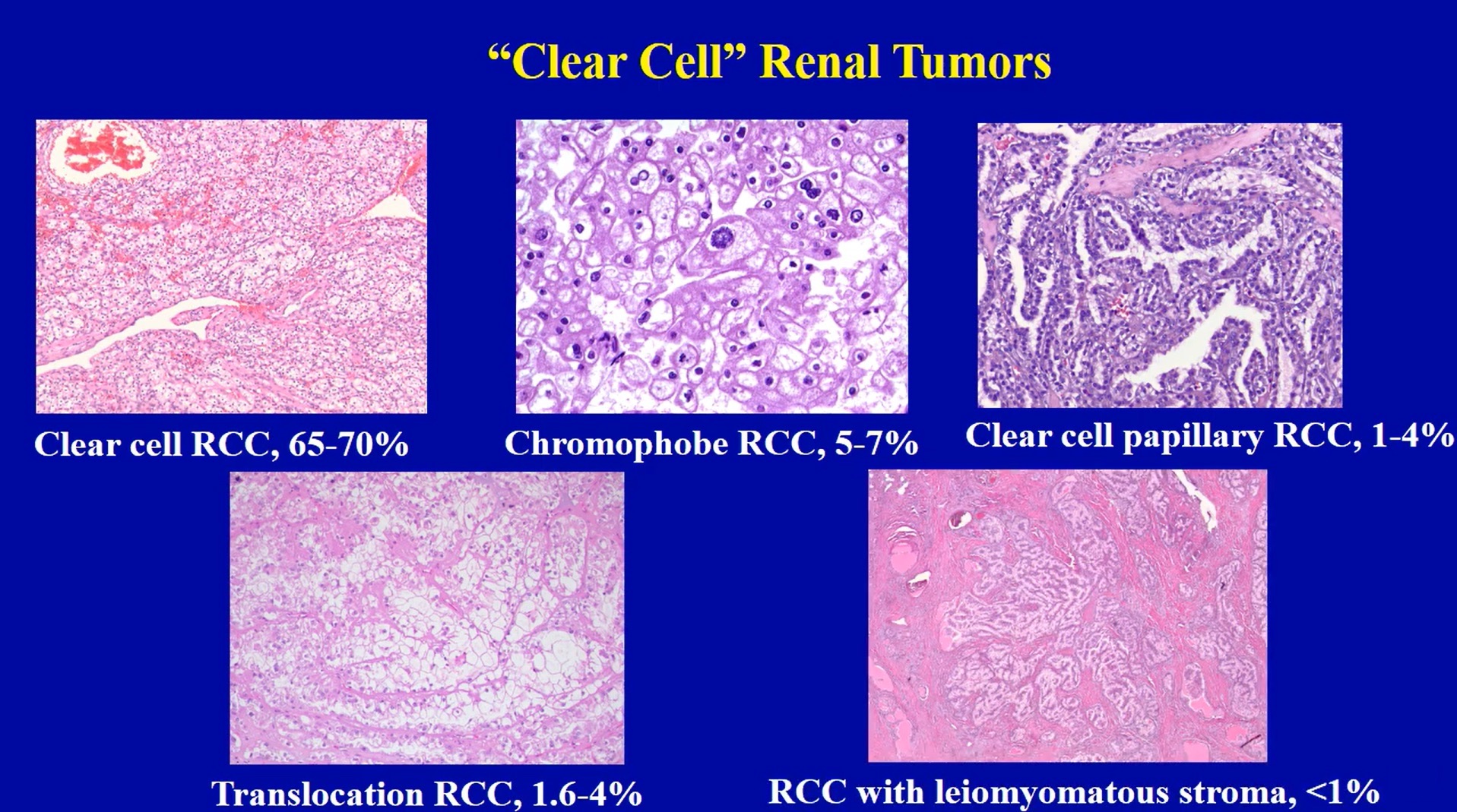
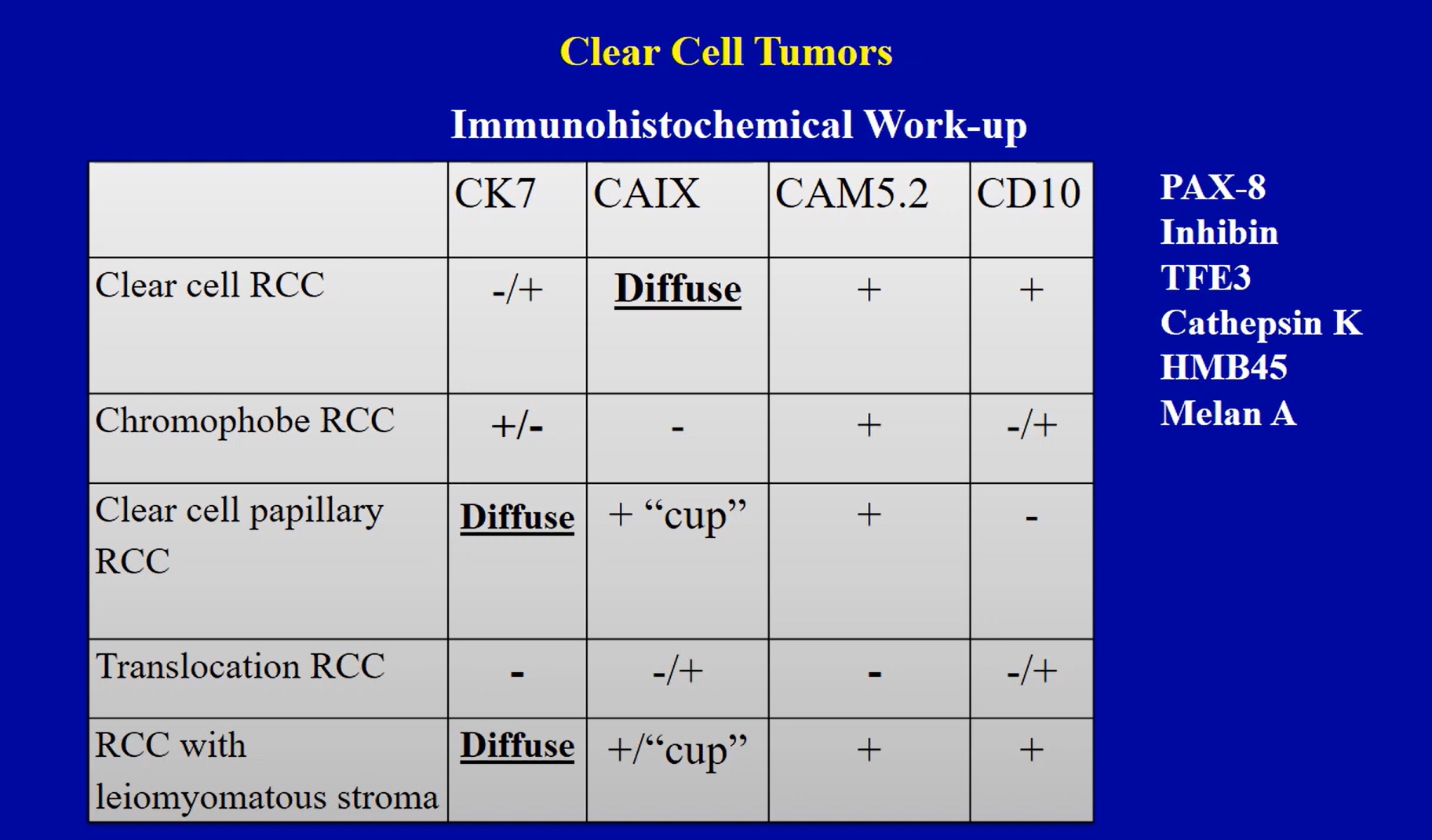
- pTX: primary tumor cannot be assessed
- pT0: no evidence of primary tumor
- pT1a: ≤ 4 cm, limited to the kidney
- pT1b: > 4 cm and ≤ 7 cm, limited to the kidney
- pT2a: > 7 cm and ≤ 10 cm, limited to the kidney
- pT2b: > 10 cm, limited to the kidney
- pT3a: invades renal vein / branches, perirenal fat, renal sinus fat or pelvicaliceal system
- pT3b: extends into vena cava below the diaphragm
- pT3c: extends into vena cava above the diaphragm or invades vena cava wall
- pT4: invades beyond Gerota fascia, including direct extension to adrenal gland
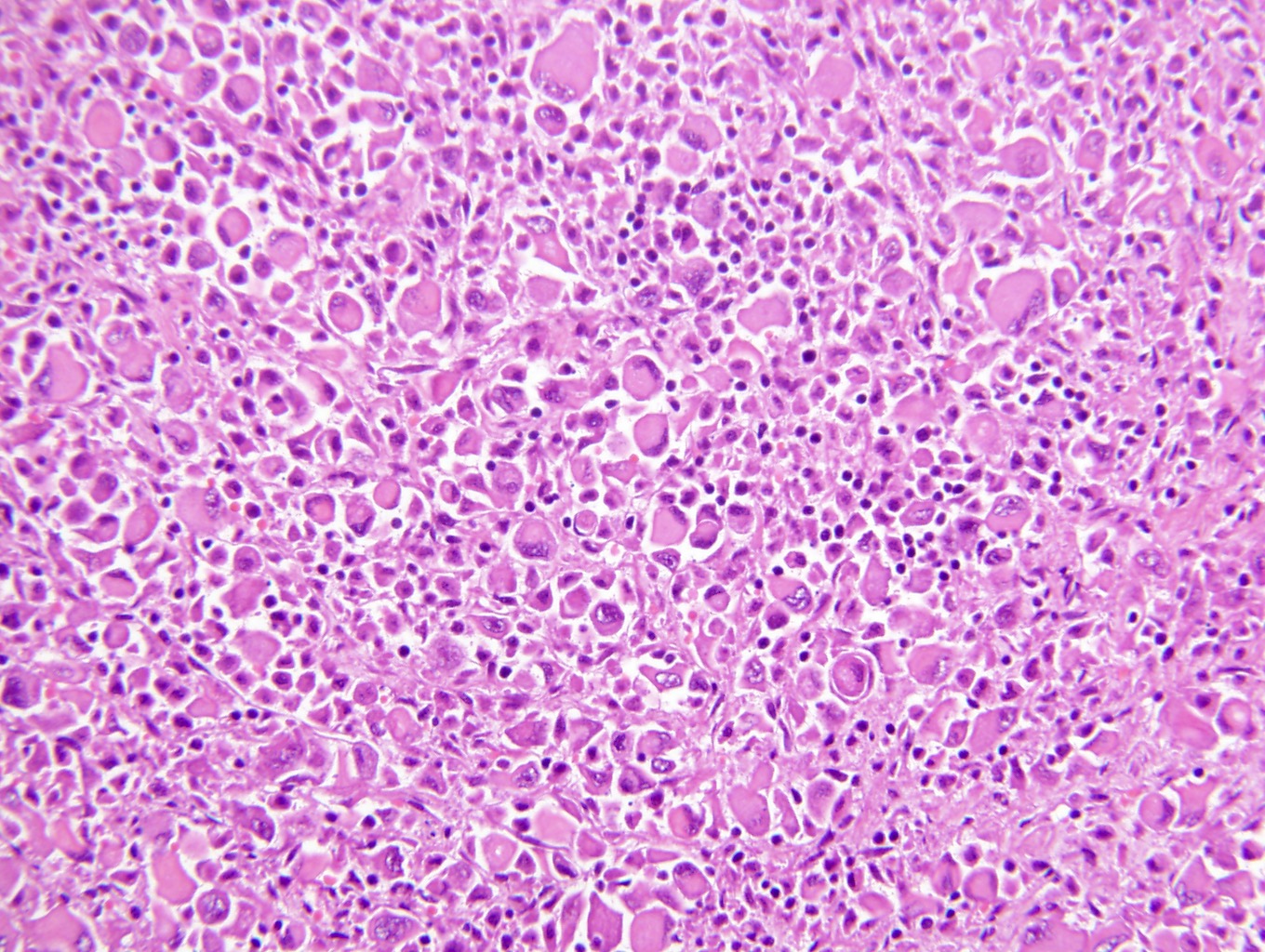
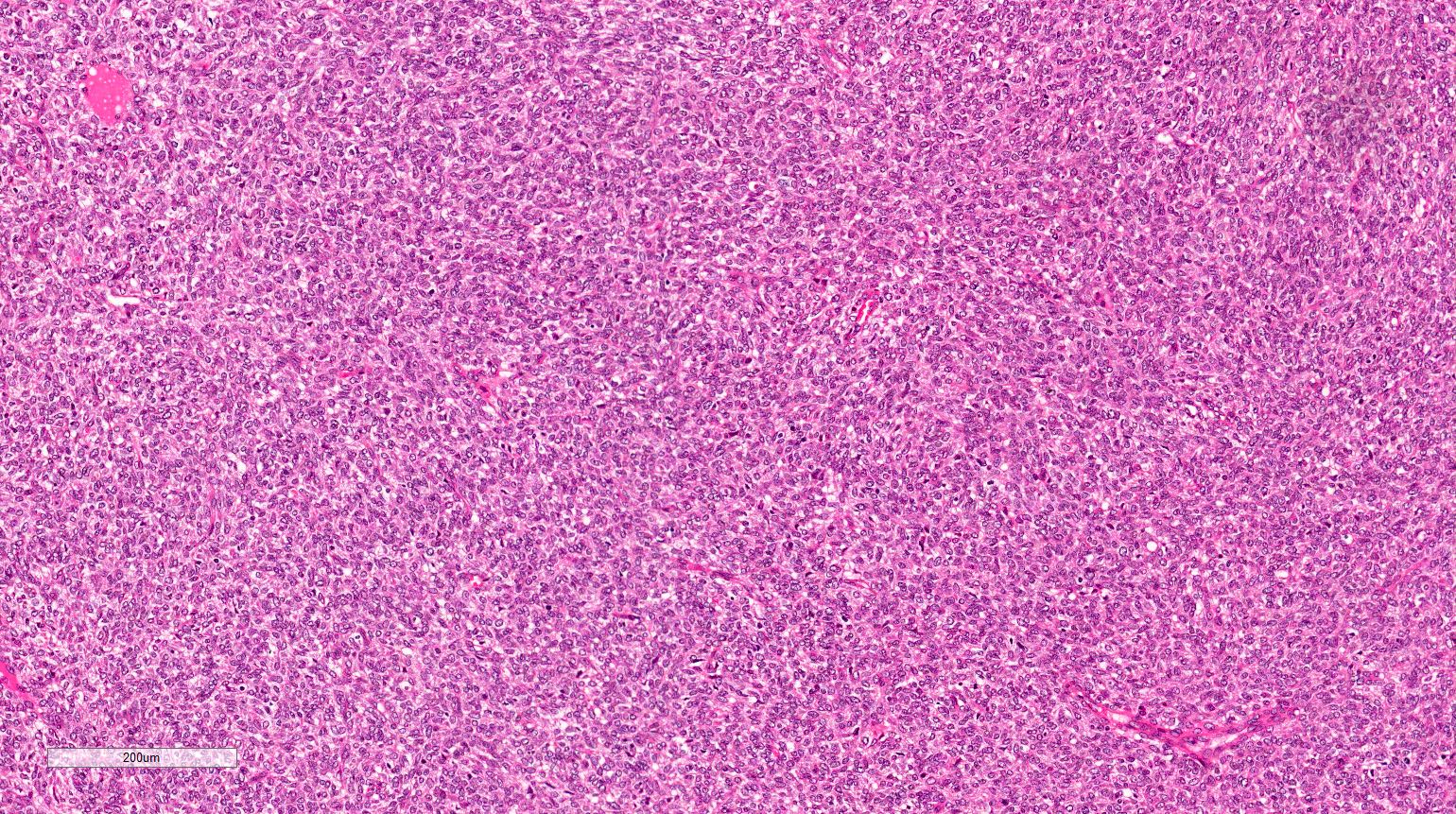
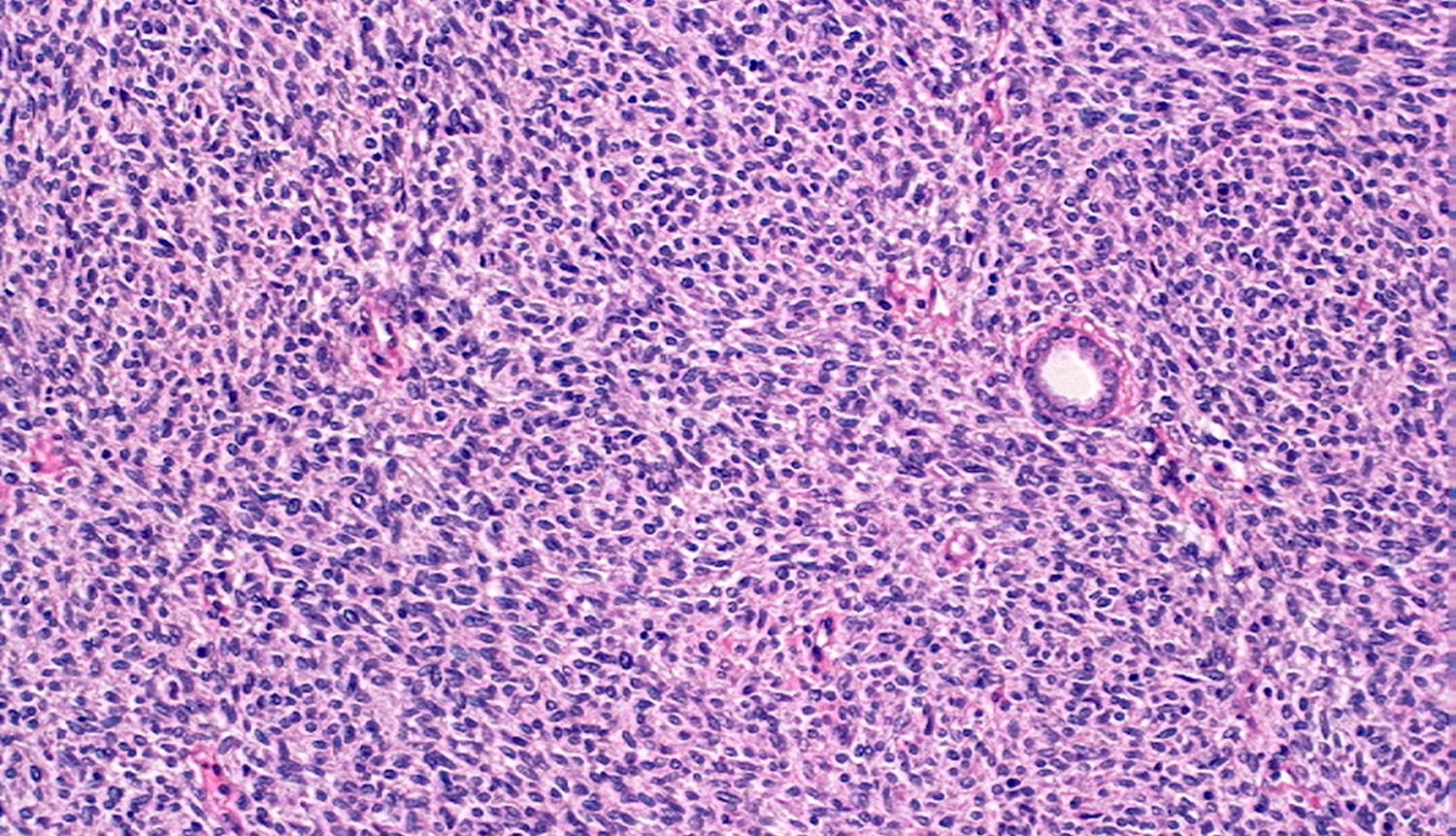
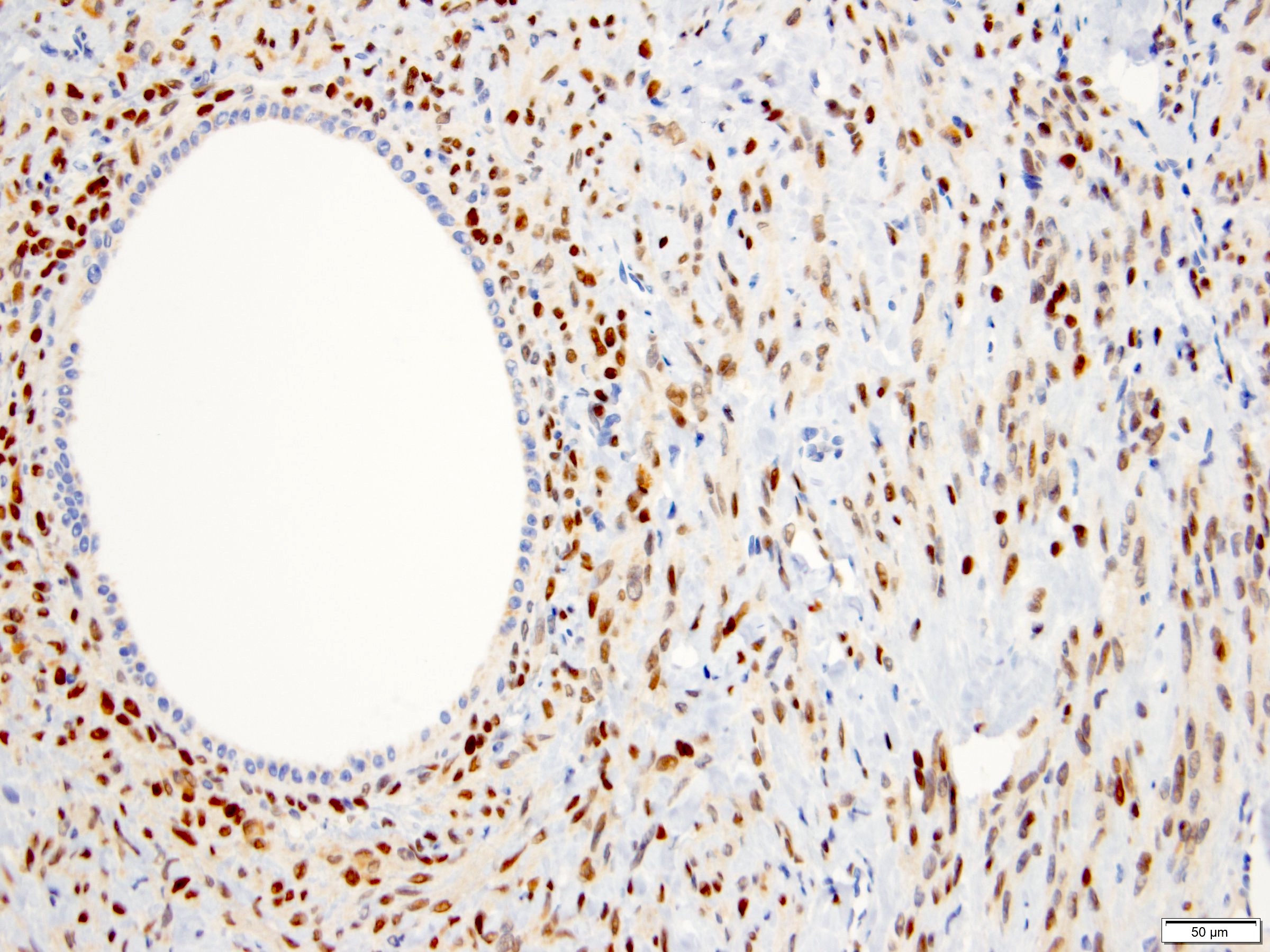
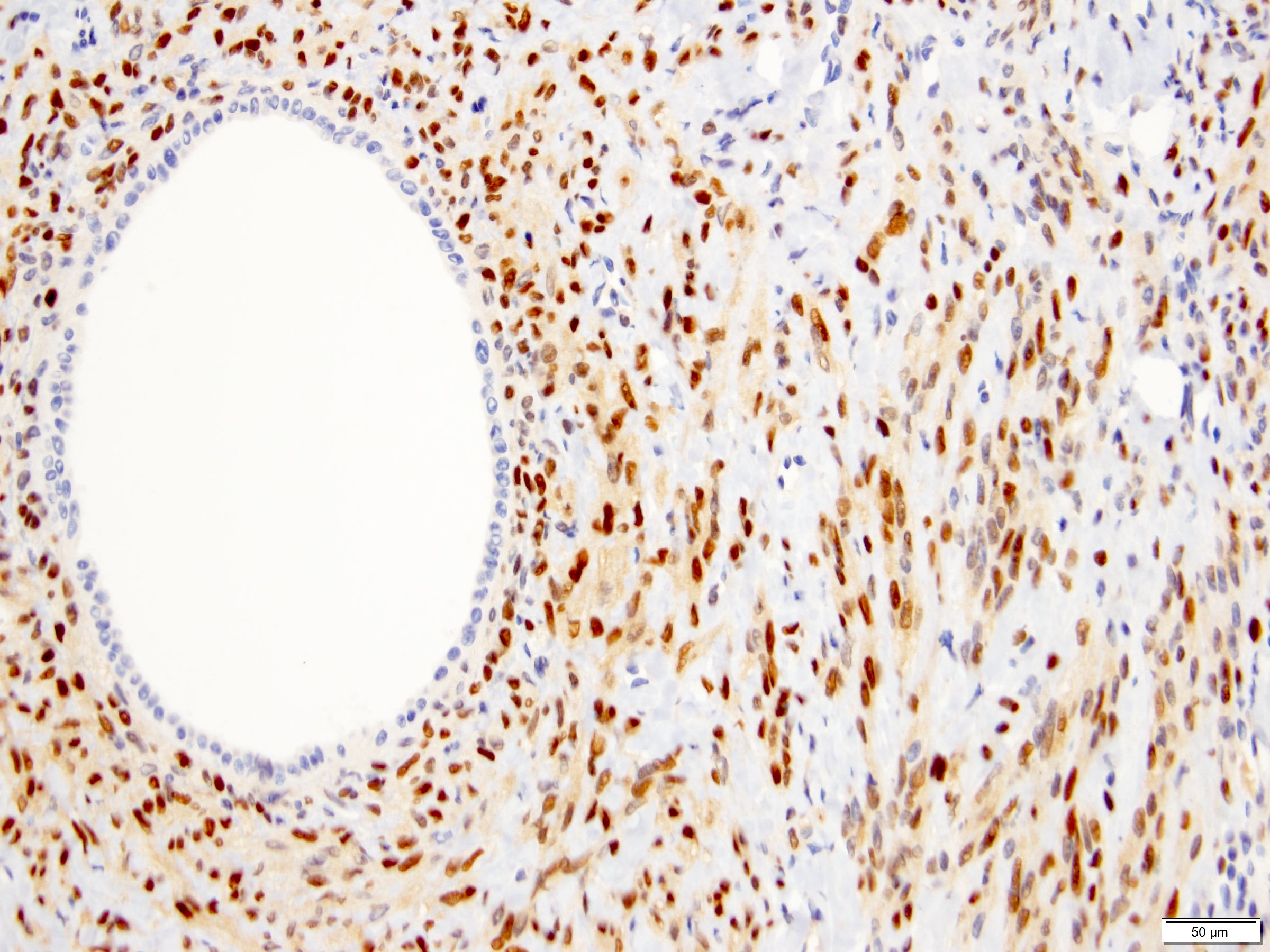
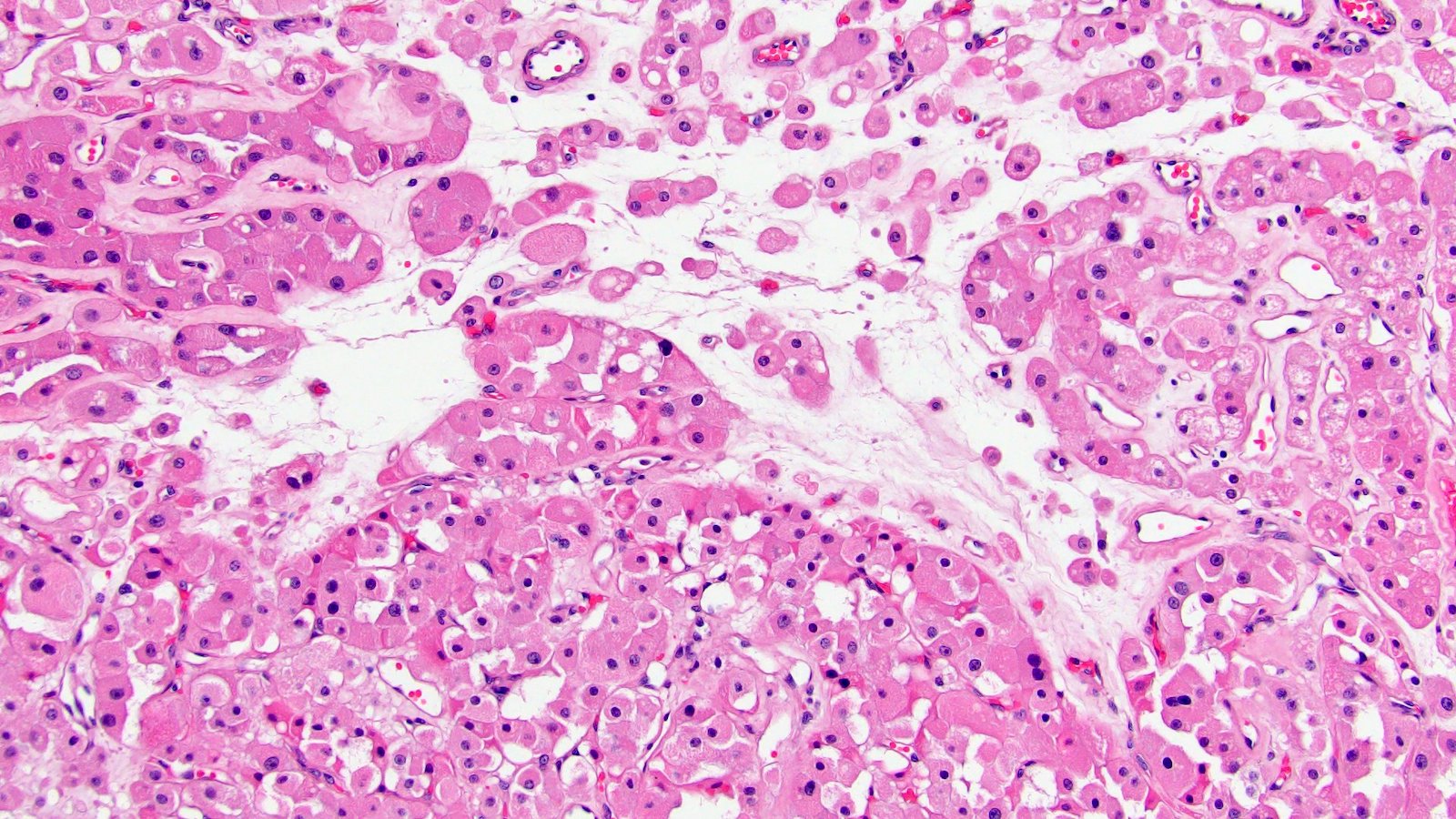
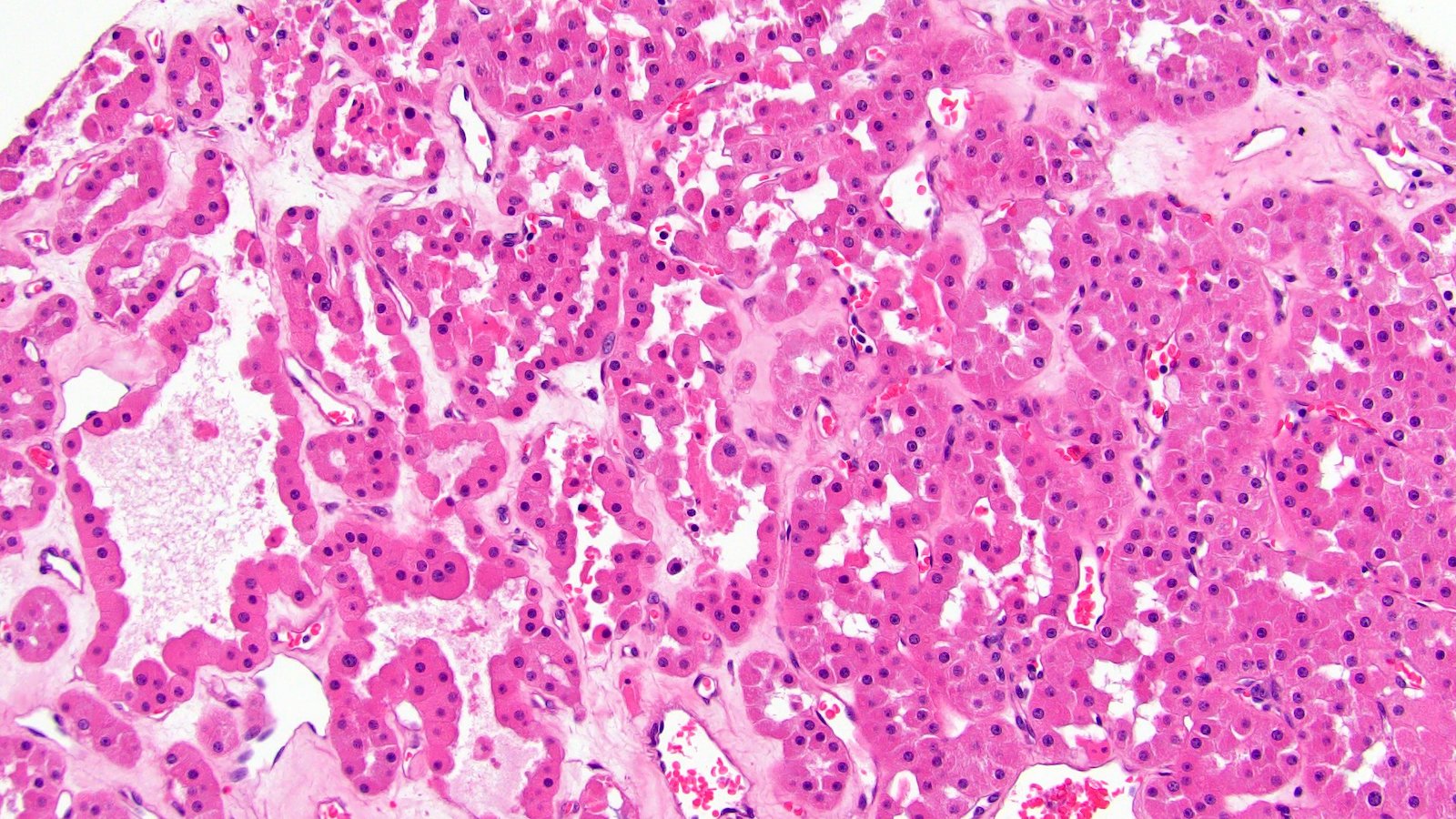
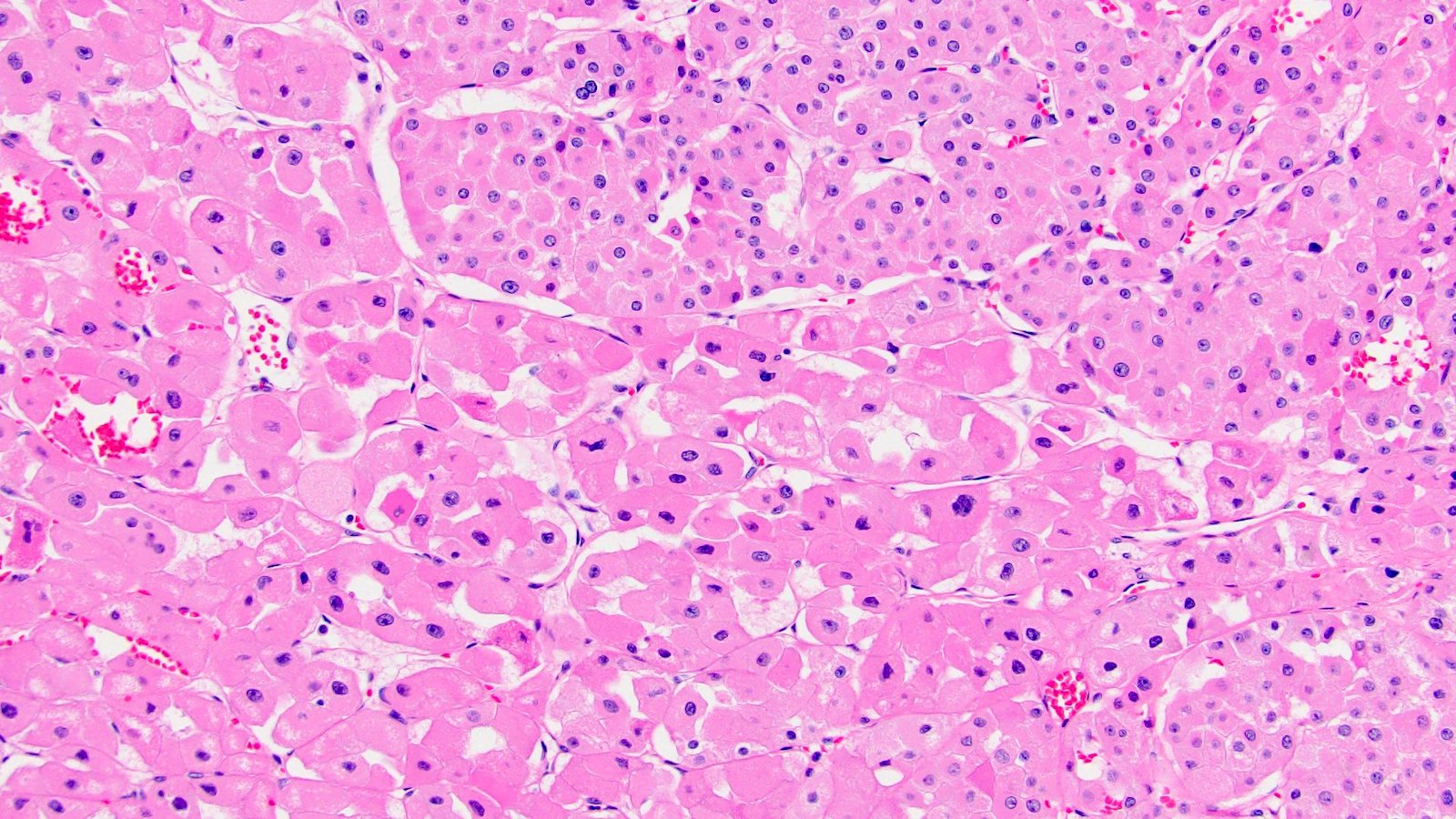
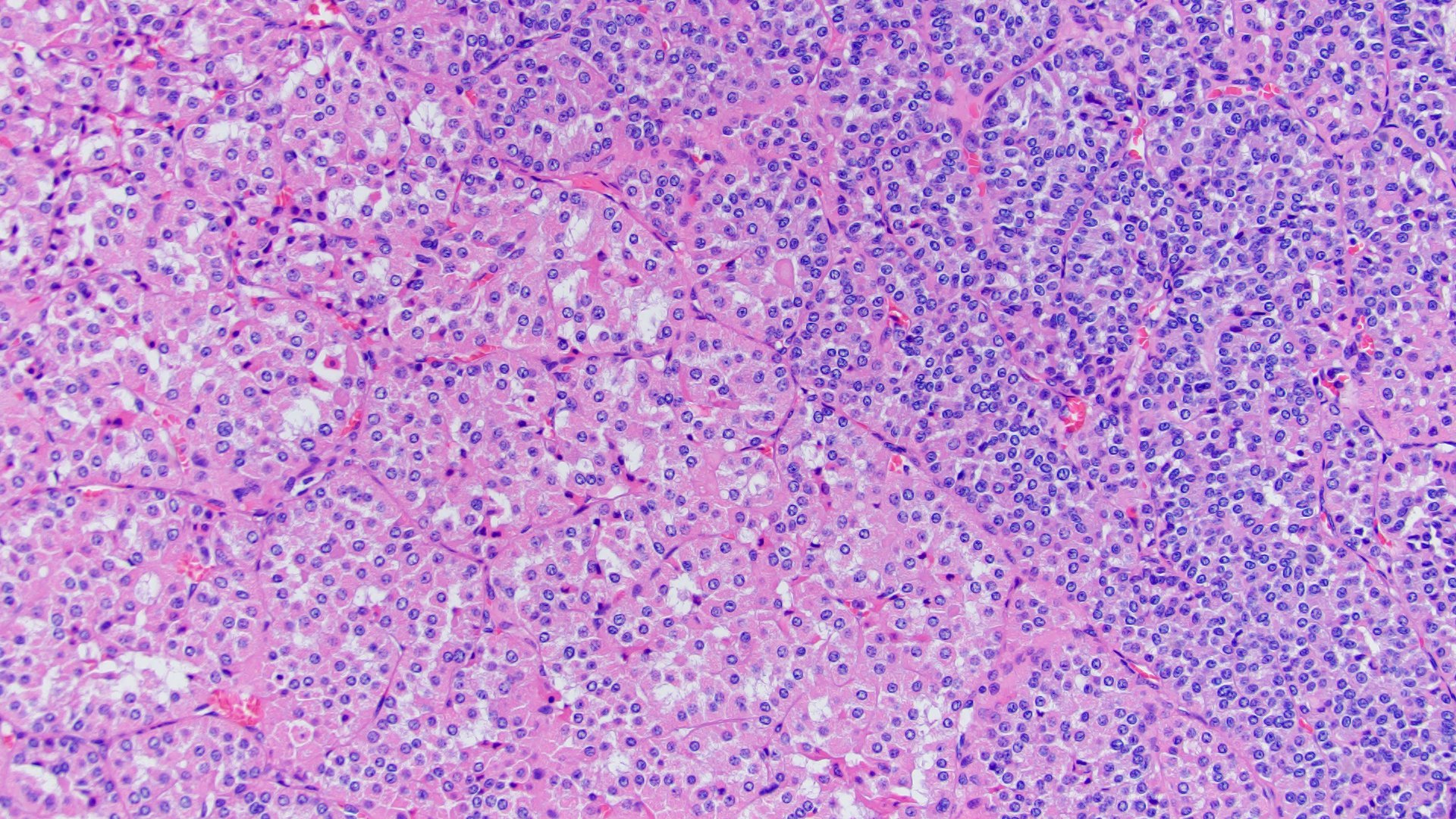
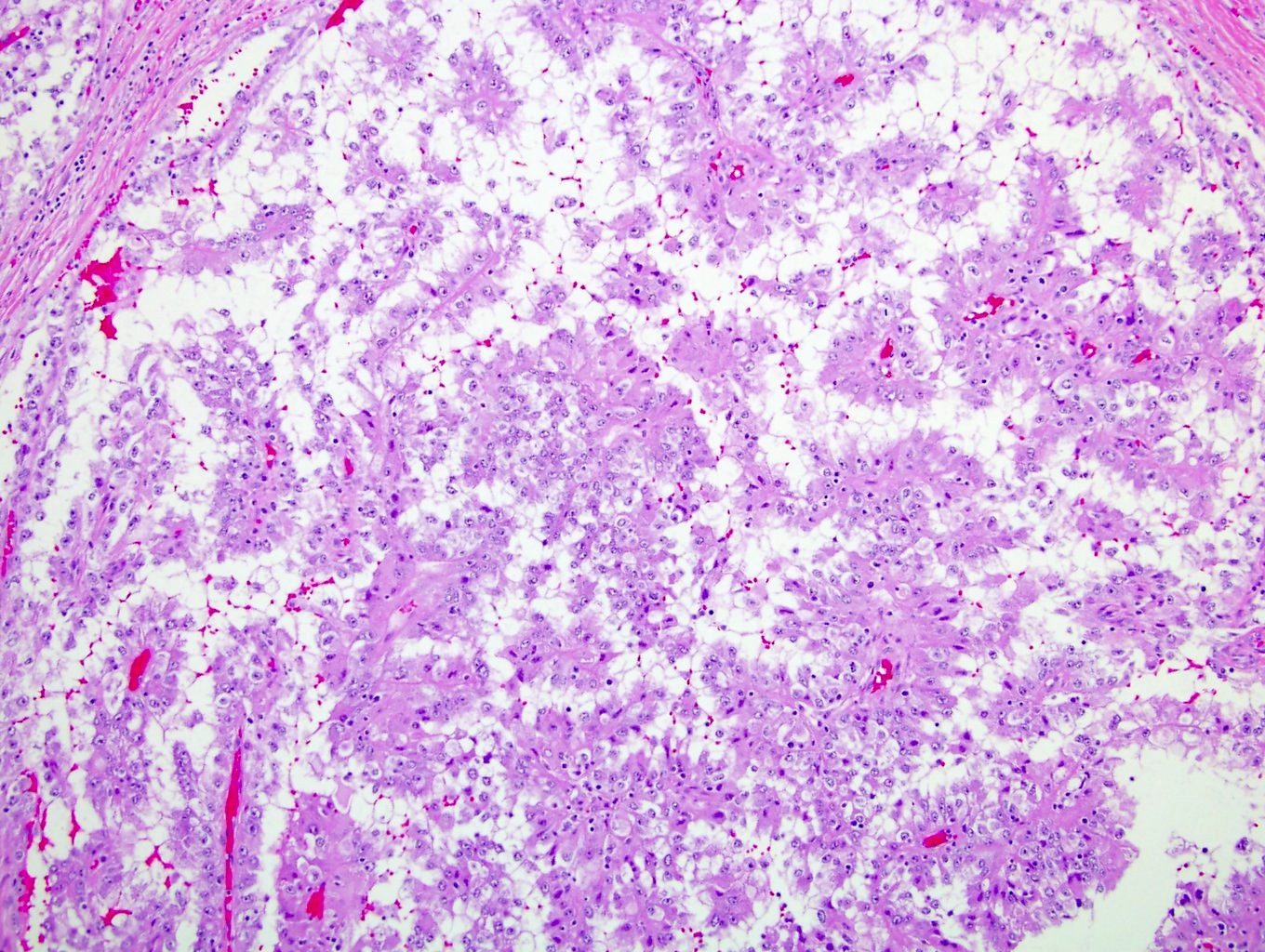
- Renal cell carcinoma (RCC) associated with anaplastic lymphoma kinase (ALK) gene rearrangement (chromosome 2p23)
- Affects children with sickle cell trait or adults without sickle cell trait
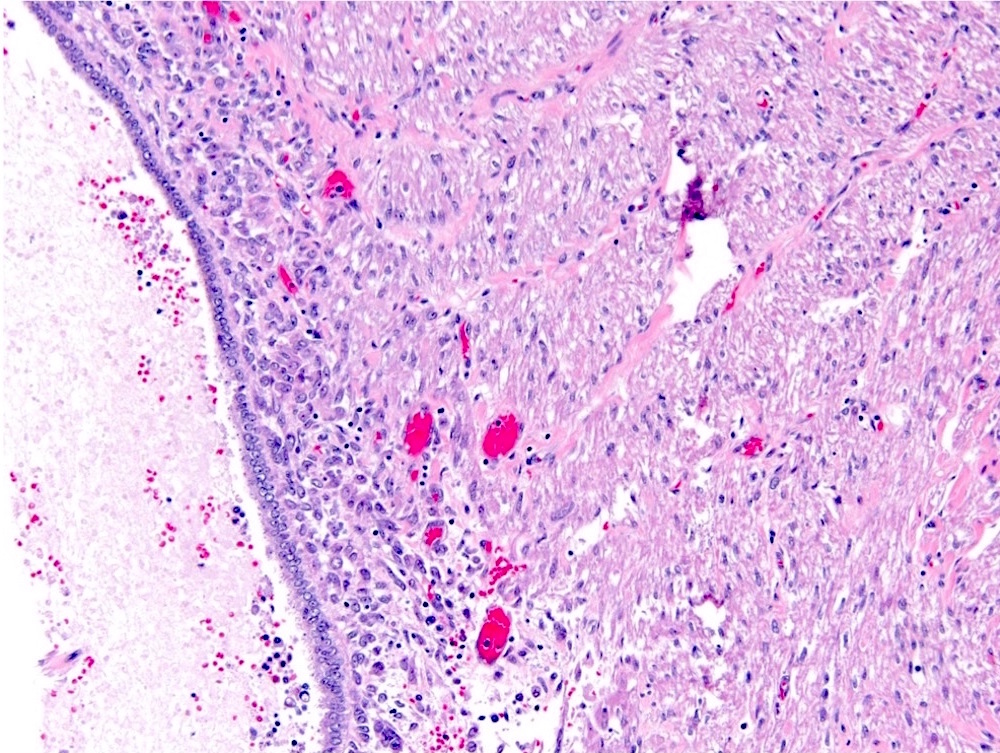
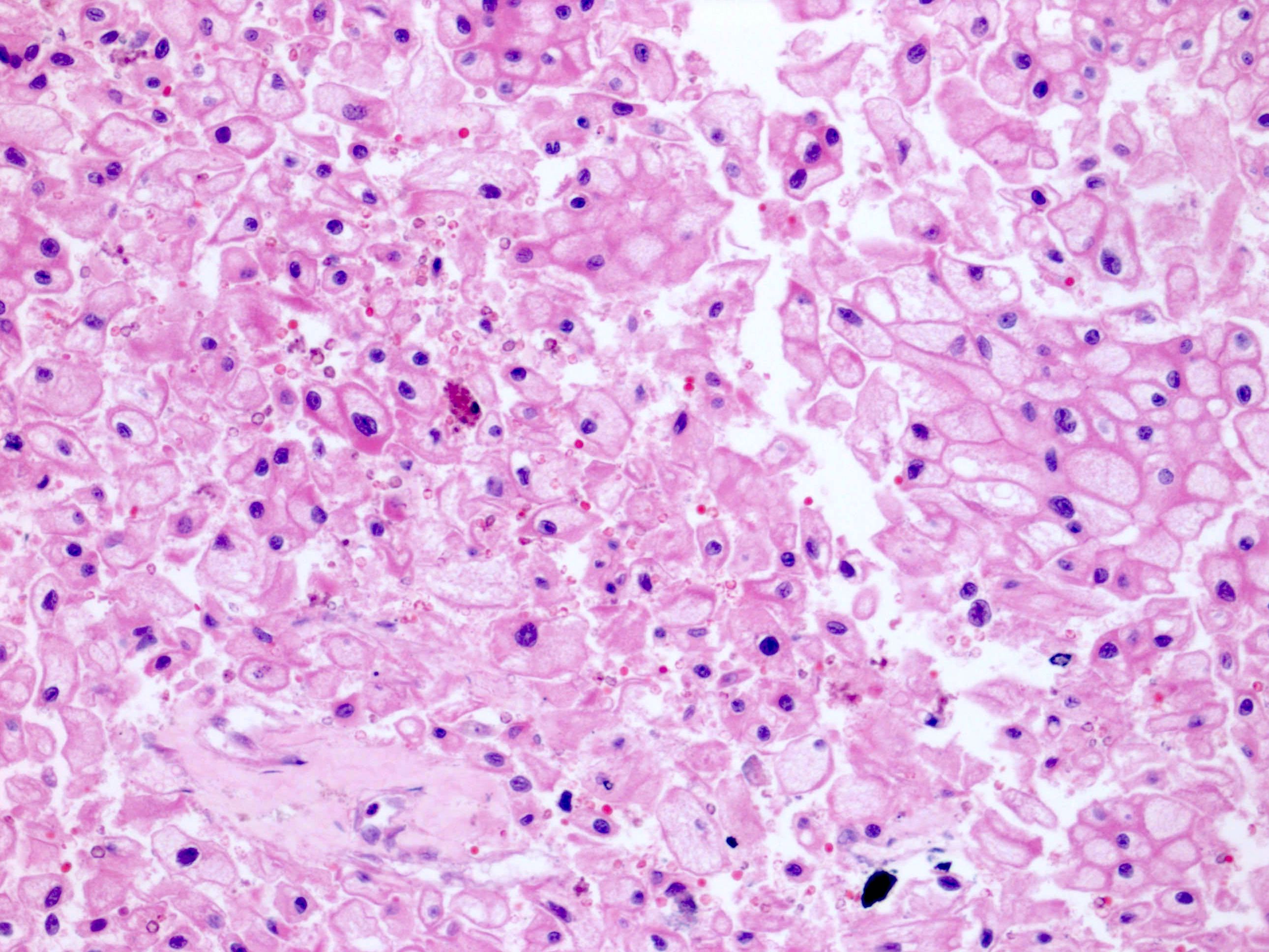
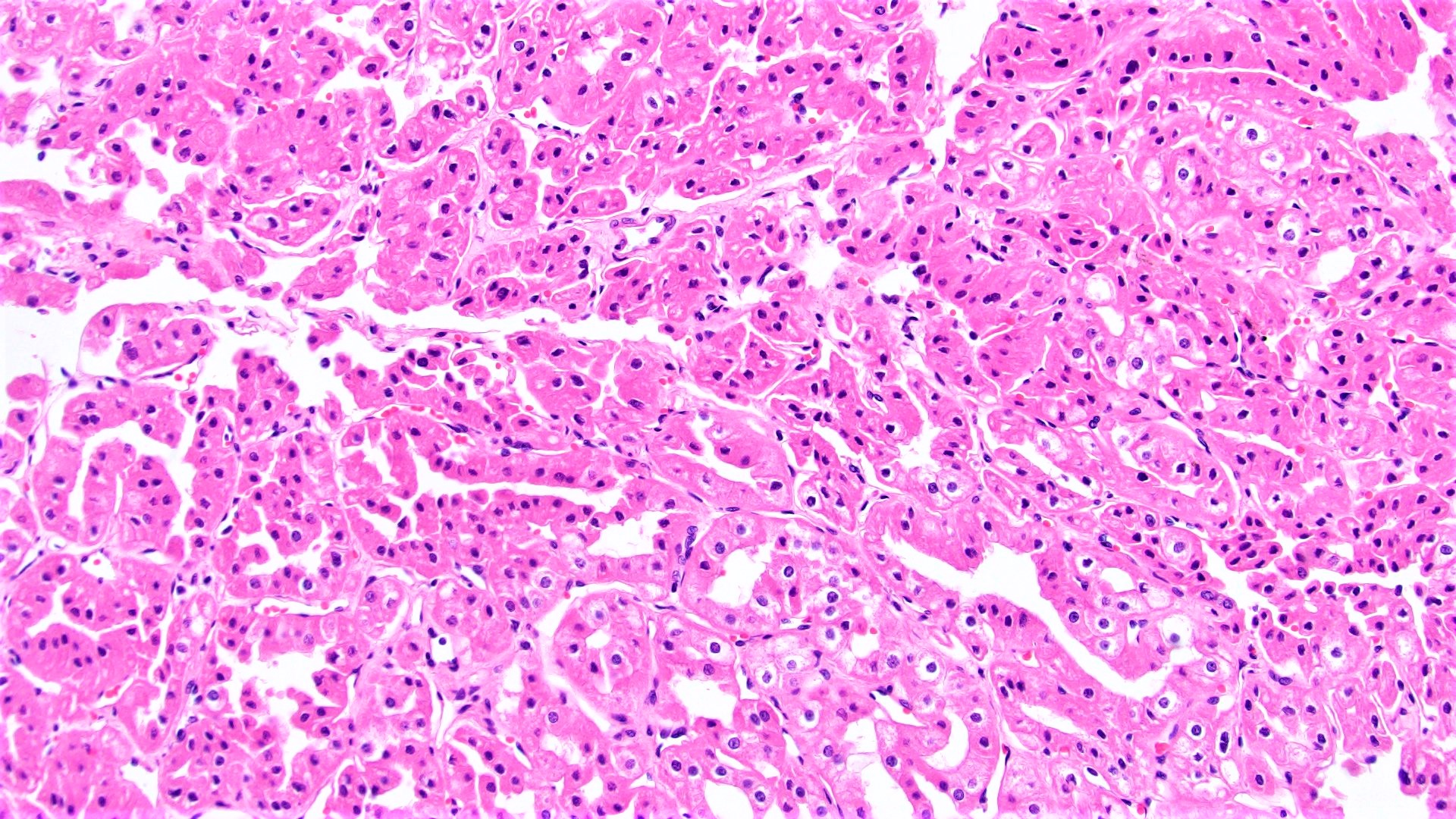
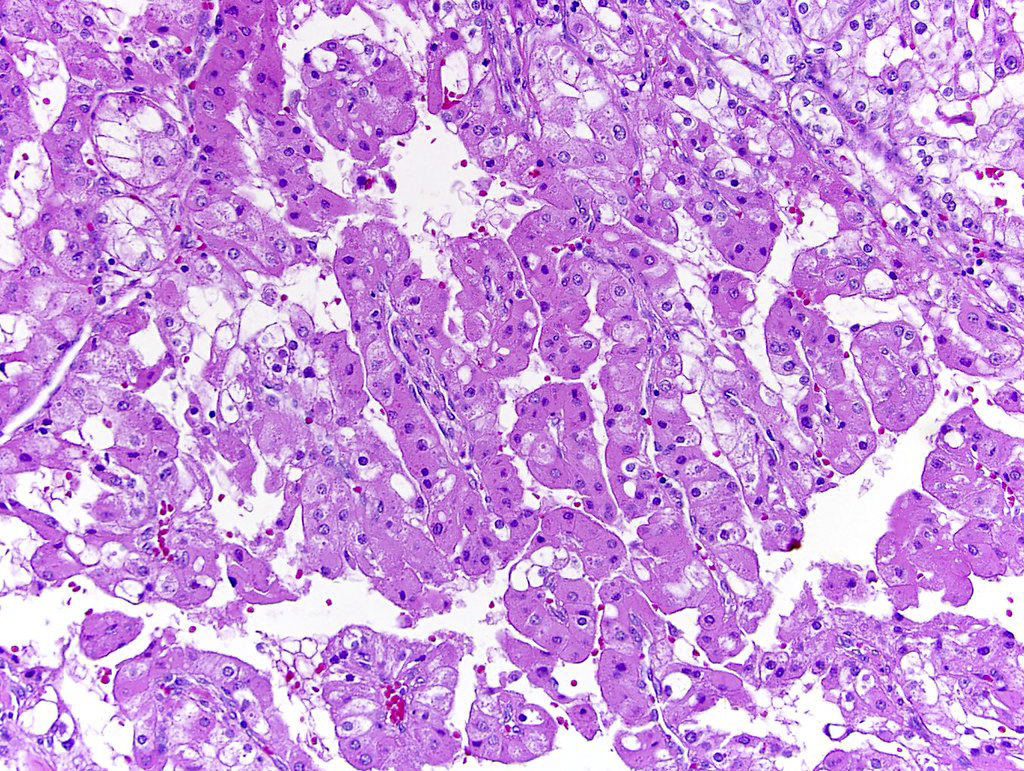
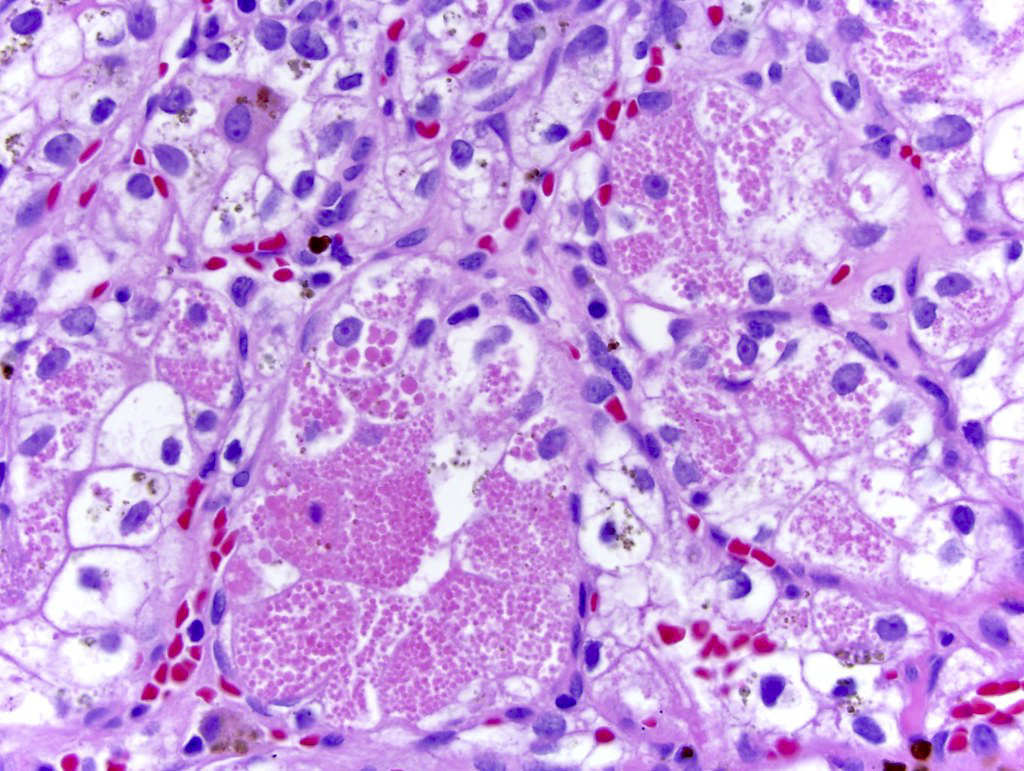
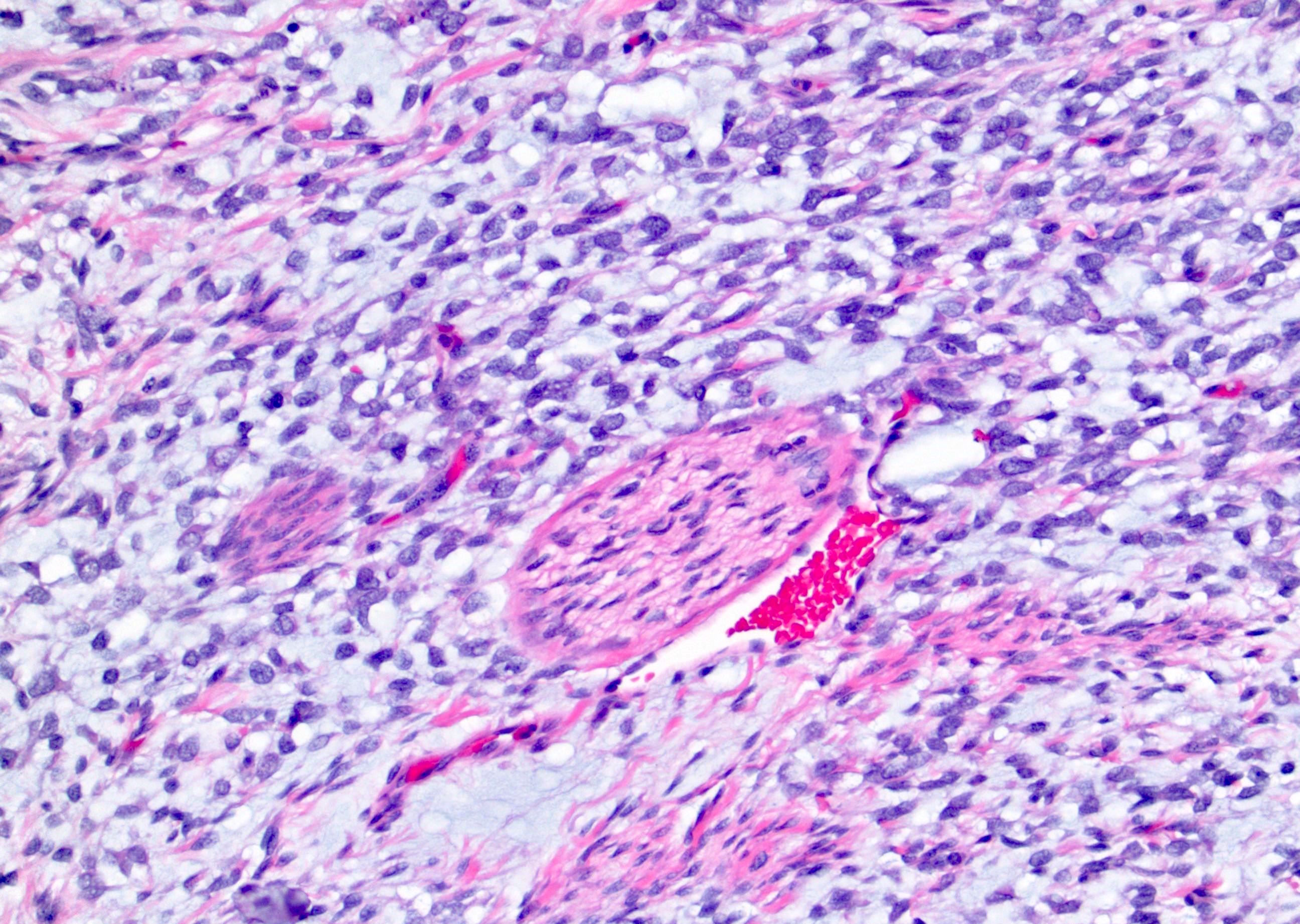
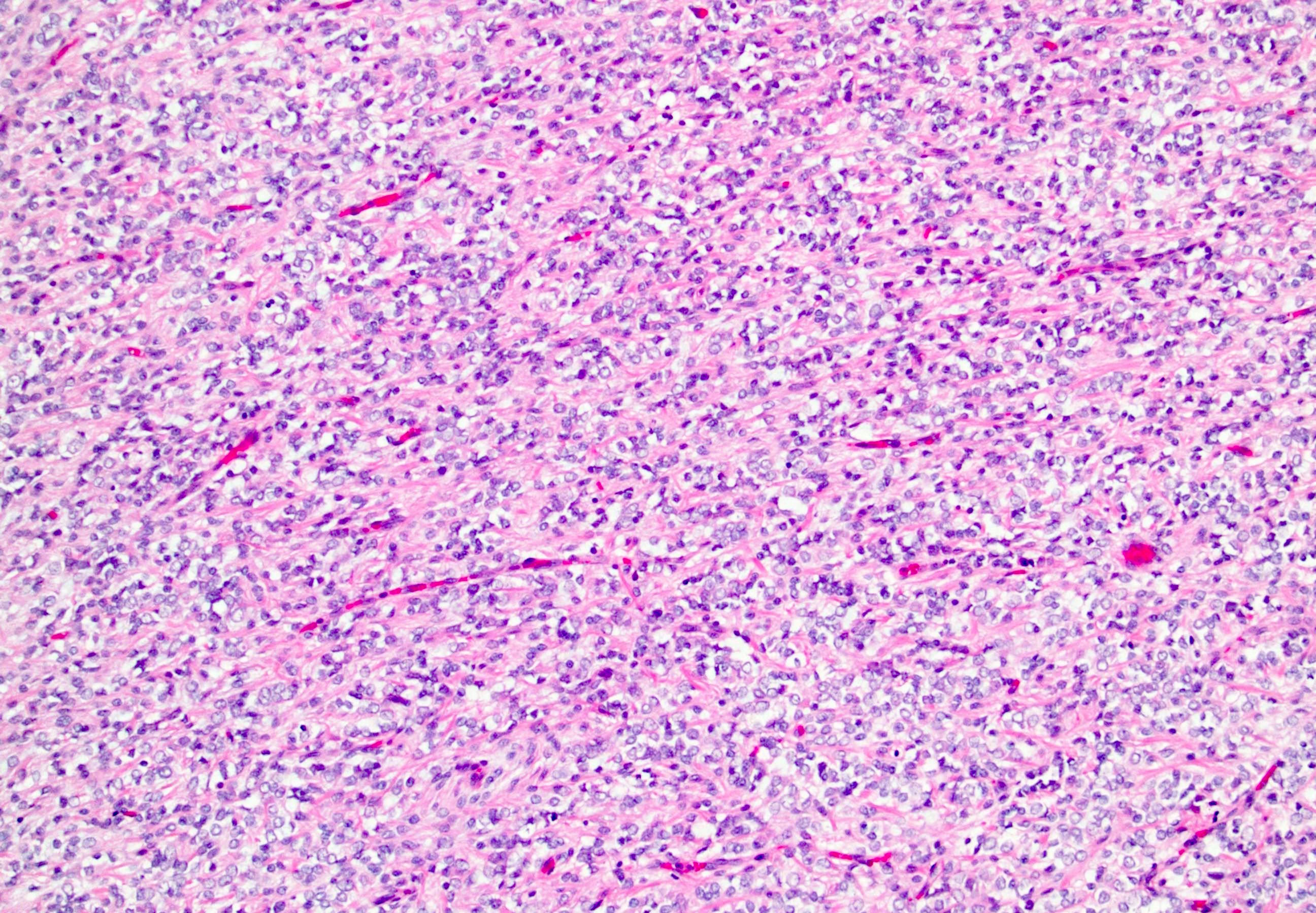
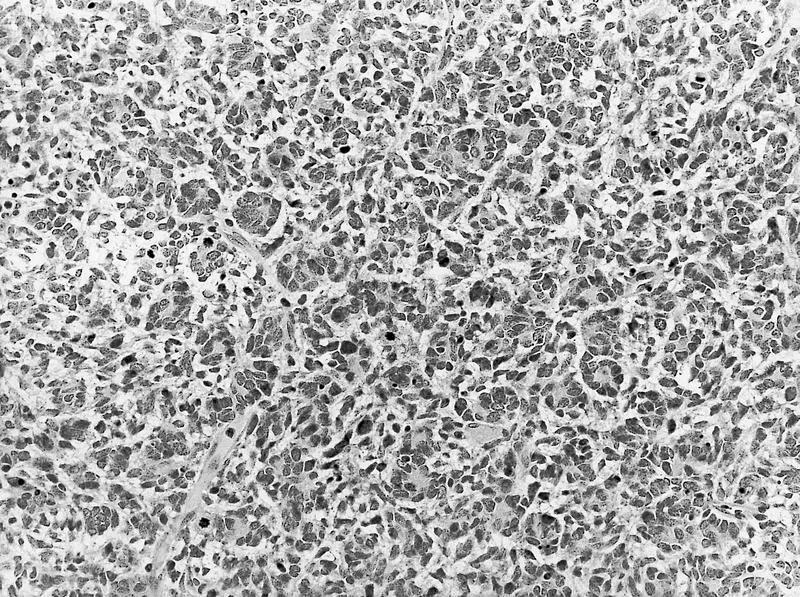
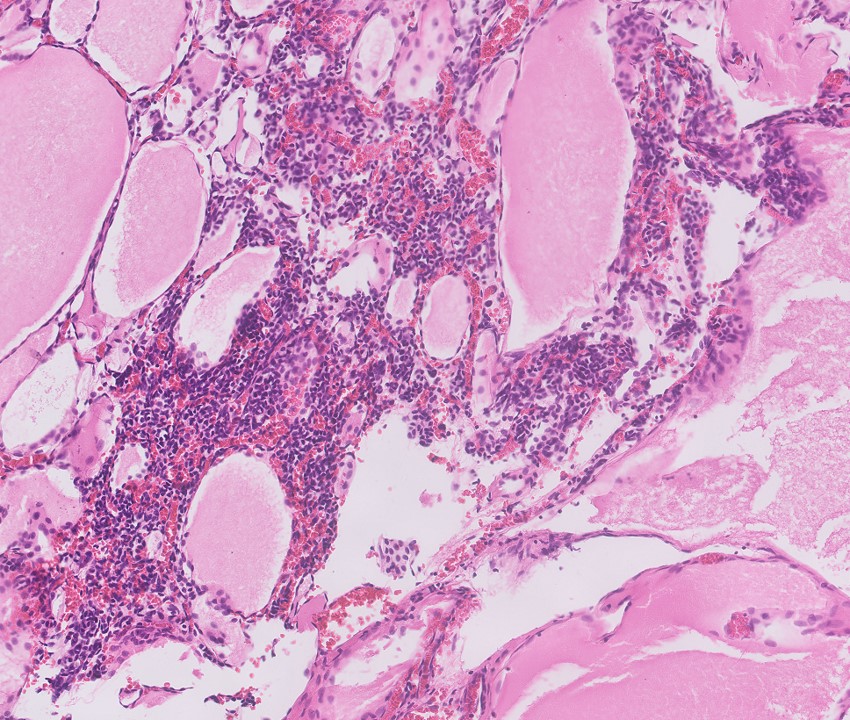
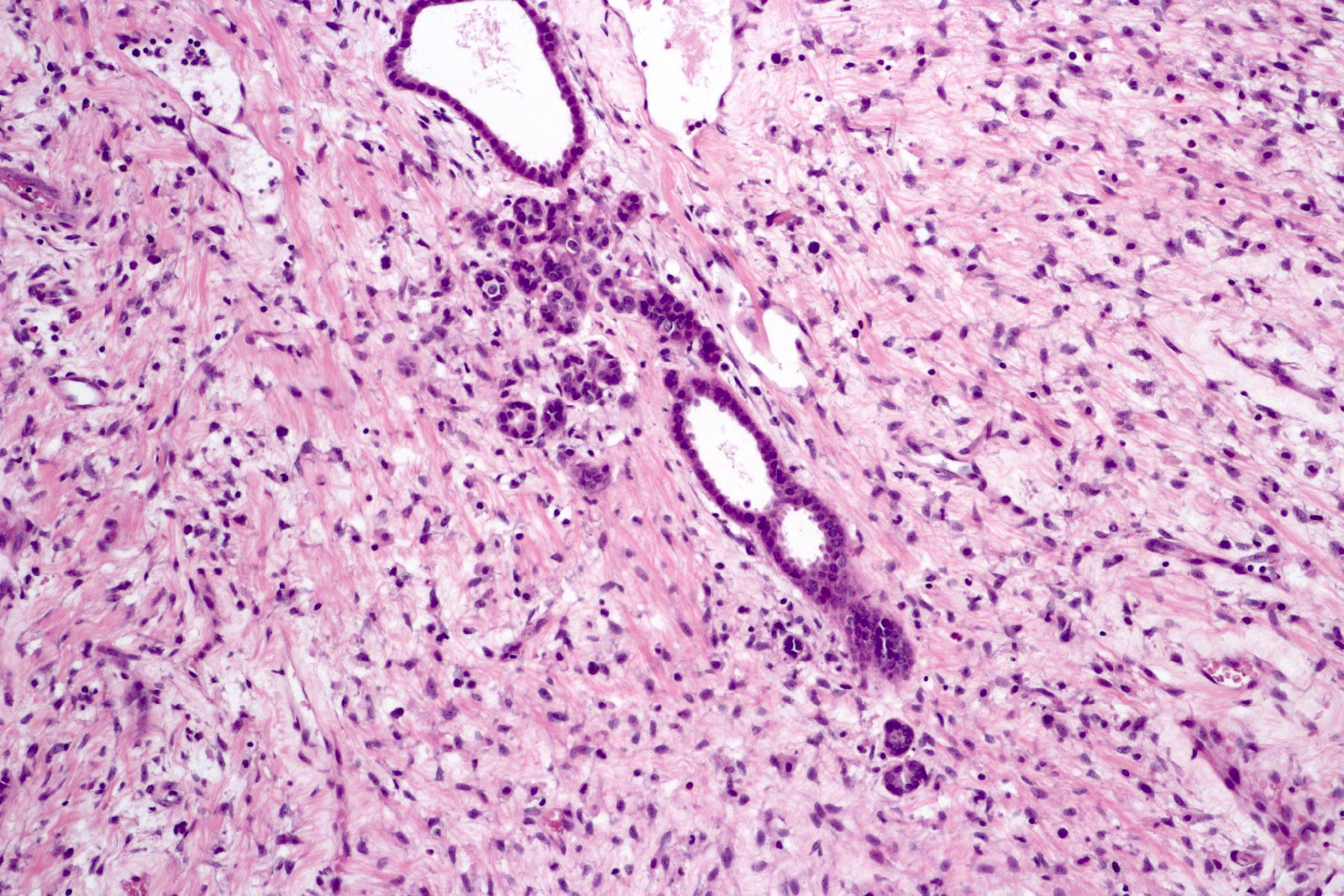
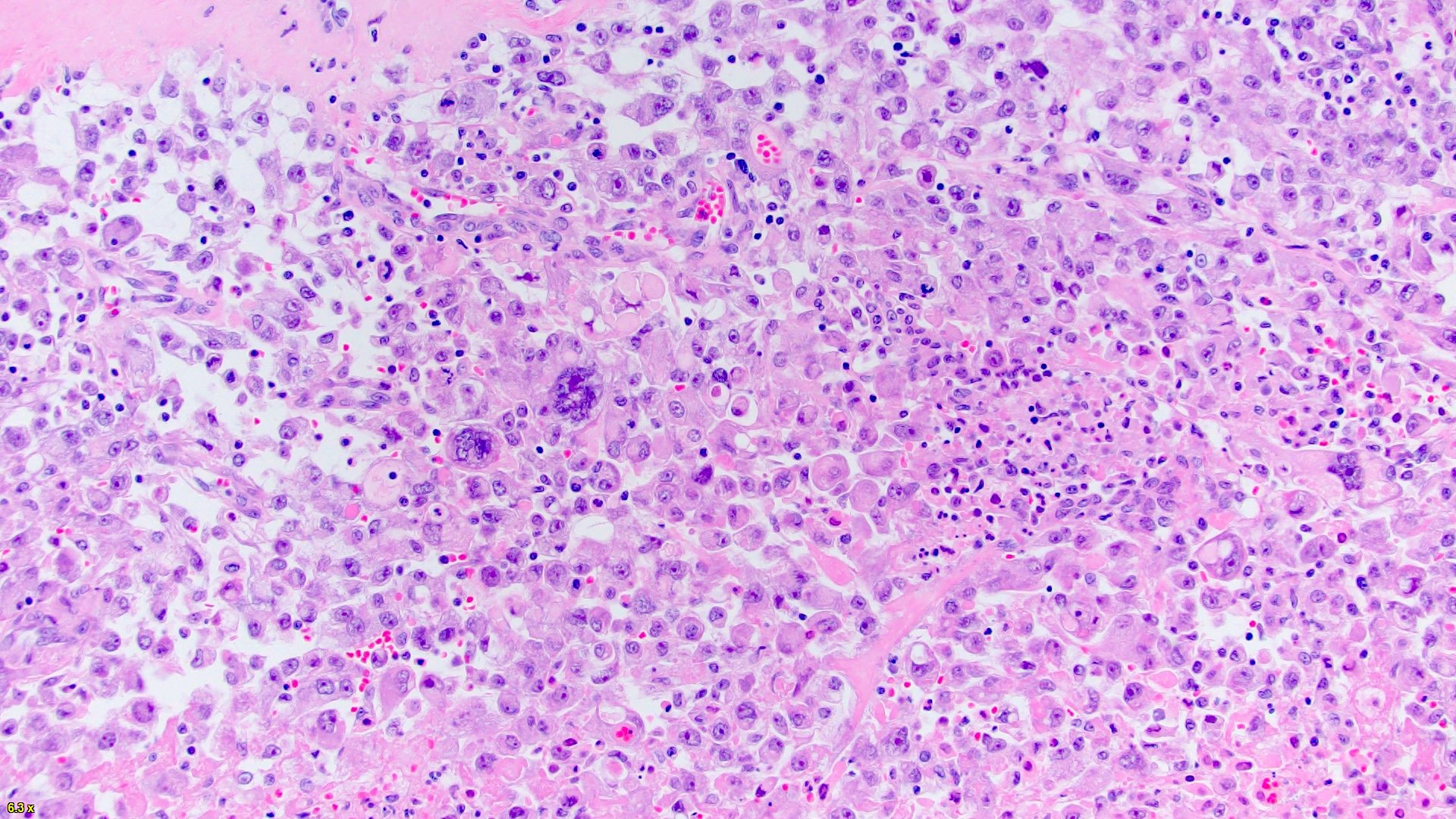
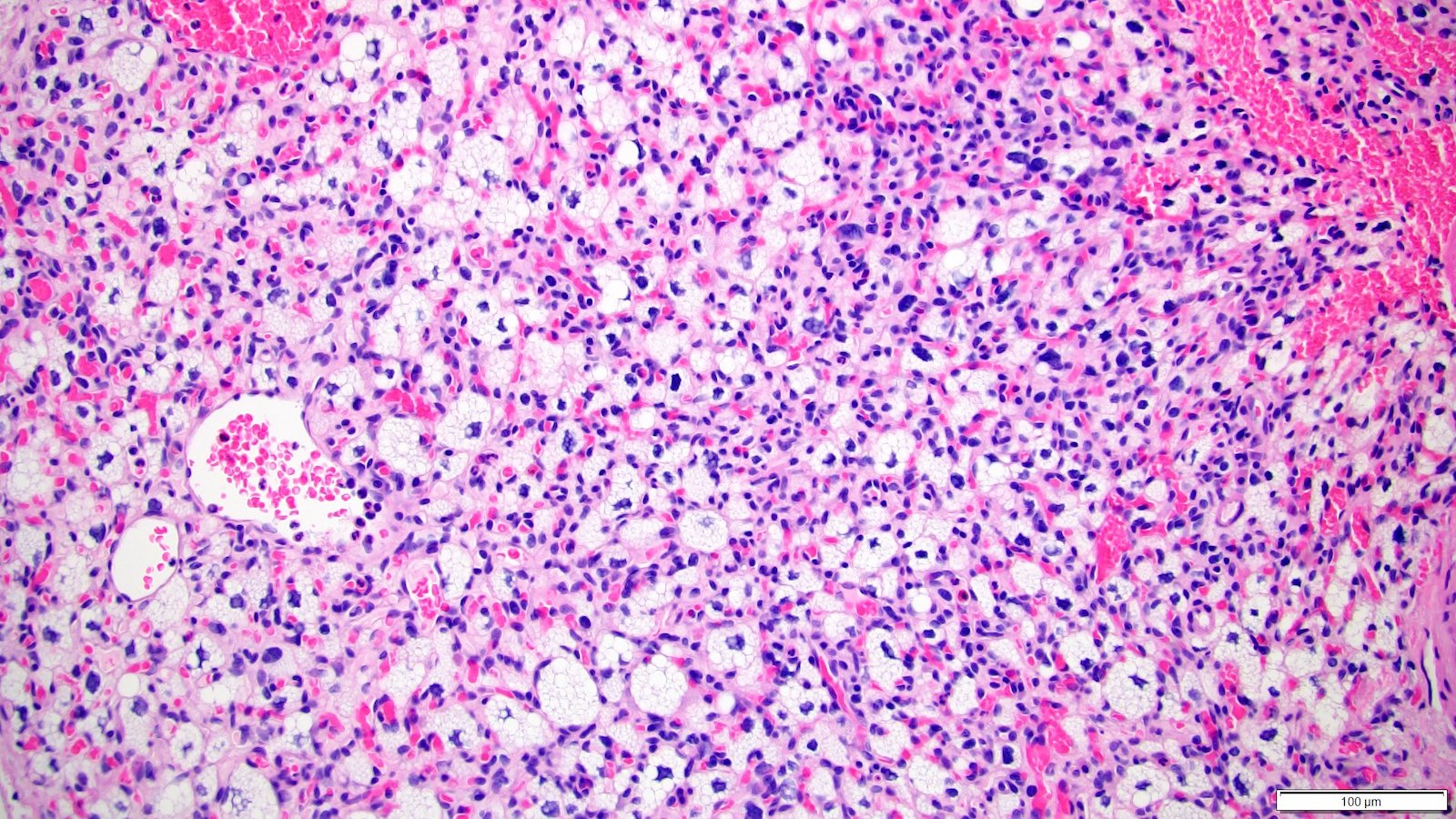
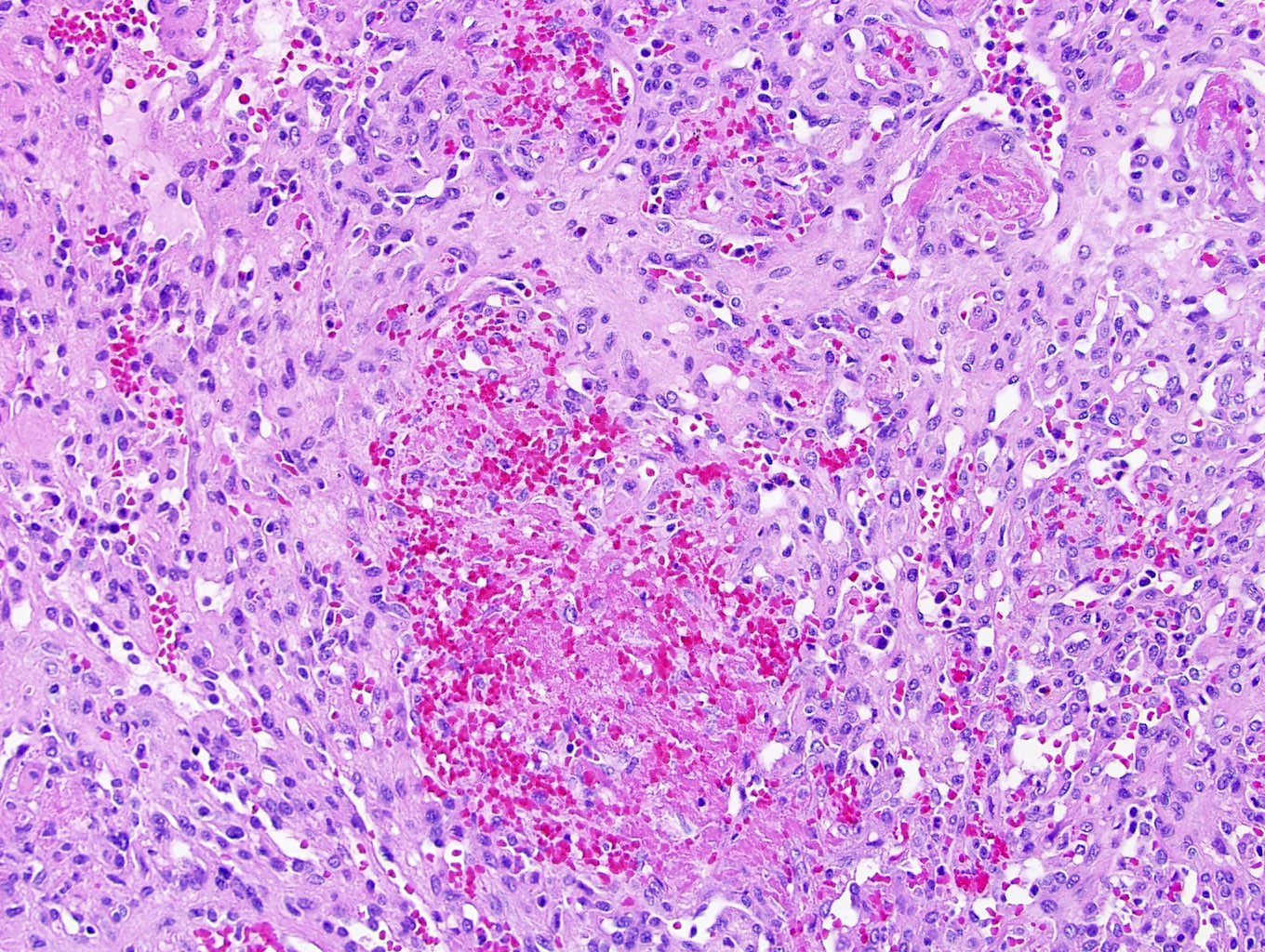
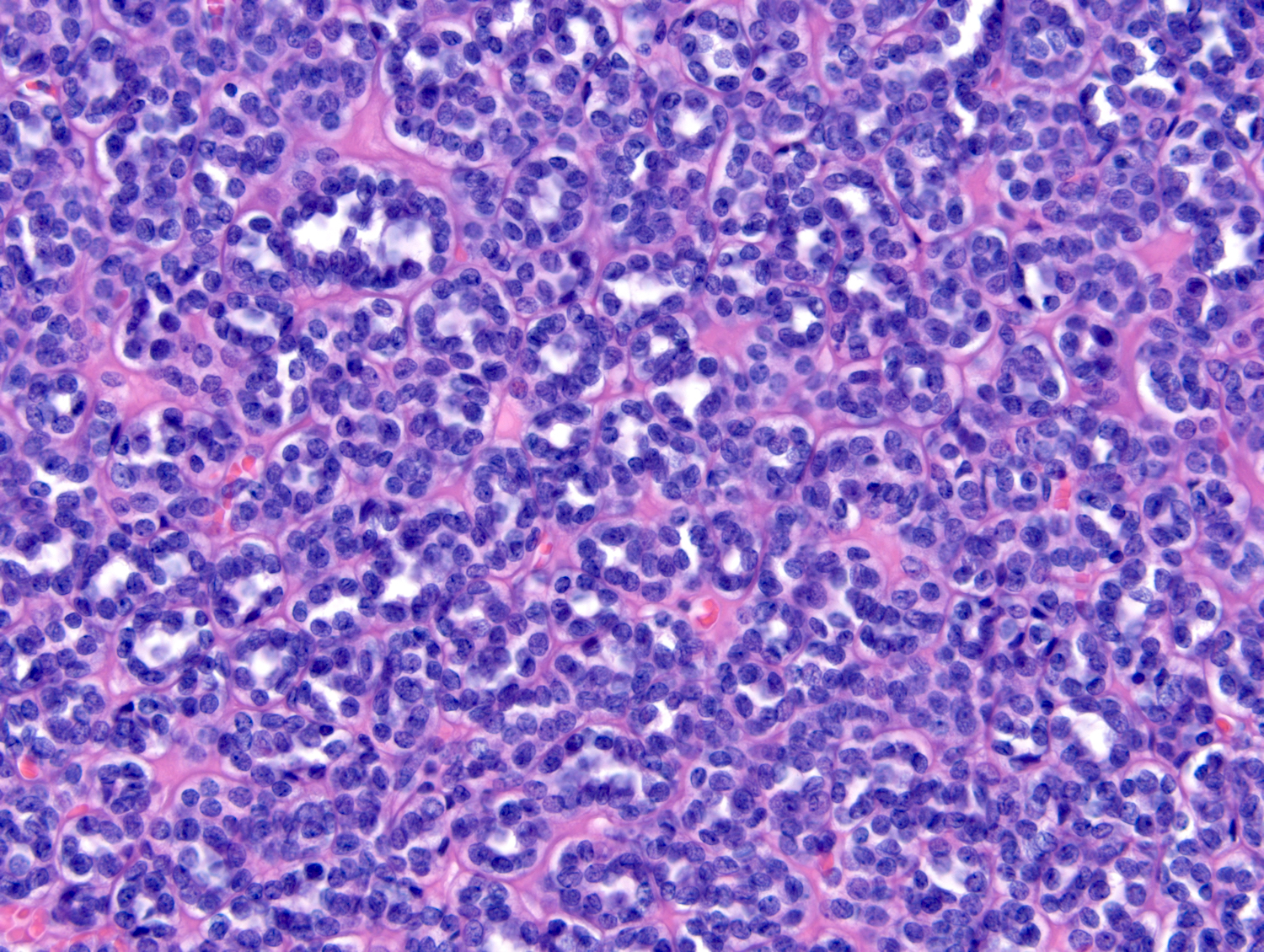
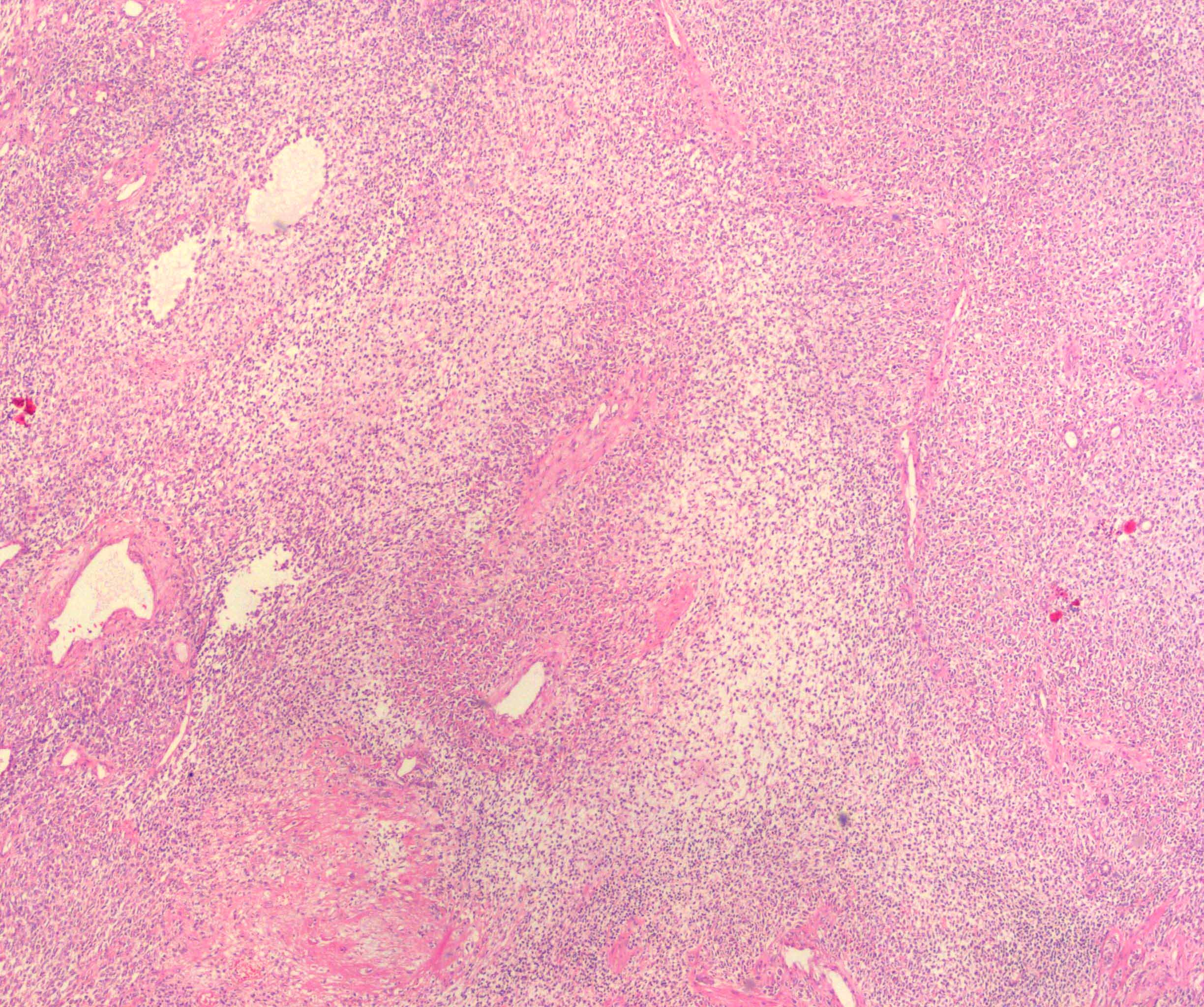
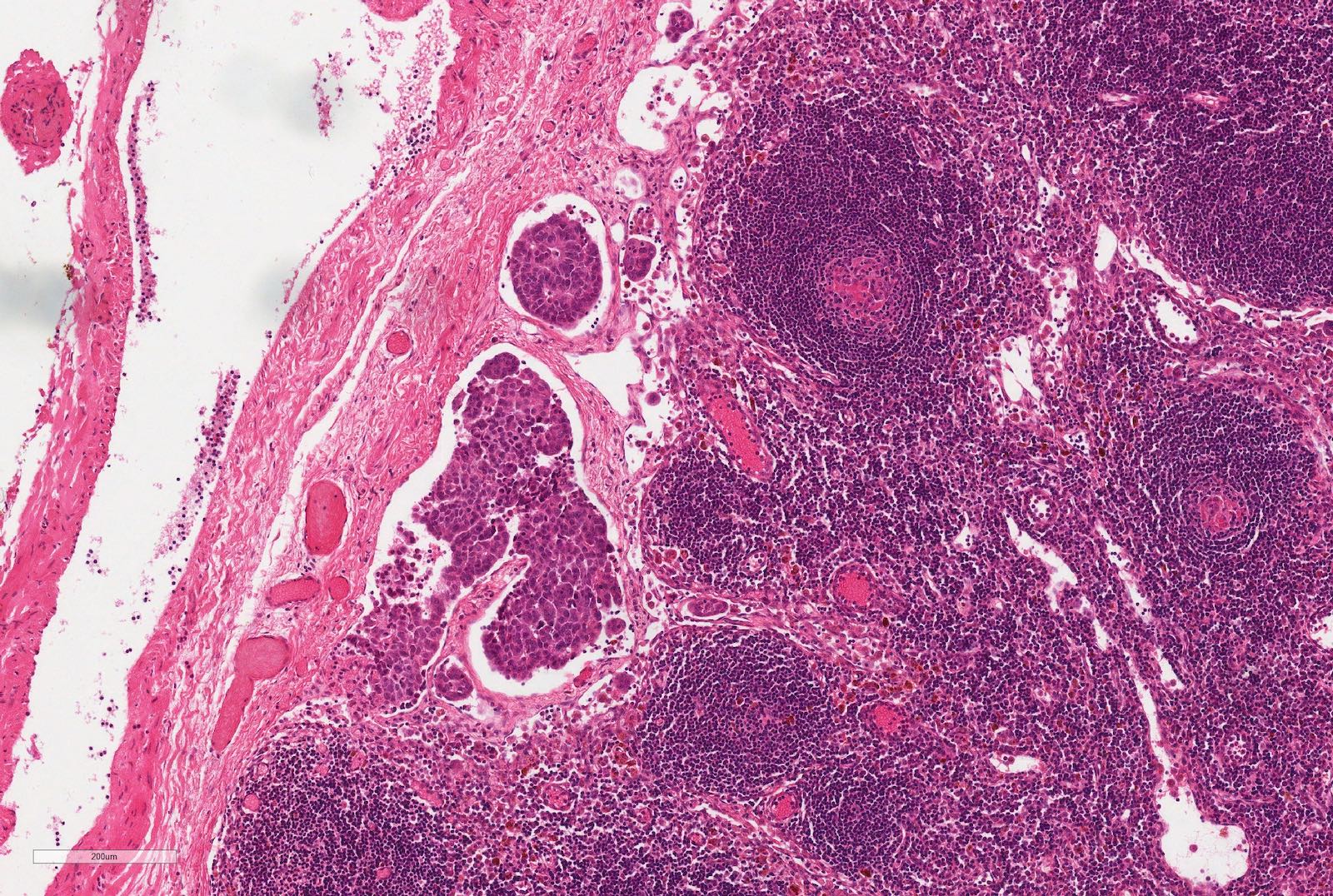
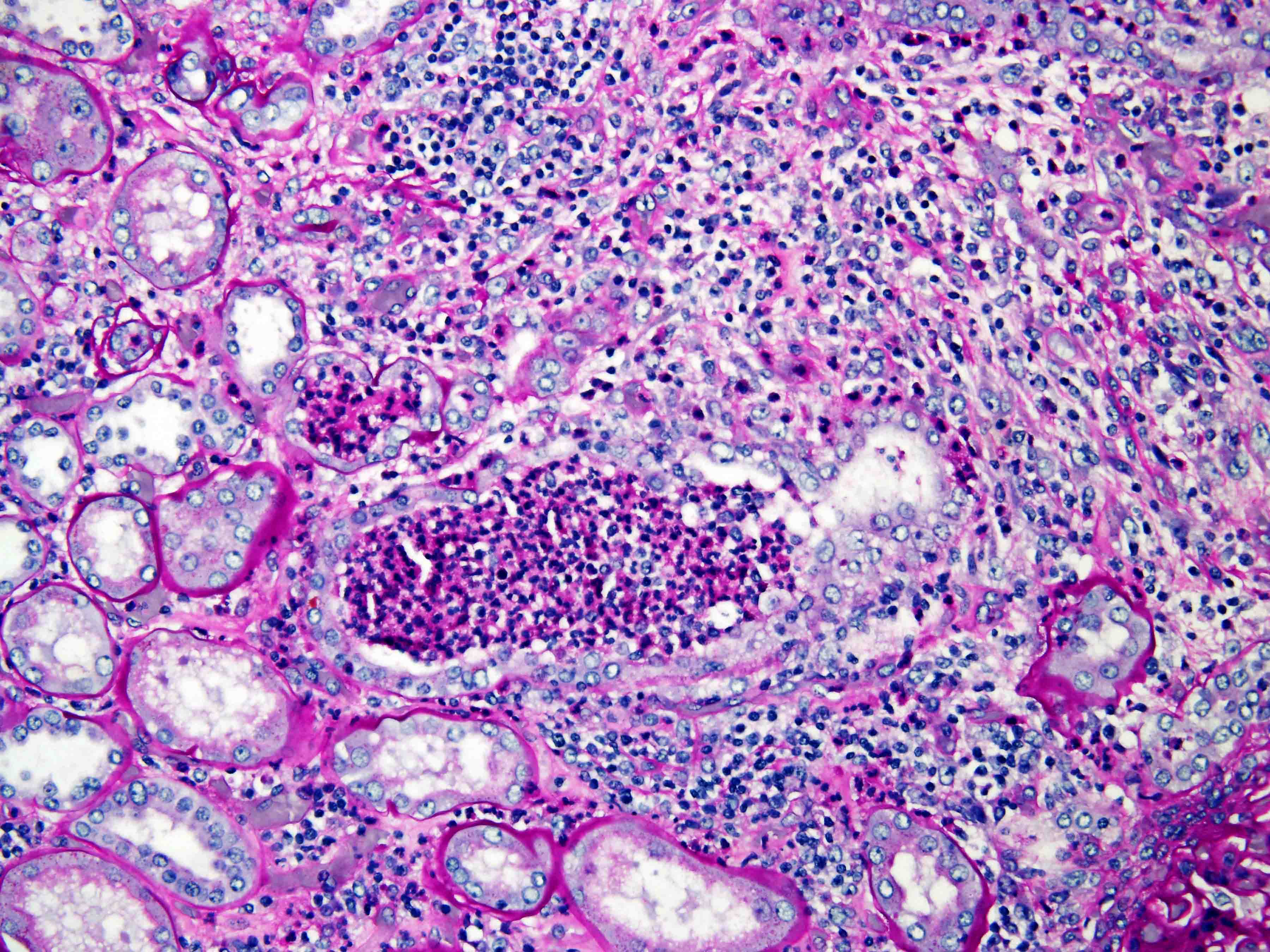
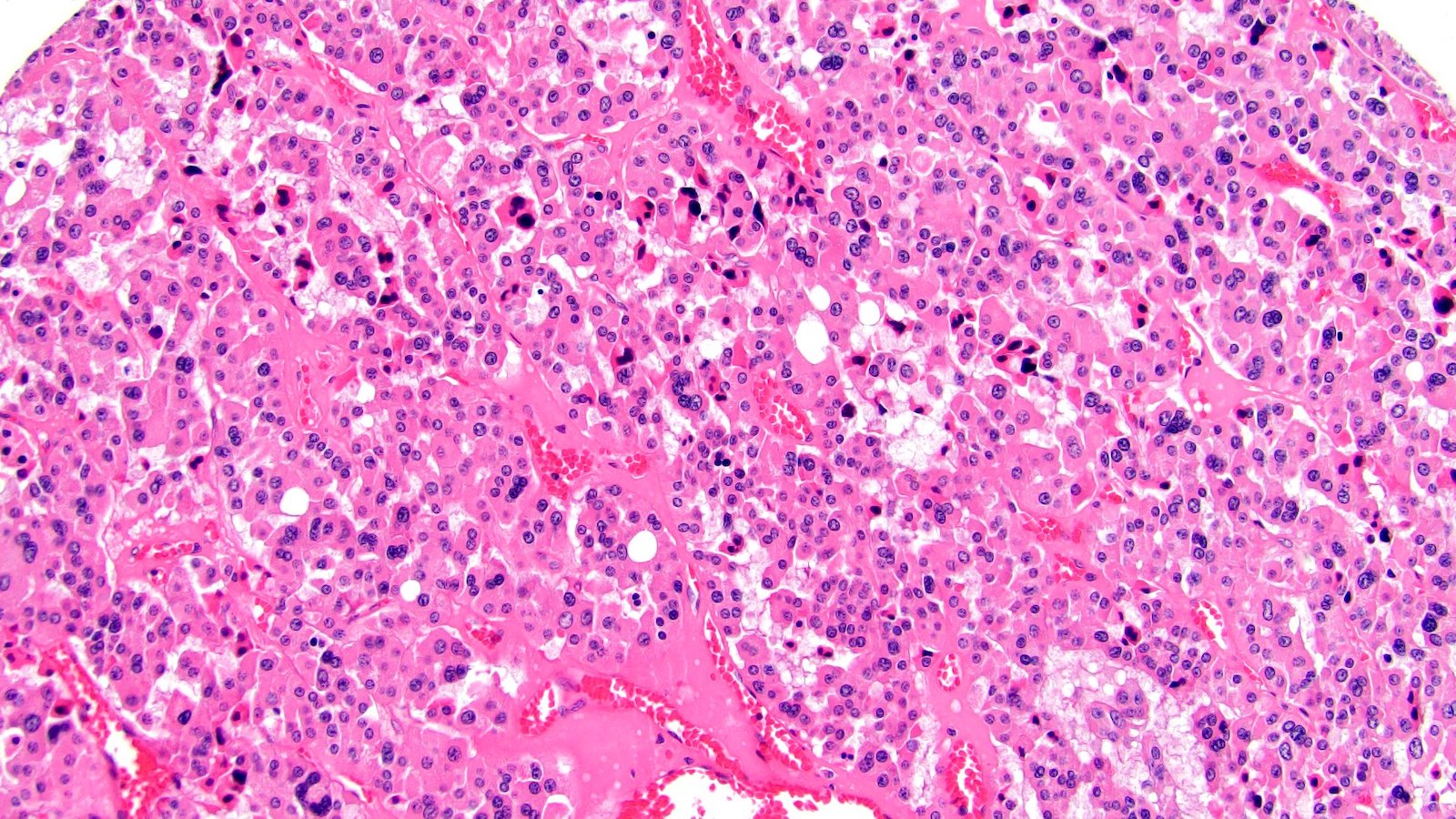
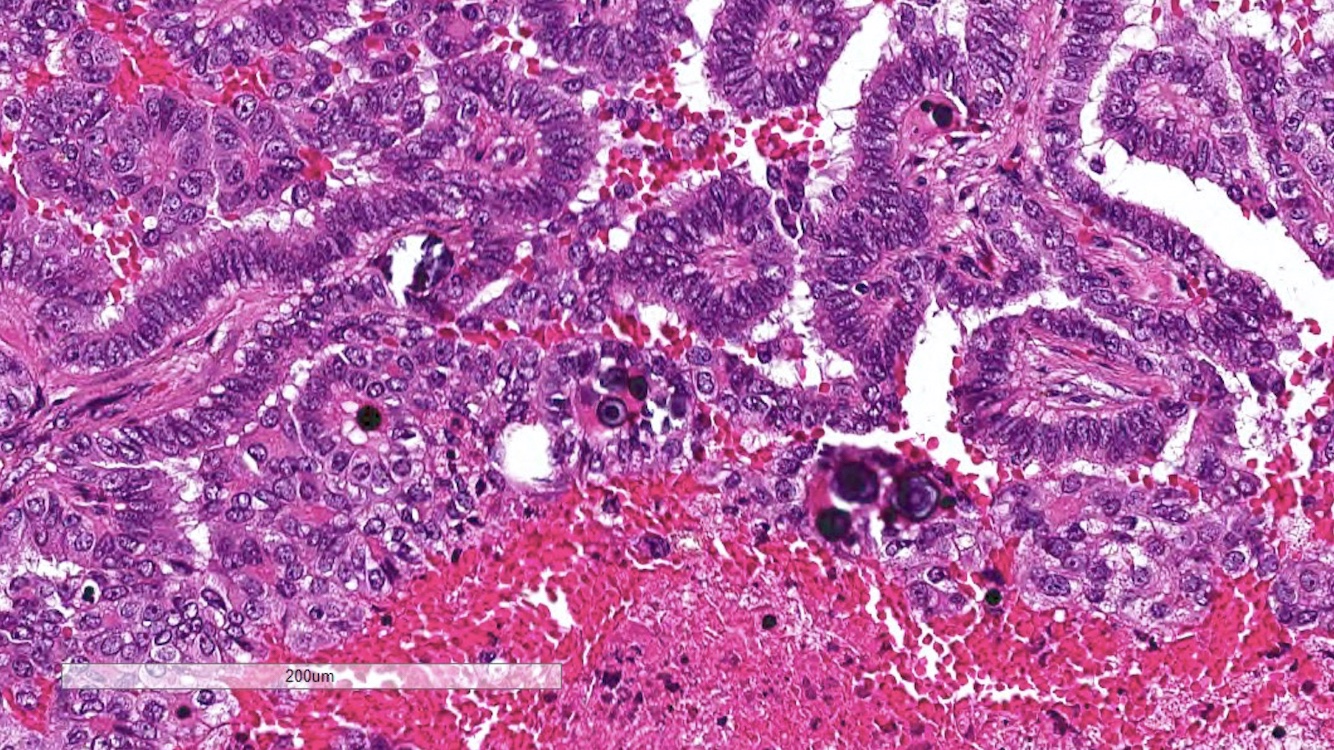
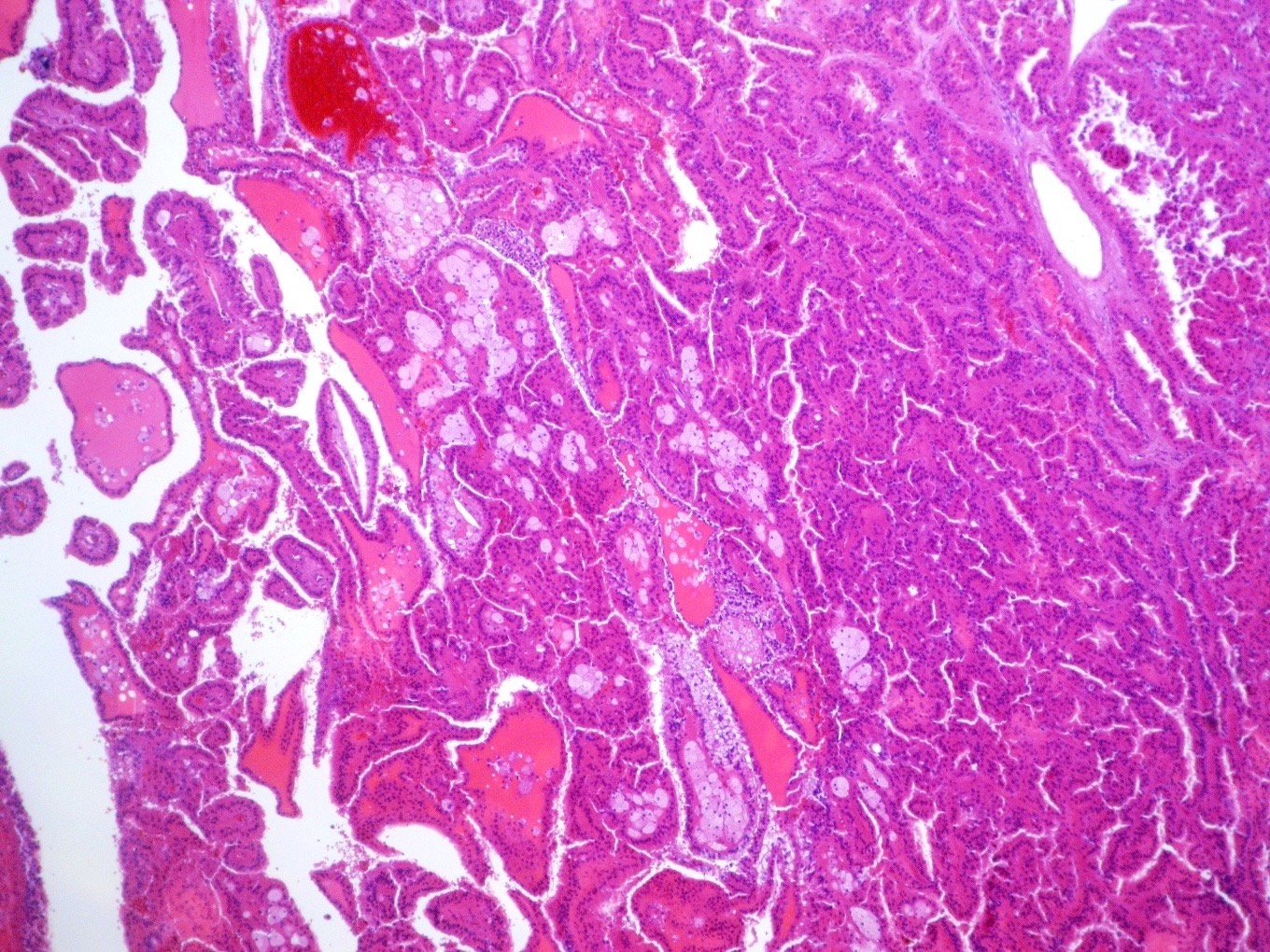
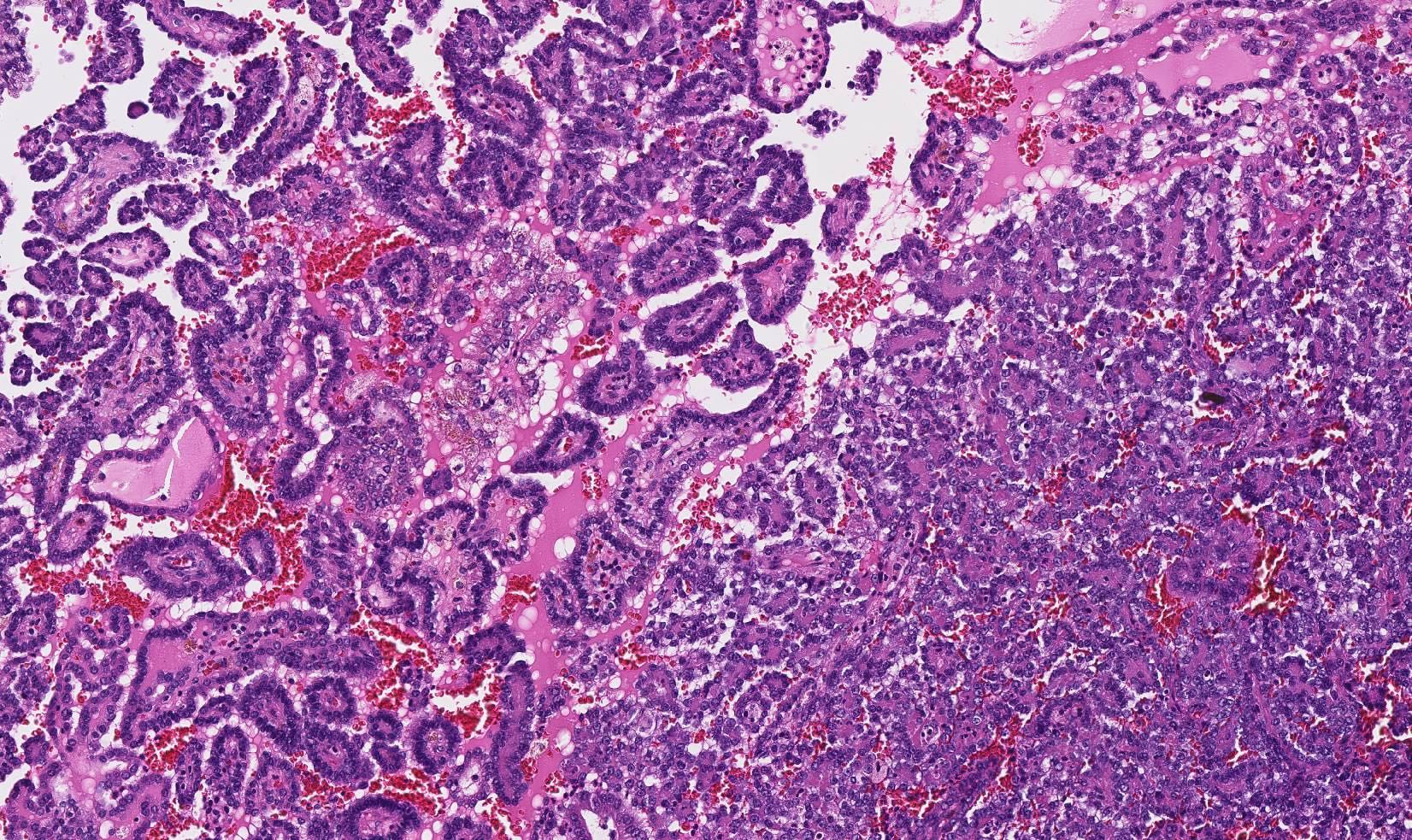
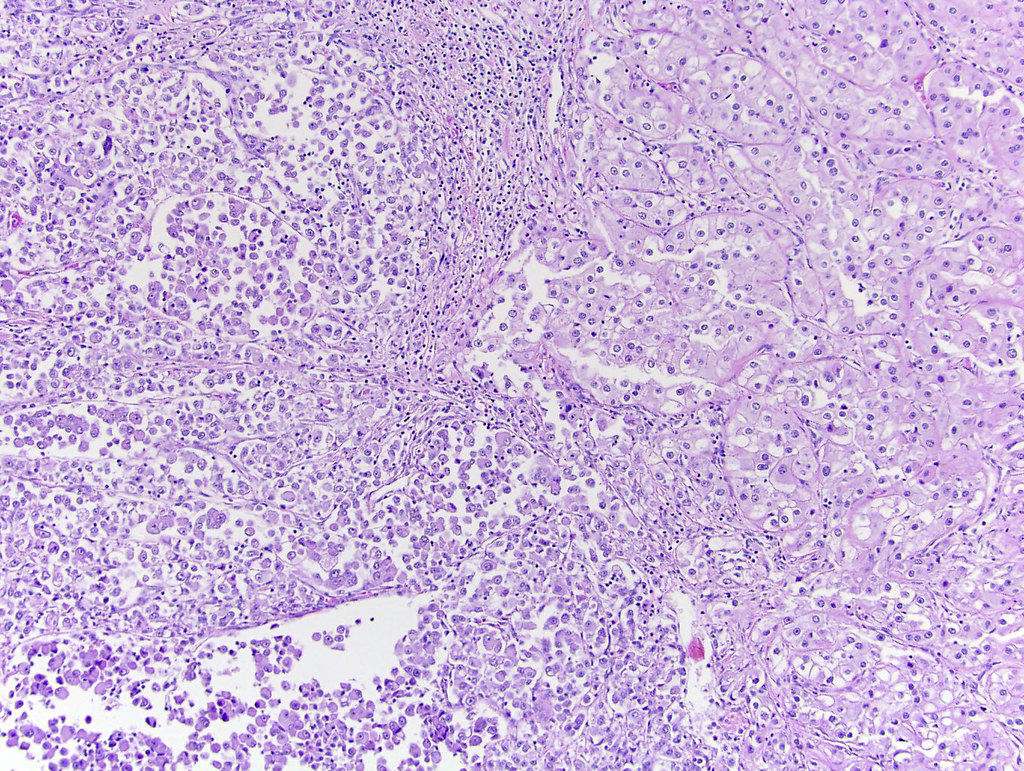
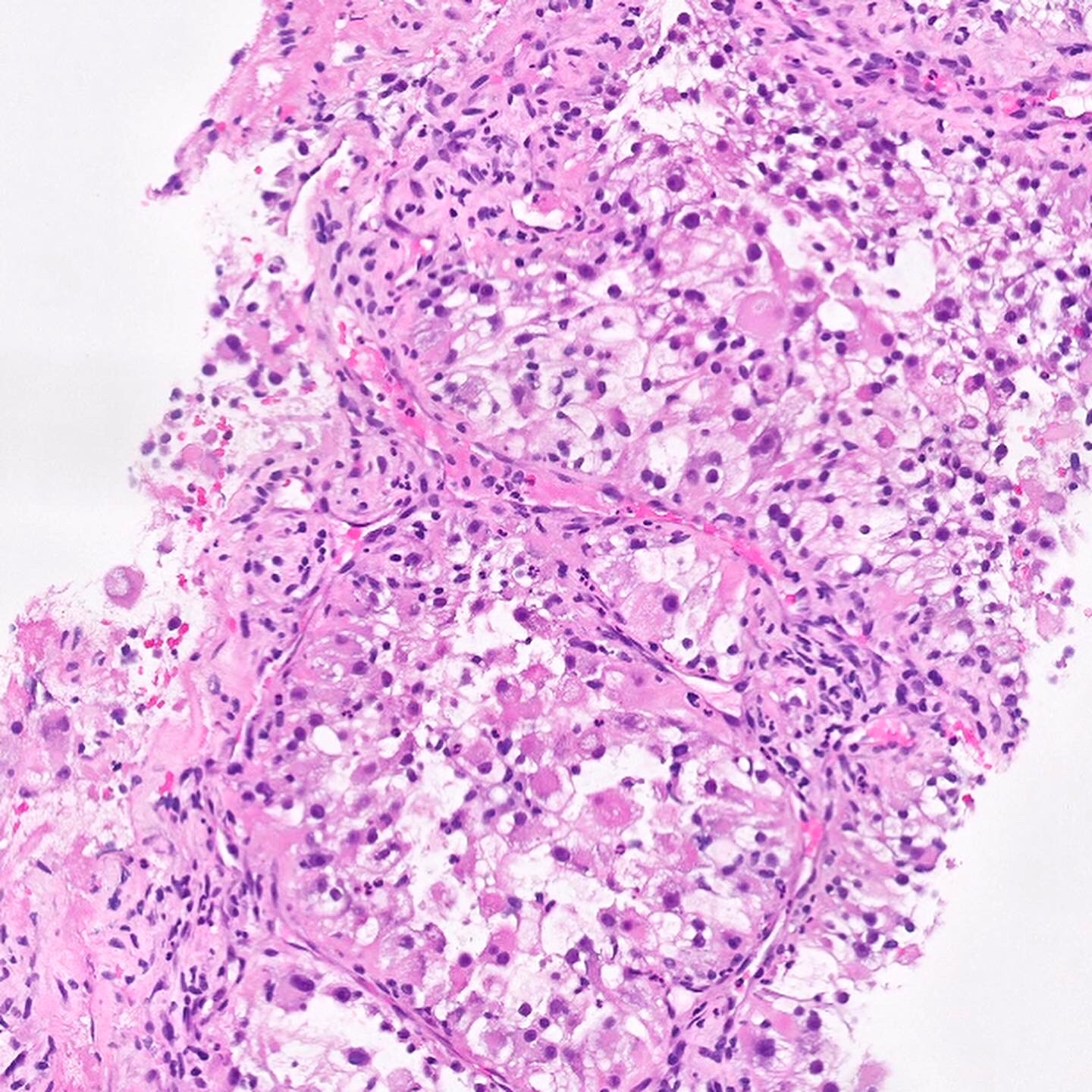
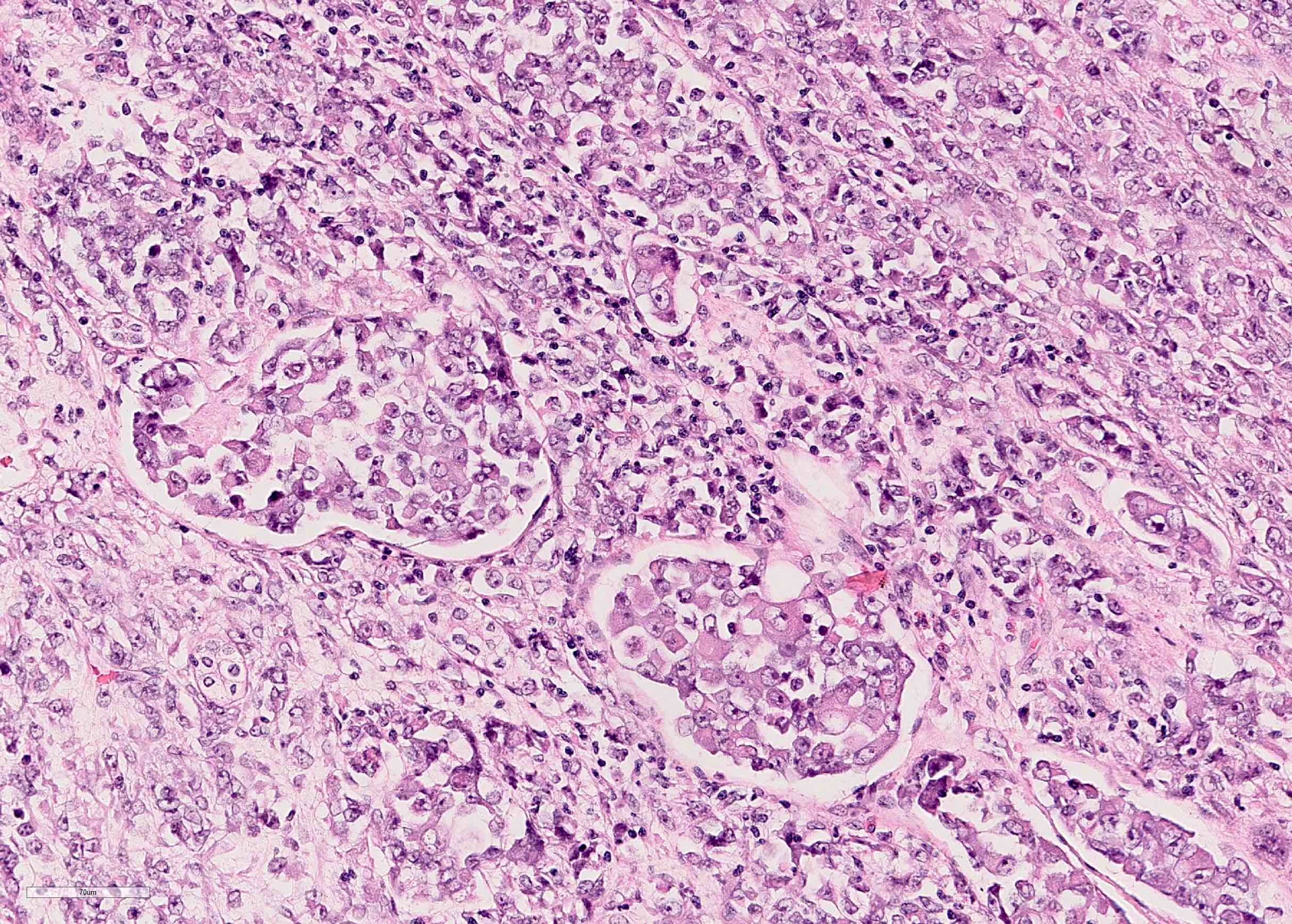
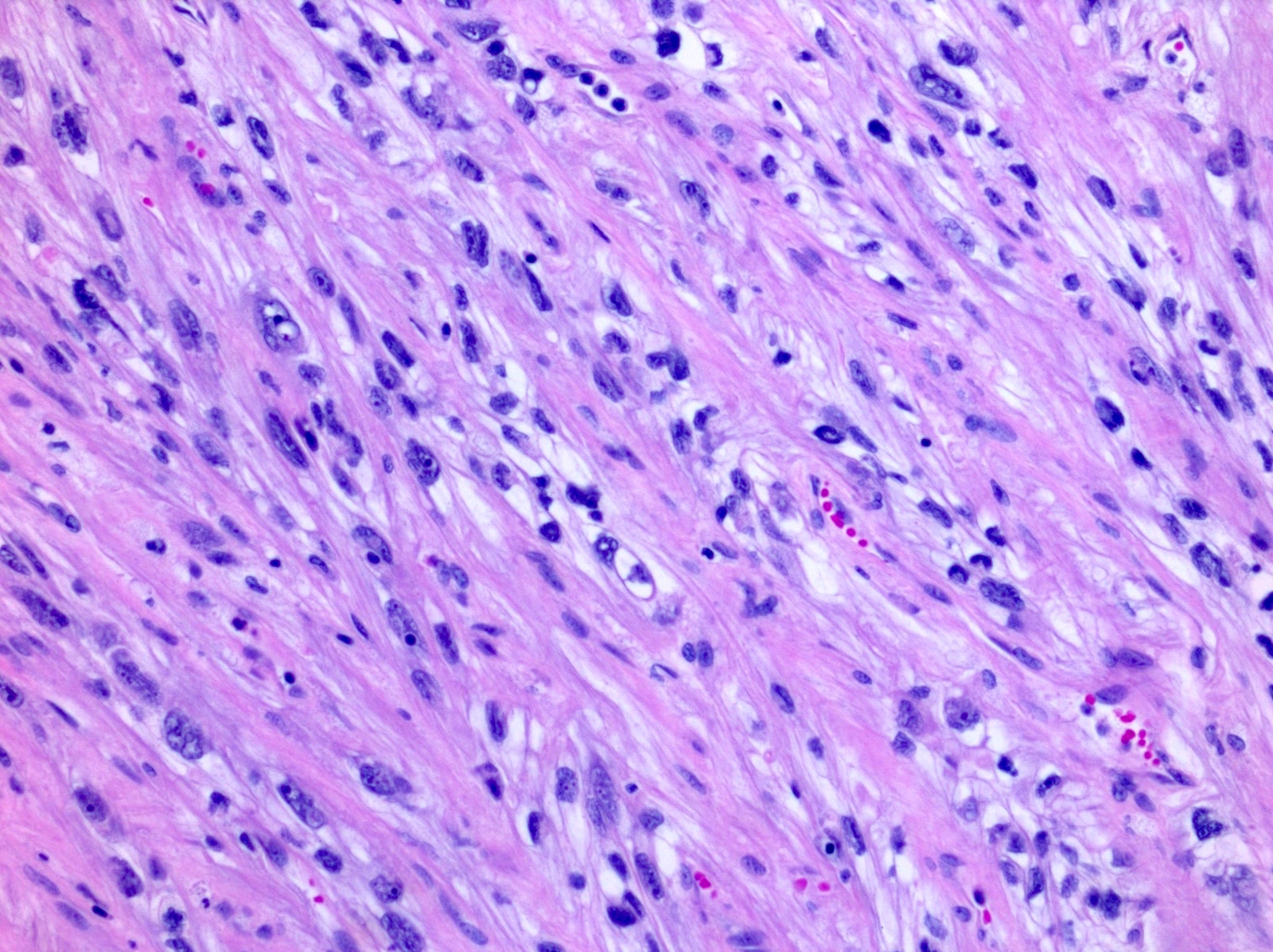
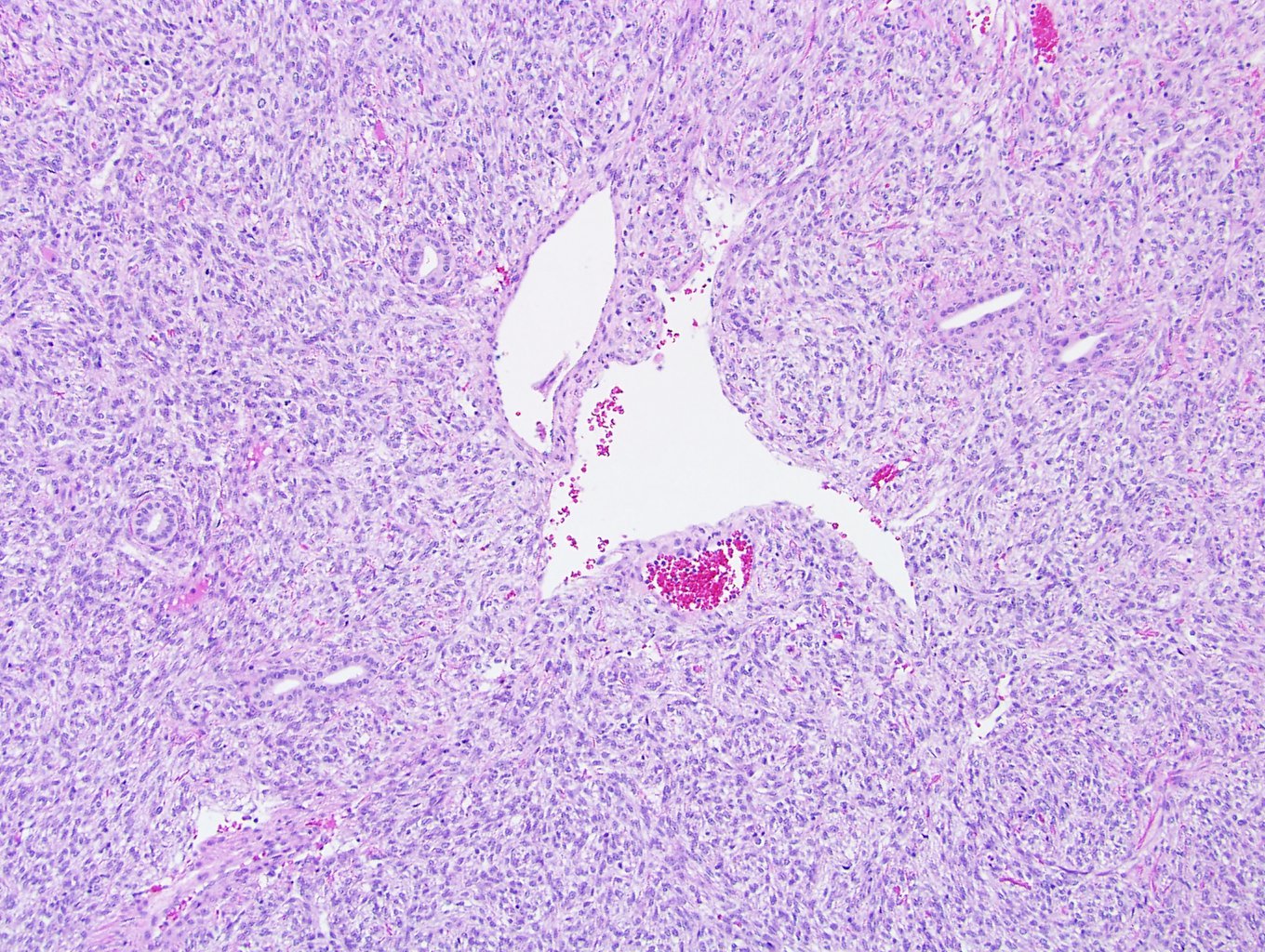
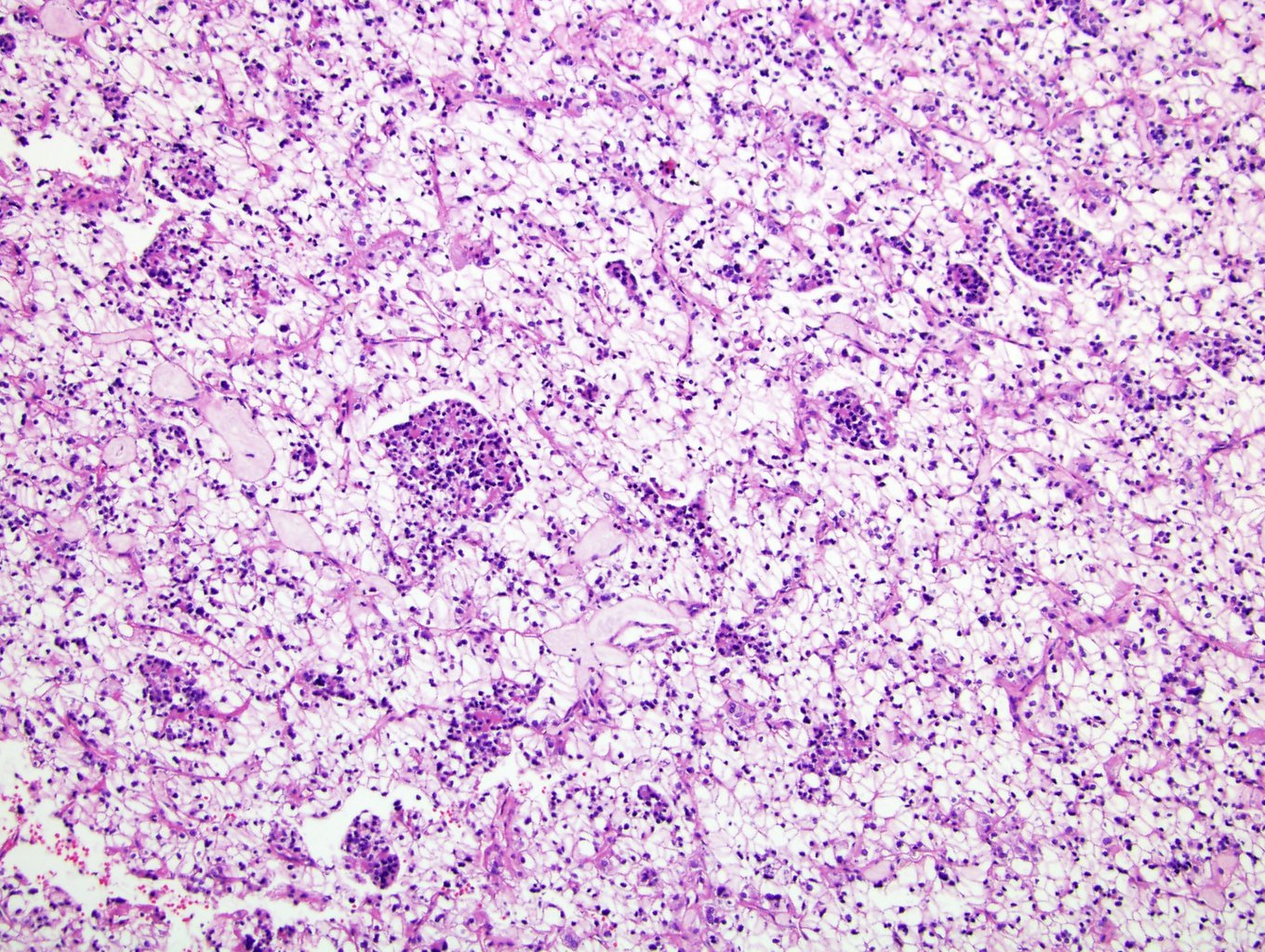
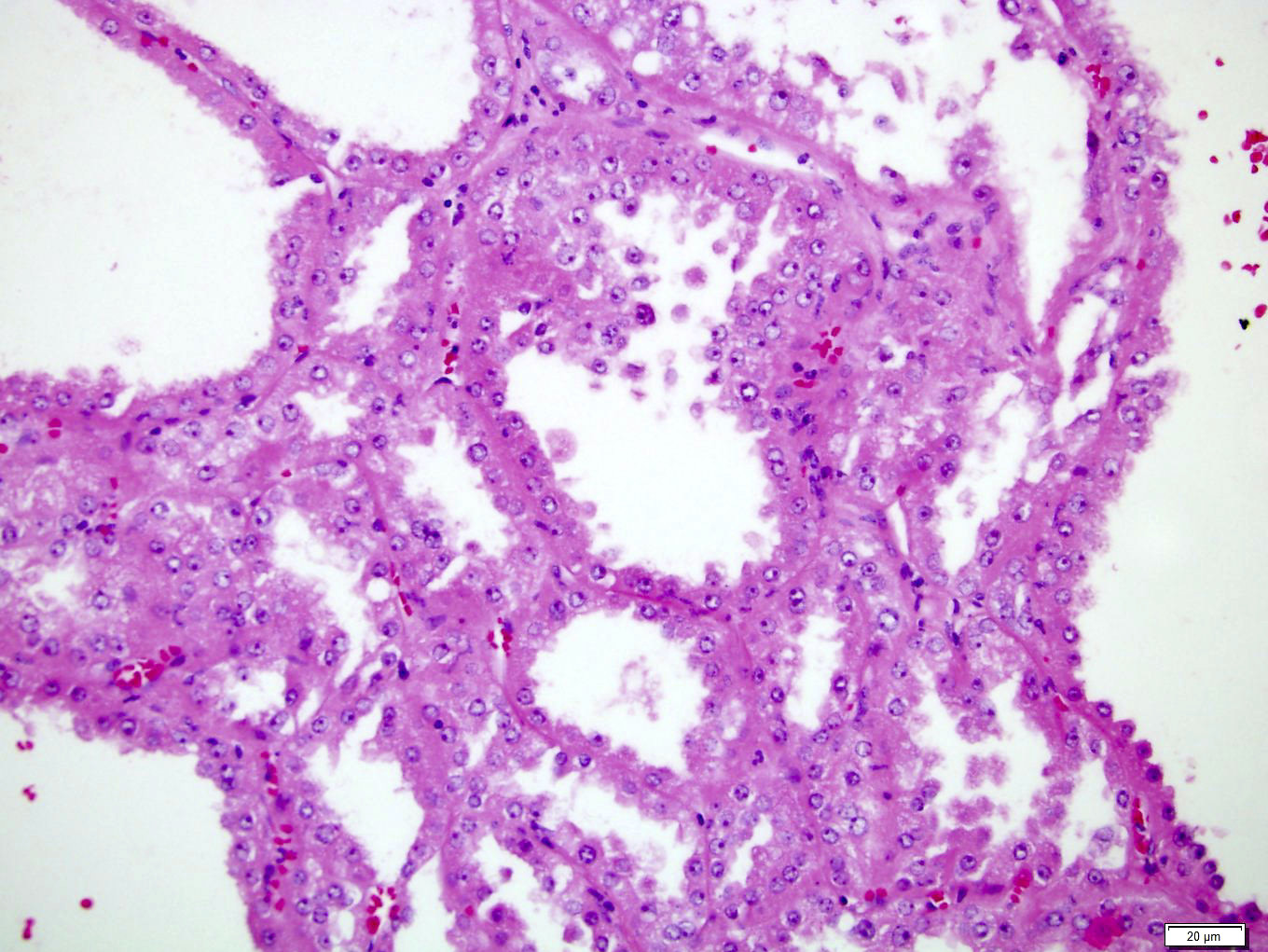
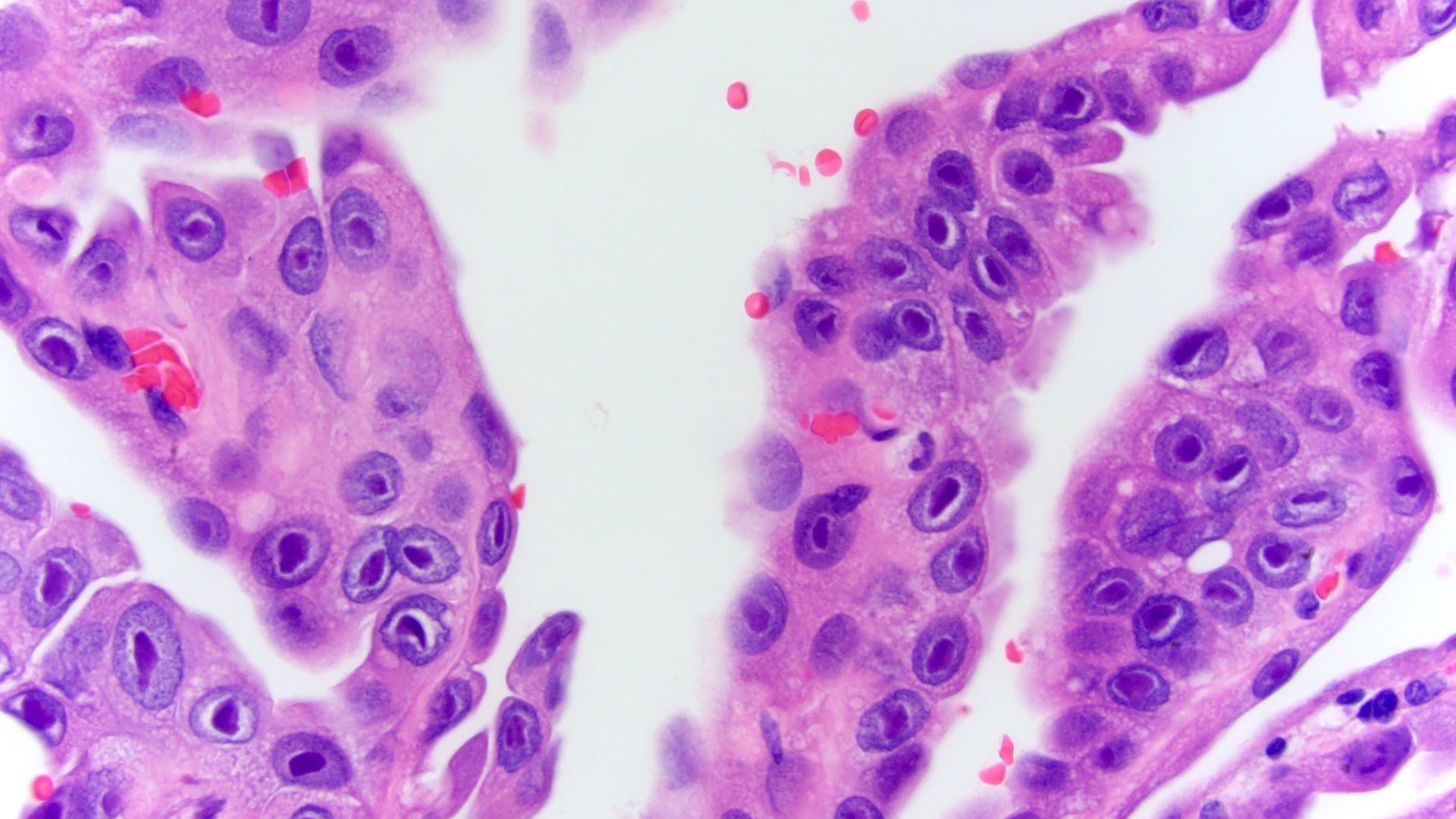
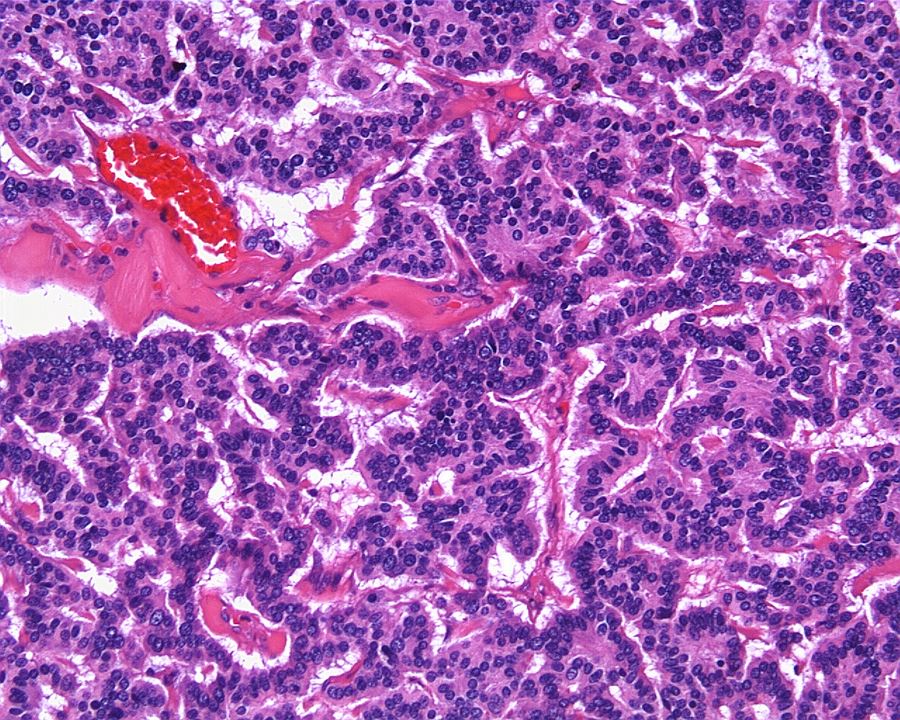
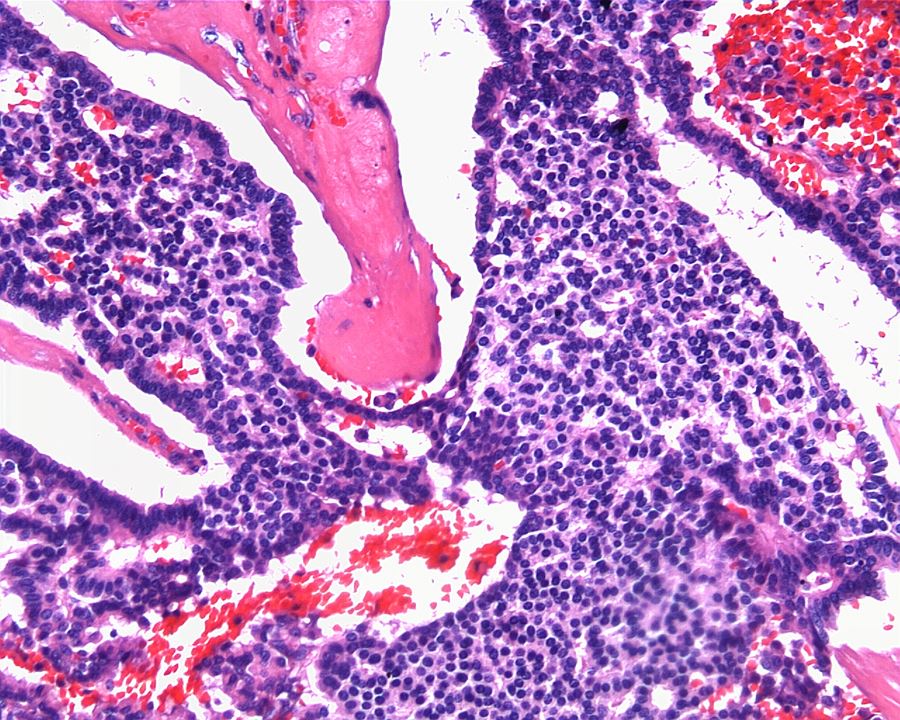
- In children, resembles renal medullary carcinoma: medulla centric with diffuse infiltrating growth, lymphoplasmacytic infiltrate, large polygonal discohesive or spindled cells, cytoplasmic vacuoles and vesicular nuclei
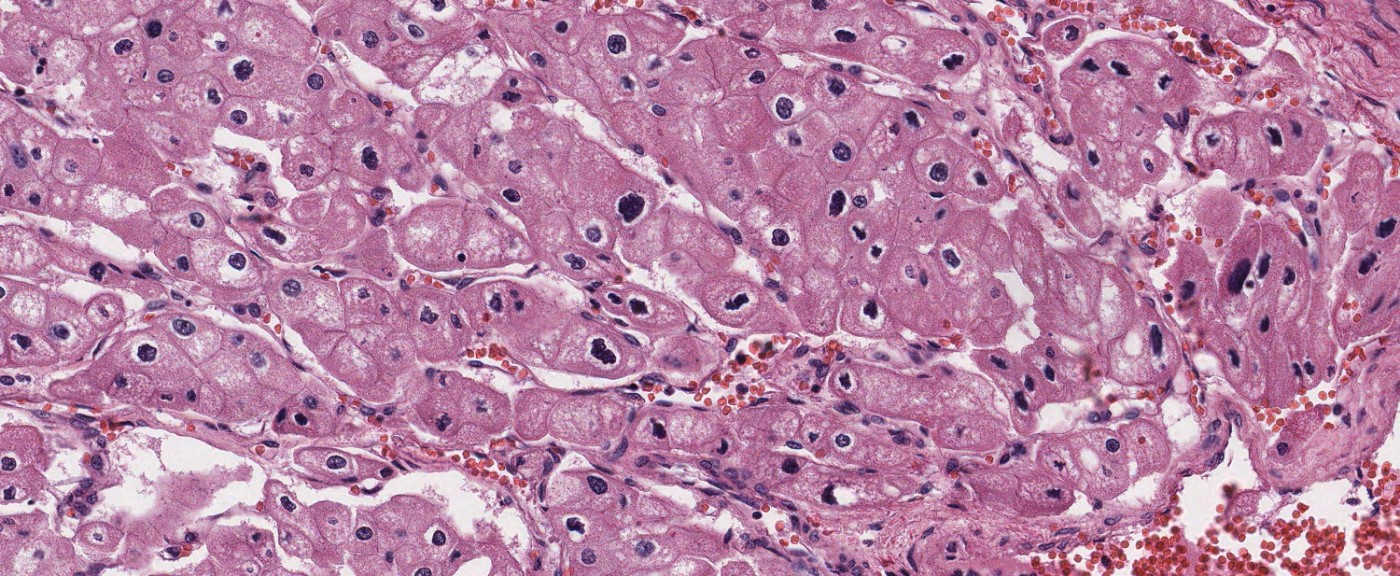
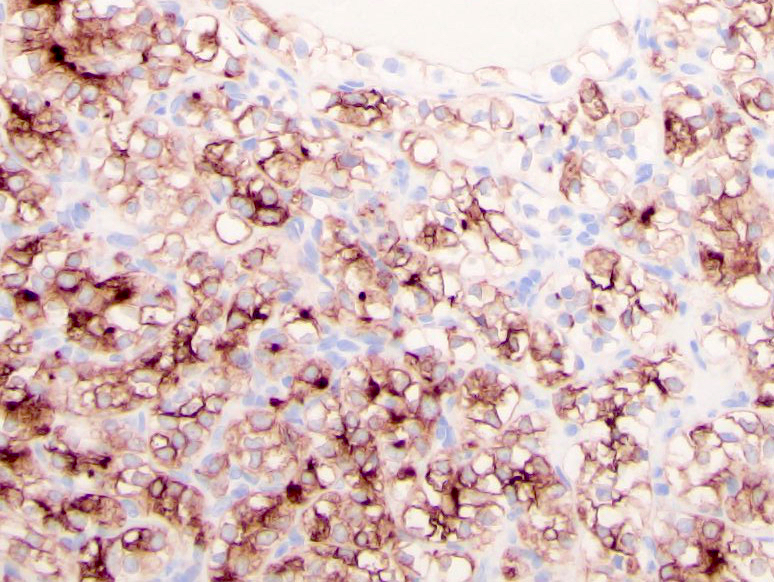
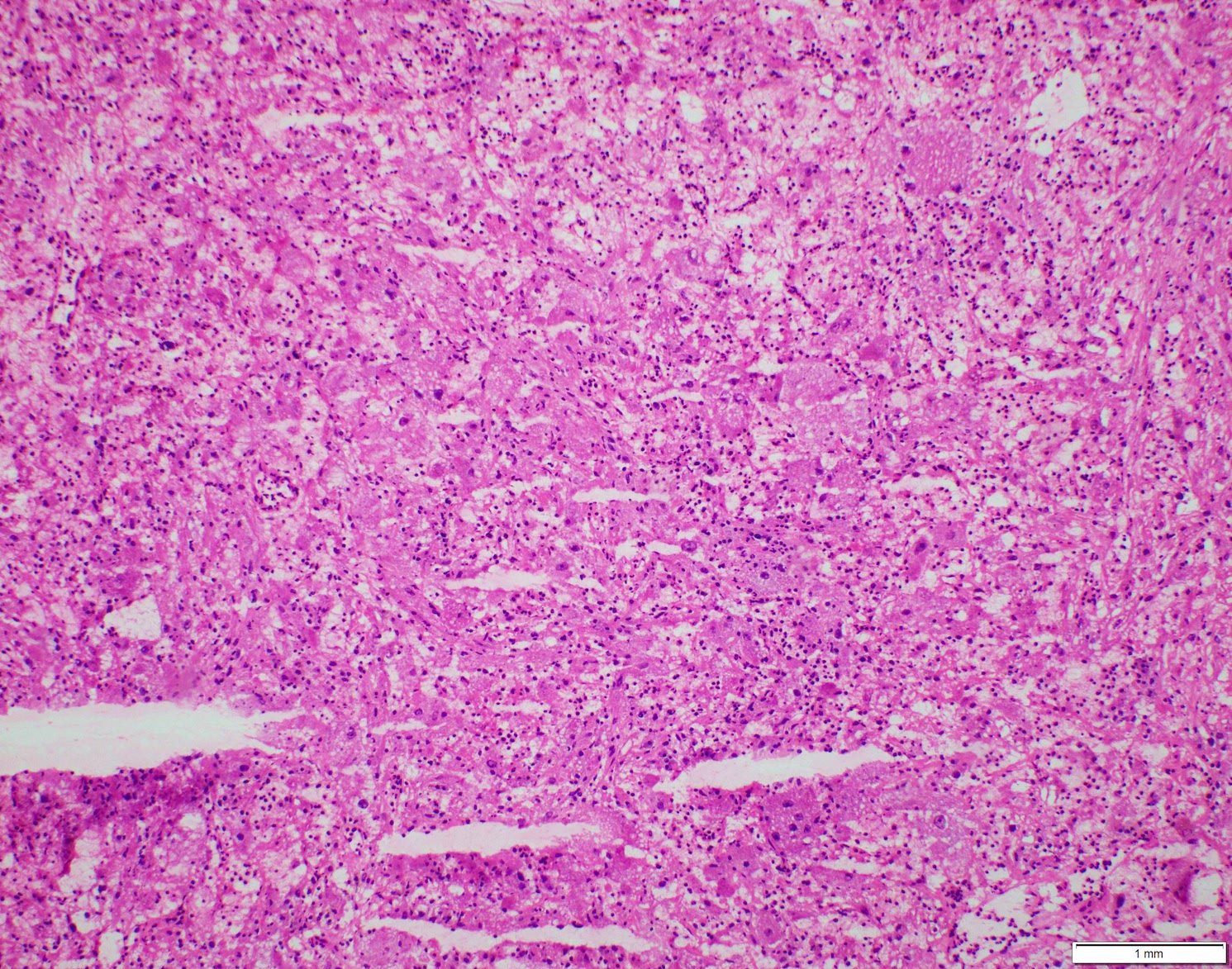
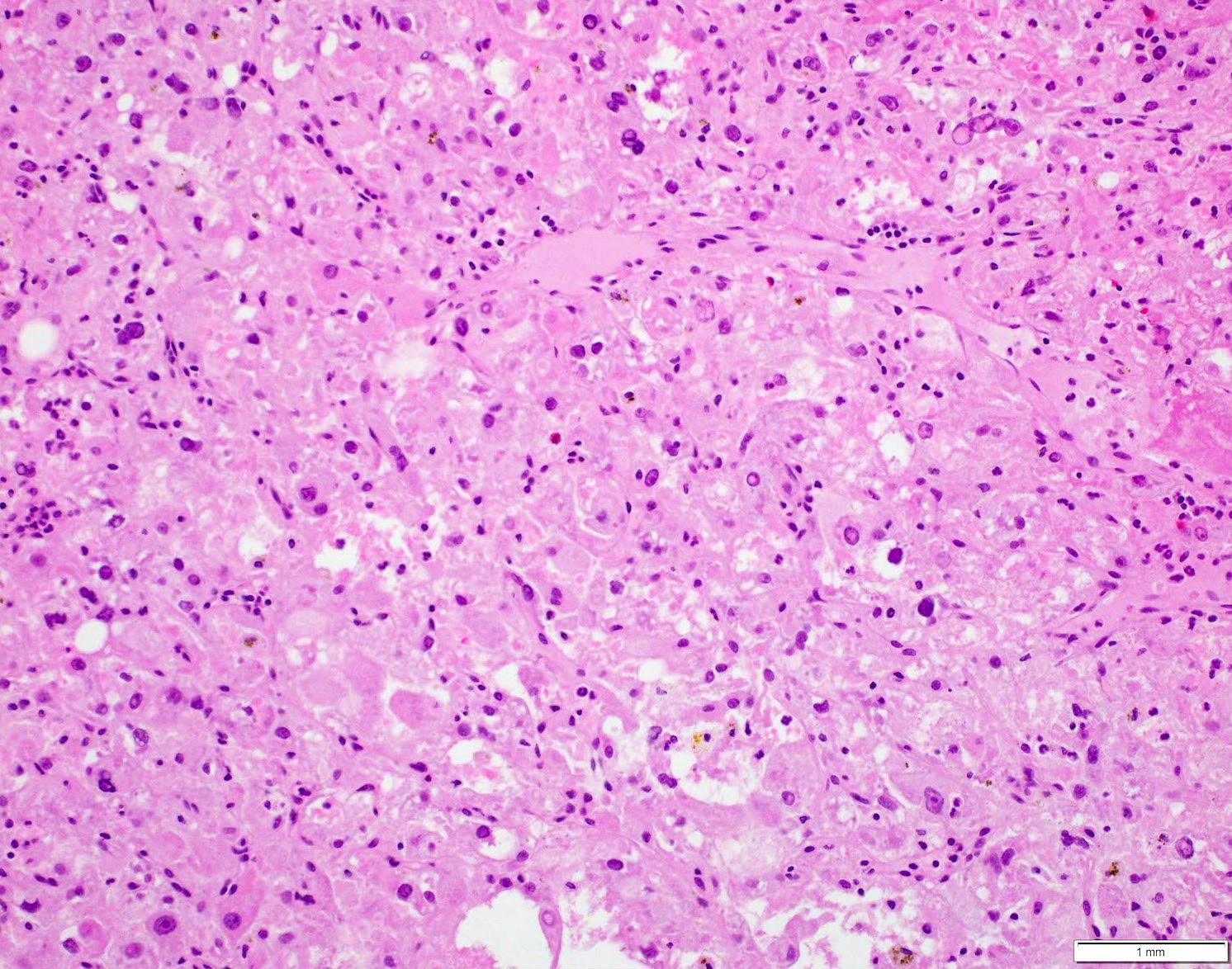
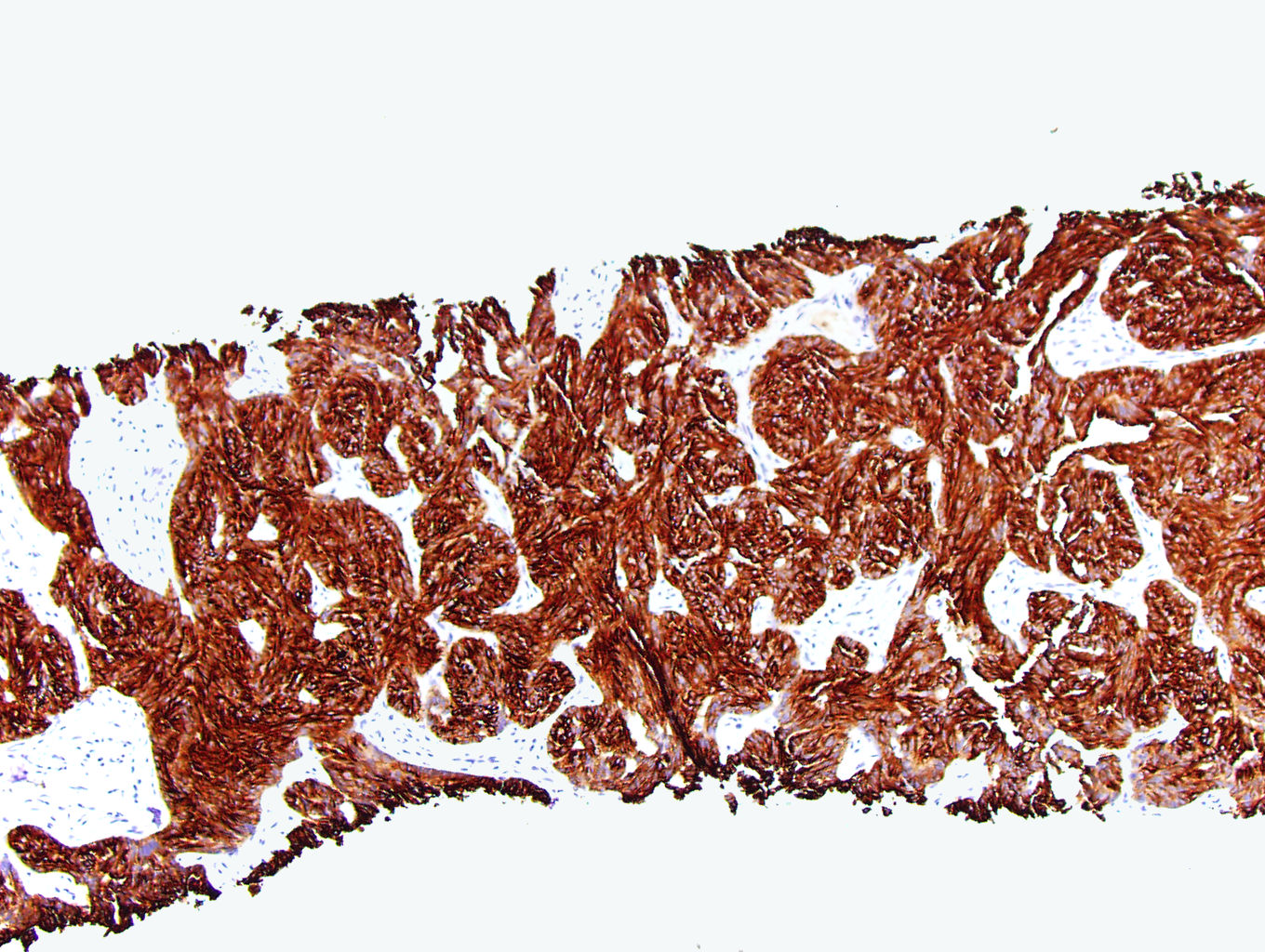
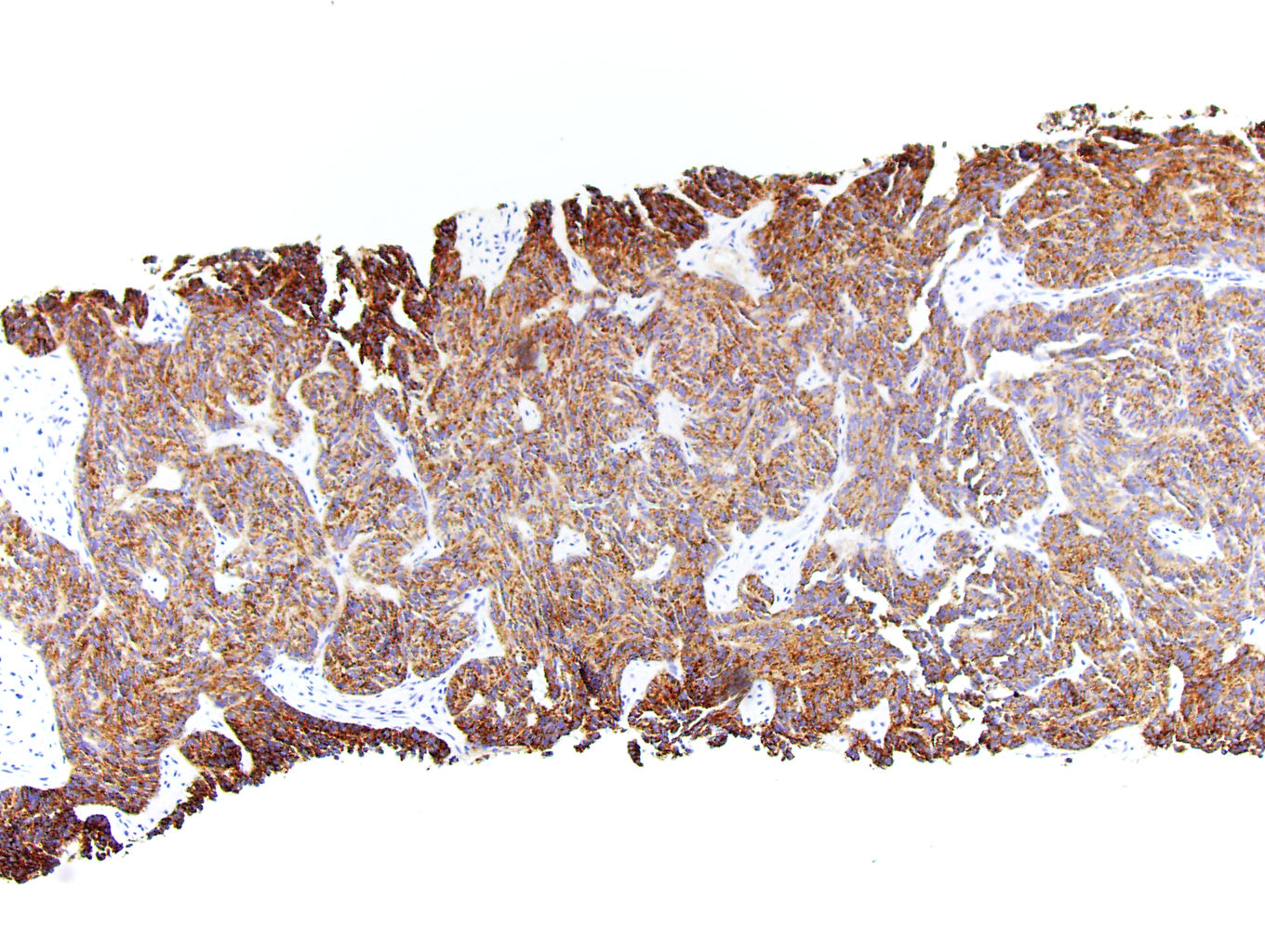
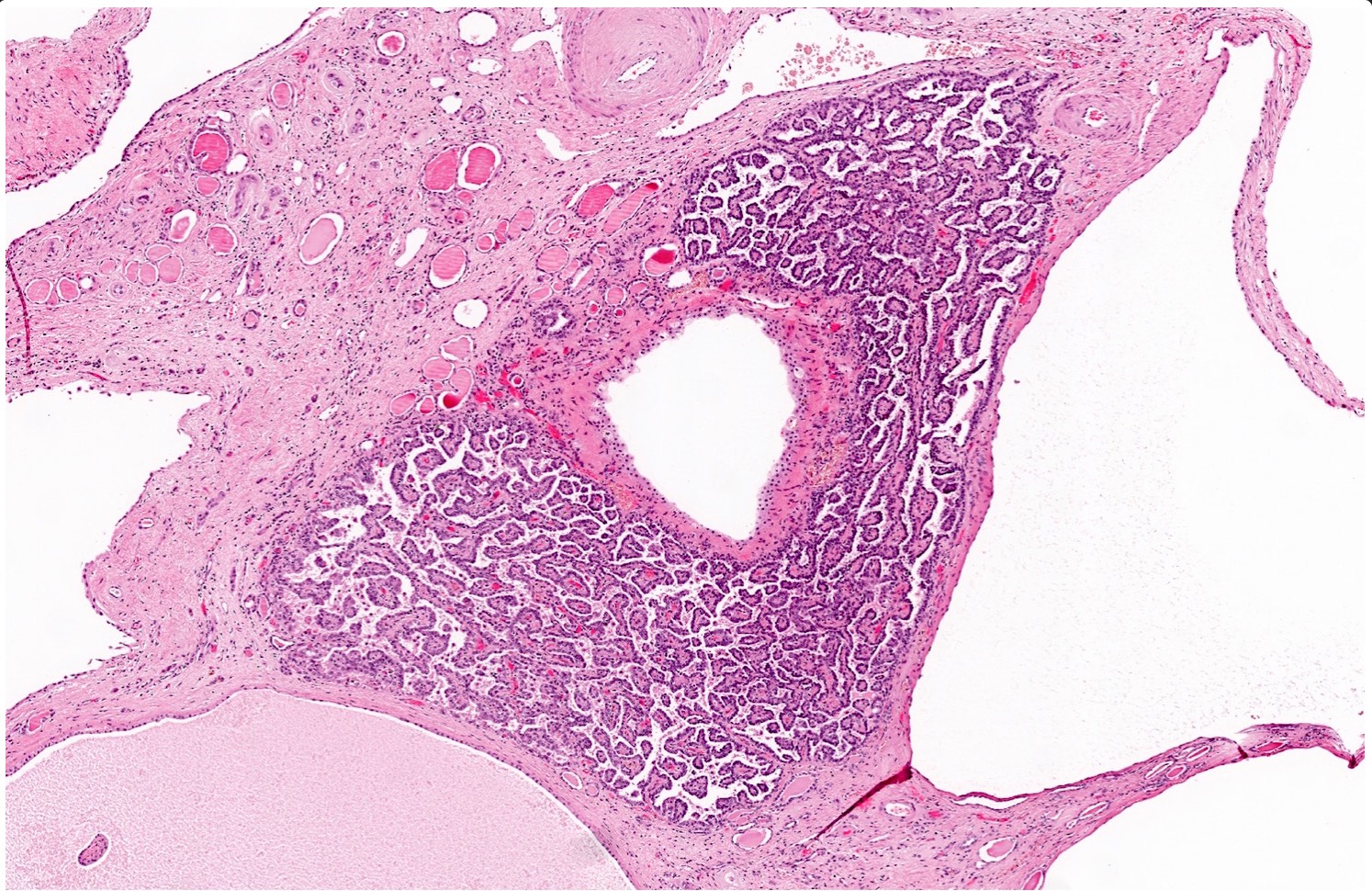
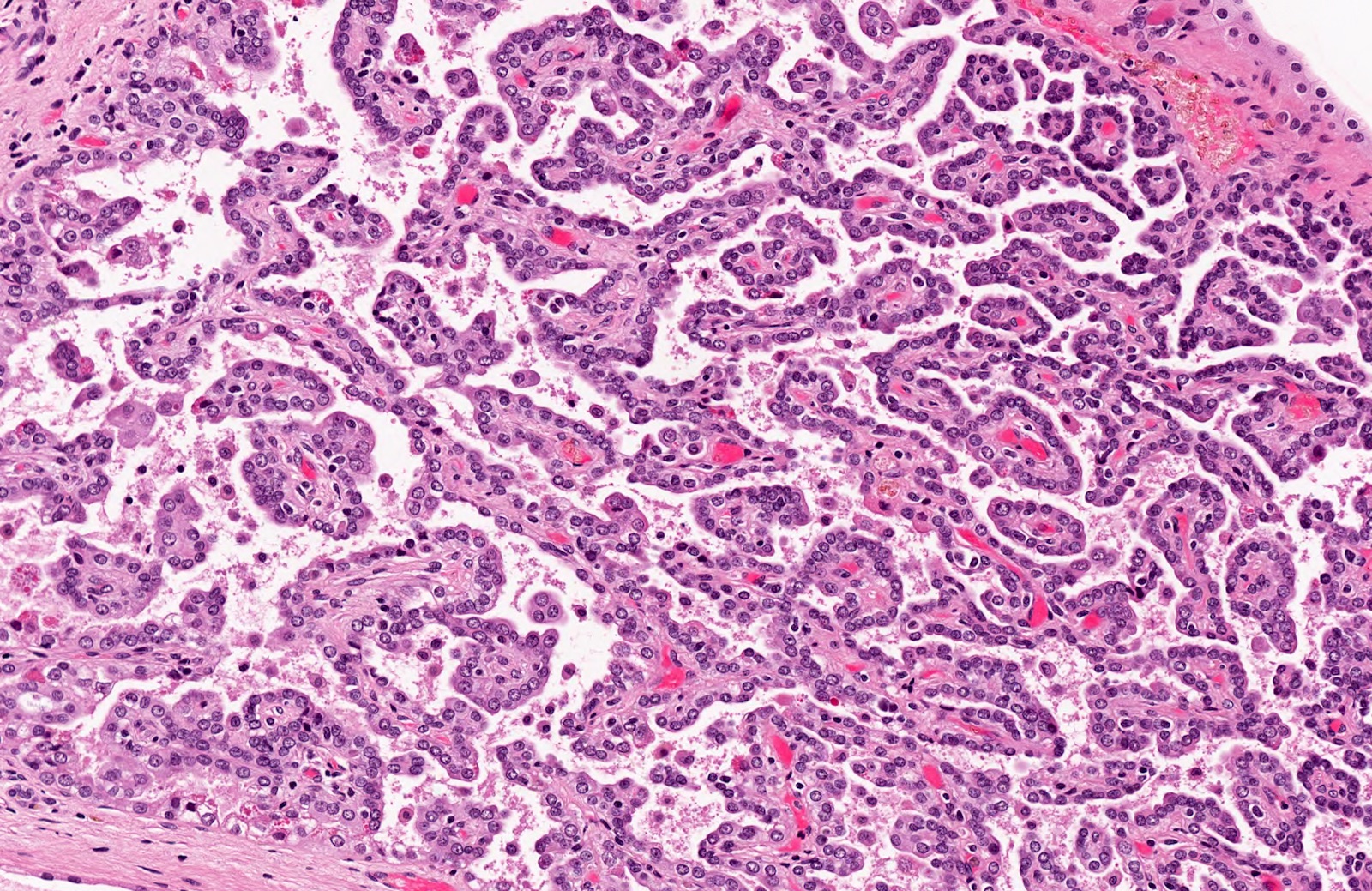
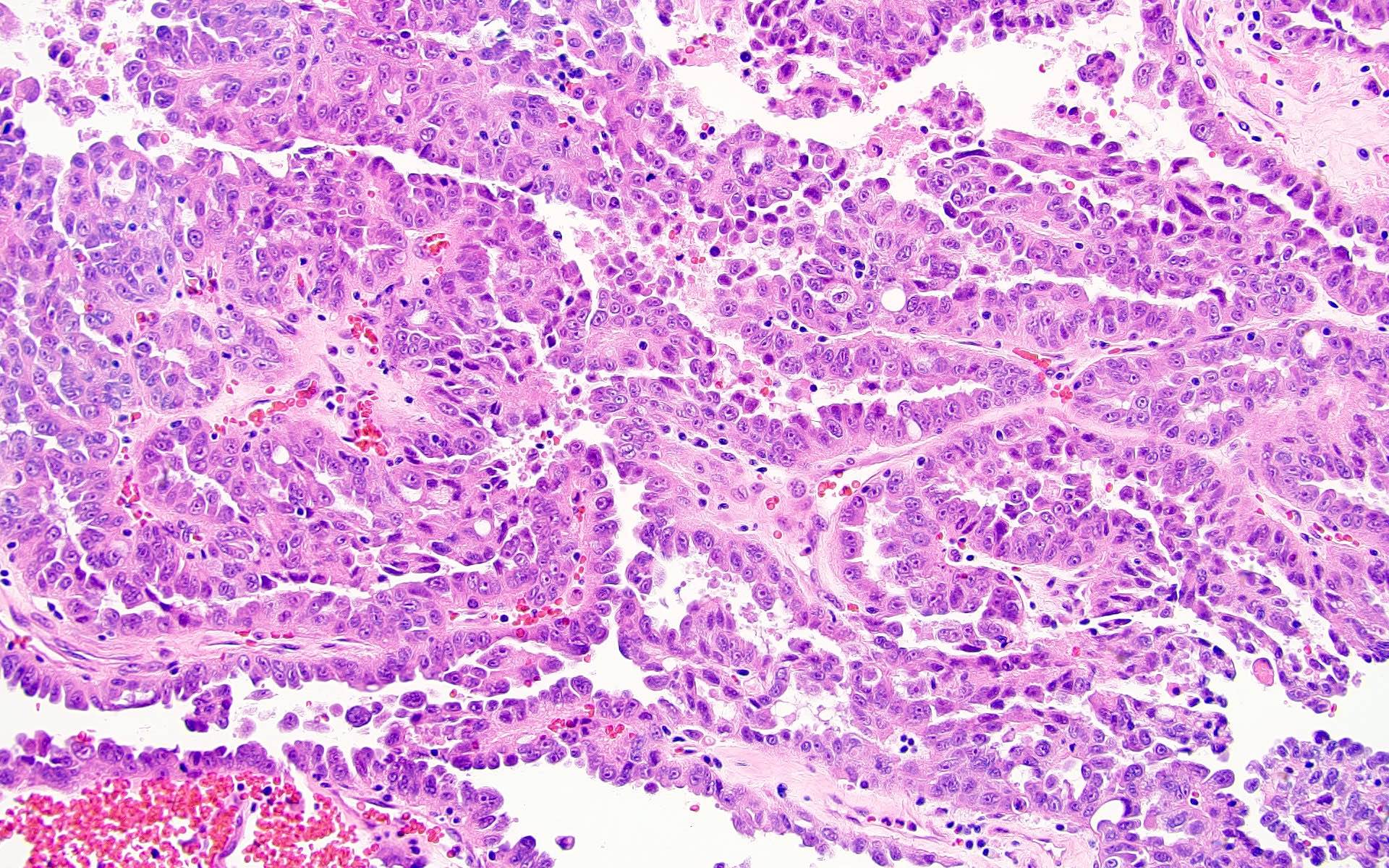
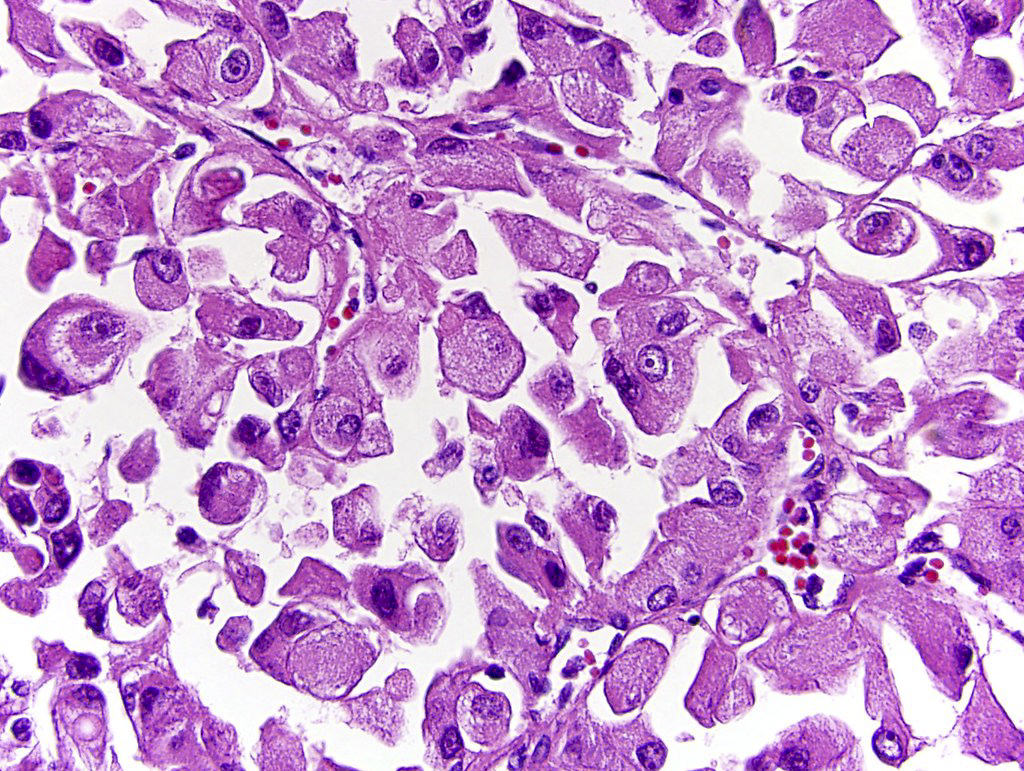
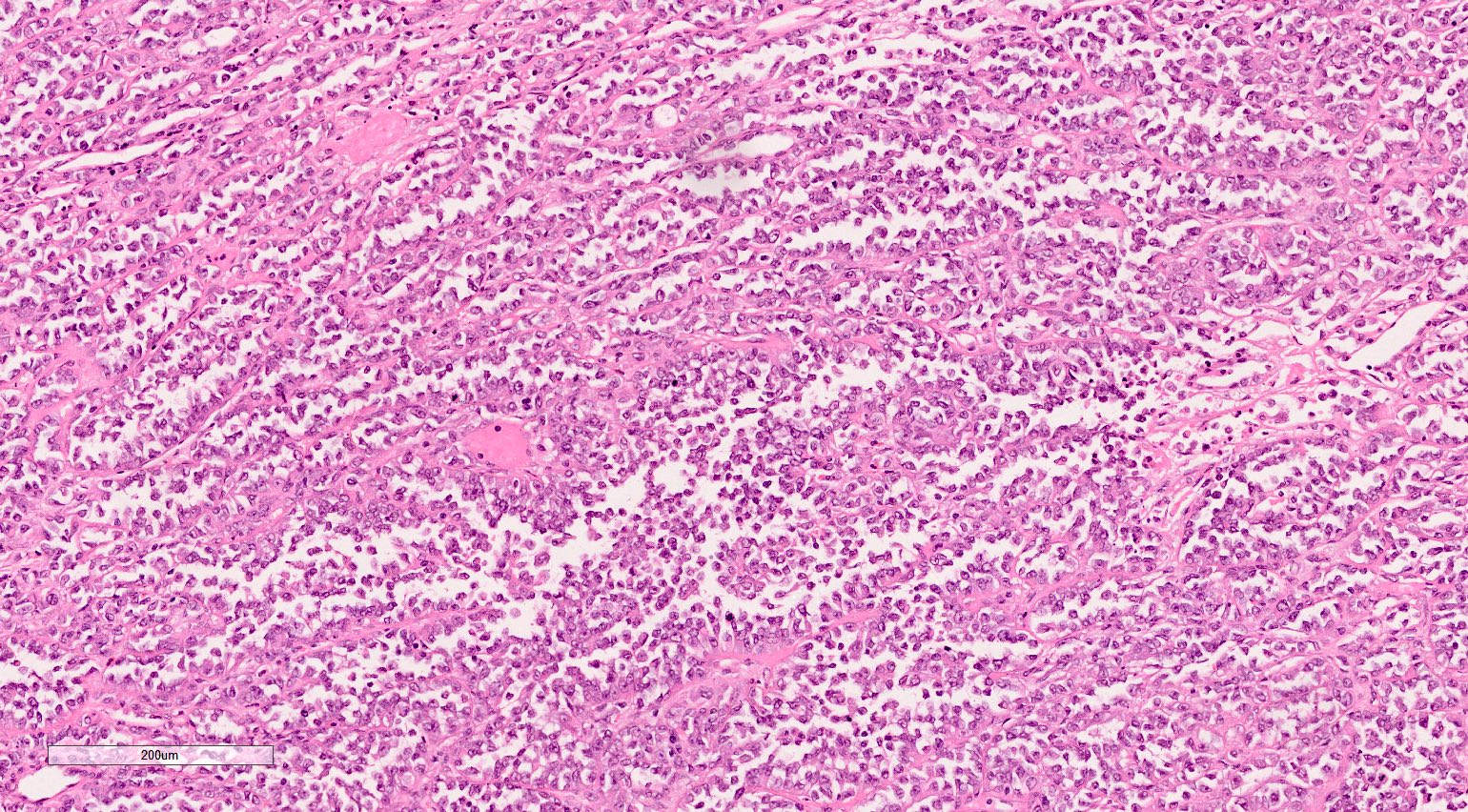
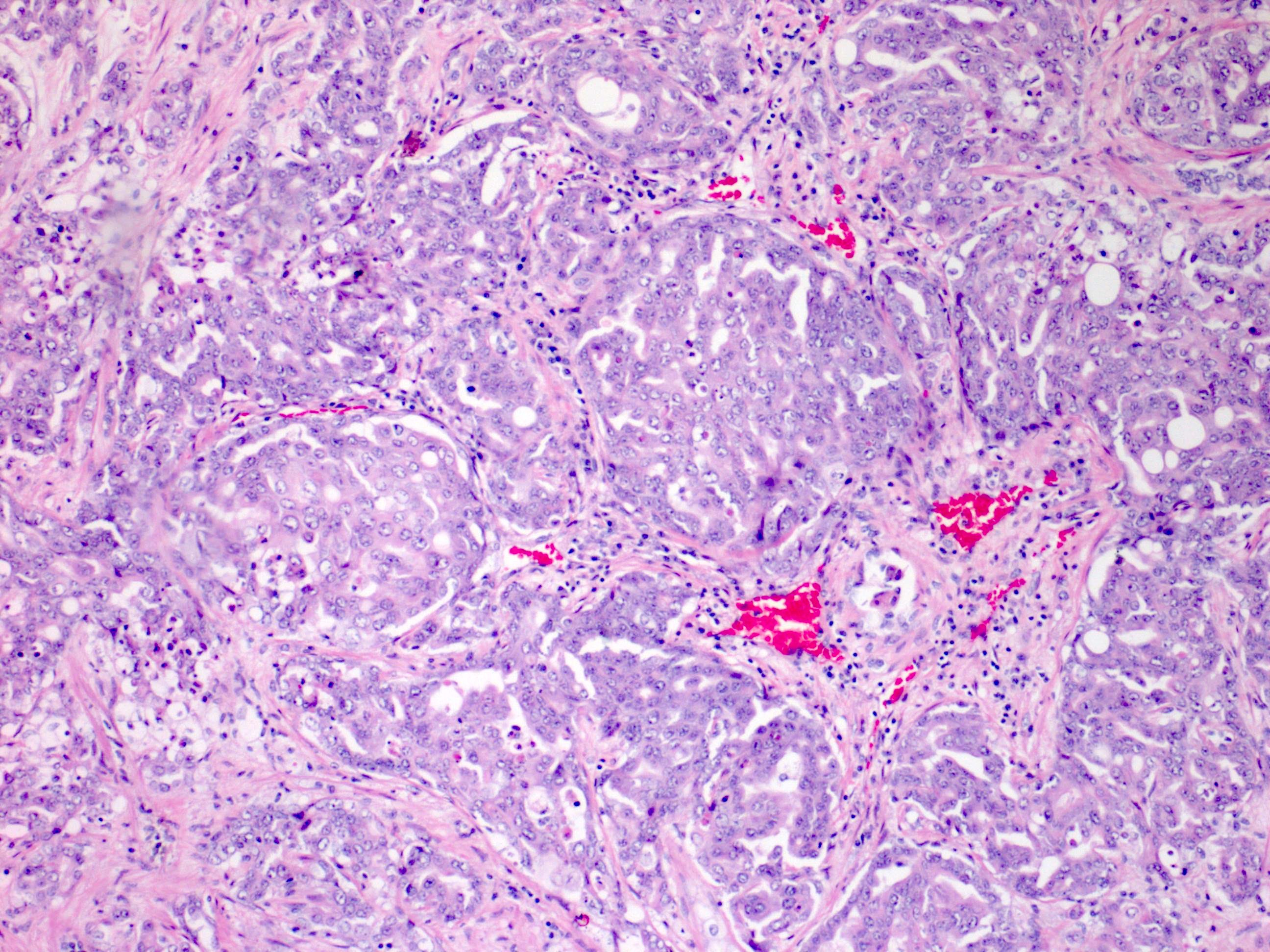
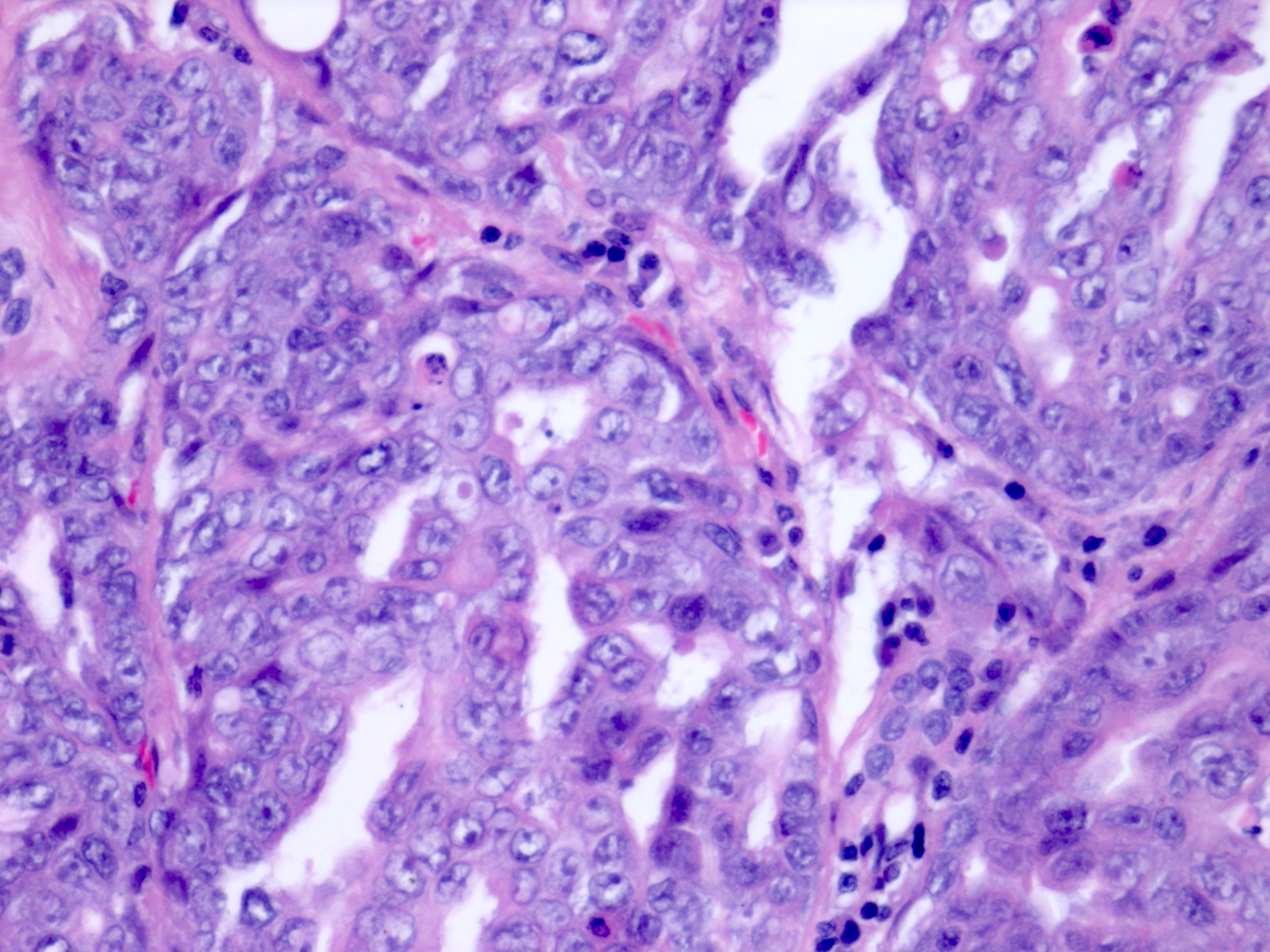
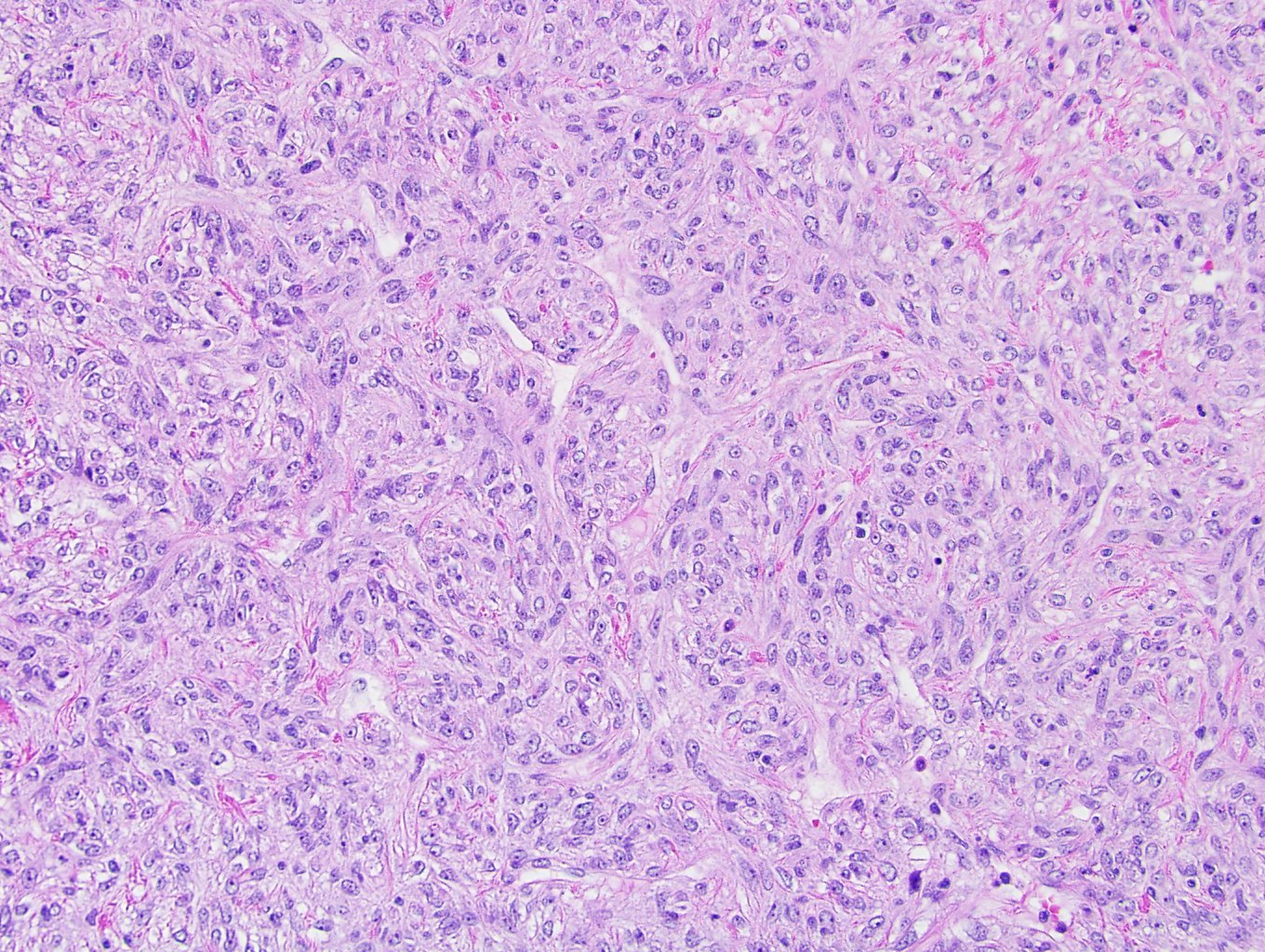
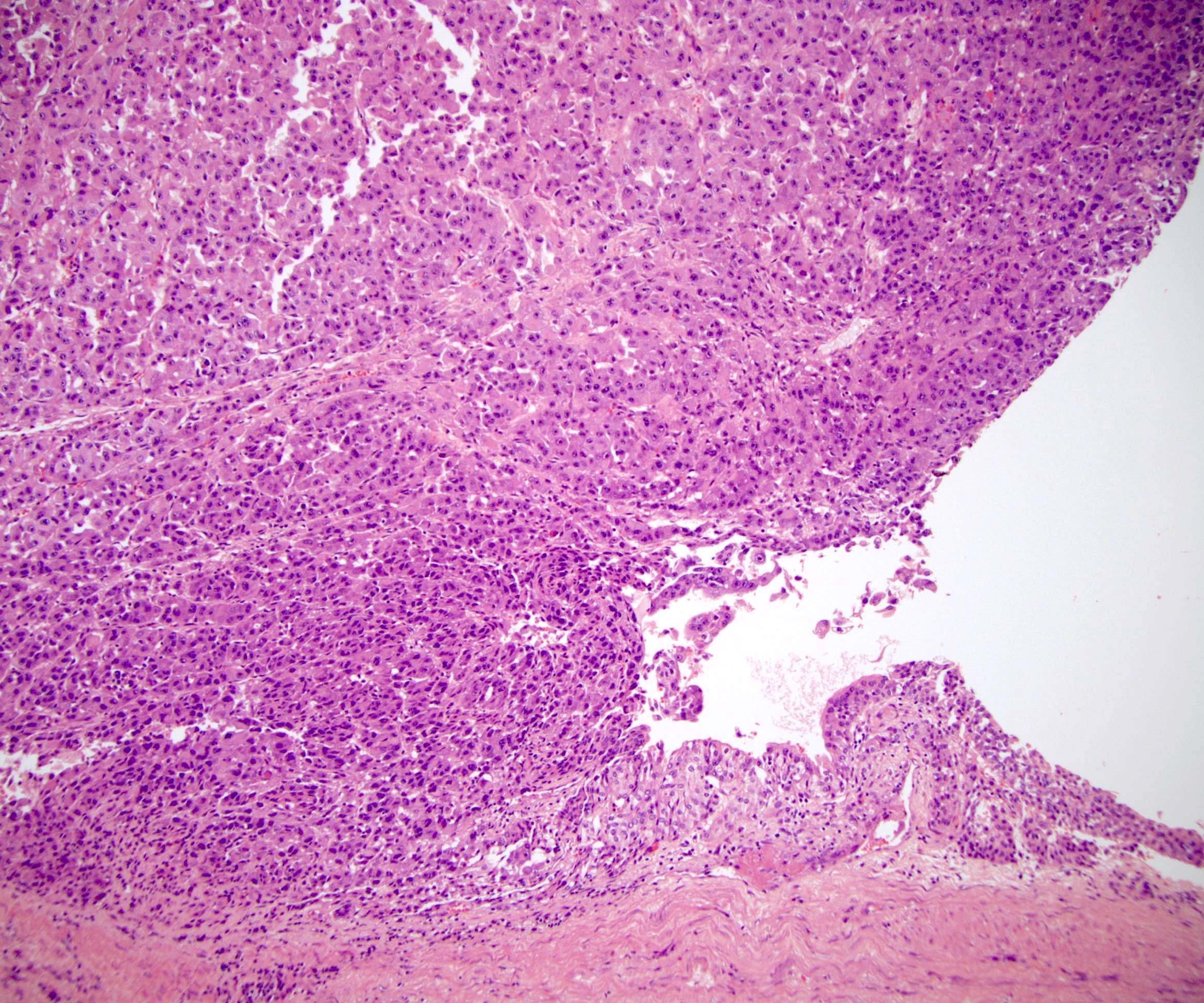
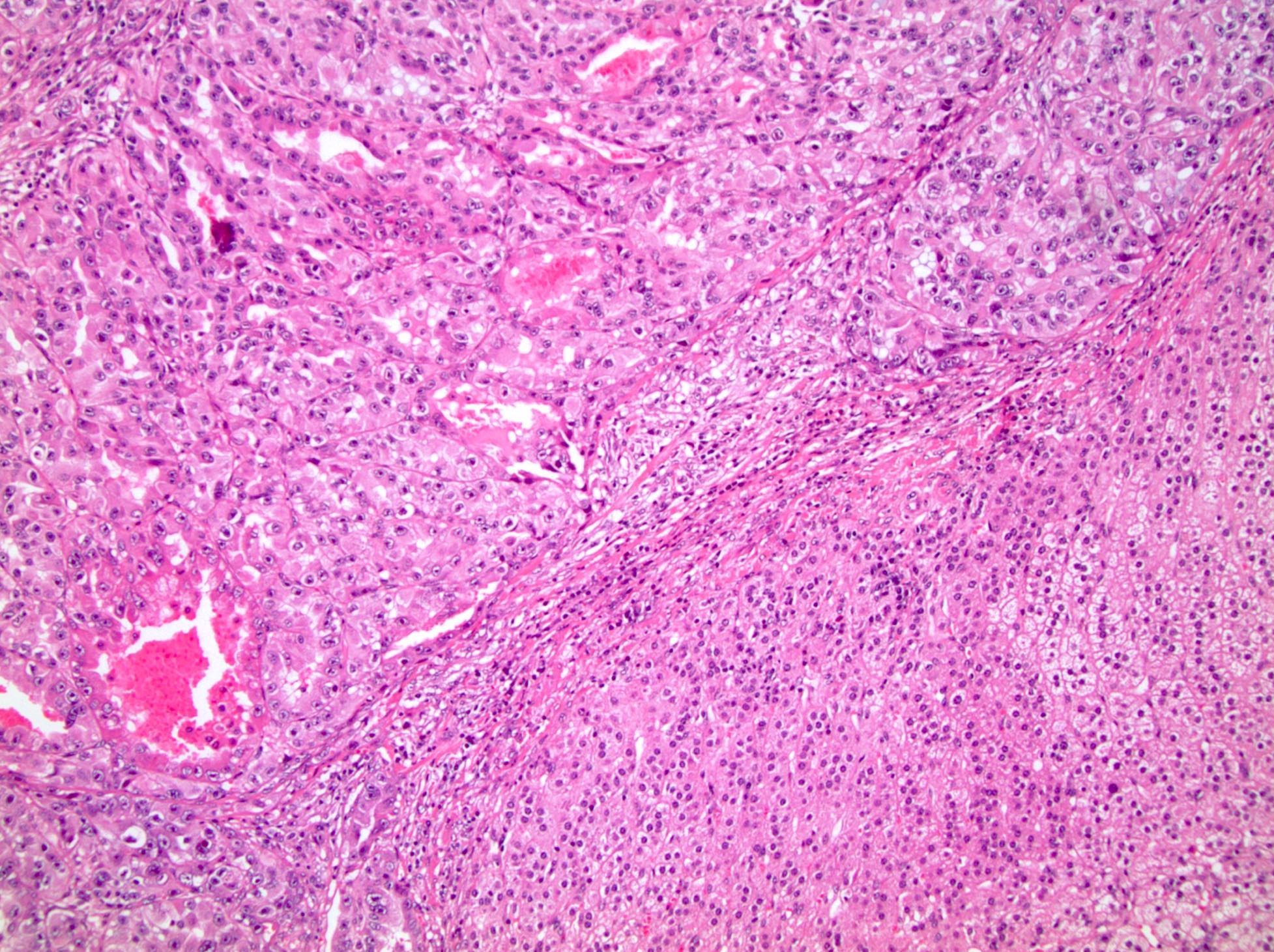
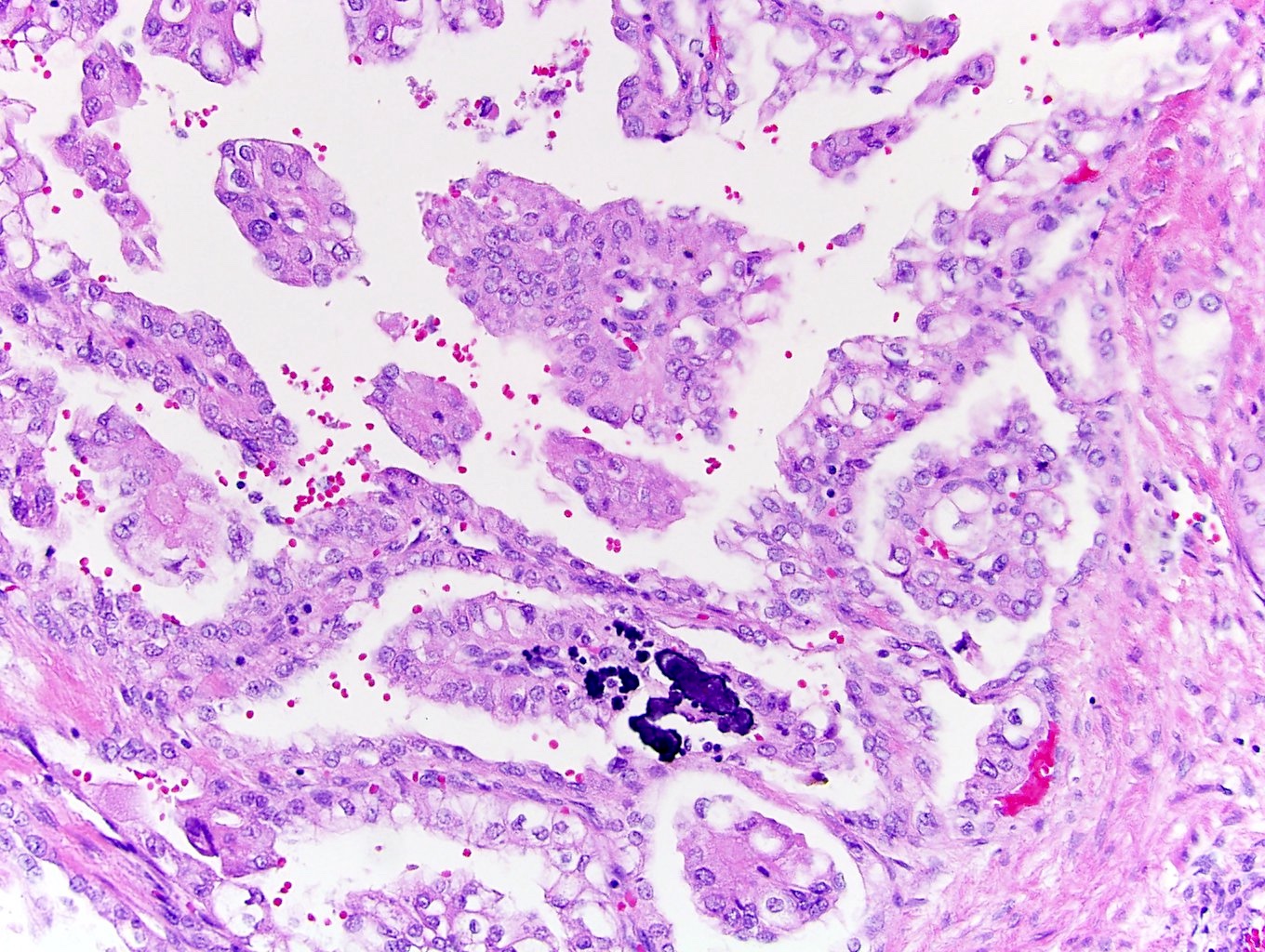
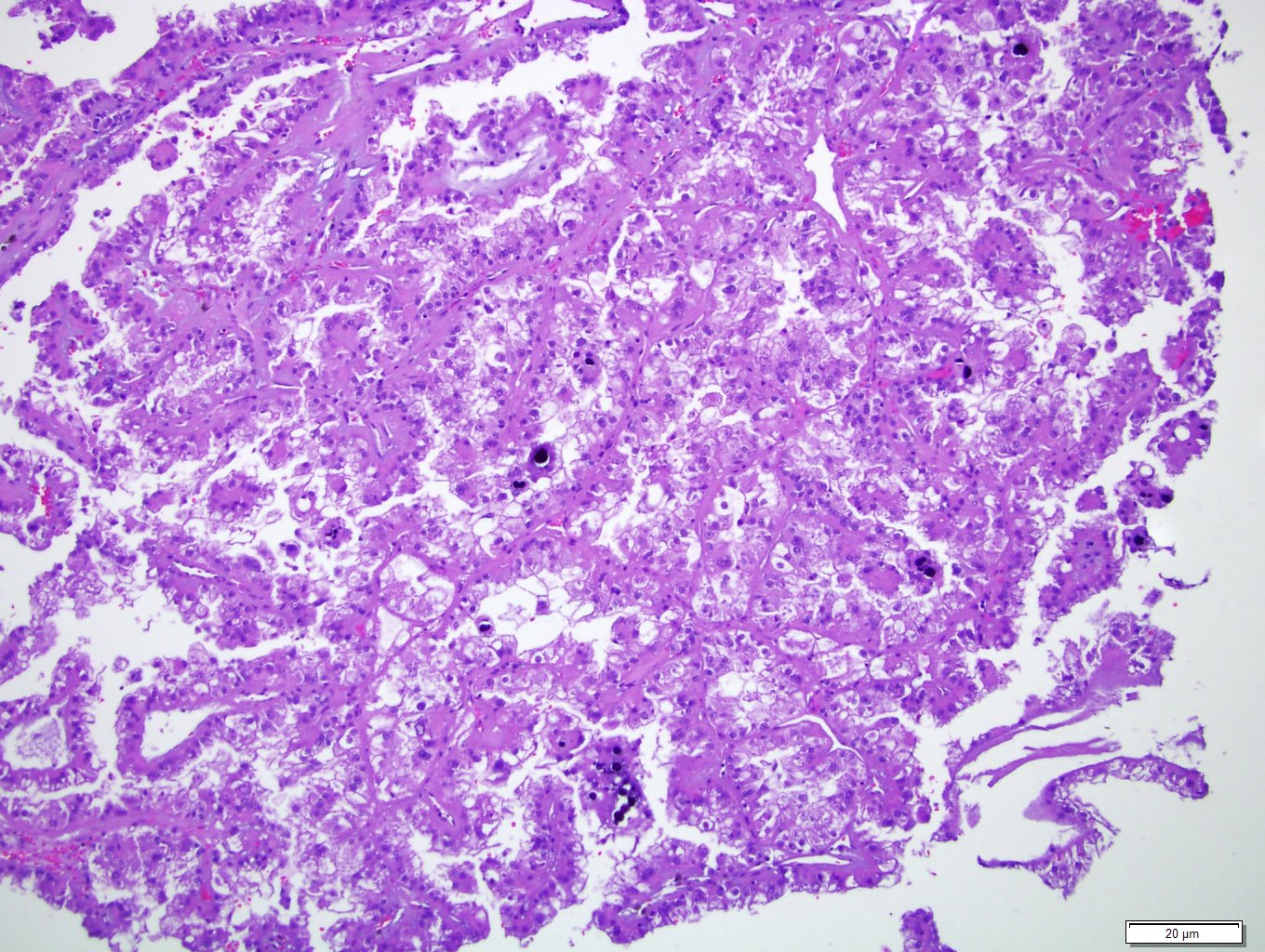
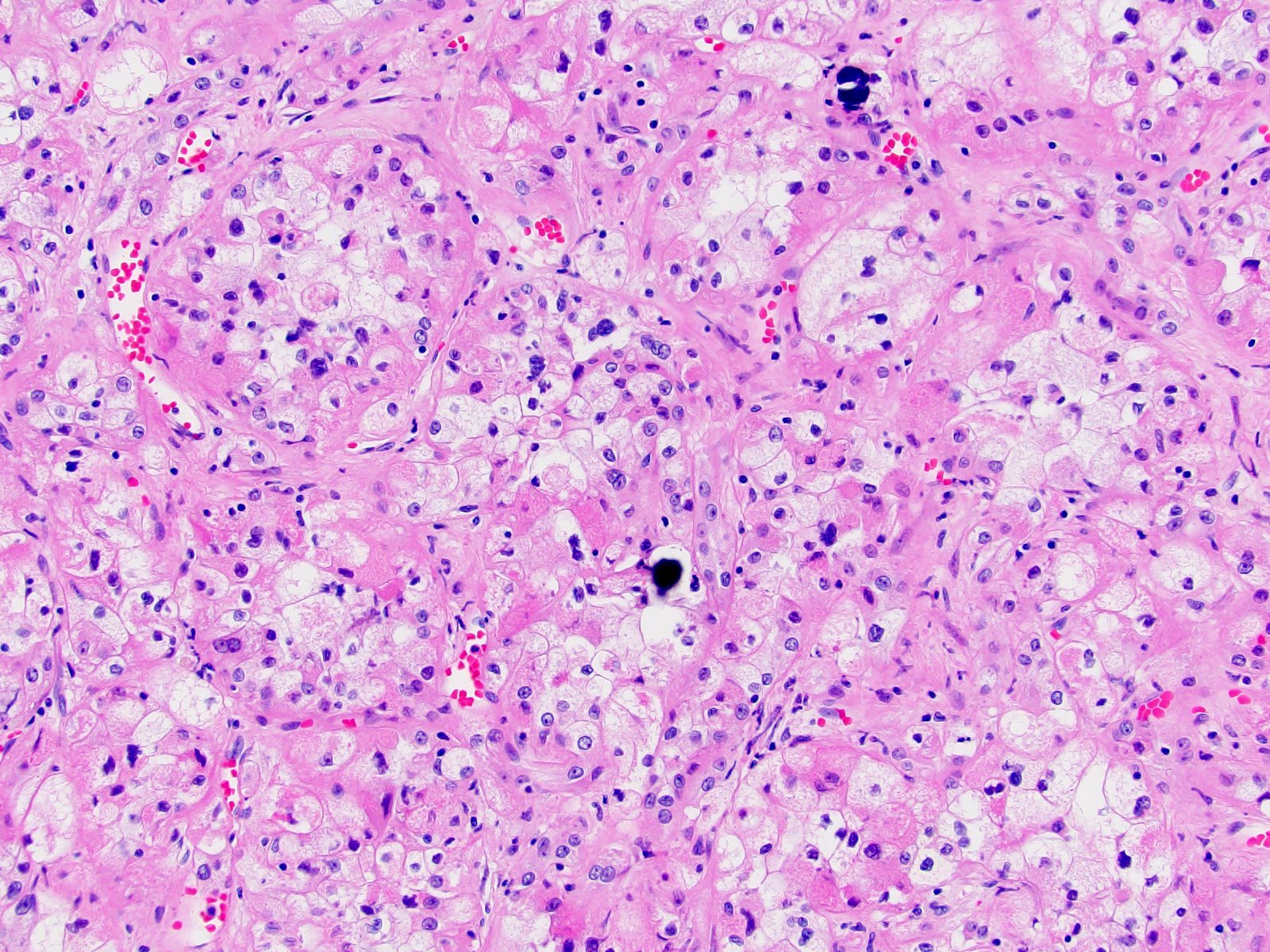
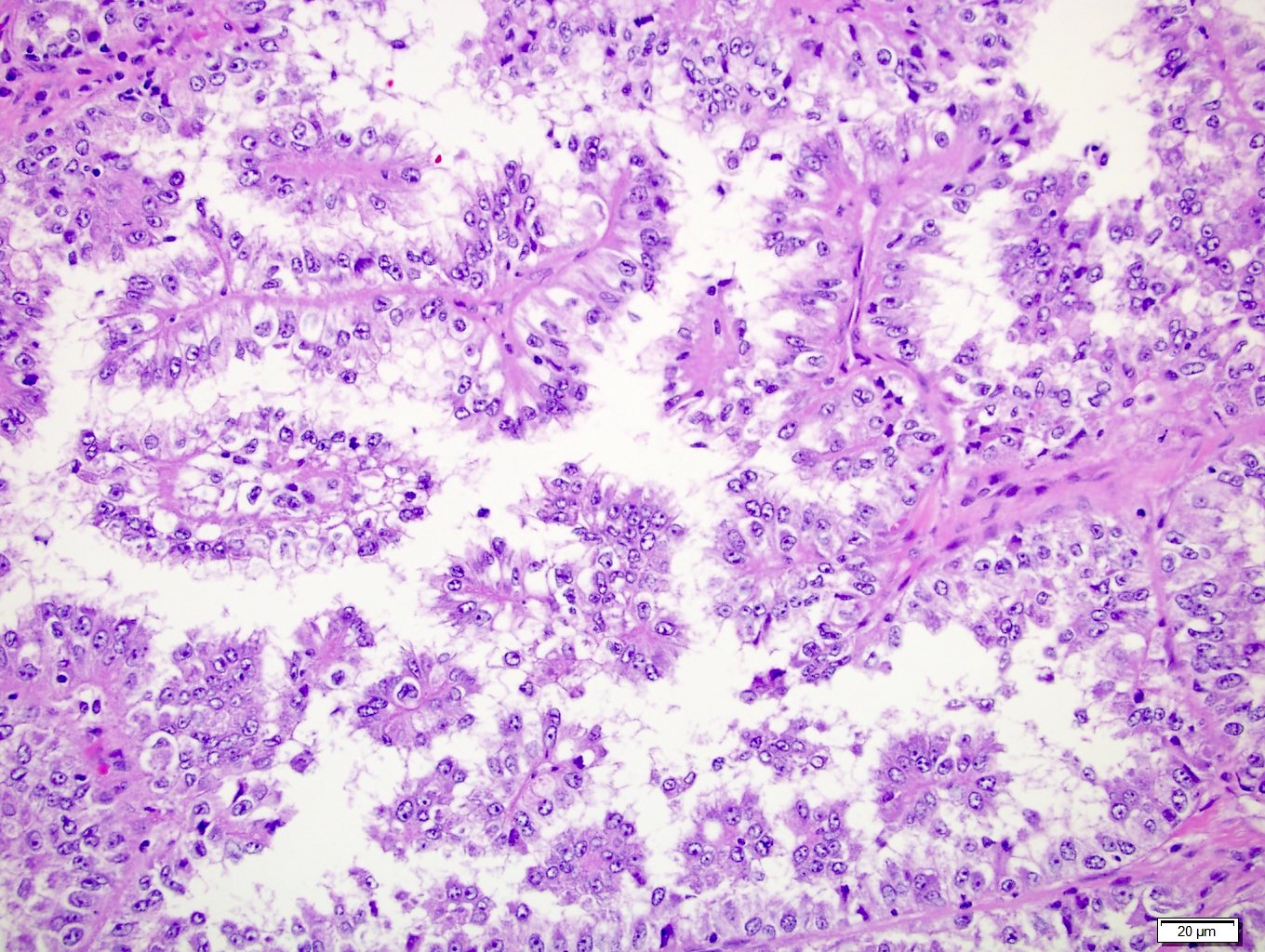
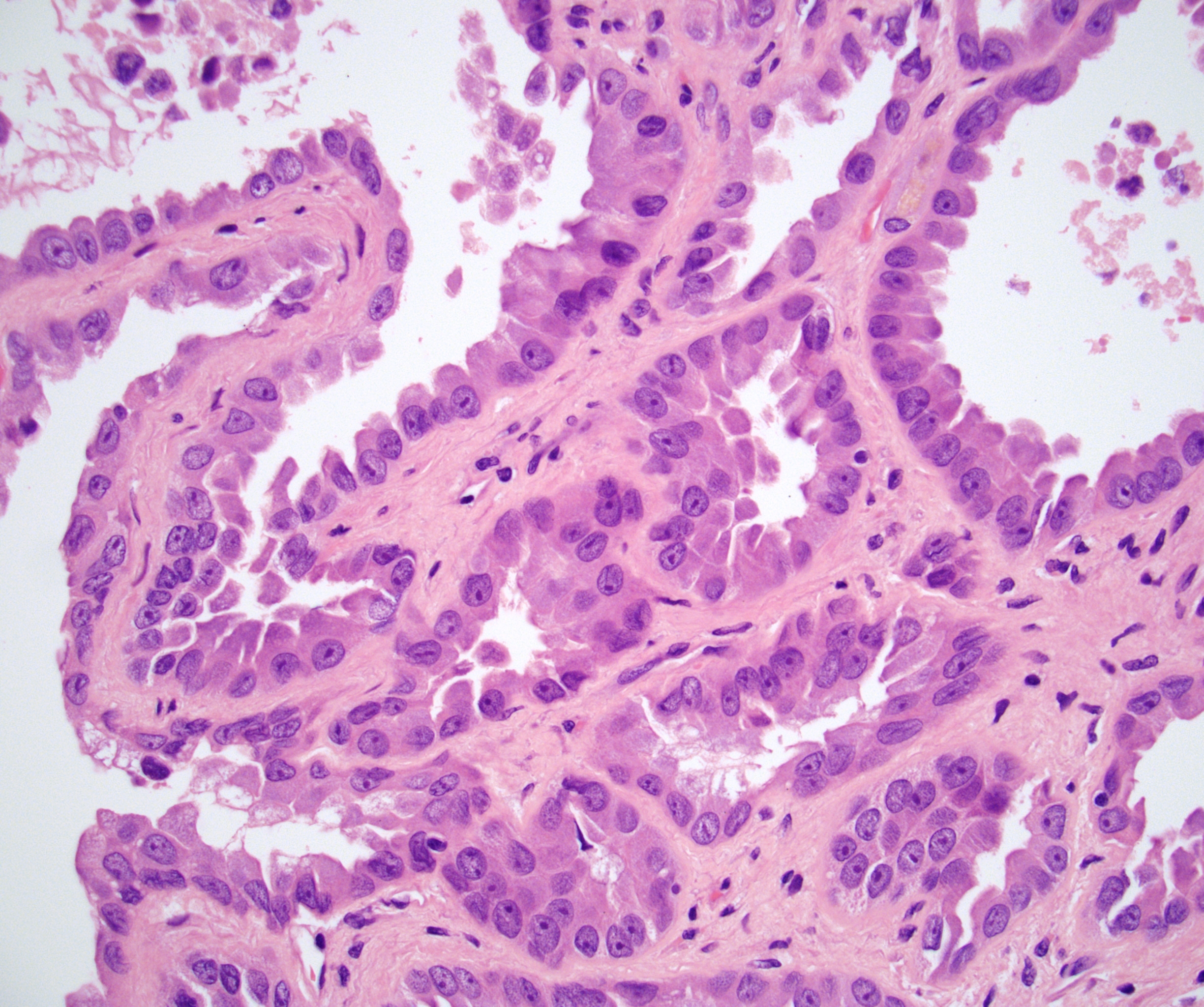
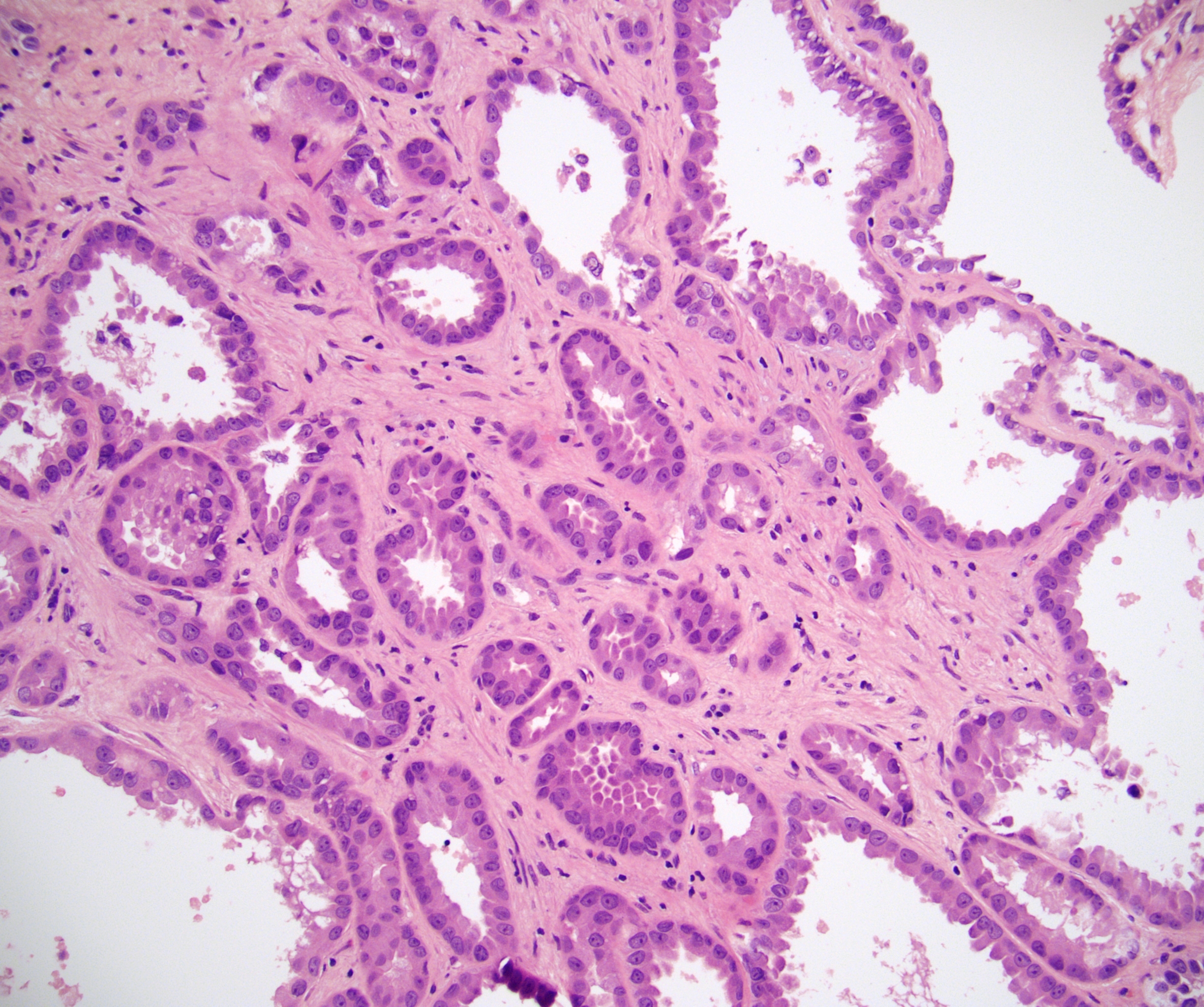
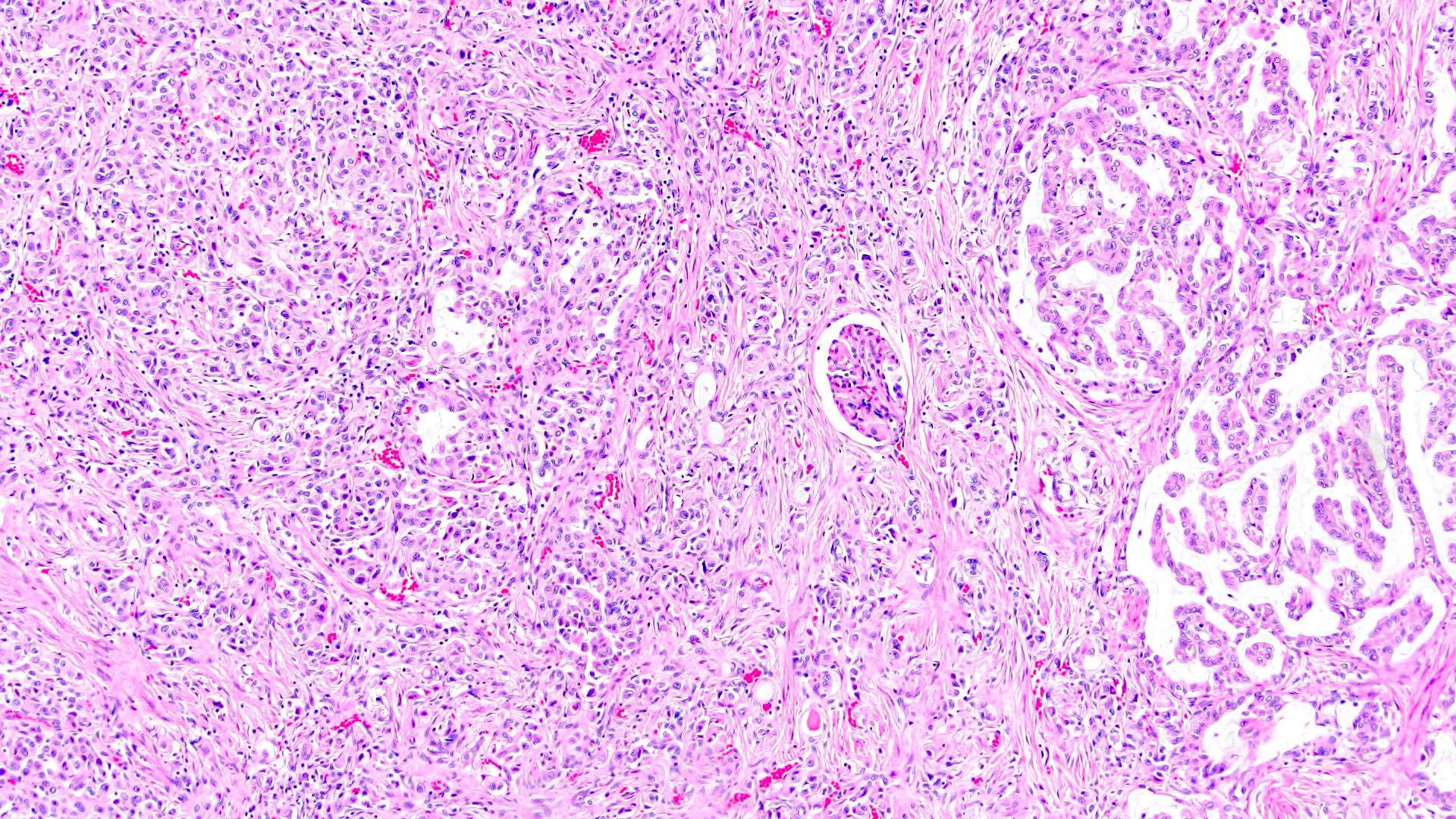
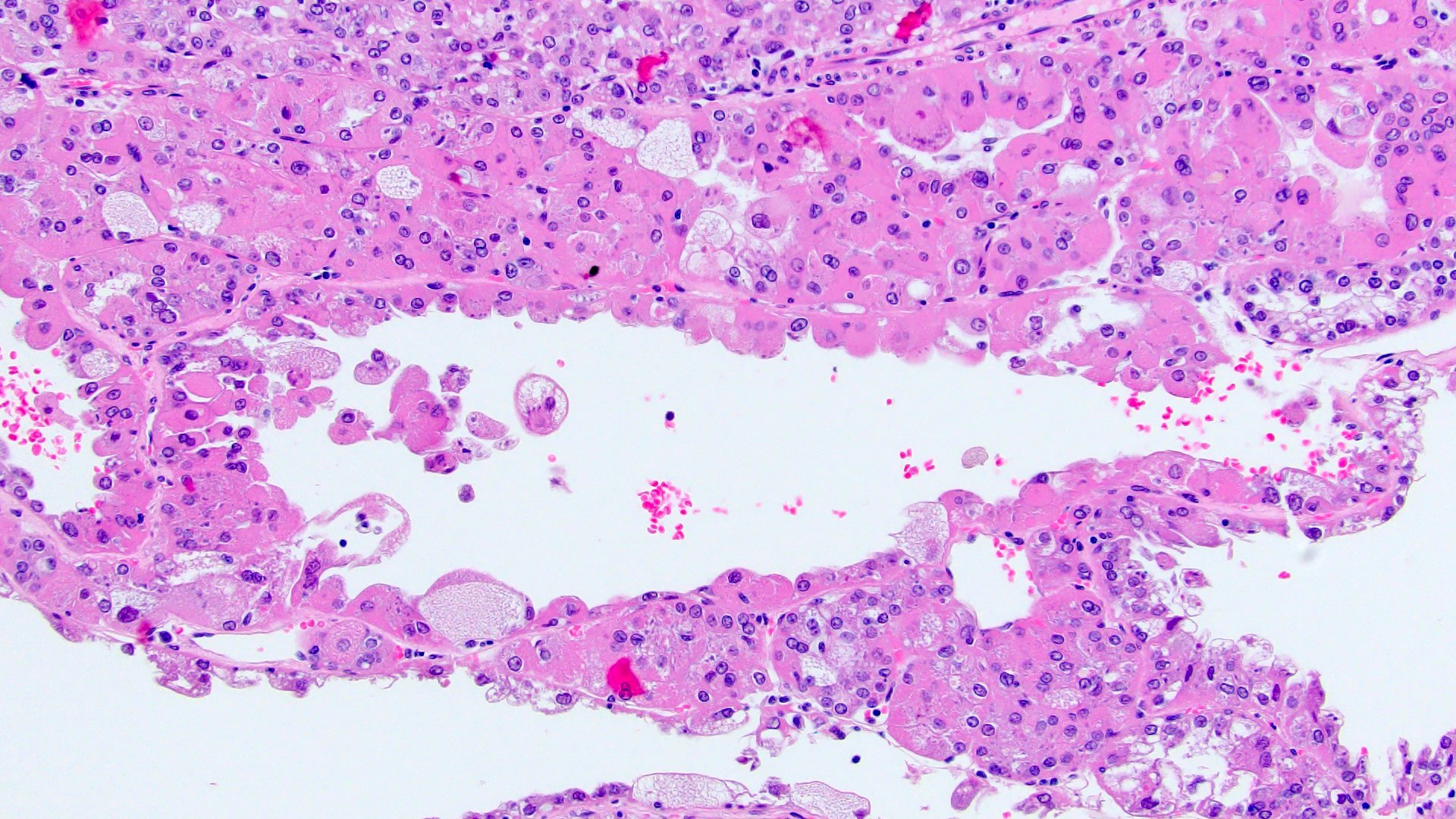
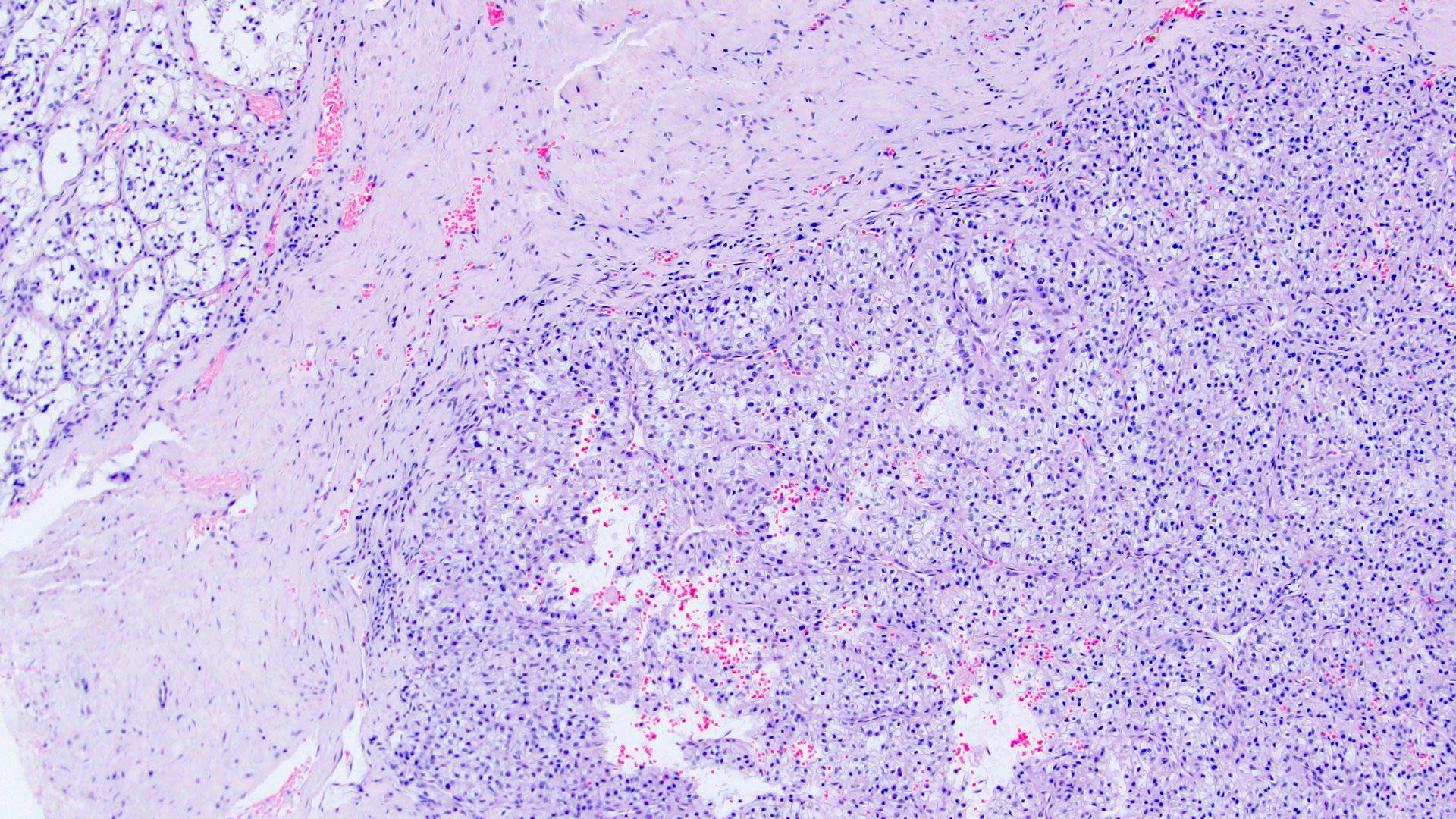
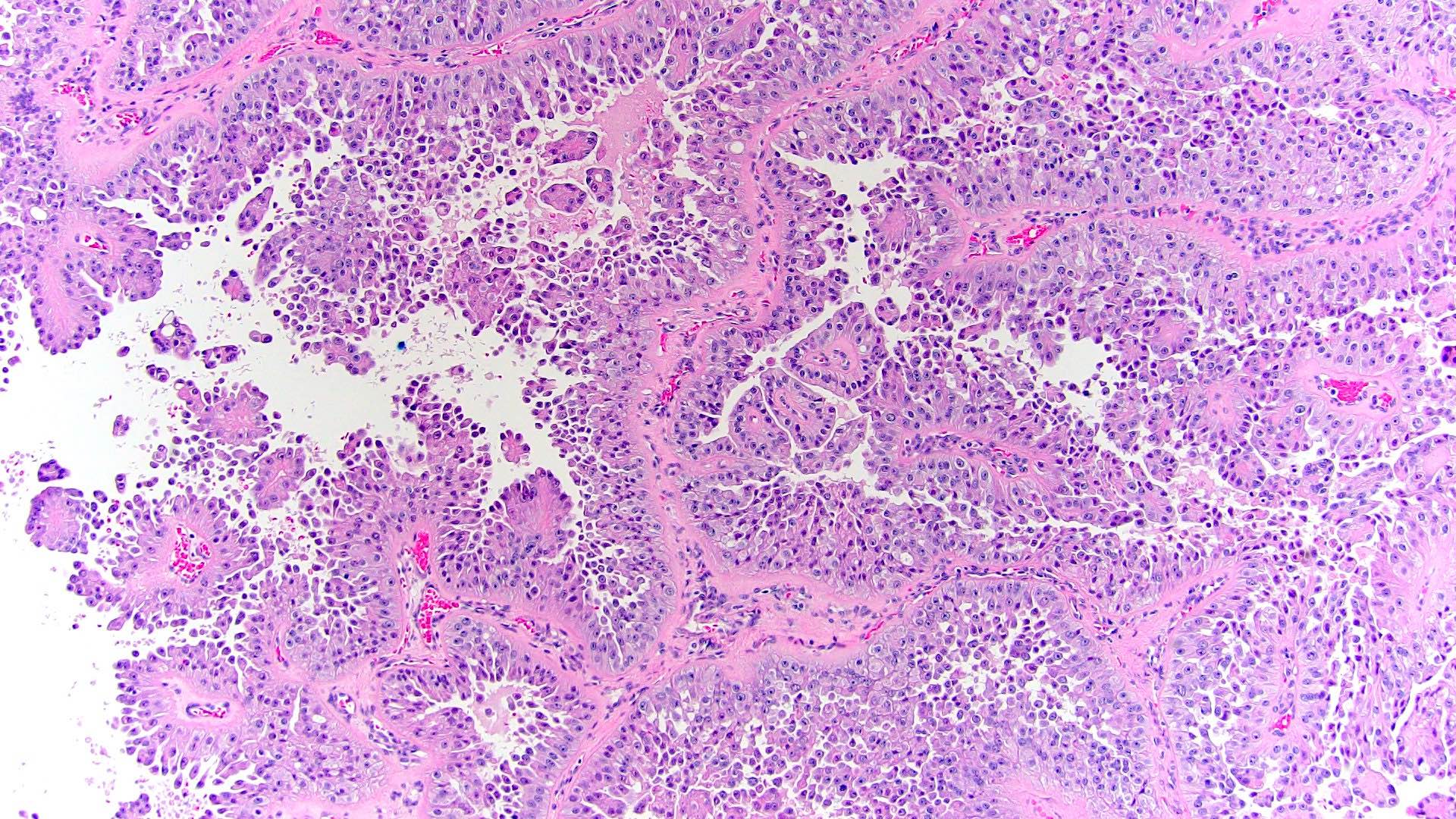
- In adults, heterogeneous solid architecture, mucinous cribriform, signet ring and solid rhabdoid patterns, high grade eosinophilic cells with intracytoplasmic lumina (Pol J Pathol 2018;69:109, Semin Diagn Pathol 2015;32:90, Histopathology 2019;74:31)
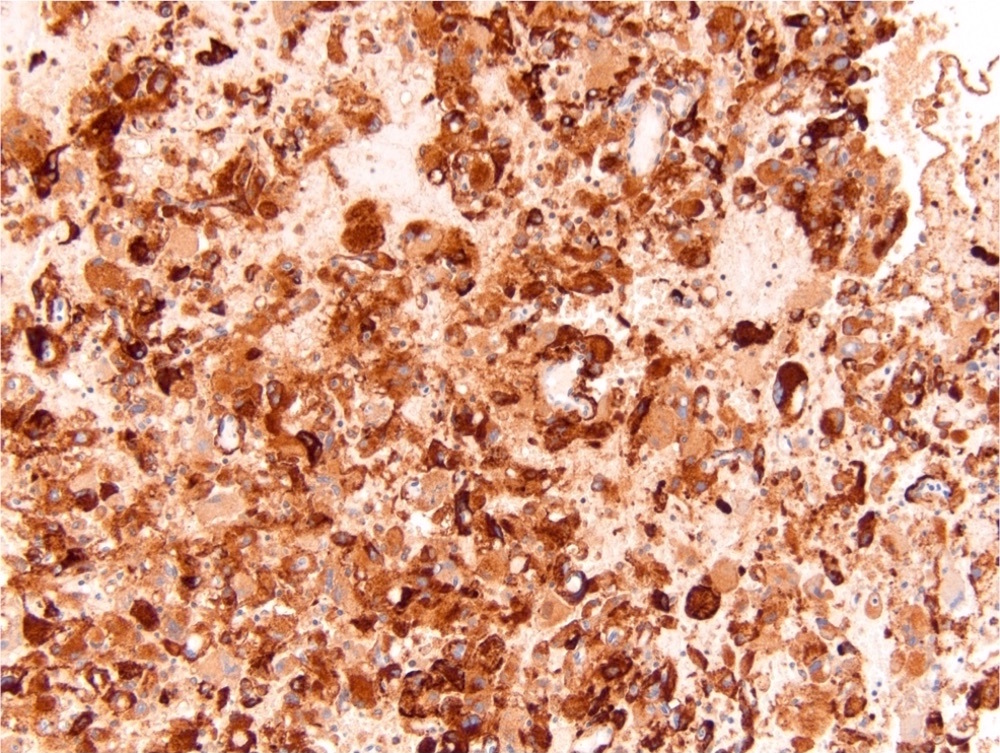
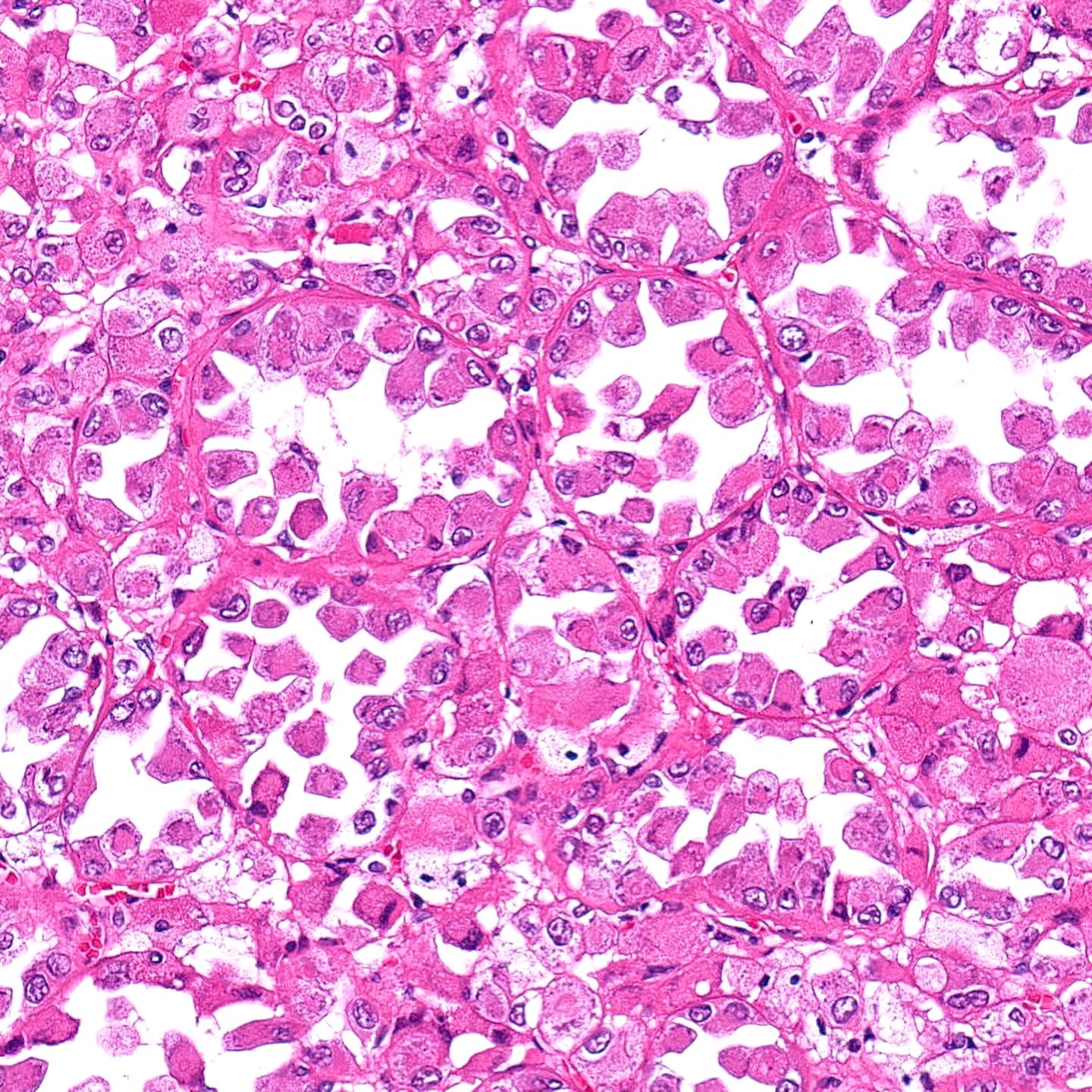
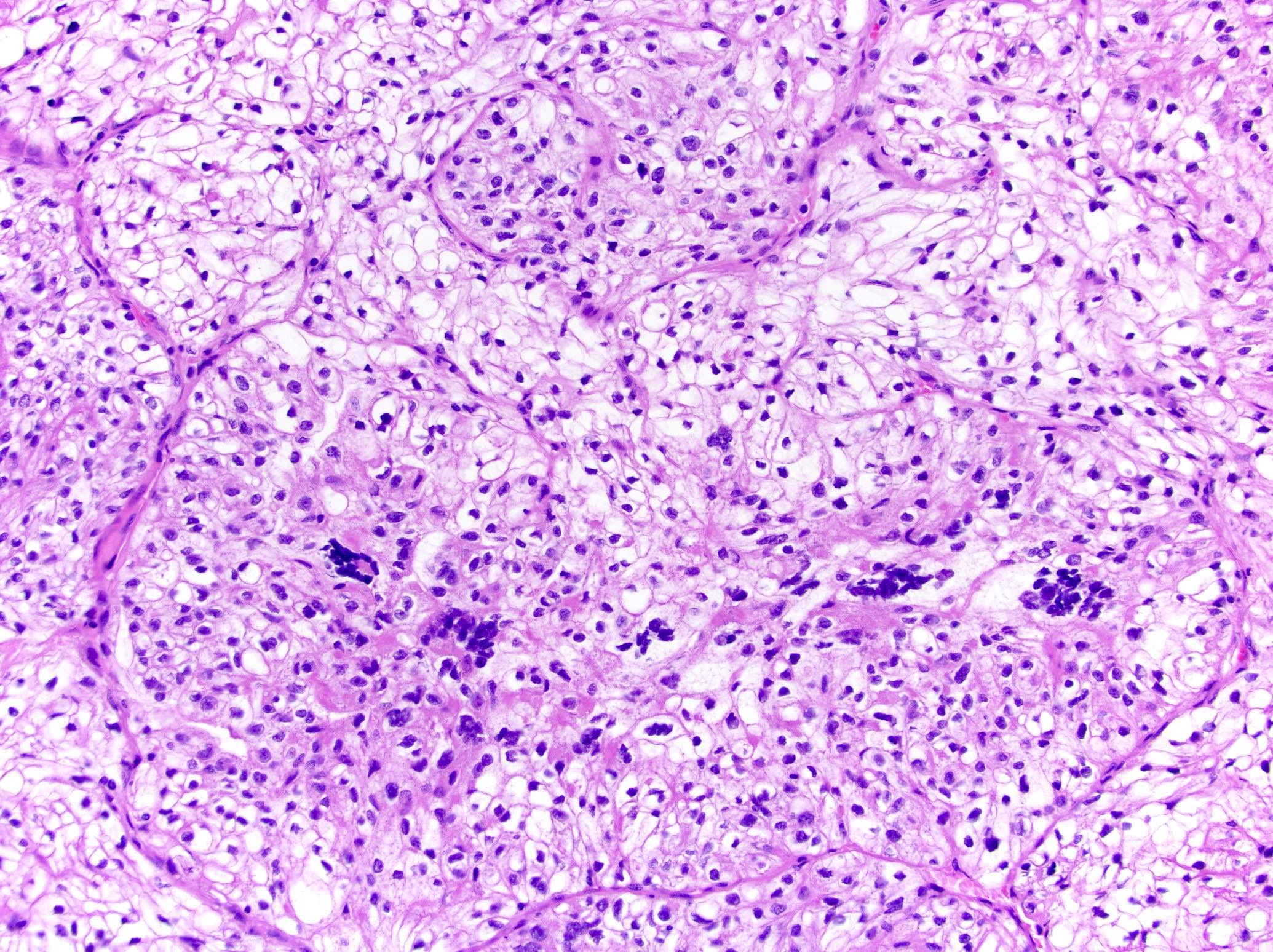
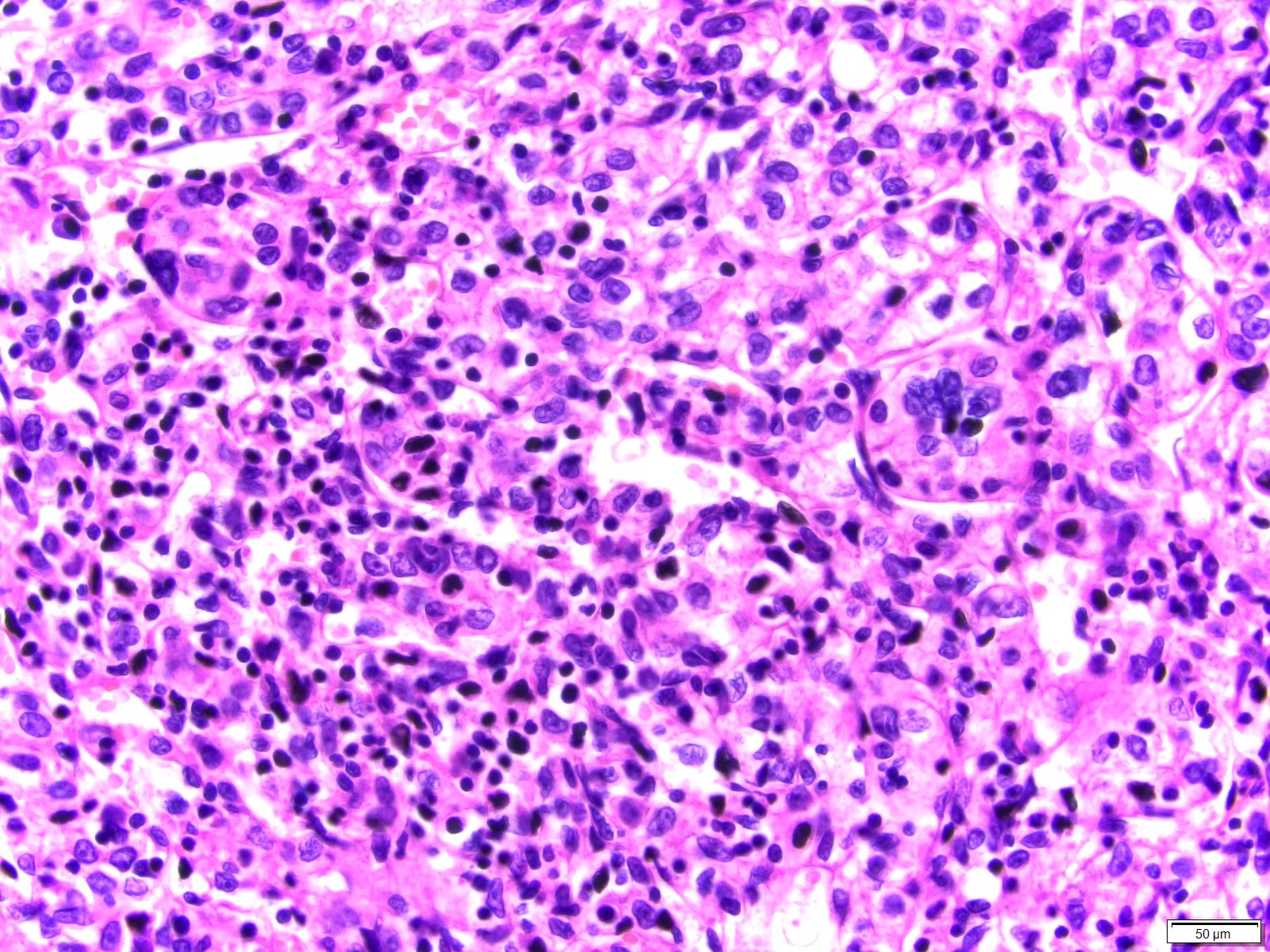
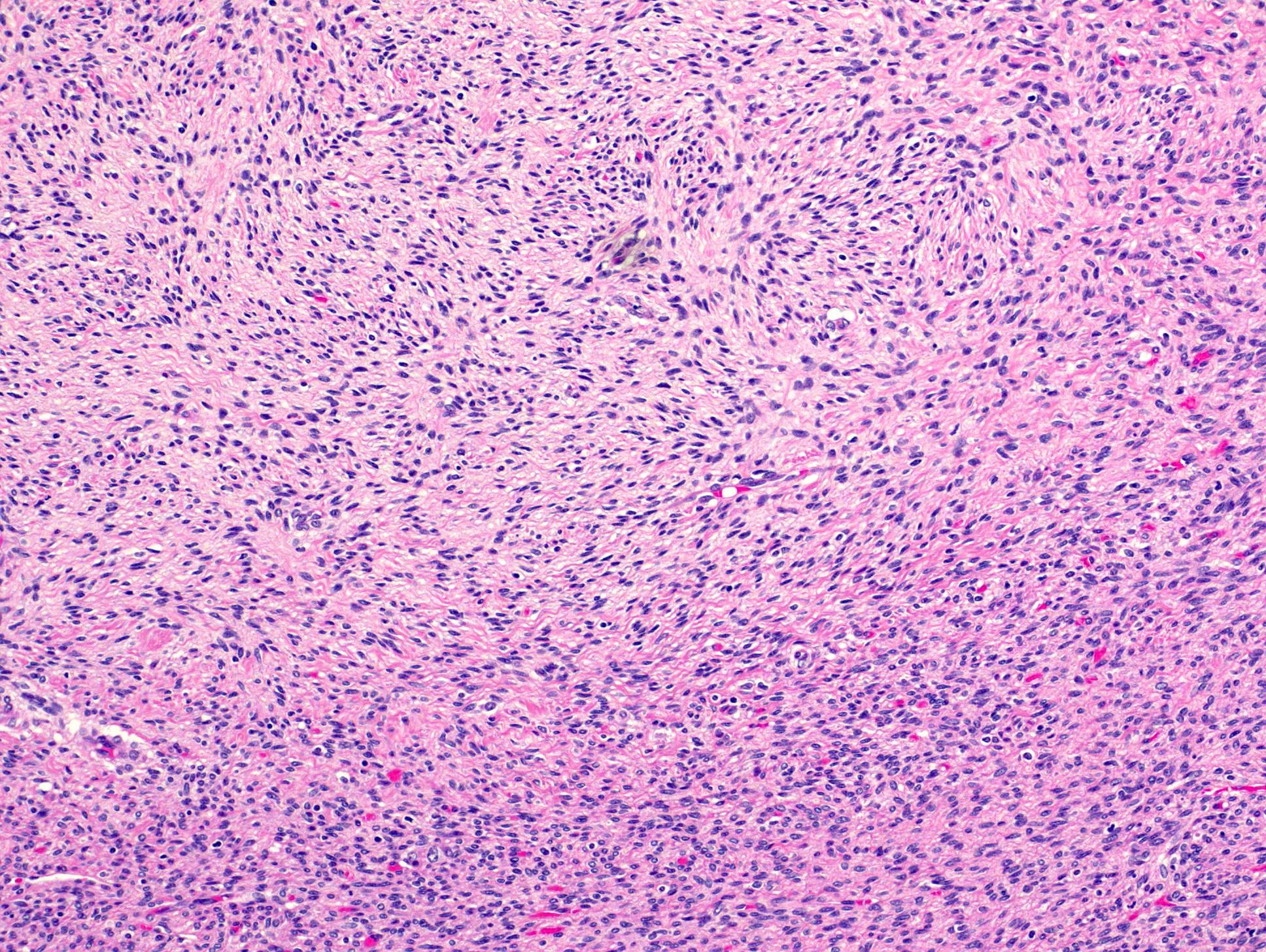
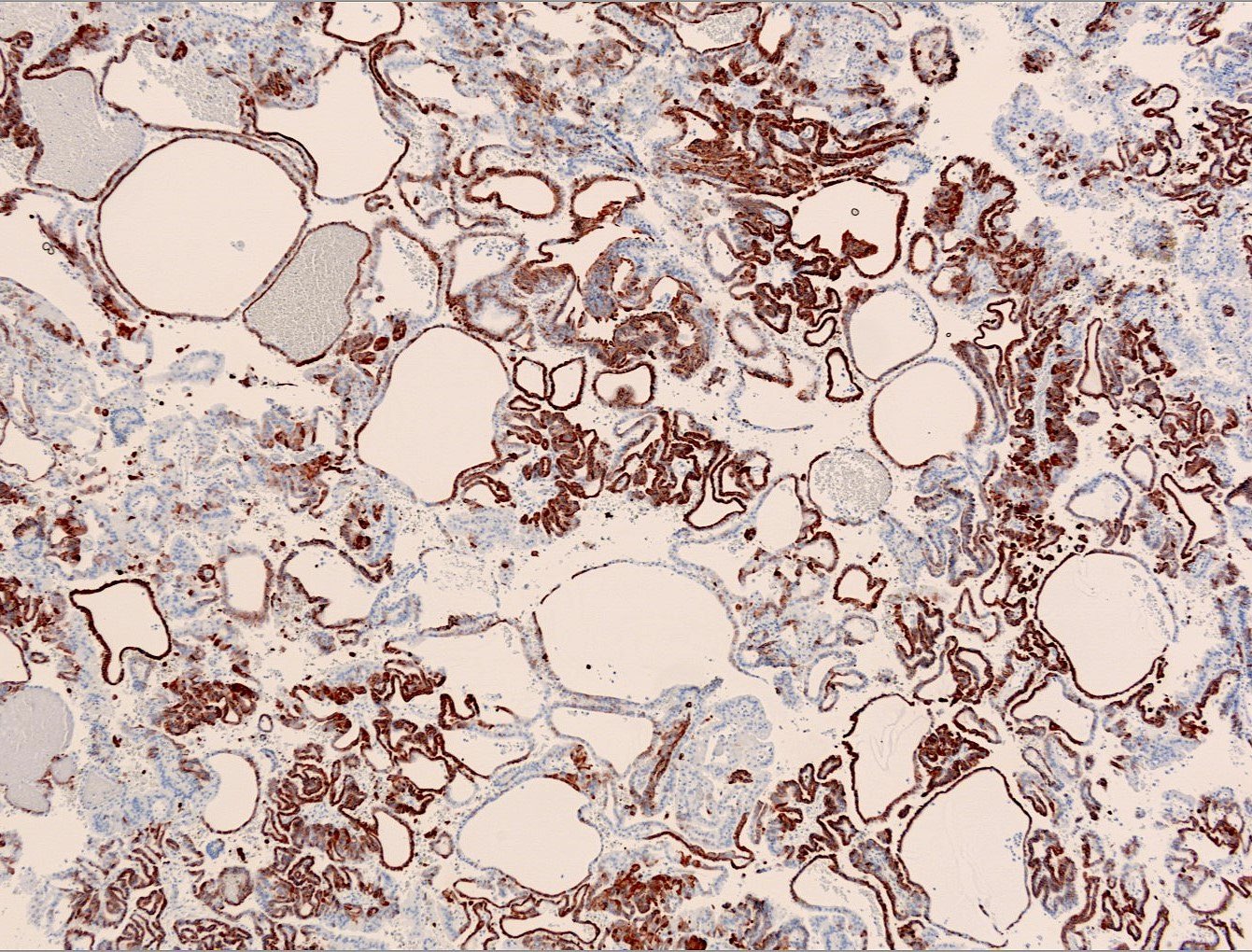
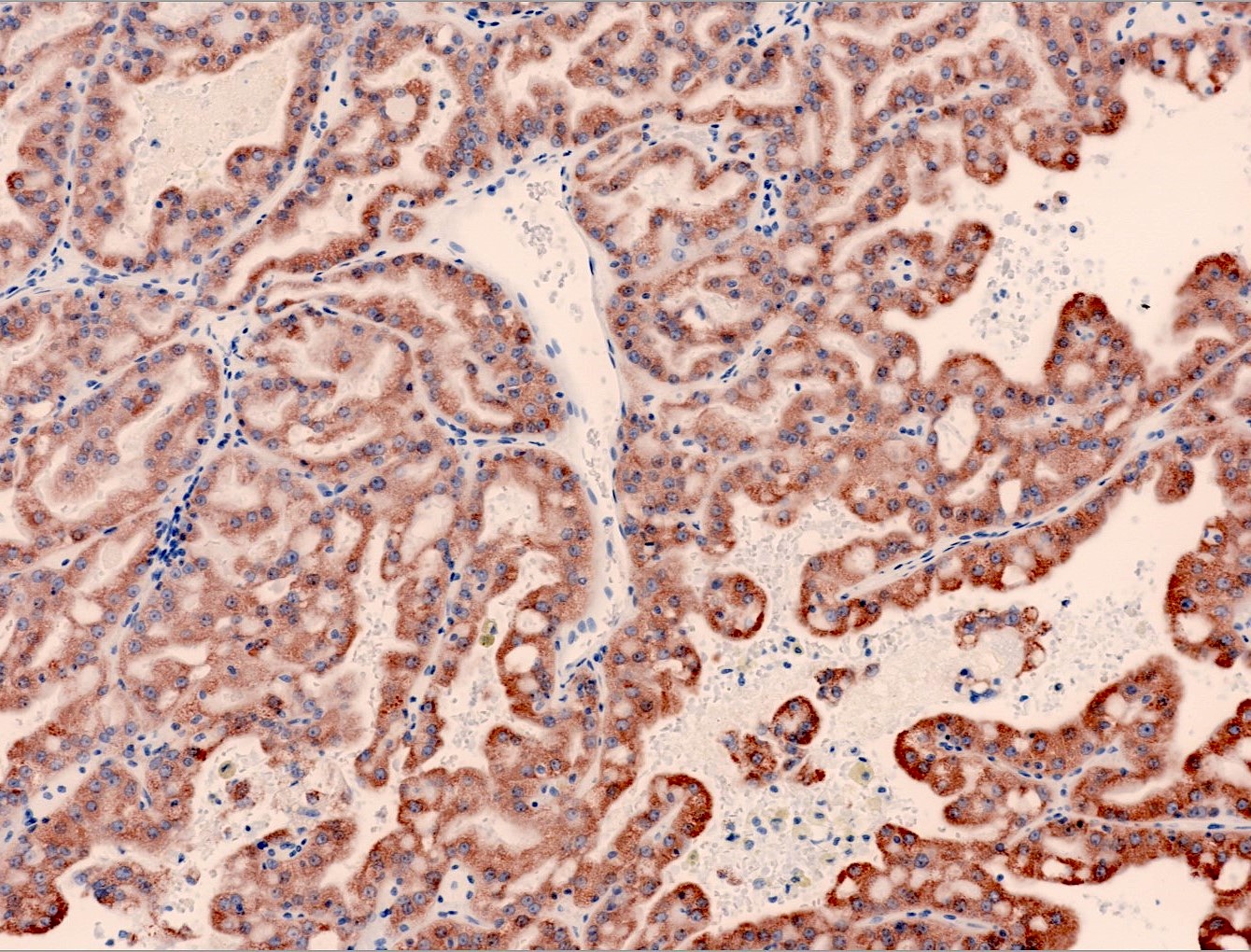
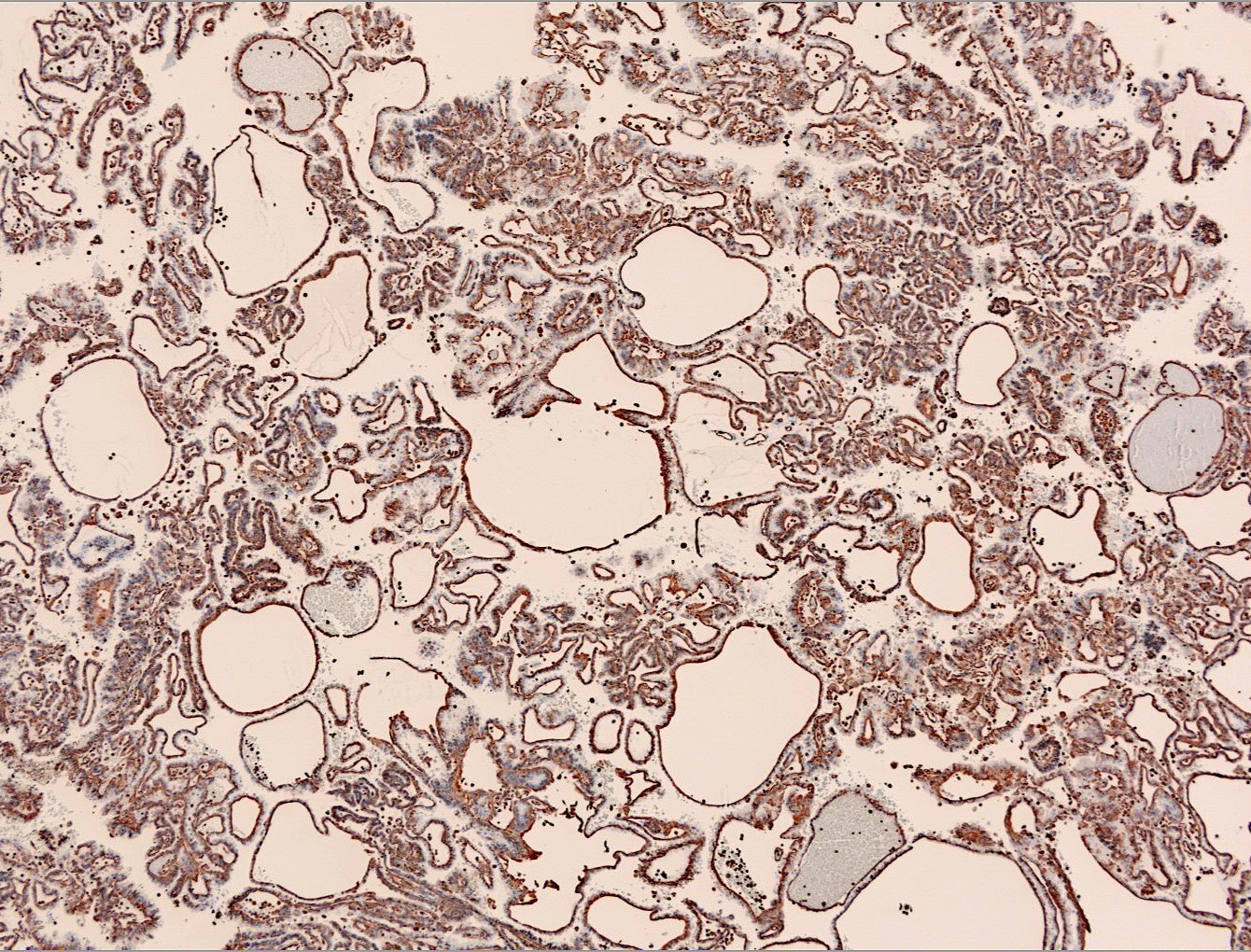
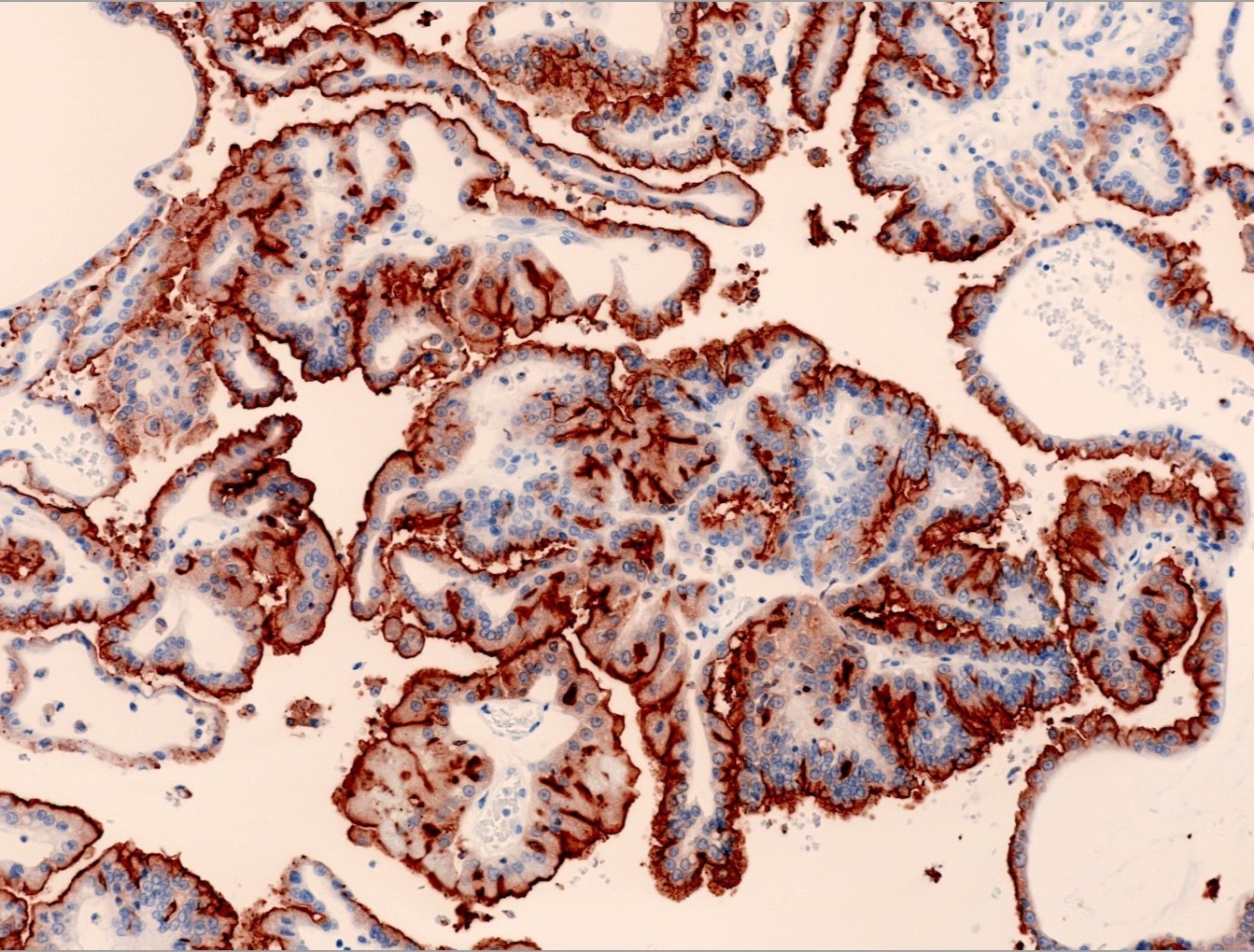
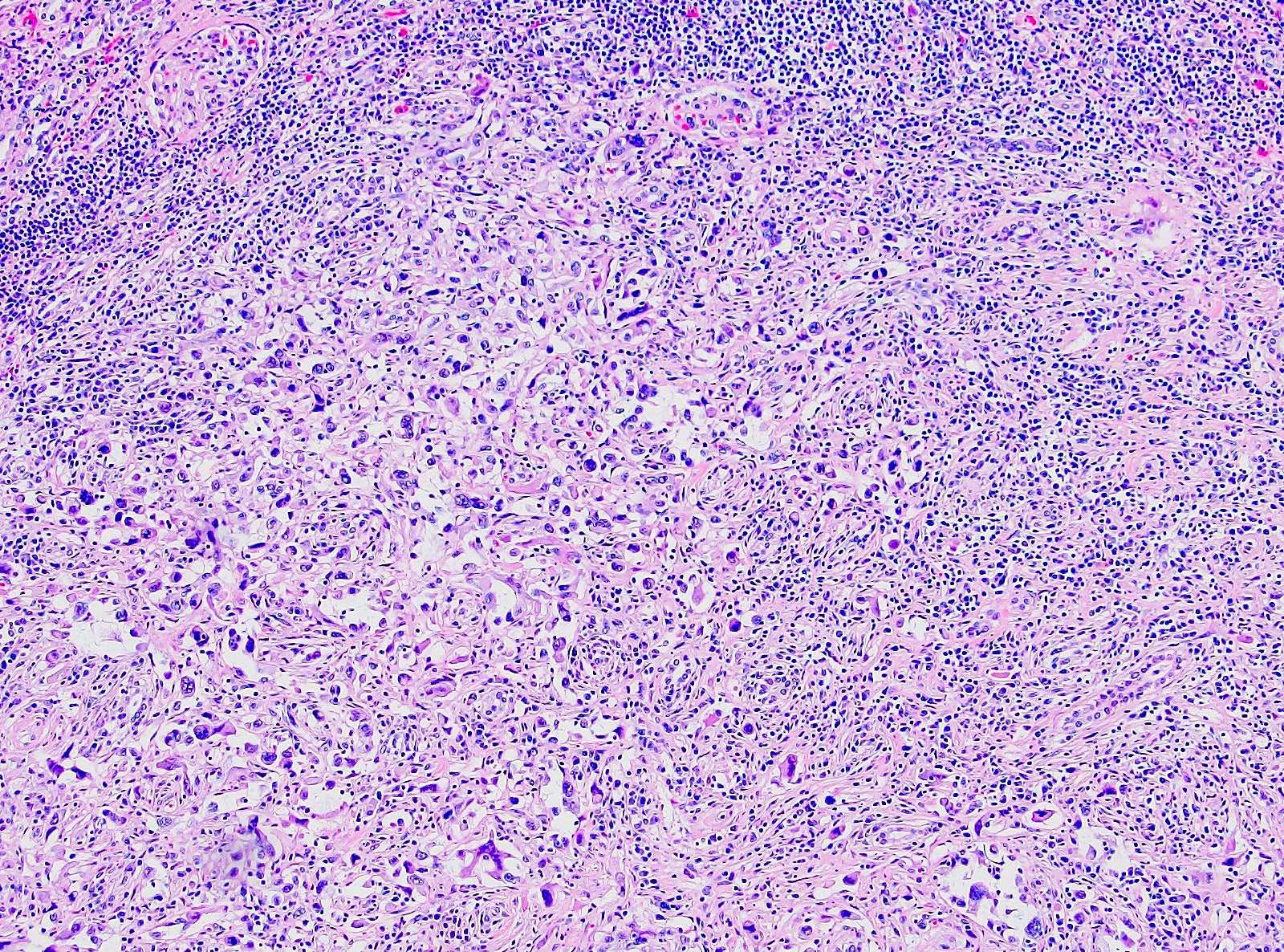
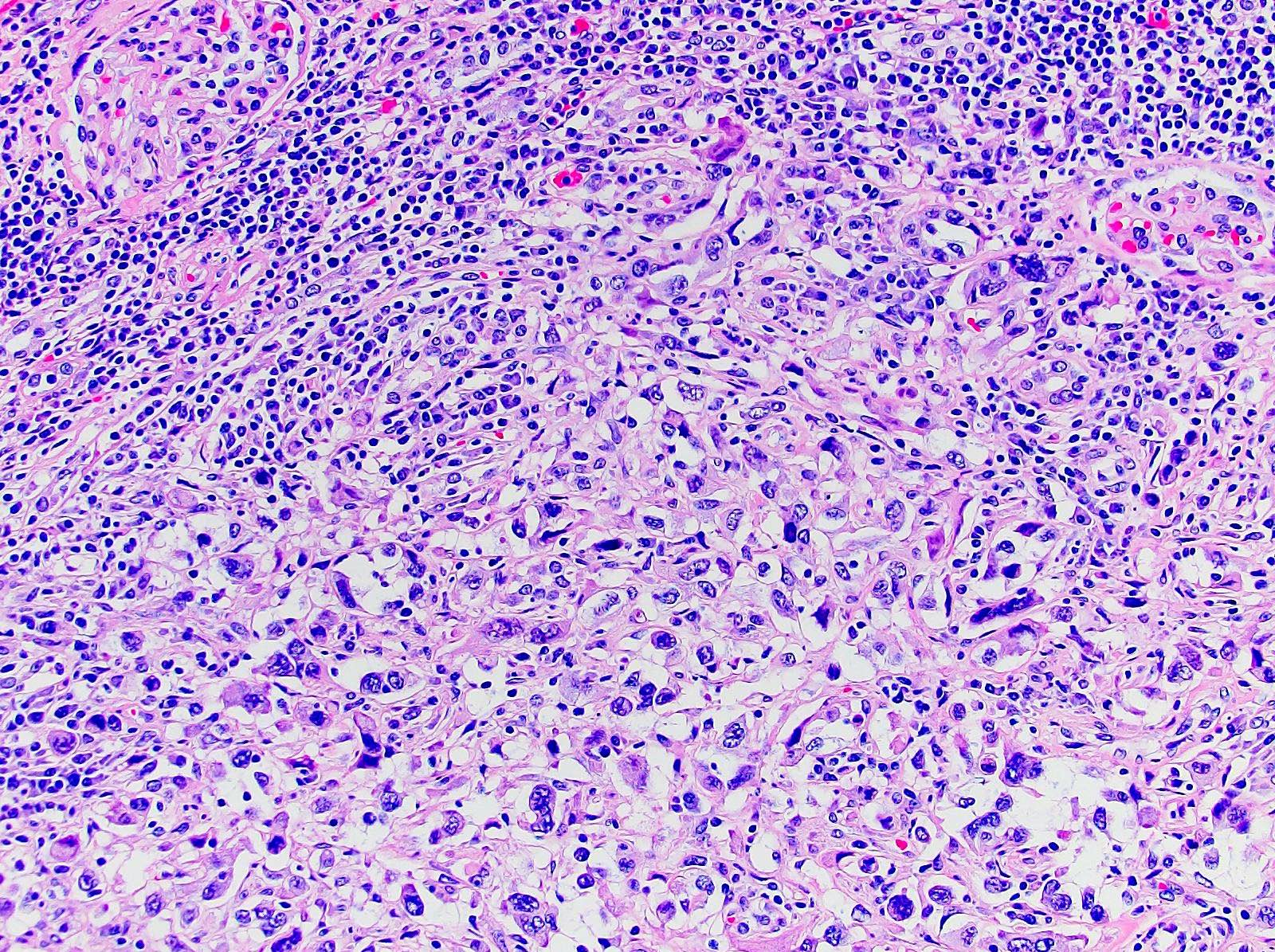
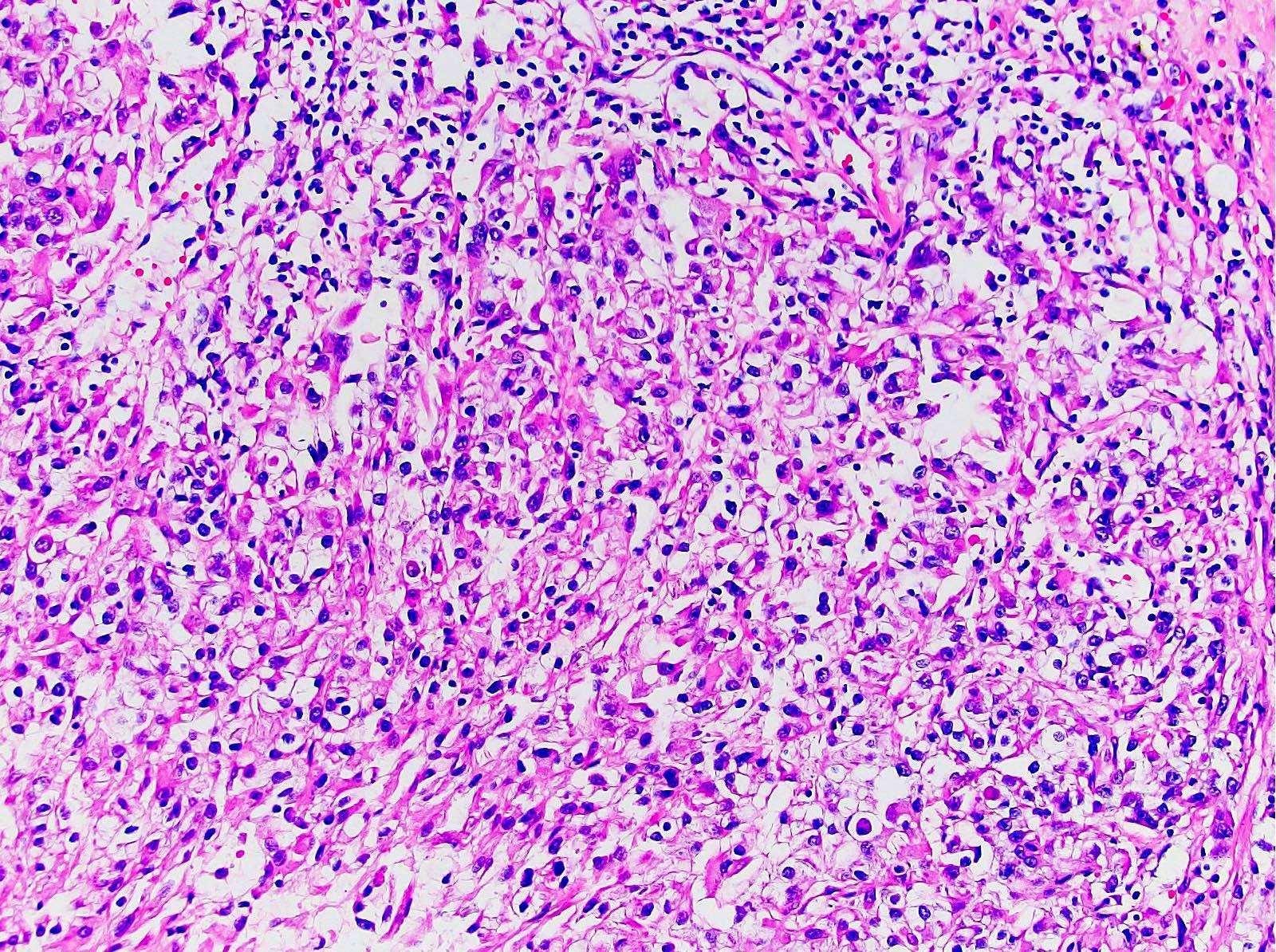
- Architecturally heterogeneous eosinophilic tumor with polygonal, rhabdoid, signet ring and spindled cells, mucin production and cytoplasmic vacuoles
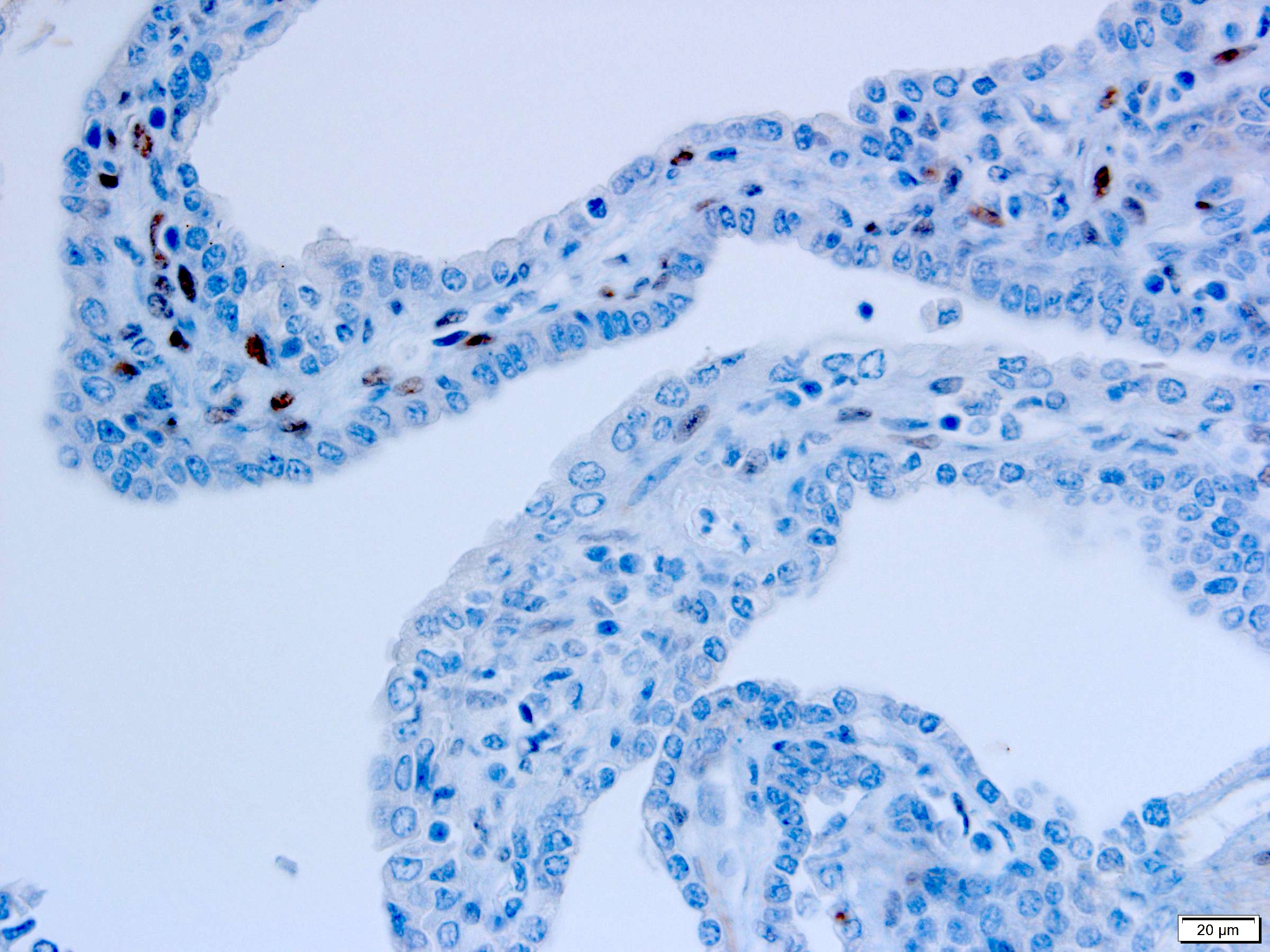
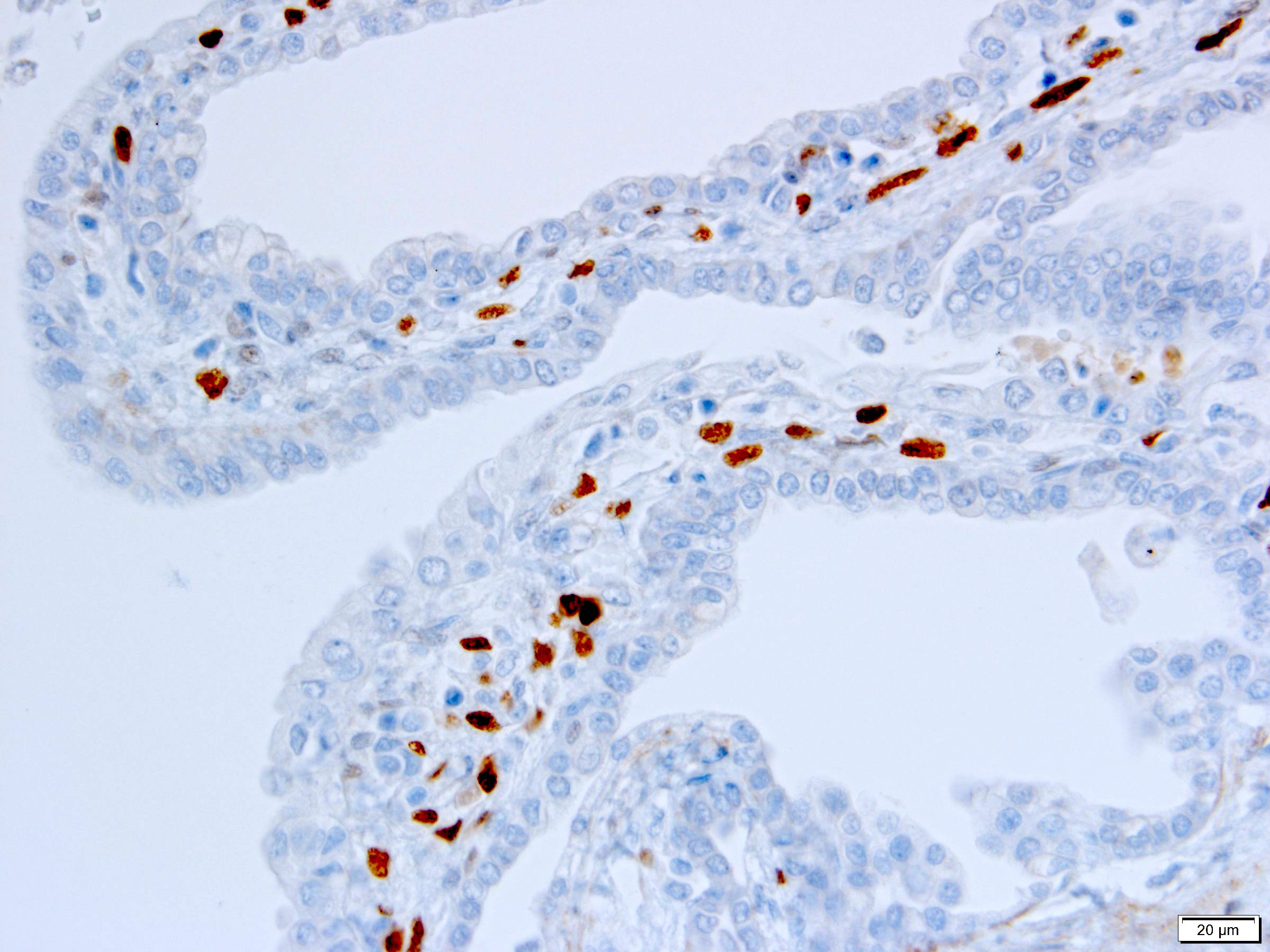
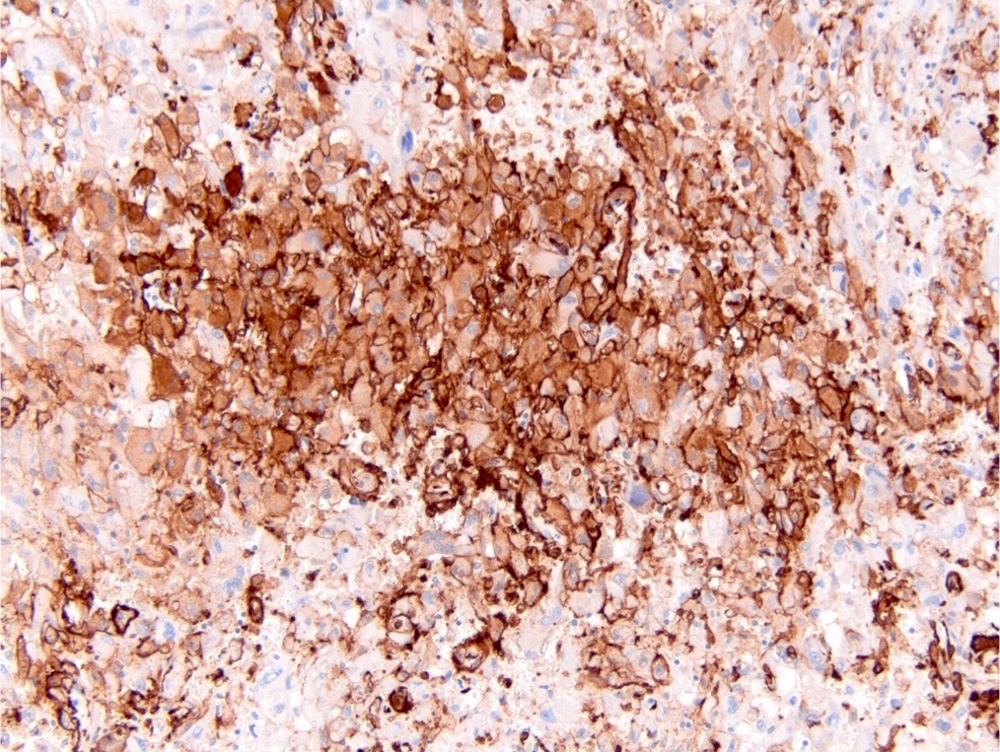
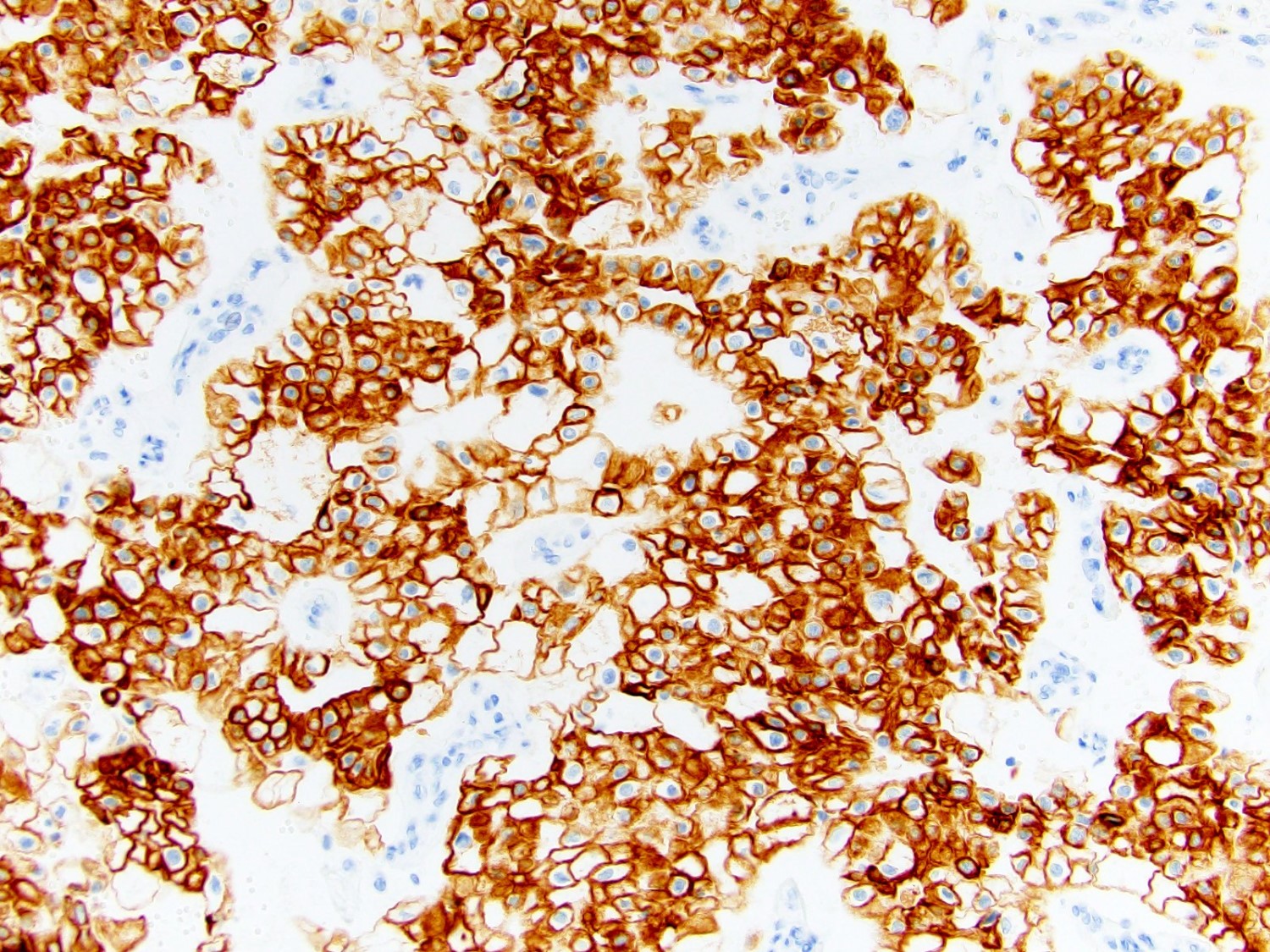
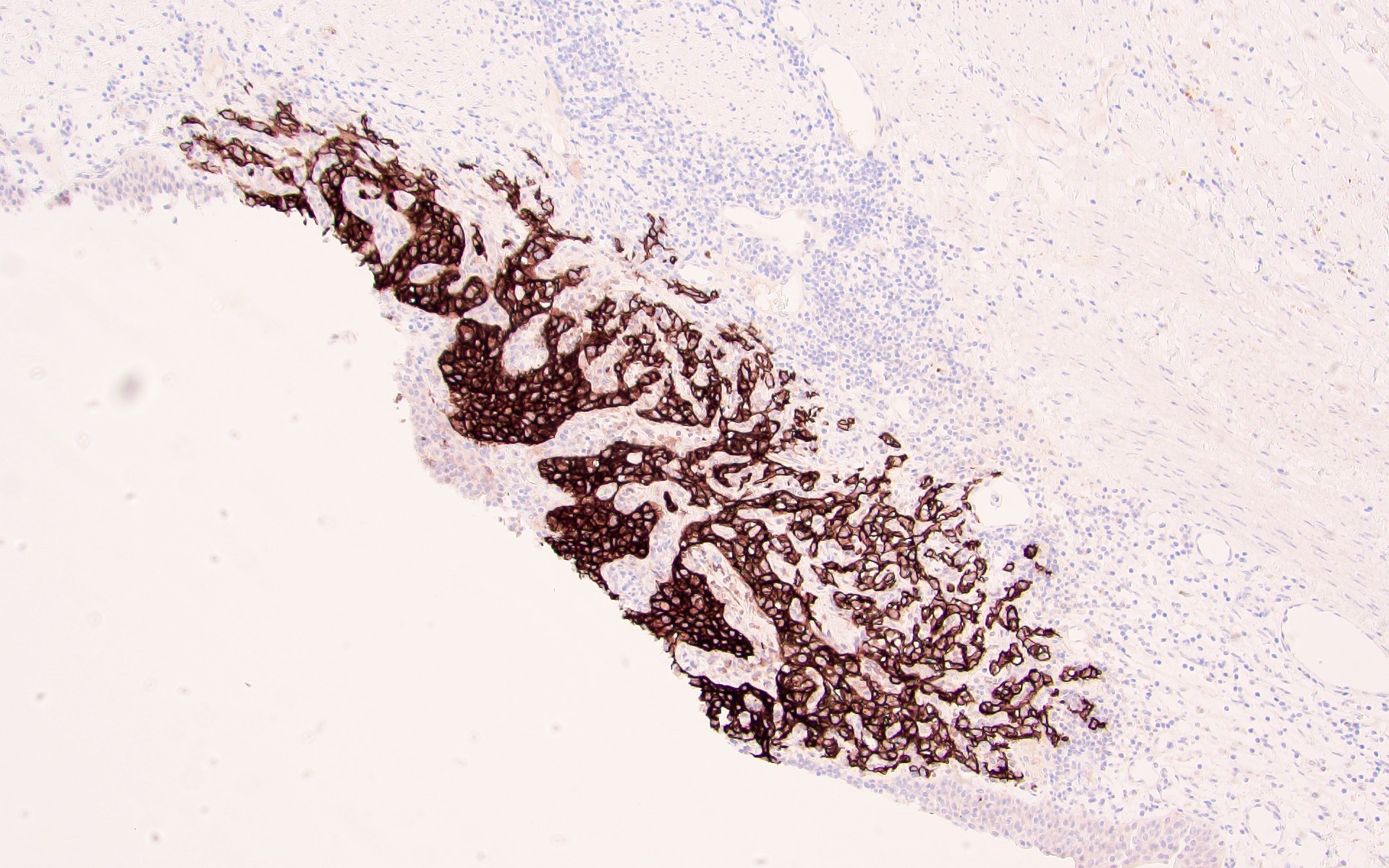
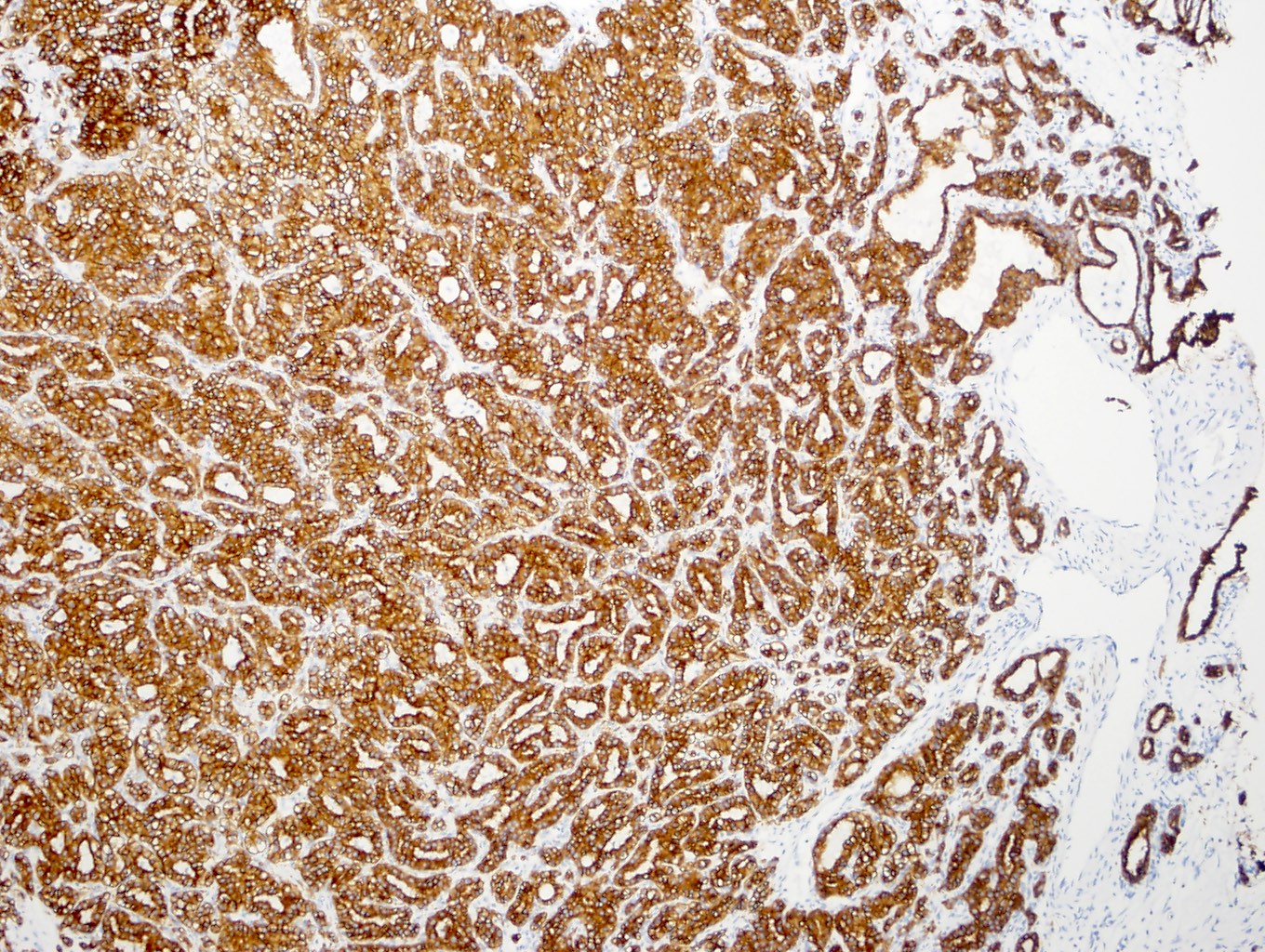
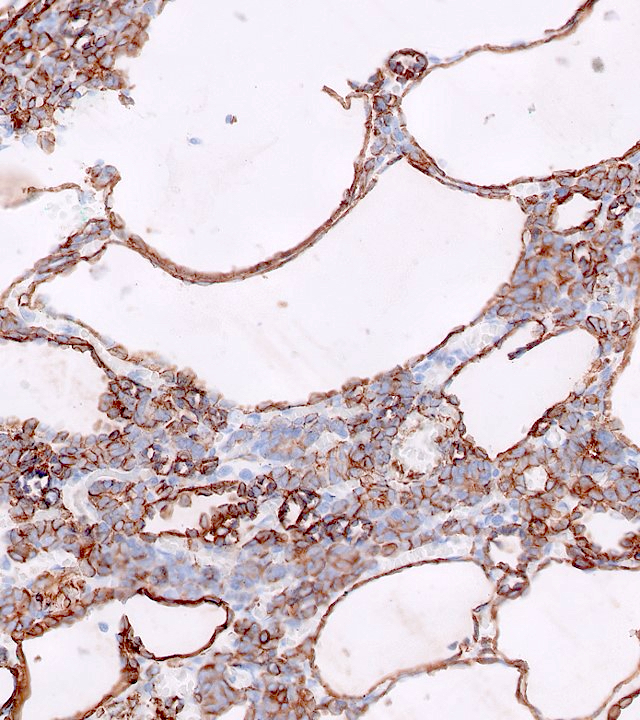
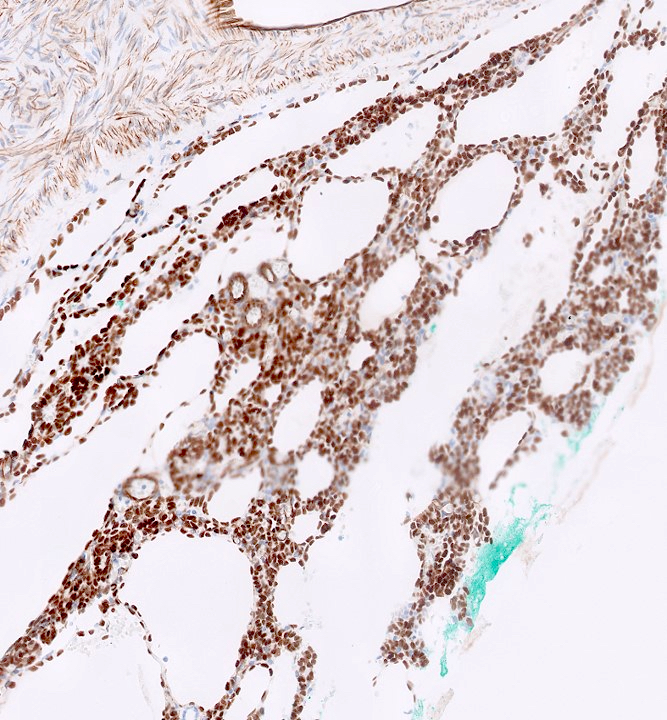
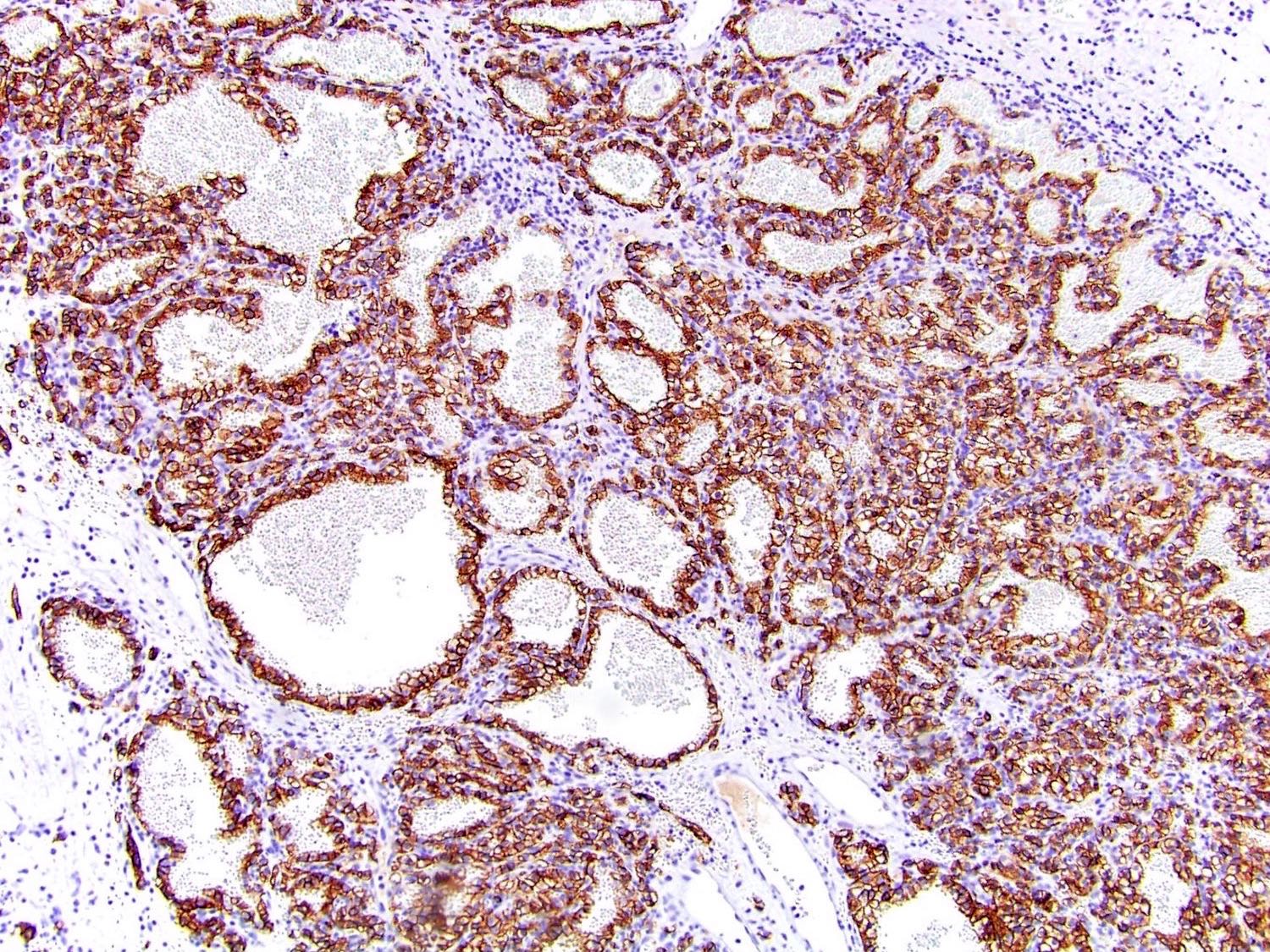
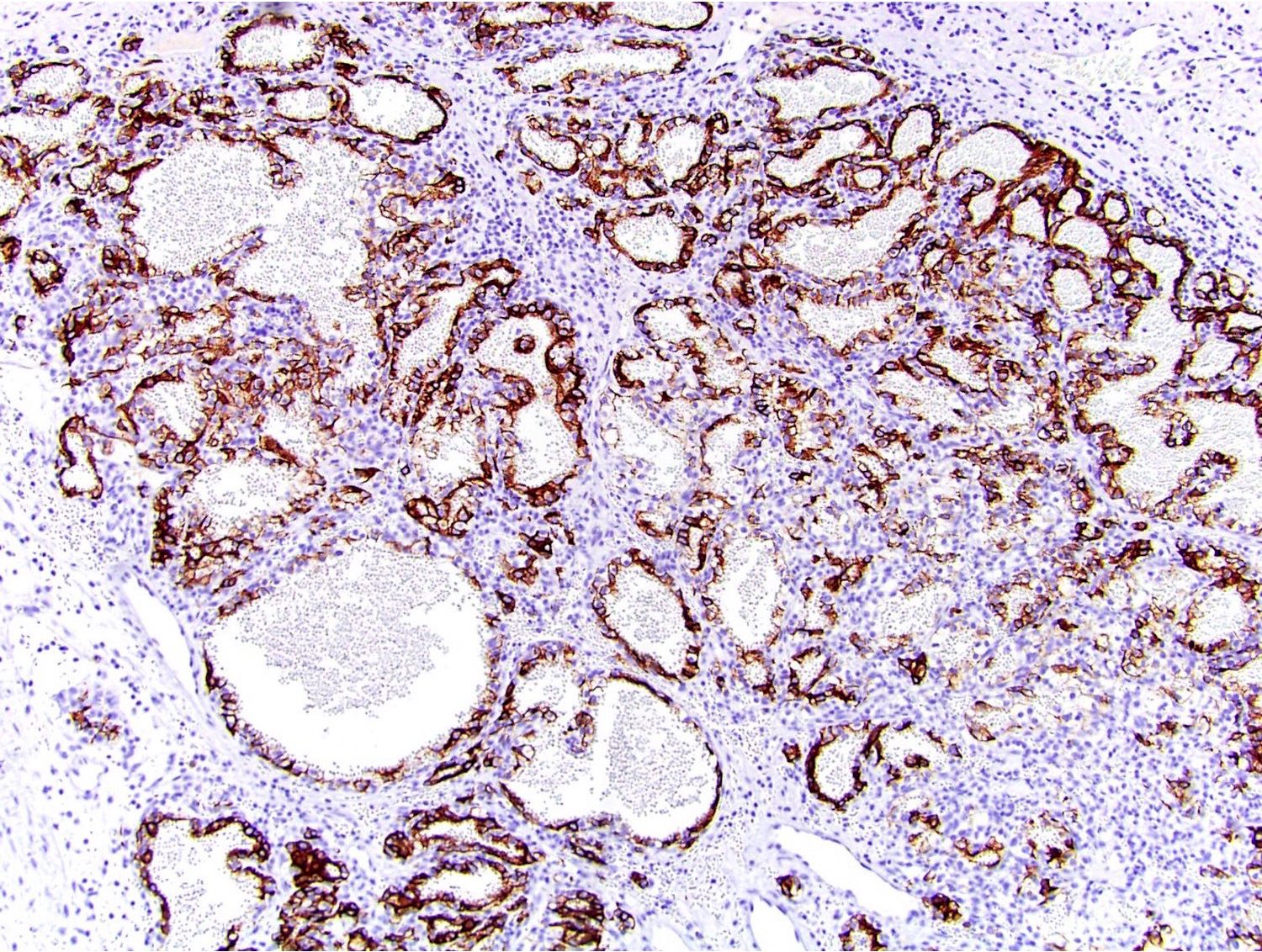
