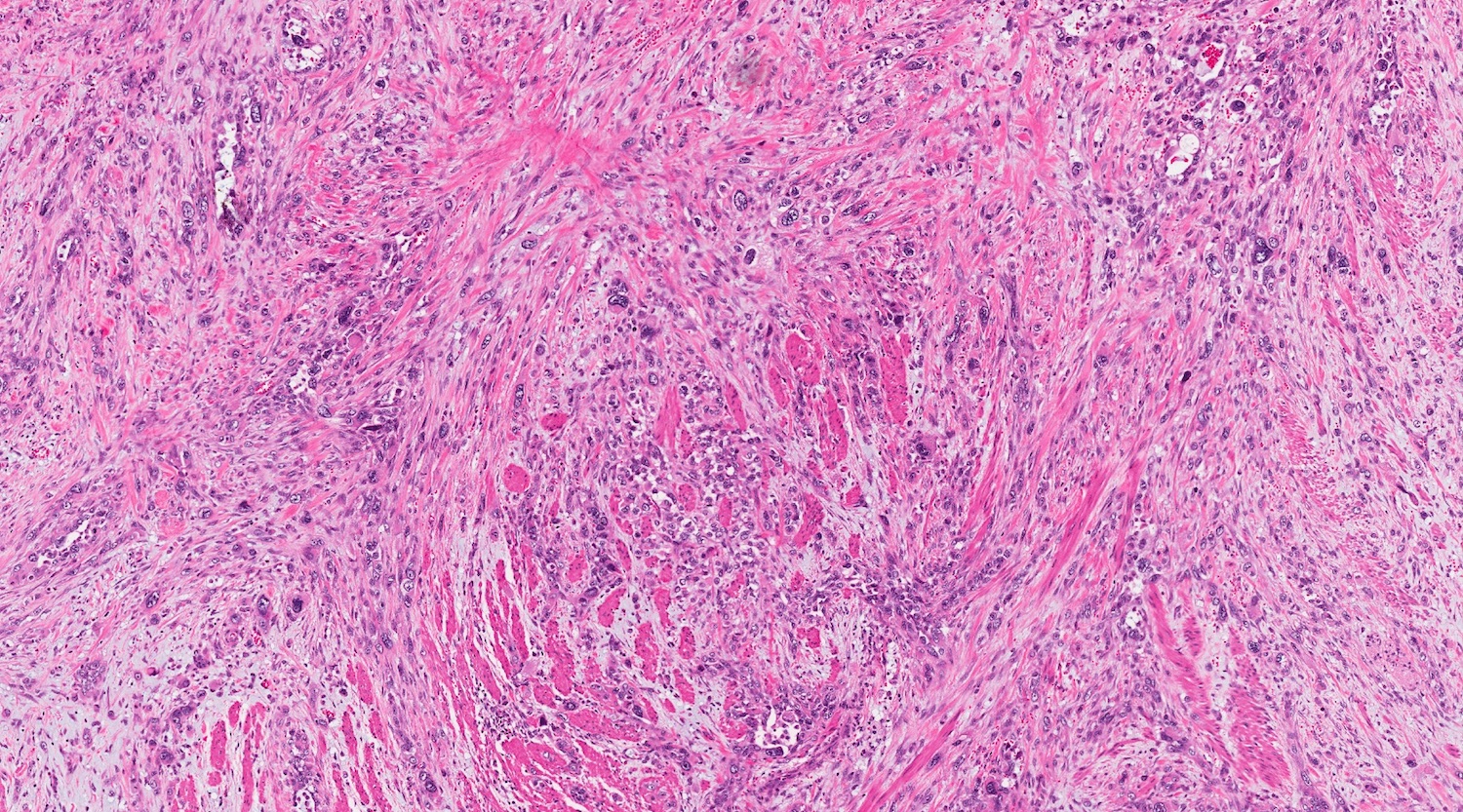
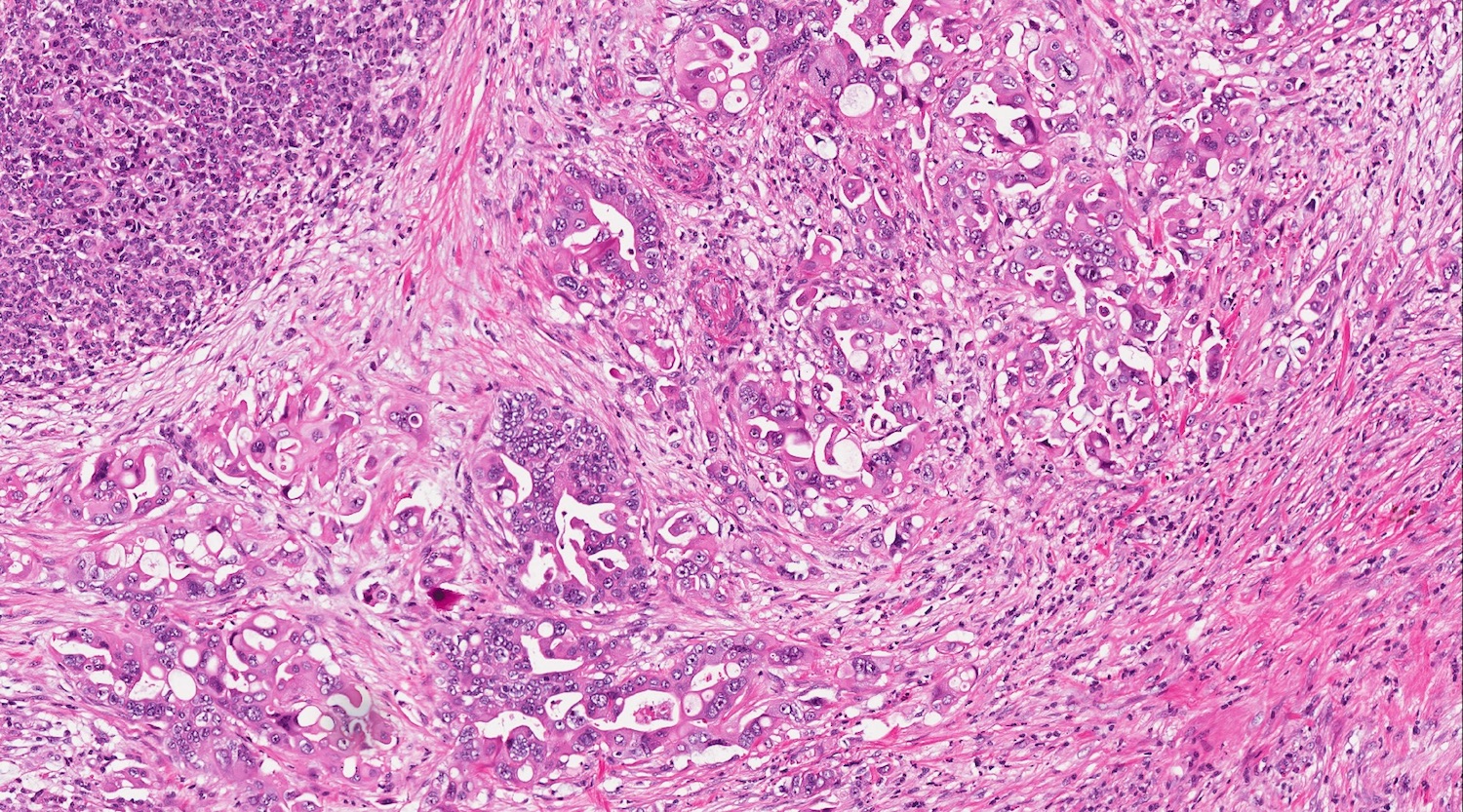
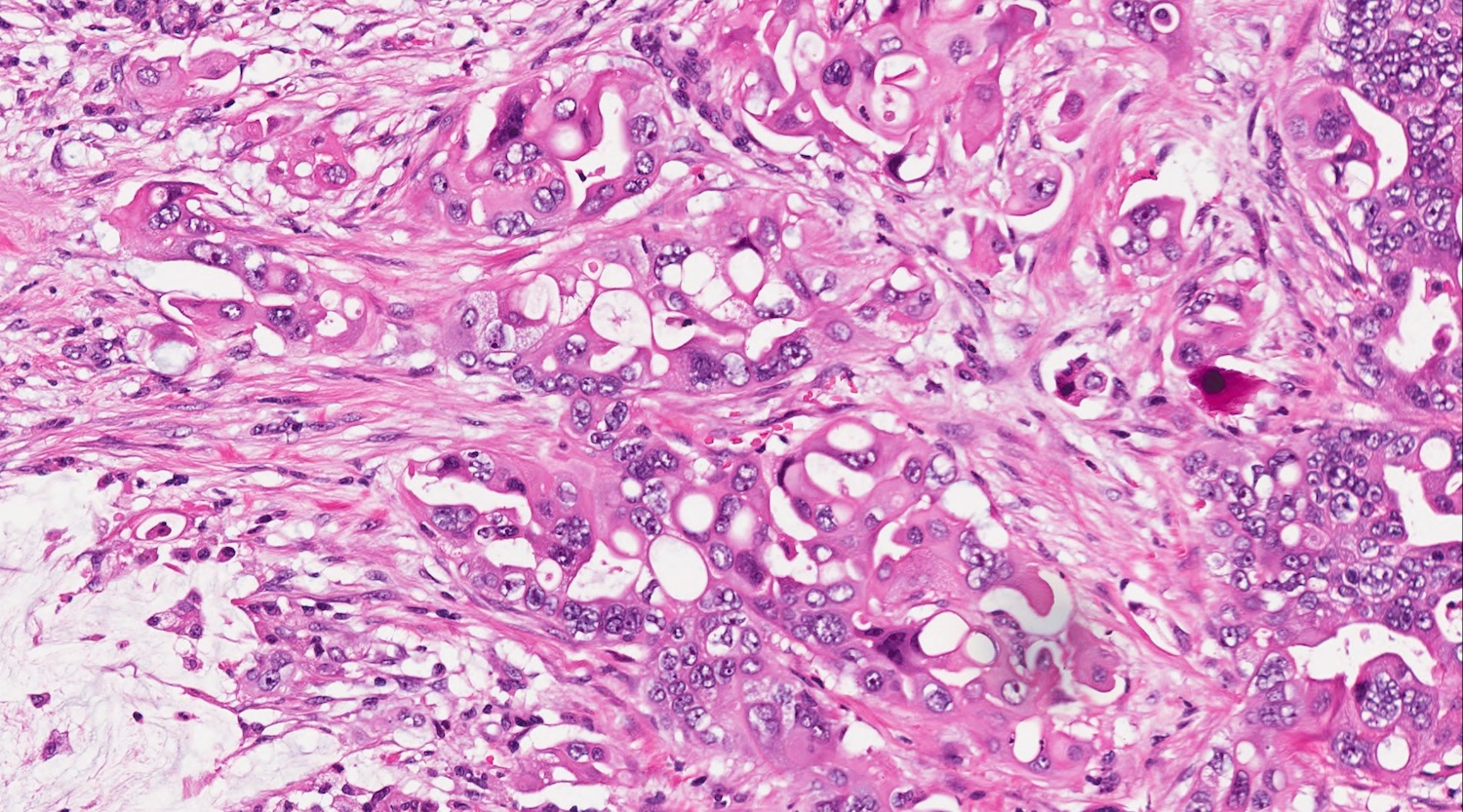
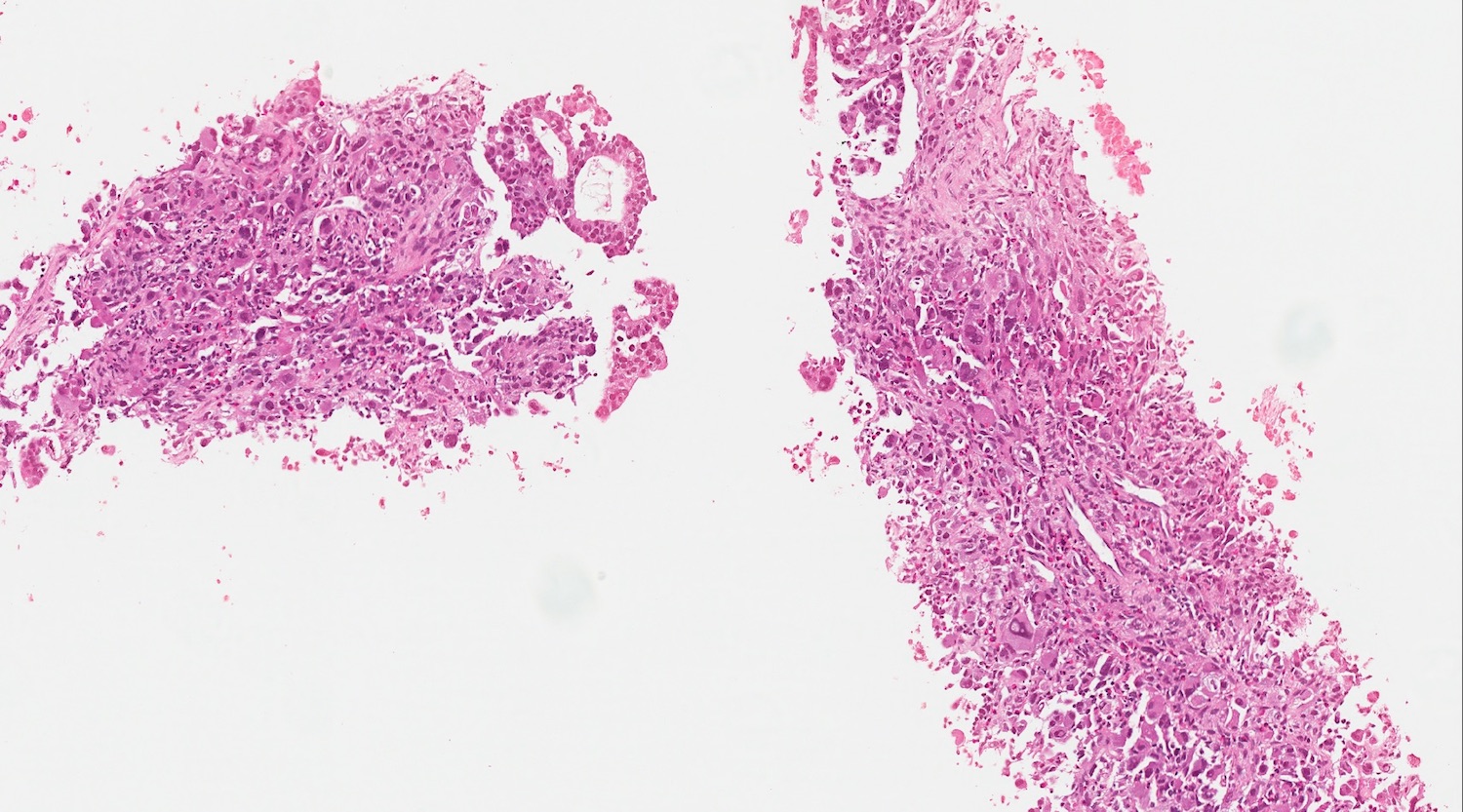
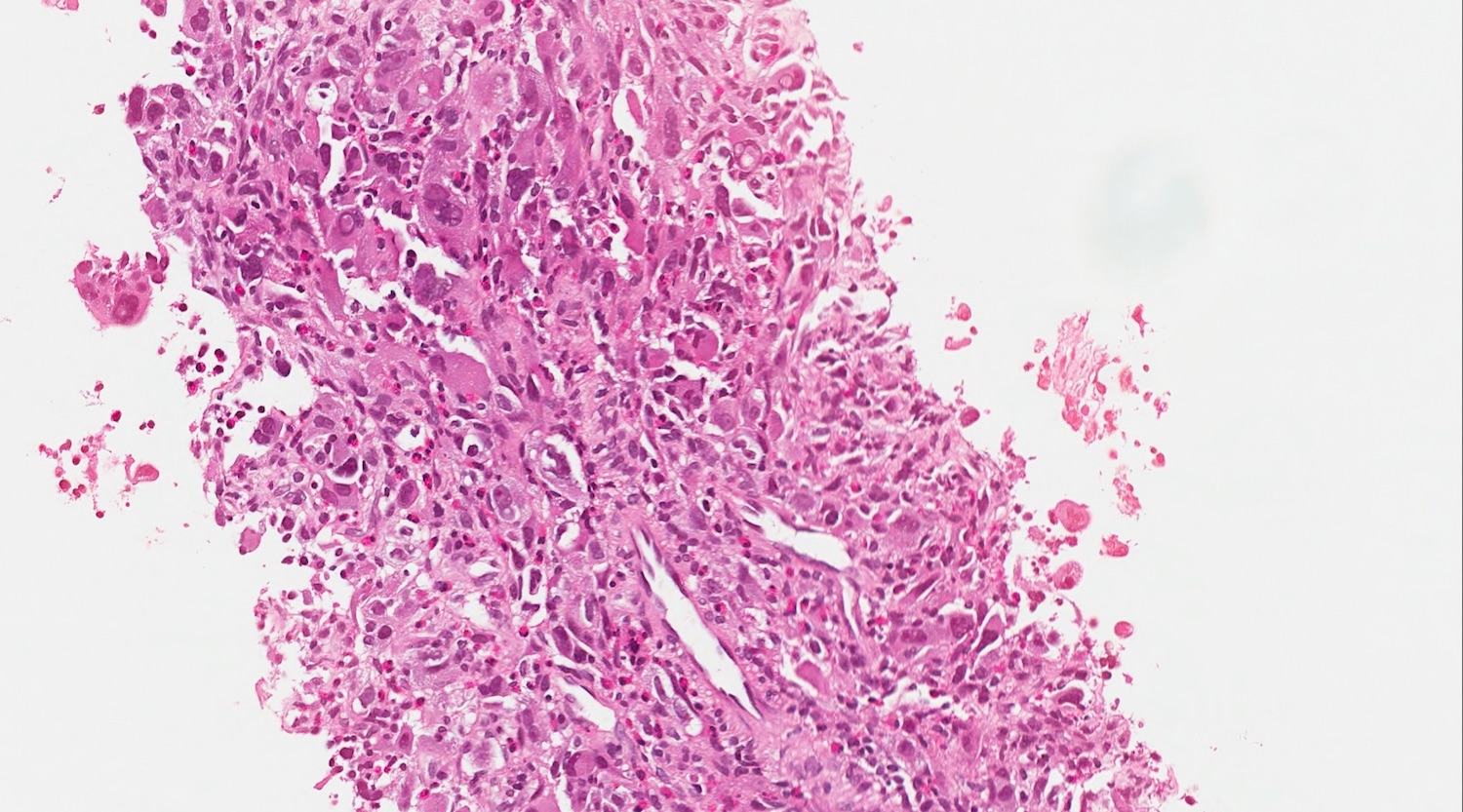
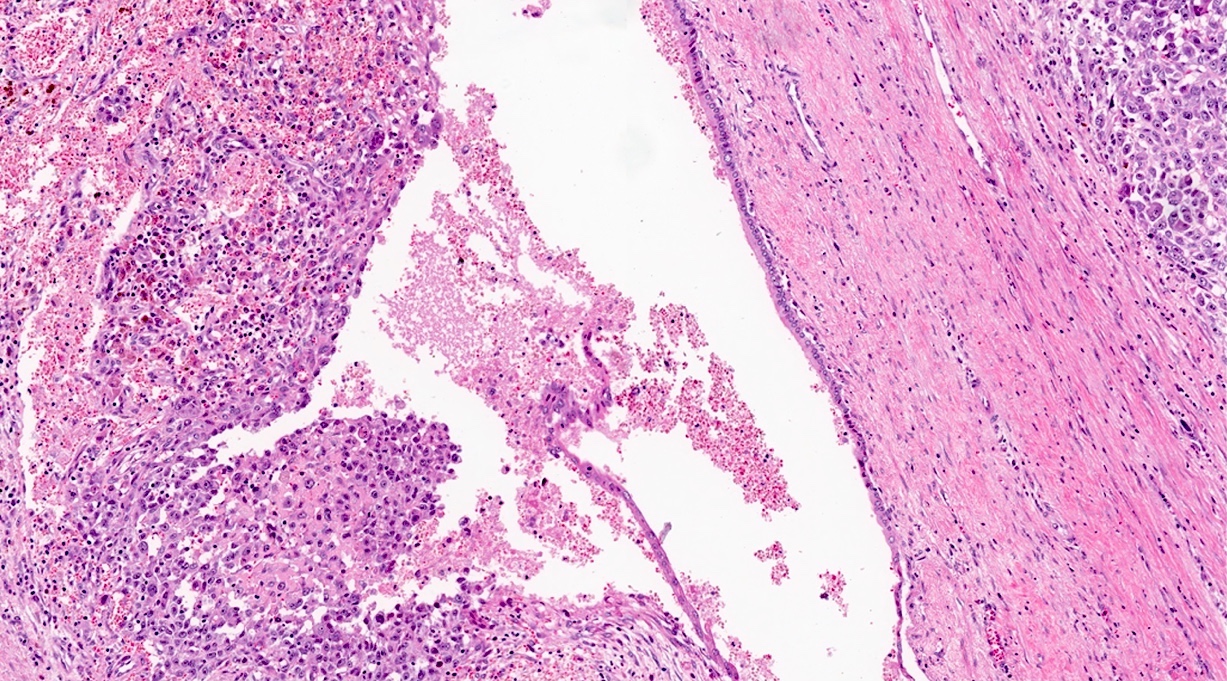
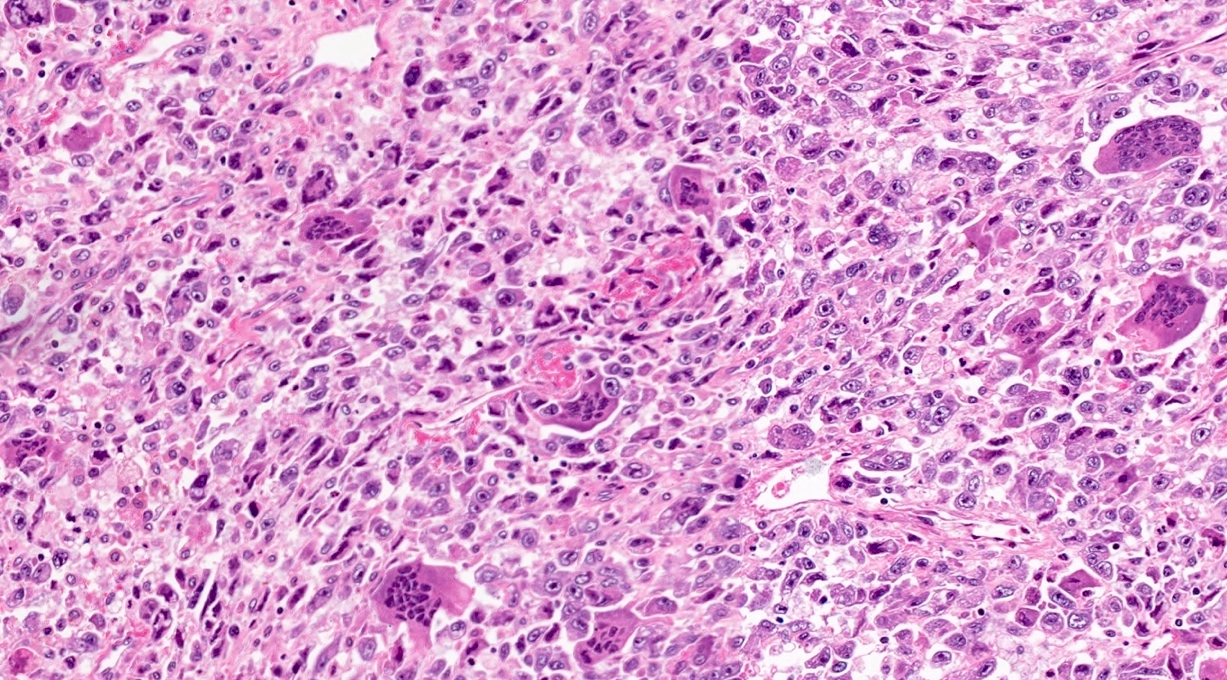
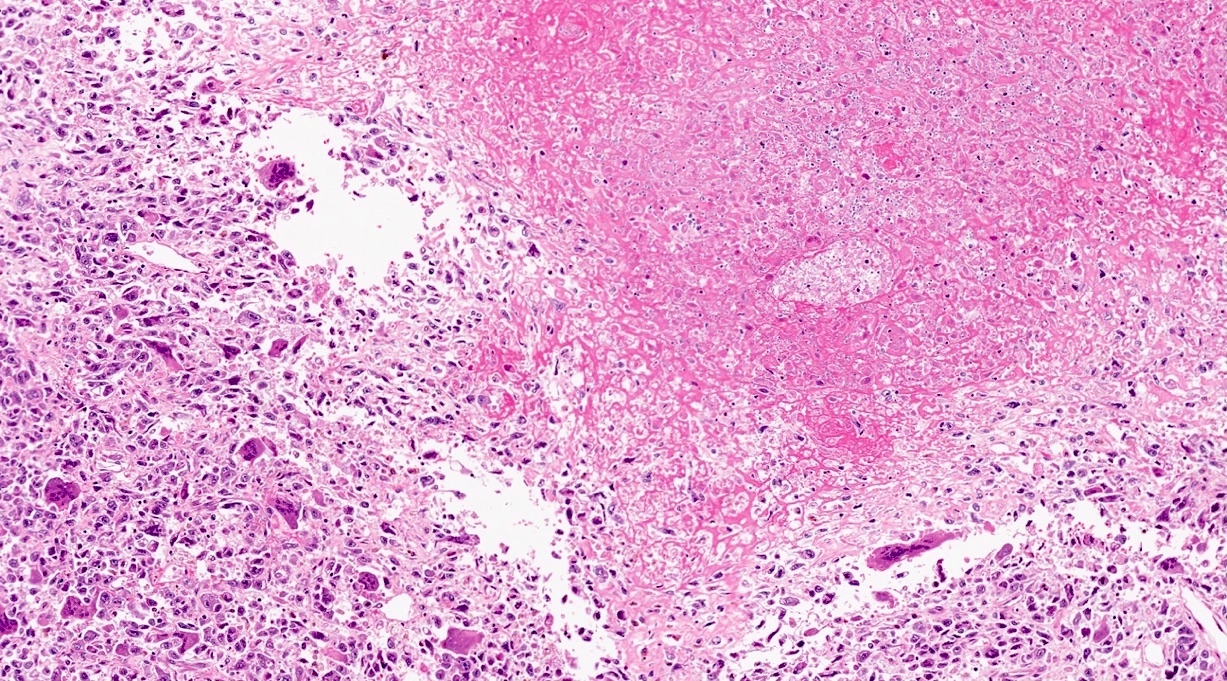
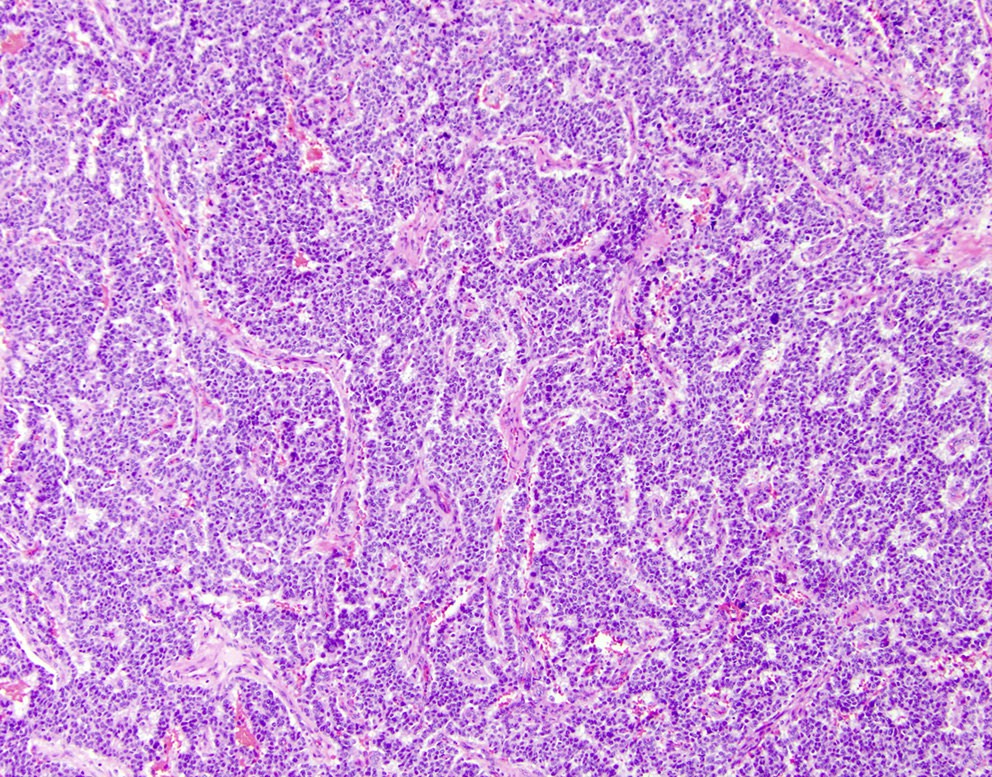
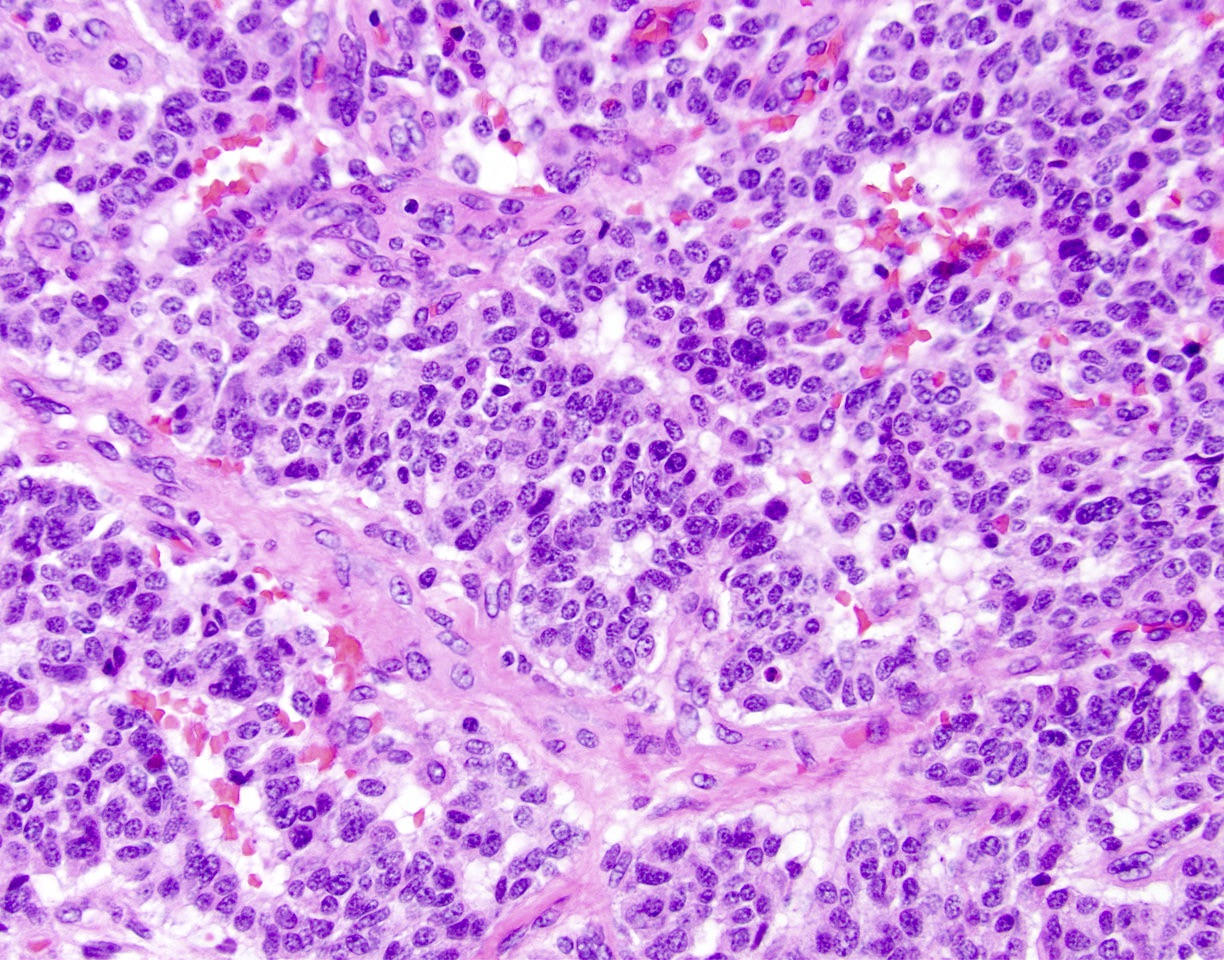
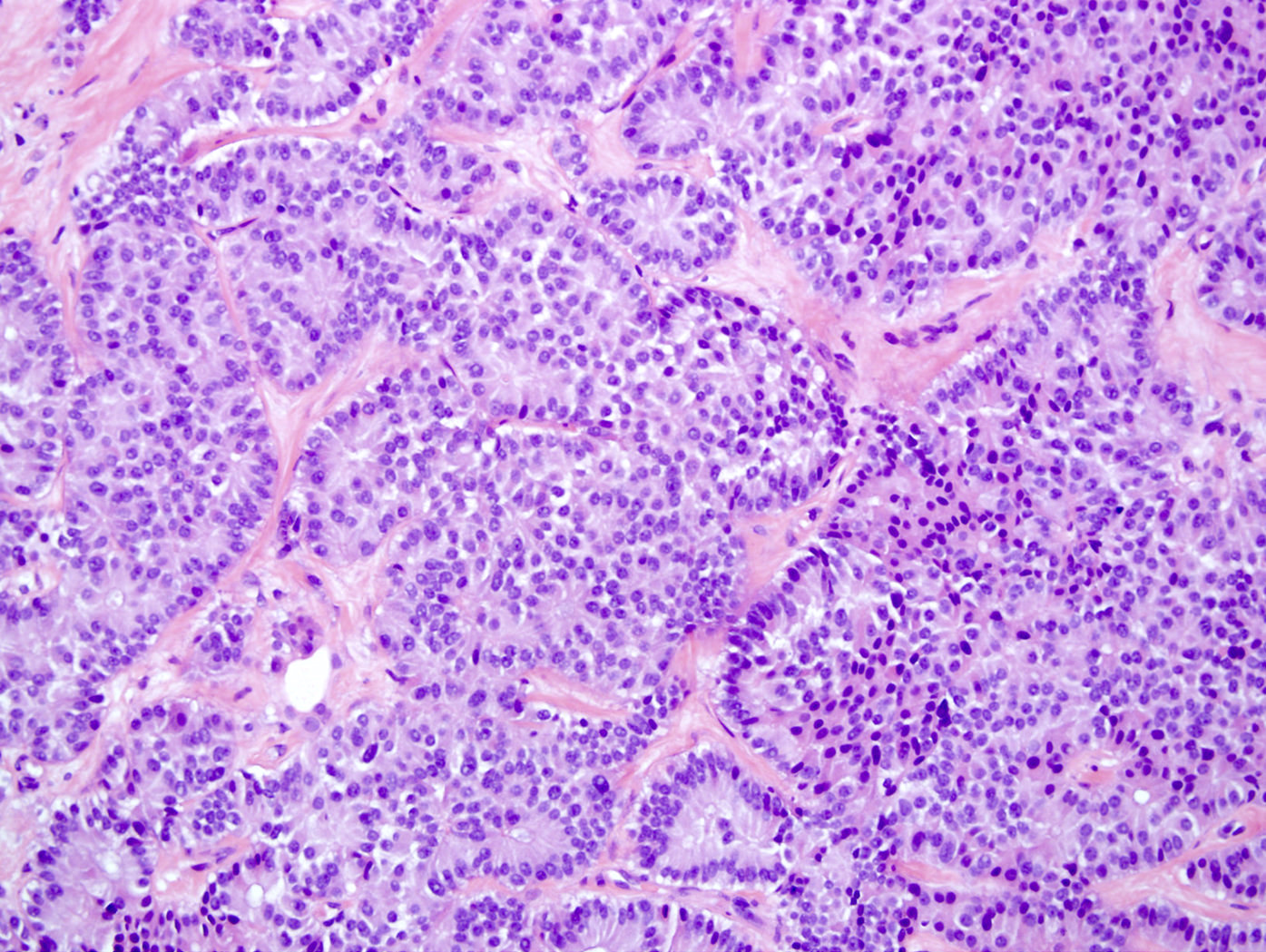
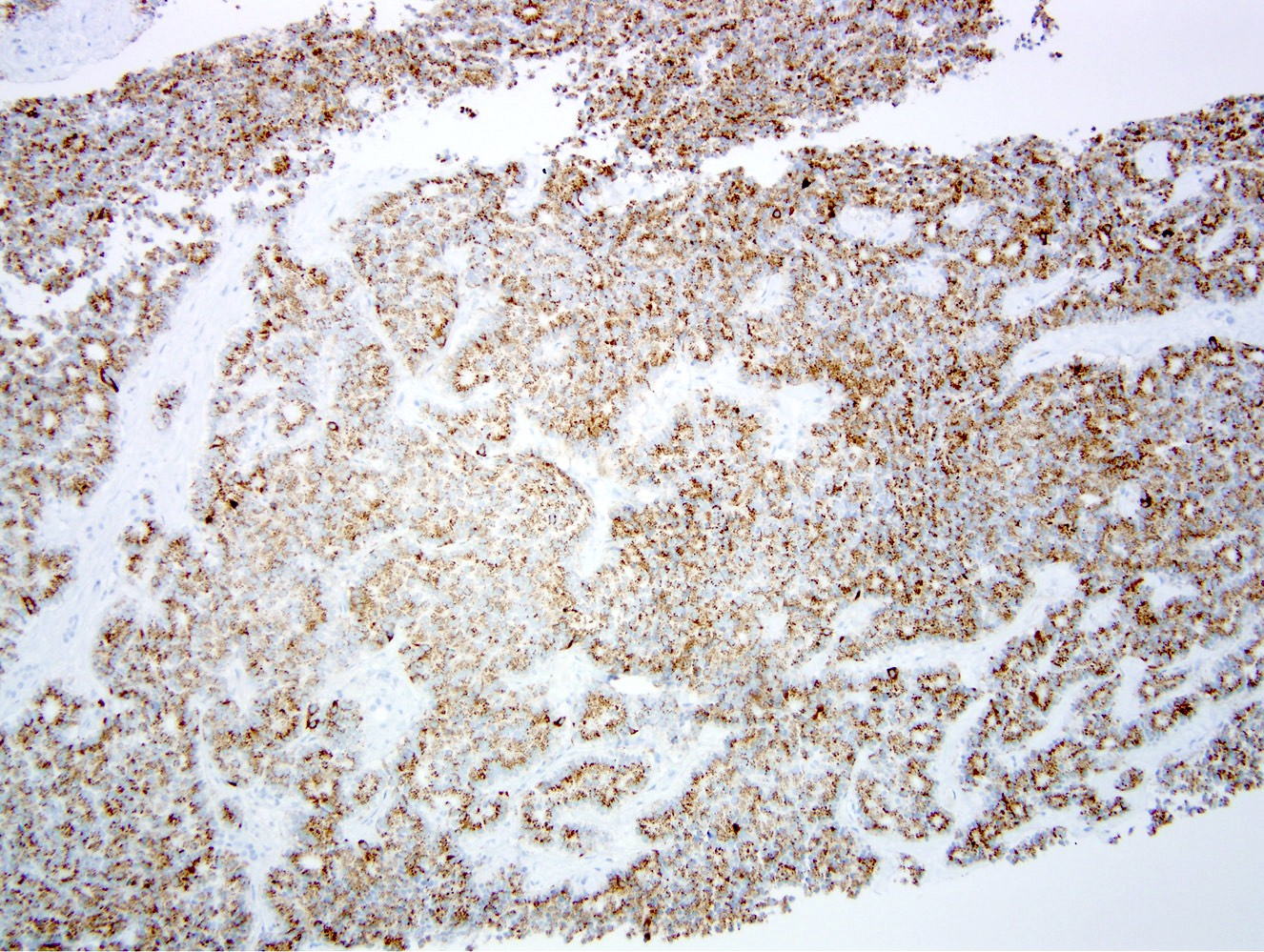
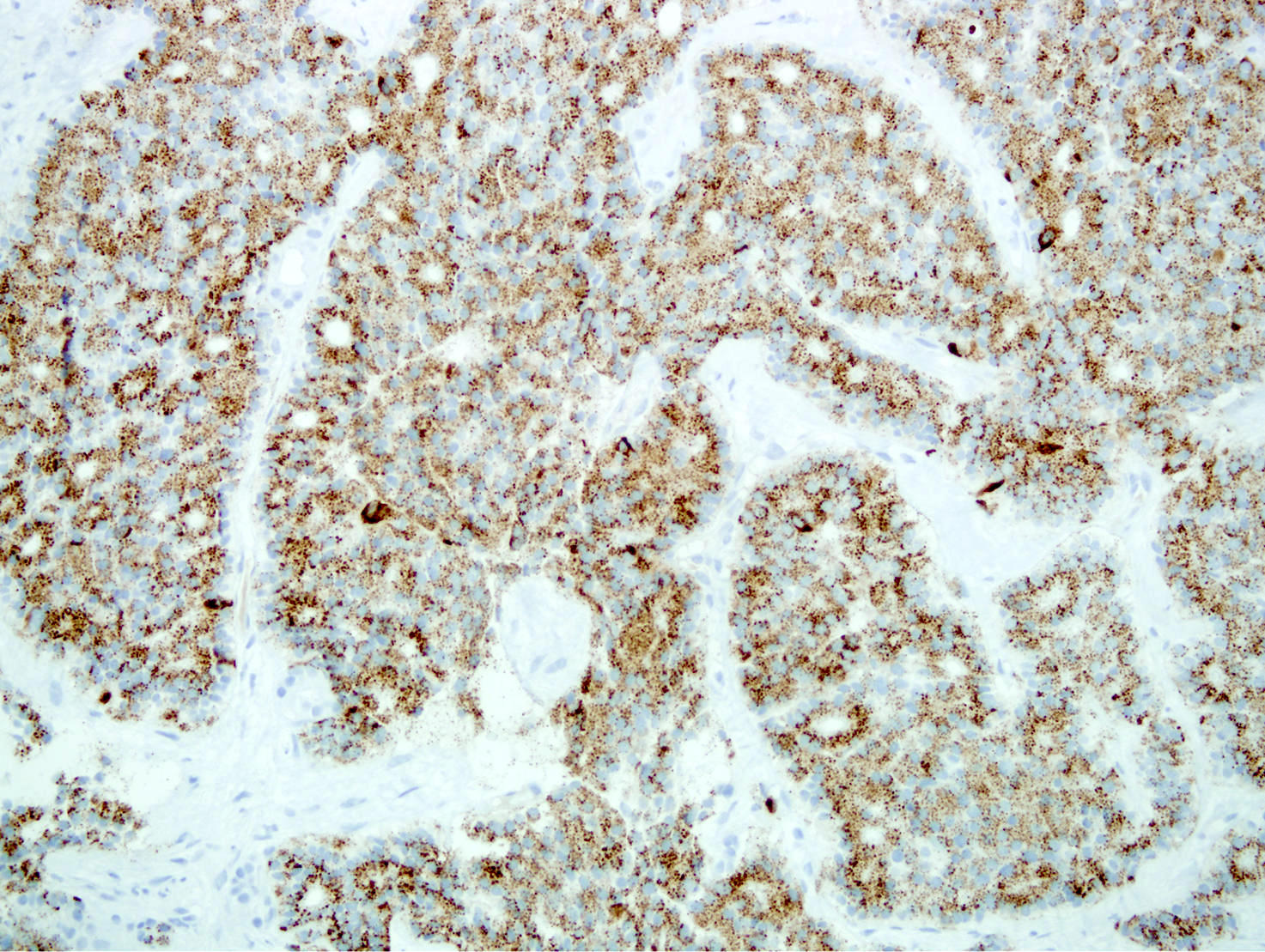
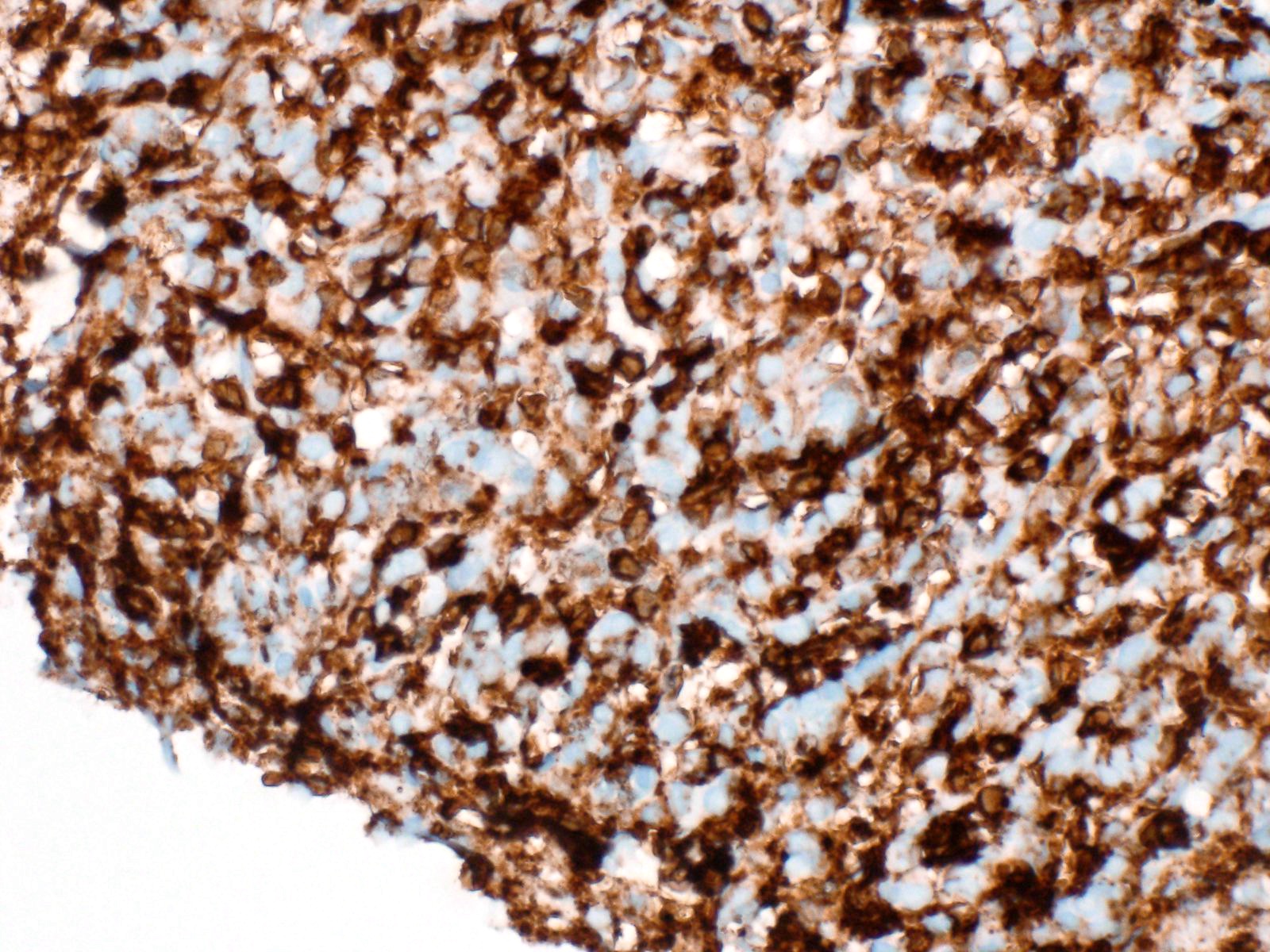
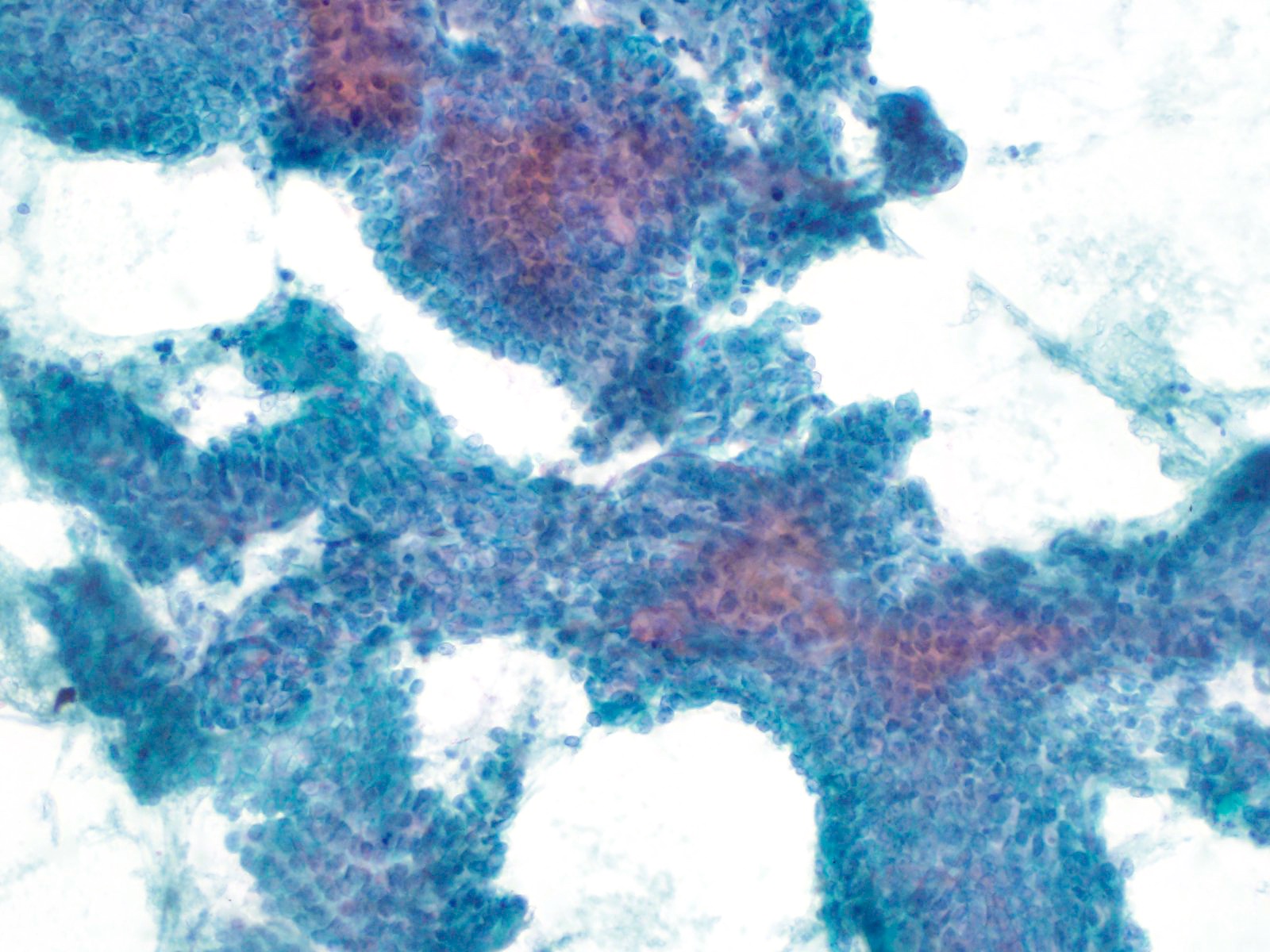
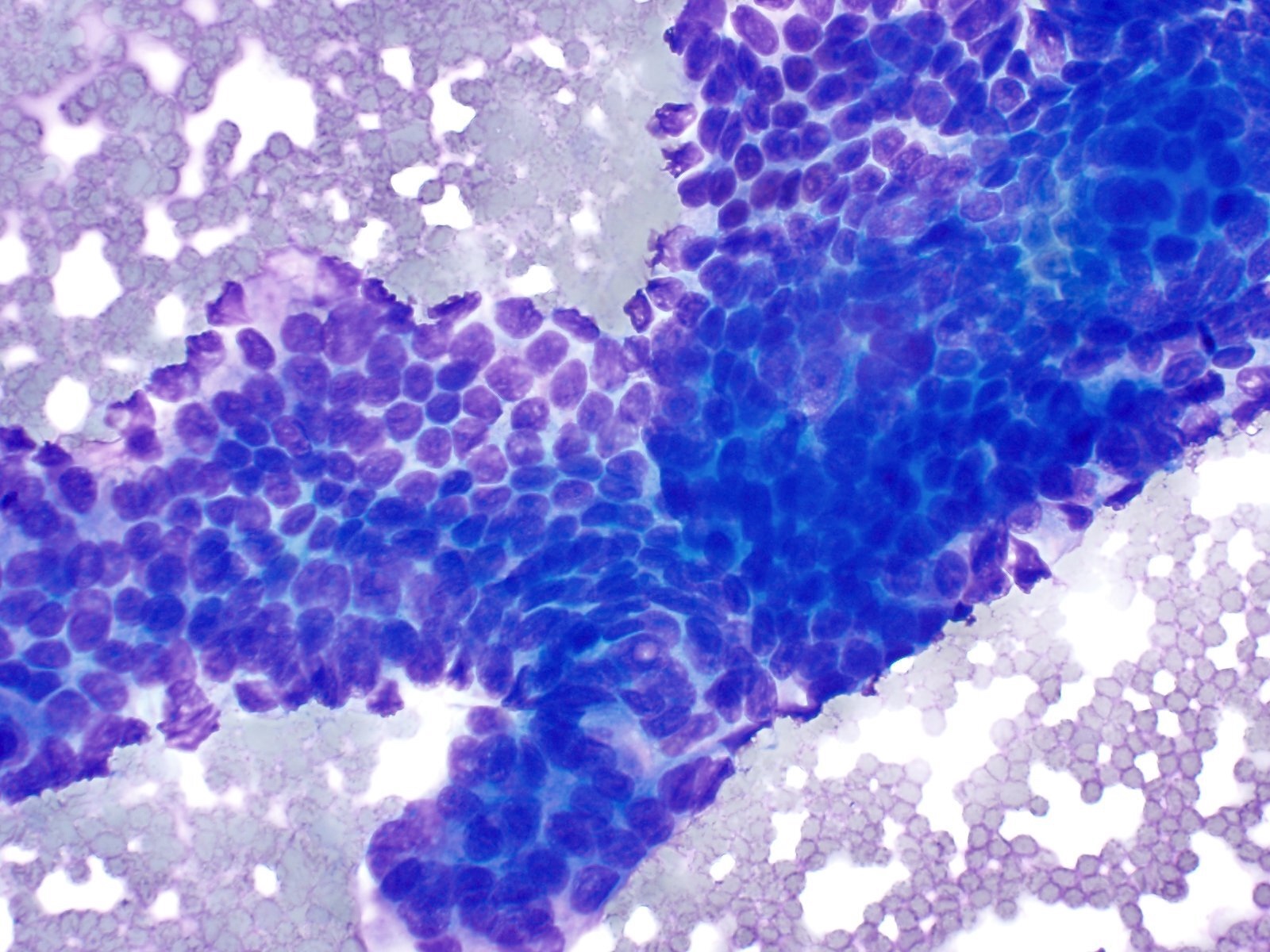
- Unlike conventional ductal adenocarcinoma, this tumor is highly cellular and with scant fibrous stroma
- Cells show moderate amounts of granular eosinophilic cytoplasm containing PAS positive diastase resistant zymogen granules
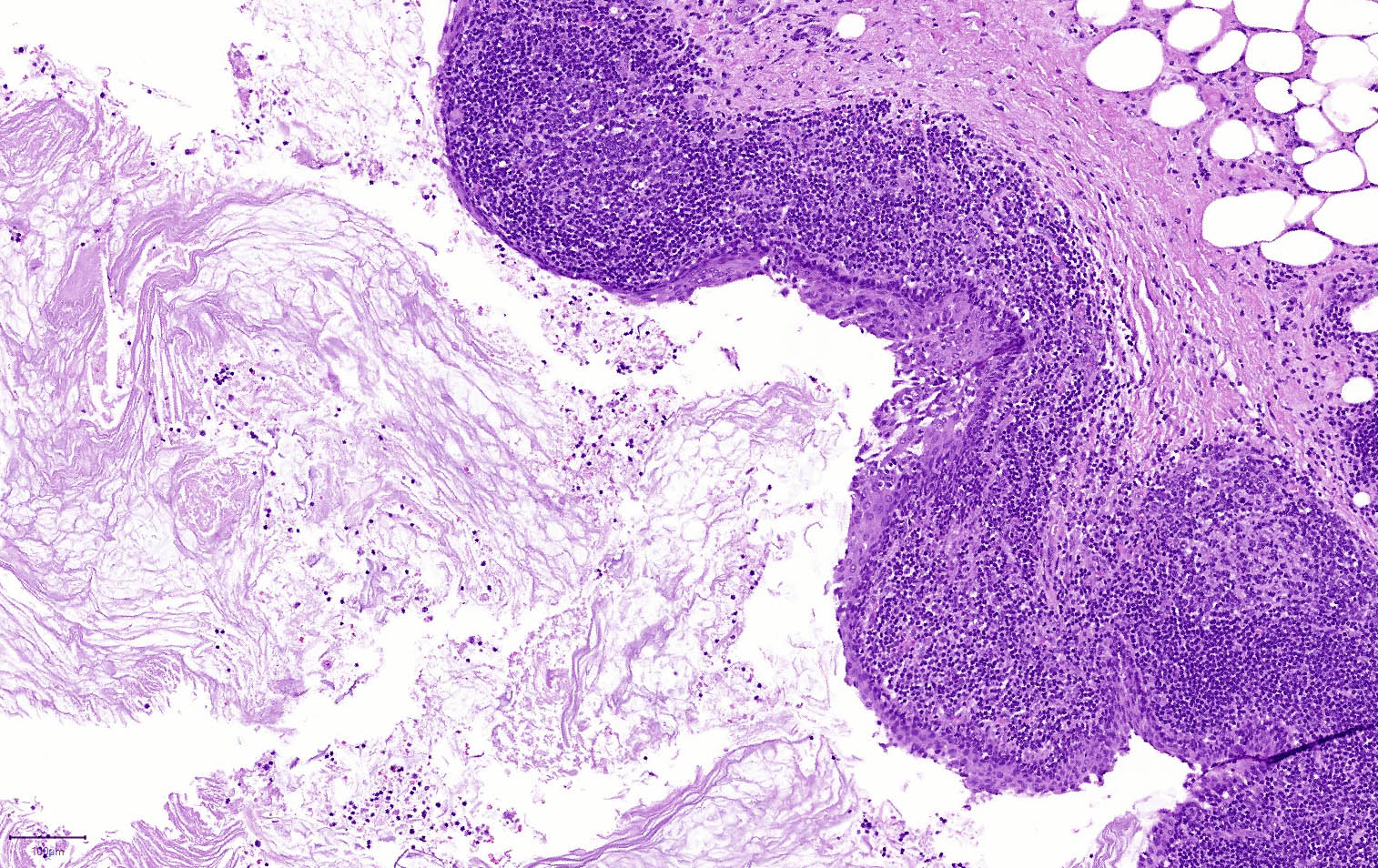
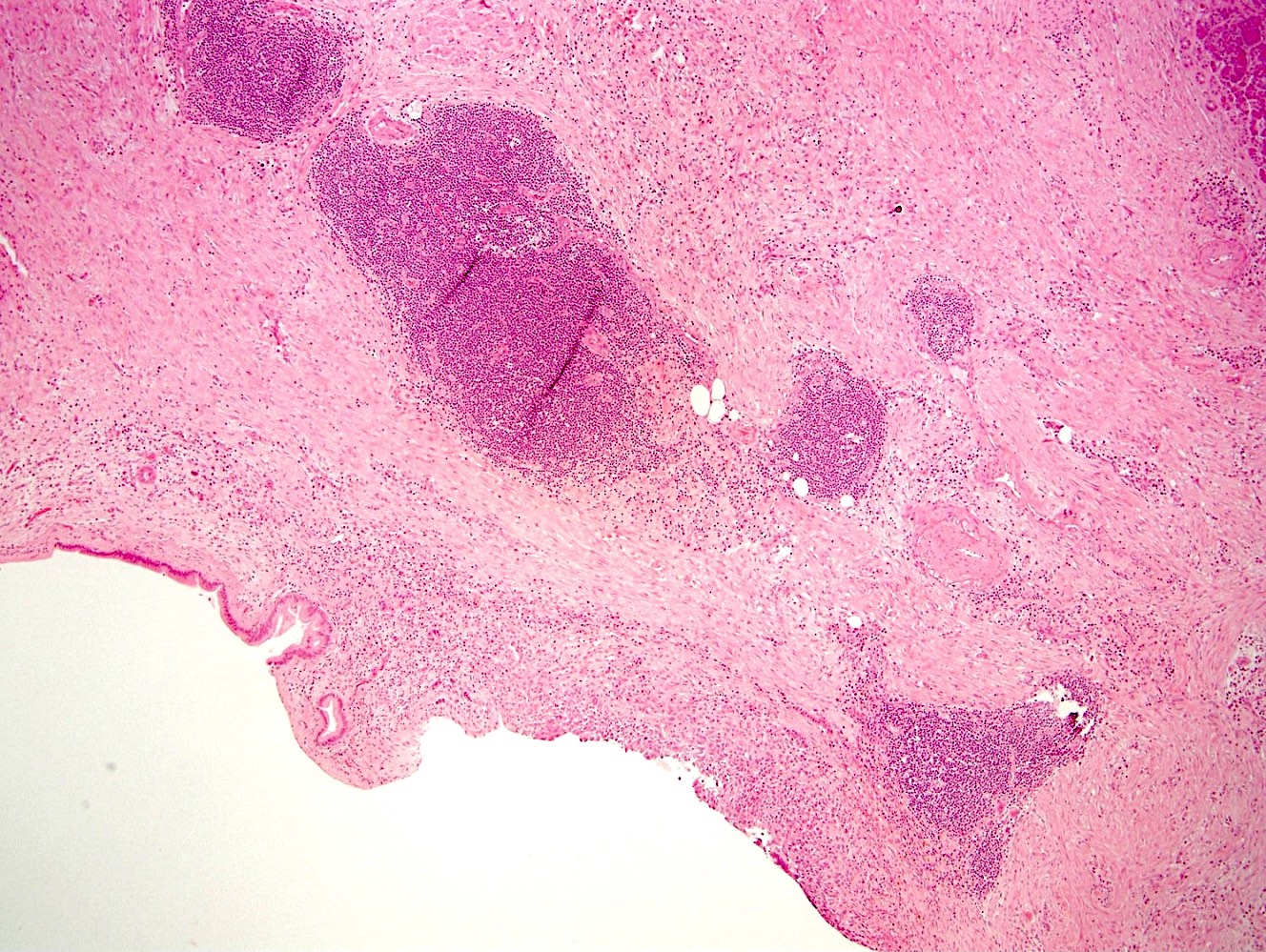
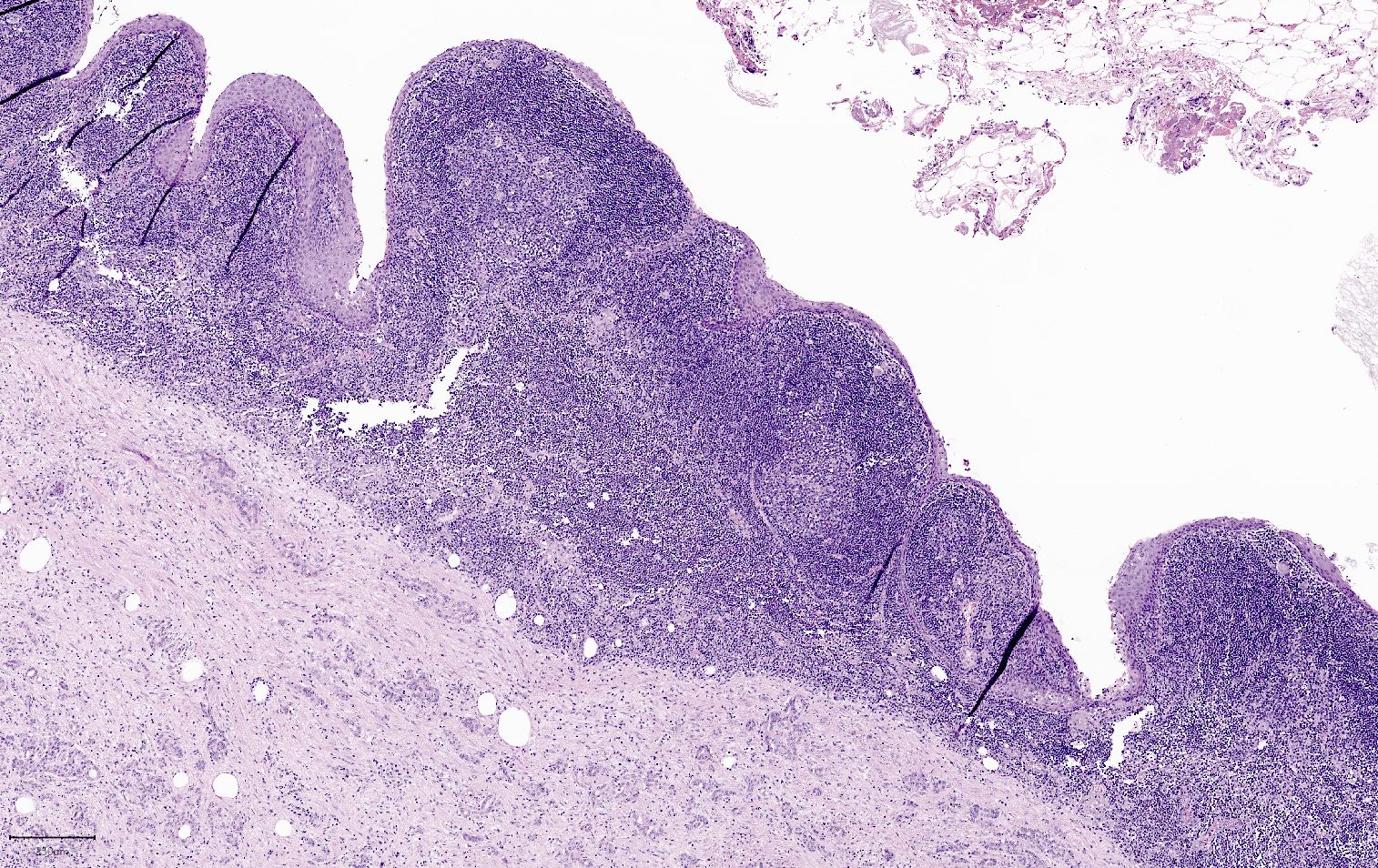
- Nuclei are uniform with a typically present, single and prominent nucleolus (Semin Diagn Pathol 2016;33:307)
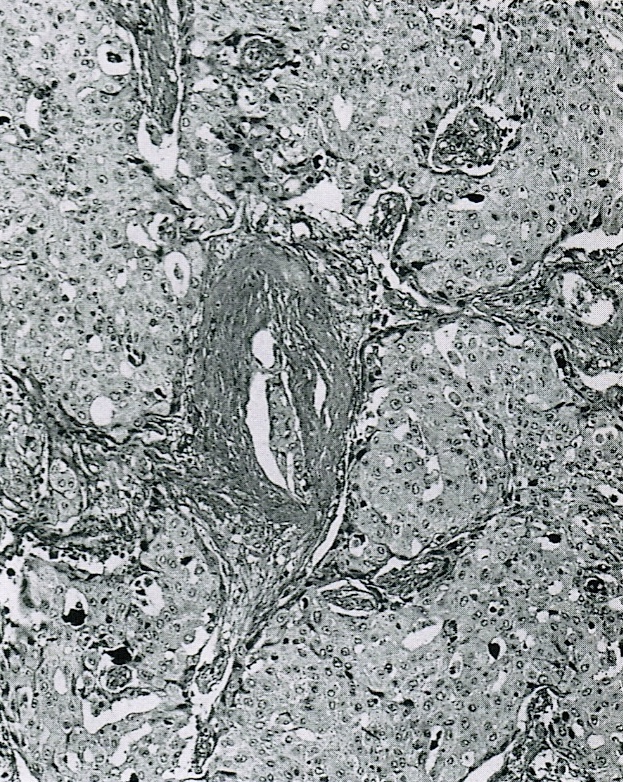
- Perineural invasion and vascular invasion are very common
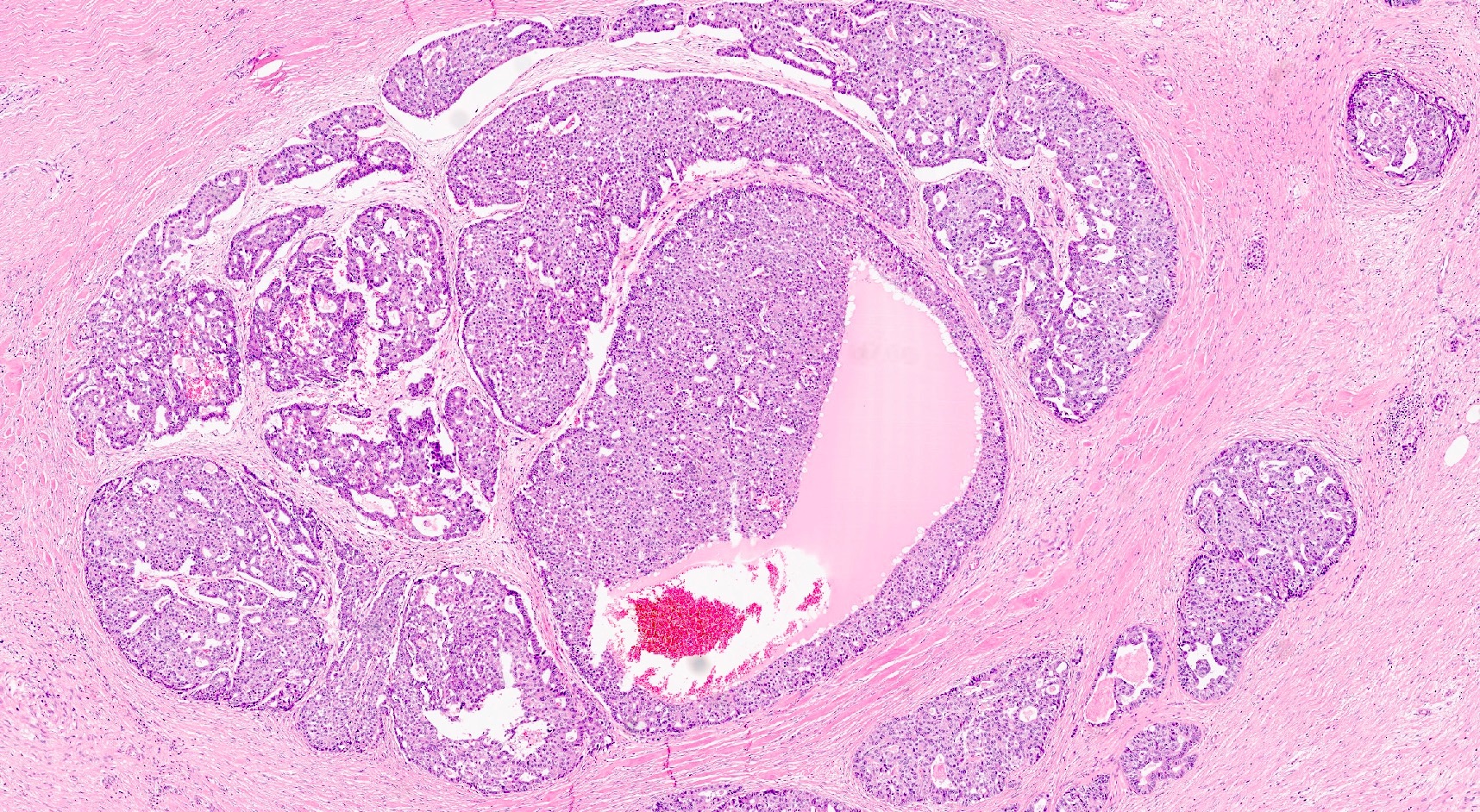
- Can have different architectures and growth patterns, including cystic, acinar, glandular and intraductal
- Nonneuroendocrine component of mixed neuroendocrine nonneuroendocrine neoplasms (MiNEN) in the pancreas can be represented by acinar cell carcinoma; MiNEN diagnosis should be based on both morphology and immunohistochemistry
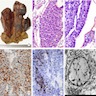
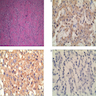
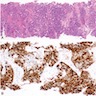
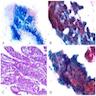
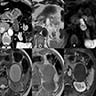
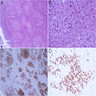
Microscopic (histologic) images
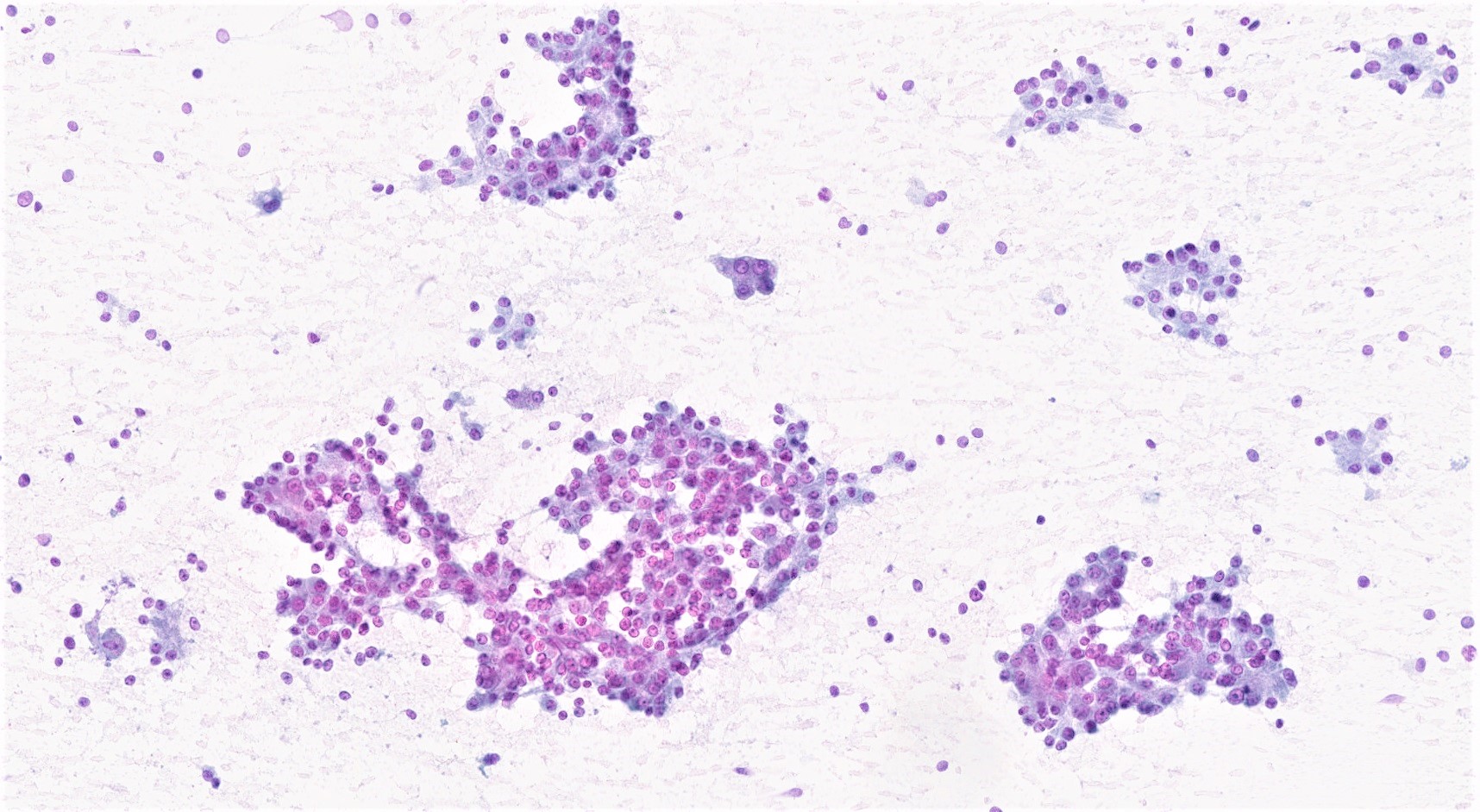
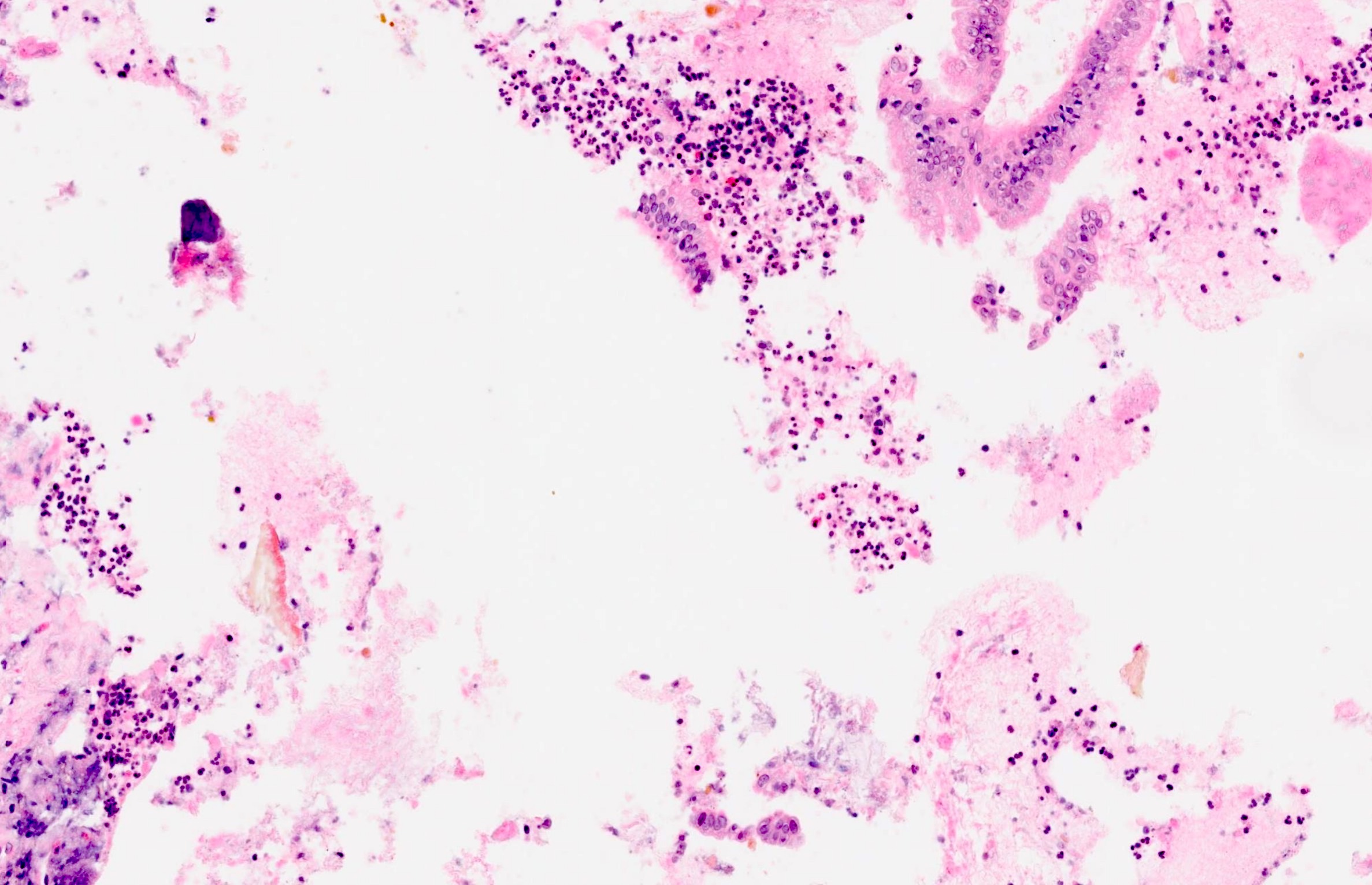
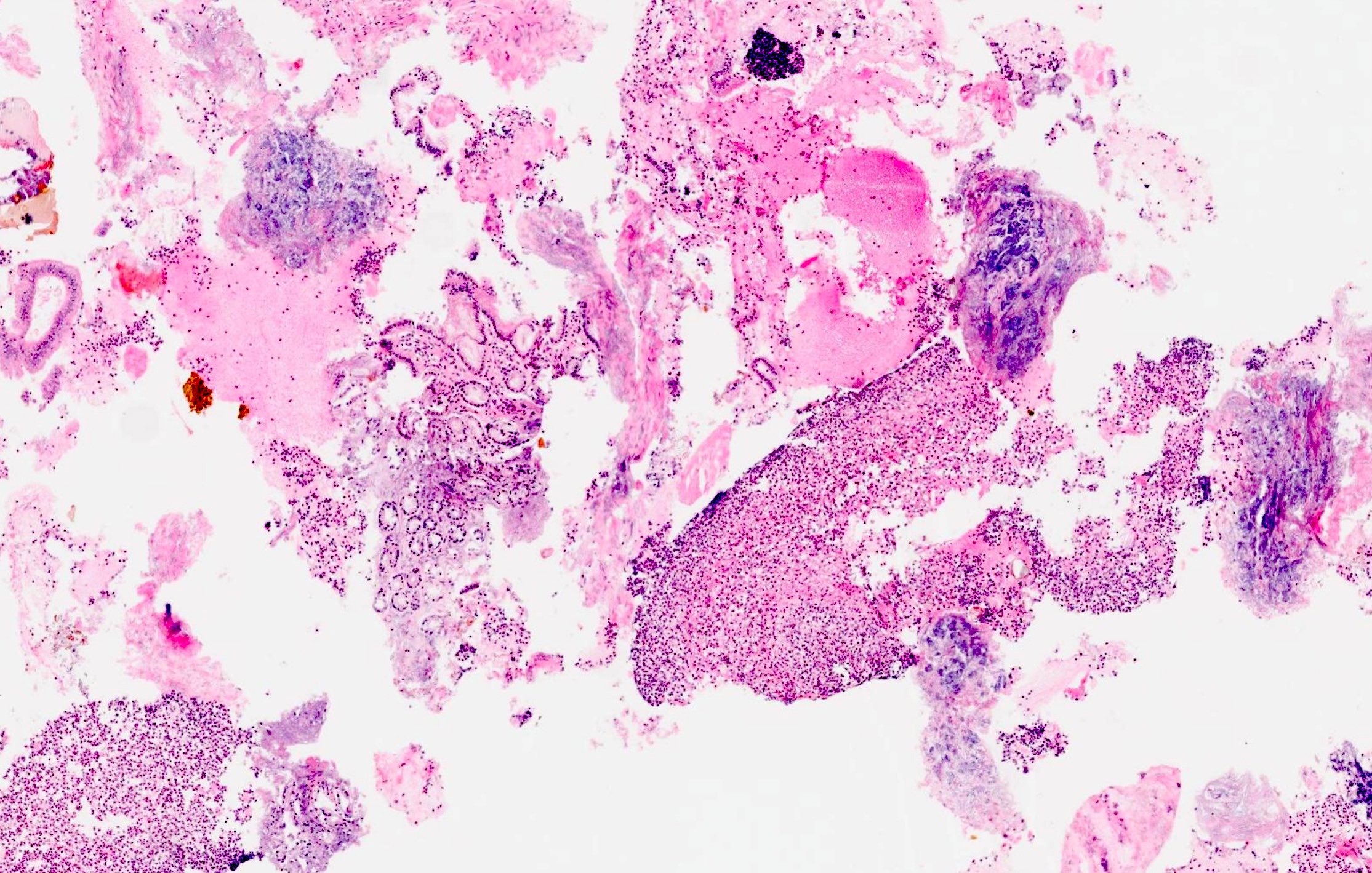
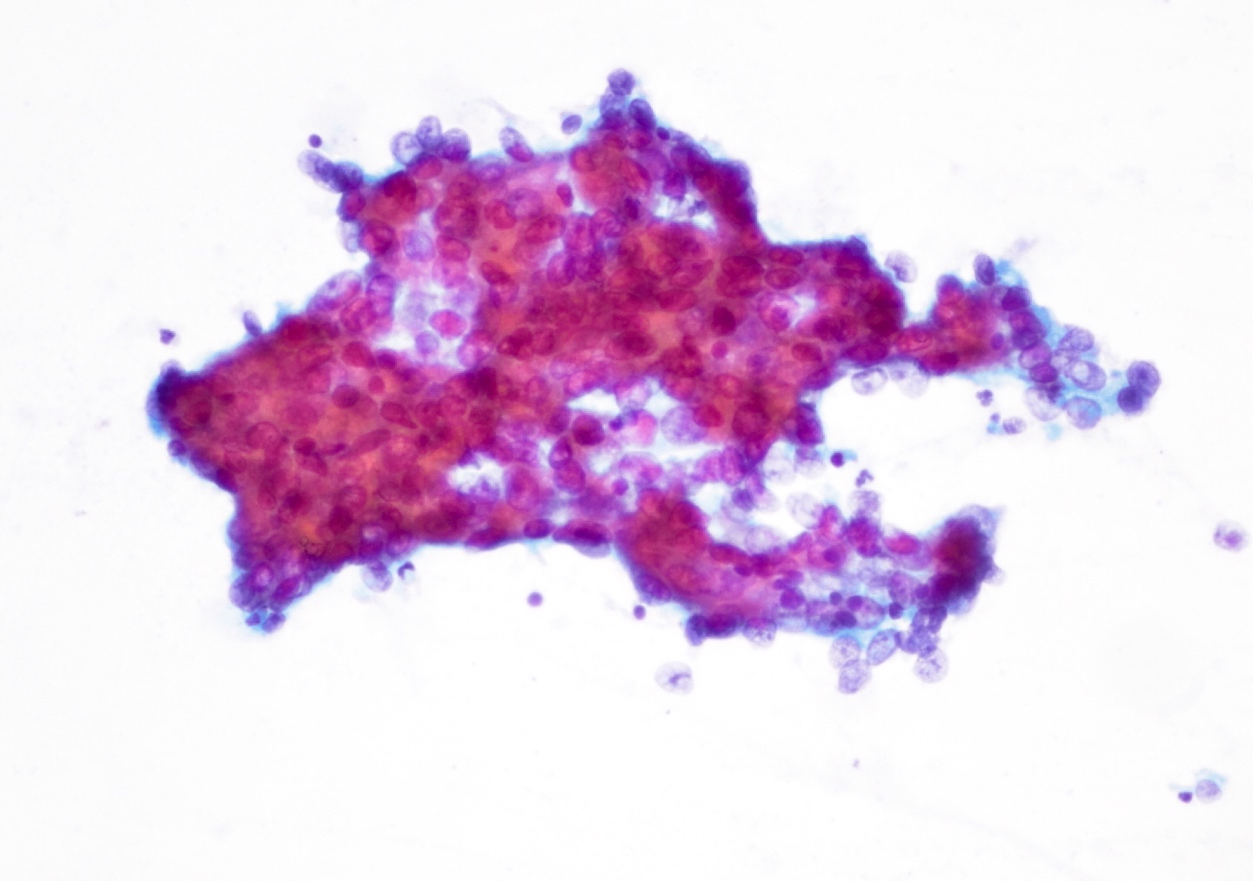
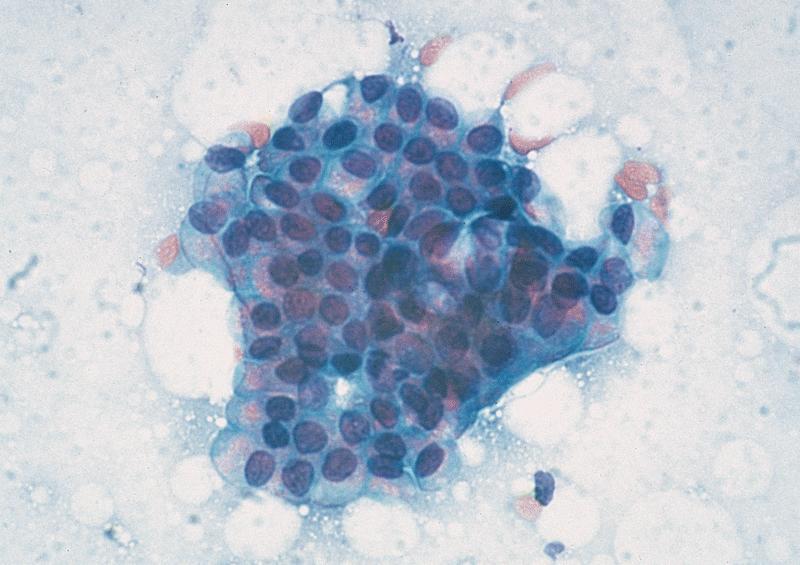
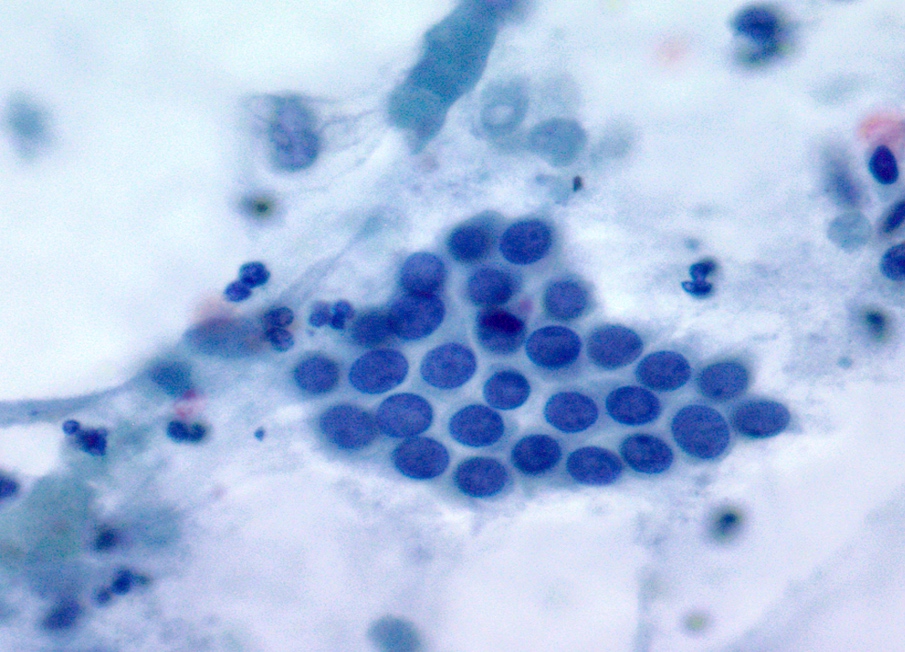
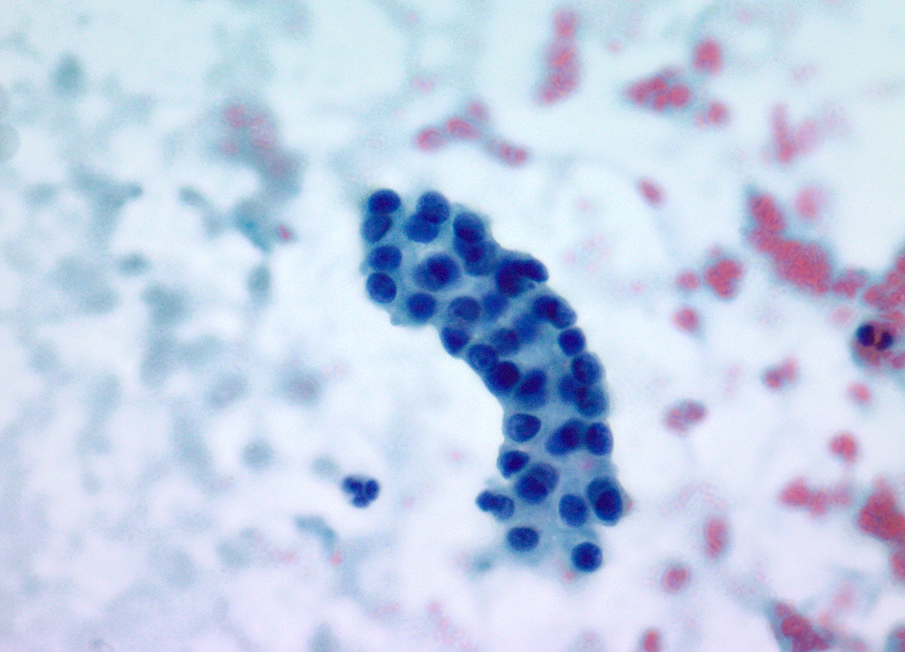
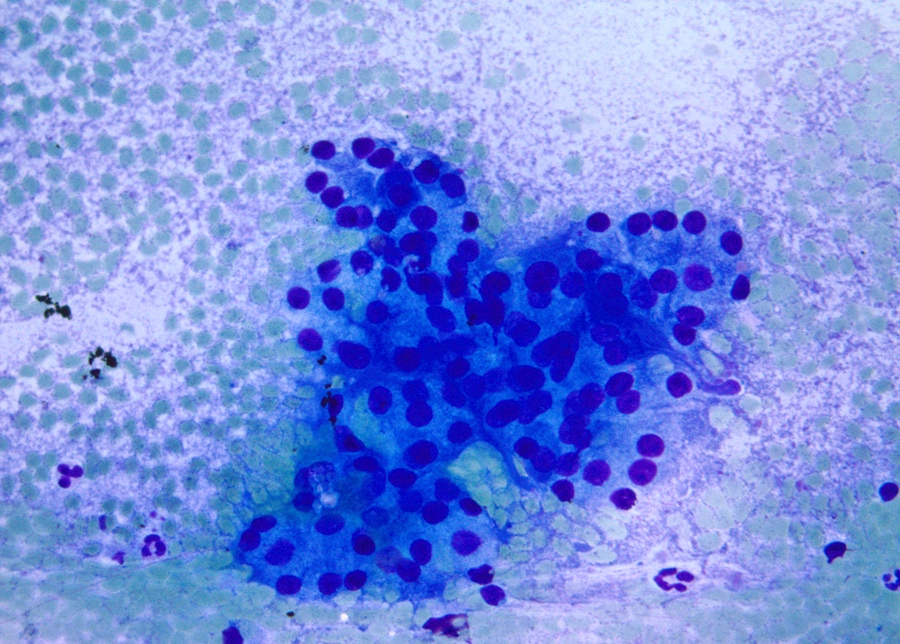
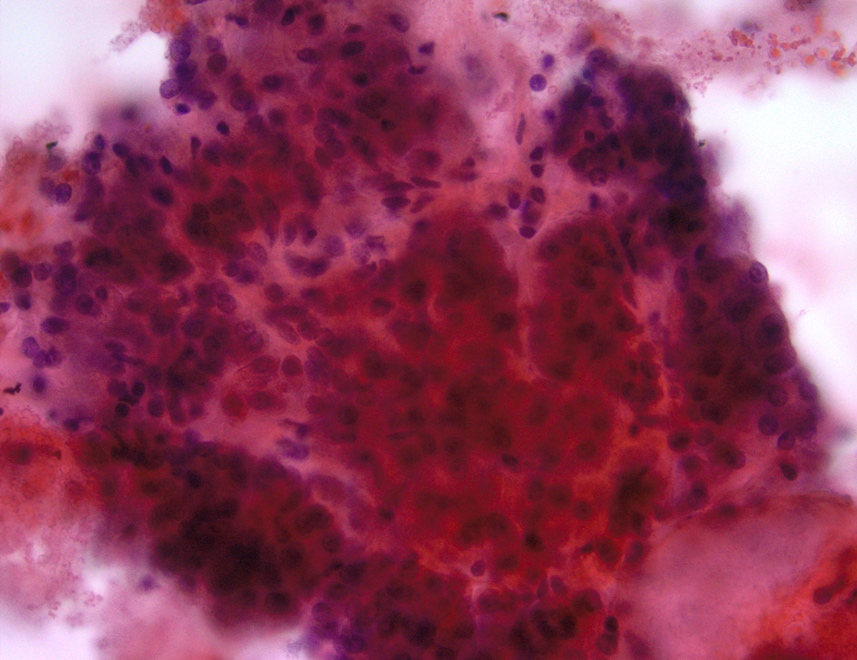
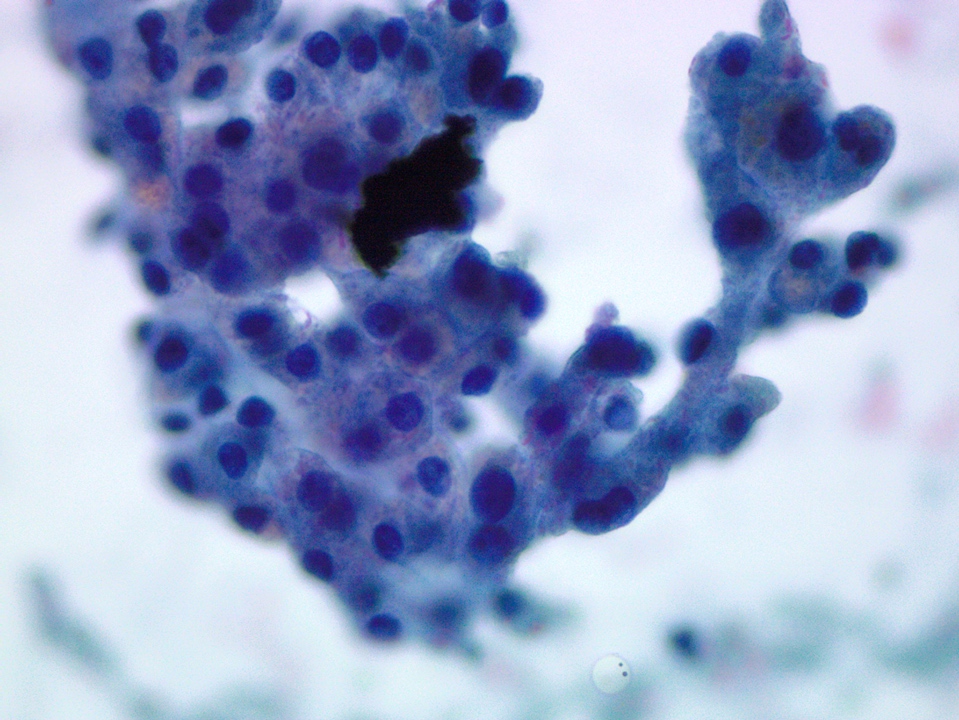
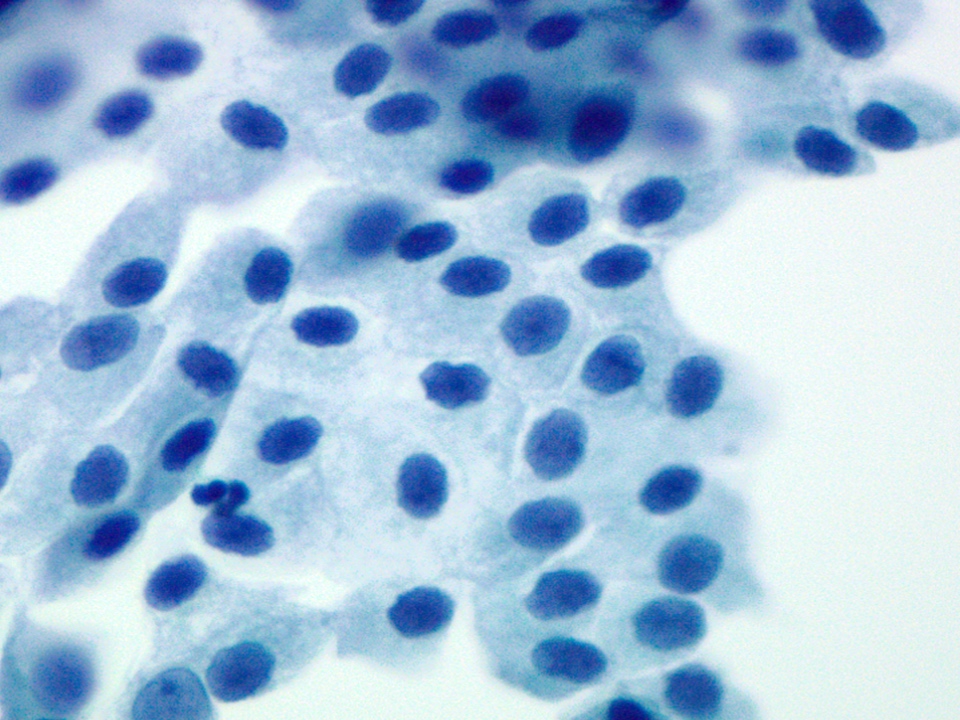
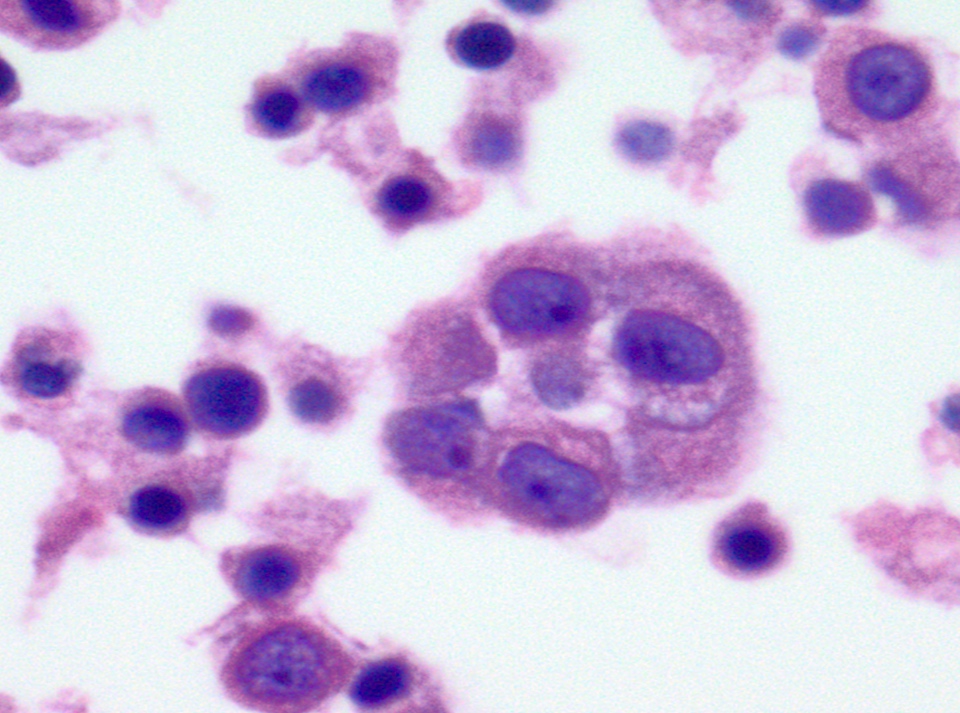
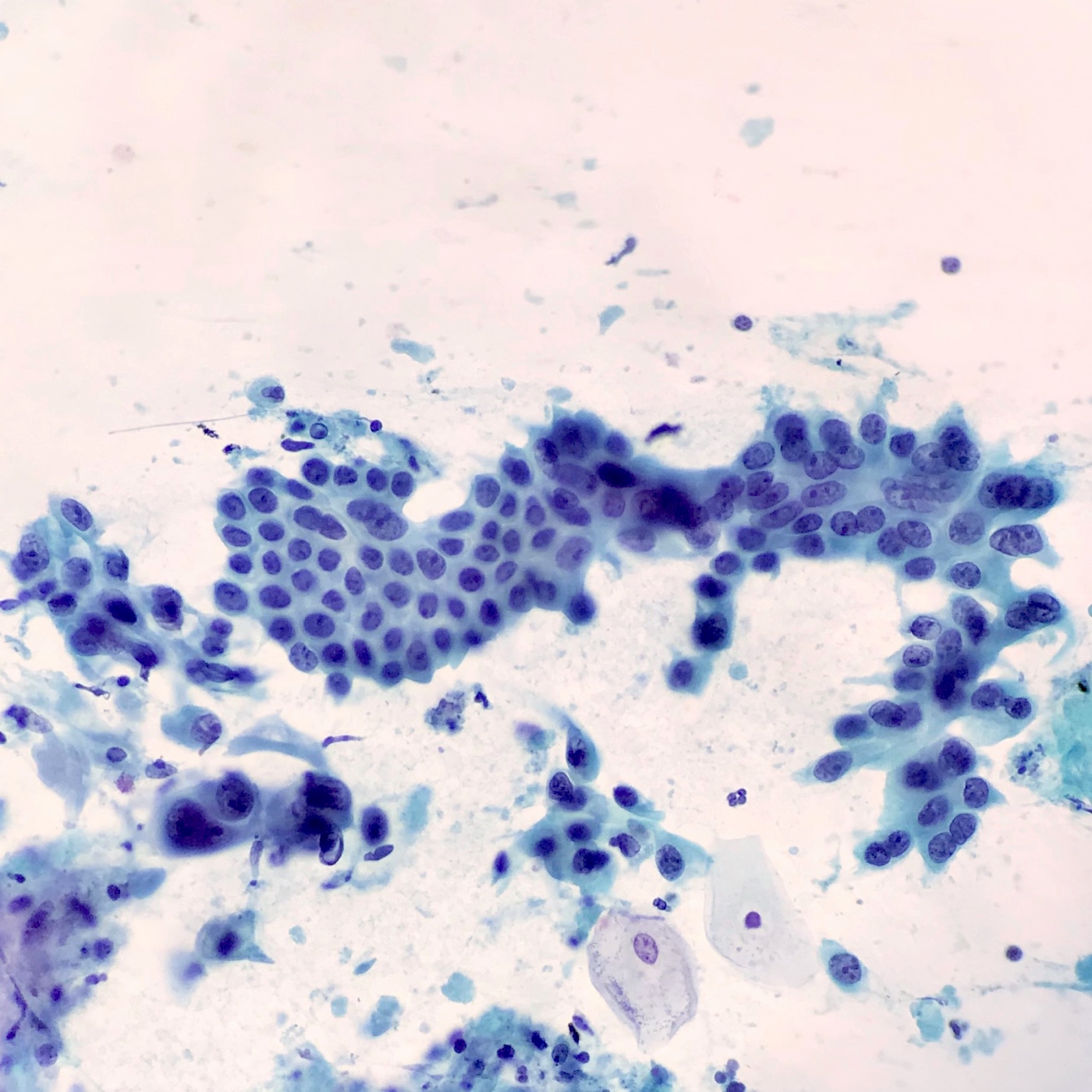
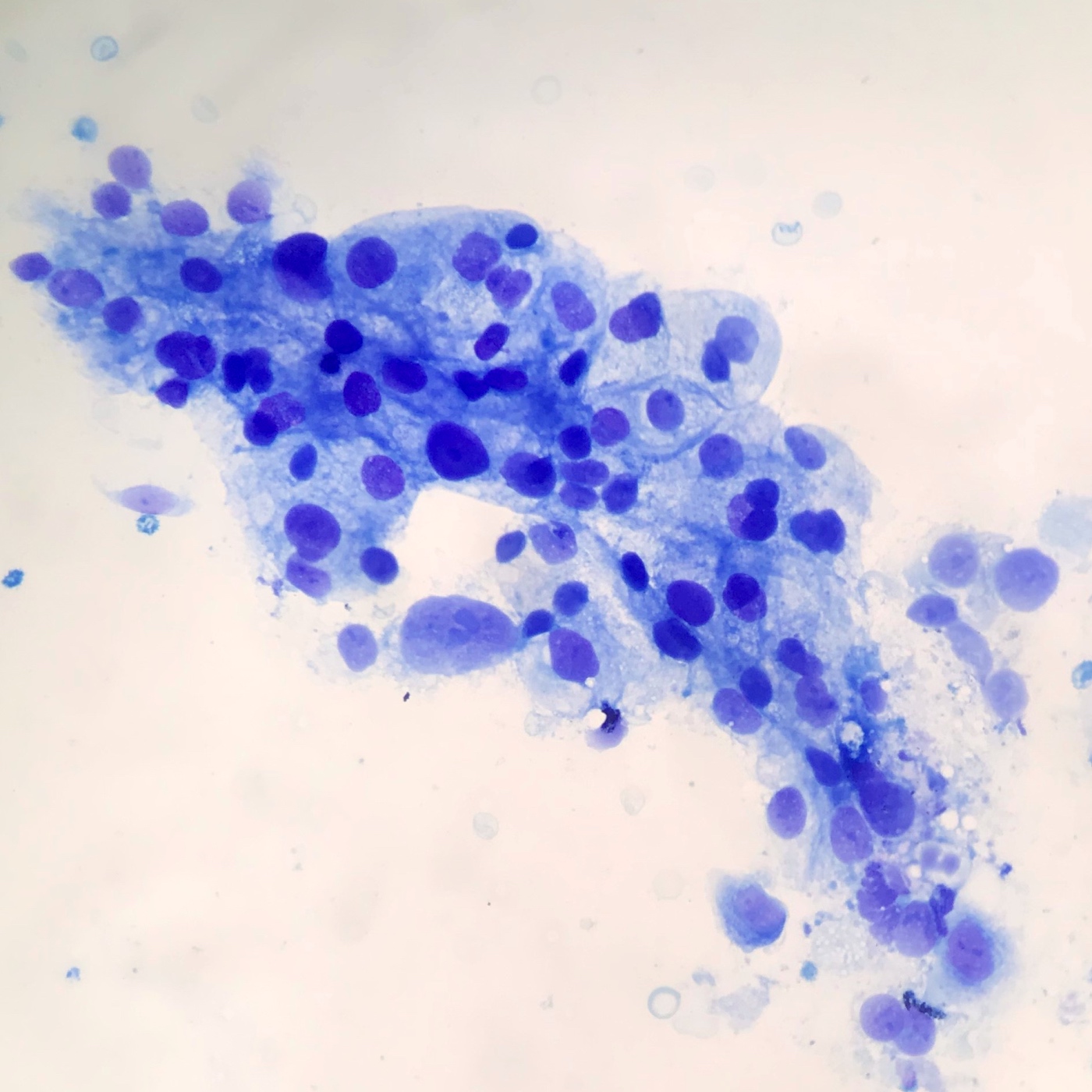
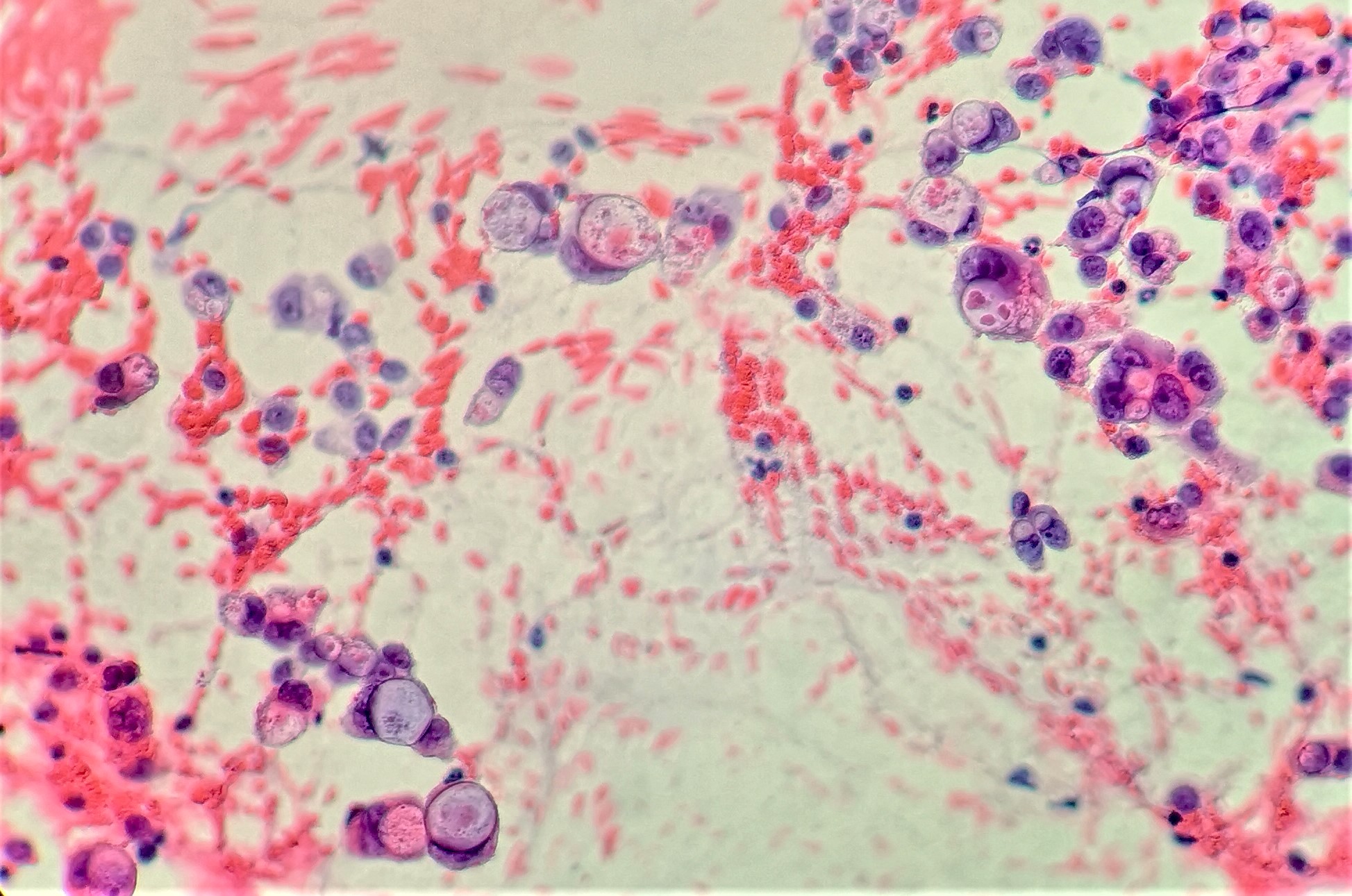
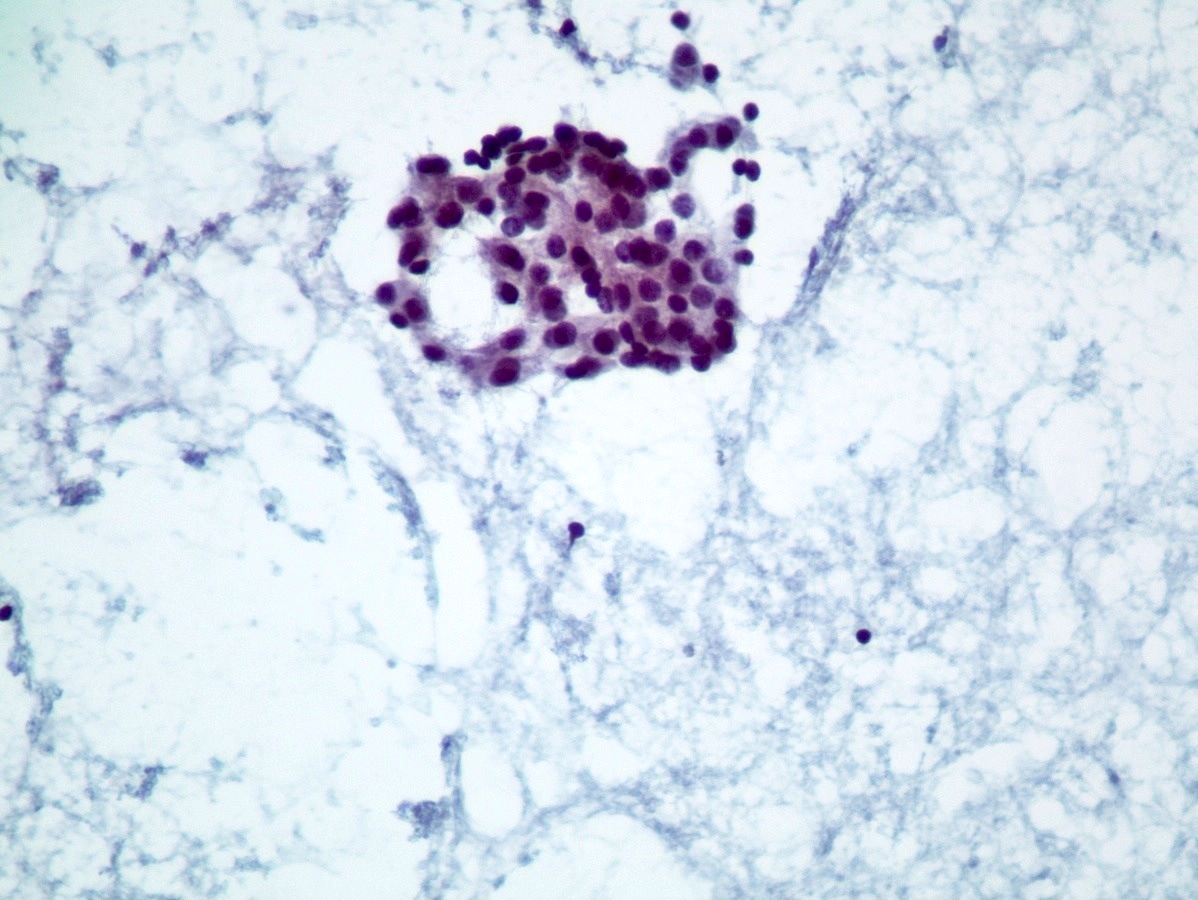
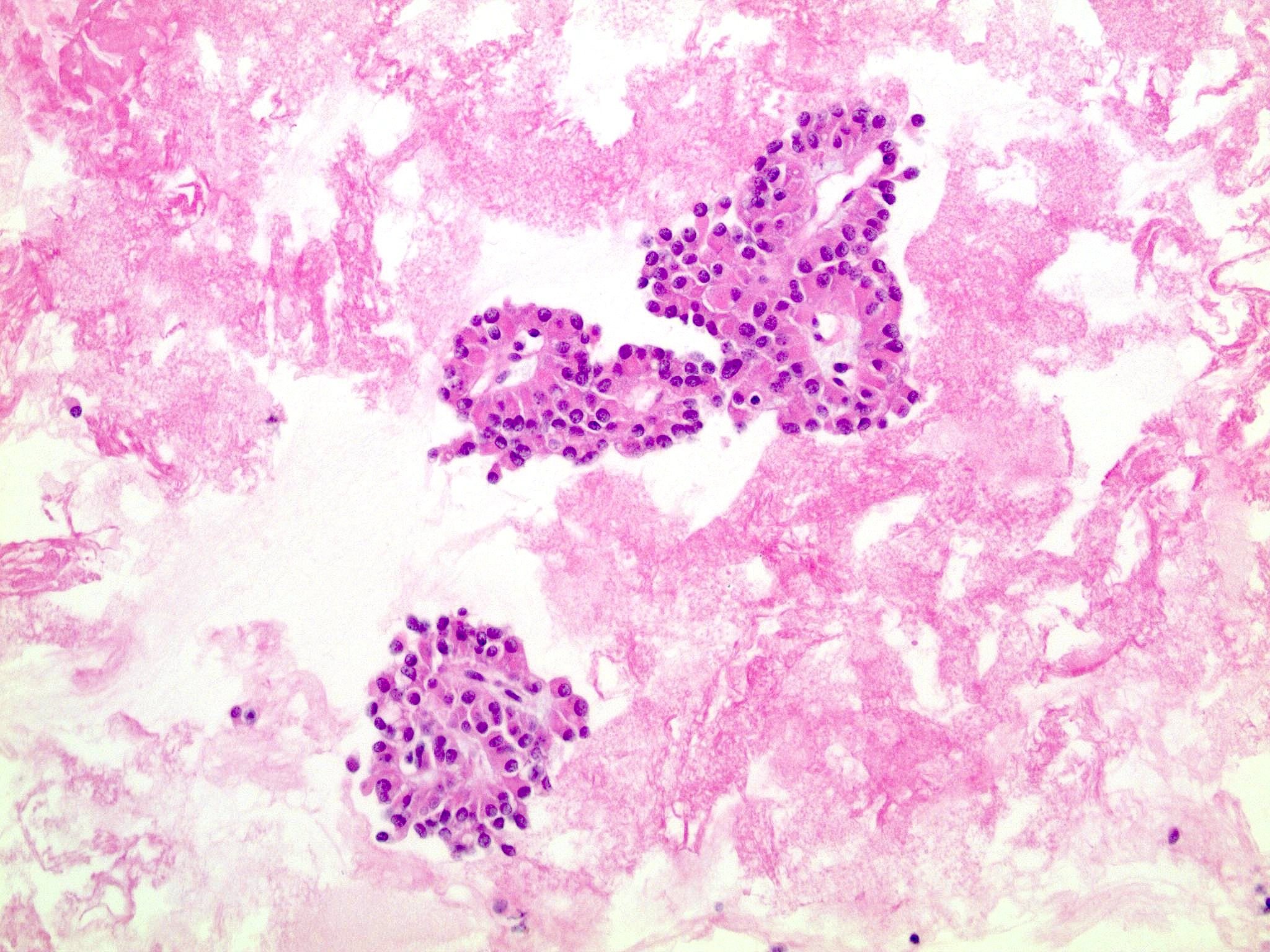
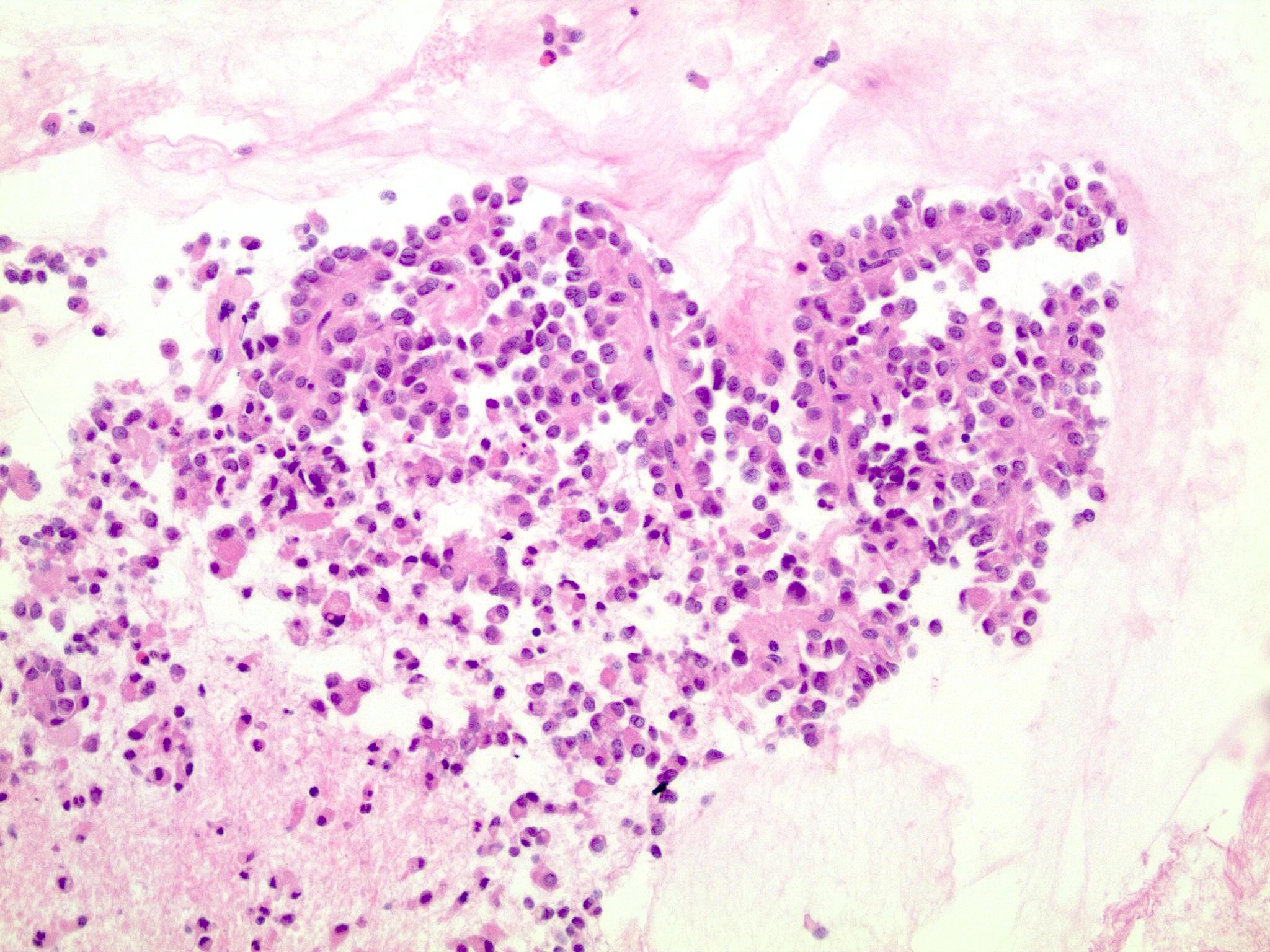
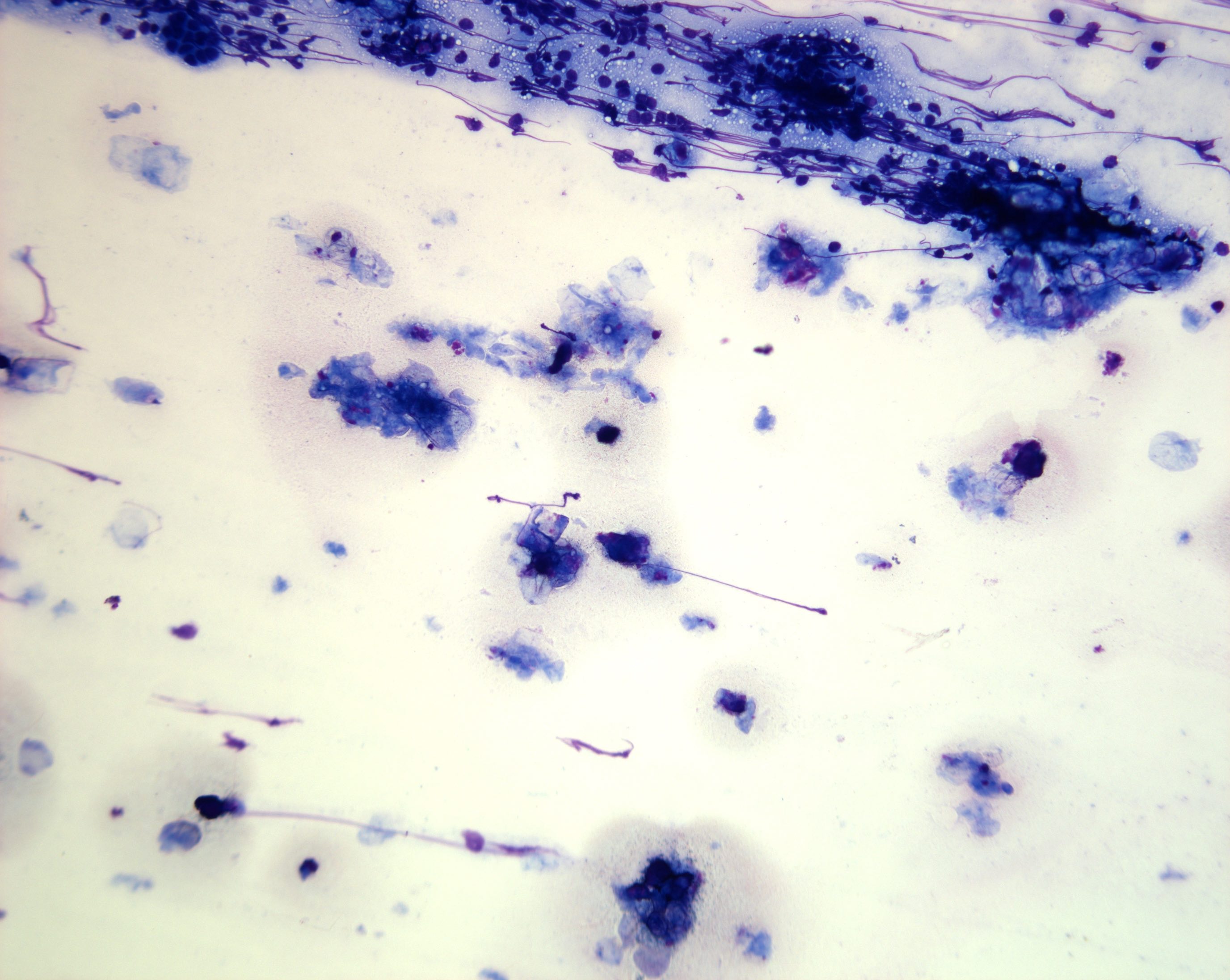
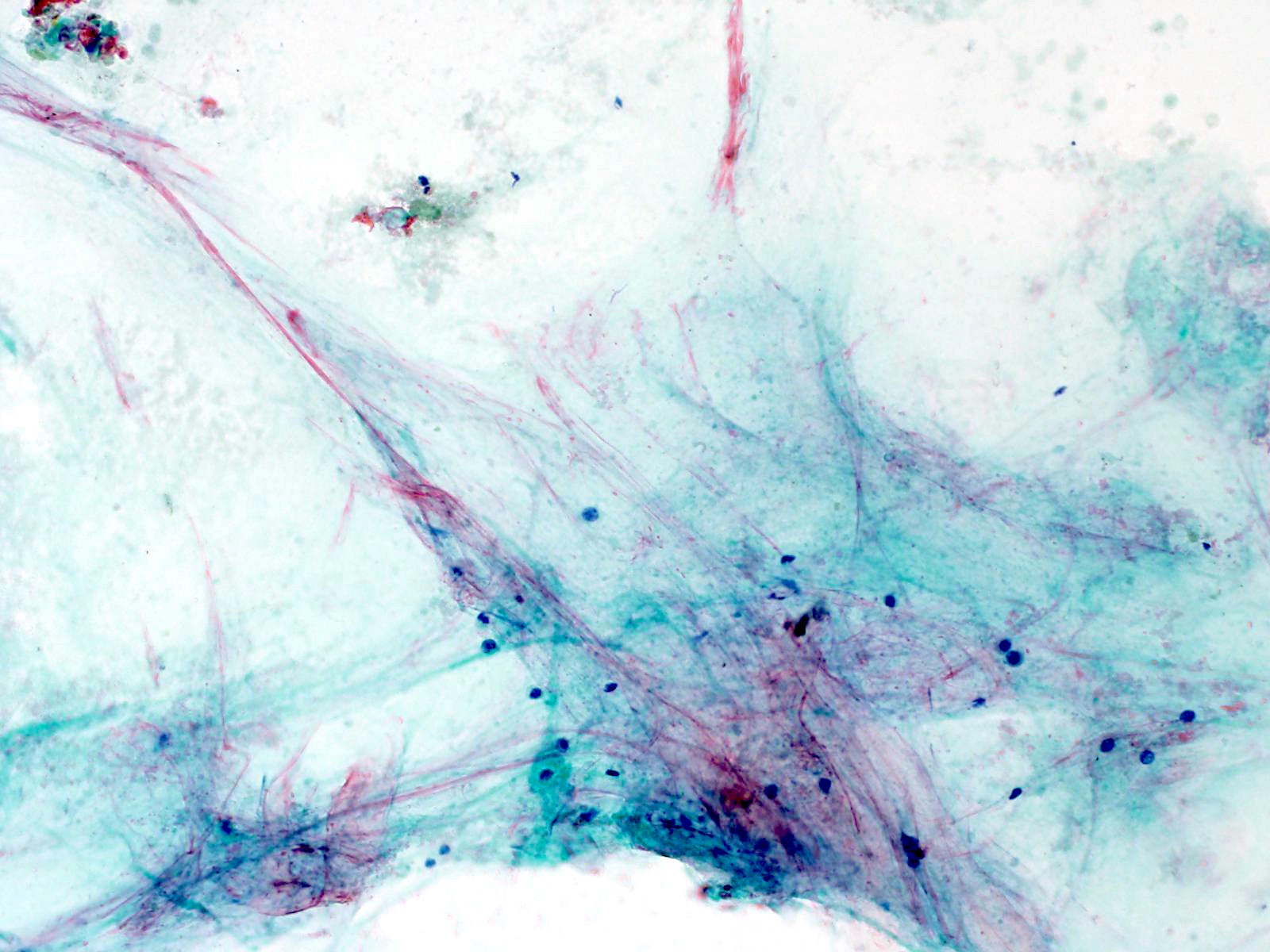
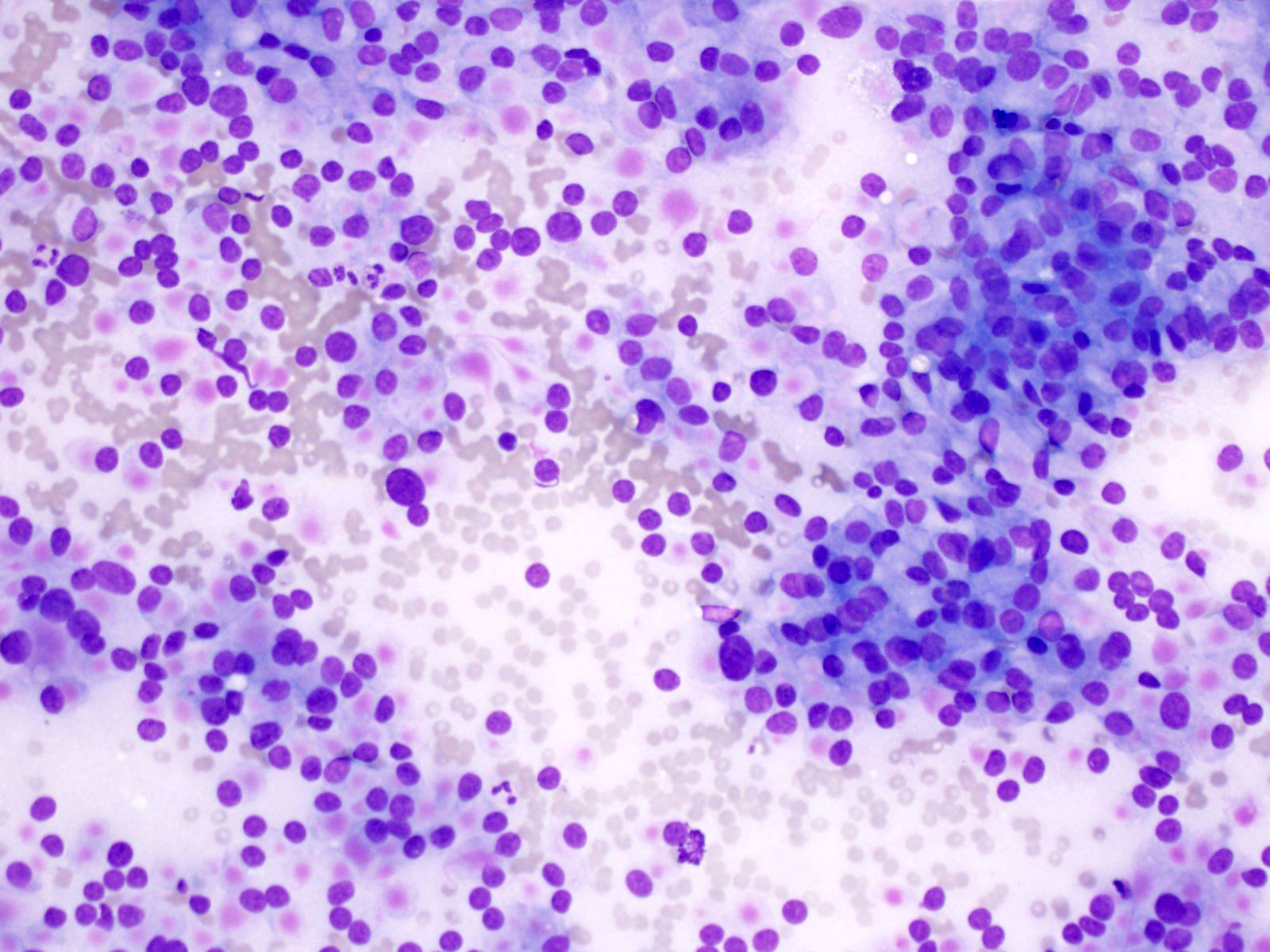
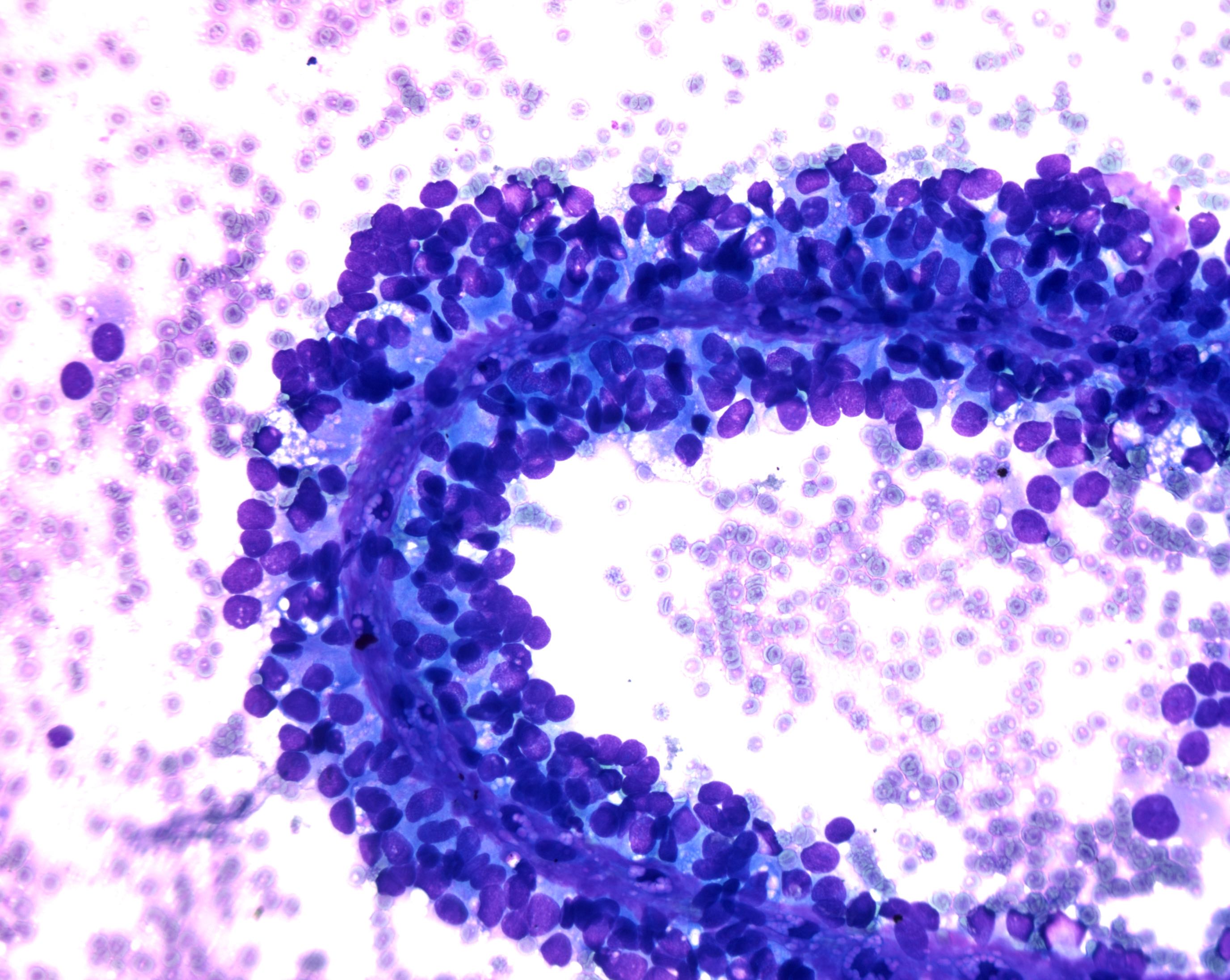
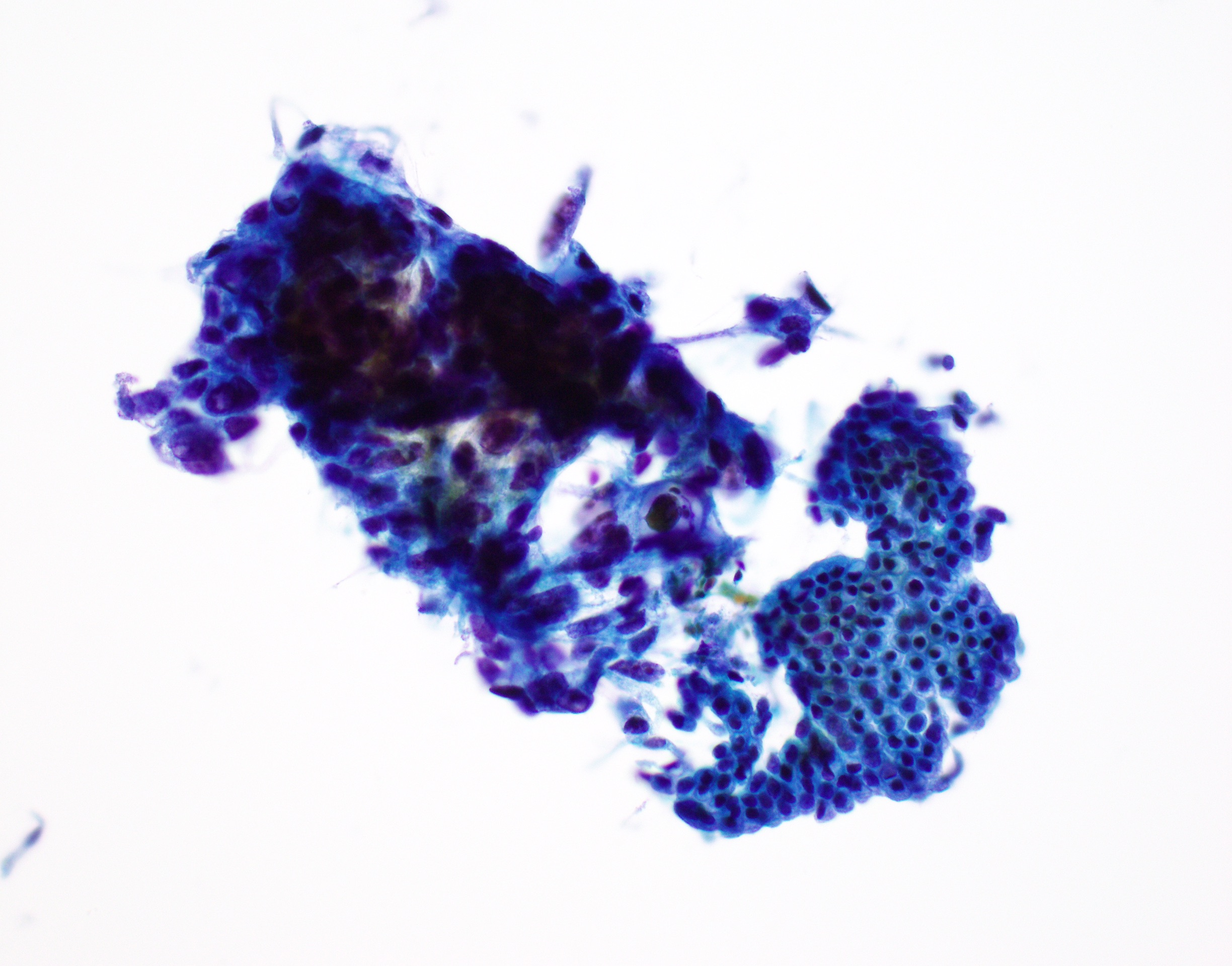
Cytology description
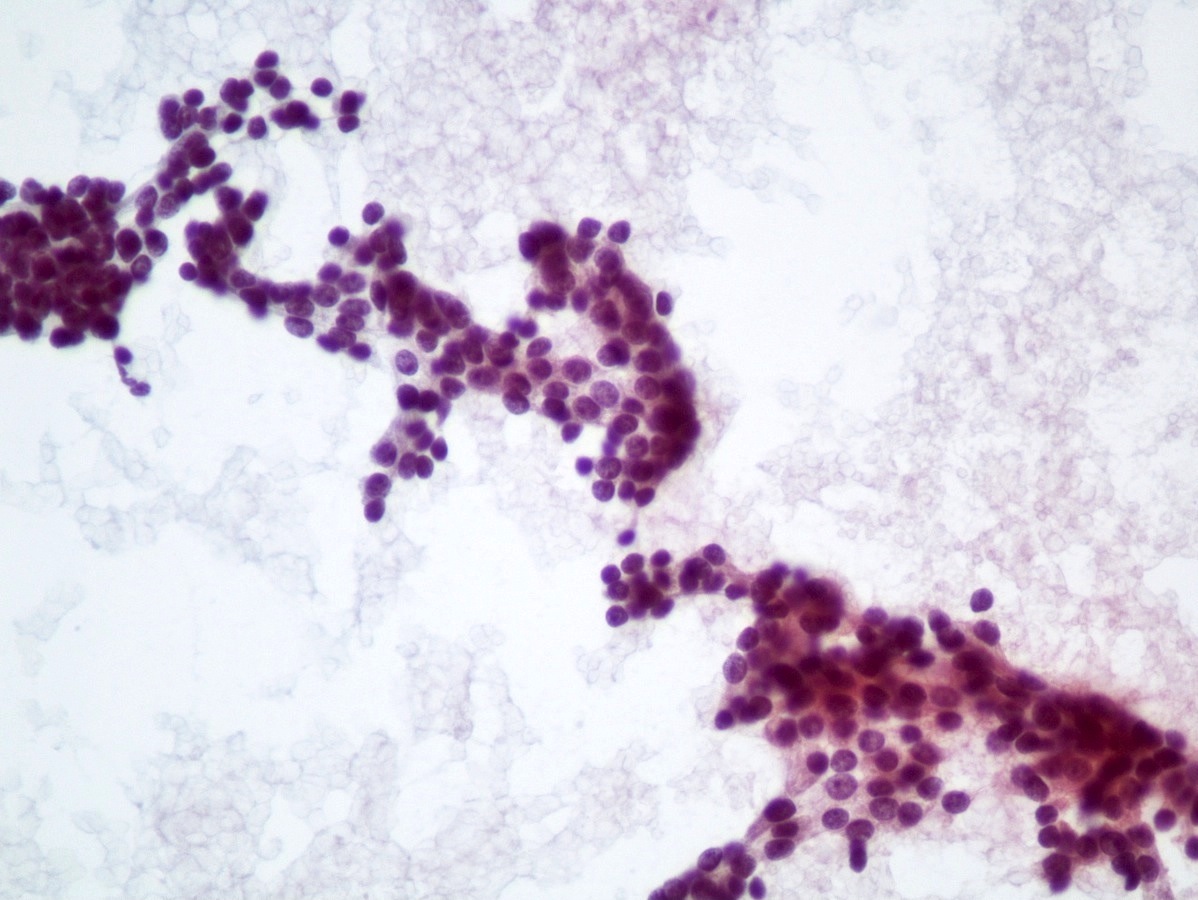
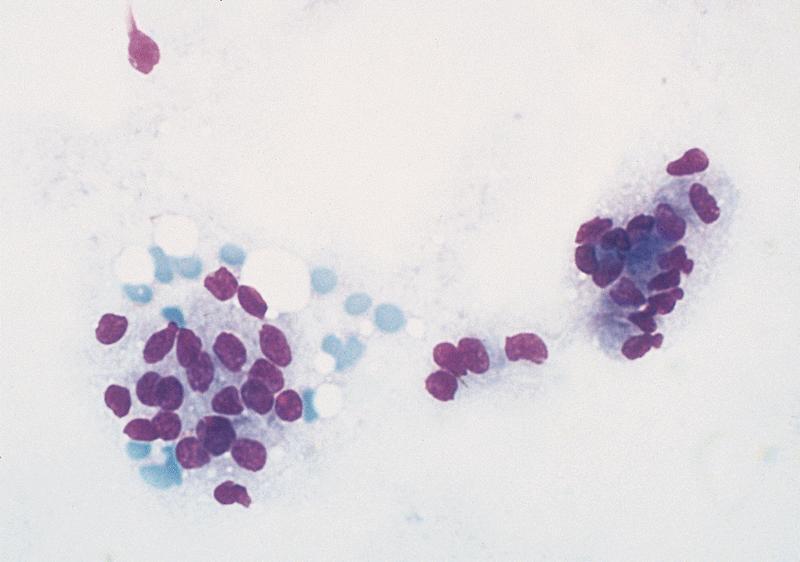
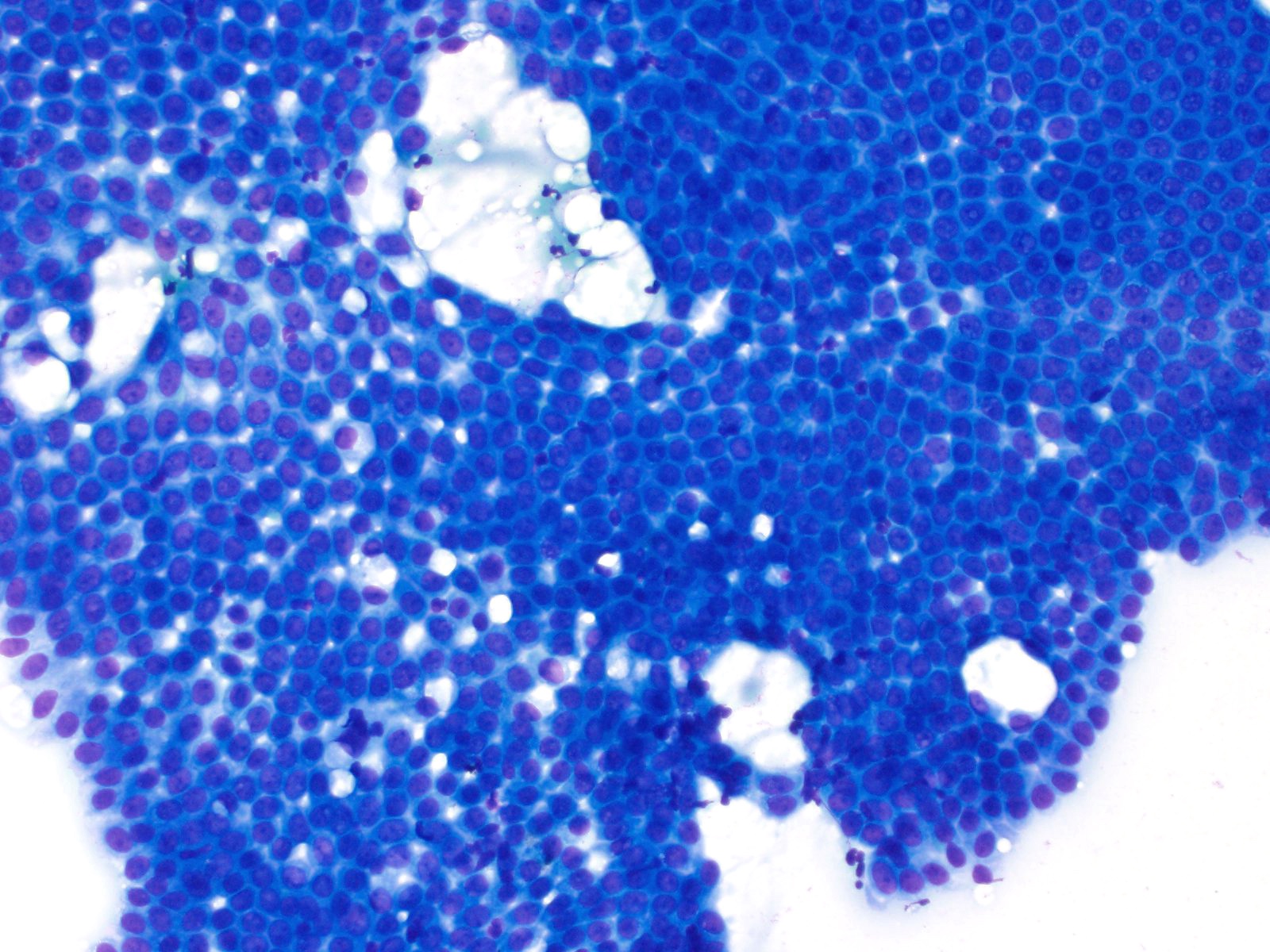
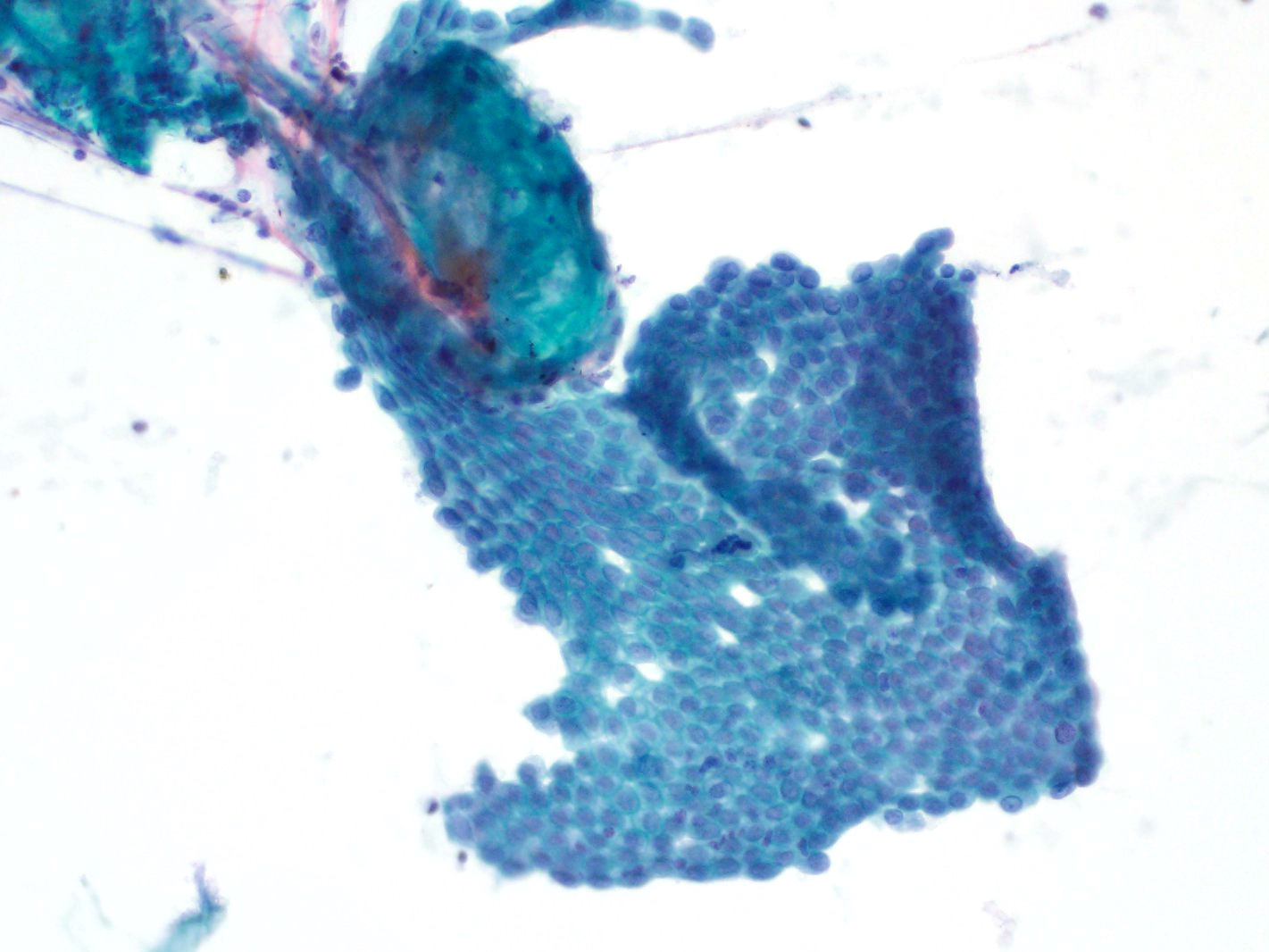
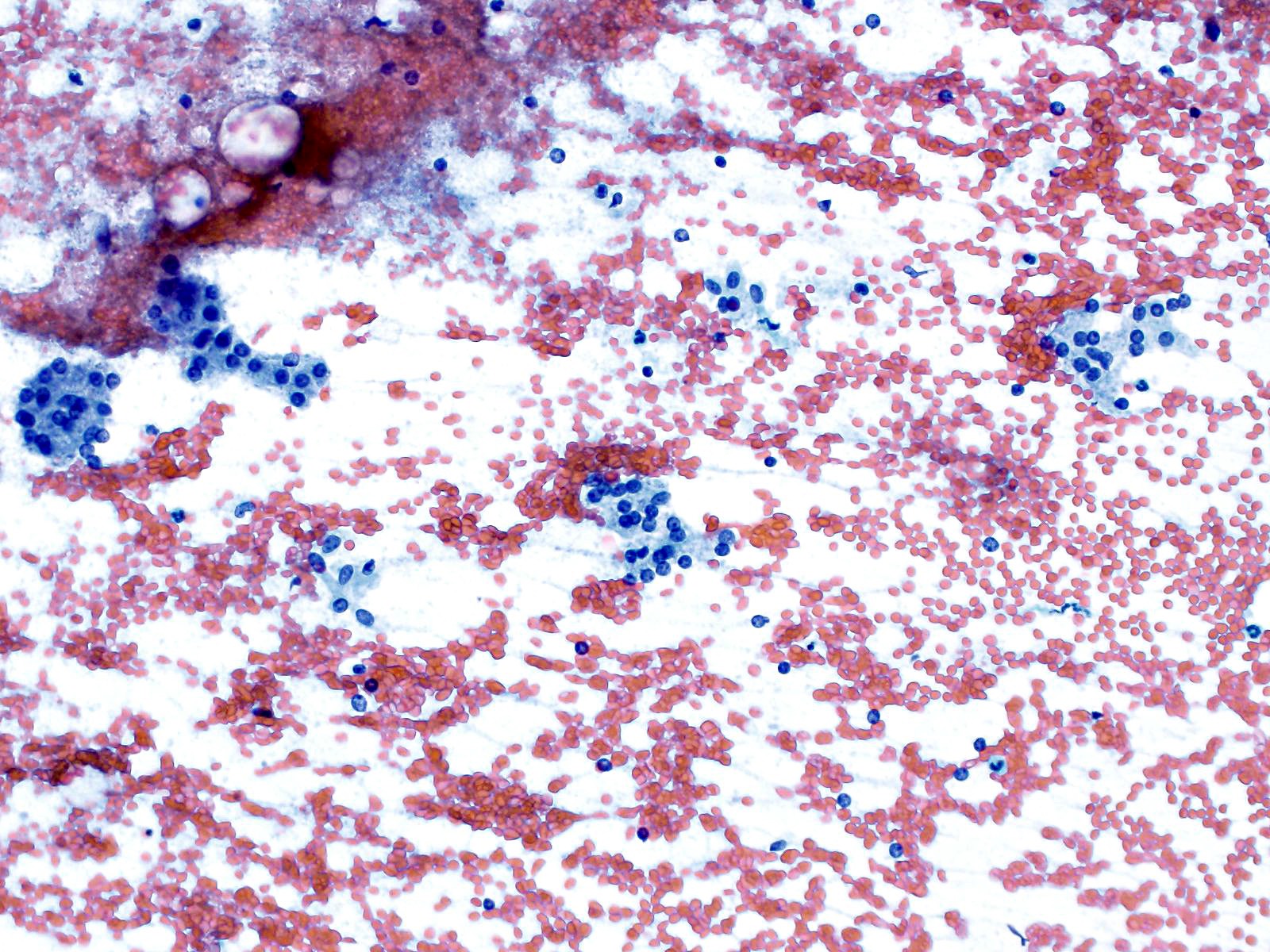
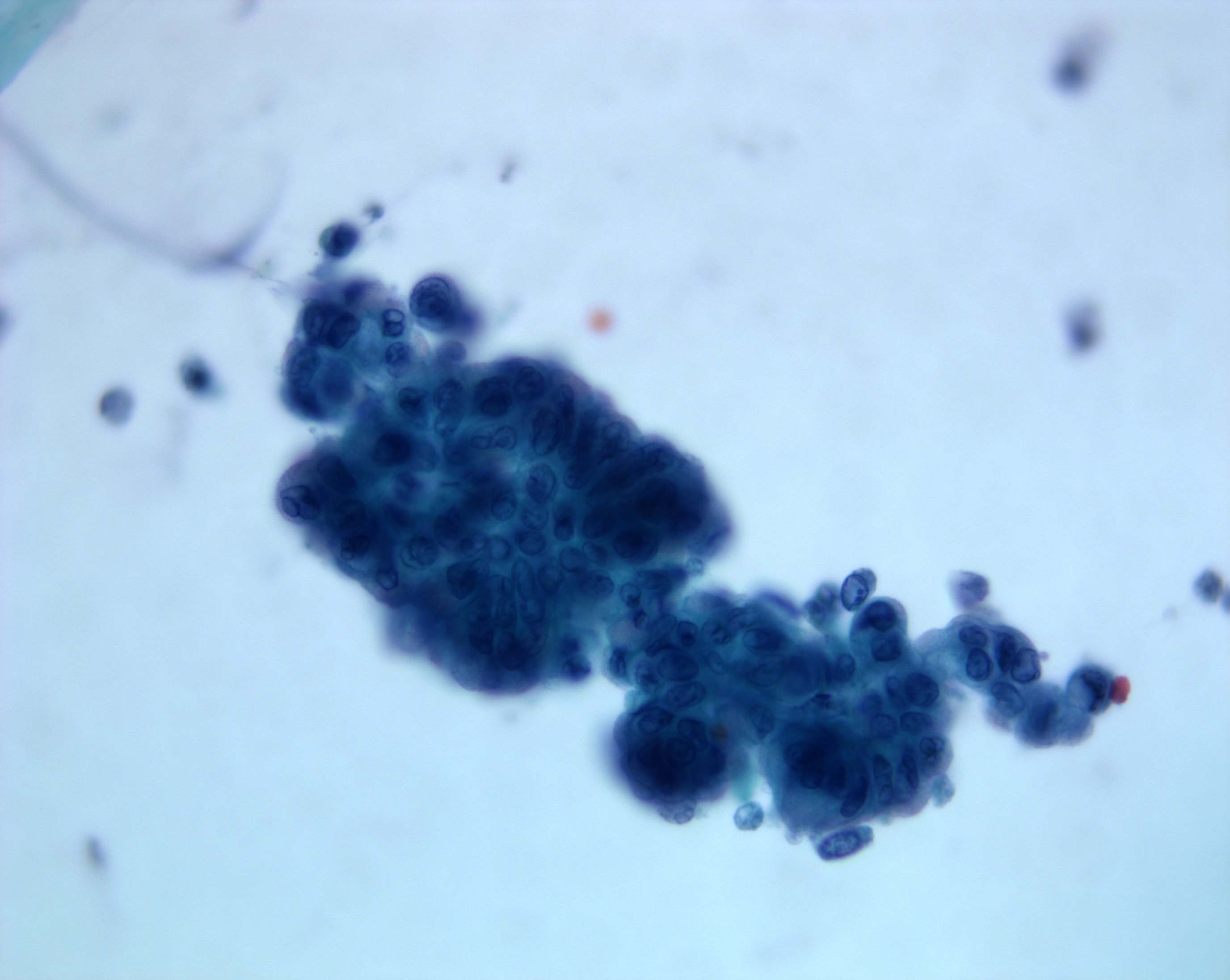
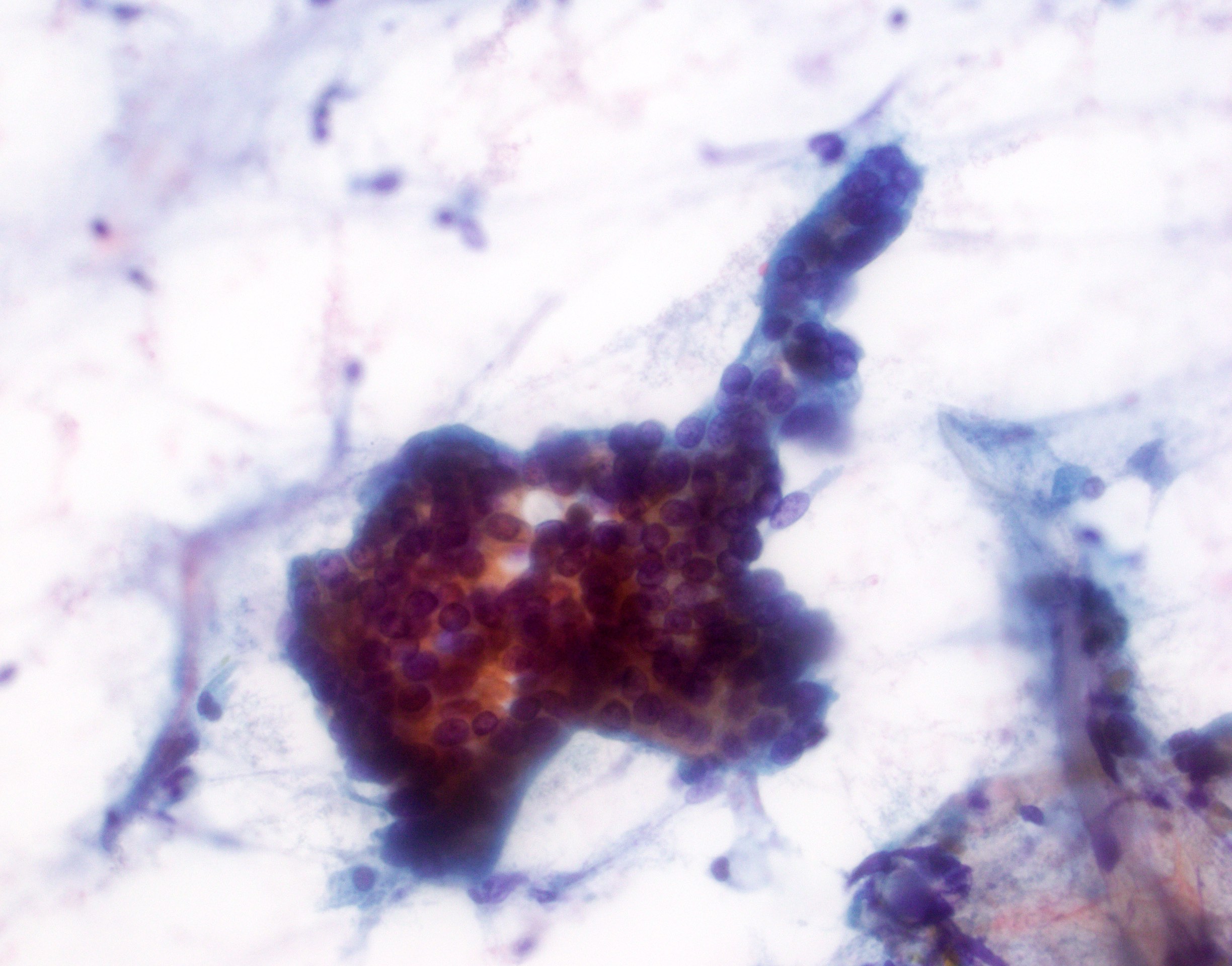
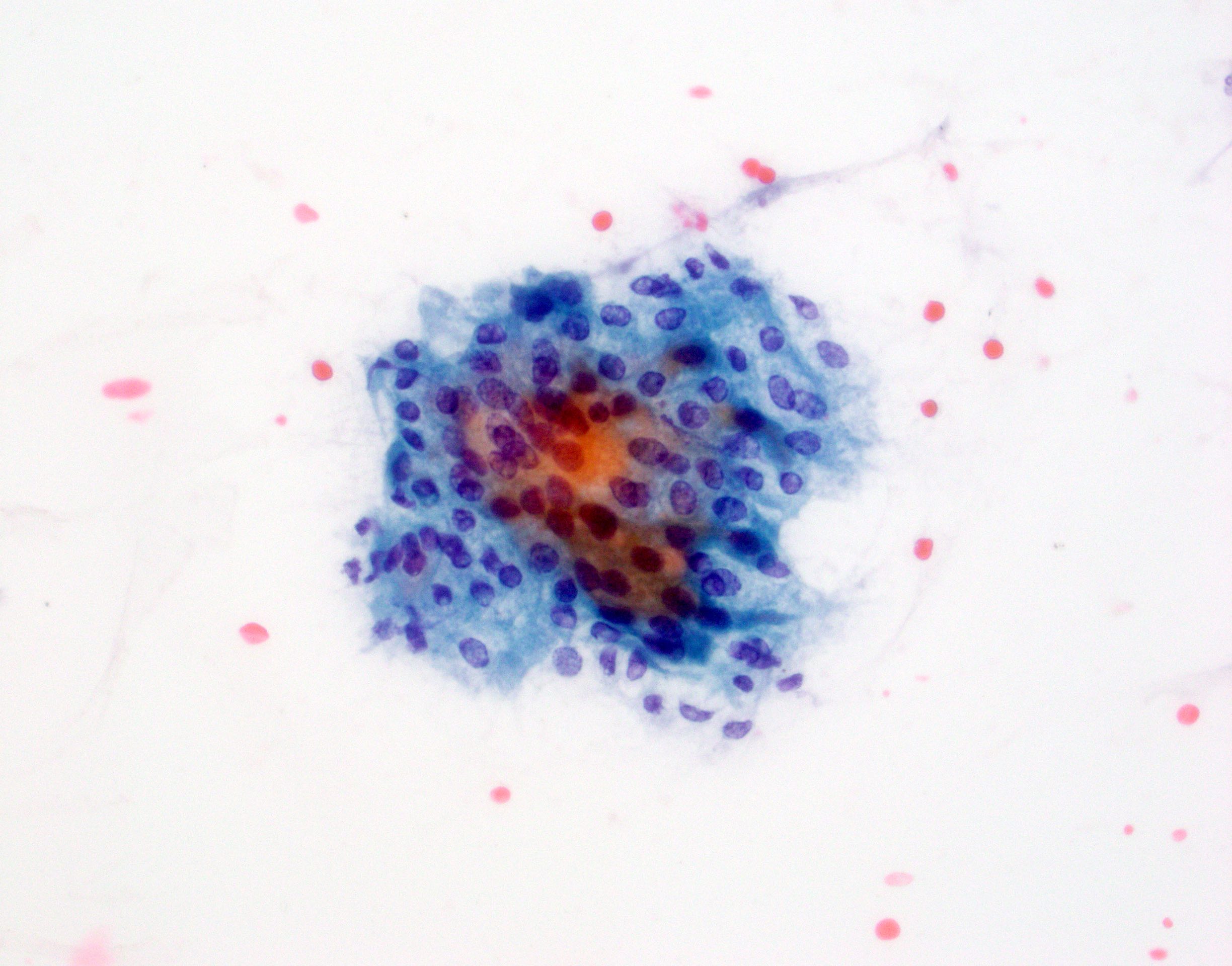
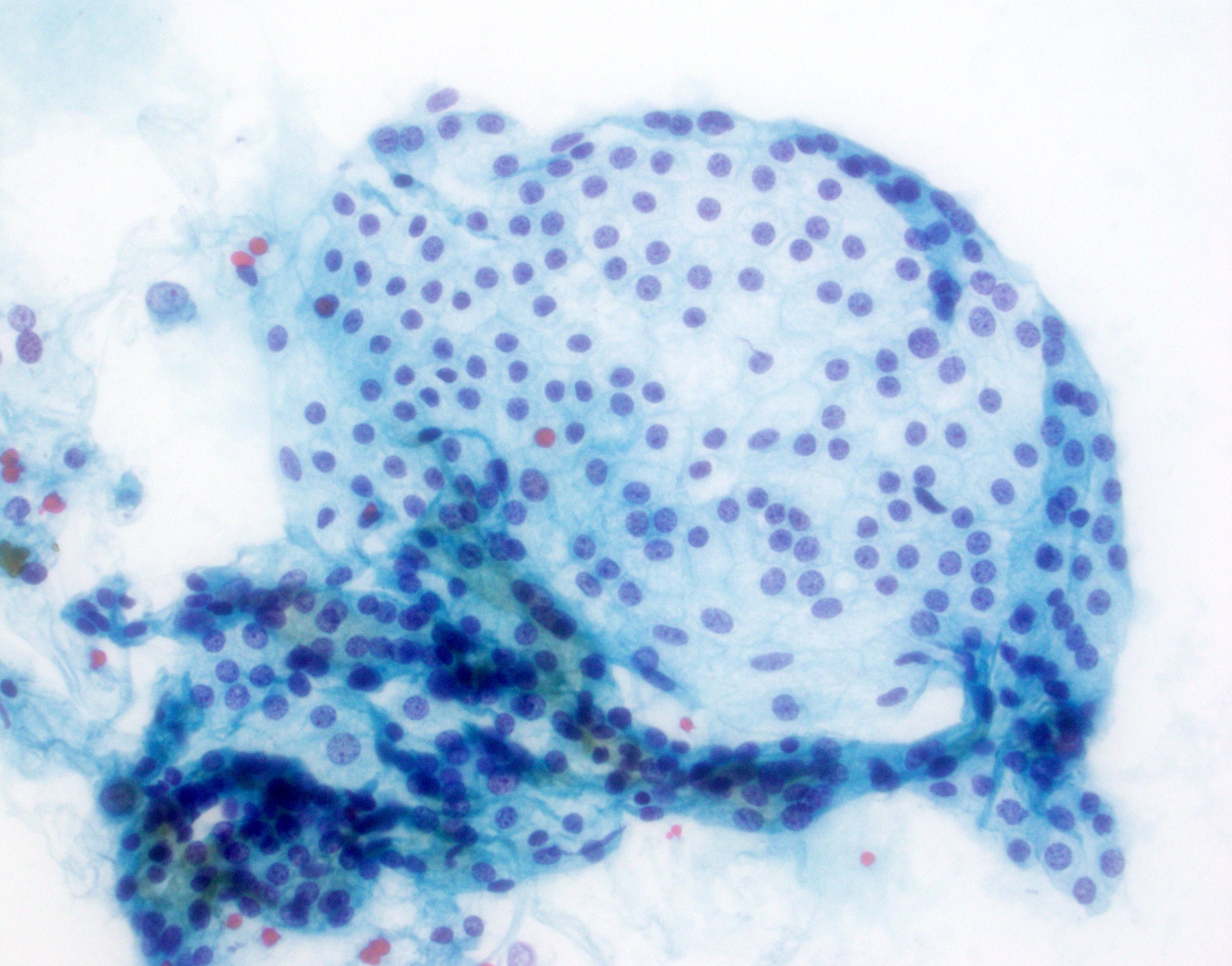
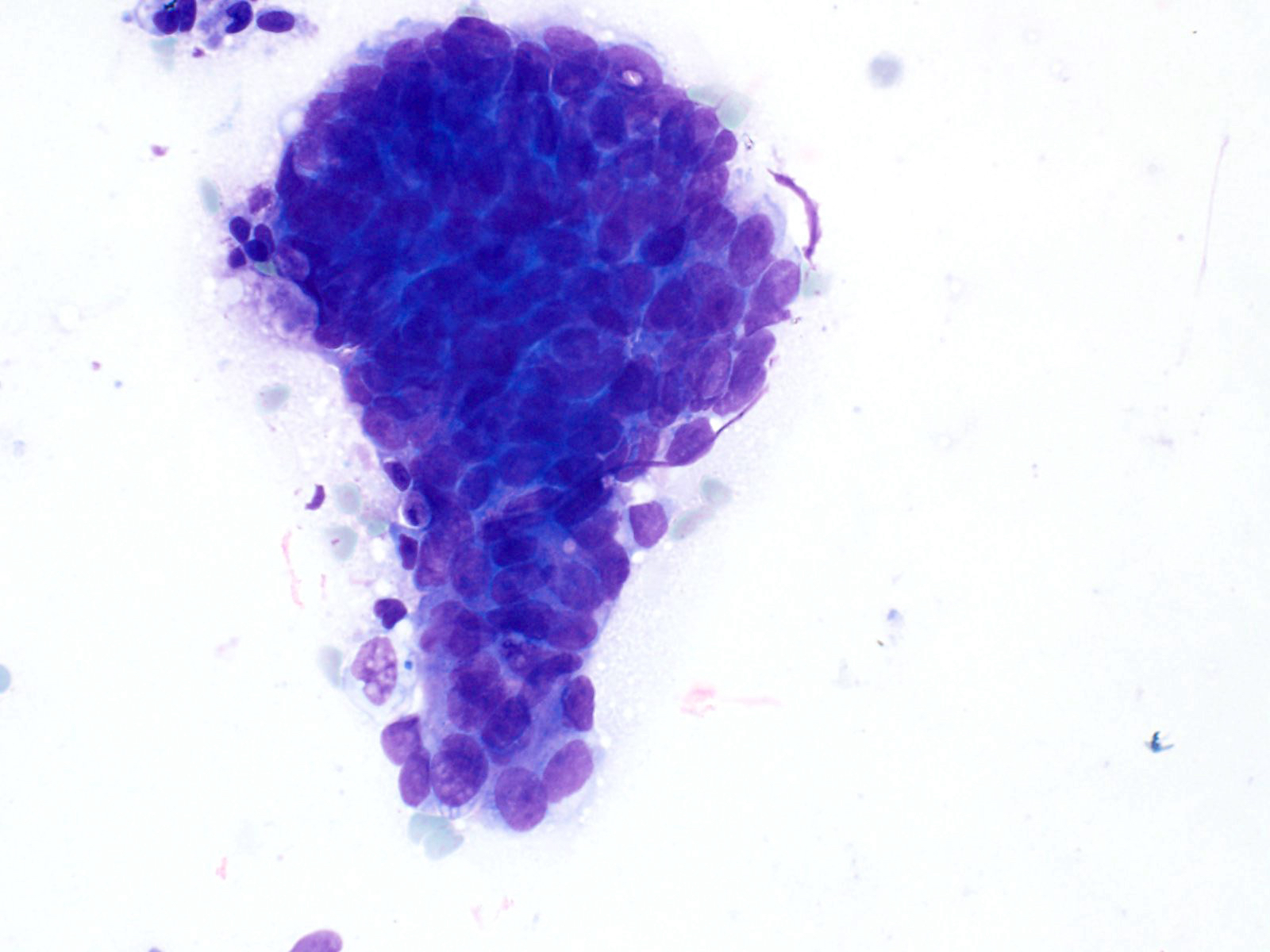
- Hypercellular / moderately cellular neoplasm, monomorphic nuclei with prominent nucleoli (Diagn Cytopathol 2017;45:247)