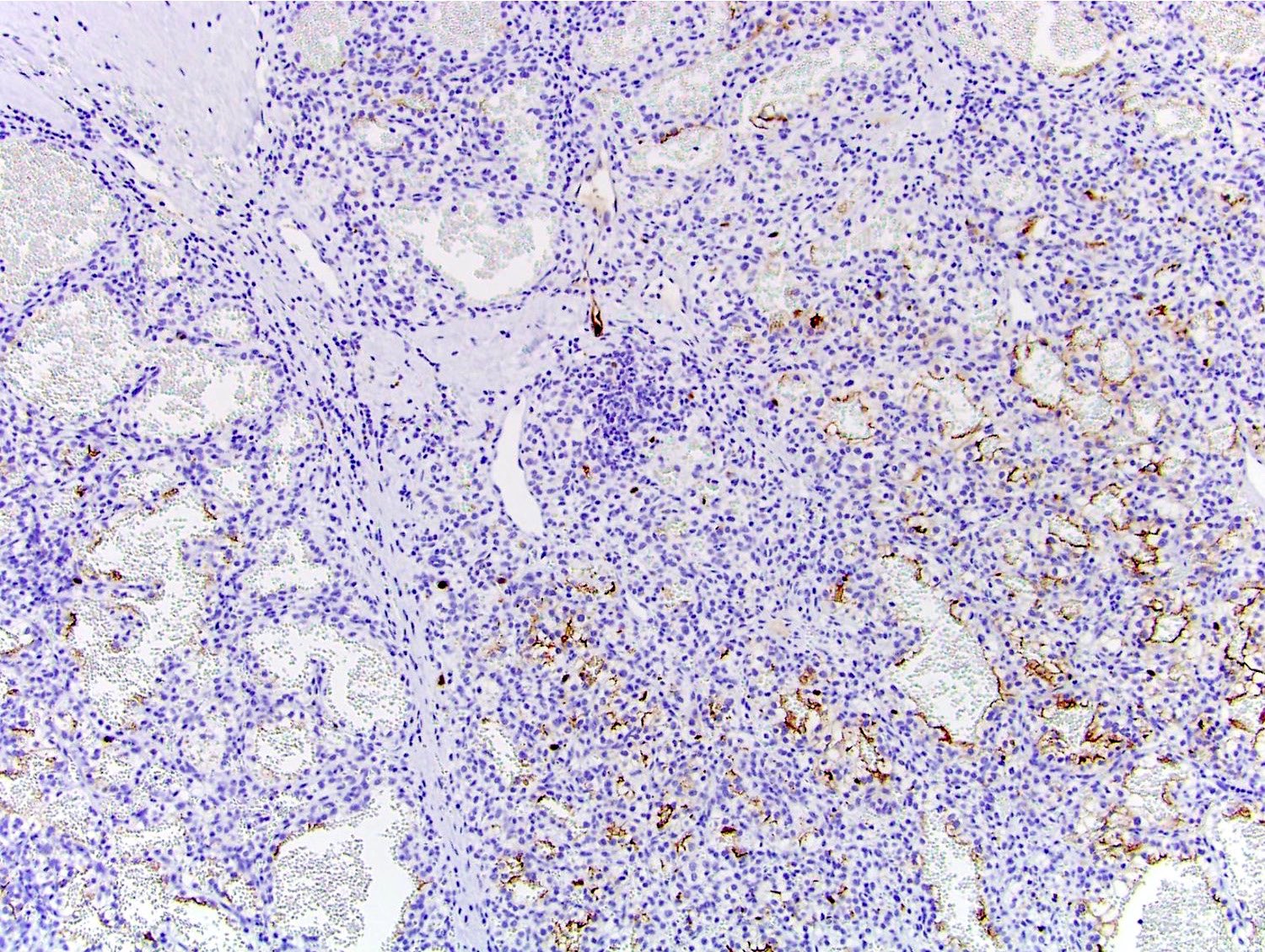
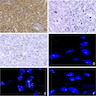
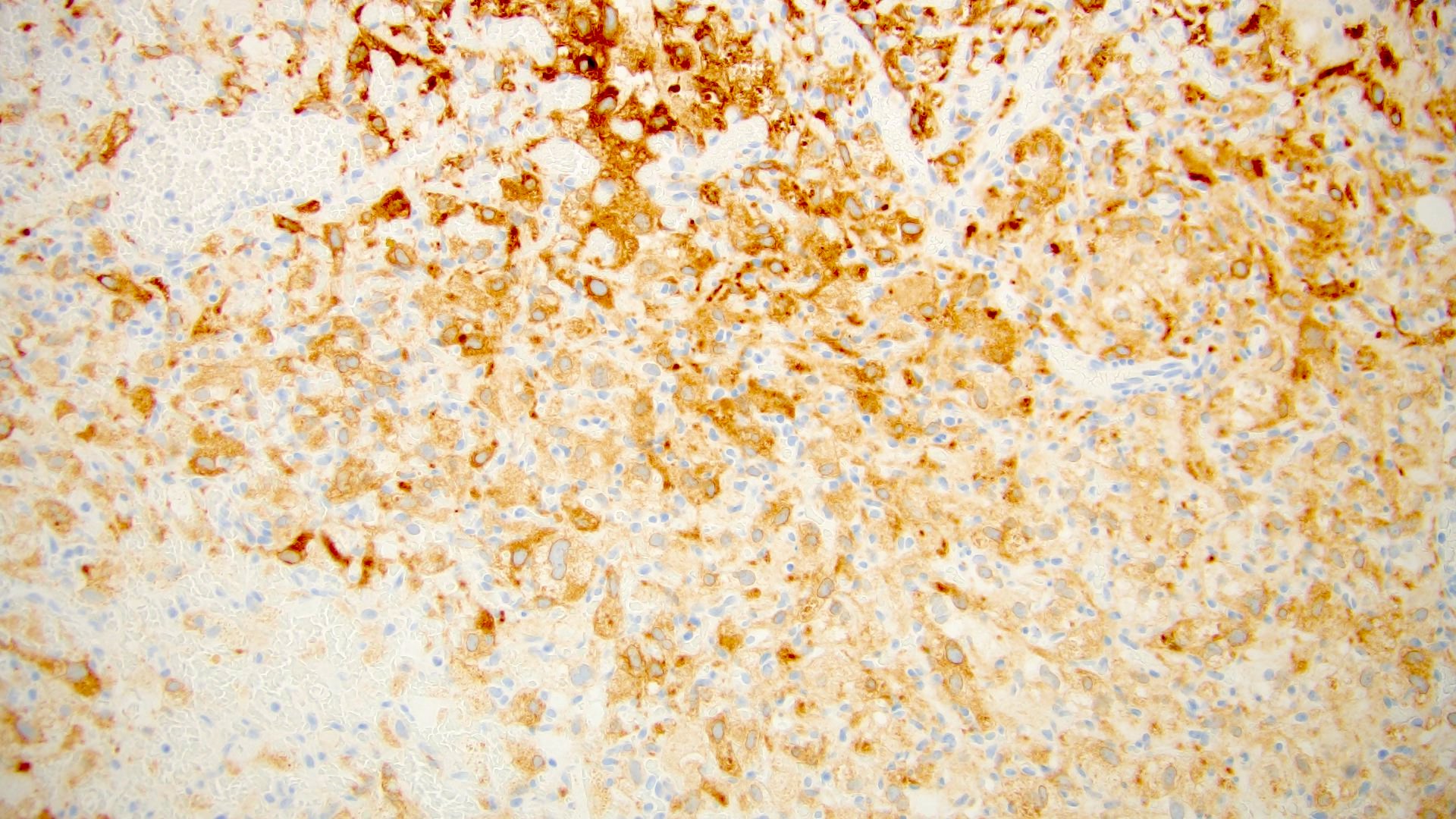
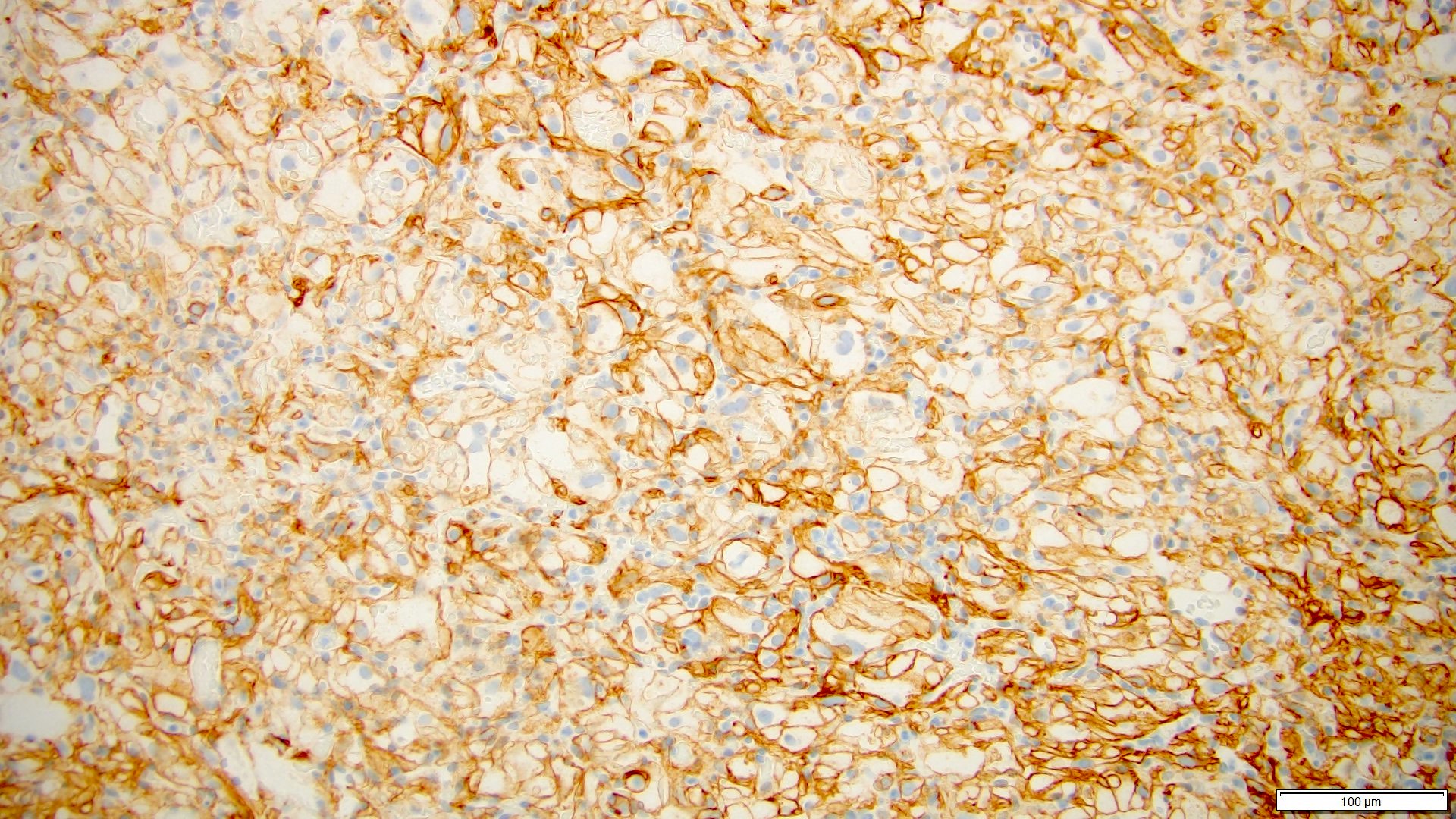
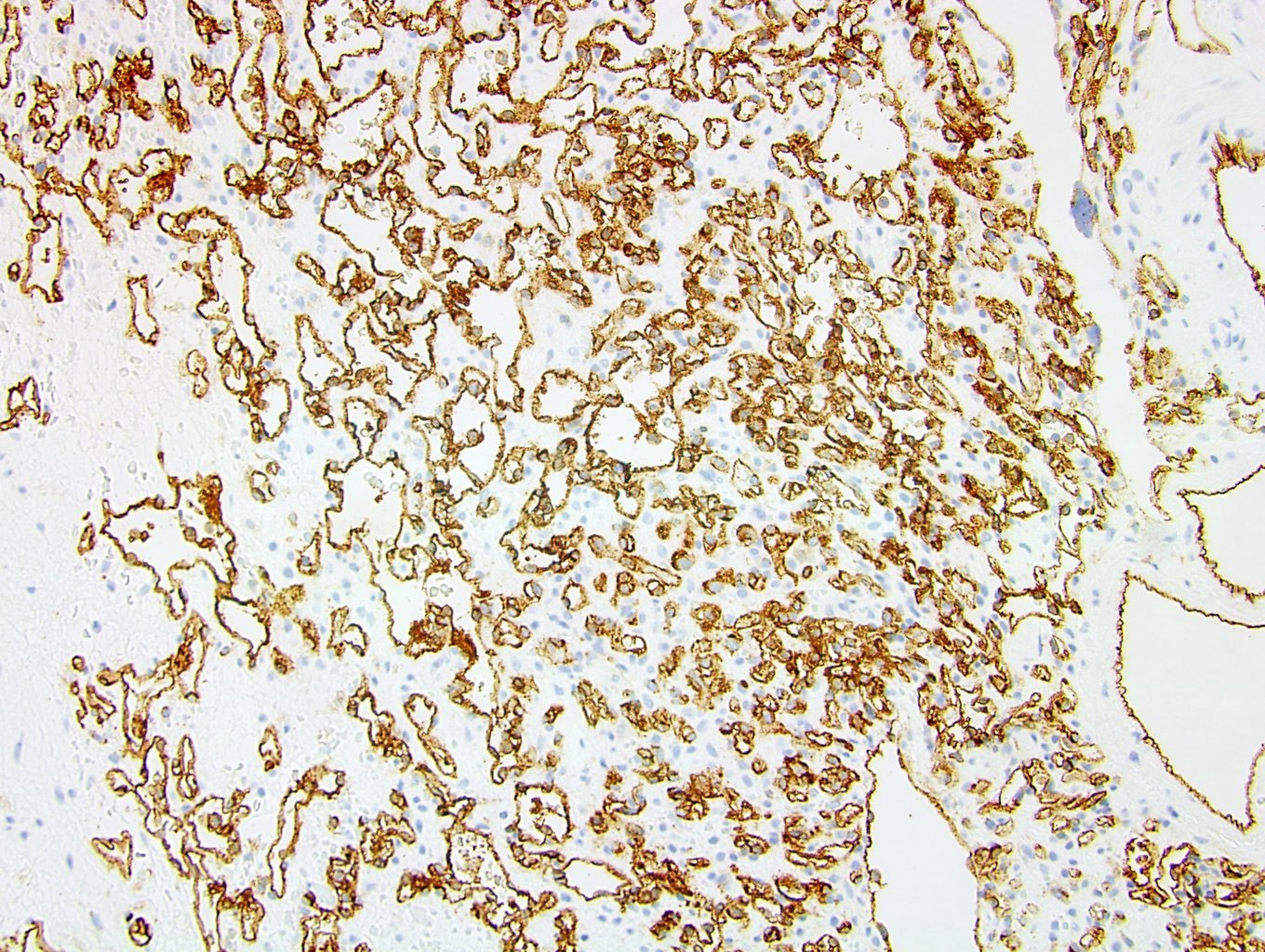
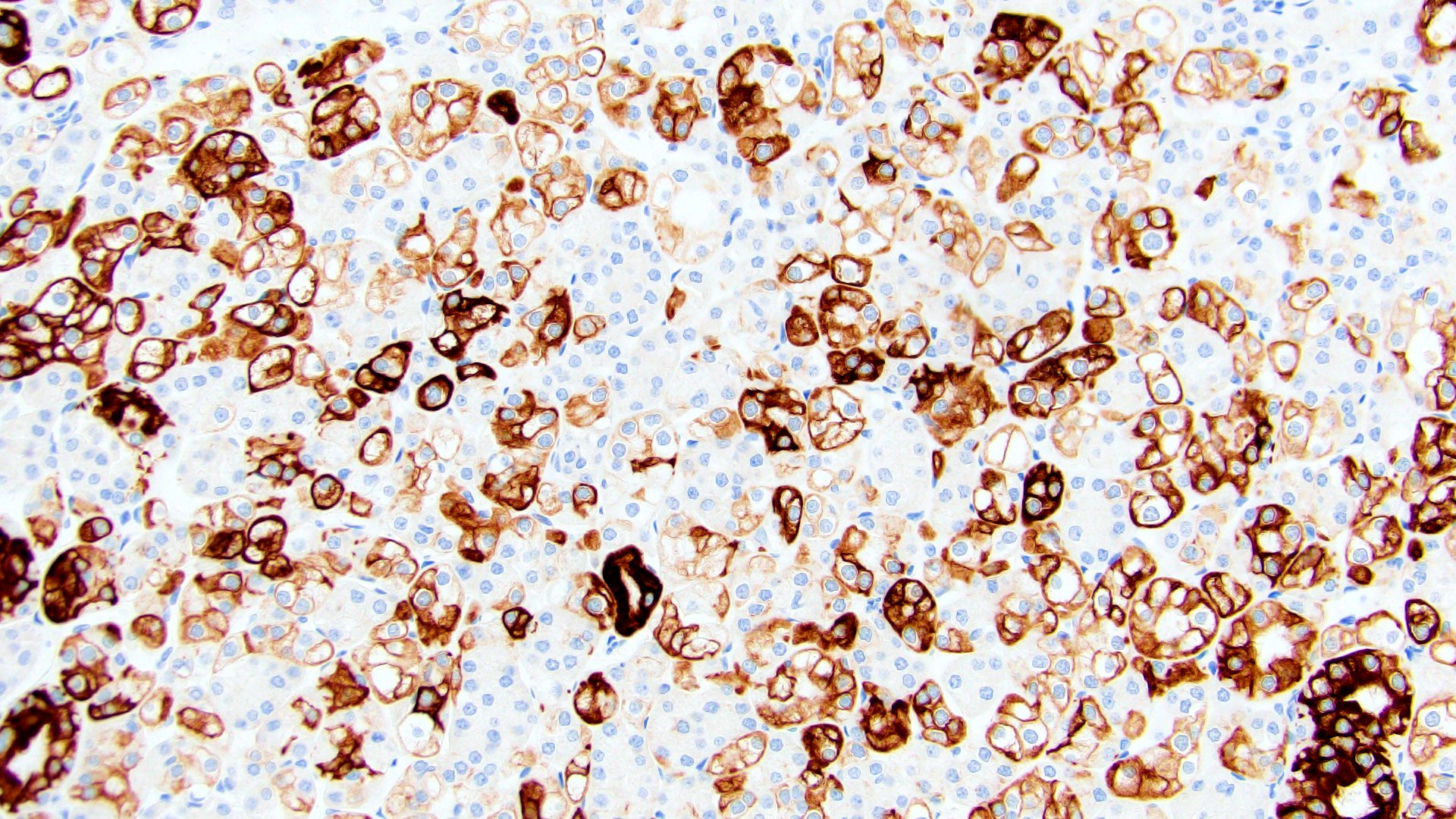
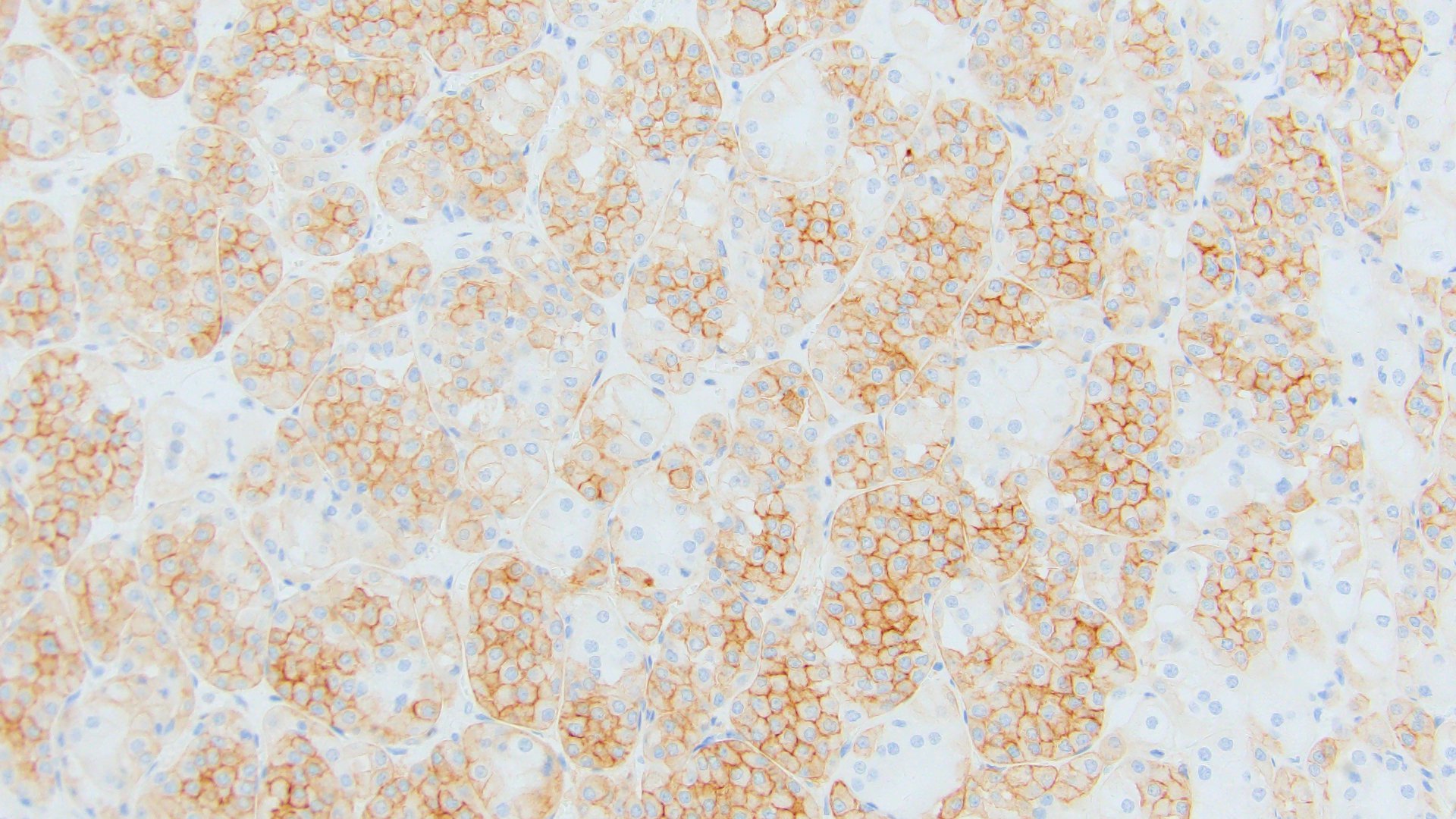
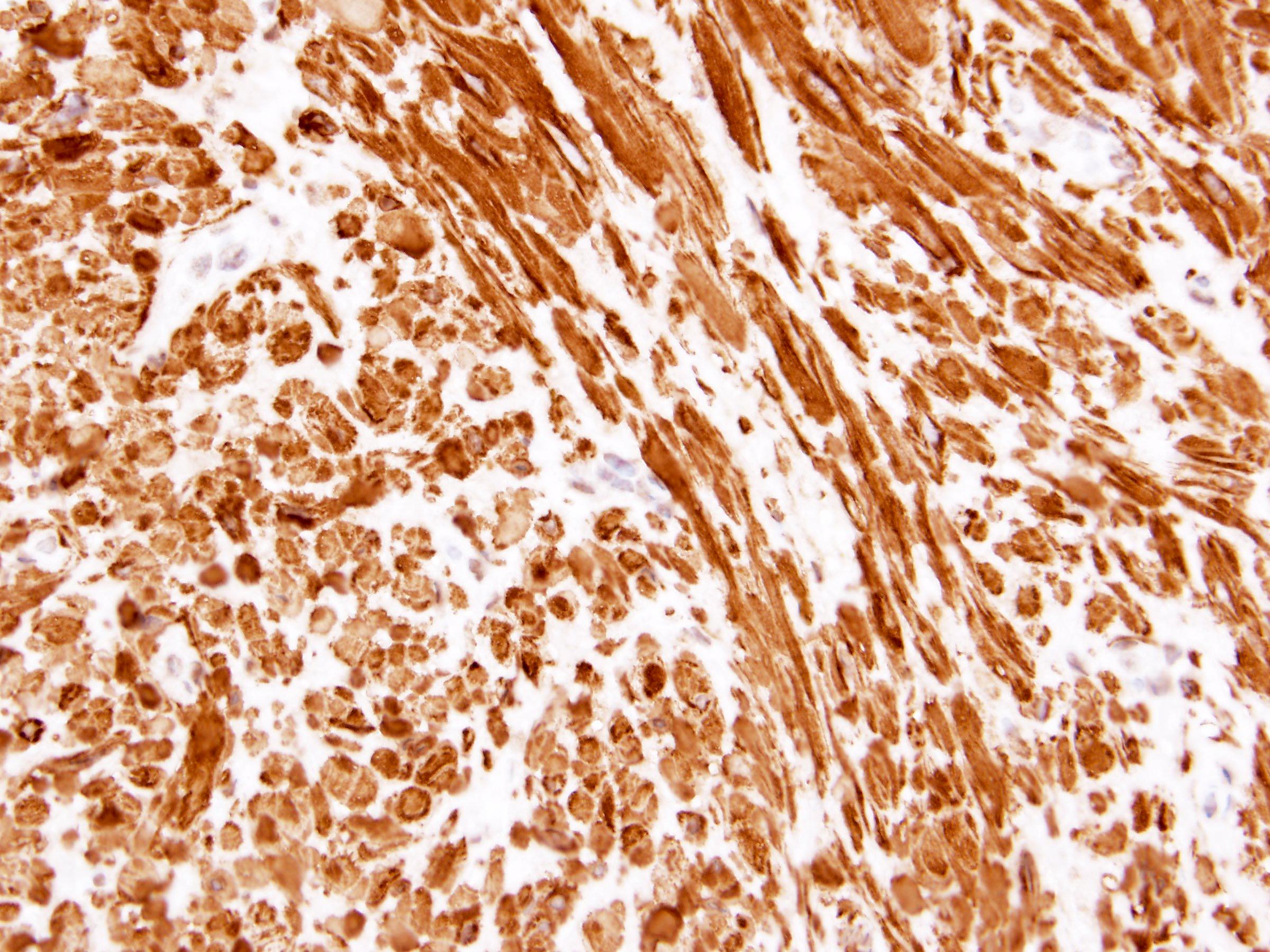
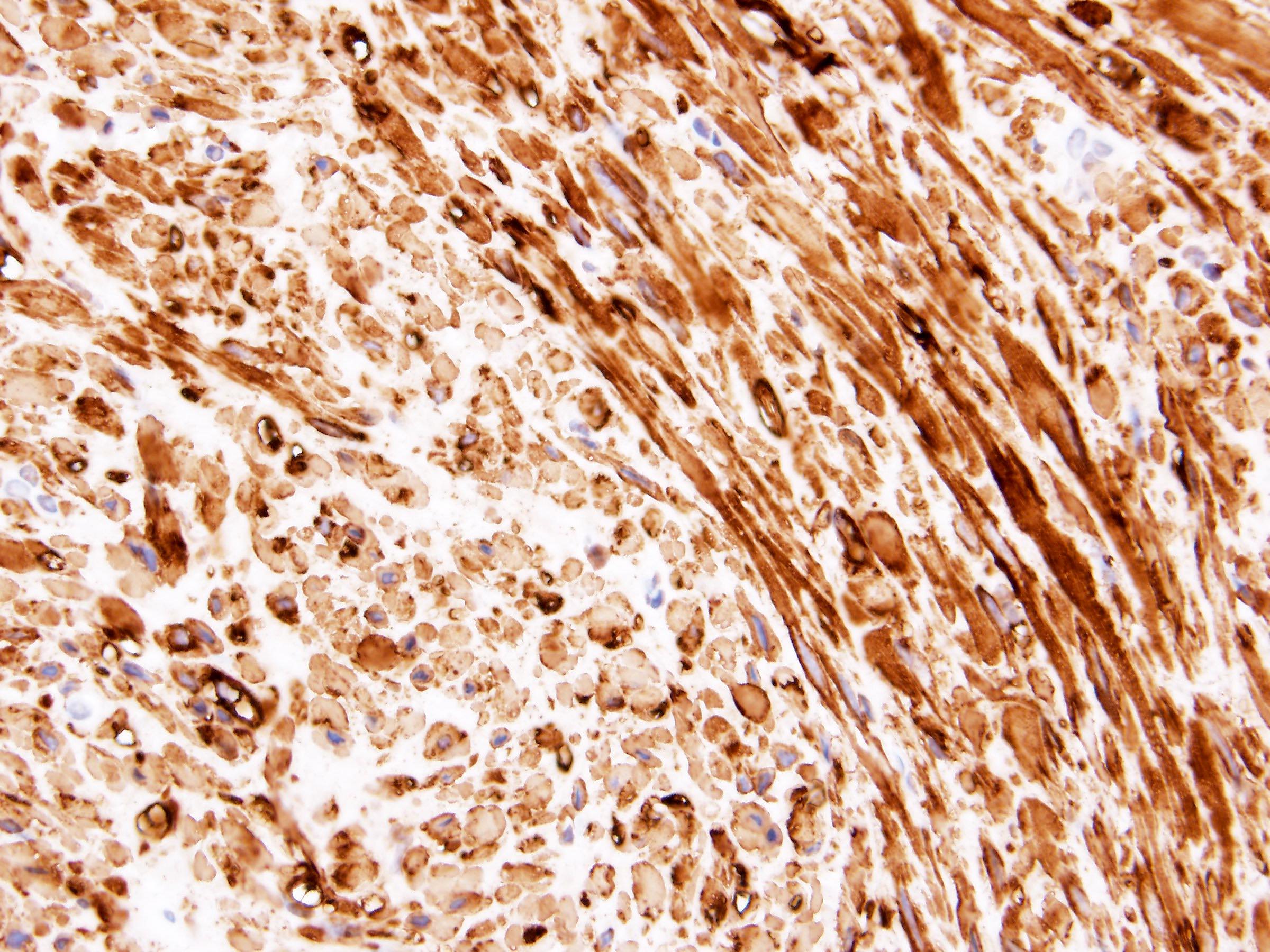
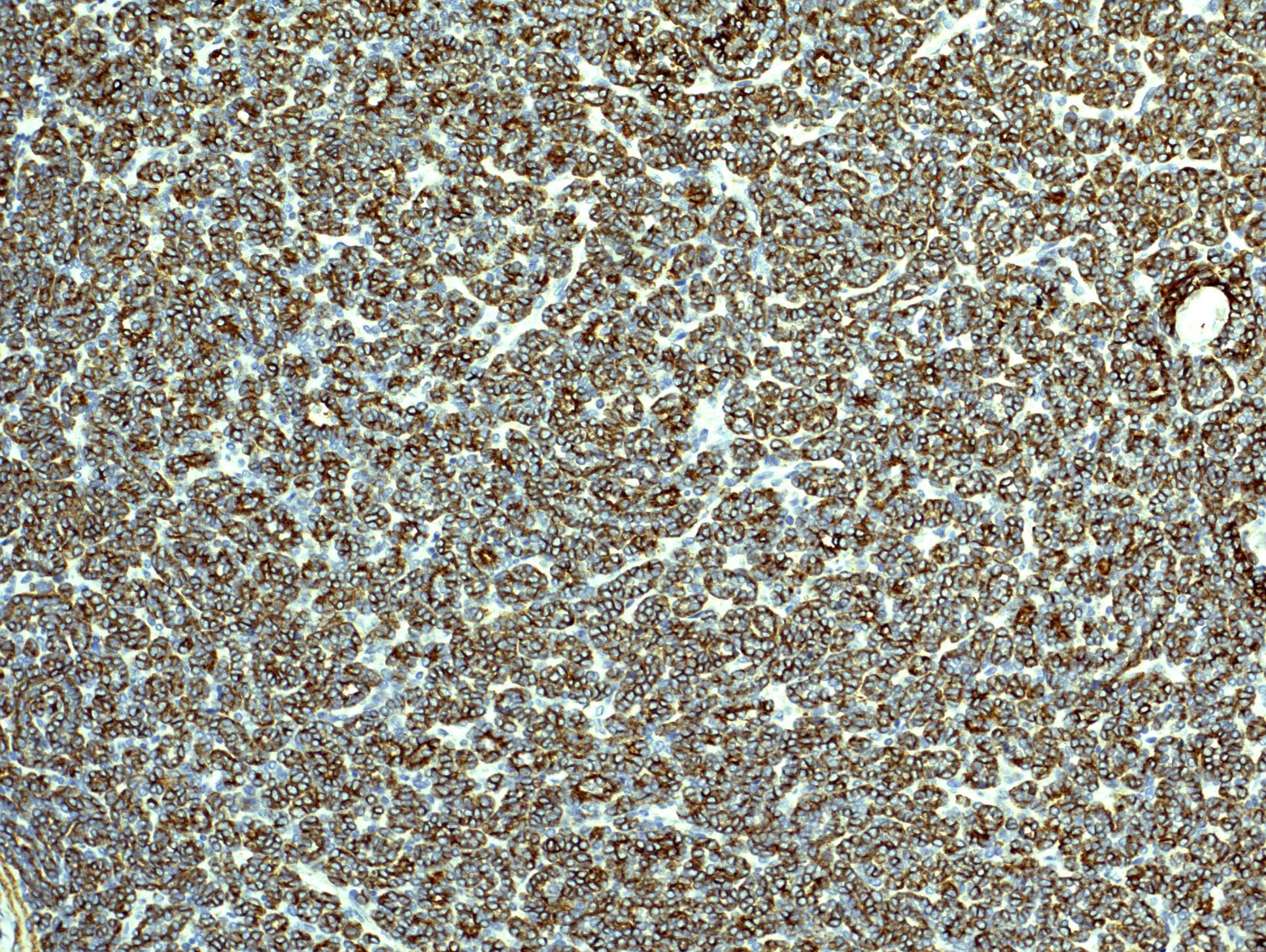
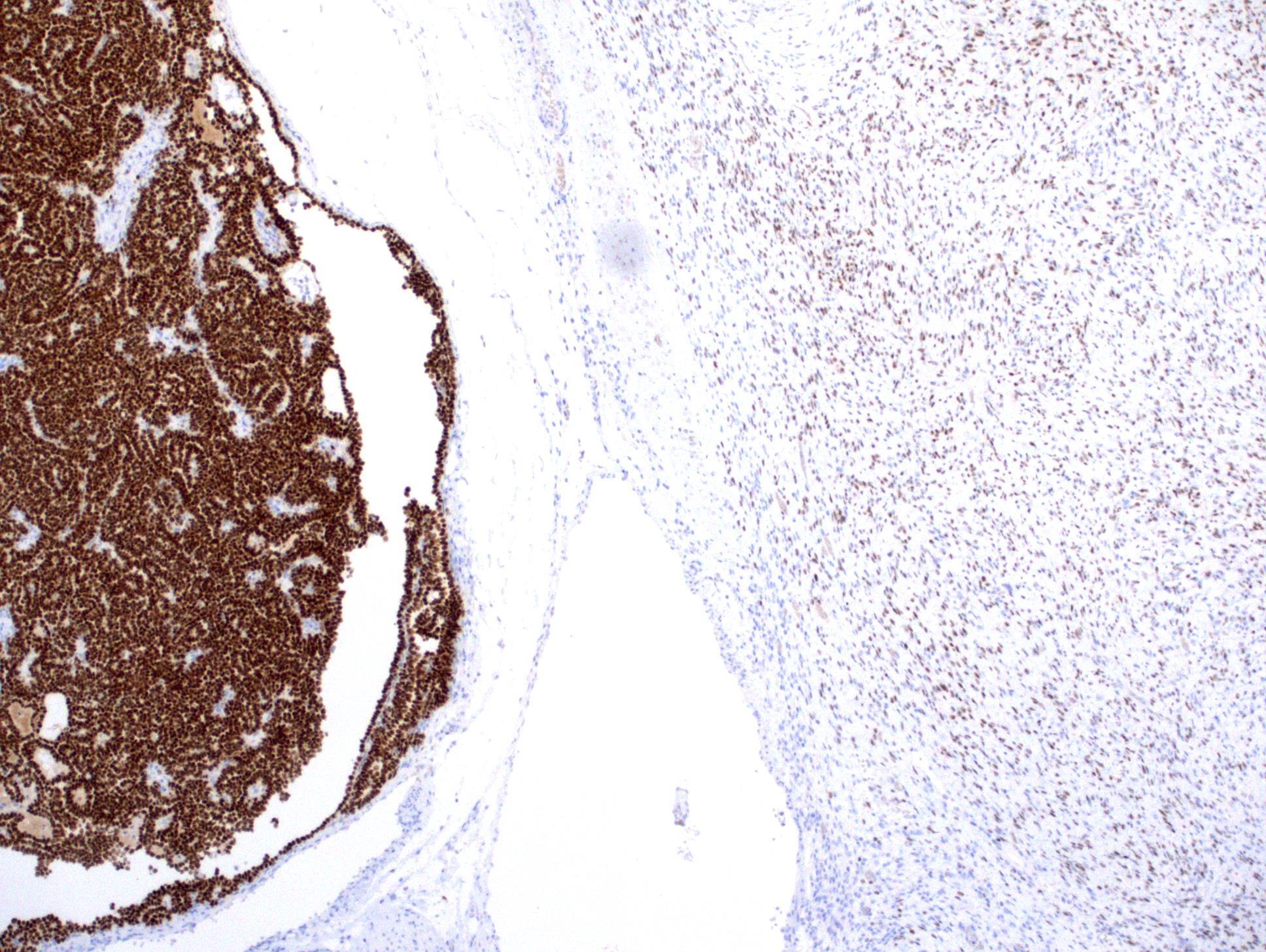
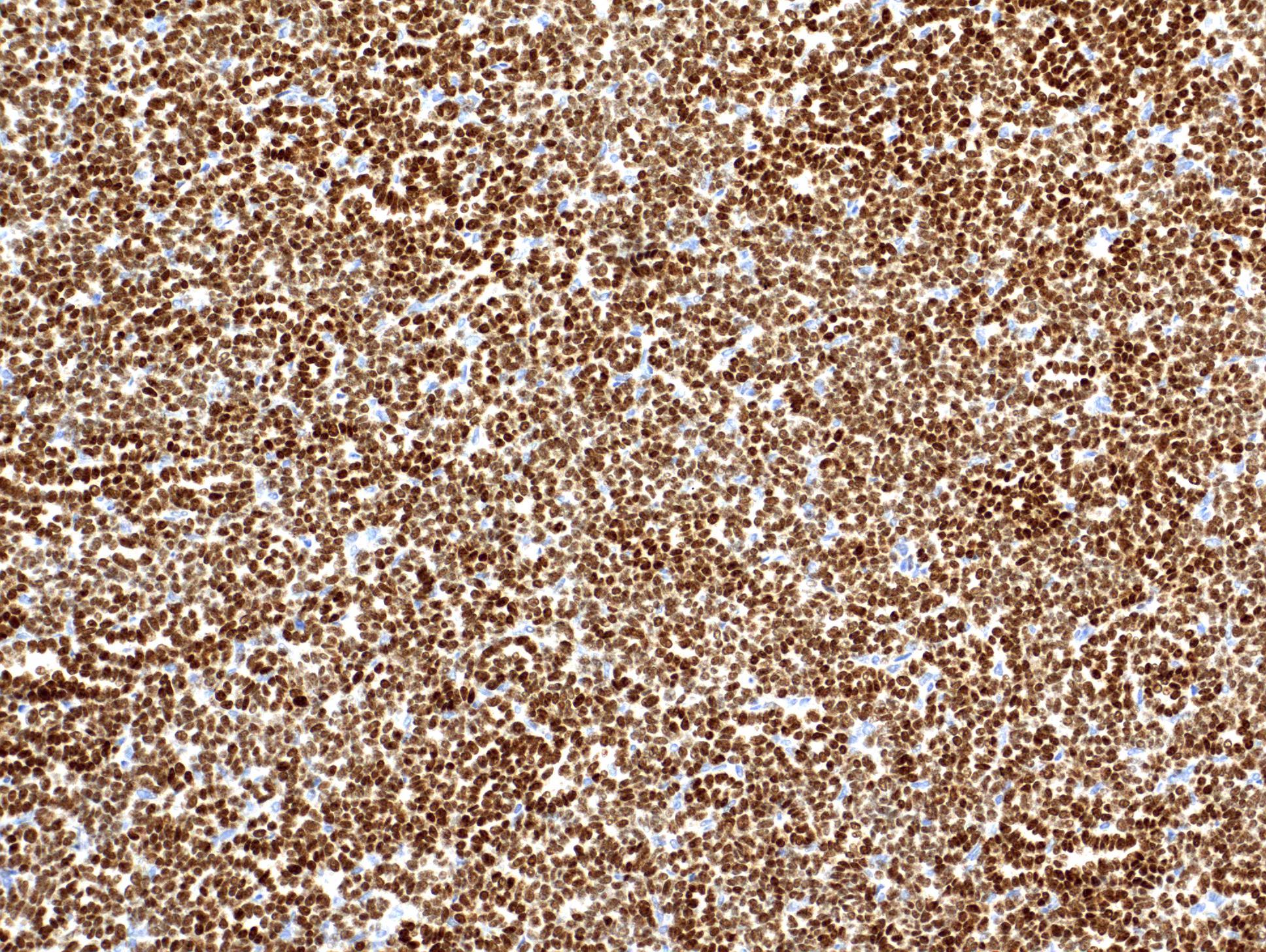
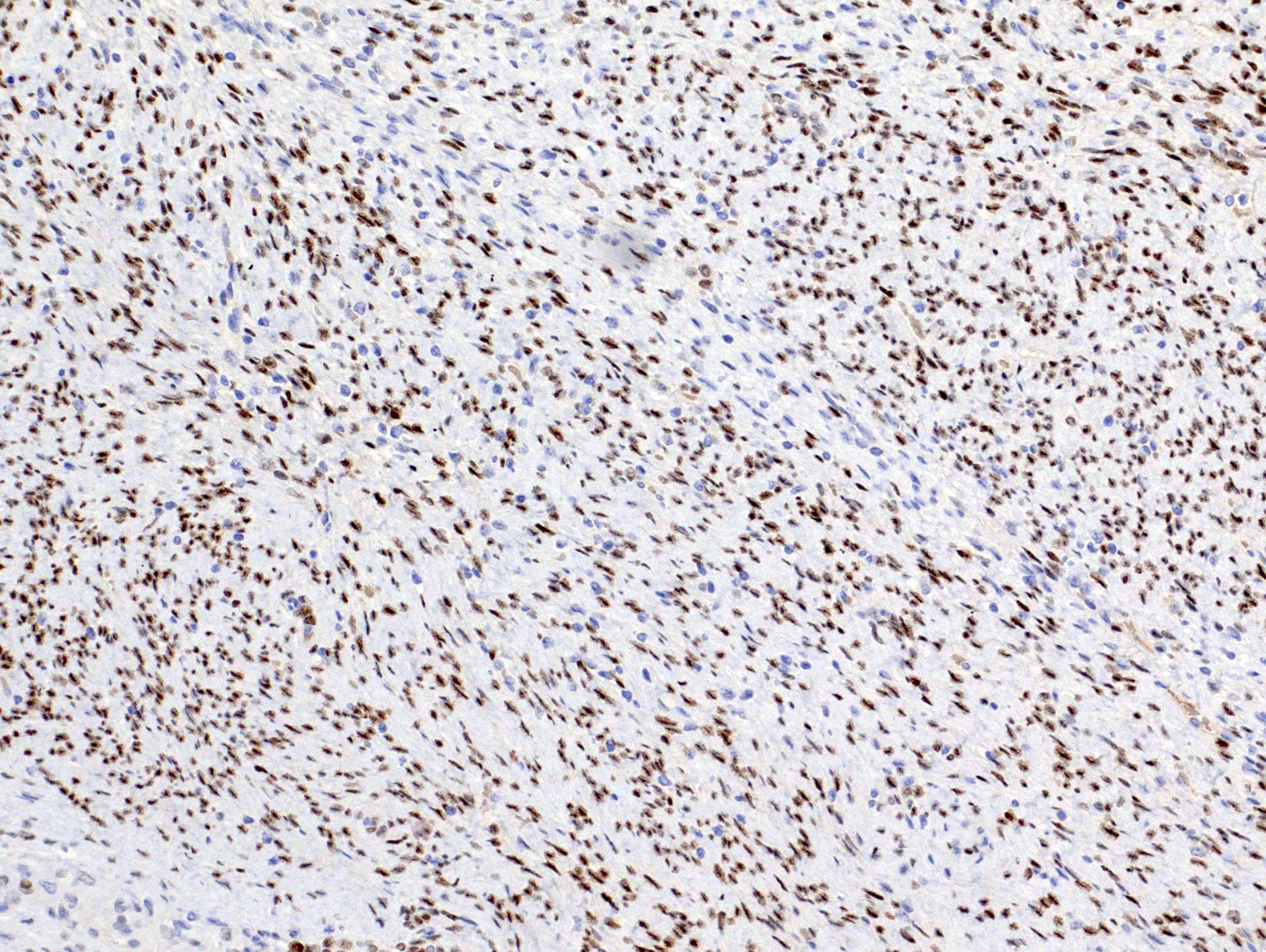
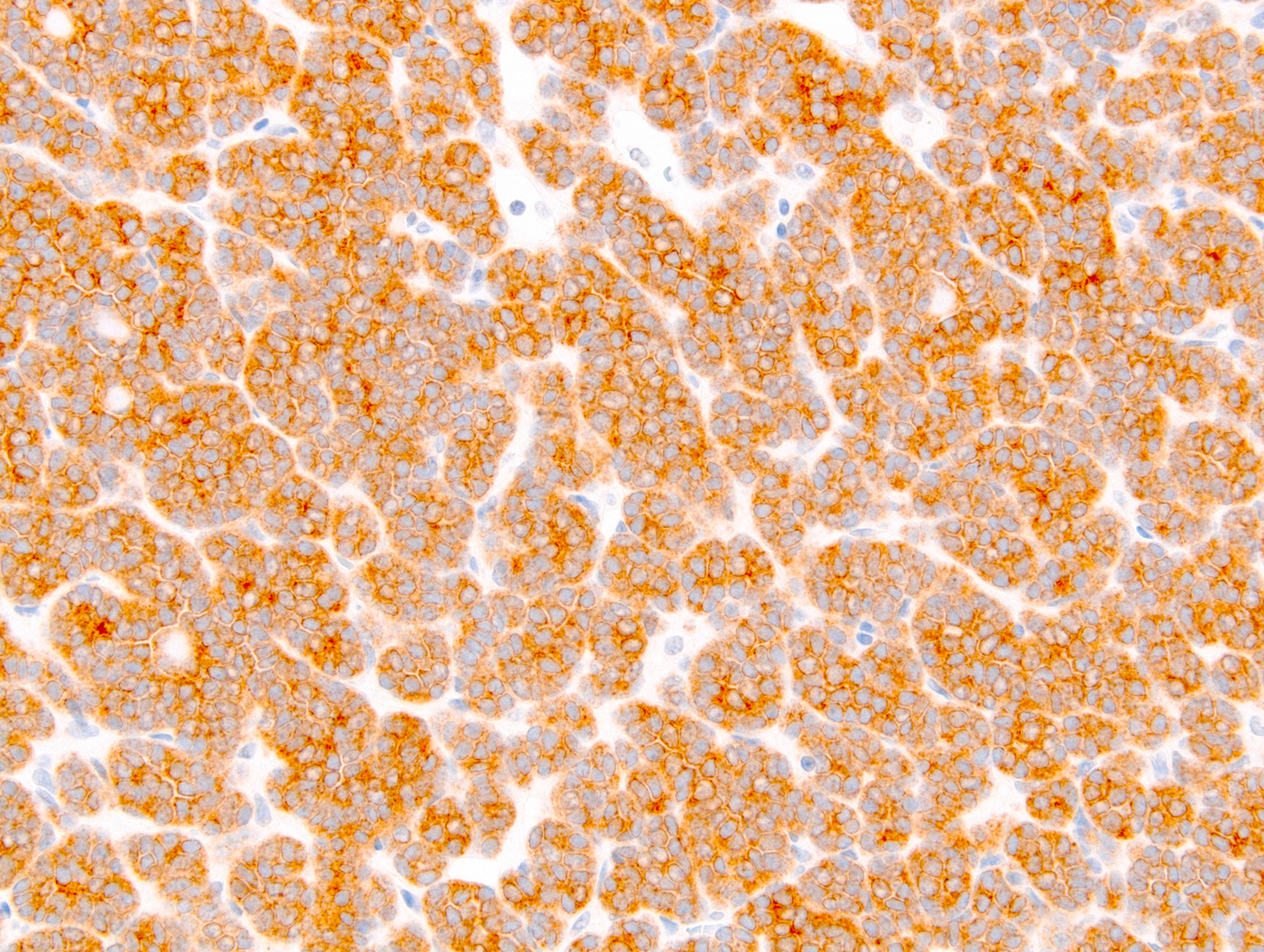
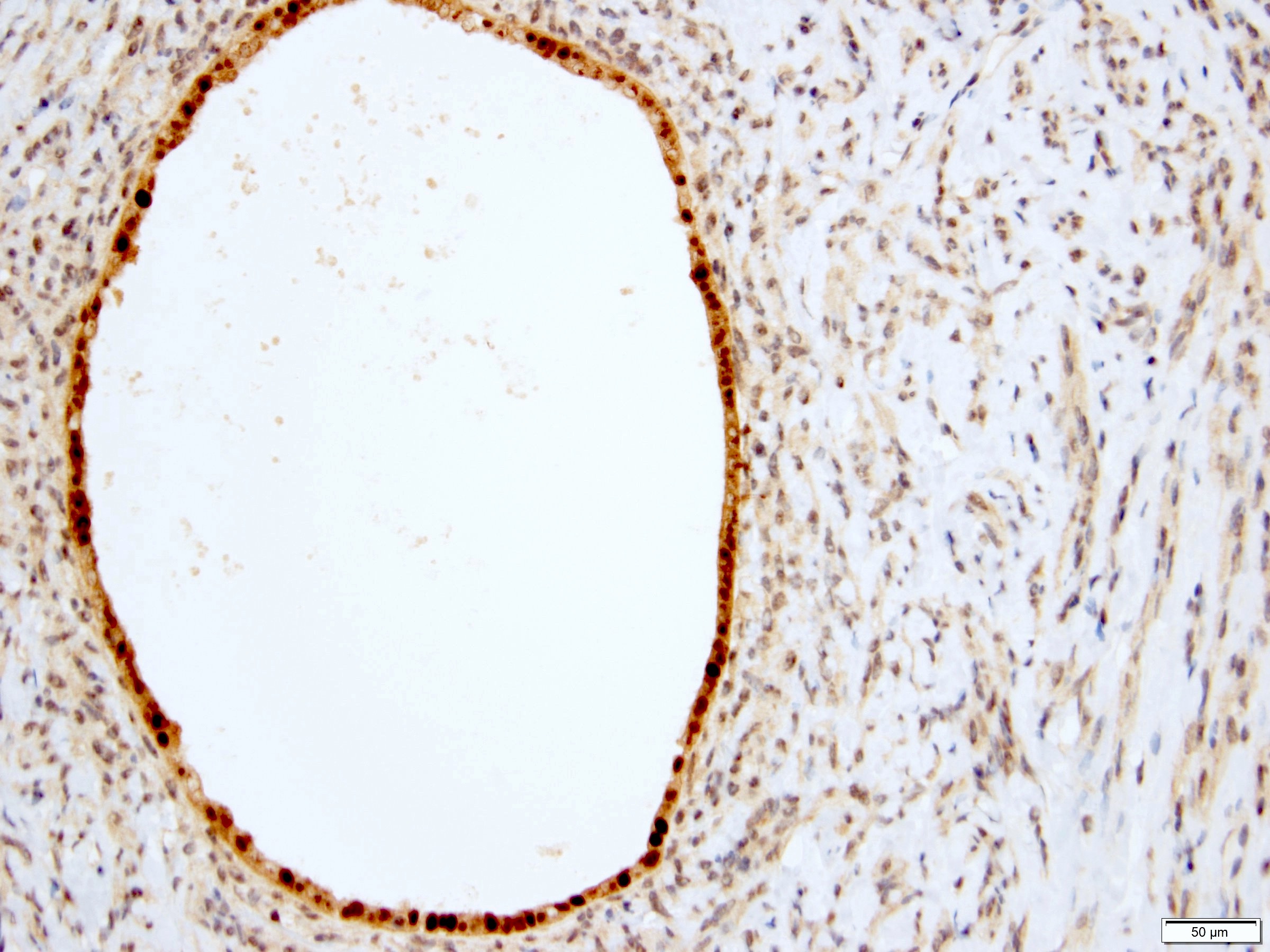
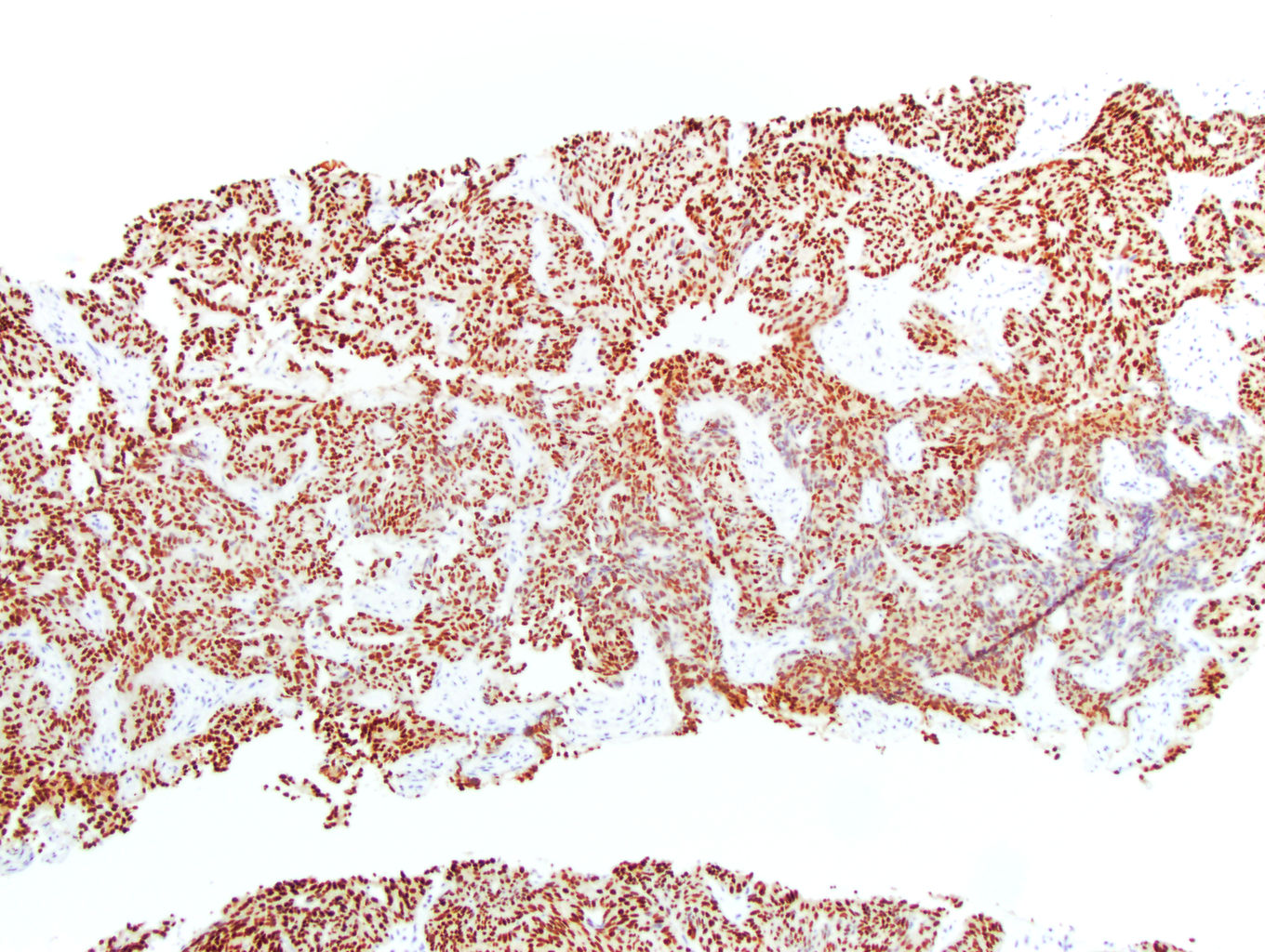
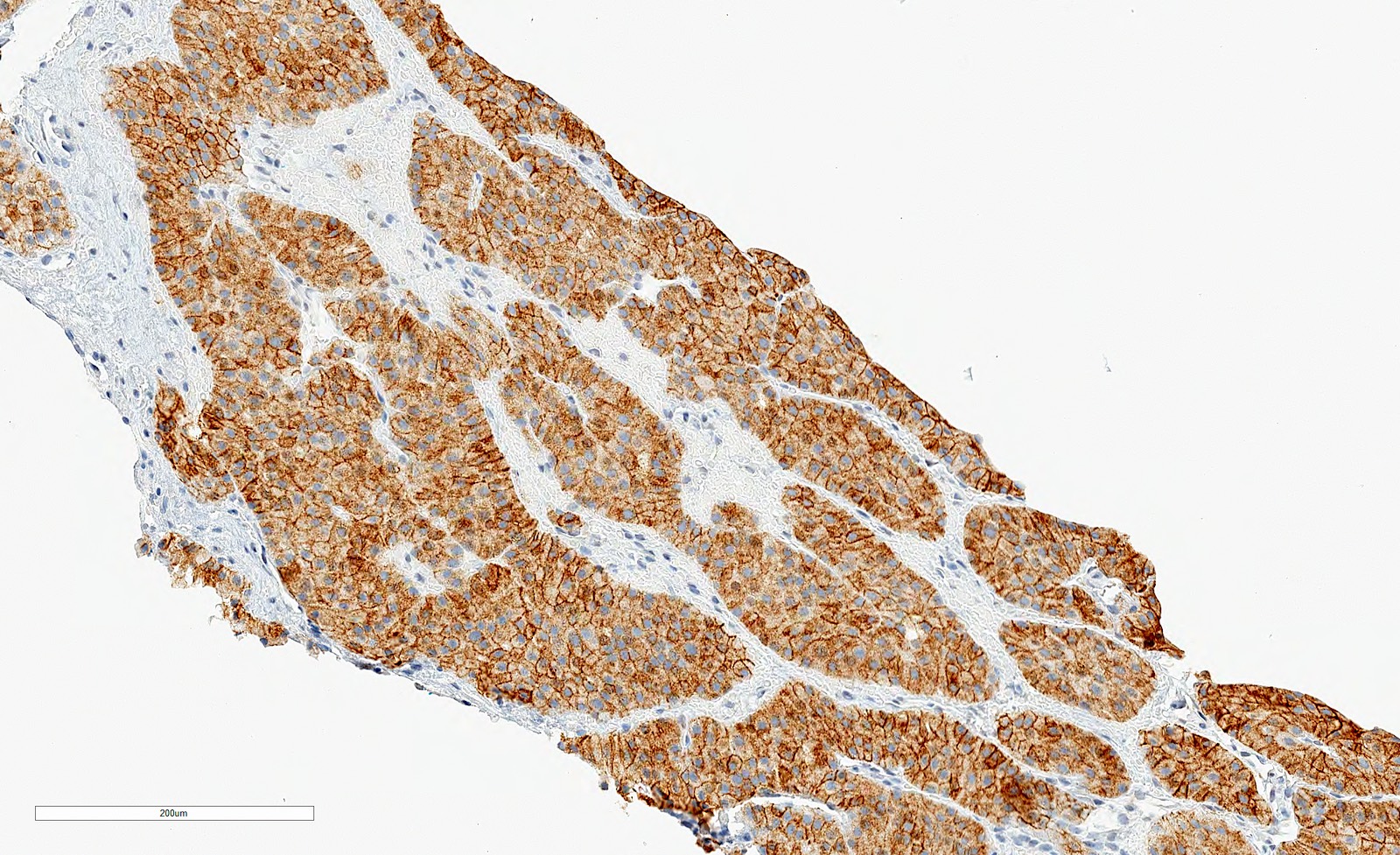
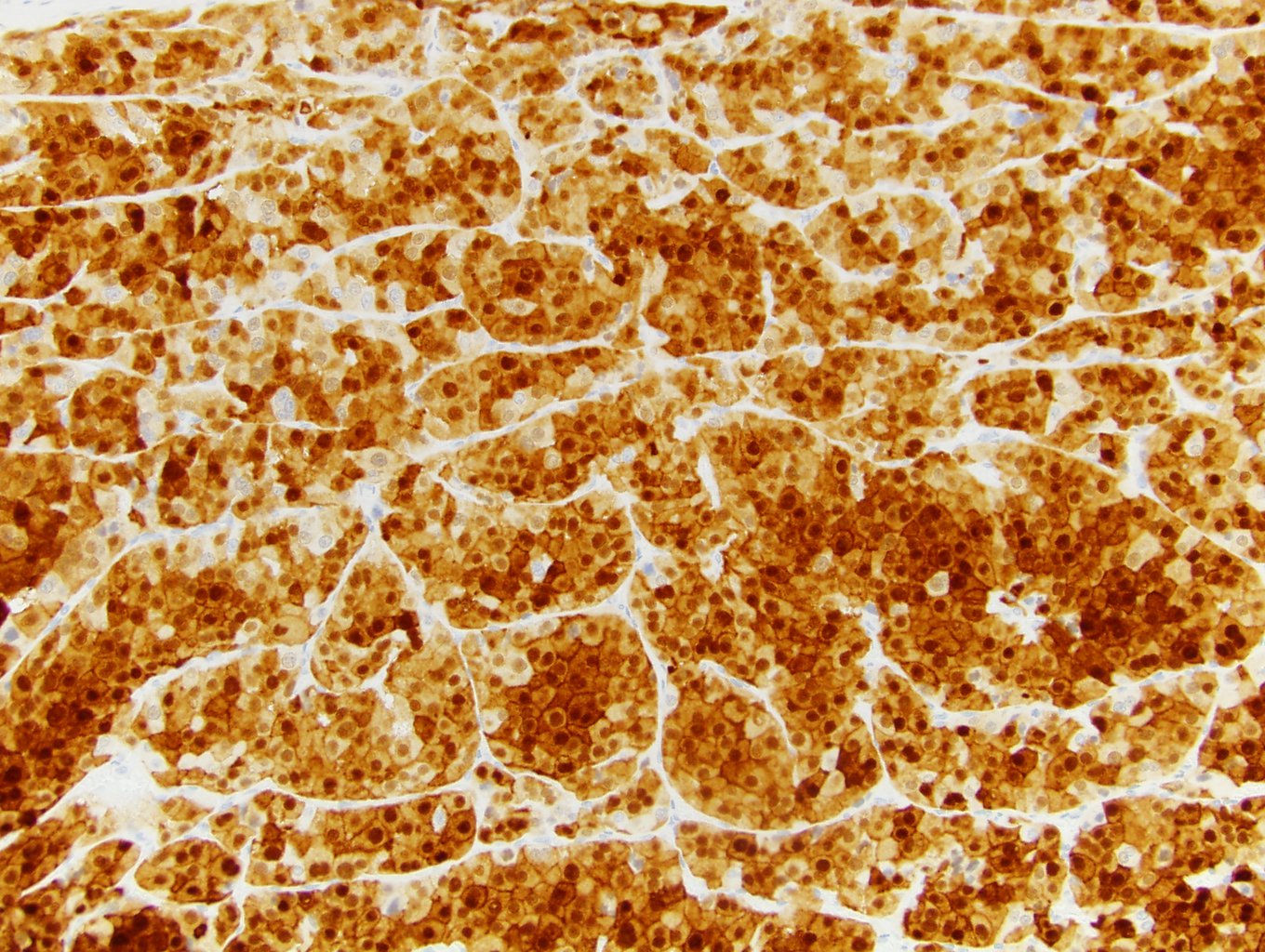
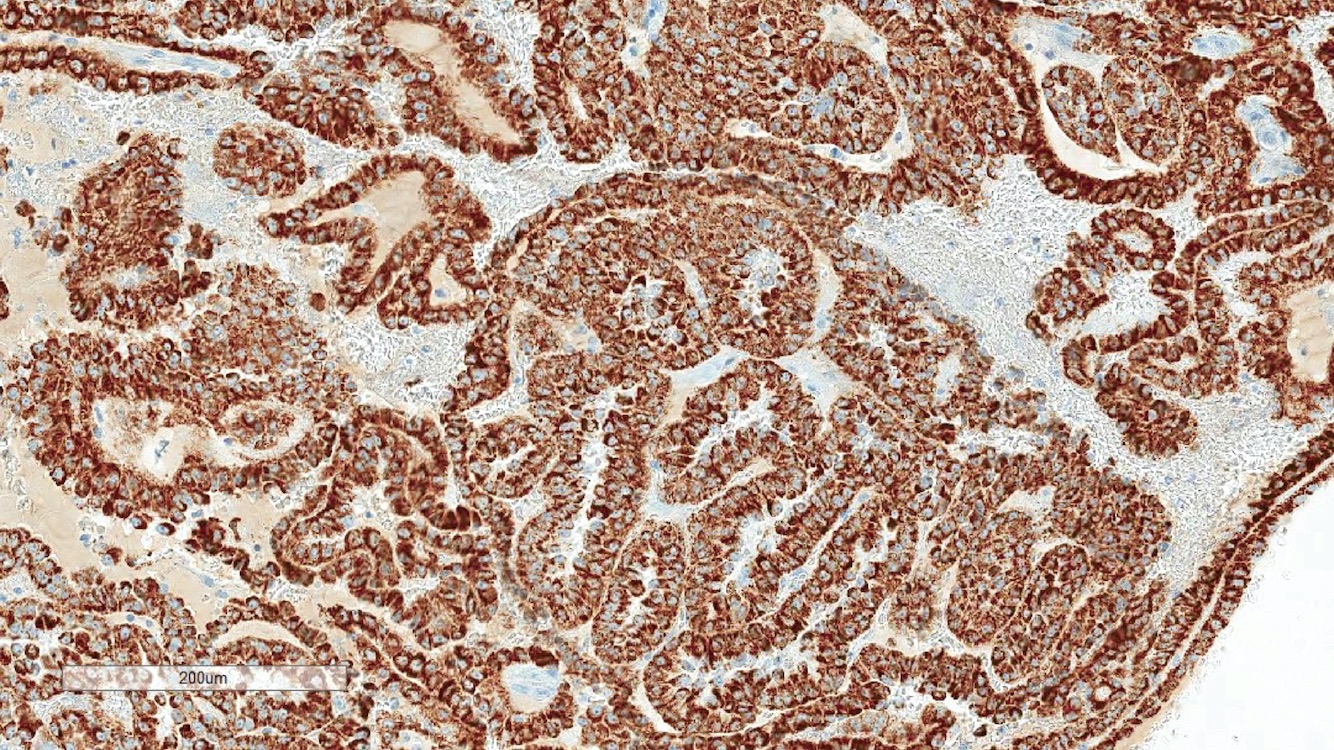
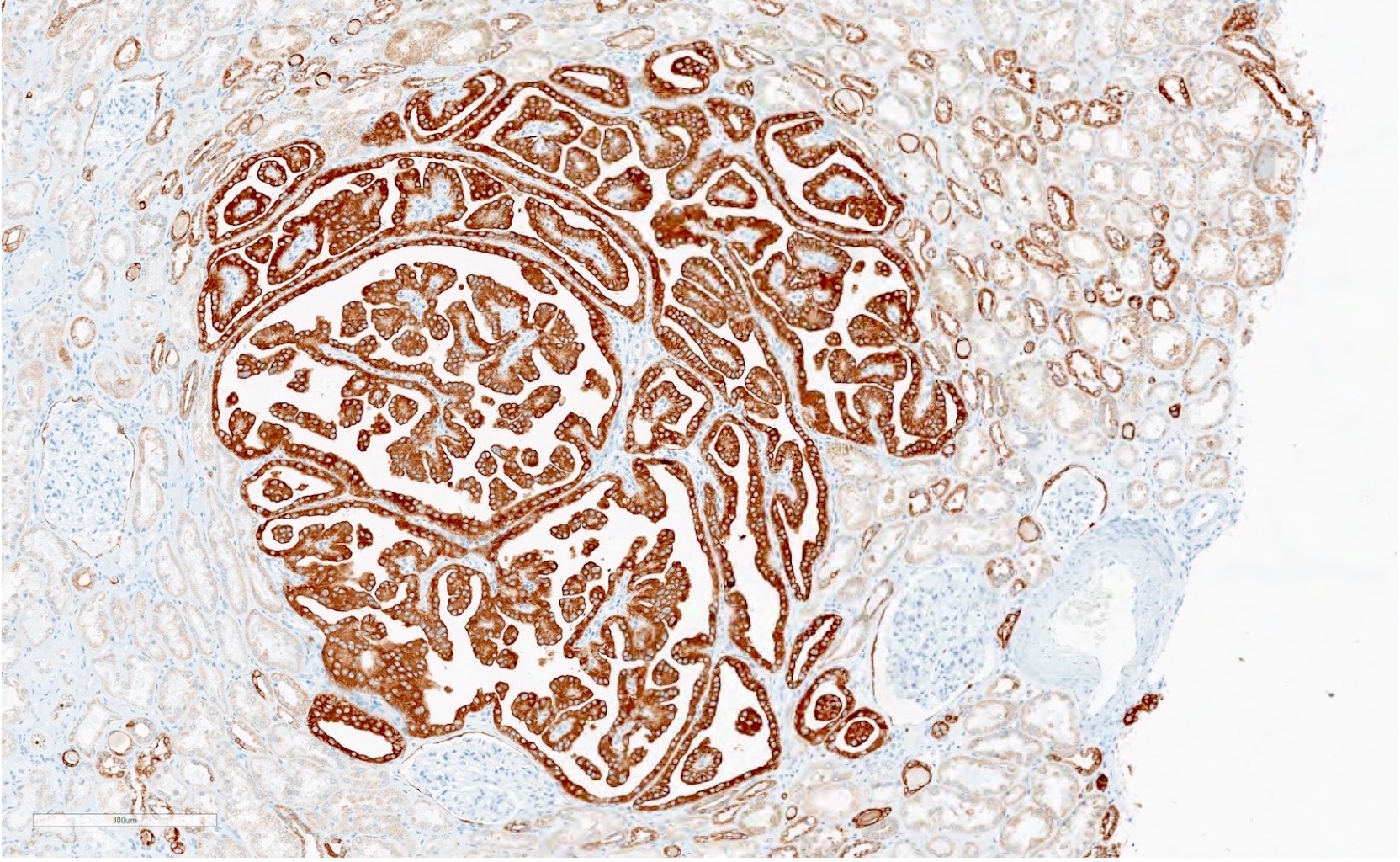
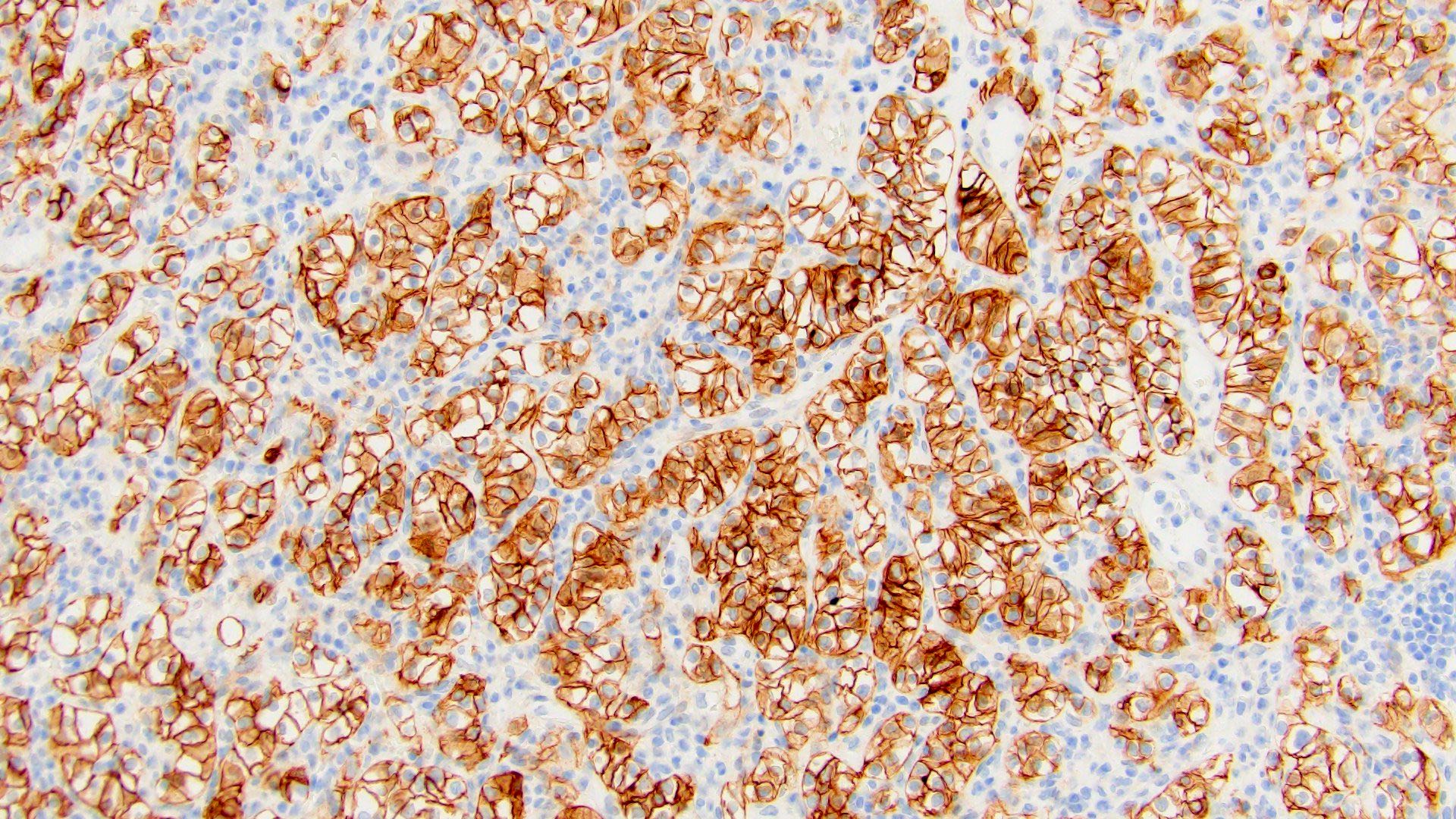
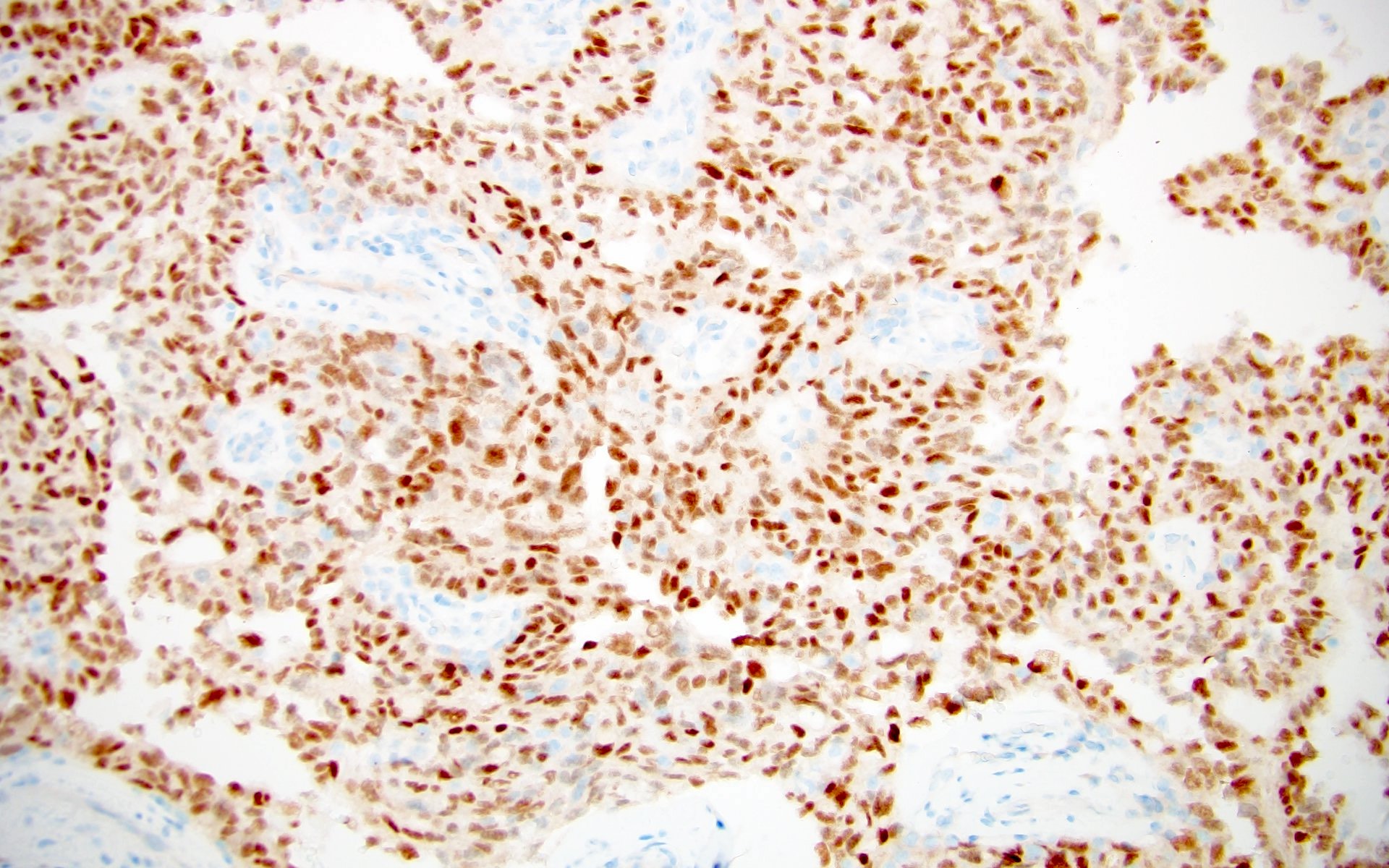
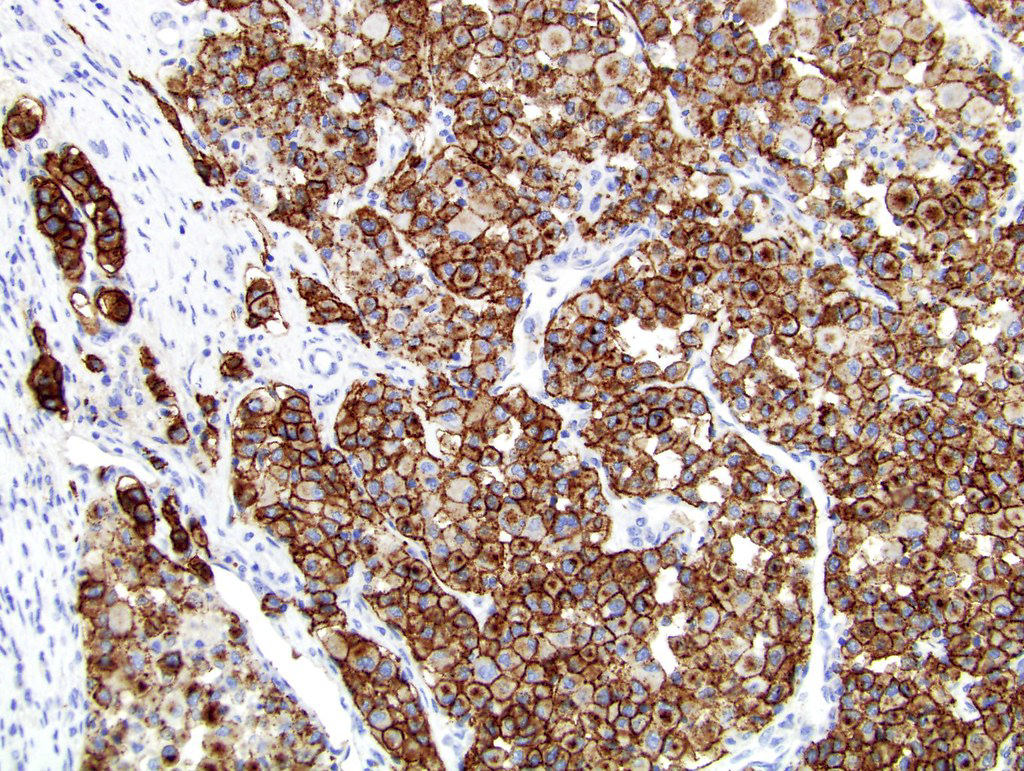
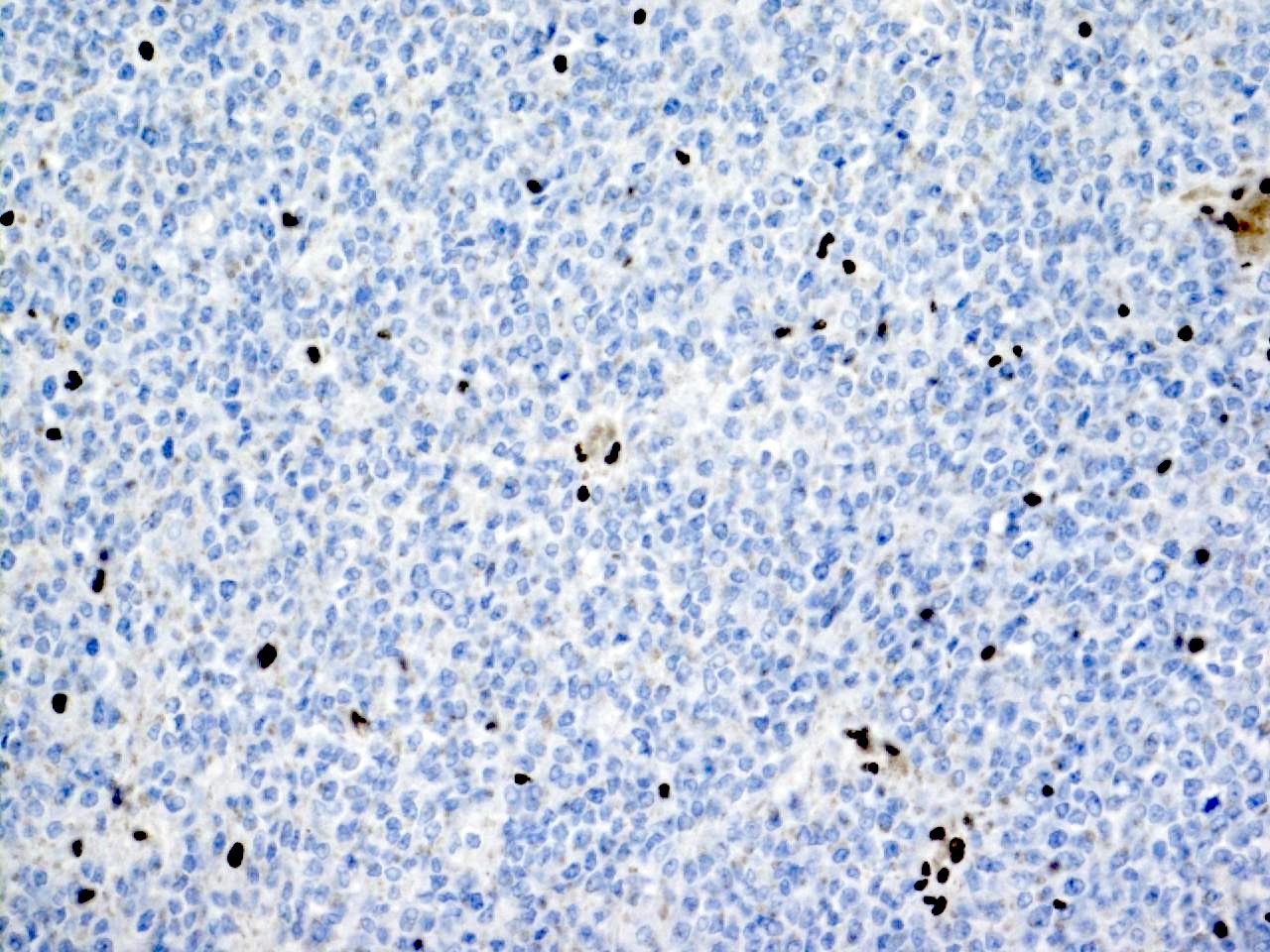
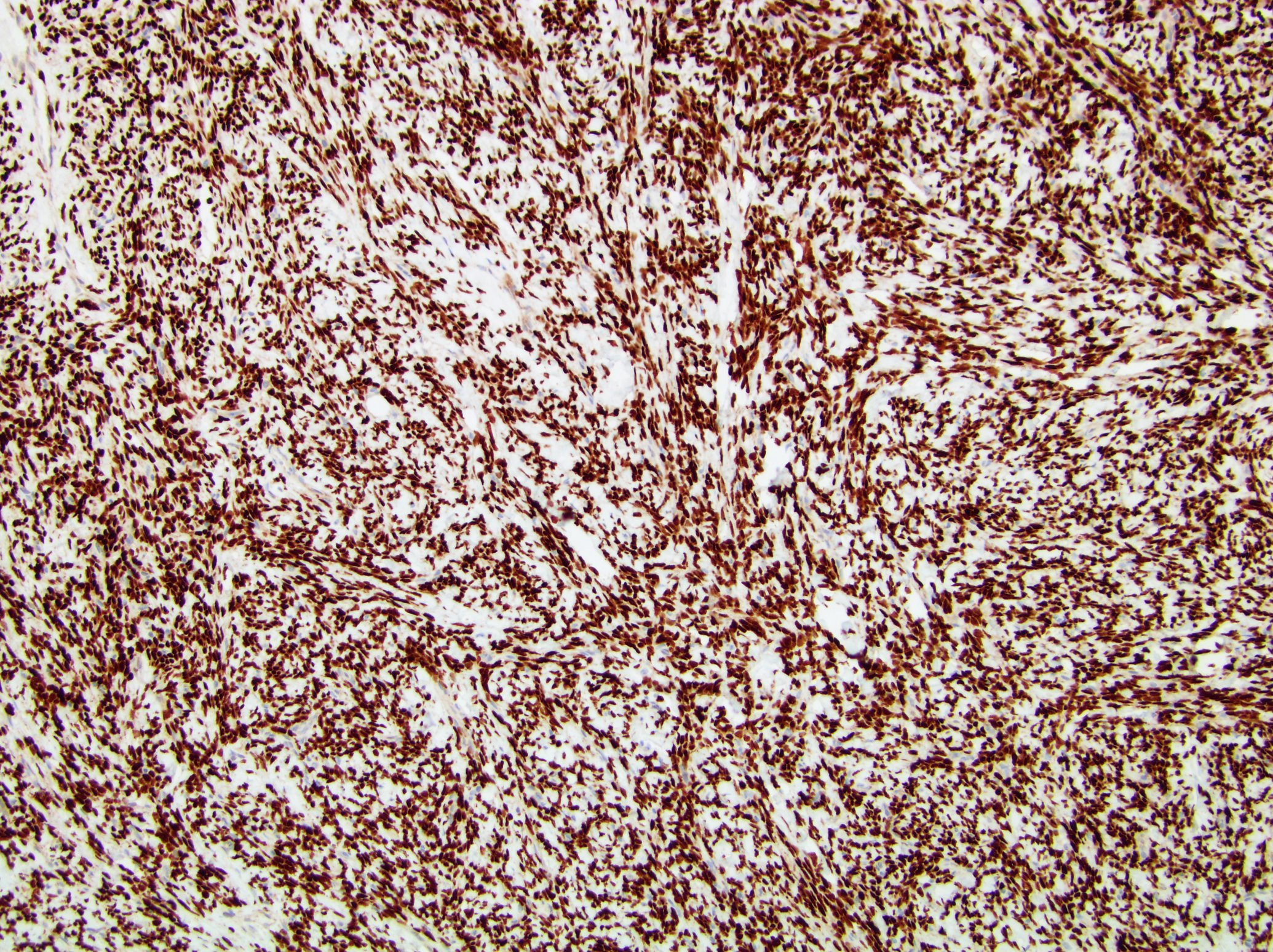
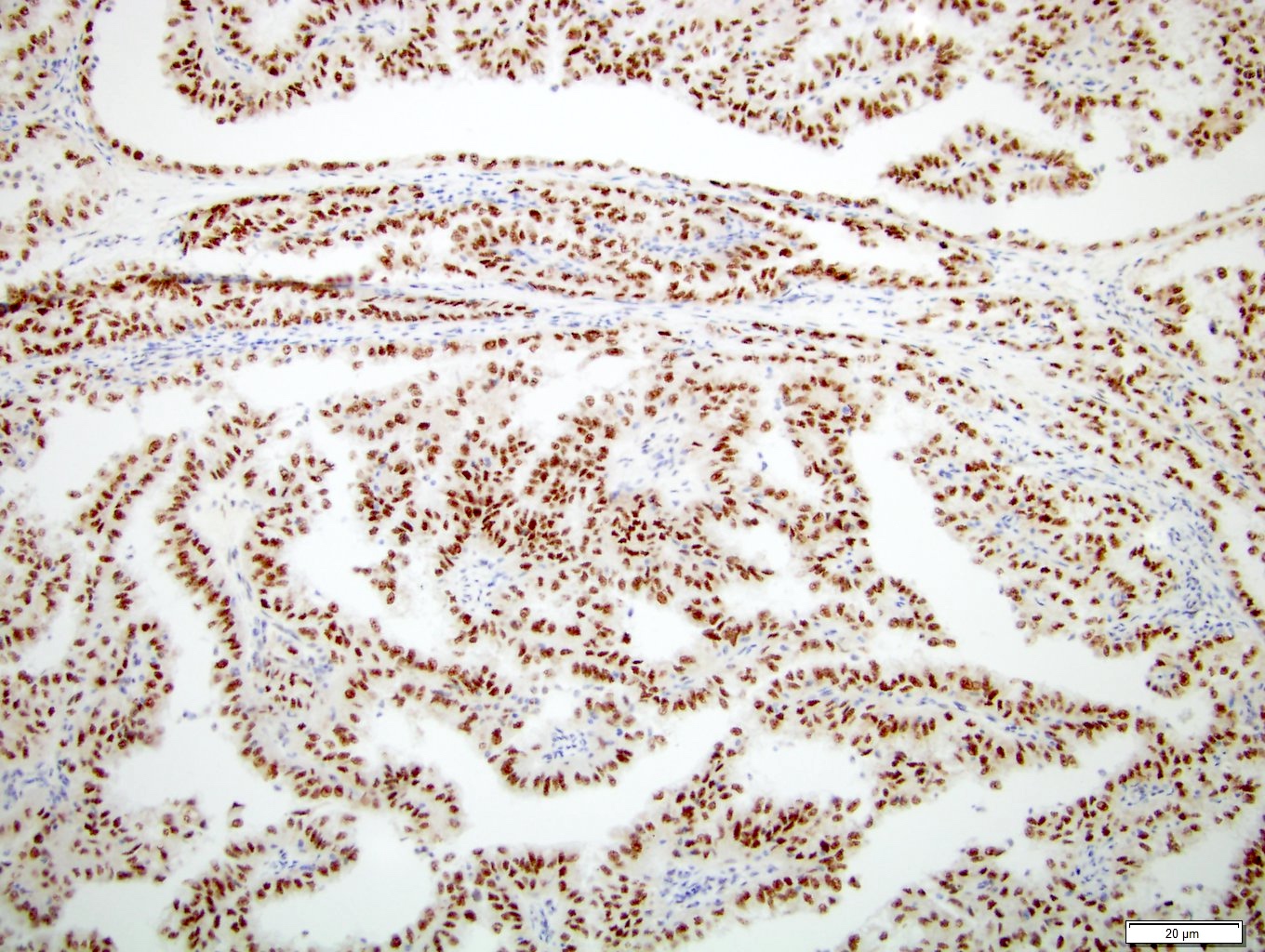
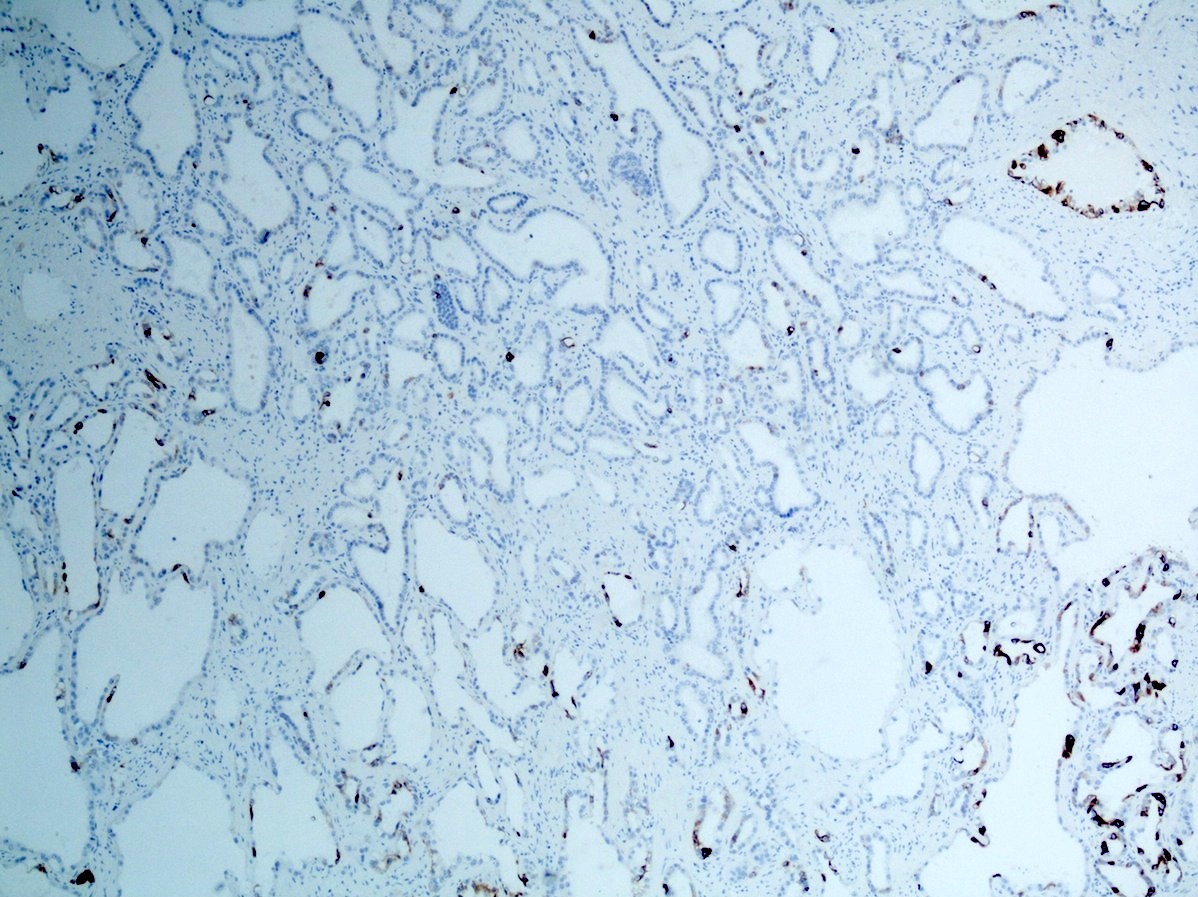
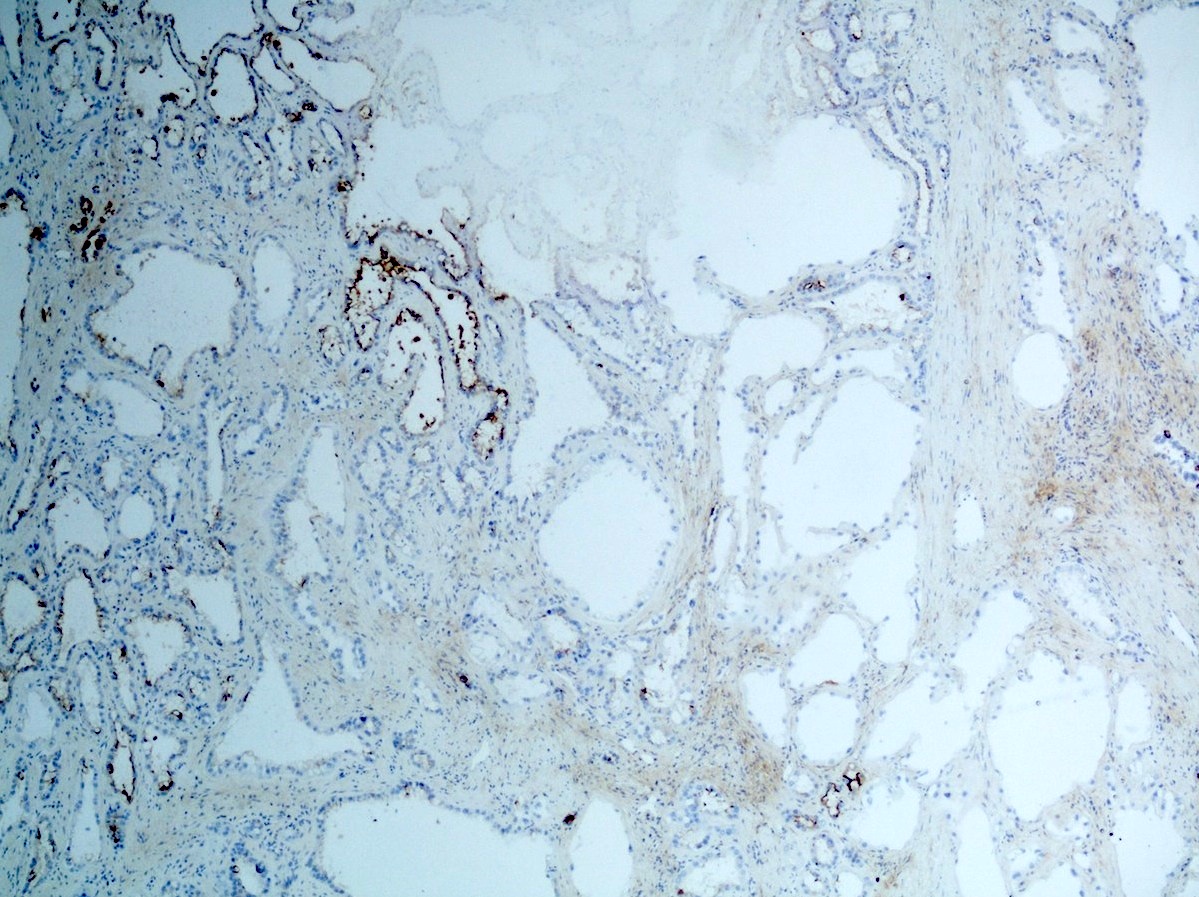
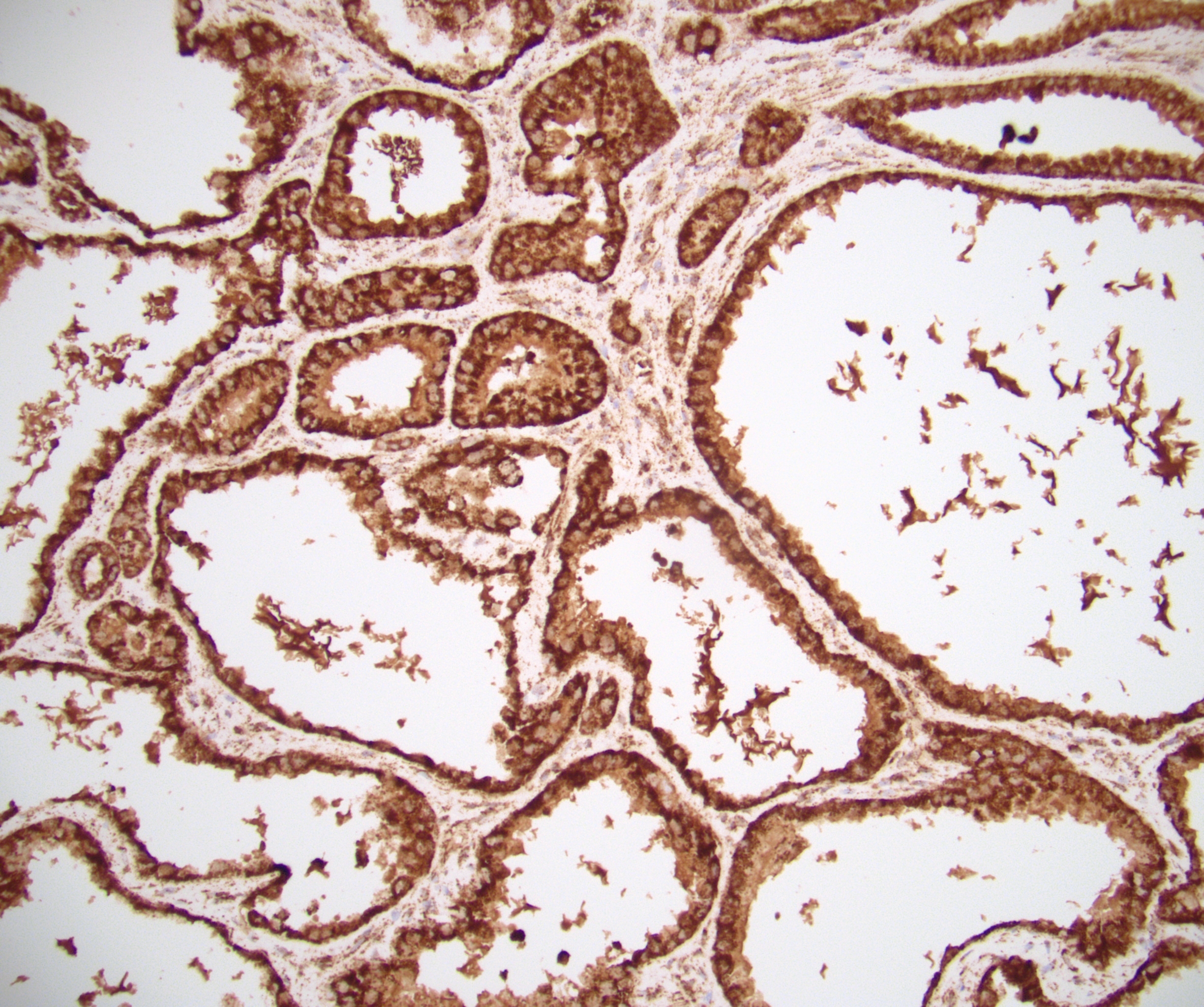
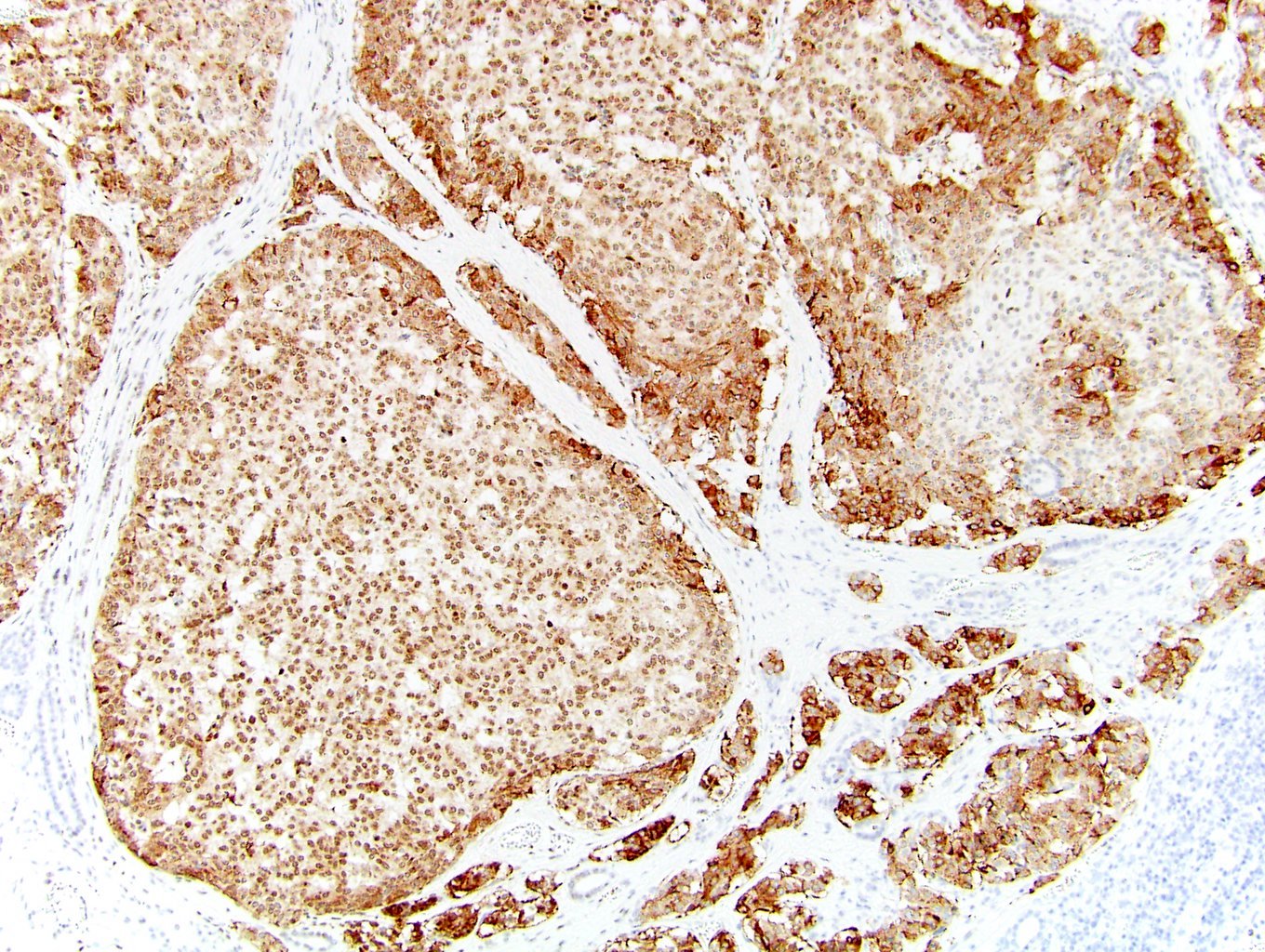
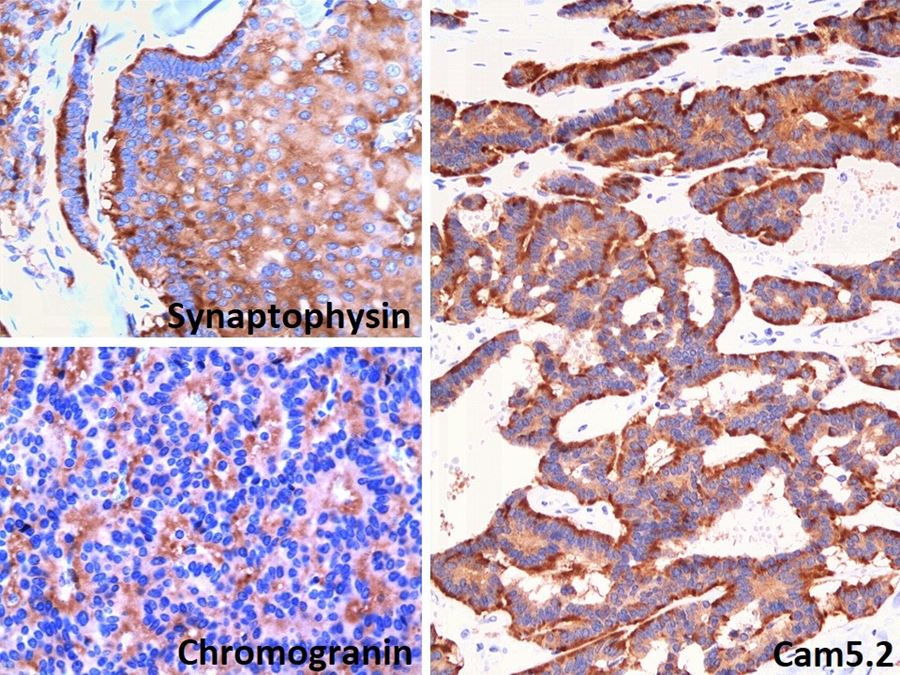
- Diffuse ALK protein expression
- Detection of ALK gene rearrangement by FISH, RT-PCR or RNA sequencing
- First 2 cases of VCL-ALK fusion RCC described in 2010 (Mod Pathol 2011;24:430)
- Synonyms: ALK rearrangement associated RCC, ALK translocation RCC, ALK-RCC
- Provisional entity in the WHO classification (2016)
- ICD-10: C64 - malignant neoplasm of kidney, except renal pelvis
- Rare; < 25 cases described
- Bimodal age distribution:
- Children with sickle cell trait, often African descent (range: 6 - 19 years)
- Adults without sickle cell trait (range: 33 - 61 years)
- Incidence: 3.5 - 3.8% of pediatric renal cancers, 0.4 - 0.5% of adult renal cancers (Cancer 2018;124:3381, Histopathology 2017;71:53, Genes Chromosomes Cancer 2016;55:442, Cancer 2012;118:4427, Histopathology 2019;74:31)
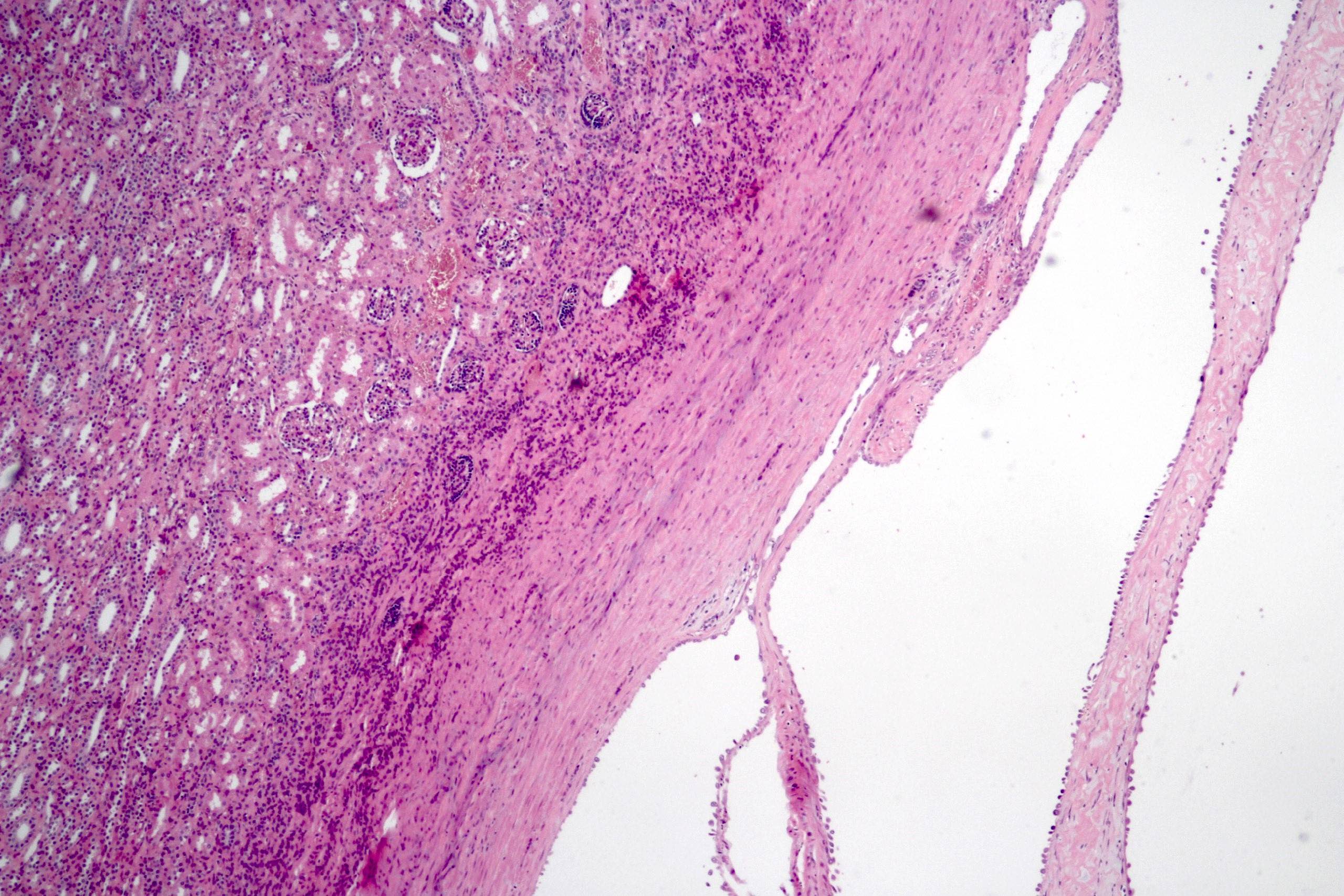
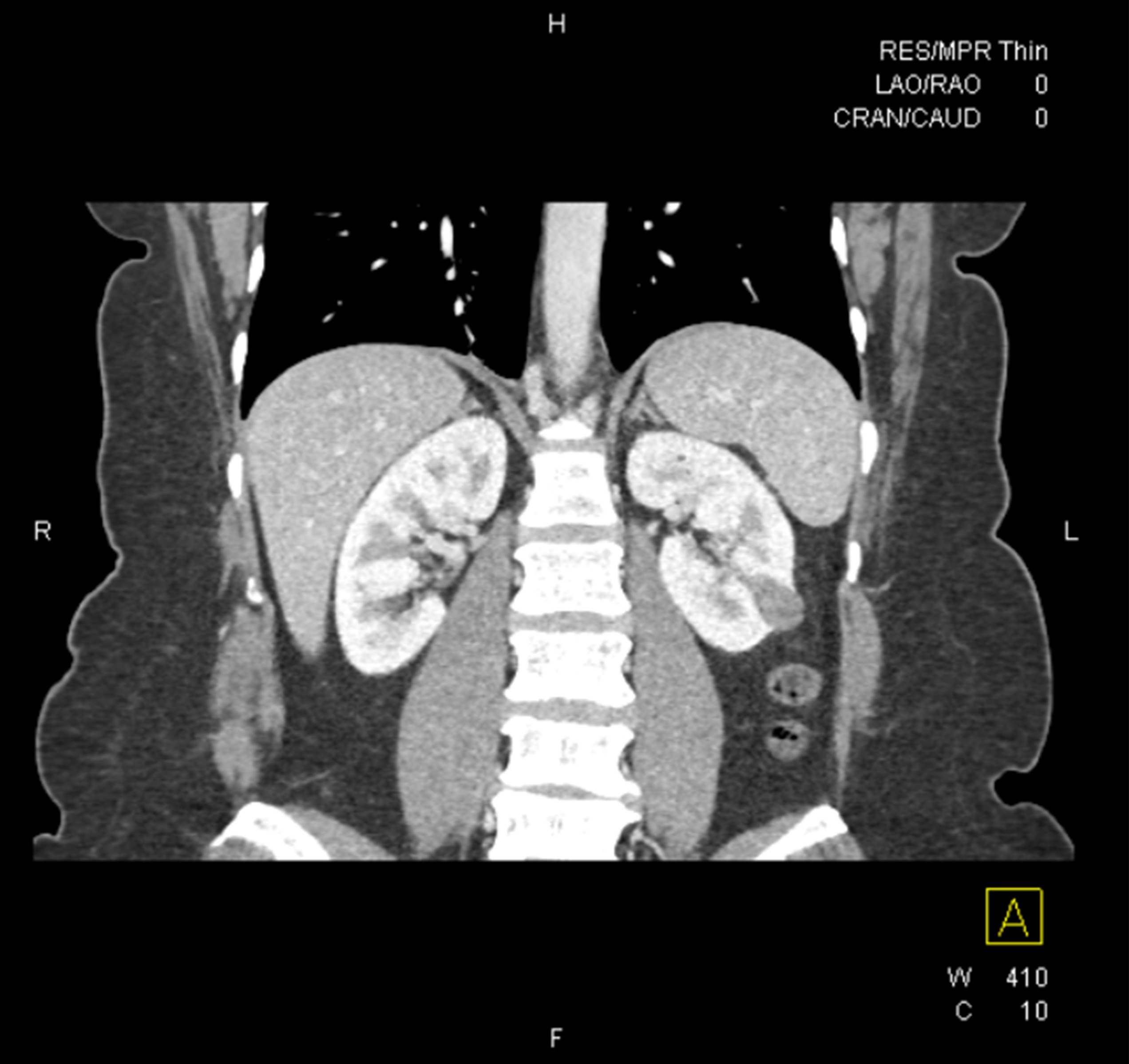
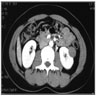
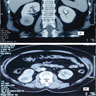
- Solitary kidney mass
- Most commonly in renal medulla, renal pelvis or mid kidney
- ALK belongs to the insulin receptor tyrosine kinase superfamily and is normally expressed at a low level in the central nervous system
- ALK rearrangement represents an oncogenic driver mutation that probably occurs during the early stage of carcinogenesis (Histopathology 2019;74:31)
- In children, related to sickle cell trait in a subset of patients (Am J Surg Pathol 2014;38:858)
- Majority with hematuria, flank, abdominal or periumbilical pain (Pol J Pathol 2018;69:109, Histopathology 2017;71:53)
- Incidental in ~33% of cases
- Most pT1a or pT1b
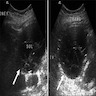
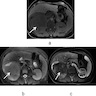
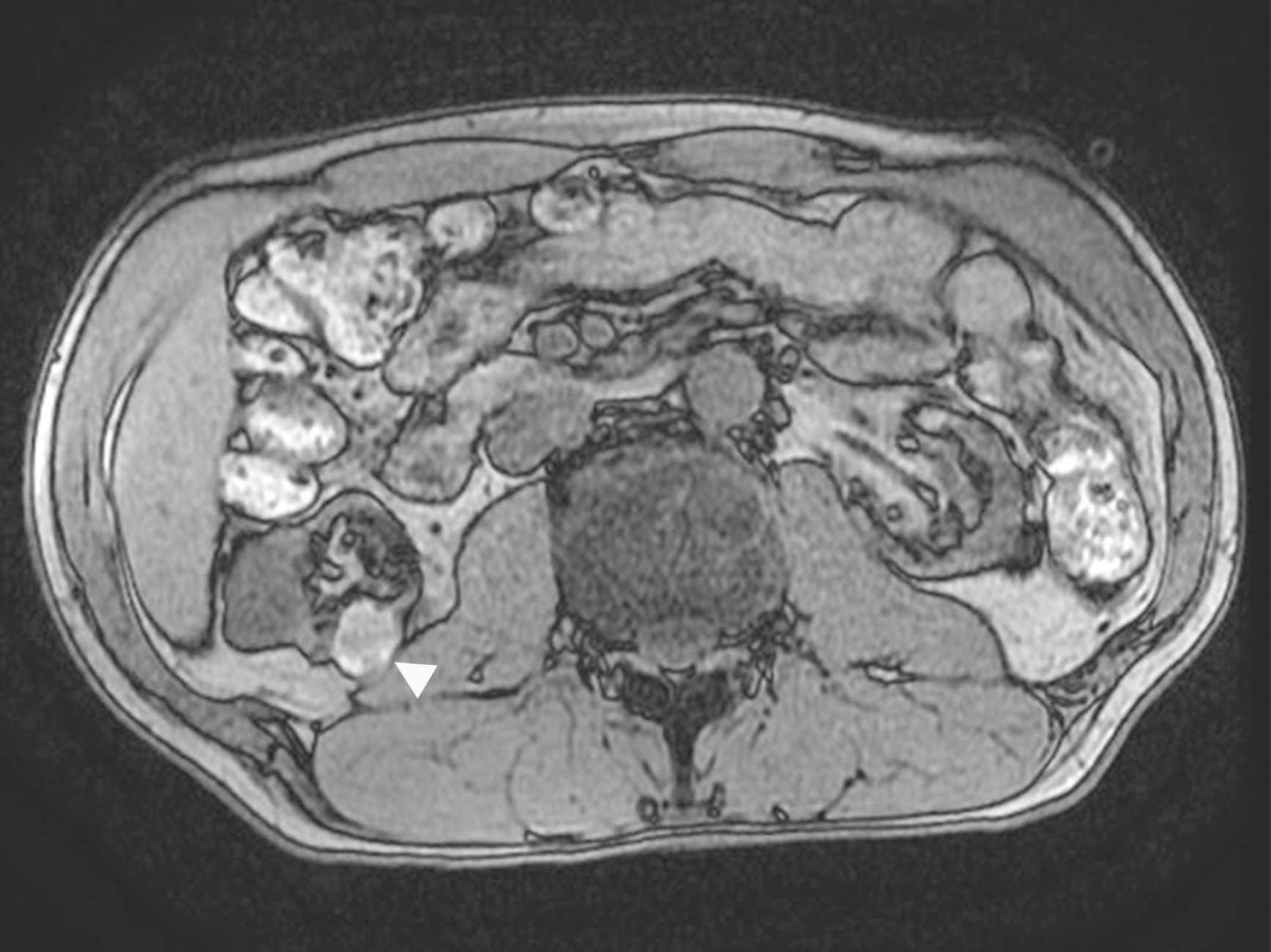
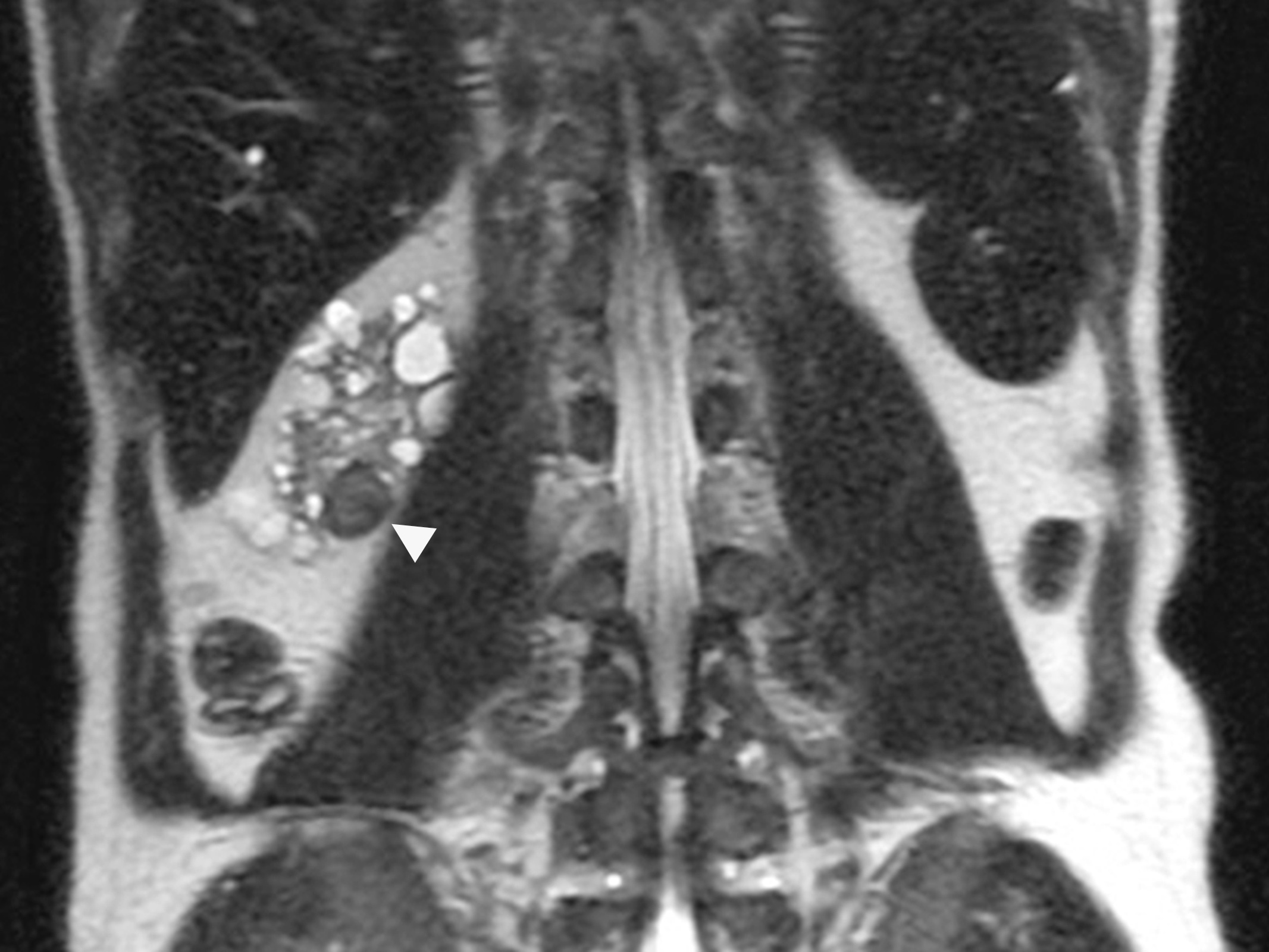
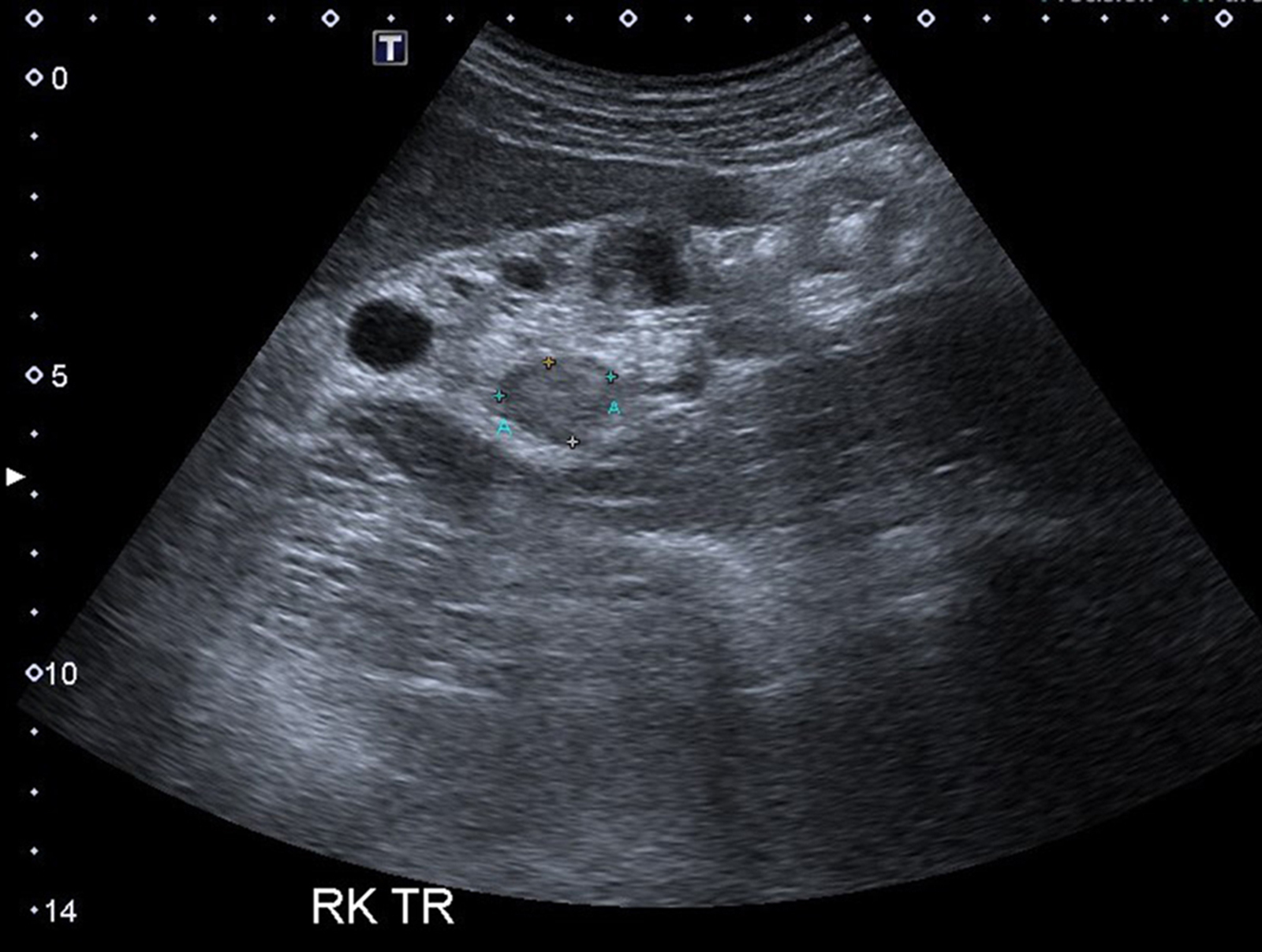
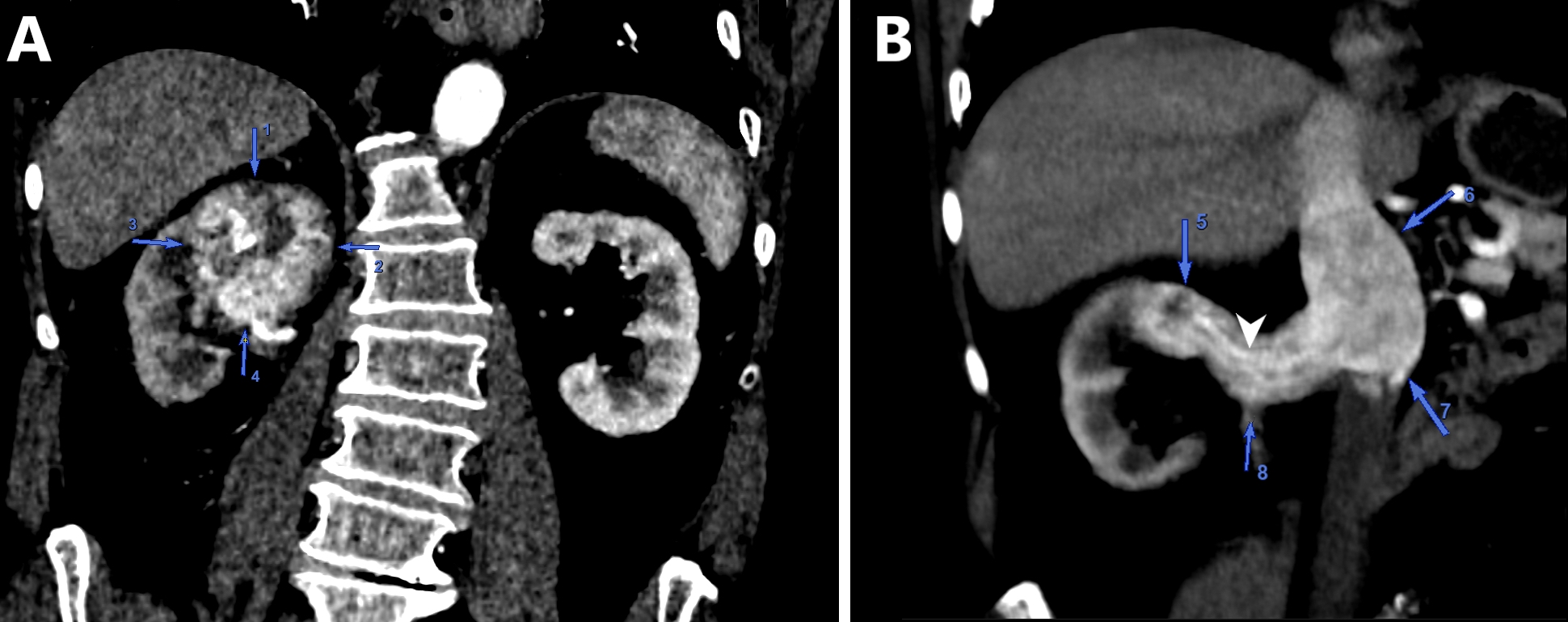
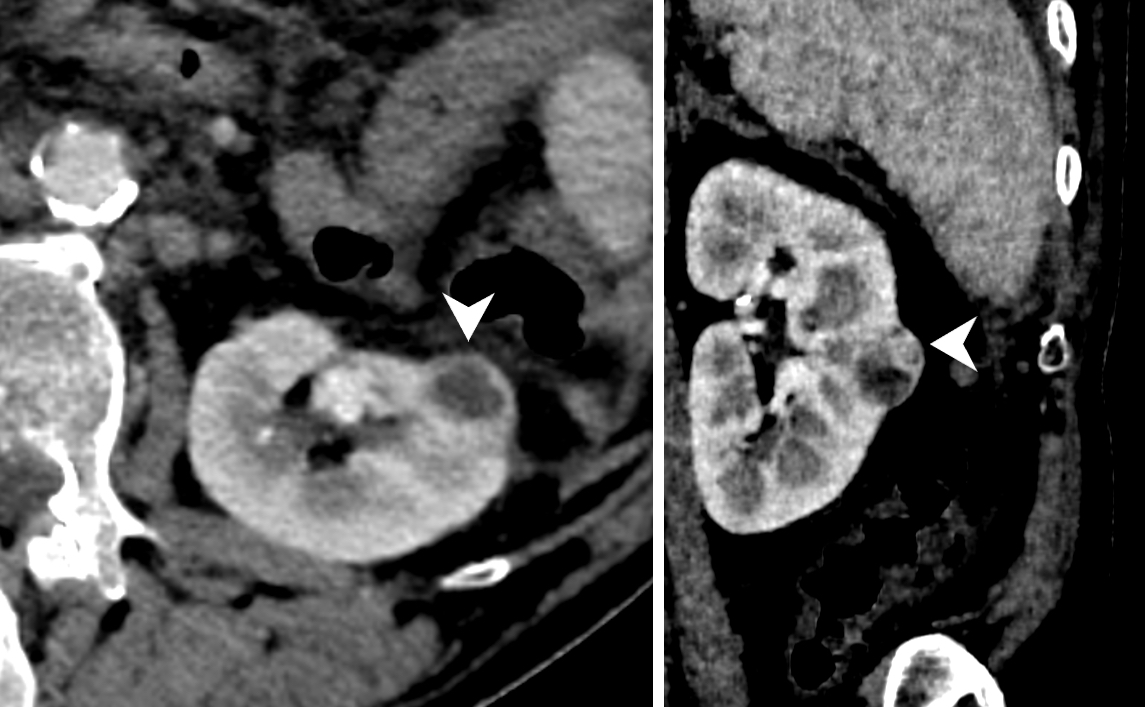
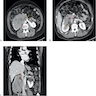
- Ultrasound sonography demonstrates a hypoechoic medulla centric mass
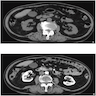
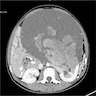
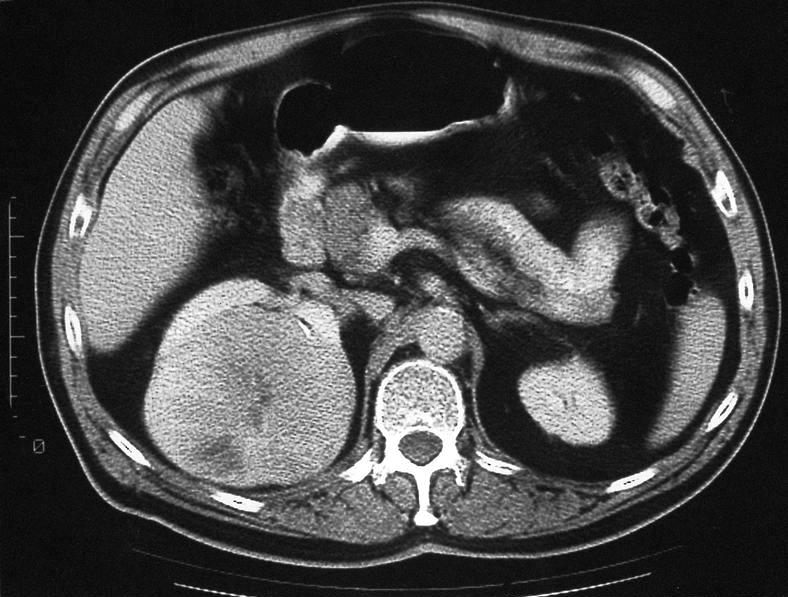
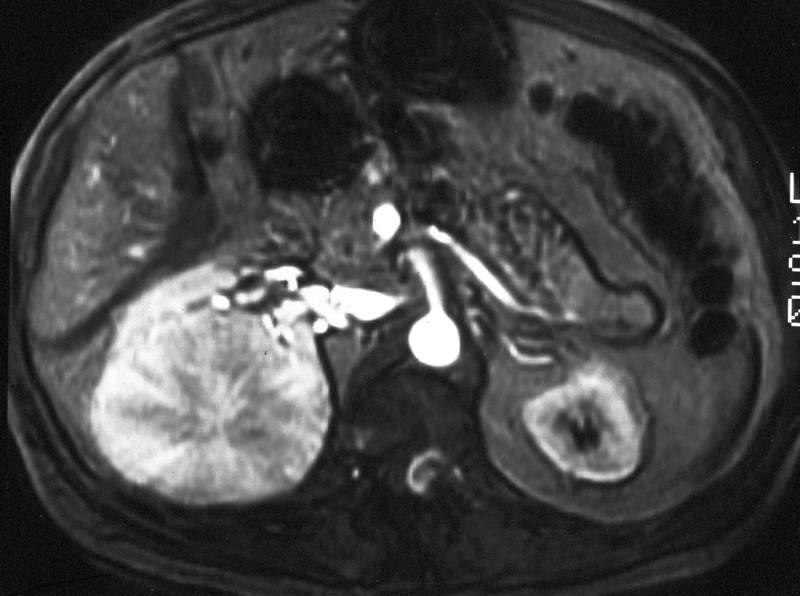
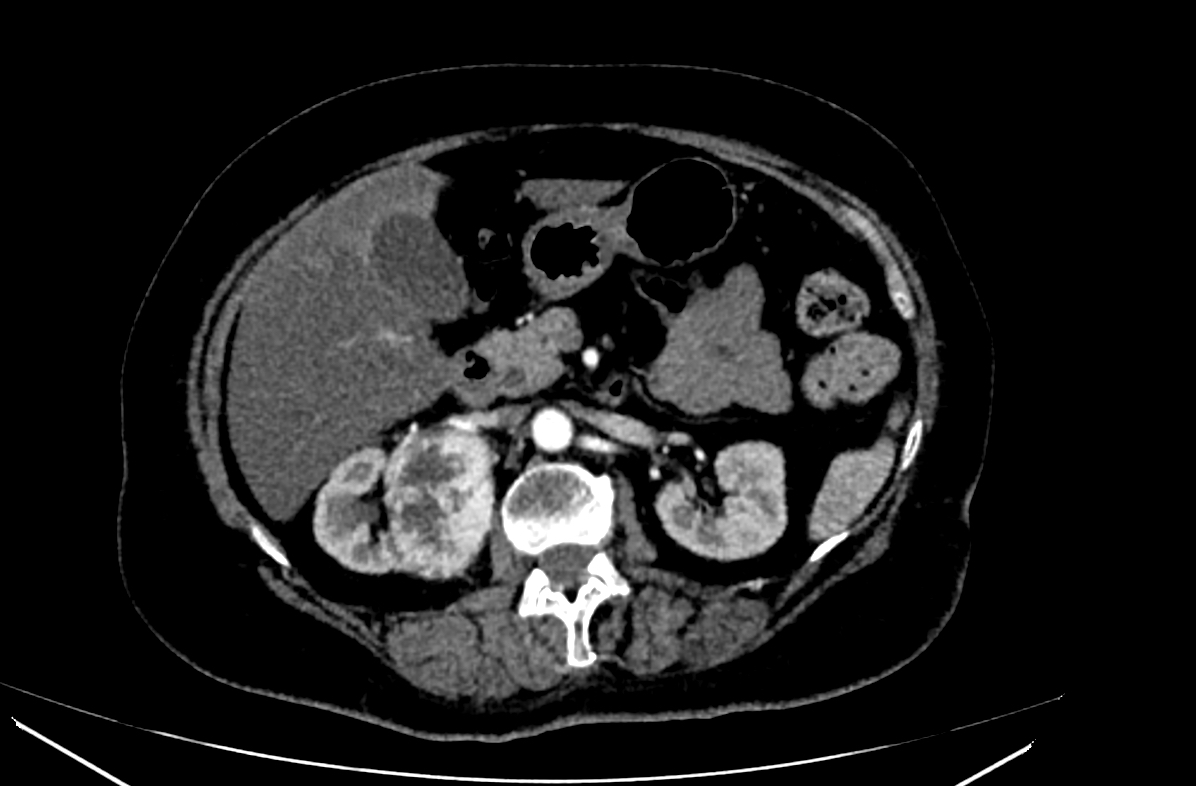
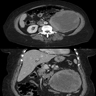
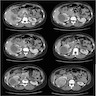
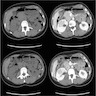
- Simple computed tomography scan shows an isodense mass
- Contrast computed tomography scan shows a slightly enhancing or heterogeneous enhancing mass (Pol J Pathol 2018;69:109)
- Children with VCL-ALK: no recurrence or distant metastasis reported to date
- Adult non-VCL-ALK RCC: ~33% have adverse prognosis (Histopathology 2017;71:53, Histopathology 2019;74:31)
- Small number of cases and short followup in the majority of them precludes from accurate assessment of risk factors
- 16 year old boy with novel HOOK1-ALK fusion (Genes Chromosomes Cancer 2016;55:814)
- 19 year old woman with ALK-RCC and Hodgkin lymphoma (Pathol Int 2017;67:626)
- 33 year old woman and 38 year old man with RCC harboring novel STRN-ALK fusion (Am J Surg Pathol 2016;40:761)
- 55 year old woman with ALK-TPM3 rearrangement and loss of chromosome 3 (Pol J Pathol 2018;69:109)
- Increased ALK1 copy number and RCC (Virchows Arch 2014;464:241)
- Primary tumor, early stage: radical nephrectomy or nephroureterectomy
- Metastatic ALK-RCC responds to ALK inhibitor alectinib (Eur Urol 2018;74:124)
- Possibly responds to crizotinib, similar to ALK rearrangement lung cancer (Semin Diagn Pathol 2015;32:90)
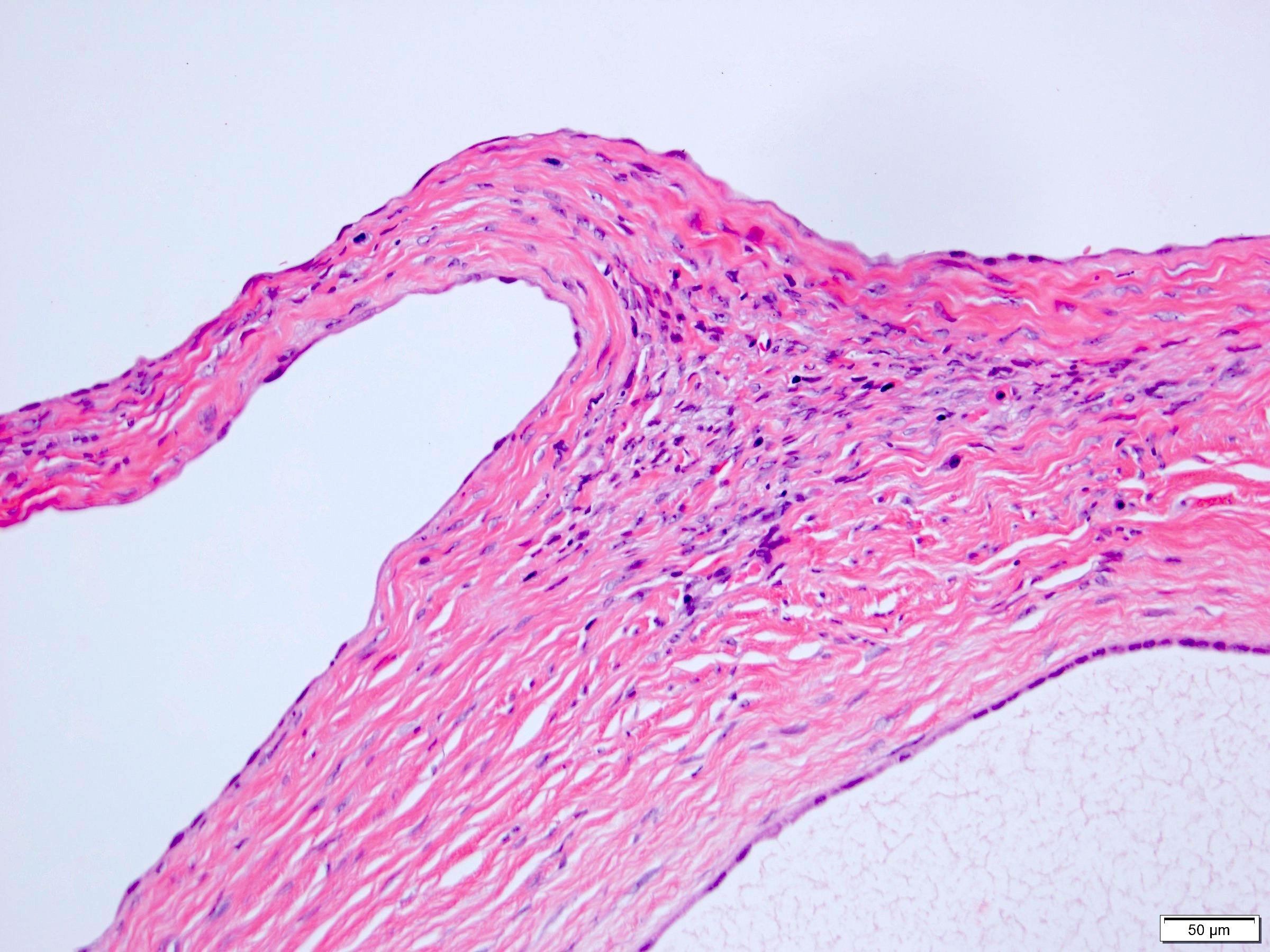
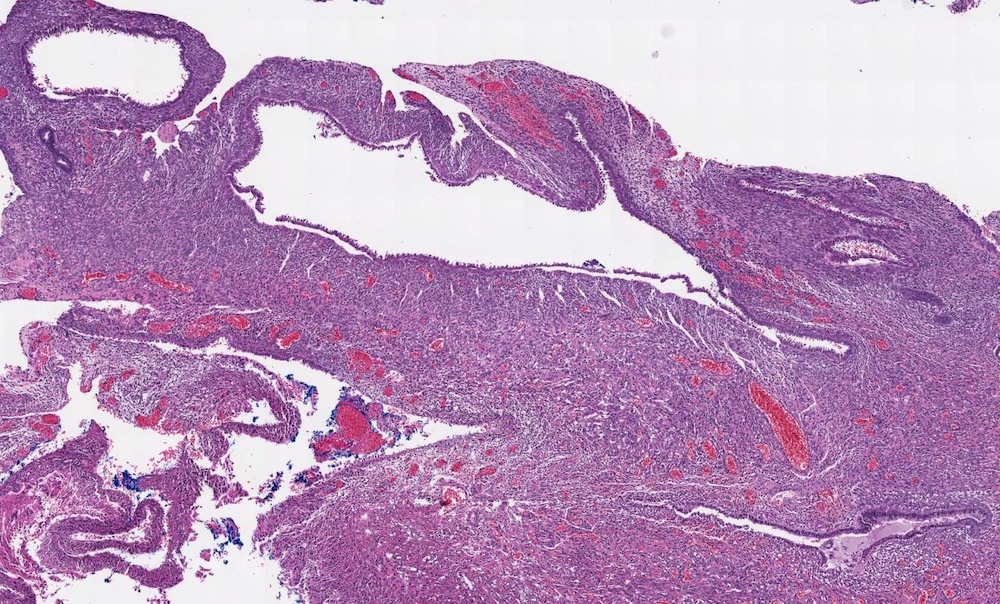
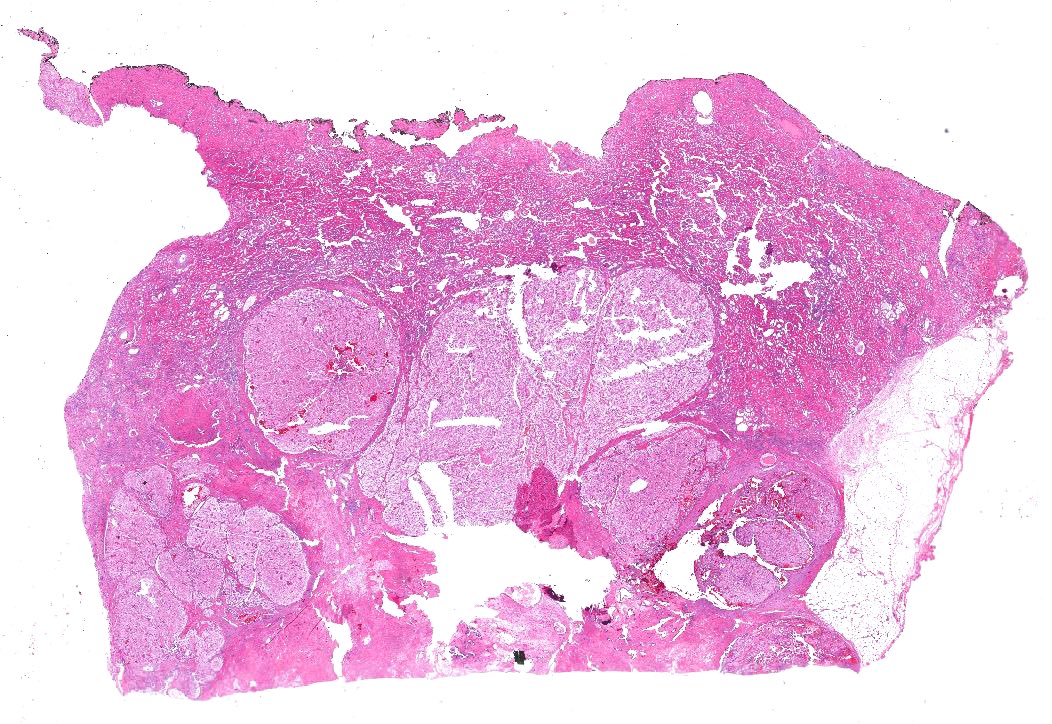
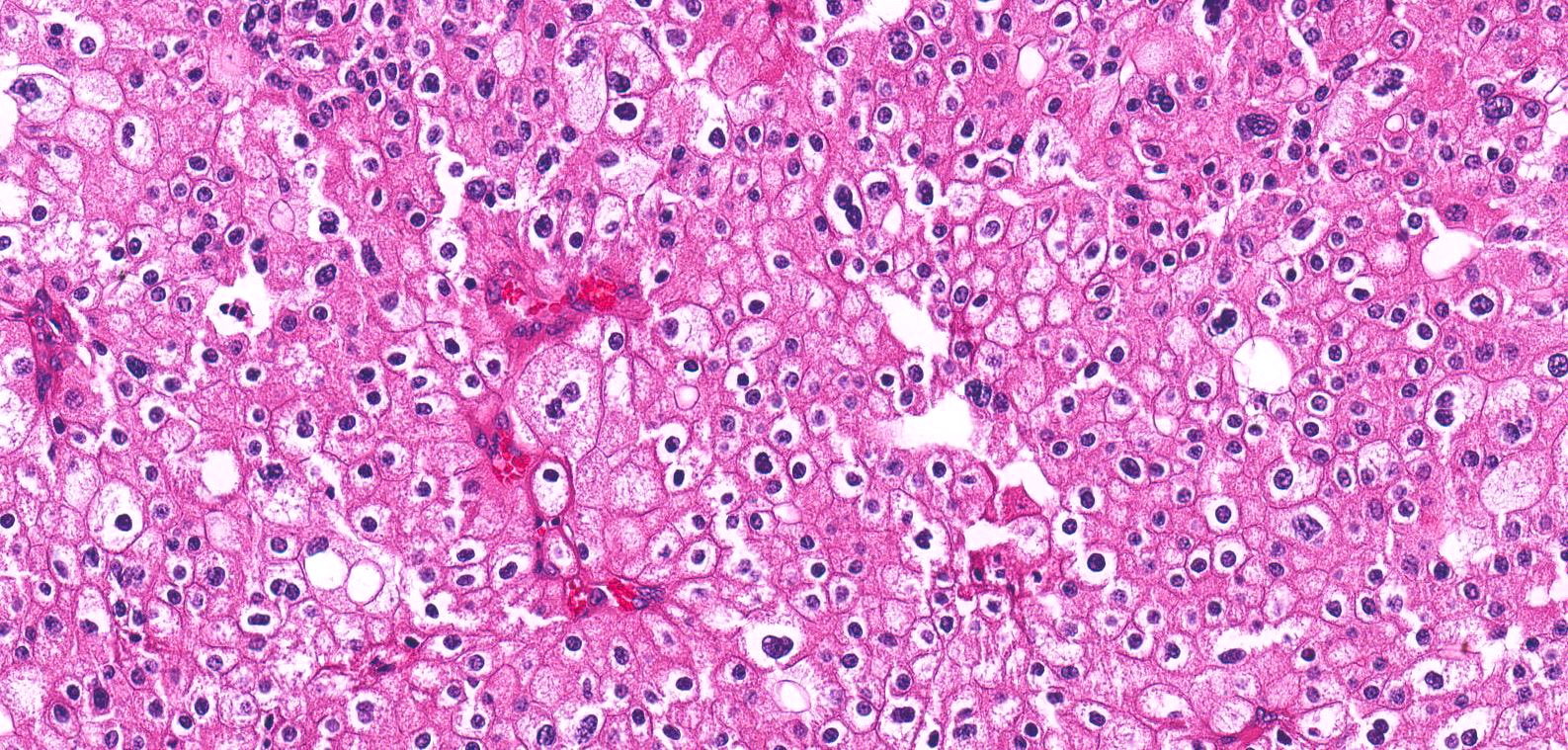
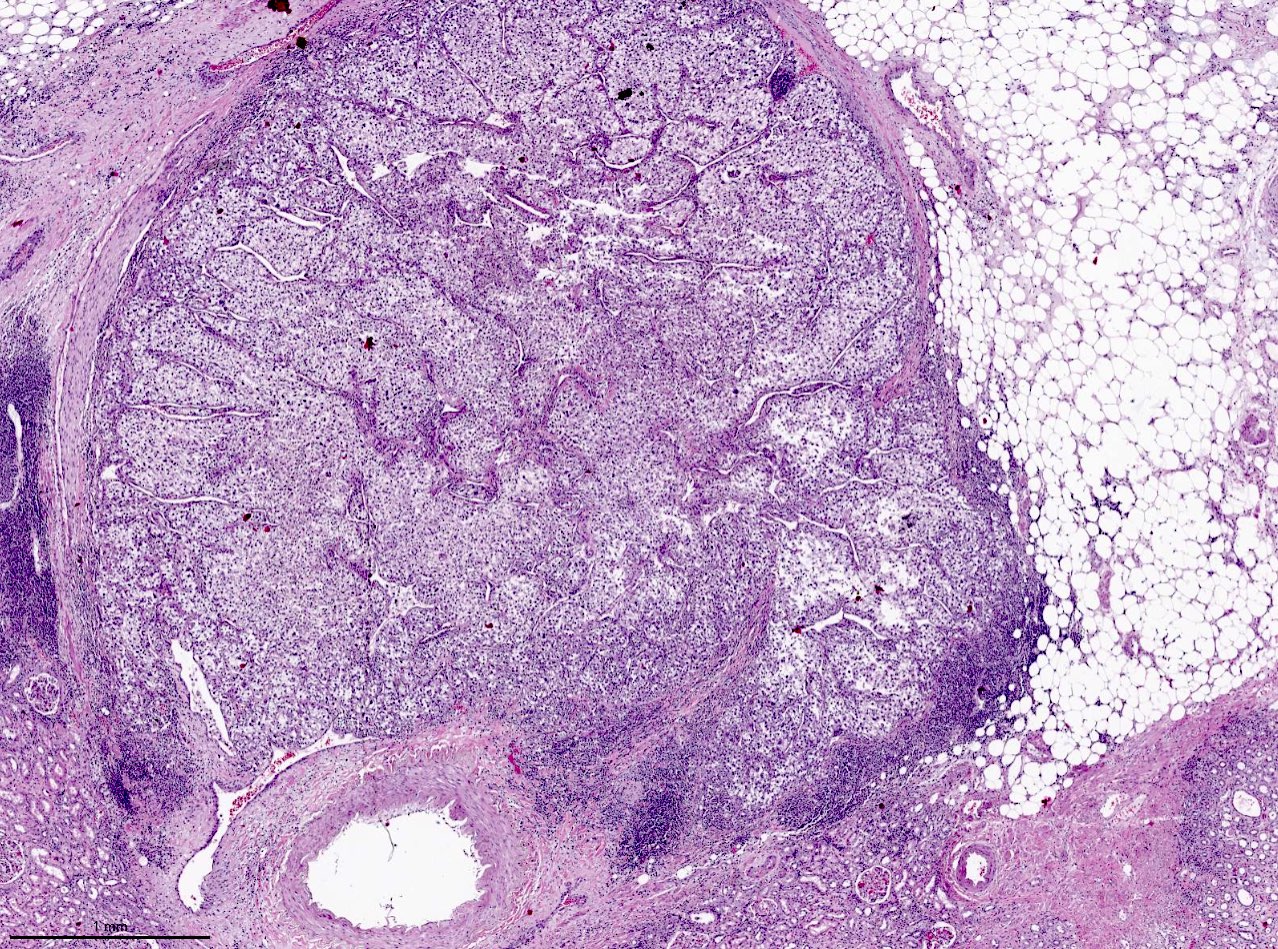
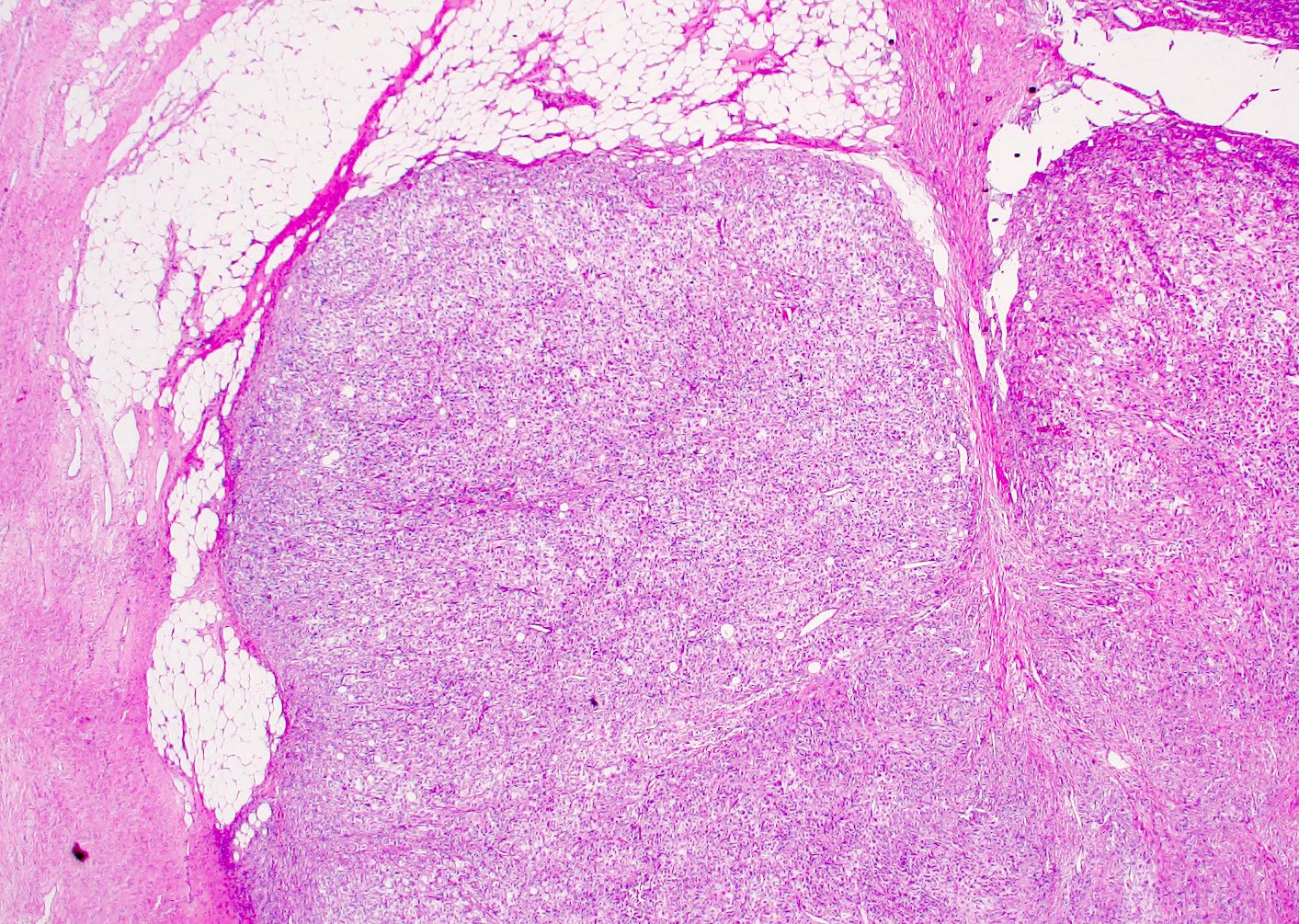
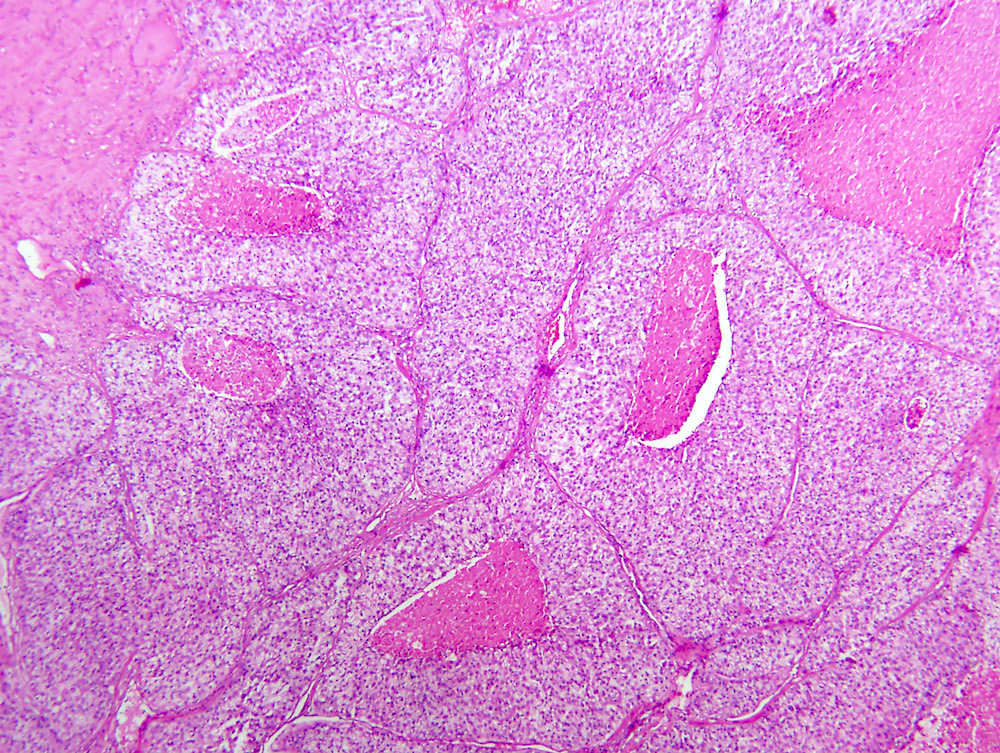
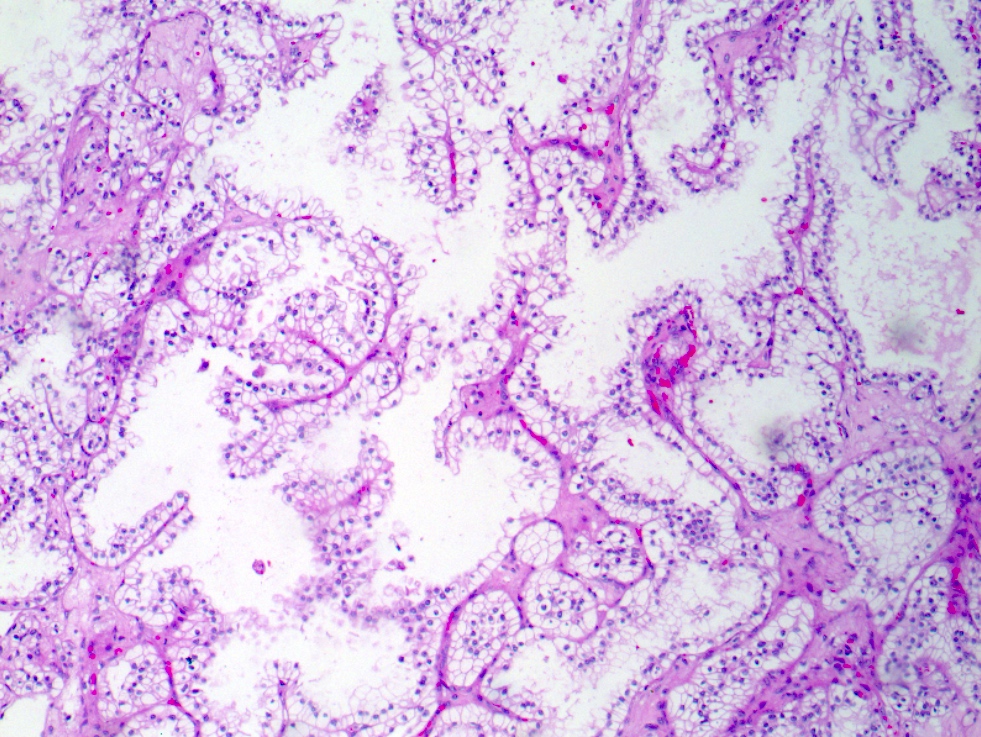