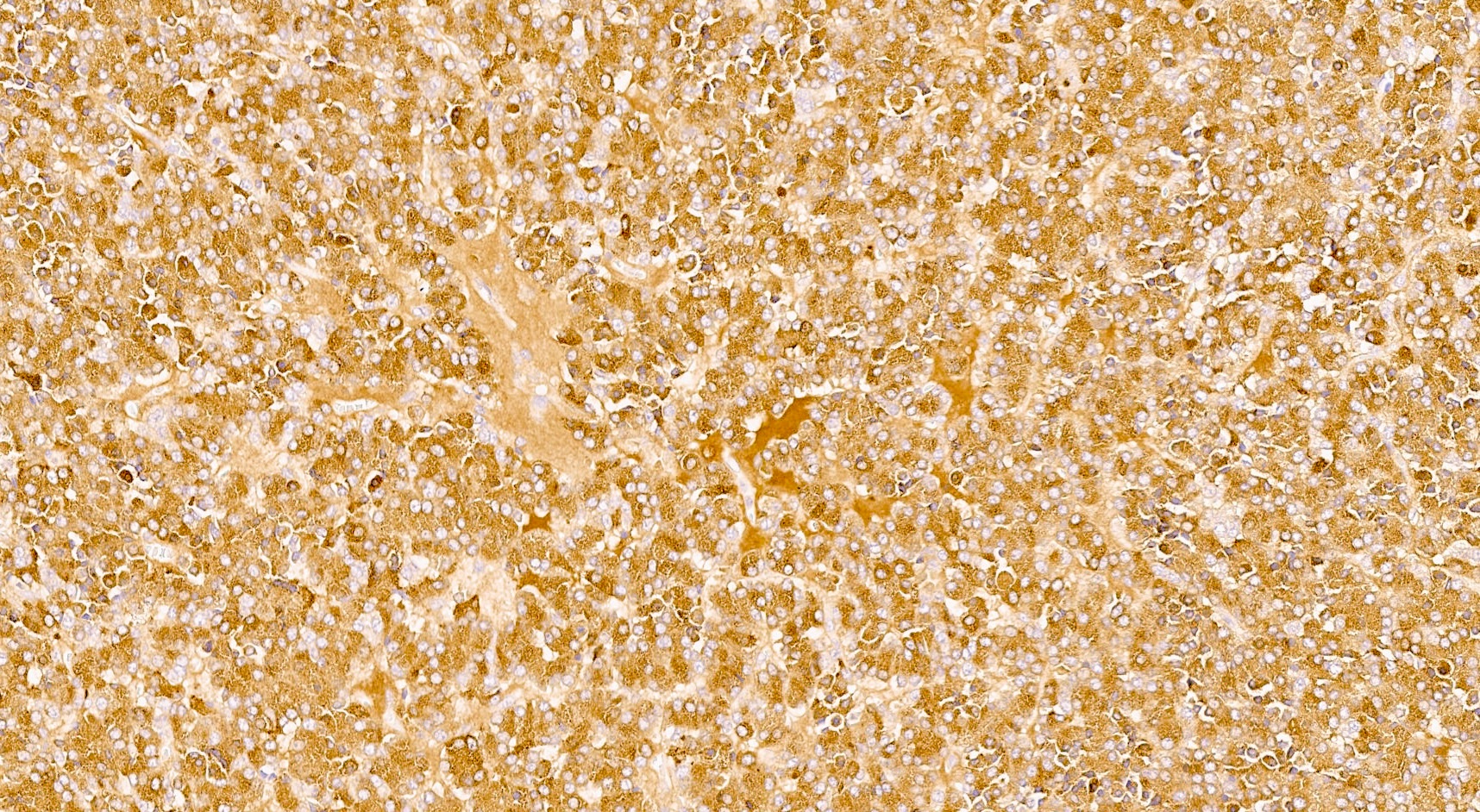
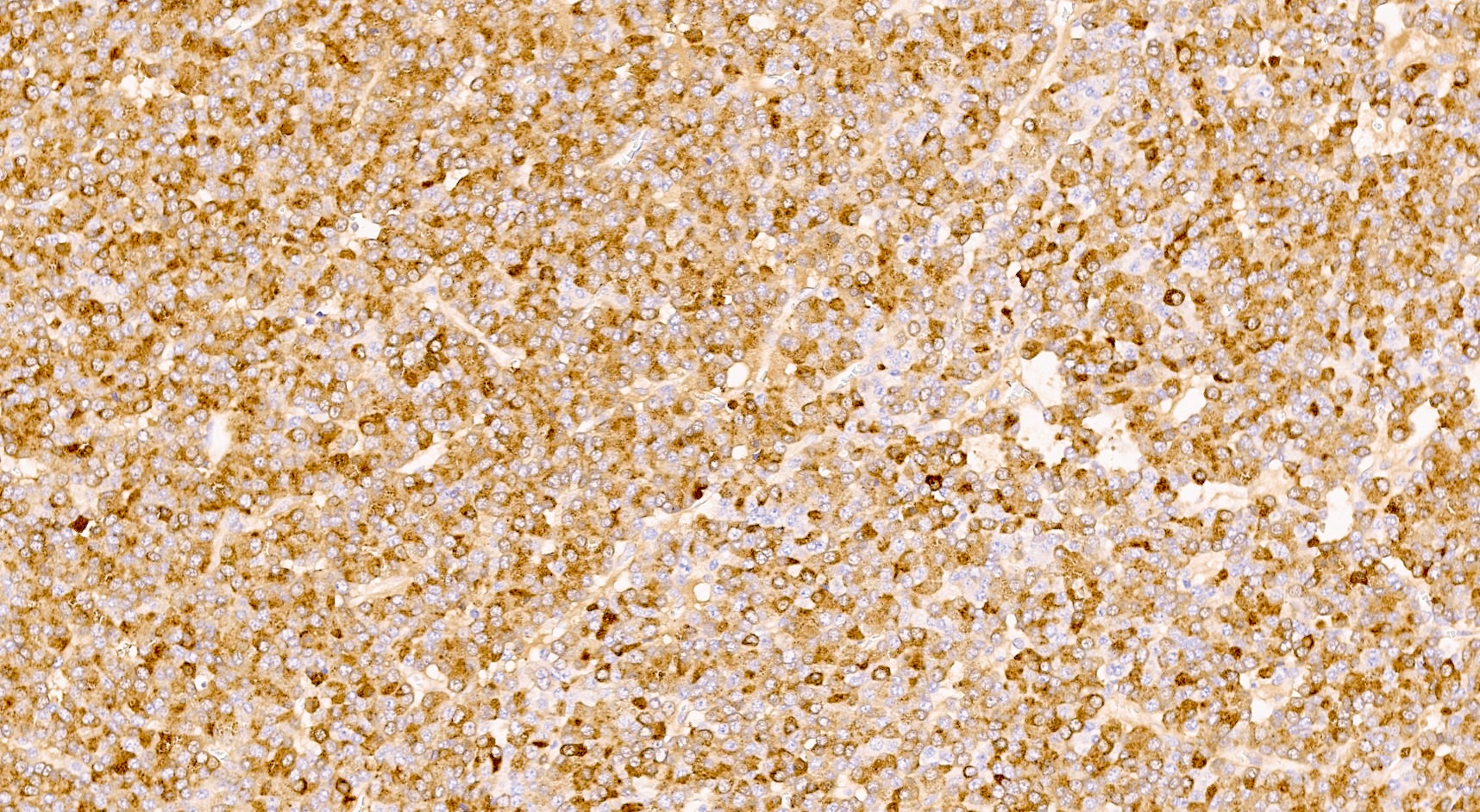
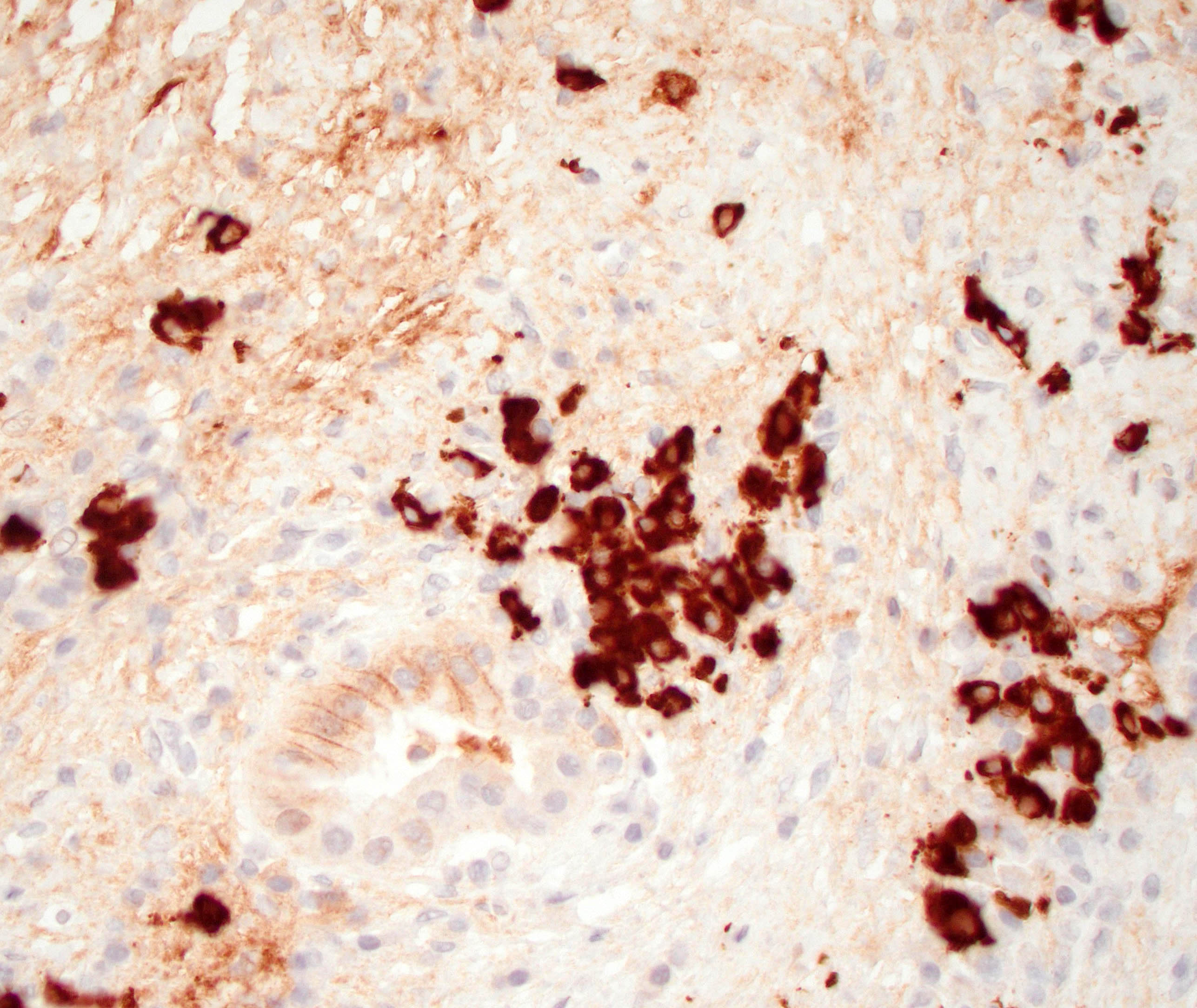
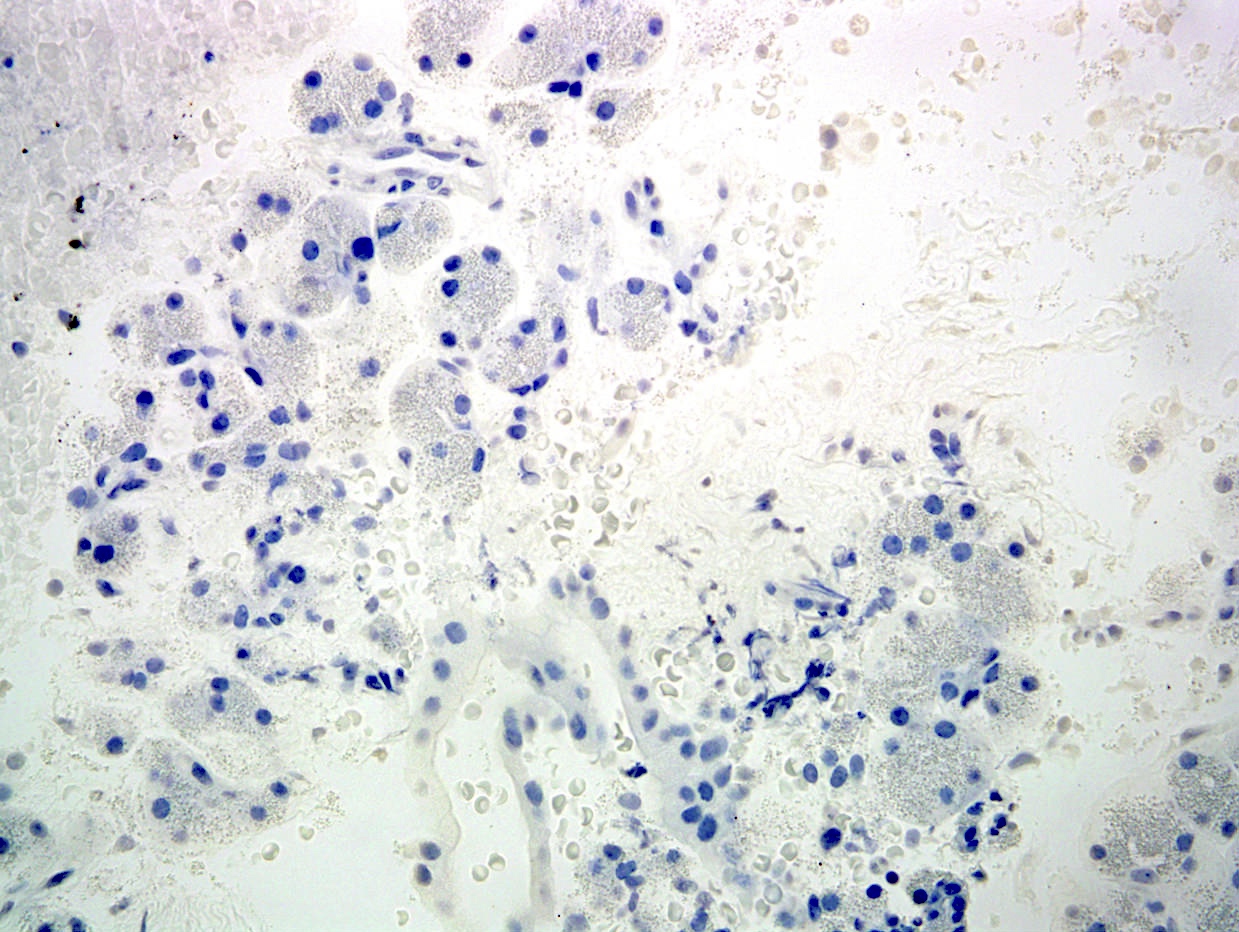
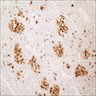
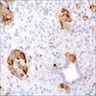
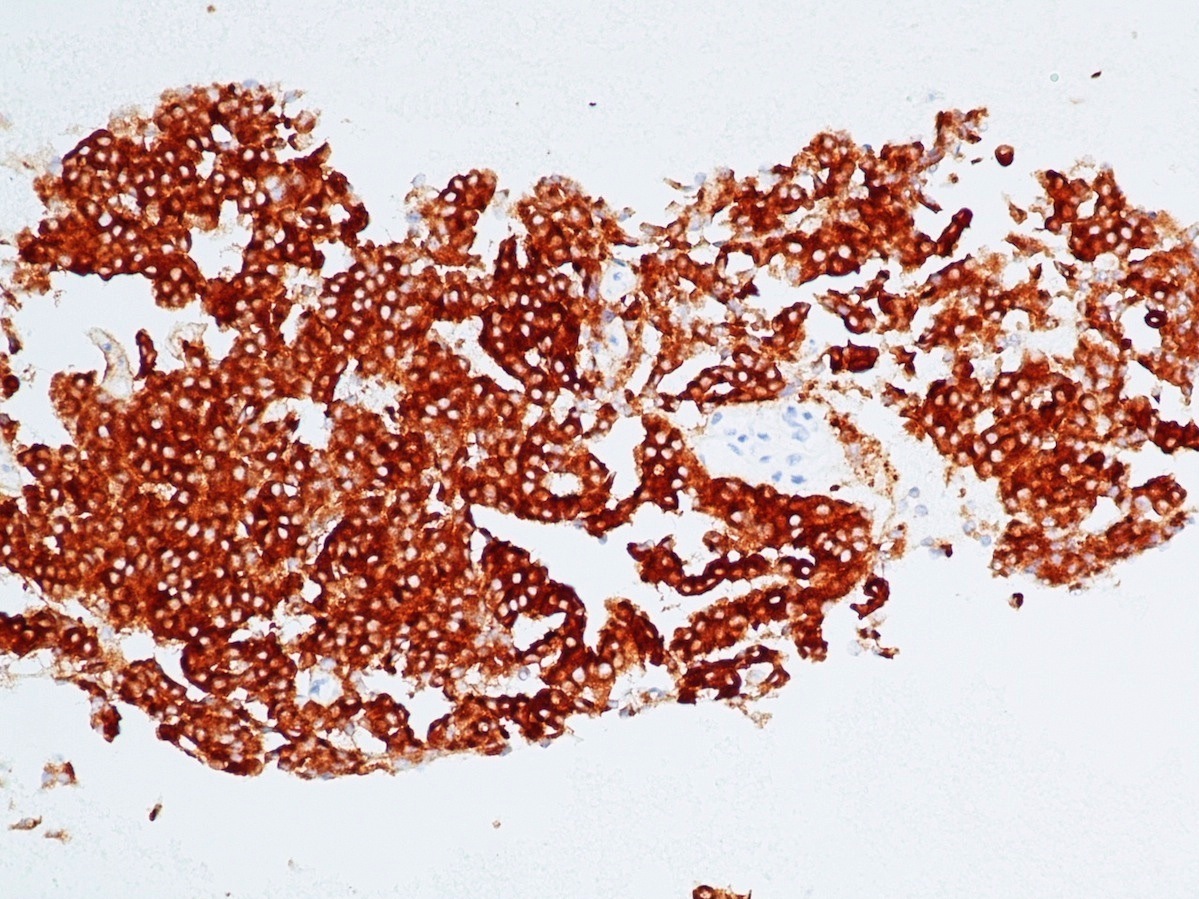
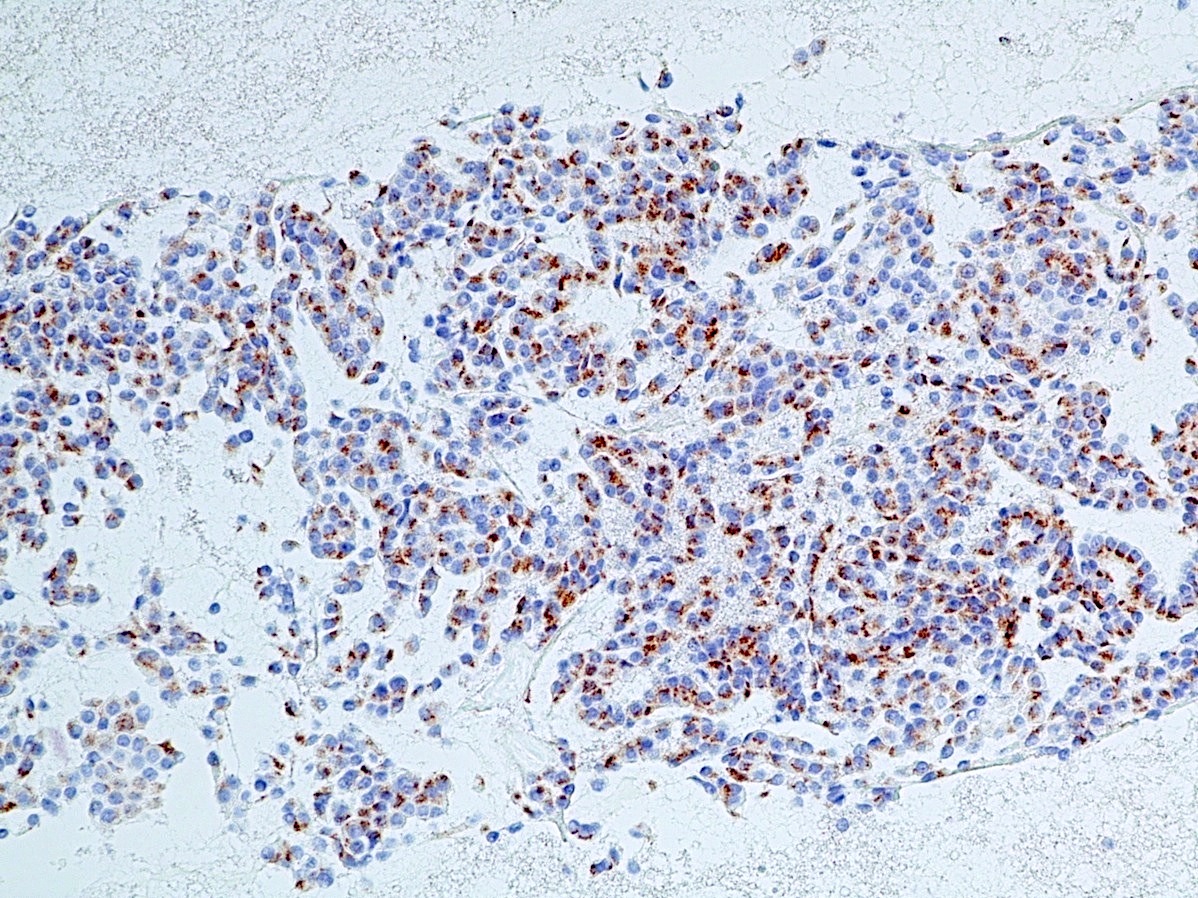
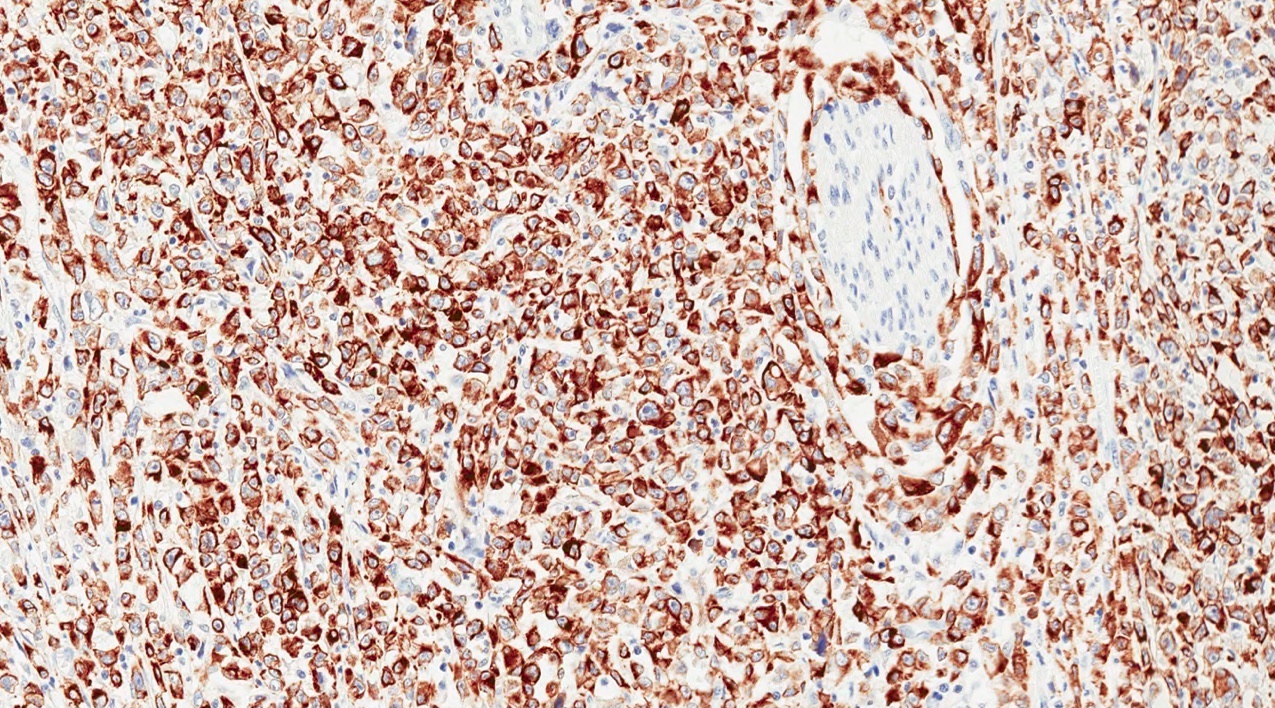
Positive stains
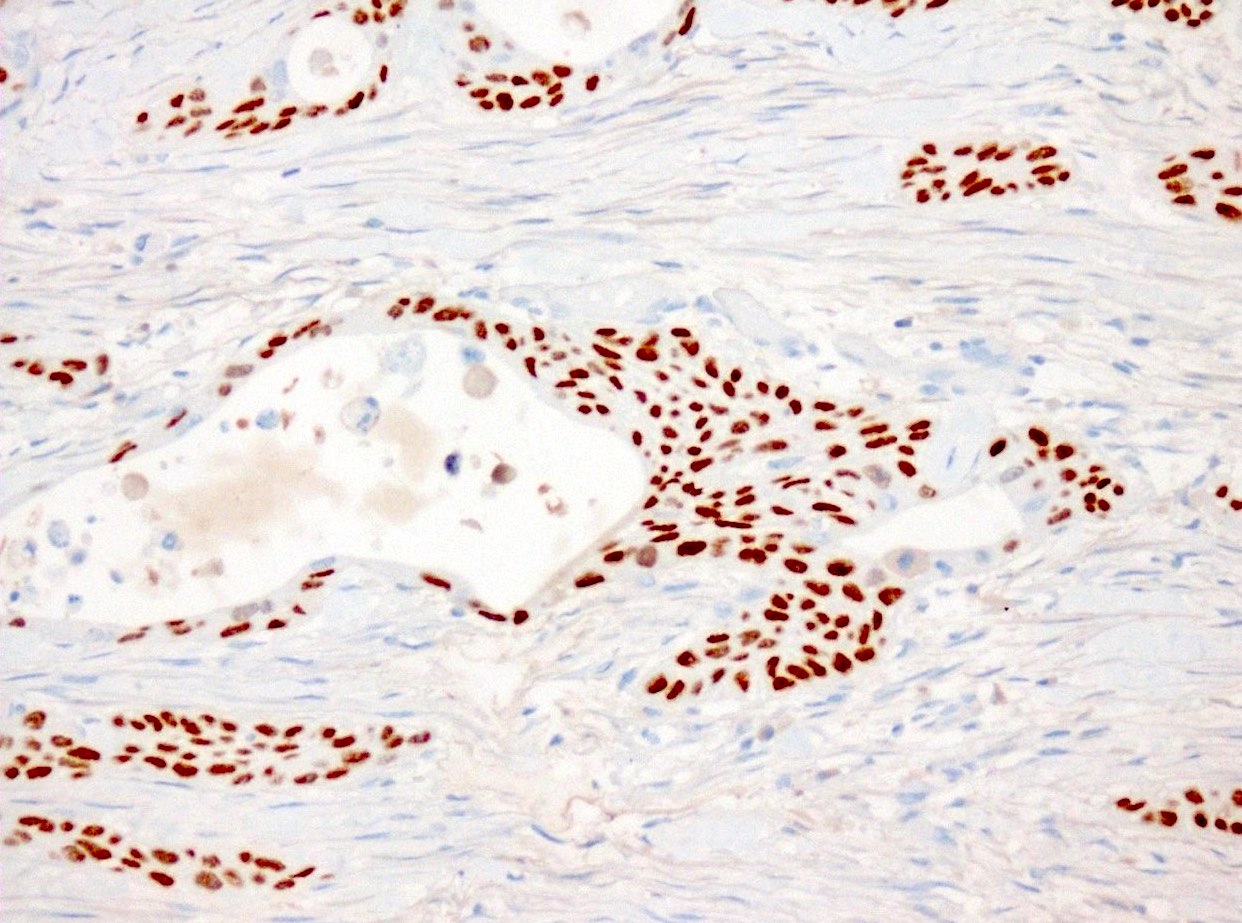
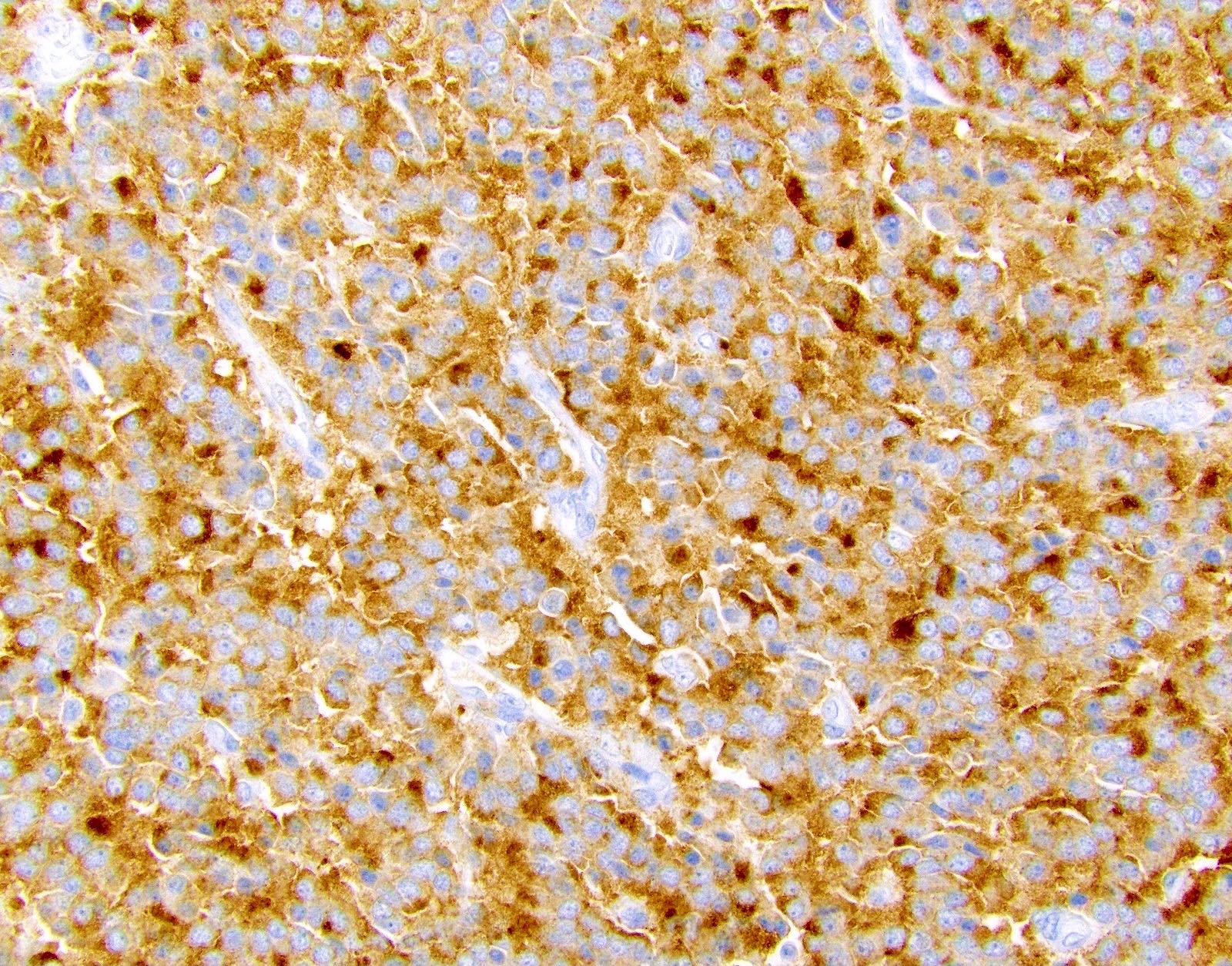
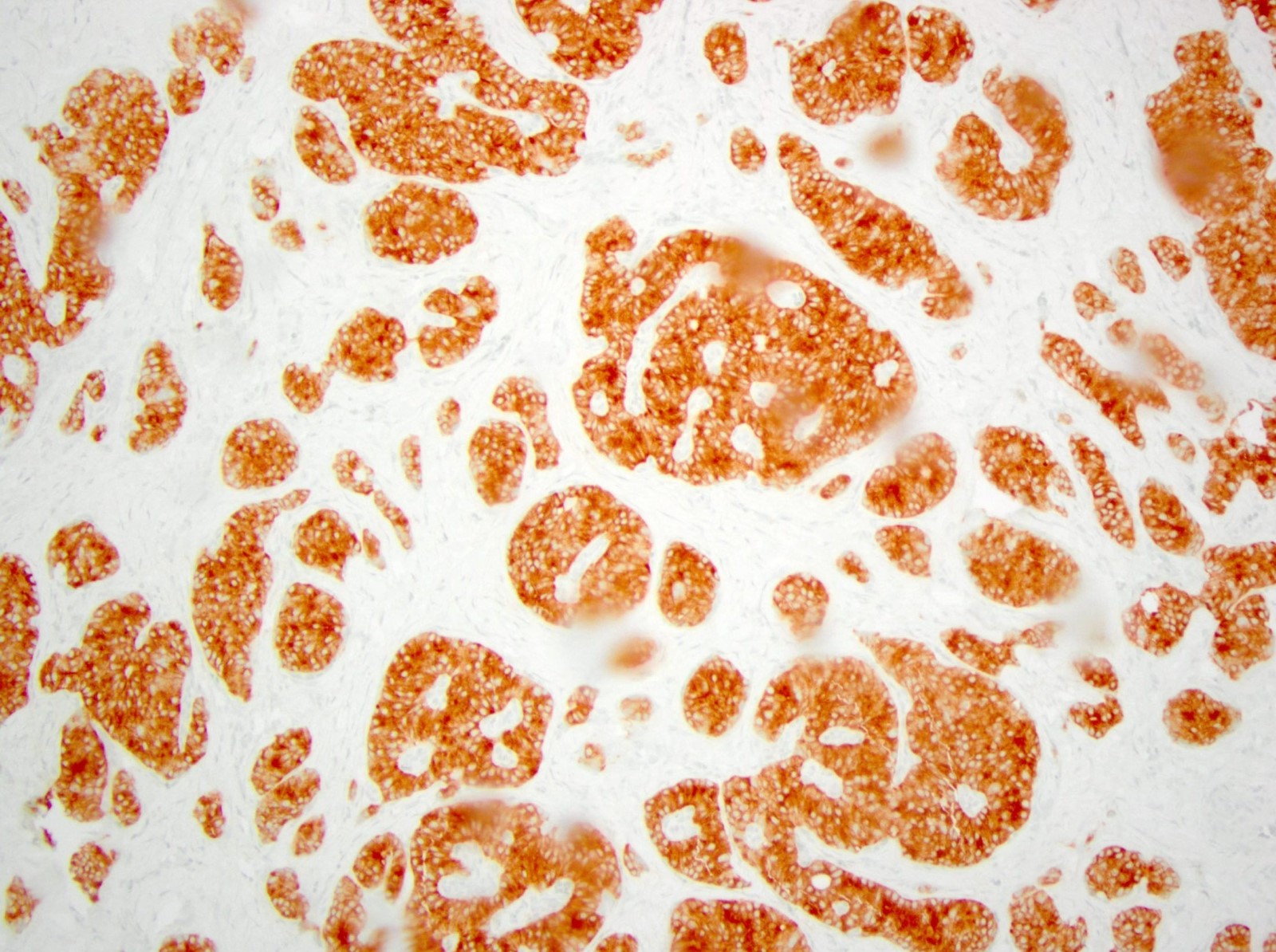
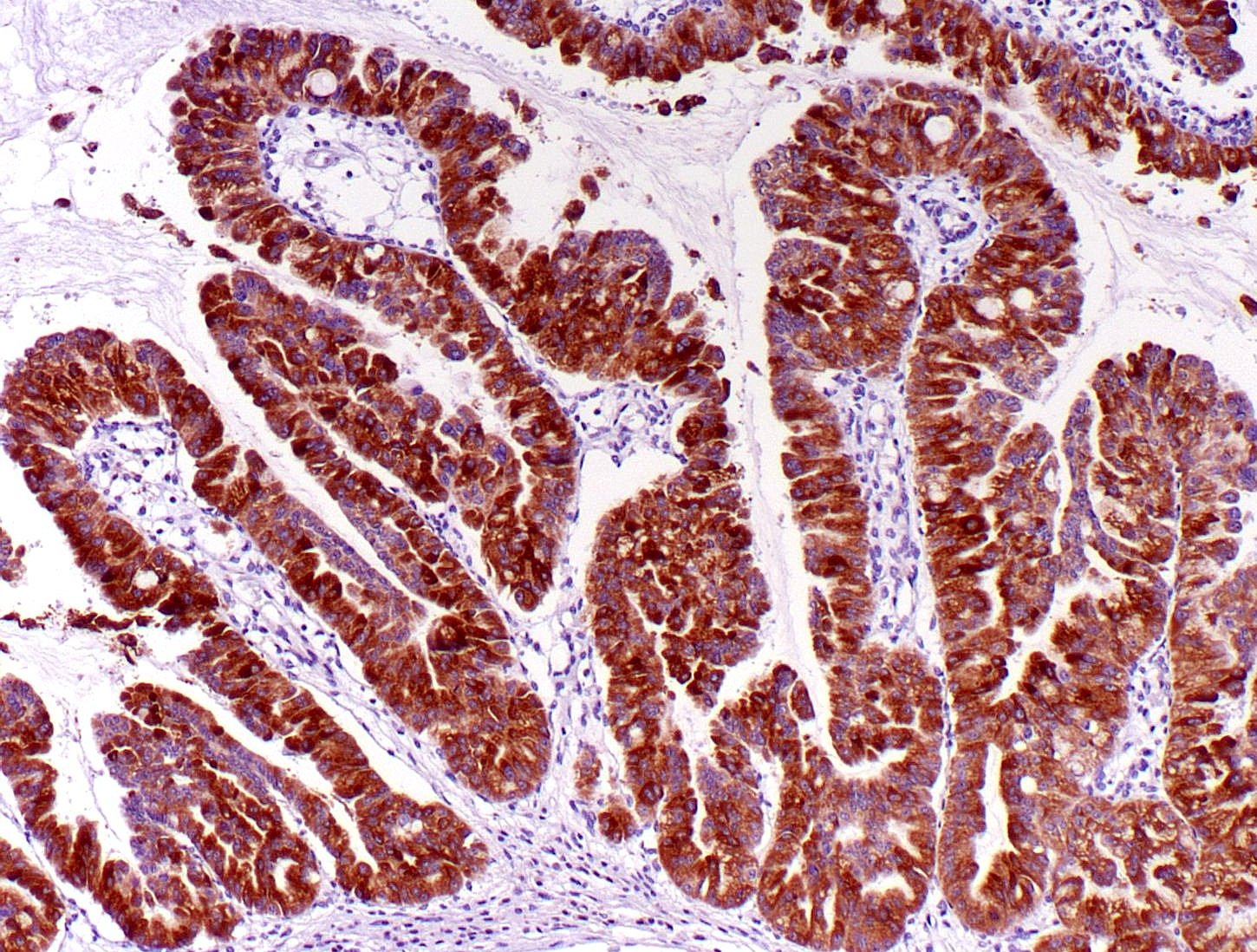
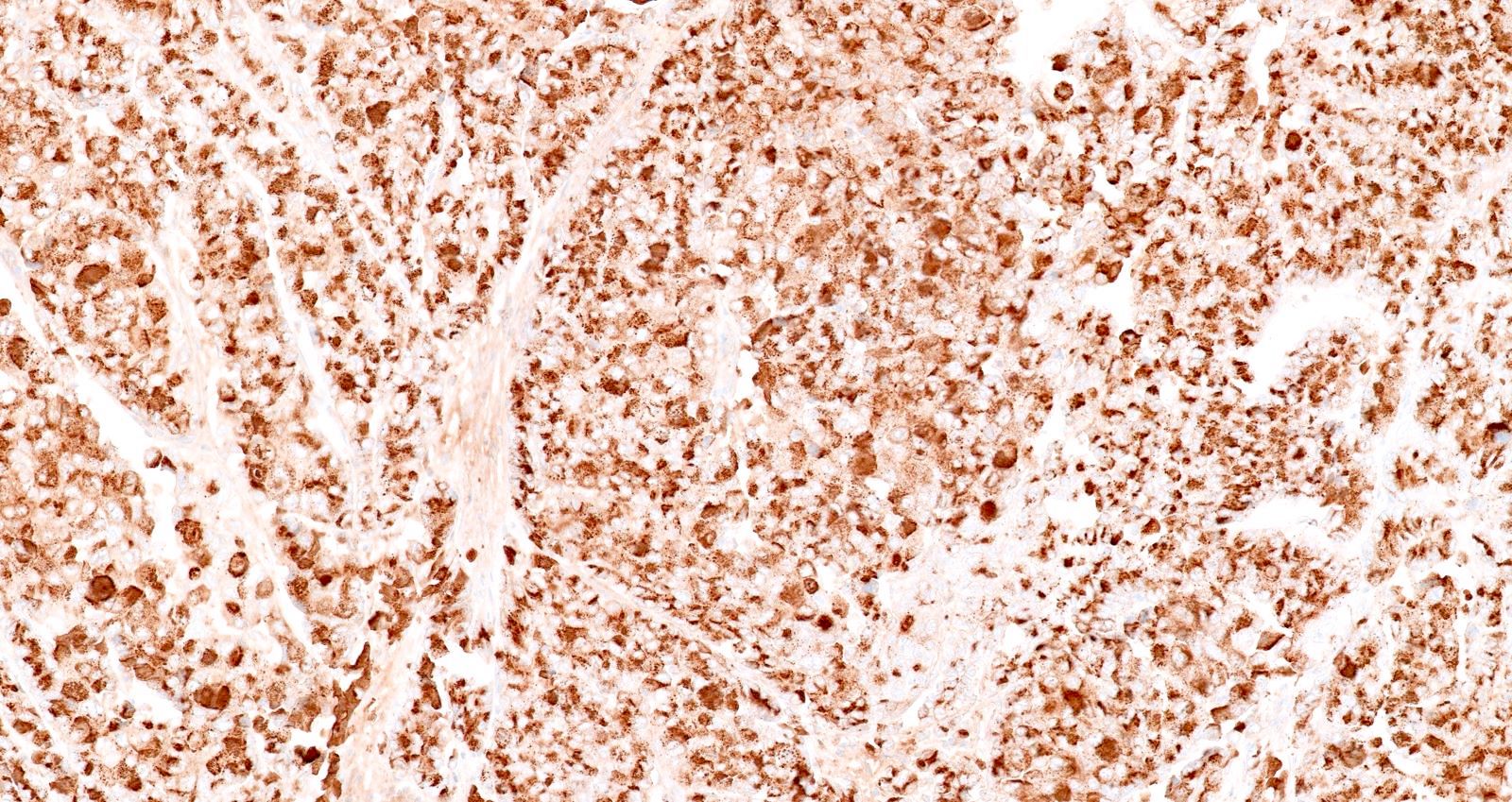
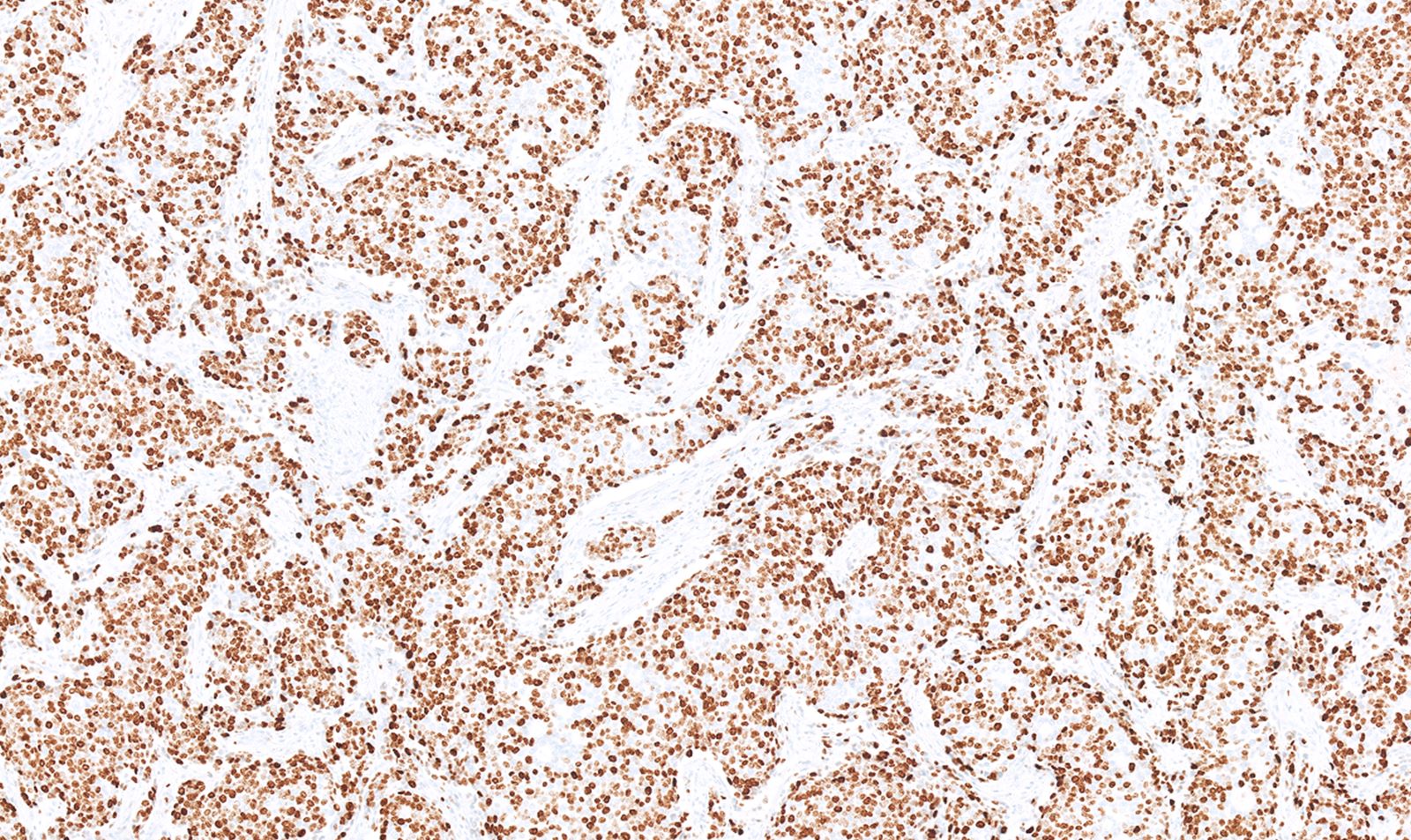
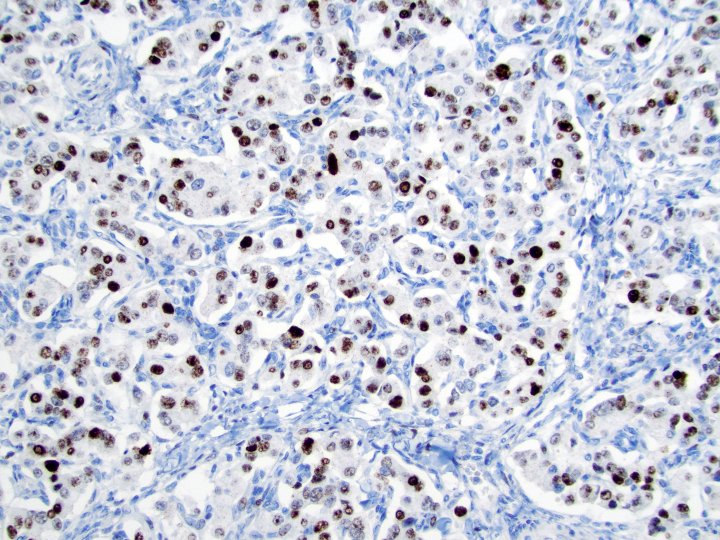
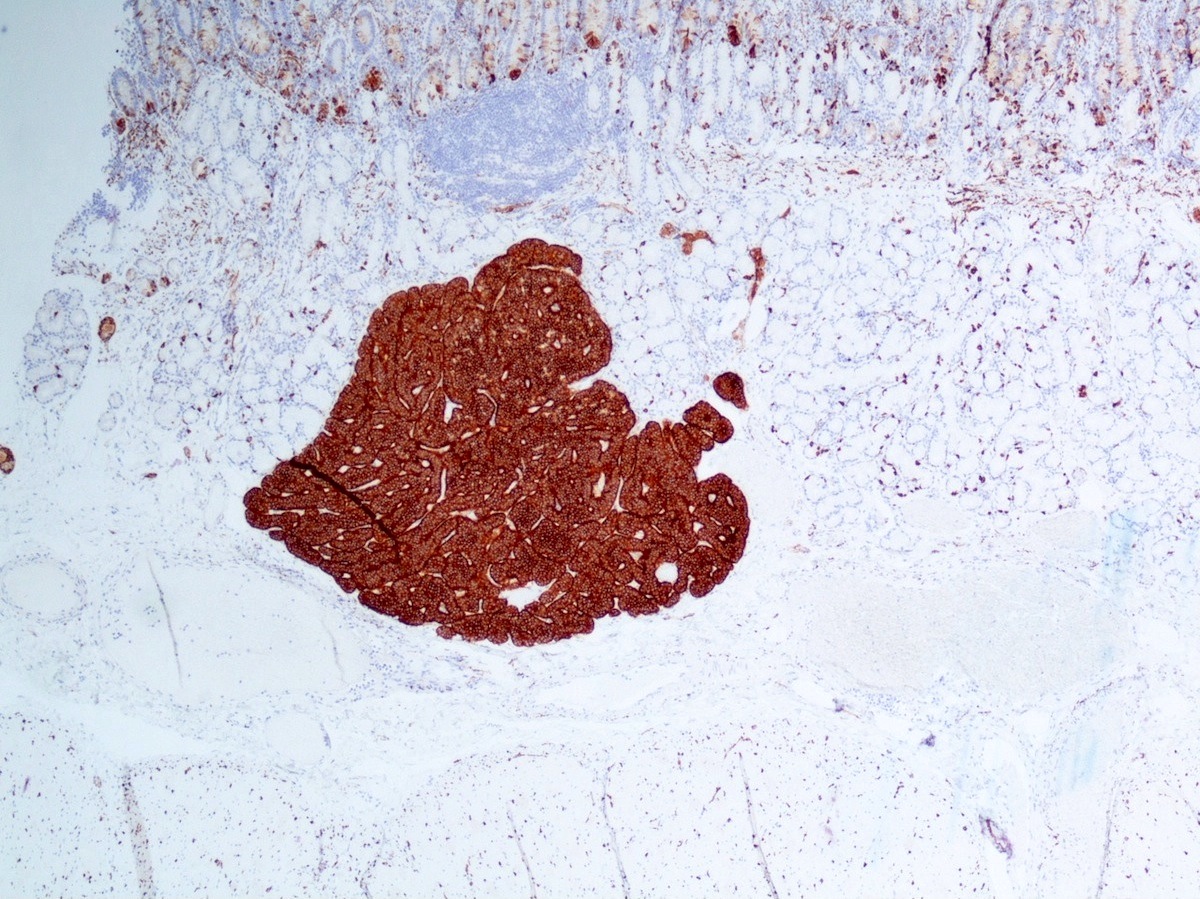
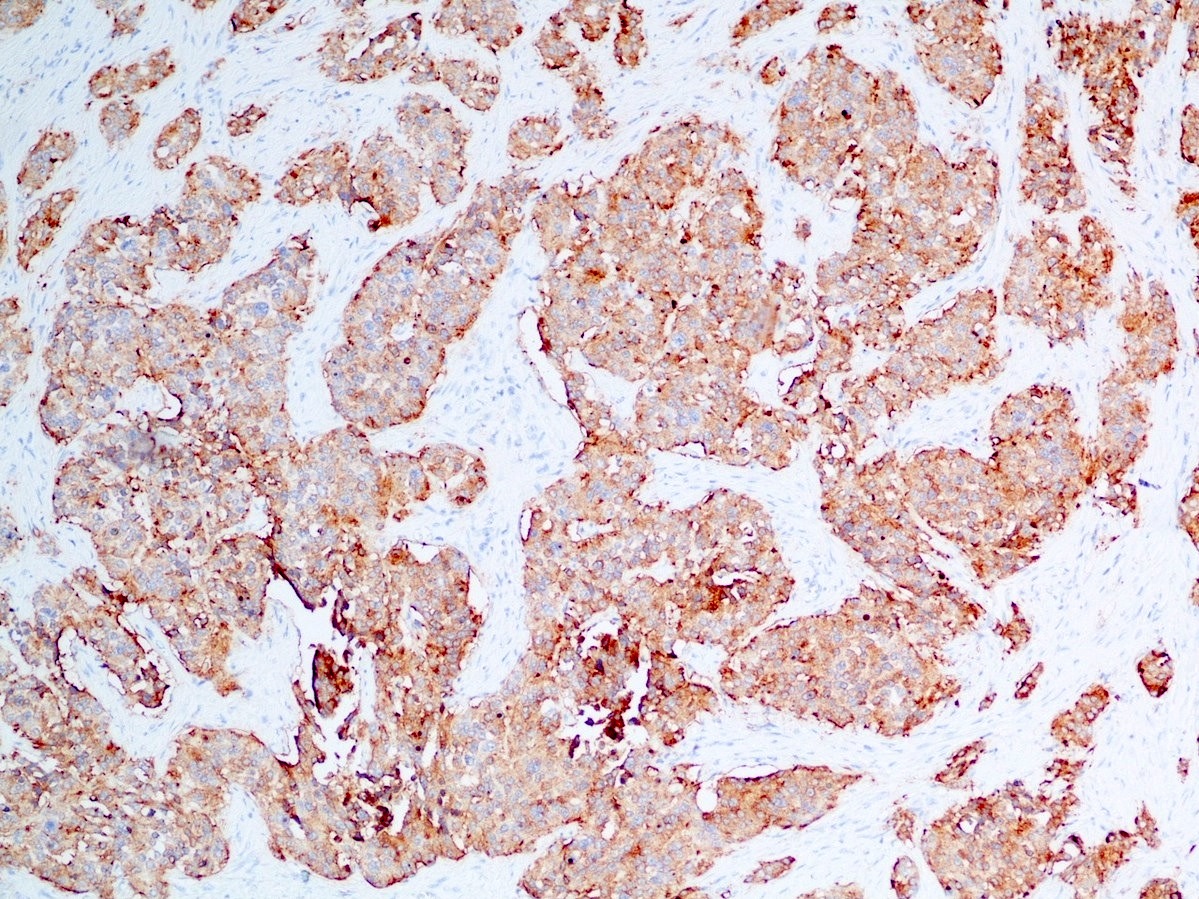
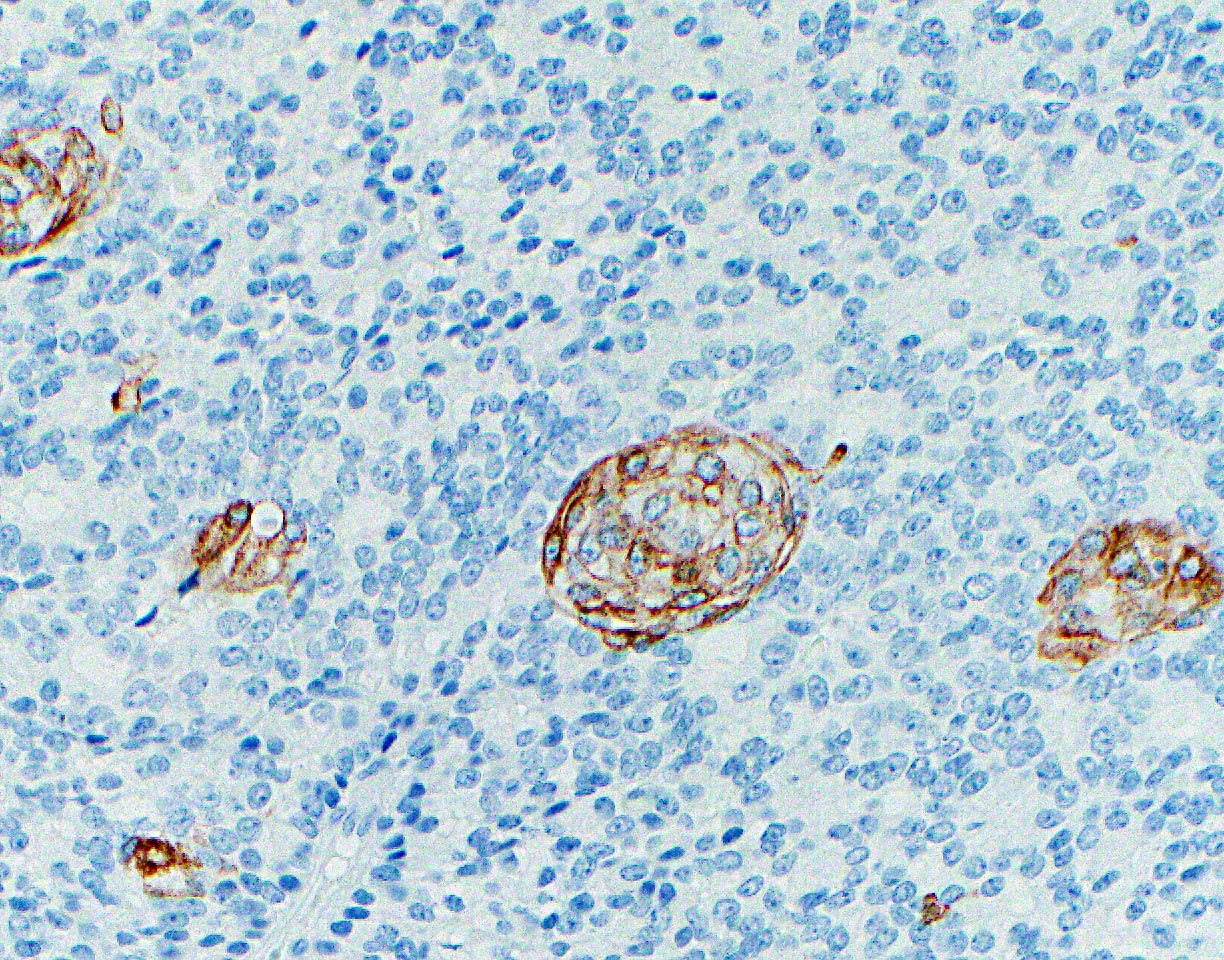
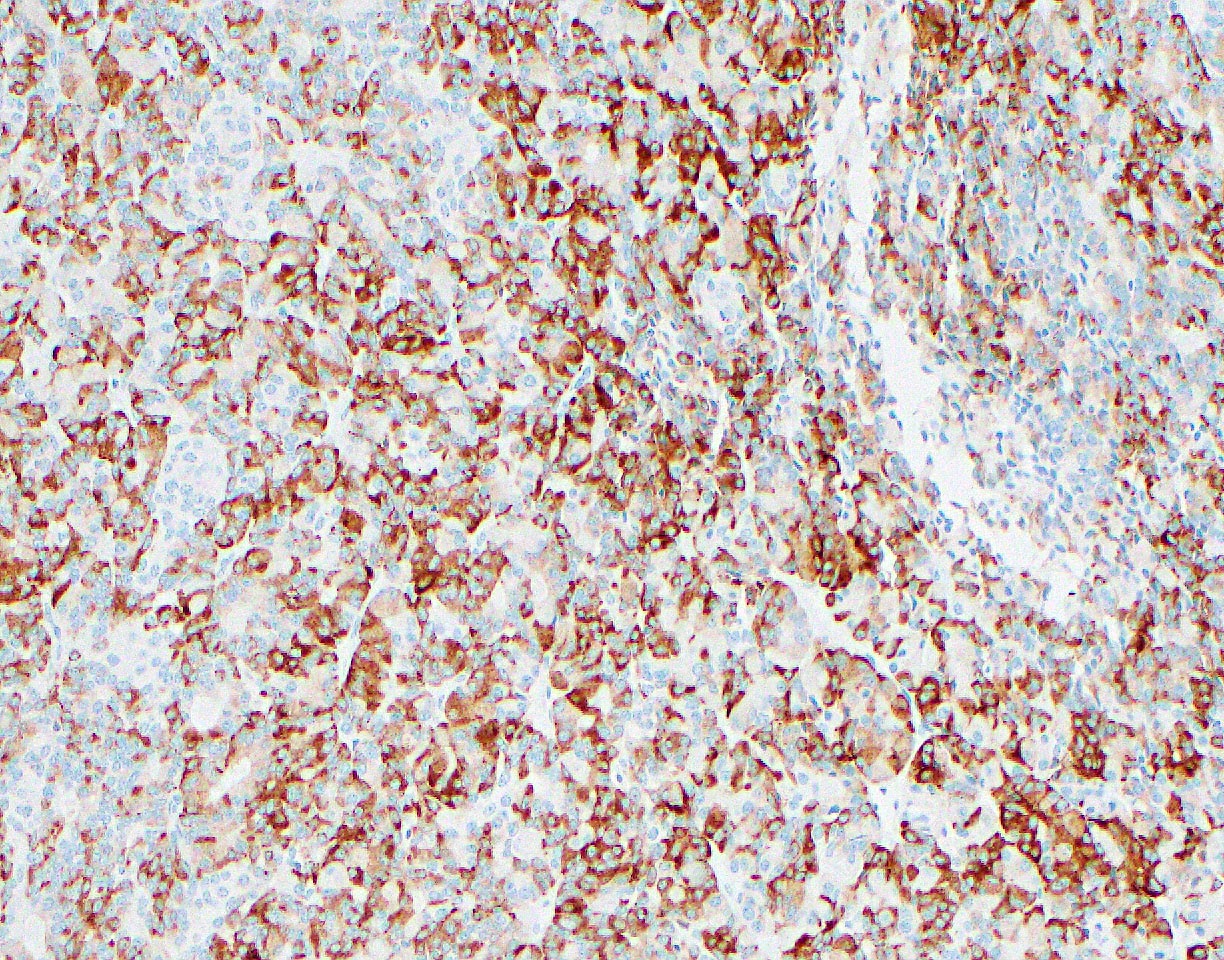
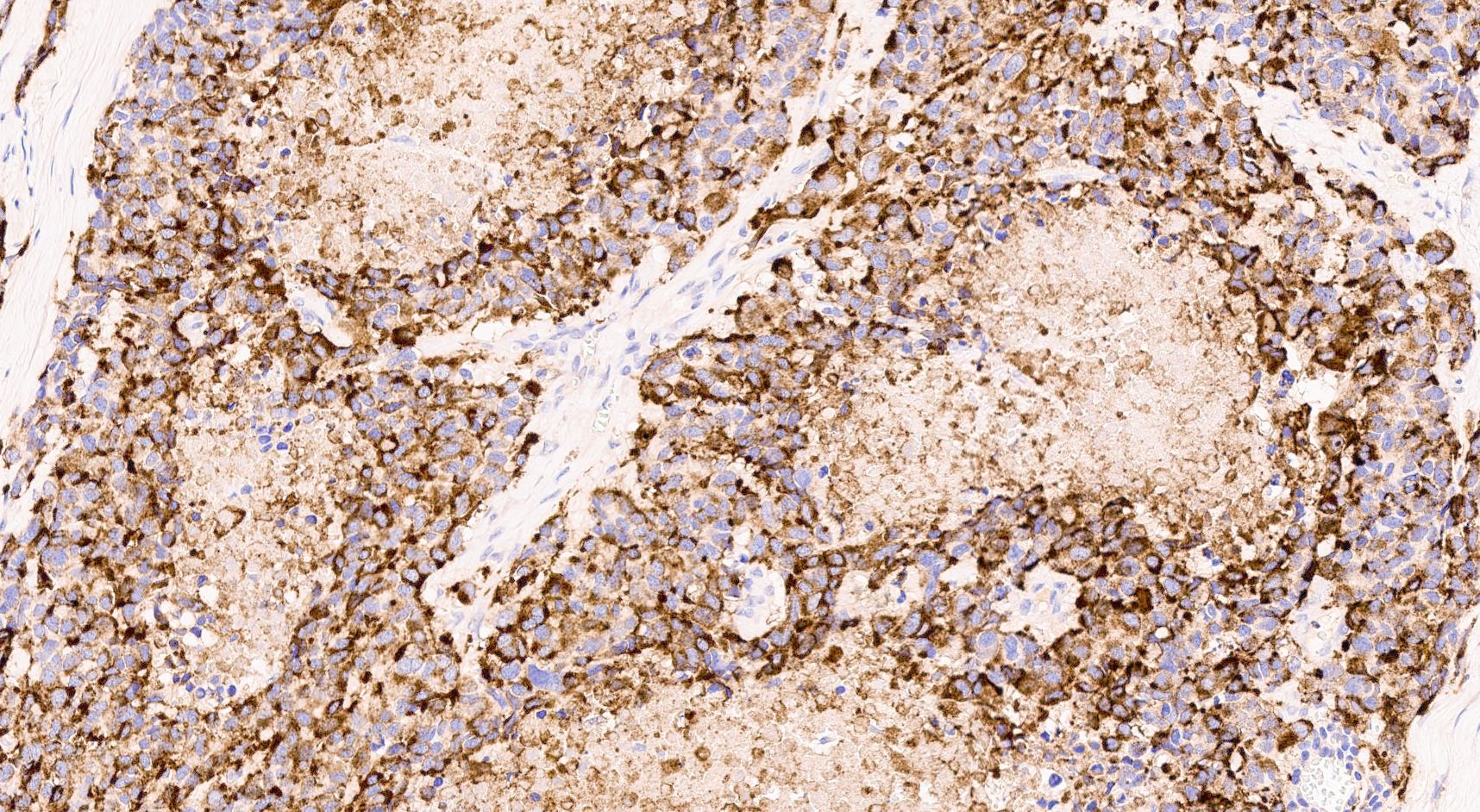
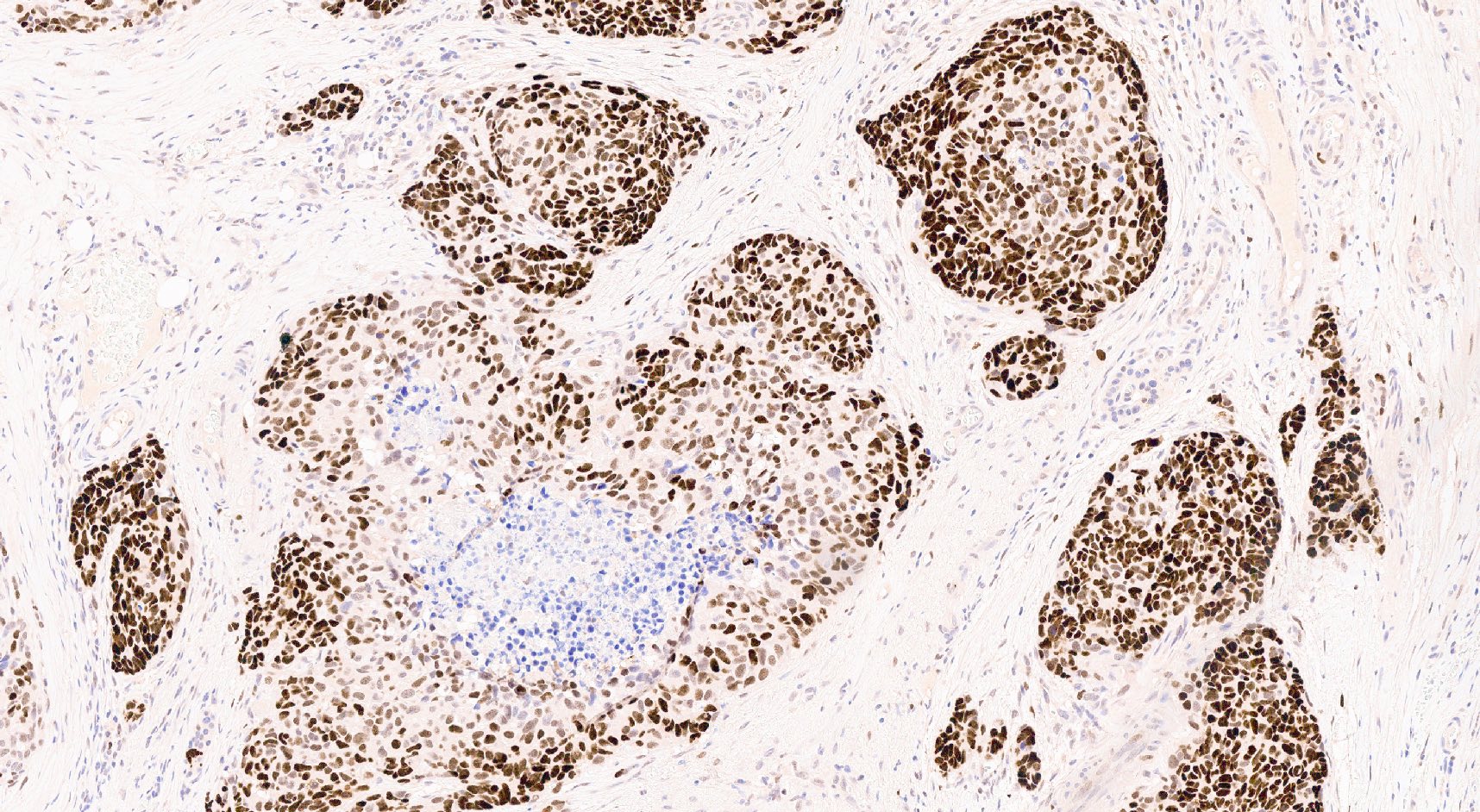
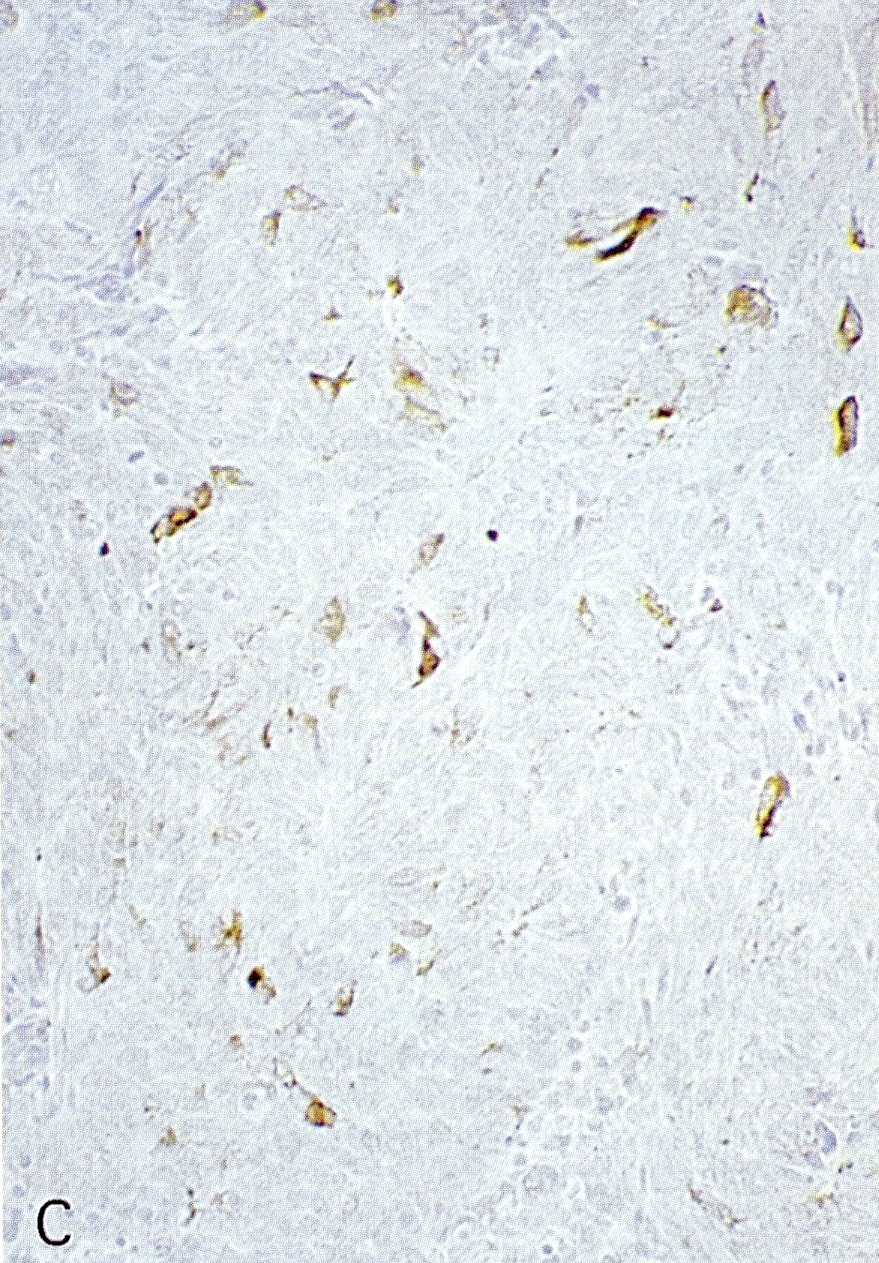
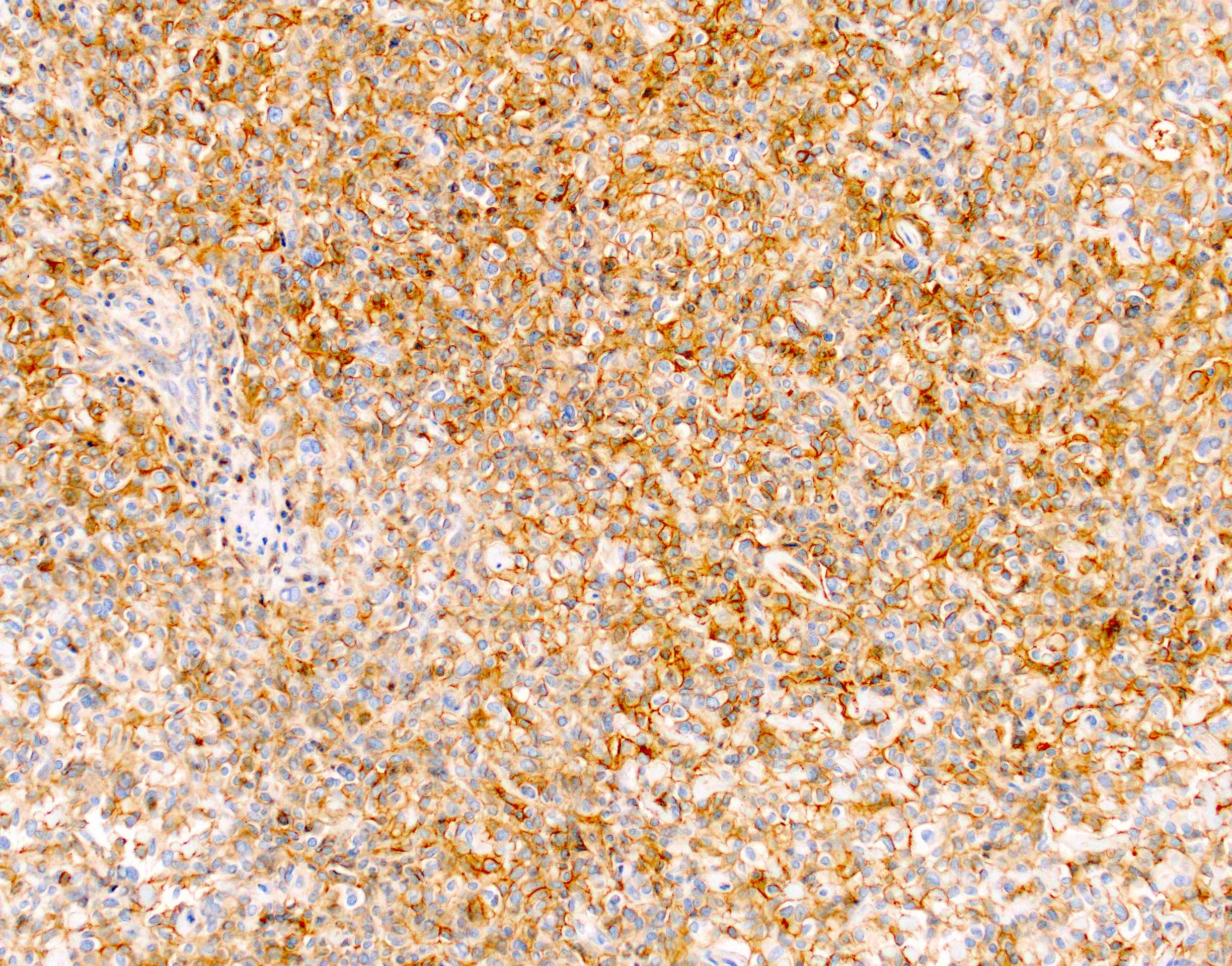
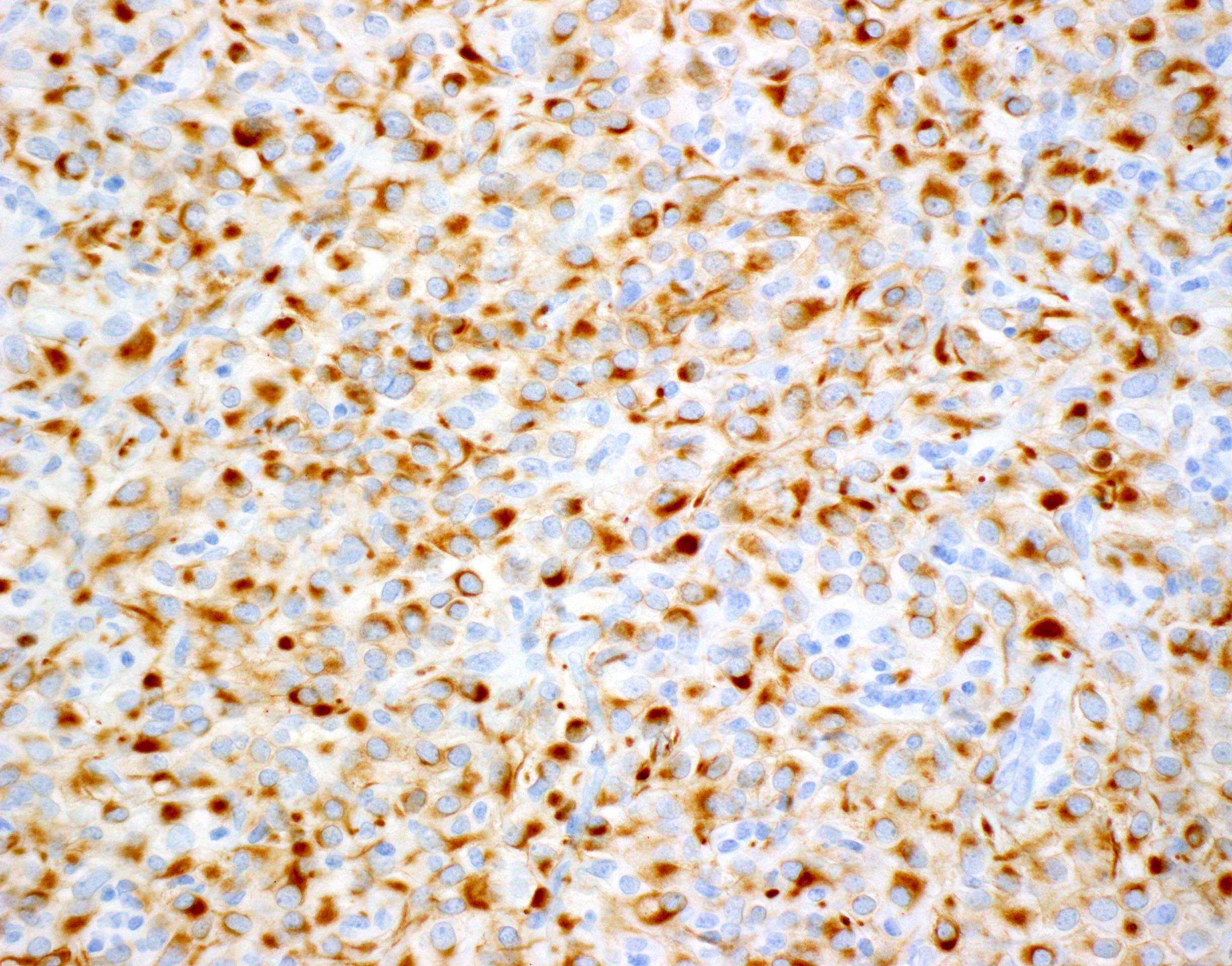
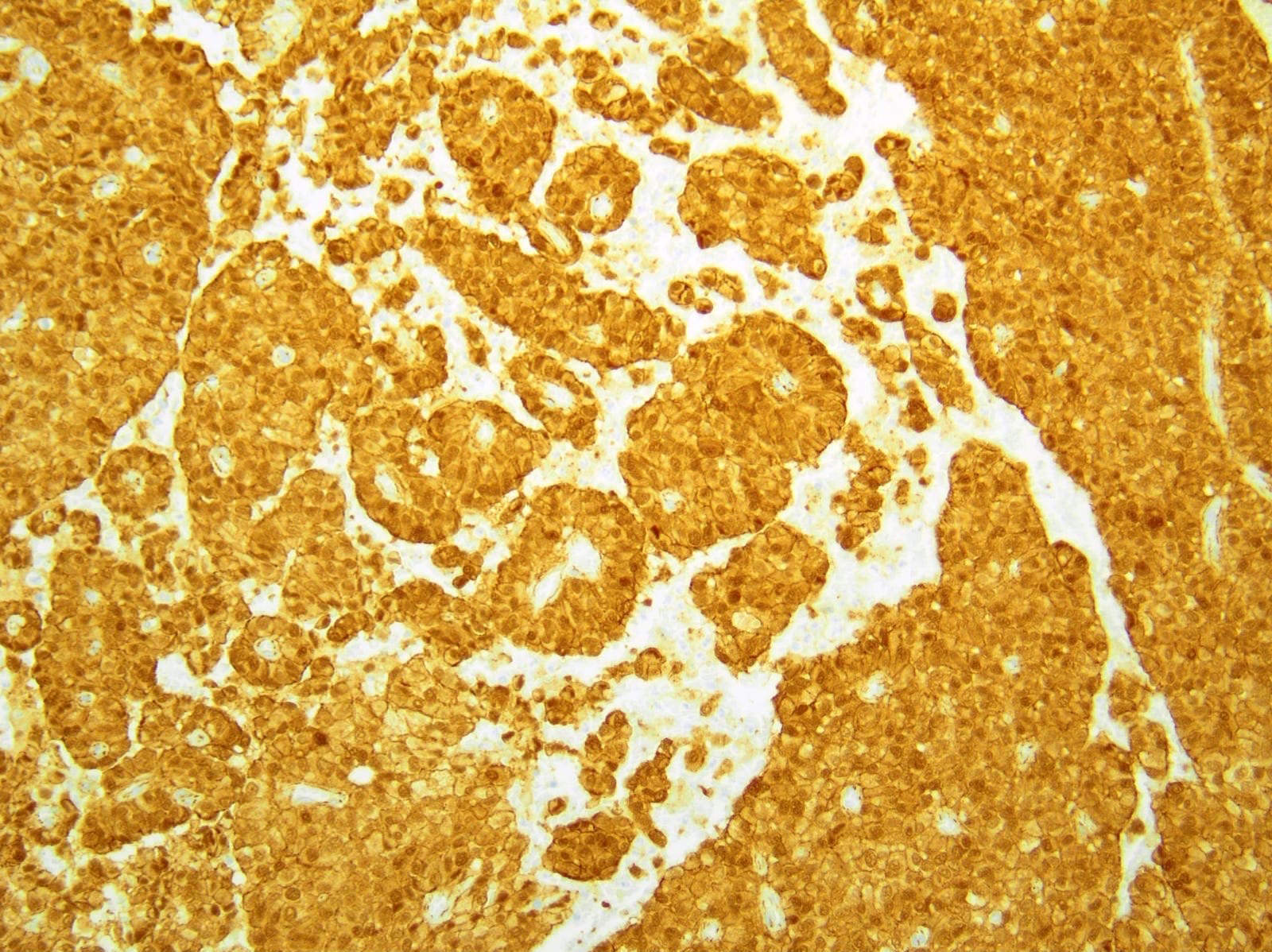
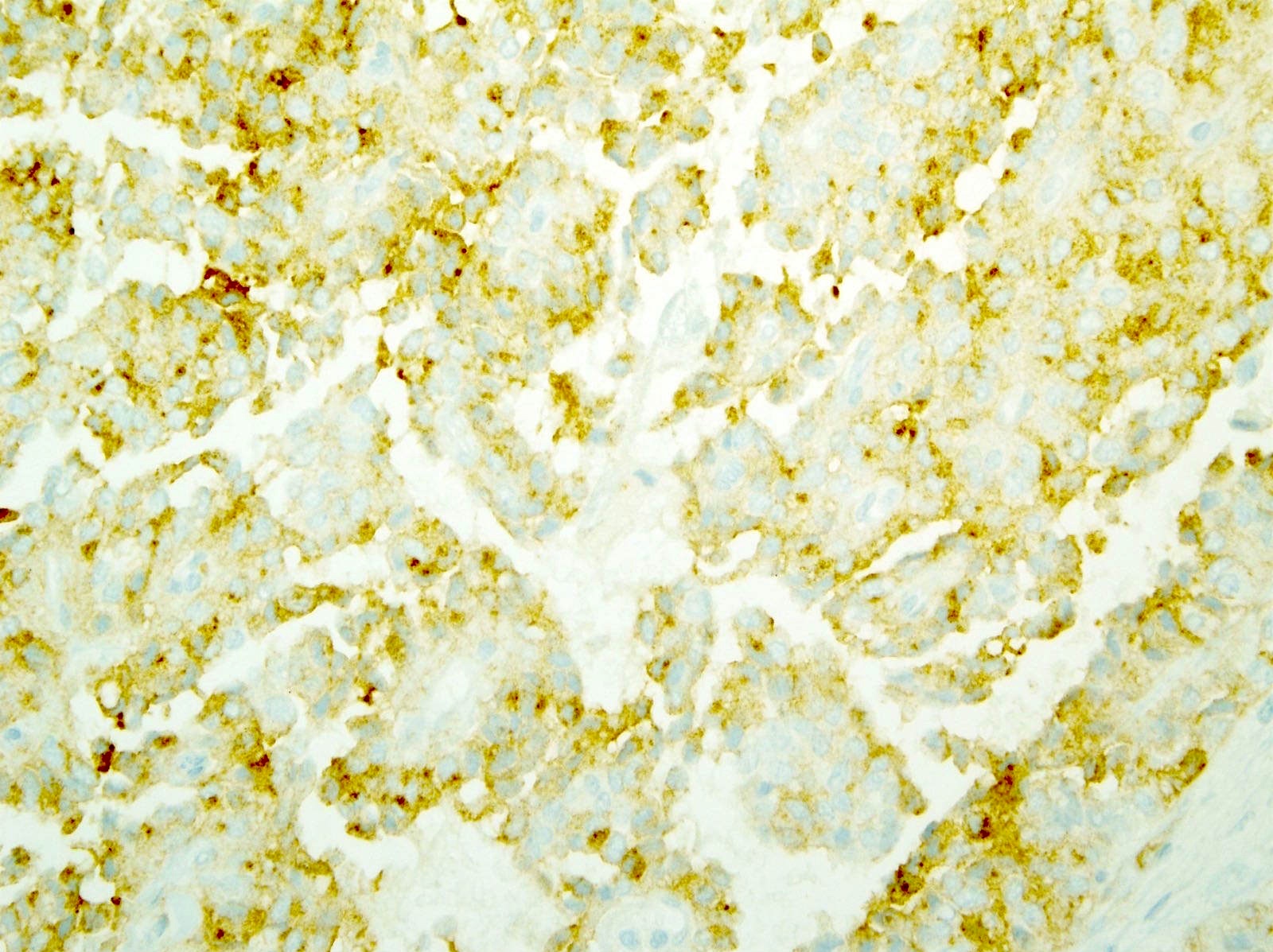
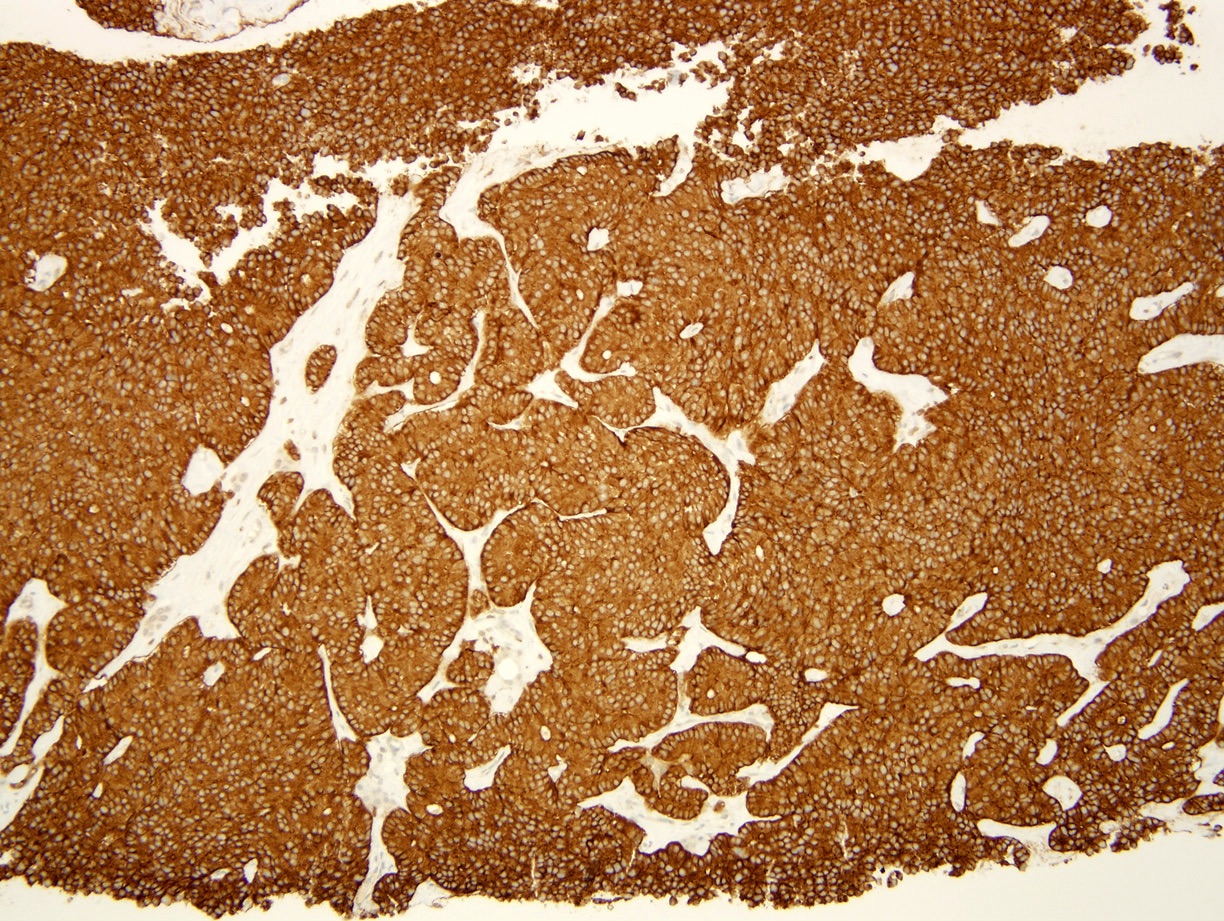
- Keratins (particularly CK7 and CK8 / CK18 / CK19), BCL10 (particularly the clone 331.3) and trypsin (Virchows Arch 2009;454:133, Cancer Cytopathol 2013;121:459, Pathologica 2020;112:210)
- Nuclear expression of beta catenin and CD200 expression is found in about 10% of cases (Am J Surg Pathol 2012;36:1782, Virchows Arch 2019;474:105)
Negative stains
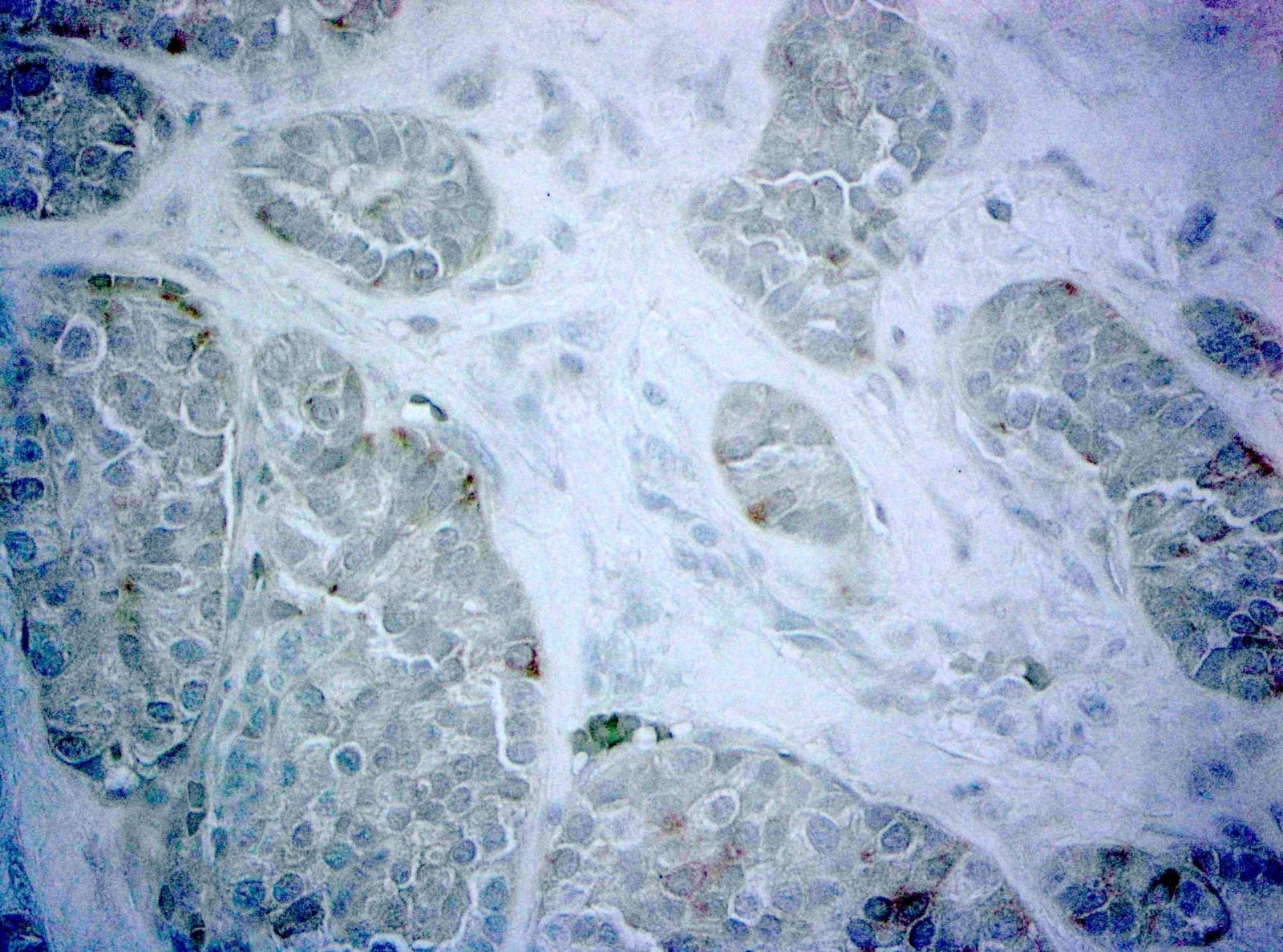
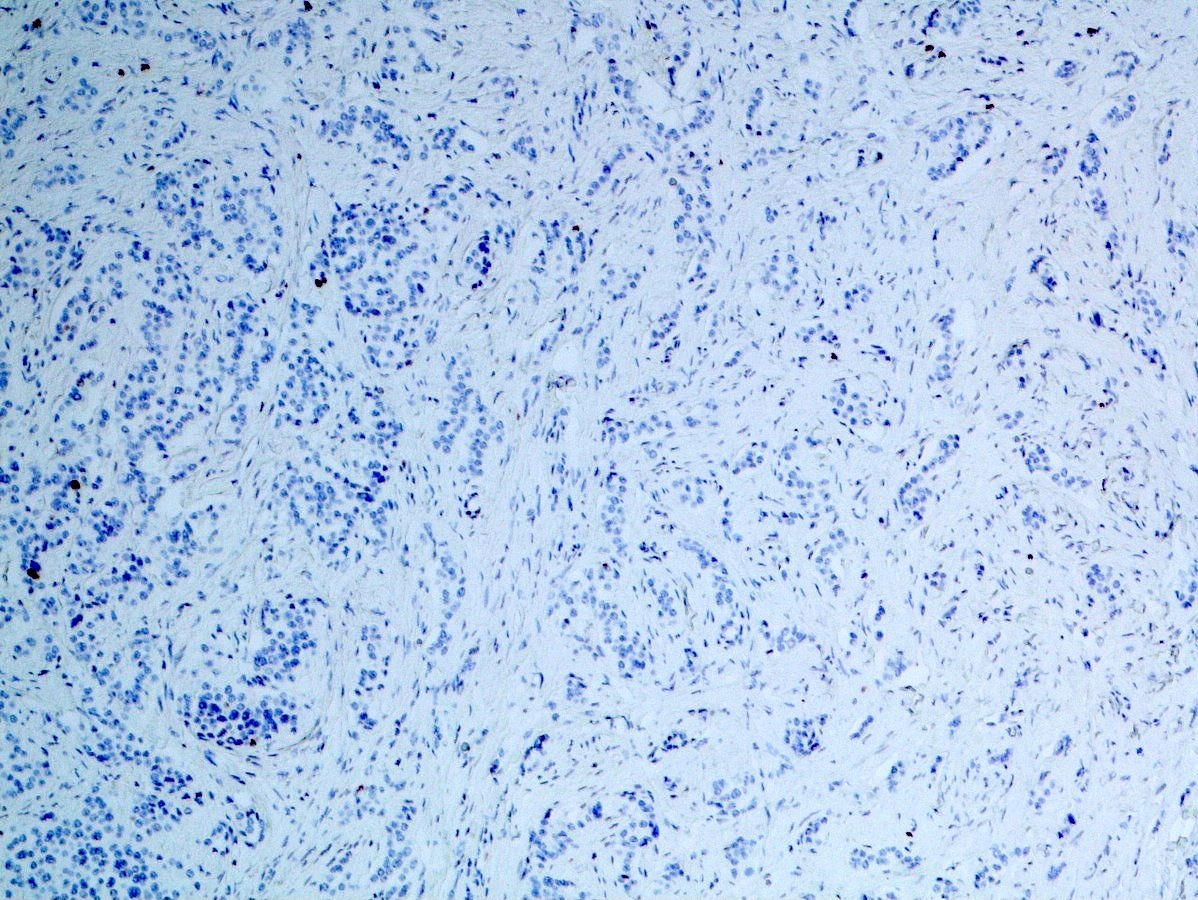
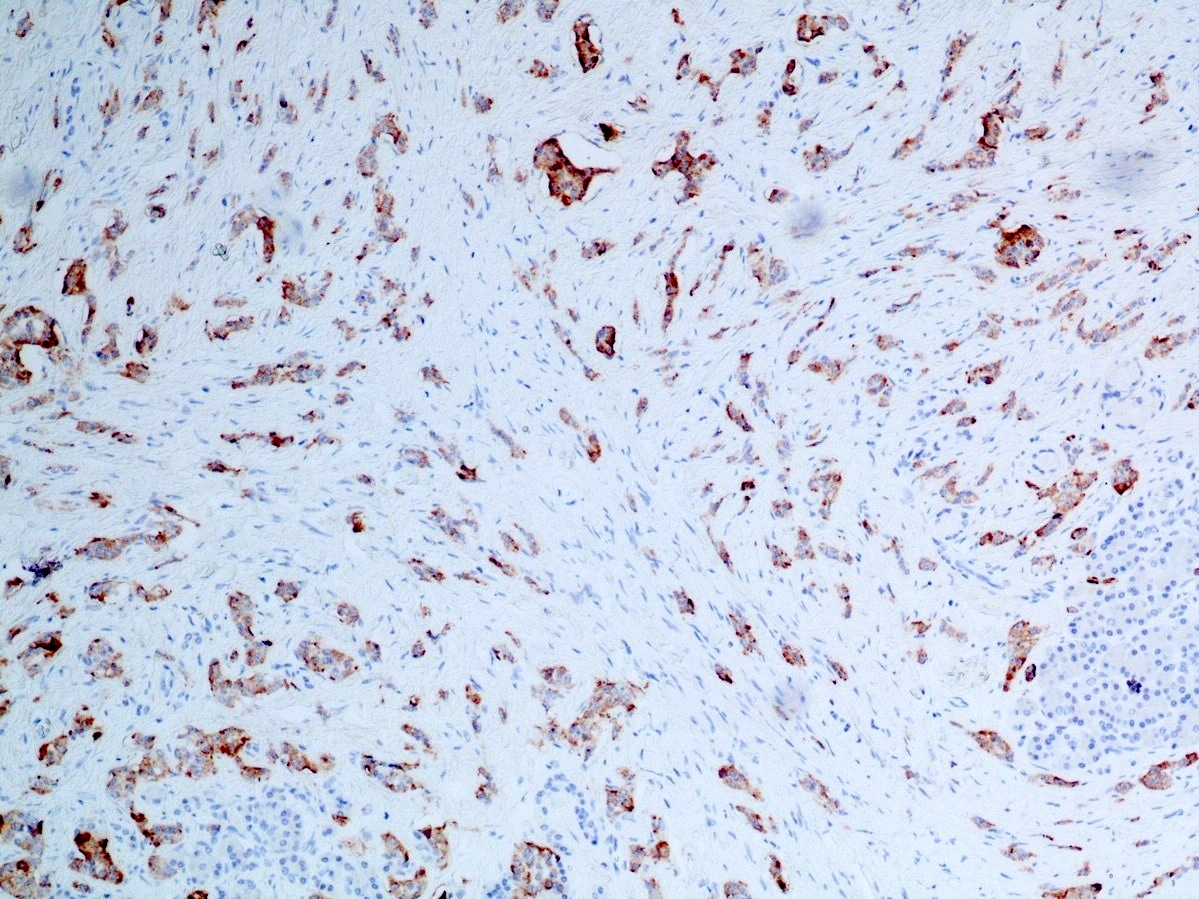
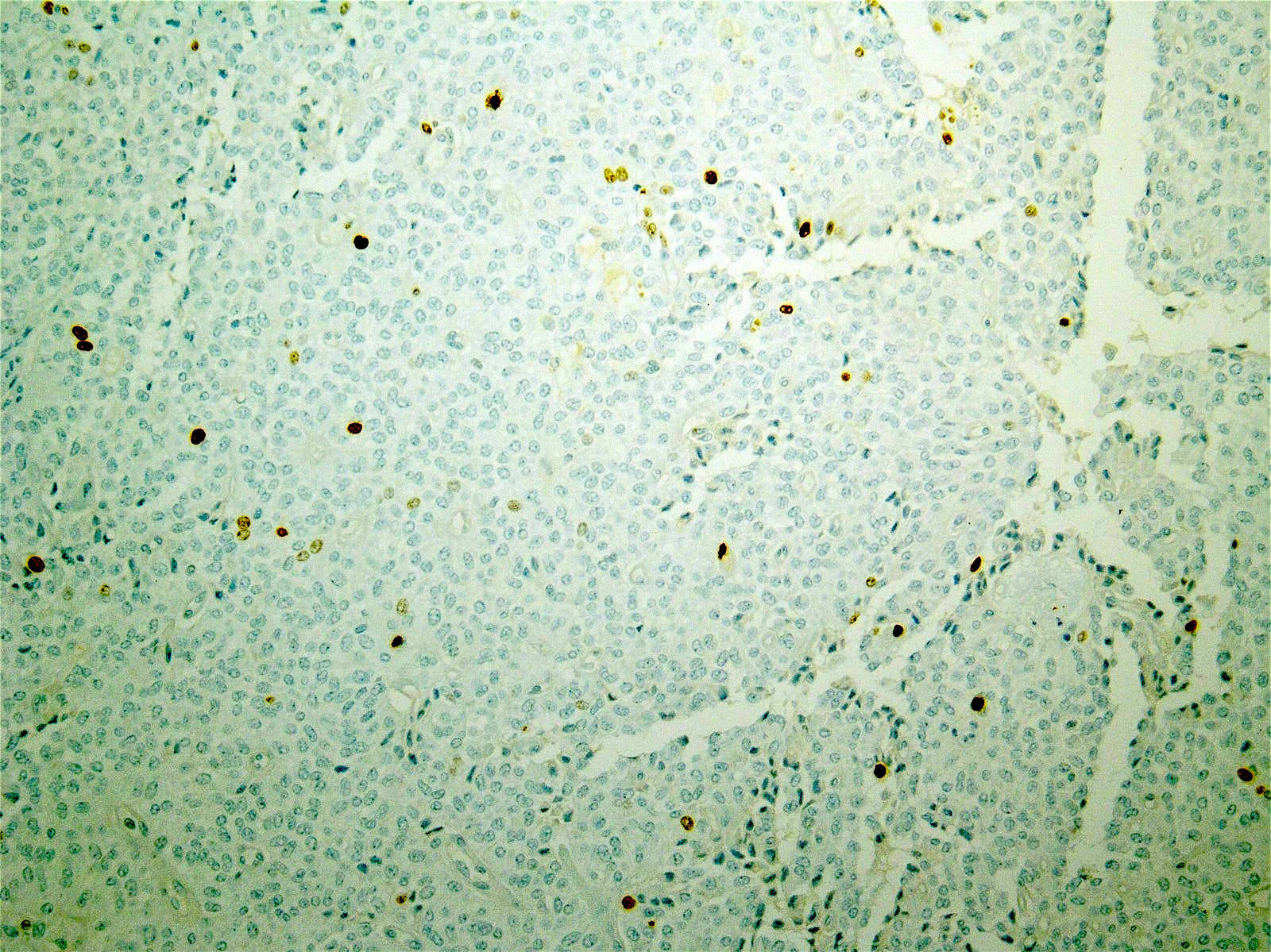
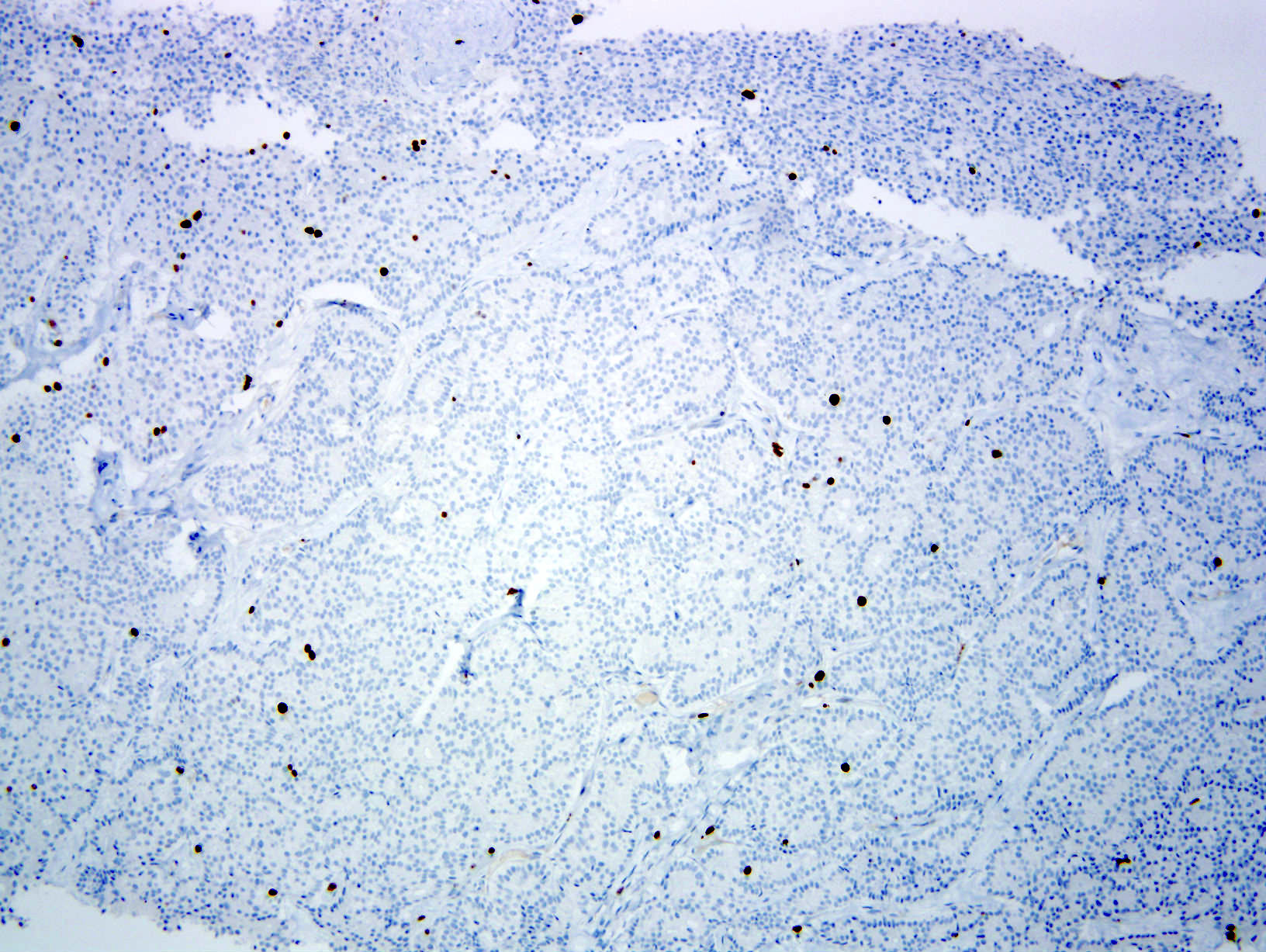
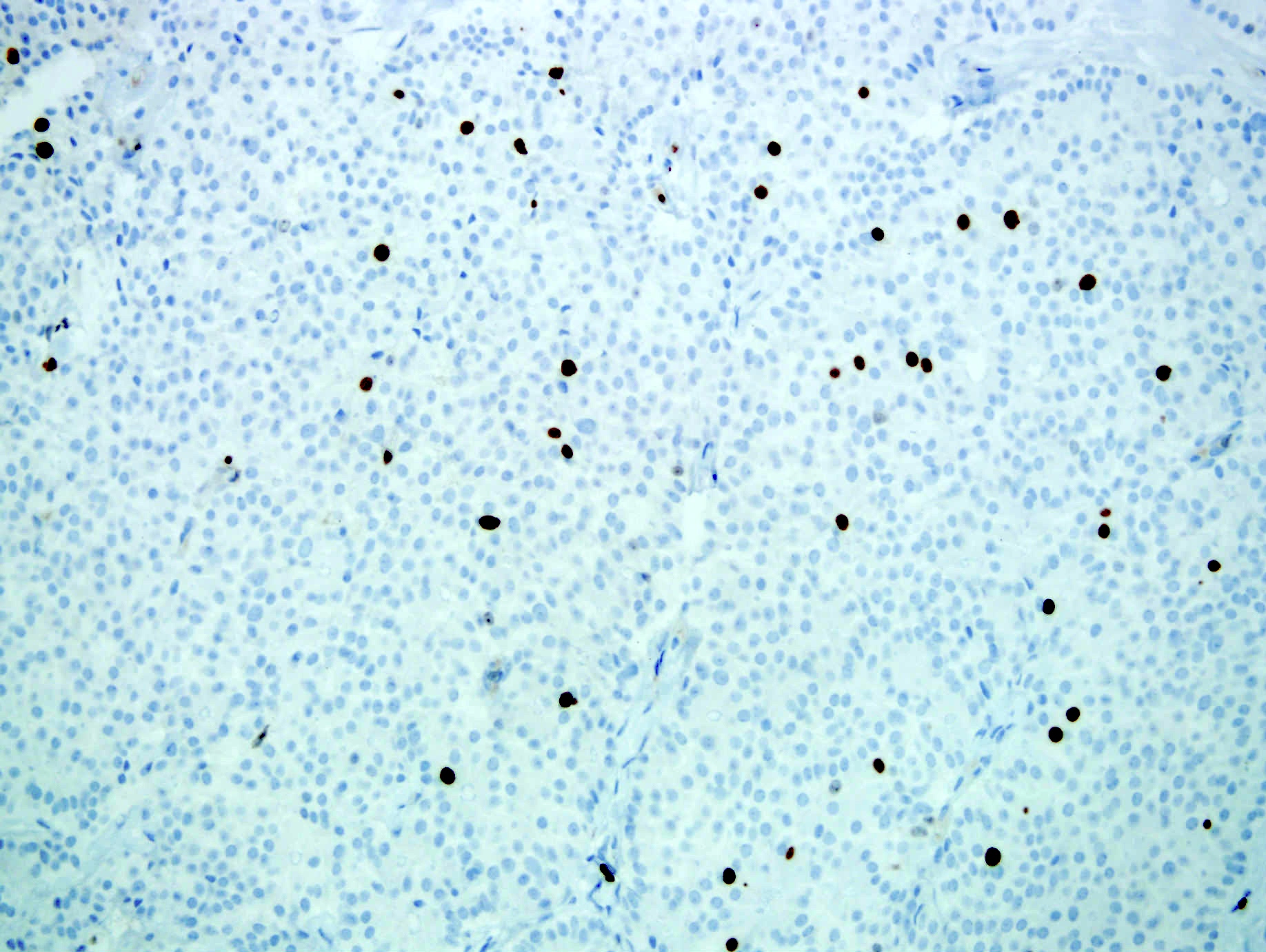
- Neoplastic cells are negative for chromogranin and synaptophysin (rarely, scattered positive cells may be present)
Molecular / cytogenetics description
- Most common molecular alterations of pancreatic ductal adenocarcinoma (e.g. KRAS and SMAD4 mutations) are usually absent
- Recurrent presence of BRAF, RAF1 and RET rearrangements (Cancer Discov 2014;4:1398, Mod Pathol 2020;33:1811, Mod Pathol 2020;33:657)
- Wnt pathway alterations with APC / CTNNB1 mutations can be present in a subset of cases
Sample pathology report
- Distal stomach, duodenum and pancreatic head, pancreaticoduodenectomy (Whipple resection):
- Acinar cell carcinoma of the pancreas, 2.2 cm
- Carcinoma involves distal bile duct
- All margins negative for carcinoma
- Positive for lymphovascular invasion and perineural invasion
- Metastatic carcinoma involving 1 of 27 lymph nodes
- Portion of benign stomach and duodenum with no significant pathologic change
Differential diagnosis
- Neuroendocrine neoplasms:
- BCL10 negative, chromogranin and synaptophysin positive
- Salt and pepper chromatin
- No evident nucleoli
- Pancreatoblastoma:
- Pancreatic neoplasm with acinar differentiation: the presence of squamoid nests is diagnostic for pancreatoblastoma
- Intraductal tubulopapillary neoplasm (ITPN):
Additional references
Board review style question #1
A high magnification field of a tumor within the pancreas is shown above. Can the diagnosis of acinar cell carcinoma be based on morphology alone or are other analyses required?