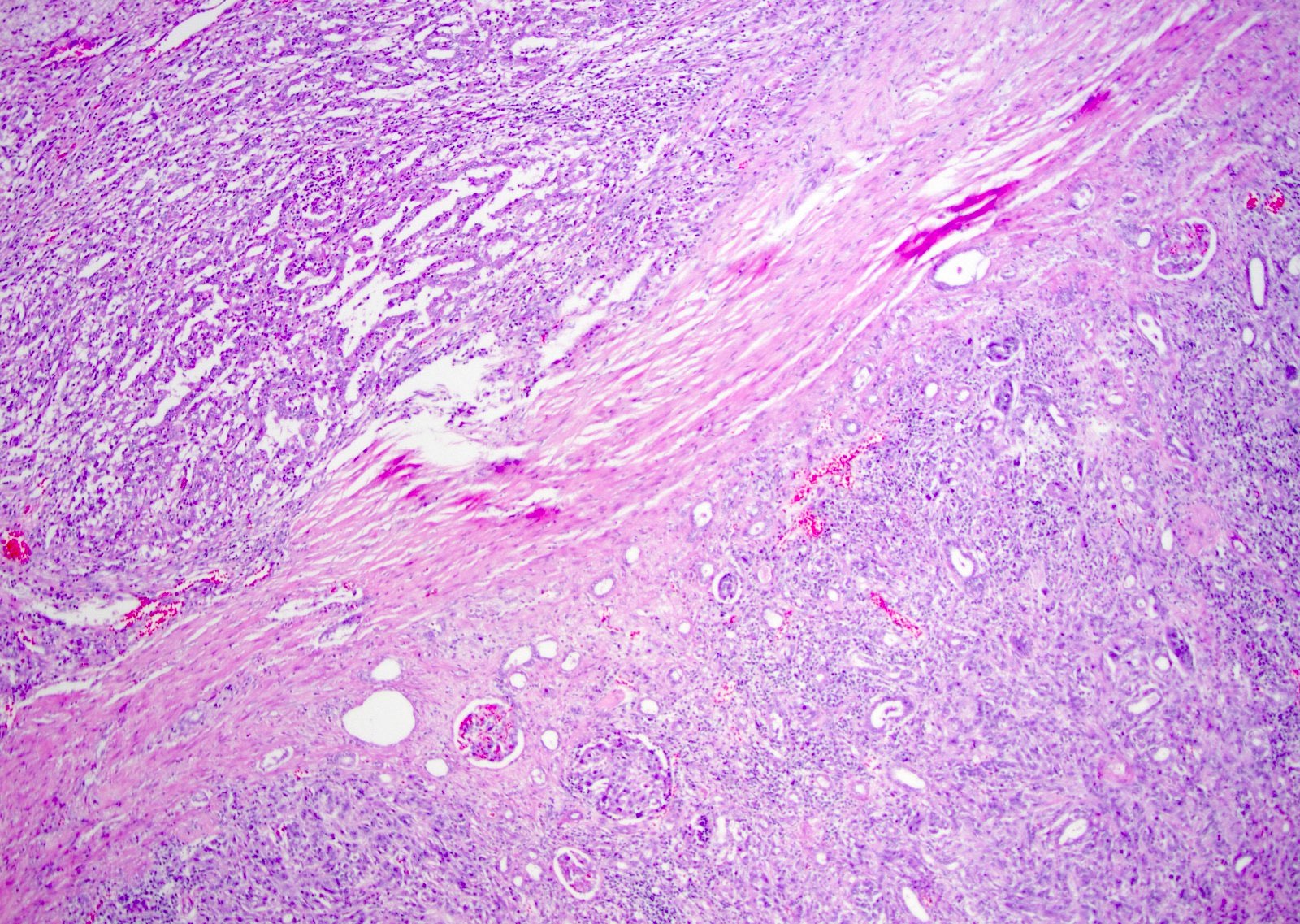
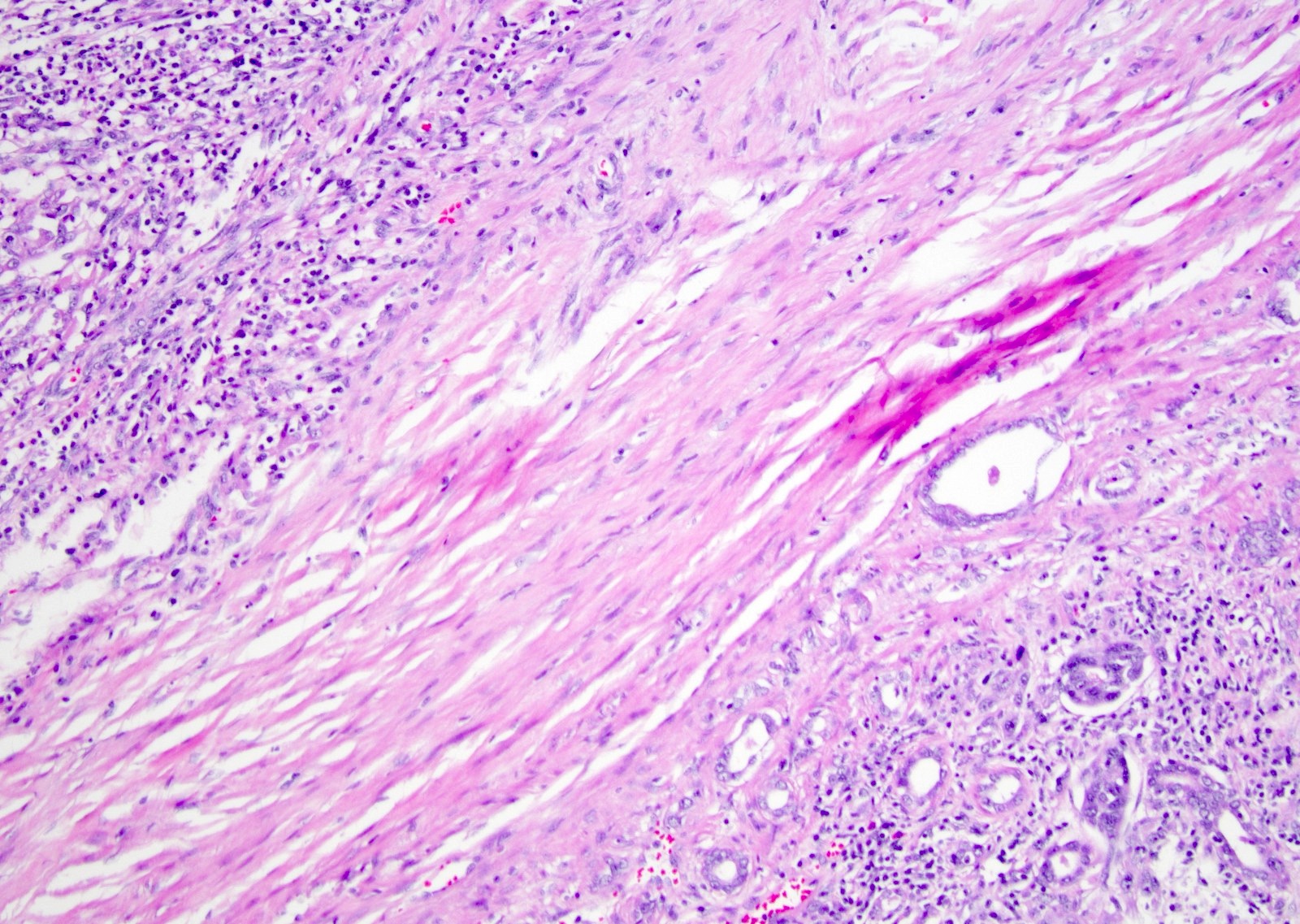
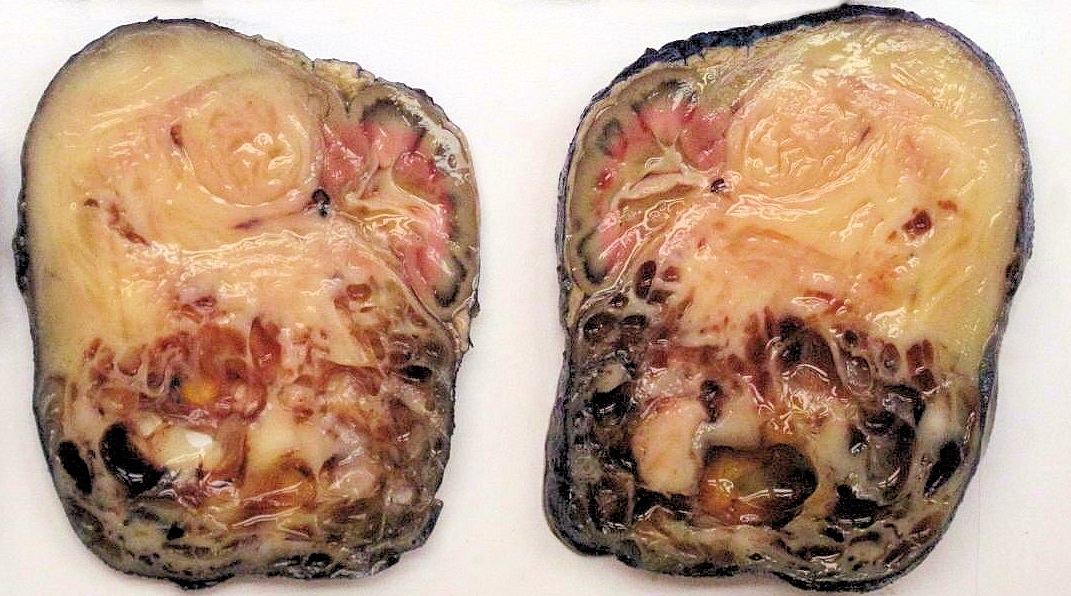
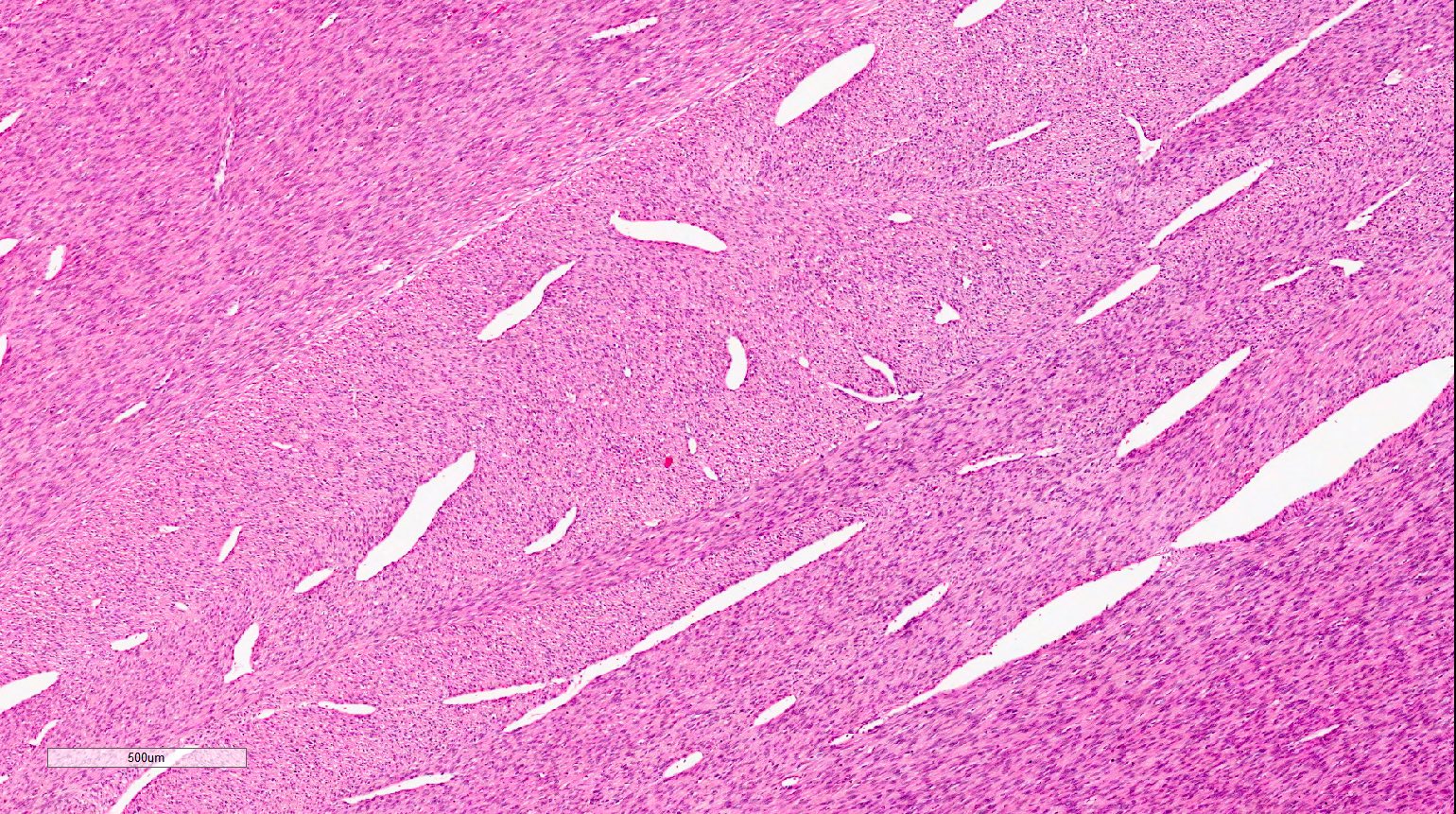
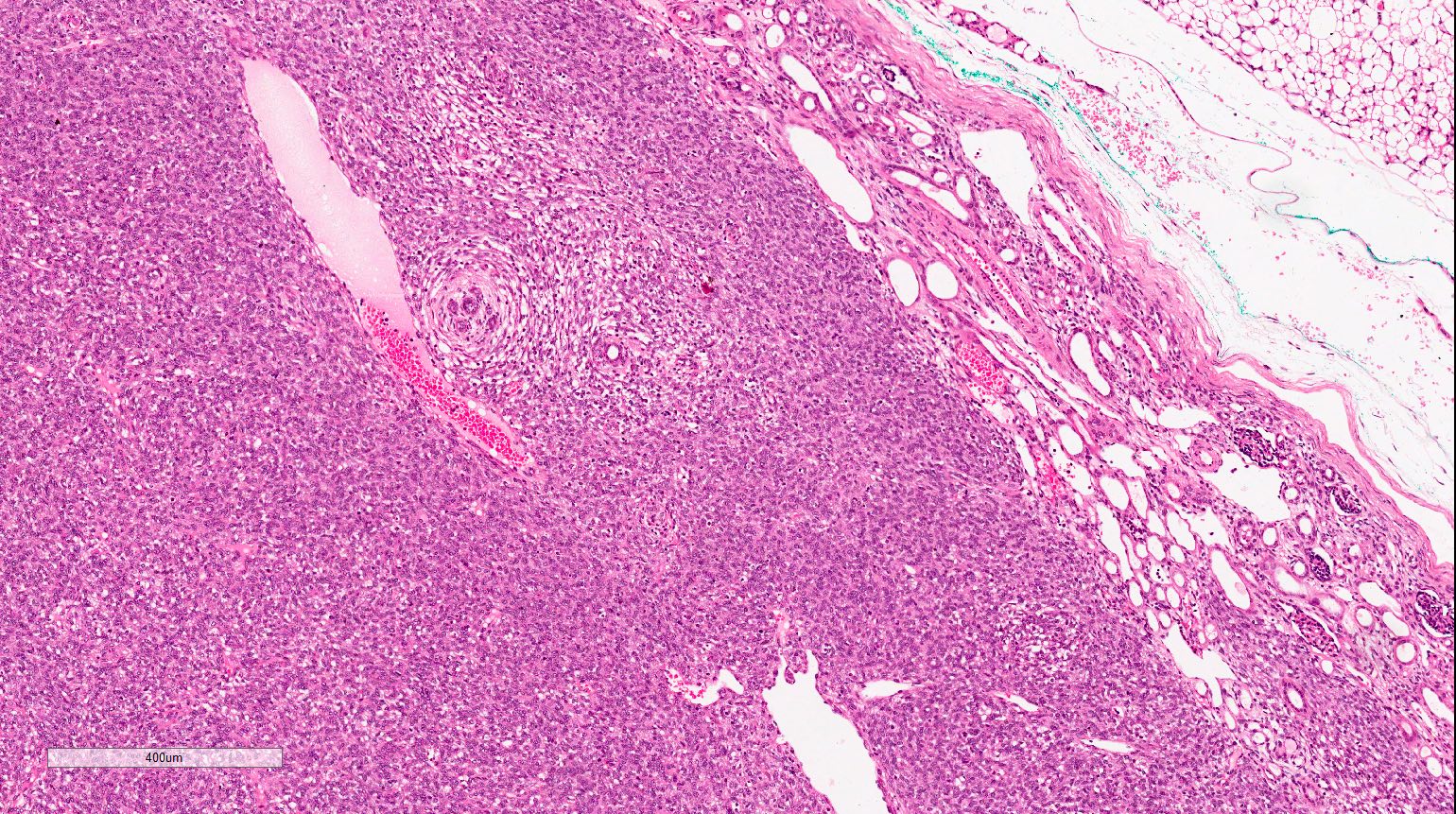
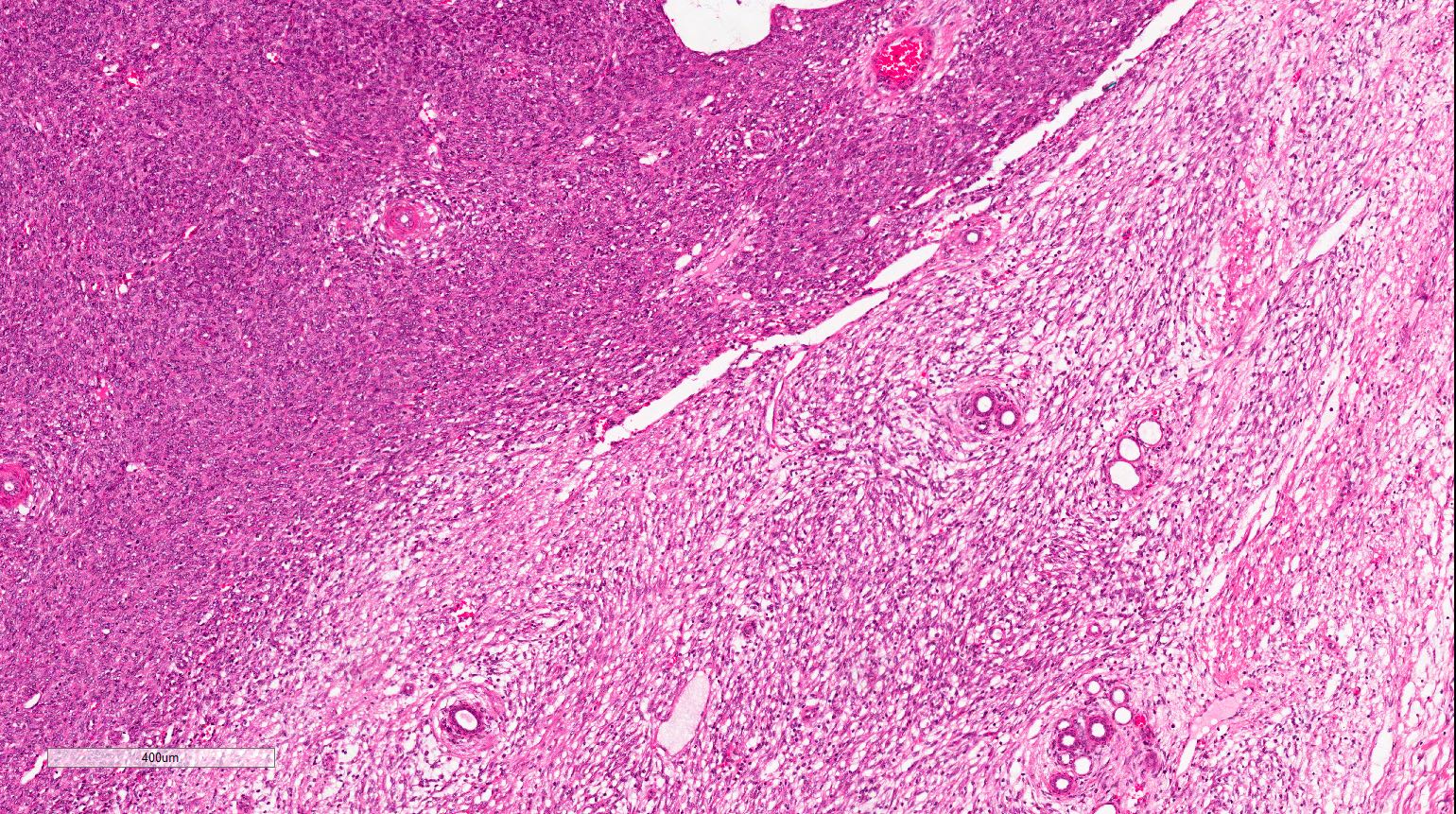
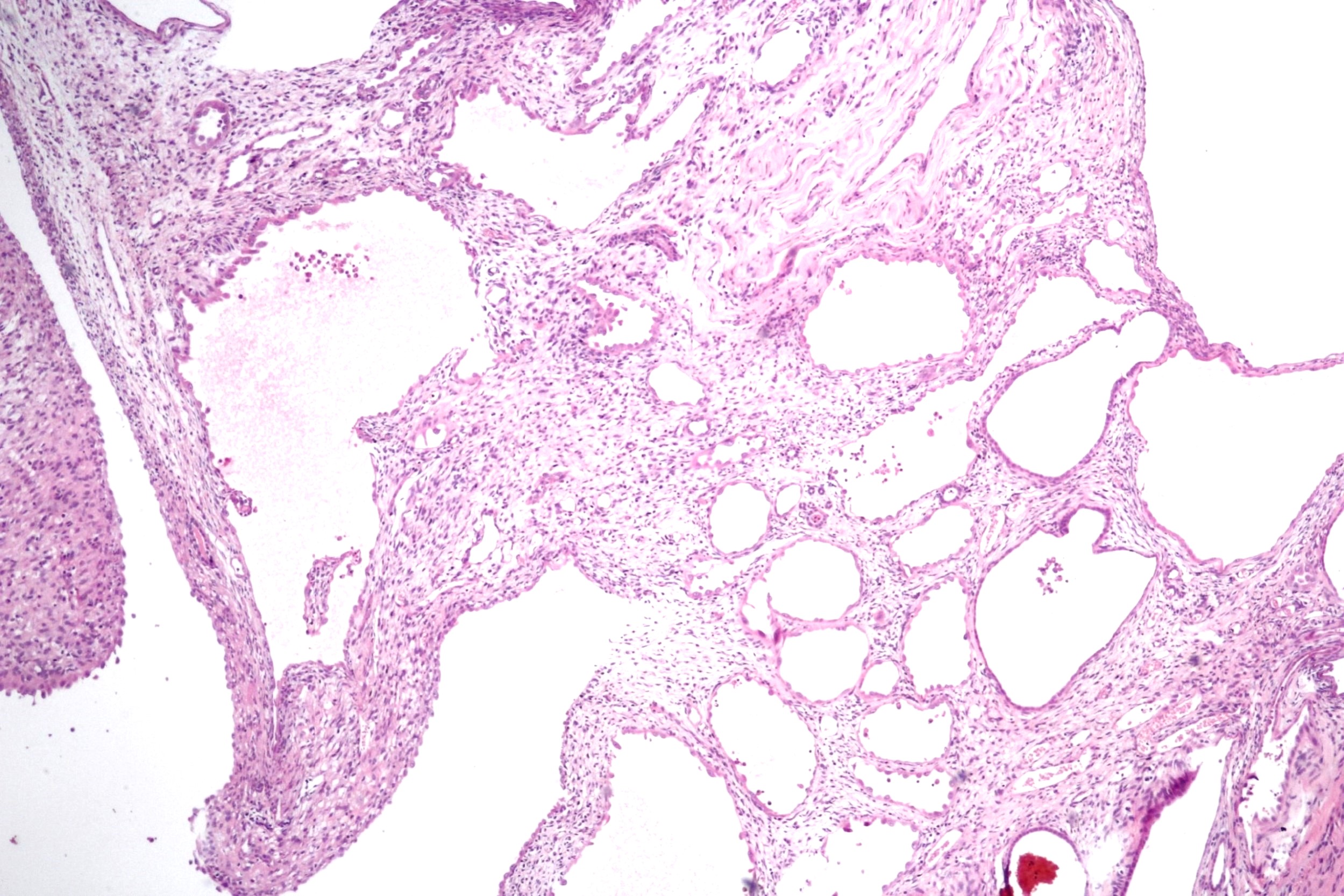
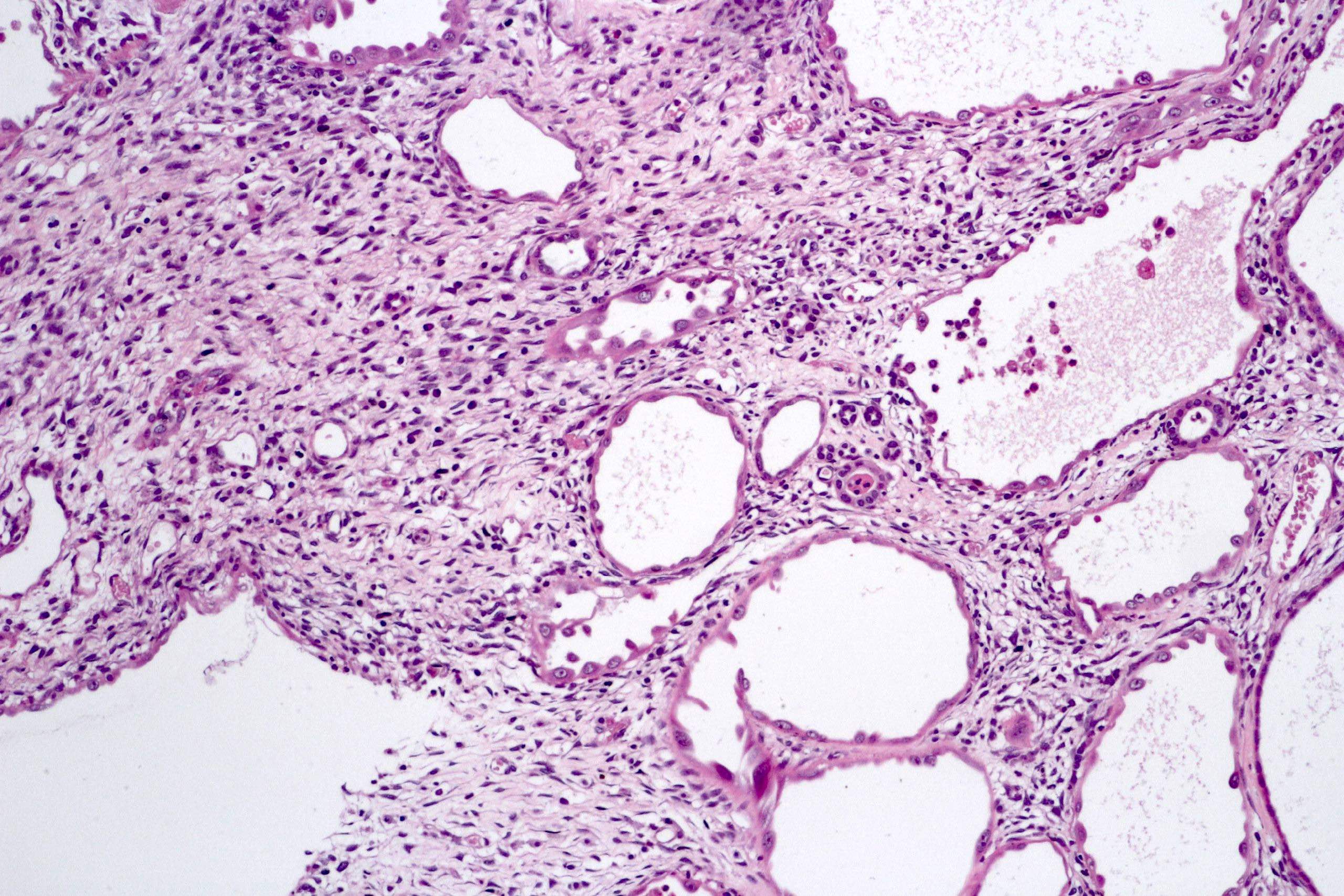
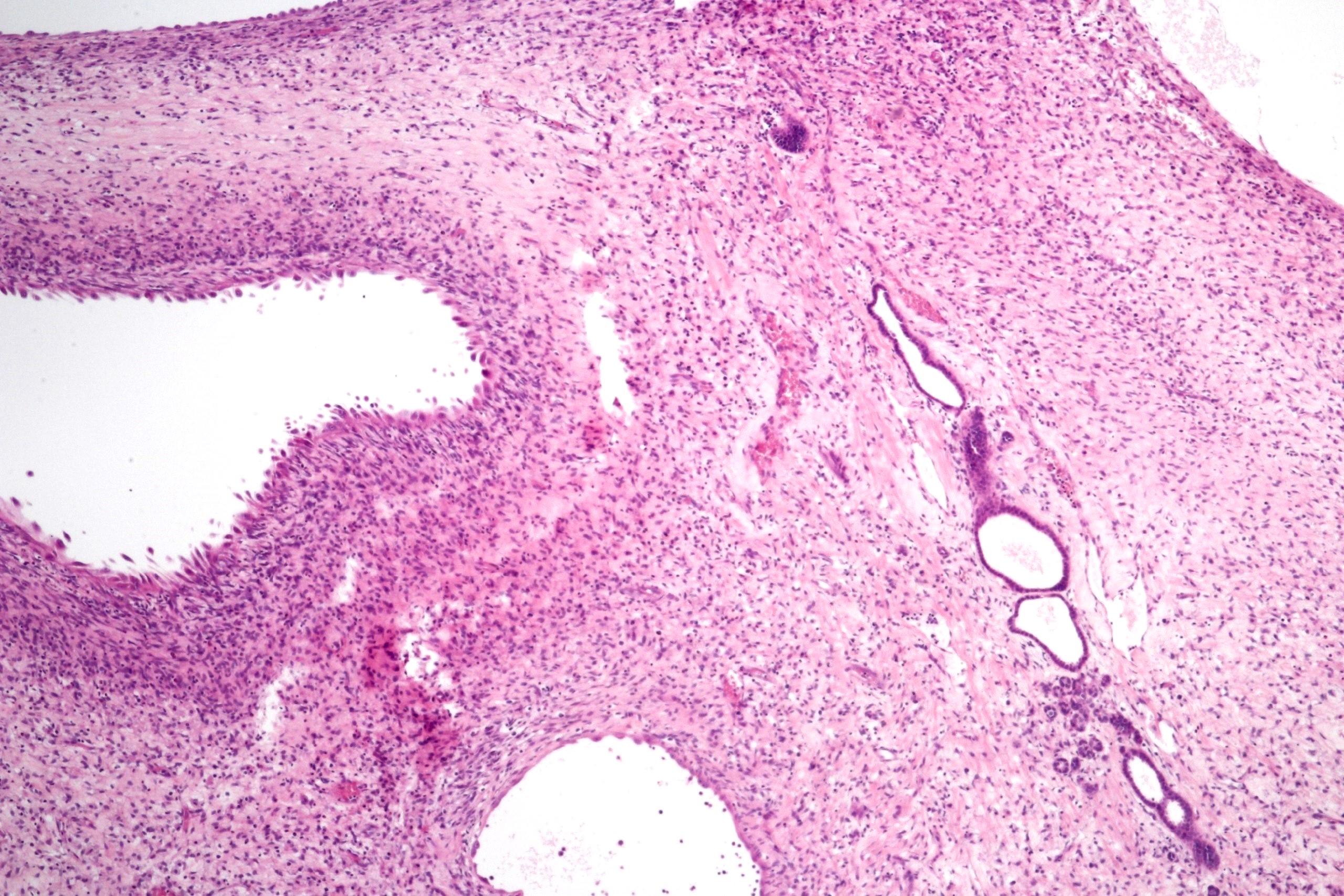
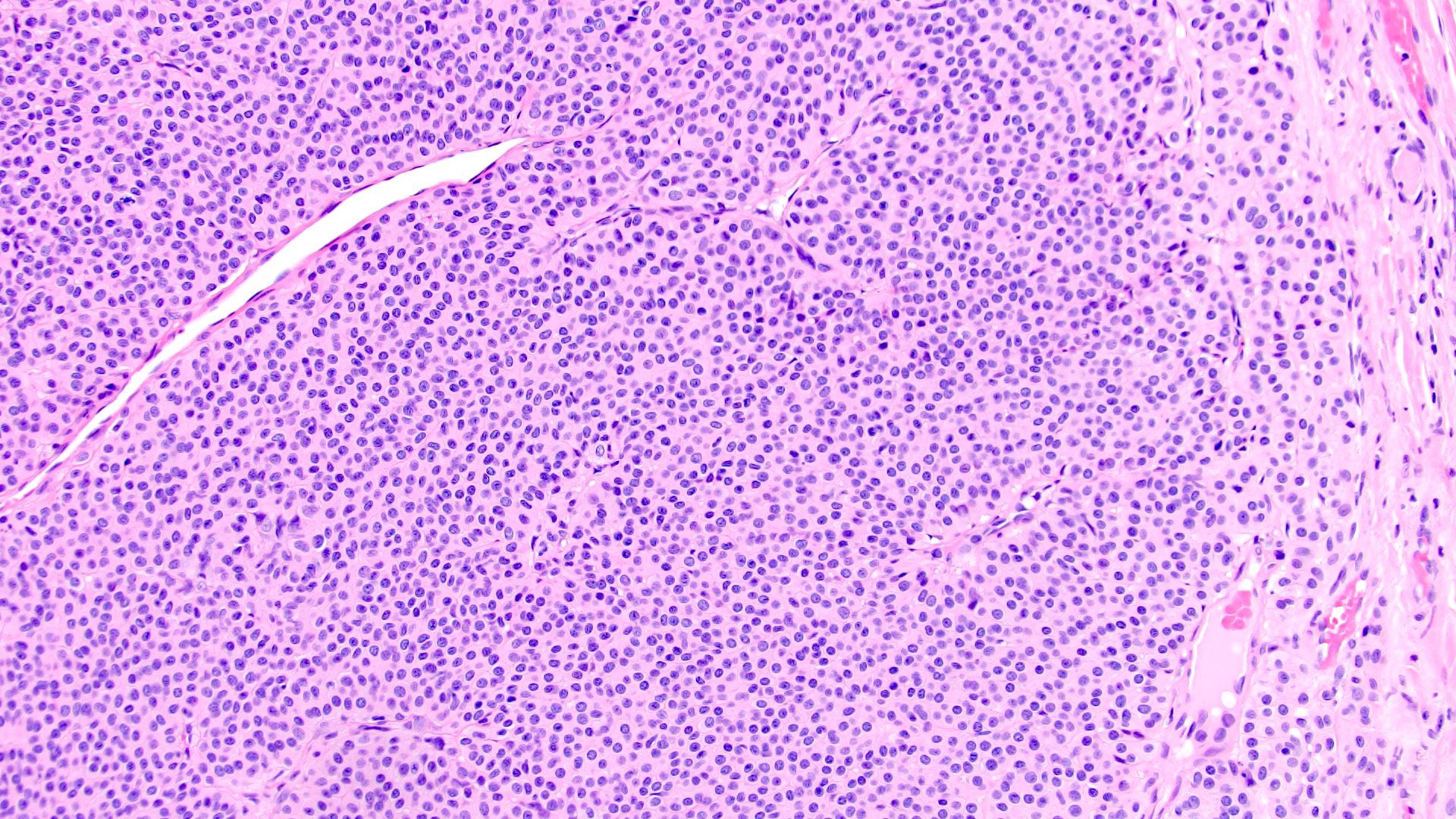
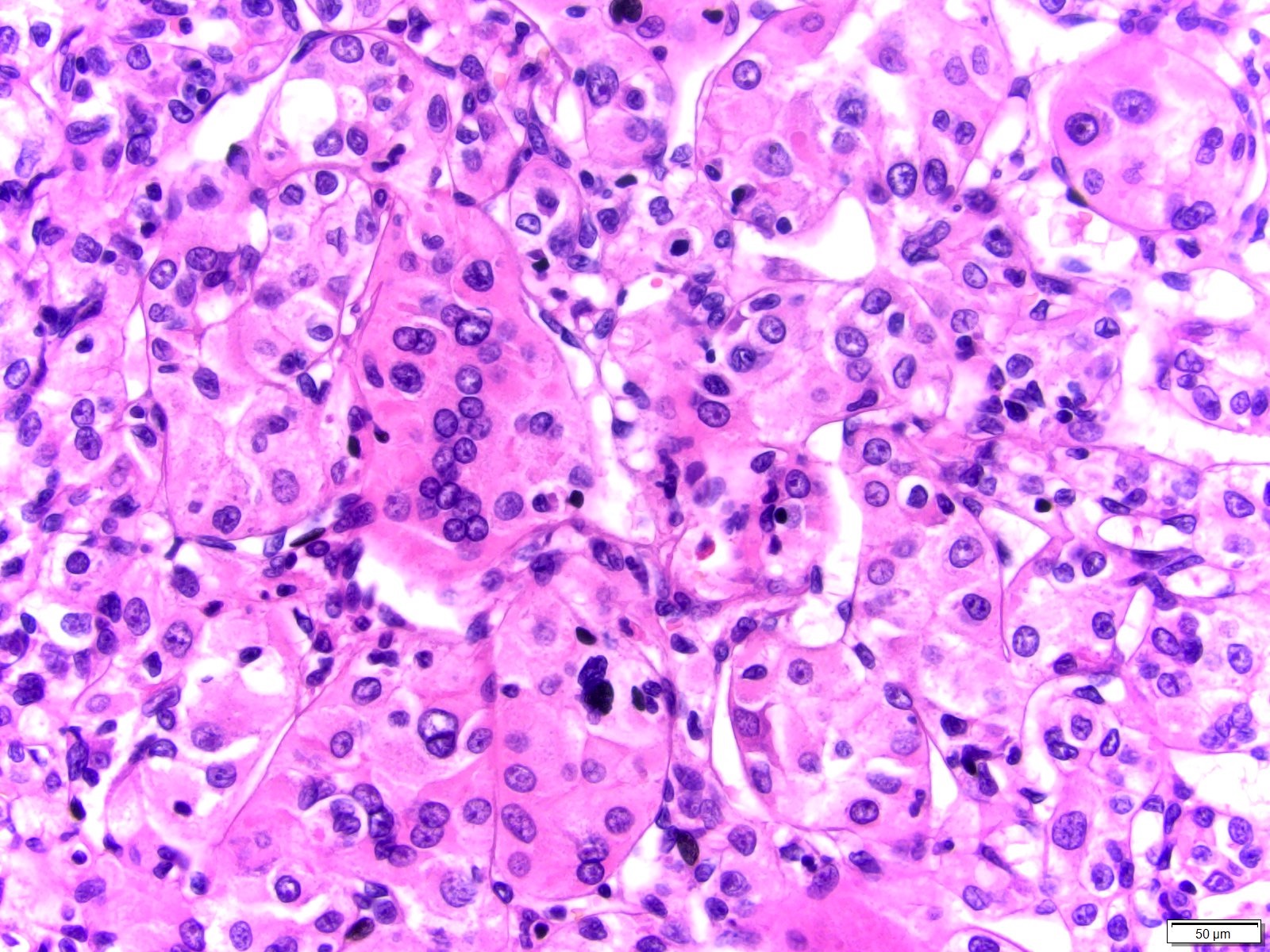
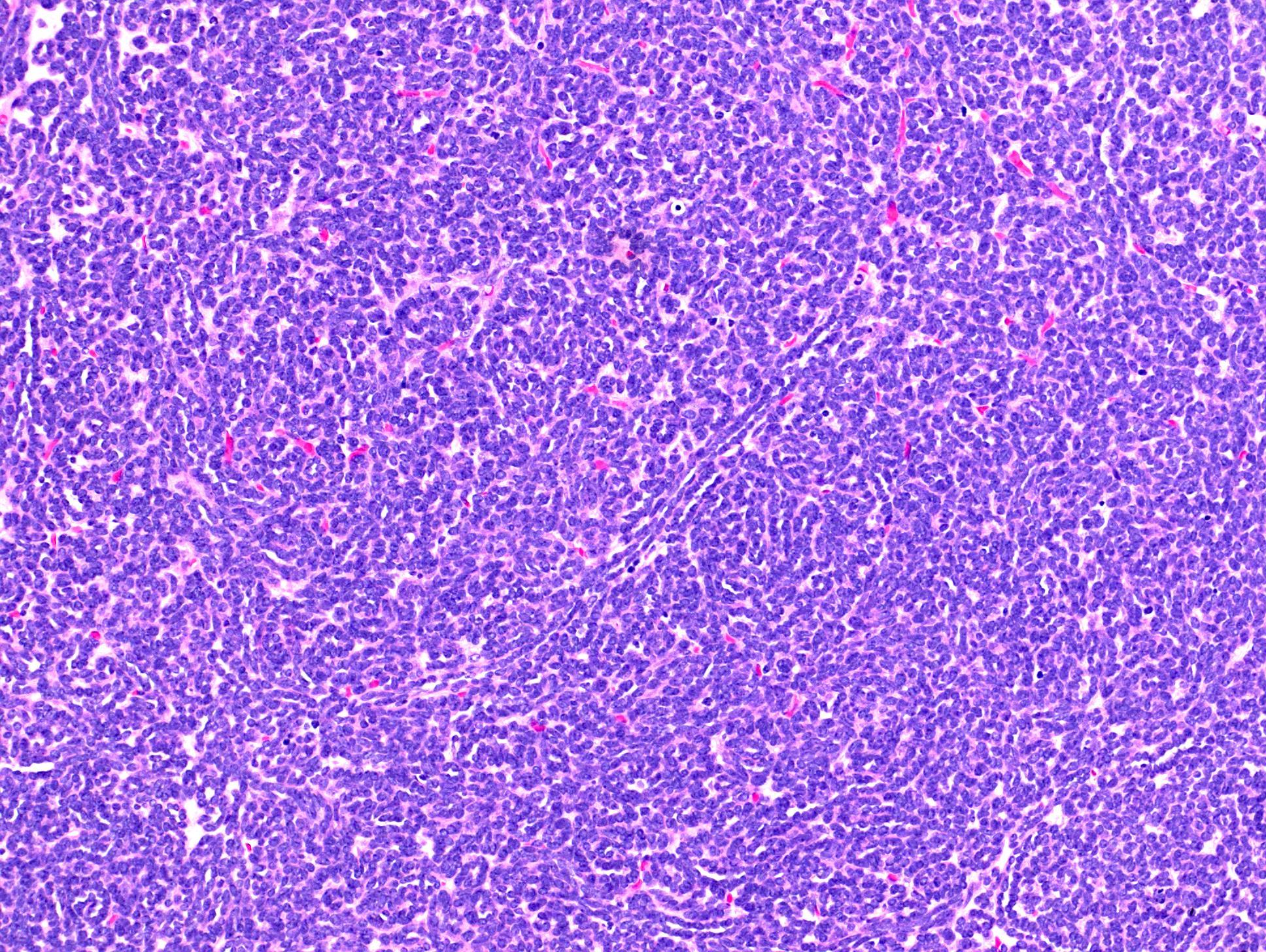
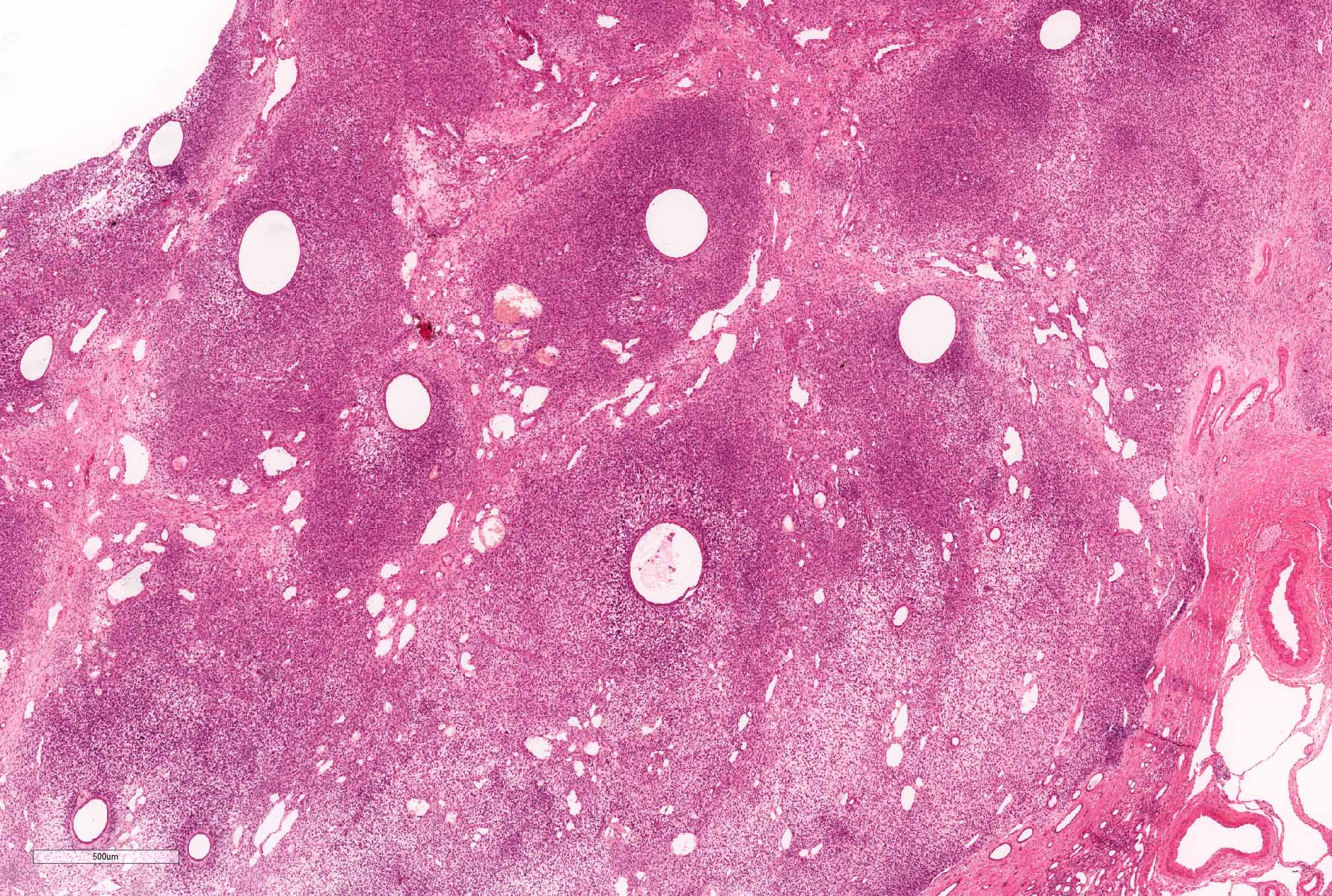
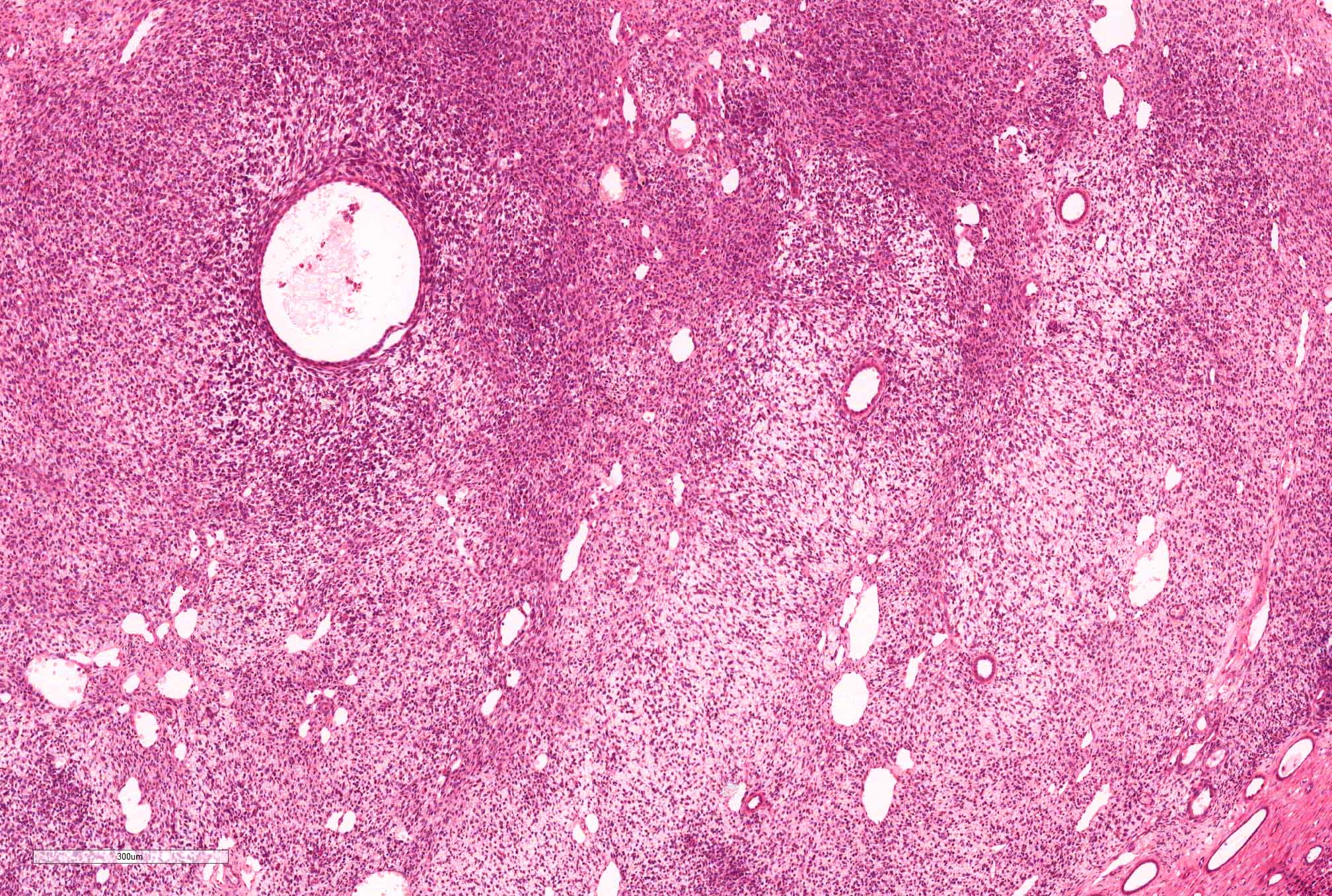
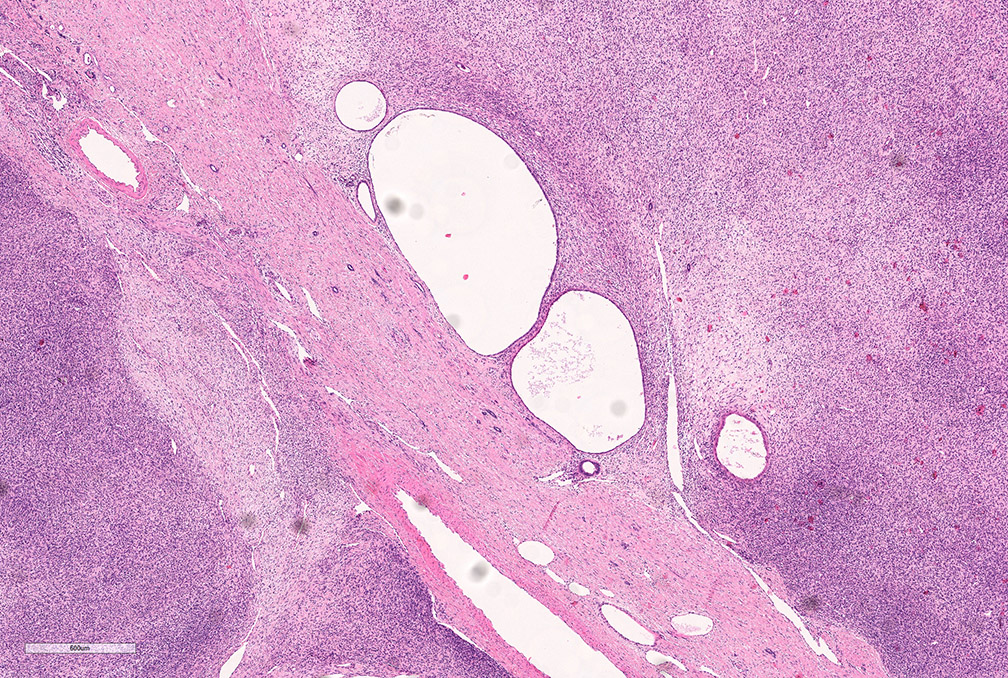
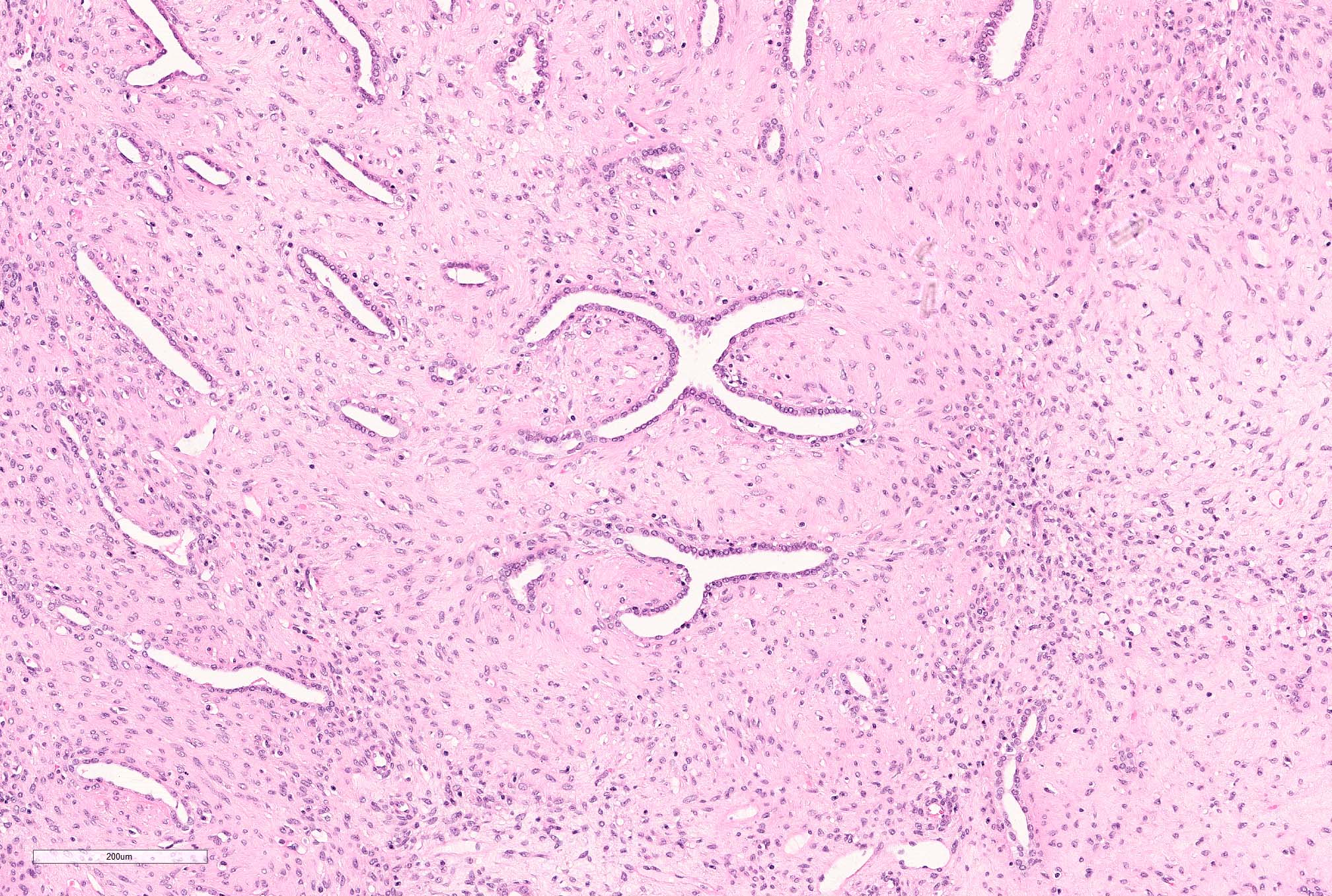
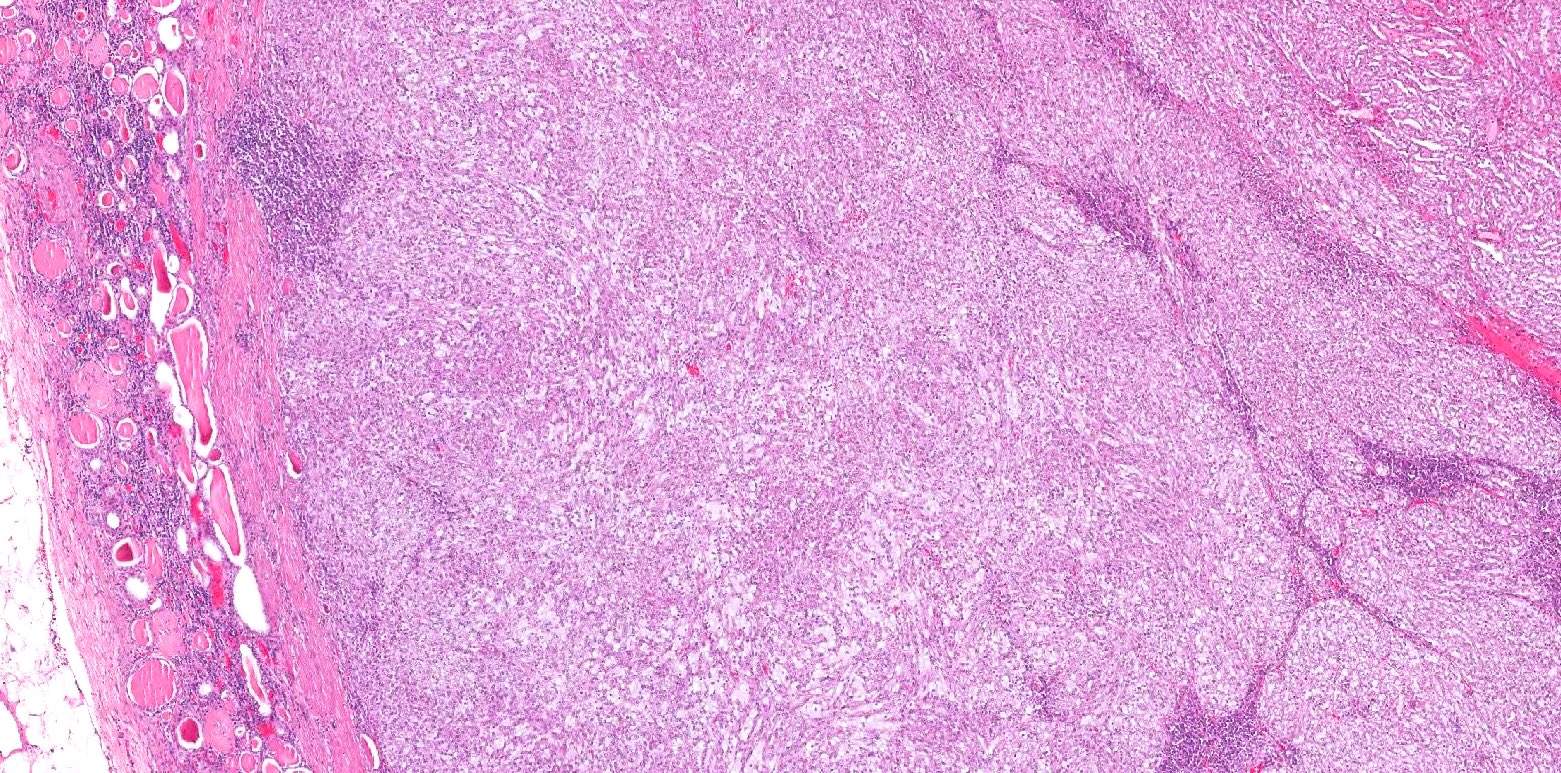
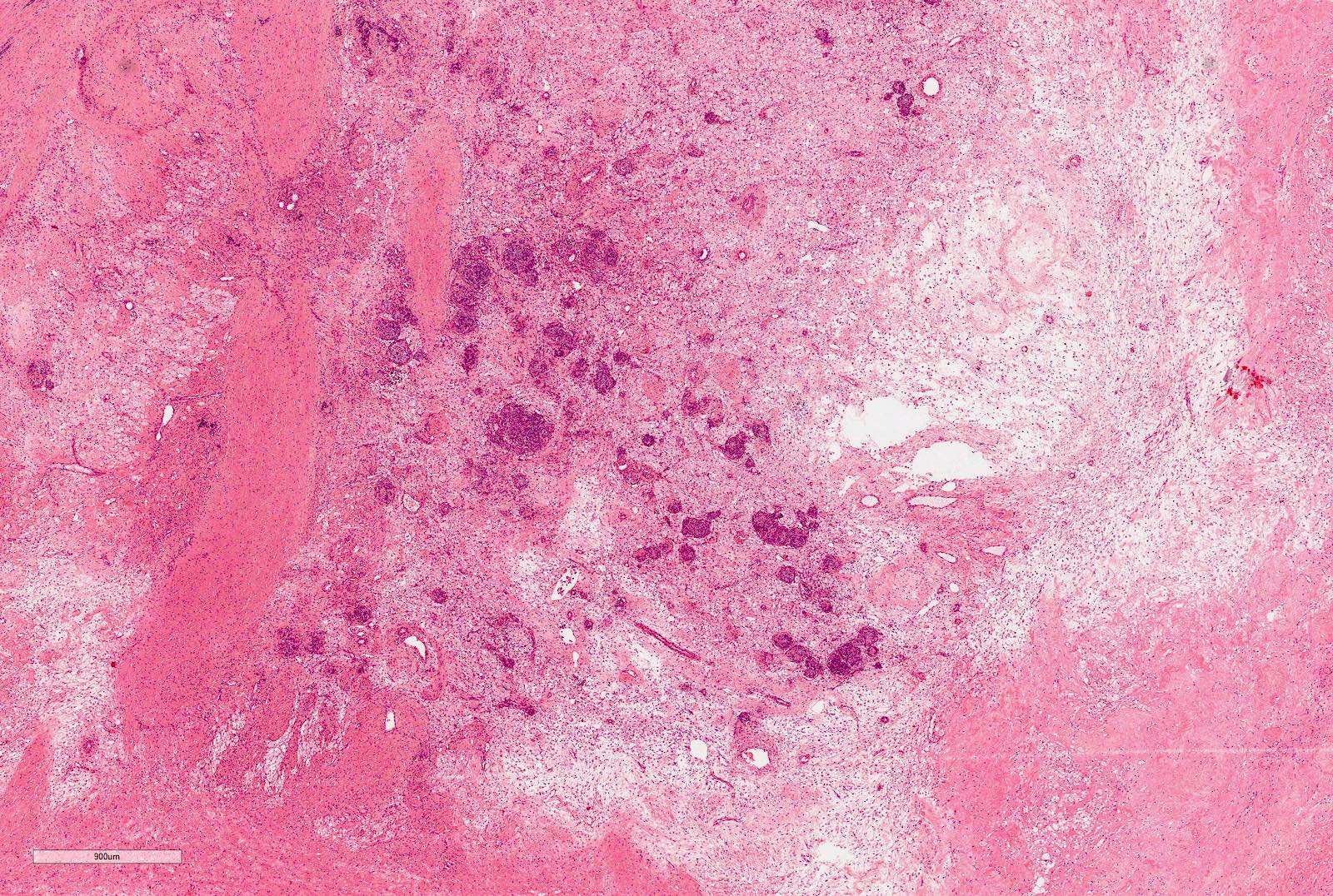
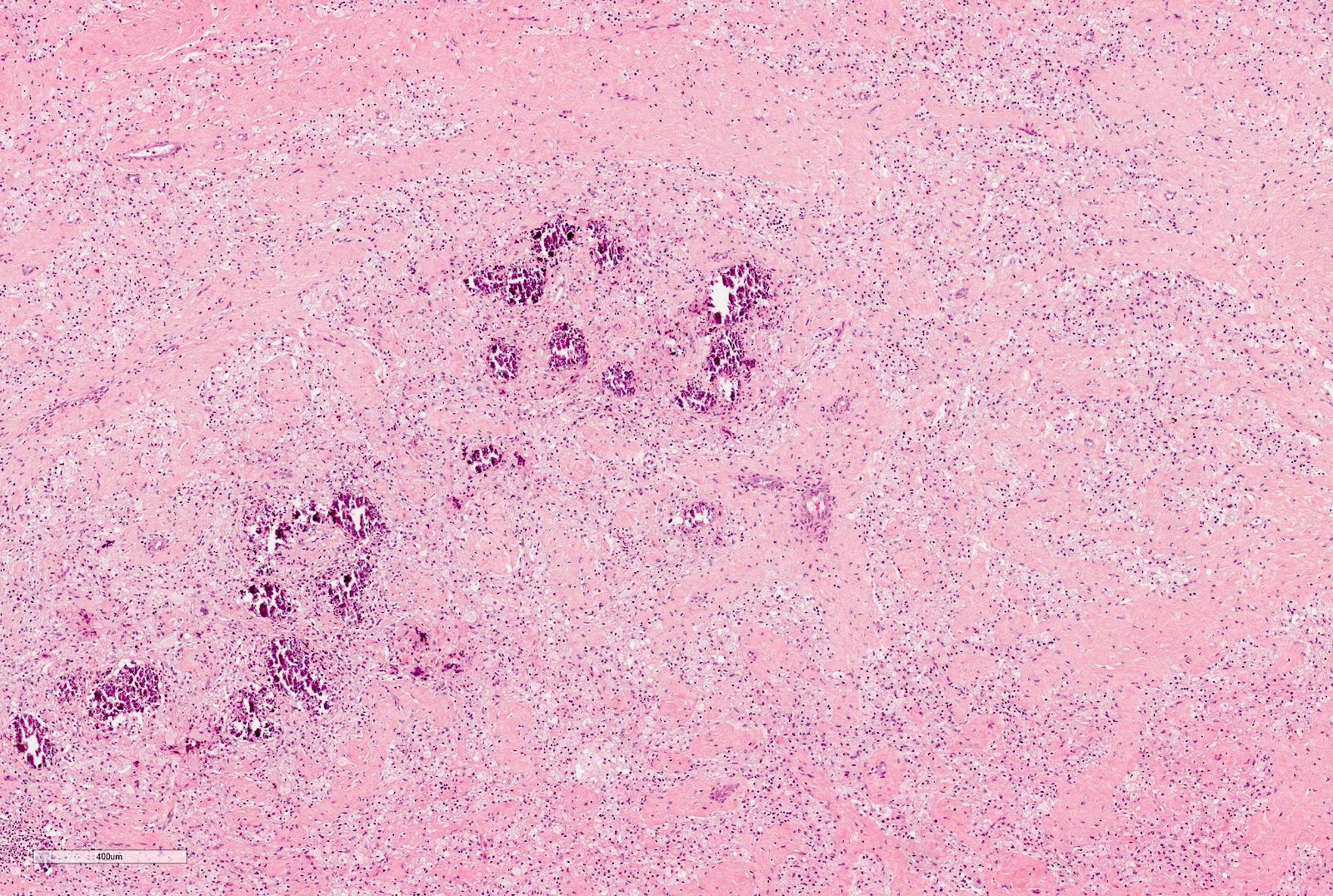
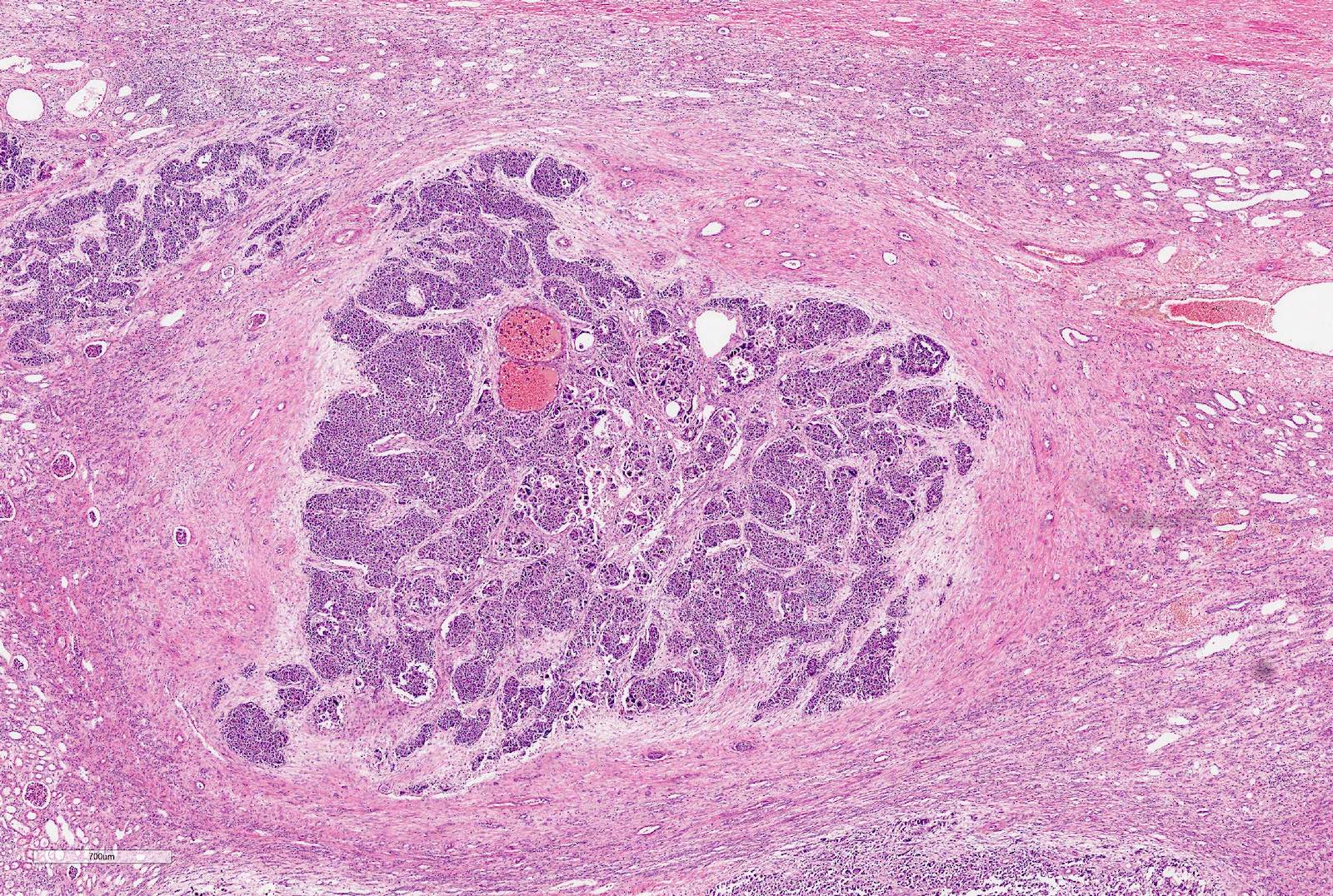
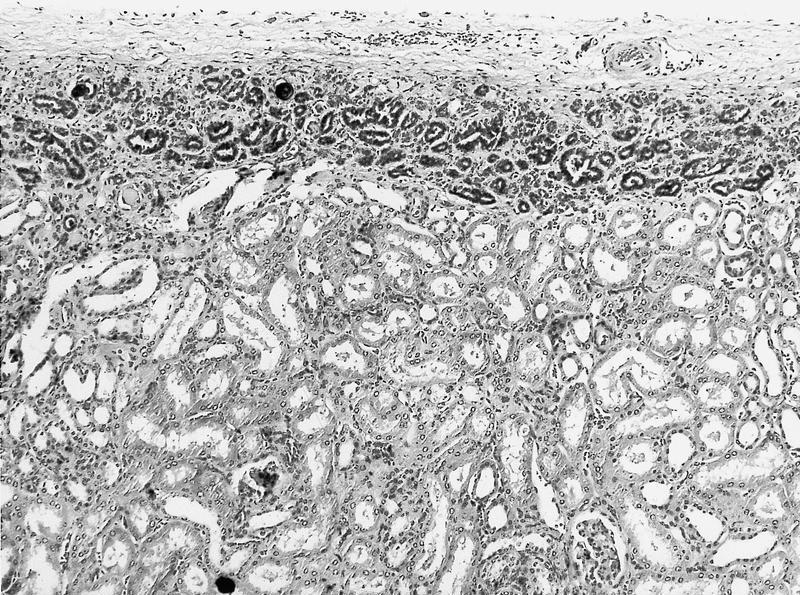
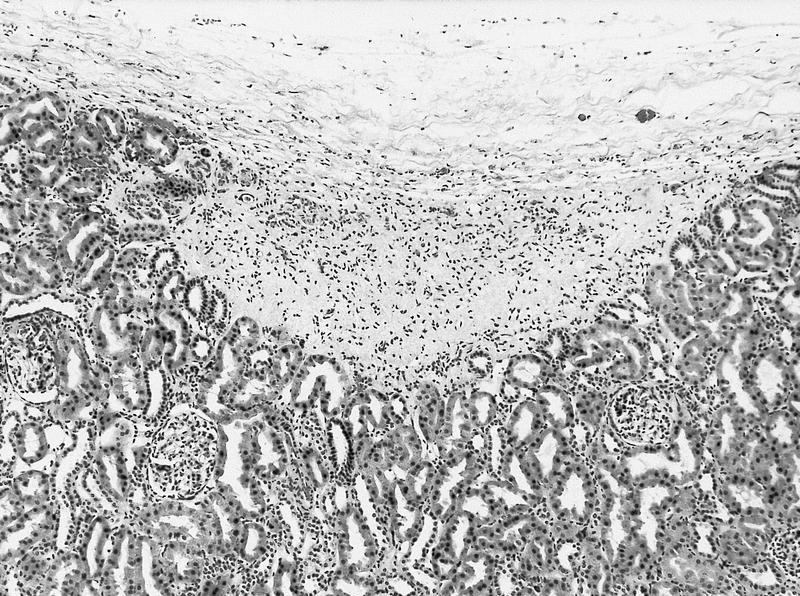
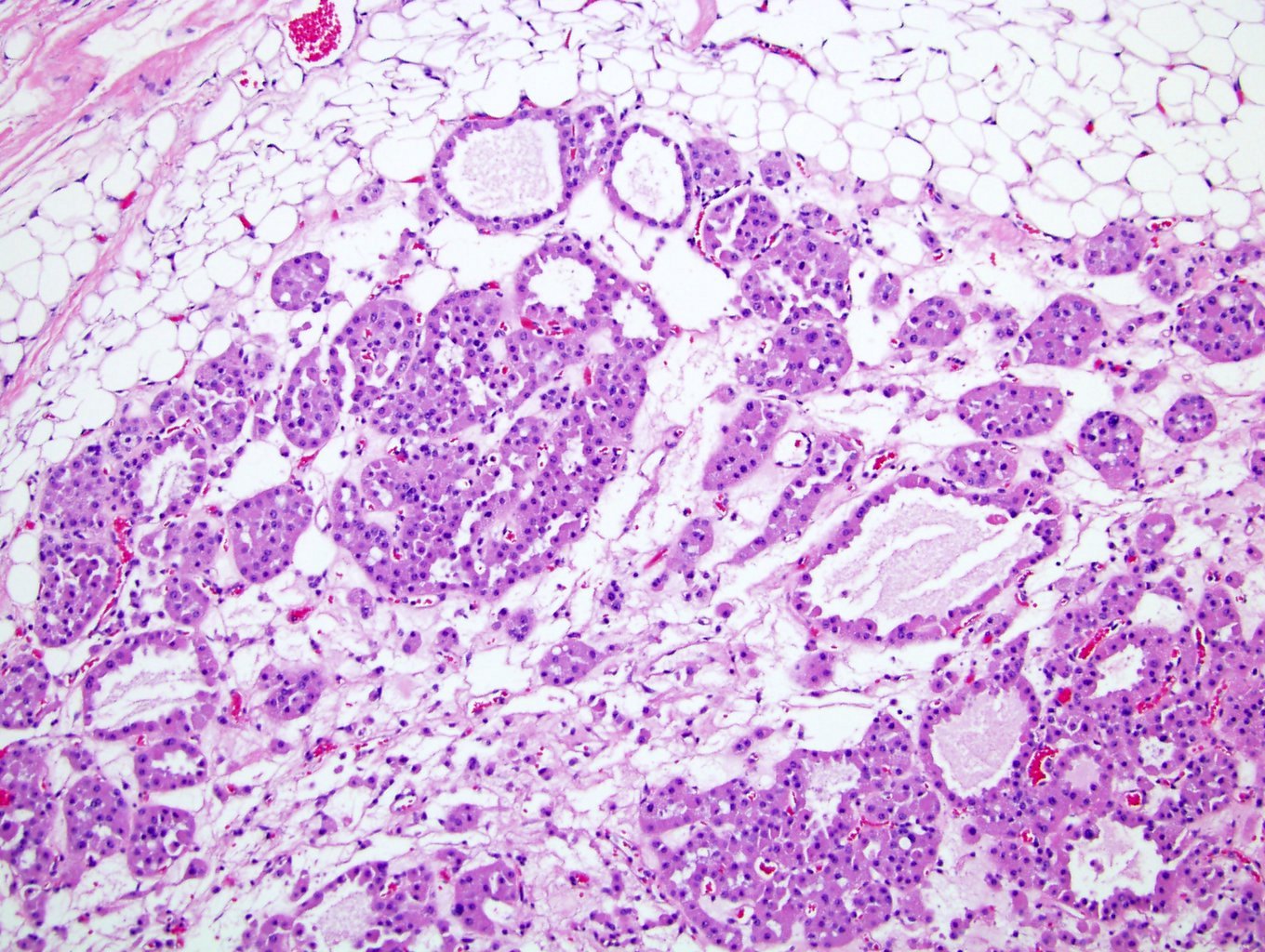
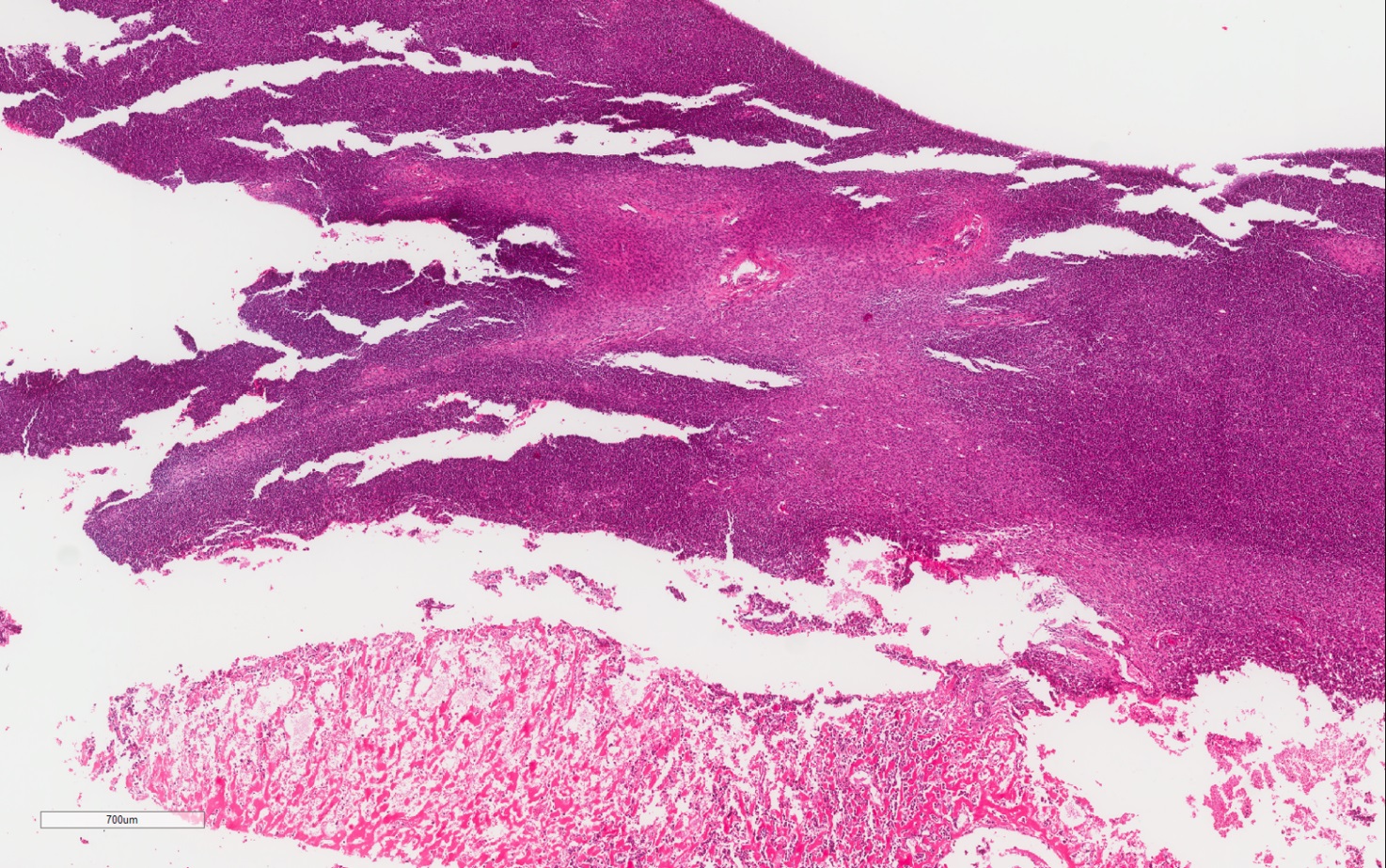
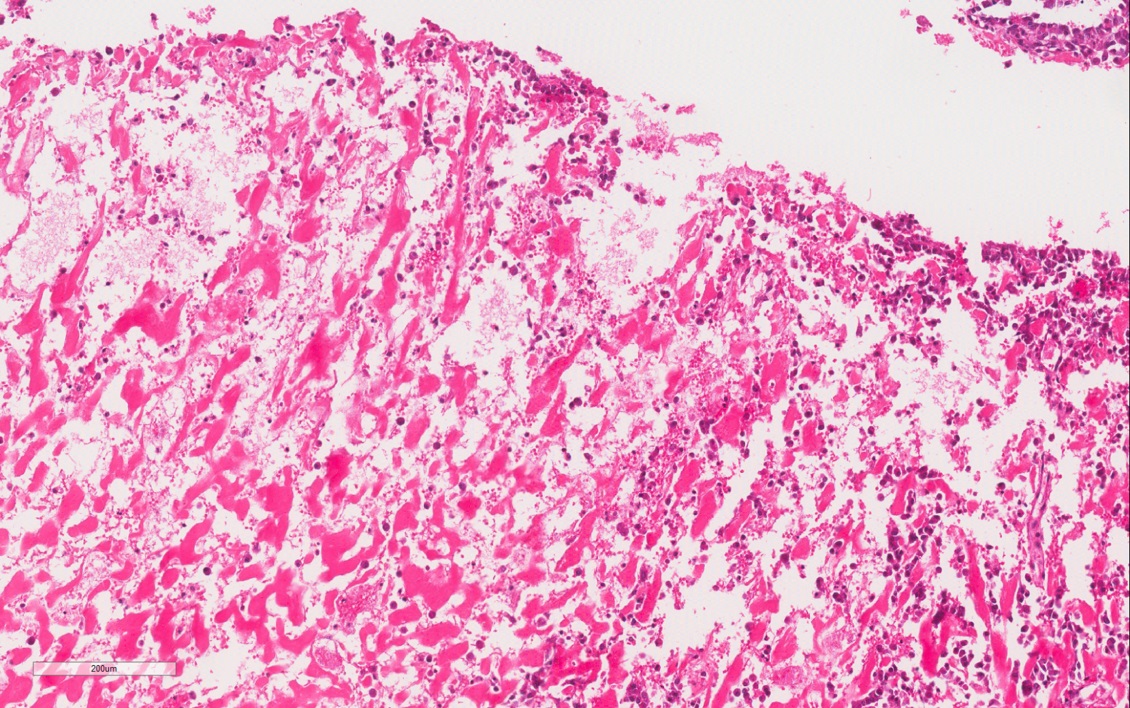
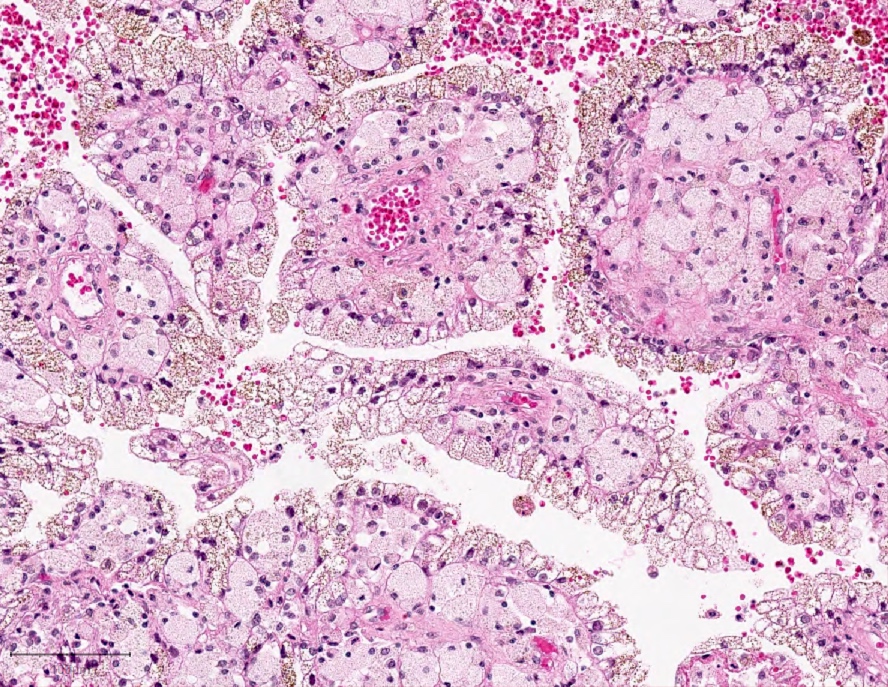
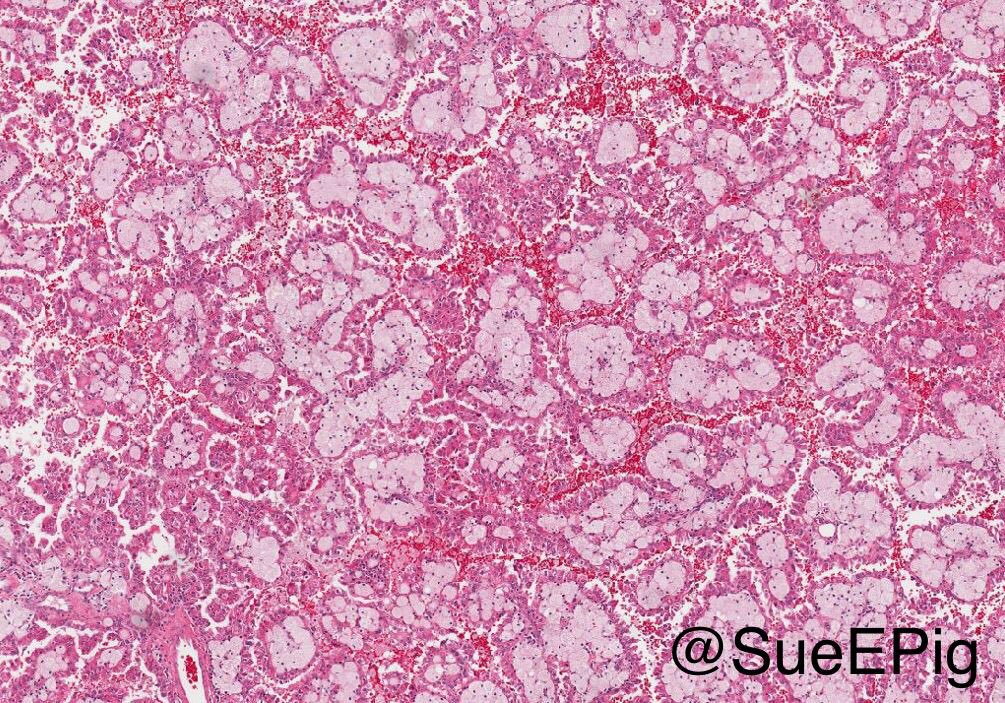
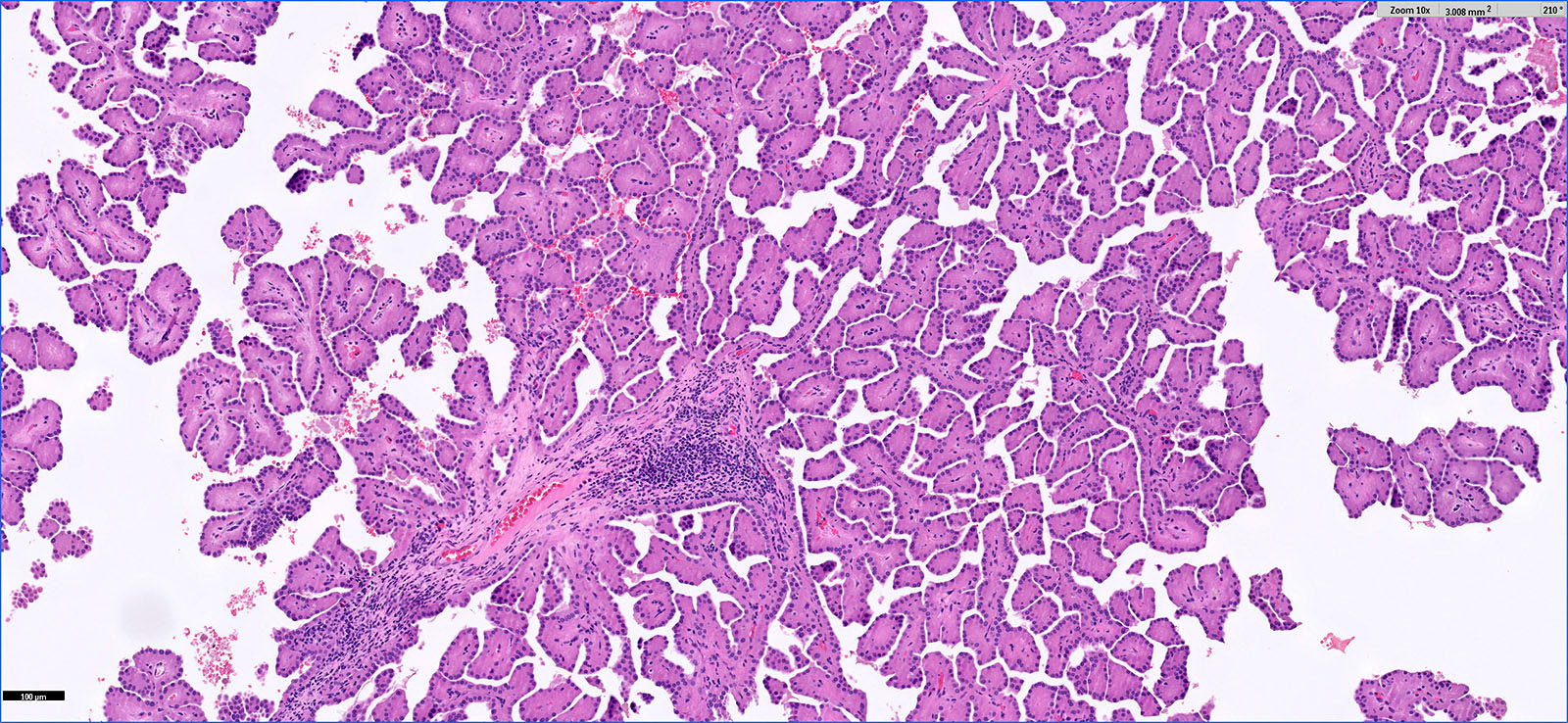
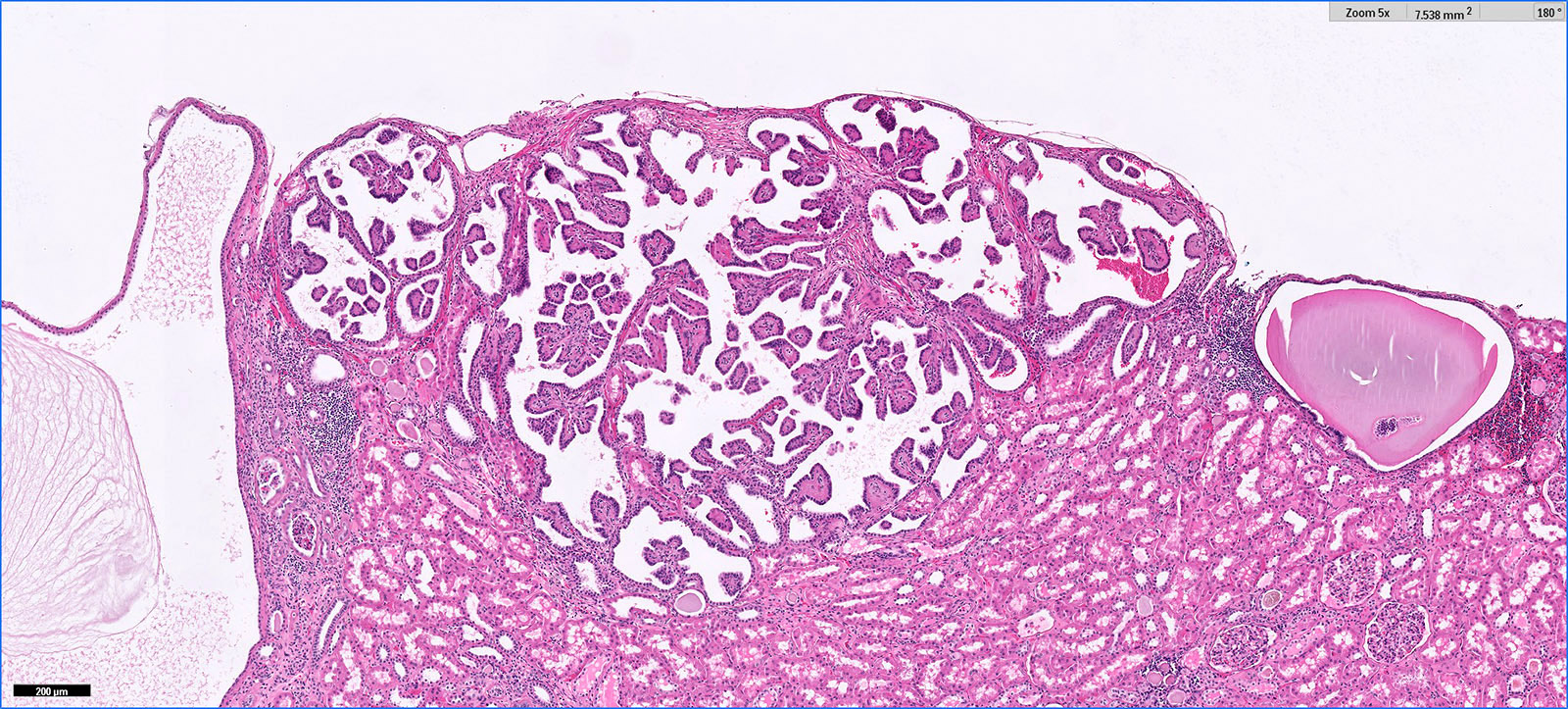
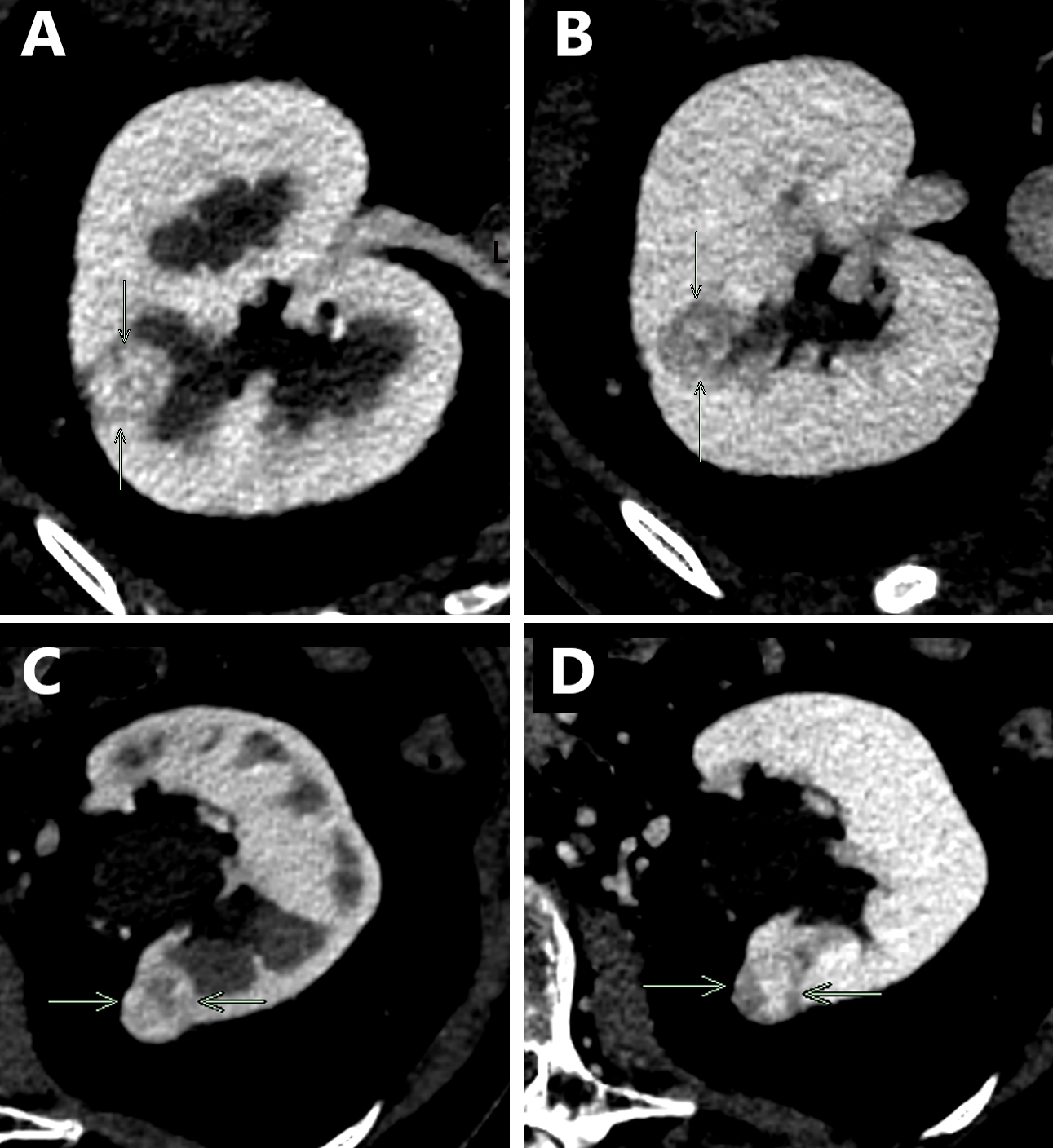
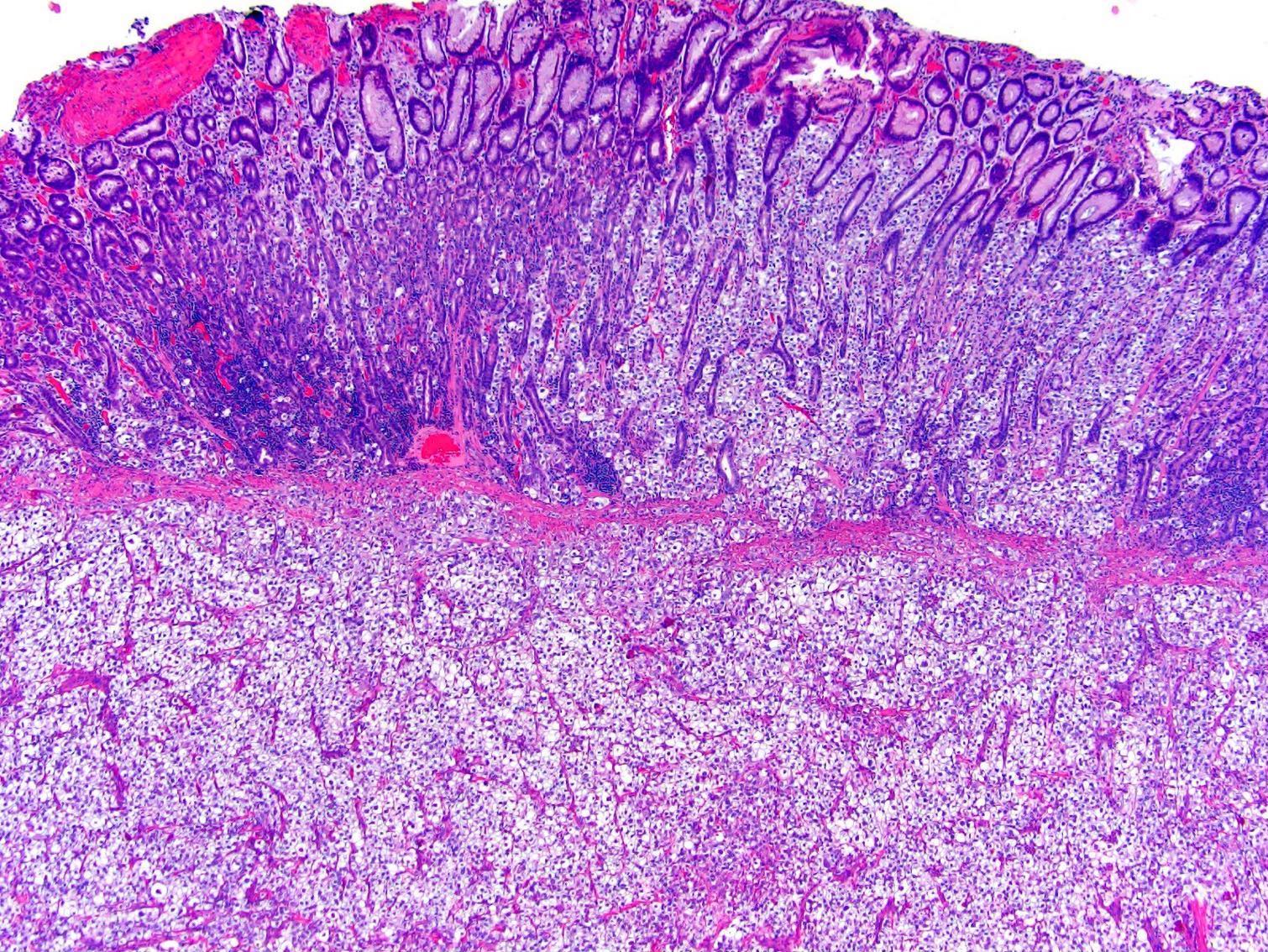
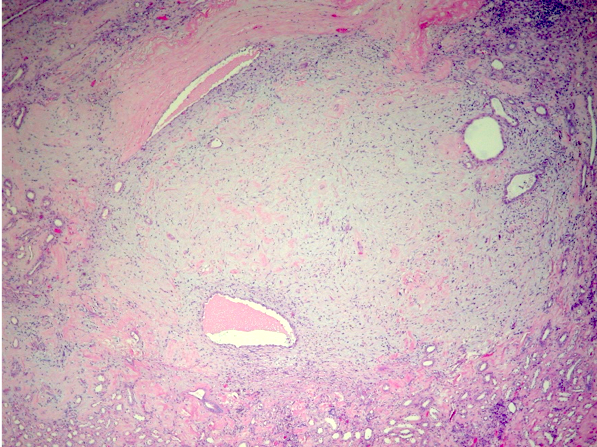
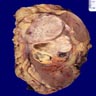
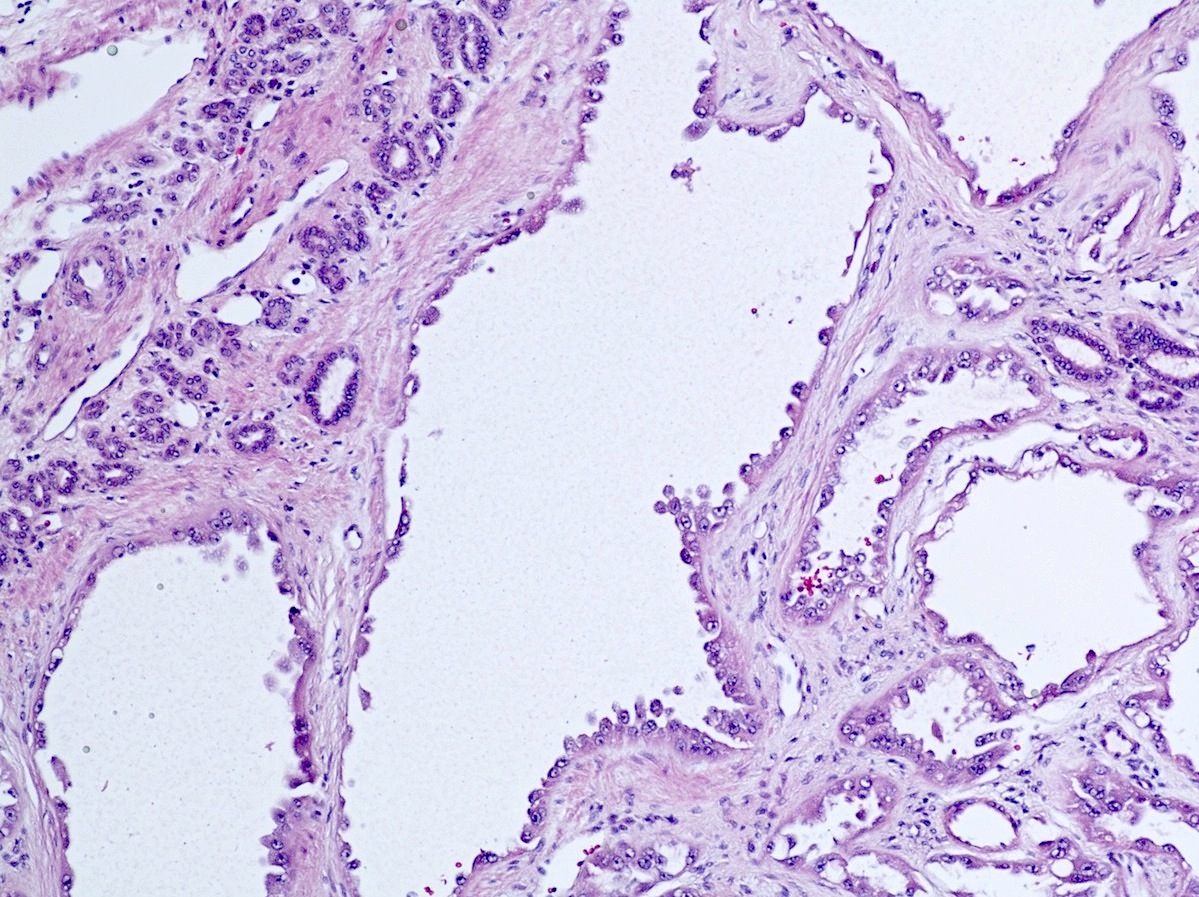
- Pediatric patients: medulla centric, irregularly shaped solid tumor mass with infiltrative borders (Genes Chromosomes Cancer 2016;55:442, Pol J Pathol 2018;69:109)
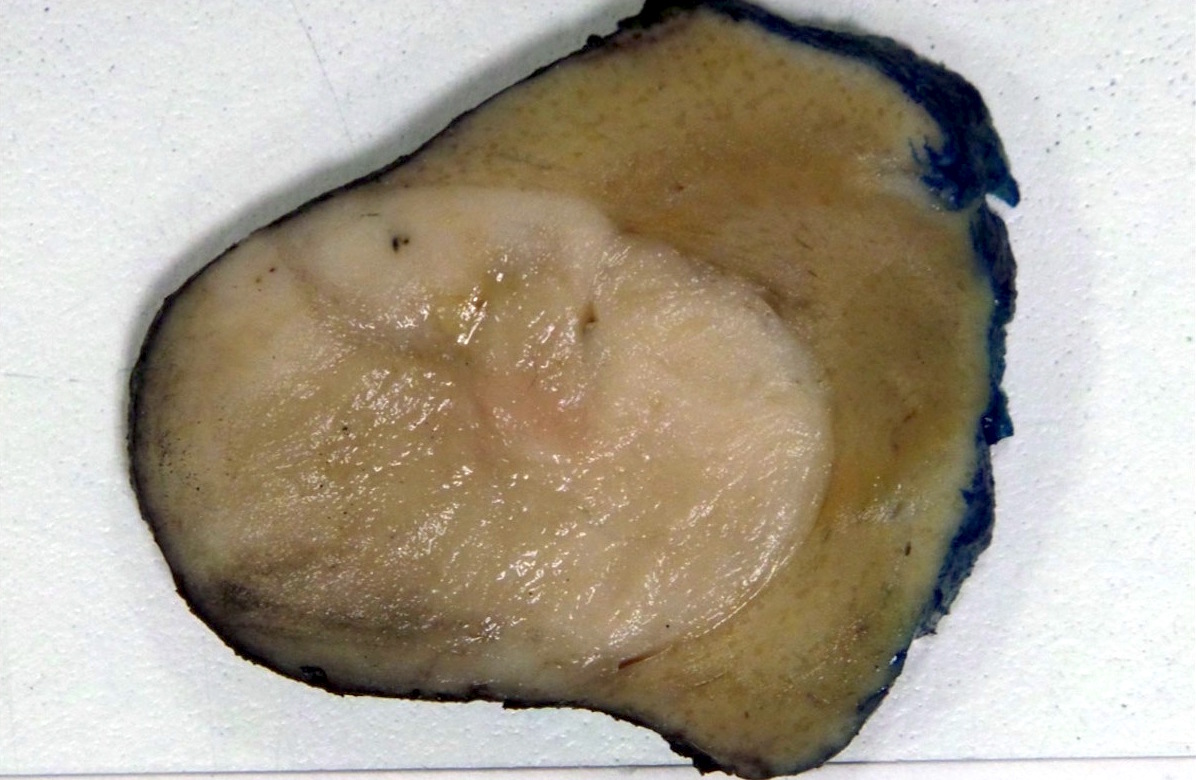
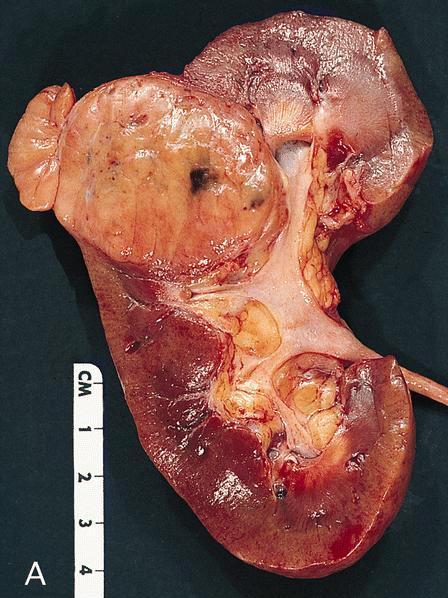
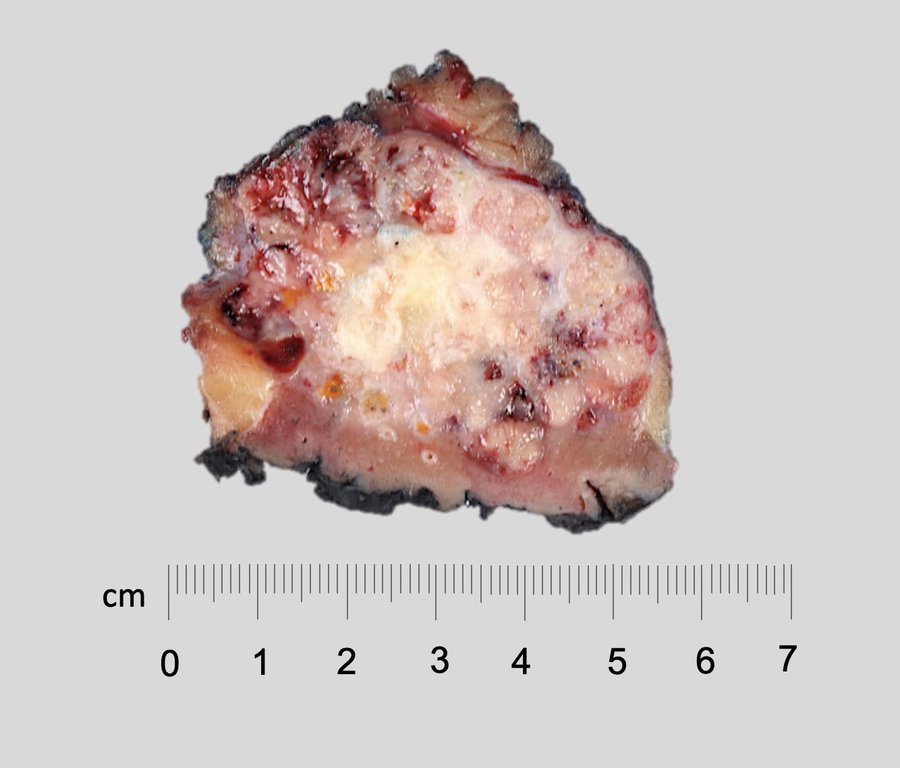
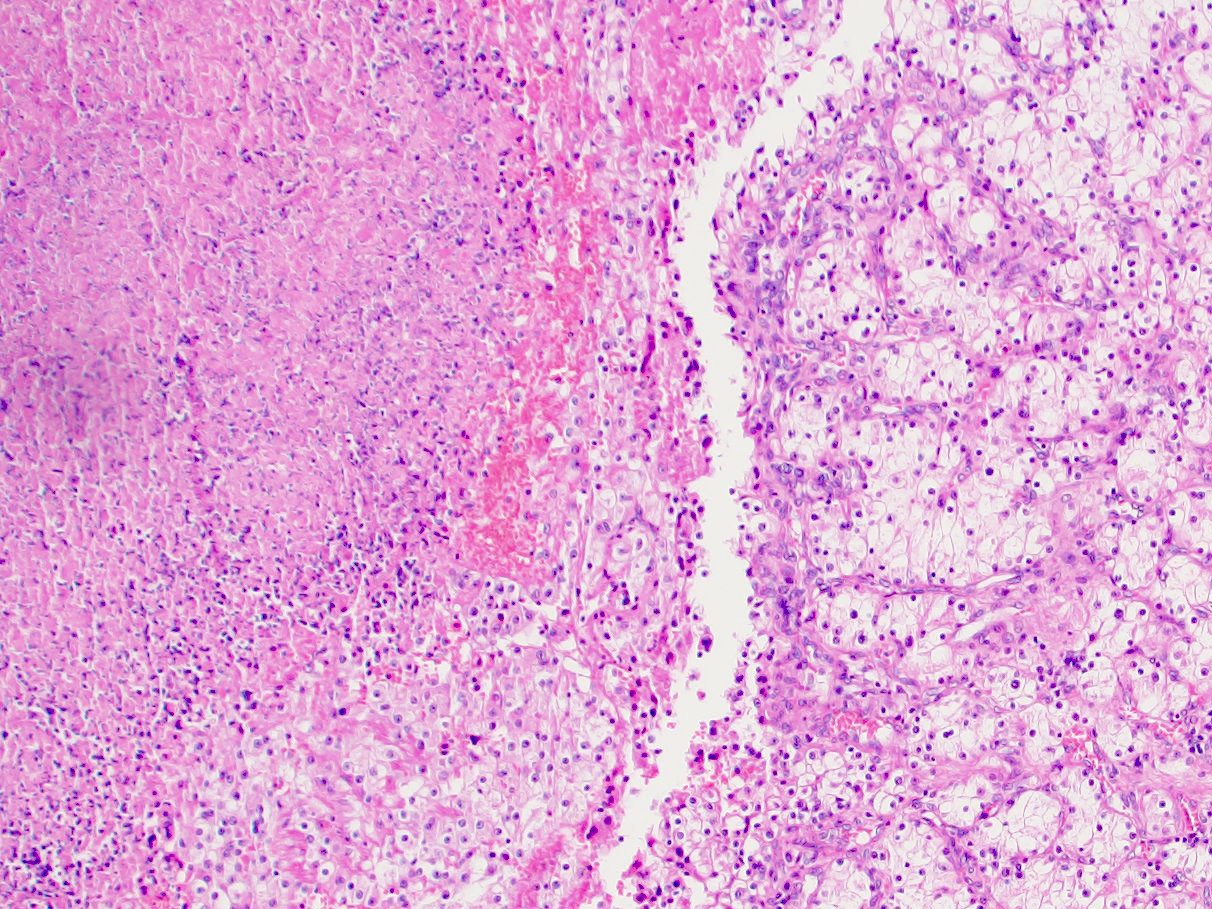
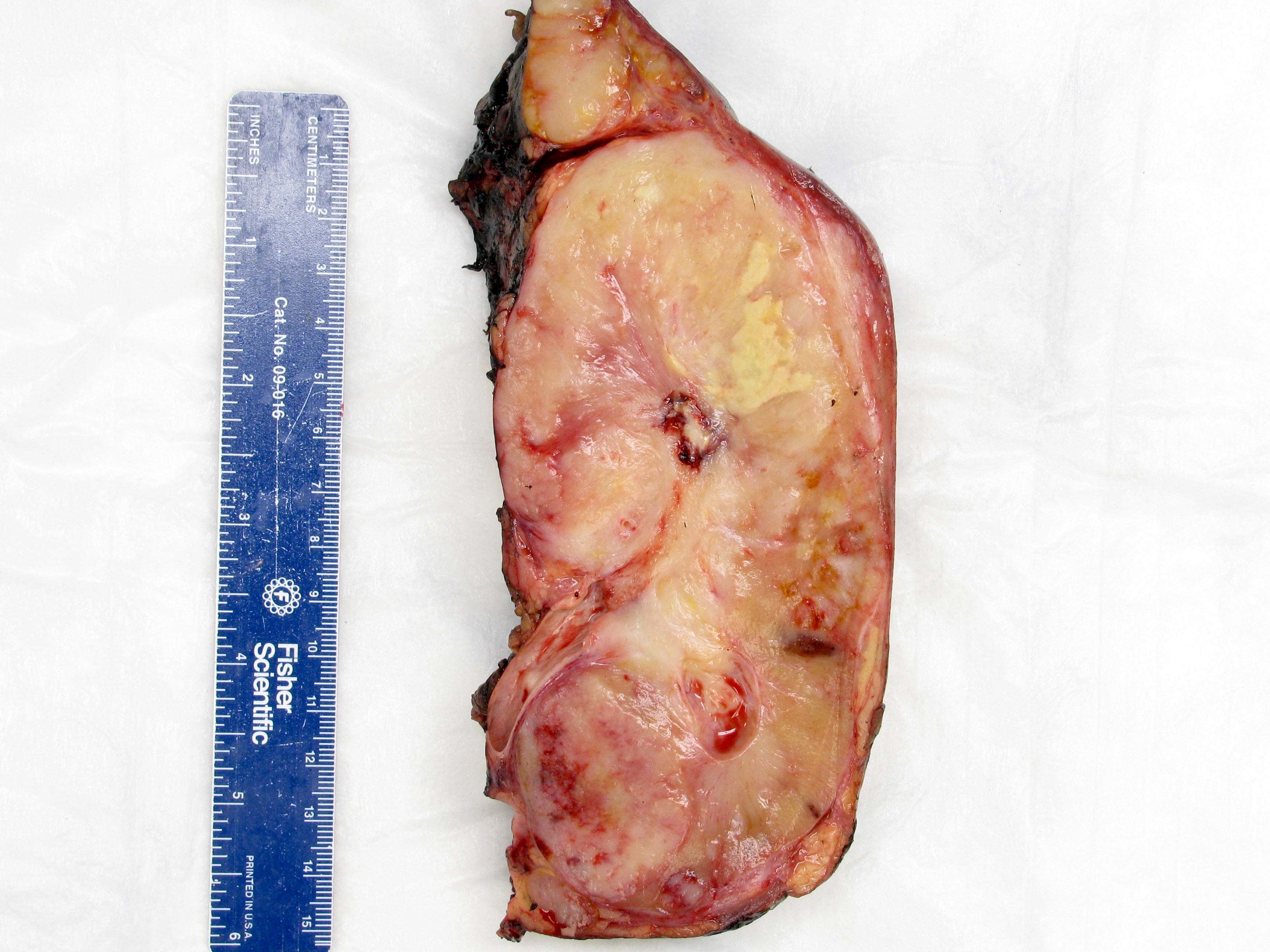
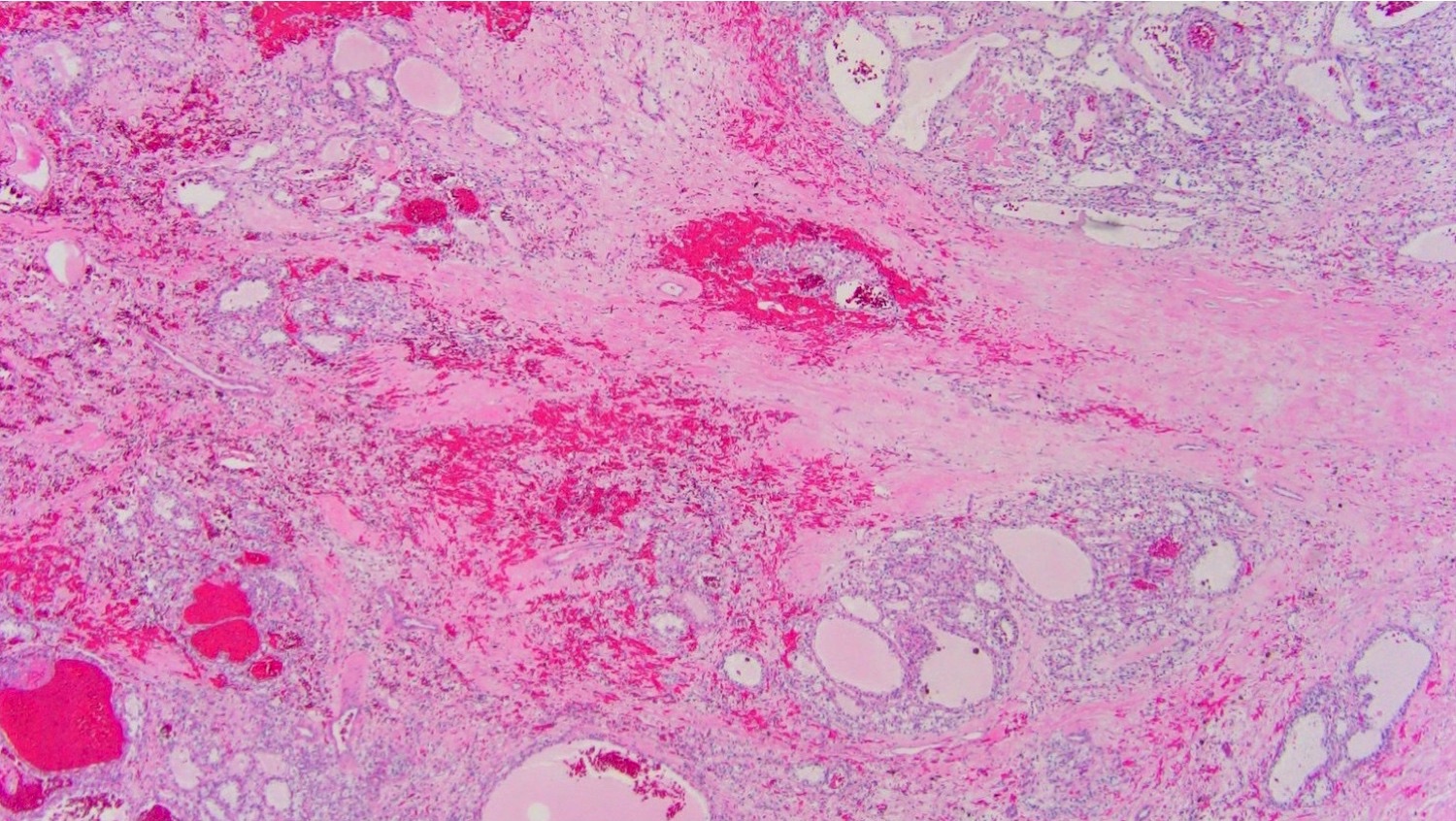
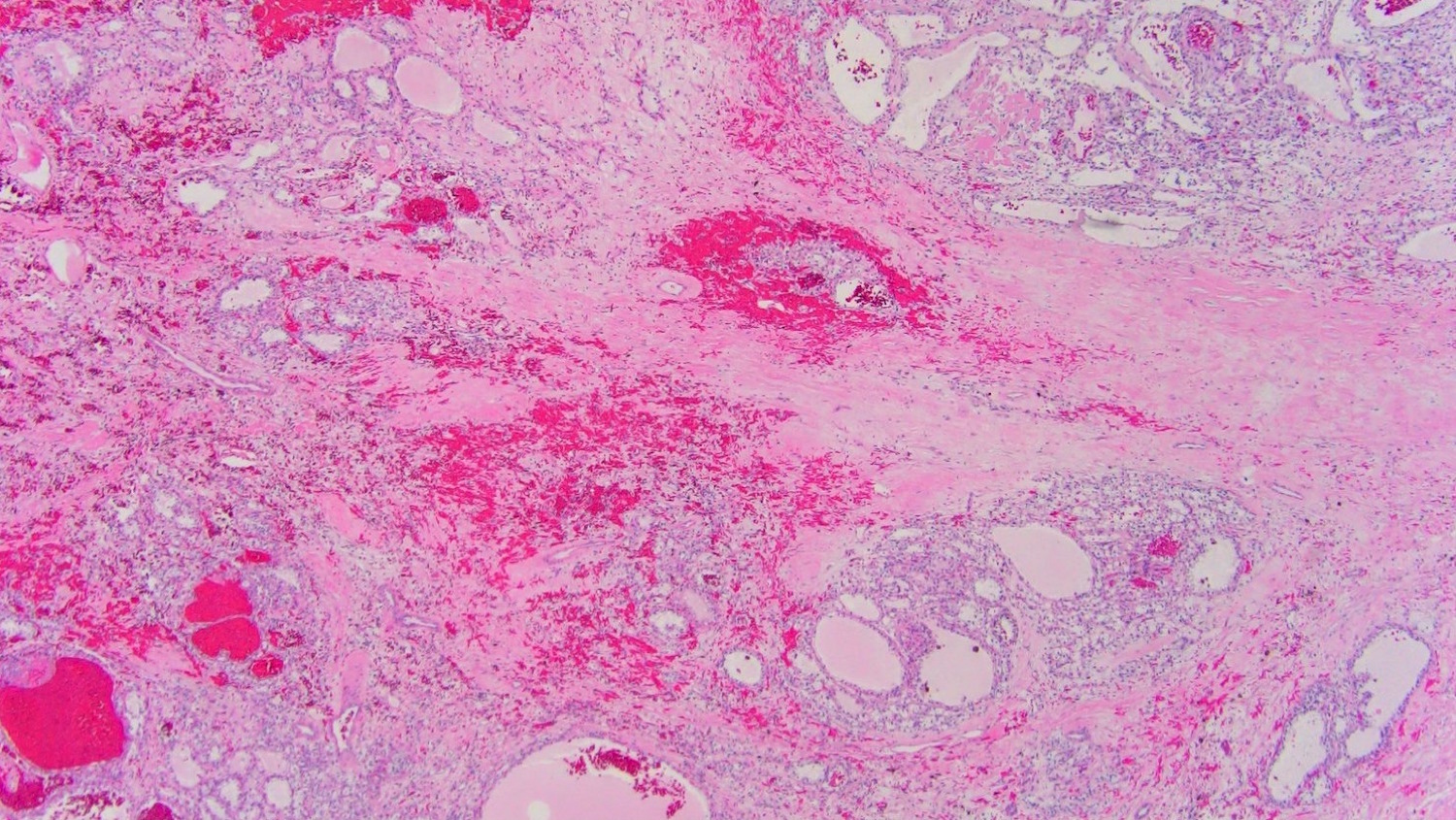
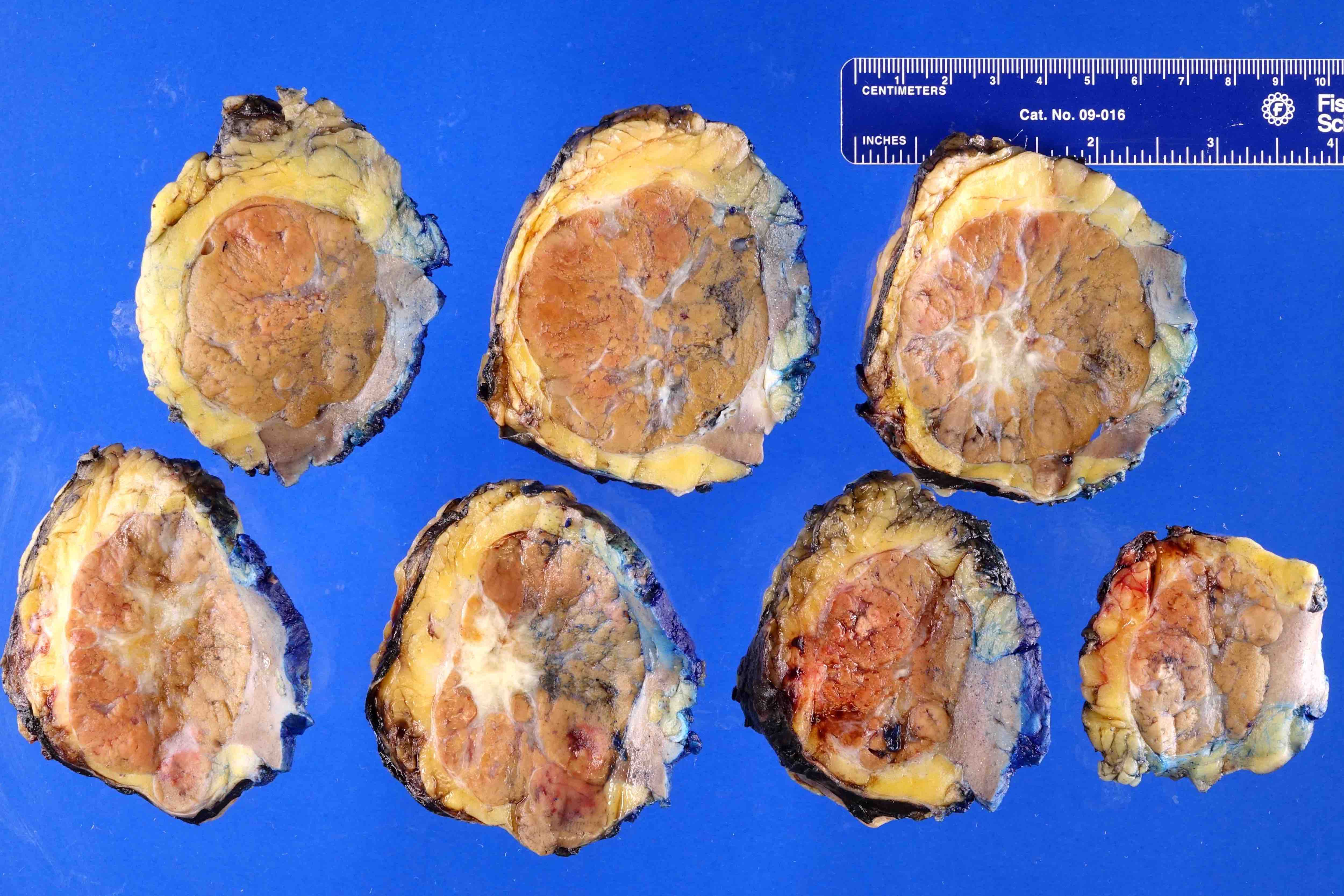
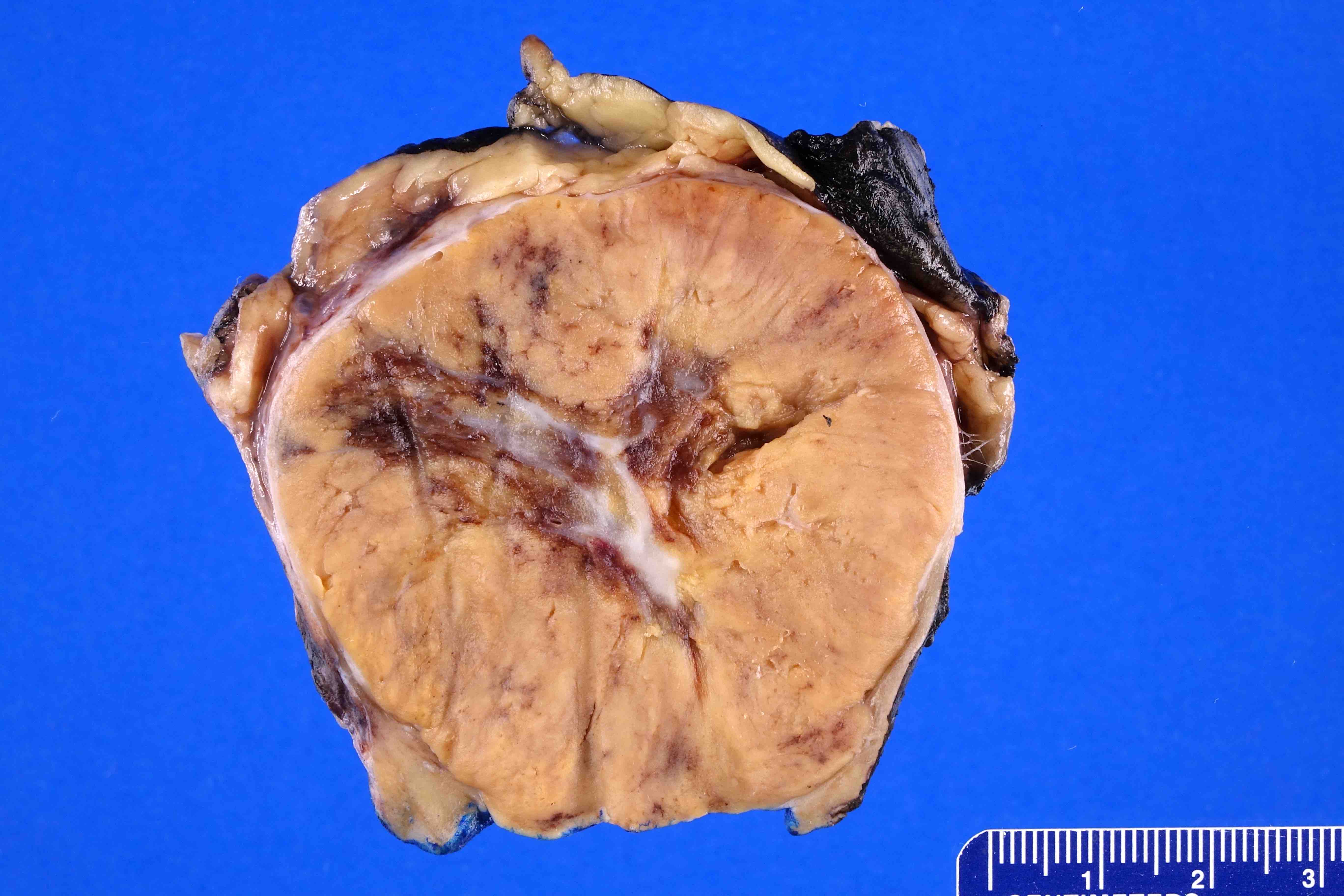
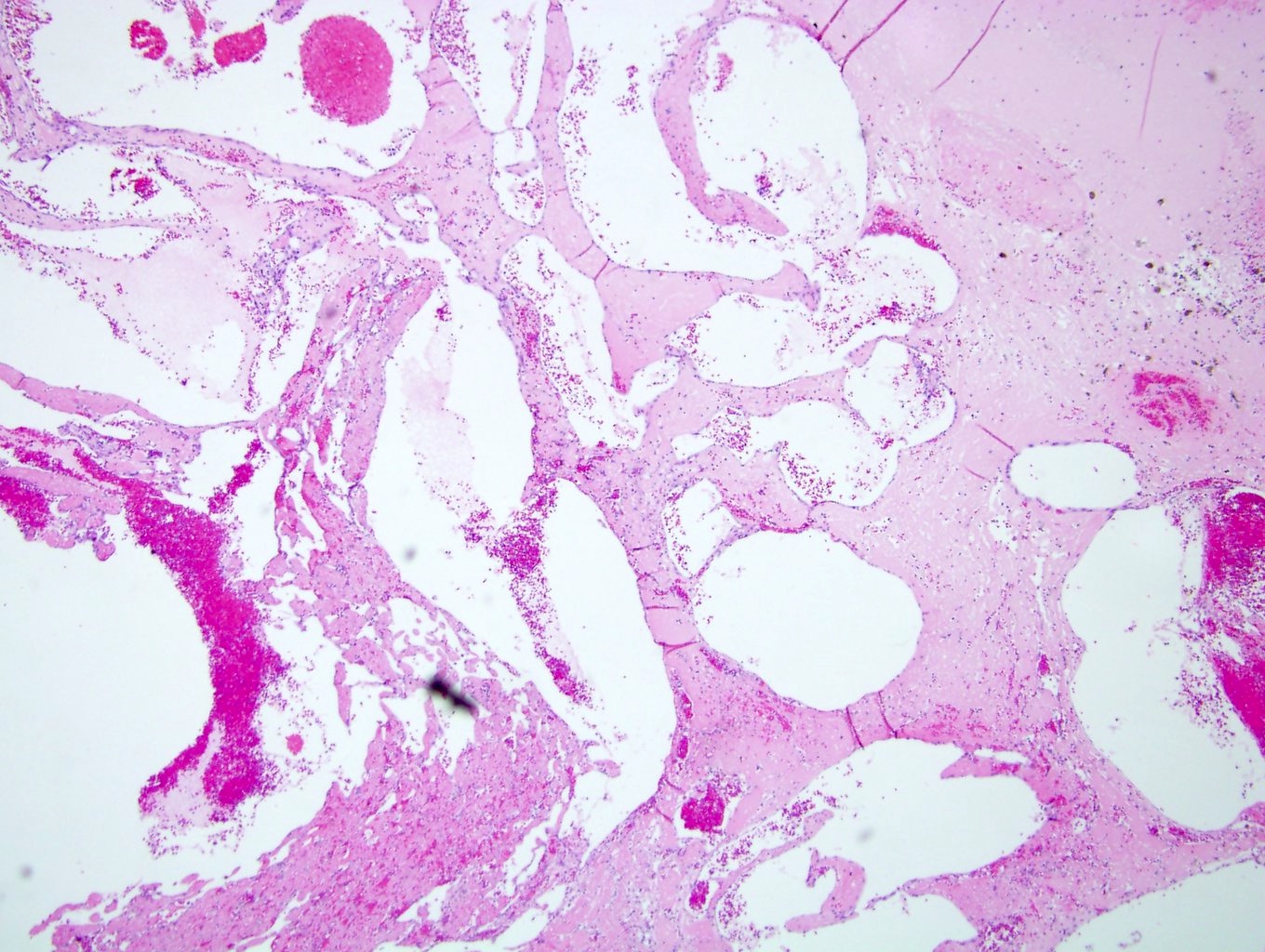
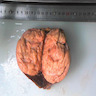
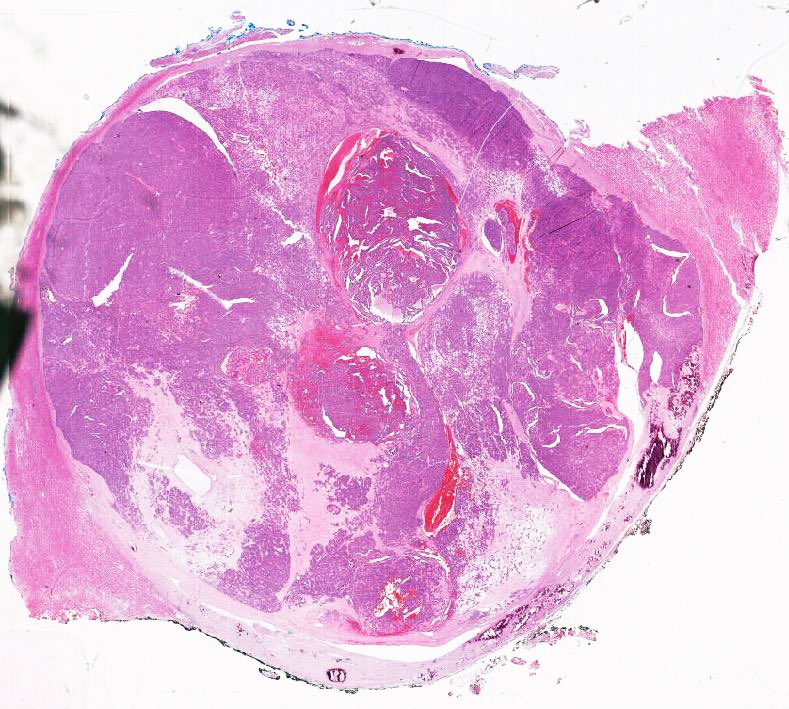
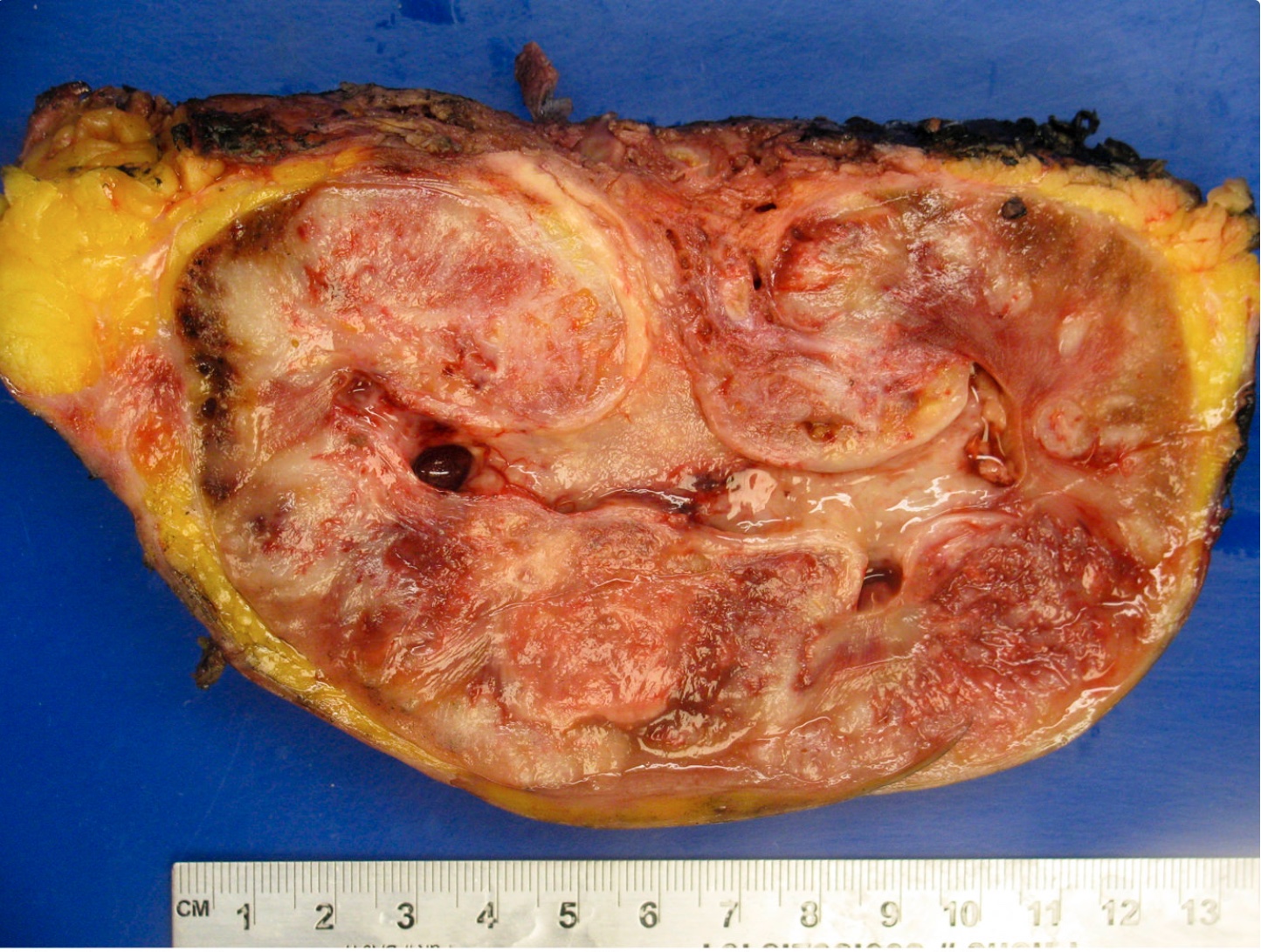
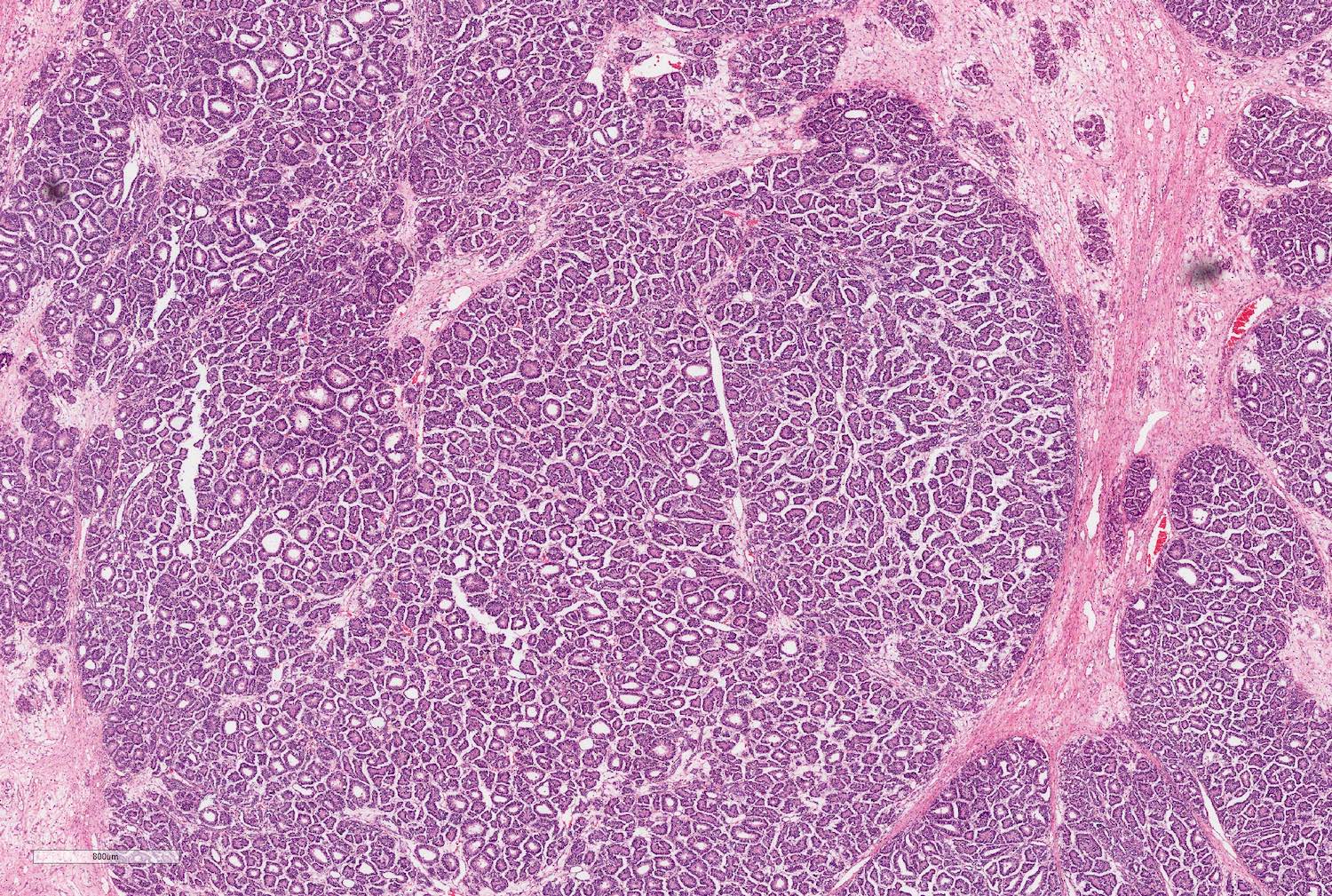
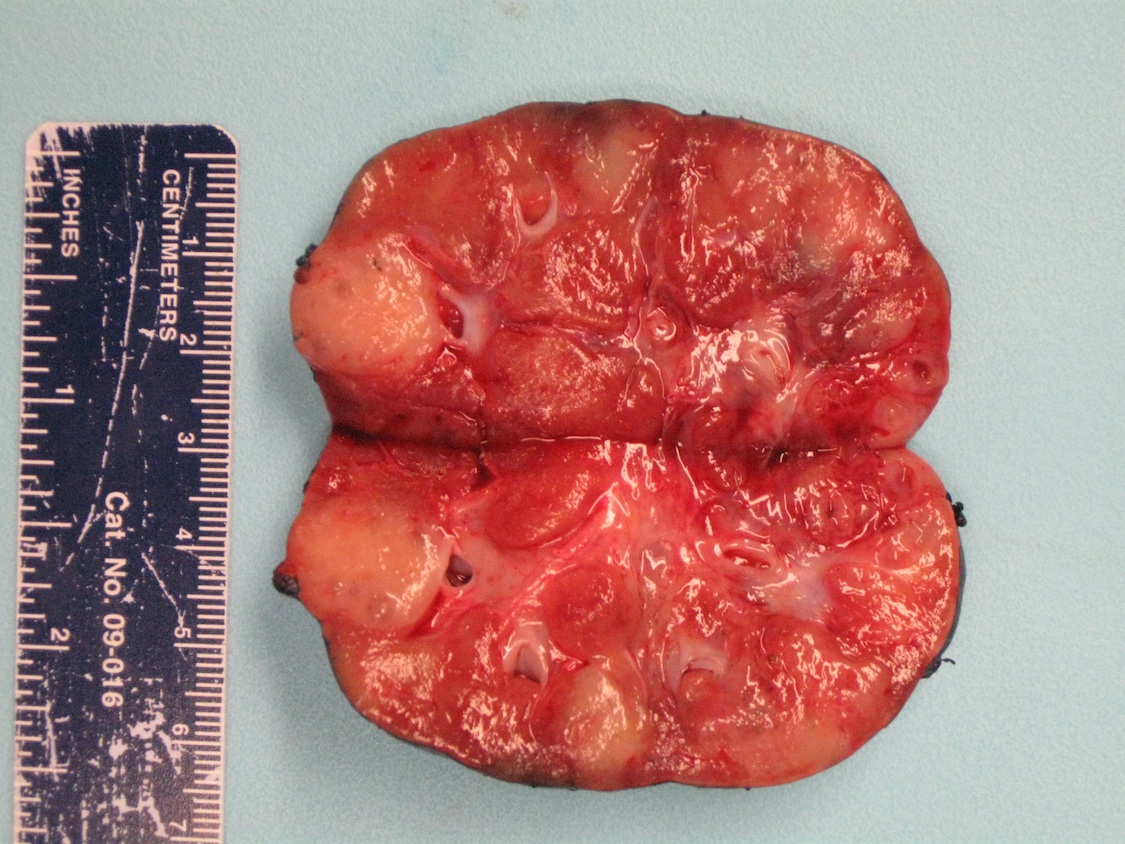
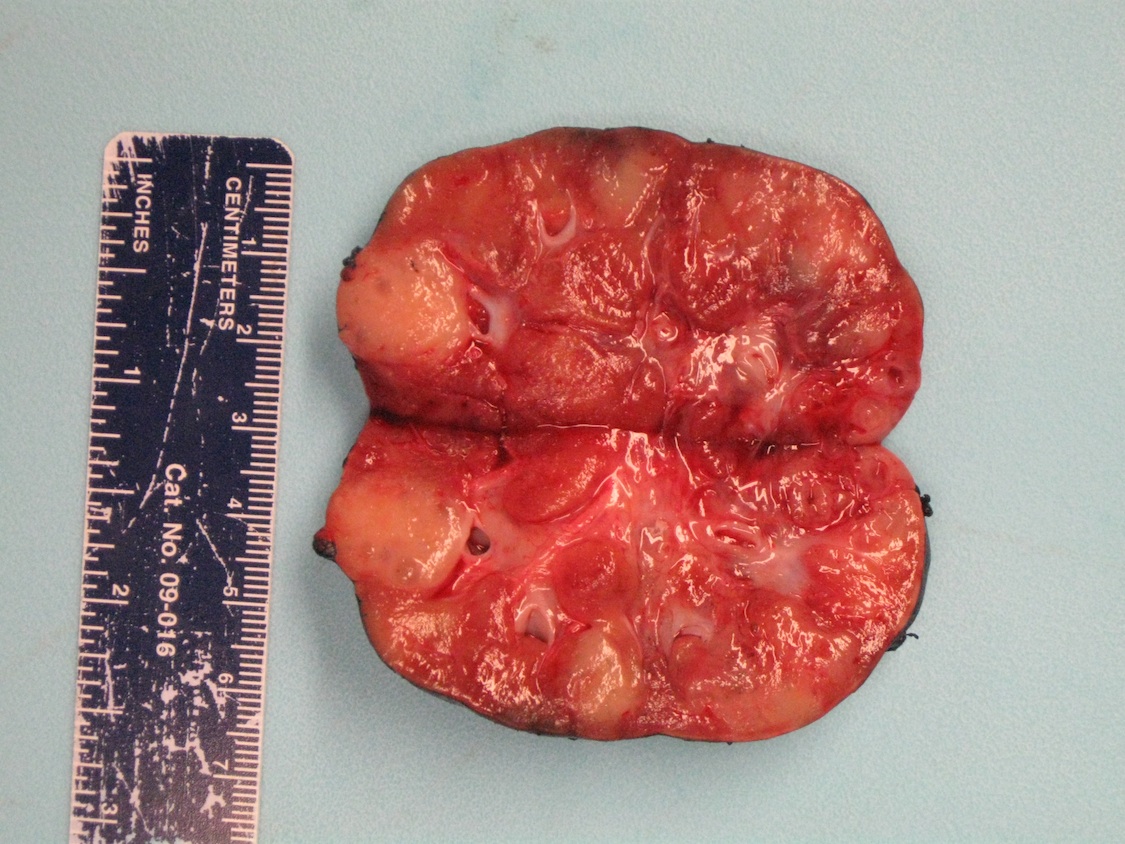
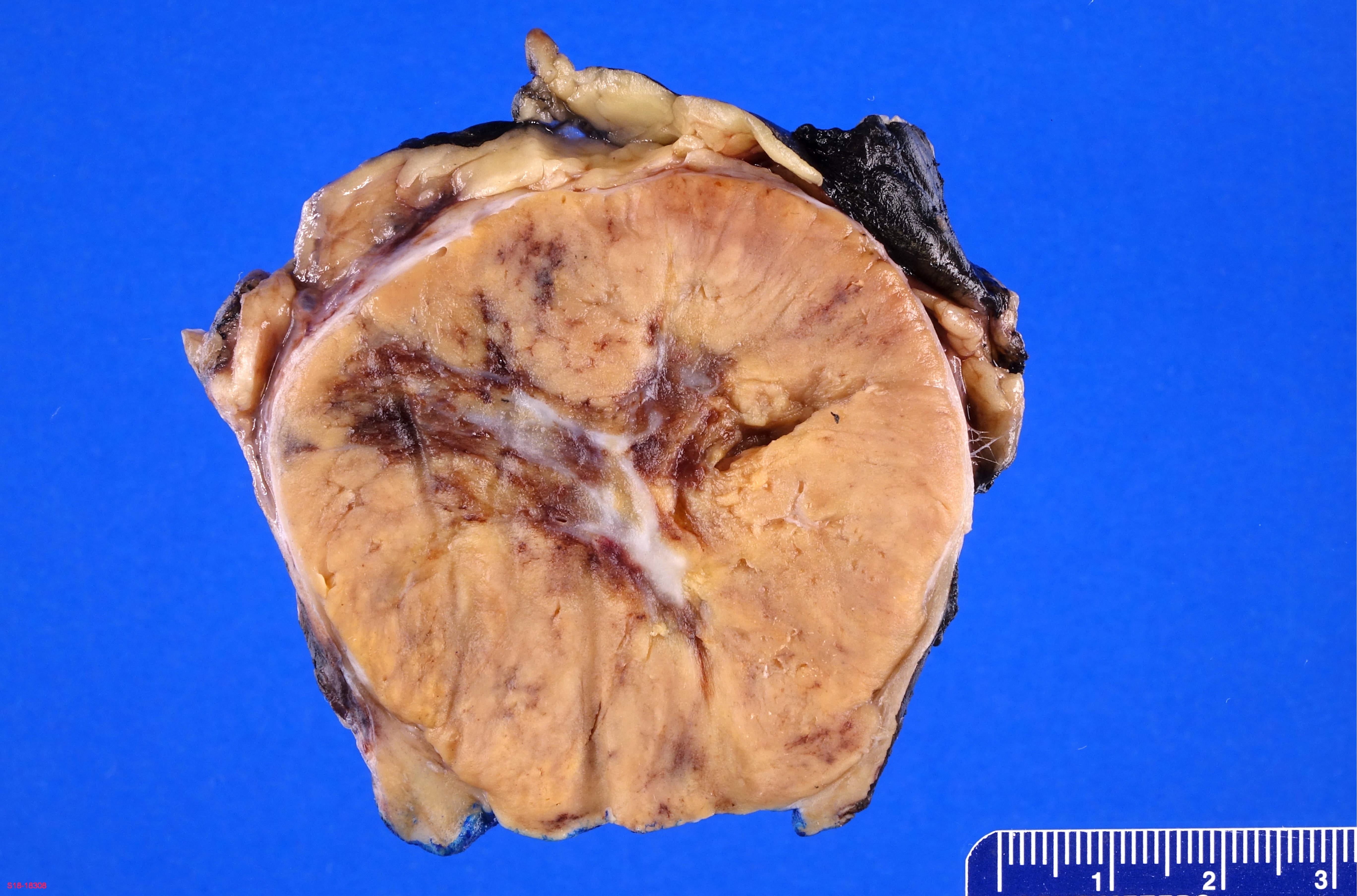
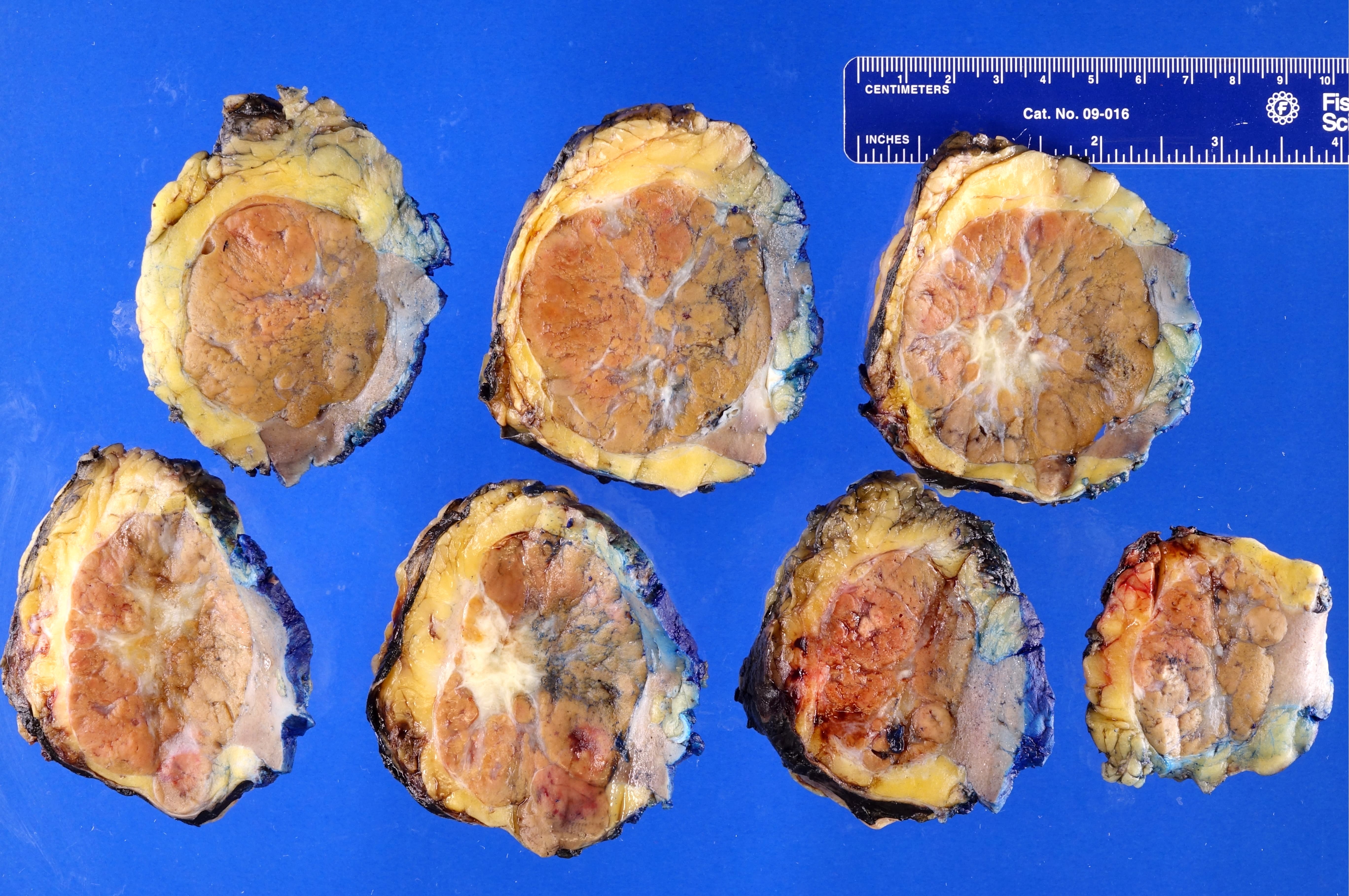
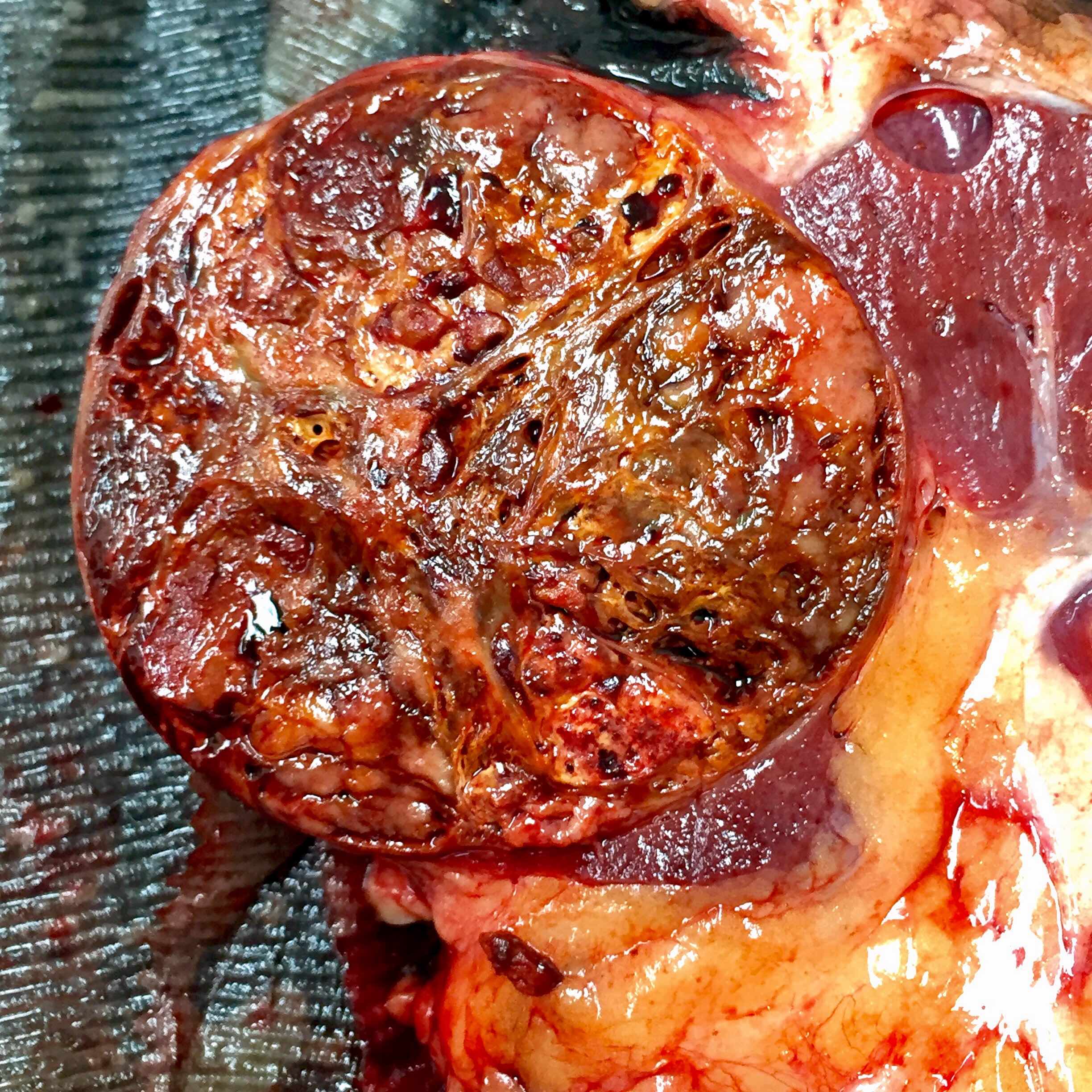
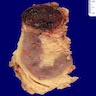
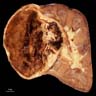
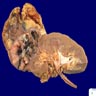
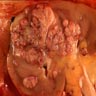
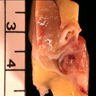
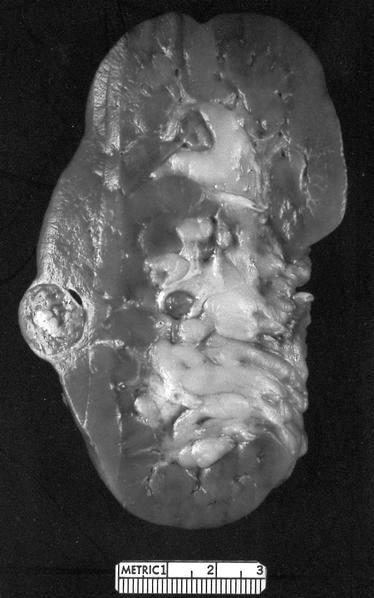
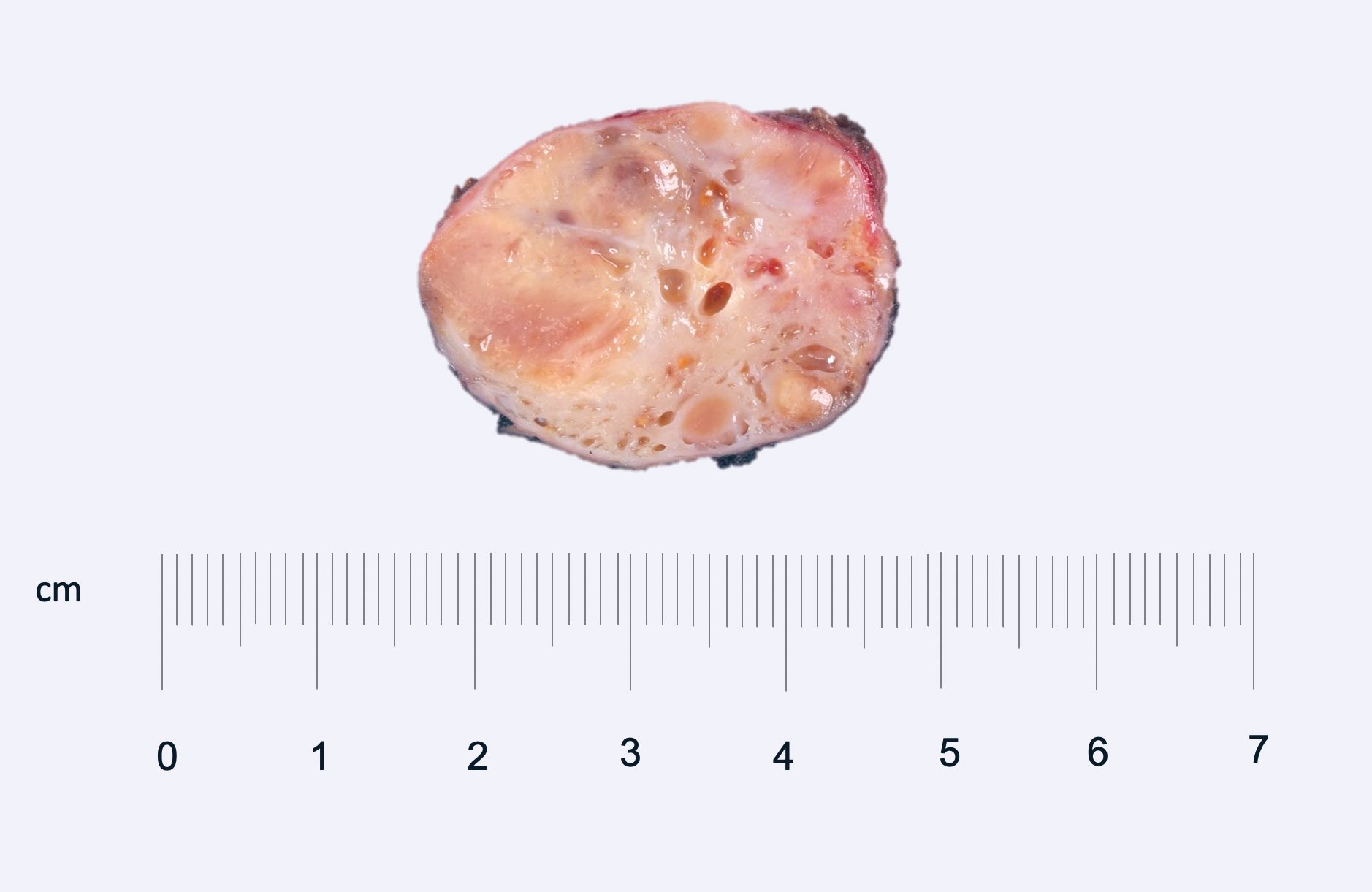
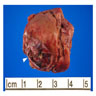
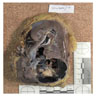
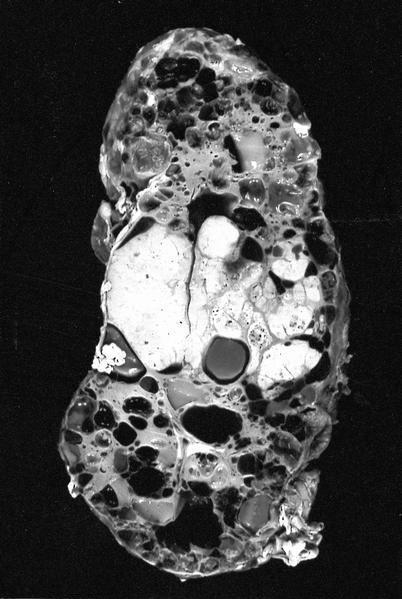
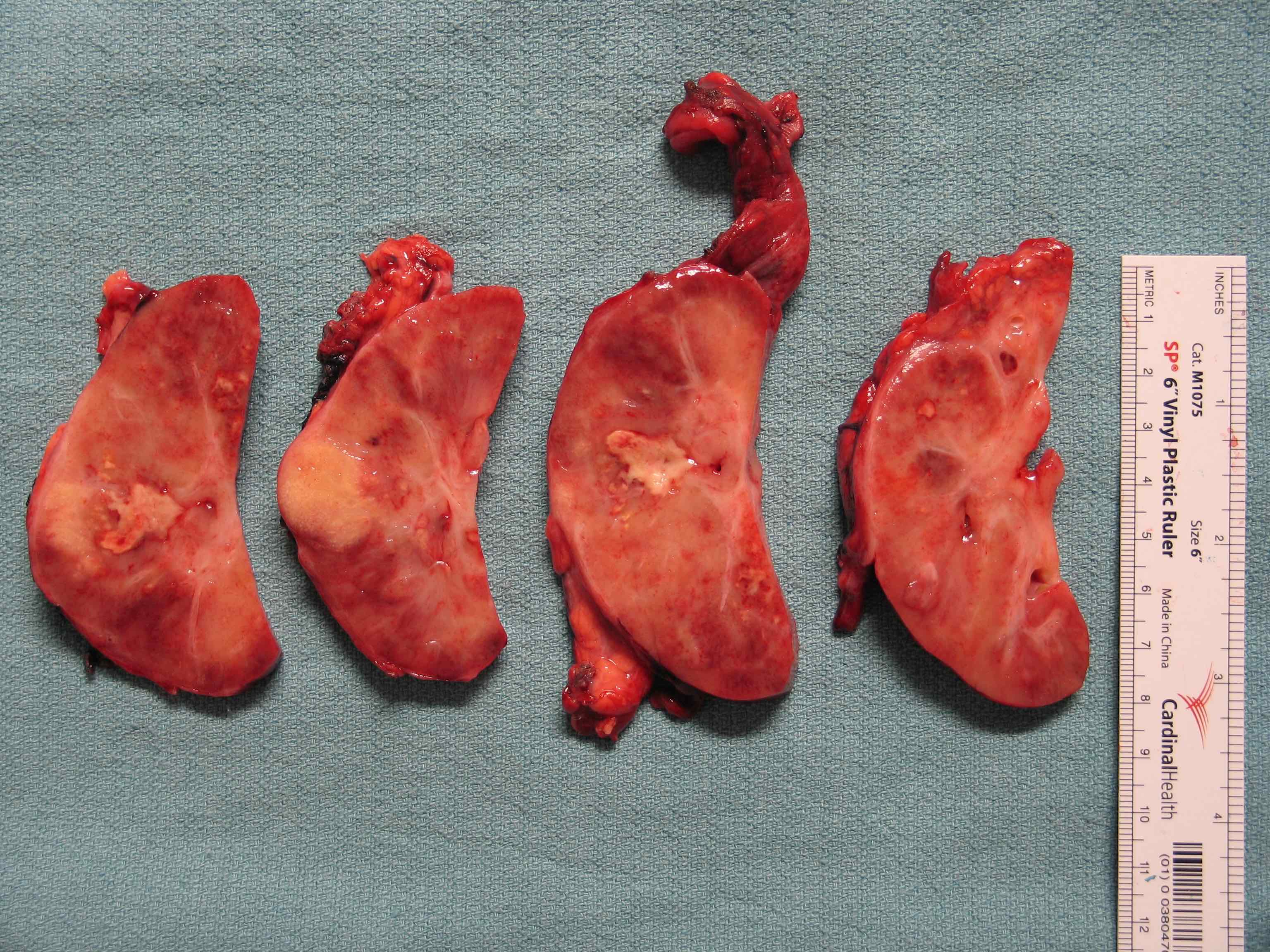
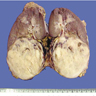
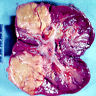
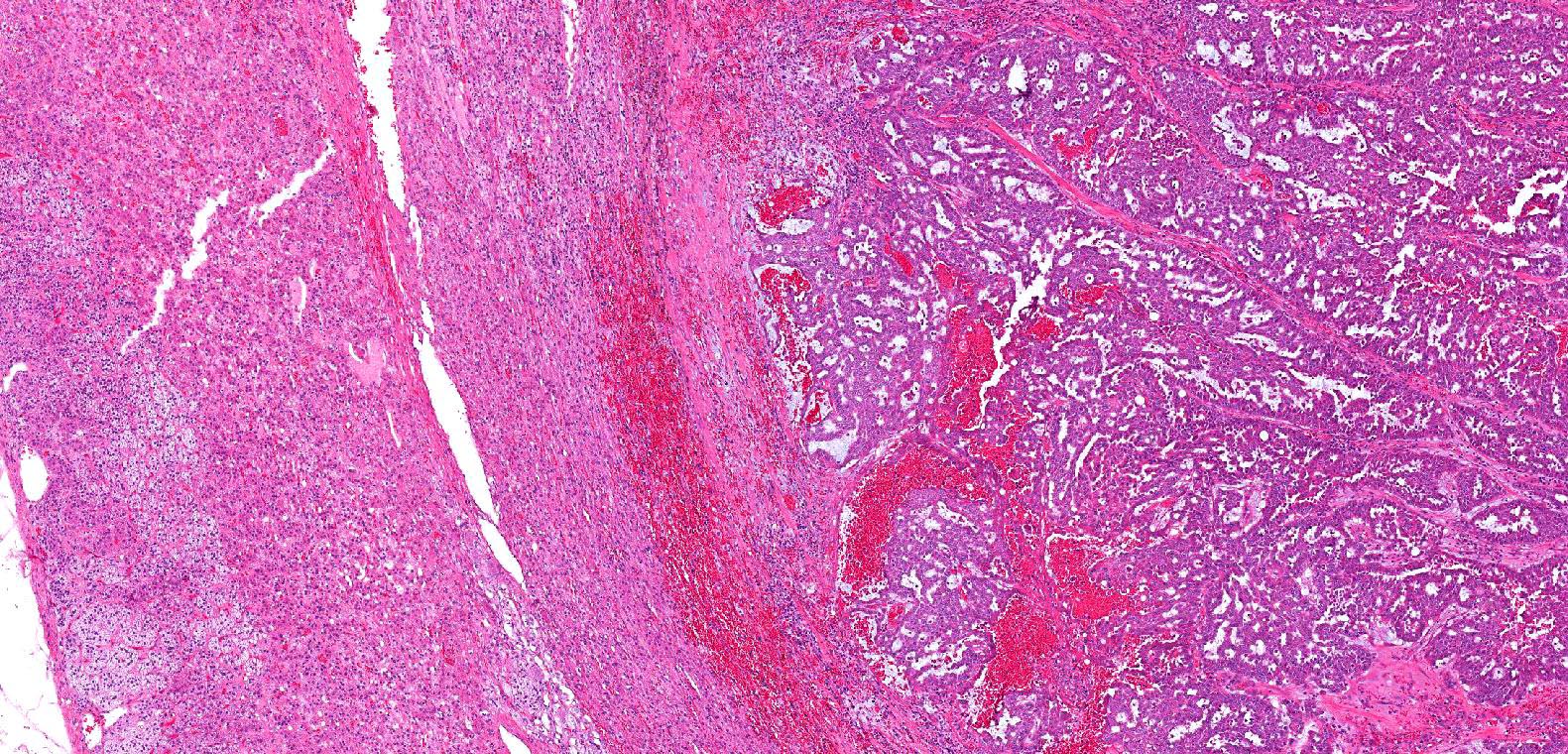
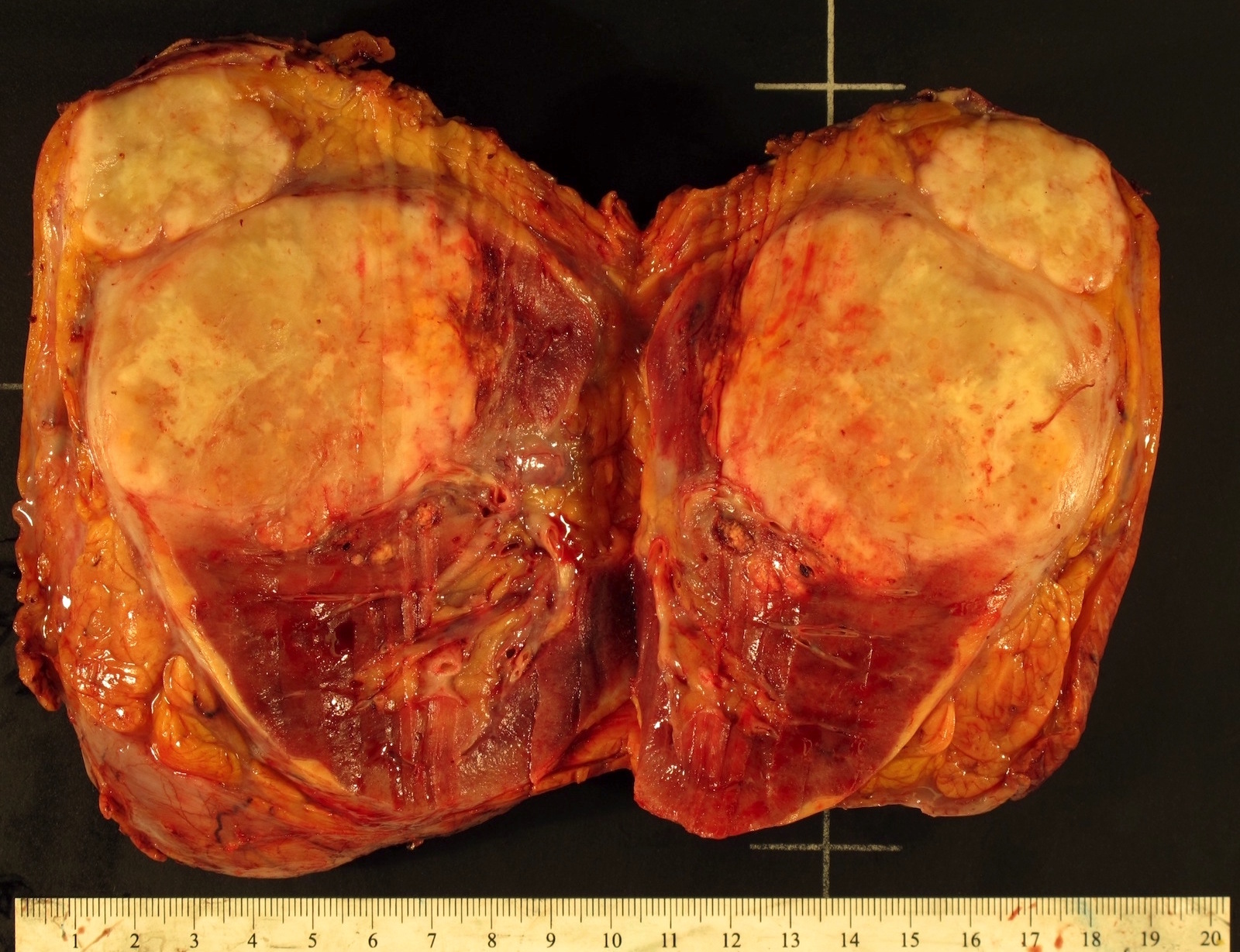
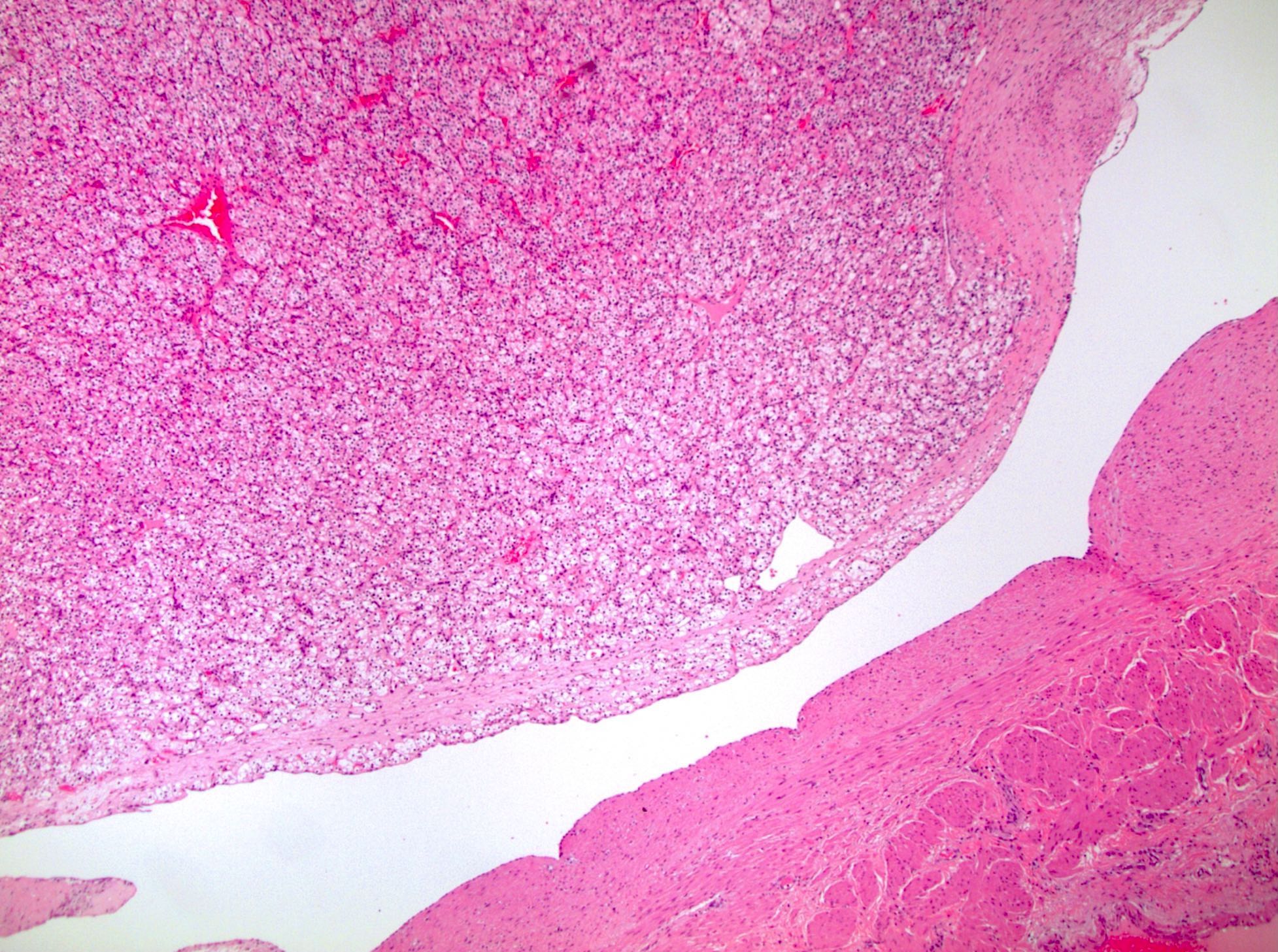
- Adult patients: well demarcated solid tumor in mid kidney, tan to brown or white to gray-white color, cystic change or hemorrhage may be present but pseudocapsule is absent
- Size: 3 - 7 cm
- Pediatric patients (Genes Chromosomes Cancer 2016;55:442, Cancer 2018;124:3381):
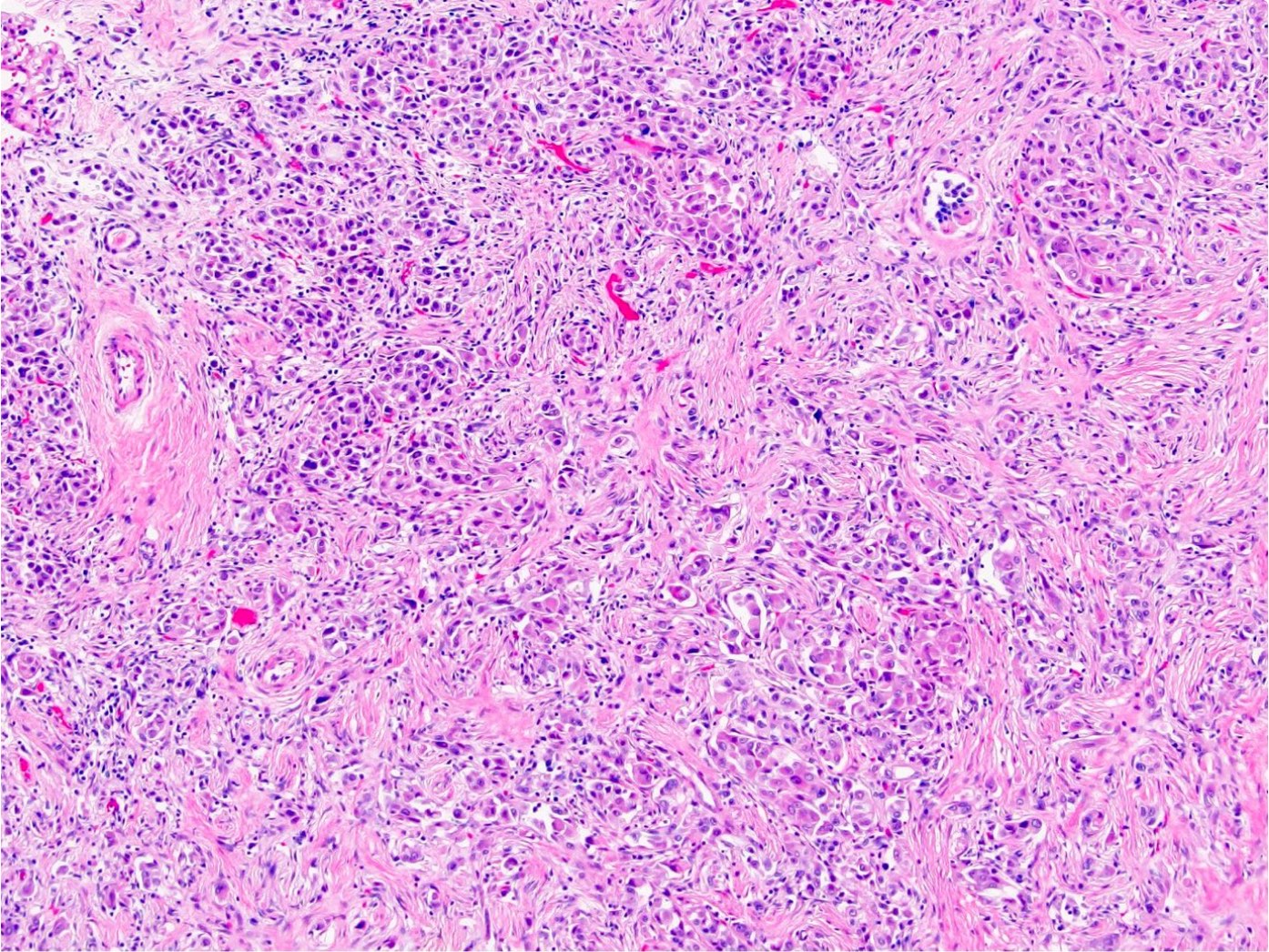
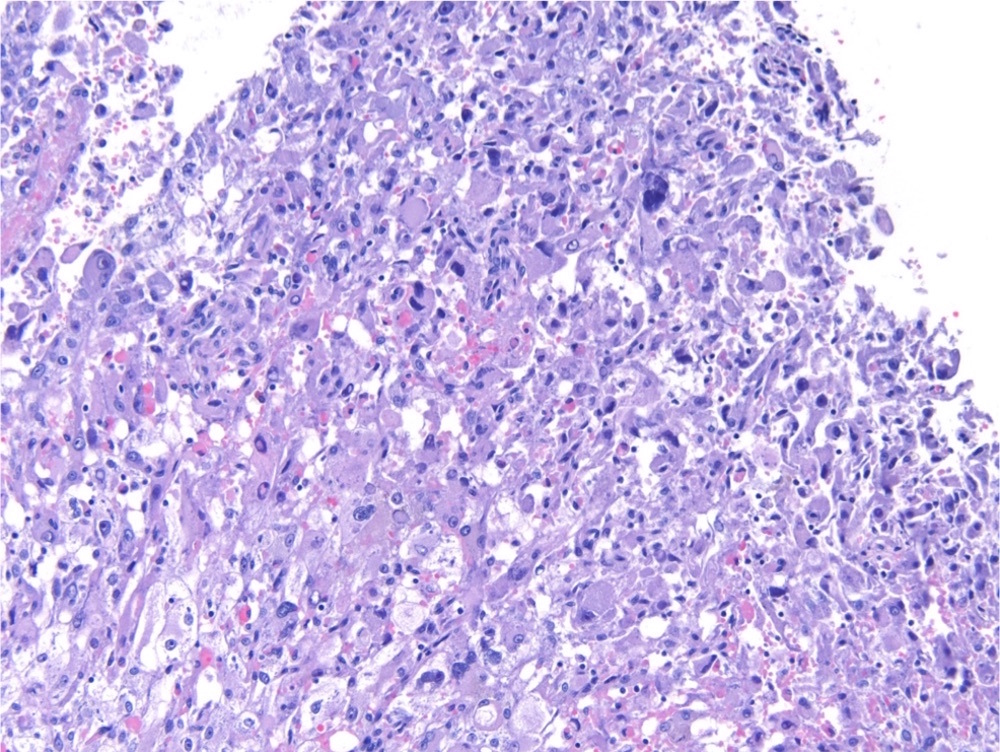
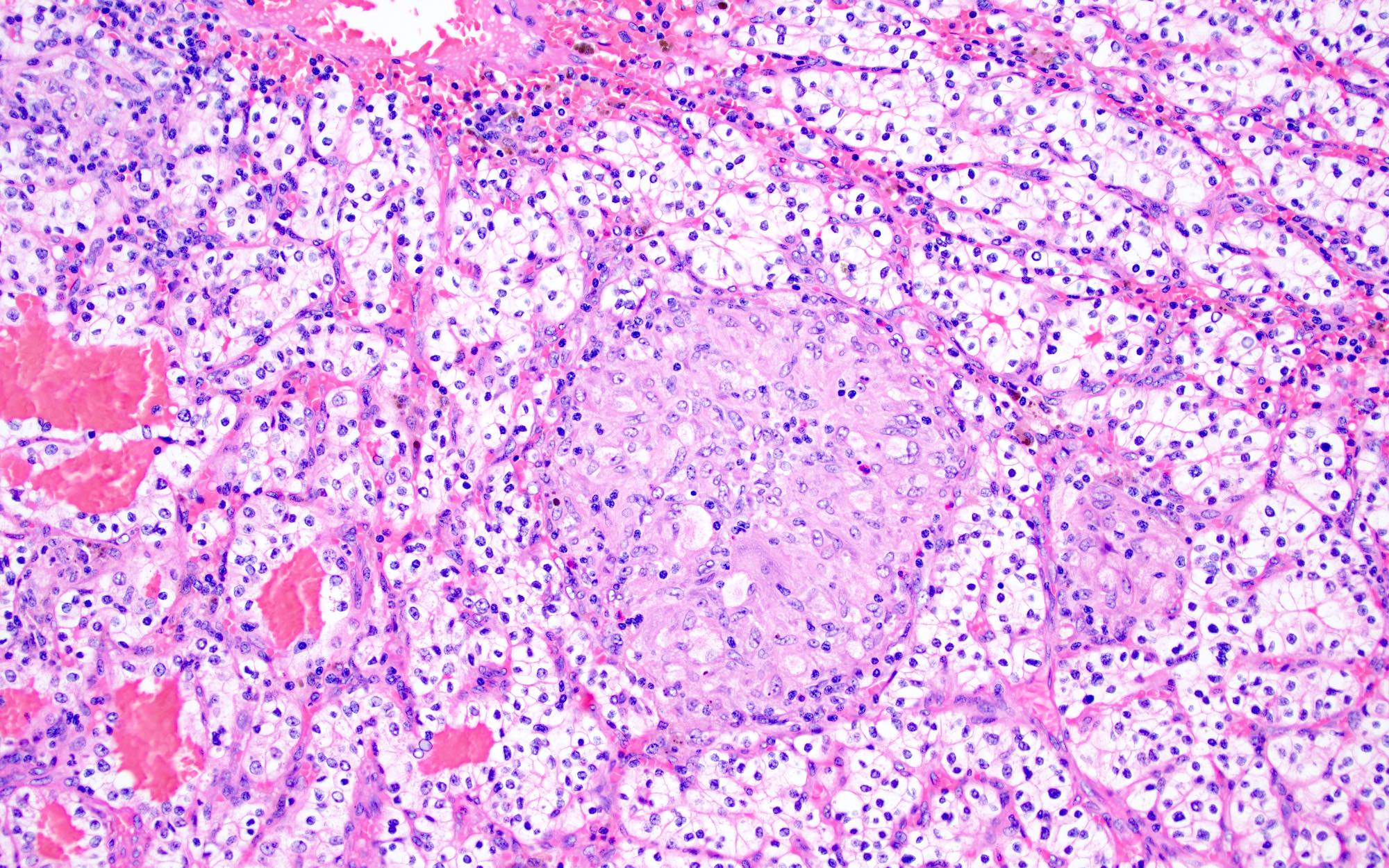
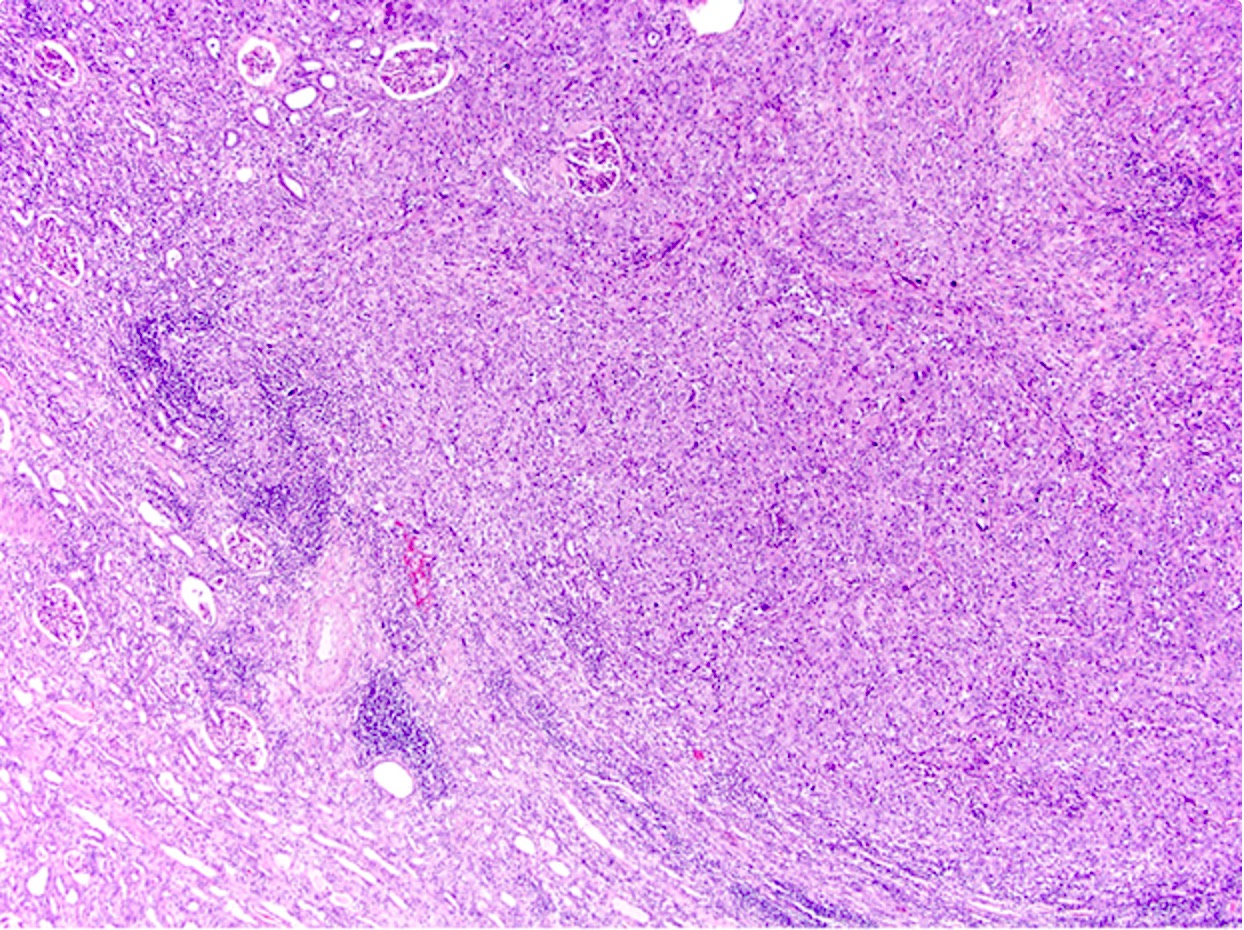
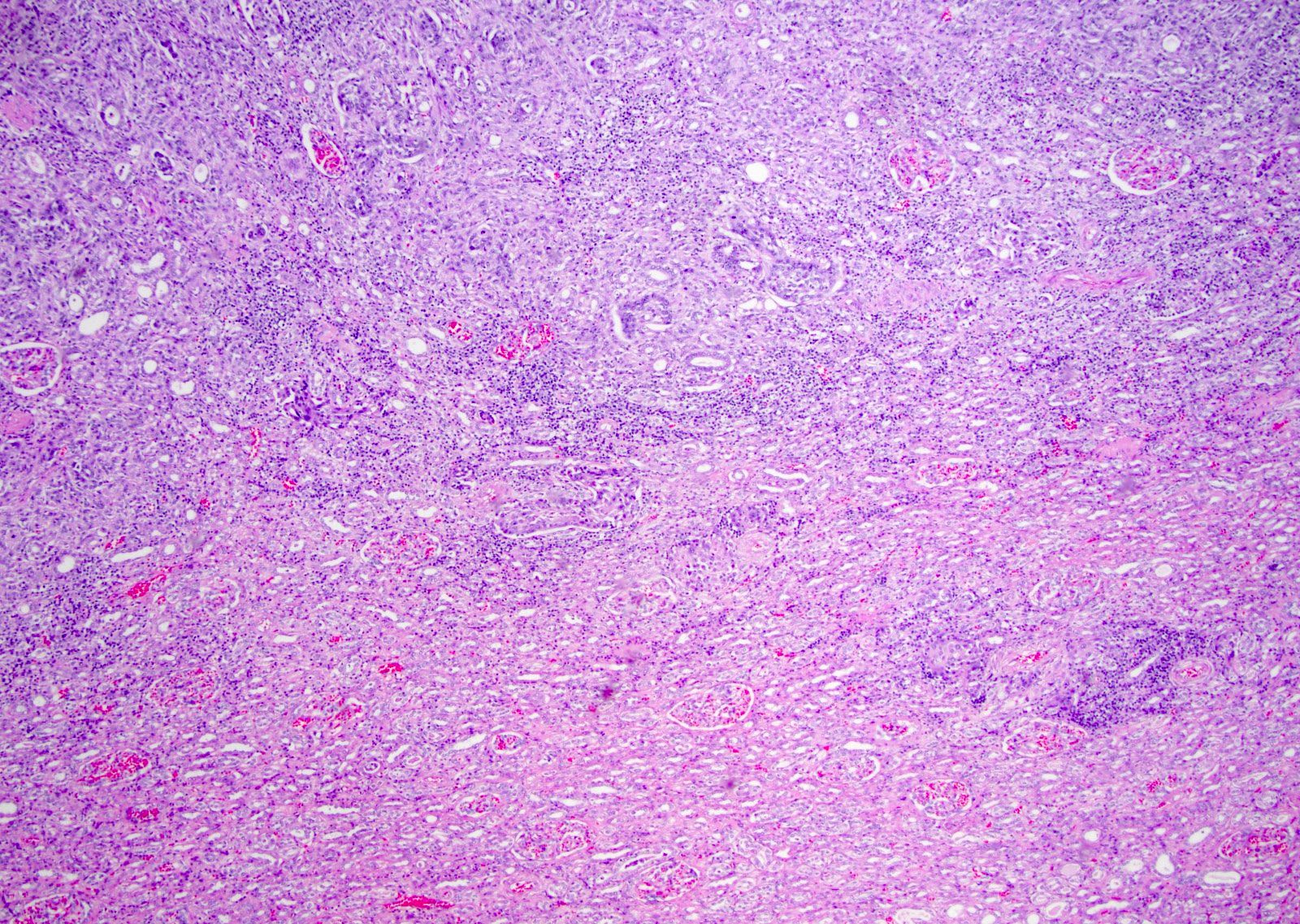
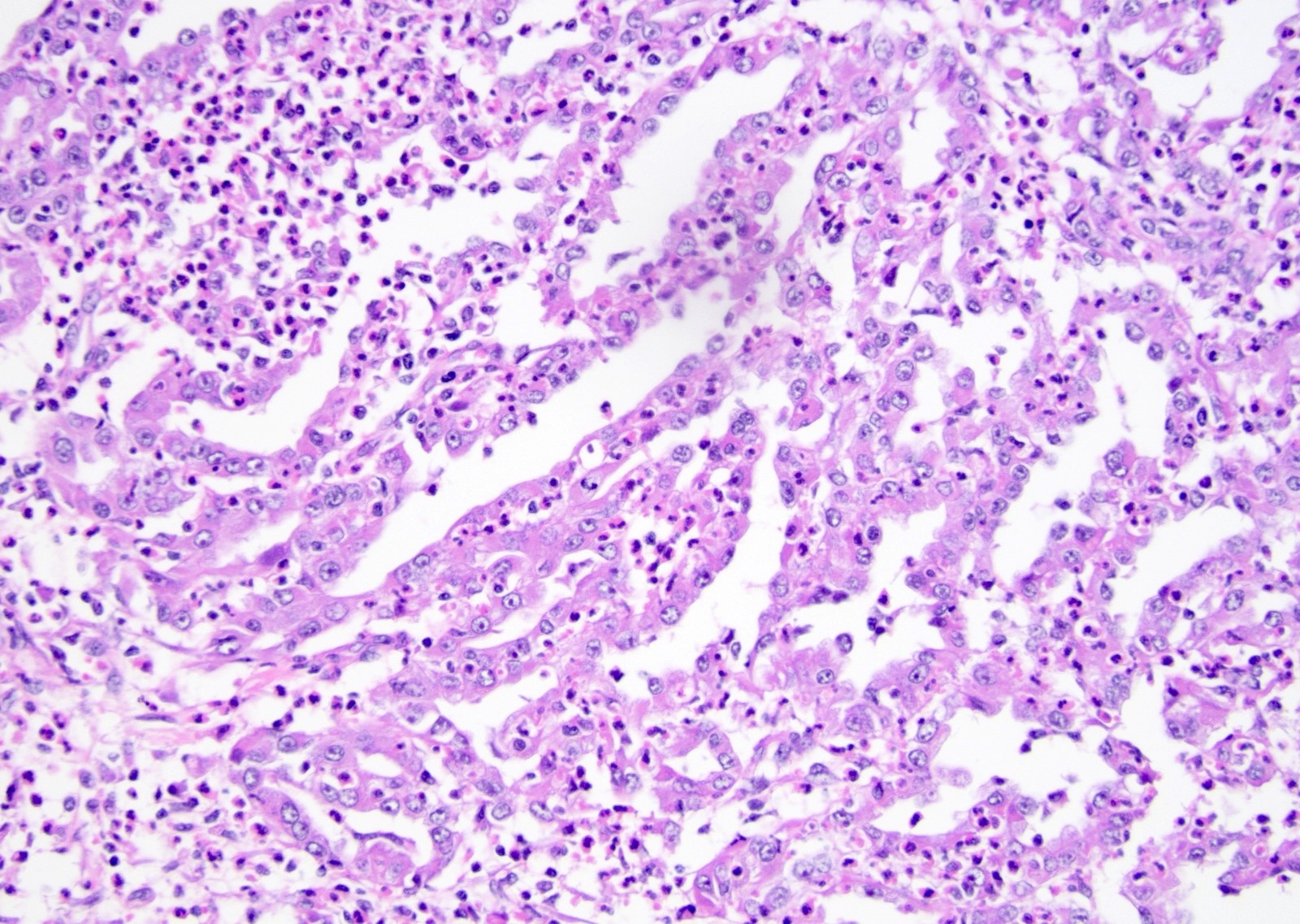
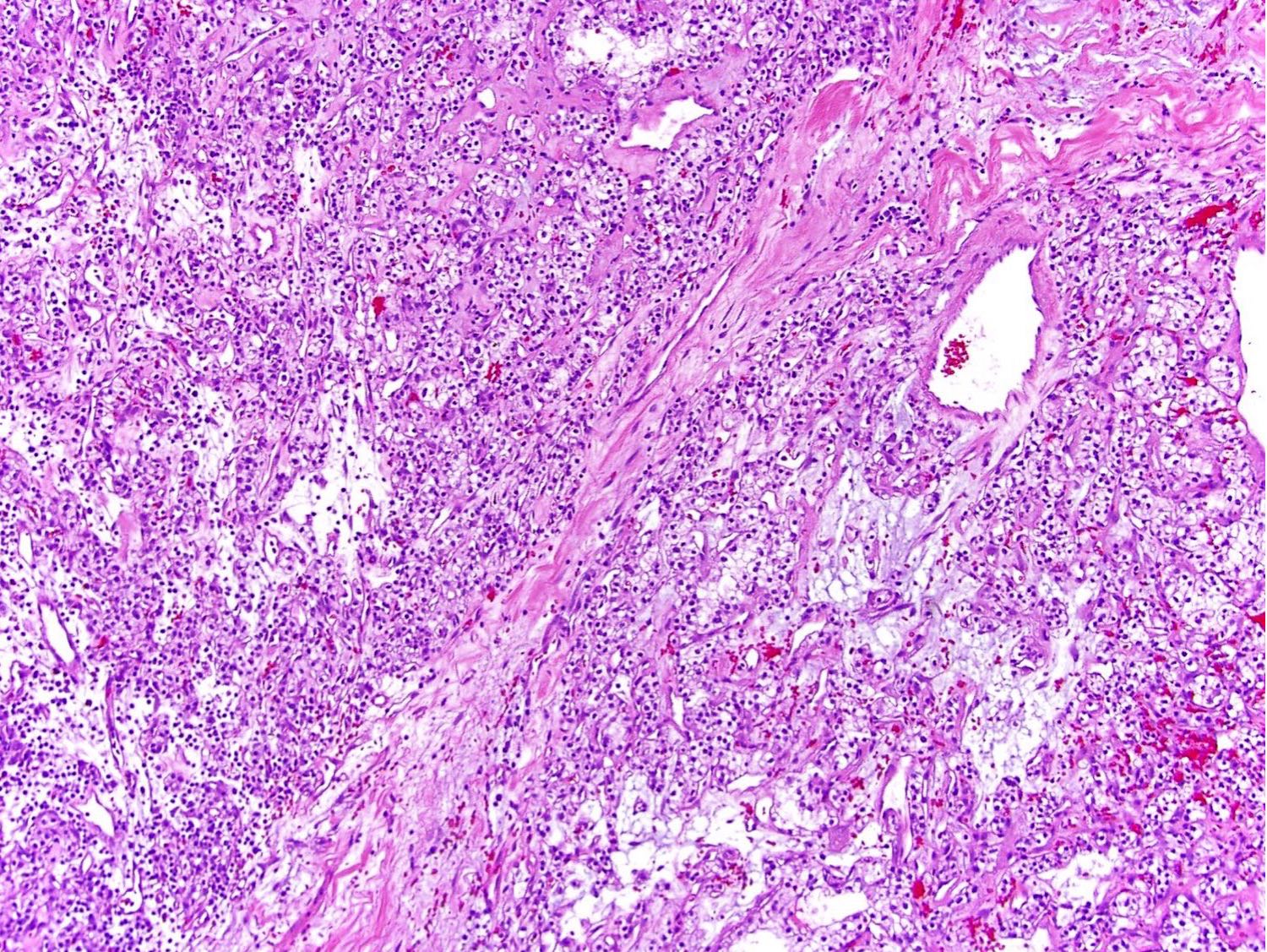
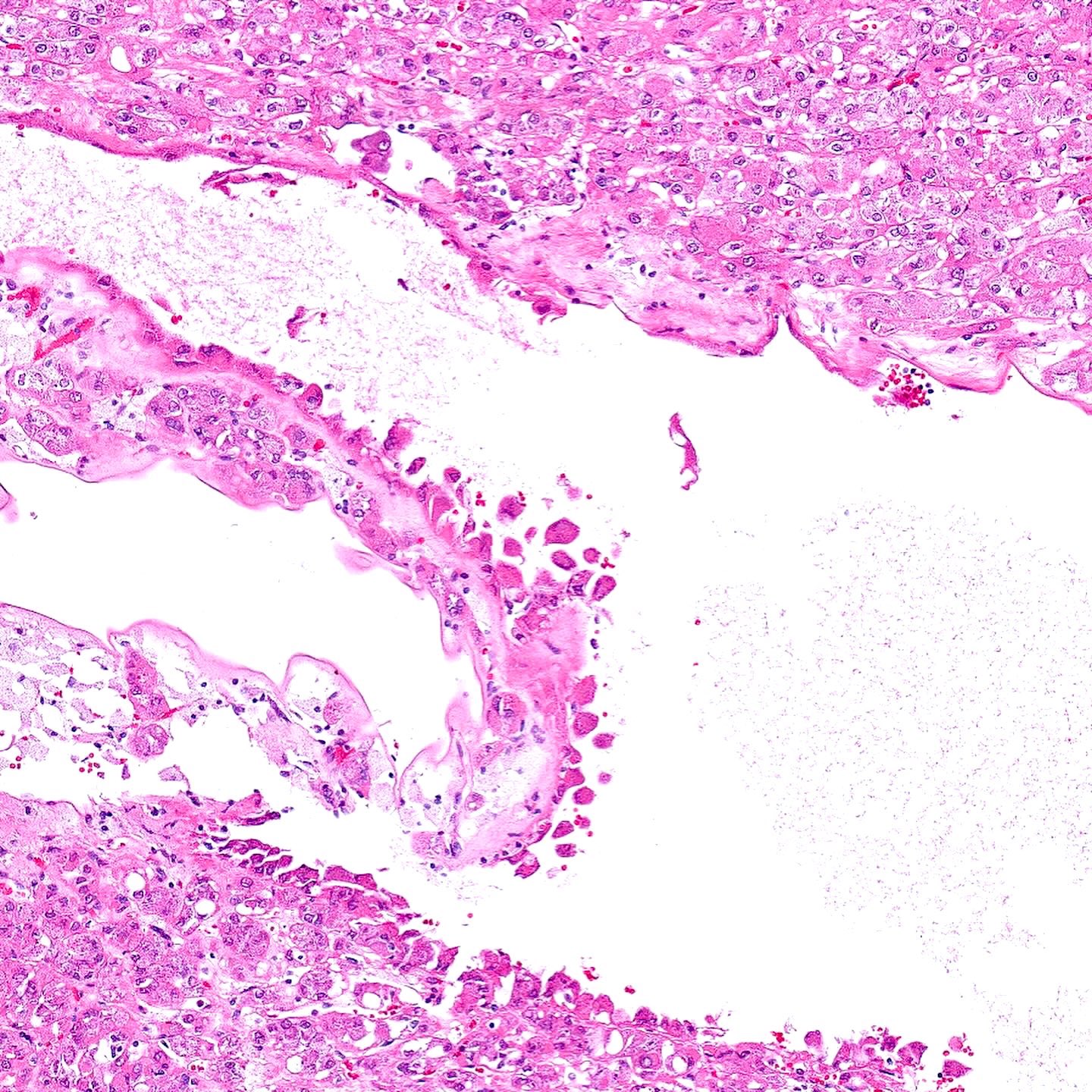
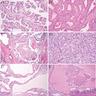
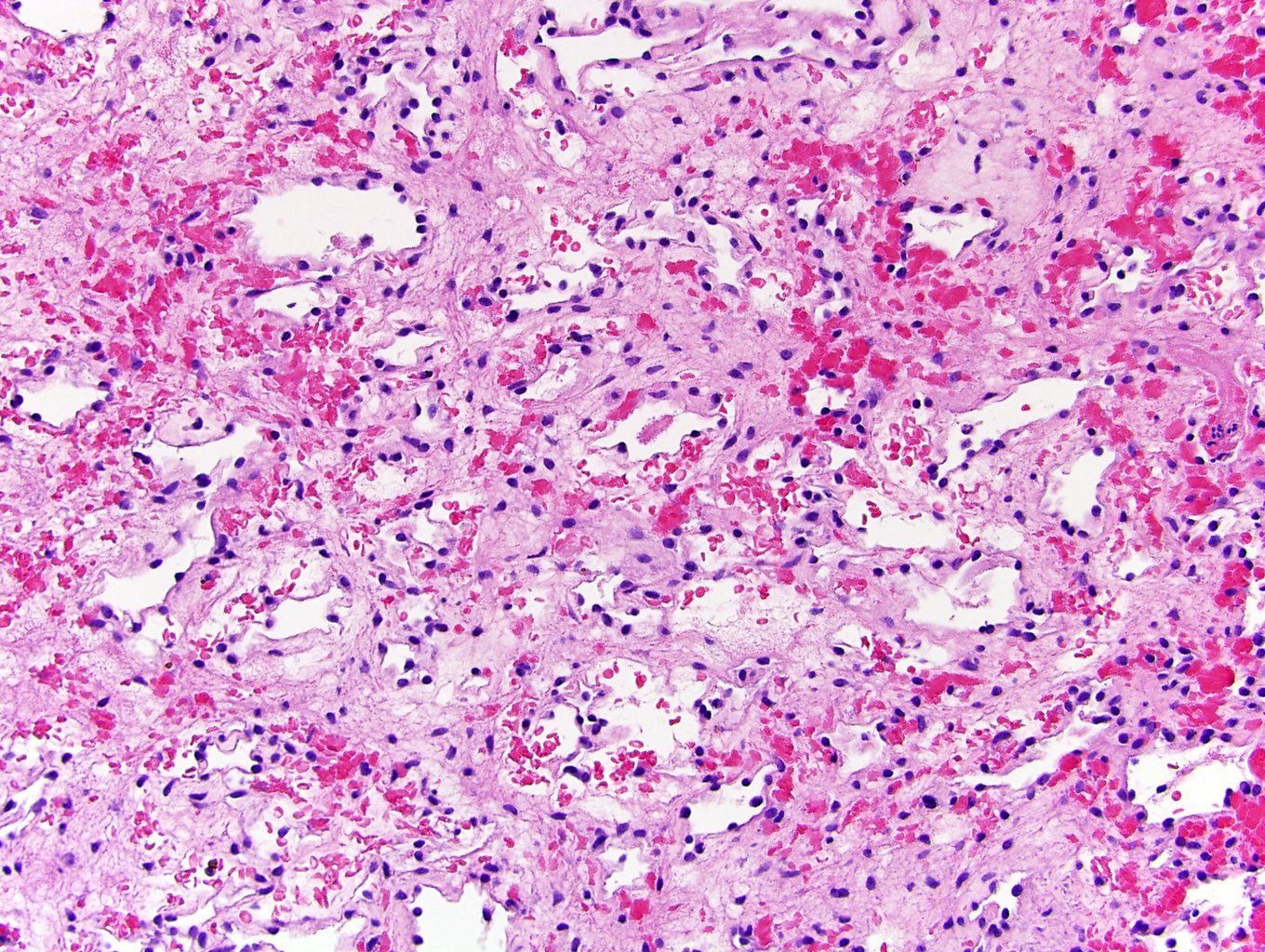
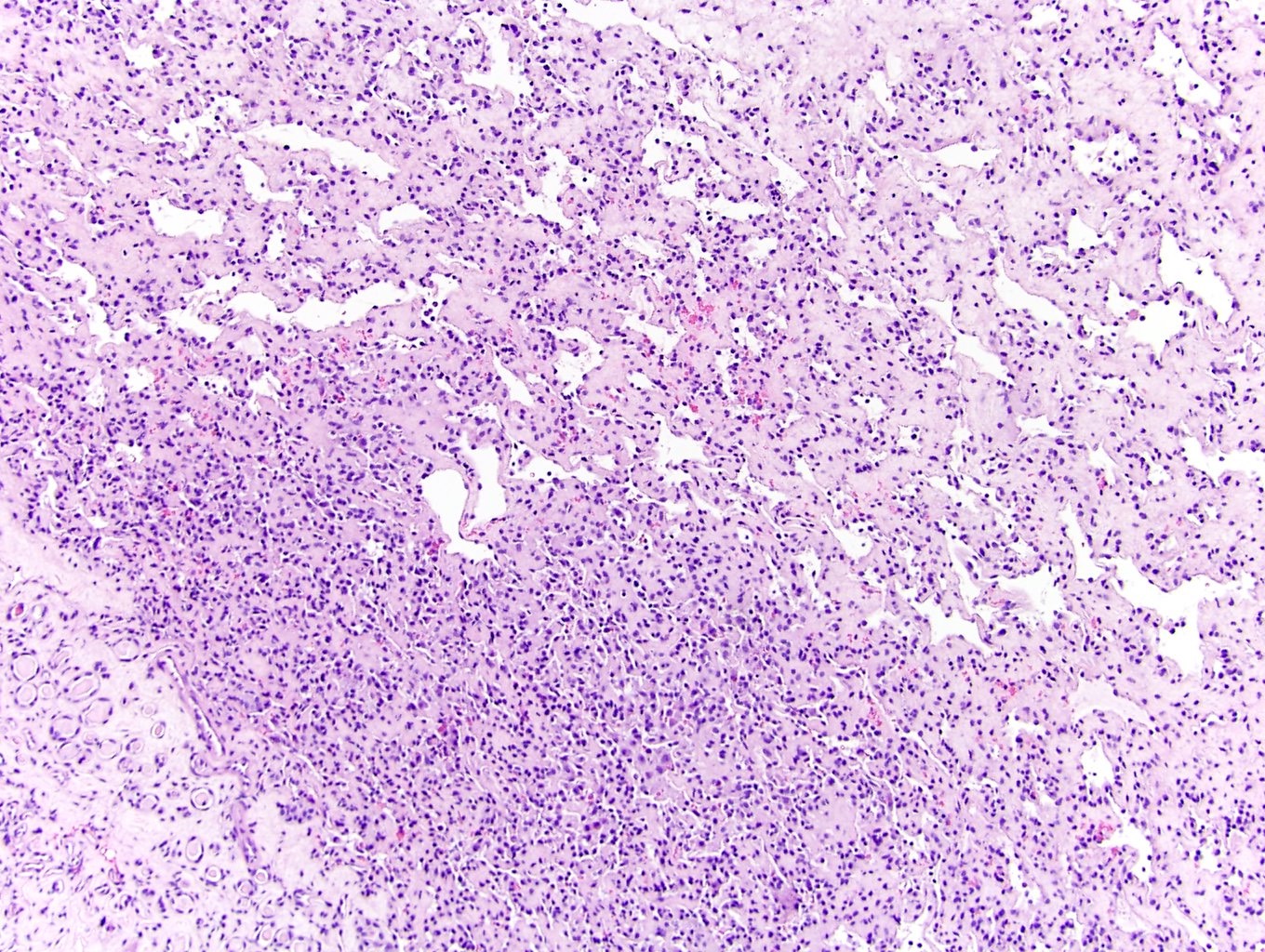
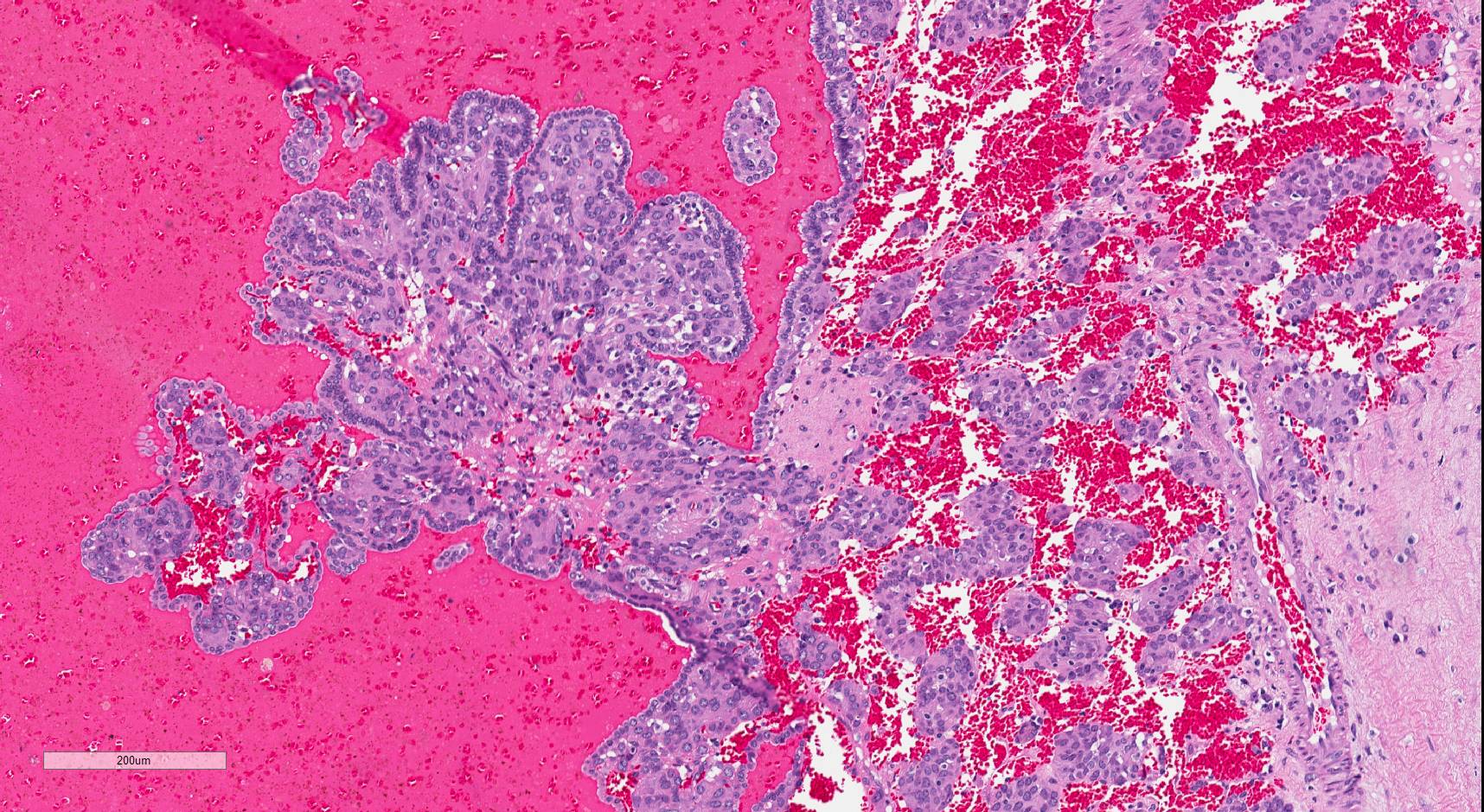
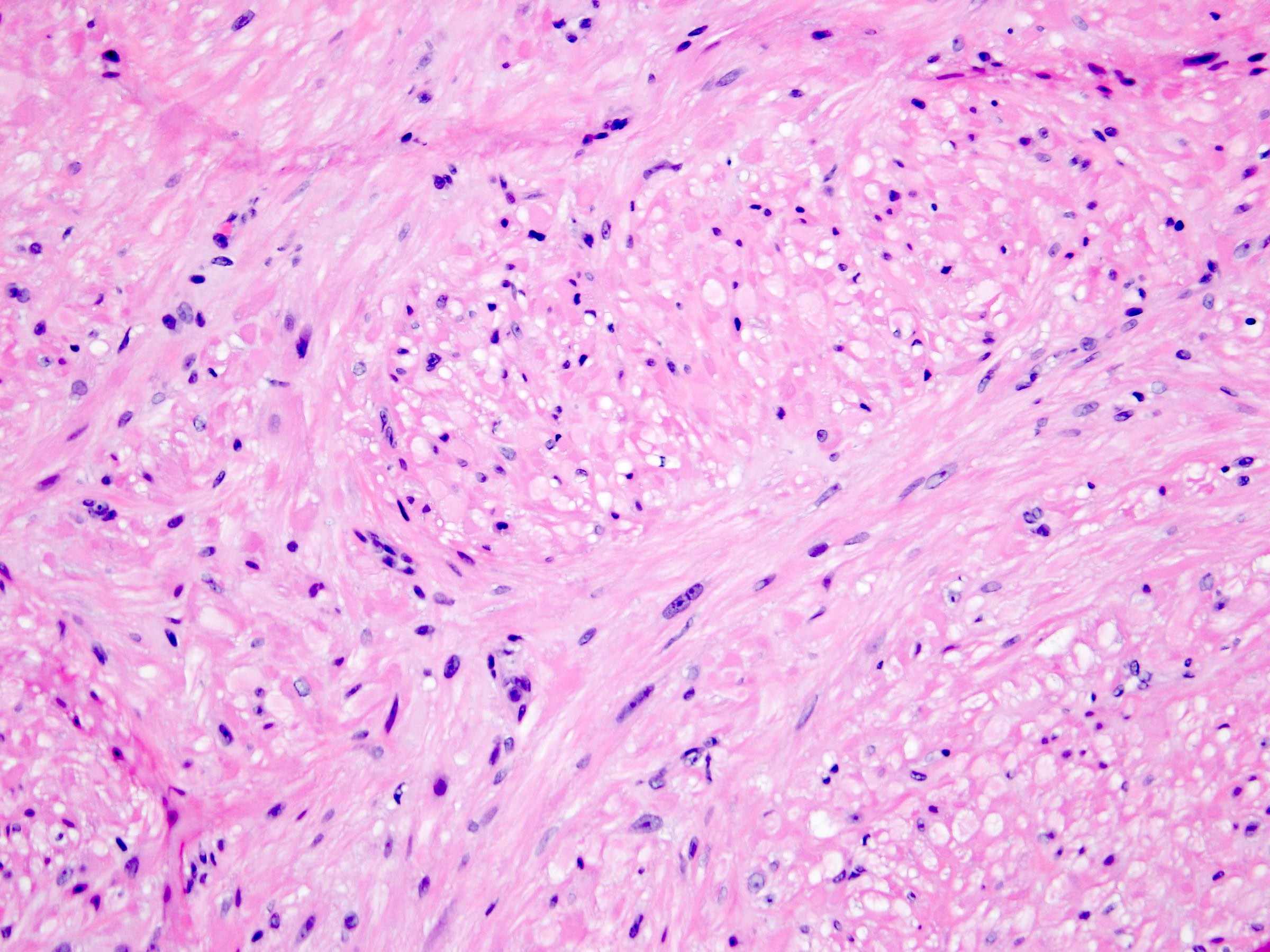
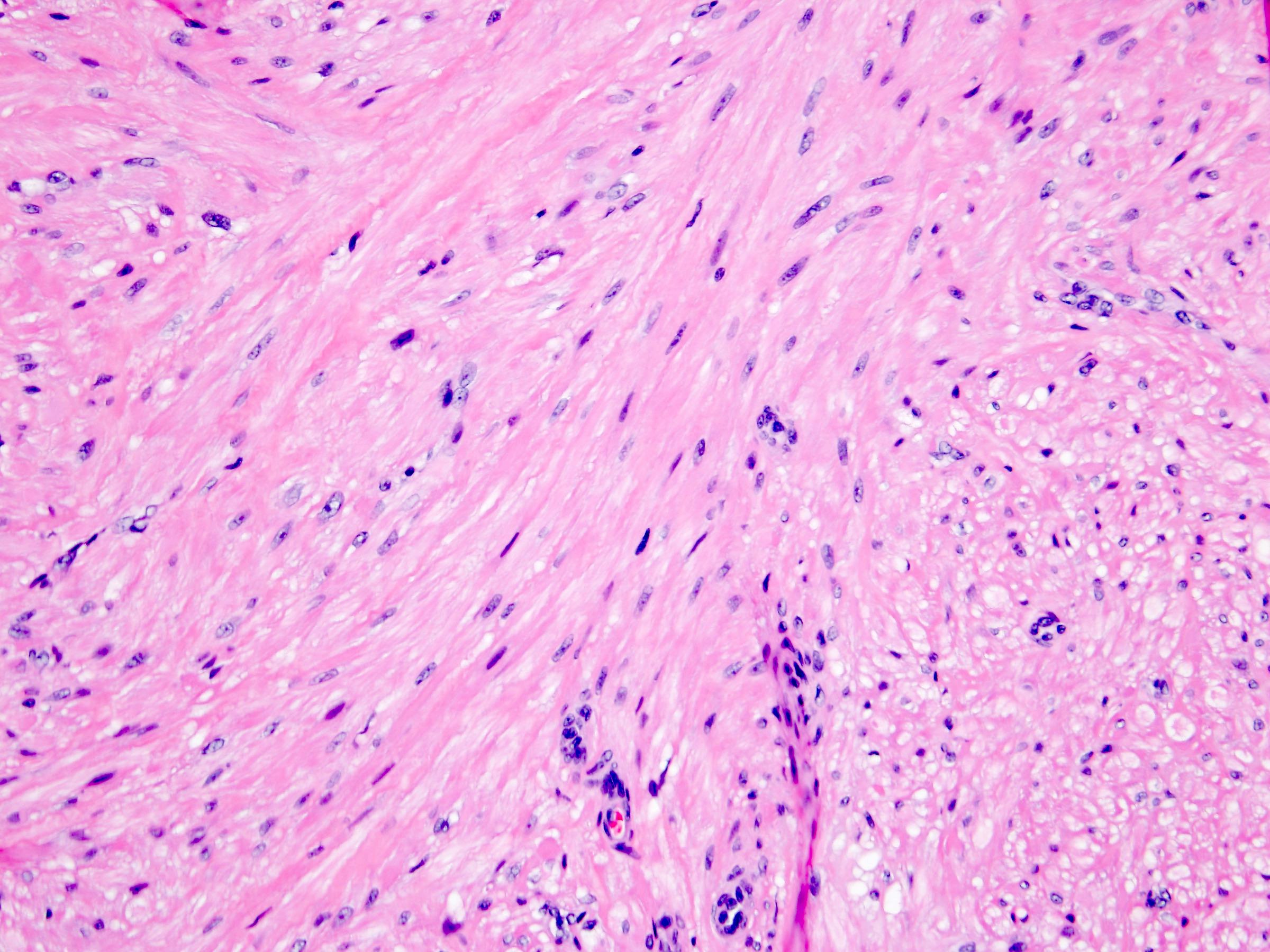
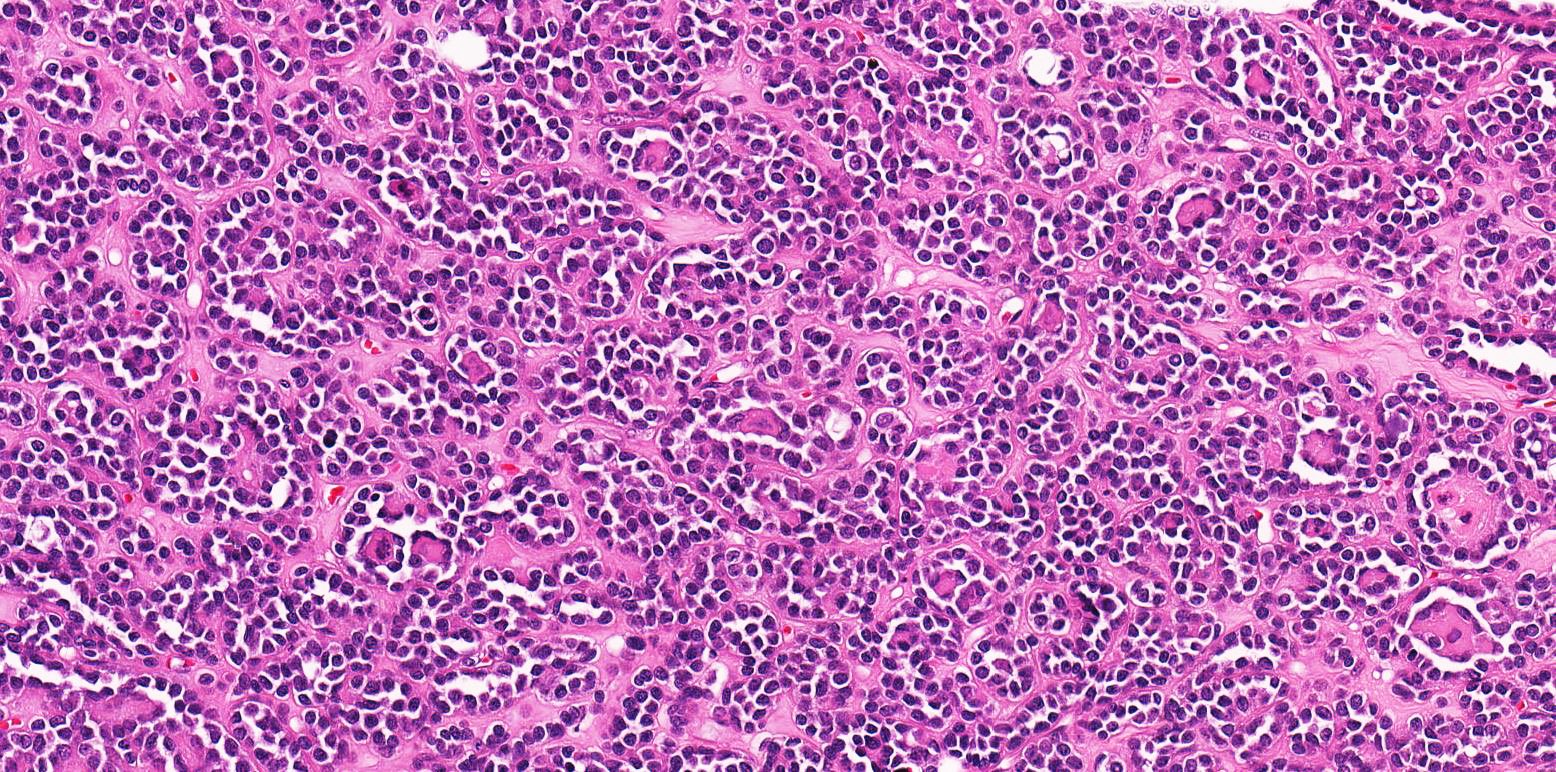
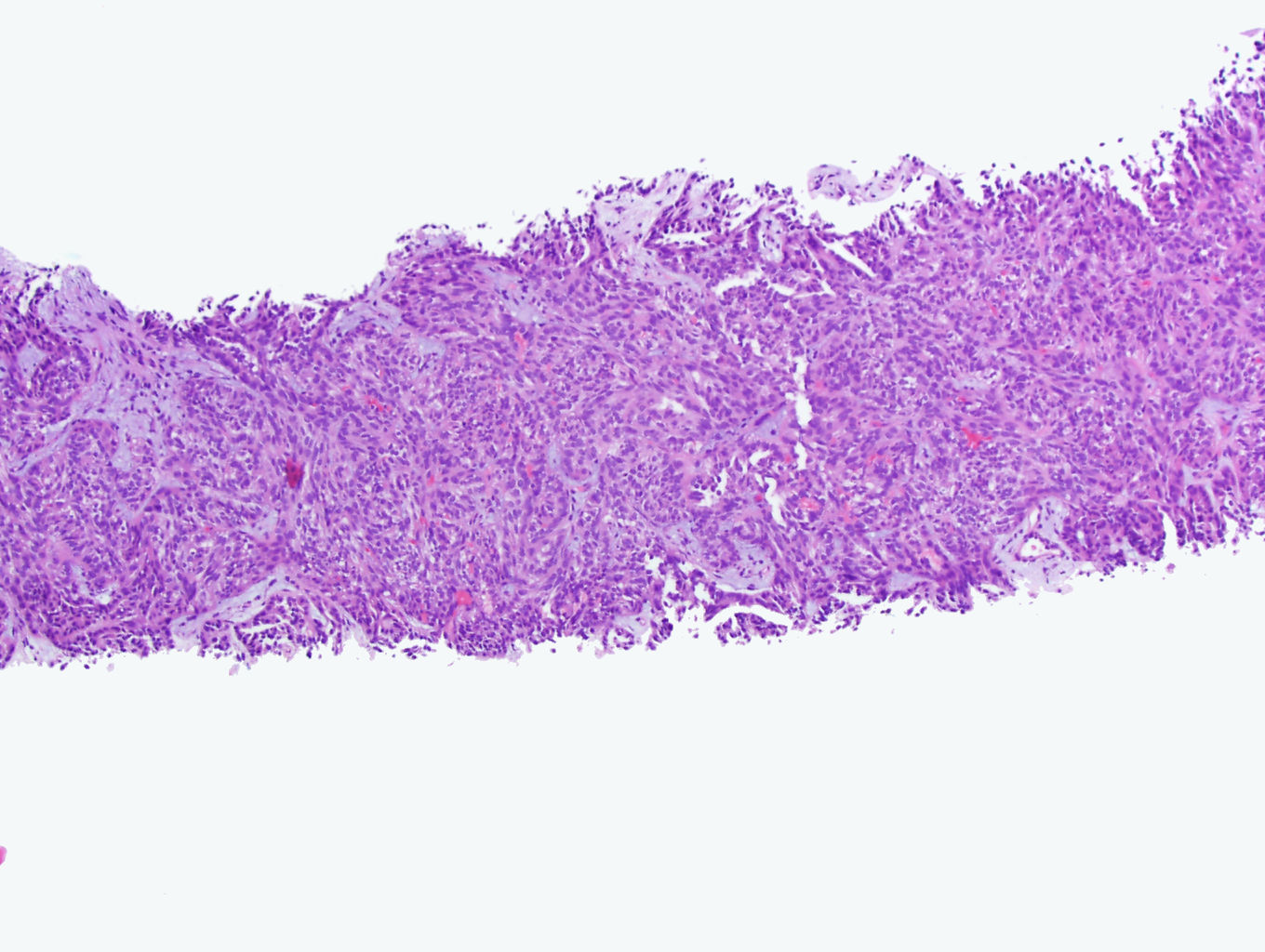
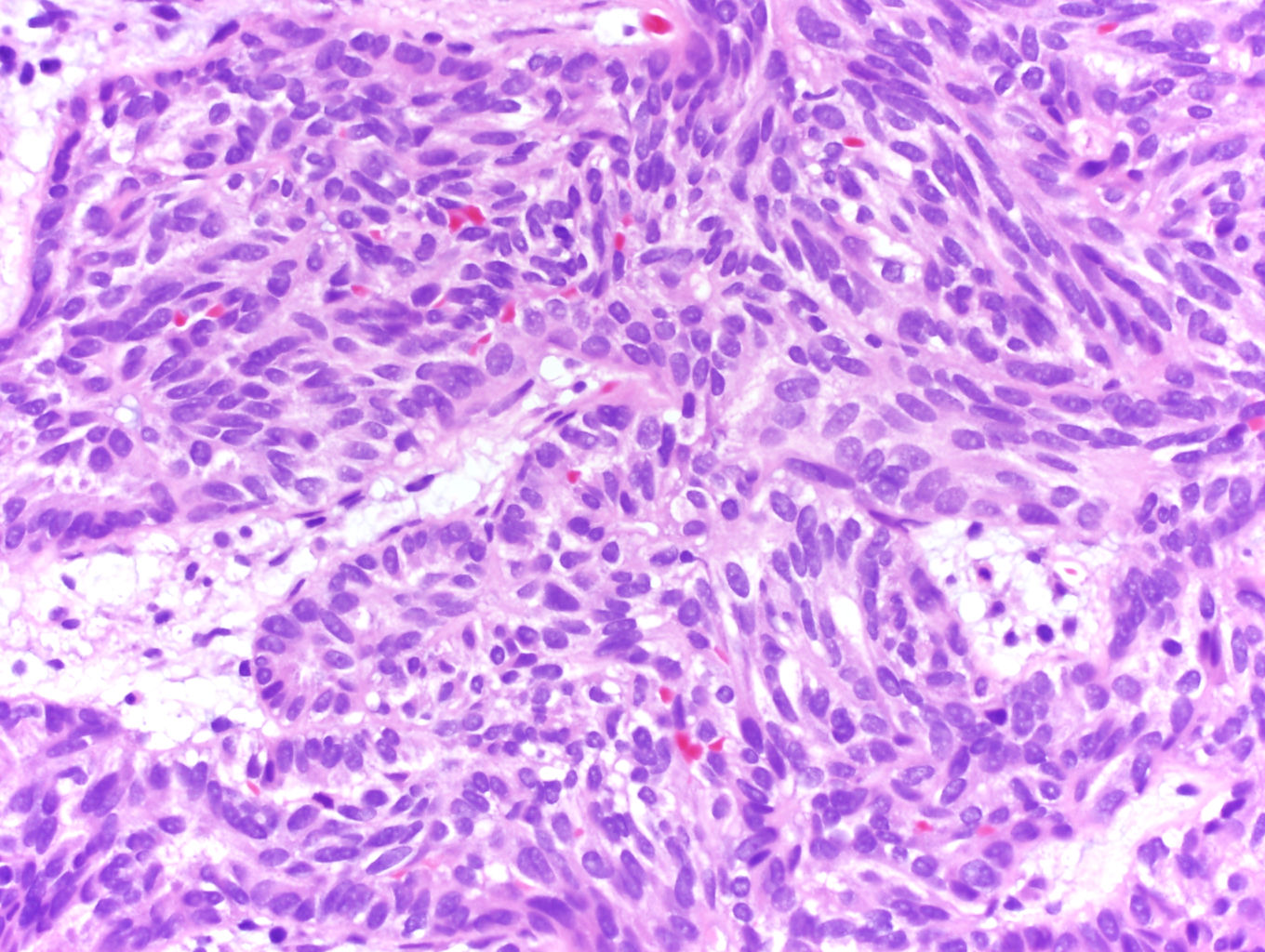
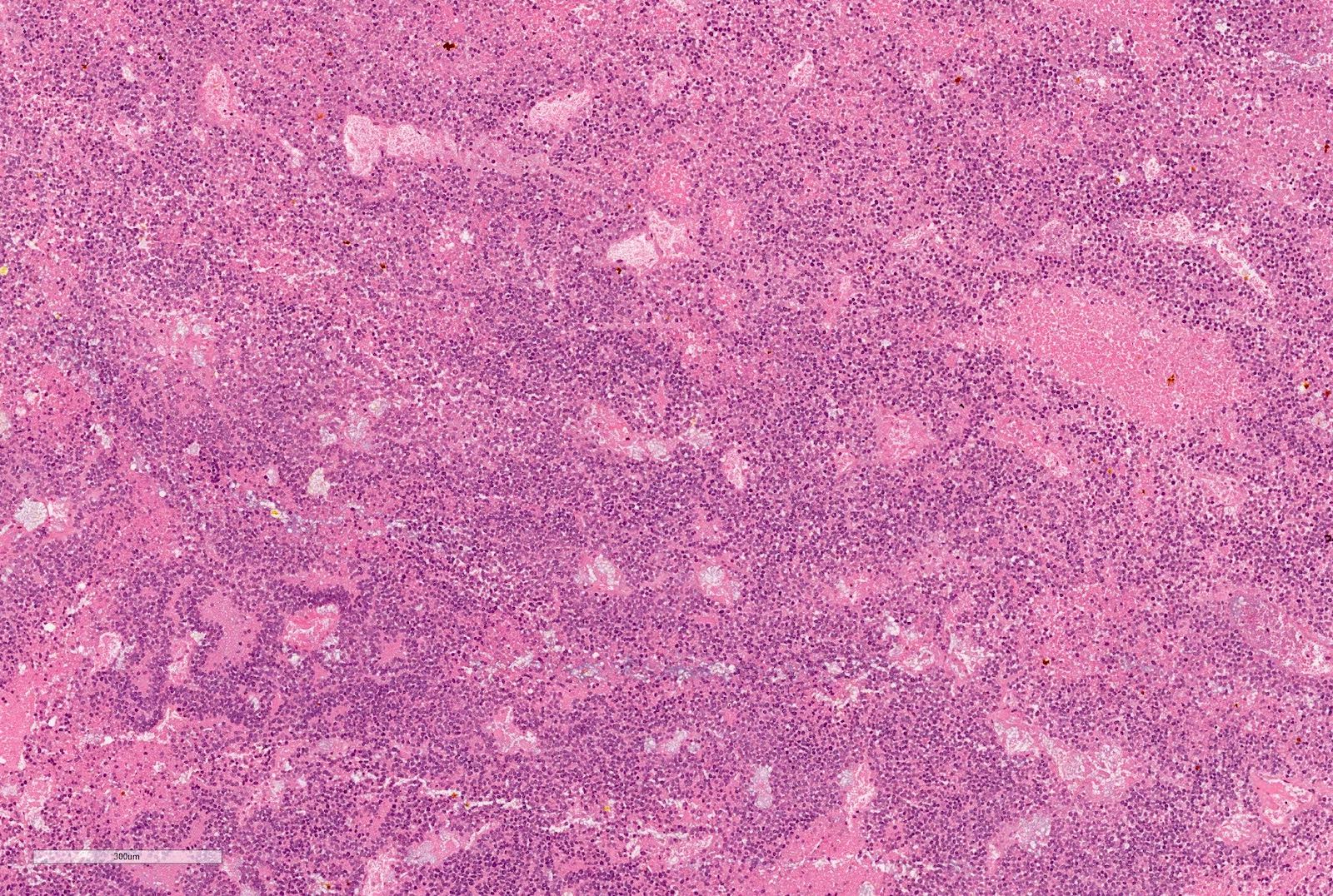
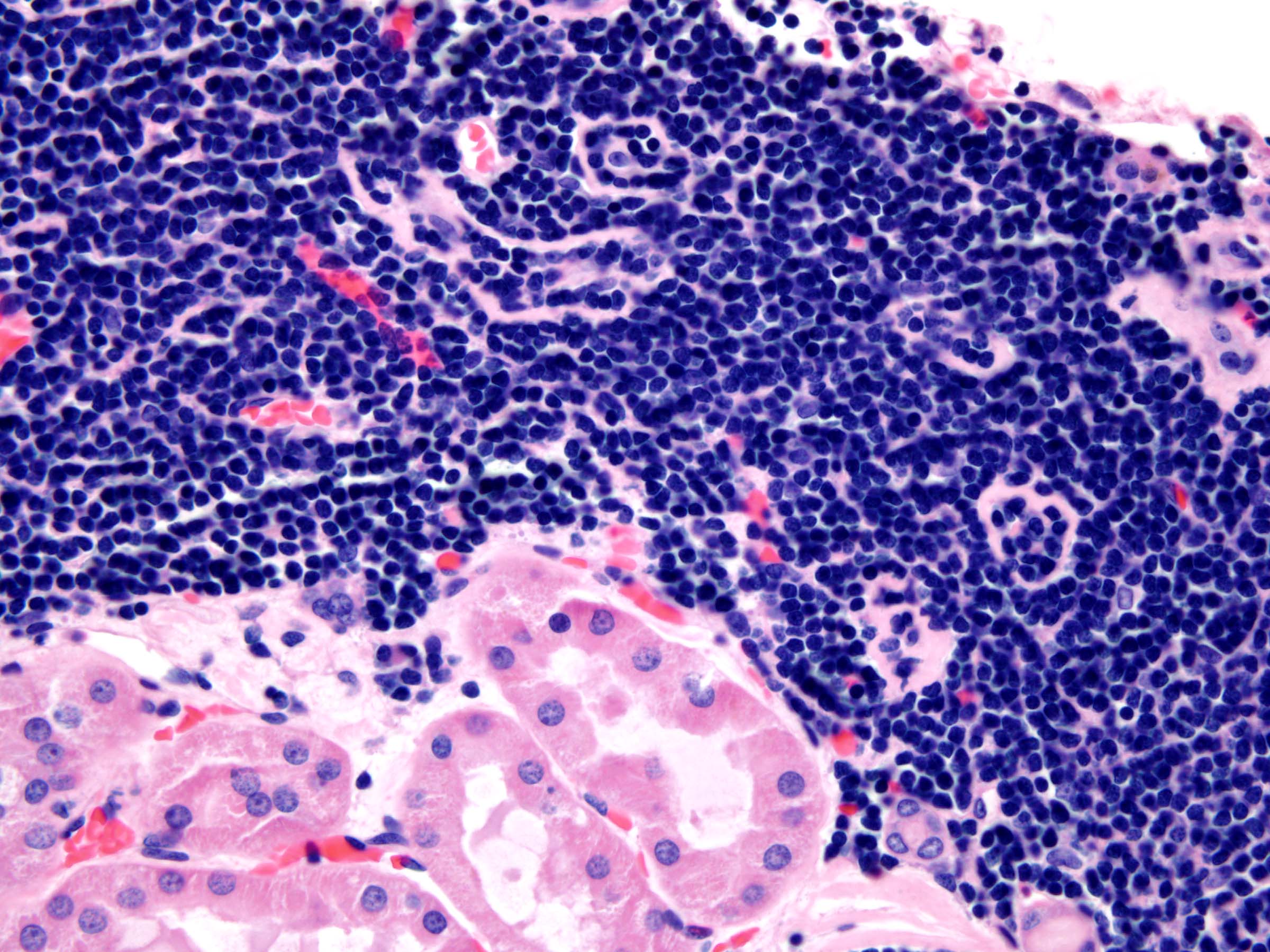
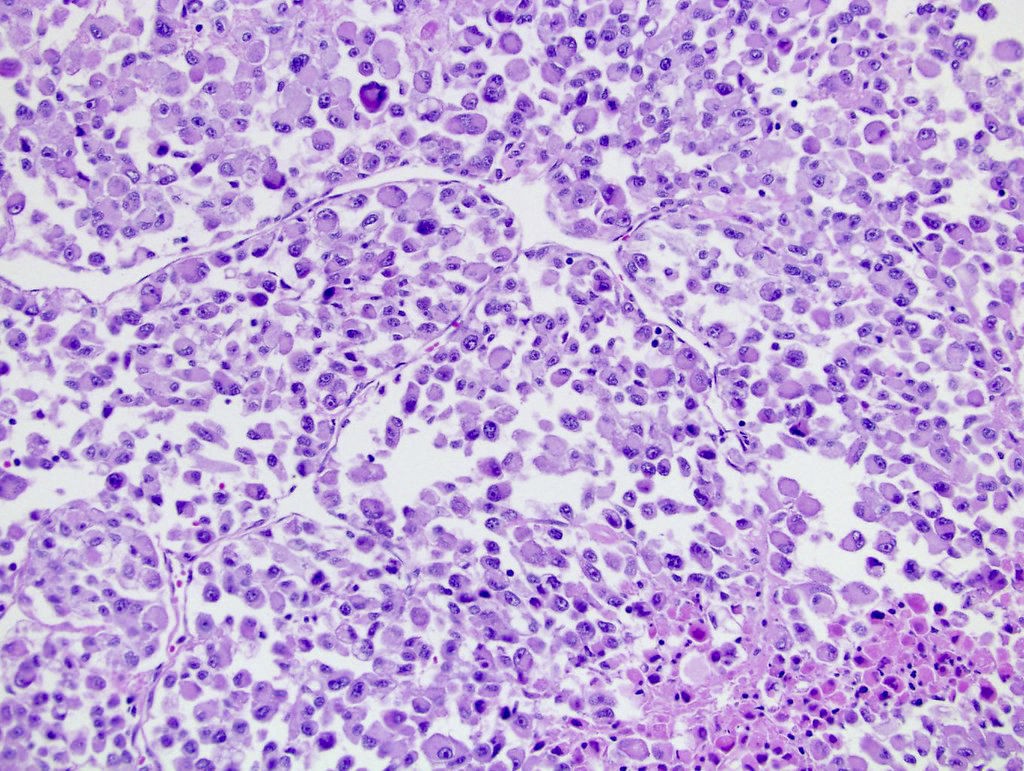
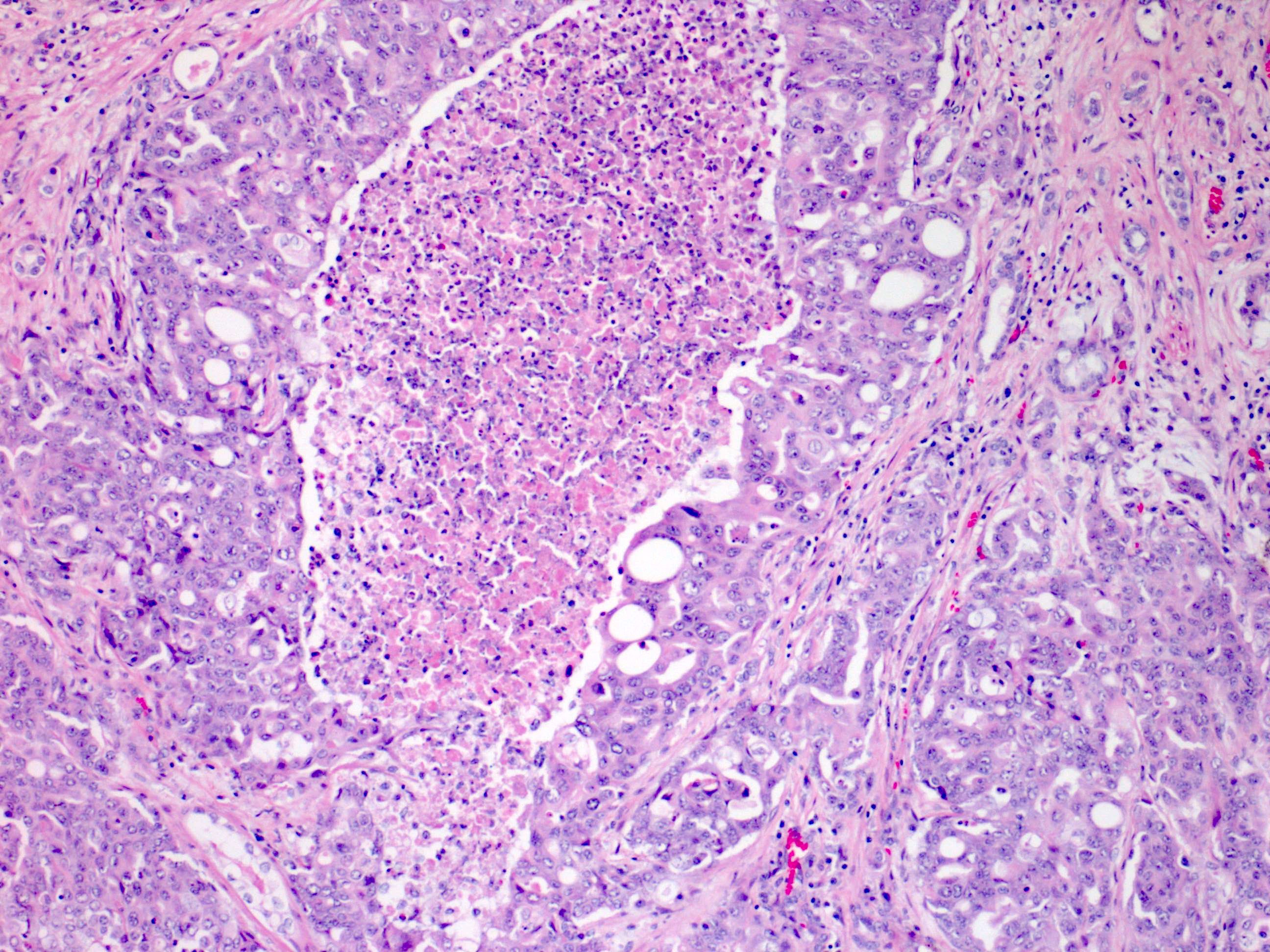
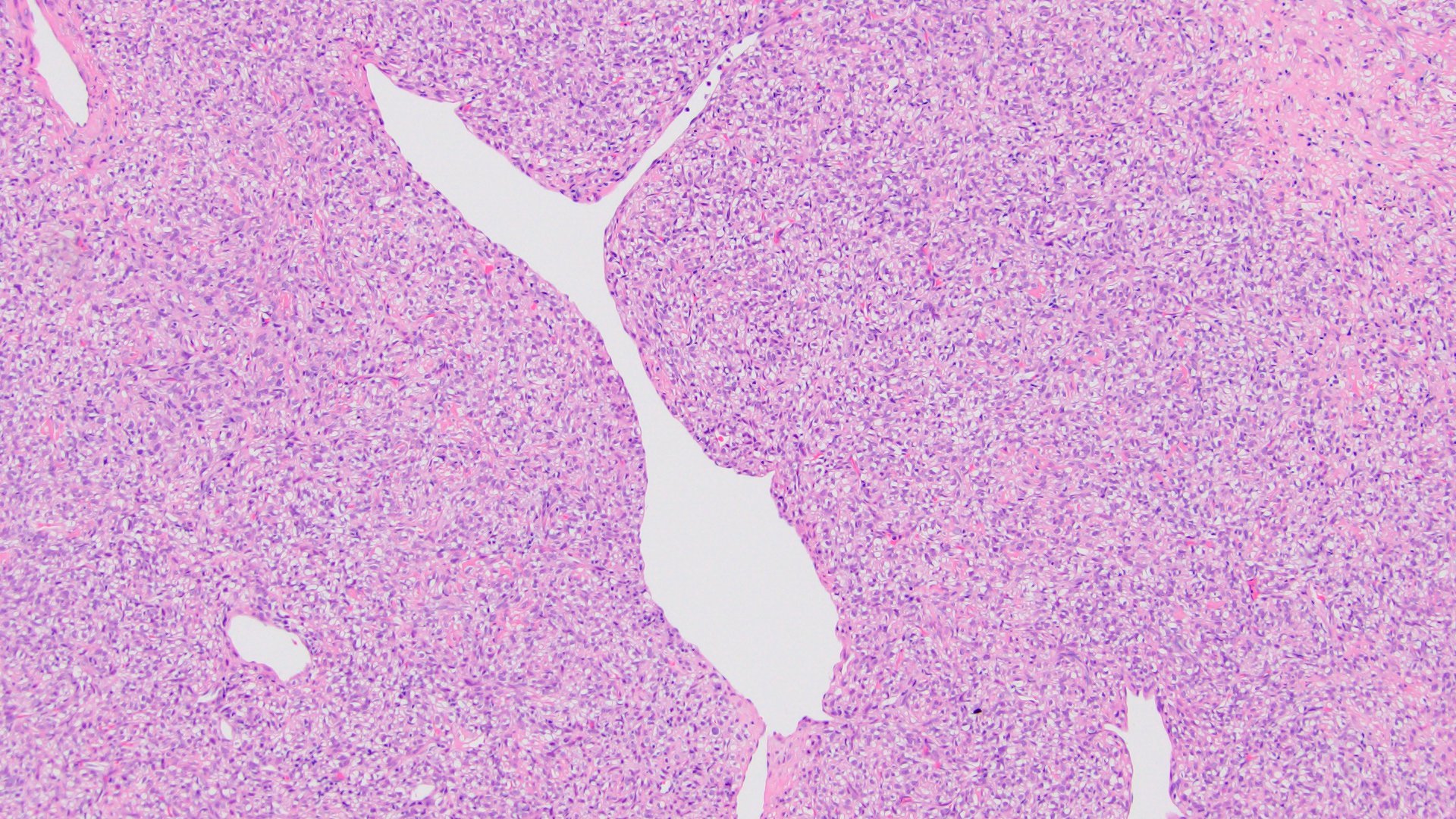
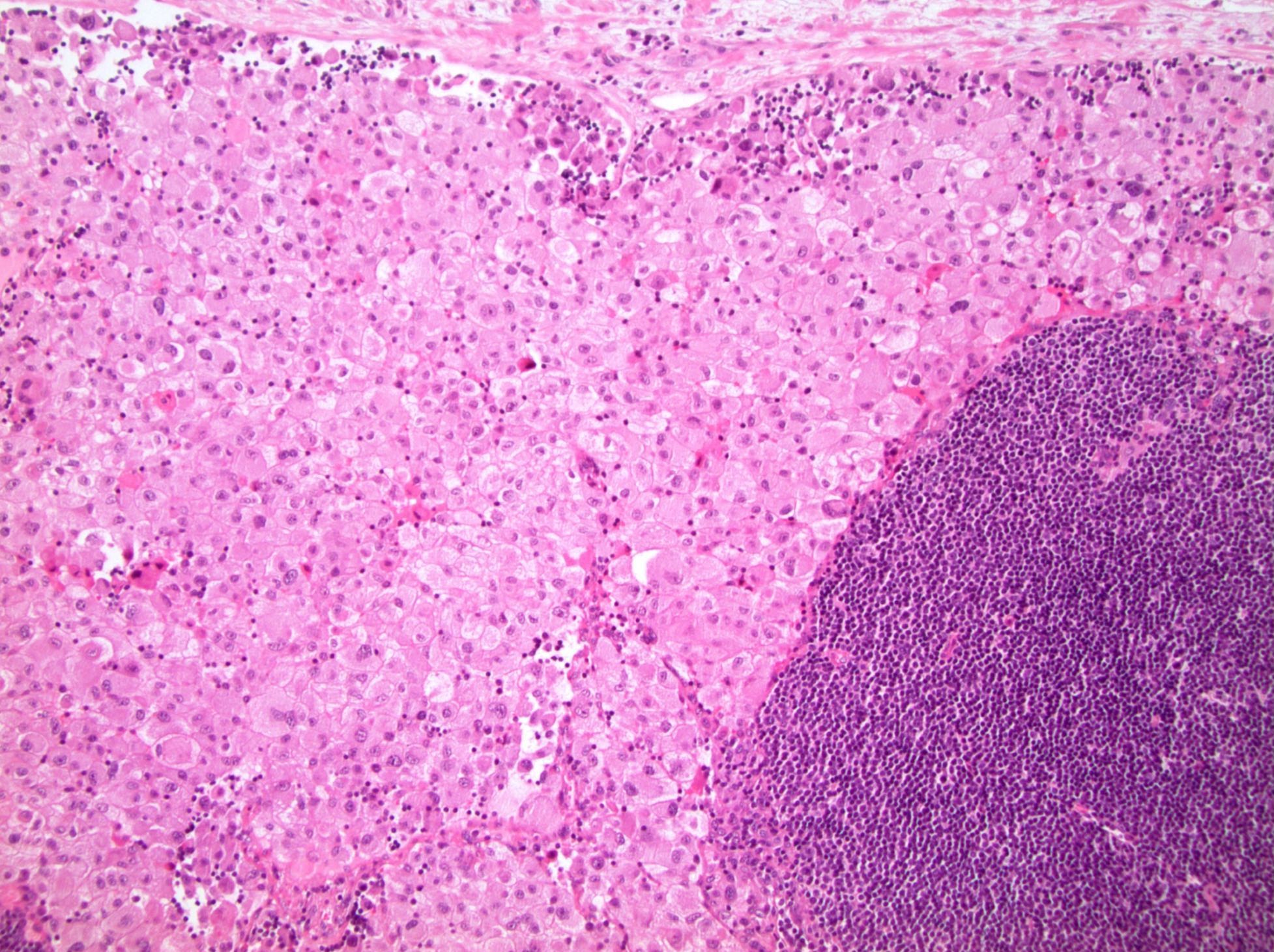
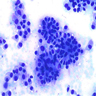
- Diffuse sheet-like infiltrating growth pattern
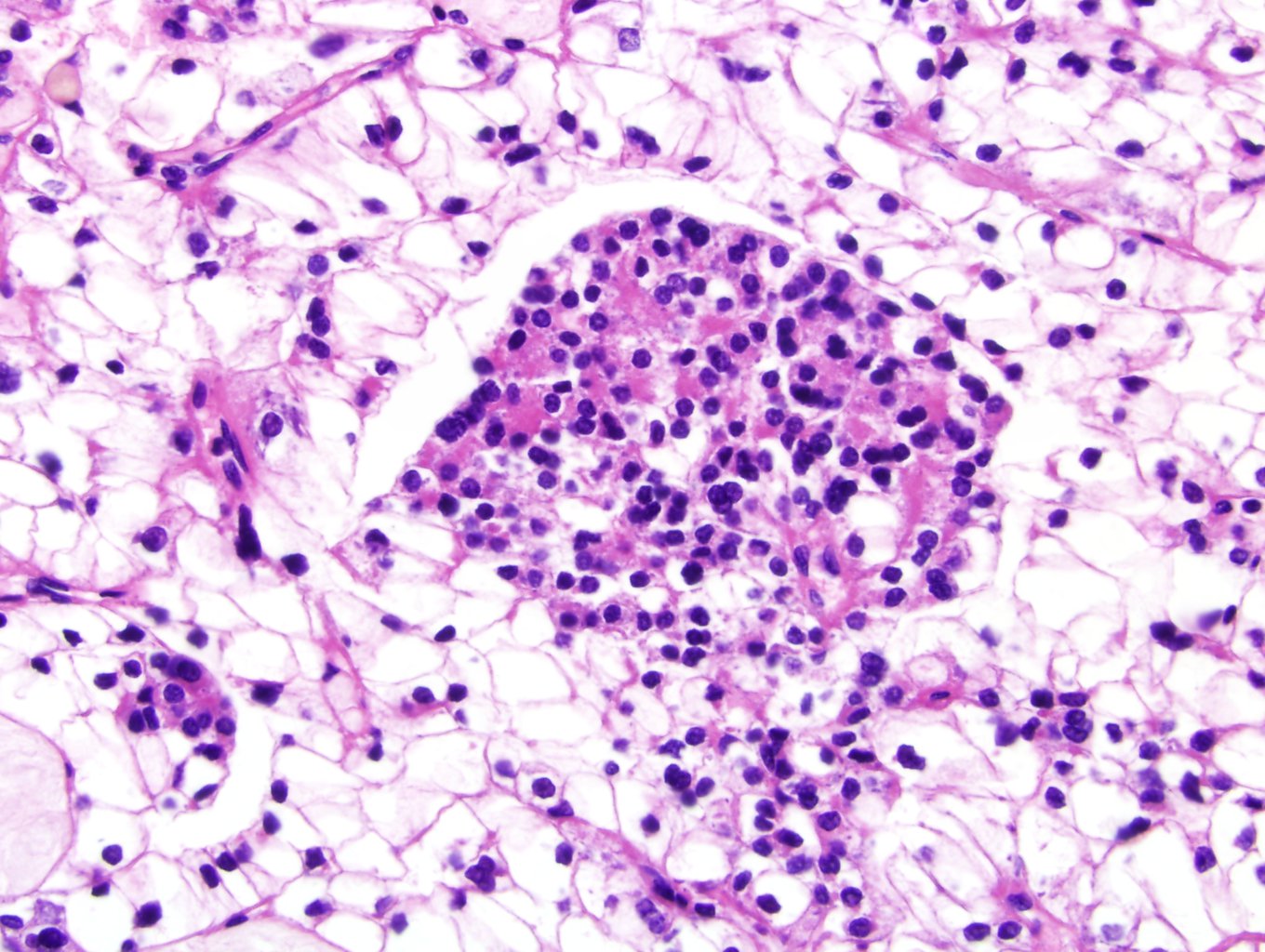
- Lymphoplasmacytic infiltrate and intravascular sickling
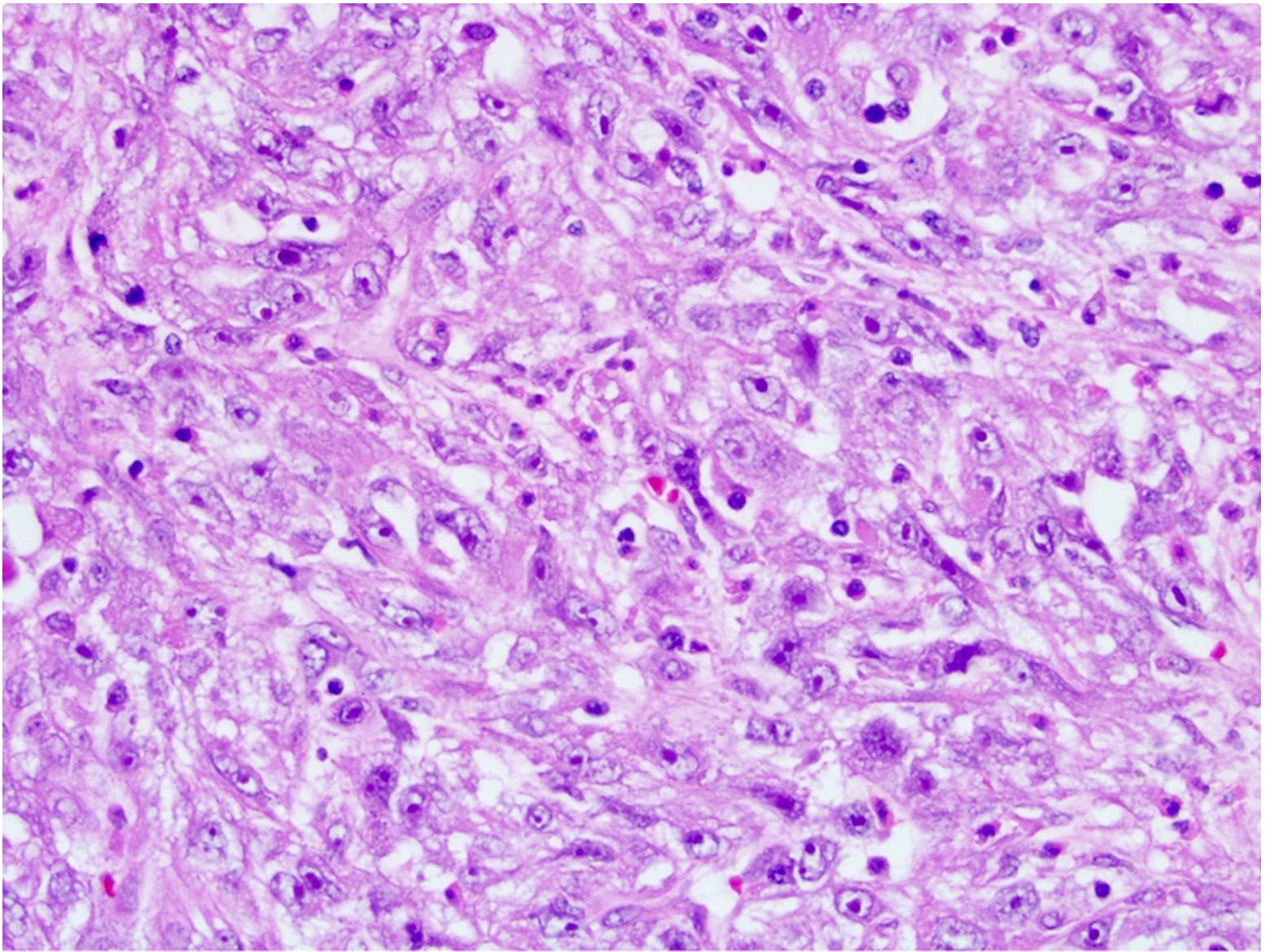
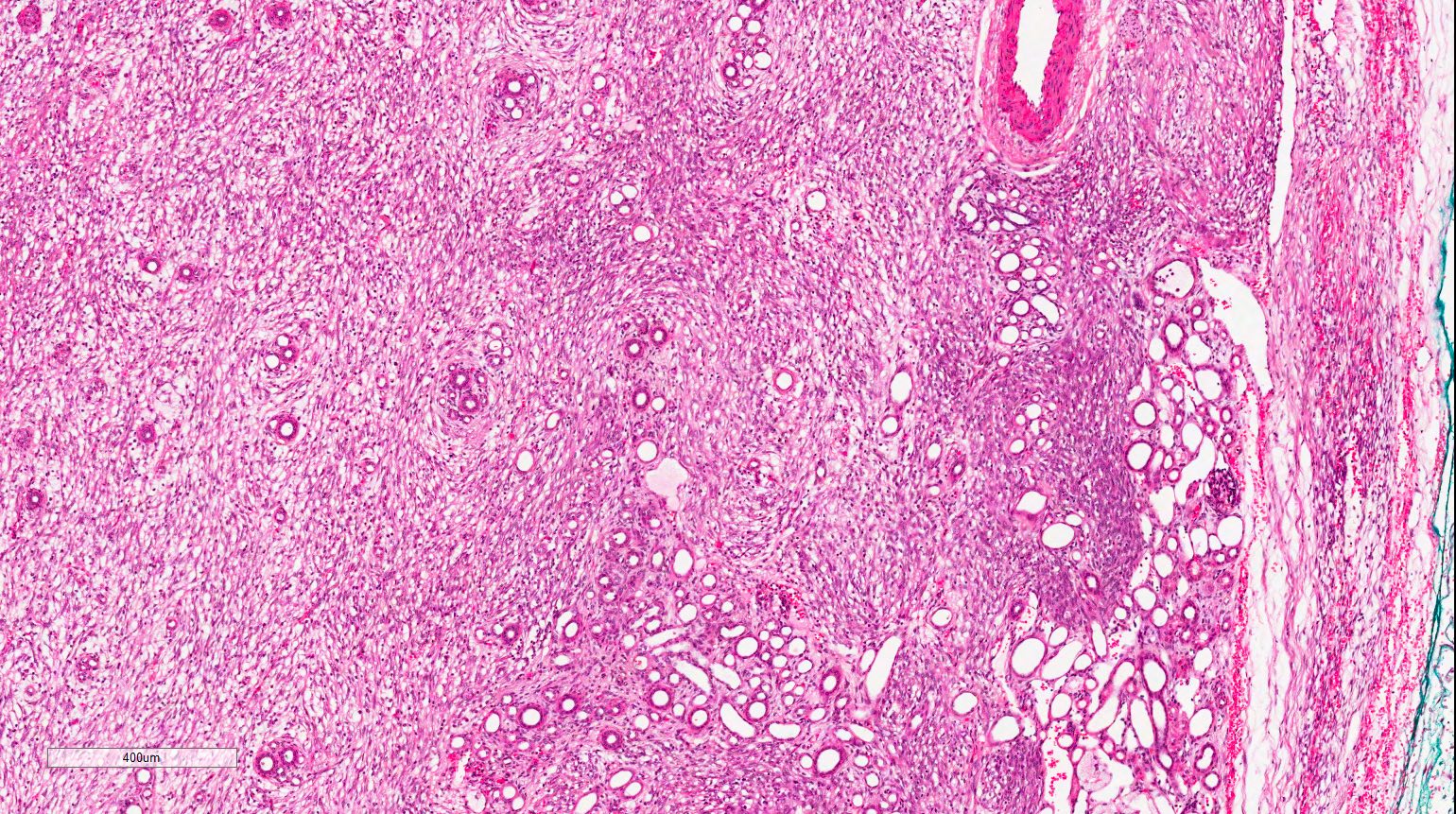
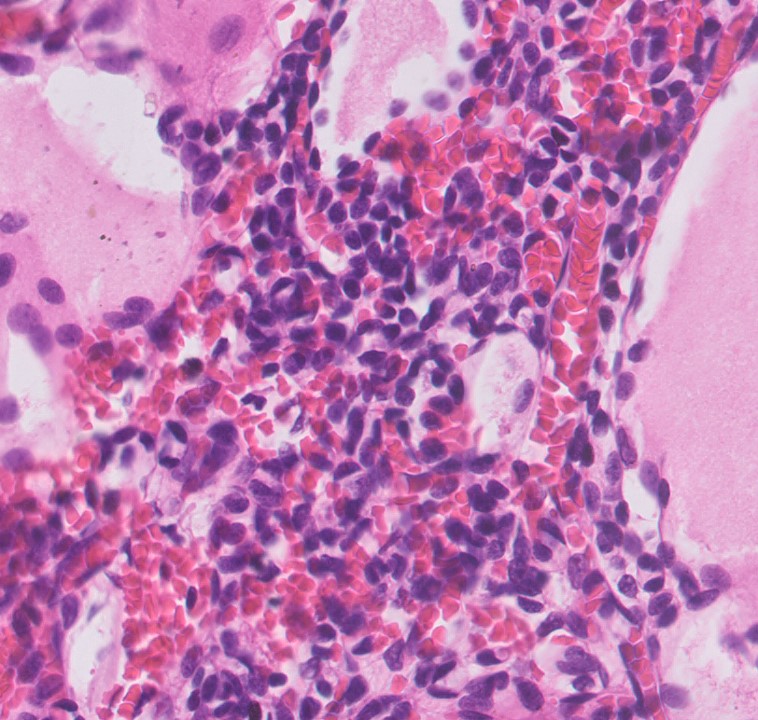
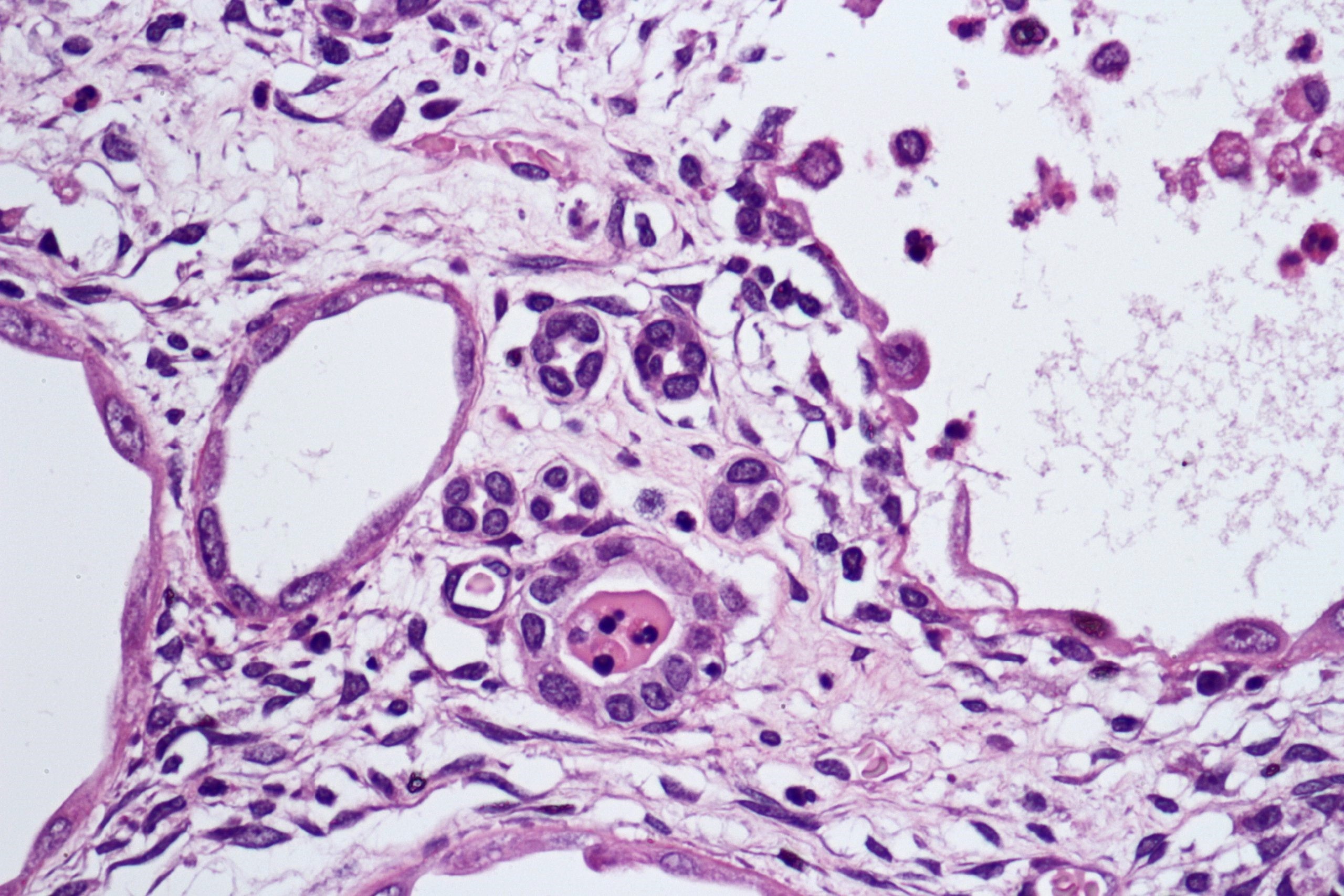
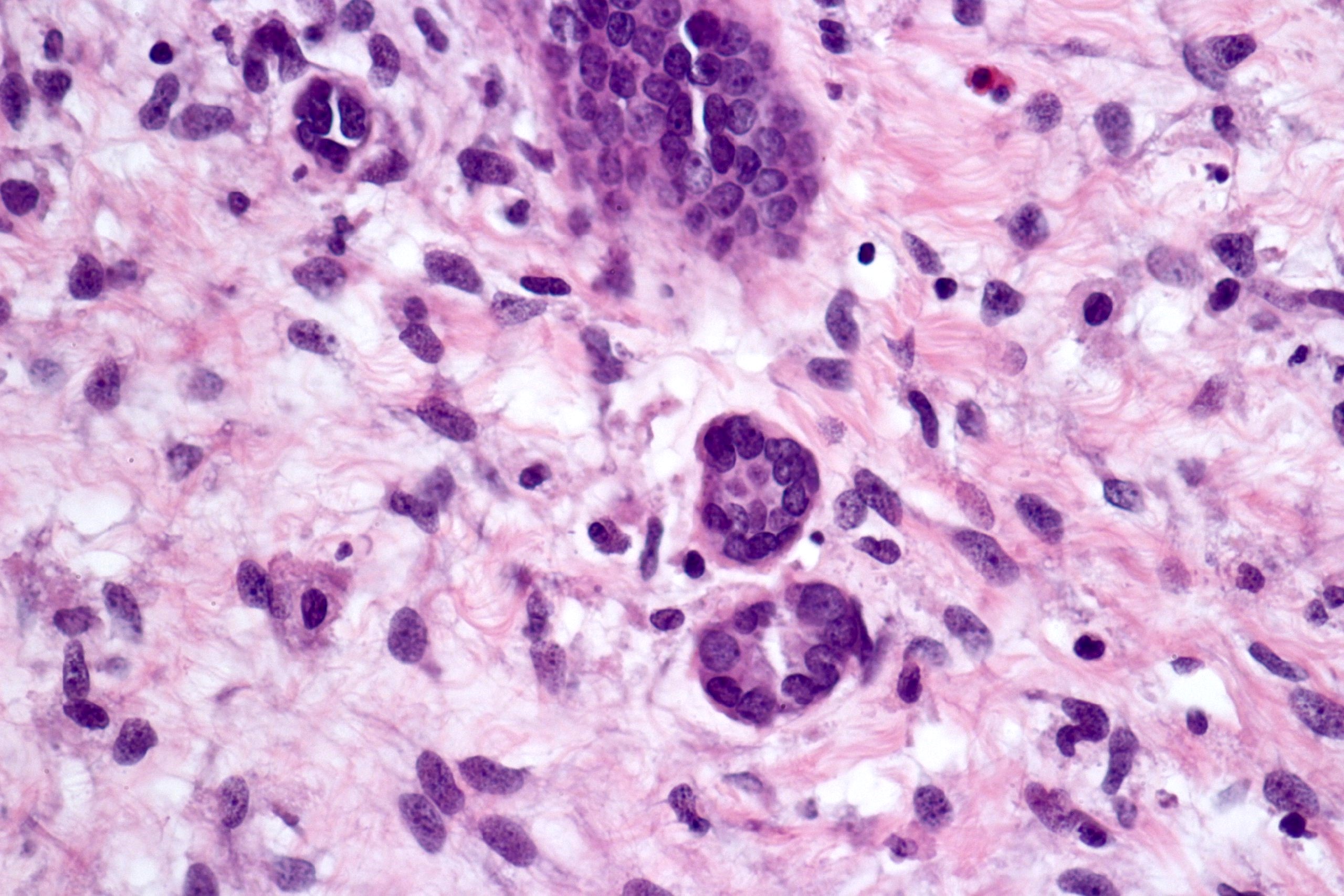
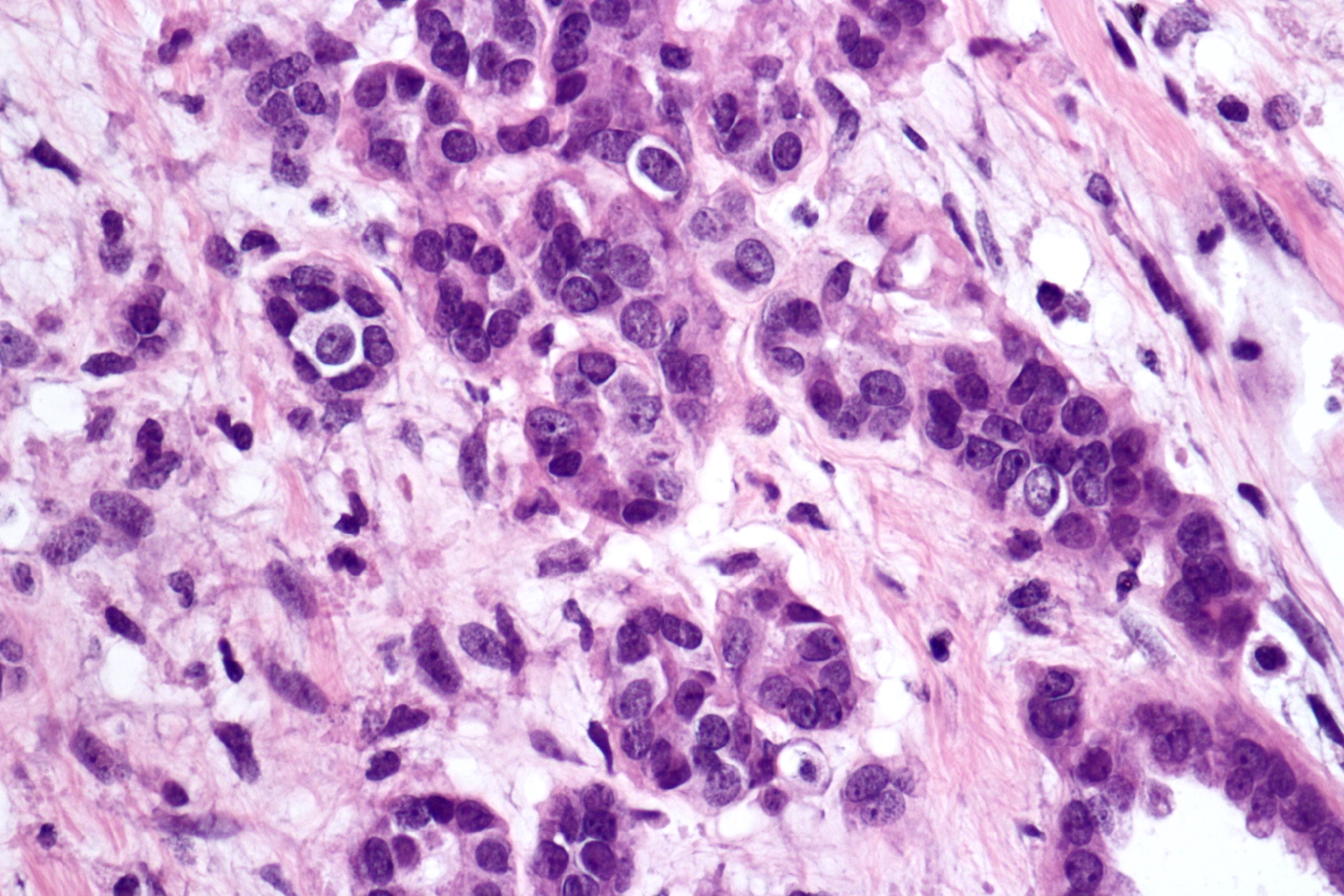
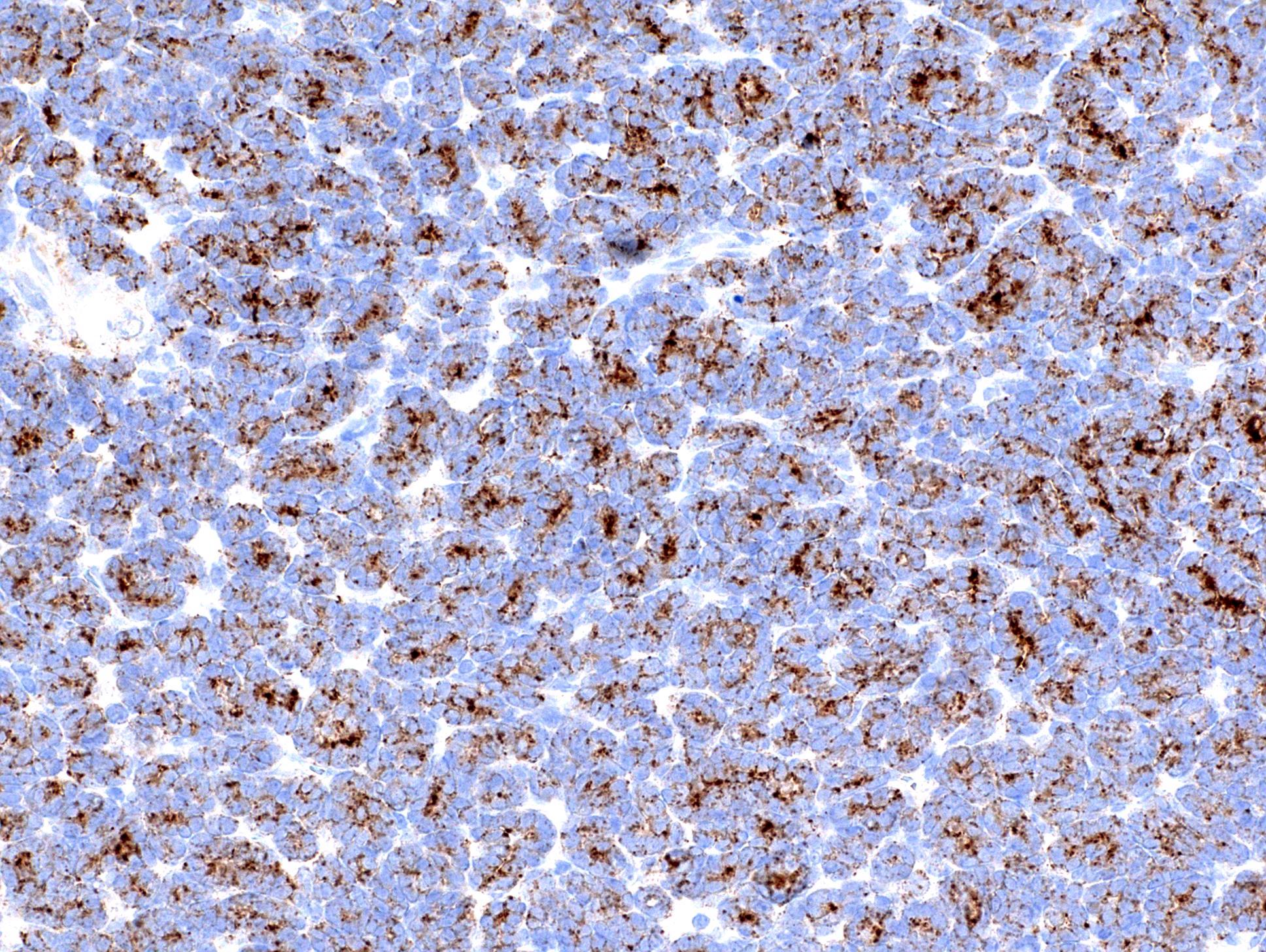
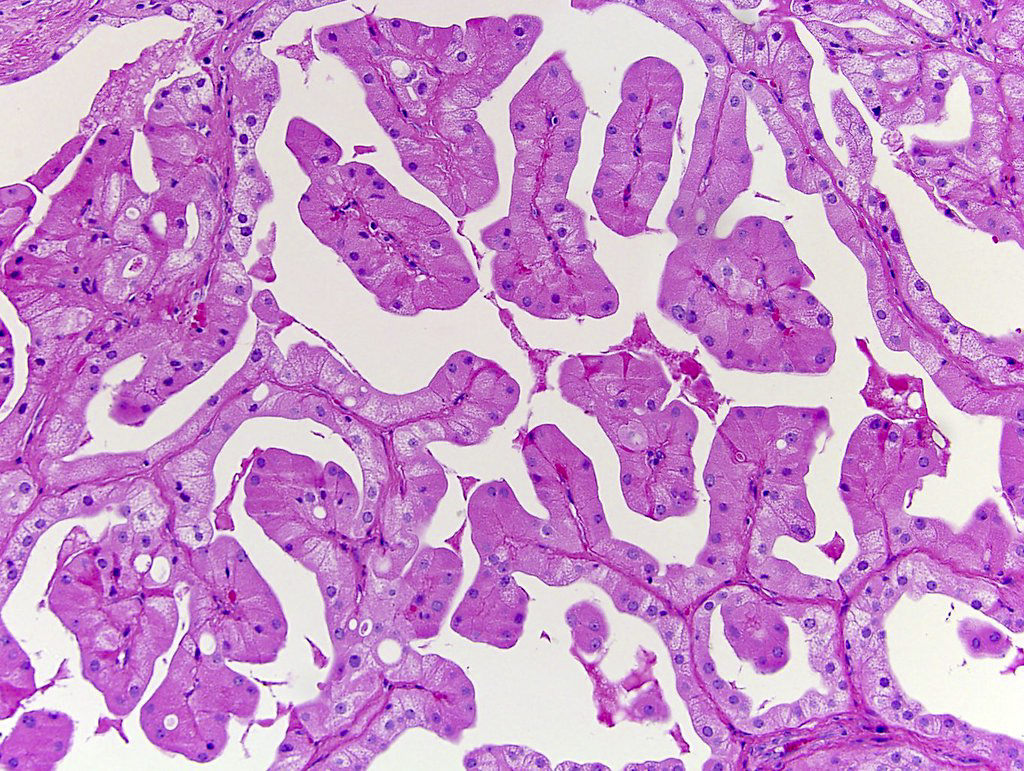
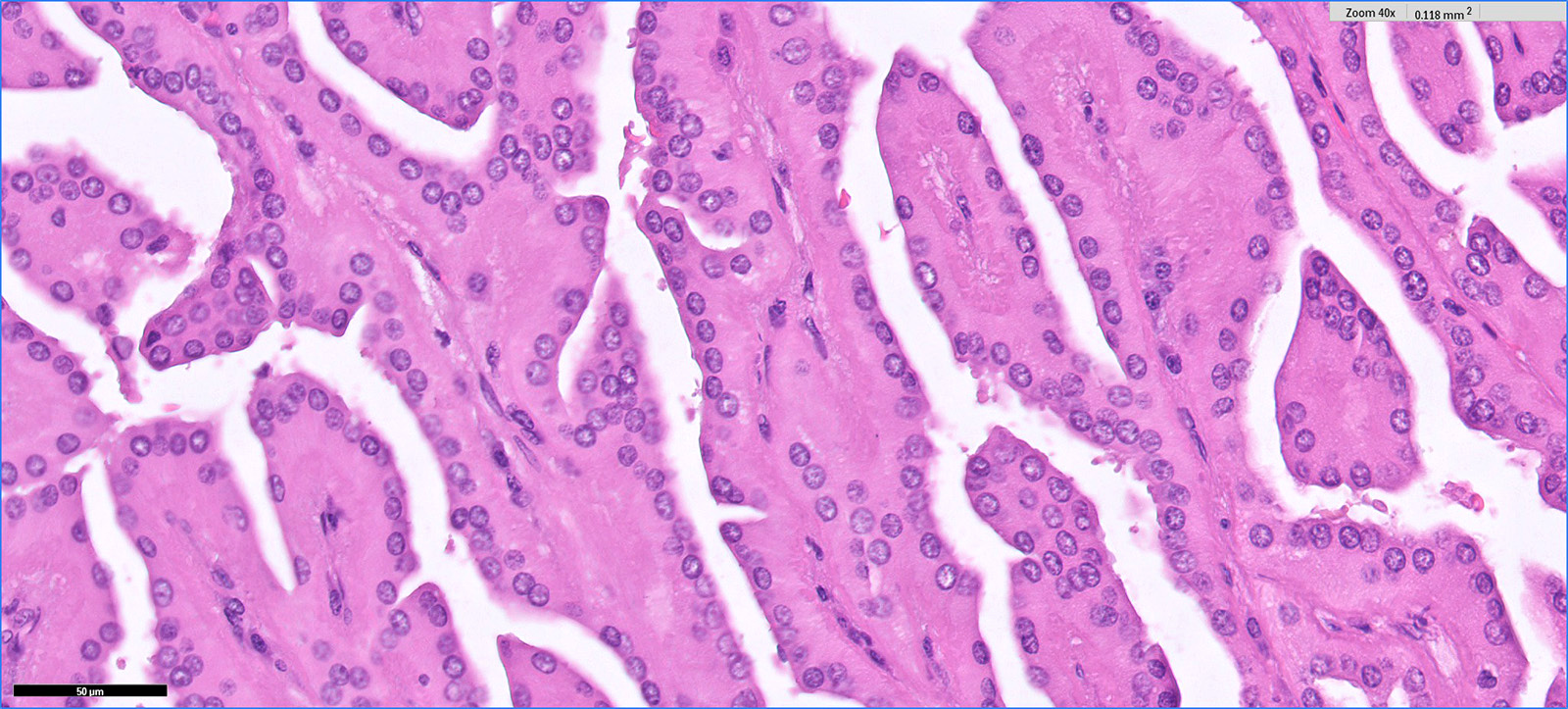
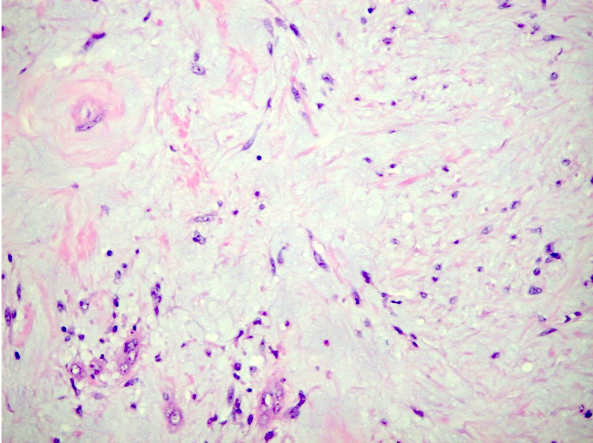
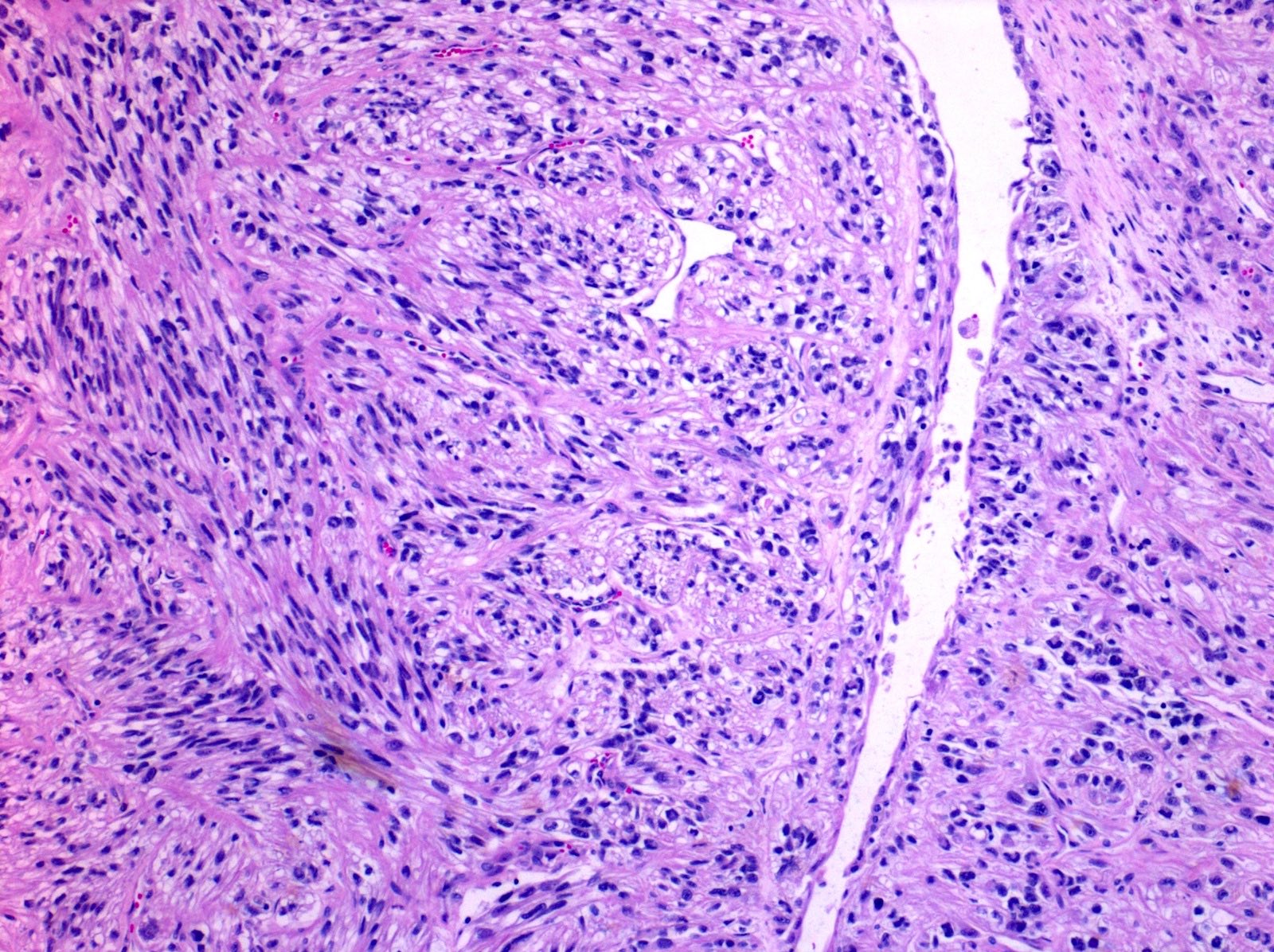
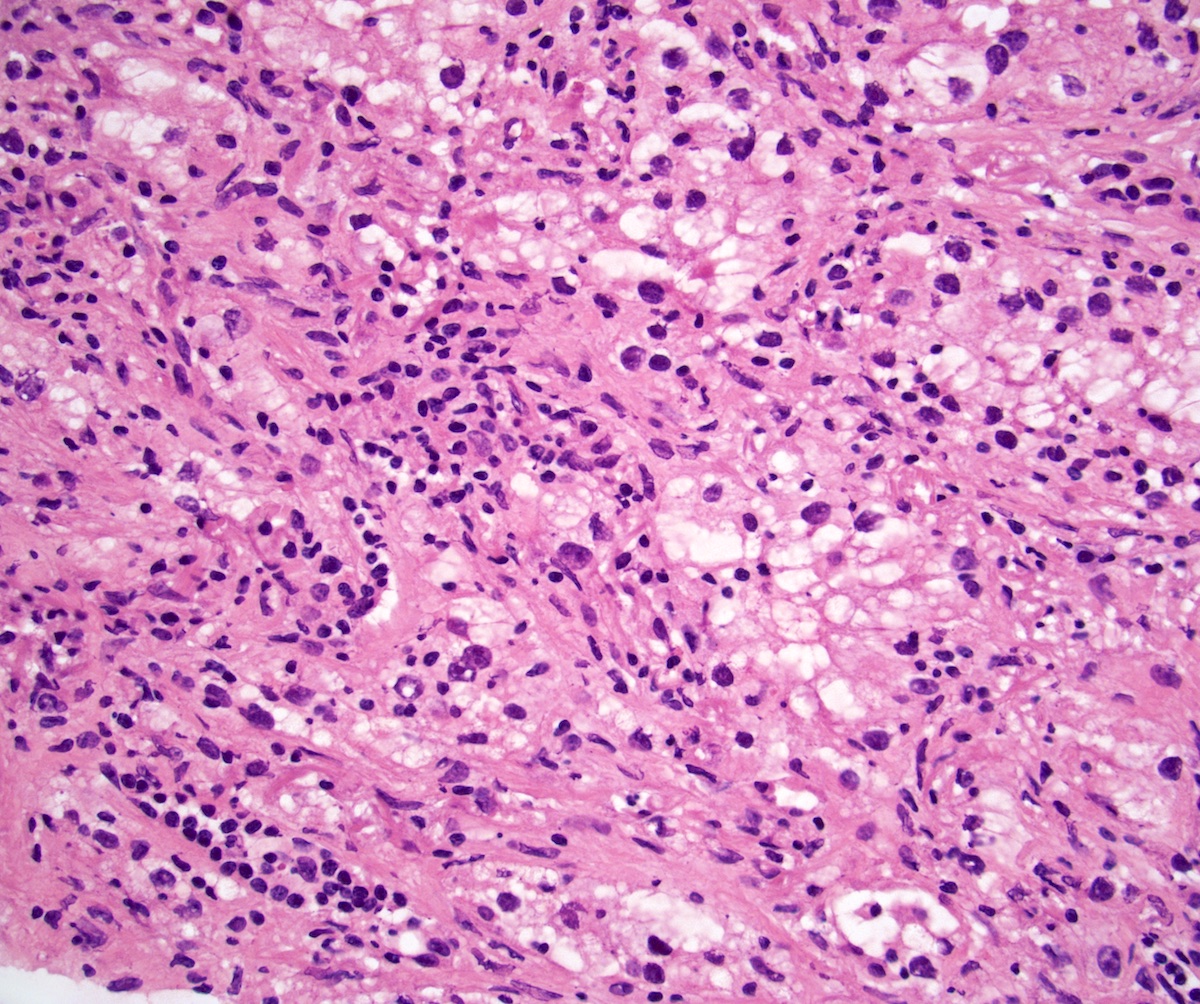
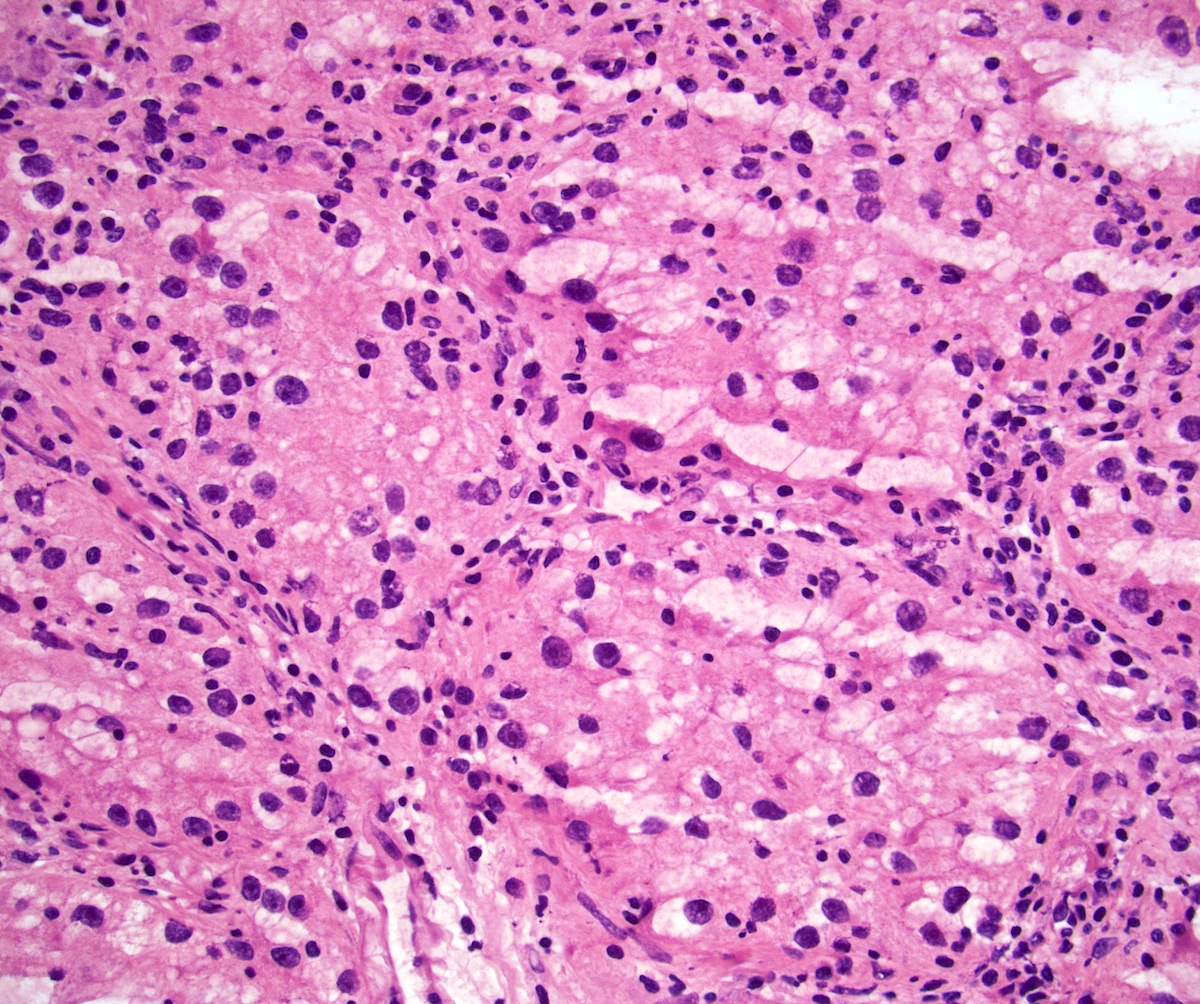
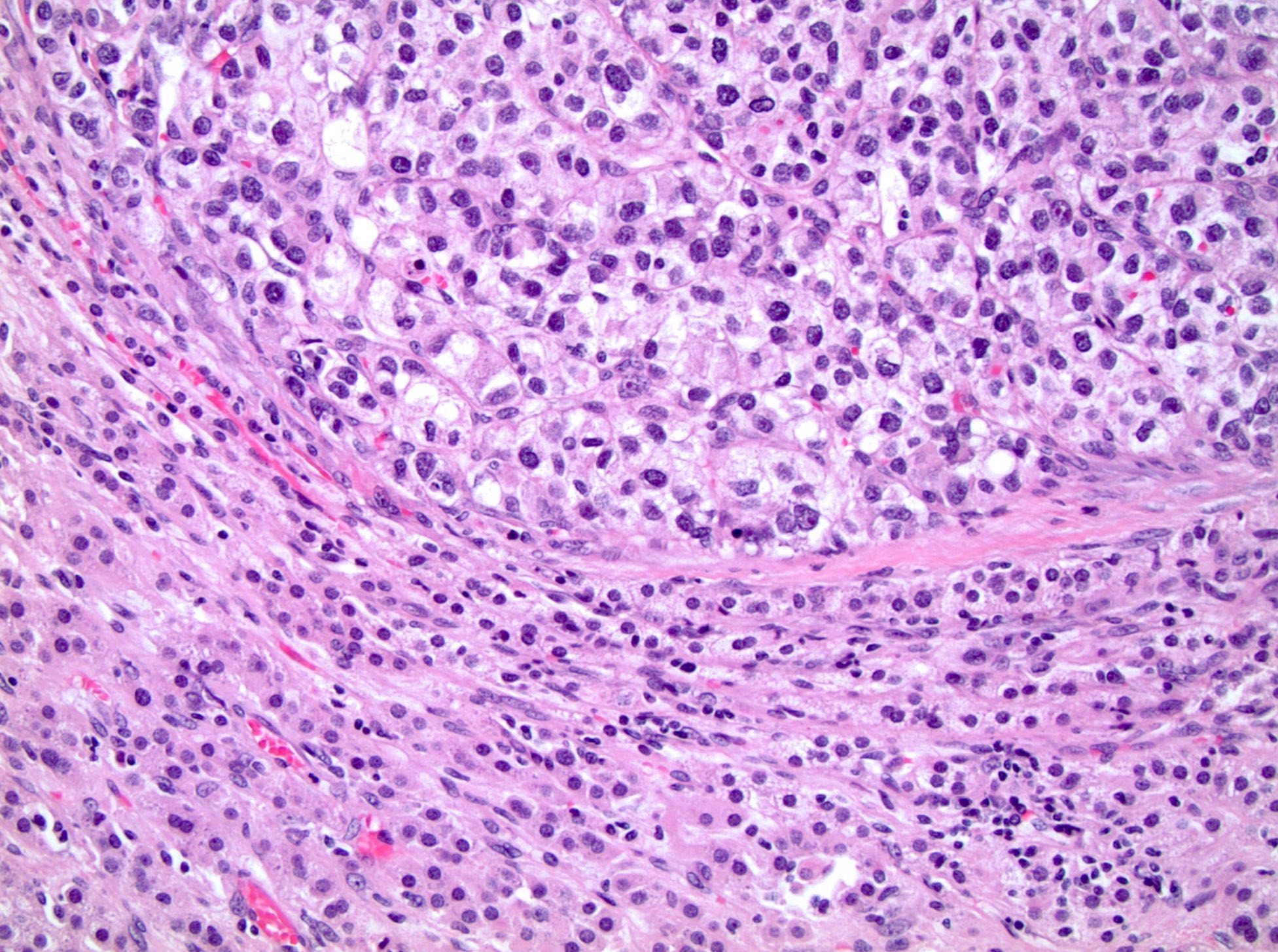
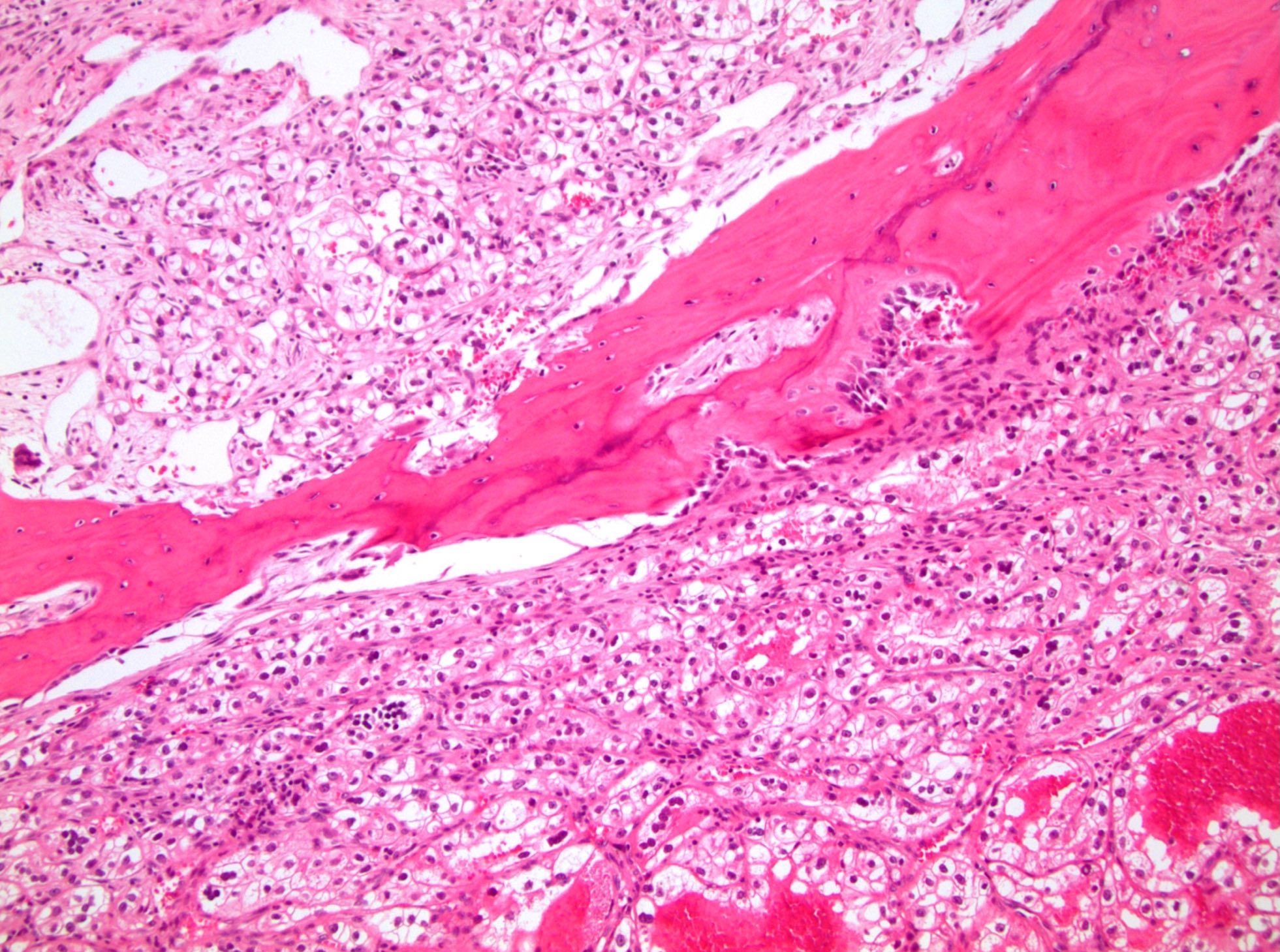
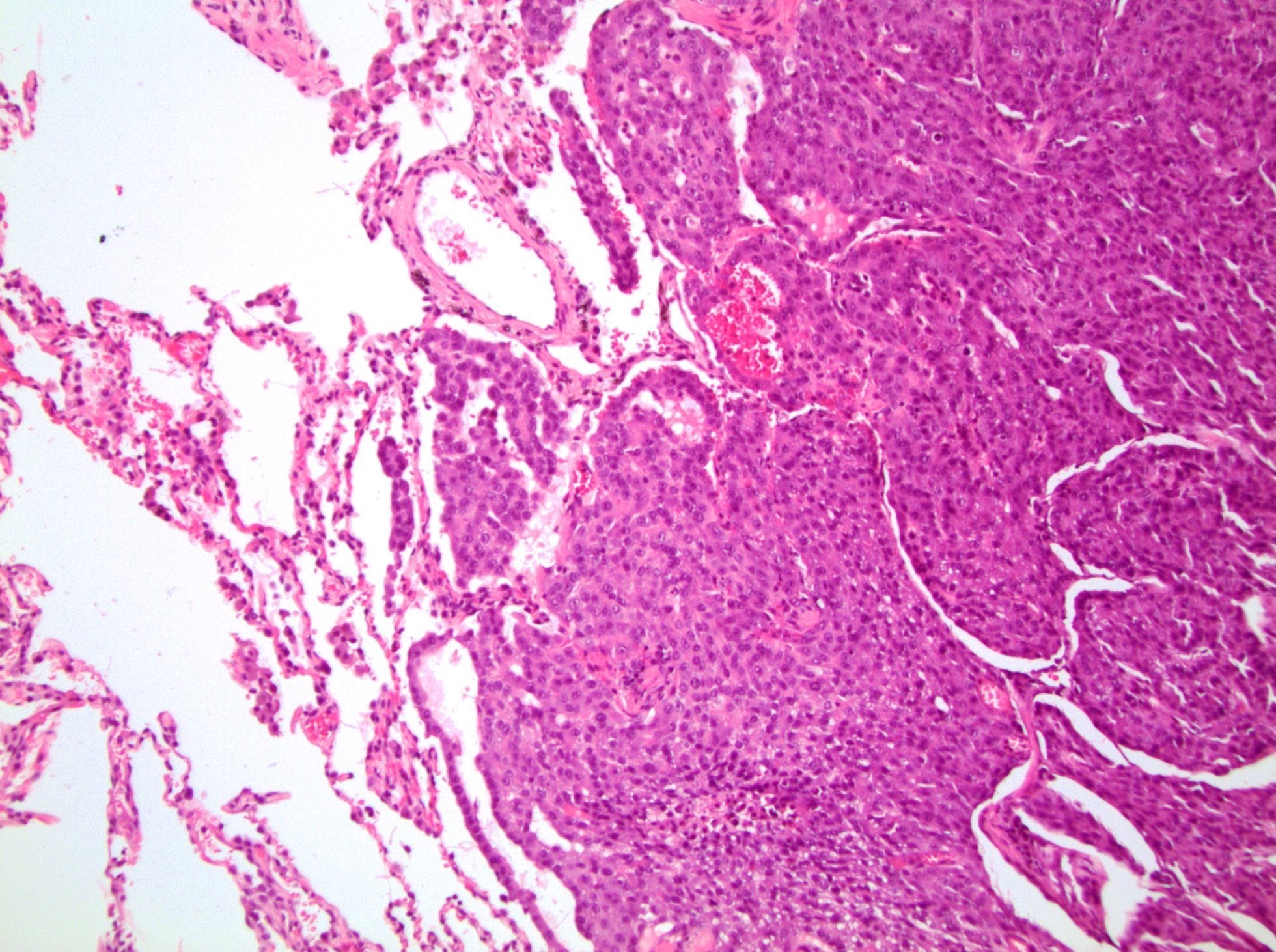
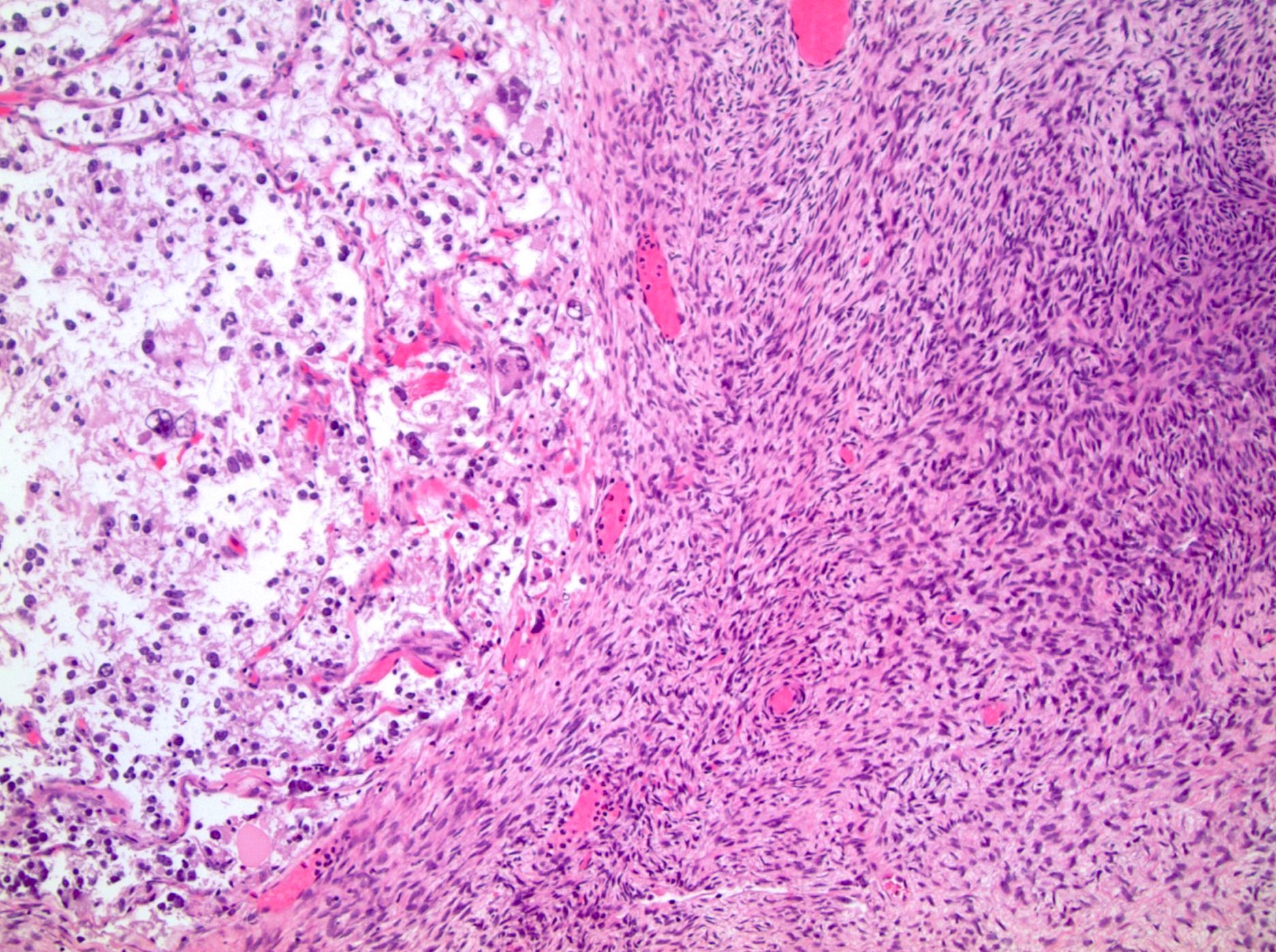