- No, morphology alone is always sufficient
- Yes, electron microscopy is of great help to rule out a neuroendocrine tumor and is always required
- Yes, in addition to morphology, the immunohistochemical demonstration of the acinar differentiation represents the diagnostic gold standard
- Yes, molecular analysis is mandatory
- Yes, the demonstration of a BRAF rearrangement is required for the diagnosis
Board review style answer #1
C. Yes, in addition to morphology, the immunohistochemical demonstration of the acinar differentiation represents the diagnostic gold standard. Morphology and immunohistochemistry represent the 2 most important tools for the diagnosis. The acinar differentiation seen at histology should be supported with BCL10 and trypsin positivity. Tumor cells are also positive for PASD.
Comment Here
Reference: Acinar cell carcinoma
Comment Here
Reference: Acinar cell carcinoma
Board review style question #2
Is it important to report the intraductal growth pattern of pancreatic acinar cell carcinoma?
- No, it is only a descriptive finding
- No, this pattern does not belong to the morphological spectrum of acinar cell carcinoma
- Yes, this pattern is associated with a better prognosis
- Yes, this pattern is associated with a very poor prognosis
- Yes, this pattern is associated with intraductal papillary mucinous neoplasm
Board review style answer #2
C. Yes, this pattern is associated with a better prognosis. This pattern represents a potential diagnostic pitfall with intraductal neoplasms (above all, intraductal tubulopapillary neoplasm) and is associated with a better prognosis.
Comment Here
Reference: Acinar cell carcinoma
Comment Here
Reference: Acinar cell carcinoma
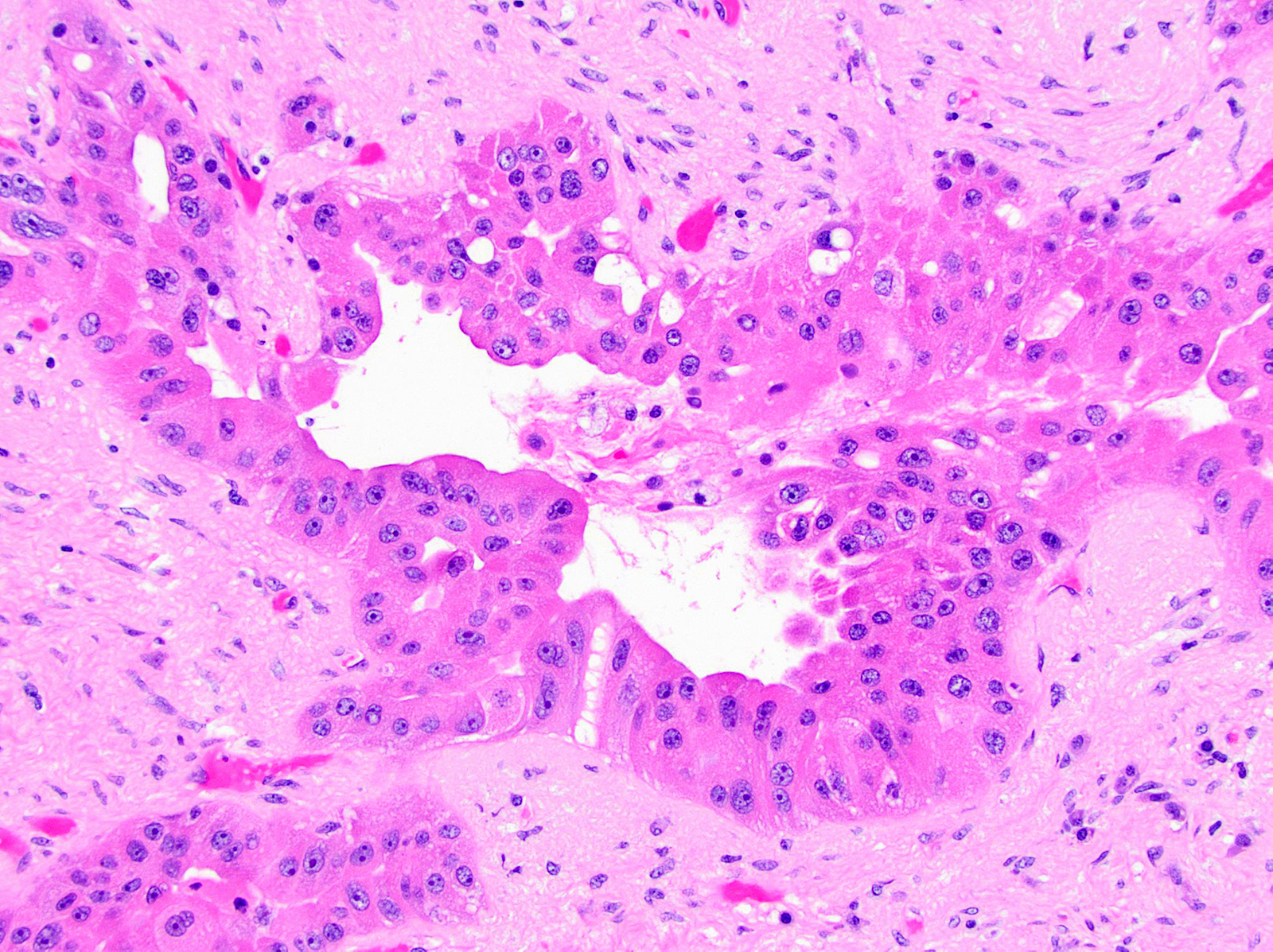
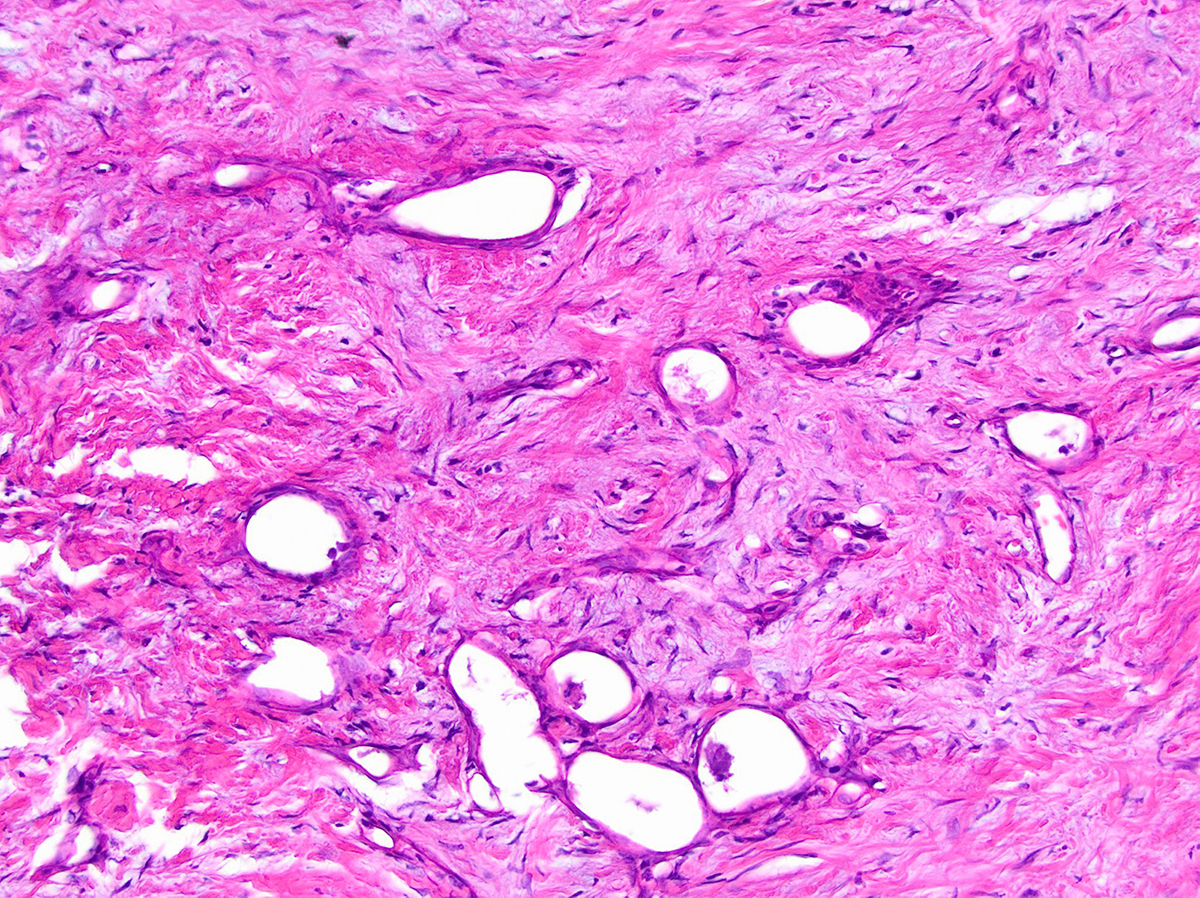
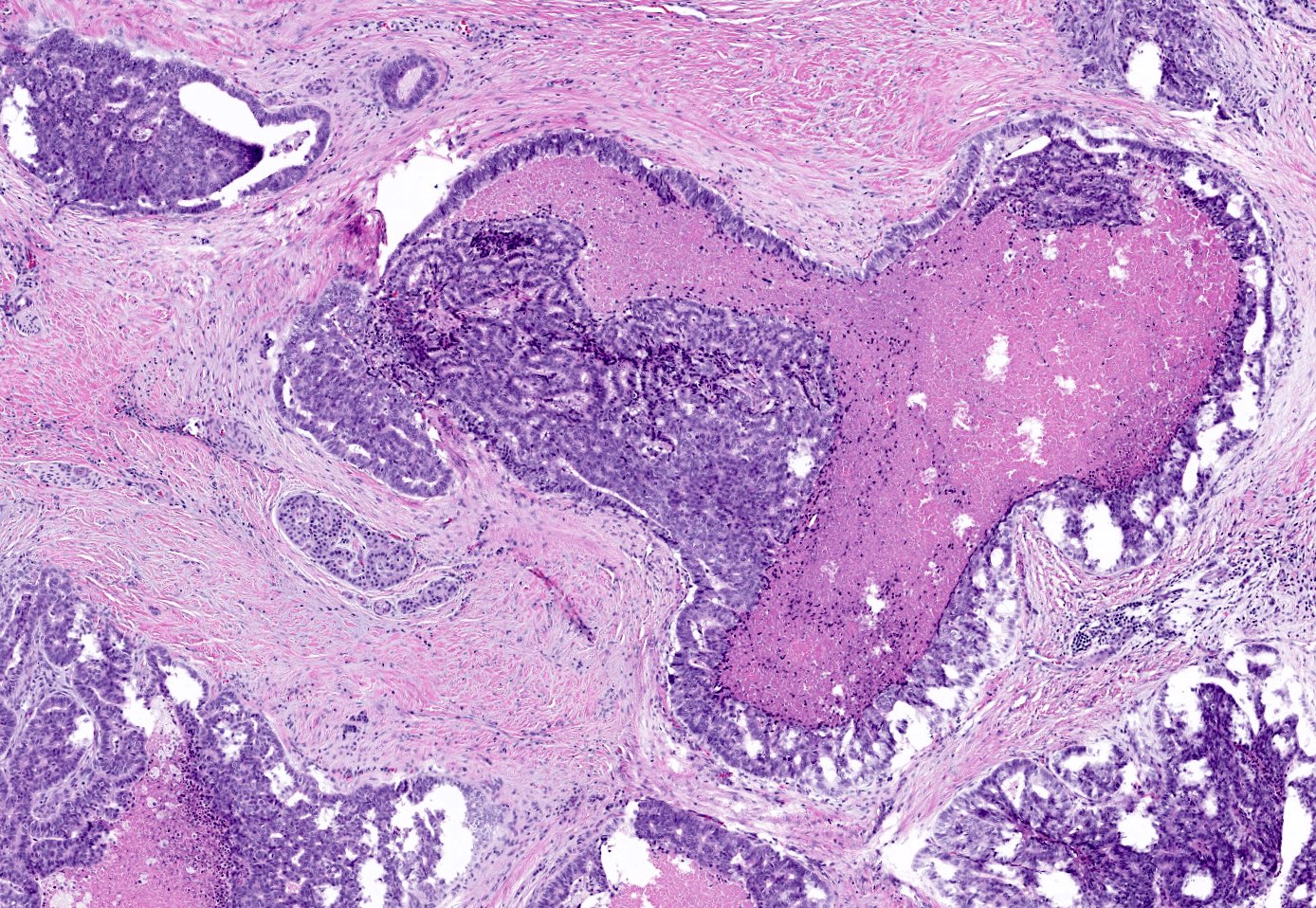
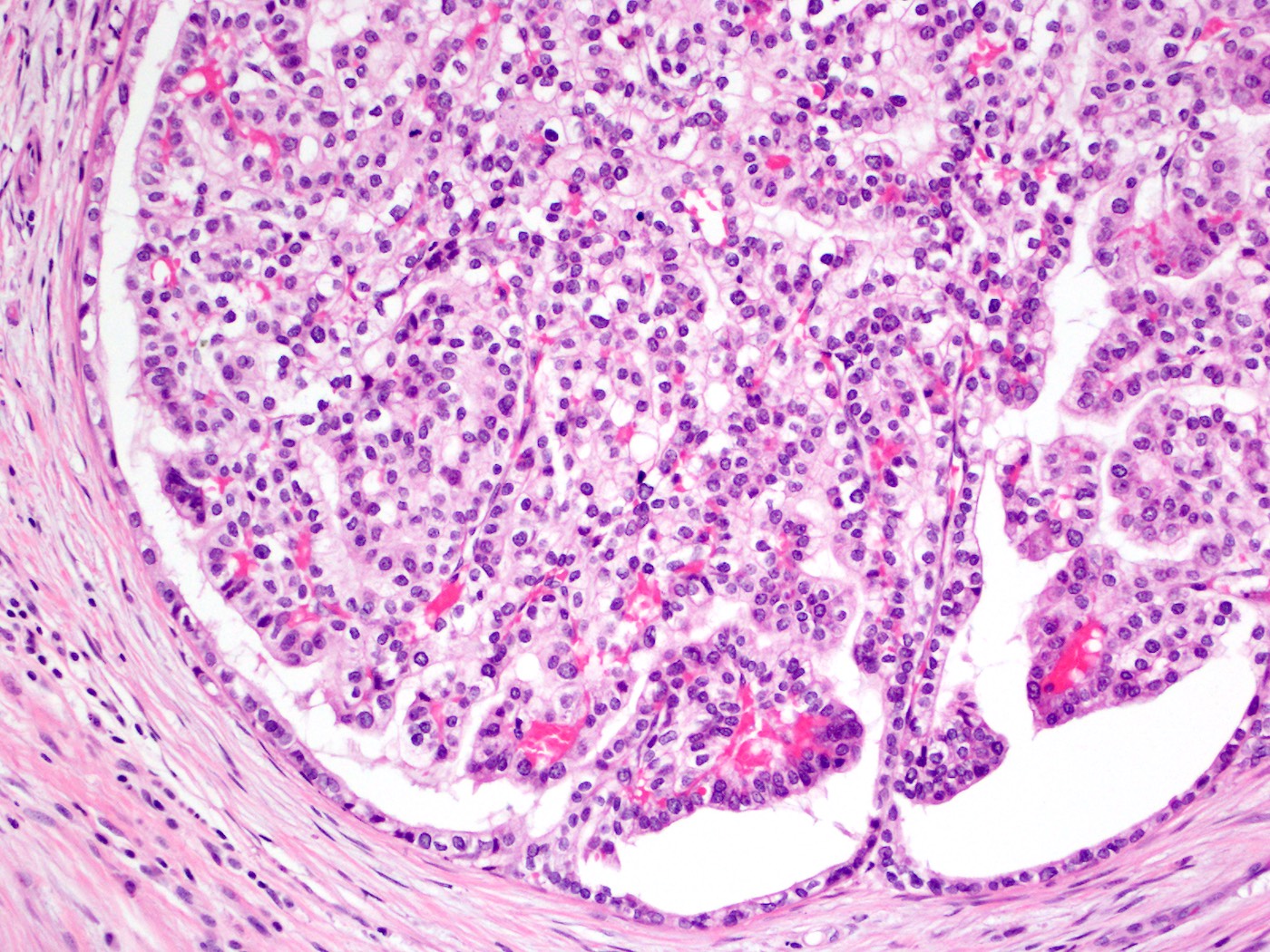
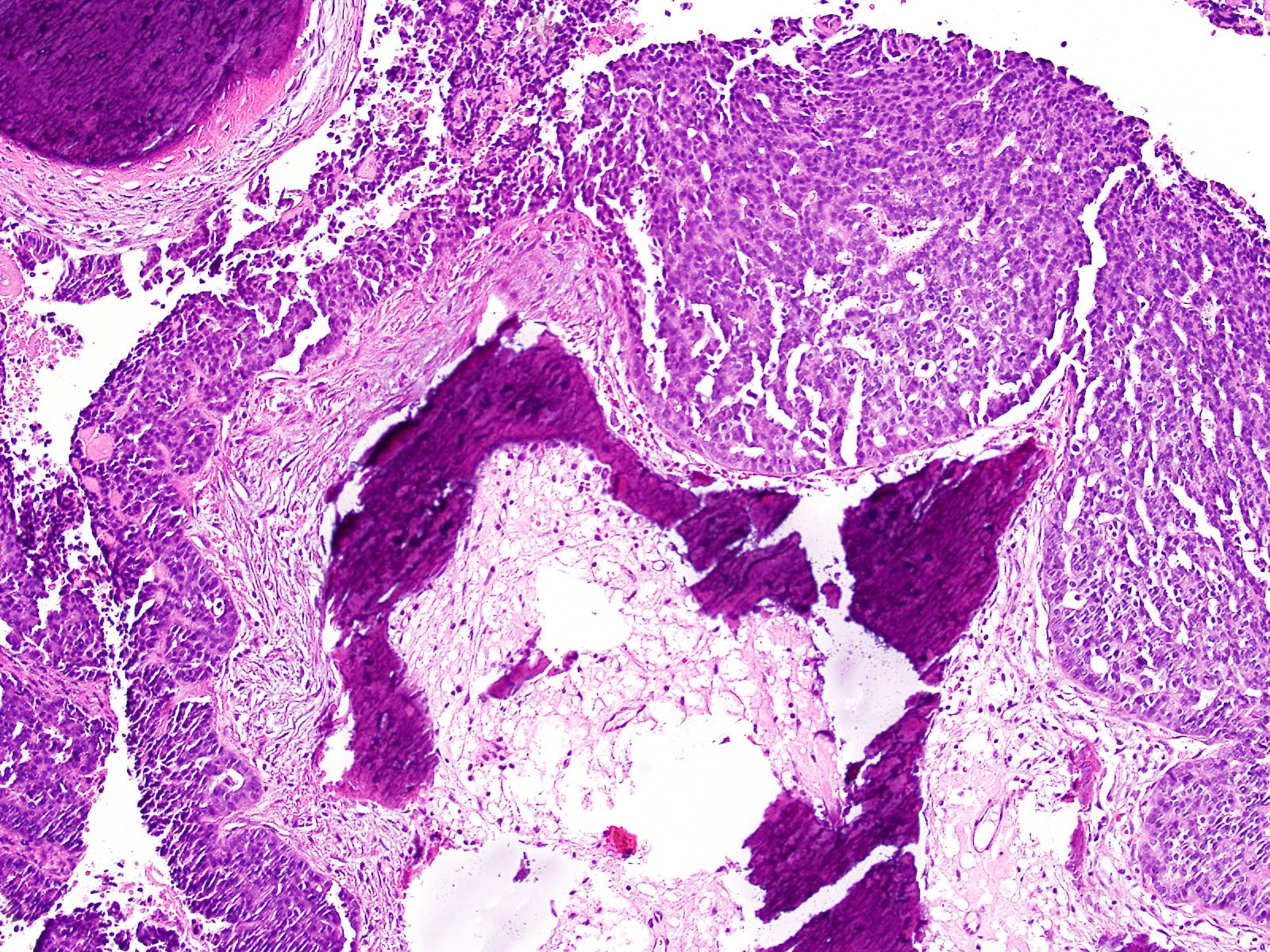
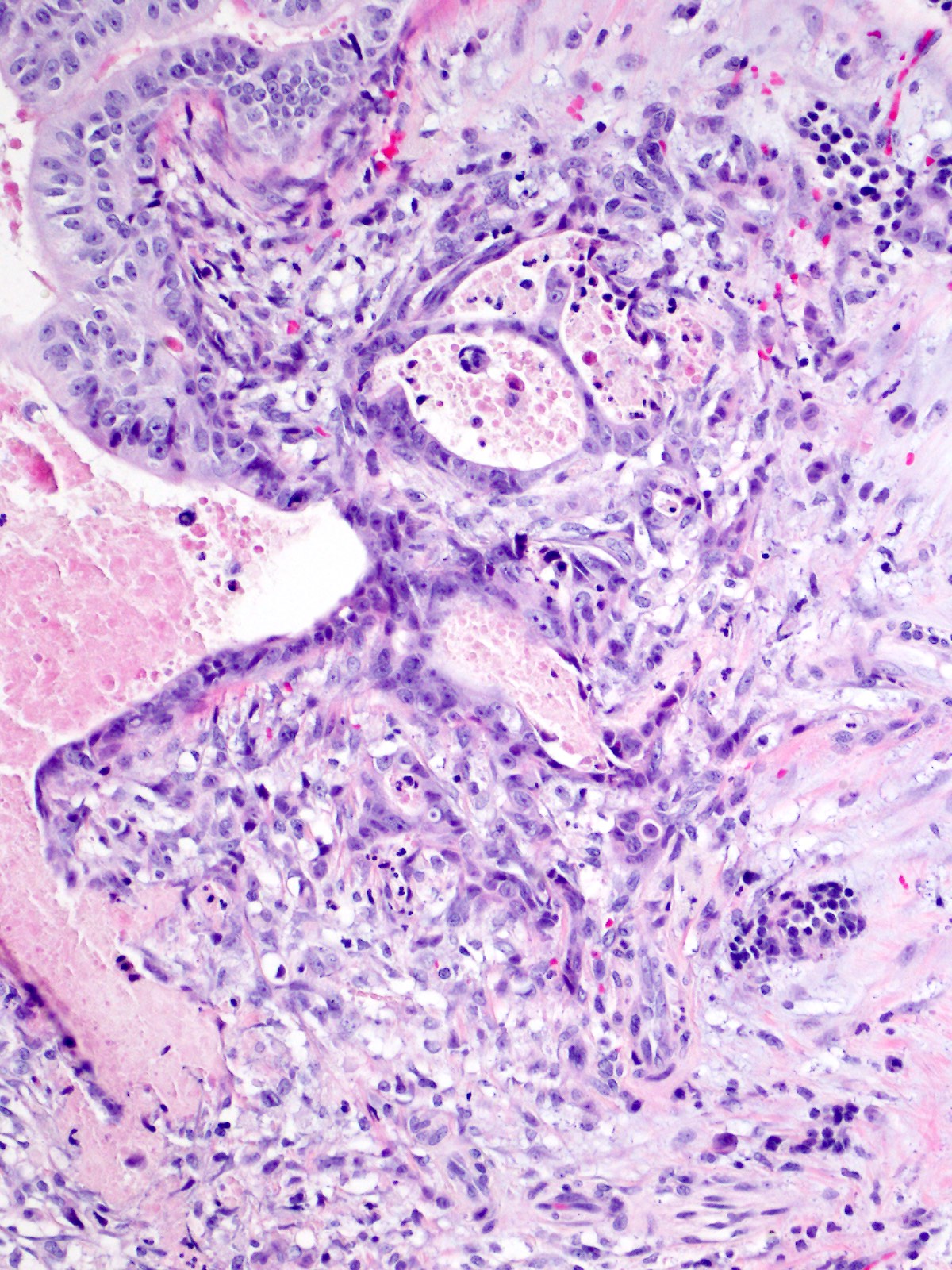
Acinar cystic transformation
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Definition / general
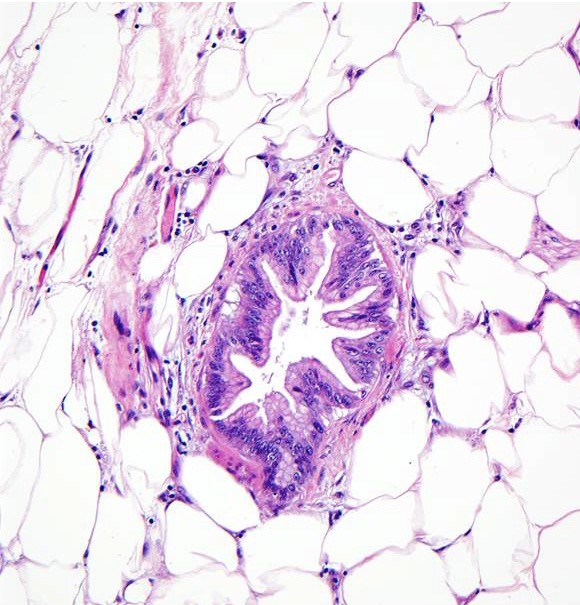
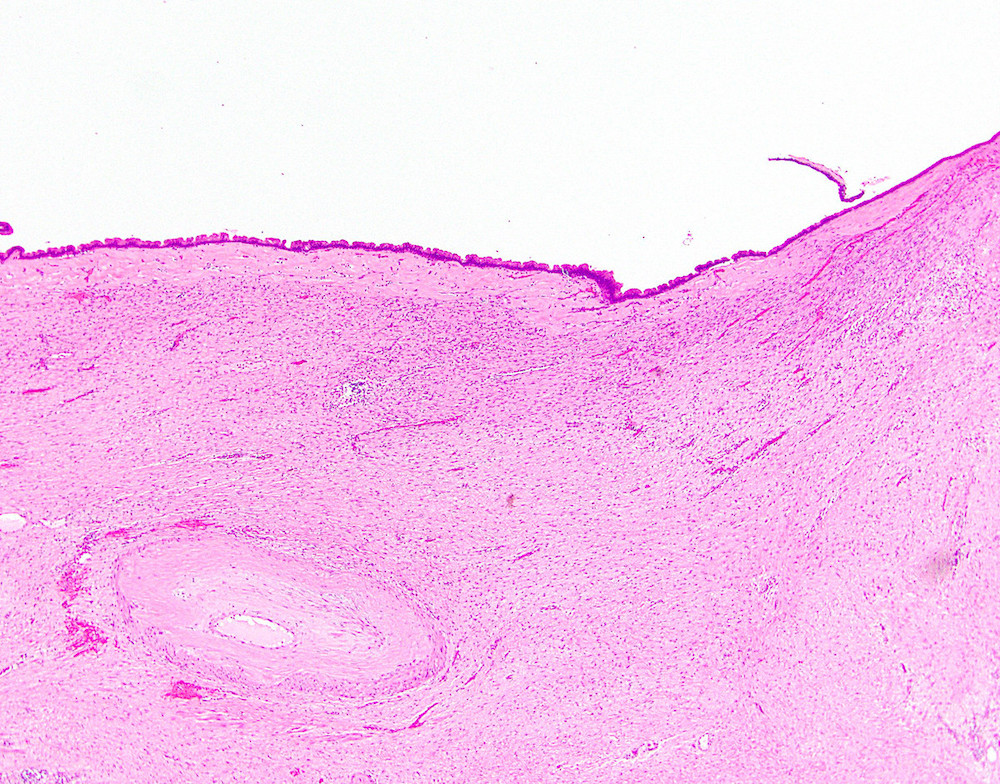
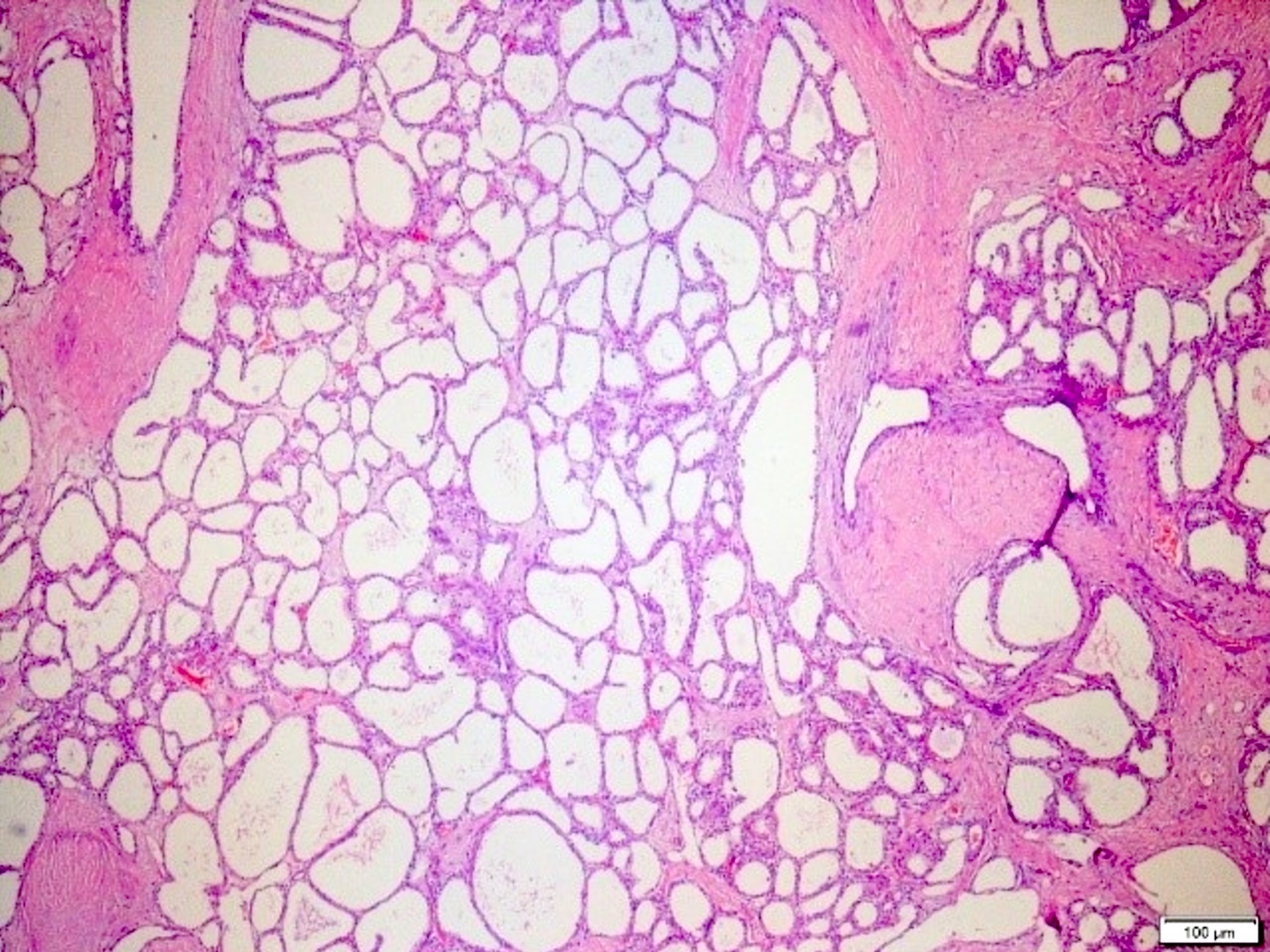
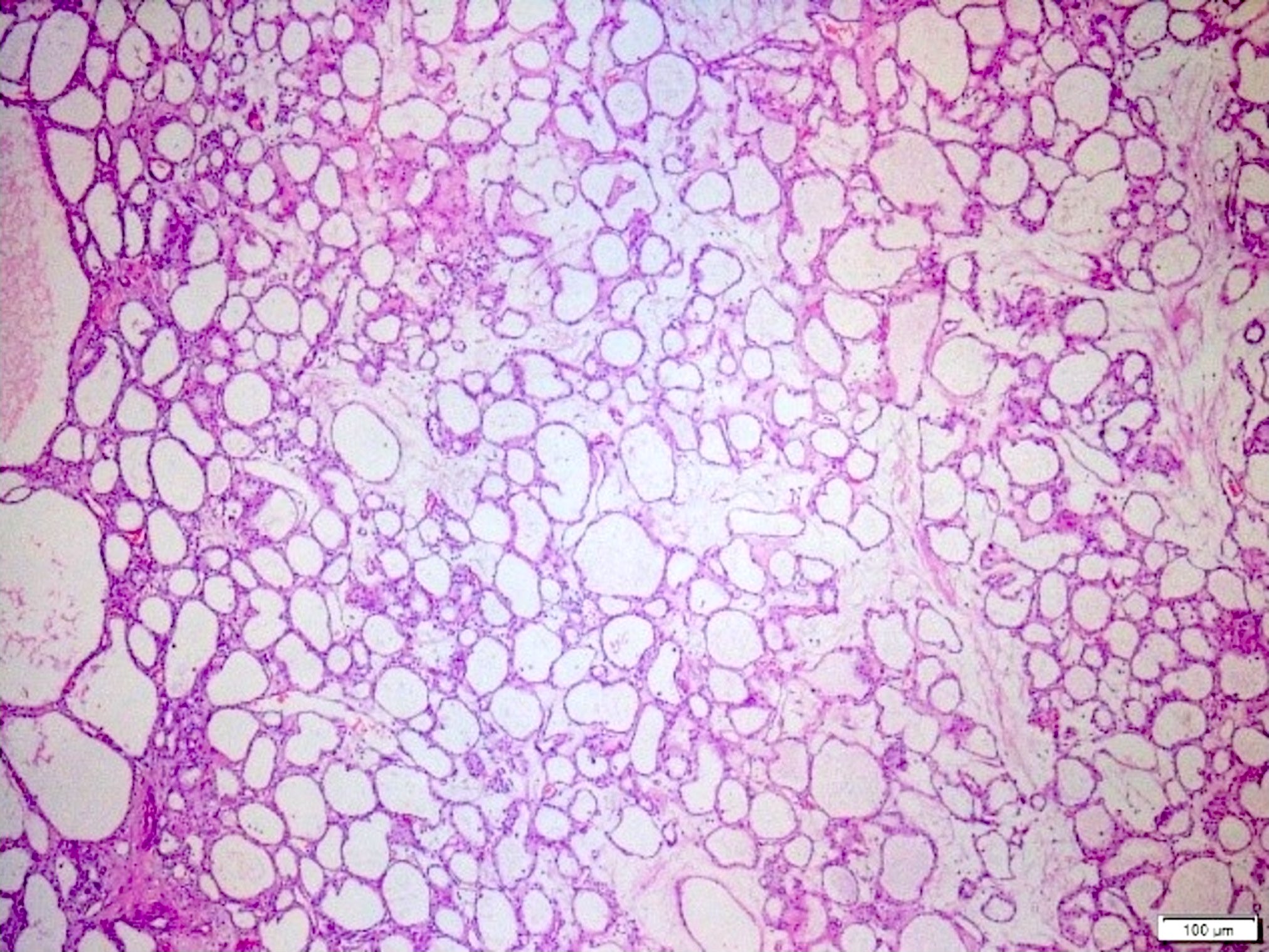
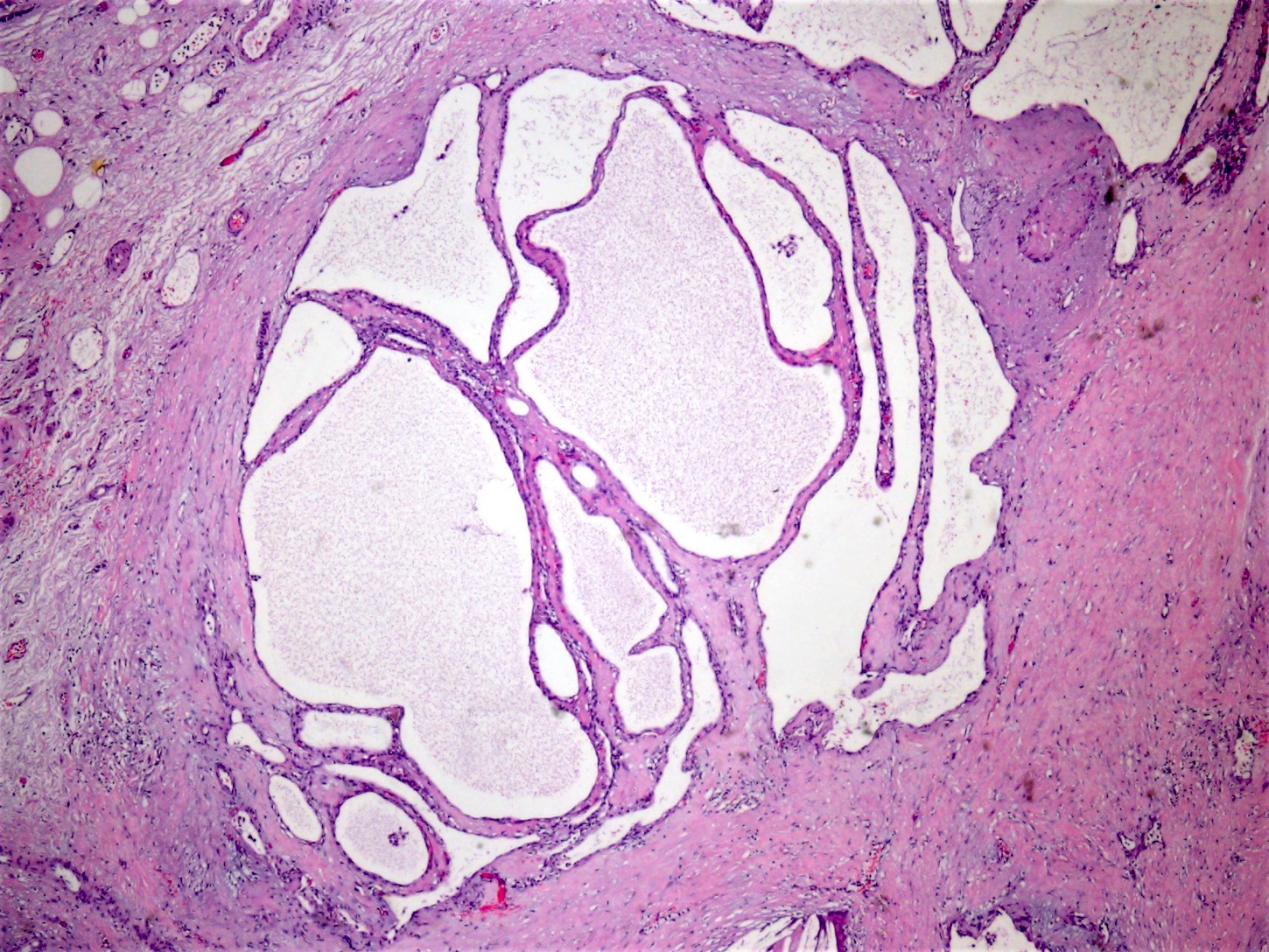
- Rare, nonneoplastic cystic lesion of the pancreas lined by benign appearing acinar and ductal epithelium
Essential features
- First described in 2002; recognized by WHO in 2010 (Am J Surg Pathol 2002;26:698)
- Clinically indolent; no cases of metastasis have been reported
- Most cases found incidentally; though may be symptomatic
- Cyst epithelium of acinar cystic transformation (ACT) consists of bland acinar and ductal epithelium, without mitoses, atypia and necrosis
Terminology
- Other names include acinar cell transformation and acinar cell cystadenoma
ICD coding
- ICD-11: DC30.0 - cyst of pancreas
Epidemiology
- Rare; so far < 130 cases reported in the literature (J Clin Pathol 2023;76:740)
- Female predominance (65.3%) with no age predilection
Sites
- Found in all sites of the pancreas; can diffusely involve the gland
- More common in head of pancreas
Pathophysiology
- Most studies report nonneoplastic dilatation of the acinar and ductal epithelium (Oncol Lett 2014;8:852, J Clin Pathol 2023;76:740)
- May arise from a heterogeneous background (Am J Surg Pathol 2023;47:379)
- Evolving from acinar microcysts
- Secondary to underlying obstructive lesions
- Subset may possibly be neoplastic
Etiology
- Unknown; some may occur due to obstruction
- No longer thought to represent the benign counterpart to acinar cell cystadenocarcinoma
Clinical features
- Often found incidentally on imaging
- Abdominal pain (42.1%), weight loss (4.9%), pancreatitis (4.9%), palpable mass (3.3%), jaundice (2.5%) (J Clin Pathol 2023;76:740)
Diagnosis
- Requires clinical, radiologic and pathologic correlation
Radiology description
- Nonspecific but the presence of 5 or more cysts, clustered peripheral small cysts, presence of cyst calcifications and absence of communication with the main pancreatic duct are supportive (Eur Radiol 2014;24:2128)
Prognostic factors
- Clinically benign; there is no evidence of recurrence, malignant transformation or association with acinar cell carcinoma
- Minority of cases involve controversial / high risk histomolecular features, including intralesional pancreatic intraepithelial neoplasia (PanIN) (4%), chromosomal gains and somatic mutations of KRAS and SMO genes (J Clin Pathol 2023;76:740)
Case reports
- 22 year old woman with epigastric pain and 5 cm cystic lesion in tail of pancreas (Surg Case Rep 2016;2:39)
- 43 year old woman with recurrent attacks of acute pancreatitis found to have diffuse involvement of pancreas by small multiple cysts (< 1 cm in maximum diameter) (Int J Surg Pathol 2022;30:697)
- 52 year old man with a pancreatic tail mass found incidentally on routine imaging for renal cell carcinoma followup (Korean J Radiol 2011;12:129)
- Largest systematic review of literature (J Clin Pathol 2023;76:740)
Treatment
- Benign; no need of surgical resection (J Clin Pathol 2023;76:740)
- Some are resected for symptomatic relief or to exclude other cystic neoplasms associated with malignancy
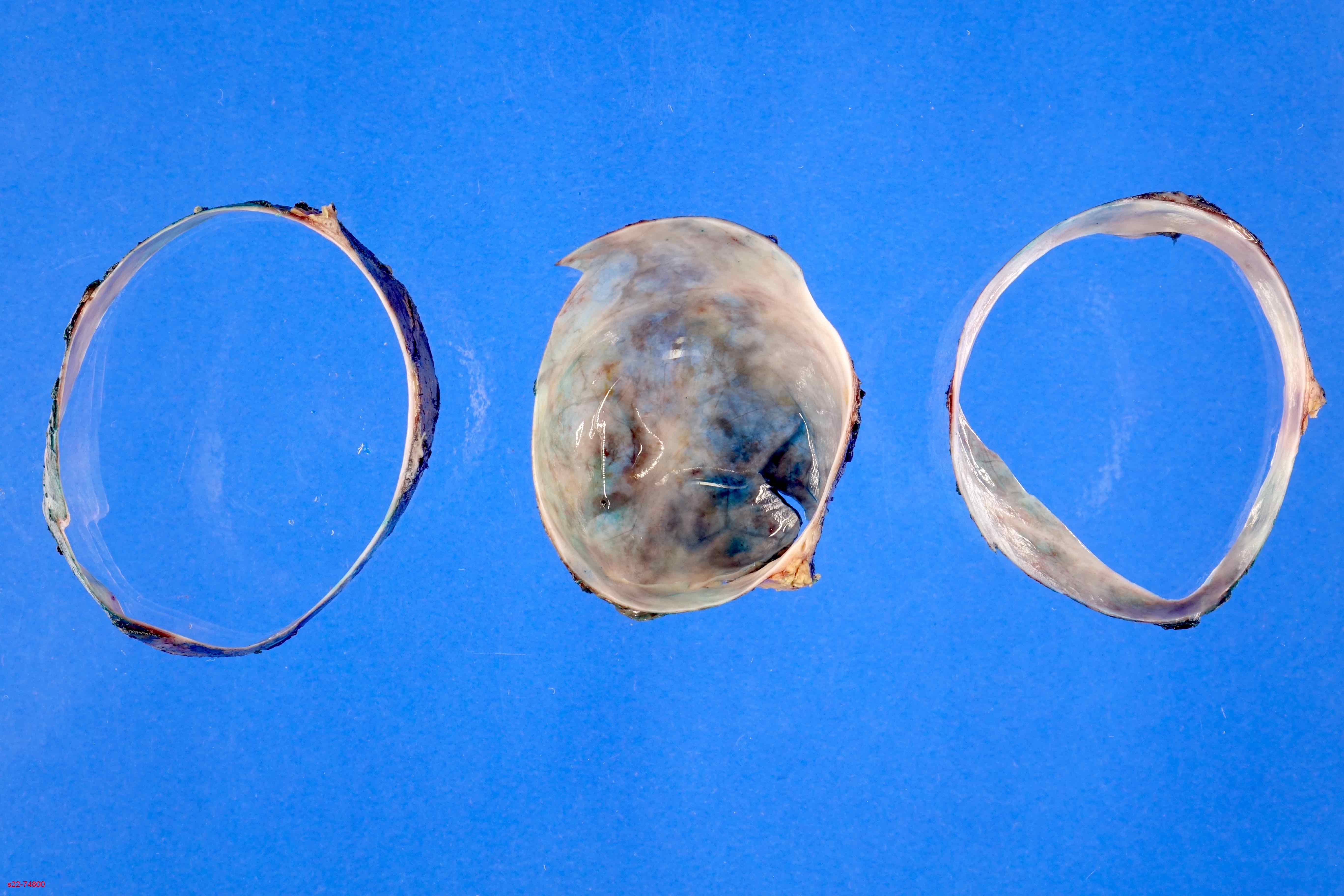
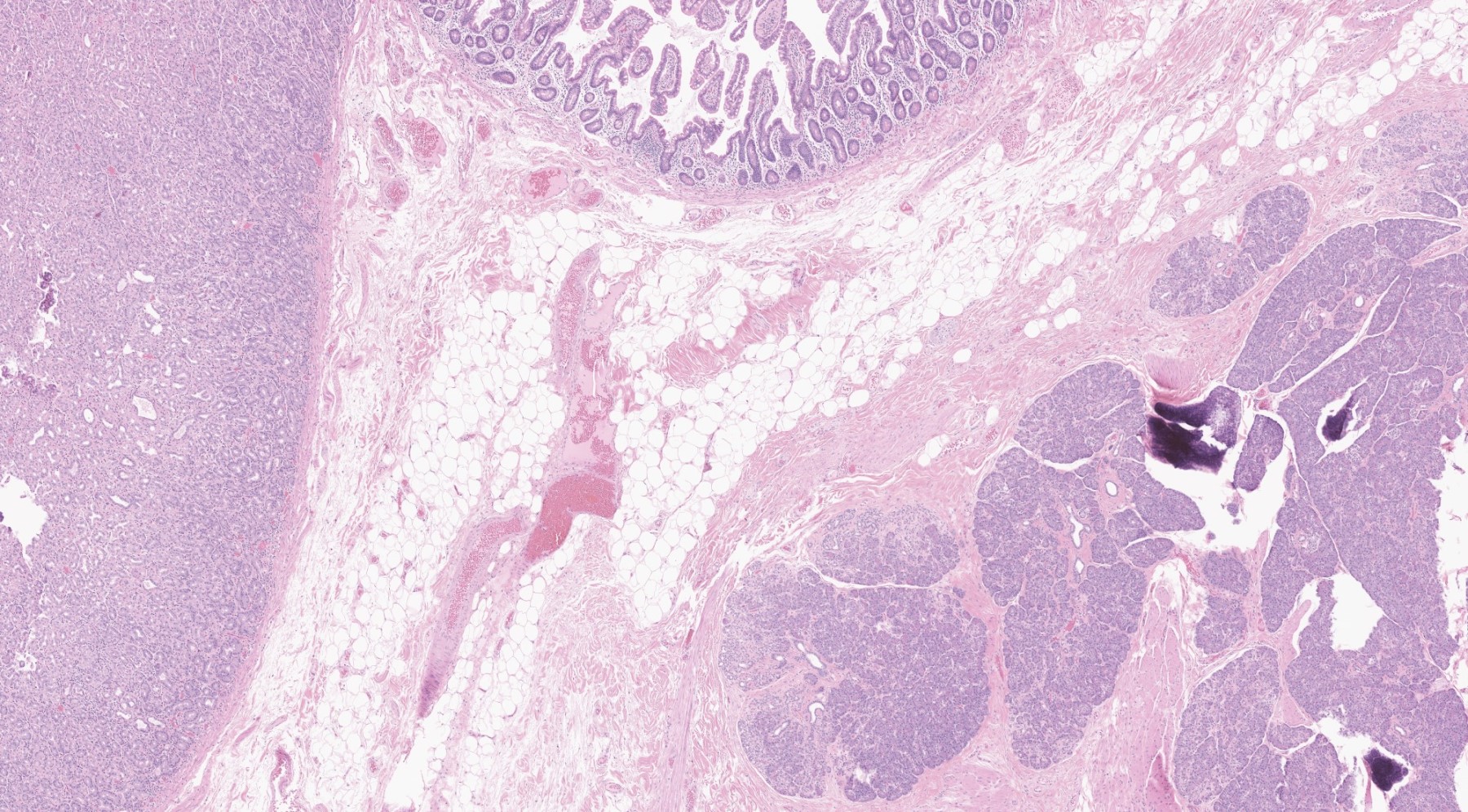
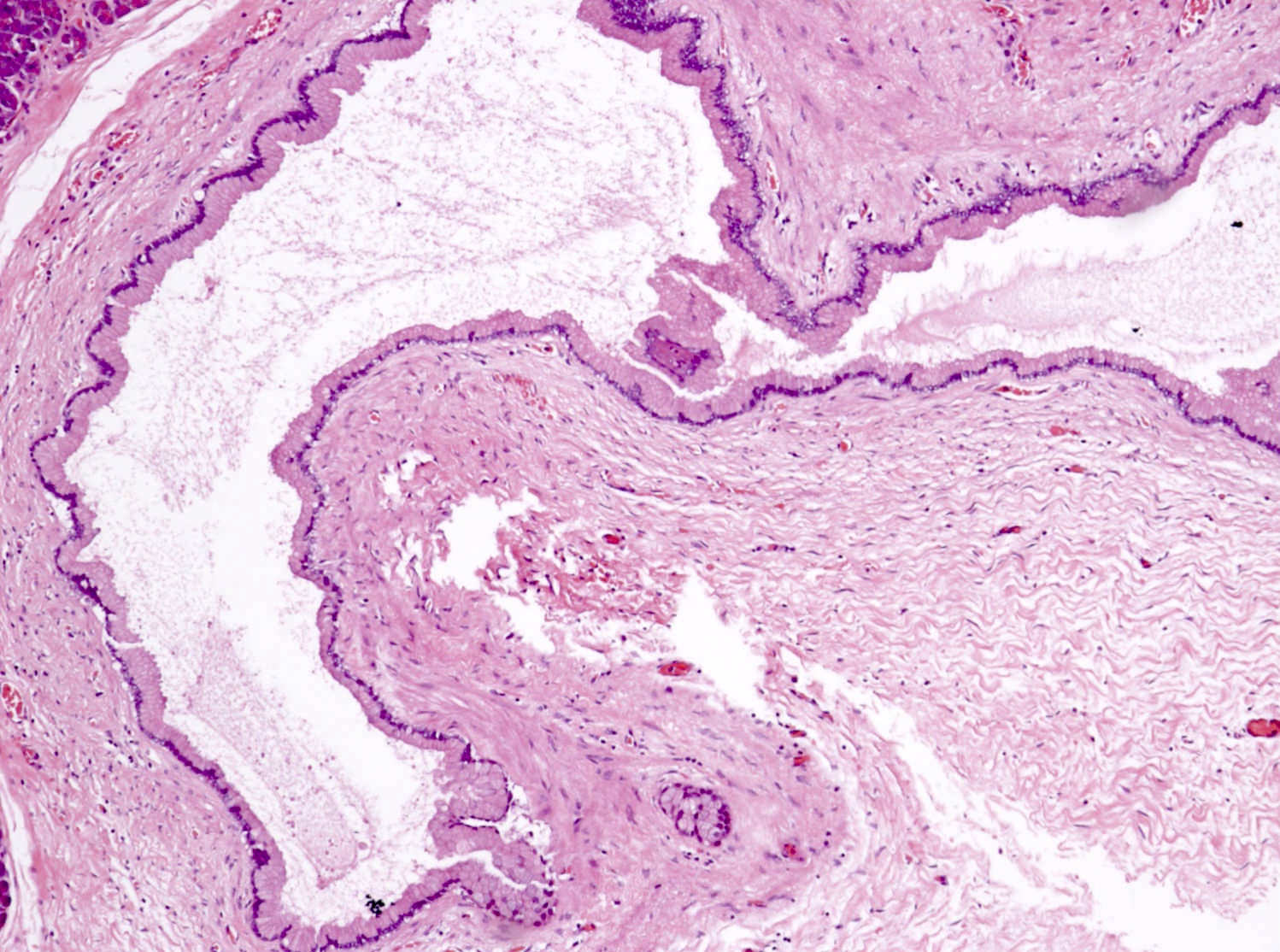
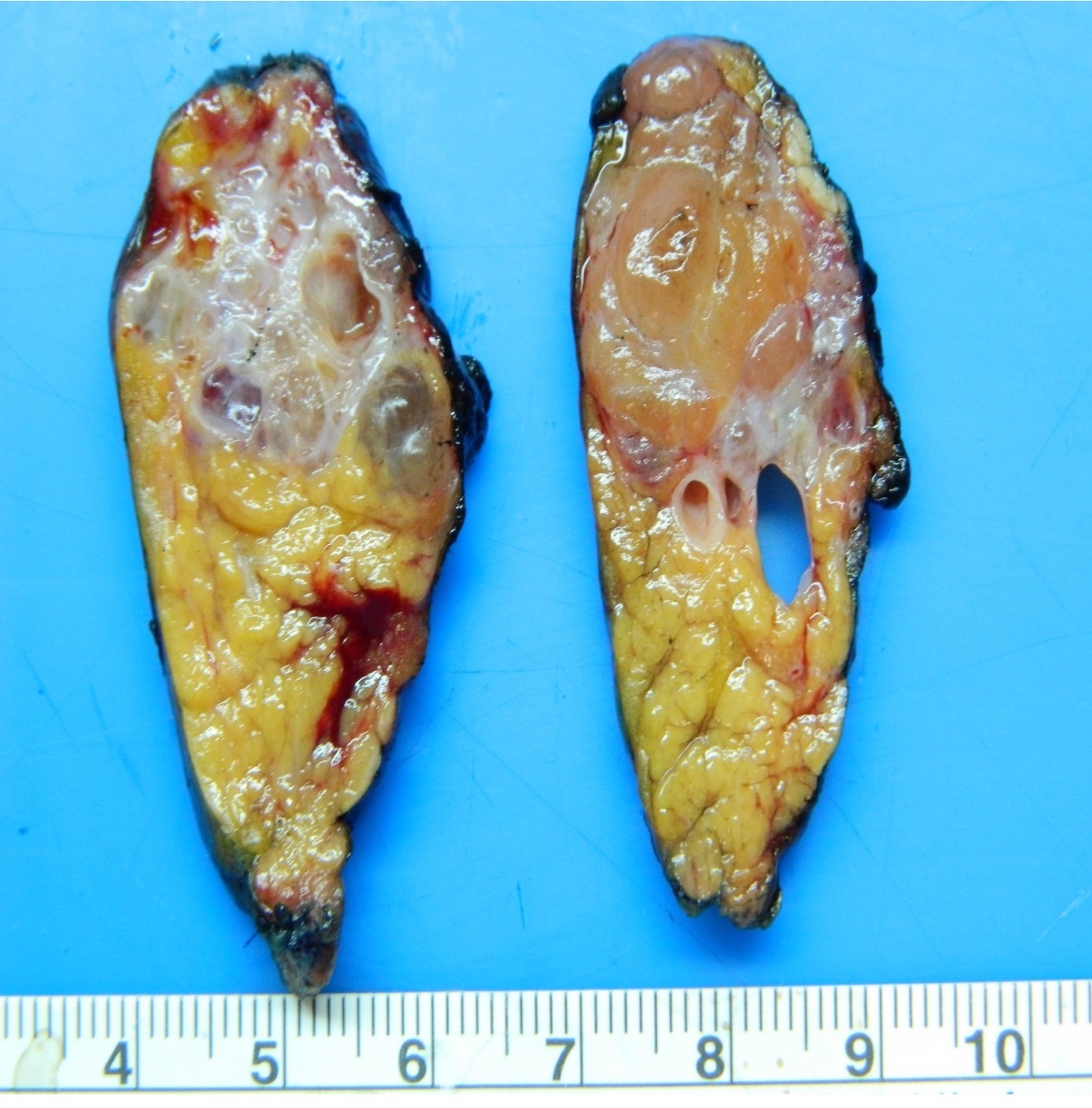
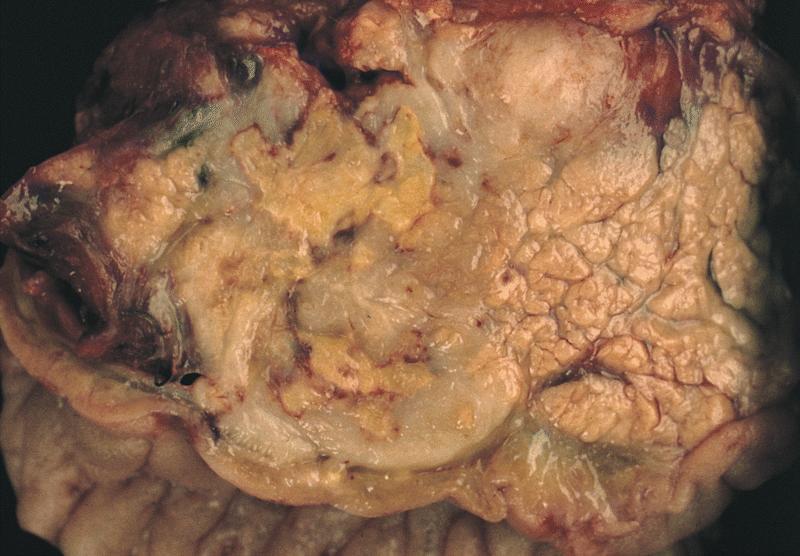
Gross description
- Unifocal > multifocal, multilocular > unilocular
- Average size ~5 cm (range: 2 - 20 cm)
- Typically does not communicate with ductal system, though rare cases have been reported
- Thin walled cyst containing clear white serous watery fluid
- Solid areas or papillary excrescences on cyst wall are typically absent
- References: J Clin Pathol 2023;76:740, Am J Surg Pathol 2023;47:379, Esposito: Pathology of the Pancreas, 1st Edition, 2022
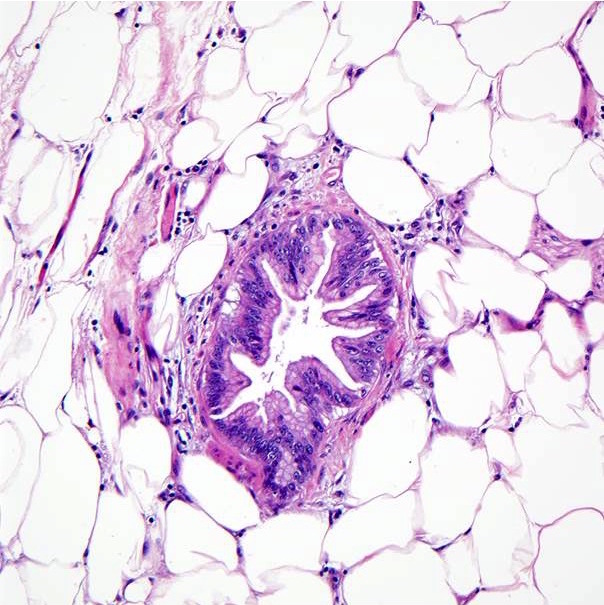
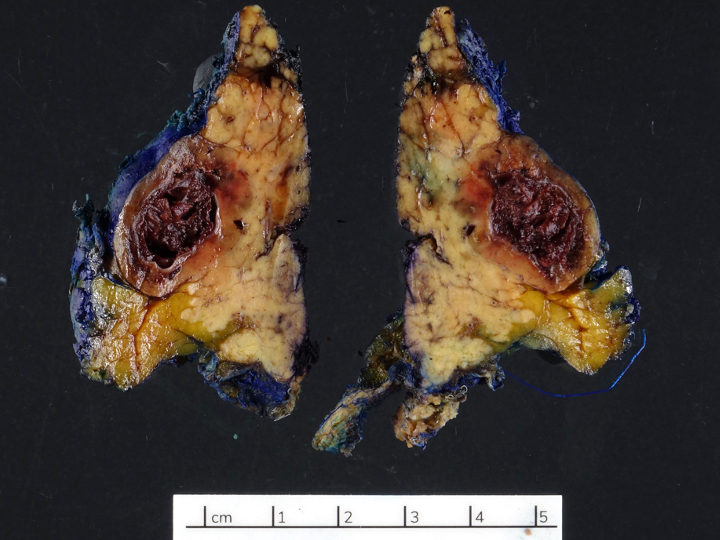
Gross images
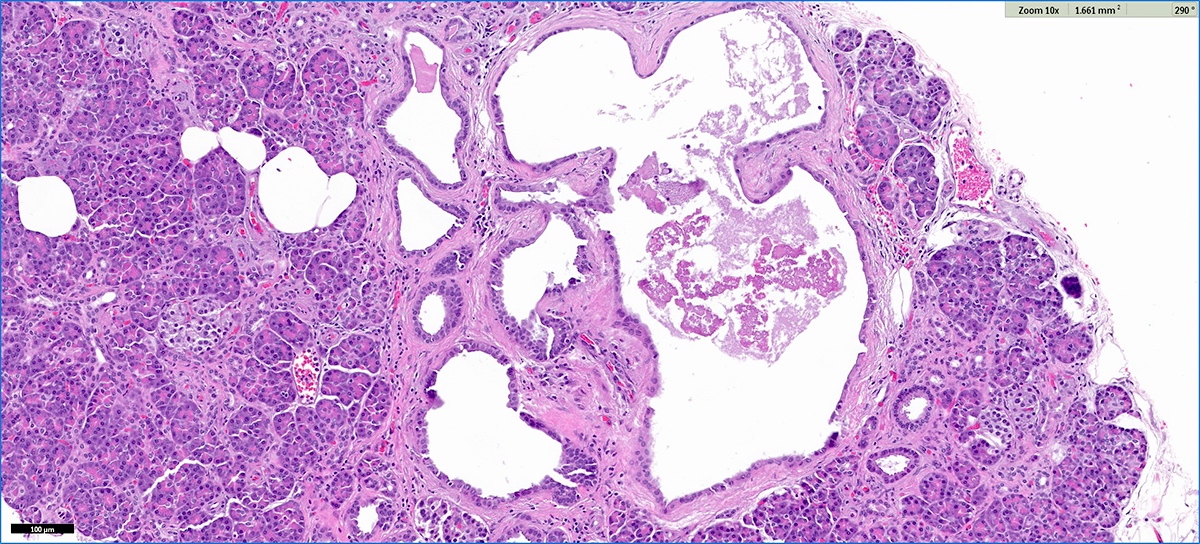
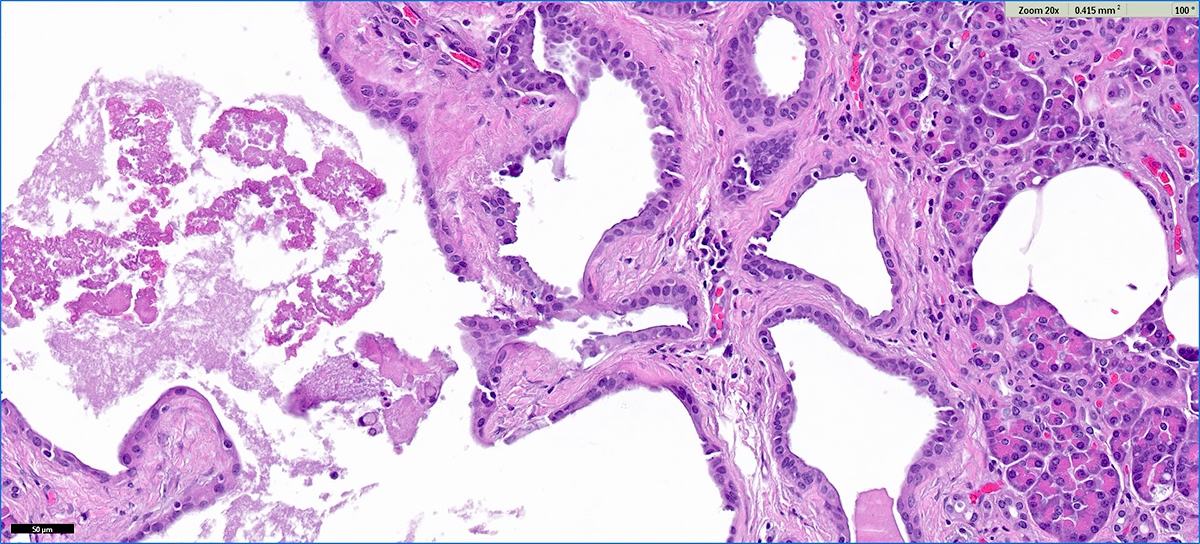
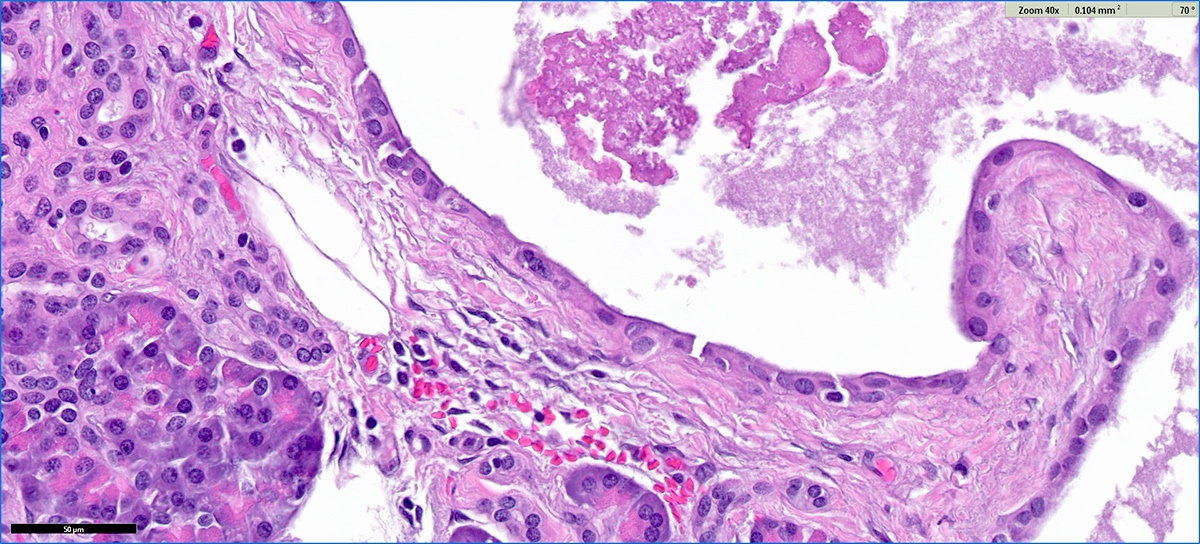
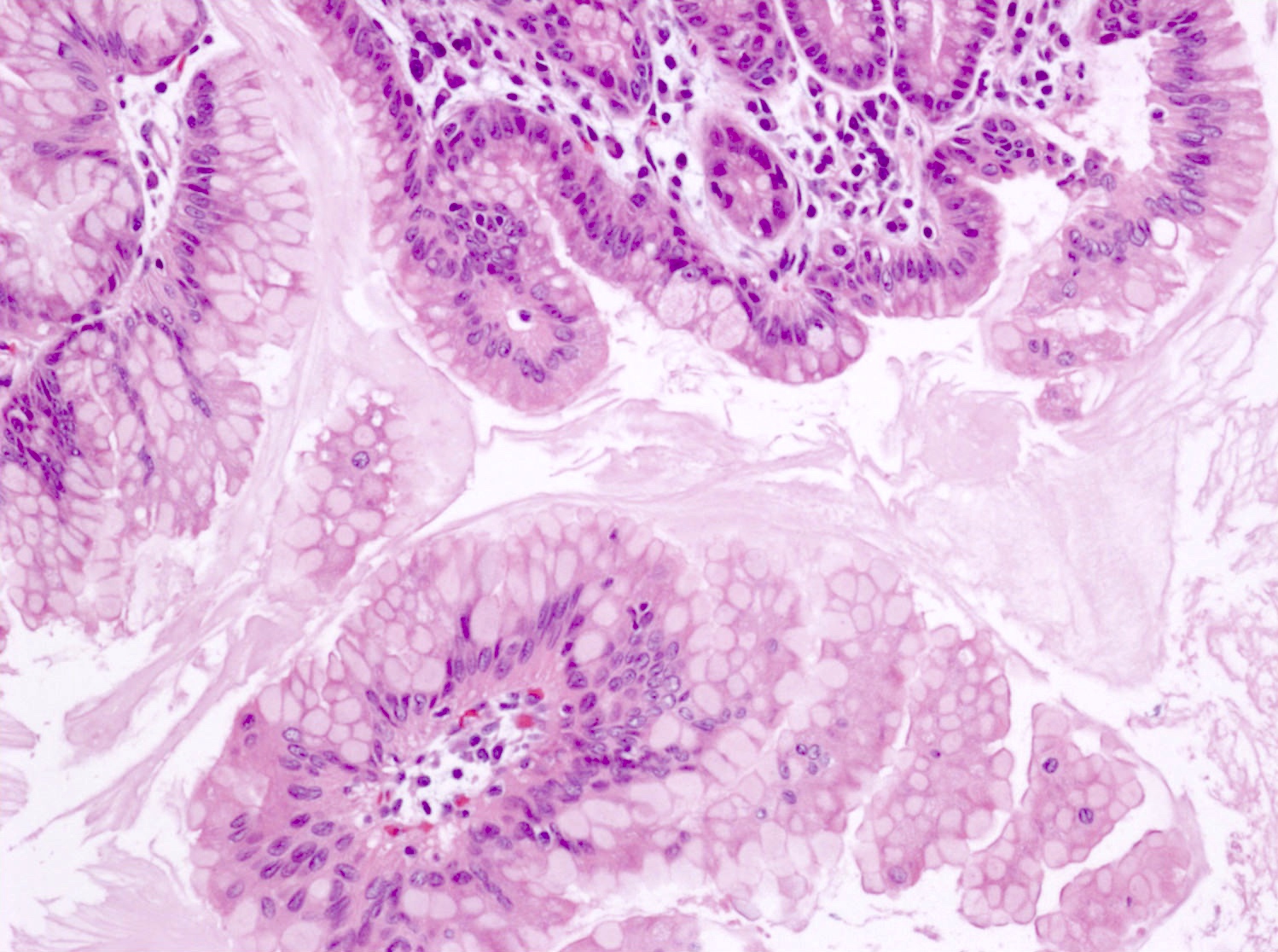
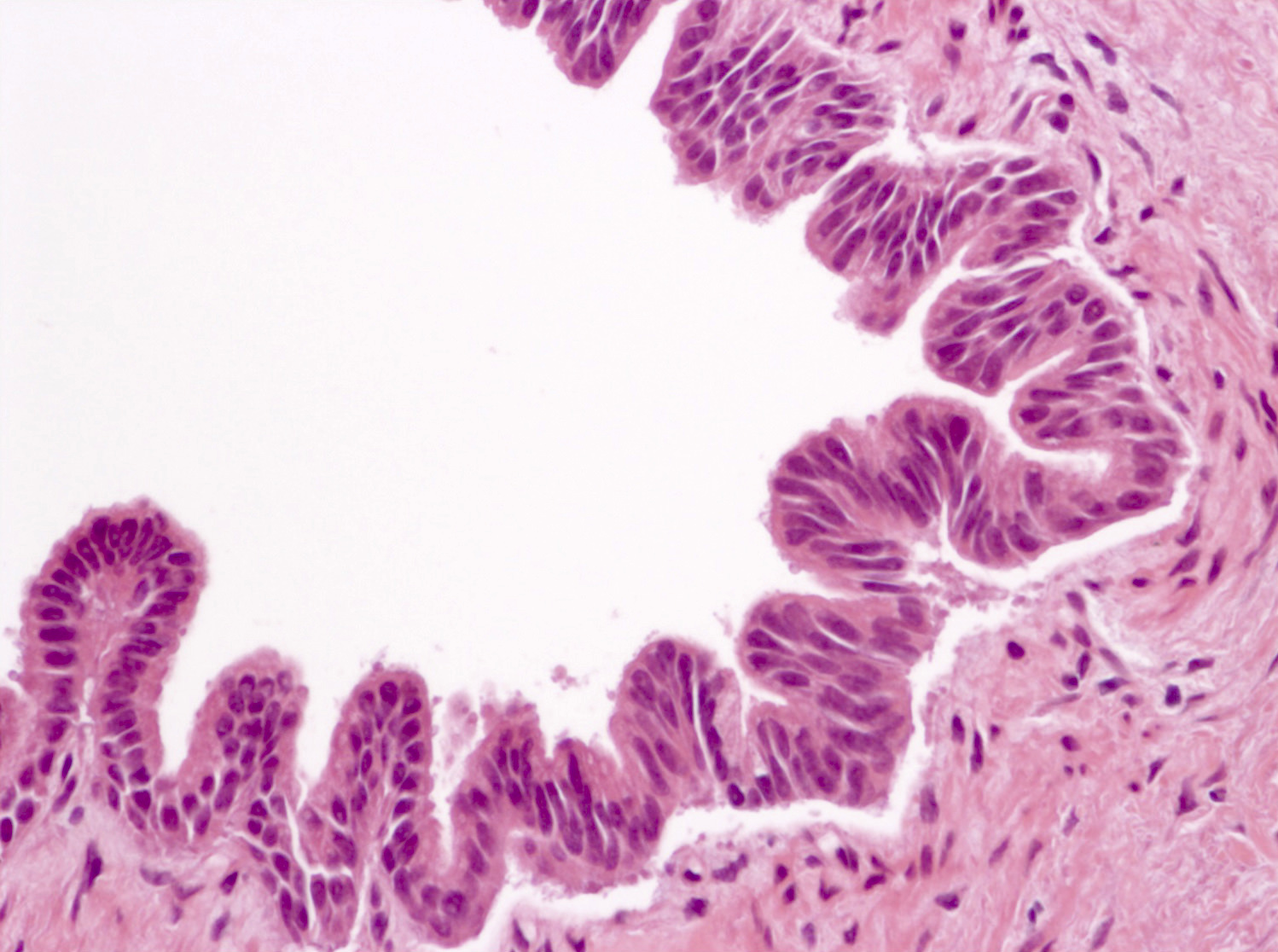
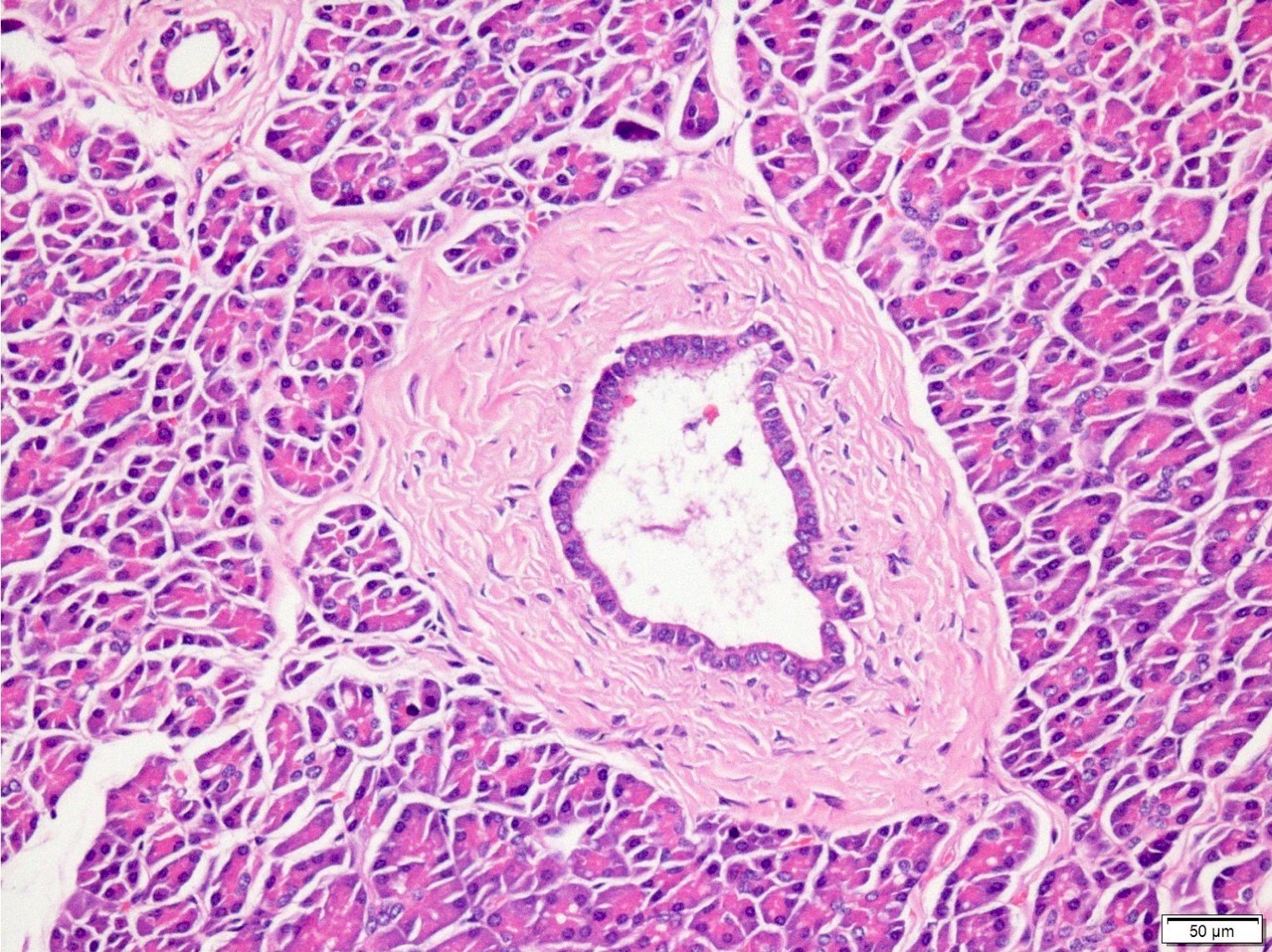
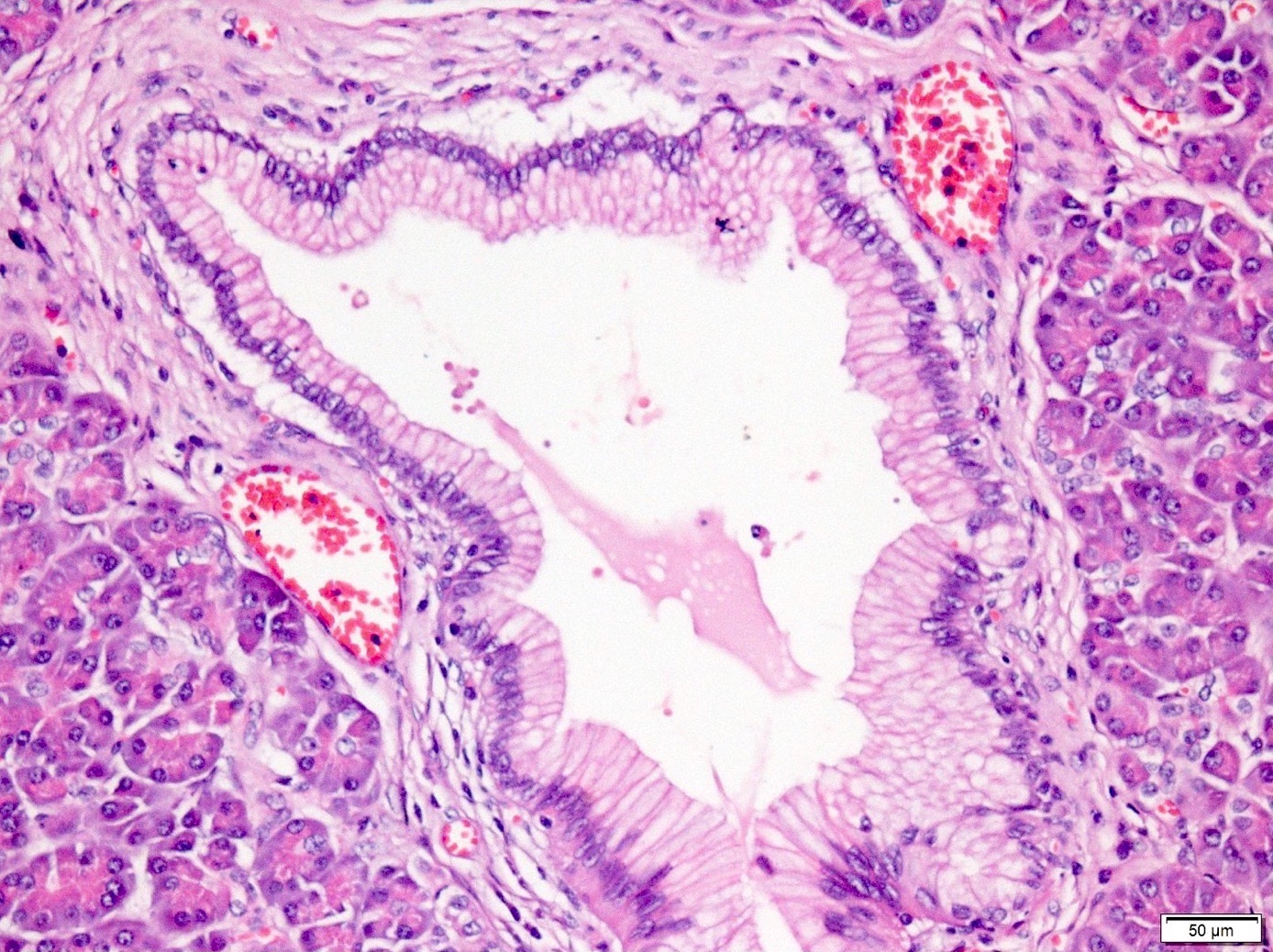
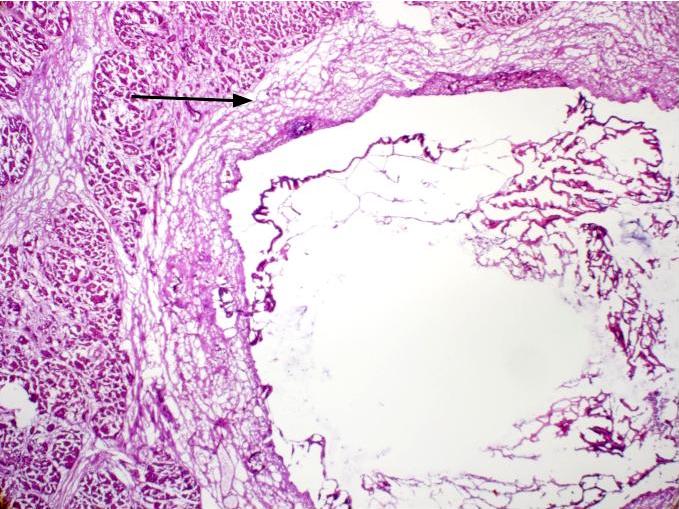
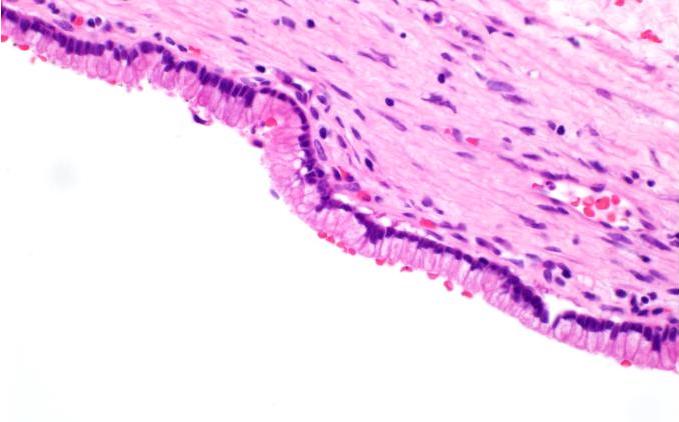
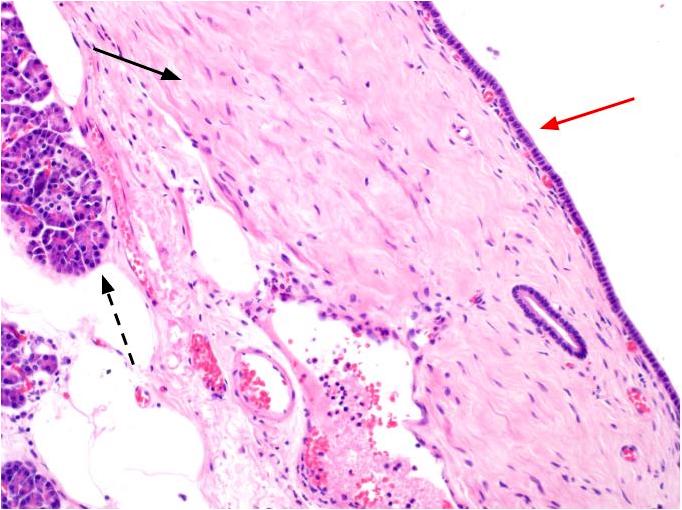
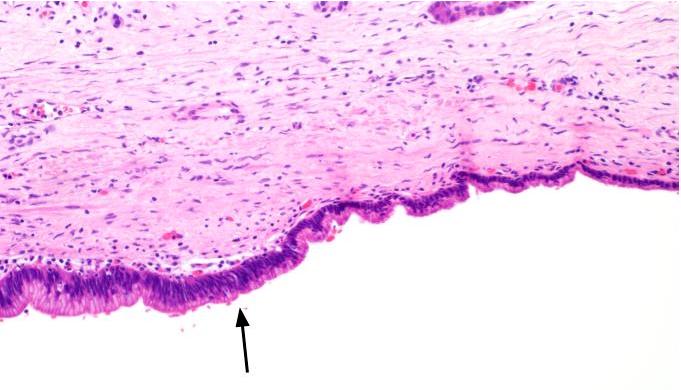
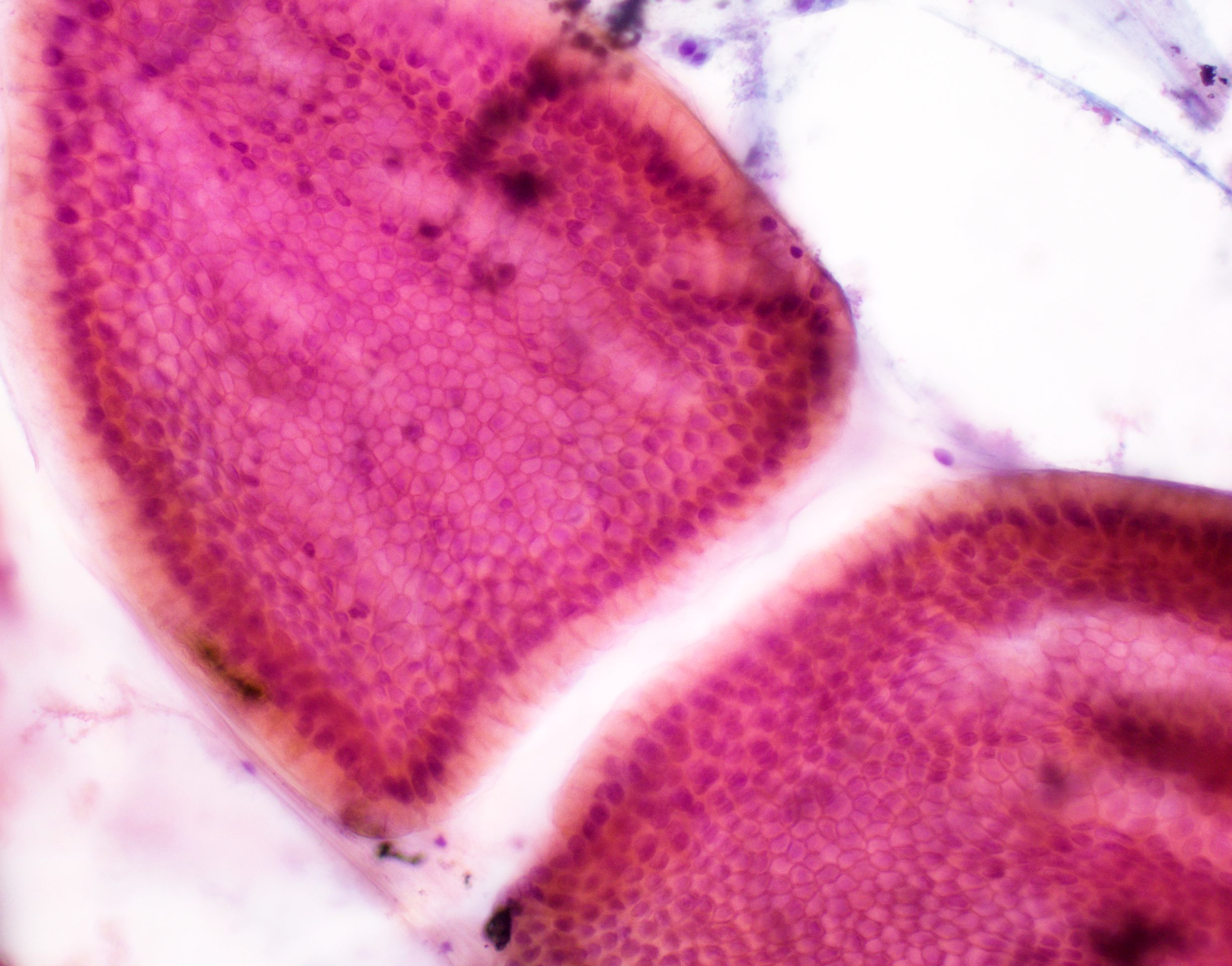
Microscopic (histologic) description
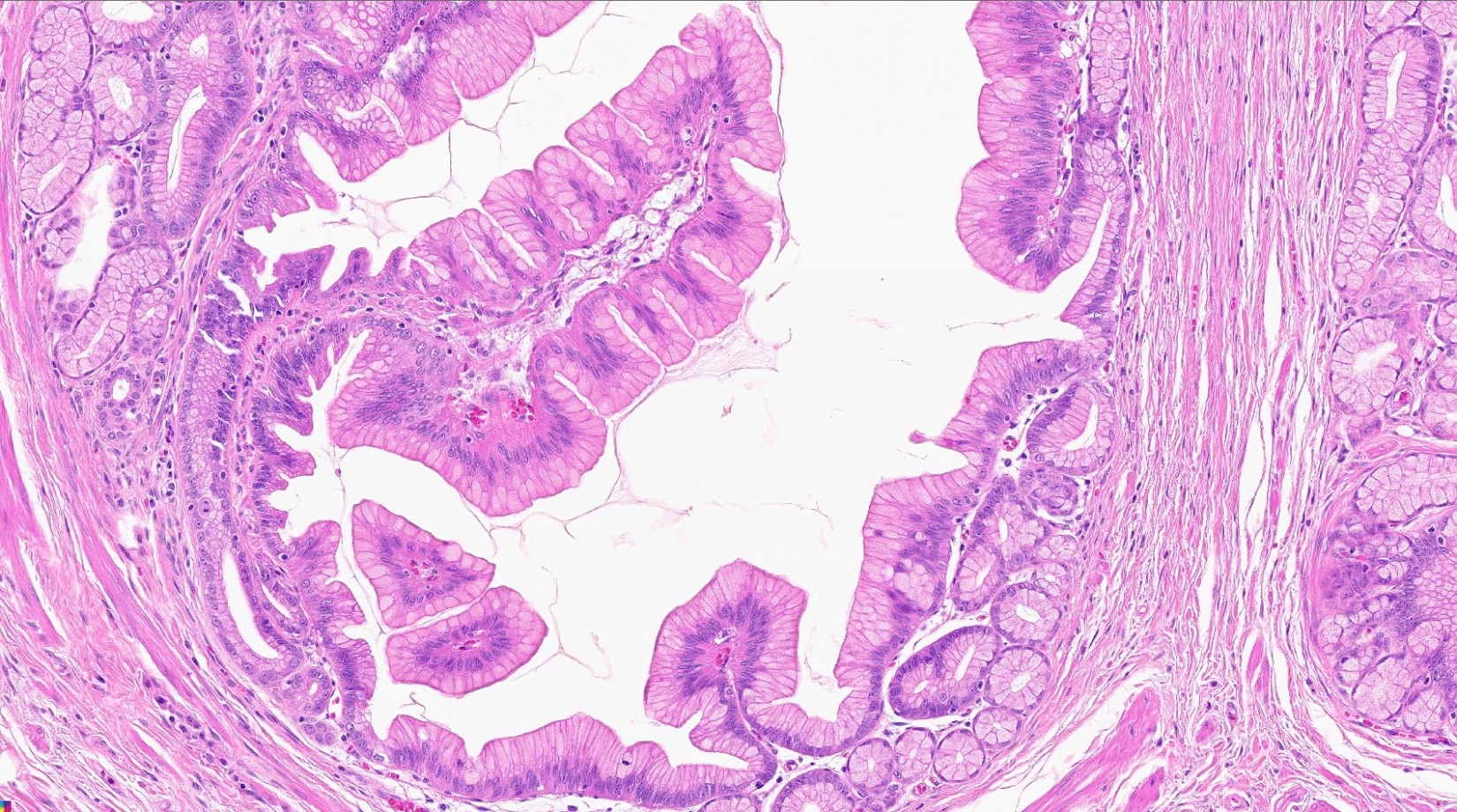
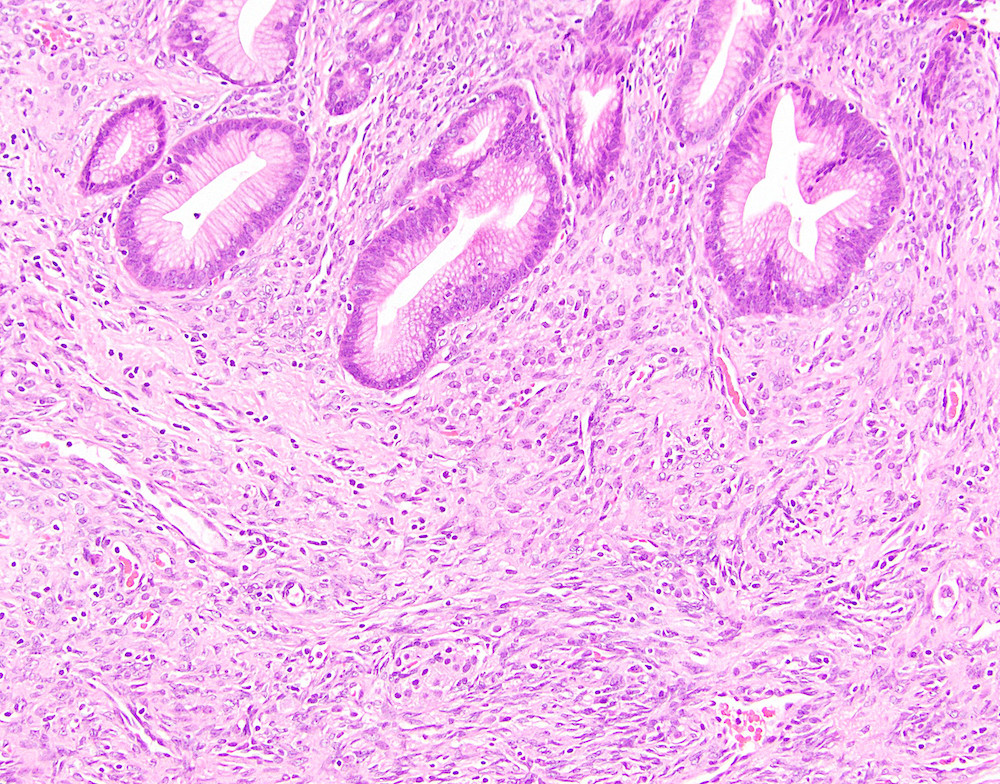
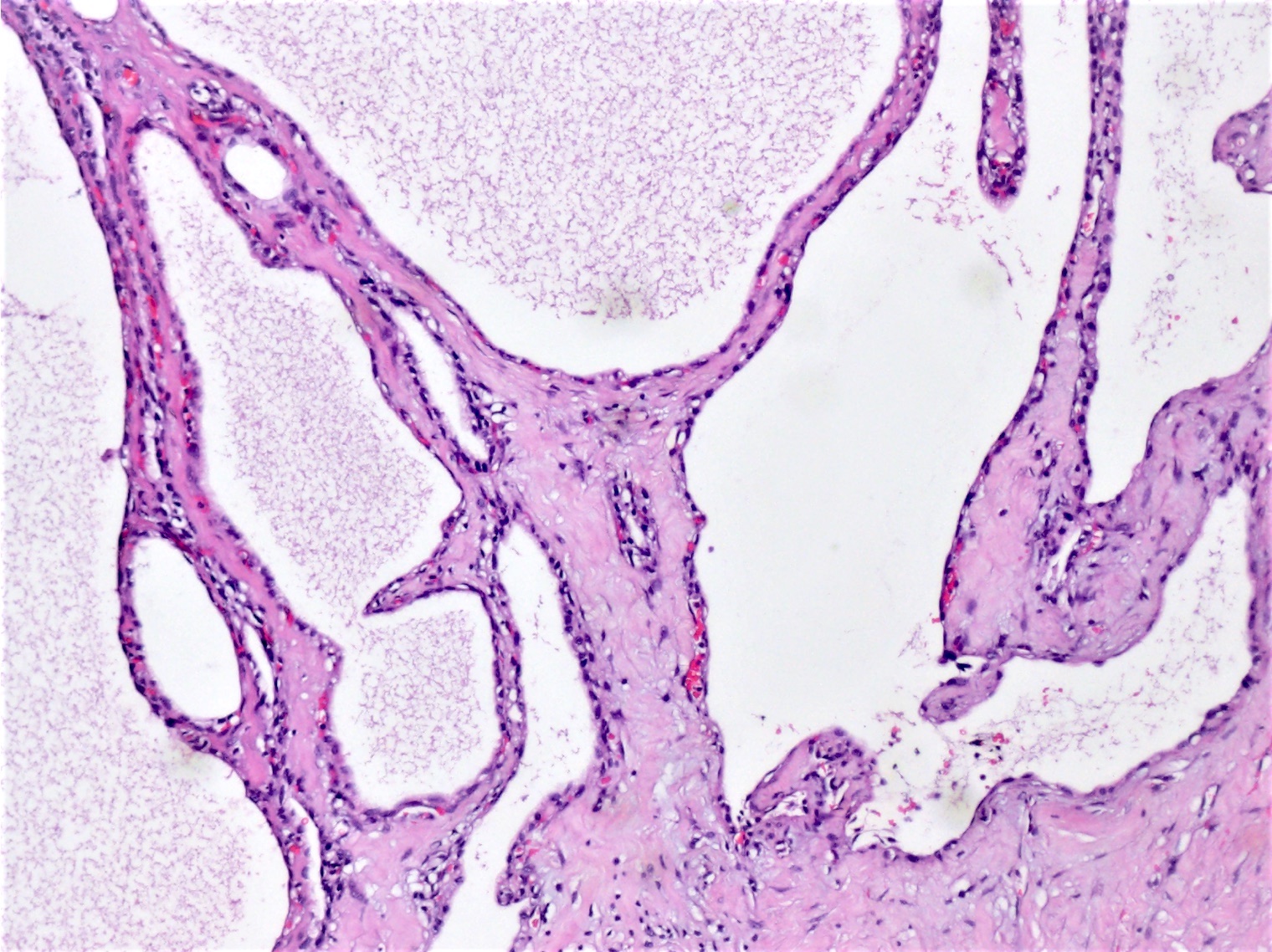
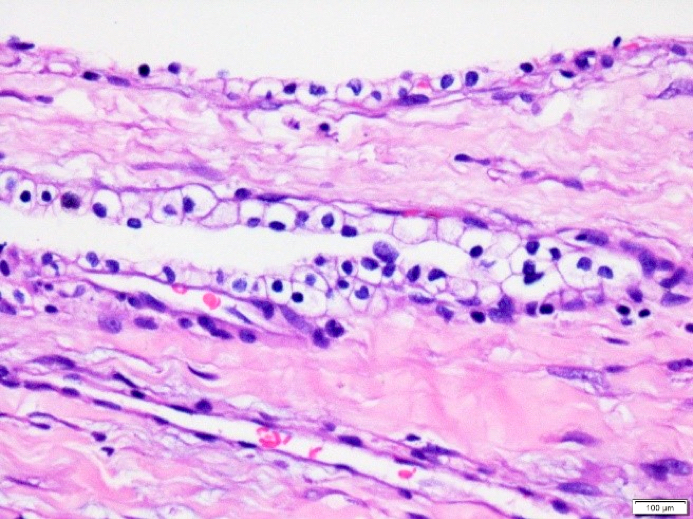
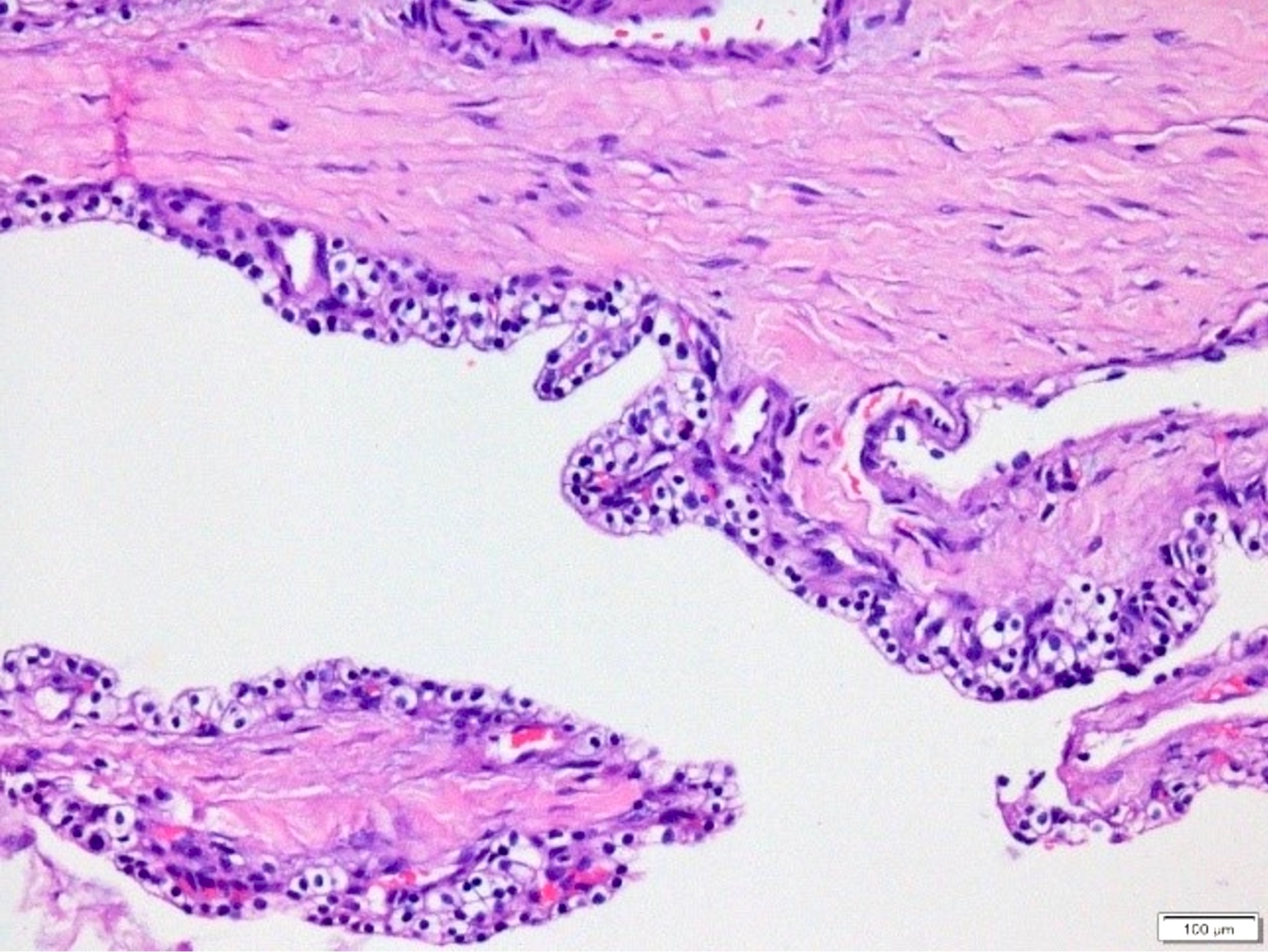
- Cysts of variable sizes lined by bland flattened cuboidal acinar cells with apical granular eosinophilic cytoplasm and dense basophilic basal cytoplasm
- Ductal epithelium can be admixed with the acinar cells
- May contain corpora amylacea-like dense eosinophilic lamellar concretions
- Multilocular cyst often demonstrates incomplete septa and club-like pseudopapillae
- Low Ki67 proliferation (≤ 3%) and nuclear atypia is minimal
- Cyst lining may show focal mucinous or clear cell change
- Negative for ovarian type stroma, necrosis or infiltrative growth
- References: J Clin Pathol 2023;76:740, Am J Surg Pathol 2023;47:379, Esposito: Pathology of the Pancreas, 1st Edition, 2022
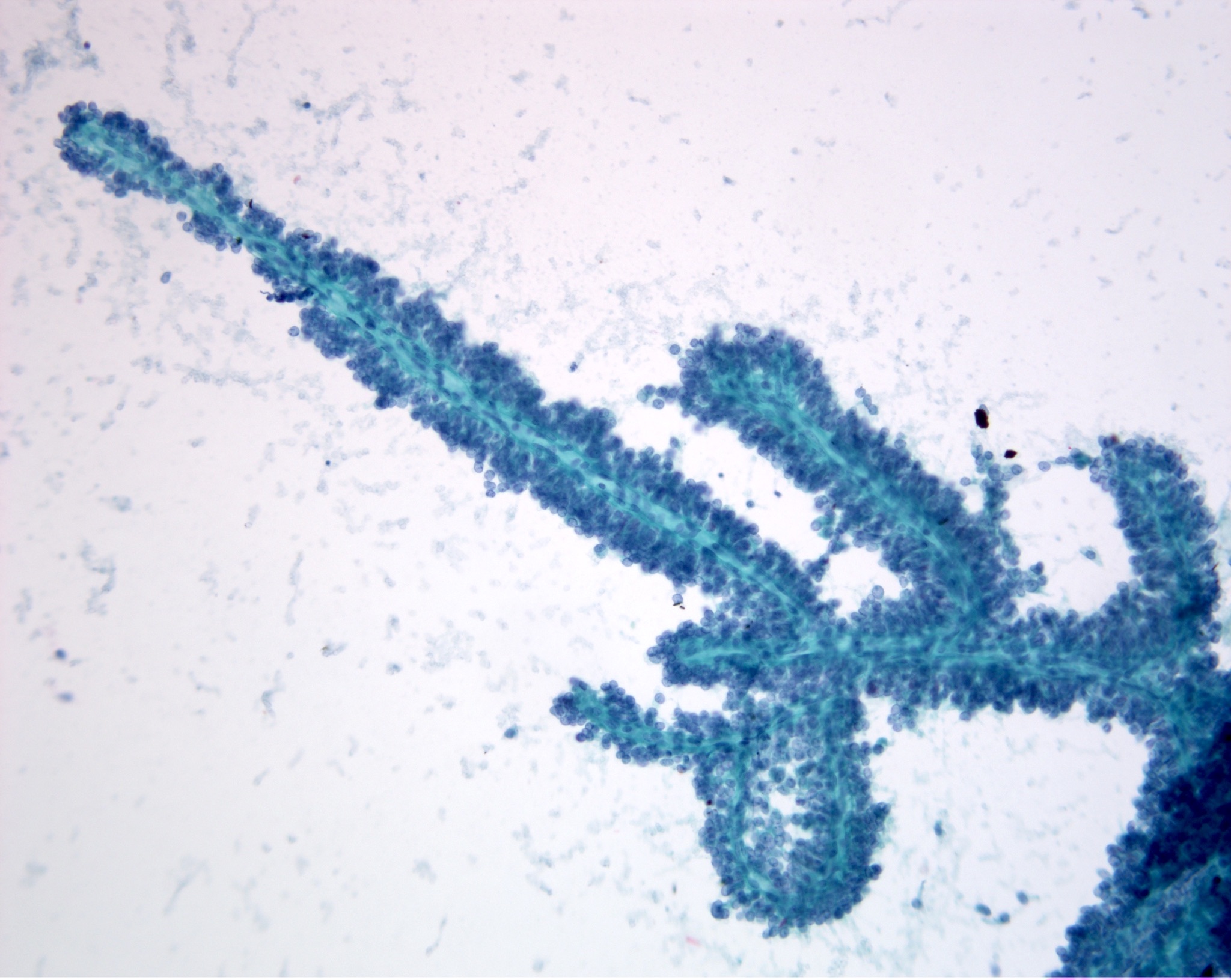
Microscopic (histologic) images
Contributed by Vidya Arole, M.D. and Wei Chen, M.D., Ph.D.
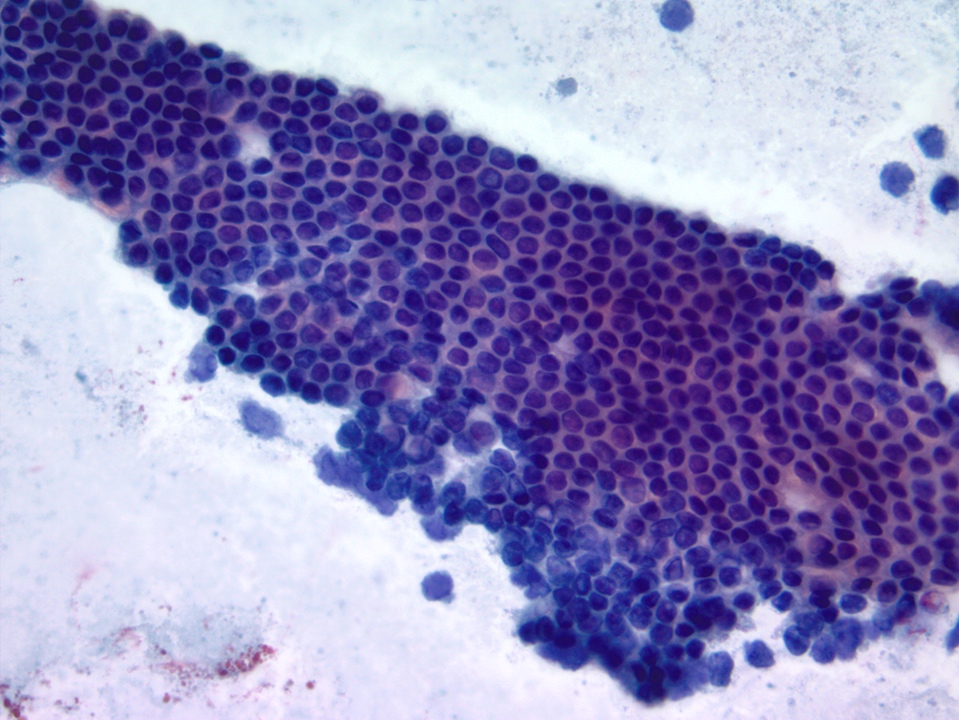
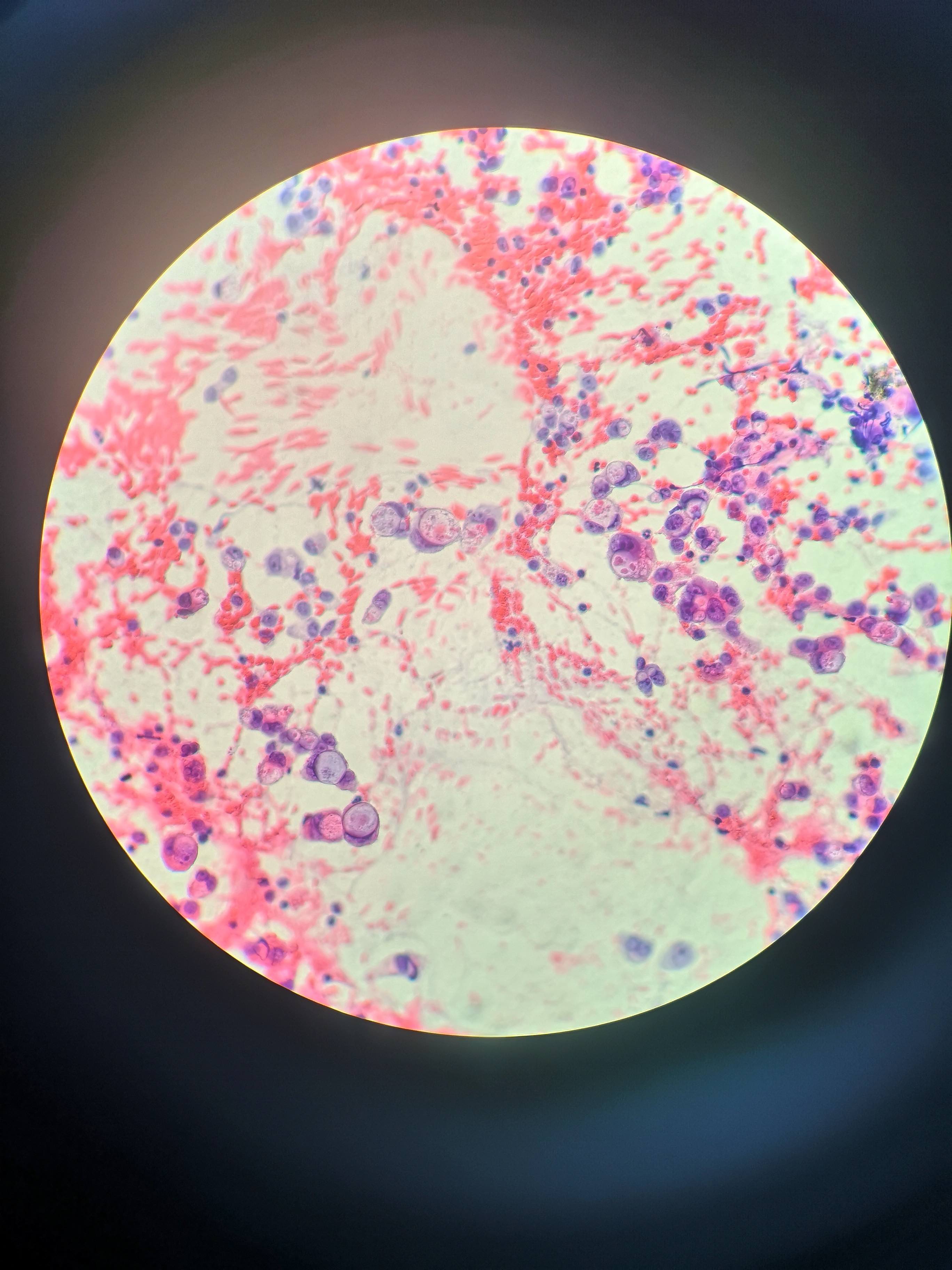
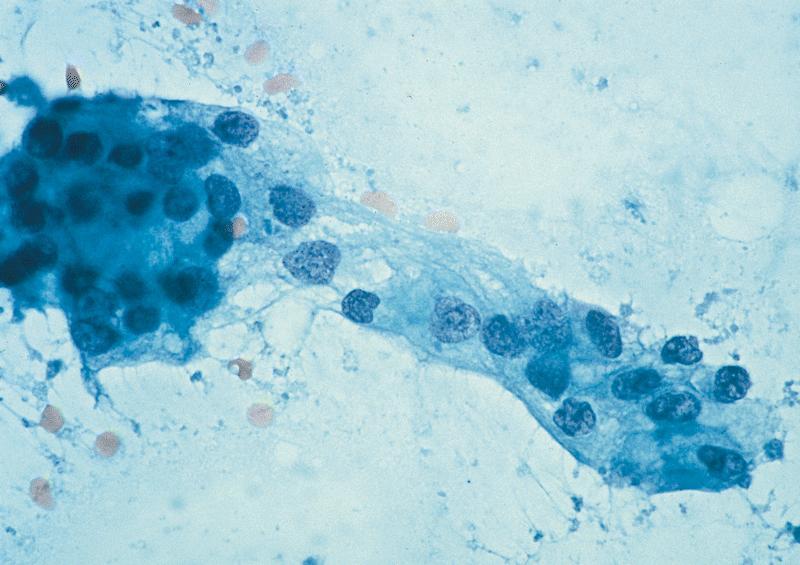
Cytology description
- Lesional epithelium indistinguishable from normal acinar and ductal cells
- Smears usually low cellularity, containing cells resembling benign acinar or ductal epithelial cells
- Smears / FNA specimens often interpreted as benign or nondiagnostic
- Eosinophilic concretions can mimic mucinous secretions
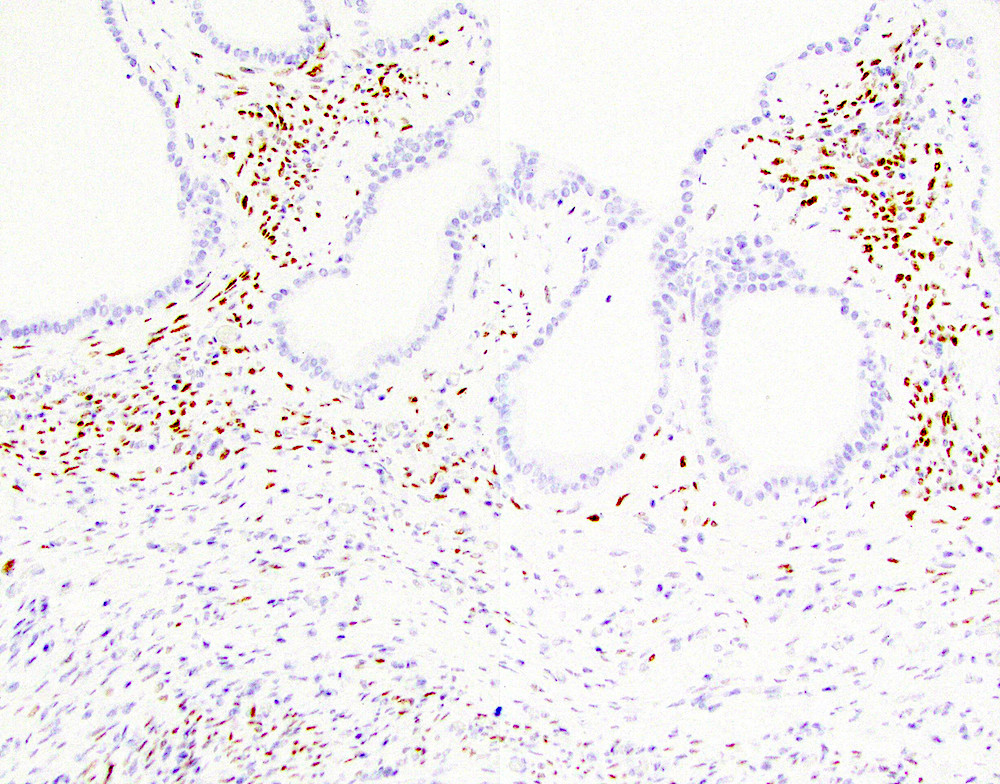
Positive stains
- Trypsin, chymotrypsin, lipase, CAM 5.2, CK7, CK8, CK18
- Apical cytoplasm with PAS positive, diastase resistant granules
- CK19 will stain intervening patches of the ductal epithelium
- References: J Clin Pathol 2023;76:740, Am J Surg Pathol 2023;47:379, Esposito: Pathology of the Pancreas, 1st Edition, 2022
Negative stains
- Alpha amylase, synaptophysin, chromogranin
Molecular / cytogenetics description
- Single report of array comparative genomic hybridization (CGH) reported the following chromosome gains: 1p, 3p, 5q, 6p, 7q, 8, 10q, 11, 14, 20 and X (Am J Surg Pathol 2012;36:1579)
- Random X chromosome inactivation observed in 5/5 cases, favoring nonneoplastic origin (Am J Surg Pathol 2013;37:1329)
- In molecular analysis of 4 ACT cases, all cases had wild type status for KRAS and CTNNB1 genes (Oncol Lett 2014;8:852)
- Next generation sequencing (NGS) performed on 9 ACT cases demonstrated driver mutations in 2 cases: one case a likely pathogenic mutation in SMO gene and the other a pathogenic mutation in KRAS gene (Am J Surg Pathol 2023;47:379)
Sample pathology report
- Distal pancreas and spleen, distal pancreatectomy and splenectomy:
- Acinar cystic transformation, 3.8 cm, pancreatic tail (see comment)
- Proximal pancreatic resection margin is uninvolved
- Benign spleen with no diagnostic abnormality
- Comment: Sections show a multilocular cyst lined by bland cuboidal cells with granular cytoplasm. Eosinophilic concretions are present in the cyst. On immunohistochemical staining, the cyst epithelium is positive for both trypsin and CK19. The findings support the diagnosis of acinar cystic transformation.
Differential diagnosis
- Cystic variant of acinar cell carcinoma (acinar cell cystadenocarcinoma):
- May have similar histology but mitotic activity is conspicuous and there is clear atypia
- Intraductal papillary mucinous neoplasms:
- Mucinous, papillary lining and connection to ductal systems favors IPMN
- Mucinous cystic neoplasm:
- Ovarian type stroma is diagnostic; absent in cystic acinar cell lesions
- Cyst lining is typically mucinous but may be nonmucinous; does not contain acinar cells
- Serous cystadenoma:
- Areas with attenuated cytoplasm may mimic ACT
- Serous cystadenoma contains rich subepithelial vascular network, which is typically absent in ACT
- Should be negative for trypsin
- Squamoid cyst of pancreatic ducts:
- Cystically dilated ducts are lined by a squamous / transitional epithelium, not acinar cells
- May contain acidophilic concretions
- Retention cyst:
- Cyst > 1 cm lined by flat (not papillary) epithelium and connected to pancreatic ductal system; secondary to obstruction (Am J Surg Pathol 2015;39:1730)
- Cyst epithelium may show mucinous change, negative for acinar cells / trypsin
Additional references
Board review style question #1
This is an incidental cyst found in the distal pancreas. On immunohistochemical staining, the cyst epithelium is positive for both CK19 and trypsin. Ki67 proliferation is low (< 3%). What is the diagnosis?
- Acinar cystic transformation
- Cystic acinar cell carcinoma
- Cystic solid pseudopapillary neoplasm
- Intraductal papillary mucinous neoplasm
Board review style answer #1
A. Acinar cystic transformation. The photograph demonstrates a cystic lesion lined by bland nonmucinous epithelium and filled with eosinophilic concretions. The positive CK19 and trypsin indicate ductal and acinar differentiation. The overall histomorphology and immunoprofile support the diagnosis of acinar cystic transformation of the pancreas. Answer D is incorrect because the cyst epithelium is nonmucinous. Answer B is incorrect because acinar cell carcinoma would have a much higher proliferation rate than 3%. Answer C is incorrect because solid pseudopapillary neoplasm should be negative for trypsin.