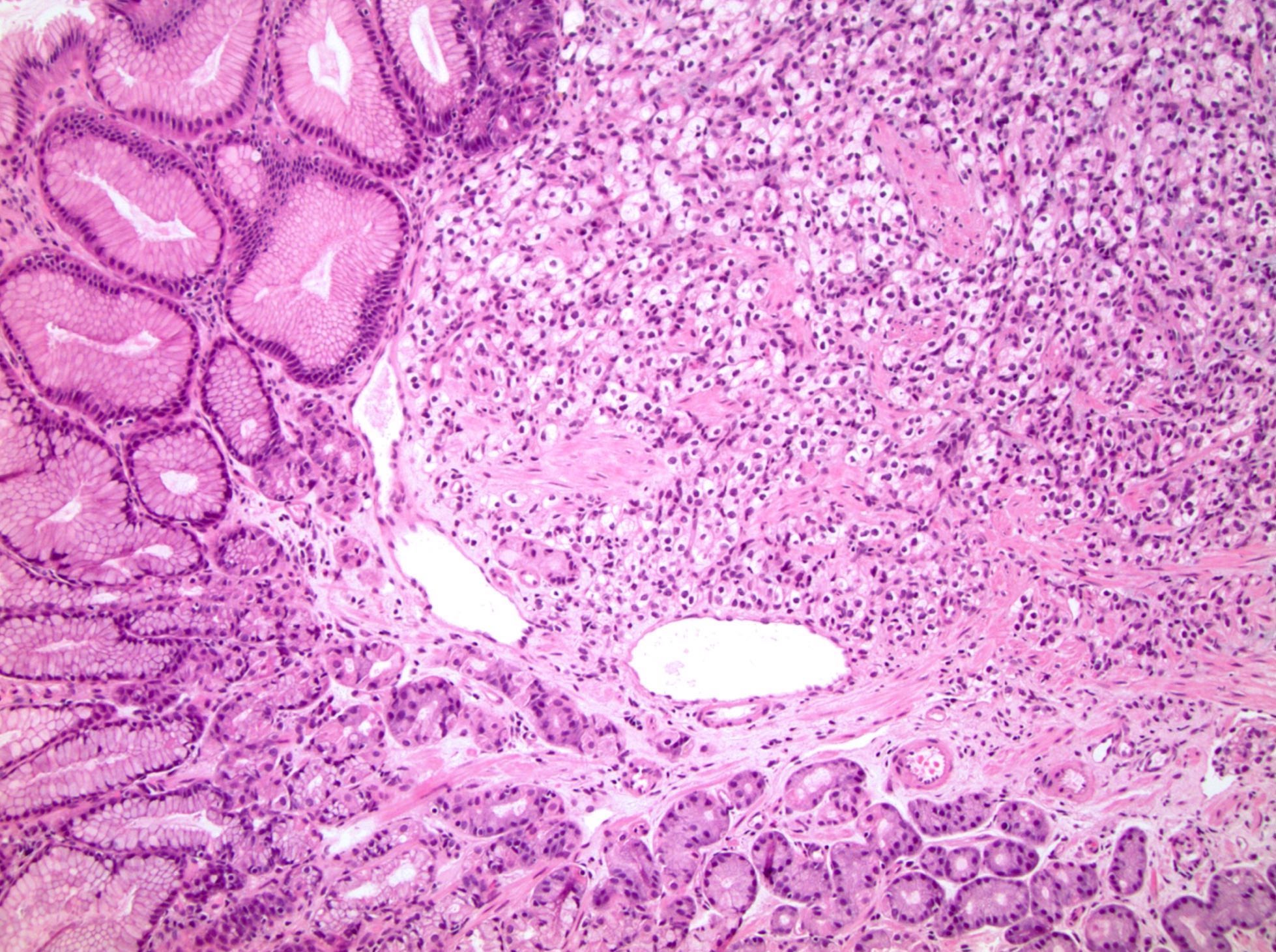
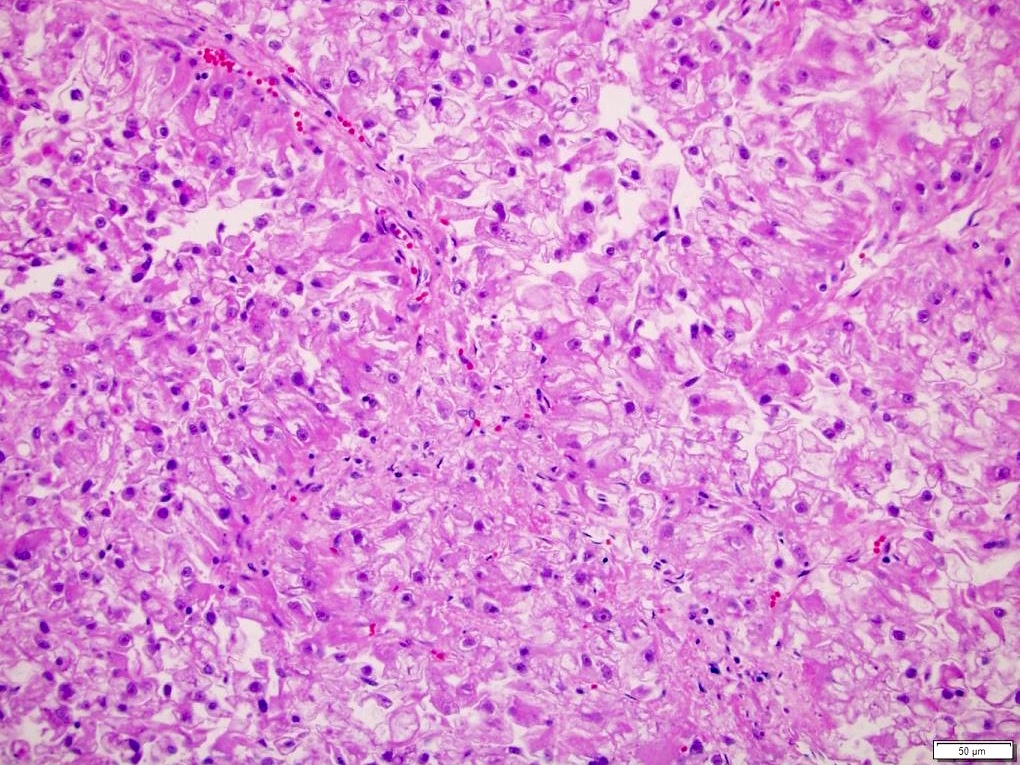
- Round, oval and polygonal tumor cells with abundant vaguely granular eosinophilic cytoplasm and frequent intracytoplasmic lumina
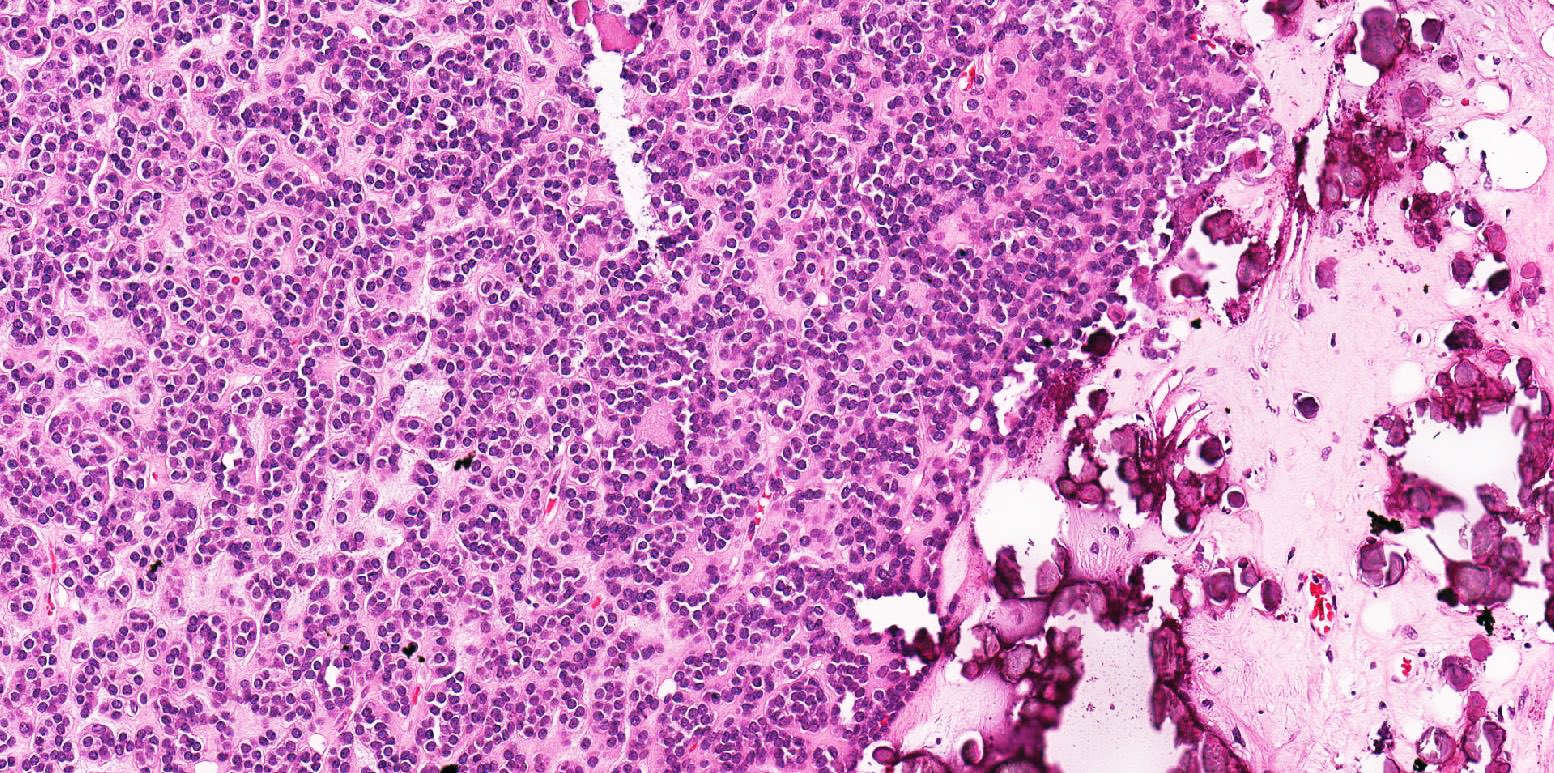
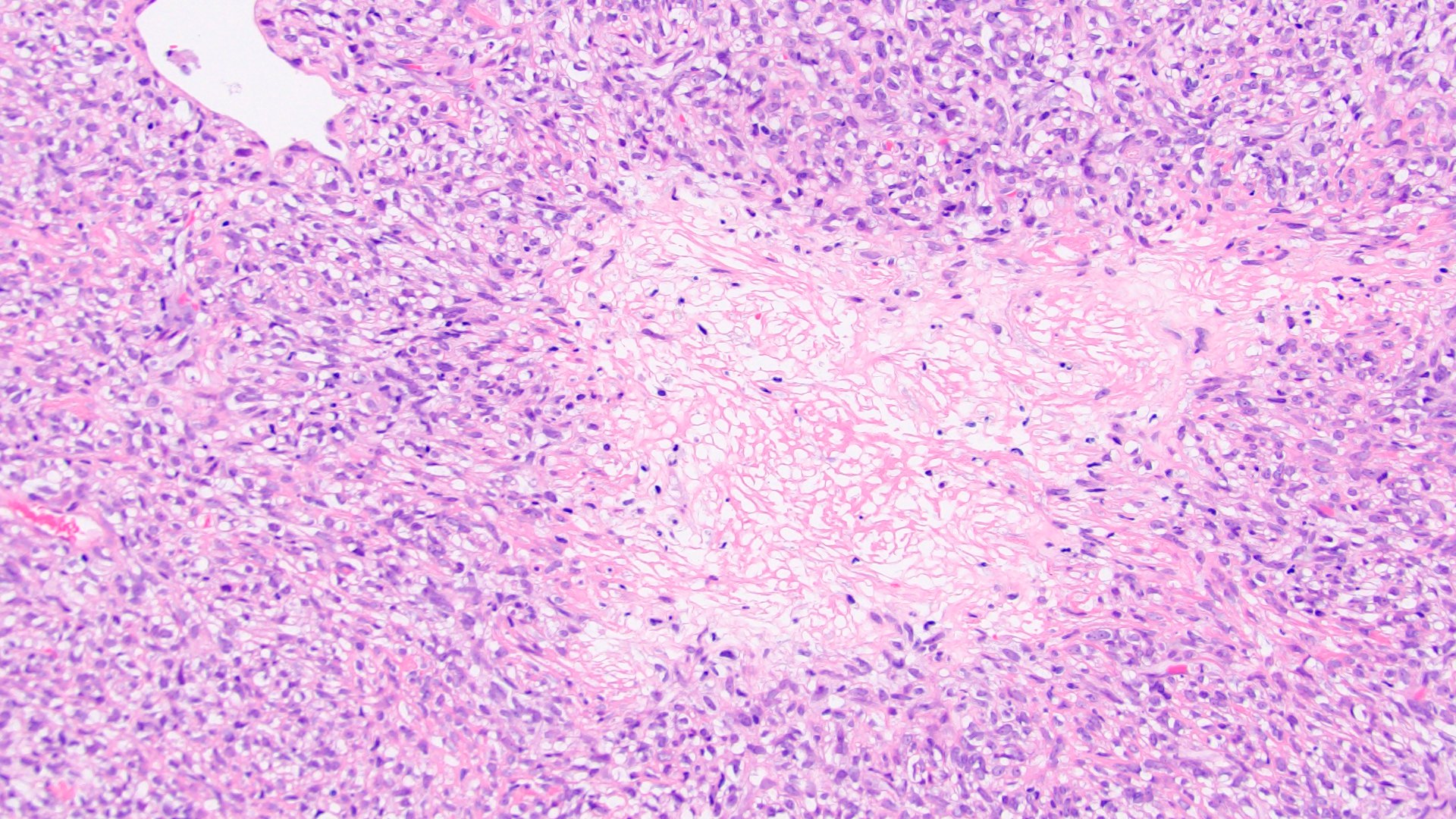
- Abundant background mucin and intracytoplasmic mucin
- Moderately polymorphic, predominantly vesicular nuclei with small nucleoli, occasional grooves and rare vacuoles
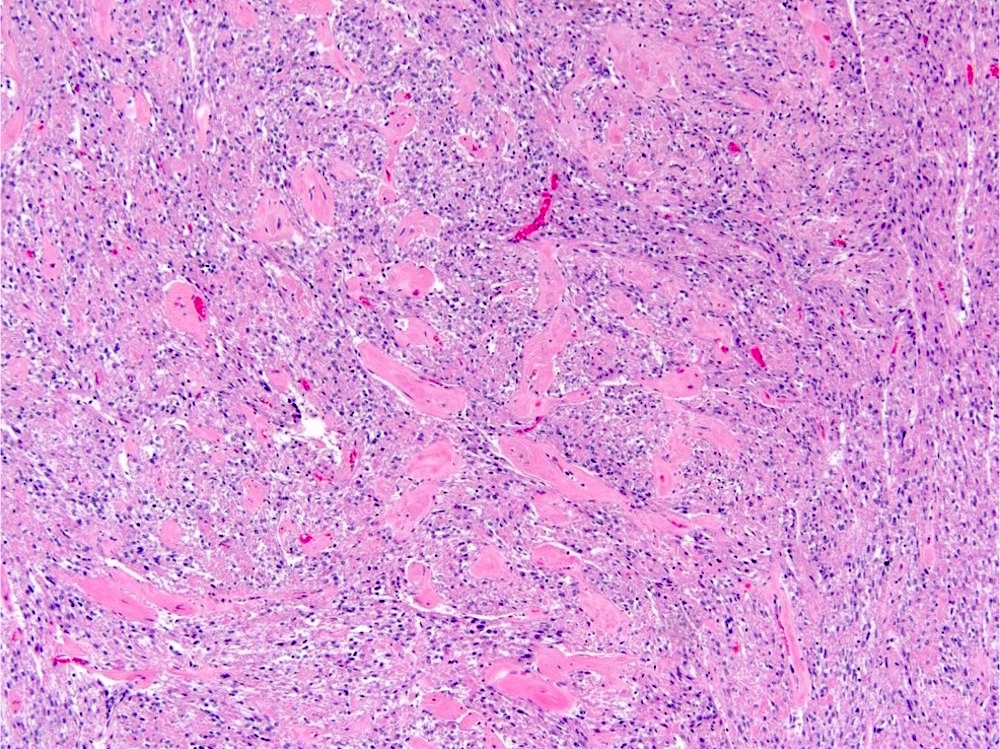
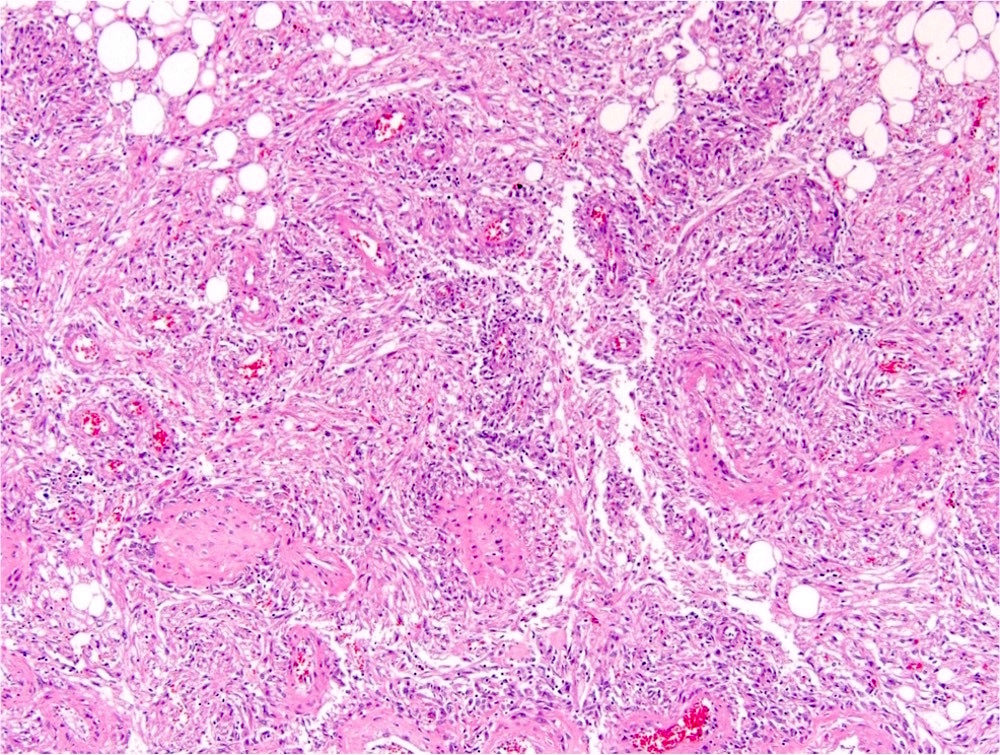
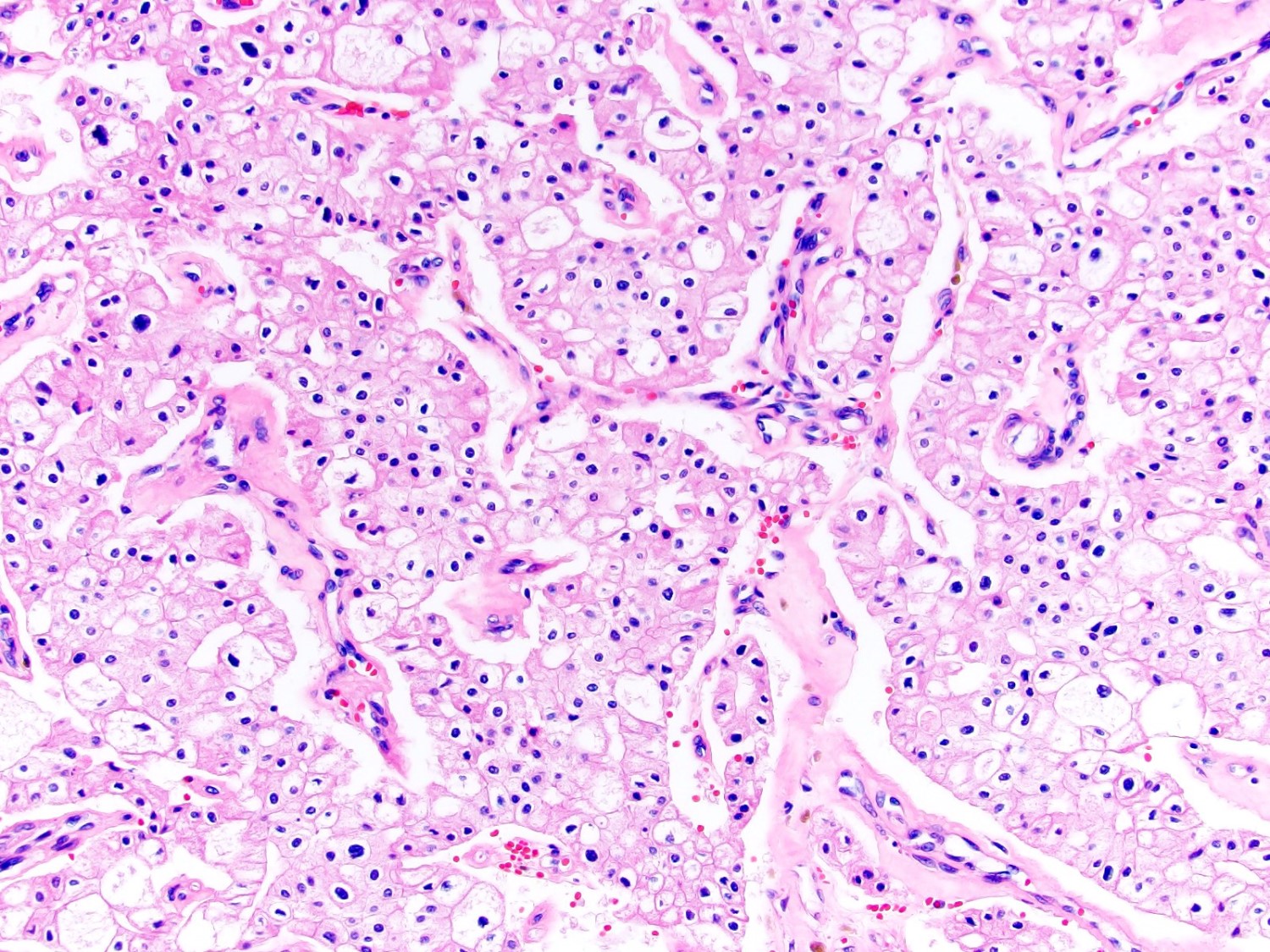
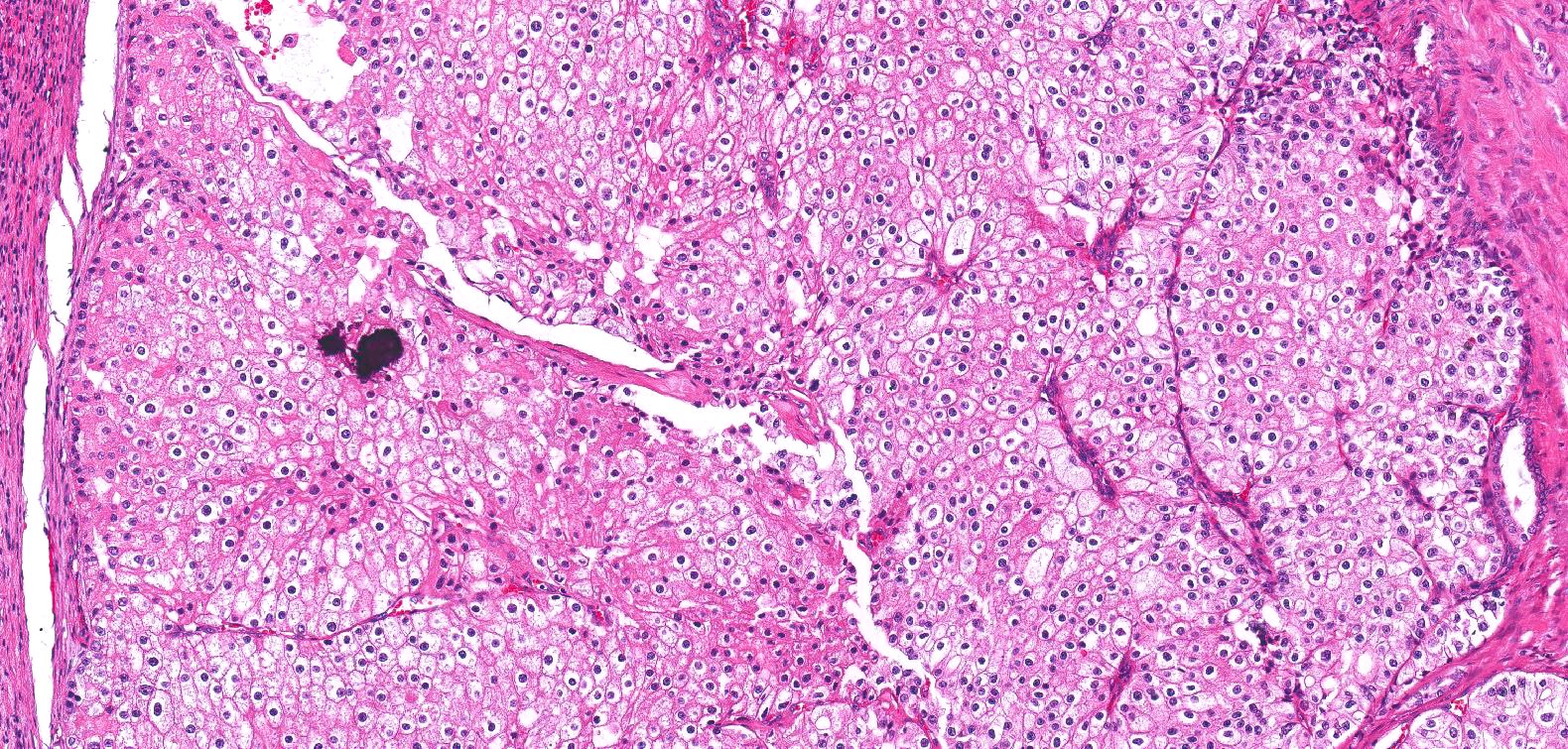
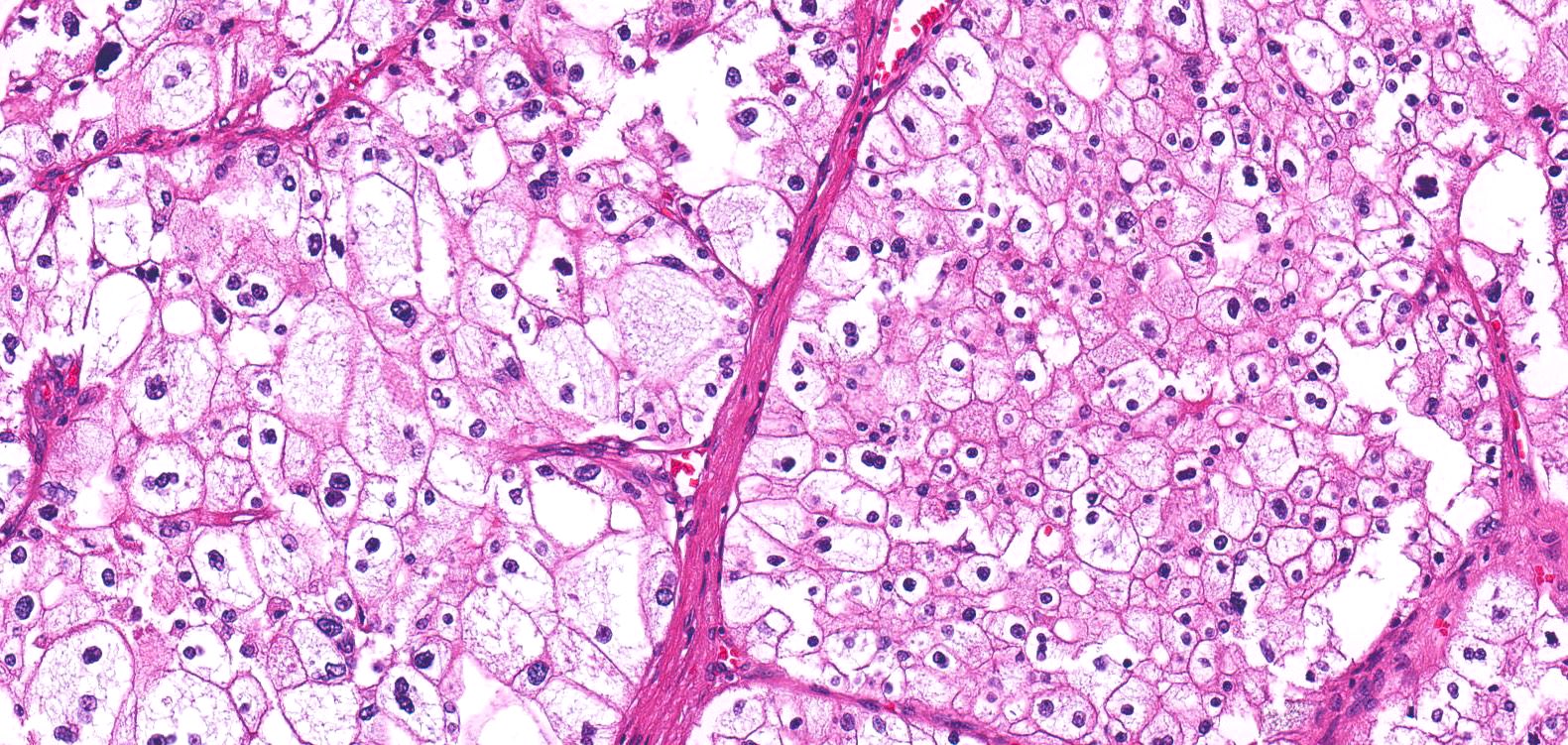
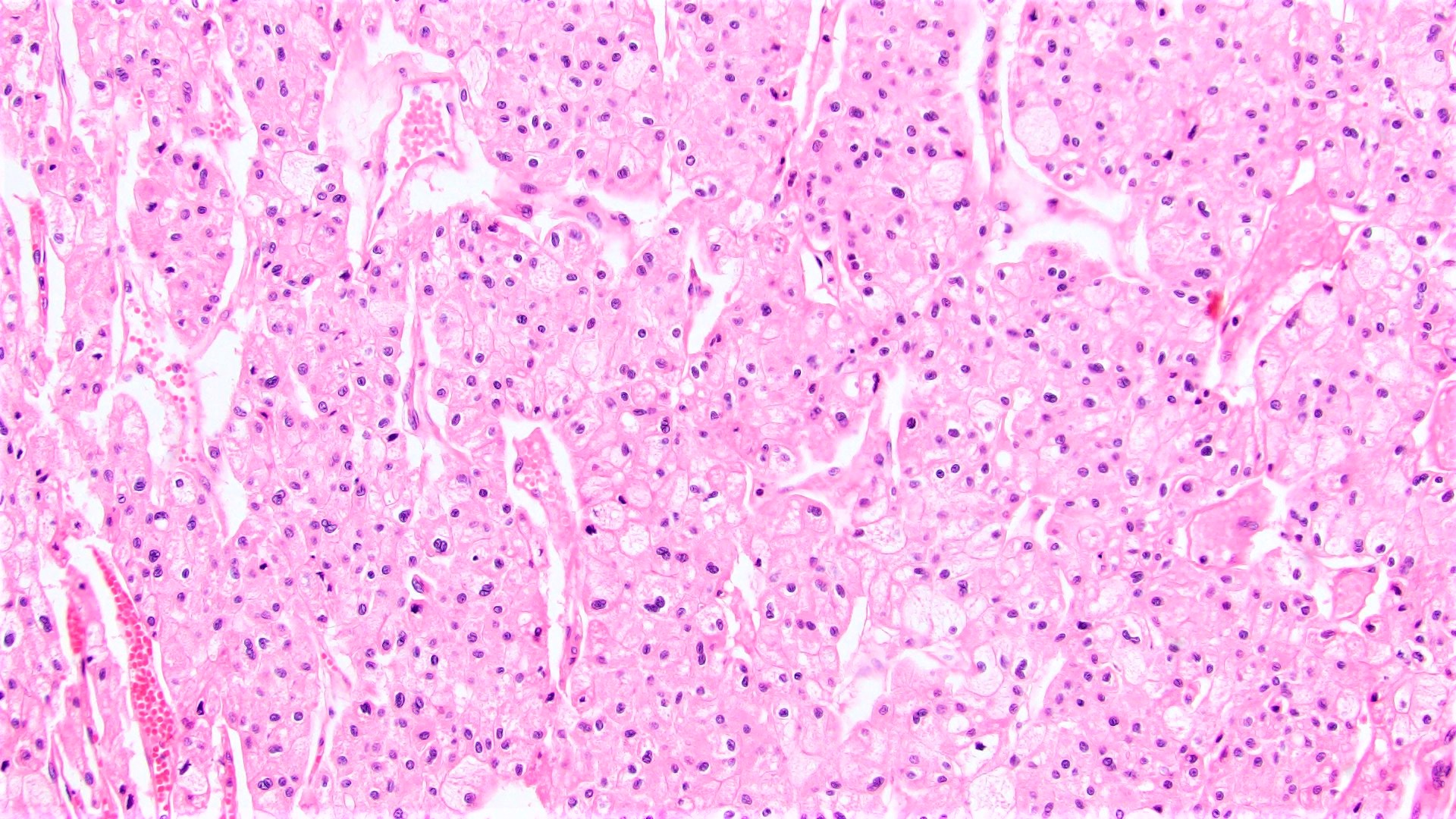
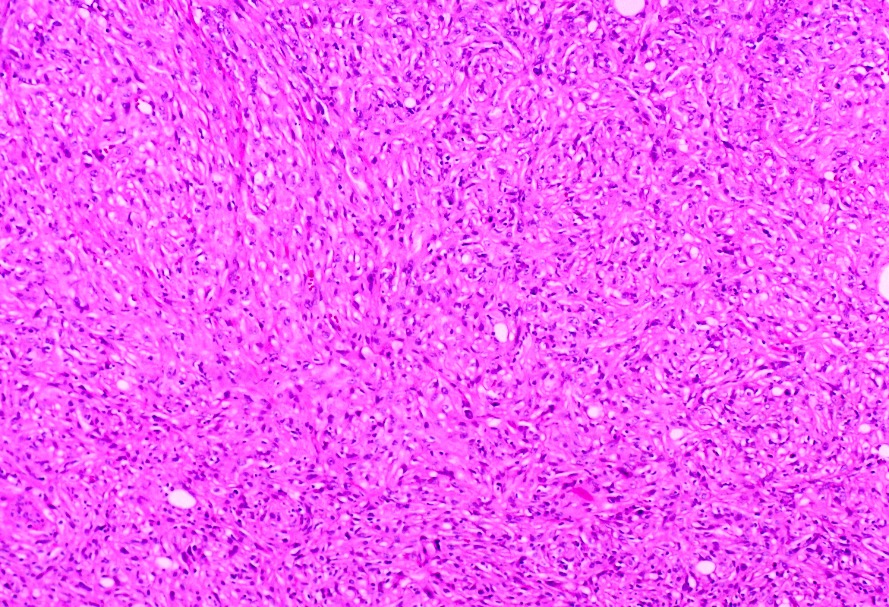
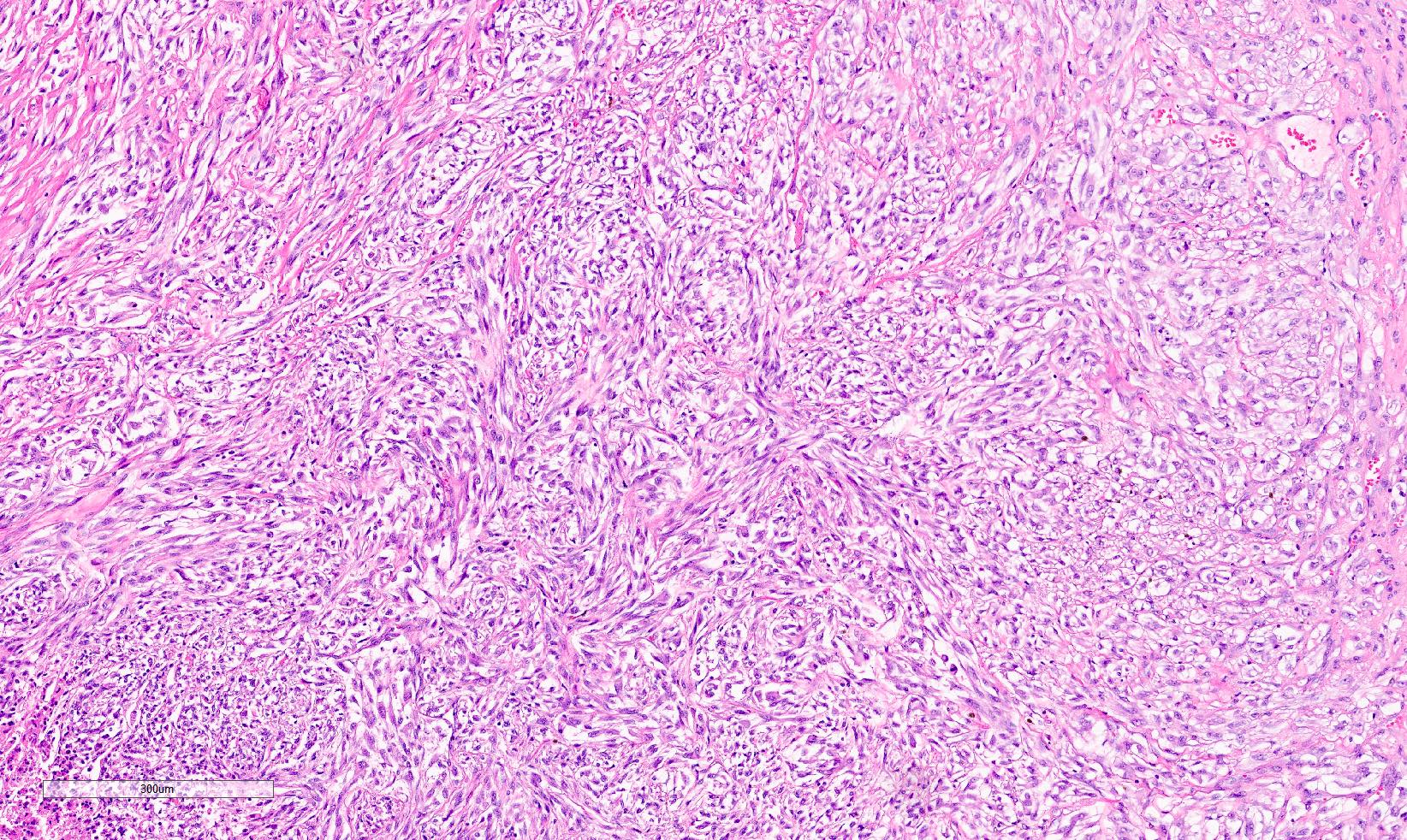
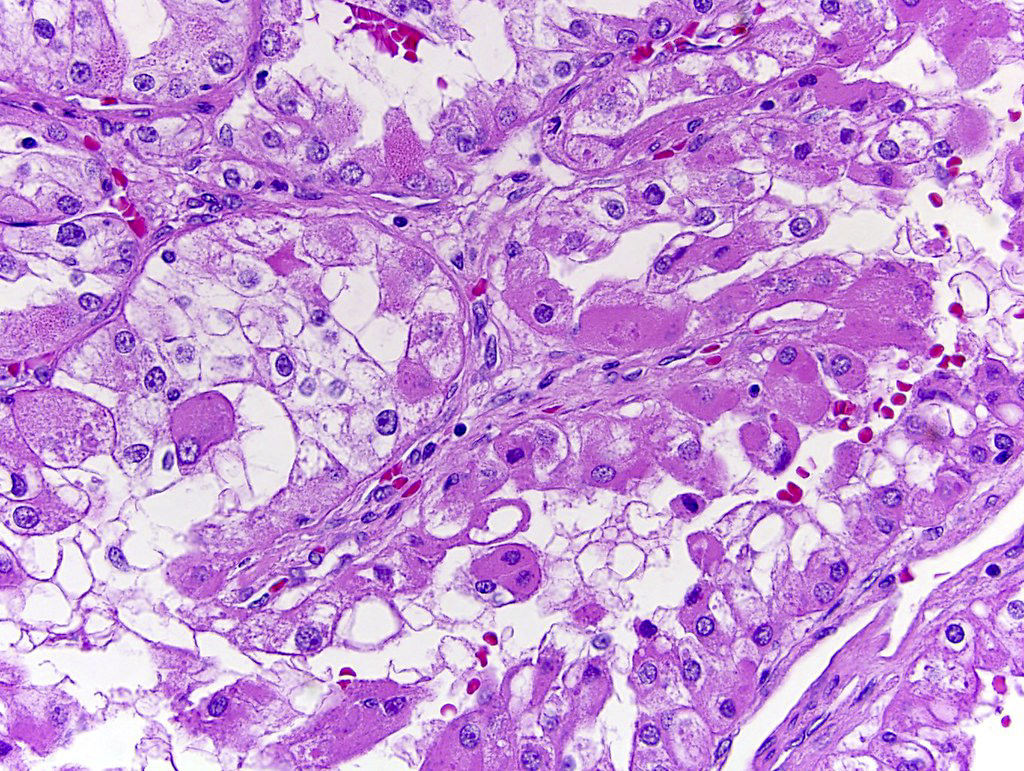
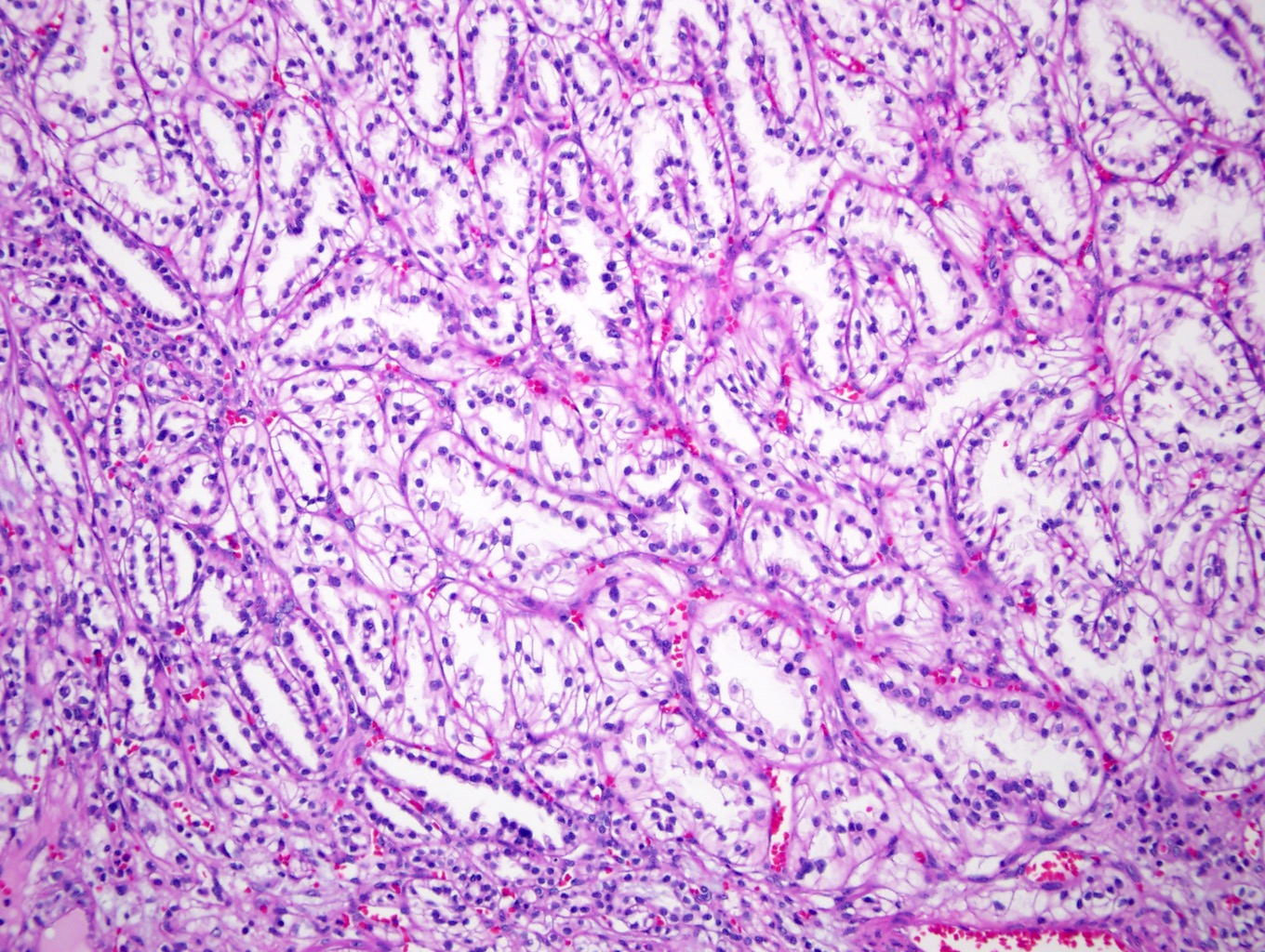
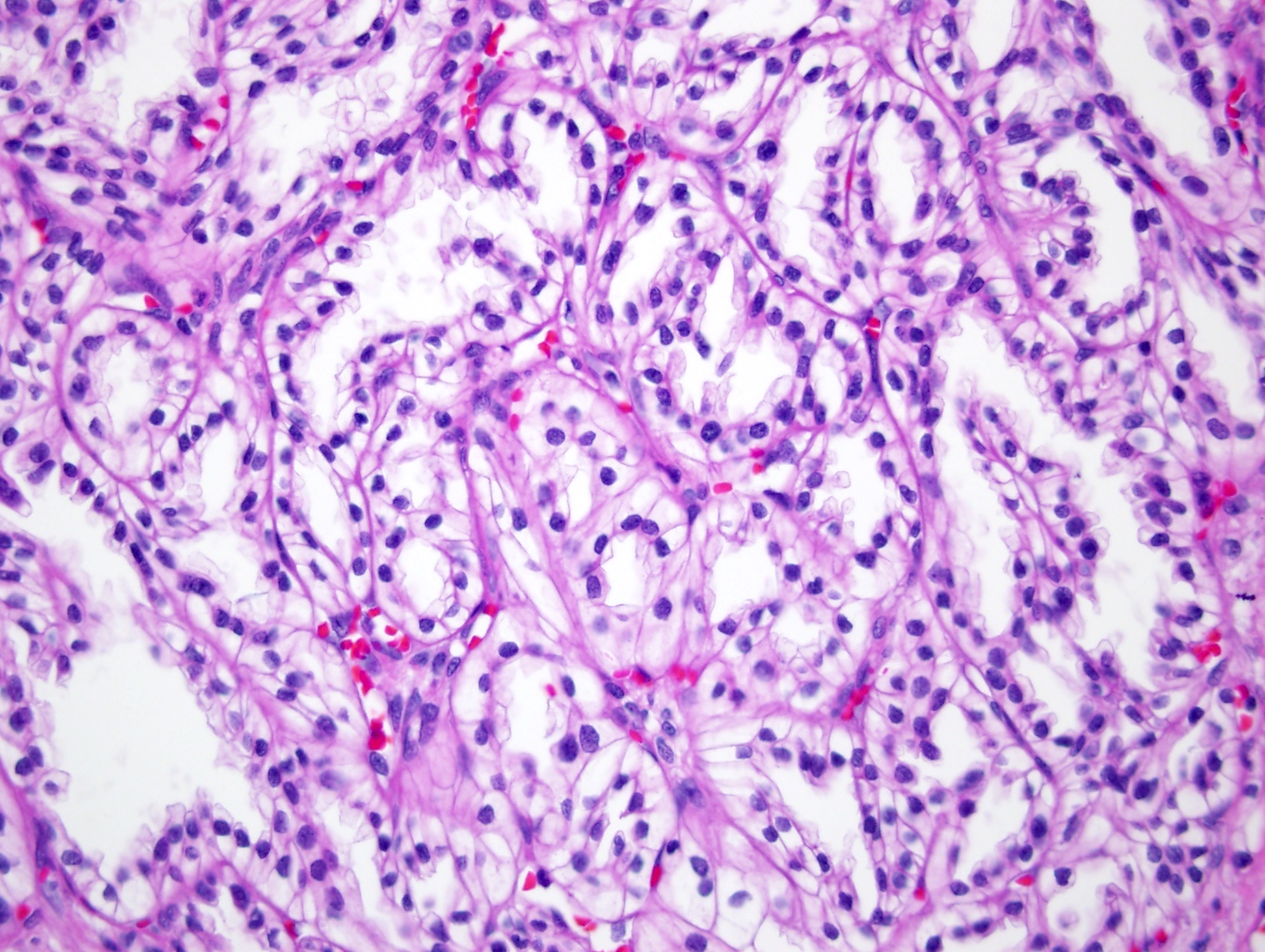
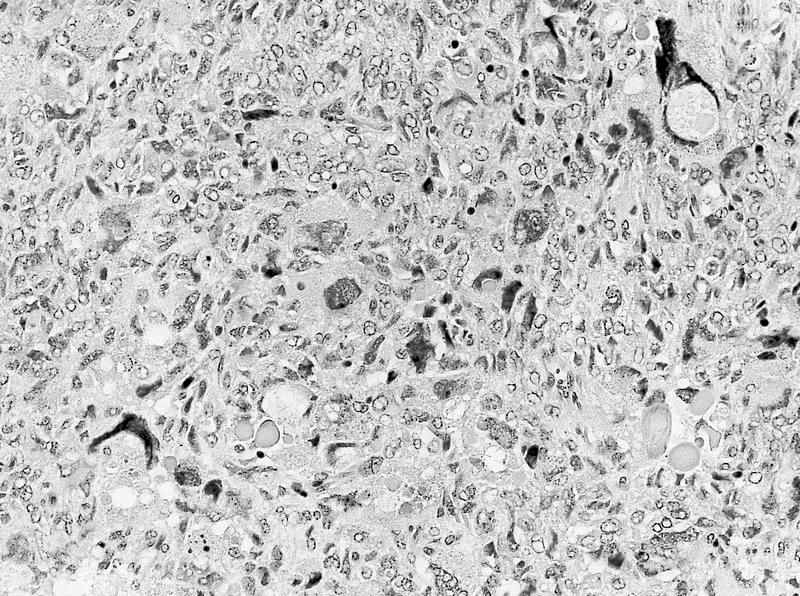
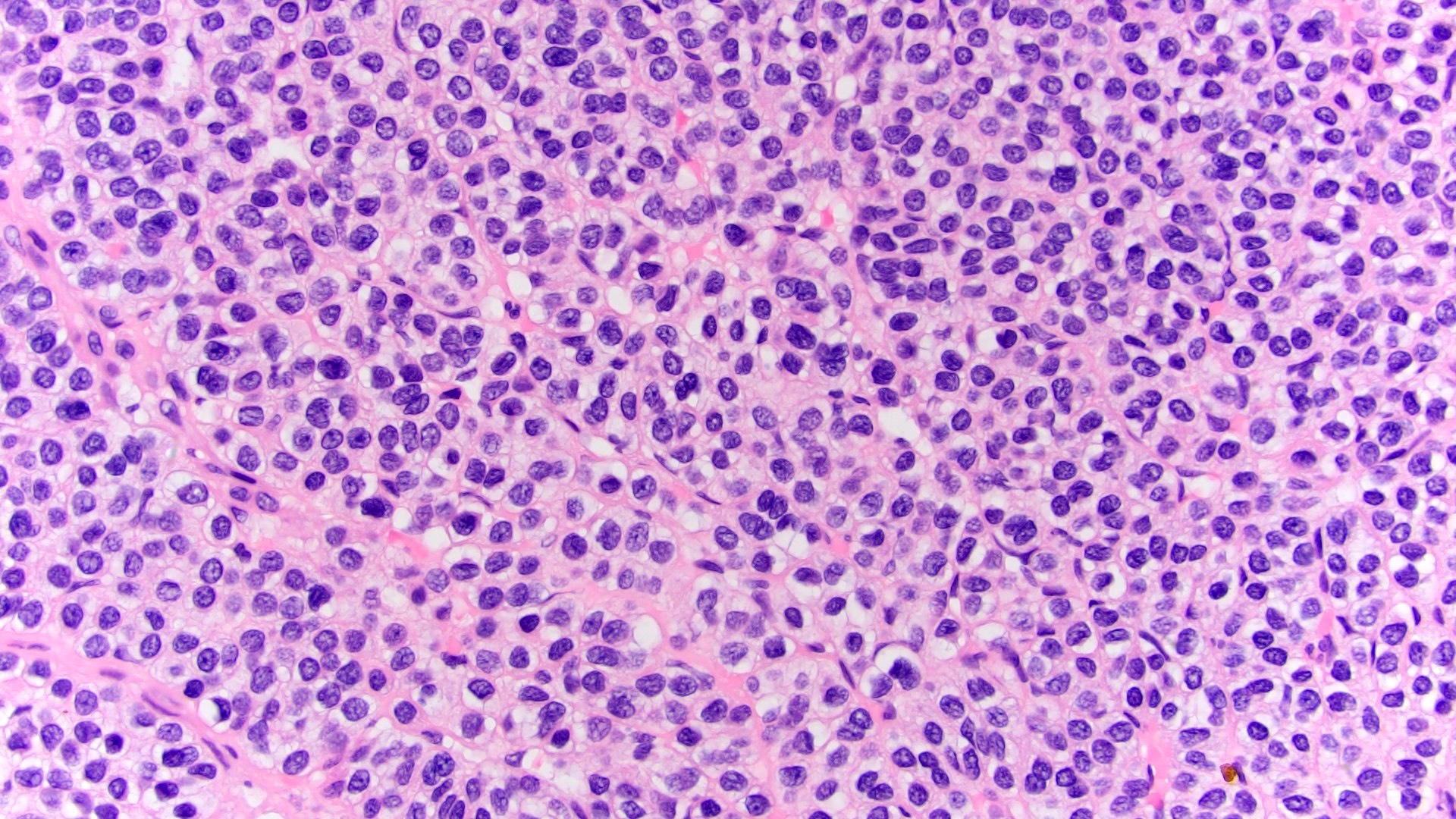
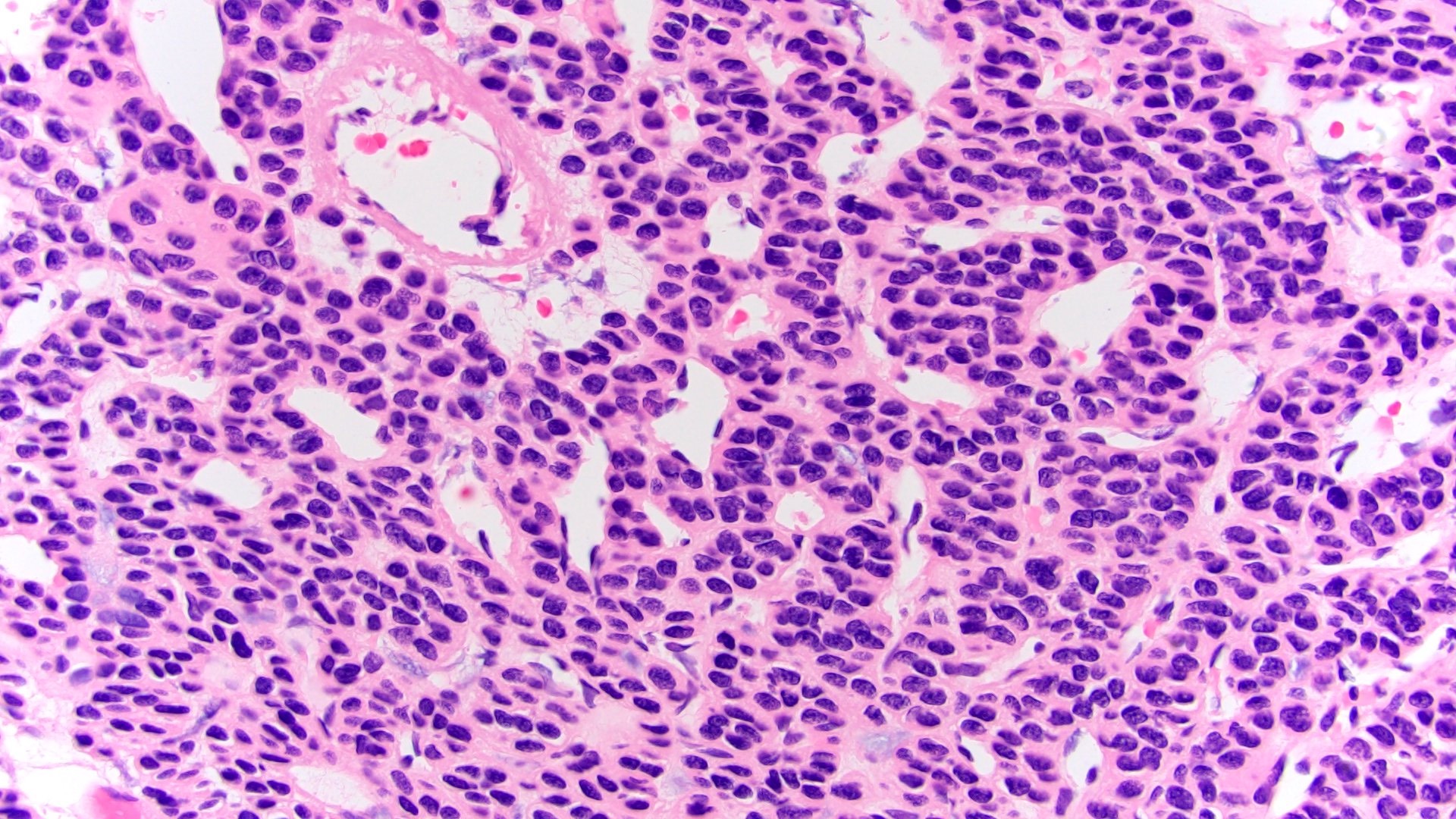
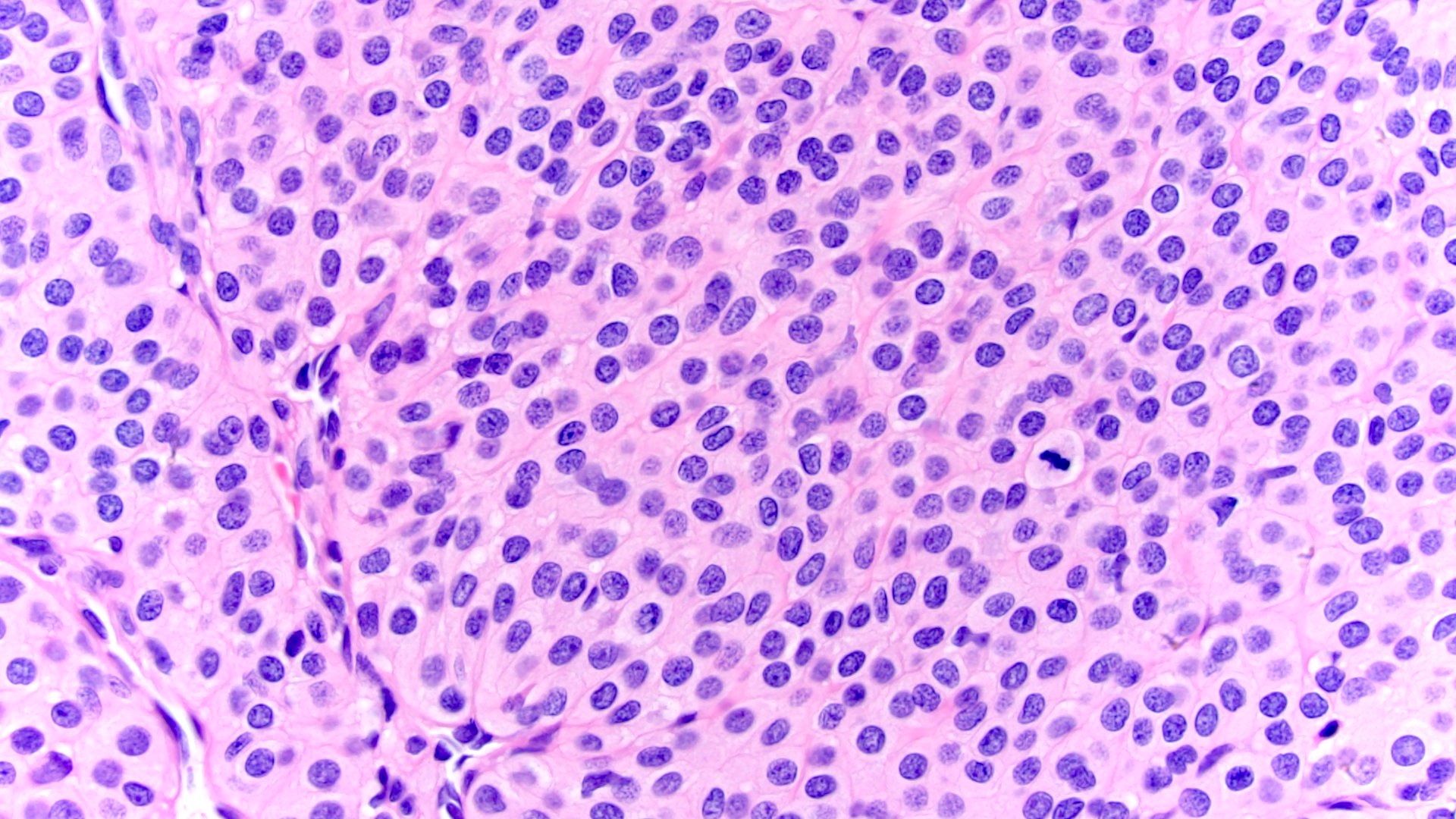
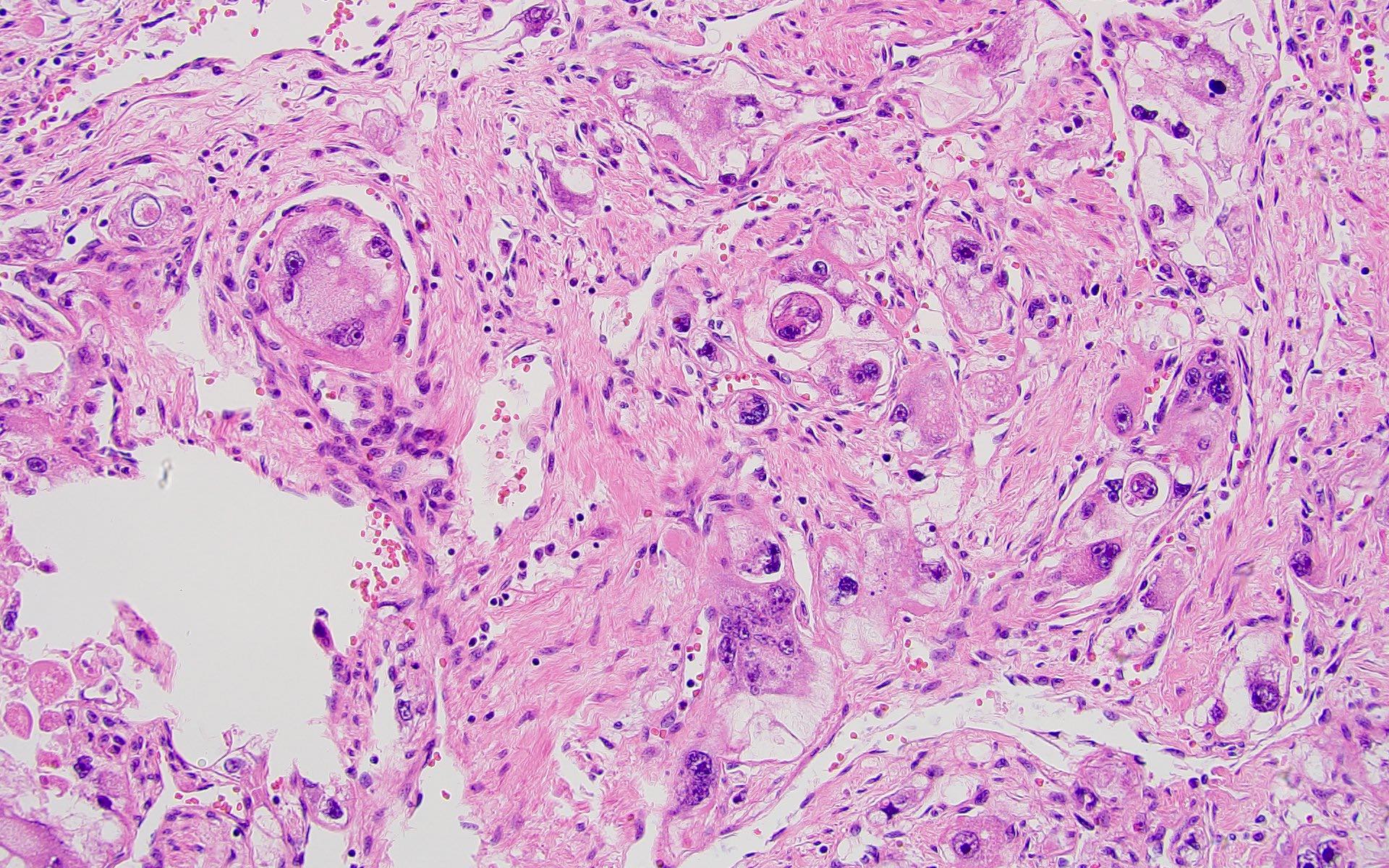
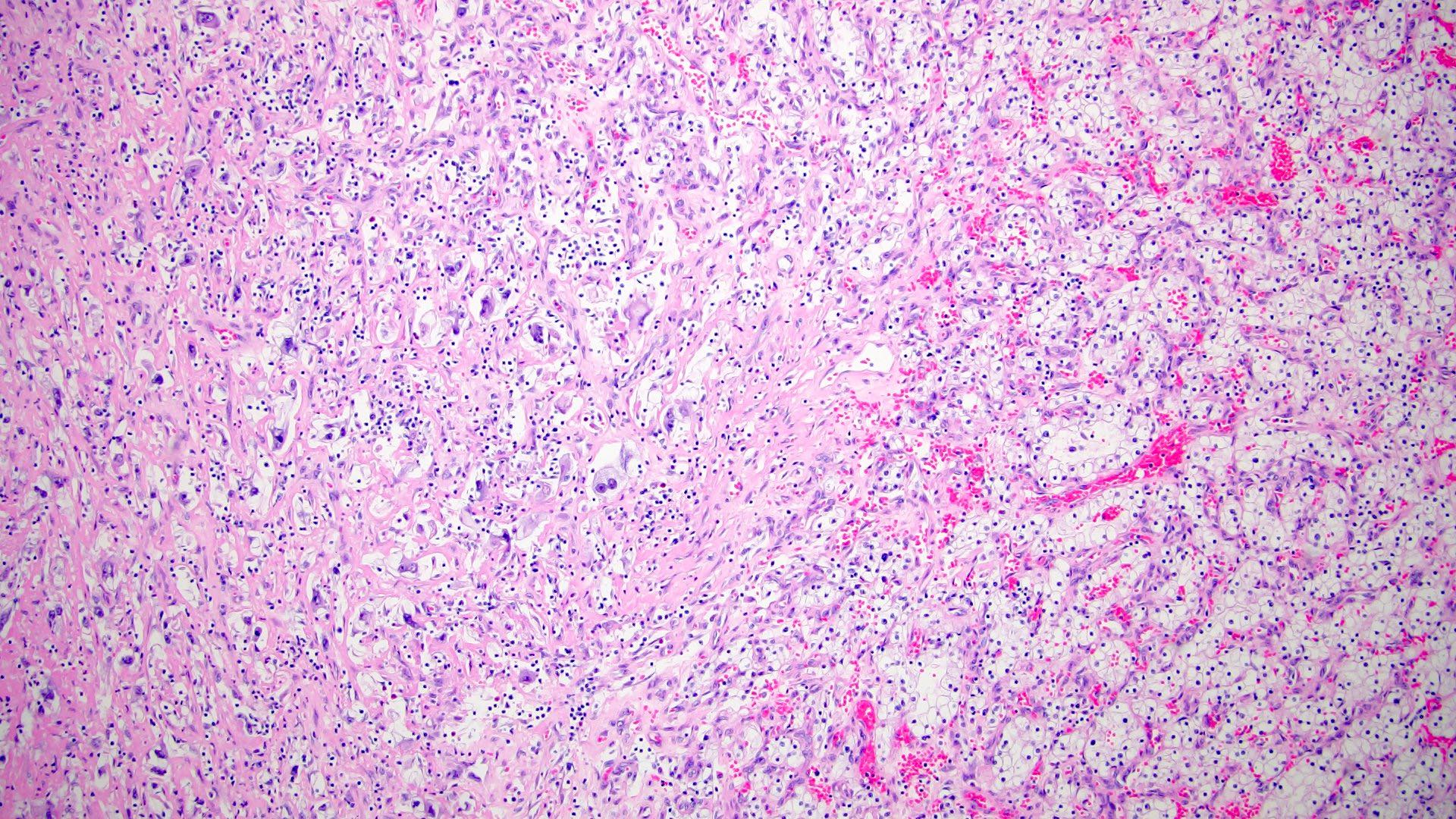
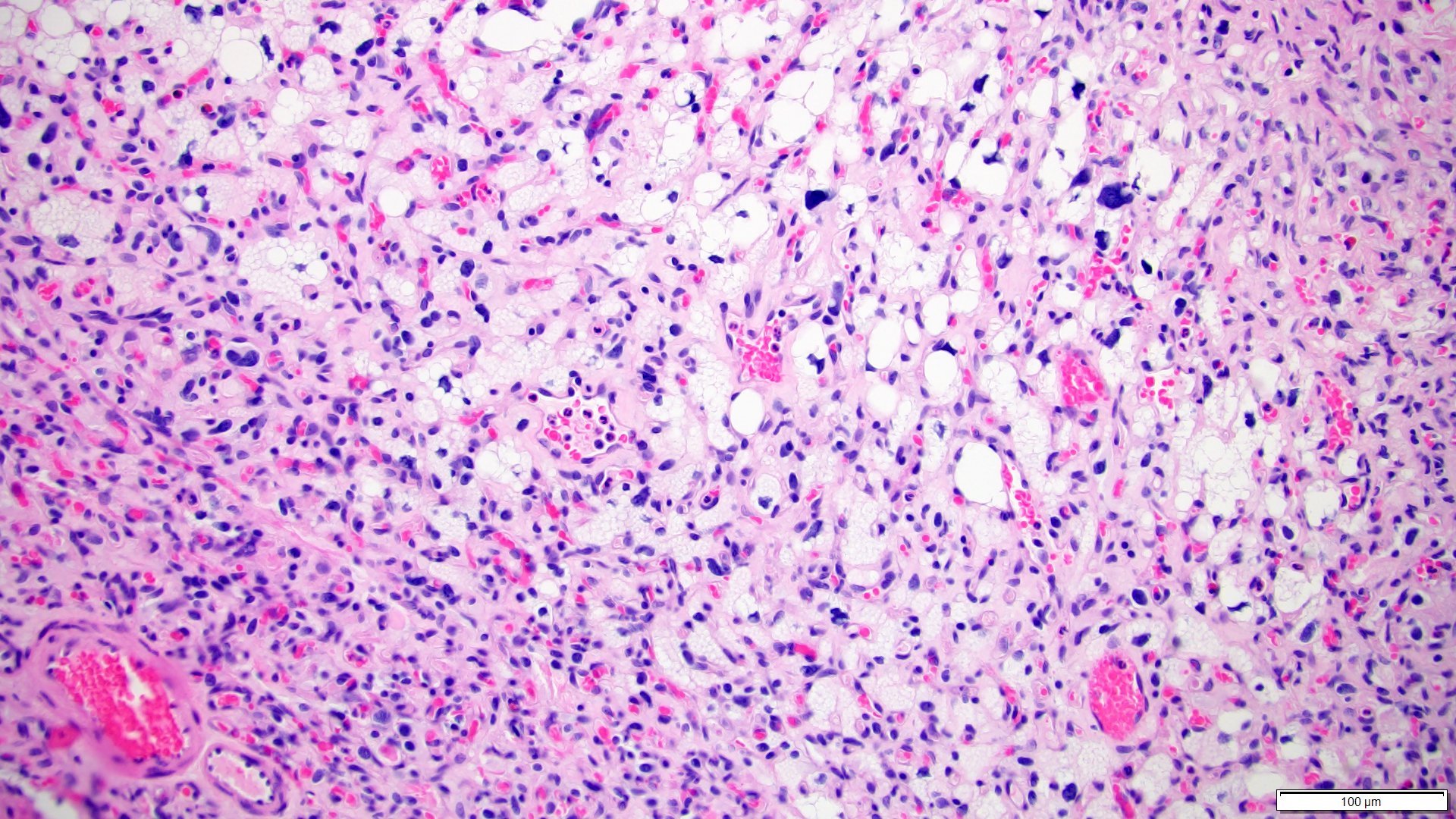
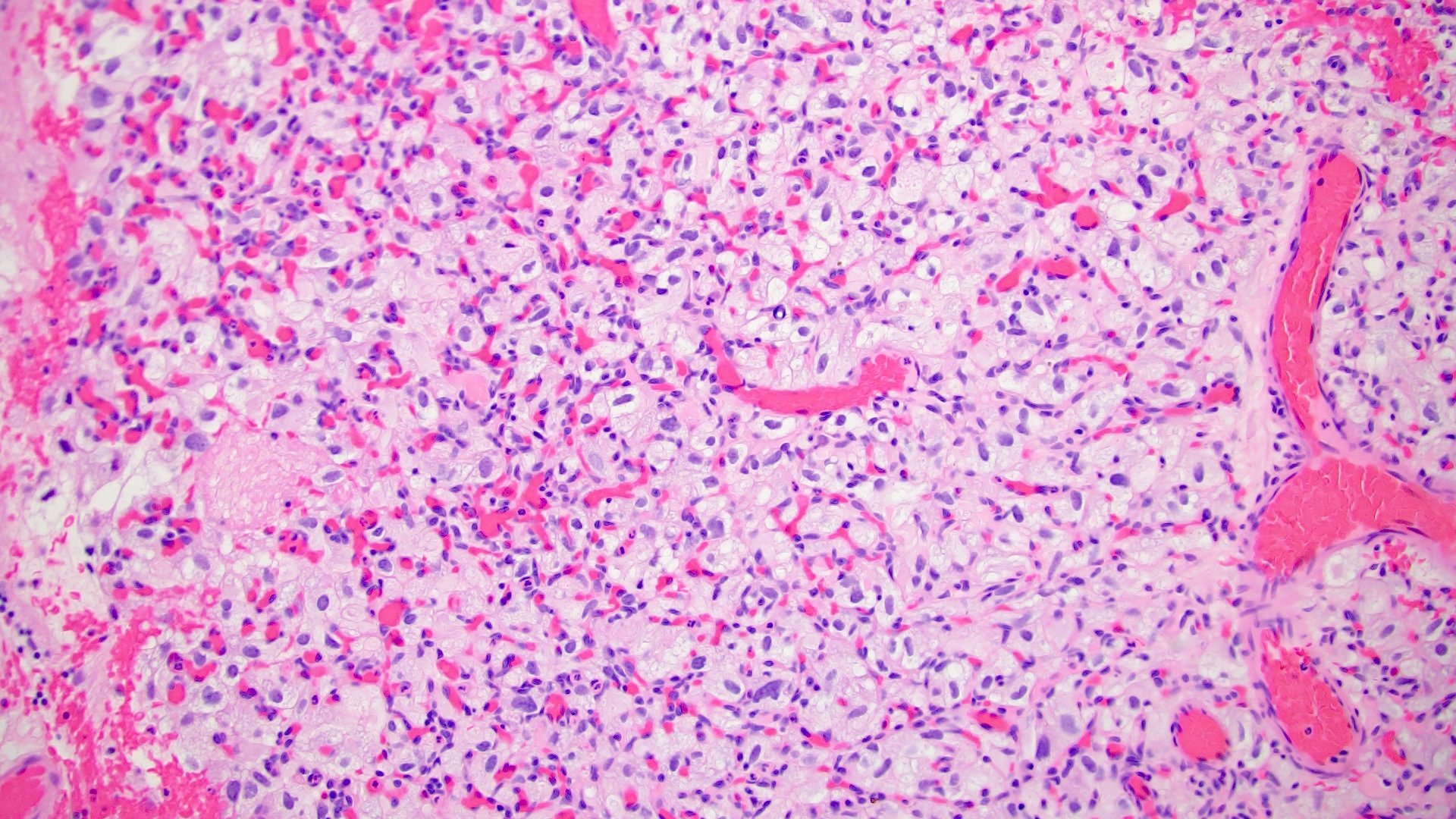
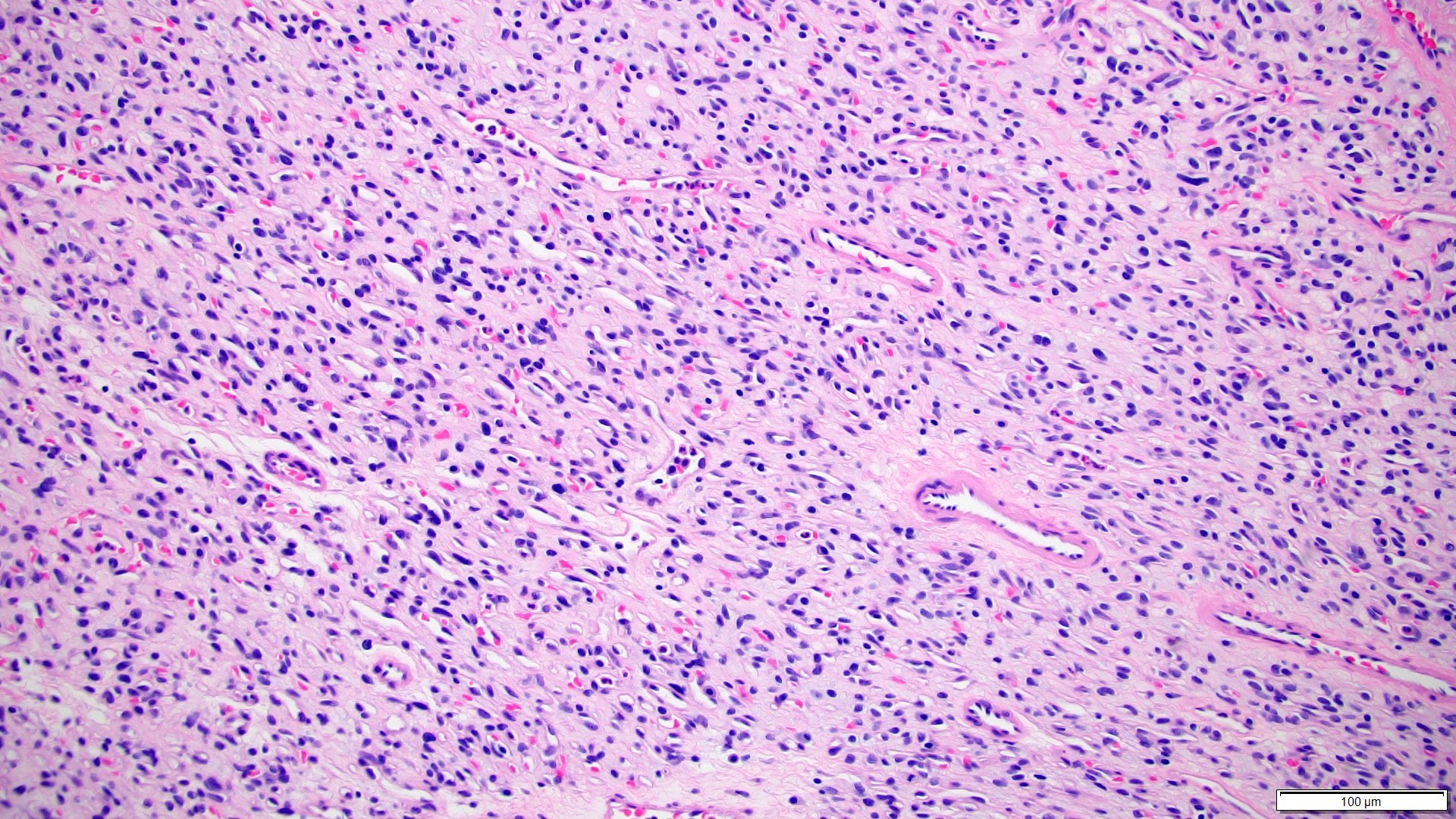
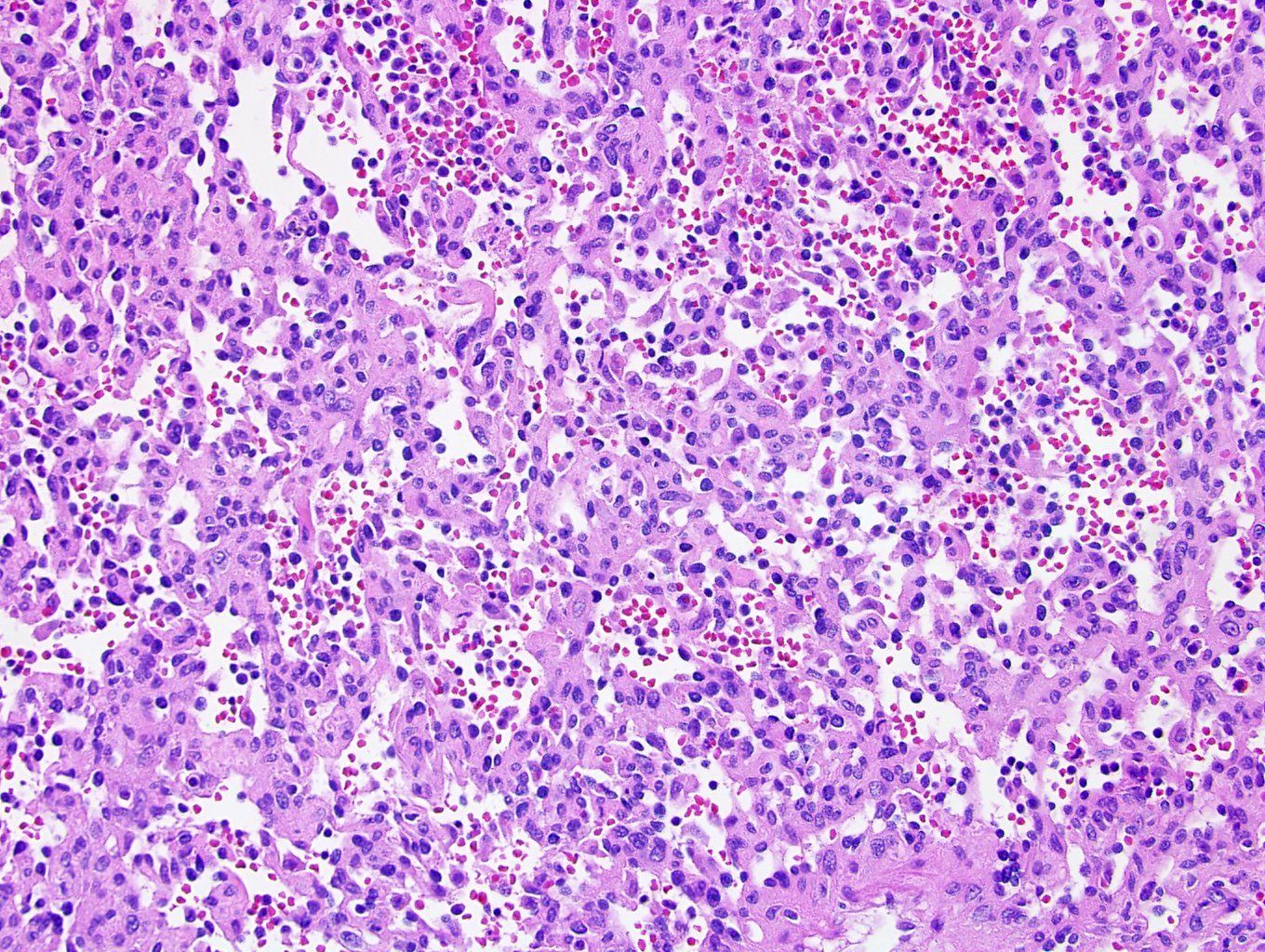
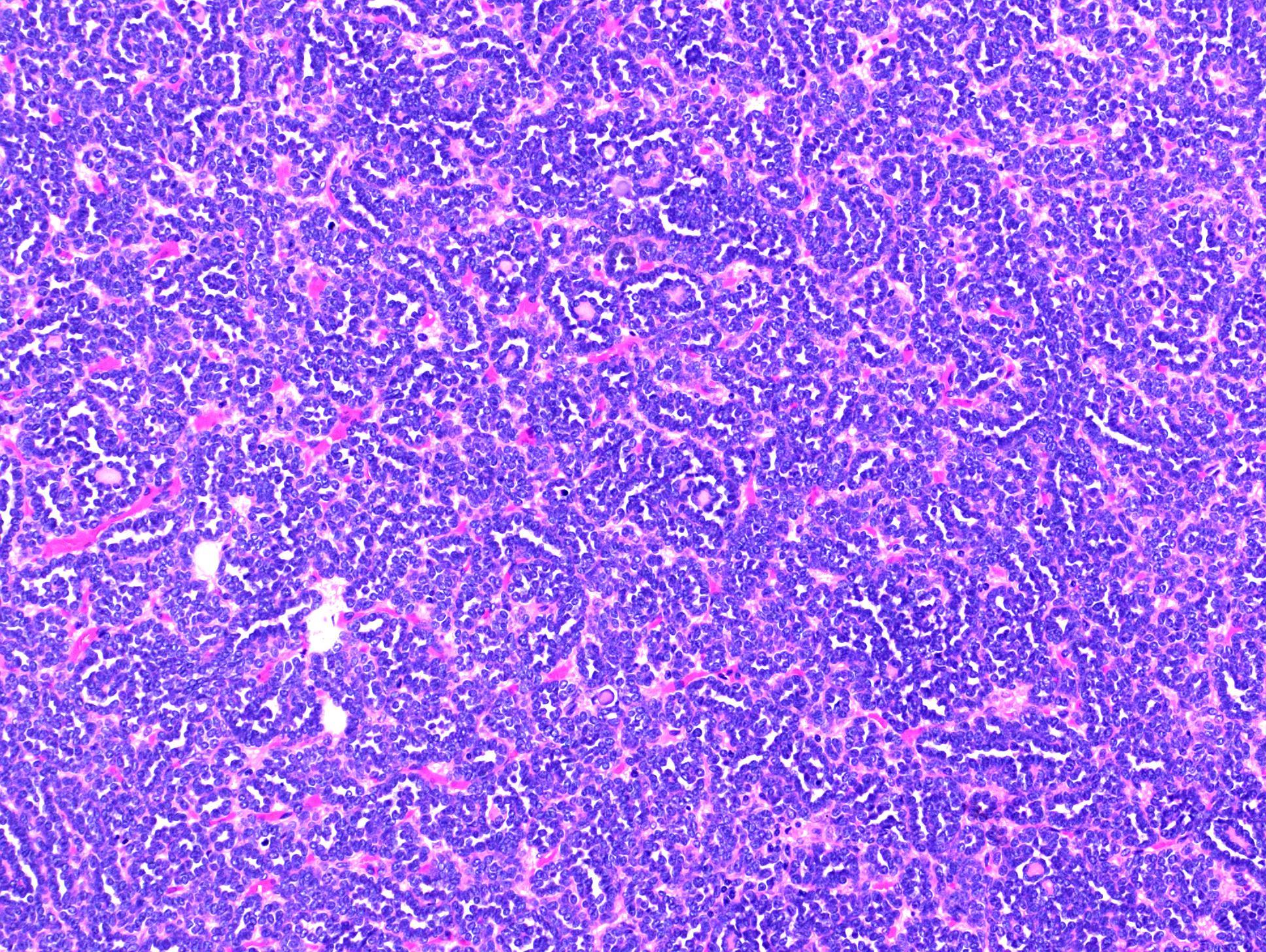
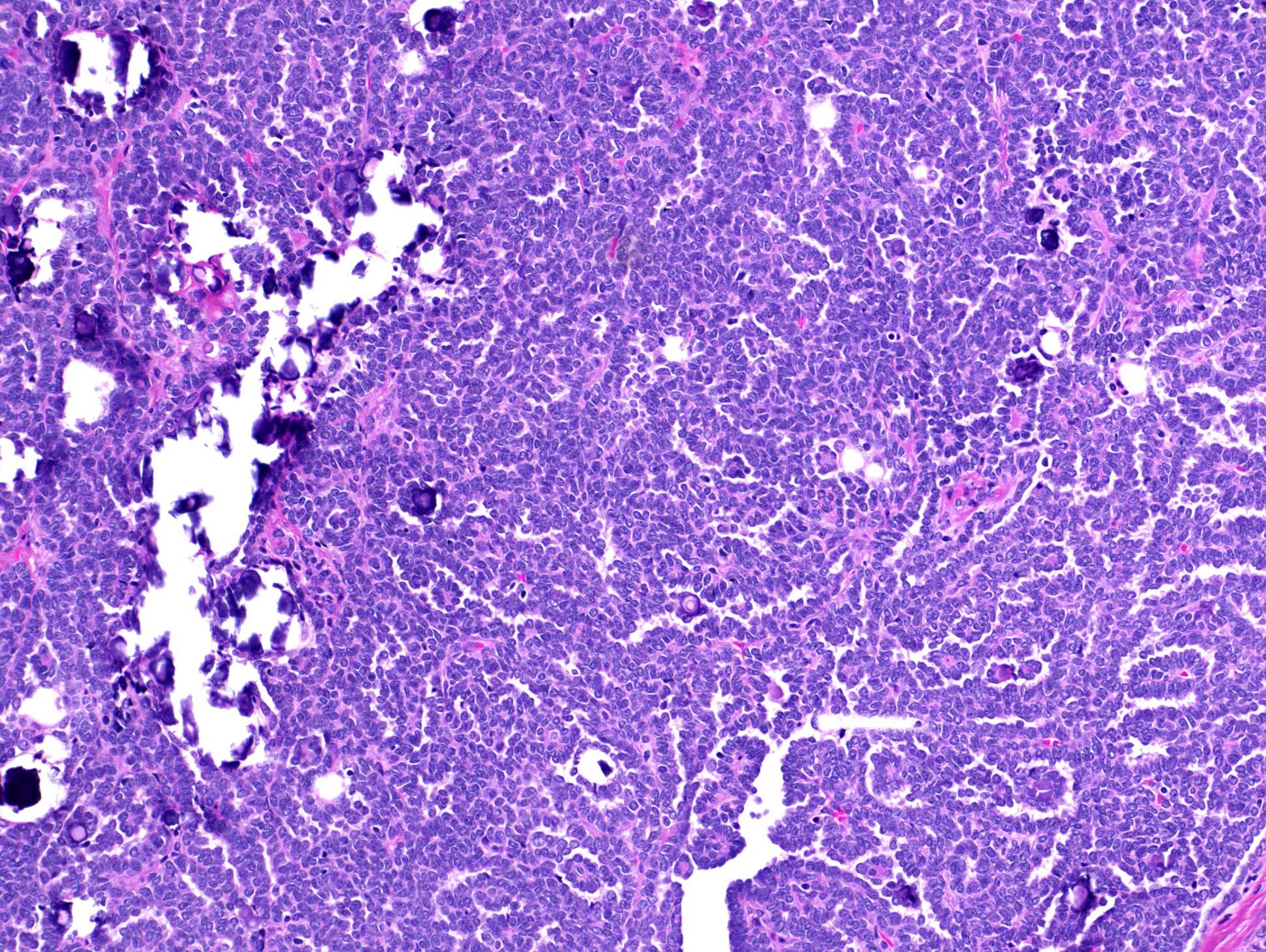
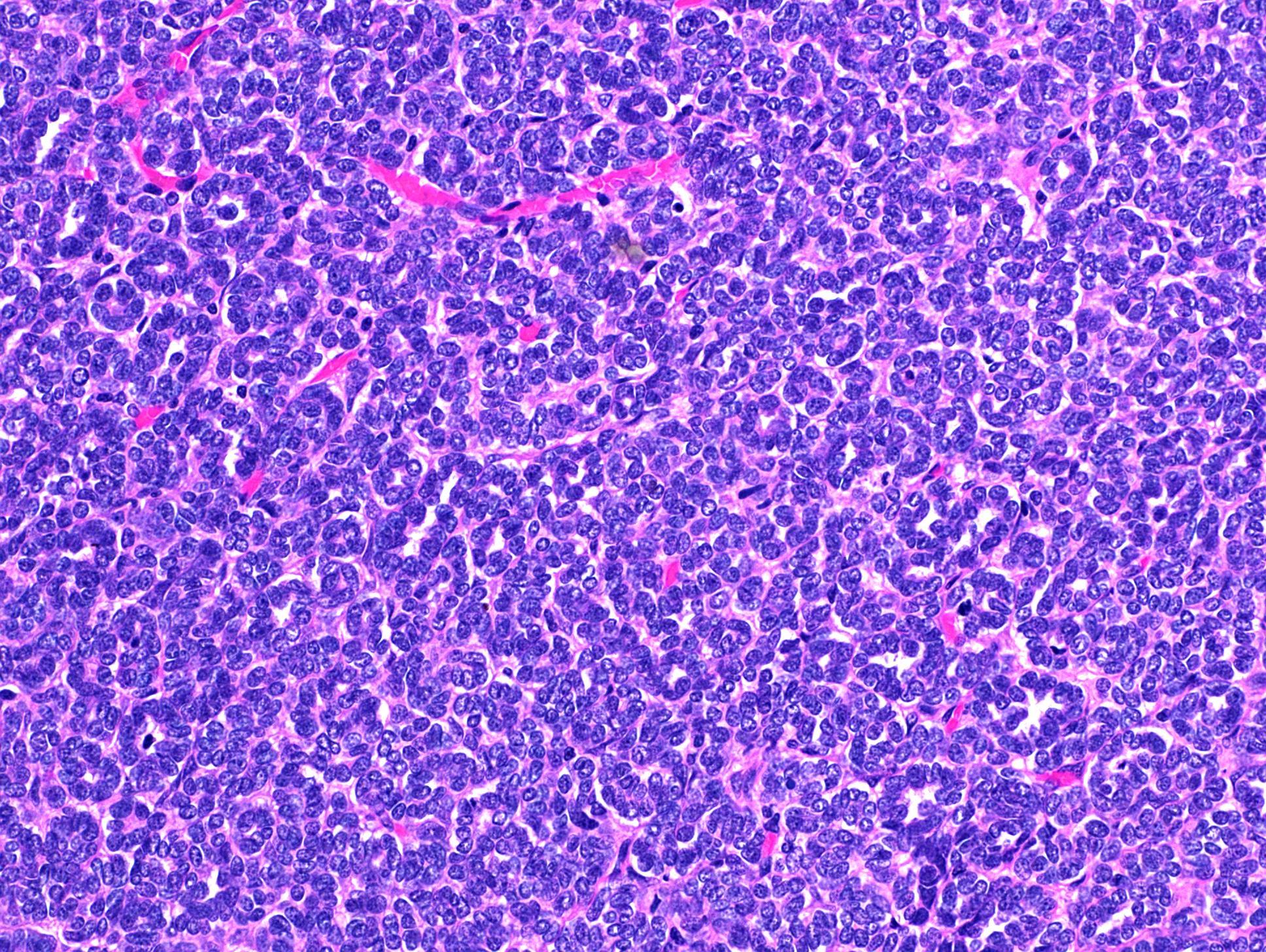
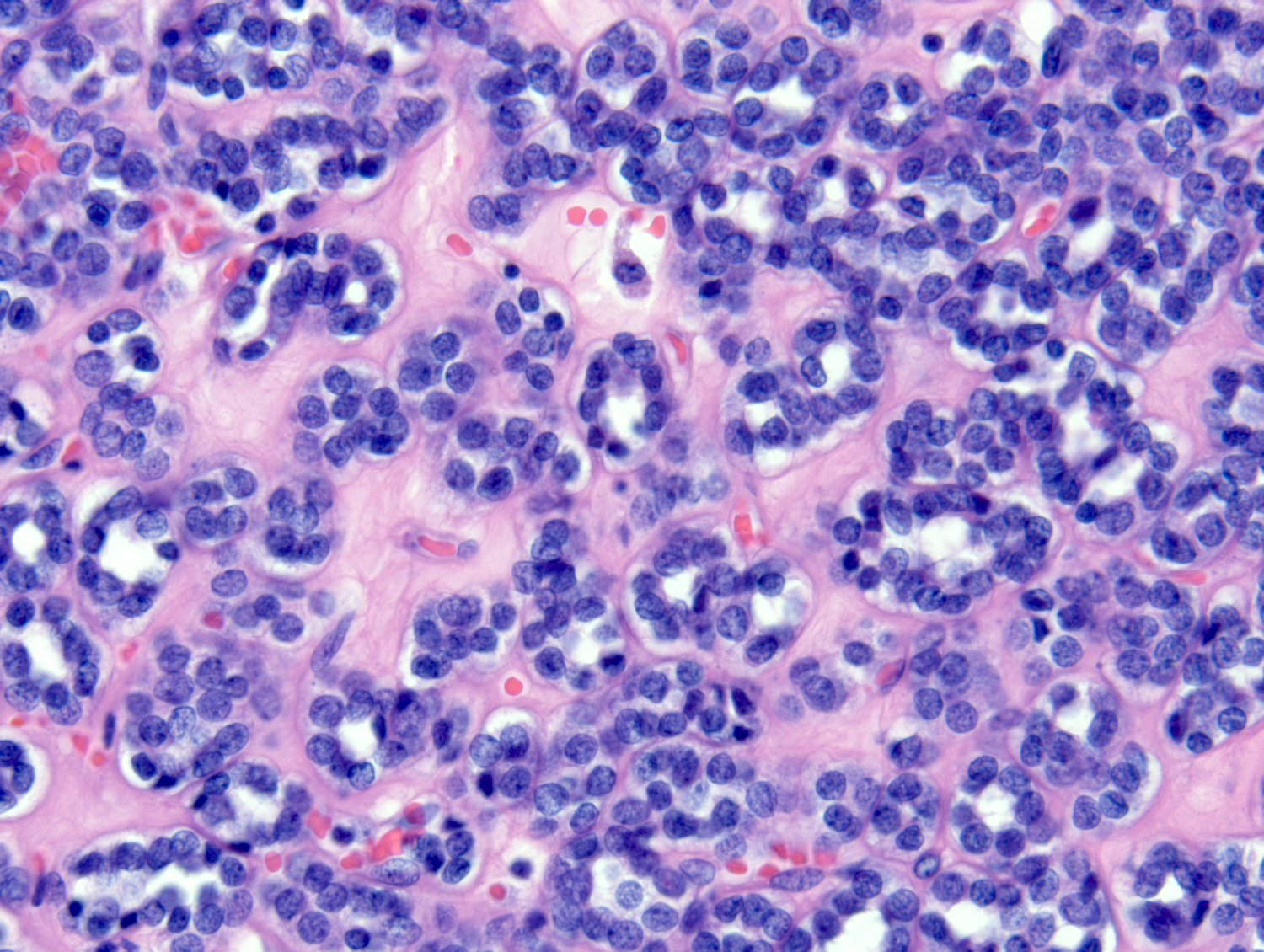
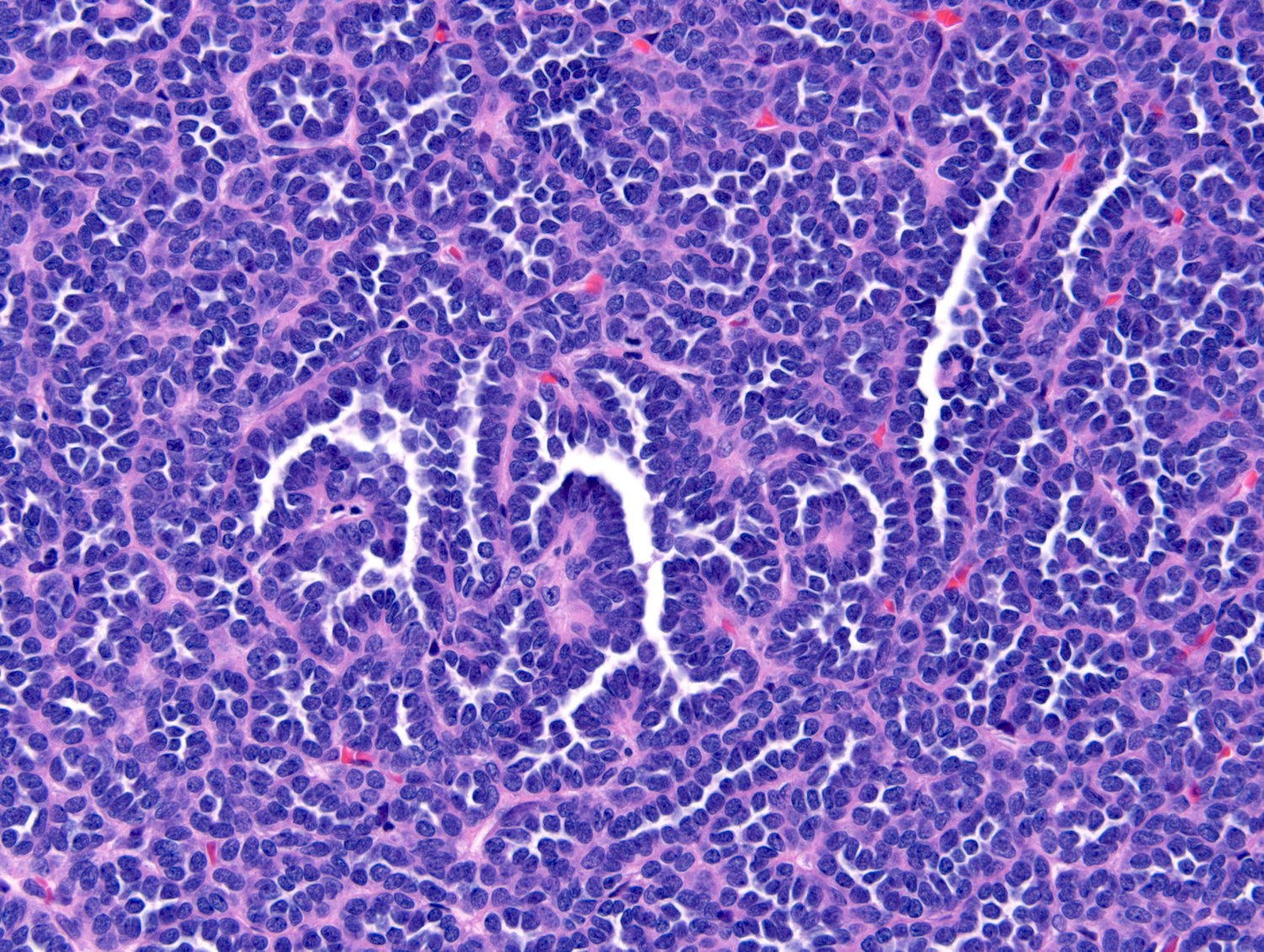
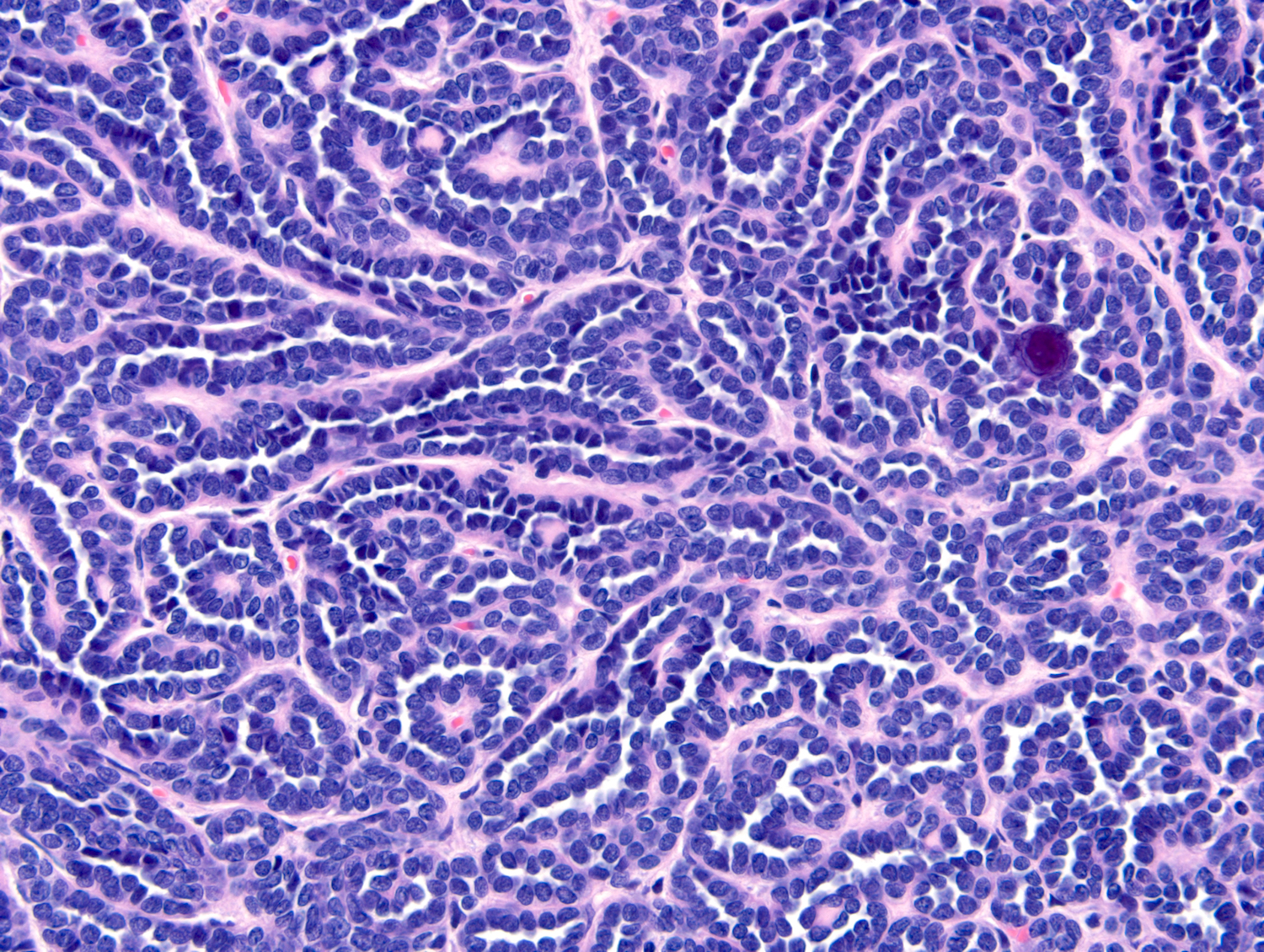
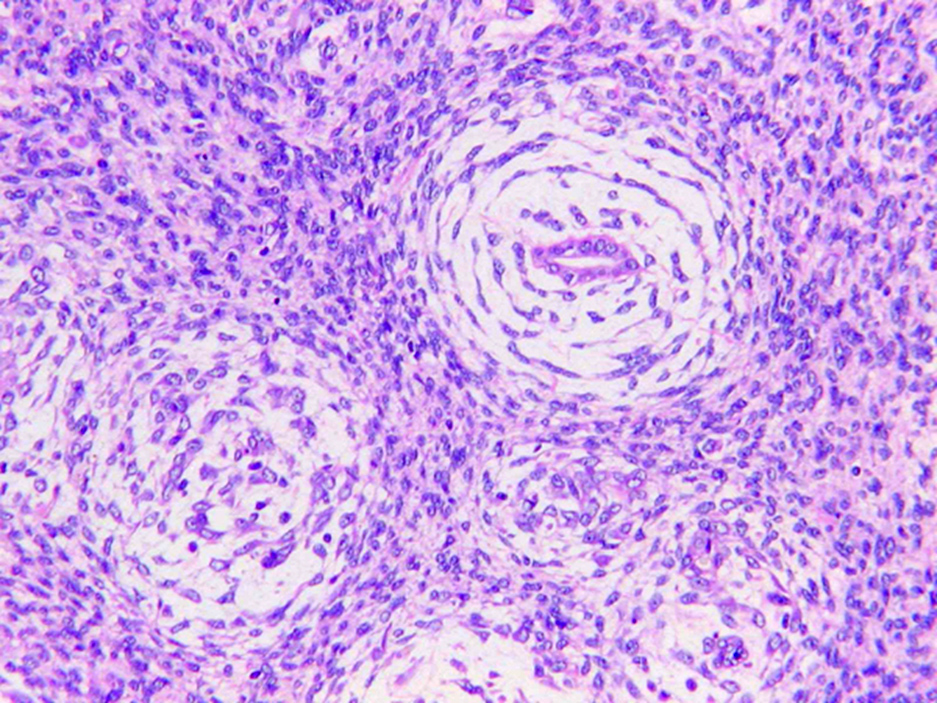
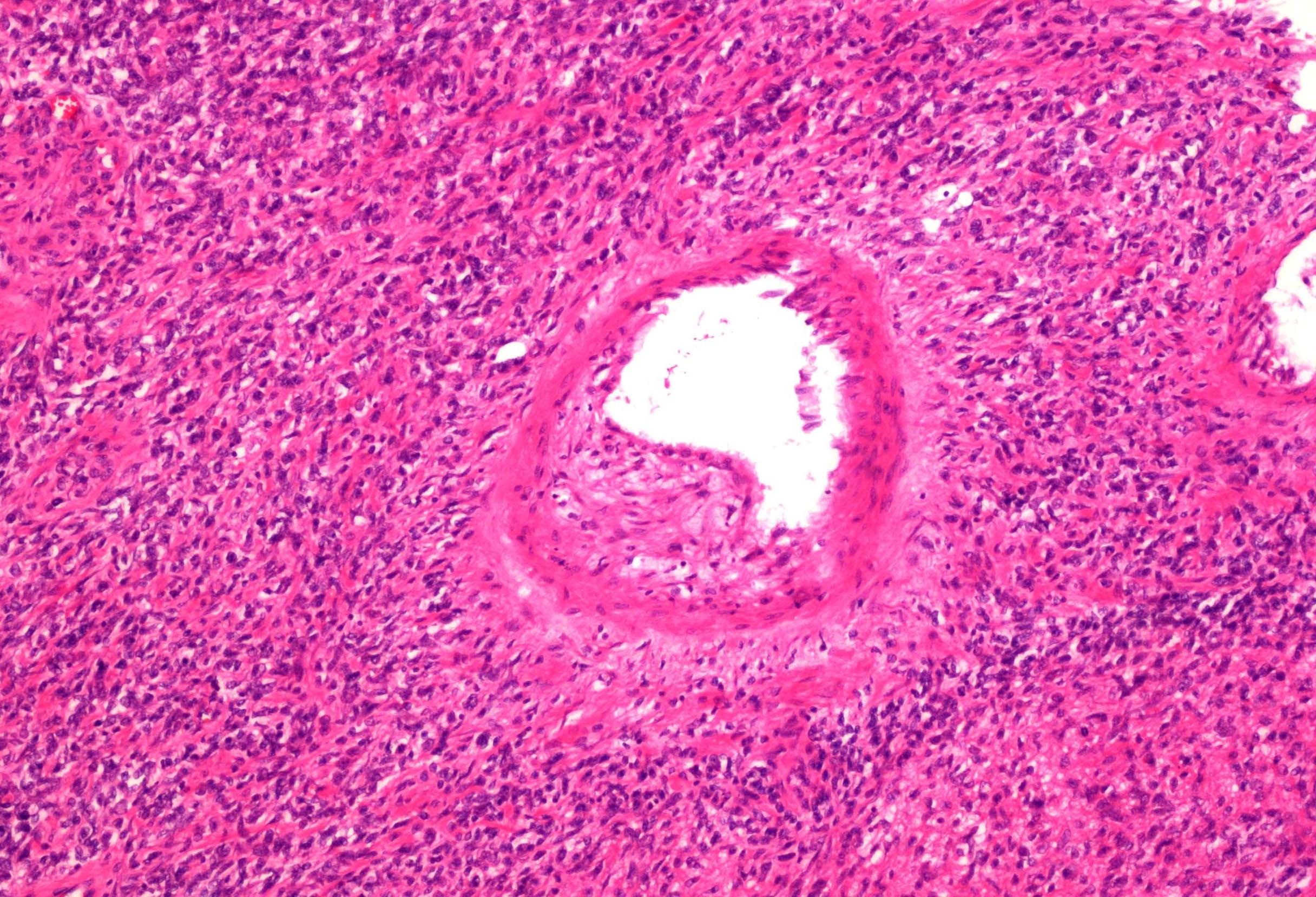
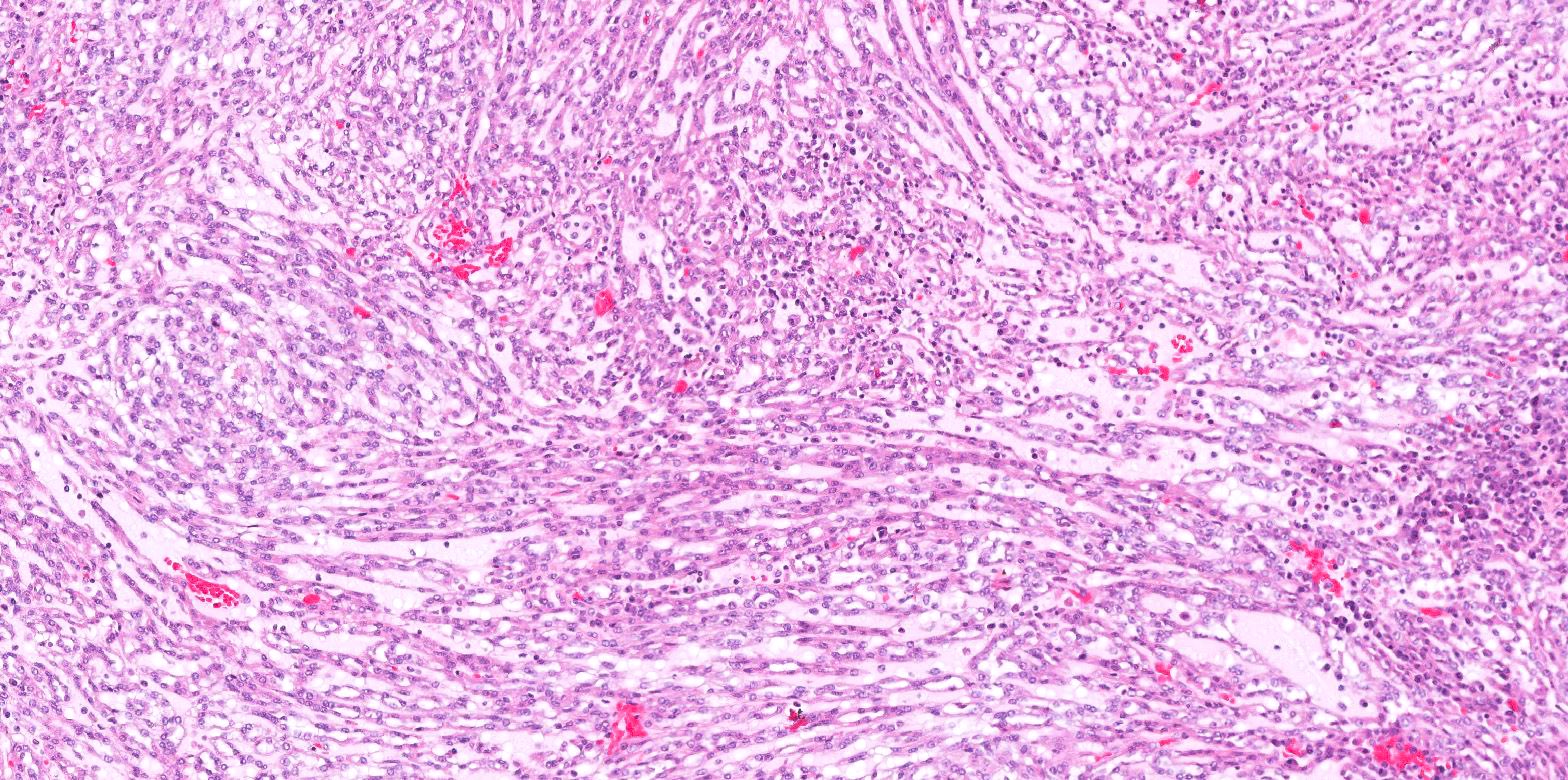
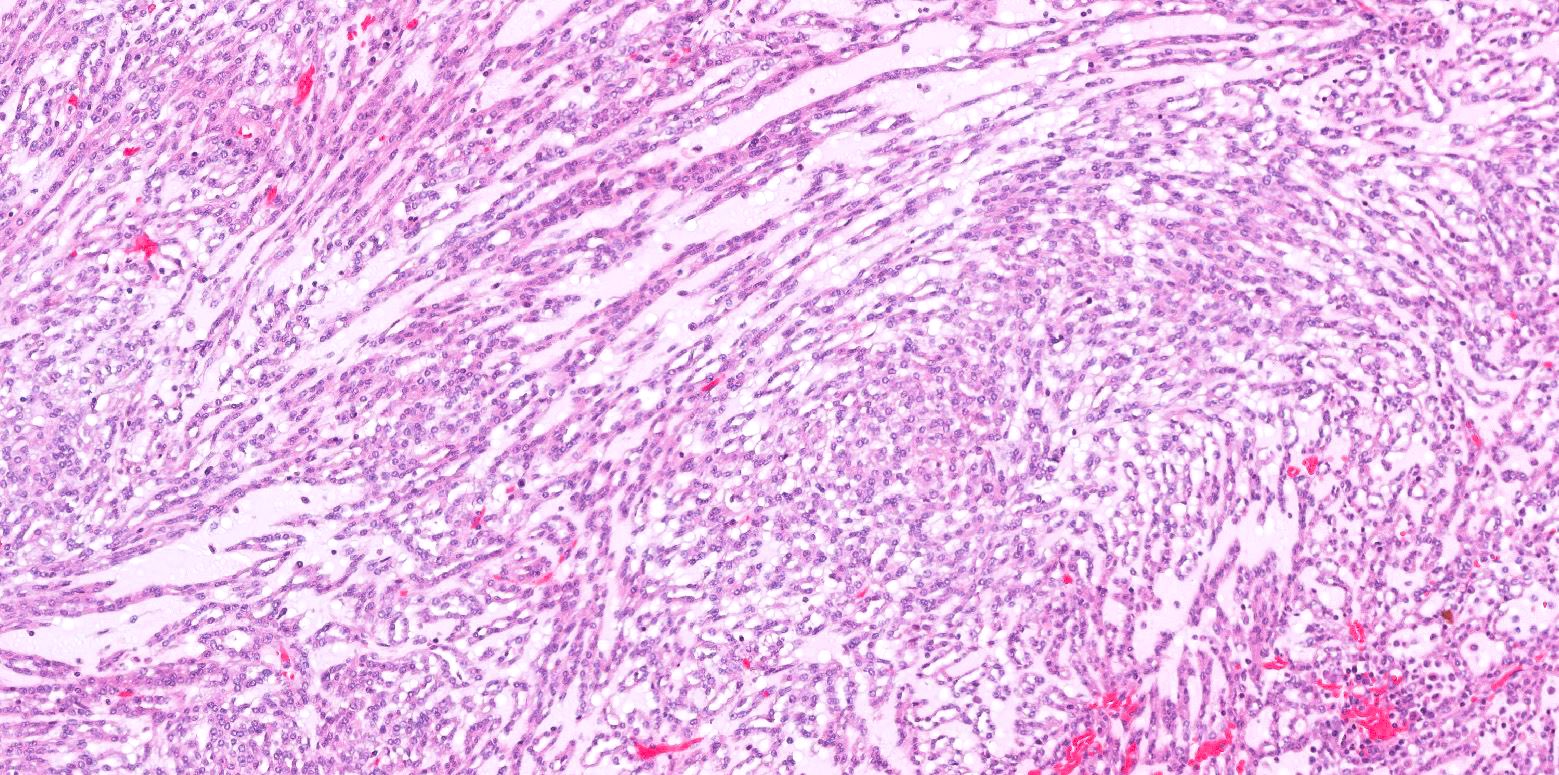
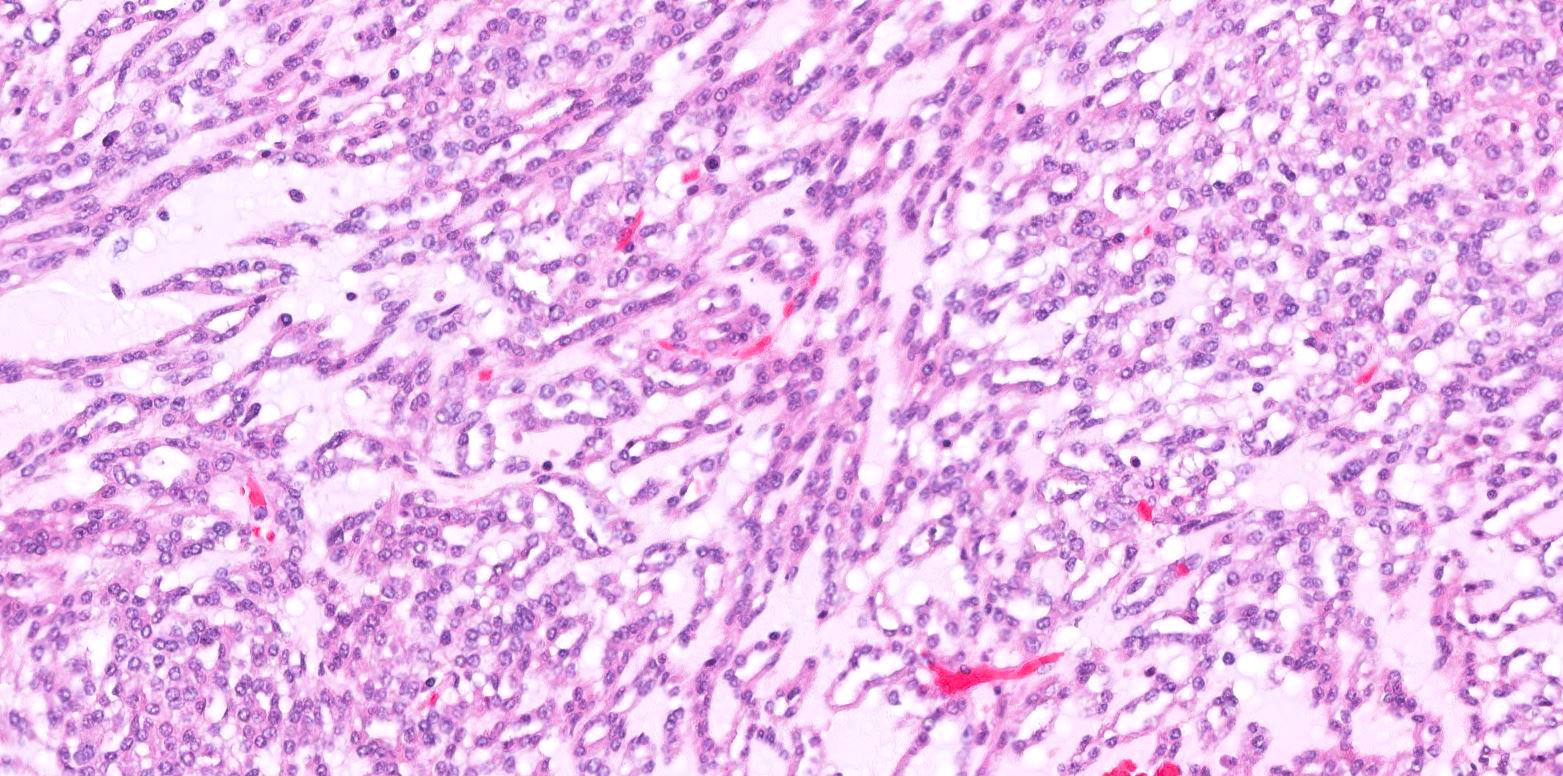
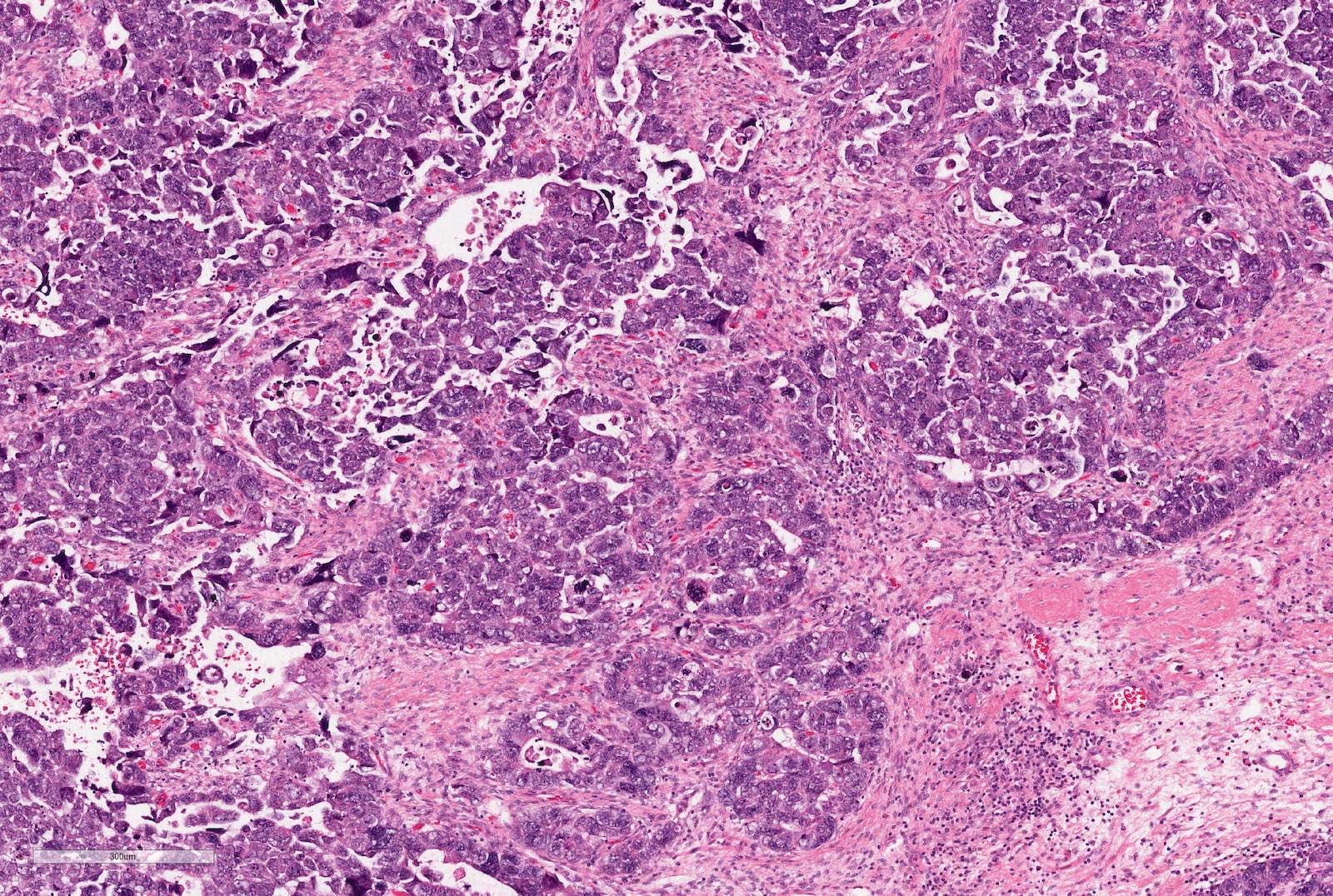
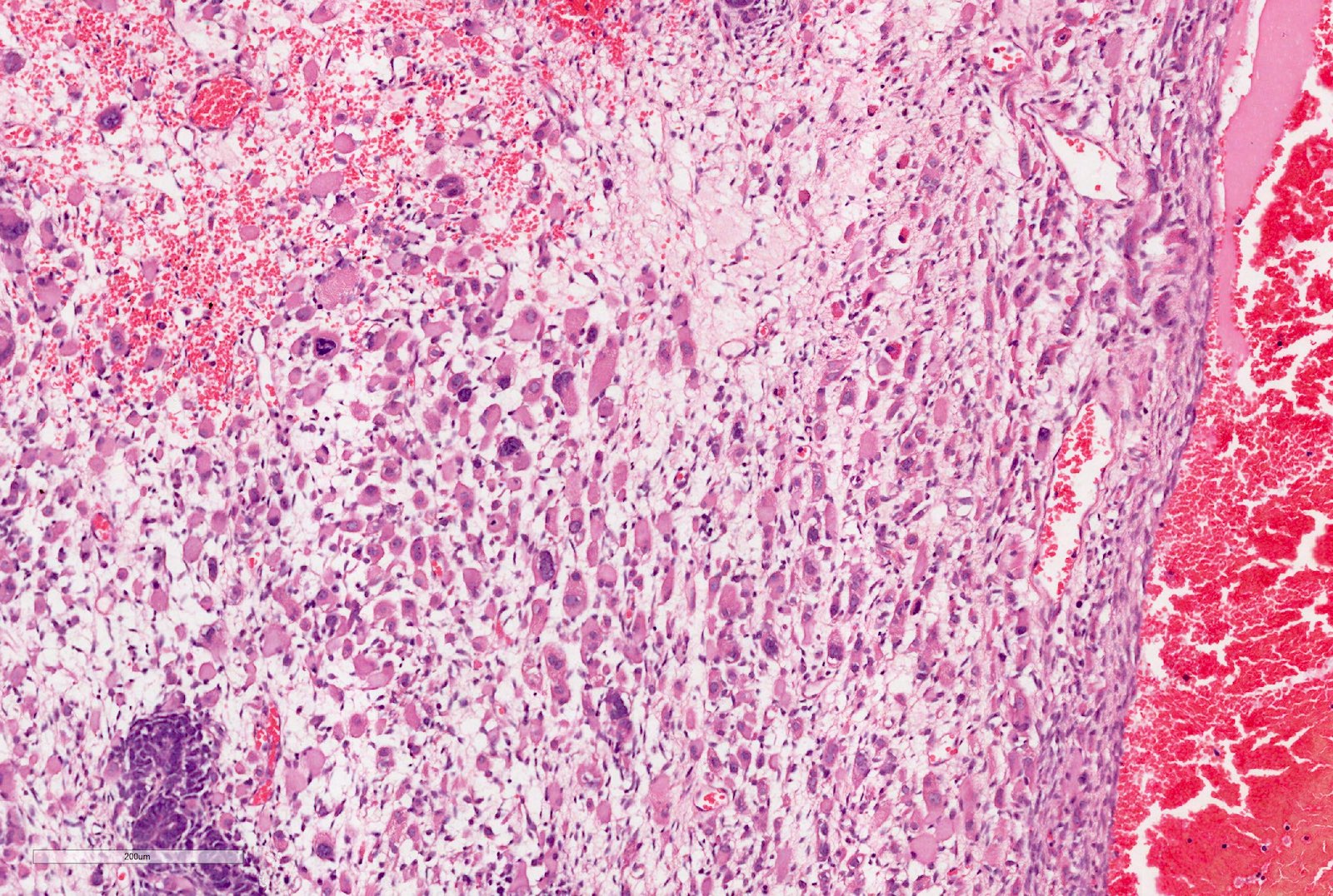
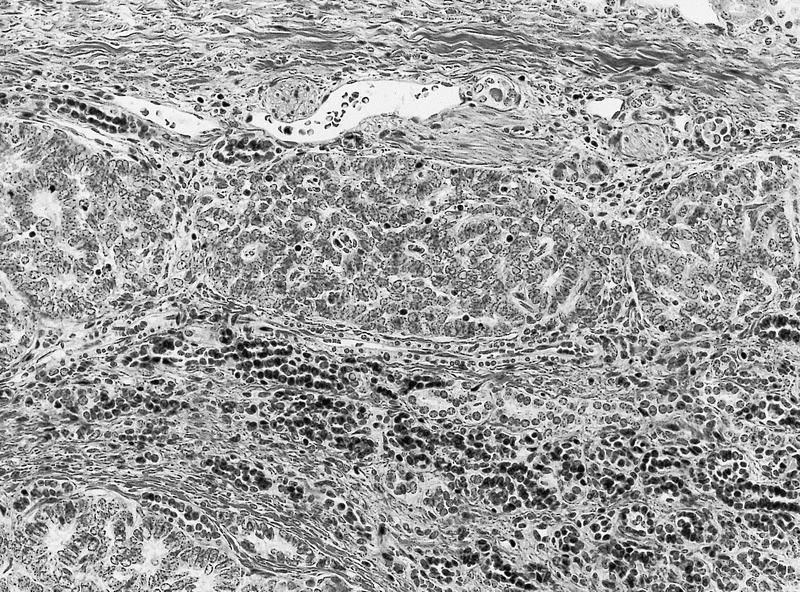
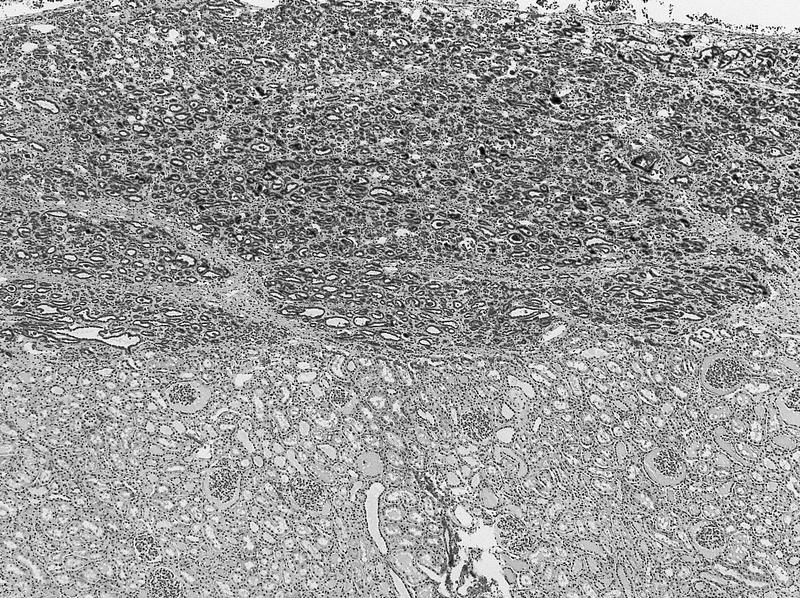
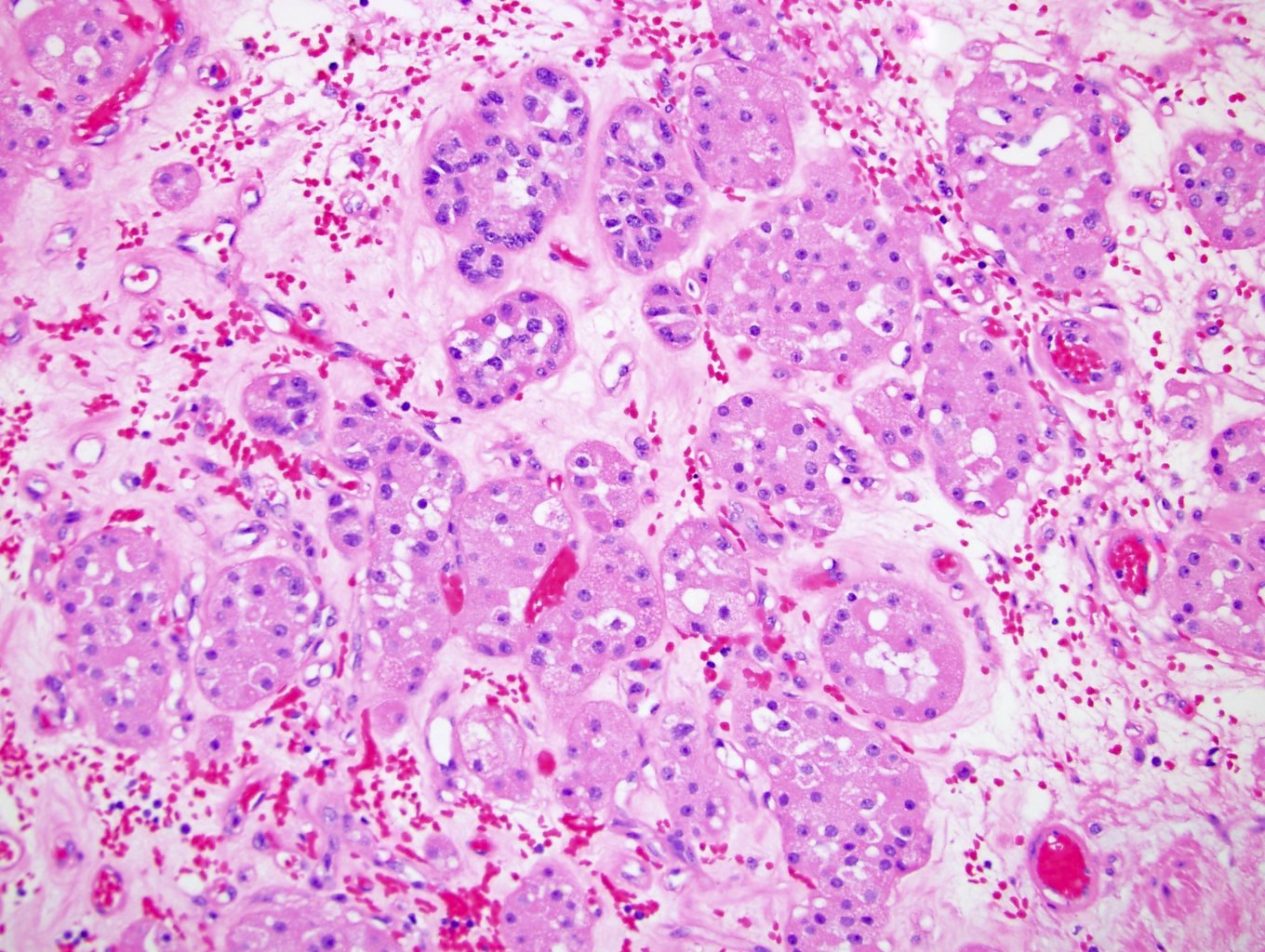
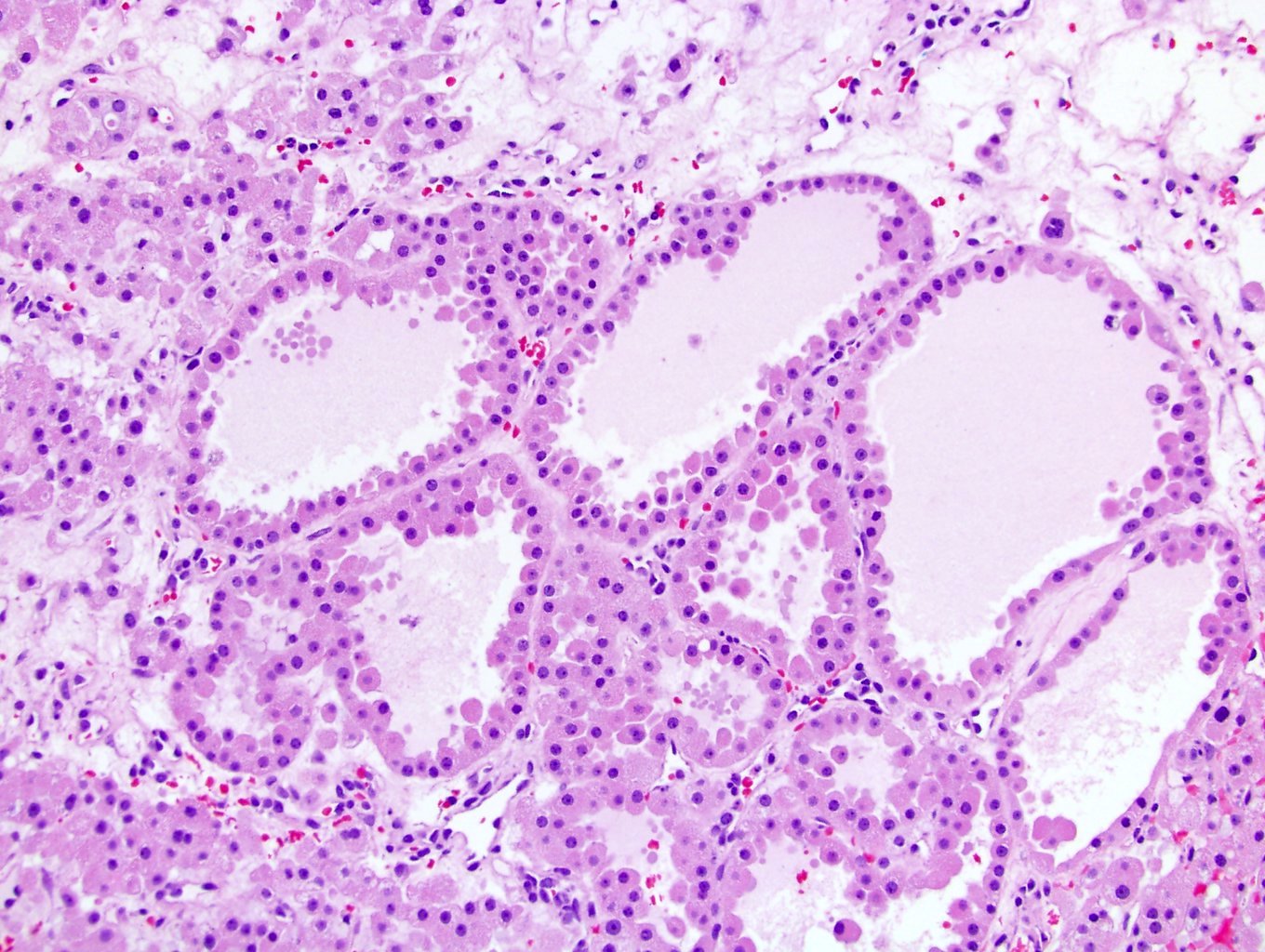
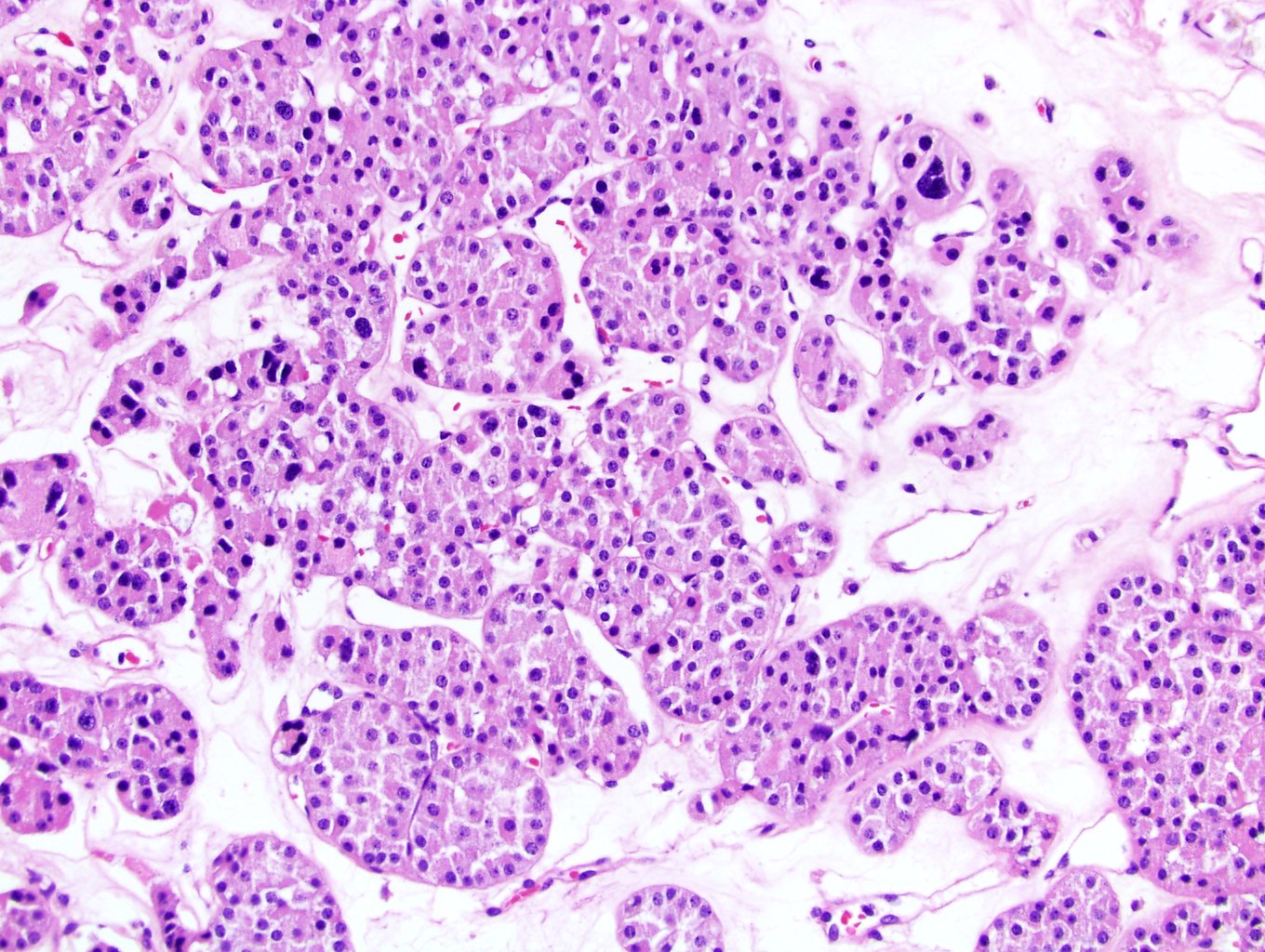
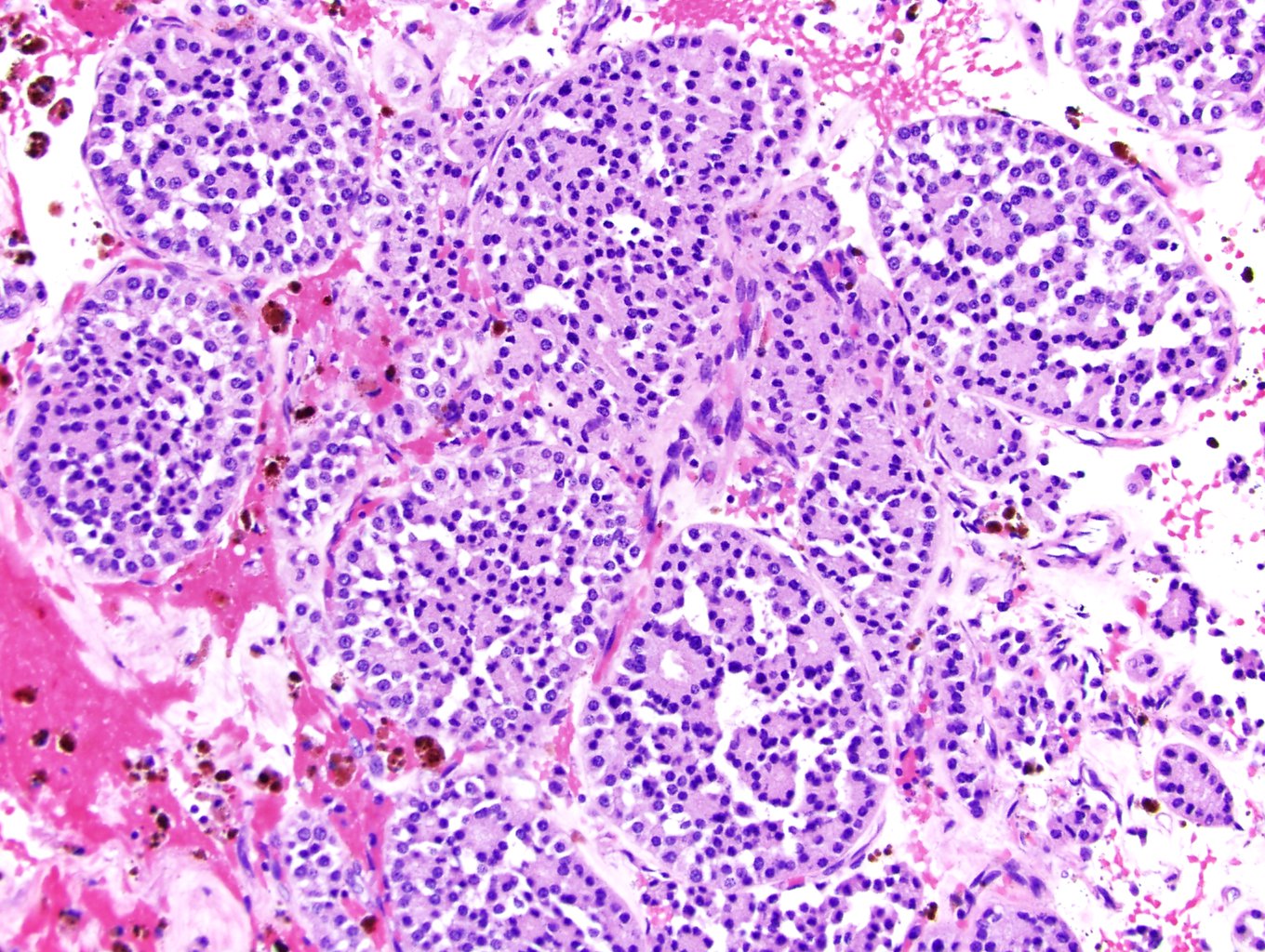
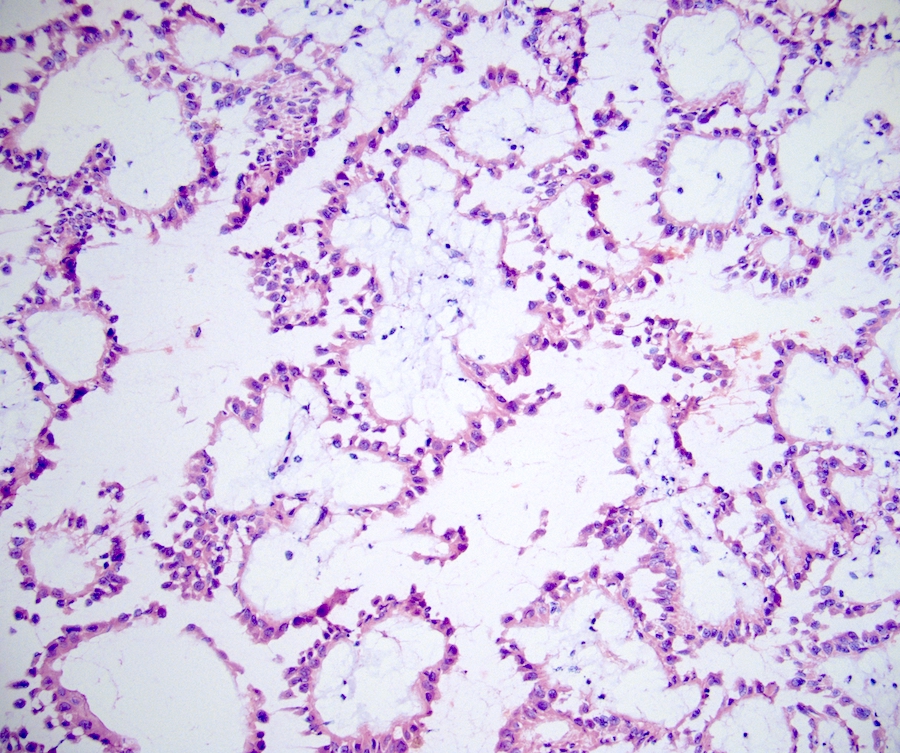
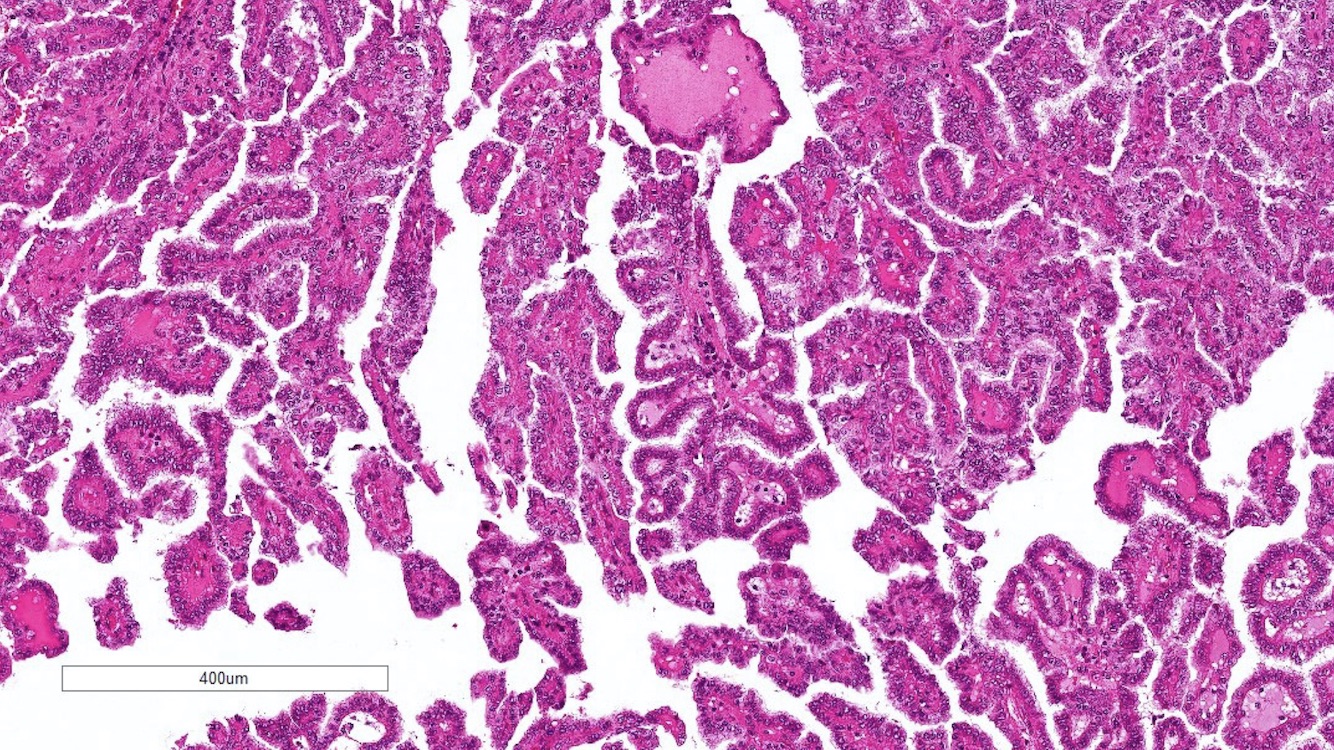
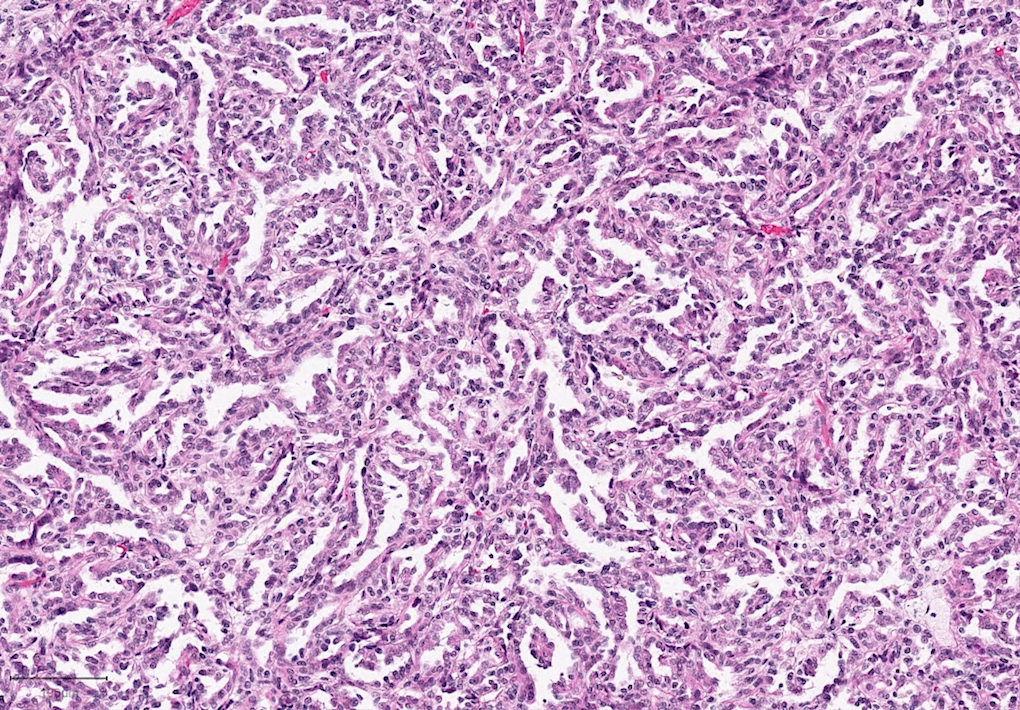
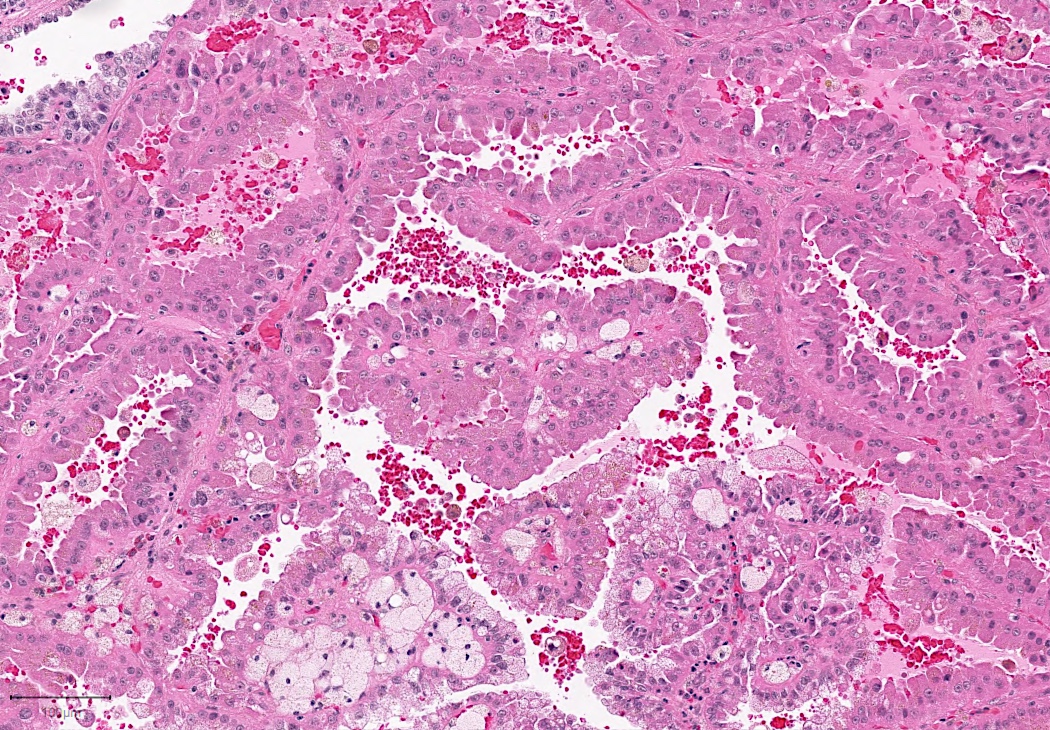
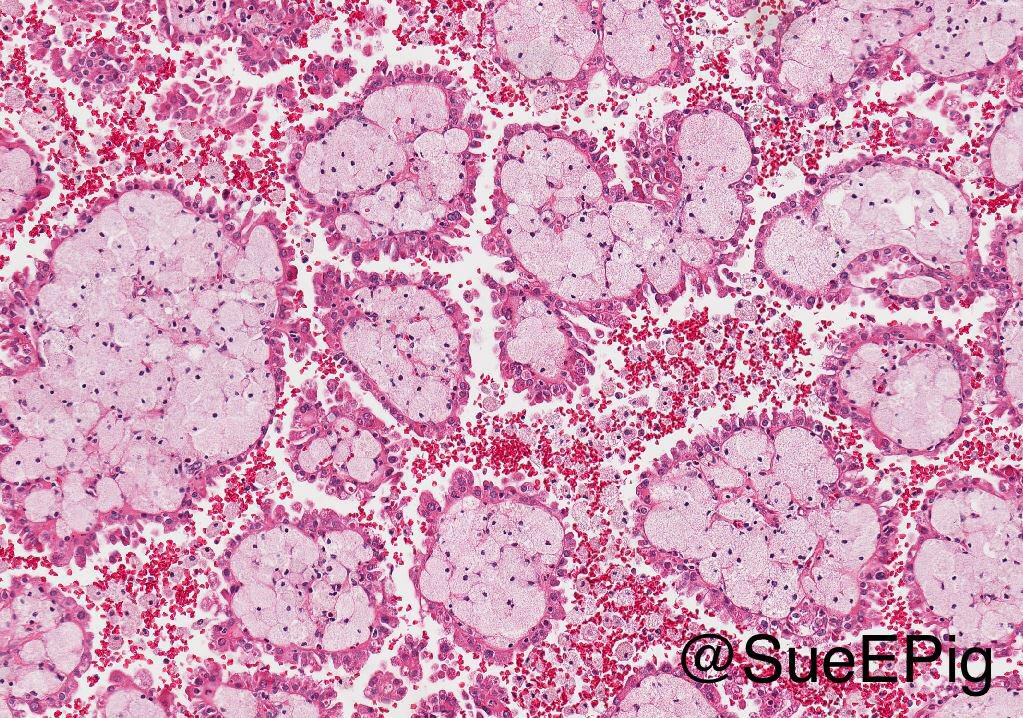
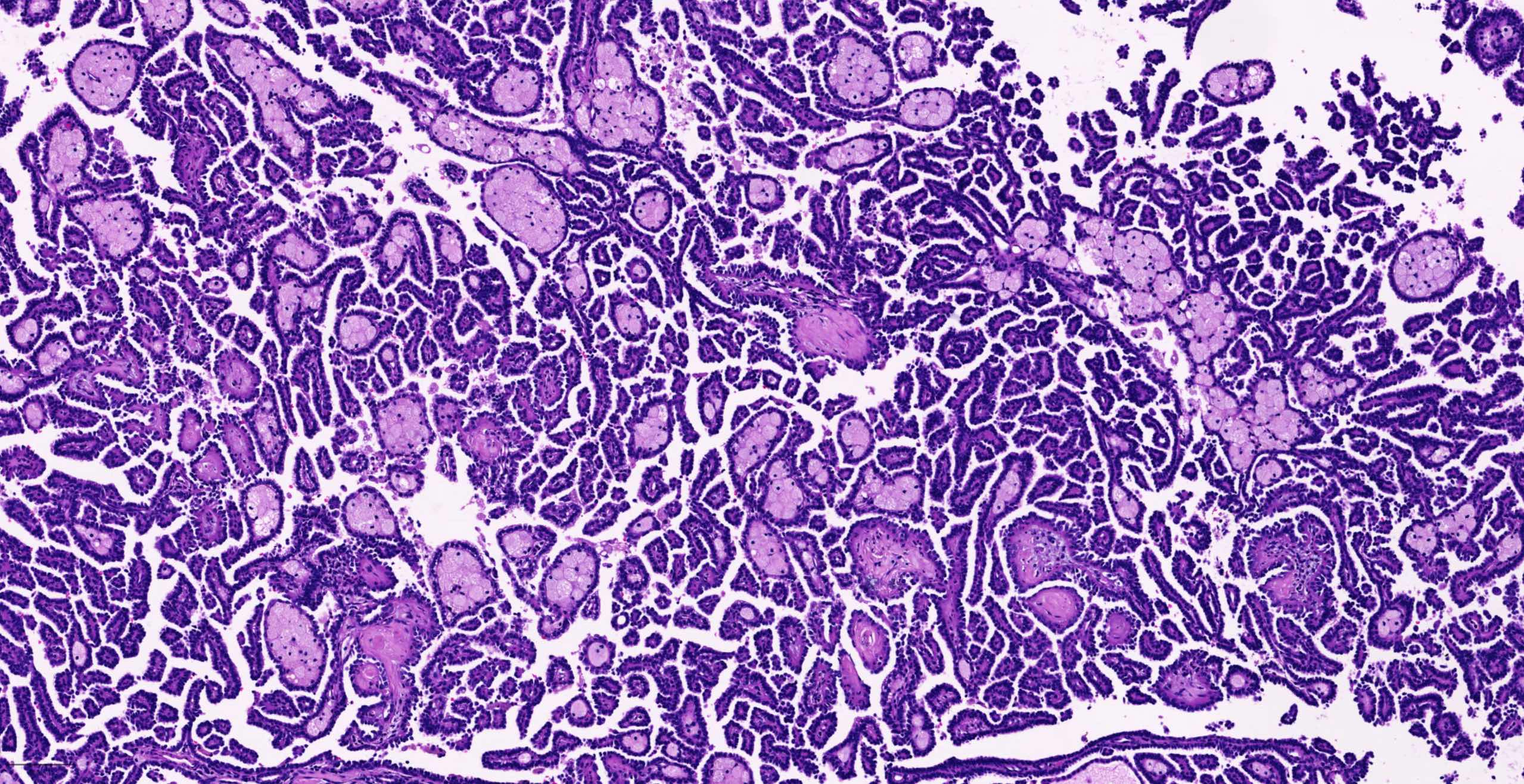
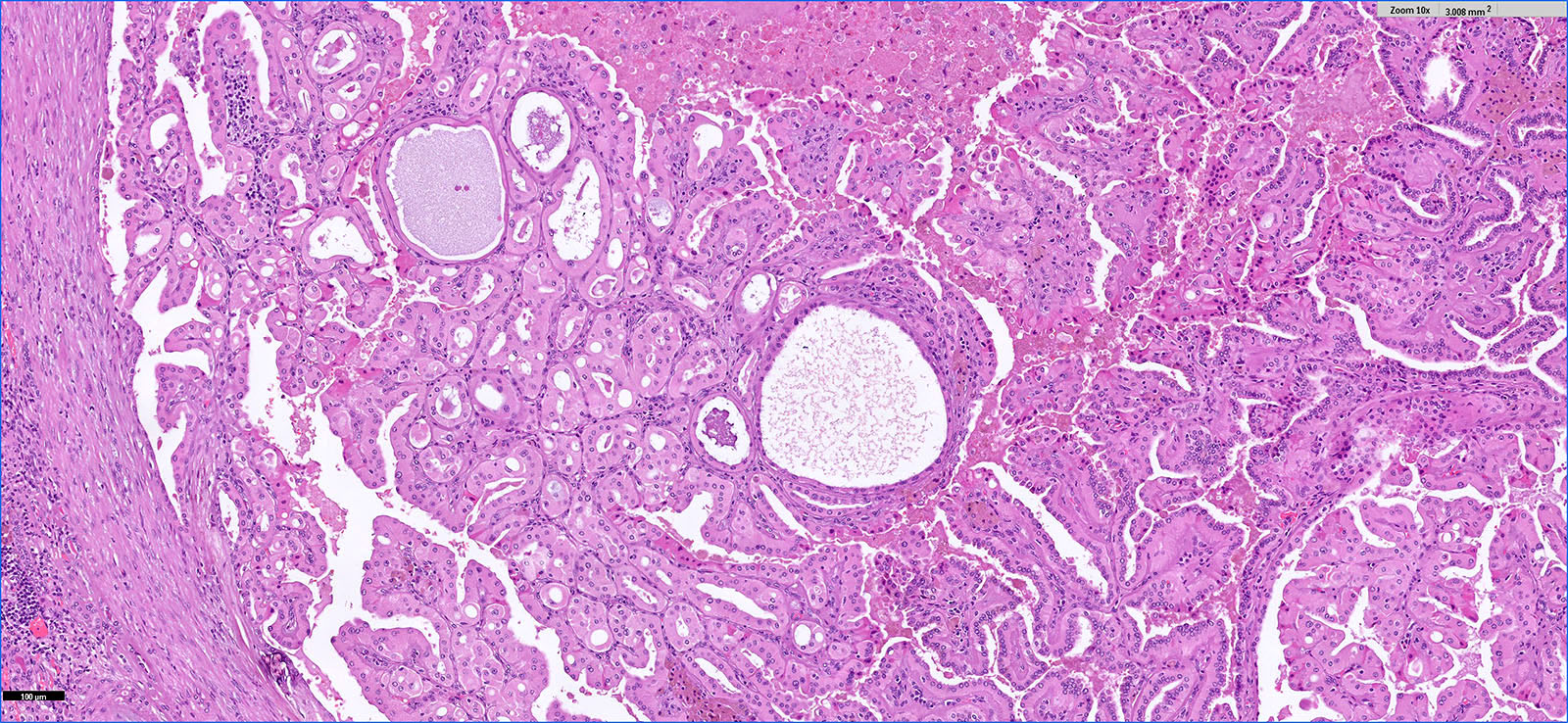
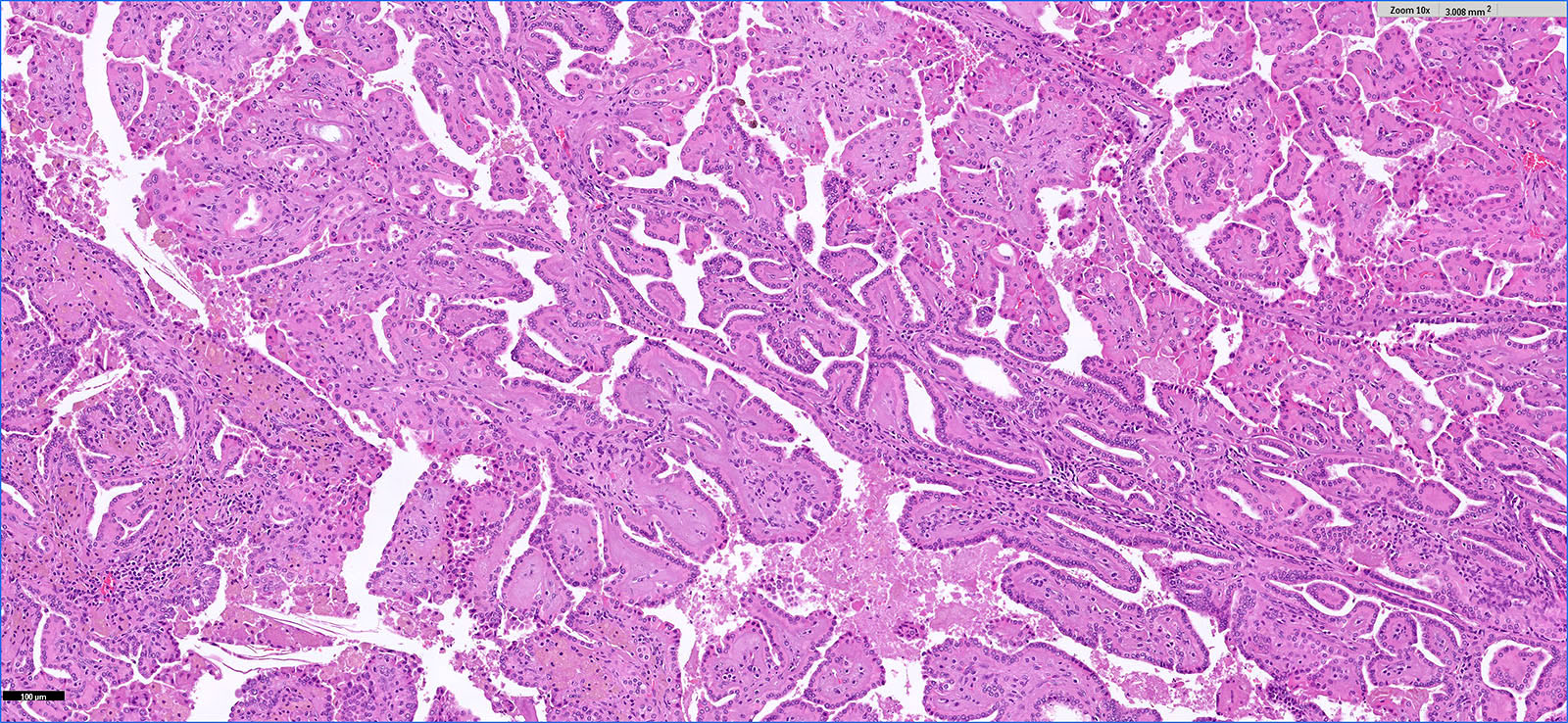
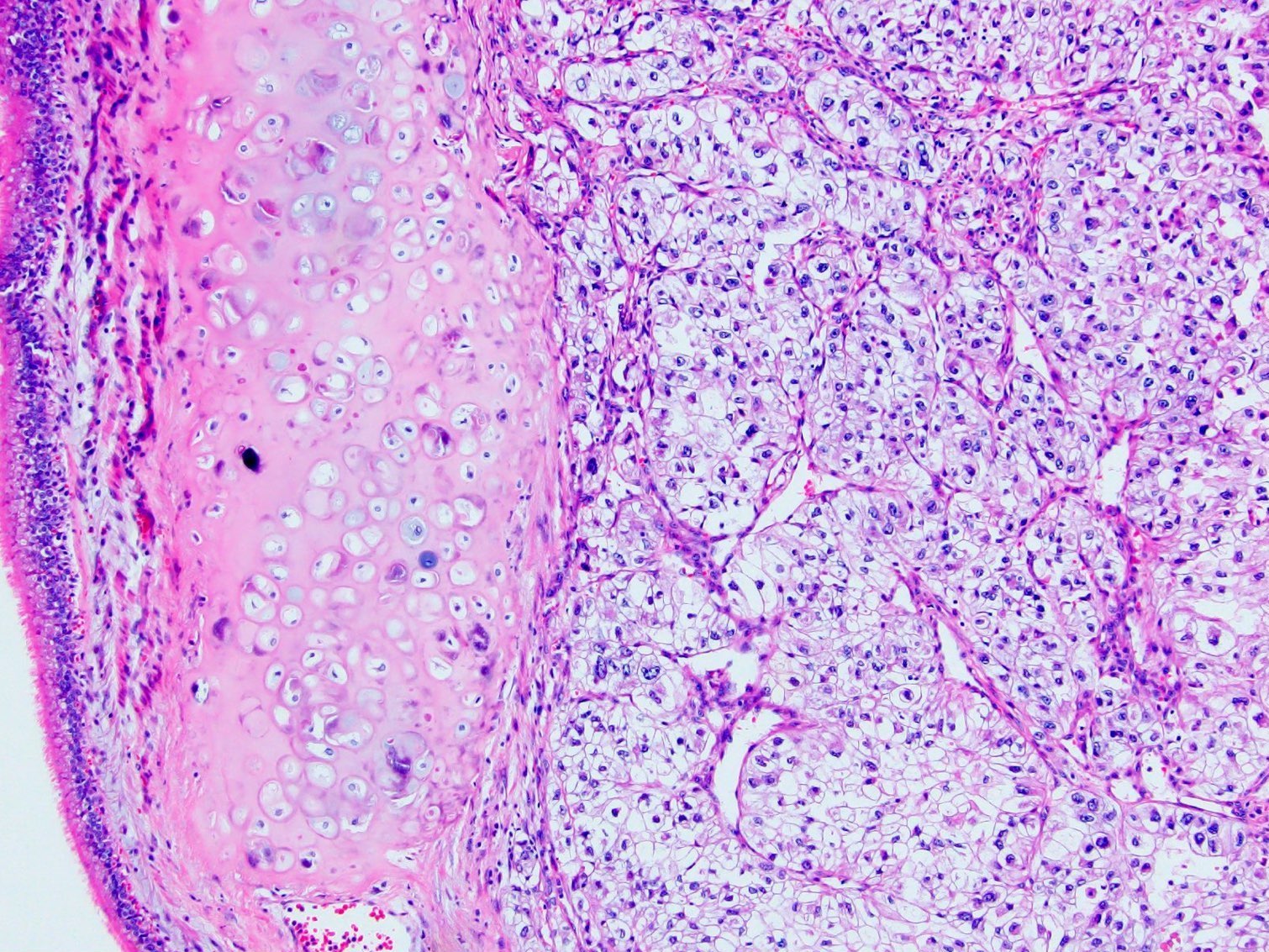
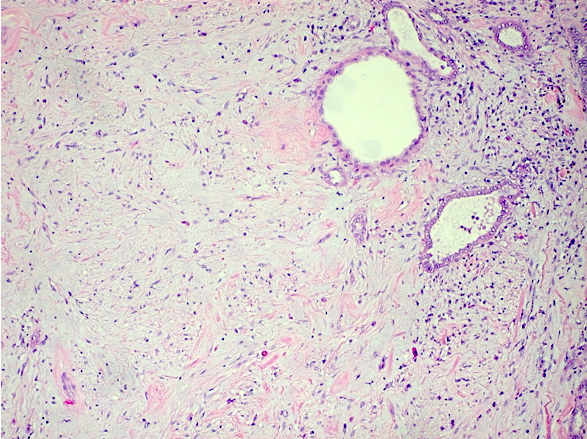
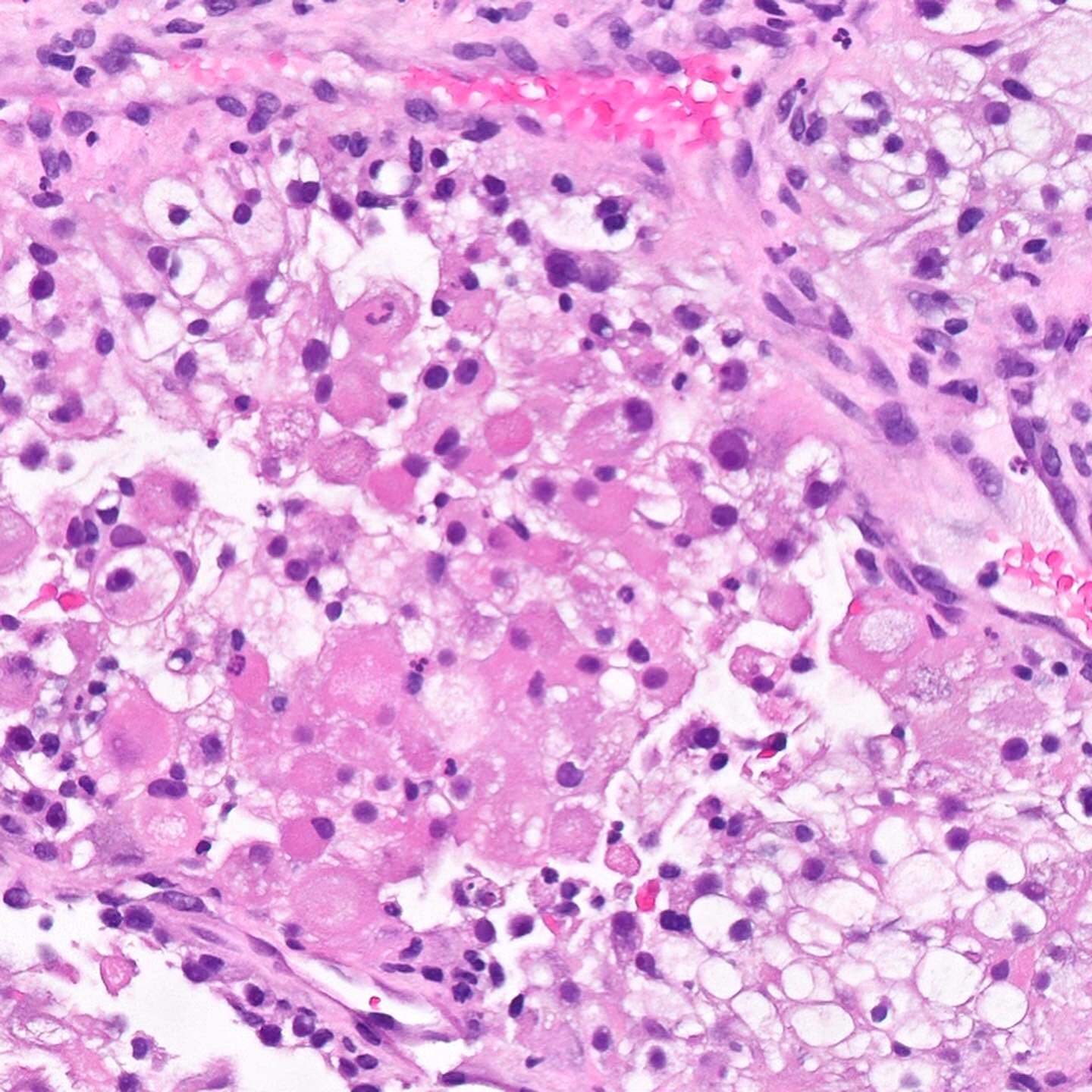
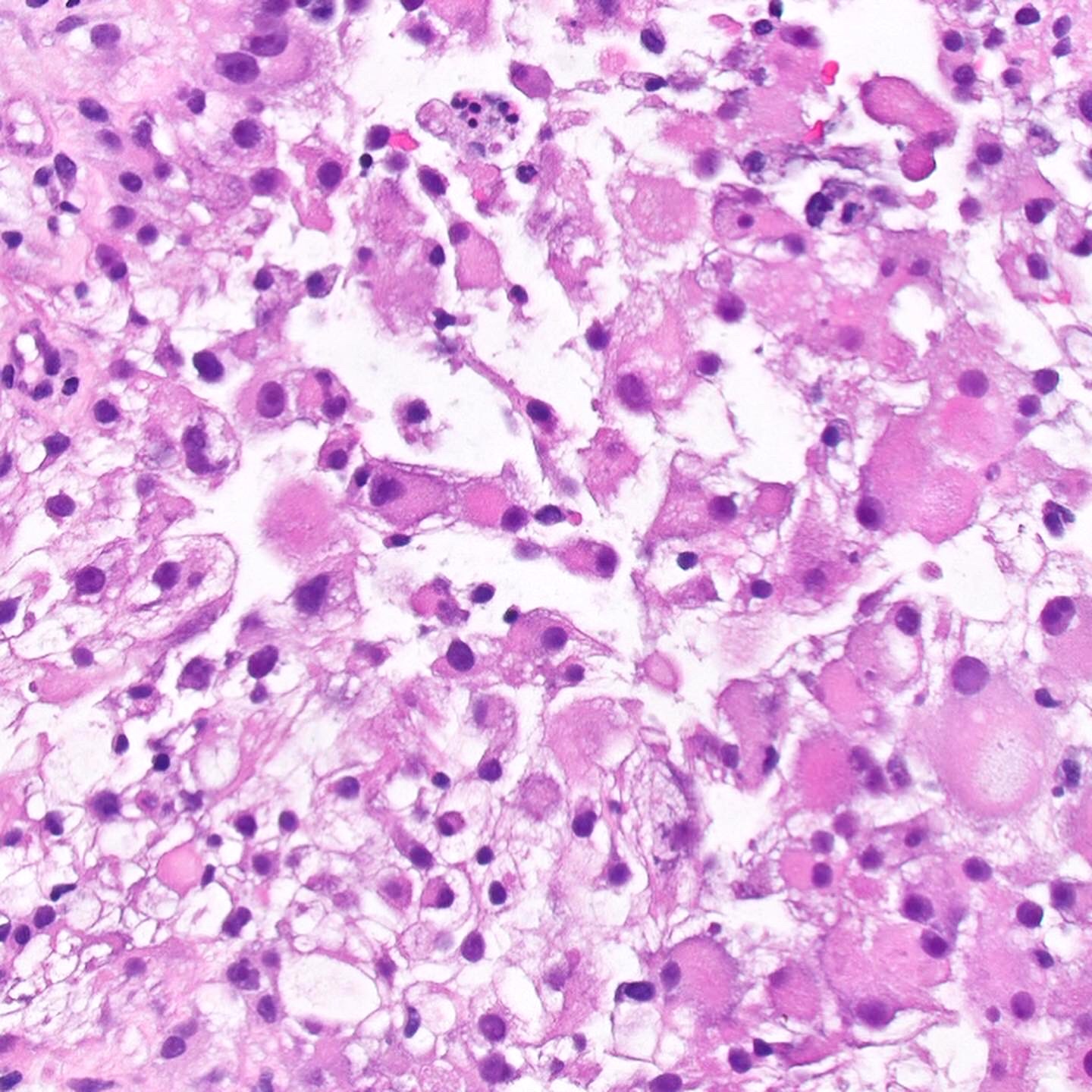
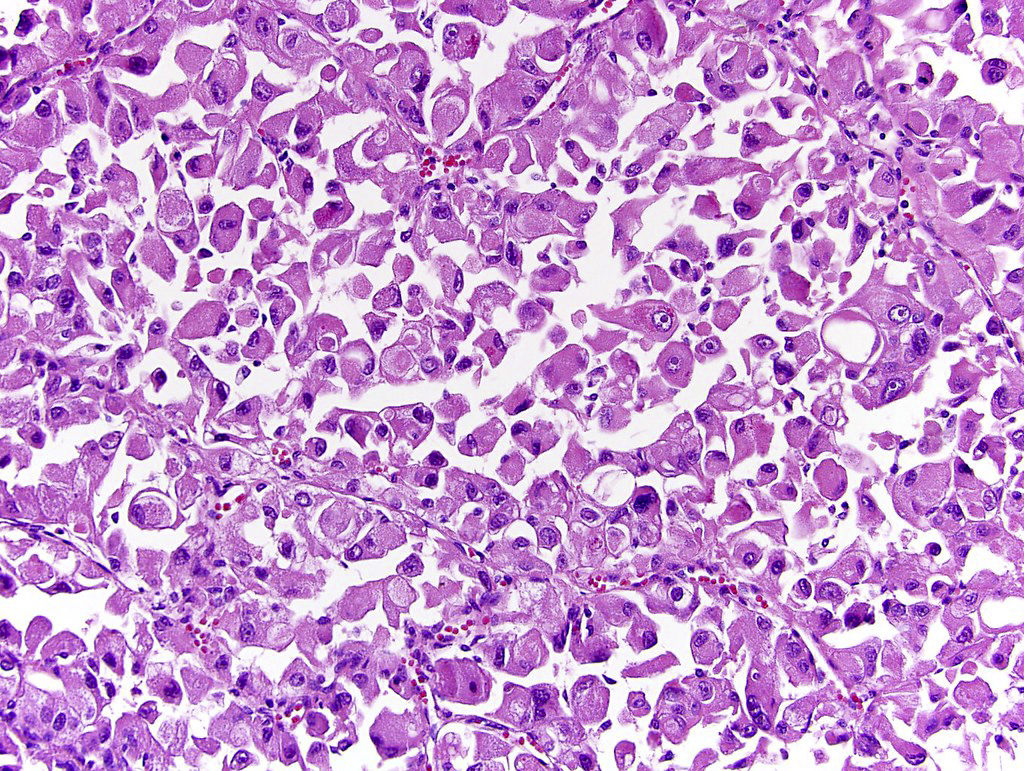
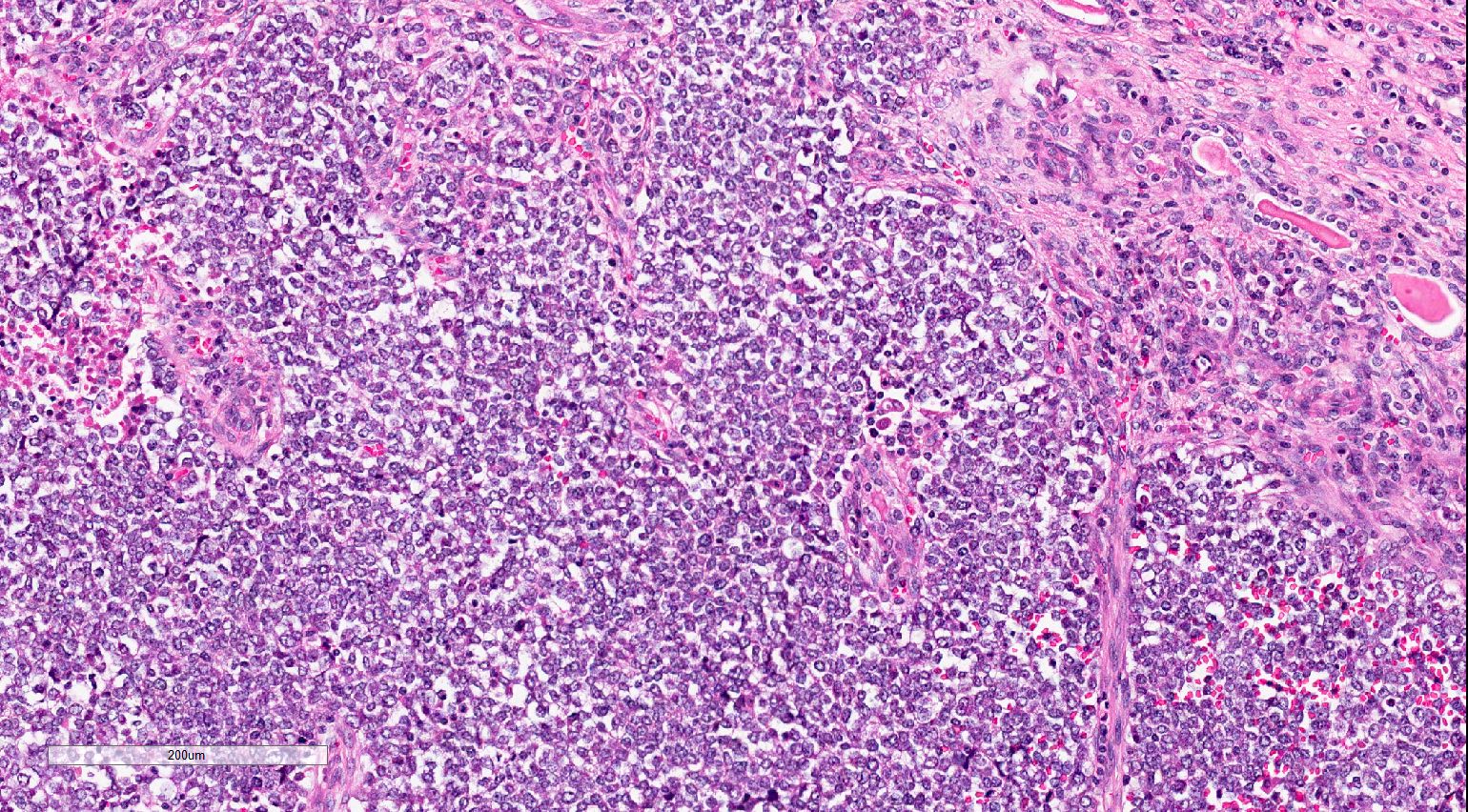
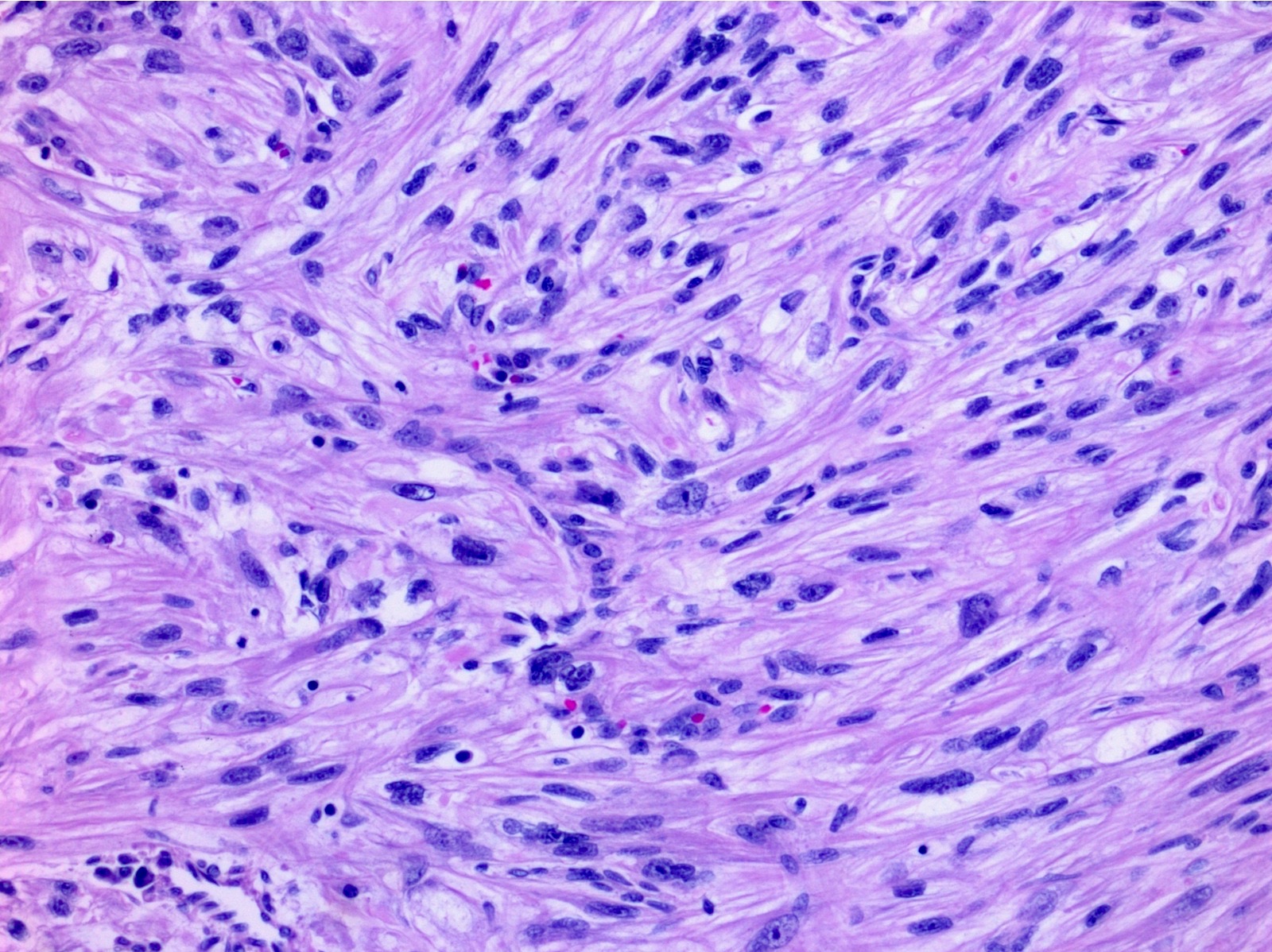
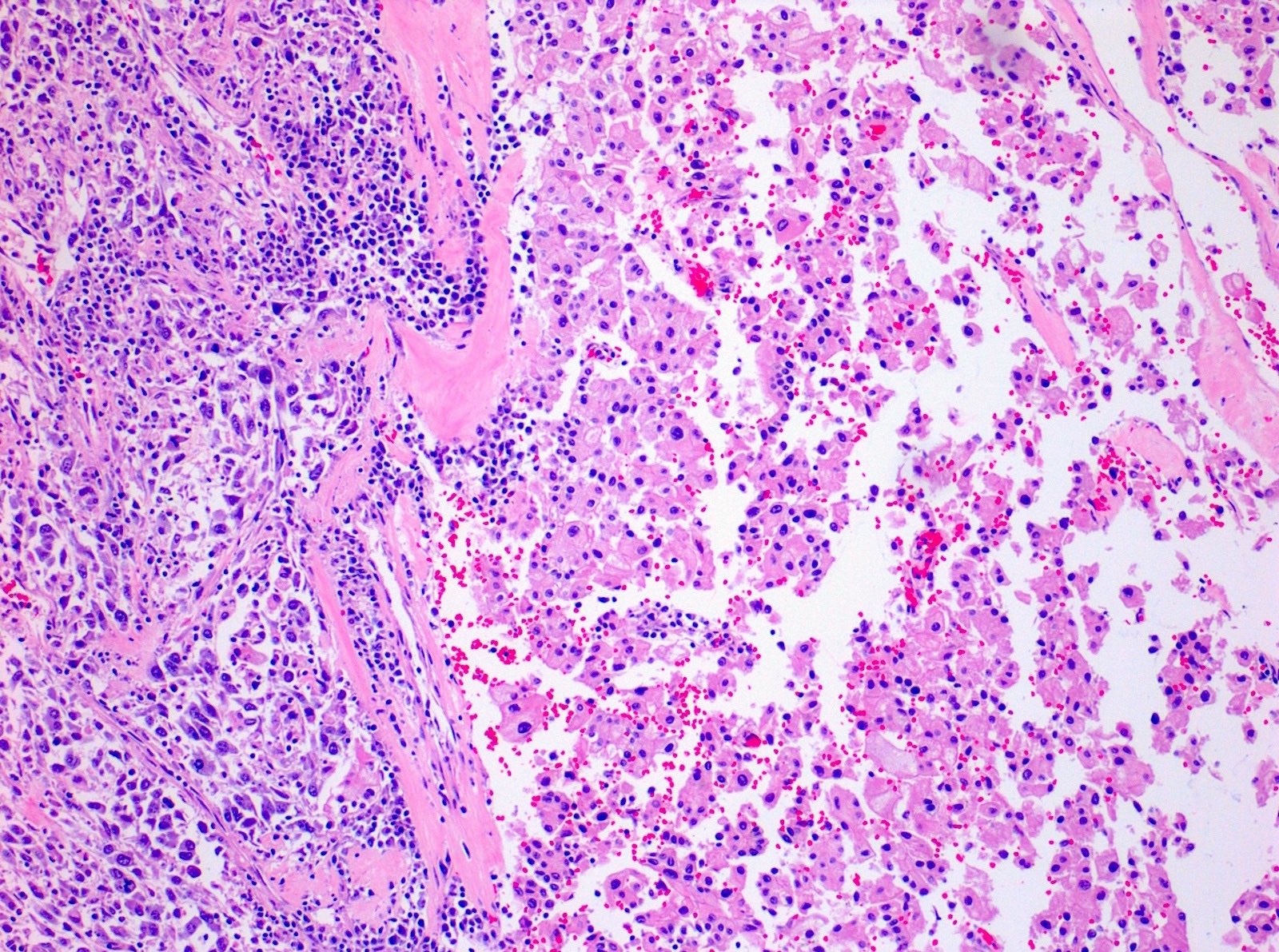
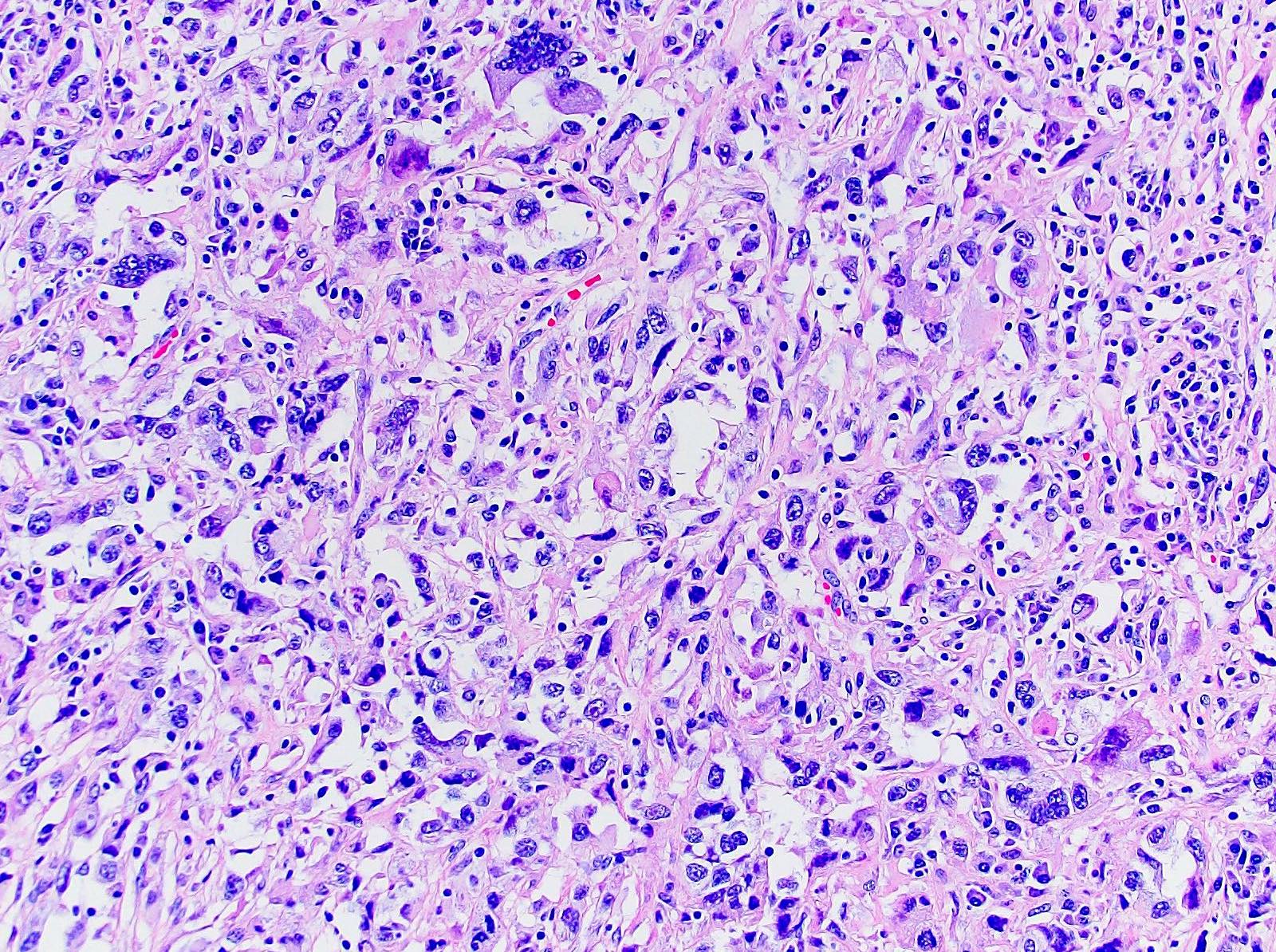
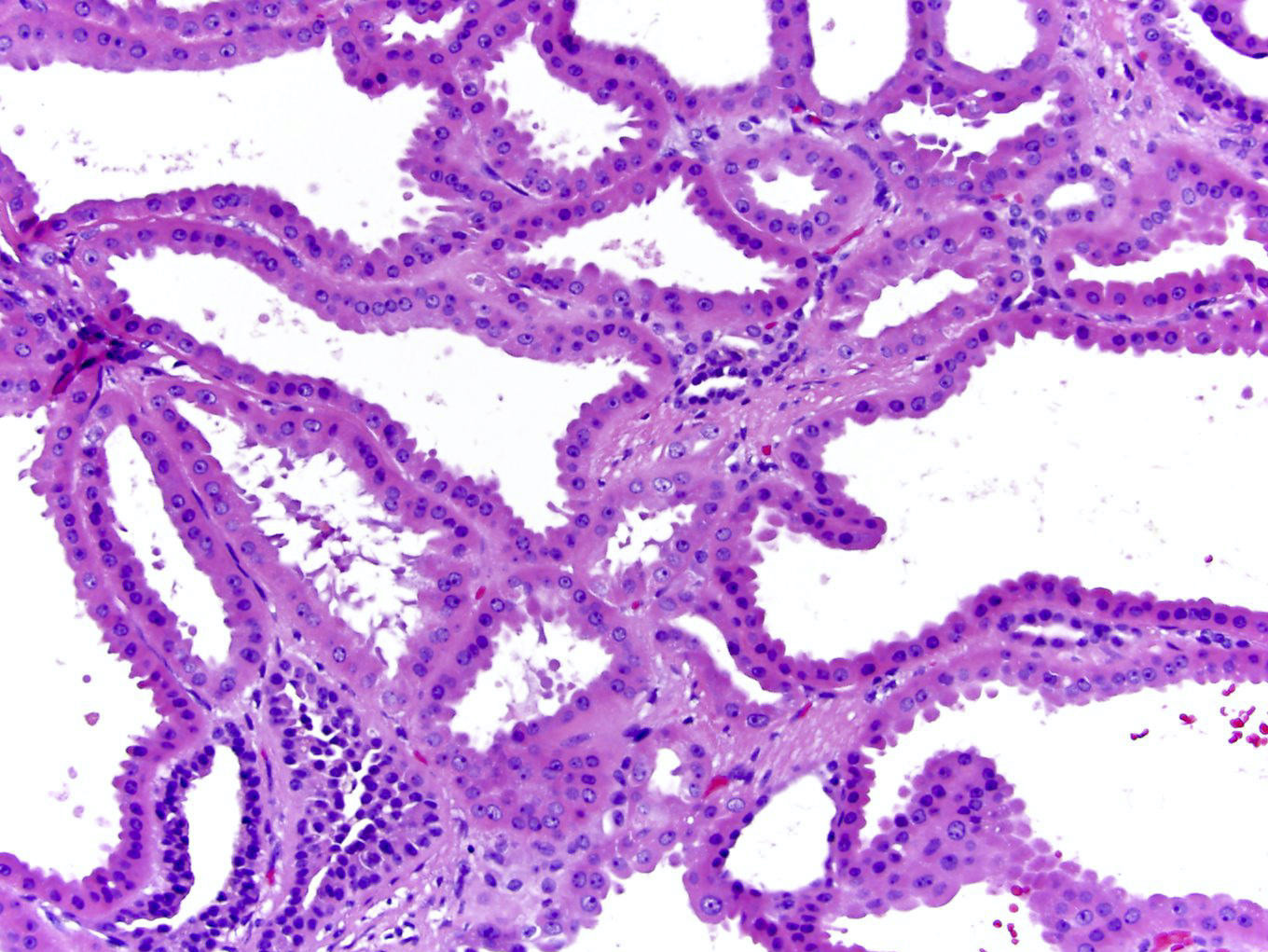
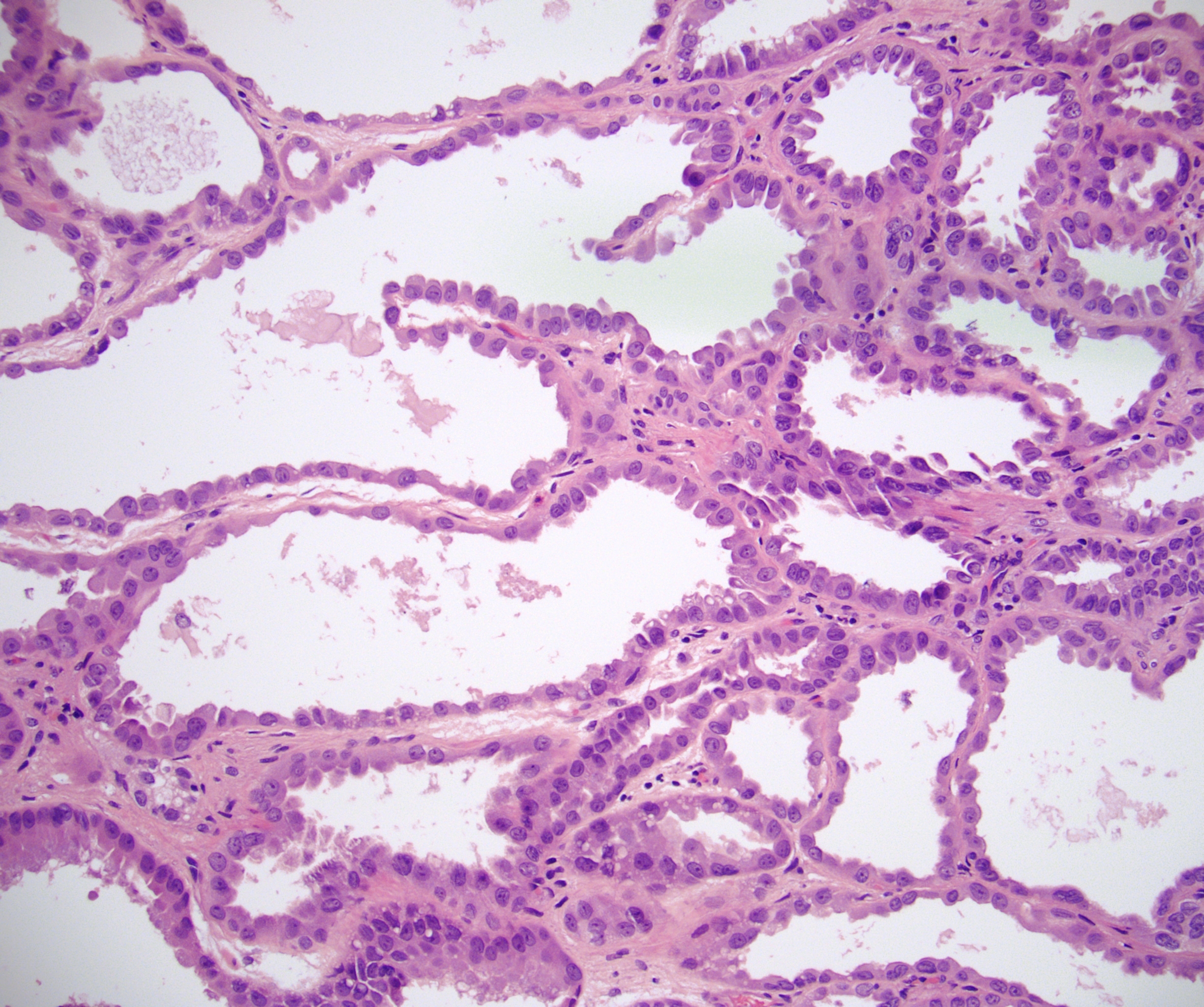
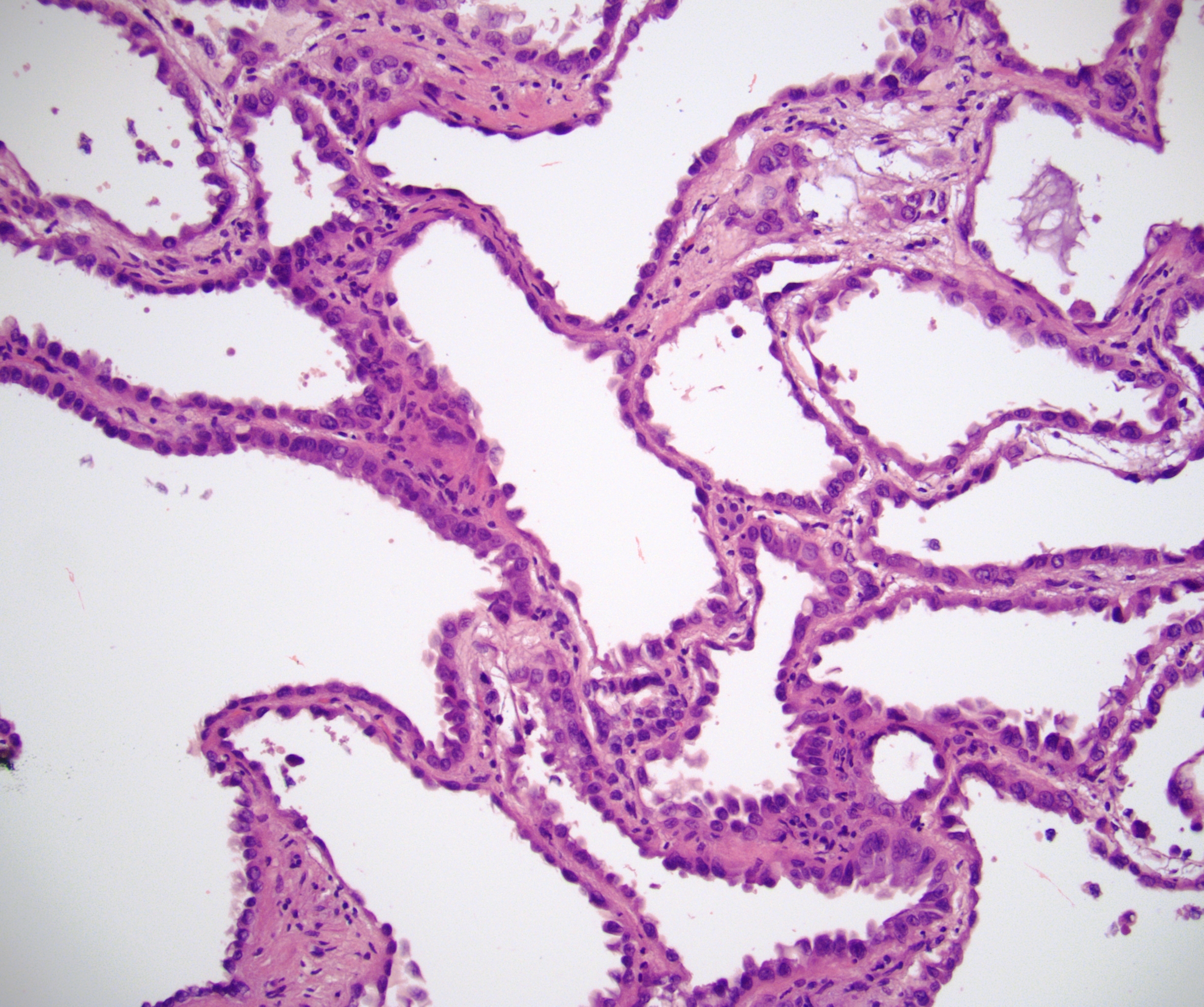
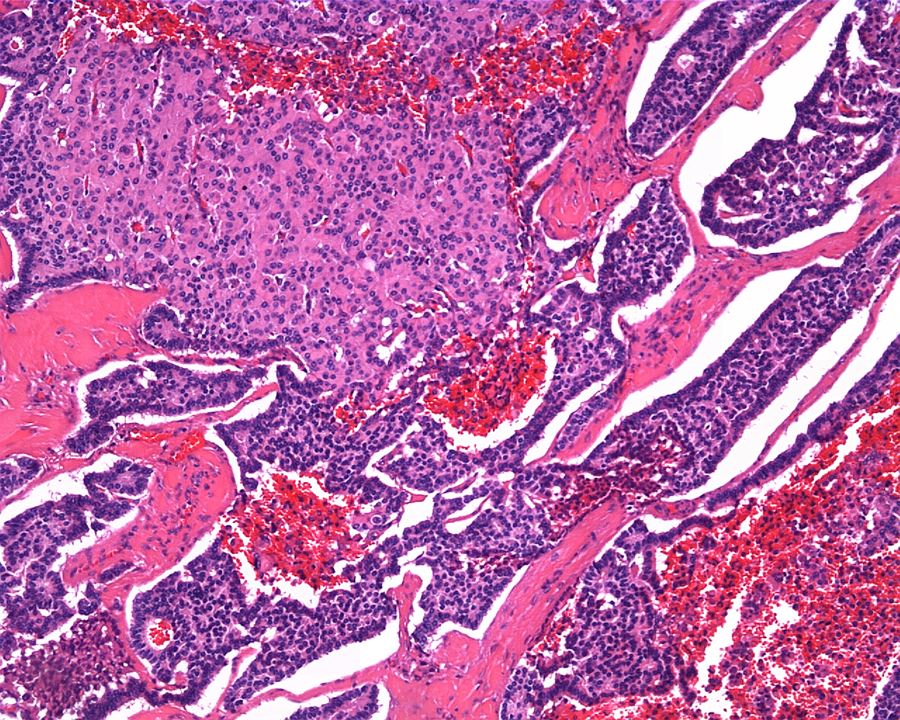
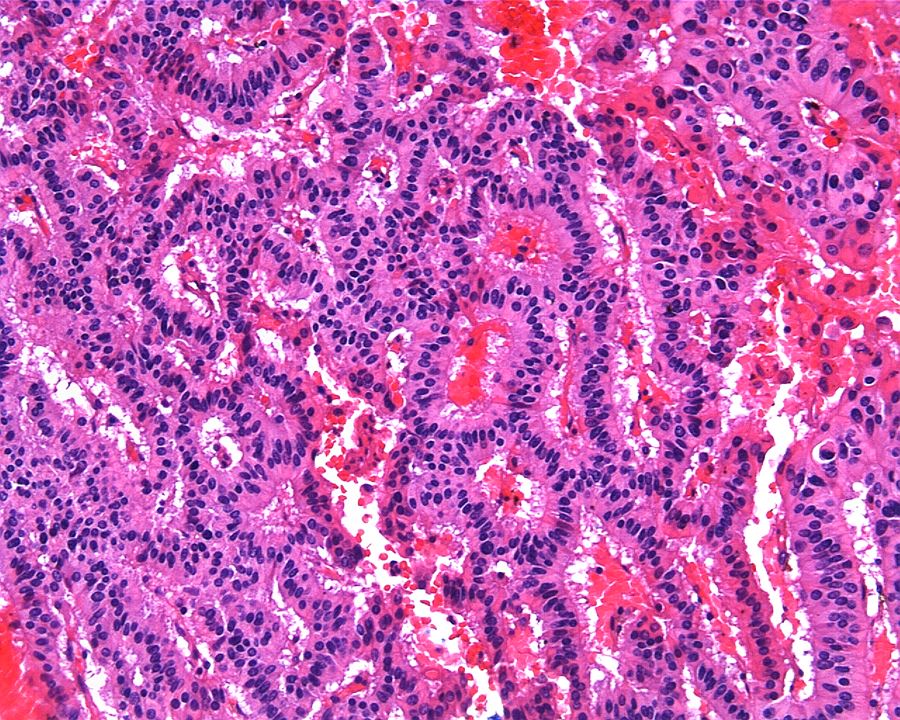
- Adult patients (Histopathology 2019;74:31, Semin Diagn Pathol 2015;32:90):
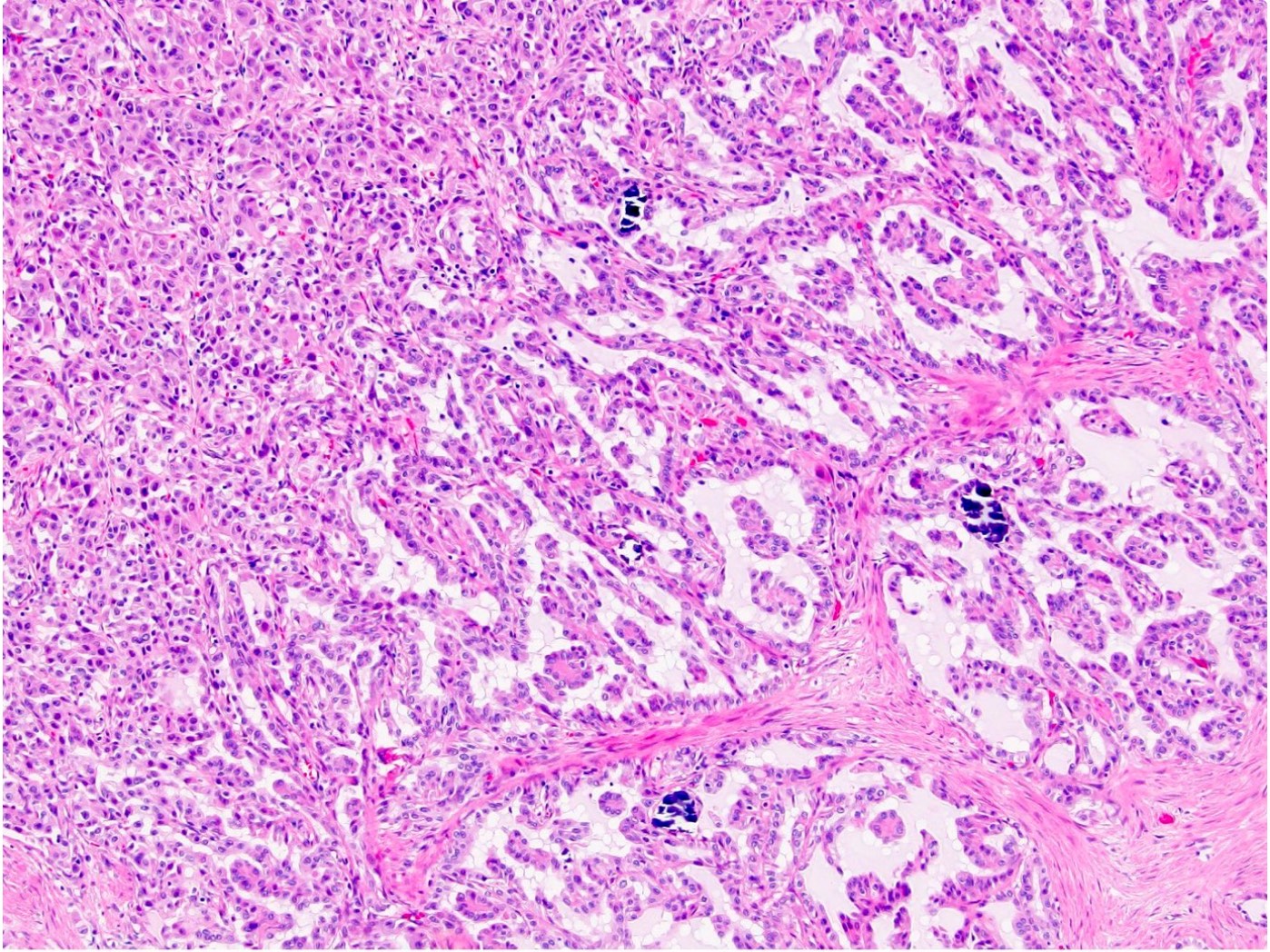
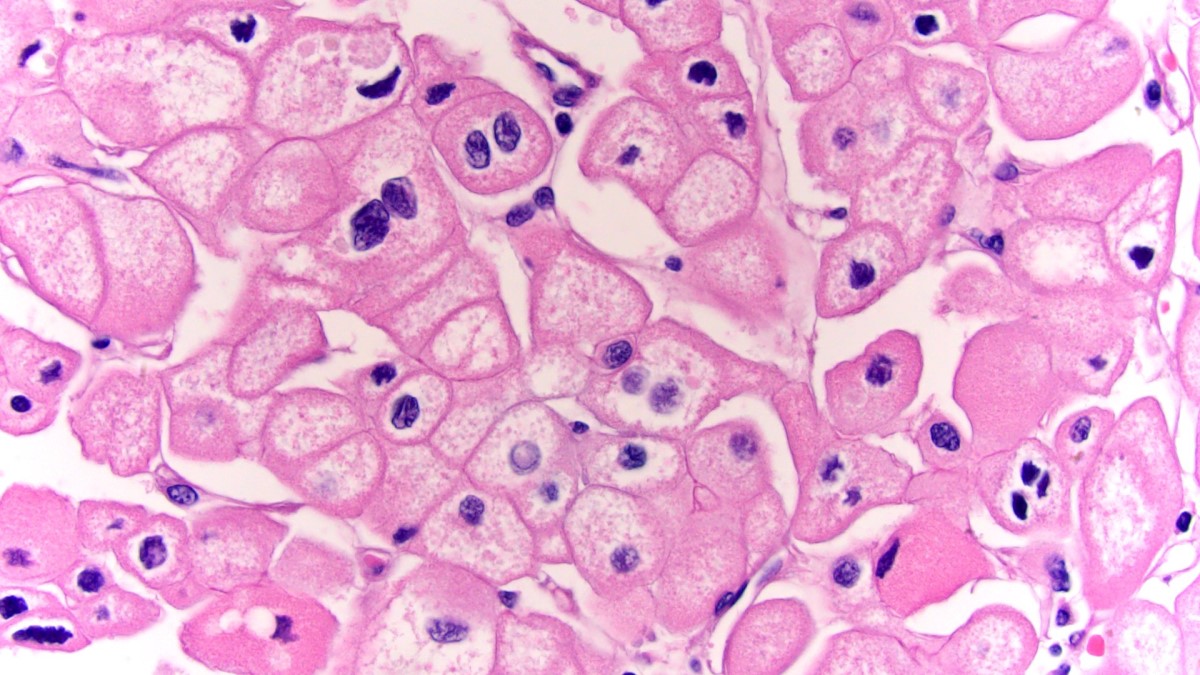
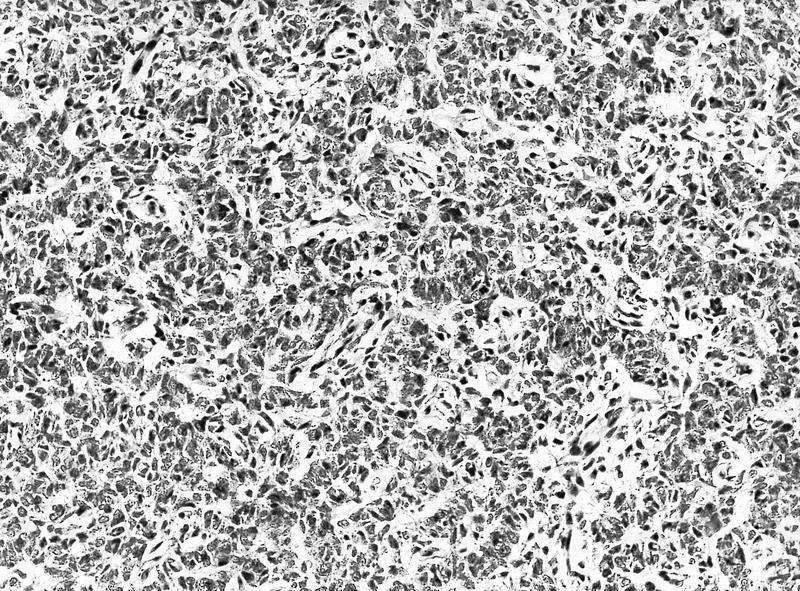
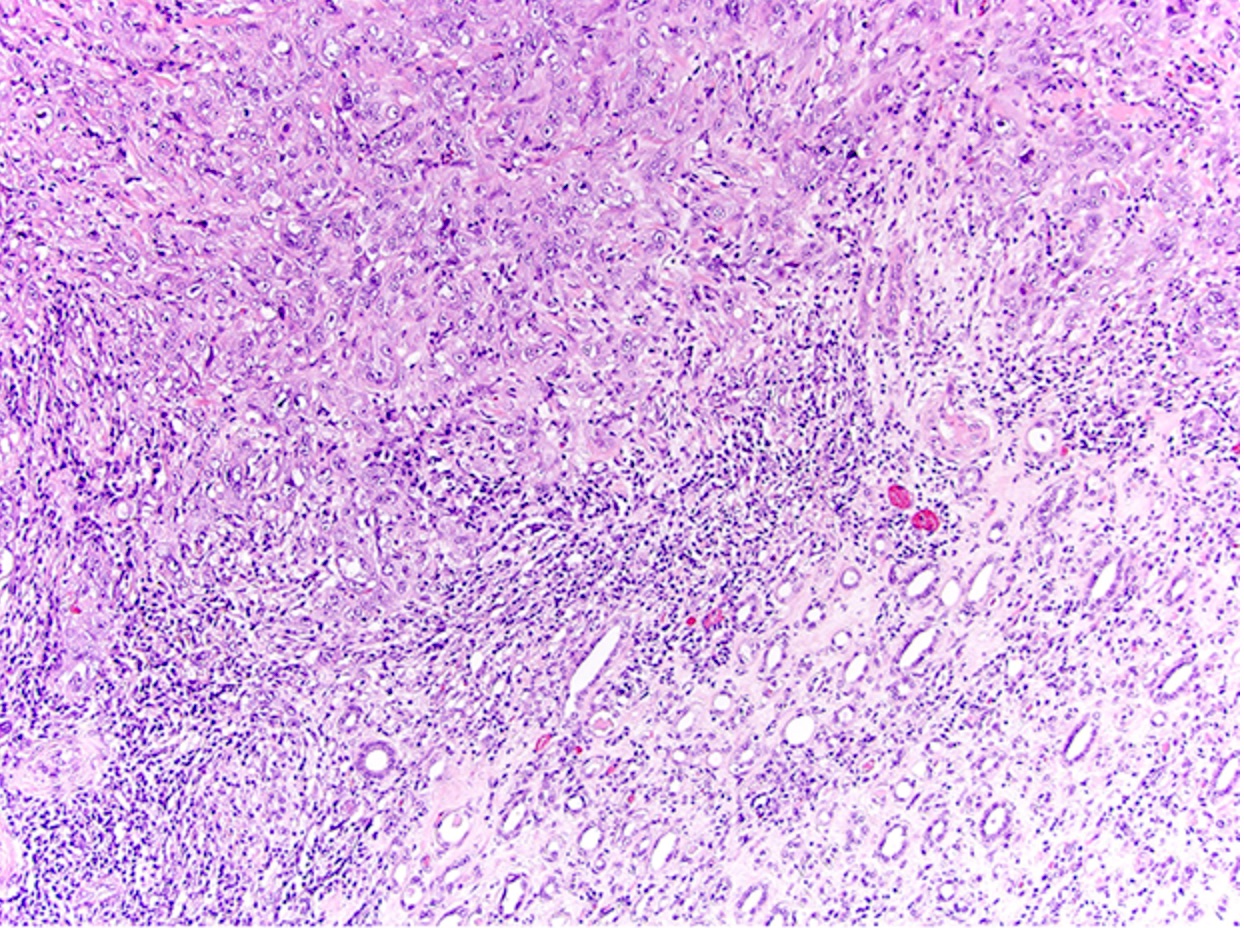
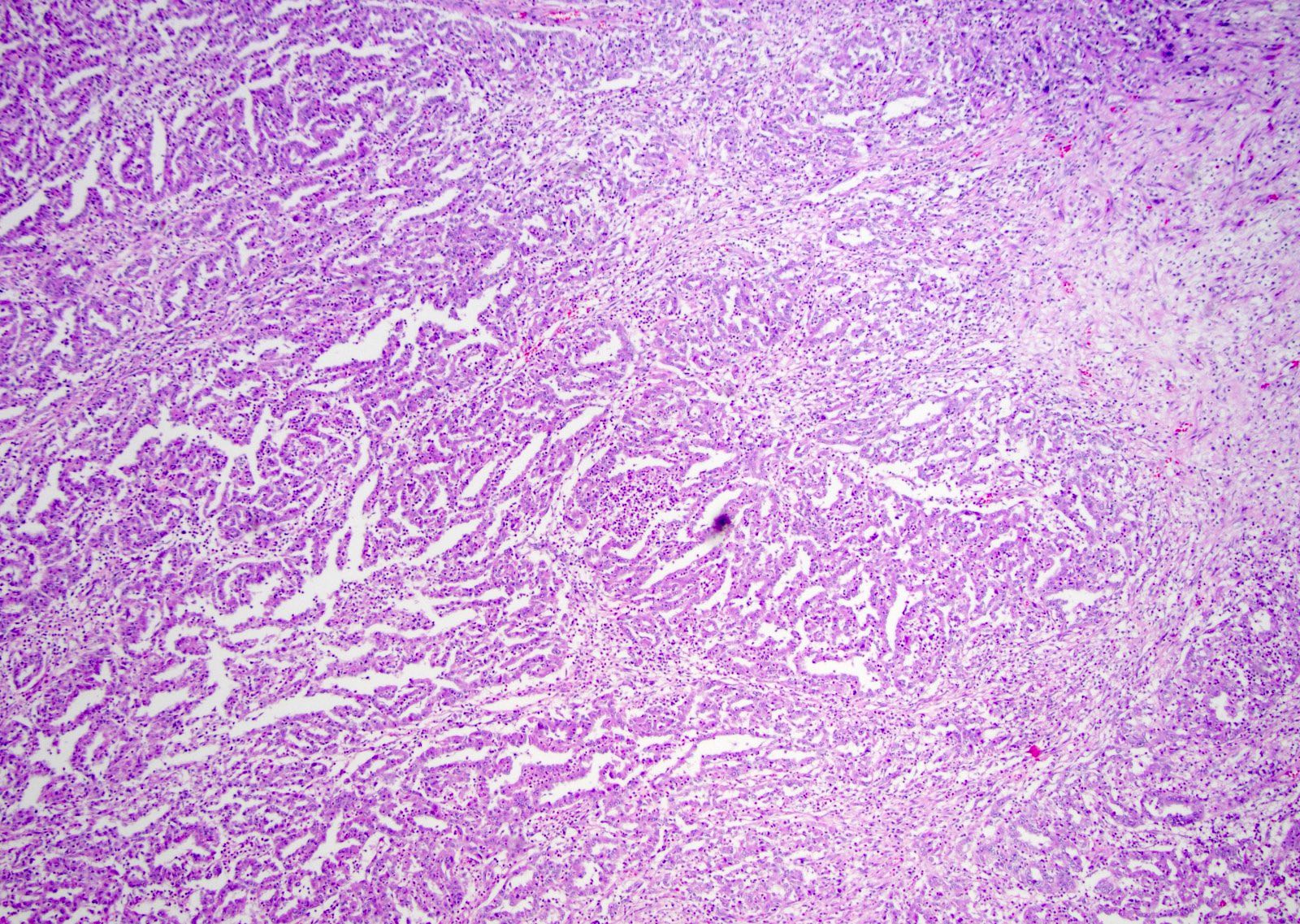
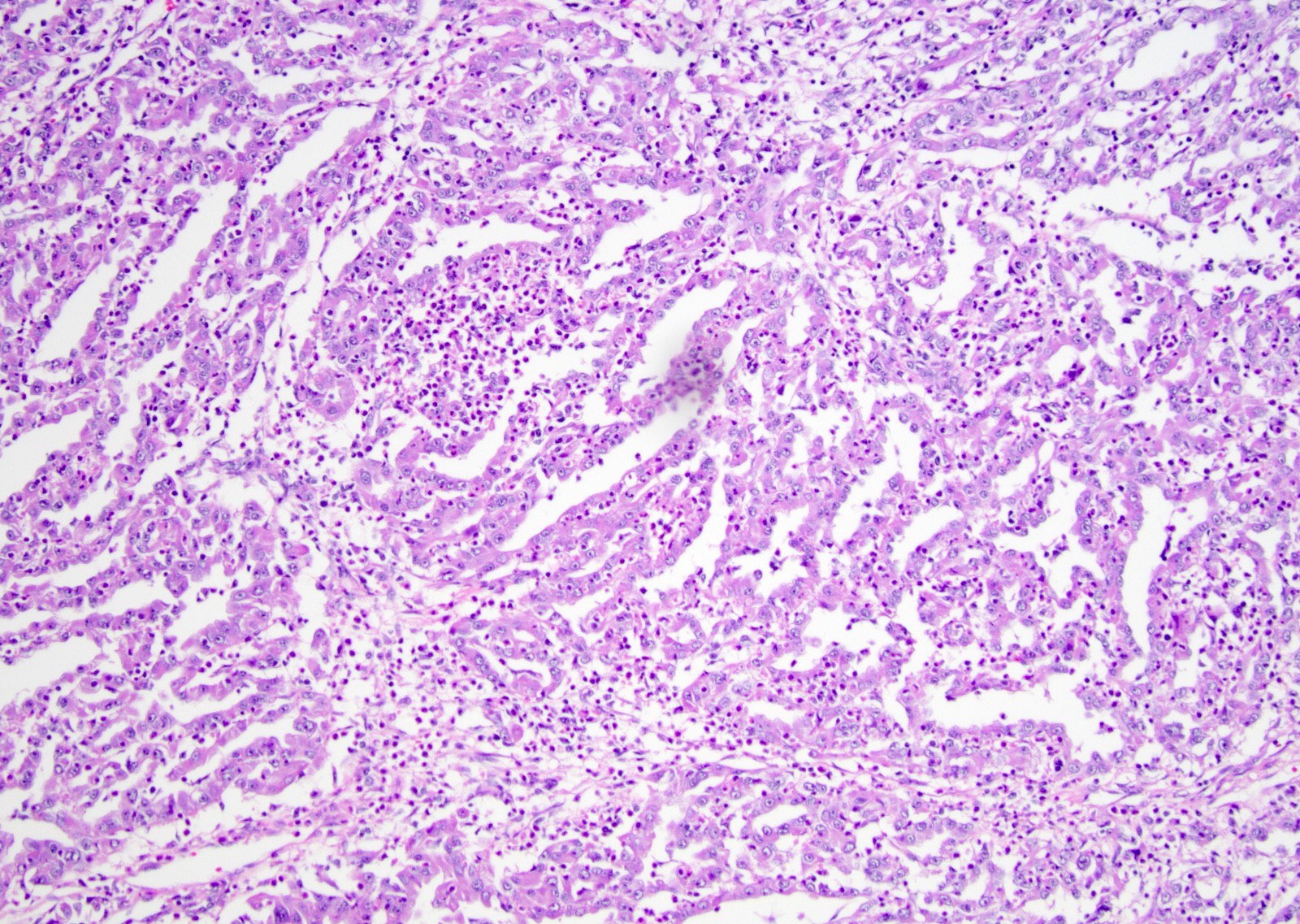
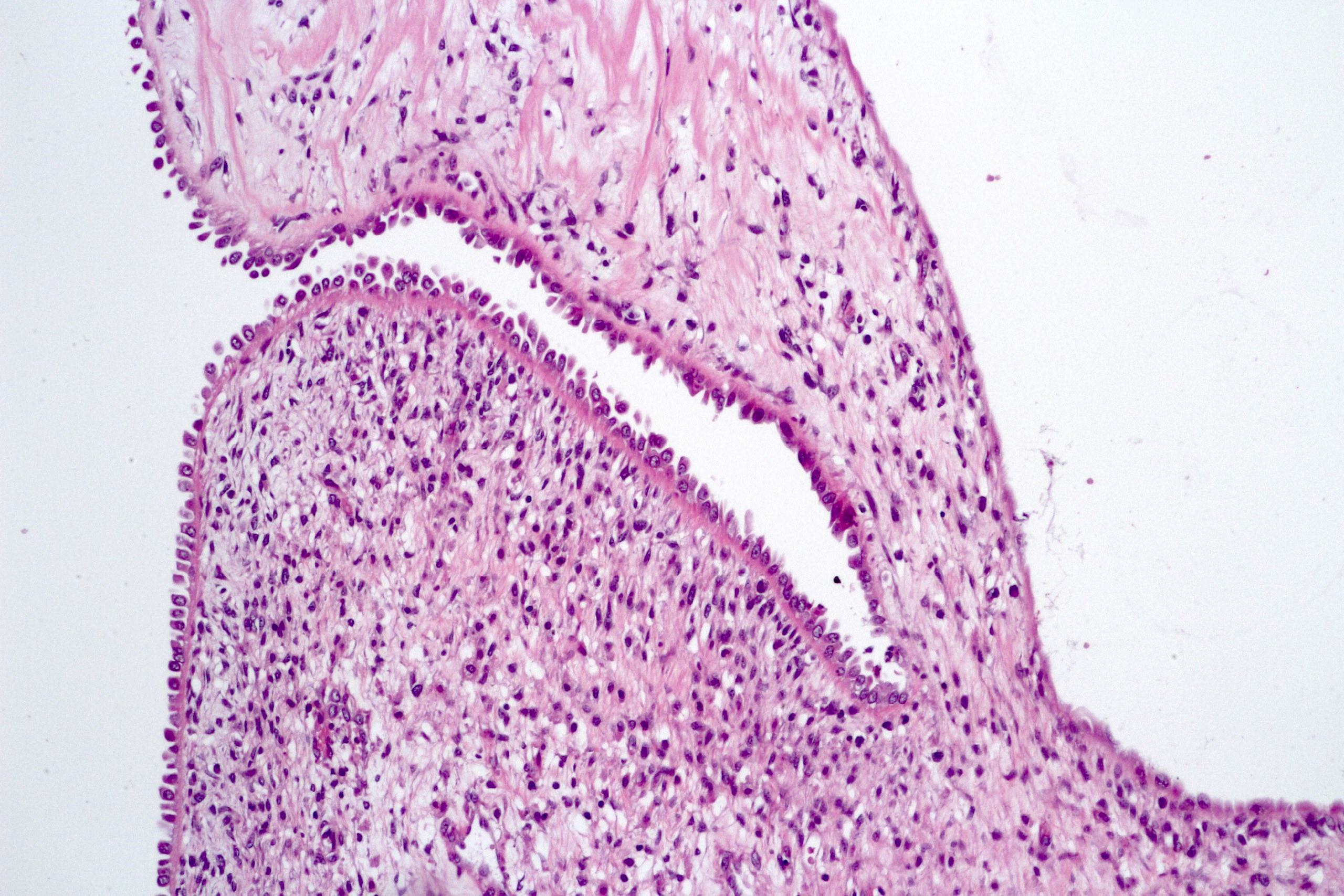
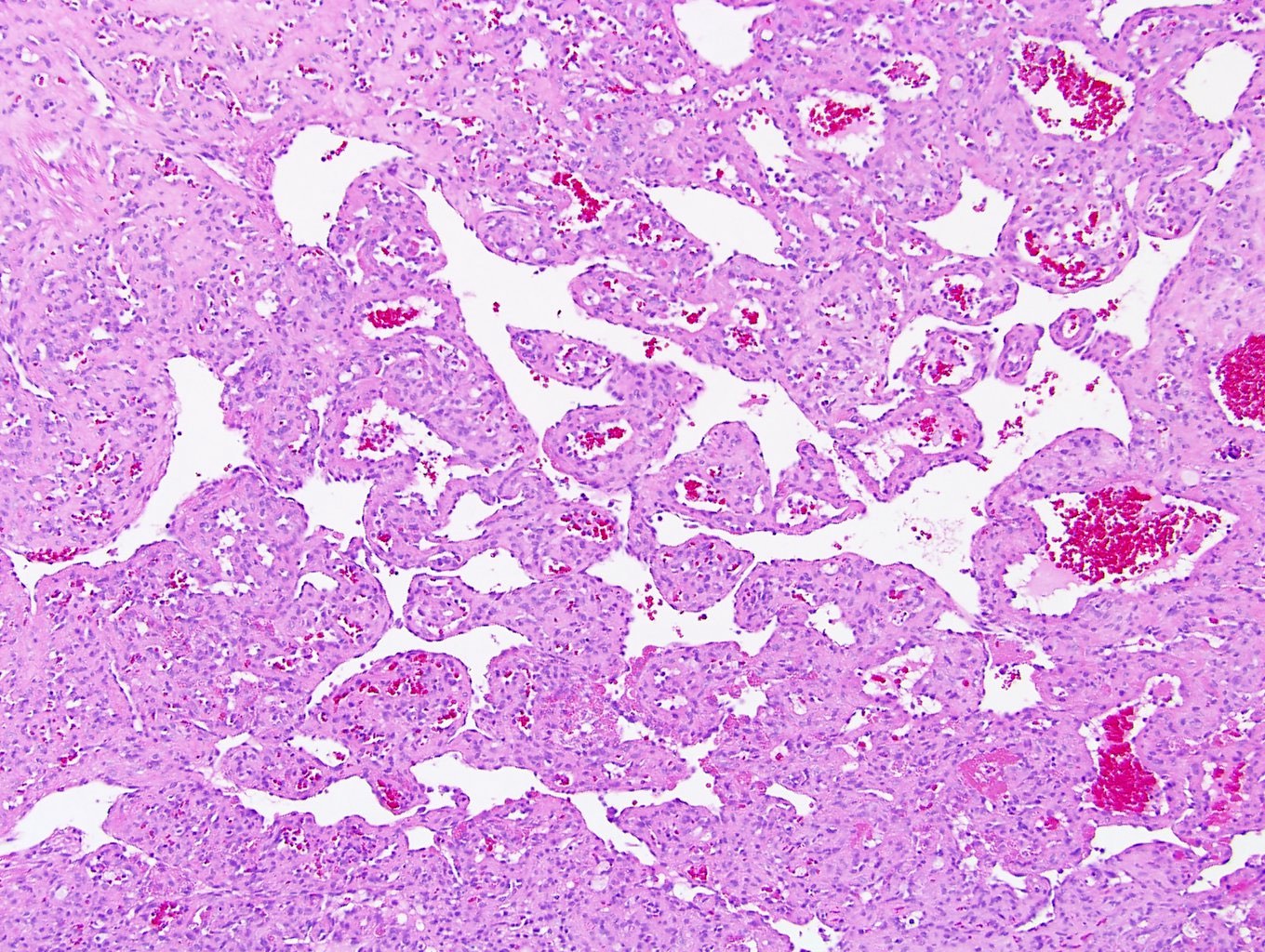
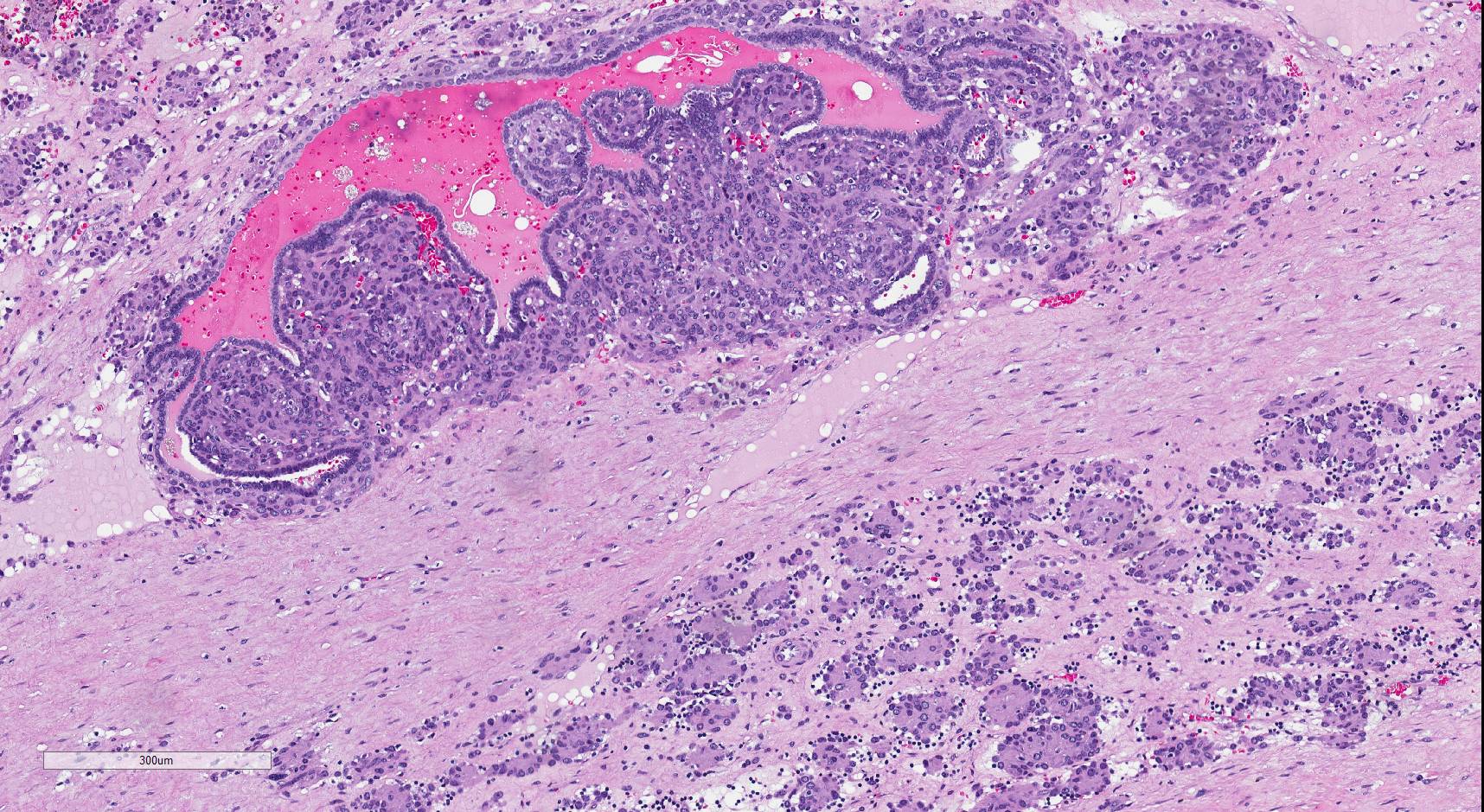
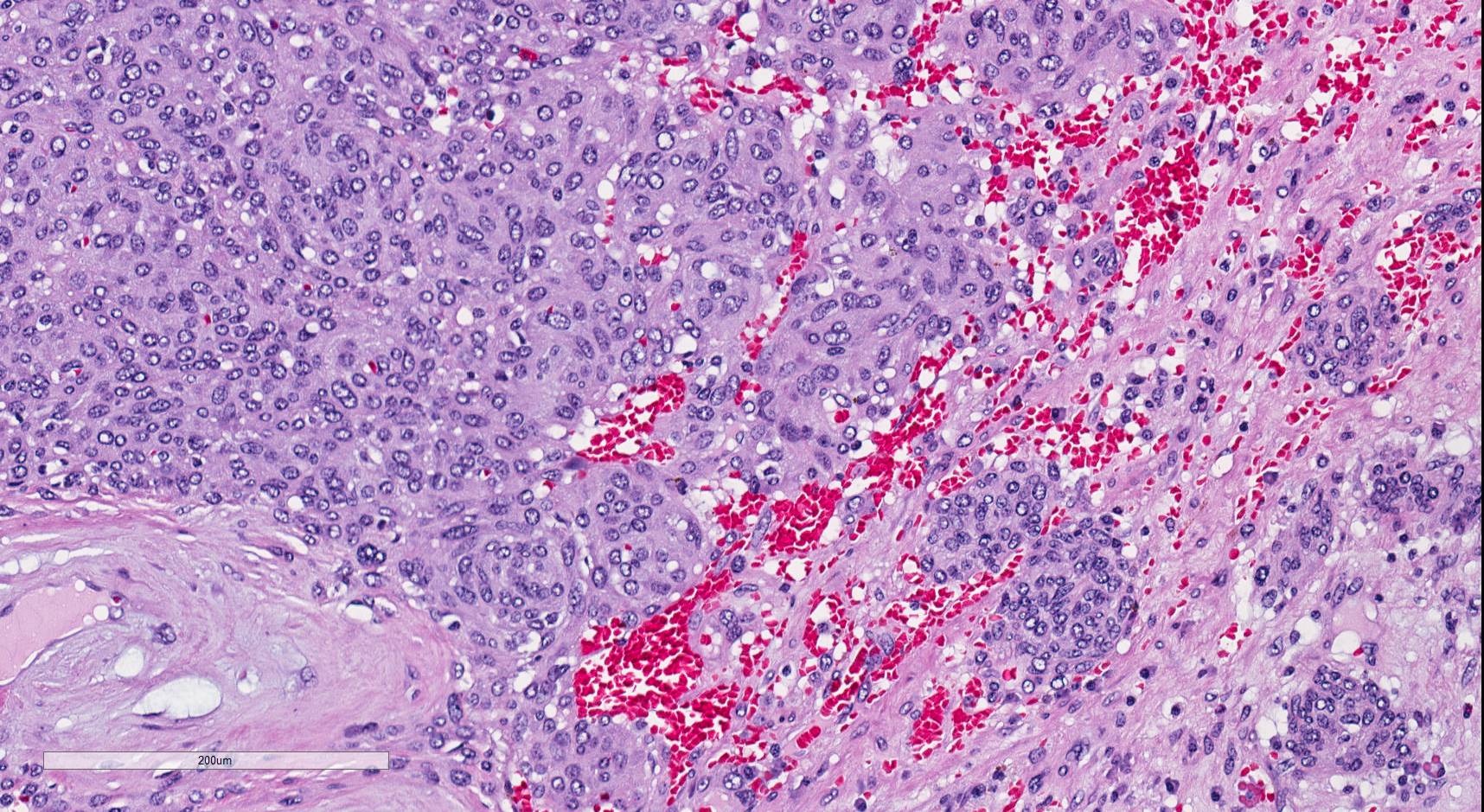
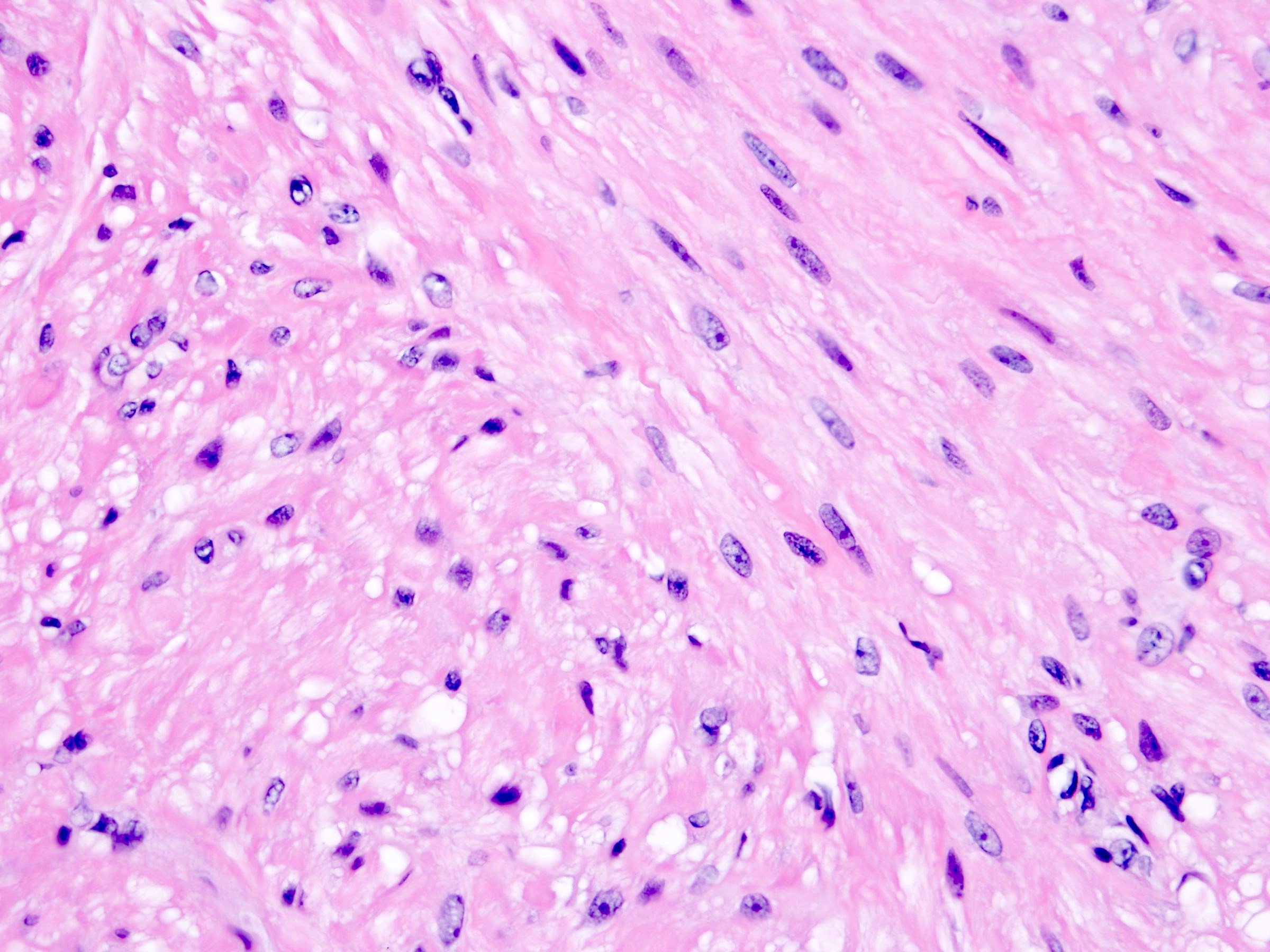
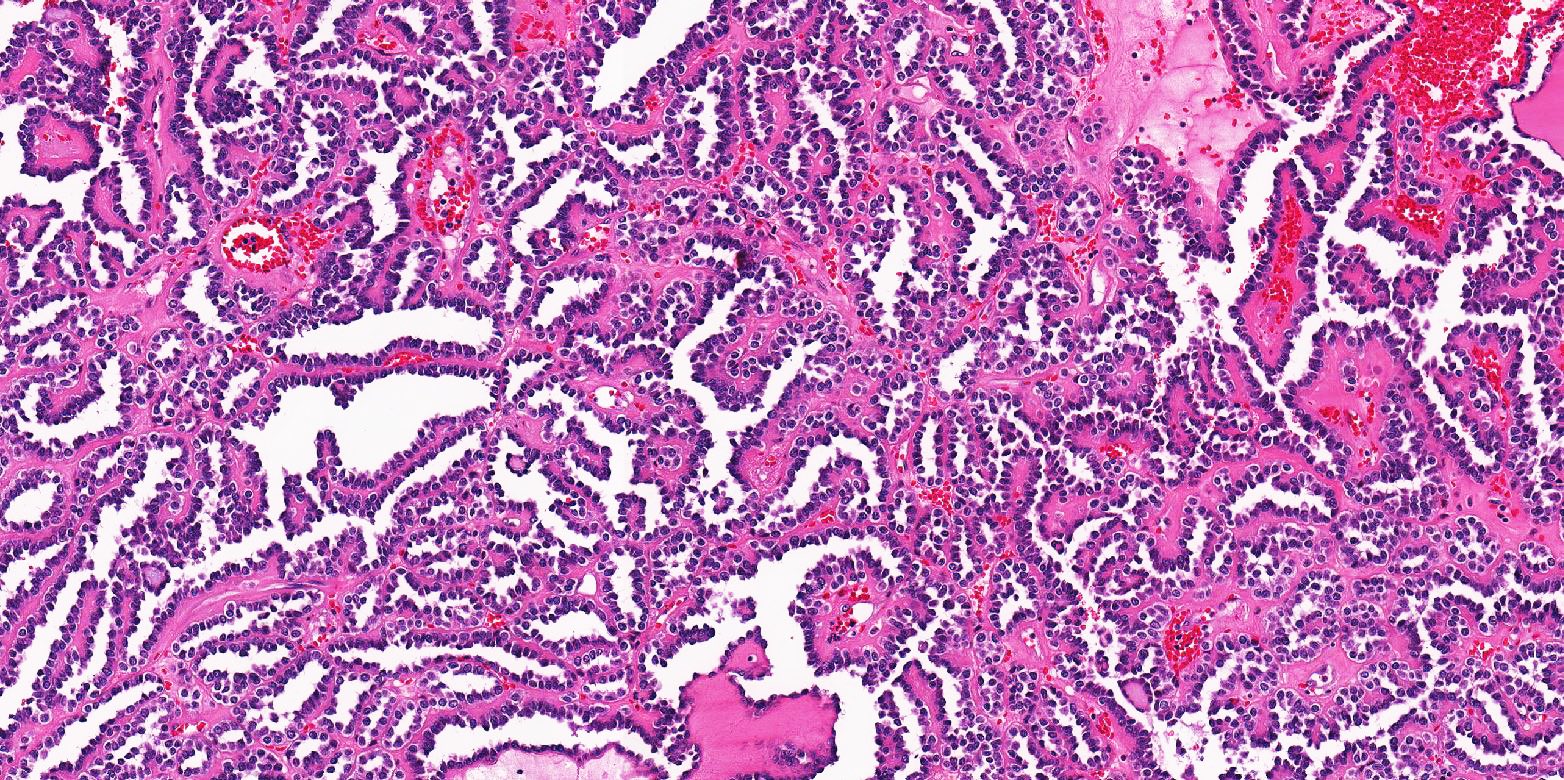
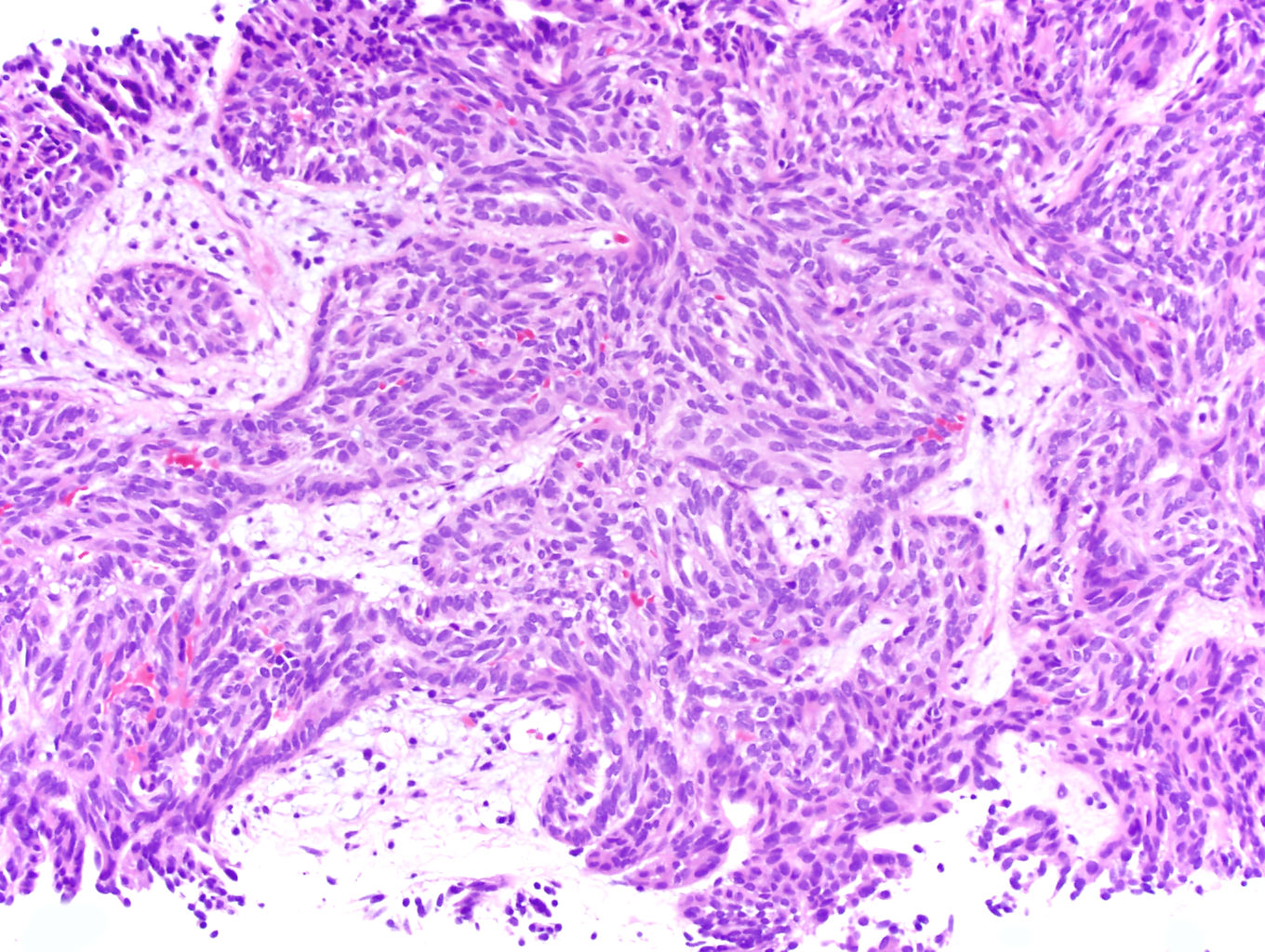
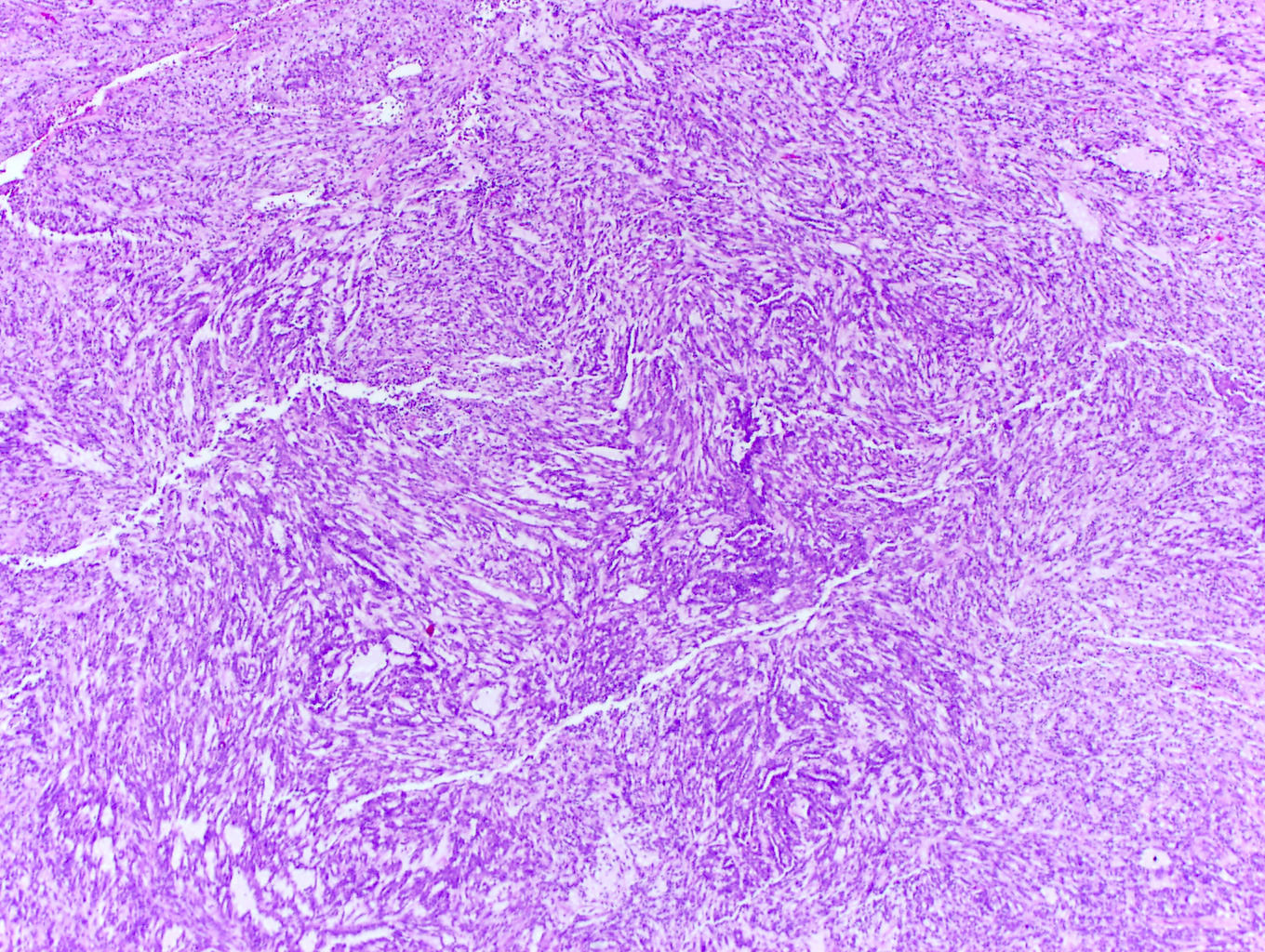
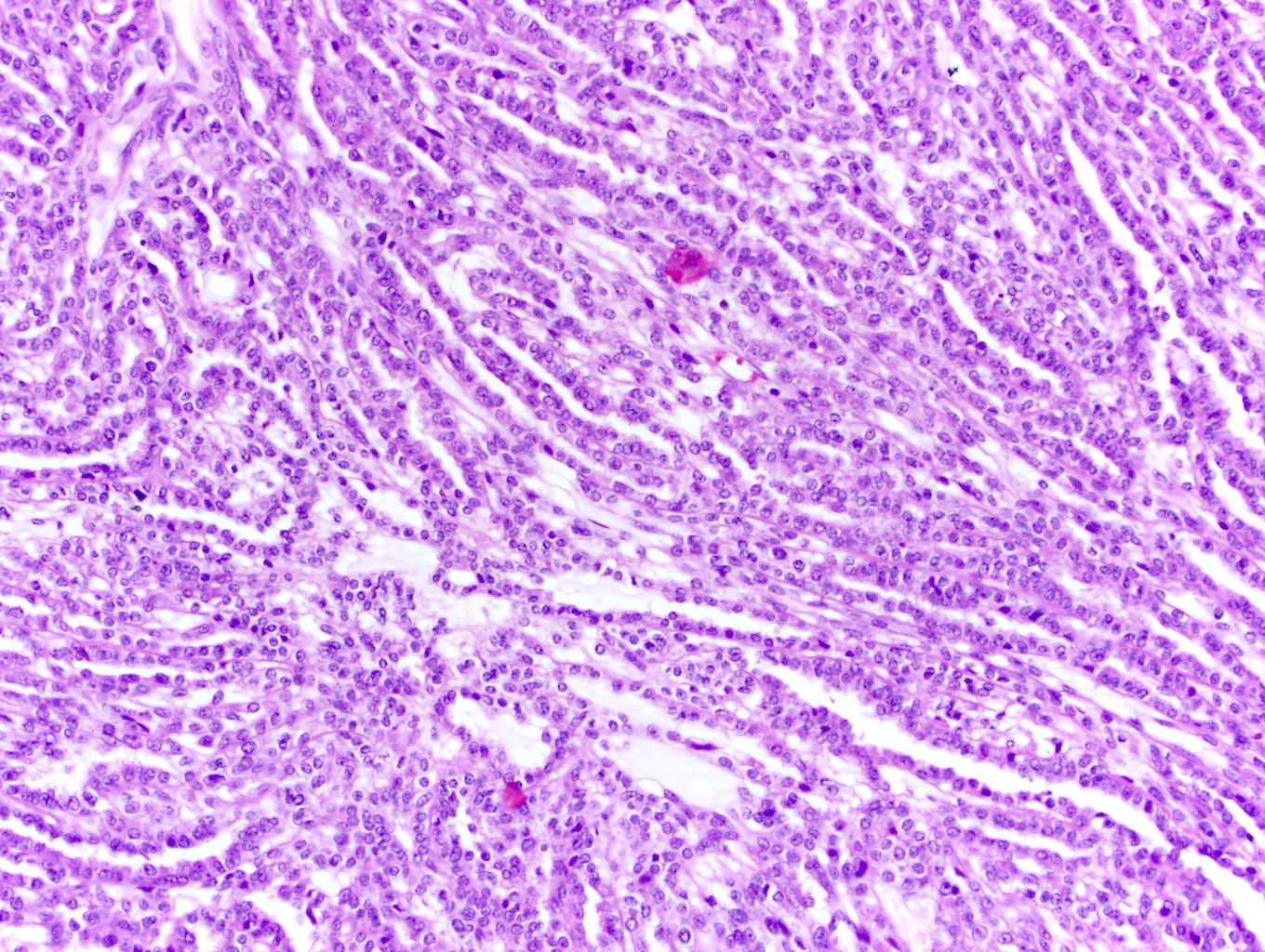
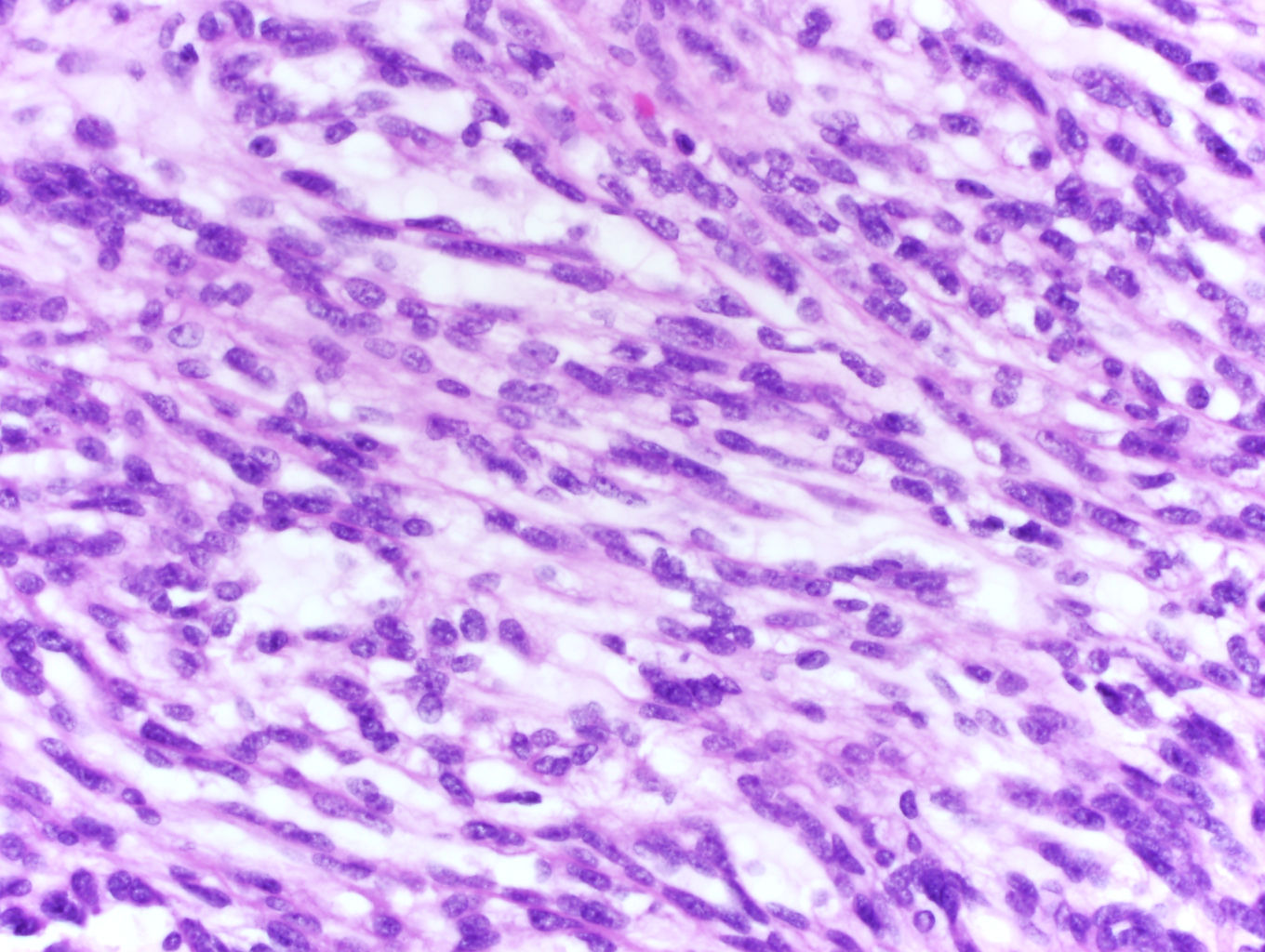
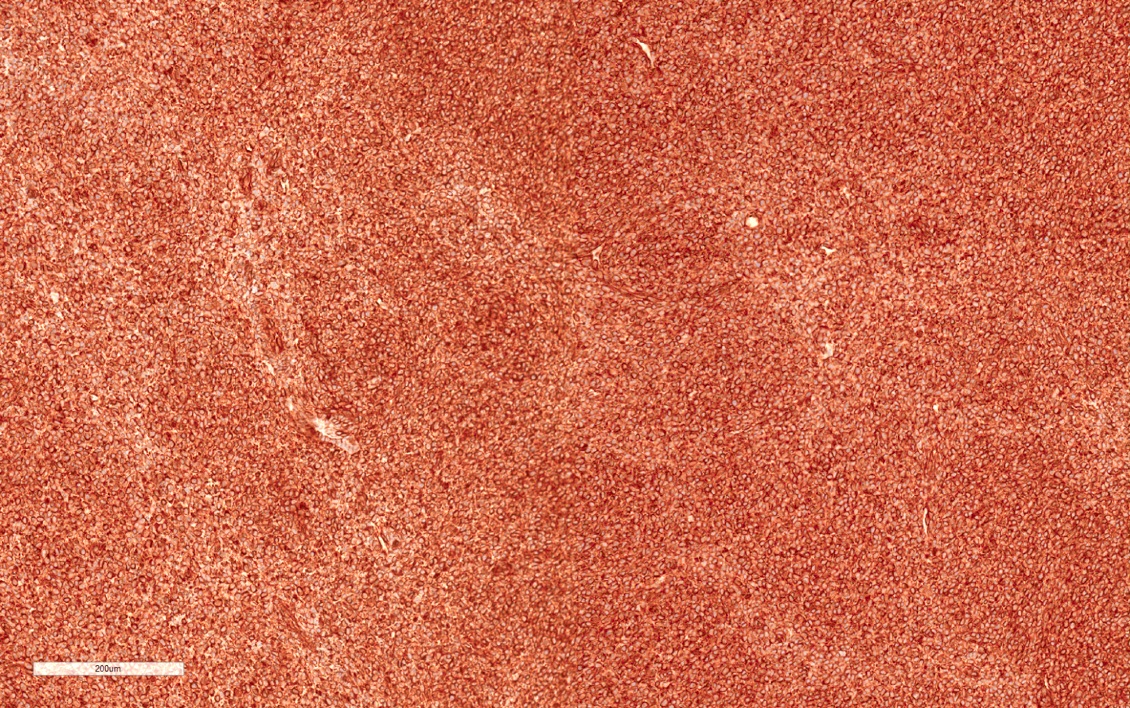
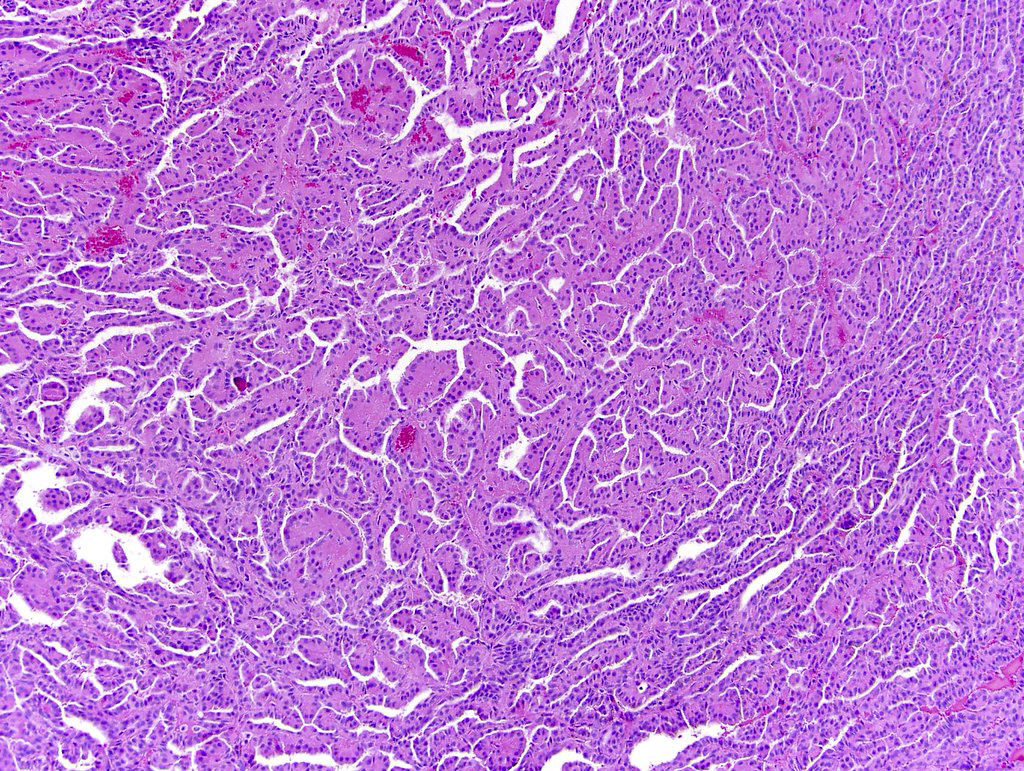
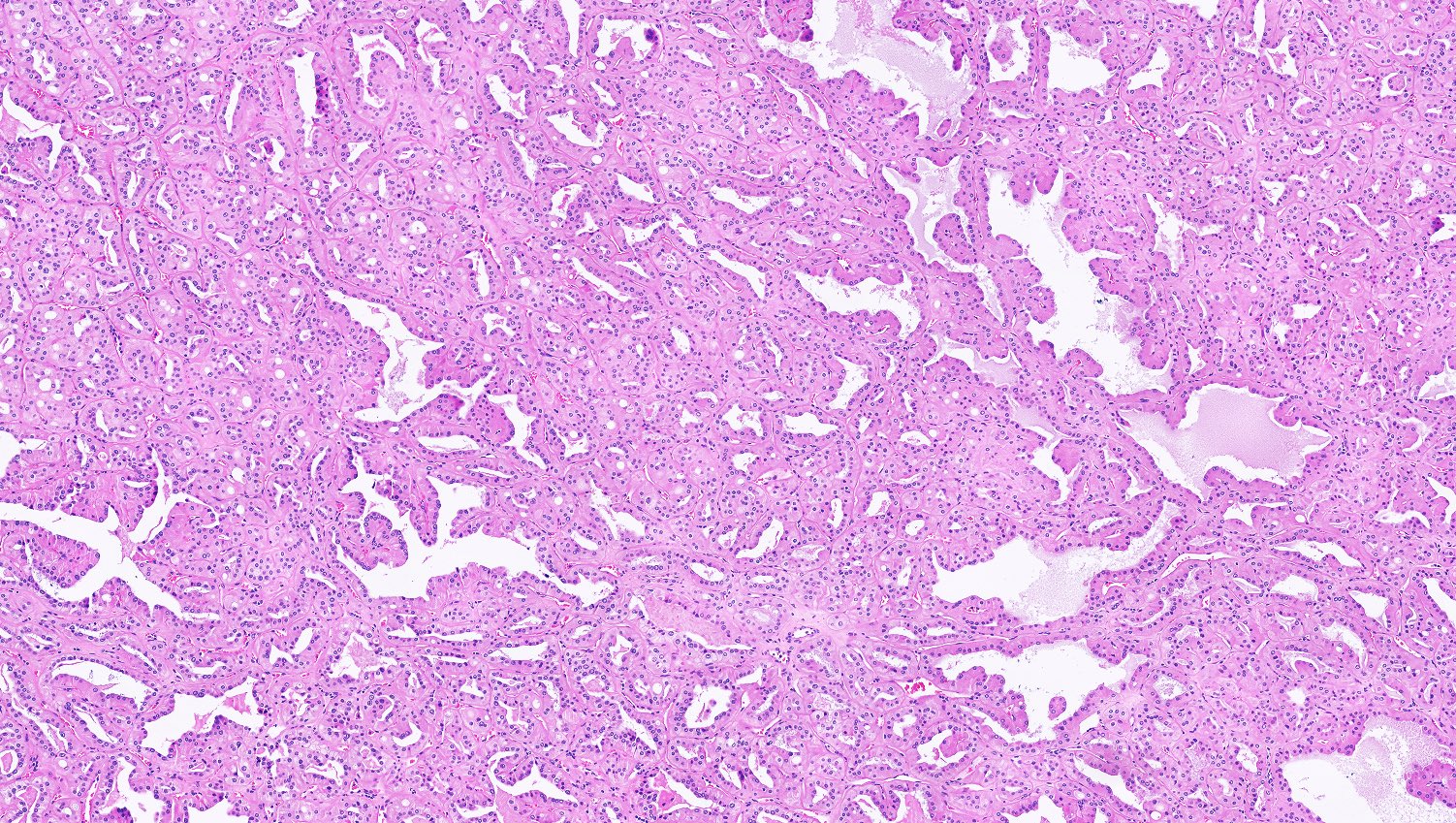
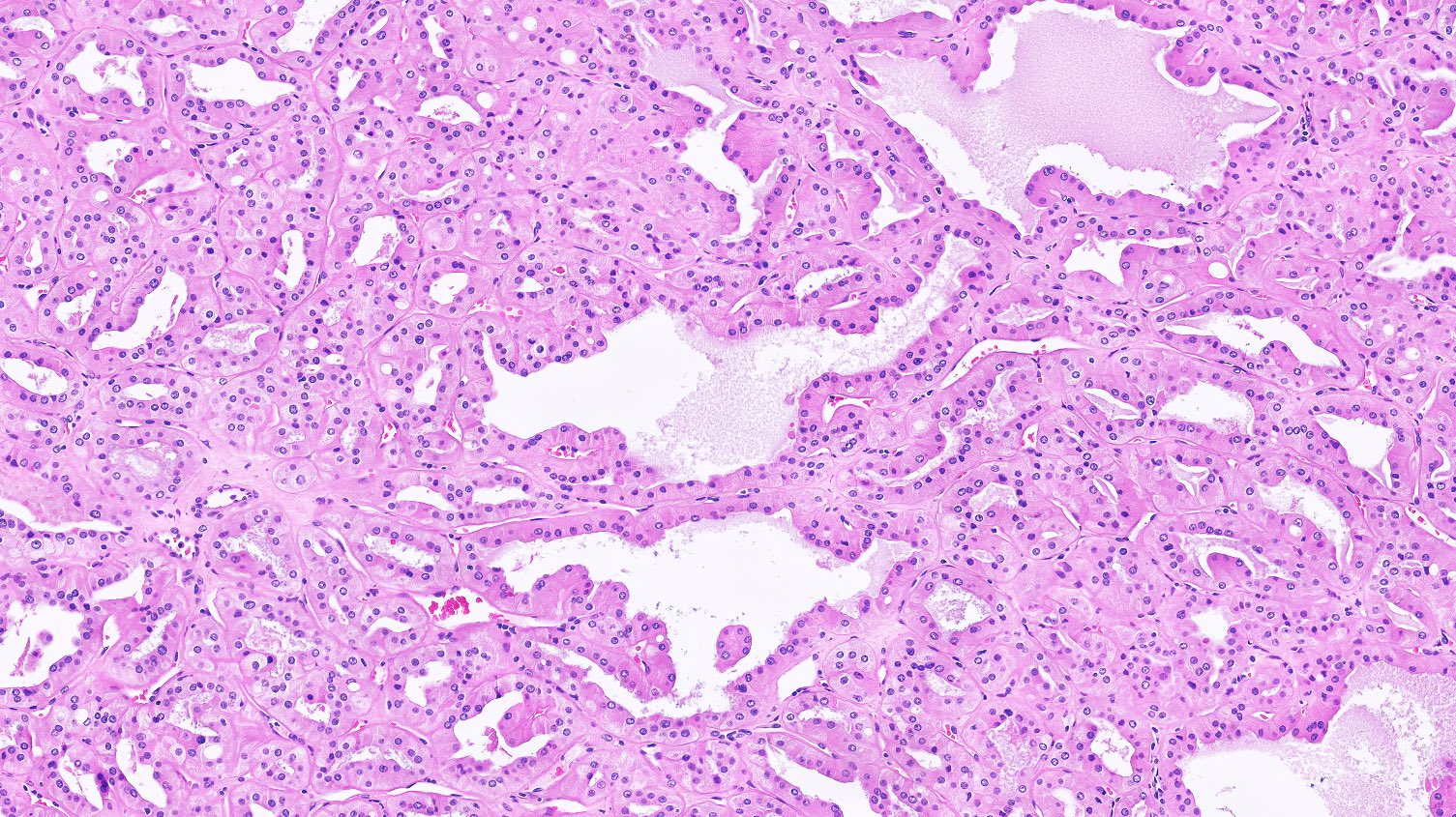
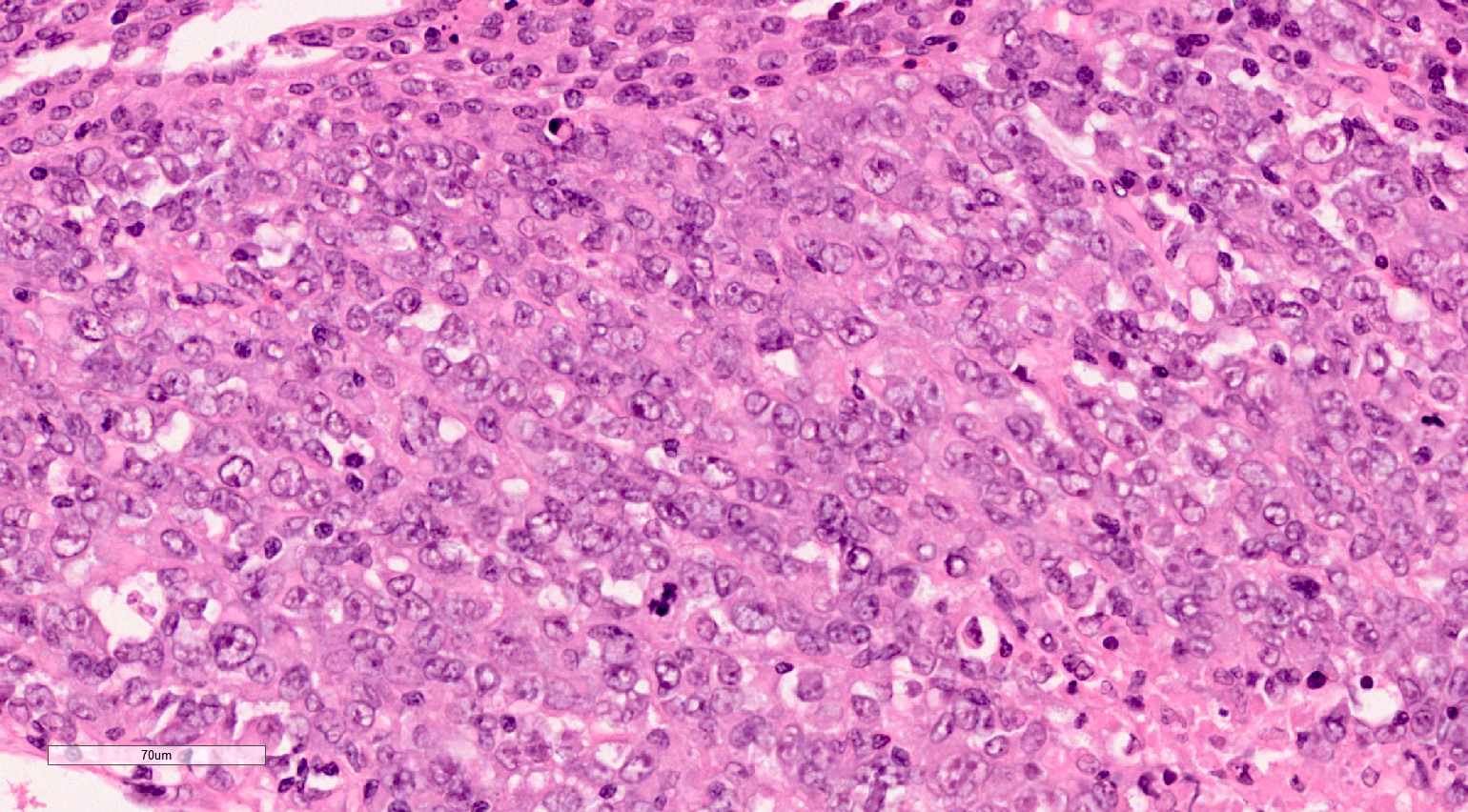
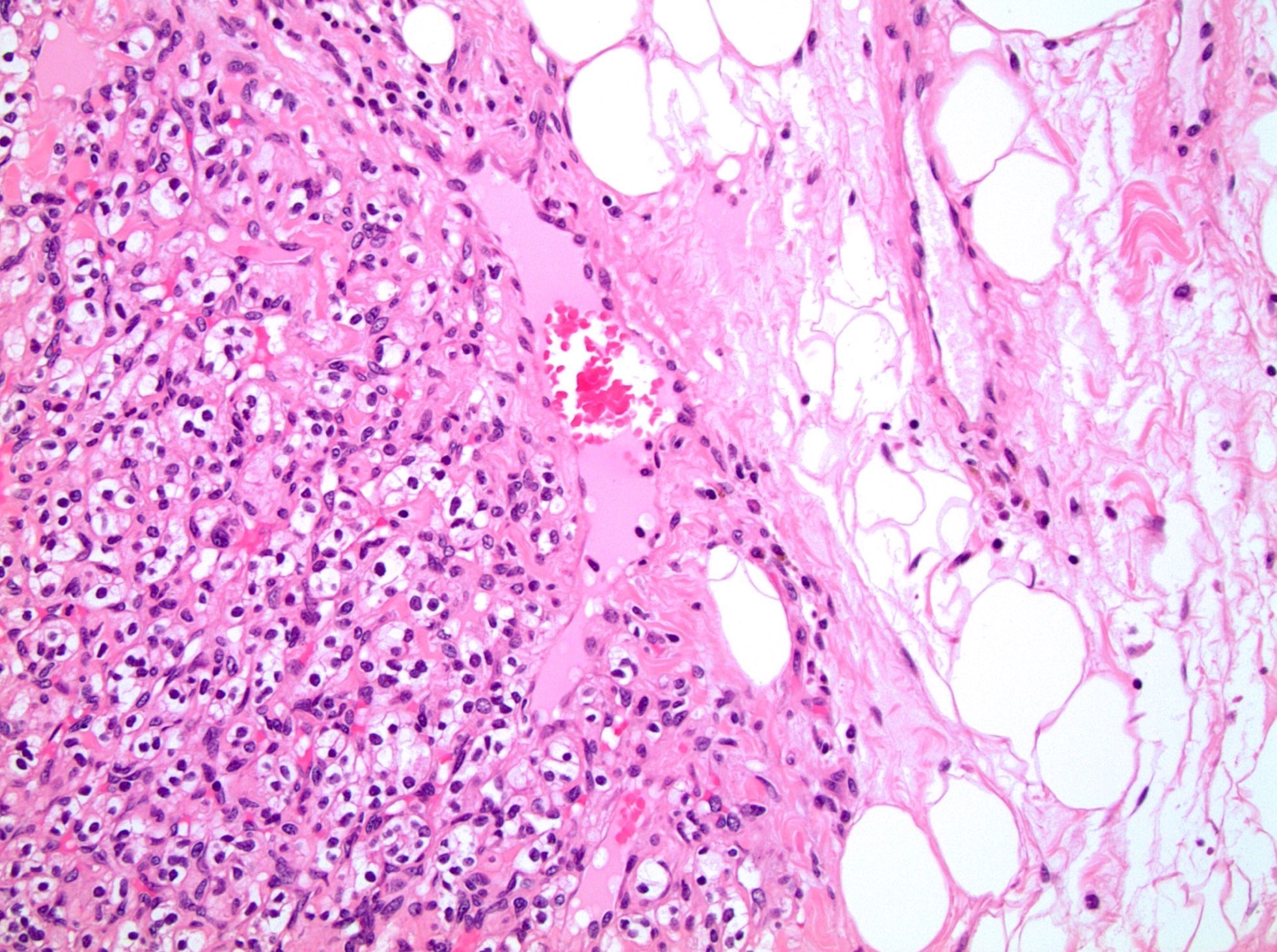
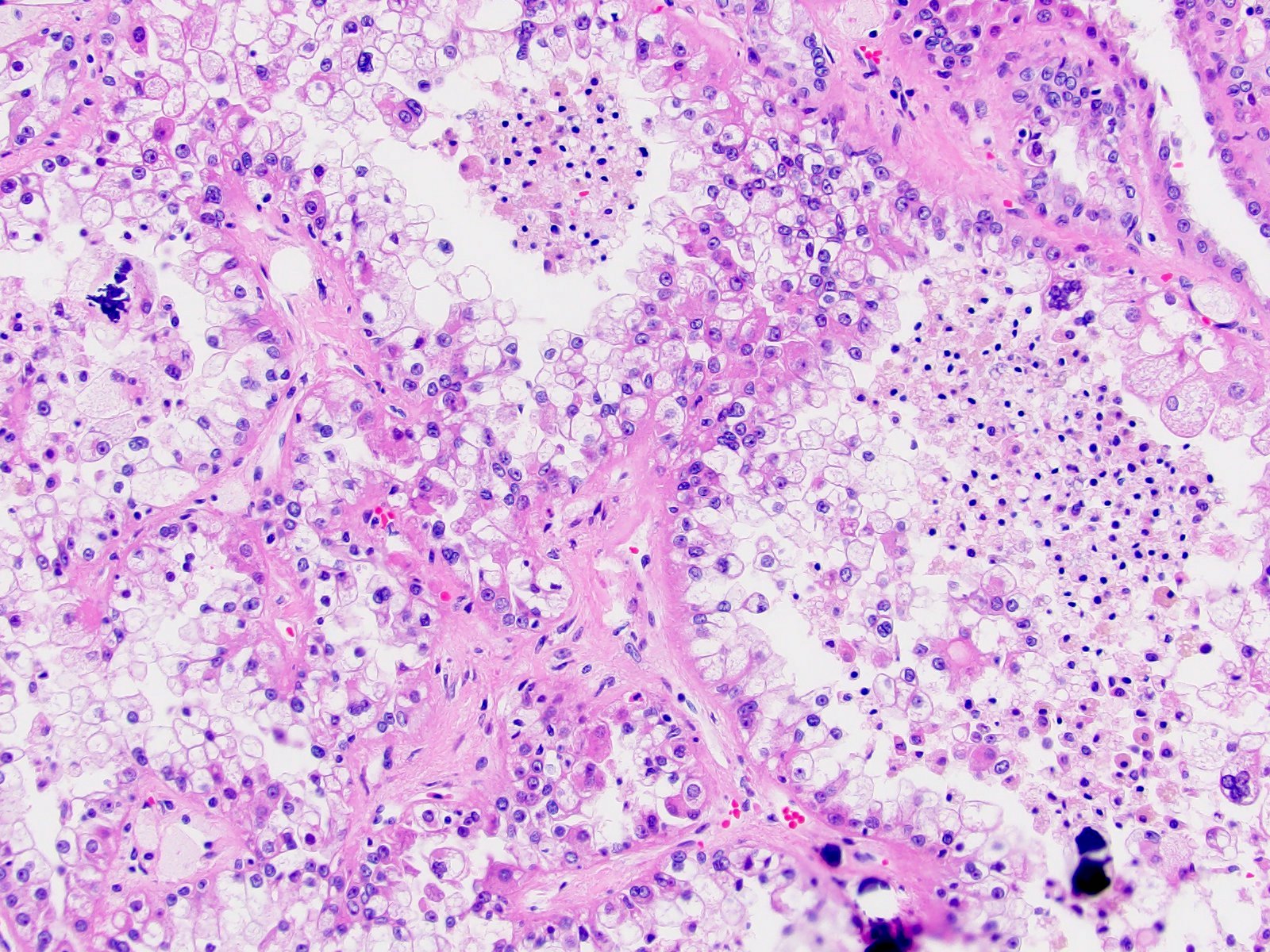
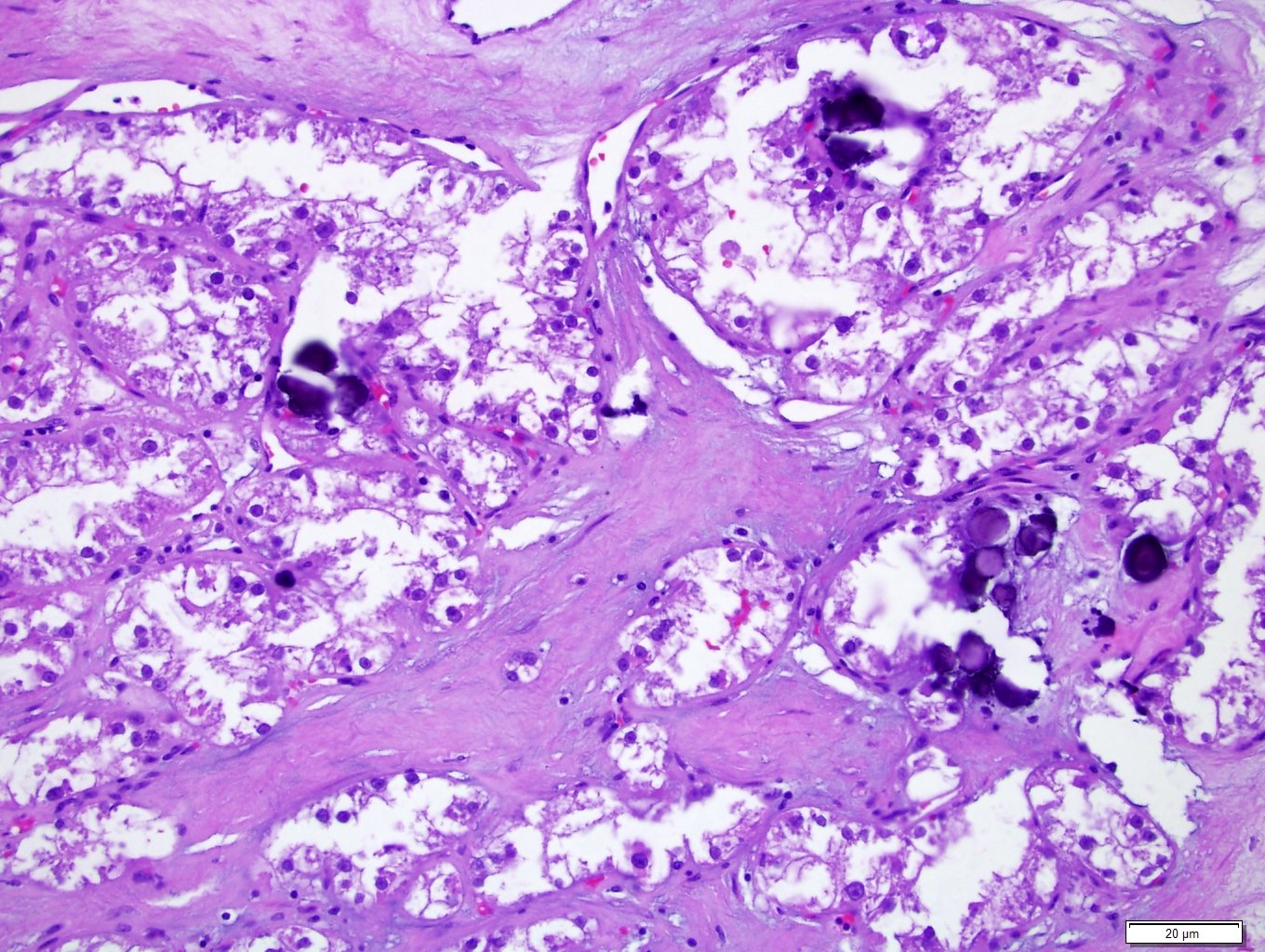
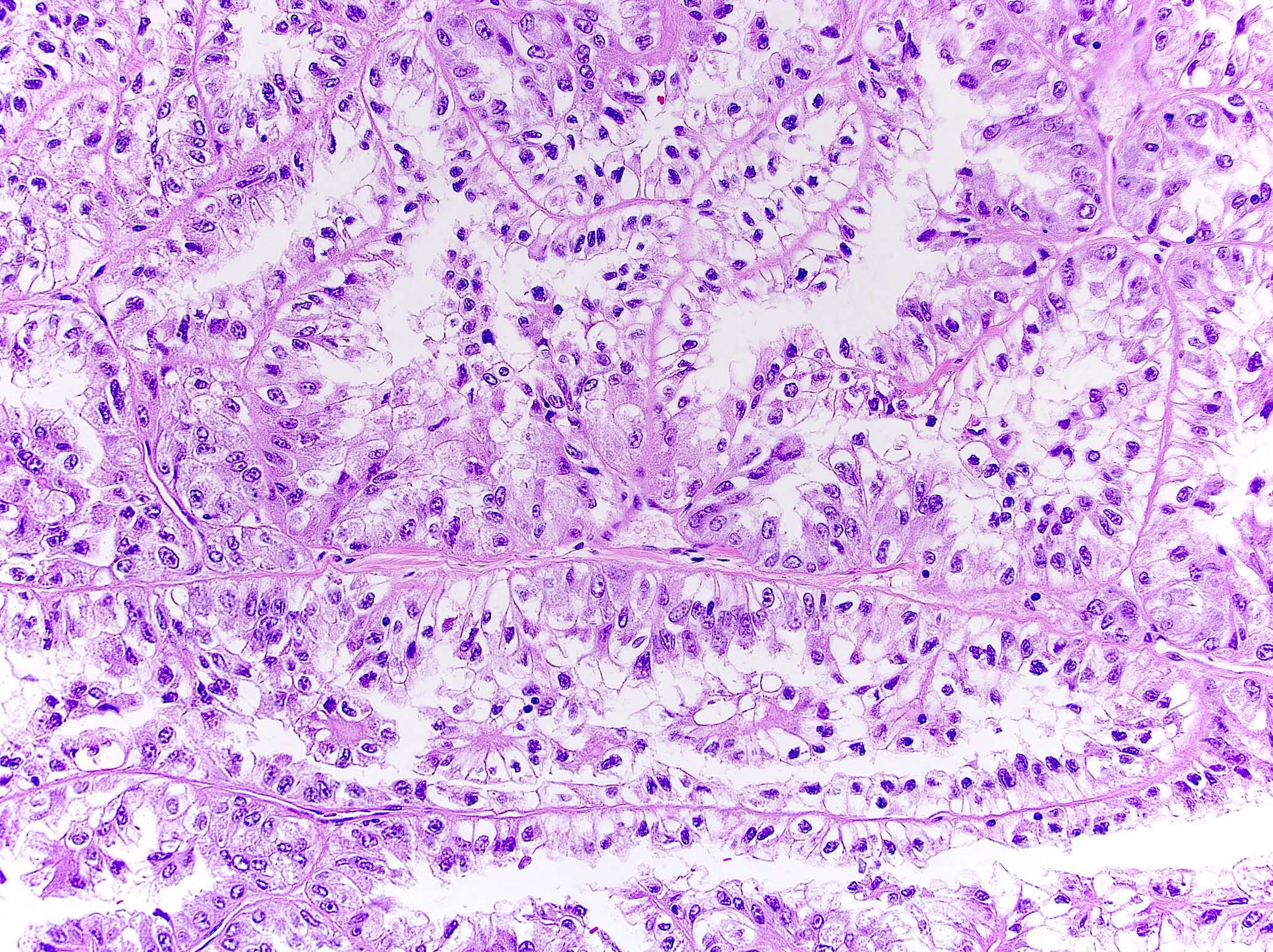
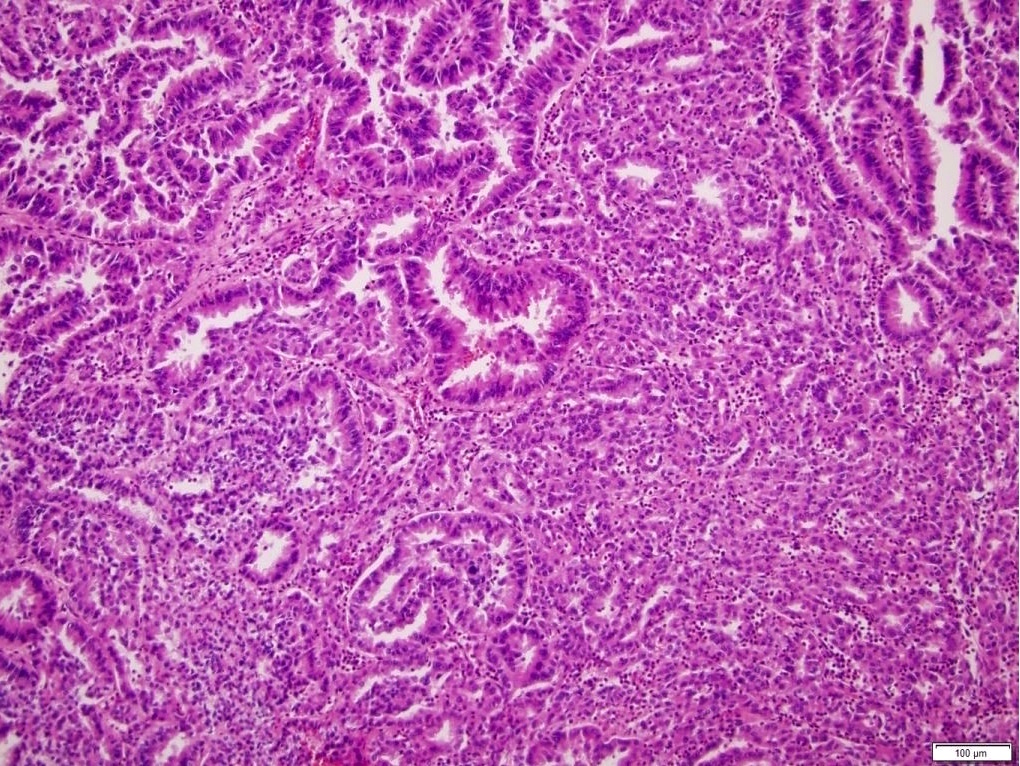
- More heterogeneous architecture: solid, mucinous cribriform, reticular, tubular, papillary growth; discohesive sheets or infiltrating single cells
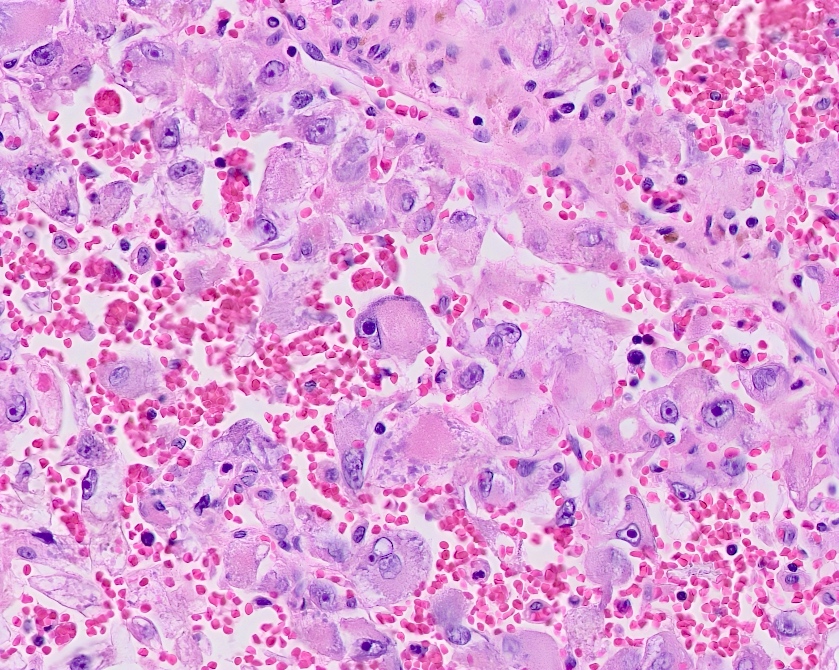
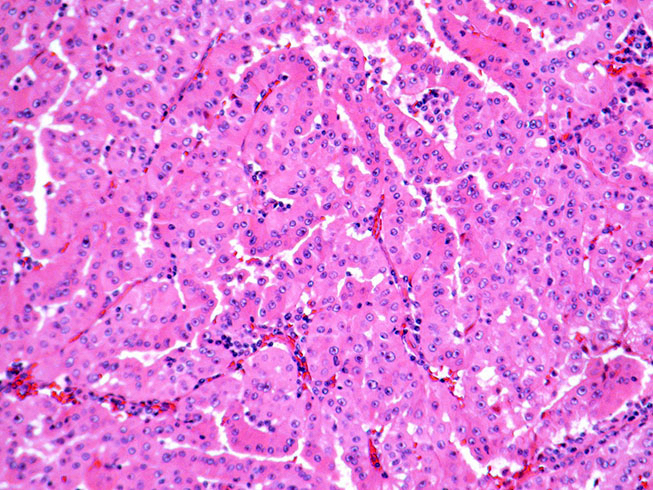
- Eosinophilic polygonal cells, rhabdoid or signet ring due to intracytoplasmic vacuolization
- May contain psammoma bodies and foamy macrophages
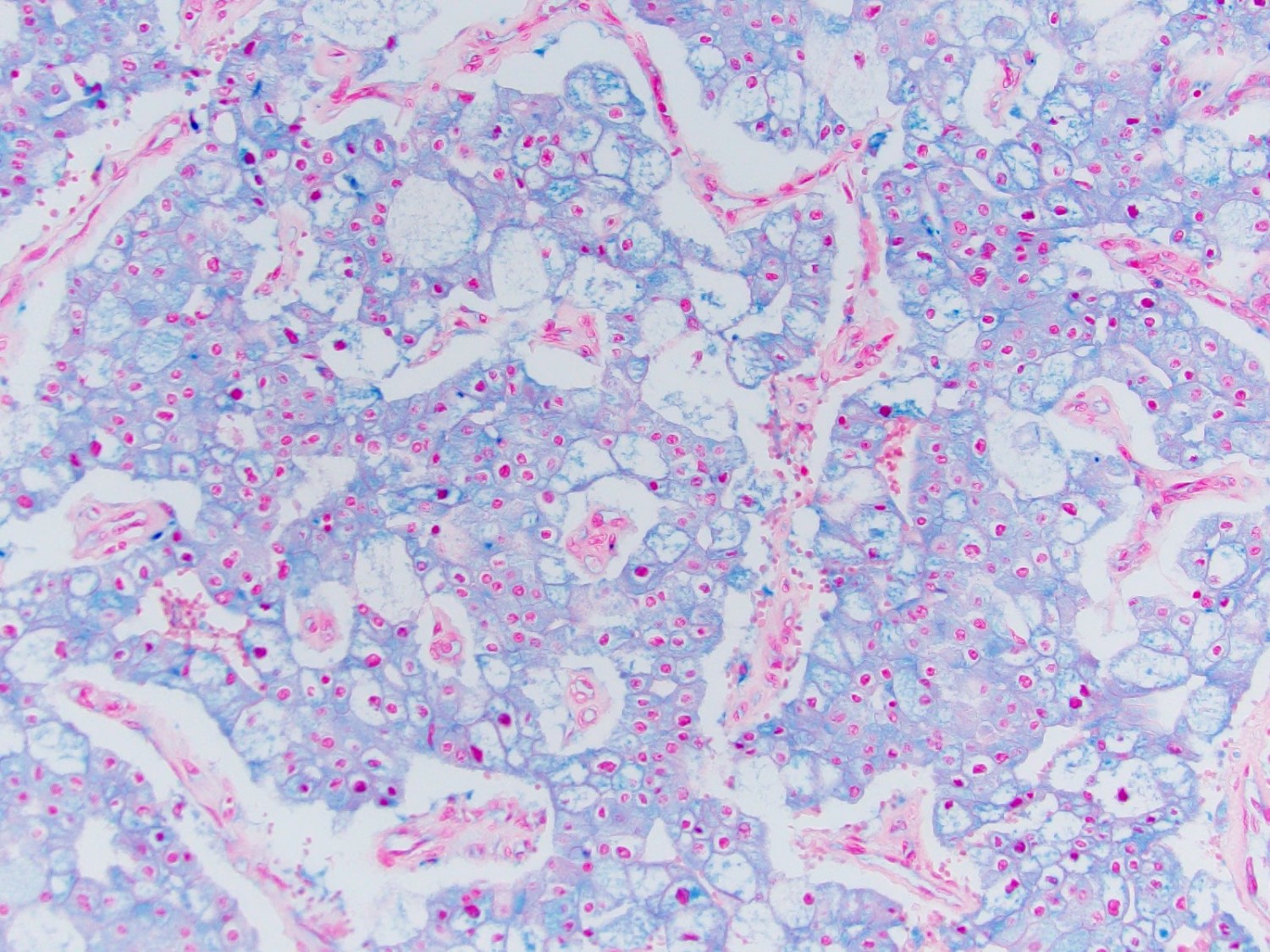
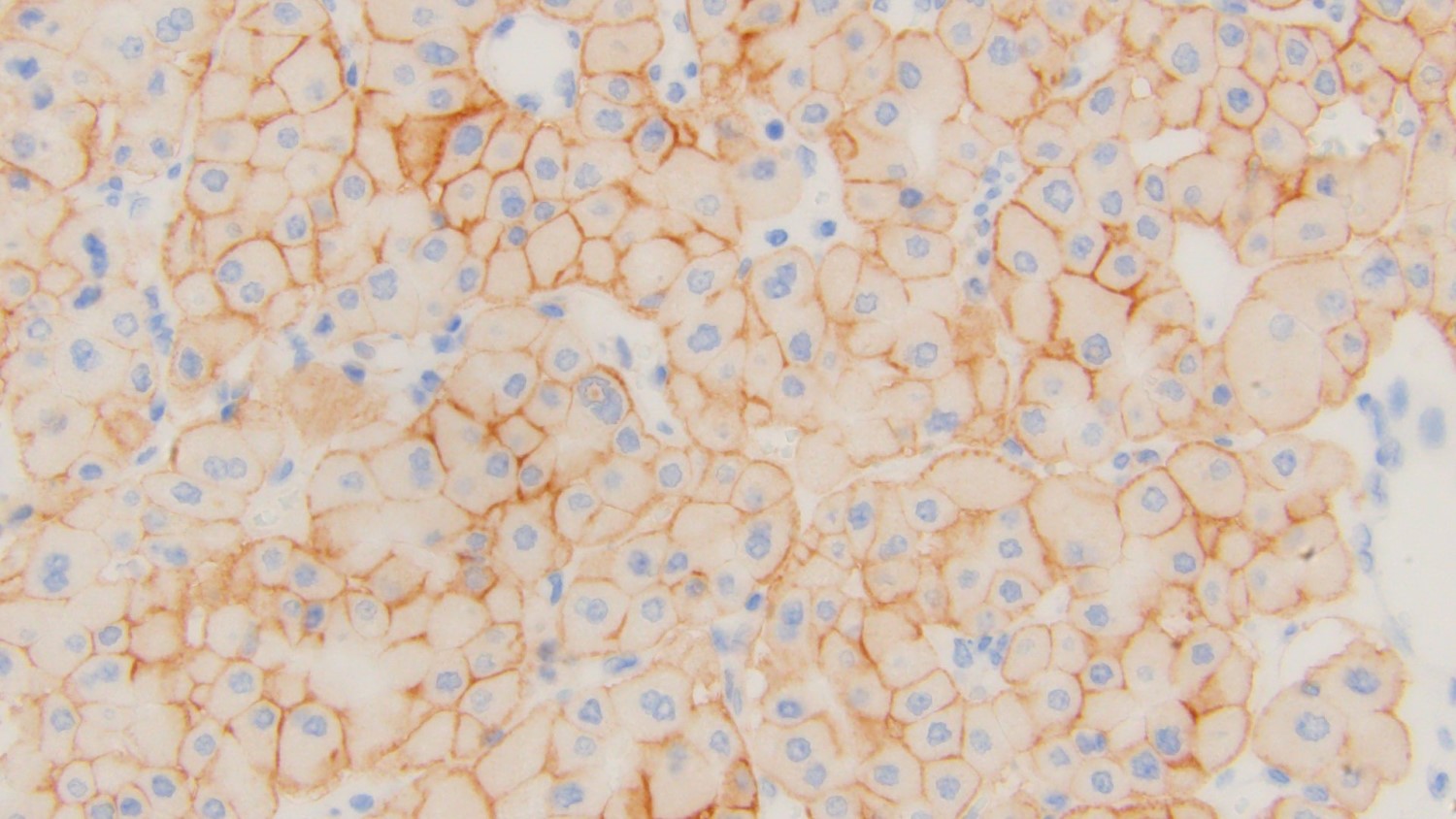
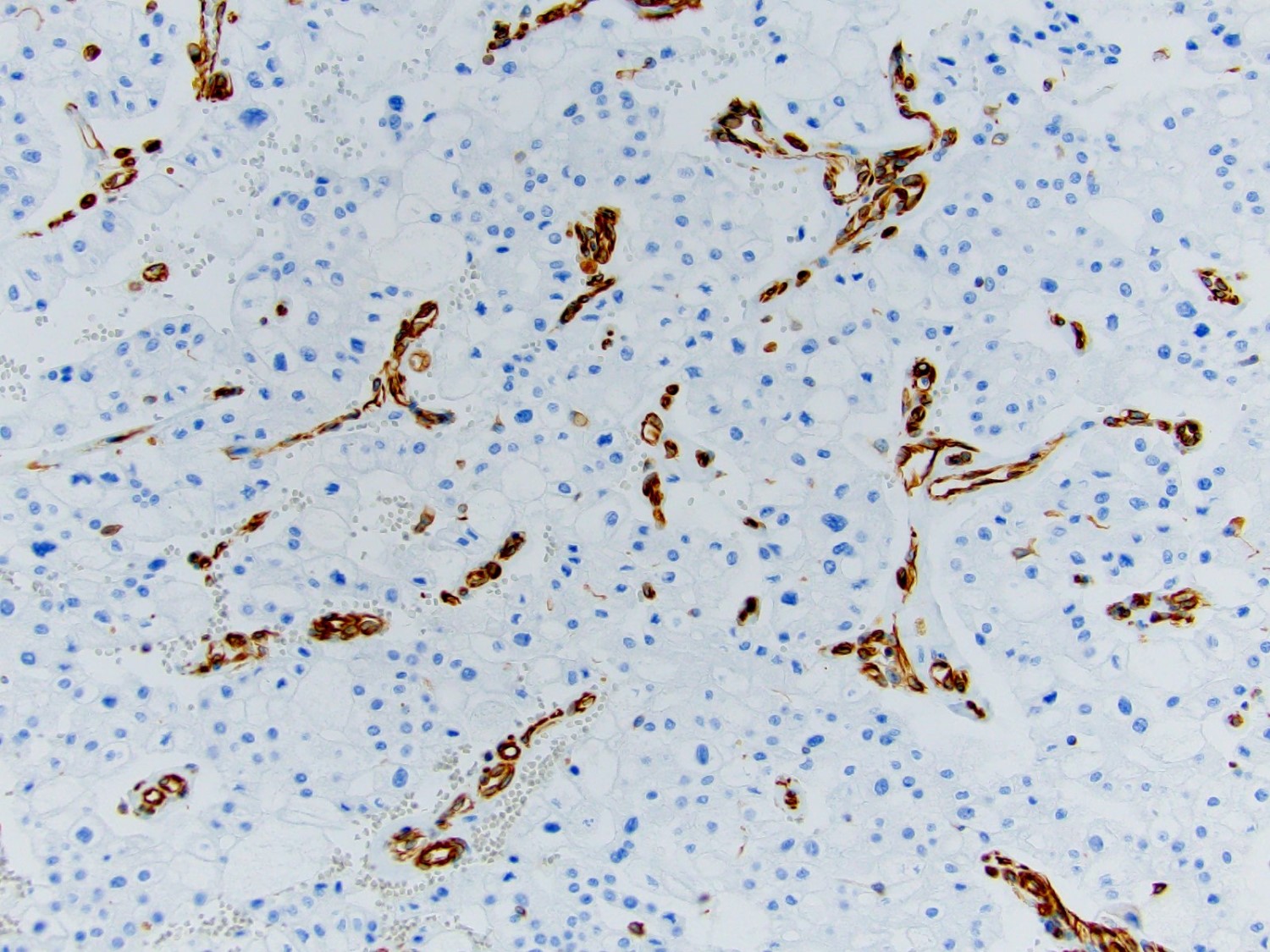
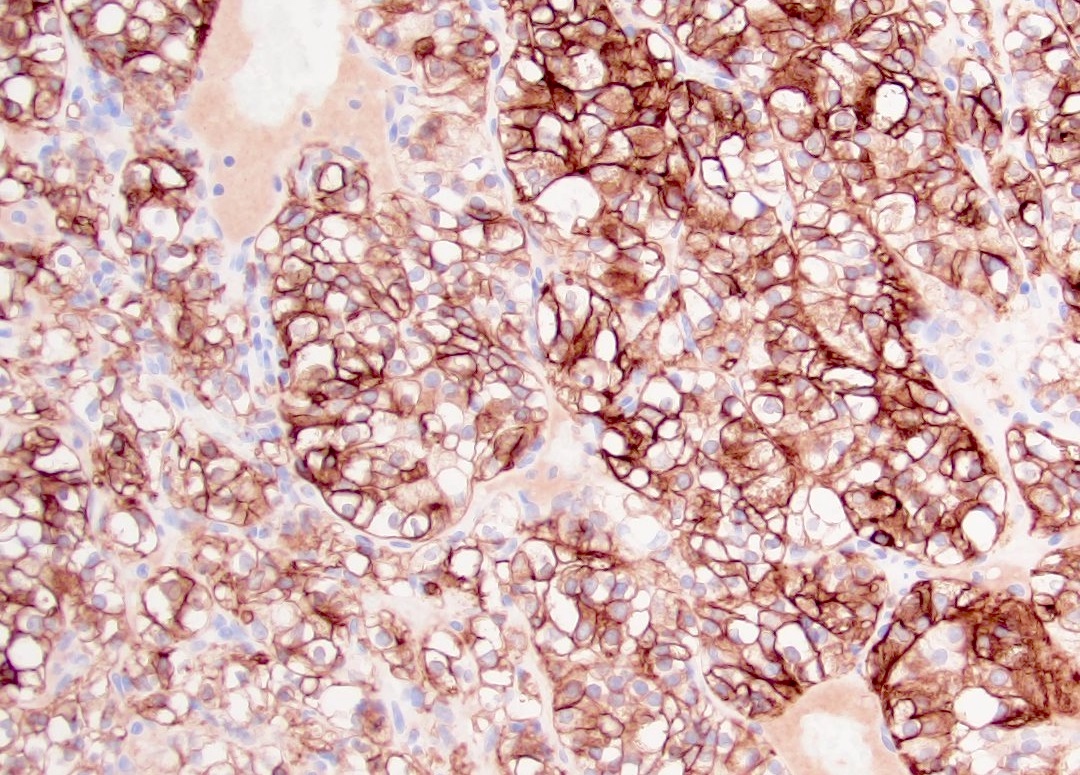
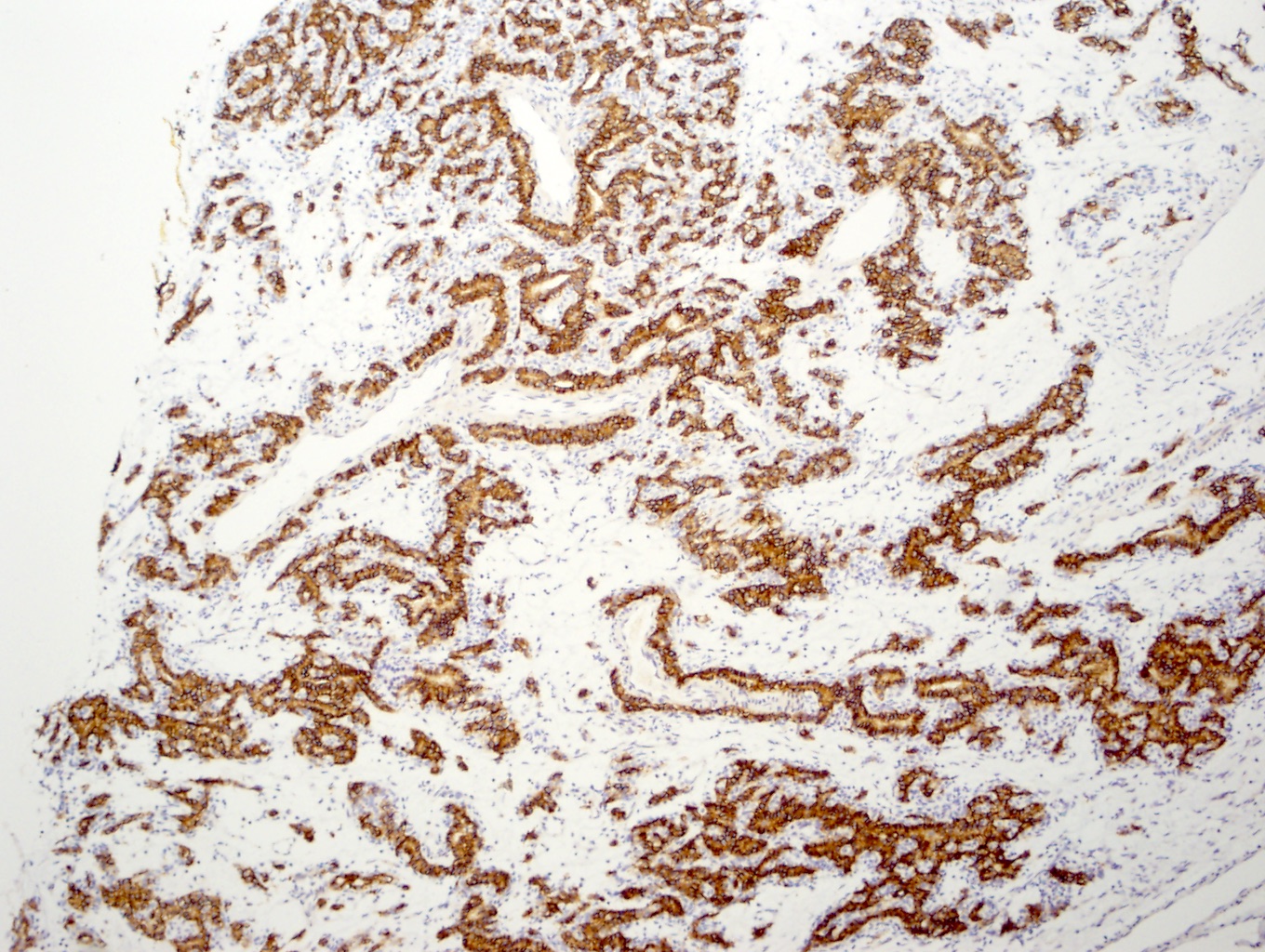
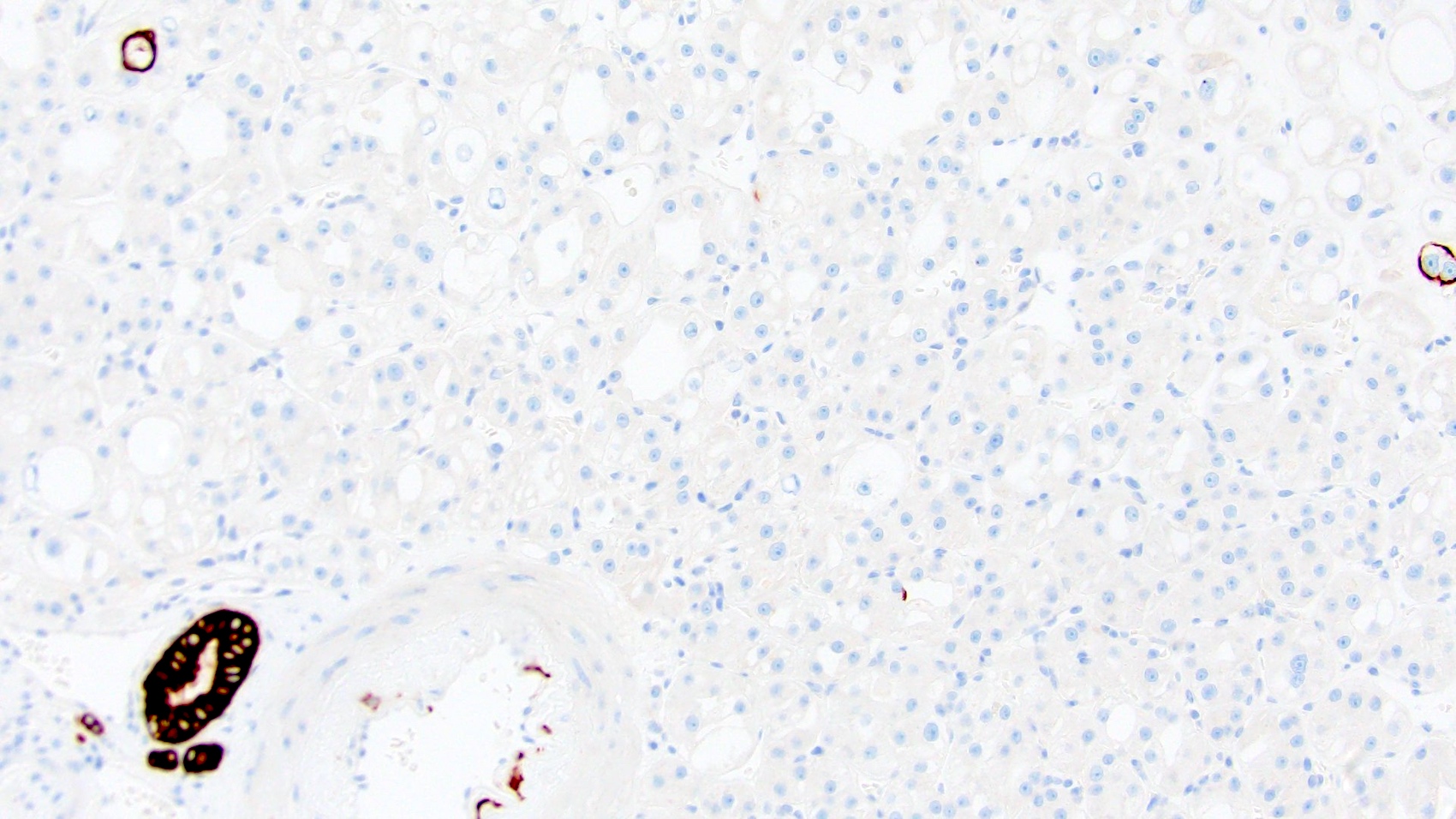
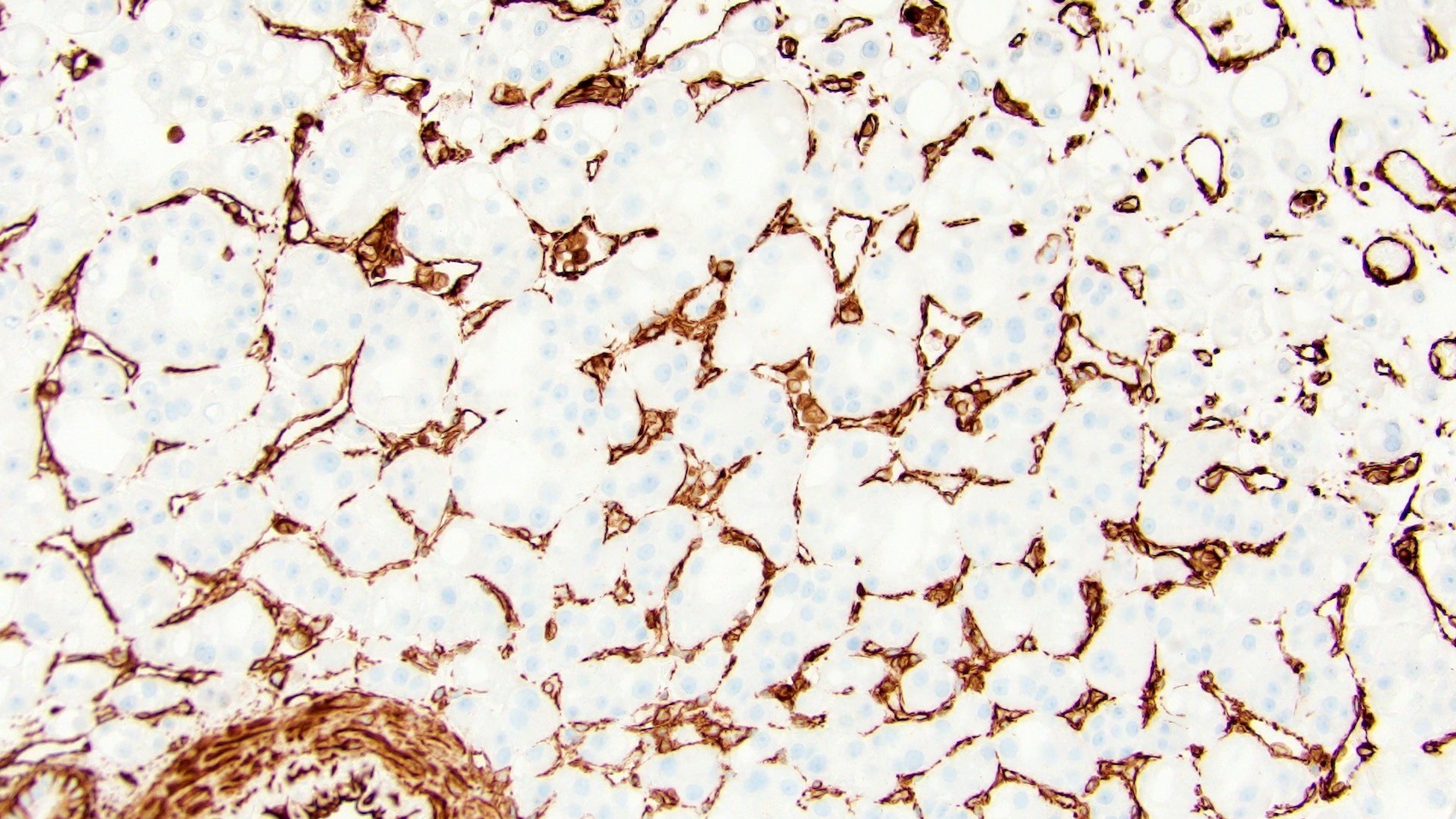
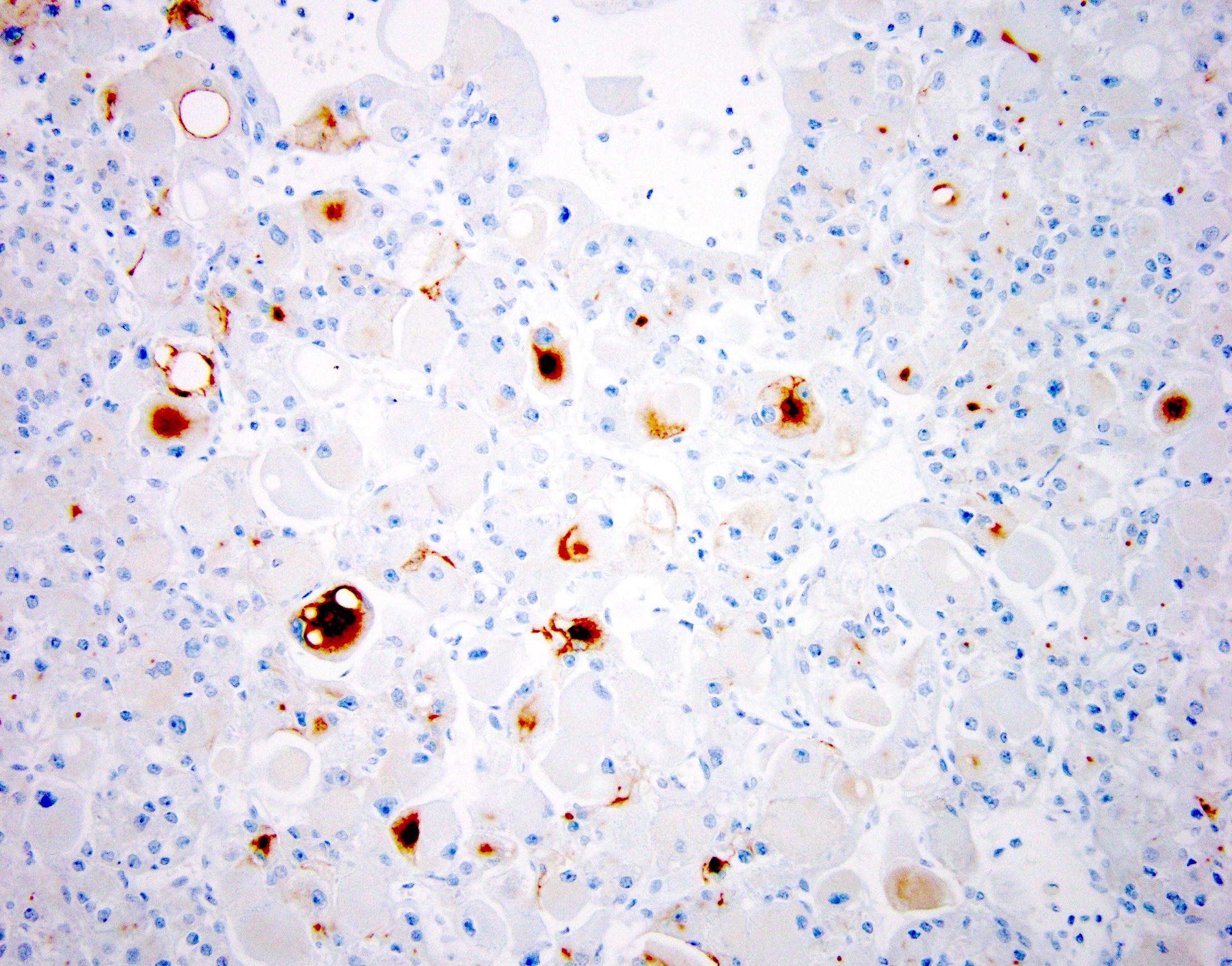
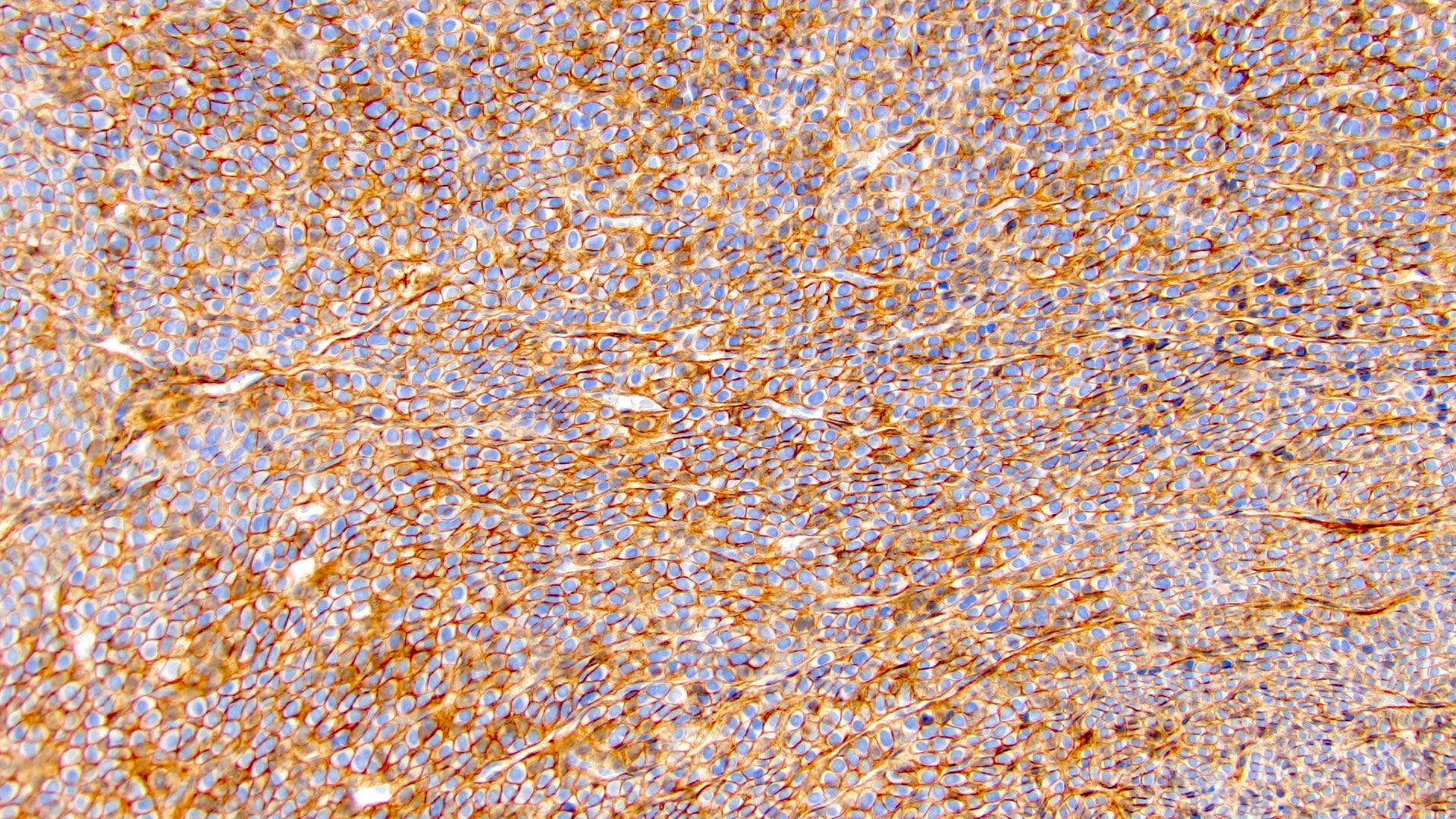
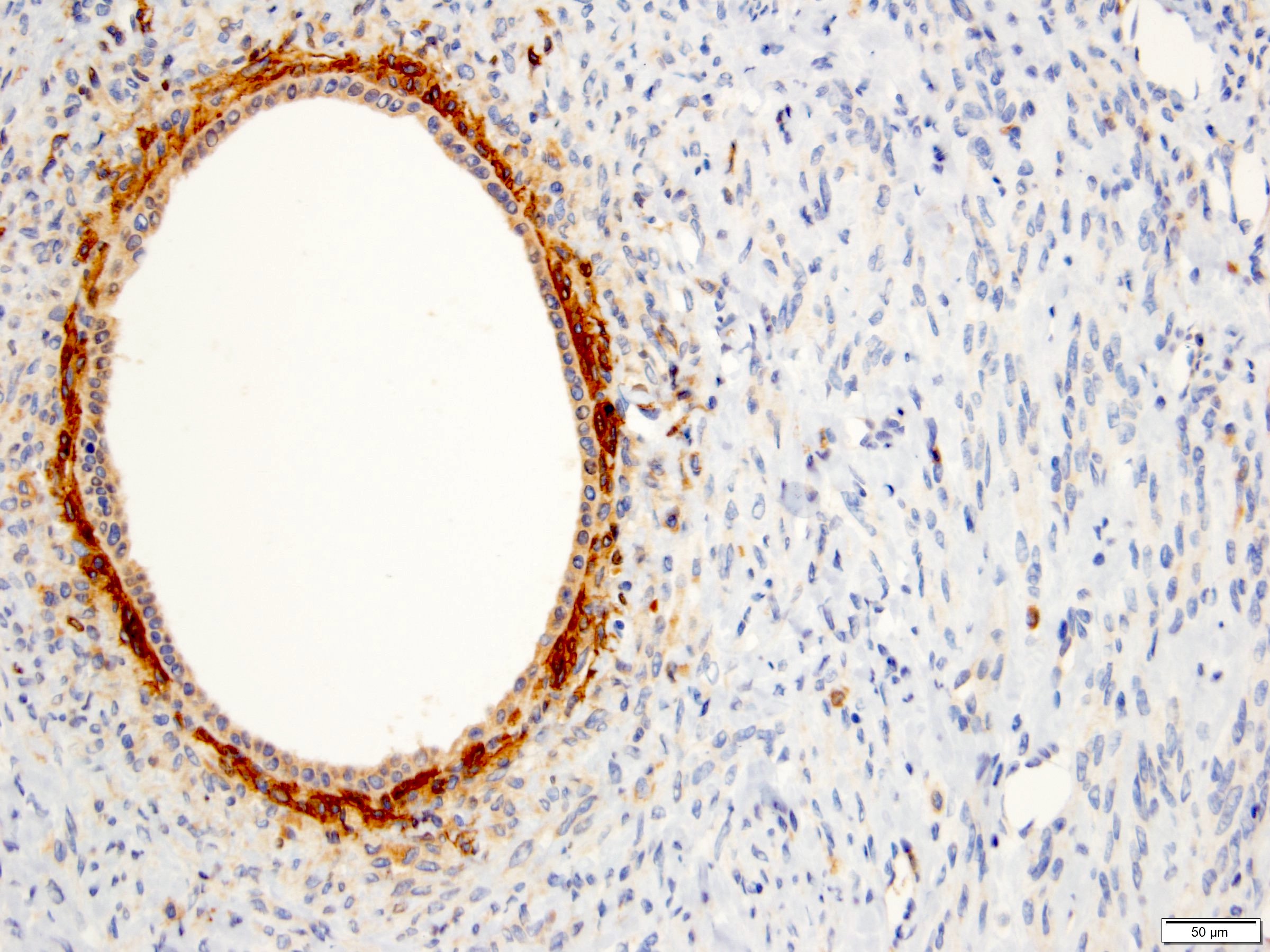
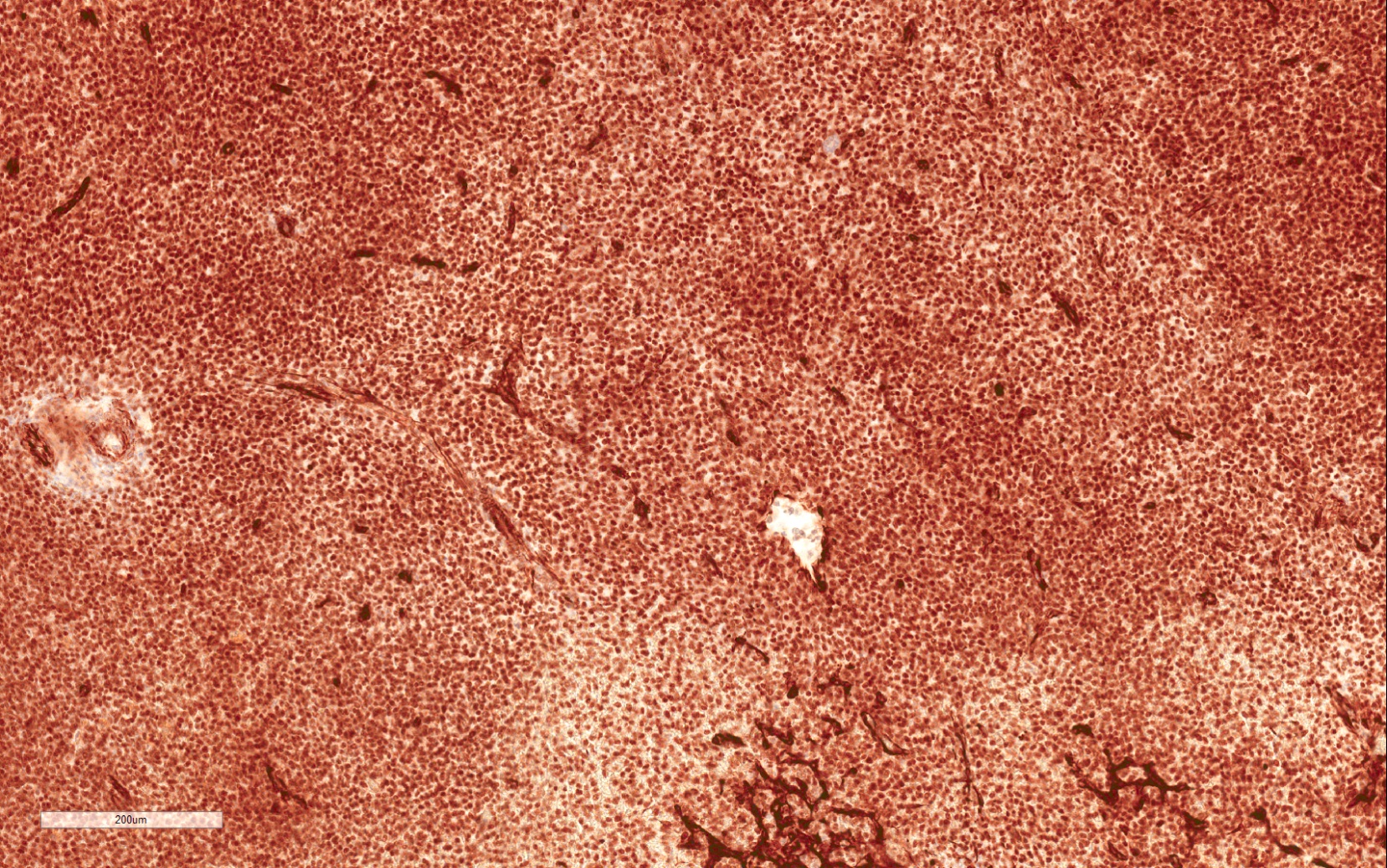
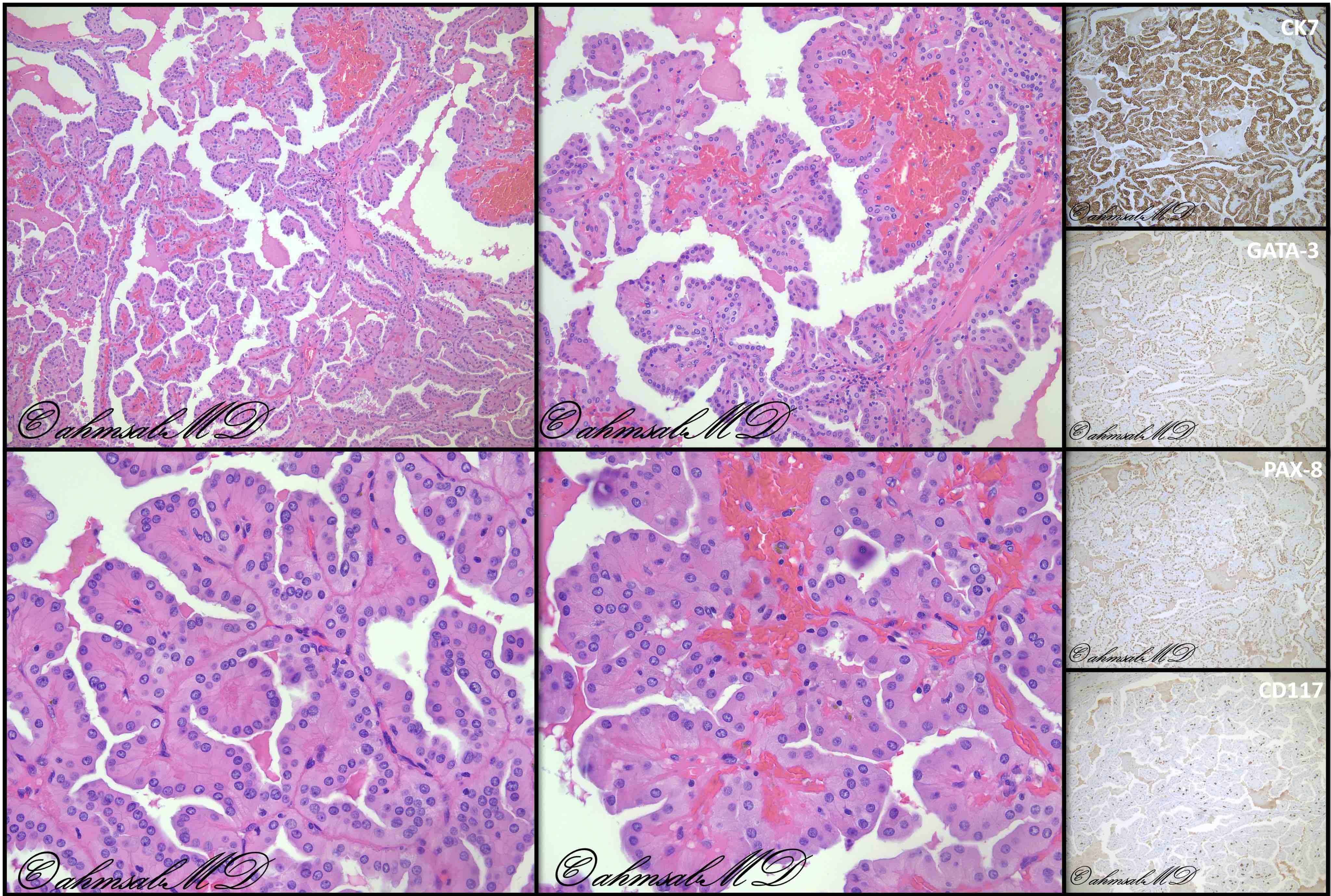
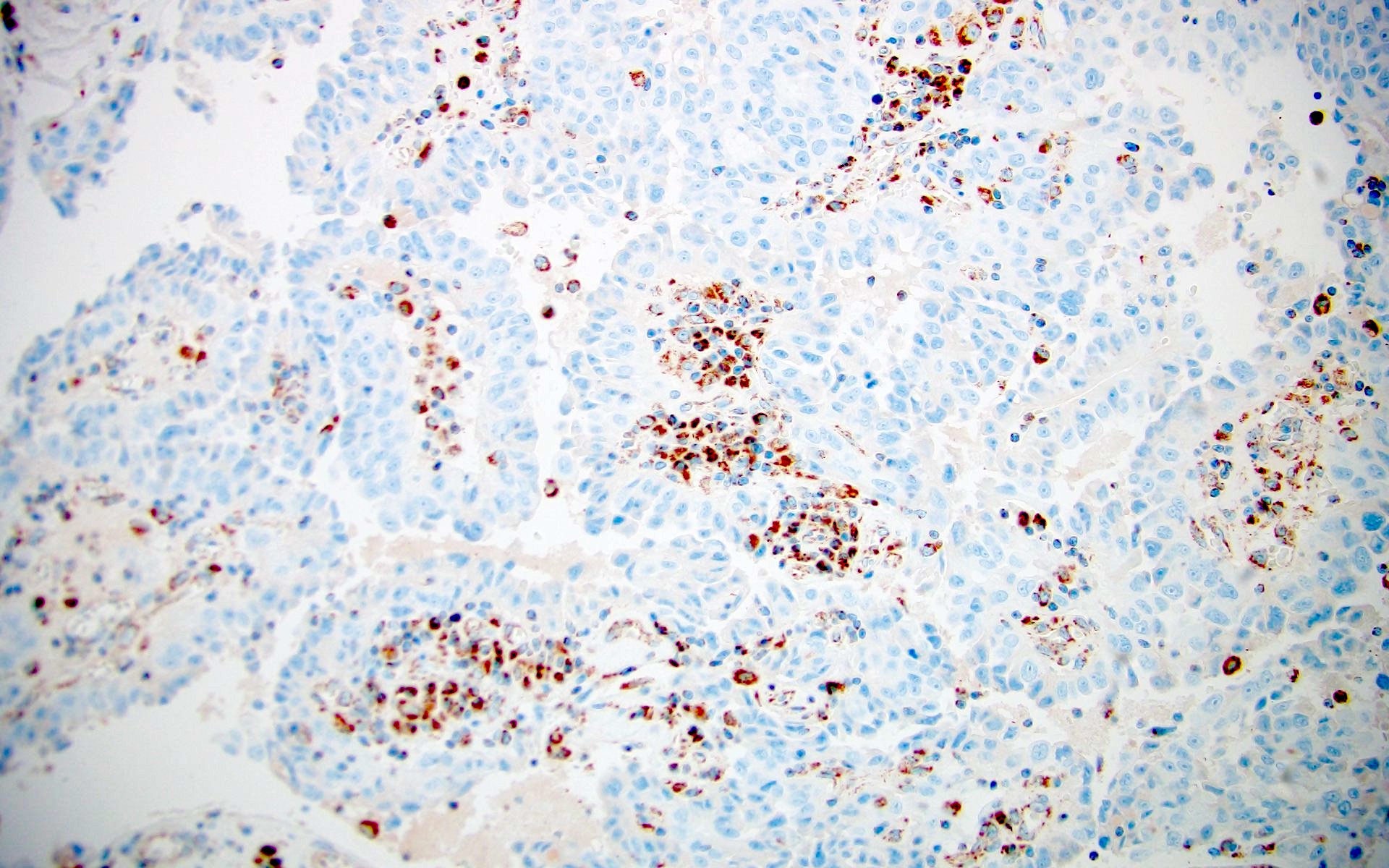
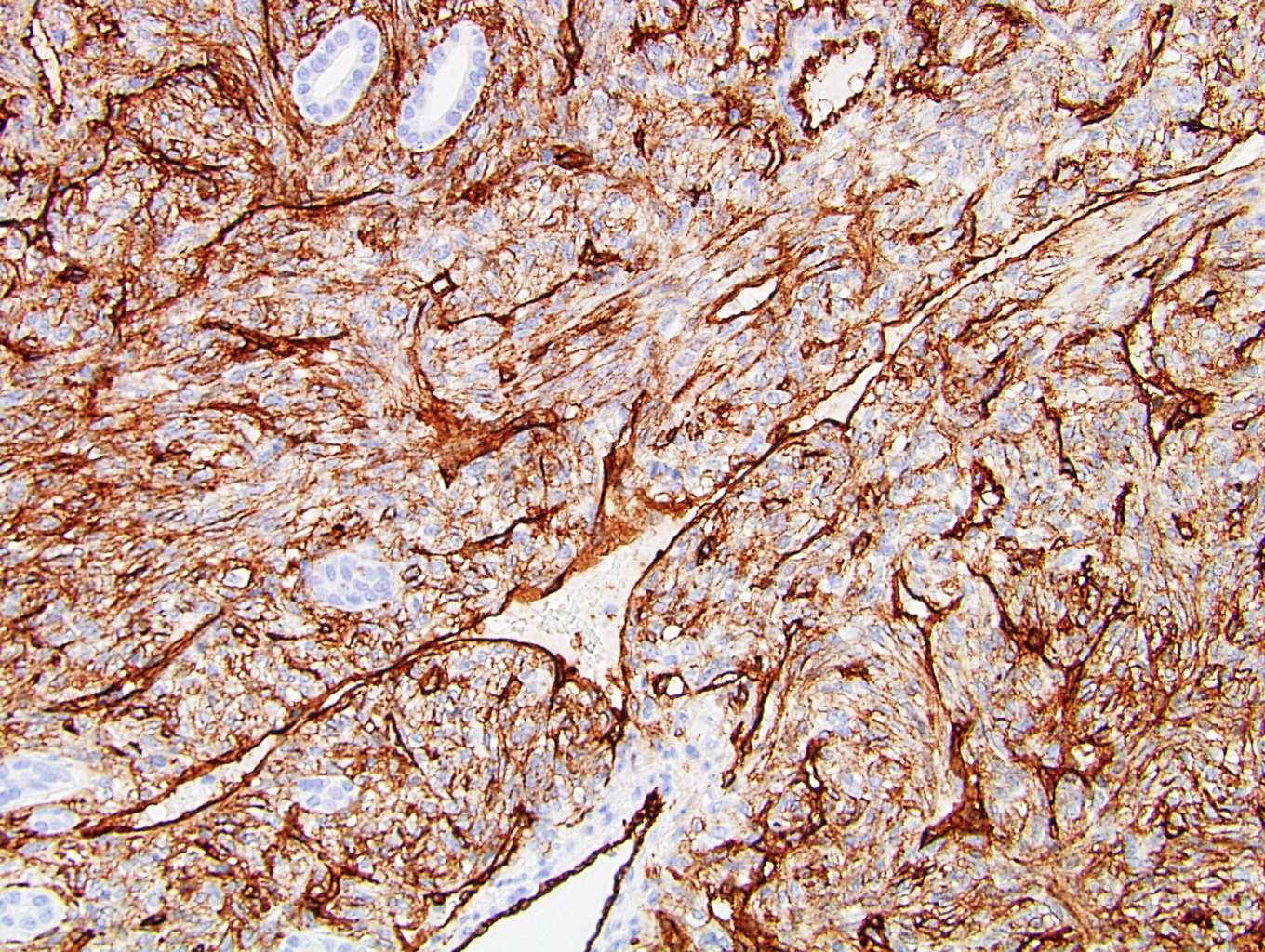