Comment Here
Reference: Acinar cystic transformation
Comment Here
Reference: Acinar cystic transformation
ACTH secreting tumors
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Clinical features | Diagnostic criteria | Radiology images | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology images | Positive stains | Electron microscopy description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
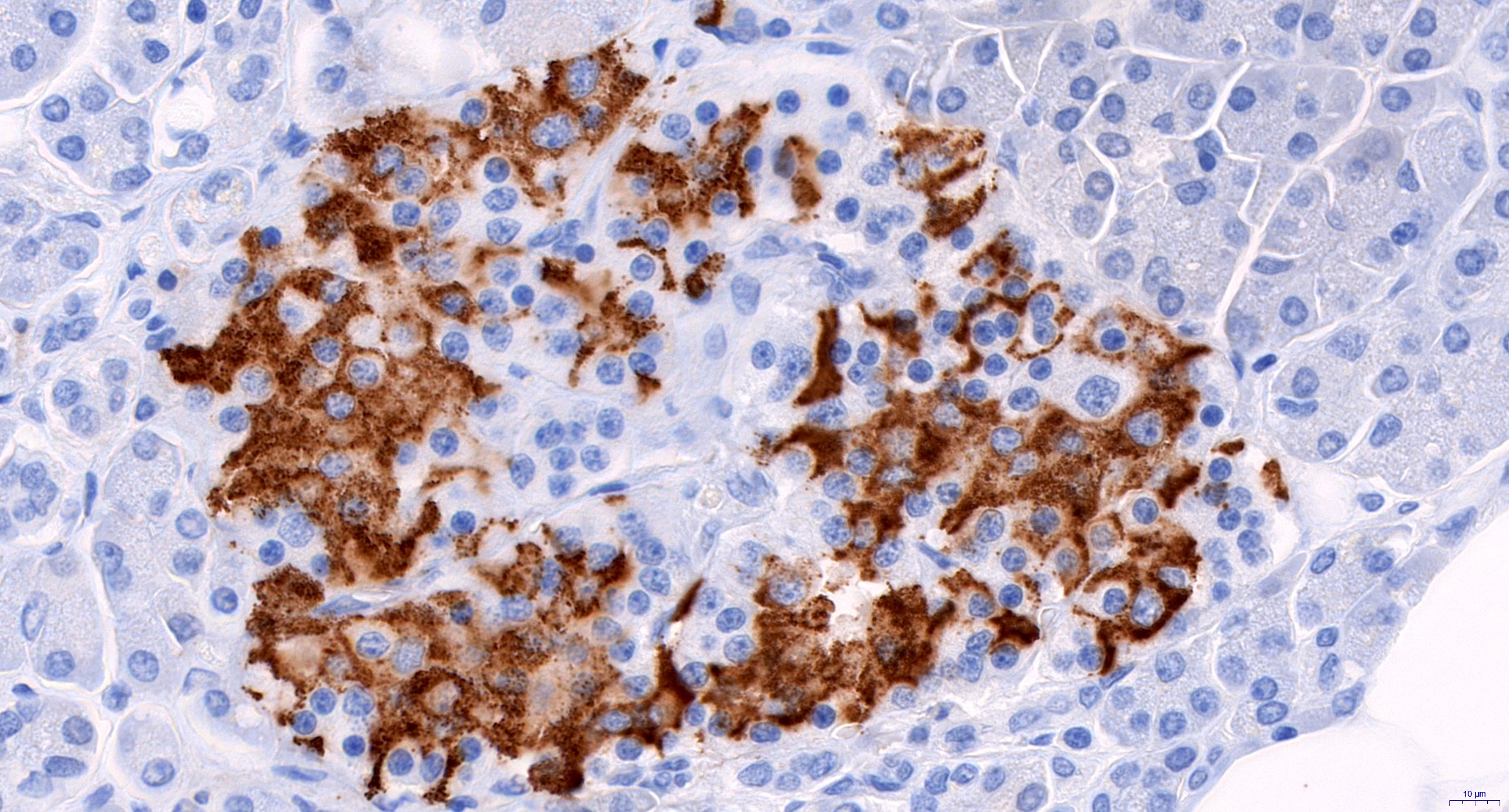
- Rare pancreatic neuroendocrine neoplasm producing adrenocorticotrophic hormone (ACTH)
- ACTH can also rarely be produced by acinar cell carcinoma or pancreatoblastoma (Am J Surg Pathol 2015;39:374)
Essential features
- Rare lesion mostly described in case reports
- Patients develop Cushing syndrome clinically
- Aggressive, with poor prognosis
Terminology
- Recognized by IARC as ACTH producing tumor with Cushing syndrome (Lloyd: WHO Classification of Tumours of Endocrine Organs, 4th Edition, 2017)
ICD coding
- ICD-10: E24.3 - ectopic ACTH syndrome
Epidemiology
- Responsible for about 15% of cases of ectopic Cushing syndrome
Sites
- May be more common in tail of pancreas
Clinical features
- Mean age 42 years, with a female predominance (Am J Surg Pathol 2015;39:374)
- Causes Cushing syndrome: central obesity, muscle weakness, glucose intolerance, hypertension
- 33% of patients also have Zollinger-Ellison syndrome
- 10 year survival is roughly 15% (Am J Surg Pathol 2015;39:374)
Diagnostic criteria
- Determined clinically, not by immunohistochemical positivity for ACTH
Case reports
- 27 year old woman with ovarian and pelvic metastases (Int J Clin Exp Pathol 2015;8:15396)
- 40 year old woman with Cushing syndrome (Ann Hepatobiliary Pancreat Surg 2017;21:61)
- 41 year old woman with bilateral ovarian metastases (Int J Gynecol Pathol 2002;21:276)
- 48 year old woman with tumor secreting ACTH and possibly corticotropin releasing hormone (CRH) (Endocr Pathol 2015;26:239)
- 54 year old woman with ACTH secretion only at second relapse (J Clin Endocrinol Metab 2004;89:3731)
Treatment
- Surgical excision
Gross description
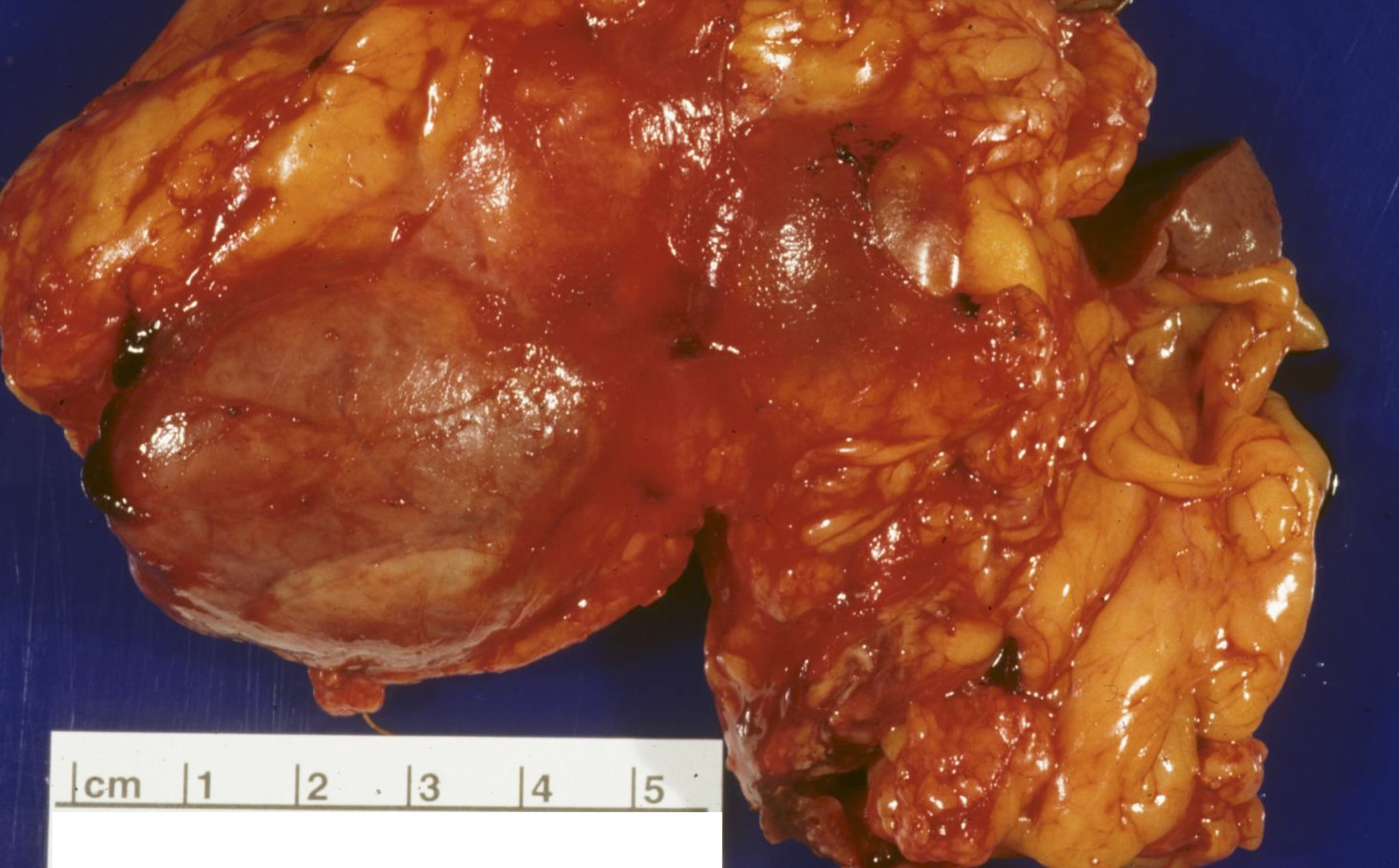
- Firm, gray-white, well circumscribed lesions that may measure up to 5 cm
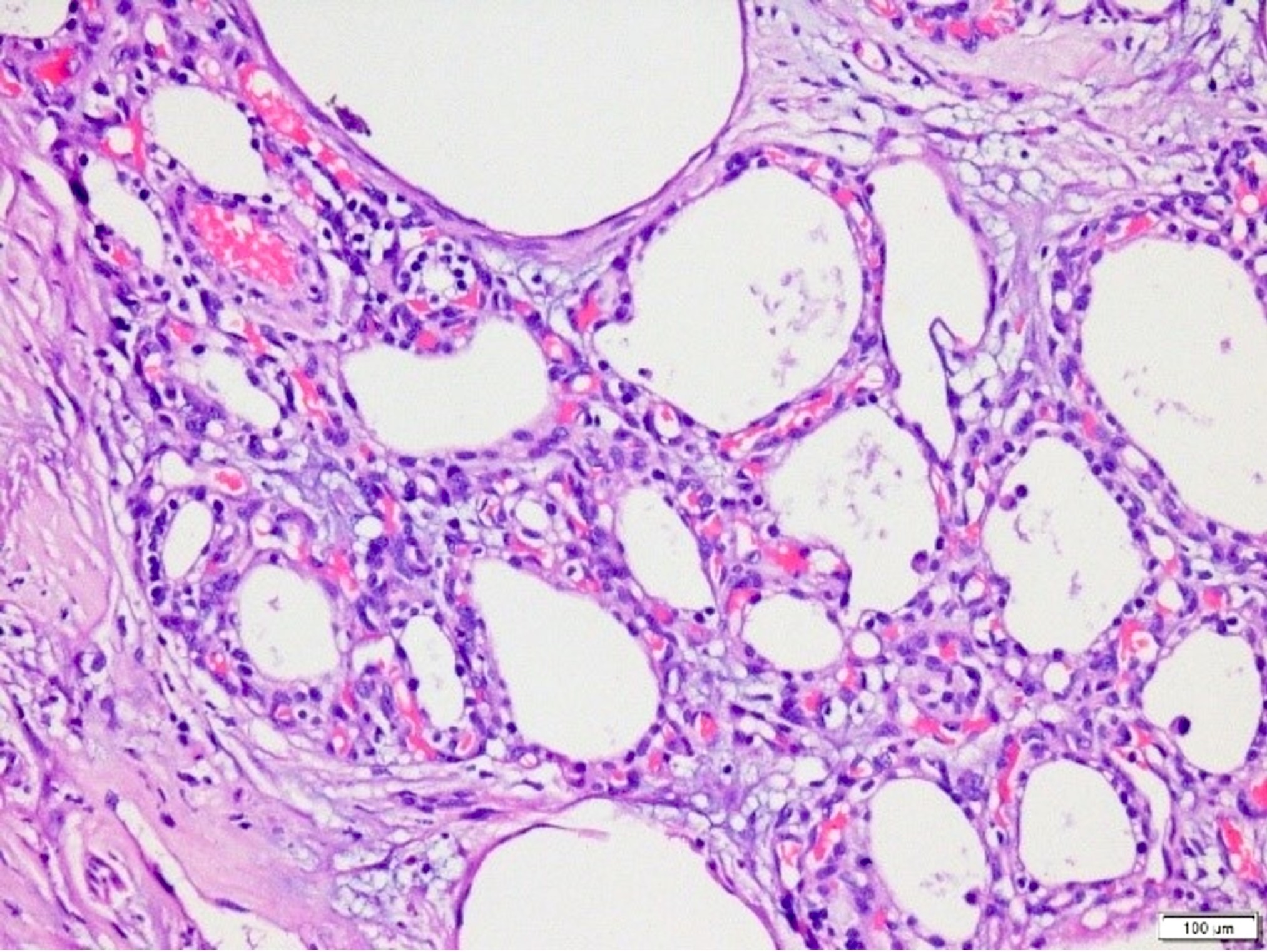
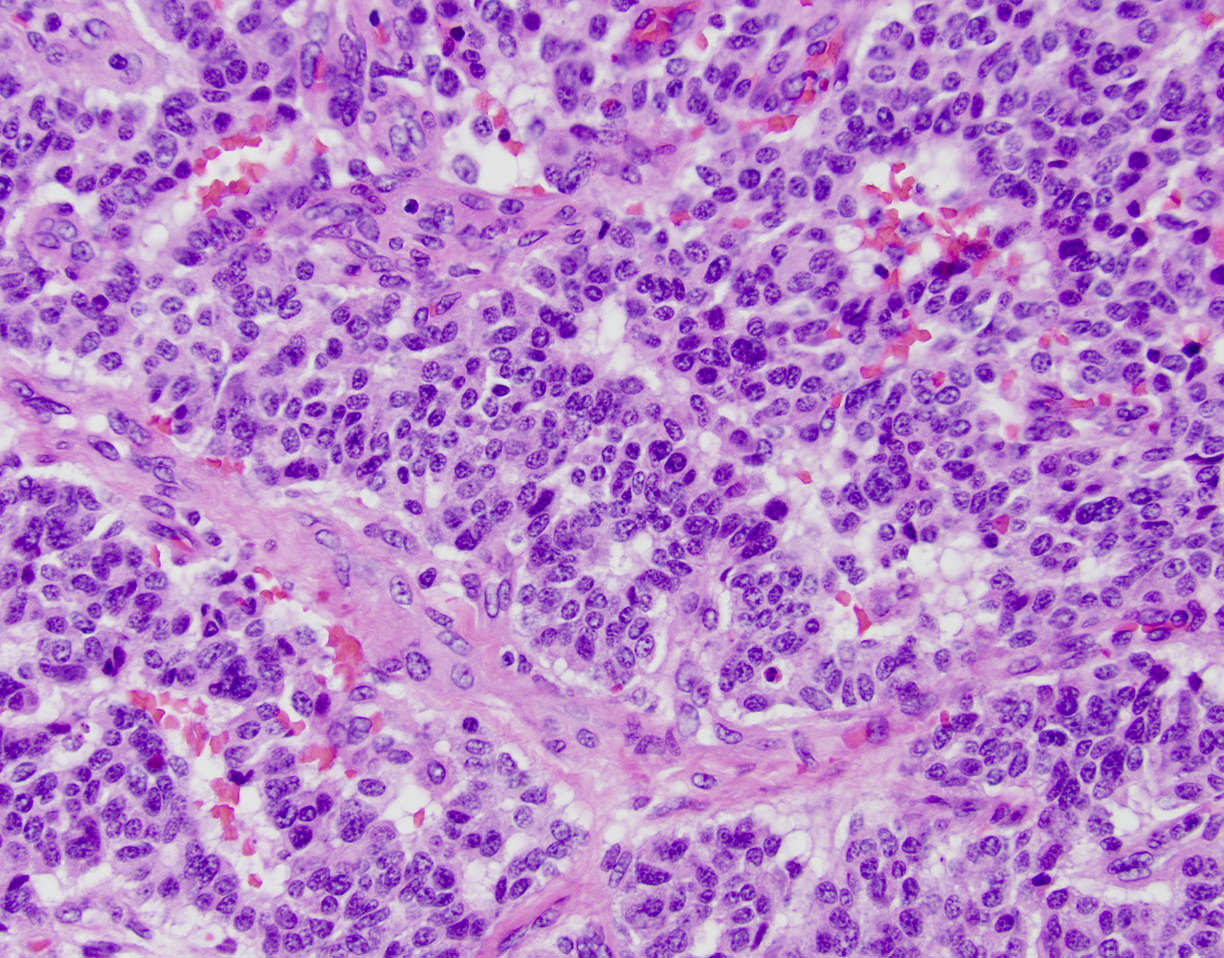
Microscopic (histologic) description
- Appears similar to other well differentiated neuroendocrine tumors of the pancreas
- Lymphovascular and perineural invasion often present
- Focal necrosis is rare
Microscopic (histologic) images
Positive stains
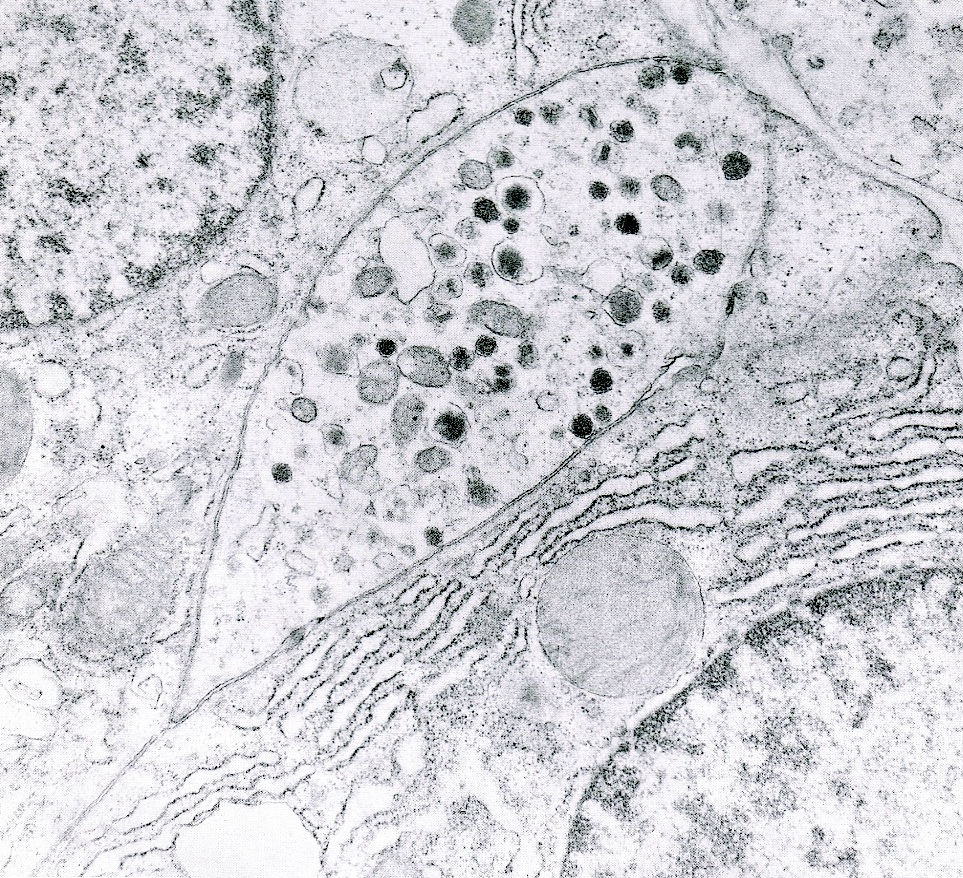
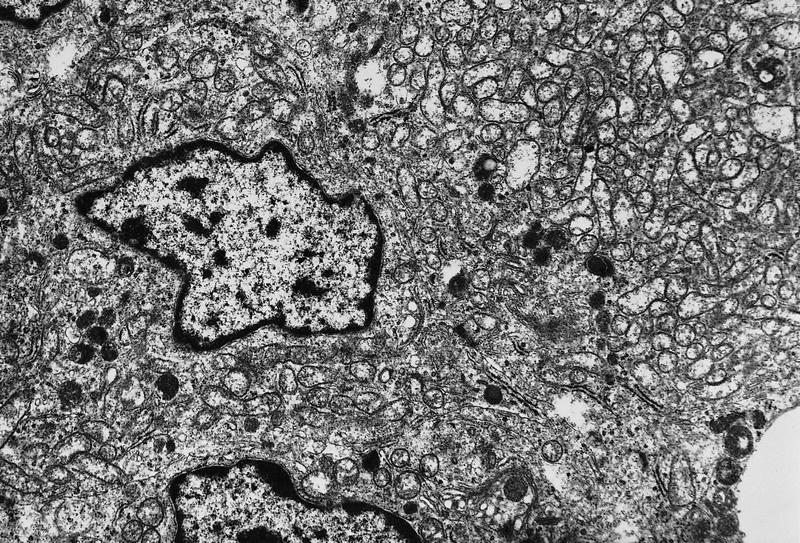
Electron microscopy description
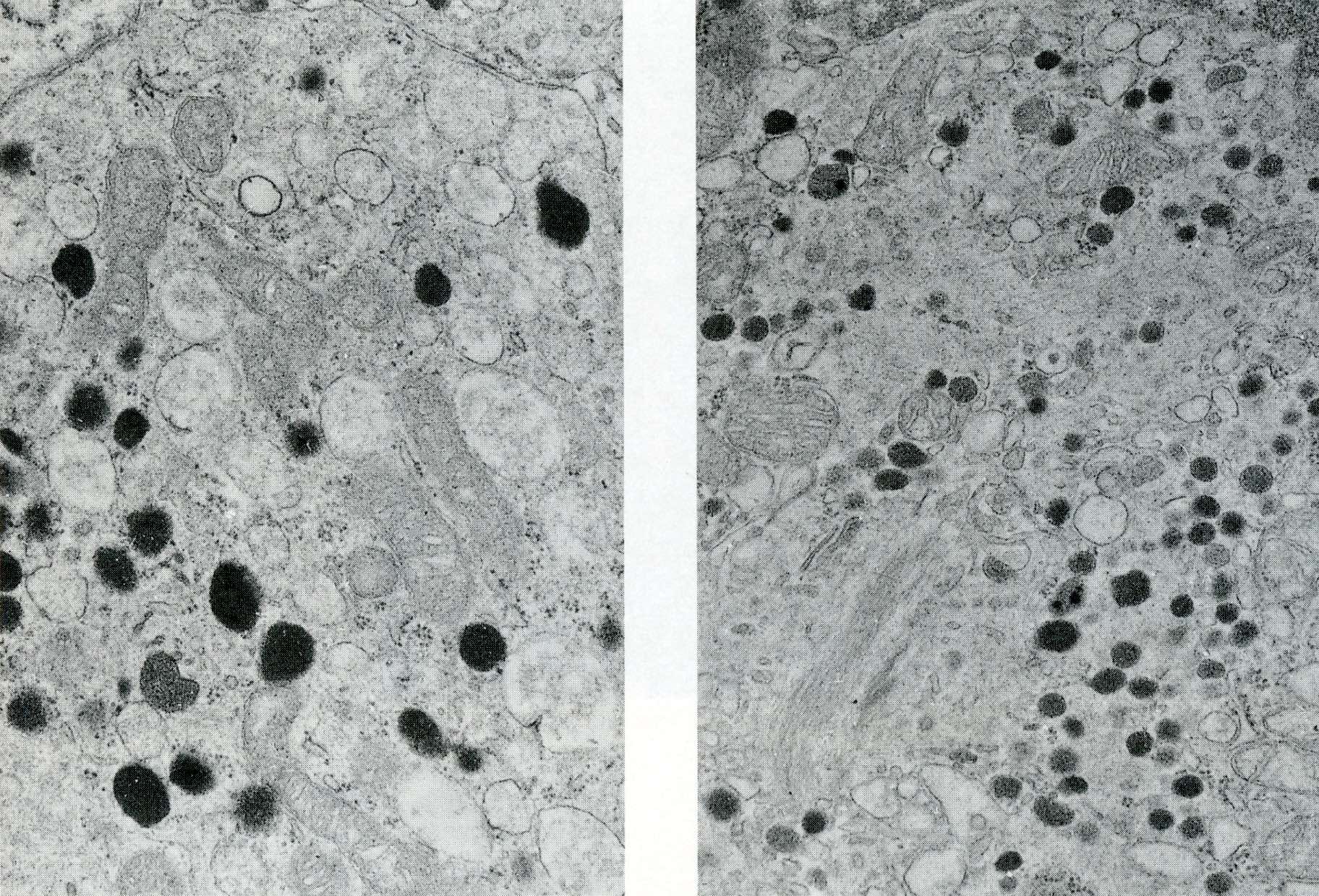
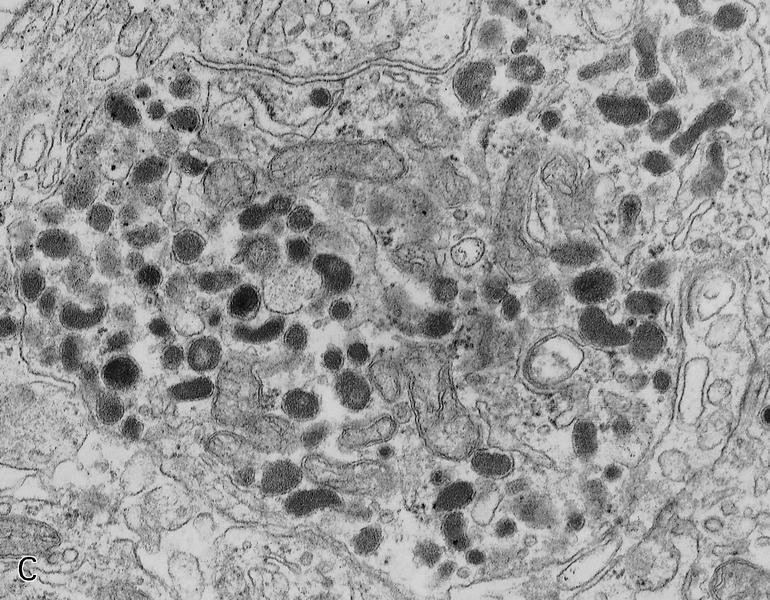
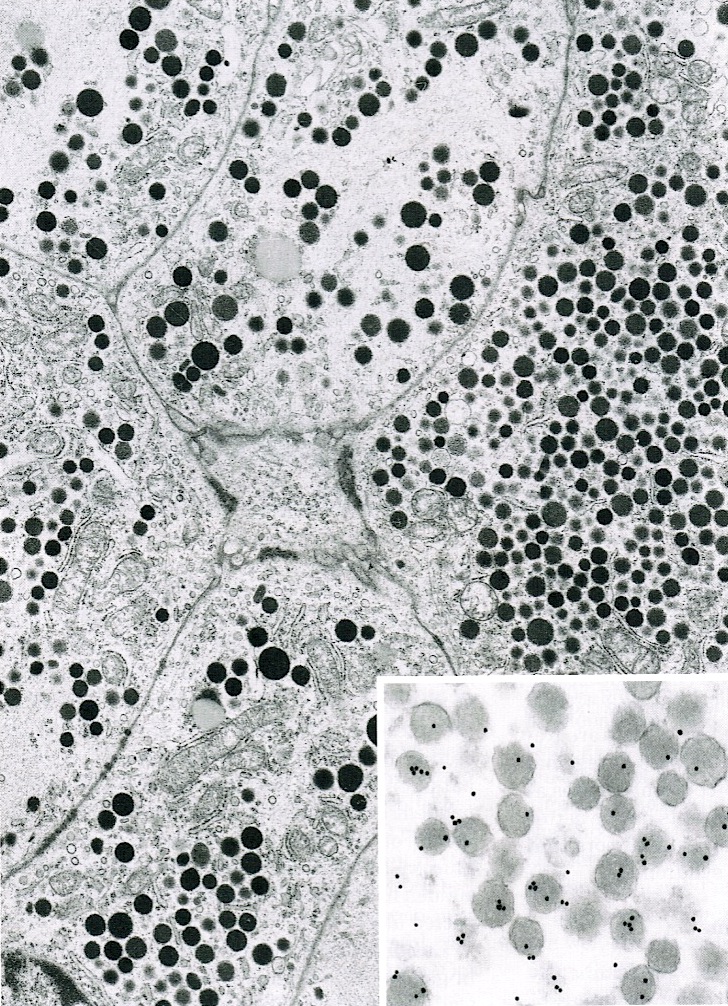
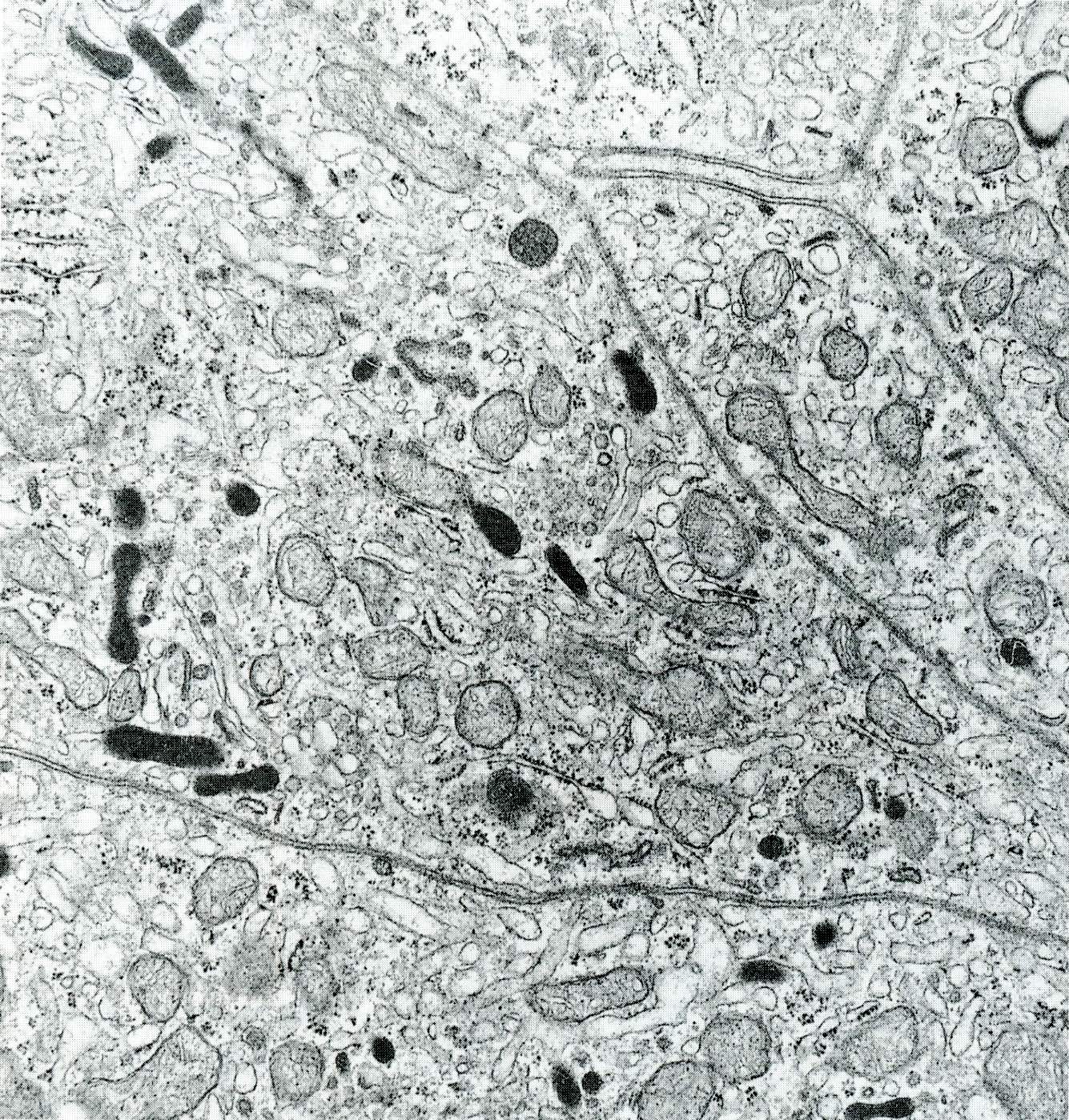
- Round cytoplasmic secretory granules, diameter of 100 - 300 nm, with an electron dense core (Int J Clin Exp Pathol 2015;8:15396)
Sample pathology report
- Pancreas, tail, resection:
- Pancreatic neuroendocrine tumor, WHO grade 1 (see synoptic report and comment)
- Comment: The patient’s clinical history of Cushing syndrome is noted. This may be due to ACTH secretion by this tumor. Immunohistochemical stains for synaptophysin and chromogranin are positive and the Ki67 index is approximately 2.3%.
Differential diagnosis
- Non-ACTH secreting pancreatic neuroendocrine tumor:
- Must be determined on clinical grounds, though ACTH positivity by immunohistochemistry is rare in these (Proc (Bayl Univ Med Cent) 2015;28:46)
- Pancreatic acinar cell carcinoma
- Pancreatoblastoma
Board review style question #1
- A 45 year old woman presents with complaints of weight gain, increased facial hair, striae on her arms and easy bruising. An abdominal CT scan shows a 4 cm mass in the tail of her pancreas. What hormone is the tumor likely secreting?
- ACTH
- Cortisol
- Gastrin
- Glucagon
Board review style answer #1
A. ACTH. The patient is presenting with Cushing syndrome, secondary to ACTH production by her pancreas lesion (likely a neuroendocrine tumor), which leads to increased cortisol production by the adrenal glands. Pancreatic tumors do not themselves secrete cortisol.
Comment Here
Reference: ACTH secreting tumors
Comment Here
Reference: ACTH secreting tumors
Board review style question #2
- Which of the following is often seen microscopically in well differentiated pancreatic neuroendocrine tumors that secrete ACTH?
- Abundant necrosis
- Grade 3 mitotic rate
- Lymphocytic infiltration
- Perineural invasion
Board review style answer #2
D. Perineural invasion. These tumors, which are commonly WHO grade 2, often show lymphovascular and perineural invasion. Focal necrosis may sometimes be seen.
Comment Here
Reference: ACTH secreting tumors
Comment Here
Reference: ACTH secreting tumors
Acute pancreatitis
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Inflammatory disease of the exocrine pancreas that causes acute onset of severe abdominal pain and might lead to pancreatic necrosis
Essential features
- Reversible pancreatic parenchymal injury
- Causes include gallstones, alcohol and hypertriglyceridemia
- Main histological features include acute inflammatory cells with hemorrhage and necrosis
- Treatment is supportive
Terminology
- Interstitial edematous pancreatitis: often referred to simply as acute pancreatitis
- Necrotizing pancreatitis
- Other terminologies: bile pancreatitis, infected pancreatic necrosis and postoperative pancreatitis
ICD coding
Epidemiology
- Incidence of acute pancreatitis is similar among men and women; male sex is associated with higher mortality
- Peak incidence of acute pancreatitis is in the fifth and sixth decades
- In the U.S., incidence has been cited as 600 - 700 per 100,000 people, with 200,000 - 250,000 discharges occurring yearly for acute pancreatitis
- Gallstone disease is the leading cause and accounts for 20 - 70% of all cases in the West
- Alcohol is the second most common cause of acute pancreatitis, accounting for up to 30% of cases
- Hypertriglyceridemia accounts for ~9% of cases, making it the third most common cause
- Mortality of acute pancreatitis ranges from 3% in patients with mild edematous pancreatitis to as high as 20% in patients with pancreatic necrosis
- References: StatPearls: Acute Pancreatitis [Accessed 11 July 2023], United European Gastroenterol J 2018;6:649, J Clin Med 2021;10:300
Sites
- Pancreas
Pathophysiology
- Acute pancreatitis is triggered by various factors that disrupt normal intracellular calcium signaling in pancreas cells, which normally maintains stimulus secretion coupling
- Increasing intracellular calcium leads to premature activation of the enzyme trypsinogen to trypsin within the acinar cell instead of the duct lumen
- Intracellular calcium also overwhelms mitochondria leading to impaired production of adenosine triphosphate (ATP)
- Diminished ATP affects cellular process leading cytokine release, cellular necrosis and inflammation
- Proinflammatory cytokines mediate powerful immune response, leading to systemic inflammatory response syndrome, multiorgan dysfunction and higher susceptibility to infections
- References: Drugs 2022;82:1251, StatPearls: Acute Pancreatitis [Accessed 11 July 2023], Int J Mol Sci 2020;21:4005
Etiology
- Gallstone disease: the leading cause of acute pancreatitis worldwide; accounts for 20 - 70% of cases in the West and is more common in women
- Alcohol: second most common cause of acute pancreatitis in North America and Europe, contributing to up to 33% of cases
- Hypertriglyceridemia: elevated triglyceride levels in the blood can cause acute pancreatitis, accounting for ~9% of cases
- Drugs such as antiretrovirals, chemotherapeutic agents, antibiotics, steroids and others
- Infectious causes: most commonly mumps, cytomegalovirus (CMV), Epstein-Barr virus (EBV)
- Genetic factors also play a role, such as hereditary pancreatitis associated with specific gene mutations in PRSS1, SPINK1, CFTR and CTRC
- Other causes: there are various less common causes of acute pancreatitis, including trauma, hypercalcemia, tumors, anatomical variants, cardiac bypass surgery, scorpion bites and organophosphate poisoning
- References: Drugs 2022;82:1251, World J Clin Pediatr 2022;11:27
Clinical features
- Symptoms: mild to severe epigastric pain, nausea and vomiting
- Signs: high white blood count, diffuse fat necrosis, peripheral vascular collapse, acute tubular necrosis, shock (blood loss, electrolyte disturbances, endotoxemia, release of cytokines), hypocalcemia, hyperglycemia
- Complications: pancreatic pseudocyst, walled off necrosis, peripancreatic fluid collection
- Systemic complications include: acute respiratory distress syndrome (ARDS), acute kidney injury (AKI), disseminated intravascular coagulation (DIC)
- References: Drugs 2022;82:1251, World J Emerg Surg 2019;14:27
Diagnosis
- Abdominal pain consistent with pancreatitis
- Serum amylase or lipase 3 or more times the upper limit of normal
- Findings consistent with pancreatitis on CT, MRI or in some cases transabdominal ultrasound (TUS)
- References: Drugs 2022;82:1251
Laboratory
- High amylase and lipase
- High blood glucose
Radiology description
- Abdominal ultrasound (US): first line modality
- Pancreatic enlargement and decreased parenchymal echogenicity due to interstitial edema
- Limitations of US is the inability to make distinction between interstitial and necrotizing pancreatitis
- Computed tomography (CT): gold standard
- Enlargement of the pancreas
- Ill defined parenchymal contours and decreased density and inhomogeneity of the pancreatic parenchyma
- Fluid collections in the peripancreatic region and the inflammatory reaction can produce increased attenuation of the peripancreatic fat tissue commonly described as stranding
- Reference: Diagn Interv Imaging 2015;96:151
Prognostic factors
- Bed side index of severity of acute pancreatitis (BISAP)
- Blood urea and nitrogen level > 8.9 mmol/L
- Systemic inflammatory response syndrome is present
- Age > 60
- Pleural effusion on radiology
- Reference: World J Emerg Surg 2019;14:27
Case reports
- 35 year old man with acute pancreatitis due to over the counter calcium carbonate (J Investig Med High Impact Case Rep 2020;8:2324709620922724)
- 50 year old man with acute pancreatitis with normal lipase and amylase (Medicine (Baltimore) 2019;98:e15138)
- 57 year old woman with steroids induced acute pancreatitis (Cureus 2021;13:e19132)
- 71 year old woman with acute pancreatitis following COVID-19 vaccination (Medicine (Baltimore) 2022;101:e28471)
- 75 year old woman with acute pancreatitis due to cytomegalovirus infection (IDCases 2020;22:e00932)
Treatment
- Oxygen supplementation
- Intravenous fluid resuscitation to correct third space volume loss and tissue hypoperfusion
- Pain management: NSAIDs or opioids, depending on the severity
- Reference: Drugs 2022;82:1251
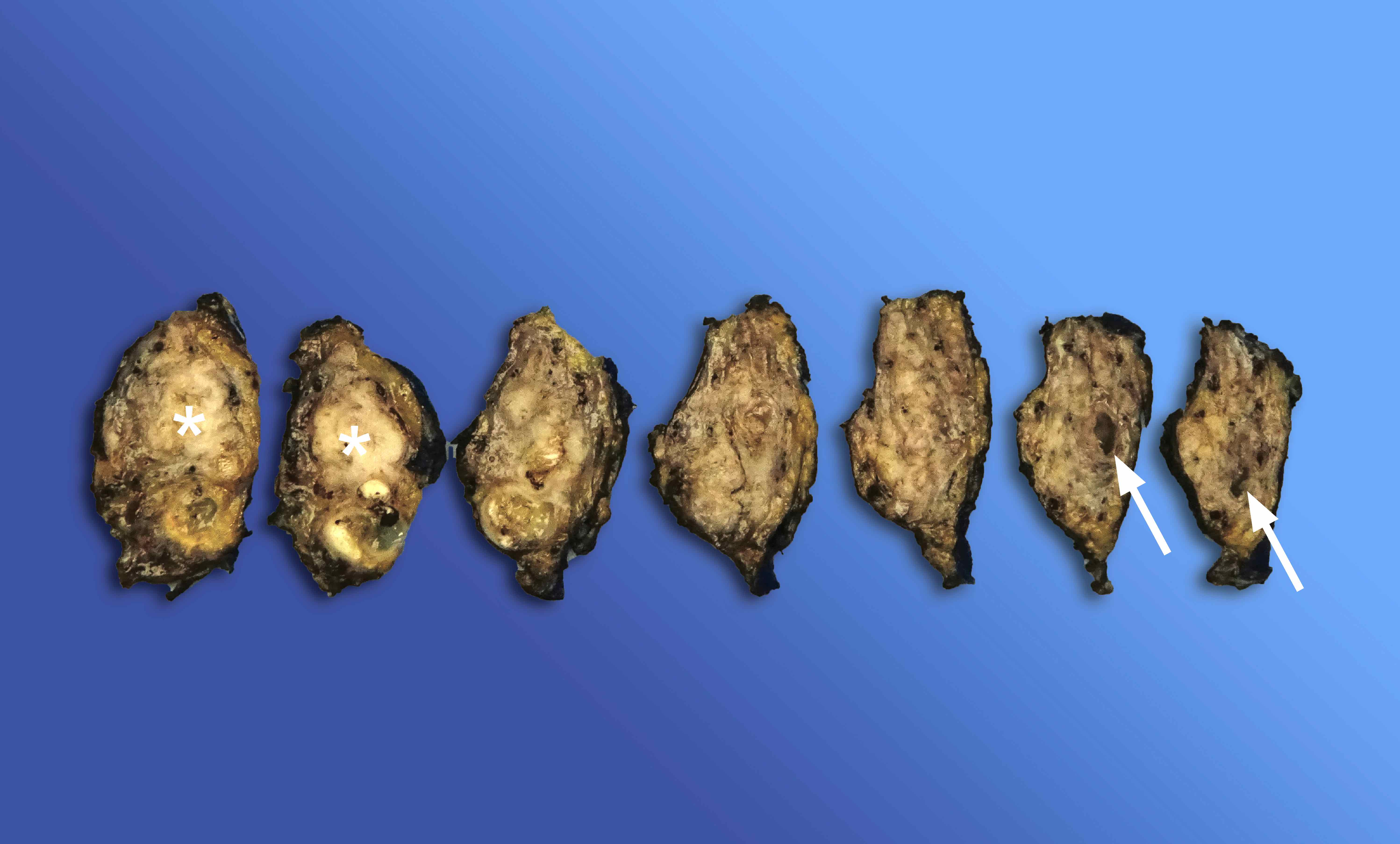
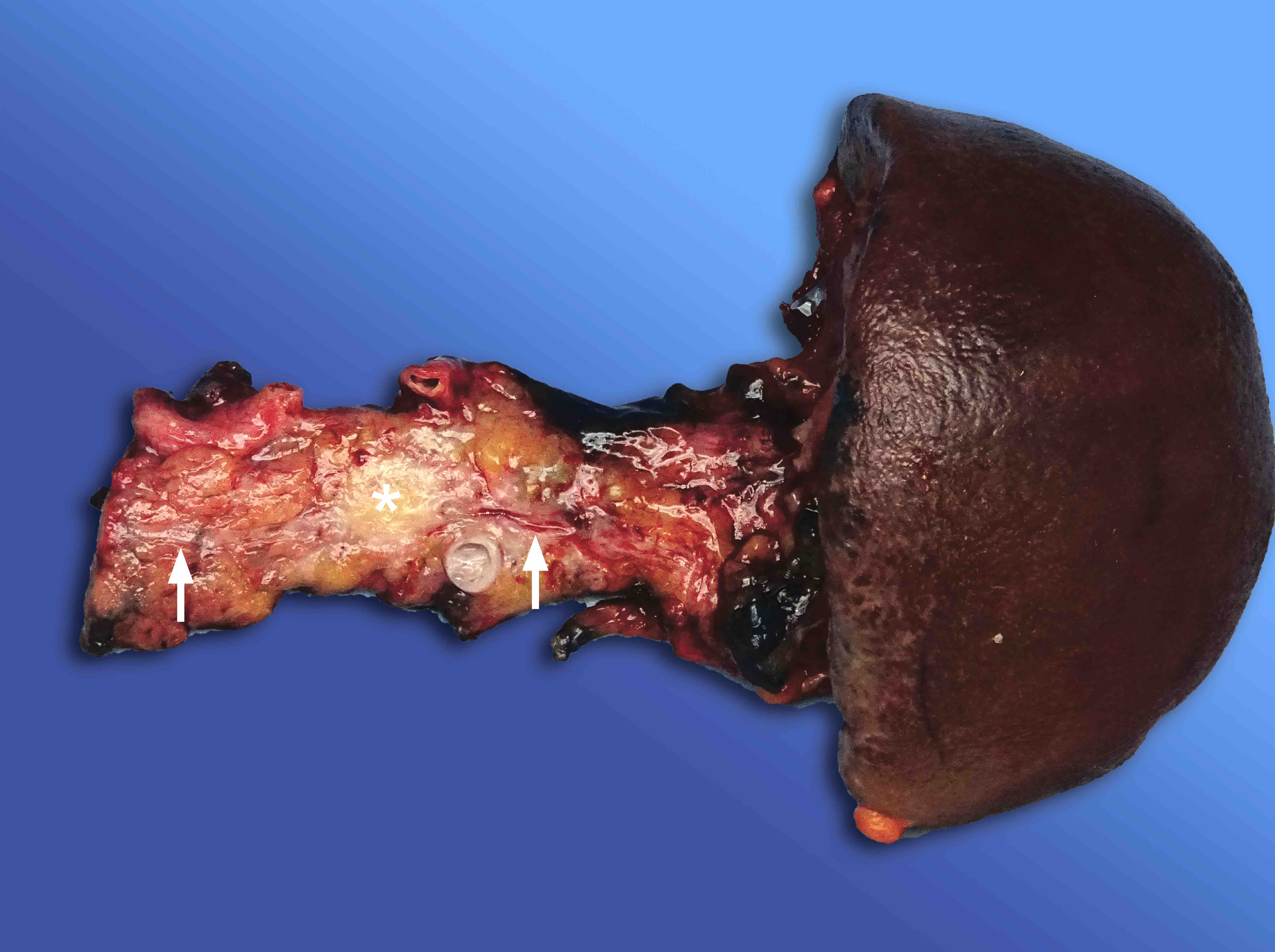
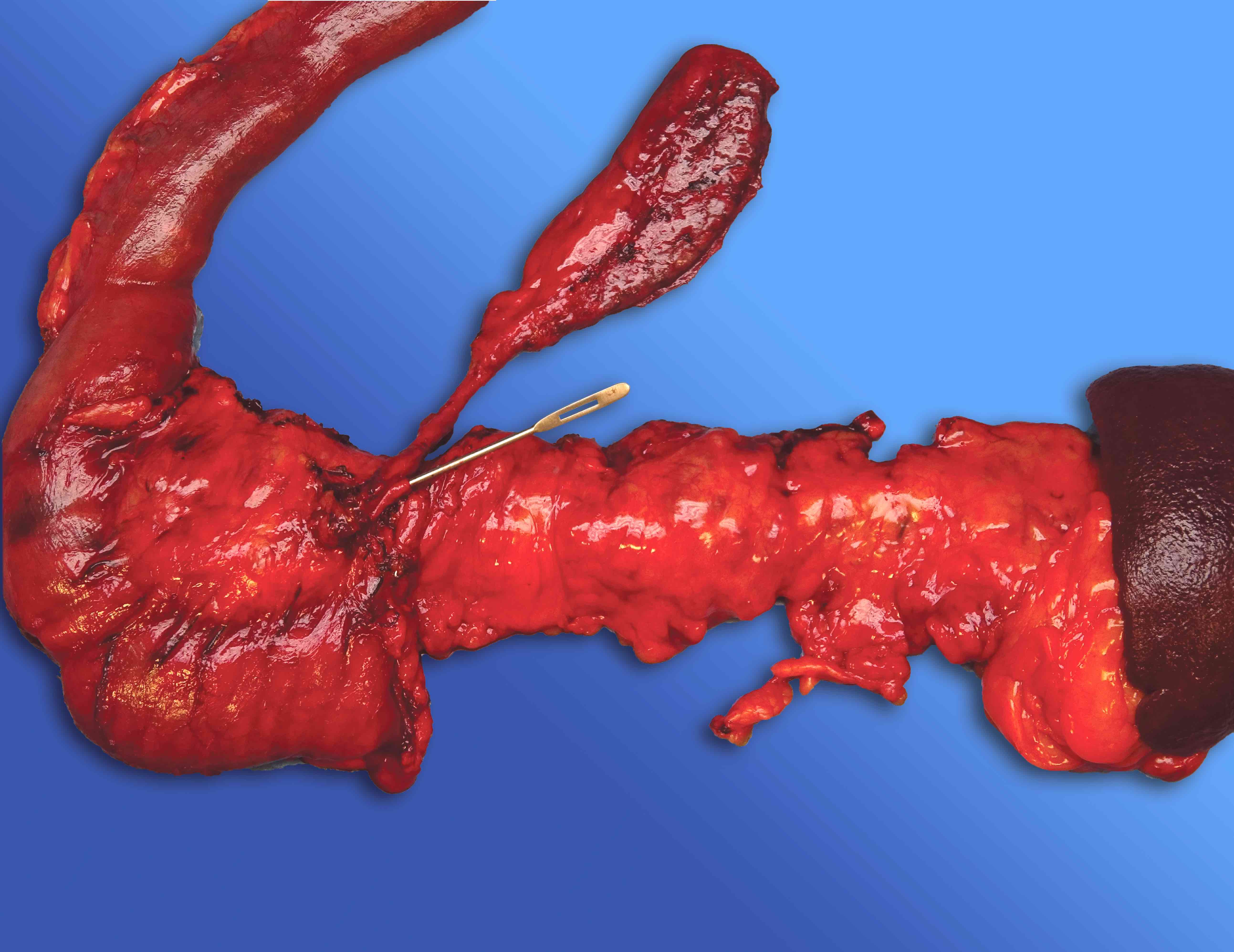
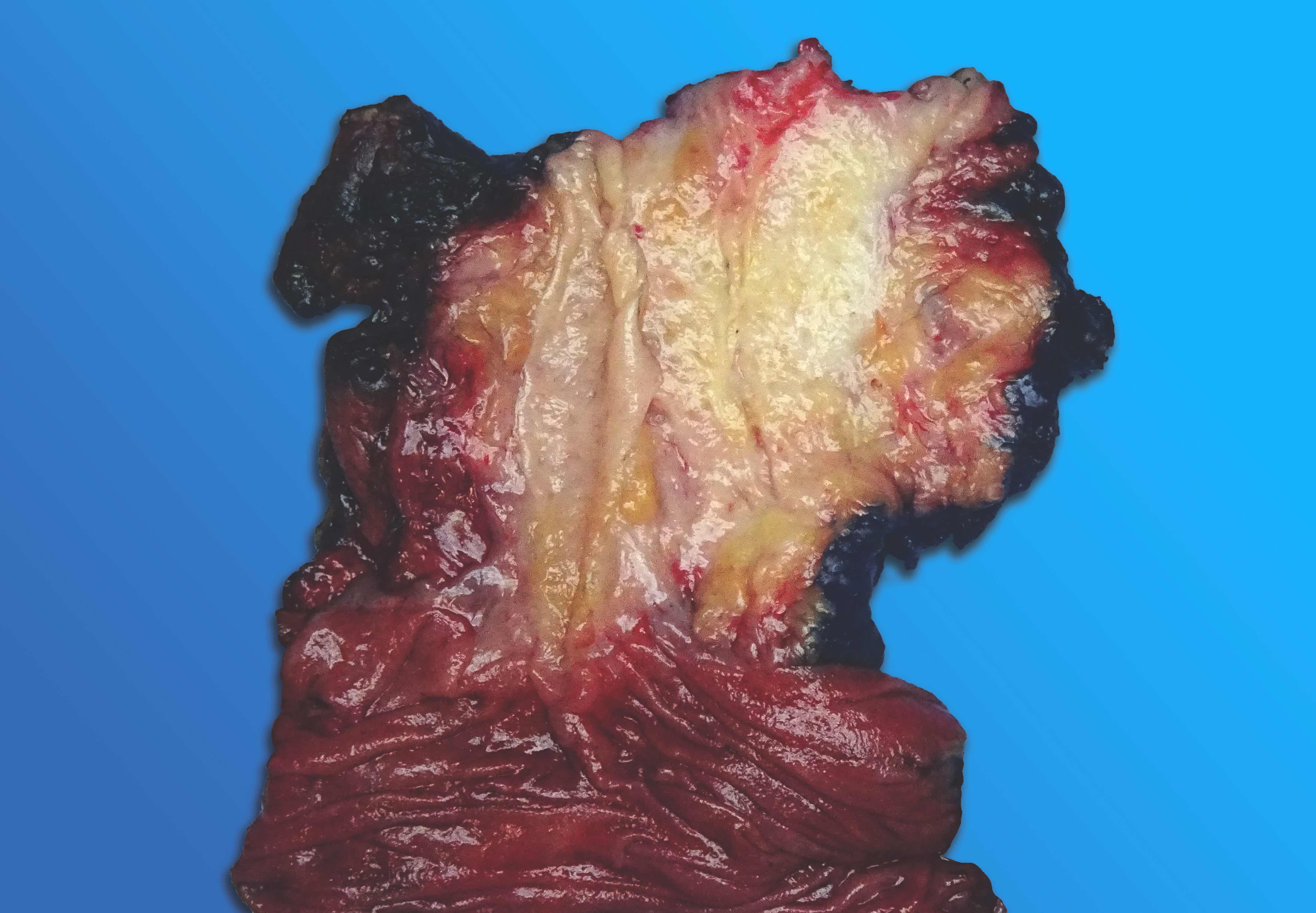
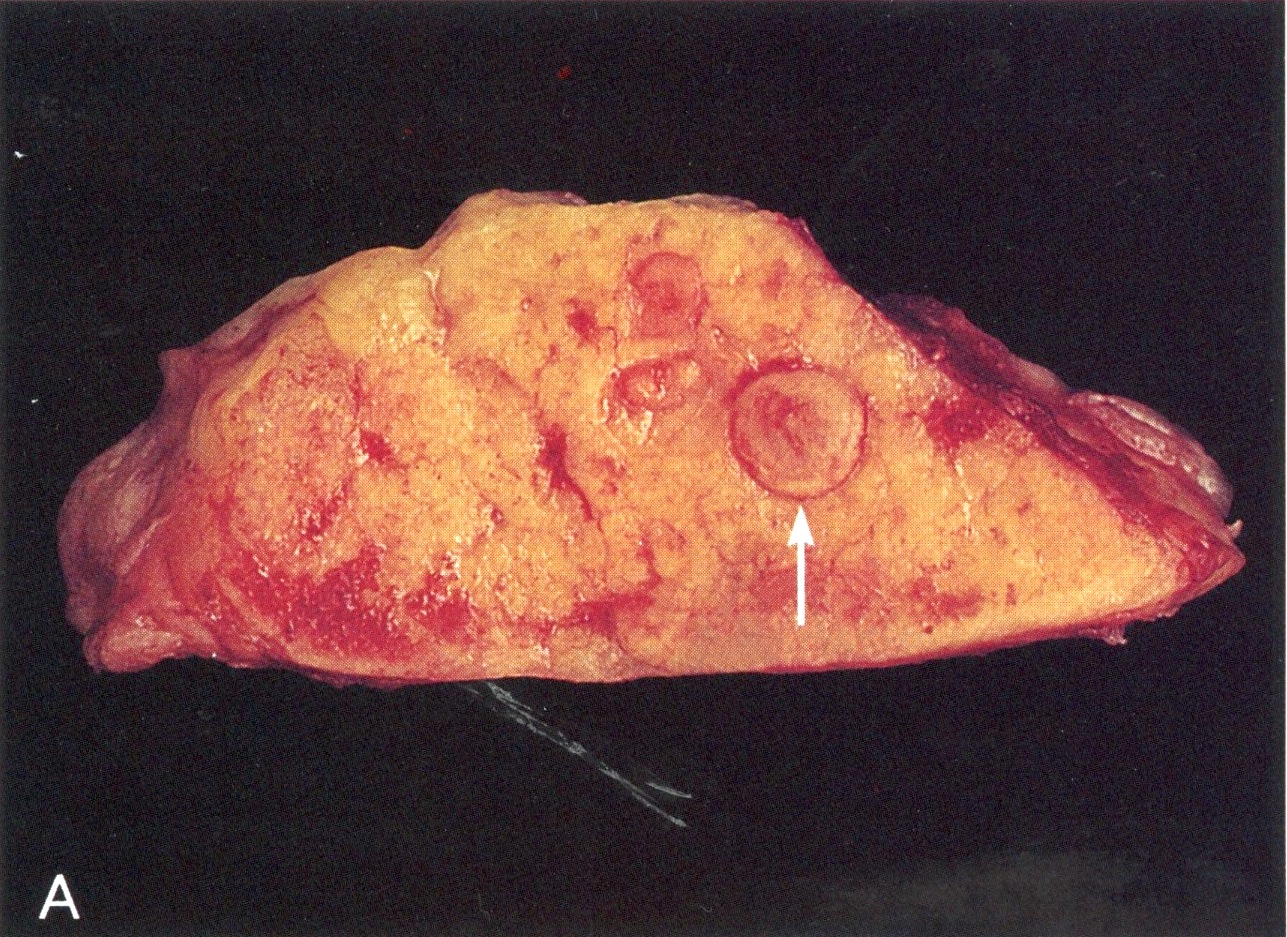
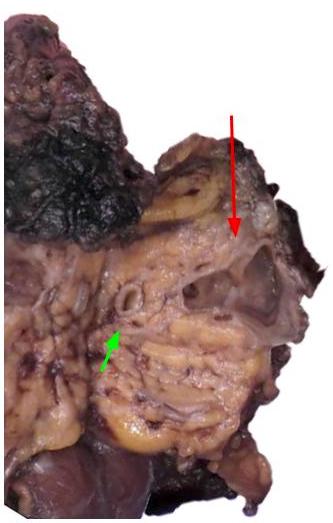
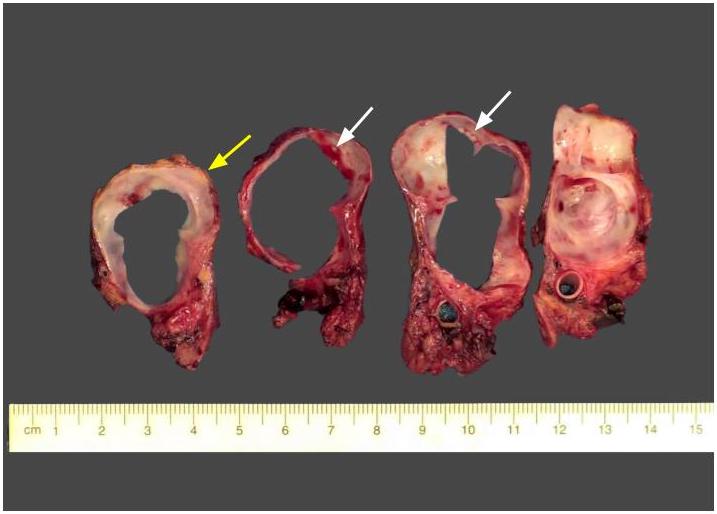
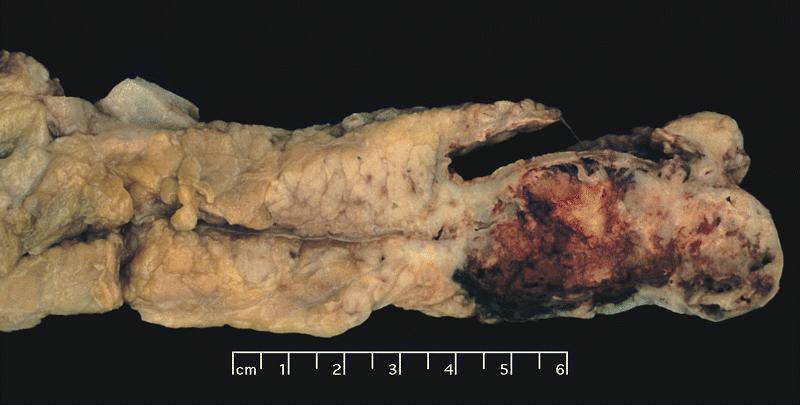
Gross description
- Diffuse stippled necrosis of the pancreatic parenchyma and peripancreatic fat
- Hemorrhagic, black-brown necrosis of the pancreatic parenchyma and peripancreatic fat
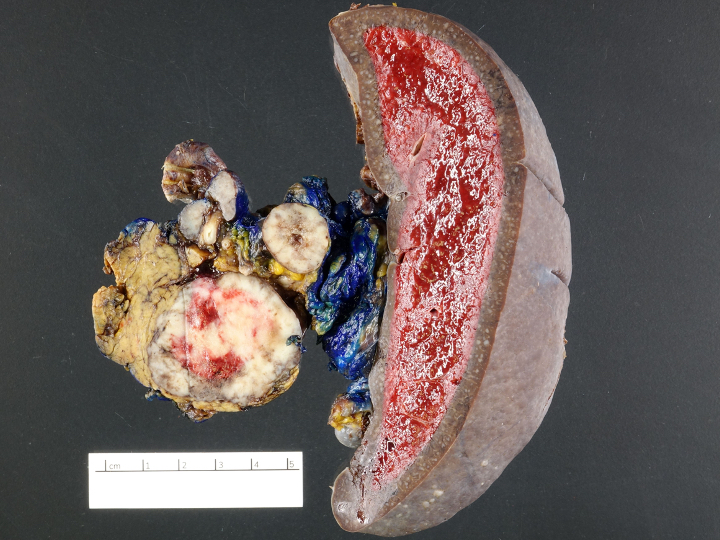
Gross images
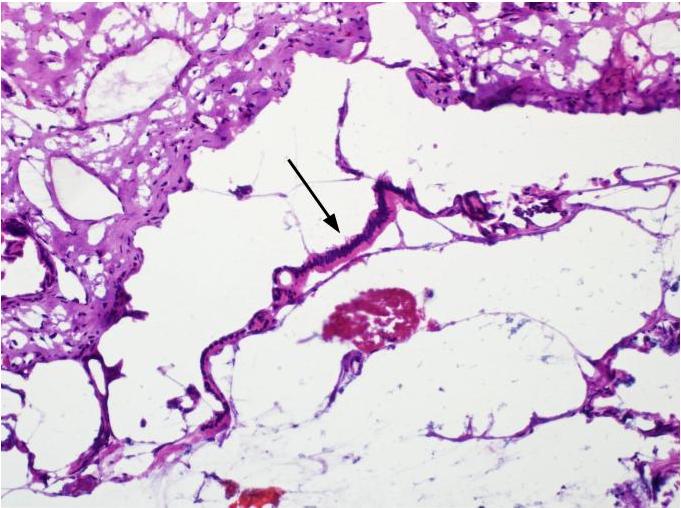
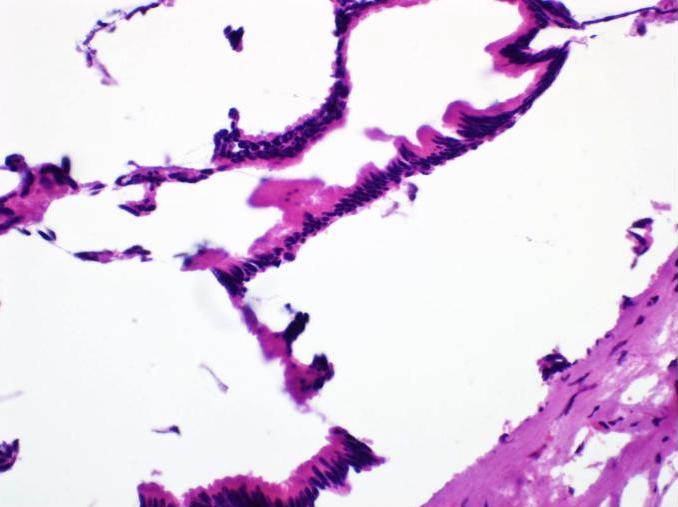
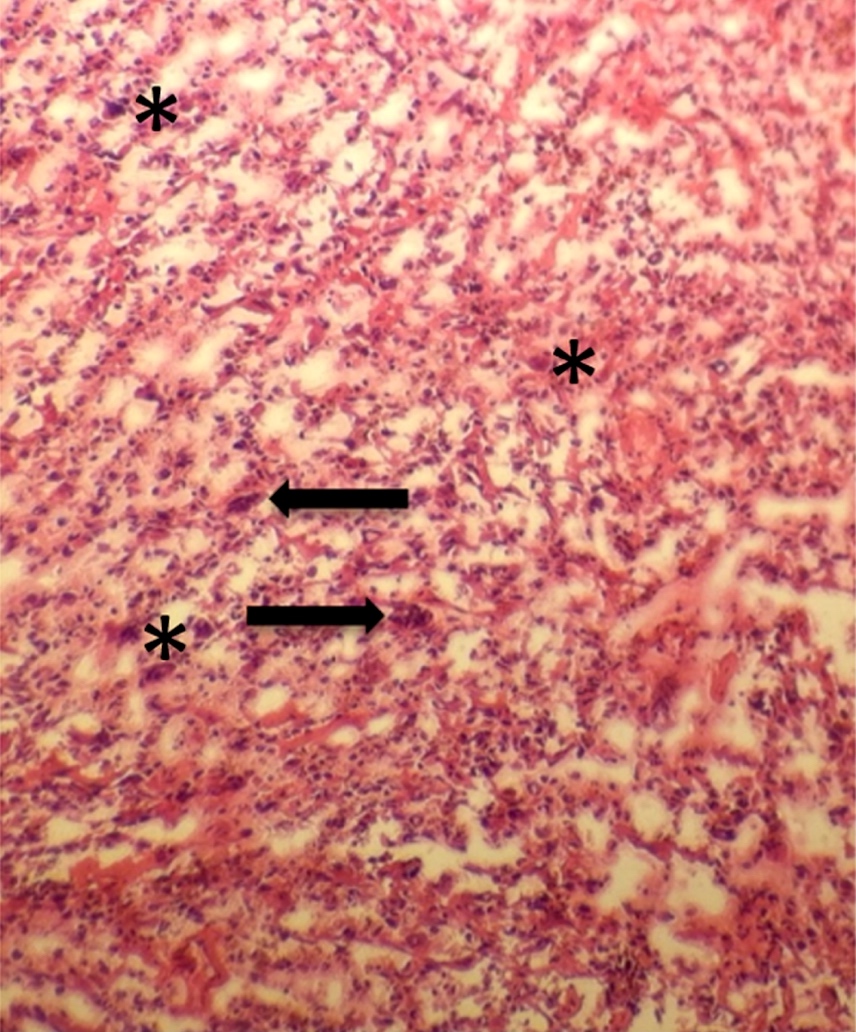
Microscopic (histologic) description
- Acute interstitial or edematous: the interstitial fibrous tissue, adipose tissue and pancreatic parenchyma are edematous and contain acute inflammatory cells with limited fat necrosis
- Acute necrotizing: marked hemorrhage, panlobular coagulative necrosis and extensive fat necrosis of peripancreatic tissues, presence of intravascular thrombi
- Initially neutrophils are present, then macrophages and lymphocytes
- Calcification occurs early and extensively
- Fat necrosis, which is characterized by lipid laden macrophages and foreign body giant cells, can progress to fat saponification, which involves calcium deposition
Microscopic (histologic) images
Cytology description
- Benign pancreatic acinar tissue and ducts
- Fat necrosis, fibrotic stromal tissue
- Acute and chronic inflammatory infiltrate
Molecular / cytogenetics description
- Most common genes associated with acute pancreatitis include
- PRSS1 mutations: inhibits trypsin self destruction, mutations lead to pancreatic autodigestion and pancreatitis
- SPINK1 mutations: encodes a trypsin inhibitor and mutations lead to increased intrapancreatic trypsin activity
- References: Diagnostics (Basel) 2020;11:31, Gastroenterology 2019;156:1951
Videos
Pancreas: acute pancreatitis, gross and microscopy
Sample pathology report
- Pancreas, resection:
- Fragments of necrotic pancreatic and adipose tissues with fat necrosis, hemorrhage, bacterial colonies and fibrinopurulent exudate, consistent with acute hemorrhagic pancreatitis
Differential diagnosis
- Acute cholecystitis:
- Severe abdominal pain with jaundice
- Imaging studies are utilized to make a definite diagnosis
- Acute mesenteric ischemia:
- Severe, sudden abdominal pain in patients with history of atherosclerotic disease
- Angiography
- Acute cholangitis:
- Clinical features include right upper quadrant pain, fever, malaise, chills and jaundice
- Imaging studies including US, CT scan and magnetic resonance cholangiopancreatography (MRCP)
- Chronic pancreatitis:
- Less severe pain
- Malabsorption syndrome (fatty stool and weight loss)
- Histological features of chronic pancreatitis include loss of acinar cells, islet cell hyperplasia, presence of intralobular fibrosis and periductal chronic inflammation
- Myocardial infarction:
- Chest and upper abdominal pain in patients with history of atherosclerotic disease
- ECG with characteristic changes of ischemic injury
- Peptic ulcer disease:
- Bouts of mid epigastric pain associated with eating
- Upper gastrointestinal endoscopy
Board review style question #1
Board review style answer #1
C. Elevated serum lipase and amylase levels. Amylase and lipase are digestive enzymes normally released from the acinar cells of the exocrine pancreas into the duodenum. Following injury to the pancreas, these enzymes are released into the circulation and the pancreas will continue to release these enzymes form injured cells even when the normal outflow of these enzymes is obstructed. This will result in elevated serum lipase and amylase levels in the blood. Answer A is incorrect because elevated bilirubin is associated with gall stones and acute cholecystitis. Answer B is incorrect because high blood alcohol levels have no immediate association with acute pancreatitis and only indicate recent drinking. Answer C is incorrect because acute pancreatitis is an inflammatory condition that leads to high white blood count.
Comment Here
Reference: Acute pancreatitis
Comment Here
Reference: Acute pancreatitis
Board review style question #2
Why is the body more susceptible to secondary infections during cases of pancreatitis?
- Activation of leukocytes in response to proinflammatory cytokine production
- Formation of pseudocysts
- Production of anti-inflammatory cytokines and specific cytokine inhibitors
- Release of cortisol in response to stress
Board review style answer #2
C. Production of anti-inflammatory cytokines and specific cytokine inhibitors.
As a result of the systemic inflammatory response during acute pancreatitis, anti-inflammatory cytokines and specific cytokine inhibitors are produced, increasing the body's risk for infection. Answer A is incorrect because increased leucocytes lead to activation of the immune response and less susceptibility to infections. Answer B is incorrect because pseudocyst cyst formation is a result of the ongoing inflammation and has no relation with susceptibility to infections. Answer D is incorrect because cortisol is ordinarily anti-inflammatory and contains the immune response, so acute increase does not increase infection susceptibility.
Comment Here
Reference: Acute pancreatitis
Comment Here
Reference: Acute pancreatitis
Adenosquamous carcinoma
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Etiology | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Malignant tumor that consists of both squamous cell carcinoma and ductal adenocarcinoma components (Digestion 2005;72:104, Cancer 2003;99:372, Int J Pancreatol 1999;26:85, Arch Surg 1999;134:599)
Essential features
- Malignant tumor consisting of both squamous cell carcinoma and ductal adenocarcinoma components (Digestion 2005;72:104, Cancer 2003;99:372, Int J Pancreatol 1999;26:85, Arch Surg 1999;134:599)
- Closely related to and admixed with pancreatic ductal adenocarcinoma (PDAC); some require 30% squamous differentiation for the diagnosis of adenosquamous carcinoma
- Cases with < 30% of squamous component also behave like adenosquamous carcinoma and can be acknowledged as PDAC with focal (< 30%) squamous differentiation
- Metastatic tumor can be solely composed of the glandular component (Mod Pathol 2001;14:443)
- Often has necrotic and cystic components (Arch Surg 1999;134:599)
- Behaves even worse than ordinary PDAC
ICD coding
- ICD-O: 8560/3 - adenosquamous carcinoma
Epidemiology
- Accounts for about ~2% of exocrine pancreatic malignancies
- M:F = 2:1
- Mean age of 65 years (Arch Surg 1999;134:599)
Sites
- Body and tail of the pancreas is more often affected than the head (J Surg Res 2012;174:12)
Etiology
- Ionizing radiation of the pancreas may predispose to the occurrence of adenosquamous carcinoma (Mod Pathol 2001;14:443)
Diagnosis
- Generally discovered with radiology and confirmed on fine needle aspiration biopsy (FNAB) or resection
Radiology description
- Similar to pancreatic ductal adenocarcinoma but many cases present with more round, lobulated lesion with extensive central necrosis
- Portal venous system may contain tumor thrombus (Abdom Radiol (NY) 2016;41:508)
Prognostic factors
- Similar to PDAC, stage is the most important prognostic factor
Case reports
- 63 year old man and 69 year old woman with adenosquamous carcinoma of the pancreas (Annu Rev Pathol 2020;15:97)
- 66 year old woman with adenosquamous carcinoma of the pancreas (Clin Case Rep 2022;10:e6181)
- 80 year old Japanese woman with adenosquamous carcinoma coexisting with intraductal papillary mucinous neoplasm of the pancreas (J Med Case Rep 2023;17:72)
Treatment
- Similar to pancreatic ductal adenocarcinoma
- If the tumor is operable, complete surgical resection, followed by adjuvant chemotherapy; increasingly neoadjuvant therapy is utilized just as in PDAC (Hum Pathol 2010;41:113, J Gastrointest Oncol 2015;6:115)
- Even more aggressive than ordinary PDAC with a median survival of < 1 year (Gastrointest Cancer Res 2013;6:75)
- Based on the immunohistochemical results, 11% of patients are assumed to be potential candidates for therapy with antibodies against PD-1 / PDL1 (Pancreatology 2021;21:920)
Gross description
- Large, firm mass of the pancreas, with ill defined borders, often with necrotic component, with or without cystic areas
- Some cases are more demarcated
- Reference: World J Gastrointest Oncol 2015;7:132
Microscopic (histologic) description
- Adenocarcinoma component can bare all the characteristics of classic pancreatic ductal adenocarcinoma
- Squamous component can show keratinization with intercellular bridges or sheets of squamous cells with keratohyaline granules or pearls
- Some are adenoacanthoma-like, with bland mature squamous elements, whereas others are more basal-like (with high N:C ratio and basophilic appearance)
- Adenocarcinoma and squamous components can vary greatly in the amount and distribution
- Sarcomatous component can also be encountered (Anticancer Res 2019;39:4575)
Microscopic (histologic) images
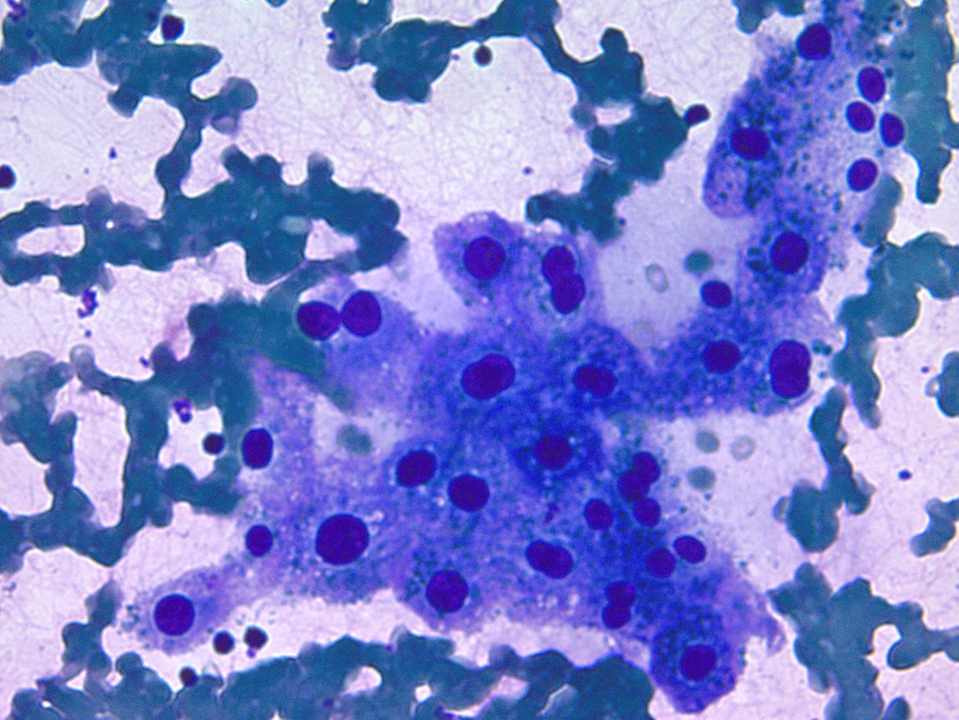
Cytology description
- Squamous component may be undersampled but malignant squamous cells are highly significant for the diagnosis (Acta Cytol 2013;57:139)
- Glandular and squamous components can both be distinguished
- Dense globules, silhouettes of squamous cells, anucleate squames, atypical cytoplasm and enlarged pyknotic nuclei are present in the prominent necrotic background
- Sheets and clusters of atypical cells with nuclei of variable sizes and shapes (Cancer 2003;99:372)
Positive stains
- Immunoreactivity for AE1 / AE3 is identified
- p63, p40, high molecular weight cytokeratin specifically highlights the squamous component (Mod Pathol 2005;18:1193, Mod Pathol 2009;22:651)
Negative stains
- Loss of SMAD4 / DPC4 protein expression
Molecular / cytogenetics description
- KRAS mutations occur in almost all cases (Mod Pathol 2001;14:443, Mod Pathol 2009;22:651)
- TP53 mutations and 3p loss are commonly encountered
- Recently discovered basal-like molecular subtype of PDAC is believed to also represent adenosquamous carcinoma (Nature 2016;531:47)
- Both components are believed to originate from the same progenitor cell (J Pathol 2017;243:155)
- Susceptibility genes including MAP3K1, PDE4DIP and BCR are frequently mutated in the germlines of patients (Cancer Biomark 2020;27:389)
Sample pathology report
- Pancreas, resection:
- Adenosquamous carcinoma (see synoptic report)
Differential diagnosis
- Pure squamous cell carcinoma:
- Extremely rare; most cases contain a small amount of glandular component, which is enough to call the tumor adenosquamous carcinoma
- Metastasis of adenosquamous carcinoma of the lung:
- Needs to be carefully excluded (Virchows Arch 2004;444:527)
Board review style question #1
A 65 year old man presented with back pain and severe weight loss. Radiologic images showed a 4 cm relatively round, lobulated mass with extensive central necrosis in the pancreatic body. He underwent resection. Histologic sections demonstrate conventional ductal adenocarcinoma admixed with sheets of squamous cells with keratohyaline granules and intercellular bridges. Which of the following immunohistochemical stains would be positive in this case?
- Beta catenin nuclear expression
- Chromogranin A
- p63
- Synaptophysin
Board review style answer #1
C. p63. The histology described in this case is consistent with pancreatic adenosquamous carcinoma. The squamous components should be positive with p63 immunohistochemistry. Neuroendocrine neoplasms are positive for chromogranin and synaptophysin. Beta catenin nuclear expression is observed in solid pseudopapillary neoplasm.
Comment Here
Reference: Adenosquamous carcinoma
Comment Here
Reference: Adenosquamous carcinoma
Board review style question #2
Board review style answer #2
D. p63 specifically highlights the squamous component. The squamous components of the tumor should be positive with p63 immunohistochemistry. Answer A is incorrect as adenosquamous carcinomas behave worse than pancreatic ductal adenocarcinomas. Answer B is incorrect as pancreatic ductal adenocarcinoma is the most common pancreatic malignancy. Answer C is incorrect as adenosquamous carcinomas occur in elder patients (mean age of 65 years).
Comment Here
Reference: Adenosquamous carcinoma
Comment Here
Reference: Adenosquamous carcinoma
Allograft rejection
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Electron microscopy description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Definition / general
- Pancreas transplant rejection is an immunological reaction directed by the host immune system against the allograft, leading to allograft damage and requiring immunosuppressive treatment to prevent permanent disruption of the allograft function
Essential features
- Pancreas transplantation is the only method to achieve long term insulin independence and euglycemia (Transplant Proc 2022;54:1918, Am J Transplant 2011;11:1792, Am J Transplant 2024;24:362)
- Although the serum level of pancreatic enzymes tends to increase in pancreas transplants undergoing rejection, a pancreas transplant biopsy is the gold standard in the diagnosis of pancreas rejection and the only method to differentiate between cell and antibody mediated rejection
- Classification and grading of pancreas transplant rejection are performed according to the Banff guidelines, which take into consideration the type, location and numbers of inflammatory cells, involvement of the pancreatic structures, the presence of complement deposition and donor specific antibodies and chronic changes
Terminology
- Histological definitions used in the diagnosis of pancreas rejection are listed in the 2008 Banff Schema for Grading Pancreas Allograft Rejection and updated in the Banff 2022 pancreas transplantation multidisciplinary report (Am J Transplant 2008;8:1237, Am J Transplant 2024;24:362)
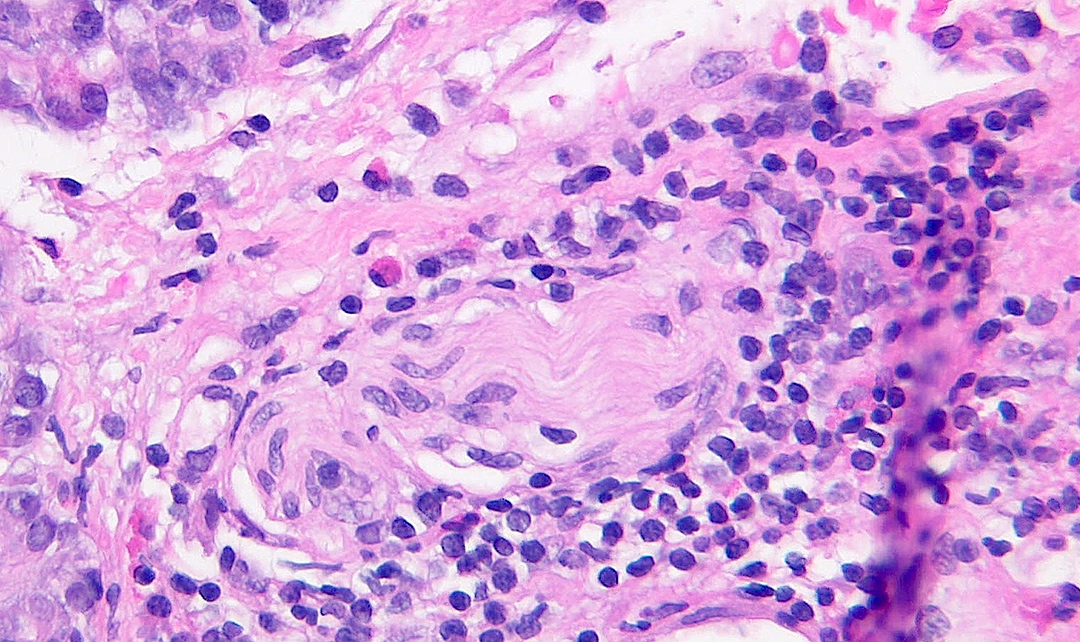
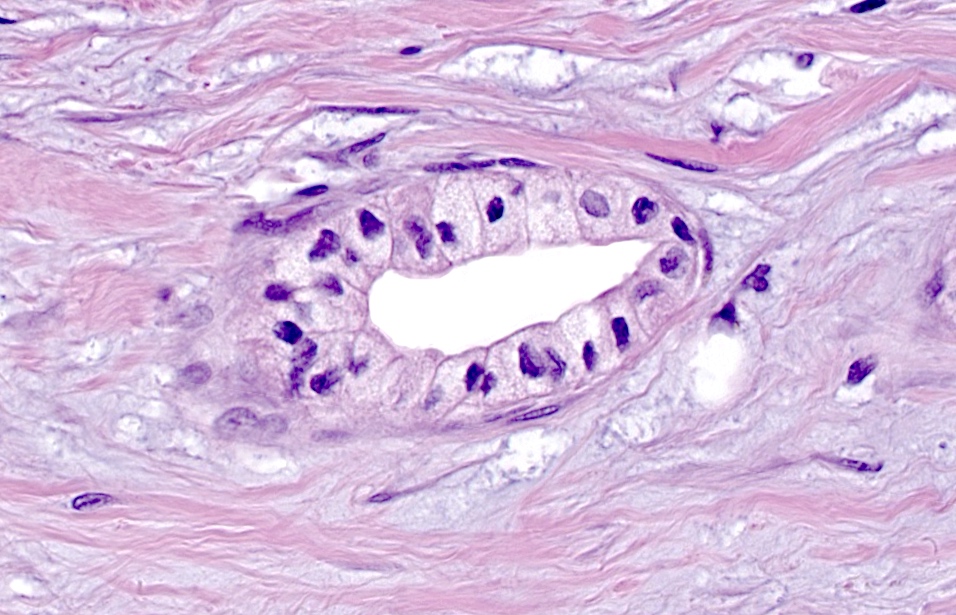
- Venulitis: circumferential cuffing of septal veins with subendothelial accumulation of inflammatory cells and endothelial injury, activation, swelling or lifting (Am J Transplant 2008;8:1237, Am J Transplant 2024;24:362)
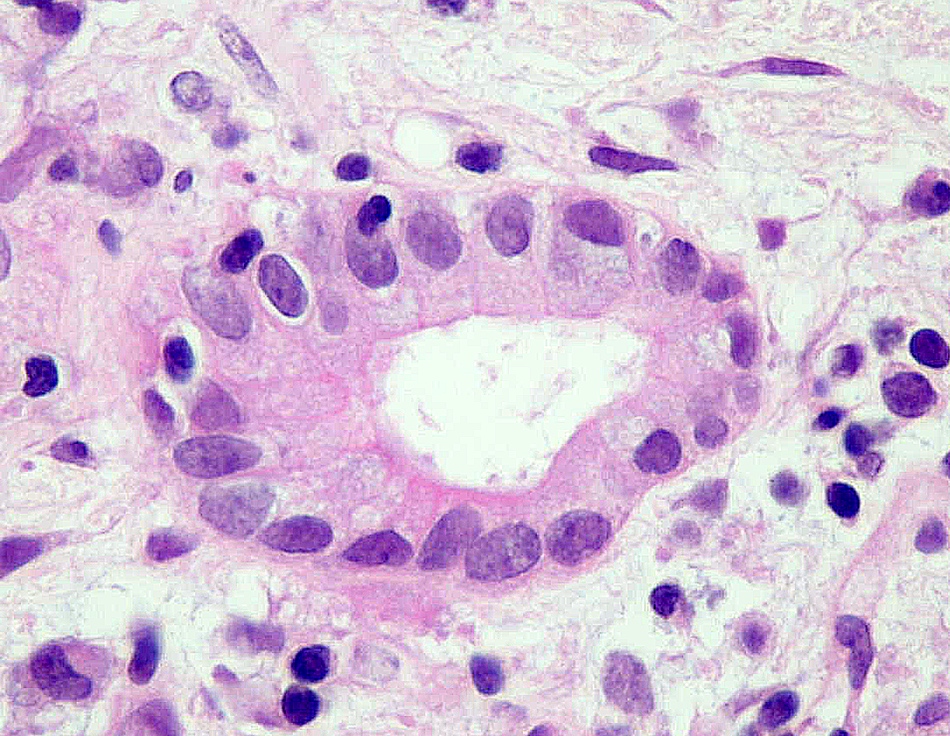
- Ductitis: infiltration of ductal epithelium by mononuclear cells or eosinophils and ductal epithelial cell injury, reactive features, disarray, sloughing (Am J Transplant 2008;8:1237, Am J Transplant 2024;24:362)
- Activated lymphocyte: lymphocyte that is activated by antigen binding to its receptor; it shows more abundant cytoplasm and an enlarged nucleus with more open chromatin
- Active inflammation: activated lymphocytes with or without eosinophils
- Neural and perineural inflammation: septal inflammatory infiltrates in and around the nerve branches (Am J Transplant 2008;8:1237)
- Acinar inflammatory focus: collection of ≥ 10 lymphocytes / eosinophils within an acinar area (Am J Transplant 2008;8:1237)
- Focal acinar inflammation: ≤ 2 inflammatory foci per lobule with no evidence of acinar cell injury (Am J Transplant 2008;8:1237)
- Multifocal acinar inflammation: ≥ 3 foci of inflammation per lobule with isolated cell injury / necrosis
- Severe acinar inflammation: confluent diffuse acinar inflammation with focal or diffuse acinar cell injury or necrosis (Am J Transplant 2008;8:1237)
- Acinar cell injury: cytoplasmic swelling and vacuolization or karyopiknosis, apoptotic bodies, cell dropout (Am J Transplant 2008;8:1237)
- Active antibody mediated rejection components
- Confirmed donor specific antibody
- Morphologic evidence of active tissue injury (interacinar inflammation with neutrophilic infiltrates / capillaritis, acinar cell damage, vasculitis, thrombosis)
- Complement component 4d (C4d) positivity in interacinar capillaries (Am J Transplant 2024;24:362)
- Chronic rejection: progressive fibrosis resulting from persistent, repeated or untreated allograft rejection (Am J Transplant 2024;24:362)
- Chronic active cell mediated rejection: ongoing inflammation in acini or ducts or arteries with features of chronic injury, fibrosis or remodeling (Am J Transplant 2024;24:362)
- Chronic allograft arteriopathy: arterial intimal fibrosis with mononuclear cell infiltration in fibrosis, formation of neointima (Am J Transplant 2008;8:1237)
- Active allograft arteriopathy: narrowing of the arterial lumen by a subendothelial proliferation of fibroblasts, myofibroblasts and smooth muscle cells with infiltration of the subintimal fibrous proliferation by mononuclear cells (Am J Transplant 2008;8:1237)
ICD coding
Epidemiology
- Pancreas transplantation is a surgical treatment for diabetes mellitus
- Most transplant recipients have diabetes mellitus type 1 and 18.4% have diabetes mellitus type 2 (Transplant Proc 2022;54:1918)
- Median age for pancreas transplant recipients is 42 (12 - 75) years; pancreas transplants in pediatric recipients are done rarely (Transplant Proc 2022;54:1918)
- Between years 2015 and 2019, 5,369 pancreas transplants were performed in the United States and Canada and 3,923 in Europe (Transplant Proc 2022;54:1918)
- Number of living donor transplants in the United States is very low to none (Transplant Proc 2022;54:1918)
- Most pancreas transplants (73.4%) in the United States were simultaneous pancreas and kidney (SPK); 14.6% were pancreas after kidney (PAK) and 7.8% were pancreas transplant alone (PTA) (Transplant Proc 2022;54:1918)
- 59% of SPK transplants are a 5 or 6 HLA antigen mismatch (Transplant Proc 2022;54:1918)
- Preferred duct management technique is enteric drainage (Transplant Proc 2022;54:1918)
- Patient survival at 1 year is > 96%
- Incidence of acute rejection by 1 year is 12.5% for PAK, 21.8% for PTA and 10.6% for SPK (Am J Transplant 2022;22:137)
- Risk factors for acute rejection include a solitary pancreas transplant (PAK or PTA), race mismatch, HLA mismatch, transplantation involving a male donor and a female recipient and increasing donor age (UpToDate: Pancreas Allograft Rejection [Accessed 1 March 2024])
Sites
- Pancreas
Pathophysiology
- Both major and minor histocompatibility antigens activate the immune system against the allograft
- Major histocompatibility complex (MHC) encodes the human leukocyte antigens
- MHC molecules present foreign antigens to the T cells
- MHC class I molecules are constitutively expressed on the surface of all nucleated cells
- MHC class II molecules are constitutively expressed on the surface of professional antigen presenting cells but many cell types upon activation express MHC class II
- T cells directly recognize intact nonself MHC molecules present on the surface of donor cells, eliciting the strongest of responses to allogeneic tissues (Pediatr Nephrol 2010;25:61)
- Processed MHC peptides and minor histocompatibility antigens elicit a slower, less intense immune response (Pediatr Nephrol 2010;25:61)
- T cell activation is central to graft rejection
- Graft tissue destruction occurs due to direct T cell mediated lysis of graft cells, T cell activation of accessory cells (cell mediated rejection), alloantibody production and complement activation (Pediatr Nephrol 2010;25:61)
- Arteries, ducts and acini are the preferred targets of cell mediated rejection
- Islets of Langerhans are not immediately or directly affected (Am J Transplant 2008;8:1237)
- Presence of allograft infiltrating B cells and plasma cells correlates with irreversible acute and chronic rejection (Pediatr Nephrol 2010;25:61)
- B cells damage grafts by producing donor specific antibodies (antibody mediated rejection), which bind to HLA or non-HLA molecules on endothelial cells within the graft and activate complement dependent and independent mechanisms that recruit leukocytes and macrophages leading to interacinar capillaritis and acinar damage
- Islets may be susceptible to microvascular injury in antibody mediated rejection (Am J Transplant 2008;8:1237)
Etiology
- Solid organ allograft rejection is initiated when recipient T lymphocytes recognize donor derived antigens, resulting in T lymphocyte activation
- Activated T cells undergo clonal expansion, differentiate into effector cells and migrate into the graft where they promote tissue destruction
- Traditionally, acute rejection is classified as either cell mediated rejection or antibody mediated rejection
- Activation and differentiation of CD4+ T helper cells (Th) into specific Th subsets plays a central role in the acute cell mediated rejection (Transplantation 2023;107:2341)
- Th1 cells secrete interleukins IL2, IL12, IFNγ and TNFα, promoting leukocyte recruitment and cytotoxin T lymphocyte priming (Transplantation 2023;107:2341)
- Th2 cells secrete cytokines IL4, IL5, IL6, IL9 and IL13, inducing macrophage activity and shaping T cell responses (Transplantation 2023;107:2341)
- Tfh cells activate the alloantigen specific B cells, contributing to antibody responses (Transplantation 2023;107:2341)
- After direct activation by MHC class I alloantigen, graft infiltrating CD8+ cells play a central role in allograft injury via, e.g., secretion of TNFα, IFNγ, perforin and granzyme secretion, natural killer (NK) cell activation (Transplantation 2023;107:2341)
Clinical features
- Some patients with pancreas graft rejection occasionally present with mild graft tenderness or fever, whereas many patients are asymptomatic (UpToDate: Pancreas Allograft Rejection [Accessed 1 March 2024])
Diagnosis
- Primary means of monitoring for pancreas allograft rejection is periodic laboratory testing (UpToDate: Pancreas Allograft Rejection [Accessed 1 March 2024])
- Elevated serum amylase / lipase prompt CT or MRI abdomen and further testing (see Laboratory) and pancreas allograft biopsy (see Curr Transplant Rep 2015;2:169 and UpToDate: Pancreas Allograft Rejection [Accessed 1 March 2024] for the pancreas allograft diagnosis and treatment algorithm)
- Pancreas allograft biopsy is the gold standard in the diagnosis of pancreas rejection
- In SPK recipients, renal allograft rejection is a poor surrogate for pancreas allograft status due to high biopsy discordance rate (Transplantation 2010;90:75)
- Likewise, sampling of the duodenal mucosa from the pancreaticoduodenal graft is not widely accepted due to potential for discordant findings (Curr Transplant Rep 2015;2:169)
Laboratory
- Elevated pancreatic enzymes (amylase and lipase) is the most common presentation of pancreatic allograft rejection and correlates with the grade of acute rejection (Curr Transplant Rep 2015;2:169, Transplantation 1998;66:1741)
- However, their lack of specificity precludes their use as sole markers of acute rejection (Transplantation 1998;66:1741)
- Lipase is considered to be more specific than amylase (Curr Transplant Rep 2015;2:169)
- If the pancreas allograft has received bladder drainage, a decrease in urinary amylase is observed
- As early rejection usually does not affect the islets of Langerhans (see Etiology), fasting blood glucose is not a useful parameter in the early diagnosis of rejection (Transplantation 1998;66:1741)
- Elevated fasting blood glucose, elevated hemoglobin A1C or decrease in fasting C peptide levels are a late finding in acute rejection and are usually associated with significant graft tissue damage (Transplantation 1998;66:1741, UpToDate: Pancreas Allograft Rejection [Accessed 1 March 2024])
- Clinical parameters cannot discriminate between acute cell and antibody mediated rejection (Am J Transplant 2011;11:1792)
- Patients may develop chronic rejection and graft failure without significant increases in the pancreatic enzymes (Curr Transplant Rep 2015;2:169)
- In SPK transplant recipients, an elevated serum creatinine concentration may be a surrogate marker for pancreas allograft rejection; however, stable kidney function does not consistently guarantee the absence of pancreas allograft rejection (UpToDate: Pancreas Allograft Rejection [Accessed 1 March 2024], Curr Transplant Rep 2015;2:169)
- De novo donor specific antibodies (DSA) or rising DSA levels in a patient with preexisting DSA have been associated with antibody mediated rejection and graft loss (UpToDate: Pancreas Allograft Rejection [Accessed 1 March 2024], Am J Transplant 2002;2:134)
- Donor derived cell free DNA shows promise for rejection surveillance in SPK transplant recipients (Transplant Direct 2022;8:e1321)
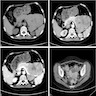
Radiology description
- Imaging findings are nonspecific and they may look similar to other complications, such as pancreatitis; contrast enhanced CT or MRI may show heterogeneous parenchymal enhancement, allograft swelling or stranding (Insights Imaging 2010;1:329, UpToDate: Pancreas Allograft Rejection [Accessed 1 March 2024])
- In chronic rejection, the graft appears small on imaging studies (Curr Transplant Rep 2015;2:169)
Prognostic factors
- Acute rejection episodes have a negative impact on the transplant pancreas and kidney survival in SPK recipients (Am J Transplant 2003;3:439)
- Early diagnosis and successful treatment of pancreas allograft rejection results in preservation of graft endocrine function
- Pancreatic enzyme levels frequently recover to the previous baseline level, although not always (UpToDate: Pancreas Allograft Rejection [Accessed 1 March 2024])
Case reports
- 31 year old man with a history of diabetes mellitus 1 received an SPK and underwent severe rejection, damaging the kidney only (J Am Soc Nephrol 1994;4:1841)
- 40 year old man with a history of diabetes mellitus 1 and HIV infection received an SPK (Transplant Proc 2010;42:3887)
- 45 year old man with a history of diabetes mellitus 1 received an SPK and developed thrombotic microangiopathy (Nephrology (Carlton) 2017;22:23)
Treatment
- Induction therapy is the initial high dose bolus of immunosuppression given perioperatively to transplant patients (Transpl Int 2013;26:704)
- In particular, high risk patients, such as sensitized patients, recipients of solitary pancreas transplants, repeat transplants, African American patients or patients receiving positive crossmatch organs, are thought to benefit from induction therapy (Transpl Int 2013;26:704)
- Induction therapy involves the use of depleting (e.g., antithymocyte globulin, alemtuzumab) or nondepleting antibodies (daclizumab, basiliximab) ( Transplant Proc 2022;54:1918)
- Most used combinations for maintenance therapy are cyclosporine A with azathioprine and later tacrolimus with mycophenolate mofetil with or without (due to their diabetogenicity) steroids (Transplant Proc 2022;54:1918)
- For patients with acute cell mediated rejection grade 1, 2 or 3, treatment with rabbit antithymocyte globulin plus glucocorticoids is recommended; if high dose glucocorticoids alone are used, a follow up pancreas biopsy should be considered to confirm the resolution of rejection (UpToDate: Pancreas Allograft Rejection [Accessed 1 March 2024])
- For patients with acute antibody mediated rejection, various combinations of plasmapheresis, intravenous immune globulin and rituximab are used (UpToDate: Pancreas Allograft Rejection [Accessed 1 March 2024])
- Patients with mixed acute rejection are treated for both acute cell and antibody mediated rejection
Frozen section description
- Pancreas transplant rejection cannot be diagnosed on frozen sections
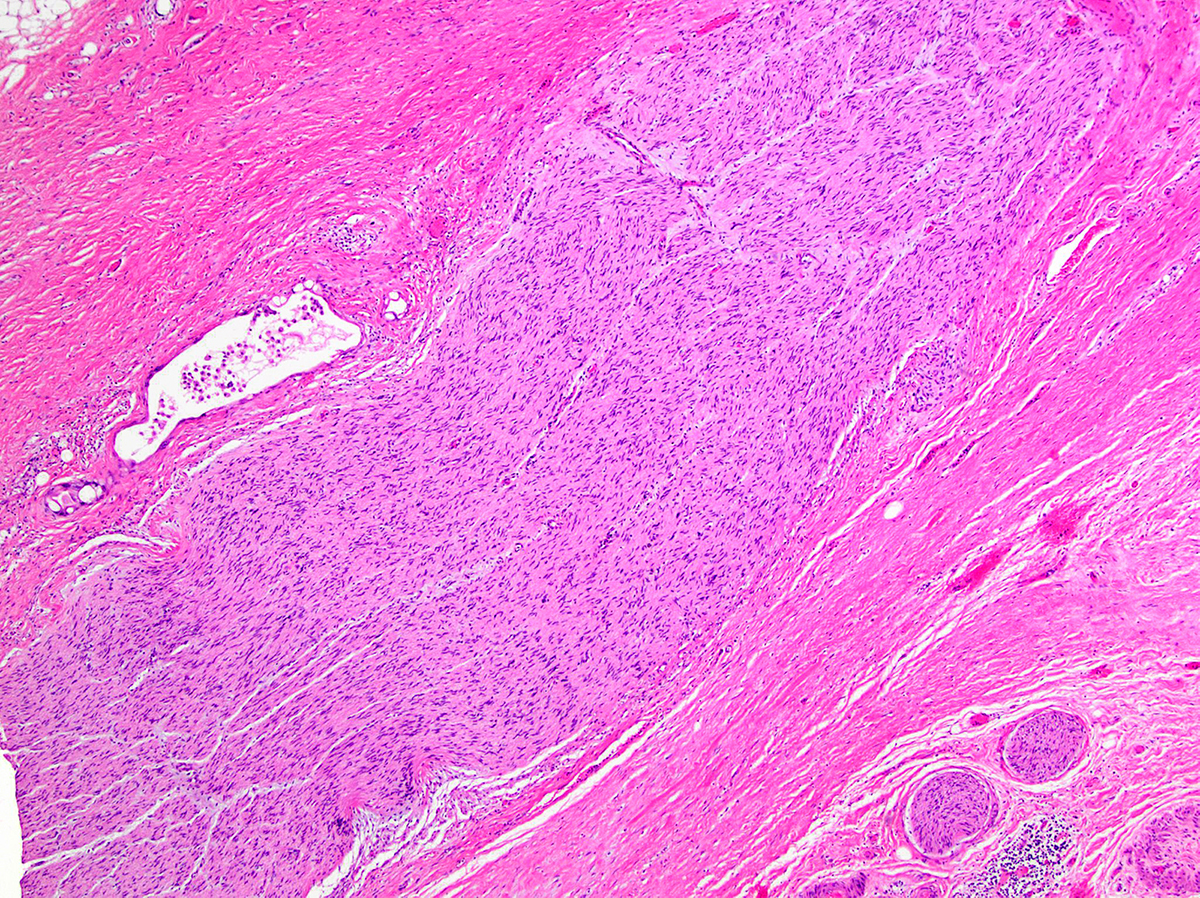
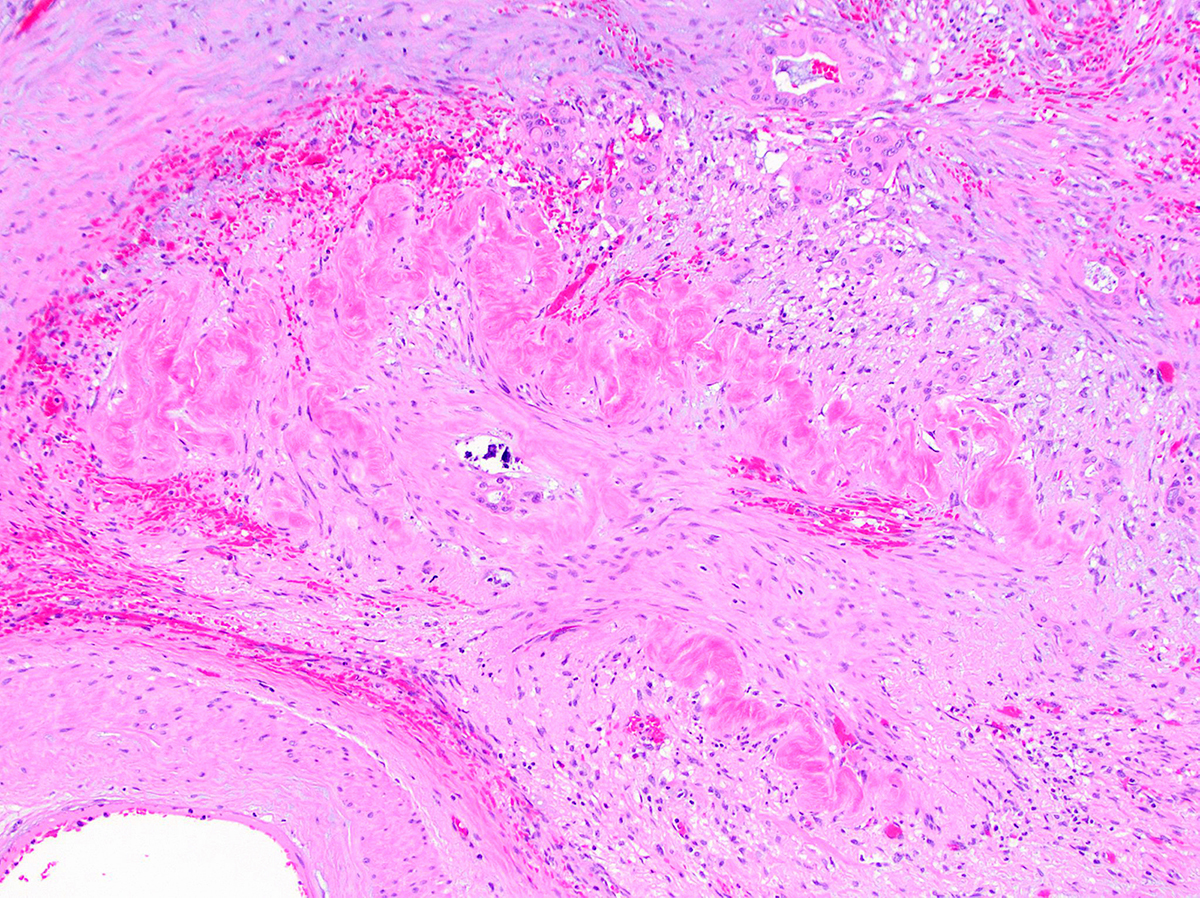
Microscopic (histologic) description
- Modern histological criteria and histological grading of pancreas allograft rejection were established by the multidisciplinary international consensus panel in the reports of 2008, 2011 and 2022
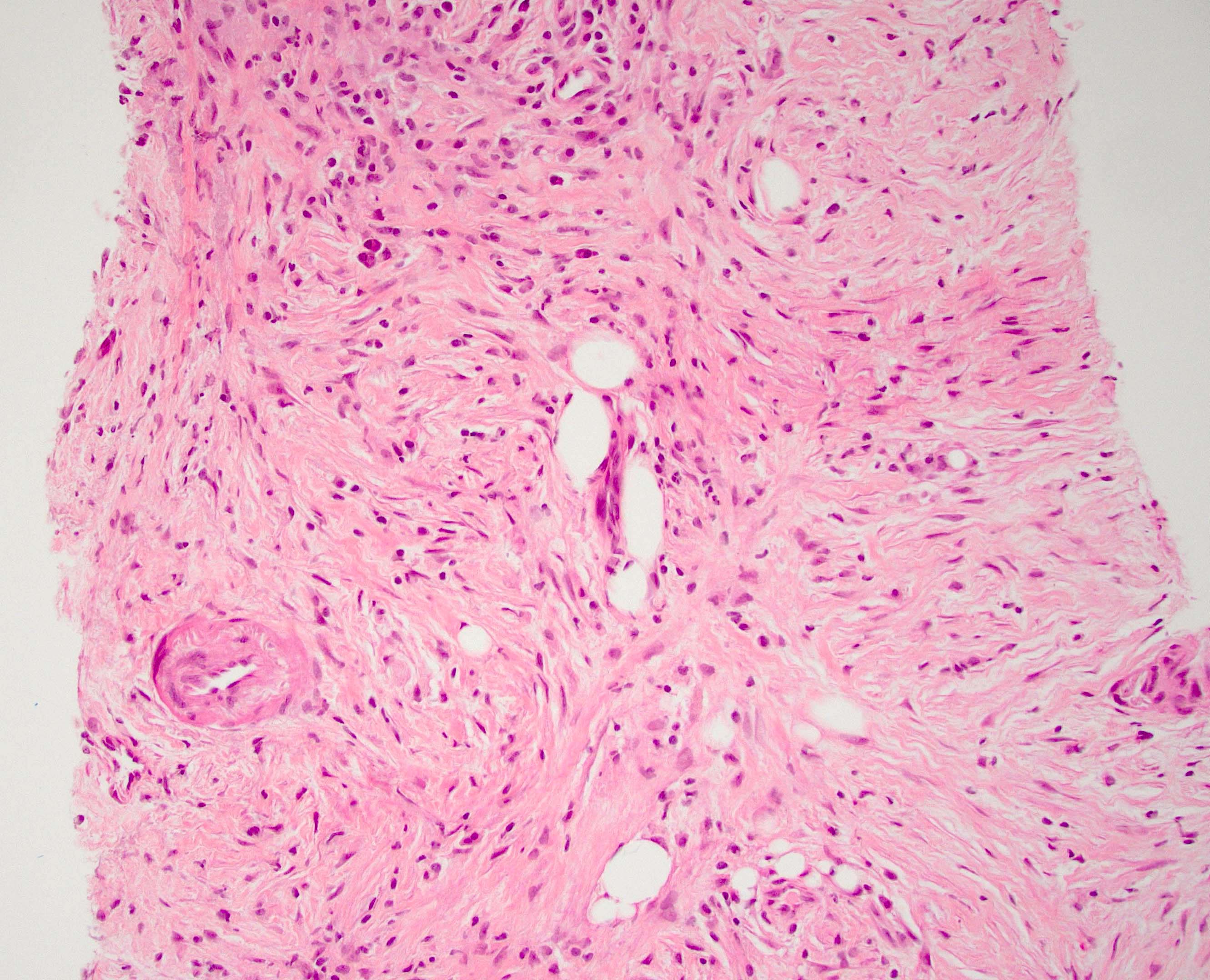
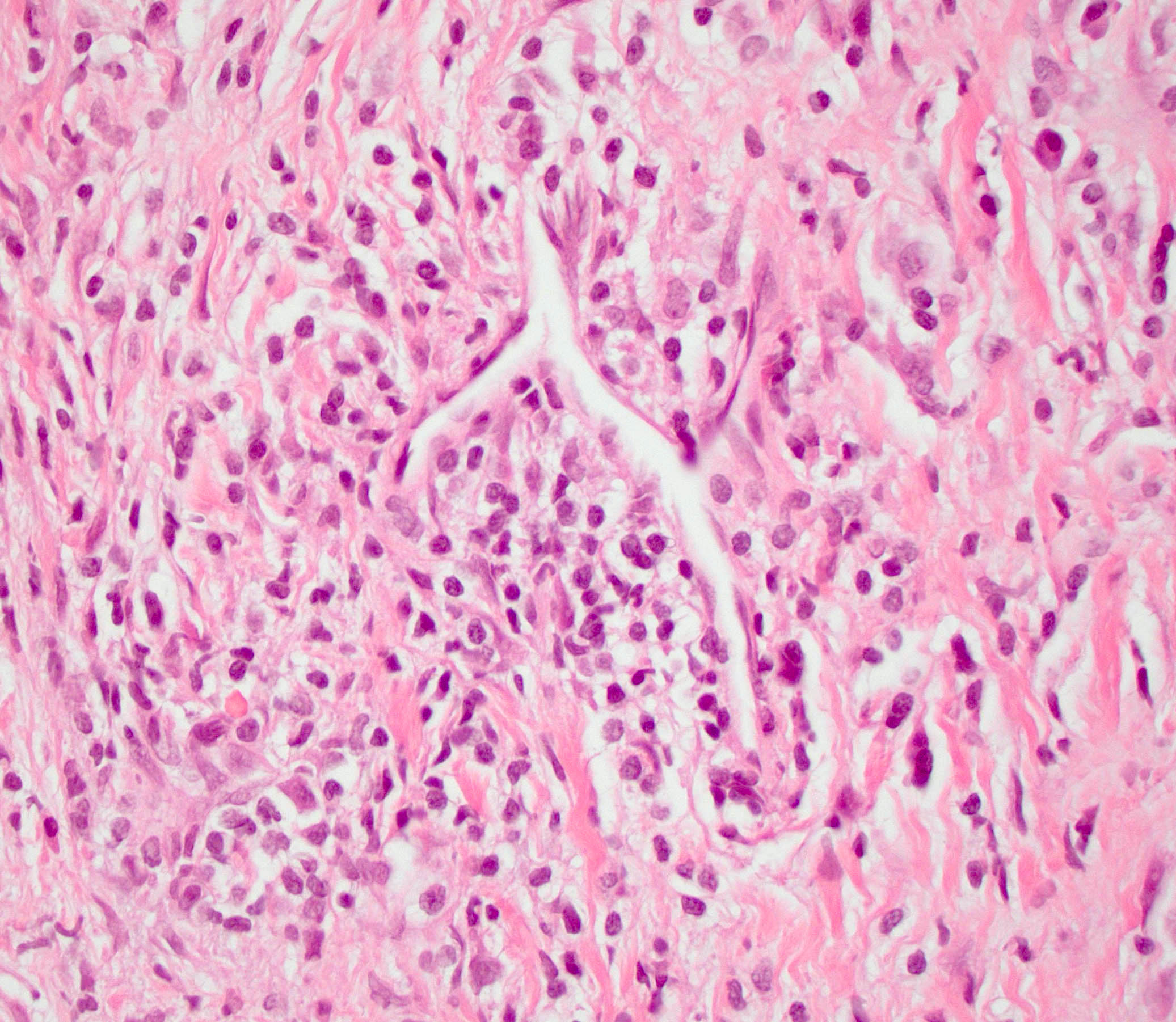
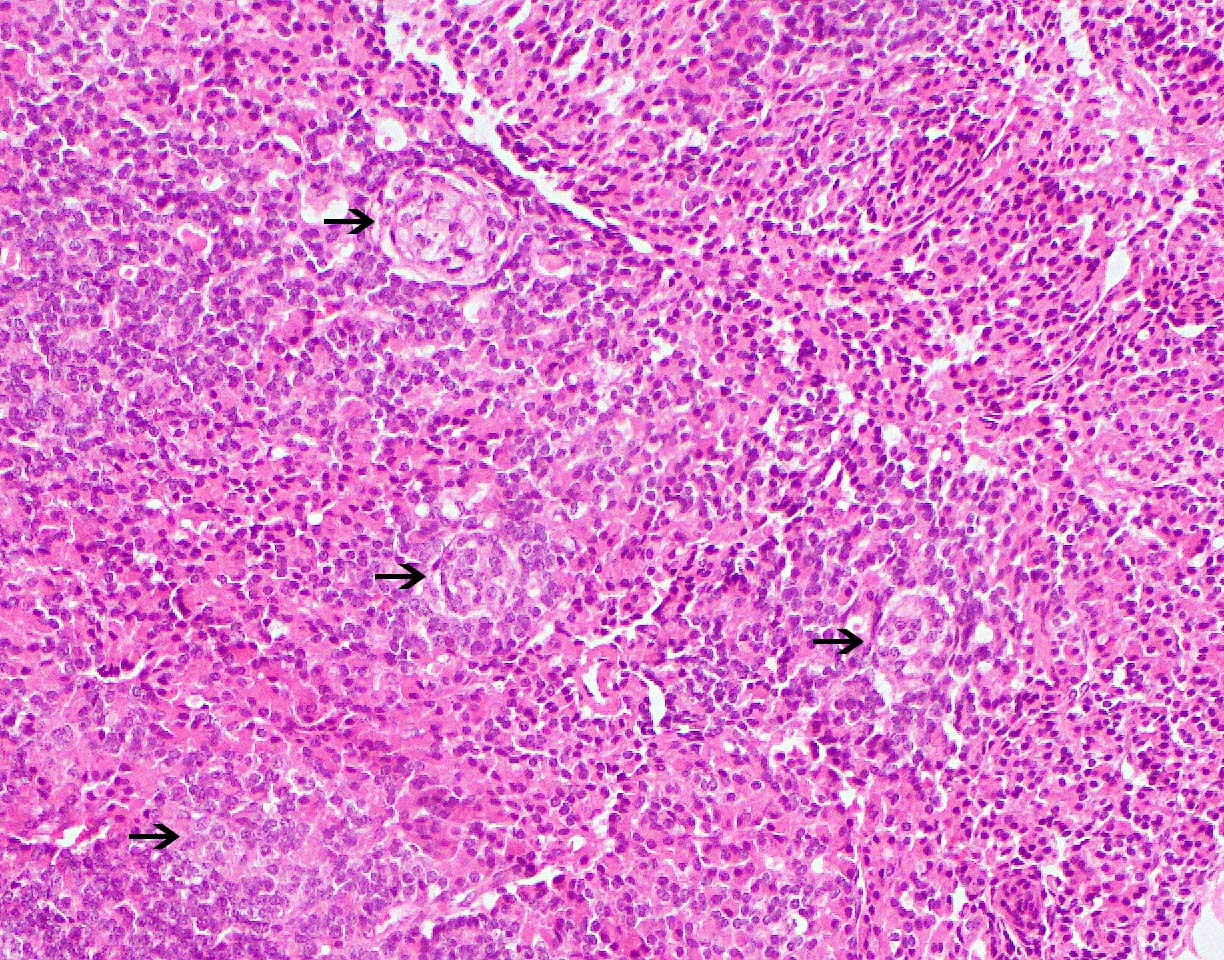
- Adequate pancreas biopsy includes at least 3 lobular areas and their associated interlobular septa
- Arteries should be present; their absence should be noted in the pathology report (Am J Transplant 2008;8:1237)
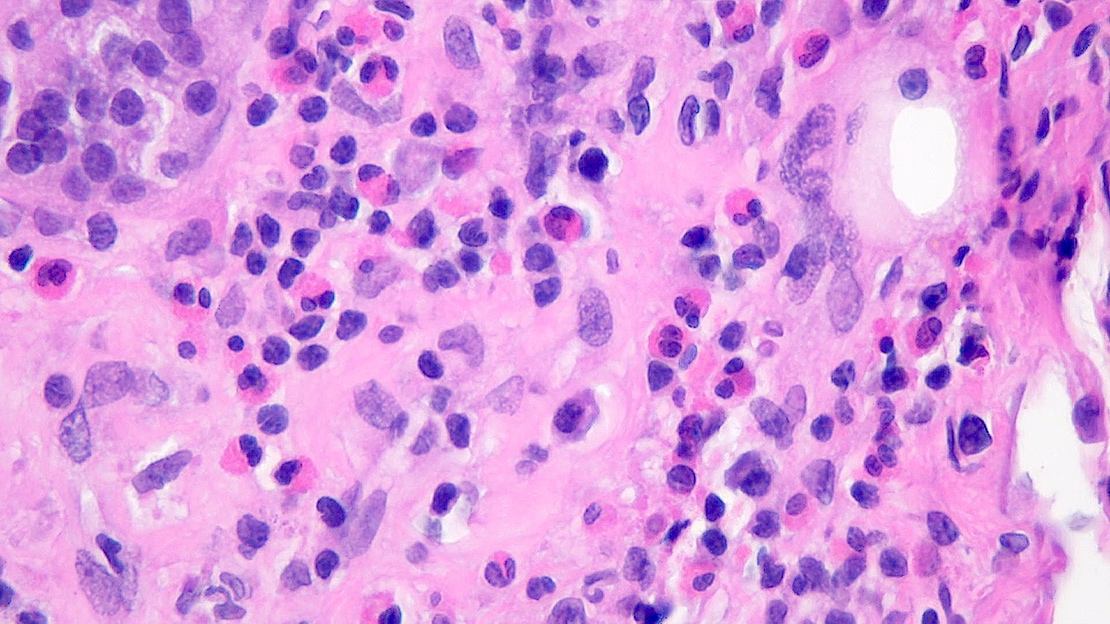
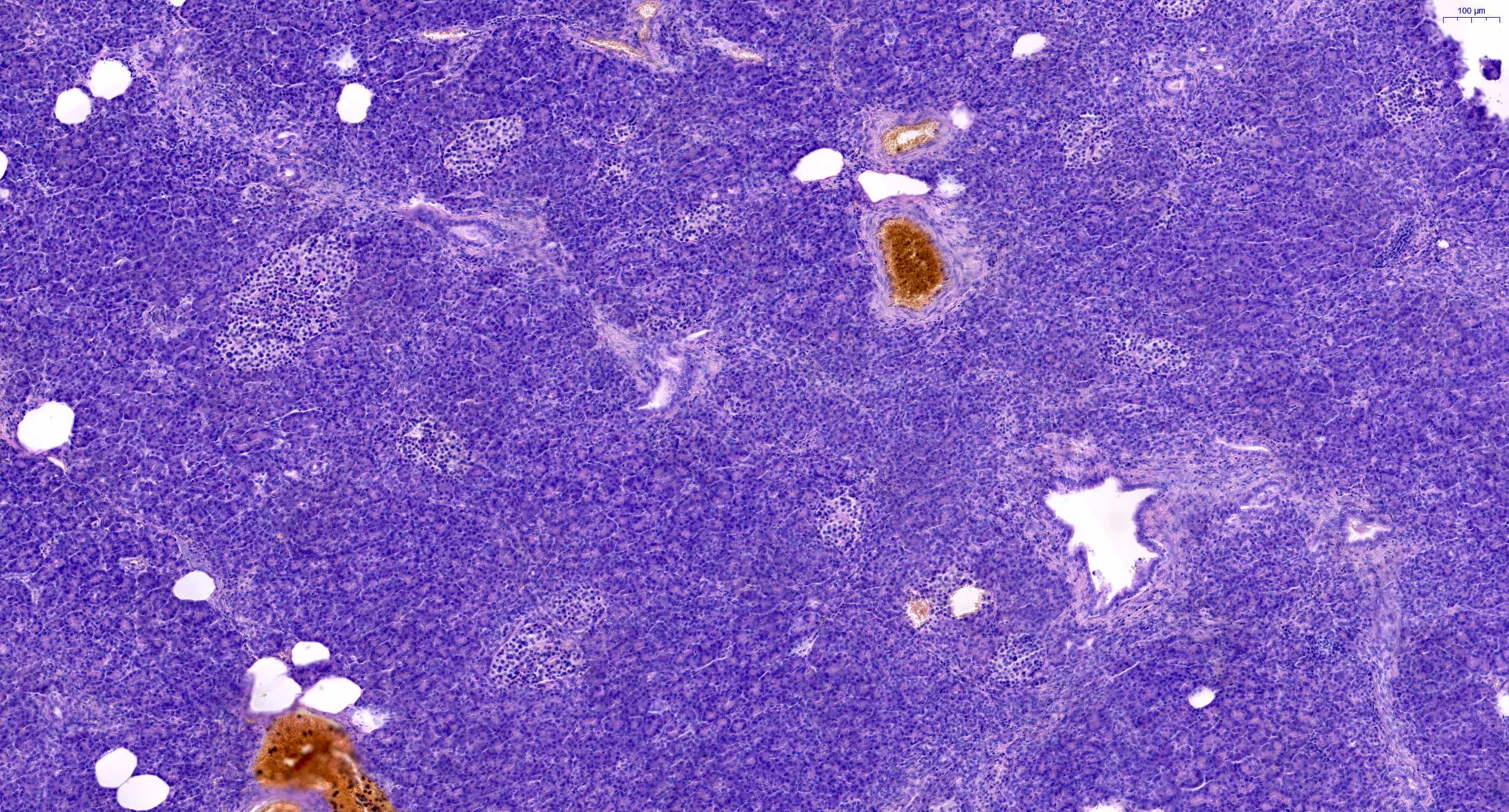
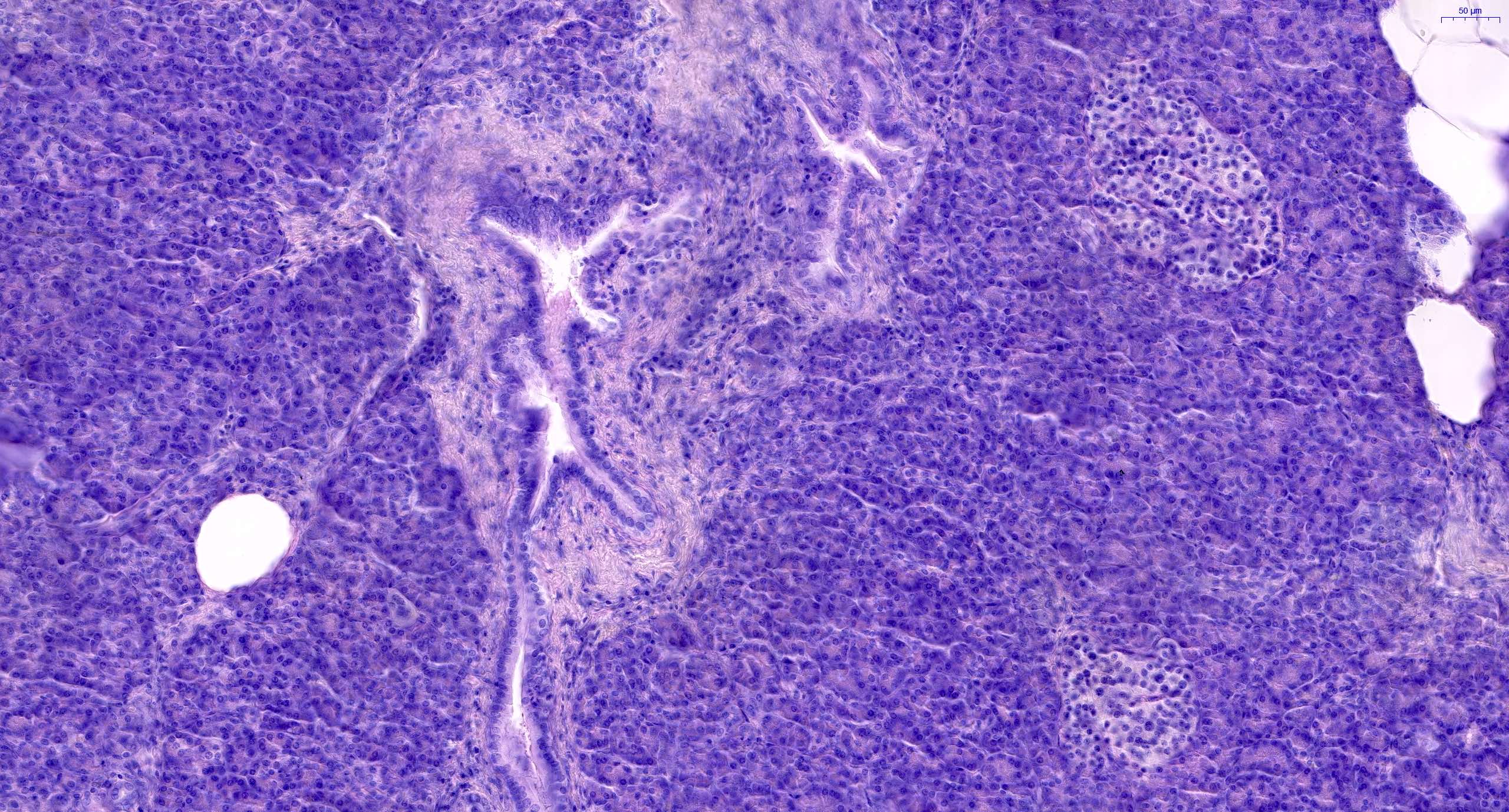
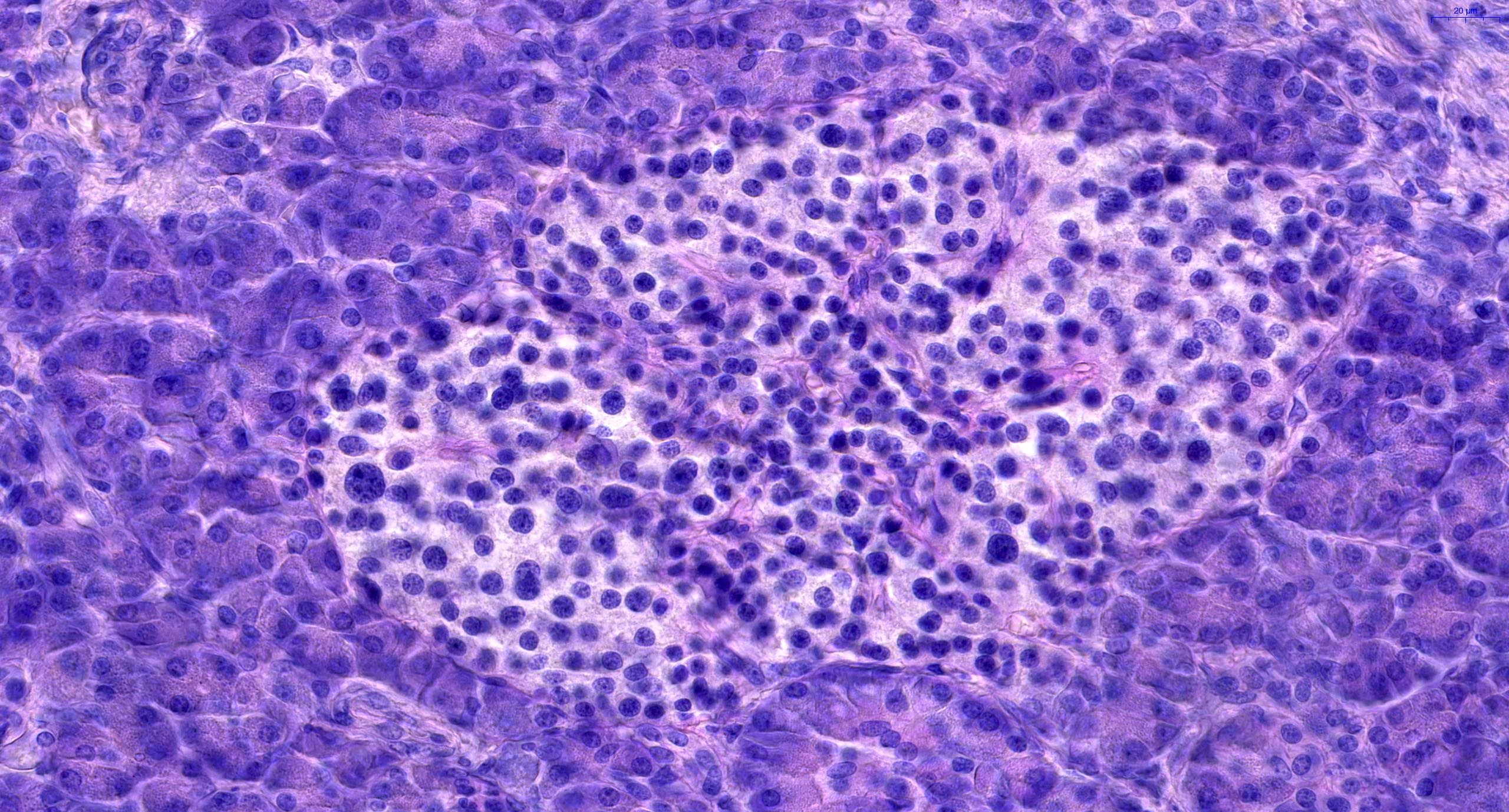
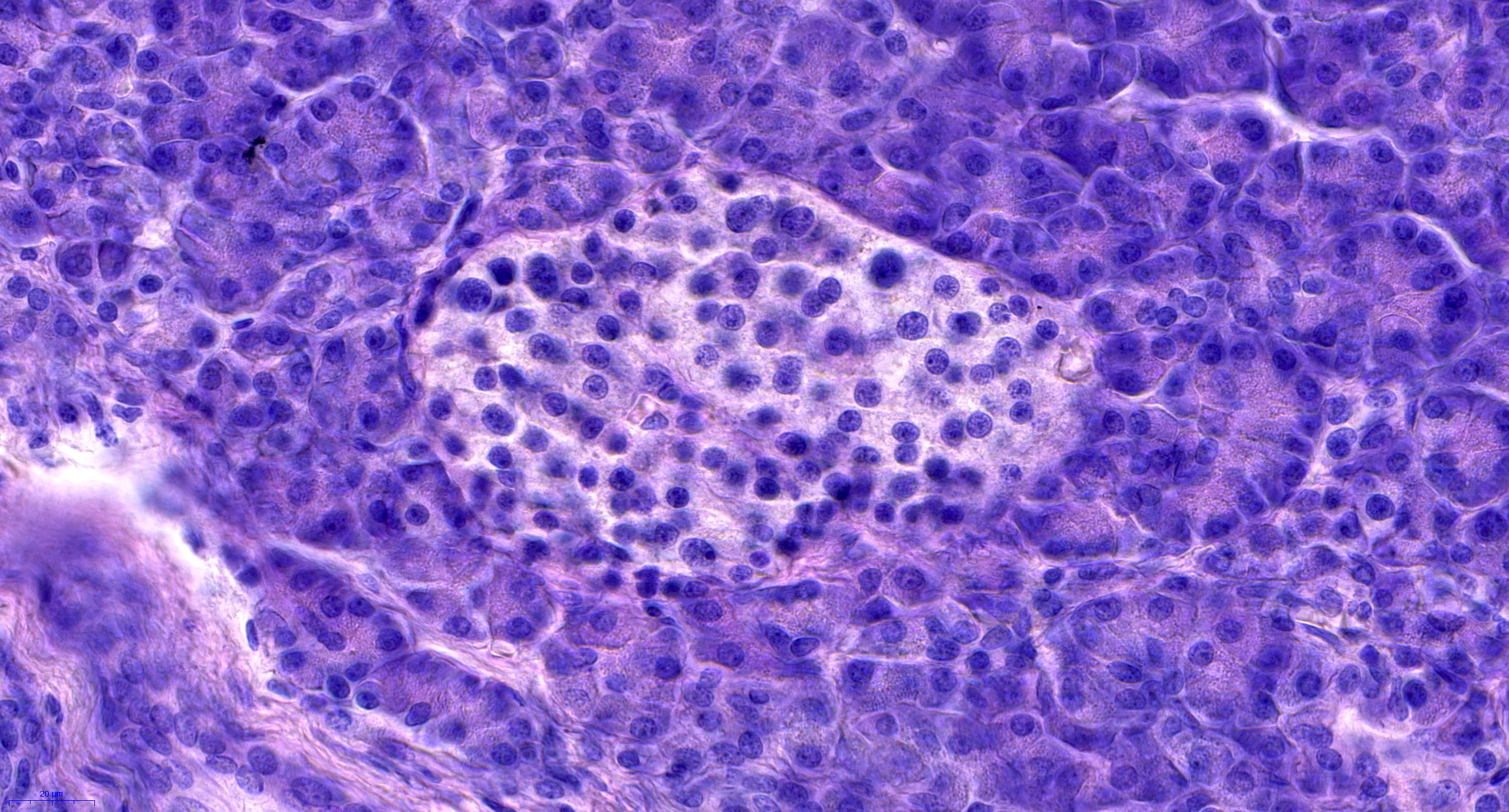
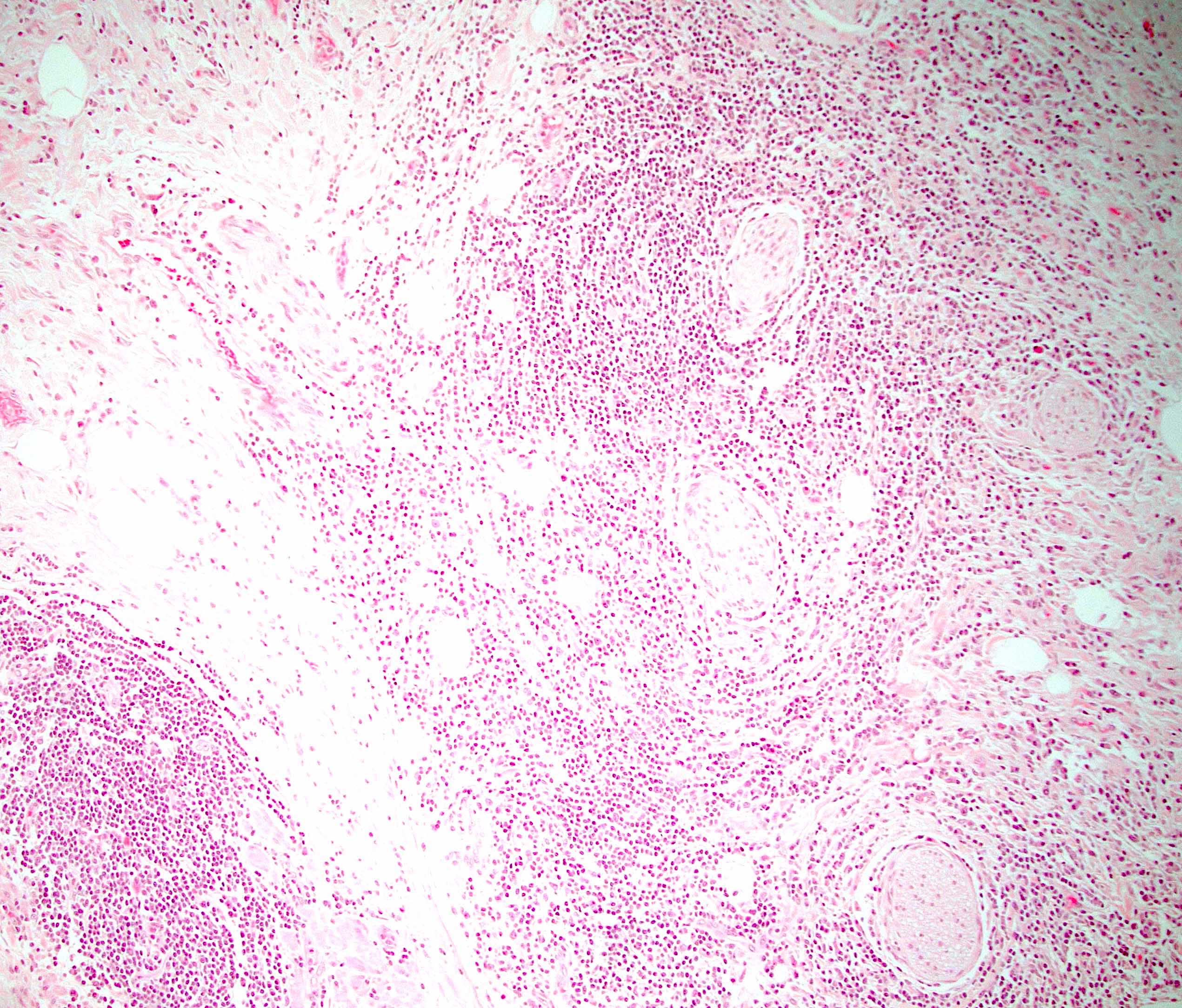
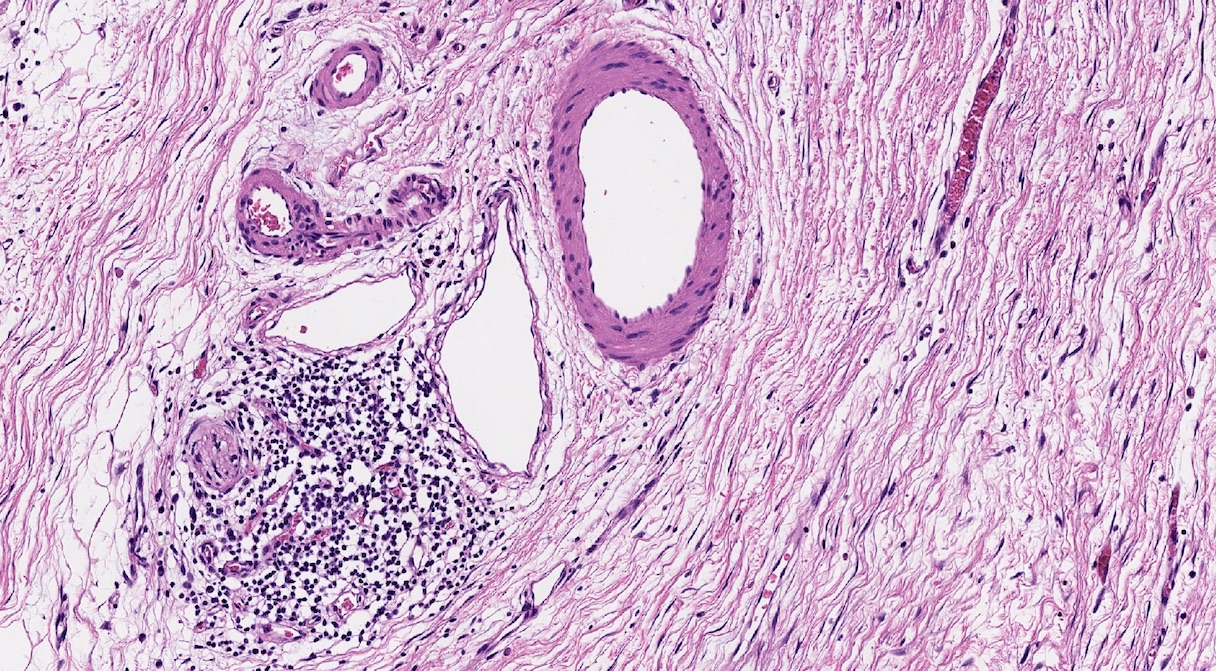
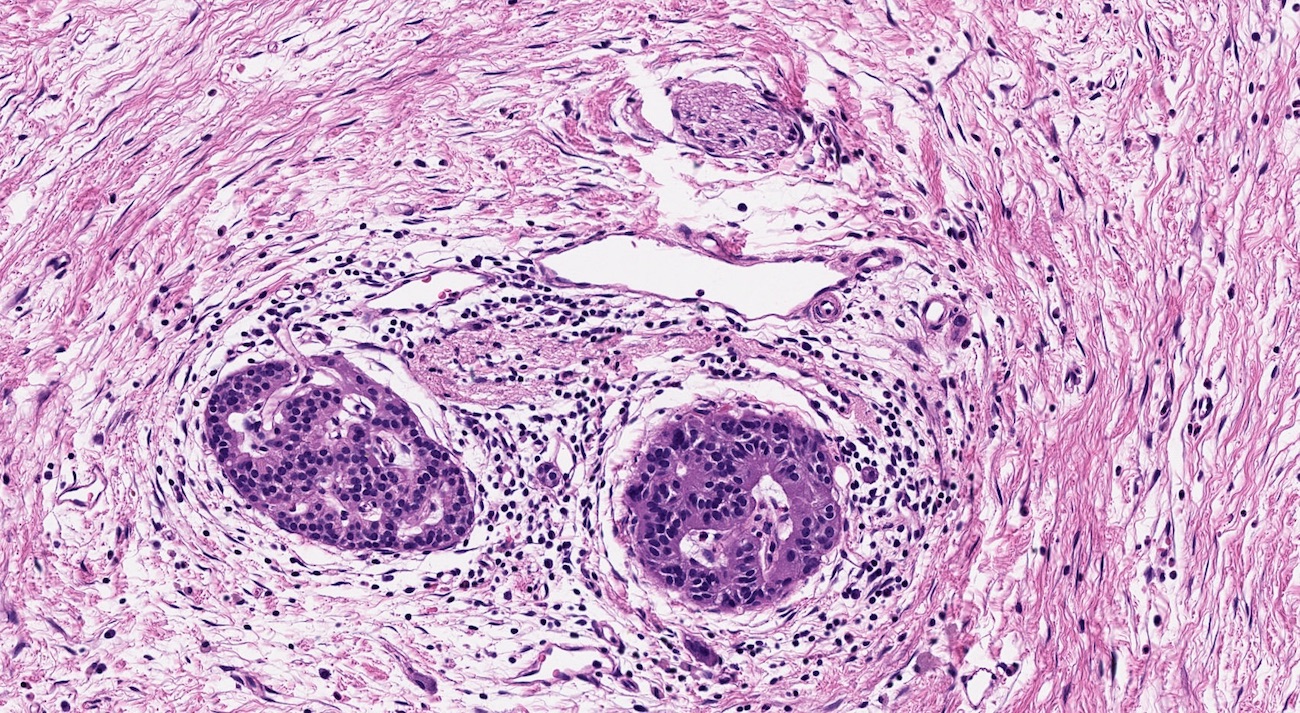
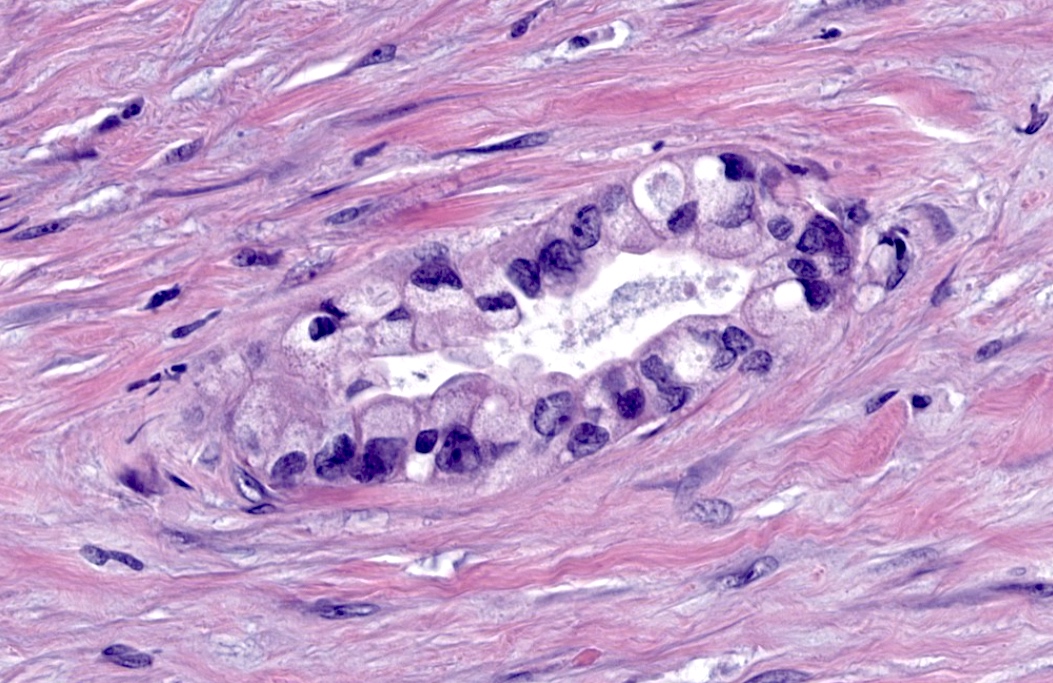
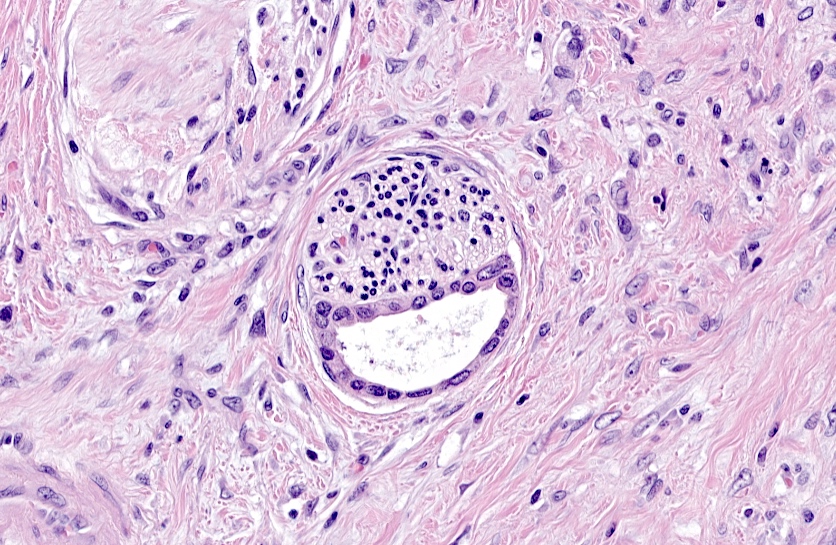
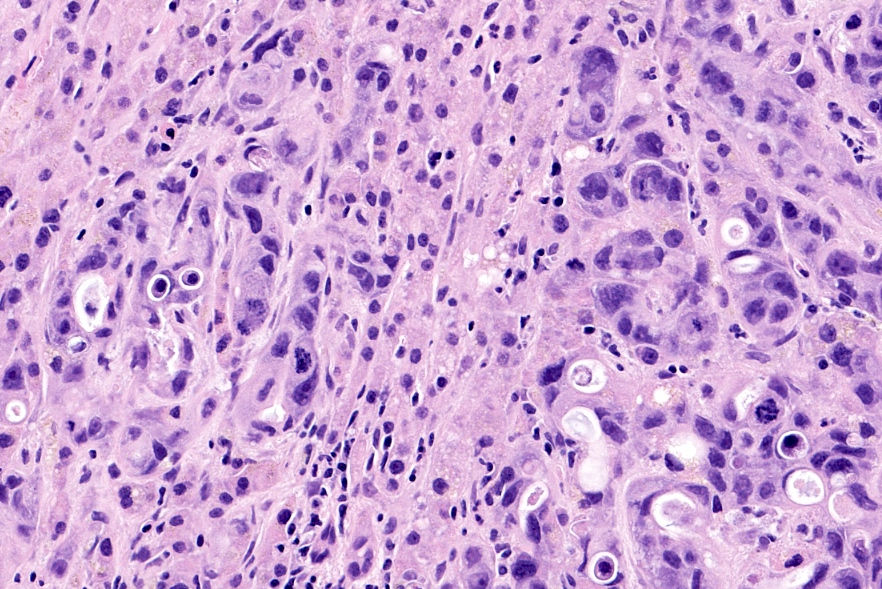
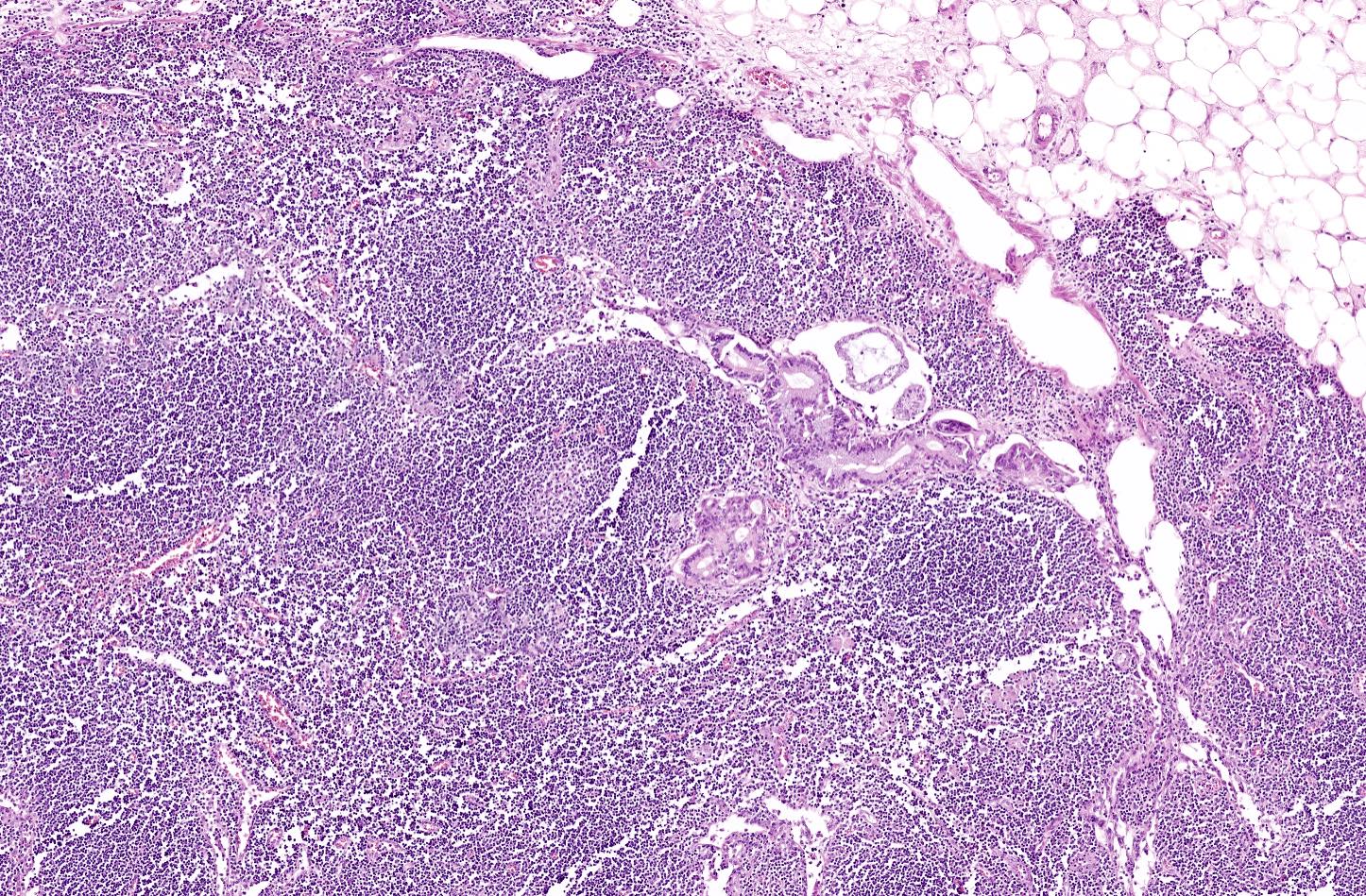
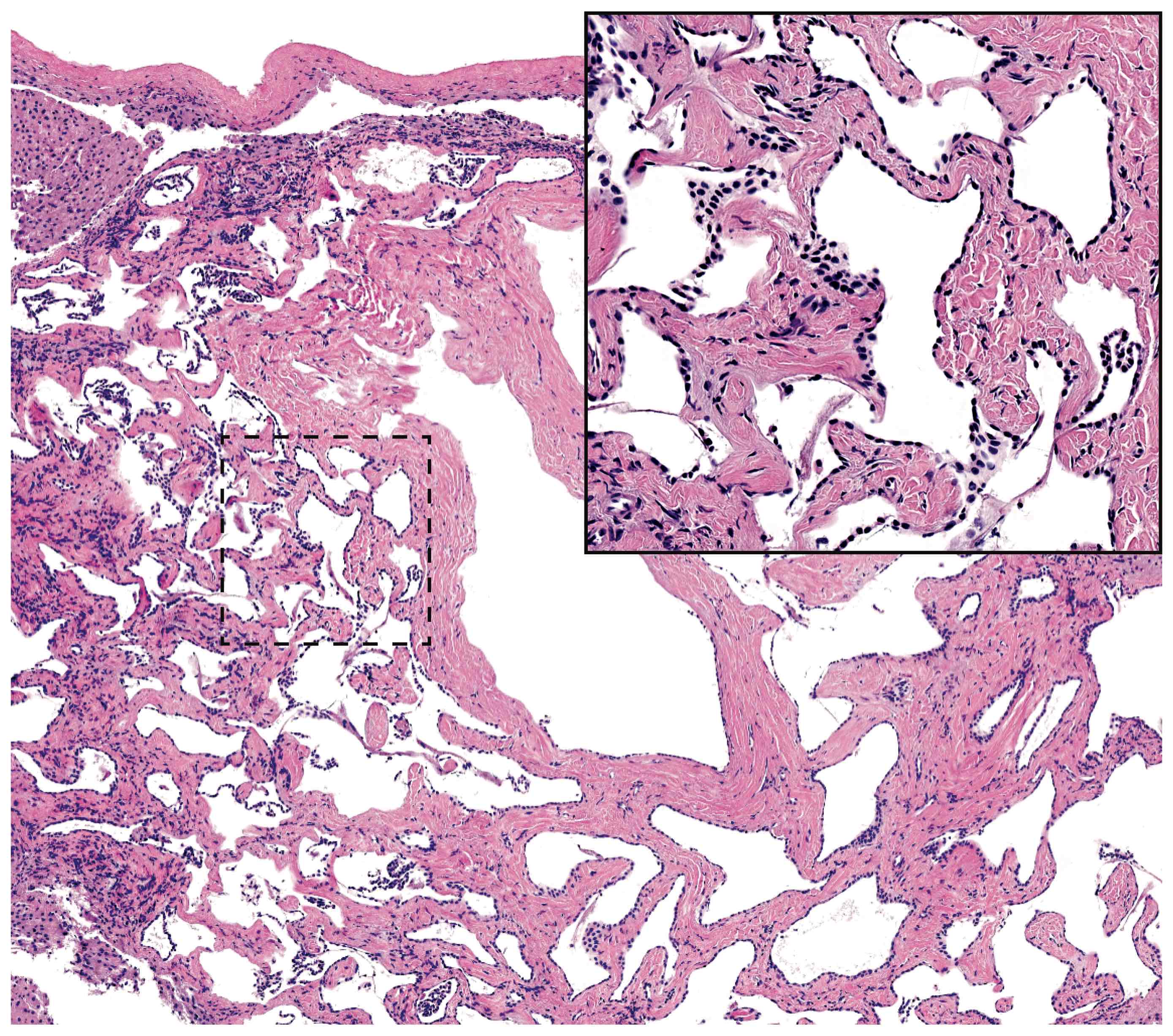
- As a general guideline, histologic features favoring active cell mediated rejection are septal infiltrates, eosinophils, venulitis, mononuclear acinar inflammation (Am J Transplant 2024;24:362)
- Histologic features favoring active antibody mediated rejection: neutrophilic acinar / septal infiltrates, capillaritis, necrotizing vasculitis, interstitial hemorrhage, hemorrhagic necrosis (Am J Transplant 2024;24:362)
- 6 diagnostic categories are defined as follows
- Normal: absent or inactive septal inflammation not involving ducts, veins, arteries or acini; no graft sclerosis (fibrosis) (Am J Transplant 2024;24:362)
- Indeterminate: septal inflammation that has focal features of activity but there is no ductitis, venulitis or acinar inflammation (Am J Transplant 2024;24:362)
- Cell mediated rejection
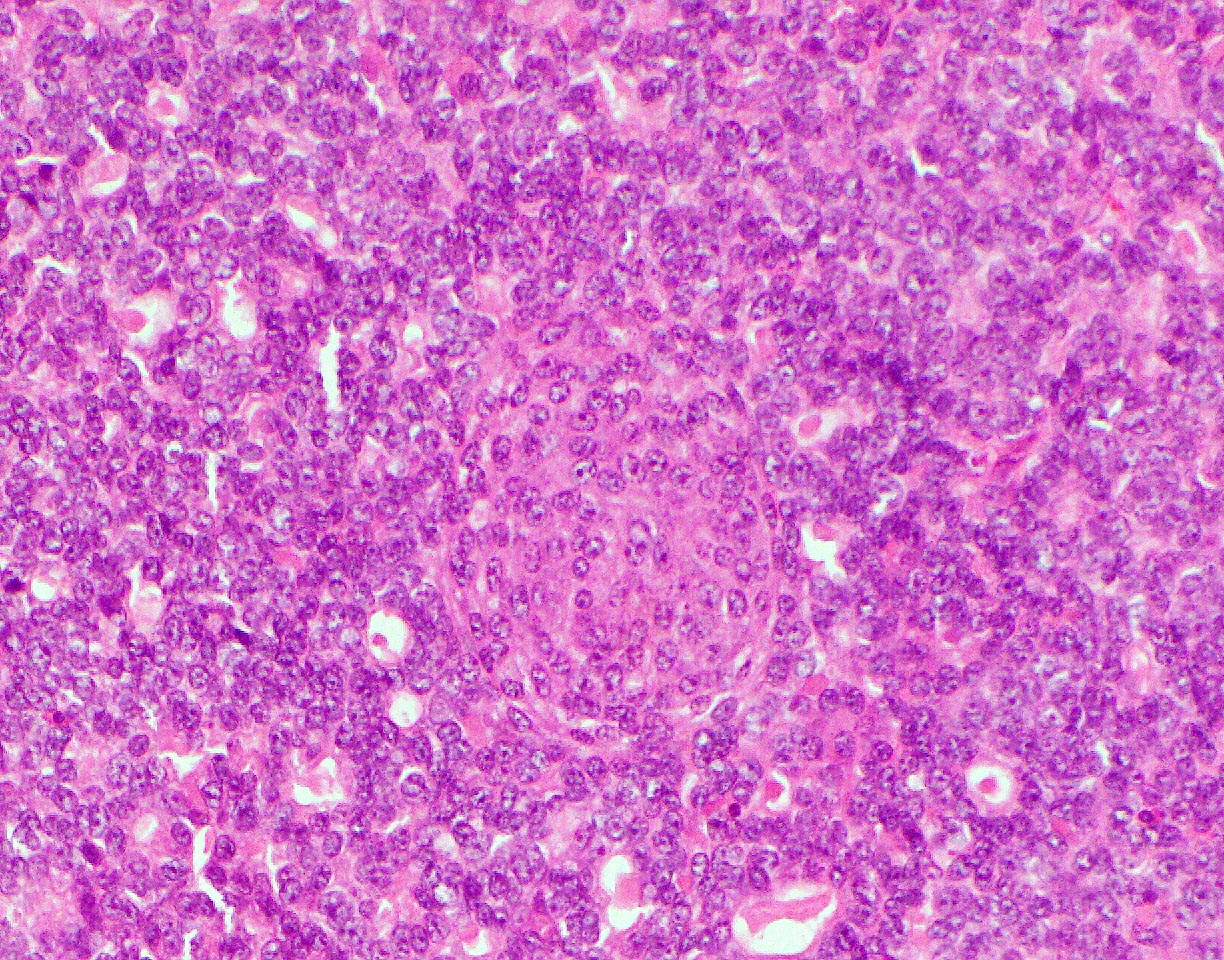
- Acute cell mediated rejection grade 1 / mild active cell mediated rejection: active septal inflammation with venulitis, ductitis, neural / perineural inflammation or focal acinar inflammation (Am J Transplant 2008;8:1237)
- Acute cell mediated rejection grade 2 / moderate active cell mediated rejection: features of mild acute cell mediated rejection or multifocal acinar inflammation with spotty acinar cell injury / dropout or mild intimal arteritis with < 25% luminal compromise (Am J Transplant 2024;24:362)
- Acute cell mediated rejection grade 3 / severe active cell mediated rejection: features of mild acute cell mediated rejection or severe acinar inflammation with focal or diffuse / confluent acinar cell necrosis or moderate or severe intimal arteritis (> 25% luminal compromise) with or without transmural inflammation - necrotizing arteritis (Am J Transplant 2008;8:1237, Am J Transplant 2024;24:362)
- Chronic active cell mediated rejection: predominantly mononuclear inflammation in fragmented acini with ≥ stage 1 graft fibrosis; ductal inflammation with periductal fibrosis or degenerative atrophic or metaplastic epithelial changes; chronic transplant arteriopathy (Am J Transplant 2024;24:362)
- Concurrent active cell mediated rejection should be graded separately (Am J Transplant 2024;24:362)
- Antibody mediated rejection: active has all 3 diagnostic components (see Terminology) present and probably active has 2 of 3 components present (Am J Transplant 2024;24:362)
- Chronic active antibody mediated rejection: active antibody mediated rejection and chronic tissue injury with ≥ stage 1 graft fibrosis or vascular changes (e.g., chronic transplant arteriopathy) (Am J Transplant 2024;24:362)
- Probable chronic active antibody mediated rejection (see Am J Transplant 2024;24:362)
- Graft fibrosis (sclerosis)
- No graft fibrosis: the fibrous component is limited to normal septa and is proportional to the size of ducts and vessels (Am J Transplant 2008;8:1237)
- Mild graft fibrosis / fibrosis stage 1: fibrosis involves < 30% of the core surface; expansion of the fibrous septa; acinar lobules have eroded at the periphery (atrophy) (Am J Transplant 2024;24:362)
- Moderate graft fibrosis / fibrosis stage 2: fibrosis involves 30 - 60% of the core surface, the acinar atrophy affects the majority of the locules at the periphery and in the center, thin fibrous strands crisscross between individual acini (Am J Transplant 2024;24:362)
- Severe graft fibrosis / fibrosis stage 3: fibrosis involves > 60% of the core surface with only isolated residual acinar tissue and islets present (Am J Transplant 2024;24:362)
- Islet pathology
- Islet injury due to parenchymal ischemia
- β cell injury due to calcineurin inhibitor toxicity
- Recurrence of autoimmune diabetes mellitus (insulitis, selective β cell loss)
- Features of diabetes mellitus type 2 (islet amyloid deposition)
- Nonspecific reactive / regenerative changes (nesidioblastosis, islet hyperplasia)
- Other histologic diagnosis
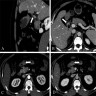
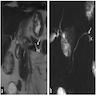
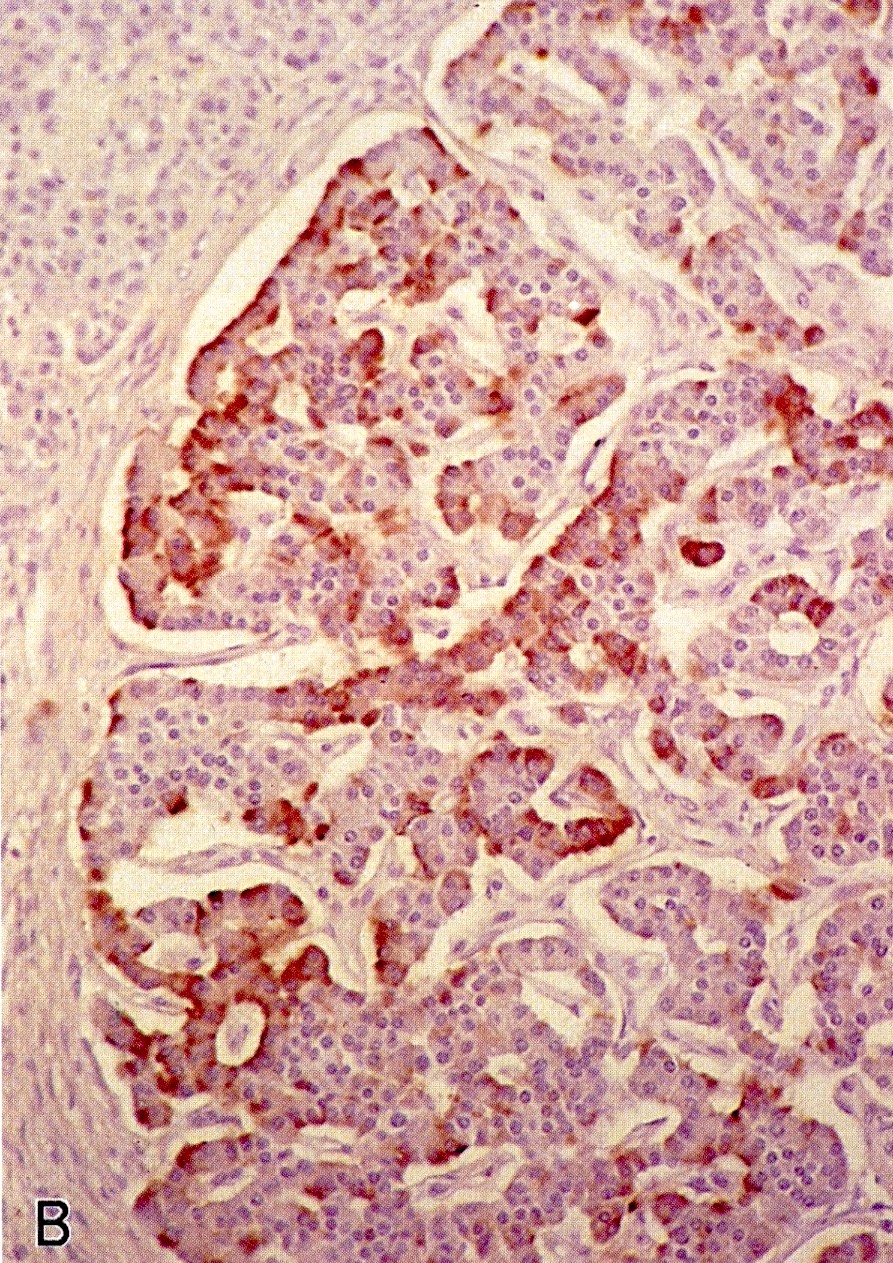
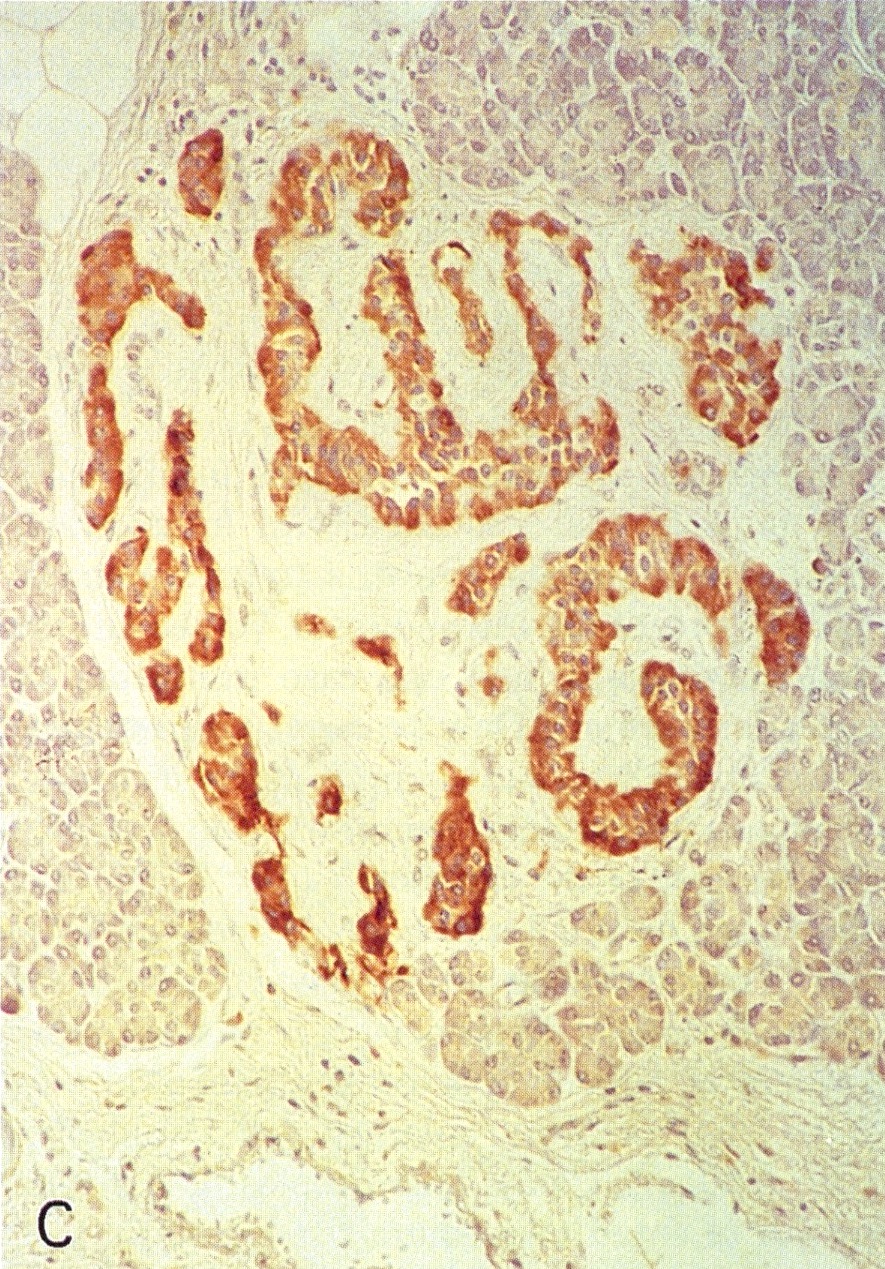
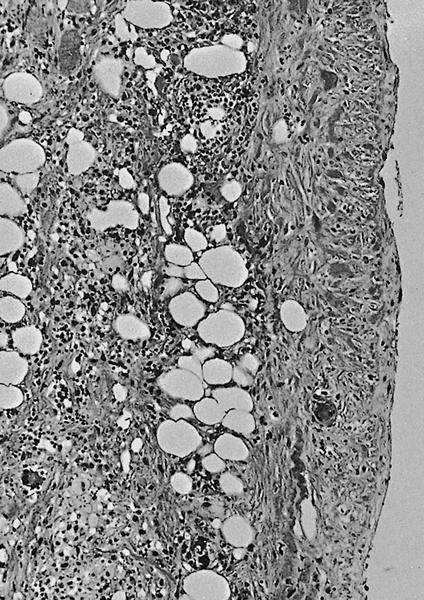
Microscopic (histologic) images
Contributed by Alexei Mikhailov, M.D., Ph.D. and Cinthia Drachenberg, M.D.