- RCC, CD117 / KIT, S100, HMB45, MelanA, cathepsin K, WT1
- Ki67 very low (< 5%)
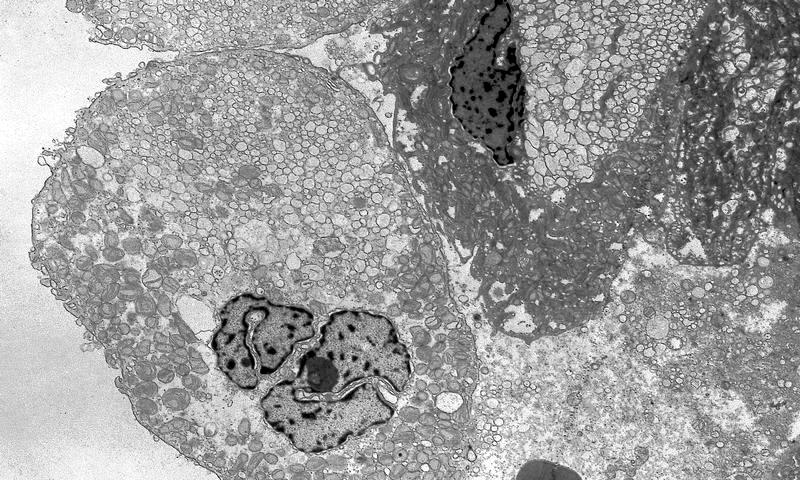
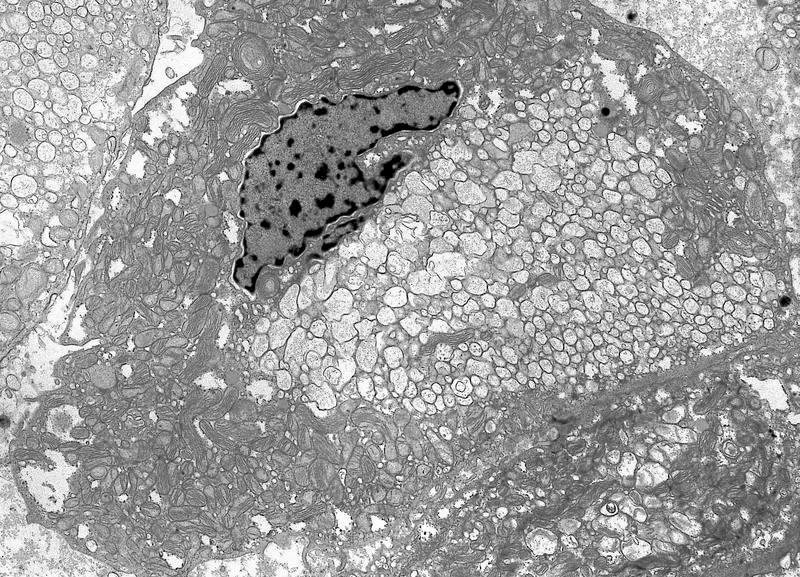
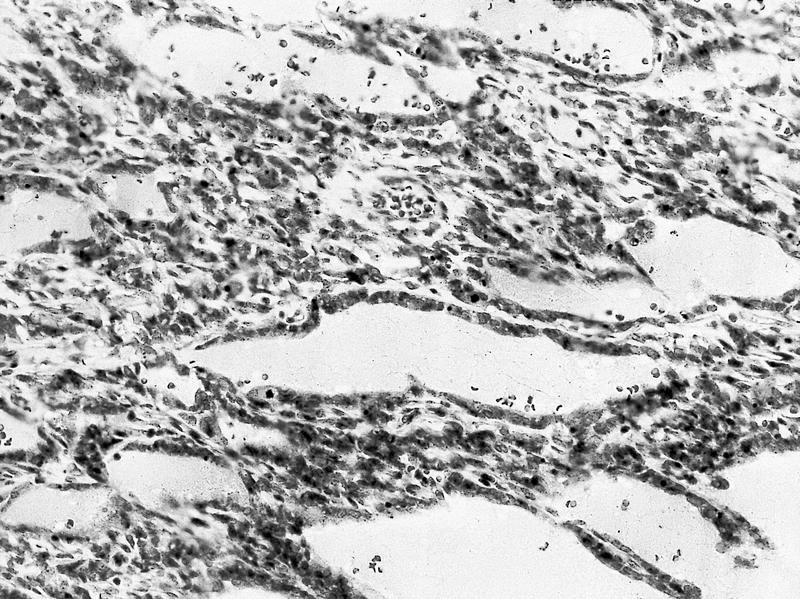
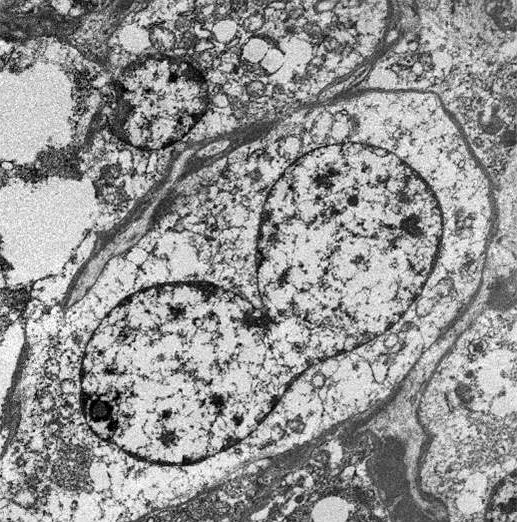
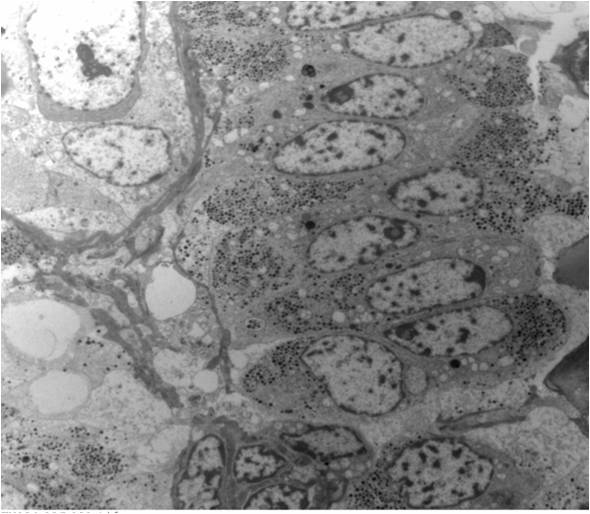
- Tumor cells with bundles of tonofilaments, intercellular junctions, desmosomes, intracytoplasmic lumina lined by microvilli and lipofuscin-like lysosomal structures (Genes Chromosomes Cancer 2016;55:442)
- VCL-ALK fusion (strong association with sickle cell trait) (Genes Chromosomes Cancer 2011;50:146)
- TPM3-ALK fusion (no association with sickle cell trait) (Genes Chromosomes Cancer 2016;55:442)
- Other less common fusions: STRN-ALK, EML4-ALK, HOOK1-ALK (Am J Surg Pathol 2016;40:761, Pol J Pathol 2018;69:109)
- Clonal inversion involving 2p23 (Mod Pathol 2011;24:430)
- No fusion but increased copy number of ALK1 (Virchows Arch 2014;464:241)
- ALK copy number gain could be identified in up to 10% of clear cell RCC and associated with worse cancer specific survival (Mod Pathol 2012;25:1516)
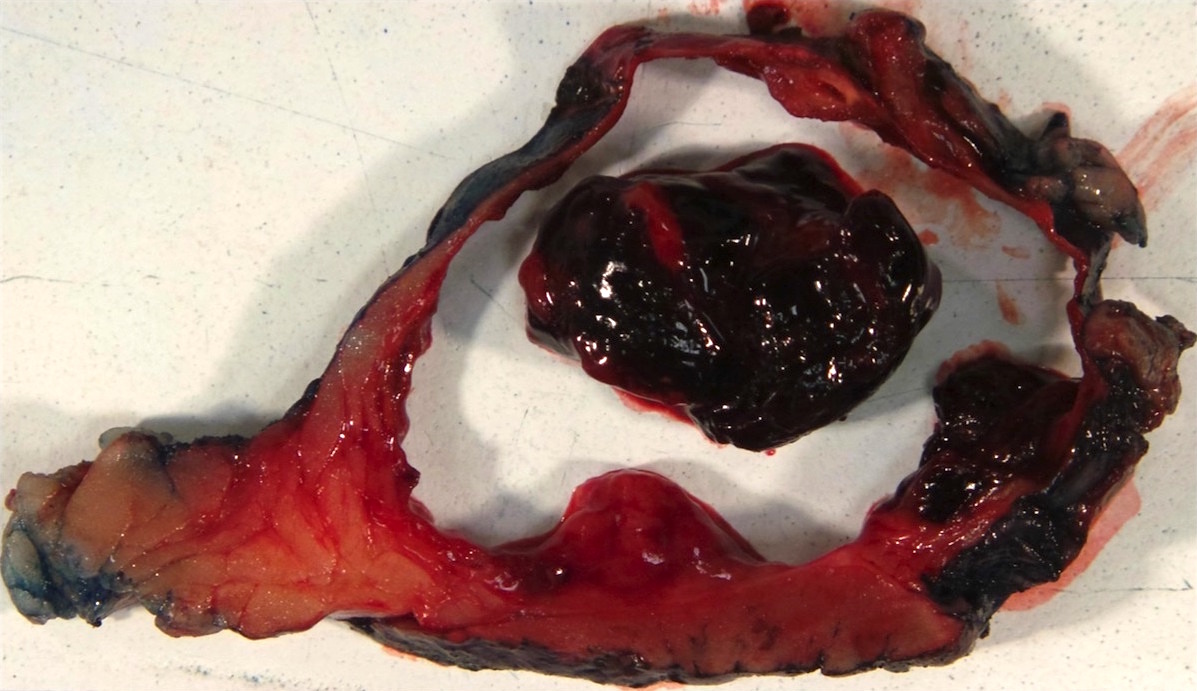
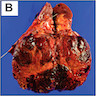
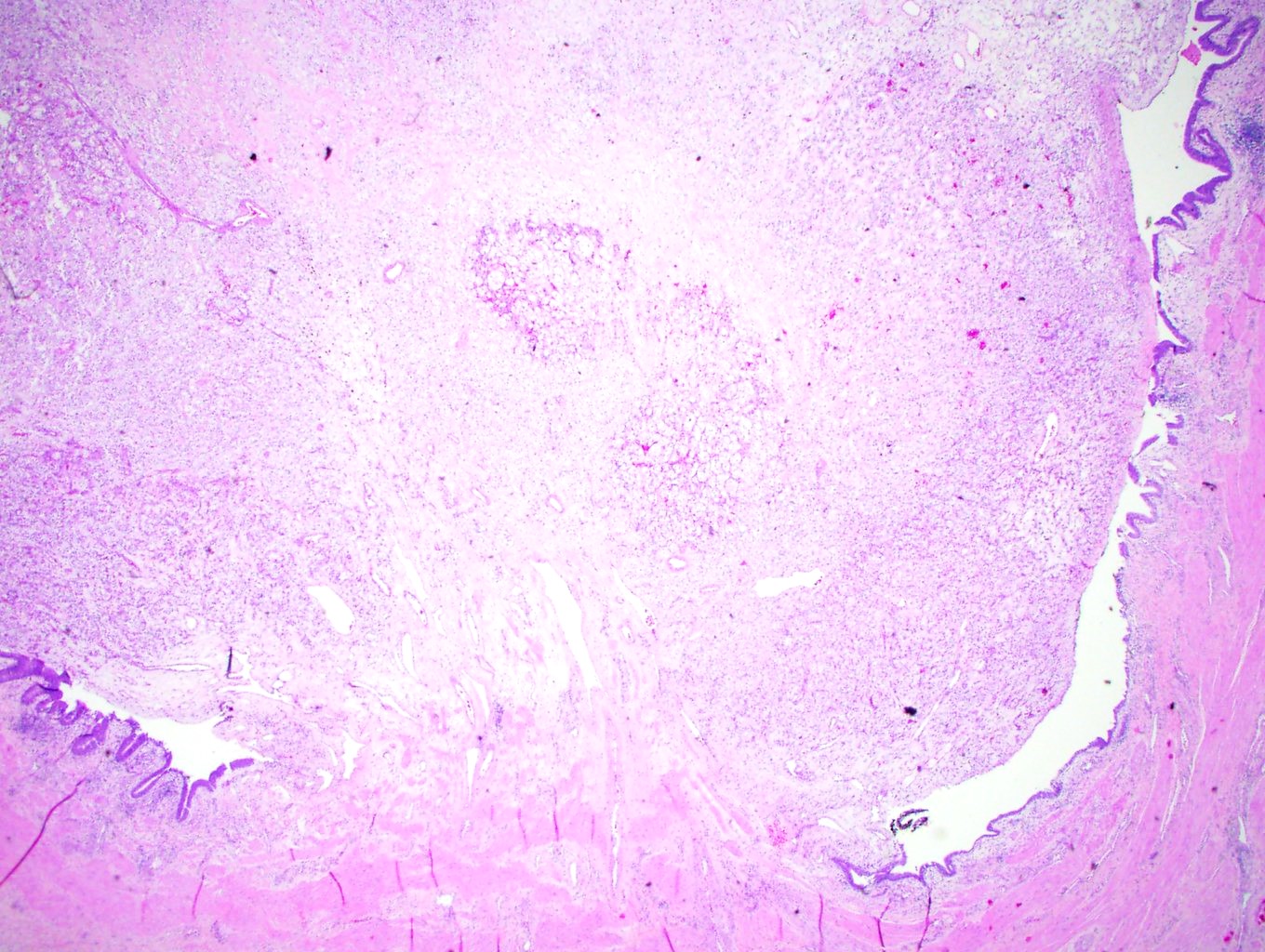
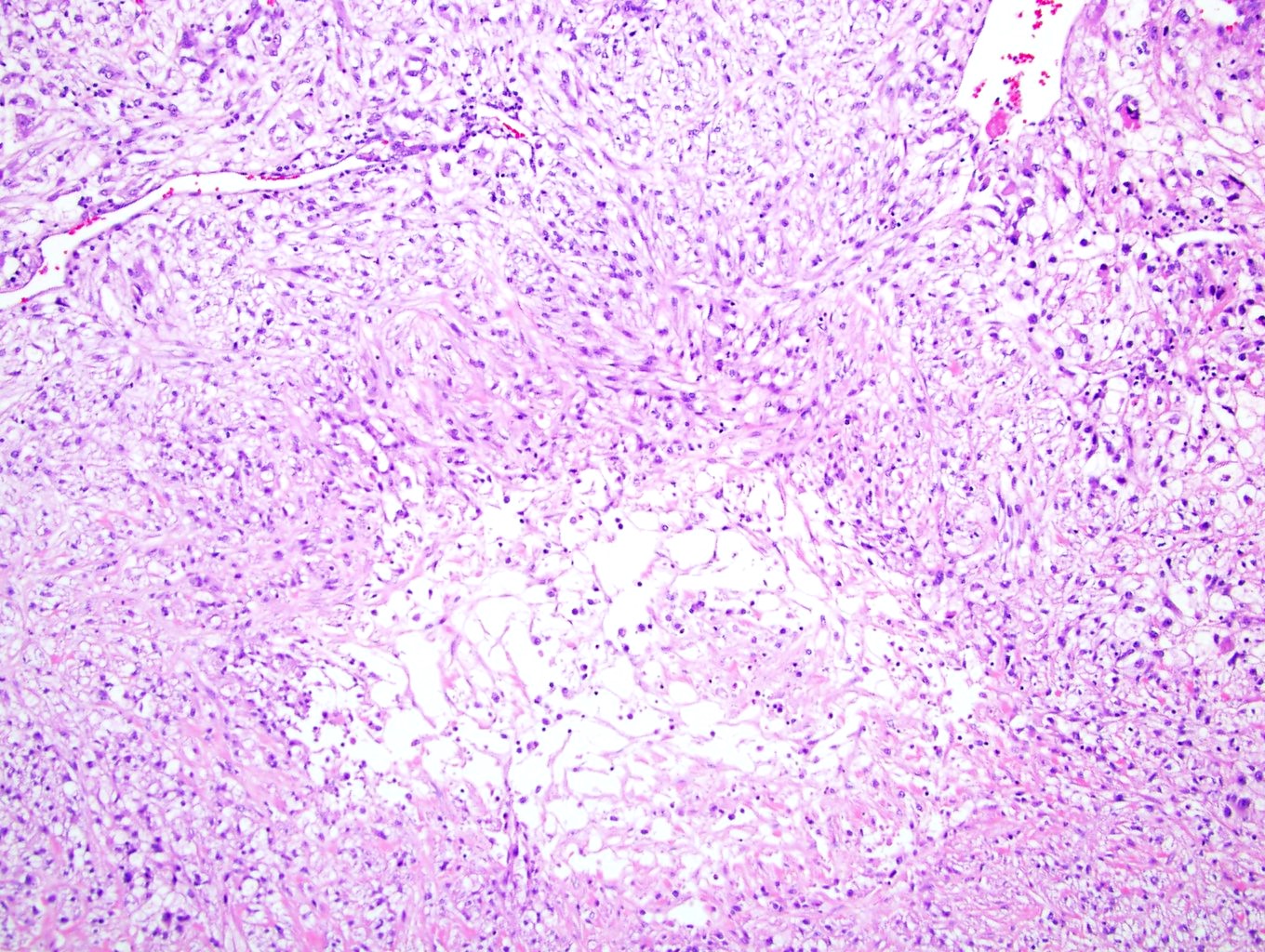
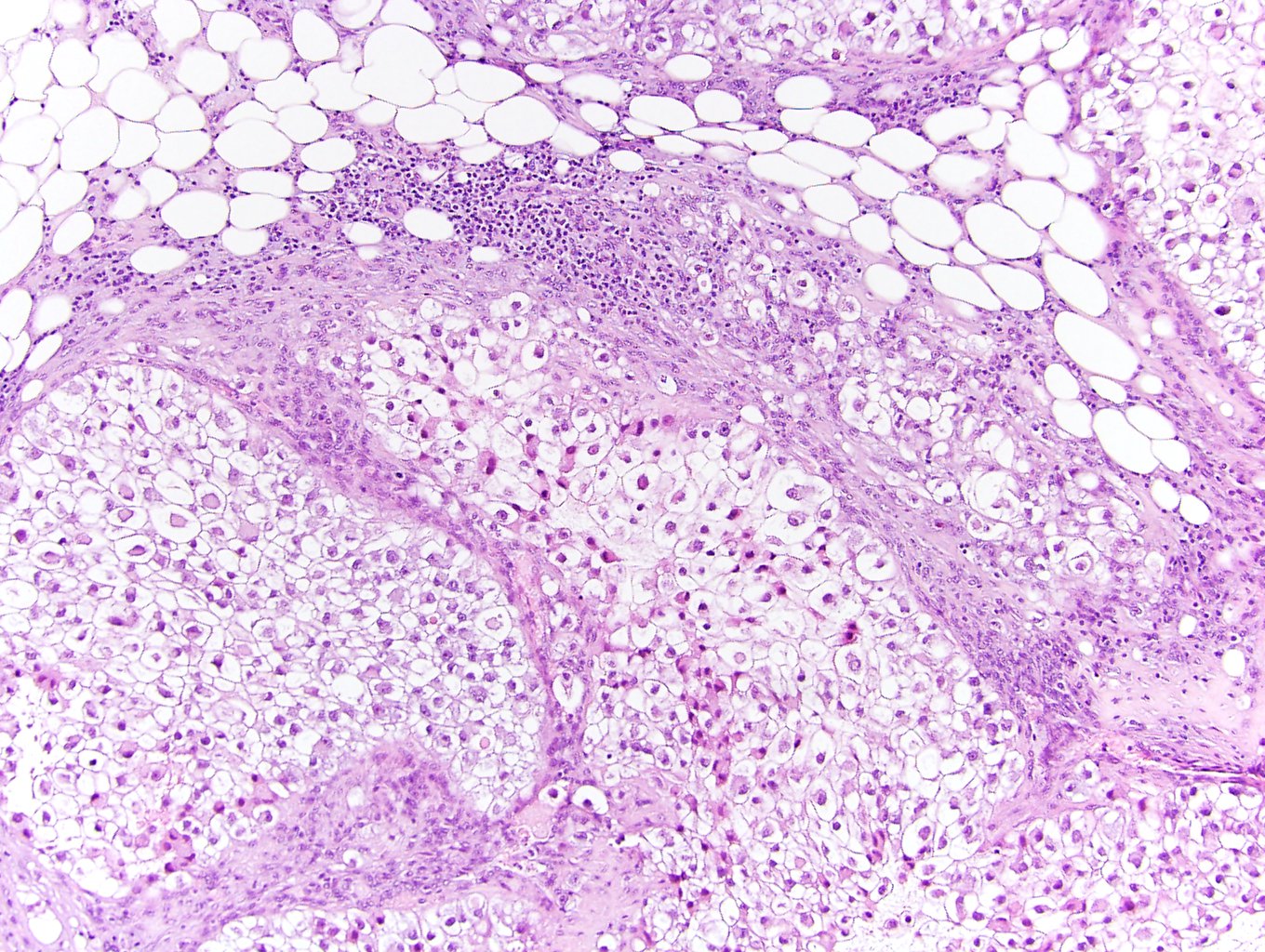
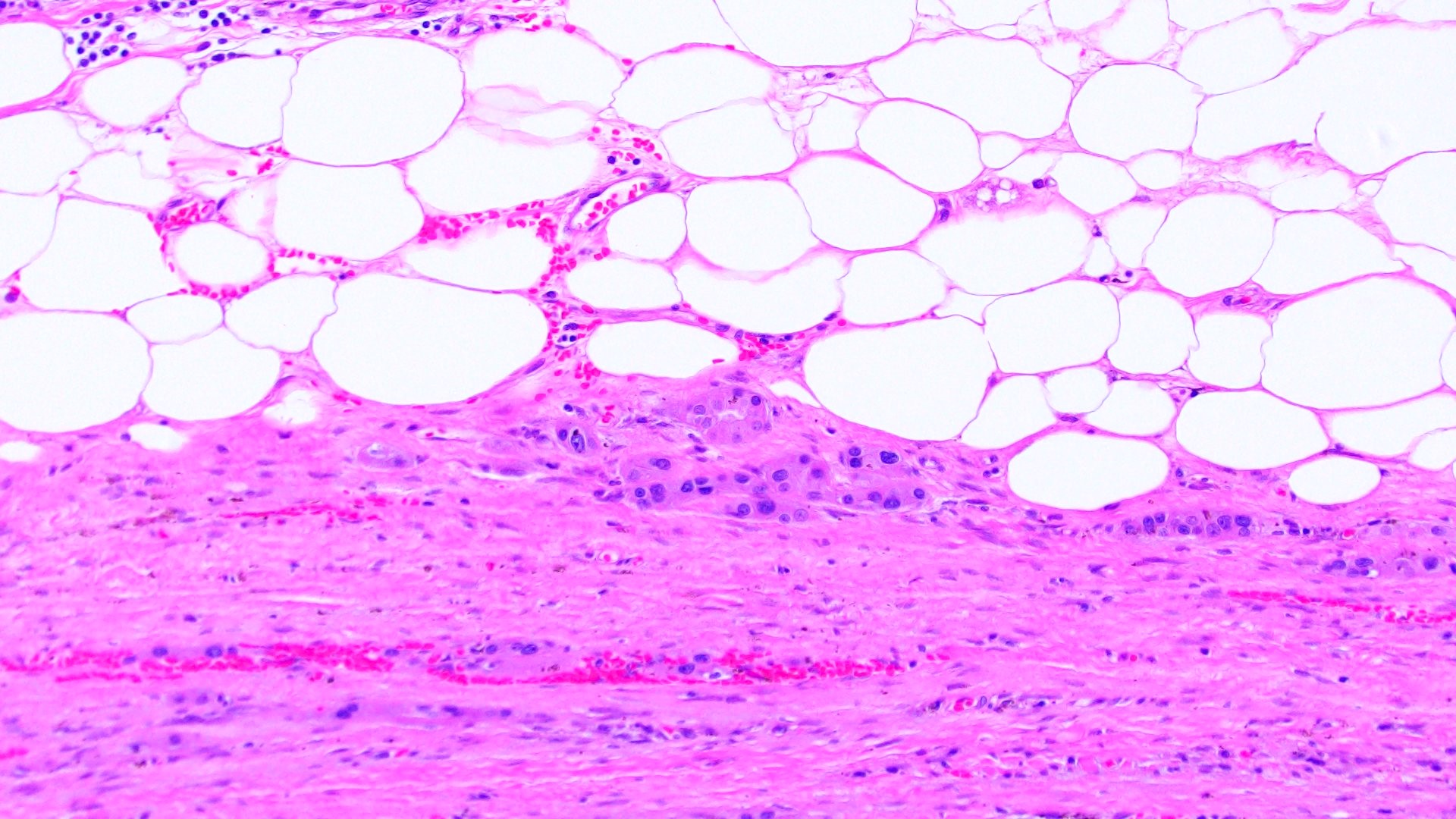
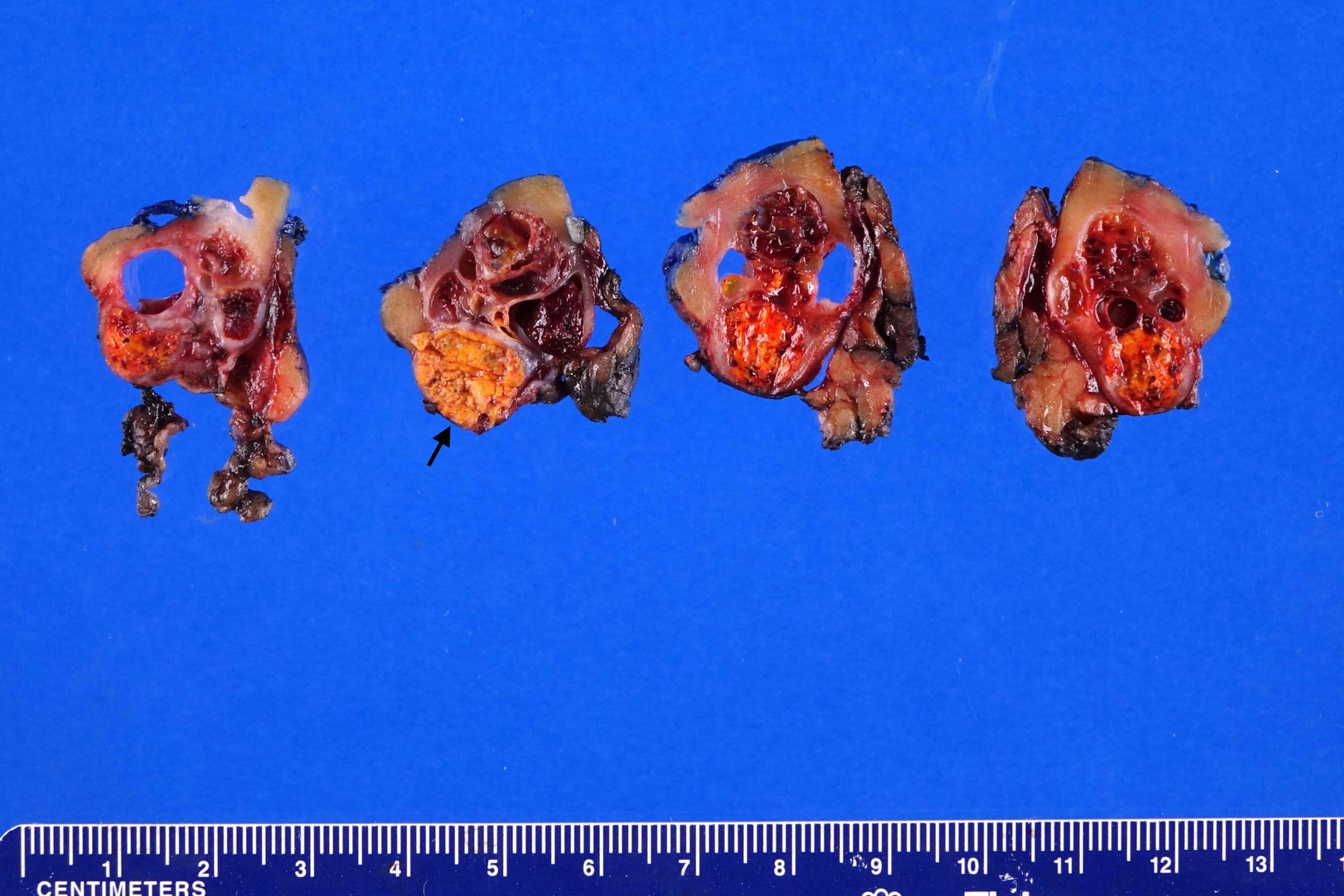
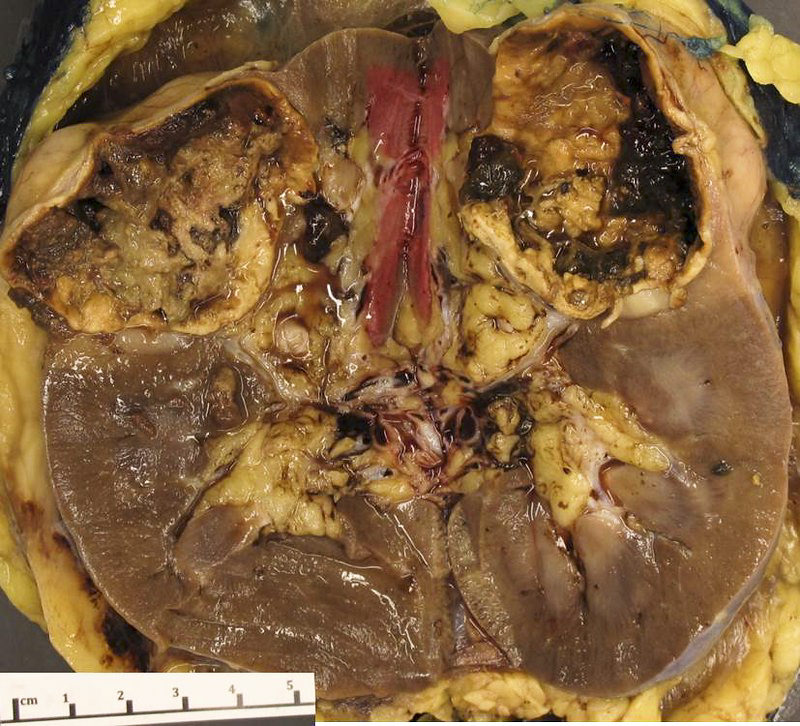
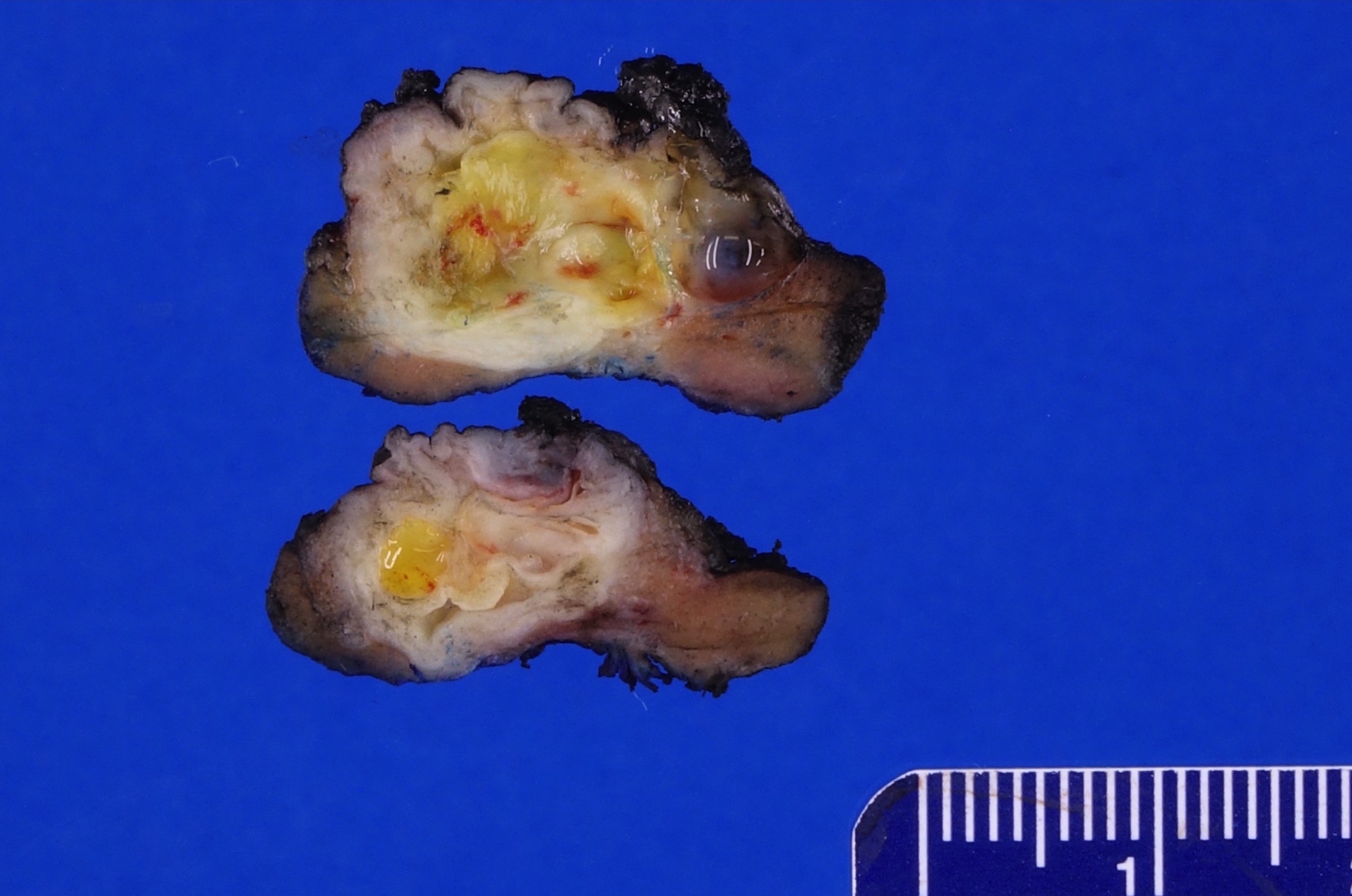
- Left kidney, nephrectomy:
- Renal cell carcinoma with ALK gene rearrangement (see comment and synoptic report)
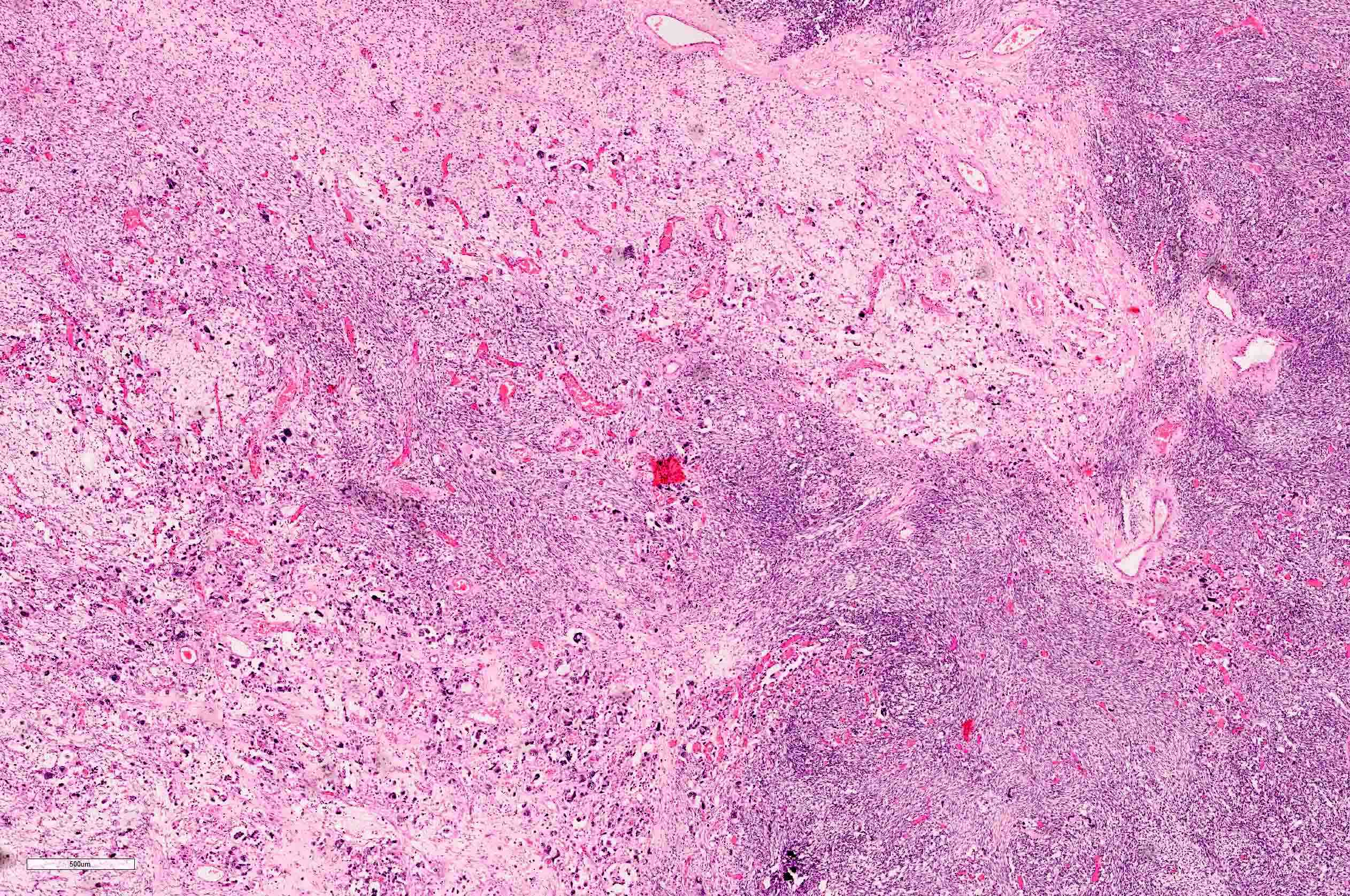
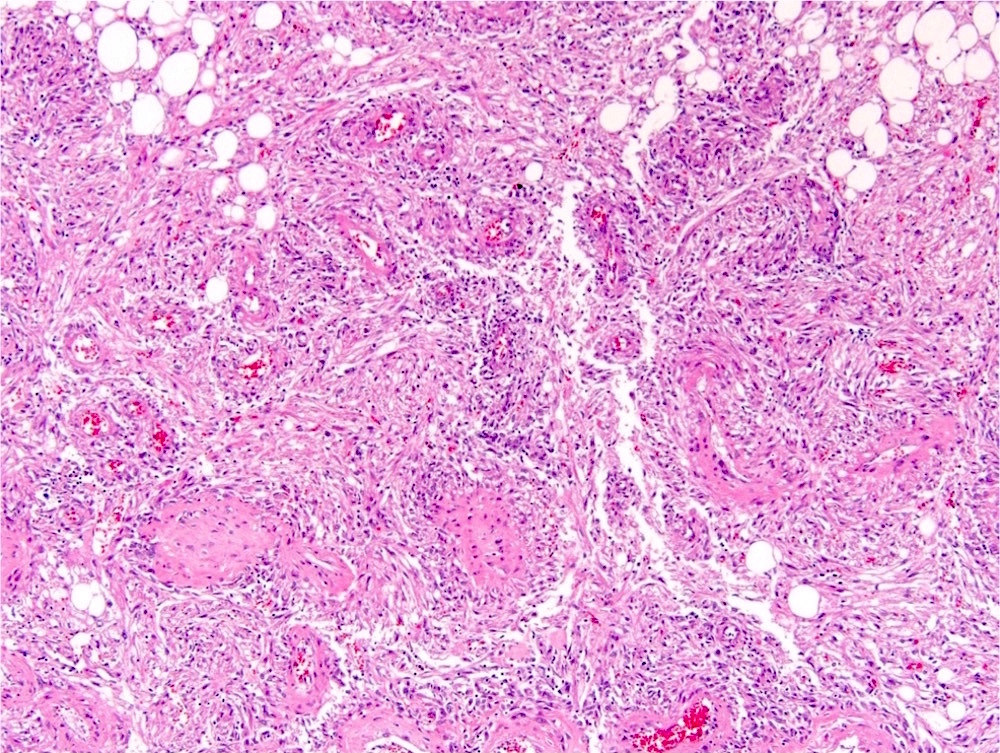
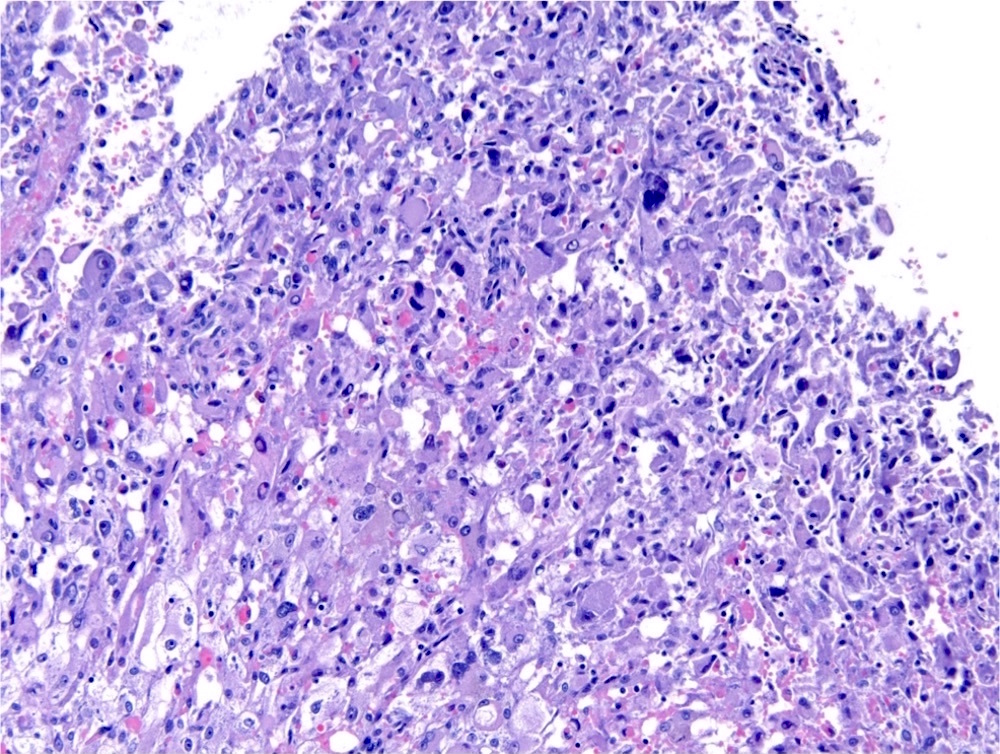
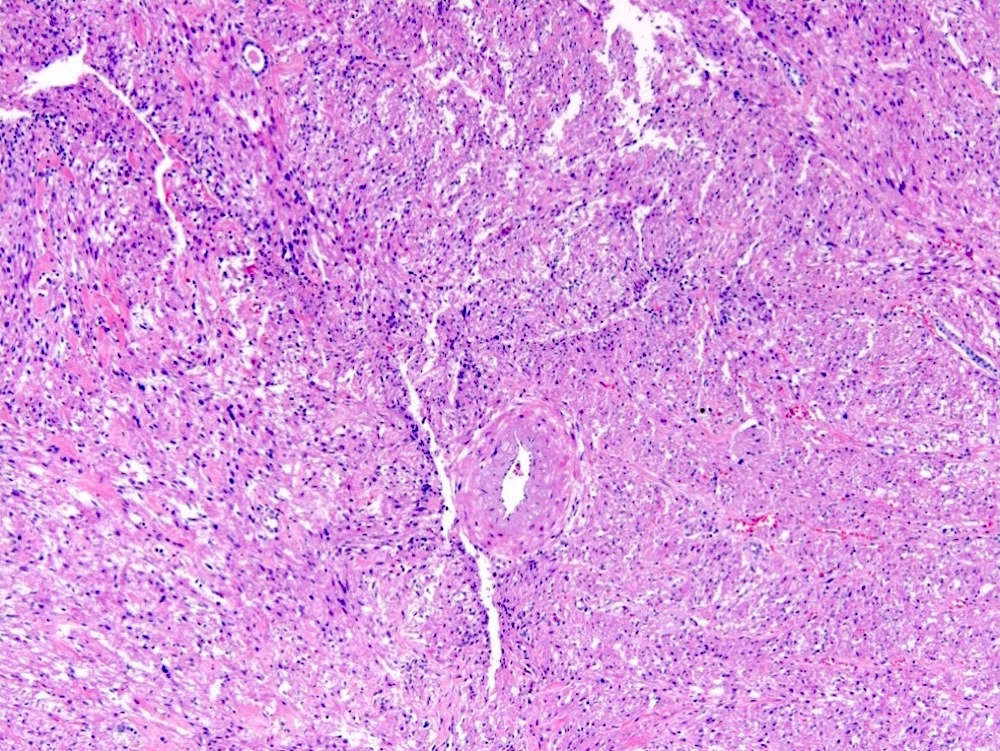
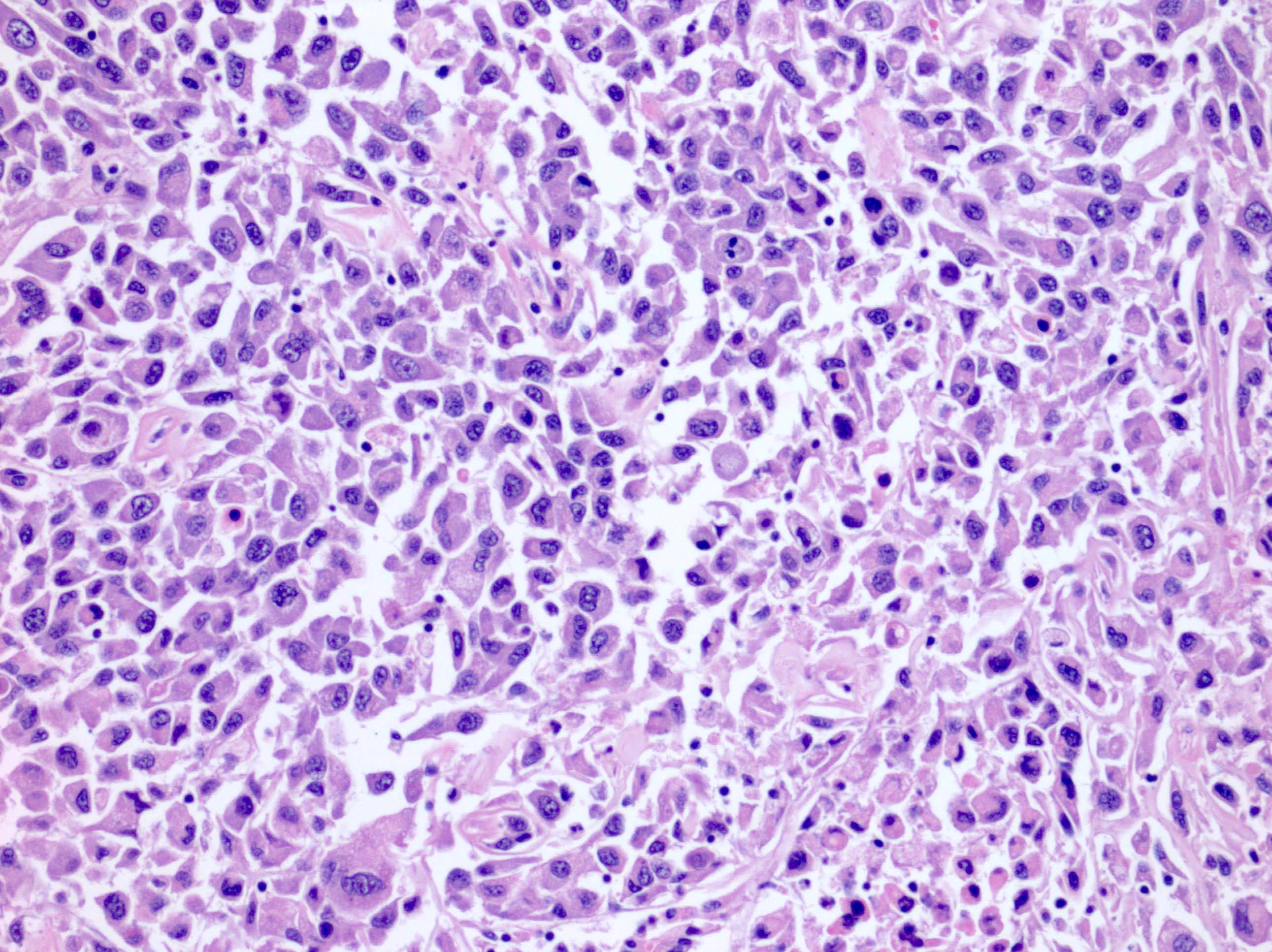
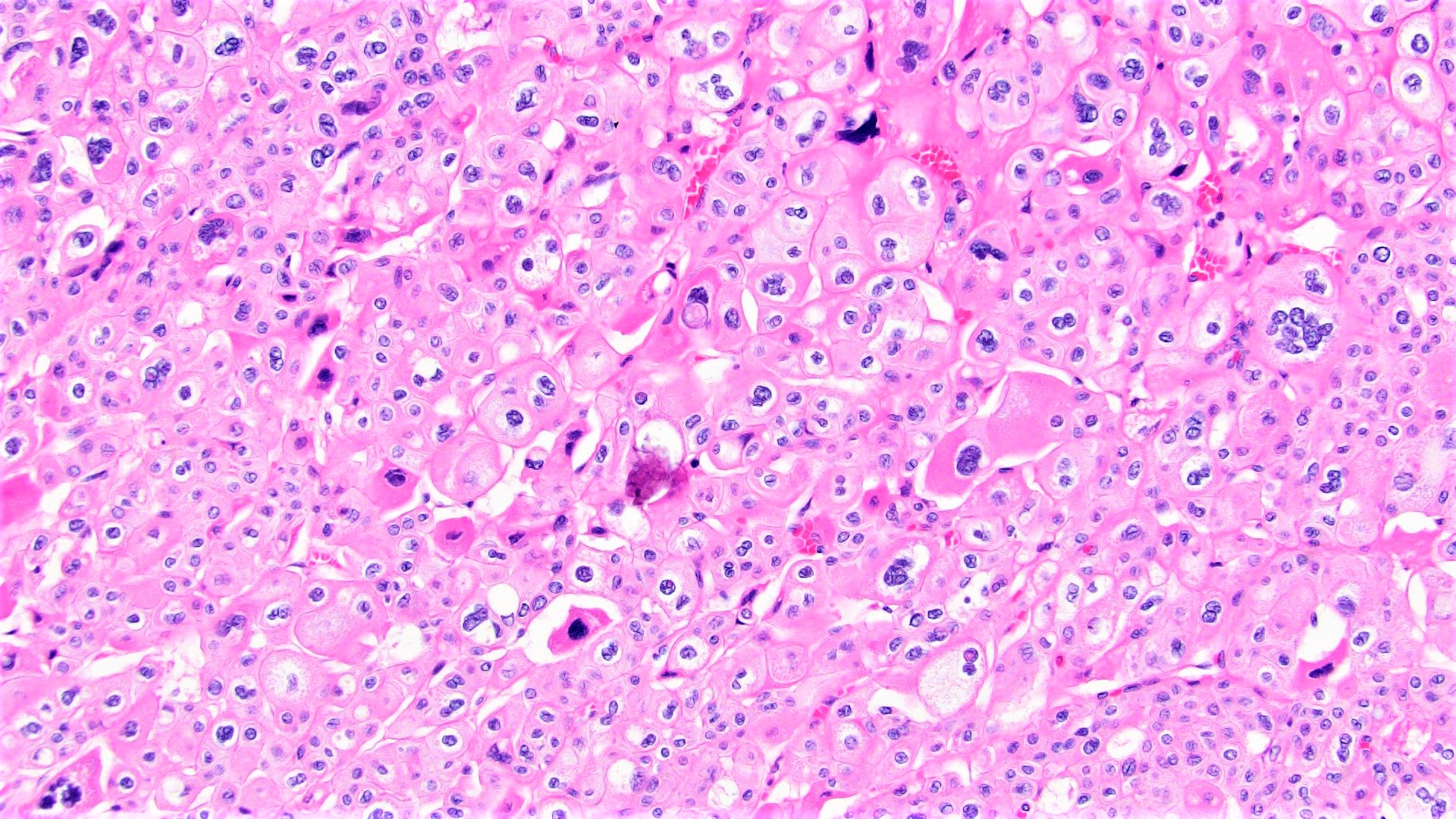
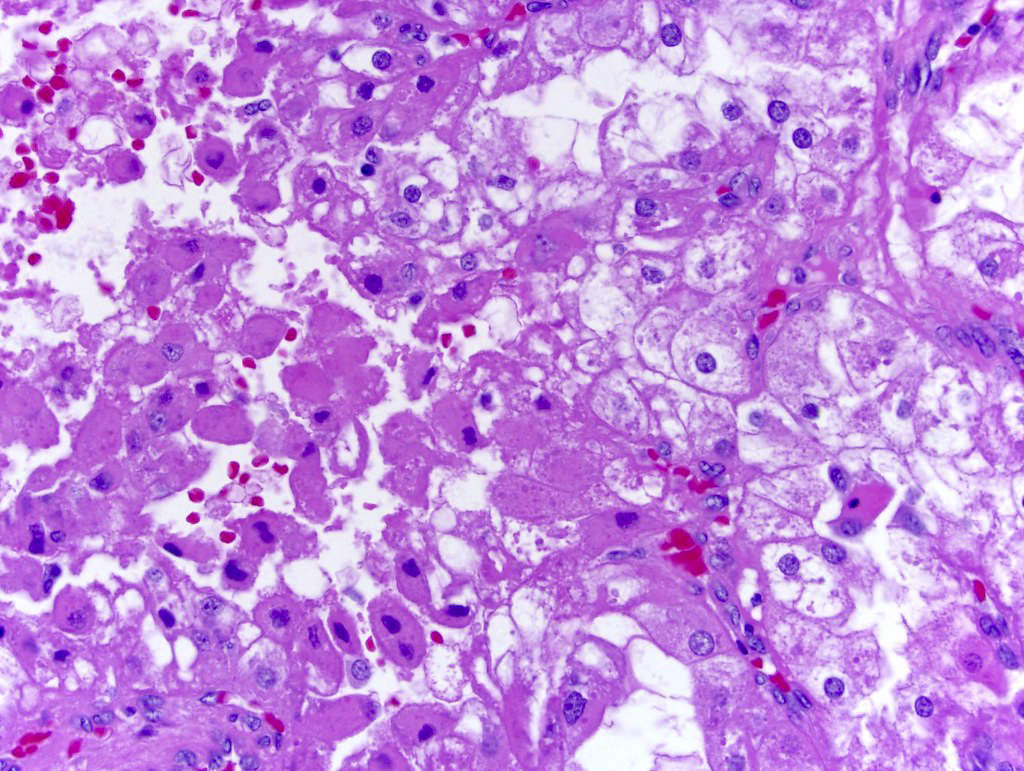
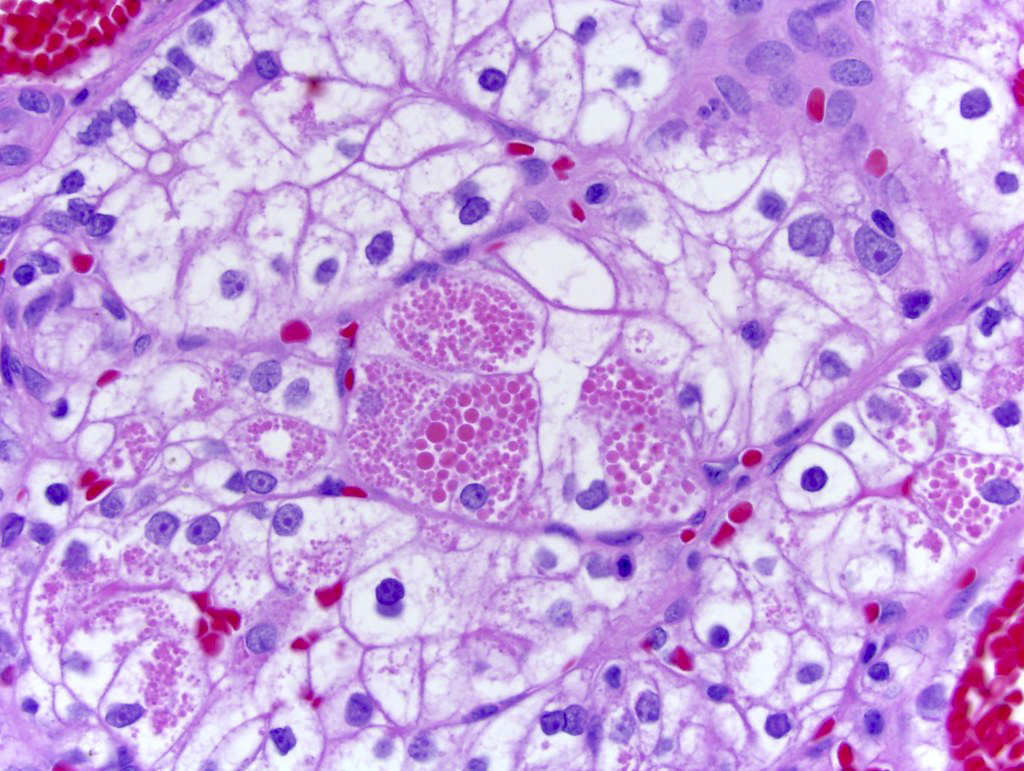
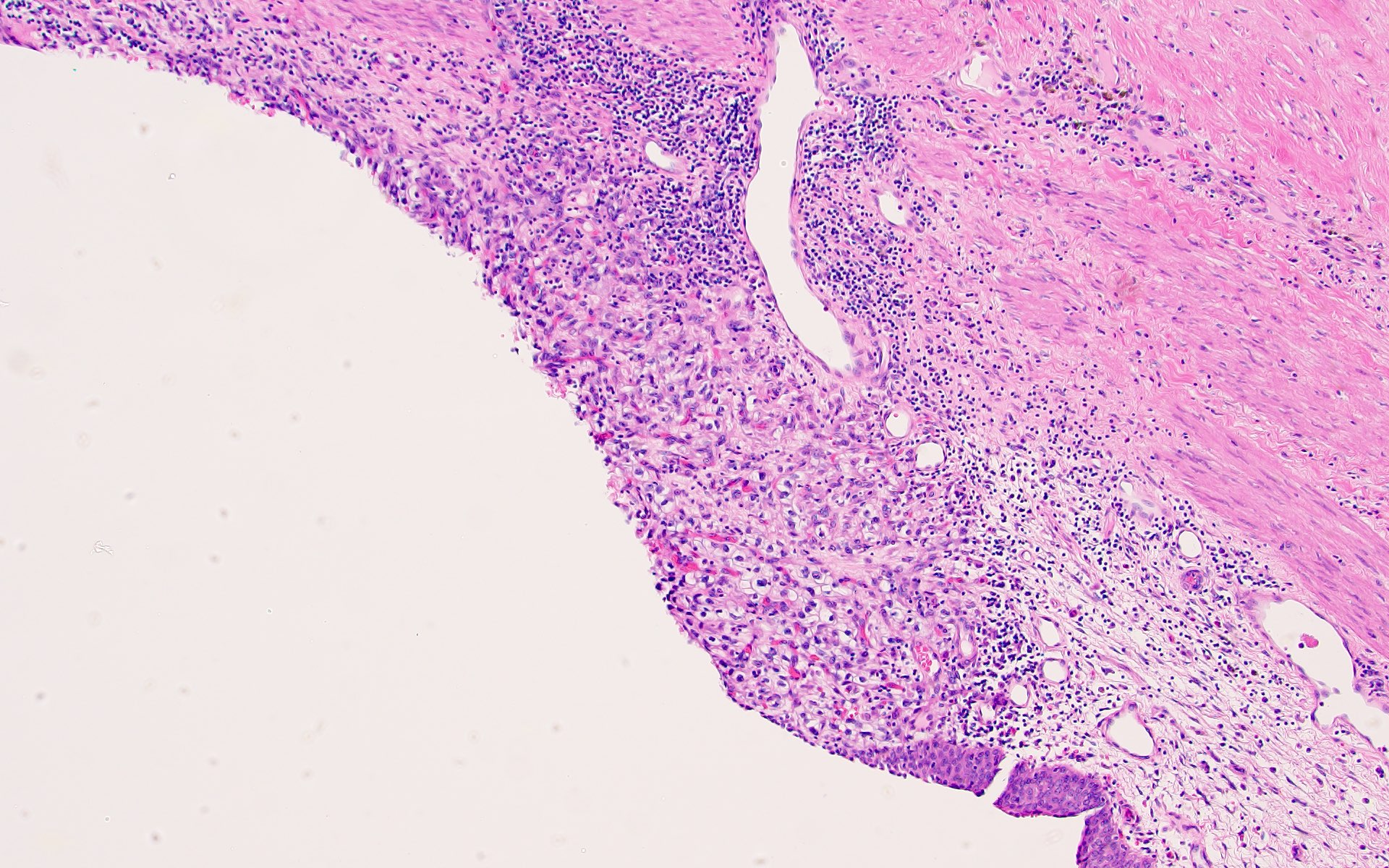
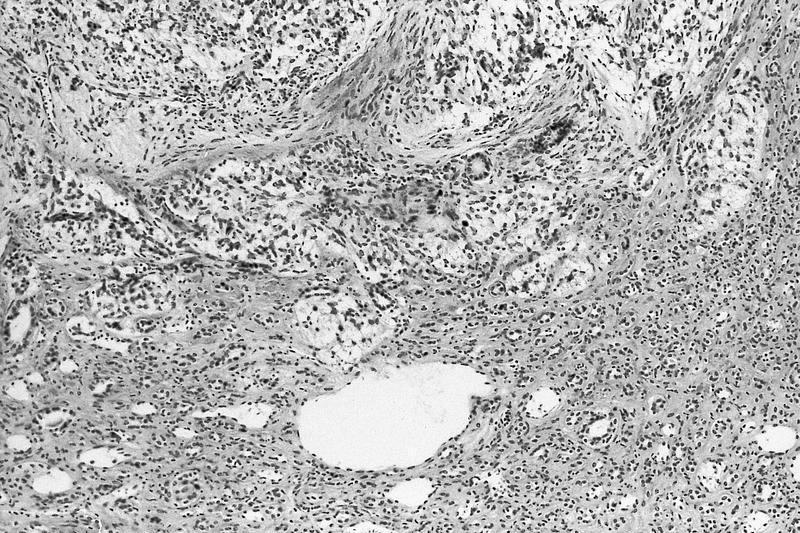
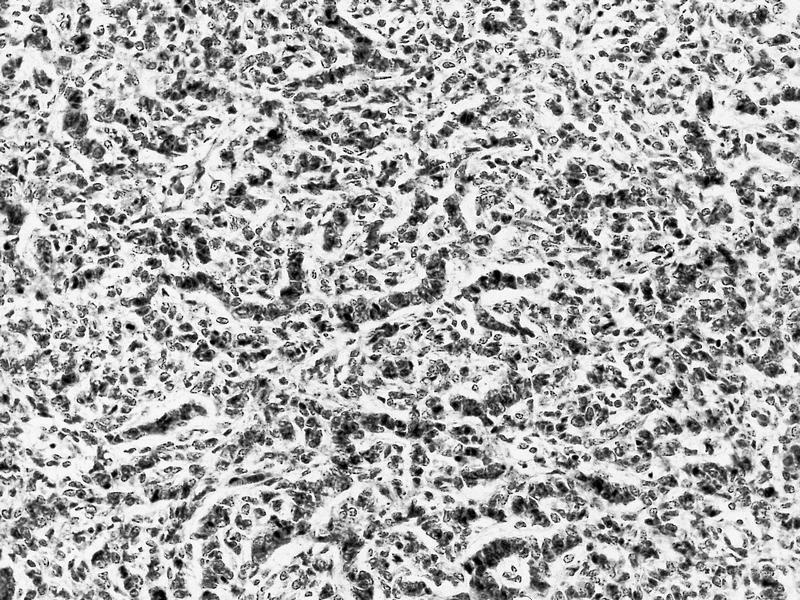
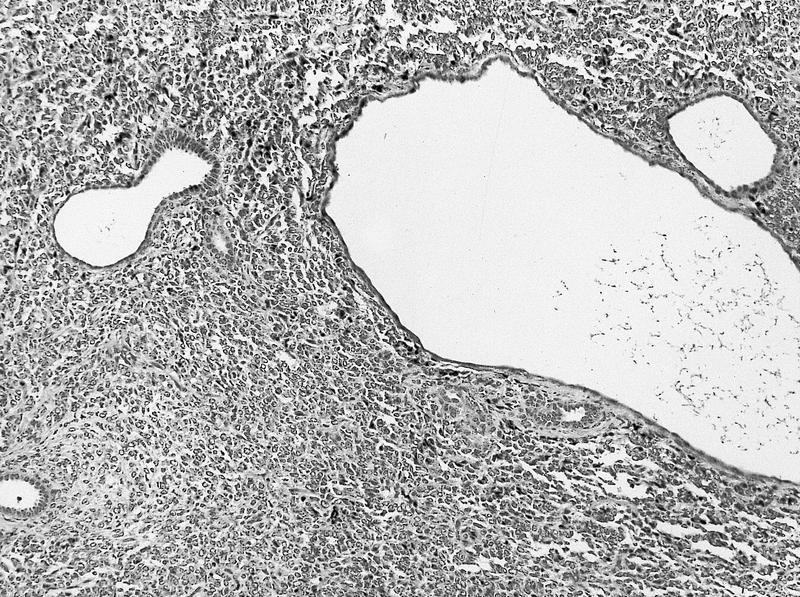
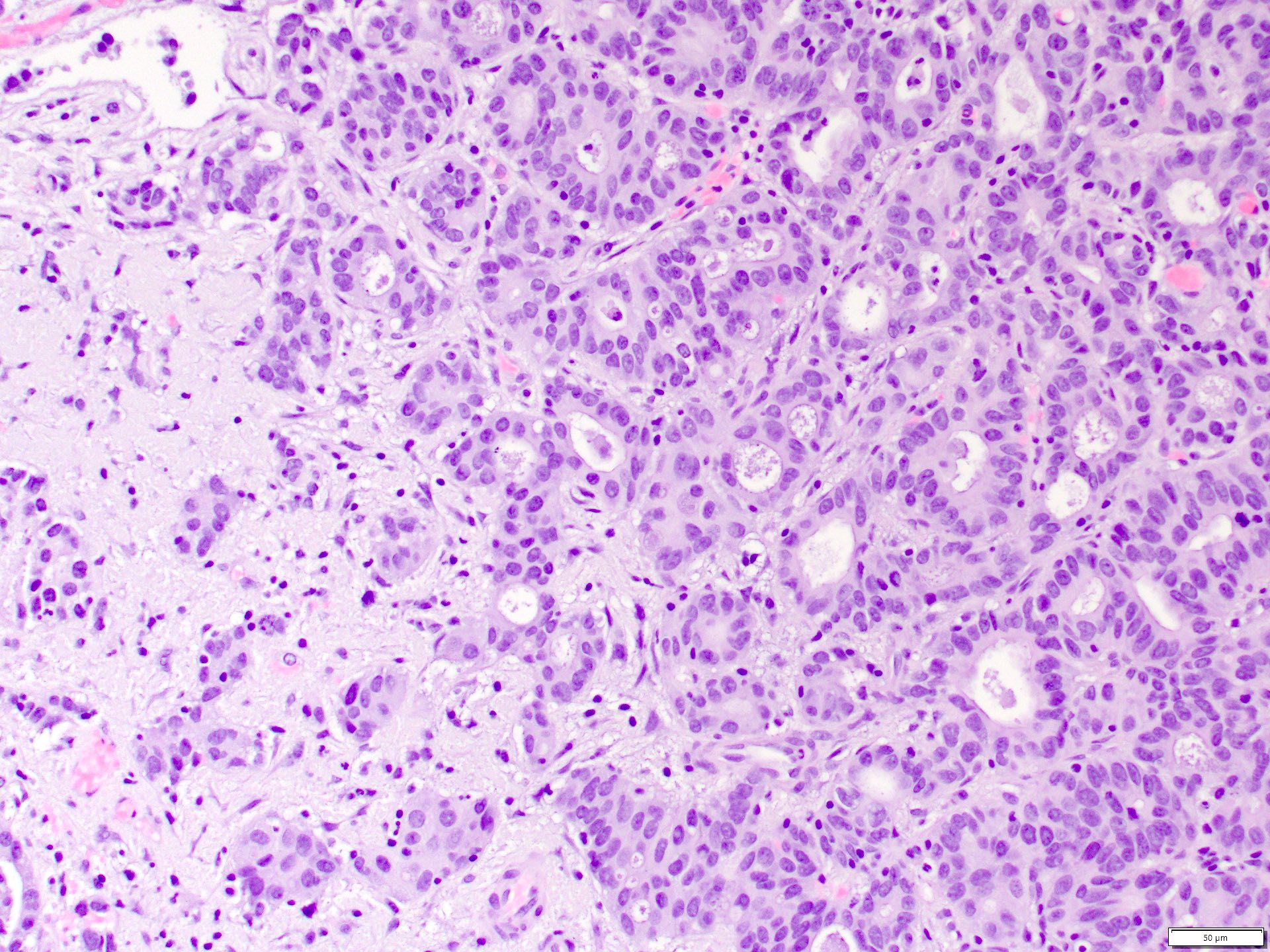
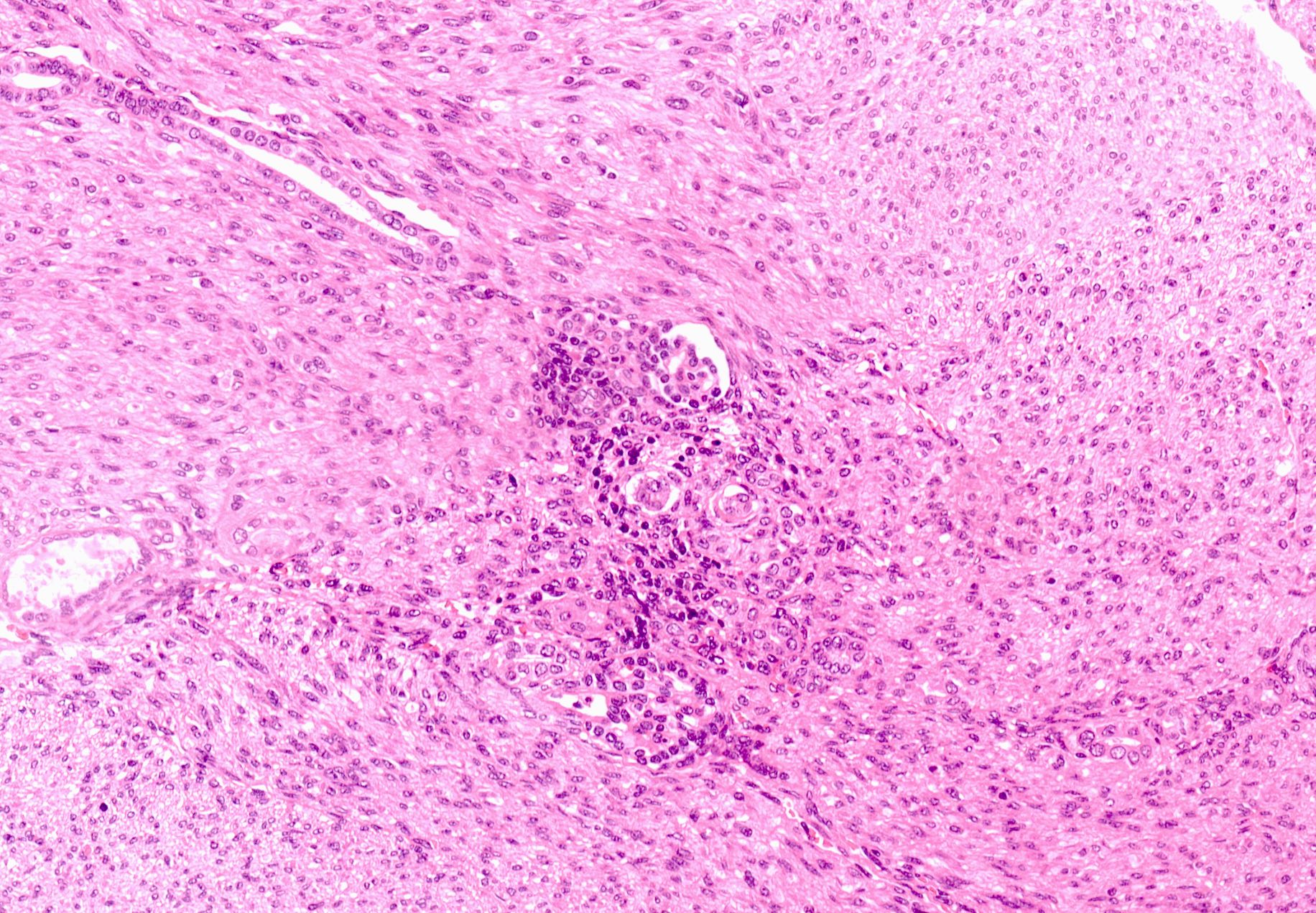
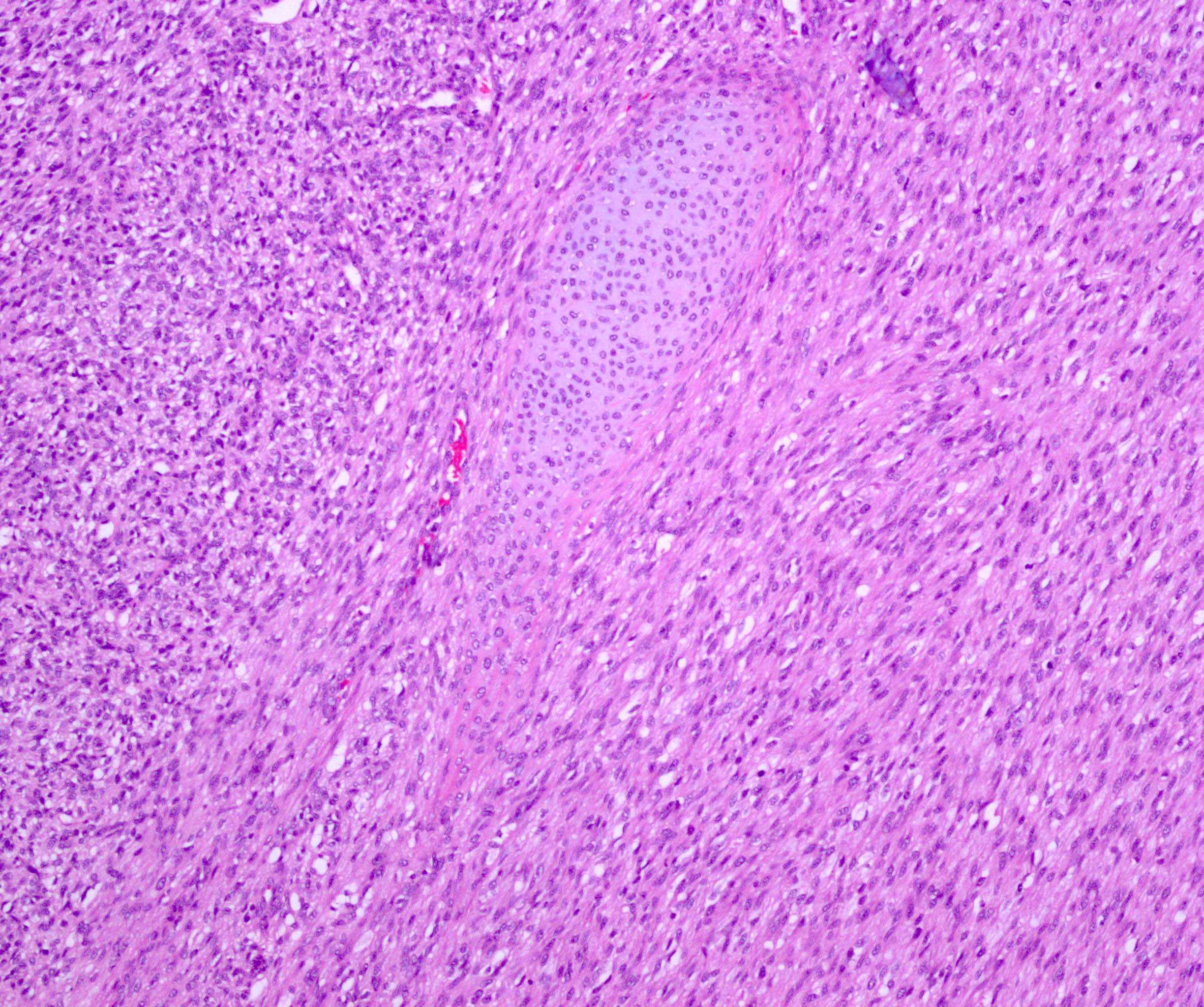
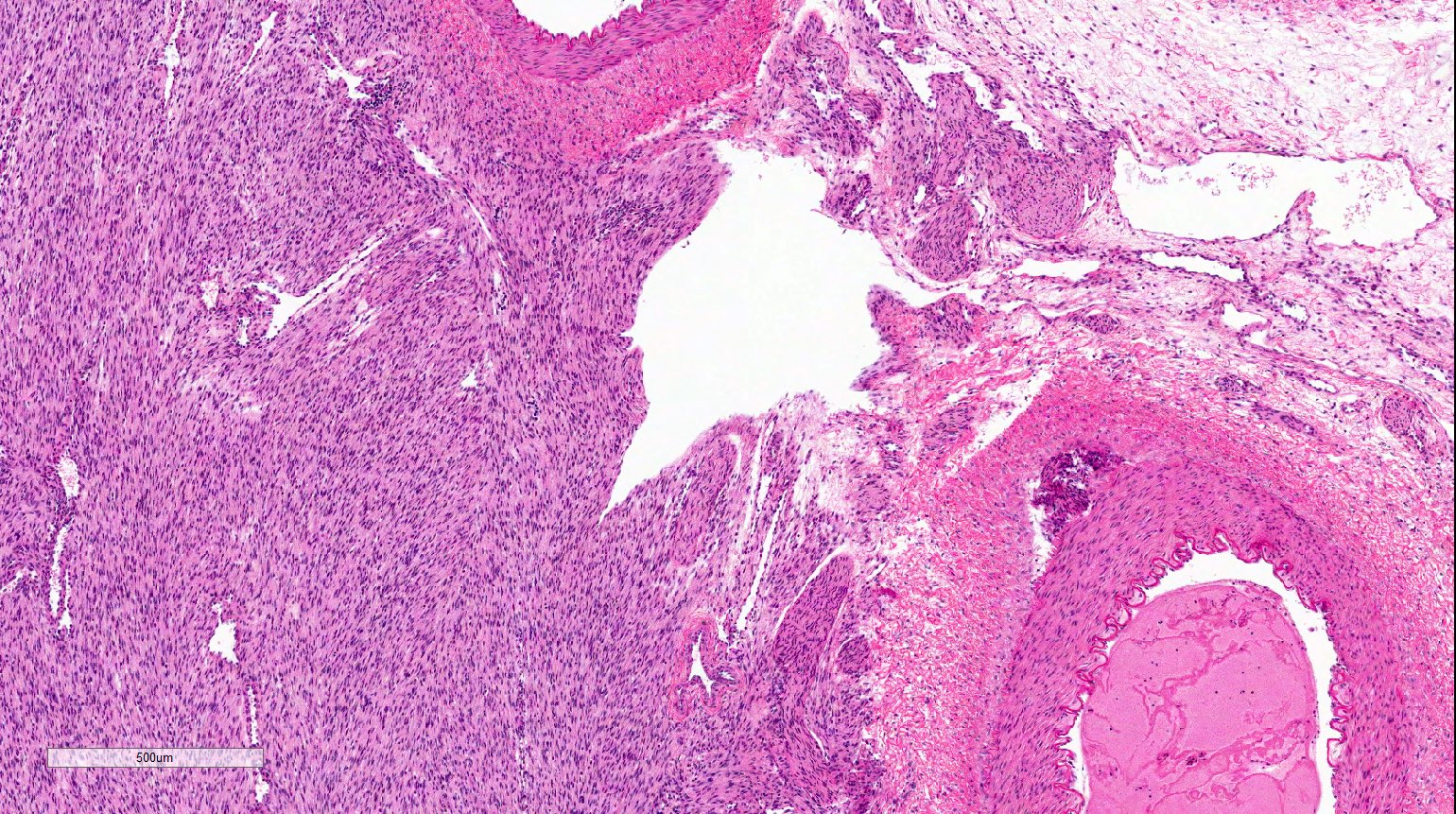
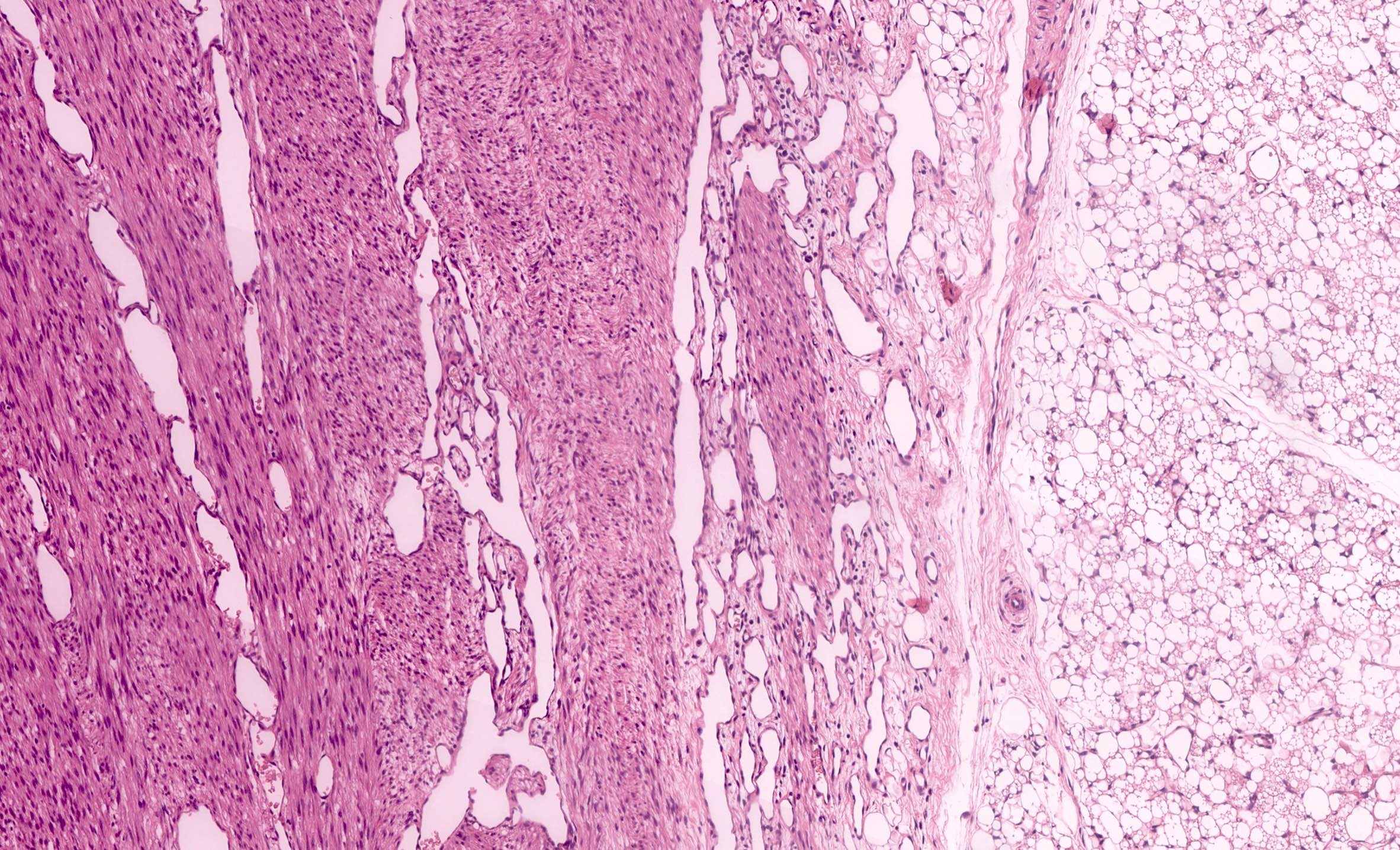
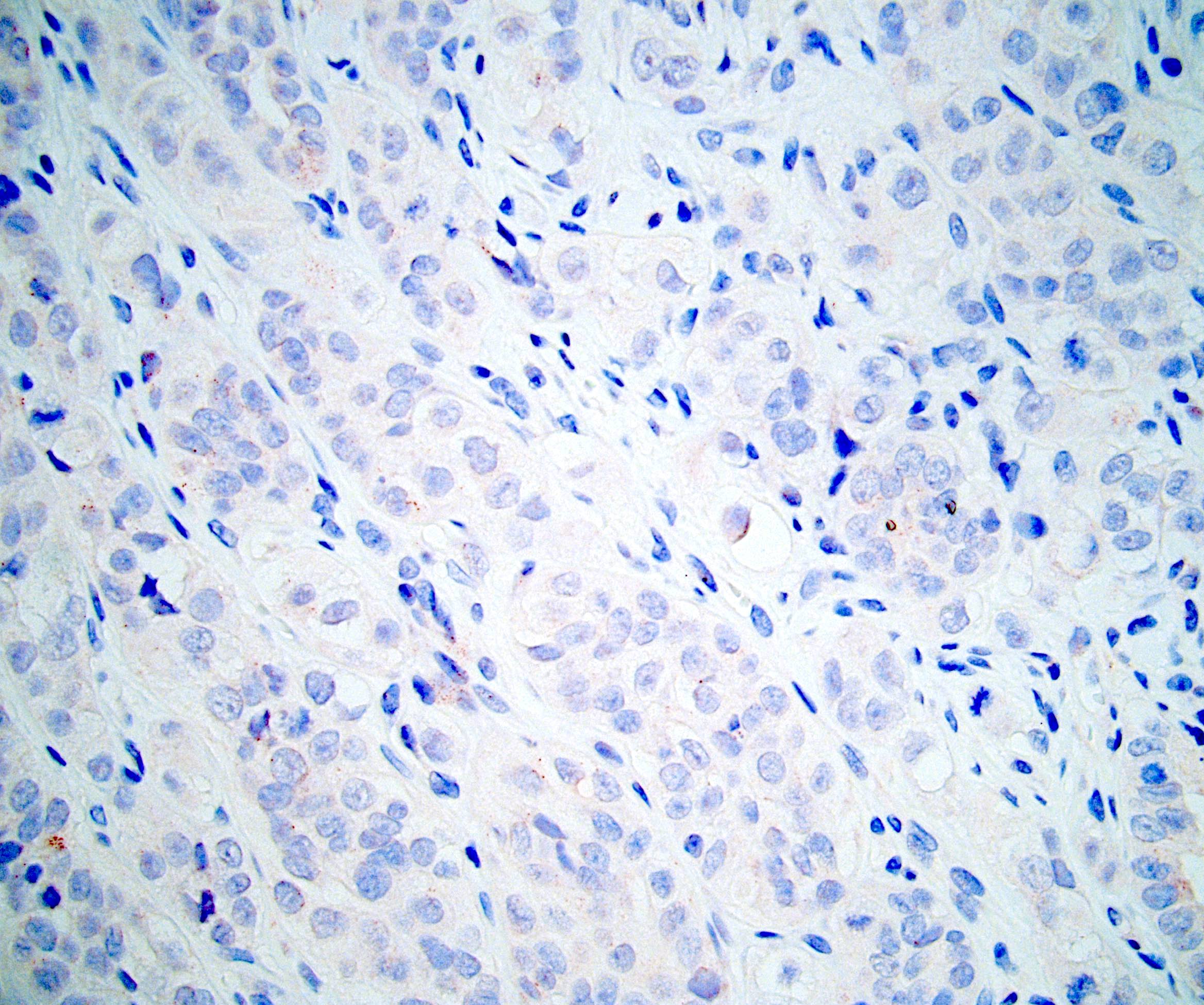
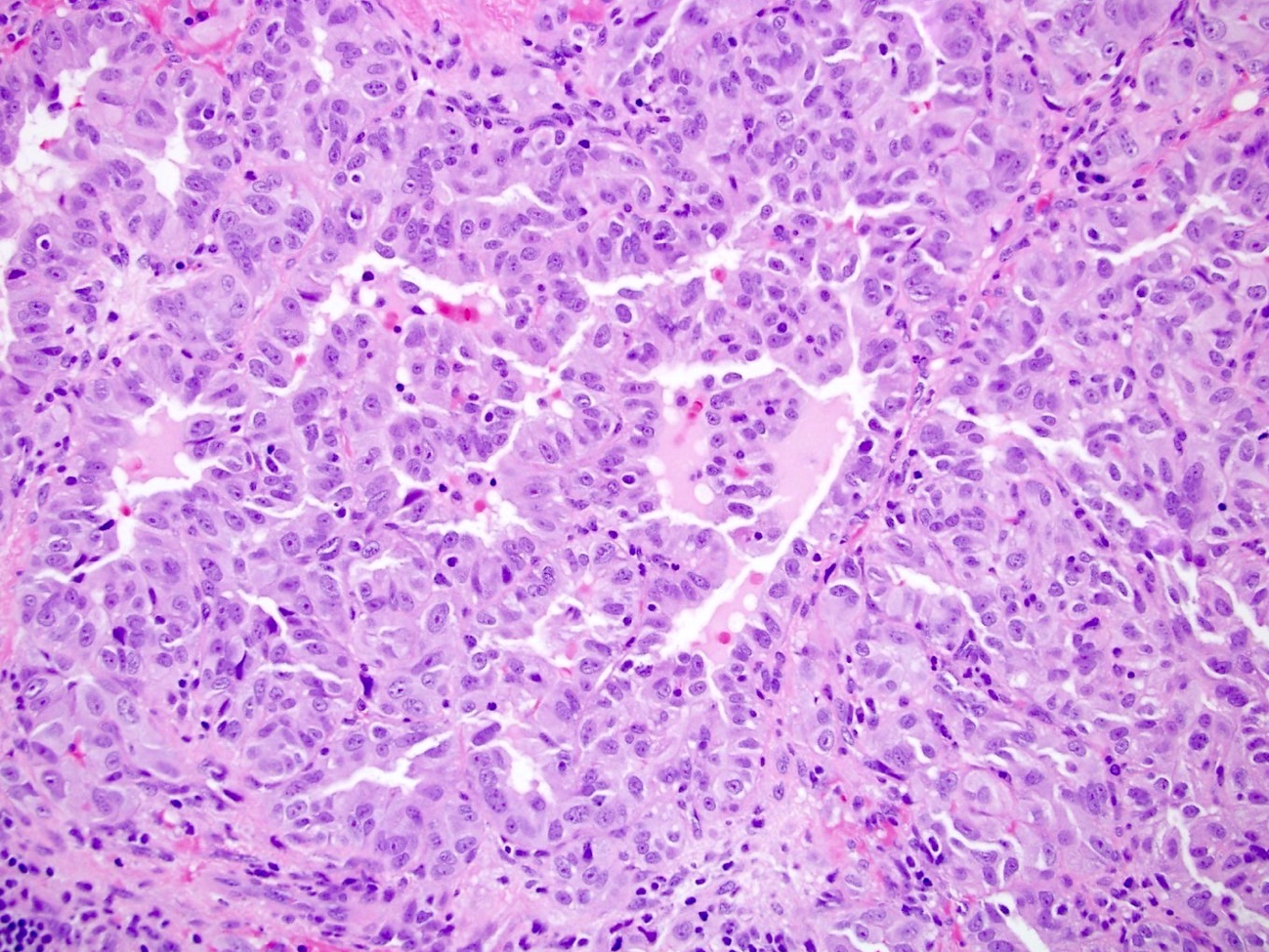
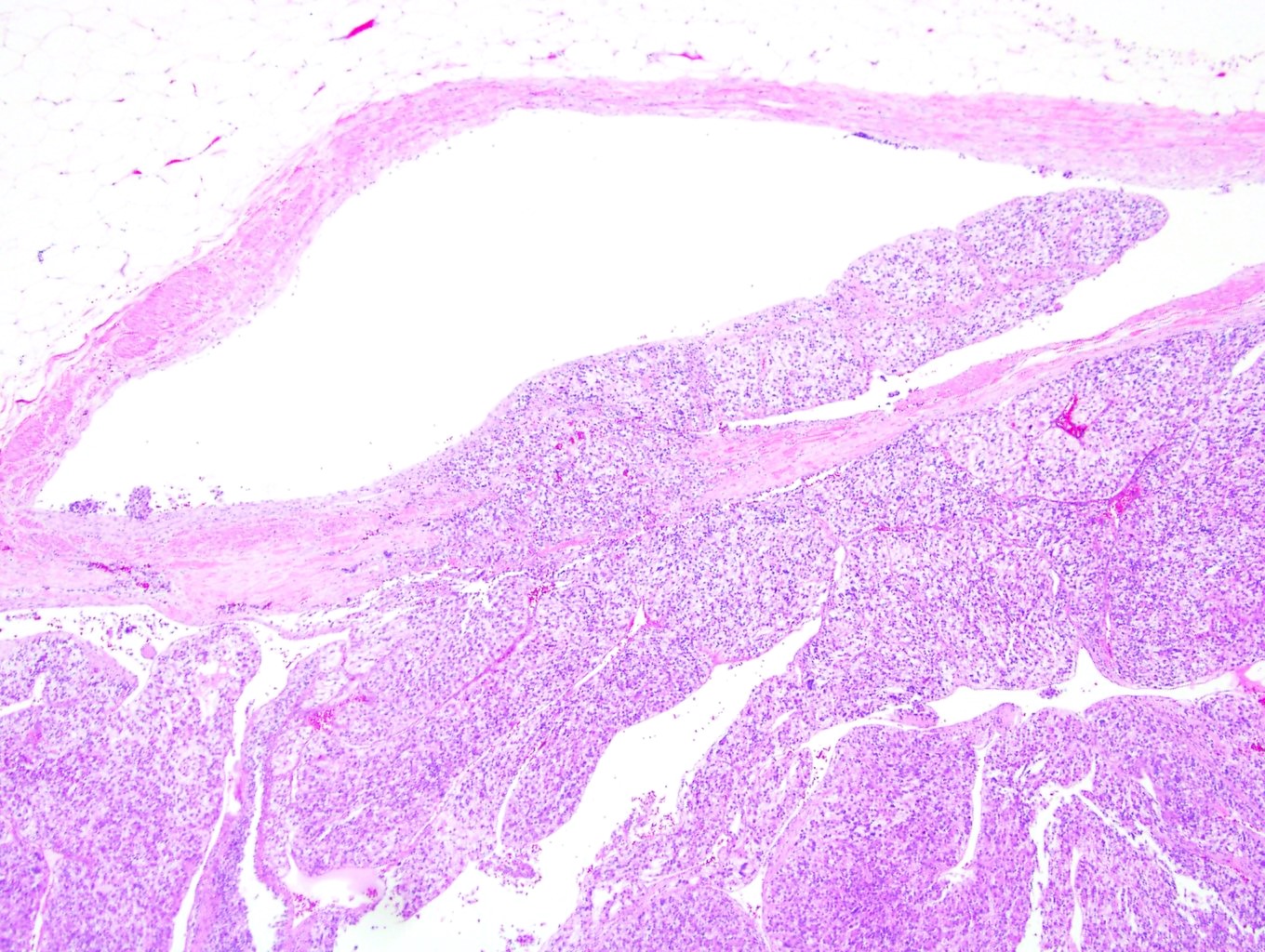
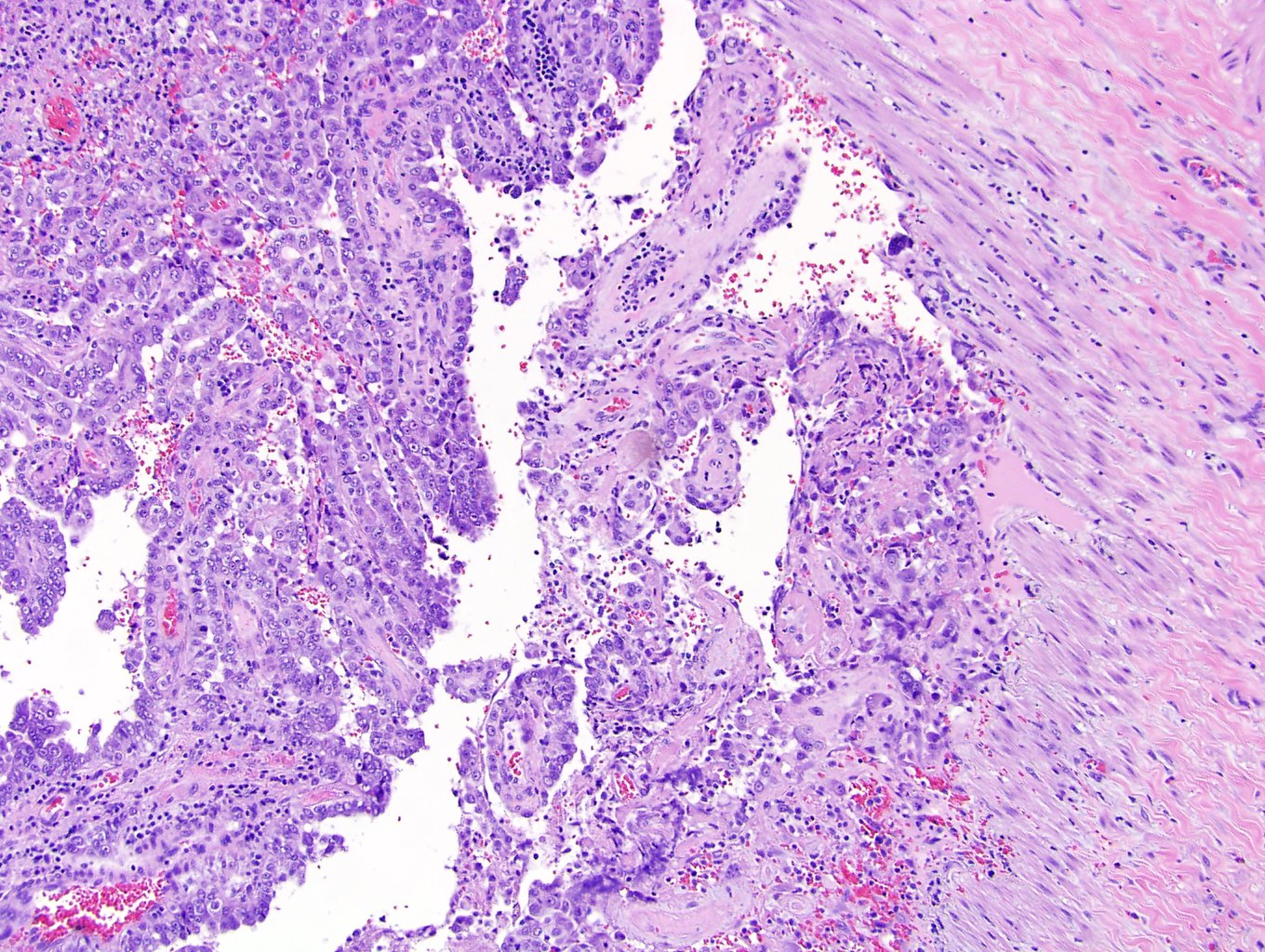
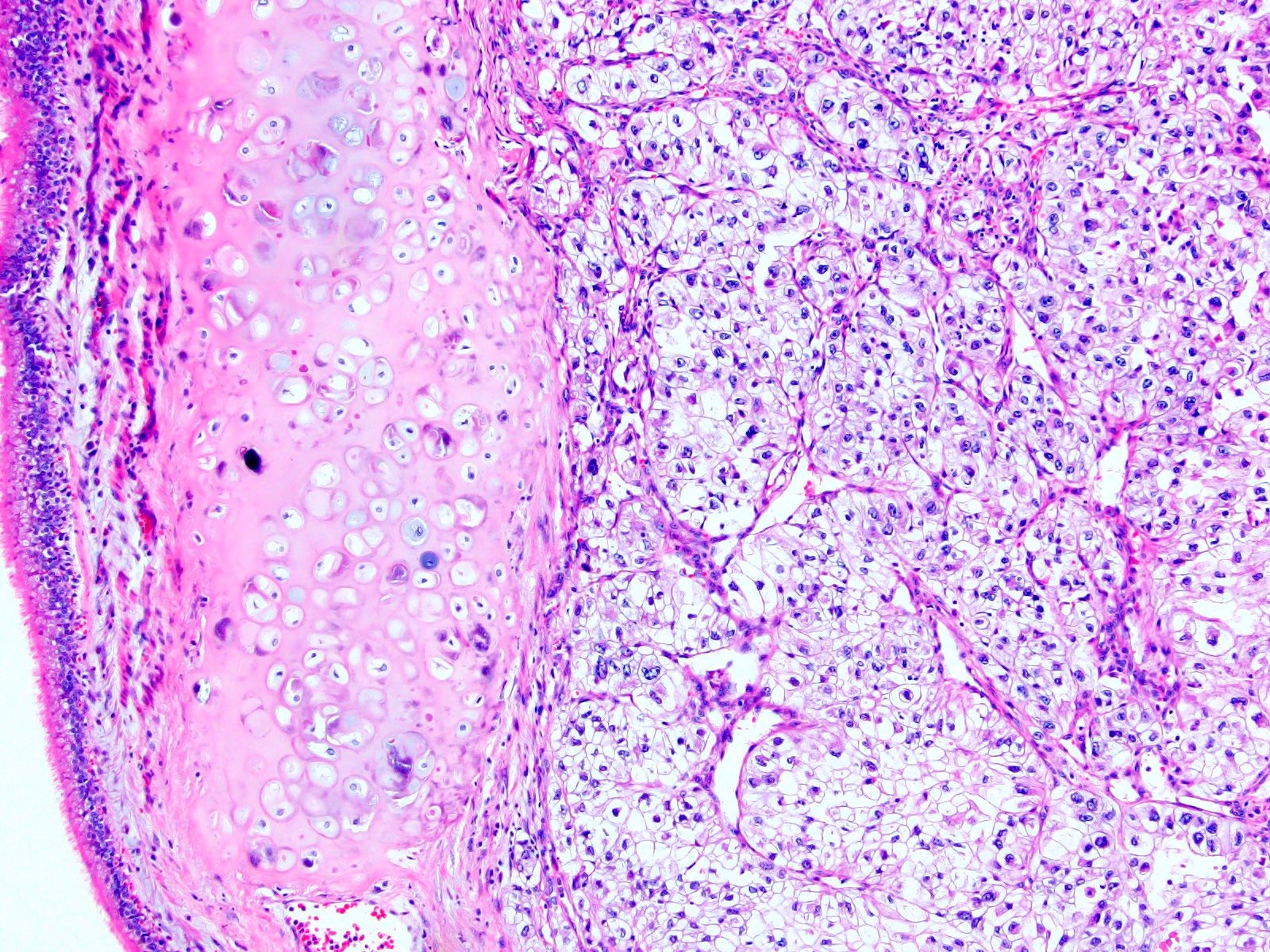
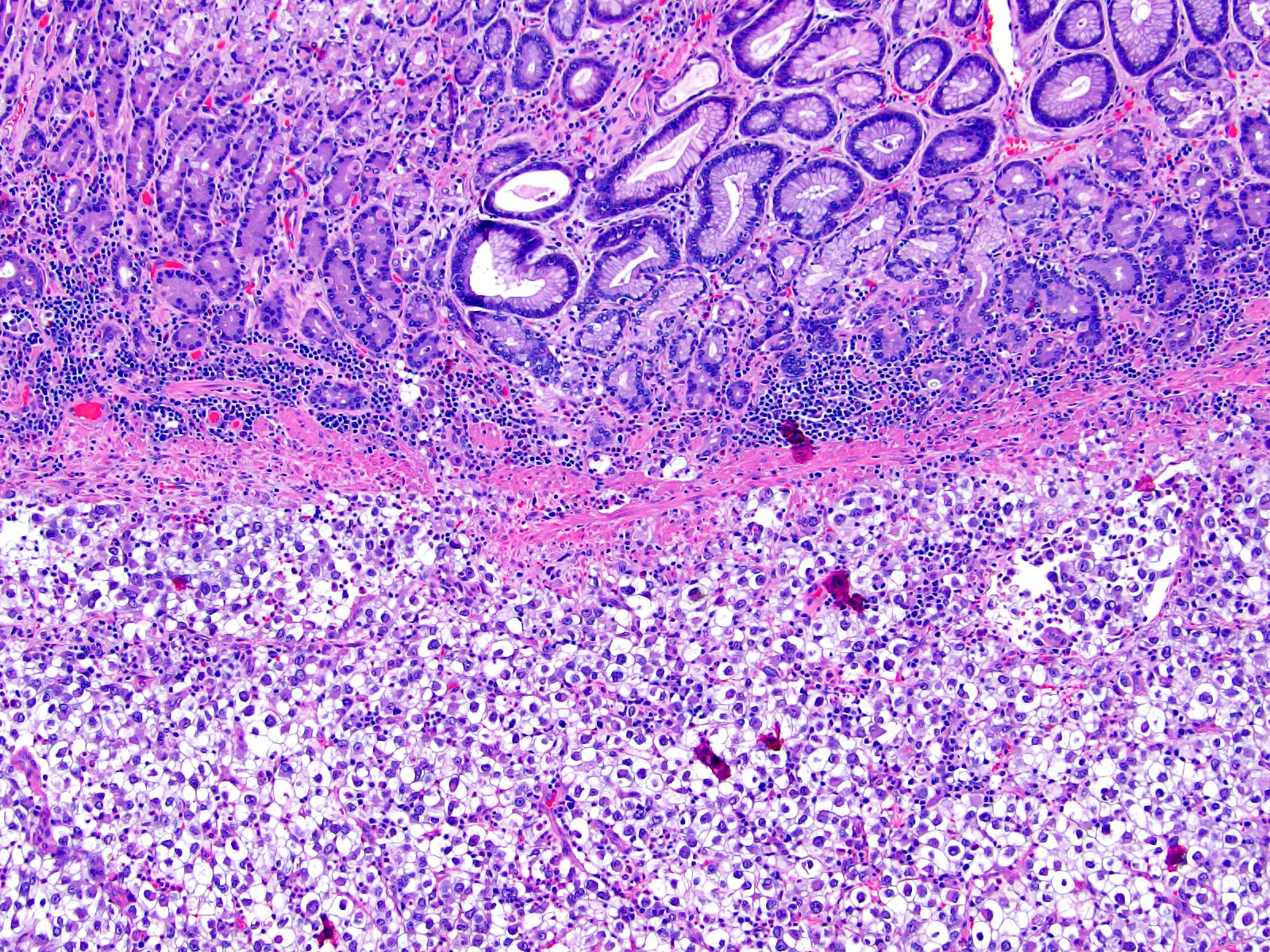
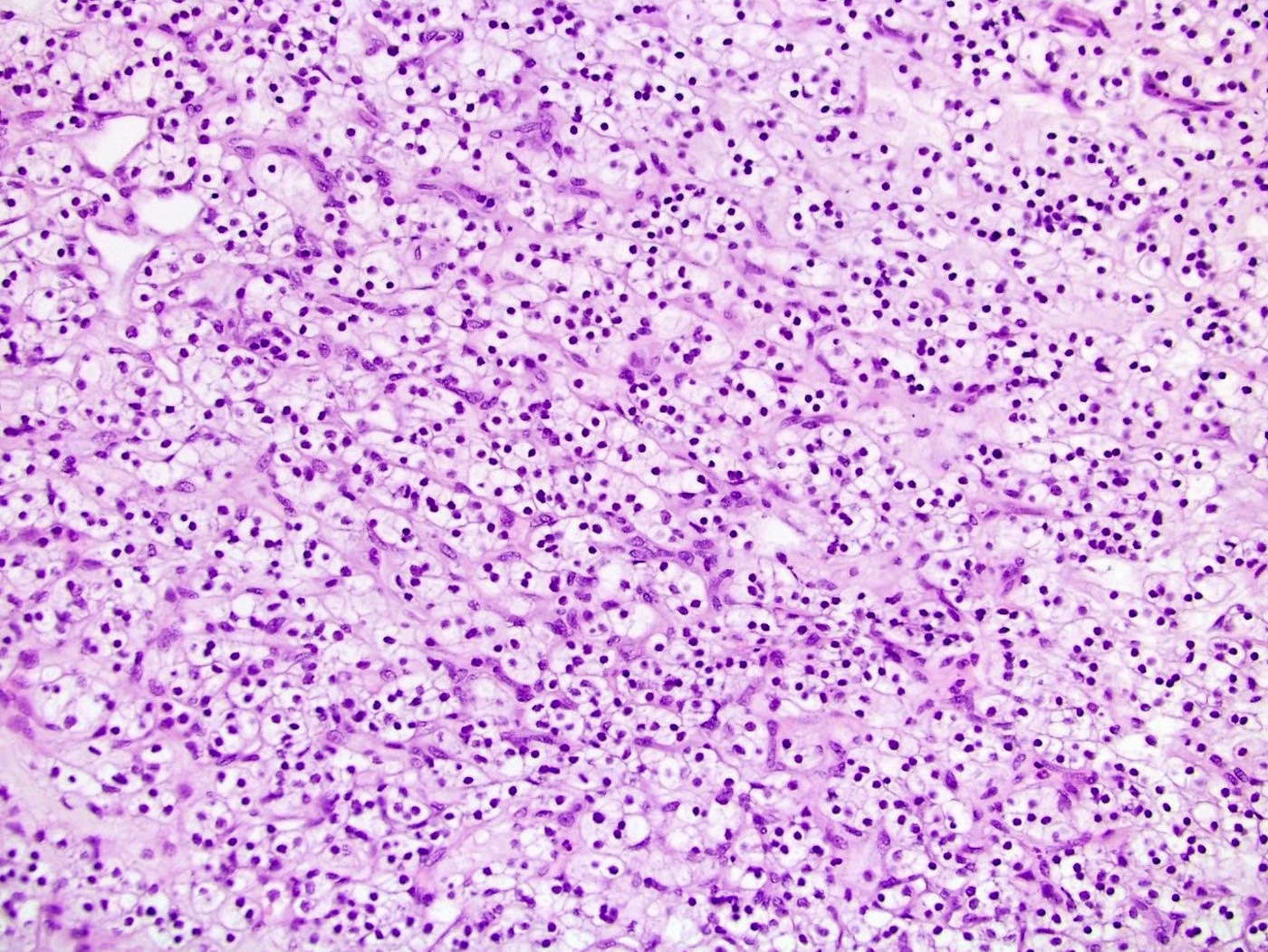
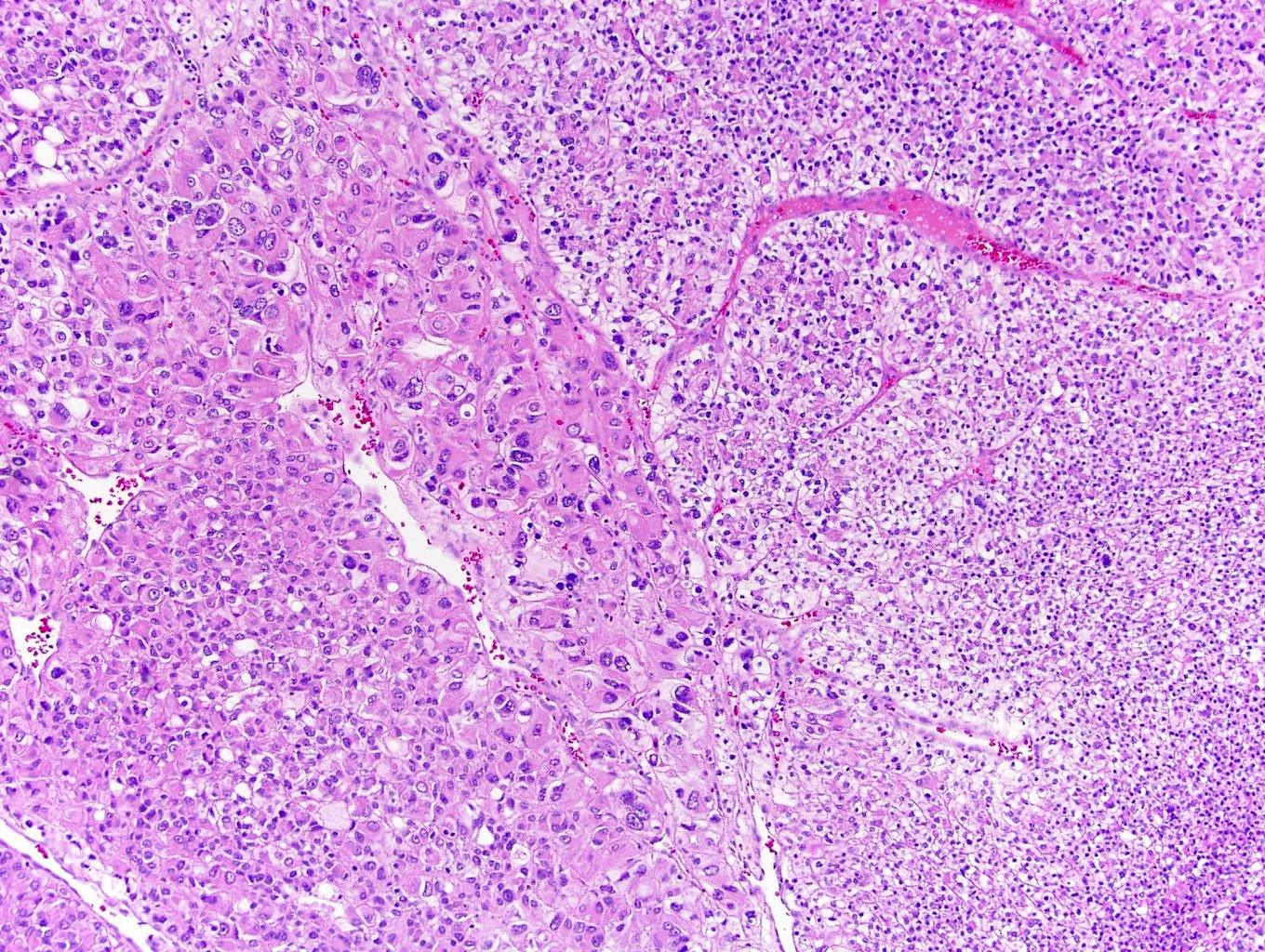
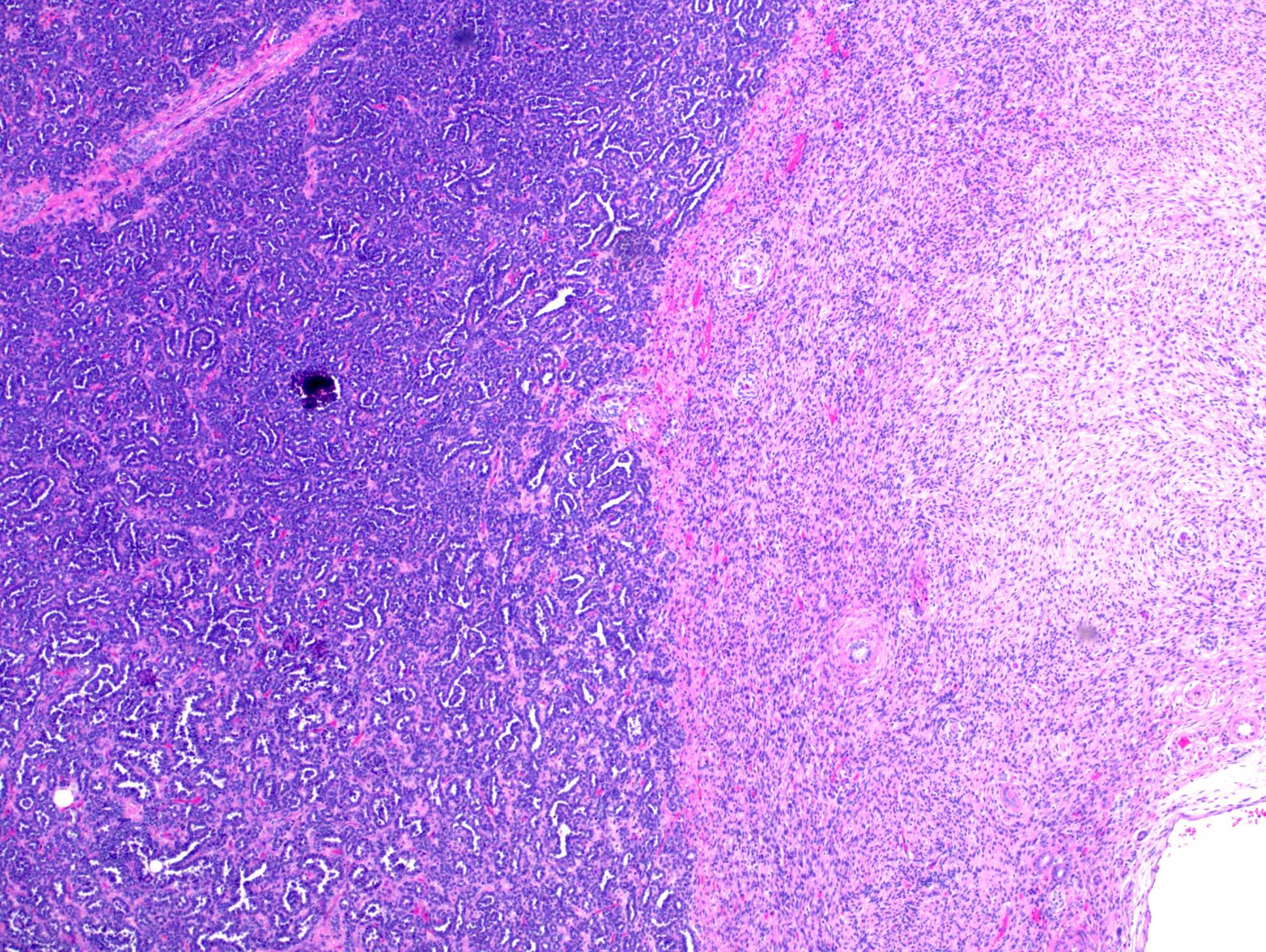
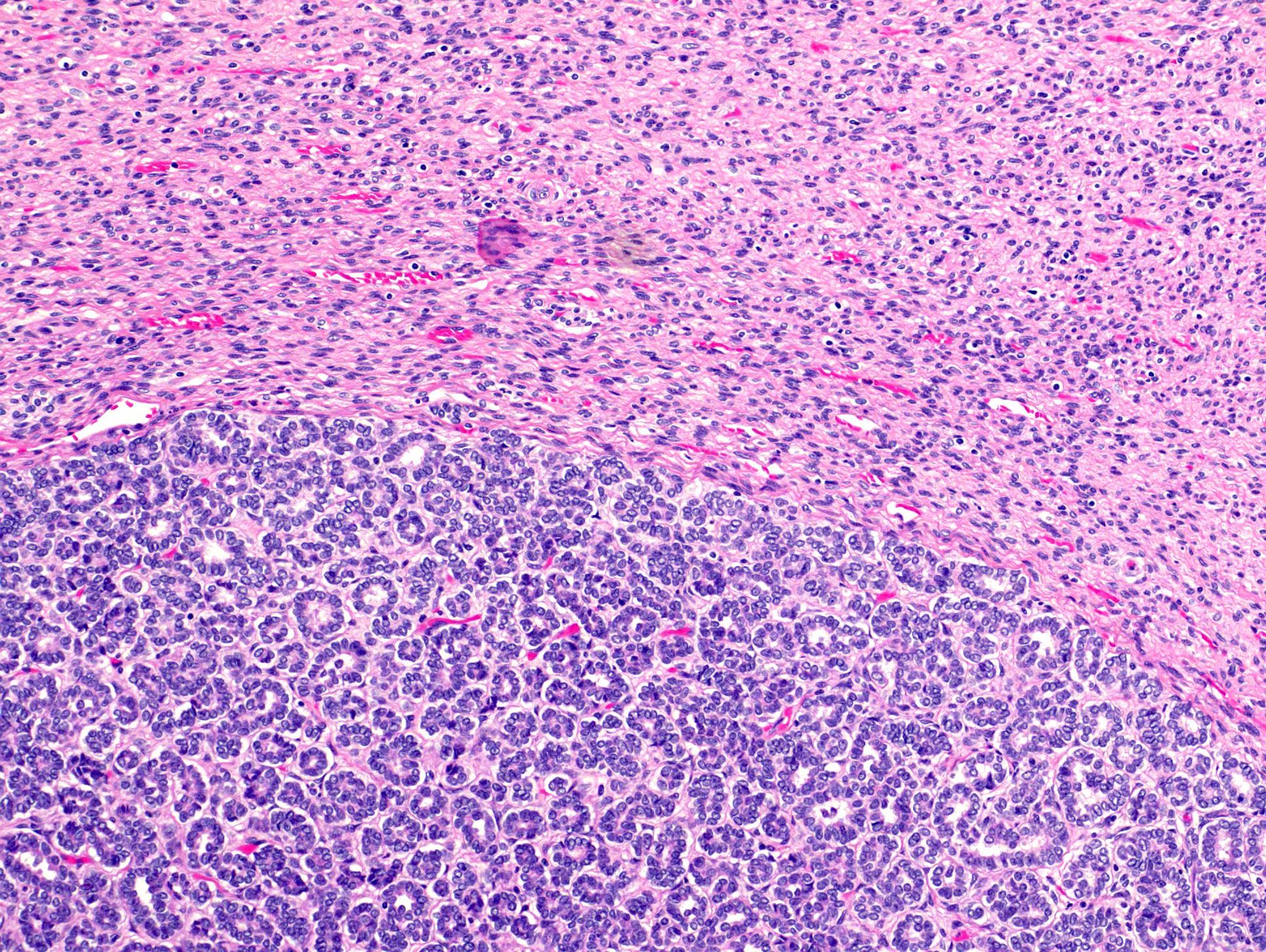
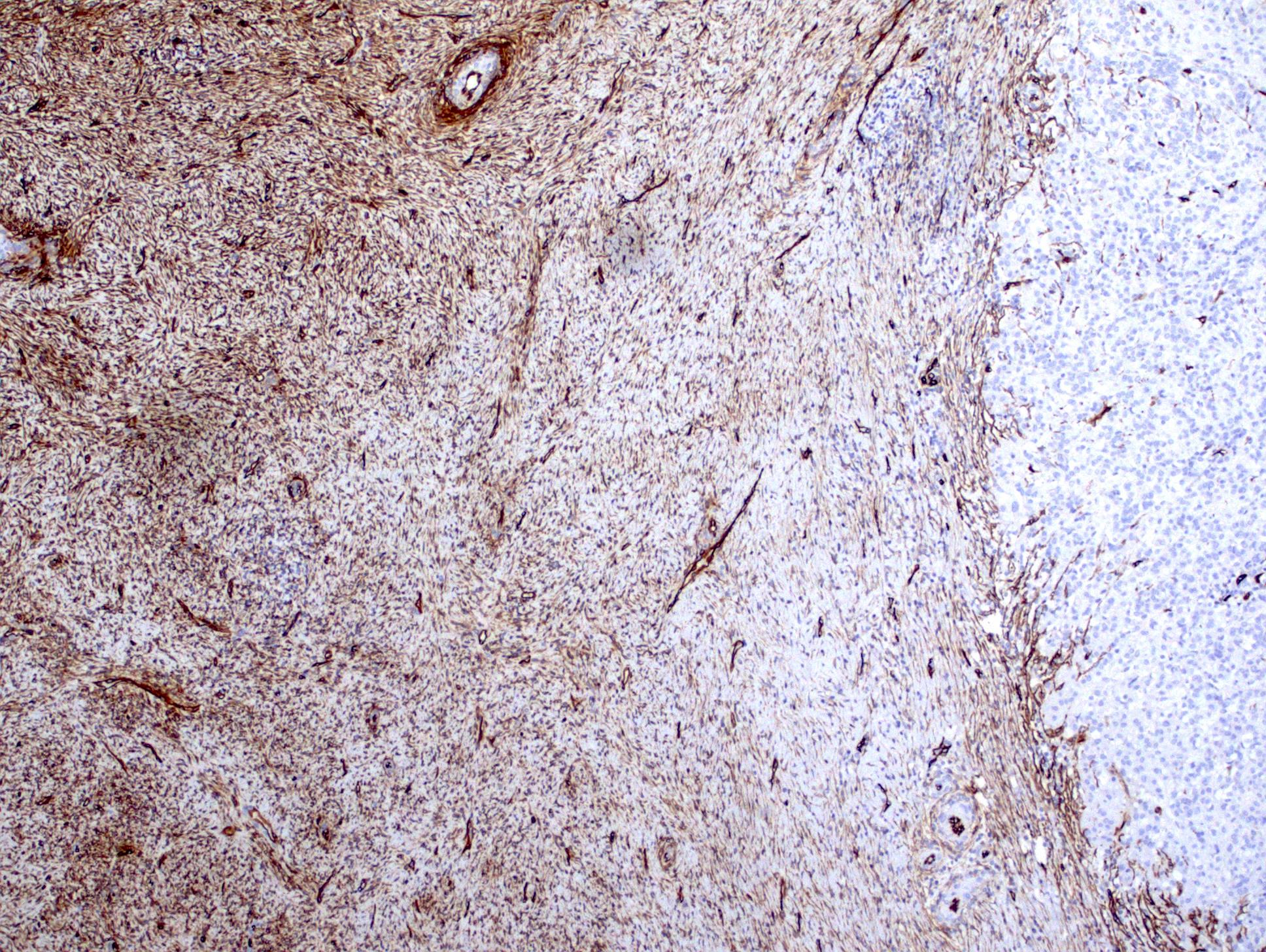
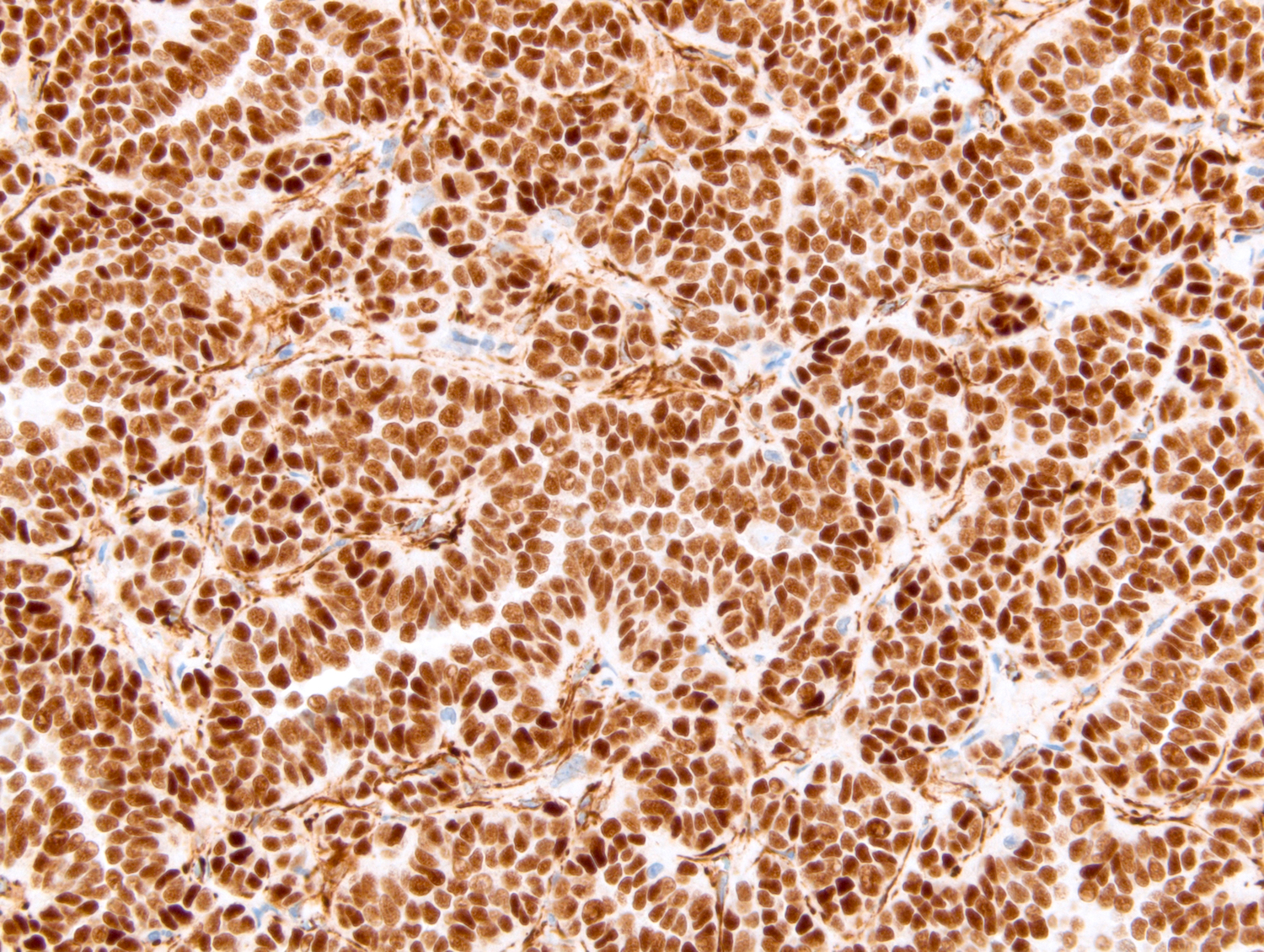
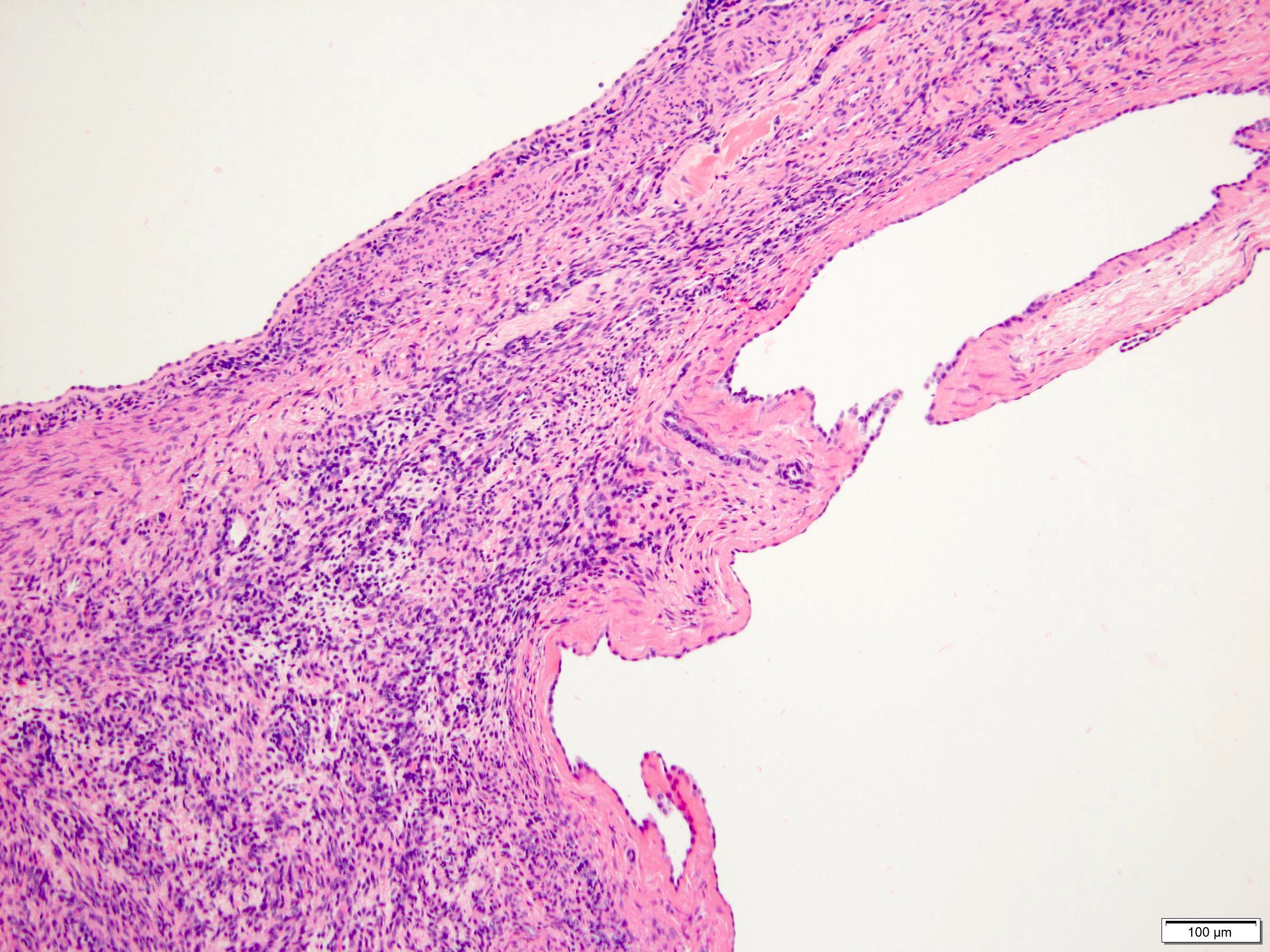
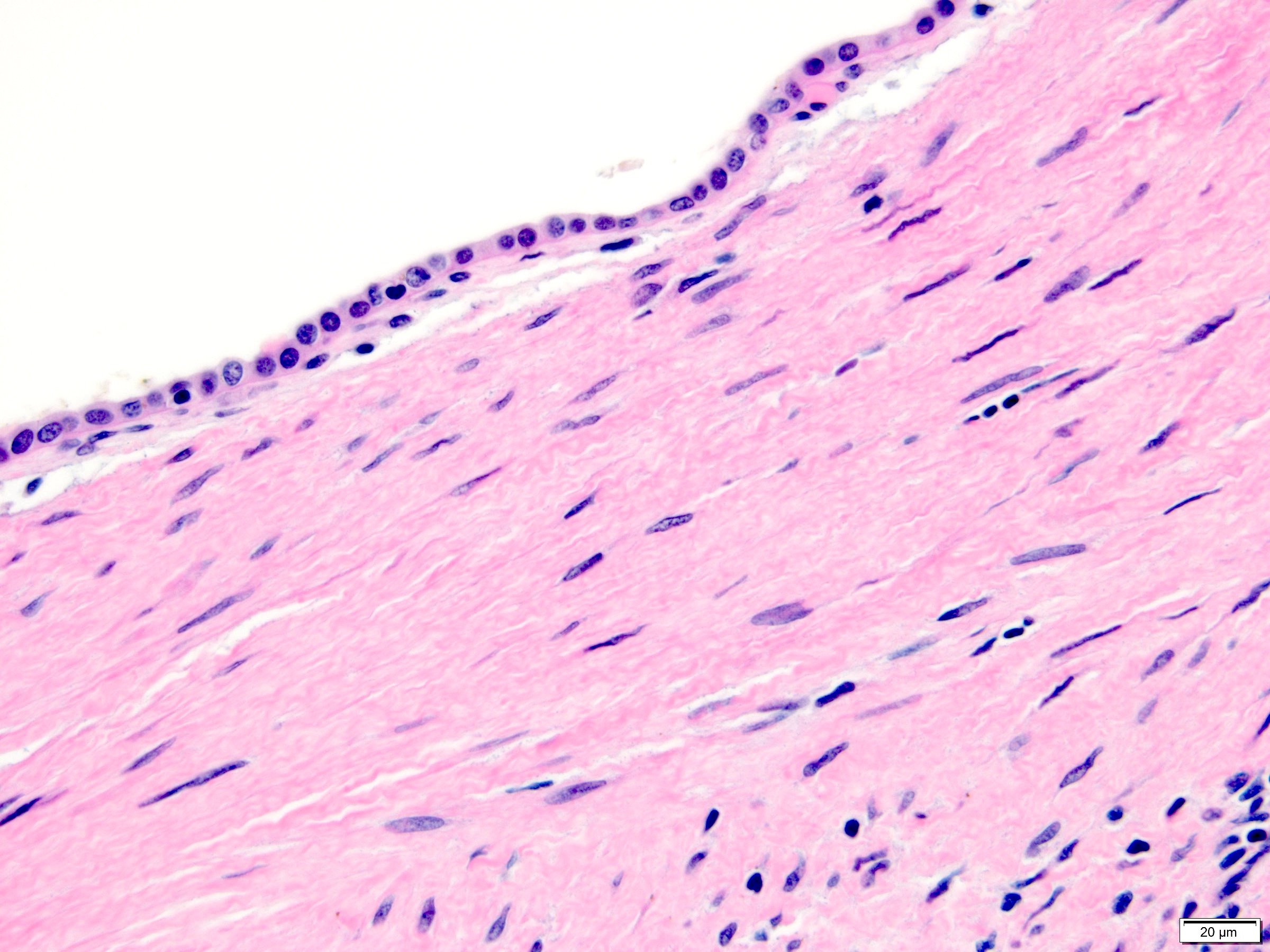
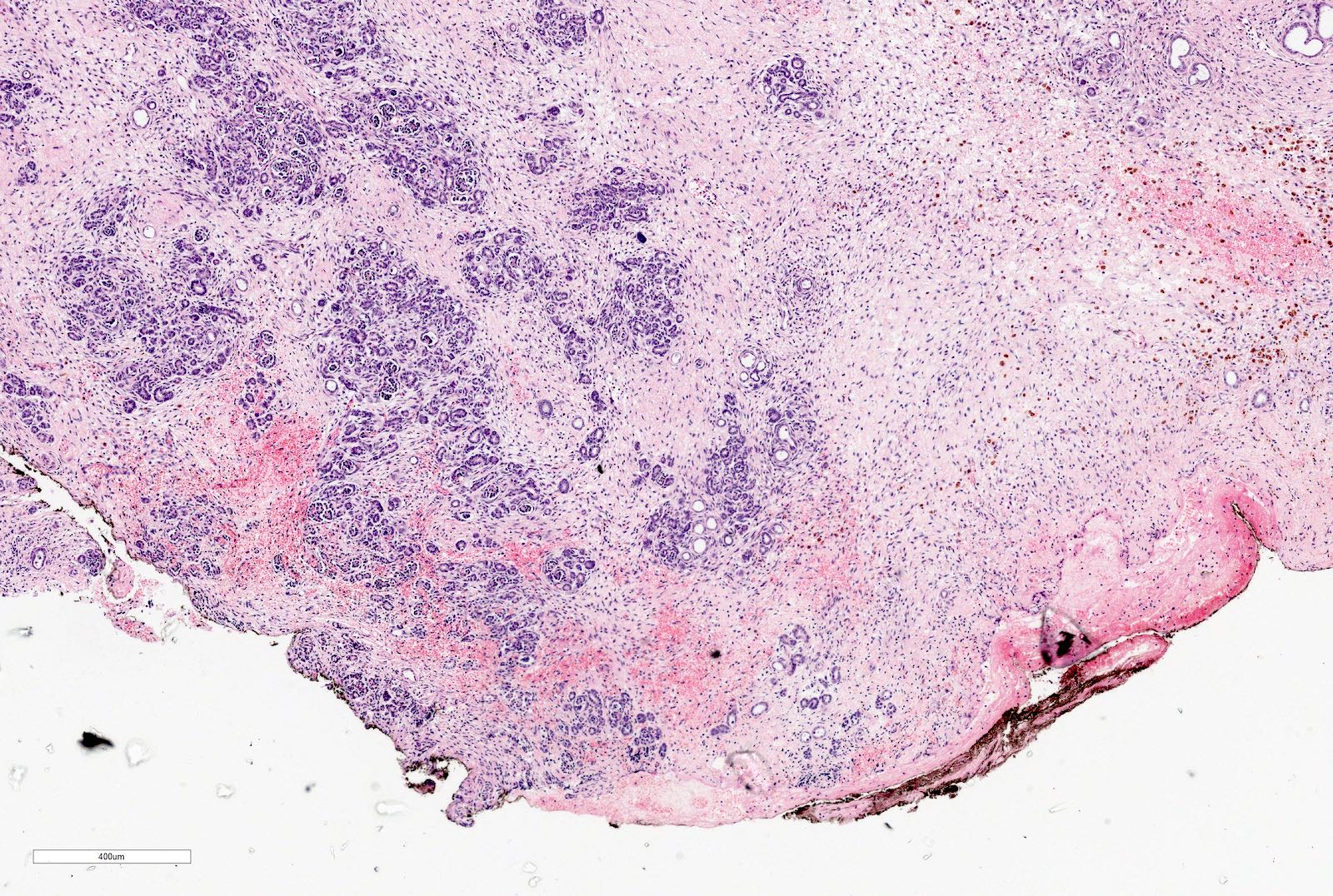
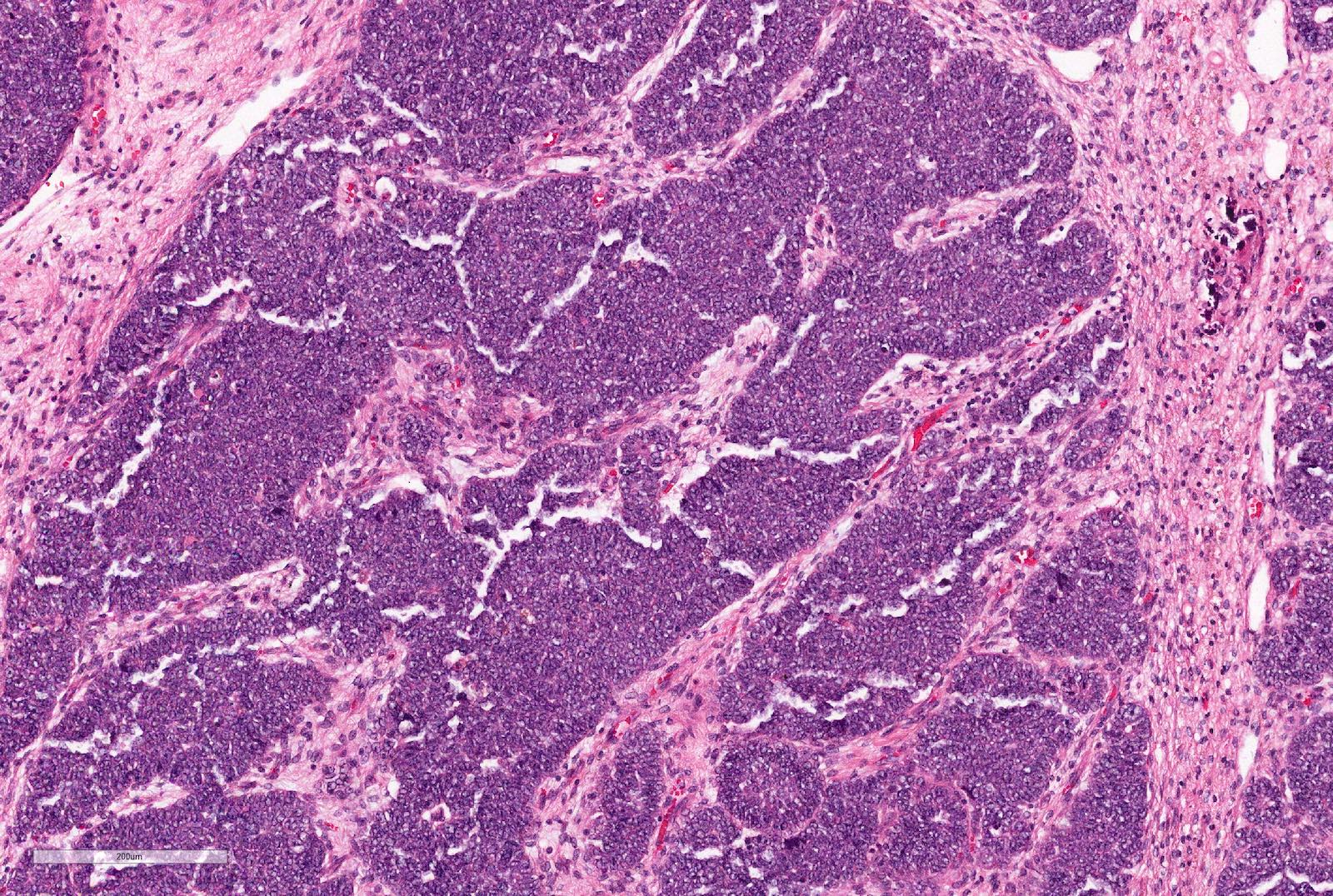
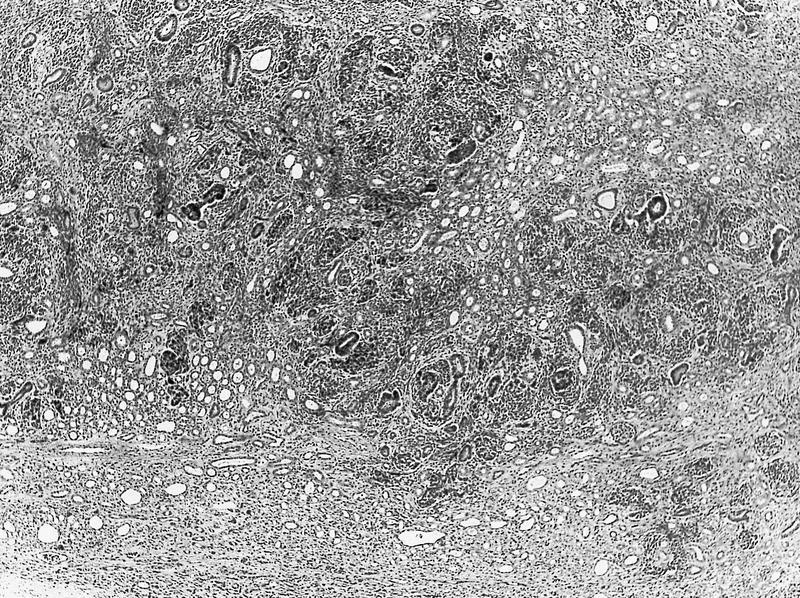
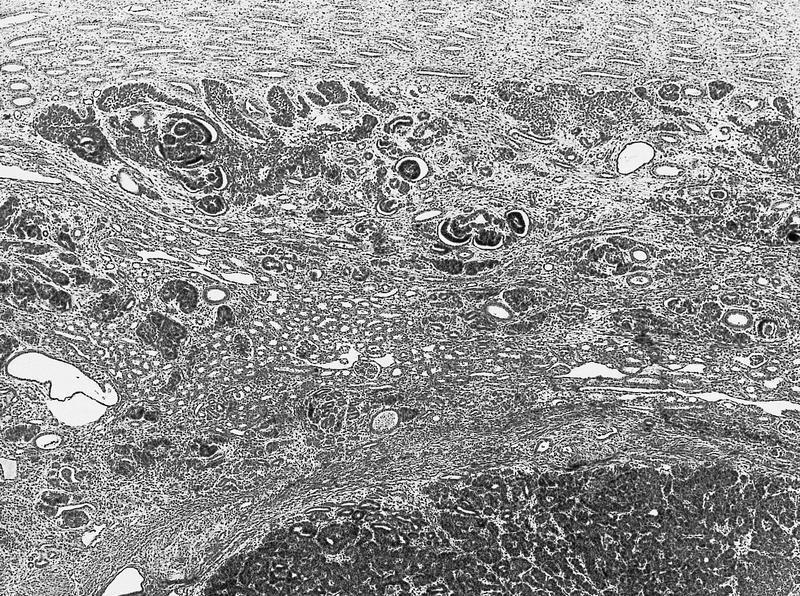
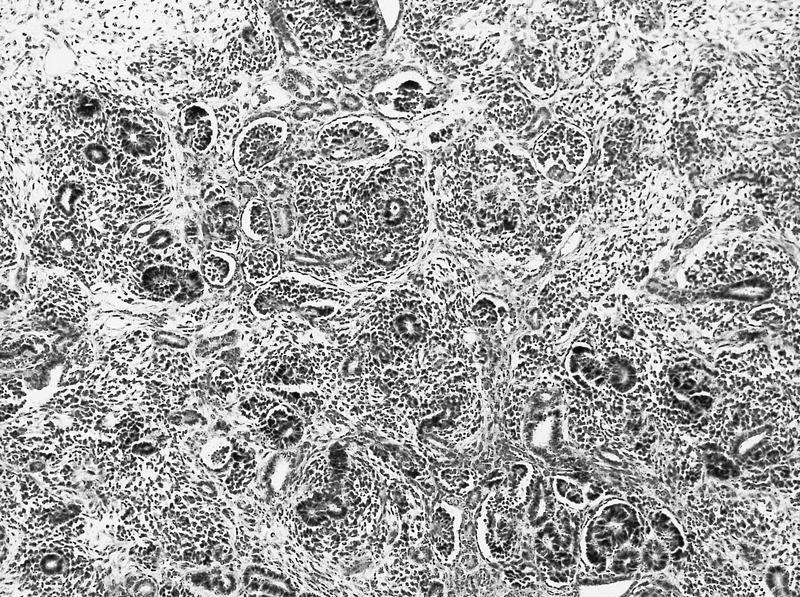
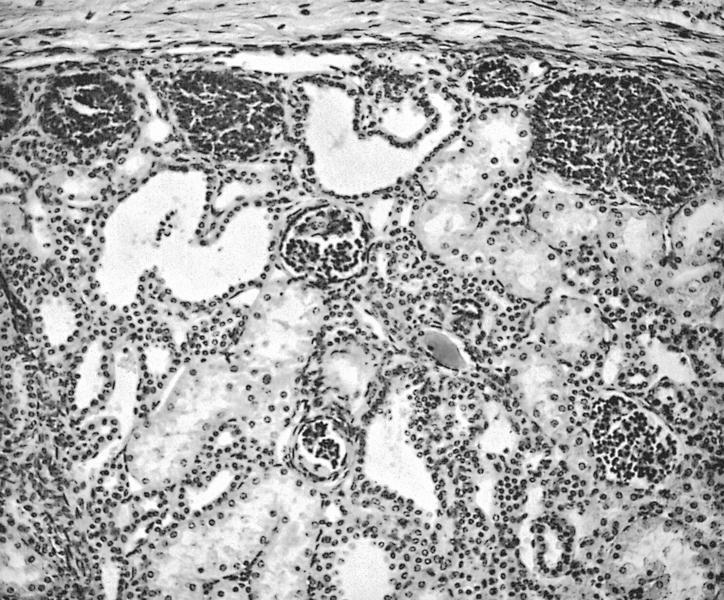
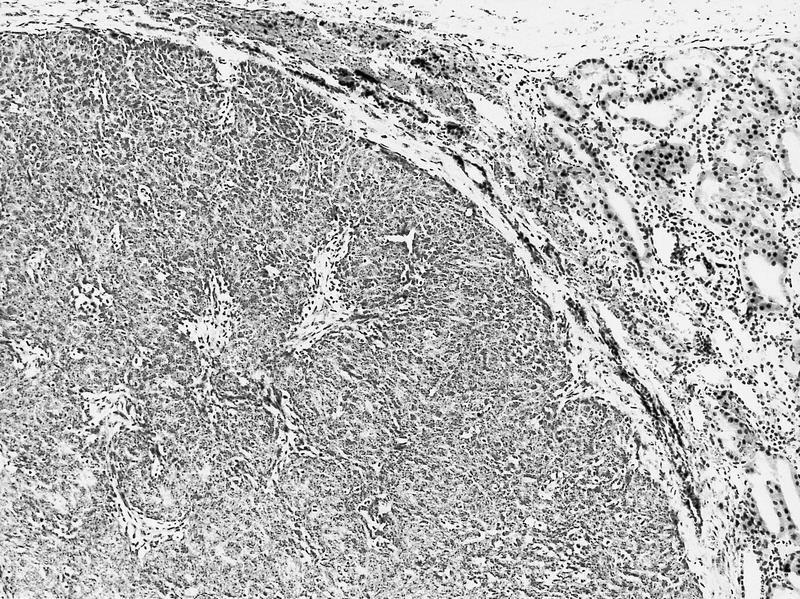
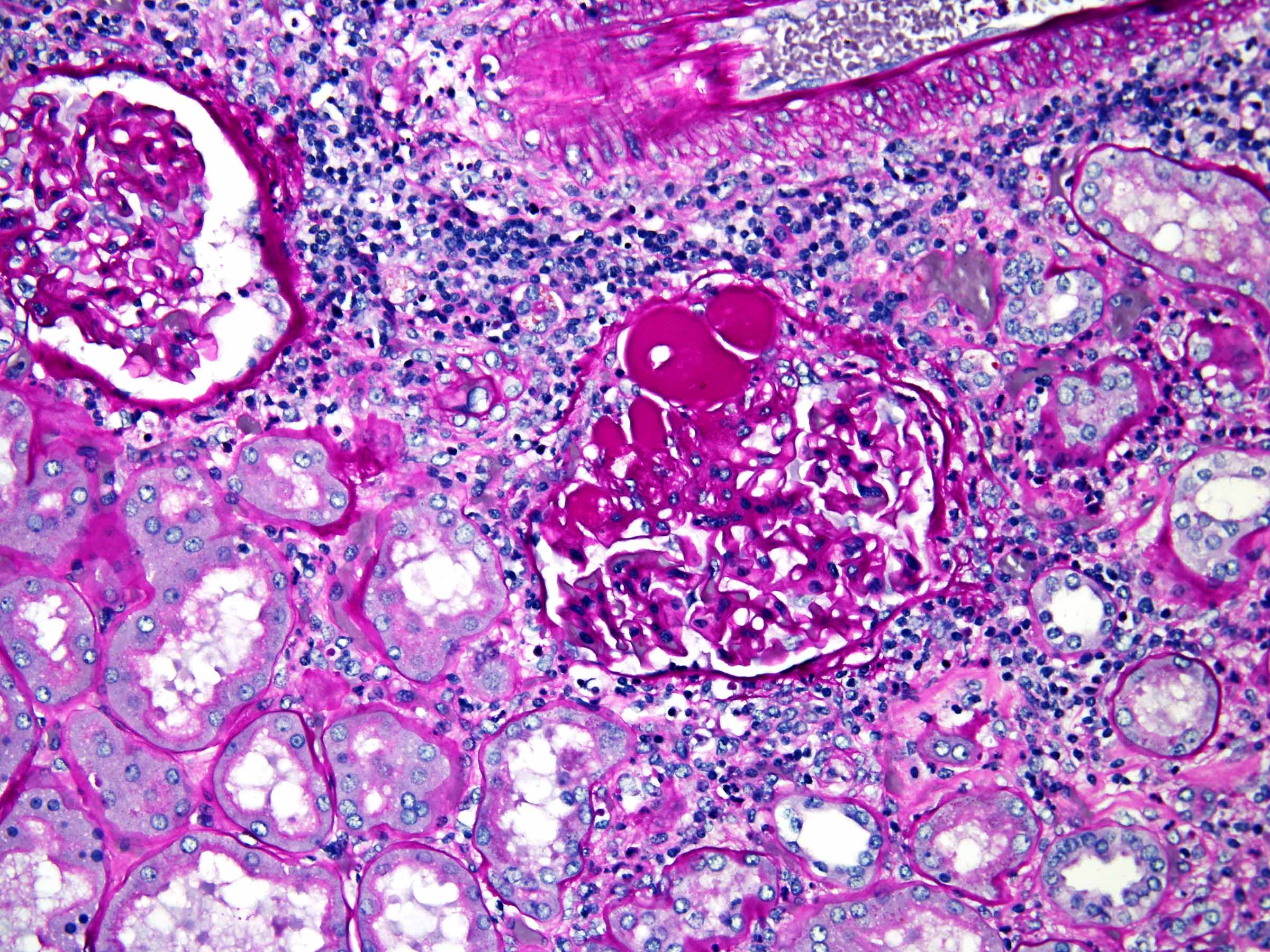
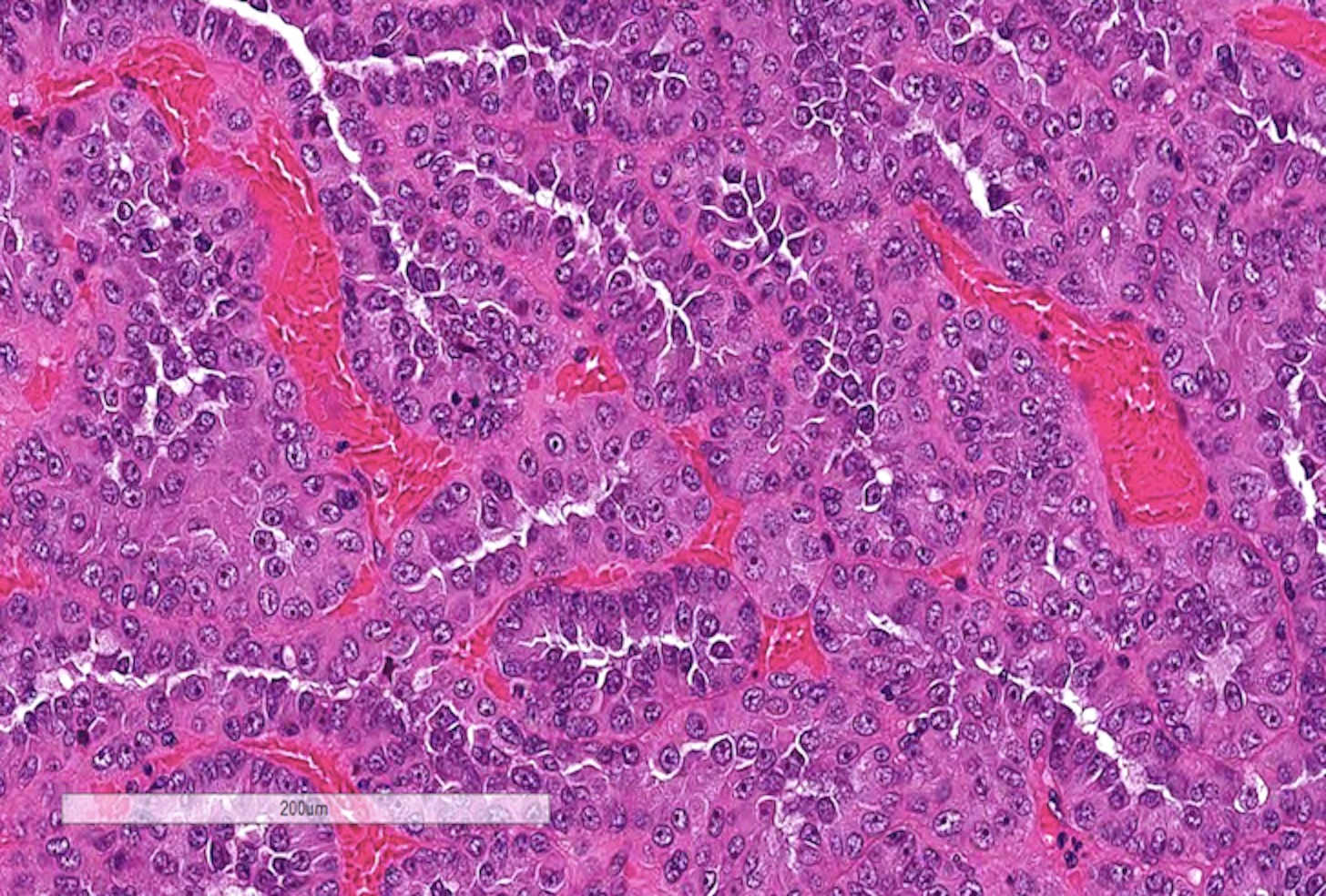
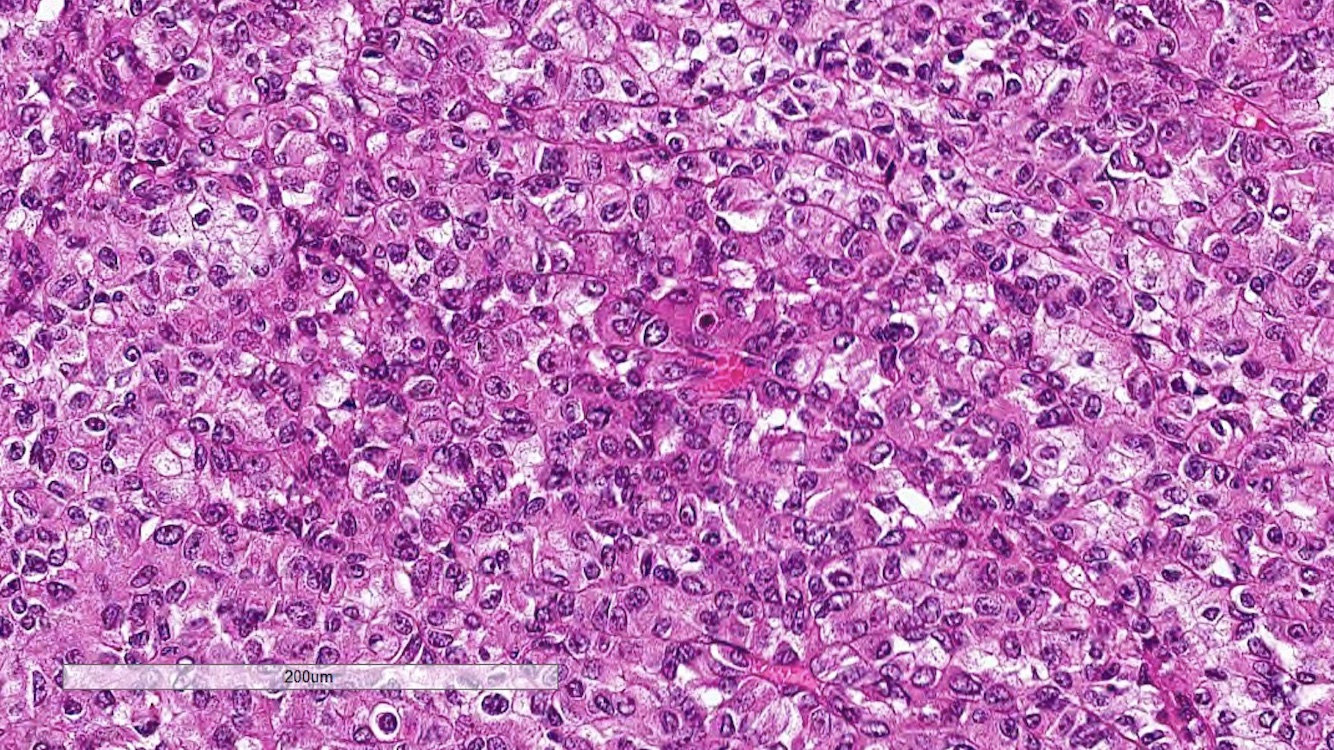
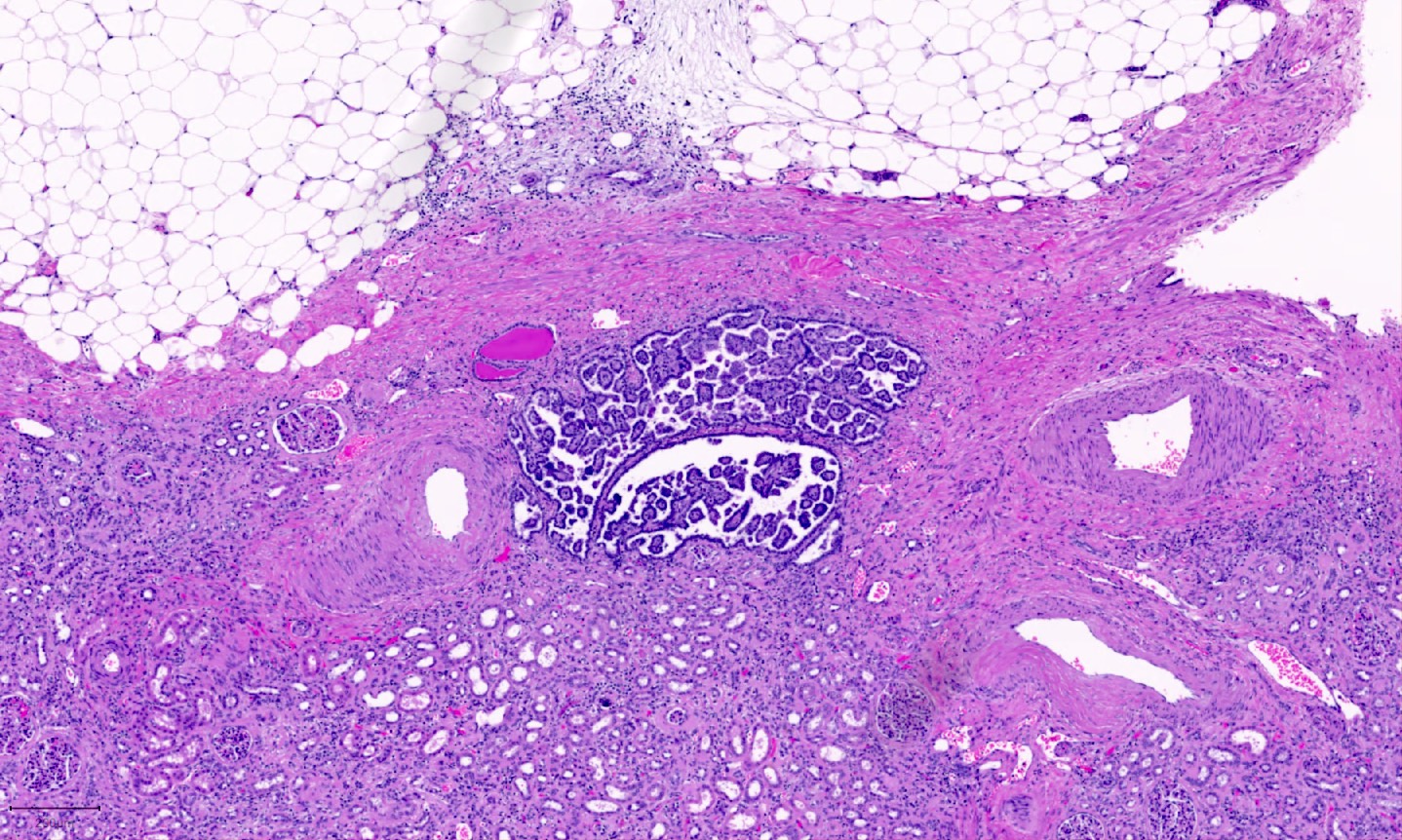
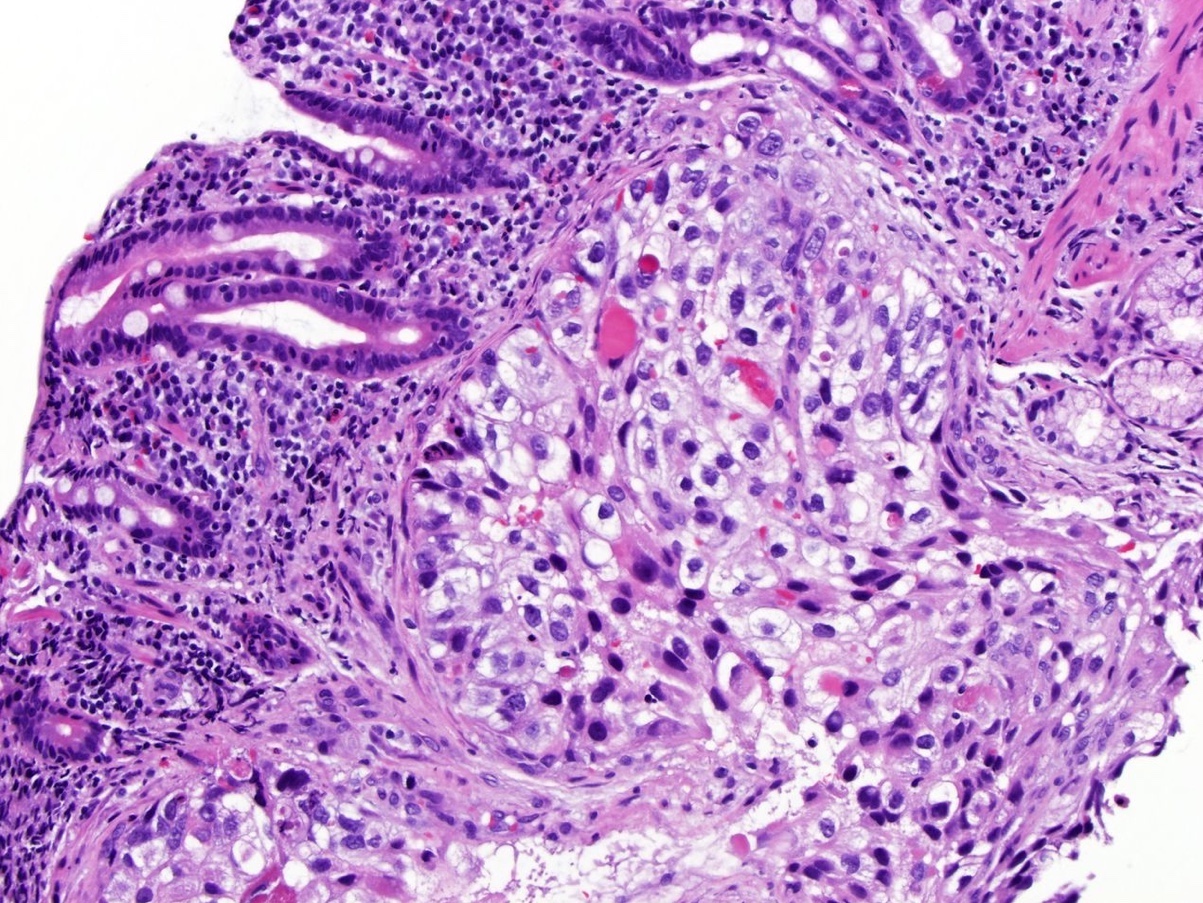
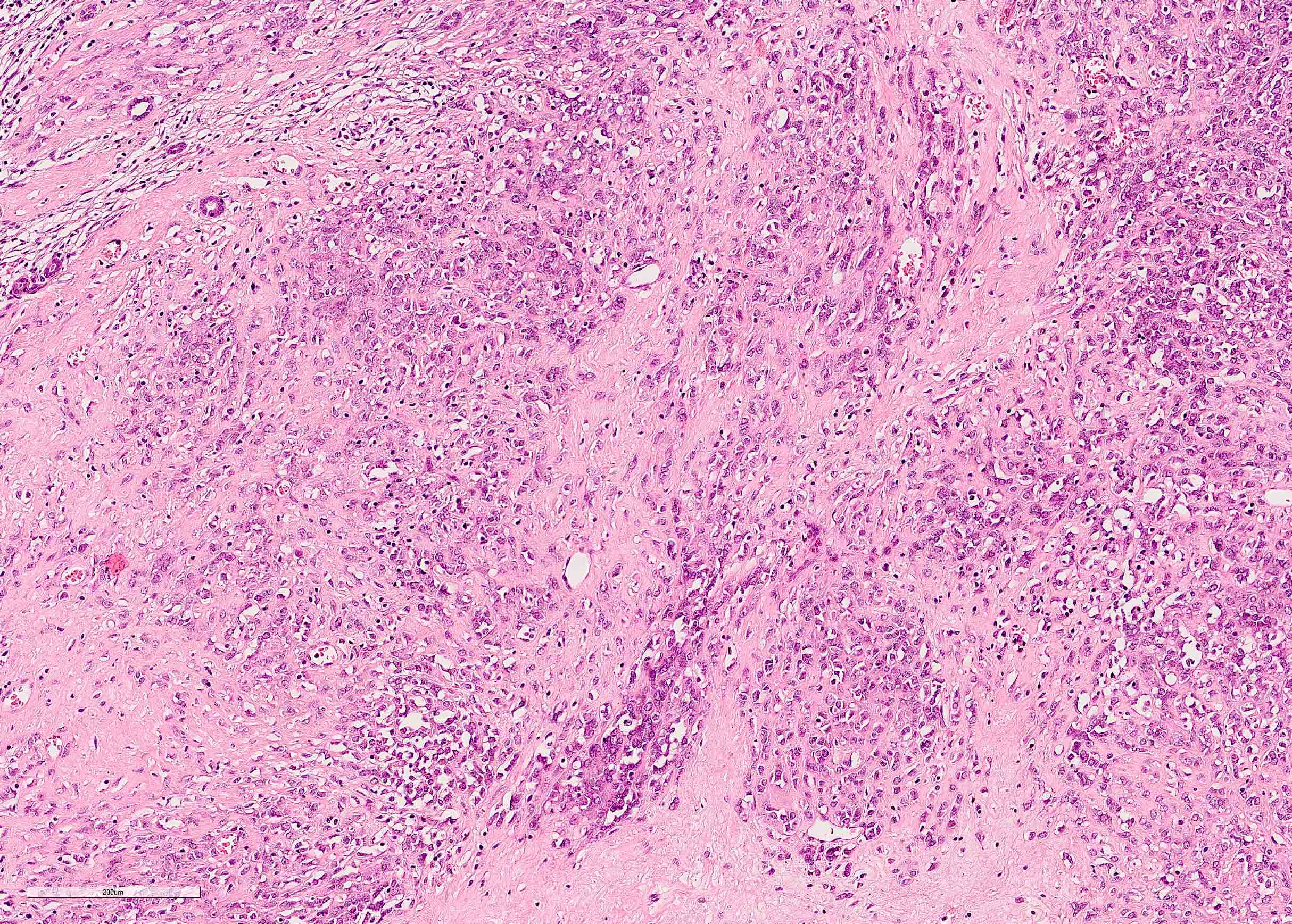
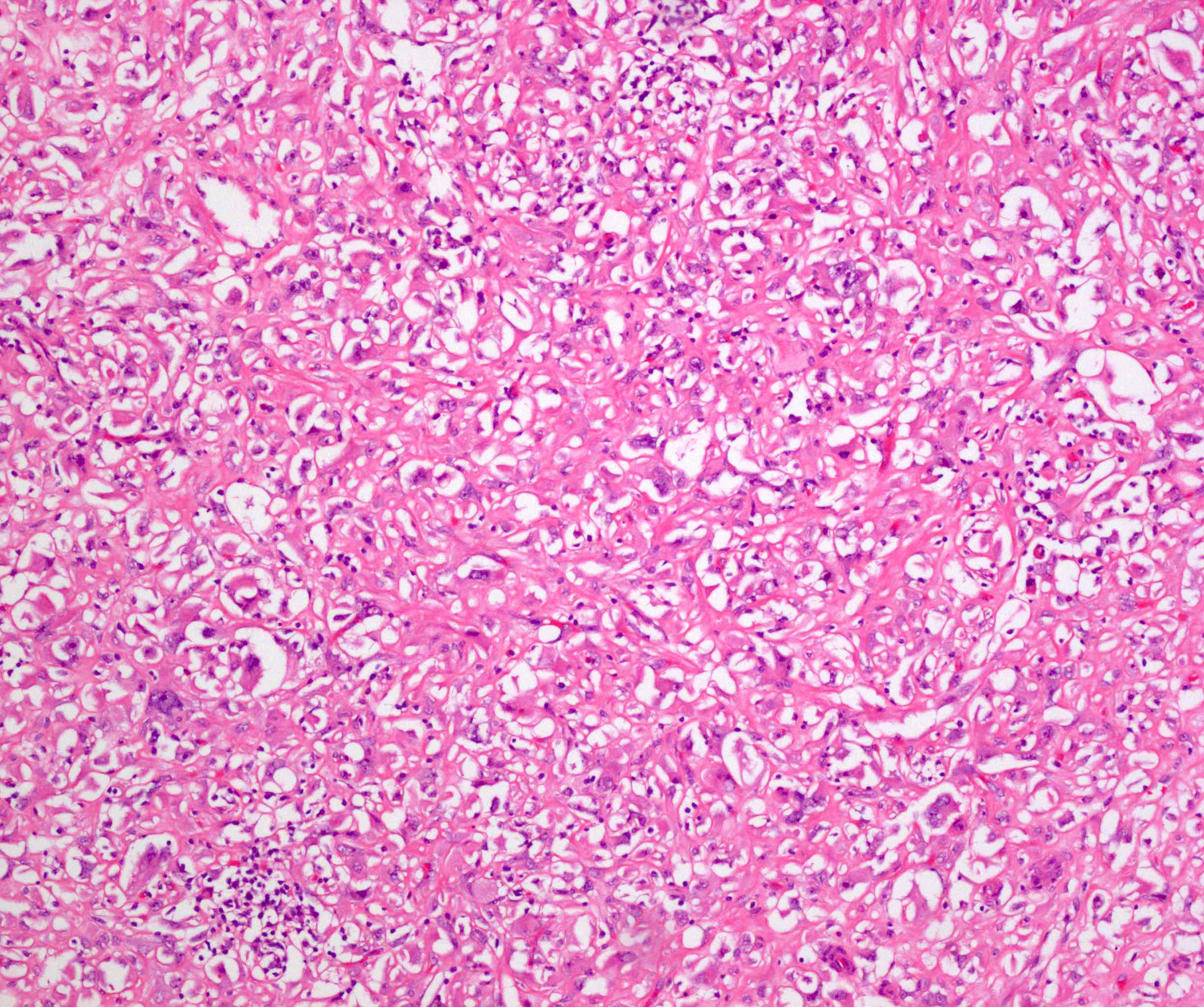
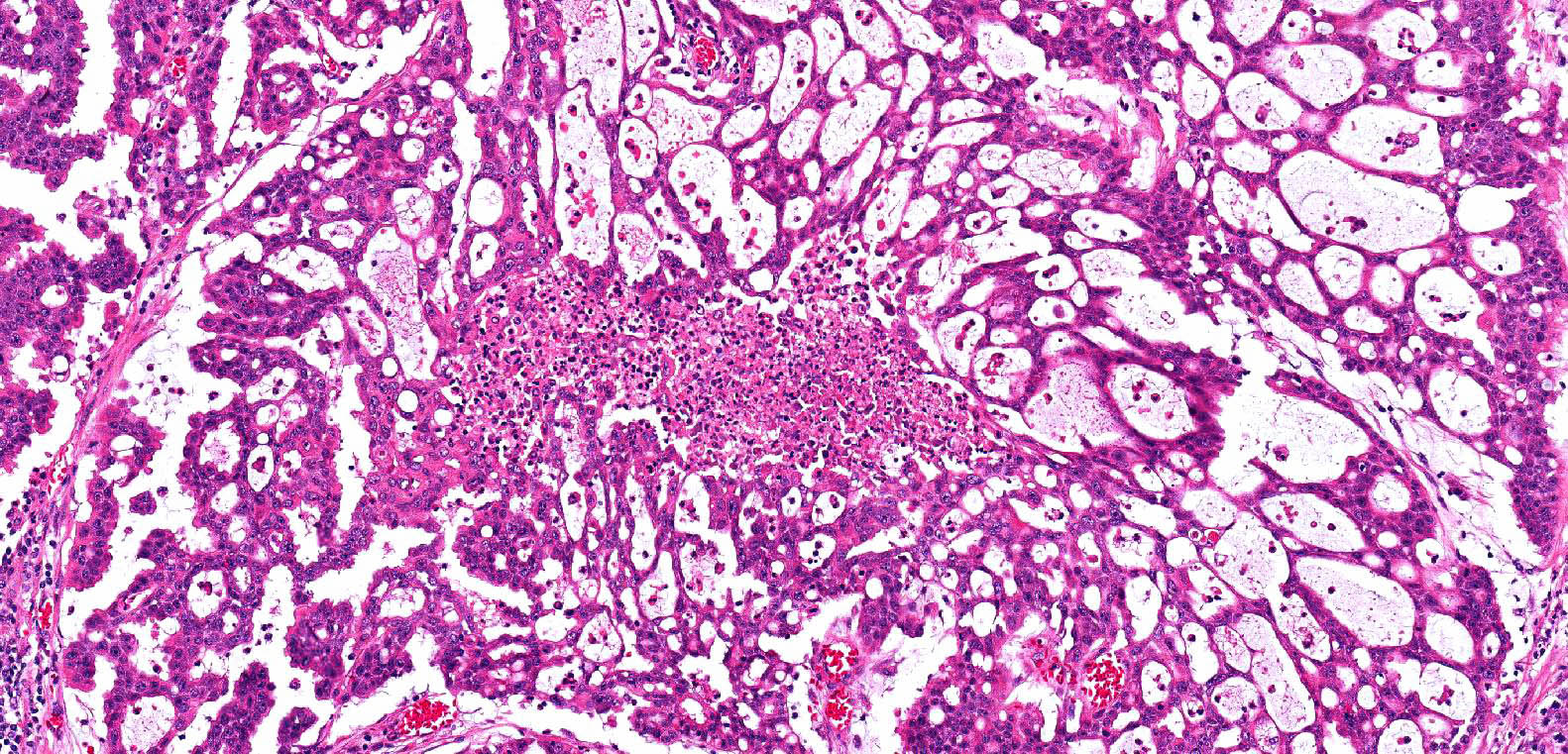
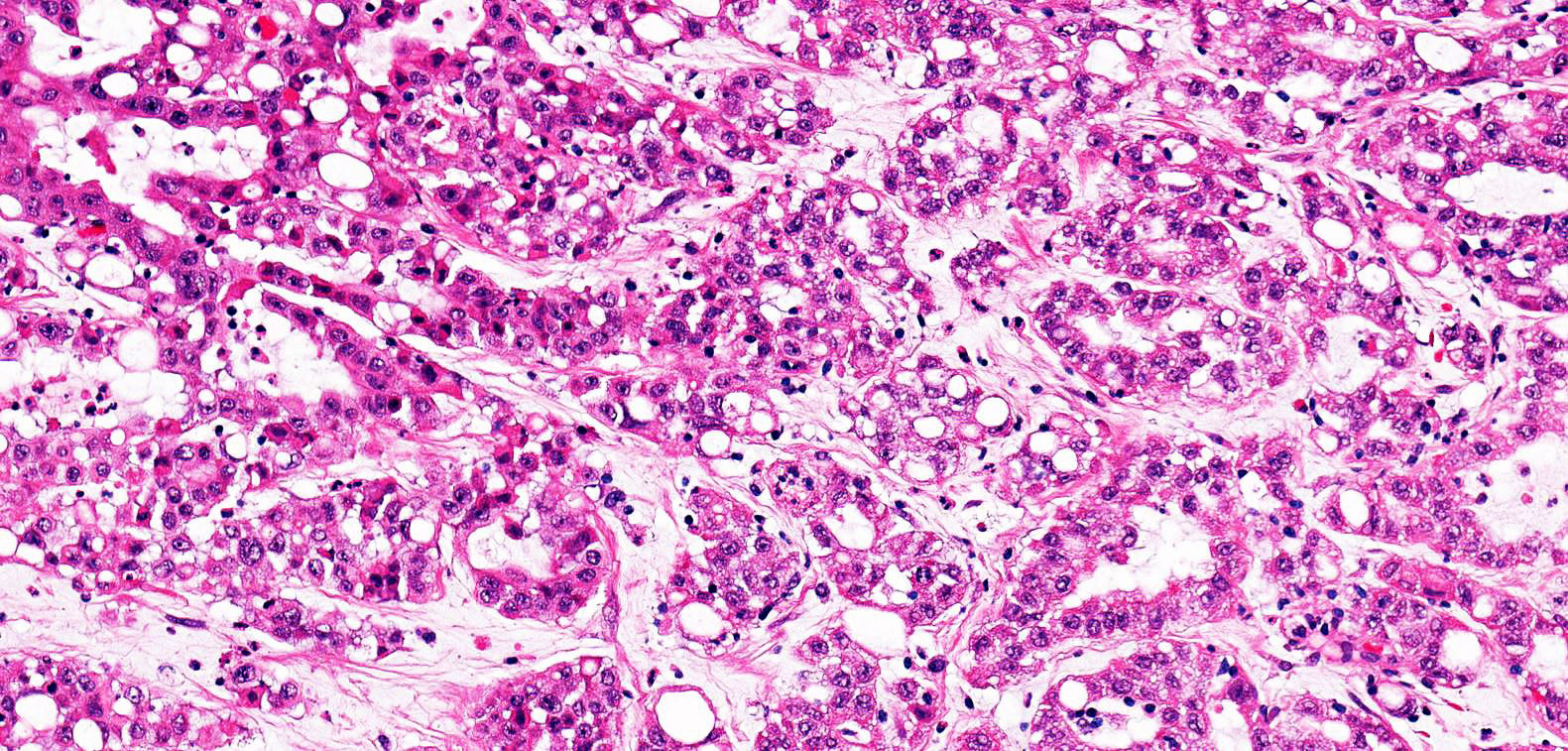
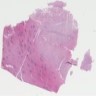
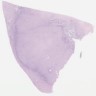
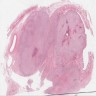
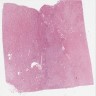
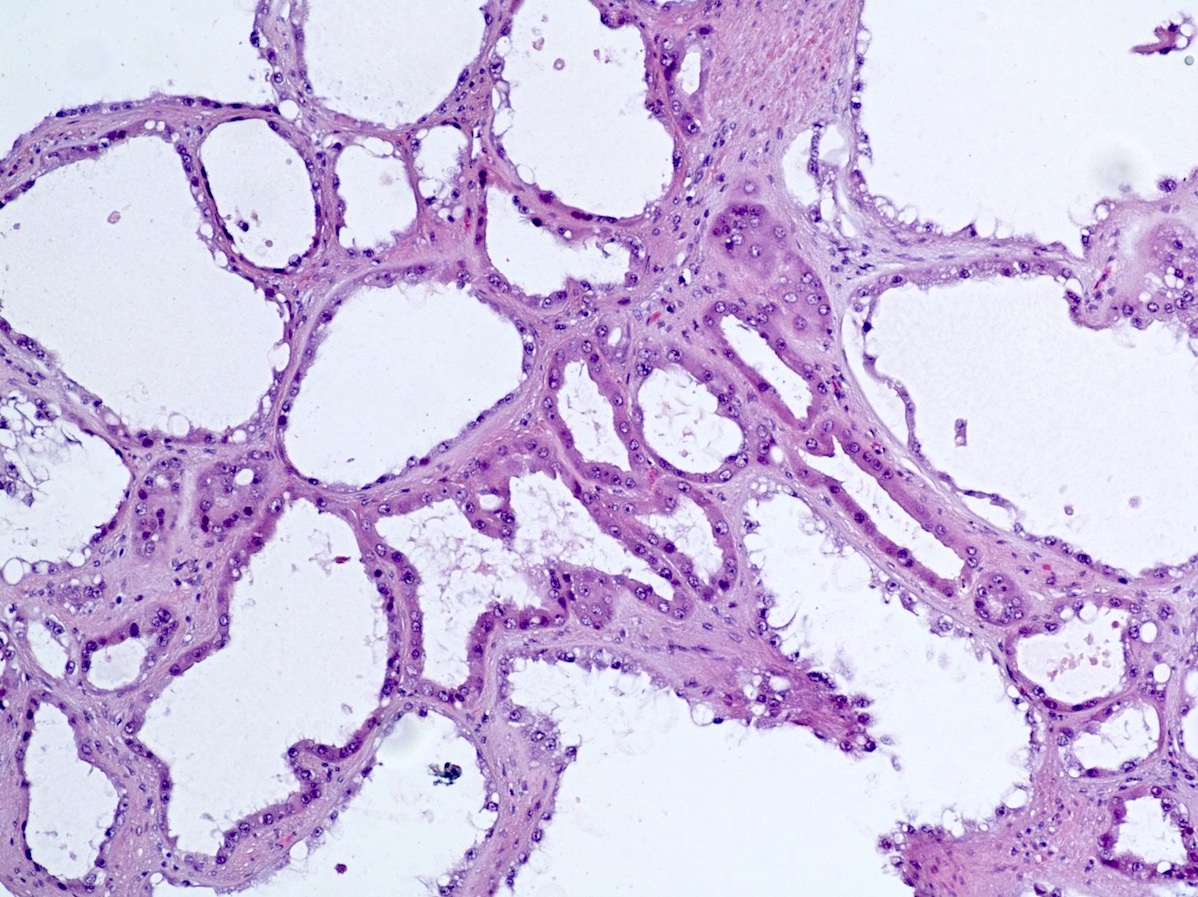
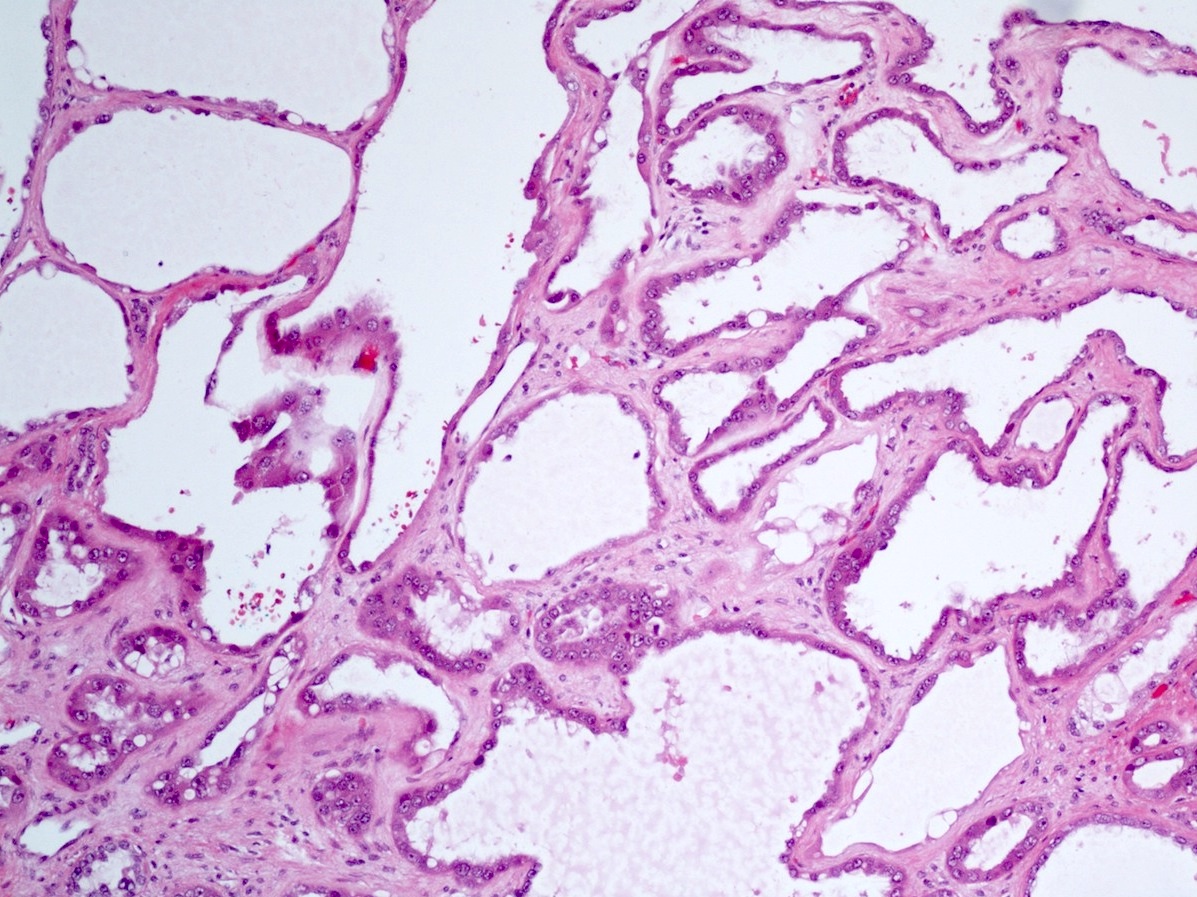
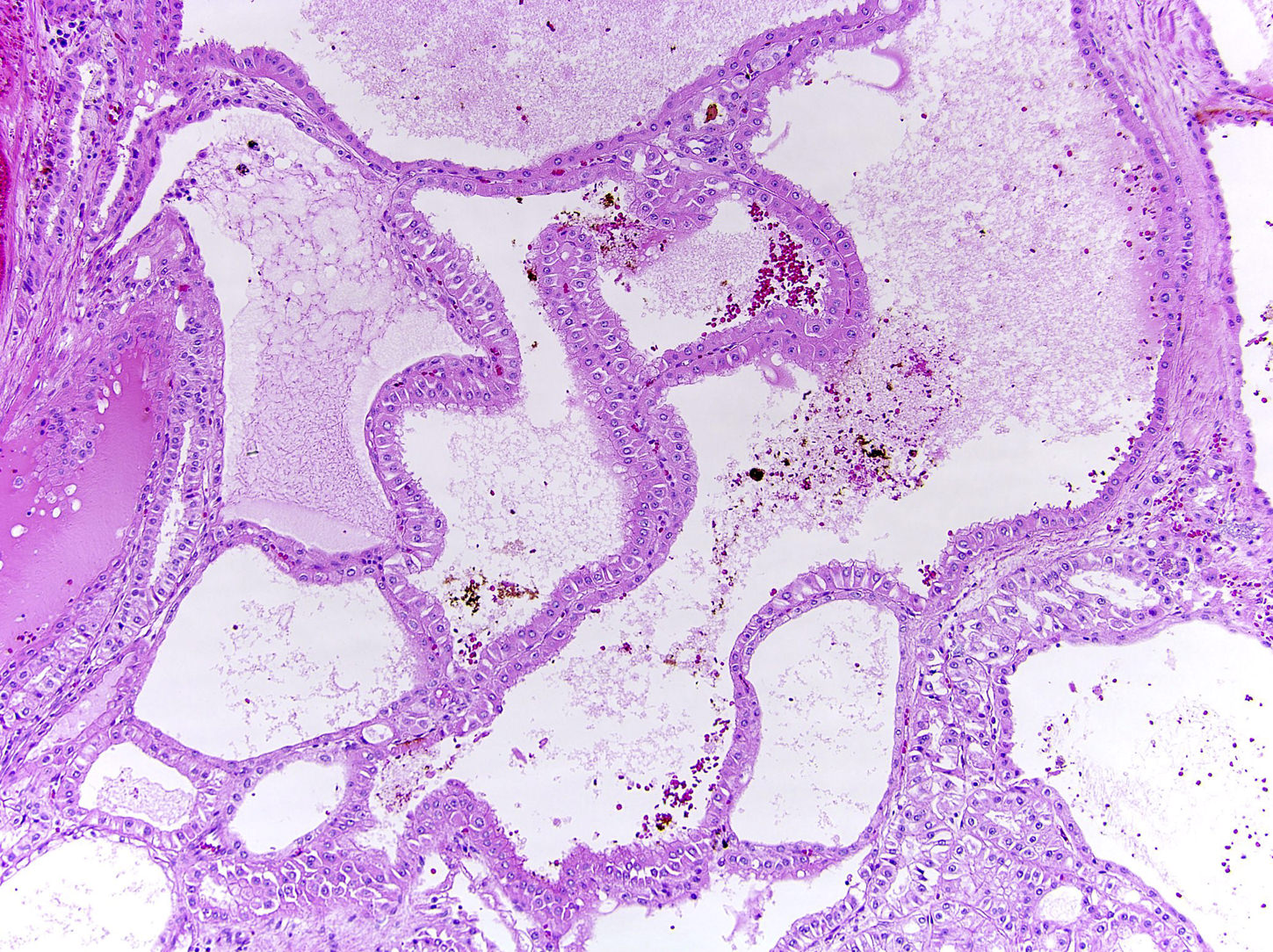
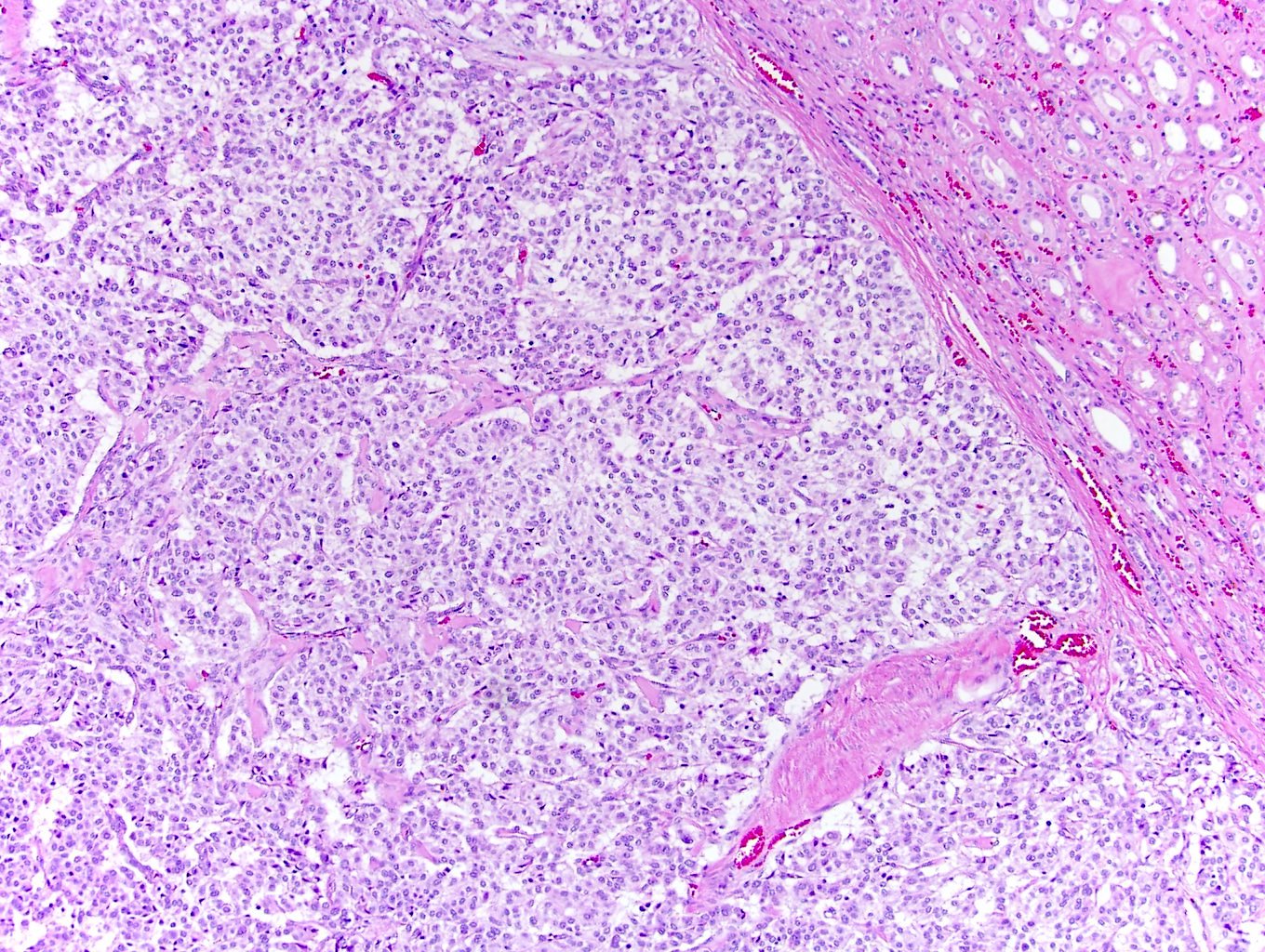
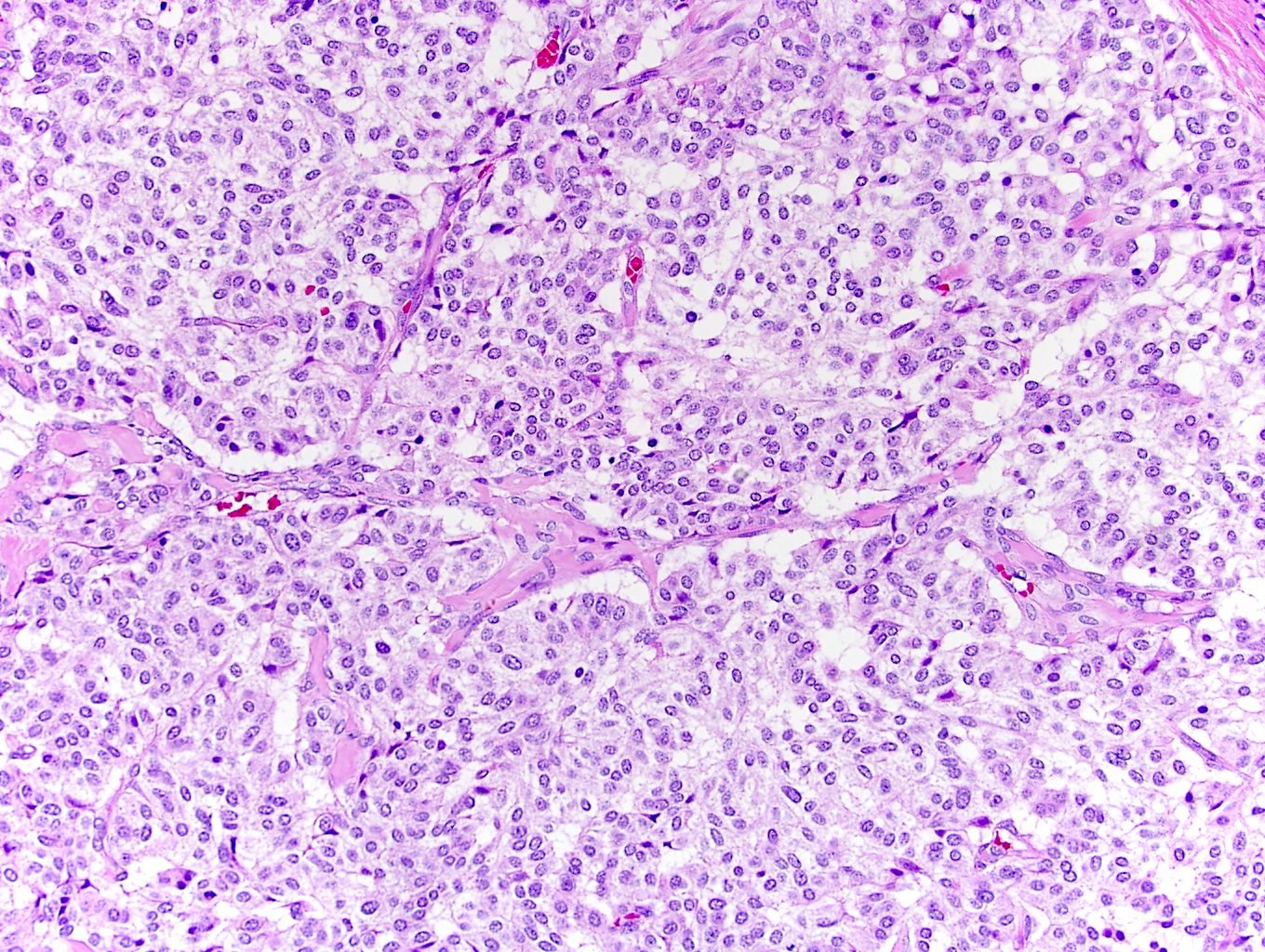
- Comment: The sections show an infiltrating epithelioid neoplasm in the kidney, arranged as lobules, small nests and ducts, some with blue mucinous material in the lumina. Rare, dispersed apparent mucin containing cells are present. The neoplastic cells have an eosinophilic cytoplasm and slightly pleomorphic, variably enlarged and hyperchromatic nuclei, which have a generally low grade cytology. Many nuclei have intranuclear vacuoles. Cells with rhabdoid and signet ring features are noted but there are virtually no mitoses. Our differential diagnosis of this infiltrating high grade adenocarcinoma is very broad, including collecting duct carcinoma, translocation carcinoma, urothelial carcinoma, metastatic carcinoma, unclassified renal cell carcinoma and renal medullary carcinoma. Immunohistochemical stains showed that the tumor cells are positive for keratin, PAX8, INI1 and negative for GATA3 and TTF1, which is characteristic of a primary renal cell carcinoma. Additionally performed immunostaining with ALK was diffusely positive. FISH study showed evidence of a translocation or rearrangement involving the ALK gene (100% split apart signals). These findings are consistent with ALK rearranged RCC, a rare subtype of renal cell carcinoma.
- Very broad, thus immunostaining with ALK antibody and molecular studies confirming ALK translocation are critical for diagnosis
- Renal medullary carcinoma:
- Collecting duct carcinoma:
- Also in medullary location but usually larger size, predominant tubular growth, stromal desmoplasia, high Ki67
- MiT family translocation renal cell carcinoma:
- Also TFE3 positive and can have similar morphology
- Can be positive for melanocytic markers and cathepsin K
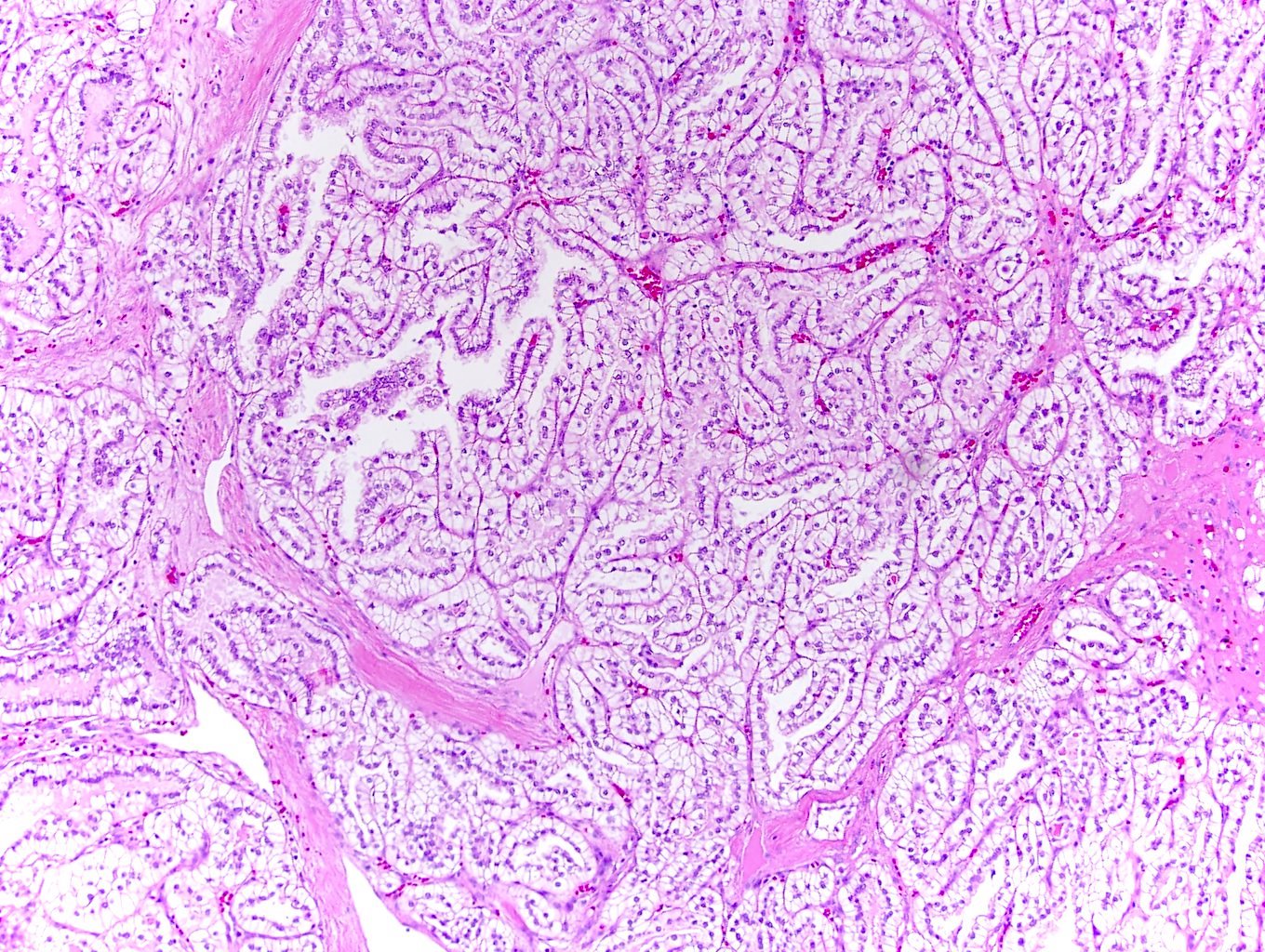
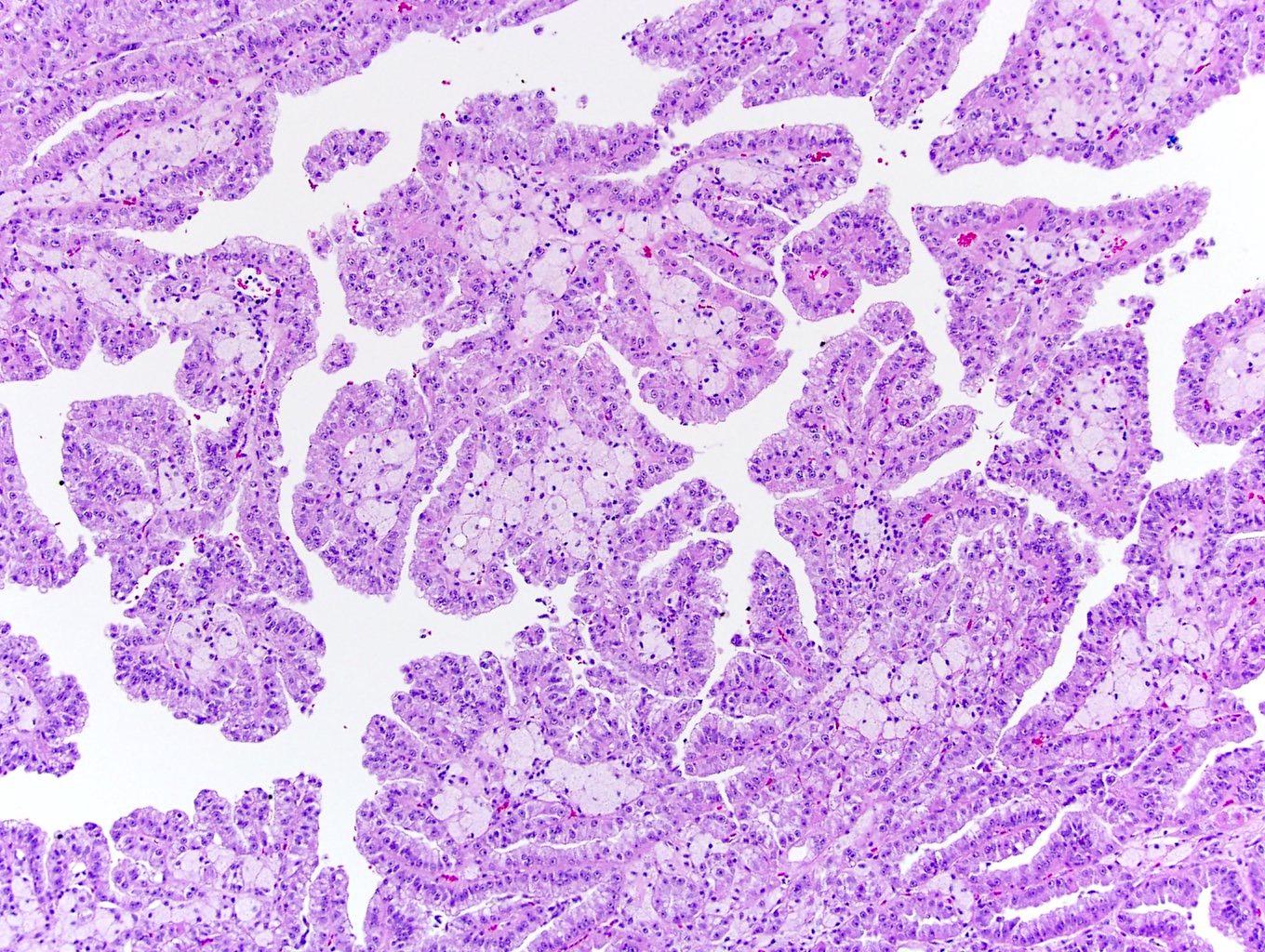
- Papillary renal cell carcinoma and mucinous tubular and spindle cell carcinoma:
- Also has tubulopapillary architecture, mucin production, psammoma bodies, foamy macrophages and overlapping immunoprofile
- Lack ALK immunohistochemical expression
- Unclassified renal cell carcinoma:
- ALK immunohistochemistry negative
- Metastases, especially ALK rearranged lung adenocarcinoma:
- Usually positive for TTF1 / napsin A and negative for PAX8
- Also check clinical history (Semin Diagn Pathol 2015;32:90, Pol J Pathol 2018;69:109)
- Can be easily distinguished from other renal carcinomas due to heterogeneous solid, tubular, cribriform and rhabdoid morphology
- Detection of ALK rearrangement is critical for diagnosis
- It affects only pediatric patients
- Typically has aggressive course
Comment Here
Reference: ALK translocation
Comment Here
Reference: ALK translocation
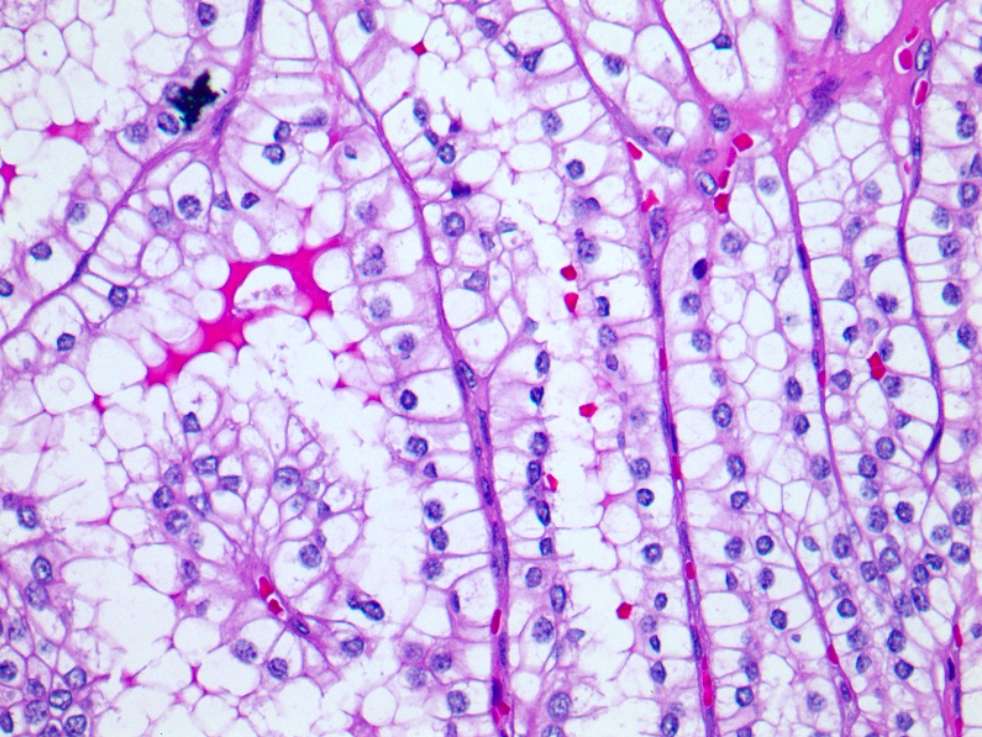
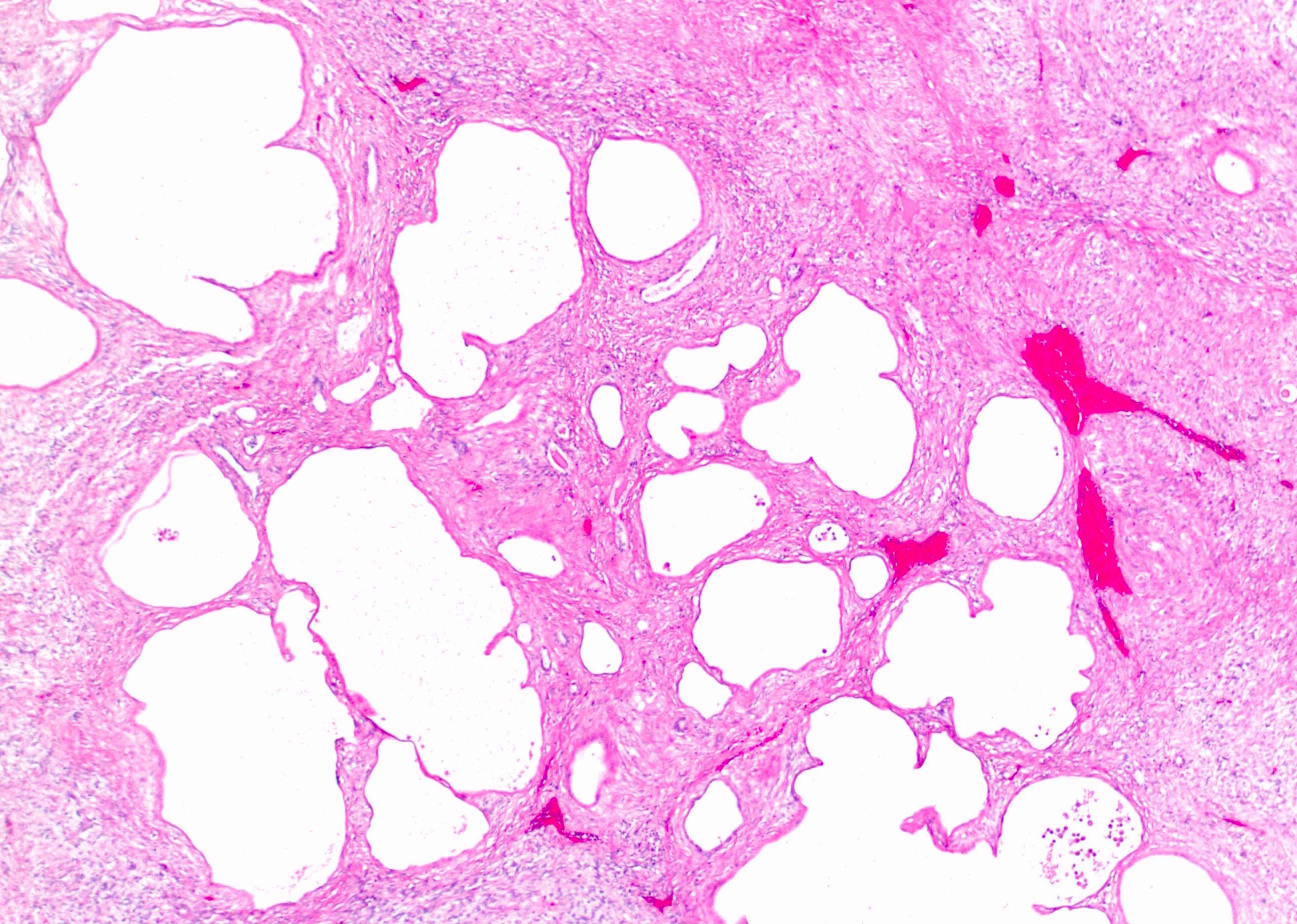
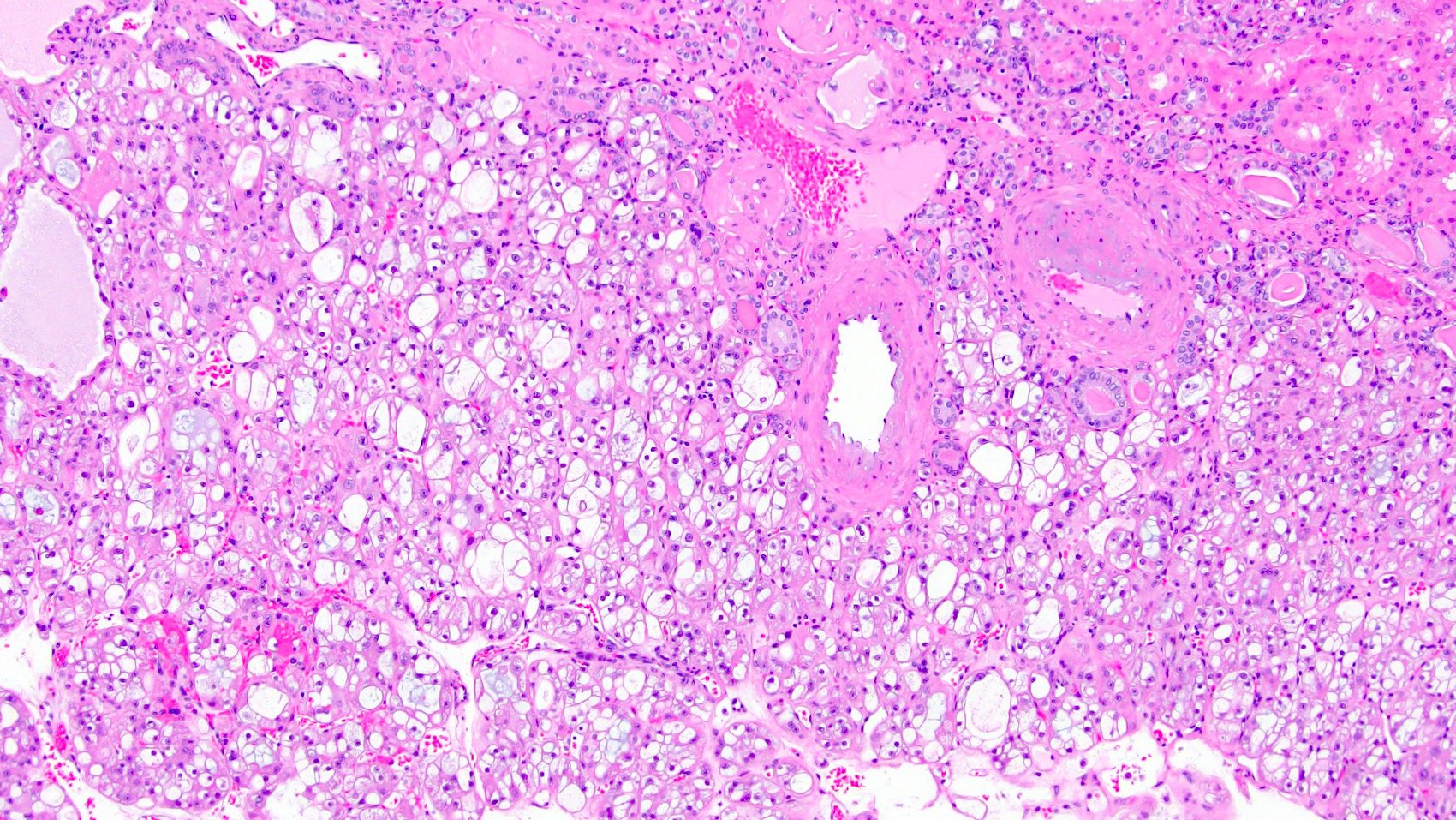
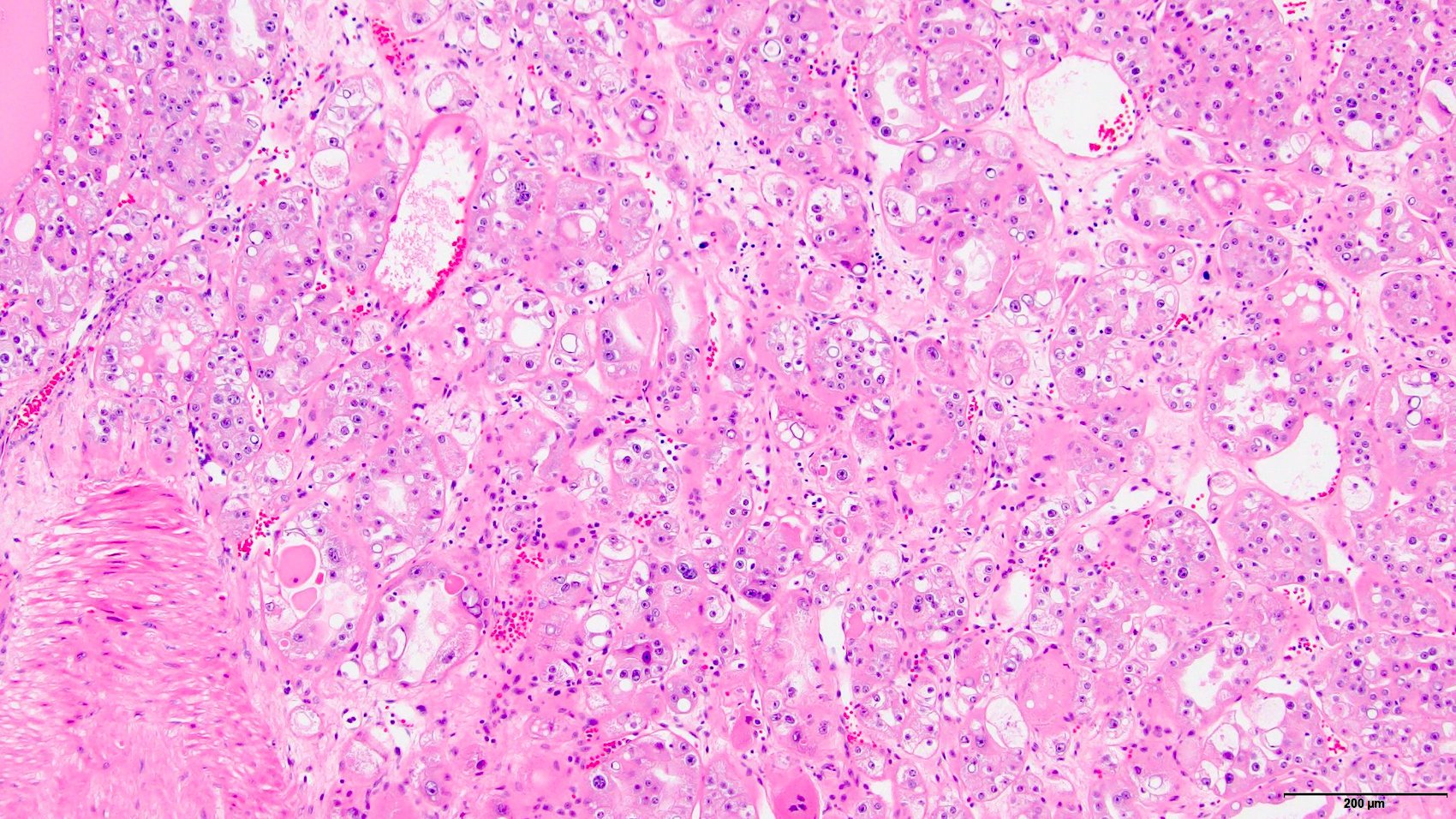
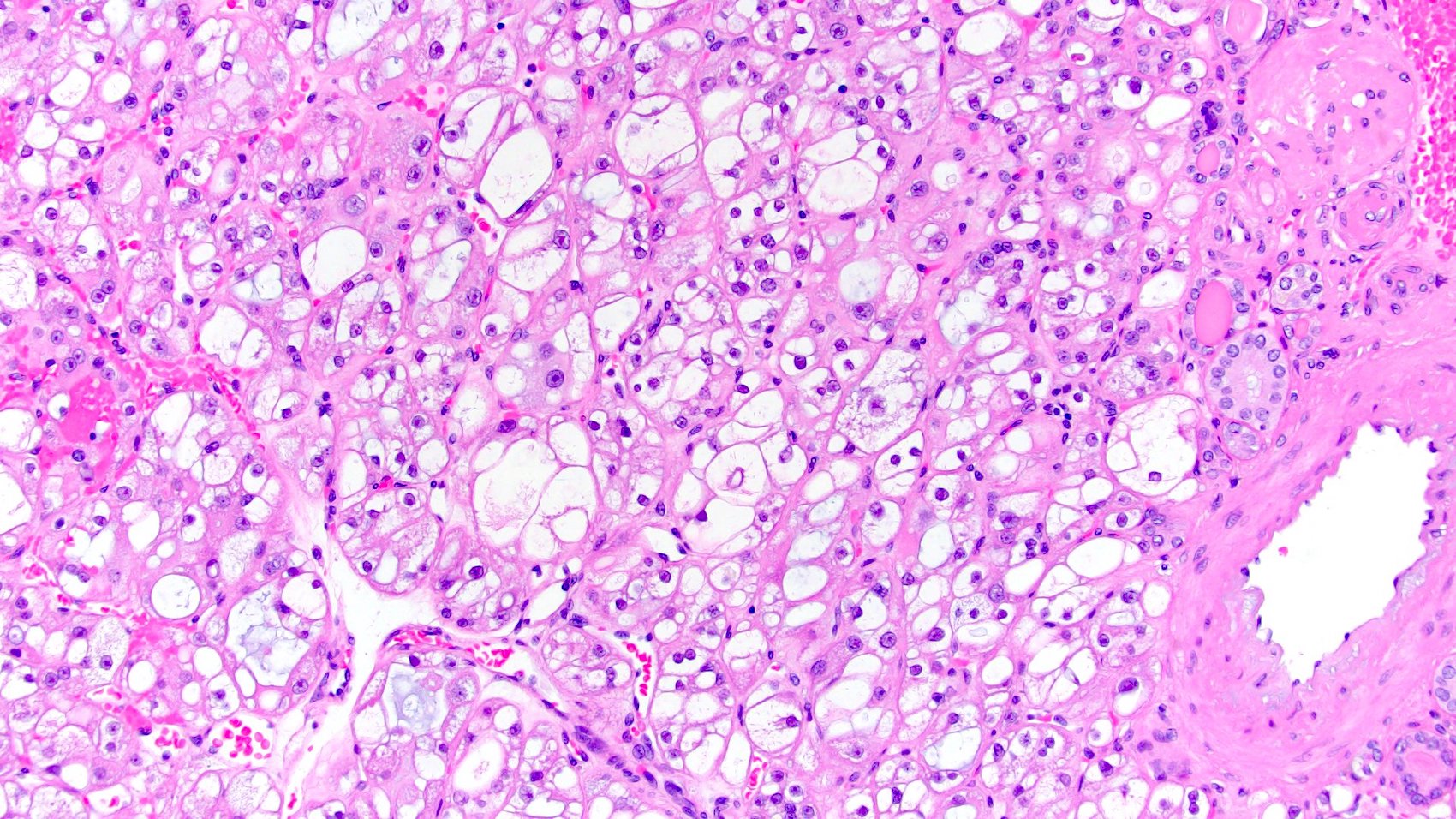
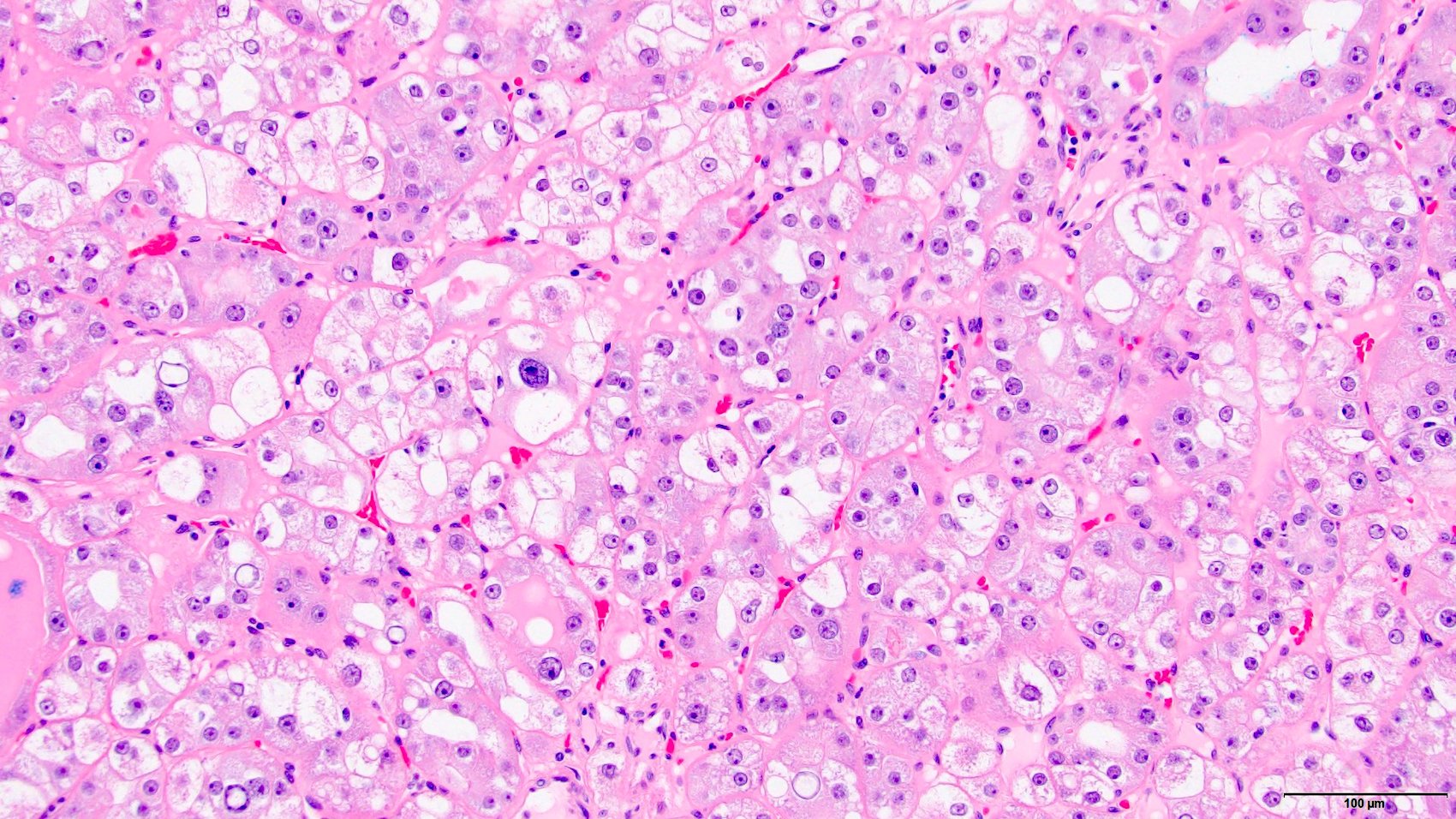
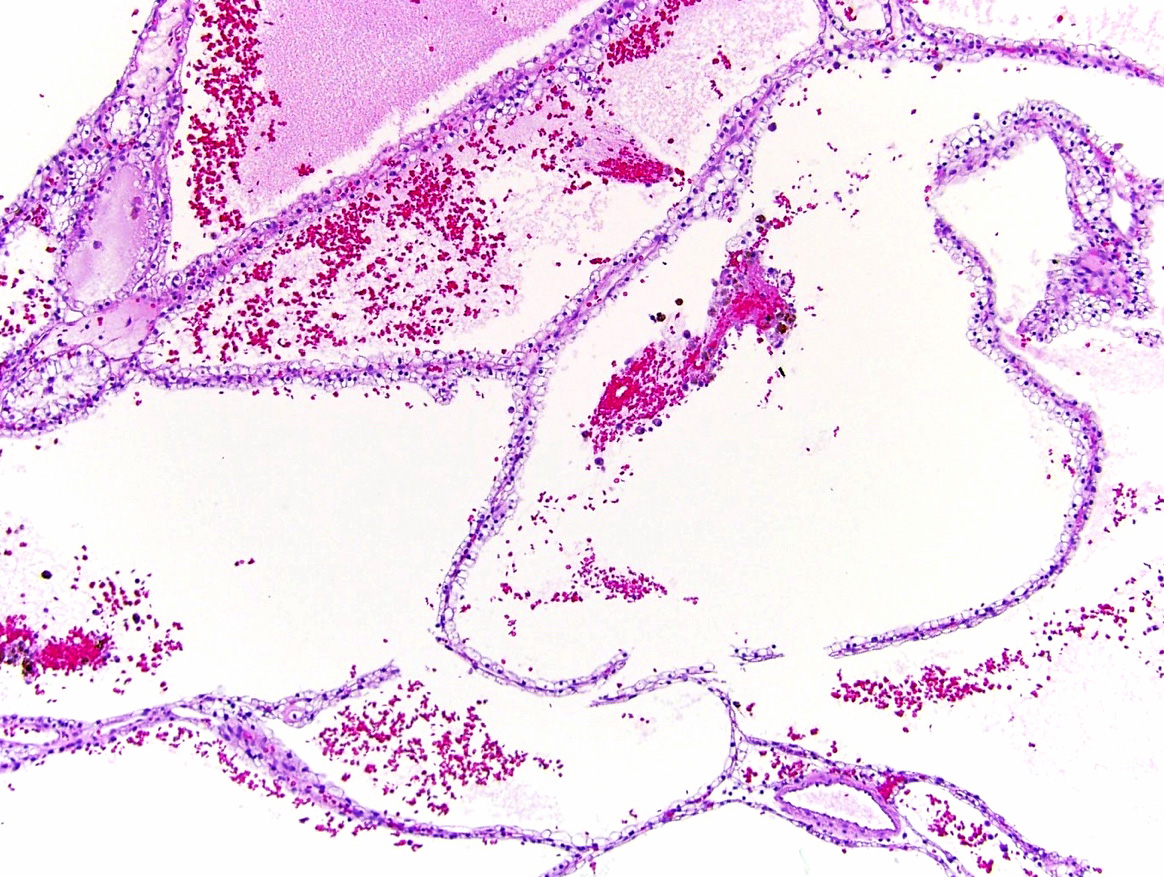
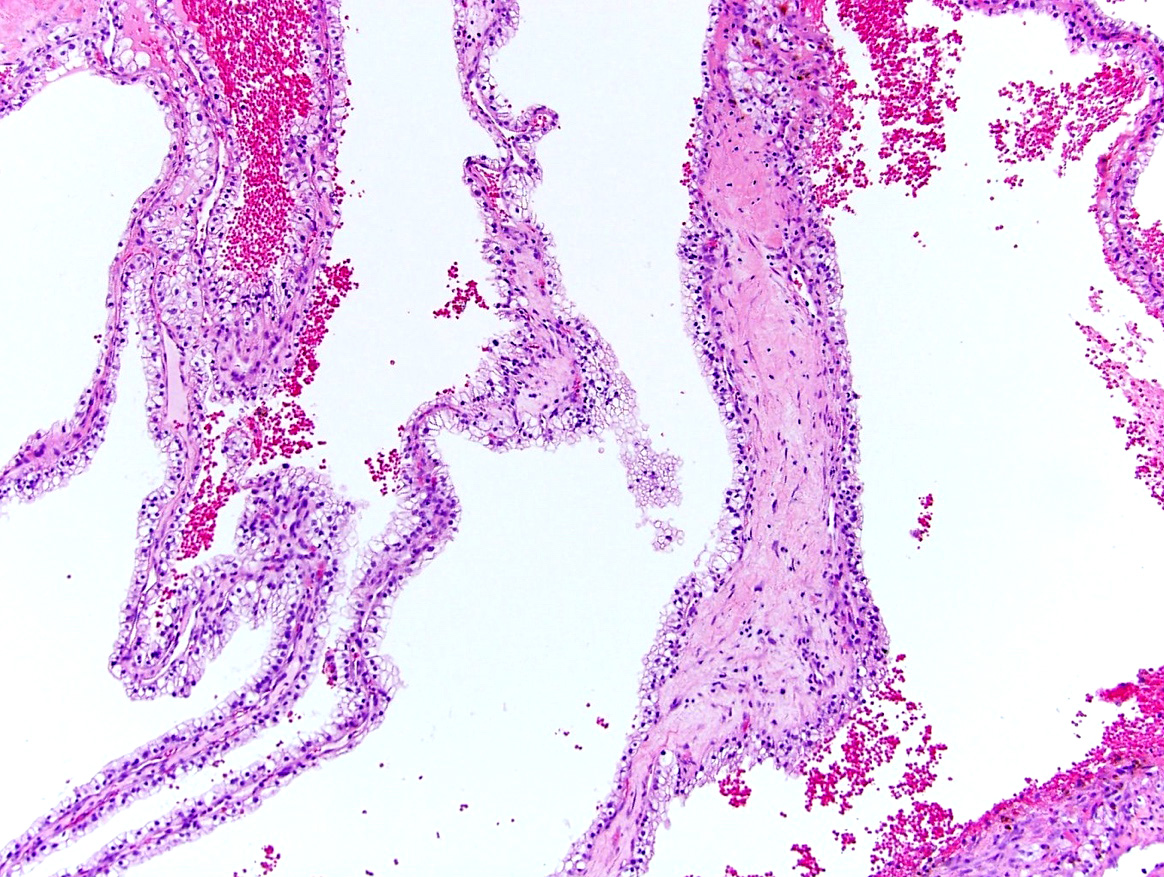
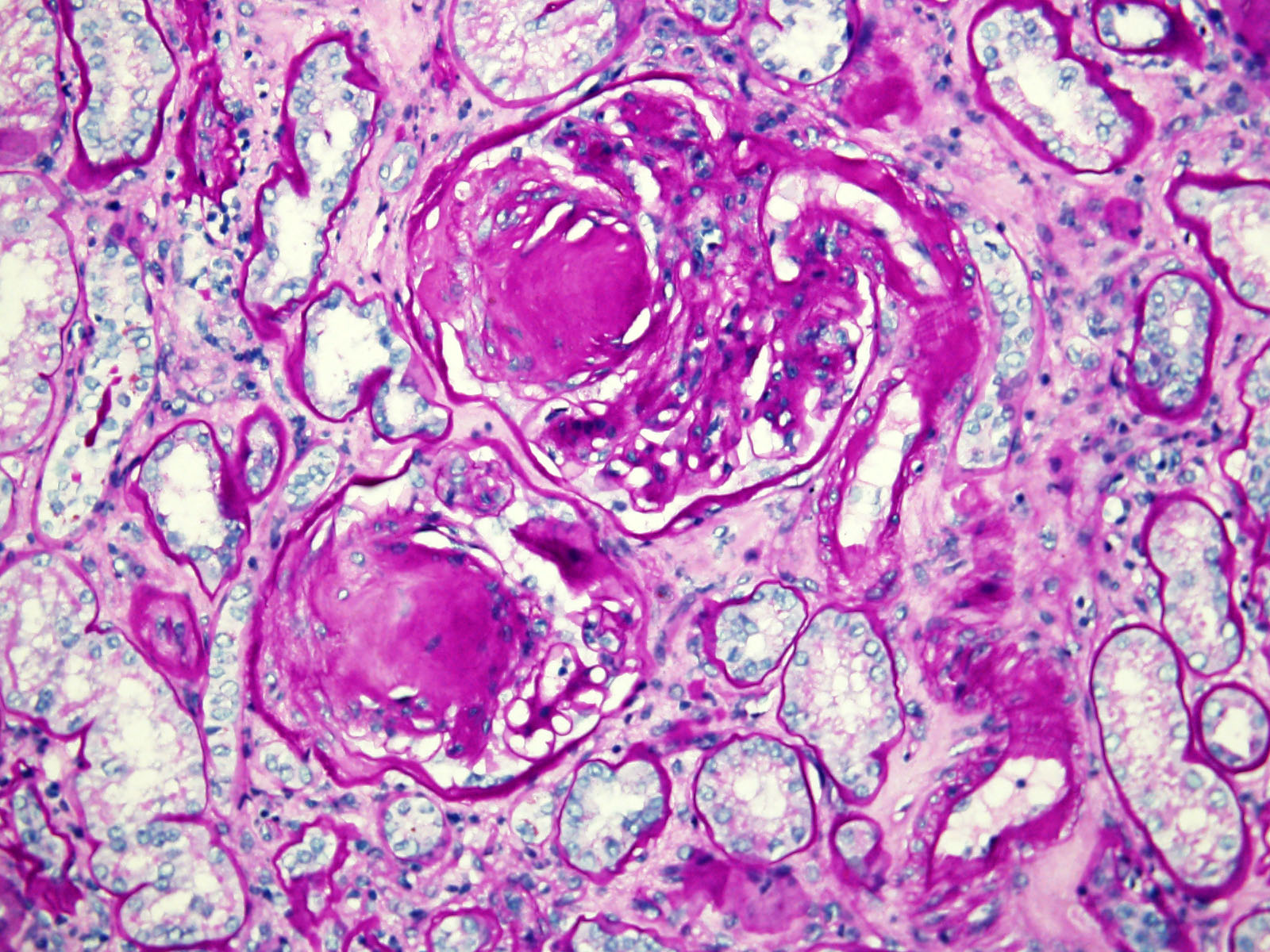
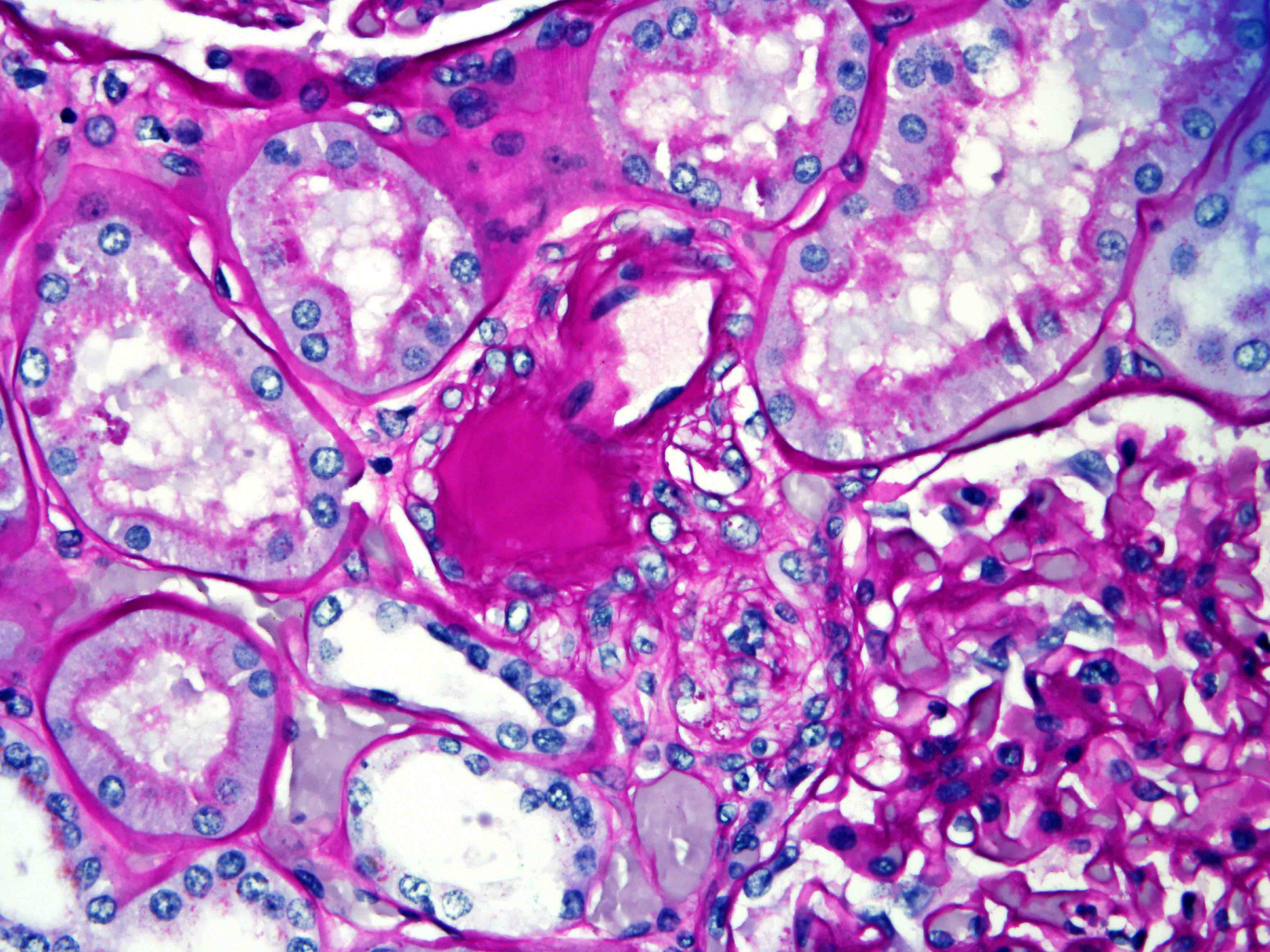
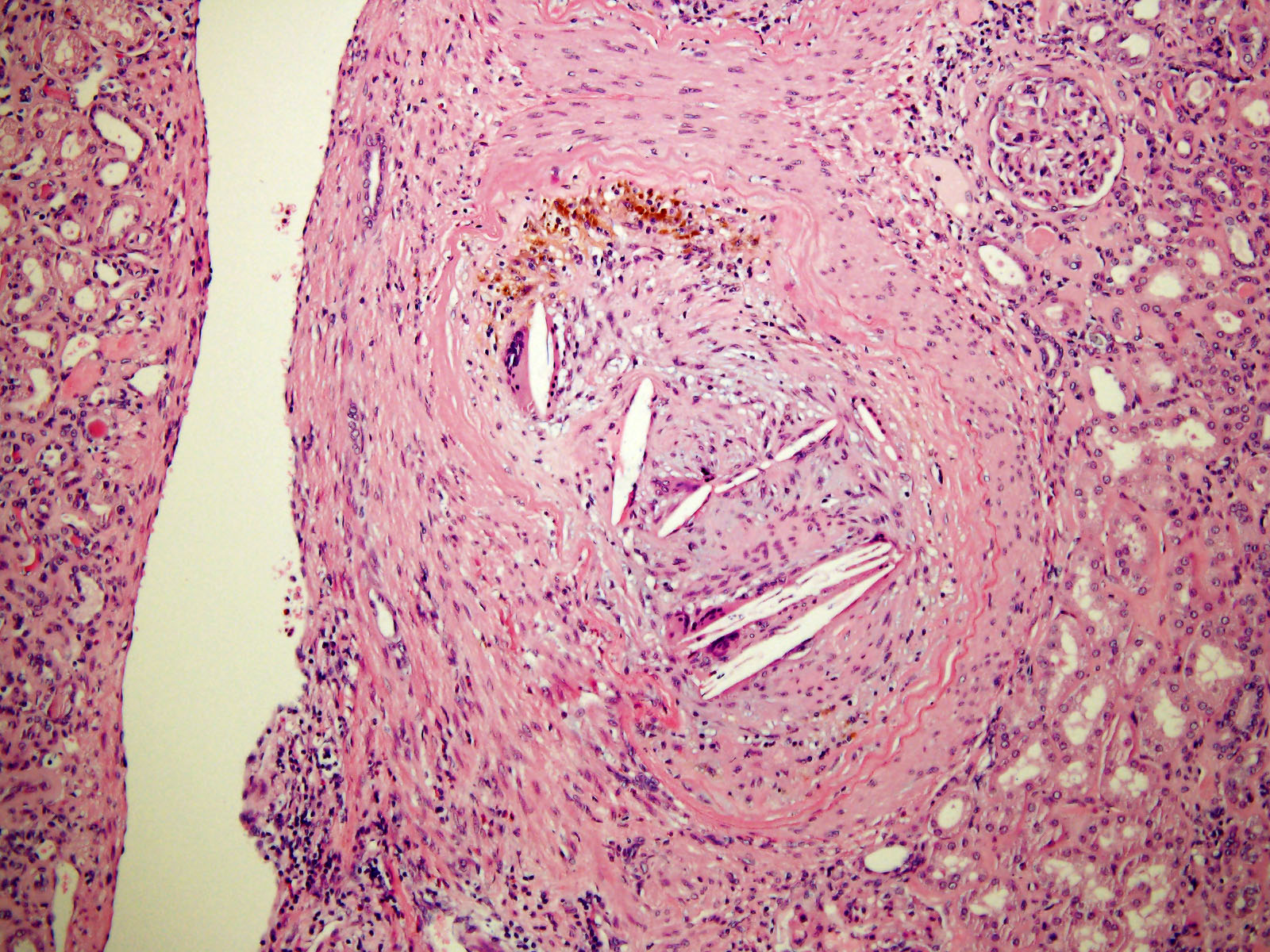
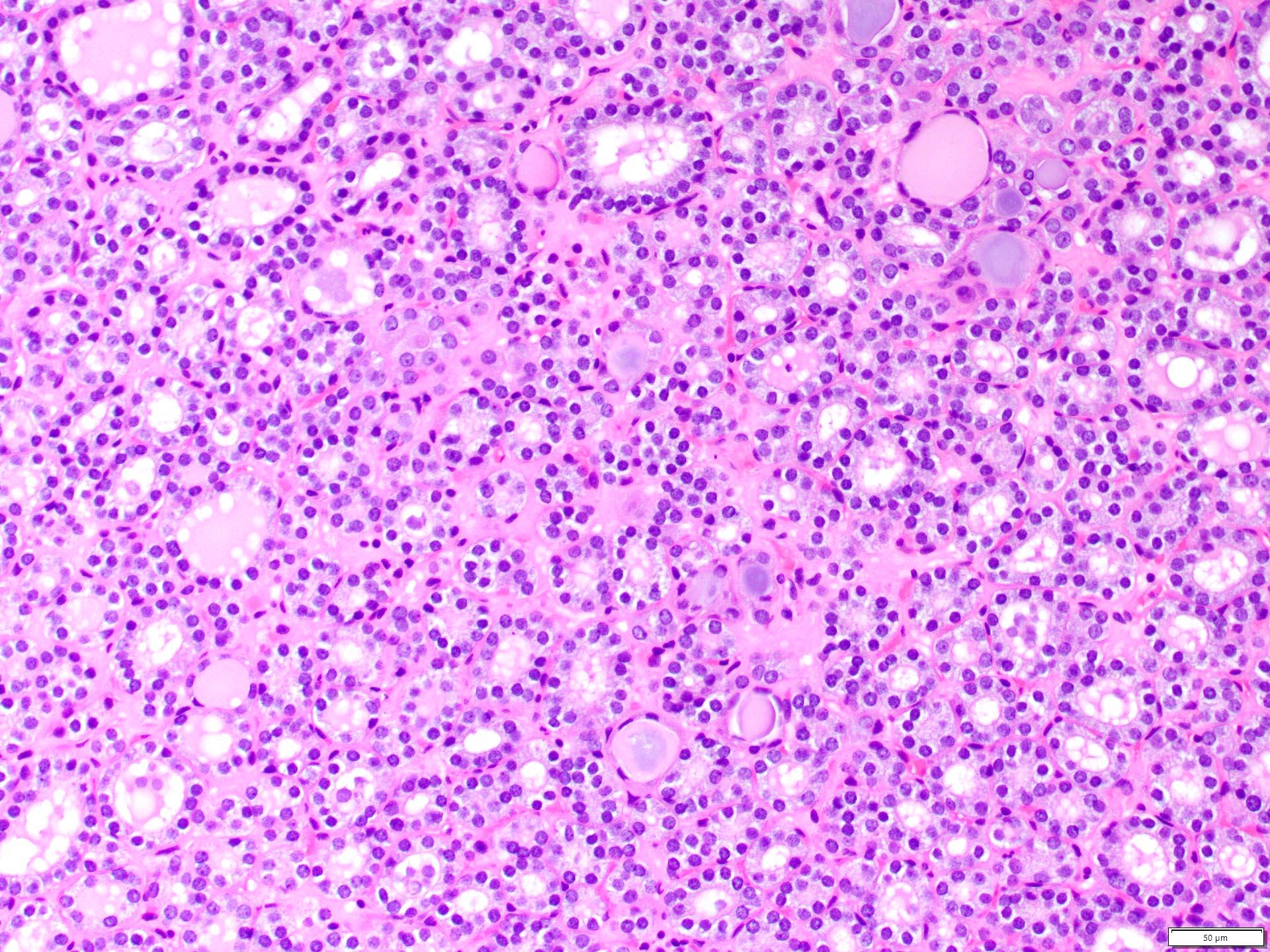
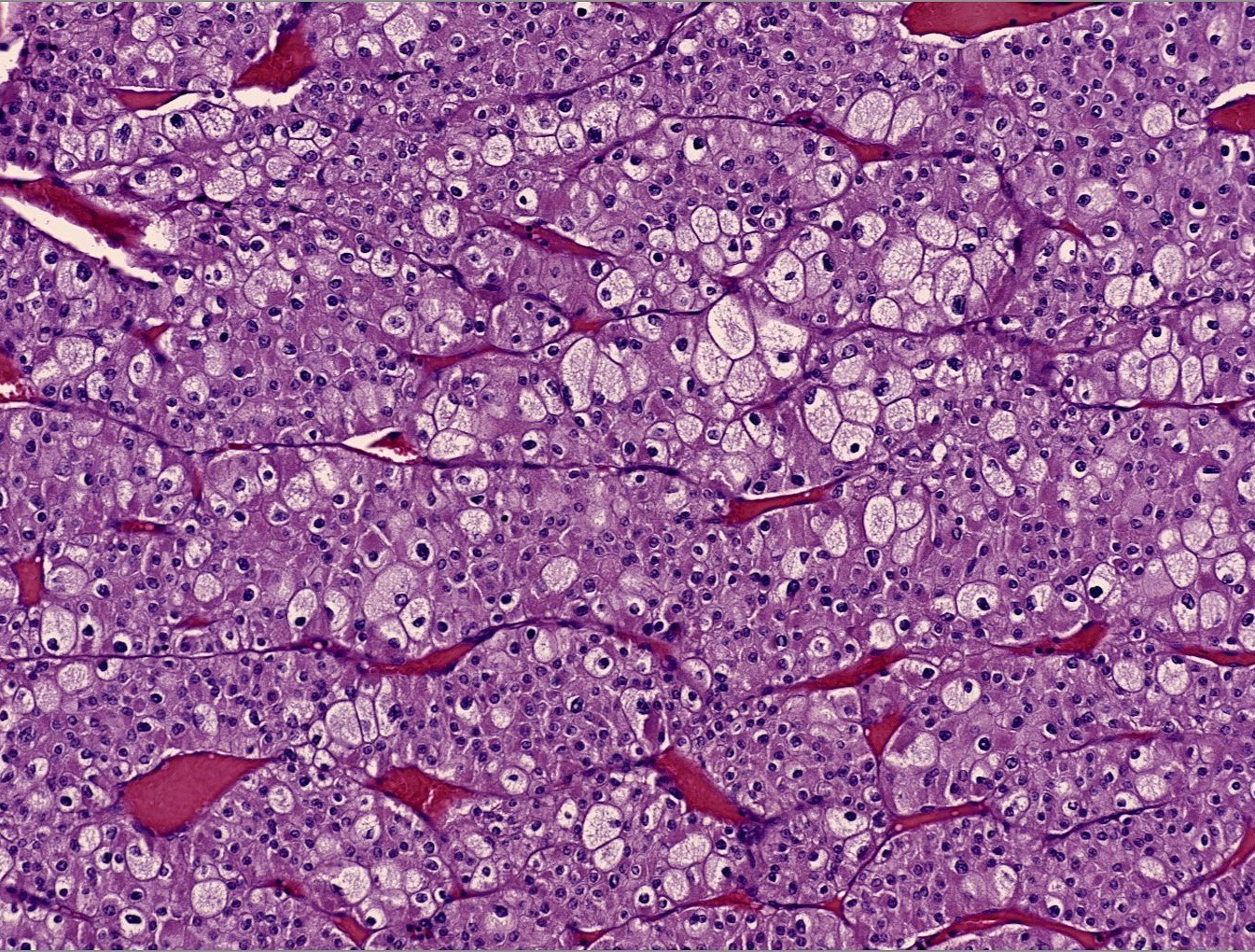
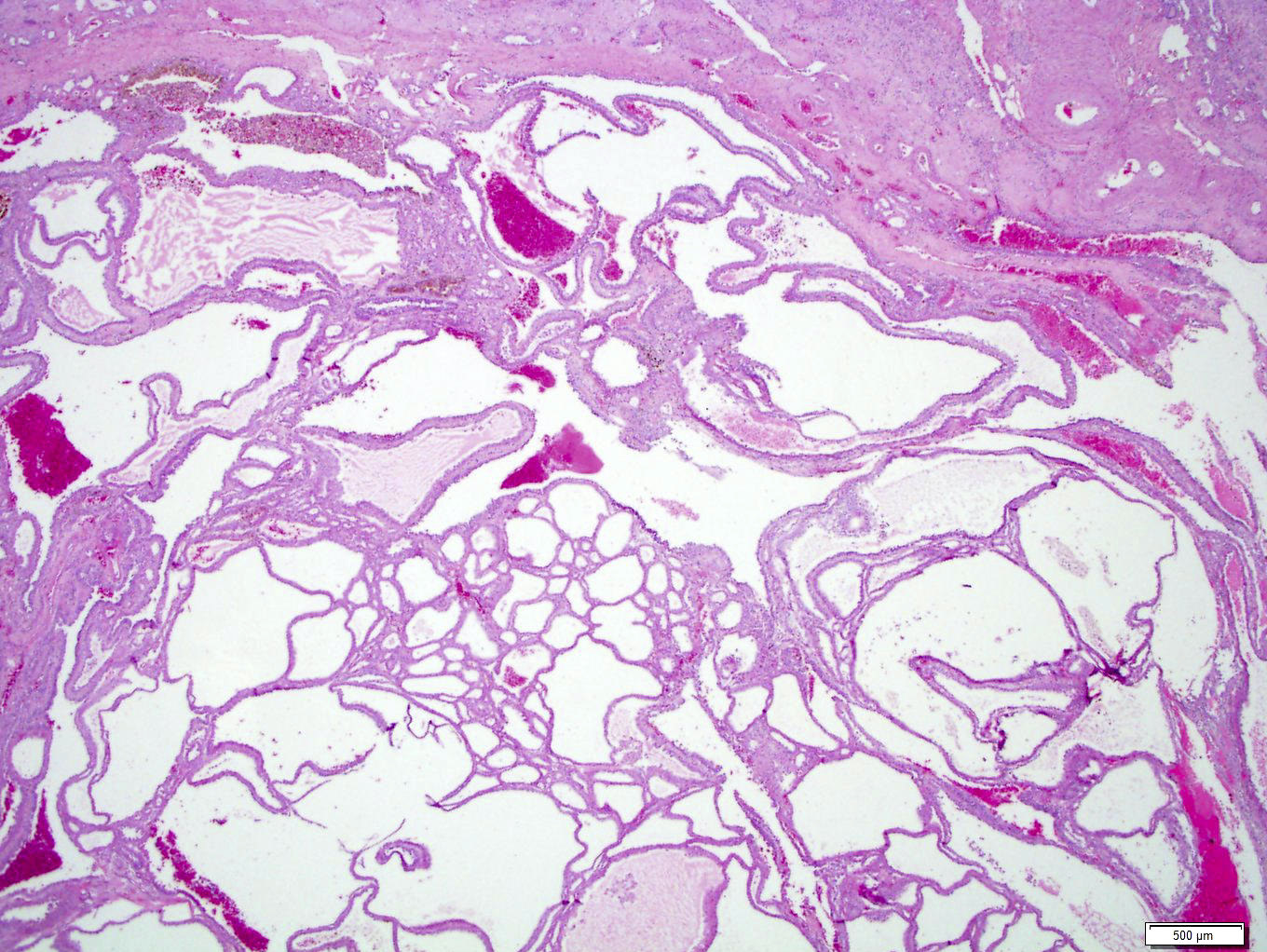
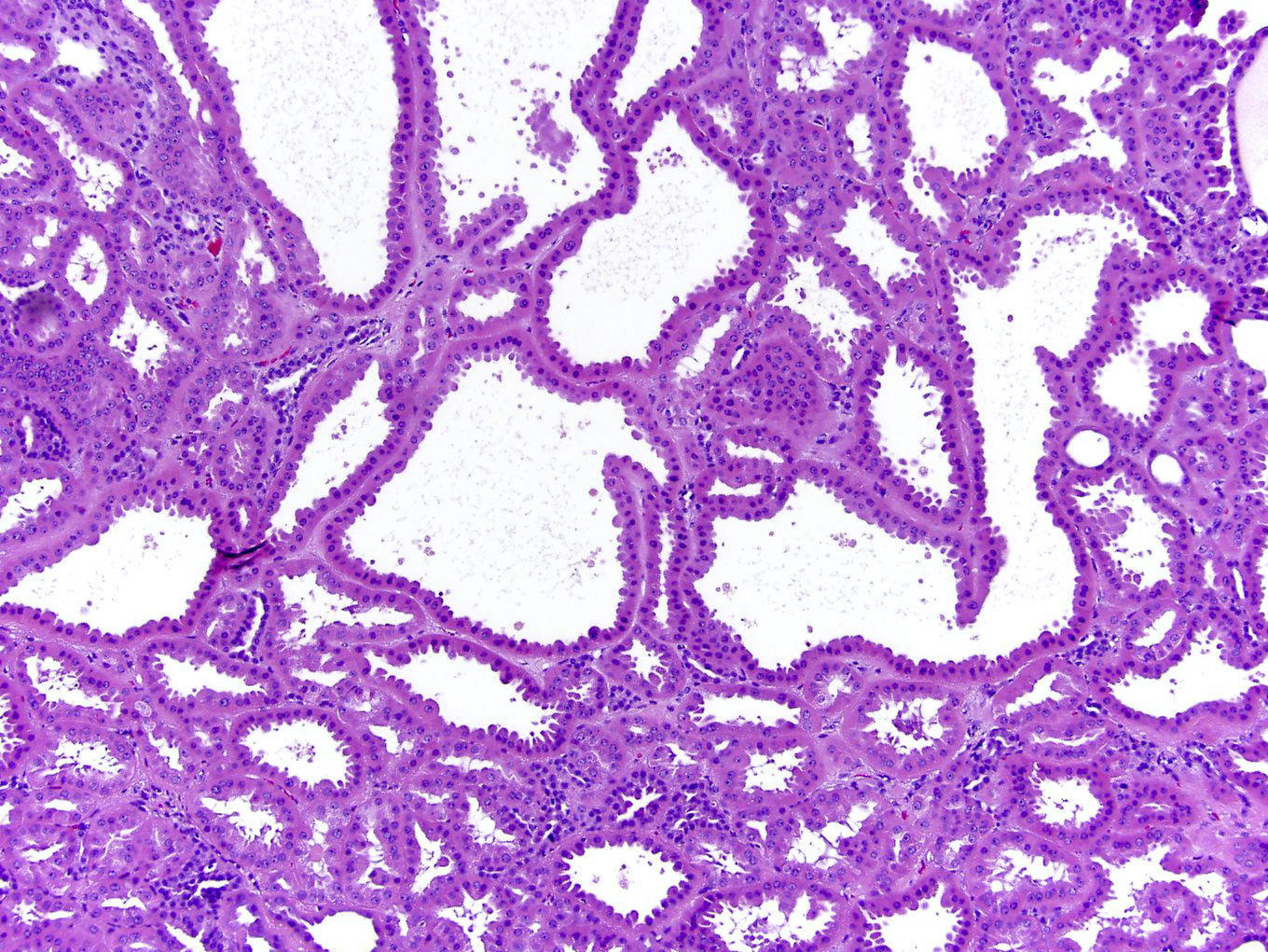
- Renal cell carcinoma (RCC) arising exclusively in patients with acquired cystic disease (ACD)
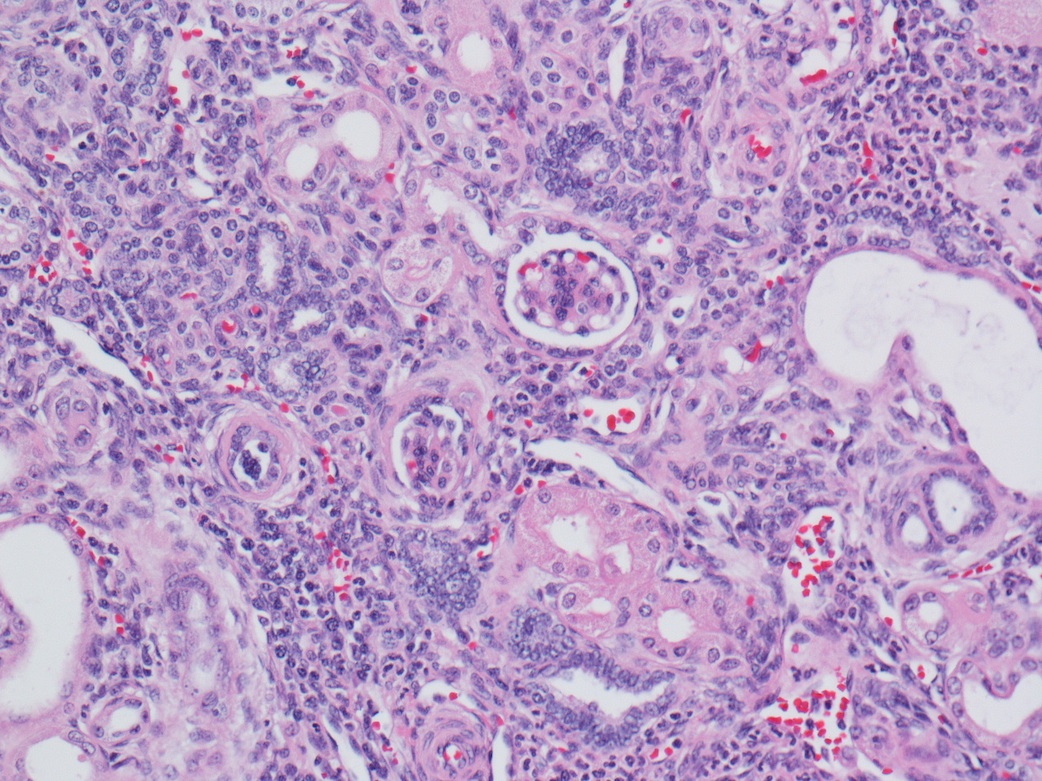
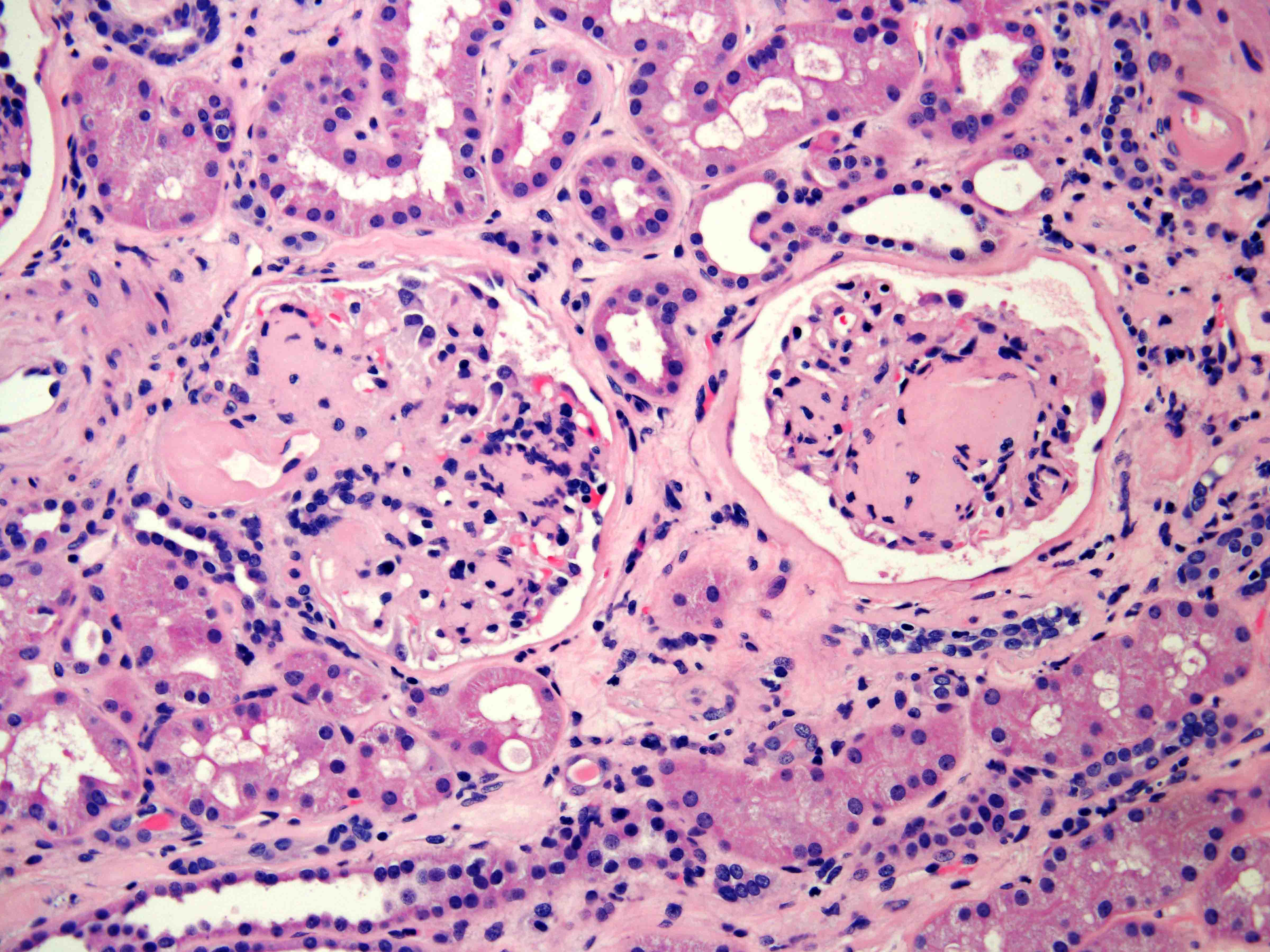
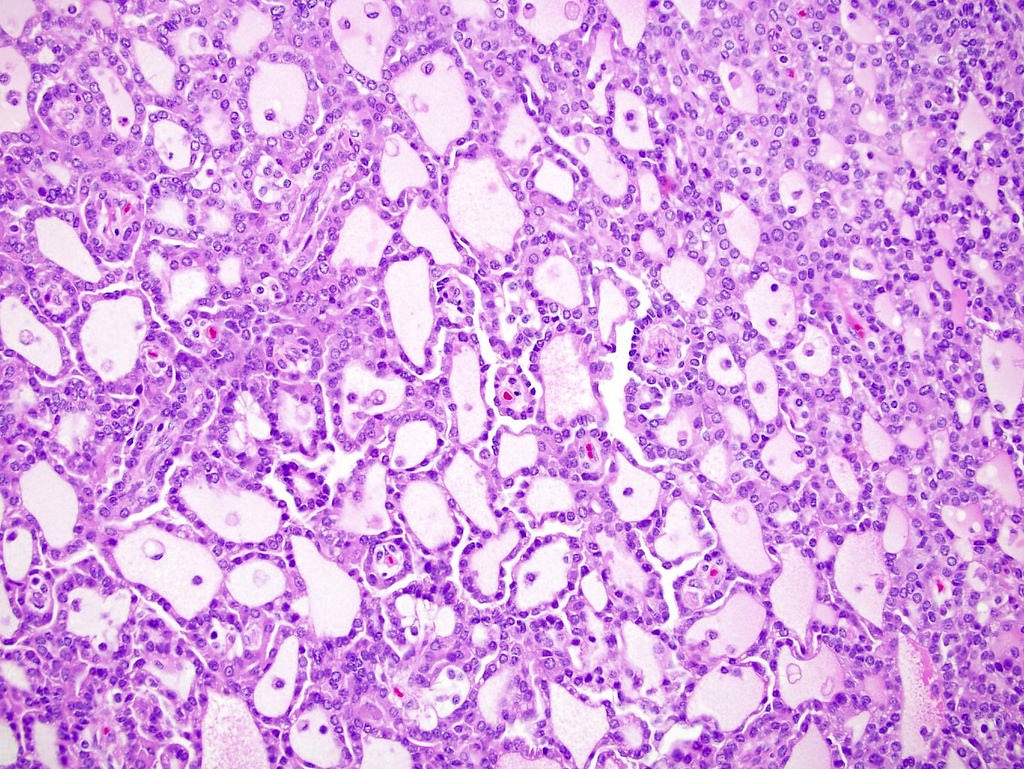
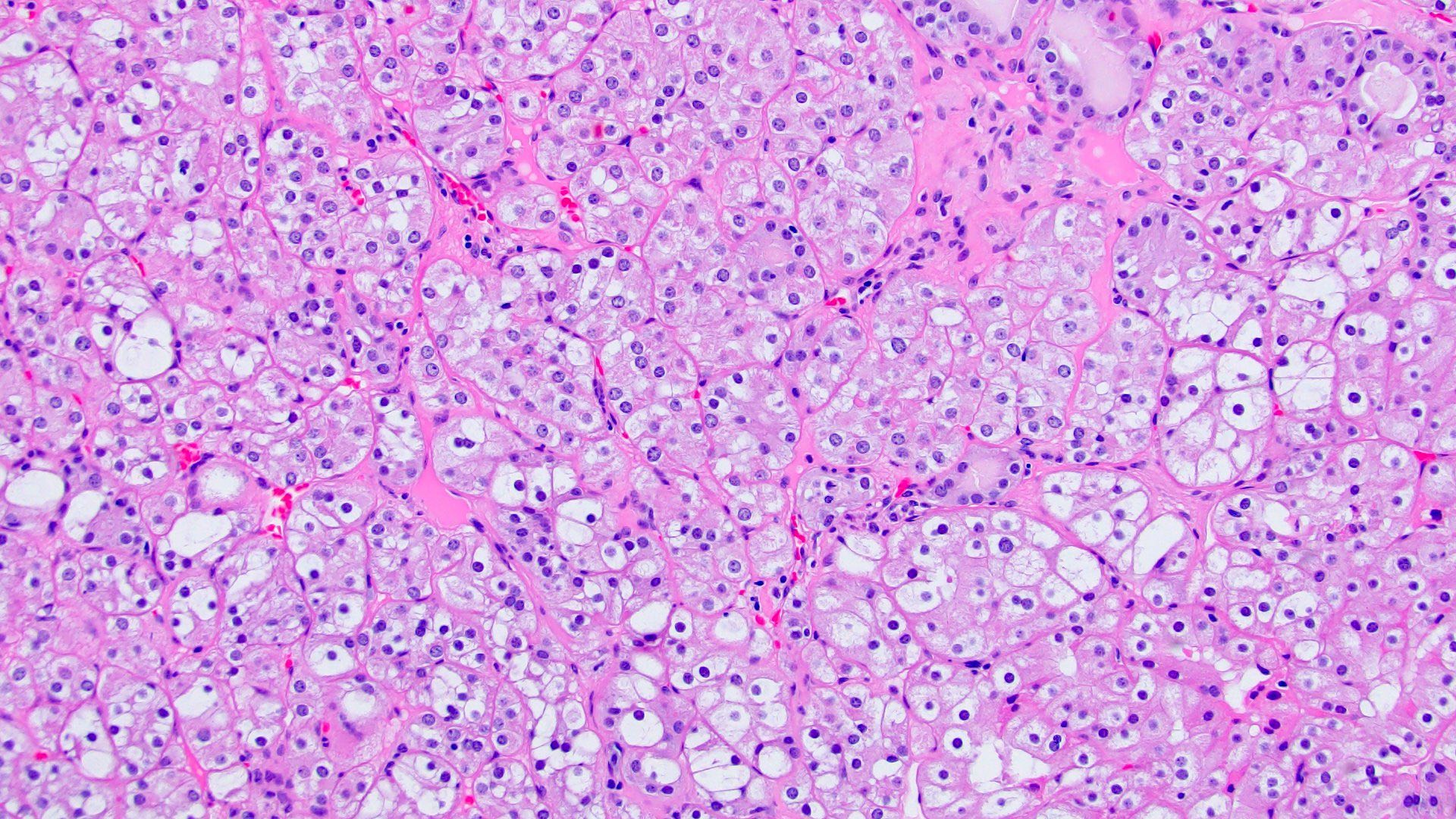
- Characterized by microcystic sieve-like, papillary and solid architecture, eosinophilic and clear cells and abundant calcium oxalate crystals (Am J Surg Pathol 2006;30:141)
- Most common RCC in patients with ACD (Eur Urol 2016;70:93)
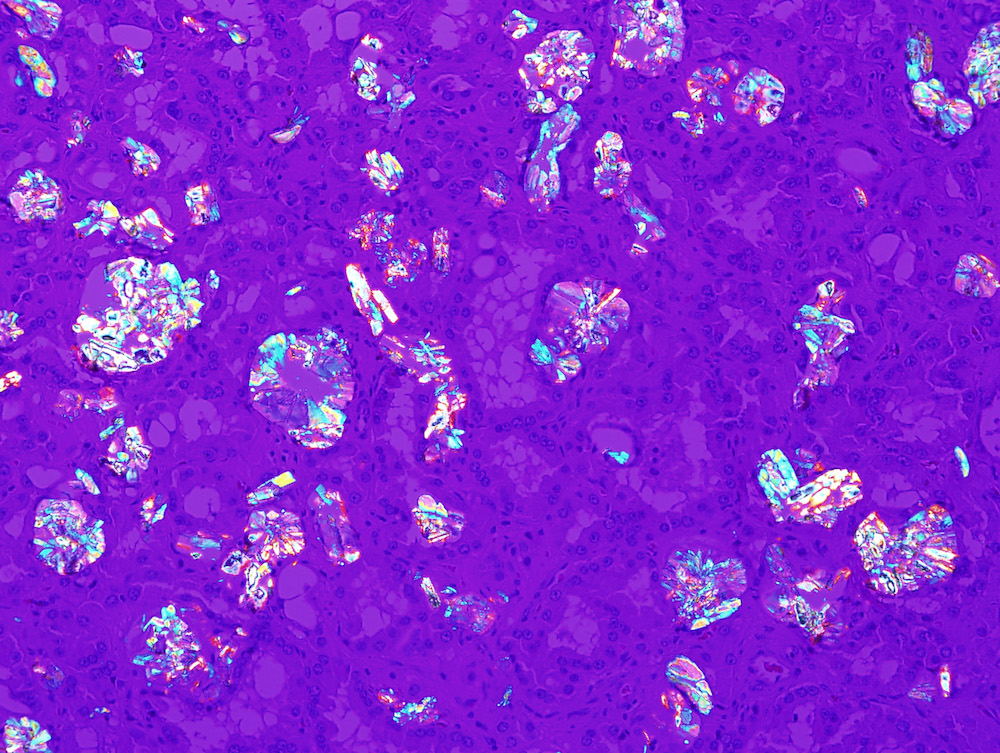
- Abundant intratumoral oxalate crystals
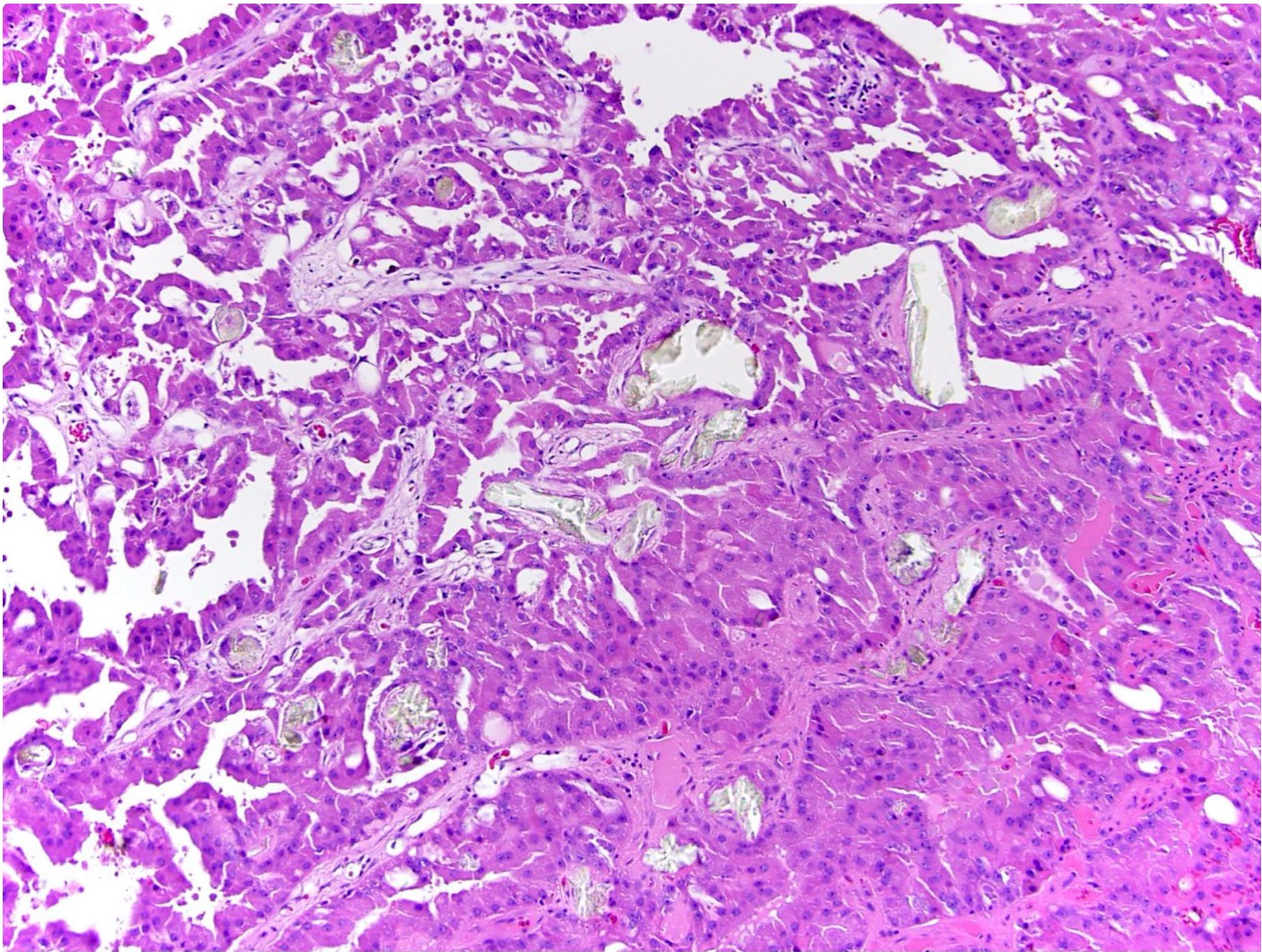
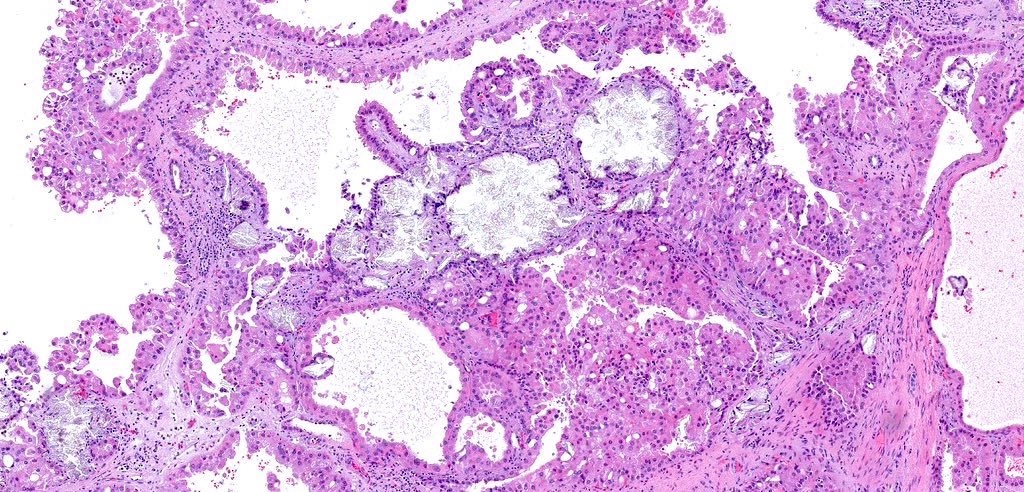
- Heterogenous morphology with mixed sieve-like, solid, microcystic, papillary architecture and eosinophilic to clear cells
- Acquired cystic disease associated renal cell carcinoma (ACD-RCC)
- ICD-O: 8316/3 - acquired cystic disease associated renal cell carcinoma
- ICD-11: 2C90.0 & XH0RU3 - renal cell carcinoma of kidney, except renal pelvis & acquired cystic disease associated renal cell carcinoma
- ACD typically occurs after dialysis for end stage renal disease (ESRD)
- Incidence of ACD progressively increases with duration of dialysis: from 10 - 20% after 3 years to > 90% after 10 years of hemodialysis or peritoneal dialysis
- Overall incidence of ACD-RCC is 3 - 7% in end stage kidney, which is 100 times greater than in the general population (Kidney Int 2002;61:2201)
- ACD-RCC is the most common RCC subtype in both ACD (40%) and ESRD (30%)
- Other tumors that occur in end stage kidneys include clear cell papillary renal cell tumors and clear cell, papillary and chromophobe RCCs
- Kidney
- Causes of increased tumorigenesis in end stage kidney and ACD are multifactorial, including
- Depressed humoral and cellular immunity in renal failure
- Increased production of reactive oxygen species and impaired antioxidant defense (Virchows Arch 2013;463:553)
- Use of immunosuppressive medications
- Oxalate crystals induced tubular proliferative activity (Arch Pathol Lab Med 2017;141:600)
- See Pathophysiology
- ACD-RCC has overall indolent clinical behavior, likely because of early detection with periodic imaging of ESRD patients (Eur Urol 2016;70:93)
- 10 - 15% of tumors present with recurrence and metastases, commonly associated with sarcomatoid and rhabdoid phenotypes (Am J Surg Pathol 2006;30:141)
- Majority are asymptomatic tumors detected incidentally on routine imaging (Eur Urol 2011;60:e35)
- A minority of patients present with microscopic hematuria or flank pain
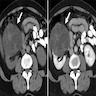
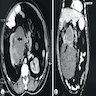
- On computed tomography (CT) scan: well defined lesions of variable size, rounded and often exophytic
- Often isodense on nonenhanced exam; can be solid, cystic or mixed in attenuation on enhanced exam (Front Oncol 2023;13:1187495)
- May have numerous calcifications
- 41 year old man and 46 year old woman with clear cell and papillary RCCs with abundant calcium oxalate crystals arising in acquired cystic disease of the kidney (Arch Pathol Lab Med 2003;127:E89)
- 56 year old man with sarcomatoid and rhabdoid change (Ann Diagn Pathol 2011;15:462)
- 58 year old man with PTCH1 mutation (Front Oncol 2024;14:1349610)
- 78 year old woman with sarcomatoid change (Histol Histopathol 2008;23:1327)
- Standard treatment for ACD-RCC is nephrectomy, either partial or total
- Chemotherapeutic agents have not been evaluated specifically for ACD-RCC
- Imaging for masses or large cysts is recommended for patients with ESRD and frequent screening is recommended, especially after ≥ 10 years on dialysis (Nephrol Dial Transplant 2011;26:1677)
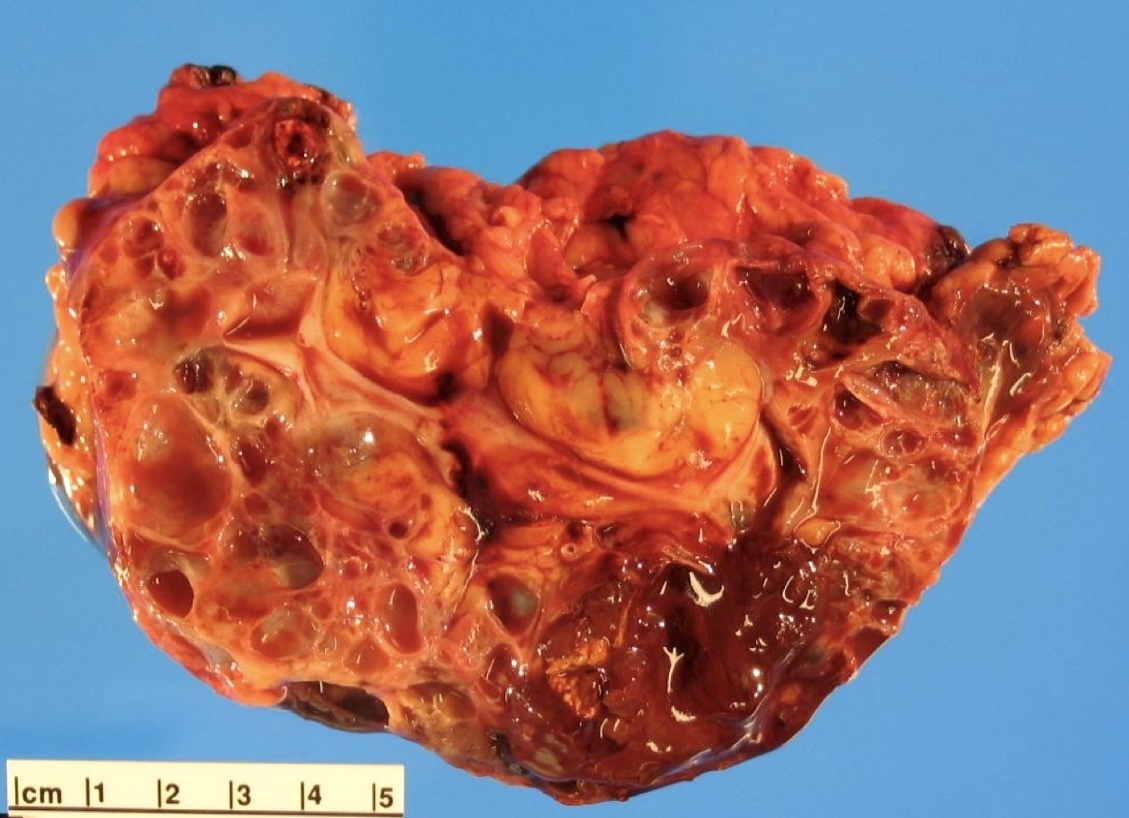
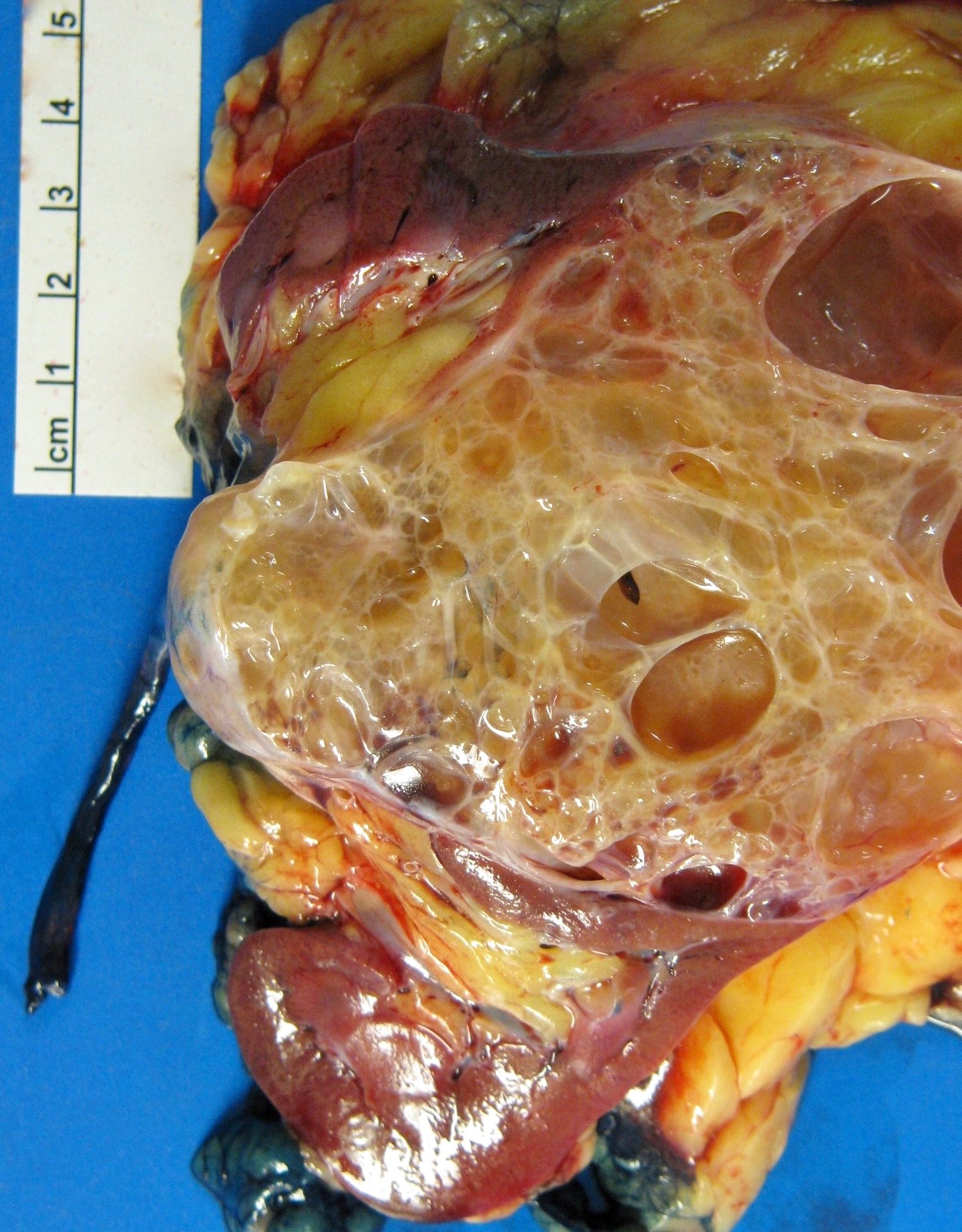
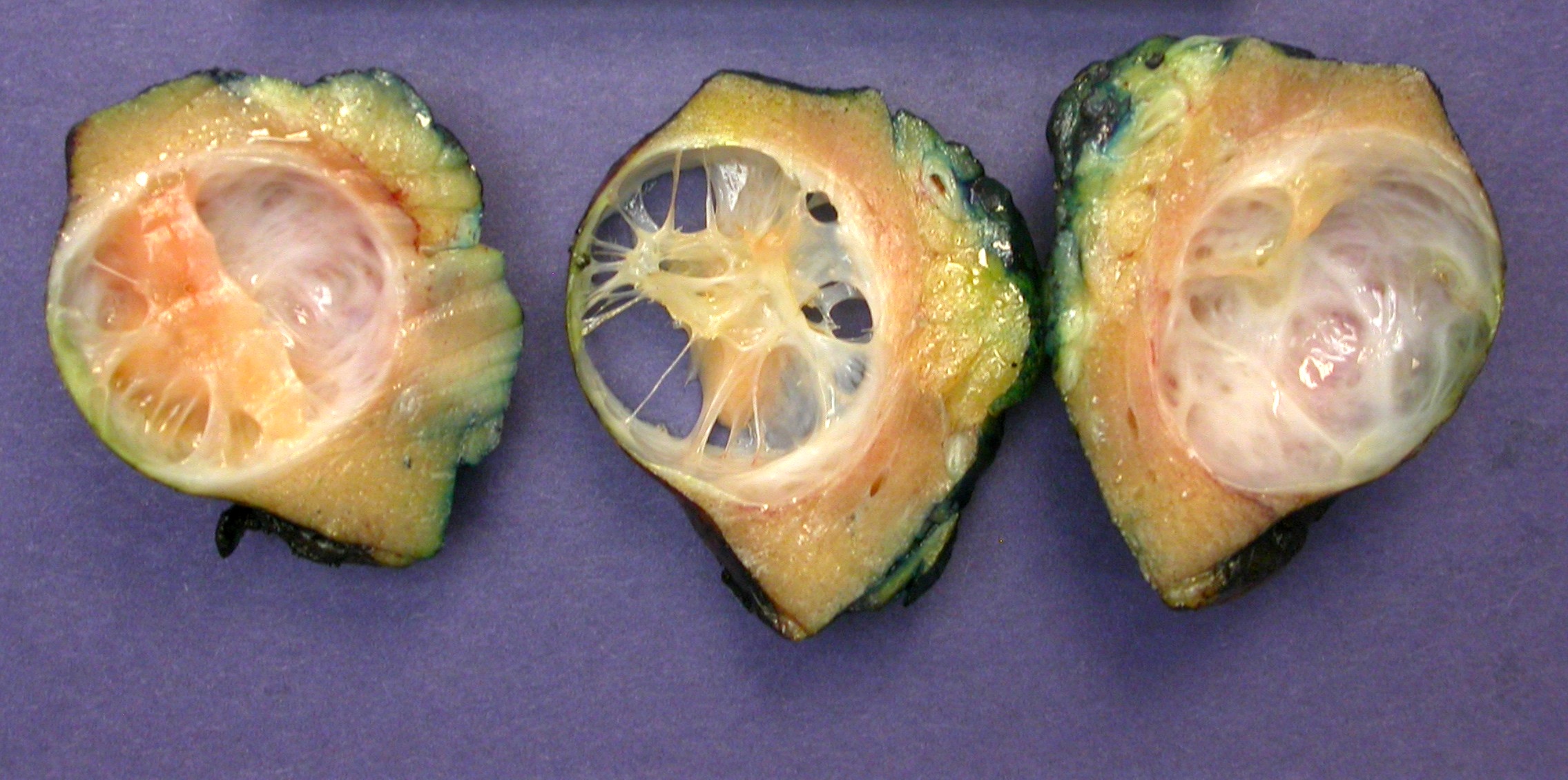
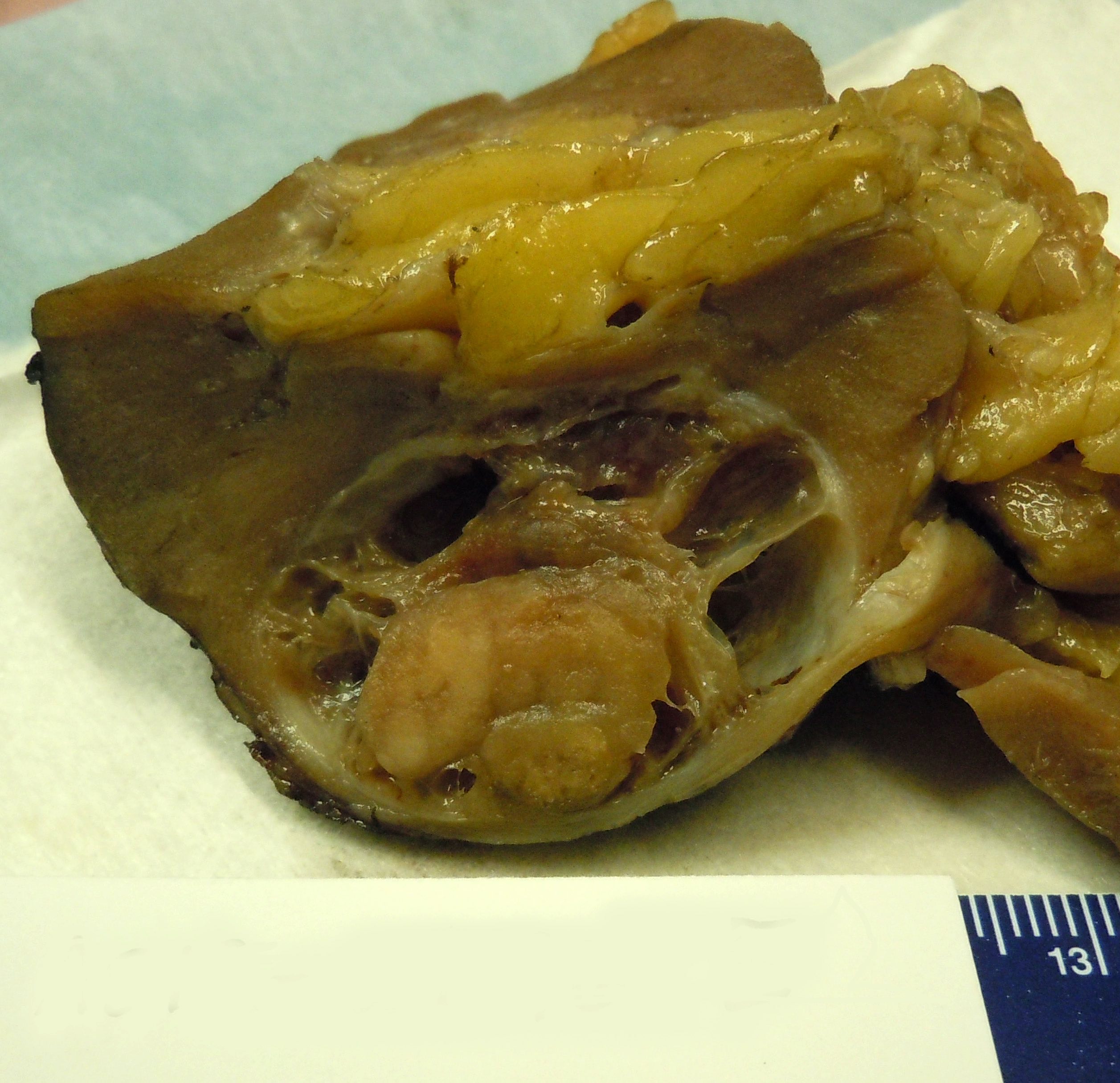
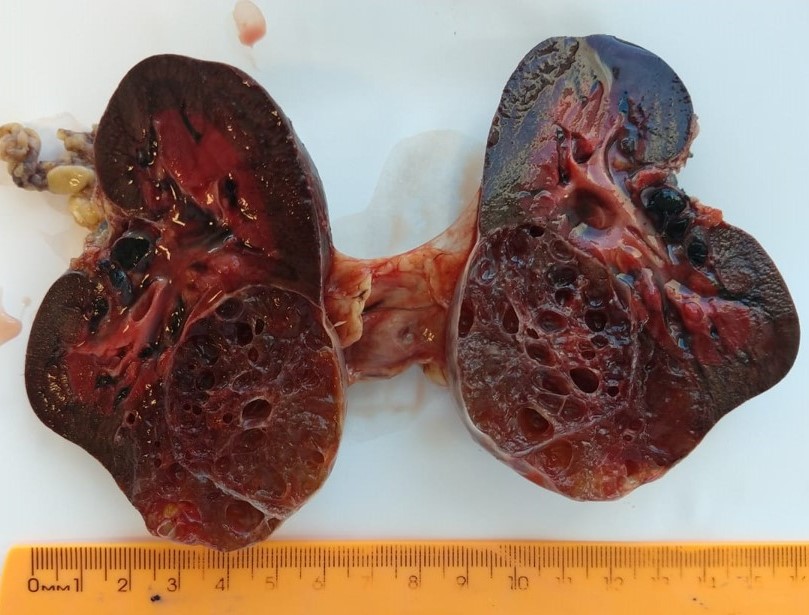
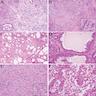
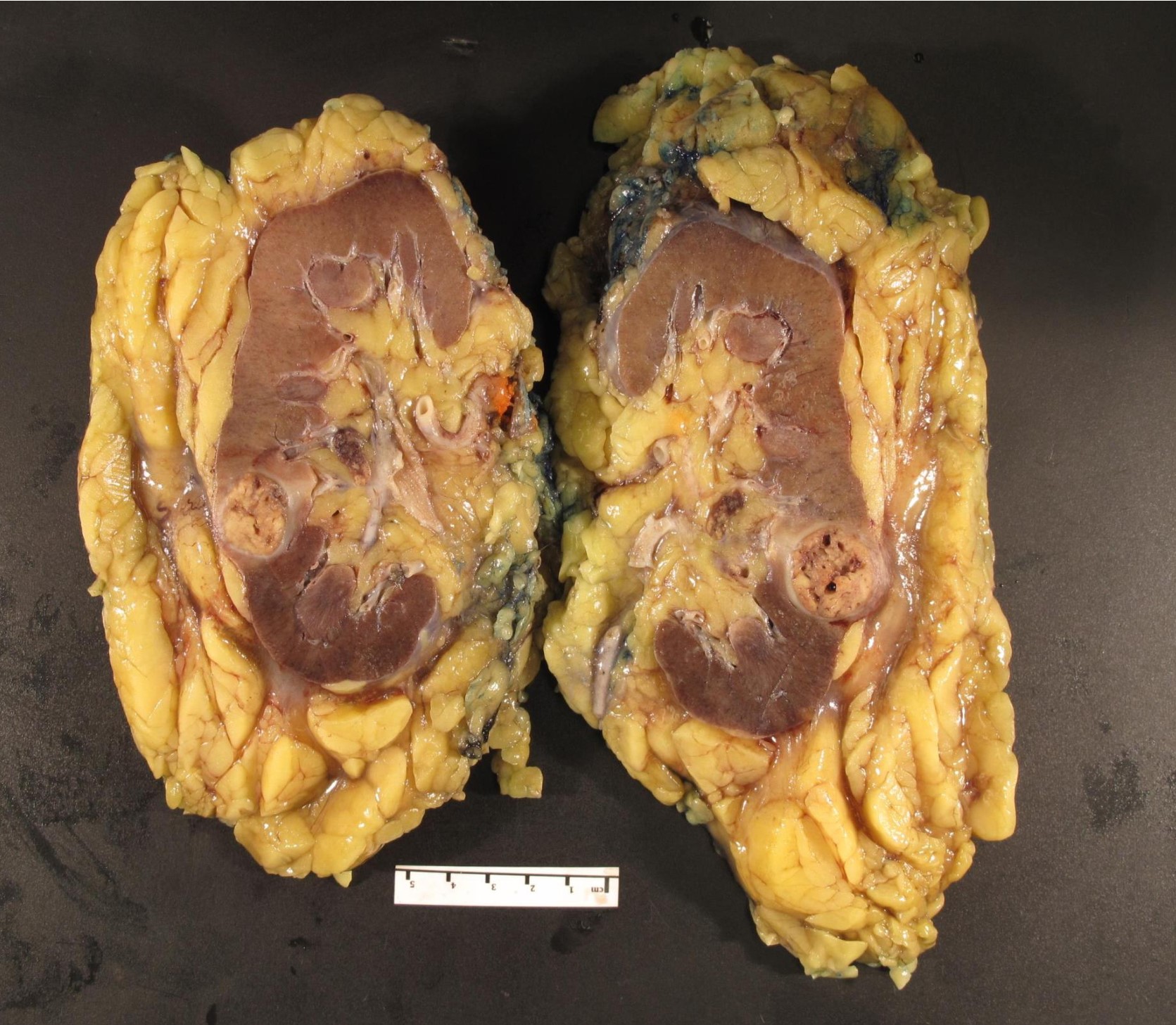
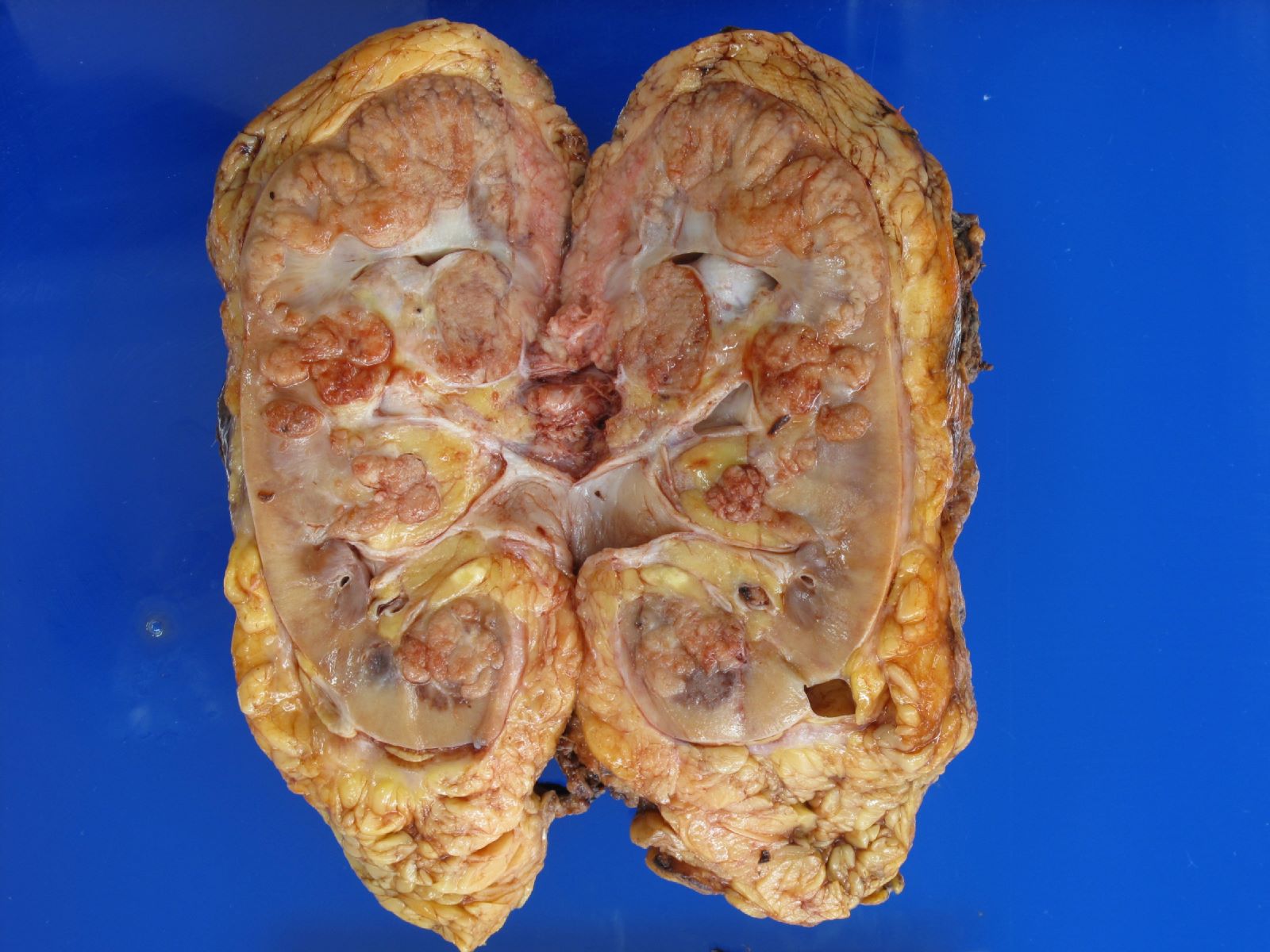
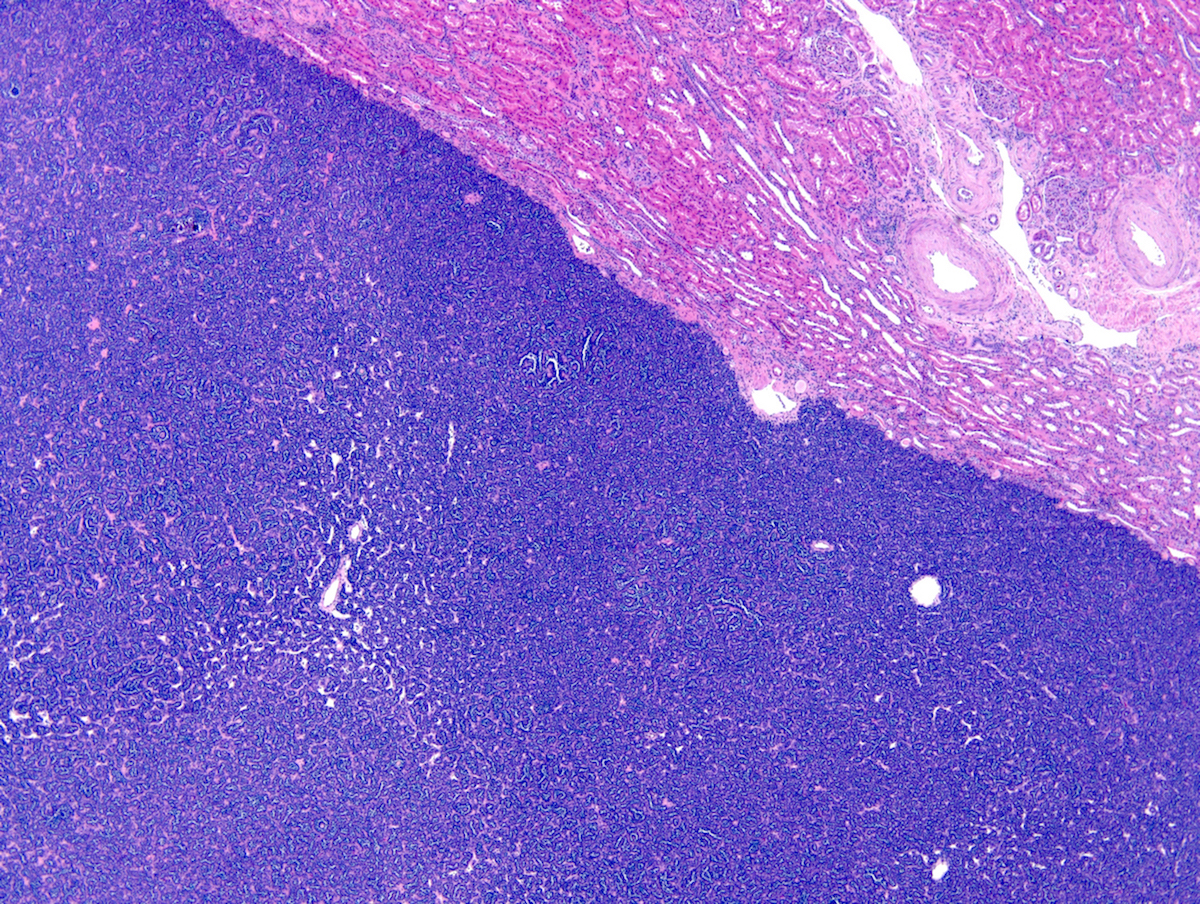
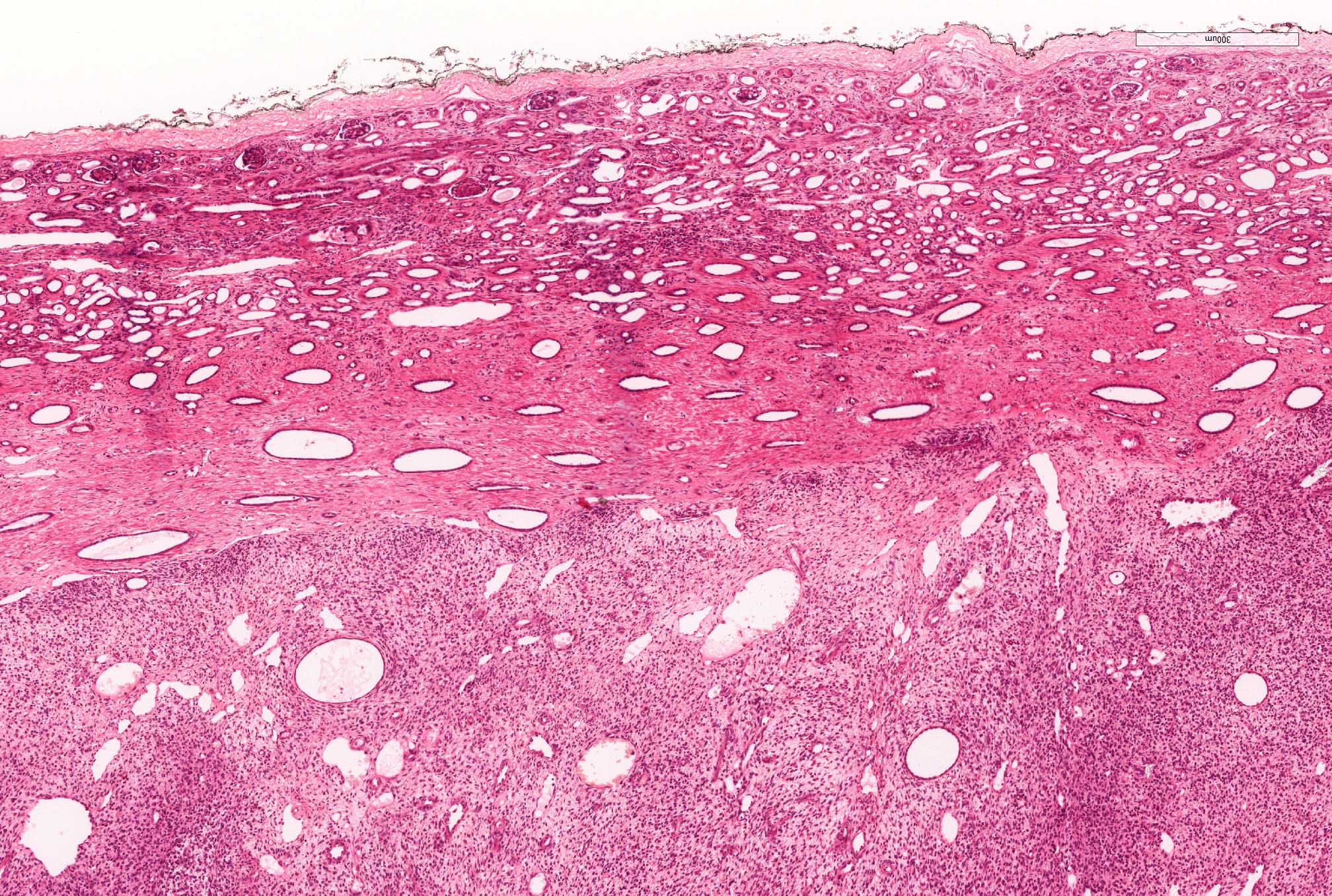
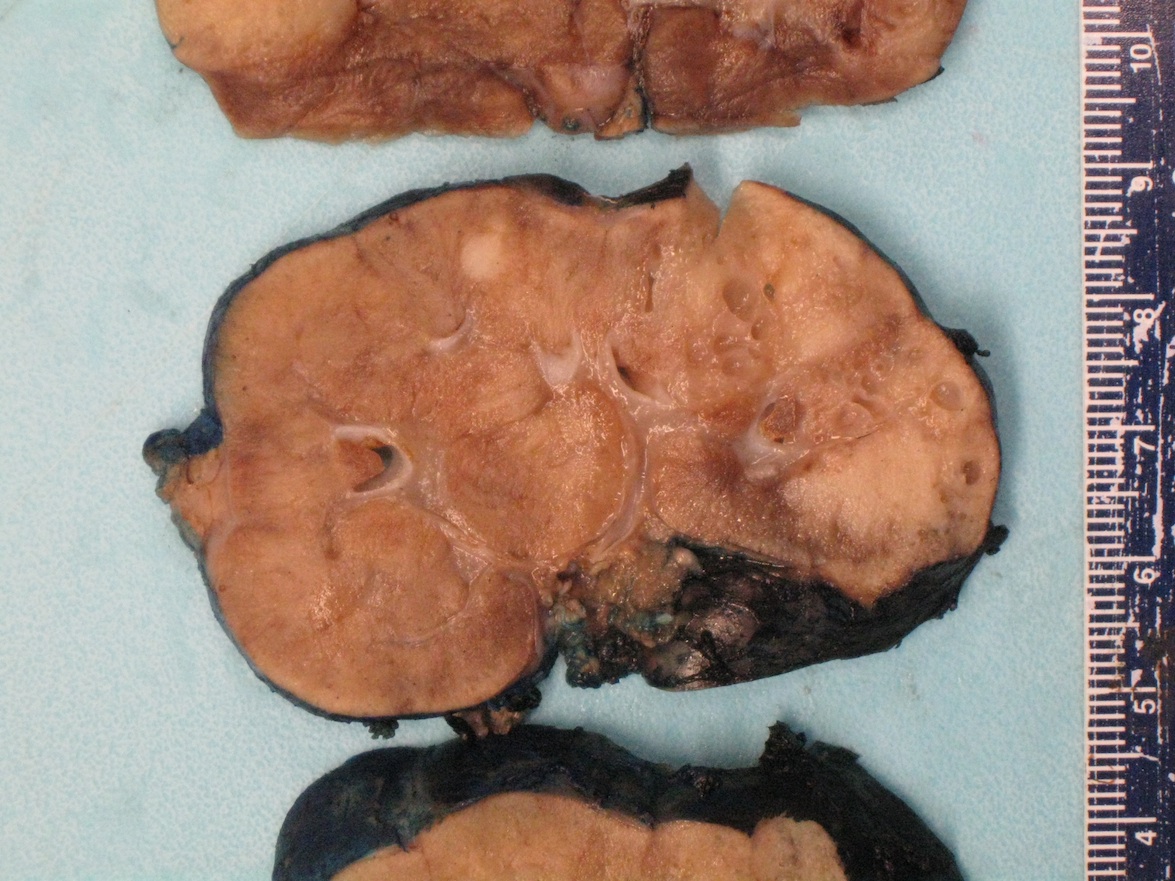
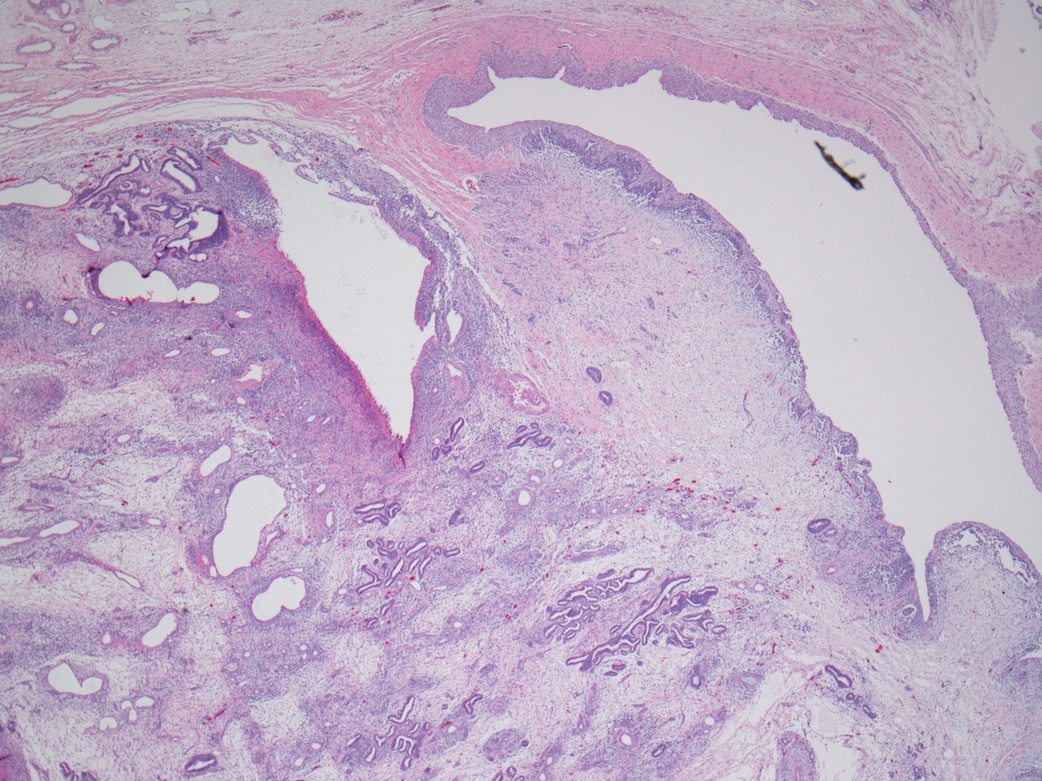
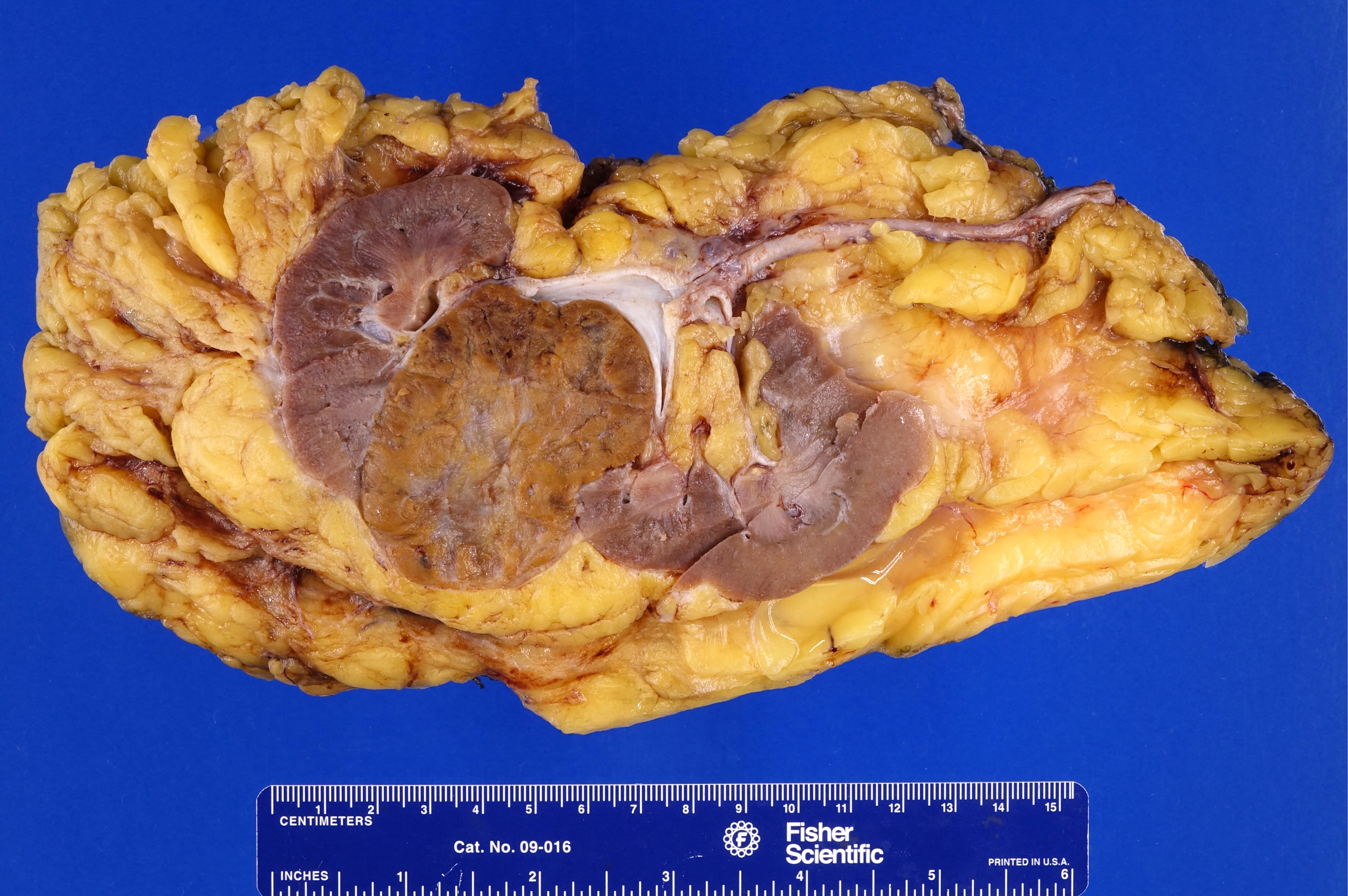
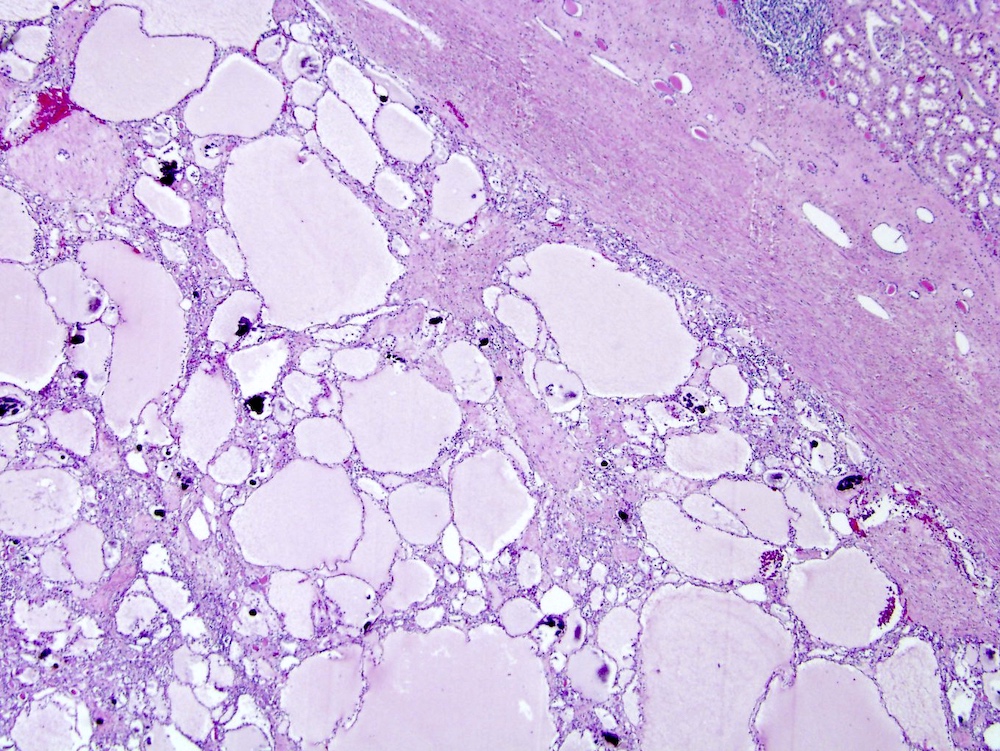
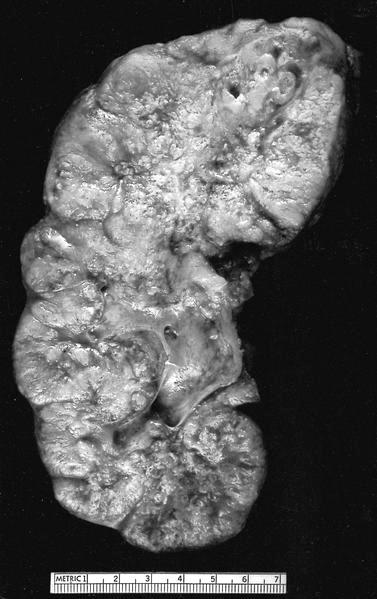
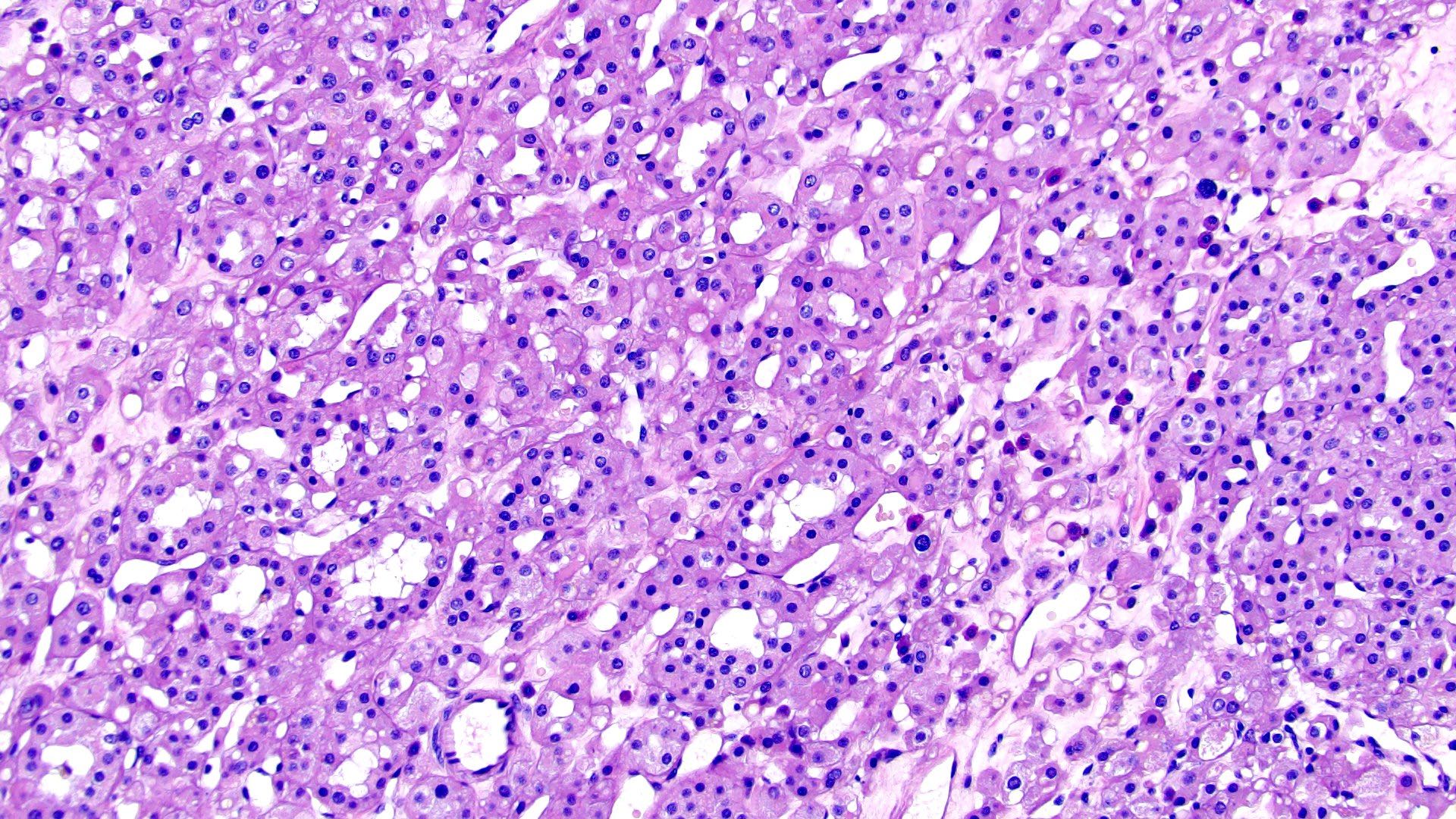
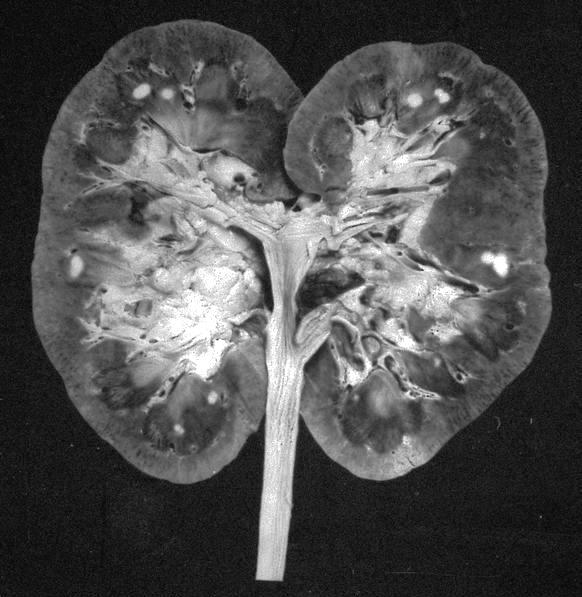
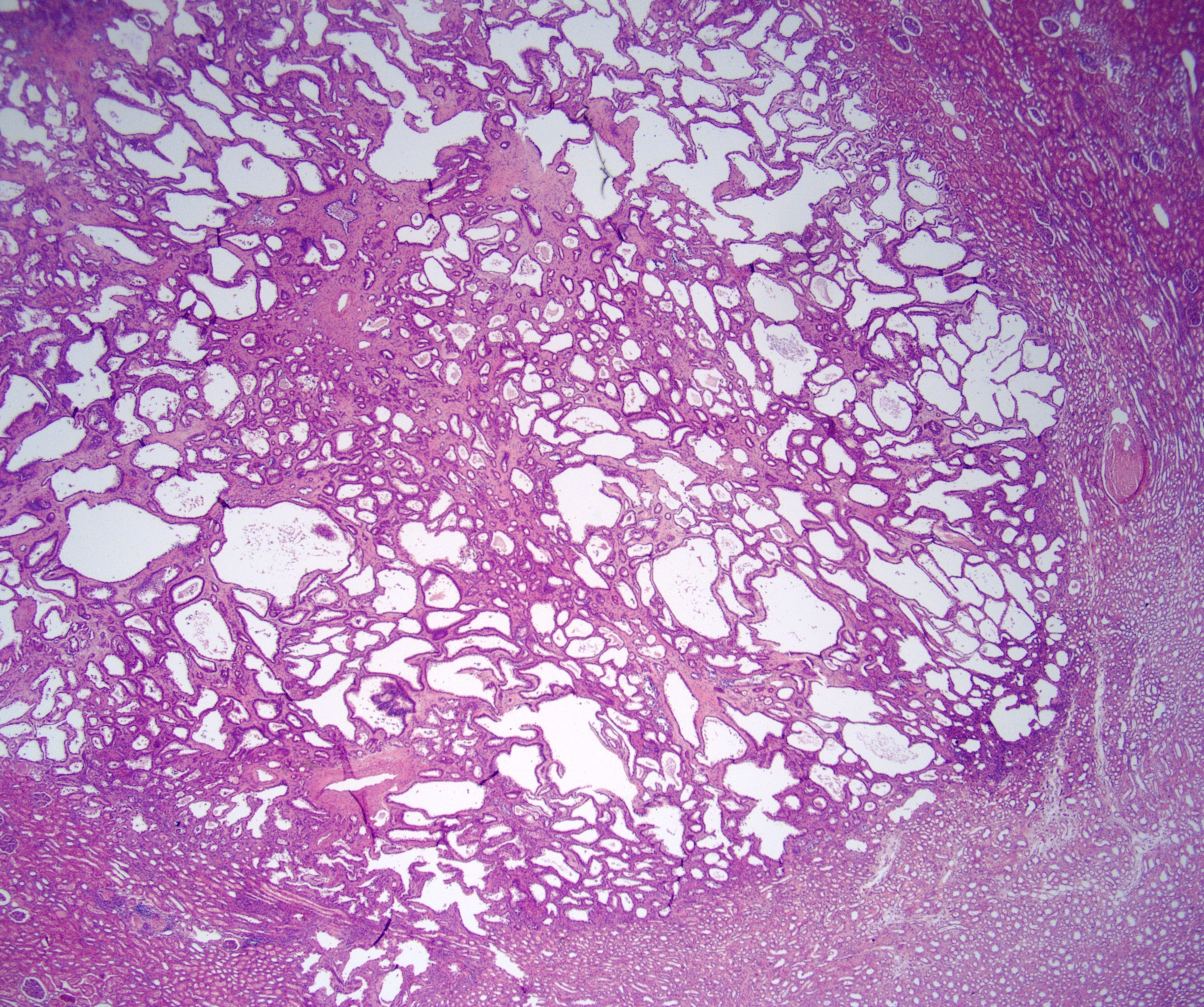
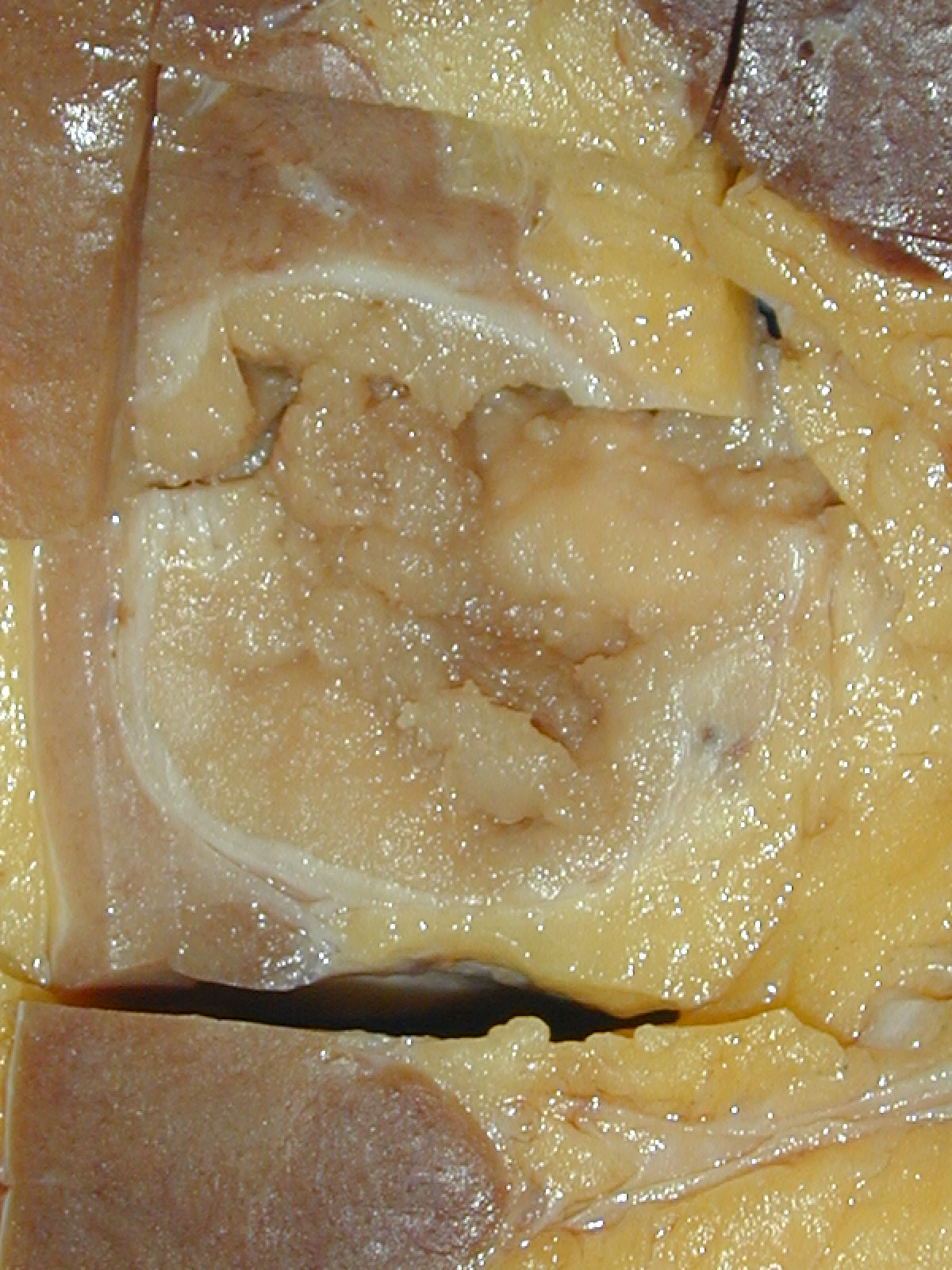
- Mass lesion: usually ≤ 3 cm or less in ACD background of atrophic kidney with corticomedullary junctions obscured by diffuse, small cortical cysts
- Multifocal (~50%) and bilateral (~25%)
- May be found in association with atypical cysts, adenomas or additional neoplasms
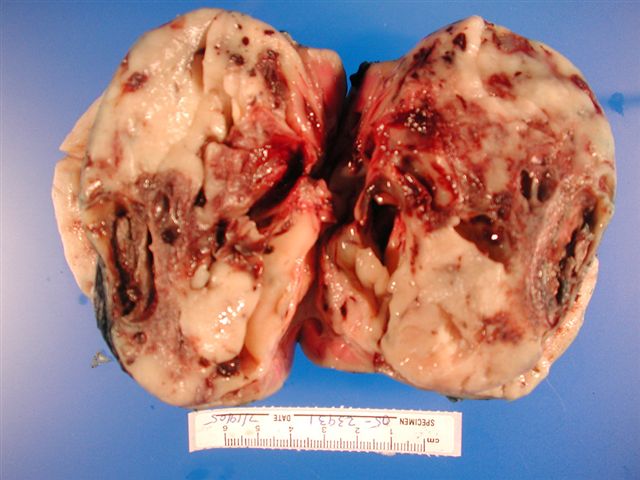
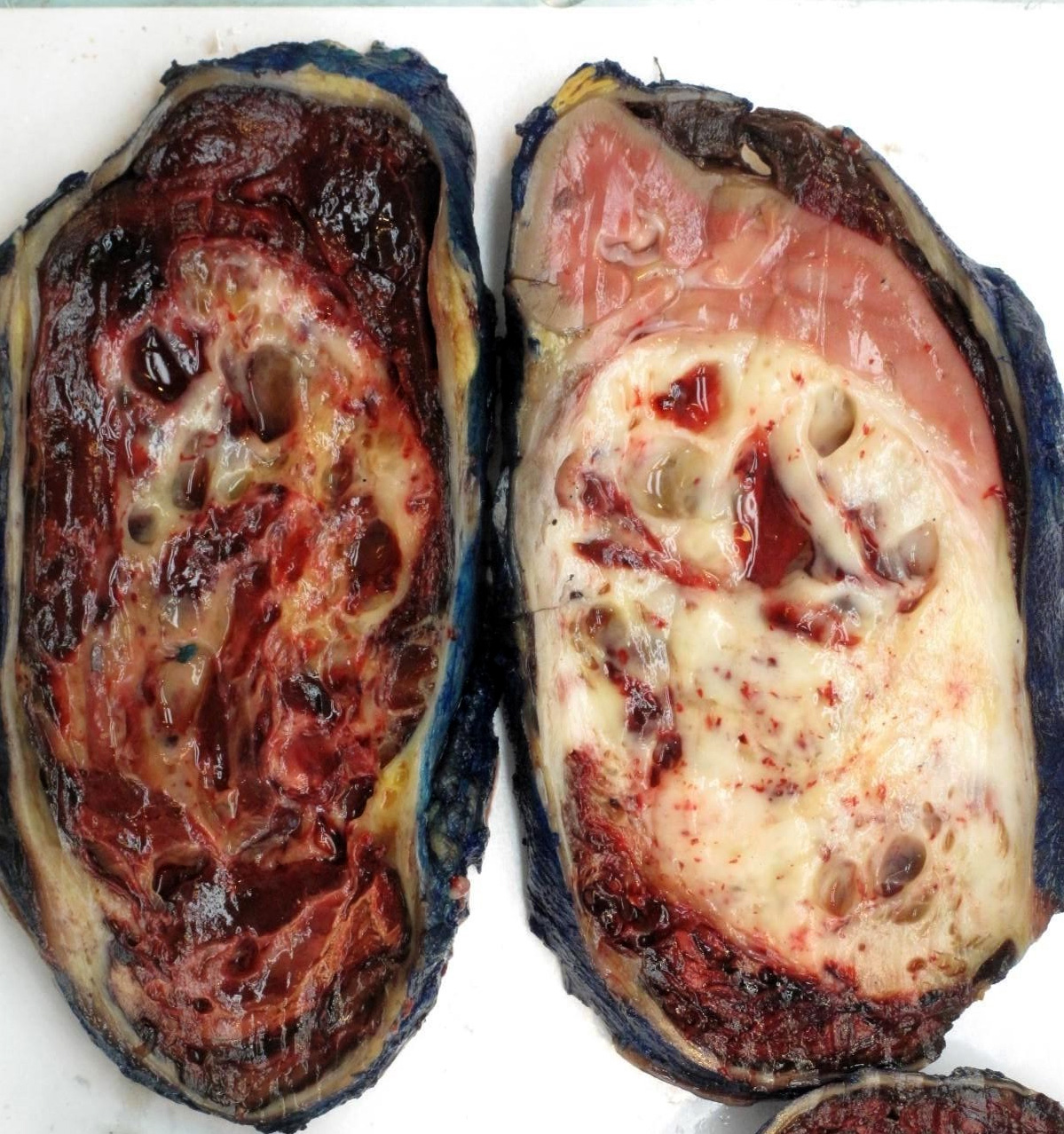
- Generally circumscribed and often arises in a cyst; larger tumors with thick, fibrous capsules
- Brown to yellow cut surface with focal hemorrhage and necrosis (Eur Urol 2016;70:93)
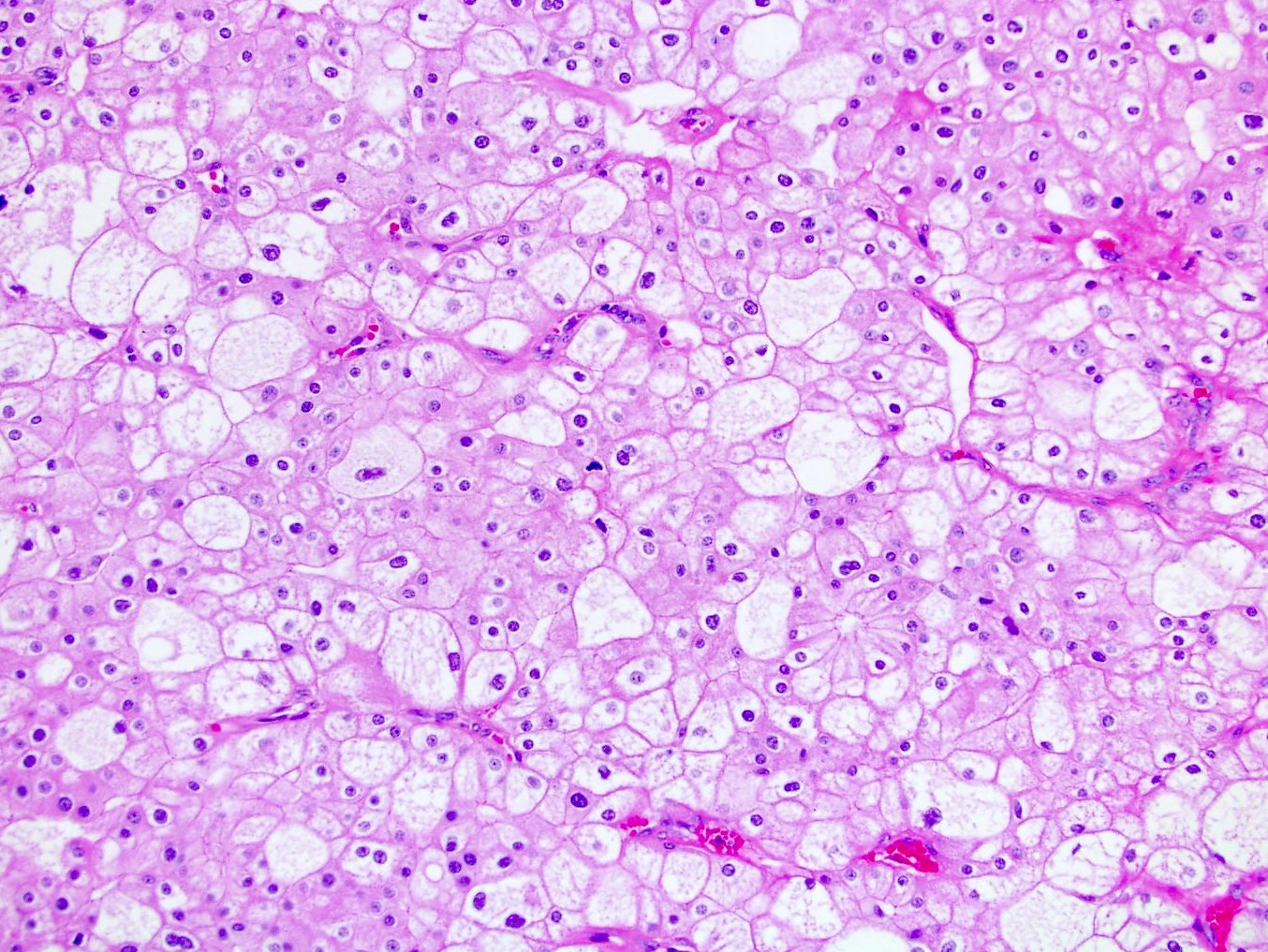
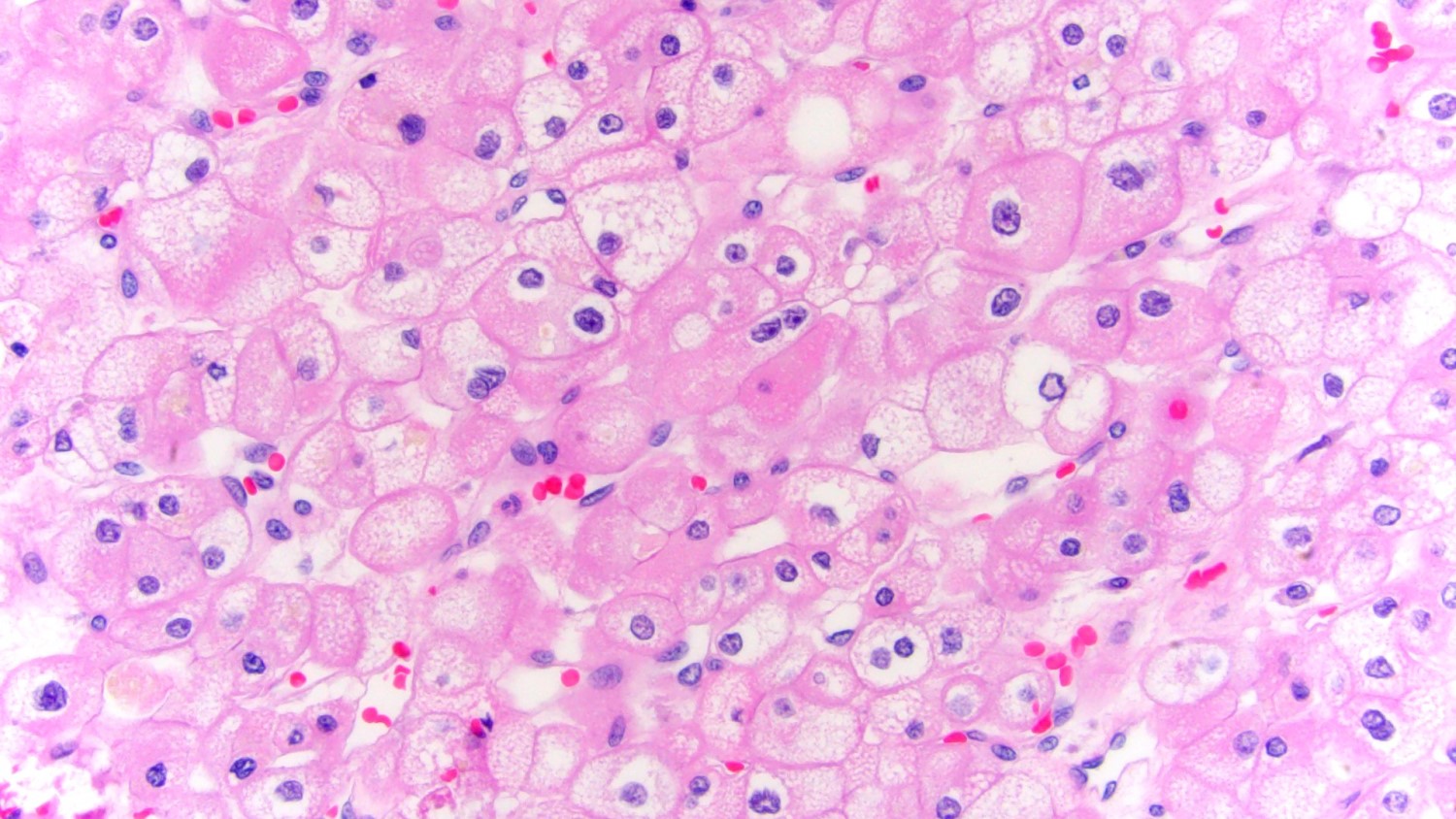
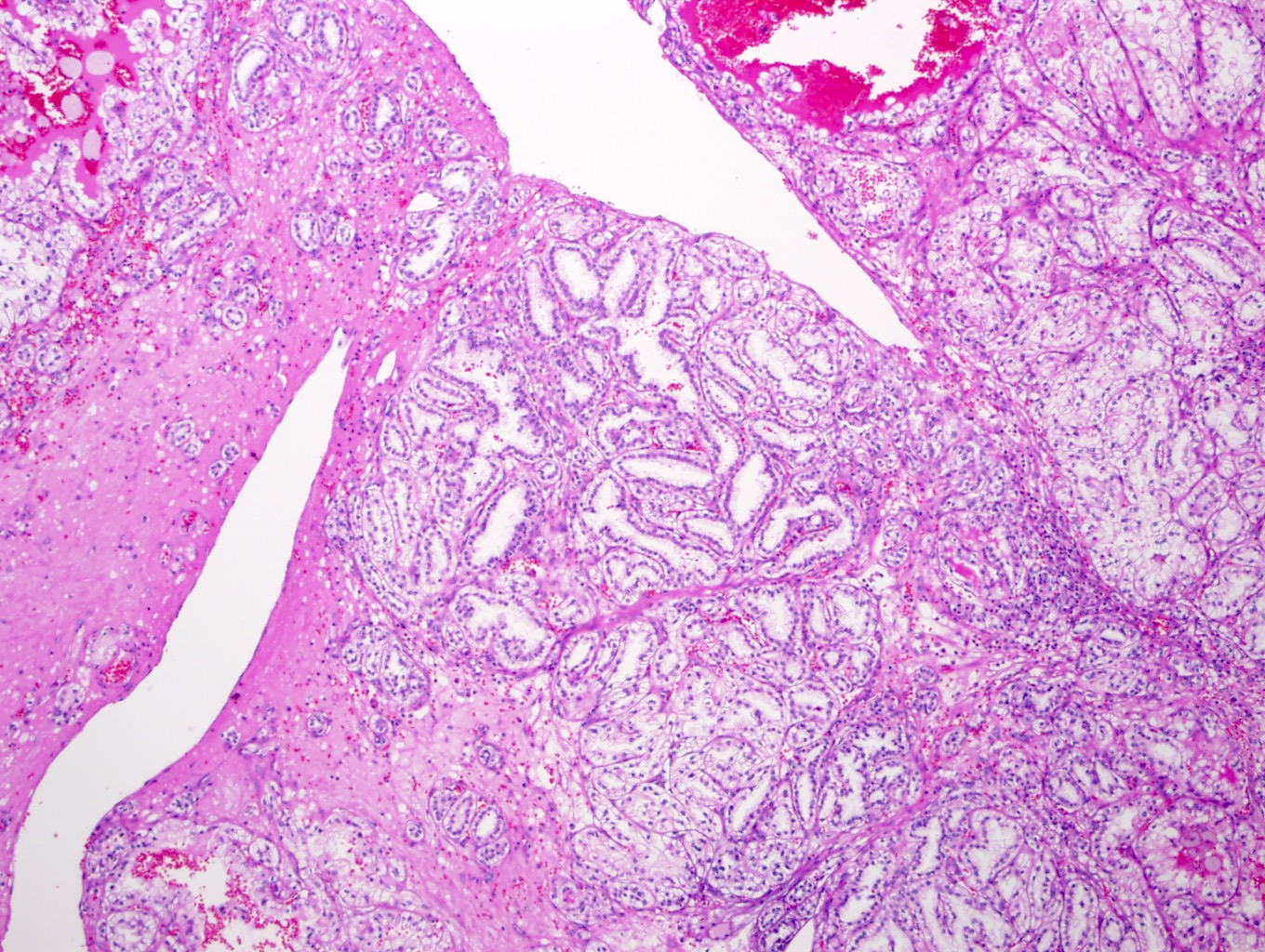
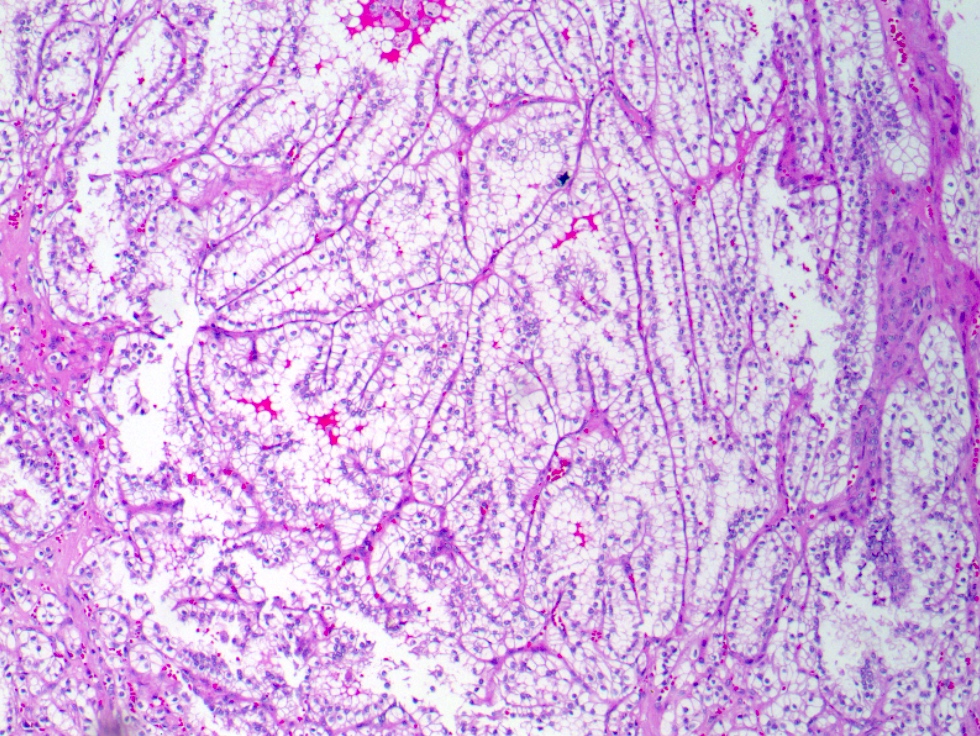
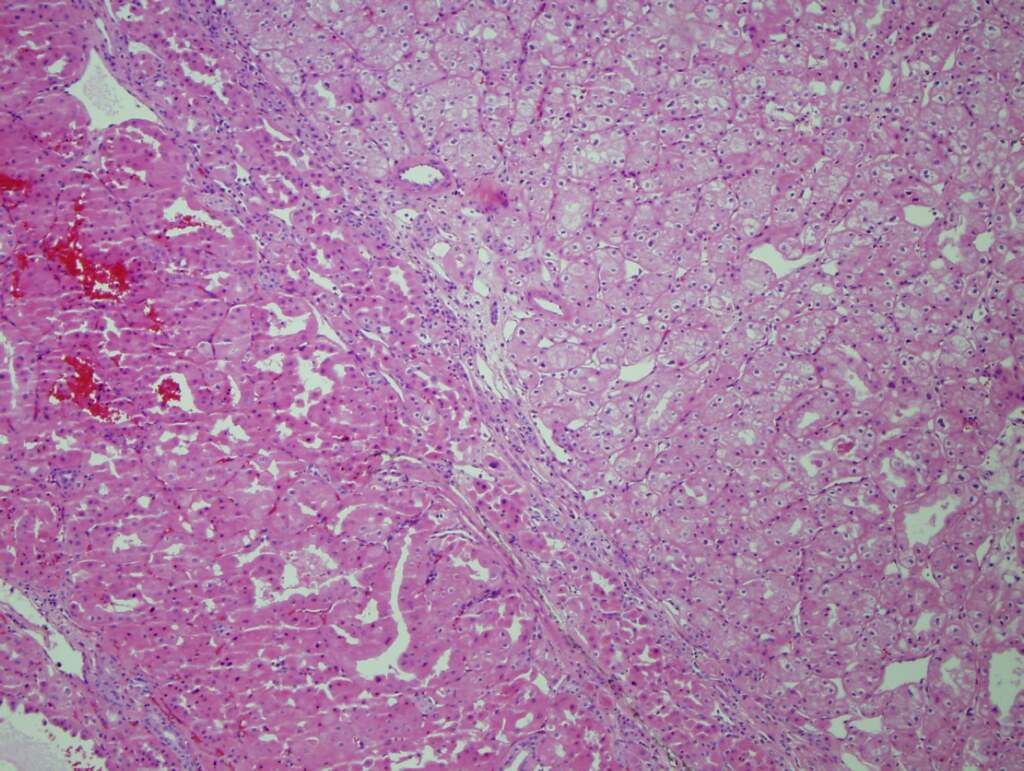
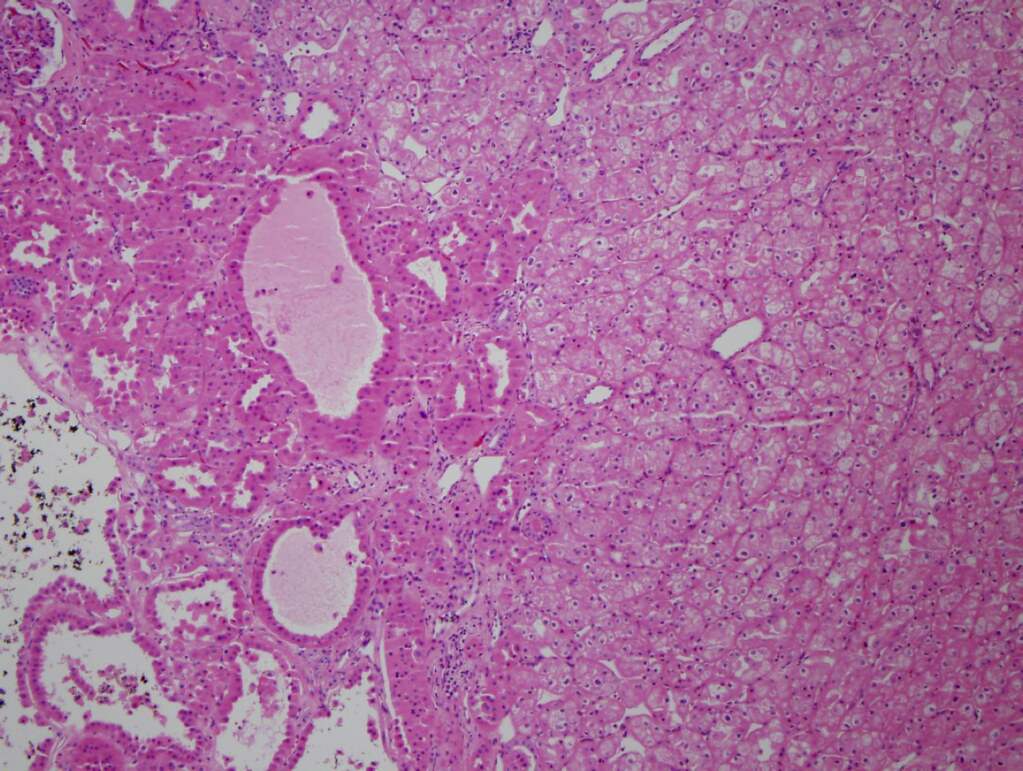
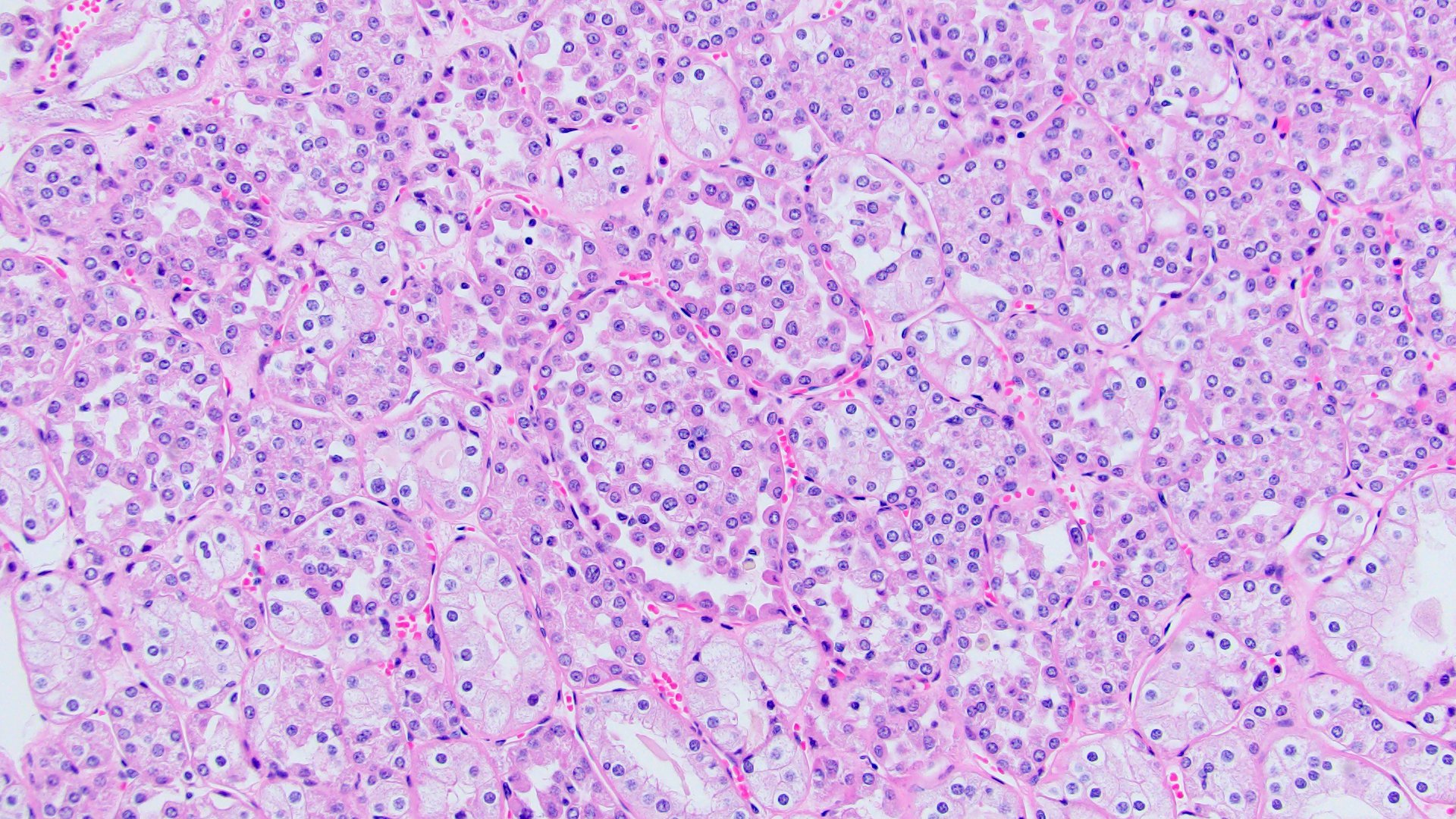
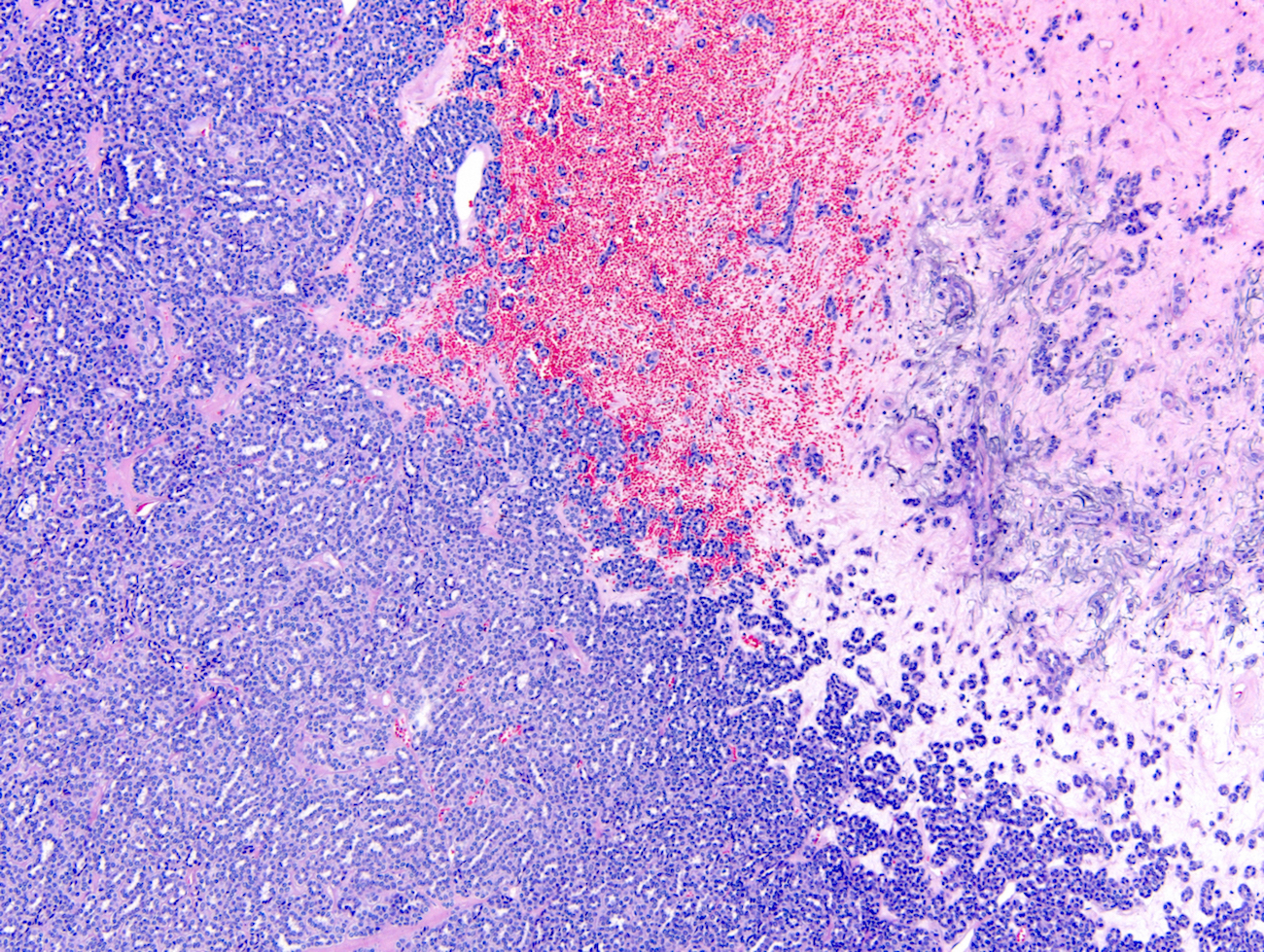
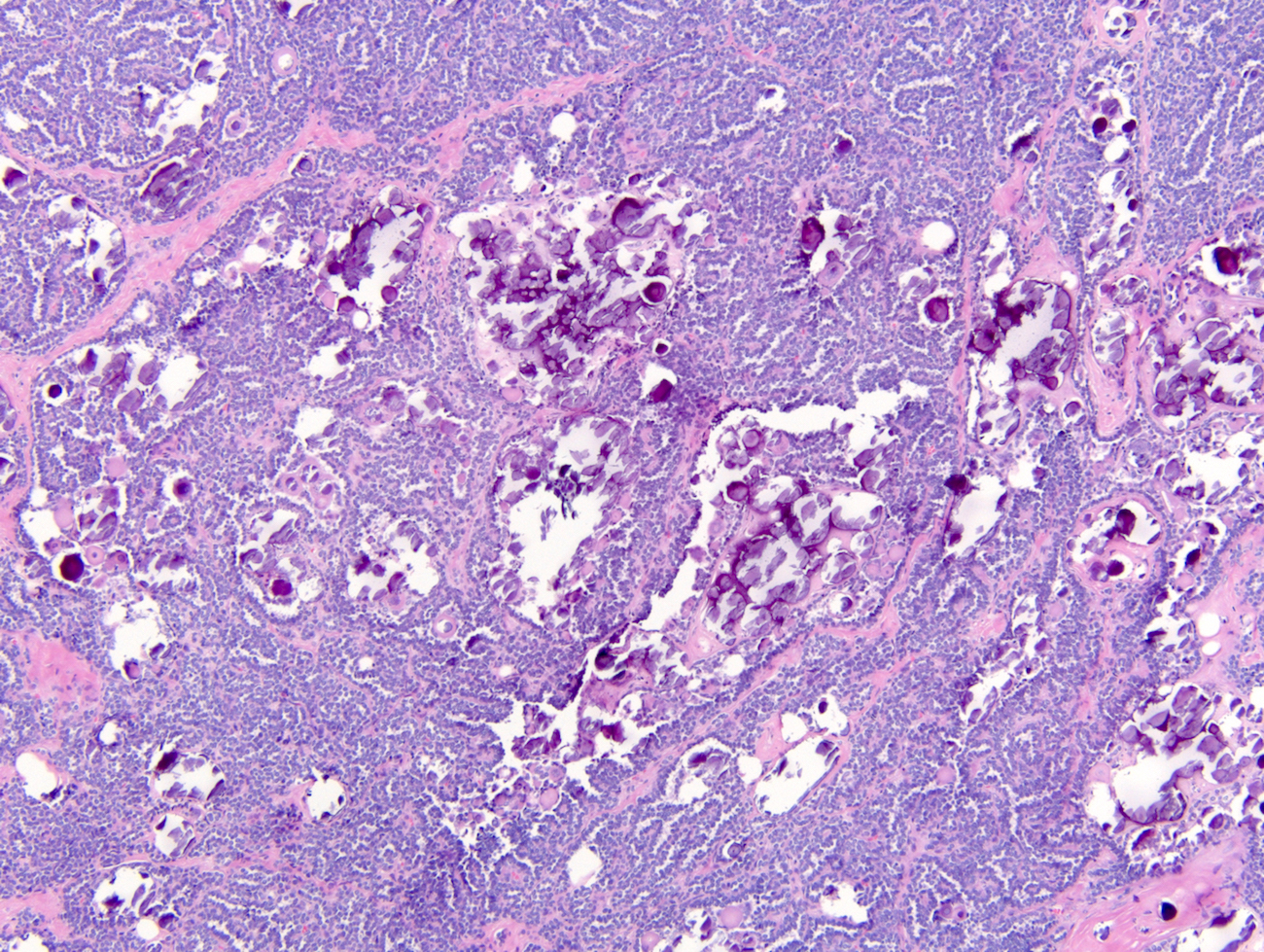
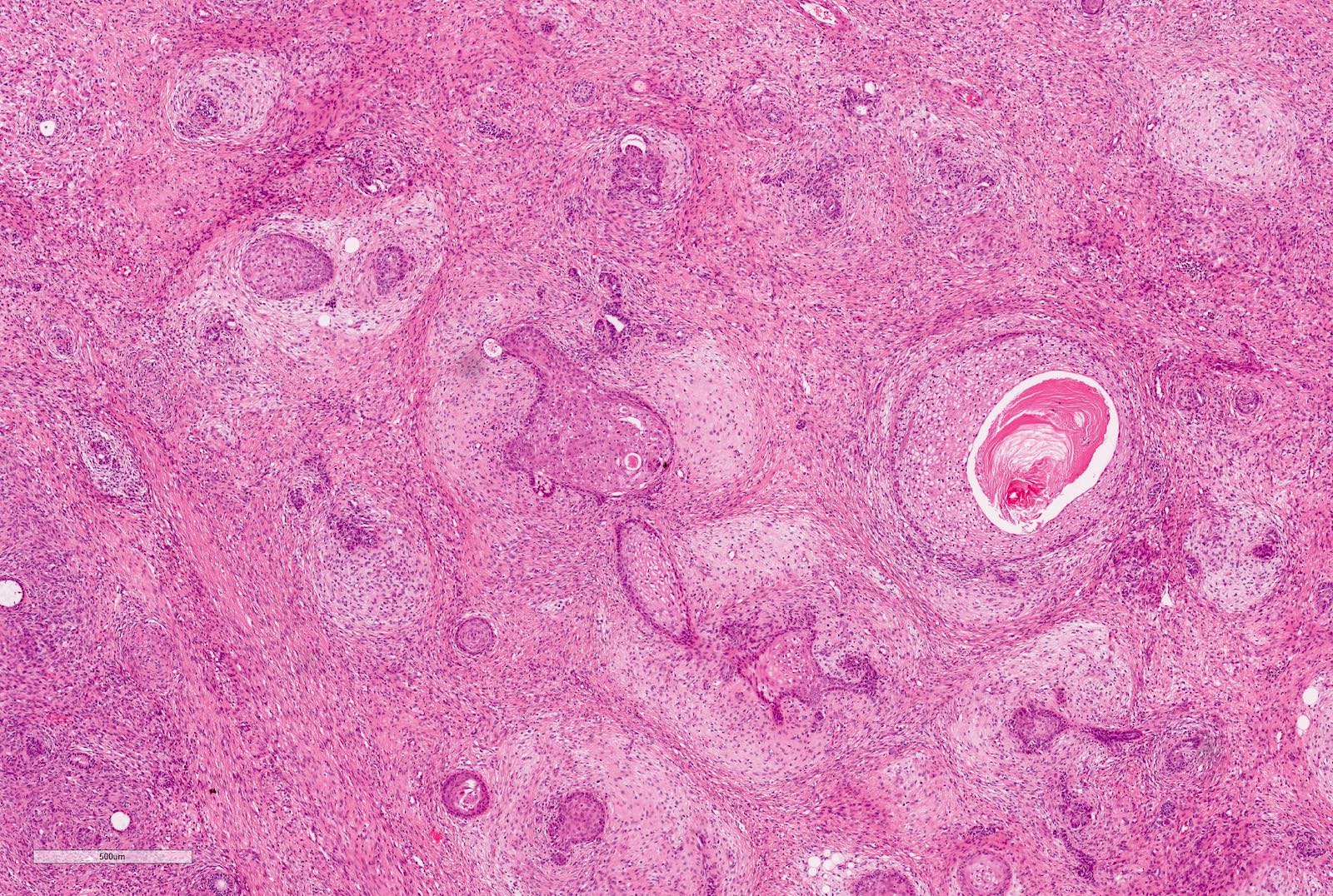
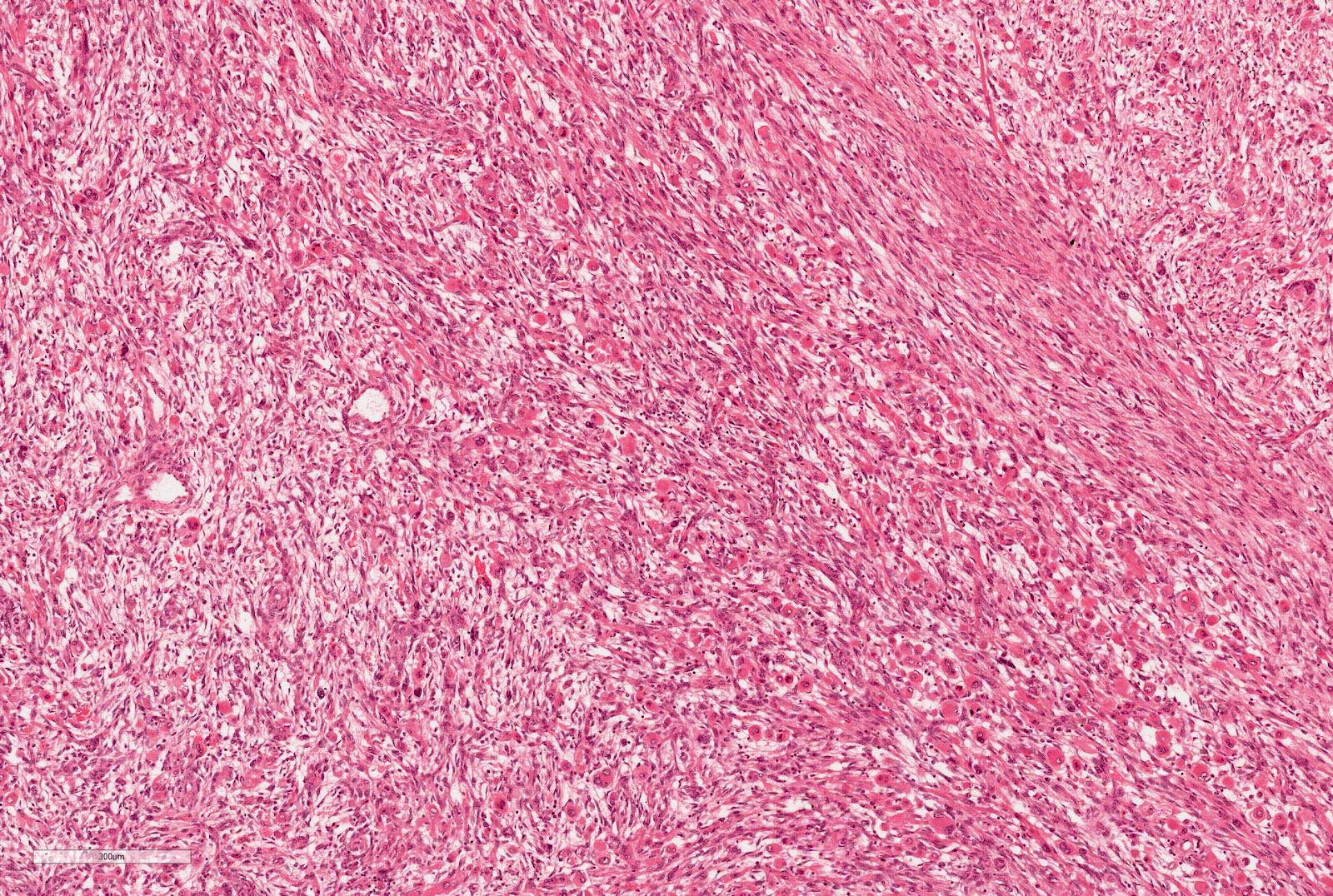
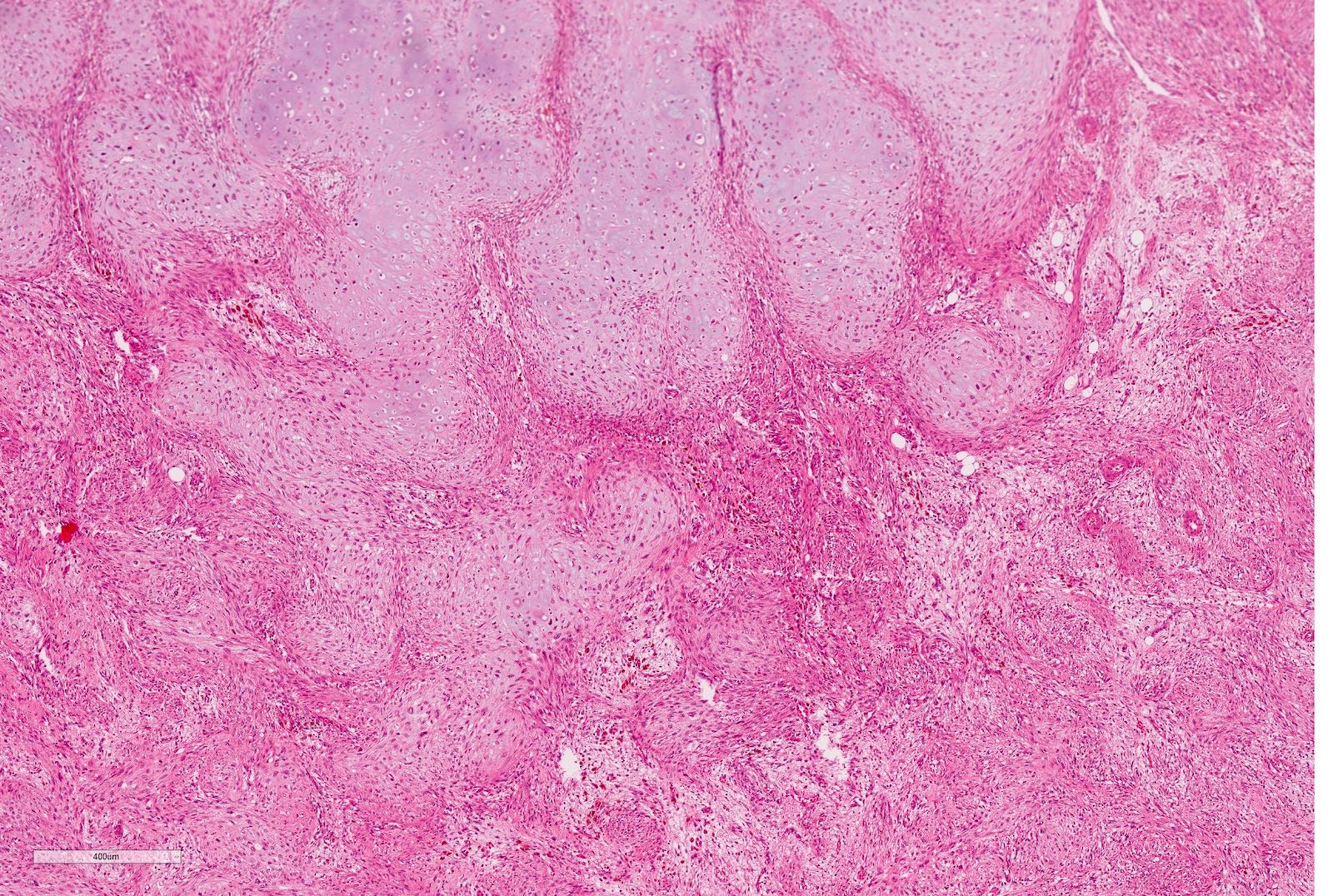
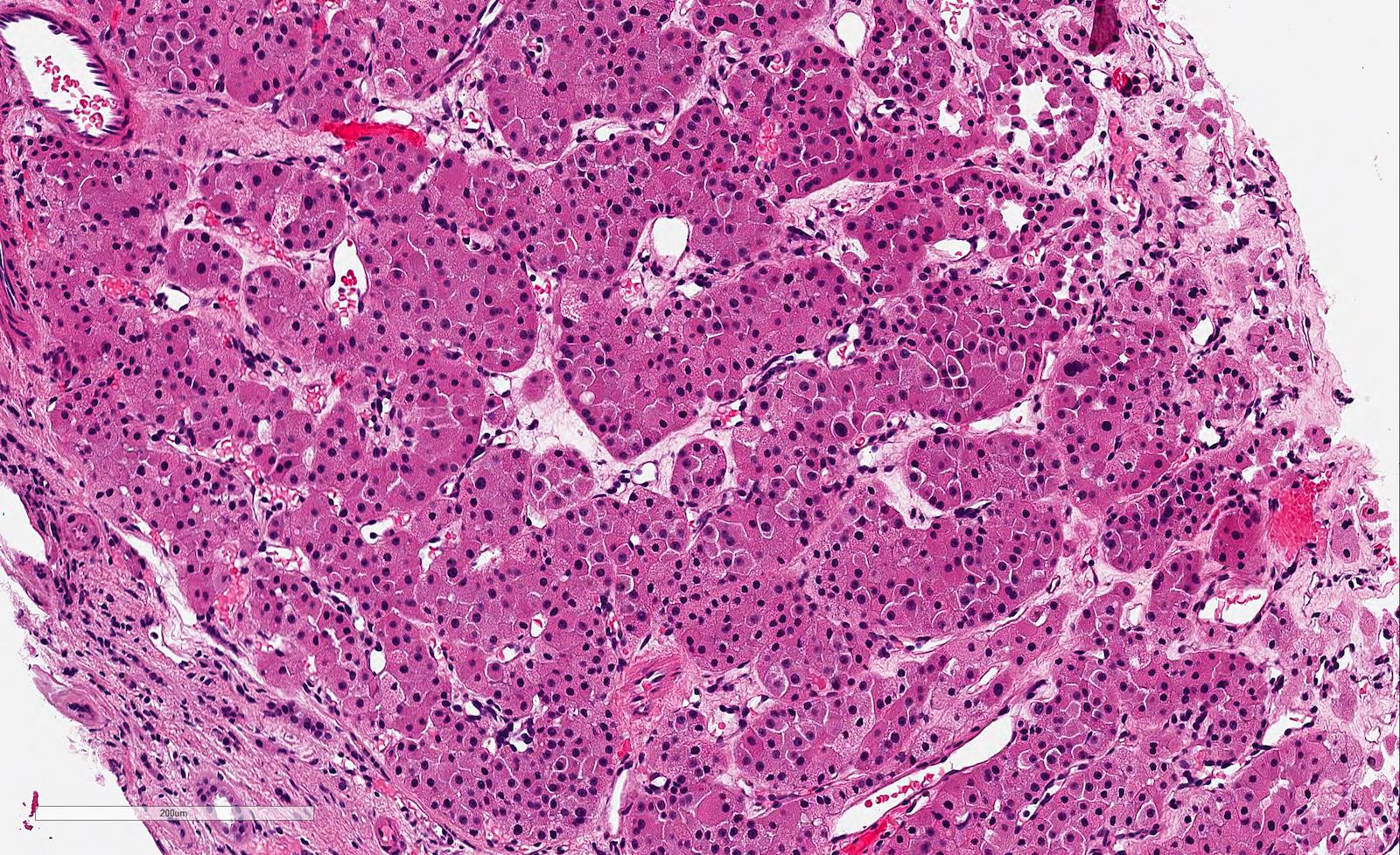
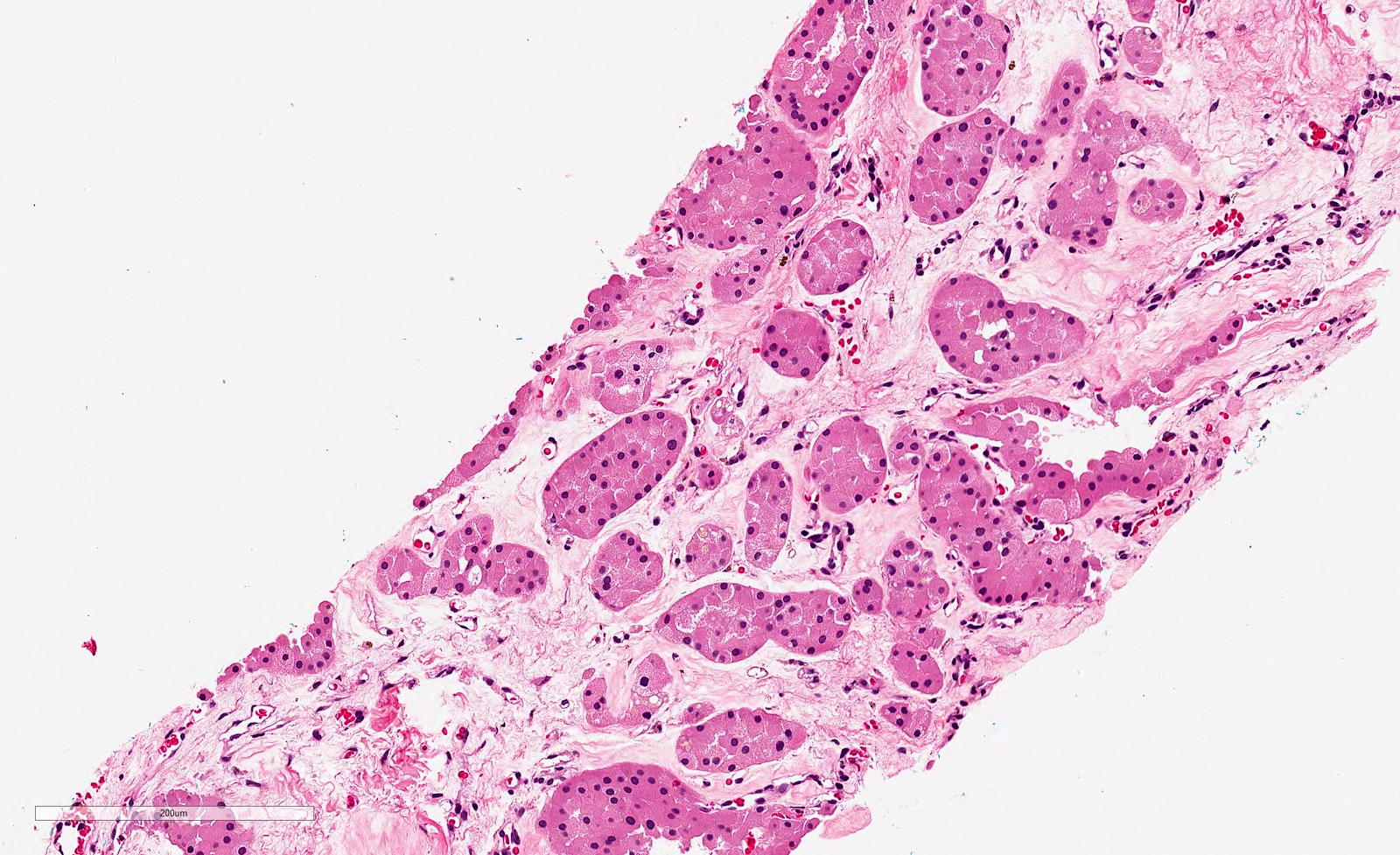
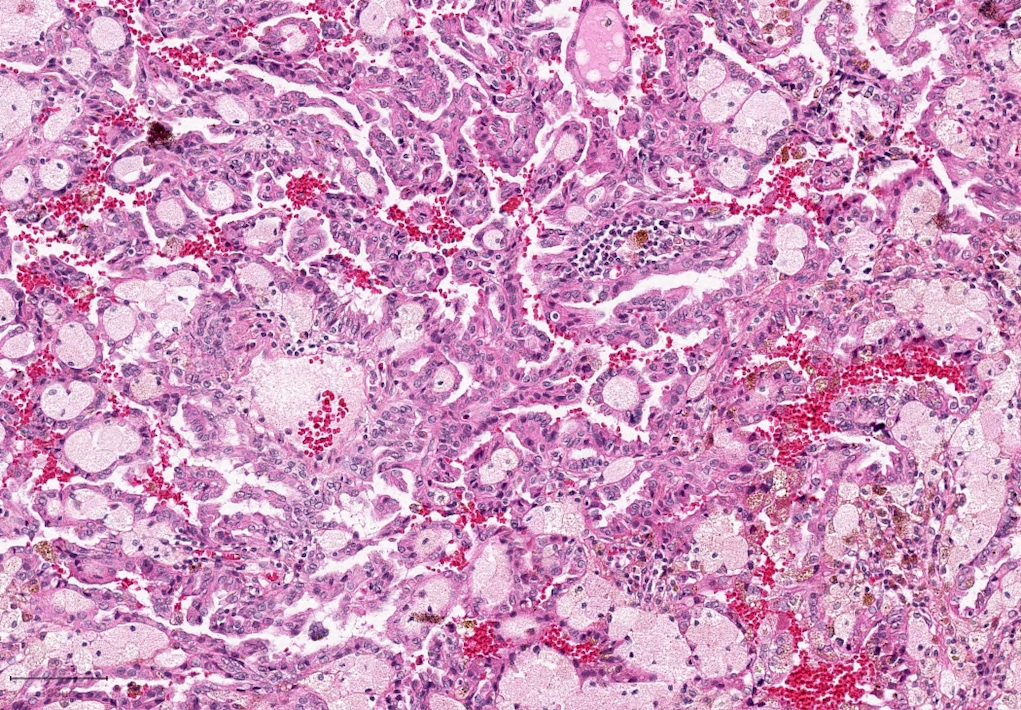
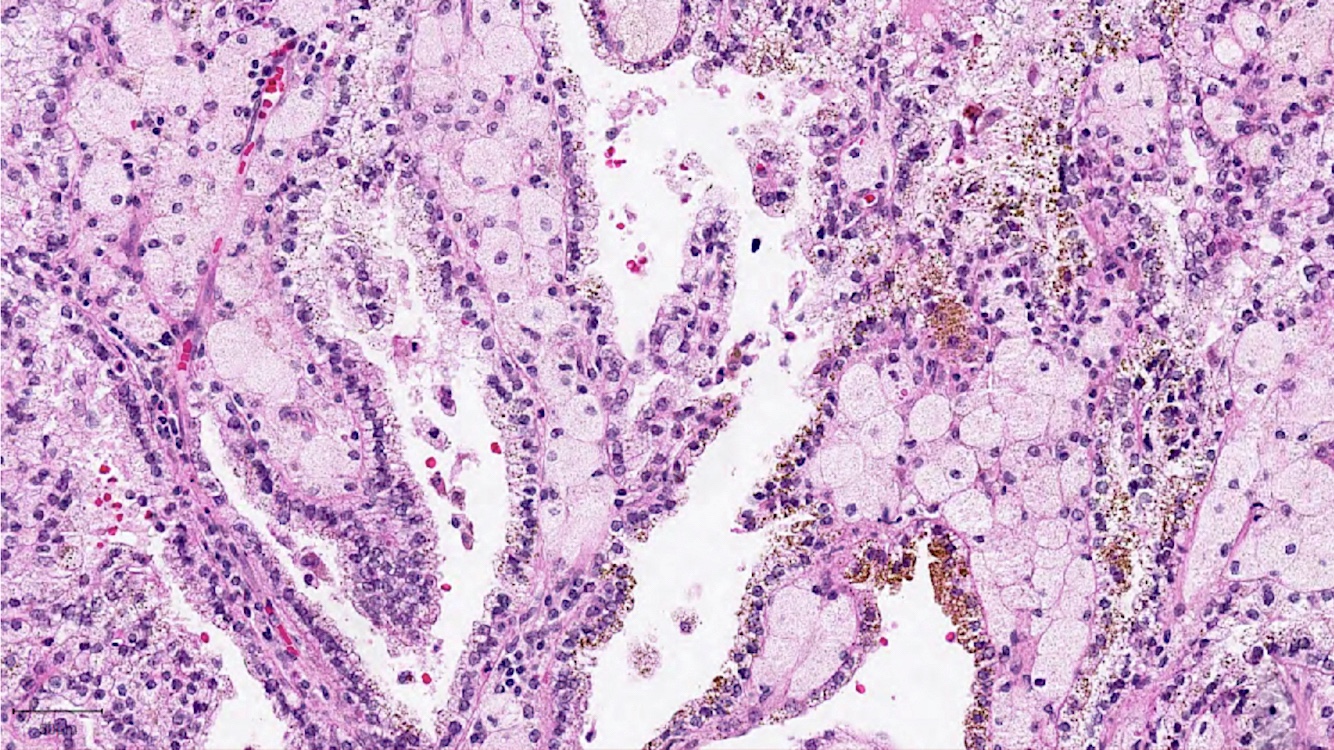
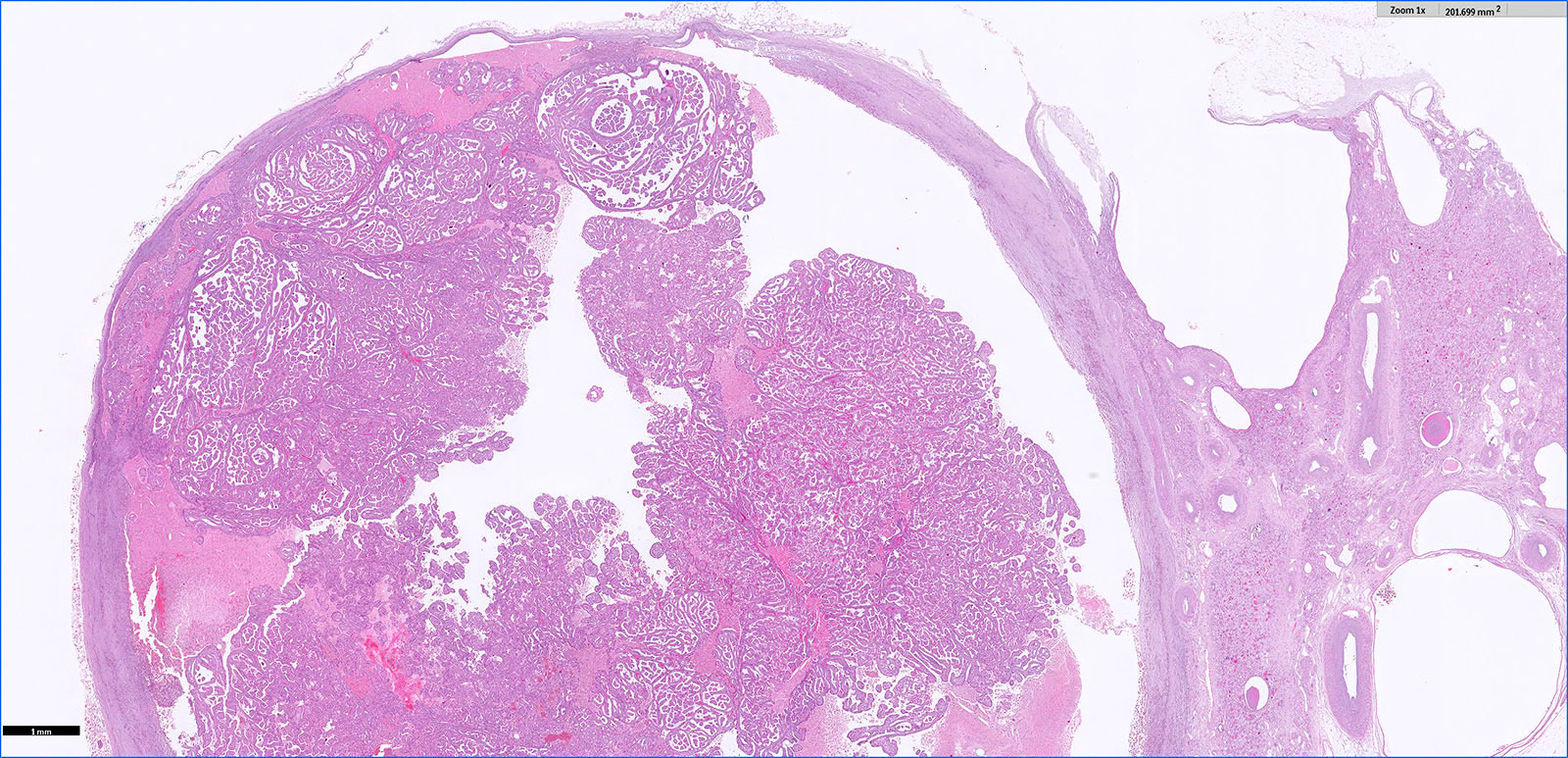
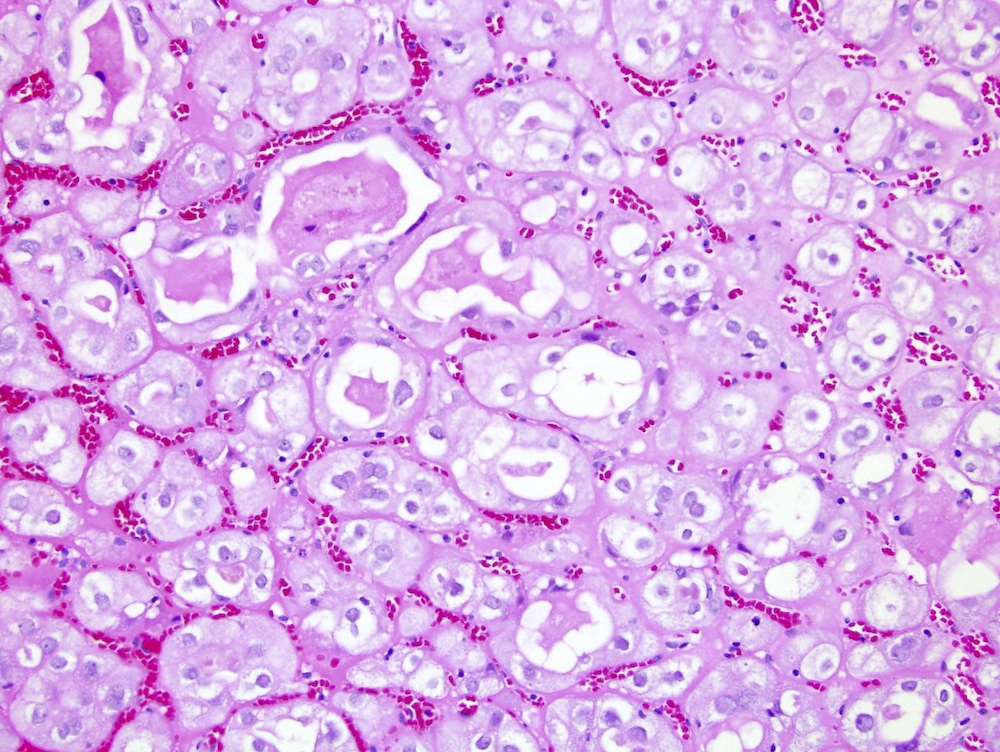
- Highly variable architecture: cribriform, microcystic, sieve-like, tubulopapillary and solid patterns
- Cells with large nuclei, prominent nucleoli and abundant granular eosinophilic cytoplasm with intracytoplasmic holes and intercytoplasmic lumina due to crystal depositions
- Intratumoral calcium oxalate crystals are very common but not necessary for diagnosis (Am J Surg Pathol 2005;29:443)
- May be nodules arising from cyst walls or solid masses separate from cysts (Am J Surg Pathol 2013;37:1469)
- Sometimes prominent clear cell cytology
Contributed by Nicole Andeen, M.D., Maria Tretiakova, M.D., Ph.D., Gregory T. MacLennan, M.D. and @katcollmd on Twitter
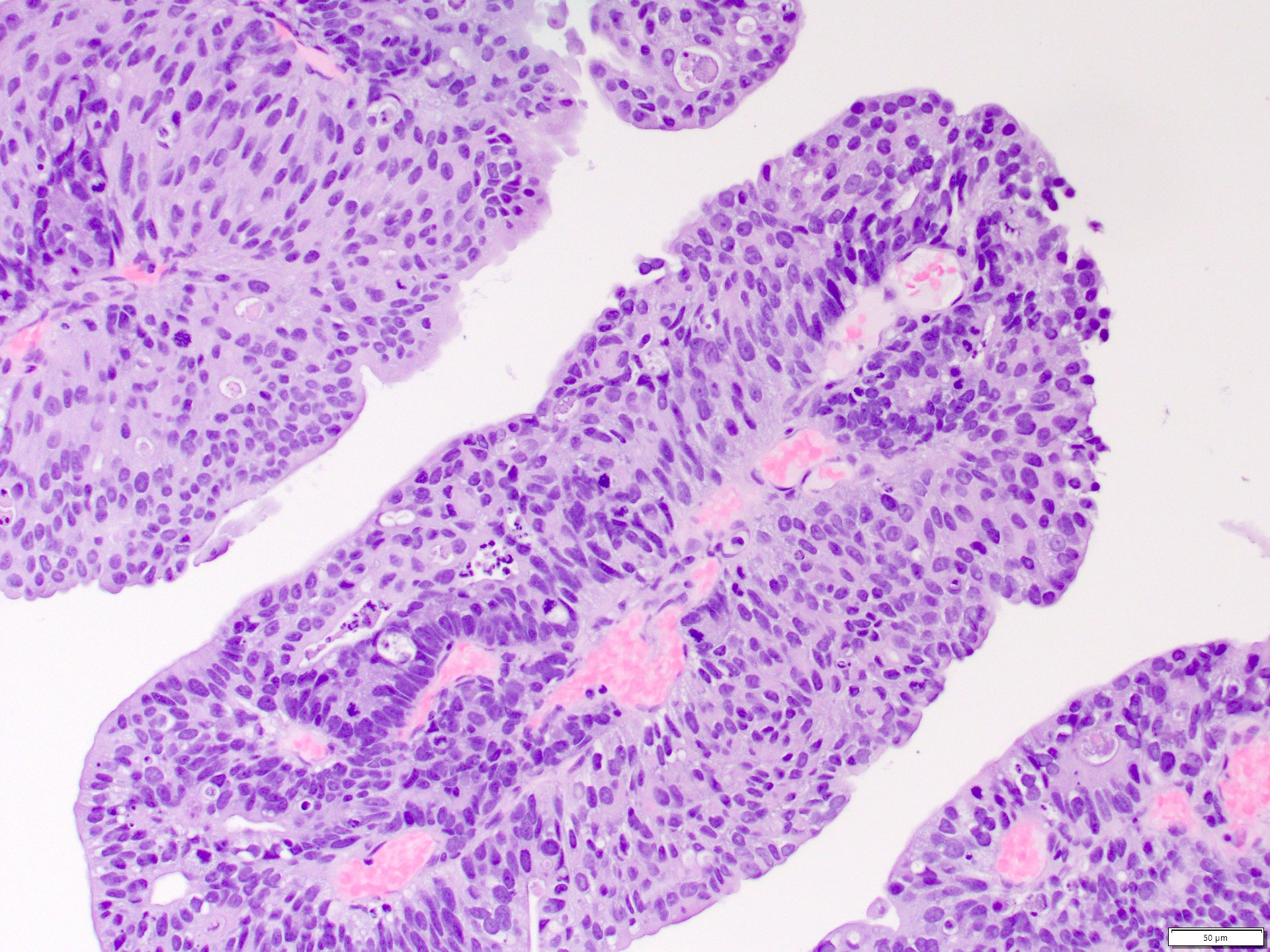
- Moderately cellular, papillary clusters of polygonal to columnar cells with abundant eosinophilic granular cytoplasm, round and central nuclei, finely granular chromatin, prominent central grade 3 nucleoli (Diagn Cytopathol 2008;36:344)
- PAX8, CD10, AE1 / AE3, RCC, AMACR (Mod Pathol 2006;19:780, Eur Urol 2016;70:93)
- Specific immunohistochemical profile is not required for diagnosis (Am J Surg Pathol 2013;37:1469)
- Comparative genomic microarray and FISH studies reveal gains and losses of multiple chromosomes (Am J Surg Pathol 2013;37:1469)
- Gains of sex chromosomes and gains of 3, 7, 16, 17 (Eur Urol 2016;70:93)
- High prevalence of gains of Y, 3 and 16 distinguishes from papillary RCC, which also has gains in chromosomes 7 and 17 (Eur Urol 2016;70:93)
- Recurrent KMT2C or TSC2 gene mutations (Am J Surg Pathol 2020;44:1479)
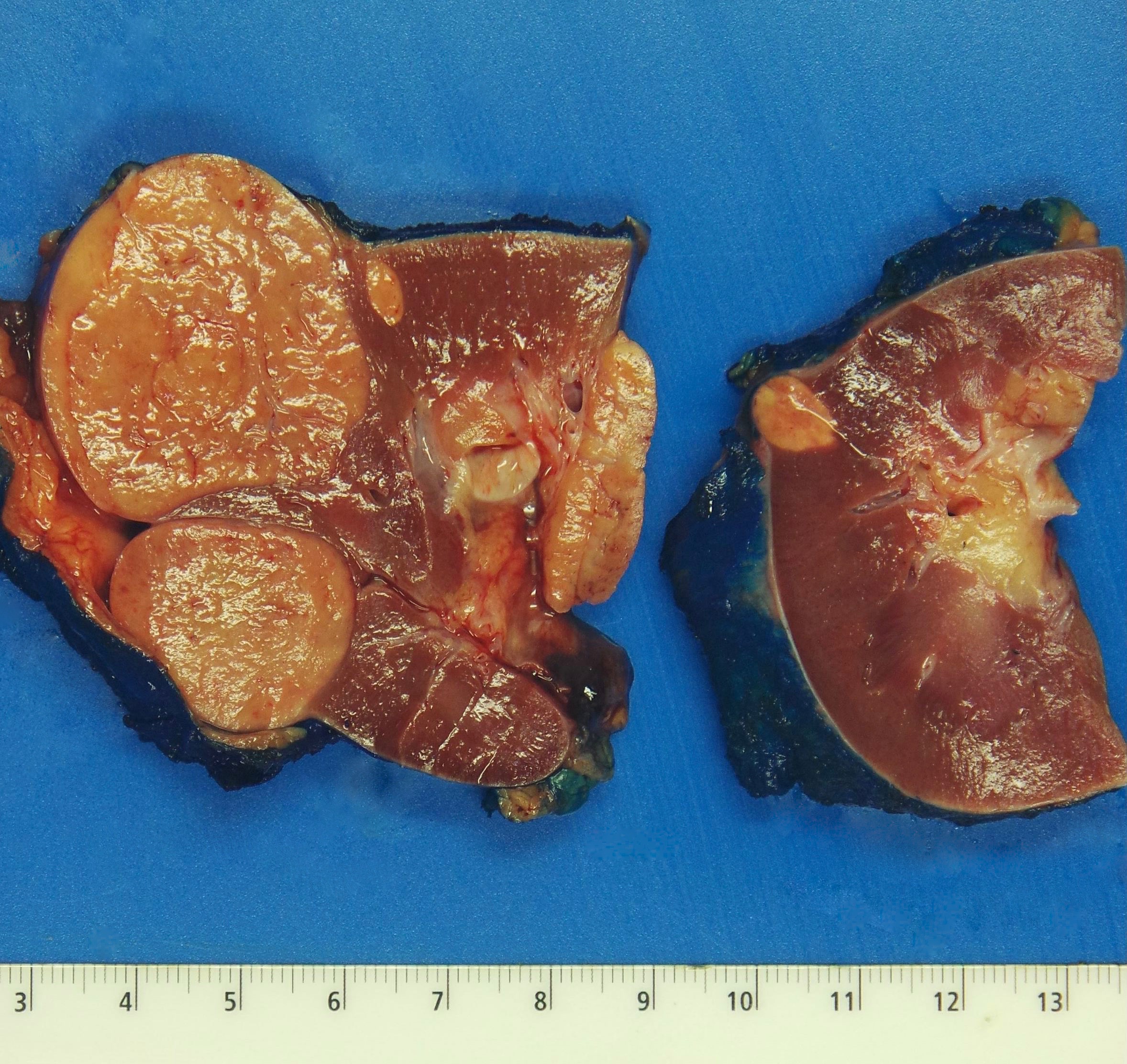
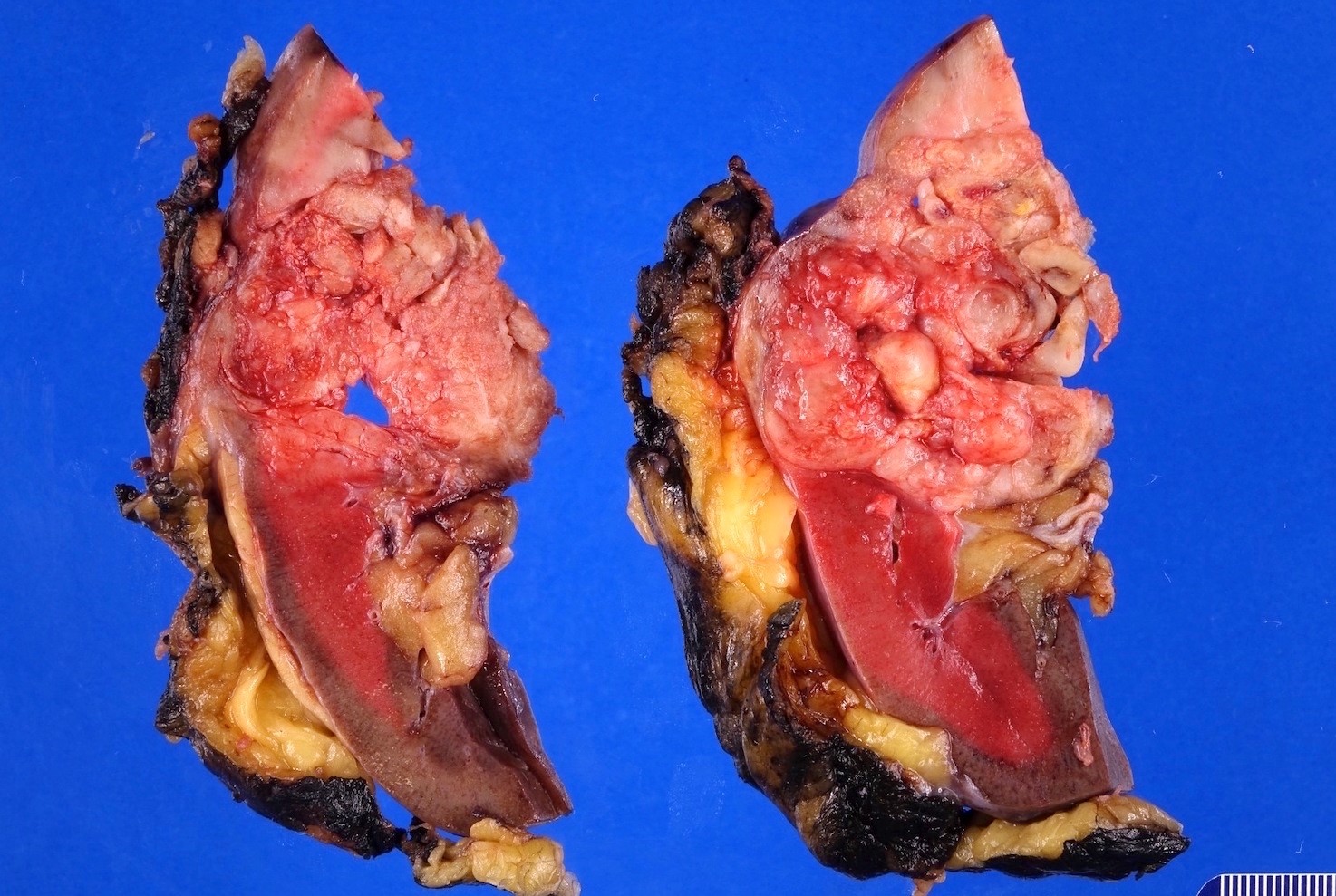
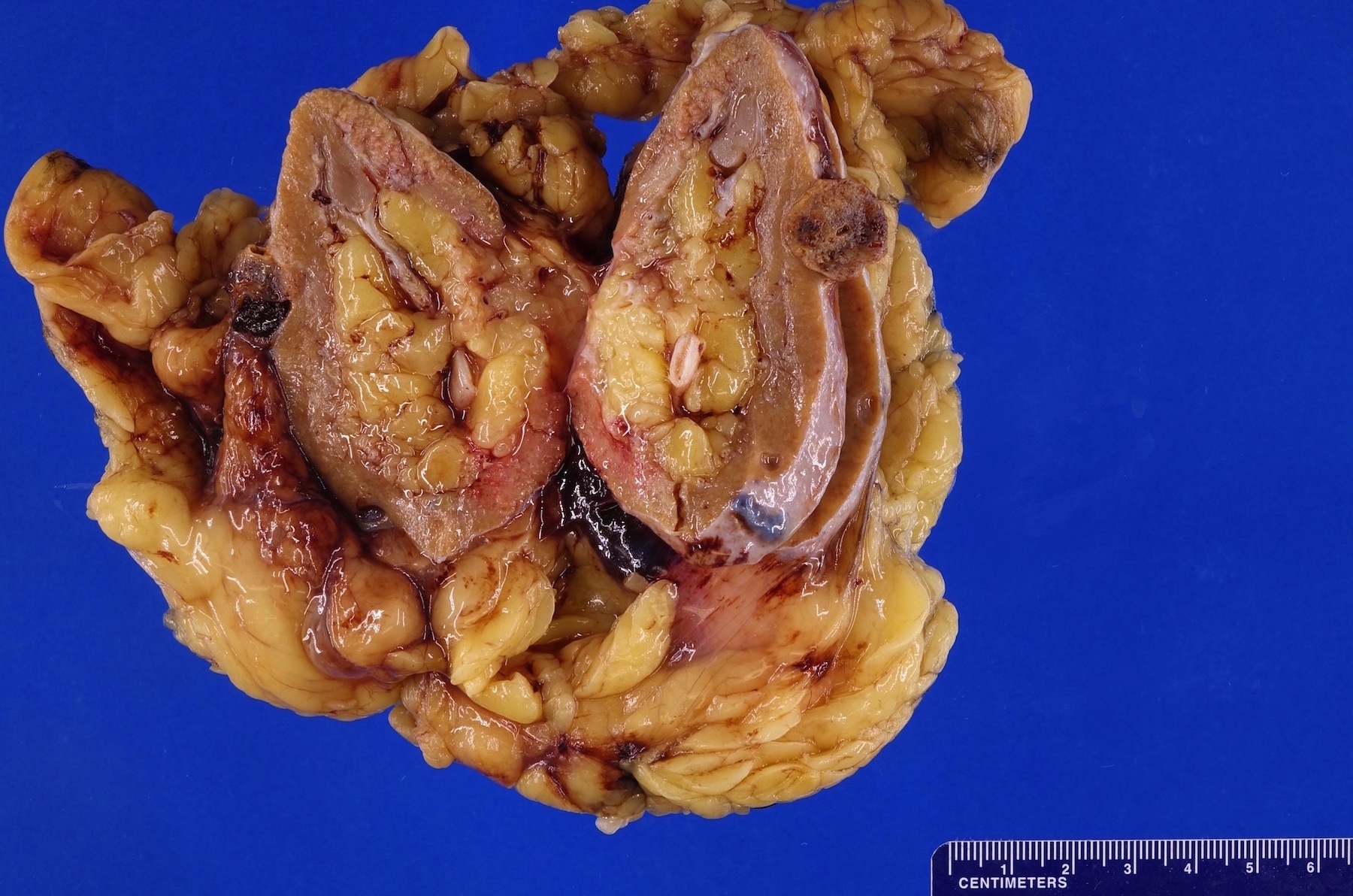
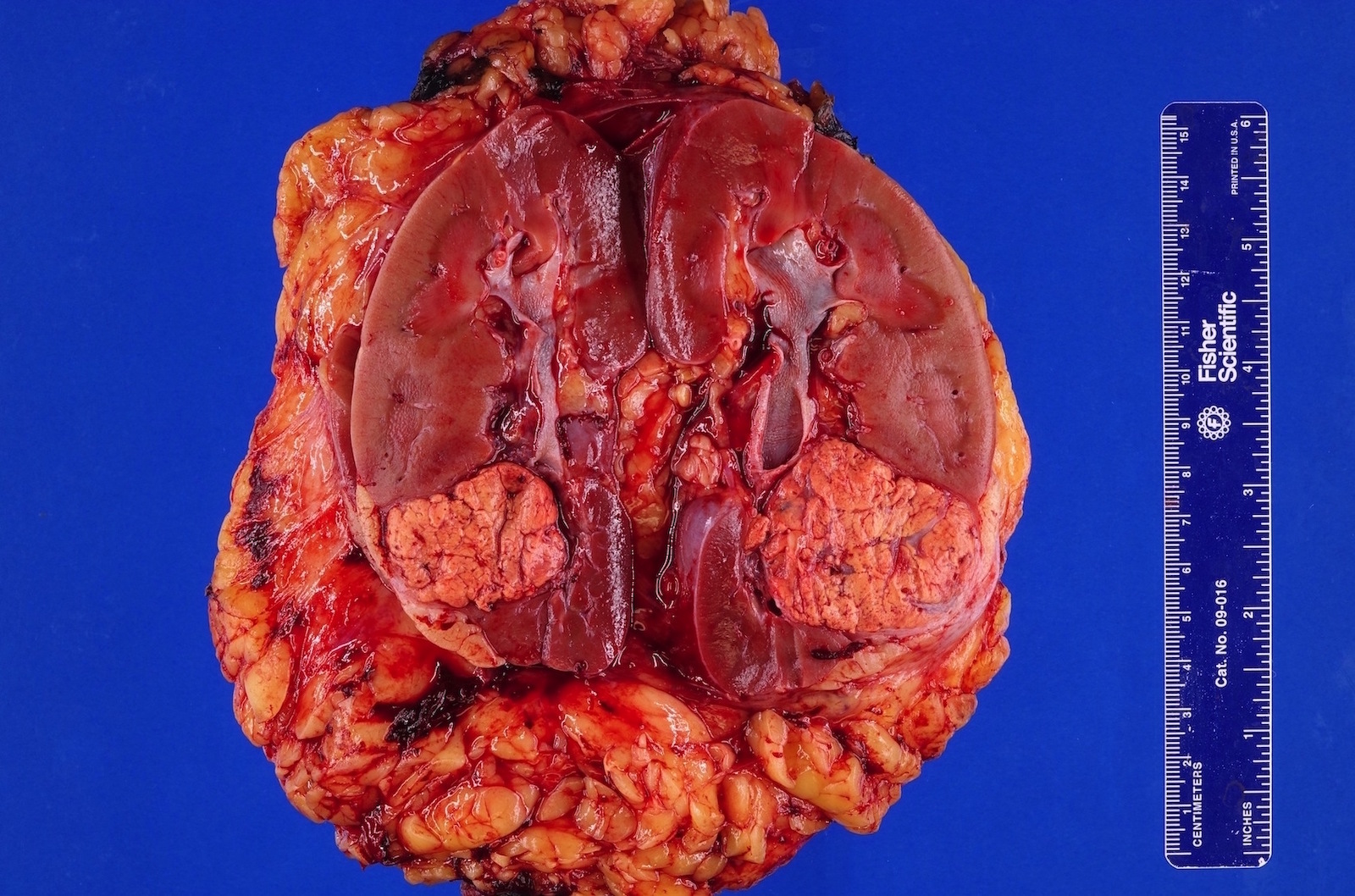
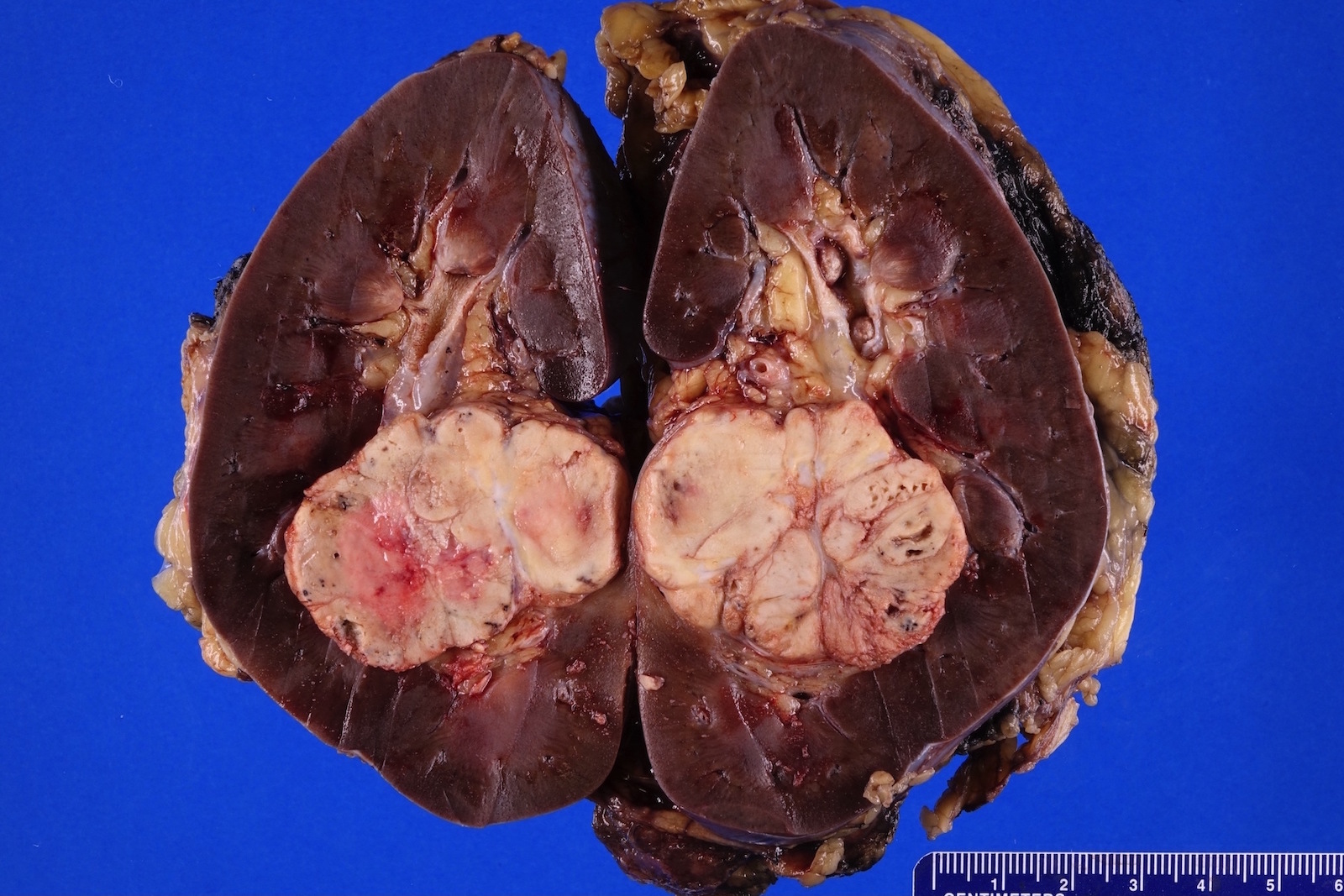
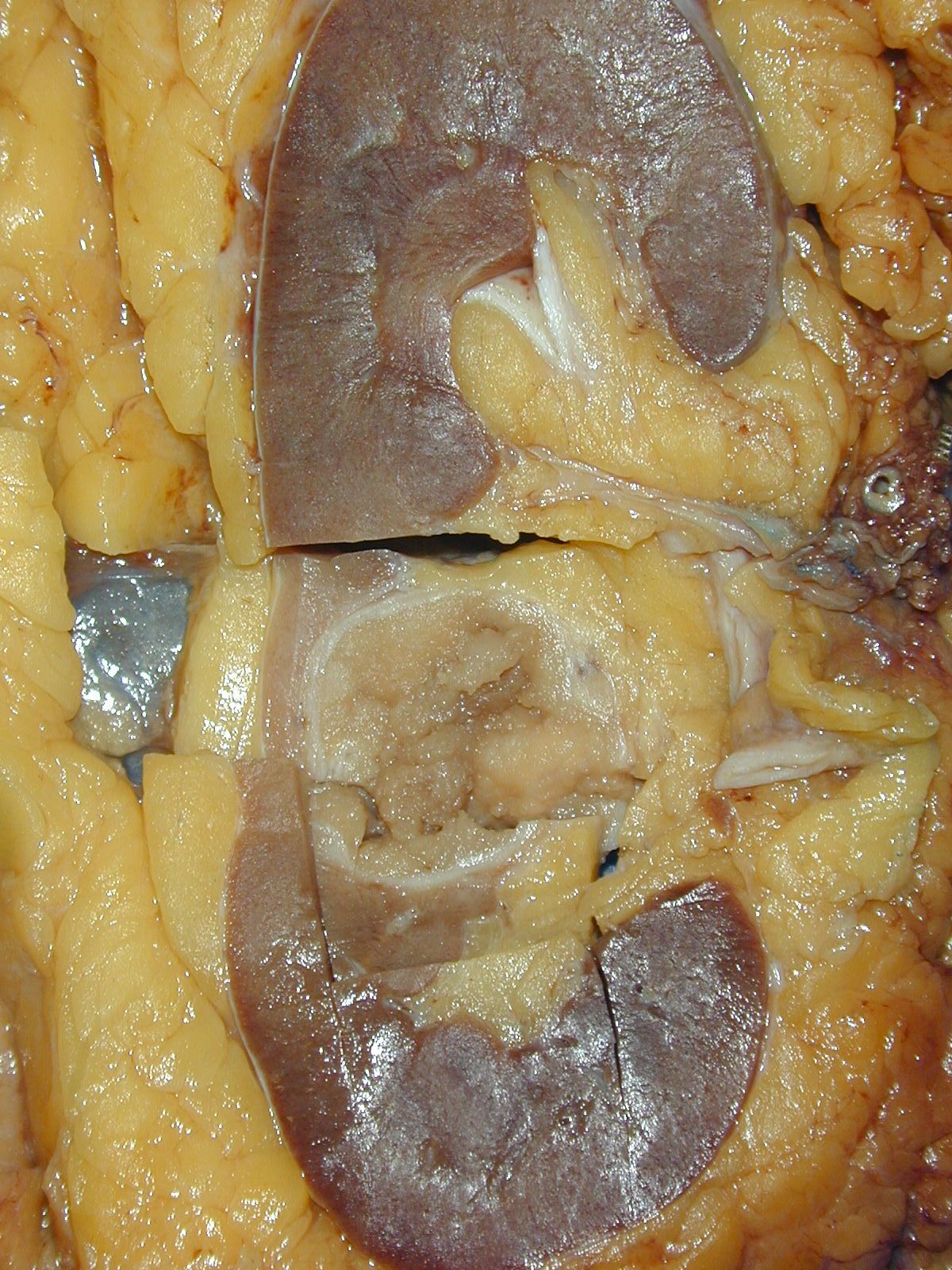
- Right kidney, radical nephrectomy:
- Acquired cystic disease associated renal cell carcinoma with the following features (see cancer summary report and comment)
- Size: 2.5 cm in greatest dimension
- Tumor stage: organ confined (pT1a)
- Margins: all surgical margins are negative for carcinoma
- Background end stage kidney with acquired cystic disease
- Comment: Intramural oxalate crystals and background of cystic end stage kidney are characteristic of this diagnosis. Immunohistochemical stains are positive for CD10, AMACR and AE1 / AE3 and negative for CK7, supportive of this diagnosis.
- Acquired cystic disease associated renal cell carcinoma with the following features (see cancer summary report and comment)
- Clear cell renal cell carcinoma:
- Both entities may have clear cells and nested architecture
- ACD-RCC has predominantly eosinophilic cells, variable cribriform, solid and papillary architecture, oxalate crystals and background cystic renal parenchyma
- CAIX is negative in ACD-RCC but positive in clear cell RCC
- Papillary renal cell carcinoma:
- Both entities may have papillary architecture and eosinophilic cells
- ACD-RCC has characteristic sieve-like architecture, oxalate crystals and background cystic renal parenchyma
- CK7 is usually negative in ACD-RCC but positive in papillary RCC
- Chromophobe cell renal cell carcinoma and oncocytoma:
- These tumors characterized by granular eosinophilic cells and solid architecture also observed in ACD-RCC but
ACD-RCC has characteristic sieve-like architecture, oxalate crystals and background cystic renal parenchyma
- CKIT is negative in ACD-RCC but positive in chromophobe RCC and oncocytoma
- These tumors characterized by granular eosinophilic cells and solid architecture also observed in ACD-RCC but
ACD-RCC has characteristic sieve-like architecture, oxalate crystals and background cystic renal parenchyma
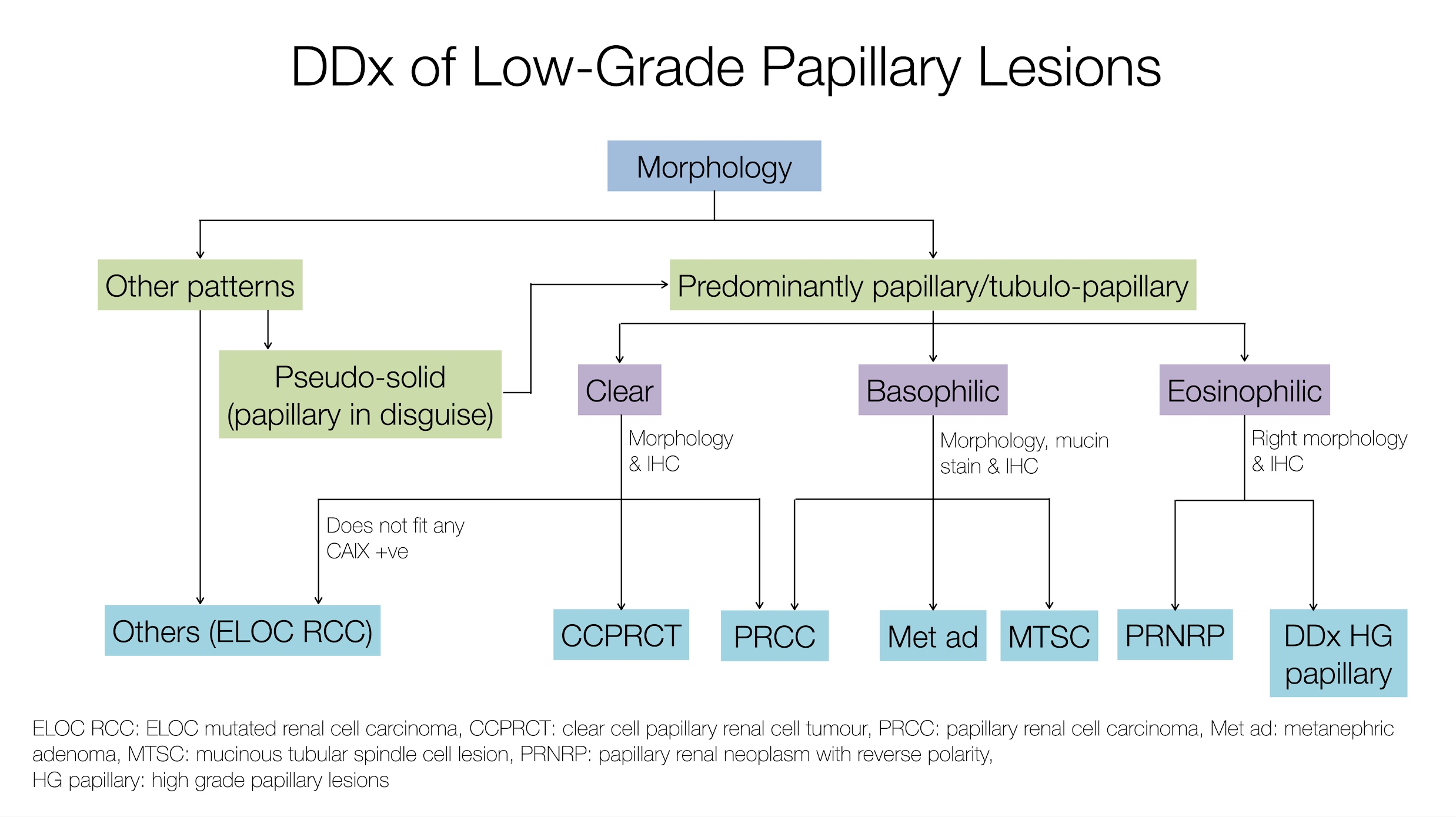
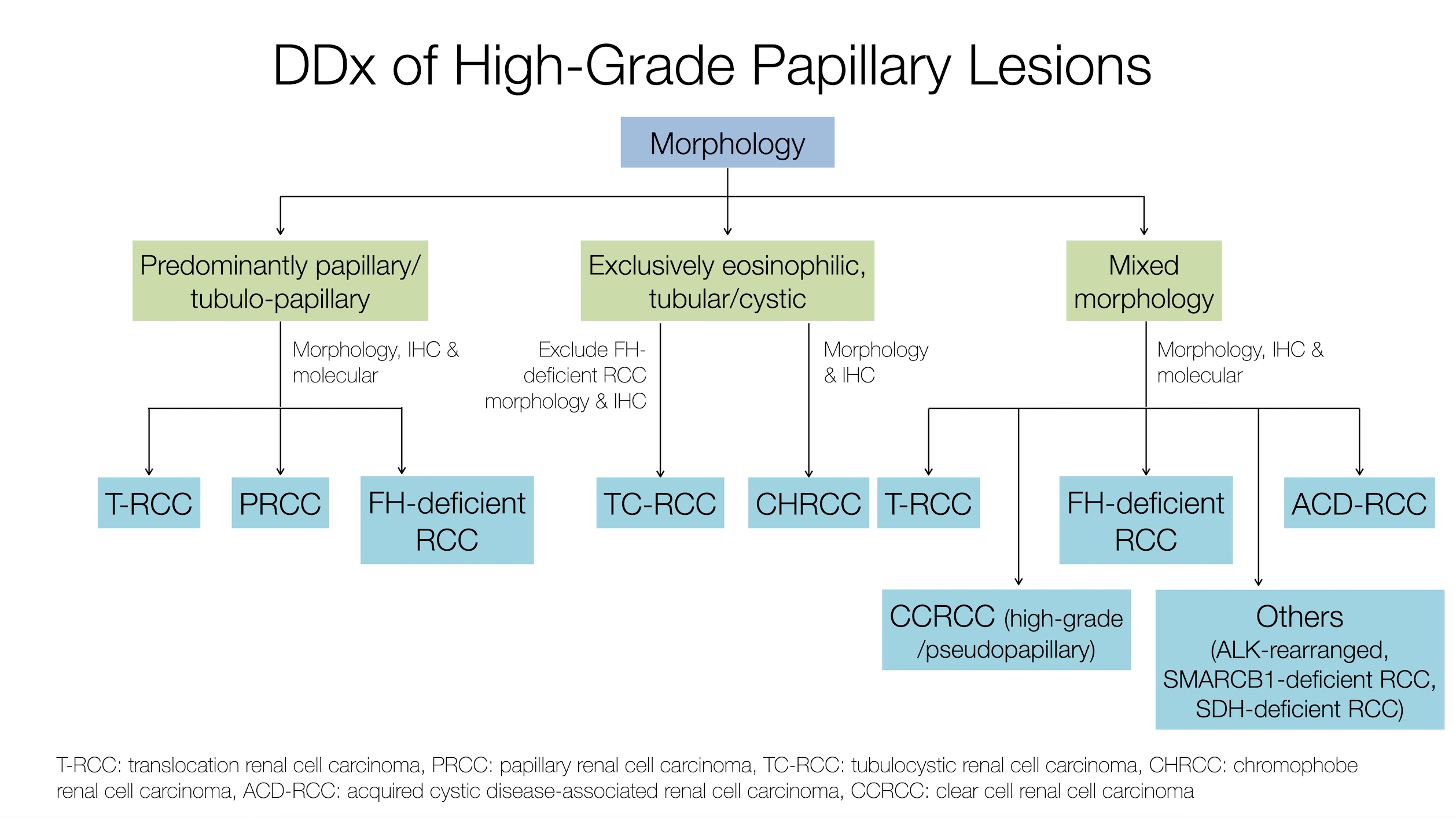
What is the most helpful feature to distinguish the tumor captured on this image from other RCCs?
- Intratumoral oxalate crystals and background of end stage renal disease (ESRD) with cysts
- Presence of papillary architecture and eosinophilic cells
- Specific recurrent cytogenetic abnormality
- Unique immunohistochemical profile
Comment Here
Reference: Acquired cystic disease associated renal cell carcinoma
- Aggressive tumors usually diagnosed at advanced stage
- CAIX can be used to differentiate it from clear cell RCC
- Intratumoral oxalate crystals are required to make the diagnosis
- The only tumor that arises in end stage kidney
Comment Here
Reference: Acquired cystic disease associated renal cell carcinoma
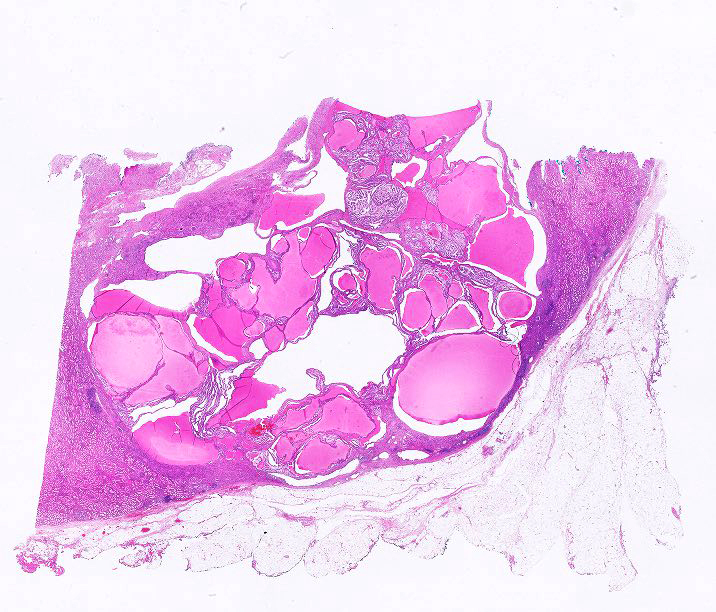
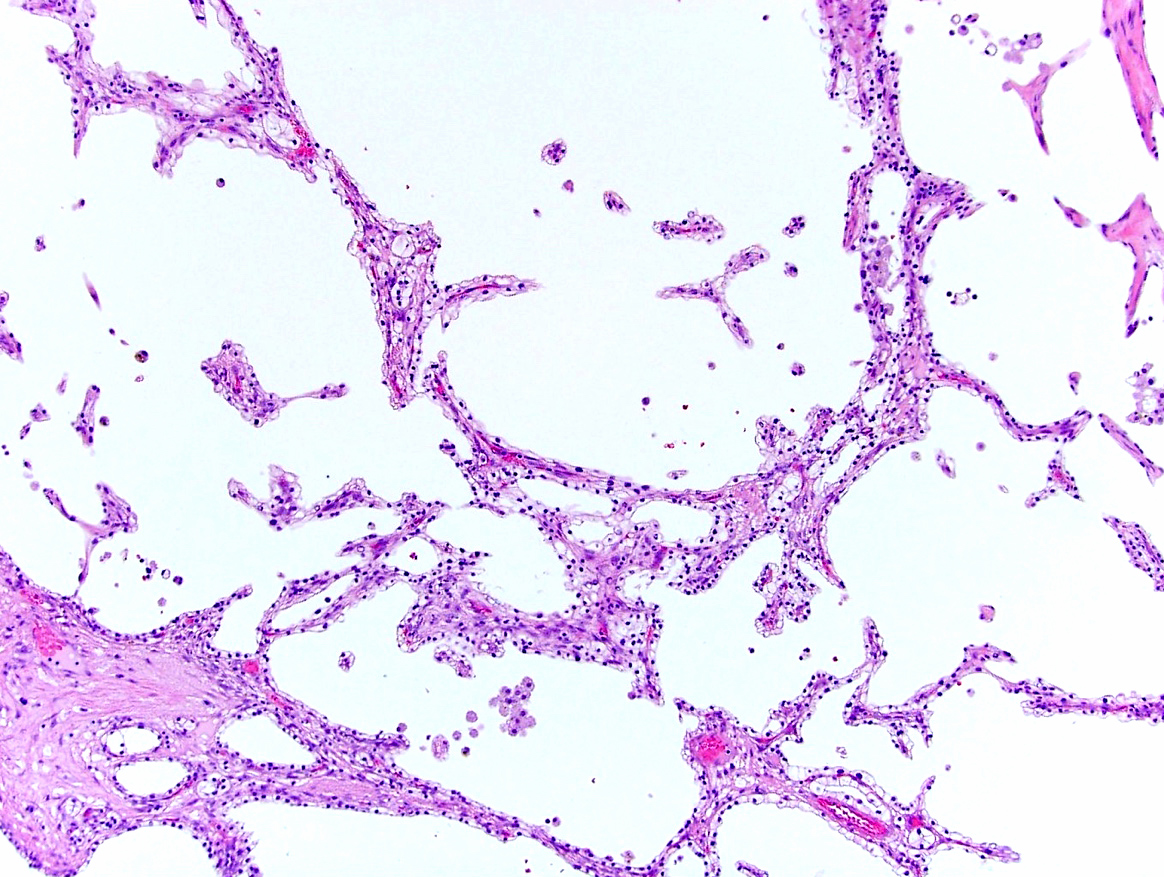
- Uncommon multilocular cystic tumor without a grossly appreciable solid component
- Classified as part of mixed epithelial and stromal tumor (MEST) family with MEST at opposite end of spectrum, by the 2016 World Health Organization (WHO) Classification
- Mainly occurs in adult women
- Well circumscribed, multicystic tumors with no apparent solid component
- Versus MEST - variable solid and cystic components
- Noncommunicating cysts lined by single layer epithelium separated by septa with hypocellular fibrous to hypercellular spindle cell stroma
- Most benign but very rare local recurrence and malignant transformation
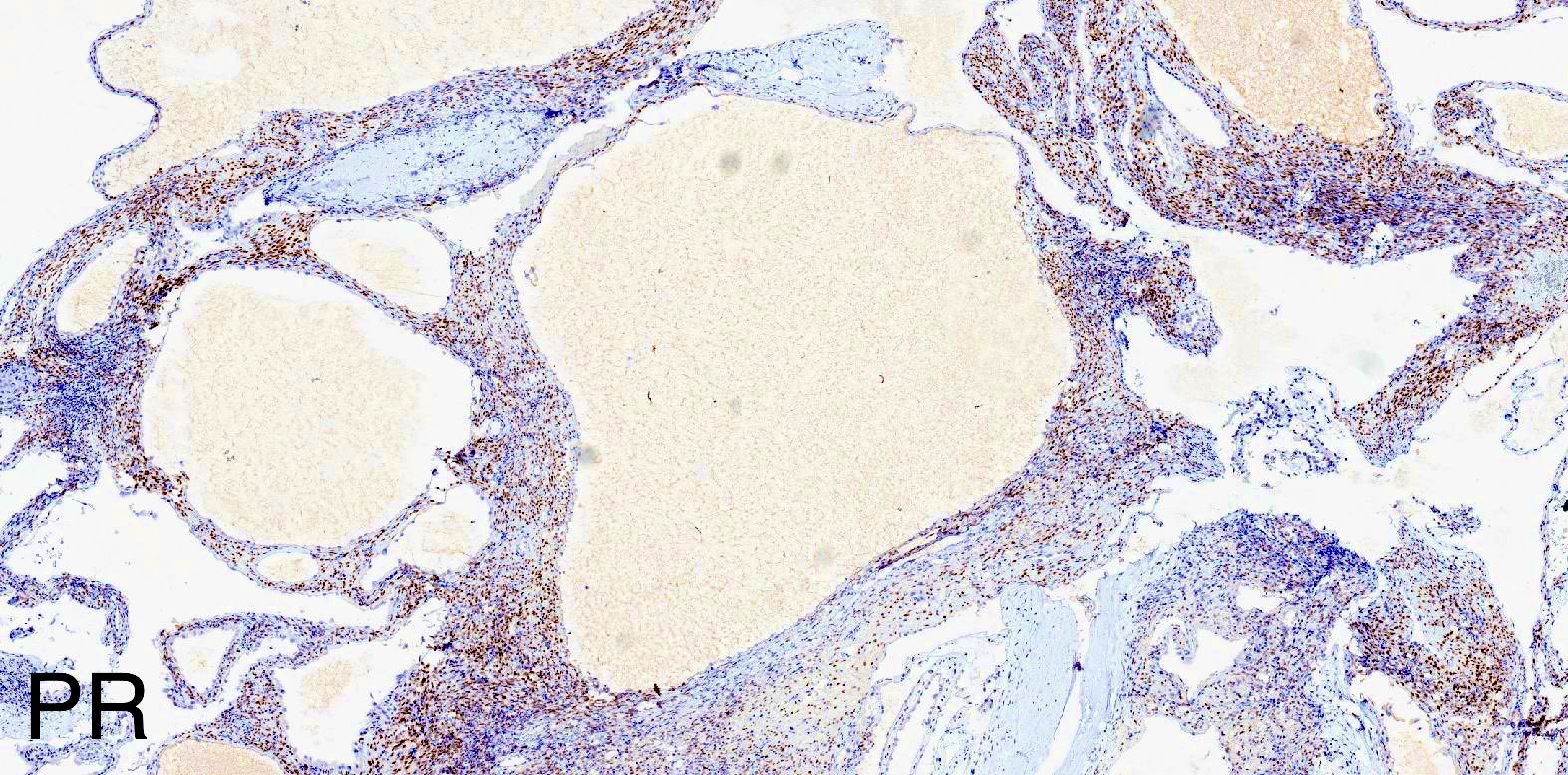
- Stromal cells typically positive for ER / PR
- Renal epithelial and stromal tumor (REST) (Am J Surg Pathol 2007;31:489)
- Multilocular cystic nephroma
- Multilocular renal cyst (term not recommended, does not reflect neoplastic nature of entity)
- ICD-O: 8959/0 - benign cystic nephroma
- M:F = 1:8 (Semin Diagn Pathol 1998;15:2, Arch Pathol Lab Med 2004;128:1404, Am J Surg Pathol 2007;31:489, Am J Surg Pathol 2016;40:1591)
- Age, peak incidence 50 - 60 years (Semin Diagn Pathol 1998;15:2, Am J Surg Pathol 2016;40:1591)
- Confined to kidney
- Located at poles of kidney, close to hilum or closely associated with pelvicalyceal system (Arch Pathol Lab Med 2004;128:1404, Urology 2008;71:1142)
- Possible role of hormones in pathogenesis (Am J Surg Pathol 2007;31:489)
- Often incidentally found
- Others present with abdominal / flank pain, hematuria, urinary tract infection, palpable mass (Urology 2008;71:1142)
- Diagnosis by histologic examination of tissue
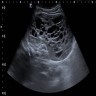
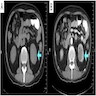
- Ultrasound (Urol Ann 2013;5:13, J Kidney Cancer VHL 2017;4:1):
- Multiple anechoic spaces traversed by thin septa and without vascularity
- Rarely, calcifications seen in septa
- CT (Urology 2008;71:1142, Urol Ann 2013;5:13):
- Well circumscribed, fluid density mass with multiple septations
- Enhancement of septations following contrast but contrast does not accumulate within individual loculi and calcification may be seen
- Most classified into Bosniak category III
- MRI (Urol Ann 2013;5:13):
- T1 hypointensity and T2 hyperintensity
- Mostly benign with rare local recurrence reported (Int J Surg Pathol 2015;23:238)
- Rarely aggressive
- Sarcomatous transformation (Semin Diagn Pathol 1998;15:2, Hum Pathol 2007;38:1432)
- Carcinomatous transformation (Int Braz J Urol 2006;32:187)
- 48 year old woman with adult cystic nephroma (Turk J Urol 2018;44:373)
- 57 year old woman with local recurrence of adult cystic nephroma (Int J Urol 2004;11:329)
- 59 year old woman with ureteral invagination of multilocular cystic nephroma (adult cystic nephroma) (Int J Clin Exp Pathol 2014;7:5271)
- 65 year old woman with cystic renal cell carcinoma arising from multilocular cystic nephroma (adult cystic nephroma) (Int Braz J Urol 2006;32:187)
- 66 year old man with adult cystic nephroma (Rare Tumors 2015;7:5860)
- Nephron sparing surgery whenever feasible
- Unilateral but rarely bilateral (Urology 2008;71:1142, Am J Surg Pathol 2009;33:72)
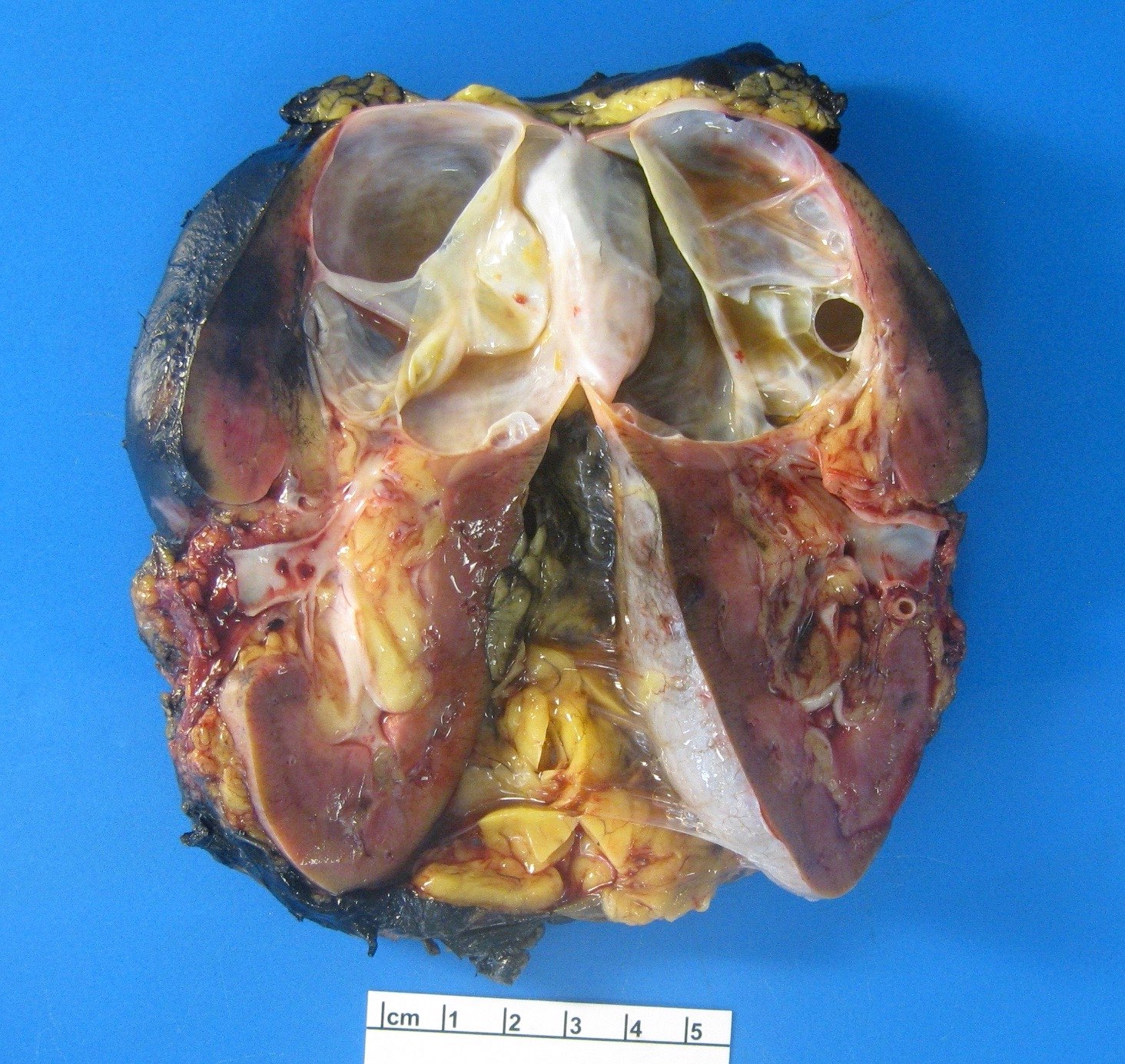
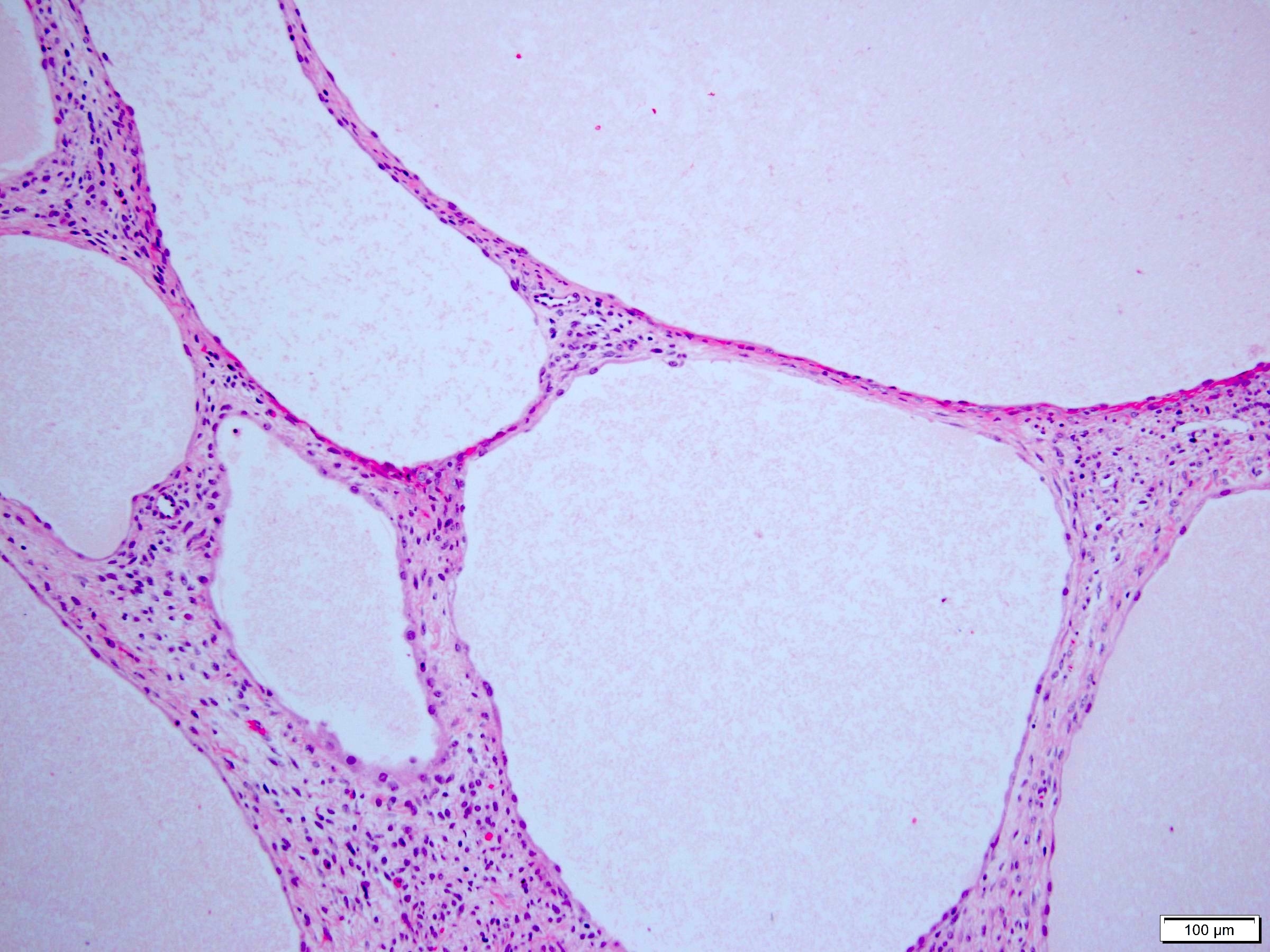
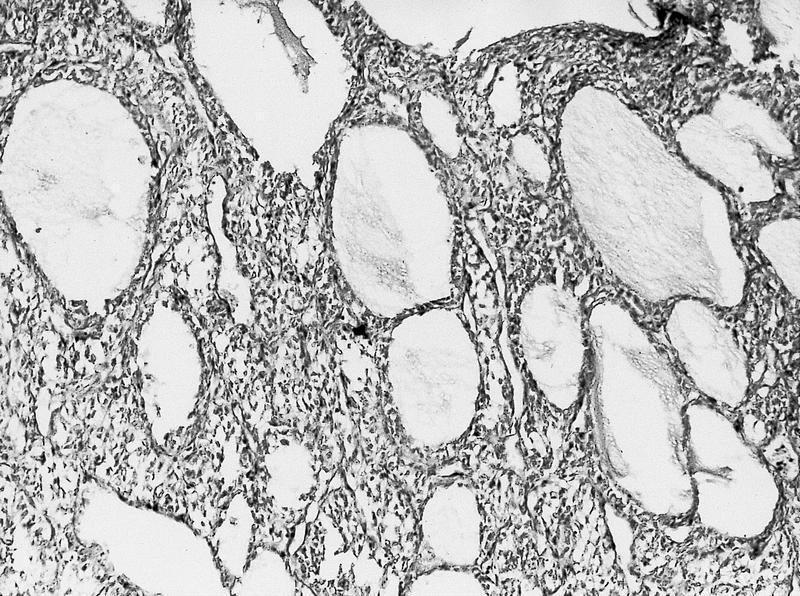
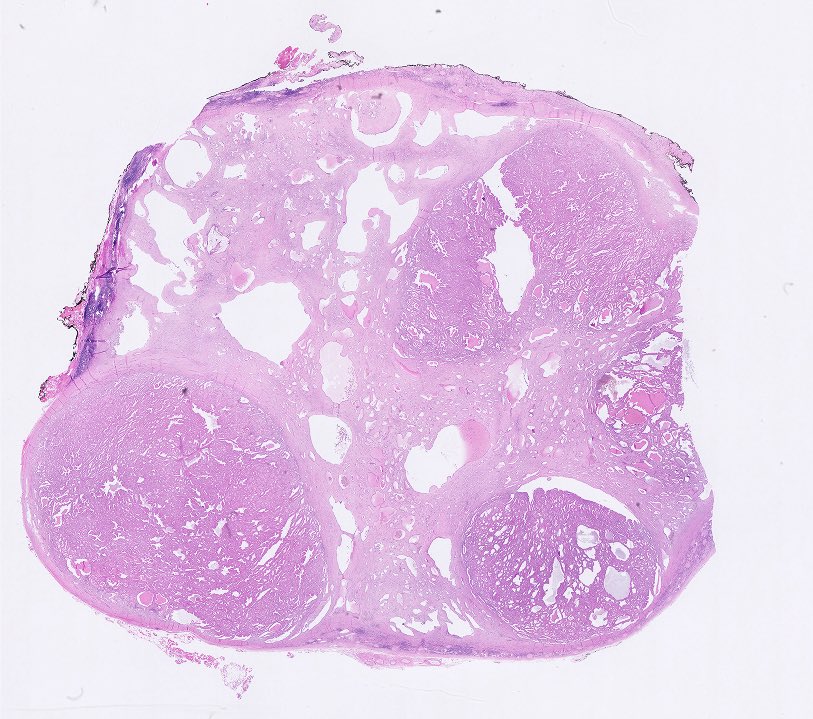
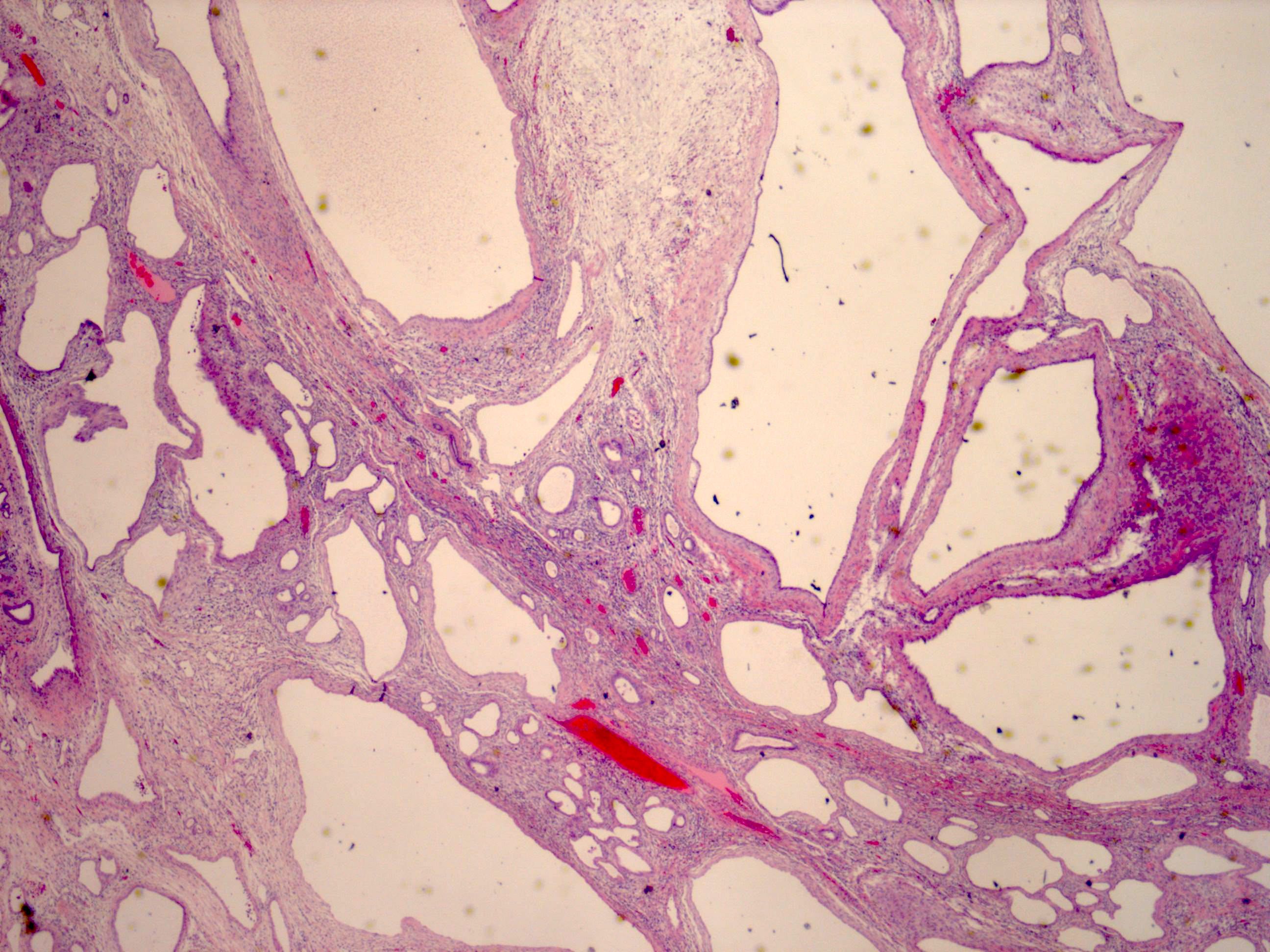
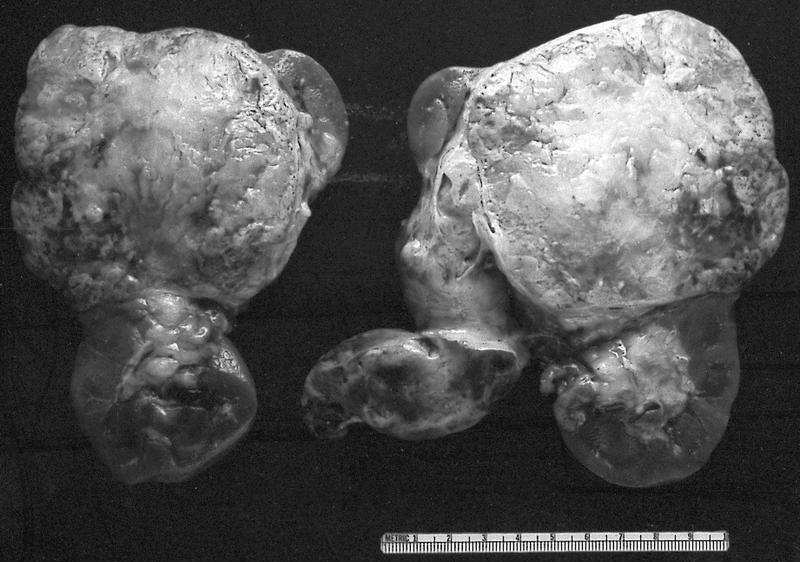
- Well circumscribed, multilocular cystic mass
- Median size: 6.8 cm (Am J Surg Pathol 2016;40:1591)
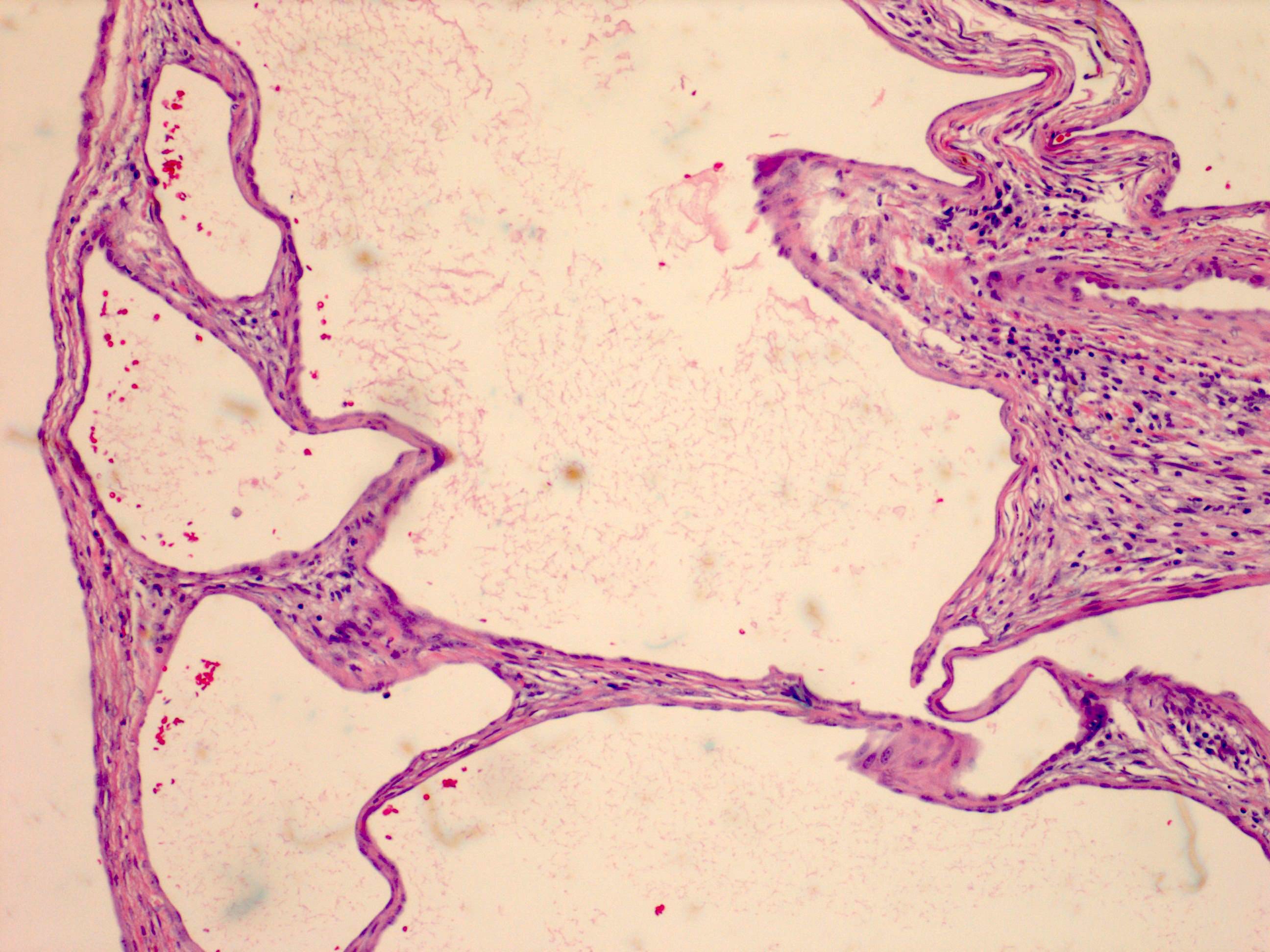
- Noncommunicating thin walled cysts of varying sizes
- Smooth and glistening linings
- Filled with serous to serosanguinous fluid
- Many with at least a partial pseudocapsule
- Entirely composed of cysts separated by septa (Semin Diagn Pathol 1998;15:2, Arch Pathol Lab Med 2004;128:1404, Am J Surg Pathol 2007;31:489, Eur Urol 2008;54:1237, Am J Surg Pathol 2016;40:1591)
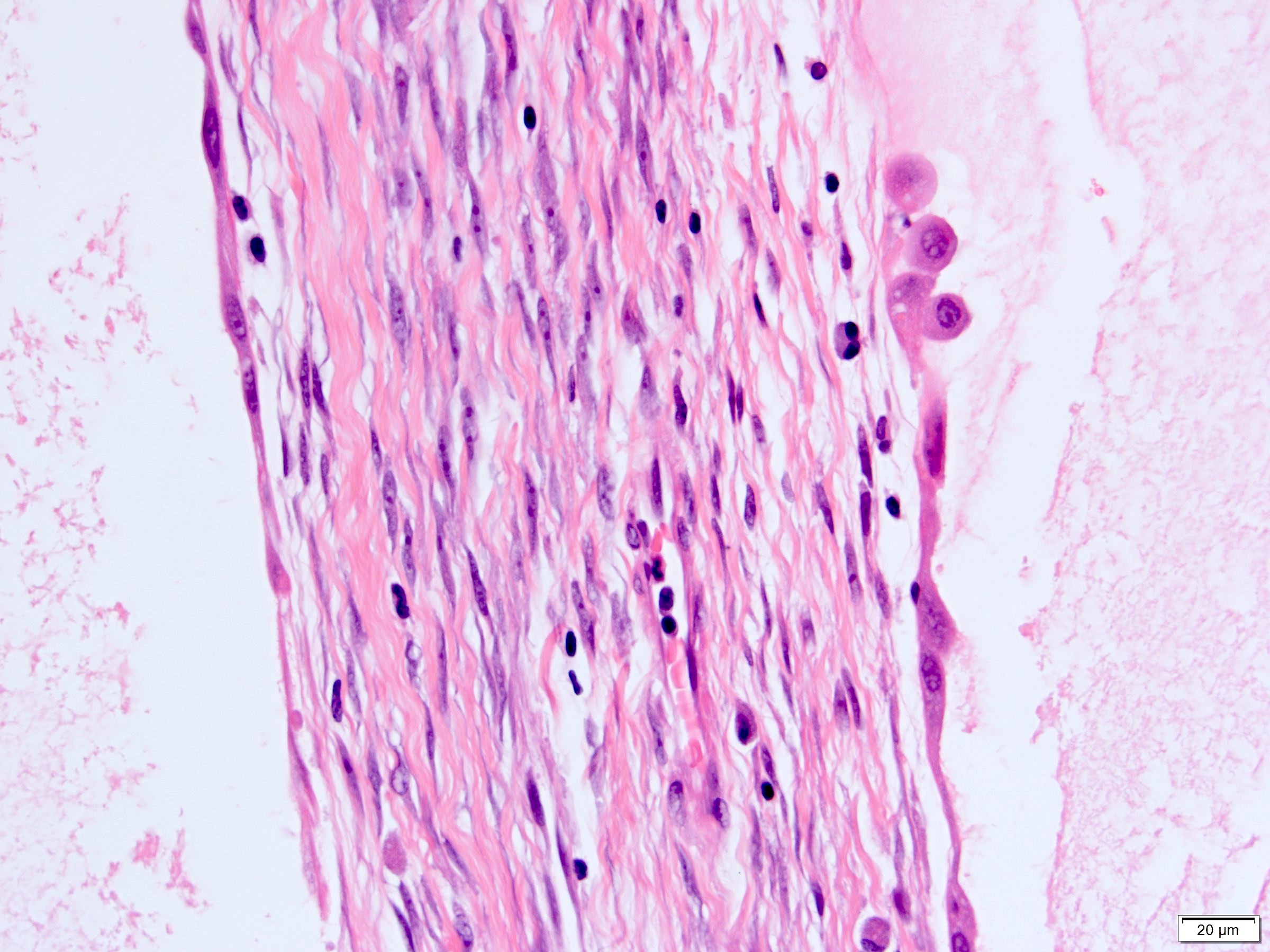
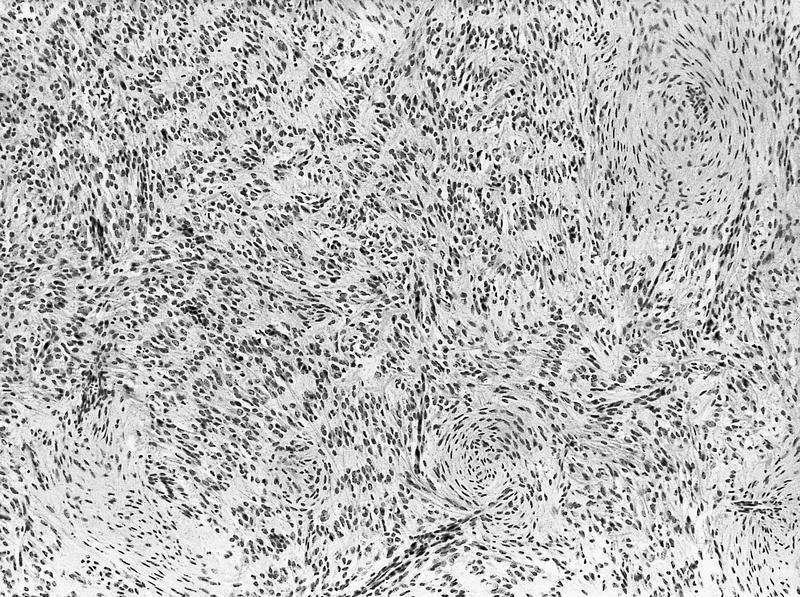
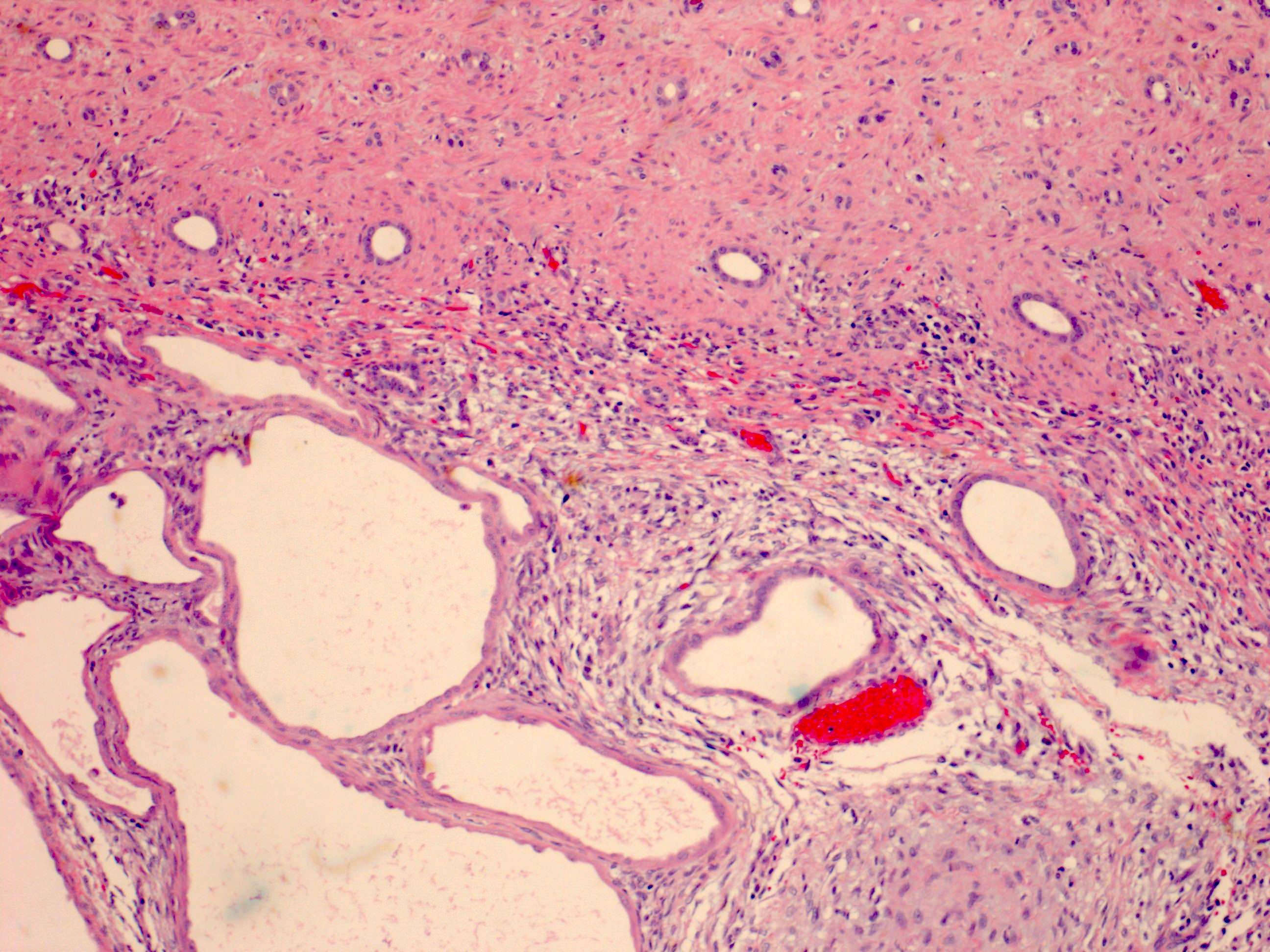
- Stroma:
- Hypocellular to hypercellular
- Collagenous and fibrous to edematous and myxoid
- Areas of hyalinized stroma with contours resembling ovarian corpora albicantia
- Spindle cells; closely packed areas resemble ovarian stroma
- Cellular foci embedded with epithelial elements ranging from handful of cells with no lumen to tiny cysts with pinpoint lumens and to slightly larger cysts
- Steroidogenic cells: small clusters of polygonal cells with amphophilic cytoplasm and round nuclei, frequently around epithelial component
- Calcifications, multinucleated giant cells, foamy or hemosiderin laden macrophages and focal chronic inflammation
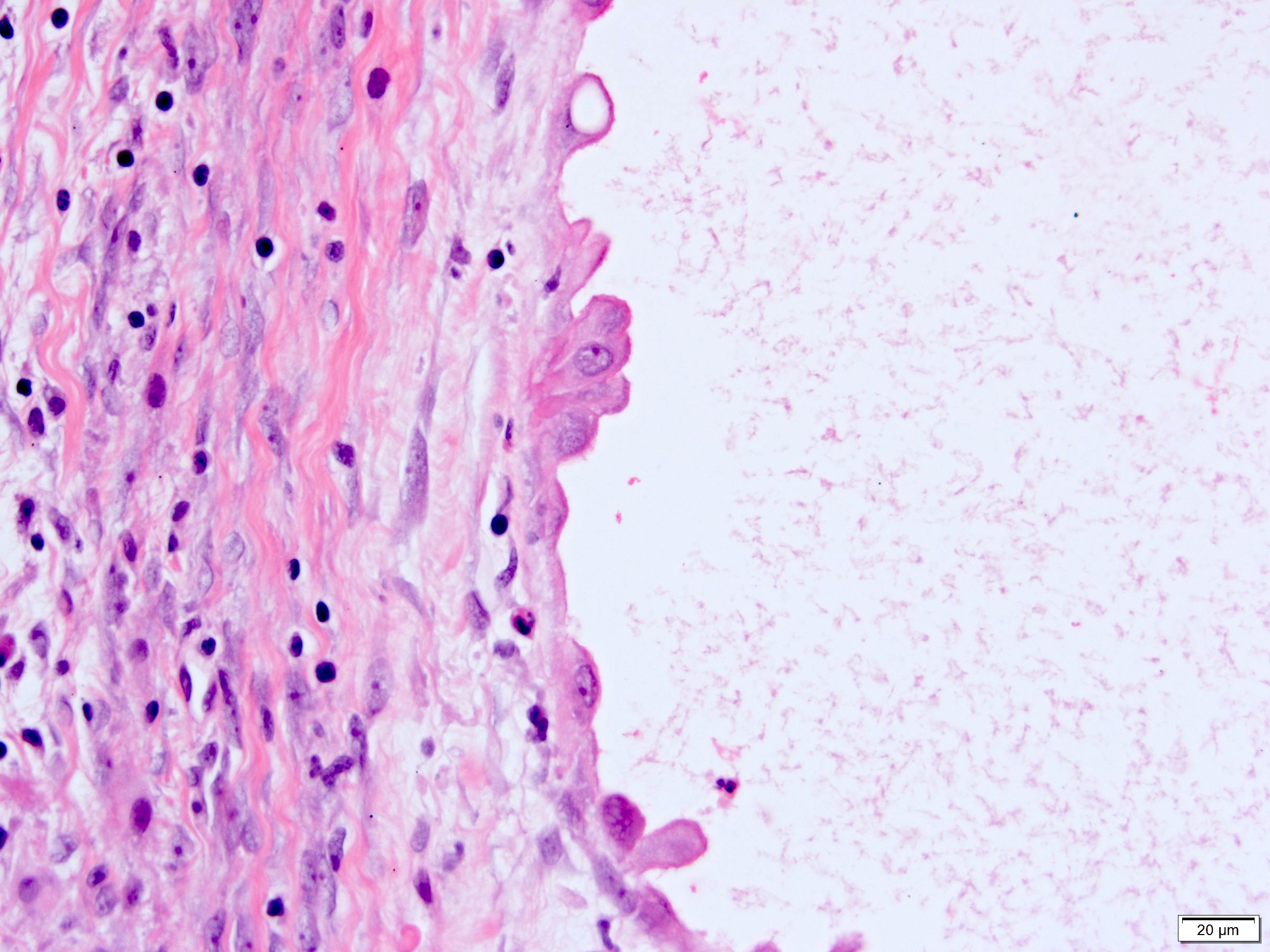
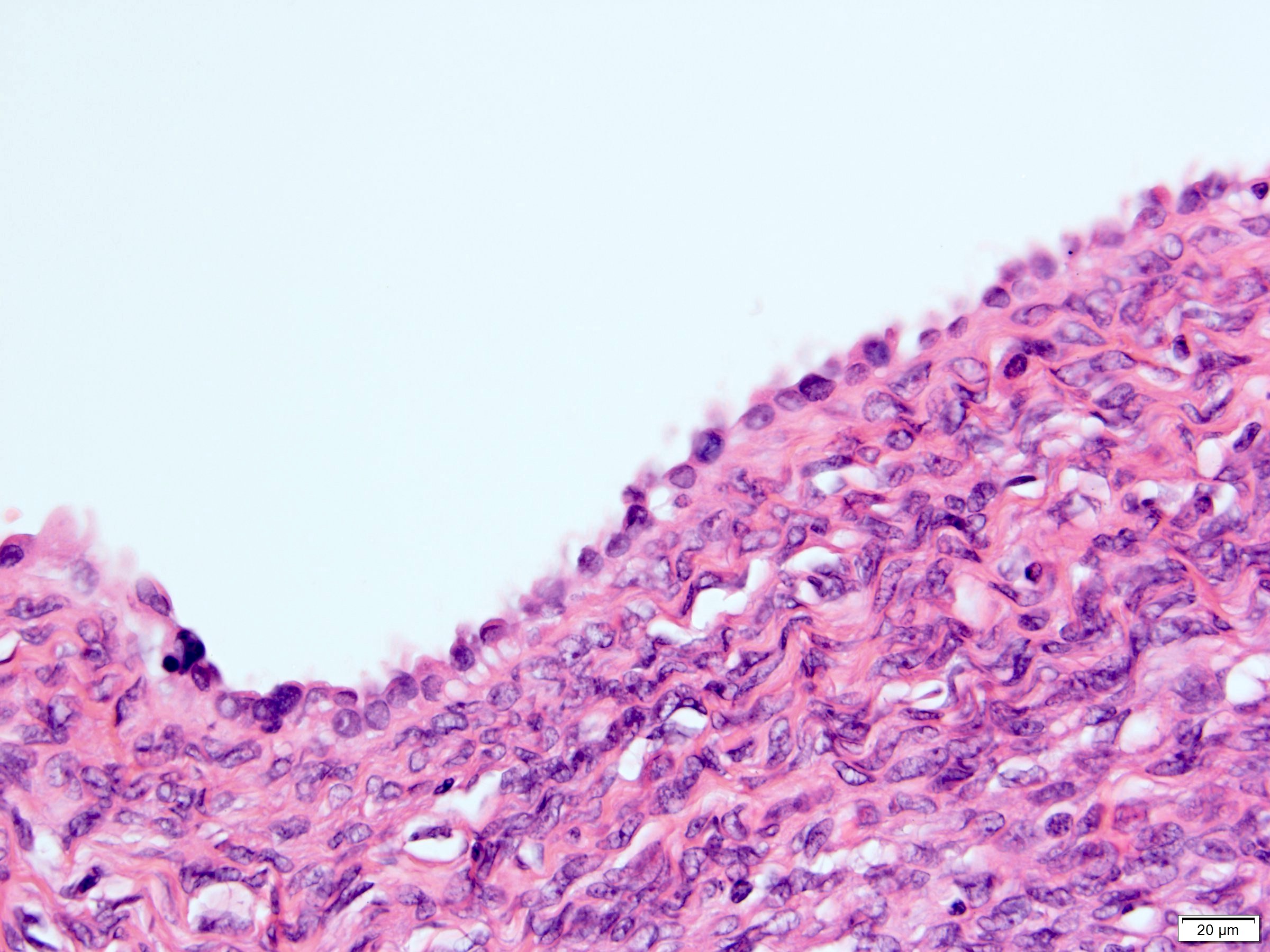
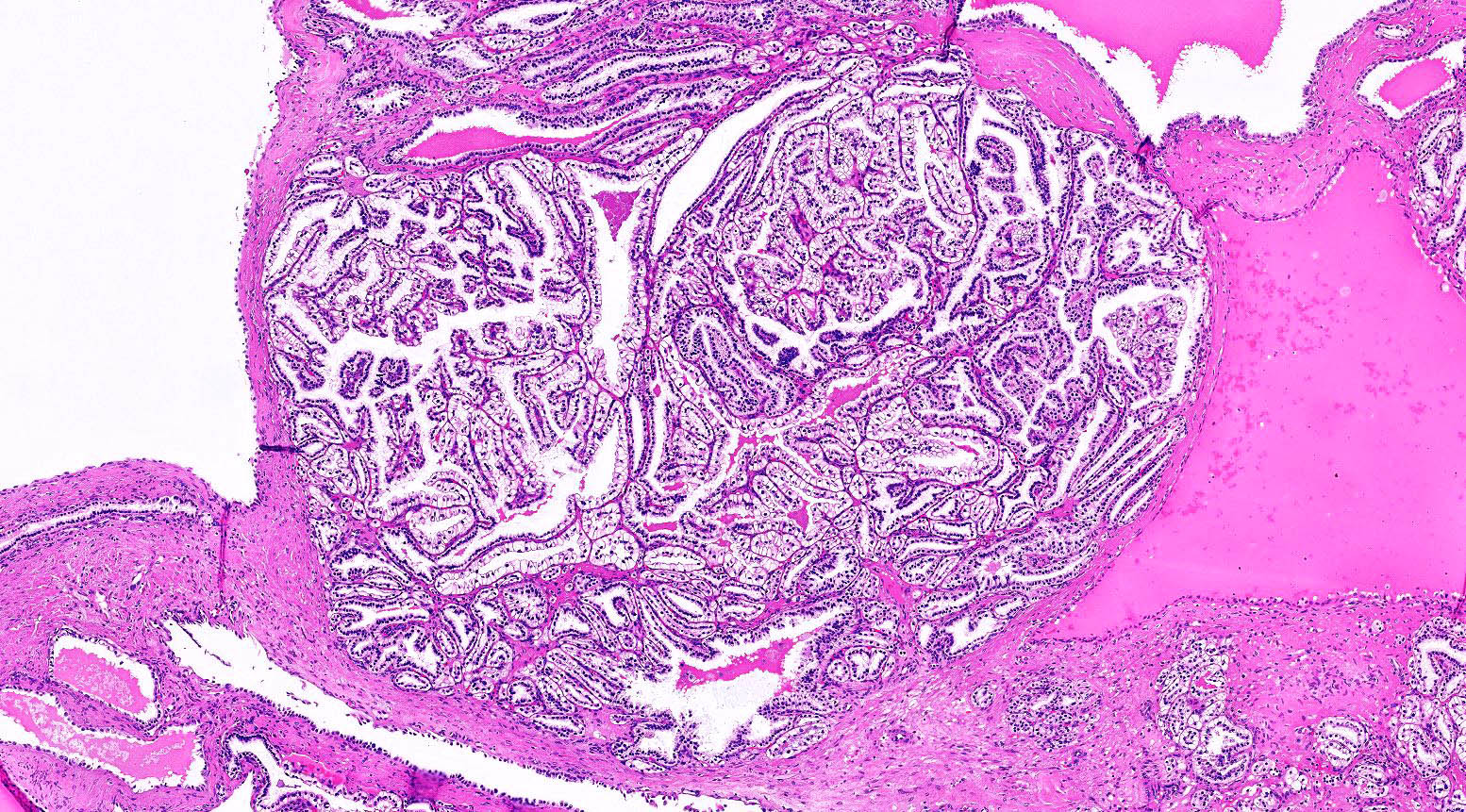
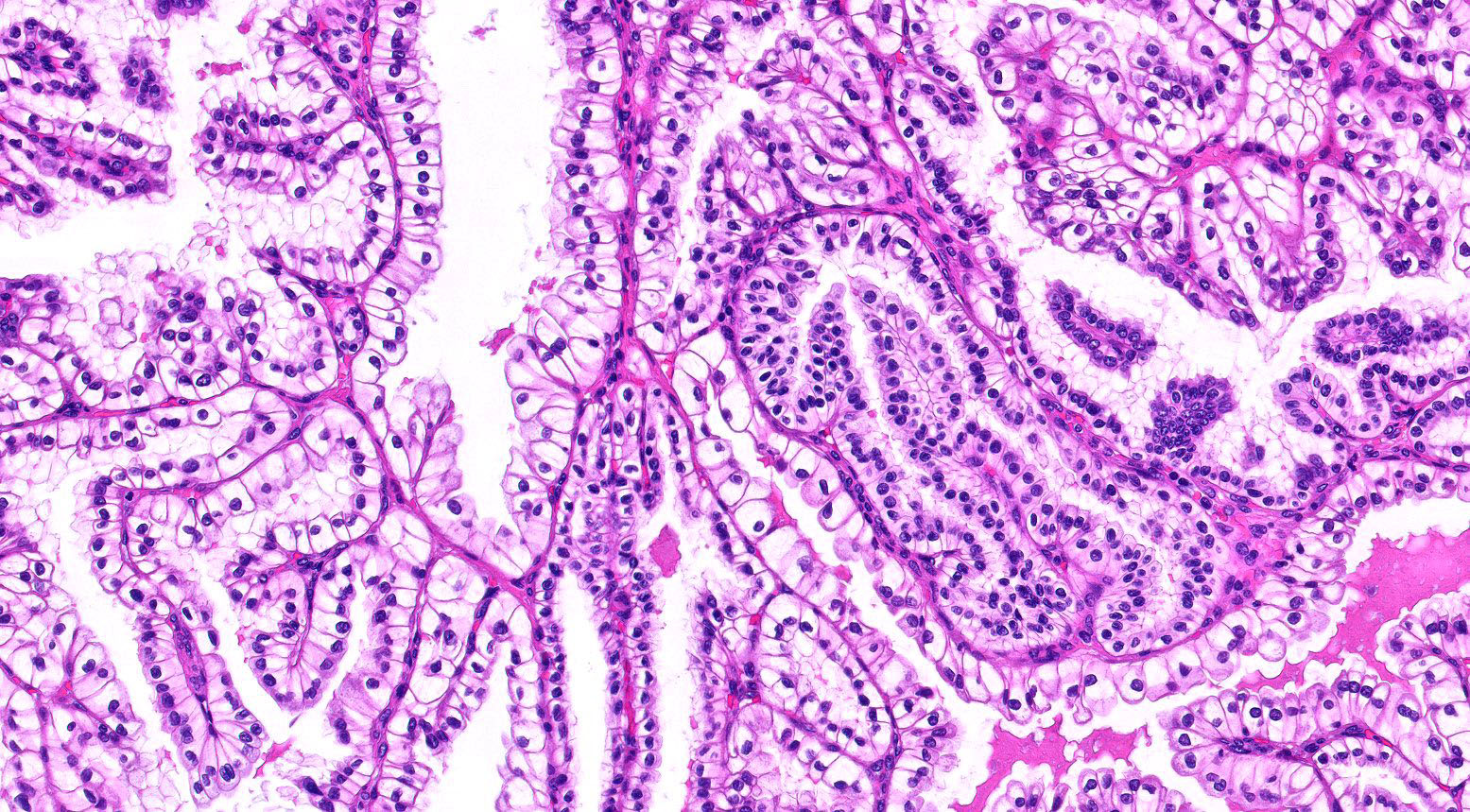
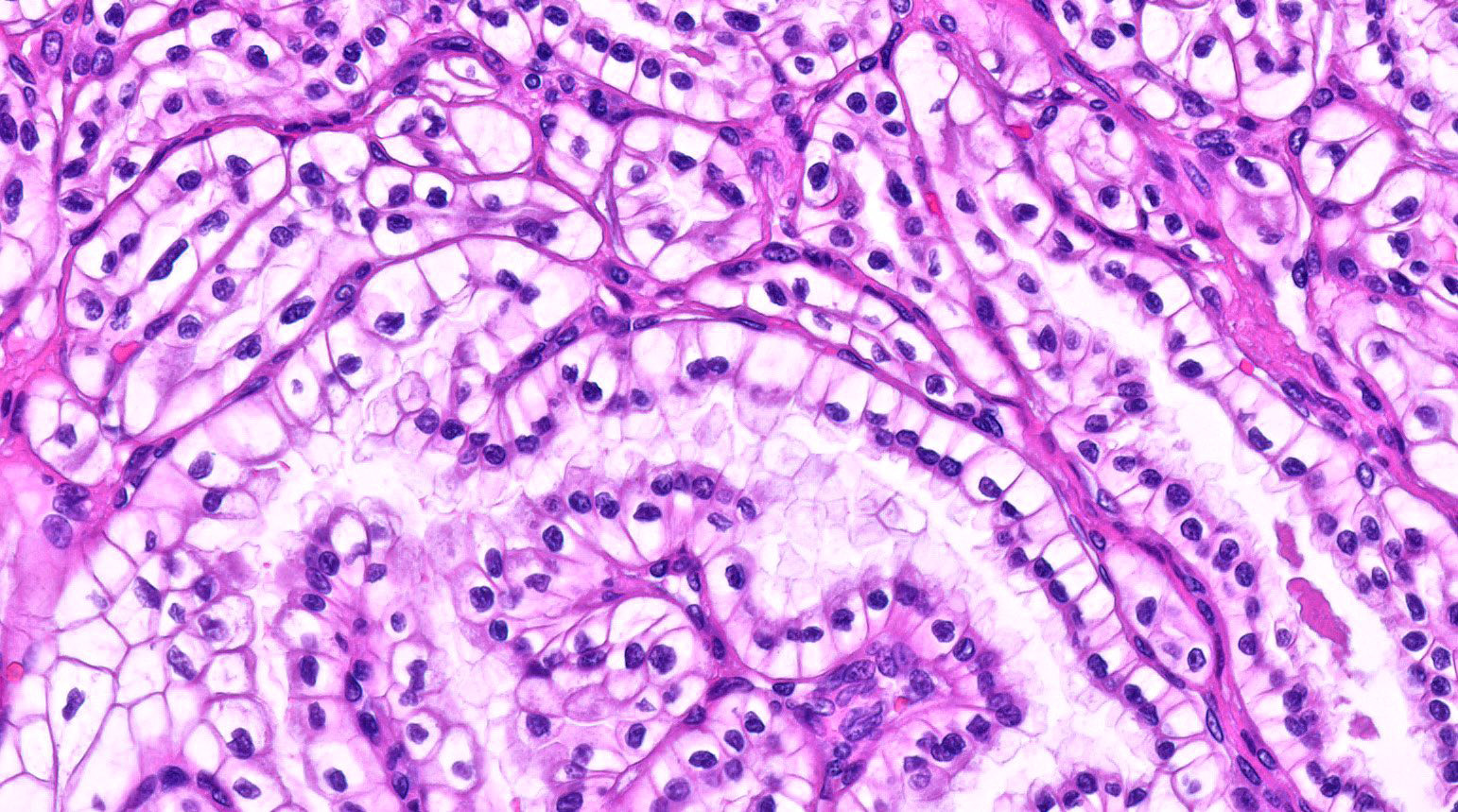
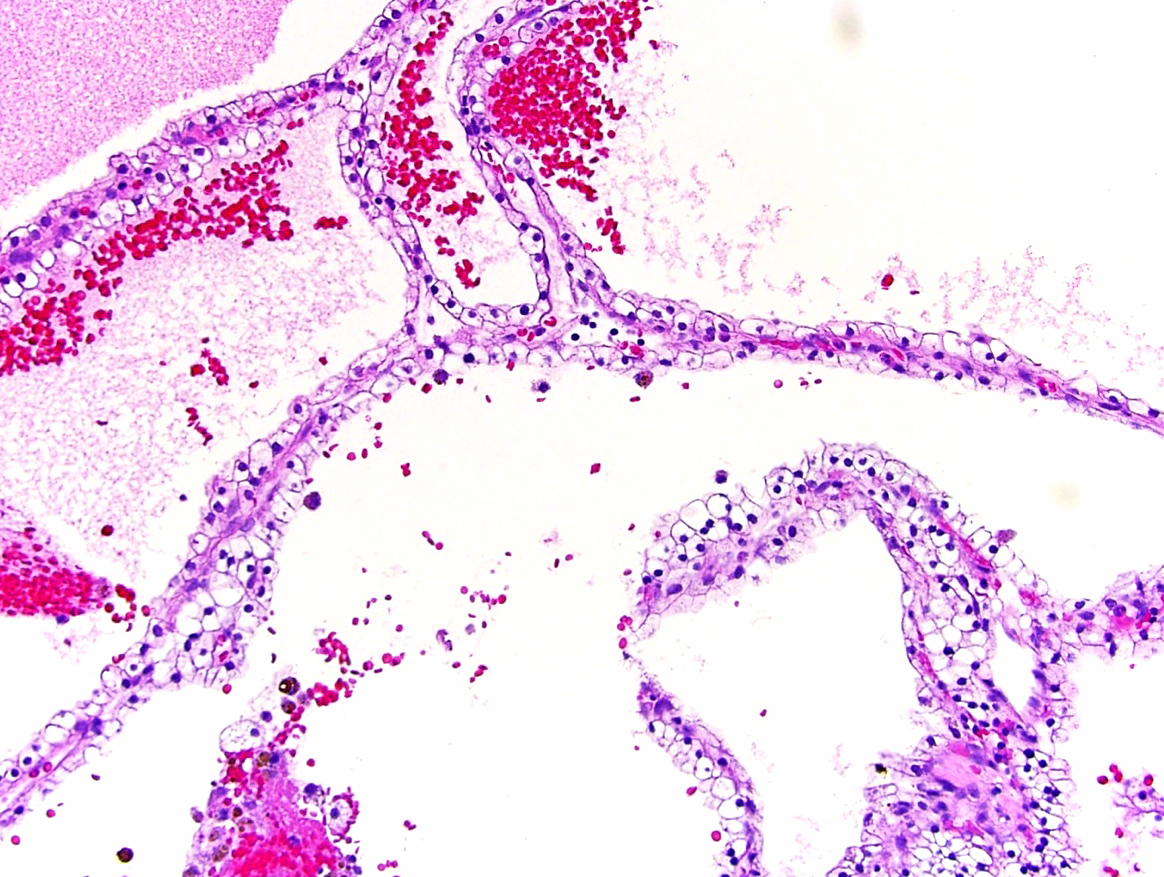
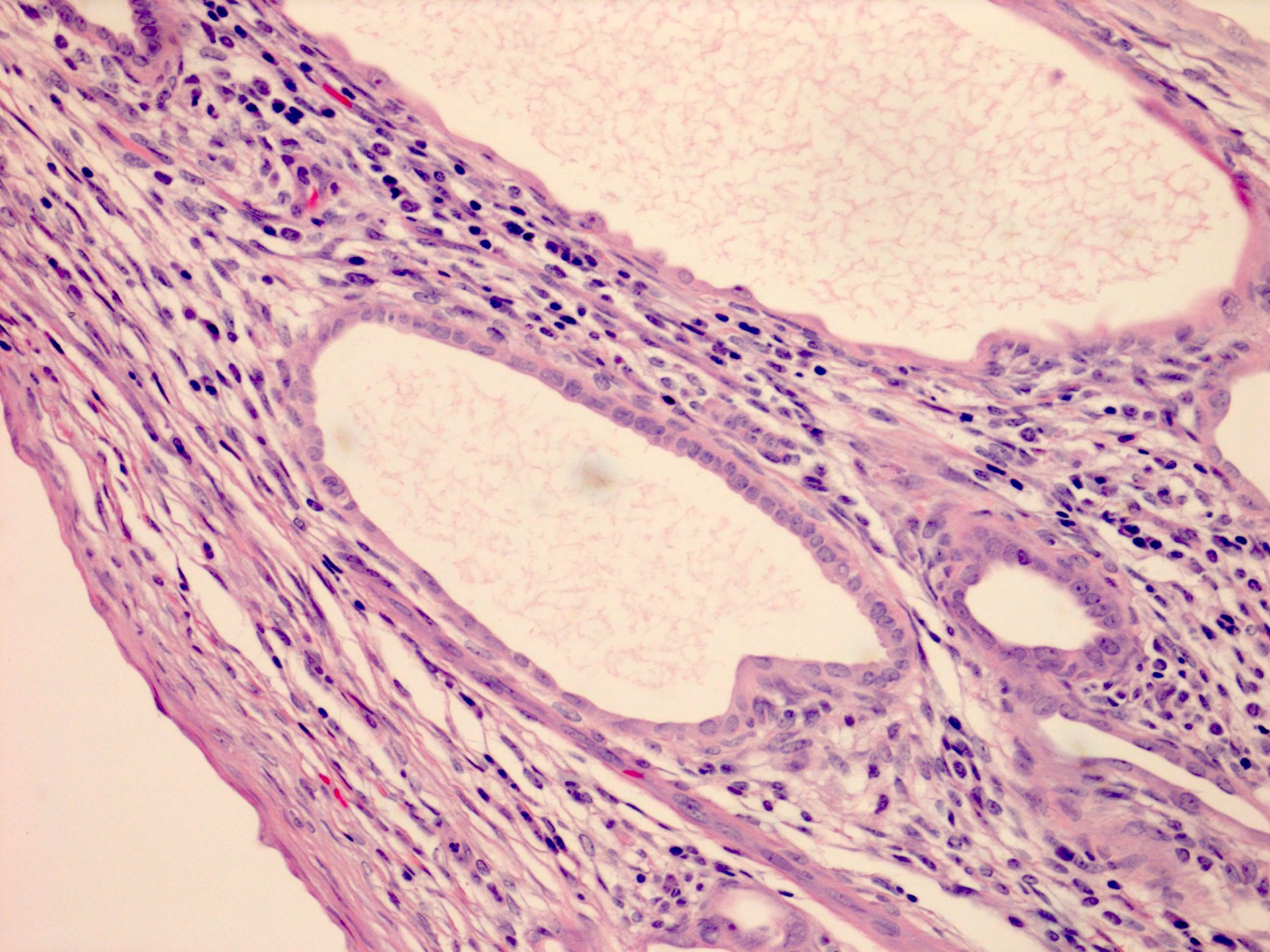
- Epithelium:
- Cells lining cysts
- Mostly arranged in single layer with various morphology: flat, cuboidal, hobnail, clear cell
- Rarely, foci of blunt and delicate papillae or foci of multiple layers of epithelium
- Minimal cytologic atypia
- Rare necrosis, no mitosis
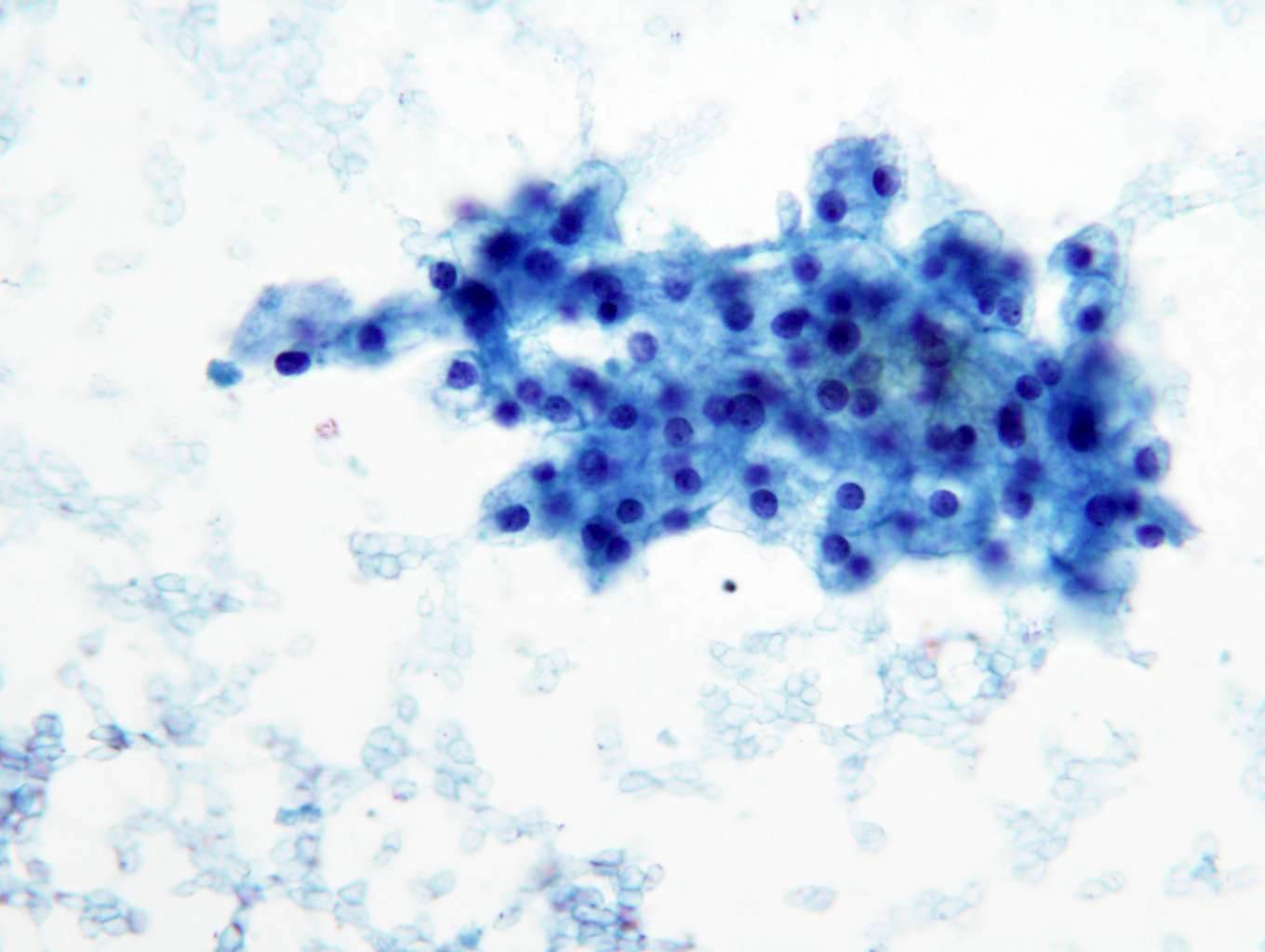
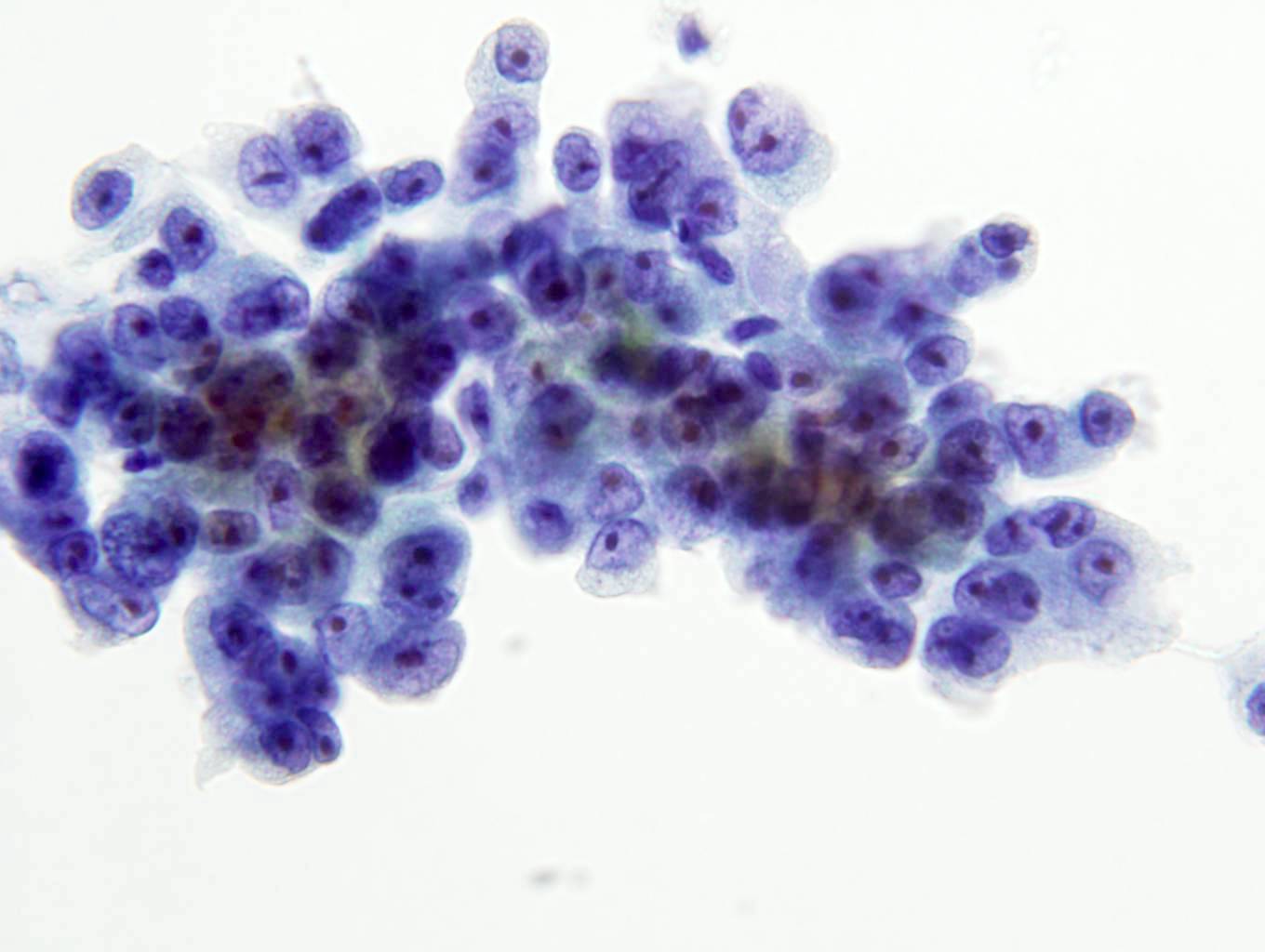
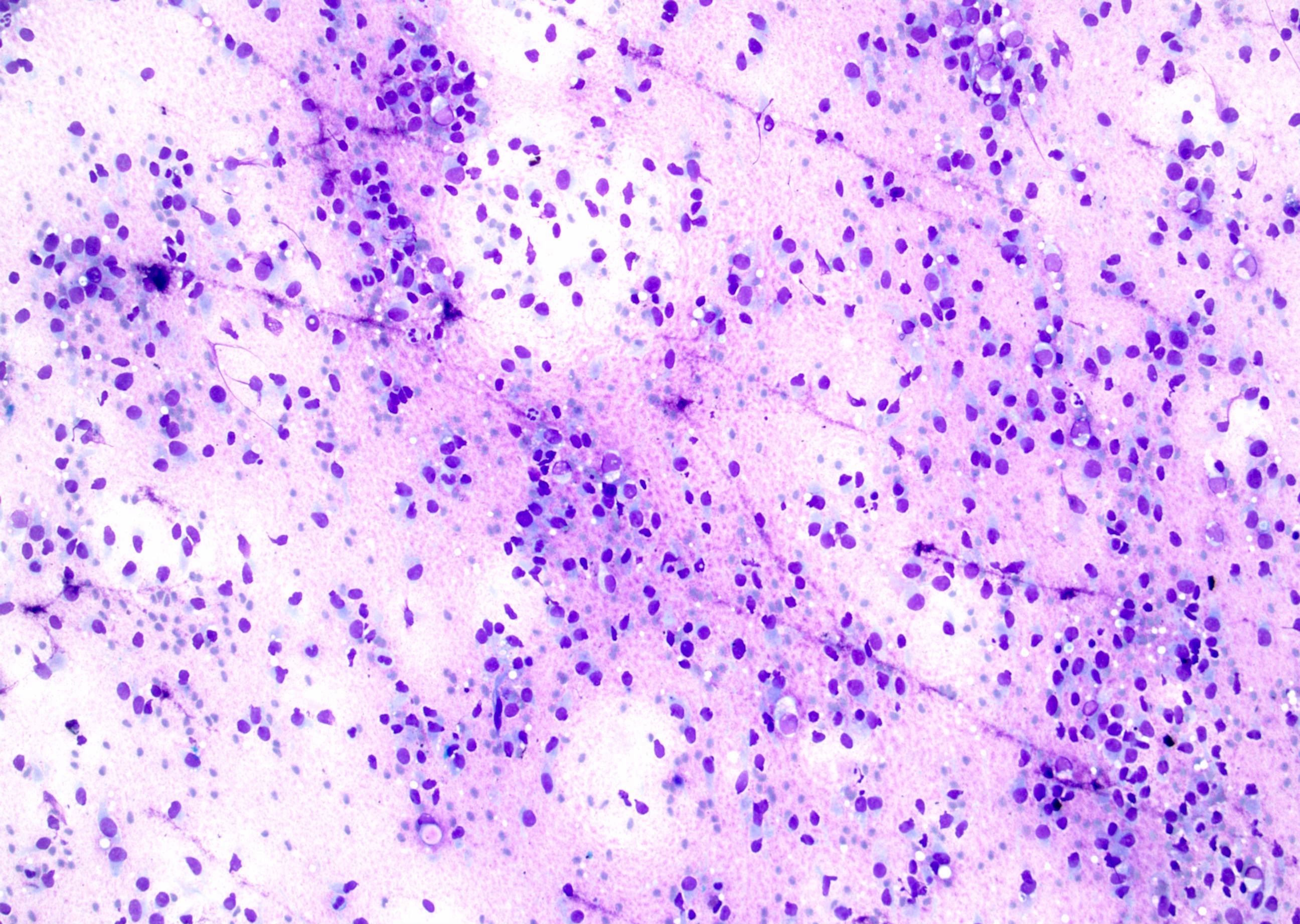
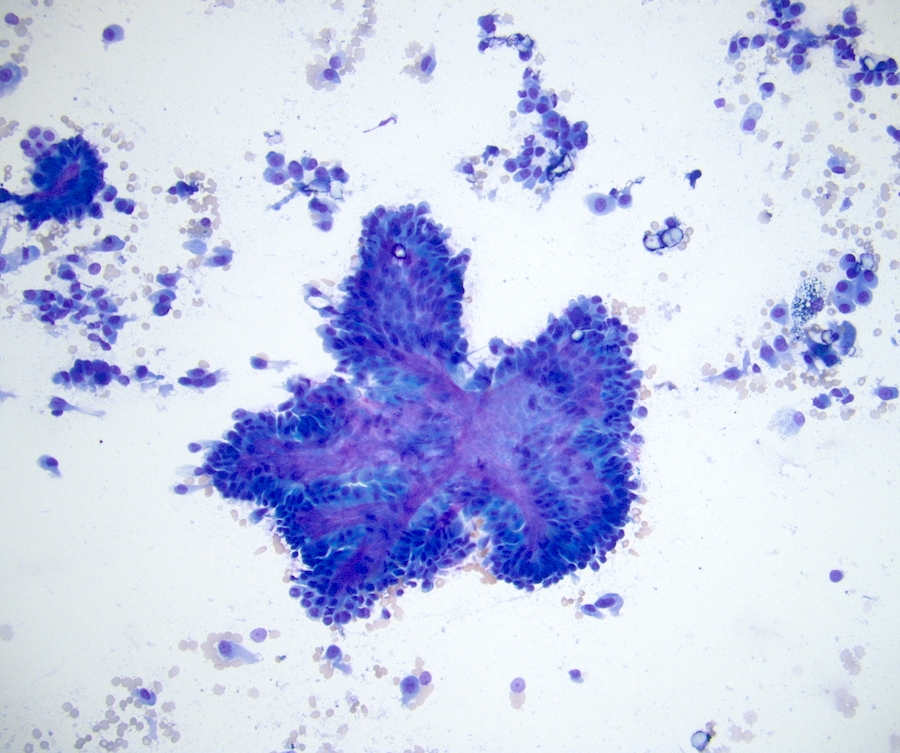
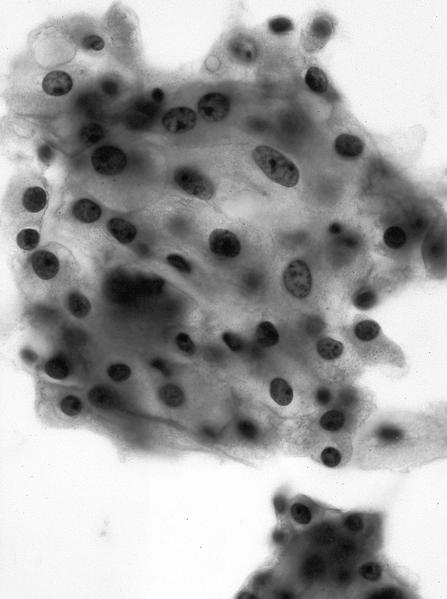
- FNA (Diagn Cytopathol 1992;8:349, Diagn Cytopathol 1996;14:60, Acta Cytol 2008;52:91, Acta Cytol 2010;54:233):
- Hypocellular specimen
- Small papillary clusters of epithelial cells
- Cells with eccentrically placed, hyperchromatic, irregular nuclei, prominent nucleoli and cytoplasmic vacuoles
- Background of red blood cells, histiocytes and rare neutrophils
- No necrosis
- Stroma:
- SMA: strong and diffuse, 95% (Am J Surg Pathol 2016;40:1591, Arch Pathol Lab Med 2004;128:1404, Arch Pathol Lab Med 2006;130:80)
- Desmin and caldesmon: more variable, 42% and 60%, respectively (Am J Surg Pathol 2016;40:1591, Arch Pathol Lab Med 2004;128:1404)
- ER and PR, 50% and 95%, respectively (Am J Surg Pathol 2016;40:1591, Arch Pathol Lab Med 2004;128:1404, Arch Pathol Lab Med 2006;130:80, Am J Surg Pathol 2007;31:489)
- CD10: mainly in spindle cells around epithelial elements, 81% (Am J Surg Pathol 2016;40:1591, Arch Pathol Lab Med 2004;128:1404, Am J Surg Pathol 2007;31:489)
- FOXL2: in ovarian type stroma, 90% (Hum Pathol 2014;45:1010)
- Epithelium:
- HMB45 (Arch Pathol Lab Med 2004;128:1404, Arch Pathol Lab Med 2006;130:80, Am J Surg Pathol 2016;40:1591)
- MelanA: may show positivity in steroidogenic cells (Am J Surg Pathol 2016;40:1591)
- Inhibin and SF1: may show positivity in steroidogenic cells (Am J Surg Pathol 2007;31:489, Am J Surg Pathol 2016;40:1591)
- GATA3: epithelial coexpression with PAX8, 32% (Am J Surg Pathol 2016;40:1591)
- S100 (Arch Pathol Lab Med 2004;128:1404)
- Neuron specific enolase (NSE) (Arch Pathol Lab Med 2004;128:1404)
- Cathepsin K (Am J Surg Pathol 2016;40:1591)
- WT1: may show positivity in stromal cells (Arch Pathol Lab Med 2006;130:80, Am J Surg Pathol 2016;40:1591)
- CD34: may show positivity in pericystic stromal cells (Arch Pathol Lab Med 2006;130:80, Am J Surg Pathol 2016;40:1591)
- Similar mRNA expression profile between adult cystic nephroma and MEST supports these tumors to represent opposite ends of same disease spectrum (Am J Surg Pathol 2009;33:72)
- Highest differentially expressed gene: insulin-like growth factor 2
- Lowest differentially expressed gene: carbonic anhydrase II
- No DICER1 mutations as seen in pediatric cystic nephroma (Am J Surg Pathol 2017;41:472)
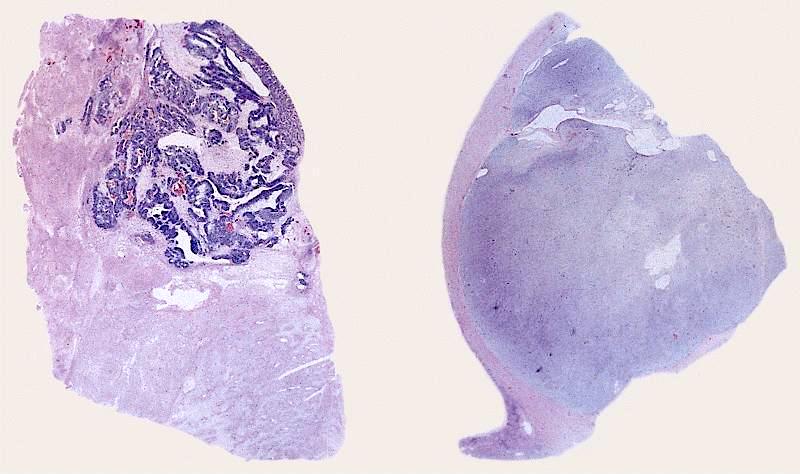
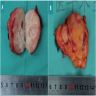
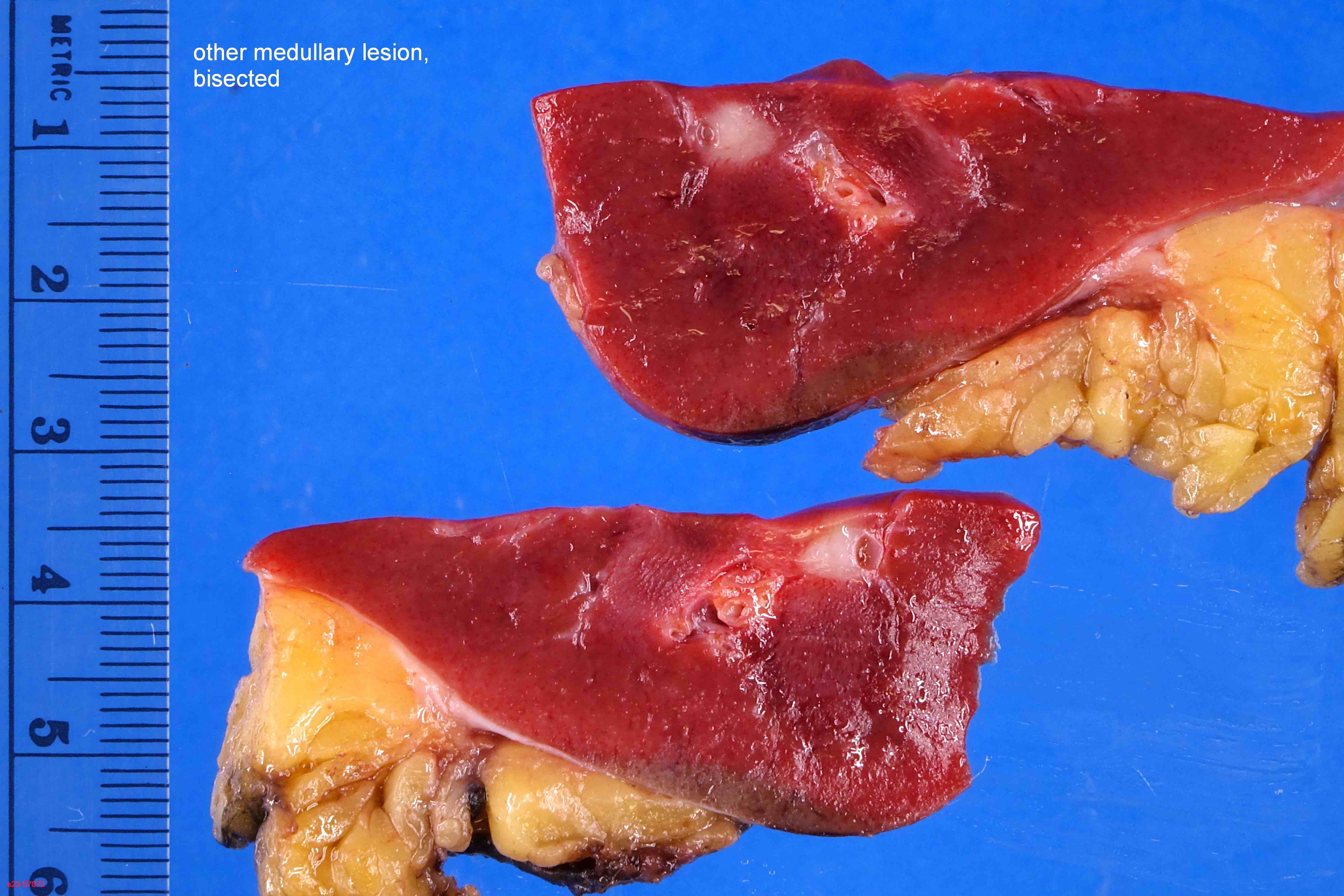
- Left kidney, mass, partial nephrectomy:
- Adult cystic nephroma, measuring 6.5 cm in greatest dimension (see comment)
- Surgical margins, negative for tumor
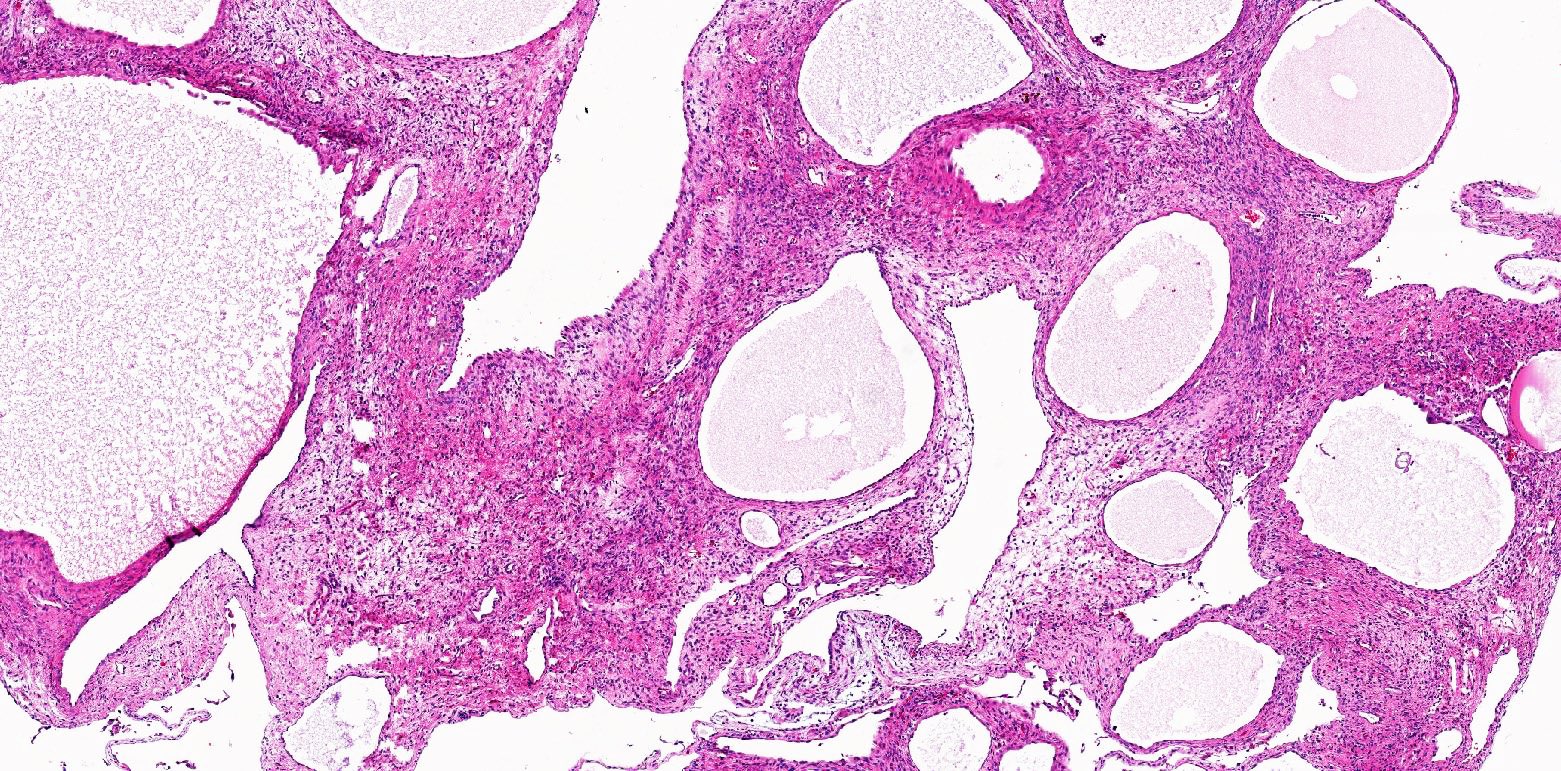
- Comment: The sections show a well circumscribed tumor composed of multiple cysts lined by flattened or cuboidal epithelium. Immunohistochemistry was performed to show the septal stroma is positive for ER and PR. The morphologic and immunohistochemical findings support the diagnosis of adult cystic nephroma.