Cytology description
- Cytology studies are not used in the diagnosis of pancreas transplant rejection
Positive stains
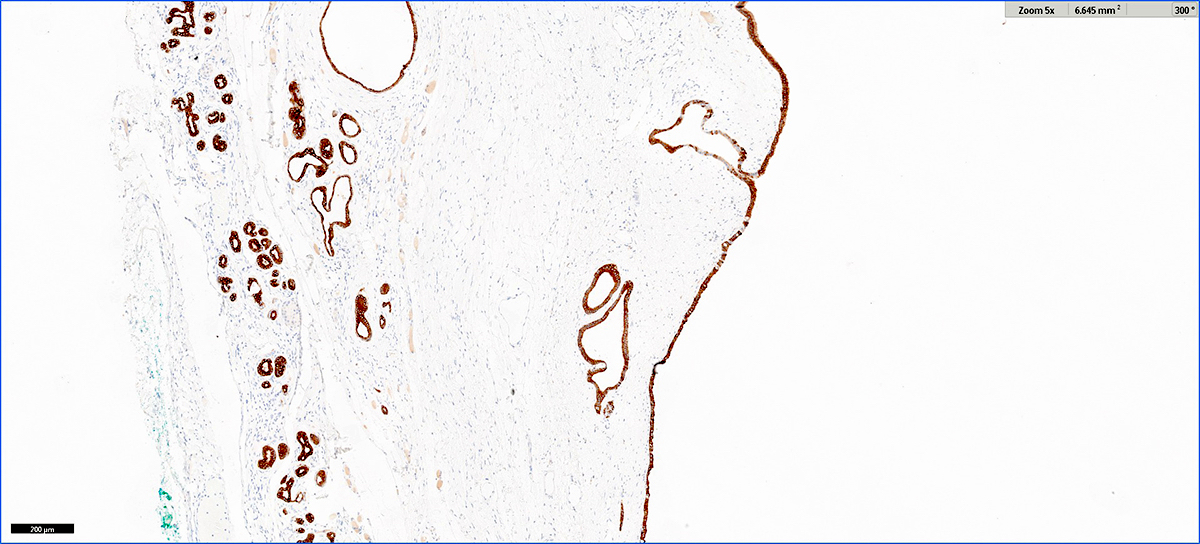
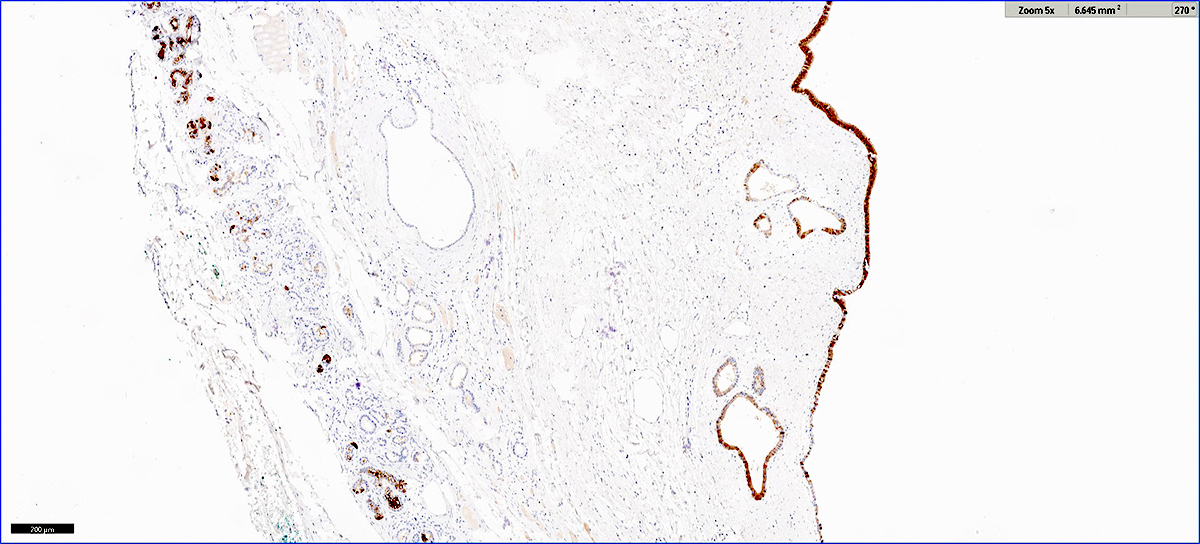
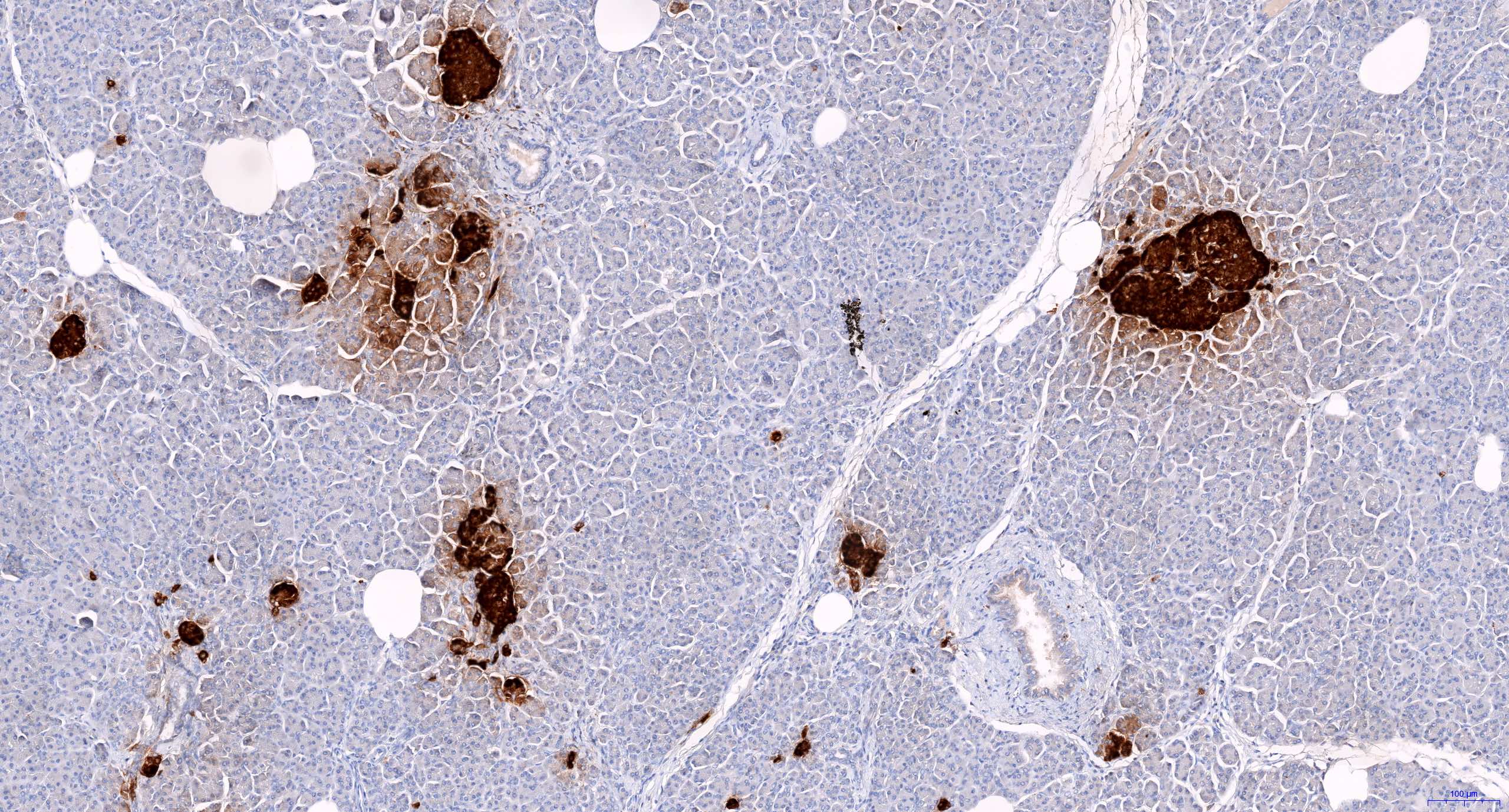
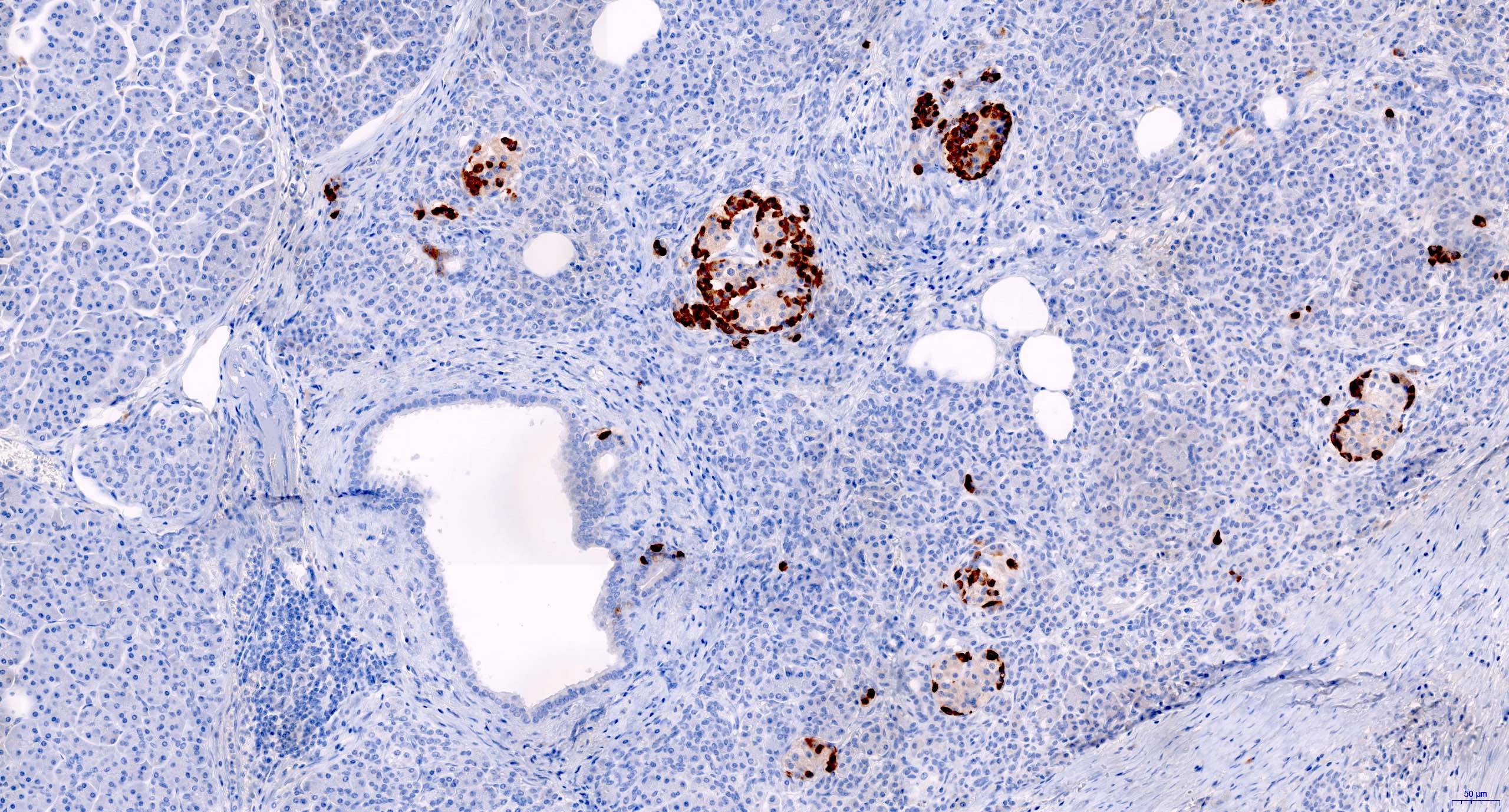
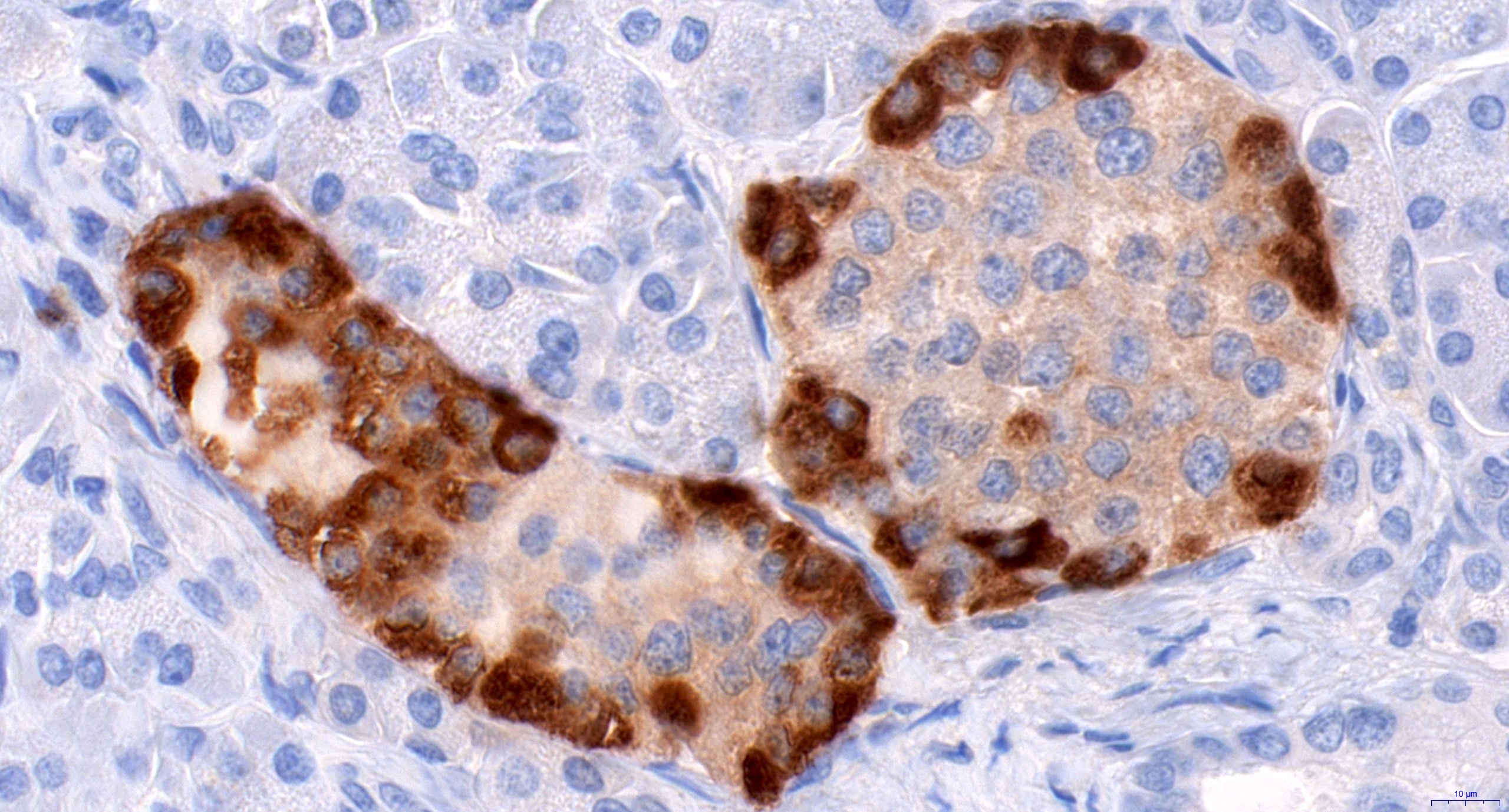
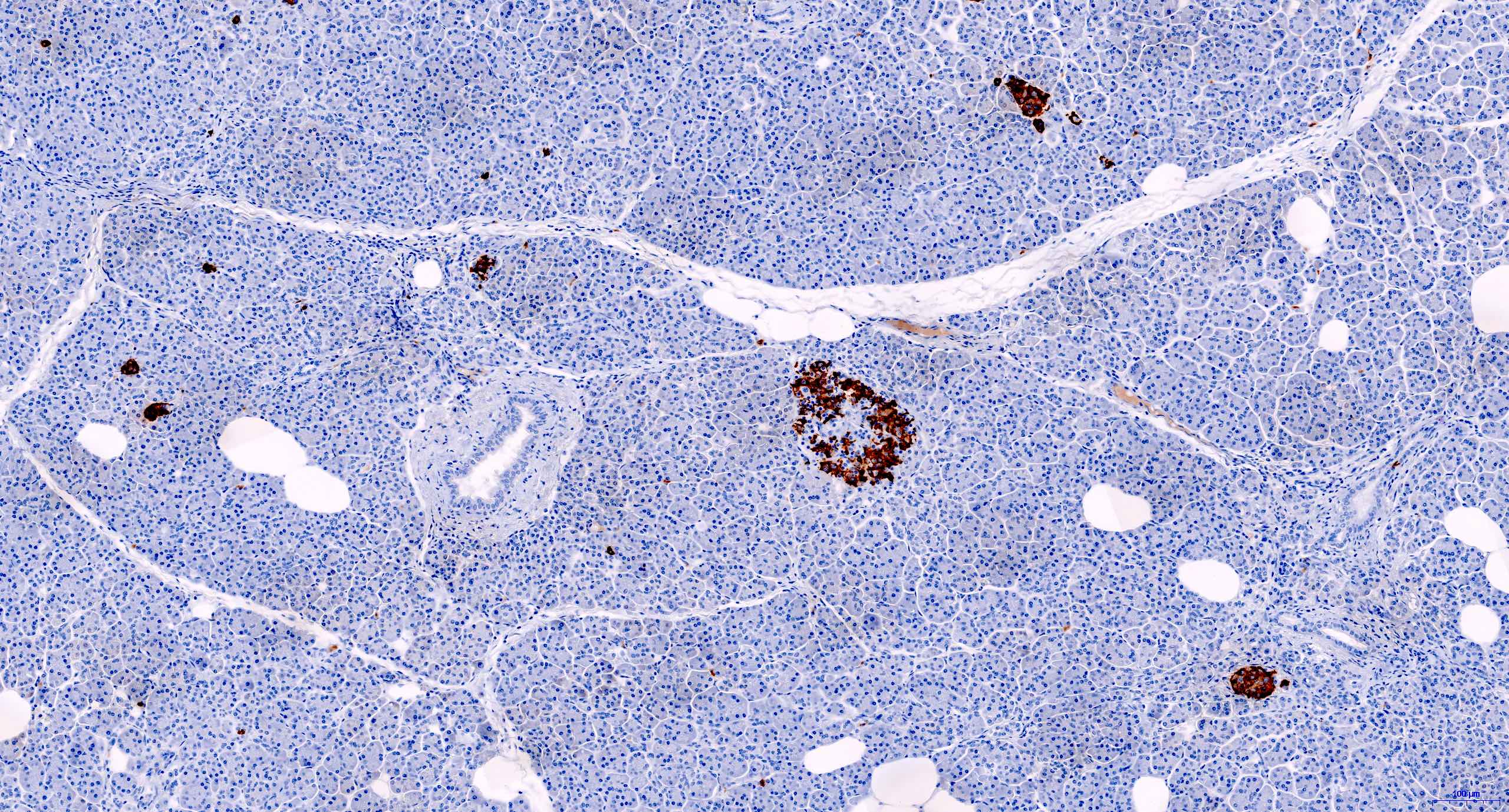
- CD3 and CD68 immunohistochemical stains highlight the presence of T lymphocytes and macrophages in the lobules and facilitate the detection of lymphocytes in the venules and ducts
- At present, however, the Banff pathology working group recommends that the diagnosis and grading of rejection remain based on the evaluation of routine stains (Am J Transplant 2024;24:362)
- C4d immunohistochemical staining if positive in the interacinar capillaries (≥ 1% of the acinar lobular surface) becomes 1 of the 3 diagnostic components in the detection of active or chronic active antibody mediated rejection
Negative stains
- Insulin and glucagon immunohistochemical stains demonstrate diminished secretion in both β and α cells in ischemic graft injury
- Diagnosis of type 1 diabetes mellitus recurrence requires immunohistochemical demonstration of a lack of β cells in the islets (Am J Transplant 2024;24:362)
Electron microscopy description
- Electron microscopy is not used in the diagnosis of pancreas transplant rejection
Sample pathology report
- Pancreas, transplant, biopsy:
- Acute cell mediated rejection with ductitis, venulitis and foci of mild acinar inflammation, consistent with Banff grade 1
- Focal mild septal fibrosis stage 1
- Clinical information
- The patient is a 34 year old man with a history of end stage renal disease secondary to diabetes mellitus type 1. He received a simultaneous pancreas kidney biopsy 1 year ago. Laboratory data: serum amylase 123 IU/mL, serum lipase 170 IU/mL, serum creatinine 1.4 mg/dL (baseline 0.9 mg/dL).
- Microscopic description
- 6 lobules of pancreatic parenchyma are sampled. The septa show variable numbers of lymphocytes, including activated lymphocytes, with admixed eosinophils, from scattered cells to groups and clusters. Several ducts show intraepithelial lymphocytes with associated vacuolization and detachment of ductal epithelium. A few venules display circumferential cuffing, activated endothelium and subendothelial lymphocytes. Some lobules display small foci of lymphocytes with no appreciable acinar cell damage. There is mild fibrosis involving ~15% of the biopsy core surface, with peripheral acinar erosion. Unremarkable medium caliber arteries are present.
- Special stains
- The C4d immunoperoxidase stain is negative
- The immunoperoxidase stains for CD3 and CD68 demonstrate increased numbers of septal and acinar T lymphocytes and macrophages
Differential diagnosis
- Ischemia reperfusion injury:
- Seen in the early postoperative period, significant inflammation is absent (Am J Transplant 2024;24:362)
- Pancreatitis / peripancreatic fluid collection:
- Septa and periphery of lobules show mixed (lymphocytes, plasma cells, eosinophils, neutrophils) inflammation; center of lobules is preserved (Am J Transplant 2008;8:1237)
- Peripancreatitis:
- Fibrosis may appear very pronounced in the superficial areas of the pancreas and it does not affect the deeper areas, which may lead to errors in determining the fibrosis stage
- Cytomegalovirus pancreatitis:
- Cytomegalovirus cytopathic changes in acinar, stromal and endothelial cells (Am J Transplant 2008;8:1237)
- Polyomavirus nephropathy:
- Develops in the kidney of the SPK transplants only; the pancreas is spared from clinical evidence of infection (Transplant Proc 2004;36:1097, Transplant Proc 2004;36:1095)
- Posttransplant lymphoproliferative disorder:
- Infiltrates are nodular and expansile, containing a significant proportion of atypical plasmacytoid B cells
- In situ hybridization with Epstein-Barr encoded RNAs is positive (Hum Pathol 1998;29:569)
- Bacterial or fungal infection:
- Purulent, necrotizing, granulomatous
- Systemic or localized infectious symptoms are seen (Am J Transplant 2008;8:1237)
- Calcineurin inhibitor toxicity:
- Pathologic changes are limited to islet β cells, which demonstrate cytoplasmic clearing / vacuolization and weak insulin staining (Am J Transplant 2024;24:362)
- Findings of rejection are absent
- Diabetes type 1 recurrence:
- Insulitis with progressive and selective loss of β cells (Am J Transplant 2024;24:362)
- Findings of rejection are absent
- Diabetes type 2 development:
- Often associated with amyloid deposition and degenerative changes in islets (Am J Transplant 2024;24:362)
- Findings of rejection are absent
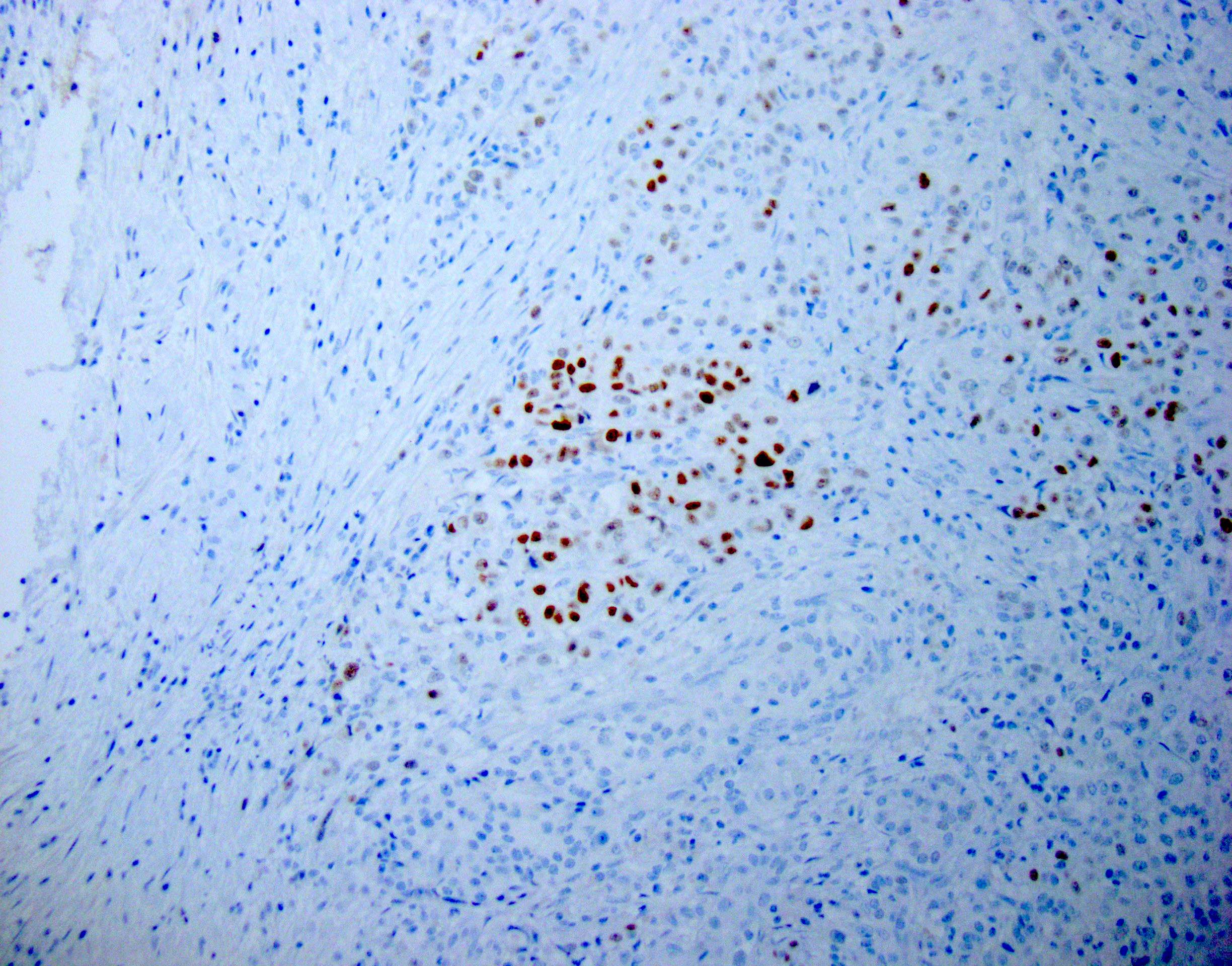
Board review style question #1
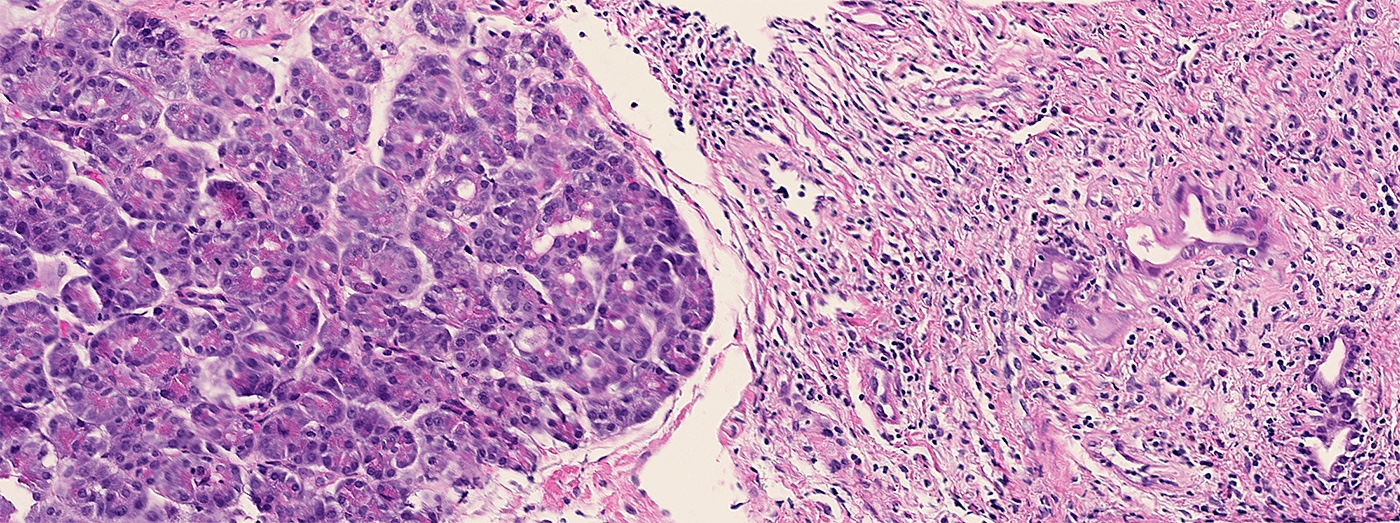
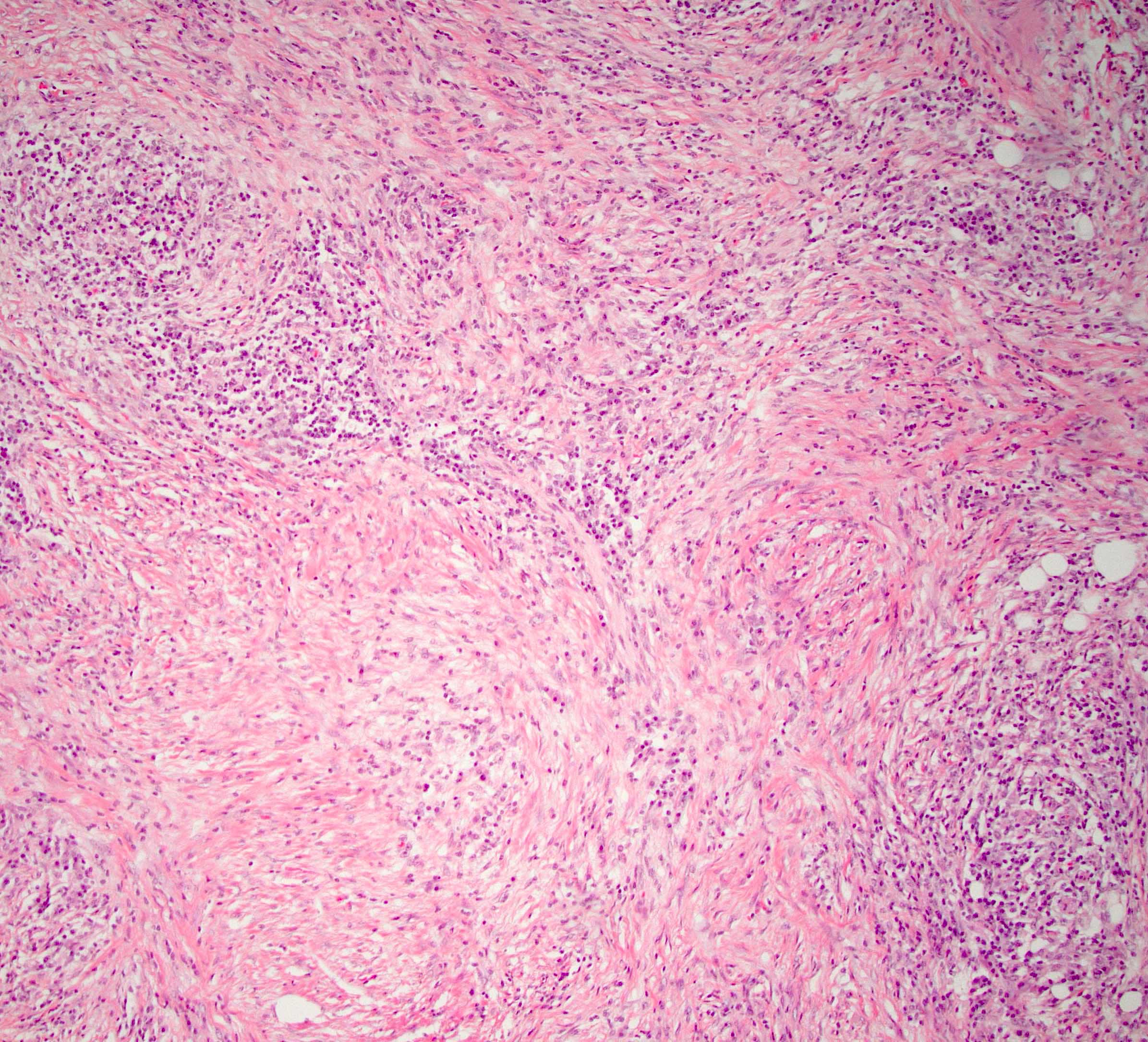
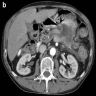
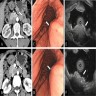
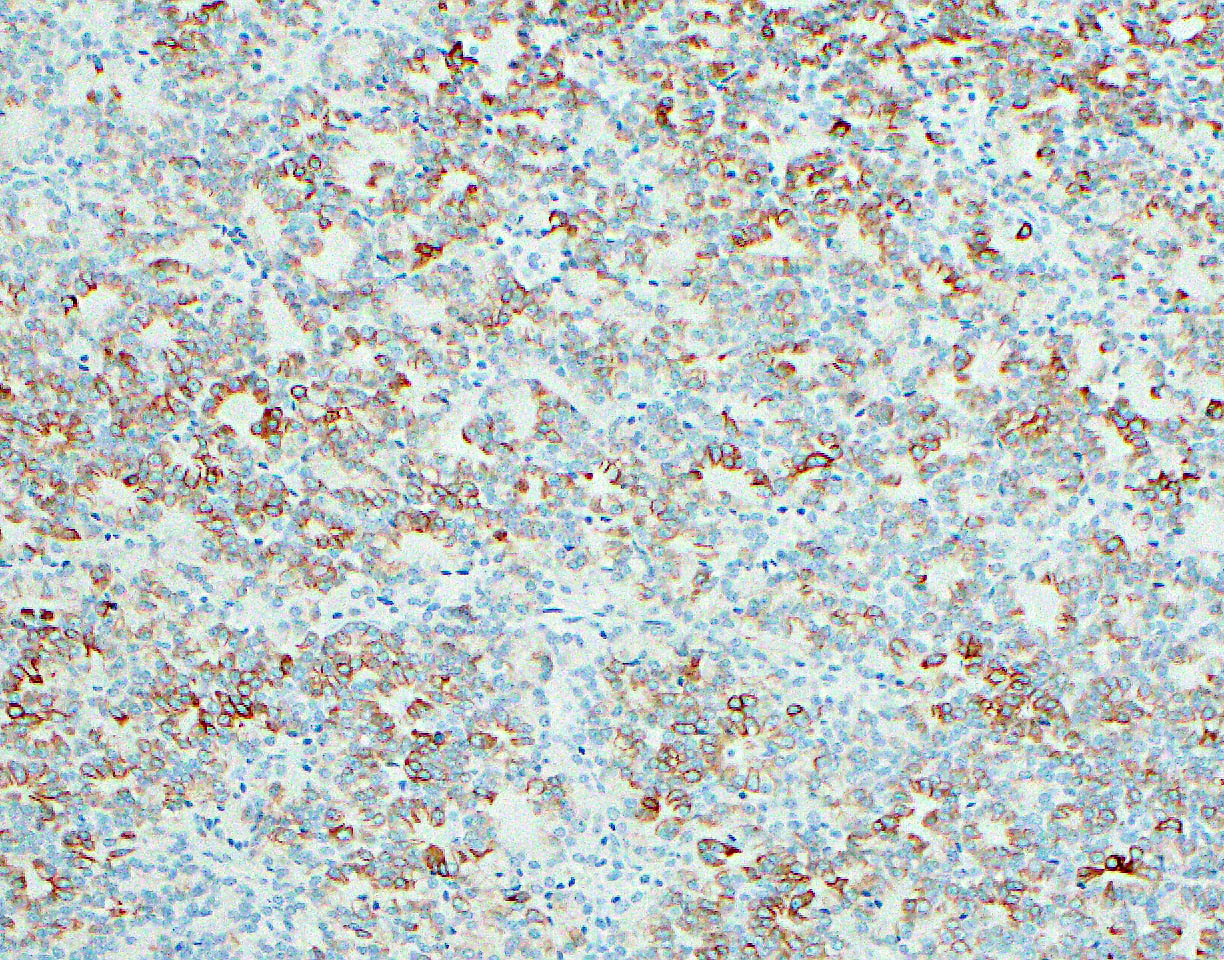
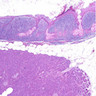
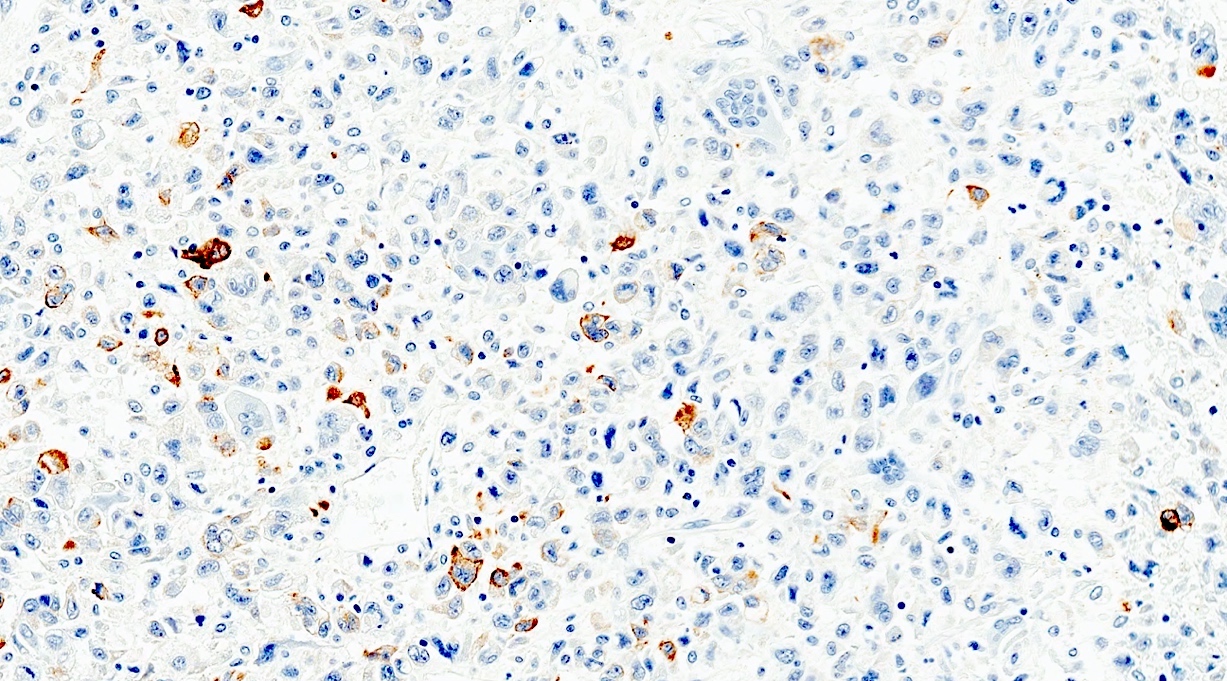
The representative image above is from the pancreas biopsy of a simultaneous pancreas kidney transplant recipient. Biopsy was performed due to elevated serum amylase and lipase. Based on this H&E stained section alone, how would you grade this biopsy?
- Acute antibody mediated rejection
- Acute cell mediated rejection, grade 1
- Acute cell mediated rejection, grade 2
- Acute cell mediated rejection, grade 3
- Indeterminate for rejection
Board review style answer #1
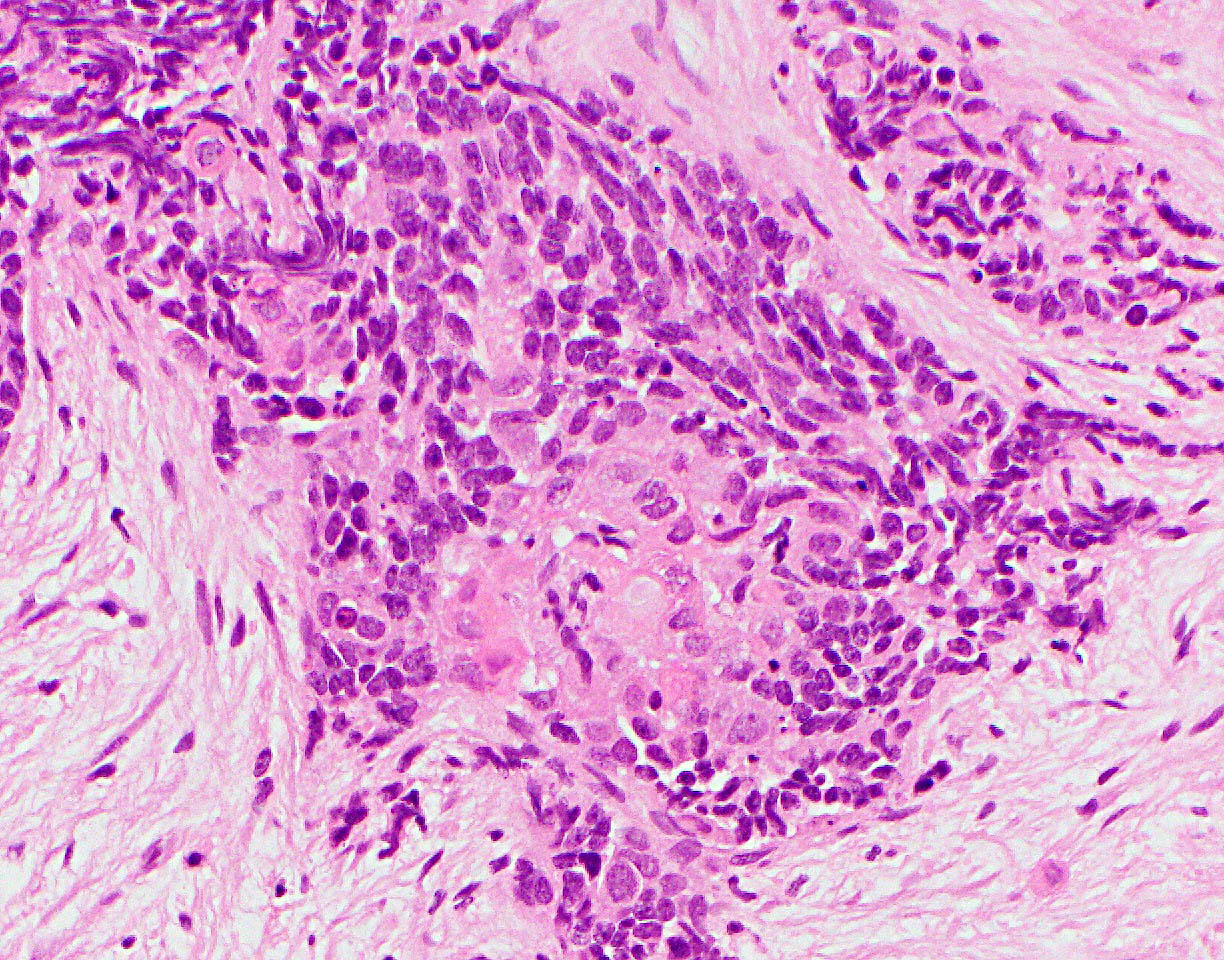
B. Acute cell mediated rejection, grade 1. The interlobular septum is diffusely involved with a mononuclear infiltrate containing activated lymphocytes and eosinophils; there is ductitis with significant epithelial damage, defining this process as acute cell mediated rejection, grade 1.
Answer E is incorrect because the septal infiltrate is active and shows features of acute cell mediated rejection. Answers C and D are incorrect because acute cell mediated rejection grades 2 and 3 require multifocal or severe acinar inflammation. Answer A is incorrect because there are no histological (interacinar capillaritis) or laboratory (donor specific antibodies) features of acute antibody mediated rejection.
Comment Here
Reference: Allograft rejection
Comment Here
Reference: Allograft rejection
Board review style question #2
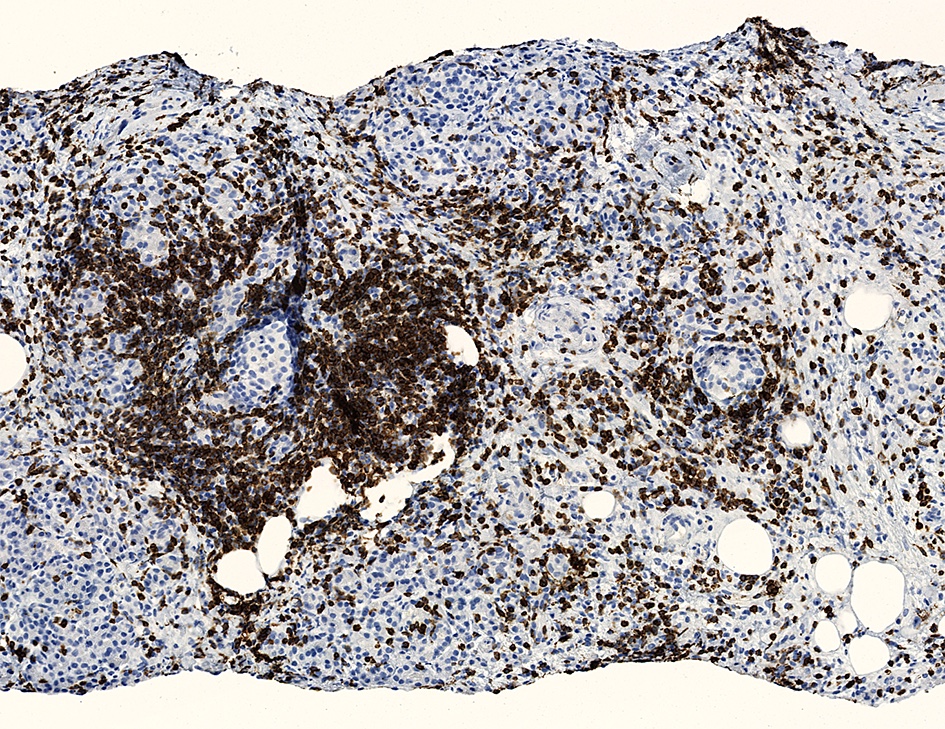
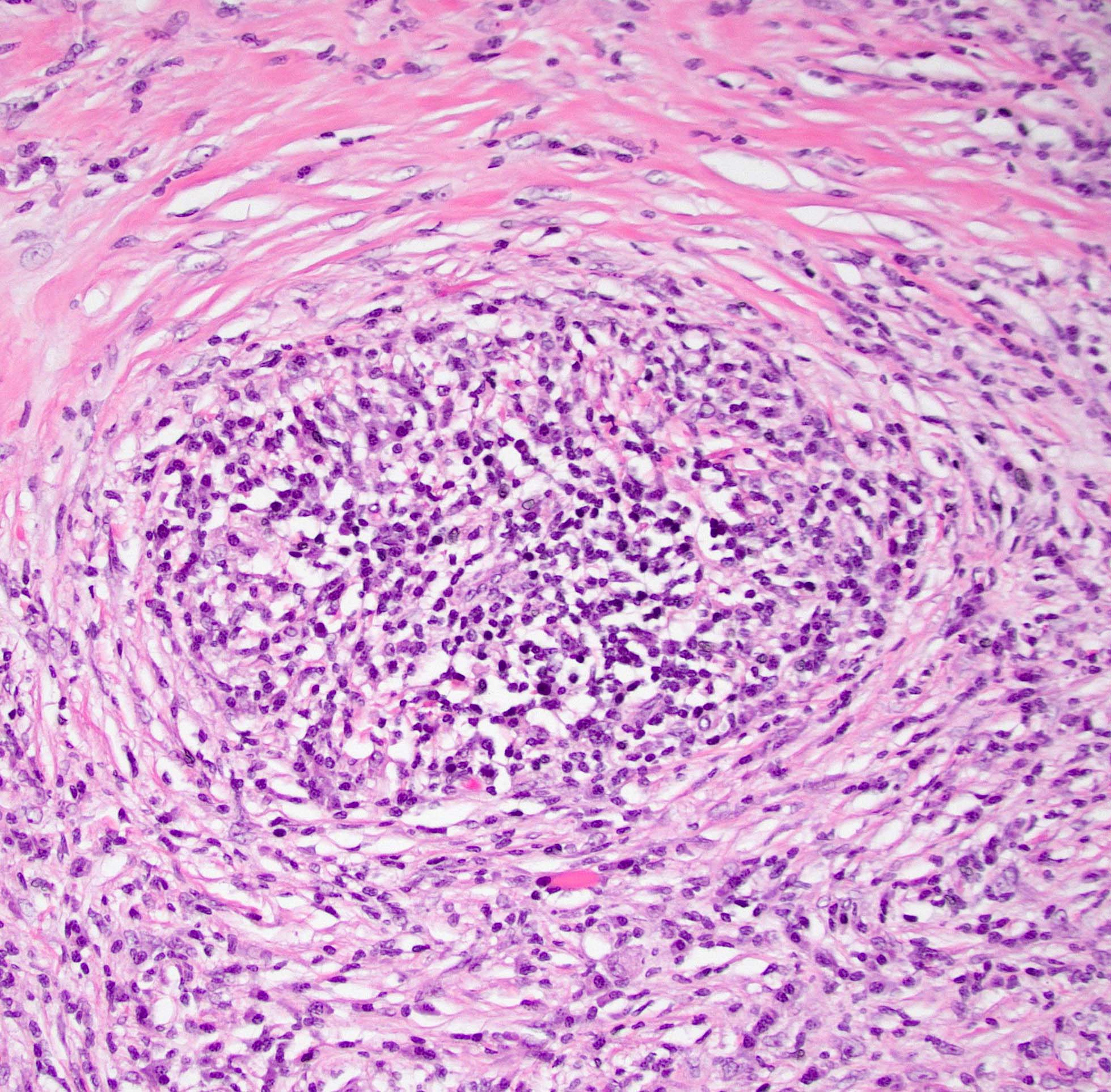
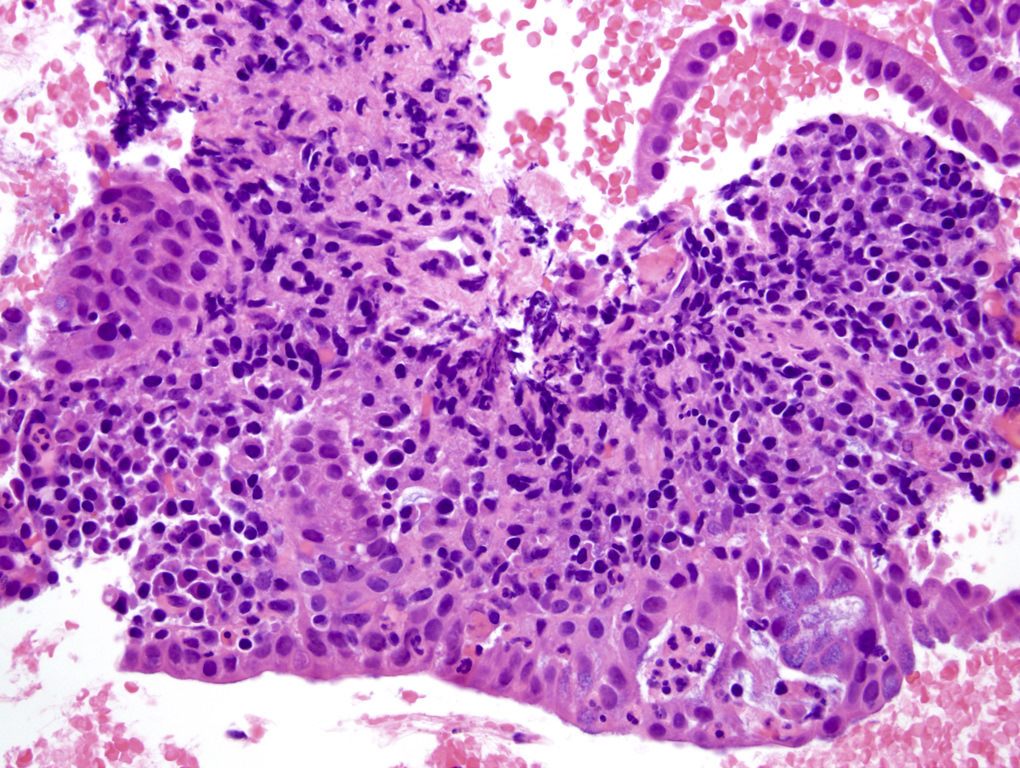
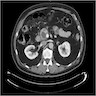
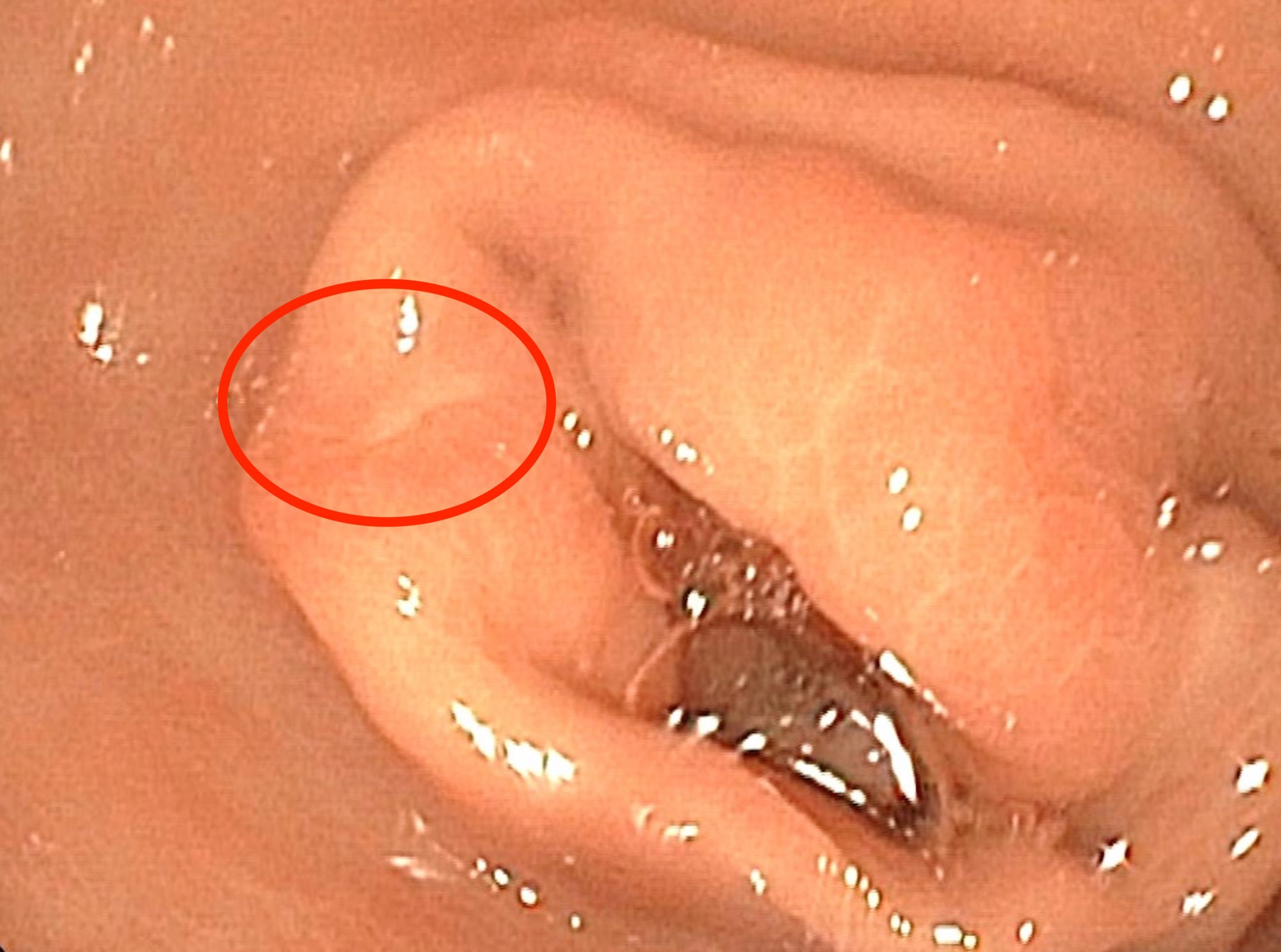
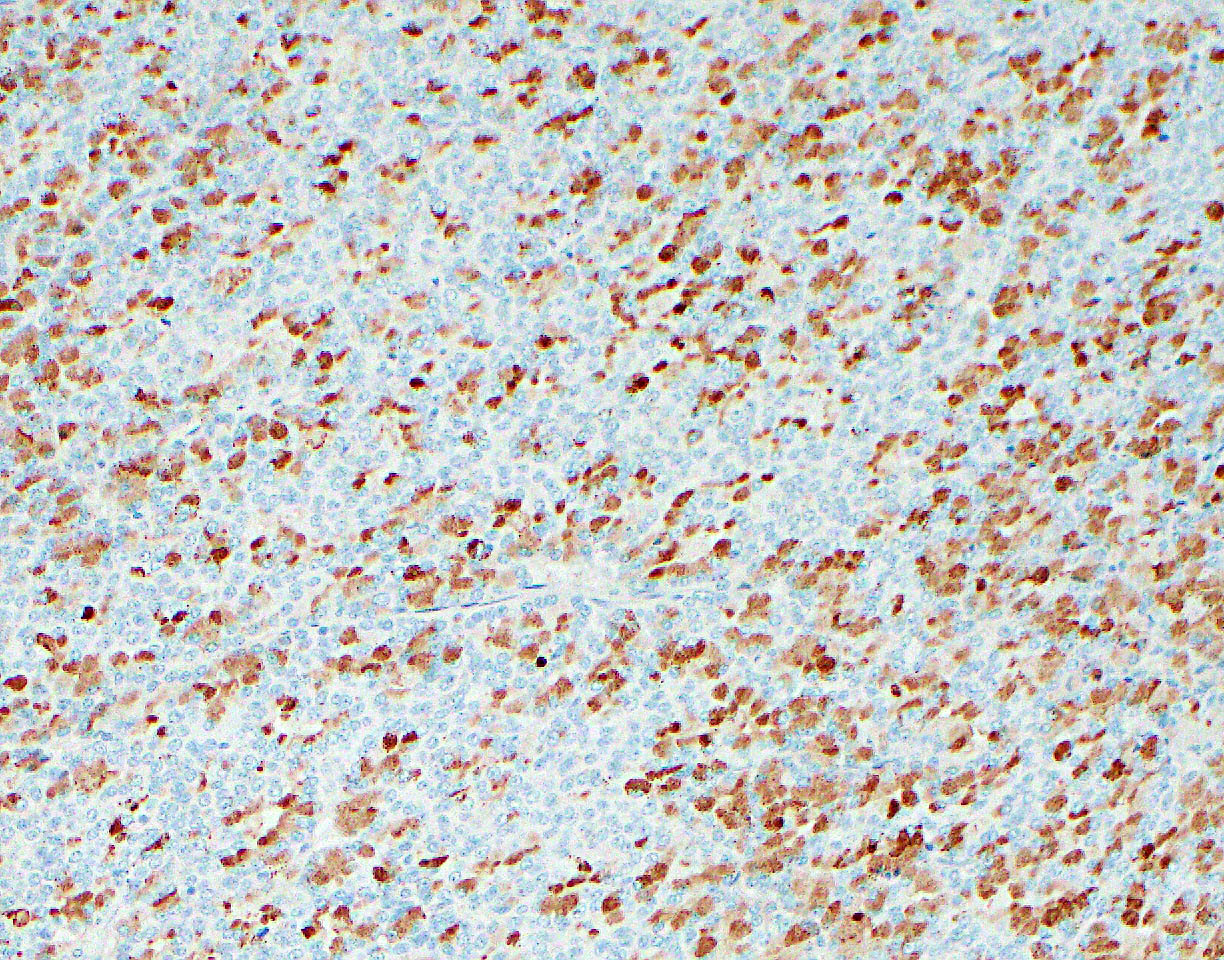
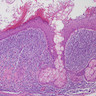
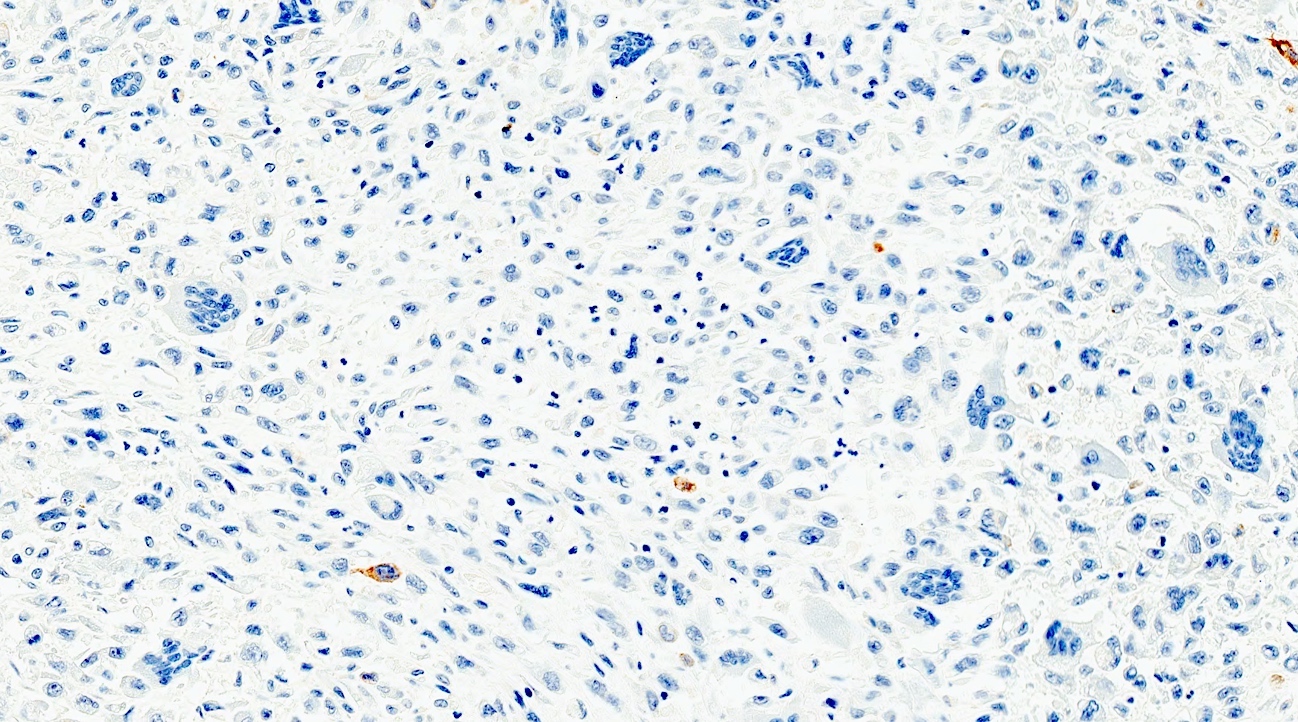
A pancreas transplant recipient underwent an allograft biopsy. A representative image of the biopsy core stained with antibodies to CD3 is shown above. Based on this image alone, what is your diagnosis?
- Acute cell mediated rejection, grade 1
- Acute cell mediated rejection, grade 2
- Acute cell mediated rejection, grade 3
- Indeterminate for rejection
- Normal pancreas
Board review style answer #2
A. Acute cell mediated rejection, grade 1. Most of the septum shown is occupied by an unremarkable artery and the remaining portion shows only scattered T cells. Focal acinar inflammation (≤ 2 foci of T cells with at least 10 cells in each focus), however, is present, defining this as acute cell mediated rejection, grade 1.
Answers D and E are incorrect because the lobule clearly shows several foci of lymphocytic accumulation. Answers B and C are incorrect because ≥ 3 foci of lymphocytic inflammation with at least 10 cells in each focus are not seen.
Comment Here
Reference: Allograft rejection
Comment Here
Reference: Allograft rejection
Board review style question #3
A patient with diabetes mellitus 1 received a pancreas transplant. Maintenance therapy included tacrolimus; FK506 level was higher than expected. Serum amylase and lipase were normal but the blood glucose level became higher. An allograft biopsy demonstrated islet cell swelling, vacuolization, islet cell dropout and formation of empty spaces; no islet centered inflammation was found. What additional pathological changes were most likely seen on this biopsy?
- Acinar cells with enlarged nuclei with viral cytopathic changes
- Ductitis, venulitis and acinar inflammation
- Endarteritis
- Mixed septal and peripheral lobular inflammatory infiltrate containing polymorphonuclear neutrophils
- Weak or absent insulin staining
Board review style answer #3
E. Weak or absent insulin staining. Isolated β cell damage in the absence of islet centered inflammation is characteristic for calcineurin inhibitor toxicity; note also the high FK506 (tacrolimus) level and high blood glucose in this patient.
Answers B and C are incorrect because these are manifestations of acute cell mediated rejection. Selective islet cell damage is not characteristic for acute cell mediated rejection; note also high levels of immunosuppressant tacrolimus and normal pancreatic enzymes. Answer D is incorrect because it points to the histological features of pancreatitis and answer A is incorrect because it points to cytomegalovirus pancreatitis; again, selective islet cell damage is not seen under these conditions.
Comment Here
Reference: Allograft rejection
Comment Here
Reference: Allograft rejection
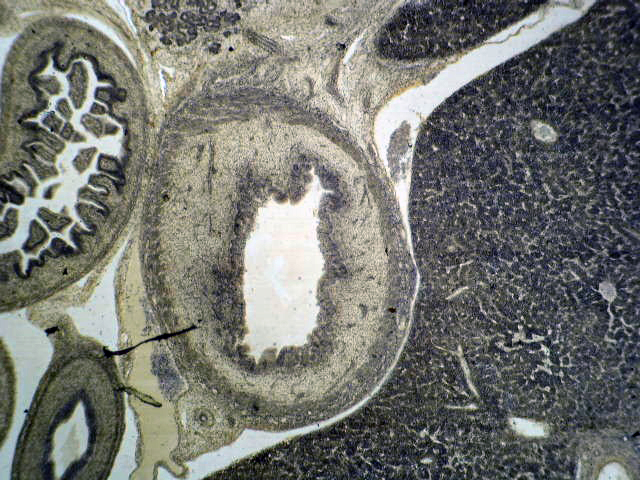
Anatomy & histology
Table of Contents
Definition / general | Essential features | Terminology | Physiology | Anatomy | Embryology | Exocrine pancreas | Endocrine pancreas | Drawings / diagrams | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Electron microscopy images | Board review style question #1 | Board review style answer #1Definition / general
- The pancreas is a mixed exocrine and endocrine gland located in the retroperitoneum behind the stomach, in front of the inferior vena cava and the aorta
Essential features
- Retroperitoneal organ with exocrine and endocrine functions
- Histology: serous ductal / acinar (exocrine cells) gland with tubuloalveolar arrangement with interspersed islets of Langerhans (endocrine cells)
Terminology
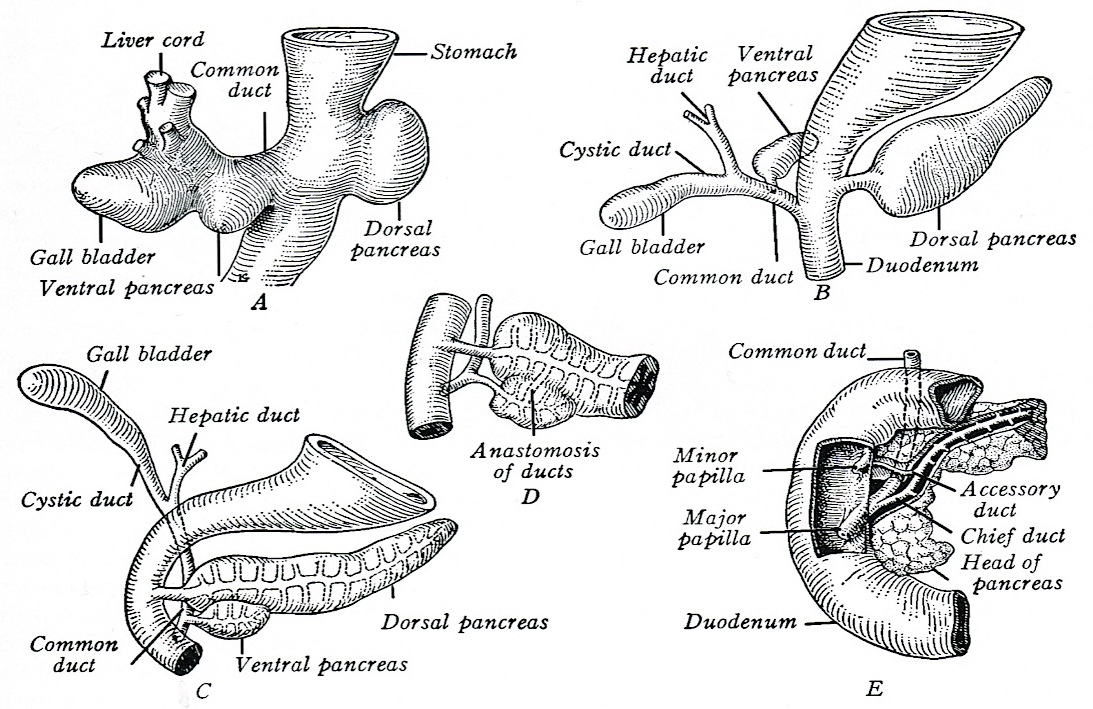
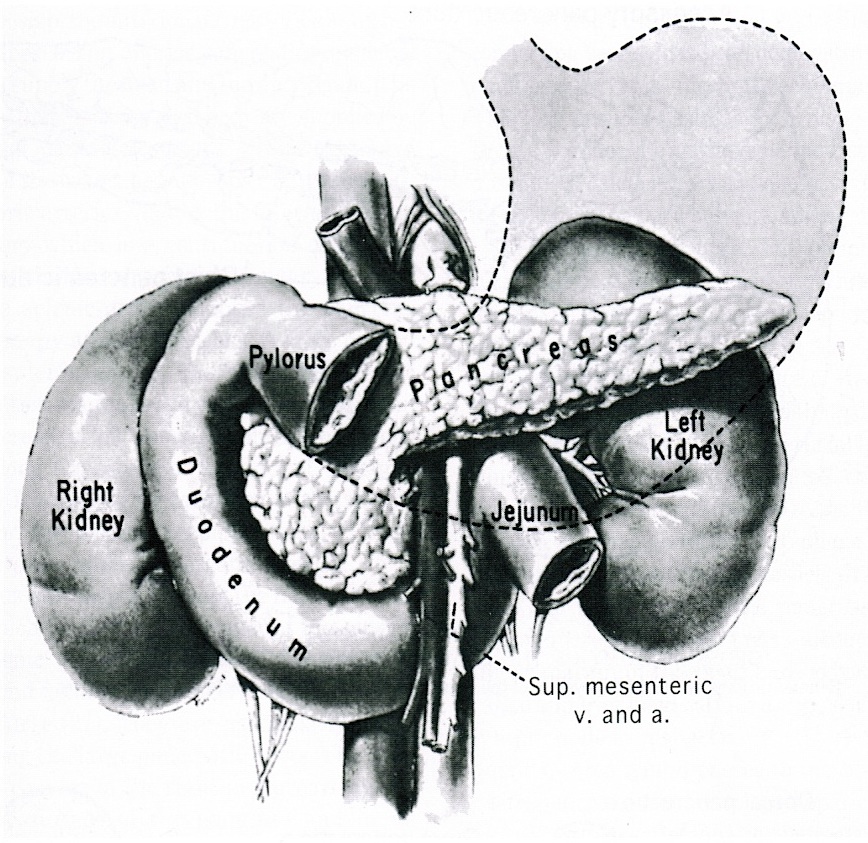
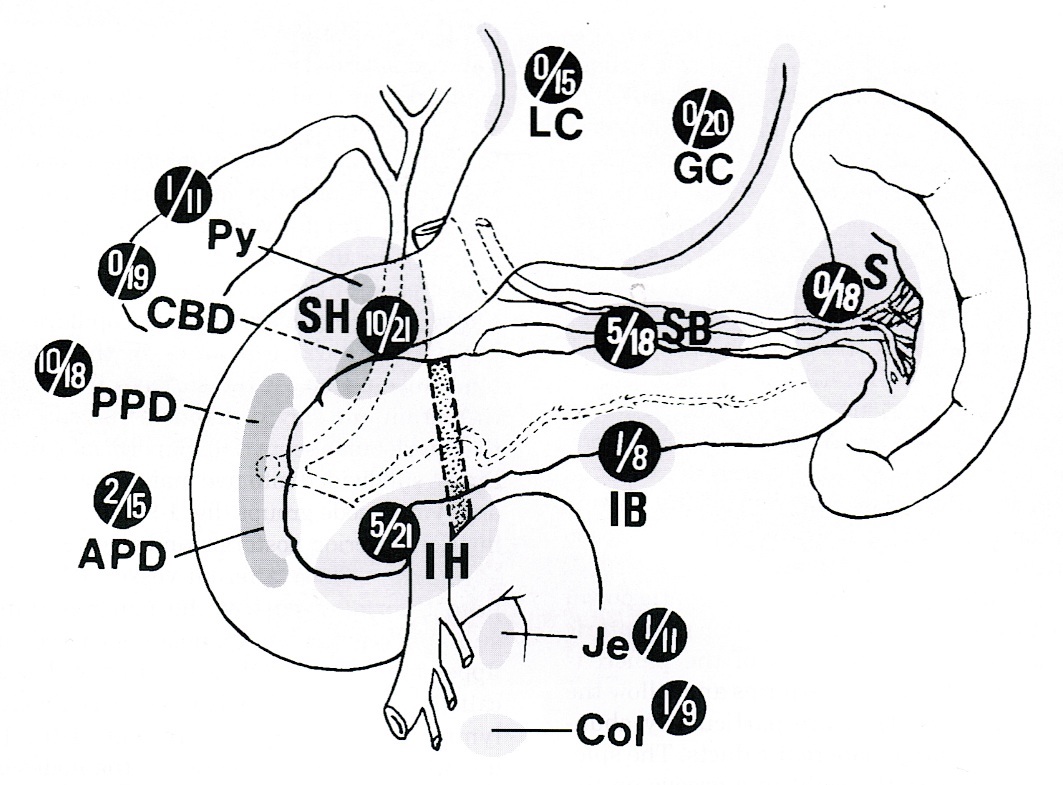
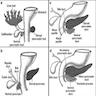
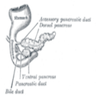
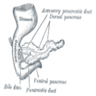
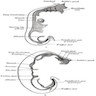
- Anatomically, from right to left, the pancreas is divided into the caput (head), collum (neck), corpus (body) and cauda (tail)
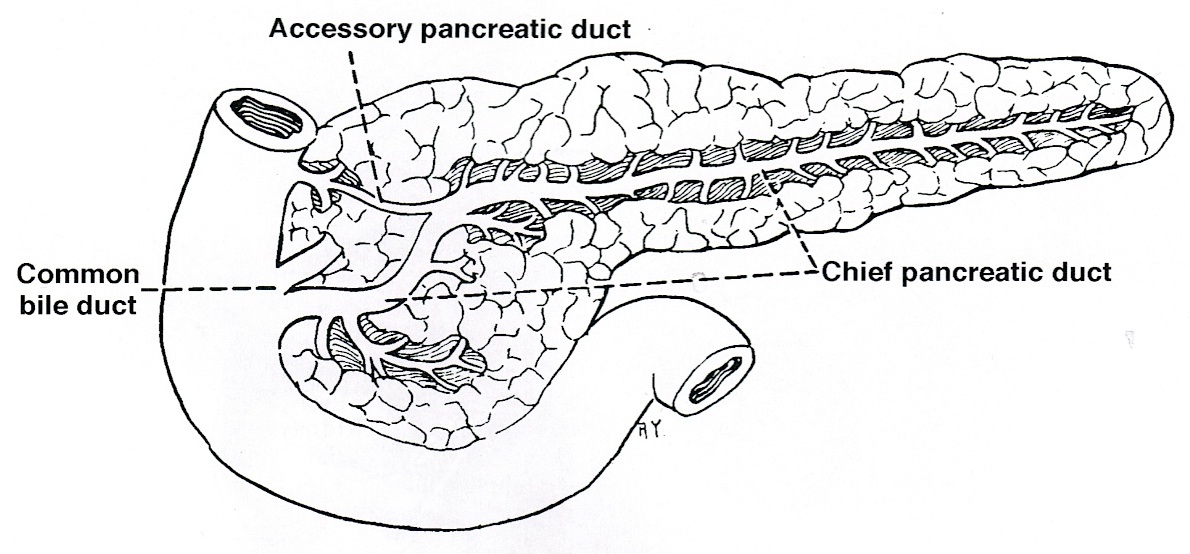
- Pancreatic secretion is drained by the main pancreatic duct (Wirsung duct), with orifice in major duodenal papilla (papilla of Vater), mostly together with the main bile duct
- ~5 - 10% of people have an accessory pancreatic duct (Santorini duct), with orifice in minor duodenal papilla
- Pancreas is embedded in fibrous capsule passing in fibrous septa, separating pancreatic lobuli
- Lobuli consist of multiple acini (the exocrine part) drained by intralobular ducts (leading the pancreatic juice into the small bowel)
- Interlobular ducts originate from the confluence of many intralobular ducts
- Interlobular ducts lead to the main pancreatic duct
Physiology
- Exocrine function (99% of cells): pancreatic juice is produced by acinar cells
- Pancreatic juice contains bicarbonate and proteolytic enzymes (trypsinogen, chymotrypsinogen, carboxypeptidase, elastase), lipolytic enzymes (lipase, esterase, phospholipase), saccharide degrading enzyme (amylase), nucleic acid degrading enzymes (DNAse, RNAse)
- Enzymes are activated in the intestine; enterocyte glycocalyx enzymes (enterokinase) turns trypsinogen to trypsin
- Trypsin catalyzes activation of the other enzymes
- Endocrine function (1% of cells): islets of Langerhans consist of several cell types producing various hormones, which are released into the blood
- A cells (20% of the islets): in the periphery of the islets, glucagon production - increase of blood glucose concentration
- B cells (70% of the islets): in the center of the islets, insulin production - decrease of blood glucose, glucose intake in adipocytes and muscle cells
- D cells (5% of the islets): somatostatin production - inhibition of smooth muscle motility, antagonist of several gastrointestinal hormones
- F cells (1% of the islets): pancreatic polypeptide production - regulation of pancreatic juice and bile release
- D1 cells (less than 1%): vasoactive intestinal polypeptide - glycogenolysis and hyperglycemia, stimulates GI fluid secretion and causes secretory diarrhea
- EC (enterochromaffin) cells (less than 1%): P substance, serotonin - stimulation of gastrointestinal motility
- G cells (less than 1%): gastrin - stimulation of gastric juice release
- PP cells: pancreatic polypeptide - stimulates secretion of gastric and intestinal enzymes and inhibits intestinal motility
- Ghrelin producing cells: ghrelin - regulation of hunger and energetic metabolism
Anatomy
- 15 cm long, 60 - 140 g
- Shape is akin to letter J turned sideways, with loop of J around the duodenum
- Divided into head (right of left border of superior mesenteric vein; contains uncinate process), body (between left border of superior mesenteric vein and left border of aorta) and tail
- Retroperitoneal organ (except for tail), lies within duodenal curve, close to superior mesenteric artery and portal vein
- Anterior body of pancreas touches posterior wall of stomach
- Posterior of pancreas touches aorta, splenic vein and left kidney
- Pancreatic tail extends to the splenic hilum
- Pancreatic head has close anatomic relationship to the common bile duct (choledochus) - distal part (ca. 4 - 5 cm) passes through the pancreatic head into the small intestine
- Orifice of the bile duct is called ampulla of Vater (major papilla), which is equipped with circular smooth muscle (sphincter of Oddi) releasing bile and pancreatic juice into the duodenum
- Pathology of pancreatic head, common bile duct and papilla share clinical symptoms
Embryology
- Forms from embryonic foregut, of endodermal origin (Wikipedia: Pancreas [Accessed 7 December 2017])
- Pancreas forms from ventral and dorsal buds that rotate and fuse
- Ventral bud (anlage) develops from hepatic duct, forms posterior / inferior head and uncinate process
- Dorsal bud (anlage) develops from foregut and extends into dorsal mesentery; forms body, tail, anterior head
- Fusion of ducts at week 7 creates main pancreatic duct (Wirsung), which extends to papilla of Vater, usually with common bile duct
- Proximal portion of dorsal duct persists as accessory duct of Santorini, empties into minor duodenal papilla
- Usually numerous anastomotic connections between ducts of Wirsung and Santorini; if not, result is pancreas divisum (10% of individuals), in which duct of Santorini is major drainage duct
- Percentage of acinar cells decreases after birth
- Anomalies:
- In 67% of adults, pancreatic duct empties into common bile duct, not into ampulla directly
- Abnormal fusion of ventral and dorsal buds causes annular pancreas or heterotopic pancreas
Exocrine pancreas
- Acini comprise 80% of pancreas
- Composed of columnar to pyramidal epithelial cells with minimal stroma
- Basophilic due to prominent rough endoplasmic reticulum
- Pancreas produces 2 liters/day of bicarbonate rich fluid containing digestive enzymes and proenzymes, regulated by neural stimulation (vagus nerve) and humoral factors (secretin, cholecystokinin)
- Cholecystokinin: released from duodenum in response to fatty acids, peptides and amino acids; promotes discharge of digestive enzymes by acinar cells
- Secretin: stimulates water and bicarbonate secretion by duct cells; is stimulated by acid from stomach and luminal fatty acids
- Pancreatic self digestion is prevented by:
- Packaging of most proteins as inactive proenzymes, enzyme sequestration in zymogen granules
- Proenzymes activated only by trypsin, which is activated only by duodenal enterokinase
- Trypsin inhibitors are present in ductal and acinar secretions
- Intrapancreatic release of trypsin activates enzymes, which degrade other digestive enzymes before they can destroy pancreas
- Lysosomal hydrolases can degrade zymogen granules to prevent autodestruction if acinar secretion is impaired
- Acinar cells themselves are highly resistant to trypsin, chymotrypsin and phospholipase A2
- Pancreatic ductal epithelia secrete the bulk of secretagogue induced bicarbonate ions by moving HCO3 from the blood into the pancreatic fluid (Said: Physiology of the Gastrointestinal Tract, 6th Edition, 2018)
- Mucin producing cells are also found among pancreatic ductal epithelia - mucin lubricates, hydrates and protects epithelial cells (Johnson: Encyclopedia of Gastroenterology, 1st Edition, 2004)
Endocrine pancreas
- Consists of islets of Langerhans; represents 1% of pancreas (percentage higher at birth)
- Round, compact, highly vascularized with scant connective tissue
- More irregular outline and trabecular arrangement in posterior head of pancreas, with cells producing pancreatic polypeptide
- Size of islets usually 0.1 - 0.2 mm; endodermal origin; 1 million islets present in pancreas
- Postgastrectomy, may get islet hypertrophy, then beta cell proliferation, then atrophy and amylin deposits (Hum Pathol 2000;31:1368)
- Alpha cells: peripherally dense and round on electron microscopy
- Beta cells: crystalline appearance on electron microscopy, with surrounding halo
- Delta cells: large pale granules on electron microscopy
- PP cells: also scattered in exocrine pancreas; more PP cells in posterior head of pancreas (from ventral bud)
- Nesidioblastosis:
- Islets in intimate association with ducts, with formation of ductuloinsular complexes
- Nesidiodysplasia (Hum Pathol 1988;19:1215):
- Loss of the usual centrilobular concentration of larger islets, with increased small irregularly distributed aggregates of islet cells
- Also increase in beta cell nuclear size and DNA content
- May be associated with endocrine neoplasms
- Peliosis:
- Selective congestion and dilation of vessels of islets only, not seen in vessels elsewhere
Drawings / diagrams
Microscopic (histologic) description
- Vast majority of pancreas volume is composed of exocrine tissue (pancreatic acini)
- Acini show alveolar (round) shape and lobulated architecture
- Acini are packed closely together, drained by series of ductules and ducts
- Acinar cells stain blue by hematoxylin at their base because of high RNA content and presence of nuclei
- Luminal aspect stains pink due to high content of digestive enzymes (zymogen granula, staining with PAS)
- Ductules and ducts are lined by a single layer of cuboidal or cylindrical epithelia
- There is a significant amount of collagen within the wall of large ducts, including main pancreatic duct
- Embedded within the pancreatic exocrine tissue are the islets of Langerhans
- Islets display rich vascularization and consist of endocrine cells
- Endocrine cells have polygonal to round shape, voluminous pale to slightly eosinophilic cytoplasm and monomorphic round nuclei with heterogenous (salt and pepper) chromatin
Microscopic (histologic) images
Contributed by Jan Hrudka, M.D., Ph.D.
Contributed by Grigory Demyashkin, M.D., Ph.D. and AFIP images
Cytology description
- Smear, cytospins or cytoblock from brushings or needle aspirates from ducts; eventually fine needle aspirations (FNAs) from solid tissue
- FNA - predominant cell type is acinic: pyramidal or triangular cells with abundant granular cytoplasm and round eccentric or central nucleus with fine chromatin and often distinct nucleolus
- Ductal cells = 2 dimensional flat sheets (honeycomb), picket fence appearance, tall or cuboidal cells with scant pale cytoplasm and basally located bland appearing nuclei
- Contaminants = mesothelia or hepatocytes in transcutaneous biopsies, duodenal or gastric mucosa in endoscopic aspirations (more often)
Positive stains
- Exocrine cells: cytokeratin 8 / 18, trypsin, BCL10, PAS (zymogen granules)
- Endocrine cells: chromogranin, synaptophysin, neuron specific enolase, neurofilament, CD56
Electron microscopy images
Board review style question #1
Elastase, trypsin and DNAse are placed in the
- Basophilic basal part of acinic cells
- Cytoplasmic vacuoles in the ductal cells
- Eosinophilic cytoplasm of beta cells
- Eosinophilic granula in the acinic cells
Board review style answer #1
D. Eosinophilic granula in the acinic cells. As basic substances, proteins are usually eosinophilic in H&E staining. The eosinophilic granula are called zymogenic in pancreas acinic cells. Answer A is incorrect because the basophilic part of the acinic cell cytoplasm contains ribosomes (rough endoplasmic reticulum). Answer B is incorrect because vacuoles in the ductal cells contain mucin. Answer C is incorrect because beta cells are a major part of Langerhans islets; eosinophilic cytoplasm contains endocrine granula (insulin).
Comment Here
Reference: Pancreas - Anatomy & histology
Comment Here
Reference: Pancreas - Anatomy & histology
Autoimmune pancreatitis type 1
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- 1 of 2 types of autoimmune pancreatitis
- Pancreatic manifestation of IgG4 related disease (IgG4 RD)
Essential features
- Autoimmune pancreatitis is the pancreatic manifestation of IgG4 RD so patients typically have increased IgG4 on serology and other organ involvement by the disease
- Diagnosis can be difficult due to rarity, vague presentation and being a mimic of pancreatic ductal adenocarcinoma
- International Consensus Diagnostic Criteria (ICDC) for autoimmune pancreatitis has set a standard diagnostic approach and criteria for diagnosis, which includes imaging, serology and histology
- Characteristic histologic findings are periductal lymphoplasmacytic infiltrate without granulocytic infiltration, obliterative phlebitis, storiform fibrosis and abundant IgG4 positive plasma cells
Terminology
- Autoimmune pancreatitis (AIP) type 1 or IgG4 related pancreatitis (IgG4 RP)
- Historically termed lymphoplasmacytic sclerosing pancreatitis (LPSP) or AIP without granulocyte epithelial lesions (GELs) (Pancreas 2011;40:352)
Epidemiology
- Patients are generally male and older than 50 years old (Pathologica 2020;112:197, World J Gastroenterol 2022;28:6867)
- Increased prevalence in Asia (Adv Med Sci 2020;65:403)
- Rare; prevalence is < 1% (World J Gastroenterol 2022;28:6867)
- Incidence has been increasing due to better description and recognition of the disease but overall numbers are most likely still underestimated due to the complexity of the disease (World J Gastroenterol 2022;28:6867)
Sites
- Pancreatic manifestation of IgG4 RD
- Extrapancreatic organ sites can be affected, including salivary and lacrimal glands, retroperitoneum, biliary tree, prostate and kidneys (Pathologica 2020;112:197, World J Gastroenterol 2022;28:6867, Adv Med Sci 2020;65:403)
- Other organ involvement is defined as the manifestation of IgG4 related systemic disease and may be diagnosed through imaging, clinical exam or histology (Pancreas 2011;40:352)
Pathophysiology
- Currently unknown
- Still unclear whether the IgG4 antibodies are a reflection of overexpression caused by an unknown inflammatory stimulus or whether they are self reactive autoantibodies
- Has been linked to an increase in interferon type I (IFN I) produced by plasmacytoid dendritic cells but this is nonspecific to autoimmune pancreatitis
- H. pylori infection may be the trigger mechanism for an autoimmune response against pancreatic acini via molecular mimicry but this hypothesis is not supported at this time
- Reference: Adv Med Sci 2020;65:403
Etiology
- Multifactorial, including genetic, environmental and immunological factors
- HLA region genes, HLA DRB1 and ABCF1, are associated with disease susceptibility
- Repeated exposure to certain antigens, including mineral oils, solvents and industrial or metal dusts, may contribute
- Reference: Adv Med Sci 2020;65:403
Diagrams / tables
Clinical features
- Patients most commonly present with obstructive jaundice (Pathologica 2020;112:197, World J Gastroenterol 2022;28:6867)
- Patients can also present with a pancreatic mass, weight loss or abdominal pain / discomfort but do not present with acute pancreatitis symptoms (Adv Med Sci 2020;65:403)
- Diabetes commonly occurs with autoimmune pancreatitis (World J Gastroenterol 2022;28:6867)
- Other manifestations and symptoms of IgG4 RD can be present, including enlargement of the lacrimal and salivary glands as well as retroperitoneal fibrosis and inflammation of the biliary tree (Adv Med Sci 2020;65:403)
Diagnosis
- ICDC for autoimmune pancreatitis was published in 2011, which uses the following combination of categories for diagnosis (Pancreas 2011;40:352)
- Imaging
- Serology
- Other organ involvement (OOI)
- Histology
- Response to steroid therapy
- Can be diagnosed without histology (Pancreas 2011;40:352)
- Other diagnostic criteria can be used, including the HISORt criteria published by Mayo Clinic; however, the ICDC is the most widely used (Adv Med Sci 2020;65:403)
- See Diagrams / tables
Laboratory
- IgG4 > 2 times the upper limit of normal on serology (Pancreas 2011;40:352)
- Serum IgG4 elevation is the single best marker of autoimmune pancreatitis (Pancreas 2011;40:352)
- Elevated IgG4 can also be seen in pancreatic carcinoma patients (10%) and so elevation is not sufficient to make a diagnosis unless seen in the setting of typical imaging or histologic findings (Adv Med Sci 2020;65:403, Pancreas 2011;40:352)
- Positive autoimmune markers are common, including antinuclear antibodies (ANA) (40%), anticarbonic anhydrase II antibodies (55%), antipancreatic secretory trypsin inhibitor (PSTI) antibodies (55%) and antilactoferrin antibodies (75%) (Adv Med Sci 2020;65:403)
- May have elevated CA 19-9, which can raise suspicion of malignancy (Pathologica 2020;112:197)
Radiology description
- Parenchymal imaging
- Typical: diffuse enlargement with delayed enhancement (sometimes associated with rim-like enhancement)
- Indeterminate (including atypical): segmental / focal enlargement with delayed enhancement
- Ductal imaging (endoscopic retrograde cholangiopancreatography [ERCP])
- Level 1: long (> one - third length of the main pancreatic duct) or multiple strictures without marked upstream dilatation
- Level 2: segmental / focal narrowing without marked upstream dilatation (duct size
- Diffuse / non-mass forming autoimmune pancreatitis
- Diffuse pancreas enlargement ("sausage" pancreas), often with low density edge (halo ring sign or capsule sign) on computed tomography (CT) (World J Clin Cases 2022;10:12458)
- Contrast can highlight the capsule and shows mild enhancement during the venous or delayed phase (World J Clin Cases 2022;10:12458)
- Mass forming autoimmune pancreatitis
- Often shows a low density or isodense mass in the head of the pancreas on CT (World J Clin Cases 2022;10:12458)
- Contrast enhanced CT shows slight enhancement of the lesion in the arterial phase and obvious uniformity and delayed enhancement in the venous phase (World J Clin Cases 2022;10:12458)
- Ducts
- Smooth stenosis at the lower end of the common bile duct can be shown in autoimmune pancreatitis patients with obstructive jaundice (World J Clin Cases 2022;10:12458)
- Pancreatic duct is usually irregularly narrow and interrupted and often exceeds one - third of the total length of the main pancreatic duct or appears as a jumping stenosis (World J Clin Cases 2022;10:12458)
- Contrast enhanced CT and magnetic resonance imaging (MRI) show similar results; ultrasound can be variable in diagnostic quality (Adv Med Sci 2020;65:403)
- Other organ involvement can also be seen on imaging and help support the diagnosis of autoimmune pancreatitis
Radiology images
Prognostic factors
- Patients commonly relapse (40%) despite remarkable responses to treatment (Pathologica 2020;112:197, Adv Med Sci 2020;65:403)
Case reports
- 49 year old woman with weight loss, abdominal pain and jaundice (Cureus 2023;15:e47471)
- 54 year old man with weight loss and abdominal pain status post-COVID vaccine (ACG Case Rep J 2023;10:e00950)
- 55 year old man with weight loss, jaundice and pruritus (Cureus 2023;15:e45970)
- 64 year old Japanese woman with new onset bleeding due to acquired hemophilia A with known longstanding disease (Int J Hematol 2018;108:335)
Treatment
- Steroids are the main treatment strategy
- Steroid trial of prednisone 0.6 - 1 mg/kg for 2 weeks with improvement on repeat imaging and serology can be confirmatory in the appropriate context (Pancreas 2011;40:352)
- Steroid trial should not be done in isolation for diagnosis of autoimmune pancreatitis (Pancreas 2011;40:352)
- Immunosuppressants or rituximab can be started if corticosteroids are not effective or cannot be tolerated
- Patients with asymptomatic disease should be treated to prevent the progression of fibrotic changes and decrease the risk of developing pancreatic insufficiency or relapse
- Follow up should consist of a combination of methods (symptoms, serology, imaging) and not IgG4 levels alone
- Endoscopic procedures may be needed in cases of insufficient drug effect or obstructive jaundice
- Surgery should be reserved for cases where malignancy cannot be excluded, extensive fibrosis is present or endoscopic biliary drainage cannot be performed
- Reference: Adv Med Sci 2020;65:403
Gross description
- Disease is localized mainly in the pancreatic head, less frequently in the body / tail
- Diffuse enlargement or "sausage" pancreas may be observed without a discrete mass
- Segmental stenosis of the main duct may be seen
- References: Pathologica 2020;112:197, Abdom Radiol (NY) 2016;41:1003, World J Gastroenterol 2022;28:6867
Gross images
Microscopic (histologic) description
- Characteristic findings (at least 3 for level 1 criteria) (Pancreas 2011;40:352)
- Periductal lymphoplasmacytic infiltrate without granulocytic infiltration
- Obliterative phlebitis
- Storiform fibrosis
- Abundant (> 10 cells/high power field) IgG4 positive plasma cells
- Biopsy showing some but not all of the above features can be used as supportive evidence for the diagnosis of autoimmune pancreatitis (Pancreas 2011;40:352)
- Inflammation is localized within the pancreatic parenchyma and is centered around / within medium to large interlobular ducts, which causes shrinkage of the ductal lumen (Pathologica 2020;112:197)
- Inflammation can also be seen between the pancreatic parenchyma and peripancreatic adipose tissue (Pathologica 2020;112:197)
- Inflammation of the venous wall can progress to obliterative phlebitis with fibrosis of the lumen (Pathologica 2020;112:197)
- As the inflammation progresses, fibrosis becomes more diffuse, assuming a whorled or storiform pattern (Pathologica 2020;112:197)
- Perineural inflammation can also be present (Pathologica 2020;112:197)
- Involvement of the pancreatic neck margin or biliary resection margin should be clearly stated in the pathology report for therapeutic purposes (Pathologica 2020;112:197)
Microscopic (histologic) images
Cytology description
- FNA has variable results with diagnosing autoimmune pancreatitis type 1 but can be used to rule out malignancy in mass forming lesions (Diagnostics (Basel) 2021;11:1653)
- Lymphoplasmacytic infiltration, storiform fibrosis and obliterative phlebitis can be seen alone or in combination (Diagnostics (Basel) 2021;11:1653, World J Gastroenterol 2012;18:3883)
Positive stains
- IgG and IgG4
- > 10 IgG4 positive cells per high power field in biopsy specimens (Pancreas 2011;40:352)
- > 50 IgG4 positive cells per high power field in resection specimens (Virchows Arch 2018;472:545)
- IgG4/IgG ratio > 40% (Virchows Arch 2018;472:545)
- Elastic: highlights remnant veins in longstanding autoimmune pancreatitis
- Masson trichrome: highlights storiform fibrosis
- Normal / intact expression of DPC4 in entrapped ducts
Sample pathology report
- Pancreas, mass, core needle biopsy:
- Chronic pancreatitis with storiform fibrosis and increased IgG4 positive plasma cells (see comment)
- Negative for malignancy
- Comment: H&E sections demonstrate pancreatic parenchyma with dense storiform fibrosis and numerous chronic inflammatory cells including abundant plasma cells. A partially obliterated vein is seen. Immunohistochemical stain for IgG4 shows increased IgG4 positive plasma cells (up to 20 - 25 per high power field). The combined findings are most consistent with autoimmune pancreatitis type 1.
Differential diagnosis
- Pancreatic ductal adenocarcinoma:
- Can have similar presentation (vague abdominal symptoms, jaundice, pancreatic mass) with similar radiographic findings and serology (increased CA 19-9 with or without increased IgG4)
- Infiltrating well to poorly formed glandular / ductal structures surrounded by remarkably desmoplastic stroma
- Perineural or lymphovascular invasion
- Molecular alterations not present in AIP type 1 (World J Gastroenterol 2023;29:2241)
- Autoimmune pancreatitis type 2:
- Younger patients with no sex predilection
- Associated with chronic inflammatory bowel disease
- Serum IgG4 antibody levels are within normal ranges
- Requires histological specimen to make definitive diagnosis (Pancreas 2011;40:352)
- Duct centric granulocytic abscesses and lobular (but not storiform) fibrosis
- Follicular pancreatitis:
- Inflammation with numerous lymphoid follicles with or without prominent germinal centers
- Lacks storiform fibrosis, obliterative phlebitis and IgG4+ cells
- Obstructive chronic pancreatitis:
- Groups of acini and tubules scattered in fibrous tissue
Board review style question #1
Which of the following clinical and histologic features would support a diagnosis of autoimmune pancreatitis (AIP) type 1 over AIP type 2?
- 25 year old patient
- Abundant IgG4 positive plasma cells on histologic section
- Female patient
- Lobular fibrosis
Board review style answer #1
B. Abundant IgG4 positive plasma cells on histologic section is the most supportive of a diagnosis of AIP type 1. AIP type 1 is the pancreatic manifestation of IgG4 related disease (IgG4 RD) and so patients will typically have elevated IgG4 levels on serology as well as demonstrate abundant IgG4 positive plasma cells on histologic section. Answers A and C are incorrect because AIP type 1 patients are more commonly over 50 years old and male. Answer D is incorrect because AIP type 1 also shows characteristic storiform fibrosis, not lobular fibrosis, which can be seen in AIP type 2.
Comment Here
Reference: Autoimmune pancreatitis type 1
Comment Here
Reference: Autoimmune pancreatitis type 1
Board review style question #2
Board review style answer #2
C. Elevated IgG4 on serology. The histologic section shows the characteristic findings of autoimmune pancreatitis (AIP) type 1 (storiform fibrosis and a lymphoplasmacytic infiltrate). Most AIP type 1 patients have elevated IgG4 on serology. Answer A is incorrect because patients do not present with symptoms of acute pancreatitis (acute abdominal pain with elevated lipase on serology). Answer B is incorrect because AIP type 1 patients can present with elevated CA 19-9 on serology but this is not as common as elevated IgG4. Answer D is incorrect because inflammatory bowel disease is associated with AIP type 2, not type 1. Answer E is incorrect because a pancreas mass can be seen in AIP type 1 but this is variable.
Comment Here
Reference: Autoimmune pancreatitis type 1
Comment Here
Reference: Autoimmune pancreatitis type 1
Autoimmune pancreatitis type 2
Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnostic criteria | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- 1 of 2 types of autoimmune pancreatitis, characterized by clinical evidence of jaundice, with or without formation of a pancreatic mass on radiographic imaging, with excellent clinical response to steroid treatment
- Unlike type 1 autoimmune pancreatitis, type 2 is IgG4 negative and does not show extra pancreatic organ involvement and is not associated with increased IgG4 in serum or IgG4 positive plasma cells
- Diagnosis requires histologic confirmation of granulocytic epithelial lesions (Pancreas 2011;40(3):352, Am J Surg Pathol 2003;27:1119)
Essential features
- Characterized by clinically nonspecific signs and symptoms, radiographic evidence of pancreas enlargement, histologic evidence of destructive duct centric inflammation with granulocytic epithelial lesions, with an excellent response to treatment with steroids
Terminology
- Autoimmune pancreatitis type 2, idiopathic duct centric pancreatitis, autoimmune pancreatitis with granulocyte epithelial lesions, nonalcoholic duct destructive pancreatitis
Epidemiology
- Incidence largely unknown; requirement of tissue for definitive histologic diagnosis limits estimates of true incidence; thought to be less common than type 1 autoimmune pancreatitis (< 1 per 100,000) (Int J Rheumatol 2012;2012:358371)
- Age: mean 40 - 44 years (Gut 2013;62:1771, Pancreas 2011;40:809)
- Sex: M = F
Sites
- Pancreas
- No significant extra pancreatic involvement
Pathophysiology
- Largely unknown
Etiology
- Largely unknown
Clinical features
- Numerous, nonspecific signs and symptoms
- Jaundice, weight loss, abdominal pain, diffuse enlargement or mass forming lesion on imaging
- No biochemical evidence of pancreatitis, absent serum IgG4, no extra pancreatic organ involvement (Pancreas 2011;40:809)
- An association with inflammatory bowel disease, especially ulcerative colitis, is observed in up to 15% of cases (Gastroenterology 2015;149:39)
Diagnostic criteria
- Histologic examination of tissue is critical for diagnosis per International Consensus Diagnostic Criteria 2011 (Pancreas 2011;40(3):352)
- Categorization as "definitive" or "probable" based on histologic criteria:
- Level 1
- Granulocytic infiltration of duct wall [aka granulocytic epithelial lesion(s)] with or without granulocytic acinar inflammation
- Absent or scant IgG4 positive cells (0 - 10 cells/high powered fields)
- Level 2
- Granulocytic and lymphoplasmacytic acinar infiltrate
- Absent or scant IgG4 positive cells (0 - 10 cells/high powered fields)
- Level 1
- Definitive type 2 autoimmune pancreatitis:
- Level 1 histologic findings with supportive findings on imaging or level 2 histologic findings, supportive findings on imaging, clinical inflammatory bowel disease and response to steroid treatment
- Probable type 2 autoimmune pancreatitis:
- Level 2 histologic findings, supportive findings on imaging, variable inflammatory bowel disease and response to steroid treatment
Laboratory
- No serum biomarkers have been elucidated (Pancreas 2011;40:809)
- No biochemical evidence of pancreatitis (normal serum amylase, lipase)
Radiology description
- No specific radiologic features to distinguish type 2 from type 1
- Contrast enhanced ultrasound (Endosc Ultrasound 2018;7:196):
- Noninvasive imaging study; dynamic contrast enhancement improved discrimination between autoimmune pancreatitis and pancreatic ductal adenocarcinoma
- Shows iso or hyperenhancement during the arterial and phases
- CT (AJR Am J Roentgenol 2015;205:2):
- Important imaging modality for differentiation from pancreatic cancer
- Diffuse parenchymal involvement, sausage shaped
- Delayed enhancement studies
- Rim-like enhancement
- Magnetic resonance (Pancreas 2018;47:1115, Radiology 2011;260:428)
- T1 weighted: hypointense
- T2: predominantly hyperintense
- Dynamic study shows the effect of fibrosis widening the extracellular and extravascular spaces, resulting in hypointensity during pancreatic phase and contrast retention / hyperintensity during the delayed phase
- Magnetic resonance cholangiopancreatography (Pancreas 2018;47:1115)
- Stenosis of the main pancreatic duct, however with less upstream dilatation than seen in type 1 autoimmune pancreatitis
- Rare / absent common bile duct stenosis and rare / absent stenosis of intrahepatic bile ducts, consistent with no extra pancreatic organ involvement in type 2 autoimmune pancreatitis compared to type 1 autoimmune pancreatitis
Prognostic factors
- Excellent prognosis (up to 99% remission) with steroid treatment
- Low risk of recurrence (10 - 15%) (Gut 2013;62:1771)
Case reports
- 9 year old girl with intrahepatic and extrahepatic biliary ductal dilatation (Clin J Gastroenterol 2015;8:421)
- 20 year old man with Crohn's disease (Intern Med. 2018 Oct 15;57(20):2957)
- 64 year old man with inflammatory bowel disease and dilated common bile duct (J Investig Med High Impact Case Rep 2017;5:2324709617734245)
Treatment
- Steroids, up to 99% response (Gastrointest Endosc Clin N Am 2018;28:493)
- Immune modulation (rituximab, methotrexate)
- Surgical resection of pancreas in rare cases unresponsive to medical management
Microscopic (histologic) description
- Confined to pancreas
- Key histologic features:
- Lymphoplasmacytic inflammation, predominantly periductal, although acini are also involved
- Epithelium of medium and small sized duct walls infiltrated by neutrophils [(granulocytic
epithelial lesion(s)] with or without granulocytic acinar inflammation
- Often causes destruction of the pancreatic duct epithelium
- Lobular fibrosis (but not storiform) and obliterative phlebitis, however less prominent than in type 1 autoimmune pancreatitis
- Absent or scant IgG4 positive cells (plasma cells) (0 - 10 cells / high powered fields) (Gastroenterology 2015;149:39)
Microscopic (histologic) images
Positive stains
- IHC not necessary for diagnosis
- One study has suggested a role for evaluating IL8 expression in ductal epithelium (85% versus 0% in type 1 autoimmune pancreatitis) and infiltrating neutrophils (100% versus 0% in type 1 autoimmune pancreatitis) (Am J Surg Pathol 2017;41:1129)
Negative stains
- Scant or absent IgG4
Differential diagnosis
- Pancreatic ductal adenocarcinoma
- Autoimmune pancreatitis type 1
- Associated with an older age group
- Serum IgG4 levels may be elevated
- Histology shows dense lymphoplasmacytic infiltrate, storiform fibrosis and obliterative phlebitis
- IgG4 positive plasma cells may be present, considered IgG4 related if IgG4/IgG ratio > 40% (Virchows Arch 2018;472:545)
- Alcoholic / gallstone pancreatitis
- Acute pancreatitis that occurs in any age group
- Elevated amylase and lipase
- Histology is not needed for diagnosis, shows acute interstitial inflammation with spotty peripancreatic fat necrosis (mild cases) or large areas of saponification, hemorrhage and spotty widened viable acinar lumens (severe cases)
- Chronic pancreatitis
- Progressive inflammation and fibrosis of the pancreas, resulting in waxing and waning nausea, weight loss, fat malabsorption (steatorrhea) and diabetes
- May have elevated serum CA 19-9
- Histology reveals atrophy of acini and ducts with preservation of pancreatic lobular architecture, variable amount of fibrosis centered around ducts and between lobules and mild chronic inflammatory cell infiltrate (Mod Pathol 2007;20:S113)
Additional references
Board review style question #1
A 40 year old man presented with jaundice and abdominal imaging showed a pancreatic mass. Serum IgG4 levels were not elevated. Endoscopic ultrasound guided fine needle biopsy was performed and a representative photomicrograph is shown. Which of the following statements is true?
- Although serum IgG4 levels are not elevated, will have IgG4 positive cells
- Despite steroid therapy, the patient's symptoms will persist and this will support the most likely diagnosis
- Histopathologic findings in the photomicrograph are required for definitive diagnosis
- Most likely diagnosis is associated with systemic inflammation, with frequent involvement of extra pancreatic organs
Board review style answer #1
C.
According to the International Consensus Diagnostic Criteria established in 2011, the definitive diagnosis autoimmune pancreatitis type 2 requires tissue biopsy of the pancreas demonstrating granulocytic infiltration of the pancreatic duct wall [(granulocytic epithelial lesion(s)].
Answer choice A: IgG4 positive cells are absent in autoimmune pancreatitis type 2. This finding is more characteristic of autoimmune pancreatitis type 1.
Answer choice B: Both autoimmune pancreatitis types 1 and 2 respond to the steroid therapy.
Answer choice D: Autoimmune pancreatitis type 2 is not associated with extra pancreatic organ manifestations. This finding is more characteristic of autoimmune pancreatitis type 1.
Comment Here
Reference: Autoimmune pancreatitis type 2
Answer choice A: IgG4 positive cells are absent in autoimmune pancreatitis type 2. This finding is more characteristic of autoimmune pancreatitis type 1.
Answer choice B: Both autoimmune pancreatitis types 1 and 2 respond to the steroid therapy.
Answer choice D: Autoimmune pancreatitis type 2 is not associated with extra pancreatic organ manifestations. This finding is more characteristic of autoimmune pancreatitis type 1.
Comment Here
Reference: Autoimmune pancreatitis type 2
Board review style question #2
A 45 year old woman presented with abdominal pain and jaundice. Abdominal imaging showed a
diffusely enlarged sausage shaped pancreas and stenosis of the main pancreatic duct. Serum
amylase, lipase and IgG4 levels were not elevated. During the workup, endoscopic ultrasound guided fine needle biopsy was performed
and histologic sections showed pancreatic duct centric inflammation including ductal epithelium with
intra-epithelial neutrophils. Upon further gathering of past medical history, the patient was most likely
found to have which of the following?
- Ankylosing spondylitis
- Inflammatory bowel disease
- Salivary gland enlargement and thyroiditis
- Systemic lupus erythematosus
Board review style answer #2
B.
There is a known association between autoimmune pancreatitis type 2 and inflammatory bowel disease, especially ulcerative colitis, occurring in 15 - 20% of biopsy proven autoimmune pancreatitis type 2. The basis of this association is unclear.
Answer choice A: Ankylosing spondylitis is an inflammatory disease that is associated with HLA-B27 and is not known to be associated with autoimmune pancreatitis type 2.
Answer choice C: Autoimmune pancreatitis type 1 is an IgG4 related disease. Patients often present with extra pancreatic manifestations of systemic IgG4 related inflammation including enlarged salivary glands and Riedel thyroiditis or the sclerosing variant of Hashimoto thyroiditis.
Answer choice D: Systemic lupus erythematosus is not known to be associated with autoimmune pancreatitis type 2.
Comment Here
Reference: Autoimmune pancreatitis type 2
Answer choice A: Ankylosing spondylitis is an inflammatory disease that is associated with HLA-B27 and is not known to be associated with autoimmune pancreatitis type 2.
Answer choice C: Autoimmune pancreatitis type 1 is an IgG4 related disease. Patients often present with extra pancreatic manifestations of systemic IgG4 related inflammation including enlarged salivary glands and Riedel thyroiditis or the sclerosing variant of Hashimoto thyroiditis.
Answer choice D: Systemic lupus erythematosus is not known to be associated with autoimmune pancreatitis type 2.
Comment Here
Reference: Autoimmune pancreatitis type 2
Chronic pancreatitis
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology / etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Case reports | Treatment | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Pancreatic fibroinflammatory syndrome histologically characterized by parenchymal fibrosis, atrophy and duct changes and clinically by pain and symptoms of pancreatic insufficiency (Pancreatology 2020;20:586)
Essential features
- Pancreatic fibroinflammatory disease with fibrosis, loss of acinar tissue and duct changes
- Histology alone is not sufficient; clinical and radiological data are required for diagnosis (Pancreatology 2020;20:586)
Terminology
- Main category: chronic pancreatitis
- Subtypes: alcoholic pancreatitis, obstructive pancreatitis, hereditary pancreatitis, paraduodenal (groove) pancreatitis (PGP)
ICD coding
Epidemiology
- Alcoholic pancreatitis: young to middle aged males (Pathologica 2020;112:197)
- Obstructive pancreatitis: M = F; wide age range (Pathologica 2020;112:197)
- Hereditary pancreatitis: M = F; PRSS1 and CFTR related: very young patients (SPINK1 associated: adult patients (Nat Genet 1996;14:141, Gastroenterology 1981;81:1143, Nat Genet 2000;25:213)
- Paraduodenal pancreatitis: middle aged males with a history of alcohol abuse and smoking (World J Surg 2009;33:2664)
Sites
- Alcoholic and hereditary chronic pancreatitis affects the whole pancreas
- Obstructive pancreatitis affects the gland distal to the site of obstruction
- Paraduodenal pancreatitis is centered around the minor papilla, in the so called groove area between the common bile duct, the superior margin of the pancreatic head and the duodenal wall (Pathologica 2020;112:197)
Pathophysiology / etiology
- Alcoholic pancreatitis: prolonged alcohol abuse and other cofactors such as smoking (Alcohol Health Res World 1997;21:13)
- Obstructive pancreatitis: obstruction of main or secondary pancreatic ducts due to mass forming lesions or intraductal stones
- Hereditary pancreatitis: germline mutations in the genes serine protease 1 (PRSS1) or serine peptidase inhibitor kazal type 1 (SPINK1); autosomal recessive mutations in cystic fibrosis transmembrane conductance regulator (CFTR)
- Paraduodenal pancreatitis: chronic obstruction of the minor papilla (Pathologica 2020;112:197)
Clinical features
- Severe abdominal pain, often radiated to the interscapular region
- Signs and symptoms of exocrine and endocrine pancreatic dysfunction: steatorrhea, weight loss, bloating, diabetes (J Clin Gastroenterol 2022;56:e1)
- Paraduodenal pancreatitis may be associated with signs and symptoms of duodenal stenosis such as postprandial vomit
- Risk of developing pancreatic ductal adenocarcinoma is increased tenfold in patients with chronic pancreatitis
Diagnosis
- Histology alone is not gold standard; clinical and radiological data are required for a definitive diagnosis of chronic pancreatitis (Pancreatology 2020;20:586)
- Typical clinical picture
- History of alcohol abuse or smoking
- Pancreatic calcifications on CT scans
- Morphological triad of fibrosis, loss of acinar tissue and duct changes
Laboratory
- Lab tests alone are of limited utility in the diagnosis of chronic pancreatitis
- Low plasma amylase levels, particularly in advanced cases (Alcohol Health Res World 1997;21:13)
Radiology description
- Ultrasound: diffuse hyperechogenicity
- CT: parenchymal atrophy, duct dilation, presence of calcification and pseudocysts
- Paraduodenal pancreatitis may mimic cystic lesions or malignancy and may be associated with the so called double duct sign, due to dilation of the main pancreatic duct and the common bile duct (Alcohol Health Res World 1997;21:13)
Case reports
- 34 year old man with a mass forming chronic pancreatitis mimicking a cystic neoplasm (World J Gastroenterol 2018;24:297)
- 39 year old woman with hereditary pancreatitis who developed a pancreatic anaplastic carcinoma (Am J Case Rep 2021;22:e928993)
- 51 year old woman with paraduodenal pancreatitis mimicking undifferentiated carcinoma with osteoclastic giant cells (UCOCGC) (Cytopathology 2015;26:122)
- 57 year old man with chronic pancreatitis with ductal stones in the pancreatic head, successfully treated by surgery (J Surg Case Rep 2020;2020:rjaa352)
Treatment
- Therapeutic strategies are mostly supportive
- Paraduodenal pancreatitis may require pancreaticoduodenectomy
- Mass forming / symptomatic cases may be amenable to surgical resection
- Reference: Dis Mon 2021;67:101225
Gross description
- Whitish parenchyma with extensive fibrosis, dilated ducts and calculi
- Fibrotic parenchyma retains a lobulated appearance
- In advanced stages, a reduction in the volume of the pancreatic gland may occur
- Parenchymal and intraductal calcification are typical and often grossly appreciable
- Pseudocysts are more common in alcoholic pancreatitis, whereas obstructive pancreatitis shows retention cysts (Pathologica 2020;112:197)
- Paraduodenal pancreatitis may show cystic change near the minor ampulla and trabeculated fibrosis centered around the duodenal wall (Semin Diagn Pathol 2004;21:247)
Gross images
Frozen section description
- Frozen sections may show extensive fibrosis of the parenchyma and architectural distortion of pancreatic ducts
- To identify invasive carcinoma on frozen sections with chronic pancreatitis, look for ruptured glands, high grade dysplasia, mitoses, perineural invasion, naked gland in peripancreatic adipose tissue
- References: Arch Pathol Lab Med 2005;129:1610, Arch Pathol Lab Med 2022;146:84
Frozen section images
Microscopic (histologic) description
- Triad of cardinal features: fibrosis, loss of acinar tissue, duct changes (Pancreatology 2020;20:586)
- No universally accepted and reproducible grading system (Pancreatology 2020;20:586)
- No unique morphologic features to distinguish different etiologies of nonautoimmune chronic pancreatitis
- Fibrosis is initially perilobular; during disease progression, it involves the pancreatic lobular units, eventually replacing the acinar parenchyma (so called intralobular fibrosis)
- Acinar tissue may be replaced by fibrosis or fatty tissue; the latter process (lipomatous atrophy) is more frequently seen in hereditary pancreatitis (Pathologica 2020;112:197)
- Loss of acinar tissue predates that of pancreatic islets, which are often seen isolated in fibrosis or fatty tissue in advanced cases
- Ductal changes include distortion of ductal profiles, ectasia, presence of intraluminal concretions of amorphous material (so called protein plugs), squamous metaplasia, intraductal calcification
- Foci of low and high grade pancreatic intraepithelial neoplasia (PanIN) may be encountered
- Foci of periductal chronic inflammation and fat necrosis represent a common finding
- Pseudocysts lined by granulation tissue are common in alcoholic chronic pancreatitis
- Paraduodenal pancreatitis shows Brunner gland hyperplasia, myofibroblastic proliferation in the duodenal wall, cysts lined by cuboidal ductal epithelium, which may be replaced by granulation tissue; multinucleated giant cells may be found (Cytopathology 2015;26:122)
Microscopic (histologic) images
Cytology description
- Clusters of acinar cells with mild atypia and no atypical mitotic figures
- Chronic inflammatory elements: lymphocytes, macrophages
- Clusters of activated fibroblasts may be found
- Proteinaceous material may be present
- Neutrophils may be present in case of associated acute inflammation
- Multinucleated giant cells may be present in paraduodenal pancreatitis (Cytopathology 2015;26:122)
Cytology images
Positive stains
- Ductal epithelium is positive for keratins CK7 and CK19
- Nuclear expression of SMAD4 is retained
- Reference: World J Gastrointest Surg 2021;13:406
Negative stains
- Staining for p53 does not show a mutated pattern of expression
Molecular / cytogenetics description
- Most common mutations in PRSS1 related hereditary pancreatitis are R122H, R122C, N29I and A16V (Am J Physiol Gastrointest Liver Physiol 2014;306:G466)
- SPINK1 related pancreatitis is associated with the presence of high risk SPINK1 N34S haplotype (PLoS One 2008;3:e2003)
Sample pathology report
- Duodenum and pancreatic head, pancreaticoduodenectomy:
- Pancreatic head with diffuse fibrous replacement of acinar tissue, foci of chronic inflammation, pseudocysts lined by granulation tissue, duct ectasia with protein plugs and squamous metaplasia of the lining epithelium (see comment)
- No evidence of infiltrating carcinoma
- 12 lymph nodes and duodenal margins with no significant pathologic change
- Comment: Histologic picture is consistent with chronic pancreatitis.
Differential diagnosis
- Pancreatic ductal adenocarcinoma:
- Ruptured glands, gland in peripancreatic adipose tissue without pancreatic islets, mitotic figures, perineural and vascular invasions are useful tips in the differential diagnosis
- Immunohistochemistry for p53 and SMAD4 may be of aid in the differential diagnosis between chronic pancreatitis and foci of ductal adenocarcinoma
- Autoimmune pancreatitis type 1:
- Chronic inflammation centered around ducts and venules
- Presence of ≥ 10 IgG4 positive plasma cells/high power field (HPF), although a widely accepted and standardized threshold is still lacking
- Integration of clinical, laboratory and radiological data is required for differential diagnosis
- Autoimmune pancreatitis type 2:
- Granulocytic epithelial lesions (GEL) with formation of neutrophil microabscesses within the duct lumen
- Integration of clinical, laboratory and radiological data is required for differential diagnosis
Additional references
Board review style question #1
What are the cardinal features of chronic pancreatitis?
- Fat necrosis, chronic inflammation and loss of Langerhans islets
- Fibrosis, loss of acinar tissue and duct changes
- Giant cell granulomas, perineural inflammation and plasma cell infiltrate
- Intraductal proliferative lesions, lipomatous atrophy and perivenulitis
- Necrosis, granulocytic epithelial lesions and pseudocysts
Board review style answer #1
Board review style question #2
Is there a reproducible grading system for chronic pancreatitis?
- No, there is no universally accepted grading system
- Yes, grading is based on the degree of acinar tissue loss
- Yes, grading is based on the entity of fibrosis
- Yes, grading is based on the presence of pseudocysts
- Yes, grading is based on the severity of chronic inflammation
Board review style answer #2
A. No, there is no universally accepted grading system. However, classification as mild, moderate or severe may be applied.
Comment Here
Reference: Chronic pancreatitis
Comment Here
Reference: Chronic pancreatitis
Clear cell pancreatic endocrine tumor
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Uncommon morphologic variant of pancreatic well differentiated neuroendocrine tumor; associated with von Hippel-Lindau syndrome (VHL) and multiple endocrine neoplasia type I (MEN I) syndrome
Essential features
- Clear, vacuolated cytoplasm due to accumulation of lipid, glycogen or mucin
- Characteristic salt and pepper nuclear chromatin features
- Can be easily misdiagnosed due to morphologic similarities with primary and metastatic tumors of the pancreas
Terminology
- Also known as lipid rich variant
ICD coding
- ICD-10: C25.4 - malignant neoplasm of endocrine pancreas
Epidemiology
- Adults ages 40 - 60
- No sex predilection
- Syndromic patients may present at an earlier age (Pathol Res Pract 2016;212:747)
Sites
- Most commonly in the head of the pancreas
Pathophysiology
- In VHL, the disease results from deletions or mutations in the VHL tumor suppressor gene on chromosome 3p25.5 (Mod Pathol 2011;24:S66)
- Mutations in MEN1 on chromosome 11q result in loss of function of tumor suppressor protein menin (Mod Pathol 2011;24:S66)
- Sporadic tumors consistently result from allelic loss of chromosome 11q
Clinical features
- Patients with VHL or MEN I may be screened following clinical suspicion
- Patients may present for evaluation of abdominal symptoms or abnormal laboratory results
- Some cases may be found incidentally on imaging (Mod Pathol 2011;24:S66)
- Pancreatic neuroendocrine tumors in VHL or MEN I are typically nonfunctional (Arch Pathol Lab Med 2006;130:963)
Diagnosis
- Imaging may identify a suspicious mass
- Definitive diagnosis is made following endoscopic biopsy or surgical resection
Radiology description
- Hyperintense, enhancing mass, well circumscribed with sharp borders on computed tomography (Radiographics 2010;30:1445)
Prognostic factors
- Worse prognosis with high Ki67 proliferative index, tumor size > 2 cm, nonfunctioning tumors, tumor necrosis and poor response to chemotherapy (J Hepatobiliary Pancreat Sci 2015;22:586, Mod Pathol 2011;24:S66)
Case reports
- 29 year old man with liver and pancreatic lesions (Diagn Cytopathol 2009;37:365)
- 32 year old woman with abdominal pain and a pancreatic mass (Cytojournal 2016;13:7)
- 47 year old woman presenting with a mass in the pancreatic tail (Neuro Endocrinol Lett 2017;38:83)
- 60 year old woman with pancreatic cysts and octreotide avid lesions (Virchows Arch 2013;463:593)
- 64 year old man with a nodule in the head of the pancreas (Cytopathology 2013;24:197)
Treatment
- Surgical resection and chemotherapy (Mod Pathol 2011;24:S66)
Gross description
- Most often solid, solitary, homogenous, tan to yellow (Am J Surg Pathol 2001;25:602)
- If extensive fibrosis present, may be gray and firm (J Hepatobiliary Pancreat Sci 2015;22:586)
- Hereditary syndromes may present with multiple tumors
- Rarely a cystic component can be seen (Neuro Endocrinol Lett 2017;38:83)
Microscopic (histologic) description
- Solitary, well circumscribed
- Widely variable architectural patterns: nested, trabecular, glandular, ribbon-like, tubuloacinar, mixed patterns are common; however, there is usually a dominant pattern (Arch Pathol Lab Med 2006;130:963)
- Small / medium round, polygonal cells with clear, vacuolated cytoplasm due to lipid accumulation
- Central nucleus with characteristic salt and pepper chromatin pattern and inconspicuous nucleoli (Arch Pathol Lab Med 2009;133:350)
Microscopic (histologic) images
Cytology description
- Isolated or small clusters of round monomorphic tumor cells (Cytopathology 2013;24:197)
- Numerous small cytoplasmic vacuoles imparting a foamy appearance
- Eccentric nuclei with fine nuclear chromatin, without prominent nucleoli (J Hepatobiliary Pancreat Sci 2015;22:586)
Positive stains
Negative stains
Electron microscopy description
- Variably sized cytoplasmic lipid globules and dense neurosecretory granules (Arch Pathol Lab Med 2009;133:350)
Molecular / cytogenetics description
- Von Hippel-Lindau disease
- Autosomal dominant mutation in VHL tumor suppressor gene on chromosome 3p25.5 (Arch Pathol Lab Med 2010;134:1080)
- Multiple endocrine neoplasia type I
- Autosomal dominant germline mutation in MEN1 tumor suppressor gene on chromosome 11q13
- Sporadic tumors may arise from chromosomal alterations, epigenetic changes or functional genomic alterations
Videos
Pancreatic neuroendocrine neoplasms: overview
Sample pathology report
- Pancreas and duodenum, Whipple procedure:
- Clear cell pancreatic neuroendocrine tumor, WHO grade 1 (see synoptic report and comment)
- Comment: The patient's clinical history of von Hippel-Lindau syndrome is noted. The clear cell variant of pancreatic neuroendocrine tumor is more common in this setting. Immunohistochemical stains for synaptophysin and chromogranin are positive and the Ki67 index is approximately 2.0%.
Differential diagnosis
- Metastatic:
- Renal cell carcinoma, clear cell type:
- Positive for AE1/AE3, CD10, PAX8, CAIX
- Negative for neuroendocrine markers (Arch Pathol Lab Med 2010;134:1080)
- Renal cell carcinoma, clear cell type:
- Primary:
- Ductal adenocarcinoma with clear cell features:
- Positive for AE1 / AE3
- Negative for neuroendocrine markers
- Serous cystadenoma, solid variant:
- Positive for PAS, AE1 / AE3, vimentin
- Negative for synaptophysin
- Solid pseudopapillary neoplasm, clear cell variant:
- Positive for beta catenin (nuclear and cytoplasmic), CD10, CD56, vimentin
- Variable neuroendocrine markers
- Loss of E-cadherin (J Hepatobiliary Pancreat Sci 2015;22:586)
- Clear cell sugar tumor / PEComa:
- Ductal adenocarcinoma with clear cell features:
- Peripancreatic region:
- Adrenocortical tumors:
- Positive for inhibin, MelanA
- Weak with AE1 / AE3
- Negative for chromogranin A (J Hepatobiliary Pancreat Sci 2015;22:586)
- Clear cell hepatocellular carcinoma:
- Positive for Arginase1, HepPar1
- Negative for neuroendocrine markers
- Adrenocortical tumors:
Additional references
Board review style question #1
- Which of the following statements regarding clear cell pancreatic endocrine tumor is true?
- A diagnosis should not stimulate genetic evaluation for the VHL syndrome
- Frequently immunoreactive for cytokeratins
- Immunohistochemistry can distinguish from histologically similar metastatic clear cell renal cell carcinoma in VHL disease
- Only seen in the setting of VHL disease
- Tumors associated with VHL syndrome and MEN1 syndrome are often functional tumors
Board review style answer #1
C. Immunohistochemistry can distinguish clear cell pancreatic endocrine tumors from histologically similar metastatic clear cell renal cell carcinoma in VHL disease
Comment Here
Reference: Clear cell pancreatic endocrine tumor
Comment Here
Reference: Clear cell pancreatic endocrine tumor
Board review style question #2
- A patient is diagnosed with a clear cell pancreatic endocrine tumor. What genetic mutation is likely to be present?
- Mutation in APC tumor suppressor gene on chromosome 5q21-22
- Mutation in c-KIT proto-oncogene gene on chromosome 4q11-4q13
- Mutation in CDH1 gene on chromosome 16q22.1
- Mutation in MET proto-oncogene located on chromosome 17q31.1-34
- Mutation in VHL tumor suppressor gene on chromosome 3p25.5
Board review style answer #2
E. Mutation in VHL tumor suppressor gene on chromosome 3p25.5
Comment Here
Reference: Clear cell pancreatic endocrine tumor
Comment Here
Reference: Clear cell pancreatic endocrine tumor
Colloid carcinoma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Infiltrating ductal epithelial neoplasm of the pancreas characterized by the presence (in at least 80% of the neoplasm) of large extracellular stromal mucin pools containing suspended neoplastic cells (WHO)
Essential features
- > 80% of the tumor volume is composed of mucin pools with scanty floating tumor cells
- Associated with intraductal papillary mucinous neoplasm (almost always arises in an intestinal type IPMN), mucinous cystic neoplasm (MCN) and ampullary / duodenal tubulovillous adenomas
- Thought to have more favorable prognosis than conventional pancreatic ductal adenocarcinoma (cPDAC) (World J Gastroenterol 2018;24:4846)
Terminology
- Colloid carcinoma
- Mucinous noncystic carcinoma
- Gelatinous carcinoma
ICD coding
- ICD-10: C25 - malignant neoplasm of pancreas
Epidemiology
- 1 - 3% of malignant neoplasms of the exocrine pancreas
- 27 - 70% of IPMNs with associated invasive adenocarcinoma have a colloid component (Pathology 2008;40:655)
Sites
- Usually in the head of the pancreas
Pathophysiology
- Inverse polarization of cells (mucin glycoproteins in stroma facing surfaces instead of luminal surface)
- Expression of MUC2 (gel forming mucin, rare in cPDAC) and the absence of external lamina or basement membrane may lead to accumulation of extracellular mucin, which limits tumor spread and appears to have tumor suppressor activity (Am J Surg Pathol 2003;27:571, Mod Pathol 2002;15:1087)
Clinical features
- Mean age: 61 years
- M = F
- Abdominal / epigastric pain (in contrast to back pain of cPDAC), pancreatitis (50% of patients), diarrhea, jaundice, weight loss
- Larger (6.0 cm) compared with cPDAC, lower stage, better survival
- Incisional biopsy may contribute to thromboembolic complications (Am J Surg Pathol 2001;25:26)
- Pseudomyxoma peritonei can be a rare complication
Diagnosis
- Based on extensively sampled resection specimen
Radiology description
- Dilated ducts (sometimes filled with nodular lesions)
Prognostic factors
- 5 year survival is 57 - 72% (versus 12% for resectable cPDAC) (Am J Surg Pathol 2001;25:26, Dig Dis Sci 2011;56:1295)
- Tumor diameter, presence of IPMN / MCN precursor lesion, surgical margin status, vascular or perineural invasion, lymph node status, KRAS mutation or TP53 mutation are not prognostic factors (Am J Surg Pathol 2001;25:26)
Case reports
- 52 year old woman with history of necrotizing pancreatitis (Arch Pathol Lab Med 2005;129:255)
- 58 year old man with recurrent pancreatitis and 72 year old woman with pancreatic exocrine and endocrine insufficiency (Case Rep Pancreat Cancer 2016;2:40)
- 64 year old man with 15 cm tumor (JOP 2012;13:219)
- 65 year old man with pancreatic head tumor (Oncol Lett 2015;10:3195)
Treatment
- No specific treatment guidelines
Gross description
- Large (mean 5 cm), well demarcated tumor
- Solid, firm, gelatinous cut surface
Microscopic (histologic) description
- At least 80% of the neoplasm consists of large extracellular stromal mucin pools
- Scanty carcinoma cells suspended within these mucin pools
- Usually cuboidal or columnar cells
- Cribriform or stellate clusters, strips of columnar cells, small tubules or signet ring cells
- Incomplete lining of mucin lakes common
- Mucin tends to be retained during histologic processing (in contrast to IPMN and MCN)
- Muconodular invasive component of 1 cm or more
- Usually arise in association with IPMN, MCN or tubular / tubulovillous adenoma
- Perineural invasion and regional lymph node metastasis common
- Reference: Am J Surg Pathol 2001;25:26
Microscopic (histologic) images
Cytology description
- Difficult to spread thinly on slides due to abundant mucus
- Cellularity of malignant component may be low
Positive stains
- Strong expression of CDX2 and MUC2 (indicating intestinal differentiation); cPDAC usually MUC2-
- CEA shows accentuated staining in basal aspects of tumor cells (luminalization), in addition to luminal staining
- May show focal reactivity for synaptophysin or chromogranin
- Reference: Am J Surg Pathol 2001;25:26
Electron microscopy description
- Mucigen granules on stromal surface, no basement membrane
Molecular / cytogenetics description
- KRAS codon 12 mutation (33%); less frequent than cPDAC (> 90%)
- TP53 mutation (22%)
- High prevalence of somatic mutations in GNAS
- Microsatellite stable, unlike mucinous carcinomas of colon (Mod Pathol 2003;16:537)
Sample pathology report
- Distal stomach, duodenum and pancreatic head, pancreaticoduodenectomy:
- Colloid carcinoma of the pancreas, arising in a background of high grade intraductal papillary mucinous neoplasm (IPMN)
- Carcinoma involves the muscularis propria of the duodenum
- Positive for perineural invasion
- Negative for lymphovascular invasion
- All margins of resection, negative for carcinoma or high grade dysplasia
- Metastatic carcinoma involving one of seventeen lymph nodes (1/17)
- Portion of benign stomach with chronic inactive gastritis, negative for Helicobacter pylori on H&E stained sections
Differential diagnosis
- Extravasated benign stromal mucin:
- Mucus lakes limited to periductal location, devoid of neoplastic cells
- Frequently inflamed
- Intraductal papillary mucinous neoplasm:
- Smooth contours, complete epithelial lining, intraluminal mucin lost during processing
- Mucinous cystic neoplasm:
- Ovarian type stroma
- Usually occurs in women
- Conventional ductal adenocarcinoma:
- Intracytoplasmic and luminal mucin
- No or scant stromal mucin
Additional references
Board review style question #1
A 62 year old woman presented with a pancreatic head mass. Histologic sections demonstrate areas of irregular mucin pools with suspended clusters of malignant cells and also floating signet ring cells. However, no individual infiltrating signet ring cells are identified in the stroma. There is an intestinal type intraductal papillary mucinous neoplasm (IPMN) in the background; no conventional ductal adenocarcinoma component is seen. What is the diagnosis?
- Colloid carcinoma
- IPMN with rupture and extruded stromal mucin
- Mucinous cystadenocarcinoma
- Signet ring cell carcinoma
Board review style answer #1
Cystic endocrine tumors
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains (IHC and special stains) | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Distinctive subgroup of pancreatic neuroendocrine tumors that are well differentiated with a low mitotic rate and low Ki67 proliferative index as well as have a cystic component (Am J Surg Pathol 2012;36:1666)
Essential features
- Histologically resembles typical pancreatic neuroendocrine tumor but with cystic component
- Improved prognosis
- Occurs more frequently in patients with MEN1
ICD coding
- ICD-10: C25.4 - malignant neoplasm of endocrine pancreas
Epidemiology
- Up to 17% of pancreatic neuroendocrine tumors are purely or partially cystic, due to cystic degeneration of originally solid tumor (J Am Coll Surg 2008;206:1154, Rev Esp Enferm Dig 2018;110:130)
Sites
- More common in tail of pancreas
Pathophysiology
- 10 - 25% are associated with MEN1, occur in younger patients and are more often multiple and functional (Rev Esp Enferm Dig 2017;109:778)
Clinical features
- Usually (85%) nonfunctional (Rev Esp Enferm Dig 2017;109:778)
- If functional, usually insulinoma
- Often (45%) diagnosed incidentally (Rev Esp Enferm Dig 2017;109:778)
Diagnosis
- Can be confirmed by octreoscan scintigraphy
Radiology description
- Cystic, making diagnosis difficult; may be confused with other pancreatic cystic lesions (including intraductal papillary mucinous neoplasm and mucinous cystic neoplasm)
- Usually shows peripheral enhancement (AJR Am J Roentgenol 2013;200:W283)
Prognostic factors
- Better prognosis than noncystic neuroendocrine tumors of pancreas
- 60% disease free survival at 10 years (Rev Esp Enferm Dig 2017;109:778)
Case reports
- 20 year old woman with incidental tumor (J Cancer Res Ther 2012;8:289)
- 42 year old woman with cystic mass and tumorlets (World J Gastroenterol 2017;23:6911)
- 51 year old woman with multicystic pancreas mass (Clin J Gastroenterol 2018;11:87)
- 66 year old woman with lesion mimicking mucinous cystic neoplasm (Clin J Gastroenterol 2018;11:333)
- 78 year old man with epigastric pain (BMC Res Notes 2014;7:510)
Treatment
- Surgical excision
Gross description
- Mean 5 cm
- Generally unilocular cystic lesion filled with straw colored fluid
Gross images
Microscopic (histologic) description
- Typically appears as a thick fibrous wall with neuroendocrine cells lining the central cystic space and sometimes forming nests or vesicular structures within the fibrosis (Am J Surg Pathol 2001;25:752)
- Necrosis and lymphovascular invasion are uncommon
Microscopic (histologic) images
Cytology description
- Loosely cohesive aggregates and single cells
- Cells are small and plasmacytoid with occasional cytoplasmic vacuoles; nuclei are round / oval and uniform with finely and evenly distributed chromatin (Cancer 2009;117:203)
Positive stains (IHC and special stains)
- Neuroendocrine markers (synaptophysin / chromogranin)
- Often express glucagon but do not behave clinically as glucagonomas
Sample pathology report
- Pancreas, tail, resection:
- Pancreatic neuroendocrine tumor with cystic degeneration, WHO grade 1 (see synoptic report and comment)
- Comment: Immunohistochemical stains for synaptophysin and chromogranin are positive and the Ki67 index is approximately 1.1%.
Differential diagnosis
- Acinar cell cystadenoma / cystadenocarcinoma:
- Cysts are lined by acinar cells without associated acinar cell nests
- Negative for synaptophysin and chromogranin
Board review style question #1
- A patient is found to have an incidental cystic pancreatic mass on CT. Fine needle aspiration is performed and shows loosely cohesive tumor cells with a plasmacytoid appearance. Immunostains performed on the cell block show the cells are positive for synaptophysin and chromogranin. What is true about this lesion?
- Almost never incidental, despite this case
- More common in patients with MEN1 syndrome
- Probably arose in the head of the pancreas
- Very poor prognosis
Board review style answer #1
B. More common in patients with MEN1 syndrome. This is a cystic pancreatic neuroendocrine tumor.
Comment Here
Reference: Cystic endocrine tumors
Comment Here
Reference: Cystic endocrine tumors
Board review style question #2
Board review style answer #2
C. Chromogranin and synaptophysin. This is a cystic pancreatic neuroendocrine tumor.
Comment Here
Reference: Cystic endocrine tumors
Comment Here
Reference: Cystic endocrine tumors
Cystic fibrosis
Table of Contents
Definition / general | Gross images | Microscopic (histologic) description | Positive stains | Videos | Differential diagnosisDefinition / general
- Autosomal recessive disorder affecting most critically the lungs; also pancreas, liver, intestines; characterized by abnormal transport of chloride and sodium across an epithelium, leading to thick, viscous secretions (Wikipedia: Cystic Fibrosis [Accessed 11 December 2017])
- Incidence: 1 in 20 in U.S. are carriers
- Most common mutation is #708 (seen in 70% with disease)
- Mutations cause reduced chloride ion in secretions, thicker respiratory secretions, upper respiratory infections
- Complications: pancreatic insufficiency late in disease course in 90%, diabetes, malabsorption, pancreatitis
- Mutations also cause defective cilia and infertility; may cause meconium ileus (5 - 10%), intussusception
- Cysts form secondary to ductal obstruction due to thick, tenacious secretions, variable numbers of cysts range from 1 to 3 mm, very rarely entire pancreas replaced by multiple macroscopic cysts, termed "pancreatic cystosis"
Gross images
Microscopic (histologic) description
- Grade I: accumulation of secretion
- Grade II: exocrine atrophy
- Grade III: atrophy with lipomatosis
- Grade IV: fibrosis with total obliteration of the exocrine glands and ducts with scattered islets of Langerhans
- Pancreatic ducts diffusely dilated and filled with numerous lamellated concretions
- Associated with fibrosis
- Nondiabetic patients have fibrocystic changes with normal islets, prominent nesidioblastosis, some persisting exocrine tissue
- Young adult diabetic patients have total loss of exocrine pancreas with fat replacement, no nesidioblastosis, reduced islets (Hum Pathol 1984;15:278)
Positive stains
- PAS+ diastase resistant, CEA, alpha-1-antitrypsin (Arch Pathol Lab Med 1989;113:1142)
Videos
Histopathology pancreas: cystic fibrosis
Differential diagnosis
- Kartagener syndrome:
- Defective cilia syndrome
Cytology
Table of Contents
Definition / general | Essential features | Sites | Clinical features | Diagnosis | Cytology description | Cytology images | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Normal and nonneoplastic findings in pancreas cytology
- Preparations:
- Conventional smears or touch preparation stained with Diff-Quik, Papanicolaou
- Liquid based (thin prep)
- Cytospin
- Cell block (for immunohistochemistry and molecular)
- Cyst fluid (chemistry, tumor markers, molecular)
Essential features
- Normal cellular elements:
- Acinar
- Ductal
- Islet cells (rare)
- Contaminants during procedure:
- Bile
- Gastric epithelium
- Duodenal epithelium
- Hepatocytes
- Mesothelial cells
- Procedures:
- Pancreas image guided fine needle aspiration (FNA)
- Bile duct brushing
- Percutaneous interventional guided FNA
Sites
- Head / uncinate, body and tail of pancreas
Clinical features
- General settings: patients with suspected neoplasia of the pancreaticobiliary tract should receive the following (Diagn Cytopathol 2014;42:325)
- Clinical history and physical examination, including identifying risk factors for malignant causes and for benign explanations for a stricture or cyst (e.g. chronic pancreatitis, primary sclerosing cholangitis / inflammatory bowel disease, autoimmune disease, etc.)
- Laboratory studies (e.g. serum bilirubin and alkaline phosphatase)
- Selective use of serum tumor markers (CA19-9 and CEA)
- Imaging studies
- Serum IgG4 when autoimmune disease is suspected
- Clinical indications for pancreas FNA
- Pancreatic mass / cyst on imaging (Diagn Cytopathol 2014;42:325)
- Unexplained acute pancreatitis in older patients
- Newly diagnosed late onset diabetes mellitus
- Jaundice, pruritus, cholestasis unexplained by underling hepatobiliary disease or choledocholithiasis
- Anorexia, weight loss, abdominal pain radiating to the back
- Clinical indications for bile duct brushing
- Obstructive jaundice (Acta Cytol 2016;60:167)
- Distal common bile duct stricture
- Proximal / complex hilar stricture
- Screening patients with primary sclerosing cholangitis for biliary neoplasia (Endoscopy 2016;48:432)
Diagnosis
- Common diagnostic procedures utilized in the procurement of pancreatic cytology samples:
- Endoscopic ultrasound (EUS) guided FNA for pancreatic mass / lesions
- Endoscopic retrograde cholangiopancreatography (ERCP) and percutaneous transhepatic cholangiography (PTC) are used to obtain brushing or aspiration from the common bile duct and pancreatic duct stricture / mass
Cytology description
Normal
Nonneoplastic contaminants
- Bile duct
- Monolayered flat sheets arranged in honeycomb pattern
- Cuboidal to columnar cells
- Bland appearing basally located nuclei imparting a picket fence appearance
- Scant pale cytoplasm
- Acinar cells
- Pyramidal to triangle shaped cells arranged singly, in clusters or grape-like appearance occasionally embedded in fibroconnective tissue
- Abundant granular cytoplasm
- Round central to eccentric nuclei
- Occasional nucleoli
- Islet cells
- Infrequently seen in normal pancreas
Nonneoplastic contaminants
- Bile: seen in biliary specimens
- Duodenal epithelium: seen in EUS guided transduodenal FNA for lesions in pancreatic head and uncinate
- Flat sheets (honeycomb) arrangement, occasional singly scattered cells
- Uniformly round, bland appearing evenly spaced nuclei
- Scant pale cytoplasm
- Goblet cells present (Diagn Cytopathol 2005;33:381)
- Gastric epithelium: present in transgastric EUS FNA for pancreatic lesions in the body and tail
- Flat sheets arranged in monolayered (honeycomb) pattern (Diagn Cytopathol 2005;33:381)
- Cells with uniformly round, evenly spaced bland appearing nuclei
- Pale cytoplasm with well defined cell border
- Surface epithelium lined by tall columnar cells with basally located nuclei and cytoplasm filled with abundant mucin
- Hepatocytes: contaminant in procedures using percutaneous interventional guided FNA
- Polygonal cells with well defined cell borders and abundant granular cytoplasm
- May have intracytoplasmic pigment (bile, lipofuscin or iron)
- Centrally located nuclei, prominent nucleoli
- Occasional intranuclear inclusions
- Mesothelial cells: contaminant in procedures using percutaneous interventional guided FNA
- Polygonal cells arranged in flat sheets
- Moderate amount of cytoplasm (dense in the center and pale in periphery)
- Clear spaces in between cells intercellular windows
- Single to occasionally binucleated to multinucleated round to oval nuclei
Cytology images
Contributed by Maria Luisa C. Policarpio-Nicolas, M.D.
Sample pathology report
- Pancreas, head, endoscopic ultrasound guided fine needle aspiration:
- Nondiagnostic
- Normal acinar and ductal epithelium. The biopsy does not explain the well defined pancreatic mass seen on imaging (Cytojournal 2014;11:3).
Differential diagnosis
- Acinar carcinoma:
- Highly cellular, small to moderate sized loose groups, numerous single cells and stripped round nuclei, prominent acinar formation, little anisonucleosis, prominent nucleoli (Diagn Cytopathol 2006;34:367)
- Ductal adenocarcinoma:
- 3 dimensional to glandular arrangement of tumor cells, loss of polarity, moderate to marked variation in nuclear size (4:1 ratio), small to large prominent nucleoli, singly scattered tumor cells, mitotic figures (few to many)
- Low grade neoplastic mucinous cyst (intraductal papillary mucinous neoplasm, mucinous cystic neoplasm):
- On cytomorphology alone, it is extremely difficult to distinguish gastric contamination from a neoplastic mucinous cyst
- If background mucin is present, a note / comment can be added (see below)
- Note / comment: Benign appearing mucinous epithelium is present from this transgastric FNA in a background of extracellular mucin. The morphology of the cells is nonspecific and may represent low grade dysplasia or gastric contamination. No high grade atypia is identified and no necrosis is present (Pitman: The Papanicolaou Society of Cytopathology System for Reporting Pancreaticobiliary Cytology, 2015).
Board review style question #1
Board review style answer #1
Board review style question #2
Which is the method used to procure a cytology sample from a bile duct stricture?
- Endoscopic ultrasound guided
- CT guided
- Endoscopic retrograde cholangiopancreatography
- MRI guided
Board review style answer #2
Diabetes mellitus
Table of Contents
Definition / general | Epidemiology | Etiology | Type 1 | Type 2 (NIDDM) | Maturity onset diabetes of the young | Diagrams / tables | Clinical complications | Diagnosis | Case reports | Treatment | Microscopic (histologic) description | VideosDefinition / general
- Chronic disorder of carbohydrate, fat and protein metabolism due to defective or deficient insulin secretory response (Wikipedia: Diabetes Mellitus [Accessed 8 December 2017])
Epidemiology
- 3% of world population, 26 million in U.S. but only 75% are clinically diagnosed
- #7 leading cause of death in 2007 (underlying cause on 71,382 death certificates) (American Diabetes Association: Statistics About Diabetes [Accessed 8 December 2017])
- Lifetime risk of diabetes: type 1 - 0.5%, type 2 - 5%
- Numerous variations, all with hyperglycemia
Etiology
- Destruction of islets due to drugs (steroids, thiazides, pentamidine), hemochromatosis ("bronze diabetes" due to hemosiderin deposition in pancreas), hereditary ceruloplasmin deficiency (Hum Pathol 1997;28:499), infections (congenital rubella, CMV, coxsackievirus [Arch Pathol Lab Med 1980;104:438], enteroviruses [Diabetologia 2004;47:225]), pancreatitis, surgery, tumors, endocrinopathies (pituitary, adrenal, pregnancy) or idiopathic
Type 1
- Chronic disease of carbohydrate, fat and protein metabolism due to reduction in beta cell mass causing severe, absolute lack of insulin (eMedicine: Type 1 Diabetes Mellitus [Accessed 8 December 2017])
- 10% of all cases
- Without insulin, patients develop diabetic ketoacidosis (DKA), coma and death
Etiology
- Presumed autoimmune cause for islet cell destruction but precise etiology unclear (Wikipedia: Diabetes Mellitus Type 1 [Accessed 8 December 2017])
- Usually Northern European descent
- 70% concordance in identical twins, HLA-D linked
- Genetic predisposition may affect immune responsiveness to a beta cell autoantigen or method of presentation to T cells
Viruses and IDDM:
- Viruses may damage beta cells, exposing antigens which trigger an autoimmune response
- May be due to molecular mimicry (immune response develops against shared amino acid sequences): GAD and Coxsackie B4 virus share a six amino acid sequence
- Retrovirus may serve as a superantigen
Autoimmune aspects:
- Islet cell autoantibodies present in 70%; also CD8+ T cell infiltrate in islets
- Antigens are glutamic acid decarboxylase (GAD), islet autoantigen 2, insulin associated antibody, gangliosides
- GAD antibodies precede clinical symptoms, present in most newly diagnosed patients and 80% of first degree relatives
- GAD antibody also causes stiff man syndrome, whose patients often have a history of IDDM
- Many IDDM patients also have antithyroid peroxidase, antiparietal cell and antiadrenocortical antibodies
- Some NIDDM patients have autoantibodies but no other features of IDDM
- Usually chronic (years)
- Clinical disease when 90% of islet cells are destroyed
Clinical features
- Onset at age < 20 years, normal weight (unlike most NIDDM)
- Characterized by PPP (polyuria, polydipsia, polyphagia) and ketoacidosis (DKA)
- Polyphagia combined with weight loss is specific for IDDM; type 2 patients rarely have either
- Severe fasting hypoglycemia is due to cessation of glycogen storage in fat and muscle
- Glycosemia causes glycosuria with depletion of water and electrolytes
- Also: low / absent plasma insulin, high plasma glucagon, unstable glucose tolerance (very sensitive to changes in insulin, diet, exercise, infection, stress), presence of free fatty acids (due to breakdown of adipose stores), which produces ketone bodies (acetoacetic acid and beta hydroxybutyric acid)
- May get hyperosmotic nonketotic coma - dehydration due to hyperglycemic diuresis with failure to drink enough fluids to compensate, often in an elderly person with diabetes and stroke / infection
- "Dead in bed syndrome": sudden death in young people with type 1 diabetes, cause unknown (Hum Pathol 2010;41:392)
Type 2 (NIDDM)
- Also called adult onset, non insulin dependent diabetes mellitus / NIDDM, type 2
- 80 - 90% of cases of diabetes (Wikipedia: Diabetes Mellitus Type 2 [Accessed 8 December 2017])
- Usually > 30 years old, obese (80% of cases, abdominal obesity more important than subcutaneous obesity), normal or increased blood insulin, rare diabetic ketoacidosis, no anti-islet antibodies
Pathophysiology
Early:
- Normal insulin secretion and plasma levels but loss of pulsatile, oscillating pattern of secretion
- Also loss of rapid first phase of insulin secretion triggered by glucose
- NO insulinitis is present
Later:
- Mild / moderate insulin deficiency, may be due to beta cell damage
- Beta cells may be "exhausted" due to chronic hyperglycemia and persistent beta cell stimulation
Amylin:
- 37 amino acid peptide, normally produced by beta cells, packaged and cosecreted with insulin
- In NIDDM patients, tends to accumulate outside beta cells and resembles amyloid
Clinical features
- 90%+ concordance in twins, apparently due to multiple genetic polymorphisms (no HLA association)
- Due to insulin resistance (associated with obesity and pregnancy) or derangement in beta cell secretion of insulin
- Associated with amyloid deposits in islets (amyloid associated with basement membrane heparan sulfate proteoglycan) (Arch Pathol Lab Med 1992;116:951) and pituitary (Arch Pathol Lab Med 1995;119:1055)
Maturity onset diabetes of the young
- 1 - 2% of all cases of diabetes (Diabetes Metab Syndr Obes 2012;5:101)
- Also called monogenic diabetes (Wikipedia: Maturity Onset Diabetes of the Young [Accessed 8 December 2017])
- Type 2 diabetes-like condition which occurs in 2+ generations, with autosomal dominant inheritance (Diabetes Care 2011;34:1878)
- Autosomal dominant but not a single entity - mutations in 9 genes identified to date (see Diagrams / tables below)
- Common genes affected are hepatic nuclear factor 1 or 4 alpha, glucokinase
- Onset before age 25, normal weight, mild hypoglycemia
- No GAD antibodies, no insulin resistance, no beta cell loss but impaired beta cell function
Diagrams / tables
Clinical complications
General
Nonenzymatic glycosylation
Vascular complications
Diabetic renal disease
Ocular
Neuropathy
- Main complications are microangiopathy, retinopathy, nephropathy, neuropathy - all due to hyperglycemia
- Kidneys transplanted into diabetic patients develop nephropathy within 3 - 5 years but kidneys from diabetic patients transplanted into normal patients have remission of nephropathy
- Strict control of diabetes delays progression of microvascular complications
- Complications are due to nonenzymatic glycosylation and disturbances in polyol pathways
Nonenzymatic glycosylation
- Glucose + protein => Schiff base (protein - NH = CH (CHOH)4-CH2OH) => Amadori product
- (protein - NH-CH2-C = 0-(CHOH)3-CH2OH) => protein - protein cross linking via N-C-N bonding
- Early reactions are reversible and related to HbA1c level
- Advanced glycosylation end products (AGE) are not reversible
- AGE traps LDL in blood vessels, enhances cholesterol deposition, accelerating atherosclerosis
- AGE inhibition antagonizes diabetic complications in experimental models
Vascular complications
- Relative risk is 100:1
- Accelerated atherosclerosis in aorta and large / medium sized vessels
- Myocardial infarction: most common cause of death, no gender preference
- Gangrene of lower extremities
- Micro description:
- Hyaline arteriolosclerosis, associated with hypertension, more common / severe in diabetes but not specific
- Amorphous hyaline thickening in arteriolar wall
- Related to severity of disease and hypertension
- Microangiopathy: diffuse basement membrane thickening with protein leakage in capillaries of skin, skeletal muscle, retina, renal glomeruli, renal medulla, renal tubules, Bowman capsule, peripheral nerves, placenta
Diabetic renal disease
Ocular
- #4 cause of blindness in U.S.
- Associated with retinopathy, cataracts, glaucoma
- Polyol pathways: important in lens and other tissues (nerves, kidney, blood vessels) that don't require insulin for glucose transport
- High intracellular glucose plus aldose reductase produces sorbitol and later fructose, causing water influx and osmotic cell injury
- In lens, causes swelling and opacity
- Inhibition of sorbitol may reduce formation of cataracts and neuropathy
Neuropathy
- Peripheral, symmetric neuropathy of lower extremity most common, sensory more common than motor
Diagnosis
- High fasting glucose or impaired glucose tolerance (without diabetes, oral glucose loads cause only slight rise in blood glucose due to brisk insulin response; with diabetes, blood glucose rises markedly for a sustained period)
Case reports
- Adult with acute onset, death within 3 days, T cell pancreatic infiltrate (Arch Pathol Lab Med 1994;118:84)
- Islet inflammation with Coxsackie B5 infection (Hum Pathol 1982;13:661)
Treatment
- Type 1: immunosuppressive therapy is effective in children with new onset disease
- Type 2: diet, exercise and education (eMedicine: Type 2 Diabetes Mellitus [Accessed 8 December 2017])
- Lifestyle intervention and metformin delay onset of diabetes (N Engl J Med 2012;367:1177)
Microscopic (histologic) description
- Type 1: inconsistent reduction in number and size of islets, uneven insulinitis (T lymphocytes)
- Type 1: early insulinitis with marked islet atrophy and fibrosis and severe beta cell depletion (Islets 2011;3:131)
- Type 2: subtle reduction in islet cell mass, amyloid replacement of islets due to amylin fibrils (also seen in aging nondiabetics); associated with marked fatty replacement
- Type 2: amyloid in the islets of Langerhans is the uniform pathologic feature
- Gestational diabetes: lower total insulin+ area due to smaller islets (Islets 2011;3:231)
- Infants of diabetic mothers: islet cell hypertrophy / hyperplasia
Videos
Histopathology pancreas: type 2 diabetes mellitus
Ductal adenocarcinoma, NOS
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Pancreatic ductal adenocarcinoma (PDAC) is an invasive pancreatic epithelial neoplasm with glandular (ductal) differentiation, usually demonstrating luminal or intracellular mucin and without a substantial component of any other histological type
Essential features
- Adenocarcinoma derived from pancreatic ductal epithelia, with randomly arranged epithelial elements, intense stromal desmoplasia and variable necrosis
- Poor prognosis: 5 year survival rate of 6% (ranges from 2 - 9%) (Cancer Res 2014;74:2913, World J Gastroenterol 2016;22:9694)
- Precursors of invasive ductal adenocarcinoma:
Terminology
- Sometimes called tubular adenocarcinoma
ICD coding
- ICD-11: 2C10.0 - adenocarcinoma of pancreas
Epidemiology
- Fourth leading cause of cancer related deaths in the U.S. (predicted to rise to the second most common cause by 2030) (CA Cancer J Clin 2021;71:7, Cancer Res 2014;74:2913)
- Globally, 495,773 new cases and 466,003 new deaths in 2020 (CA Cancer J Clin 2021;71:209)
- M:F = 1.13:1 (CA Cancer J Clin 2021;71:209)
Sites
- Head of the pancreas: 60 - 70%; body: 5 - 15%; tail: 10 - 15%
- Head tumors: 50% have distention of biliary tree and progressive jaundice; 85% have extension beyond pancreas at diagnosis
- Body / tail tumors: typically larger at diagnosis since these tumors do not cause symptoms until late; 25% have peripheral venous thrombi; metastases common
- Rarely arises from heterotopic pancreatic tissue in the gastrointestinal tract
- Reference: Surg Pathol Clin 2016;9:547
Pathophysiology
- Invasive ductal adenocarcinoma arises in association with precursor lesions: PanIN, IPMN or MCN
Etiology
- Risk factors: smoking, alcohol abuse, obesity, high intake of dietary saturated fat, chronic pancreatitis, diabetes
- Hereditary syndromes: Peutz-Jeghers syndrome, hereditary pancreatitis, familial atypical multiple mole melanoma (FAMMM), familial pancreatic cancer, Lynch syndrome, familial breast cancer and other Fanconi anemia genes, familial adenomatous polyposis (FAP)
- Reference: World J Gastroenterol 2018;24:4846
Clinical features
- Back pain, weight loss, malaise, jaundice, diabetes mellitus
- Trousseau sign: migratory thrombophlebitis in 10% due to tumor or tumor necrosis producing platelet aggregating factors and procoagulants; causes arterial and venous thrombi
- Coexisting pancreatitis in 10%
- Metastases:
- Local lymph nodes (microscopic metastases found in 75% with T1 / T2 disease)
- Liver, lung, peritoneum, adrenal, bone, distal nodes
- Supraclavicular node metastasis may be a presenting symptom
- Tumor may track along biopsy needle path
- Metastases to ovary may simulate primary mucinous ovarian tumors (Am J Surg Pathol 1989;13:748)
Diagnosis
- Preoperative / pretreatment by endoscopic ultrasound guided fine needle aspiration (EUS-FNA)
- Surgical resection specimen
Laboratory
- Serum tests: CA19-9, CEA
Radiology description
- Hypodense mass on CT imaging in 92% of cases
- Double duct sign (dilation of both the biliary and pancreatic ducts) in pancreatic head mass
- Reference: Gastroenterology 2014;146:291
Prognostic factors
- Dependent on stage and tumor size
- Resectable and size < 4.5 cm are favorable factors
- Perineural invasion and vascular invasion are adverse prognostic factors (Eur J Surg Oncol 2007;33:892)
- High histologic grade unfavorable
- Any squamous component indicates poorer prognosis (median survival: 7 - 11 months)
Case reports
- Woman in her 50s with faint hypoechoic area in the body of the pancreas (J Med Ultrason 2018;45:617)
- 63 year old man with lobulated cystic lesion in the head of the pancreas with diffuse dilatation of the main pancreatic duct (Pancreas 2019;48:e24)
- 78 year old man with two solid tumors in the remnant pancreas after pancreaticoduodenectomy for acinar cell carcinoma (J Nippon Med Sch 2019;86:279)
Treatment
- Most (85%) tumors are not amenable to curative surgery
- For head / periampullary tumors: Whipple resection (subtotal pancreaticoduodenectomy); perioperative mortality: ~2%
- For body / tail tumors: distal pancreatectomy
- Resect retroperitoneal nerves and nodes if stage I / II to reduce local recurrence
- Palliative treatment: bypass operations, chemotherapy and radiation therapy
- Reference: World J Gastroenterol 2018;24:4846
Gross description
- White gray, sclerotic, poorly defined mass
- > 75% are solid tumors
- 25% of head tumors extend to duodenal wall
- If advanced, may be difficult to determine site of origin between pancreas, ampulla and common bile duct
- 20% have multiple tumors
- Reference: Surg Pathol Clin 2016;9:547
Frozen section description
- Frozen sections used to confirm diagnosis and determine vascular resectability, margin positivity and status of extraregional lymph nodes / other metastatic sites
- Common margins evaluated: pancreatic neck / distal pancreatic parenchyma, bile duct and uncinate
- Helpful features of PDAC: anisonucleosis in a single gland (at least 4:1), perineural invasion, tumor gland immediately next to large vessel, single infiltrating cells, low power evaluation for disorganized duct distribution with ill defined borders, necrosis and mitoses (Arch Pathol Lab Med 2021 Mar 26 [Epub ahead of print])
Microscopic (histologic) description
- Infiltrating well to poorly formed glandular / ductal structures surrounded by remarkably desmoplastic stroma
- Mucin production is specific for ductal origin versus acinar or neuroendocrine differentiation
- Perineural invasion present in 90%, typically with better differentiated glands
- Angiolymphatic invasion in 50%; vascular invasion may mimic PanIN (Am J Surg Pathol 2012;36:235)
- Well differentiated: pink apical band composed of mucin granules, irregular shape and distribution; desmoplasia, marked nuclear pleomorphism with nucleoli, loss of polarity, mitotic figures
- Moderately to poorly differentiated (most tumors); abortive tubular structures, deeply infiltrative growth pattern, frequent mitosis, irregular and abortive mucin production
- TNM histologic grading system (College of American Pathologists), based on extent of glandular differentiation:
- G1 = well differentiated (> 95% tumor composed of glands)
- G2 = moderately differentiated (50 - 95% glands)
- G3 = poorly differentiated (< 50% glands)
- Post neoadjuvant therapy (Hum Pathol 2021;109:1, Mod Pathol 2018;31:4, Arch Pathol Lab Med 2020;144:838):
- Variable amount of residual tumor in a densely fibrotic tumor bed
- Attention to perineural space and duodenal wall for residual tumor glands
- Blue-grey fibrosis is a useful clue to suggest the possibility of adjacent residual tumor (Hum Pathol 2021;109:1)
- May show vacuolated or eosinophilic tumor cell changes
- WHO variants:
- Adenosquamous carcinoma and squamous cell carcinoma
- Colloid carcinoma
- Hepatoid carcinoma
- Medullary carcinoma
- Invasive micropapillary carcinoma
- Signet ring cell (poorly cohesive cell) carcinoma
- Undifferentiated carcinoma (anaplastic, sarcomatoid, carcinosarcoma)
- Undifferentiated carcinoma with osteoclast-like giant cells
- Non-WHO variants / patterns:
- Clear cell:
- Glandular, ductal or nested structures with single layer of polygonal cells, distinct cell borders and variable nuclear atypia
- Not due to accumulation of glycogen or mucin
- Overexpression of HNF1β by IHC (Mod Pathol 2008;21:1075)
- Foamy gland:
- Deceptively benign appearing pattern
- Well formed glands with bland cells but subtle infiltration
- Cells have abundant microvesicular (white and crisply foamy) cytoplasm with distinct pink brush border-like zone at apical / luminal portion of the cell, nuclei are basal oriented, dense or wrinkled (raisinoid)
- Foamy material due to evenly sized mucigen granules that are mucin negative
- Foamy PanIN proposed to be the precursor lesion (Am J Surg Pathol 2000;24:493, Ann Diagn Pathol 2008;12:252)
- Large duct:
- Seen in < 10% of PDAC
- Clustered ducts with irregular jagged contours (diameter: 0.5 mm - 1 cm)
- Desmoplastic and myxoid stroma, intraluminal neutrophils and granular debris
- Focal microcystic appearance due to marked ectasia of infiltrating neoplastic glands, particularly near duodenal muscularis propria
- May have papillary pattern (Mod Pathol 2012;25:439, Am J Surg Pathol 2012;36:696)
- Vacuolated:
- High grade tumors, clusters of tumor cells containing large vacuoles imparting cribriform architecture (reminiscent of adipocytes or signet ring cells)
- Microcysts contain cellular debris and mucin
- Can resemble fat necrosis or lipogranulomas in lymph node
- Recognizing the atypical, enlarged, hyperchromatic nuclei is key (Virchows Arch 2010;457:643)
- Clear cell:
Microscopic (histologic) images
Contributed by Wei Chen, M.D., Ph.D.
Cytology description
- EUS-FNA has sensitivity and specificity of > 90% and 100%, increased sensitivity with ThinPrep, brushings are 50% sensitive (repeat if inconsistent with clinical or radiologic findings) (Arch Pathol Lab Med 2000;124:387)
- Aspirates are cellular, without acinar cells, with atypical ductal cells in sheets (drunken honeycomb), clusters or singly; anisonucleosis (4:1 variation)
- Signet ring cells and mitotic figures are helpful when present
- Papanicolaou Society of Cytopathology's 6 tiered system for pancreatobiliary cytology: nondiagnostic, negative for malignancy, atypical, neoplastic, suspicious and positive / malignant (Cytojournal 2014;11:3)
- Duodenal secretions are 80% sensitive in head tumors, 33% sensitive in tail tumors; endoscopic retrograde cholangiopancreatography (ERCP) juice is 50 - 85% sensitive
Cytology images
Positive stains
Negative stains
- pVHL negative / loss seen in > 90% PDACs (Arch Pathol Lab Med 2012;136:601, Hum Pathol 2013;44:503)
- Loss of SMAD4 / DPC4 seen in 55%; specific for malignancy (in situ or invasive) (Am J Clin Pathol 2001;116:831)
- CK20, MUC2, vimentin, synaptophysin, chromogranin, trypsin, chymotrypsin, lipase
Electron microscopy description
- Mucigen granules
Molecular / cytogenetics description
- 4 key driver gene mutations (Arch Pathol Lab Med 2011;135:716, Hum Pathol 2009;40:612, Cancer Cell 2017;32:185)
- KRAS: > 95%, early event during carcinogenesis (Cancer 2007;109:1561)
- CDKN2A (p16): 95%
- TP53: 50 - 80%
- SMAD4: 55%
- 50% overexpress HER2
- Many core signaling pathways involved
- ~10% of pancreatic cancers have a familial basis
Sample pathology report
- Distal stomach, duodenum and pancreatic head, pancreaticoduodenectomy:
- Moderately differentiated pancreatic ductal adenocarcinoma, 2.2 cm
- Adenocarcinoma involves distal bile duct
- All margins negative for carcinoma or high grade dysplasia
- Positive for lymphovascular invasion and perineural invasion
- Metastatic adenocarcinoma involving 2 of 23 lymph nodes (2/23)
- Portion of benign stomach with chronic inactive gastritis, negative for Helicobacter pylori on H&E
- Benign duodenum with no significant pathologic change
Differential diagnosis
- Ampullary adenocarcinoma:
- Epicenter at ampulla, presence of preinvasive component at ampulla
- Chronic pancreatitis:
- Lobular architecture at low power with central ectatic branched ductules and clusters of round ductules surrounded by cuff of stroma (Arch Pathol Lab Med 2009;133:382)
- Features of PDAC to differentiate from chronic pancreatitis (Arch Pathol Lab Med 2015;139:848):
- Haphazard architecture and glands at abnormal locations in interlobular areas, next to vessels and naked glands in fat
- Anisonucleosis (4:1 variation), loss of cell polarity, perineurial invasion, individual cell infiltration, budding into lumen; has p53 mutations and loss of SMAD4 / DPC4
- Distal bile duct adenocarcinoma:
- Epicenter at bile duct, circumferential / symmetrical involvement of the bile duct, presence of in situ component (BilIN or biliary intraductal papillary neoplasm)
Additional references
Board review style question #1
Among the entities below, which one is considered a precursor lesion for invasive ductal adenocarcinoma of pancreas?
- Intraductal papillary mucinous neoplasm
- Lymphoepithelial cyst
- Serous cystadenoma
- Solid pseudopapillary neoplasm
Board review style answer #1
Board review style question #2
- The above microphotograph demonstrates a section from peripancreatic adipose tissue in a patient with pancreatic ductal adenocarcinoma. What is the lesion in the center of the microphotograph?
- Intraductal papillary mucinous neoplasm
- Pancreatic ductal adenocarcinoma
- Pancreatic intraepithelial neoplasia, high grade
- Pancreatic intraepithelial neoplasia, low grade
Board review style answer #2
Familial pancreatic neoplasms (pending)
[Pending]
Features to report-pancreatectomy
Table of Contents
Definition / general | Essential features | Features to report by organization | Features to report (including pancreatic neuroendocrine neoplasms) | Tips | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- This topic describes which features to report for exocrine and endocrine pancreatic neoplasms obtained from pancreaticoduodenectomy, distal pancreatectomy and total pancreatectomy procedures
- Essential clinical history: none
Essential features
- Essential features for reporting the tumors of the exocrine pancreas:
- Core data elements by CAP: procedure, tumor site, histologic type, histologic grade, greatest dimension of the tumor (cm), site(s) involved by direct tumor extension (except for pancreatic surfaces), treatment effect, lymphovascular invasion, perineural invasion, margin status, margin(s) involved by tumor, regional lymph node status, number of lymph nodes with tumor, number of lymph nodes examined, distant metastasis, TNM descriptors, pT / pN / pM categories
- Optional data elements by CAP: additional dimensions of tumor (cm x cm), involvement of pancreatic surface(s), closest margins, distance to closest margin (cm), additional findings, special studies, comments
- Essential features for reporting the tumors of the endocrine pancreas:
- Core data elements by CAP: procedure, tumor site, histologic type, histologic grade, mitotic rate (number of mitoses per 2 mm2), Ki67 index (%), greatest dimension of tumor (cm), tumor focality, site(s) involved by direct tumor extension, lymphovascular invasion, perineural invasion, margin status, margin(s) involved by tumor, regional lymph node status, number of lymph nodes with tumor, number of lymph nodes examined, distant metastasis, TNM descriptors, pT / pN / pM categories
- Optional data elements by CAP: clinical history, functional type, additional dimensions of the tumor (cm x cm), tumor necrosis, closest margins, distance to closest margin (cm), additional findings, comments
Features to report by organization
Features to report (including pancreatic neuroendocrine neoplasms)
- Procedure
- Tumor location
- Histologic type
- Histologic grade
- Precursor preinvasive neoplastic lesions; including their location and histologic grade
- Tumor size
- Document overall tumor size and size of the invasive component separately for the carcinomas associated with preinvasive neoplastic lesions: i.e., intraductal papillary mucinous neoplasm (IPMN), intraductal tubulopapillary neoplasm (ITPN), intraductal oncocytic papillary neoplasm (IOPN), mucinous cystic neoplasm (MCN)
- Tumor focality (for pancreatic neuroendocrine neoplasms)
- Microscopic direct tumor extension
- Lymphovascular invasion
- Perineural invasion
- Mitotic index and Ki67 proliferation index (for pancreatic neuroendocrine neoplasms)
- Surgical margin status
- Free pancreatic surface status
- Treatment effect, if applicable
- Regional lymph node status
- Distant metastasis status
- TNM descriptors, if applicable
- pT stage
- pN stage
- pM stage
- Additional findings, including adjacent pancreas parenchyma and other organs
- Special studies, if any
- Notes, if needed
Tips
- pT stage should be reported based on the size of the invasive component only for the carcinomas associated with preinvasive neoplastic lesions
- For cases with more than one tumor, pT stage should be reported based on the largest tumor
- Additionally, localization and characteristics of the other (smaller) tumor(s) should be documented
- Macroscopic tumor size should be confirmed by microscopic examination for the postneoadjuvant treatment pancreatectomy specimens
- The largest dimension of the entire area defined by viable neoplastic cells including intervening stroma and nonneoplastic pancreatic tissue should be measured microscopically (Am J Surg Pathol 2022;46:754)
- There are several methods for Ki67 proliferation index assessment for pancreatic neuroendocrine tumors, including automated counting by image analyzer
- Manual counting of camera captured / printed image is encouraged since it is highly reliable and easily performed (Mod Pathol 2015;28:686)
- For pancreaticoduodenectomy (Whipple) specimens, status of bile duct margin, pancreatic neck margin, uncinate (retroperitoneal) margin, proximal margin of the stomach / duodenum and distal margin of the duodenum should be reported
- For distal pancreatectomy specimens, proximal pancreatic parenchyma margin should be reported
Additional references
- CAP: Protocol for the Examination of Specimens from Patients with Carcinoma of the Pancreas [Accessed 2 December 2022], CAP: Protocol for the Examination of Specimens from Patients with Tumors of the Endocrine Pancreas [Accessed 2 December 2022], WHO Classification of Tumours Editorial Board: Digestive System Tumours, 5th Edition, 2019, WHO Classification of Tumours Editorial Board: Endocrine and Neuroendocrine Tumours, 5th Edition, 2022, Am J Surg Pathol 2005;29:724, Ann Surg 2016;263:162
Board review style question #1
Which of the following is a core (mandatory) data element when reporting the resection specimens with pancreatic neuroendocrine tumors according to CAP?
- Distance of the tumor from all negative margins
- Functional type of the tumor
- Histologic grade of the tumor
- Patient’s clinical history
- Tumor necrosis
Board review style answer #1
Frozen section
Table of Contents
Definition / general | Procedure | Tips | Frozen section images | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Click here for the frozen section procedure topic
- This topic describes frozen section (intraoperative consultation) procedures for pancreaticoduodenectomy and distal pancreatectomy specimens
- Essential clinical history: none
Procedure
- Frozen section for primary diagnosis:
- Preoperative diagnostic modalities have mostly replaced frozen section practice for primary diagnosis
- However, it is still performed on occasional cases when the preoperative cytology or biopsy was inconclusive or when there are unusual intraoperative findings (Arch Pathol Lab Med 2022;146:84)
- Frozen section for distant metastasis:
- Since presence of any distant metastasis means unresectable disease for pancreatic ductal adenocarcinoma (PDAC) cases, surgeons may send suspected tissues (e.g., liver, peritoneum, distant lymph nodes, etc.) for frozen section examination
- If it reveals metastasis, the procedure will most likely be aborted (J Clin Pathol 2007;60:975)
- Frozen section for margin status:
- Main purpose for this request is to evaluate the completeness of the resection (Ann Surg Oncol 2013;20:3626)
- If the margin (i.e., pancreatic parenchyma / duct, biliary duct) is positive, the surgeon will resect additional tissue to achieve a clear margin
- For the pathologist, the main question is whether there is an invasive carcinoma or high grade dysplasia in the margin or not
- Presence of low grade dysplasia does not require further resection
- Frozen section for intraductal neoplasms:
- Sometimes surgeons may request intraoperative examination of an intraductal neoplasm to investigate if there is high grade dysplasia or invasive carcinoma
- However, both high grade dysplasia and invasive carcinoma are usually focal and an invasive carcinoma component may not be easy to identify grossly
- Therefore, it is not possible to fully exclude high grade dysplasia or invasive carcinoma during the frozen section and a discrepancy may occur between frozen section and permanent assessment (Ann Surg 2016;263:162)
Tips
- It can be challenging to differentiate pancreatic ductal adenocarcinoma from PanIN or a reactive process (e.g., chronic pancreatitis) in frozen section
- Findings in favor of pancreatic ductal adenocarcinoma include:
- Loss of normal lobular organization and haphazardly distributed ducts / glands (Semin Diagn Pathol 2004;21:268)
- Irregular shaped ducts with angular contours and incomplete lumen formation (Arch Pathol Lab Med 2002;126:1169, Semin Diagn Pathol 2004;21:268)
- Complex and atypical arrangement (e.g., cribriform, cord-like or single cell infiltration) (Arch Pathol Lab Med 2002;126:1169)
- Abnormal location of glands (i.e., adjacent to a thick walled, medium - small sized muscular artery) (Am J Surg Pathol 2004;28:613)
- Naked (solitary) duct in adipose tissue without an intervening stroma (Semin Diagn Pathol 2004;21:268)
- Perineural or vascular invasion
- Nuclear atypia, pleomorphism (i.e., variation in nuclear size of 4:1 or more within an individual gland), hyperchromatism, nuclear membrane irregularities, nuclear grooves, raisinoid / wrinkled nuclei (especially in the foamy gland pattern), mitotic figures, atypical mitosis, loss of polarity and large nucleoli (Arch Pathol Lab Med 2002;126:1169, Semin Diagn Pathol 2004;21:268)
- Cytoplasmic characteristics (e.g., foamy gland pattern pancreatic ductal adenocarcinoma is characterized by abundant foamy / microvesicular cytoplasm) (Am J Surg Pathol 2000;24:493)
- Intraluminal necrotic / cellular debris and plugs (Arch Pathol Lab Med 2002;126:1169, Semin Diagn Pathol 2004;21:268)
- Disorganized, basophilic and even myxoid stroma (Semin Diagn Pathol 2004;21:268)
- Sometimes, low grade gastric-like mucinous epithelium may be identified in the pancreatic parenchyma margin
- For such cases, it is difficult (if not impossible) and unnecessary to distinguish pancreatic intraepithelial neoplasia (PanIN) and intraductal papillary mucinous neoplasm (IPMN) during the intraoperative consultation
- It is sufficient to document that there is no high grade dysplasia or invasive carcinoma in the margin; low grade mucinous epithelium is identified in the margin and differential diagnoses include low grade PanIN and low grade IPMN (Ann Surg Oncol 2013;20:3626)
Frozen section images
Additional references
Board review style question #1
Which of the following is a typical reason for frozen section requests in the surgical management of pancreatic tumors?
- To evaluate a suspicious liver lesion
- To evaluate extranodal tumor extension in a metastatic peripancreatic lymph node
- To evaluate neoadjuvant treatment response
- To evaluate the histologic grade of the tumor
- To evaluate the mitotic index of the tumor
Board review style answer #1
Gastrinoma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology / etiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Pancreatic well differentiated neuroendocrine tumor that causes clinical symptoms secondary to secretion of gastrin
Essential features
- Functionally active and usually aggressive neuroendocrine tumor
- Gastrin secretion causes Zollinger-Ellison syndrome
- May occur sporadically or in the setting of MEN1
Terminology
- Pathology diagnosis should be well differentiated neuroendocrine tumor, as gastrinoma is a clinical determination
- Sometimes called G cell neoplasm
ICD coding
Epidemiology
- Roughly 20% of functional pancreatic neuroendocrine tumors are gastrinomas, though only about 5% of overall pancreatic neuroendocrine tumors are gastrinomas (Surg Pathol Clin 2014;7:559, World J Gastroenterol 2006;12:5440)
Sites
- Gastrinomas are more common in duodenum than in pancreas
- Rare examples of so called primary lymph node gastrinoma can occur:
- Gastrin producing tumors in lymph nodes with no GI or pancreatic primary, generally in patients with MEN1 (Am J Surg Pathol 2008;32:1101)
- Occur in gastrinoma triangle: from cystic and common bile ducts to the second and third portion of the duodenum to neck and body of the pancreas
- May be due to gastrin secreting neuroendocrine cells within these nodes or due to occult duodenal microgastrinomas with lymph node metastasis (Arch Pathol Lab Med 2000;124:832, Am J Surg Pathol 2008;32:1101)
Pathophysiology / etiology
- Hypersecretion of gastrin stimulates secretion of gastric acid by parietal cells, leading to ulcer formation
Etiology
- 80% of cases are sporadic; 20% are familial due to MEN1 (Hepatogastroenterology 2005;52:1668)
Clinical features
- Associated with hypersecretion of gastric acid and severe peptic ulceration
- 90% have ulcers (85% in duodenum / jejunum, 15% in stomach)
- 33% of patients have diarrhea
- May be metastatic at time of diagnosis
Diagnosis
- Glucagon provocative test is useful (Surg Today 2013;43:1281)
Radiology description
- Highly vascular and well circumscribed, as with other neuroendocrine tumors of pancreas
- Imaging may detect positive lymph nodes within the gastrinoma triangle (Diagn Interv Imaging 2016;97:1241)
- Octreotide scans have high sensitivity
Prognostic factors
- Sporadic tumors are usually solitary, aggressive and located in pancreas
- MEN1 cases are often multicentric, less aggressive and arise in duodenal wall (Gut 2007;56:606)
Case reports
- 25 year old woman with mass blocking the pancreatic duct (Surgery 2005;138:111)
- 37 year old woman with von Hippel-Lindau syndrome and 2 pancreas lesions (Rev Esp Enferm Dig 2017;109:154)
- 68 year old woman with abdominal pain and diarrhea (Oncol Lett 2016;11:3433)
- 73 year old man with pancreatic gastrinoma and endobronchial metastasis (Am J Respir Crit Care Med 2012;185:590)
Treatment
- H2 blockers for symptom management
- Surgical resection of tumor
Gross description
- Single or multiple lesions, most commonly in head of pancreas
- Firm, homogeneous, well circumscribed
Microscopic (histologic) description
- Nests of monotonous low grade neuroendocrine cells with salt and pepper nuclei and ample amphophilic cytoplasm, as with any other well differentiated neuroendocrine tumor
- Associated with pancreatic polypeptide cell hyperplasia (Hum Pathol 1997;28:149)
- Nonneoplastic pancreas may show large islets and nesidioblastosis
Microscopic (histologic) images
Positive stains
- Gastrin (though this does not establish the diagnosis)
- Synaptophysin, chromogranin
- PDX1, INSM1 (Am J Surg Pathol 2012;36:737)
Negative stains
Electron microscopy description
- Tumor cells may contain small, electron dense granules
Sample pathology report
- Pancreas and duodenum, Whipple procedure:
- Pancreatic neuroendocrine tumor, WHO grade 2 (see synoptic report and comment)
- Comment: The patient’s clinical history of Zollinger-Ellison syndrome is noted. This may be due to gastrin secretion by this tumor, which would make it a gastrinoma. Immunohistochemical stains for synaptophysin and chromogranin are positive and the Ki67 index is approximately 4.5%.
Differential diagnosis
- Nongastrin secreting pancreatic neuroendocrine tumor:
- No histopathologic differences
- Must be determined on clinical grounds
Board review style question #1
- Which of the following types of neuroendocrine tumor may arise primarily within a lymph node near the duodenum and pancreas?
- Gastrinoma
- Glucagonoma
- Insulinoma
- Somatostatinoma
- VIPoma
Board review style answer #1
Board review style question #2
- Gastrinomas arising sporadically, compared to those arising in the setting of MEN1, are more likely to be
- Benign clinically
- Cystic
- Located in the pancreas
- Multifocal
- WHO grade 1
Board review style answer #2
Glucagonoma (alpha cell tumors)
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Electron microscopy description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Rare, poorly characterized pancreatic neuroendocrine tumor that produces glucagon
Essential features
- WHO recognized diagnosis
- Produces glucagon, akin to alpha cells of the pancreas
- Clinical glucagonoma symptoms include necrolytic migratory erythema, diabetes, weight loss and anemia
Terminology
- Necrolytic migratory erythema: skin rash of legs, perineum, groin; rash becomes blisters with central clearing, heals with hyperpigmentation but without scarring in 7 - 14 days (Am J Surg Pathol 1986;10:445)
ICD coding
- ICD-10: D37.8 - Neoplasm of uncertain behavior of pancreas
Epidemiology
- Incidence rate of approximately 2.5/100,000,000 (Oncol Lett 2018;15:2749)
- Mean age 54 years
- More common in women
Sites
- More common in tail of pancreas
Pathophysiology
- Generally sporadic
- Patients with multiple endocrine neoplasia type 1 may have multiple small glucagonomas (with better prognosis)
Clinical features
- Causes glucagonoma syndrome (necrolytic migratory erythema, diabetes mellitus, weight loss, anemia, angular stomatitis)
- Necrolytic migratory erythema may become infected
- Roughly half of cases metastasize, often to liver
Diagnosis
- Determined clinically, not by immunohistochemical positivity for glucagon
Laboratory
- Elevated blood glucagon levels
Radiology description
- MRI: low signal intensity on T1, slightly high signal intensity on T2
Case reports
- 50 year old woman with pruritic rash (Oncol Lett 2015;10:1113)
- 51 year old woman with heart failure (Endocrinol Diabetes Metab Case Rep 2014;2014:140061)
- 62 year old woman with ovarian mass and history of glucagonoma (JOP 2013;14:510)
- 64 year old woman with erythematous rash (Case Rep Surg 2016;2016:1484089)
- 68 year old man with anemia and diabetes (J Hepatobiliary Pancreat Surg 2003;10:101)
Treatment
- Surgery is only curative option
- Somatostatin analogues may relieve symptoms (Clin Endocrinol (Oxf) 2011;74:593)
Clinical images
Gross description
- Average gross tumor size is 5.0 cm
Microscopic (histologic) description
- Nests of monotonous low grade neuroendocrine cells with salt and pepper nuclei and ample amphophilic cytoplasm
Microscopic (histologic) images
Positive stains
- Glucagon (though this does not establish the diagnosis)
- Synaptophysin and chromogranin
Electron microscopy description
- Single type secretory granules similar to normal alpha cell granules (Ultrastruct Pathol 1989;13:15)
Sample pathology report
- Pancreas and duodenum, Whipple procedure:
- Pancreatic neuroendocrine tumor, WHO grade 1 (see synoptic report and comment)
- Comment: The patient’s clinical history of necrolytic migratory erythema is noted. This may be due to glucagon secretion by this tumor, which would make it a glucagonoma. Immunohistochemical stains for synaptophysin and chromogranin are positive and the Ki67 index is approximately 1.2%.
Differential diagnosis
- Nonglucagon secreting pancreatic neuroendocrine tumor:
- Must be determined on clinical grounds (Proc (Bayl Univ Med Cent) 2015;28:46)
Board review style question #1
- Which of the following is a potential clinical symptom of pancreatic glucagonoma?
- Angular stomatitis
- Hypochlorhydria
- Hypoglycemia
- Water diarrhea
Board review style answer #1
A. Angular stomatitis (though the most common finding is necrolytic migratory erythema). Hypochlorhydria is sometimes seen in somatostatinoma. Hypoglycemia is typical of insulinoma. Watery diarrhea is typical of VIPoma.
Comment Here
Reference: Glucagonoma (alpha cell tumors)
Comment Here
Reference: Glucagonoma (alpha cell tumors)
Board review style question #2
- Which of the following is necessary to confirm a diagnosis of glucagonoma in a patient with a pancreatic mass?
- Classic radiologic findings
- Clinical glucagonoma type symptoms
- Immunohistochemical positivity for glucagon
- Specific histologic findings
Board review style answer #2
B. Clinical glucagonoma type symptoms. The diagnosis of glucagonoma is made on clinical grounds in the setting of a pancreatic mass lesion.
Comment Here
Reference: Glucagonoma (alpha cell tumors)
Comment Here
Reference: Glucagonoma (alpha cell tumors)
Grossing
Table of Contents
Definition / general | Procedure | Sections to obtain | Tips | Gross description | Gross images | Gross template samples | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- This topic describes how to gross specimens obtained from pancreaticoduodenectomy, distal pancreatectomy and total pancreatectomy procedures
- Essential clinical history: none
Procedure
Pancreaticoduodenectomy (Whipple) specimens:
Distal pancreatectomy specimens:
Total pancreatectomy specimens:
- Orientation and external examination
- Specimen consists of pancreatic head and duodenum and occasionally distal stomach and gallbladder
- Examine the specimen freshly
- Measure the overall specimen and each organ / structure
- Identify the trapezoid:
- There is a trapezoid shaped area in the posteromedian aspect of the pancreatic head (Am J Surg Pathol 2014;38:480)
- The center of the trapezoid is a concave, smooth and shiny surfaced firm area, on which the superior mesenteric vein / portal vein and superior mesenteric artery lie
- This area is referred to as the vascular groove or vascular bed (see figure 1)
- Identify and ink the important structures and margins:
- Trapezoid is formed by the pancreatic neck margin on the left, the uncinate margin on the right and the common bile duct (CBD) on the top (see figure 1)
- Pancreatic duct (PD) is in the upper half of the pancreatic neck margin towards the vascular groove
- Ink the pancreatic neck margin, uncinate margin and common bile duct margin with different colors (e.g., green, black and red, respectively) (see figure 2)
- Although it is not a surgical margin, ink the vascular groove area (e.g., orange) as well, after checking if it includes a portion of the portal vein (Am J Surg Pathol 2014;38:480)
- Check if a portion of the portal vein is present in the vascular groove area: if present, ink the superior and inferior margins using different colors
- Identify and ink the anterior and posterior free peritoneal surfaces:
- Anterior free surface is convex shaped and contains abundant fat tissue
- Pancreatic head - duodenum transition is abrupt and easily identifiable on this surface (see figure 3)
- However, the posterior free surface is flat and shiny in appearance while the pancreatic head - duodenum transition in this area is smoother and barely identifiable (see figure 4)
- If not sutured or stapled, the common bile duct margin can be seen readily on this aspect
- Although they are not surgical margins, it is encouraged to ink the anterior and posterior free surfaces in different colors (e.g., blue for anterior, black for posterior) (Am J Surg Pathol 2014;38:480)
- Removing the margins
- Bile duct margin:
- Shave and submit enface (see figure 5) (Am J Surg Pathol 2014;38:480)
- Bile duct margin corresponds to the CBD margin in most of the specimens
- However, if gallbladder is attached to the specimen, it corresponds to common hepatic duct (CHD) margin
- If the specimen includes cystic duct (CD) without attached gallbladder, it corresponds to both CHD and CD margins
- Pancreatic neck margin (including PD): shave and submit enface (see figure 6) (Am J Surg Pathol 2014;38:480)
- Uncinate (retroperitoneal or posteroinferior or superior mesenteric artery) margin:
- This margin is formed by blunt dissection of the pancreatic head from the retroperitoneal soft tissues in the posterior inferior aspect of the uncinate process, rather than a surgical cut
- Shave and submit entirely as 3 - 4 mm thick bread loaf sections in 2 or 3 cassettes (see figure 7) (Am J Surg Pathol 2014;38:480)
- Portal vein margins (if present): superior and inferior margins of the vein can be shaved and submitted enface or can be submitted as a part of the main perpendicular sections of the vessel wall
- Vascular groove (vascular bed or superior mesenteric / portal vein bed) surface:
- Submitting 1 perpendicular section that shows the area closest to the tumor is enough for most of the cases
- However, shaving and submitting bread loaf sections just like the uncinate margin is another option
- Proximal (stomach / duodenum) and distal (duodenum) margins: submit 1 shaved section from each margin
- Bile duct margin:
- Opening the duodenum and examining intraduodenal aspect
- Open through the antipancreatic (antiampullary) border (Am J Surg Pathol 2014;38:480)
- Inspect the ampullary region to identify and describe any lesions (e.g., intra / periampullary localization; mass forming, papillary or mucinous features, etc.)
- Inspect the minor (accessory) ampulla, if present:
- It is located ~2 cm anterior and superior to the main ampulla and looks like a small bump / nodule / polyp (see figure 8)
- In some cases, the PD might open into minor ampulla (Am J Surg Pathol 2014;38:480)
- Bivalving and sectioning
- Although there are several approaches in sectioning the pancreatic head, bivalving is the most commonly used method as it allows for assessment of the relation of tumor with both the CBD and PD and for proper evaluation and documentation of ampullary tumors (Am J Surg Pathol 2014;38:480)
- Bivalve the pancreatic head by placing the probes in the CBD and PD to the ampulla; probe the CBD all the way to the ampulla and the PD at least as far as it can be traced
- Start sectioning using a long knife (e.g., 6 inches)
- Knife needs to be angled as if cutting the probes themselves (see figure 9)
- Carefully examine and re-angle after each stroke (Am J Surg Pathol 2014;38:480)
- Finally, the pancreatic head should be separated into anterior and posterior halves (see figure 10)
- Identifying the tumor(s)
- Inspect and describe the following features of the tumor(s): localization, size (measurements), characteristics (e.g., color, solid / cystic, necrotic / hemorrhagic / papillary areas, soft / firm, well circumscribed / ill defined), association with / distance from the CBD, PD, ampulla, margins and surfaces
- Harvest fresh tumor samples for the tumor bank as needed
- Identifying variant anatomies (i.e., low union)
- Normal (most common) insertion of the CD into the CHD is at least 5 mm above the pancreatic parenchyma border
- Other levels of insertion (i.e., within the 5 mm of pancreatic parenchyma border or even intrapancreatic) are considered as low union, which have been shown to play a significant role in pancreatobiliary and periampullary carcinogenesis (HPB (Oxford) 2020;22:1675)
- Therefore, when present, it should be documented (see figure 11)
- Lymph node dissection
- Peripancreatic lymph nodes are separated into 8 regions as peri-CBD, anterior pancreatic, anterior pancreatoduodenal, superior pancreatic, inferior pancreatic, posterior pancreatic, posterior pancreatoduodenal and uncinate margin lymph nodes; however, these regions currently do not play any role in pN staging
- Shave off all peripancreatic soft tissues using the orange peeling method (see figures 12 and 13) including the priorly inked anterior and posterior free surfaces (Am J Surg Pathol 2014;38:480, Mod Pathol 2009;22:107)
- Submit entirely in subsequent cassettes
- Make a note of sectioned lymph nodes to prevent overcounting
- Dissect and submit perigastric and periduodenal lymph nodes using conventional methods
- Fixation and harvesting
- Let the specimen be properly fixed in formalin for 24 - 48 hours
- After fixation, submit at least 1 section per centimeter of the tumor for solid tumors
- Increase the number of the sections or even submit it entirely if the tumor was treated (Am J Surg Pathol 2022;46:754)
- Unless there is a grossly visible invasion, cystic / intraductal tumors should be submitted entirely to rule out invasion (Ann Surg 2016;263:162)
- Include proper sections to show any association between the tumor and important structures (i.e., ampulla, CBD, PD, vascular bed, duodenum, etc.)
- Include nontumoral tissue sections from the pancreas and the adjacent organs
- Taking gross images
- Always take several images of the specimen in both fresh (before and after inking, bivalving and sectioning) and fixed states
Distal pancreatectomy specimens:
- Orientation, external examination and removing the margin
- Specimen consists of body and tail of the pancreas with or without spleen attached
- Freshly examine the specimen
- Measure the overall specimen and each organ / structure
- Identify splenic artery on the superior and splenic vein on the posterior surface of the pancreas (see figures 14 and 15)
- Ink anterior and posterior surfaces in different colors (e.g., blue for anterior, black for posterior)
- Identify, ink and remove the pancreatic parenchymal (duct) margin; shave and submit enface
- Sectioning
- One approach is serial sectioning of the entire pancreas and spleen at 0.5 - 1 cm intervals starting from the proximal edge of the pancreas (see figure 16)
- Alternatively, entire pancreas and spleen can be bivalved and sectioned with the guidance of a probe placed in the PD, starting from the proximal edge of the pancreas (see figure 17)
- Then, perpendicular (to the surfaces) sections are submitted from each half
- Identifying the tumor(s)
- Inspect and describe the following features of the tumor(s): localization, size (measurements), characteristics (e.g., color, solid / cystic, necrotic / hemorrhagic / papillary areas, soft / firm, well circumscribed / ill defined), association with / distance from the PD, pancreatic parenchymal margin, anterior and posterior surfaces and spleen
- Harvest fresh tumor samples for the tumor bank as needed
- Lymph node dissection
- Dissect the peripancreatic and splenic hilar lymph nodes using conventional methods
- Fixation and harvesting
- Let the specimen be properly fixed in formalin for 24 - 48 hours
- After fixation, submit at least 1 section per centimeter of the tumor for solid tumors
- Increase the number of the sections or even submit it entirely if the tumor was treated (Am J Surg Pathol 2022;46:754)
- Unless there is a grossly visible invasion, cystic / intraductal tumors should be submitted entirely to rule out invasion (Ann Surg 2016;263:162)
- Include proper sections to show any association between the tumor and important structures (i.e., PD, spleen, splenic hilum, etc.)
- Include nontumoral tissue sections from the pancreas and the spleen
- Taking gross images
- Always take several images of the specimen in both fresh (before and after inking, bivalving and sectioning) and fixed states
Total pancreatectomy specimens:
- Specimen consists of pancreatic head, neck, body and tail, in addition to duodenum, spleen and occasionally distal stomach and gallbladder (see figure 18)
- Follow the steps for pancreaticoduodenectomy and distal pancreatectomy for the appropriate portions of the specimen (see above)
Sections to obtain
- Tumor:
- Submit representative tumor sections for a total of 1 cassette per 1 cm of the largest tumor dimension; increase the number as needed (e.g., if the tumor is cystic / intraductal, if the patient had neoadjuvant therapy, etc.) (Am J Surg Pathol 2022;46:754, Ann Surg 2016;263:162)
- Include proper sections to show any association between the tumor and adjacent structures
- Margins: submit the proper margins for pancreaticoduodenectomy, distal pancreatectomy and total pancreatomy specimens as described above
- Lymph nodes: submit all dissected lymph nodes (and the entire peripancreatic soft tissue for pancreaticoduodenectomy specimens) properly (Am J Surg Pathol 2014;38:480)
- Nontumoral adjacent organs: submit uninvolved tissue sections from the pancreas and adjacent organs in 1 cassette for each organ
Tips
- Distinguishing CBD and PD:
- Probing the PD:
- If the PD orifice is not easily identified in the pancreatic neck margin, squishing the pancreatic head and looking for the pancreatic fluid coming from the PD might be useful
- However it needs to be done carefully, as the pancreatic fluid can only be seen in the first few attempts
- In some cases, probing of the PD is problematic, especially in the distal part, because of its narrow and kinked nature in contrast to the CBD which is always probe-patent
- One option is retroprobing the PD from the ampulla
- If it does not work, another option is to probe the PD as far as it can be traced and past that point using the guidance of the CBD alone
- If the PD orifice is not easily identified in the pancreatic neck margin, squishing the pancreatic head and looking for the pancreatic fluid coming from the PD might be useful
- Cystic tumors:
- Examine the nature of cystic fluid (e.g., mucinous, clear, hemorrhagic, etc.)
- Assess the association of the cyst with main / branch pancreatic ducts
- Pay attention to the solid / granular / papillary / nodular areas of the cyst, if any, since they have value in evaluating the invasion
- Ampullary tumors:
- Ampullary carcinoma is defined by the bulk of the tumor (> 75%) being located in the ampulla
- Classification of ampullary carcinomas (i.e., intra-ampullary, ampullary ductal, periampullary duodenal and ampullary, NOS) depends on a proper gross examination (Am J Surg Pathol 2012;36:1592)
- Sections should be taken perpendicularly to make measurement of the invasion depth possible; they should also include the ampulla, pancreas, periampullary duodenum and periampullary soft tissues
- Nonampullary duodenal tumors:
- By definition, the epicenter of a nonampullary duodenal carcinoma is entirely located in the duodenum and the ampulla is uninvolved
- Distal CBD tumors:
- Distal CBD carcinoma is defined by whether the bulk of the tumor is located circumferentially around the CBD or if an associated precursor intraductal neoplasm is identified in the CBD (Mod Pathol 2016;29:1358, J Gastrointest Surg 2016;20:953)
- Taking gross images of the specimen in both fresh and fixed states is helpful for microscopic examination and reporting
Gross description
- Pancreatic ductal adenocarcinoma (PDAC):
- Ill defined solid mass with irregular / infiltrating borders, gray-white-tan greenish in color, firm and scirrhous in consistency (Am J Surg Pathol 2014;38:480)
- Necrosis or cystic degeneration may be present
- Pancreatic neuroendocrine carcinoma (NEC):
- Ill defined or vaguely nodular solid mass with relatively irregular / infiltrating borders, tan-white-brown-red or yellowish in color, usually fleshier than PDAC
- Necrosis and hemorrhage are often easily identified (Am J Surg Pathol 2014;38:437)
- Pancreatic neuroendocrine tumor (PanNET):
- Well circumscribed solid mass with pushing borders, gray-tan pinkish in color, soft and fleshy in consistency (Endocr Pathol 2014;25:65)
- Cystic degeneration or accompanying fibrosis, calcification and ossification may be present
- Acinar cell carcinoma (ACC):
- Well circumscribed and sometimes encapsulated, solid, bulky mass, gray-tan pinkish reddish in color, fleshy in consistency (Semin Diagn Pathol 2016;33:307)
- Solid pseudopapillary tumor (SPN):
- Well circumscribed and encapsulated mass, which reveals solid and cystic areas, tan-brown pinkish in color, fleshy and friable in consistency
- Hemorrhage and necrosis are observed in most cases (Arch Pathol Lab Med 2009;133:423)
- Intraductal papillary mucinous neoplasm (IPMN):
- Cystic mass in the main or branch pancreatic ducts, may reveal mucin or papillary structures (Am J Surg Pathol 2014;38:480, Semin Diagn Pathol 2014;31:452)
- Intraductal oncocytic papillary neoplasm (IOPN):
- Multilocular or unilocular cystic mass in the main pancreatic duct with friable papillary / nodular projections (Mod Pathol 2016;29:1058, Am J Surg Pathol 2019;43:656)
- Intraductal tubulopapillary neoplasm (ITPN):
- Multinodular / polypoid / solid mass in the main pancreatic duct, without a very distinct cyst formation and papillary projections (Semin Diagn Pathol 2014;31:452, Mod Pathol 2017;30:1760)
- Mucinous cystic neoplasm (MCN):
- Well circumscribed and large cystic mass without any association with ductal system, may reveal papillary / trabecular / nodular / solid areas (Arch Pathol Lab Med 2009;133:423, J Gastrointest Surg 2008;12:401)
- Serous cystadenoma (SCA):
- Well circumscribed and multicystic (sponge-like) tumor, usually with a central scar and without any association with ductal system (Am J Surg Pathol 2015;39:1597, Semin Diagn Pathol 2014;31:475)
- Rare cases may reveal oligocystic or even solid appearance
Gross template samples
- Pancreaticoduodenectomy (Whipple) specimens:
- The specimen is received (fresh), labeled as pancreatoduodenectomy and consists of pancreatic head, duodenum and (other organs). The pancreas measures ( ) x ( ) x ( ) cm, the duodenum measures ( ) cm in length and ( ) cm in circumference, (other organs) measures ( ) cm. External surface of the pancreas is ( ). The pancreatic neck / duct margin is inked (green), the bile duct margin is inked (red), the uncinate margin is inked (black), the vascular groove is inked (orange), posterior surface of the pancreas is inked (black) and anterior surface of the pancreas is inked (blue). The bile duct and pancreatic neck / duct margins are shaved and submitted enface. The uncinate margin is shaved, serially sectioned and entirely submitted.
- Proximal and distal margins of the duodenum are submitted. Duodenum is opened through the antipancreatic border. Ampulla reveals ( ). The bile duct and pancreatic duct are probed.
- Bivalving of the pancreas reveals that the pancreatic duct is ( ) cm in length with a luminal circumference of ( ) cm, the bile duct is ( ) cm in length with a luminal circumference of ( ) cm. Cystic duct is present / absent in the specimen. If cystic duct is present, it is ( ) cm in length, with a luminal circumference of ( ) cm and it adjoins the common hepatic duct at ( ). There is a ( ) x ( ) x ( ) cm solid / cystic mass, ( ) in color, with a firm / soft / fleshy cut surface, ill defined / well circumscribed borders, located at the ( ). The tumor is in association with ( ). ( ) are involved by the tumor. The tumor is in ( ) cm distance from the ( ). Representative sections of the tumor submitted. Ampulla is submitted. Entire peripancreatic soft tissues and dissected periduodenal lymph nodes are submitted. The remaining pancreatic cut surface reveals ( ) / unremarkable. The remaining duodenal mucosal surface reveals ( ) / unremarkable. Other organs on the specimen reveal ( ) / unremarkable. Representative sections of the remaining pancreas and adjacent organs are submitted.
- Distal pancreatectomy specimens:
- The specimen is received (fresh), labeled as distal pancreatectomy and splenectomy, and consists of distal pancreas and spleen. The distal pancreas measures ( ) x ( ) x ( ) cm. Spleen is () gr and measures ( ) x ( ) x ( ) cm. The pancreatic parenchymal margin is shaved and submitted enface. The anterior surface of the pancreas is inked (blue) and the posterior surface is inked (black). Serially sectioning (or bivalving) of the specimen reveals a ( ) x ( ) x ( ) cm solid / cystic mass, ( ) in color, with a firm / soft / fleshy cut surface, ill defined / well circumscribed borders, located at the ( ). The tumor is in association with ( ). ( ) are involved by the tumor. The tumor is in ( ) cm distance from the ( ). Representative sections of the tumor submitted. Peripancreatic and perihilar lymph nodes are dissected and submitted. The remaining pancreatic cut surface reveals ( ) / unremarkable. Sections of the spleen reveal ( ) / unremarkable. Representative sections of the remaining pancreas and spleen are submitted.
- Total pancreatectomy specimens:
- See the gross template samples for pancreaticoduodenectomy and distal pancreatectomy specimens
Additional references
Board review style question #1
Gross examination of a pancreaticoduodenectomy (Whipple) specimen reveals a white-tan colored, firm, solid mass with ill defined borders, located in the pancreatic head. Which of the following would be included in the initial differential diagnosis?
- Acinar cell carcinoma
- Pancreatic ductal adenocarcinoma
- Solid pseudopapillary tumor
- Well differentiated pancreatic neuroendocrine tumor, grade 1
- Well differentiated pancreatic neuroendocrine tumor, grade 3
Board review style answer #1
Heterotopic pancreas
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Positive stains | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Pancreatic tissue that is anatomically separate from the main pancreatic gland and has no vascular or ductal connection to it
Essential features
- Pancreatic tissue that is anatomically separate from the main pancreatic gland and has no ductal or vascular connections to it the main pancreatic gland
- Composed of a variable mixture of pancreatic acini, ducts and islets
- Most common in stomach but may occur throughout GI tract; and rarely in extragastrointestinal sites
- Often incidental finding
- May develop the same diseases as the main pancreatic gland (e.g., pancreatitis or pancreatic neoplasms)
- May be asymptomatic or depending on size and location, may present with symptoms such as abdominal pain, GI bleed or obstruction
- Surgical management if symptomatic or if diagnosis is unknown
Terminology
- Ectopic pancreas, heterotopic pancreas, pancreatic rest, accessory pancreas, aberrant pancreas, pancreatic choristoma
ICD coding
- ICD-10: Q45.3 - other congenital malformations of pancreas and duct
Epidemiology
- M:F = 3:1
- Most commonly identified in fifth to sixth decades of life
- Incidence of 0.5 - 13% in autopsy studies
- Incidental finding in 0.2 - 0.9% of upper abdominal surgeries and gastrectomies, respectively
- Reference: Gastroenterology Res 2021;14:45
Sites
- Can occur anywhere in the gastrointestinal tract (GI)
- Most common in upper GI tract (stomach, duodenum and proximal jejunum) (Gastroenterology Res 2021;14:45)
- Less common in Meckel diverticulum, esophagus, ileum, colon, biliary tree, mesentery, spleen and lung (Gastroenterology Res 2021;14:45, Saudi Med J 2018;39:834)
Pathophysiology
- Exact embryologic mechanism unknown
- Misplacement theory: portion(s) of pancreas become separated during rotation of foregut and develop into mature pancreatic tissue (Radiographics 2017;37:484, Arch Pathol Lab Med 1999;123:707)
- Metaplasia theory: pancreatic metaplasia of endodermal tissue that has migrated to submucosa (Radiographics 2017;37:484, Arch Pathol Lab Med 1999;123:707)
- Totipotent cell theory: endodermal cells in GI tract differentiate into pancreatic tissue (Radiographics 2017;37:484)
Clinical features
- Often asymptomatic (Gastroenterology Res 2021;14:45, Radiographics 2017;37:484)
- Depending on location and size, can present with symptoms of abdominal pain, melena, obstruction or intussusception (Gastroenterology Res 2021;14:45, Radiographics 2017;37:484)
- May develop the same diseases as the main pancreas, including acute or chronic pancreatitis and neoplasms (Radiographics 2017;37:484)
Diagnosis
- Frequently is an incidental finding
- Radiographically, typically appears as ovoid intramural mass (Radiographics 2017;37:484)
- Endoscopically presents as a solid submucosal mass (Radiographics 2017;37:484)
- Definitive diagnosis requires histologic examination
Laboratory
- Most cases lack specific lab abnormalities
- Rare cases may show lab abnormalities associated with disease of the heterotopic pancreatic tissue:
- Microcytic anemia related to GI bleed caused by heterotopic tissue (BMC Gastroenterol 2019;19:57)
- Elevated serum amylase in heterotopic pancreas with acute pancreatitis (Case Rep Gastrointest Med 2018;2018:5640379)
- Elevated CA 19-9 in adenocarcinoma arising in heterotopic pancreas (Int Surg 2012;97:351)
Radiology description
- Endoscopically appears as a round, subepithelial lesion, often with a central depression that represents the opening of the duct (Insights Imaging 2021;12:144, Gastroenterol Rep (Oxf) 2017;5:237)
- Endoscopic ultrasound (EUS) shows solid, submucosal mass that is hypoechoic relative to the hyperechoic submucosa and isoechoic relative to the muscularis propria (Insights Imaging 2021;12:144)
- Typical computed tomography (CT) findings include small (< 3 cm), oval, intramural mass with microlobulated margins and an endoluminal growth pattern (Insights Imaging 2021;12:144, Radiographics 2017;37:484)
- Acinus dominant lesions show avid homogenous enhancement with intravenous contrast
- Duct dominant lesions are hypovascular and heterogenous
- By magnetic resonance imaging (MRI), heterotopic pancreatic tissue will show similar characteristics to the native gland (Insights Imaging 2021;12:144, Radiographics 2017;37:484)
- Magnetic resonance cholangiopancreatography (MRCP) can show ectopic duct sign (i.e., a dilated ectopic pancreatic duct) (Clin Radiol 2010;65:403)
Radiology images
Prognostic factors
- Overall good prognosis but with potential to vary, depending on disease state of heterotopic tissue
- Patients with adenocarcinoma arising in pancreatic heterotopia may do better, compared to those with tumors arising in the main gland (BMC Surg 2017;17:53)
Case reports
- 9 month old girl with pancreatic heterotopia in omphalomesenteric duct remnant (Diagn Pathol 2017;12:49)
- 9 year old boy with inflamed Meckel diverticulum with elevated serum amylase mimicking acute pancreatitis (Indian J Crit Care Med 2017;21:789)
- 27 year old woman with large pancreatic heterotopia presenting with gastric outlet obstruction (Gastroenterol Rep (Oxf) 2017;5:237)
- 48 year old man with pancreatic heterotopia in jejunum and elevated serum chromogranin A (Cureus 2021;13:e15409)
- 71 year old woman with gastric pancreatic heterotopia with intraductal papillary mucinous neoplasm (IPMN) with GNAS mutation (World J Surg Oncol 2021;19:309)
Treatment
- Surgery, if symptomatic
Clinical images
Gross description
- Usually is a submucosal based lesion but may involve other layers of GI tract (Hum Pathol 2016;55:135)
- Well demarcated borders with vaguely lobular appearance (Hum Pathol 2016;55:135)
- On cut surface, typically appears pale yellowish white in color with solid and firm consistency (Hum Pathol 2016;55:135)
- Central mucosal dimple (opening of pancreatic ducts) may be visible
Gross images
Microscopic (histologic) description
- In GI tract, typically centered in submucosa but may involve other layers
- Rarely may involve extragastrointestinal sites
- Variable mixture of pancreatic acini, ducts, islets
- May contain hypertrophic smooth muscle bundles
- 4 types of pancreatic heterotopia, originally described by Heinrich in 1909 and modified in 1973 by Gasper-Fuentes (Radiographics 2017;37:484):
- Type 1:
- Contains all elements of normal pancreatic tissue (acini, ducts and islets)
- Type 2:
- Acini and ducts (no islets) in Heinrich classification
- Ducts only in Gasper-Fuentes classification
- Type 3:
- Ducts only in Heinrich classification
- Acini only in Gasper-Fuentes classification
- Type 4:
- Does not exist in Heinrich classification
- Islet cells only in Gasper-Fuentes classification (very rare) (Arch Pathol Lab Med 2002;126:464)
- Type 1:
- May show the same pathologic changes as the main gland, including acute or chronic pancreatitis, pseudocyst formation or neoplasia
Microscopic (histologic) images
Contributed by Kenechukwu Ojukwu, M.D., M.P.P. and Danielle Hutchings, M.D.
Pancreatic heterotopia in the stomach
Cytology description
- Predominantly pancreatic acini: polygonal cells with abundant granular cytoplasm and eccentric nuclei
- Ductal structures: cuboidal to columnar cells, honeycomb sheets
- Islet cells usually not seen
- Reference: World J Gastroenterol 2015;21:2367
Cytology images
Positive stains
- Heterotopic pancreas shows staining similar to the native gland
- Acinar cells: positive for trypsin, chymotrypsin, PAS and BCL10 (Virchows Arch 2009;454:133)
- Ductal epithelial cells: positive for CK7, CK8 / CK18
- Islet cells: positive for synaptophysin, chromogranin A and INSM1 (Am J Clin Pathol 2015;144:579)
Sample pathology report
- Gastric antrum, endoscopic mucosal resection:
- Pancreatic heterotopia involving submucosa and focally mucosa
- Overlying unremarkable antral mucosa
Differential diagnosis
- Pancreatic metaplasia:
- Involves mucosa
- Acinar cells only, no ducts or islet cells present
- Adenocarcinoma:
- Duct predominant heterotopia may show branching pattern and be mistaken at low power for adenocarcinoma
- Adenocarcinoma shows infiltrative growth, irregular glands, desmoplasia and cytologic atypia
Additional references
Board review style question #1
Board review style answer #1
C. Meckel diverticulum with pancreatic and gastric heterotopia
Comment Here
Reference: Heterotopic pancreas
Comment Here
Reference: Heterotopic pancreas
Board review style question #2
A 55 year old man presents with persistent anemia, abdominal pain and intermittent melena. Endoscopy reveals a subepithelial gastric antral mass with overlying umbilicated mucosa. Endoscopic mucosal resection shows the images above. What is the most likely diagnosis?
- Gastric adenocarcinoma
- Heterotopic pancreas
- Metastatic adenocarcinoma
- Pancreatic metaplasia
Board review style answer #2
Insulinoma (beta cell tumor)
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Rare neuroendocrine neoplasm of pancreas with functional production of insulin
Essential features
- Tumor secretes insulin (analogous to beta cell production in normal pancreatic islets) and thus clinical hyperinsulinemic hypoglycemia is required for this diagnosis
- Majority of insulinomas are small (< 2 cm) and benign
- Multiple insulinomas associated with multiple endocrine neoplasia type 1 (MEN1) syndrome
Terminology
- Beta cell tumor
ICD coding
Epidemiology
- Usually seen in older adults
- More common in females (Ann Surg 2008;247:165)
- Overall most common functioning pancreatic neuroendocrine neoplasm (J Gastroenterol 2015;50:58)
- Majority are benign; 5 - 10% are malignant (Best Pract Res Clin Gastroenterol 2005;19:783)
Sites
- Pancreas, evenly distributed (Pancreas 2014;43:675, J Hepatobiliary Pancreat Sci 2019;26:383)
- Extrapancreatic sites, such as duodenum or hilum of the spleen, are exceedingly rare (J Hepatobiliary Pancreat Sci 2019;26:383)
Pathophysiology
- Unknown
Etiology
- Mostly sporadic; familial cases are associated with MEN1 or MAFA germline mutations (Endocr J 2012;59:859, Proc Natl Acad Sci U S A 2018;115:1027)
Clinical features
- Whipple triad: symptoms of hypoglycemia (stupor, confusion, loss of consciousness), glucose < 45 mg/dL, symptoms relieved by glucose or symptoms caused by fasting or exercise
- 10 - 15% associated with MEN1 syndrome; age < 20 years is suggestive of MEN1 (usually multiple tumors are seen) (Endocr J 2012;59:859, Best Pract Res Clin Gastroenterol 2005;19:783)
Diagnosis
- Clinical diagnosis with demonstration of hyperinsulinemic hypoglycemia
- 72 hour fast is the gold standard for diagnosis (Endocrinol Metab Clin North Am 1999;28:519)
- Imaging based modalities such as CT, ultrasound, MRI, PET or radiolabeled scintigraphy (Neuroendocrinology 2009;90:167, Best Pract Res Clin Endocrinol Metab 2005;19:311, J Clin Endocrinol Metab 2009;94:4398)
- Arteriography was once the gold standard but has been obviated by other, less invasive, modalities and generally is restricted for difficult cases; transhepatic portal venous sampling and selective arterial calcium stimulation with venous sampling are additional more invasive methods (PLoS One 2019;14:e0224928)
Laboratory
- Demonstration of hyperinsulinemic hypoglycemia
- Blood glucose ≤ 45 mg/dL, insulin ≥ 43 pmol/L, C peptide ≥ 200 pmol/L, no sulfonylurea in plasma (Best Pract Res Clin Gastroenterol 2005;19:783)
Radiology description
- CT shows higher attenuation within the lesion than surrounding uninvolved pancreas in venous contrast enhancement phase
- On ultrasound, the lesion is characterized by low echogenicity and hypervascularity
- MRI and radiolabeled glucagon-like peptide 1 receptor (GLP-1R) scintigraphy has been used to localize small insulinomas (J Clin Endocrinol Metab 2009;94:4398)
- Reference: Neuroendocrinology 2009;90:167
Prognostic factors
- Stage and grade are important prognostic factors (Neoplasma 2015;62:484)
- Increased risk for metastasis and reduced survival in the following:
- Tumor ≥ 2 cm (Neoplasma 2015;62:484, Endocr Pathol 2020;31:108)
- ARX immunohistochemical (IHC) protein expression (Endocr Pathol 2020;31:108)
- Loss of DAXX or ATRX by IHC stain and alternative lengthening of telomeres (ALT) (Gastroenterology 2014;146:453, Endocr Pathol 2020;31:108)
- High Ki67 labeling index and high mitotic count are unfavorable prognostic factors (see well differentiated neuroendocrine tumor for additional details on grading)
- Insulinomas generally have a more favorable prognosis compared with nonfunctional tumors, as well as other functioning tumors (Surg Pathol Clin 2016;9:595, Front Med 2016;10:444)
- Proteomic study revealed low tumor protein D52 expression as a strong independent prognostic factor for both recurrence free and overall disease related survival (Mod Pathol 2015;28:69)
Case reports
- 20 year old slender, lean man complained of increased hunger, tremor and frequent seizures with worsening symptoms for 4 months (Cureus 2022;14:e23414)
- 32 year old woman with long history of prolactinoma and secondary amenorrhea presented with hypoglycemia (BMC Endocr Disord 2022;22:108)
- 50 year old man transiently lost consciousness while piloting a helicopter rescue (Med Lav 2022;113:e2022007)
- 83 year old woman with recurrent episodes of delirium occurring overnight, associated with hypoglycemia (Age Ageing 2022;51:afac055)
- 84 year old nondiabetic woman presented with recurrent falls and hypoglycemic episodes (Clin Med (Lond) 2022;22:90)
Treatment
- Definitive surgical resection, enucleation is preferred if possible (J Laparoendosc Adv Surg Tech A 2022 Apr 19 [Epub ahead of print])
- Symptomatic control with somatostatin analogs (octreotide) (Neoplasma 2015;62:484)
Gross description
- Most commonly a solitary, well delineated lesion with tan-yellow homogenous cut surface, with or without hemorrhage (Surg Pathol Clin 2016;9:595)
Gross images
Microscopic (histologic) description
- Trabecular, nested, gyriform or solid architecture
- Monotonous cells demonstrating round nuclei with salt and pepper-like chromatin and abundant cytoplasm
- Amyloid stromal deposition may be seen (nonspecific) (Arch Pathol Lab Med 1978;102:227, Endocr Pathol 2021;32:318)
Microscopic (histologic) images
Cytology description
Positive stains
- Synaptophysin, chromogranin, ISL1, INSM1 (Am J Surg Pathol 2013;37:399)
- Insulin (positivity is not required for diagnosis), proinsulin (50%), amylin, islet amyloid polypeptide (Am J Surg Pathol 2013;37:399)
- PDX1 (beta cell marker) (Endocr Pathol 2020;31:108):
- Positive in indolent insulinoma; variable staining pattern in aggressive insulinoma
- ARX (alpha cell marker) (Endocr Pathol 2020;31:108):
- Negative in indolent insulinoma; positive in aggressive insulinoma
Negative stains
- Beta catenin (normal membranous expression)
- PDX1 (beta cell marker) (Endocr Pathol 2020;31:108):
- Positive in indolent insulinoma; variable staining pattern in aggressive insulinoma
- ARX (alpha cell marker) (Endocr Pathol 2020;31:108):
- Negative in indolent insulinoma; positive in aggressive insulinoma
Electron microscopy description
- Round secretory granules with paracrystalline content (similar to granules seen in beta cells of normal pancreatic islets) (Nouv Presse Med 1977;6:3713)
Molecular / cytogenetics description
- MEN1, DAXX, ATRX, PTEN and TSC2 mutations as those seen in PanNET, are rare in insulinoma (J Hepatobiliary Pancreat Sci 2019;26:383)
- A subset (30%) showed recurrent p.T372R mutation in YY1 gene (Nat Commun 2013;4:2810, J Clin Endocrinol Metab 2015;100:E776)
- A subset showed alternative lengthening of telomere phenotype, loss of DAXX / ATRX and loss of CDKN2A, which is associated with metastatic disease and closely related to PanNET (Endocr Pathol 2020;31:108)
Sample pathology report
- Pancreas, distal pancreatectomy:
- Well differentiated neuroendocrine tumor, WHO grade __, functional, clinically consistent with insulinoma
Differential diagnosis
- Noninsulin secreting pancreatic neuroendocrine tumor (see well differentiated neuroendocrine tumor and neuroendocrine neoplasms - general):
- Does not demonstrate clinical hyperinsulinemic hypoglycemia (i.e., are nonfunctional)
- Histologic features are essentially identical
- Solid pseudopapillary neoplasm:
- Usually seen in young females
- No associated clinical syndrome of hyperinsulinemic hypoglycemia
- Pseudopapillae formation
- Positive nuclear beta catenin
Additional references
Board review style question #1
Which syndrome is associated with multiple insulinomas?
- MEN1
- MEN2A
- MEN2B
- NF2
- Peutz-Jeghers
Board review style answer #1
Board review style question #2
A midbody pancreatic enucleation is performed (see image above). Histologically, the neoplasm is positive for insulin, chromogranin and synaptophysin, with a Ki67 of < 2%. The patient has no clinical syndrome of hyperinsulinemic hypoglycemia. What is the most appropriate diagnosis?
- Glucagonoma
- Insulinoma
- VIPoma
- Well differentiated neuroendocrine tumor, G1
- Well differentiated neuroendocrine tumor, G2
Board review style answer #2
D. Well differentiated neuroendocrine tumor, G1. In the absence of a clinical syndrome, the term insulinoma should not be used; the Ki67 of < 2% classifies this as a grade 1 neuroendocrine tumor.
Comment Here
Reference: Insulinoma
Comment Here
Reference: Insulinoma
Intraductal oncocytic papillary neoplasm (IOPN)
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Etiology | Clinical features | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style answer #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Intraductal pancreatic lesion with papillary architecture and characteristic oncocytic epithelium
- First described in 1996 by Adsay et al (Am J Surg Pathol 1996;20:980)
- 2019 WHO recognizes as a distinct type of intraductal neoplasm
- Accounts for 4.5% of intraductal neoplasms of the pancreas (Pancreas 2016;45:1233); also occurs in bile ducts (Surg Today 2012;42:1240)
Essential features
- A rare and distinct type of intraductal neoplasm of pancreas
- Distinctive cytologic features are oncocytic cells with large prominent nucleoli
- Less mucin production than intraductal papillary mucinous neoplasms (IPMNs)
- Lack genetic alterations commonly seen in IPMN
- Indolent behavior even if associated with invasive carcinoma
Terminology
- Intraductal oncocytic papillary neoplasm
- Older term is oncocytic type of intraductal papillary mucinous neoplasm (WHO 2010)
ICD coding
-
ICD-O:
- 8455/2 - Intraductal oncocytic papillary neoplasm NOS
- 8455/3 - Intraductal oncocytic papillary neoplasm with associated invasive carcinoma
Epidemiology
- Mean age 59 years
- F > M (Am J Surg Pathol 2019;43:656)
- Accounts for 4.5% of intraductal neoplasms of the pancreas (Pancreas 2016;45:1233)
Sites
- 70% occur in the head, 10% involve whole gland (Am J Surg Pathol 1996;20:980)
- Also occurs in bile ducts (Surg Today 2012;42:1240)
Etiology
- No known etiological factors
Clinical features
- Some are incidentally discovered (Pancreas 2016;45:1233)
- Others present with abdominal pain and jaundice (Am J Surg Pathol 1996;20:980, J Am Coll Surg 2015;220:839)
Radiology description
- Complex mass with both solid and cystic components that may lead to misdiagnosis of pancreatic ductal adenocarcinoma (Cancer Cytopathol 2016;124:122)
Prognostic factors
- Exhibits indolent behavior even if associated with invasive carcinoma (Am J Surg Pathol 2019;43:656, J Am Coll Surg 2015;220:839)
Case reports
- 45 year old woman with intermittent nausea and vomiting (Can J Gastroenterol 2013;27:387)
- 53 year old woman with vague abdominal pain (JOP 2013;14:77)
- 69 year old woman presented with chest pain (J Pancreat Cancer 2017;3:5)
Treatment
- Treated primarily by surgical resection
Gross description
- Cystic with friable papillary or exophytic nodular projections often within the main pancreatic duct
- Median size 4.5 cm (Am J Surg Pathol 2019;43:656)
Gross images
Contributed by @liverwei on Twitter
Contributed by @liverwei on Twitter (see original post here)">
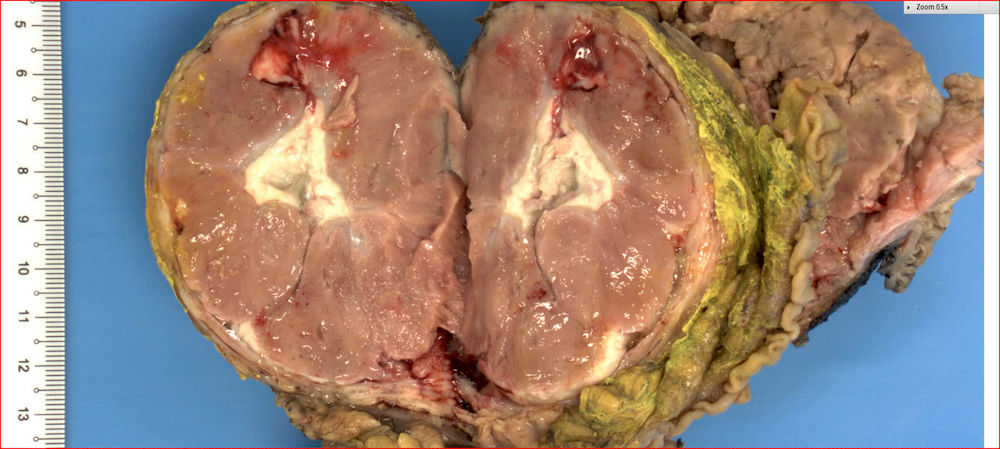

Intraductal oncocytic papillary neoplasm
Microscopic (histologic) description
- Multilocular or unilocular cysts containing complex and arborizing papillae with delicate fibrovascular cores (Virchows Arch 2016;469:523)
- Papillae are lined by multiple layers of neoplastic cells with voluminous granular oncocytic cytoplasm and prominent, large, eccentric nucleoli (Cancer Cytopathol 2016;124:122)
- Cribriform structure and intraluminal mucin formation are not uncommon
- Papillae may fuse and form solid growth pattern
- Invasive carcinoma is seen in about 30% of cases (Am J Surg Pathol 2019;43:656)
- True invasion seen in different patterns: small infiltrative tubules, mucinous and solid nests of oncocytic cells (Am J Surg Pathol 2019;43:656)
- Tangential sectioning may mimic true invasion (Am J Surg Pathol 2019;43:656)
- Abrupt transition from a normal epithelium to oncocytic epithelium, within the same duct, favors pseudoinvasion
Microscopic (histologic) images
Contributed by Gokce Askan, M.D. and Olca Basturk, M.D.
Contributed by @liverwei on Twitter
Contributed by @liverwei on Twitter (see original post here)">
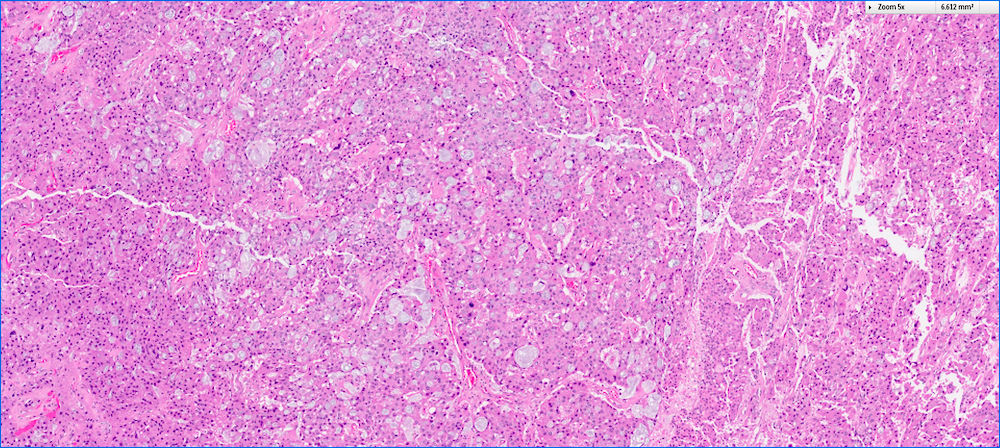 Contributed by @liverwei on Twitter (see original post here)">
Contributed by @liverwei on Twitter (see original post here)">
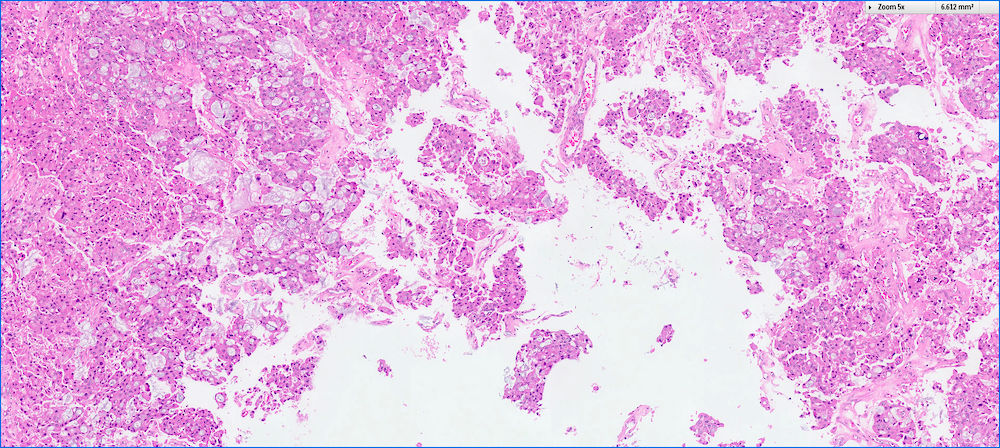
 Contributed by @liverwei on Twitter (see original post here)">
Contributed by @liverwei on Twitter (see original post here)">

Intraductal oncocytic papillary neoplasm
Cytology description
- Oncocytic cells with abundant granular cytoplasm, well defined cell borders, large central nuclei, prominent eccentric nucleoli and focal intercellular punched out spaces (Mod Pathol 2016;29:1058, Cancer Cytopathol 2016;124:122)
Cytology images
Positive stains
- MUC1 and MUC6 (diffuse) (Virchows Arch 2016;469:523, Am J Surg Pathol 2010;34:364)
- MUC2 and MUC5AC (restricted to goblet cells) (Virchows Arch 2016;469:523)
- HepPar1, CDX2 (Virchows Arch 2016;469:523)
Negative stains
- Trypsin, chymotrypsin and neuroendocrine markers (Virchows Arch 2016;469:523, Am J Surg Pathol 2010;34:364, Am J Surg Pathol 2001;25:942, J Pathol 2002;197:632)
Electron microscopy description
- Cells frequently packed with mitochondria (Am J Surg Pathol 1996;20:980)
Molecular / cytogenetics description
- IOPNs lack KRAS, GNAS and RNF43 mutations (Mod Pathol 2016;29:1058)
- ARHGAP26, ASXL1, EPHA8 and ERBB4 are recurrently mutated in some IOPNs (Mod Pathol 2016;29:1058)
- Recently it has been shown that a subset of IOPNs harbors DNAJB1-PRKACA fusions proving DNAJB1-PRKACA fusion is no longer specific for fibrolamellar hepatocellular carcinoma (Mod Pathol 2020;33:648, Gastroenterology 2020;158:573)
Sample pathology report
- Pancreas and duodenum, pancreaticoduodenectomy:
- Intraductal oncocytic papillary neoplasm of pancreass (IOPN) (see comment)
- Comment: The pancreas was submitted entirely for microscopic evaluation and no associated invasive carcinoma is identified. The neoplasm involves the main pancreatic duct. The neoplasm measures 3.5 cm in greatest dimension. Adjacent pancreas reveals atrophy. Surgical margins are free of neoplasm. Twelve benign lymph nodes are identified (0/12).
Differential diagnosis
- Intraductal papillary mucinous neoplasms (IPMNs), pancreatobiliary type
- More delicate papillae that are lined with highly atypical cuboidal cells
- No intraepithelial lumina
- Positive for MUC1
- KRAS mutations are common
- Acinar cell carcinoma
- No intracellular mucin or cytoplasmic vacuoles
- Mitotically very active
- Positive for trypsin and chymotrypsin
- Neuroendocrine neoplasms
- Salt and pepper chromatin pattern
- Positive for synaptophysin, chromogranin and CD56
Additional references
Board review style answer #1
- Which of the following is true about intraductal oncocytic neoplasms of pancreas (IOPNs)?
- Cribriform structure is one of the most common histologic features
- They commonly occur in the tail of the pancreas
- They exhibit indolent behavior even if associated with invasive carcinoma
- They have mucin production as much as seen in intraductal papillary mucinous neoplasms (IPMNs)
- They have same genetic alterations that commonly seen in intraductal papillary mucinous neoplasms (IPMNs)
Board review style answer #1
C. They exhibit indolent behavior even if associated with invasive carcinoma
Comment Here
Reference: Intraductal oncocytic papillary neoplasm (IOPN)
Comment Here
Reference: Intraductal oncocytic papillary neoplasm (IOPN)
Board review style question #2
- Which one of the following is positive in intraductal oncocytic neoplasms of pancreas (IOPNs)?
- Chromogranin
- Chymotrypsin
- MUC6
- Synaptophysin
- Trypsin
Board review style answer #2
Intraductal papillary mucinous neoplasm (IPMN)
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Grossly visible noninvasive mucinous epithelial neoplasm arising from main pancreatic duct or branch ducts, usually > 5 mm, composed of various cell types with various cytologic and architectural atypia (Virchows Arch 2005;447:794, Pancreatology 2017;17:738)
- Further classified as:
- Low grade (encompasses low or intermediate grade dysplasia) (Am J Surg Pathol 2015;39:1730)
- High grade (Am J Surg Pathol 2015;39:1730)
- With an associated invasive carcinoma (Am J Surg Pathol 2015;39:1730)
- Further classified as:
- 1 of 3 precursor lesions of pancreatic adenocarcinoma (see also mucinous cystic neoplasm)
Essential features
- Grossly visible (> 5 mm) cystic pancreatic neoplasm, usually in head of pancreas
- > 90% 5 year survival with complete resection
- Roughly 33% of cases have an associated invasive carcinoma
- Further subtyped into gastric, intestinal and pancreaticobiliary types based on epithelium (note: intraductal oncocytic papillary neoplasm (IOPN) is now considered a distinct and separate entity)
Terminology
- Low grade to intermediate grade dysplasia previously termed: intraductal papillary mucinous adenoma
- High grade dysplasia previously termed: intraductal papillary mucinous carcinoma, noninvasive
- With an associated invasive carcinoma previously termed: intraductal papillary mucinous carcinoma, invasive
- Other previous terms include: mucin producing tumor, mucinous duct ectasia, ductectatic mucinous cystadenoma / cystadenocarcinoma, villous adenoma or papillary adenoma / carcinoma
ICD coding
- ICD-10: D13.6 - benign neoplasm of pancreas
Epidemiology
- Rising incidence likely secondary to better characterization of this entity along with use of radiologic imaging studies (Clin Gastroenterol Hepatol 2010;8:213)
- More common in men, usually ages 60 - 70 years (Hum Pathol 2012;43:1)
Sites
- Main duct intraductal papillary mucinous neoplasm (IPMN) mostly involves head of pancreas, 33% in body and tail (Hum Pathol 2012;43:1)
- Branch duct IPMN mostly involves head of pancreas or uncinate process, with multiple distinct lesions seen in ~33% of cases (Hum Pathol 2012;43:1)
Pathophysiology
Etiology
- No well established factors; cigarette smoking implicated in one series (Adv Anat Pathol 1999;6:65)
- Reported in Peutz-Jeghers syndrome and familial adenomatous polyposis (Gut 2002;51:446, Am J Pathol 1999;154:1835)
Clinical features
- Clinically divided into main duct IPMN, branch duct IPMN and mixed IPMN (most mixed type IPMN present and behave as main duct IPMN) (Hum Pathol 2012;43:1)
- Main duct IPMN tends to be symptomatic, with symptoms related to duct obstruction (pancreatitis) (Hum Pathol 2012;43:1)
- Signs and symptoms include epigastric pain, weight loss, jaundice, diabetes, pancreatitis (Arch Pathol Lab Med 1996;120:981)
- Branch duct IPMN tends to be asymptomatic and is generally discovered incidentally on imaging (Hum Pathol 2012;43:1)
- Peutz-Jeghers syndrome (autosomal dominant inherited syndrome, mutations in STK11 / LKB1I) is also associated with IPMN (Hum Pathol 2012;43:1)
Diagnosis
- Radiographic / endoscopic findings
- Fine needle aspiration
- Surgical specimen
Laboratory
- Pancreatic cyst fluid analysis can assist in determining type of cyst (Am J Gastroenterol 2008;103:2871, Gastrointest Endosc 2009;69:1095, Gastrointest Endosc 2016;83:140)
- Elevated CEA and identification of KRAS / GNAS mutations supports diagnosis of mucinous cyst
Radiology description
- CT:
- Main duct IPMN causes distention of main pancreatic duct
- Branch duct IPMN produces multilocular grape-like cystic appearance
- ERCP (endoscopic retrograde cholangiopancreatography): pancreatic ductal filling defects may be seen / ductal dilation
- MRCP (magnetic resonance cholangiopancreatography): additional imaging option which does not produce radiation
- EUS (endoscopic ultrasound): can also allow FNA and cyst fluid analysis
- References: Diagn Interv Imaging 2016;97:1275, World J Gastroenterol 2016;22:9562
Radiology images
Prognostic factors
- Without an invasive carcinoma, has > 90% 5 year survival; those associated with an invasive carcinoma carry a worse prognosis (about half die of the disease) (Ann Surg 2016;263:162)
- Main duct IPMN: 60% have high grade dysplasia and 45% are associated with an invasive carcinoma (Hum Pathol 2012;43:1)
- Branch duct IPMN: most are low grade, 25% have high grade dysplasia and 20% are associated with an invasive carcinoma (Hum Pathol 2012;43:1)
- Invasive carcinoma associated with IPMN includes:
- Tubular (ductal) adenocarcinoma: seen in about half of cases, with slightly better prognosis than non IPMN associated pancreatic ductal adenocarcinoma
- Colloid carcinoma: seen in half of cases, with much better prognosis than pancreatic ductal adenocarcinoma (Ann Surg 2016;263:162)
Case reports
- 52 year old man with recurrent upper abdominal pain (Int J Clin Exp Med 2015;8:3332)
- 53 year old man with multiple pancreatic cystic lesions (World J Gastroenterol 2013;19:3358)
- 55 year old woman with abdominal pain and anemia; pancreatic masses are found on CT (World J Surg Oncol 2018;16:83)
- 68 year old man with cystic pancreatic mass with mural nodule (Int J Surg Case Rep 2017;40:69)
- 74 year old man with cystic pancreatic lesion on ultrasound (Intern Med 2018;57:1093)
Treatment
- Main duct IPMN: surgical resection indicated if main pancreatic duct > 10 mm, jaundice or presence of mural nodules (Pancreatology 2017;17:738)
- Branch duct IPMN: surgical resection indicated if symptomatic, presence of mural nodule ≥ 5 mm, suspicious / positive cytology, obstructive jaundice or main pancreatic duct ≥ 10 mm (Pancreatology 2017;17:738)
- See Diagrams / tables
Gross description
- Main duct involvement:
- Usually diffusely dilated, tortuous and irregular, filled with mucin
- Typically arises in head and progresses along path of main duct, may involve entire pancreas; may involve major or minor papillae leading to mucin extrusion from ampulla
- Uninvolved pancreas is often pale and firm, reflecting extensive chronic obstructive pancreatitis
- Branch duct involvement:
- Often in uncinate process
- Forms multicystic, grape-like structures; cystically dilated ducts, filled with tenacious mucin; cyst walls are usually thin with flat or papillary lining
- Cysts separated by normal pancreas, suggesting that cysts are separate on cut sections
- Multicentricity seen in up to 40% of cases (Am J Gastroenterol 2007;102:1759)
- Extensive sampling / complete submission of cyst for microscopic evaluation important to rule out an associated invasive carcinoma (Am J Surg Pathol 2014;38:480, Ann Surg 2016;263:162)
- Greater than 5 mm in diameter
- Incipient IPMN is a term that can be used for lesions 0.5 - 1.0 cm in size
Frozen section description
- Typically pancreatic resection margin status will be evaluated to exclude IPMN; additional margins will be submitted if invasive carcinoma is present or possibly for the presence of high grade dysplasia (Am J Surg Pathol 2015;39:1730, Pancreatology 2017;17:738)
- If low grade dysplasia is seen, typically no additional resection done; this may represent pancreatic intraepithelial neoplasia (PanIN) versus IPMN (Am J Surg Pathol 2015;39:1730, Pancreatology 2017;17:738)
Frozen section images
Microscopic (histologic) description
- Mucin producing epithelial cells with varied degrees of dysplasia (Am J Surg Pathol 2015;39:1730)
- Low grade dysplasia is characterized by a flat epithelial lining with basal located nuclei without significant pleomorphism, while intermediate dysplasia has features between those of low and high grade dysplasia (note: low and intermediate dysplasia are now both grouped as low grade dysplasia) (Am J Surg Pathol 2015;39:1730)
- High grade dysplasia is characterized by complex architectural features (i.e. irregular branching, cribiforming) with loss of nuclear polarity along with increased nuclear hyperchromasia and nuclear irregularities (Am J Surg Pathol 2015;39:1730)
- Epithelial cells show variable differentiation and can be subclassified into: intestinal, gastric and pancreaticobiliary subtypes (oncocytic lining suggests intraductal oncocytic papillary neoplasm)
- Associated with pancreatic intraepithelial neoplasia (PanIN), chronic pancreatitis (Am J Surg Pathol 2004;28:1184)
- No ovarian type stroma
- Assess presence or absence of invasive carcinoma (most important prognostic factor) (Hum Pathol 2012;43:1)
- Type of invasion is associated with MUC1 / MUC2 pattern; see below (Mod Pathol 2002;15:1087)
- Gastric type IPMN:
- Cells resemble gastric foveolae
- Intestinal metaplasia may be seen, usually with low grade dysplasia and branch duct IPMN
- If associated invasive carcinoma present, typically ductal (tubular) adenocarcinoma (Ann Surg 2016;263:162, Virchows Arch 2005;447:794)
- Intestinal type IPMN:
- Cells with tall columnar epithelium (resembling intestinal villous adenomas)
- Usually low or high grade dysplasia and main duct IPMN
- If associated invasive carcinoma present, typically mucinous (colloid) carcinoma
- Pancreaticobiliary type IPMN:
- Complex, thin branching papillae resembling cholangiopapillary neoplasms
- Cuboidal cells with prominent nucleoli
- Usually high grade dysplasia and main duct IPMN
- If associated invasive carcinoma present, typically ductal (tubular) adenocarcinoma
Microscopic (histologic) images
Cytology description
- Cannot distinguish IPMN from mucinous cystic neoplasm on cytology (neoplastic mucinous cyst)
- Disordered mucinous epithelial clusters with nuclear overlap and variable cytologic atypia
- Distinguishing a low grade mucinous neoplasm from gastrointestinal tract contaminant (particularly gastric mucosa) can be challenging
Cytology images
Positive stains
- General ductal markers are positive, including CK7, CK19, CA 19-9, B72.3 and CEA
- Subtypes show various mucin glycoprotein (MUC) expression (as below):
- Gastric type IPMN: MUC5AC+, MUC6 variable (Am J Surg Pathol 2006;30:1561, Mod Pathol 2016;29:977)
- Intestinal type IPMN: MUC2+, CDX2+, MUC5AC+; similar staining pattern for associated invasive colloid carcinoma (Mod Pathol 2016;29:977)
- Pancreaticobiliary type IPMN: MUC1+, MUC6+, MUC5AC+; similar staining pattern for associated invasive ductal adenocarcinoma (Am J Surg Pathol 2006;30:1561, Mod Pathol 2016;29:977)
Negative stains
- Gastric type IPMN: MUC1-, MUC2- (Am J Surg Pathol 2006;30:1561, Mod Pathol 2016;29:977)
- Intestinal type IPMN: MUC1-; similar staining pattern for associated invasive colloid carcinoma (Mod Pathol 2016;29:977)
- Pancreaticobiliary type IPMN: MUC2-; similar staining pattern for associated invasive ductal adenocarcinoma (Am J Surg Pathol 2010;34:364, Mod Pathol 2016;29:977)
Molecular / cytogenetics description
- KRAS, GNAS and RNF43 mutations seen in decreasing frequency in noninvasive IPMNs and IPMNs with an associated invasive carcinoma (J Am Coll Surg 2015;220:845)
- GNAS mutations more commonly associated with IPMNs, which harbor an invasive colloid carcinoma
- KRAS mutations more commonly associated with IPMNs, which harbor an invasive tubular (ductal) adenocarcinoma
- With increasing grades of dysplasia, increased mutations in KRAS, TP53, CDKN2A (p16); also hypermethylation and reduced BRG1 protein (Hum Pathol 2012;43:585)
- Loss of programmed cell death 4 (PDCD4) and CD24 expression associated with tumor progression and proliferation (Hum Pathol 2010;41:1507, Hum Pathol 2010;41:1466)
- Cyst fluid shows mutations in KRAS2 and GNAS
Sample pathology report
- Pancreas, small bowel and stomach, pancreaticoduodenectomy (Whipple resection):
- Intraductal papillary mucinous neoplasm (IPMN), low grade, gastric phenotype, branch duct type, 3.0 cm (see comment)
- Negative for high grade dysplasia or malignancy.
- Margins are negative for IPMN.
- 23 lymph nodes with no significant histologic abnormality.
- Comment: The entire cyst is submitted for histologic examination.
Differential diagnosis
- Intraductal oncocytic papillary neoplasm:
- Lined by oncocytic cells with complex growth patterns and arborizing papillae
- Distinct molecular pathway with ARHGAP26, ASXL1, EPHA8 and ERBB4 genetic alterations (Mod Pathol 2016;29:1058)
- DNAJB1-PRKACA fusions (Mod Pathol 2020;33:648)
- MUC1+, MUC6+
- Intraductal tubulopapillary neoplasm:
- Recently recognized subtype with potential origin from peribiliary cysts (Am J Surg Pathol 2009;33:1164)
- Confluent epithelial cells with tubular and papillary architectural patterns and usually high grade dysplasia
- Necrotic foci, more solid growth without visible mucin or scanty cytoplasmic mucin, no KRAS2 gene mutations
- MUC1+, MUC2-, MUC6+
- Mucinous cystic neoplasm:
- Epithelial mucinous lining with ovarian type stroma
- No communication to pancreatic ductal system
- Pancreatic intraepithelial neoplasia (PanIN):
- Only detectable microscopically
- No mass lesion, unlike IPMN
- Almost always has gastric foveolar differentiation
- Simple mucinous cyst:
- Cyst > 1 cm with flat (not papillary) gastric mucinous lining and no significant atypia (Am J Surg Pathol 2015;39:1730, Am J Surg Pathol 2017;41:121)
- Retention cyst:
- Cyst > 1 cm with flat (not papillary) ductal lining and no significant atypia in a background of obstruction (Am J Surg Pathol 2015;39:1730)
Additional references
Board review style question #1
Board review style answer #1
A. Gastric. The histologic features are typical of the gastric type of IPMN, no features of intestinal or pancreatobiliary differentiation are seen (choices B and E); choice D is not a morphologic type of IPMN; rather, intraductal oncocytic papillary neoplams are a distinct separate entity.
Comment Here
Reference: Intraductal papillary mucinous neoplasm (IPMN)
Comment Here
Reference: Intraductal papillary mucinous neoplasm (IPMN)
Board review style question #2
Which of the following clinical scenarios would most likely represent an intraductal papillary mucinous neoplasm (IPMN)?
- 29 year old woman with 8 cm solid and cystic pancreatic tail mass
- 45 year old woman with 3 cm cystic pancreatic tail mass
- 58 year old man with 3 cm solid pancreatic head mass
- 66 year old man with 2 cm cystic pancreatic head mass
Board review style answer #2
D. 66 year old man with 2 cm cystic pancreatic head mass. IPMNs are more often seen in older men and are cystic; thus choices A - C are incorrect (choice A would be more typical of a solid pseudopapillary neoplasm; choice B would more typical of a mucinous cystic neoplasm; choice C would be more typical of pancreatic ductal adenocarcinoma or a pancreatic neuroendocrine tumor).
Comment Here
Reference: Intraductal papillary mucinous neoplasm (IPMN)
Comment Here
Reference: Intraductal papillary mucinous neoplasm (IPMN)
Intraductal tubulopapillary neoplasm (ITPN)
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Etiology | Clinical features | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Intraductal, grossly solid and cystic, tubule forming, epithelial neoplasm with high grade dysplasia and no overt mucin production (Am J Surg Pathol 2017;41:313)
- First described in 2004 by Tajiri et al. under the name of intraductal tubular carcinoma (Pancreas 2005;30:115)
- Renamed in 2009 by Jamaguchi et al. as intraductal tubulopapillary neoplasm (Am J Surg Pathol 2009;33:1164)
- Previous (2010) and current (2019) WHO classify as a distinct intraductal neoplasm of the pancreas
- Accounts for 3% of intraductal neoplasms of the pancreas (Am J Surg Pathol 2011;35:1812)
Essential features
- A rare and distinct type of pancreatic intraductal neoplasm
- Highly cellular, complex tubular or cribriform growth pattern
- Focal papillary growth may be seen
- Intracellular mucin is typically not detectable or is very minimal
- Positive for MUC1 and MUC6; negative for MUC5AC
- Lacks genetic alterations commonly seen in intraductal papillary mucinous neoplasms (IPMNs)
- Favorable overall outcome even if associated with invasive carcinoma (Am J Surg Pathol 2017;41:313)
Terminology
- Older term is intraductal tubular carcinoma (not recommended)
ICD coding
Epidemiology
- Mean age: 55 years (Am J Surg Pathol 2017;41:313)
- F:M = 17:14 (Am J Surg Pathol 2017;41:313)
- Accounts for 3% of intraductal neoplasms of the pancreas (Am J Surg Pathol 2011;35:1812)
Sites
- 50% occur in head, 15% occur in tail and 30% involve entire gland of the pancreas (Am J Surg Pathol 2017;41:313, Int J Clin Exp Pathol 2015;8:9672)
- Also occurs in bile ducts (Mod Pathol 2015;28:1249)
Etiology
- No known etiological factors
Clinical features
- Nonspecific symptoms: abdominal pain, weight loss, nausea, vomiting, steatorrhea
- Jaundice is not common
Radiology description
- 2 tone duct sign on CT image and cork of wine bottle sign on MRCP examination as characteristic imaging findings that represent their intraductal growth (J Comput Assist Tomogr 2012;36:710, Medicine (Baltimore) 2019;98:e14426)
- Intraductal solid tumor within dilated main pancreatic duct mimicking IPMN (Diagn Interv Radiol 2019;25:251)
- Irregular main pancreatic duct dilatation may lead to misdiagnosis of chronic pancreatitis (Diagn Interv Radiol 2019;25:251)
Prognostic factors
- Exhibits relatively indolent behavior even if associated with invasive carcinoma (Am J Surg Pathol 2017;41:313)
Case reports
- 23 year old woman with incidental asymptomatic retroperitoneal cystic lesion (Int J Clin Exp Pathol 2019;12:1041)
- 43 year old woman with epigastric pain (Diagn Pathol 2014;9:11)
- 62 year old man with epigastric pain, nausea and vomiting (J Clin Diagn Res 2017;11:PD14)
- 72 year old man with recurrent pancreatitis (Int J Surg Case Rep 2018;48:122)
- 73 year old man with nausea and persistent abdominal pain (World J Surg Oncol 2017;15:203)
Treatment
- Treated primarily by surgical resection
Gross description
- Multinodular, polypoid, solid or fleshy mass within dilated main pancreatic duct (Am J Surg Pathol 2017;41:313)
- Cyst formation is less common
- Median size: 4.5 cm (Am J Surg Pathol 2017;41:313)
- Multifocality may be seen (Int J Clin Exp Pathol 2015;8:9672)
Microscopic (histologic) description
- Circumscribed nodules of back to back tubular glands surrounded by fibrotic stroma; however, the intraductal location of at least some of the nodules is evidenced by continuity of the neoplastic epithelium with nonneoplastic ductal epithelium
- Tubules are lined by cuboidal or columnar epithelium with a modest amount of eosinophilic or amphophilic cytoplasm
- Nuclei are small, round to oval and moderately to markedly atypical with readily identifiable mitotic figures
- Intracellular mucin production is typically not detectable or is very minimal
- Intraluminal secretions and necrosis, often with comedo-like pattern, are present
- Clear cell changes, cartilaginous or osseous metaplasia may be seen (Diagn Pathol 2014;9:11, Pathol Int 2012;62:339, Am J Surg Pathol 2017;41:313, Pathol Int 2015;65:501)
- Associated invasive carcinoma is seen in about 70% of cases as individual cells or small angulated nonmucinous glands surrounded by usually desmoplastic stroma (Am J Surg Pathol 2017;41:313)
Microscopic (histologic) images
Contributed by Gokce Askan, M.D. and Olca Basturk, M.D.
Cytology description
- Large cribriform and tubular clusters with luminal spaces and scanty intracytoplasmic mucin (Diagn Cytopathol 2014;42:314, Arch Pathol Lab Med 2017;141:366)
Positive stains
Negative stains
- MUC2 and MUC5AC (Pancreas 2004;29:116, Am J Surg Pathol 2017;41:313, Pancreas 2005;30:115, Am J Surg Pathol 2009;33:1164)
- Acinar markers (trypsin, chymotrypsin) (Am J Surg Pathol 2017;41:313)
- Neuroendocrine markers (a few scattered cells may be positive for chromogranin or synaptophysin) (Am J Surg Pathol 2017;41:313)
Molecular / cytogenetics description
- In general, ITPNs lack KRAS, GNAS and BRAF mutation (Mod Pathol 2017;30:1760, Am J Surg Pathol 2009;33:1164, Am J Surg Pathol 2011;35:1812, J Pathol 2013;231:335)
- PIK3CA, PIK3CB, INPP4A, PTEN may be mutated in ITPNs (Mod Pathol 2017;30:1760, Am J Surg Pathol 2009;33:1164, Am J Surg Pathol 2011;35:1812, J Pathol 2013;231:335)
- Recently, it has been shown that certain chromatin remodeling genes (MLL1, MLL2, MLL3, BAP1, etc.) may be mutated in ITPNs and a subset of the cases harbors FGFR2 and STRN-ALK fusions (Mod Pathol 2017;30:1760)
Sample pathology report
- Pancreas and duodenum, pancreaticoduodenectomy:
- Intraductal tubulopapillary neoplasm of pancreas (ITPN) (see comment)
- Comment: The pancreas was submitted entirely for microscopic evaluation and no associated invasive carcinoma is identified. The neoplasm involves the main pancreatic duct and measures 4 cm in greatest dimension. Adjacent pancreas reveals atrophy. Surgical margins are free of neoplasm. 15 benign lymph nodes are identified (0/15).
- Pancreas and duodenum, pancreaticoduodenectomy:
- Intraductal tubulopapillary neoplasm of pancreas (ITPN) associated with an invasive ductal carcinoma (see comment)
- Comment: ITPN involves the main pancreatic duct. The entire tumor measures 5 cm in greatest dimension; the invasive carcinoma component measures 0.7 cm in greatest dimension (suggestion for if multifocal invasion is present: the invasive carcinoma accounts for 25% of entire tumor and is estimated to measure 1.25 cm in greatest dimension). Adjacent pancreas reveals atrophy. Surgical margins are free of ITPN and invasive carcinoma. 14 benign lymph nodes are identified (0/14).
- Reference: Ann Surg 2016;263:162
Differential diagnosis
- Intraductal papillary mucinous neoplasm, pancreatobiliary type:
- More delicate papillae that are lined with highly atypical cuboidal cells
- Positive for MUC5AC
- KRAS mutations are common
- Acinar cell carcinoma intraductal variant:
- Eosinophilic zymogen granule containing cytoplasm and single prominent central nucleolus
- Mitotically very active
- Positive for trypsin, chymotrypsin and BCL10
- Neuroendocrine neoplasm, with intraductal growth pattern:
- Salt and pepper chromatin pattern
- Positive for chromogranin and synaptophysin
Additional references
Board review style question #1
Which of the following is true about intraductal tubulopapillary neoplasm of the pancreas?
- Jaundice is one of the most common symptoms
- Papilla and mucin formation are common histologic features
- They are positive for MUC5AC
- They commonly have KRAS, GNAS and BRAF mutations
- They have relatively indolent prognosis even when associated with invasive carcinoma
Board review style answer #1
E. They have relatively indolent prognosis even when associated with invasive carcinoma
Comment Here
Reference: Intraductal tubulopapillary neoplasm (ITPN)
Comment Here
Reference: Intraductal tubulopapillary neoplasm (ITPN)
Board review style question #2
Which one of the following stains is negative in intraductal tubulopapillary neoplasm of the pancreas?
- CK7
- CK19
- MUC5AC
- MUC1
- MUC6
Board review style answer #2
Lymphoepithelial cysts
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Rare, nonneoplastic, true cysts lined by squamous epithelium surrounded by abundant, reactive lymphoid tissue
- Histologically similar to branchial cleft cysts arising in the neck
- First described in 1985 (Pathologe 1985;6:217)
- No association with salivary gland lymphoepithelial cysts
Essential features
- Consists of a squamous, epithelial lined cyst with surrounding reactive lymphoid tissue
ICD coding
- ICD-10: K86.2 - cyst of pancreas
Epidemiology
- Mean age: 56 years
- Range: 20 - 82 years
- 80% male
- 0.5% of resected pancreatic cysts (Diagnostics (Basel) 2022;13:65)
Sites
- Occurs anywhere in pancreas (head, body or tail) or extrapancreatic location
Pathophysiology
- May occasionally develop from epithelial remnants in lymph nodes or from accessory spleen in pancreas (Hum Pathol 1991;22:924, Mod Pathol 1998;11:1171)
Etiology
- Several proposed hypotheses (Case Rep Gastrointest Med 2016;2016:5492824)
- May originate from epithelial remnants within a lymph node
- Arise via cystic transformation and squamous metaplasia of pancreatic ducts
- May derive from a misplaced branchial cleft cyst that fused with the pancreas during embryogenesis
Clinical features
- Most common symptom is abdominal pain (~48%), particularly with larger cysts
- Significant portion are asymptomatic (~43%)
- Can occasionally present with fatigue or weight loss, mimicking malignancy (Case Rep Gastroenterol 2016;10:181)
- Not associated with immunosuppression or autoimmune diseases
Diagnosis
- Can be difficult to differentiate preoperatively from other pancreatic cystic lesions
- Cyst fluid chemistry may show elevated CA 19-9 or elevated carcinoembryonic antigen (CEA)
- Cytology / histology examination for final confirmation of diagnosis
- Reference: Arch Pathol Lab Med 2020;144:47
Laboratory
- Cyst fluid may have elevated CA 19-9 levels (50% of cases) and elevated CEA levels (up to 32% of cases) (diagnostic pitfall) (Case Rep Gastrointest Med 2016;2016:5492824)
Radiology description
- Imaging varies and is similar to other pancreatic cystic lesions but more likely to be extrapancreatic or exophytic
- CT: multilocular or unilocular cyst with possible solid component and slight hyperintensity of keratinized material (Case Rep Gastrointest Med 2016;2016:5492824)
- MRI: multilocular or unilocular cyst with possible solid component, hyperintensity on T1 weighted images, hypointensity on T2 weighted images and water restriction in diffusion weighted images (Jpn J Radiol 2021;39:118)
Radiology images
Prognostic factors
- Benign; does not recur or progress
Case reports
- 41 year old woman who presented with abdominal discomfort, bloating and dyspepsia (Acta Chir Belg 2022 Mar 17 [Epub ahead of print])
- 43 year old man with a lymphoepithelial cyst occurring in a pancreatic accessory spleen (Clin Case Rep 2021;9:e04241)
- 49 year old man with diarrhea and weight loss (Int J Surg Case Rep 2019;55:192)
- 62 year old man with abdominal pain and a pancreatic mass (Case Rep Med 2020;2020:4590758)
- 65 year old man with prior pancreatoduodenectomy for carcinoma arising from the ampulla of Vater and a new pancreatic mass in the remnant pancreatic tail (Surg Case Rep 2021;7:108)
Treatment
- Asymptomatic patients with a confirmed diagnosis by FNA may be followed by serial imaging
- Symptomatic patients with a confirmed diagnosis by FNA may be treated with enucleation or drainage (Indian J Surg 2010;72:427)
- Resection is curative
Gross description
- Mean size: 5 cm (range: 1 - 17 cm) (Mod Pathol 2002;15:492)
- Often round and well demarcated from surrounding pancreas
- Either multilocular (60%) or unilocular (40%)
- Cysts contain clear serous fluid or caseous / cheese-like material (Diagnostics (Basel) 2022;13:65)
Gross images
Microscopic (histologic) description
- Unilocular or multilocular cyst lined by squamous epithelium (nucleated or anucleated), with cyst wall containing lymphocyte rich cuff with germinal centers
- Combinations of squamous lining with other epithelial types (i.e., simple columnar) have been reported
- Lymphoid tissue, often a dense band
- With or without keratinous debris
- Occasional solid lymphoepithelial islands, rarely mucinous goblet cells
- Rare sebaceous differentiation, keratin granulomas, cholesterol clefts, multinucleated giant cells and foamy histiocytes may be present
- Reference: Diagnostics (Basel) 2022;13:65
Microscopic (histologic) images
Cytology description
- Predominantly mature squamous cells, anucleated squamous cells and keratin debris
- Lymphocytes, macrophages and cholesterol crystals are occasionally seen (Arch Pathol Lab Med 2020;144:47)
- May have mildly atypical mucinous glandular and parakeratotic epithelium
Cytology images
Positive stains
Negative stains
- Squamous epithelial lining is negative for MUC2 and MUC6 (100%) (Mod Pathol 2010;23:1467)
Molecular / cytogenetics description
- No recurrent molecular / cytogenetic abnormalities identified
Sample pathology report
- Pancreas, cyst, excision:
- Lymphoepithelial cyst
Differential diagnosis
- Dermoid cyst (cystic teratoma):
- Requires the presence of microscopic elements such as dermal appendages, cartilage, respiratory mucosa or neural tissue
- Younger mean age
- Epidermoid cyst in intrapancreatic spleen:
- Lined by squamous epithelium and surrounded by splenic tissue (Acta Pathol Jpn 1991;41:916)
- Squamoid cysts:
- Smaller (median size: 1.5 cm)
- Lined exclusively by nonkeratinizing squamous epithelium without lymphoid tissue (Am J Surg Pathol 2007;31:291)
- Lymphangioma:
- Lined by a single flat or cuboidal epithelium that will be positive for endothelial and lymphatic markers (such as CD31 and D2-40)
- Typically contains small aggregates of lymphoid tissue rather than a dense band (Arch Pathol Lab Med 2020;144:47)
- Pseudocyst:
- No epithelial lining or lymphoid stroma
- Presence of granulation tissue and fibrosis
- Of note, while the imaging or clinical differential diagnosis may include mucinous cystic neoplasm, intraductal papillary mucinous neoplasm (IPMN) and intraductal oncocytic papillary neoplasm (IOPN), these neoplasms are not similar appearing on histologic examination and are not on the histologic differential diagnosis
Board review style question #1
Which of the following statements about the pancreatic lesion shown above is true?