- Mixed epithelial and stromal tumor (MEST):
- Part of the MEST family with adult cystic nephroma at the opposite end of spectrum, on basis of comparable clinical features (sex and age distribution), overlapping morphologic findings and similar immunohistochemical profile
- Variable solid and cystic components
- Morphologically diverse epithelial and stromal elements
- Pediatric cystic nephroma:
- Almost exclusively occurs in children with higher prevalence in males
- Similar macroscopic and microscopic findings
- Most harbor DICER1 mutations (J Med Genet 2010;47:863, Mod Pathol 2014;27:1267, Hum Pathol 2016;48:81)
- Angiomyolipoma (AML) with epithelial cysts (Am J Surg Pathol 2006;30:593, Arch Pathol Lab Med 2016;140:594):
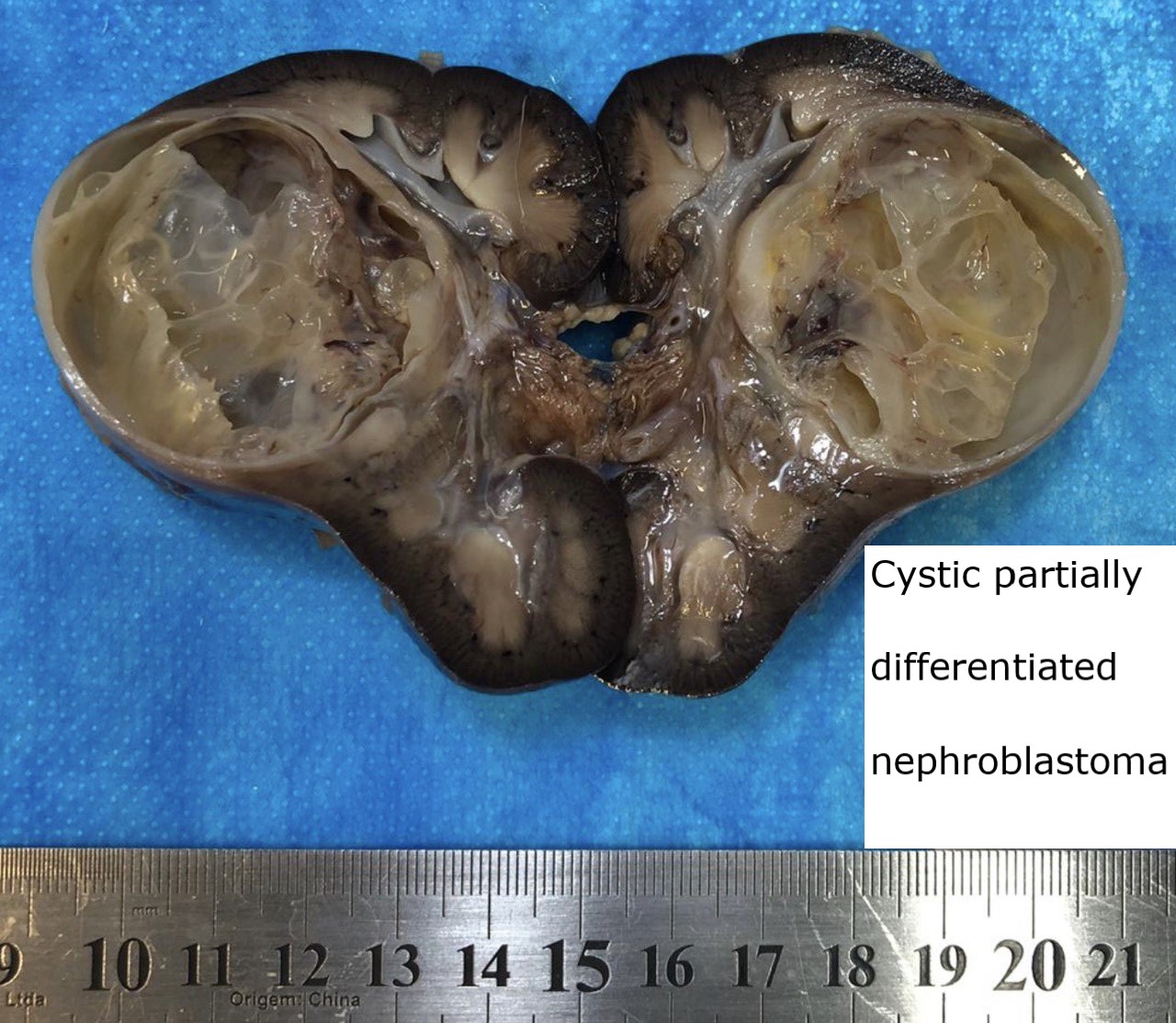
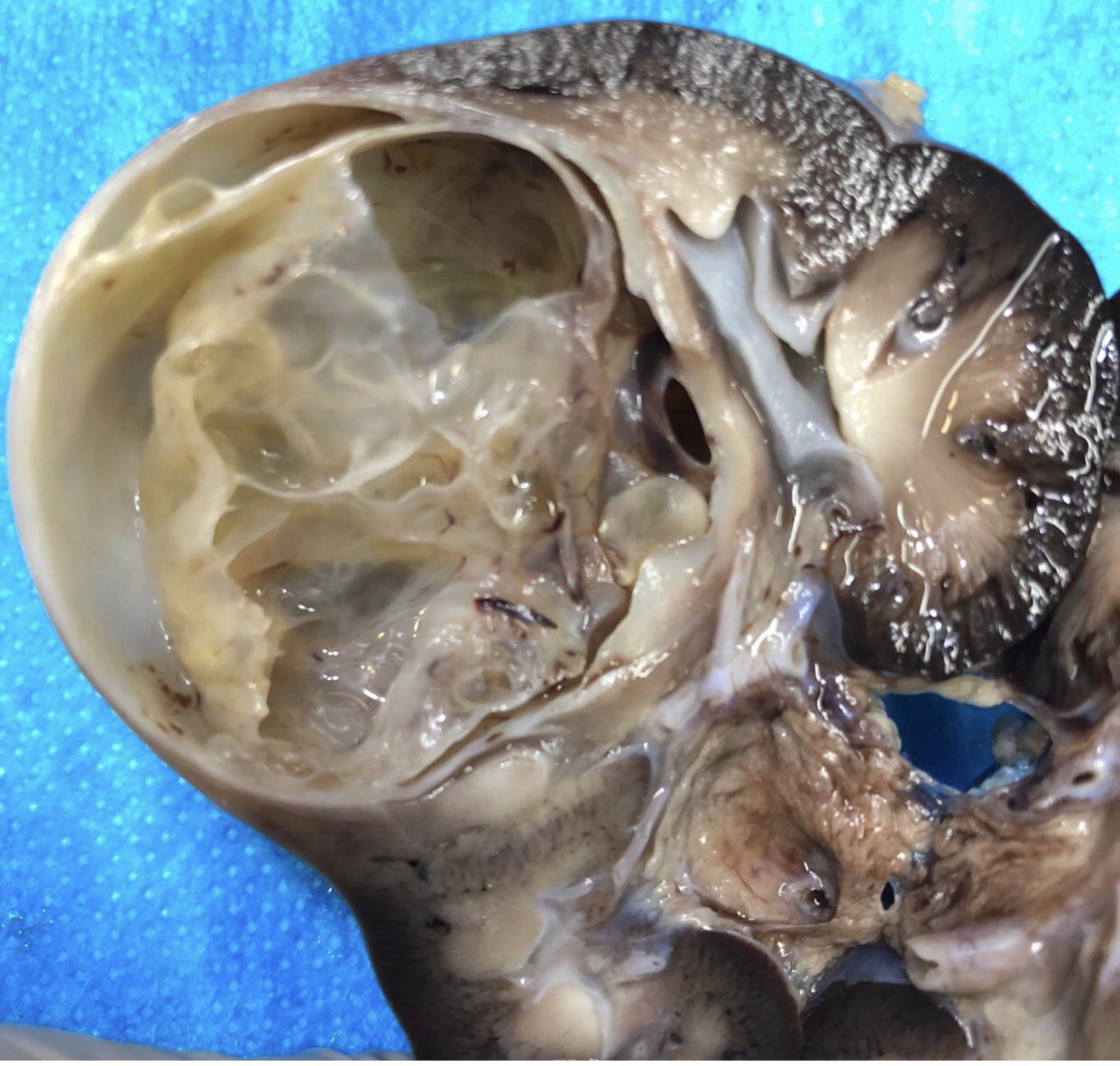
- Cystic partially differentiated nephroblastoma:
- Most occur in patients < 24 months old
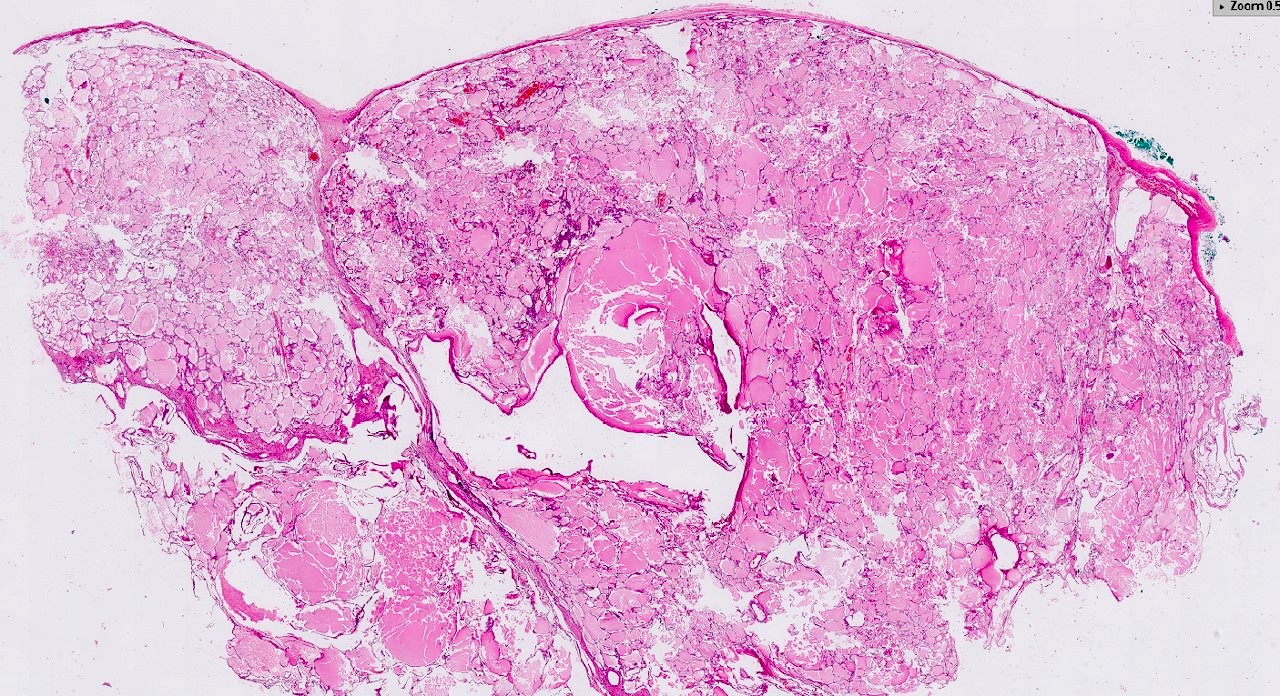
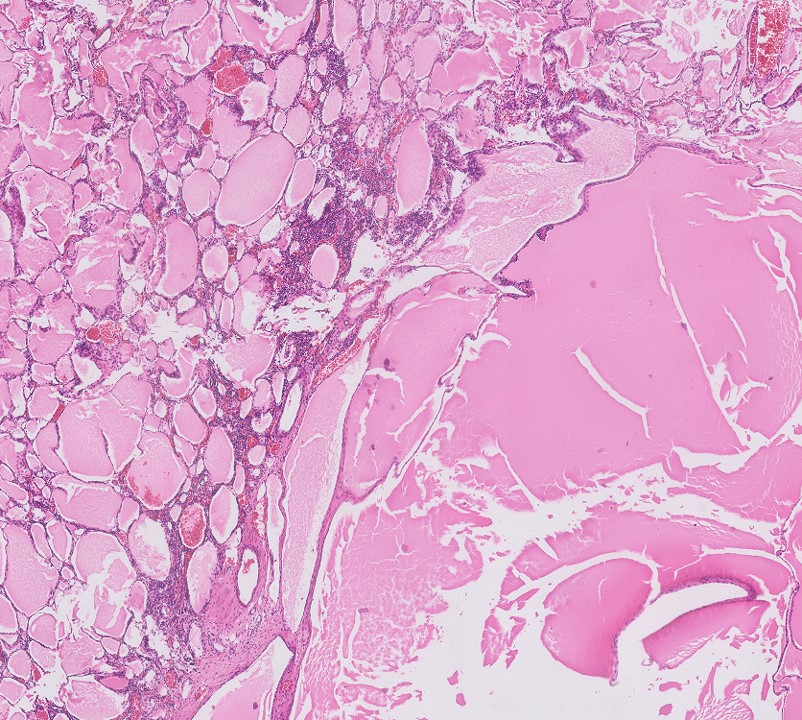
- Nephroblastematous tissue (e.g. blastema, immature stromal cells, primitive epithelial elements) present
- Multilocular cystic neoplasm of low malignant potential:
- Clusters or nests of tumor cells with abundant clear cytoplasm and small nuclei without prominent nucleoli (WHO / ISUP grade 1 or 2) present in cystic septa
- Occasional small papillae may be seen
- No cellular stroma
- Carbonic anhydrase IX+, PR-
- Tubulocystic renal cell carcinoma:
- Strong male predominance (≥ 7:1)
- Small to medium sized tubules admixed with cystically dilated larger tubules lined by cells with enlarged irregular nuclei and prominent nucleoli (WHO / ISUP grade 3)
- Clear cell renal cell carcinoma with extensive cystic change:
- Expansile solid nodules of tumor cells with clear cytoplasm altering septal configuration
- Carbonic anhydrase IX+, PR-
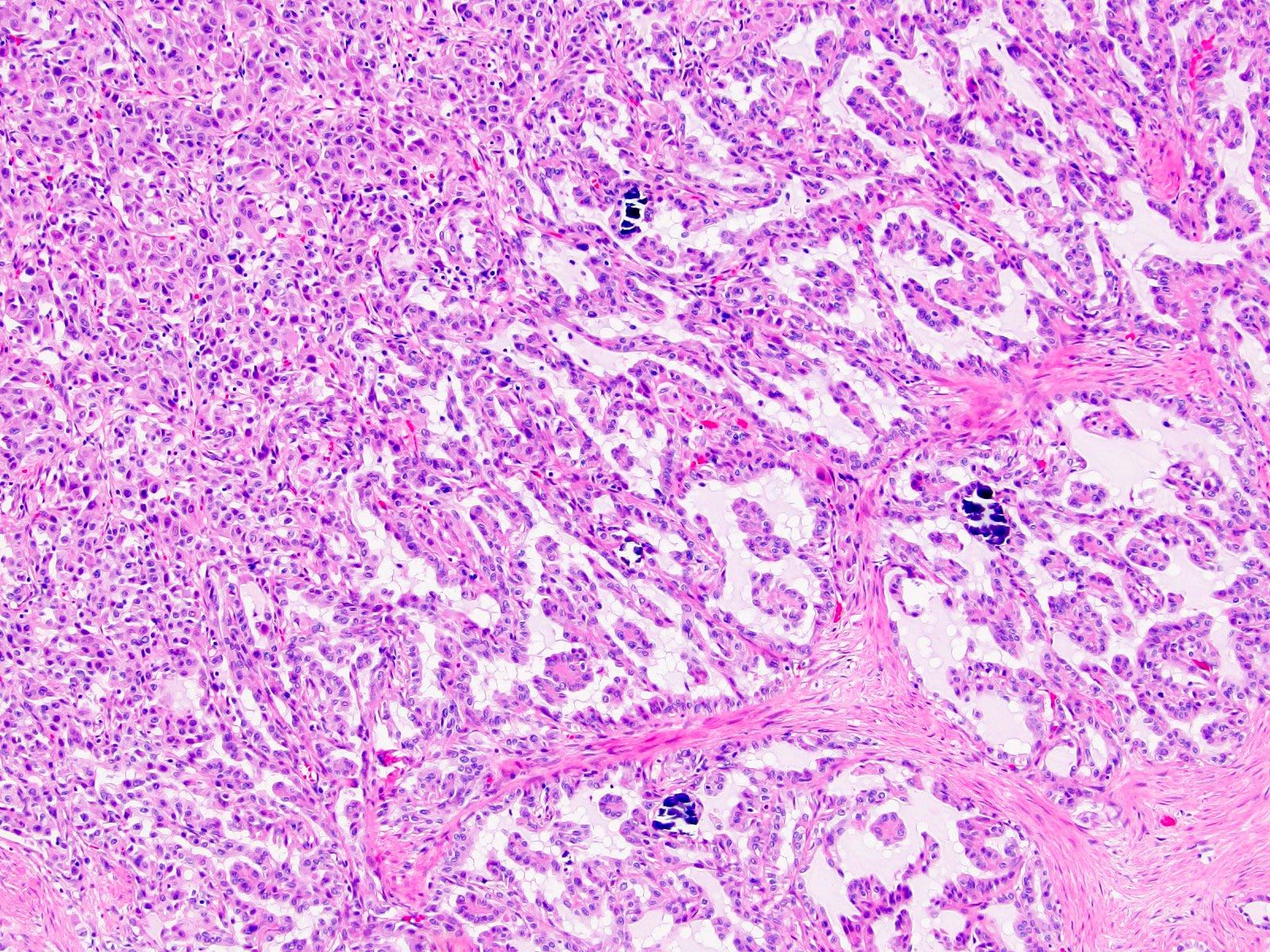
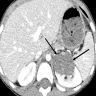
A 6 cm predominantly well circumscribed, multicystic mass was incidentally found in a 55 year old woman. Sections of the partial nephrectomy showed the above histologic features. The stromal component is positive for ER and PR (shown above). What is the likely diagnosis?
- Adult cystic nephroma
- Angiomyolipoma with epithelial cysts
- Cystic partially differentiated nephroblastoma
- Multilocular cystic neoplasm of low malignant potential
Comment Here
Reference: Adult cystic nephroma
Comment Here
Reference: Adult cystic nephroma
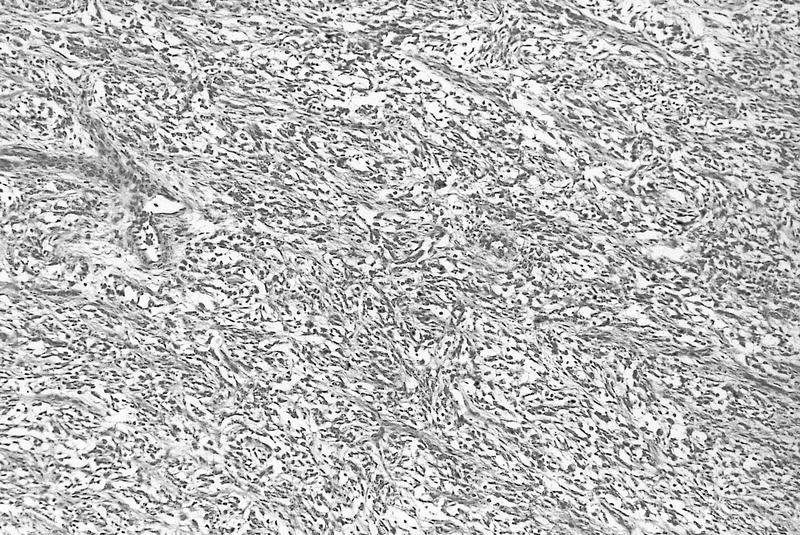
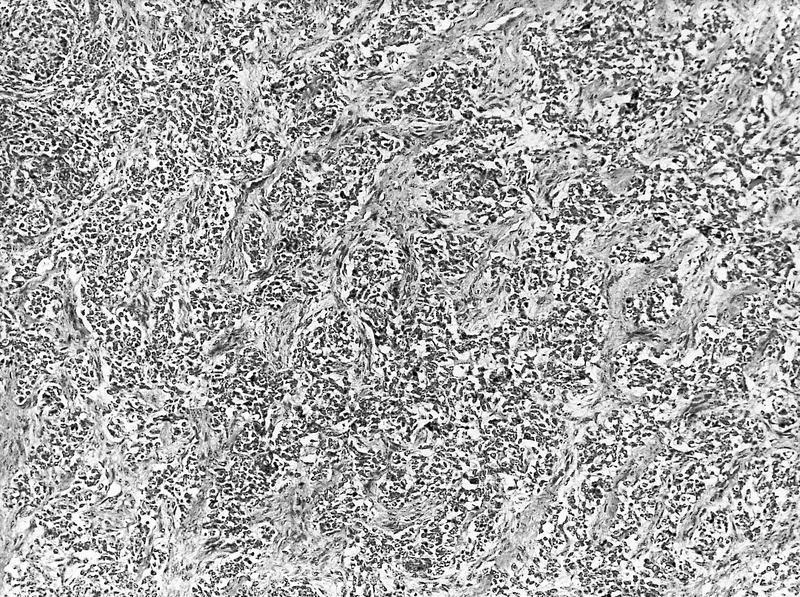
- Anaplastic sarcoma of the kidney is a polyphenotypic sarcoma associated with DICER1 mutations
- First described by Vujanić et al. in 2007 (Am J Surg Pathol 2007;31:1459)
- Rare malignant tumor, with ~50 cases reported in the literature
- Belongs to DICER1 associated tumors
- Occurs in children and adults (age: 7 months - 41 years)
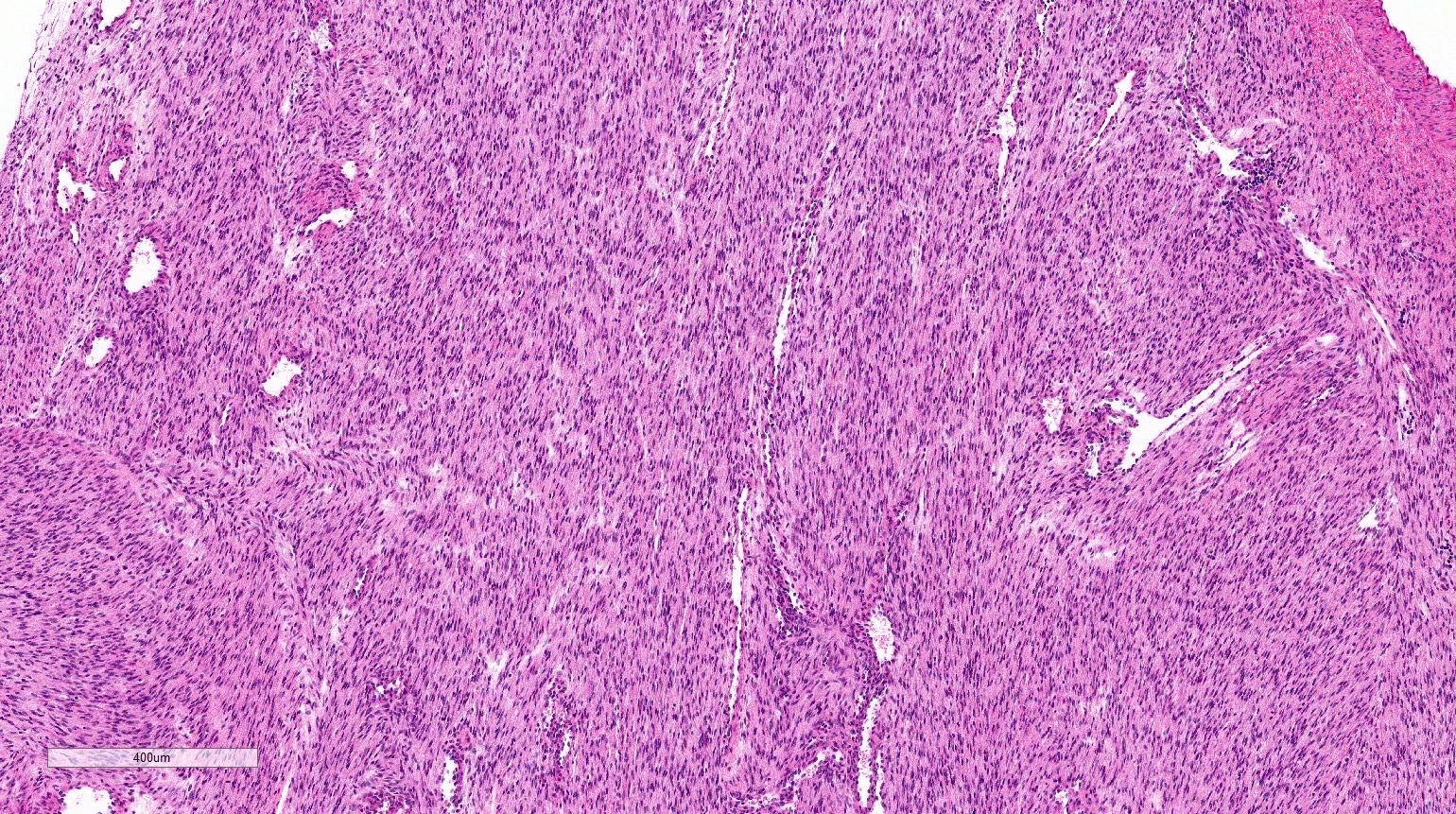
- Histologically characterized by sheets of spindle cells and undifferentiated morphology, marked anaplasia and islands of benign or malignant cartilage or chondroid differentiation
- Often contains a cystic component (cystic nephroma-like)
- DICER1 sarcoma of the kidney
- ICD-O: 8802/3 - anaplastic sarcoma of the kidney
- Only ~50 cases reported in the literature (Pediatr Blood Cancer 2024;71:e31090, World J Clin Cases 2020;8:1495)
- Age at presentation: 7 months - 41 years (Am J Surg Pathol 2007;31:1459, Pediatr Blood Cancer 2024;71:e31090)
- Median age: 8 years; 20/28 (71%) patients under the age of 15 years (World J Clin Cases 2020;8:1495)
- Female predominance (F:M = 2:1) (Pediatr Blood Cancer 2024;71:e31090, World J Clin Cases 2020;8:1495)
- Kidney
- Unilateral tumors
- Only 1 bilateral case reported (JCO Precis Oncol 2018;2:PO.17.00113)
- Majority (or even all) have a somatic RNase IIIb DICER1 mutation
- DICER1 is an endoribonuclease central to generating microRNAs (miRNAs)
- DICER1 utilizes its RNase IIIa and IIIb endonuclease domains to cleave precursor (pre)miRNA stem loops, thus releasing the mature single strand miRNA (Mod Pathol 2018;31:169)
- Co-occurrence of a TP53 mutation with DICER1 was confirmed in some cases but it is not a useful molecular marker of the disease (Mod Pathol 2018;31:169)
- DICER1 mutations are the major genetic event in the development of anaplastic sarcoma of the kidney (Mod Pathol 2018;31:169)
- Large (and palpable) tumors
- Hematuria
- Flank pain
- Stage distribution (Pediatr Blood Cancer 2024;71:e31090)
- Stage I: 32.5%
- Stage II: 30%
- Stage III: 25%
- Stage IV: 12.5%
- Histologic features (see Microscopic description) are usually diagnostic
- DICER1 mutations should be done to confirm the diagnosis
- No histological features associated with outcome
- Older age at presentation associated with worse outcomes (Pediatr Blood Cancer 2024;71:e31090)
- Out of 28 reported cases, 22 had documented followup data (World J Clin Cases 2020;8:1495)
- 68% of patients had no recurrence
- 4.5% had local recurrence
- 27% had distant metastases
- Genetic testing for DICER1 mutation and counseling should be offered (Mod Pathol 2014;27:1267)
- 3 year old girl with a large abdominal mass (Int J Surg Pathol 2016;24:556)
- 12 year old girl with buttock pain and urinary incontinence (Hum Pathol 2010;41:1495)
- 13 year old boy with a large renal mass (Pediatr Int 2013;55:e129)
- 27 year old woman with a palpable mass over the abdomen (World J Clin Cases 2020;8:1495)
- No standardized treatment
- Treated with primary or delayed surgery, followed by chemotherapy and radiotherapy (for higher stages)
- 2 year event free survival
- Stage I - II: 82%
- Stage III - IV: 47%
- 2 year overall survival (Pediatr Blood Cancer 2024;71:e31090)
- Stage I - II: 89%
- Stage III - IV: 70%
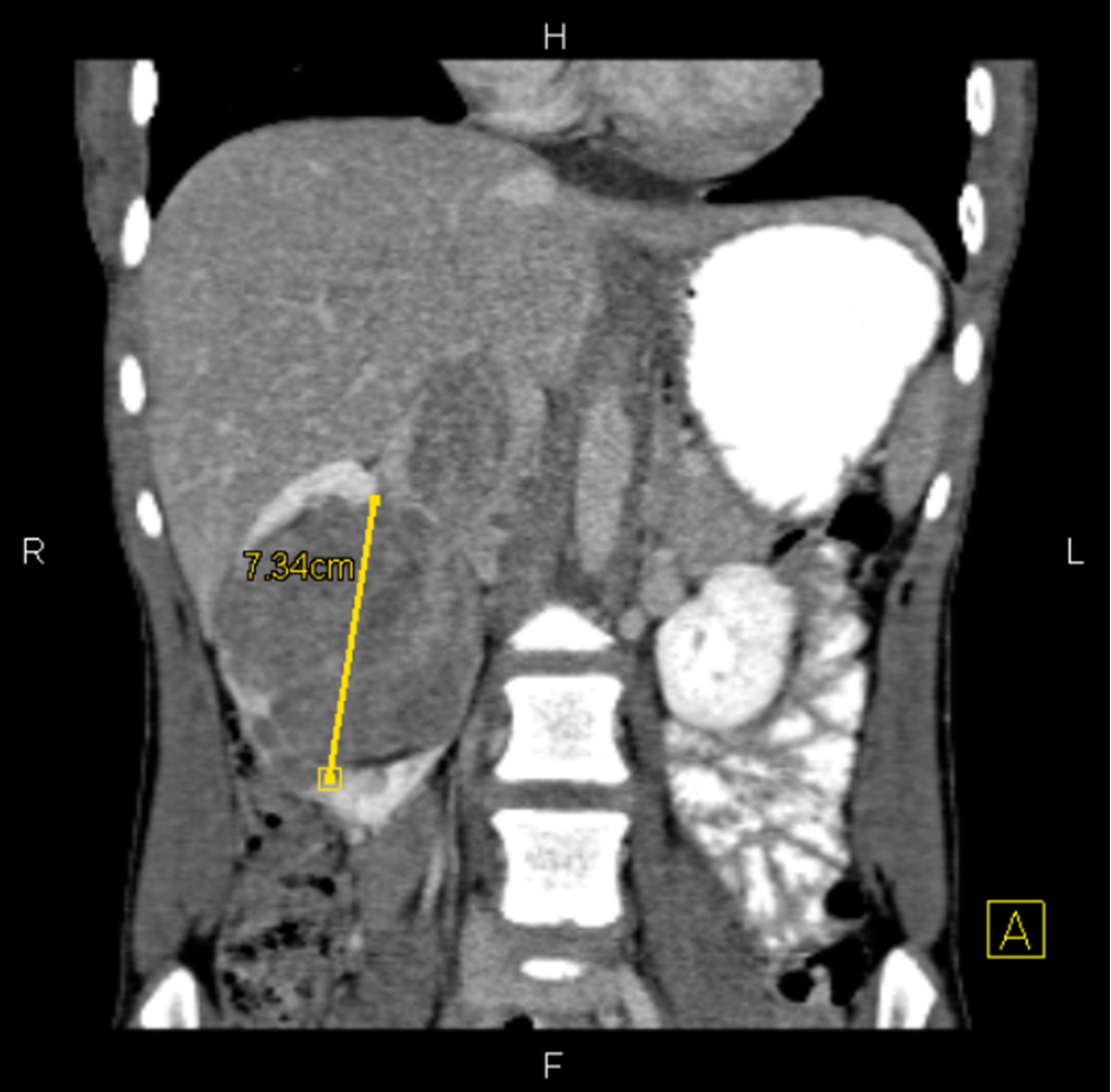
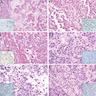
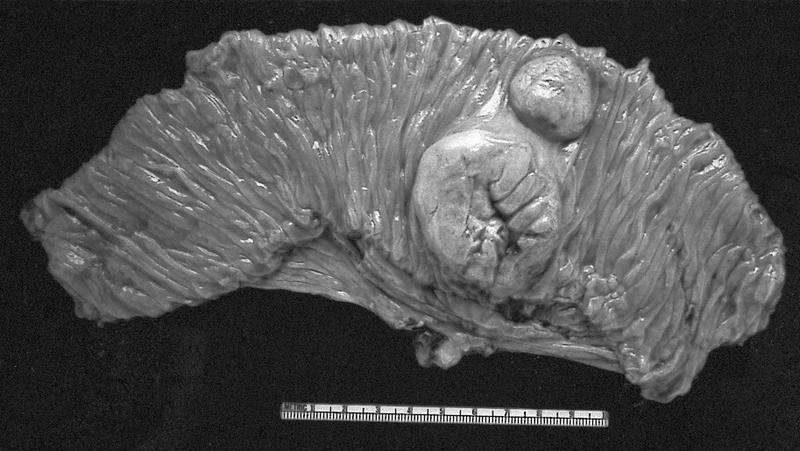
- Typically large (median size: 12.5 cm)
- Centrally located in the kidney
- Most tumors have a distinct cystic component
- Reference: JCO Precis Oncol 2022;6:e2100554
- Typical features
- Undifferentiated cells
- Spindle cell areas
- Marked diffuse anaplasia
- Benign or malignant cartilage or chondroid differentiation
- Rhabdomyoblastic differentiation (desmin and myogenin positivity)
- Cystic nephroma-like cystic component
- May have
- Osteoid differentiation
- Ganglioneuroblastic differentiation (Pediatr Dev Pathol 2022;25:186)
- Staging criteria are the same as nephroblastoma (Histopathology 2022;80:1026)
- Limited series tested; variable results
- p53
- Desmin
- Myogenin
- Vimentin
- PGP9.5
- References: Am J Surg Pathol 2007;31:1459, Pediatr Blood Cancer 2024;71:e31090
- WT1
- NB84a
- CD34
- CD99
- CK AE1 / AE3
- EMA
- BCOR
- PAX8
- CD56
- References: Am J Surg Pathol 2007;31:1459, Pediatr Blood Cancer 2024;71:e31090
- Genetic pathogenesis of cystic nephroma to anaplastic sarcoma of the kidney parallels that of type I to type II / III malignant progression of pleuropulmonary blastoma (Mod Pathol 2014;27:1267)
- Cystic nephroma in patients with DICER1 syndrome may progress to anaplastic sarcoma of the kidney (Pediatr Blood Cancer 2016;63:1272)
- Kidney, total nephrectomy:
- Anaplastic sarcoma of the kidney, stage I (see comment)
- Comment: Tumor comprises solid and cystic components. The solid component shows spindle cell areas with marked, diffuse anaplasia and islands of cartilage with benign and malignant features. The cystic component looks like a cystic nephroma. It is advisable to do a DICER1 mutation test.
- Wilms tumor with diffuse anaplasia:
- Presence of (immature or mature) epithelial structures
- WT1 is negative in anaplastic sarcoma of the kidney
- Nephrogenic rests are not present in anaplastic sarcoma of the kidney
- Primary renal synovial sarcoma:
- Usually exhibits a moderate to strong nuclear staining for TLE1 (but can be patchy positive in anaplastic sarcoma of the kidney) (JCO Precis Oncol 2022;6:e2100554)
- SS18::SSX fusion specific antibody: 95% sensitive, 100% specific
- SSX C terminus antibody: 100% sensitive, 96% specific (Am J Surg Pathol 2020;44:922)
- Mesenchymal chondrosarcoma:
- No DICER1 mutation
A 9 year old boy presents with a painless macroscopic hematuria. Imaging studies revealed a cystic and solid mass in the right kidney. A total right nephrectomy was performed. Which of the following is the most characteristic pathologic finding for this tumor?
- Epithelial elements
- Marked, diffuse anaplasia
- Rhabdomyoblastic differentiation
- WT1 positive immunostaining
Comment Here
Reference: Anaplastic sarcoma of the kidney
- BCOR internal tandem duplication (ITD)
- BRAF mutation
- DICER1 mutation
- MYCN amplification
Comment Here
Reference: Anaplastic sarcoma of the kidney
- Member of perivascular epithelioid cell (PEC) tumor family
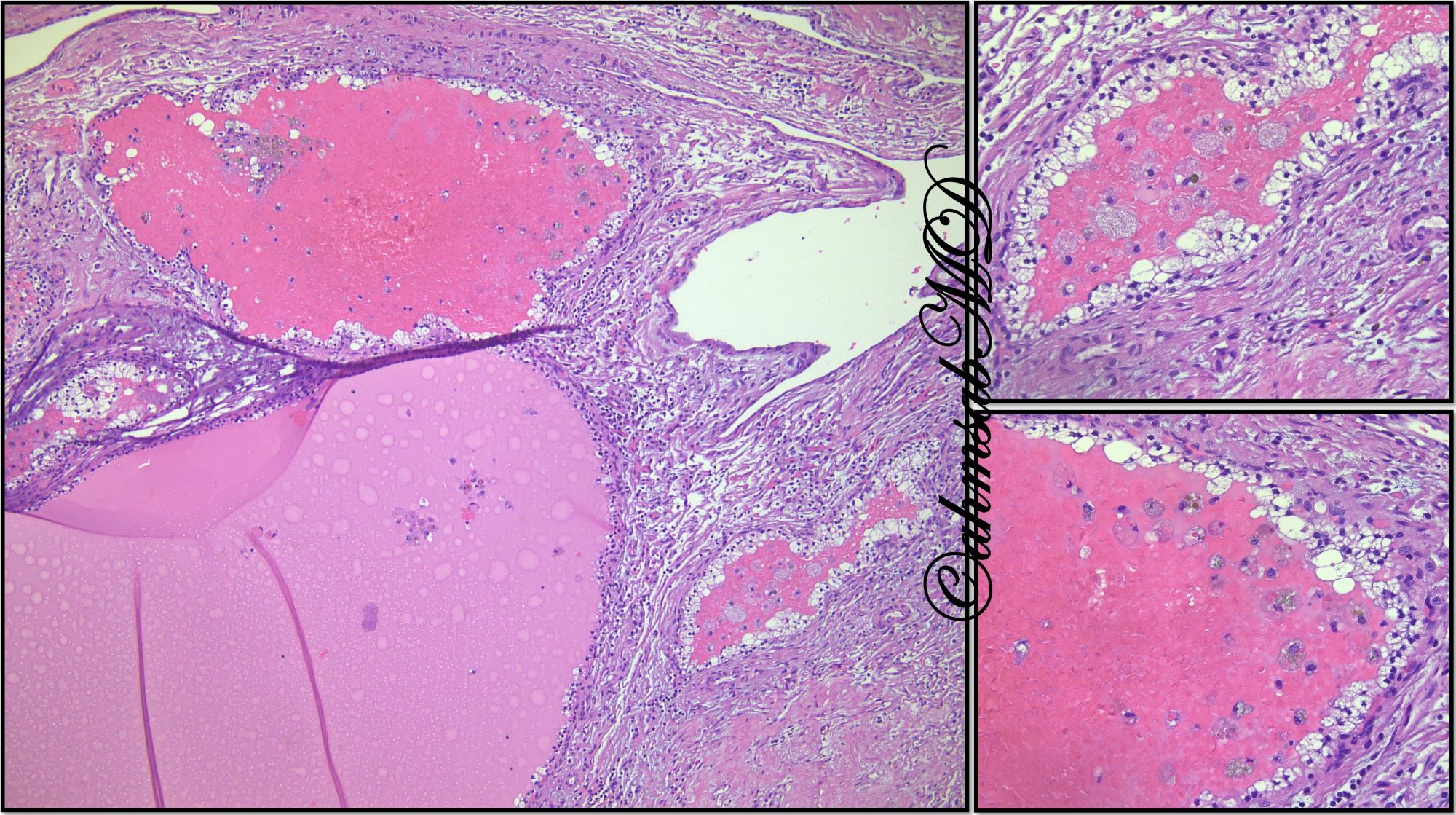
- Angiomyolipoma, classic variant, is a benign mesenchymal neoplasm composed of admixture of thick dysmorphic blood vessels, smooth muscle and adipose tissue
- Amount of each component is variable
- Some cases show significant sclerosis
- Epithelioid variant has potential to metastasize
- Angiomyolipoma can occur in extrarenal sites
- PEComa: perivascular epithelioid cell (PEC) tumor family
- ICD-O: 8860/0 - angiomyolipoma
- Reported in 0.13 - 2.2% of asymptomatic adults who underwent imaging studies (Eur Urol 1995;27:124, Am J Kidney Dis 2012;59:611)
- Occurs sporadically or in patients with tuberous sclerosis
- Majority are sporadic cases (80%) and usually diagnosed in middle aged adults (F > M) (Yonsei Med J 2010;51:728)
- Tuberous sclerosis cases occur in young patients and have no sex predilection (J Urol 2017;197:500)
- Constitutes ~1% of all resected renal tumors
- A large autopsy study demonstrated that 9% of an unselected population had angiomyolipomas (Hum Pathol 2016;58:41)
- Kidney; can occur in extrarenal sites such as liver and lymph nodes
- Rarely occurs in the ovary (Int J Gynecol Pathol 2002;21:69, Internet Journal of Pathology 2006;6:1)
- Presumably arises from perivascular epithelioid cells, which have no known body counterpart
- Usually asymptomatic in screened tuberous sclerosis patients due to smaller size when discovered
- May coexist with renal cell carcinoma in nontuberous sclerosis patients, particularly clear cell carcinoma (Mod Pathol 2001;14:157)
- Tumors can be quite large, multifocal and can extend into the renal vein or vena cava
- Classic variant is benign but may be complicated by hemorrhage if the tumor is large
- Imaging is often sufficient to render the diagnosis for classic angiomyolipoma (Radiology 2011;260:158, Int J Urol 2011;18:727, AJR Am J Roentgenol 2012;198:377, Radiology 2012;263:160)
- Definitive diagnosis by light microscopic examination of tissue
- So called fat poor and epithelioid variants may additionally require immunohistochemistry
- Routine laboratory within normal limits
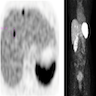
- Characteristic radiologic appearance with fat content in the classic variant
- Radiologic findings depend on the amount of fat within the tumor (AJR Am J Roentgenol 2017;209:826)
- Benign course in angiomyolipoma, classic variant
- Tumors with epithelioid and pleomorphic features can have a more aggressive course (Mod Pathol 2013;26:1355, Ann Diagn Pathol 2020;47:151538, Urol Oncol 2022;40:18)
- Sarcomatous transformation with distant metastasis is extremely rare
- Retroperitoneal hemorrhage is an important complication (Urology 2008;72:1077)
- Patients with bilateral disease can have renal failure
- Death can occur due to involvement of contiguous organs particularly blood vessels
- 33 year old woman with retroperitoneal hemorrhage, tuberous sclerosis and bilateral tumors (Am J Case Rep 2017;18:1309)
- 34 year old woman with a giant angiomyolipoma (Mol Clin Oncol 2017;7:298)
- 41 year old man with extension into vena cava and pulmonary arteries (J Thorac Dis 2018;10:E166)
- 48 year old woman and 62 year old man with malignant epithelioid variant (Medicine (Baltimore) 2018;97:e11805)
- 64 year old woman with angiosarcoma arising in angiomyolipoma (Diagn Pathol 2018;13:53)
- Tumors may be embolized or undergo surgical excision (Eur Urol 2009;55:1155)
- mTOR inhibitors such as everolimus can be used if large or extending into the vena cava (Urol Case Rep 2017;11:11)
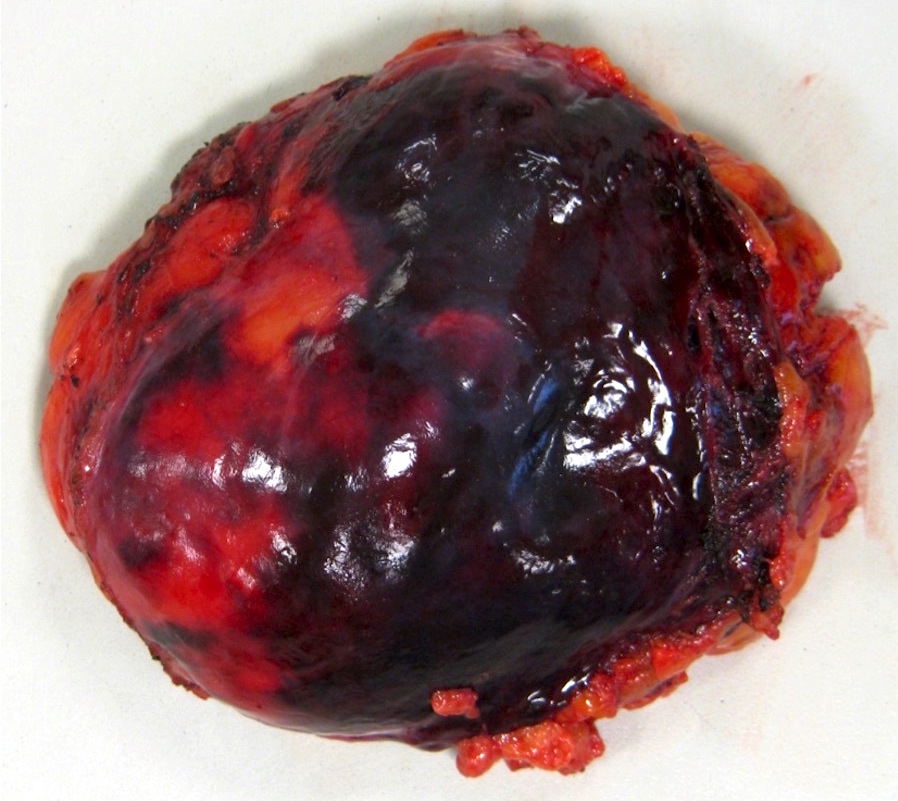
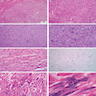
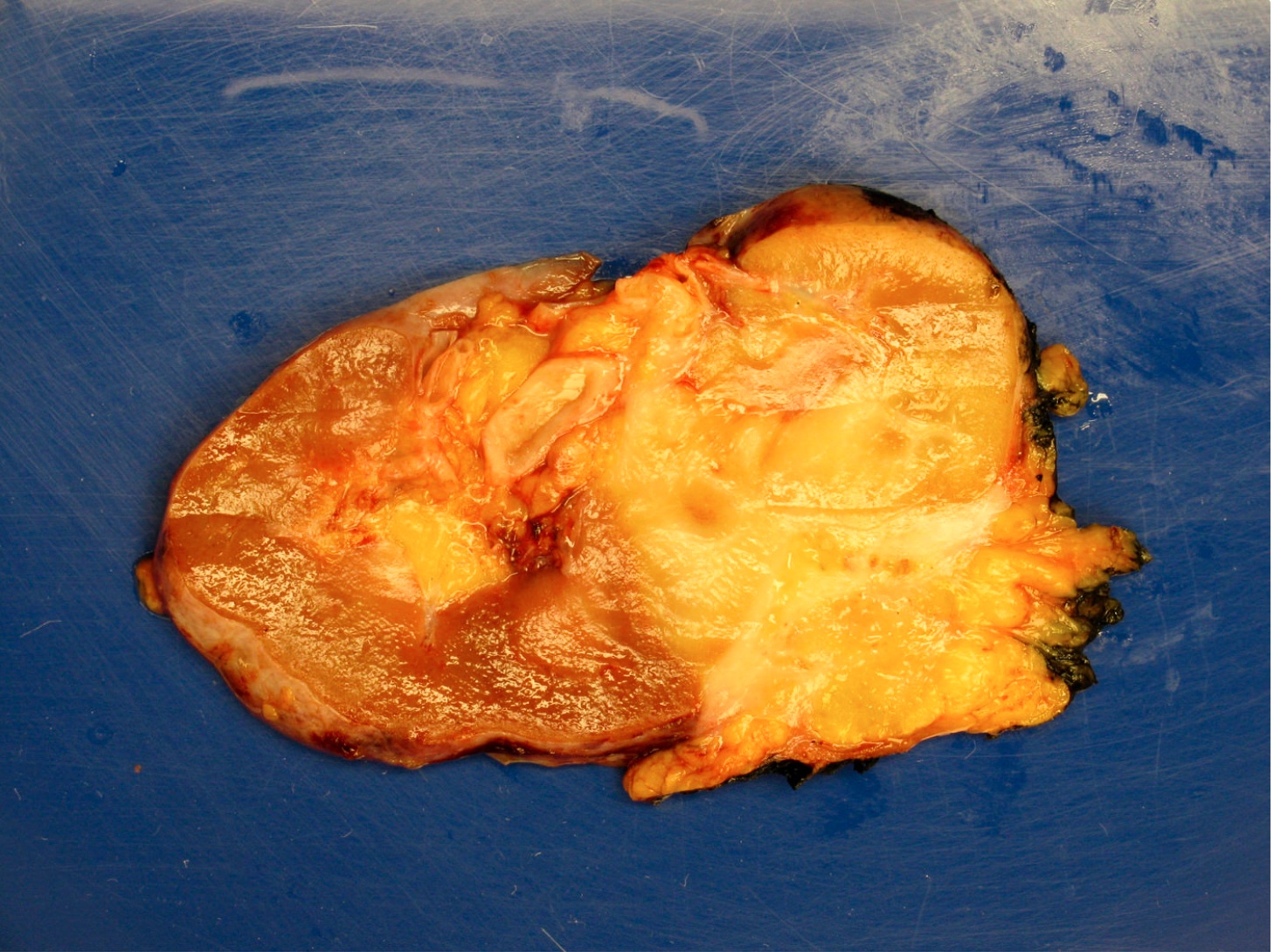
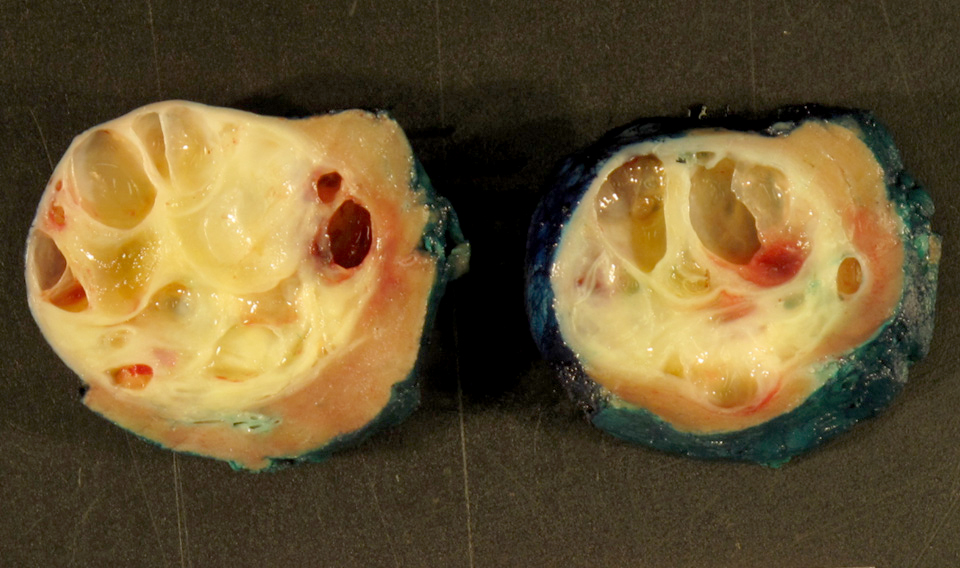
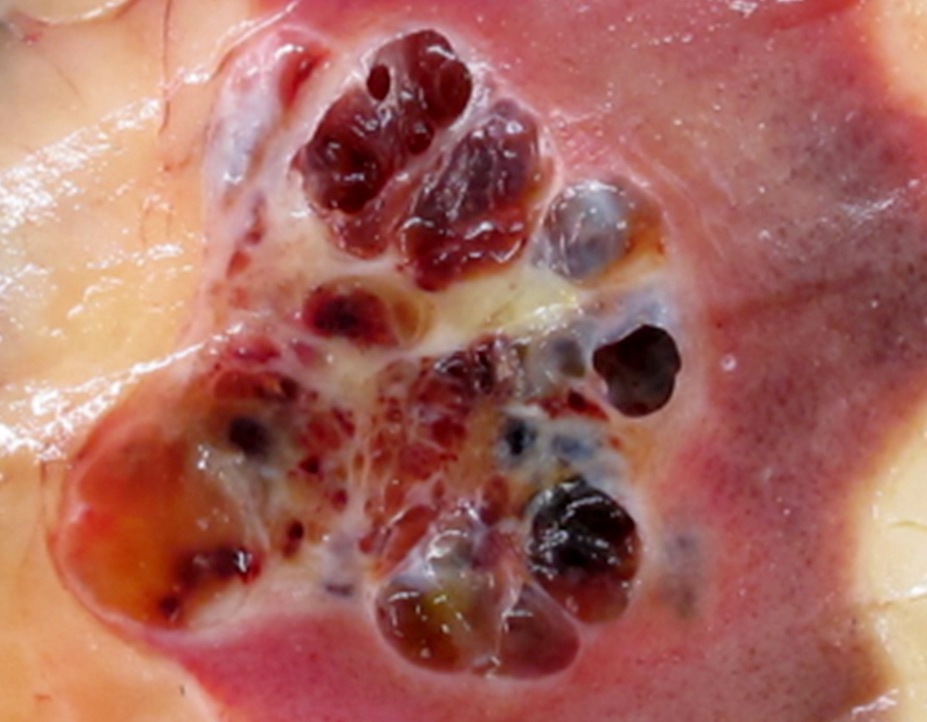
- Tumor ranges from 0.5 to 25 cm with a mean of 6 cm
- Circumscribed, not encapsulated with pushing border
- Cut surface can have red (vascular component), gray-white (smooth muscle component) or yellow (adipose component) appearance
- May involve the intrarenal venous system, the renal vein or the vena cava despite being benign (Arch Pathol Lab Med 1990;114:65)
- Tumors rarely have a cystic component (Mod Pathol 2006;19:669)
- Tumors are usually unilateral and unifocal
- Multiple (~33%) or bilateral (15%) tumors suggest underlying tuberous sclerosis
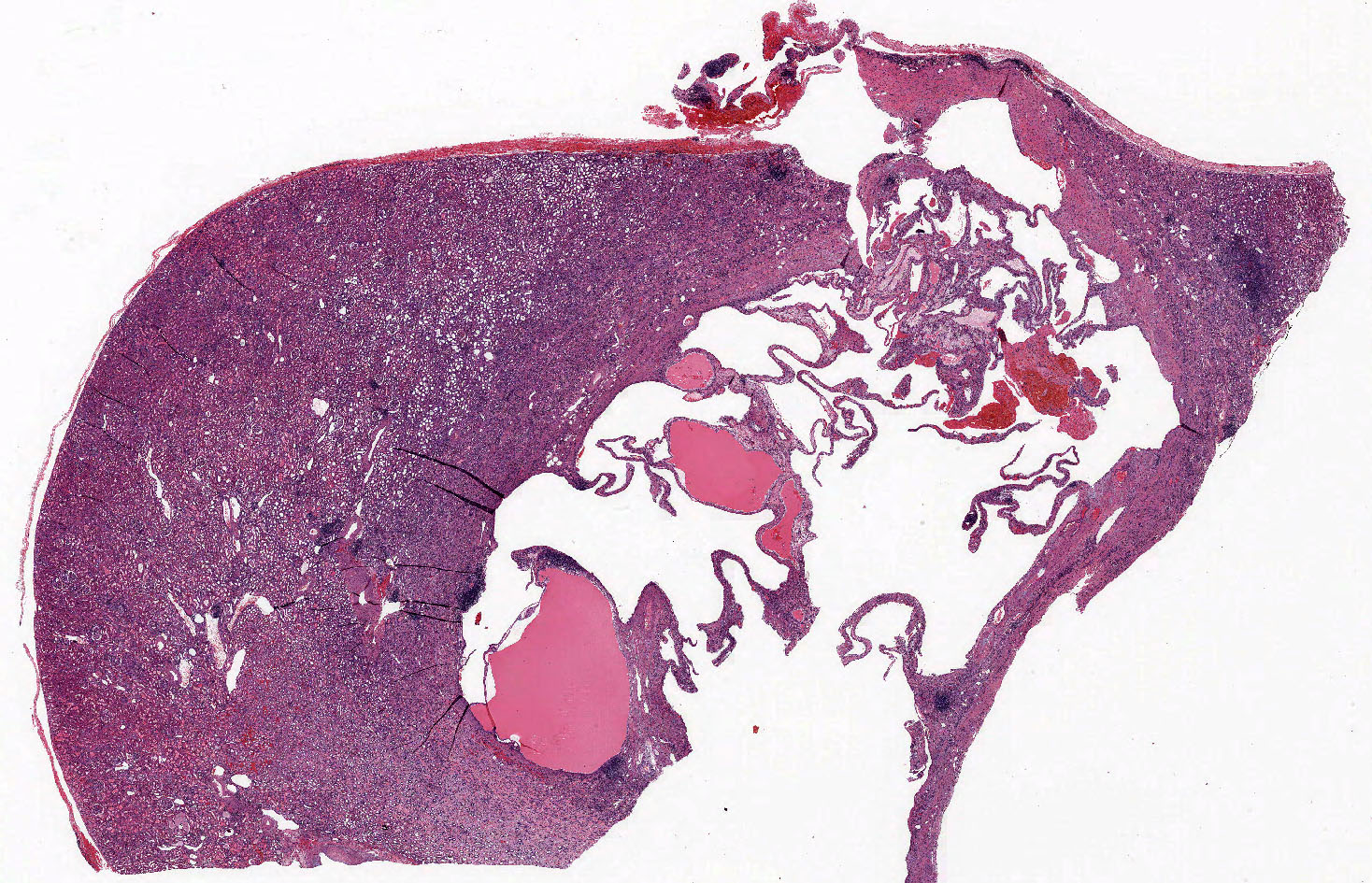
- Classic triphasic with myoid spindle cells, mature adipose tissue and dysmorphic thick walled blood vessels without elastic lamina
- Smooth muscle component appears to originate from vessel walls and may be hypercellular, atypical, pleomorphic or epithelioid
- Vascular component is in the form of thick walled hyalinized vessels
- Fat component is in the form of mature adipose tissue and is seen in > 90% of tumors
- Epithelioid variant (Mod Pathol 2013;26:1355):
- Pure or predominant population of polygonal cells with clear or densely eosinophilic cytoplasm, large hyperchromatic bizarre nuclei
- Multilobated nuclei and multinucleation is common
- Hemorrhage, mitotic figures and necrosis are common
- Angiomyolipoma with epithelial cysts (AMLEC) represents a morphologic spectrum of the tumors that is characterized by cysts lined by cuboidal or hobnail epithelial cells reminiscent of renal tubular epithelium
- Oval to spindled cells and cohesive stromal fragments, adipose tissue and branching blood vessels in a hemorrhagic background (Diagn Cytopathol 2019;47:1190)
- No mitotic figures (Cytopathology 2007;18:250)
- Coexpression of melanocytic and smooth muscle markers in myoid and lipoid components can be seen with stronger expression of melanocytic markers in epithelioid areas and stronger expression of muscle markers in spindled areas
- HMB45 (adipose 90%, smooth muscle 85%, vessel 80%) (Arch Pathol Lab Med 2007;131:122)
- MART1 / MelanA (adipose 70%, smooth muscle 60%, vessel 40%) (Arch Pathol Lab Med 2007;131:122)
- Cathepsin K, SMA, MSA, calponin, caldesmon (Mod Pathol 2012;25:100, Arch Pathol Lab Med 2001;125:751, Am J Surg Pathol 2009;33:289, Am J Surg Pathol 2015;39:349)
- Cytokeratin
- PAX8
- CAIX
- GATA3
- Inhibin
- CD117 (adipose 20%, smooth muscle 40%) (Arch Pathol Lab Med 2007;131:122)
- Premelanosomes
- TSC2 (most commonly) or TSC1 mutations in sporadic angiomyolipomas (PLoS One 2011;6:e24919)
- Copy neutral LOH on chromosome 16p (PLoS Genet 2016;12:e1006242)
- 5q (Hum Pathol 1999;30:295)
- P53 mutation in epithelioid angiomyolipomas (Urol Oncol 2022;40:18)
- Rare cases of MDM2 amplification by fluorescence in situ hybridization in fat predominant angiomyolipomas (Virchows Arch 2020;477:661)
- Right kidney, mass, partial nephrectomy:
- Angiomyolipoma, classic variant, measuring 3.5 cm in greatest dimension (see comment)
- Surgical margins, negative for tumor
- Comment: The sections show well circumscribed tumor composed of adipose tissue, thick walled blood vessels and smooth muscle. There are vessel walls with prominent sclerosis. Immunohistochemical stain SMA is diffusely positive in the vessel walls and the smooth muscle component. HMB45 is focally staining adipose tissue and smooth muscle.
- Renal cell carcinoma, clear cell type:
- Well differentiated liposarcoma:
- No vascular component
- Negative for melanocytic markers and SMA
- Leiomyoma:
- No vascular or adipose component
- Negative for melanocytic markers
- Leiomyosarcoma:
- Prominent atypia, infiltrative, usually no vascular or adipose component
- Negative for melanocytic markers
- Pleomorphic rhabdomyosarcoma:
- Smooth muscle component is markedly atypical, tumor is infiltrative, no vascular or adipose component
- Negative for melanocytic markers
- Melanoma:
- Marked atypia, no adipose or vascular component
- Negative for SMA
- Adrenal cortical carcinoma:
- Oncocytoma:
- Cytologically bland oncocytes
- No adipose or vascular component
- Negative for melanocytic markers and SMA
- Mixed epithelial and stromal tumor of the kidney (MEST):
- Solid component of MEST can resemble a spindle cell component of so called fat poor angiomyolipoma
- Ovarian stroma-like spindle cells of the solid MEST component are positive for SMA, desmin, ER, PR and FOXL2 and negative for melanocytic markers
- Epithelial component of MEST is similar to AMLEC and is positive for PAX8
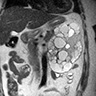
- A 16 year old girl with history of seizures was found to have bilateral renal masses containing a fat component on the CT imaging study. Histology is shown in the image above. Which hereditary disease is this patient likely to have?
- Birt-Hogg-Dubé
- Familiar papillary renal cell carcinoma
- Hereditary leiomyomatosis
- Tuberous sclerosis
- von Hippel-Lindau
Comment Here
Reference: Angiomyolipoma
- A renal tumor from a 43 year old woman shows proliferation of epithelioid cells with abundant eosinophilic cytoplasm and prominent nuclear atypia. What is the best initial immunohistochemical panel for this case?
- Cytokeratin, PAX8, CAIX, CD10, vimentin
- Cytokeratin, PAX8, CK7, CK20, p53
- Cytokeratin, PAX8, MDM2, MyoD1, desmin
- Cytokeratin, PAX8, MITF, S100, tyrosinase
- Cytokeratin, PAX8, SMA, HMB45, inhibin
Comment Here
Reference: Angiomyolipoma
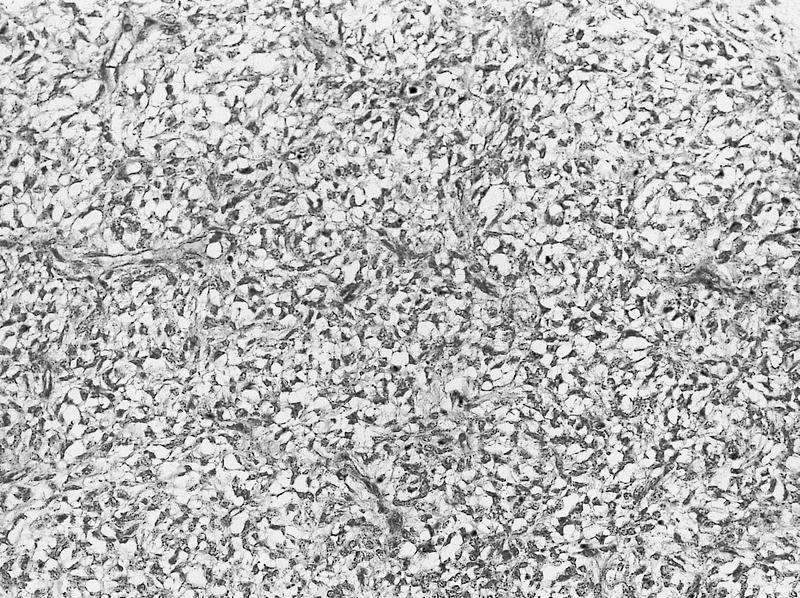
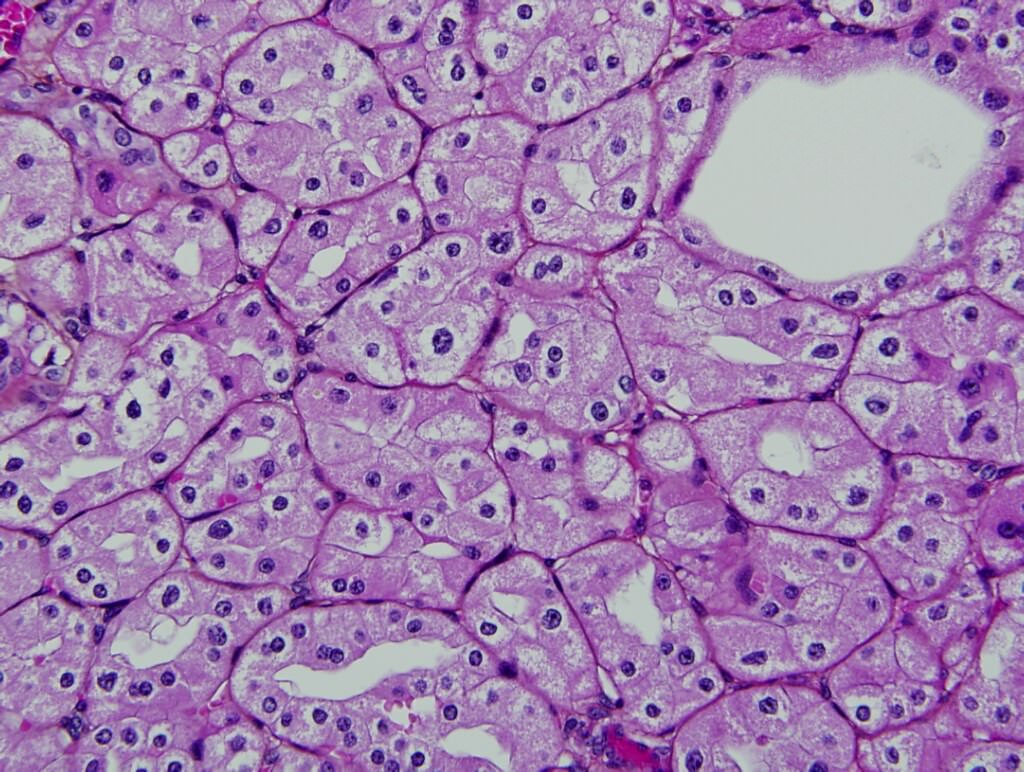
- Solid tumor composed of granular pale cells with prominent cell borders, finely reticular cytoplasm, perinuclear halos and wrinkled hyperchromatic nuclei
- First described in 1985 (J Pathol 1988;155:277)
- Cell of origin: intercalated cells of distal convoluted tubules
- Solid sheet-like architecture
- Sharply defined cell membranes (plant-like)
- Wrinkled irregular nuclei (raisinoid)
- Perinuclear halos (koilocytic)
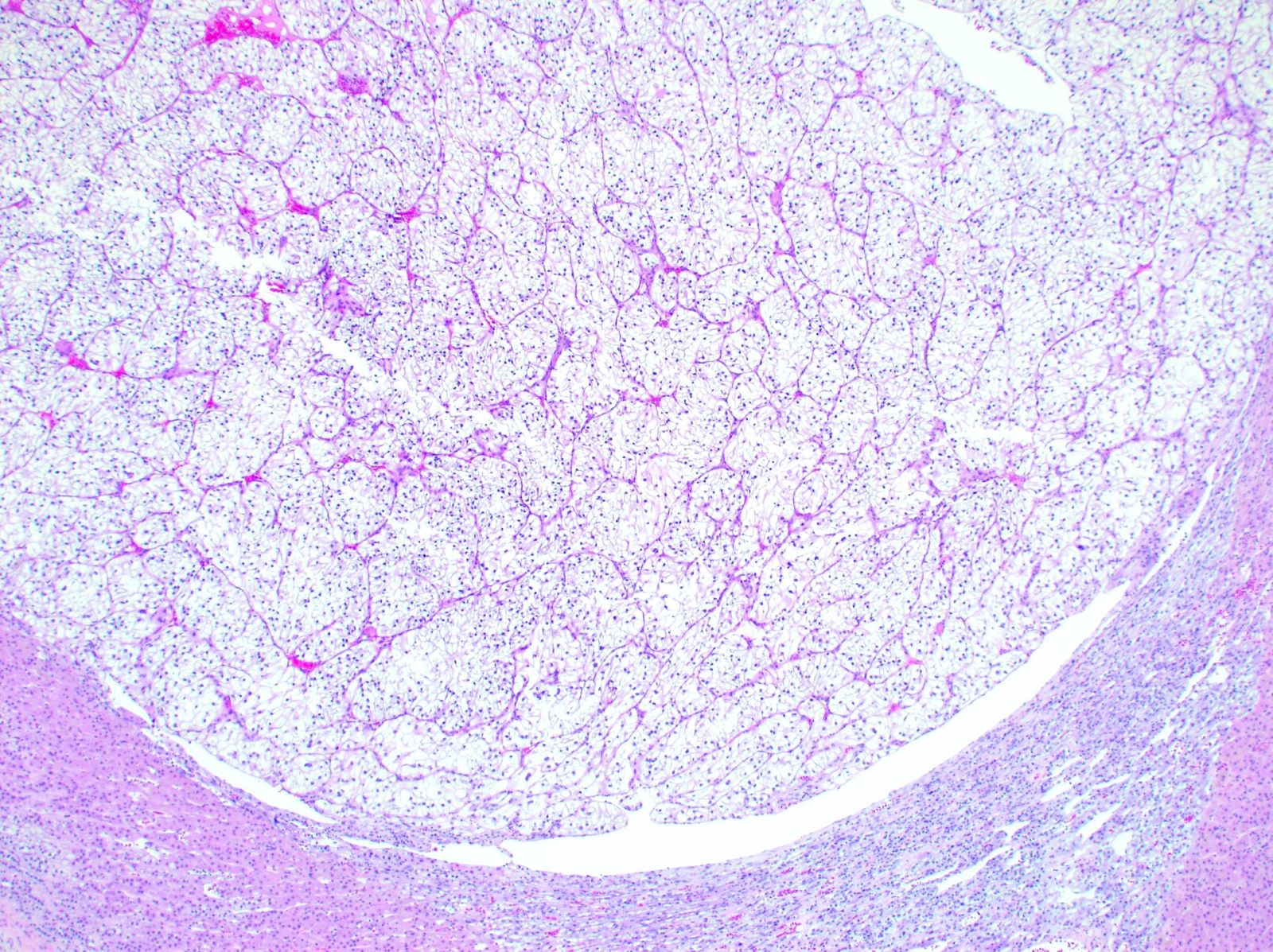
- Chromophobe renal cell carcinoma (ChRCC), classic variant
- ICD-10: C64 - malignant neoplasm of kidney, except renal pelvis
- Third most common renal cell carcinoma (RCC) subtype; 7% of adult renal epithelial tumors (Arch Pathol Lab Med 2019;143:1455)
- Majority are sporadic and incidental, no gender preference
- Mean age 58 years (Cancer 2004;100:1406)
- Solitary kidney mass
- Most commonly in renal cortex
- Multiple tumors (mean 5.3); mean age 51 years at first renal tumor diagnosis
- Bilateral multifocal ChRCC, oncocytomas or hybrid oncocytic chromophobe tumor (HOCT), also may have oncocytosis (Am J Surg Pathol 2002;26:1542)
- Autosomal dominant syndrome: small dome shaped papular fibrofolliculomas of face, neck and upper trunk, renal tumors, lung cysts and spontaneous pneumothorax
- Mutations in the folliculin gene (FLCN) at 17p11.2, leading to premature truncation and loss of function of the folliculin protein (Cancer Cell 2002;2:157, Hum Mutat 2010;31:E1043)
- Most are organ confined T1 - T2, N0, M0 and have good prognosis (Cancer 2004;100:1406)
- Recurrence or metastatic disease developing in 4 - 10% cases (Eur J Cancer 2017;80:55)
- 5 and 10 year cancer specific survival are 93% and 88.9%, respectively (BJU Int 2012;110:76)
- Usually large, well circumscribed, hypovascular mass with relatively homogeneous contrast enhancement; may show central scar
- Poor prognostic factors
- Sarcomatoid change (~5%), microscopic necrosis, vascular invasion (Am J Surg Pathol 2008;32:1822, Am J Surg Pathol 2011;35:962)
- Tumor size (> 7 cm)
- Higher clinical T category and pathologic TNM stage
- Male gender (BJU Int 2012;110:76)
- Tumor grading is not prognostically relevant and not recommended (J Clin Pharm Ther 2019;44:268)
- 41 year old woman with significant response to nivolumab for sarcomatoid metastatic ChRCC (BMC Urol 2018;18:26)
- 46 year old man with de novo ChRCC in the graft 3 decades after renal transplantation (Saudi J Kidney Dis Transpl 2020;31:271)
- 61 year old man with sarcomatoid heterologous component (Iran J Pathol 2020;15:57)
- 65 year old man with retrograde venous invasion and gain of chromosome 21 (Int J Surg Pathol 2018;26:536)
- 70 year old man with neuroendocrine differentiation (Urol Ann 2015;7:383)
- Surgery, cryoablation and targeted systemic chemotherapy for metastatic disease with antiangiogenic, TK and mTOR inhibitors (Eur J Cancer 2017;80:55)
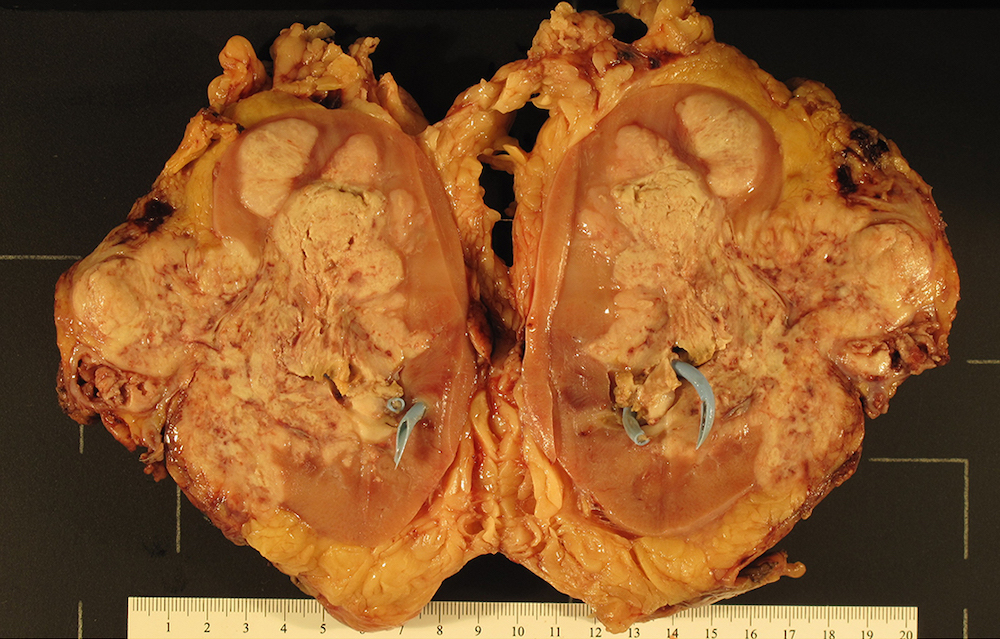
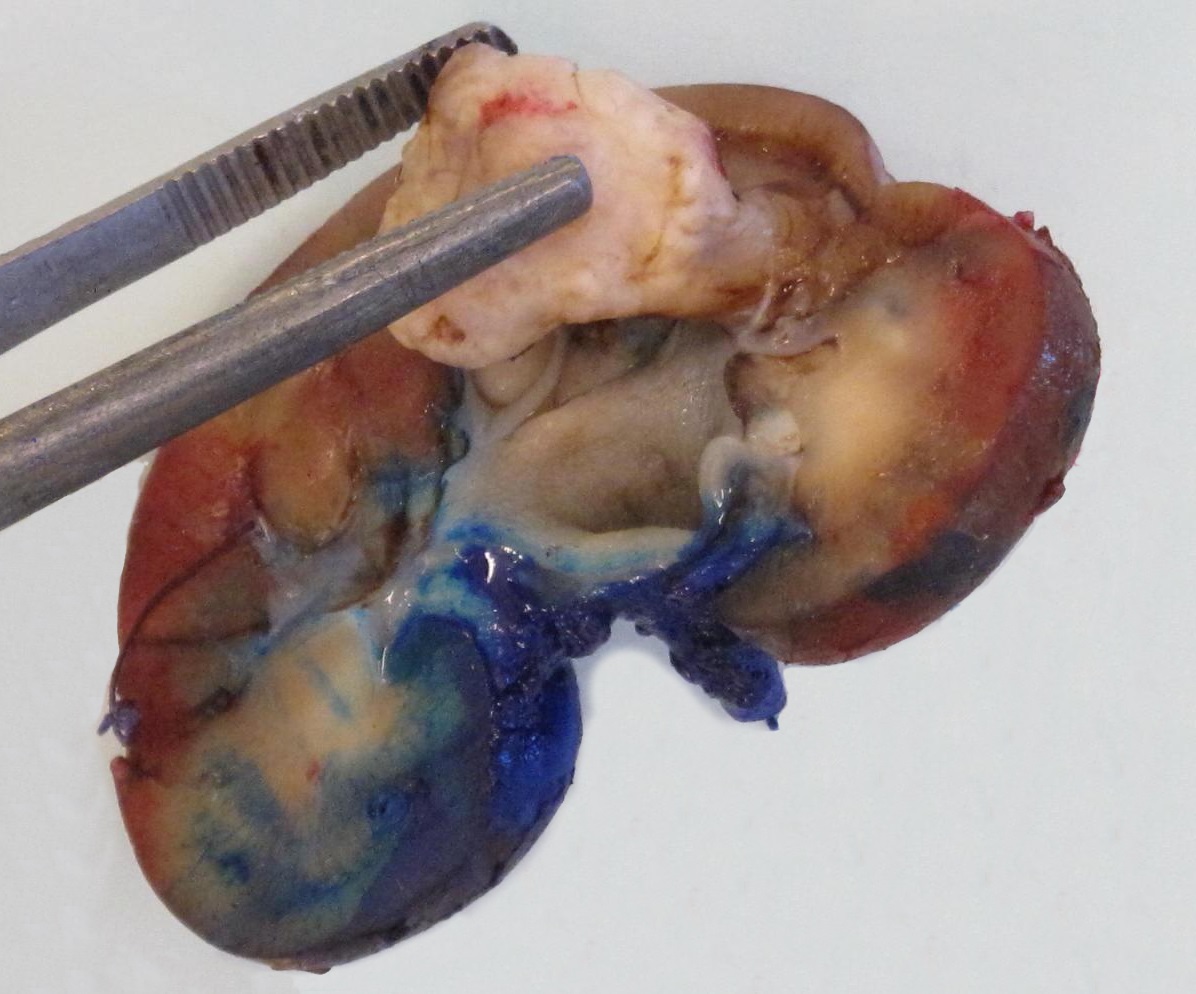
- Well circumscribed, unencapsulated, tan to light brown
- Average size 8 cm (BJU Int 2012;110:76)
- Necrosis, hemorrhage and small cysts (25 - 30%)
- Occasionally central scar (~15%); multifocal (10%)
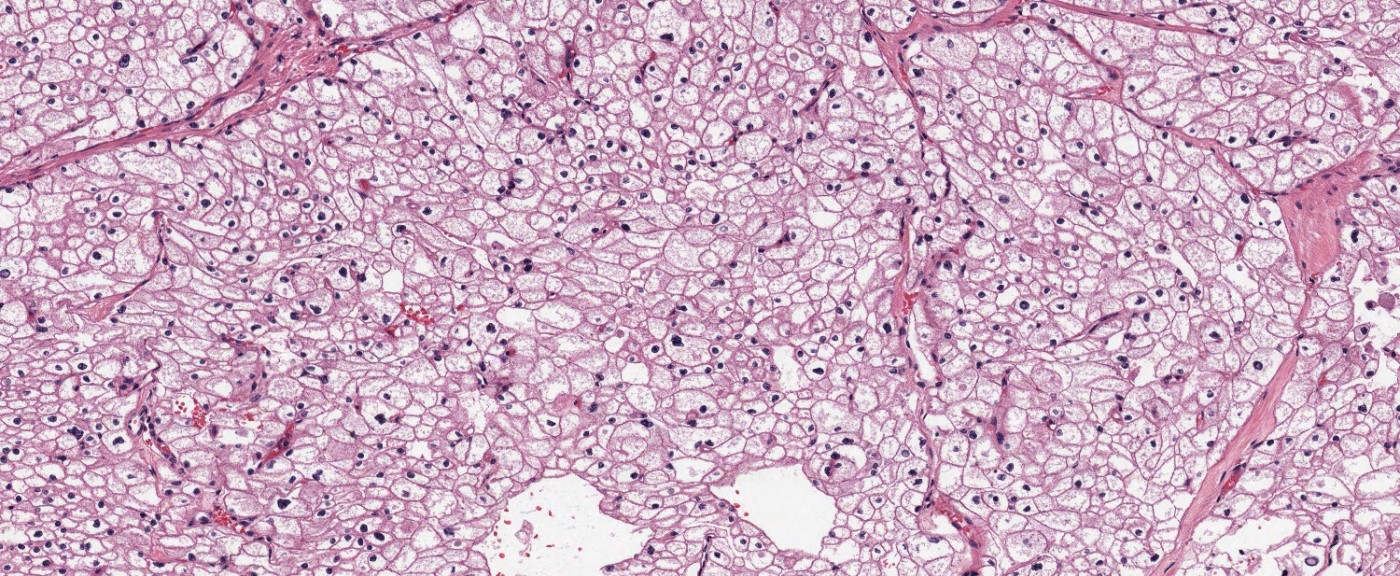
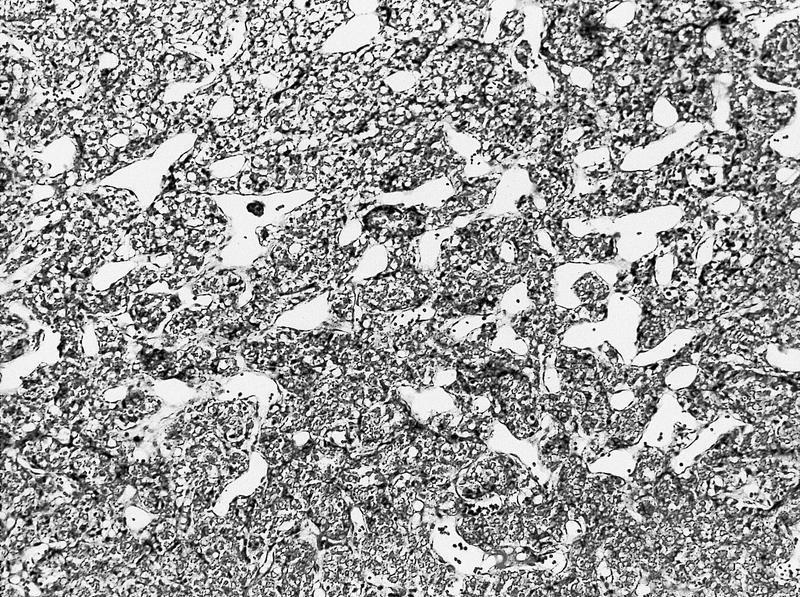
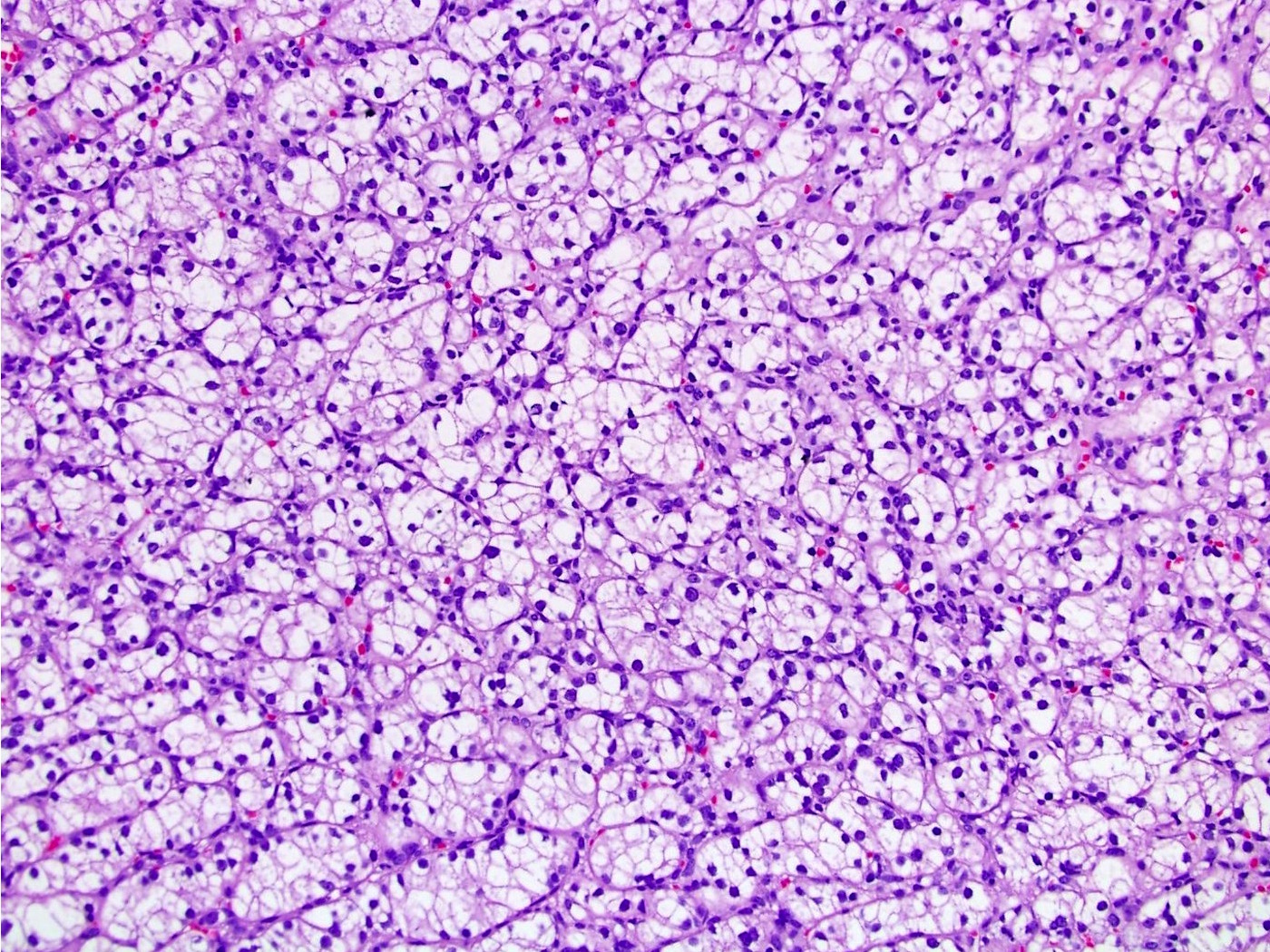
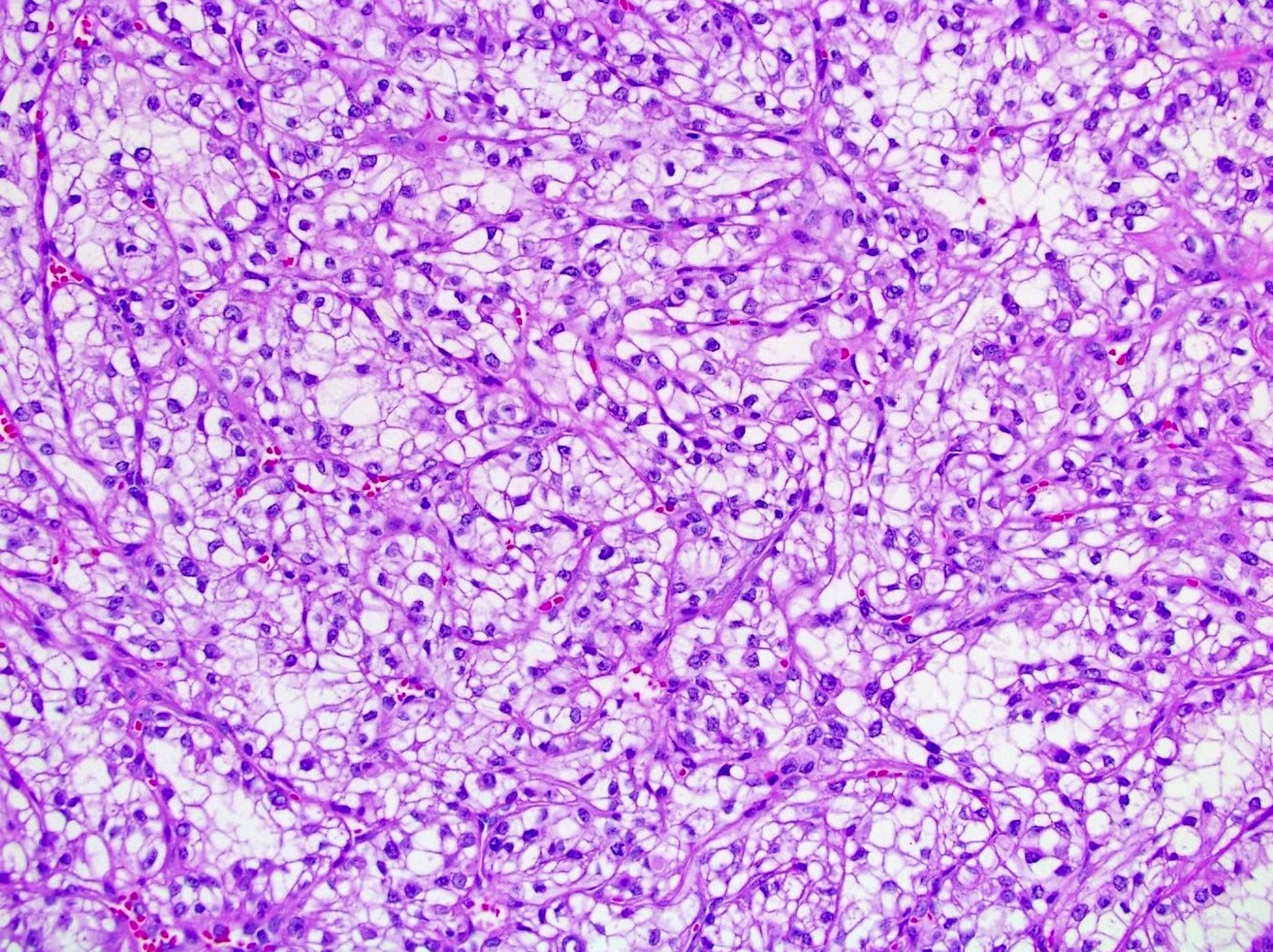
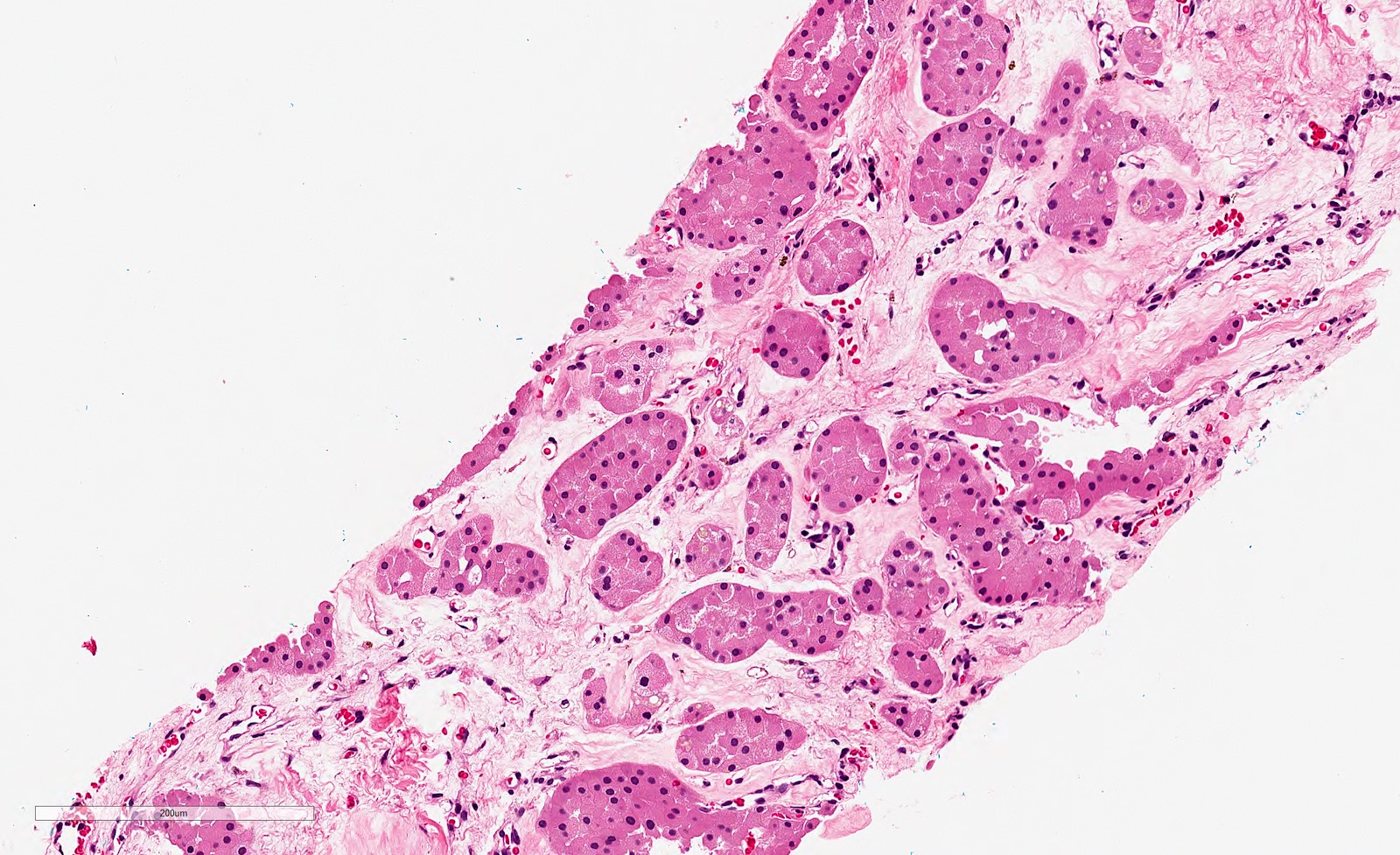
- Typically confluent solid growth with nests, sheets or alveoli / trabeculae composed of pale cells with sharply defined plant-like cell borders (vegetable cells)
- 2 types of tumor cells:
- Type 1: large, polygonal cells with hard cell border; abundant cytoplasm with reticular pattern
- Type 2: smaller cells with finely granular eosinophilic cytoplasm (predominant in eosinophilic variant)
- Nuclei are irregular, wrinkled and angulated with coarse chromatin (raisinoid)
- Common binucleation, multinucleation and perinuclear halos (koilocytic atypia)
- Mitotic figures present but usually scant
- Stroma minimal, composed of incomplete fibrovascular septae around solid sheets
- No chicken wire vasculature (more fibrovascular than vascular)
- Fuhrman / WHO grading has no prognostic value and is discouraged (Histopathology 2019;74:4)
- Paner grading system adds no prognostic value after considering TNM stage and sarcomatoid differentiation (Am J Surg Pathol 2010;34:1233, Am J Surg Pathol 2012;36:851)
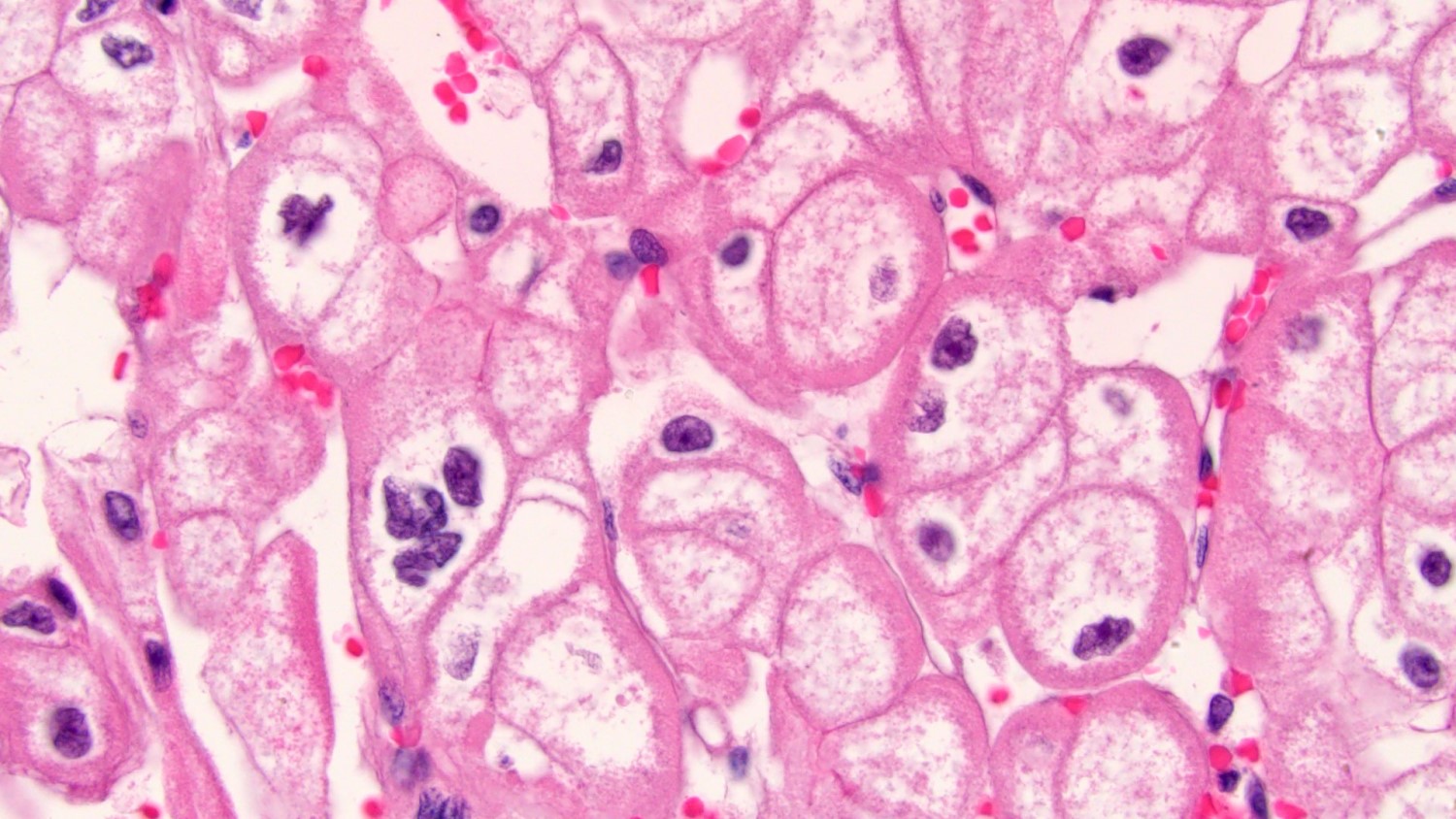
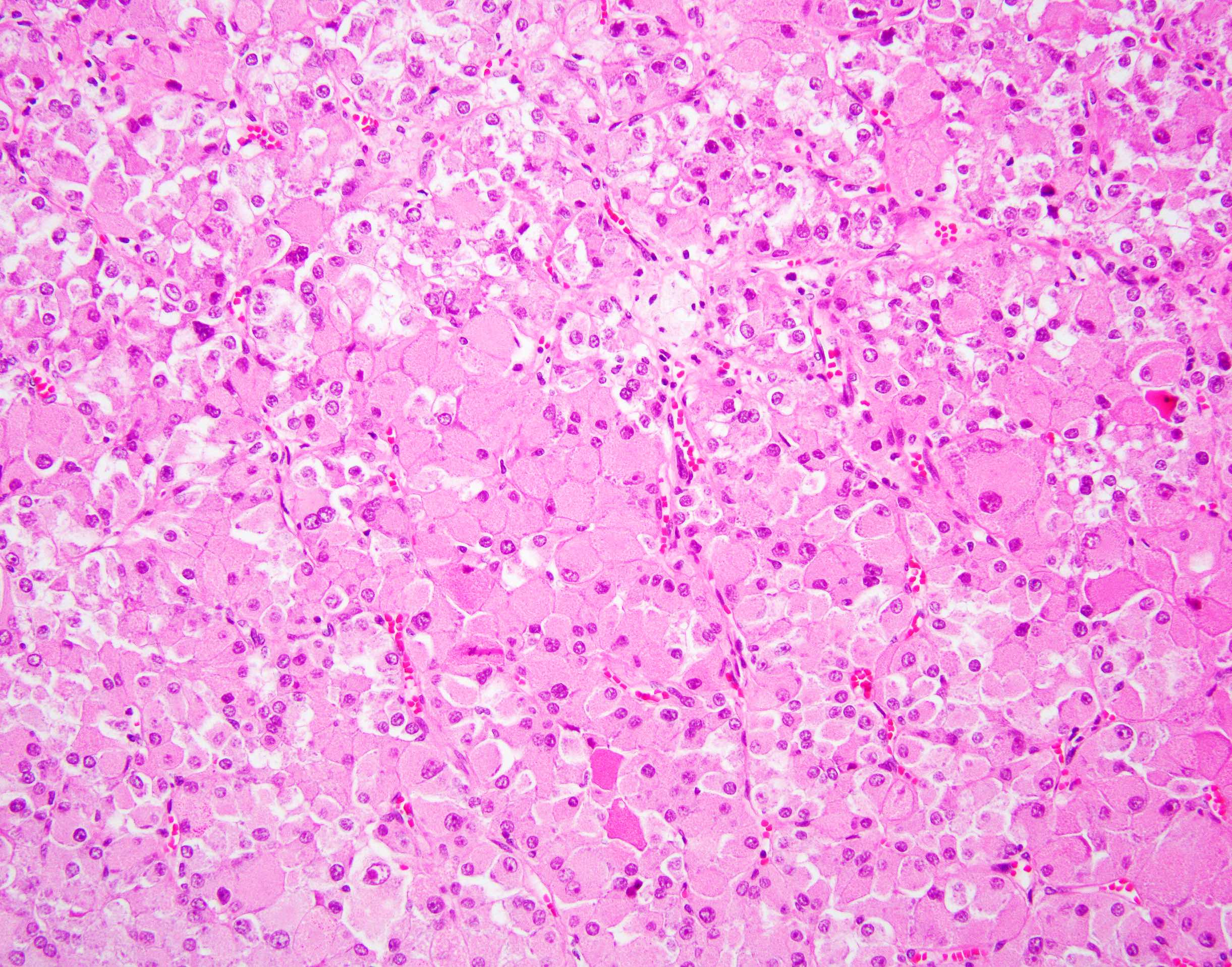
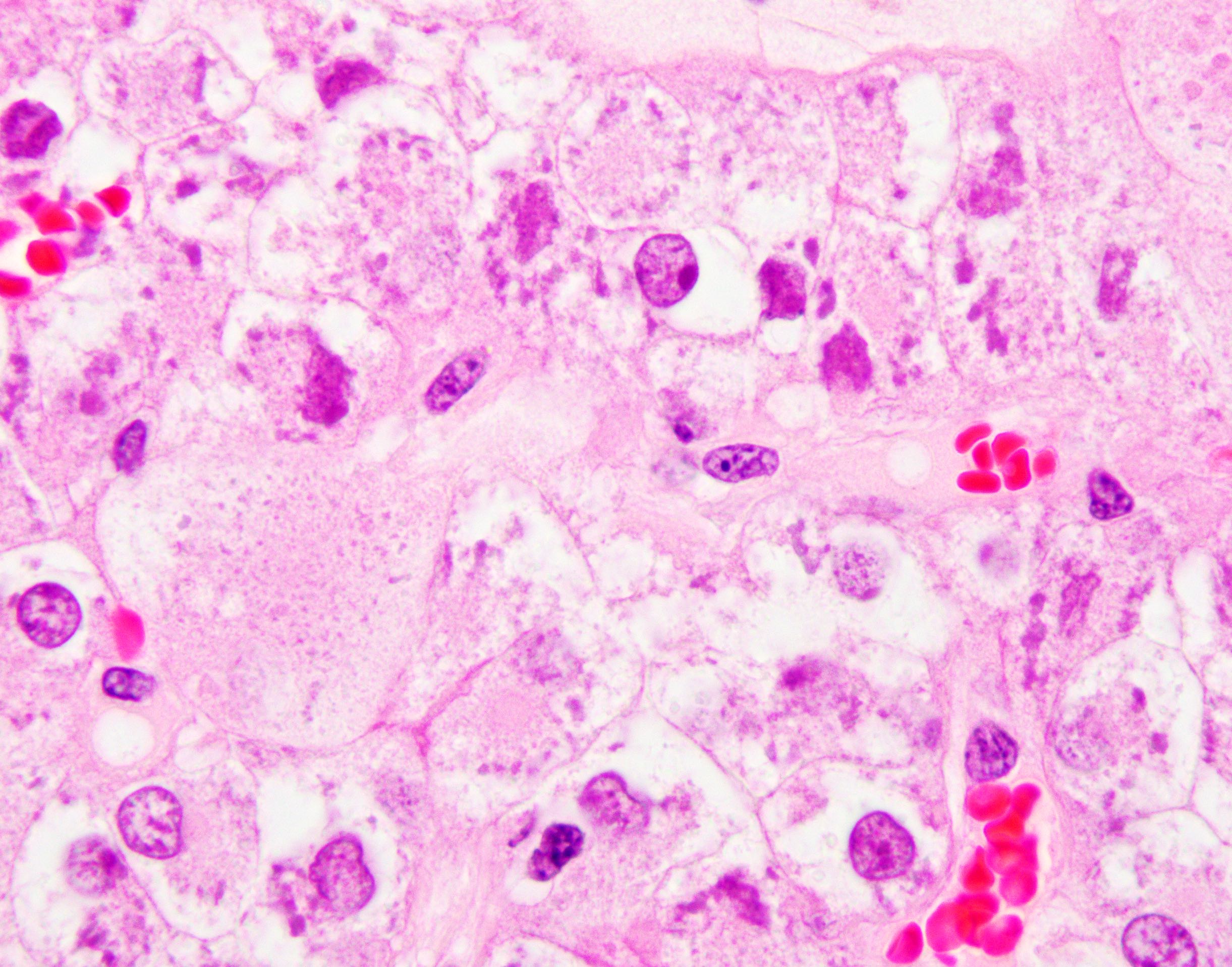
- Eosinophilic variant of chromophobe renal cell carcinoma: when composed entirely of smaller eosinophilic cells
Contributed by Maria Tretiakova, M.D., Ph.D., Daniel Anderson, M.D., M.B.A. and @katcollmd on Twitter
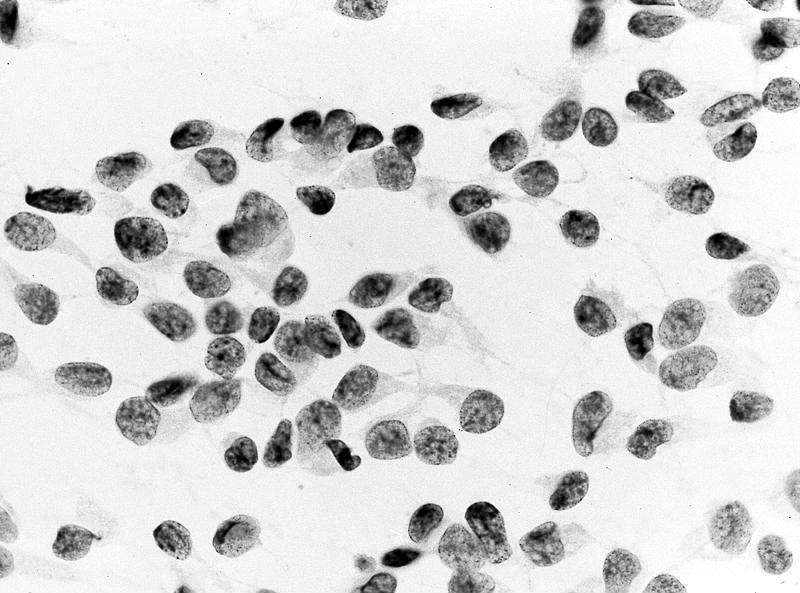
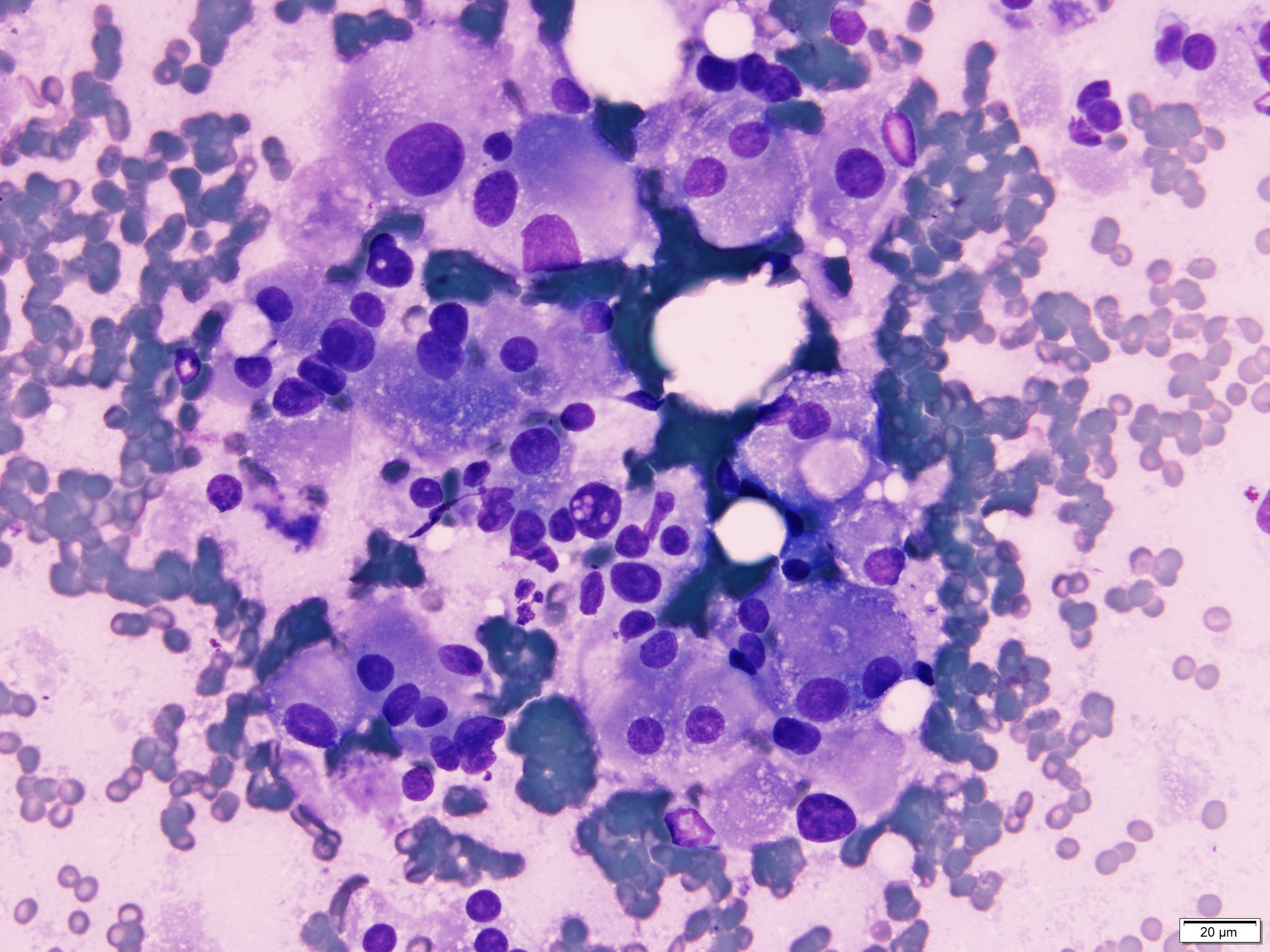
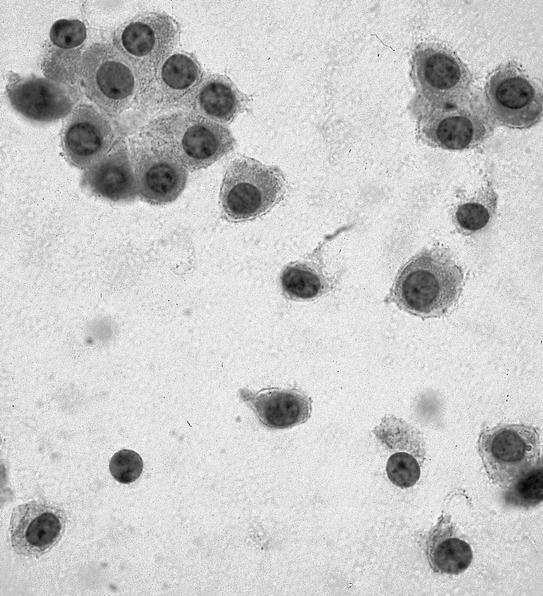
- Single cells and small, discohesive, monolayered groups; cells vary in size from small to large
- Large cells show clear, flocculent cytoplasm with small, eccentric nuclei and frequent binucleation, occasional nuclear pseudoinclusions
- Small cells usually have dense, homogeneous cytoplasm, clear cytoplasmic spaces resembling perinuclear halos, binucleation and marginal nuclear location
- No necrosis, no basement membrane or other stromal material (Cytopathology 2009;20:44, Cancer 1997;81:122)
- Most commonly used:
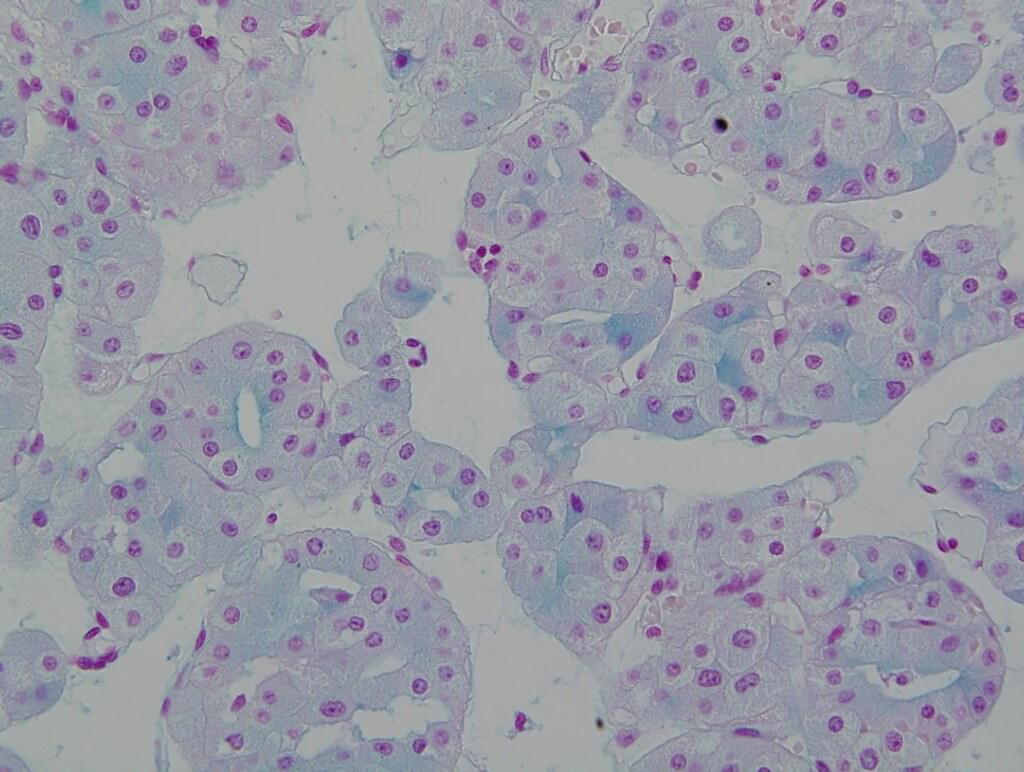
- Hale colloidal iron: stains acid mucopolysaccharides in microvesicles, diffuse and strong, reticular (J Pathol 1988;155:277, Am J Surg Pathol 1998;22:419)
- CK7: diffuse and strong (compared with oncocytoma with scattered single cells) (Am J Clin Pathol 2007;127:225)
- CD117 / KIT: membranous (Hum Pathol 2005;36:262)
- Other positive stains:
- E-cadherin, claudin7 (distal nephron marker) (Arch Pathol Lab Med 2007;131:1541, Hum Pathol 2009;40:206)
- Kidney specific cadherin (Am J Clin Pathol 2006;126:79, Mod Pathol 2005;18:933)
- EMA / MUC1 (diffuse cytoplasmic) (Mod Pathol 2004;17:180)
- Low molecular weight keratin (CK8 / CK18), RCC, CD10 (Mod Pathol 2004;17:1455)
- Parvalbumin (calcium binding protein) (Mod Pathol 2001;14:760)
- Cytochrome c oxidase positive versus negative in oncocytoma (Appl Immunohistochem Mol Morphol 2015;23:54)
- DOG1 (Pathol Res Pract 2015;211:303)
- PR (90% cells) (Arch Pathol Lab Med 2019;143:1455)
- PDL1 22C3 expressed in a minority of cases (Med Oncol 2016;33:120)
- Vimentin (or weak), CAIX, AMACR, N-cadherin, low Ki67 labeling index (Mod Pathol 1999;12:310, Mod Pathol 1998;11:1115)
- Cyclin D1 (Pathol Res Pract 2015;211:303)
| Hale | KIT | CK7 | S100A1 | VIM | CAIX | AMACR | SDH | TFE3 | |
| Chromophobe RCC | +++ | +++ | +++ | - | - | - | - | +++ | - |
| Clear cell RCC | - | - | - | - | +++ | +++ | - | +++ | - |
| Oncocytoma | - | +++ | rare | +++ | - | - | - | +++ | - |
| Papillary RCC | - | - | +++ | - | +++ | - | +++ | +++ | - |
| Translocation RCC | - | - | - | - | - | - | ++ | +++ | +++ |
| SDH deficient RCC | - | - | - | - | - | - | - | - | - |
References: Pathol Res Pract 2015;211:303, Ann Diagn Pathol 2020;44:151448, Am J Surg Pathol 2014;38:e6, Arch Pathol Lab Med 2019;143:1455, Transl Androl Urol 2019;8:S123, Hum Pathol 2020 Jul 13 [Epub ahead of print]
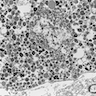
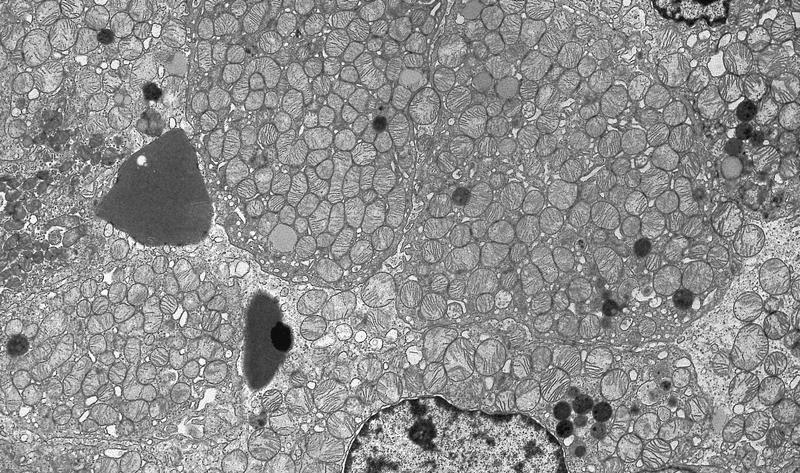
- All contain cytoplasmic microvesicles, which are considered a unique and consistent ultrastructural feature (Am J Surg Pathol 2000;24:1247)
- These 150 - 350 nm membrane bound microvesicles do not stain with H&E stain (chromophobe), often concentrated in perinuclear location corresponding to perinuclear halos on light microscopy and reticular pale cytoplasm
- Microvesicle origin is uncertain but possibly from mitochondrial outpouchings or defective mitochondriogenesis
- Eosinophilic cells are similar but with more mitochondria; mitochondria often show tubulocystic cristae, rare short and stubby microvilli (Am J Surg Pathol 2000;24:1247)
- Multiple losses of whole chromosomes, most often 1, 2, 6, 10, 13, 17, 21 or Y (versus no loss in oncocytoma) (Hum Pathol 1998;29:1181, Mod Pathol 2005;18:161)
- FISH and SNP arrays may be more helpful than karyotyping (Am J Clin Pathol 2010;133:116)
- DNA rearrangement breakpoints within the TERT promoter region
- TP53 and PTEN mutated in 10 - 30% cases (TCGA cohort) (Cancer Cell 2014;26:319)
- NRAS, mTOR and TSC1 / TSC2 in ~5% cases (JCI Insight 2017;2:e92688)
- Mitochondrial (mt) mutations DNA most commonly affect MT-ND5 (Cancer Cell 2014;26:319)
- Increased expression of genes encoding enzymes in the Krebs cycle (expression is suppressed in clear cell RCC) (Cancer Cell 2014;26:319)
- Sarcomatoid tumors have different genetic abnormalities (Mod Pathol 2007;20:303)
- MiRNA expression patterns may be associated with progression, recurrence free survival and overall survival (Sci Rep 2015;5:10328)
Which of the following is true about chromophobe (ChRCC)?
- Binucleation is pathognomonic
- Cellulose deposition explains plant-like prominent membranes
- Cytoplasmic clearing is due to high concentration of lipids
- Multinucleation is an unfavorable prognostic feature
- Perinuclear halos are due to accumulation of cytoplasmic microvesicles
Comment Here
Reference: Chromophobe
- Can be reliably distinguished from clear cell RCC by CK7, CKIT (CD117), CAIX and vimentin
- Classic autosomal dominant genetic correlation is Birt-Hogg-Dubé syndrome, which is associated with a mutation of the HOGG gene on chromosome 3p
- Fuhrman / WHO grading is useful prognostic indicator
- Sarcomatoid dedifferentiation is very common
- To establish the diagnosis, a documentation of monosomies involving chromosomes 1, 2, 6, 10, 13, 17, 21 or Y is needed
Comment Here
Reference: Chromophobe
- Histologic variant of chromophobe renal cell carcinoma (ChRCC) with at least 80% of cells with eosinophilic cytoplasm
- At least 80% of the neoplastic cells must be eosinophilic
- No difference in prognosis compared with classic / mixed chromophobe renal cell carcinoma
- Must distinguish from benign mimic oncocytoma
- Chromophobe renal cell carcinoma is ~ 5% of all renal cell carcinomas (Int J Urol 2012;19:894)
- In large study case series, 41 - 51% of ChRCC were eosinophilic variant (Am J Surg Pathol 2008;32:1822, Eur J Surg Oncol 2008;34:687)
- Studies do not mention other epidemiologic differences from classic ChRCC
- Kidney in corticomedullary parenchyma
- Same as classic ChRCC: neoplasm of intercalated cells of collecting duct (Virchows Arch B Cell Pathol Incl Mol Pathol 1989;56:237)
- Eosinophilic cytoplasm is due to increased mitochondria
- Potential presenting symptoms: hematuria, pain, flank mass, anemia, pyrexia, cachexia, fatigue and weight loss (Int J Urol 2012;19:894)
- Large case series found eosinophilic more likely to be bilateral (11%) and multifocal (22%) (Am J Surg Pathol 2008;32:1822)
- Can be part of Birt-Hogg-Dubé syndrome (BHD) (FLCN mutation) (Am J Surg Pathol 2002;26:1542)
- Fibrofolliculomas, spontaneous pneumothorax, other renal neoplasms
- Mass seen on imaging with confirmatory renal biopsy or mass resection
- Biopsy may not be diagnostic, especially if limited
- On CT scan, ChRCC more likely to have homogenous enhancement (69%) and can have calcifications (38%) (AJR Am J Roentgenol 2002;178:1499)
- No studies identified differentiating findings in eosinophilic from classic ChRCC
- No difference in outcome from classic ChRCC (Eur J Surg Oncol 2008;34:687, Cancers (Basel) 2019;11:1492, Am J Surg Pathol 2008;32:1822)
- 31 year old man, long term renal transplant recipient, with 1.8 cm mass in allograft (Case Rep Transplant 2017;2017:4232474)
- 55 year old man with painless hematuria, 9.0 cm upper pole renal mass extending into the pelvicalyceal system and concurrent right atrial myxoma (J Lab Physicians. 2011;3:116)
- 60 year old woman with intermittent painless hematuria, 9.0 cm upper pole renal mass (J Lab Physicians. 2011;3:116)
- 73 year old woman with 1.8 cm upper pole renal mass and 67 year old man with incidental 3.0 cm lower pole renal mass (Proc (Bayl Univ Med Cent) 2015;28:57)
- 80 year old man with incidental 2.5 cm renal mass with partial papillary growth (Int J Clin Exp Pathol 2015 Oct;8:13590)
- Typically resection via partial or total nephrectomy, the same as classic ChRCC
- Usually well circumscribed, lobulated and with beige to brown to yellow coloration
- Eosinophilic variant more likely to have brown coloration compared with classic, which is typically more beige-yellow (Am J Surg Pathol 2008;32:1822)
- Can mimic oncocytoma on frozen section (Biomedicine (Taipei) 2019;9:6)
- At least 80% of the chromophobe neoplastic cells must have eosinophilic cytoplasm to classify as eosinophilic variant
- Cells are often smaller than classic chromophobe cells
- Besides eosinophilic cytoplasm and smaller size, have otherwise similar histology to classic ChRCC including
- Well defined / thickened cell border
- Wrinkled, raisinoid nuclei
- Frequent binucleation
- Perinuclear halos / clearing
- Rare mitoses
- Lacking prominent vasculature
- Nested, alveolar or sheet-like architecture
- Nested architecture more common in eosinophilic than classic (Am J Surg Pathol 2008;32:1822)
- Grading is not recommended
- Fuhrman grading system not predictive of outcome and is obsolete (Am J Surg Pathol 2007;31:957)
- WHO / ISUP system has not been validated for ChRCC (Am J Surg Pathol 2013;37:1490)
- Several grading schemes proposed but no overall consensus (Eur Urol 2016;70:93)
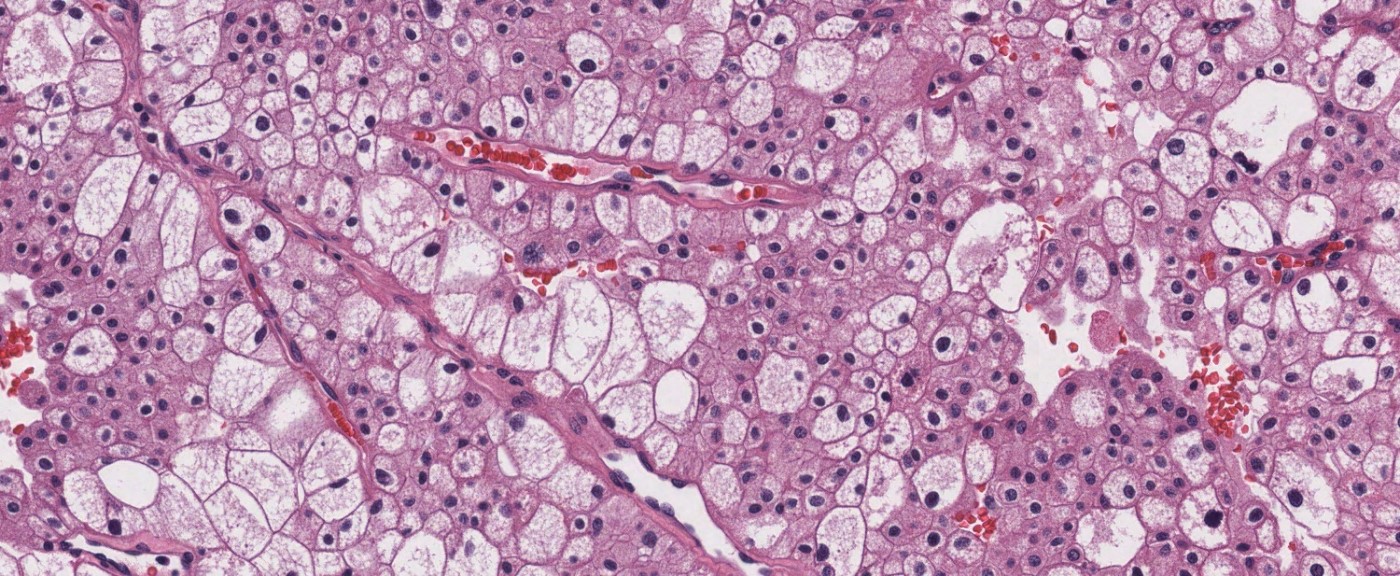
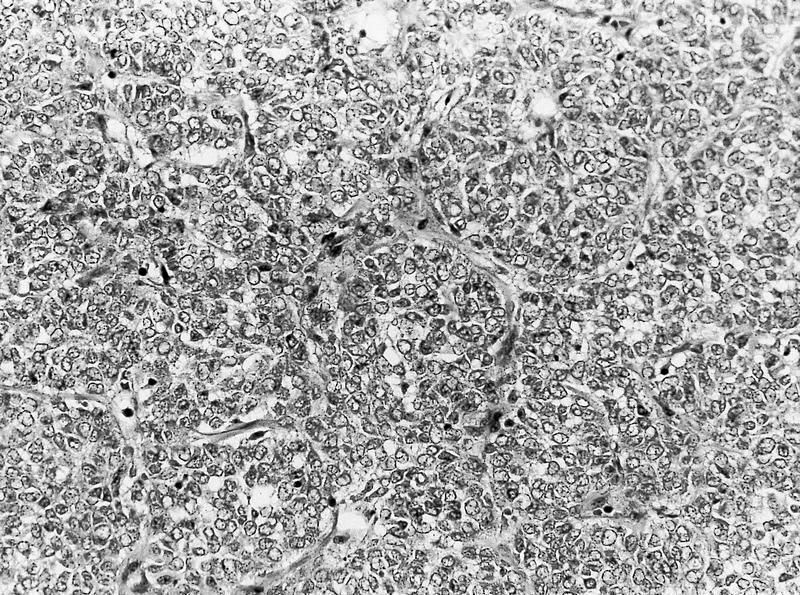
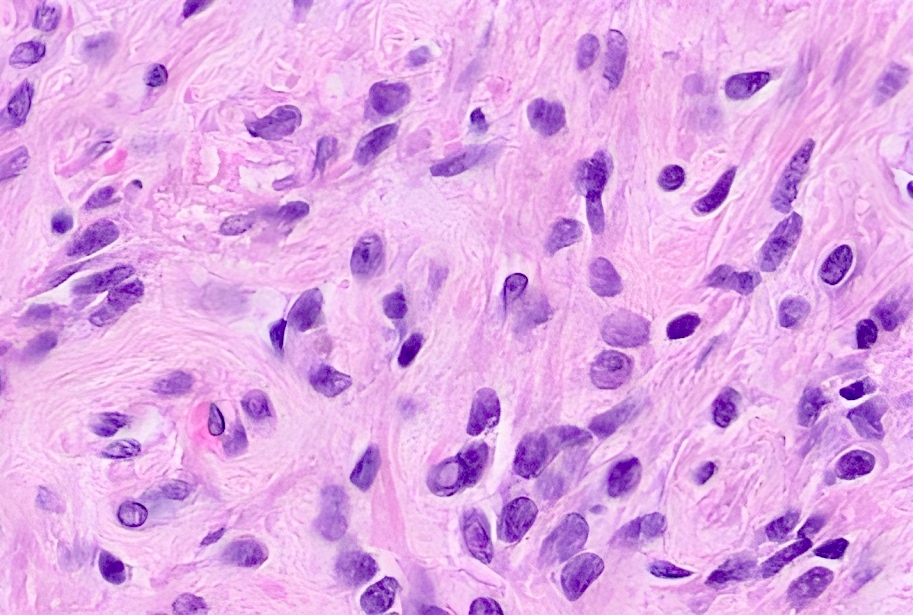
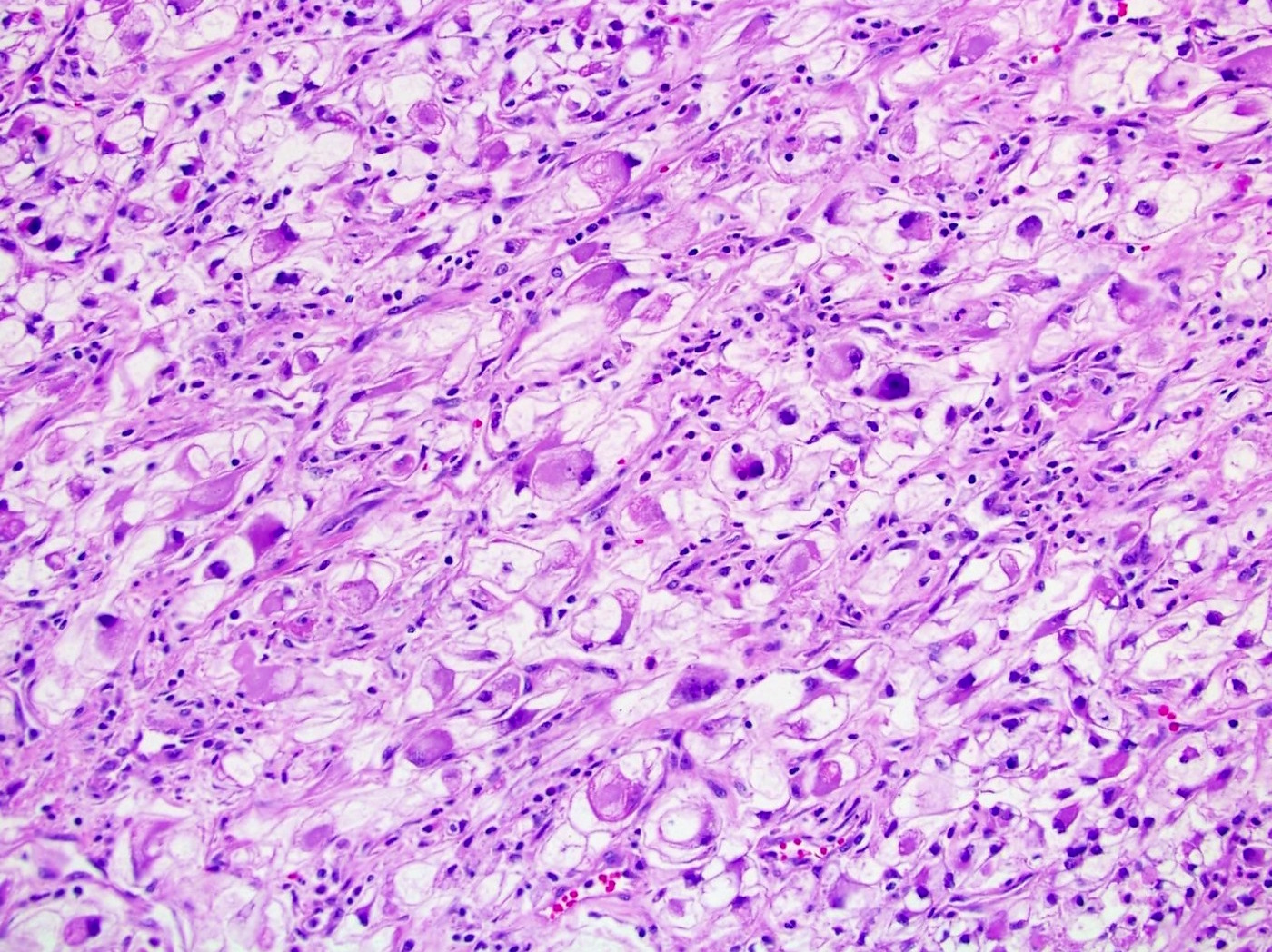
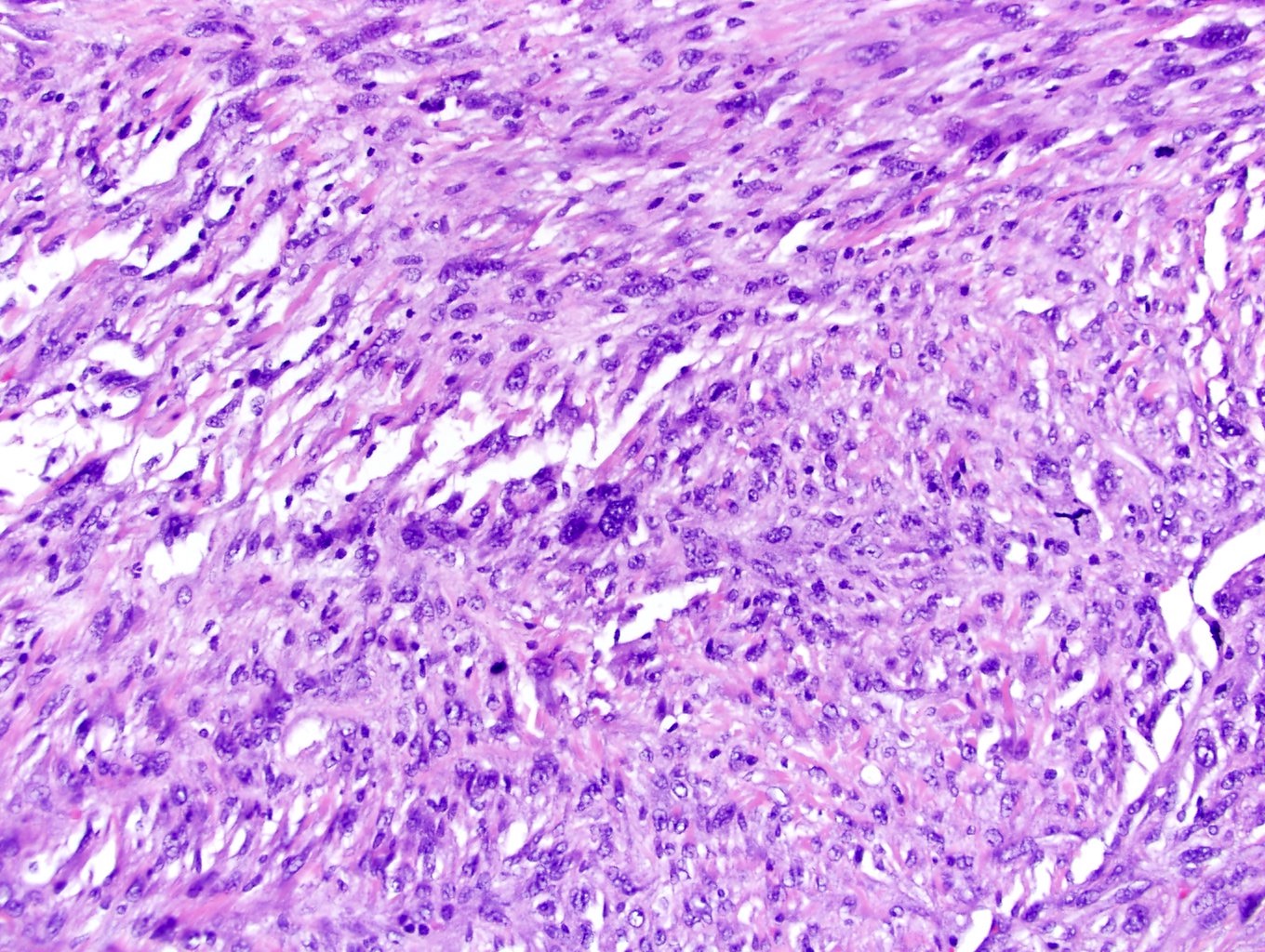
- Single or clusters of large cells with moderate pleomorphism
- Abundant granular eosinophilic cytoplasm
- Well defined / accentuated cell border
- Perinuclear clearing / vacuolization
- Binucleation may be seen
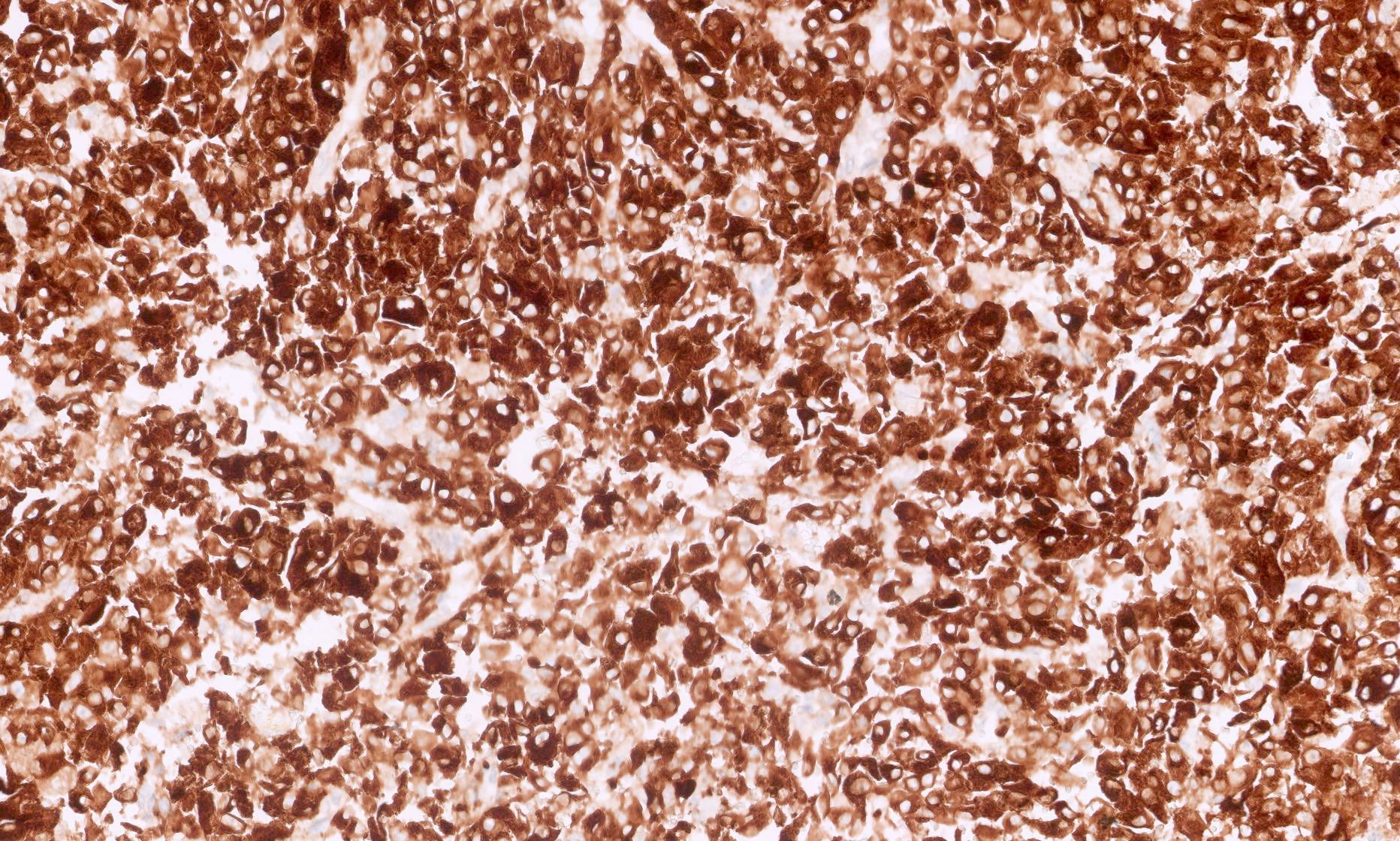
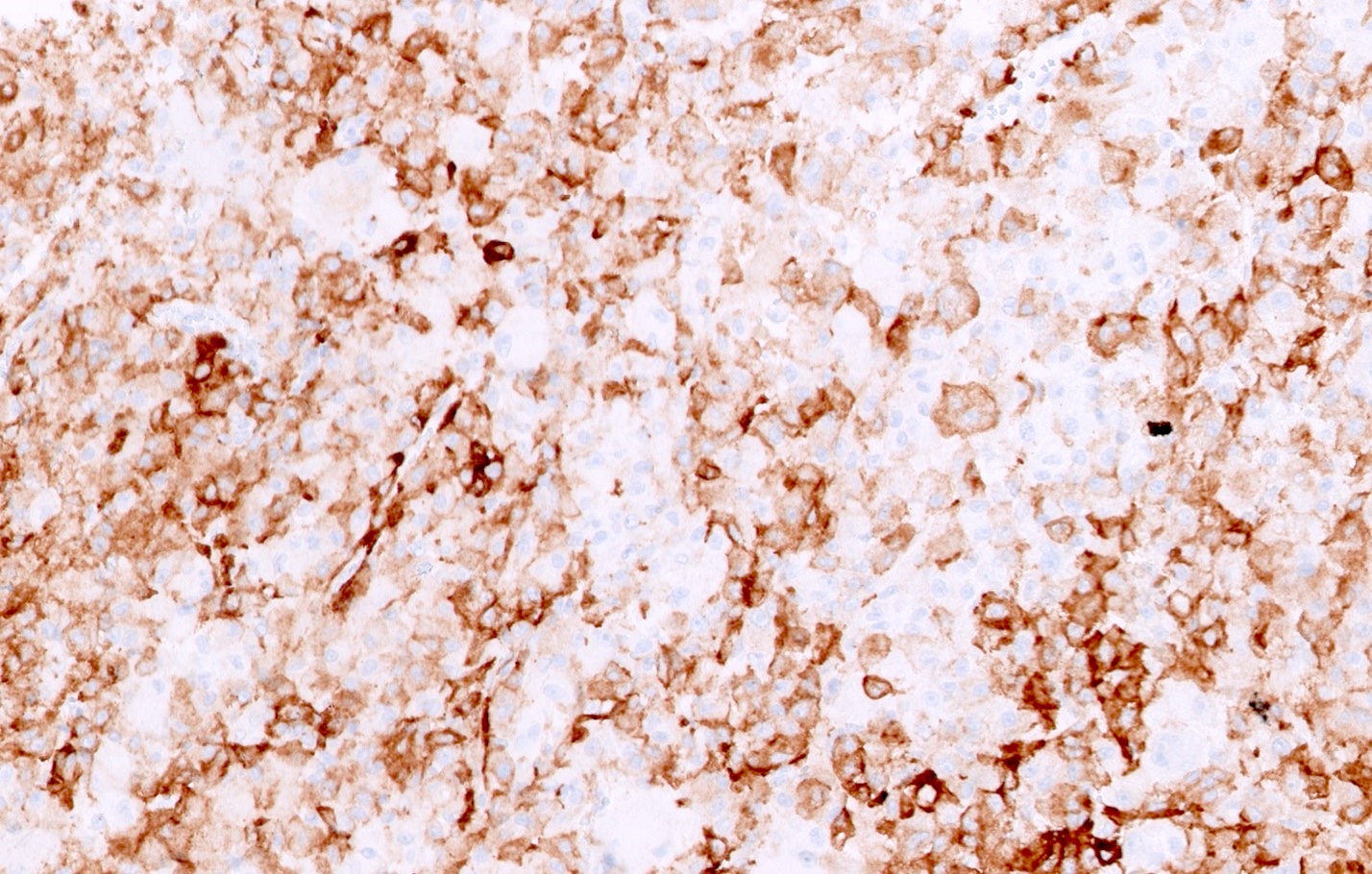
- CK7, KIT, Hale colloidal iron (diffuse granular cytoplasmic positivity)
- Claudin 7, EMA, E-cadherin, CK8, CK18, parvalbumin, EpCAM, ESA, ERA, KAI1, FXYD2 (J Clin Pathol 2016;69:661, Mod Pathol 1999;12:310, Am J Surg Pathol 2005;29:747, Mod Pathol 2001;14:760, Bratisl Lek Listy 2020;121:663, Med Mol Morphol 2012;45:98)
- Distinguishing classic versus eosinophilic variants of ChRCC: LMP2 (100% of eosinophilic) (Exp Mol Pathol 2013;94:29)
- Vimentin, CAIX, S100A1, RCC, CD10, GSTa, N-cadherin, amylase α1A, Wnt5a, HNF1β, AMACR, ARPP (Transl Androl Urol 2019;8:S123, Am J Surg Pathol 2000;24:203, Cancers (Basel) 2020;12:602, Bratisl Lek Listy 2020;121:663, Mod Pathol 1999;12:310, Am J Surg Pathol 2013;37:1824, Tumori 2010;96:304, Mod Pathol 2007;20:199)
- Numerous cytoplasmic microvesicles (Am J Surg Pathol 2000;24:1247)
- Mitochondria with tubulocystic cristae (Am J Surg Pathol 2000;24:1247)
- Eosinophilic variant has more abundant mitochondria (Pathol Int 2000;50:872)
- Compared with classic ChRCC, less frequent losses of chromosomes 1, 2, 6, 10, 13 and 17, suggesting classic has greater chromosomal instability (Cancers (Basel) 2019;11:1492)
- Most common chromosomal losses (in order of most to least frequent): 1, 2, 17, 6, 10, 13 and 21; no chromosomal gains (Adv Anat Pathol 2021;28:8)
- Doubled hypodiploidy by whole genome endoduplication is a common phenomenon in eosinophilic ChRCC (Hum Pathol 2020;104:18)
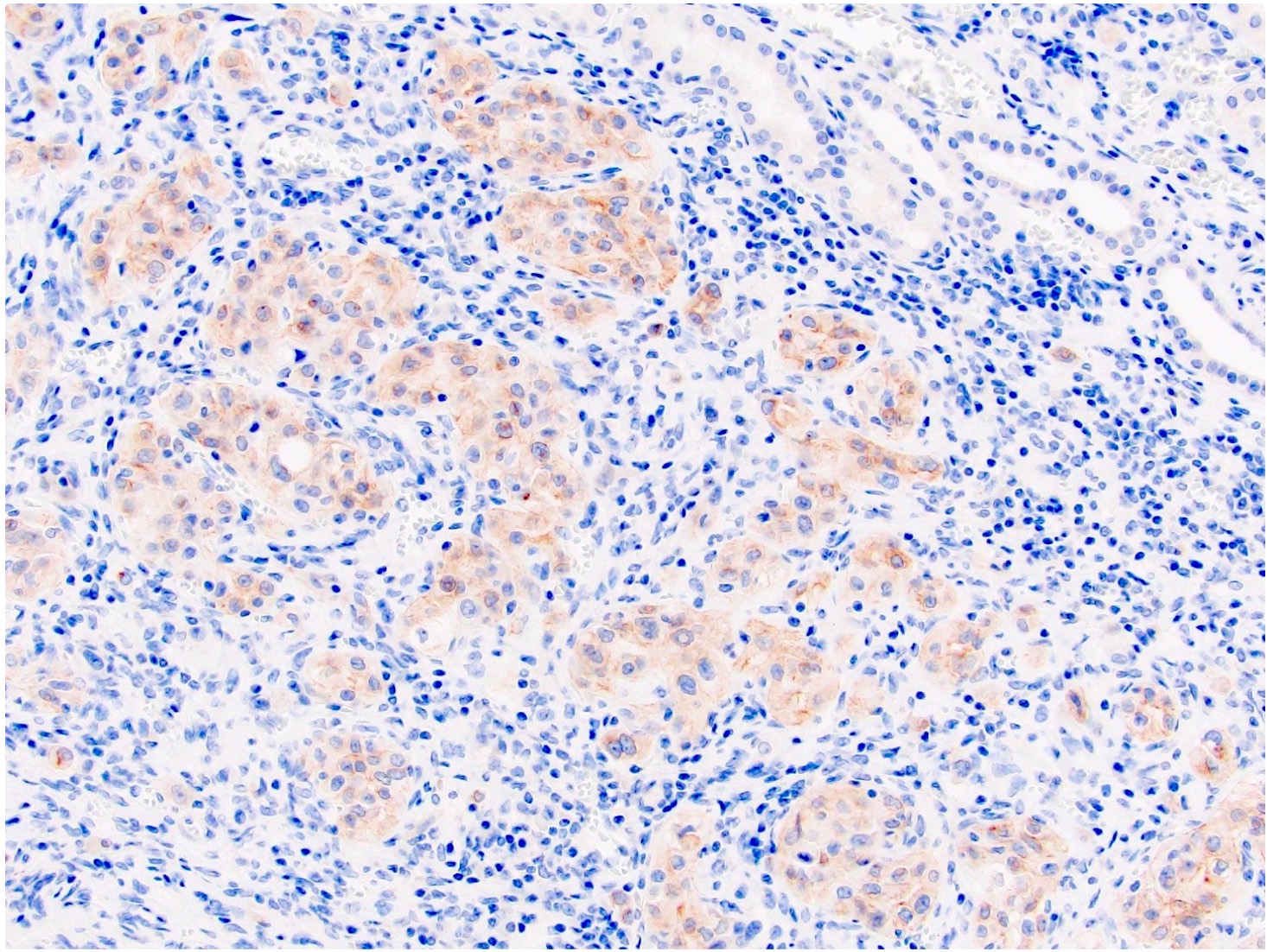
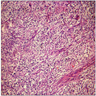
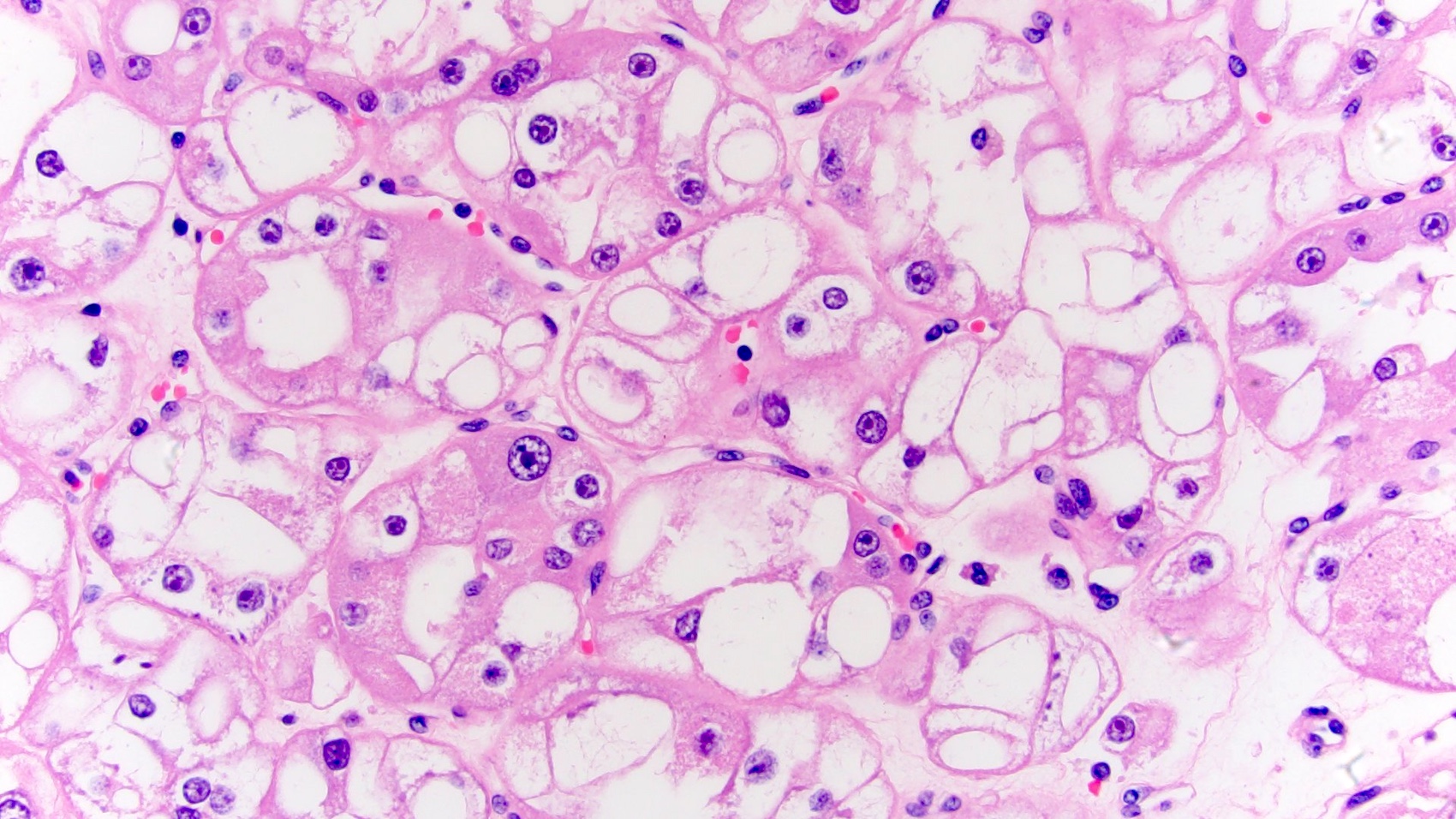
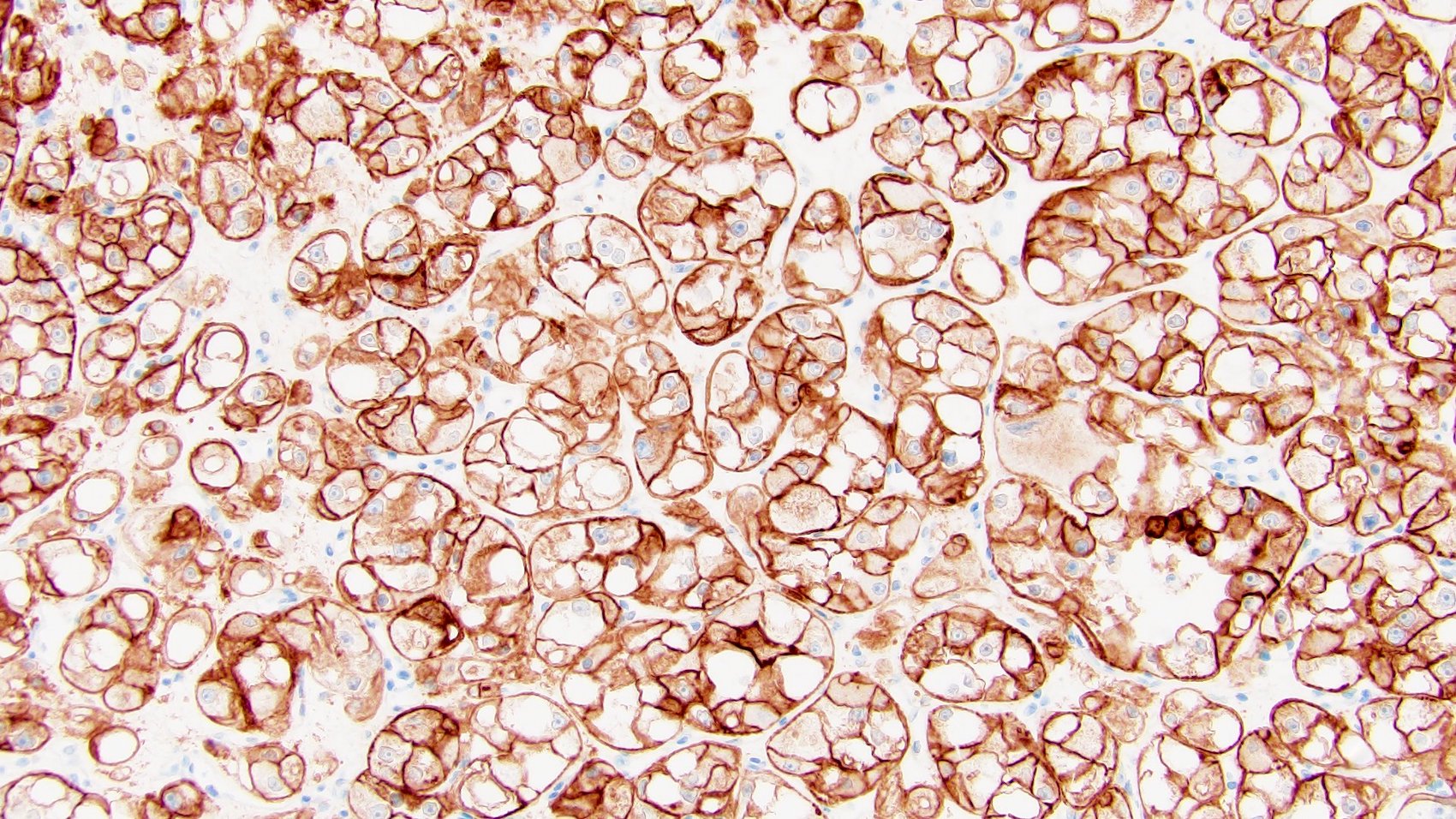
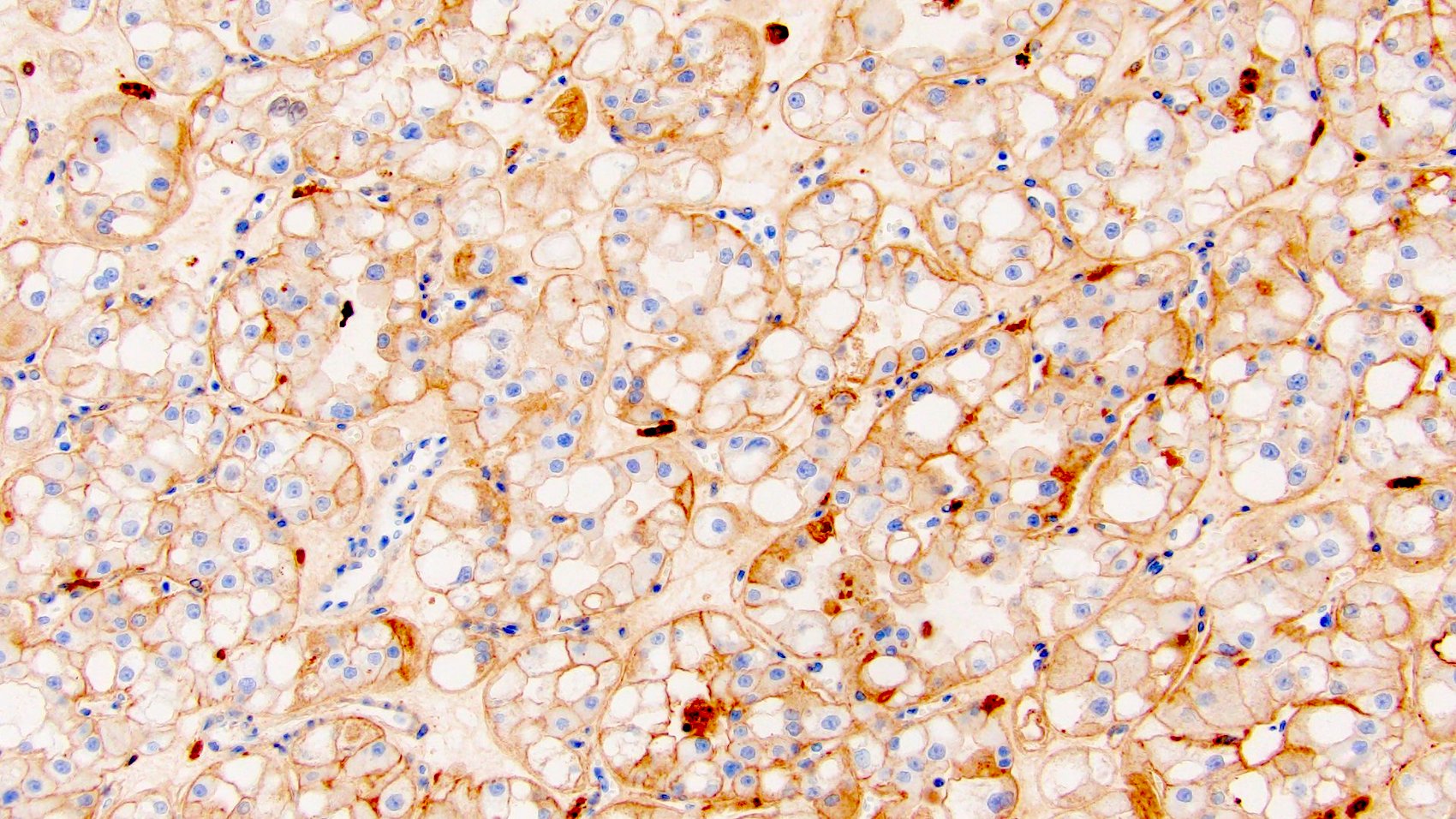
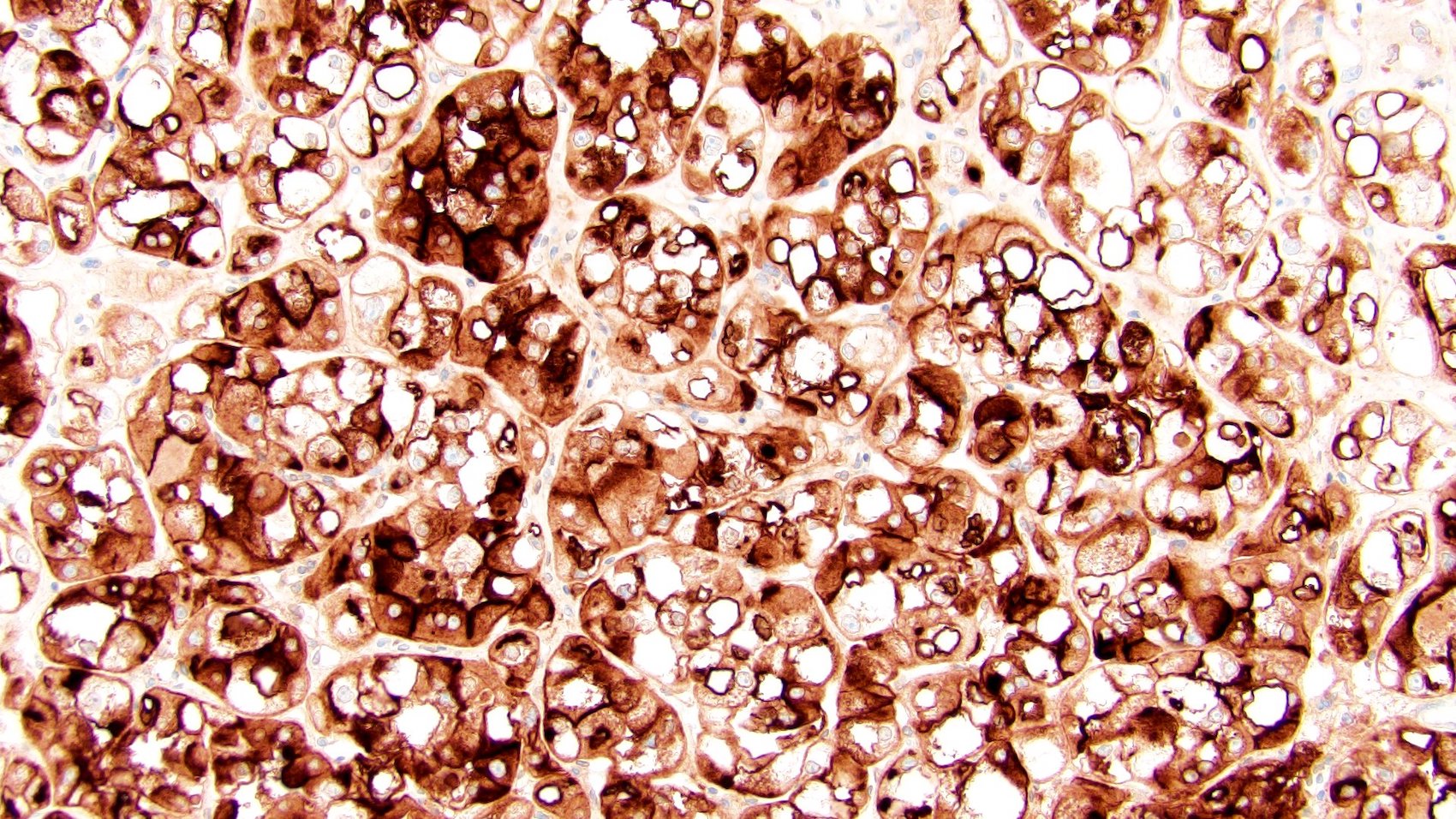
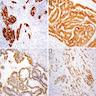
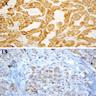
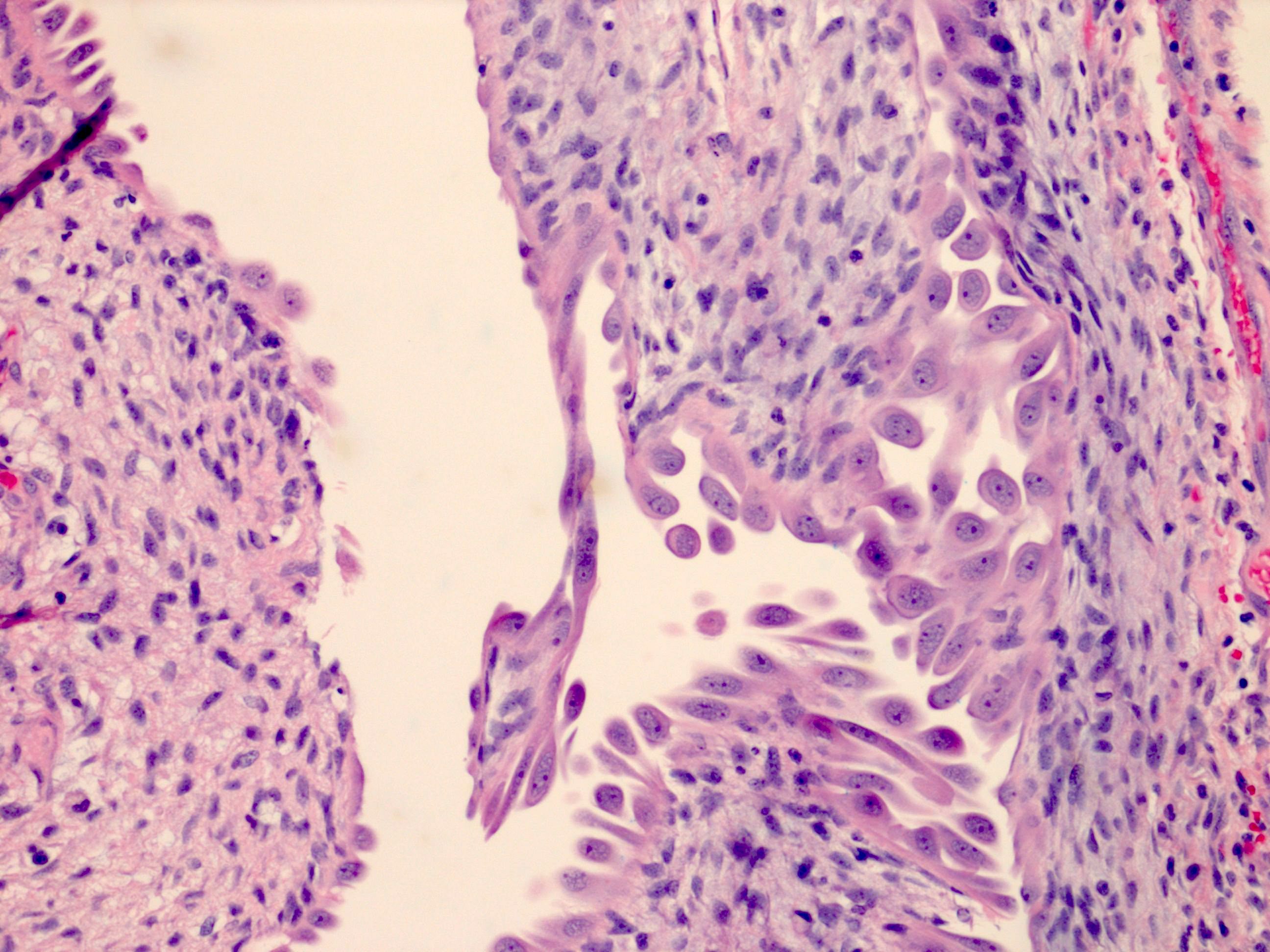
Distinguishing eosinophilic chromophobe RCC from oncocytoma
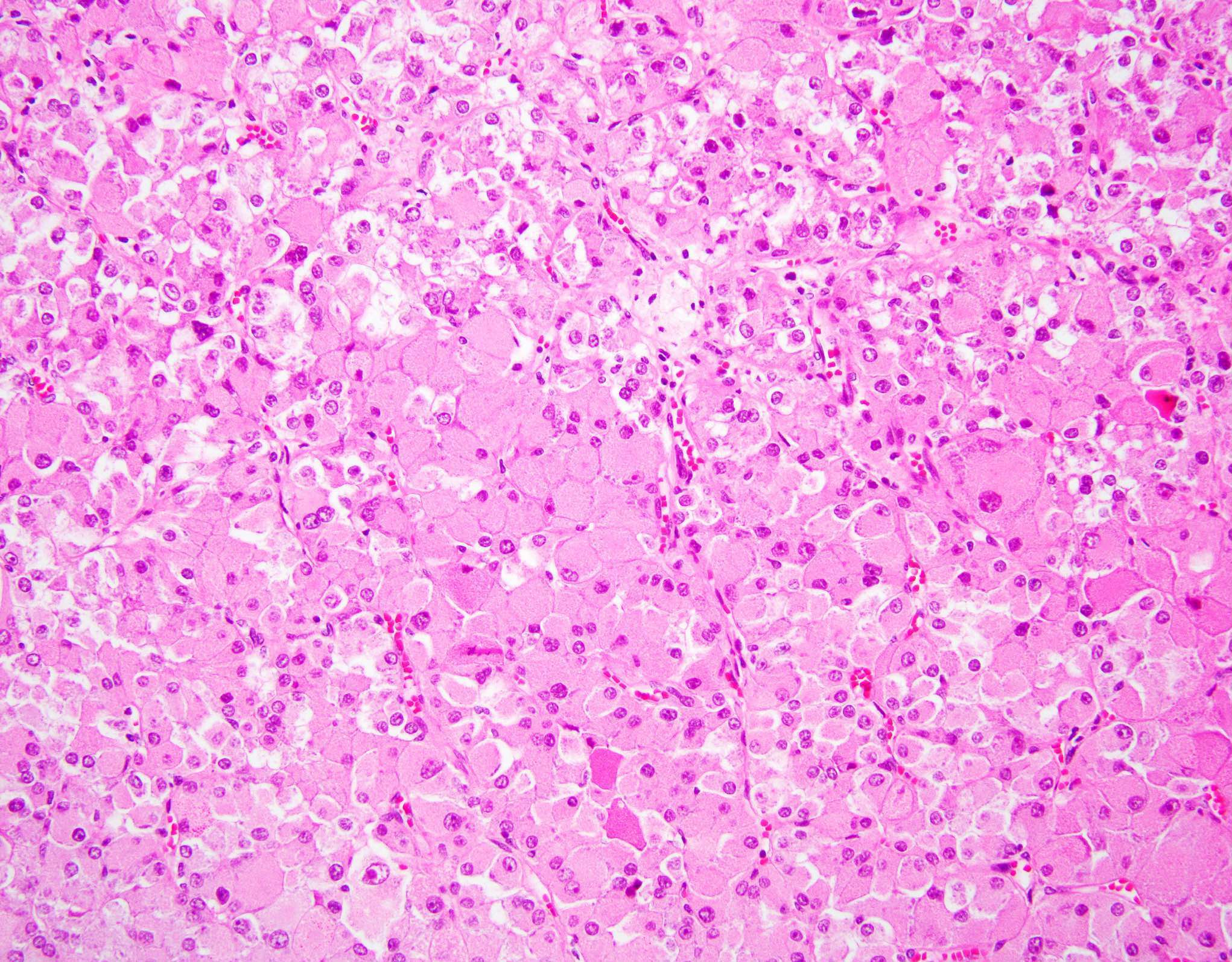
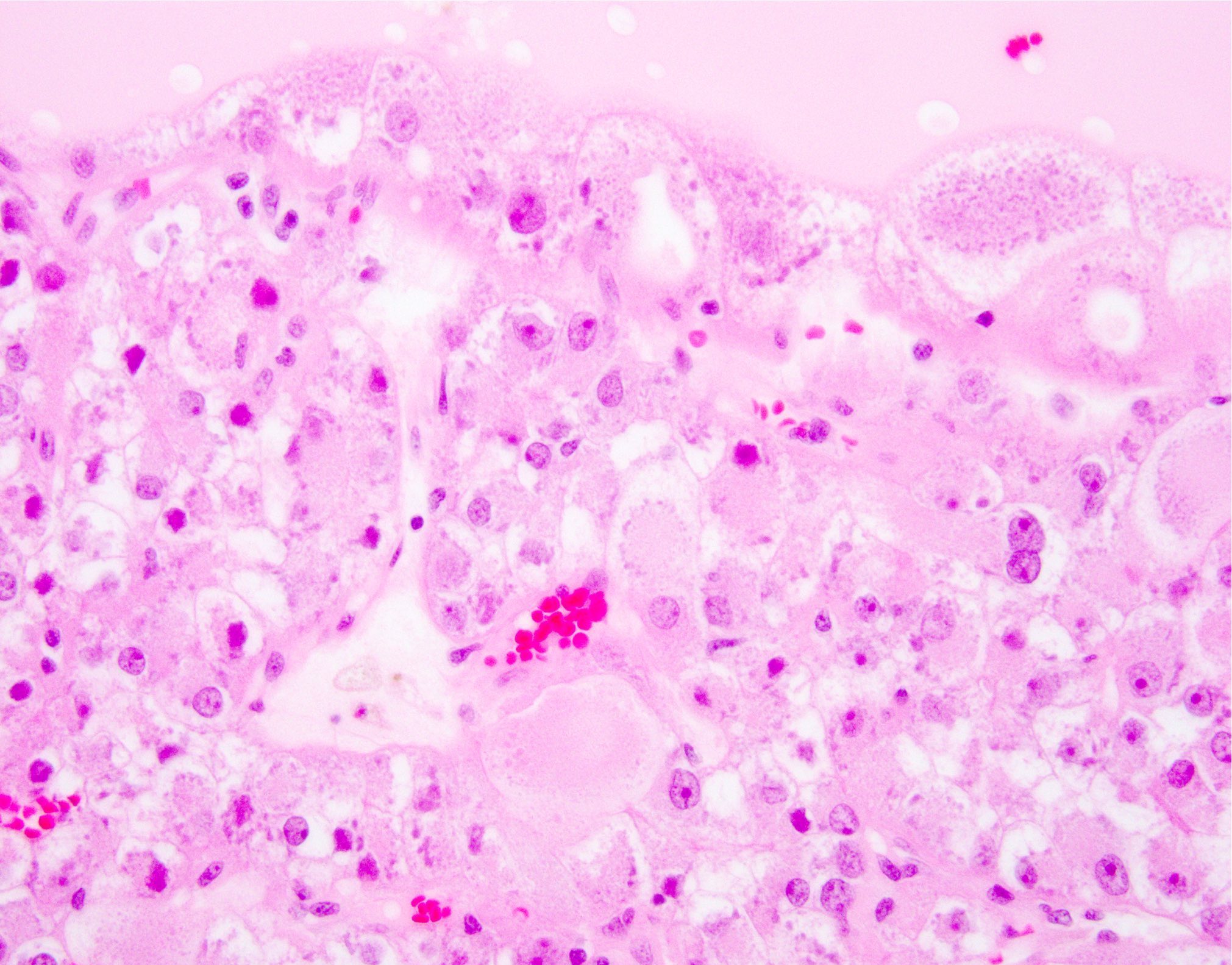
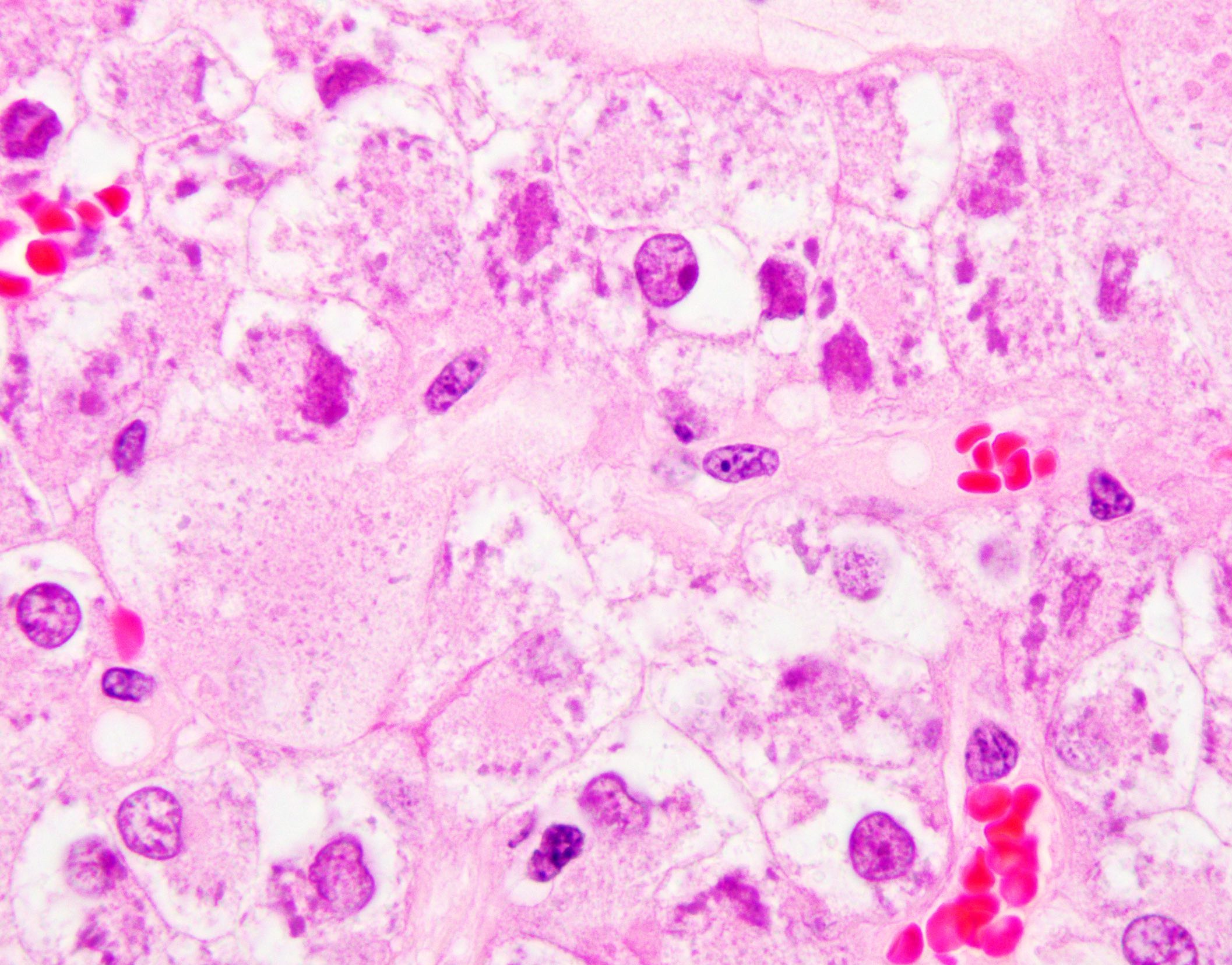
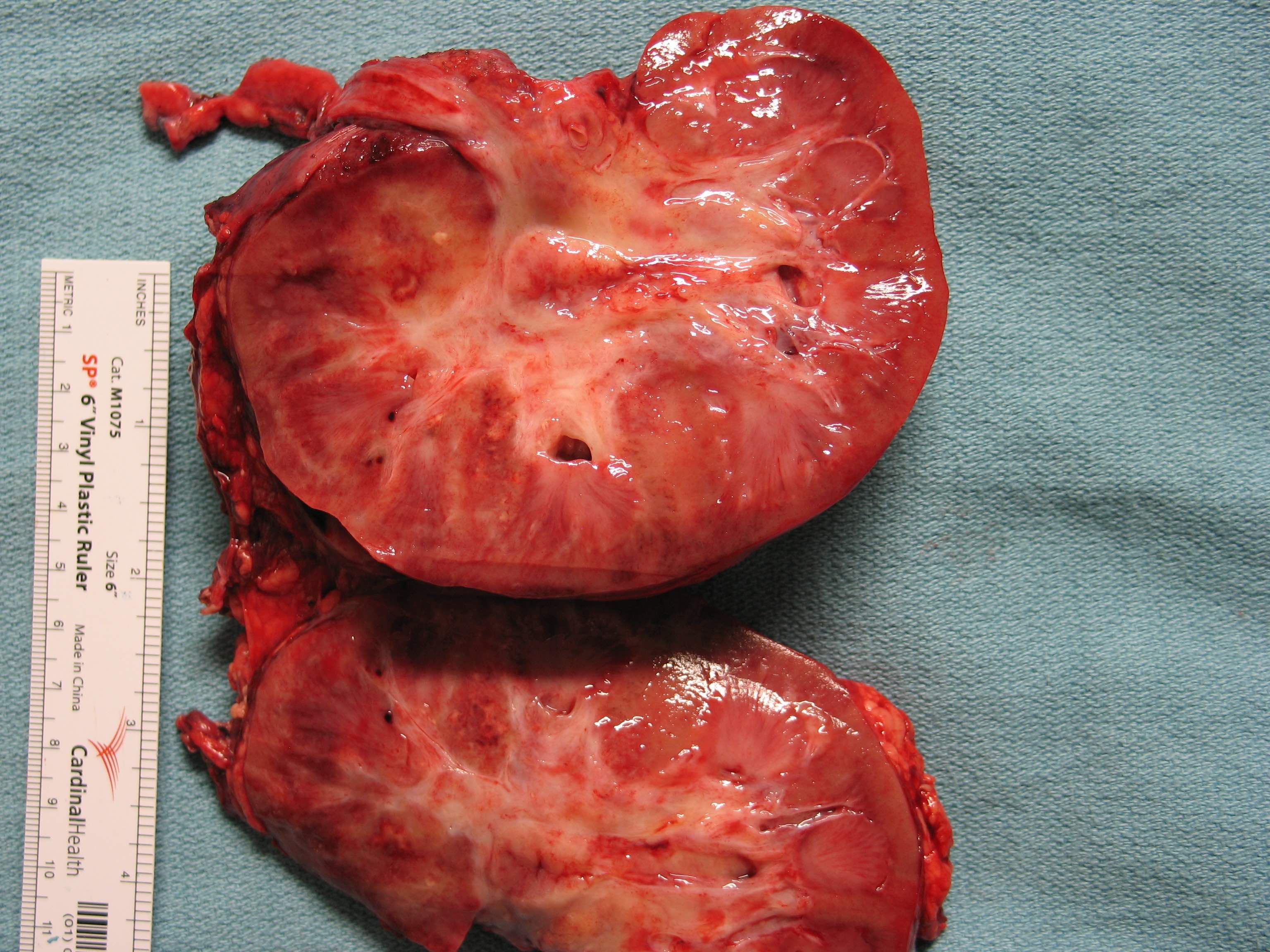
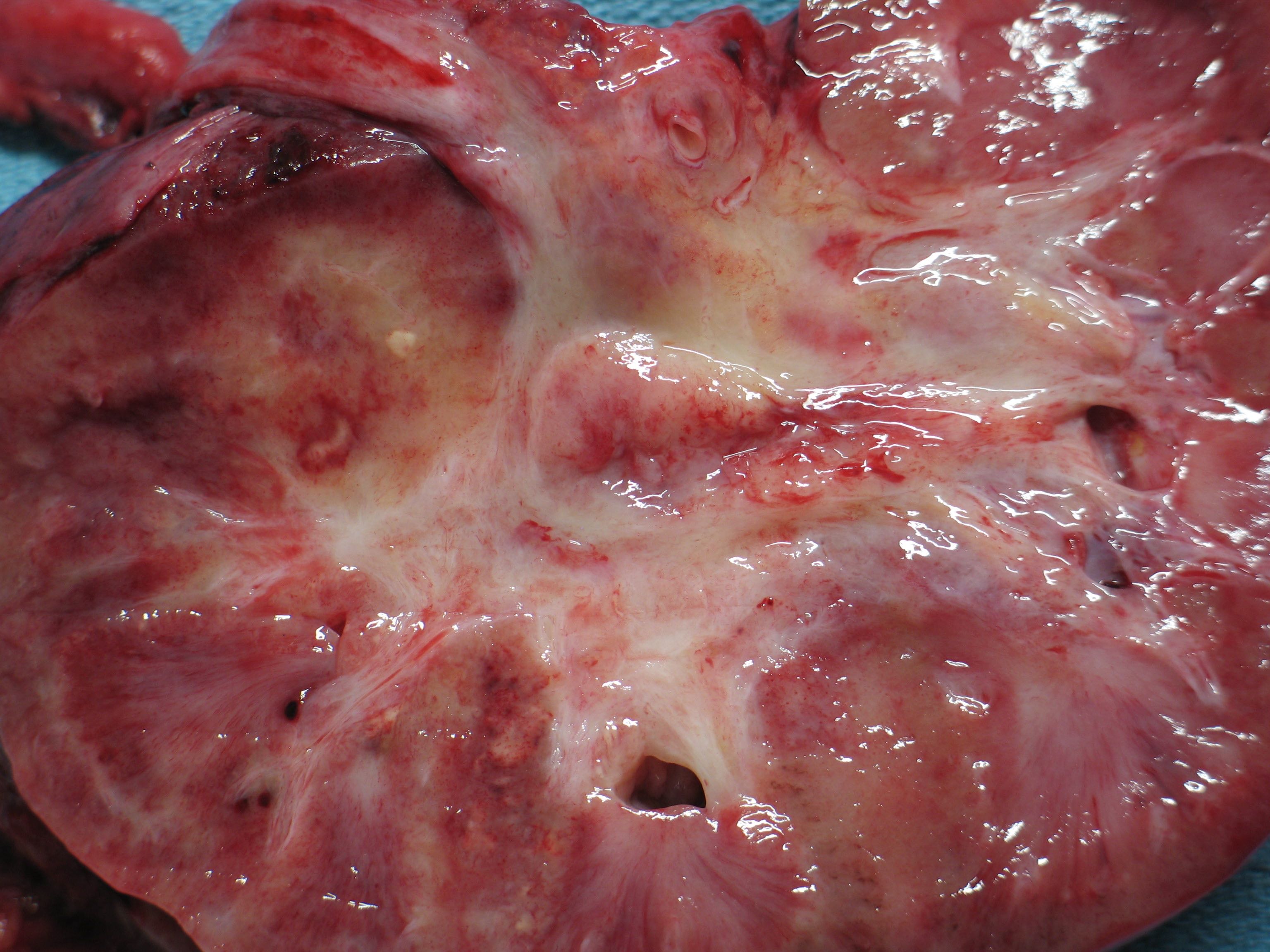
- Left kidney, mass, partial nephrectomy:
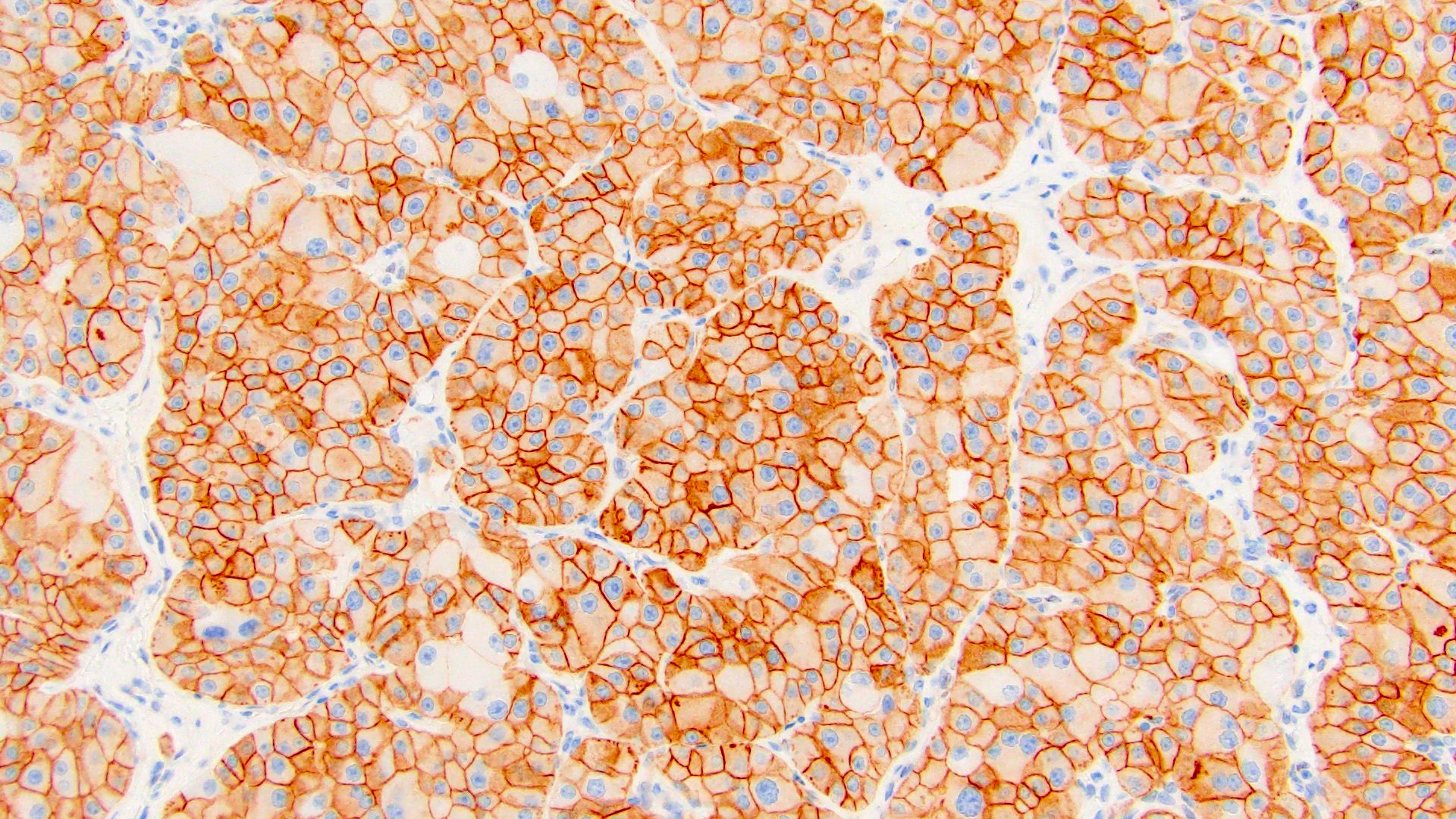
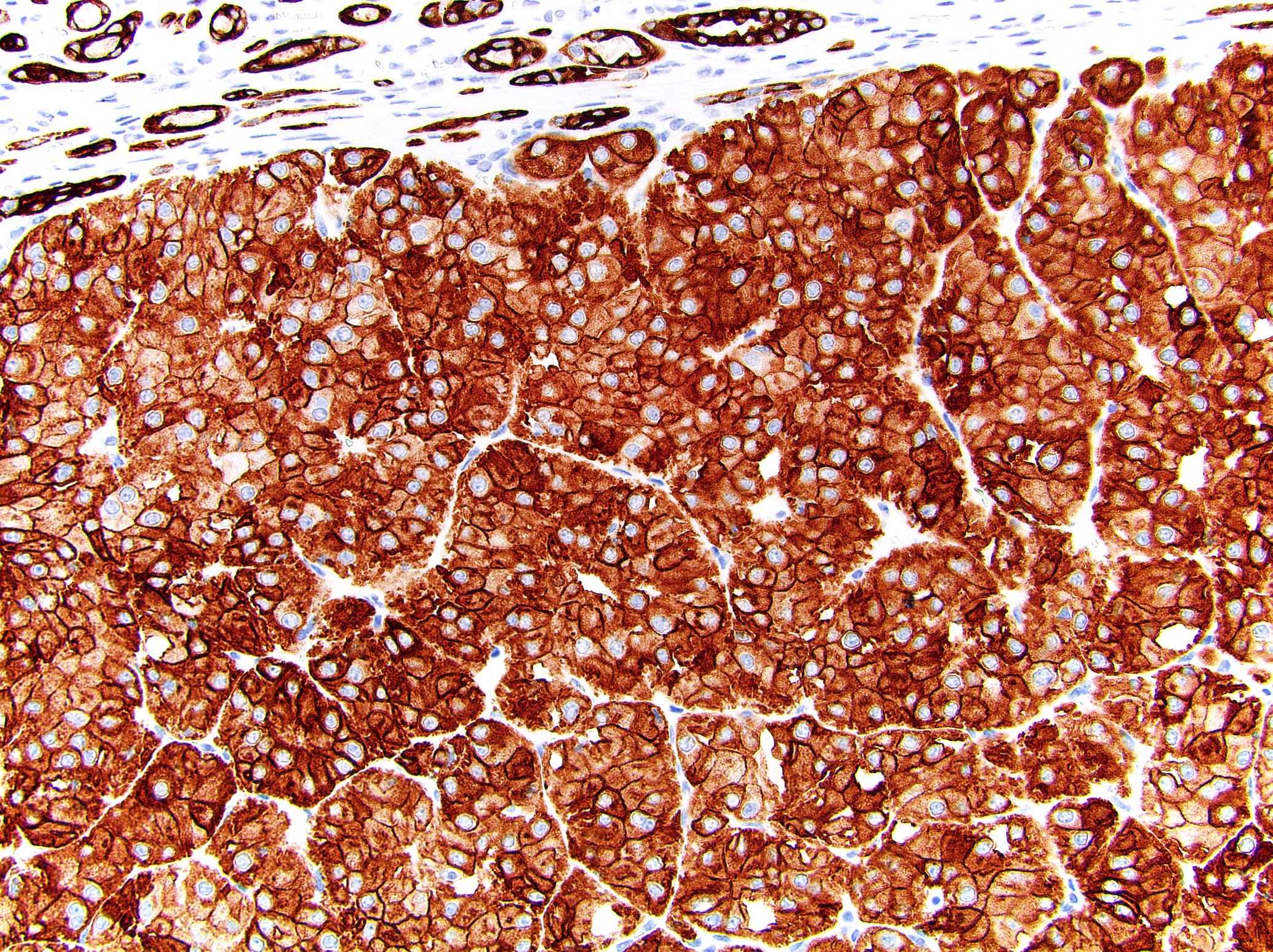
- Chromophobe renal cell carcinoma, eosinophilic variant (see synoptic report)
- Oncocytoma:
- Round, hyperchromatic nuclei; smooth nuclear border
- Cells arranged in a nested or tubular pattern
- Lacks nuclear pleomorphism, oval nuclei, multiple nucleoli
- CK7 usually negative
- Hale colloidal iron focal positive staining confined to luminal borders
- No copy number alterations or losses of chromosomes 1 and X / Y (Hum Pathol 2020;104:18)
- Hybrid oncocytic / chromophobe tumors (HOCT) (Histol Histopathol 2013;28:1257):
- Can be sporadic but also often found in association with renal oncocytomatosis or Birt-Hogg-Dubé (BHD) syndrome
- Sporadic / oncocytomatosis: solid alveolar pattern, scattered cells with perinuclear halos but with no raisinoid nuclei
- BHD: admixed areas of oncocytoma and ChRCC, scattered chromophobe cells, intracytoplasmic vacuoles
- No aggressive behavior
- Usually parvalbumin, antimitochondrial antigen and CK7 positive
- Sporadic cases do not exhibit mutations in genes that are recurrently mutated in oncocytoma or ChRCC; syndromic cases with folliculin gene mutations (Mod Pathol 2019;32:1698)
- Eosinophilic variant of clear cell renal cell carcinoma:
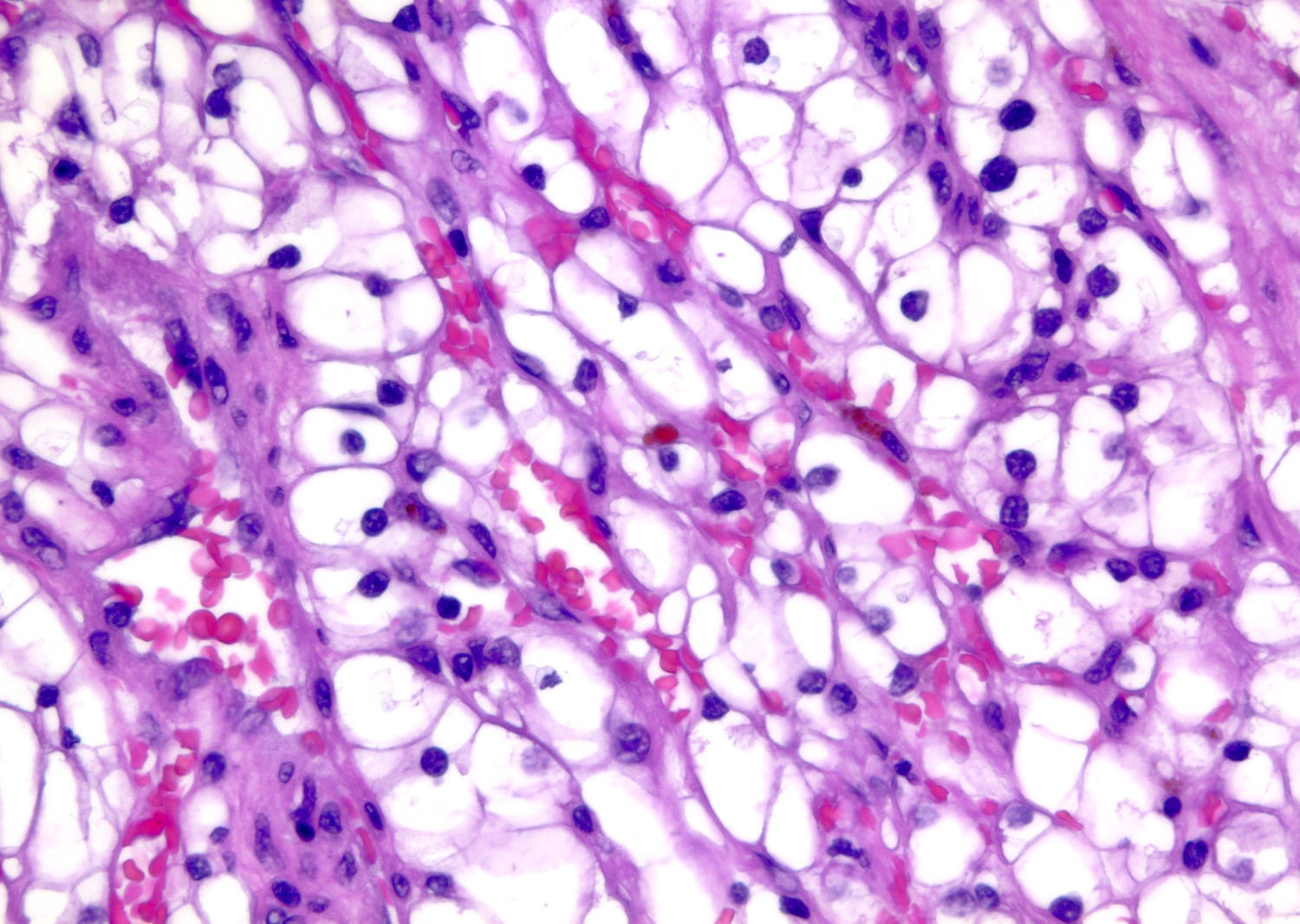
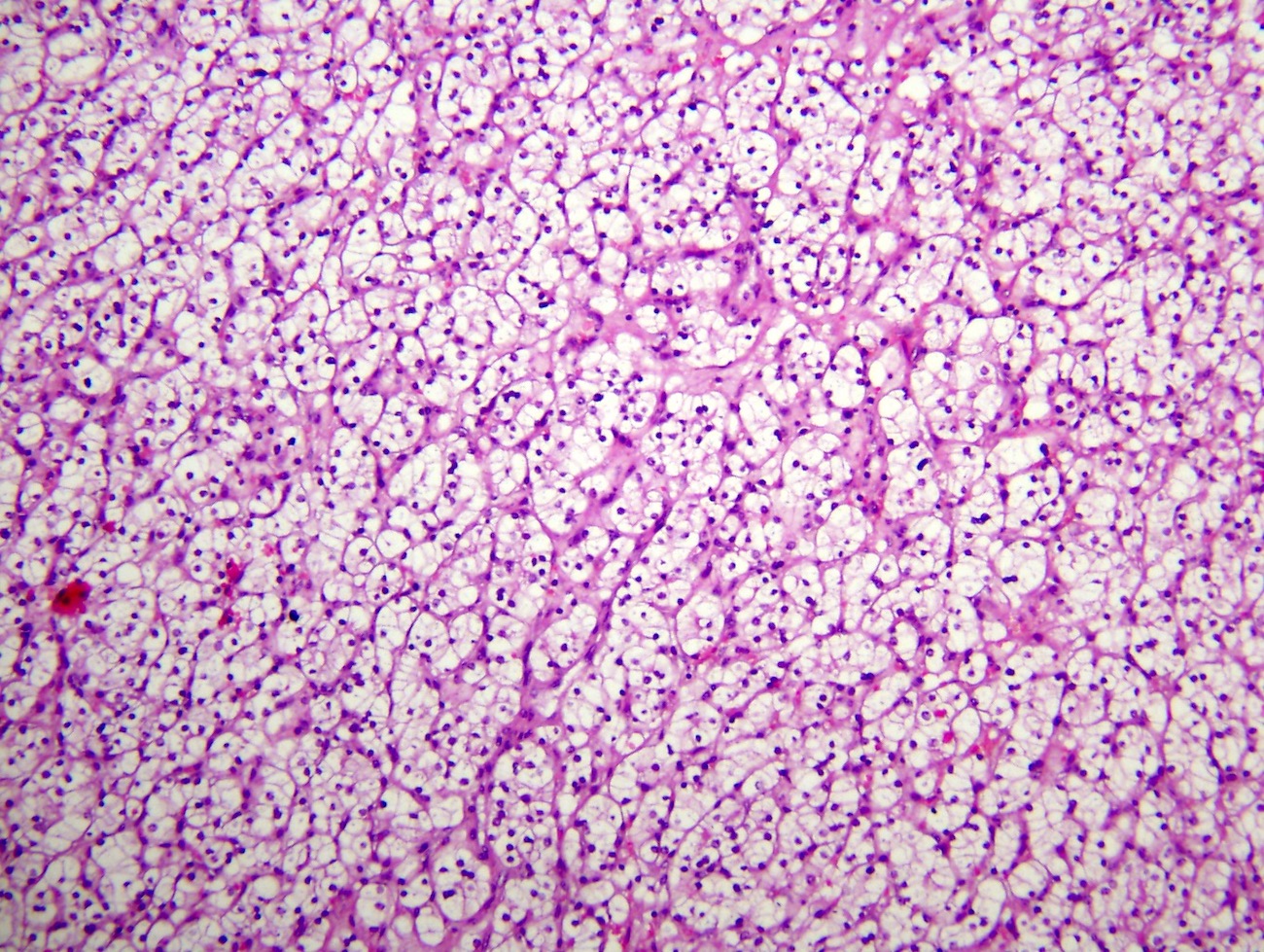
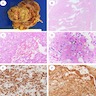
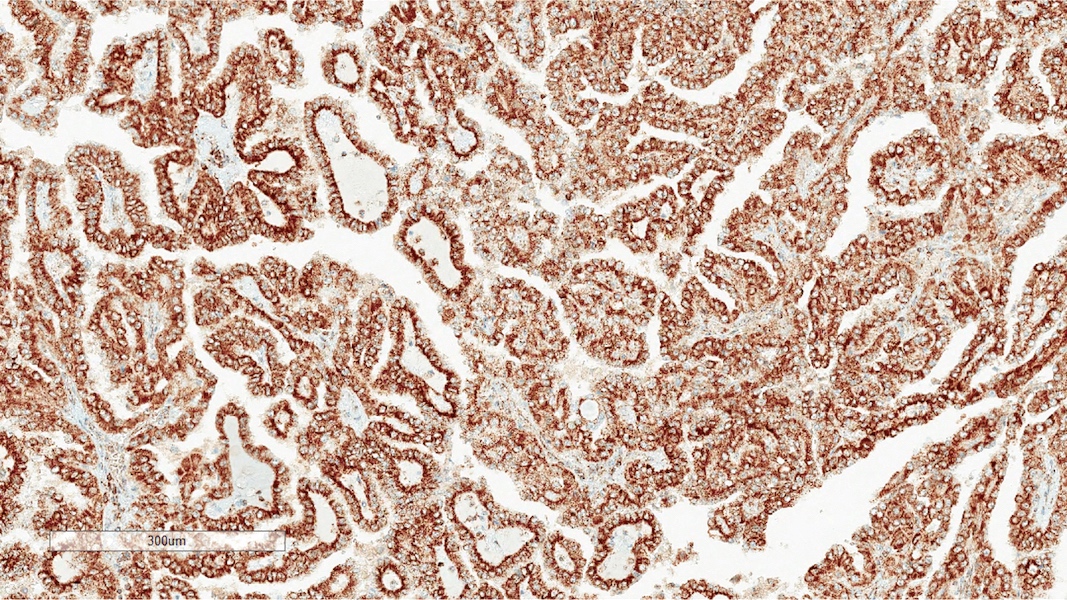
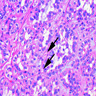
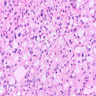
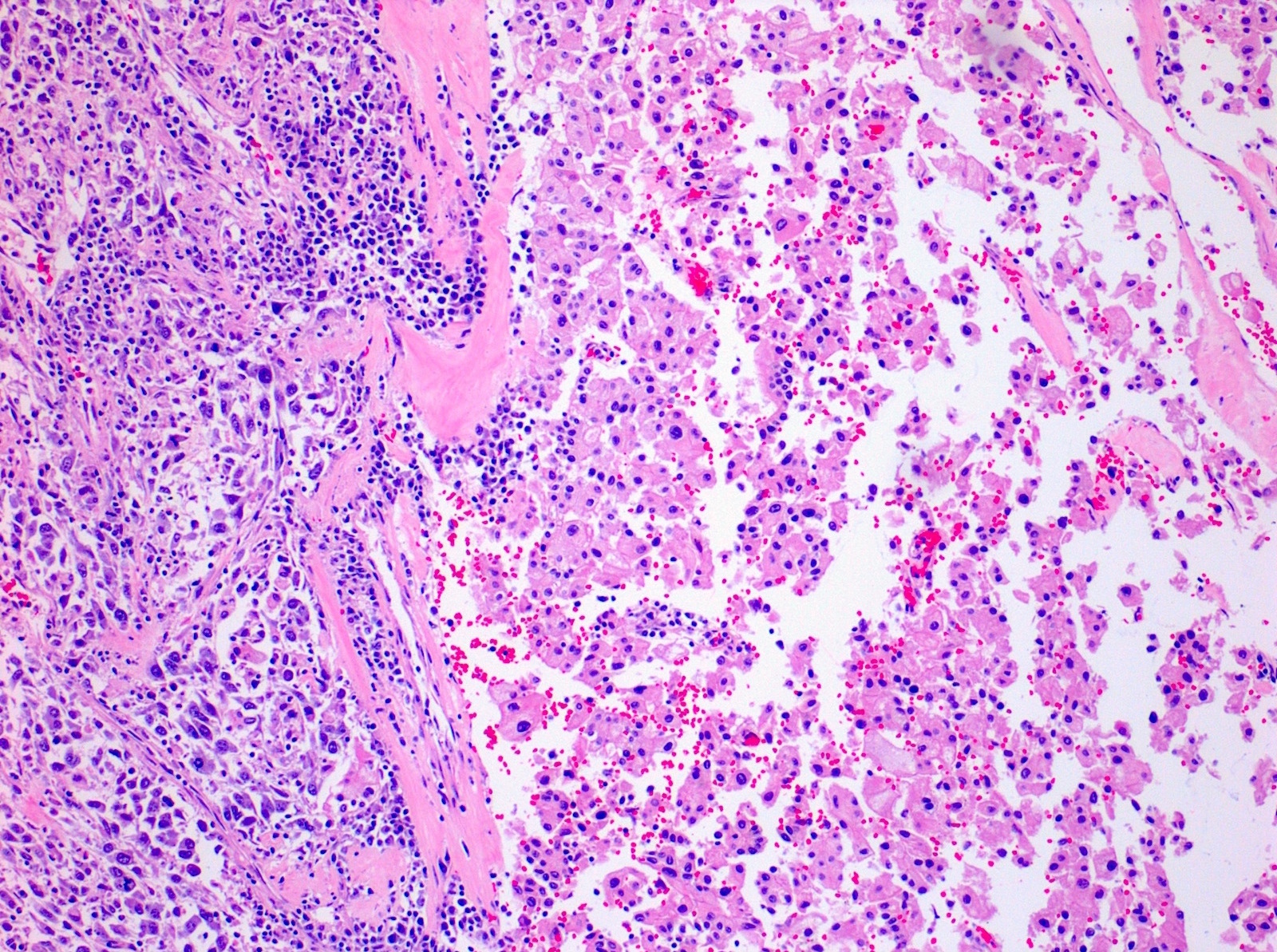
The image shown is chromophobe renal cell carcinoma (ChRCC), eosinophilic variant. Histologic clues to the diagnosis of ChRCC are the raisinoid nuclei, perinuclear clearing / halos and well defined cell borders. Furthermore, > 80% of the cells have an eosinophilic cytoplasm, thus making it the eosinophilic variant of ChRCC. The immunoprofile of ChRCC is typically CK7+, KIT+, vimentin- and CAIX-. The other entity to consider is oncocytoma, but in this case the degree of nuclear pleomorphism (with enlarged, wrinkled and oval nuclei) favors ChRCC. Answer D (CK7-, KIT+, vimentin-, CAIX-) would be the immunohistochemical profile most typical of oncocytoma, although vimentin can often be focally positive. Answer E is the immunoprofile most typical of clear cell renal cell carcinoma; while there is an eosinophilic variant of this condition, the perinuclear clearing and raisinoid nuclei are still most characteristic of ChRCC (Pathol Res Pract 2015;211:303).
Comment Here
Reference: Chromophobe eosinophilic variant
- No differences in cytogenetics have been found between the eosinophilic variant and classic ChRCC
- The classic variant is more likely to have a brown coloration grossly
- The classic variant typically has smaller cells than the eosinophilic variant
- The eosinophilic variant must have 100% eosinophilic cells
- The prognosis of the eosinophilic variant is the same as classic ChRCC
Studies have shown no difference in prognosis between the eosinophilic variant of ChRCC and classic ChRCC. Answer A is wrong as a recent study showed that the eosinophilic variant frequently has less chromosomal instability compared with classic ChRCC and is often characterized by doubled hypodiploidy (Eur J Surg Oncol 2008;34:687, Cancers (Basel) 2019;11:1492, Am J Surg Pathol 2008;32:1822, Cancers (Basel) 2019;11:1492, Hum Pathol 2020;104:18, Am J Surg Pathol 2008;32:1822). Answer B is wrong as the eosinophilic variant is more likely to be brown in coloration grossly. Answer C is wrong as the eosinophilic variant typically has smaller cells compared with classic ChRCC. Answer D is wrong because, by definition, the eosinophilic variant only needs to have > 80% eosinophilic cells.