- Carcinoembryonic antigen (CEA) is never elevated in cyst fluid
- This lesion does not undergo malignant transformation
- This lesion is more common than mucinous cystic neoplasm
- This lesion is more commonly found in women
Board review style answer #1
B. This lesion does not undergo malignant transformation. Lymphoepithelial cysts are benign and there have been no reported incidences of malignant transformation. Answer C is incorrect because lymphoepithelial cysts of the pancreas are rare (0.5% of resected pancreatic cysts) and are much less common than pancreatic mucinous cystic neoplasms (~8% of resected pancreatic cysts). Answer D is incorrect because lymphoepithelial cysts are more common in men than women (80% men). Answer A is incorrect because CEA levels (as well as CA 19-9) are commonly elevated in lymphoepithelial cysts. This is a diagnostic pitfall that increases the clinical concern for malignancy.
Comment Here
Reference: Lymphoepithelial cysts
Comment Here
Reference: Lymphoepithelial cysts
Board review style question #2
Which of the following statements about the pancreatic lesion shown above is true?
- Foamy histiocytes and multinucleated giant cells can be present
- This lesion is associated with immunosuppression or autoimmune dysfunction
- This lesion is associated with ovarian type stroma
- This lesion is the most common cystic lesion of the pancreas
Board review style answer #2
A. Foamy histiocytes and multinucleated giant cells can be present. Lymphoepithelial cysts are composed of squamous epithelium and reactive lymphoid tissue but may rarely show sebaceous differentiation, keratin granulomas, cholesterol clefts, multinucleated giant cells and foamy histiocytes. Answer D is incorrect because lymphoepithelial cysts of the pancreas are rare (0.5% of resected pancreatic cysts). The most common cystic lesion of the pancreas is intraductal papillary mucinous neoplasm (IPMN). Answer B is incorrect because while the etiology of lymphoepithelial cysts is not fully understood, it may originate from epithelial remnants within a lymph node, arise through cystic transformation of pancreatic ducts or be derived from a misplaced branchial cleft cyst that fused with the pancreas during embryogenesis. No association with immunosuppression or autoimmune dysfunction has been identified. Answer C is incorrect because lymphoepithelial cysts are composed of a squamous epithelial lined cyst with surrounding reactive lymphoid tissue. Mucinous cystic neoplasms (MCN) contain ovarian stroma.
Comment Here
Reference: Lymphoepithelial cysts
Comment Here
Reference: Lymphoepithelial cysts
Medullary carcinoma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1Definition / general
- Rare histologic variant of pancreatic ductal adenocarcinoma (PDAC) characterized by poor differentiation, pushing borders and a syncytial growth pattern
Essential features
- Main histologic features are poor differentiation with limited gland formation, syncytial growth pattern and pushing borders
- Prominent tumor infiltrating lymphocytes (TILs) are common
- Associated with microsatellite instability (MSI) and wild type KRAS status
- Represents < ~4% of PDAC cases
- 26 reported cases in the literature
- References: Am J Pathol 1998;152:1501, Front Oncol 2023;12:1082927
Terminology
- Pancreatic medullary carcinoma
- Medullary pancreatic carcinoma
ICD coding
Epidemiology
- Cancer screening in the patient's immediate family following diagnosis of medullary pancreatic carcinoma has been suggested (Am J Pathol 2000;156:1641)
- Accumulated risk of pancreatic cancer in Lynch syndrome patients is 3.7% and tumors often have characteristic medullary morphology (J Cancer 2017;8:3667)
- No known associations with race, gender, age, alcohol use or tobacco use
Pathophysiology
- Possible association with PanIN as the precursor lesion (Am J Pathol 2000;156:1641)
Etiology
- Hereditary syndromes: Lynch syndrome
Clinical features
- Epigastric pain, back pain, jaundice, weight loss, diabetes mellitus
Diagnosis
- Abdominal CT scan or MRI
- Endoscopic ultrasound guided fine needle aspiration (EUS FNA)
- Histologic evaluation of surgical resection specimen
Laboratory
- Serum elevation of CA19-9 or CEA
Radiology description
- PDAC typically appears as a hypoattenuating mass compared to normal pancreatic parenchyma (Insights Imaging 2020;11:58)
Prognostic factors
- Poor prognosis (overall survival: 29% at 2 years, 13% at 5 years) (Am J Pathol 2000;156:1641)
- 15 out of 20 reported cases died of disease (Surg Case Rep 2018;4:80)
- Tumor with certain genetic mutations may show improved survival (Surg Case Rep 2018;4:80, Pancreas 2020;49:999)
- Limited data is available to draw meaningful conclusion about prognosis
Case reports
- Woman in her 60s with head of the pancreas mass with POLE mutation (Pancreas 2020;49:999)
- 64 year old man with medullary pancreatic cancer and the first patient derived cell line (Front Oncol 2023;12:1082927)
- 73 year old woman with pancreatic mass with loss of MSH2 and MSH6 expression (Diagn Pathol 2021;16:117)
- 73 year old man with asymptomatic pancreatic duct cyst (Surg Case Rep 2018;4:80)
Treatment
- Neoadjuvant chemotherapy (World J Gastroenterol 2022;28:3297)
- Whipple resection or distal pancreatectomy depending on tumor location in the pancreas (World J Gastroenterol 2022;28:3297)
- Due to the presence of MSI, may be responsive to treatment with immunotherapy (Front Oncol 2023;12:1082927)
- Due to its rarity, data regarding drug response and treatment outcome is limited (Front Oncol 2023;12:1082927)
Gross description
- There are no definitive diagnostic criteria to grossly distinguish medullary carcinoma from conventional PDAC; it may show circumscribed borders as compared to infiltrative borders of conventional PDAC (Pathologica 2020;112:210)
Microscopic (histologic) description
- Well circumscribed tumor with pushing / expansile borders
- Syncytial growth pattern with poorly defined cell borders
- Solid pattern of growth (sheets and nests) with limited gland formation
- Tumor cells with pleomorphic nuclei, prominent nucleoli and abundant amphophilic cytoplasm
- Large areas of necrosis may be present
- Abundant lymphocytes within and around tumor
- Focal clear cells and squamoid differentiation have also been reported (Am J Pathol 2000;156:1641)
Microscopic (histologic) images
Positive stains
Negative stains
- Similar to nonmedullary PDAC: CK20, MUC2, trypsin, chymotrypsin, lipase
- Neuroendocrine markers: chromogranin A, synaptophysin, CD56
- Acinar cell marker: BCL10
- Reference: Pathologica 2020;112:210
Molecular / cytogenetics description
- Association with MSI (22%) and wild type KRAS (67%)
- Most commonly associated with loss of MLH1 (Am J Pathol 2000;156:1641)
- Possible genetic associations with KRAS mutation and somatic POLE mutation (Surg Case Rep 2018;4:80, Pancreas 2020;49:999)
Sample pathology report
- Pancreas, Whipple resection:
- Poorly differentiated carcinoma, consistent with medullary carcinoma (x cm in maximum dimension)
- Resection margins are negative for carcinoma (mention the positive margin, if any)
- Negative / positive for perineural and lymphovascular invasion
- All lymph nodes are negative for carcinoma
Differential diagnosis
- Poorly differentiated PDAC:
- Infiltrative growth pattern, well to poorly formed glands and desmoplastic stroma, KRAS mutated, microsatellite stable
- EBV associated lymphoepithelioma-like carcinoma:
- EBV encoded RNA (EBER) in situ hybridization positive
Board review style question #1
Which of the following is characteristic of medullary carcinoma in the pancreas?
- EBV encoded RNA (EBER) in situ hybridization positive
- Infiltrative growth pattern with well to poorly formed glands
- KRAS mutations
- MSS (microsatellite stable)
- Pushing borders with syncytial growth pattern, limited to no gland formation and tumor infiltrating lymphocytes
Board review style answer #1
E. Pushing borders with syncytial growth pattern, limited to no gland formation and tumor infiltrating lymphocytes. Expansile invasion (pushing borders), poorly defined cell borders (syncytial growth) and little to no glandular formation (poor differentiation) are the 3 main characteristics of medullary carcinoma in the pancreas. Abundant tumor infiltrating lymphocytes are also present. Answer D is incorrect because medullary carcinoma of the pancreas is associated with microsatellite instability (MSI), which may aid in the diagnosis. Answer C is incorrect because unlike conventional PDAC, medullary carcinoma of the pancreas is associated with wild type KRAS status. Answer A is incorrect because EBV encoded RNA (EBER) in situ hybridization positivity describes EBV associated lymphoepithelioma-like carcinoma. Answer B is incorrect because medullary carcinoma of the pancreas lacks well formed glands and is defined by syncytial growth pattern.
Comment Here
Reference: Medullary carcinoma
Comment Here
Reference: Medullary carcinoma
MEN1 syndrome
Table of Contents
Definition / general | Essential features | Epidemiology | Sites | Pathophysiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Gross images | Microscopic (histologic) images | Molecular / cytogenetics description | Board review style question #1 | Board review style answer #1Definition / general
- Syndrome characterized by mutations in MEN1 gene and development of multiple endocrine tumors (and other tumor types)
- Formerly known as Wermer syndrome
- Autosomal dominant inheritance
Essential features
- Defined by MEN1 mutation (chromosome 11q13, menin, tumor suppressor gene, 100% penetrance)
- Classic constellation of pituitary, parathyroid and pancreatic tumors (the 3 P's)
- Variety of nonendocrine tumors are also part of MEN1 (meningioma, ependymoma, angiofibroma)
Epidemiology
- Prevalence of MEN1 is 1:30,000 to 1:500,000 (geographic variability due to familial clustering) (F1000Res 2017;6:73)
- MEN1 cases are 90% familial and 10% sporadic (F1000Res 2017;6:73)
- 100% penetrance (100% with parathyroid adenomas by age 50) (F1000Res 2017;6:73)
- Most frequent cause of MEN1 related death is complications secondary to pancreatic / gastrointestinal neuroendocrine tumors (J Pediatr Genet 2016;5:89)
Sites
- Variety of neuroendocrine and nonneuroendocrine tumors (J Pediatr Genet 2016;5:89, Cancer Genet 2016;209:36, Endocr Relat Cancer 2017;24:T119)
- Parathyroid (90% of patients)
- Anterior pituitary (30 - 40% of patients)
- Pancreas / gastrointestinal tract (30 - 70% of patients)
- Adrenal cortex
- Thymus
- Facial skin (angiofibromas, collagenomas)
- Testis (epididymomas)
- Meninges (meningiomas)
-
Of note, nonsyndromic tumors have somatic MEN1 mutations at the following frequencies (Endocr Relat Cancer 2017;24:T119):
- Glucagonoma 60%
- VIPoma 57%
- Nonfunctioning PanNETs 44%
- Gastrinoma 38%
- Bronchial carcinoid 35%
- Parathyroid adenoma 35%
- Lipoma 28%
- Insulinoma 2 - 19%
- Angiofibroma 10%
- Anterior pituitary tumor 3.5%
- Adrenocortical tumor 2%
Pathophysiology
- MEN1 gene first identified in 1997, found on chromosome 11q13 (Science 1997;276:404)
- Menin, the protein product of MEN1, is a tumor suppressor gene
- Twelve mutations were initially identified and more than 1,800 mutations have been identified to date
- Most of these are private (family specific)
- Seventy five percent are inactivating (F1000Res 2017;6:73)
- 5 - 20% may not harbor mutations in the MEN1 gene coding region (F1000Res 2017;6:73, Cancer Genet 2016;209:36)
- There is no genotype phenotype correlation with the type of MEN1 mutation and the type of tumors which develop (F1000Res 2017;6:73)
- Menin does not show homology with any other known protein but is conserved across species from Drosophila to humans (F1000Res 2017;6:73, Cancer Genet 2016;209:36)
- Normal functions of menin: cell proliferation, apoptosis and genome integrity (F1000Res 2017;6:73)
- Mechanism by which a nonfunctional menin protein causes tumor development remains unknown
Clinical features
- Endocrine tumors found in parathyroid, anterior pituitary, pancreas / gastrointestinal tract, adrenal cortex and thymus
- Non endocrine tumors: angiofibromas, collagenomas, epididymomas, meningiomas
- Increased frequency of renal stones compared with the general population
- Penetrance is 100%, with all patients developing parathyroid adenomas by age 50
- Patients with gastrinomas, typically found in the duodenum, may have Zollinger-Ellison syndrome
- Primary gastrinomas may be microadenomas but the lymph node metastases can be much larger than the primary (J Pediatr Genet 2016;5:89)
- Patients with parathyroid adenomas have hypersecretion of parathyroid hormone (PTH) with resultant symptoms of hypercalcemia (GI pain, renal stones, clouded mental functioning)
- Cushing disease, Nelson syndrome, acromegaly or hyperprolactinemia in cases of functional pituitary adenomas
- Pituitary adenomas can also cause a number of visual field defects due to compression of the optic chiasm or extraocular muscle compression
- Syndromes of other functioning pancreatic and gastrointestinal neuroendocrine neoplasms can be found on Neuroendocrine Neoplasms - General
Diagnosis
- Identification of a MEN1 mutation by PCR amplification of exons and splicing sites
- Notably, this fails to identify the subset of MEN1 patients that do not have the mutation within the coding region (World J Exp Med 2015;5:124)
- Next generation sequencing would bypass these limitations
- Mutational analysis should be offered to:
- Asymptomatic first degree relatives of a known MEN1 patient
- Patients with at least two typical MEN1 tumors or one MEN1 tumor and a positive family history
- Patients with early onset (MEN1 manifestation (hyperparathyroidism, Zollinger-Ellison syndrome) (Endocr Relat Cancer 2017;24:T209)
Laboratory
- Relevant to the type of tumor present and only functional tumors
Radiology description
- Osteoporosis, terminal tuft erosion (distal phalanges) and nephrolithiasis in patients with parathyroid adenomas and hypercalcemia
- Calvarial thickening, frontal bossing, enlarged sinuses (especially frontal sinuses), enlarged sella turcica, prognathic mandible and increased vertebral fractures in patients with acromegaly due to pituitary adenoma
Prognostic factors
- As described for pancreatic and gastrointestinal tumors in the WHO classification
Case reports
- 18 year old woman with hyperprolactinemia and hypercalcemia since age 13 (Clinical Chemistry 2015;61:1328)
- 36 year old man with pancreatic neuroendocrine tumor, pituitary adenoma and acromegaly (W V Med J 2012;108:26)
- 51 year old man with urolithiasis and 2 jejunal perforations 9 months apart (Korean J Gastroenterol 2013;61:333)
Treatment
- Surgical resection of neuroendocrine tumors
- Minimally invasive trans sphenoidal approach for pituitary adenomas
- Increased screening and surveillance in known kindreds: insulinoma starting age 5, pituitary adenoma starting age 5, parathyroid adenoma starting age 8, gastrinoma starting age 20, adrenal gland starting before age 10, thymic tumors starting before age 15 (World J Exp Med 2015;5:124)
Microscopic (histologic) images
Molecular / cytogenetics description
- MEN1 mutation (chromosome 11q13)
Board review style question #1
- Which of the following patients would NOT be representative of a patient with MEN1 syndrome?
- A patient with facial angiofibromas, trichodiscomas and achrochordons
- A patient with MEN1 mutation and no tumor development at time of evaluation
- A patient with parathyroid adenoma and nephrolithiasis
- A patient with parathyroid adenoma, meningioma and epididymoma
- A patient with parathyroid adenoma, pituitary adenoma and pancreatic neuroendocrine tumor
Board review style answer #1
A. The patient NOT representative of a patient with MEN1 syndrome is the patient with angiofibromas, trichodiscomas and achrochordons. Angiofibromas are a part of MEN1 syndrome but trichodiscomas and achrochordons are not (they are part of Birt-Hogg-Dubé syndrome).
Comment Here
Reference: MEN1 syndrome
Comment Here
Reference: MEN1 syndrome
Mixed neuroendocrine nonneuroendocrine neoplasms (MiNENs)
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Pancreatic mixed neuroendocrine nonneuroendocrine neoplasms (MiNENs) are rare and heterogeneous malignancies characterized by histologically recognizable neuroendocrine (i.e., carcinoma and rarely, a well differentiated neuroendocrine tumor) and exocrine (usually ductal carcinoma, acinar carcinoma or both) components, each composing ≥ 30% of the neoplasm volume (Endocr Pathol 2016;27:284)
Essential features
- Histologically recognizable neuroendocrine and nonneuroendocrine components each comprise ≥ 30% of the neoplasm
- Neuroendocrine component is mostly a poorly differentiated neuroendocrine carcinoma (NEC); rarely a well differentiated neuroendocrine tumor (NET)
- Exocrine component can either be pancreatic ductal adenocarcinoma (PDAC) or acinar cell carcinoma (ACC); very rarely, both acinar and ductal carcinoma
- Neuroendocrine and nonneuroendocrine components are confirmed with immunohistochemistry (IHC)
- Poor prognosis
Terminology
- Subtypes: mixed ductal neuroendocrine carcinoma, mixed acinar neuroendocrine carcinoma, mixed acinar neuroendocrine ductal carcinoma
- Not recommended: composite carcinoid, mucin producing carcinoid, argentaffin cell adenocarcinoma, goblet cell carcinoid, adenocarcinoid, small cell undifferentiated carcinoma (Cancers (Basel) 2012;4:11)
ICD coding
- ICD-O: 8154/3 - mixed pancreatic endocrine and exocrine tumor, malignant
- ICD-10:
- ICD-11: 2C10.0 & XH8E54 - mixed neuroendocrine nonneuroendocrine neoplasm (MiNEN)
Epidemiology
- M = F
- Average age at presentation is 68 years (ranges from 21 - 84 years) (Am J Surg Pathol 1994;18:765)
- Mixed ductal neuroendocrine carcinomas comprise 0.5 - 2% of all ductal adenocarcinomas (Virchows Arch 2003;442:258)
- Mixed acinar neuroendocrine carcinomas comprise 15 - 20% of all acinar carcinomas (Am J Surg Pathol 2012;36:1782)
Sites
- Anywhere in the pancreas
- Mixed ductal neuroendocrine carcinomas are more common in the head of the pancreas
Pathophysiology
- Unclear
- May arise as the combination of 2 neoplastic clones or as the proliferation of 1 precursor cell with divergent differentiation; however, molecular and genetic studies point towards a monoclonal origin of both components (Endocr Pathol 2016;27:284, J Clin Med 2020;9:273)
- Behavior is thought to be driven by the neuroendocrine component (J Clin Med 2020;9:273)
Etiology
- Unknown
Clinical features
- Presentation is nonspecific and depends on the tumor size and degree of metastatic disease
Diagnosis
- Histomorphology and IHC on surgical resection specimens
- Biopsy may be used but is vulnerable to limited sampling and inadequate quantification of tumor components (Int J Surg Pathol 2017;25:585)
Laboratory
- Similar to pancreatic adenocarcinomas
- Increase in biliary and pancreatic enzymes may occur with obstruction of the common bile duct or main pancreatic duct (BMJ Case Rep 2020;13:e234855, Int J Surg Pathol 2017;25:585)
Radiology description
- Radiologic features of MiNENs can resemble either pancreatic adenocarcinoma or pancreatic neuroendocrine neoplasms on abdominal CT scan with contrast (Pancreatology 2021;21:224)
- Imaging findings similar to pancreatic adenocarcinomas (including hypodense hypoenhancing mass with ill defined border) are commonly seen with mixed ductal neuroendocrine carcinomas
- Imaging findings similar to pancreatic neuroendocrine neoplasms (including heterogeneously enhancing mass with or without cystic component and well defined margins) are commonly seen with mixed acinar neuroendocrine carcinomas
Radiology images
Prognostic factors
- Tumor size and resectability are the most important prognostic factors
- Mixed ductal neuroendocrine carcinoma: 5 year survival rates are similar to PDAC and NEC
- Mixed acinar neuroendocrine carcinoma: similar to 5 year survival rate (36 - 72%) of pure acinar cell carcinoma (Am J Surg Pathol 2012;36:1782)
- Recent study demonstrated that acinar MiNEN patients have a worse prognosis than ACC and NEC (Pancreatology 2021;21:224)
- A poorly differentiated neuroendocrine carcinoma component comprising > 50% of the tumor is associated with distant metastasis and poor prognosis (J Chin Med Assoc 2015;78:454)
Case reports
- 33 year old man with uncinate mass and a 66 year old man with pancreatic tail mass (Diagn Cytopathol 2018;46:971)
- 49 year old Indian man presented with constant, dull, aching epigastric pain (Int J Surg Case Rep 2021;88:106524)
- 61 year old man was admitted to the hospital with incidental pancreatitis (Asian J Surg 2022;45:520)
- 63 year old man presented with hyperglycemia (Surg Case Rep 2016;2:133)
- 70 year old man who was asymptomatic underwent a FNA after an incidental pancreatic mass was found on ultrasound (Clin J Gastroenterol 2022;15:244)
- 81 year old man presented with painful progressive jaundice for 1 month (BMJ Case Rep 2020;13:e234855)
Treatment
- No clear treatment guidelines due to rarity of tumors
- Some recommend treatment based on the neuroendocrine component since it seems to be the most aggressive (Neuroendocrinology 2016;103:186, J Clin Med 2020;9:273)
- Radical surgical resection with or without adjuvant chemotherapy for localized cases
- Chemotherapy (platinum based) for extensively metastatic cases
Gross description
- Mixed ductal neuroendocrine carcinomas are solid tumors with infiltrative margins similar to ductal adenocarcinomas
- Mixed acinar neuroendocrine carcinomas are usually nodular, fleshy, hemorrhagic and necrotic, similar to neuroendocrine carcinoma and acinar cell carcinoma (Am J Surg Pathol 1994;18:765)
Microscopic (histologic) description
- Neuroendocrine and nonneuroendocrine components are intimately intermixed and sometimes can only be differentiated with IHC (Int J Surg Pathol 2017;25:585)
- Both components must comprise ≥ 30% of the neoplasm (Endocr Pathol 2016;27:284)
- Neuroendocrine component can either be poorly differentiated neuroendocrine carcinoma or well differentiated neuroendocrine tumor (Surg Pathol Clin 2020;13:377)
- Poorly differentiated neuroendocrine carcinoma (90% of MiNENs) (J Clin Med 2020;9:273):
- Poorly formed trabeculae and nests or diffuse sheets of cells
- Can be large cell types (moderate to abundant eosinophilic cytoplasm, fine to vesicular chromatin and prominent nucleoli) or small cell types (high nuclear to cytoplasmic ratio, hyperchromatic nuclei with fine chromatin, inconspicuous nucleoli)
- Multiple foci of necrosis or geographic necrosis and numerous apoptotic bodies
- Mitoses are frequent
- Perineural and vascular invasion is common
- Well differentiated neuroendocrine tumor:
- Nested, lobular or trabecular architecture composed of monomorphic, regularly arranged cells
- Nuclei with stippled (salt and pepper) chromatin
- Inconspicuous nucleoli
- Poorly differentiated neuroendocrine carcinoma (90% of MiNENs) (J Clin Med 2020;9:273):
- Ductal adenocarcinoma component:
- Identical to conventional pancreatic ductal adenocarcinoma
- Grade ranges from well differentiated to poorly differentiated adenocarcinoma
- Infiltrating, well to poorly formed glandular, cribriform, solid or trabecular structures surrounded by desmoplastic stroma
- Mucin production
- Perineural and vascular invasion are common
- Acinar cell carcinoma component:
- Identical to acinar cell carcinoma
- Highly cellular
- Cystic, acinar or glandular architecture
- Abundant eosinophilic granular cytoplasm
- Monomorphic nuclei with a single prominent nucleolus
- Perineural and vascular invasion are common
Microscopic (histologic) images
Cytology description
- Diagnosis of MiNENs on FNA is exceedingly challenging due to cytomorphologic variability, sampling bias and the often minimal quantity of tissue available for cell block and (IHC (Diagn Cytopathol 2013;41:164, Acta Cytol 2013;57:296, Diagn Cytopathol 2018;46:971)
- Mixed cytologic features of neuroendocrine and exocrine component (adenocarcinoma or acinar) should raise suspicion of MiNEN
- IHC is helpful to demonstrate the endocrine and exocrine components
Positive stains
- IHC expression is similar to the respective component of MiNENs (Endocr Pathol 2016;27:284)
- Neuroendocrine component (Pathology 2020;52:336):
- Positive stains:
- Synaptophysin (diffusely and strongly positive)
- Chromogranin A (may be reduced in poorly differentiated neuroendocrine components)
- INSM1 (Am J Clin Pathol 2015;144:579)
- Cytokeratins CAM5.2, AE1 / AE3, CK8/18
- Ki67: proliferation index is typically high in poorly differentiated NEC (> 50%); required for grading of well differentiated NET component, grade 1 ( 20%)
- Panel to differentiate poorly differentiated neuroendocrine carcinoma from well differentiated neuroendocrine tumor components (Arch Pathol Lab Med 2019;143:1317)
- Positive stains:
- Ductal adenocarcinoma component:
- Common expression of CEA, CAM5.2, AE1 / AE3, CK7, CK8/18, CK19, EMA (MUC1), MUC3, MUC4, MUC5AC, CA 19-9, CA125
- Maspin, placental S100 (S100P), IMP3 (Arch Pathol Lab Med 2012;136:601, Hum Pathol 2013;44:503)
- Acinar cell carcinoma component (Pathology 2020;52:336):
Negative stains
- Neuroendocrine component:
- Trypsin (Am J Surg Pathol 2002;26:893)
- Chymotrypsin, BCL10 (Arch Pathol Lab Med 2019;143:1317)
- Ductal adenocarcinoma component:
- CK20, synaptophysin, chromogranin, trypsin, chymotrypsin, lipase
- pVHL (Hum Pathol 2013;44:503)
- Loss of SMAD4 / DPC4 (Am J Clin Pathol 2001;116:831)
- Acinar cell carcinoma component:
- Chromogranin and synaptophysin (synaptophysin can be focally positive)
Electron microscopy description
- Electron microscopy diagnostic findings (Am J Surg Pathol 1994;18:765, Pathol Int 2021;71:485):
- Secretory zymogen granules in acinar cell component; usually > 500 nm in size
- Neuroendocrine granules in neuroendocrine component; usually 100 - 200 nm in size
- Mucinous granules in ductal adenocarcinoma component
Molecular / cytogenetics description
- Not well understood but may show molecular alterations similar to conventional neuroendocrine neoplasms, ductal adenocarcinomas and acinar carcinomas, as highlighted below (Endocr Pathol 2016;27:284)
- Neuroendocrine carcinoma component:
- TP53 and RB1 (Am J Surg Pathol 2012;36:173)
- Ductal adenocarcinoma component:
- KRAS, CDKN2A (P16), TP53 and SMAD4 (DPC4) (J Clin Med 2020;9:273, Cancer Cell 2017;32:185, Hum Pathol 2009;40:612)
- Acinar carcinoma component:
- APC / beta catenin alterations, BRAF fusions (Virchows Arch 2014;464:553, Cancer Discov 2014;4:1398, J Clin Med 2020;9:273)
- Neuroendocrine carcinoma component:
- No known association with genetic syndromes
Videos
MiNEN basics
Sample pathology report
- Pancreas tail and spleen, distal pancreatectomy:
- Mixed neuroendocrine and nonneuroendocrine neoplasm (MiNEN) of pancreas composed of ductal adenocarcinoma and neuroendocrine carcinoma, 3.5 cm (see comment and synoptic report)
- Tumor infiltrates the peripancreatic soft tissue
- Prominent lymphovascular and perineural invasion is identified
- Pancreatic parenchymal resection margin is negative for tumor
- Metastatic neuroendocrine carcinoma is identified in 8 of 21 lymph nodes
- Pathology stage: pT3 / N2
- Spleen with no significant diagnostic alterations
- Comment: The sections of the pancreatic tail mass show a biphasic appearance with histologically distinct ductal and neuroendocrine carcinoma components. The neuroendocrine component comprises approximately 60% of the tumor and is characterized by infiltrating sheets and nests of high grade tumor cells with moderate eosinophilic cytoplasm, vesicular chromatin and viably prominent nucleoli. Frequent mitoses are noted (up to 25/HPF). Areas of tumor necrosis are identified. A well differentiated ductal adenocarcinoma component, characterized by infiltrating well formed tubules, is seen intricately associated with the neuroendocrine carcinoma component. Neuroendocrine carcinoma shows diffuse and strong expression for neuroendocrine markers chromogranin and synaptophysin. The ductal carcinoma component is negative for neuroendocrine markers. Neuroendocrine carcinoma shows a Ki67 proliferative index of approximately 90%. Overall, the findings are consistent with mixed ductal neuroendocrine carcinoma of the pancreas.
- Mixed neuroendocrine and nonneuroendocrine neoplasm (MiNEN) of pancreas composed of ductal adenocarcinoma and neuroendocrine carcinoma, 3.5 cm (see comment and synoptic report)
Differential diagnosis
- Amphicrine carcinoma (Pathol Int 2021;71:485):
- May represent a variant of MiNEN but does not fit under the current definition of MiNEN
- Rare tumor where each neoplastic cell displays exocrine and endocrine differentiation confirmed with IHC
- Mixed intraductal papillary mucinous neoplasm (IPMN) neuroendocrine tumor (Virchows Arch 2021;478:1215):
- Rare tumor characterized by low or high grade IPMN associated with a well differentiated neuroendocrine tumor in the papillae
- Recent evidence suggests common clonal origin with shared molecular abnormalities in both components
- May represent a variant of MiNEN
- Ductal adenocarcinoma with neuroendocrine component:
- Neuroendocrine component
- Pancreatic neuroendocrine tumor with entrapped ductules (Am J Surg Pathol 2004;28:813):
- Entrapment of preexisting nonneoplastic ductules by neuroendocrine tumor
- Acinar cell carcinoma with a neuroendocrine component:
- Neuroendocrine cells comprises May be focally positive for synaptophysin but should be negative for chromogranin; in contrast, in acinar MiNEN, immunohistochemistry for acinar and neuroendocrine cell markers demonstrates distinct compartments in the tumor tissue
- Collision tumors:
- Neuroendocrine and nonneuroendocrine components are distinct from each other and not intimately intermixed
- Pancreatoblastoma:
- Commonly a childhood malignant pancreatic neoplasm with predominantly acinar differentiation and squamoid nests
- Can have neuroendocrine component
- Squamoid nests are critical for diagnosis
Additional references
Board review style question #1
A 55 year old man presented with a 4 cm large pancreatic head mass. The sections from the resected tumor showed a mixed neoplasm with varied histologic findings, as highlighted in the image above. What is the most common component of such neoplasms?
- Acinar carcinoma
- Ductal adenocarcinoma
- Poorly differentiated neuroendocrine carcinoma
- Squamous cell carcinoma
- Well differentiated neuroendocrine tumor
Board review style answer #1
C. Poorly differentiated neuroendocrine carcinoma. The tumor is likely pancreatic mixed neuroendocrine nonneuroendocrine neoplasms (MiNENs) with ductal adenocarcinoma and poorly differentiated neuroendocrine carcinoma components. Poorly differentiated neuroendocrine carcinoma is the most common component seen in around 90% of MiNENs.
Comment Here
Reference: Mixed neuroendocrine nonneuroendocrine neoplasms pancreas (MiNENs)
Comment Here
Reference: Mixed neuroendocrine nonneuroendocrine neoplasms pancreas (MiNENs)
Board review style question #2
In a mixed neuroendocrine nonneuroendocrine neoplasm (MiNEN), what is the lowest percentage of the neoplasm the neuroendocrine component can comprise in order to classify as a MiNEN?
- 20%
- 30%
- 40%
- 50%
- 60%
Board review style answer #2
Molecular genetics of pancreatic ductal carcinoma
Table of Contents
Definition / general | Essential features | Somatic mutations | Germline mutations / genetic syndromes | Transcriptomic subtypes | Other relevant genetic alterations | Clinical features | Prognostic factors | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Pancreatic ductal adenocarcinoma (PDAC) is the most common type of pancreatic cancer, representing > 90% of all pancreatic malignancies
- From a genetic point of view, PDAC is a complex disease with several genes altered through different mechanisms, which include point mutations, chromosomal aberrations and epigenetic changes
Essential features
- Somatic mutations affect, above all, four genes: KRAS, which is an oncogene and TP53, CDKN2A and SMAD4, which are tumor suppressor genes (Lancet 2016;388:73)
- A subset of pancreatic cancer arises in the background of genetic syndromes with germline mutations affecting different genes, such as CDKN2A, TP53, STK11, BRCA1 / 2, ATM and MMR (J Clin Oncol 2017;35:3382)
- Transcriptomic profile divides pancreatic cancer into different groups; among them, the most important distinction involves the identification of the so called squamous subtype, which has the worst prognosis (Nature 2016;531:47)
- Only a few molecular alterations have a predictive value in pancreatic cancer
- BRCA mutated tumors show a good response to platinum based therapy and to poly ADP ribose polymerase (PARP) inhibitors; tumors with microsatellite instability can be treated with immunotherapy (N Engl J Med 2019;381:317, J Clin Oncol 2020;38:1)
Somatic mutations
- Four genes are the most commonly mutated in PDAC: KRAS, which is an oncogene and TP53, CDKN2A and SMAD4, which are tumor suppressor genes (Lancet 2016;388:73)
- KRAS
- Most frequently mutated gene in PDAC (90 - 92% of cases)
- Oncogene
- Located on chromosome 12
- Downstream pathways: MAPK and PI3K
- TP53
- Mutated in 50 - 60% of PDAC
- Tumor suppressor gene
- Located on chromosome 17
- Encodes for P53, a crucial protein that controls cell growth, metabolism, senescence, DNA repair and apoptosis
- CDKN2A
- Mutated in > 60% of PDAC
- Tumor suppressor gene
- Located on chromosome 9
- Encodes for P16, a protein encoded that acts as a mediator of the retinoblastoma signaling pathway
- SMAD4
- Mutated in 30 - 55% of PDAC
- Tumor suppressor gene
- Located on chromosome 18
- Protein encoded by SMAD4 is an effector of the transforming growth factor (TGF) β signaling pathway
- KRAS
- In addition to these four main genetic drivers, other important genes can be altered by somatic mutations in PDAC with a lower but significant prevalence (Lancet 2016;388:73, Gastroenterology 2019;156:2242):
- ARID1A (AT rich interaction domain 1A, 5 - 10% of cases), a tumor suppressor located on chromosome 3 involved in chromatin remodeling
- BRCA1 (breast cancer gene-1, up to 2% of cases) and BRCA2 (breast cancer gene-2, up to 7% of cases) are tumor suppressor genes located on chromosome 17 and 13, respectively, involved in the process of homologous recombination repair pathway
- BRAF (B-Raf proto-oncogene, up to 2% of cases), an oncogene located on chromosome 7, involved in MAPK pathway and with mutations that are almost always mutually exclusive with those affecting KRAS
- GNAS (guanine nucleotide binding protein, alpha stimulating activity polypeptide) is an oncogene located on chromosome 20; is typically mutated in the case of a cancer derived from an intraductal papillary mucinous neoplasm (IPMN)
Germline mutations / genetic syndromes
- A subset of pancreatic cancer arises in the background of genetic syndromes with germline mutations most commonly affecting the following genes
(J Clin Oncol 2017;35:3382, Ann Gastroenterol Surg 2020;4:229):
- CDKN2A: germline mutations of this gene characterize the familial atypical multiple mole melanoma (FAMMM) syndrome, an autosomal dominant syndrome in which the cumulative risk for PDAC development is up to 15%
- TP53: germline mutations of this gene characterize the Li-Fraumeni syndrome, an autosomal dominant syndrome in which the cumulative risk for PDAC development is up to 5%
- BRCA1 / 2 and PALB2: germline mutations of these genes characterize the hereditary breast and ovarian cancer syndrome, an autosomal dominant syndrome in which the cumulative risk for PDAC development is up to 7%
- ATM: germline mutations of this gene characterize the ataxia telangiectasia, familial breast cancer syndrome, an autosomal recessive syndrome in which the cumulative risk for PDAC development is up to 5%
- MMR genes: germline mutations of these genes characterize Lynch syndrome, an autosomal dominant syndrome in which the cumulative risk for PDAC development is up to 9%
- STK11: germline mutations of this gene characterize the Peutz-Jeghers syndrome, an autosomal dominant syndrome in which the cumulative risk for PDAC development is up to 30%
- PRSS1 and SPINK1: germline mutations of these gene characterize the hereditary pancreatitis syndrome through an autosomal dominant (PRSS1) or recessive (SPINK1) inheritance, in which the cumulative risk for PDAC development is up to 50%
- CFTR: germline mutations of this gene characterize the cystic fibrosis syndrome, an autosomal recessive syndrome in which the cumulative risk for PDAC development is up to 5%
Transcriptomic subtypes
- Different studies have tried to address transcriptomic profiles and, along this line, the most reliable classification indicates the following four categories (Nat Med 2011;17:500, Nature 2016;531:47):
- Squamous subtype: characterized by a strong upregulation of TP63 and downregulation of GATA6; such tumors show the worst prognosis
- Pancreatic progenitor subtype: expresses transcriptional networks involving PDX1, FOXA2 and FOXA3, crucial for pancreatic cell fate determination
- Aberrantly differentiated endocrine exocrine (ADEX) subtype: expresses transcriptional networks crucial for more advanced stage of normal pancreatic differentiation
- Immunogenic subtype: correlates with a marked immune infiltrate, mainly B and T cells, with upregulation of immune checkpoints CTLA4 and PD1, suggesting a possible role for immunotherapy drugs in this tumor subtype
Other relevant genetic alterations
- Microsatellite instability (MSI): a genetic condition due to mutations of the mismatch repair (MMR) genes, leading to a hypermutated phenotype with mutations clustered in specific regions known as microsatellites (Ann Oncol 2019;30:1232)
- POLE mutation: a rare occurrence in pancreatic cancer associated with an ultra hypermutated phenotype due to mutations affecting polymerase ε; associated with prolonged survival (Pancreas 2020;49:999)
- Kinase fusion genes: genetic alterations present in KRAS wild type pancreatic cancer; the genes most commonly involved are FGFR2, RAF, ALK, RET, MET and NTRK1 (Gastroenterology 2019;156:2242)
Clinical features
- Some genetic alterations have been recently associated with specific clinical features (Gastroenterology 2019;156:2242):
- Mutations in KRAS, SMAD4 are more frequent in older patients (≥ 50 years)
- Alterations in BRCA1 / 2 are more frequent in younger patients (Mutations in KRAS, SMAD4 and CDKN2A are more frequent in female patients
- Mutations in GNAS and ATM are more frequent in male patients
Prognostic factors
- SMAD4 mutations are associated with more aggressive behavior and with a widespread metastasis pattern (J Clin Oncol 2009;27:1806)
- MSI tumors do not always have a more favorable prognosis, as in colorectal or gastric cancer (Gut 2020 Apr 29 [Epub ahead of print])
Treatment
- Tumors with germline BRCA mutations can be treated with platinum based therapy and with PARP inhibitors (N Engl J Med 2019;381:317)
- MSI tumors may be susceptible to immune checkpoint blockade (J Clin Oncol 2020;38:1)
- Cancer with ALK fusion may be treated with ALK inhibitors (J Natl Compr Canc Netw 2017;15:555)
Microscopic (histologic) description
- MSI tumors more often show a medullary or colloid morphology (Gut 2020 Apr 29 [Epub ahead of print])
- Germline mutated ATM tumors more often show a mucinous / colloid histology (Mod Pathol 2019;32:1806)
- Tumors with squamous features show a very poor prognosis (Nat Med 2011;17:500, Nature 2016;531:47)
Microscopic (histologic) images
Board review style question #1
Board review style answer #1
C. Mucinous colloid histology is a classic histology encountered in MSI pancreatic cancer.
Comment Here
Reference: Molecular genetics of pancreatic ductal carcinoma
Comment Here
Reference: Molecular genetics of pancreatic ductal carcinoma
Board review style question #2
Which is the most important and frequently mutated gene in pancreatic ductal adenocarcinoma?
- ARID1A
- CDKN2A
- KRAS
- SMAD4
- TP53
Board review style answer #2
C. KRAS is the most important and frequent mutated gene in pancreatic ductal adenocarcinoma.
Comment Here
Reference: Molecular genetics of pancreatic ductal carcinoma
Comment Here
Reference: Molecular genetics of pancreatic ductal carcinoma
Mucinous cystic neoplasm (MCN)
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1Definition / general
- Benign or potentially low grade malignant cystic epithelial neoplasm composed of cells which contain intracytoplasmic mucin (ICD-O: 8470/0 [Accessed 18 February 2021])
- WHO classification:
- MCN with low grade dysplasia (adenoma)
- MCN with high grade dysplasia (carcinoma in situ)
- MCN with invasive carcinoma
Essential features
- Cystic neoplastic lesion that is a precursor to pancreatic adenocarcinoma
- May harbor invasive carcinoma
- Presence of associated ovarian type stroma
ICD coding
Epidemiology
- Almost always women (> 95%); mean age of 45 years (Ann Surg 2008;247:571)
Sites
- Distal pancreas (> 95%) (Ann Surg 2008;247:571)
- Can also occur in the liver and gallbladder
- Metastases usually restricted to abdominal cavity; metastases to ovary may simulate primary ovarian tumors
Pathophysiology
- Ectopic ovarian stroma is thought to be seeded from primordial ovarian cells at early stages of embryonic development
- Cysts are later formed by hormones and growth factors released by the ovarian stroma (Am J Surg Pathol 1999;23:410)
Etiology
- No known etiology
Clinical features
- Usually a single lesion (Gut Liver 2015;9:571)
- May present as abdominal pain or acute pancreatitis (Ann Surg 2008;247:571)
- Majority are slow growing and asymptomatic (Gastroenterology Res 2014;7:44)
Diagnosis
- Cytology and lab analysis of pancreatic cyst fluid from endoscopic ultrasound guided FNA
- Histology of pancreatic resection
Laboratory
- Elevated carcinoembryonic antigen (CEA) and presence of KRAS mutation in cyst fluid supports a mucinous cyst (includes MCN and intraductal papillary mucinous neoplasm) (Ann Gastroenterol;26:122)
Radiology description
- Thick walled, single, septated cyst in the body or tail of the pancreas (Gut Liver 2015;9:571)
- May have nodules or calcifications
Prognostic factors
- Prognosis is excellent unless there is invasive carcinoma with extracapsular or diffuse intracapsular infiltration (Am J Surg Pathol 2015;39:179, Ann Surg 2008;247:571)
- Features associated with invasive carcinoma include increased cyst size (> 5 cm), intracystic papillary nodules > 1 cm in size and elevated serum CA19-9 (Am J Surg Pathol 2015;39:179)
- Less than 20% of cases have invasive carcinoma (Gut Liver 2015;9:571, Ann Surg 2008;247:571)
Case reports
- 38 year old woman with anaplastic carcinoma and mucinous cystic neoplasm of the pancreas during pregnancy (World J Gastroenterol 2008;14:132)
- 46 year old man with pancreatic mucinous cystic neoplasm with sarcomatous stroma metastasizing to liver (World J Surg Oncol 2013;11:100)
- 52 year old Japanese woman with anaplastic carcinoma combined with mucinous cystadenocarcinoma of the pancreas (Arch Pathol Lab Med 1997;121:1104)
- 65 year old man (JOP 2012;13:687)
Treatment
- Surgical resection is indicated for all MCNs (Gut Liver 2015;9:571, Gastroenterology Res 2014;7:44)
Gross description
- Large (mean 10 cm)
- Typically unilocular megacysts that do not communicate with ductal system, though up to 15% communicate with main pancreatic duct (Gut Liver 2015;9:571)
- Cyst wall is papillary, trabecular or thickened
- Has mucoid / watery cyst contents
- Must sample solid areas within the cyst
Gross images
Contributed by Diana Agostini-Vulaj, D.O. and AFIP images
Images hosted on other servers:
Microscopic (histologic) description
- Large cyst lined by intestinal, pseudopyloric or gastric foveolar type epithelium that often form papillae, surrounded by characteristic dense ovarian type stroma (Gut Liver 2015;9:571)
- Epithelial lining has variable atypia (none, low grade, high grade); scattered neuroendocrine cells may be present
- Invasive adenocarcinoma may or may not be present; must sample extensively to rule out an invasive component (Gut Liver 2015;9:571, Am J Surg Pathol 1999;23:1320)
- Calcifications are common
- May have mural nodules with features of giant cell tumor, malignant fibrous histiocytoma or anaplastic carcinoma
Microscopic (histologic) images
Cytology description
- Cyst aspirates are usually acellular with thick, gelatinous mucus
- Clusters of 3 dimensional atypical glandular cells with hyperchromasia predict at least moderate dysplasia (Arch Pathol Lab Med 2009;133:388)
Positive stains
- Ovarian type stroma: CD10, ER, inhibin, PR, smooth muscle actin (SMA) and vimentin (Gut Liver 2015;9:571)
- Epithelium stains carcinoembryonic antigen (CEA), CK7, CK8, CK18 and CK19 (Gut Liver 2015;9:571)
- Gastric type epithelium stains MUC5AC (Gut Liver 2015;9:571)
- DPC4 (MADH4, SMAD4), MUC5AC present in situ areas (usually lost in invasive disease) (Am J Surg Pathol 2000;24:1544, Gut Liver 2015;9:571, Arch Pathol Lab Med 2015;139:24)
- Invasive component stains MUC1
- Cyst fluid may stain positive for Alcian blue and mucicarmine
- S100P in epithelium (Arch Pathol Lab Med 2015;139:24)
- SF1 in stroma (Pathol Int 2016;66:281)
Negative stains
- MUC1 (except in invasive components) (Am J Surg Pathol 2002;26:466, Gut Liver 2015;9:571)
- MUC2 (except for faint staining of goblet cells)
- DPC4 (MADH4, SMAD4) staining is lost in invasive MCNs (Gut Liver 2015;9:571)
- pVHL (Arch Pathol Lab Med 2015;139:24)
Molecular / cytogenetics description
- KRAS mutations noted in in situ or invasive areas, inactivating SMAD4 and TP53 mutations in more advanced MCNs (Gut Liver 2015;9:571)
- Negative for GNAS mutations
Sample pathology report
- Pancreas and duodenum, Whipple resection:
- Mucinous cystic neoplasm with low grade intraepithelial neoplasia (8.3 cm) (see comment)
- Negative for high grade intraepithelial neoplasia or malignancy.
- Focal background chronic pancreatitis
- Margins of resection unremarkable.
- Seven benign lymph nodes.
- Comment: The gross cystic lesion was entirely submitted for microscopic analysis.
- Pancreas and duodenum, Whipple resection:
- Focal adenocarcinoma arising from a mucinous cystic neoplasm (see synoptic report)
Differential diagnosis
- Intraductal papillary mucinous neoplasm:
- Usually at the head of pancreas and communicates with the duct system (MCNs usually do not communicate with the main pancreatic duct)
- IPMNs may be positive for GNAS mutations while MCNs will be negative (Gut Liver 2015;9:571)
- Both have elevated CEA and KRAS mutation in cyst fluid
- Ovarian mucinous tumors:
- Similar clinical and histologic appearance
- Pancreatic ductal adenocarcinoma, large duct variant:
- Will have smaller cysts, clustering of ducts and myxoid stroma (Arch Pathol Lab Med 2009;133:423)
- Pancreatic ductal adenocarcinoma:
- Usually not cystic, shows irregular infiltrative glands with nuclear atypia, no ovarian type stroma
- Pancreatic pseudocyst:
- Mimics MCN when MCN has denuded cyst lining
- MCN cystic fluid has high CEA content and viscosity, high expression of microRNAs, lower amylase (< 250 U/L) and elastase I than pseudocyst, although values may vary within different loculi of same neoplasm (Am J Clin Pathol 1993;100:425, Ann Gastroenterol 2013;26:122, Gastroenterology Res 2014;7:44)
- Serous cystadenoma:
- Has low levels of CEA
Board review style question #1
Which of the following findings on fine needle aspiration of a pancreatic cyst are most consistent with a mucinous cystic neoplasm?
- Decreased CEA, decreased amylase, no KRAS mutation, no GNAS mutation
- Elevated CEA, elevated amylase, KRAS mutation, GNAS mutated
- Elevated CEA, highly elevated amylase, no KRAS mutation, no GNAS mutation
- Elevated CEA, variable amylase, KRAS mutation, no GNAS mutation
Board review style answer #1
D. Mucinous cystic neoplasms have elevated CEA, variable amylase, KRAS mutation and no GNAS mutation. If a GNAS mutation is present, then the findings will favor an IPMN (answer B). Decreased CEA and amylase with no KRAS or GNAS mutations favor a serous cystadenoma (answer A). An elevated CEA and amylase without KRAS or GNAS mutations will favor a pancreatic pseudocyst (answer C).
Comment Here
Reference: Mucinous cystic neoplasm (MCN)
Comment Here
Reference: Mucinous cystic neoplasm (MCN)
Mucinous pancreatic tumor overview
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Neoplasms within the pancreas characterized by predominant mucin production by the tumor cells
- Mucus: a substance that is 95 - 98% water and 2 - 5% solids, with > 60% of the solid component consisting of mucin (World J Gastroenterol 2011;17:4757)
- Mucin: a glycoprotein consisting of a polypeptide backbone with attached oligosaccharide conjugates
Essential features
- General term for benign, low or high grade dysplastic or malignant mucin producing epithelium within the pancreas
- 4 most prevalent types
- MCN and IPMN may harbor or develop into pancreatic ductal adenocarcinoma (small percentage); MCN may undergo transformation to cystadenocarcinoma as well (Pancreas 2017;46:745)
| Simple mucinous cyst / MNC | MCN | IPMN | Pancreatic ductal adenocarcinoma | Age, decade | Sixth - seventh | Fourth - fifth | Sixth - seventh | Sixth - eighth |
| Location | Head = body = tail | Body / tail > head | Head > body / tail | Head > body / tail |
| Sex predilection | F > M | F >>> M | Main duct: M > F Branch duct: F > M |
M > F |
| CEA | ↑ | ↑ | ↑ | N / A |
| Amylase | ↓ | ↓ | ↑ | N / A |
| Pancreatic duct communication | Rare | No | Yes | Yes |
| Precursor for invasive carcinoma | No | Yes | Yes | N / A |
| Cytologic atypia | No | Low grade / high grade | Low grade / high grade | N / A |
| Epithelium type | Flat | Flat or papillary | Papillary | Flat or papillary |
| Ovarian stroma | No | Yes | No | N / A |
| Molecular mutations | KMT2C, KRAS, BRAF, RNF43, CDKN2A, TP53, SMAD4 | KRAS, RNF43 (low grade); SMAD4, TP53 (high grade) | GNAS, KRAS, RNF43, TP53 | KRAS, TP53, CDKN2A, SMAD4, BRCA1/2 |
Terminology
- Simple mucinous cyst / mucinous nonneoplastic cyst
- Mucinous cystic neoplasm / mucinous cystadenoma
- Intraductal papillary mucinous neoplasm / carcinoma in situ
- Pancreatic ductal adenocarcinoma / duct cell adenocarcinoma / infiltrating duct carcinoma / tubular adenocarcinoma
ICD coding
Epidemiology
- Simple mucinous cyst / mucinous nonneoplastic cyst (MNC)
- 2 - 7% of pancreatic cyst resections and 8 - 10% of mucinous cysts of the pancreas
- More common in women (F:M = 2.8:1), mean age of 64 years old (Arch Pathol Lab Med 2017;141:1330)
- Mucinous cystic neoplasm (MCN)
- Comprises ~25% of resected pancreatic cystic neoplasms
- Almost exclusively in women (> 95%)
- Rare; 2 - 5% exocrine pancreatic tumors
- Most common in fourth through fifth decade (Arch Pathol Lab Med 2017;141:1330, Gut Liver 2015;9:571)
- Intraductal papillary mucinous neoplasm (IPMN)
- May account for between 18 and 50% of resected cystic pancreatic lesions (Arch Surg 2009;144:448, Pancreas 2008;37:254, Pancreas 2011;40:779)
- Main duct IPMN is slightly more common in men (M:F = 1.1:1), while branch duct IPMN is more common in women (M:F = 0.76:1) (Diagnostics (Basel) 2022;13:65)
- Most common in sixth through seventh decade (Arch Pathol Lab Med 2017;141:1330)
- Pancreatic ductal adenocarcinoma
- Most common pancreatic tumor (~90% of pancreatic cancer diagnoses)
- 80% occur in patients 60 - 80 years old
- Slightly more common in men (CA Cancer J Clin 2018;68:394)
- Colloid carcinoma / mucinous carcinoma is an uncommon variant with > 50% extracellular mucus production, accounting for 1 - 3%
Sites
- Simple mucinous cyst / mucinous nonneoplastic cyst (MNC)
- Occur equally in head / neck / uncinate and body / tail (Arch Pathol Lab Med 2017;141:1330)
- Mucinous cystic neoplasm (MCN)
- > 95% arise in the distal pancreas as a single lesion (Gut Liver 2015;9:571)
- Intraductal papillary mucinous neoplasm (IPMN)
- Can occur anywhere in the pancreas, most common in the head (~58%) (Am J Clin Pathol 2020;154:559)
- Pancreatic ductal adenocarcinoma
- 60 - 70% occur in pancreatic head
- Usually solitary, occasionally multifocal (Surg Pathol Clin 2016;9:547)
Pathophysiology
- Simple mucinous cyst / mucinous nonneoplastic cyst (MNC)
- Unknown pathogenesis but may develop from acinar ductal mucinous metaplasia (Hum Pathol 2010;41:513, Arch Pathol Lab Med 2017;141:1330)
- Mucinous cystic neoplasm (MCN)
- Stromal component possibly derived from ovarian primordium / Müllerian type stroma or primary yolk cells implanted in the pancreas during embryogenesis (Am J Surg Pathol 1999;23:410, Dig Surg 2006;23:186)
- Intraductal papillary mucinous neoplasm (IPMN)
- Histologically arising in the pancreatic main duct, branch duct or combined (Clin Gastroenterol Hepatol 2010;8:213)
- Pancreatic ductal adenocarcinoma
- Arises from pancreatic duct epithelia
- Precursor lesions include intraductal papillary mucinous neoplasm (IPMN), mucinous cystic neoplasm (MCN) and pancreatic intraepithelial neoplasia (PanIN)
Etiology
- Simple mucinous cyst / mucinous nonneoplastic cyst (MNC)
- Unclear etiology
- Mucinous cystic neoplasm (MCN)
- No known etiology
- Intraductal papillary mucinous neoplasm (IPMN)
- In one study, most patients with IPMN were cigarette smokers (Adv Anat Pathol 1999;6:65)
- Patients with Peutz-Jeghers syndrome, familial adenomatous polyposis and McCune-Albright syndrome are at increased risk (Adv Anat Pathol 1999;6:65, Gut 2002;51:446, Virchows Arch 2017;470:391)
- Pancreatic ductal adenocarcinoma
- Strongly associated with cigarette smoking (2 - 3x increased risk)
- History of pancreatitis (2 - 10 fold increased risk)
- Obesity / diet (risk increases with increasing body mass index [BMI])
- Hereditary pancreatic cancer syndrome (due to an underlying germline mutation)
- Familial pancreatic cancer syndrome (defined as pancreatic cancer in at least 2 first degree relatives without an underlying germline mutation)
- Other possible factors: high alcohol consumption, previous gastric surgery and diabetes (Lancet Gastroenterol Hepatol 2016;1:298, Lancet Gastroenterol Hepatol 2016;1:226, Medicine (Baltimore) 2017;96:e5908)
Clinical features
- Simple mucinous cyst / mucinous nonneoplastic cyst (MNC)
- Half of patients are asymptomatic; others report abdominal pain or jaundice (Arch Pathol Lab Med 2017;141:1330)
- Mucinous cystic neoplasm (MCN)
- More than half of patients are asymptomatic
- Causes compression of adjacent structures
- Symptoms include a palpable mass in the epigastric region and nonspecific abdominal pain or distension (World J Gastrointest Oncol 2020;12:642)
- Intraductal papillary mucinous neoplasm (IPMN)
- Majority are asymptomatic; often incidental
- Symptoms present in 2 - 20% of patients and include epigastric pain, weight loss, diabetes and jaundice (Gut Liver 2015;9:571, Arch Pathol Lab Med 2022;146:298)
- Pancreatic ductal adenocarcinoma
- Most commonly reported symptoms: loss of appetite (~45%), jaundice (~41%) and abdominal pain (40%)
- Other symptoms include weight loss, back pain, new onset diabetes, depression, migratory thrombophlebitis and acute pancreatitis (Clin Gastroenterol Hepatol 2004;2:510)
Diagnosis
- Overall, diagnosis of mucinous cysts and neoplasms of the pancreas relies on
- Cyst fluid analysis obtained from endoscopic ultrasound guided fine needle aspiration (EUS FNA)
- Cytology analysis (EUS FNA)
- Histologic analysis of surgical resection
- Reference: Front Physiol 2022;13:856803
Laboratory
- Simple mucinous cyst / mucinous nonneoplastic cyst (MNC)
- Cyst fluid often shows variably elevated carcinoembryonic antigen (CEA) and amylase
- Mucinous cystic neoplasm (MCN)
- Serum tumor markers include CEA, CA 19-9, TAG-72, CA 15-3 or MCA and low levels of amylase
- Cyst fluid often shows elevated CEA, CA 19-9 and low amylase
- Intraductal papillary mucinous neoplasm (IPMN)
- Serum amylase and lipase are elevated
- Cyst fluid shows elevated CEA and amylase
- Pancreatic ductal adenocarcinoma
- Serum tumor markers include CA 19-9, CEA
- Cyst fluid has high levels of CA 19-9, DUPAN-2, CEA, Span-1 (Arch Pathol Lab Med 2017;141:1330, Front Physiol 2022;13:856803)
Radiology description
- Simple mucinous cyst / mucinous nonneoplastic cyst (MNC)
- Single unilocular or multilocular cyst (Front Physiol 2022;13:856803)
- Mucinous cystic neoplasm (MCN)
- Well defined multilocular or unilocular thick walled cyst
- No connection with the main pancreatic duct (Front Physiol 2022;13:856803)
- Intraductal papillary mucinous neoplasm (IPMN)
- Dilated main pancreatic duct in main duct type IPMN
- Branch duct IPMN typically produces a grape-like cyst
- Mural nodules may correspond to high grade dysplasia (Front Physiol 2022;13:856803)
- Pancreatic ductal adenocarcinoma
- Irregular, solid hypodense mass
- Double duct sign (dilation of both the biliary and pancreatic ducts) is pathognomonic (Radiology 2011;260:446)
Prognostic factors
- Simple mucinous cyst / mucinous nonneoplastic cyst (MNC)
- Not associated with progression or invasive carcinoma (Arch Pathol Lab Med 2017;141:1330)
- Mucinous cystic neoplasm (MCN)
- 5 year survival rate of 96 - 100% after surgical resection (Am J Surg Pathol 2015;39:179, World J Surg Oncol 2020;18:287)
- No survival difference between MCNs with low grade or high grade dysplasia (Am J Surg Pathol 2018;42:578)
- Invasion limited to the ovarian type stroma, cystic septa or capsule has a favorable outcome (5 year survival 100%), while extracapsular invasion is associated with poor survival (J Pathol Clin Res 2021;7:507)
- Intraductal papillary mucinous neoplasm (IPMN)
- Absence of invasive carcinoma is the most important prognostic factor (Hum Pathol 2012;43:1)
- Main duct type shows highest incidence of invasive adenocarcinoma (30 - 50%)
- Branch type without mural nodules are more commonly found with low grade dysplasia (~24% with invasion) (Dig Liver Dis 2012;44:257, Am J Surg Pathol 2000;24:1372, Arch Pathol Lab Med 2022;146:298)
- Pancreatic ductal adenocarcinoma
- Most ductal adenocarcinomas are fatal (5 year survival ~5%)
- Survival time is longer in patients with tumors confined to the pancreas or Tumors in the body or tail tend to present at a more advanced stage (Cancer Res 2014;74:2913, J Gastrointest Surg 2000;4:567)
- Colloid carcinomas have a better prognosis than conventional adenocarcinomas with a 5 year survival rate of > 55% (Am J Surg Pathol 2003;27:571)
Case reports
- 32 year old woman with a large mucinous cystic neoplasm of the pancreas arising during pregnancy (J Surg Case Rep 2020;2020:rjaa085)
- 63 year old man with a 2 year history of abdominal pain without weight loss and a simple mucinous cyst (Virchows Arch 2021;479:179)
- 72 year old woman with pancreatic ductal carcinoma arising in pancreatic remnant 13 years after resected IPMN (Mol Clin Oncol 2018;8:417)
- 78 year old woman with a bowel obstruction and an intraductal papillary mucinous neoplasm (Radiol Case Rep 2023;18:1103)
- 91 year old woman with new onset diabetes mellitus and a pancreatic ductal adenocarcinoma (Cureus 2022;14:e31608)
Treatment
- Simple mucinous cyst / mucinous nonneoplastic cyst (MNC)
- Typically resected, may be treated conservatively with continued imaging follow up
- Mucinous cystic neoplasm (MCN)
- Surgical resection is typical
- Can follow with close imaging surveillance for poor surgical candidates with MCN without high risk imaging findings (Gut Liver 2015;9:571)
- Intraductal papillary mucinous neoplasm (IPMN)
- Surgical resection for main duct IPMNs
- Can follow with close imaging surveillance for branch duct IPMN < 3 cm without high risk imaging findings (Arch Pathol Lab Med 2022;146:298)
- Pancreatic ductal adenocarcinoma
- Radical resection with or without chemotherapy
Gross description
- Simple mucinous cyst / mucinous nonneoplastic cyst (MNC)
- Macroscopic cyst > 1 cm, usually 3 - 12 cm
- Unilocular or multilocular
- May contain clear, serous or mucinous fluid (Arch Pathol Lab Med 2017;141:1330)
- Mucinous cystic neoplasm (MCN)
- 2 - 35 cm in greatest dimension
- Unilocular or multilocular, thick walls
- Round mass with smooth surface and fibrous pseudocapsule, can have papillary projections in higher grade lesions
- Calcifications may be present
- May contain thick mucin, hemorrhage or necrotic debris (Am J Surg Pathol 2015;39:179)
- Intraductal papillary mucinous neoplasm (IPMN)
- 1 - 8 cm in greatest dimension
- Cystic, multiloculated
- Grape-like clusters in branch type
- Pancreatic ductal adenocarcinoma
- Firm, poorly defined mass, with yellow to white cut surface
- Can be difficult to differentiate from background pancreatitis (HPB (Oxford) 2014;16:83)
Gross images
Microscopic (histologic) description
- Simple mucinous cyst / mucinous nonneoplastic cyst (MNC)
- Cyst lined by monolayer of cuboidal to columnar mucinous epithelium
- Usually has gastric phenotype
- No ovarian type stroma present
- Little to no cytologic atypia and no / minimal papillary excrescences in epithelial lining
- No malignant potential
- Rare cases of focal high grade dysplasia reported (Arch Pathol Lab Med 2017;141:1330)
- Mucinous cystic neoplasm (MCN)
- Cyst lined by 2 components
- Inner epithelial layer composed predominantly of mucin producing cells
- Outer densely cellular Müllerian / ovarian type stroma
- No connection to ducts
- Stroma can undergo luteinization, can have hilar-like cells and immunophenotypic sex cord stromal differentiation
- 2 tiered grading system
- Low grade: mild moderate atypia, mitoses and papillary projections uncommon
- High grade: severe atypia, papillae formation with irregular branching, loss of nuclear polarity, frequent mitoses
- Invasion seen in ~16% of MCN (Arch Pathol Lab Med 2022;146:298)
- Cyst lined by 2 components
- Intraductal papillary mucinous neoplasm (IPMN)
- Cyst lined by neoplastic columnar / mucinous epithelium that can be flat or form papillae
- 3 types of epithelium
- Intestinal type (~20%): resembles villous adenomas of the gastrointestinal tract; often contains high grade dysplasia
- Gastric type (~70%): resembles gastric foveolar epithelium; often low grade dysplasia
- Pancreatobiliary type (~10%): frequently involves the main duct, shows complex branching of papillae and often contains high grade dysplasia
- 2 tiered grading system
- Low grade: mild moderate atypia, mitoses are uncommon
- High grade: severe atypia, irregular branching and budding of papillae, loss of nuclear polarity, frequent mitoses (Arch Pathol Lab Med 2022;146:298)
- Pancreatic ductal adenocarcinoma
- Classically shows well developed neoplastic glands that imitate normal pancreatic ducts in the background of desmoplastic stroma
- Neoplastic glands show an irregular and haphazard distribution with irregular contours and frequently contain neutrophils and necrotic debris
- Nuclear pleomorphism, prominent nucleoli and mitoses are typically frequent
- Mucin containing glands may be ruptured or poorly formed
- Most are well to moderately differentiated
- Grade (College of American Pathologists) based on extent of glandular differentiation
- Well differentiated (> 95% of tumor composed of glands)
- Moderately differentiated (50 - 95% of tumor composed of glands)
- Poorly differentiated (≤ 49% of tumor composed of glands)
- Colloid carcinoma is a subtype that contains ≥ 80% of neoplastic cells suspended in pools of mucin
Microscopic (histologic) images
Cytology description
- Simple mucinous cyst / mucinous nonneoplastic cyst (MNC)
- Cuboidal or columnar cells in strips or honeycomb sheets without significant atypia
- Background mucin and macrophages are frequently present (Pancreas 2013;42:27)
- Mucinous cystic neoplasm (MCN)
- Cannot be distinguished from IPMN on cytology
- Low grade MCN will have low cellularity with a variable amount of mucin with histiocytes and bland epithelial cells
- High grade MCN will have low to moderate cellularity, variable amount of mucin, pleomorphic, atypical cells with a high nuclear to cytoplasmic ratio and frequent necrosis
- Ovarian type stroma is not typically present (Cancer Cytopathol 2017;125:169)
- Intraductal papillary mucinous neoplasm (IPMN)
- Cannot be distinguished from MCN on cytology
- Low grade IPMN will have low cellularity with minimal nuclear atypia in epithelial cells and no background necrosis
- High grade IPMN will be more cellular with small epithelial cells with hyperchromatic, irregular nuclei and prominent background inflammation and necrosis in most cases (Cancer Cytopathol 2014;122:40)
- Pancreatic ductal adenocarcinoma
- Typically highly cellular, often with background necrosis
- Tissue fragments are composed of cells with irregular nuclear spacing (drunken honeycomb), hyperchromatic nuclei with irregular contours and occasional atypical mitotic figures (Cancer 2003;99:44)
Positive stains
Negative stains
- Simple mucinous cyst / mucinous nonneoplastic cyst (MNC)
- Trypsin, CEA, synaptophysin, chromogranin A, calretinin, alpha inhibin, MUC1 (35% positive), MUC2 (1% positive), PR (8% positive)
- CDX2 (can be focally positive) (Arch Pathol Lab Med 2017;141:1330, Am J Surg Pathol 2017;41:121, Virchows Arch 2021;479:179)
- Mucinous cystic neoplasm (MCN)
- MUC1 (can be aberrantly expressed in invasion), MUC2, pVHL (Arch Pathol Lab Med 2015;139:24)
- Intraductal papillary mucinous neoplasm (IPMN)
- MUC2 (in biliary and gastric types)
- MUC1 (in gastric and intestinal types) (Front Physiol 2022;13:856803)
- Pancreatic ductal adenocarcinoma
- CK20, vimentin, synaptophysin, chromogranin, MUC2
- pVHL (positive in reactive ducts) (Arch Pathol Lab Med 2012;136:601, Hum Pathol 2013;44:503)
- SMAD4 / DPC4 loss seen in 55%; specific for malignancy (Am J Clin Pathol 2001;116:831)
Molecular / cytogenetics description
- Simple mucinous cyst / mucinous nonneoplastic cyst (MNC)
- KMT2C (62%), KRAS (15%), TP53 (15%), BRAF (8%), RNF43 (8%), CDKN2A (8%), SMAD4 (8%)
- No mutations detected in 31% of cases (Hum Pathol 2020;101:1)
- Mucinous cystic neoplasm (MCN)
- KRAS (21 - 46%) and RNF43 (~50%) mutations in low grade lesions
- Inactivating and TP53 mutations in more advanced MCN
- No GNAS mutations (Gut Liver 2015;9:571, Proc Natl Acad Sci U S A 2011;108:21188, J Gastrointest Surg 2020;24:1201, Virchows Arch 2022;480:1189)
- Intraductal papillary mucinous neoplasm (IPMN)
- Commonly mutated: KRAS (90%), GNAS (40 - 60%), RNF43 (25%), TP53 (10%)
- Lower frequency: CDKN2A, CTNNB1, IDH1, STK11, PTEN (World J Gastroenterol 2021;27:2710)
- Pancreatic ductal adenocarcinoma
- Key drivers
- TP53 is inactivated in 50 - 75%
- KRAS: 70 - 95%, early event
- CDKN2A (p16): > 95%
- SMAD4: ~50 - 55%
- BRCA1 / BRCA2: 5 - 10%
- Other less common alterations: microsatellite instability (MSI), BRAF mutations, MGMT promotor hypermethylation (Cancer Cell 2017;32:185, World J Gastroenterol 2021;27:2710)
- Key drivers
Sample pathology report
- Distal pancreas and spleen, distal pancreatectomy and splenectomy:
- Intraductal papillary mucinous neoplasm (IPMN) (3.0 cm), low grade, involving branch ducts (see comment)
- Comment: Pancreatic resection margin, negative for dysplasia. No high grade dysplasia or invasive component was identified. 13 lymph nodes with reactive changes (0/13).
Differential diagnosis
Additional references
Board review style question #1
A 55 year old woman presents with a pancreatic cyst incidentally detected on abdominal imaging. The cyst is located in the head of the pancreas and measures ~3 cm in size. Endoscopic ultrasound guided fine needle aspiration (EUS FNA) is performed and analysis of the cyst fluid reveals the following laboratory values: high CEA level, elevated amylase and low viscosity. A picture of the resection specimen is shown above. Which of the following statements is true about the most likely diagnosis?
- This lesion can display multiple histologic subtypes that resemble gastric, intestinal or pancreatobiliary epithelium
- This lesion does not commonly connect with the pancreatic duct system
- This lesion is not a precursor for invasive ductal adenocarcinoma
- VHL is the most commonly mutated gene in this lesion
Board review style answer #1
A. This lesion can display multiple histologic subtypes that resemble gastric, intestinal or pancreatobiliary epithelium. This lesion in the head of the pancreas with elevated CEA and amylase levels in the cyst fluid is an intraductal papillary mucinous neoplasm (IPMN). The epithelium in IPMNs most commonly resembles gastric epithelium, with intestinal and pancreatobiliary types being less common.
Answer B is incorrect because IPMNs connect with the pancreatic duct system, which causes an increase in amylase levels in the cyst fluid. Other pancreatic cysts such as mucinous cystic neoplasm (MCN) and simple mucinous cysts do not typically connect with the pancreatic duct system.
Answer C is incorrect because invasive carcinomas are found in ~30 - 50% of main duct IPMNs and ~24% of branch duct IPMNs.
Answer D is incorrect because VHL is commonly mutated in serous cystadenomas, not IPMNs.
Comment Here
Reference: Mucinous pancreatic tumor overview
Comment Here
Reference: Mucinous pancreatic tumor overview
Board review style question #2
A 45 year old woman presents with abdominal discomfort and a large cystic lesion in the tail of the pancreas on abdominal imaging. Endoscopic ultrasound guided fine needle aspiration (EUS FNA) is performed and analysis of the cyst fluid reveals elevated levels of CEA, CA 19-9 and low amylase. A picture of the resection specimen is shown above. Which of the following statements about this pancreatic lesion is true?
- This lesion contains ovarian type stroma and is most commonly seen in females
- This lesion is most commonly seen in older men with a history of chronic pancreatitis
- This lesion is typically a benign cystic tumor that contains serous fluid
- These lesions are typically associated with elevated levels of amylase and CEA in the cyst fluid
Board review style answer #2
A. This lesion contains ovarian type stroma and is most commonly seen in females. This female patient with a cystic lesion in the tail of the pancreas with elevated CEA, CA 19-9 and low amylase has a mucinous cystic neoplasm (MCN). MCNs are characterized by the presence of ovarian type stroma and are seen almost exclusively in females (> 95%).
Answer B is incorrect because pancreatic ductal adenocarcinomas are more commonly associated with older men with a history of chronic pancreatitis. MCNs are seen in female patients and are not associated with chronic pancreatitis.
Answer C is incorrect because serous cystadenomas are benign cystic tumors that contain serous fluid. MCNs contain mucin and are characterized as low grade or high grade lesions with ~16% associated with invasive carcinoma.
Answer D is incorrect because the cyst fluid of MCNs typically has elevated CEA and CA 19-9 but has low amylase levels because they do not connect to the pancreatic duct system. The presence of elevated amylase and CEA levels in pancreatic cyst fluid is most often associated with an intraductal papillary mucinous neoplasm (IPMN).
Comment Here
Reference: Mucinous pancreatic tumor overview
Comment Here
Reference: Mucinous pancreatic tumor overview
Nesidioblastosis
Table of Contents
Definition / general | Clinical features | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) imagesDefinition / general
- Islets in intimate association with ducts, with formation of ductuloinsular complexes
- Also called congenital islet hyperplasia
- Indicates active formation of endocrine cells by multipotential cells in basal layer of ducts
Clinical features
- Normal in infants, exaggerated in neonatal hyperglycemia (infants of diabetic mothers)
- In adults, is rare cause of persistent hyperinsulinemic hypoglycemia
- Associated with Beckwith-Weidemann syndrome, chronic pancreatitis, cystic fibrosis, endocrine neoplasms, gastric bypass patients (Mod Pathol 2009;22:239), Zollinger-Ellison syndrome
- May be due to increase in expression of growth factors IGF2, IGF1Ra and TGFBR3 in islets (Mod Pathol 2009;22:239)
- Must rule out mutation in HADH gene (J Clin Endocrinol Metab 2011;96:E498)
- Either focal or diffuse (Am J Surg Pathol 1989;13:766):
- Focal: nodular hyperplasia of islet-like cell clusters, including ductuloinsular complexes and hypertrophied insulin cells with giant nuclei
- Diffuse: involves entire pancreas; irregularly sized islets and ductuloinsular complexes present, both contain hypertrophied insulin cells, peliosis type vascular ectasia
Case reports
- 44 year old man with symptomatic hypoglycemia and localized nesidioblastosis (Hum Pathol 2010;41:447)
Treatment
- Focal nesidioblastosis: partial pancreatectomy with excision of diseased areas; diffuse: near total pancreatectomy
Microscopic (histologic) description
- Islets in intimate association with ducts, with formation of ductuloinsular complexes
- Beta cell hypertrophy (Am J Surg Pathol 2005;29:524)
Microscopic (histologic) images
Neuroendocrine neoplasms-general
Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Electron microscopy images | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Includes well differentiated neuroendocrine tumors (WDNET) and poorly differentiated neuroendocrine carcinomas (PDNEC)
- Express neuroendocrine markers (synaptophysin and chromogranin A) (see Positive stains)
- Mixed neuroendocrine nonneuroendocrine neoplasms (MiNEN) includes both neuroendocrine and nonneuroendocrine component (e.g., adenocarcinoma, acinar carcinoma), each ≥ 30%
- Functional tumors are associated with elevated serum hormone levels and a clinical hormonal syndrome (see Clinical features)
- Nonfunctional tumors are more common and are not associated with a clinical hormonal syndrome
- May still be associated with elevated serum hormone levels or tissue hormone expression on immunohistochemistry
- Nonfunctional tumors < 0.5 cm are termed pancreatic neuroendocrine microadenomas
Essential features
- Well differentiated neuroendocrine tumors (WDNET):
- Clumped chromatin (salt and pepper) nuclei
- Cellular uniformity, central ovoid nucleus
- Organoid architecture: ribbons / trabeculae, nesting, glandular, gyriform, pseudoacinar
- Necrosis is rare
- Amyloid deposition in insulinomas
- Psammoma bodies in somatostatinomas (also associated with NF1)
- Background of microadenomas in multiple endocrine neoplasia type 1 (MEN1) and von Hippel-Lindau (VHL) syndromes
- Clear cell morphology in von Hippel-Lindau syndrome
- Poorly differentiated neuroendocrine carcinomas (PDNEC):
- Diffuse sheets or solid nests of markedly atypical malignant cells with small cell or large cell morphology
- Abundant mitoses and apoptotic bodies
- Necrosis is frequent
Terminology
- Antiquated terms: islet cell tumor / carcinoma, carcinoid, APUDoma
- Classification based on WHO system:
- Per WHO 2019, neuroendocrine neoplasms with Ki67 > 20% are now regarded in 2 distinct groups, WDNET grade 3 or PDNEC, based on the morphologic characteristics
- Well differentiated neuroendocrine tumor grading:
- WHO grade 1: < 2 mitoses/2 mm2 or Ki67 index < 3%
- WHO grade 2: 2 - 20 mitoses/2 mm2 or Ki67 index 3 - 20%
- WHO grade 3: > 20 mitoses/2 mm2 or Ki67 > 20%
- Ki67 count should be done on a minimum of 500 cells in hot spot areas
- Both mitotic count and Ki67 should be performed and grade is assigned based on largest value; usually Ki67 is higher than mitotic count (Am J Surg Pathol 2013;37:1671)
Epidemiology
- 1 - 5% of all pancreatic neoplasms; increasing incidence due to improved imaging techniques (Arch Pathol Lab Med 2019;143:1317, Cancer 2015;121:589)
- Incidence of 1 per 100,000 persons (Cancer 2015;121:589)
- Wide age range but most common in third to sixth decades (Arch Pathol Lab Med 2019;143:1317)
- M = F (Arch Pathol Lab Med 2019;143:1317)
- Most are sporadic
- 10 - 20% associated with syndrome (Arch Pathol Lab Med 2019;143:1317):
- 20 - 70% of multiple endocrine neoplasia 1 (MEN1) patients
- 20% von Hippel-Lindau (VHL) patients
- 10% neurofibromatosis type 1 (NF1) patients
- 1% of tuberous sclerosis complex (TSC) patients
Sites
- WDNET is more common in tail and body (Arch Pathol Lab Med 2019;143:1317)
- PDNEC is more common in head (Arch Pathol Lab Med 2019;143:1317)
- See Clinical hormonal syndromes
Pathophysiology
- Varies with clinical syndrome (see Clinical features)
Etiology
- Risk factors include smoking, diabetes and first degree relative with history of cancer (Sci Rep 2016;6:36073)
- Subset associated with MEN1 (MEN1 gene on 11q13), von Hippel-Lindau (VHL tumor suppressor gene on 3p25), neurofibromatosis 1 (microdeletion in neurofibromin gene on 17q11.2) and tuberous sclerosis complex (TSC1 tumor suppressor gene on 9q34 or TSC2 tumor suppressor gene on 16p13.3) (Arch Pathol Lab Med 2019;143:1317)
Clinical features
- Nonfunctional tumors are encountered incidentally and are usually larger at time of diagnosis
- Local obstruction / mass effect, if located in the pancreatic head
- Other clinical features of MEN1, VHL, NF1 or TSC (if applicable)
- Clinical hormonal syndromes in functioning tumors, 10 - 30% of tumors (Arch Pathol Lab Med 2009;133:350)
- Insulinoma:
- Most common
- Insulin secretion
- Whipple triad: symptoms of hypoglycemia, low plasma glucose, relief of symptoms with glucose administration
- Solitary tumor < 2 cm
- 5 - 10% MEN1 and are usually multiple
- Generally indolent
- Gastrinoma:
- Second most common functioning pancreatic neuroendocrine tumor
- Gastrin secretion
- Zollinger-Ellison syndrome (peptic / duodenal ulcers, gastroesophageal reflux, diarrhea)
- Located in gastrinoma triangle (common bile duct, duodenum, pancreatic head)
- More commonly found in duodenum than pancreas
- 20 - 30% associated with MEN1
- Glucagonoma:
- 4 Ds: diabetes, dermatitis (necrolytic migratory erythema), deep vein thrombosis, depression
- Solitary, large
- Tail > head
- > 50% have metastasis at presentation
- VIPoma:
- Verner-Morrison syndrome: watery diarrhea, hypokalemia, achlorhydria / hypochlorhydria
- Solitary, large
- Tail > head
- Somatostatinoma:
- Diabetes mellitus, diarrhea or steatorrhea, anemia, malabsorption, cholelithiasis
- Very rare
- Solitary, large
- > 50% have metastasis at presentation
- Ectopic hormone producing neuroendocrine tumor:
- ACTH (Cushing syndrome), serotonin (atypical carcinoid syndrome), growth hormone
- Solitary, large
Laboratory
- Elevated serum hormone in functional tumors (see Clinical features)
- Elevated serum chromogranin A in 50 - 100%; correlates with tumor burden and metastasis but has limited utility due to low specificity and sensitivity (Arch Pathol Lab Med 2019;143:1317)
Radiology description
- Round, solid, hypervascular
- Subset may be cystic
- Well circumscribed
- Contrast enhanced computed tomography (CT) has decreased sensitivity in smaller (< 2 cm) tumors (Clin Endosc 2017;50:537)
- Magnetic resonance imaging (MRI) has better sensitivity, especially for small tumors compared with CT (Clin Endosc 2017;50:537)
- Gallium 68 dotatate positron emission tomography (PET) (Clin Endosc 2017;50:537):
- Uses radiolabeled somatostatin analog
- Useful for WDNETs, most of which express somatostatin receptor
- High sensitivity and specificity for somatostatin receptor expressing tumors
Radiology images
Prognostic factors
- WDNETs are usually slow growing with prolonged clinical course
- PDNECs are usually aggressive and rapidly progressive
- Ki67 index and mitotic count, as described for WHO grading
- Prognostic immunohistochemical markers (not widely used in routine clinical practice):
- Expression of COX2, p27, CD99 and PR immunostains (Pathol Int 2001;51:770, Am J Clin Pathol 2003;120:685, Am J Surg Pathol 2006;30:1588, Cancer 1992;70:2268)
- Expression of CK19 is associated with aggressive behavior (Am J Surg Pathol 2006;30:1588, Histopathology 2007;50:597, Am J Surg Pathol 2004;28:1145)
Case reports
- 45 year old woman with possible VHL syndrome with mixed serous neuroendocrine neoplasm (World J Clin Cases 2019;7:4119)
- 48 year old man with cystic pancreatic WDNET radiographically mimicking cystic mucinous neoplasm (Autops Case Rep 2020;10:e2020171)
- 53 year old man with pancreatic WDNET with rhabdoid features (Virchows Arch 2018;473:247)
- 58 year old woman with systemic mastocytosis incidentally found to have pancreatic WDNET (Int J Surg Case Rep 2020;77:397)
- 73 year old woman with breast carcinoma with unrecognized neuroendocrine differentiation metastasizing to the pancreas (Int J Surg Pathol 2016;24:463)
Treatment
- Surgical resection is the mainstay for WDNET, with resolution of both mass effect symptoms and symptoms associated with hormone secretion (Surg Clin North Am 2019;99:793)
- Benefit for both localized and metastatic disease
- Other therapies for metastatic WDNET (Surg Clin North Am 2019;99:793):
- Somatostatin analog therapy
- Molecular therapies: tyrosine kinase inhibitor, sunitinib; mTOR inhibitor, everolimus
- Chemotherapy (capecitabine and temozolomide [CAPTEM])
- Peptide receptor radionuclide therapy (PRRT)
- Liver directed therapy: ablation or hepatic artery embolization of liver metastasis
- PDNECs usually treated with platinum based chemotherapy (Surg Clin North Am 2019;99:793)
Gross description
- Usually well circumscribed, homogeneous
- May have cystic change
- Color and consistency varies according to degree of vascularity and stroma, ranges from white to pink to tan to brown; may be yellow if necrosis present (Arch Pathol Lab Med 2009;133:350)
- Pigmented black pancreatic neuroendocrine tumor contains intracytoplasmic lipfuscin and mimics metastatic melanoma (Arch Pathol Lab Med 2009;133:350)
- Lipid rich pancreatic neuroendocrine tumor appears yellow and may mimic adrenal cortical neoplasia (Arch Pathol Lab Med 2009;133:350)
- Large tumors may appear lobulated and infiltrative
- Can invade adjacent fibroadipose tissue, other organs and large vessels
Gross images
Microscopic (histologic) description
- Well differentiated neuroendocrine tumors:
- Organoid architecture: solid nests, trabeculae, gyri, cords, festoons, ribbons, glandular, tubuloacinar
- Small to medium cells with eosinophilic to amphophilic and finely granular cytoplasm; nuclei are uniform, central, round / oval, with salt and pepper (finely stippled) chromatin; no or inconspicuous nucleoli
- Rich vascular network
- Amyloid deposition in insulinomas
- Psammoma bodies in somatostatinomas
- May have hyaline globules (Am J Surg Pathol 2011;35:981)
- Variants include clear cell, lipid rich, oncocytic, rhabdoid and pigmented black neuroendocrine neoplasm (Arch Pathol Lab Med 2019;143:1317, Virchows Arch 2018;473:247, Cancer 2001;92:1984)
- Poorly differentiated neuroendocrine carcinomas:
- Diffuse sheets or solid nests of markedly atypical malignant cells
- Salt and pepper chromatin is lost
- Abundant mitoses and apoptotic bodies
- Necrosis is frequent
- May be small cell (high N/C ratio, nuclear hyperchromasia and molding) or large cell (moderate to abundant amphophilic cytoplasm, may have prominent nucleoli) morphology
Microscopic (histologic) images
Contributed by Danielle Hutchings, M.D. and Sabrina Sopha, M.D.
Cytology description
- Well differentiated neuroendocrine tumors (Diagn Cytopathol 2006;34:649):
- Cellular specimen with clean background
- Clusters and individual cells
- Uniform round oval, small / medium sized cells
- Frequently plasmacytoid
- Amphophilic, finely granular cytoplasm (neurosecretory capability)
- Poorly differentiated neuroendocrine carcinomas (Diagn Cytopathol 2006;34:649):
- Overtly malignant cells with small cell or large cell morphology
- Necrosis
Cytology images
Positive stains
- Epithelial markers: CK8 / CK18, CAM 5.2, OSCAR, AE1 / AE3 (labels 50% of tumors) (Virchows Arch A Pathol Anat Histopathol 1986;409:609)
- Neuroendocrine markers:
- Synaptophysin: strong and diffuse in WDNET; may be weak, patchy or negative in PDNEC (Hum Pathol 2020;96:8)
- Chromogranin A: may be more focal and 10 - 20% of WDNET may be negative; may be focal or negative in PDNEC (Hum Pathol 2020;96:8)
- INSM1: newer marker with high sensitivity and specificity (Am J Clin Pathol 2015;144:579)
- CD56, neuron specific enolase (NSE), CD57: less specific, limited utility (Hum Pathol 2020;96:8)
- Panel to determine pancreatic origin for WDNET: Islet1+, PAX6+, TTF1-, CDX2 usually negative, SATB2-
- Panel is nonspecific in the setting of PDNEC
- TTF1 is positive in 40% of extrapulmonary PDNEC (Hum Pathol 2020;96:8)
- Panel to differentiate WDNET, grade 3 from PDNEC (Arch Pathol Lab Med 2019;143:1317)
- WDNET: somatostatin receptor+, loss of DAXX / ATRX
- PDNEC: abnormal expression of p53, loss of RB1, loss of SMAD4 / DPC4
- Hormone expression (insulin, glucagon, gastrin, somatostatin, VIP, pancreatic polypeptide): may be seen but does not indicate functional tumor
- Trypsin: scattered positive cells may be seen but if > 25%, the tumor is classified as mixed (Am J Surg Pathol 1994;18:765, Am J Surg Pathol 2002;26:893, Dig Dis Sci 2002;47:2254)
Negative stains
- Beta catenin (labels cell membrane only) (Arch Pathol Lab Med 2019;143:1317)
- Trypsin (negative or only few positive cells) (Am J Surg Pathol 1994;18:765, Am J Surg Pathol 2002;26:893, Dig Dis Sci 2002;47:2254)
- Chymotrypsin (Arch Pathol Lab Med 2019;143:1317)
- BCL10 (Arch Pathol Lab Med 2019;143:1317)
Molecular / cytogenetics description
- WDNETs:
- MEN1 inactivation 44% (Arch Pathol Lab Med 2019;143:1317)
- DAXX / ATRX mutation or loss 43% (Arch Pathol Lab Med 2019;143:1317)
- mTOR pathway alterations (PTEN, TSC2, PIK3CA) 14% (Arch Pathol Lab Med 2019;143:1317)
- VHL small subset (Semin Diagn Pathol 2014;31:498)
- Poorly differentiated neuroendocrine carcinomas and mixed neuroendocrine nonneuroendocrine neoplasms:
- TP53, RB, KRAS, APC, BRAF, SMAD4 / DPC4 (more like adenocarcinomas) (Arch Pathol Lab Med 2019;143:1317, Am J Surg Pathol 2012;36:173)
Videos
Solid tumors of the pancreas
Sample pathology report
- Pancreas, distal pancreatectomy:
- Well differentiated neuroendocrine tumor, WHO grade 1 of 3 (3 cm) (see comment)
- Ki67 proliferation index: 2%
- 1 mitosis per 2 mm2
- Tumor confined to the pancreas
- Lymphovascular and perineural invasion identified
- Proximal pancreatic parenchyma margin is uninvolved
- Metastatic tumor in 2/17 lymph nodes
- Comment: The tumor cells are positive for synaptophysin and chromogranin A.
- Well differentiated neuroendocrine tumor, WHO grade 1 of 3 (3 cm) (see comment)
Differential diagnosis
- Solid pancreatic tumors:
- Acinar cell carcinoma:
- Adults, high mitotic rate, labeling with BCL10, trypsin, chymotrypsin
- May have focal positivity with synaptophysin but chromogranin is negative
- Pancreatoblastoma:
- Children, squamoid nests
- Solid pseudopapillary neoplasm:
- Women, degenerated pseudopapillae, hyaline globules
- Nuclear labeling with beta catenin
- May have focal positivity with synaptophysin but chromogranin is negative
- Usual ductal adenocarcinoma:
- Desmoplastic stroma
- Invasive mucin producing glands
- Paraganglioma:
- Very rare, nested zellballlen architecture
- Cytokeratin negative, S100 highlights sustentacular cells, GATA3 positive (Mod Pathol 2013;26:1365)
- Acinar cell carcinoma:
- Clear cell tumors:
- Clear cell sarcoma:
- Labels with melanoma markers and has t(12;22)
- Metastatic renal cell carcinoma:
- PAX8+
- PEComa:
- Labels with melanoma markers, inhibin, cathepsin K and TFE3, melanoma and other clear cell tumors
- Solid variant of serous cystadenoma:
- Labels with inhibin (Am J Surg Pathol 2004;28:339)
- Clear cell sarcoma:
Additional references
Board review style question #1
A 58 year old man presents with a 2.8 cm solid mass in the tail of the pancreas. Based on the morphology below, you suspect a well differentiated neuroendocrine tumor (WDNET). Which of the following immunoprofiles would be most characteristic of pancreatic WDNET?
- Synatophysin-, chromogranin-, BCL10+, trypsin+, beta catenin cytoplasmic
- Synatophysin-, chromogranin-, BCL10-, trypsin-, beta catenin nuclear
- Synatophysin+, chromogranin+, BCL10-, trypsin-, beta catenin cytoplasmic
- Synatophysin+, chromogranin-, BCL10-, trypsin-, cytokeratin-
Board review style answer #1
C. Synatophysin+, chromogranin+, BCL10-, trypsin-, beta catenin cytoplasmic
Comment Here
Reference: Neuroendocrine neoplasms-general
Comment Here
Reference: Neuroendocrine neoplasms-general
Board review style question #2
A 45 year old woman presents recurrent episodes of dizziness, palpitations and sweating, which she reports usually resolve with drinking orange juice. She is found to have a 2.0 cm solid mass in the body of the pancreas. Resection specimen is shown in the image above. What is the most likely diagnosis based on H&E and congo red stains?
- Acinar cell carcinoma
- Gastrinoma
- Insulinoma
- Pancreatic ductal adenocarcinoma
- Well differentiated pancreatic neuroendocrine tumor, nonfunctional
Board review style answer #2
Neuroendocrine tumor with sarcomatous differentiation
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Radiology images | Case reports | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Electron microscopy description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1Definition / general
- Well differentiated neuroendocrine tumor with poorly differentiated myosarcomatous component
Essential features
- Extremely rare lesion (two cases reported in English literature)
- Lesion may transition from neuroendocrine to sarcomatous component or may have two discrete admixed components
Terminology
- Both reported cases use outdated term islet cell tumor, as they were published in 1989 and 2001
ICD coding
- ICD-10: C25.4 - malignant neoplasm of endocrine pancreas
Case reports
- 61 year old woman with 11 cm pancreas mass (Mod Pathol 2001;14:1187)
- Patient with widely metastatic lesion with rhabdomyosarcomatous component (Am J Surg Pathol 1989;13:422)
Microscopic (histologic) description
- Lesion is primarily composed of typical well differentiated neuroendocrine tumor
- Sarcomatous component may have rhabdomyosarcomatous features or may show spindled cells with enlarged nuclei arranged in bundles
- Sarcomatous component is admixed with neuroendocrine component but transition may be gradual or abrupt (with sarcomatous component appearing as discrete nodules)
Microscopic (histologic) images
Positive stains
- Neuroendocrine markers (synaptophysin, chromogranin) in neuroendocrine component
- Desmin in sarcomatous component
Electron microscopy description
- Z lines and thick filaments (if rhabdomyosarcomatous component)
Sample pathology report
- Pancreas and duodenum, Whipple procedure:
- Pancreatic neuroendocrine tumor with focal sarcomatous differentiation (see synoptic report and comment)
- Comment: Sarcomatous differentiation is extremely rare in pancreatic neuroendocrine tumors. Immunohistochemical stains for synaptophysin and chromogranin are positive in the typical neuroendocrine component and the Ki67 index in that component is approximately 2.7% (WHO grade 1).
Differential diagnosis
- Leiomyosarcoma:
- Most common sarcoma of pancreas
- Would not demonstrate neuroendocrine component
Board review style question #1
A neuroendocrine tumor of the pancreas with a sarcomatous component would be most likely to show what sort of differentiation within that component?
- Angiosarcomatous
- Leiomyosarcomatous
- Liposarcomatous
- Osteosarcomatous
Board review style answer #1
B. Leiomyosarcomatous. Additionally, leiomyosarcoma is the most common primary sarcoma of the pancreas.
Comment Here
Reference: Neuroendocrine tumor with sarcomatous differentiation
Comment Here
Reference: Neuroendocrine tumor with sarcomatous differentiation
Pancreatic cystic fluid analysis (pending)
[Pending]
Pancreatic polypeptide secreting tumors
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Sites | Pathophysiology | Clinical features | Diagnostic criteria | Radiology images | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Electron microscopy images | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1Definition / general
- Neuroendocrine tumor with high proportion of pancreatic polypeptide (PP) cells
- Not a WHO diagnosis
Essential features
- Pancreatic neuroendocrine tumor that sometimes causes watery diarrhea, hypokalemia, achlorhydria (WDHA) syndrome (due to PP secretion)
- May metastasize to liver
Terminology
- Sometimes called PPoma
ICD coding
- ICD-10: C25.4 - malignant neoplasm of endocrine pancreas
Sites
- May arise anywhere within pancreas
Pathophysiology
- Pancreatic polypeptide regulates endocrine and exocrine secretion by the pancreas
- Vasoactive intestinal polypeptide (VIP) and PP are derived from a common precursor and thus pancreatic polypeptide secreting tumors can also cause watery diarrhea, hypokalemia, achlorhydria (WDHA) syndrome
- It has been proposed that most / all nonfunctioning pancreatic neuroendocrine tumors represent pancreatic polypeptide secreting tumors (Best Pract Res Clin Gastroenterol 2012;26:737)
Clinical features
- Can cause nonspecific abdominal pain or watery diarrhea, hypokalemia, achlorhydria (WDHA) syndrome
- Patients may have multiple endocrine neoplasia 1 syndrome (MEN1) (Arch Surg 1984;119:508)
Diagnostic criteria
- Pancreas mass and elevated levels of fasting pancreatic polypeptide hormone
Case reports
- 46 year old woman with WDHA syndrome (Case Rep Gastroenterol 2008;2:238)
- 46 year old woman with MEN1 (Int J Gastrointest Cancer 2002;32:153)
Treatment
- Surgery is generally curative (World J Surg 2008;32:1815)
Microscopic (histologic) description
- Resembles all other well differentiated neuroendocrine tumors of the pancreas
Microscopic (histologic) images
Positive stains
Electron microscopy images
Sample pathology report
- Pancreas, tail, resection:
- Pancreatic neuroendocrine tumor, WHO grade 2 (see synoptic report and comment)
- Comment: The patient’s clinical history of watery diarrhea, hypokalemia and achlorhydria is noted. This may be due to pancreatic polypeptide secretion by this tumor. Immunohistochemical stains for synaptophysin and chromogranin are positive and the Ki67 index is approximately 3.2%.
Differential diagnosis
- Nonfunctional well differentiated neuroendocrine tumor:
- Same microscopic appearance
- Some PP secretion may be seen by immunohistochemistry
- Distinction is based on serum PP levels
- VIPoma:
- Same microscopic appearance
- Can also cause watery diarrhea, hypokalemia, achlorhydria (WDHA) syndrome
- Distinction is based on serum PP levels
- Pancreatic polypeptide cell hyperplasia:
- Neuroendocrine hyperplasia admixed with elements of normal pancreas (J Clin Pathol 2006;59:1087)
Board review style question #1
Which of the following pancreatic neuroendocrine tumors can cause watery diarrhea, hypokalemia and achlorhydria?
- Glucagonoma
- Insulinoma
- Pancreatic polypeptide secreting tumor (PPoma)
- Somatostatinoma
Board review style answer #1
C. Pancreatic polypeptide secreting tumor (PPoma). Watery diarrhea, hypokalemia, achlorhydria
(WDHA) syndrome can also be caused by VIPomas.
Comment Here
Reference: Pancreatic polypeptide secreting tumors
Comment Here
Reference: Pancreatic polypeptide secreting tumors
Pancreatoblastoma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Rare pancreatic malignancy that is seen most often in children and displays multiple lines of differentiation
Essential features
- Displays at least 2 pancreatic lines of differentiation, including acinar, ductal and neuroendocrine elements
- Presence of squamoid nests is a defining diagnostic feature
- Tumors are aggressive, with complete surgical resection being the most important prognostic factor
Terminology
- Infantile pancreatic carcinoma
- Pancreatic carcinoma in childhood (Cancer Cytopathol 2019;127:708)
ICD coding
Epidemiology
- Children > adults
- Most common pancreatic tumor of children < 10 years old
- Median age in this group is 4 - 5 years (Pediatr Surg Int 2019;35:1231)
- Mean age of presentation of adult tumors is 41 years
- M = F (Gland Surg 2015;4:322)
- Most common pancreatic tumor of children < 10 years old
- Only about 200 cases reported in the literature
Sites
- About 50% occur in pancreatic head, with the rest relatively equally split between tail and body (Gland Surg 2015;4:322)
- 1 reported case found in ampulla of Vater (Arch Pathol Lab Med 2003;127:1501)
Pathophysiology
- Unknown
- Loss of chromosome 11p or mutations in APC / B catenin pathway possibly implicated in pathogenesis (Gland Surg 2015;4:322)
Etiology
- Unknown
- Some cases associated with Beckwith-Wiedemann syndrome, familial adenomatous polyposis (Gland Surg 2015;4:322)
Clinical features
- Generally nonspecific presentation; most common symptom is abdominal pain (Sci Rep 2020;10:11285)
- Commonly advanced at presentation with local extension and metastases
- Commonly metastasizes to liver > lymph nodes > lung
- Associated with Beckwith-Wiedemann syndrome, familial adenomatous polyposis (Gland Surg 2015;4:322)
Diagnosis
- Can be identified on CT or MRI
- Difficult to diagnose by FNA due to variety of cell lines expressed (Gland Surg 2015;4:322)
Laboratory
- Up to 50% of pediatric patients can show elevations in carcinoembryonic antigen (CEA) and alpha fetoprotein (AFP); less helpful in adults (Gland Surg 2015;4:322)
- 66% of children will have AFP > 1000 μg/L
- Some tumors reported to secrete adrenocorticotropic hormone (ACTH), resulting in Cushing syndrome (J Cancer Res Ther 2015;11:1027)
Radiology description
- Generally solid, irregular lesion but can be cystic or degenerative
- Can grow outward, as branches growing from a tree trunk (Sci Rep 2020;10:11285)
Prognostic factors
- Prognosis largely dependent on resectability of tumor (Pancreatology 2004;4:441)
- 5 year survival for resectable tumors: 65%
- 5 year survival for nonresectable tumors: 0%
- Presence of distant metastases portends poorer prognosis
- Prognosis worse in adults, as children tend to have well encapsulated tumors without metastases
Case reports
- 4 year old boy with multiple pancreatic and liver masses (Ecancermedicalscience 2018;12:861)
- 27 year old woman with a 3.6 cm pancreatic mass and metastasis to the liver and lungs (JOP 2012;13:301)
- 69 year old man with a pancreatic head mass (BMJ Case Rep 2020;13:e233884)
Treatment
- Complete surgical resection is critical to prognosis
- Chemotherapy or radiotherapy may be used to ensure complete resection
Gross description
- Large (average size is 10 cm), lobulated, fleshy mass
- Can have cystic components or soft areas of necrosis
- Predominantly cystic tumors can be seen in Beckwith-Wiedemann syndrome (Gland Surg 2015;4:322)
Microscopic (histologic) description
- Multilineage components resembling embryologic pancreas
- Geographic hypercellular solid tumor lobules separated by fibrous bands
- Acinar differentiation is the predominant pattern, mimicking acinar cell carcinoma:
- Acinar appearances resembling normal acini, with small lumen and luminal secretion
- Focal solid, trabecular or pseudoglandular patterns
- Cells with eosinophilic or amphophilic granular cytoplasm, mild atypical nuclei and small nucleoli
- Squamoid nests:
- Defining feature, present in every case
- Plump to spindle shaped cells with abundant eosinophilic / clear cytoplasm arranged in whorls or nests
- Typically bland cytology
- May show keratinization
- Neuroendocrine component:
- More than 50% of cases show focal neuroendocrine differentiation
- Neuroendocrine cells diffusely intermix with acinar component, being highlighted by immunostains or form focal organoid or trabecular growth
- Ductal component:
- Very focal glandular formation
- May be highlighted by a mucicarmine stain
- Primitive component:
- Solid sheets of immature small round cells
- Stroma:
- Variable cellularity
- Sometimes showing heterologous elements (e.g. bone, cartilage)
- Reference: Am J Surg Pathol 1995;19:1371
Microscopic (histologic) images
Contributed by Lizhi Zhang, M.D.
Cytology description
- Loosely cohesive, blast-like tumor cells are small, with fine chromatin, granular cytoplasm and atypia with nucleoli (Cancer Cytopathol 2019;127:708)
- Mimic acinar cell carcinoma
- Squamoid nest cells can be well defined, whorled clusters or syncytial appearing groups (Cancer Cytopathol 2019;127:708)
- Better appreciated on cell block preparations
Positive stains
- Pancytokeratin
- Squamoid nests positive for beta catenin (nuclear staining), CK5 and EMA
- Acinar markers (Gland Surg 2015;4:322):
- Ductal markers (positive in up to 65% of cases) (Gland Surg 2015;4:322):
- CEA
- B72.3
- DUPAN-2
- CK19
- Mucicarmine
- Neuroendocrine markers (if present) (Gland Surg 2015;4:322):
- Synaptophysin
- Chromogranin
- INSM1
- AFP, if serum levels are elevated
Negative stains
- Germ cell markers
- p53
- Rare cases loss of DPC4 expression (Am J Pathol 2001;159:1619)
Electron microscopy description
- All showing zymogen granules (400 - 800 nm) for acinar differentiation (Am J Surg Pathol 1995;19:1371)
- Some with neuroendocrine granules (125 - 250 nm) and mucigen granules (500 - 900 nm)
Molecular / cytogenetics description
- Loss of chromosome 11p (Gland Surg 2015;4:322)
- Alterations in the APC / beta catenin pathway (Am J Pathol 2001;159:1619)
Sample pathology report
- Pancreas, duodenum and gallbladder, Whipple procedure:
- Pancreatoblastoma, forming an 8 x 6 x 4 cm mass in the pancreatic head (see comment)
- Tumor is confined within the pancreas without extension to peripancreatic soft tissue, common bile duct and duodenum
- Tumor necrosis is present
- Angiolymphatic and perineural invasion are present
- All surgical margins are negative for tumor
- Background pancreatic parenchyma is unremarkable
- Multiple (3 of 14) regional lymph nodes are positive for metastatic tumor
- Gallbladder is without diagnostic abnormality
- Comment: Immunoperoxidase stains show the tumor cells are positive for trypsin with beta catenin nuclear staining in the scattered squamoid nests, confirming the diagnosis. Focal area is also positive for synaptophysin and chromogranin, representing a minor component of neuroendocrine tumor. No convincing component of ductal adenocarcinoma is seen.
Differential diagnosis
- Acinar cell carcinoma:
- No squamoid nests or other lines of differentiation
- No nuclear beta catenin staining
- Neuroendocrine tumor / carcinoma:
- May have focal acinar appearance but no squamoid nests
- Nested or trabecular growth patterns
- Diffuse positive staining of neuroendocrine markers (synaptophysin, chromogranin, INSM1)
- No nuclear beta catenin staining
- Solid pseudopapillary neoplasm:
- No acinar differentiation
- No squamoid nests
- Pseudopapillary structures
- Diffuse nuclear beta catenin staining
- Undifferentiated carcinoma or anaplastic carcinoma:
- No acinar differentiation
- No squamoid nests
- Highly atypical and pleomorphic tumor cells
- May have multinucleated giant tumor cells or osteoclast-like giant cells
Additional references
Board review style question #1
A 40 year old man presents with abdominal pain and unintentional weight loss. Subsequent imaging reveals an 8 cm mass in the pancreatic head invading into the duodenum and a likely metastasis in the liver. The patient undergoes neoadjuvant chemoradiation and a pancreaticoduodenectomy. A representative histology section is shown above. The cell population that most definitively identifies this diagnosis would be most specifically highlighted by which of the following immunohistochemical stains?
- Alpha fetoprotein
- Beta catenin
- Chromogranin
- Trypsin
Board review style answer #1
B. Beta catenin. Nuclear staining of beta catenin is a marker of alterations in the APC / beta catenin pathway, which commonly occurs in pancreatoblastoma associated with loss of chromosome 11p.
Comment Here
Reference: Pancreatoblastoma
Comment Here
Reference: Pancreatoblastoma
Board review style question #2
A 5 year old boy presents with weight loss, abdominal pain and distention and hepatomegaly. An abdominal ultrasound reveals a pancreatic mass and multiple liver masses. A pancreas biopsy is performed. Based on the findings in the image shown above, what is the most likely diagnosis?
- Acinar cell carcinoma
- Malignant germ cell tumor
- Pancreatic neuroendocrine tumor
- Pancreatoblastoma
Board review style answer #2
D. Pancreatoblastoma. The characteristic squamoid nest shown in the image is a defining feature of pancreatoblastoma, present in every case.
Comment Here
Reference: Pancreatoblastoma
Comment Here
Reference: Pancreatoblastoma
PanIN
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Diagrams / tables | Clinical features | Prognostic factors | Case reports | Gross description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Pancreatic intraepithelial neoplasia (PanIN) is a microscopic neoplastic lesion of the pancreas that can progress to invasive ductal adenocarcinoma (Arch Pathol Lab Med 2017;141:1606)
- Regarded as the main precursor lesion to invasive pancreatic carcinoma
- Small, papillary or flat, noninvasive epithelial neoplasm characterized by variable mucin production and a spectrum / variable degrees of cytologic and architectural atypia (Am J Surg Pathol 2015;39:1730)
- By definition, it is a nontumoral form of dysplasia (intraepithelial neoplasia) (see intraductal papillary mucinous neoplasm for the tumoral version of intraepithelial neoplasia)
- As such, almost by default, it doesn't have any clinical manifestation
- Other noninvasive precursors of pancreatic ductal adenocarcinoma such as intraductal papillary mucinous neoplasms (and similar intraductal neoplams) and mucinous cystic neoplasms, all of which are defined as grossly visible (tumoral) forms of intraepithelial neoplasia, are classified separately (Am J Surg Pathol 2015;39:1730, Am J Surg Pathol 2004;28:977)
Essential features
- Usually < 0.5 cm (very small to be seen grossly or by radiologic imaging)
- 2 tiered classification, low grade versus high grade, was recommended to replace the former 3 tiered classification for PanIN, intraductal papillary mucinous neoplasm and mucinous cystic neoplasm at the Baltimore consensus meeting (2014) (Am J Surg Pathol 2015;39:1730)
- Only carcinoma in situ type lesions are regarded high grade and warrant clinical attention
ICD coding
- ICD-O: 8148/2 (for high grade only)
Epidemiology
- Increase in frequency by age
- Low grade lesion is a very common incidental finding (found in over half of the population > 50 years) (Ann Surg Oncol 2013;20:3643, Mod Pathol 2003;16:996)
- In contrast, high grade is rarely seen without invasive ductal adenocarcinoma (Mod Pathol 2003;16:996)
- It can occasionally be seen incidentally in pancreatectomy performed for other lesions
Sites
- Anywhere in the pancreas, mostly in cases with resected pancreatic ductal adenocarcinoma
- It is more common in branch ducts rather than in main pancreatic duct
- Can be present in heterotopic pancreas (Am J Surg Pathol 2017;41:833)
Clinical features
- Does not manifest clinically
- PanINs are very common adjacent to resected pancreatic ductal adenocarcinoma (Ann Surg Treat Res 2018;94:247)
Prognostic factors
- Low grade is inconsequential
- High grade believed to have a high frequency of progression into pancreatic ductal adenocarcinoma and therefore needs careful evaluation
- PanIN of any grade at a margin of a resected pancreas that already has invasive carcinoma doesn't seem to have any prognostic implications; however, if PanIN 3 (carcinoma in situ) is encountered at a margin in a pancreas without carcinoma, it needs to be carefully evaluated (Am J Surg Pathol 2015;39:1730)
Case reports
- 47 year old woman with PanIN in heterotopic pancreas that was incidentally diagnosed on endoscopic mucosal resection of a duodenal polyp (BMJ Case Rep 2018 Jun 23;2018)
- 58 year old man with PanIN 2 in heterotopic pancreas (J Gastrointestin Liver Dis 2017;26:199)
- 63 year old man and 77 year old man with high grade PanIN over long time periods (Oncology 2017;93:81)
- 82 year old man with concurrent PanIN and type 1 autoimmune pancreatitis with marked pancreatic duct dilatation (Clin J Gastroenterol 2016;9:266)
Gross description
- Not detectable grossly
- Occasionally obstructs a duct and lead to subtle fibrosis of upstream pancreatic tissue
Microscopic (histologic) description
- Usually < 0.5 cm
- 2 tiered classification, low grade versus high grade, was recommended to replace the former 3 tiered classification for PanIN, intraductal papillary mucinous neoplasm and mucinous cystic neoplasm at the Baltimore consensus meeting (2014) (Am J Surg Pathol 2015;39:1730)
- PanIN 1A / low grade:
- Earliest precursor lesion
- Flat epithelium composed of tall columnar mucin producing cells with basally located small round to oval nuclei
- Cells show essentially no cytologic atypia
- PanIN 1B / low grade:
- Identical to PanIN 1A but epithelium shows papillary, micropapillary or basally pseudostratified architecture
- PanIN 2 / low grade:
- Flat to papillary mucinous epithelial proliferations with focal or mild nuclear abnormalities, such as loss of polarity, nuclear enlargement, nuclear crowding, hyperchromatism and pseudostratification
- Rare mitoses but not apical or atypical
- PanIN 3 / high grade / carcinoma in situ:
- Predominantly papillary or micropapillary architecture, rarely flat
- Cribriforming, tufting pattern and luminal necrosis suggests PanIN 3
- Cytologically, these lesions have loss of polarity, dystrophic mucinous cells, enlarged irregular nuclei and prominent (macro) nucleoli
- Increased mitotic figures, including atypical forms
- Only carcinoma in situ type lesions are regarded as PanIN 3 / high grade (Am J Surg Pathol 2015;39:1730)
| Pancreatic intraepithelial neoplasia classification | ||
| Oldest terminology | WHO 2010 classification | Baltimore consensus meeting 2014 |
| Mucinous (goblet cell) metaplasia, flat duct lesion without atypia, mucinous ductal hyperplasia, simple hyperplasia, mucinous cell hyperplasia, flat duct hyperplasia, nonpapillary epithelial hypertrophy | PanIN 1A | Low grade PanIN |
| Papillary hyperplasia, papillary duct lesion without atypia, ductal hyperplasia, adenomatoid hyperplasia | PanIN 1B | |
| Atypical hyperplasia, papillary duct lesion with atypia, low grade dysplasia, moderate dysplasia | PanIN 2 | |
| Carcinoma in situ, intraductal carcinoma, high grade dysplasia, severe dysplasia | PanIN 3 | High grade PanIN |
Microscopic (histologic) images
Cytology description
- PanINs are, by definition, microscopic incidental lesions, not visible clinically or radiologically and therefore are not amenable to targeting by fine needle aspiration biopsy
- On rare occasions that they come to aspiration material, they would appear as mucinous glandular epithelium with atypical changes but their nature as PanIN cannot be affirmed
- The diagnosis of a PanIN should not be rendered on cytologic preparations
Positive stains
- MUC5AC, MUC6, HER2 nonbreast and fascin
- MUC1 (luminal membranous; cytoplasmic in high grade) and cyclin D1
- Ki67 labeling increases with PanIN grade
- In high grade lesions, a subset with p53 overexpression or SMAD4 / DPC4 loss
Molecular / cytogenetics description
- Early molecular changes: telomere shortening, KRAS oncogene activation (can be seen in PanIN 1 / low grade), p21 upregulation
- Intermediate steps: inactivation of tumor suppressor gene p16 CDKN2A
- Late stages: BRCA2, SMAD4 (DPC4) and TP53 tumor suppressor gene inactivation (Am J Clin Pathol 2014;141:168, Arch Pathol Lab Med 2017;141:366)
Sample pathology report
- Pancreas, tail, distal pancreatectomy:
- Incidental low grade pancreatic intraepithelial neoplasm (see comment)
- Comment: Incidental pancreatic intraepithelial neoplasms that are low grade are of no known clinical significance to an extent that consensus is that it may not even have to be reported. They are very common in general population.
- Pancreas, tail, distal pancreatectomy:
- High grade pancreatic intraepithelial neoplasm (carcinoma in situ) (see comment)
- Comment: High grade pancreatic intraepithelial neoplasms are seldom detected in isolation. Invariably they represent carcinoma elsewhere in the gland. In fact, this finding may represent pagetoid spread of invasive carcinoma from somewhere else in the pancreas which is now showing intraductal spread ("cancerization") and creating the image of high grade pancreatic intraepithelial neoplasm. This possibility needs to be excluded with careful evaluation of the patient before this is accepted as a mere precursor lesion.
Differential diagnosis
- Normal mucosal elements and peribiliary glands in ampulla and bile ducts:
- In the vicinity of the ampulla and bile ducts, normal mucosal elements often show cytologic findings that would otherwise qualify as low grade PanIN if they occur in the pancreatic ducts
- Transitional / squamous metaplasia:
- Shows stratified cells with high N/C ratio and this can closely mimic high grade PanIN
- Cancerization of ducts:
- Invasive carcinomas may re-enter and grow within the pancreatic ducts in a pagetoid spreading pattern, mimicking PanIN 3
- Invasive carcinoma near a duct lesion transitioning from high grade atypia to normal duct epithelium suggests cancerization but it can sometimes be impossible to make this determination
- In a case with invasive carcinoma, high grade lesion in a duct may have to be considered cancerization of ducts, especially if it is spatially close to invasive glands (Am J Surg Pathol 2015;39:1730)
- Tumoral intraepithelial neoplasia:
- Defined as clinically and grossly visible intramucosal / intraepithelial tumors that are generally > 1 cm
- These include the branch duct spread of tumors like intraductal papillary mucinous neoplasm
- If taken in isolation at microscopic level, especially when they have more gastric / endocervical-like morphology, these tumors can be indistinguishable from PanINs
- Gastric type intraductal papillary mucinous neoplasms have significant morphologic overlap with PanINs at a microscopic level (Ann Surg 2016;263:162)
- Though not widely employed, for cysts > 0.5 cm and < 1 cm lined by gastric-like mucinous epithelium, the term incipient intraductal papillary mucinous neoplasm has been proposed (Am J Surg Pathol 2014;38:360)
- In indeterminate cases, should be classified as PanIN, not intraductal papillary mucinous neoplasm
- Simple mucinous cysts with flat mucinous lining:
- Cysts with usually flat, gastric phenotype, lack ovarian stroma and are > 1 cm (Am J Surg Pathol 2017;41:121)
- Can show dysplastic changes of variable degrees and sometimes are diagnosed as simple mucinous cyst with PanIN
- Vascular invasion in pancreatic ductal adenocarcinoma:
- If the invasive neoplastic cells form complete luminal structures inside of the blood vessels, classify the case as having PanIN-like vascular invasion (Am J Surg Pathol 2012;36:235)
Board review style question #1
A 60 year old man, who is an alcoholic, presented with back pain, weight loss and jaundice. Gross sections showed firm, white-beige appearance in the head of the pancreas. Initial histologic sections demonstrated chronic pancreatitis and this lesion in a focal area. Which of the following statements concerning this case and this pancreatic lesion is true?
- p63 / p40 often labels this lesion
- This does not need to be reported
- This is low grade pancreatic intraepithelial neoplasia (PanIN 1)
- This is probably accompanied by carcinoma and warrants a thorough examination of the remaining pancreas
- This lesion is a very common incidental lesion in the general population
Board review style answer #1
D. This is probably accompanied by carcinoma and warrants a thorough examination of the remaining pancreas
Comment Here
Reference: PanIN (pancreatic intraepithelial neoplasia)
Comment Here
Reference: PanIN (pancreatic intraepithelial neoplasia)
Board review style question #2
Which of the following statements concerning pancreatic intraepithelial neoplasia is true?
- High grade PanIN (PanIN 3) is usually seen in pancreas without invasive ductal adenocarcinoma
- PanIN 2 is a high grade pancreatic intraepithelial neoplasia by new classification
- PanIN of any grade (including low grade) at a margin of a resected pancreas has very important prognostic implications
- PanINs are large lesions that can be seen grossly or by radiologic imaging
- Recommended latest terminology from Baltimore consensus meeting is a 2 tiered system: low grade and high grade PanIN
Board review style answer #2
E. Recommended latest terminology from Baltimore consensus meeting is a 2 tiered system: low grade and high grade PanIN
Comment Here
Reference: PanIN (pancreatic intraepithelial neoplasia)
Comment Here
Reference: PanIN (pancreatic intraepithelial neoplasia)
Poorly differentiated neuroendocrine carcinoma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology / etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Grading | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Poorly differentiated, high grade, malignant epithelial neoplasm with neuroendocrine differentiation
- Poorly differentiated pancreatic neuroendocrine carcinomas (PanNECs) are divided into small cell type (SC PanNEC) and large cell type (LC PanNEC)
Essential features
- Poorly differentiated neuroendocrine carcinomas represent a distinct entity from well differentiated (G1, G2, G3) neuroendocrine tumors (Am J Surg Pathol 2012;36:173)
- By morphology, PanNECs can be divided into small cell or large cell subtypes
- At the immunohistochemical level, the expression of neuroendocrine markers can be partial or weak (chromogranin A expression can even be absent)
- Tumor necrosis and high grade cytology
- Ki67 proliferation index > 20% (usually uniform and > 60%) (Ann Oncol 2013;24:152)
- Mitotic rate of > 20 mitoses/2 mm2
Terminology
- Neuroendocrine carcinoma refers to poorly differentiated neuroendocrine neoplasms, high grade by definition
- Neuroendocrine tumor is now reserved for well differentiated neoplasms with typical pathologic features of neuroendocrine lineage, truly resembling their nonneoplastic counterpart (cells of pancreatic islet), regardless of grade
- The current WHO (2019) classification groups pancreatic neuroendocrine neoplasms as follows:
- Pancreatic neuroendocrine microtumor (< 5 mm); previously known as neuroendocrine microadenoma
- Well differentiated pancreatic neuroendocrine tumor (WD PanNETs), including nonfunctional PanNETs and functional PanNETs (with clinical evidence of hormone release, such as insulinoma, glucagonoma, gastrinoma, VIPoma)
- Poorly differentiated pancreatic neuroendocrine carcinomas (PD PanNECs), including SC PanNECs and LC PanNECs
- Mixed neuroendocrine nonneuroendocrine neoplasm (MiNEN)
- Mixed neoplasm with a neuroendocrine component combined with a nonneuroendocrine component (typically ductal adenocarcinoma or acinar cell carcinoma)
- Each component must account for ≥ 30% of the tumor cell population and both components should be morphologically recognizable
- Neuroendocrine component may be a tumor (less frequently) or a carcinoma (more frequently)
ICD coding
- ICD-O:
- 8246/3 - neuroendocrine carcinoma, NOS
- ICD-11:
- 2C10.1 & XH0U20 - neuroendocrine neoplasms of pancreas & neuroendocrine carcinoma, NOS
- XH9SY0 - small cell neuroendocrine carcinoma
- XH0NL5 - large cell neuroendocrine carcinoma
Epidemiology
- Rare (accounting for < 1% of all pancreatic tumors and no more than 2 - 3% of pancreatic neuroendocrine neoplasms)
- Mean patient age is 59 years (patients are usually aged 50 - 60 years but can occur in younger patients as well)
- M:F = 1.4:1
- Large cell variant is more commonly encountered (60%) (Am J Surg Pathol 2012;36:173)
Sites
- Pancreatic gland (head, neck, body, tail)
- Head of the pancreas is the most common site (Am J Surg Pathol 2012;36:173)
Pathophysiology / etiology
- Largely unknown
- Tobacco smoking
Diagrams / tables
Clinical features
- Back pain, jaundice (for PanNECs of pancreatic head) or nonspecific abdominal symptoms, likewise pancreatic ductal adenocarcinomas
- Neoplastic syndromes secondary to ectopic hormone production, such as ACTH (rare)
- Vast majority (> 90%) of patients present with metastasis at the time of diagnosis (Am J Surg Pathol 2012;36:173)
Diagnosis
- CT scan, MRI and ultrasonography are the preferred imaging modality
- FDG PET scans present high standardized uptake value
- Somatostatin receptor scintigraphy (SSRS) is often negative (or focal avidity) due to lack of expression of SSTR2 and SSTR5 (Arch Pathol Lab Med 2020;144:816)
- Diagnosis is by cytology / fine needle biopsy or on surgical specimens (Am J Surg Pathol 2016;40:1192)
Laboratory
- Serum hormone activity (such as chromogranin A) is very unusual (Cancer 1973;31:1523)
- Serum carcinoma associated markers (e.g., CEA, CA19-9, CA125) levels may be elevated (Am J Surg Pathol 2016;40:1192)
Radiology description
- CT and MRI features: irregular margins and frequent presence of necrotic foci within tumor mass
Grading
- PanNECs are, by WHO definition high grade, based on Ki67 (MIB1) index > 20% and mitotic rate > 20 mitoses/2 mm2
- WD PanNETs are graded from G1 to G3; but G3 PanNETs represent a distinct entity from PanNECs (Endocr Pathol 2022;33:115, Pathologica 2021;113:28)
Prognostic factors
- Very poor prognosis, compared to WD PanNETs
- Metastatic spread is present in most patients at the time of diagnosis
- MiNEN including a NEC component show an aggressive behavior: in these tumors, the Ki67 index of the NEC component is the most important prognostic driver (Endocr Relat Cancer 2018;25:583)
- Even in cases amenable to surgical resection and treated with adjuvant platinum based therapy, the median survival time is very short (< 1 year) and < 25% of patients survive beyond 2 years (Am J Surg Pathol 2012;36:173)
Case reports
- 27 year old woman with ACTH secreting PanNEC causing Cushing syndrome with pelvic and bilateral ovarian metastases (Int J Clin Exp Pathol 2015;8:15396)
- 56 year old man presenting with pure, alpha fetoprotein producing PanNEC without another coexisting malignant component, such as adenocarcinoma or hepatoid carcinoma (BMC Gastroenterol 2015;15:16)
- 65 year old man with SC PanNEC with similar genetic alterations to invasive ductal adenocarcinoma (KRAS mutation, altered expressions of TP53 and SMAD4 / DPC4) (Clin J Gastroenterol 2016;9:261)
- 67 year old woman with cutaneous metastases of a PanNEC with CK20 positivity (G Ital Dermatol Venereol 2018;153:722)
Treatment
- Surgical resection
- Chemotherapy (no established protocol): platinum based regimen (Pancreas 2021;50:138)
Gross description
- Large neoplasms with infiltrative borders
- Brownish to whitish color, fleshy or hard consistency and typically with grossly visible necrotic areas (Endocr Pathol 2022;33:115, Pathologica 2021;113:28)
Frozen section description
- High grade and hypercellular neoplasm, with necrotic areas
- Diagnosis should be postponed to definitive examination after formalin fixation and also supported by ancillary methods (Endocr Pathol 2022;33:115, Pathologica 2021;113:28)
Microscopic (histologic) description
- Hypercellular neoplasm, with large and irregular nests, infiltrative growth pattern with randomly oriented large vascular structures and desmoplastic type fibrosis
- Necrosis with geographic pattern and comedo-like appearance
- High rate of cellular turnover (high mitotic rate and high apoptotic rate) (Front Oncol 2013;3:2)
- SC PanNEC: diffuse sheets of relatively small cells with round or elongated hyperchromatic nuclei, finely granular chromatin and lacking nucleoli; typical feature of nuclear molding (sharing with the pulmonary counterpart) (Front Oncol 2013;3:2)
- LC PanNEC: nesting / trabecular pattern, with round to polygonal medium to large sized cells, with amphiphilic cytoplasm and atypical nuclei with either coarse chromatin or conspicuous nucleoli; the nuclear to cytoplasmic (N/C) ratio of LCNECs is lower than that of SCNECs
Microscopic (histologic) images
Contributed by Claudio Luchini, M.D., Ph.D. and AFIP images
Cytology description
- Scant cytoplasm, slightly granular and high N/C ratio
- SC PanNEC: small cells with large nuclei, nuclear molding, finely speckled and dark chromatin with inconspicuous nucleoli, prominent background degeneration and typical crush artifact with nuclear streaming (Am J Surg Pathol 2012;36:173)
- LC PanNEC: large undifferentiated cells with bizarre forms or syncytial aggregates, irregular overlapping nuclei with prominent nucleoli, vesicular chromatin and abundant cytoplasm (delicate, dense or granular) (Am J Surg Pathol 2012;36:173)
Positive stains
- Cytokeratins (CK8 / CK18, CK AE1 / AE3)
- Synaptophysin: usually positive, may be rarely weak or focal (Am J Surg Pathol 2012;36:173)
- Chromogranin A: usually positive or weakly positive; can be also almost absent; SC PanNEC can also show a paranuclear dot-like immunoreactivity (Endocr Pathol 2018;29:150)
- CD56, neuron specific enolase (NSE), protein gene product 9.5 (PGP9.5) / ubiquitin C terminal hydrolase 1 protein (UCHL1): general neuroendocrine markers, less specific, limited utility
- Insulinoma associated protein 1 (INSM1) has emerged as an additional general neuroendocrine marker (Am J Clin Pathol 2015;144:579)
- Achaete-scute homolog 1 (ASH1) (Hum Pathol 2013;44:1391)
- p53: abnormal expression pattern / nuclear accumulation (Pathologica 2021;113:28)
- Useful (but neither ideal nor absolute) panel for determining pancreatic origin is PDX1+, ISL1+, PAX8+, CDX2+, TTF1- (Pathologica 2021;113:28)
Negative stains
- Rb (classic loss of Rb protein) (Am J Surg Pathol 2012;36:173)
- SMAD4 (Am J Surg Pathol 2016;40:1192)
- BCL10 (helping in excluding an acinar cell carcinoma or the presence of a focal acinar differentiation)
- Beta catenin (no nuclear staining; normal positivity of cell membrane)
Molecular / cytogenetics description
- Alterations of TP53 and Rb represent the most typical molecular events in PanNEC (Cancer Treat Rev 2017;56:28, Cancer Discov 2022;12:692)
- KRAS is also mutated in a significant proportion of cases (up to 30%) (Am J Surg Pathol 2012;36:173)
- Inactivation of SMAD4 / DPC4 is also reported (5%) (Am J Surg Pathol 2012;36:173)
- Microsatellite instability can be present in up to 5 - 8% of cases and should be assessed at the time of diagnosis (Hum Pathol 2022 Jun 14 [Epub ahead of print])
Sample pathology report
- Duodenum and pancreatic head, pancreaticoduodenectomy:
- Pancreatic neuroendocrine carcinoma, large cell type (see comment)
- Pancreatic head with the presence of a high grade neuroendocrine neoplasm
- Ki67 (MIB1) index 70%; mitotic rate: 50 mitoses/2 mm2
- Presence of tumor necrosis
- The neoplasm infiltrates distal choledochus, adipose tissue and duodenum
- Metastasis in 3/12 lymph nodes
- Surgical margins without tumor involvement
- Comment: The integration of morphology with the immunohistochemical profile (and in particular, CK AE1 / AE3 and CK8 / CK18 positive, synaptophysin positive, chromogranin A weakly positive, TP53: aberrant pattern; Rb: negative) is consistent with the diagnosis of pancreatic neuroendocrine carcinoma.
Differential diagnosis
- Well differentiated G3 neuroendocrine tumor (WD PanNET):
- Nonfunctional PanNETs are identified incidentally and have no symptoms
- PanNETs G3 show avidity on SSRS imaging (Octreoscan and Gallium 68 Dotatate PET / CT)
- Cytology: PanNETs G3 show abundant granular cytoplasm, low N/C and stippled chromatin
- Resection specimens: in PanNETs G3, the G3 component is usually not homogenous and mixed with a lower grade counterpart and the same for Ki67, which is heterogeneous
- Positive stains: in PanNETs, there are diffuse and strong chromogranin A (more specific) and synaptophysin (more sensitive) staining
- Molecular profile: in PanNETs, TP53 and Rb are usually wild type; there are recurrent and mutually exclusive mutations of DAXX (death domain associated protein, 25%) and ATRX (alpha thalassemia / intellectual disability syndrome X linked, 17.6%), usually wild type in PanNECs (Science 2011;331:1199)
- High grade neuroendocrine carcinomas of other sites (pancreatic metastasis):
- Other pancreatic primaries:
- Acinar cell carcinoma:
- Mixed neuroendocrine nonneuroendocrine neoplasms:
- Different components should be identified by morphology and not only by immunohistochemistry
- Medullary carcinoma:
- Syncytial growth pattern, intratumor inflammatory cells
- Negative for neuroendocrine markers
- Lymphoma:
- Melanoma:
- Small blue cell tumors in young adults:
- Desmoplastic small cell tumor (Am J Surg Pathol 2004;28:808):
- Large nests and broad bands of small blue cells, separated by fibrous stroma
- Positive stains: cytokeratins (AE1 / AE3 and CAM5.2), NSE, desmin and WT1
- Negative stains: chromogranin A, S100 and CD99
- Primitive neuroectodermal tumors (Am J Surg Pathol 2002;26:1040):
- Sheets and lobules of small cells with round to oval nuclei, nuclear molding and scant cytoplasm; no Homer-Wright rosettes
- Positive stains: CD99, cytokeratins (AE1 / AE3 and CAM5.2), NSE, chromogranin A or synaptophysin (may be focal or diffuse)
- Negative stans: desmin, actin, S100 protein, insulin, glucagon or somatostatin
- Desmoplastic small cell tumor (Am J Surg Pathol 2004;28:808):
Additional references
Board review style question #1
Which are the molecular hallmarks of PanNEC?
- CCND1 amplification
- DAXX and ATRX mutation
- EGFR alteration
- KRAS mutation
- TP53 and Rb alteration
Board review style answer #1
E. TP53 and Rb alteration. Indeed, the most typical molecular events in PanNEC are TP53 and Rb alteration. Immunohistochemistry is a good surrogate of their molecular status and in PanNEC it generally shows an aberrant expression pattern (usually a hyperaccumulation) for p53 and Rb loss.
Comment Here
Reference: Neuroendocrine carcinoma
Comment Here
Reference: Neuroendocrine carcinoma
Board review style question #2
Board review style answer #2
B. Focal tumor necrosis and nuclear features. Tumor necrosis is a very important morphological feature for supporting the diagnosis of PanNEC but the immunohistochemical confirmation of the neuroendocrine nature is also very important.
Comment Here
Reference: Neuroendocrine carcinoma
Comment Here
Reference: Neuroendocrine carcinoma
PRSS1 hereditary pancreatitis
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Pathophysiology | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Autosomal dominant disorder caused by mutations of serine protease 1 (PRSS1), also known as the cationic trypsinogen gene, characterized by progressive irreversible lipomatous atrophy of the pancreatic parenchyma leading to pancreatic insufficiency (Am J Surg Pathol 2014;38:346)
- Most common subtype of familial hereditary pancreatitis (80%); other gene mutations associated with chronic pancreatitis include SPINK1, CFTR and CTRC
- Incomplete penetrance; 40 - 93% of mutation carriers develop chronic pancreatitis
- Patients may develop pancreatic intraepithelial neoplasia (PanIN) and have an increased lifetime risk of pancreatic cancer
Essential features
- Autosomal dominant mutation of PRSS1 with incomplete penetrance
- Recurrent episodes of acute pancreatitis that progress to chronic pancreatitis and lipomatous atrophy of the pancreas
- Increased risk of pancreatic cancer
Terminology
- Familial pancreatitis, hereditary chronic pancreatitis, autosomal dominant hereditary pancreatitis, recurrent or relapsing acute or chronic pancreatitis
ICD coding
- ICD-10: K86.1 - other chronic pancreatitis
Epidemiology
- Age of onset ranges from infancy to sixth decade of life
- Highest prevalence in the mid and southeastern regions of the United States, as well as parts of France, England and Germany (Clin Gastroenterol Hepatol 2004;2:252, Pancreatology 2001;1:439)
Pathophysiology
- R122H and N29I mutations in PRSS1 result in an increased propensity for trypsin-mediated trypsinogen activation, referred to as autoactivation, resulting in increased inappropriate intrapancreatic trypsin activity and digestion of the pancreatic parenchyma (Clin Exp Gastroenterol 2016;9:197)
- R122H mutation is a dual gain of function mutation that also prevents the autodegradation of active trypsin (Biochem Biophys Res Commun 2000;278:286)
- Obstruction from intraductal calculi may be responsible for lipomatous atrophy; exact mechanism unclear
Clinical features
- Recurrent episodes of acute pancreatitis, typically starting in childhood, with progression to chronic pancreatitis over time
- Presentation of acute and chronic pancreatitis is clinically indistinguishable from other etiologies for pancreatitis
Diagnosis
- Patients should be suspected of PRSS1 associated hereditary pancreatitis with the following: acute pancreatitis occurring in childhood; recurrent acute pancreatitis of unknown etiology; chronic pancreatitis of unknown cause (especially among individuals before age 25); a family history of recurrent acute pancreatitis and chronic pancreatitis following an autosomal dominant pattern of inheritance; and a family history of pancreatitis and pancreatic cancer
- Considering the aforementioned suspected clinical findings, a diagnosis is established by the identification of a heterozygous pathologic mutation in PRSS1
- Rare cases of PRSS1 R122H and A16V mutations have been reported in nonhereditary pancreatitis; therefore, family history prior to genetic testing is important to avoid misclassification and unnecessary testing (Clin Genet 2001;59:189, Pancreas 2001;22:18)
Prognostic factors
- R122H mutation may be associated with a more aggressive course, with earlier onset and increased severity of disease (Br J Surg 2000;87:170)
Case reports
- 38 month old boy with pancreaticopleural fistula (Pediatr Gastroenterol Hepatol Nutr 2019;22:601)
- 12 year old boy with recurrent abdominal pain (Tohoku J Exp Med 2001;195:191)
- 15 year old girl with painless, progressive abdominal distention (Postgrad Med J 1986;62:873)
- 11 family members with chronic pancreatitis (Arch Dis Child 1999;80:473)
Treatment
- Management is the same as for sporadic disease
- Total pancreatectomy may be considered in older patients due to the increased risk of cancer (World J Gastroenterol 2003;9:1)
- Relatives should undergo genetic counseling
Gross description
- Pediatric specimens are typically unremarkable
- Adult specimens are soft and shrunken with marked atrophy and fat replacement, ductal dilatation and multiple intraductal calcifications
Microscopic (histologic) description
- Childhood (early) changes
- Patchy loss of acinar and ductal tissue
- Loose perilobular and interlobular fibrosis
- Relative preservation and aggregation of islets of Langerhans (resulting in a hyperplastic appearance)
- Young adult (second to third decade of life) changes
- Progressive loss of acinar cell mass and ductal epithelium and increased fibrosis over time
- Fibrosis is increasingly intermingled with adipocytes
- Islets of Langerhans are still preserved
- Older adult (fourth to sixth decade of life) changes
- Near complete replacement of pancreatic parenchyma by mature adipose tissue with scattered islets of Langerhans and nerves
- Primarily periductal fibrosis
- Main pancreatic duct and larger interlobular ducts are more likely to be dilated, while smaller ducts are reduced or scarred
- Amyloid deposition may be seen
- Nonspecific ductal changes such as atrophy, dilatation, squamous metaplasia and intraductal calcifications
- Can be associated with pancreatic intraepithelial neoplasia at any age (Am J Surg Pathol 2014;38:346, Dig Liver Dis 2012;44:8)
Microscopic (histologic) images
Molecular / cytogenetics description
- 2 most common PRSS1 mutations are the R122H and N29I missense mutations
- > 30 mutations within PRSS1 have been identified; other less common mutations include A16V, R122C, N29T, D22G and K23R (Clin Gastroenterol Hepatol 2004;2:252)
Sample pathology report
- Pancreas, biopsy:
- Pancreatic parenchyma with intralobular and interlobular fibrosis (history of hereditary pancreatitis)
- Pancreas, total pancreatectomy:
- Pancreatic parenchyma with intralobular and interlobular fibrosis, marked acinar parenchymal loss and pseudolipomatous atrophy (history of hereditary pancreatitis)
- Low grade pancreatic intraepithelial neoplasia
- Benign cystic dilation of small and large pancreatic ducts with associated reactive epithelial changes including squamous metaplasia
- Reactive bile duct mucosal changes with associated fibrosis
- 15 benign lymph nodes
- Negative for malignancy
Differential diagnosis
- Cystic fibrosis:
- Autosomal recessive disorder caused by CFTR mutation
- Progressive pancreatitis also characterized by lipomatous pseudohypertrophy; may share a common pathophysiological mechanism
- Majority of patients present with pancreatic insufficiency in utero or early infancy
- Alcoholic / obstruction associated chronic pancreatitis:
- Fibrosis in PRSS1 hereditary pancreatitis is relatively thinner and less compact (Am J Surg Pathol 2014;38:346)
- Lipomatous pseudohypertrophy of the pancreas:
- Common degenerative change in the obese and elderly
- Typically presents as a focal lesion and not as extensive as seen in PRSS1 pancreatitis
- Other conditions associated with lipomatous pseudohypertrophy include Cushing syndrome, steroid therapy, malnutrition, viral infection, main pancreatic duct obstruction and other genetic syndromes
Additional references
Board review style question #1
- A 20 year old man with a family history of recurrent pancreatitis and diabetes was found to have a PRSS1 mutation after being tested at age 5 during his first documented episode of pancreatitis. Regarding this entity, which of the following statements is true?
- Diagnosis is made based primarily on radiology
- Most common PRSS1 mutations are N29I and A16V
- Patients are at an increased risk of pancreatic cancer
- Pattern of inheritance for this entity is autosomal recessive
Board review style answer #1
C. Patients are at an increased risk of pancreatic cancer. This picture shows a pancreas with partial replacement of normal parenchyma with loose perilobular and interlobular fibrosis with scattered adipocytes. In a patient with a family history of recurrent pancreatitis, identification of a pathologic mutation in PRSS1 in this patient establishes a diagnosis of PRSS1 hereditary pancreatitis. As the patient's disease progresses, the pancreas will continue to undergo replacement largely by adipocytes with primarily perilobuductal fibrosis. PanIN has been seen in several cases of hereditary pancreatitis and these patients are at an increased risk of pancreatic cancer.
Inheritance is in an autosomal dominant pattern with variable penetrance. The most common PRSS1 mutations are R122H and N29I. Patients' clinical, laboratory and radiological presentations of acute and chronic pancreatitis are the same as for other etiologies. Careful family history taking and testing for a PRSS1 mutation in a suspected individual is what establishes the diagnosis.
Comment Here
Reference: PRSS1 hereditary pancreatitis
Inheritance is in an autosomal dominant pattern with variable penetrance. The most common PRSS1 mutations are R122H and N29I. Patients' clinical, laboratory and radiological presentations of acute and chronic pancreatitis are the same as for other etiologies. Careful family history taking and testing for a PRSS1 mutation in a suspected individual is what establishes the diagnosis.
Comment Here
Reference: PRSS1 hereditary pancreatitis
Board review style question #2
- Which of the following statements is true regarding the histologic findings in PRSS1 hereditary pancreatitis?
- Definitive diagnosis can be made if lipomatous atrophy is present
- Islets of Langerhans are preferentially lost in early stages of the disease while acinar tissue is preserved
- Pancreatic intraepithelial neoplasia can be present at any age
- Presence of intraductal calcifications is associated with an increased risk of pancreatic cancer
Board review style answer #2
C. Pancreatic intraepithelial neoplasia can be present at any age. PRSS1 hereditary pancreatitis is a progressive disease. In early stages, fibrosis and loss of acinar and ductal tissue is mild and patchy while islets of Langerhans are relatively preserved, which can result in a hyperplastic appearance. As the disease progresses, the pancreatic parenchyma undergoes near complete replacement by mature adipose tissue and fibrosis. Lipomatous atrophy is a nonspecific finding, which can be seen as a degenerative change in the obese and elderly or in patients with cystic fibrosis or other genetic syndromes. Intraductal calcifications are not associated with an increased risk of cancer and are one of several nonspecific microscopic changes seen in hereditary pancreatitis, along with ductal dilatation, squamous metaplasia and amyloid deposition. Patients with PanIN are at an increased risk of pancreatic cancer and PanIN can be seen at any age.
Comment Here
Reference: PRSS1 hereditary pancreatitis
Comment Here
Reference: PRSS1 hereditary pancreatitis
Pseudocysts
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Negative stains | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Encapsulated collection of homogenous fluid with a well defined fibrous / inflammatory wall
- Localized collections of pancreatic secretions that usually develop after pancreatitis (acute or chronic), trauma, ductal calculi or obstructive neoplasms
Essential features
- There is no epithelial lining
- Fluid content
- Nonneoplastic nature
Terminology
- Pseudocyst is the only standard term to indicate this condition
- Older terms (e.g., false cyst) are discouraged
ICD coding
- ICD-10: K86.3 - pseudocyst of pancreas
Epidemiology
- Male prevalence (M:F = 4:1)
- Peak of incidence in fourth to fifth decade
- Virtually all patients with pancreatic pseudocyst have symptoms and show an history of acute or chronic pancreatitis (Am J Gastroenterol 2018;113:464)
- Typically, pancreatic pseudocyst is unilocular and does not display calcification
- Involvement of main pancreatic duct is common and it might be irregularly dilated with stones (Pancreatology 2017;17:738)
Sites
- All parts of the pancreatic gland may be involved
- Distal location (body / tail) is more frequent than the cephalic presentation (65% of cases show body / tail involvement)
Etiology
- Thought to arise from disruption of the main pancreatic duct (or its intrapancreatic branches) and the consequent leakage of pancreatic juice that results in a persistent, localized fluid collection (Gut 2013;62:102)
- May also arise in the setting of acute necrotizing pancreatitis as a result of a disconnected duct syndrome, whereby pancreatic parenchymal necrosis of the neck or body of the gland isolates a still viable distal pancreatic remnant (Gastrointest Endosc 2008;68:91)
Clinical features
- Pseudocysts are detectable at least 4 weeks after the clinical presentation of acute pancreatitis, which requires at least 2 of the 3 following features:
- Abdominal pain consistent with acute pancreatitis (acute onset of a persistent, severe, epigastric pain, often radiating to the back)
- Serum lipase activity (or amylase activity) at least 3 times greater than the upper limit of normal
- Characteristic findings of acute pancreatitis on contrast enhanced CT; less commonly on MRI or transabdominal ultrasonography (Gut 2013;62:102)
Diagnosis
- First line of imaging to study pancreatic lesions is represented by CT / MRI
- Second line is represented by endoscopic ultrasound (EUS) with cyst fluid analysis
- Histopathology (Pathologica 2020;112:197)
Laboratory
- EUS could be easily associated with additional investigation, such as
- Cyst fluid analysis; in particular, in pseudocyst, there is usually a marked increase in amylase activity and a very low carcinoembryonic antigen (CEA) concentration in the cyst fluid (Gut 2013;62:102, Gastrointest Endosc Clin N Am 2018;28:549)
- Pathology: see Cytology description and Microscopic (histologic) description
Radiology description
- In particular, CT is indicated for the detection of parenchymal, mural or central calcification, especially when differentiating pseudocysts associated with chronic pancreatitis from pancreatic cystic neoplasm (Gut 2018;67:789)
- If CT / MRI is not sufficient, EUS examination is recommended
- Presence of hyperenhancing solid components within cystic lesion rules out the diagnosis of pseudocyst
Case reports
- 7 year old boy with pancreatic pseudocyst caused by blunt abdominal trauma (J Med Case Rep 2017;11:217)
- 13 year old boy with pancreatic pseudocyst in a setting of incomplete pancreas divisum and recurrent acute pancreatitis (J Int Med Res 2021;49:3000605211014395)
- 40 year old woman with pseudocyst arising in gastric ectopic pancreas (Clin Res Hepatol Gastroenterol 2014;38:389)
- 41 year old woman with pancreatic pseudocyst treated with endoscopic management after stent displacement (World J Gastroenterol 2015;21:2249)
- 49 year old man with pancreatic pseudocyst that was suspected to be a cystic neoplasm, specifically a solid pseudopapillary neoplasm (Cureus 2020;12:e9883)
Treatment
- For evacuation of the material that sustains the pseudocyst, 2 major approaches (endoscopic or laparoscopic) are used
- Endoscopic drainage consists of stent implantation by endoscopic retrograde cholangiopancreatography or by EUS
- Advantages: short operation time, low intraoperative blood loss, low to null intra-abdominal sewing, low chances of injury to blood vessels and nerves, short hospital stay, low opioid demand
- Disadvantages: complications, such as hemorrhage, recurrence and stent exchange, occur in 5 - 19% of procedures
- Laparoscopic drainage
- Advantages: removal of more consistent quantities of necrotic tissue and better exploration of cyst wall structure than with the endoscopic approach
- Disadvantages: complications such as abdominal contamination, incomplete anastomosis, gastric perforation (Pancreas 2021;50:788)
- Endoscopic drainage consists of stent implantation by endoscopic retrograde cholangiopancreatography or by EUS
- Current guidelines for treatment of cystic pancreatic lesions discourage surgical treatment of pancreatic cystic lesions, unless there is a high probability of neoplastic degeneration or in order to relieve symptoms of clinical pancreatitis (Pancreatology 2017;17:738)
- Patients with asymptomatic cysts do not require treatment or surveillance if they are diagnosed as pseudocysts on initial imaging and clinical history or if they have a very low risk of malignant transformation (e.g., serous cystadenoma of the pancreas) (Am J Gastroenterol 2018;113:464)
Gross description
- Typically unilocular
- Diameter is variable; can be > 20 cm in size
- Thick, irregular wall that contains turbid, sometimes blood tinged fluid (Pathologica 2020;112:197)
Gross images
Contributed by Paola Mattiolo, M.D. and Claudio Luchini, M.D., Ph.D.
Images hosted on other servers:
Microscopic (histologic) description
- By definition, there is no epithelial lining; please note that large pancreatic ducts may interface between pseudocyst and adjacent pancreas
- Cyst wall may contain histiocytes, giant cells, granulation tissue, rarely eosinophils
- To rule out the presence of neoplastic epithelium or dysplastic changes, examination of the entire cyst wall is strongly suggested (Pathologica 2020;112:197, Am J Gastroenterol 2018;113:464)
Microscopic (histologic) images
Cytology description
- Cyst fluid cytology typically demonstrates hemosiderin laden macrophages, acute inflammatory cells or lymphocytes, fibrin, debris, bile pigment and lack of neoplastic cells
- Through the needle microforceps biopsy: modality that allows the collection of samples from pancreatic cystic wall for microscopic histological examination through a 19 gauge needle
- Advantages: diagnostic accuracy is high (up to around 80%) (Gastrointest Endosc 2019;90:784)
- Disadvantages: the procedure is burdened by the occurrence of adverse effect in ~9% of cases, 4% of cases with severe adverse events and 1% of cases with fatality, while it changes the clinical management in ~11% of cases; thus, patient selection should be strictly managed and limited to those in whom sampling has a high probability of changing the clinical management (Endoscopy 2021;53:44)
Negative stains
- Cytokeratin (all types) are negative: by definition, no epithelial cells are present in pseudocyst lining
Sample pathology report
- Duodenum and pancreatic head, pancreaticoduodenectomy:
- Pancreatic head with the presence of cyst without an epithelial lining (see comment)
- The pancreatic cyst has been entirely analyzed
- Regional lymph nodes with reactive changes; duodenum without pathological modifications
- Comment: The histological appearance is consistent with the diagnosis of pancreatic pseudocyst. Correlation of histology with clinical history and radiology is strongly suggested.
Differential diagnosis
- Pancreatic cystic neoplasm:
- Particularly if multiloculated, may display significant denudation of the lining epithelium (Dig Liver Dis 2020;52:547)
- Intraductal papillary mucinous neoplasms (IPMNs)
- Serous cystic neoplasms:
- Even if completely denudated, it displays a subepithelial network of delicate capillary channels (Am J Surg Pathol 2012;36:726)
- Mucinous cystic neoplasms (MCNs):
- Even if completely denudated, it displays ovarian type stroma
- Cystic neuroendocrine neoplasms:
- Display a neuroendocrine epithelial lining, positive at immunohistochemistry for chromogranin A and synaptophysin
- Solid pseudopapillary neoplasms:
- Present neoplastic tissue, with necrotic / hemorrhagic cystic changes and tumor cells that are positive at immunohistochemistry for beta catenin (nuclear), LEF1 and CD10
- Cystic pancreatic ductal adenocarcinoma:
- Particularly if multiloculated, may display significant denudation of the lining epithelium (Dig Liver Dis 2020;52:547)
- Acute necrotic collection:
- Differs from pseudocyst because it is not well defined and it is filled by a commixture of fluid and necrosis, with different density
- Appears during the clinical manifestation of the necrotizing pancreatitis
- Walled off necrosis:
- Differs from pseudocyst because it is filled by a commixture of fluid and necrosis, with different density
- Infective cysts:
- Secondary bacterial infection may produce cyst
- Rarely, Echinococcus spp. may cause hydatid cyst in the pancreas and it should be suspected in endemic region (Diagn Cytopathol 1992;8:65, World J Gastrointest Surg 2014;6:190)
- Cystic lymphangiomas:
- Lesion associated with prominent lymphoid tissues that demonstrates ectatic spaces lined by endothelial type cells
- Lymphoepithelial cysts:
- Radiologically and sonographically indistinguishable from pseudocyst
- Smears show abundant anucleated squamous cells, keratinous debris, nucleated squamous cells and it is possible to recognize squamous cell block with preserved granular layer (Cancer 2006;108:501)
- Cysts associated with paraduodenal pancreatitis:
- Differential diagnosis is given by the clinicopathological context
- Squamoid cysts of the pancreas:
- Extremely uncommon cyst that is lined exclusively by squamous epithelium, lacking in solid areas
- Acinar cystic transformation of the pancreas:
- Cystic lesion lined by acinar epithelium
- Not excluded the presence of foci of mucinous or squamous epithelium
- Congenital cysts of the pancreas:
- Rare cysts that arise by developmental errors of pancreatic ducts, presumably related to localized obstruction of the duct in the utero
- Typically, these cysts are symptomatic before the second year of age and histologically, they present nonmucin producing cuboidal, columnar or flattened cells (Semin Diagn Pathol 2000;17:7)
- Enteric duplication cysts:
- Rare congenital malformations that are most commonly diagnosed in children
- These cysts display gastric or intestinal type lining epithelium and a well developed, bilayered muscular wall
- Ciliated foregut cysts:
- Cytology is characterized by amorphous debris, rare macrophages and ciliated columnar cells and detached ciliary tufts, which distinguish ciliated foregut cyst from pseudocyst
- Epidermoid cysts:
- Presence of squamous epithelium and keratin flakes
- Colliquated metastasis:
- Renal cell carcinoma is the most common source of metastasis to the pancreas
- Other malignancies that involve the pancreatic district are melanoma, pulmonary, mammary, gastric and colonic adenocarcinoma
Additional references
Board review style question #1
Is this histological image of pancreatic parenchyma sufficient to support the diagnosis of pseudocyst?
- No, it is necessary to examine the entire lesion in order to exclude the presence of epithelium
- Yes, the image proves the absence of dysplastic epithelium
- Yes, the image proves the presence of a fibrotic wall
- Yes, the image shows a fibrotic wall with bona fide disepithelization
- Yes, the image shows the presence of residual pancreatic parenchyma
Board review style answer #1
A. No, it is necessary to examine the entire lesion in order to exclude the presence of epithelium. For the diagnosis of pseudocysts, first it is mandatory to exclude the presence of an epithelial cell lining. A random sampling is not sufficient; the sampling should allow the examination of the entire lesion.
Comment Here
Reference: Pseudocysts
Comment Here
Reference: Pseudocysts
Board review style question #2
Which analysis of cystic fluid is most suspicious for pseudocyst?
- High carcinoembryonic antigen (CEA), high amylase
- High CEA, high lipase
- High CEA, low amylase
- Low CEA, high amylase
- Low CEA, low amylase
Board review style answer #2
D. Low CEA, high amylase. Typically, the cyst fluid analysis of a pseudocyst usually shows a very low CEA concentration in the cyst fluid (
Comment Here
Reference: Pseudocysts
Comment Here
Reference: Pseudocysts
Risk stratification of IPMNs (pending)
[Pending]
Sclerosing epithelioid mesenchymal neoplasm
Table of Contents
Definition / general | Essential features | Epidemiology | Sites | Clinical features | Laboratory | Prognostic factors | Case reports | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Molecular / cytogenetics images | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- A novel indolent pancreatic neoplasm with unique morphologic / immunophenotypic features and a distinct methylation pattern, different from both epithelial and mesenchymal neoplasms described in other anatomical sites (Mod Pathol 2020;33:456)
- First described in 2020 (Mod Pathol 2020;33:456)
Essential features
- Indolent
- Well circumscribed tumor with a geographic pattern of epithelioid cell nests alternating with spindle cell fascicles
- Vimentin (diffuse), CD99 (diffuse) and cytokeratin (AE1 / AE3 and CK18, dot-like) expression
- Lack of abnormalities in any of key genetic drivers
- Mesenchymal transcriptomic features and distinct methylation signature
Epidemiology
- Mean age: 43 years (Mod Pathol 2020;33:456)
- F:M = 3:1 (Mod Pathol 2020;33:456)
Sites
- Mostly occurs in head and neck of the pancreas (Mod Pathol 2020;33:456)
Clinical features
- Nonspecific symptoms
Laboratory
- Serum tumor markers (CA19.9 and CEA) within normal limits
Prognostic factors
- None known; exhibits indolent behavior (Mod Pathol 2020;33:456)
Case reports
- 31 year old woman with suspected solid pseudopapillary neoplasm of the pancreatic tail (Pancreas 2021;50:e47)
Gross description
- Well circumscribed and solid, usually confined to the pancreatic parenchyma
- White-tan and firm cut surface
- Median tumor size: 1.8 cm (Mod Pathol 2020;33:456)
Microscopic (histologic) description
- Solid neoplasms associated with extensive fibrosis and dense lymphoid aggregates at the periphery (Mod Pathol 2020;33:456)
- Entrapped pancreatic parenchyma may be seen at the periphery or within the tumor
- Density of tumor cells varies significantly, not only among tumors but also throughout each tumor, creating a geographic appearance of hypercellular and hypocellular areas with collagenous stroma
- No true epithelial structures such as glands, papillae or pseudopapillae (Mod Pathol 2020;33:456)
- Most cells are epithelioid to spindled with scant cytoplasm and round to oval nuclei with open chromatin
- Others display more irregular, hyperchromatic nuclei
- Occasional mitotic figures may be seen
Microscopic (histologic) images
Contributed by Olca Basturk, M.D.
Positive stains
- Vimentin, CD99, AE1 / AE3, CK18 (dot-like) (Mod Pathol 2020;33:456)
Negative stains
- Synaptophysin, chromogranin, abnormal beta catenin, progesterone receptor, CD10, trypsin, chymotrypsin, MUC4, desmin, myogenin, INI1 (retained), ERG, CD31, CD34, S100, HMB45, MelanA, CD117, DOG1, TTF1, HepPar1, BCL2, ALK, STAT6, CD21, CD35, CD45 (Mod Pathol 2020;33:456)
Electron microscopy description
- Nonspecific findings; occasional desmosomes, perinuclear tonofilaments and abundant rough endoplasmic reticulum (Mod Pathol 2020;33:456)
Molecular / cytogenetics description
- No recurrent somatic mutations, amplifications or deletions in any known oncogenes or suppressor genes (Mod Pathol 2020;33:456)
- Activation or enrichment of pathways related to the extracellular matrix, tight junctions, adherens junctions and TGFβ signaling by Gene Set Enrichment Analysis (Mod Pathol 2020;33:456)
- A unique cluster by unsupervised analysis of the methylation profiles (Mod Pathol 2020;33:456)
Molecular / cytogenetics images
Sample pathology report
- Pancreas and duodenum, pancreaticoduodenectomy:
- Sclerosing epithelioid mesenchymal neoplasm (see comment)
- Comment: The tumor is confined to the pancreatic parenchyma and measures 2.2 cm in greatest dimension. Adjacent pancreas reveals atrophy. Surgical margins are free of tumor. 16 benign lymph nodes are identified (0/16).
Differential diagnosis
- Solid pseudopapillary neoplasm:
- Pseudopapillae, areas of macrophages, hyaline globules and nuclear grooves
- Abnormal beta catenin and nuclear progesterone receptor expression
- CTNNB1 mutations
- Sarcomatoid carcinoma:
- Larger infiltrative tumors with considerable pleomorphism, necrosis and proliferative activity
- Glandular component may be present
- KRAS, TP53, SMAD4, CDKN2A mutations
- Sclerosing epithelioid fibrosarcoma:
- MUC4 expression
- EWSR1-CREB3L1, FUS-CREB3L21 or FUS-CREB3L2 fusions
- Solitary fibrous tumor:
- Inflammatory myofibroblastic tumor:
- Perivascular epithelioid cell neoplasm (PEComa):
- Gastrointestinal stromal tumor:
Board review style question #1
Board review style answer #1
Board review style question #2
Which one of the following microscopic features is seen in sclerosing epithelioid mesenchymal neoplasms of the pancreas?
- No entrapped pancreatic parenchyma is seen within the tumors.
- They are composed of epithelioid cells only.
- They are predominantly hypocellular tumors.
- They have well circumscribed borders.
- True epithelial structures such as glands, papillae and pseudopapillae are common.
Board review style answer #2
D. They have well circumscribed borders.
Comment Here
Reference: Sclerosing epithelioid mesenchymal neoplasm
Comment Here
Reference: Sclerosing epithelioid mesenchymal neoplasm
Serous cystadenoma
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Etiology | Clinical features | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Benign pancreatic neoplasm of presumed ductal origin but of nonmucinous nature (Semin Diagn Pathol 2014;31:475, Am J Surg Pathol 2015;39:1597, Pancreatology 2009;9:182)
- Characterized by glycogen rich (nonmucinous; serous) epithelial cells with distinctive cytology showing uniform round nuclei with homogenous dense chromatin
- A prominent microvascular network hugging the epithelium as in other clear cell tumors with VHL pathway alterations (Am J Surg Pathol 2015;39:1597, Pancreatology 2009;9:182, Surgery 2012;152:S4)
- Microcystic pattern, which is the most common, creates a unique sponge-like configuration diagnostic of this tumor type
Essential features
- Rare, benign pancreatic neoplasm, more common in females and elderly patients (Semin Diagn Pathol 2014;31:475, Am J Surg Pathol 2015;39:1597, Pancreatology 2009;9:182)
- Characterized by numerous, small cystic spaces (synonym: microcystic adenoma) lined by distinctive glycogen rich epithelial cells (synonym: glycogen rich adenoma) with uniform round nuclei and a prominent microvascular network hugging the epithelium (Am J Surg Pathol 2015;39:1597, Pancreatology 2009;9:182, Surgery 2012;152:S4)
- Microcystic pattern, which is the most common, creates a unique sponge-like configuration diagnostic of this tumor type
Epidemiology
- Rare pancreatic tumor that often presents as a cystic mass occurring predominantly in women (F:M = 3:1)
- Mean age of patients is 58 years (Am J Surg Pathol 2015;39:1597)
Sites
- Can be found anywhere but more frequently in the tail of the pancreas
Etiology
- VHL pathway alterations (and can occur in VHL syndrome patients) but no known etiology
Clinical features
- Mean age: 58
- F:M = 3:1
- Frequently occurs sporadically but rarely arises in von Hippel-Lindau (VHL) syndrome
- VHL associated cases are sometimes patchy, multifocal (Pancreas 2012;41:380, Surgery 2012;152:1106)
- Often asymptomatic; rarely can be symptomatic due to obstruction of the biliary or GI tract (Am J Gastroenterol 2011;106:1521)
- Abdominal pain is occasionally attributed to this tumor
- Solid variant is often clinically misdiagnosed as pancreatic neuroendocrine tumor (PanNET)
- Macrocystic / oligocystic variants are often misdiagnosed as other cystic tumors
- Association with other tumors is common (13%), especially PanNET
- Typically, these minute cysts / glands are back to back and form a distinct mass but sometimes they can be dispersed in between native pancreatic elements
Radiology description
- CT typically demonstrates a demarcated, lobulated mass in the pancreas that is often interpreted as cystic (J Family Med Prim Care 2019;8:2744)
Prognostic factors
- Most serous tumors of the pancreas are benign with excellent prognosis (World J Surg 2003;27:319)
- Some of these tumors may attain a large size and push into (invade) the neighboring organs but overt malignant transformation and mortality is exceedingly uncommon (Dig Surg 2016;33:240)
- Synchronous or metachronous liver lesions are debated as to whether metastatic or multifocality (Am J Surg Pathol 2015;39:1597)
Case reports
- 17 year old girl with unilocular macrocystic serous cystadenoma of pancreas (Rev Med Brux 2017;38:39)
- 67 year old woman with serous cystic neoplasm at the pancreatic body and high grade PanIN lesions at the main and branch pancreatic ducts (Int J Surg Pathol 2018;26:551)
- 67 year old woman with macrocystic multilocular cystic lesion in the pancreatic head with proximal dilatation of Wirsung duct (J Gastrointest Surg 2019;23:176)
- 72 year old woman with hypervascular solid mass in the tail of the pancreas (Case Rep Surg 2016;2016:3730249)
- 75 year old woman with duodenal neuroendocrine tumor and pancreatic serous cystic neoplasm (Int J Surg Pathol 2018;26:551)
Treatment
- Watchful waiting because invariably benign (Zhonghua Wai Ke Za Zhi 2018;56:591)
- Surgical treatment should be proposed in a minority of patients, only for uncertain diagnosis remaining after complete workup, including CT scan, MRI and endoscopic ultrasound, significant and related symptoms or exceptionally, when concern for malignancy exists (Gut 2016;65:305)
- Rarely, local adhesion / infiltration into the adjacent lymph nodes or adjacent organs (colon, stomach) is seen in larger tumors
- Occurrence of similar tumors in the liver is being debated as whether synchronicity, multifocality or metastasis; behavior of those is also excellent
- True malignant degeneration or mortality due to this tumor is virtually nonexistent (exceedingly uncommon, dubious cases)
Gross description
- Favors the pancreatic body / tail region over the head / neck (ratio of 1.6:1) (Am J Surg Pathol 2015;39:1597)
- Well demarcated, large mass lesion (mean: 6 cm; range: 1 - 30 cm)
- Often has a central stellate scar
- Microcystic type: most common; characterized by a collection of innumerable microcysts (each within millimeters) creating the pathognomonic sponge-like configuration with gray to beige / cream color; rare large loculi measuring within centimeters are often seen dispersed within the lesion
- Oligocystic (macrocystic; megacystic) type: composed of fewer and larger loculi, with the overall picture closely resembling other megacystic tumors such as mucinous cystic neoplasms
- Solid type: homogenous, demarcated, beige-tan nodule with shiny gelatinous appearance; often mistaken as PanNETs clinically and grossly
- In the cystic types, the fluid is clear and watery; hemorrhage can occur
- Rarely multifocal or dispersed (especially VHL associated cases) (J Gastrointest Cancer 2010;41:197)
Gross images
Microscopic (histologic) description
- Innumerable small, irregularly contoured tubular structures of variable shapes ranging from submillimeter to centimeters
- Cystic spaces lined by bland appearing cuboidal to low columnar epithelial cells with abundant clear, glycogen rich (PAS+, diastase sensitive) cytoplasm
- Nuclei are round and remarkably uniform with dense, homogenous chromatin and inconspicuous nucleoli
- Degenerative atypia leading to pleomorphic cells can be encountered as scattered cells
- Mitoses are typically absent
- Stroma ranges from edematous to fibrotic, myxoid or hyalinized with an intimately admixed capillary network; calcifications can be seen (Pancreatology 2009;9:182)
- Occasionally, the neoplastic cells form intraluminal minute papillary projections, usually without a fibrovascular stalk
- Some cases have more oncocytic cells with granular cytoplasm
- Solid variant is composed of tightly packed, back to back, uniform, small glands with only minimal or no lumen formation, showing the typical cytologic characteristics described above
Microscopic (histologic) images
Cytology description
- Scant amount of serous fluid
- Smears with very low cellularity, rich in protein; seldom diagnostic (Acta Cytol 2017;61:27)
- Epithelial cells arranged in flat sheets or singly (Cancer Cytopathol 2014;122:33)
- Stripped nuclei, clear cytoplasm
- Cytoplasmic borders well defined
- Round nuclei, evenly distributed chromatin
- Capillarization may be helpful
Positive stains
- Epithelial markers (keratins, EMA)
- MUC1 (often), MUC6 (common) (Am J Surg Pathol 2004;28:339)
- Inhibin A (Am J Surg Pathol 2004;28:339)
- GLUT1, HIF1α, CAIX, VEGF (Pancreatology 2009;9:182)
- Endothelial markers (highlight the rich periepithelial capillary network) (Pancreatology 2009;9:182)
Negative stains
Electron microscopy description
- Tumor tissue consists of cysts lined by columnar epithelium
- Tumor cells have intervening cell junctions and desmosomes
- Many small blood vessels in the subepithelial layer, the latter being intimately associated with the basal portions of epithelial tumor cells (Ultrastruct Pathol 2006;30:119)
- Organelle deficient and lack mucin, zymogen and dense glycogen granules; surface microvilli and cilia have rarely been reported (Pathology 2002;34:148)
- Myoepithelial cells have also rarely been reported (Ultrastruct Pathol 2006;30:119)
Molecular / cytogenetics description
- VHL gene alterations; loss of heterozygosity of the chromosome 3p region is most common in patients with VHL (Am J Pathol 2001;158:317)
- VHL gene alterations may also be detected in sporadic cases
Sample pathology report
- Partial distal pancreatectomy:
- Serous cystadenoma (see comment)
- Comment: There are multiple cystic spaces lined by a simple cuboidal to flattened epithelium with round, uniform nuclei and clear cytoplasm. Significant nuclear atypia or mitotic activity was not seen. There is some acellular fibrous tissue with a variable degree of hyalinization and edematous change in the stroma. Immunohistochemically the tumor cells are positive with EMA and MUC1 while negative for HMB45. The morphologic and immunohistochemical features are consistent with pancreatic serous cystadenoma.
Differential diagnosis
- Lymphangioma:
- Peripancreatic tissue, lymphoid aggregates between the cystic spaces attenuated cells with pale chromatin
- Cytokeratin-
- Metastatic renal cell carcinoma:
- Solid and acinar regions, prominent nuclear atypia, sinusoidal vascular pattern
- PAX8+
- Mucinous cystic neoplasm:
- Ovarian type stroma, mucinous or nonmucinous ductal cells (Am J Surg Pathol 2017;41:121)
- Clear cell (sugar) tumor (PEComa):
- Epitheloid spindle cells with cytoplasmic vacuolization
- HMB45+
Board review style question #1
Which of the following is true about pancreatic serous cystadenoma?
- It affects males and females equally
- It usually carries risk of malignant transformation
- Macrocystic type is the most frequent histologic subtype
- Some cases are associated with von Hippel-Lindau (VHL) syndrome
- Synaptophysin and chromogranin are positive for immunohistochemistry
Board review style answer #1
D. Some cases are associated with von Hippel-Lindau (VHL) syndrome. Pancreatic serous cystadenoma frequently occurs sporadically but rarely arises in von Hippel-Lindau (VHL) syndrome. VHL associated cases are sometimes patchy or multifocal.
Comment Here
Reference: Serous cystadenoma
Comment Here
Reference: Serous cystadenoma
Board review style question #2
A 47 year old woman presented with abdominal pain. CT images showed a 4 cm well circumscribed mass in the tail of the pancreas. Repeated fine needle aspiration biopsy did not yield specific diagnostic findings. The tumor was resected and showed a well demarcated tumor with innumerable tiny cystic spaces that created a sponge-like appearance, with beige-tan color and a central stellate scar. On microscopic examination, there were tightly packed small glandular / cystic elements of variable sizes lined by clear cells with nuclei that were small, round and uniform with dense homogeneous chromatin and devoid of mitotic activity (as shown in the picture). What is the most likely diagnosis and expected immunoprofile of this tumor?
- Mucinous cystic neoplasm: progesterone receptor in the stroma, mucicarmine, MUC5AC
- Pancreatic ductal adenocarcinoma: CEA, SMAD4 loss, p53
- Pancreatic serous cystadenoma: pancytokeratin, inhibin, GLUT1
- Solid pseudopapillary neoplasm: diffuse nuclear beta catenin, synaptophysin, CD10
- Solid serous adenoma: pancytokeratin, MUC6
Board review style answer #2
C. Pancreatic serous cystadenoma: pancytokeratin, inhibin, GLUT1
Comment Here
Reference: Serous cystadenoma
Comment Here
Reference: Serous cystadenoma
Simple mucinous cyst
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Mucinous pancreatic cyst with minimal cytologic atypia, grossly > 1 cm, lined by single layer of gastric type flat epithelium without ovarian type stroma
Essential features
- Unilocular or multilocular mucinous cyst (> 1 cm) with single layer of columnar or cuboidal cells and low grade dysplasia
- Paucicellular fibrotic wall with no ovarian type stroma or lymphoid band
- Cyst rarely communicates with pancreatic duct
- High cyst fluid CEA, low amylase
- KRAS and KMT2C mutations in a subset of cases
- Follows benign clinical course, no reported progression to carcinoma
Terminology
- Also called mucinous nonneoplastic cyst, first proposed by Kosmahl et al. in 2002 (Mod Pathol 2002;15:154)
ICD coding
- ICD-10: K86.2 - cyst of pancreas
Epidemiology
- 2 - 3.4% of pancreatic resections, 2.2 - 6.7% of pancreatic cyst resections and 7.6 - 10.3% of pancreatic mucinous cyst resections (Arch Pathol Lab Med 2017;141:1330)
- Mean age: 64 (range, 20 - 88) (Arch Pathol Lab Med 2017;141:1330)
- More common in females, F:M = 2.8:1 to 4:1 (Arch Pathol Lab Med 2017;141:1330, Am J Surg Pathol 2017;41:121)
Sites
- Body, tail (53 - 69%)
- Head, neck, uncinate (31 - 47%) (Arch Pathol Lab Med 2017;141:1330, Am J Surg Pathol 2017;41:121)
Clinical features
- Asymptomatic or incidental (41 - 49%), abdominal pain (34 - 44%), jaundice (4 - 7%), back pain (11%) (Arch Pathol Lab Med 2017;141:1330, Am J Surg Pathol 2017;41:121)
- Size: 1 - 12 cm (mean 2.5 cm) (Arch Pathol Lab Med 2017;141:1330)
Diagnosis
- CT or MRI of abdomen
- EUS with FNA or biopsy
- Cyst fluid analysis
- Cytology
- Surgical specimen (StatPearls: Pacreatic Cysts [Accessed 8 June 2020])
Laboratory
- Cyst fluid: often elevated CEA, mean 6,797 ng/mL (range, 11 - 64,000 ng/mL) (Arch Pathol Lab Med 2017;141:1330, Am J Surg Pathol 2017;41:121)
- Low amylase (unless connected to duct)
Radiology description
- CT / MRI:
- Unilocular (55 - 59%), multilocular or septated (41 - 45%)
- Rare communication with pancreatic duct (3 - 7%) or mural nodule (Arch Pathol Lab Med 2017;141:1330, Am J Surg Pathol 2017;41:121)
- Upstream main pancreatic duct dilatation (Abdom Radiol (NY) 2017;42:2827)
- Low signal intensity of cyst fluid on T1 weighted, high on T2 weighted and low on diffusion weighted
- Most with thin cyst wall but can have cyst wall enhancement
- EUS:
- Septate in 26.3%, solid component in 10.5%, possible communication with pancreatic duct in 21% (Pancreas 2012;41:813)
Radiology images
Prognostic factors
- Overall benign behavior with no recurrence or malignant transformation (Am J Surg Pathol 2015;39:1730, Arch Pathol Lab Med 2017;141:1330)
Case reports
- 52 year old man with hematochezia and active bleeding from ampulla (Korean J Gastroenterol 2017;70:301)
- 71 year old woman with a cystic tumor in the body of the pancreas (World J Gastroenterol 2005;11:2045)
- 75 year old man with pancreatic cyst (J Med Case Rep 2019;13:264)
Treatment
- Often treated with surgical resection due to preoperative imaging indistinguishable from other mucinous neoplasms
- Surveillance
Clinical images
Gross description
- Unilocular or multilocular cyst, mean size 2.5 cm, range 1.0 - 12 cm (Arch Pathol Lab Med 2017;141:1330, Am J Surg Pathol 2017;41:121)
- No or rare communication with pancreatic duct (Arch Pathol Lab Med 2017;141:1330, Am J Surg Pathol 2017;41:121)
- Usually clear or serous fluid, ~33% with mucinous fluid, rarely serosanguinous or brown-green thick fluid (Arch Pathol Lab Med 2017;141:1330, Am J Surg Pathol 2017;41:121)
- Smooth cyst lining, no or minimal focal papillary excrescences (Arch Pathol Lab Med 2017;141:1330, Am J Surg Pathol 2017;41:121)
- Submit entire lining (or extensive sampling if large cyst) to rule out high grade dysplasia or invasive carcinoma
Gross images
Frozen section description
- > 1 cm unilocular or multilocular cyst
- Flat gastric type mucinous epithelium
- No significant atypia
- No ovarian type stroma
Frozen section images
Microscopic (histologic) description
- Flat monolayer of columnar mucinous epithelium or attenuated cuboidal epithelial cells, often resembling gastric type mucinous epithelium (Arch Pathol Lab Med 2017;141:1330, Am J Surg Pathol 2017;41:121)
- Low grade dysplasia, which may mimic low grade pancreatic intraepithelial neoplasia (PanIN)
- Focal high grade dysplasia in 8% cases in one study (Am J Surg Pathol 2017;41:121)
- No or minimal papillary architecture (Arch Pathol Lab Med 2017;141:1330, Am J Surg Pathol 2017;41:121)
- Paucicellular fibrotic wall with no ovarian type stroma or lymphoid band (Arch Pathol Lab Med 2017;141:1330, Am J Surg Pathol 2017;41:121)
- Degenerative changes in cyst wall common:
- Hyalinization, myxoid stroma, hemorrhage, calcifications, macrophage clusters, granulation-like tissue (Arch Pathol Lab Med 2017;141:1330, Am J Surg Pathol 2017;41:121)
Microscopic (histologic) images
Virtual slides
Cytology description
- Flat honeycomb sheets / nests of cuboidal or columnar cells without significant atypia (100%) (Pancreas 2013;42:27, Pancreas 2012;41:813)
- Round to oval nuclei, small to slightly enlarged, 1 - 2 inconspicuous nucleoli, fine granular chromatin, smooth nuclear contour, nuclear grooves (43.5%), nuclear pseudoinclusions (26.1%) (Pancreas 2013;42:27)
- Delicate, vacuolated cytoplasm (60.9%), variable in amount (Pancreas 2013;42:27)
- May show papillary architecture (10.5%), acini formation (10.5%), 3D clusters (5.3%), single cell pattern (5.3%), goblet cells (17.4%) (Pancreas 2013;42:27)
- Background mucin, macrophages (43.5%), rarely stroma (17.4%) (Pancreas 2013;42:27)
- Limitations: may not be representative of entire cyst lining
- Unremarkable mucin
Positive stains
- MUC5AC (77 - 90%), MUC6 (74 - 93%), MUC1 (35 - 37%), CK7 (Arch Pathol Lab Med 2017;141:1330, Am J Surg Pathol 2017;41:121)
Negative stains
- MUC2, retained SMAD4 (Arch Pathol Lab Med 2017;141:1330, Am J Surg Pathol 2017;41:121)
- CDX2 (can be focal)
- p53
Molecular / cytogenetics description
- KRAS mutations in 13 - 55% (Arch Pathol Lab Med 2017;141:1330, Am J Surg Pathol 2017;41:121)
- KMT2C (MLL3) mutations in 62% (Hum Pathol 2020;101:1)
- BRAF, RNF43, CDKN2A, SMAD4 mutations in 8% (Hum Pathol 2020;101:1)
- TP53 mutations in 15% (Hum Pathol 2020;101:1)
- Loss of heterozygosity at 10q (PTEN) or 17q (ubiquitin E3 ligase ring finger 43 / RNF43) reported in 2 cases (Hum Pathol 2016;55:159)
- Clonality analysis with HUMARA gene showed polymorphism (Hum Pathol 2010;41:513)
Sample pathology report
- Pancreas, resection:
- Simple mucinous cyst (see comment)
- Comment: Many of these lesions have KRAS mutation. Although there is no consensus, many authors consider this entity a neoplastic process. However, they tend to have a benign behavior with no reported recurrence or malignant transformation after resection.
Differential diagnosis
- Intraductal pancreatic mucinous neoplasm (IPMN):
- Increased amylase in cyst fluid (Arch Pathol Lab Med 2017;141:1330, Am J Surg Pathol 2017;41:121)
- More commonly head of pancreas
- More papillary architecture
- Communicates with pancreatic duct
- Can have high grade dysplasia or be associated with invasive carcinoma
- Mucinous cystic neoplasm (MCN):
- Almost exclusively in women
- Body and tail of pancreas
- Can have papillary epithelium
- Can have high grade dysplasia
- Ovarian type stroma (progesterone receptor / PR positive) (Arch Pathol Lab Med 2017;141:1330, Am J Surg Pathol 2017;41:121)
- Can be associated with invasive carcinoma
- Retention cyst:
- Cystic dilation of pancreatic duct
- Intraluminal obstruction (Clin Endosc 2015;48:31)
- Lymphoepithelial cyst:
- Squamous or columnar epithelium
- Lymphocytes and germinal centers within the wall
- Epidermoid cyst:
- Keratinizing squamous epithelium
- Squamoid cyst:
Board review style question #1
- A 62 year old woman presents with an incidental cystic lesion in the head / uncinate process of the pancreas on imaging. The cyst does not communicate with the pancreatic duct. Upon grossing of the Whipple resection specimen, a 4.6 cm multiloculated cyst with smooth lining is present in the head / uncinate. Representative H&E image of the cyst lining is shown. Which of the following is true regarding this entity?
- High grade dysplasia is often present
- KRAS mutations have been demonstrated in a subset of cases
- Often communicate with pancreatic duct
- Ovarian type stroma is diagnostic of this entity
- Papillary architecture is common
Board review style answer #1
B. KRAS mutations have been demonstrated in a subset of cases
Comment Here
Reference: Simple mucinous cyst
Comment Here
Reference: Simple mucinous cyst
Board review style question #2
- A 2.3 cm multiloculated cyst with smooth lining located in the head of the pancreas was removed during surgery. It does not connect with the main pancreatic duct. The pathologist observes that the cyst lining is composed of a single layer of columnar mucinous cells with no papillary architecture or high grade dysplasia. The surrounding cyst wall is fibrotic and paucicellular with no ovarian type stroma. No ductal obstruction is present. What is the best diagnosis for this entity?
- Intraductal pancreatic mucinous neoplasm
- Mucinous cystic neoplasm
- Pancreatic pseudocyst
- Retention cyst
- Simple mucinous cyst
Board review style answer #2
Solid pseudopapillary neoplasm
Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Sites | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Low malignant potential tumors with unclear cell of origin and pathogenesis
- May be derived from pluripotent stem cells of the genital ridges that become attached to the pancreas during embryogenesis (Semin Diagn Pathol 2014;31:484)
- First described by V.K. Frantz in 1959; incorporated into the WHO classification in 1996 (Br J Surg 1959;47:334)
Essential features
- Presents in younger women, classically as solitary body / tail mass
- Variable amount of solid and cystic areas grossly
- Papillary fronds on myxoid or hyalinized vascular stalk lined by poorly cohesive, uniform cells with nuclear grooves comprising solid and cystic areas beta catenin nuclear / cytoplasmic positive
Terminology
- Also known as solid pseudopapillary tumor, papillary epithelial neoplasm, papillary cystic neoplasm, solid and papillary neoplasm, low grade papillary neoplasm and Hamoudi or Frantz tumor
Epidemiology
- Represents 1 - 2% of pancreatic neoplasms
- F:M = 10:1 (accounts for 30% tumors in < 40 year old women) (Arch Pathol Lab Med 2020;144:829)
- Usually presents in the third to fourth decade of life (mean age 35 years)
- In men, tends to occur at an older age with more aggressive behavior (Surgery 2008;143:29)
- Occasional cases have been reported in patients with familial adenomatous polyposis (Intern Med 2015;54:1349, Surg Case Rep 2021;7:35)
Sites
- Located throughout the pancreas but more frequently in the body or tail in adults, while more frequent in the head of pancreas in children (Arch Surg 2008;143:1218)
- Rarely reported in extrapancreatic sites, such as mesocolon, omentum, ovary and testis (Virchows Arch A Pathol Anat Histopathol 1991;418:179, Am J Surg Pathol 2010;34:1514, Int J Gynecol Pathol 2011;30:539, Ann Diagn Pathol 2018;35:42)
Clinical features
- Most common symptoms are abdominal pain and a palpable, nontender, upper abdominal mass
- Also symptoms related to an intra abdominal mass effect, such as discomfort, nausea, vomiting and early satiety
- However, most cases are incidentally discovered on imaging
- Reference: Arch Pathol Lab Med 2017;141:990
Diagnosis
- Solid / solid cystic mass in the tail of pancreas on imaging, especially in young women (Diagn Interv Radiol 2017;23:94)
- Cyst fluid chemistry shows low CEA and amylase (Ann Gastroenterol 2013;26:122)
- Cytology / histology are necessary for confirmation of diagnosis
Laboratory
- Cyst fluid is often bloody with low CEA and low amylase (Ann Gastroenterol 2013;26:122)
Radiology description
- Well circumscribed, encapsulated, heterogeneous pancreatic lesion with cystic degeneration on CT or MRI
Radiology images
Prognostic factors
- Poor prognostic factors include: size > 5 cm, male gender, necrosis, cellular atypia, vascular invasion, perineural invasion and invasion into adjacent structures (Dig Liver Dis 2013;45:703)
Case reports
- 14 year old Hispanic girl with silent presentation of a solid pseudopapillary neoplasm of the pancreas (Am J Case Rep 2017;18:656)
- 16 year old boy with tonic convulsions (J Clin Res Pediatr Endocrinol 2017;9:375)
- 40 year old man with subsequent multiple metastases (World J Gastrointest Oncol 2017;9:497)
- 45 year old woman with situs invertus (Medicine (Baltimore) 2018;97:e0205)
- 62 year old woman with IgG4 related pancreatitis (Int J Surg Pathol 2017;25:271)
Treatment
- Surgical resection is curative in > 95% of cases
Gross description
- Range from 0.5 to 34.5 cm, with a mean diameter of 6 cm
- Well defined, encapsulated, with variable amount of solid and cystic patterns
- Smaller lesions tend to be more solid but less sharply circumscribed
- Larger tumors demonstrate a fibrous pseudocapsule and have a variegated and friable cut surface
- Cystic degeneration and hemorrhage are common findings in larger specimens
- Rarely, may extend into adjacent structures, such as duodenum
- Reference: Arch Pathol Lab Med 2020;144:829
Gross images
Microscopic (histologic) description
- Tumors are heterogeneous, with variable admixture of solid and pseudopapillary areas
- Solid areas are comprised of uniform cells admixed with capillary sized blood vessels
- Pseudopapillae are formed due to tumor cells getting detached from blood vessels forming fibrovascular stalks or rosette-like structures (Arch Pathol Lab Med 2020;144:829)
- Stroma usually shows various degrees of hyalinization or evidence of degeneration, such as hemorrhage, foamy macrophages, calcification and cholesterol clefts
- Tumor cells usually have a moderate amount of eosinophilic cytoplasm with intracytoplasmic hyaline globules (PAS+ and diastase resistant, positive for alpha-1-antitrypsin) and perinuclear vacuoles (Am J Surg Pathol 2011;35:981)
- Relatively uniform nuclei with finely textured chromatin, inconspicuous nucleoli and characteristic longitudinal grooves
- Variants include clear cell, oncocytic and pleomorphic
- Rare mitotic figures
- Although grossly well circumscribed, microscopic finding of infiltration to the surrounding pancreatic tissue is not uncommon
- Rare cases of highly aggressive behavior; histological features in those cases included diffuse growth pattern, extensive necrosis, significant nuclear atypia, high mitotic count (35 - 70/50 high power fields) or sarcomatoid features (Am J Surg Pathol 2005;29:512)
Microscopic (histologic) images
Contributed by Monika Vyas, M.D., Omid Savari, M.D. and Raul S. Gonzalez, M.D.
Cytology description
- Cellular smears with delicate papillary fronds
- Tumor cells are usually bland and uniform with a moderate amount of cytoplasm, which usually contains variable sized clear perinuclear vacuoles or cytoplasmic eosinophilic hyaline globules
- Nuclei are round to oval with grooves and finely granular chromatin (J Cytol 2010;27:118, Arch Pathol Lab Med 2017;141:990)
- Cercariform cells, cytoplasmic vacuolation, reniform nuclei, hyaline globules and degenerative features, such as cholesterol crystals, calcifications, foam cells or giant cells, are more commonly seen in solid pseudopapillary neoplasms, compared with acinar cell carcinomas / neuroendocrine tumors (Cancer Cytopathol 2013;121:298)
Cytology images
Positive stains
- Beta catenin (98%): aberrant nuclear expression
- E-cadherin (loss of membranous expression) (Hum Pathol 2008;39:251)
- p120 (cytoplasmic staining with loss / reduction of membranous expression) (Am J Clin Pathol 2008;130:71)
- Alpha-1-antichymotrypsin (95%), alpha-1-antitrypsin (82%)
- Vimentin (88%)
- Cyclin D1
- CD10 (63%)
- SOX11 (100%)
- Androgen receptor (81%), TFE3 (75%), LEF1 (93%), FUS (85%), progesterone receptor (63%), claudin7, claudin5 (Am J Surg Pathol 2009;33:768)
- CD56 (96%), neuron specific enolase (70%), synaptophysin (55%)
- Cytokeratin (52%)
- CD99 (dot-like) (Am J Surg Pathol 2011;35:799)
Negative stains
- Chromogranin A (positive in only 9%)
- CEA
- Estrogen receptor
- Reference: Arch Pathol Lab Med 2017;141:990
Electron microscopy description
- Mitochondria rich cytoplasm with electron dense granules and rough endoplasmic reticulum
Molecular / cytogenetics description
- Point mutation in exon 3 of β catenin gene (CTNNB1) is present in > 90%
- Gene mutation results in the accumulation of β catenin in the cytoplasm and formation of a β catenin Tcf / Lef complex, through which the Wnt signaling pathway activates several oncogenic genes, such as MYC and cyclin D1 (Am J Clin Pathol 2017;149:67)
- Rarely, mutations in APC gene (also associated with familial adenomatous polyposis) have been described (Pathol Int 2019;69:193)
Sample pathology report
- Pancreas and spleen, distal pancreatectomy and splenectomy:
- Solid pseudopapillary neoplasm (XX cm), confined to pancreas (see comment)
- Resection margins are free of tumor
- Unremarkable spleen, (x g)
- X benign lymph nodes (0/12)
- Comment: Immunohistochemical stains show the neoplastic cells are positive for CD10 and exhibit nuclear expression of beta catenin while negative for synaptophysin and chromogranin.
- Pancreas, mass, biopsy:
- Epithelial neoplasm, most consistent with solid pseudopapillary neoplasm (see comment)
- Comment: By immunohistochemistry, the neoplastic cells are positive for CD56, pancytokeratin (focal) and show rare focal positivity for synaptophysin while being negative for chromogranin. Aberrant nuclear expression of beta catenin is present. The histologic and immunohistochemical findings are in keeping with a solid pseudopapillary neoplasm.
- Pathological staging (AJCC 8th edition) - similar to exocrine pancreatic tumors
Differential diagnosis
- Pancreatic neuroendocrine tumor:
- Small to medium sized cells with uniform nuclei and finely granular chromatin (salt and pepper appearance), arranged in organoid, trabecular or nested patterns
- Positive for neuroendocrine markers and negative for nuclear beta catenin
- Acinar cell carcinoma:
- Prominent acinar formation and cells with finely granular cytoplasm
- Positive for pancreatic enzymes (trypsin, chymotrypsin), BCL10 and variable staining for beta catenin
- Pancreatoblastoma:
- Primitive pancreatic elements with acinar, ductal and neuroendocrine differentiation
- Also squamoid corpuscles; more common in children younger than 10 years
- Positive for beta catenin (80%), neuroendocrine markers and pancreatic enzymes (trypsin, chymotrypsin and lipase) and BCL10
Board review style question #1
What is the most common gene mutation in pancreatic solid pseudopapillary neoplasm?
- CTNNB1
- KIT
- PDGFRA
- RAS
Board review style answer #1
A. Somatic point mutation of β catenin gene (CTNNB1) is present in > 90% of pancreatic solid pseudopapillary neoplasms
Comment Here
Reference: Solid pseudopapillary neoplasm
Comment Here
Reference: Solid pseudopapillary neoplasm
Board review style question #2
Board review style answer #2
A. The hyaline globules are immunoreactive for alpha-1-antitrypsin and are also PAS+ and diastase resistant
Comment Here
Reference: Solid pseudopapillary neoplasm
Comment Here
Reference: Solid pseudopapillary neoplasm
Somatostatinoma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Clinical features | Diagnosis | Radiology images | Case reports | Treatment | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Neuroendocrine neoplasm (may be well differentiated tumor or poorly differentiated carcinoma) that secretes somatostatin, leading to diabetes, cholecystolithiasis, steatorrhea and diarrhea (J Hepatobiliary Pancreat Sci 2015;22:578)
Essential features
- Rare functional neuroendocrine neoplasm that secretes somatostatin
- May appear microscopically as a well differentiated tumor or a poorly differentiated carcinoma
Terminology
- Also called delta cell tumor
ICD coding
Epidemiology
- Rare; fewer than 1% of gastroenteropancreatic neuroendocrine neoplasms (J Gastroenterol Hepatol 2008;23:521)
Sites
- Can occur in the pancreas or in the ampulla / duodenum
Clinical features
- Rare; may be more common in women
- May occur in patients with NF1 (Clin Gastroenterol Hepatol 2009;7:A28)
- Most lesions that stain for somatostatin by immunohistochemistry do not produce somatostatinoma syndrome and arguably do not qualify to be diagnosed as somatostatinomas (Endocr Relat Cancer 2008;15:229)
- Often metastasizes but still usually has good prognosis
Diagnosis
- Imaging to detect pancreatic mass
- Elevated plasma somatostatin levels
Case reports
- 57 year old woman with tumors in pancreas and duodenum (World J Gastroenterol 2009;15:5859)
- 72 year old woman with pancreatic tumor and lymph node metastasis (JOP 2014;15:66)
- 4 patients with tumor in the pancreas and peripancreas (Ann R Coll Surg Engl 2011;93:356)
Treatment
- Surgical resection of primary lesion, if possible
- Hepatic metastases can be resected or embolized
- Octreotide for metastatic disease
Microscopic (histologic) description
- Some cases are well differentiated neuroendocrine tumor, with nests of monotonous low grade neuroendocrine cells with salt and pepper nuclei and ample amphophilic cytoplasm
- Other cases are poorly differentiated neuroendocrine carcinoma, with nests and sheets of mildly / moderately pleomorphic cells with high mitotic rate, small cell or large cell features (no salt and pepper nuclei) and foci of necrosis
- May rarely feature psammoma bodies (which are common in duodenal somatostatinomas) (J Surg Oncol 1995;59:67)
Positive stains
- Somatostatin (though this does not establish the diagnosis)
- Synaptophysin, chromogranin
- PDX1, INSM1 (Am J Surg Pathol 2012;36:737)
Negative stains
Electron microscopy description
- Tumor cells resemble normal D cells (N Engl J Med 1977;296:963)
Sample pathology report
- Pancreas and duodenum, Whipple procedure:
- Well differentiated neuroendocrine tumor of pancreas (see comment and synoptic report)
- Comment: Based on the patient’s clinical symptoms, this neuroendocrine tumor is best considered a somatostatinoma.
Differential diagnosis
- Nongastrin secreting pancreatic neuroendocrine tumor or carcinoma:
- No histopathologic differences
- Must be determined on clinical grounds
Additional references
Board review style question #1
- Patients with a somatostatinoma of the pancreas may experience which symptoms?
- Cholelithiasis and steatorrhea
- High volume watery diarrhea
- Hypoglycemia
- Nausea, vomiting and peptic ulcer disease
- Necrolytic migratory erythema
Board review style answer #1
Board review style question #2
- Which of the following is true of pancreatic somatostatinomas?
- They are common in patients with von Hippel-Lindau syndrome
- They are the most common functional pancreatic neuroendocrine neoplasm
- They are typically localized and rarely metastasize
- They may be neuroendocrine tumors or carcinomas
- They usually contain psammoma bodies
Board review style answer #2
Staging-exocrine
Table of Contents
Definition / general | Essential features | ICD coding | Primary tumor (pT) | Regional lymph nodes (pN) | Distant metastasis (pM) | Prefixes | Stage grouping | Registry data collection variables | Histologic grade | Histopathologic type | Board review style question #1 | Board review style answer #1Pathologic TNM staging of exocrine tumors of the pancreas, AJCC 8th edition
Definition / general
- All exocrine tumors of the pancreas (including any lesion designated as carcinoma, as well as solid papillary neoplasm and pancreaticoblastoma) are covered by this staging system
- Not covered by this staging system are well differentiated neuroendocrine tumors at this location (use the pancreas neuroendocrine staging system instead)
- The 8th edition staging has been shown to be more prognostically relevant than the 7th edition staging for pancreatic ductal adenocarcinoma (Pancreas 2018;47:742)
Essential features
- AJCC 7th edition staging was sunset on December 31, 2017; as of January 1, 2018, use of the AJCC, 8th Edition, 2018 is mandatory
ICD coding
- ICD-10: C25.9 - malignant neoplasm of pancreas, unspecified
Primary tumor (pT)
Regional lymph nodes (pN)
- pNX: regional lymph nodes cannot be assessed
- pN0: no regional lymph node involvement
- pN1: metastasis in one to three regional lymph nodes
- pN2: metastasis in four or more regional lymph nodes
Notes:
- Regional lymph nodes depend on tumor site
- For tumors of the head or neck of the pancreas, regional nodes include common bile duct, common hepatic artery, portal vein, pyloric, posterior and anterior pancreatoduodenal arcades, superior mesenteric vein and right lateral wall of superior mesenteric vein nodes
- For tumors of the body or tail of the pancreas, regional nodes include common hepatic artery, celiac axis, splenic artery and splenic hilum nodes
- Minimum of 12 lymph nodes must be recovered for lymph node staging to be considered accurate in curative resections
Distant metastasis (pM)
- pM0: no distant metastasis
- pM1: distant metastasis
Prefixes
- y: preoperative radiotherapy or chemotherapy
- r: recurrent tumor stage
Stage grouping
- Stage IA:T1 N0 M0
- Stage IB:T2 N0 M0
- Stage IIA:T3 N0 M0
- Stage IIB:T1 - 3 N1 M0
- Stage III:T1 - 3 N2 M0
- T4 any N M0
- Stage IV:any T any N M1
Registry data collection variables
- Preoperative CA 19-9
- Preoperative carcinoembryonic antigen (CEA)
Histologic grade
- GX: grade cannot be assessed
- G1: well differentiated
- G2: moderately differentiated
- G3: poorly differentiated
Histopathologic type
- Ductal adenocarcinoma
- Adenosquamous carcinoma
- Hepatoid carcinoma
- Medullary carcinoma
- Mucinous noncystic carcinoma (colloid carcinoma)
- Signet ring cell carcinoma
- Undifferentiated carcinoma
- Undifferentiated carcinoma with osteoclast-like giant cells
- Acinar cell carcinoma
- Acinar cell cystadenocarcinoma
- Intraductal papillary mucinous neoplasm with associated invasive carcinoma
- Intraductal tubulopapillary neoplasm with associated invasive carcinoma
- Mucinous cystic neoplasm with associated invasive carcinoma
- Pancreaticoblastoma
- Serous cystadenocarcinoma
- Solid pseudopapillary neoplasm
- Neuroendocrine carcinoma
- Small cell neuroendocrine carcinoma
- Large cell neuroendocrine carcinoma
- Mixed acinar ductal carcinoma
- Mixed acinar neuroendocrine carcinoma
- Mixed acinar neuroendocrine ductal carcinoma
- Mixed neuroendocrine ductal carcinoma
Board review style question #1
Under 8th edition AJCC staging criteria, which of the following would categorize a pancreatic ductal adenocarcinoma as pT3?
- Involvement of the duodenum
- Involvement of the peripancreatic fat
- Involvement of the superior mesenteric artery
- Size > 4 cm
Board review style answer #1
Staging-neuroendocrine
Table of Contents
Definition / general | Essential features | ICD coding | Primary tumor (pT) | Regional lymph nodes (pN) | Distant metastasis (pM) | Prefixes | Primary tumor suffix | Regional lymph nodes suffix | AJCC prognostic stage groups | Prognostic tumor characteristics | Registry data collection variables | Histologic grade (G) | Histopathologic type | Residual tumor (operative factor) | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- All well differentiated neuroendocrine tumors of the pancreas are covered by this staging system
- Not covered by this staging system are poorly differentiated neuroendocrine carcinomas at this location (use the pancreas exocrine staging system instead)
Essential features
- AJCC 7th edition staging was sunset on December 31, 2017; as of January 1, 2018, use of the 8th edition is mandatory
- AJCC 9th edition was released in 2023, with minimal changes from the 8th edition
ICD coding
- ICD-10: C25.4 - malignant neoplasm of endocrine pancreas
Primary tumor (pT)
- pTX: tumor cannot be assessed
- pT1: tumor limited to the pancreas, ≤ 2 cm in greatest dimension
- pT2: tumor limited to the pancreas, > 2 cm but ≤ 4 cm in greatest dimension
- pT3: tumor limited to the pancreas, > 4 cm in greatest dimension or tumor invading the duodenum, ampulla of Vater or common bile duct
- pT4: tumor invading adjacent organs (stomach, spleen, colon, adrenal gland) or the wall of large vessels (celiac axis, superior mesenteric artery / vein, splenic artery / vein, gastroduodenal artery / vein, portal vein)
Notes:
- Limited to the pancreas means no extension into adjacent organs or structures; invasion of peripancreatic adipose tissue does not impact staging
- When multiple tumors are present, the largest tumor should be used to assign the T category
- Use T(#) (e.g., pT3[4] N0 M0) or use the m suffix, T(m) (e.g., pT3[m] N0 M0)
Regional lymph nodes (pN)
- NX: regional lymph nodes cannot be assessed
- N0: no tumor involvement of regional lymph node(s)
- N1: tumor involvement of regional lymph node(s)
Notes:
- Regional lymph nodes depend on tumor site
- For tumors of the head of the pancreas, regional nodes include common bile duct, common hepatic artery, portal vein, posterior and anterior pancreatoduodenal arcades, superior mesenteric vein and right lateral wall of superior mesenteric vein nodes
- For tumors of the body or tail of the pancreas, regional nodes include common hepatic artery, celiac axis, splenic artery and splenic hilum nodes
Distant metastasis (pM)
- cM0: no distant metastasis
- cM1: distant metastasis
- cM1a: metastasis confined to liver
- cM1b: metastasis in at least 1 extrahepatic site (e.g., lung, ovary, nonregional lymph node, peritoneum, bone)
- cM1c: both hepatic and extrahepatic metastasis
- pM1: microscopic confirmation of distant metastasis
- pM1a: microscopic confirmation of metastasis confined to liver
- pM1b: microscopic confirmation of metastasis in at least 1 extrahepatic site (e.g., lung, ovary, nonregional lymph node, peritoneum, bone)
- pM1c: microscopic confirmation of both hepatic and extrahepatic metastasis
Prefixes
- c: clinical
- p: pathological
- yc: posttherapy clinical
- yp: posttherapy pathological
Primary tumor suffix
- (m): multiple synchronous primary tumors
Regional lymph nodes suffix
- (f): fine needle aspiration (FNA) or core needle biopsy
AJCC prognostic stage groups
| Stage I: | T1 | N0 | M0 |
| Stage II: | T2, T3 | N0 | M0 |
| Stage III: | T4 | N0 | M0 |
| any T | N1 | M0 | |
| Stage IV: | any T | any N | M1 |
Prognostic tumor characteristics
- Mitotic count
- Ki67 index
- Associated genetic syndrome
- Chromogranin A (CgA)
- Functionality
- DAXX / ATRX
- ARX, PDX1 expression
Registry data collection variables
- Size of tumor (value, unknown)
- Presence of invasion into adjacent organs / structures
- If yes, which ones (pick all that apply)
- Stomach (yes / no)
- Duodenum (yes / no)
- Spleen (yes / no)
- Colon (yes / no)
- Other:
- If yes, were multiple adjacent organs involved (yes / no)
- If yes, which ones (pick all that apply)
- Presence of necrosis
- Number of tumors (multicentric disease at primary site)
- Lymph node status (including number of lymph nodes assessed and number of positive nodes)
- Grade (based on Ki67 or mitotic count): G1, G2, G3, unknown
- Mitotic count (value; unknown)
- Ki67 index (value; unknown)
- Perineural invasion
- Lymphovascular invasion
- Margin status
- Functional status (yes / no, type of syndrome)
- Genetic syndrome (yes / no, type of syndrome)
- Location in pancreas (head, tail, body, junction body / tail, junction body / head, unknown)
- Type of surgery (enucleation, distal pancreatectomy with or without splenectomy, central pancreatectomy, pancreaticoduodenectomy / Whipple procedure, unknown, other)
- Preoperative CgA level (absolute value with upper limit of normal; unknown)
- Age of patient
Histologic grade (G)
- G1: mitotic rate 2 and Ki67 G2: mitotic rate 2 - 20 per 2 mm2 or Ki67 3 - 20%
- G3: mitotic rate > 20 per 2 mm2 or Ki67 > 20%
Histopathologic type
- Neuroendocrine tumor (NET), NOS
- Neuroendocrine tumor, grade 1
- Neuroendocrine tumor, grade 2
- Neuroendocrine tumor, grade 3
- Pancreatic neuroendocrine tumor, nonfunctioning
- Insulinoma
- Gastrinoma
- Vasoactive intestinal peptide secreting tumor (VIPoma)
- Glucagonoma
- Somatostatinoma
- Adrenocorticotropic hormone (ACTH) producing neuroendocrine tumor
- Serotonin producing neuroendocrine tumor
- Growth hormone (GH) producing neuroendocrine tumor
Residual tumor (operative factor)
- R0: complete resection, margins histologically negative, no residual tumor left after resection
- R1: incomplete resection, margins histologically involved, microscopic tumor remains after resection of gross disease (relevant to resection margins that are microscopically involved by tumor)
- R2: incomplete resection, margins involved or gross disease remains
Board review style question #1
Invasion of which of the following structures would upstage a pancreatic neuroendocrine tumor to pT4 disease?
- Common bile duct
- Duodenum
- Peripancreatic fat
- Spleen
Board review style answer #1
D. Spleen. Direction invasion of this organ is specifically noted as upstaging a pancreatic well differentiated neuroendocrine tumor to pT4 disease. Answers A and B are incorrect because invasion of these structures is noted as signifying pT3 disease. Answer C is incorrect because invasion of peripancreatic fat does not affect the staging of these tumors.
Comment Here
Reference: Pancreas - Staging-neuroendocrine
Comment Here
Reference: Pancreas - Staging-neuroendocrine
Board review style question #2
Which of the following is true regarding staging and prognosis of pancreatic well differentiated neuroendocrine tumors?
- ARX and PDX1 expression are considered prognostic indicators
- More than 5 positive lymph nodes indicates pN2 disease
- Regional lymph nodes are the same regardless of tumor location within the pancreas
- Tumor functionality is not considered a prognostic indicator
Board review style answer #2
A. ARX and PDX1 expression are considered prognostic indicators per the AJCC, among multiple additional factors. Answer B is incorrect because there is no pN2 designation for pancreatic neuroendocrine tumors. Answer C is incorrect because the regional lymph nodes are different depending on whether the tumor arises in the head or the body / tail. Answer D is incorrect because tumor functionality is considered a prognostic indicator.
Comment Here
Reference: Pancreas - Staging-neuroendocrine
Comment Here
Reference: Pancreas - Staging-neuroendocrine
True cysts
Table of Contents
Congenital / dysgenetic cysts | Cysts of cystic fibrosis | Dermoid cysts | Epidermoid cysts | Foregut cysts attached to pancreas | Mesothelial cysts | Pancreatic hamartoma | Nonepithelial cysts | Pancreatic tissue derived cysts | Tumors that are cysticCongenital / dysgenetic cysts
Definition / general
Case reports
Microscopic (histologic) images
AFIP images
- By definition are intrapancreatic, do not communicate with duct system, lined by a single layer of cuboidal, columnar or flattened atrophic epithelium with a fibrous wall
- Most congenital pancreatic cysts are multiple, almost all are associated with underlying congenital diseases that primarily affect other organ systems (AJR Am J Roentgenol 2002;179:1375)
- Often associated with polycystic disease affecting kidney and liver, von Hippel-Lindau syndrome or oral-facial-digital syndrome type I (GeneReviews: Oral-Facial-Digital Syndrome Type I [Accessed 11 December 2017])
- Single cysts may be due to abnormal duct development
- Other syndromes: Elejalde syndrome (eMedicine: Elejalde Syndrome [Accessed 11 December 2017]), glutaric aciduria II (NIH: Glutaric Acidemia Type II [Accessed 11 December 2017]), Ivemark syndrome (NORD: Ivemark Syndrome [Accessed 11 December 2017]), Jeune syndrome (NIH: Jeune Syndrome [Accessed 11 December 2017]), Meckel-Gruber syndrome (eMedicine: Meckel-Gruber Syndrome [Accessed 11 December 2017]), short rib polydactyly syndrome type 1 (OMIM: Short Rib Thoracic Dysplasia 3 With or Without Polydactyly [Accessed 11 December 2017]), trisomy 13, 14, 15, tuberous sclerosis
Case reports
- Multilocular cysts associated with choledochal cyst (Hum Pathol 2003;34:99)
Microscopic (histologic) images
AFIP images
Cysts of cystic fibrosis
Dermoid cysts
Definition / general
Case reports
Gross images
Images hosted on other servers:
Microscopic (histologic) images
Images hosted on other servers:
- Seen in young patients (2nd - 3rd decade)
Case reports
- 64 year old man (World J Surg Oncol 2007;5:85)
Gross images
Images hosted on other servers:
Microscopic (histologic) images
Images hosted on other servers:
Epidermoid cysts
Definition / general
Case reports
- May be present within an intrapancreatic spleen (Pancreas 2011;40:956)
- In tail, unilocular, typically contain concentric enzymatic concretions, may communicate with acinar system
- Squamoid cyst of pancreatic ducts; has thin wall lined by transitional / squamous cells without keratinization or granular layer
Case reports
- 62 year old man with intrapancreatic spleen containing epidermoid cyst (JOP 2011;12:279)
Foregut cysts attached to pancreas
Definition / general
Case reports
Microscopic (histologic) images
Images hosted on other servers:
Cytology images
Images hosted on other servers:
- Rare; unilocular, smooth surface with clear mucoid material, ciliated epithelium resembles bronchogenic cyst but no respiratory glands, no cartilage and two smooth muscle layers (Am J Surg Pathol 1996;20:476)
Case reports
- 34 year old woman with pancreatic mass (JSLS 2008;12:183)
- 37 year old woman with acute cholangitis (JOP 2011;12:420)
Microscopic (histologic) images
Images hosted on other servers:
Cytology images
Images hosted on other servers:
Mesothelial cysts
Definition / general
Case reports
- May be multiple and involve pancreas, liver, kidney or other abdominal structures
Case reports
- 36 year old man with 3 cm cyst (Ann Diagn Pathol 2006;10:371)
Pancreatic hamartoma
Case reports
Microscopic (histologic) images
Images hosted on other servers:
- Initial report in 20 month old girl with 9 cm cystic mass (Hum Pathol 1992;23:1309)
- 52 year old woman with 2.5 cm mass in pancreatic head (J Korean Surg Soc 2012;83:330)
- Well demarcated and composed of cystic ductal structures embedded in focally inflamed stromal tissue (Am J Surg Pathol 2005;29:797)
Microscopic (histologic) images
Images hosted on other servers:
Nonepithelial cysts
Definition / general
- Includes endometrial cyst, lymphangioma, pseudocysts, parasitic cyst
Pancreatic tissue derived cysts
Definition / general
Gross images
Images hosted on other servers:
Microscopic (histologic) images
Images hosted on other servers:
- May appear in thorax or mediastinum, as components of gastroenteric duplication cysts, intralobar pulmonary sequestrations, teratomas or rarely from ectopic pancreatic tissue (Mod Pathol 1996;9:210)
- Paraduodenal wall cyst (cystic dystrophy): patients with paraduodenal (groove) pancreatitis, cysts may enlarge and adhere to duodenal wall, mimicking duodenal duplication; lined by granulation tissue, may be partly lined by ductal epithelium (Semin Diagn Pathol 2004;21:247)
Gross images
Images hosted on other servers:
Microscopic (histologic) images
Images hosted on other servers:
Tumors that are cystic
Definition / general
- Cystic tumors include ductal adenocarcinoma, IOPN, IPMN, lymphoepithelial cyst, mucinous cystic neoplasm, mucinous nonneoplastic cyst, serous cystadenoma, solid papillary neoplasm
Undifferentiated carcinoma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Carcinoma of the pancreas that does not show a definitive direction of differentiation
- Under this definition, there are 4 variants: anaplastic, sarcomatoid, rhabdoid and carcinosarcoma (Mod Pathol 2015;28:248)
Essential features
- Undifferentiated carcinoma with atypical neoplastic cells and without gland formation
- Absence of osteoclast-like giant cells
- Very poor prognosis
Terminology
- Undifferentiated carcinoma of the pancreas
- Old terminology, not in use: spindle cell carcinoma, pleomorphic carcinoma
ICD coding
Epidemiology
- M:F = 2:1 (World J Gastroenterol 2016;22:8631)
- Commonly presents in the sixth to seventh decades (World J Gastroenterol 2016;22:8631, J Am Coll Surg 2012;215:627)
- 1 - 5% of all pancreatic cancers (World J Gastroenterol 2016;22:8631, J Am Coll Surg 2012;215:627)
Sites
- Head of the pancreas (50% of cases), body / tail (45%), entire pancreas (5%) (World J Gastroenterol 2016;22:8631)
Pathophysiology
- The process of epithelial to mesenchymal transition may play an important role (Pancreas 2019;48:36, Virchows Arch 2021;478:319)
- Chronic inflammation with histiocytes is not a crucial mechanism as in undifferentiated carcinoma with osteoclast-like giant cells (Hum Pathol 2018;81:157)
Clinical features
- Presenting symptoms include jaundice (tumors in the pancreatic head), abdominal or back pain, weight loss, nausea and anemia (Diagn Cytopathol 2016;44:538, World J Gastroenterol 2016;22:8631, World J Clin Cases 2019;7:236)
Diagnosis
- CT scan and MRI are the preferred imaging modality (J Med Imaging Radiat Oncol 2019;63:580)
- Diagnosis is by biopsy or surgical resection
Radiology description
- Usually mass lesions with delayed enhancement, intratumor calcifications (J Med Imaging Radiat Oncol 2019;63:580)
Prognostic factors
- Prognosis is very poor, with an average survival time of just 5 months, making it difficult to describe significant prognostic factors (J Am Coll Surg 2012;215:627)
- Absence of osteoclast-like giant cells is important for the diagnosis of real, undifferentiated carcinoma (i.e., to avoid misdiagnosis) but is essential for the prognosis as well, since the prognosis of real, undifferentiated carcinoma is very poor, compared with that of carcinoma with osteoclast-like giant cells
Case reports
- 59 year old man with undifferentiated carcinoma of the pancreas, sarcomatoid type (World J Clin Cases 2019;7:236)
- 63 year old man with undifferentiated carcinoma of the pancreas, rhabdoid type, with multiple metastases (autopsy report) (Pathol Int 2015;65:264)
- 64 year old woman with undifferentiated carcinoma of the pancreas, anaplastic type, associated with cystic degeneration of the lesion (World J Gastroenterol 2016;22:8631)
- 65 year old man with undifferentiated carcinoma of the pancreas, anaplastic type, involving the ampullary region (Am J Case Rep 2019;20:597)
- 73 year old woman with undifferentiated carcinoma of the pancreas, carcinosarcoma type (Pancreas 2017;46:1225)
Treatment
- Surgical resection if possible; gemcitabine based chemotherapy / radiotherapy (J Surg Res 2017;219:238)
- A recent study highlighted a possible role of immunotherapy in selected cases, PDL1 positive (Expert Opin Ther Targets 2021;25:1007)
Gross description
- Very often similar to conventional pancreatic ductal adenocarcinoma
- May show cystic degeneration and necrosis (World J Gastroenterol 2016;22:8631)
Gross images
Frozen section description
- Hypercellular neoplasm with atypical cells
Microscopic (histologic) description
- Unlike conventional ductal adenocarcinoma, this tumor is composed of poorly cohesive cells
- Undifferentiated carcinoma of the pancreas is a hypercellular tumor with scant stroma and scant desmoplastic reaction; this differs from conventional pancreatic ductal adenocarcinoma which has a large amount of desmoplastic stroma with few neoplastic cells / glands
- This is a major point in the differential diagnosis between these 2 entities
- Perineural invasion and vascular invasion are very common
- Different subtypes are characterized at the histological level by different morphologic hallmarks:
- Anaplastic subtype is a tumor composed of > 80% highly atypical cells that have pleomorphic nuclei and lack gland formation
- Sarcomatoid subtype is composed of > 80% atypical spindle cells, resembling a sarcoma (Int J Mol Sci 2022;23:1283, Hum Pathol 2022;128:124)
- Rhabdoid subtype is a very rare sarcomatoid variant which is composed of large atypical cells with eosinophilic cytoplasm and eccentrically located nuclei
- Carcinosarcoma is a subtype in which there is a mixture of roundish epithelioid cells and spindle sarcomatous cells; each component should arbitrarily constitute 30% of the neoplasm to qualify as carcinosarcoma
Microscopic (histologic) images
Contributed by Claudio Luchini, M.D., Ph.D. and AFIP
Cytology description
- Hypercellular neoplasm: presence of highly atypical cells
Negative stains
- Neoplastic cells are negative for E-cadherin
- ~50% of undifferentiated rhabdoid carcinomas typically present a nuclear loss of SMARCB1 / INI1 at immunohistochemistry (J Gastrointest Oncol 2021;12:874, Mod Pathol 2015;28:248)
Molecular / cytogenetics description
- Overlaps with the genetic profile of conventional ductal adenocarcinoma
- In the rhabdoid variant, KRAS alterations and SMARCB1 expression status define 2 subtypes, with possible translational implications (Mod Pathol 2015;28:248)
- There is an enrichment in KRAS amplification in both rhabdoid and sarcomatoid variants, if compared with conventional ductal adenocarcinoma (Mod Pathol 2015;28:248, Hum Pathol 2022;128:124)
- SMAD4 alterations appear as a rare molecular event in the sarcomatoid variant, if compared with conventional ductal adenocarcinoma (Hum Pathol 2022;128:124)
Sample pathology report
- Pancreas, pancreatectomy:
- Pancreatic undifferentiated carcinoma
- Pancreas, biopsy / cytology:
- Pancreatic undifferentiated carcinoma (see comment)
- Comment: Cellular neoplasm with highly atypical cells is consistent with undifferentiated carcinoma of the pancreas.
- Surgical resection: percentage of the undifferentiated component; infiltration, if any, of duodenum, common bile duct, neck margin, intestinal margin and retroperitoneal margin if Whipple resection, spleen and pancreatic margin if distal pancreatectomy; vascular / perineural invasion if any; nodal metastasis (if any); pTNM staging.
Differential diagnosis
- Undifferentiated carcinoma of the pancreas with osteoclast-like giant cells:
- Presence of osteoclast-like giant cells
- Sarcoma:
- Keratin is negative
- Melanoma:
Additional references
Board review style question #1
This is a high magnification field of a tumor within the pancreas. Can the diagnosis of undifferentiated carcinoma be made based on morphology only?
- No, electron microscopy must be used to rule out a melanoma
- No, immunohistochemistry is mandatory
- No, molecular analysis is mandatory
- No, special histochemical stains are mandatory
- Yes, morphology only is sufficient
Board review style answer #1
B. No, immunohistochemistry is mandatory. Morphology is the most important step for the diagnosis; however, immunohistochemistry for keratin and vimentin should be performed in most cases to rule out sarcoma, melanoma and metastasis. This is particularly true for undifferentiated carcinoma with sarcomatoid features, to avoid misdiagnosis with sarcomas and for anaplastic type, to rule out melanoma or metastases.
Comment Here
Reference: Undifferentiated carcinoma
Comment Here
Reference: Undifferentiated carcinoma
Board review style question #2
How do the fibrosis and desmoplastic reactions seen in conventional ductal adenocarcinoma of the pancreas compare to that of undifferentiated carcinoma?
- Conventional ductal adenocarcinoma typically has more fibrosis and desmoplasia
- Only anaplastic type undifferentiated carcinoma shows more fibrosis / desmoplasia than conventional ductal adenocarcinoma
- There is always more fibrosis in undifferentiated carcinoma
- There is the same quantity of fibrosis in both diagnoses
- They are highly variable in both diagnoses
Board review style answer #2
A. Conventional ductal adenocarcinoma usually has much more fibrosis / desmoplasia than undifferentiated carcinoma. This is a major point in the differential diagnosis between the 2 diagnoses.
Comment Here
Reference: Undifferentiated carcinoma
Comment Here
Reference: Undifferentiated carcinoma
Undifferentiated carcinoma with osteoclast-like giant cells
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Undifferentiated carcinoma of the pancreas with no glandular differentiation and with prominent infiltration by histiocytes and osteoclast-like giant cells
- May be found in association with a differentiated component of pancreatic ductal adenocarcinoma
Essential features
- Undifferentiated carcinoma with atypical neoplastic cells and lacking gland formation
- Presence of multinucleated giant cells resembling osteoclasts
Terminology
- Undifferentiated carcinoma with osteoclast-like giant cells (UCOGC, UCOCGC)
- Old terminology, not in use: giant cell tumor of the pancreas, osteoclastoma
ICD coding
Epidemiology
- M:F = 0.9:1 (Am J Surg Pathol 2016;40:1203, J Pathol 2017;243:148)
- Commonly presents in the sixth to seventh decades (Am J Surg Pathol 2016;40:1203, J Pathol 2017;243:148)
- 1.4% of all pancreatic cancers (Am J Surg Pathol 2016;40:1203)
Sites
- Head of the pancreas (60 - 75% of cases), body / tail (25 - 40%) (Am J Surg Pathol 2016;40:1203, J Pathol 2017;243:148)
Pathophysiology
- Unknown; a role may be played by hemorrhagic / chronic inflammatory foci with recruitment of tumor supporting macrophages within the pancreas or within a pancreatic ductal adenocarcinoma (Hum Pathol 2018;81:157)
Clinical features
- Presenting symptoms include jaundice (tumors in the pancreatic head), abdominal or back pain, weight loss and nausea (Am J Surg Pathol 2016;40:1203, J Pathol 2017;243:148)
Diagnosis
- CT scan and MRI are the preferred imaging modalities
- Diagnosis is by biopsy or surgical resection (more difficult with cytology but may be possible) (Cancer Cytopathol 2017;125:563)
Radiology description
- Usually mass lesions with delayed enhancement (Am J Surg Pathol 2016;40:1203)
Prognostic factors
- Presence of an associated conventional ductal adenocarcinoma component is associated with a poorer prognosis and is one of the most important prognostic moderators (Am J Surg Pathol 2016;40:1203, J Pathol 2017;243:148)
- PDL1 expression by tumor cells is associated with poor prognosis (Hum Pathol 2018;81:157)
Case reports
- 54 year old man with a huge (28 cm) mass for which radical resection was possible (Int Cancer Conf J 2017;6:193)
- 54 year old woman with a large cystic mass in the pancreas (Int J Clin Exp Pathol 2015;8:11785)
- 61 year old woman with long term survival after surgical resection and chemotherapy (Case Rep Gastroenterol 2016;10:472)
- 62 year old woman with metastatic disease showing durable response to immunotherapy (tumor with high tumor mutational burden) (J Natl Compr Canc Netw 2021;19:247)
- 65 year old man with weight loss (Medicine (Baltimore) 2018;97:e13516)
- 68 year old woman with a prominent invasion of pancreatic ductal tree (JOP 2011;12:170)
Treatment
- Surgical resection if possible, gemcitabine based chemotherapy (Case Rep Gastroenterol 2016;10:472)
- Immunotherapy is a novel opportunity to be explored in this type of cancer (BMC Gastroenterol 2020;20:220, J Natl Compr Canc Netw 2021;19:247)
Gross description
- Larger than conventional ductal adenocarcinoma (J Pathol 2017;243:148)
- Brownish (not whitish) and with pushing borders
- Frequent cyst formation
- Hemorrhage or necrosis is typically present
Gross images
Frozen section description
- Hypercellular neoplasm with atypical cells
- Multinucleated giant cells, although they cannot always be documented in small biopsy sent for frozen section (may be focal component)
Frozen section images
Microscopic (histologic) description
- Composed of 3 cell types:
- Mononuclear neoplastic cells, sometimes very atypical
- Mononuclear histiocytes (nonneoplastic)
- Multinucleated giant cells (osteoclast-like giant cells) (nonneoplastic)
- Foci of hemorrhage and necrosis are very common
- Typical involvement of the pancreatic ductal tree, sometimes with a pseudopolypoid growth pattern (J Pathol 2017;243:148)
- May arise in association with mucinous cystic neoplasm of the pancreas and intraductal papillary mucinous neoplasm of the pancreas (Am J Surg Pathol 2016;40:1203, World J Surg Oncol 2011;9:100, Am J Surg Pathol 2015;39:179)
- Osteoid material may be found (J Pathol 2017;243:148)
Microscopic (histologic) images
Cytology description
- 3 cell types (osteoclast-like giant cells, neoplastic cells and histiocytes) are present in most cases (~80%)
- Cytology has a diagnostic value for this tumor in ~80% of cases (Cancer Cytopathol 2017;125:563)
Positive stains
- Neoplastic cells:
- Cytokeratin AE1 / AE3, CK7, CK8/18 are usually positive but may be totally negative
- p53 in > 50% of cases
- Ki67 high proliferation index
- Histiocytes:
- Multinucleated giant cells:
Negative stains
Electron microscopy description
- Neoplastic cells: abundant mitochondria, no microvilli or desmosomes (J Korean Med Sci 2005;20:516)
- Multinucleated osteoclast-like giant cells: multiple nuclei with rim of chromatin; abundant mitochondria; free ribosomes; dilated, empty, rough endoplasmic reticulum cisternae (J Korean Med Sci 2005;20:516)
Molecular / cytogenetics description
- Overlaps with the genetic profile of conventional ductal adenocarcinoma, with the most common mutated genes being KRAS, TP53, CDKN2A and SMAD4 (J Pathol 2017;243:148)
- GLI3, a gene belonging to the hedgehog signaling pathway, may be found mutated in this tumor
- The same mutation (290 M > L) affecting the gene SERPINA3 has been documented in 25% of cases in a whole exome sequencing analysis (J Pathol 2017;243:148)
Sample pathology report
- Pancreas, pancreatectomy:
- Pancreatic undifferentiated carcinoma with osteoclast-like giant cells (see synoptic report)
Differential diagnosis
- Undifferentiated / anaplastic carcinoma of the pancreas:
- Absence of osteoclast-like giant cells
- Paraduodenal (groove) pancreatitis:
- Multinucleated giant cells may be abundant but neoplastic cells are lacking (Cytopathology 2015;26:122)
- Melanoma:
- Contains melanin pigment instead of or in addition to hemosiderin
- S100+
Additional references
Board review style question #1
This is an immunohistochemical slide (CK8/18) from a pancreatic tumor in an elderly woman with jaundice. S100 was negative throughout and the multinucleated cells were CD68 positive. Which of the following is the most likely diagnosis?
- Anaplastic lymphoma
- Melanoma
- Pancreatic undifferentiated carcinoma with osteoclast-like giant cells
- Pancreatitis
- Primitive mesenchymal tumor of the pancreas
Board review style answer #1
C. This is a pancreatic undifferentiated carcinoma with osteoclast-like giant cells. These tumors may be composed of neoplastic cells that are totally negative for cytokeratin. This finding, which is not uncommon, cannot be used to exclude this tumor entity.
Comment Here
Reference: Undifferentiated carcinoma with osteoclast-like giant cells
Comment Here
Reference: Undifferentiated carcinoma with osteoclast-like giant cells
Board review style question #2
Which cell types usually form an undifferentiated carcinoma with osteoclast-like giant cells?
- Osteoclast-like giant cells, which are tumor cells
- Tumor cells and histiocytes
- Tumor cells arranged in glands and osteoclast-like giant cells
- Tumor cells, histiocytes and osteoclast-like giant cells
- Tumor cells, lymphocytes and osteoclast-like giant cells
Board review style answer #2
D. Undifferentiated carcinoma of the pancreas with osteoclast-like giant cells typically contains 3 types of cells: tumor cells, histiocytes and osteoclast-like giant cells.
Comment Here
Reference: Undifferentiated carcinoma with osteoclast-like giant cells
Comment Here
Reference: Undifferentiated carcinoma with osteoclast-like giant cells
VIPoma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Electron microscopy images | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Well differentiated neuroendocrine tumor that secretes vasoactive intestinal peptide (VIP), leading to chronic watery diarrhea
Essential features
- Functional well differentiated neuroendocrine tumor (WD-NET) that secretes vasoactive intestinal peptides
- Histology is same as other well differentiated neuroendocrine tumors in pancreas
- Clinical diarrhea can be severe, possibly fatal (may exceed 8 liters of stool in 24 hours) (South Dartmouth (MA): MDText.com, Inc;2017 Jun 5)
Terminology
- VIPoma syndrome is also termed Verner-Morrison syndrome and WDHA syndrome (watery diarrhea, hypokalemia, achlorhydria) (Rom J Morphol Embryol 2017;58:371)
ICD coding
Epidemiology
- Incidence is roughly 1 per 10 million persons per year (Surg Clin North Am 1987;67:379)
- Mean age 51 years
Sites
- 90% of VIPomas arise from pancreas (Am J Med 1987;82:37)
- Most common pancreatic location is tail (Surgery 1998;124:1050)
Pathophysiology
- VIP stimulates intestinal secretion of water and electrolytes, causing VIPoma syndrome when excessive
Etiology
- Most cases are sporadic but about 5% occur in the setting of multiple endocrine neoplasia 1 syndrome (MEN1)
Clinical features
- Chronic secretory diarrhea hypokalemia, achlorhydria, flushing, hypercalcemia (Hepatogastroenterology 2005;52:1259)
Diagnosis
- Imaging to detect pancreatic mass
- Elevated plasma vasoactive intestinal levels
Prognostic factors
- Often metastatic at time of diagnosis (Surgery 1998;124:1050)
- Median survival is roughly 6 years (Pancreas 2019;48:934)
- Poor prognostic factors: age < 40 years or > 60, size > 4 cm, poor clinical management of water, electrolyte and acid base profiles, metastatic disease, unresectability (J Clin Case Rep 2012,2:15)
Case reports
- 13 year old girl with multiple endocrine neoplasia 1 syndrome and pancreas mass (Indian J Endocrinol Metab 2013;17:S215)
- 46 year old woman with 18 cm tumor (World J Surg Oncol 2008;6:80)
- 47 year old man with sporadic tumor (Hematol Oncol Stem Cell Ther 2014;7:109)
- 74 year old man with diarrhea, weight loss and liver metastasis (Case Rep Gastroenterol 2019;13:225)
Treatment
- Surgical resection of primary lesion, if possible
- Hepatic metastases can be resected or embolized
- Octreotide for metastatic disease
Gross description
- Single or multiple lesions, most commonly in tail of pancreas
- Firm, homogeneous, well circumscribed
Microscopic (histologic) description
- Nests of monotonous low grade neuroendocrine cells with salt and pepper nuclei and ample amphophilic cytoplasm, as with any other well differentiated neuroendocrine tumor
Positive stains
- VIP (though this does not establish the diagnosis)
- Synaptophysin, chromogranin
- PDX1, INSM1 (Am J Surg Pathol 2012;36:737)
Negative stains
Molecular / cytogenetics description
- Overexpression of CXCR4, down regulation of MSH2 in one case (World J Surg Oncol 2012;10:264)
Sample pathology report
- Pancreas and duodenum, Whipple procedure:
- Well differentiated neuroendocrine tumor of pancreas (see comment and synoptic report)
- Comment: Based on the patient's clinical symptoms, this neuroendocrine tumor is best considered a VIPoma.
Differential diagnosis
- Nongastrin secreting pancreatic neuroendocrine tumor:
- No histopathologic differences; must be determined on clinical grounds
Board review style question #1
What is the most common symptom of a VIPoma?
- Abdominal pain
- Acromegaly
- Diarrhea
- Necrotizing migratory erythema
- Weakness
Board review style answer #1
Board review style question #2
Board review style answer #2
Well differentiated neuroendocrine tumor
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Molecular / cytogenetics images | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Definition / general
- Low, intermediate or high grade subset of neuroendocrine tumors (NET) that lack necrosis and express neuroendocrine markers (synaptophysin or chromogranin A)
Essential features
- WHO Classification of Tumours of Endocrine Organs (2017) and WHO Classification of Tumours of the Digestive System (2019) classifies well differentiated pancreatic neuroendocrine tumors (PanNETs) as grade 1, grade 2 or grade 3 (see Diagnosis)
- Classification uses criteria similar to other gastrointestinal neuroendocrine neoplasms
- May be nonfunctioning or secrete 1 or more peptides; multiple tumors in the same patient may secrete the same or different peptides
Terminology
- Synonyms: well differentiated neuroendocrine tumor (WD NET), pancreatic neuroendocrine tumor (PanNET), pancreatic endocrine tumor, islet cell tumor
- Specific functional terms (glucagonoma, insulinoma, gastrinoma) not recommended for use unless hormonal syndrome exists clinically
- Microscopic lesions < 0.5 cm are termed microadenomas
- Carcinoid is a term that should be avoided if possible
ICD coding
- ICD-10: C25 - malignant neoplasm of pancreas
Epidemiology
- M = F
- Peak incidence: 30 - 60 years
- Comprise ~2 - 5% of pancreatic neoplasms (J Gastrointest Surg 2010;14:541)
- Most are sporadic; risk factors include (Ann Oncol 2016;27:68):
- Family history of cancer (not site dependent; lung, colon, among others)
- Elevated BMI
- Diabetes
- Cigarette smoking
- Elevated alcohol consumption
- 10 - 20% are associated with syndromes:
- Multiple endocrine neoplasia type 1 (MEN1): nearly 100% pancreatic involvement at autopsy (Surgery 1979;86:475)
- Von Hippel-Lindau (VHL): 12.3 - 17% of patients (Surgery 2007;142:814, Gastroenterology 2000;119:1087)
- Neurofibromatosis type 1: 10% of patients (Crit Rev Oncol Hematol 2022;172:103648)
- Tuberous sclerosis: 1 - 9% of patients (Crit Rev Oncol Hematol 2022;172:103648)
- Nonfunctional tumors represent 60 - 90% of all PanNETs (Neuroendocrinology 2016;103:153, Biomed Res Int 2017;2017:9856140)
- Functional tumors (10 - 40%):
- Insulinoma (most common, account for 4 - 20% of PanNETs) (J Gastroenterol 2015;50:58)
- Gastrinoma (second most common, account for 4 - 8% of PanNETs) (J Gastroenterol 2015;50:58)
- VIPoma (Endocr J 2022;69:1201)
- Glucagnoma (1 - 2% of PanNETs) (Ann Surg Oncol 2007;14:3492)
- Somatostatinoma (J Gastroenterol Hepatol 2008;23:521)
- ACTH producing neuroendocrine tumor (Am J Surg Pathol 2015;39:374)
- Serotonin producing neuroendocrine tumor (up to 3.9%) (Am J Surg Pathol 2015;39:592)
Sites
- Found throughout the pancreas
- Nonfunctional, gastrinomas or serotonin producing tumors are more likely to be found in the head of the pancreas
- Insulinomas, glucagonomas and VIPomas are more likely to be found in the tail (Ann Surg Oncol 2007;14:3492)
Pathophysiology
- Theorized to arise from totipotent stem cells in the pancreatic ductal system and islets of Langerhans (Endotext: Pathophysiology and Treatment of Pancreatic Neuroendocrine Tumors [Accessed 30 September 2022])
Etiology
- Majority are sporadic and nonsyndromic
- Minority are associated with multiple endocrine neoplasia syndrome (80 - 100% incidence), tuberous sclerosis (rare), von Hippel-Lindau (VHL, 10 - 17% incidence), among others (Cancer 2008;113:1807)
- Hyperplastic and preneoplastic lesions were removed from the WHO 2017; PanNEN precursor lesions have not been clearly associated with sporadic neoplasms (but are described in MEN1 and VHL)
Clinical features
- Demonstrate a spectrum of clinical behavior dependent on hormones produced
- Commonly causes abdominal pain, jaundice
- Asymptomatic pancreatic neuroendocrine tumors are increasingly common, likely because of an increase in detection due to increased imaging frequency and improvement in imaging modalities (Cancer 2015;121:589)
- Functional tumors may have elevated serum peptides corresponding to tumor cell type
- May be associated with carcinoid syndrome (flushing, diarrhea) if metastatic to liver
- Most nonfunctional tumors are large and detected at an advanced stage at diagnosis, with 60 - 85% having liver metastases (Cancer 2008;113:1807)
- Liver is the most common site of metastasis
- Insulinoma:
- Composed of insulin producing cells with uncontrolled secretion of insulin
- Clinical: Whipple triad (hypoglycemic symptoms, plasma glucose Typically, small (Neoplasma 2015;62:484)
- Gastrinoma:
- Composed of gastrin producing cells with uncontrolled secretion of gastrin
- Clinical: Zollinger-Ellison syndrome (reflux symptoms and duodenal ulcers)
- Typically, large size (average 3.8 cm diameter), more common in the duodenum than in the pancreas (Wien Klin Wochenschr 2007;119:579)
- VIPoma:
- Composed of uncontrolled vasoactive intestinal peptide (VIP) secreting cells
- Clinical: WDHA syndrome (watery diarrhea [persists when fasting], hypokalemia and achlorhydria)
- Typically, large (average ~5 cm diameter) and solitary (Ann N Y Acad Sci 1988;527:508)
- Glucagonoma:
- Composed of cells with uncontrolled secretion of glucagon and preglucagon derived peptide
- Clinical: triad of skin rash (necrolytic migratory erythema), diabetes mellitus and weight loss
- Typically, variable in size (average 3 - 7 cm diameter) and solitary (Medicine (Baltimore) 1996;75:53)
- Somatostatinoma:
- Composed of cells that demonstrate D cell differentiation with uncontrolled secretion of somatostatin
- Clinical: less distinctive than other functioning PanNETs (glucose intolerance, cholelithiasis and diarrhea)
- Typically, large (average 5 - 6 cm diameter) and multinodular (J Exp Clin Cancer Res 1999;18:13)
- ACTH producing neuroendocrine tumor:
- Composed of cells secreting uncontrolled adrenocorticotropic hormone (ACTH)
- Clinical: features of Cushing syndrome (central obesity, weight gain, moon face, hypertension, hyperglycemia, osteoporosis, hypokalemia and cutaneous hyperpigmentation)
- Typically, large (average 4.8 cm diameter) and solitary (Am J Surg Pathol 2015;39:374)
- Composed of cells secreting uncontrolled adrenocorticotropic hormone (ACTH)
- Serotonin producing neuroendocrine tumor:
- Composed of cells secreting uncontrolled serotonin
- Clinical (usually only after metastasizing to the liver): carcinoid syndrome (abdominal pain, flushing, diarrhea and weight loss)
- Typically large (average ~5 cm diameter) and solitary (Pancreas 2011;40:883)
Diagnosis
- Requires neuroendocrine histology and positive staining for neuroendocrine and cytokeratin makers (and the presence of clinical symptoms if functional)
- Contains mixed neuroendocrine nonneuroendocrine neoplasms [MiNEN])
- Does not contain necrosis or severe atypia (otherwise, see neuroendocrine carcinoma [PanNEC])
- Well differentiated tumors are classified by mitotic count and proliferative index (Ki67):
- G1 (NET G1): mitotic count < 2/2 mm2 and G2 (NET G2): mitotic count 2 - 20/2 mm2 or 3 - 20% Ki67 index
- G3 (NET G3): mitoses > 20/2 mm2 or > 20% Ki67 index
Laboratory
- Elevated serum chromogranin A levels (> 15 ng/mL) show a sensitivity of 66% and a specificity of 95% for the diagnosis pancreatic neuroendocrine tumors (World J Gastroenterol 2020;26:2305)
- Elevated serum neuron specific enolase (NSE) and pancreatic polypeptide (PP) are respectively seen in up to 30 - 50% and 50% of PNET patients (Front Oncol 2020;10:831)
- Specific biomarkers for functional PNET are not currently recommended due to their poor sensitivity, specificity and lack of standardization between laboratories (Surg Oncol Clin N Am 2020;29:165)
Radiology description
- General: highly vascular, well circumscribed enhancing lesion that can be solid or cystic
- Ultrasound: circumscribed masses with smooth margins, suboptimal visualization of the body and tail due to bowel gas obstruction, sensitivity of 20 - 80%
- CT: well defined, uniformly hypervascular masses on arterial phase CT, most widely available technique
- MRI: hyperintense on T1 weighted imaging due to an abundance of proteinaceous material and variable signal on T2 weighted images; better resolution than CT (Semin Ultrasound CT MR 2019;40:469)
- 68Ga SRS (stimulated Raman scattering) is a type of PET imaging of somatostatin receptor, current gold standard for functional imaging of NETs (Nat Rev Endocrinol 2018;14:656)
Prognostic factors
- Relatively indolent but with variable outcome
- Features associated with adverse outcome (Am J Surg Pathol 2007;31:1677, HPB (Oxford) 2009;11:422, Sci Rep 2018;8:7271):
- Size > 2 cm
- Tumor necrosis
- Lymph node positivity
- Mitoses > 2/10 high powered fields
- Vascular invasion
- Perineural invasion
- High Ki67 index
- CK19 positivity
- Loss of DAXX / ATRX expression and alternative lengthening of telomeres suggest poor outcome (Clin Cancer Res 2017;23:600, Front Endocrinol (Lausanne) 2021;12:691557)
Case reports
- 23 year old woman with VHL and SDHA pathogenic variant co-occurrence in PanNET (Front Oncol 2022;12:925582)
- 43 year old man with MEN1 and metastatic grade 3 PanNET (J Endocr Soc 2022;6:bvac122)
- 44 year old woman with recurrent pancreatic glucagonoma (Ann Med Surg (Lond) 2022;77:103604)
- 62 year old man with metastases (J Med Life 2018;11:57)
- 69 year old man with pancreatic insulinoma presenting with postprandial hypoglycemia (AACE Clin Case Rep 2022;8:154)
Treatment
- Primarily surgical (benefits shown for primary and metastatic disease)
- Enucleation can be used for small and localized tumors
- First line treatments for metastatic disease are somatostatin analogs (octreotide and lanreotide)
- Second line treatments include tyrosine kinase inhibitors (sunitinib) and mTOR inhibitors (everolimus)
- Novel chemotherapy regime of capeciabine and temozolomide (CAPTEM) and peptide receptor radionuclide therapy (PRRT) has been approved
- Reference: Surg Clin North Am 2019;99:793
Gross description
- Solid, well circumscribed mass, typically 2 - 5 cm in diameter
- Larger tumors often show heterogenous and lobulated cut surfaces
- Color can range from red-tan to brown-yellow
- May show extensive fibrosis, calcification or ossification
- 5% are cystic (unilocular, less commonly multilocular) but necrosis is uncommon in well differentiated PanNETs
- Reference: Arch Pathol Lab Med 2019;143:1317
Gross images
Microscopic (histologic) description
- Architecture can be solid, organoid, nesting, gyriform, trabecular or glandular
- Small, round monotonous cells with coarse, salt and pepper nuclear chromatin and minimal atypia
- Occasional small nucleoli are most common; large nucleoli can be seen
- Cytoplasm can be pale pink, oncocytic, granular or lipid rich / vacuolated
- Rarely, nuclei can be eccentrically located (rhabdoid cell appearance) (Am J Surg Pathol 2003;27:642)
- Islet amyloid polypeptide (amylin) deposition is specific for insulinoma but is only seen in ~5% of cases
- Psammoma bodies can be seen in somatostatinomas
Microscopic (histologic) images
Contributed by Katrina Krogh, M.D.
Contributed by @RaulSGonzalezMD on Twitter
bit.ly/3zIR8Xf for a nice review). Here's a great example of the ductulo-insular subtype, with admixed benign glandular structures. Good prognosis. #pathology #gipath #PathTwitter #PathOutPic"
Contributed by @RaulSGonzalezMD on Twitter (see original post here)">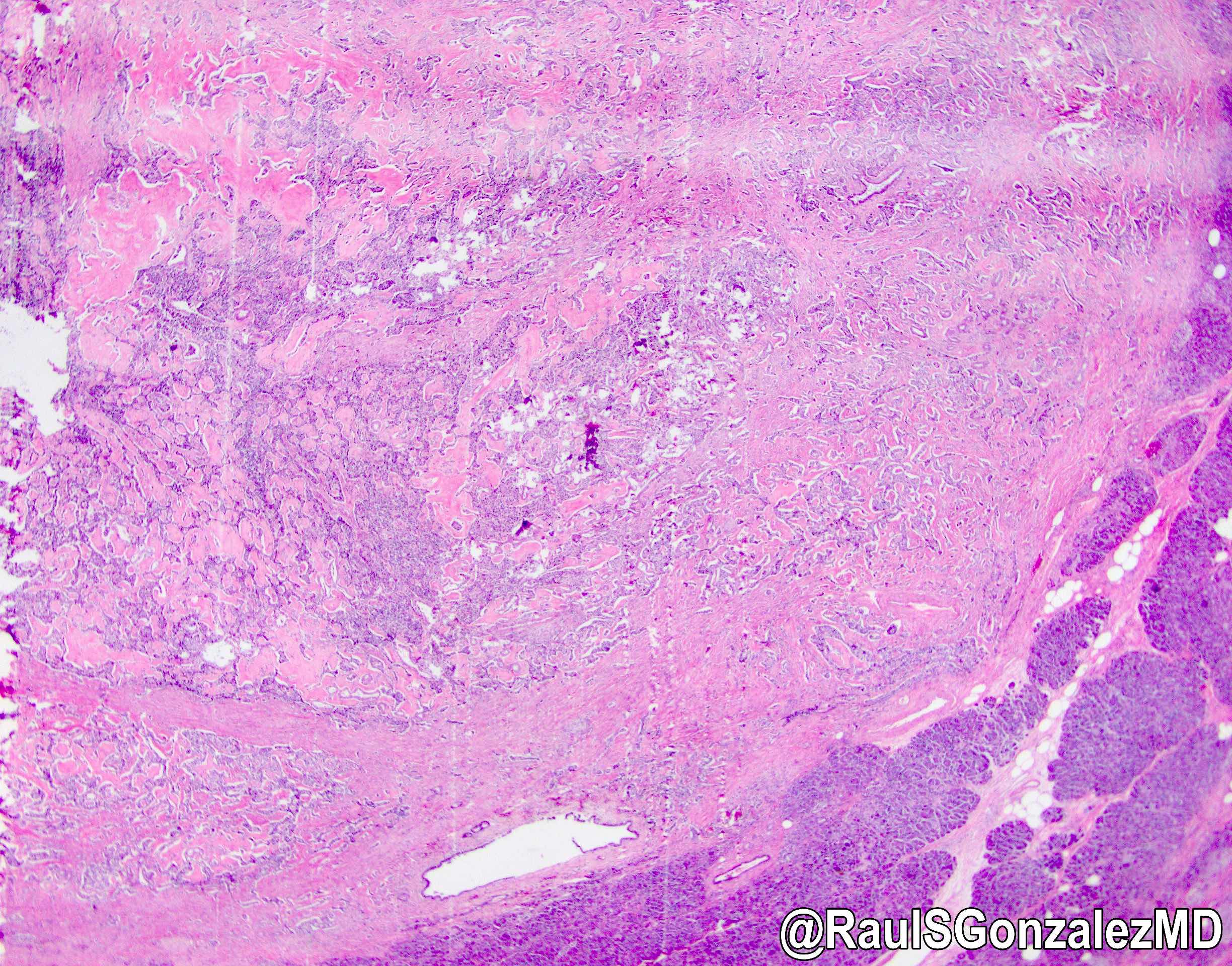 bit.ly/3zIR8Xf for a nice review). Here's a great example of the ductulo-insular subtype, with admixed benign glandular structures. Good prognosis. #pathology #gipath #PathTwitter #PathOutPic"
bit.ly/3zIR8Xf for a nice review). Here's a great example of the ductulo-insular subtype, with admixed benign glandular structures. Good prognosis. #pathology #gipath #PathTwitter #PathOutPic"
Contributed by @RaulSGonzalezMD on Twitter (see original post here)">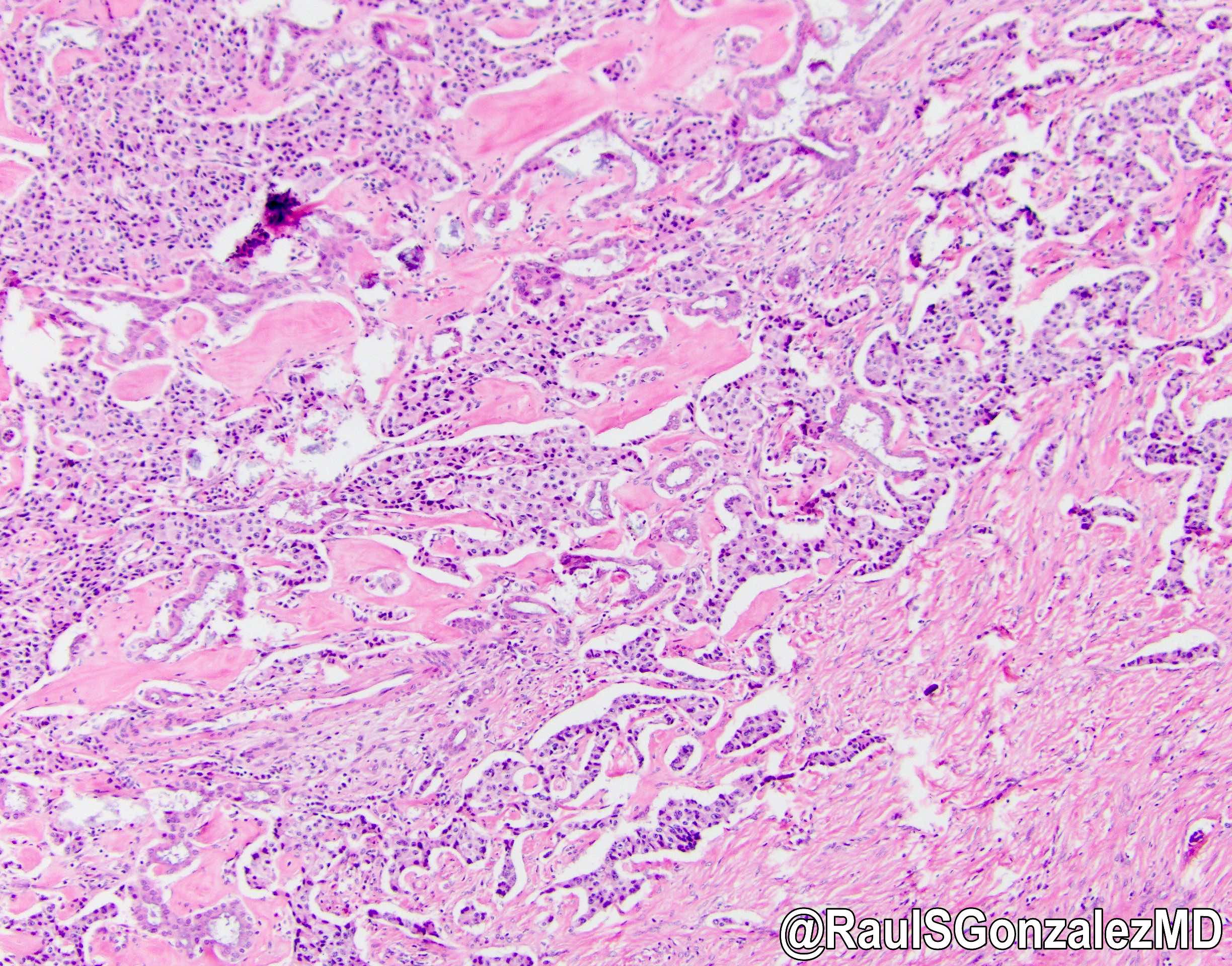 bit.ly/3zIR8Xf for a nice review). Here's a great example of the ductulo-insular subtype, with admixed benign glandular structures. Good prognosis. #pathology #gipath #PathTwitter #PathOutPic"
bit.ly/3zIR8Xf for a nice review). Here's a great example of the ductulo-insular subtype, with admixed benign glandular structures. Good prognosis. #pathology #gipath #PathTwitter #PathOutPic"
Contributed by @RaulSGonzalezMD on Twitter (see original post here)">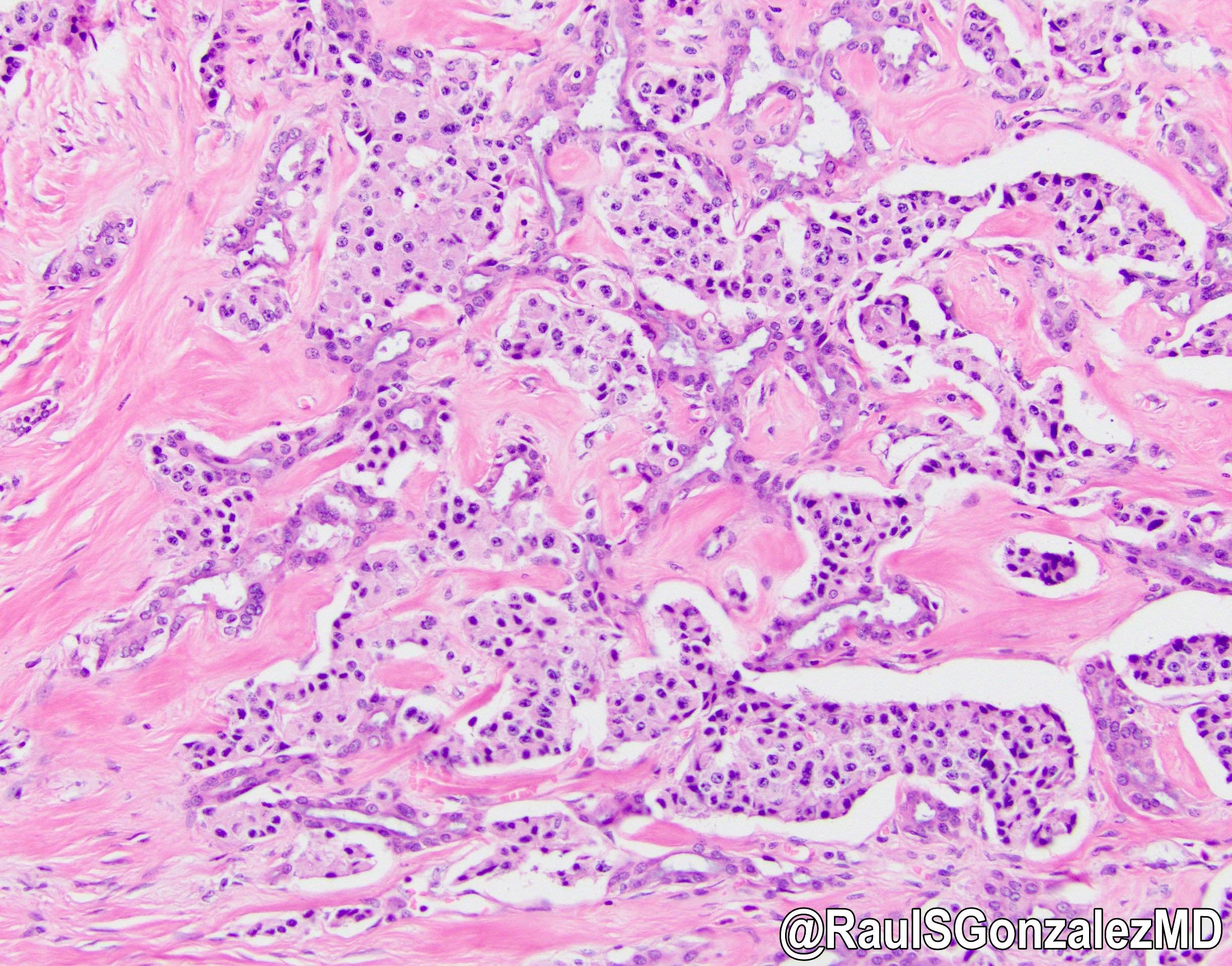
Contributed by @RaulSGonzalezMD on Twitter (see original post here)">
 bit.ly/3zIR8Xf for a nice review). Here's a great example of the ductulo-insular subtype, with admixed benign glandular structures. Good prognosis. #pathology #gipath #PathTwitter #PathOutPic"
bit.ly/3zIR8Xf for a nice review). Here's a great example of the ductulo-insular subtype, with admixed benign glandular structures. Good prognosis. #pathology #gipath #PathTwitter #PathOutPic"Contributed by @RaulSGonzalezMD on Twitter (see original post here)">
 bit.ly/3zIR8Xf for a nice review). Here's a great example of the ductulo-insular subtype, with admixed benign glandular structures. Good prognosis. #pathology #gipath #PathTwitter #PathOutPic"
bit.ly/3zIR8Xf for a nice review). Here's a great example of the ductulo-insular subtype, with admixed benign glandular structures. Good prognosis. #pathology #gipath #PathTwitter #PathOutPic"Contributed by @RaulSGonzalezMD on Twitter (see original post here)">

Well differentiated neuroendocrine tumor
Cytology description
- Single cell type; monotonous plasmacytoid cells with moderate amount of cytoplasm and distinctive neuroendocrine chromatin (Clin Cancer Res 2017;23:600)
- Can have rosette-like aggregates and eccentric nuclei
Positive stains
- Chromogranin A (more specific) and synaptophysin (more sensitive): diffuse and strong
- NSE, CD56, CD57
- AE1 / AE3, CAM5.2 and CK8/18
- Somatostatin receptor is positive in 80 - 90% and PR is positive in 58% of PanNETs (Hum Pathol 2020;96:8)
- Immunohistochemistry for peptide hormones (insulin, glucagon, VIP, etc.) is rarely required and nonfunctional tumors may be positive
- SMAD4 and Rb retained
- In metastatic setting: Isl1 (most sensitive) and PAX6 positivity is supportive
- PAX8 (polyclonal) is positive in 50 - 60% of cases metastatic to the liver (monoclonal PAX8 is negative) (Hum Pathol 2020;96:8)
- NESP55+ and PDX1+, in the presence of negative CDX2 and TTF1, is 97% specific for pancreatic origin (Clin Cancer Res 2017;23:600)
Negative stains
- CDX2, SATB2 and TTF1 (Surg Oncol Clin N Am 2020;29:185)
- Nuclear beta catenin
- Trypsin / chymotrypsin
- BCL10
- SMA
Molecular / cytogenetics description
- Frequently mutated genes (Science 2011;331:1199, Endocr Relat Cancer 2009;16:1219, Expert Rev Gastroenterol Hepatol 2015;9:1407):
- MEN1 (44.1%)
- DAXX (death domain associated protein, 25%)
- ATDX ([alpha] thalassemia / intellectual disability syndrome X linked, 17.6%)
- TSC2 (8.8%)
- PTEN (7.3%)
- PI3KCA (1%)
- VHL (up to 25% including deletion by promoter methylation)
Sample pathology report
- Distal pancreas and spleen, distal pancreatectomy and splenectomy:
- Well differentiated neuroendocrine tumor, WHO grade 2, measuring 4.1 cm in greatest dimension (see comment)
- Lymphovascular and perineural invasion identified
- Pancreatic resection margin, negative for tumor
- 2 of 10 lymph nodes, positive for metastatic tumor (2/10)
- Spleen with no significant pathologic change
- Pathologic stage: pT3, pN1
- Comment: Immunohistochemical stains are performed and show the tumor cells are positive for cytokeratin AE1 / AE3, synaptophysin and chromogranin (patchy). The Ki67 proliferative index is 15%. Beta catenin shows membranous staining (negative result). Overall findings are consistent with 2019 WHO grade 2 (out of 3).
Differential diagnosis
- Solid pseudopapillary neoplasm:
- Positive stains: nuclear beta catenin, cytoplasmic / perinuclear CD10, nuclear PR, synaptophysin (sometimes), pankeratins (variable)
- Negative stains: membranous E-cadherin, ER, chromogranin (usually)
- Contains pseudopapillary architecture, which is rare in neuroendocrine tumors
- Mixed neuroendocrine nonneuroendocrine neoplasms (MiNEN):
- Typically, neuroendocrine component is mixed with a ductal adenocarcinoma, occasionally with acinar cell carcinoma
- Each component must account for > 30% of the tumor
- Metastatic neuroendocrine tumors of other origin:
- Negative for islet 1, PAX6, PDX1
- Positive for TTF1, SATB2, CDX2 (Surg Oncol Clin N Am 2020;29:185)
- Neuroendocrine carcinoma (NEC):
- High mitotic rate (> 20/2 mm2 or Ki67 > 20%)
- Large or small cell with corresponding high grade morphology
- Acinar cell carcinoma (Am J Surg Pathol 1992;16:815, Cancer Cytopathol 2013;121:459):
- PAS+ diastase resistant granules sometimes detected
- Positive stains: trypsin, chymotrypsin, lipase, CK8/18
- Occasionally positive for synaptophysin or chromogranin
- Pancreatoblastoma (Cancer Cytopathol 2013;121:459):
- Solid and acinar patterns, squamoid nests are present
- Trypsin, chymotrypsin, lipase, BCL10, synaptophysin and chromogranin can be focally positive
- Glomus tumor:
- Composed of 3 cell types: glomus, vasculature and smooth muscle cells
- Positive SMA, negative synaptophysin, chromogranin
Additional references
Board review style question #1
Which of the following statements is true regarding the pancreatic lesion seen above?
- Commonly associated with familial adenomatous polyposis (FAP)
- Loss of DAXX / ATRX expression are associated with better outcomes
- More common than pancreatic adenocarcinoma
- More likely to be nonsecreting than secreting
Board review style answer #1
D. More likely to be nonsecreting than secreting
Comment Here
Reference: Well differentiated neuroendocrine tumor
Comment Here
Reference: Well differentiated neuroendocrine tumor
Board review style question #2
Board review style answer #2
D. Treatment depends on Ki67 interpretation
Comment Here
Reference: Well differentiated neuroendocrine tumor
Comment Here
Reference: Well differentiated neuroendocrine tumor
Board review style question #3
A patient presents to his primary care doctor with a 1 month history of constant watery diarrhea that wakes him up several times each night. Laboratory values were remarkable for a potassium of 3.2 mmol/L (normal: 3.6 - 5.2 mmol/L). An abdominal CT showed a solitary, solid, well demarcated and vascular 5 cm lesion in the tail of the pancreas that is consistent with a neoplasm. What is the most likely gene mutation in this lesion?
- APC
- CTNNB1
- MEN1
- SMAD4
- STK11
Board review style answer #3
C. MEN1. The patient is presenting with WDHA syndrome (watery diarrhea that persists when fasting, hypokalemia and achlorhydria) and a pancreatic mass. This is likely due to a VIPoma, which is a type of pancreatic neuroendocrine tumor (PanNET). The most common mutation in these tumors is MEN1.
Comment Here
Reference: Well differentiated neuroendocrine tumor
Comment Here
Reference: Well differentiated neuroendocrine tumor
WHO classification
Definition / general
- WHO classification of tumors of the pancreas
- Currently on 5th edition, published in 2019
- Based on histologic appearance, not molecular characteristics
WHO (2019)
-
Benign epithelial tumors and precursors ICD-O codes
- Serous cystadenoma, NOS 8441/0
- Macrocystic (oligocystic) serous cystadenoma
- Solid serous adenoma
- Von Hippel-Lindau syndrome associated serous cystic neoplasm
- Mixed serous-neuroendocrine neoplasm
- Serous cystadenocarcinoma, NOS 8441/3
- Glandular intraepithelial neoplasia, low grade 8148/0
- Glandular intraepithelial neoplasia, high grade 8148/2
- Intraductal papillary mucinous neoplasm with low grade intraepithelial neoplasia 8453/0
- Intraductal papillary mucinous neoplasm with high grade intraepithelial neoplasia 8453/2
- Intraductal papillary mucinous neoplasm with associated invasive carcinoma 8453/3
- Intraductal oncocytic papillary neoplasm, NOS 8455/2
- Intraductal oncocytic papillary neoplasm with associated invasive carcinoma 8455/3
- Intraductal tubulopapillary neoplasm 8503/2
- Intraductal tubulopapillary neoplasm with associated invasive carcinoma 8503/3
- Mucinous cystic neoplasm with low grade intraepithelial neoplasia 8470/0
- Mucinous cystic neoplasm with high grade intraepithelial neoplasia 8470/2
- Mucinous cystic neoplasm with associated invasive carcinoma 8470/3
-
Malignant epithelial tumors ICD-O codes
- Duct adenocarcinoma, NOS 8500/3
- Colloid carcinoma 8480/3
- Poorly cohesive carcinoma 8490/3
- Signet ring cell carcinoma 8490/3
- Medullary carcinoma, NOS 8510/3
- Adenosquamous carcinoma 8560/3
- Epidermoid carcinoma 8576/3
- Large cell carcinoma with rhabdoid phenotype 8014/3
- Carcinoma, undifferentiated, NOS 8020/3
- Undifferentiated carcinoma with osteoclast-like giant cells 8035/3
- Acinar cell carcinoma 8550/3
- Acinar cell cystadenocarcinoma 8551/3
- Mixed acinar-neuroendocrine carcinoma 8154/3
- Mixed acinar-endocrine-ductal carcinoma 8154/3
- Mixed acinar-ductal carcinoma 8552/3
- Pancreatoblastoma 8971/3
- Solid pseudopapillary neoplasm of the pancreas 8452/3
- Solid pseudopapillary neoplasm with high grade dysplasia
-
Pancreatic neuroendocrine neoplasms ICD-O codes
- Pancreatic neuroendocrine microadenoma 8150/0
- Neuroendocrine tumor, NOS 8240/3
- Neuroendocrine tumor, grade 1 8240/3
- Neuroendocrine tumor, grade 2 8249/3
- Neuroendocrine tumor, grade 3 8249/3
- Pancreatic neuroendocrine tumor, nonfunctioning 8150/3
- Oncocytic pancreatic neuroendocrine tumor, nonfunctioning
- Pleomorphic pancreatic neuroendocrine tumor, nonfunctioning
- Clear cell pancreatic neuroendocrine tumor, nonfunctioning
- Cystic pancreatic neuroendocrine tumor, nonfunctioning
- Functioning pancreatic neuroendocrine tumors
- Insulinoma 8151/3
- Gastrinoma 8153/3
- VIPoma 8155/3
- Glucagonoma 8152/3
- Somatostatinoma 8156/3
- ACTH producing tumor 8158/3
- Enterochromaffin cell carcinoid 8241/3
- Serotonin producing tumor 8241/3
- Neuroendocrine carcinoma, NOS 8246/3
- Large cell neuroendocrine carcinoma 8013/3
- Small cell neuroendocrine carcinoma 8041/3
- Mixed neuroendocrine - nonneuroendocrine neoplasm (MiNEN) 8154/3
WHO reporting system for pancreaticobiliary cytopathology
Table of Contents
Definition / general | Essential features | Techniques for cytologic sampling of pancreatic and bile duct lesions | Metrics | Papanicolaou system for reporting pancreaticobiliary cytology | Diagrams / tables | Laboratory | Case reports | Cytology description | Cytology images | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- The Papanicolaou Society of Cytopathology (PSC) developed guidelines in 2014 for interpreting and reporting pancreaticobiliary cytology
- PSC was comprised of international, multidisciplinary pancreaticobiliary experts who met over an 18 month period to provide recommendations for:
- Clinical and imaging workup (Diagn Cytopathol 2014;42:325)
- Techniques for sampling lesions (Diagn Cytopathol 2014;42:333)
- Standardized terminology and nomenclature (Diagn Cytopathol 2014;42:338)
- Ancillary studies (Diagn Cytopathol 2014;42:351)
- Patient follow up and treatment (Diagn Cytopathol 2014;42:363)
- PSC was comprised of international, multidisciplinary pancreaticobiliary experts who met over an 18 month period to provide recommendations for:
- Standardization provides uniform diagnostic reporting, reproducibility and clinically relevant risk stratification
- Resulted in a 6 tiered reporting system: nondiagnostic, negative (for malignancy), atypical, neoplastic (benign or other), suspicious (for malignancy) and positive or malignant
- Second edition may consider the 2019 World Health Organization proposed updates (Diagn Cytopathol 2020;48:494, Cancer Cytopathol 2022;130:195)
Essential features
- Internationally adopted recommendations for sampling, diagnosing and managing pancreaticobiliary cytology lesions
- 6 tiered categorical system resulting in overall improved sensitivity / specificity and risk stratification
- Risks of malignancy inform multidisciplinary patient care
Techniques for cytologic sampling of pancreatic and bile duct lesions
- Primary method for sampling solid mass and cysts: endoscopic ultrasound fine needle aspiration (EUS FNA)
- Different biopsy devices may improve diagnostic yield of pancreatic cyst wall components (Cancer Cytopathol 2018;126:414, Endosc Ultrasound 2018;7:323, Gastrointest Endosc 2018;87:495)
- Primary method for sampling strictures: endoscopic retrograde cholangiopancreatography (ERCP) guided brush cytology
- Risk of pancreatitis
- Endoscopic forceps biopsy may improve poor sensitivity (Diagn Cytopathol 2014;42:333)
Metrics
- Risk of malignancy (ROM) = total number FNA specimens that proved malignant on follow up / total number of surgical resections and clinical follow ups (Cancer Cytopathol 2020;128:29)
- Average ROM for each diagnostic category (next section) is provided by the Saieg and Pitman review of several studies (Diagn Cytopathol 2020;48:494)
- Diagnostic variation due to:
- Operator skill, experience and medical device
- Variation in prevalence of pathology and patient populations
- Limited availability of ancillary tests (Cytopathology 2020;31:564)
- Absence of rapid on site assessment (ROSE) (Oncotarget 2017;8:8154)
Papanicolaou system for reporting pancreaticobiliary cytology
- Category I: nondiagnostic
- Provides no useful diagnostic or useful information about the solid or cystic lesion sampled
- Entities include:
- Cells obscured by artifacts such as hemorrhage or necrosis
- Pure gastrointestinal contamination indicates missed lesion
- Acellular specimens obtained from cyst with no evidence of mucinous etiology
- Benign pancreatic parenchyma with well defined mass on imaging
- Ancillary testing may result in reduced utilization of this category for cystic lesions (Diagn Cytopathol 2017;45:303)
- Average ROM: 23.1%
- Management: reassess or repeat sampling
- Category II: negative (for malignancy)
- Contains adequate cellular or extracellular tissue to evaluate or define a lesion that is identified on imaging
- Minor degree of loss of cell or nuclear polarity
- No cytologic atypia observed
- Entities include:
- Acute, chronic or autoimmune pancreatitis
- Pseudocyst
- Lymphoepithelial cyst
- Splenule or accessory spleen
- Benign pancreatic parenchyma with no discrete mass on imaging
- Ancillary tests such as amylase and carcinoembryonic antigen cyst fluid analysis can be helpful for cystic lesions (Diagn Cytopathol 2017;45:303)
- Increased false negative rate in biliary brushings due to underlying inflammatory lesions and subepithelial tissue entrapped within desmoplasia
- Average ROM: 8.2%
- Management: respective conservative care unless patients have irretractable symptoms or high risk of malignancy
- Contains adequate cellular or extracellular tissue to evaluate or define a lesion that is identified on imaging
- Category III: atypical
- Cells present with cytoplasmic, nuclear or architectural features that are not consistent with normal or reactive changes of the pancreas and bile ducts and insufficient to classify as neoplasm or suspicious for high grade malignancy
- Slight nuclear membrane irregularity, nuclear crowding with minor degree of overlap, nuclei haphazardly occupy lower 66% of the cell at the base, minimal degree of anisonucleosis (2:1), near normal N:C ratio, parachromatin clearing, background smear is clean or contains red blood cells
- Also includes entities with abundant intracytoplasmic mucin in the epithelium without overt cytologic atypia
- Absent features: necrosis, true nuclear molding, macronucleoli and features of malignancy
- Category with the widest range of reported ROM: 28 - 100%, average of 46.8%
- Management: repeat sampling, additional ancillary studies, continued conservative care or surgery referral as determined by team
- Cells present with cytoplasmic, nuclear or architectural features that are not consistent with normal or reactive changes of the pancreas and bile ducts and insufficient to classify as neoplasm or suspicious for high grade malignancy
- Category IV: neoplastic
- Neoplastic: benign
- Cytological specimen sufficiently cellular and representative (with or without the context of clinical, imaging and ancillary studies) to be diagnostic of a benign neoplasm
- Entities include:
- Serous cystadenoma (most common)
- Lymphangioma
- Cystic teratoma
- Schwannoma
- Average ROM: 20.2%
- Management: conservative unless irretractable symptoms, large sized lesions at risk for rupture (> 4 cm serous cystadenomas) or increased risk of recurrence (lymphangiomas)
- Neoplastic: other
- Neoplasm that is either premalignant or low grade malignant
- Does not define the neoplasm as benign or malignant
- Entities include:
- Premalignant
- Intraductal papillary mucinous neoplasm (IPMN) with low and high grade dysplasia
- Intraductal papillary neoplasm of the bile duct (IPNB) with low and high grade dysplasia
- Mucinous cystic neoplasm (MCN) with low grade dysplasia
- Note that cases with prominent high grade dysplasia may often end up in the suspicious for malignancy category
- Low grade malignant
- Pancreatic neuroendocrine tumor (PanNET)
- Infers well differentiated neoplasm > 0.5 cm unless otherwise classified
- Solid pseudopapillary neoplasm (SPN)
- If FNA is suggestive but not diagnostic, categorize as atypical rather than suspicious
- Gastrointestinal stromal tumor (GIST)
- Extra-adrenal paraganglioma
- Novel reports of preoperatively diagnosing pancreatic solitary fibrous tumor by FNA (Cytopathology 2022;33:222, J Am Soc Cytopathol 2020;9:272)
- Pancreatic neuroendocrine tumor (PanNET)
- Average ROM: 37.5%
- Management: conservative, especially if lesion is small (PanNET < 2 cm)
- Premalignant
- Neoplastic: benign
- Category V: suspicious for malignancy
- Some (but an insufficient number) of the typical features of a specific neoplasm are present, mainly pancreatic adenocarcinoma
- Significant cellular or architectural atypia but insufficient qualitative / quantitative findings for conclusive diagnosis, insufficient tissue for confirmative ancillary testing or rare cells with multiple major criteria, as listed below:
- Significant nuclear enlargement, nuclear membrane irregularities, course chromatin pattern, distinct nucleoli, increased N:C ratio, anisonucleosis (3:1 to 4:1), sometimes mucinous metaplasia
- Loss of cellular and architectural polarity
- Entities include:
- Pancreatic adenocarcinoma (most common)
- Solid cellular smear patterns suggestive of:
- PanNET, acinar cell carcinoma, pancreatoblastoma, solid pseudopapillary neoplasm (SPN)
- Cyst aspirates with solid mural nodule or cyst wall mass
- Lymphoma
- Metastases
- High grade biliary intraepithelial neoplasia (BilIN) sampled by brushing (not high grade PanIN)
- Average ROM: 92.1%
- Management: reassess or repeat sampling; perform ancillary testing (may refer for surgery if positive FISH or loss of SMAD4 staining); consult experts
- Category VI: positive for malignancy
- Neoplasms that unequivocally display malignant cytologic characteristics
- Pancreatic ductal adenocarcinoma (PDAC) (most common):
- Well differentiated
- Difficult to diagnose; often classified as suspicious unless clearly malignant
- Moderately and poorly differentiated:
- Nuclear overlap and molding, marked loss of nuclear polarity, anisonucleosis > 4:1, disorganization (drunken honeycomb), macronucleoli, bizarre nuclei, nuclear hyperchromasia, elevated N:C ratio > 0.6, irregular chromatin distribution, lack of glandular differentiation, presence of cell balls, papillary fragments, cell in cell arrangements, tumor diasthesis, 3 dimensional fragments, cytoplasmic mucin vacuoles, background necrosis and mitoses
- Well differentiated
- Pancreatic ductal adenocarcinoma (PDAC) (most common):
- Entities include:
- Pancreatic ductal adenocarcinoma and its variants
- Cholangiocarcinoma
- Acinar cell carcinoma
- High grade neuroendocrine carcinoma (small cell and large cell)
- Pancreatoblastoma
- Lymphomas
- Sarcomas
- Metastases to the pancreas
- Average ROM: 99.6%
- Management: surgical resection; may also consider neoadjuvant therapy or clinical trials if lesions are unresectable
- Neoplasms that unequivocally display malignant cytologic characteristics
Diagrams / tables
Table 1: Papanicolaou reporting system for pancreaticobiliary cytology
| Diagnostic categories | Average risk of malignancy | Examples of entities | Selected ancillary testing | Management |
I. Nondiagnostic | 23.1% | • Cellular artifact, obscuring hemorrhage or necrosis • Pure GI contamination • Acellular cyst aspirate with no evidence of mucinous etiology • Benign pancreatic parenchyma with clearly defined mass on imaging | Cyst fluid analysis: CEA and amylase (no mucinous etiology = lack of mucin, lack of elevated CEA, lack of KRAS or GNAS mutations) | Reassess / repeat |
II. Negative | 8.2% | • Pancreatitis • Pseudocyst • Lymphoepithelial cyst • Splenule / accessory spleen • Benign pancreatic parenchyma with no mass on imaging | Pseudocyst: high amylase + low CEA | Conservative |
III. Atypical | 46.8% (range: 28 - 100) |
• Ductal cells with indeterminate atypia (reactive versus low grade dysplasia, versus scant lesional tissue) • Abundant intracytoplasmic mucin in the epithelium | Cyst fluid analysis: CEA and amylase | Conservative, repeat or surgery |
IV. Neoplastic: benign | 20.2% |
• Serous cystadenoma (SCA) • Lymphangioma • Cystic teratoma • Schwannoma | SCA: low amylase + low CEA, VHL mutation, positive inhibin staining | Conservative |
IV. Neoplastic: other | 37.5% |
• GIST • Extra-adrenal paraganglioma • Pancreatic solitary fibrous tumor Premalignant • IPMN with dysplasia • IPNB • MCN with dysplasia Low grade malignant • PanNET • Solid pseudopapillary neoplasm (SPN) |
Solitary fibrous tumor: STAT6 mutation Intraductal papillary mucinous neoplasm: KRAS, RNF43, GNAS (specific), TP53, SMAD4 mutations Solid pseudopapillary neoplasm: CTNNB1 (beta catenin) mutation | Surgery with an option for conservative management in select scenarios |
V. Suspicious | 92.1% |
• Pancreatic ductal adenocarcinoma (PDAC) • PanNET • Acinar cell carcinoma • Pancreatoblastoma • Solid pseudopapillary neoplasm • Cyst aspirate with solid mural nodule or cyst wall mass • Lymphoma • Metastases • High grade BilIN | Immunocytochemistry, FISH molecular analysis Cyst fluid analysis: CEA and amylase | Surgery |
VI. Positive | 99.6% | • PDAC and variants • Cholangiocarcinoma • Acinar cell carcinoma • Small and large cell neuroendocrine carcinoma • Pancreatoblastoma • Lymphomas • Sarcomas • Metastases to the pancreas | Immunocytochemistry patterns in malignancy: • Positive: S100P, IMP3, MUC4, mesothelin • Negative: pVHL, CD10, clusterin beta, SMAD4 FISH significantly improves diagnostic sensitivity of adenocarcinoma (copy number abnormalities in CEP3, CEP7, CEP17 and of band 9p21) | Surgery or neoadjuvant therapy / clinical trials |
- Reference: Diagn Cytopathol 2020;48:494
Laboratory
- Pancreaticobiliary strictures (Diagn Cytopathol 2014;42:351)
- Fluorescence in situ hybridization (FISH)
- DNA probe set for biliary brush specimens; outperforms routine cytology in sensitivity, thus is the preferred complement
- Malignancy: chromosomal copy number abnormalities in CEP3, CEP7, CEP17 and of band 9p21 (CEP = centromere enumeration probe)
- Successful for evaluating negative, inconclusive and malignant specimens
- DNA probe set for biliary brush specimens; outperforms routine cytology in sensitivity, thus is the preferred complement
- Immunocytochemistry
- Marker patterns frequently observed in malignancy: S100P+, pVHL-, IMP3+, CD10-, MUC4+, mesothelin+, clusterin beta-
- KRAS mutational analysis
- Sensitive for invasive carcinomas and PDAC but not specific
- Abnormalities also observed in low grade dysplasias and chronic pancreatitis
- Shown utility in distinguishing benign from malignant in atypical specimens
- Not supported as useful in diagnosing solid pancreatic masses and bile duct strictures
- Sensitive for invasive carcinomas and PDAC but not specific
- Fluorescence in situ hybridization (FISH)
- Pancreatic cystic lesions
- Histochemical stains
- Positive staining of mucin (mucicarmine and Alcian blue / PAS)
- Diagnostically useful to identify mucinous lesions
- Biochemical tests
- Amylase and carcinoembryonic antigen (CEA) levels help diagnose:
- Pseudocysts (high amylase in the 1,000s U/L, low CEA)
- Serous cystadenomas (low amylase < 1,000 U/L, low CEA)
- Mucinous cysts (CEA > 192 ng/mL)
- Cyst fluid CEA alone reliably distinguishes mucinous from nonmucinous lesions (Diagn Cytopathol 2014;42:351)
- Not useful for predicting malignancy
- Optimal CEA cutoff of 192 is supported by meta analysis and is the most widely accepted and utilized (Dig Dis Sci 2021 Nov 16 [Epub ahead of print])
- CA125 and CA19-9 generally not useful for diagnosis
- MUC2, MUC4 and MUC7 useful in recognizing dysplasia and malignancy in cystic neoplasms
- Amylase and carcinoembryonic antigen (CEA) levels help diagnose:
- DNA analysis
- Aneuploid and tetraploid results support malignancy
- Elevated cyst fluid DNA supports malignancy
- No significantly improved diagnostic accuracy over routine cytology
- Molecular tests
- Mutations: supported diagnosis
- KRAS, GNAS, RNF43: IPMN and MCN (GNAS absent in MCN)
- TP53, SMAD4, CTTNB1, mTOR genes: advanced neoplasia (high grade dysplasia or carcinoma)
- VHL: serous cystadenomas
- CTNNB1 (beta catenin): solid pseudopapillary neoplasms
- Mutations: supported diagnosis
- Histochemical stains
- Solid pancreatic neoplasms
- Immunohistochemical tests
- Adenocarcinoma: DPC4 / SMAD4-
- PanNET: chromogranin+, synaptophysin+, INSM1+, CD56+, neuron specific enolase+, CK8/18+
- Acinar cell carcinoma: BCL10+, amylase+, trypsin+, chymotrypsin+, lipase+, CK7+
- Solid pseudopapillary neoplasm: nuclear beta catenin+, alpha-1 antitrypsin+, CD10+, SOX11+, synaptophysin+, chromogranin-, trypsin- (Cancer Cytopathol 2017;125:831)
- Pancreatic and biliary tract lymphoma: will be dependent on the type of lymphoma
- Molecular analysis
- Adenocarcinoma
- KRAS mutation not used due to low specificity for malignancy
- p16 / CDKN2A, TP53 and SMAD4 inactivation
- FISH significantly improves diagnostic sensitivity; the most reliable adjunct test for confirmation for biliary tract brushing
- Copy number abnormalities in CEP3, CEP7, CEP17 and of 9p21
- MicroRNA and loss of heterozygosity: clinical use and importance to be determined
- PanNET
- Loss of heterozygosity: malignant associations but clinical importance to be determined
- ATRX FISH performed by some labs along with DAXX IHC to identify patients with a poor prognosis (Cancer Cytopathol 2017;125:544)
- Acinar cell carcinoma
- Associated with losses of 11p; diagnostic significance unclear
- Solid pseudopapillary neoplasm
- Somatic point mutation in exon 3 of beta catenin
- Adenocarcinoma
- Immunohistochemical tests
Case reports
- 31 year old man with pancreatoblastoma (positive) (Clin J Gastroenterol 2018;11:161)
- 58 year old man with intraductal papillary neoplasm of the biliary tract (atypical) (Diagn Cytopathol 2019;47:922)
- 60 year old man with paraduodenal pancreatitis found after an initially nondiagnostic biopsy (nondiagnostic) (Case Rep Surg 2020;2020:5021578)
- 62 year old man with pancreatic lymphoepithelial cyst (negative) (Case Rep Med 2020;2020:4590758)
- 67 year old woman with cystic lymphangioma of the pancreas (neoplastic) (Gland Surg 2018;7:487)
- 84 year old man with pancreatobiliary neoplasm of the duodenum (suspicious) (Diagn Cytopathol 2019;47:1059)
Cytology description
Cytology images
Contributed by Derek Allison, M.D.
Additional references
Board review style question #1
Board review style answer #1
C. Neoplastic: other. Solid pseudopapillary neoplasm of the pancreas, characterized by small, discohesive cells surrounding branching capillaries, is considered a low grade malignant entity. The neoplastic: other category includes neoplasms that are either premalignant or low grade malignant.
Comment Here
Reference: Pancreas - Papanicolaou system
Comment Here
Reference: Pancreas - Papanicolaou system
Board review style question #2
Board review style answer #2
B. Negative DPC4 / SMAD4 by IHC. Pancreatic adenocarcinoma is supported by DPC4 / SMAD4 loss. The presence of copy number abnormalities in CEP3, CEP7, CEP17 (note the correct chromosomes) and of band 9p21 are characteristic of pancreatic and biliary malignancies. Serous cystadenoma is associated with mutated VHL. Nuclear beta catenin supports a diagnosis of solid pseudopapillary neoplasm.
Comment Here
Reference: Pancreas - Papanicolaou system
Comment Here
Reference: Pancreas - Papanicolaou system
Recent Pancreas Pathology books
Find related Pathology books: GI, GU/adrenal, head & neck/endocrine, cytopathology, liver, pediatric, molecular