Comment Here
Reference: Chromophobe eosinophilic variant
- Most common renal epithelial tumor, accounting for ~2% of all malignancies, typically with clear cytoplasm and a compact nested or acinar growth pattern, intersected by delicate vasculature and with characteristic alterations to chromosome 3p involving VHL (von Hippel-Lindau) gene inactivation
- Cortical mass with golden yellow variegated cut surface, with diverse architecture, primarily solid and nested
- Clear or granular eosinophilic cytoplasm and prominent but delicate capillary network
- > 95% sporadic, mostly single mass, in the sixth to seventh decade, commonly harboring VHL gene inactivation located on the short arm of chromosome 3 (3p25)
- Small percentage of tumors are familial, mostly VHL disease with multiple bilateral tumors and earlier onset
- Characteristic immunohistochemical profile: positive for PAX8, CAIX (box-like) and CD10; generally negative for AMACR (35% positive), CK7 (15% positive) and CD117 (2% positive)
- ICD-O: 8310/3 - clear cell renal cell carcinoma
- ~2% of all malignancies
- 65 - 70% of all renal cell carcinomas (RCC)
- Most occur after age 40, predominantly in sixth and seventh decades
- M:F = 1.5:1
- References: Eur Urol 2019;75:74, Semin Oncol 2000;27:124, CA Cancer J Clin 2019;69:7
- Kidney, typically solitary cortical mass in sporadic tumors
- Multiple tumors may represent familial syndromes but retrograde venous extension from the dominant sporadic tumor is also possible
- Renal sinus invasion is the most common pathway of spread, often accompanied by invasion of the renal vein or its segmental branches, leading to a higher risk of distant metastasis
- Metastases
- Hematogenous is more common: lung (most common), bone, liver, retroperitoneum, pleura, central nervous system (CNS), head and neck (J Urol 2008;179:474, Urol Case Rep 2019;27:100989)
- Lymphatic is less common compared to papillary RCC and chromophobe RCC: hilar, aortic, caval and thoracic lymph nodes (BJU Int 2008;102:1381, Cancer 2008;112:1480)
- Suggested to arise in epithelial cells lining the proximal convoluted tubule (EBioMedicine 2023;92:104596, Annu Rev Pathol 2015;10:263)
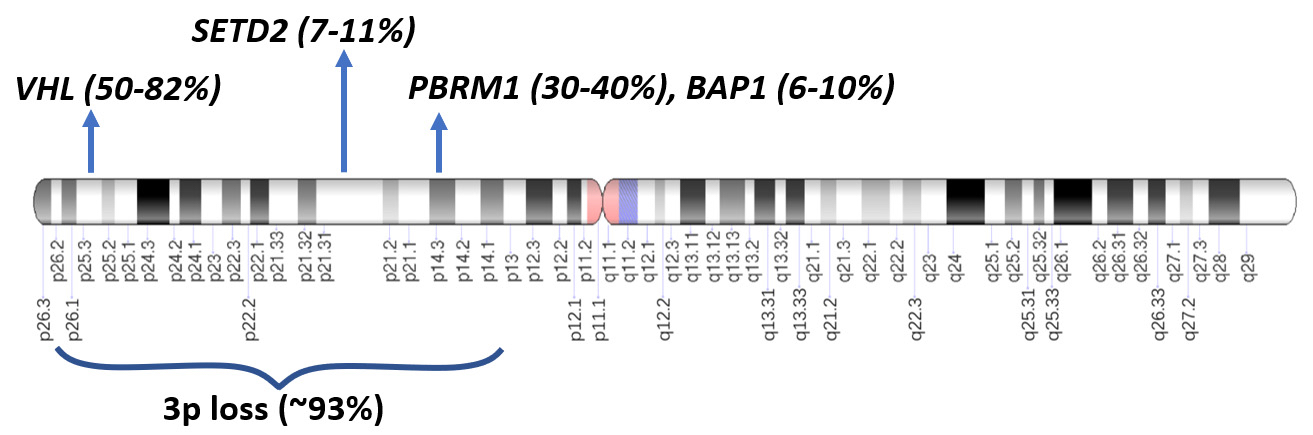
- Loss of the VHL protein function by a 2 step process in 50 - 82% of somatic clear cell renal cell carcinomas (ccRCC) leading to deletion, unbalanced translocation or biallelic alteration in VHL (von Hippel-Lindau) tumor suppressor gene (3p25-26)
- First event of 3p loss, usually by chromothripsis, occurs in childhood or adolescence in a few hundred cells (Cell 2018;173:611)
- Second allele undergoes somatic mutation or epigenetic inactivation through hypermethylation
- VHL protein loss leads to accumulation of hypoxia inducible transcription factor alpha (HIF1α)
- HIF1α accumulation drives transcription of hypoxia associated genes, including VEGF, PDFGβ, GLUT1, TGFα, CAIX, EPO and metalloproteinases (Surg Pathol Clin 2009;2:199)
- Other driver mutations are present in smaller percentages (see Molecular / cytogenetics description)
- Risk factors: smoking, obesity, hypertension, long term dialysis (particularly in acquired adult cystic kidney disease) and family history of kidney cancer
- Familial tumors: mostly von Hippel-Lindau disease, less commonly in families segregating constitutional chromosome 3 translocations, BAP1 tumor predisposition syndrome, Cowden syndrome, Birt-Hogg-Dubé syndrome and tuberous sclerosis (Genet Couns 2003;14:149, Am J Surg Pathol 2015;39:e1, Eur Urol 2019;76:754, J Urol 2013;190:1990, Adv Anat Pathol 2013;20:245)
- Most commonly: anemia, gross hematuria, flank pain and mass
- Weight loss and fever in late stages
- Classic triad of flank mass, pain and hematuria present in < 10% of cases (Urol Oncol 2002;7:135)
- 60 - 80% found incidentally on radiologic imaging
- Nephrectomy or partial nephrectomy; definitive diagnosis may be possible by needle biopsy
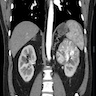
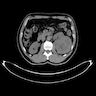
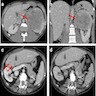
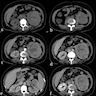
- Computed tomography (CT): exophytic with mixed enhancement and heterogeneous appearance (due to internal necrosis, cystic change or hemorrhage)
- Bosniak classification (category I to IV) for renal cysts guides the management by approximating the risk of malignancy
- Magnetic resonance imaging (MRI): similar accuracy to CT, heterogeneous in T1 and hyperintense (bright) T2 when use of contrast materials is contraindicated
- In metastatic cases, MRI and positron emission tomography (PET / CT) are the preferred methods
- Ultrasonography: useful for incidental detection of renal masses
- Reference: Radiology 2013;267:444
- 5 year survival: 50 - 70% after nephrectomy, 10% in metastatic disease
- Survival difference is mostly due to differences in TNM stage and nuclear grade, regardless of the histologic type of the RCC (J Clin Oncol 2005;23:2763, Eur Urol 2005;48:593)
- Worse prognosis within the same stage: higher histologic grade, sarcomatoid and rhabdoid differentiation and > 10% coagulative tumor necrosis (Pathology 2021;53:120, Am J Surg Pathol 2013;37:311, Pathology 2015;47:34)
- Worse prognosis than papillary and chromophobe RCC
- Better prognosis with only VHL loss or PBRM1 mutation, poor prognosis with multiple driver gene mutations or loss of 4p, 9p or 14q
- BAP1 mutated ccRCCs have distinct morphologic and immunophenotypic features associated with more aggressive behavior than typical VHL only mutated ccRCC (Am J Clin Pathol 2021;155:718)
- PBRM1 is mutually exclusive to BAP1 mutation and is associated with improved response to immunotherapy
- TNM staging: the most accurate predictor (see Kidney tumor staging)
- pT1 and pT2: limited to the kidney, classified based on size
- T1a (≤ 4 cm)
- T1b (> 4 cm to 7 cm)
- T2a (> 7 cm to 10 cm)
- T2b (> 10 cm)
- pT3: regional spread beyond kidney parenchyma
- pT3a: regional extrarenal spread into perinephric fat, renal sinus fat, involvement of the renal vein or segmental veins or invasion of the pelvicalyceal system
- pT3b: extension into the inferior vena cava below the diaphragm
- pT3c: tumor extension into the inferior vena cava above the diaphragm or invasion of the vena cava wall
- pT4: distant spread, beyond Gerota fascia, including contiguous extension into the ipsilateral adrenal gland
- pT1 and pT2: limited to the kidney, classified based on size
- Histologic grading
- WHO / ISUP grading system: 4 tiers, uses nucleolar prominence; used for clear cell and papillary renal cell carcinoma (chromophobe RCC not graded)
- G4: extreme nuclear pleomorphism, multinucleated giant cells or rhabdoid or sarcomatoid differentiation
- G3: nucleoli are conspicuous and eosinophilic at 100x magnification
- G2: nucleoli are conspicuous and eosinophilic at 400x magnification (but not prominent at 100x magnification)
- G1: nucleoli are absent or inconspicuous and basophilic at 400x magnification (Urology 2014;83:969)
- WHO / ISUP grading system: 4 tiers, uses nucleolar prominence; used for clear cell and papillary renal cell carcinoma (chromophobe RCC not graded)
- > 50% granular eosinophilic features may predict poor response to IL2 therapy (J Immunother 2005;28:488)
- Clear cell RCCs with > 40% eosinophilic component may have worse clinical outcome (Mod Pathol 2015;28:S217)
- 41, 55 and 68 year old men with clear cell RCC metastases that significantly decreased in size or resolved with no surgical or other types of treatment (Case Rep Oncol 2020;13:1285)
- 52 year old man with a metastatic clear cell RCC to the forearm without an identifiable primary renal mass (Urol Case Rep 2019;27:100989)
- 63 year old man with 15 cm clear cell RCC with syncytial giant cells (Arch Pathol Lab Med 2004;128:1435)
- 65 year old man with a 2.4 cm tumor with nonnecrotizing sarcoid-like granulomata (Clin Pathol 2020;13:2632010X20954215)
- Choice of treatment depends on the stage, overall patient health and other individualized factors
- Surgical resection of stages 1 - 3 can be curative but up to 33% will recur
- Partial nephrectomy for smaller and localized tumors; radical nephrectomy for tumors larger than 4 cm and centrally located tumors
- Cryotherapy and radiofrequency ablation for unresectable tumors or some small tumors
- Systemic chemotherapy has limited efficacy
- Immunotherapy with checkpoint inhibitors has a 15 - 20% response rate: monoclonal antibodies against PD-1 (nivolumab and pembrolizumab), PDL1 (avelumab and atezolizumab) and CTLA4 (ipilimumab) (J Natl Compr Canc Netw 2019;17:587)
- Inhibitors of mammalian target of rapamycin (mTOR) pathways (such as temsirolimus)
- Tyrosine kinase inhibitors targeting VEGFR (including lenvatinib, axitinib and sunitinib), PDGFR or multitargeted (sorafenib, tivozanib, cabozantinib, lenvatinib and pazopanib) (Surg Pathol Clin 2009;2:199, Semin Oncol 2006;33:588)
- Hypoxia inducible factor 2 alpha (HIF2α) inhibitor belzutifan for certain types of VHL disease associated tumors
- Immune checkpoint inhibitors have shown significant response in ccRCC with sarcomatoid and rhabdoid features
- Combined tyrosine kinase inhibitors and checkpoint inhibitors received Food and Drug Administration (FDA) approval for advanced RCCs (J Natl Compr Canc Netw 2022;20:71)
- IL2 therapy: used less frequently
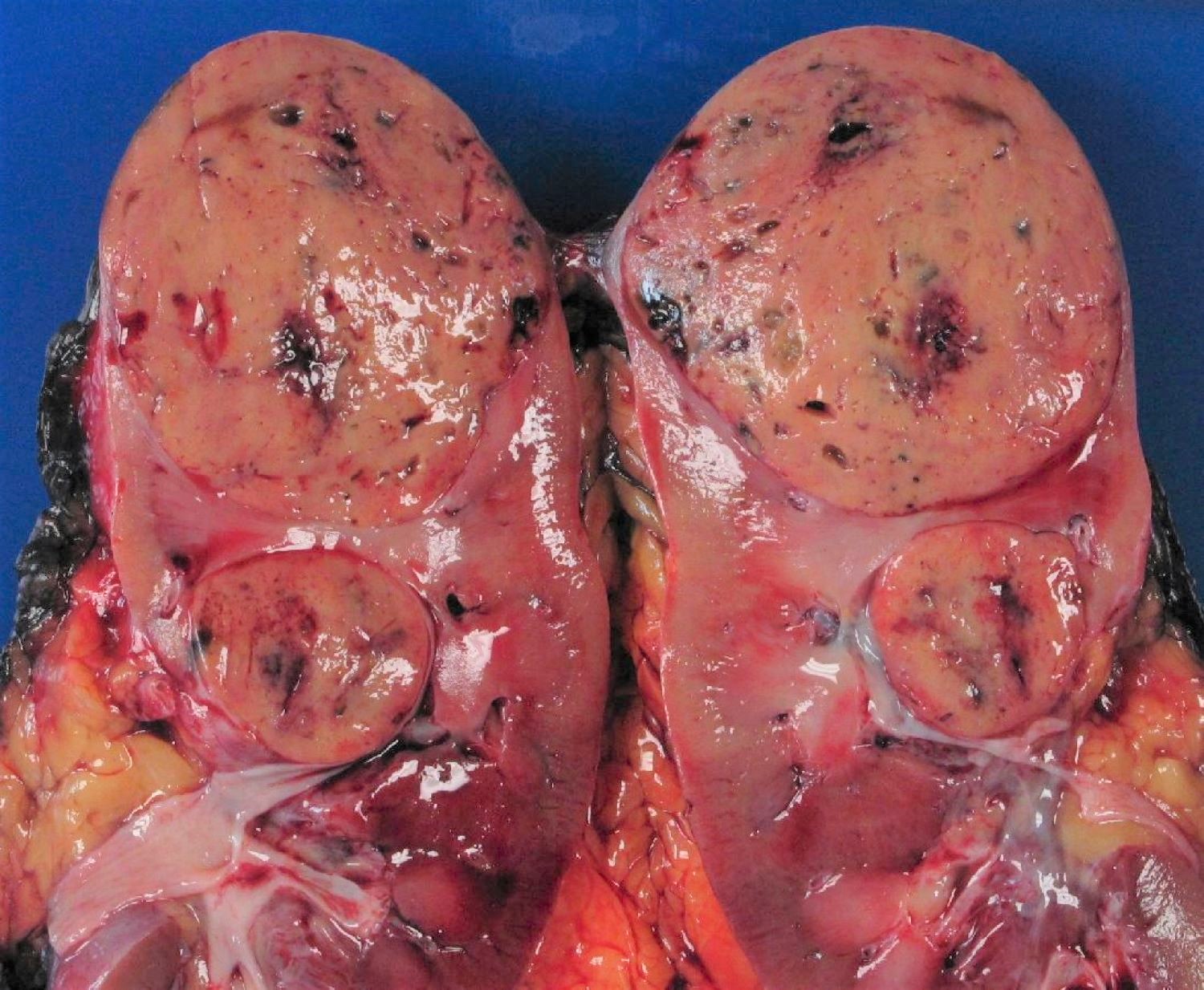
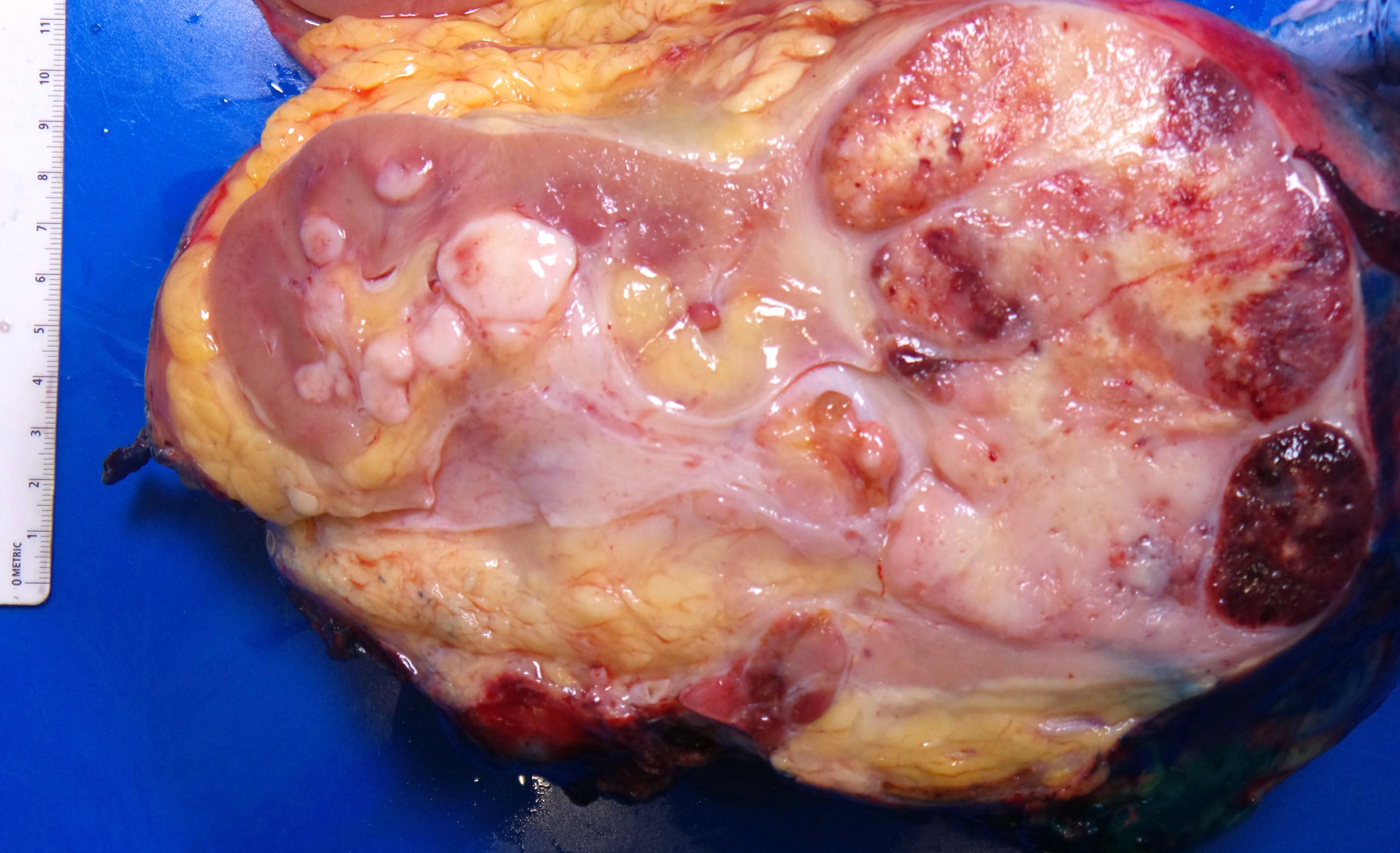
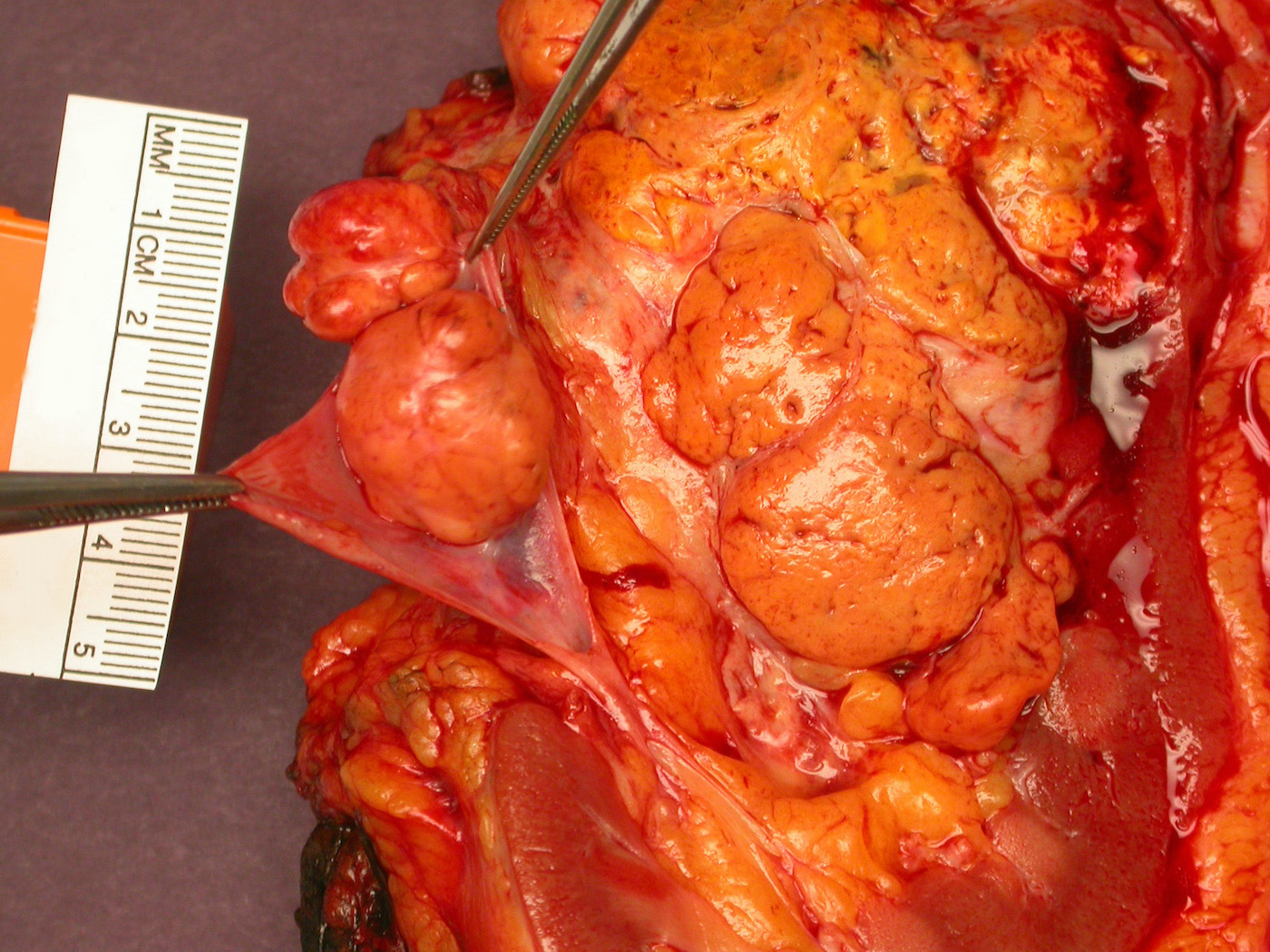
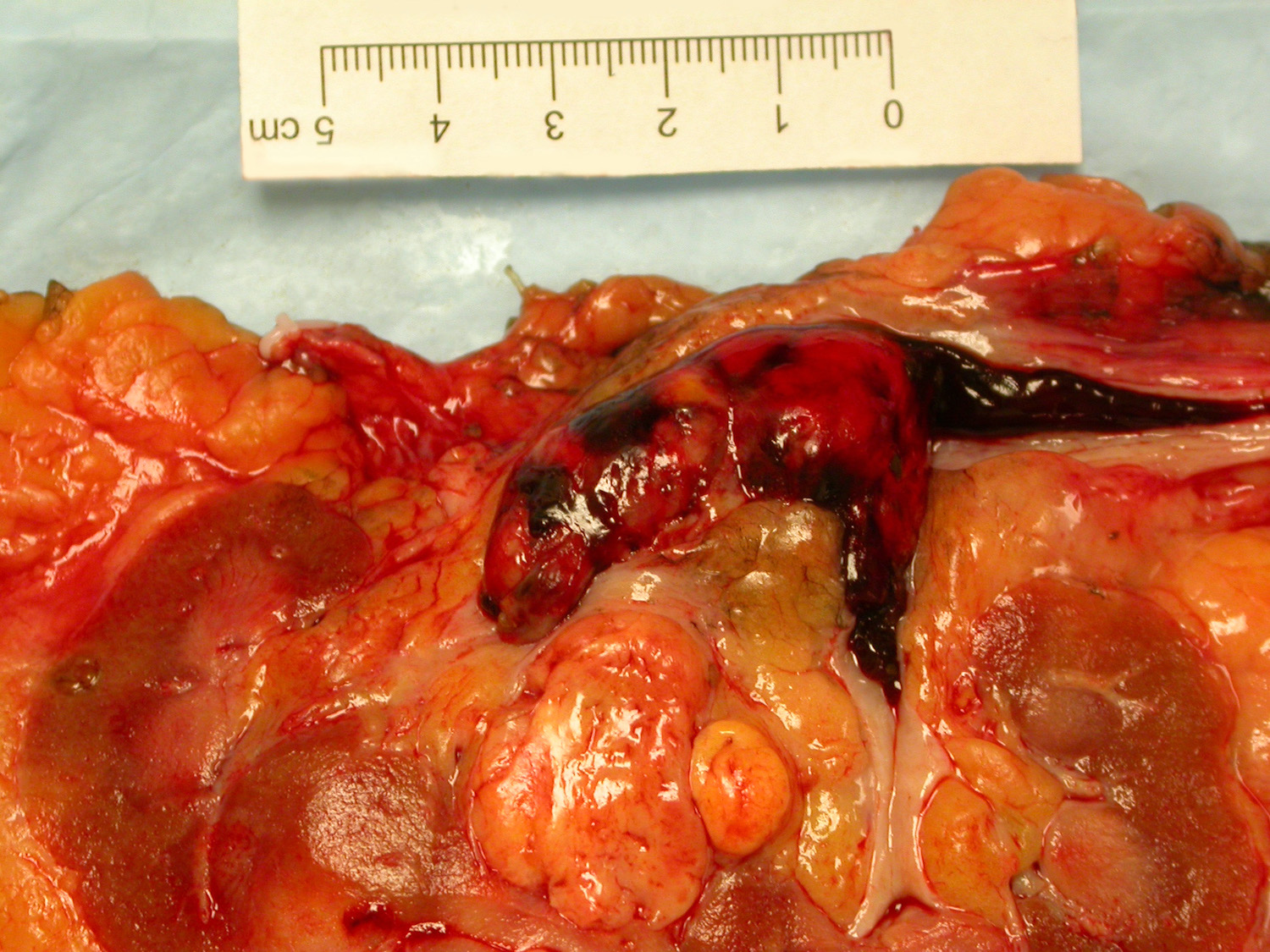
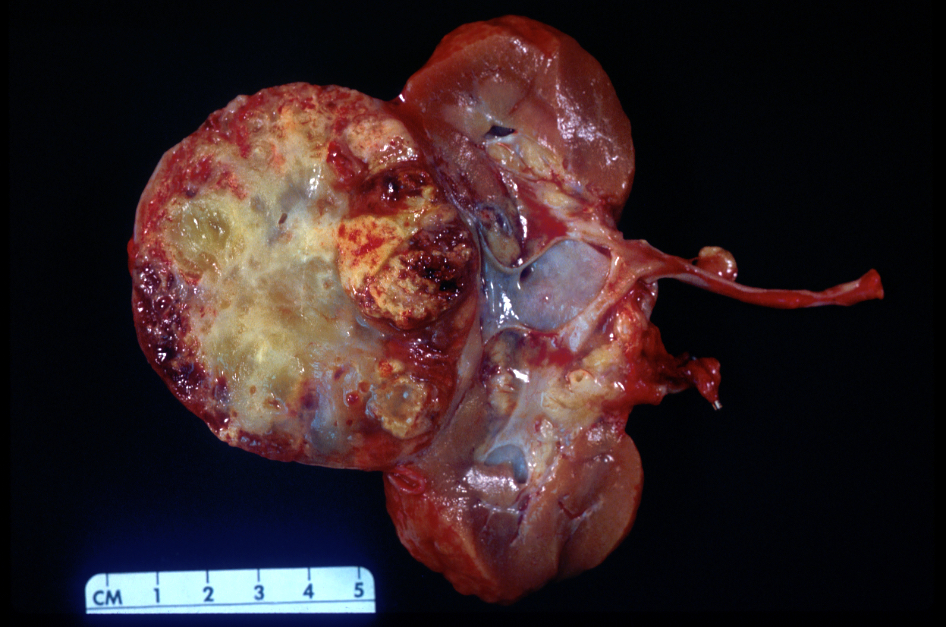
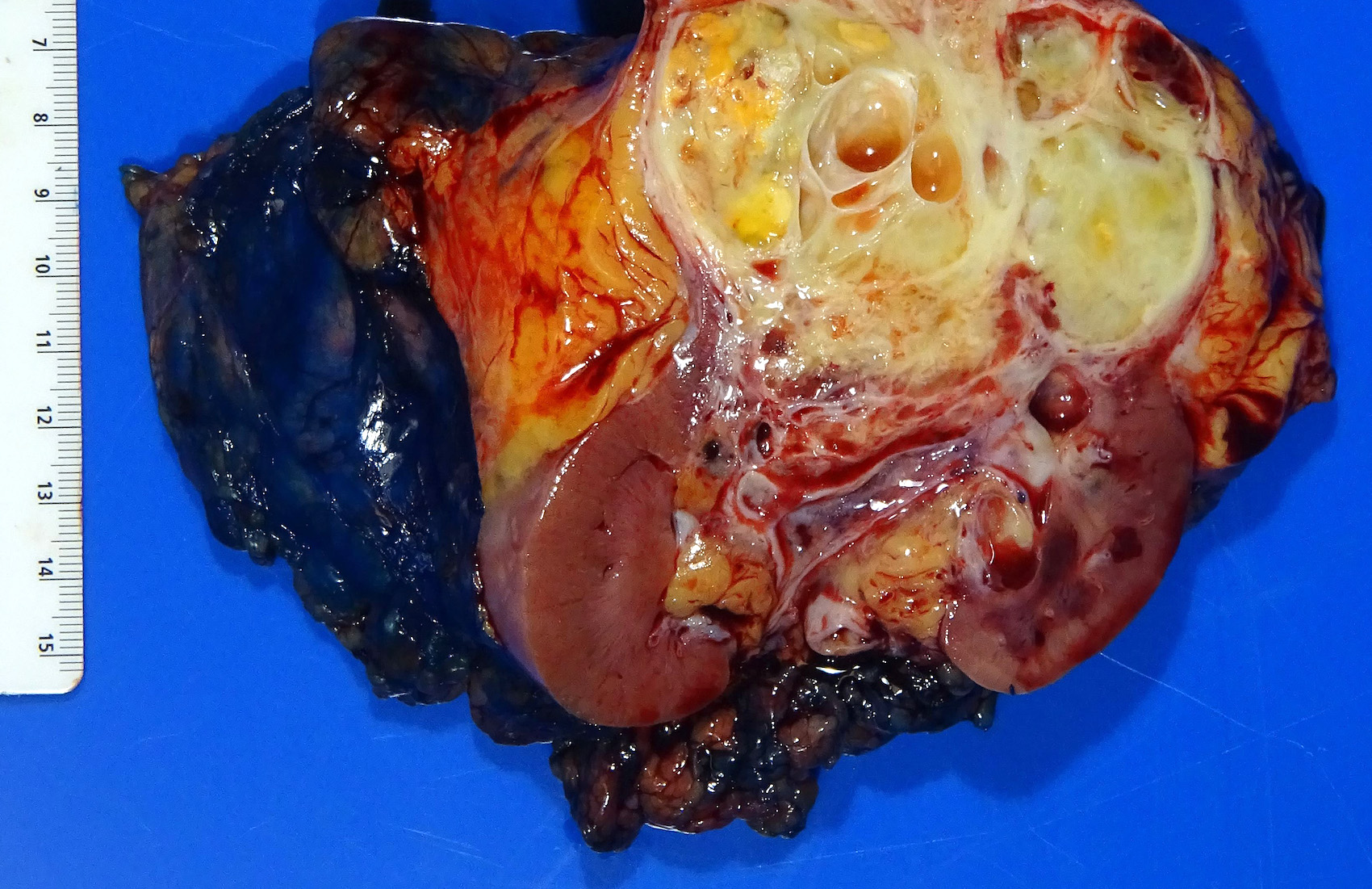
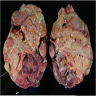
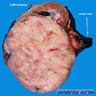
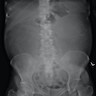
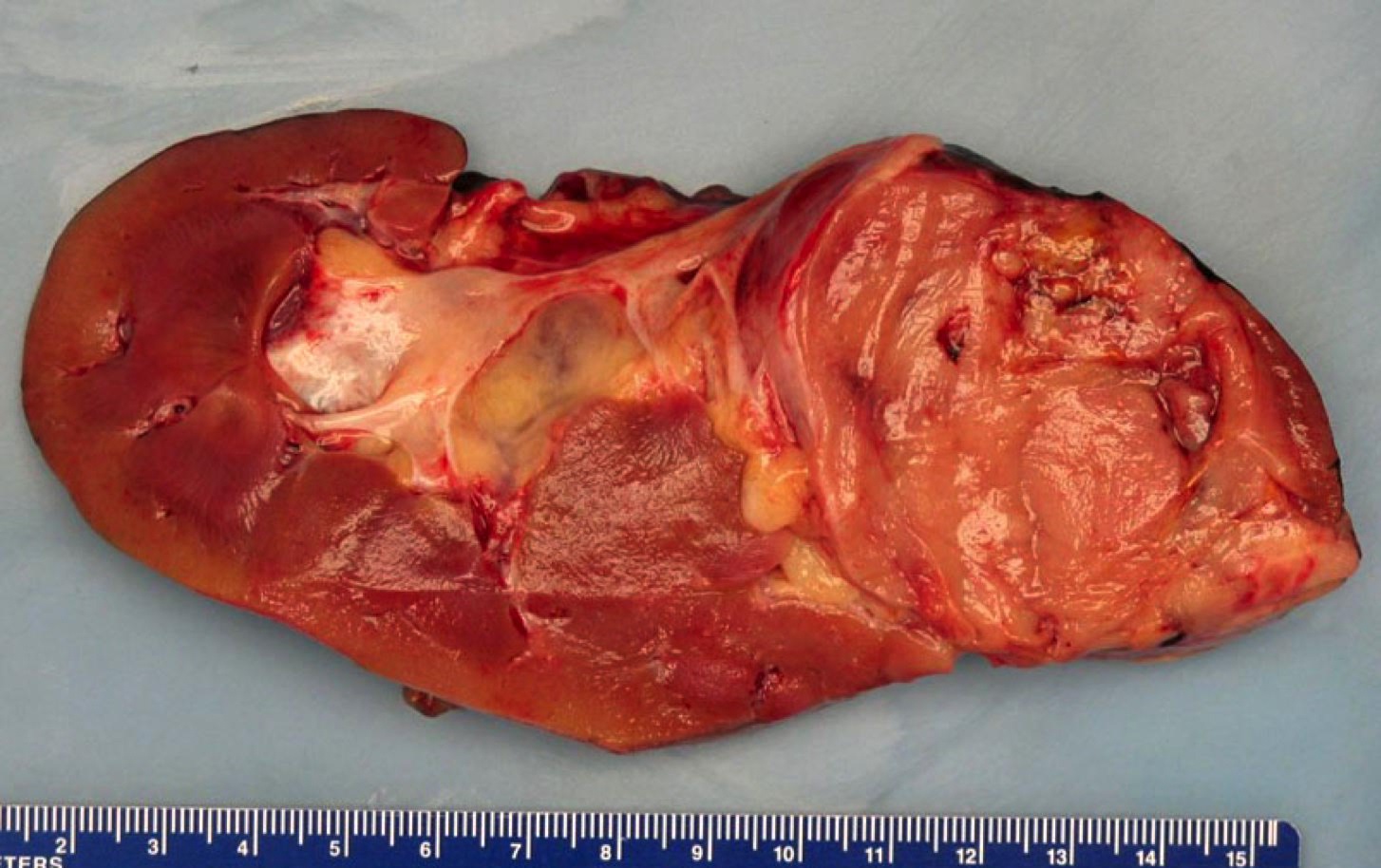
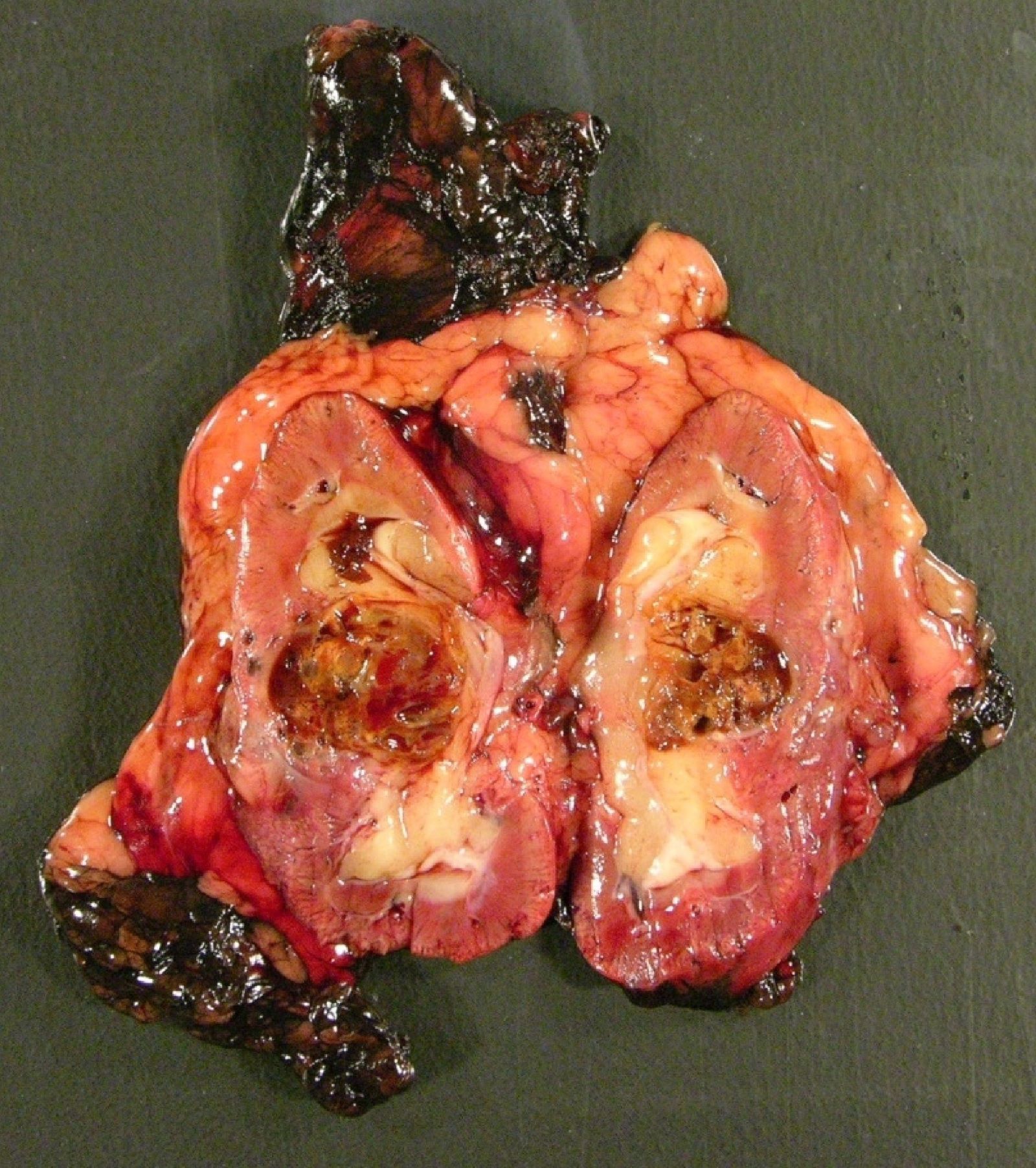
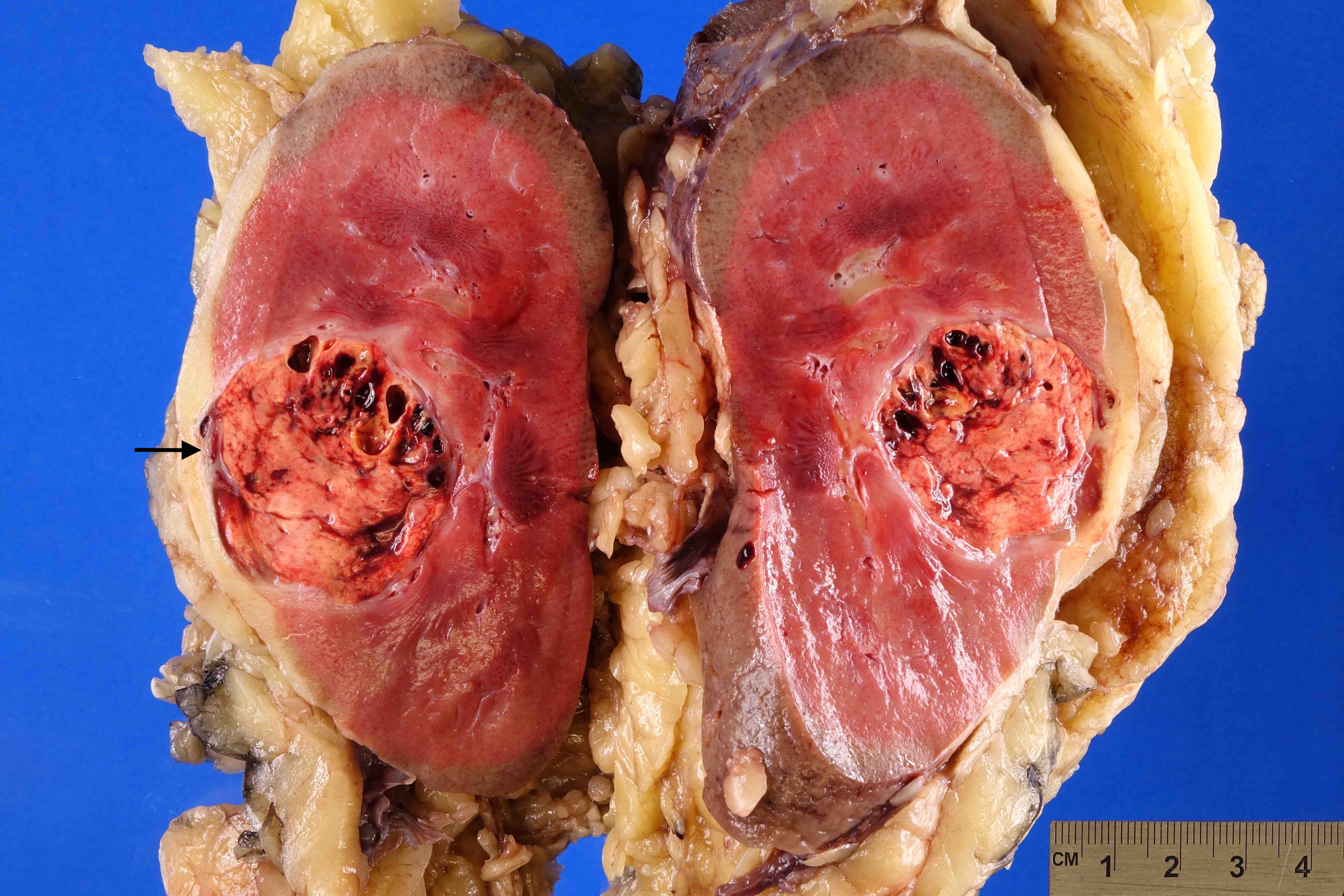
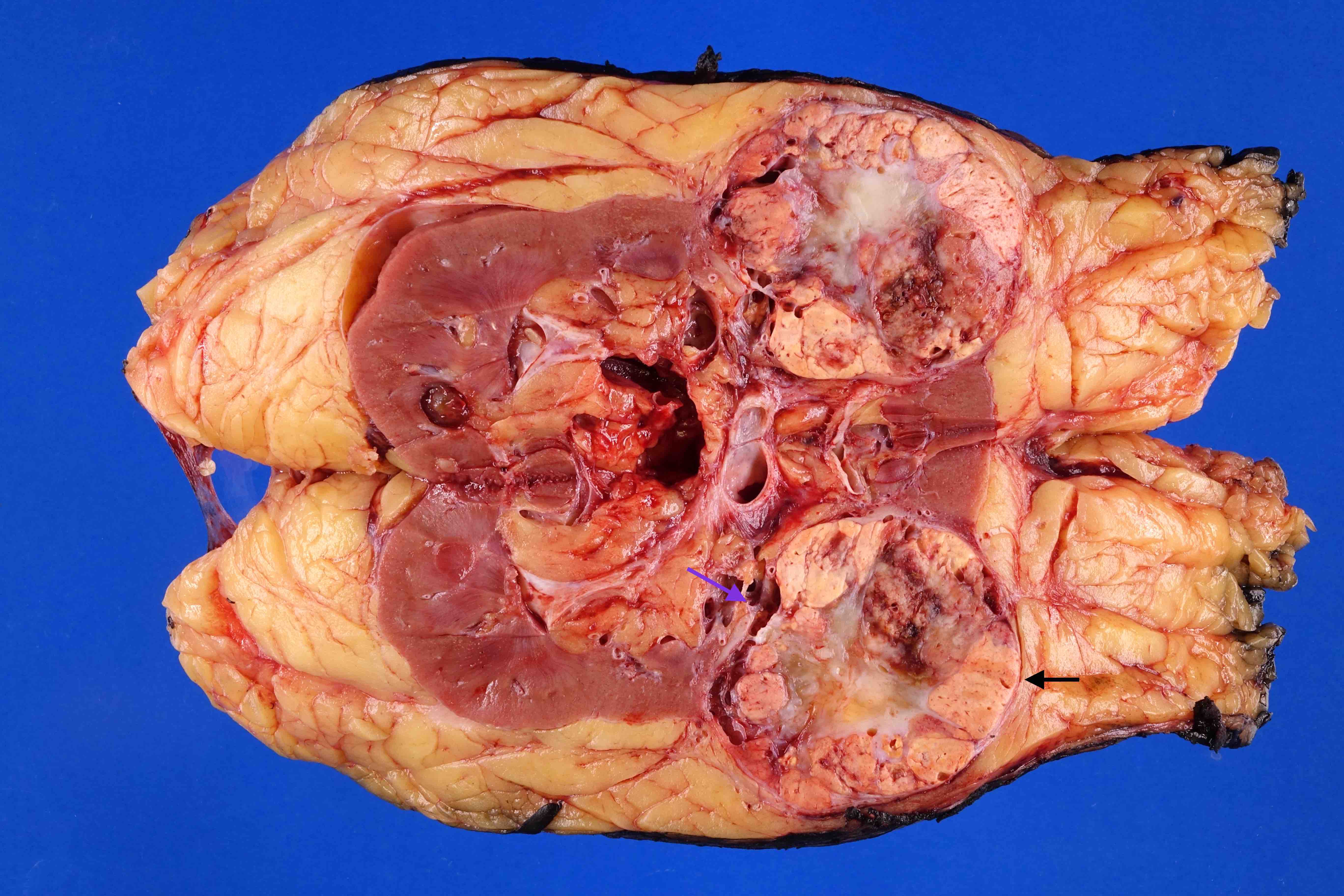
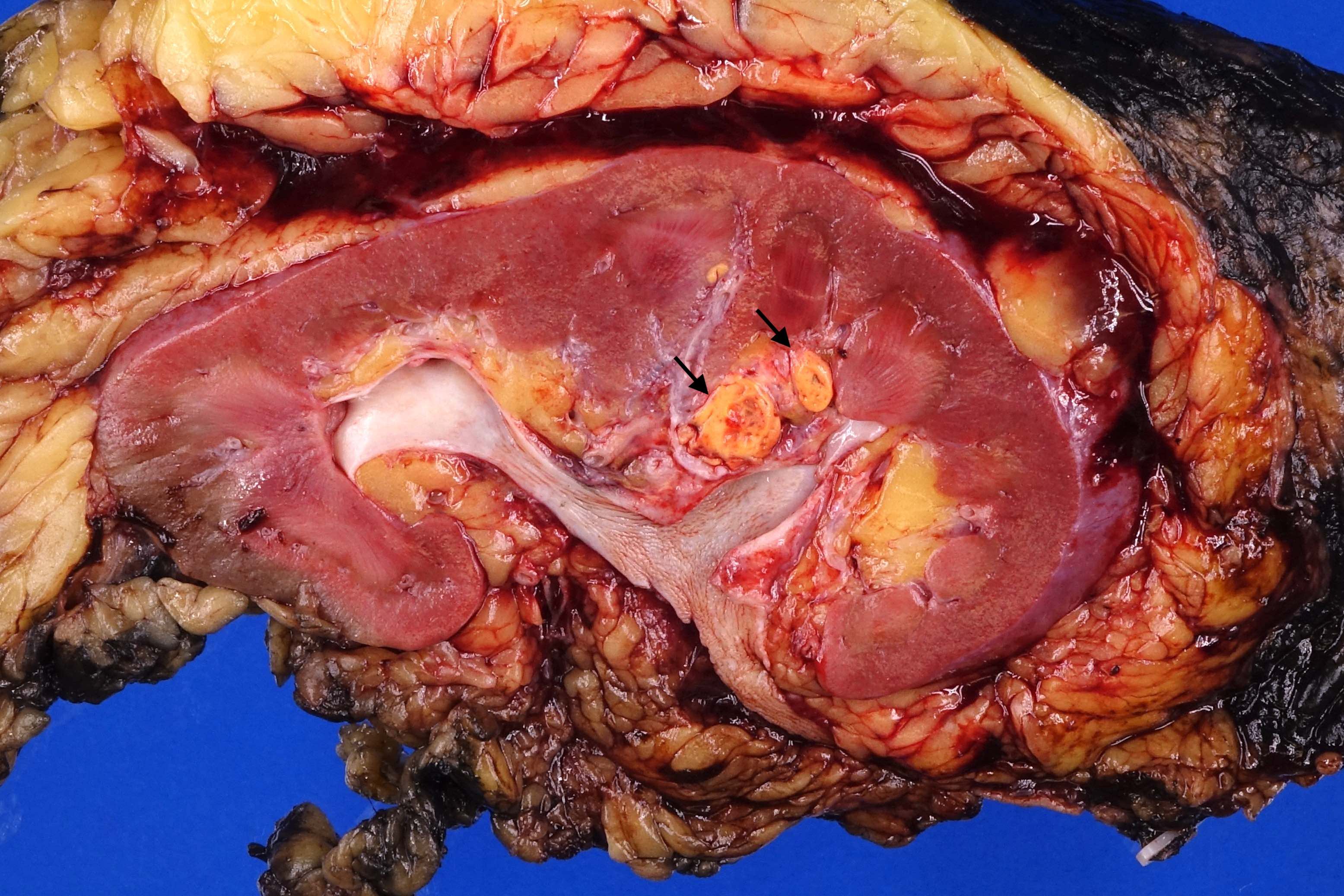
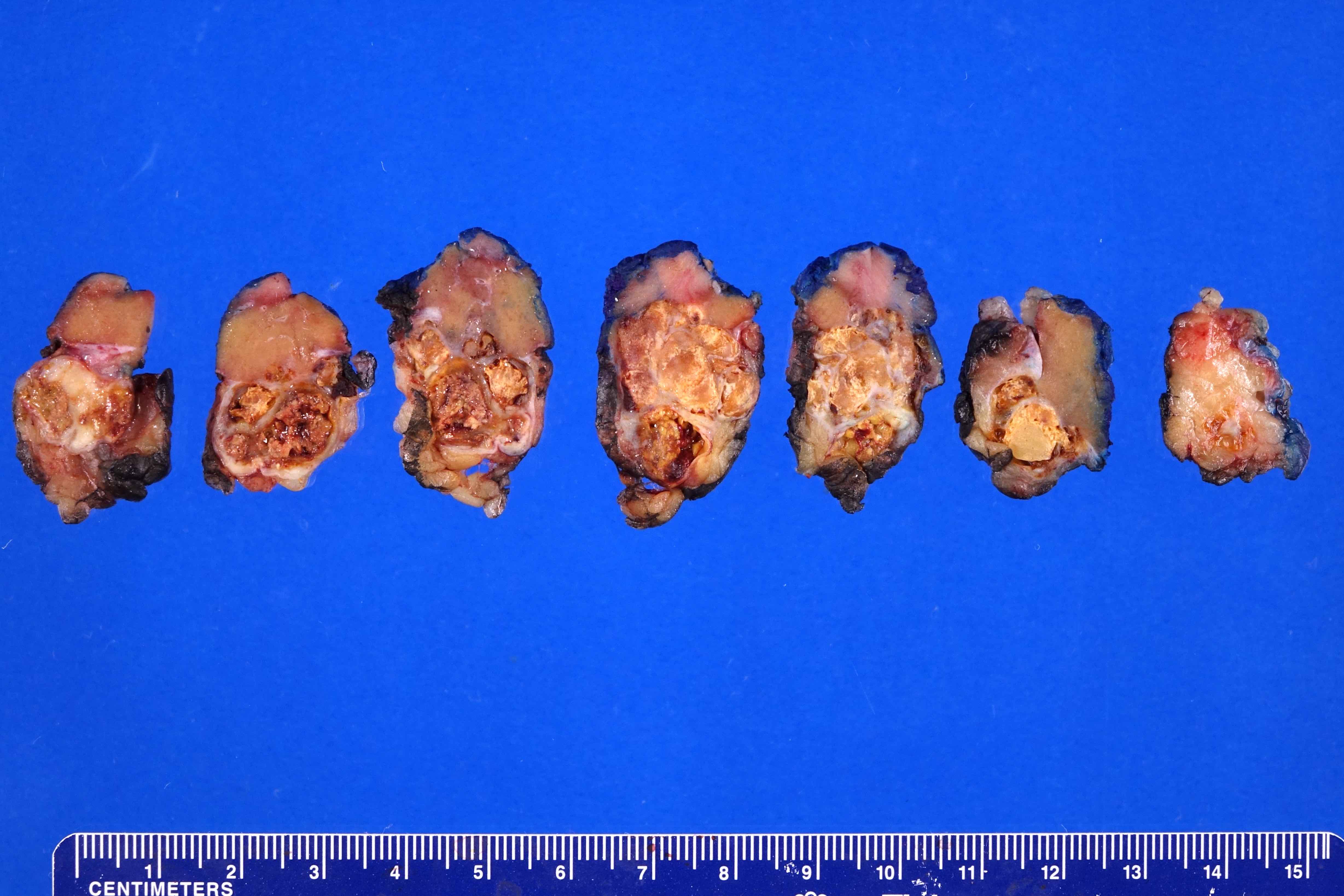
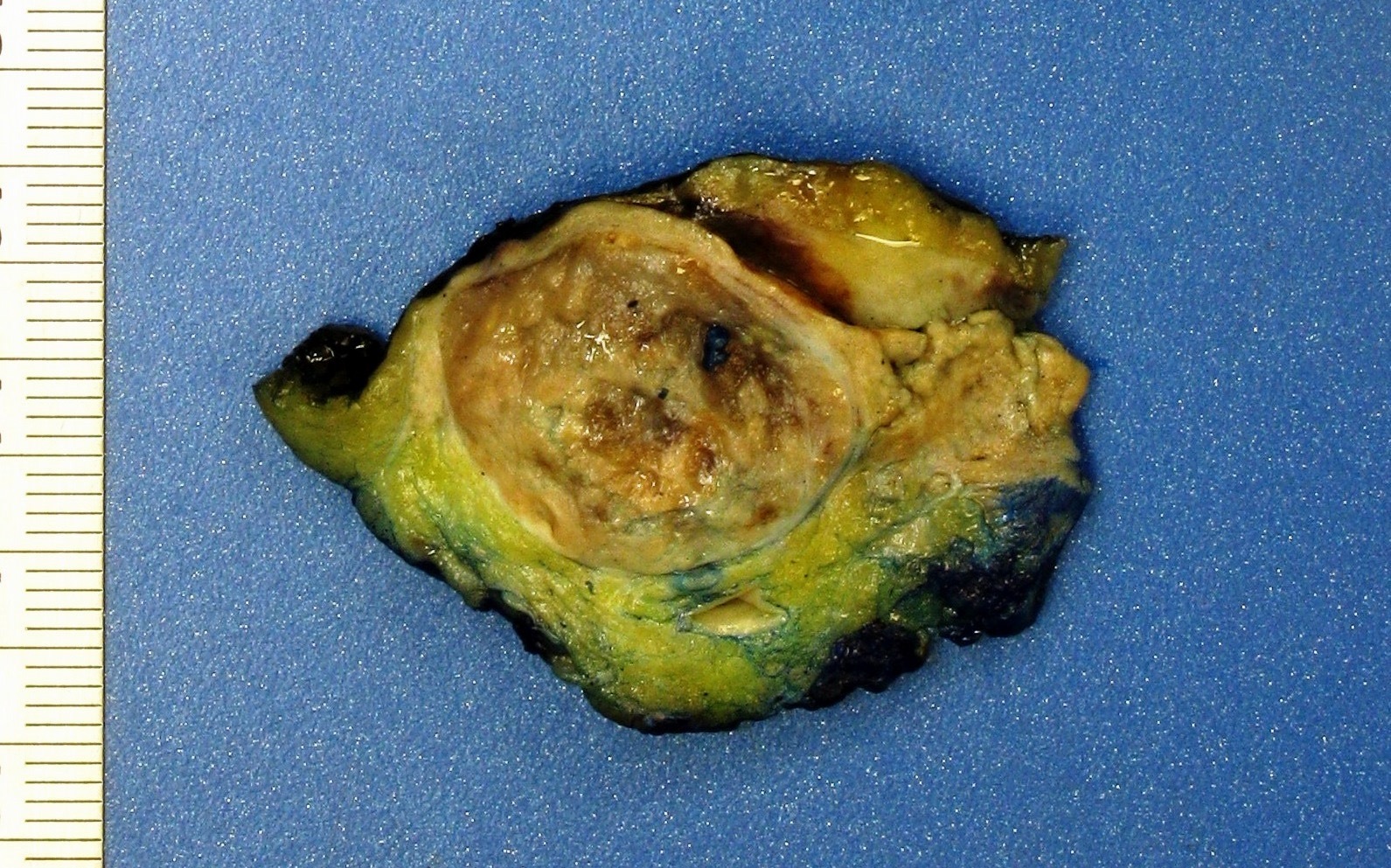
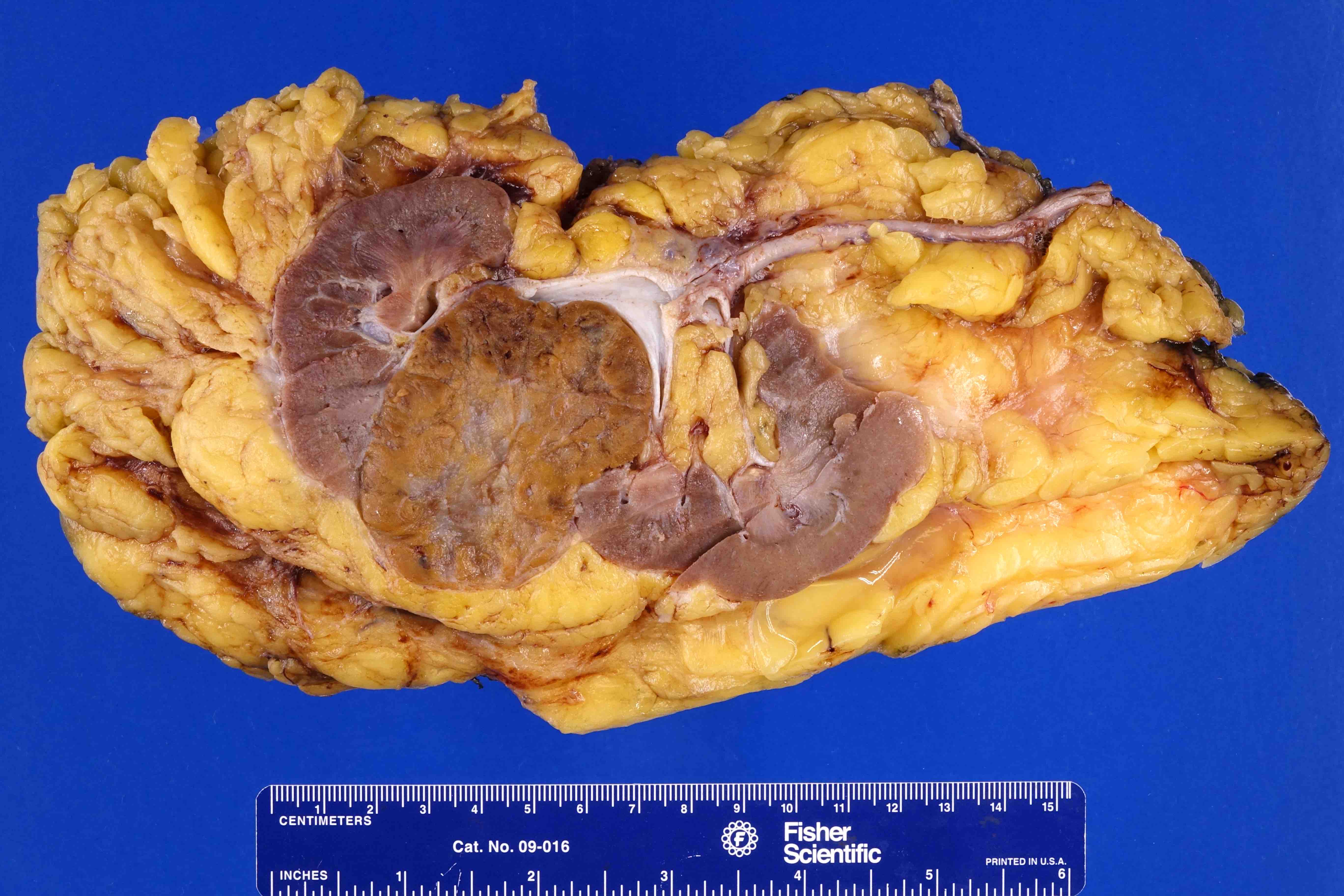
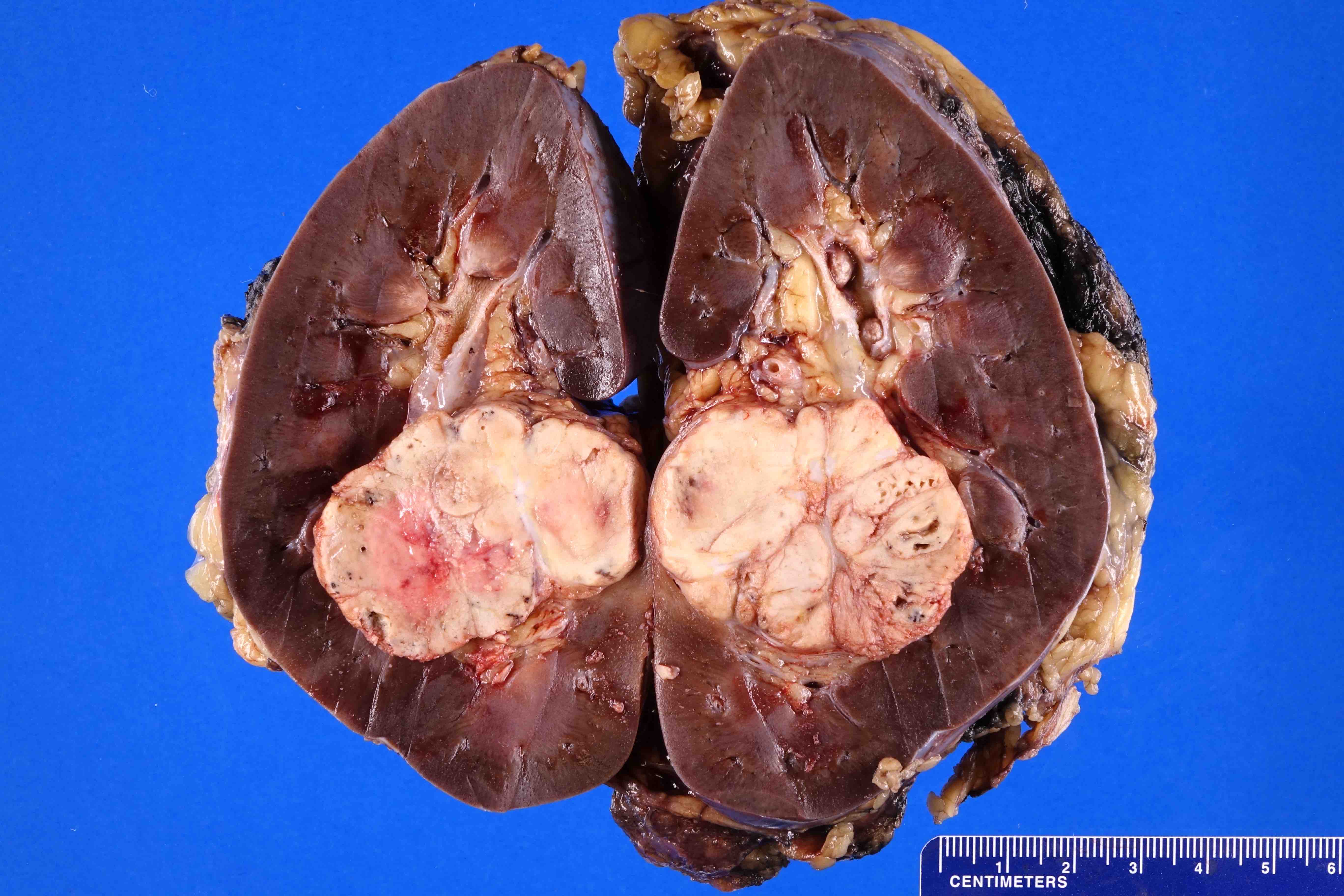
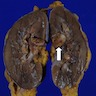
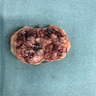
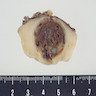
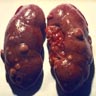
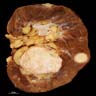
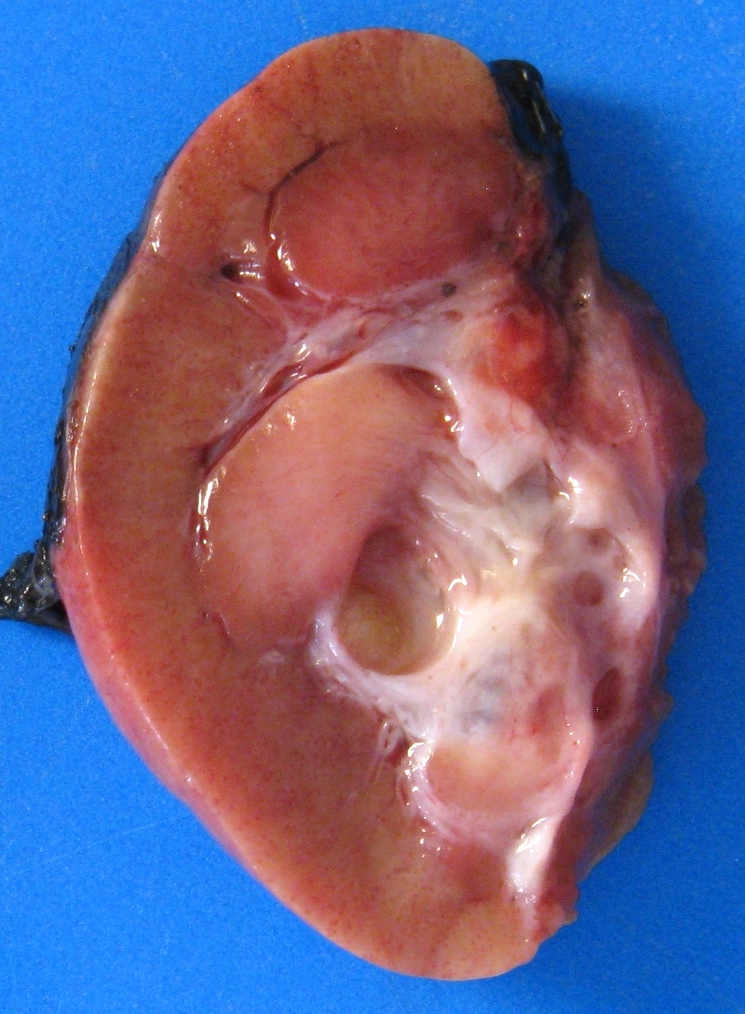
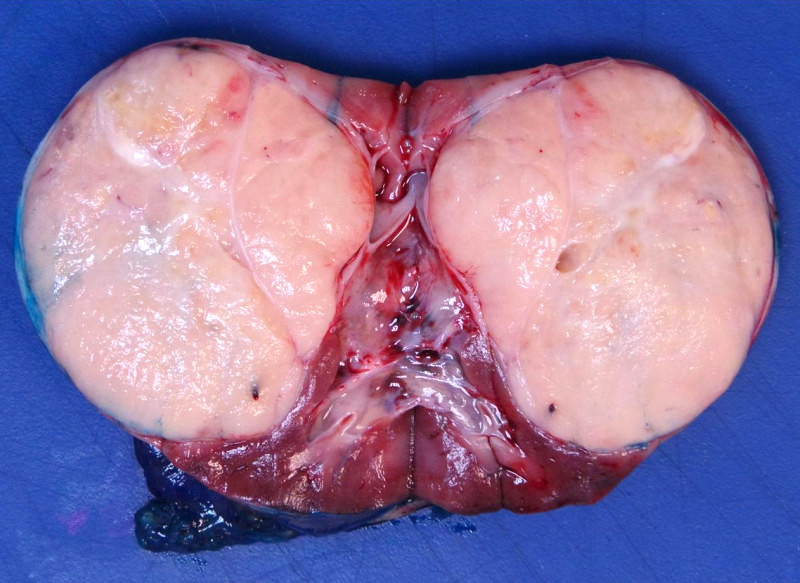
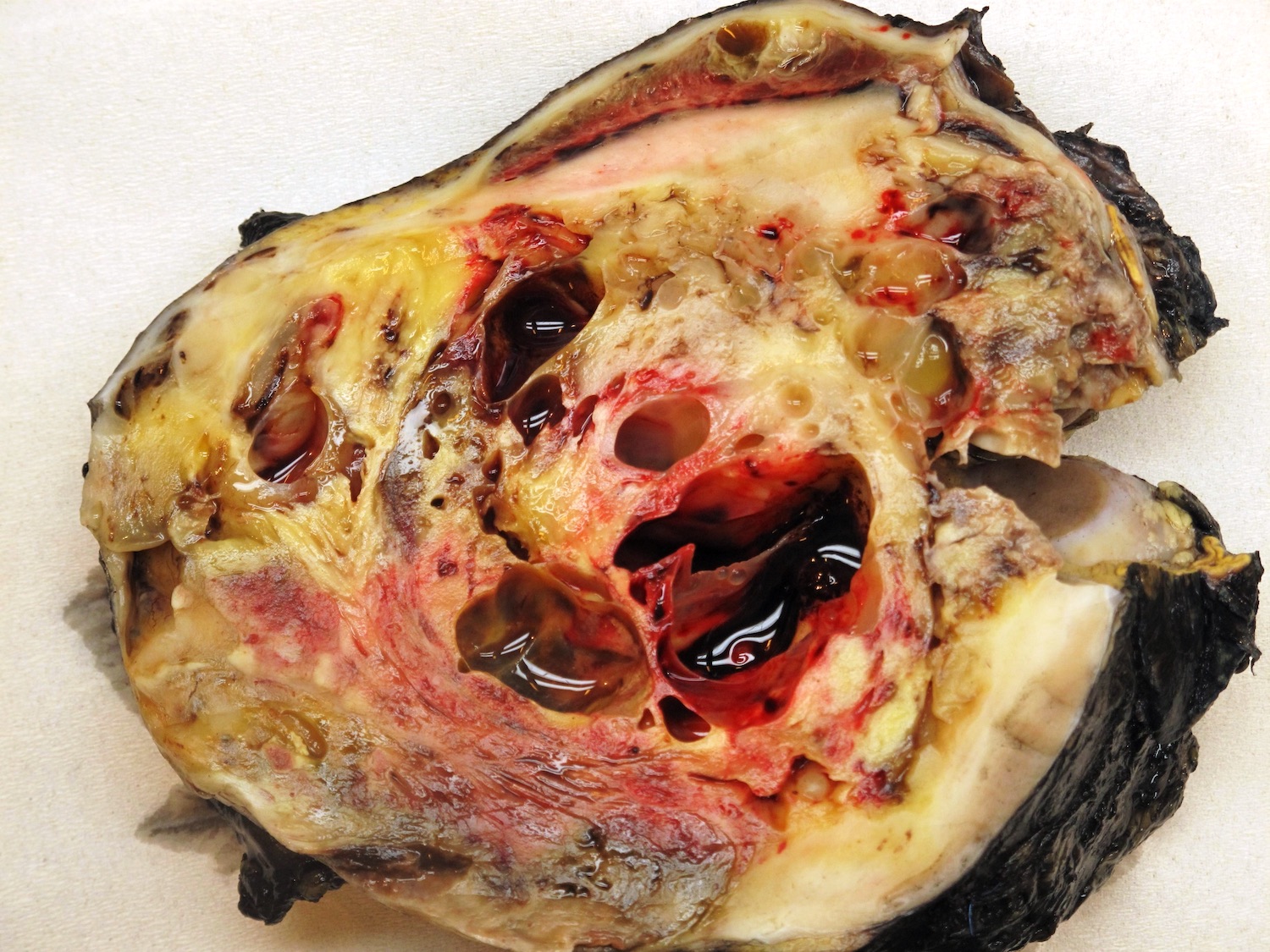
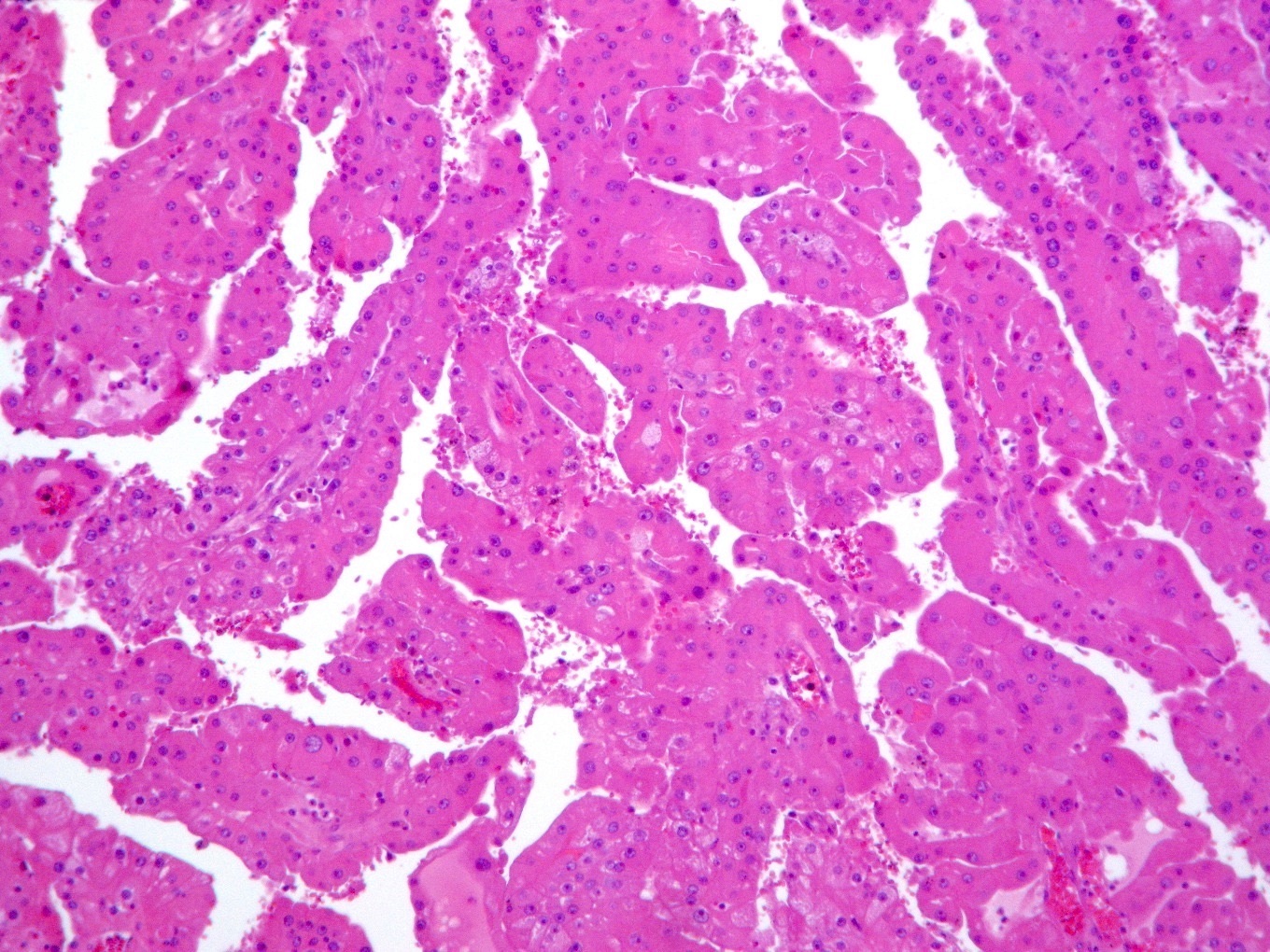
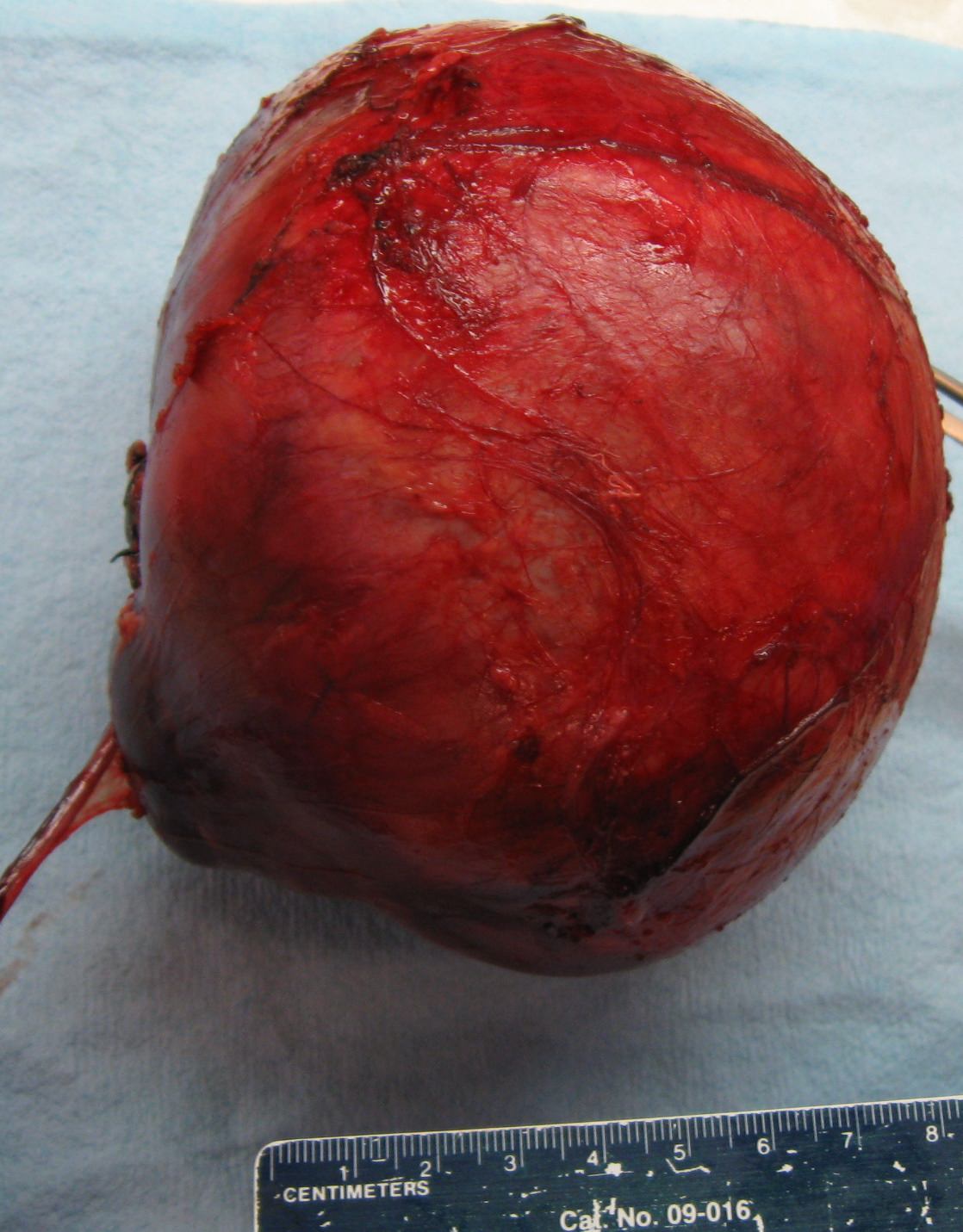
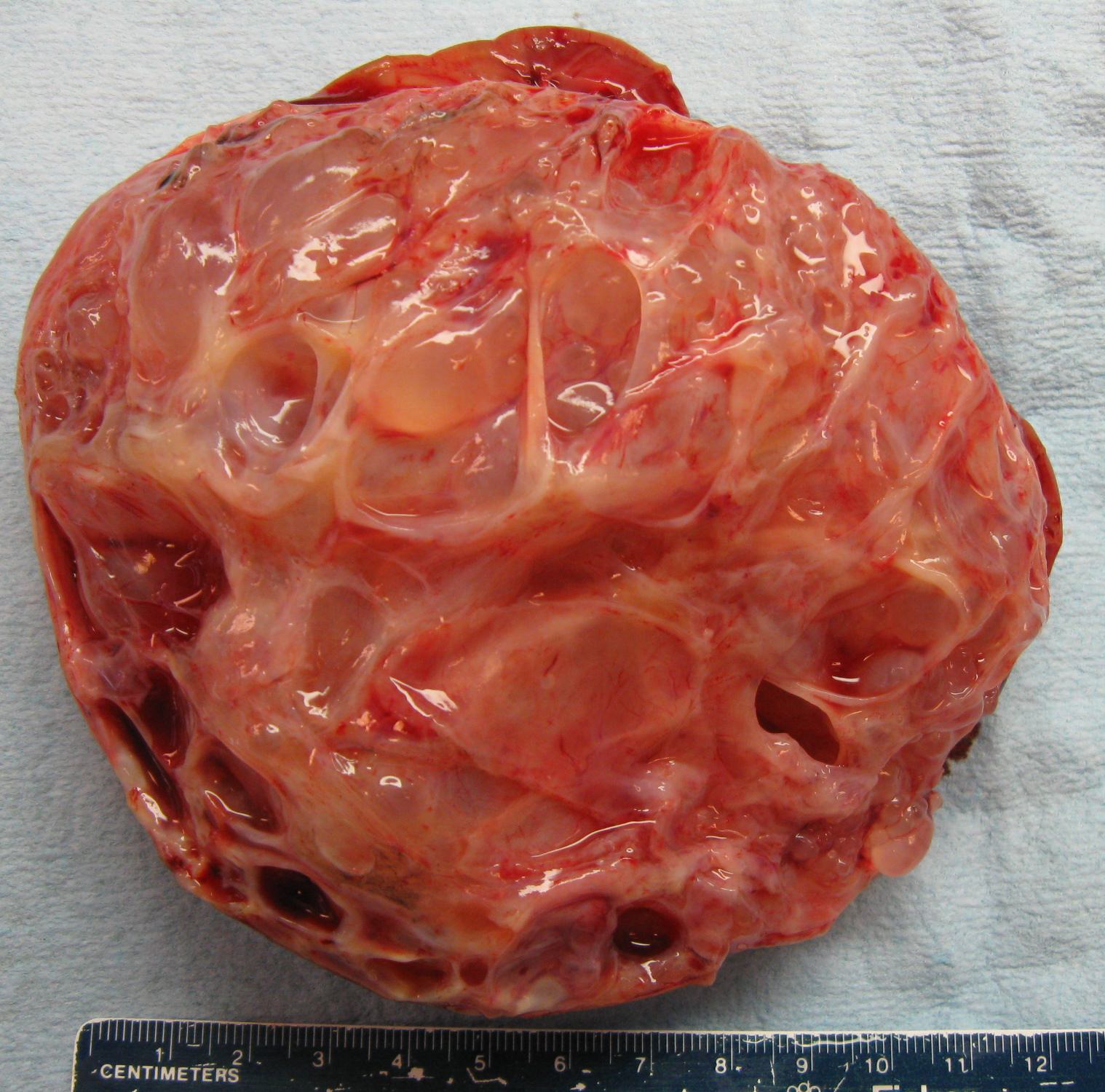
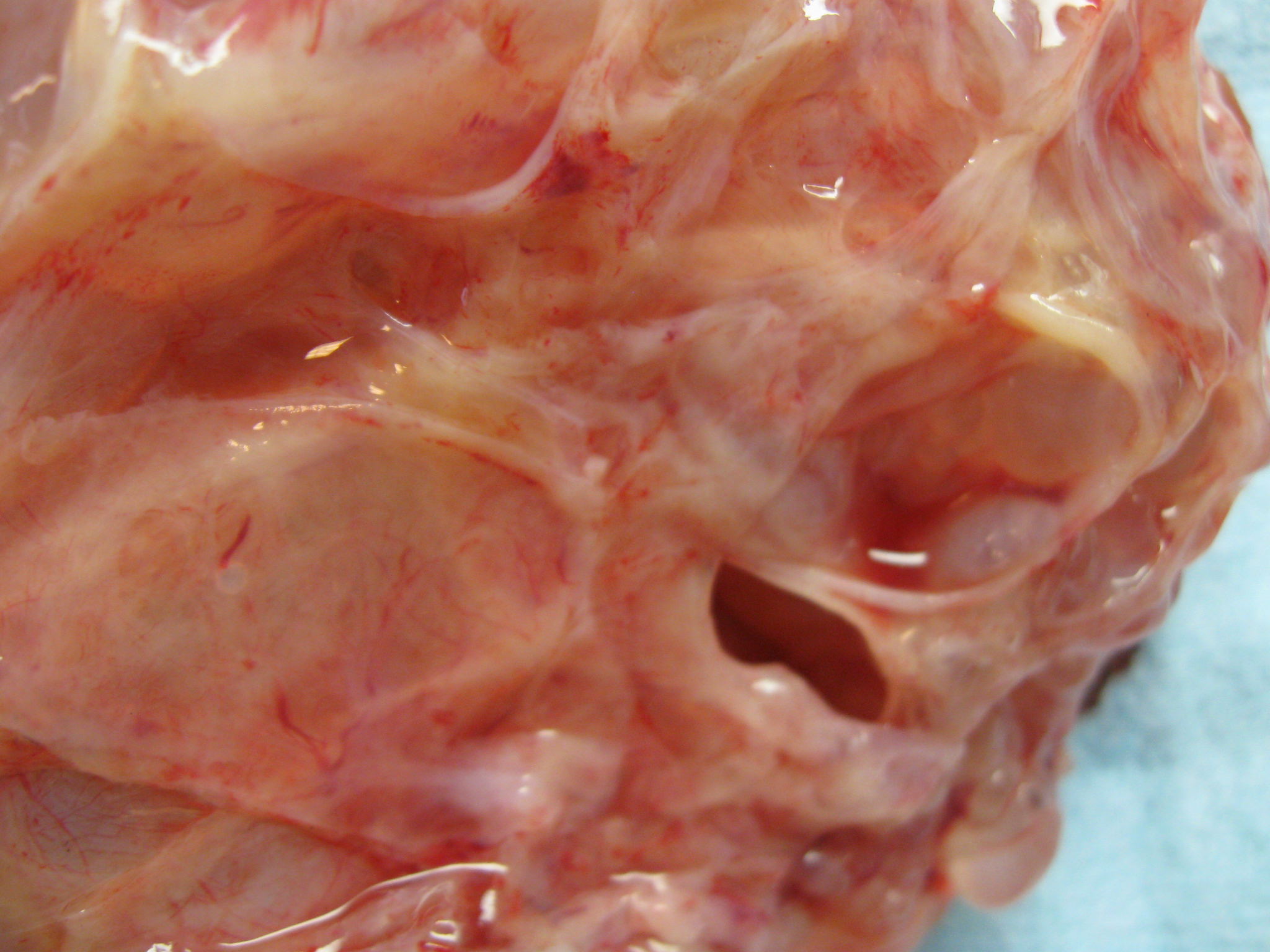
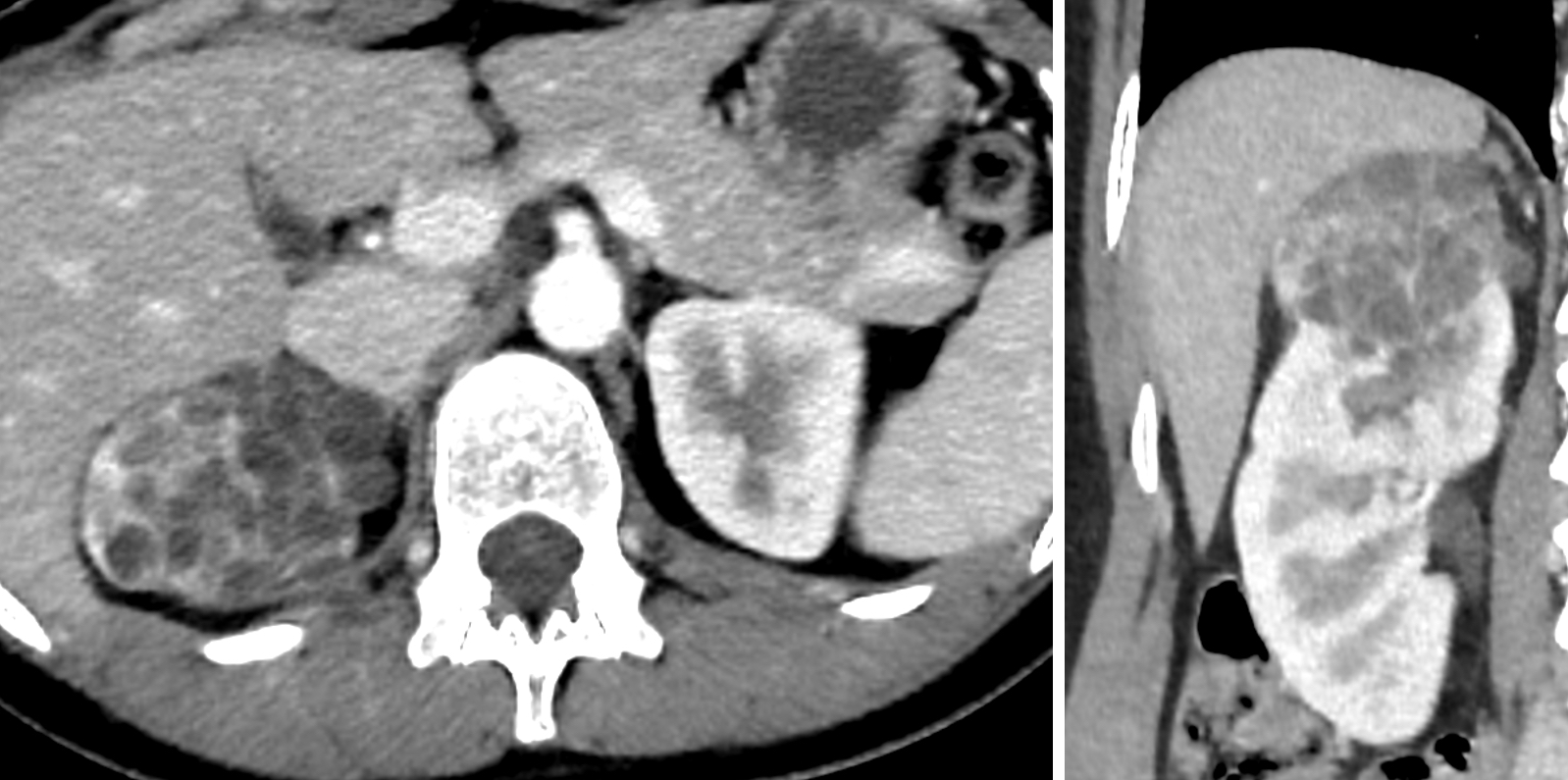
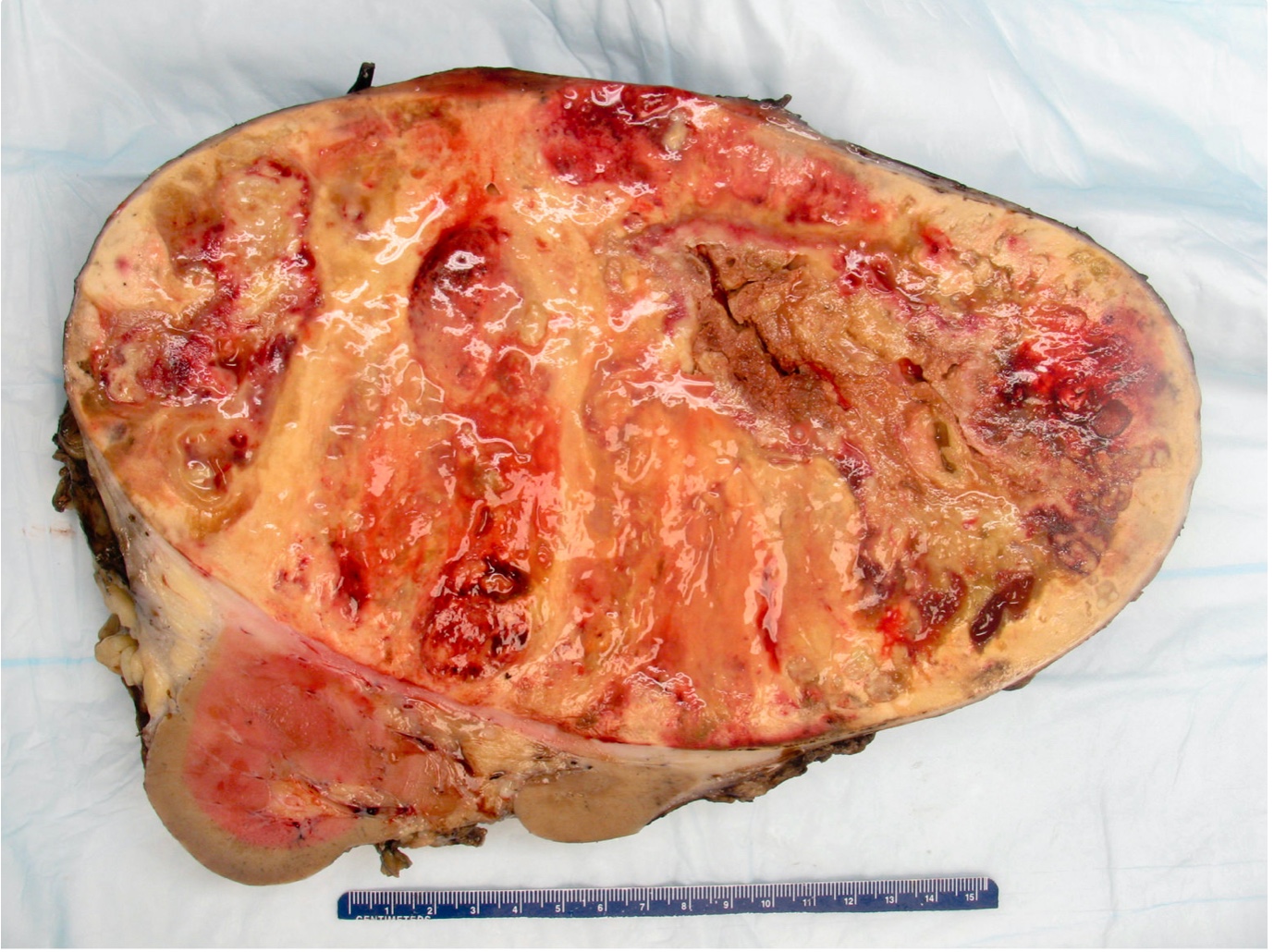
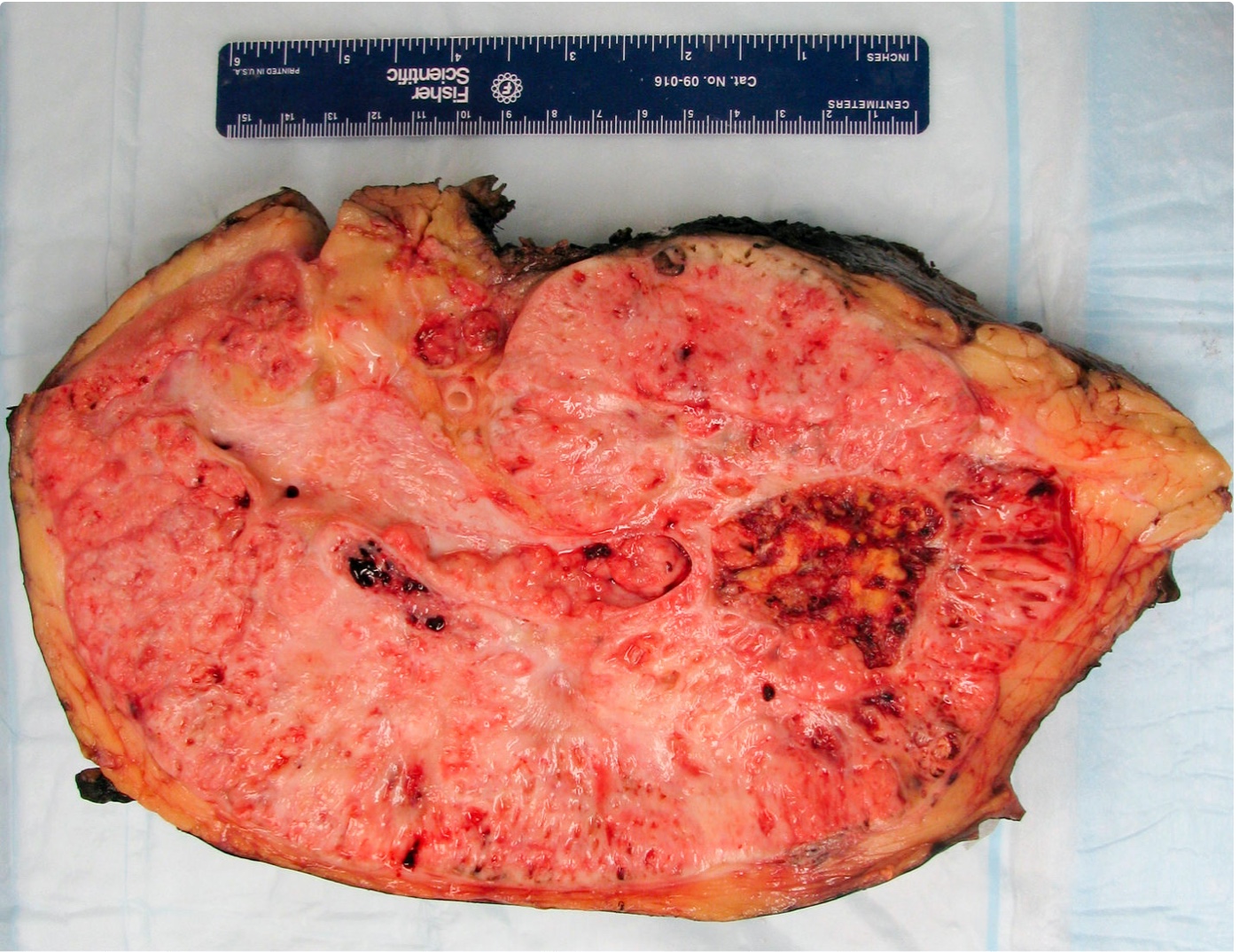
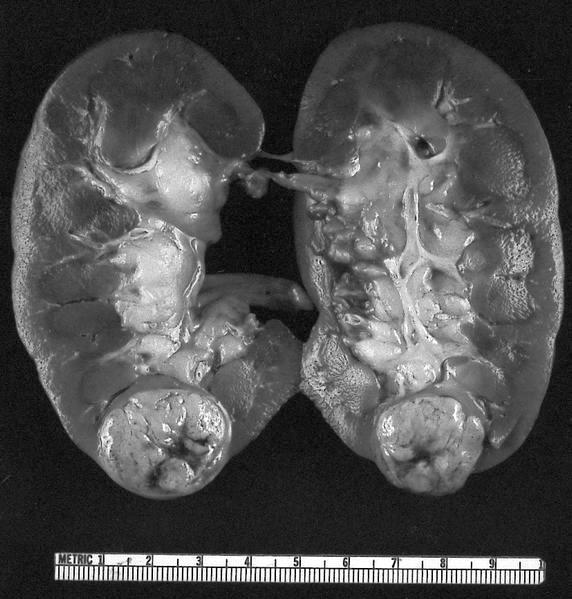
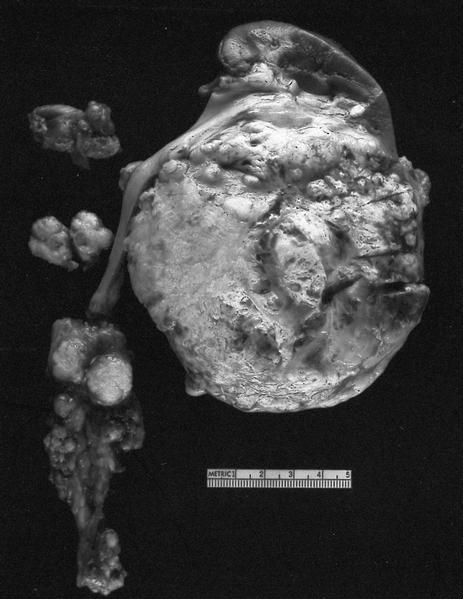
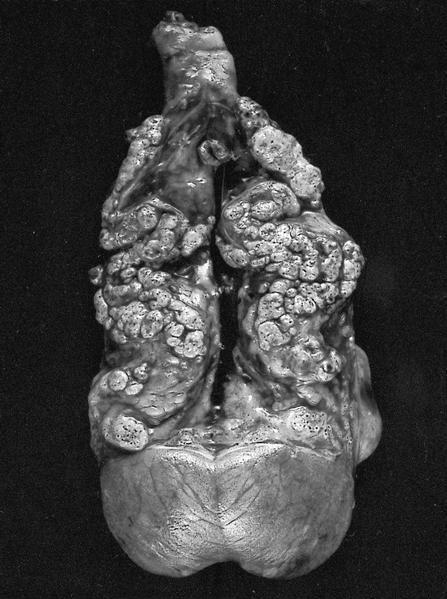
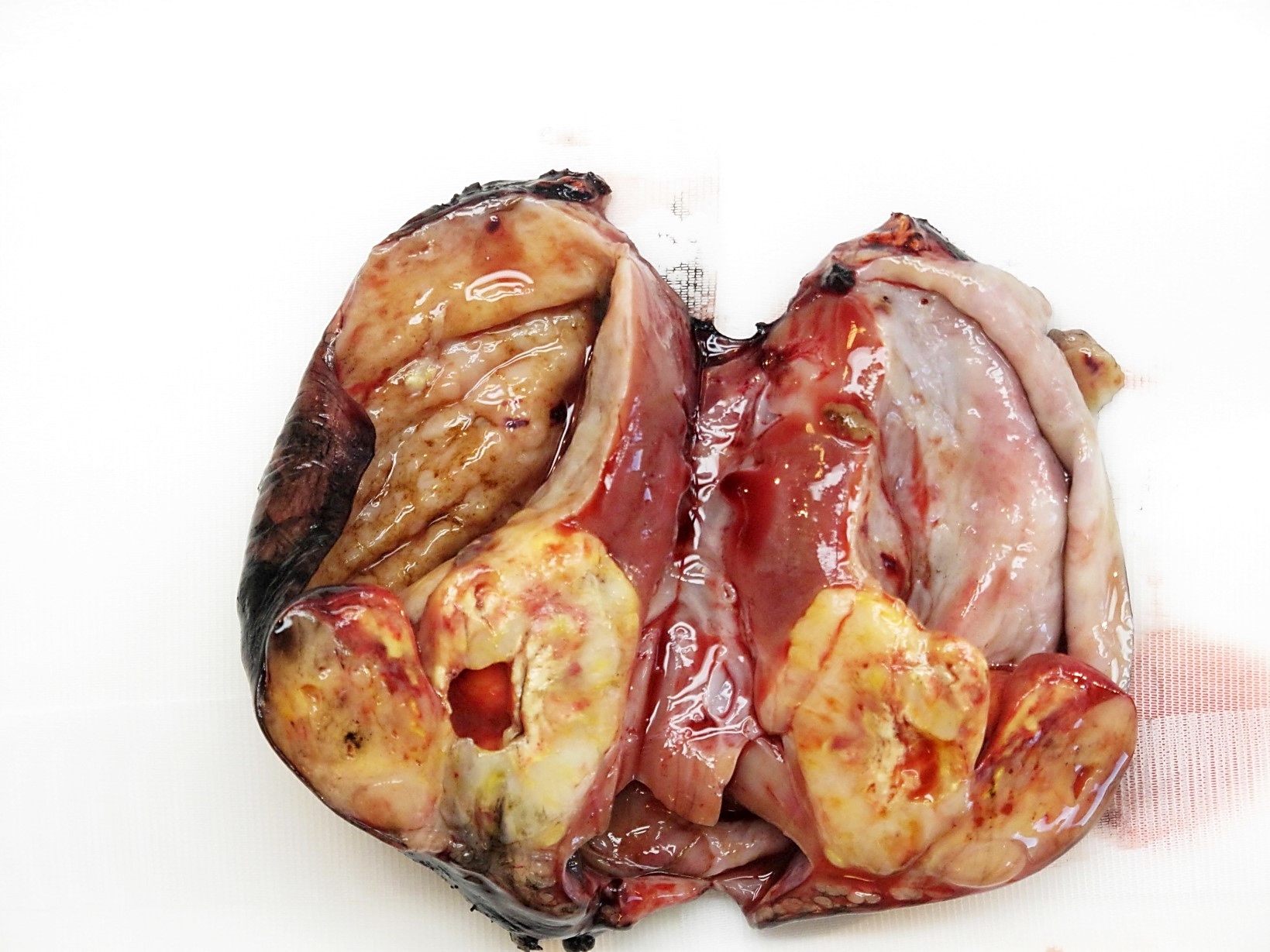
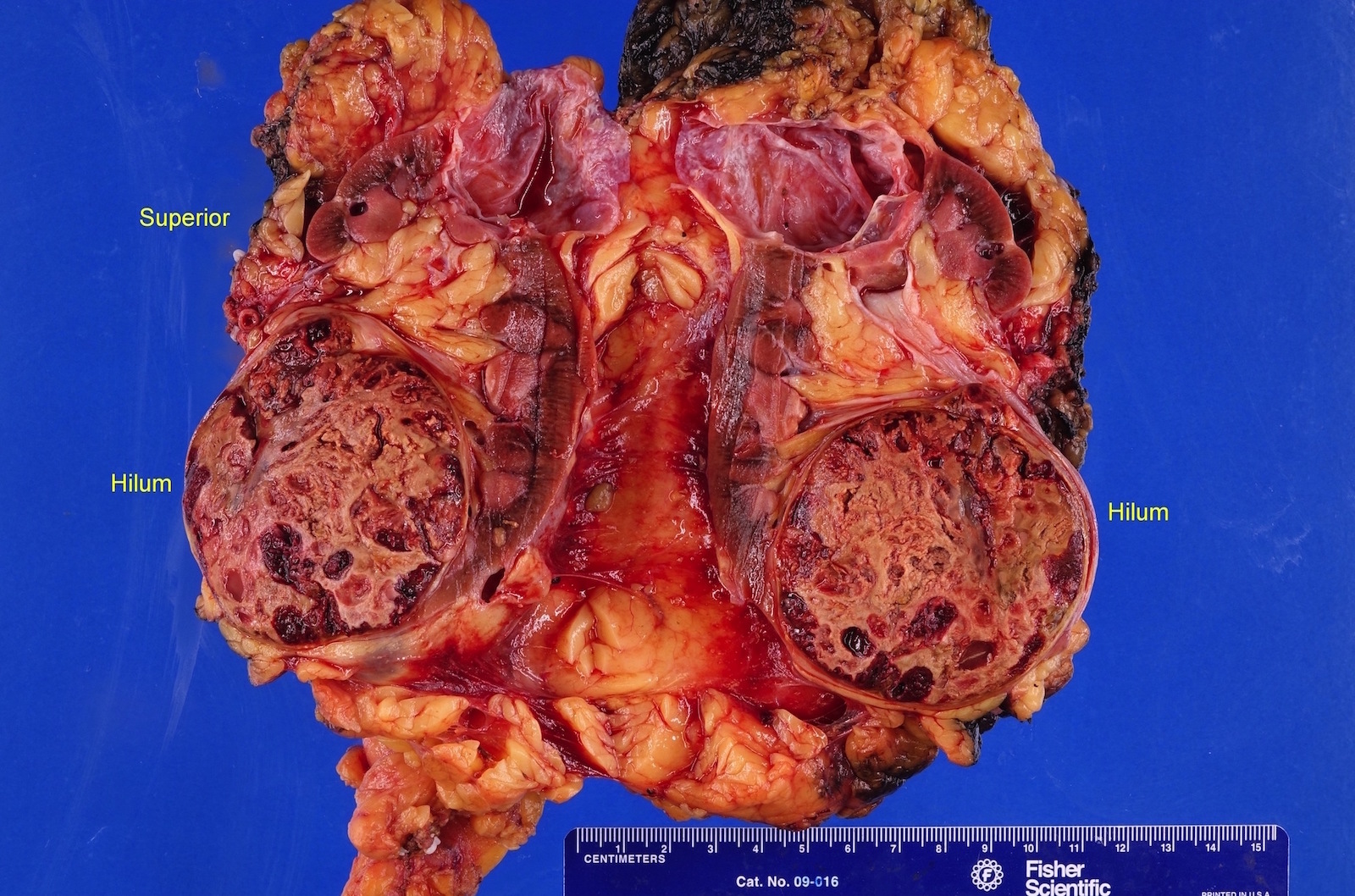
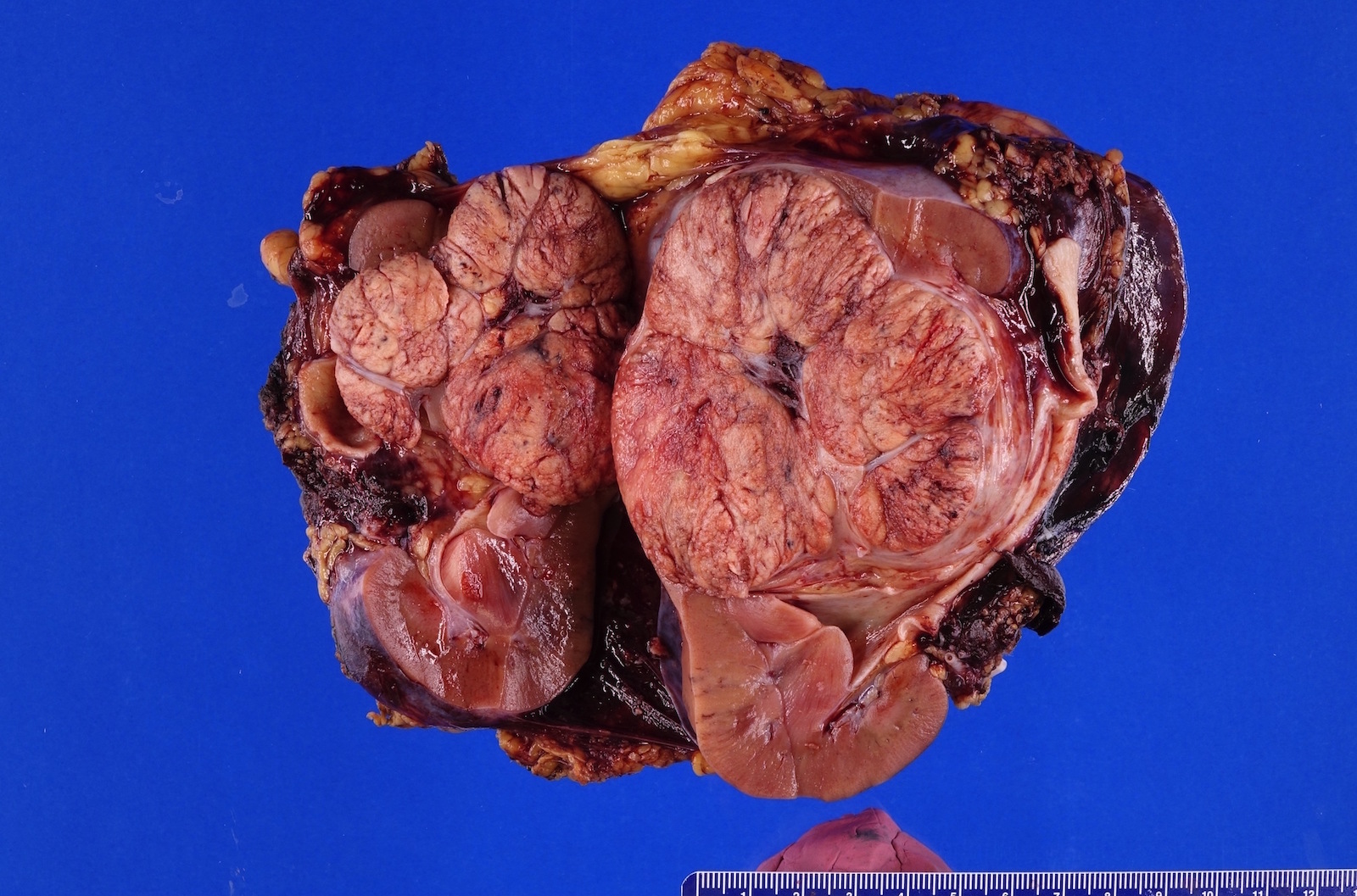
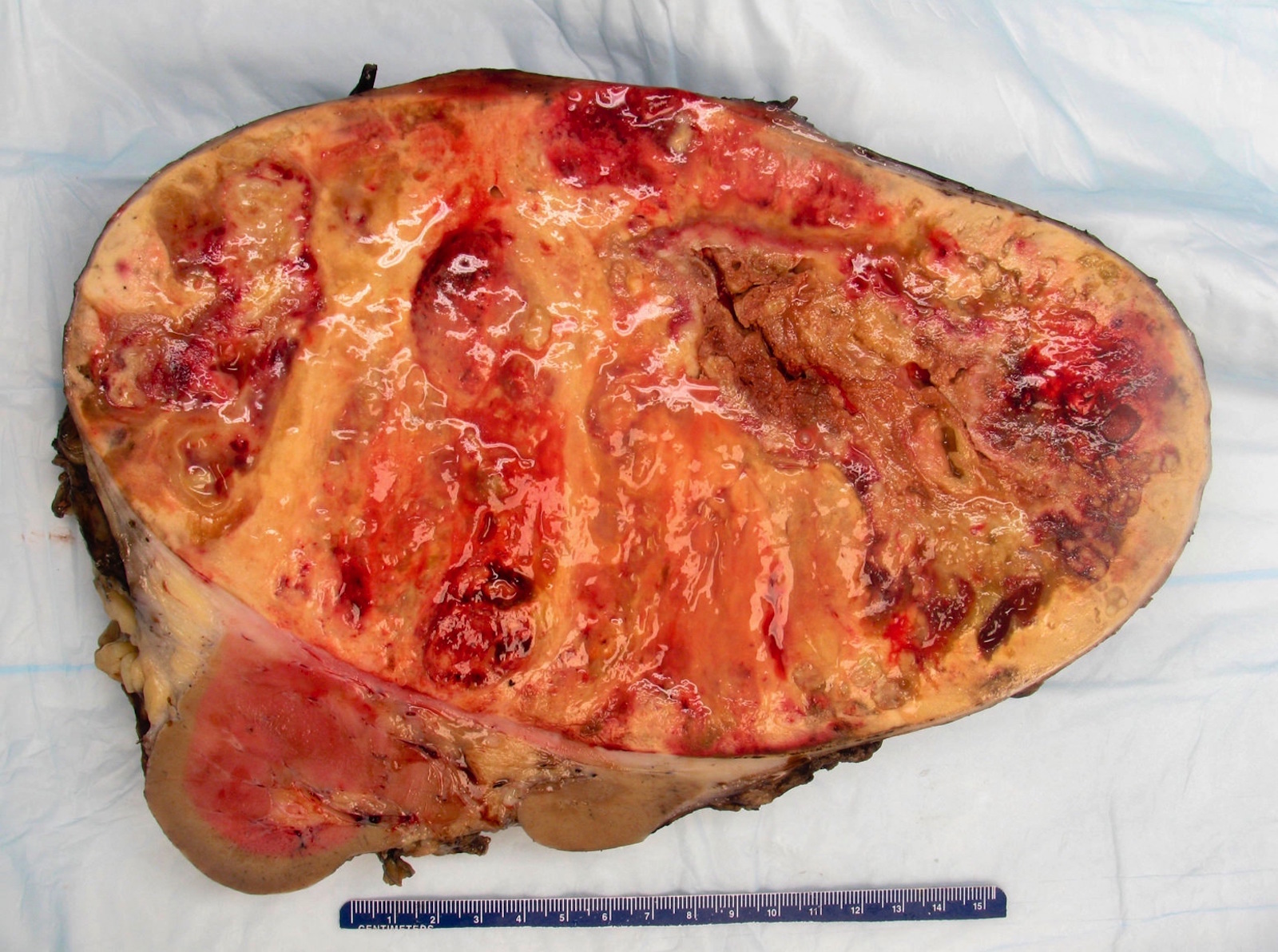
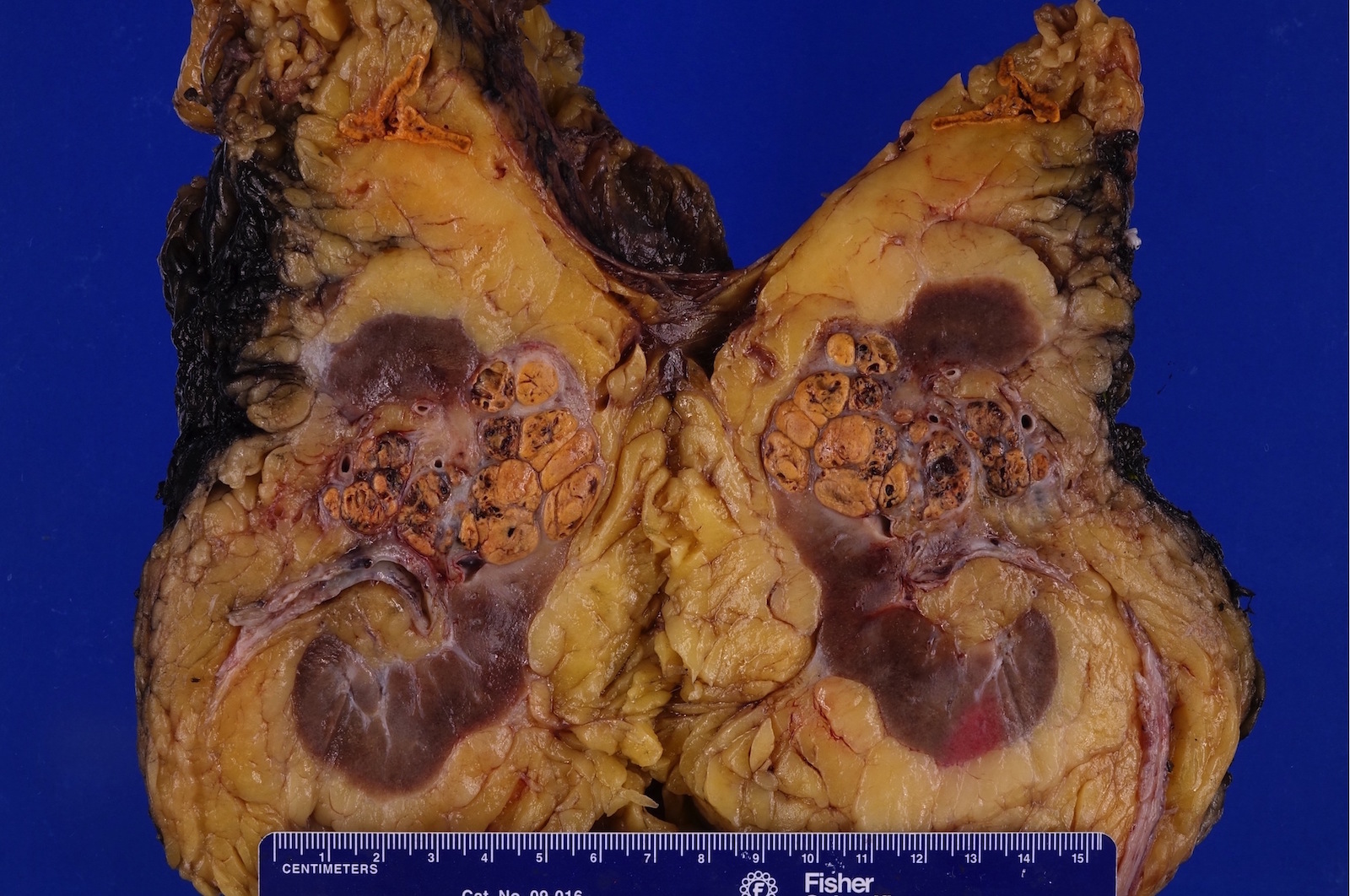
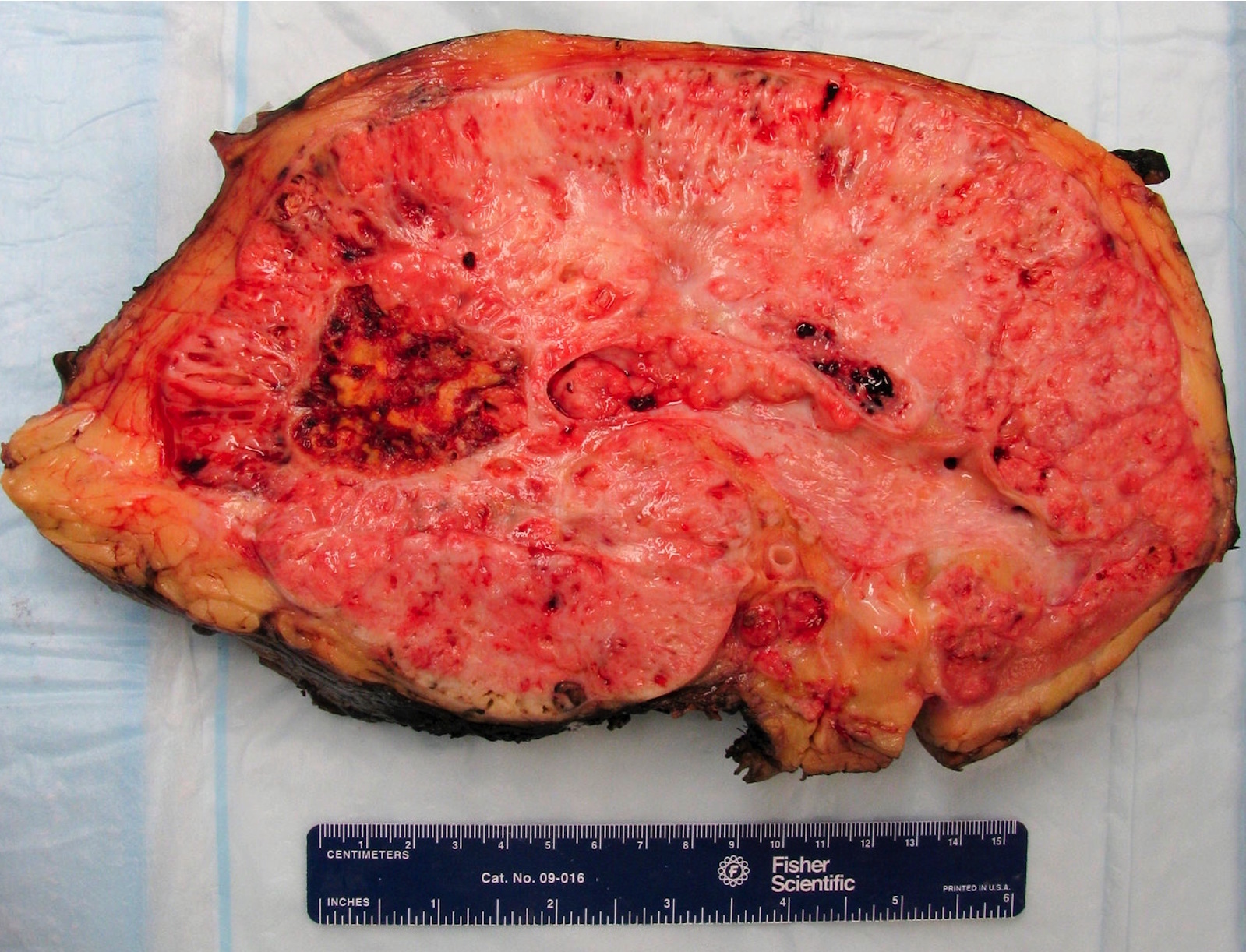
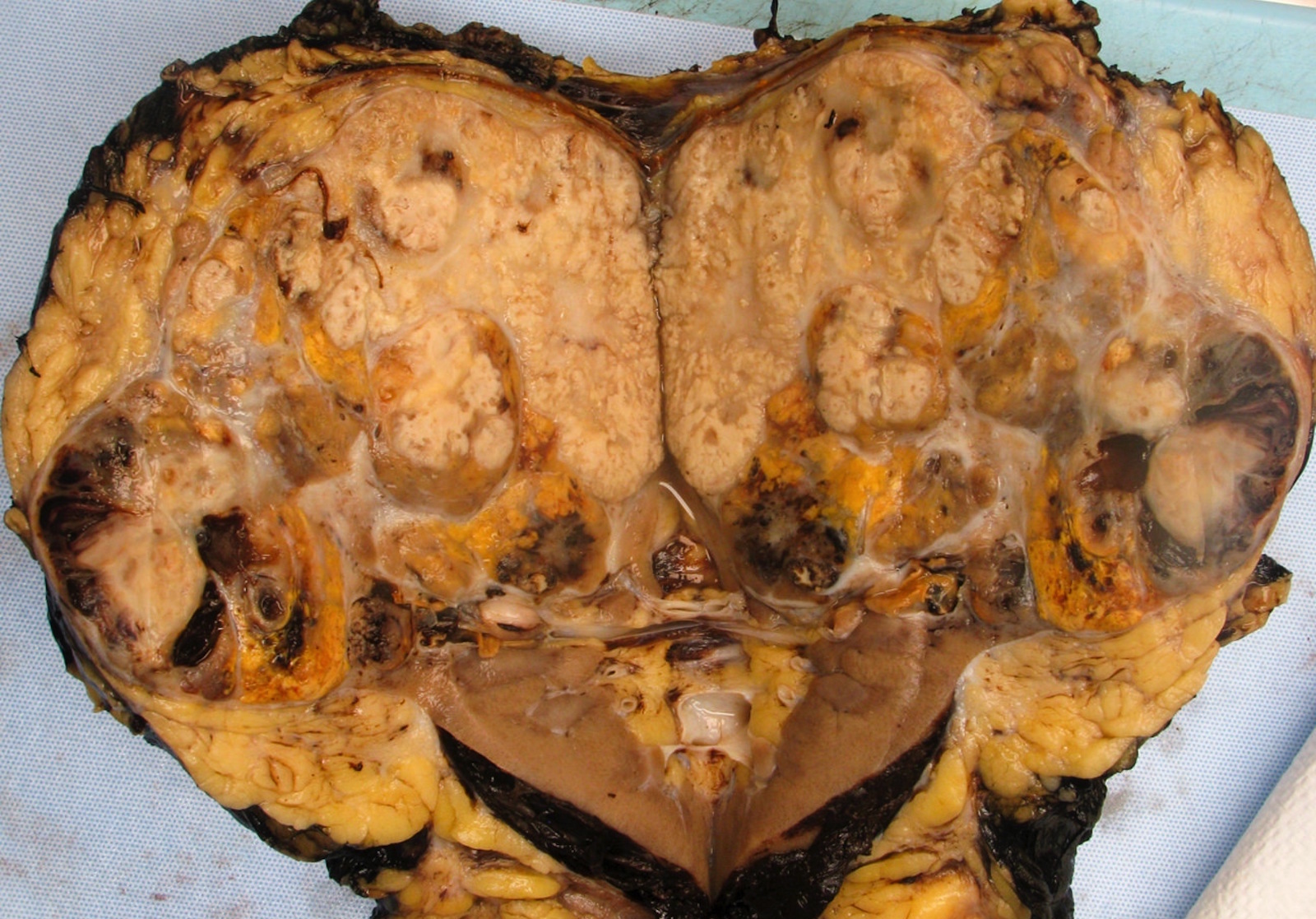
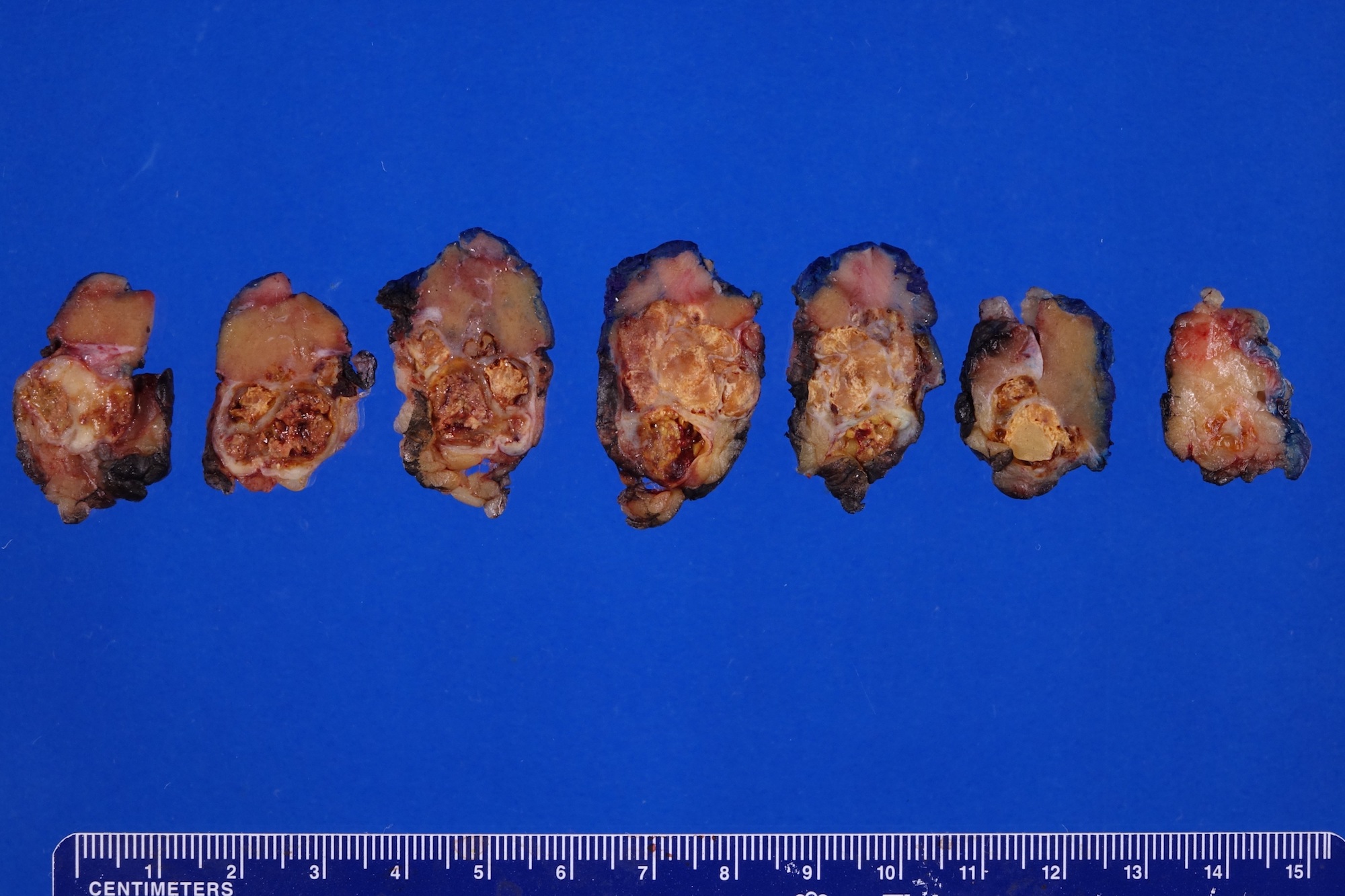
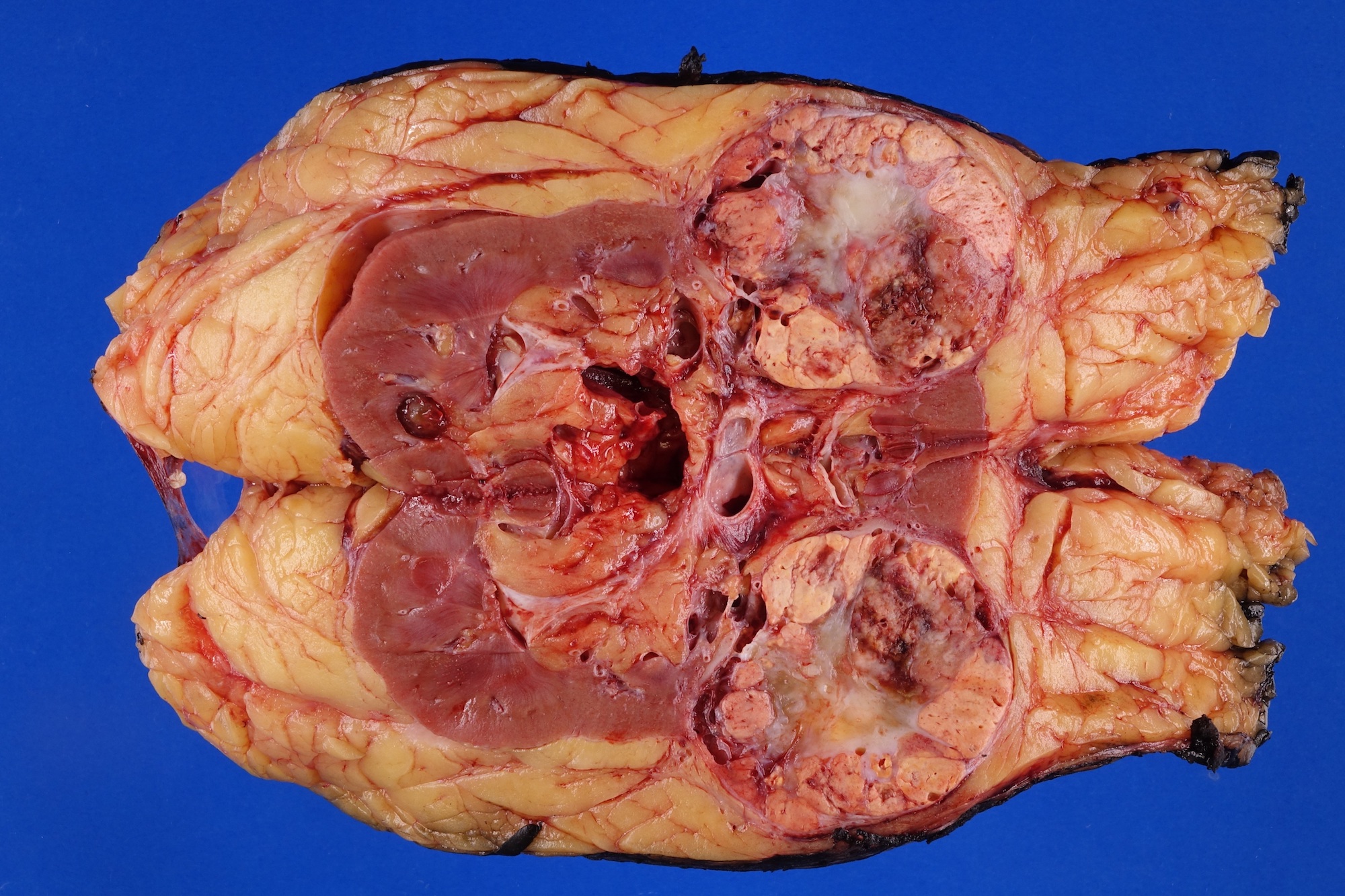
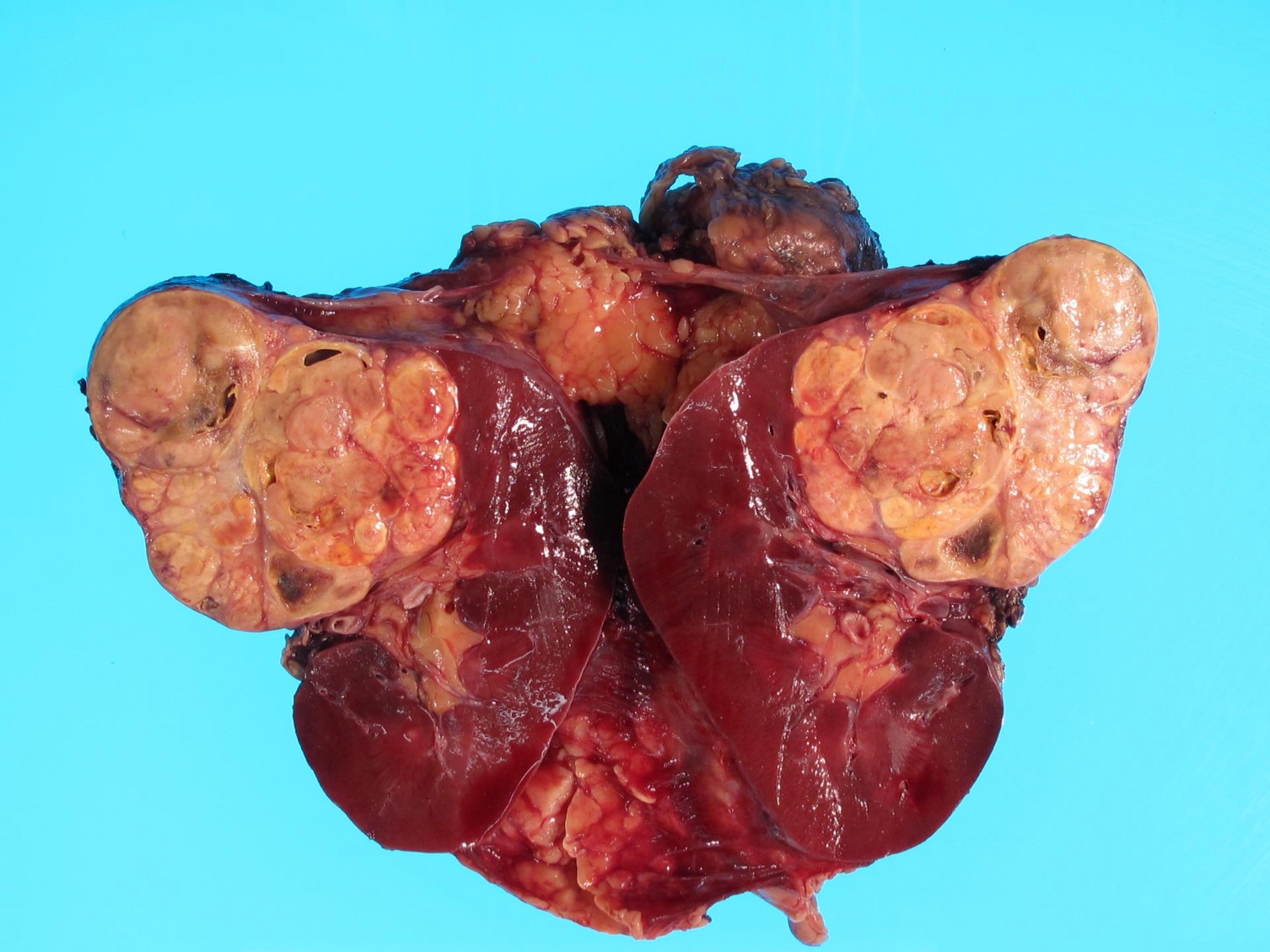
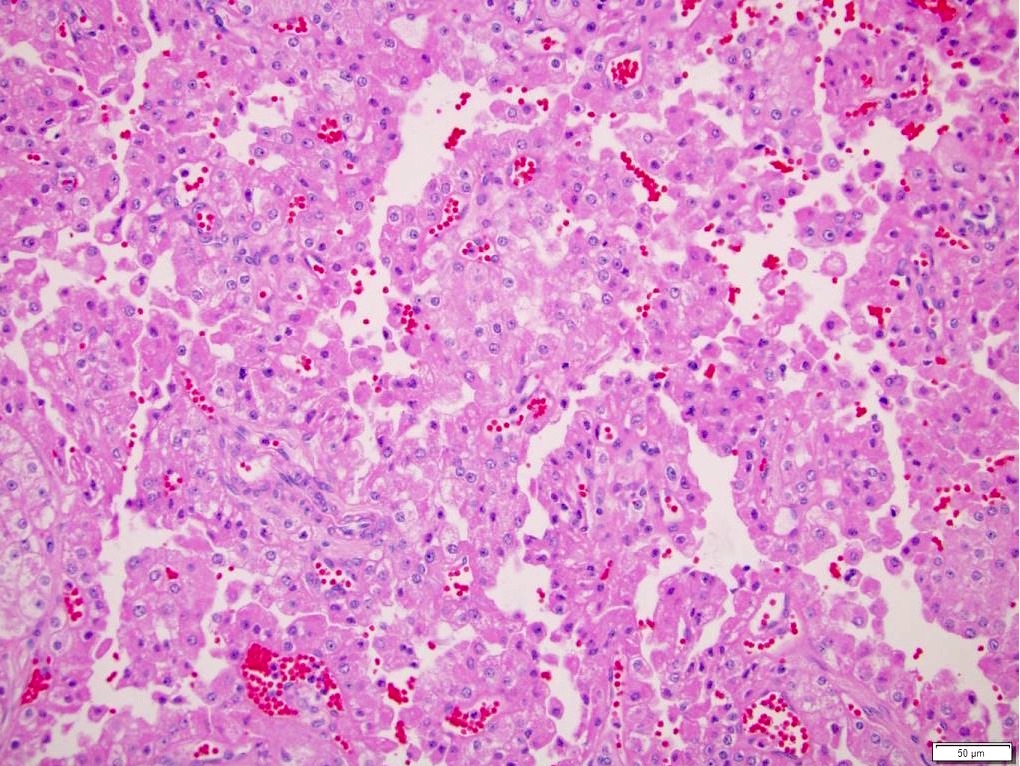
- Typically unilateral and unicentric renal cortical mass (average size: 7 cm)
- Typically well circumscribed by a pseudocapsule and expansile pushing margin protruding from the renal cortex
- Variegated solid and cystic with areas of fibrosis (gray) and recent or old hemorrhage (brown); necrosis and cystic changes are common
- Golden yellow color due to high lipid content
- Higher grade tumors may not be yellow due to less lipid and glycogen content
- Soft fleshy areas may reflect sarcomatoid differentiation
- Frequent involvement of renal vein and renal sinus
- Bilateral and multicentric masses are features of hereditary disease
- Notes on tumor staging
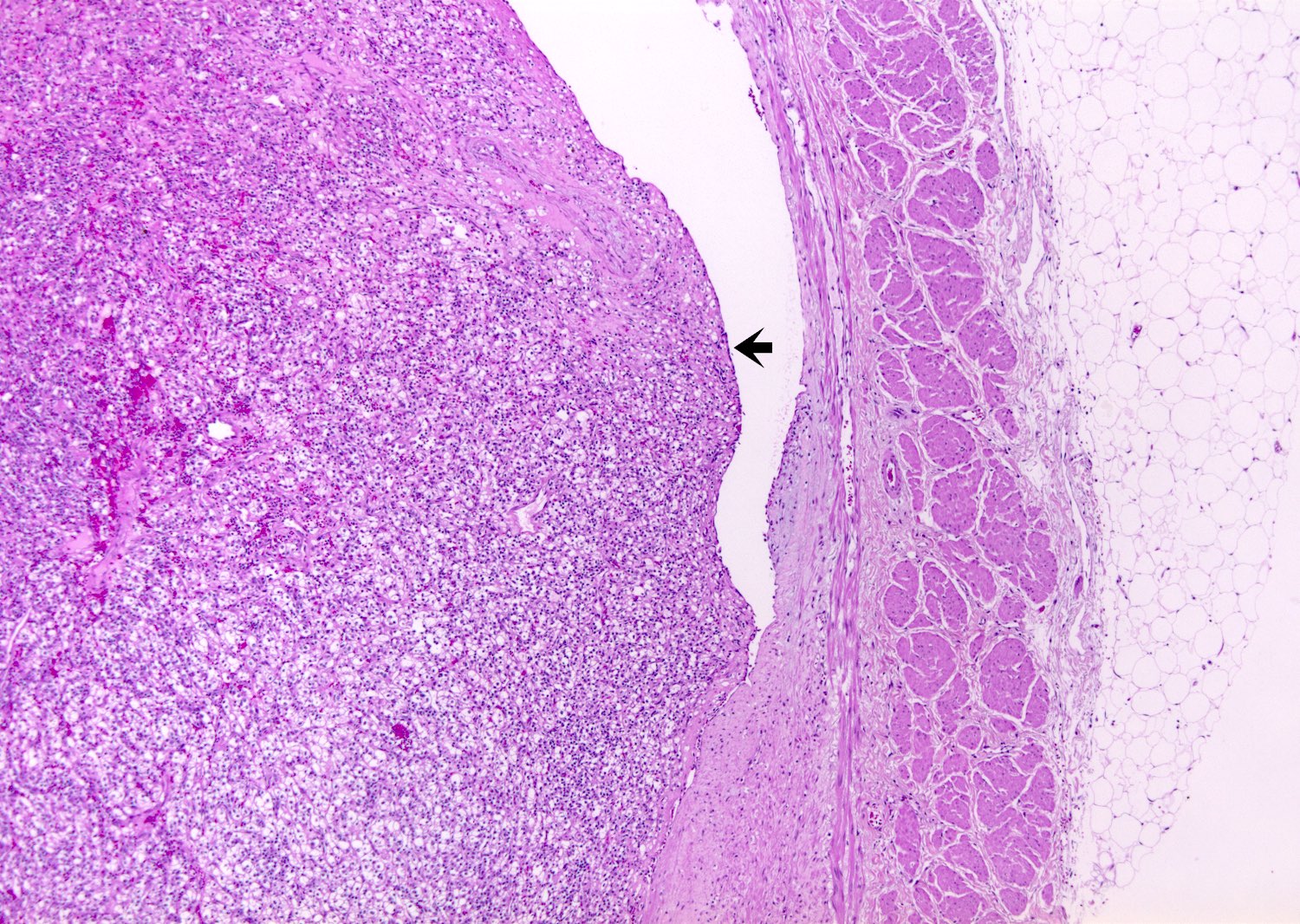
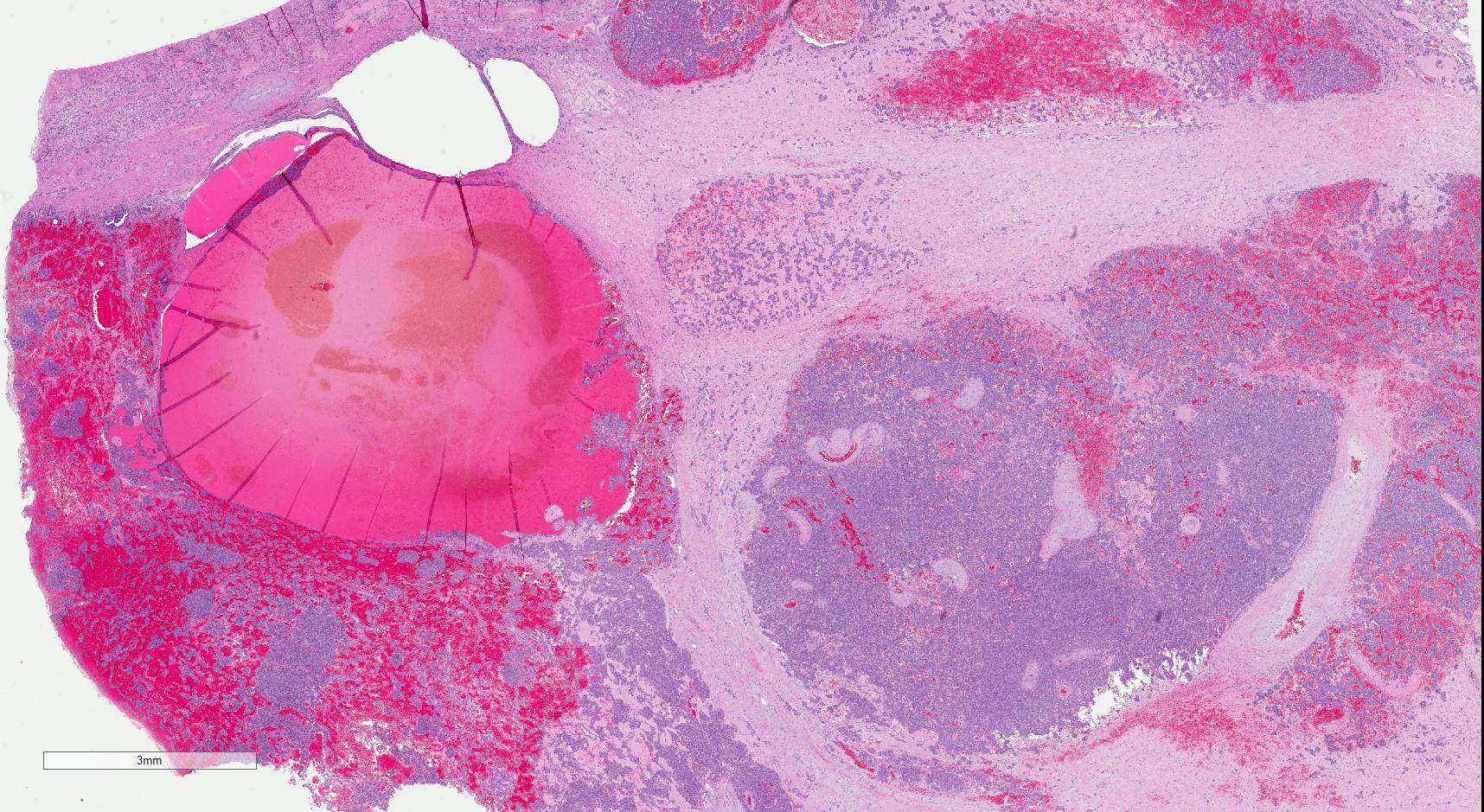
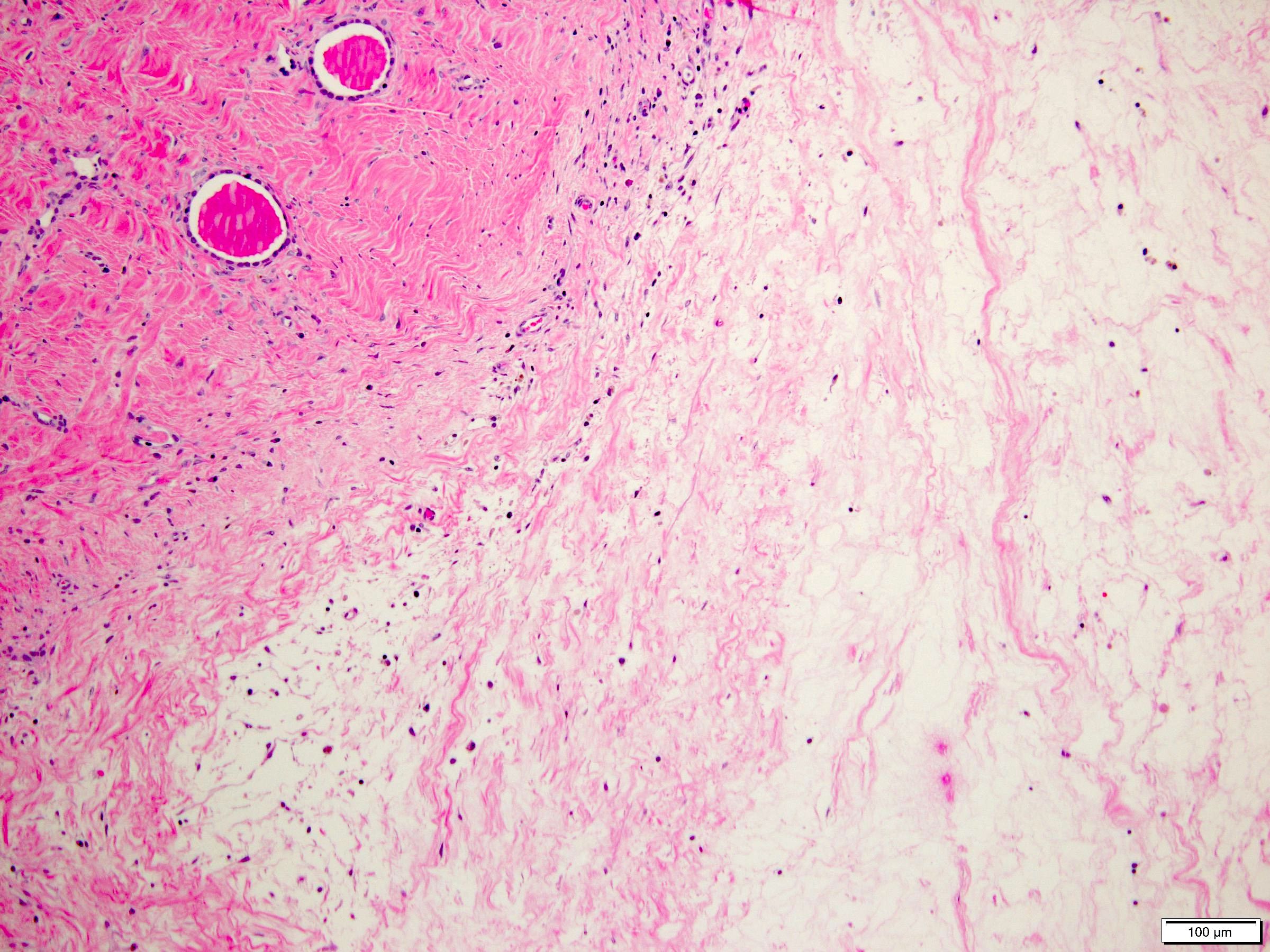
- Tumors larger than 7 cm almost always invade renal sinus fat; if no invasion is seen in larger tumors, an additional gross review is warranted (Pathology 2021;53:120)
- Capsular invasion
- Disrupted and irregular advancement of tumor into the perirenal adipose tissue and loss of the smooth convex outer contour
- Smooth bulging tumor covered by the cancer pseudocapsule is not considered perirenal fat involvement
- Tumor cell should touch fat or extend irregular tongues into perinephric tissues, with or without desmoplasia (J Kidney Cancer VHL 2014;1:26)
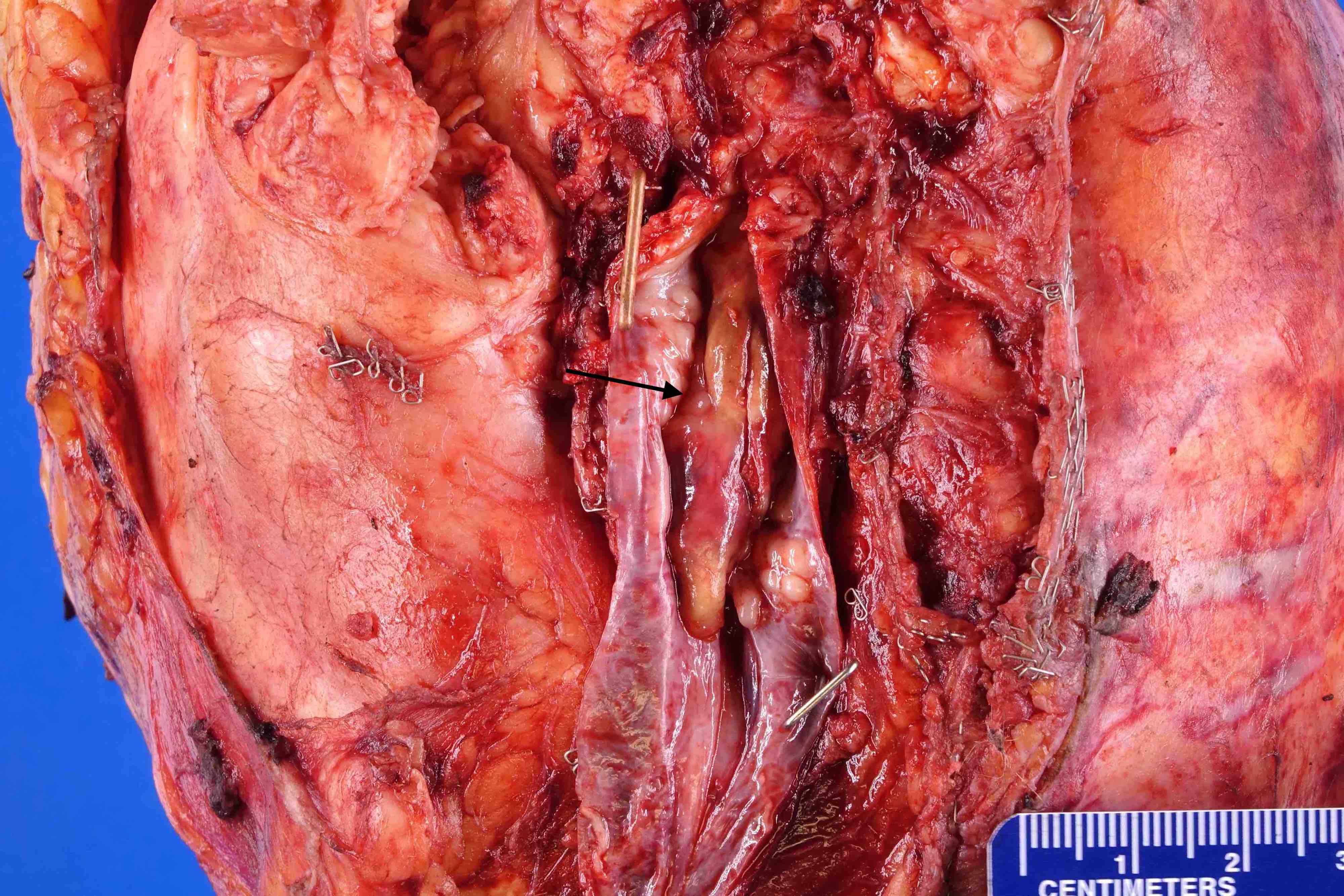
- Renal sinus invasion
- Most common route of extrarenal spread
- Usually happens before capsular invasion
- Unlike renal capsule, the renal sinus is not delineated from the renal parenchyma by a fibrous capsule
- Not regarded as true invasion if tumor is separated from sinus structures by a rim of renal parenchyma
- Regarded as sinus invasion if tumor bulges into the renal sinus fat clearly beyond renal parenchyma, even if covered by loose connective tissue (J Kidney Cancer VHL 2014;1:26)
- Tumor surrounding large lymphovascular structures is a sign of sinus fat invasion (Am J Surg Pathol 2004;28:1594, Am J Surg Pathol 2000;24:451)
- Vascular invasion
- May show as tumor nodules in renal sinus
- In partially obliterate vessels, a single layer of endothelial lining overlying the tumor does not preclude vascular invasion
- Small vein involvement usually implies large vein involvement
- Renal sinus invasion usually implies renal vein invasion, cautious examination is warranted (J Urol 2005;174:1199)
- Limited application in determining the subtype of renal cell carcinoma
- Sometimes important to distinguish urothelial carcinoma from RCC
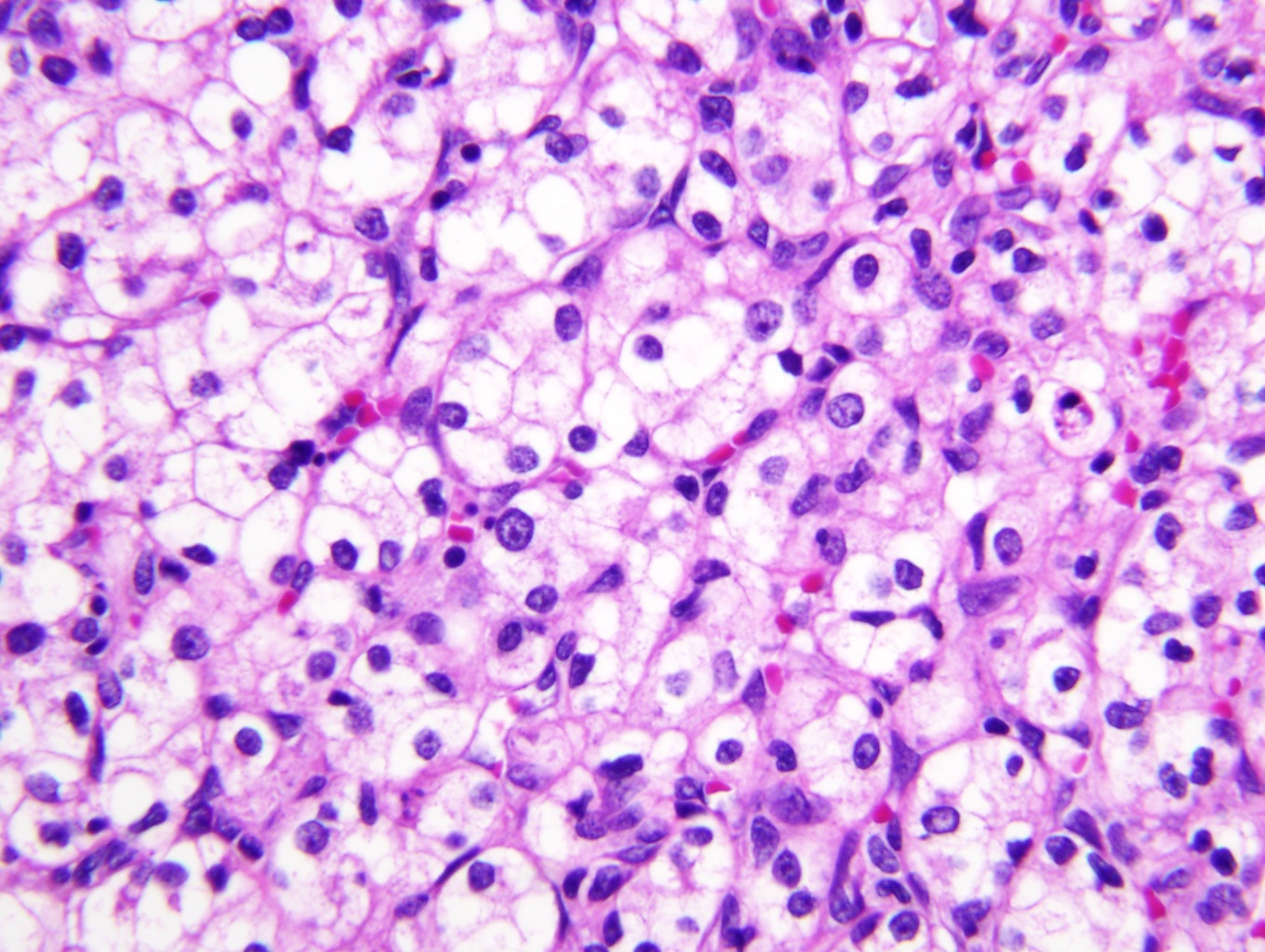
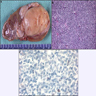
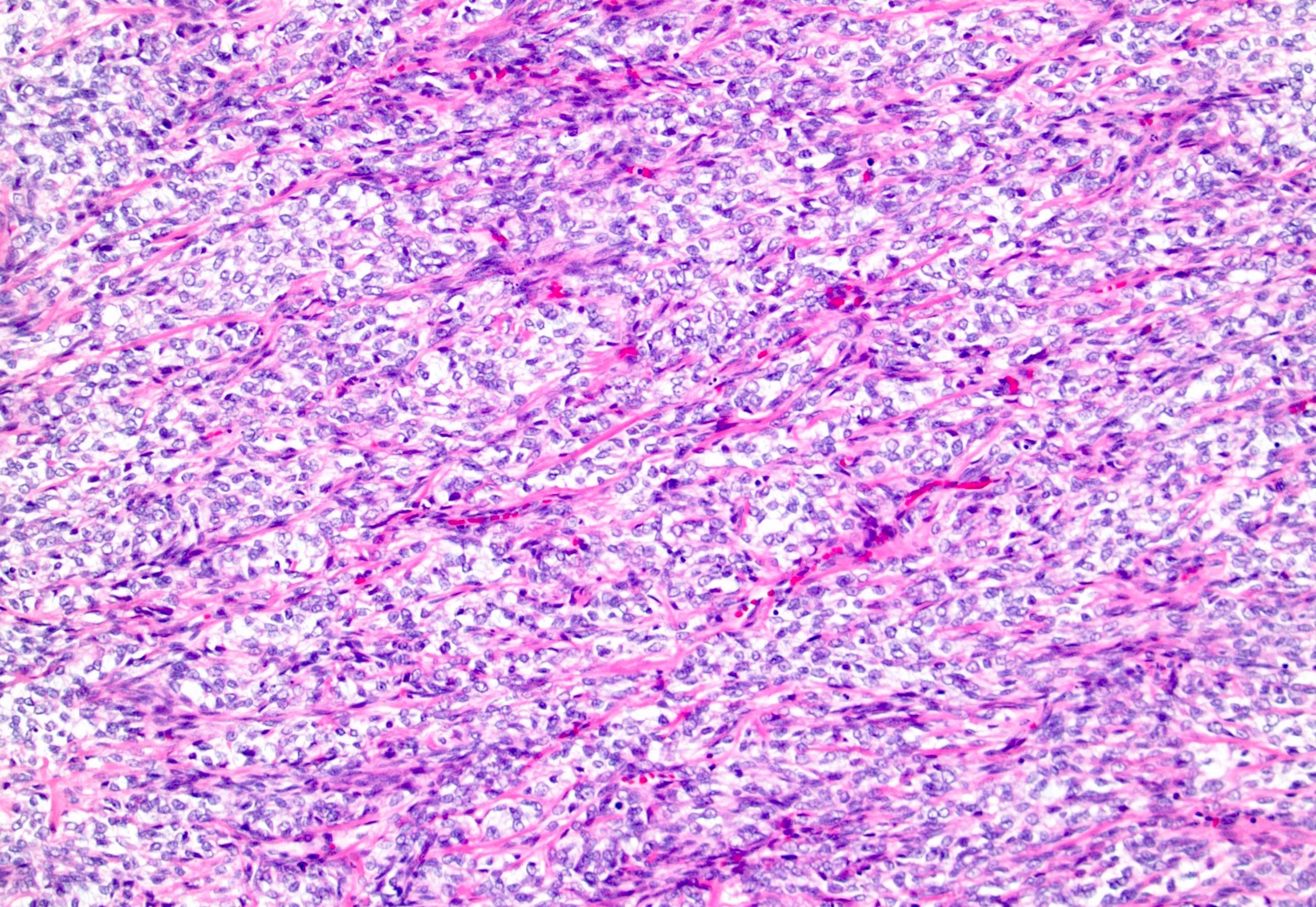
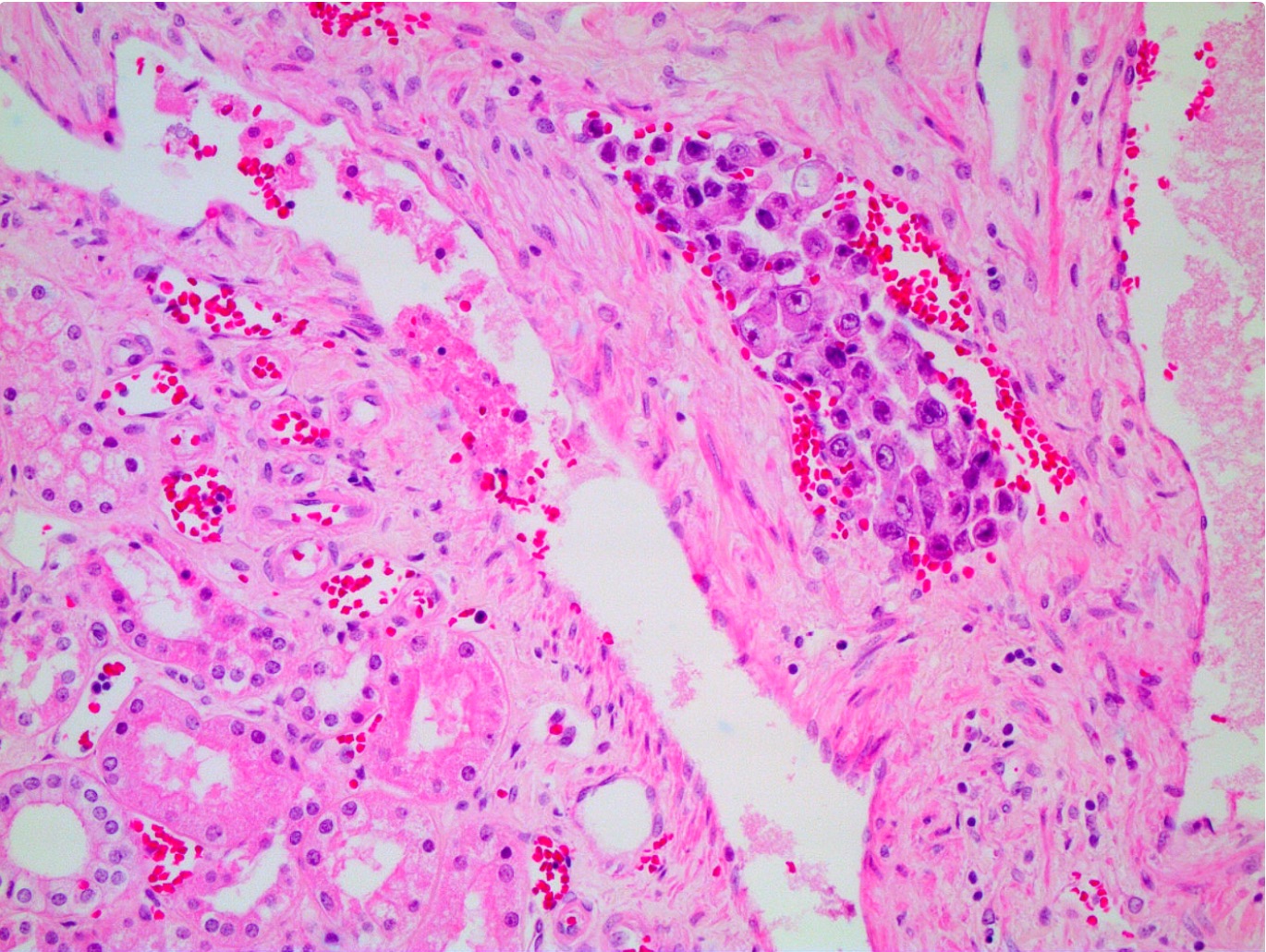
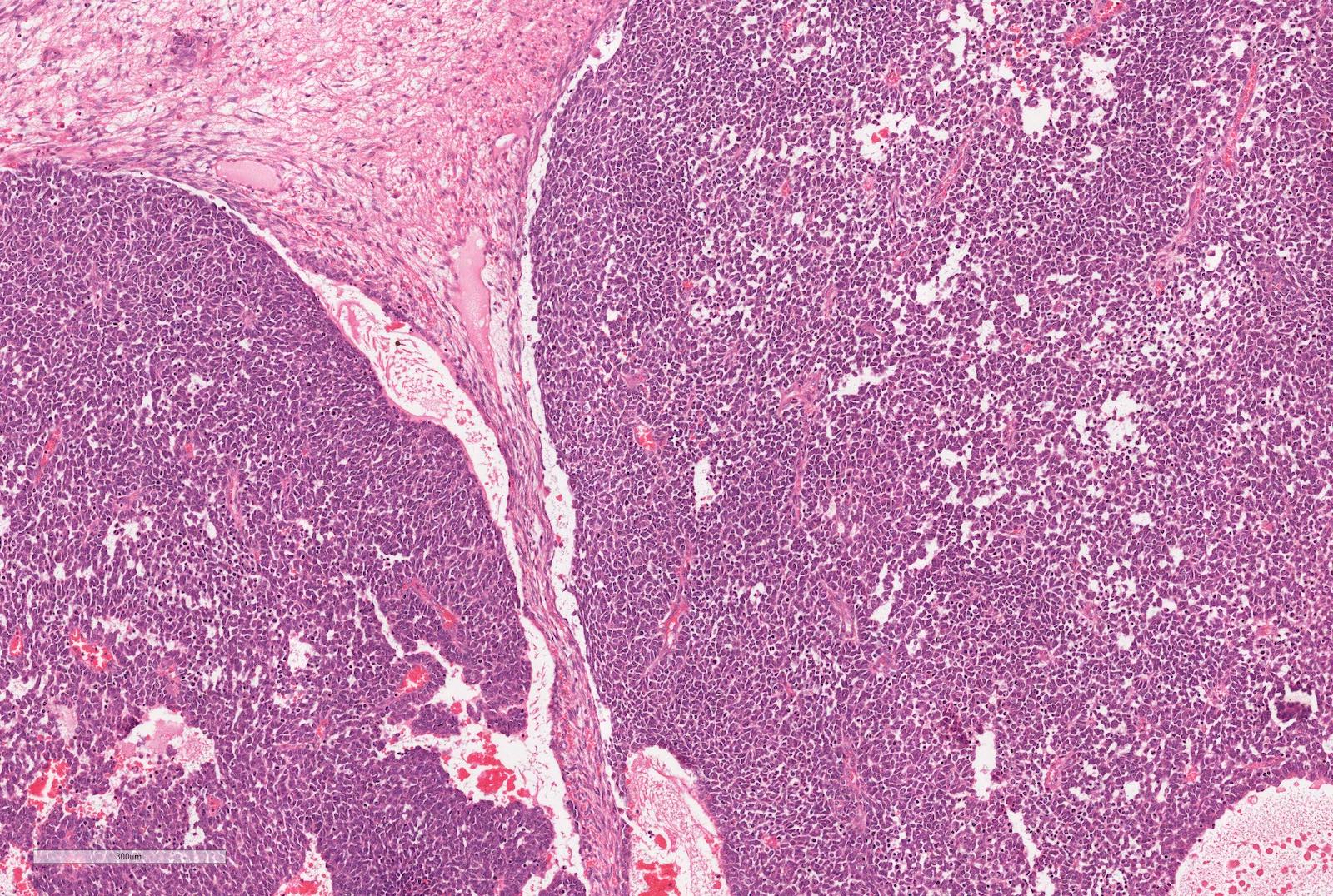
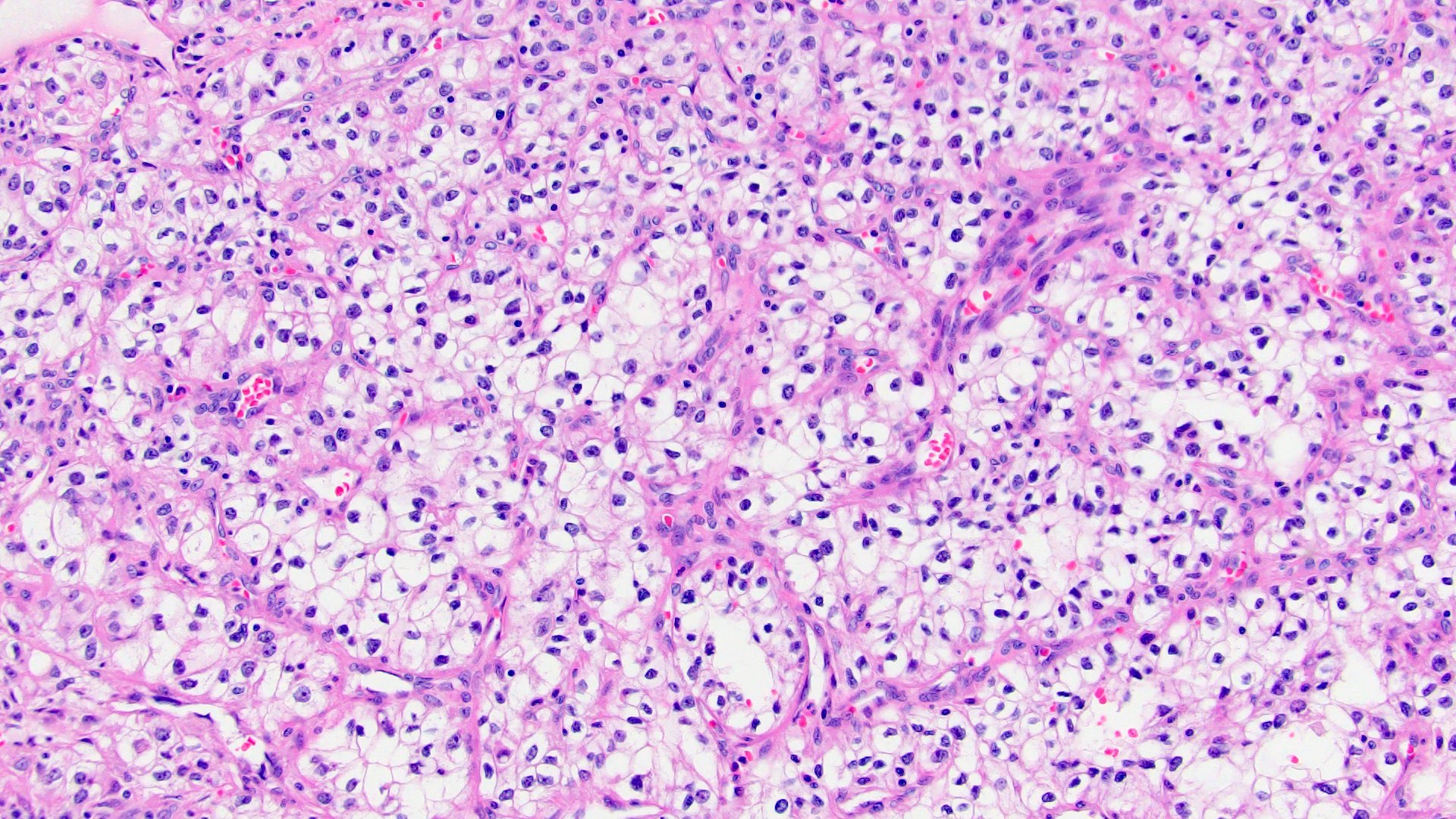
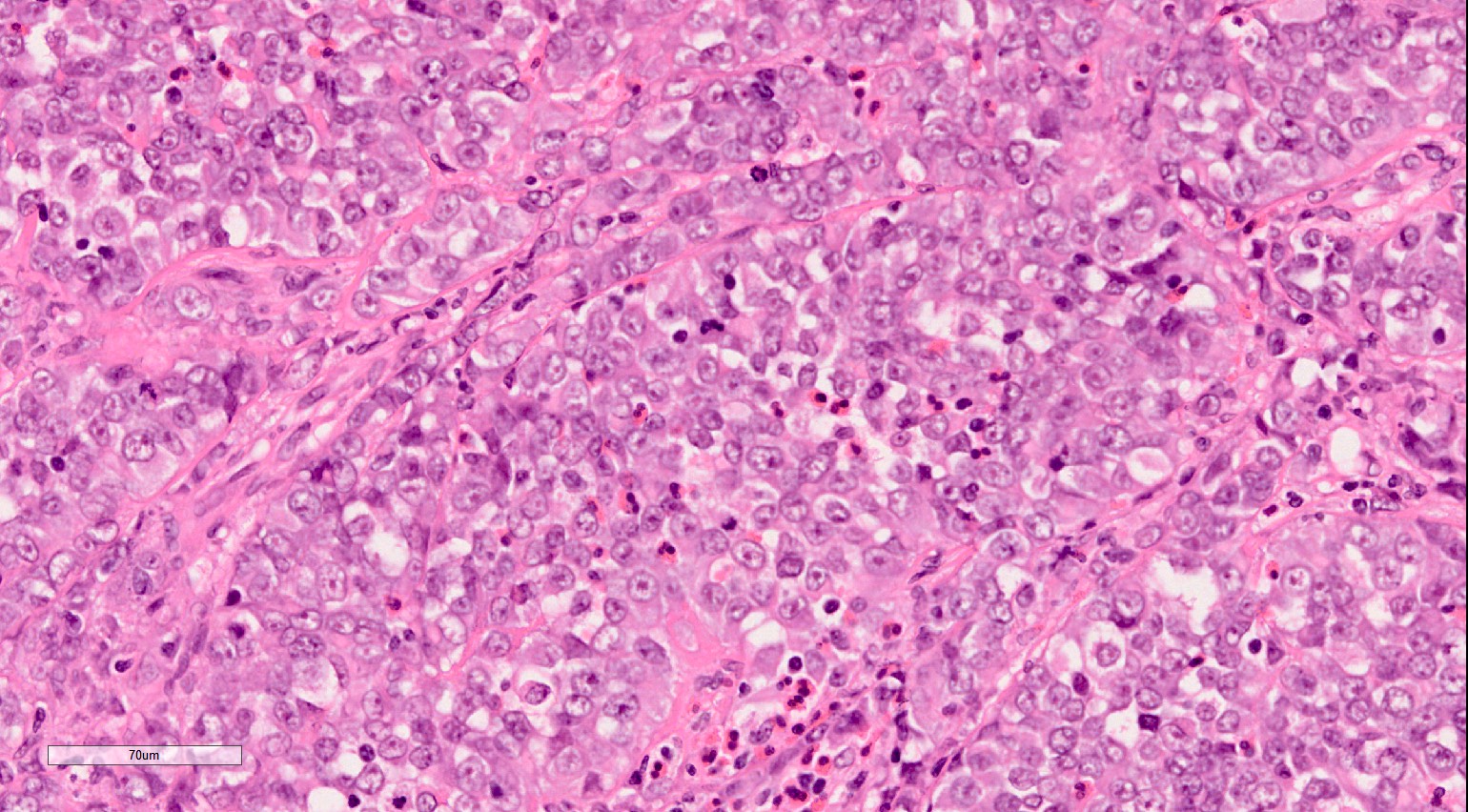
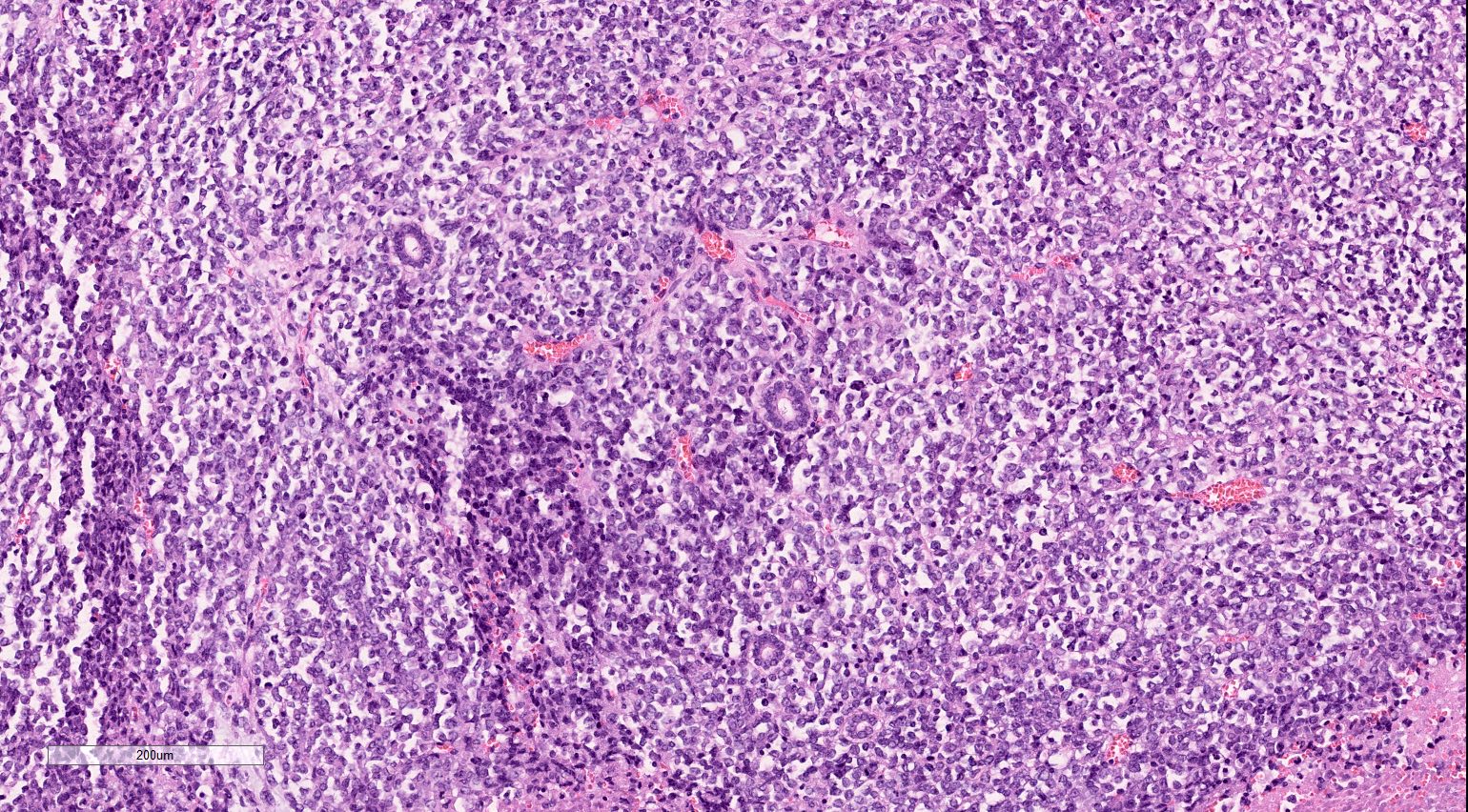
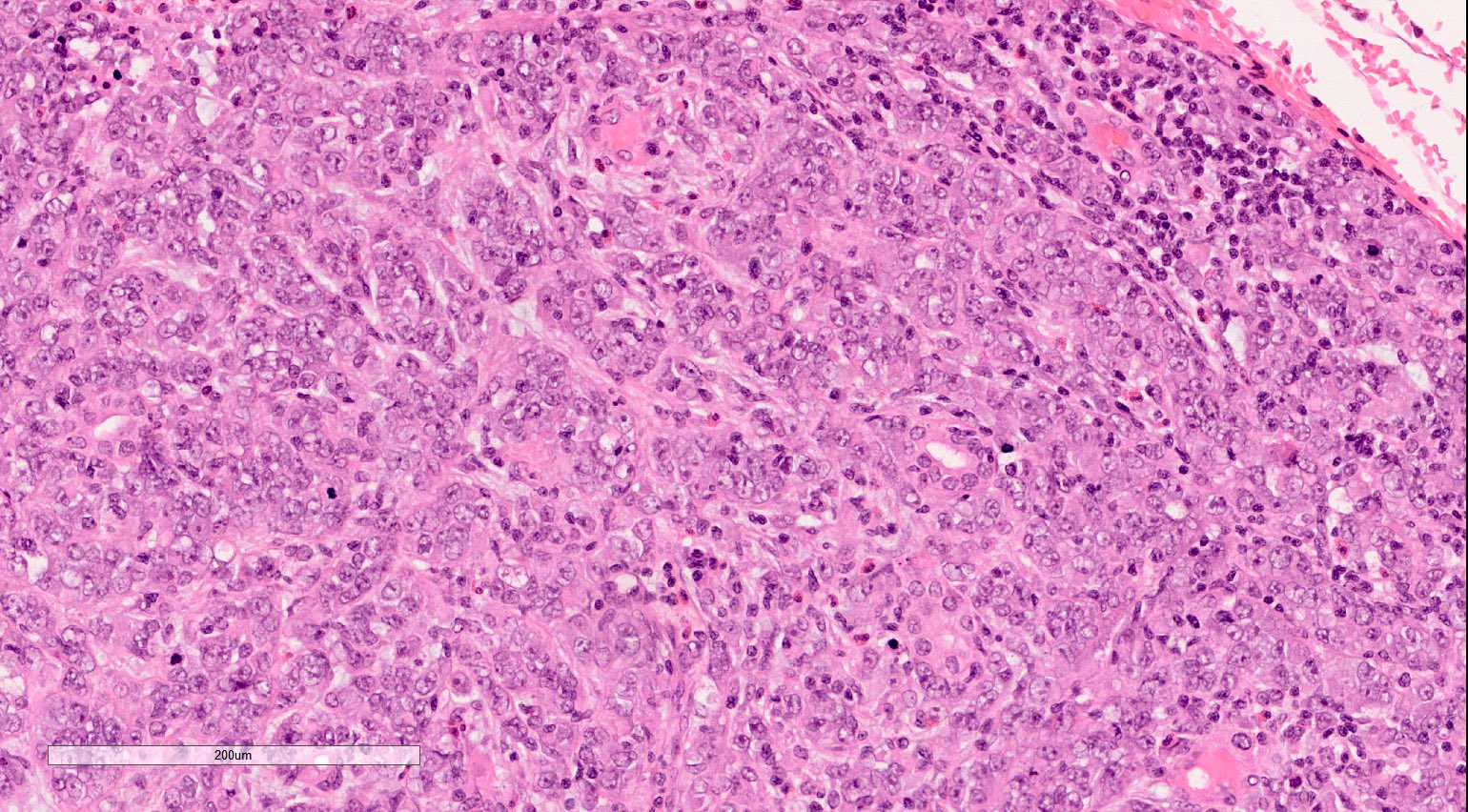
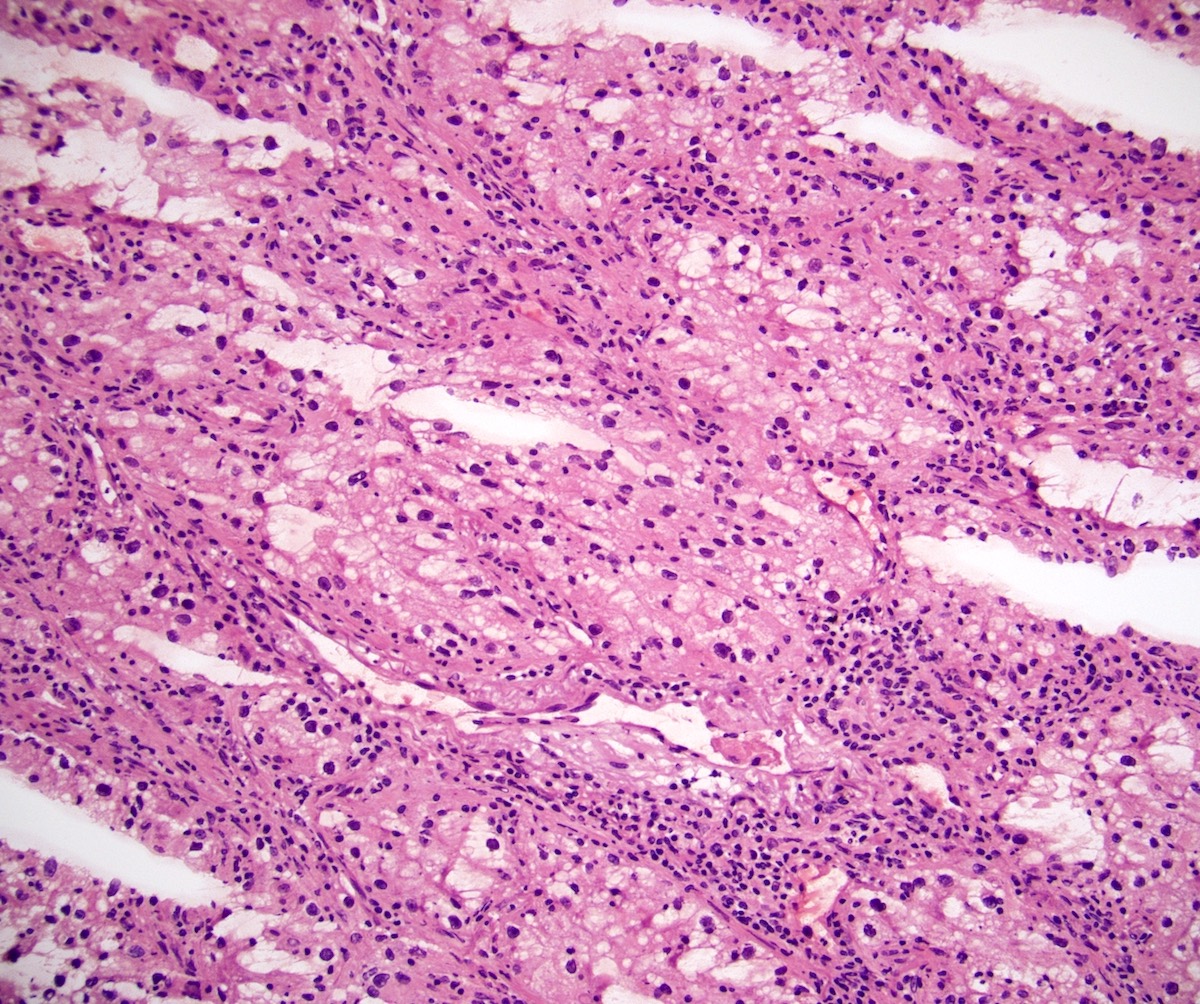
- Characteristic findings on microscopy include clear cell cytoplasm, highly dense vascularization (chicken wire) and nested growth pattern
- Sometimes only naked abnormal nuclei are present
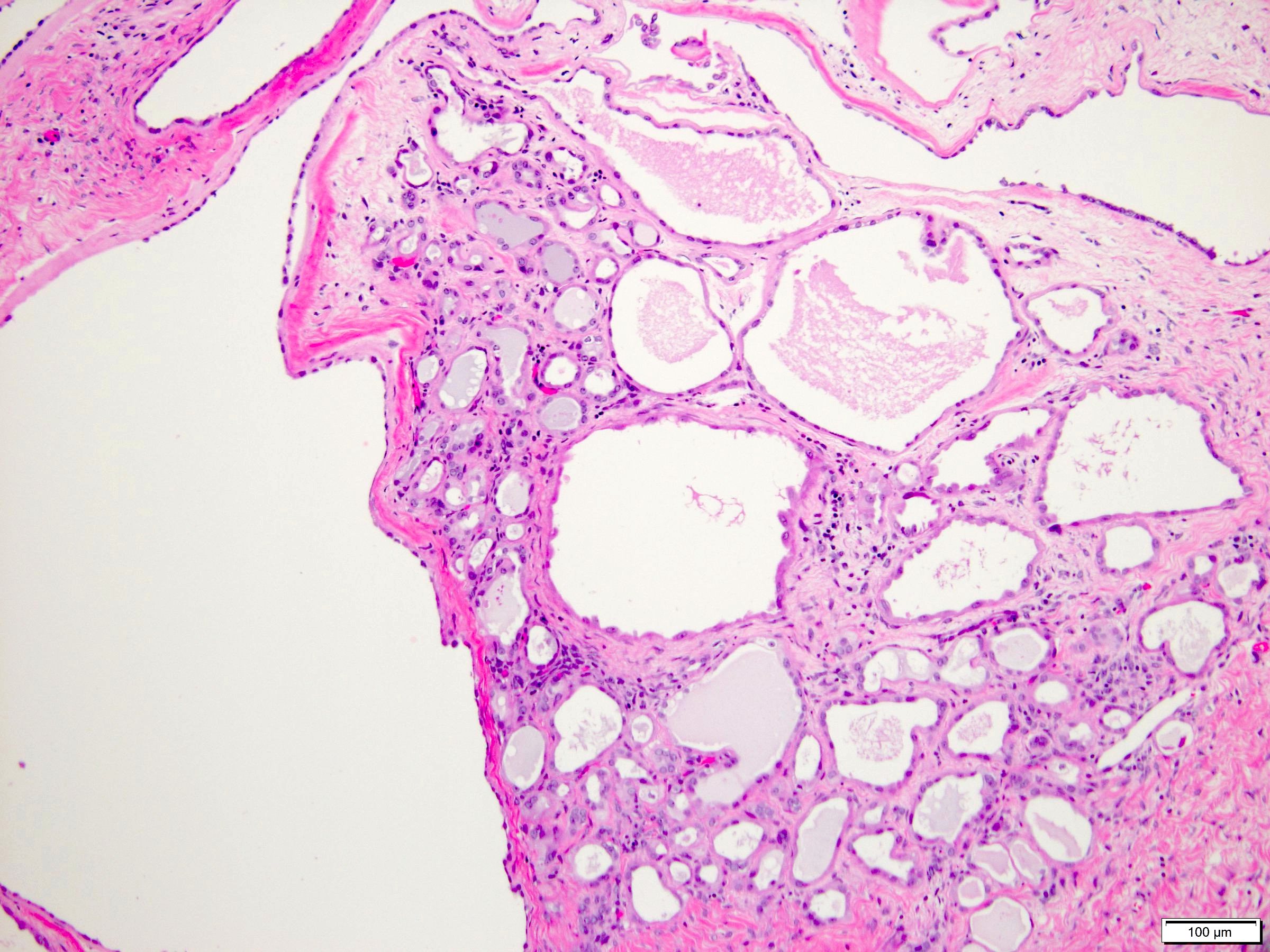
- Typically compact nests and sheets of cells with clear cytoplasm and distinct membrane
- Granular eosinophilic cytoplasm observed in high grade tumors or near areas of hemorrhage or necrosis
- Network of arborizing small, thin walled vessels (important diagnostic feature for cases with granular eosinophilic cytoplasm)
- Architectural patterns: solid, alveolar (nested), acinar (tubular), microcystic (containing extravasated red blood cells or eosinophilic fluid) and occasionally macrocystic
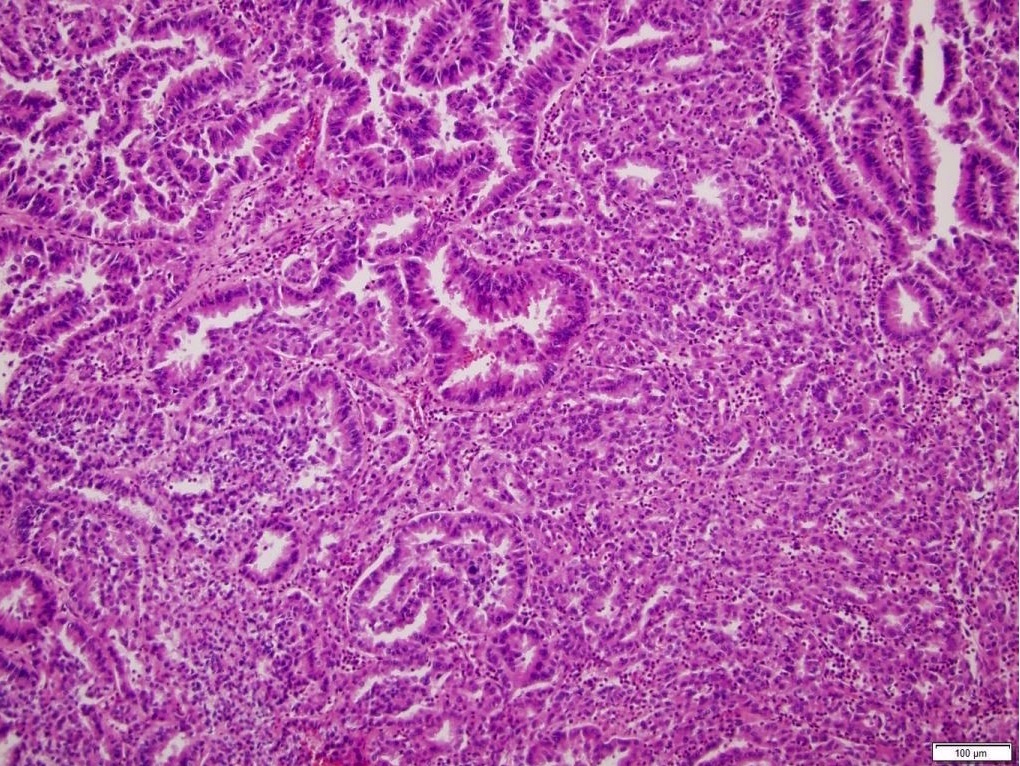
- Focal papillary architecture may be seen but prominent papillary formation raises the possibility of other subtypes (clear cell papillary renal cell tumor, TFE3 rearranged, TFEB altered, ELOC mutated RCC)
- Stroma: nondescript, no desmoplastic reaction (unlike collecting duct carcinoma or urothelial carcinoma) with little inflammatory response
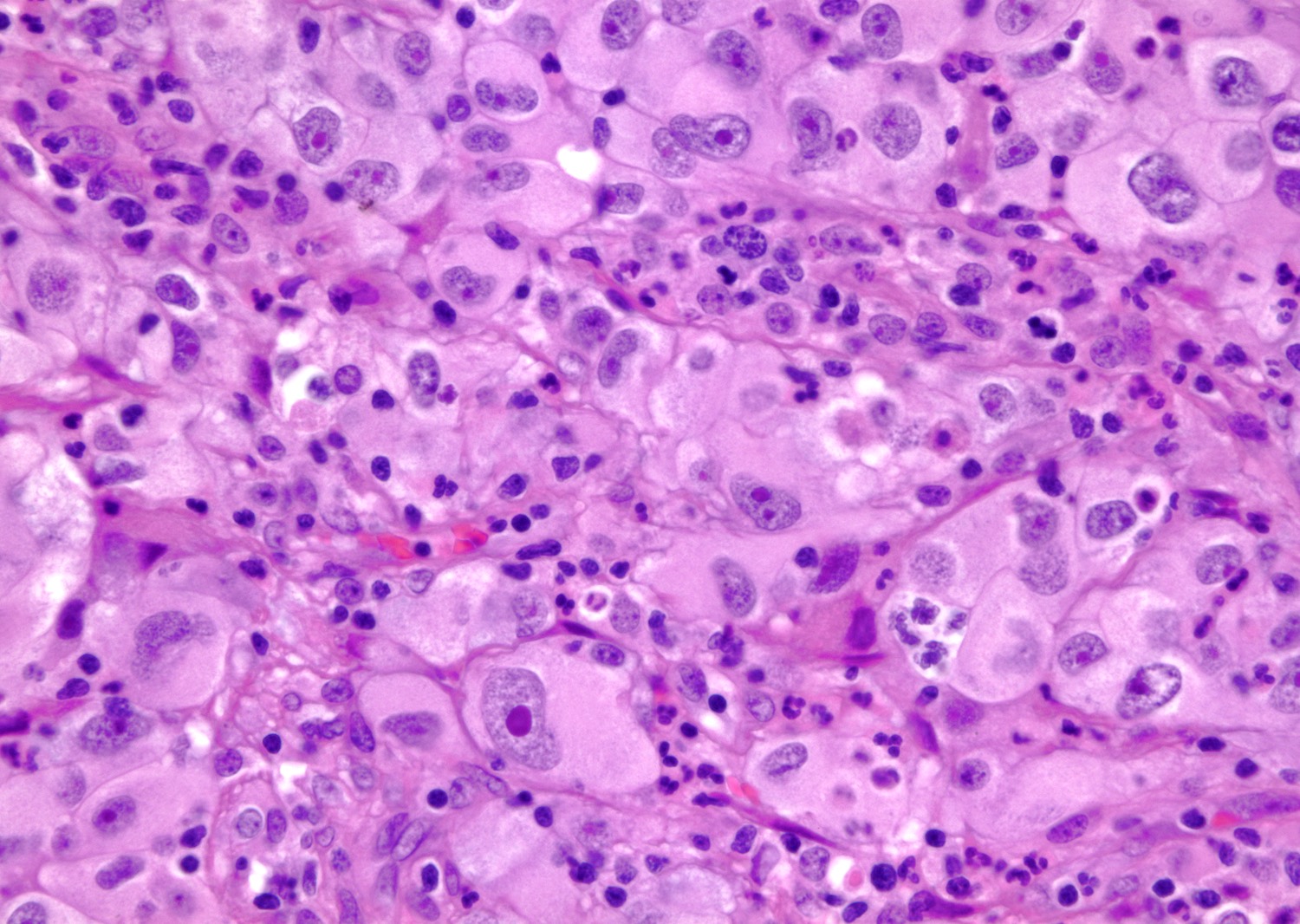
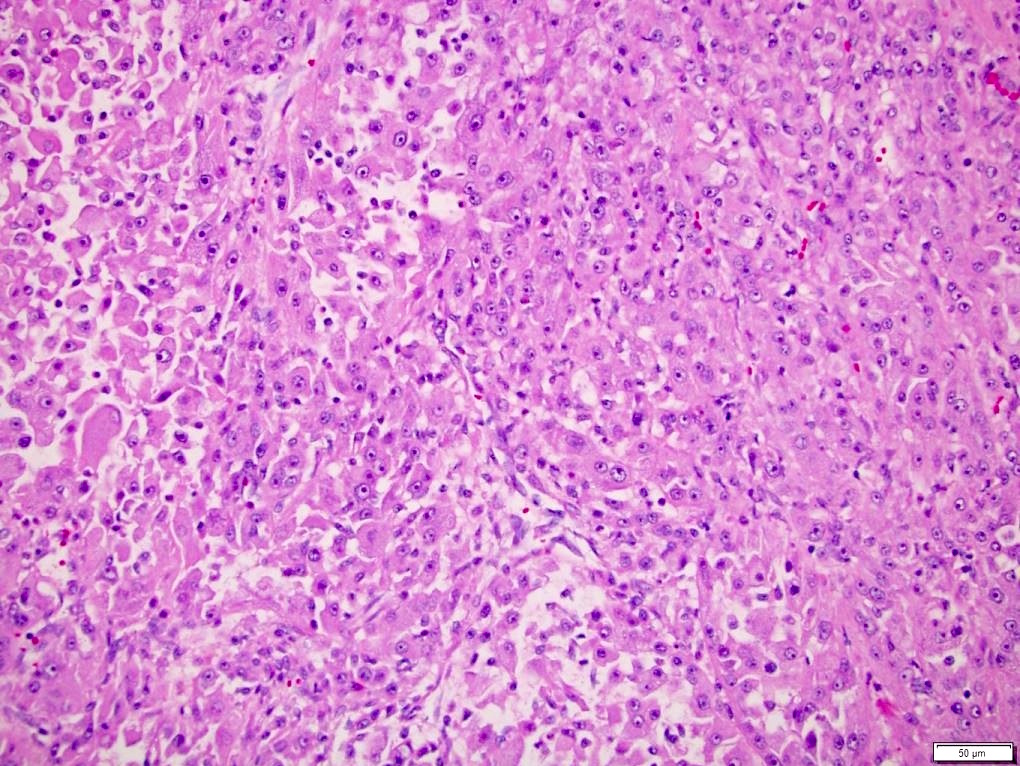
- High grade features
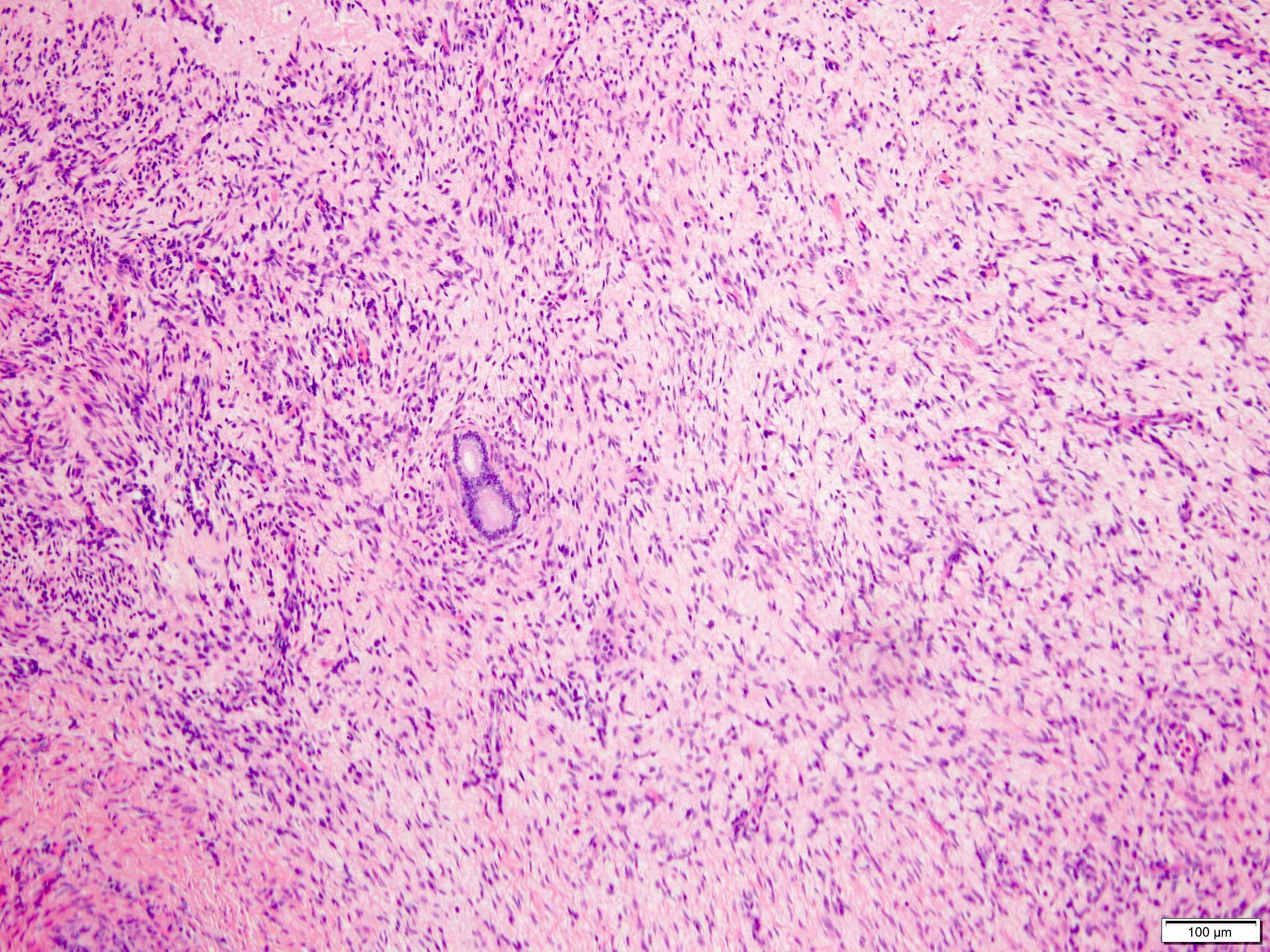
- Rhabdoid differentiation: large, high grade malignant cells with abundant homogeneous eosinophilic cytoplasm, eccentric nuclei and globular eosinophilic intracytoplasmic inclusions
- Sarcomatoid differentiation: may happen in any RCC subtype (Am J Surg Pathol 2004;28:435)
- Tumor necrosis
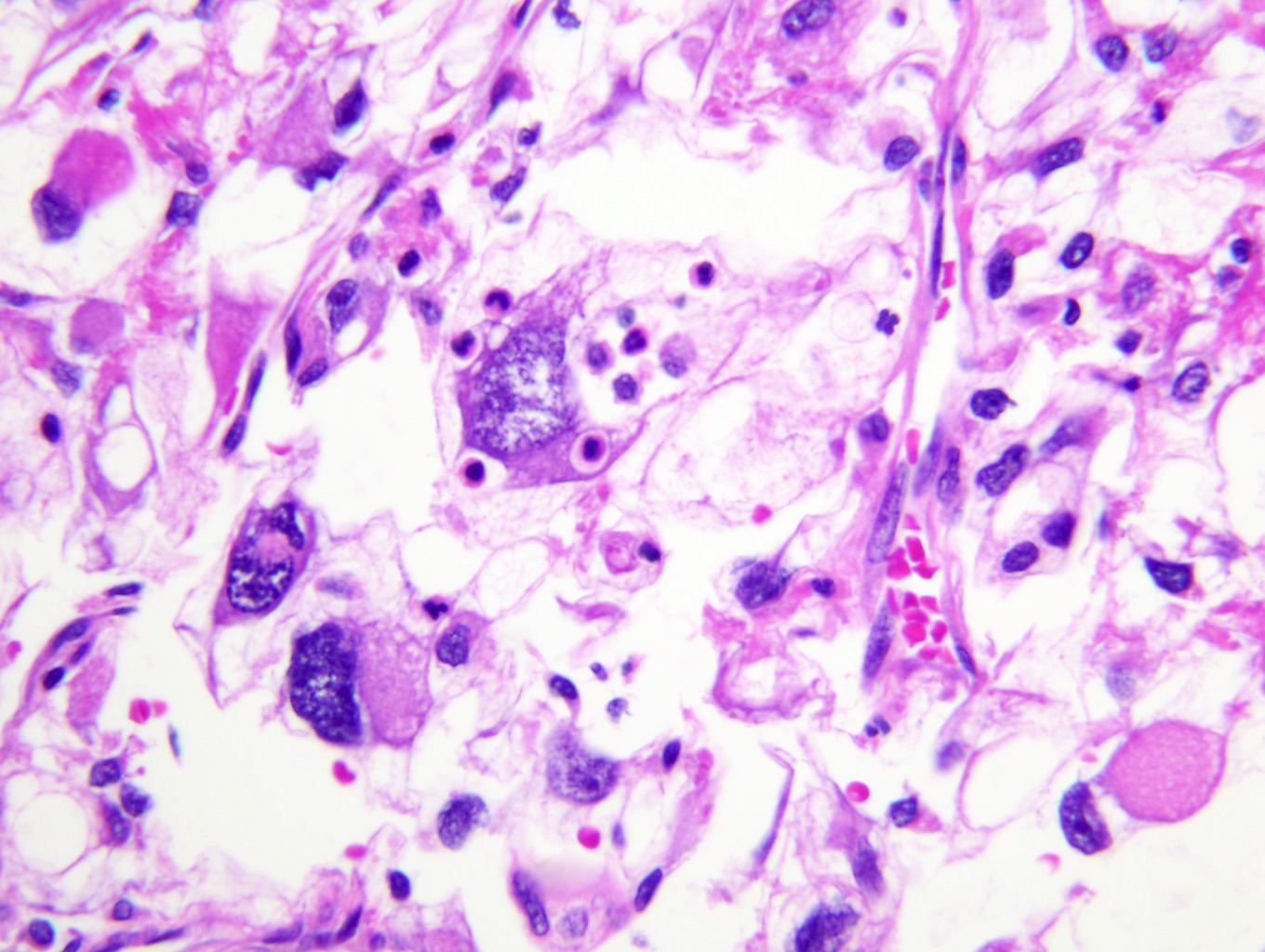
- Uncommon histologic variations (unknown prognostic significance): cystic, pseudopapillary, heterotopic bone formation, intracellular and extracellular hyaline globules, basophilic cytoplasmic inclusions, abundant multinucleated giant cells, sarcoid-like granulomas or myospherulosis features (Hum Pathol 2014;45:735, Am J Clin Pathol 2023;160:603, Histopathology 2022;80:922)
- BAP1 mutated ccRCC frequently shows papillary architecture, eosinophilic cytoplasm and cytoplasmic globules (Am J Clin Pathol 2021;155:718)
- Practically, the lower grade areas of tumor with classic ccRCC histology are most helpful for diagnosis
- Higher grade tumors can demonstrate overlapping features with other RCC types
Contributed by Gregory T. MacLennan, M.D., Behtash Nezami, M.D., Nicole K. Andeen, M.D. and Maria Tretiakova, M.D., Ph.D.
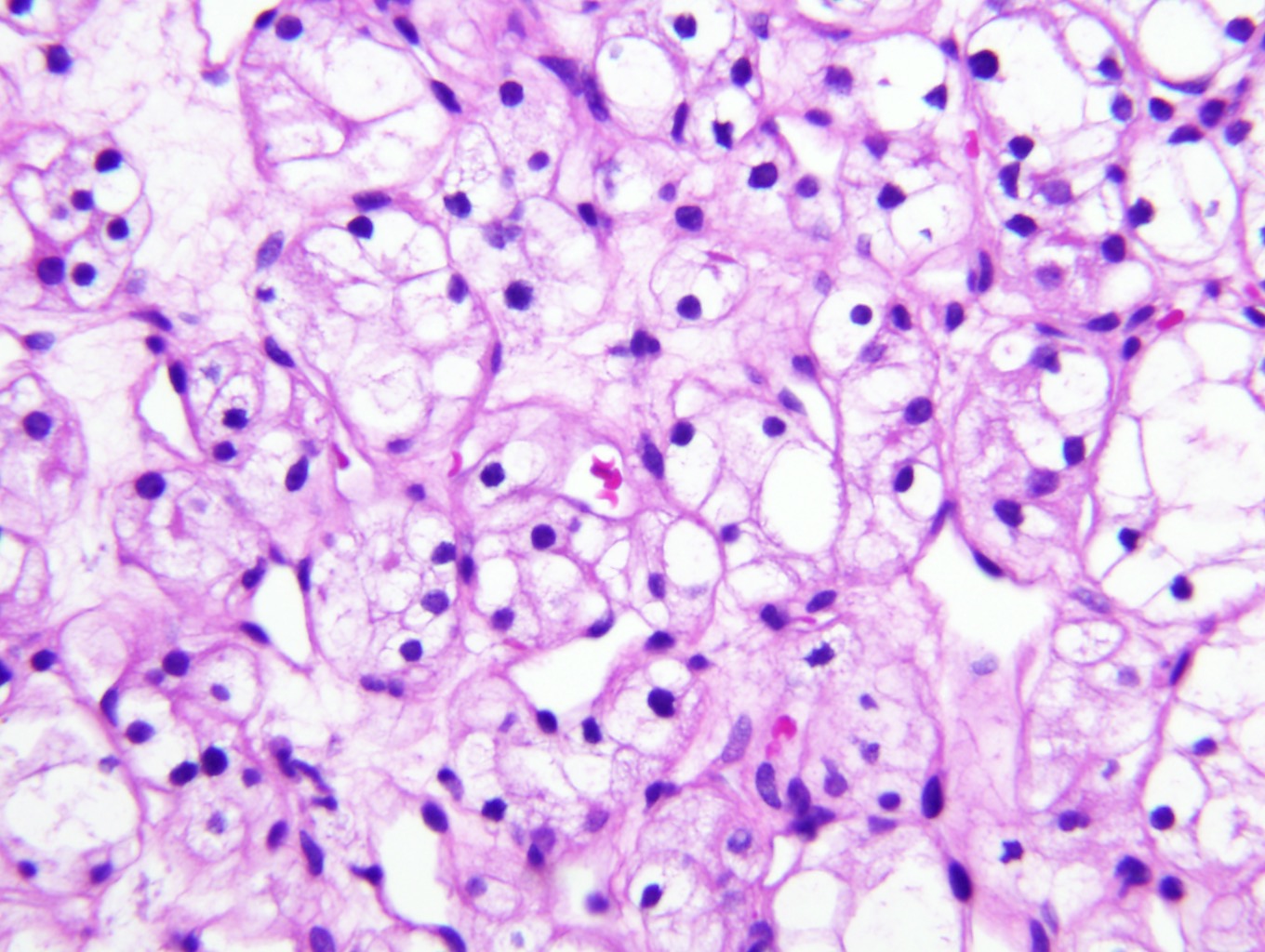
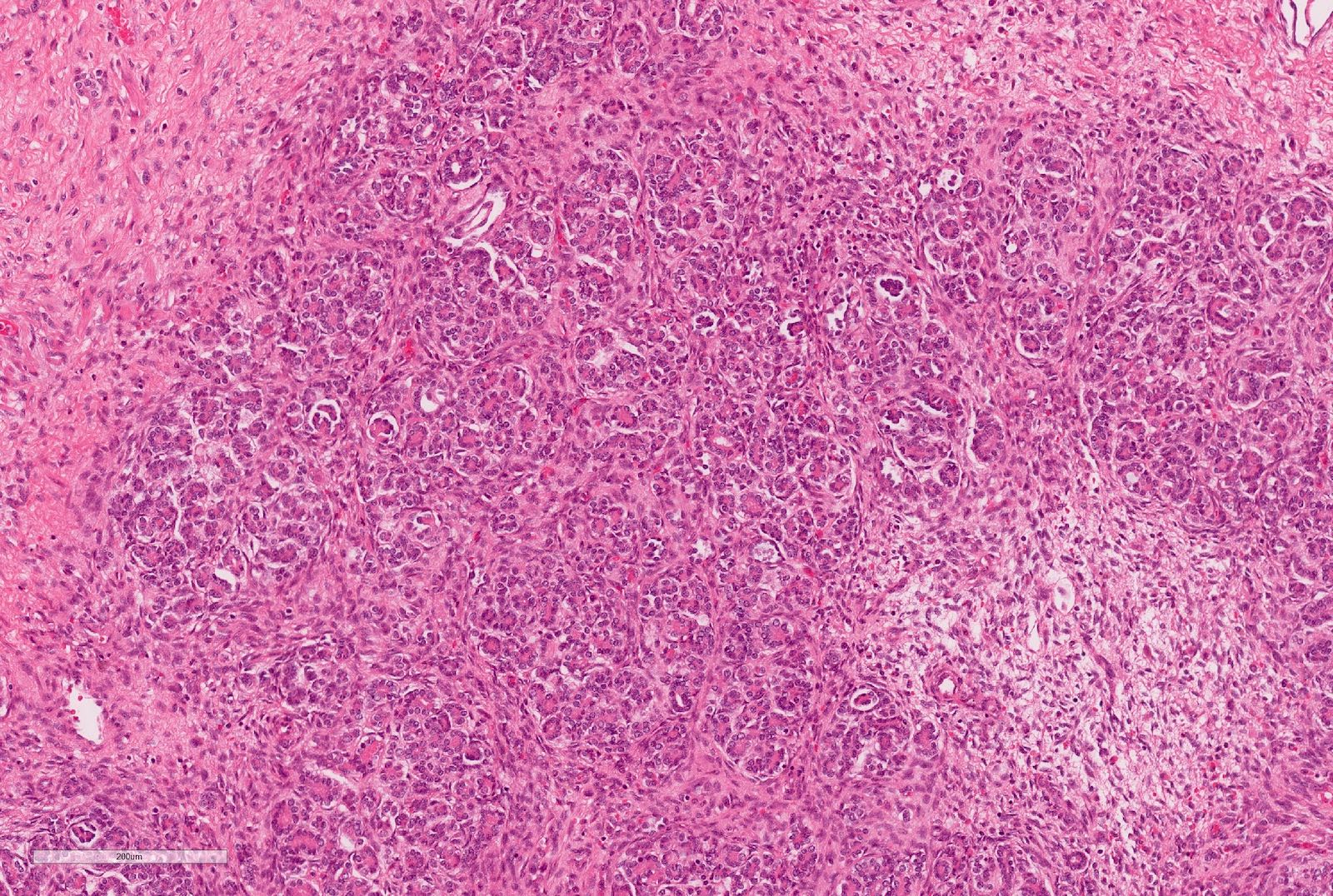
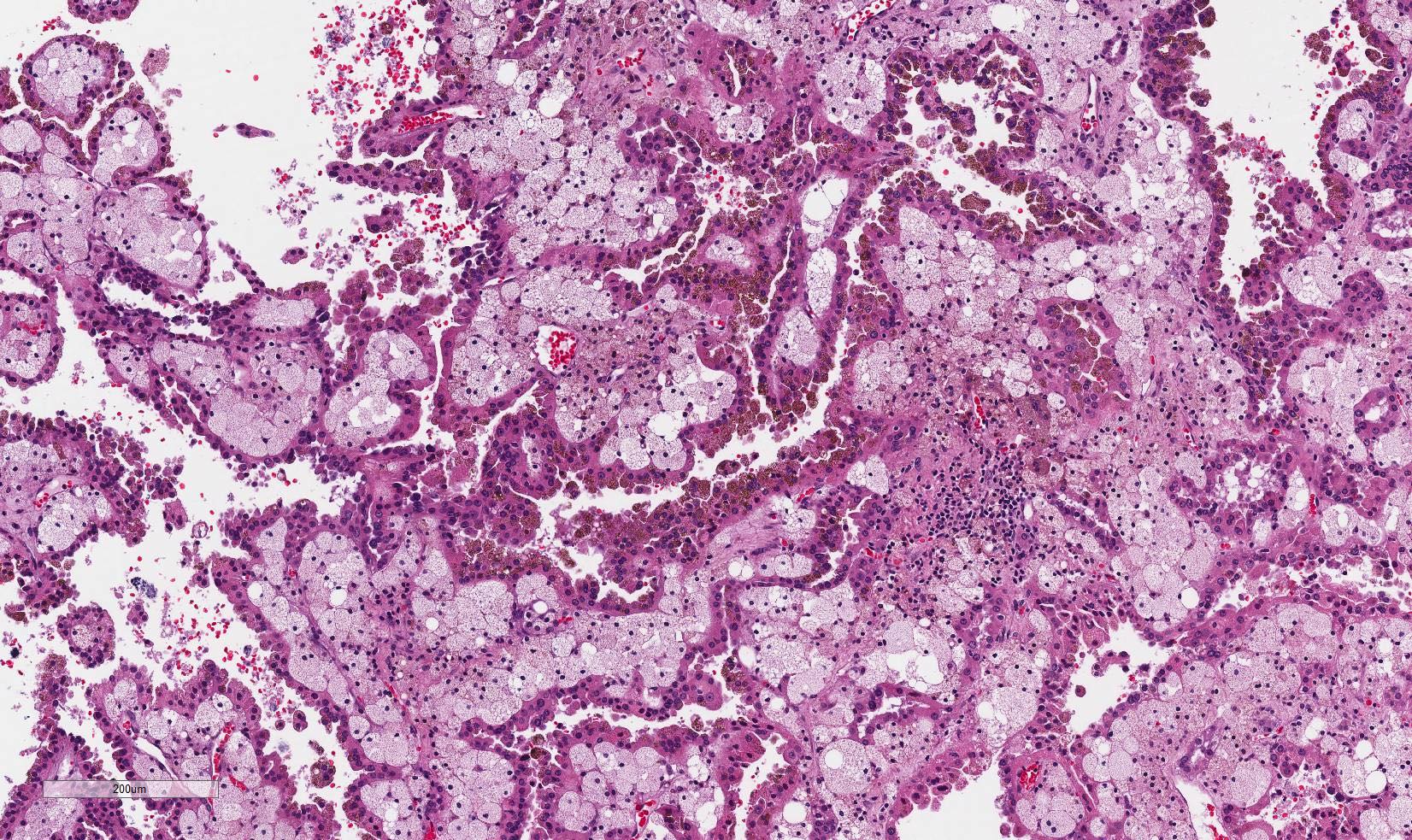
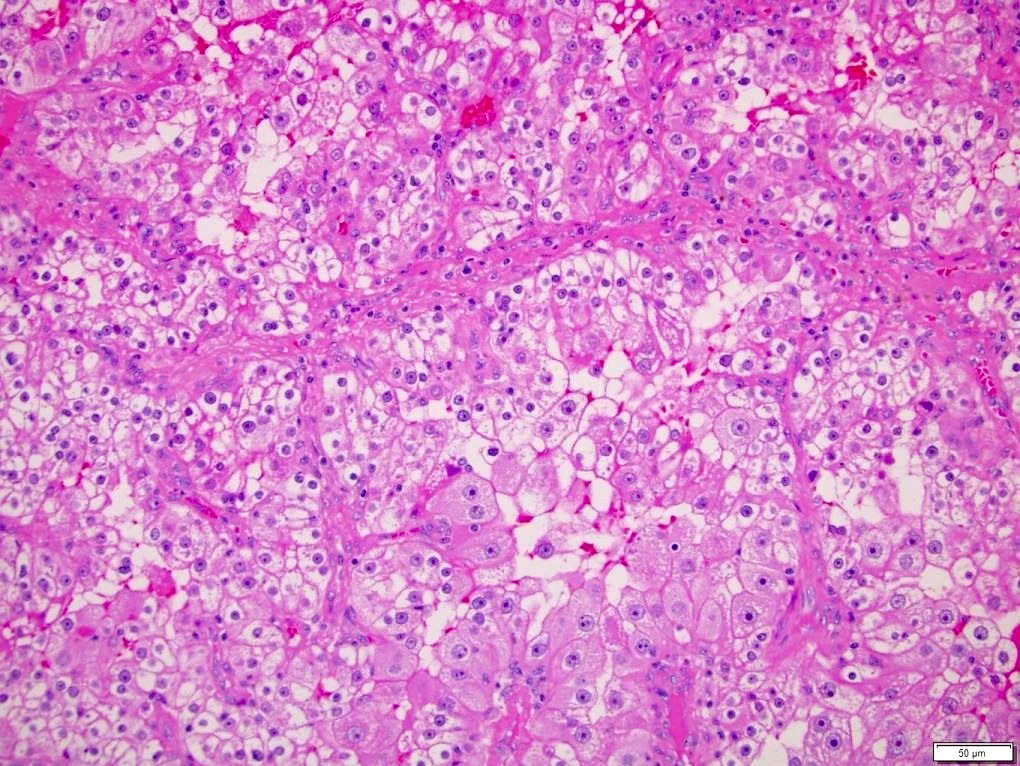
- Cohesive nests of fairly uniform cells with pale cytoplasm, mixed with stromal components and capillaries
- Numerous single cells
- Well defined cell membranes, round central or eccentric nuclei
- Prominent nucleoli in high grade
- Intranuclear vacuoles are common
- Pale, vacuolated or granular cytoplasm
- Background with blood and necrosis is frequent
- Positive and negative stains are less reliable in higher grade tumors
- PAX8: ~95%, nuclear; PAX2 similar but less sensitive (Mod Pathol 2009;22:1218, Am J Surg Pathol 2014;38:e35)
- CAIX: surrogate marker for VHL alterations, 75 - 100%, diffuse membranous (box-like), fairly specific even in tumors with sarcomatoid features (Am J Surg Pathol 2014;38:e35)
- CD10 (82 - 94%, membranous) and RCC (72 - 84%, cytoplasmic and membranous): proximal tubular brush border antigens; also present in normal cells
- Epithelial markers: AE1 / AE3, CAM 5.2, EMA / MUC1 (more sensitive than AE1 / AE3)
- Vimentin (cytoplasm and membranous)
- CK7: positive in up to 15% of cases; may be focally positive or patchy in high grade areas, cystic components or individual cells in the myxoid and degenerative tumor areas
- 34 beta E12
- CK20 (Am J Surg Pathol 2014;38:e35)
- AMACR: may be focally positive in 25 - 50% of cases (Am J Surg Pathol 2004;28:69, Cancers (Basel) 2020;12:602, Am J Surg Pathol 2015;39:1502)
- CD117
- Cathepsin K
- TFE3
- HMB45
- MelanA
- Inhibin
- BAP1 loss correlates well with BAP1 gene mutation (Nat Genet 2012;44:751)
| PAX8 | CD10 | CAIX | RCC | Melanocytic markers | Vimentin | CK7 | HMWCK | CD117 / KIT | AMACR | GATA3 | |
| Clear cell RCC | + | + | + (box-like) | + | - | + | - | - | - | -/+ | - |
| Papillary RCC | + | + | +/- | + | - | + | + | - | - | + | - |
| Clear cell papillary RCT | + | - | + (cup-like) | +/- | - | + | + | +/- | - | - | +/- |
| Chromophobe RCC | + | -/+ | - | +/- | - | - | + | - | + | - | +/- |
| Epithelioid angiomyolipoma | - | - | -/+ | - | + | -/+ | - | - | - | - | - |
| Tubulocystic carcinoma | + | + | +/- | + | - | +/- | -/+ | - | - | +/- | - |
| TFE3 rearranged / TFEB altered RCC | + | +/- | -/+ | +/- | TFEB: + TFE3: -/+ | + | - | - | - | +/- | - |
| MTSCC | + | -/+ | -/+ | +/- | - | + | + | -/+ | - | +/- | - |
| FH deficient RCC (FH loss and 2SC+) | + | - | - | - | + | - | - | - | - | -/+ | |
| TSC / mTOR / ELOC | + | + | + | - | -/+ | +/- | - | - | + | ||
Legend
| |||||||||||
- Variable cytoplasmic lipid droplets, scant organelles, microvesicles and glycogen
- Evidence of tubular differentiation: well defined long microvilli typical of the brush border seen in normal proximal tubules
- Numerous cell junctions
- Eosinophilic granular cytoplasm due to increased number of large pleomorphic mitochondria
- References: Ultrastruct Pathol 1987;11:483, Am J Surg Pathol 2000;24:1247
- VHL gene alteration in the majority of somatic cases
- 3p locus also contains other tumor suppressor chromatin remodeling genes frequently associated with ccRCC: PBRM1 (in 30 - 40% of cases), BAP1 (6 - 10%) and SETD2 (7 - 11%) (Nature 2013;499:43, Clin Cancer Res 2013;19:3259, Nat Genet 2013;45:849, Nat Genet 2013;45:860)
- Other driver alterations: mTOR pathway genes (TSC1, TSC2, MTOR, PIK3CA and PTEN), TP53, loss of 4p, 9p or 14q, mostly occurring in association with VHL alterations as subclonal drivers
- Progression of ccRCC leads to a polyploid karyotype and further gains or losses of genetic material, contributing to disease progression and aggressiveness
- Von Hippel-Lindau familial cancer syndrome
- High risk of developing renal tumors
- Patients born with a germline defect in 1 of the 2 alleles of the VHL gene
- Loss of the second functioning allele (a second hit) leads to the development of clinical disease and tumor formation
- Autosomal dominant inheritance pattern
- Tends to manifest at an early age with a lifetime risk of developing renal tumors of ~70%
- Bilateral multifocal ccRCC in 35 - 45% of patients
- As many as 1,100 cysts and 600 microscopic ccRCC foci have been reported (J Urol 2003;170:2163)
- Other familial syndromes (see Etiology)
- Kidney, core needle biopsy:
- Clear cell renal cell carcinoma (see comment)
- Comment: Tumor cells show positive immunostaining for PAX8, CAIX and keratin CAM 5.2. CK7 is negative arguing against clear cell papillary renal cell tumor.
- Kidney, radical nephrectomy:
- Clear cell renal cell carcinoma, WHO / ISUP grade 3, measuring 2.5 cm in greatest dimension (see comment)
- Tumor is confined within the renal capsule.
- All surgical margins are negative for carcinoma.
- See cancer summary report.
- Comment: Immunohistochemical stains are positive for PAX8, CAIX (box-like pattern) and CD10, supportive of this diagnosis.
- Papillary RCC with cytoplasmic clearing:
- Papillary architecture, lacks prominent delicate chicken wire vasculature
- Positive for CK7 and AMACR; negative for CAIX (opposite staining pattern to clear cell RCC) (Am J Surg Pathol 2008;32:1780)
- Clear cell papillary renal cell tumor (CCPRCT):
- Low grade tumor cells with clear cytoplasm (usually G1)
- Well circumscribed, with variable papillary, tubular, acinar and cystic architecture
- Characteristically polarized luminal nuclear arrangement (piano key) away from the basement membrane and cup-like CAIX positivity, rather than diffuse box-like membranous pattern in clear cell renal cell carcinoma
- Lacks high grade areas, tumor necrosis and local invasion
- Lack of chicken wire vasculature and presence of closed nests argue against CCPRCT
- Diffusely positive for CK7 and negative for CD10 (opposite to clear cell RCC)
- Most tumors present as pT1 stage with excellent clinical outcome
- GATA3 positive CCPRCT (Hum Pathol 2017;66:152)
- AMACR (opposite to PRCC) is usually negative
- Fumarate hydratase deficient renal cell carcinoma:
- High grade and aggressive tumor, seen in younger age than ccRCC
- Characteristic inclusion-like nucleoli with perinucleolar clearing
- Positive 2SC IHC (both nuclear and cytoplasmic) and loss of FH expression
- Associated with leiomyomas of skin and uterus in germline cases (HLRCC syndrome)
- Negative for CAIX
- Multilocular cystic renal neoplasm of low malignant potential
(MCRNLMP):
- Clear cells lining the thin fibrous septa of multiple cystic spaces or within the fibrous septa
- Uncommon, usually lower grade associated with an excellent prognosis
- Absence of expansile solid nodules
- Similar IHC staining pattern to clear cell RCC but more commonly CK7 positive and less often CD10 positive (Am J Surg Pathol 2012;36:1425)
- Epithelioid angiomyolipoma (AML):
- Characteristic abnormal AML vessels, smooth muscle and blood pools
- Lacks characteristic nests seen in ccRCC
- May resemble high grade ccRCC
- Look for areas of typical AML with fat and dysmorphic vessels
- Positive for HMB45, cathepsin K
- Negative for PAX8, CAIX, EMA and cytokeratins (Am J Surg Pathol 2001;25:65)
- TFE3 rearranged and TFEB altered renal cell carcinomas:
- TFE3 rearranged (chr Xp11) or TFEB altered (chr 6p21, rearranged or amplified)
- TFE3 and TFEB rearranged RCCs seen in a younger age range (median: 31 years)
- TFEB amplified RCCs occur in older patients (median: 64 years) (Am J Surg Pathol 2002;26:1553, Clin Cancer Res 2009;15:1170, Am J Surg Pathol 2014;38:604, Am J Surg Pathol 2016;40:1484)
- High grade nuclei, mixed clear and eosinophilic (not clear) cytoplasm
- Different architectural patterns: tubulopapillary or trabecular but lacks the classic closed nests of ccRCC
- Positive for TFE3 or TFEB, cathepsin K, CD10, AMACR and variable focal CAIX, EMA and cytokeratins (opposite to clear cell RCC)
- Clear cell RCCs with prominent fibromuscular septation and CK7 positivity, including ELOC mutated renal cell carcinoma:
- Generally exhibit indolent behavior
- Cytologically resemble ccRCC with clear cytology (mostly lower grade tumor, except for ELOC mutated RCC)
- Architecturally prominent fibromuscular stroma with thick fibromuscular bands
- Mutations in mTOR pathway or TSC1 / TSC2 genes; may be germline
- VHL alterations are not observed
- ELOC mutated RCC is mutually exclusive with VHL, PBRM1, TSC1, TSC2 or mTOR
- Positive for CK7 (strong), CD10 and CAIX complete membranous (box-like)
- Chromophobe RCC:
- Circumscribed mass with a homogeneous light tan cut surface
- Large cells with sharply defined hard cell border, finely reticulated cytoplasm, irregular nuclear border (raisinoid) and perinuclear halo
- Lacks chicken wire vasculature
- Positive for CK7, E-cadherin, CD117; negative for CAIX and vimentin (opposite staining pattern to clear cell RCC) (Am J Surg Pathol 2014;38:e35)
- Oncocytoma:
- Adrenal cortical carcinoma:
- Clear cell carcinoma of the ovary:
- Positive for keratin 34 beta E12 and CK7
- PAX8 negative at extrarenal sites but can be positive in kidney (Appl Immunohistochem Mol Morphol 2019;27:477)
- Clear cell carcinoma of the thyroid:
- Positive for thyroglobulin and TTF1
- PAX8 is positive in both
- Mesothelioma:
- Positive for calretinin, mesothelin and CK5/6
- Hemangioblastoma:
- Lack nuclear atypia, prominent nucleoli and necrosis
- PAX8 negative and inhibin positive (opposite to clear cell RCC)
- PAX8 negative at extrarenal sites but can be positive in kidney (Appl Immunohistochem Mol Morphol 2019;27:477)
Comment Here
Reference: Clear cell renal cell carcinoma
- Associated with trisomies of 7 and 17
- Has a worse prognosis than papillary renal cell carcinoma
- Minimum of 10% of sarcomatoid tumor is required to make a diagnosis of sarcomatoid carcinoma
- PAX8 staining is specific for renal cell carcinomas
- Tends to be CK7 and CAIX positive
Comment Here
Reference: Clear cell renal cell carcinoma
- Indolent tumor with clear glycogen rich cytoplasm, luminal nuclear polarization, inconspicuous nucleoli and with papillary architecture with fibrovascular stroma
- Distinctive immunohistochemical profile: positive CK7 and CAIX, with negative or patchy CD10 and AMACR
- Renamed in WHO 5th edition to reflect indolent behavior (previously was designated as a renal cell carcinoma)
- Clear cytoplasm, luminal nuclear polarization, low nuclear grade (histologic grade 1 - 2)
- Papillary architecture with fibrovascular stroma
- CK7 / CAIX positive; CD10 / AMACR patchy or negative
- Lacks features of high grade malignancies, including necrosis and vascular invasion
- No reported metastasis or recurrence following adequate resection
- Not recommended / no longer used: clear cell papillary tumor, clear cell papillary renal cell carcinoma, clear cell tubulopapillary renal cell carcinoma, renal angiomyoadenomatous tumor (Am J Surg Pathol 2010;34:1608, Ann Diagn Pathol 2000;4:311)
- ICD-O: 8323/1 - clear cell papillary renal cell carcinoma
- 3 - 4% of all renal tumors, mean age of 58.2 years (Mod Pathol 2013;26:697, Hum Pathol 2014;45:59)
- Age: 8 - 88 years
- No gender predilection
- Arises sporadically and with end stage renal disease (ESRD) (Int J Clin Exp Pathol 2014;7:7312)
- Renal cortex; predominately unifocal, with multifocality in 25% of cases (multifocality is predominantly seen in ESRD patients)
- Unknown at this time
- Etiology is unclear; lacks genetic modifications and driver mutations frequently associated with other renal cell carcinomas (RCC)
- First described in association with ESRD, particularly acquired cystic disease of the kidney (ACDK) (Am J Surg Pathol 2006;30:141)
- It is now known that most cases arise sporadically, with no evidence of somatic VHL gene inactivation or chromosome 3p loss, unlike clear cell renal cell carcinoma (CCRCC) (Arch Pathol Lab Med 2019;143:1154)
- Believed to be associated with upregulation of the hypoxia inducible factor 1 pathway, similar to other RCCs but through a non-VHL dependent mechanism
- Evidence suggests a shift away from respiratory metabolism, toward sorbitol pathway of tumorigenesis (Elife 2019;8:e38986)
- Usually discovered incidentally during imaging for unrelated disorders (80%) and occasionally with local symptoms (18%) (Eur Urol 2021;79:468)
- Some cases arise in patients with ESRD
- Indolent behavior with presentation at low tumor stage (usually pT1) (Am J Surg Pathol 2013;37:1469)
- No reported metastasis or recurrence in cases with typical pathologic features (Am J Surg Pathol 2013;37:1469)
- May present with multifocal or bilateral tumor distribution (Int J Mol Sci 2021;23:151)
- Usually initially identified by radiographic imaging (CT or ultrasound)
- Diagnosis confirmed after surgical resection and microscopic examination
- Generally 2 categories of findings: solid and cystic, with Bosniak III or IV features (Urology 2017;103:136, AJR Am J Roentgenol 2020;214:579)
- Solid: mass with relatively low level enhancement, with well defined margins
- Cystic: heterogeneous regions of hyper enhancement and predominantly unilocular
- These findings are shared with both clear cell and papillary renal cell carcinoma enhancement patterns
- Unknown at this time
- 50 year old man with glomerulonephritis on hemodialysis and 22 mm unifocal renal tumor (IJU Case Rep 2021;4:127)
- 55 year old man with bilateral tumors and concurrent unifocal neuroendocrine tumor in the setting of chronic kidney disease (Case Rep Pathol 2017;2017:9672368)
- 57 year old man with multiple renal tumors (Pathol Res Pract 2016;212:229)
- 58 year old man with concurrent ipsilateral tumor and mucinous tubular and spindle cell carcinoma (Nihon Hinyokika Gakkai Zasshi 2020;111:134)
- 63 year old woman post kidney transplant with multifocal tumor in renal allograft (Can J Urol 2019;26:9794)
- 70 year old man with incidentally found 2 cm renal mass and a history of treated fibrous histiocytoma and liposarcoma (World J Nephrol 2018;7:155)
- Radical nephrectomy or nephron sparing surgery, including partial nephrectomy or cryoablation therapy
- Mean size: 2.0 cm (0.2 - 7.5 cm)
- Solid or cystic, occasionally with flattened peripheral cysts (Int J Mol Sci 2021;23:151)
- Relatively small and well encapsulated within a fibrous capsule
- Solid tumor component cut surface appears tan-white, pink-tan, yellow or red-brown in color
- Necrosis is not observed; focal areas of hemorrhage may occasionally be present
- Various proportions of 2 broad patterns:
- Focally branched papillary architecture with hypocellular fibrovascular cores lined by cuboidal to flat cells with clear cytoplasm projecting into cystic spaces; extensively branched papillae are uncommon
- Areas of dense, compact arrangement of tubular structures lined by clear cells with characteristic luminal polarization of nuclei
- Nuclear and nucleolar size, almost always histologic grade 1 or 2
- Extrarenal extension including vascular invasion is not seen
- Stroma may be fibromyomatous but unusual or conspicuous amounts of fibromyomatous stroma should raise concern for a different entity, a distinct renal epithelial neoplasm harboring mutations in TSC1, TSC2, MTOR and ELOC / TCEB1 (see Differential diagnosis)
- Eosinophilic secretions may be seen
- Reference: Epstein: Differential Diagnoses in Surgical Pathology - Genitourinary System, 2nd Edition, 2022
- Tumor cells arranged in small nests, clusters, arrays or as single cells (Cancer Cytopathol 2017;125:48)
- Columnar tumor cells with eccentric, small, round to oval nuclei with evenly distributed, finely granular chromatin and regular nuclear membrane contour
- Clear cytoplasm containing small vacuoles, with ill defined cytoplasmic borders
- Chief value may be to provide a differential diagnosis that includes clear cell papillary renal cell tumor (Cancer Cytopathol 2021;129:190)
- CAIX: cytoplasmic, cup shaped distribution, with absent staining of luminal aspect
- CK7: diffuse membranous / cytoplasmic staining
- GLUT1: diffuse membranous staining
- GATA3: reported 42 - 76% sensitive, also may be positive in chromophobe (Hum Pathol 2017;66:152, Appl Immunohistochem Mol Morphol 2018;26:316)
- PAX2 / PAX8: nuclear staining
- Epithelial markers, including AE1 / AE3, CAM5.2, EMA, E-cadherin, CK14, CK19, cytokeratin 34 beta E12 (~92% of cases, diffuse and strong cytoplasmic staining) (Pathol Oncol Res 2018;24:447, Pathology 2017;49:10)
- Mesenchymal markers, including vimentin, actin (stromal component, focally positive ~94%), desmin (sporadic) (Mod Pathol 2013;26:697)
- Beta catenin: cytoplasmic
- Parafibromin (Appl Immunohistochem Mol Morphol 2013;21:322)
- CD70 (Histopathology 2014;64:1032)
- CD133 (Histopathology 2014;64:1032)
- p27 (Pathol Oncol Res 2018;24:447)
- Cyclin D1 (Histopathology 2014;64:1032)
- p53, HIF1, c-MET (Pathol Oncol Res 2018;24:447)
- AMACR / racemase
- CD10: typically negative in solid tumor, may be focally positive in cyst lining areas
- Cathepsin K, RCC Ma (Arch Pathol Lab Med 2012;136:391, Arch Pathol Lab Med 2019;143:1154)
- TFE3, TFEB (Arch Pathol Lab Med 2019;143:1154)
- Not well understood
- Various chromosomal aberrations identified: monosomy of chromosomes 16, 17, 20; trisomy of chromosomes 10, 12 (Int J Mol Sci 2021;23:151)
- Lower frequency of chromosome 7 and 17 trisomy than PRCC (Int J Mol Sci 2021;23:151)
- Small proportion of patients with von Hippel-Lindau (VHL) disease develop tumor with morphology and immunohistochemical expression consistent with CCPRCT; however, such tumors appear to be genomically related to CCRCC and distinct from CCPRCT (Hum Pathol 2014;45:1966, Am J Surg Pathol 2013;37:1131)
- Absence of VHL gene modifications are observed in cases with immunohistochemical coexpression of CAIX, HIF1 and GLUT1, suggesting VHL mutation independent mechanism of HIF pathway activation
- Hypothesized sorbitol accumulation as a method of HIF1 pathway activation (Int J Clin Exp Pathol 2014;7:7312)
- Mitochondrial DNA:
- Significantly lower mtDNA content compared to other RCC subtypes (Eur Urol 2021;79:468)
- Noncoding RNA:
- Overexpression of miR-200a, miR-200b, miR-200c, miR-141 and miR-429, suggesting enhanced E-cadherin mediated suppression of tumor progression (J Pathol 2014;232:32)
- Upregulation of miR-210 is associated with VHL independent manner of HIF1 pathway activation (J Vet Sci 2013;14:69)
- Right kidney, partial nephrectomy:
- Clear cell papillary renal cell tumor, 2.5 cm
- Surgical margins, negative for tumor
- Comment: Immunostaining demonstrate diffuse positivity for CAIX and CK7 and negative staining for CD10 and AMACR.
- Clear cell renal cell carcinoma:
- Papillary renal cell carcinoma:
- Xp11 translocation carcinoma:
- Younger patient demographic
- Abundant psammoma bodies
- TFE3 translocations demonstrable by FISH
- Weakly or focally positive expression of cytokeratins and epithelial membrane antigen
- Majority are cathepsin K positive
- Renal angiomyoadenomatous tumor (RAT):
- Shares similarities in the absence of VHL gene mutations / modifications and chromosome 3p loss
- Biphasic tumor with collapsed acinar architecture and angioleiomyoma-like fibrous / smooth muscle stroma
- Tumors with the appearance of clear cell papillary renal cell tumor but with conspicuous fibromyomatous stroma, may represent a distinct renal epithelial neoplasm harboring mutations in TSC1, TSC2, MTOR and ELOC / TCEB1 (Am J Surg Pathol 2015;39:889, Am J Surg Pathol 2019;43:1135, Am J Surg Pathol 2020;44:571)
- CD10+, CAIX+, CK7+, AMACR-
- CD10-, CAIX-, CK7+, AMACR-
- CD10-, CAIX+, CK7+, AMACR-
- CD10+, CAIX+, CK7-, AMACR+
- CD10+, CAIX+, CK7- AMACR-
- Clear cell sarcoma of the kidney (CCSK) is composed of uniform, small round cells with a clear appearance and evenly distributed fine chromatin separated by a delicate vascular network (eMedicine)
- First described in 1978 by three distinct groups: Beckwith and Palmer (Cancer 1978;41:1937), Morgan and Kidd (Cancer 1978;42:1916), and Marsden and coworkers (Cancer 1978;42:1922); it was initially named "bone metastasizing renal tumor of childhood" since it has predilection for skeletal metastasis (J Neonatal Surg 2014;3:35)
- Most frequently misdiagnosed renal tumor in children, as it is unusual, has varied morphology and there are no specific diagnostic markers (J Neonatal Surg 2014;3:35)
- Typically a male child, half are diagnosed at 2 - 3 years of age
- Many histological variants exist (see below), but the classic histological appearance is nests or cords of small, plump cells separated by networks of delicate vascular septa
- Clear cells occur in only 20% of cases, despite its name
- Immunohistochemistry has limited use: it is usually only positive for vimentin, although Cyclin D1 may be useful (as of 2015, see below)
- The most important drug treatment is Doxorubicin (Adriamycin®)
- Recent molecular advances in CCSK tumorigenesis have implicated the BCOR gene (see below)
- ICD-10: C64.9 - malignant neoplasm of unspecified kidney, except renal pelvis
- Rare (20 cases per year); 3% - 5% of all pediatric renal tumors
- Mean age of diagnosis is 36 months (range, 2 months - 14 years), half are diagnosed at 2 - 3 years of age (Am J Surg Pathol 2000;24:4)
- Rarely diagnosed in adolescents / adults (Am J Surg Pathol 1999;23:1455)
- 2 / 3 male
- Bone metastases occur in 17% - 40% of patients with CCSK, vs. J Neonatal Surg 2014;3:35)
- 5% of patients have metastatic disease at presentation including bone, lung, brain and liver
- Overall, survival varies with stage (5 year survival is 97% for stage I vs. 50% for stage IV, Am J Surg Pathol 2000;24:4)
- Four important prognostic factors are:
- Doxorubicin treatment
- Stage
- Age at diagnosis
- Presence of tumor necrosis (J Postgrad Med 2001;47:206)
- Stage IV disease and young age are significant adverse prognostic factors for event free survival (Eur J Cancer 2013;49:3497)
- Congenital tumor with metastases (Virchows Arch 2005;446:566)
- Total nephrectomy followed by postoperative chemotherapy and flank radiation therapy (RT) (Eur J Cancer 2013;49:3497)
- Highly responsive to combination treatment with doxorubicin, vincristine and actinomycin, with doxorubicin the most important (eMedicine)
- The essential role of doxorubicin in therapy emphasizes the need for pathologists to accurately identify CCSK (Am J Surg Pathol 2000;24:4)
- Unilateral and unicentric mass with irregular but sharp tumor / kidney interface
- Large (mean 11 cm); centered in central kidney or medulla
- Cut surface is homogenous tan / gray or gelatinous and firm with occasional cysts
- Classic pattern: nests or cords of small, plump cells separated by network of delicate vascular septa (also called chicken wire capillaries)
- This pattern is almost always focally present, but other patterns may be present
- The 8 variant patterns are myxoid (50%), sclerosing (35%), cellular (26%), epithelioid (trabecular or acinar type) (13%), palisading verocay body (11%), spindle cell (7%), storiform (4%) and anaplastic (2.6%) (Am J Surg Pathol 2000;24:4)
- Anaplasia is defined as nuclear hyperchromasia, atypical mitoses and giant nuclei (Am J Surg Pathol 2000;24:4)
- Cells may take on a spindle appearance, particularly near the vascular septae, these cells are termed "septal cells" (Zhou: Uropathology, 1st Edition, 2012)
- Although the border appears sharp at low power, high power reveals subtle infiltration of the cells into the normal parenchyma at the periphery
- Nuclei have smooth chromatin and appear washed out, similar to papillary thyroid carcinoma
- Nucleoli are not prominent; rare mitoses
- Only 20% have clear cells, and origin of clear cells varies per source: the clear nature of the cells is due to intracytoplasmic vacuoles or an extracellular mucopolysaccharide matrix that imparts a clear cell appearance (Bostwick: Urologic Surgical Pathology, 3rd Edition, 2014, Zhou: Uropathology, 1st Edition, 2012)
- Vascular invasion common
- Cells with moderate pale blue cytoplasm (Cytopathology 2008;19:80), cord cells with few stromal fragments
- Spindle cell variant has myxoid stromal fragments and septal cells
- Anaplastic variant has bizarre pleomorphic nuclei, coarse chromatin and atypical mitotic figures (Diagn Cytopathol 2005;33:83)
- Vimentin is strongly positive (45 / 45 cases) (Am J Surg Pathol 2000;24:4)
- Cyclin D1 may be useful (14 / 14 cases of CCSK are diffusely and strongly positive) (Histopathology 2015;67:306)
- CD34 highlights septal capillaries (Bostwick: Urologic Surgical Pathology, 3rd Edition, 2014)
- MIB1 (Ki67) had mean staining index of 21% in 22 cases (range among cases: 8% - 32% of cells staining), and no obvious correlation with histologic pattern was noted (Am J Surg Pathol 2000;24:4)
- p53 shows variable staining depending on histologic subtype: 86% (25 / 29 cases) of nonanaplastic CCSKs showed minimal (less than 5% of nuclei) staining; in contrast, 66% (2 of 3 cases) of anaplastic CCSKs showed intense, diffuse (> 75% of nuclei) staining (Am J Surg Pathol 2000;24:4)
- Despite clear cell sarcoma of the kidney (CCSK) being the second most common renal tumor in children, its mechanism has not yet been fully elucidated
- A 2012 study demonstrated 12% (6 / 50 cases) contain a t(10;17), resulting in YWHAE - NUTM2B/E(FAM22B) fusion, confirming an earlier report (Arch Pathol Lab Med 2007;131:446)
- The prognostic significance of this translocation is unknown (J Pathol 2012;227:72)
- The t(10;17) is the same translocation seen in high grade endometrial stromal sarcoma
- There is a correlation between BCOR gene aberration and CCSK tumorigenesis
- Internal tandem duplications in the BCOR gene (BCL6 corepressor) were identified in 100% (20 / 20) of CCSK vs 0 of 193 other pediatric renal tumors
- None of these 20 cases had the YWHAE – NUTM2 fusion (Nat Genet 2015;47:861)
- In another study, internal tandem duplications in the BCOR gene (BCL6 corepressor) were identified in 85% (23 / 27) of CCSK (Nat Commun 2015;6:8891)
- Internal tandem duplications in the BCOR gene (BCL6 corepressor) were identified in 100% (20 / 20) of CCSK vs 0 of 193 other pediatric renal tumors
- Downregulated vascular endothelial growth factor A (VEGFA) and its interacting genes may initiate clear cell sarcoma of the kidney (CCSK) development by interrupting two different pathways, kinase regulation and protein tyrosine kinase metabolism
- IIn a 2016 gene expression study of 14 CCSK and 3 fetal kidney samples, a total of 2681 differentially expressed genes were identified, of which 2138 genes were downregulated, including VEGFA, and 543 were overexpressed (Oncol Lett 2016;11:953)
- Conventional clear cell carcinoma of kidney: extremely rare in children, tumor cells are cytokeratin+
- Malignant rhabdoid tumor of the kidney: vimentin staining in a dot like pattern; CCSK is negative (Sebire: Diagnostic Pediatric Surgical Pathology, 1st Edition, 2009)
- Mesoblastic nephroma: WT1+, smooth muscle actin (SMA)+
- Wilm tumor (WT):
- Pushing border, more aggressive invasion, nephrogenic rests rule out CCSK; Wilm tumor is WT1+, epithelioid areas are keratin+
- In a pure stromal type WT treated with preoperative chemotherapy, the stroma may show a striking clear cell sarcoma-like appearance, and extensive sampling may be required to find foci with other WT components (Popov: Wilms Tumor, 2016)
- Rare (< 1% of kidney cancers) aggressive carcinoma of renal medulla arising from the principal cells of the distal collecting ducts of Bellini
- Morphology: irregular, infiltrating tubules with high grade cells often containing mucin; marked stromal desmoplasia and inflammatory infiltrate
- Essentially a diagnosis of exclusion (Am J Surg Pathol 2020;44:e47)
- Major diagnostic criteria
- Medullary involvement
- Predominantly tubular architecture
- Marked desmoplasia
- Cytologically high grade cuboidal or hobnail cells
- Infiltrative growth pattern
- Minor diagnostic criteria
- Central location (large tumors)
- Tubulopapillary architecture
- Inflammatory stroma with neutrophils
- Extensive renal, extrarenal and vascular infiltration
- Mucin positive
- Required diagnostic criteria
- Exclusion of other types of renal cell carcinoma (RCC), urothelial carcinoma and metastasis (Transl Androl Urol 2021;10:1506)
- See Differential diagnosis
- Collecting duct carcinoma (preferred)
- Bellini duct carcinoma or carcinoma of the collecting ducts of Bellini (historic name, discouraged)
- ICD-10: C64 - malignant neoplasm of kidney, except renal pelvis
- M:F = 2:1
- Mean age 55 years; median patient age in studies ranging from 43 to 63 (WHO 5th edition)
- Wide age range reported from 8 - 83 years (Pediatr Nephrol 1996;10:29, Can Urol Assoc J 2015;9:E589)
- Some debate whether it afflicts younger patients
- Questionable association with analgesic nephropathy (Am J Surg Pathol 1980;4:565)
- Arising centrally from renal medulla but often involving both the cortex and medulla because of large size
- Approximately 67% of patients present with pain, gross hematuria, weight loss, fatigue and flank mass
- Has the poorest prognosis of common renal cell carcinoma subtypes
- Often limited responses to immunotherapy and chemotherapy (J Urol 2007;177:1698)
- Diagnosis is made primarily on H&E with a focus on the major, minor and required criteria; there should be adequate sampling, including of the adjacent renal pelvis
- Presence of any component of the tumor showing classic appearances of clear cell renal cell carcinoma, papillary renal cell carcinoma or mucinous tubular and spindle cell carcinoma likely supports a diagnosis of the corresponding entity with high grade features / transformation
- Clinical history, prior cytology or pathology, and radiologic correlation may sometimes be helpful in considering a metastasis or urothelial carcinoma in the differential
- Panel of immunostains such as PAX8, 34 beta E12, GATA3, SMARCB1, OCT 3/4 and fumarate hydratase may be useful in the diagnosis
- CT imaging shows good correlation with histopathologic findings
- Mass in the renal medulla and involving renal sinus; 50% with a cystic component
- Lymphadenopathy and metastases noted in 56% and 33% of cases, respectively
- Perinephric stranding and vascular invasion present in 56% and 28% cases, respectively
- Infiltrative growth (67%) and preservation of the renal contour (61%) more common than expansile growth (33%) and exophytic configuration (39%) (Eur J Radiol 2006;57:453)
- Features distinguishing cystic renal cell carcinoma from collecting duct carcinoma (Cancer Imaging 2021;21:52)
- Median survival: 7.6 - 11 months with 67% of patients dying within the first 2 years (Can Urol Assoc J 2015;9:E589, Curr Oncol 2013;20:e223, J Urol 2009;182:2595)
- Distant metastases in most patients either at presentation or after nephrectomy; metastases are usually to lymph nodes, liver, lungs, bones, adrenal gland or contralateral kidney (J Clin Oncol 2002;20:2376)
- Approximately 40% present with metastasis (WHO 5th edition)
- One study showed that absence of lymph node metastasis, distant metastasis, sarcomatoid differentiation; earlier stage could be used to predict a better survival
- Multivariable analysis showed that sarcomatoid differentiation and absence of renal surgery were predictors of mortality (Urology 2022;164:163)
- One study associated neutrophil to lymphocyte ratio ≥ 4 (median) with worse cancer specific survival in collecting duct carcinoma (Jpn J Clin Oncol 2018;48:692)
- 39 year old man with possible BK virus associated de novo collecting duct carcinoma in renal allograft (Oncotarget 2018;9:15157)
- 54 year old man, a Caucasian engineer, referred with multifocal cardiac metastases (Urol Case Rep 2016;5:27)
- 59 year old man, a renal transplant recipient, with collecting duct carcinoma of the native kidney (Case Rep Transplant 2017;2017:4527104)
- 65 year old woman with collecting duct carcinoma masquerading as hydatid cyst (Indian J Pathol Microbiol 2018;61:410)
- 68 year old man with collecting duct carcinoma, sarcomatoid change and paraneoplastic syndrome (Urol Case Rep 2020;33:101322)
- Nephrectomy in addition to cytotoxic chemotherapy, with or without radiation (Urol Oncol 2017;35:540.e13)
- In an open label phase II trial of 23 patients treated with gemcitabine plus cisplatin, objective responses were seen in 6 patients (26%) and median overall survival was approximately 11 months (J Urol 2007;177:1698)
- May respond to palbociclib in metastatic disease bearing a CDKN2A homozygous deletion (JCO Precis Oncol 2017;1:1)
- Use of tyrosine kinase and immune checkpoint inhibitors, antiprogrammed death 1 antibody and cytotoxic T lymphocyte associated antigen 4 antibodies are investigational (Int J Clin Oncol 2011;16:153, Clin Genitourin Cancer 2018;16:e521, Anticancer Res 2014;34:1027, Eur J Cancer 2018;100:1, Case Rep Urol 2021;2021:9936330, Int Cancer Conf J 2019;9:32, Front Oncol 2021;11:764352, J Med Case Rep 2022;16:193, Cancers (Basel) 2021;13:3807, Clin Genitourin Cancer 2016;14:e431, Medicine (Baltimore) 2018;97:e13173, Mol Clin Oncol 2017;7:988)
- Other immunotherapy treatments, including neoantigen based vaccination and neoantigen reactive T cells, are experimental (J Immunother Cancer 2020;8:e000217)
- Infiltrative, firm gray or white mass
- Centered in the medulla but often involving both cortex and medulla
- Tumor size range from 2.5 - 12 cm (mean 5 cm)
- Hemorrhage, necrosis and cystic changes are common
- May have satellite nodules and renal vein invasion
- Often infiltrates perirenal and renal sinus fat
- Complex, infiltrative, poorly circumscribed, sometimes multinodular tumor
- Mainly interstitial growth patterns with preserved glomeruli
- Composed of cords, tubules or tubulopapillary structures with infiltrating glands embedded into inflamed desmoplastic stroma
- Solid, sheet-like growth, nested, cord-like, papillary, micropapillary growth or individual cells may be present
- Sieve-like / cribriform, intracystic papillary and reticular / yolk sac tumor-like patterns were identified as minor components
- Intracystic pattern demonstrated delicate fibrovascular cores and hyalinization present in 33% of cases in one study (Am J Surg Pathol 2018;42:279)
- Tubulocystic growth was not seen in any case in one study (Am J Surg Pathol 2018;42:279)
- Irregular channels are lined by high grade cuboidal to hobnail cells, usually with eosinophilic cytoplasm
- Nuclei are large, pleomorphic with prominent nucleoli and coarse chromatin
- Numerous mitotic figures; apoptotic cells and necrosis is common
- Intracytoplasmic and intraluminal mucin may be present
- May have microcystic changes from dilation of the tubular structures
- May have sarcomatoid differentiation
- Adjacent to the tumor, tubular epithelium lining collecting ducts may appear dysplastic
- Lymphovascular invasion and regional lymph node involvement are common
- Often prominent inflammatory reaction, including neutrophils, within and around the tumor
- Reference: Am J Surg Pathol 2018;42:279
Contributed by Daniel Anderson, M.D., M.B.A. and Garrison F. Pease, M.D.
- Aspirate may contain cohesive nests of tumor cells with glandular features or individual cells
- Eosinophilic, vacuolated cells with intracytoplasmic mucin; nuclei are large irregular hyperchromatic with vesicular chromatin and large nucleoli
- Ductal / tubular differentiation with benign, dysplastic and malignant features, prominent desmoplastic stroma, neutrophils (Acta Cytol 2004;48:843)
- Sarcomatoid features may be seen (Diagn Cytopathol 2010;38:603)
- Cases of collecting duct carcinoma cells in urine cytology have been reported (Diagn Cytopathol 1997;16:446)
- PAX8 and PAX2 (Am J Surg Pathol 2010;34:965)
- High molecular weight cytokeratin (34 beta E12) and CK7
- Ulex europaeus lectins (Ulex1), peanut lectin agglutinin (PNA), mucin (strong)
- Vimentin, CK8/18, CK19, EMA, S100A1
- Usually SMARCB1 / INI1 / BAF47 retained (Urology 2011;78:474.e1)
- Positive (retained) fumarate hydratase
- p63, uroplakin II, GATA3 (Am J Surg Pathol 2010;34:965)
- Usually negative for CD10, AMACR, E-cadherin, CAIX, OCT 3/4, CD117
- Features of adenocarcinoma, including intracellular and extracellular lumina
- Well formed cell junctions, prominent basal lamina and short apical microvilli
- A defining set of molecular alterations is not currently recognized
- Not associated with loss of 3p
- Not associated with trisomies 7 and 17
- Complex chromosomal aberrations, especially copy number losses or loss of heterozygosity involving multiple chromosomes, such as 1p, 6, 8, 9, 14 and 22 (WHO 5th edition)
- HER2 / neu amplifications have been reported in 45% of cases (J Urol 1997;158:245)
- Genomic alterations in NF2 (29%), SETD2 (24%), SMARCB1 (18%) and CDKN2A (12%) (Eur Urol 2016;70:516)
- Whole exome sequencing and transcriptome sequencing (RNASeq) showed recurrent somatic single nucleotide variants (SNV) in MLL in 2 samples; somatically mutated SNVs identified in ATM, CREBBP, PRDM1, CBFB, FBXW7, IKZF1, KDR, KRAS, NACA, NF2, NUP98, SS18, TP53 and ZNF521
- Single nucleotide polymorphism (SNP) array identified a CDKN2A homozygous deletion and SNV analysis showed a nonsense mutation of the CDKN2A gene with unknown somatic status
- Dysregulation in solute carrier family genes, including overexpression in SLC7A11 (cystine transporter, xCT), a cisplatin resistance associated gene (Oncotarget 2016;7:29901)
- Other gene expression study showed downregulation in 4 solute carrier genes: SLC3A1, SLC9A3, SLC26A7 and SLC47A1; also upregulated keratin 17 and downregulation MARC2, cubulin (Cancers (Basel) 2019;12:64)
- RNA sequencing study found shift towards aerobic glycolysis and overexpression of immune genes (Sci Rep 2016;6:30988, Cancers (Basel) 2021;13:2903)
- Molecularly heterogeneous tumor with at least 2 subtypes distinguished by cell signaling and metabolic and immune related alterations (Cancers (Basel) 2021;13:2903, Cancers (Basel) 2021;13:3807)
- Whole genome sequencing study of a Chinese population showed 8 recurrently somatically mutated genes: BM14, MTUS1, GAK, DST, ASPM, CDC27, RNF213 and XIRP2
- TP53 / RB1 and CDKN2A alterations documented
- Copy number variant mutation spectrum appeared unique from urothelial carcinoma and other renal cell carcinoma subtypes
- CDKN2A altered patients displayed a worse overall survival (OS) (BMC Med Genomics 2022;15:1)
Collecting duct carcinoma
- Kidney, nephrectomy:
- Collecting duct carcinoma (X cm); tumor invades perirenal fat and renal sinus fat; margins are negative (see synoptic report and comment)
- Comment: Immunohistochemistry is performed. The carcinoma cells are positive for PAX8, 34 beta E12, fumarate hydratase (retained), SMARCB1 / INI1 (retained) and negative for GATA3 and OCT 3/4.
- Metastatic carcinoma, including from GI or lung:
- Usually, well defined borders; multiple foci may be present
- Clinical history and radiologic review could be helpful
- Immunohistochemistry may be helpful in excluding a metastasis
- Urothelial carcinoma showing glandular differentiation:
- Usually associated with prior history of lower tract urothelial carcinoma
- Filling defect on imaging, positive urine cytology
- If located in renal calyces or pelvis it would favor urothelial carcinoma; however, involvement of cortex is found in higher stage disease
- Location of in situ urothelial carcinoma or residual papillary component is helpful
- Carcinoma in situ (CIS) may be present in renal pelvis
- CIS can colonize the collecting ducts with solid nests as opposed to dysplastic glandular cells in collected duct carcinoma
- Careful sampling of renal pelvis recommended
- Usually, lower stage disease at presentation
- Urothelial carcinoma typically p63+ / GATA3+ / uroplakin II+ and negative for PAX8 (Am J Surg Pathol 2010;34:965, Hum Pathol 2013;44:2651)
- Fumarate hydratase (FH) deficient renal cell carcinoma / hereditary leiomyomatosis RCC:
- Often viral inclusion-like (CMV-like) macronucleoli with perinucleolar halos are seen
- Tubulocystic and intracystic papillary with or without hyalinization patterns may favor FH deficient RCC (Am J Surg Pathol 2018;42:279)
- Loss of fumarate hydratase, nuclear and cytoplasmic expression of 2SC
- In one study, 25% of cases diagnosed previously as collecting duct carcinoma were reclassified as fumarate hydratase deficient renal cell carcinoma using fumarate hydratase and 2SC IHC (Am J Surg Pathol 2018;42:279)
- Papillary renal cell carcinoma:
- More well circumscribed, predominantly papillary, often psammoma bodies and foamy macrophages within papillae; usually no angiolymphatic invasion, no desmoplasia or inflammation, no dysplasia of collecting duct epithelium
- CEA-, HMWCK-, Ulex europaeus-, AMACR+, CD10+, trisomy 7 and 17 (Hum Pathol 2008;39:1350)
- SMARCB1 deficient renal medullary carcinoma (Am J Surg Pathol 2014;38:871):
- Occurs in those with sickle trait or related hemoglobinopathies; sickled RBCs (drepanocytes) may be found within tumor (Transl Androl Urol 2021;10:1506)
- Distinction from collecting duct not possible on morphologic grounds alone
- Sieve-like / cribriform and reticular / yolk sac tumor-like patterns favor renal medullary (Am J Surg Pathol 2018;42:279)
- Both positive for PAX8; p63 and GATA3 usually negative
- Inactivation of tumor suppressor gene INI1 on long arm of chromosome 22 is frequently detected
- INI1 negative (loss) and OCT 3/4 positive in 50% of cases (Urology 2011;78:474.e1, Histopathology 2012;61:428, Am J Surg Pathol 2018;42:279)
- Occurs in those with sickle trait or related hemoglobinopathies; sickled RBCs (drepanocytes) may be found within tumor (Transl Androl Urol 2021;10:1506)
- Mucinous tubular spindle cell carcinoma with high grade transformation
- Tubulocystic carcinoma:
- Often detected incidentally and usually without pain, hematuria, flank mass, etc.
- Cortical with involvement of medullary region at times
- Well circumscribed with multilocular sponge-like appearance without necrosis or hemorrhage (versus more solid appearance of collecting duct)
- Consists of tubules and variable sized cysts; should not display infiltrative glands, cords, solid sheets, necrosis, hemorrhage and desmoplastic stroma that is relatively common in collecting duct carcinoma
- Less mitotically active
- Will not have dysplastic medullary collecting duct structures or extension into perirenal adipose tissue
- If the size of the tumor obscures the site of origin, infiltrative growth that extends between normal structures, such as glomeruli and tubules, can be seen as a clue that the tumor is medullary based
- This pattern can be seen in collecting duct carcinoma, medullary carcinoma and invasive urothelial carcinoma; similar patterns can also be seen in metastatic tumors and lymphoma (Surg Pathol Clin 2015;8:587)
A 76 year old man with history of noninvasive low grade papillary urothelial carcinoma undergoes a nephrectomy for a 5 cm centrally located urothelial mass involving the renal pelvis, medulla and cortex. Photomicrographs are shown of the invasive component and results of renal pelvis sampling. Which of the following is true regarding this neoplasm?
- Has a multilocular, sponge-like cut surfaces on gross examination
- May show aneuploidy in chromosome 3, 7 or 17 or loss of 9p21 locus
- Will often demonstrate loss of fumarate hydratase by immunohistochemistry
- Will often show positive immunohistochemical expression for PAX8 and negative for p63 and GATA3
Comment Here
Reference: Collecting duct carcinoma
A 59 year old man is diagnosed with collecting duct carcinoma. Which of the following is true regarding this entity?
- Has no morphologic overlap with fumarate hydratase deficient
- Has prominent stromal desmoplasia, infiltrative growth and inflammatory cell reaction
- Is correlated with sickle cell trait
- Will show papillary growth with foamy macrophages in papillae
Comment Here
Reference: Collecting duct carcinoma
- Mesoblastic nephroma is a mesenchymal / myofibroblastic renal tumor of low grade malignancy
- It typically occurs in infancy, often congenital but not always
- First described by Bolande et al. in 1967 (Pediatrics 1967;40:272)
- Most common renal tumor in the first month of life
- 90% of cases diagnosed in the first 9 months of life
- Virtually never after 3 years of age
- Adult mesoblastic nephroma does not exist
- 3 histologic subtypes: classic (~25% of cases), cellular (~65%) and mixed (~10%)
- Cellular type: ETV6-NTRK3 gene fusion in ~70% of cases
- Classic type: EGFR internal tandem duplication mutation
- Most important prognostic factor is completeness of surgical resection
- Historic synonyms: renal leiomyomatous hamartoma, congenital mesoblastic nephroma
- Although "congenital" is a part of the WHO stated name for this tumor, it is sometimes a misnomer as the tumor is not always present from birth
- Represents 3 - 4% of all renal tumors of childhood (Pediatr Blood Cancer 2011;56:744)
- Most common renal tumor in the first month of life (Pediatr Blood Cancer 2008;50:1130)
- 90% of cases occur in the first 9 months of life (Pediatr Blood Cancer 2008;50:1130)
- Median age < 1 month in 63% of cases (Pediatr Blood Cancer 2017;64:e26437)
- Median age differs between classic (7 days), mixed (1.9 months) and cellular (4.6 months) types (Pediatr Blood Cancer 2011;56:744)
- Virtually never occurs after the age of 3 years (Pediatr Blood Cancer 2017;64:e26437)
- Adult mesoblastic nephroma does not exist
- Not associated with syndromes or congenital anomalies
- Kidney; never bilateral, never multifocal
- Unknown
- No recognized risk factors
- No familial cases have been reported
- Cellular type associated with t(12;15)(p13;q25) in ~70% of cases (Pediatr Blood Cancer 2018;65:e26925)
- Classic type associated with EGFR internal tandem duplication mutation (Histopathology 2020;77:611)
- Not associated with nephrogenic rests
- Abdominal mass (~75% of patients) (Pediatr Blood Cancer 2017;64:e26437)
- ~15% detected prenatally, usually associated with polyhydramnios
- Hypertension (~20%)
- Hematuria (~10%)
- Never presents as metastatic or bilateral disease
- Virtually never associated with syndromes or anomalies typical for Wilms tumor
- Imaging techniques cannot distinguish it from other renal tumors (J Pediatr Surg 2008;43:1301)
- Biopsy not recommended
- Diagnosis made on nephrectomy
- No specific laboratory findings
- Hypercalcemia (4% of cases)
- Hyperreninemia (1% of cases) (Pediatr Blood Cancer 2017;64:e26437)
- Unilateral, well demarcated, hypoechogenic mass
- Most important prognostic factor is completeness of surgical resection
- Histologic type is of no prognostic significance
- Overall survival reported as ~96% but is almost certainly higher since cases with unfavorable outcome are more likely to be reported (Pediatr Blood Cancer 2008;50:1130)
- Of 12/276 patients with mesoblastic nephroma who died, 5 died of tumor related and 7 of treatment related causes (Pediatr Blood Cancer 2017;64:e26437)
- Very rare local relapses and metastases; 38 cases reported in the literature (Pediatr Surg Int 2017;33:1183)
- Relapses and metastases occur within 12 months after the diagnosis (range 1 - 12 months, median 6 months)
- Local relapses in 27/38 (71%) of these cases
- 21/27 (76%) had incomplete resection (stage 3)
- All but 1 patient had cellular type but even then, cellular type had no significant difference in prognosis
- Metastases developed in 18 children (7 also had local recurrence)
- Lung metastases in 7/18 (39%), liver in 5/18 (28%), brain in 4/18 (22%) and heart, bone and peritoneum in 5.5% each
- 38% of patients with metastases died (all who had brain, bone, heart and peritoneum metastases)
- Prematurely born boy presented with neonatal hypertension (Turk J Pediatr 2018;60:198)
- Prematurely born boy with a prenatal diagnosis of polyhydramnios (Urol Case Rep 2015;3:157)
- 7 day old boy presented with abdominal distension and vomiting (J Neonatal Surg 2017;6:45)
- 11 month old boy presented with a renal mass (Urol Case Rep 2019;26:100979)
- Complete surgical resection (total nephrectomy) with wide resection margins (Pediatr Blood Cancer 2017;64:e26437, Pediatr Surg Int 2017;33:1183)
- Even stages II and III treated with surgery only
- Relapses also treated with surgery
- Chemotherapy treatment of relapses only in unresectable cases
- Always unilateral and solitary (Adv Anat Pathol 2003;10:243)
- Typically found in the medial renal sinus
- Classic type:
- Firm, whorled, leiomyomatous consistency
- Poorly demarcated from the normal renal parenchyma
- Cellular type:
- Usually soft
- Tumor-kidney junction relatively clearly demarcated
- Cystic areas
- Can show hemorrhage and necrosis
- Classic type (~25% of cases) (Adv Anat Pathol 2003;10:243, Histopathology 1985;9:741):
- Intermingling fascicles of spindle cells with low mitotic activity
- Collagen deposition
- Often prominent dilated, thin walled vascular spaces
- No capsule
- Tumor-kidney border is irregular with finger-like protrusions of the tumor into the renal parenchyma
- Islands of hyaline cartilage can be seen at the tumor-kidney interface
- Entrapped islands of renal parenchyma
- Foci of extramedullary hematopoiesis common
- Cellular type (~65% of cases):
- High cellularity of plump cells with vesicular nuclei, moderate amount of cytoplasm
- Sheet-like growth pattern
- High mitotic activity (which is of no prognostic significance)
- No capsule
- Clear tumor-kidney border but subtle infiltration into the renal parenchyma
- Isolated entrapped tubules, which may be mistaken for neoplastic tubules
- Mixed type (~10% of cases):
- Features of both types in a variable proportion
- Staging criteria are as for nephroblastoma
- Vast majority are stage 2 due to tumor's infiltrative growth into the renal sinus or perirenal fat
Contributed by Ellen D’Hooghe, M.D. and Gordan M. Vujanic, M.D., Ph.D.
- Fine needle aspiration is not indicated
- Cyclin D1: diffuse in ~70% of cases (Histopathology 2015;67:306)
- Pan-TRK: cytoplasmic or nuclear positivity in cellular type with ETV6-NTRK3 gene fusion (Am J Surg Pathol 2018;42:927)
- Alpha smooth muscle actin
- Vimentin
- Cellular type:
- Specific chromosomal translocation t(12;15)(p13;q25), which results in a fusion of ETV6 and NTRK3 genes in ~70% of cases (Pediatr Blood Cancer 2017;64:e26925)
- The same translocation is found in infantile fibrosarcoma (Mod Pathol 2000;13:29, Mod Pathol 2001;14:1246)
- Few cases reported with EGFR internal tandem duplication (ITD) mutation (Nat Commun 2018;9:2378)
- Rare cases with BRAF rearrangements but not BRAF V600E mutation (Histopathology 2020;77:611, Nat Commun 2018;9:2378)
- Classic type: EGFR ITD mutation is a consistent and recurrent genetic event (Histopathology 2020;77:611)
- Mixed type: may have either EGFR ITD or ETV6-NTRK3 gene fusion
- Right kidney, total nephrectomy:
- Mesoblastic nephroma, cellular type, stage II (due to renal sinus and perirenal fat invasion) (see comment)
- Comment: Tumor shows dense cellularity with plump cells with vesicular nuclei and with a high mitotic activity. Tumor is infiltrating the renal sinus and perirenal fat but is not reaching the resection margins. Lymph nodes are free of tumor.
- Metanephric stromal tumor:
- Alternating, nodular cellularity, angiodysplasia, onion skinning
- CD34+
- BRAF V600E mutation
- Stromal type Wilms tumor:
- Often rhabdomyoblasts and adipose tissue
- Often associated with nephrogenic rests
- More sampling might reveal epithelial and or blastemal elements
- BCL2+
- Clear cell sarcoma of the kidney:
- Characteristic arborising vascular pattern
- BCOR+
- Rhabdoid tumor of the kidney:
- INI1 loss
- Inflammatory myofibroblastic tumor:
- ALK+
An 11 month old boy presents with an abdominal mass noticed by parents. Imaging studies show a right sided renal mass. Total right nephrectomy is performed. The tumor is composed of undifferentiated cells with no particular pattern, shown above. Which finding is most characteristic for this entity?
- BCOR+
- BRAF V600E mutation
- ETV6-NTRK3 gene fusion
- INI1 loss
Comment Here
Reference: Congenital mesoblastic nephroma
- WHO: multilocular, exclusively cystic neoplasm of very young children, containing nephroblastomatous tissue
- The concept was introduced as a variant of nephroblastoma curable by surgery (Perspect Pediatr Pathol 1979;5:217)
- Entirely multilocular cystic tumor that lacks solid nodules on gross or microscopic examination; septa contain (undifferentiated) mesenchyme, blastemal and immature epithelial elements
- ICD-O: 8959/1 - cystic partially differentiated nephroblastoma
- ICD-11: 2C90.Y & XH1JB4 - other specified malignant neoplasms of kidney, except renal pelvis & cystic partially differentiated nephroblastoma
- Rare (21 out of 5,100 cases of nephroblastoma) (J Pediatr Surg 2003;38:897)
- Occurs in children presenting before 2 years of age (Cancers (Basel) 2021;13:997)
- Male predominance
- Palpable abdominal mass (Cancers (Basel) 2021;13:997)
- Bilateral disease in 4.4%, 3 cases with bilateral cystic partially differentiated nephroblastoma, 2 with nephroblastomatosis in the contralateral kidney and 1 with Wilms tumor in the contralateral kidney (Cancers (Basel) 2021;13:997)
- Requires microscopic examination of resected tumor
- Large multilocular cystic mass with septa of varying thickness
- No solid component
- Rare recurrences have been reported as a complication of incomplete resection or tumor rupture
- No recurrence in series with n = 21 and n = 7 (J Pediatr Surg 2003;38:897, J Urol 2007;177:294)
- Recurrences in 4 of 113 (3.5%) in a large review, however, Wilms tumor could not be ruled out in 3 cases (Cancers (Basel) 2021;13:997)
- No deaths attributable to the disease, series with n = 21, n = 7 and in a large review with n = 113 (J Pediatr Surg 2003;38:897, J Urol 2007;177:294, Cancers (Basel) 2021;13:997)
- 4 month old boy with a multicystic mass involving the whole right kidney (Clin Case Rep 2021;9:e04929)
- 18 month old boy with bilateral CPDN (Urology 2016;92:106)
- 2 year old girl with gradually increasing painless abdominal mass (Indian J Nephrol 2013;23:460)
- 3 year old girl with 4.6 cm unilocular renal cyst (Front Pediatr 2022;10:1045936)
- 45 year old man with a 3 cm multicystic kidney mass (Int J Clin Exp Pathol 2015;8:989)
- Surgery is almost always curative
- Patients with stage I disease are cured by surgery without adjuvant chemotherapy
- Questionable value of chemotherapy in cases of tumor rupture and spillage (Cancers (Basel) 2021;13:997)
- Typically, a large multilocular cystic tumor (mean diameter of 10 cm)
- Thin or variable sized septa
- Well circumscribed
- No expansive nodules
- Reference: J Postgrad Med 2006;52:45
- Cysts lined with flat / cuboidal / hobnail cells (or are denuded)
- Epithelial elements consist mainly of mature and immature / abortive tubules and small papillae resembling immature glomeruli
- Key histological findings of the variably cellular septa include
- Nephroblastomatous epithelial elements
- Islands of undifferentiated blastema and differentiated mesenchymal elements (skeletal muscle and less often cartilage and fat) (J Urol 2010;183:1585)
- Focally, the septal elements may protrude into the cystic spaces in microscopic papillary folds
Contributed by Americo Brilhante, M.D. and Daniel Athanazio, M.D., Ph.D.
- WT1: blastemal cells (J Cancer Res Ther 2022;18:209)
- Immunohistochemistry for other markers is poorly described and not relevant for the diagnosis
- Cystic partially differentiated nephroblastoma is considered part of the nephroblastoma spectrum
- Does not harbor DICER1 mutations, which are found in the morphologically similar tumor pediatric cystic nephroma (Cancers (Basel) 2021;13:997)
- No specific genetic alterations have been characterized (Cancers (Basel) 2021;13:997)
- Left kidney, nephrectomy:
- Cystic partially differentiated nephroblastoma (see synoptic report)
- Multicystic renal dysplasia:
- Typically, obstructive symptoms are present
- Usually diffuse and bilateral
- Presence of primitive ducts on histology, metaplastic cartilage and lobar disorganization (Arch Pathol Lab Med 2015;139:547)
- Pediatric cystic nephroma:
- Also an exclusively cystic renal neoplasm
- Septa do not contain nephroblastomatosis and extensive sampling is required to exclude nephroblastomatosis elements (Amin: Urological Pathology, 1st Edition, 2014)
- Does not contain ovarian type stroma
- Associated with DICER1 mutations
- Wilms tumor (nephroblastoma):
- Although it may be extensively cystic, it shows expansive nodules with nephroblastomatous component (Nat Rev Urol 2018;15:693)
- Predominately solid areas containing focally cystic areas
- Careful grossing sampling is required to identify expansive nodules (Amin: Urological Pathology, 1st Edition, 2014)
- Also WT1+
- Has malignant behavior
A large multilocular cystic renal mass was removed from an 18 month old boy. The differential diagnosis is pediatric cystic nephroma and cystic partially differentiated nephroblastoma. What finding within the septa favors cystic partially differentiated nephroblastoma?
- Fibrous tissue
- Nephroblastomatous elements
- Presence of mature renal cortex within the septa
- Tubules with flat and hobnail morphology
Comment Here
Reference: Cystic partially differentiated nephroblastoma
- Renal cell carcinoma (RCC) composed of clear cells separated by thick fibromuscular bands and associated with mutation of TCEB1 gene
- Low grade clear cells with voluminous cytoplasm
- Transecting thick fibromuscular septae imparting multilobular appearance
- Biallelic inactivation of TCEB1 gene: mutation with concurrent monosomy 8 or loss of heterozygosity at 8q21
- Lack VHL mutations, hypermethylation or loss of heterozygosity at 3p (Histopathology 2019;74:31, Mod Pathol 2015;28:845, Histopathology 2019;74:60)
- Transcription Elongation Factor B (TCEB1) mutated renal cell carcinoma
- Monosomy 8 renal cell carcinoma
- ICD-10: C64 - malignant neoplasm of kidney, except renal pelvis
- Rare; Age range: 32 - 77 year old (Mod Pathol 2015;28:845, Am J Surg Pathol 2019;43:1135, Mod Pathol 2017;30:1603, Virchows Arch 2016;469:81)
- Solitary kidney mass
- Renal cortex
- TCEB1 located at chromosome 8q21.11 and encodes 112-residue protein elongin C, subunit 1 of transcription factor B (SIII) complex
- Elongin C is part of VHL complex responsible for proteosomic degradation (ubiquitination) of hydroxylated HIF
- Loss of Elongin C leads to HIF1α stabilization, accumulation, activation of hypoxia inducible growth factors and tumor development
- Downregulate other genes in ubiquitination complex including POLR2E, POLR2C, CDK7, TCEA1 and TCEB2
- Increase the POL II transcription elongation past template encoding arresting sites (Nat Genet 2013;45:860, Mod Pathol 2015;28:845)
- Hotspot mutations in TCEB1 gene always accompanied by loss of chromosome 8 (loss of heterozygosity at 8q or monosomy 8)
- TCEB1 mutations and VHL abnormalities are mutually exclusive (Nat Genet 2013;45:860)
- Vast majority are incidental: pT1a
- Rare tumors are pT3a
- No recurrence or metastasis reported to date
- Not available at this time
- Surgical: partial or radical nephrectomy
- Unifocal, well circumscribed, partly encapsulated
- Pale-tan cut surface with traversing fibrous bands
- Could have nodular or lobulated appearance
- Size: reported 1.6 - 3.5 cm (Am J Surg Pathol 2019;43:1135, Virchows Arch 2016;469:81)
- Clear cell tumor with multinodular appearance at low power due to transecting thick fibrous or fibromuscular bands
- Variable architecture combining solid, alveolar, nested patterns with occasional cystic and tubulopapillary features
- Cells with predominantly clear voluminous cytoplasm, prominent cell membranes and low nucleolar grade (WHO / ISUP grade 1 or 2)
- No necrosis, lymphovascular invasion or heavy inflammatory infiltrate (Mod Pathol 2015;28:845, Histopathology 2019;74:31)
- CAIX (diffuse membranous, box shape pattern) like in clear cell renal cell carcinoma
- CK7 (strong in majority of cells)
- CD10 (focal weak to moderate)
- SDHB (retained but weak due to cell clearing)
- HIF1α (Virchows Arch 2016;469:81, Pathology 2019;51:453)
- TCEB1 mutations (NGS): Tyr79 (> 80%), Ala100, Ala 106 and Ser23
- Copy number analysis: loss of heterozygosity of chromosome 8, copy neutral loss of heterozygosity
- RNA expression analysis: mRNA downregulation of POLR2E, POLR2C, CDK7, TCEA1 and TCEB2 (Mod Pathol 2015;28:845, Nat Genet 2013;45:860, Am J Surg Pathol 2019;43:1135)
- Left kidney, mass, partial nephrectomy:
- Renal cell carcinoma with clear cell features, unclassified (see synoptic report and comment)
- Comment: The carcinoma is morphologically consistent with clear cell renal cell carcinoma but contains prominent fibromuscular stroma. The immunohistochemical stains demonstrate that the neoplastic cells are positive for CAIX, variably positive for CK7 and CD10 but negative for TFE3. The tumor histology and variable CK7 positivity raise the possibility of a recently described TCEB1 mutated renal cell carcinoma. Molecular characterization can be attempted to confirm the histologic impression.
- Clear cell renal cell carcinoma with prominent stromal component:
- CK7 usually negative or only focally positive
- Loss of heterozygosity at 3p or VHL alteration (biallelic loss)
- Clear cell (tubulo)papillary renal cell carcinoma:
- Renal cell carcinoma with (angio)leiomyomatous stroma:
- Provisional / emerging entity in IARC: WHO Classification of Tumours of the Urinary System and Male Genital Organs (Medicine), 4th Edition, 2016
- Prominent vascular and smooth muscle stroma, branching tubules / papillary tufts of clear cells
- Positive for CAIX, HMWCK, CK7 and CD10
- No TCEB1 abnormalities or monosomy 8; absence of VHL/3p alterations, no trisomy 7/17
- Tuberous sclerosis complex (TSC) associated renal cell carcinoma:
- Often multifocal, bilateral and associated with multiple cysts and angiomyolipomas
- TSC gene related alterations
- Family history
- MiT family translocation renal cell carcinoma:
- Usually with papillary architecture and higher nuclear grade
- Positive for TFE3
- Negative for CK7 / CAIX
- Histopathology 2019;74:31, Pathology 2019;51:453, Mod Pathol 2017;30:1603, Pathology 2017;49:10, Hum Pathol 2017;66:152, Mod Pathol 2015;28:845, Histopathology 2019;74:60
- Which is true about TCEB1 mutated renal cell carcinoma?
- Definitive diagnosis is based on documenting either TCEB1 mutation or absence of chromosome 8 (monosomy 8)
- Morphologically identical to clear cell renal cell carcinoma with prominent fibromuscular stroma
- Multiple reports indicate relatively high incidence of this tumor type
- TCEB1 and TFEB genes belong to the same family of transcription factors
- Typically pursue aggressive course
Comment Here
Reference: TCEB1 mutated renal cell carcinoma
- Which immunohistochemical results will raise concern of TCEB1 mutated renal cell carcinoma in 45 year old patient with 3 cm encapsulated tumor having prominent stroma and clear cell morphology, shown here?
- HMWCK-, CD10-, TFE3+, RCC+
- HMWCK-, CK7-, CAIX+, CD10+
- HMWCK-, CK7+, CAIX+, CD10-
- HMWCK+, CK7+, CAIX+, GATA3+
Comment Here
Reference: TCEB1 mutated renal cell carcinoma
- Emerging oncocytic renal neoplasm, not classifiable as oncocytoma, chromophobe renal cell carcinoma or other types with eosinophilic features
- Neoplastic cells characterized by abundant eosinophilic (oncocytic) cytoplasm with intracytoplasmic vacuoles and large nuclei, often with prominent nucleoli
- Included under other oncocytic tumors of the kidney in the WHO Classification of Tumours, Urinary and Male Genital Tumours (5th edition)
- Nests of eosinophilic (oncocytic) cells with large intracytoplasmic vacuoles and large nuclei, often with prominent nucleoli, imparting a high grade nuclear appearance
- Relatively consistent immunohistochemical profile
- PAX8, AE1 / AE3, cathepsin K and CD117 positive (cathepsin K and CD117 may be focal)
- Vimentin, HMB45, MelanA and TFE3 negative
- CK7 and CK20 variable (negative to focal)
- Sporadic but can occur in tuberous sclerosis complex (TSC)
- Nonoverlapping mutations in MTOR, TSC2 or TSC1, resulting in mTORC1 pathway activation
- Eosinophilic vacuolated tumor was the name proposed by Genitourinary Pathology Society (GUPS) (Mod Pathol 2021;34:1167)
- High grade oncocytic renal tumor (HOT) by He et al. (Virchows Arch 2018;473:725)
- ICD-11: 2F98 - neoplasm of unknown behavior of urinary organs
- F > M
- Wide age range (25 - 73 years)
- Indolent course
- Sporadic tumor with rare association with tuberous sclerosis
- References: Mod Pathol 2022;35:344, Mod Pathol 2021;34:1167
- Bilateral kidneys (no reported side predilection)
- Nonoverlapping mutations in MTOR, TSC2 or TSC1 are consistently observed in EVT; this results in mTORC1 pathway activation, which leads to cell growth, angiogenesis, increase in proteins, lipids and nucleotide production as well as suppression of catabolic pathways, such as autophagy
- Tumors with MTOR mutations consistently show loss of the wild type allele on chromosome 1 (locus where mTOR resides)
- 19p, 16p11 and 7q31 chromosomes losses have also been reported
- Reference: Cancers (Basel) 2023;15:4043
- Sporadic with rare association with tuberous sclerosis (Mod Pathol 2022;35:344, Histopathology 2019;75:440, Hum Pathol 2021;116:1)
- Usually found incidentally on imaging (Amin: Diagnostic Pathology - Genitourinary, 3rd Edition, 2022)
- Detected by imaging (commonly incidental), diagnosis is made by microscopic evaluation
- Unknown at this time
- Unknown at this time
- Indolent course (limited data) (Mod Pathol 2021;34:1167, Virchows Arch 2018;473:725)
- 47 year old man underwent partial nephrectomy for a suspicious kidney mass (Cureus 2022;14:e24716)
- 52 year old woman with an incidental identification of left renal mass by abdominal imaging (BMC Urol 2024 September 11 [Preprint])
- 55 year old man with an incidental renal mass identified during workup of ischemic colitis (Adv Anat Pathol 2021;28:251)
- Limited data
- Solitary, well circumscribed, unencapsulated, solid tumor with gray, tan or mahogany brown cut surface
- Size range: 1.5 - 7 cm (mean: 4.3 cm)
- Tumors lack necrosis and macrocysts
- References: Mod Pathol 2022;35:344, Virchows Arch 2018;473:725, Cureus 2022;14:e24716, Adv Anat Pathol 2021;28:251, Mod Pathol 2021;34:1167
- Solid to nested growth pattern with focal tubulocystic areas
- Tumor cells possess
- Voluminous eosinophilic (oncocytic) cytoplasm, with frequent intracytoplasmic vacuoles, which often can be large
- Cytoplasm has variable granularity, typically condensed toward the cell periphery, giving prominent cell membrane appearance of chromophobe renal cell carcinoma (RCC)
- Large round to oval nuclei, often with enlarged nucleoli
- Perinuclear halos and nuclear irregularities are typically not found
- Voluminous eosinophilic (oncocytic) cytoplasm, with frequent intracytoplasmic vacuoles, which often can be large
- Normal renal tubules and thick walled vessels are frequently found at the periphery
- References: Mod Pathol 2022;35:344, Cureus 2022;14:e24716, Mod Pathol 2021;34:1167
- Unknown at this time
- PAX8, AE1 / AE3, CD10 and antimitochondrial antigens
- Cathepsin K and CD117 (both may be focal)
- SDHB and FH retained (positive)
- References: Mod Pathol 2021;34:1167, Mod Pathol 2022;35:344, Am J Surg Pathol 2022;46:1562
- Vimentin, HMB45, MelanA, CK20 and TFE3 (Mod Pathol 2021;34:1167)
- CK7 (may be focally positive) (Mod Pathol 2021;34:1167, Mod Pathol 2022;35:344)
- Numerous intracytoplasmic mitochondria
- Dilated cisterns of rough endoplasmic reticulum (RER) corresponding to intracytoplasmic vacuoles (seen in light microscopy) (Adv Anat Pathol 2021;28:251)
- Nonoverlapping mutations in MTOR, TSC2 or TSC1, resulting in mTORC1 pathway activation
- Tumors with MTOR mutations demonstrate consistent loss of the wild type allele on chromosome 1 (where MTOR resides)
- Isolated losses of chromosomes 19p, 16p11 and 7q31 have also been reported
- References: Mod Pathol 2021;34:1167, Mod Pathol 2022;35:344, Cancers (Basel) 2023;15:4043, Am J Surg Pathol 2022;46:1562
- Right kidney, partial nephrectomy:
- Oncocytic renal neoplasm, with findings consistent with eosinophilic vacuolated tumor (see comment)
- Comment: The neoplastic cells possess abundant eosinophilic (oncocytic) cytoplasm, with numerous intracytoplasmic vacuoles and round to oval nuclei with prominent nucleoli. The cells express PAX8 (diffusely), CD117 (in a patchy manner) and CK7 (focally). They demonstrate retained immunoreactivity with SDHB and FH stains. No significant expression of CK20, HMB45, MelanA and TFE3 immunostains is observed. These findings are consistent with the recently described eosinophilic vacuolated tumor (EVT) of the kidney. This entity has been included under other oncocytic tumors of the kidney in the WHO Classification of Tumours, Urinary and Male Genital Tumours (5th edition).
- Eosinophilic, solid and cystic RCC:
- Solid and cystic architecture
- Polygonal neoplastic cells possessing abundant eosinophilic cytoplasm, with cytoplasmic stippling; stippling may have leishmania-like appearance
- Hobnail cyst lining cells
- Admixed aggregates of lymphocytes and histiocytes
- PAX8, CK20 and cathepsin K positive
- CK7 and CD117 negative
- Chromophobe renal cell carcinoma, eosinophilic variant:
- Sheet-like architecture, neoplastic cells with densely eosinophilic and granular cytoplasm
- Admixture of pale and eosinophilic cells
- Nuclei are often irregular (raisinoid) with perinuclear halos
- CD117, CK7 and cathepsin K diffusely positive
- Vimentin and CD10 negative
- Low grade oncocytic tumor:
- Epithelioid angiomyolipoma:
- TFEB altered RCC:
- Young age
- Variable architecture with a biphasic pattern of large and small cells, frequent oncocytic and papillary features, with the presence of psammoma bodies
- Underexpression of epithelial markers (cytokeratins and EMA)
- Positive expression of PAX8, HMB45, MelanA and vimentin
- Nuclear immunoreactivity for TFEB protein is highly specific but technically challenging
- TFEB rearrangement / amplification by break apart fluorescent in situ hybridization (FISH); TFEB gene fusion by RNA sequencing is the preferred diagnostic test
- References: Adv Anat Pathol 2021;28:251, Mod Pathol 2021;34:1167
- Neoplastic cells with eosinophilic cytoplasm, prominent cell borders, and raisinoid nuclei
- Nests of eosinophilic (oncocytic) cells with large intracytoplasmic vacuoles and large nuclei, often with prominent nucleoli, imparting a high grade nuclear appearance
- Polygonal neoplastic cells possessing abundant eosinophilic cytoplasm, with cytoplasmic leishmania-like stippling
- Solid nests of eosinophilic cells with round nuclei and subtle perinuclear halos
Comment Here
Reference: Eosinophilic vacuolated tumor (EVT)
- CD117+ / CK7+ / cathepsin K+ / vimentin- / CD10-
- PAX8+ / CD117+ / vimentin- / CK20-
- PAX8+ / CD117- / vimentin+ / CK20+
- PAX8+ / HMB45+ / MelanA+ / EMA-
Comment Here
Reference: Eosinophilic vacuolated tumor (EVT)
A 43 year old woman presents with a 2.3 cm left renal mass that was identified incidentally during imaging. The neoplasm highlights with PAX8 and CD117 and is negative for HMB45, TFE stains, CK7 and CK20. What is your diagnosis?
- Chromophobe renal cell carcinoma
- Eosinophilic vacuolated tumor
- Low grade oncocytic tumor
- Oncocytoma
Comment Here
Reference: Eosinophilic vacuolated tumor (EVT)
- Emerging subtype of renal cell carcinoma (RCC) with abundant eosinophilic cytoplasm and solid / cystic growth, not yet recognized in the 2013 International Society of Urological Pathology (ISUP) Vancouver Classification of Renal Neoplasia or the updated 2016 World Health Organization (WHO) renal tumor classification (Am J Surg Pathol 2013;37:1469, Eur Urol 2016;70:93)
- Solid and cystic components
- Voluminous eosinophilic cytoplasm which frequently displays basophilic intracytoplasmic stippling / inclusions
- No clinical history or features of tuberous sclerosis complex
- CK20+ / CK7- / c-kit- / PAX8+ immunoprofile
- Frequently harbors somatic loss of function mutations in TSC1 or TSC2
- ESC RCC
- F > M
- Broad age range (14 - 75 years)
- No documented clinical features, family history or germline genetic testing suggestive of tuberous sclerosis complex; see differential diagnosis below for more information
- Left = right (no reported predilection)
- Sporadic tumors believed to fall within a spectrum of renal neoplasms that harbor TSC1 / 2 genetic alterations (including the granular eosinophilic macrocystic subtype of tuberous sclerosis associated RCC and possibly the oncocytoid subtype of RCC postneuroblastoma, Am J Surg Pathol 2018;42:1166)
- TSC1 (hamartin) and TSC2 (tuberin) form a functional heterodimer in the cytoplasm involved in cell signaling and proliferation through the kinase mTOR
- Negative regulators of the AKT / mTOR signaling pathway
- Loss of function mutations in TSC1 or TSC2 lead to constitutive mTORC1 activation that is uncoupled from upstream signaling inputs
- Somatic loss of TSC1 or TSC2 tumor suppressor function (Eur Urol 2018;74:483, Am J Surg Pathol 2018;42:911, Am J Surg Pathol 2018;42:1166)
- No known predisposing factors
- Heterogeneous, well defined nodule(s) with small cystic areas (Urology 2018;114:e9)
- Cysts may display thickened irregular walls and septa
- Typically display indolent behavior
- May rarely be locally aggressive or metastasize
- Lymph node (Histopathology 2018;72:1066)
- Liver (Histopathology 2018;72:588)
- Lung (Histopathology 2018;72:588)
- Bone (Hum Pathol 2018;80:65)
- 33 and 65 year old women with loss of function mutations in TSC2 (Am J Surg Pathol 2018;42:911)
- 69 year old woman with metastasis to renal hilar lymph node (Histopathology 2018;72:1066)
Case series:
- 4 cases (3 women, 1 man; ages 27 - 50) including 1 with bone metastases and 1 with locally aggressive disease (Hum Pathol 2018;80:65)
- 6 cases (all women; ages 42 - 66), all with deleterious mutations in either TSC1 or TSC2 (biallelic alterations in 2 cases) (Am J Surg Pathol 2018;42:1166)
- 10 cases (all women; ages 32 - 85) including 1 case with multifocal disease (Am J Surg Pathol 2017;41:1299)
- 10 cases (6 women, 4 men; ages 14 - 35) including 4 cases with multifocal lesions (1 bilateral), 1 case with metastatic disease to the liver and lung and 1 case with history of sickle cell trait (Histopathology 2018;72:588)
- 16 cases, all solitary sporadic neoplasms with indolent behavior occurring in women (ages 31 - 75) (Am J Surg Pathol 2016;40:60)
- Surgery is typically curative without progression of disease (solitary, renal confined lesions)
- Possible role for mTOR inhibition in metastatic cases
- Complete clinical response in a case of metastatic TSC2 mutated tumor using first generation rapamycin analog everolimus (Am J Surg Pathol 2018;42:1166)
- Frequently solitary lesions (occasionally multifocal, rarely bilateral) (Histopathology 2018;72:588)
- Well delineated with a circumscribed pushing border
- Yellow-tan solid nodules typically interspersed with macrocystic spaces
- Occasional cases may be exclusively solid (Am J Surg Pathol 2017;41:1299)
- < 1.0 - 20.5 cm in greatest diameter (Hum Pathol 2018;80:65)
- Variable hemorrhage with associated necrosis
- Unencapsulated
- Solid or compact nested growth of eosinophilic cells admixed with macroscopic cysts or microscopic tubules / cysts (cysts may be focal to absent) (Am J Surg Pathol 2016;40:60)
- Cysts typically lined by a single hobnail layer of neoplastic cells that are often multinucleated
- May display papillary architecture
- Voluminous granular eosinophilic cytoplasm, sometimes condensed centrally with a surrounding rim or more clear or flocculent cytoplasm (solid foci may have less voluminous cytoplasm)
- Finely granular to coarse basophilic intracytoplasmic stippling / inclusions (most cases)
- Densely eosinophilic to purple cytoplasmic globules surrounded by a delicate clear rim, reminiscent of leishmaniasis
- Round to oval nuclei with mild pleomorphism and variably prominent nucleoli
- Scattered binucleation / multinucleation
- Cytoplasmic vacuolization (either macrovesicular or microvesicular) may give areas a clear cell appearance
- Foamy macrophages (common)
- Calcifications or osseous metaplasia (rare) (Hum Pathol 2018;80:65)
- PAX8 (strong, nuclear, diffuse)
- CK20 (74%, diffuse to patchy but may be focal) (Am J Surg Pathol 2017;41:1299)
- AMACR (84%, patchy to focal) (Am J Surg Pathol 2017;41:1299)
- CD10 (79%) (Am J Surg Pathol 2017;41:1299)
- SDHB (retained cytoplasmic expression)
- Fumarate hydratase (intact)
- Vimentin (90%) (Am J Surg Pathol 2017;41:1299)
- Cathepsin K (variable) (Am J Surg Pathol 2018;42:1166, Hum Pathol 2018;80:65)
- MelanA (variable) (Hum Pathol 2018;80:65)
- CK7 (may be focal in 31%) (Am J Surg Pathol 2017;41:1299)
- CD117 (may be focal in 5%) (Am J Surg Pathol 2017;41:1299)
- CAIX (no membranous staining)
- HMB45 (may show scattered positivity) (Hum Pathol 2018;80:65)
- TFE3
- Abundant rough endoplasmic reticulum, accompanied by granular material (Am J Surg Pathol 2016;40:60)
- Frequent somatic loss of function mutations in TSC1 (9q34.13) or TSC2 (16p13) (Eur Urol 2018;74:483, Am J Surg Pathol 2018;42:911, Am J Surg Pathol 2018;42:1166)
- "Second hit" genetic alterations (e.g. loss of heterozygosity or > 1 deleterious mutation) in the same gene (TSC1 or TSC2) are sometimes seen (Eur Urol 2018;74:483, Am J Surg Pathol 2018;42:911, Am J Surg Pathol 2018;42:1166)
- Copy number gain
- 16p13.3 - q23.1 (33 - 67%, including TSC2 locus in 42%) (Am J Surg Pathol 2017;41:1299)
- 7p21.2 - q36.2 (42 - 50%) (Am J Surg Pathol 2017;41:1299)
- Copy number loss
- TSC1 (33%) (Am J Surg Pathol 2017;41:1299)
- Loss of heterozygosity
- 16p11.2 - 1 (75%) (Am J Surg Pathol 2017;41:1299)
- Xq11.1 - 13.1 (75%) (Am J Surg Pathol 2017;41:1299)
- Tuberous sclerosis complex (TSC) associated RCC:
- Clinical diagnosis of tuberous sclerosis complex
- Highly penetrant autosomal dominant genetic disorder with variable expressivity
- Infantile spasms or seizures (most frequent clinical manifestation, Arch Dis Child 2011;96:1020)
- Updated 2012 clinical diagnostic criteria for TSC (Pediatr Neurol 2013;49:243)
- Major features: 1) ≥ 3 angiofibromas or fibrous cephalic plaque; 2) ≥ 2 ungual fibromas; 3) ≥ 3 hypomelanotic macules; 4) shagreen patch; 5) multiple retinal hamartomas; 6) cortical dysplasias including tubers and cerebral white matter radial migration lines; 7) subependymal nodules; 8) subependymal giant cell tumor; 9) cardiac rhabdomyoma; 10) lymphangioleiomyomatosis; 11) ≥ 2 angiomyolipomas
- Minor features: 1) "confetti" skin lesions; 2) ≥ 3 dental enamel pits; 3) ≥ 2 intraoral fibromas; 4) retinal achromic patch; 5) multiple renal cysts; 6) nonrenal hamartomas
- Definite diagnosis: 2 major features or 1 major feature with ≥ 2 minor features
- Possible diagnosis: either 1 major feature or ≥ 2 minor features
- Updated 2012 genetic diagnostic criteria for TSC (Pediatr Neurol 2013;49:243)
- Either a TSC1 or TSC2 pathogenic mutation (i.e. deleterious or loss of function) in DNA from normal tissue (definite diagnosis)
- De novo mutations (69 - 83%, mostly TSC2) (Sci Rep 2017;7:16697, Eur J Pediatr 2002;161:393, Genet Med 2007;9:88)
- Renal cell carcinoma in tuberous sclerosis complex (2 - 4% of patients, Kidney Int 2006;70:1777)
- TSC2 mutations > TSC1 mutations
- May coexist with angiomyolipoma (most common tuberous sclerosis complex renal neoplasm)
- Bilateral or multifocal (± heterogeneous morphologies, synchronous or metachronous)
- Concurrent renal cysts (common, background kidney)
- Lined by cells with prominent eosinophilic cytoplasm and round nuclei with prominent nucleoli
- Prominent cyst formation
- May indicate autosomal dominant polycystic kidney disease
- Corresponds to large deletion extending from TSC2 into adjacent PKD1 gene on chromosome 16p (may require alternative molecular testing for detection)
- Various histologic types
- Granular eosinophilic macrocystic morphology (11%): morphologically similar to sporadic eosinophilic, solid and cystic RCC (Am J Surg Pathol 2014;38:1457, Int J Surg Pathol 2010;18:409)
- Hybrid oncocytic / chromophobe tumor (HOCT) (chromophobe-like or oncocytoma-like) (59%) (Am J Surg Pathol 2014;38:1457, Am J Surg Pathol 2014;38:895)
- Renal angiomyoadenomatous tumor (RAT-like), RCC with angioleiomyoma-like stroma or RCC with a tubulopapillary architecture and clear cytoplasm separated by smooth muscle stroma (30%, Am J Surg Pathol 2014;38:1457, J Pathol Clin Res 2018;4:167)
- Clear cell RCC
- Clinical diagnosis of tuberous sclerosis complex
- RCC postneuroblastoma:
- Prior history of neuroblastoma
- Various subtypes
- Oncocytoid: morphologically similar to sporadic eosinophilic, solid and cystic RCC (Am J Surg Pathol 2016;40:989, Am J Surg Pathol 2018;42:1166, Am J Surg Pathol 1999;23:772)
- Hybrid oncocytic chromophobe tumor (HOCT)
- Clear cell RCC
- Papillary RCC-like
- MiT family translocation RCC
- TSC2 or MTOR mutated sporadic RCC with eosinophilic and vacuolated cytoplasm (Am J Surg Pathol 2019;43:121):
- 7 reported cases (Am J Surg Pathol 2019;43:121)
- Well circumscribed, unencapsulated solitary renal mass
- M = F
- Mean age at presentation, 54 years
- Nested growth pattern with dispersed single cells, extreme vacuolization and thick walled vessels
- CK7- / CK20-
- Cathepsin K+ in most cases
- Inactivating mutations of TSC2 or activating mutations of MTOR
- May be related to eosinophilic, solid and cystic RCC
- 7 reported cases (Am J Surg Pathol 2019;43:121)
- High grade oncocytic tumor (HOT) (Virchows Arch 2018;473:725):
- 14 reported cases (Virchows Arch 2018;473:725)
- F > M
- Median age, 50 years (range, 25 - 73)
- Well circumscribed and unencapsulated
- Predominantly nested growth pattern in a background of loose fibromatous oncocytoma-like stroma
- Entrapped nonneoplastic renal tubules and prominent thick walled vessels at the periphery
- Voluminous, variably granular cytoplasm with large cytoplasmic vacuoles or inclusions of flocculent eosinophilic material
- Distinct cell membranes
- Rounded nuclear contours with conspicuous nucleoli
- CD117+ in most cases
- CK7 and CK20 negative to focally positive
- Cathepsin K+ / CD10+ in most cases
- Molecular characterization limited with no sequencing data available; possibly related to TSC2 or MTOR mutated sporadic RCC with eosinophilic and vacuolated cytoplasm (see above)
- 14 reported cases (Virchows Arch 2018;473:725)
- MiT family translocation RCC:
- Epithelioid angiomyolipoma:
- Papillary RCC, solid variant, oncocytic type:
- SDH deficient RCC:
- Chromophobe RCC, eosinophilic type:
- Clear cell RCC, eosinophilic morphology:
Comment Here
Reference: Eosinophilic, solid and cystic renal cell carcinoma
- FLCN
- SDHB
- TSC1 / 2
- VHL
- Epithelioid angiomyolipoma (EAML) is a distinct rare subtype of angiomyolipoma with unique histological features
- EAML is characterized by the presence of at least 80% epithelioid cells, which is defined by round to polygonal cells with abundant pink to amphophilic to focally clear cytoplasm
- Rare mesenchymal tumor first reported in 1995 (Int J Surg Pathol 1995;2:539)
- In contrast to other angiomyolipomas, EAML have malignant potential
- Presence of at least 80% epithelial component required for EAML diagnosis
- EAML shows positive staining with melanocytic markers, smooth muscle markers and cathepsin K, while negative for PAX8 and cytokeratins
- Pure epithelioid PEComa of the kidney
- Traditionally, the term epithelioid angiomyolipoma is used for tumors in the kidney; at other sites, the term epithelioid PEComa is used
- ICD-O: 8860/1 - angiomyolipoma, epithelioid
- ICD-11
- 2F35 & XH0QR3 - benign neoplasm of urinary organs & angiomyolipoma, epithelioid
- 2B5F.2 & XH0QR3 - sarcoma, not elsewhere classified of other specified sites & angiomyolipoma, epithelioid
- Rare in clinical practice
- Incidence rate among renal tumor is 1% and among renal angiomyolipoma is 4.6 - 7.7% (Mod Pathol 2013;26:1355)
- Mean patient age is 50 years (range: 30 - 80 years) and there is no sex predilection; EAMLs represent 4.6% of all resected angiomyolipomas (Mod Pathol 2013;26:1355)
- Kidney
- Tuberous sclerosis complex (TSC)
- More than half of TSC patients have renal angiomyolipoma
- Mutations in the TSC1 gene on chromosome 9q34 and the TSC2 gene on chromosome 16p13 are identified in both TSC primary disease and sporadic EAML cases
- These proteins interact to form heterodimers with GTPase activity
- Dysfunctional hamartin and tuberin complexes lead to increased RHEB-GTP, activating the mTORC1 pathway, promoting cell growth and vascular smooth muscle differentiation (Nat Rev Neprol 2018;14:704, Nephrol Dial Transplant 2019;34:502, J Pathol 2008;214:387, Urol Case Rep 2022;45:102204)
- TFE3 gene rearrangements
- Rare EAML cases exhibit rearrangements of the TFE3 gene (Am J Surg Pathol 2010;34:1395)
- TFE3 is a transcription factor that regulates gene expression in the transforming growth factor β signaling pathway
- It activates mTOR signaling, promoting tumorigenesis
- EAML with TFE3 rearrangement is considered a unique subtype of PEComa (Oncol Clin Pract 2020;16:22, Am J Surg Pathol 2010;34:1395, J Pathol 2008;214:387, Clin Neprol Case Stud 2018;6:11)
- Genetic mutations in EAML: TSC2 is a common pathogenic factor, while TP53, ATRX and RB1 mutations are frequently noted in malignant cases (J Urol 2021;206:e708)
- Patients with small EAML may be asymptomatic but larger tumors tend to be symptomatic, often found during physical examination
- Depending on the size and location, it may present with fatigue, fever, low back pain, abdominal pain, hematuria, bleeding, dysuria and renal dysfunction; renal rupture may rarely occur (Urol Case Rep 2021;38:101645)
- It is known to cause paraneoplastic syndromes like high erythrocyte sedimentation rate (ESR), high platelet count and disruption of the immune system (J Int Med Res 2021;49:3000605211032493, Kaohsiung J Med Sci 2022;38:925)
- Imaging: CT scan, MRI (World J Surg Oncol 2015;13:280, Asian J Surg 2020;43:967)
- Biopsy
- Histopathological examination and immunostaining
- Genetic testing
- Ultrasound: lack of specific findings (Abdom Radiol (NY) 2018;43:880)
- Computed tomography (CT) scan
- Shows irregular mixed density mass shadows (usually > 45 HU) with heterogeneous enhancement, "fast in and fast out" type on contrast enhanced CT scan (Abdom Imaging 2014;39:588, World J Surg Oncol 2015;13:280)
- Features suggestive of malignancy: large tumor size > 9 cm, necrosis, metastases, extrarenal extension, formation of tumor thrombus in a vein (Sci Rep 2015;5:10030)
- Magnetic resonance imaging (MRI): T2 hypointense reticular enhancement and "fast in and fast out" attributes (Kaohsiung J Med Sci 2022;38:925)
- Unlike conventional angiomyolipomas, EAML may have a reduced amount of fat within the tumor tissue; this can make it more challenging to differentiate from other kidney tumors using imaging techniques like CT scans and MRIs
- EAML has the potential to be malignant, recur and metastasize; however, the observed variation in its aggressive behavior, ranging from 5 - 66%, should be considered (Am J Surg Pathol 2010;34:715, Am J Surg Pathol 2011;35:161, Clin Neprol Case Stud 2018;6:11, Urol Case Rep 2018;18:52)
- The following factors have been correlated with poor prognosis: epithelial nuclear atypia ≥ 70%, mitotic count ≥ 2 per 10 high power fields, atypical mitoses, necrosis, epithelial cells > 70% or atypical epithelial cells > 60% (Am J Surg Pathol 2010;34:715, Sci Rep 2015;5:10030)
- Risk stratification model, based on prognostic parameters (presence of tuberous sclerosis complex, tumor necrosis, extrarenal extension or renal vein invasion, carcinoma-like histology and tumor size > 7.0 cm), classifies tumors into 3 distinct categories (Am J Surg Pathol 2011;35:161)
- Low risk (0 - 1 parameter)
- Intermediate risk (2 - 3 parameters)
- High risk (≥ 4 parameters)
- For patients possessing at least 3 adverse parameters, a substantial 80% experienced disease progression (Am J Surg Pathol 2011;35:161)
- Recent risk model has been suggested using different parameters (pT3 - pT4 stage, presence of necrosis, severe nuclear atypia, presence of atypical mitoses, mitotic count ≥ 2, Ki67 ≥ 10% and negative SMA expression) (BMC Urol 2022;22:148)
- Mutant p53 expression may be an adverse prognostic indicator for renal EAML (Diagn Pathol 2023;18:14)
- 12 year old boy presented with episodic mild to moderate pain in the left flank for 1 month (J Kidney Cancer VHL 2021;8:20)
- 34 year old woman was complaining of acute left flank pain (Oncol Lett 2023;26:409)
- 40 year old man with a history of tuberous sclerosis presented with 5 months of painless gross hematuria (Urol Case Rep 2022;45:102204)
- 40 year old woman presented with complaints of right abdominal pain with tumor thrombus in inferior vena cava (IVC) and right atrium (Autops Case Rep 2020;10:e2020190)
- 57 year old man with lung effusion and 2 lung nodules (J Kidney Cancer VHL 2022;9:13)
- Solid renal lesions > 4 cm should be treated promptly (Onco Targets Ther 2014;7:823)
- Surgical treatment: mainstay is surgical resection
- Radical nephrectomy for tumors ≥ 4 cm; partial nephrectomy for tumors < 4 cm
- Intraoperative frozen pathological examination aids differentiation from renal cancer
- Interventional therapies (selective arterial embolization [SAE], ablation) for hemorrhage or surgery intolerance are considered when renal AML is > 4 cm or clinical symptoms are present (Asian J Surg 2020;43:967, Ann Transl Med 2019;7:766)
- Medical therapy: for noncandidate patients for surgery
- mTOR Inhibitors (e.g., rapamycin, everolimus) target TSC1 / TSC2 gene mutations but resistance and pathway changes can occur
- Combination therapies (e.g., mTOR inhibitors with exemestane) effective for specific mutations (Clin Cancer Res 2020;26:5534)
- Angiogenesis inhibitors (bevacizumab, pizopanib, sorafenib) for TFE3 associated EAML (Front Oncol 2021;11:641376, Front Oncol 2020;10:582087)
- PD-1 antibodies enhance T cell infiltration, inhibit tumor growth and are effective in EAML with TSC gene mutation (Ann Transl Med 2019;7:766, J Immunother Cancer 2018;6:97, Kaohsiung J Med Sci 2022;38:925)
- Large nodular or irregularly infiltrative in gross view, soft in texture, heterogeneous, partially cystic appearance with a grayish tan to grayish brown cut surface
- Hemorrhage and necrosis may be seen
- Potential extension into the renal vein or IVC may also occur
- EAML could involve lymph nodes; however, in some cases, it does not represent metastatic disease (Am J Surg Pathol 2010;34:715, Am J Surg Pathol 2011;35:161, Transl Androl Urol 2021;10:1506)
- Usually sheets of polygonal to plump spindle cells with abundant pink, granular cytoplasm
- Significant nuclear pleomorphism with nuclear inclusions and nucleoli
- Areas with admixed adipocytes and abnormal vessels help in the diagnosis
- References: Appl Immunohistochem Mol Morphol 2004;12:277, Int J Clin Exp Med 2015;8:21252
- According to WHO, more than 80% epithelial cells is required to diagnose EAML
- Malignant features on core needle biopsy: epithelial nuclear atypia ≥ 70%, mitotic count ≥ 2 per 10 high power fields, atypical mitoses, necrosis, epithelial cells > 70% or atypical epithelial cells > 60% (Kaohsiung J Med Sci 2022;38:925, Am J Surg Pathol 2010;34:715, Sci Rep 2015;5:10030)
- 2 distinct histologic patterns were described as follows
- Carcinoma-like growth pattern
- This pattern is characterized by the presence of large, polygonal cells with densely eosinophilic cytoplasm, diffusely atypical nuclei and prominent nucleoli
- These cells are arranged in cohesive nests, broad alveoli and compartmentalized sheets that are separated by thin vascular septa
- In some cases, intranuclear inclusions, nuclear degeneration and multinucleation are observed
- Mitotic activity is variable, with some cases showing > 2 mitotic figures per high power field (HPF), while the majority do not exhibit mitosis; atypical mitosis is not observed
- Hemorrhage and necrosis may be present to varying degrees
- Combination of prominent nucleoli, intranuclear inclusions and large, discohesive cells with eosinophilic cytoplasm gives the tumor cells a ganglion cell-like appearance
- Epithelioid and plump spindled cells in diffuse growth
- This pattern consists of epithelioid and plump spindled cells that are densely packed in diffuse sheets with homogenous growth without separation by vascular septa unlike the carcinoma-like pattern
- Cells are relatively uniform with epithelioid features, clear to granular, feathery eosinophilic cytoplasm and may lack atypia throughout; nuclei exhibit vesicular chromatin, prominent nucleoli, with intranuclear inclusion being less commonly observed
- Multinucleated giant cells may be present, either singly or in groups, areas of hemorrhage are often absent in this pattern (Am J Surg Pathol 2011;35:161)
- Carcinoma-like growth pattern
- *Besides those 2 patterns, morphology of a papillary component has been described; the papillae are by epithelioid cells containing abundant eosinophilic cytoplasm and round nuclei with conspicuous eosinophilic macronucleoli and perinucleolar halos (Int J Surg Pathol 2023 May 9 [Epub ahead of print])
- Round to plump spindle cells with indistinct cell borders
- Displaced eccentric nuclei with a voluminous, frothy cytoplasm and occasional nuclear pseudoinclusions (Diagn Cytopathol 2000;23:192, J Am Soc Cytopathol 2023;12:142)
- HMB45, HMB50, MelanA, microphthalmia transcription factor (MITF), NKI-C3, SMA (variable), tyrosinase (Histopathology 2018;72:441, Kaohsiung J Med Sci 2022;38:925)
- Cathepsin K, expressed in all PEComas (Am J Surg Pathol 2018;42:1370)
- PNL2 has a higher sensitivity for differentiating PEComa from other renal tumors (85% versus 81%) and has specificity in malignant PEComa (89% versus 81%) (Histopathology 2018;72:441)
- Ki67 ≥ 10% correlates significantly with malignancy (BMC Urol 2022;22:148)
- GPNMB (J Pathol 2022;257:158)
- Cytokeratin, S100, PAX8
- Parvalbumin, unlike in other PEComa (Virchows Arch 2021;478:785)
- Presence of glycogen, mitochondria and prominent electron dense, membrane bound granules (60 - 250 nm)
- Absence of melanosomes or premelanosomes (Am J Surg Pathol 1998;22:663)
- Alteration in mTOR signaling pathway (including TSC2, mTOR and RICTOR) (Am J Surg Pathol 1998;22:663)
- Rarely, TFE3 gene rearrangement has been described in EAMLs (Med Mol Morphol 2012;45:234, Am J Surg Pathol 2016;40:723)
- Alterations of 5 tumor suppressor genes (TP53, ATRX, RB1, APC and NF1) have been reported (Diagn Pathol 2023;18:14, Arch Pathol Lab Med 2023;147:817)
- Kidney, left, radical nephrectomy:
- Epithelioid angiomyolipoma, 6.5 cm, involving renal parenchyma and extending into sinus fat (see comment)
- Surgical margins, negative for tumor
- Comment: Focal necrosis (5%) is present. Immunohistochemical stains show the tumor to be positive for cathepsin K, MelanA and HMB45, supporting the diagnosis.
- Clear cell renal cell carcinoma:
- Chromophobe renal cell carcinoma:
- Oncocytoma:
- Usually, small solid nests of tumor cells but can also show micro and macrocystic architecture
- Abundant granular eosinophilic cytoplasm with uniformly round nuclei with smooth nuclear border
- Can show some degenerative type nuclear atypia
- Positive for PAX8, CD117
- Negative for CK7, HMB45, MelanA and cathepsin K
- MITF translocation associated renal cell carcinoma:
- Renal cell carcinoma, unclassified type with oncocytic features:
- Positive for PAX8 and usually cytokeratins, while negative for cathepsin K, HMB45 and MelanA
- Malignant melanoma:
- Adipocytes and abnormal vessels are not seen in melanoma
- Positive for S100, SOX10, MelanA, HMB45 and PRAME
- Negative for cathepsin K, SMA and cytokeratins
- Epithelioid angiosarcoma:
- Positive for CD31, CD34, ERG or FLI1; may stain for cytokeratins
- Negative for cathepsin K, MelanA and HMB45
- Dedifferentiated liposarcoma:
- Well differentiated liposarcoma component may be identified
- Positive for CDK4, MDM2 by immunohistochemical stain
- CDK4 and MDM2 amplification by FISH
- Negative for HMB45, MelanA and cathepsin K
- Urothelial carcinoma with extensive sarcomatoid differentiation:
- Positive for vimentin, OSCAR cytokeratin, CK7 and PAX8 (variable)
- Eosinophilic solid and cystic RCC (ESC RCC):
- Positive for PAX8 and cytokeratin 20, however, could also express melanA, cathepsin K or HMB45 (Hum Pathol 2018:80:65)
- Usually have substantial cystic component
- WHO Classification of Tumours Editorial Board: Urinary and Male Genital Tumours, 5th Edition, 2022, Investig Clin Urol 2018;59:357, Am J Surg Pathol 2001;25:65, Br J Radiol 2018;91:20170533, Urol Oncol 2022;40:18, Cancers (Basel) 2021;13:2441, Clin Genitourin Cancer 2020;18:e5, Pan Afr Med J 2020;37:210, Kaohsiung J Med Sci 2019;35:33
- Positive for cathepsin K, HMB45, MelanA and MITF
- Positive for PAX8
- Positive for S100, SOX10, HMB45 and MelanA
- Positivity for TFE3 immunohistochemical stain usually correlates with TFE3 gene rearrangement
Comment Here
Reference: Epithelioid angiomyolipoma
A radiological image of 40 year old man complaining of painless gross hematuria and left flank pain showed left renal mass with a central stellate scar measuring 16 x 14 x 11 cm. Left nephrectomy revealed a tumor with morphology depicted in the image above. Necrosis was present in ~30% of tumor cells. Immunohistochemistry showed diffuse strong staining for HMB45, MelanA and focal moderate staining for SMA. The tumor cells showed negative staining for EMA, AE1 / AE3, CK7, S100 and desmin. Which of the following genetic syndromes can be associated with this entity?
- Birt-Hogg-Dubé syndrome
- Succinate dehydrogenase deficiency
- Tuberous sclerosis complex
- Von Hippel-Lindau syndrome
Comment Here
Reference: Epithelioid angiomyolipoma
- Diagnosis requires at least 20% epithelioid cells
- They are a common (> 10%) variant of angiomyolipoma
- Tumors are almost always associated with tuberous sclerosis
- Tumors may have malignant behavior
Comment Here
Reference: Epithelioid angiomyolipoma
- Hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome associated renal cancer may be papillary, solid and infiltrative
- It has characteristic inclusion-like nucleoli with perinucleolar clearing
- Due to germline fumarate hydratase (FH) mutations
- Other common clinical manifestation is nonrenal leiomyomatosis
- Oncogenesis driven by metabolic derangements due to defective fumarate hydratase enzyme
- High grade, papillary, solid or infiltrative with prominent CMV inclusion-like nucleoli with perinucleolar clearing (may be only focal)
- Immunohistochemically shows overexpression of modified cysteine (2SC) and loss of fumarate hydratase
- May be confirmed by germline fumarate hydratase mutation
- HLRCC syndrome has germline mutations of fumarate hydratase gene, conferring an increased risk of uterine and cutaneous leiomyomata as well as renal cancer
- The associated renal cell carcinoma is termed HLRCC associated renal cancer
- Also called FH deficient
- Rare
- HLRCC syndrome has been reported in more than 300 families worldwide (GeneReviews 2006;2015:NBK1252)
- Kidney
- Leiomyomas in uterus and skin in HLRCC syndrome
- Mutations in the Fumarate Hydratase (FH) gene lead to a defective FH enzyme in the citric acid cycle, causing metabolic derangement, "pseudohypoxic" upregulation of hypoxia inducible factor 1 alpha (HIF1a) and nonenzymatic modification of cysteine residues in multiple proteins (succination), altering their function and ultimately driving oncogenesis (Am J Surg Pathol 2014;38:567, Oncogene 2014;33:2547)
- HLRCC associated renal cancer occurs in 1/3 with HLRCC germline mutation
- Mean age of presentation with renal cancer: 36 years (Am J Surg Pathol 2014;38:627)
- Leiomyomas of skin and uterus are the most common features (seen in 85%), usually develop in the 3rd decade
- Uterine leiomyomas are often numerous and large, 50% of women have hysterectomy before age 30 (18 - 52 years)
- Description of clinical and genetic diagnostic criteria for HLRCC syndrome (GeneReviews 2006;NBK1252:)
- HLRCC has a poor prognosis, with early and widespread metastases, even with small tumors
- Decreased fumarate hydratase activity and identification of FH mutation in circulating lymphocytes in 2 young patients with fibroids (J Obstet Gynaecol Res 2013;39:410)
- Development of cutaneous leiomyomas and diagnosis of HLRCC syndrome in pregnancy (J Cutan Med Surg 2016;20:334)
- 59 year old woman with widespread metastases (Am J Surg Pathol 2014;38:567)
- 64 year old man and his 39 year old son without cutaneous leiomyomata (BMC Res Notes 2014;7:203)
- Solitary, unilateral
- Solid or cystic, 2.5 - 12 cm
- High grade with various patterns: papillary, tubular, solid, cribriform, cystic, collecting duct carcinoma-like, sarcomatoid (Am J Surg Pathol 2014;38:627)
- Prominent eosinophilic CMV-like nucleoli with perinucleolar halo (may be only focal)
- May have hyalinized and edematous fibrovascular cores with micropapillary fronds but no foamy histiocytes (Am J Surg Pathol 2014;38:627)
Contributed by Debra Zynger, M.D., Nicole K. Andeen, M.D. and Maria Tretiakova, M.D., Ph.D.
Images hosted on other servers:
- PAX8, vimentin, GLUT1, p53, S100A1, SDHB, INI1 (Am J Surg Pathol 2018;42:279, Histopathology 2018;72:588)
- 2-succino-cysteine / 2SC overexpression (nuclear and cytoplasmic staining), which is more sensitive and specific for FH gene mutation than loss of FH by IHC (Am J Surg Pathol 2016;40:982, Am J Surg Pathol 2015;39:1529)
- Fumarate Hydratase (FH) gene is located on 1q42-43, and consists of 10 exons
- Autosomal dominant inheritance
- In HLRCC, oxidative phosphorylation is impaired, and cells undergo a metabolic shift to aerobic glycolysis (Clin Cancer Res 2013;19:3345)
- Without fumarate hydratase activity, fumarate increases and acts as an oncometabolite
- Fumarate impairs the function of hypoxia inducible factor prolyl hydroxylase, resulting in increased levels of HIF1alpha (WHO 2016)
- Overall, careful examination for characteristic nucleolar features, cytoplasmic and nuclear reactivity for S (2 succino) cysteine (2SC) and loss of fumarate hydratase expression appear to be most useful in the differential diagnosis
- Papillary type 2 RCC: unlike HLRCC, often expresses AMACR, may express CK7 (20%)
- 22% show cytoplasmic reactivity for 2SC, but nuclear staining is less likely to be diffuse compared with HLRCC (Am J Surg Pathol 2014;38:627)
- FH deficient RCC
- Remarkably similar to HLRCC clinically
- Morphologically and immunophenotypically similar, but harbor somatic and not germline mutations (Am J Surg Pathol 2016;40:865)
- Clear cell RCC: has membranous CAIX staining and no 2SC reactivity, unlike HLRCC
- Collecting duct carcinoma: CD10 and 2SC negative
- Microphthalmia transcription factor (MiT) family translocation associated RCC: TFE3 positive, 2SC negative
- Unclassified RCC: 2SC negative (Am J Surg Pathol 2014;38:627)
- The College of American Pathologists (CAP) synoptic protocol for kidney tumors outlines the essential elements that should be included in a pathology report to ensure complete and accurate communication of diagnostic and prognostic information
- A synoptic format is indicated if this is the definitive primary cancer resection specimen
- Procedure type
- Partial nephrectomy
- Can usually be performed if a tumor is ≤ 4 cm, localized to 1 pole of the kidney and without radiologic renal sinus involvement
- Radical nephrectomy
- Includes entire kidney, variable lengths of the major renal vessels at the hilus, variable length of ureter and perirenal fatty tissue
- Partial nephrectomy
- Tumor focality
- Multifocality and multinodularity is a frequent sign of intravenous renal cell carcinoma (RCC) spread with complete filling of the vascular lumina
- As intravenous nodules enlarge, they can merge with the primary tumor, leading them to be falsely included in the measurement of the primary tumor size if the process of venous invasion is not recognized (Mod Pathol 2011;24:1578)
- Recognizing venous involvement is best assessed during gross examination when the kidney is bivalved (see Grossing)
- CD31 immunohistochemical stain can be useful in demonstrating the intravenous nature of the tumor nodules, which may be partially lost as intravenous tumors enlarge and obliterate the endothelial cell lining
- If the tumor is truly multifocal, it is more likely to be associated with a syndrome
- Multifocality and multinodularity is a frequent sign of intravenous renal cell carcinoma (RCC) spread with complete filling of the vascular lumina
- Tumor size
- Greatest dimension in centimeters with cutoffs of 4, 7 and 10 cm
- pT1 and pT2 are categories based on size of tumor when limited to kidney (see Staging)
- pT1: tumor ≤ 7 cm and limited to kidney
- pT1a: tumor ≤ 4 cm, limited to kidney
- pT1b: tumor > 4 cm but ≤ 7 cm, limited to kidney
- pT2: tumor > 7 cm and limited to kidney
- pT2a: tumor > 7 cm but ≤ 10 cm, limited to kidney
- pT2b: tumor > 10 cm, limited to kidney
- pT1: tumor ≤ 7 cm and limited to kidney
- Tumor extent
- Careful gross analysis is key (see Grossing)
- Renal sinus involvement is a main route for extrarenal extension; involvement of the renal sinus predicts a more aggressive outcome than perinephric fat invasion due to increased access to lymphovascular structures in that area (J Urol 2005;174:1218)
- Renal sinus involvement usually present if renal tumor size is > 7 cm
- Multinodularity or finger-like extensions from a renal mass should be viewed with great suspicion for the possibility of vein or renal sinus invasion (Histopathology 2019;74:18)
- If invasion of the renal sinus is unclear, it is recommended that at least 3 blocks of the tumor renal sinus interface is submitted; if invasion is grossly evident or grossly absent, only 1 block is needed to confirm gross inspection (Am J Surg Pathol 2013;37:1505)
- Renal sinus involvement includes tumor in contact with renal sinus fat, tumor present in loose connective tissue of renal sinus beyond the renal parenchyma, tumor involves endothelial lined spaces
- pT3: tumor is locally invasive (see Staging)
- pT3a: involves renal vein or its segmental branches, renal sinus, pelvicalyceal system or perinephric fat; perinephric fat invasion defined as either the tumor touching the fat or extending as irregular tongues into the perinephric tissue (Am J Surg Pathol 2013;37:1505)
- T3b: involves inferior vena cava (below diaphragm)
- T3c: involves inferior vena cava (above diaphragm) or invades the wall of vena cava
- pT4: tumor is invading surrounding organs
- Invades beyond Gerota fascia
- Contiguous spread to ipsilateral adrenal gland
- If there is noncontiguous spread involving adrenal gland, this is considered pM1 disease
- Histologic subtype
- Refer to the most up to date version of WHO for tumor classifications (Pathologica 2022;115:8) (see WHO classification)
- Depending on the subtype, the clinical behavior, prognosis and management can vary widely
- Histologic grade (see Grading)
- Based on a single high power field showing the greatest degree of pleomorphism
- Grade 1 - 3: based on greatest degree of nucleolar prominence (Histopathology 2019;74:4)
- Grade 4: based on nuclear pleomorphism, anaplastic giant cells, rhabdoid morphology and sarcomatoid differentiation (Histol Histopathol 2004;19:113, Histopathology 2019;74:4)
- Tumor necrosis
- Has prognostic implications: tumors with microscopic tumor necrosis behave as tumors with 1 grade level higher (Am J Surg Pathol 2013;37:311)
- If patient has undergone presurgical renal embolization, tumor necrosis cannot be assessed
- Margin status
- In radical nephrectomy specimens, margins include ureter, major vessels (renal vein, renal artery) and soft tissue (Gerota fascia, renal sinus)
- Regional lymph nodes
- Regional lymphadenectomy is not performed in radical nephrectomies; however, a lymph node search during gross examination is recommended with a focus at the renal hilum
- Lymph nodes are found in Am J Surg Pathol 2013;37:1505)
- Additional findings in nonneoplastic kidney
- Examination of nonneoplastic renal parenchyma may reveal medical diseases and shed light on the risk of progressive renal disease in the contralateral kidney and opportunities for medical intervention (Am J Surg Pathol 2006;30:575, Arch Pathol Lab Med 2009;133:1012, Semin Nephrol 2020;40:69)
- Refer to the most up to date version of WHO for tumor classifications (Pathologica 2022;115:8) (see WHO classification)
- Left kidney, radical nephrectomy:
- Clear cell renal cell carcinoma, grade 3 (see synoptic report)
This patient (image shown above) has clear cell renal cell carcinoma with involvement of the adrenal gland. How would you classify this?
- Either pM1 (separate metastatic nodule in the adrenal gland) or pT4 (direct tumor extension to the adrenal gland)
- Neither pM1 (separate metastatic nodule in the adrenal gland) nor pT4 (direct tumor extension to the adrenal gland)
- pM1 (direct tumor extension to the adrenal gland)
- pT4 (separate metastatic nodule in the adrenal gland)
Comment Here
Reference: Kidney tumor - Features to report
Which one of the following options is most likely to be true for the image of a nephrectomy specimen containing renal cell carcinoma shown above?
- pT2a with size of dominant tumor nodule > 7 cm but ≤ 10 cm
- pT2b with aggregate tumor size > 10 cm
- pT3a multifocal tumor with likely retrograde spread via renal vein and its segmental branches
- pT4 tumor invading into Gerota fascia
Comment Here
Reference: Kidney tumor - Features to report
- Very rare pericytic (perivascular) tumor in kidney, with ~30 cases reported in English literature (Case Rep Pathol 2017;2017:7423642, Hum Pathol 2017;64:106)
- Variable proportion of glomus cells, blood vessels and smooth muscle cells
- Glomus cells are uniform, round to ovoid, with abundant cytoplasm, distinctive cell borders and centrically located nuclei
- Glomus cells forming nests or trabeculae and surrounding dilated thin walled vessels
- Subtypes include solid glomus tumor, glomangioma (glomuvenous malformation) and glomangiomyoma
- Other related names: glomangiomatosis, symplastic glomus tumor, glomus tumor of uncertain malignant potential and malignant glomus tumor (glomangiosarcoma)
- ICD-O
- ICD-10: D30.00 - benign neoplasm of unspecified kidney
- ICD-11
- 2F35 & XH47J2 - benign neoplasm of urinary organs & glomus tumor, NOS
- 2C9Y & XH21E6 - other specified malignant neoplasms of urinary tract & glomus tumor, malignant
- M:F = 1.1:1 to 2:1 (its subungual counterpart shows M:F = 1:3) (Case Rep Pathol 2017;2017:7423642, Hum Pathol 2017;64:106)
- Age range: 17 - 81
- Renal primary is very rare
- Most common extrarenal sites are subungual areas
- Uncertain in kidney given the absence of glomus bodies in visceral organs
- In soft tissue counterpart, it resembles the perivascular modified smooth muscle cells of the normal glomus body
- Not clear in renal location
- Sporadic glomus tumor in soft tissue may harbor NOTCH gene rearrangement, BRAF or KRAS mutations (Genes Chromosomes Cancer 2013;52:1075, Am J Dermatopathol 2012;34:533)
- Familial glomus tumor in soft tissue may have glomulin (GLMN) mutations, uniparental disomy of chromosome 1p or NF1 biallelic inactivation (Am J Hum Genet 2002;70:866, Am J Hum Genet 2013;92:188, Cancer Res 2009;69:7393)
- Asymptomatic or nonspecific symptoms, such as vague abdominal pain, flank pain and microscopic hematuria (Case Rep Pathol 2017;2017:7423642)
- Imaging study of renal mass and histological examination
- Ultrasound: isoechoic mass (Int J Urol 2010;17:187)
- Computed tomography (CT): well defined heterogeneous enhancing lesion (Case Rep Pathol 2017;2017:7423642)
- Magnetic resonance imaging (MRI): well circumscribed lobulated mass, isointense to muscle on T1, inhomogeneous high signal on T2 (Open Access Maced J Med Sci 2019;7:4082)
- Positron emission tomography (PET): unevenly increased FDG uptake (Am J Med Sci 2017;353:310)
- Majority of cases in kidney are benign (Case Rep Pathol 2017;2017:7423642)
- Rare cases are reported as glomus tumor of uncertain malignant potential or histologically malignant (Hum Pathol 2010;41:145, Clin Genitourin Cancer 2017;15:e151)
- Only 1 histologically malignant case showed bone metastasis (Hum Pathol 2011;42:1200)
- 31 year old woman with recurrent kidney glomus tumor, 33 year old woman with glomus tumor invading into the renal capsule and 55 year old man with renal glomangiomyoma (Clin Genitourin Cancer 2017 Sep 7 [Epub ahead of print])
- 38 year old woman with glomus tumors involving multiple organs, including bilateral kidneys (Open Access Maced J Med Sci 2019;7:4082)
- 57 year old man with left kidney mass (Case Rep Pathol 2017;2017:7423642)
- 58 year old man with 3 cm right kidney mass (Int J Surg Case Rep 2020;66:178)
- 67 year old man with right kidney mass (Cureus 2021;13:e19479)
- 69 year old woman with right kidney lesion (Urol Case Rep 2024;55:102774)
- Surgical resection
- Well circumscribed and often encapsulated, brownish or white-tan solid mass
- Usually cut surface is homogenous and may show cystic, hemorrhagic or myxoid areas (Arch Pathol Lab Med 2005;129:1172)
- Contains 3 components: glomus cells, blood vessels and smooth muscle cells
- Glomus cells: small and uniform, round to oval shaped, with distinctive cell borders, centrally located punched out nuclei, light eosinophilic or amphophilic cytoplasm (Hum Pathol 2017;64:106)
- Blood vessels: usually thin walled, capillary sized, dilated or branching
- Smooth muscle cells: elongated, mature looking (Arch Pathol Lab Med 2005;129:1172)
- Subtyping depends on the proportion of the 3 components
- Solid glomus tumor
- Glomus cells predominant
- Cuffs of uniform glomus cells forming nests or trabeculae and surrounding thin walled vessels
- Glomangioma
- Vessels predominant
- Cavernous hemangioma-like vasculature surrounded by small clusters or rare layers of glomus cells
- Glomangiomyoma
- Vessels and smooth muscle cells predominate
- Shows gradual transition from glomus cells to smooth muscle cells (Arch Pathol Lab Med 2005;129:1172)
- Solid glomus tumor
- Histological predictors of aggressive behavior (histologically malignant): atypical mitotic figures, moderate to high nuclear atypia, ≥ 5 mitotic figures/50 high power fields (Am J Surg Pathol 2001;25:1)
- Glomus tumor with uncertain malignant potential: does not meet above criteria for histologically malignant but has at least 1 atypical feature other than nuclear pleomorphism, > 2 cm in size and deep location
- Smooth muscle actin (SMA), muscle specific actin (MSA), h-caldesmon, calponin, CD57, vimentin (Int J Surg Pathol 2011;19:393)
- Collagen type IV: pericellular distribution
- CD34: 50% of cases (Hum Pathol 2017;64:106)
- S100: positive in stroma in soft tissue counterpart (J Bone Joint Surg Am 2013;95:725)
- Cells show dense basal lamina and no intracellular mucin, neurosecretory, glycogen or renin granules (Int J Surg Pathol 2011;19:393)
- Glomangiomyoma cells show dense basal lamina and numerous cytoplasmic thin microfilaments with dense bodies (Arch Pathol Lab Med 2005;129:1172)
- Right kidney, partial nephrectomy:
- Benign glomus tumor, 1.5 cm; margins of resection free of involvement (see comment)
- Comment: Clinically, this patient presented with flank pain and microhematuria. CT showed a heterogenous enhancing right renal mass. No other lesions or history of any malignancies are identified. The histological examination shows nests of small uniform tumor cells surrounding dilated capillary sized, thin walled vessels. The tumor cells are round to ovoid, with distinctive cell borders, amphophilic cytoplasm and centrally located nuclei. No mitosis is seen. Immunohistochemistry studies show that the tumor cells are positive for SMA and negative for pancytokeratin, synaptophysin and chromogranin. Collagen IV immunostain shows pericellular positivity. The overall findings are consistent with a benign glomus tumor, likely renal primary.
- Myopericytoma:
- Also belongs to pericytic / perivascular tumors
- Bland, myoid appearing spindle cells showing concentric perivascular growth
- May have PDGFRB mutations
- Juxtaglomerular cell tumor:
- Young patients with severe hypertension, hypokalemia and elevated plasma renin
- Overlapped features with glomus tumor by light microscopy
- Expresses KIT in ~50% of cases, unlike glomus tumor (Hum Pathol 2013;44:47)
- Rhomboid shaped renin crystals on electron microscopy
- Paraganglioma:
- Zellballen / organoid growth pattern
- Sustentacular cells are positive for S100 and chief cells are positive for neuroendocrine markers
- Negative for cytokeratin, SMA and collagen IV
- Misnomers (such as glomus jugulare, glomus tympanicum, etc.) are paragangliomas
- Well differentiated neuroendocrine tumor:
- Nests or organoid architecture, plasmacytoid cells with stippled chromatin
- Positive for cytokeratin and neuroendocrine markers
- Negative for SMA and collagen IV
- Solitary fibrous tumor:
- Fibroblastic spindle cell tumor with patternless pattern and staghorn vasculature
- Positive for STAT6 immunostain
- Angiomyolipoma:
Which of the following describes the histological features of a glomus tumor?
- Fibroblastic tumor with patternless pattern and staghorn vasculature
- Myoid appearing spindle cells showing concentric perivascular growth
- Nests of uniform round cells with distinctive cell borders and centrically located nuclei surrounding the dilated thin walled vessels
- Sustentacular cells and chief cells forming zellballen pattern
- Triphasic tumor with mature adipose tissue, smooth muscle and thick walled vessels
Comment Here
Reference: Glomus tumor
Comment Here
Reference: Glomus tumor
- Grading of renal carcinoma is important for prognosis and is either required by reporting agencies or recommended by professional organizations such as the College of American Pathologists (CAP), International Collaboration on Cancer Reporting (ICCR), Royal College of Pathologists Australasia (RCPA), International Society of Urological Pathology (ISUP), Genitourinary Pathology Society (GUPS) and World Health Organization (WHO)
- Important for prognosis and usually required or recommended by reporting agencies or professional organizations
- WHO / ISUP 4 tier grading system replaces Fuhrman grading system
- Based on the single high power field showing the greatest degree of pleomorphism, rather than the predominant grade
- Replaces Fuhrman grading system (Histopathology 2016;68:475, Pathologe 2016;37:355)
- Based on the single high power field showing the greatest degree of pleomorphism, rather than the predominant grade
- Validated as a prognostic parameter for clear cell and papillary renal cell carcinoma (RCC)
- Value of grading in other RCCs is yet to be determined; in the meantime, the WHO recommends grading most RCCs for descriptive purposes and for future studies
- Grades 1 - 3: degree of nucleolar prominence (Histopathology 2019;74:4)
- Not applicable
- Example: chromophobe RCC
- Grade 1: nucleoli absent or inconspicuous and basophilic at 400x magnification
- Grade 2: nucleoli conspicuous and eosinophilic at 400x magnification, visible but not prominent at 100x magnification
- Grade 3: nucleoli conspicuous and eosinophilic at 100x magnification
- Not applicable
- Grade 4: extreme nuclear pleomorphism, anaplastic giant cells, rhabdoid morphology and sarcomatoid differentiation (Histol Histopathol 2004;19:113, Histopathology 2019;74:4)
- Other factors affecting grade:
- Modified ISUP / WHO grading system has been proposed by GU experts to incorporate microscopic tumor necrosis into grading clear cell RCC (Am J Surg Pathol 2013;37:311)
- Tumors with microscopic tumor necrosis behave as tumors with 1 grade level higher (e.g., there is no difference in cancer specific survival between patients with grade 2 with necrosis and grade 3 without necrosis)
- If the patient has undergone presurgical renal embolization, tumor necrosis cannot be assessed
- Modified ISUP / WHO grading system has been proposed by GU experts to incorporate microscopic tumor necrosis into grading clear cell RCC (Am J Surg Pathol 2013;37:311)
Comparison of historic Fuhrman grading and current WHO / ISUP grading systems for renal cell carcinoma (Am J Surg Pathol 1982;6:655)
| Grading system | |
| Grade 1
| Small, round, uniform (10 um)
| Absent / inconspicuous
| Absent / inconspicuous at 400x
| Grade 2
| Larger (15 um)
| Visible at 400x
| Eosinophilic and visible at 400x
| Grade 3
| Larger, irregular (20 um)
| Visible at 100x
| Eosinophilic and prominent at 100x
| Grade 4
| Pleomorphic, bizarre, giant
| Chromatin clumps
| Pleomorphic, giant, rhabdoid, sarcomatoid
| | |||||||||||
- Chromophobe RCC (chRCC)
- Current WHO recommendation is to not grade chRCC using WHO / ISUP grading system as these tumors have innate nuclear pleomorphism, multinucleation, hyperchromasia and often lack visible nucleoli; thus, current grading does not correlate to outcomes (Virchows Arch 2020;476:409)
- Grading of chRCC is a highly controversial topic and studies are inconclusive
- A novel 3 tiered grading system discounting nuclear atypia was proposed (Am J Surg Pathol 2010;34:1233)
- Tumors with geographic nuclear crowding and anaplasia portend a higher grade
- Most recently, a 2 tiered model to predict the aggressiveness was proposed (Virchows Arch 2020;476:409)
- chRCC with necrosis or sarcomatoid differentiation of chRCC = high grade
- Although chRCC has a favorable prognosis, those that are high grade based on this definition are the ones that tend to progress (if progression were to occur)
- A novel 3 tiered grading system discounting nuclear atypia was proposed (Am J Surg Pathol 2010;34:1233)
- WHO / ISUP grading is not equally significant for all RCC subtypes; the WHO divides them into several categories based on grading value
| # | Categories | Examples |
| 1 | Validated | Clear cell RCC, papillary RCC |
| 2 | Not applicable | Chromophobe RCC, TFE3 rearranged RCC |
| 3 | Potentially useful | SDH deficient RCC, mucinous tubular and spindle cell carcinoma, ELOC mutated RCC, TFEB altered RCC, RCC (NOS), FH deficient RCC (including HLRCC RCC) |
| 4 | Inherently aggressive irrespective of grade | Collecting duct carcinoma, SMARCB1 deficient renal medullary carcinoma |
| 5 | Grading is potentially misleading | Tubulocystic carcinoma, acquired cystic disease associated RCC, eosinophilic solid and cystic RCC, eosinophilic vacuolated tumor |
| 6 | Low grade features are essential for accurate histologic classification | Papillary adenoma, multilocular cystic renal neoplasm of low malignant potential, clear cell papillary renal cell tumor |
| 7 | Neoplasms with limited data on grading or behavior | ALK rearranged RCC, other oncocytic tumors |
- Left kidney, radical nephrectomy:
- Clear cell renal cell carcinoma, grade 2 (see synoptic report)
Comment Here
Reference: Kidney tumor - Grading
- Grade 2: nucleoli absent or inconspicuous at 400x magnification
- Grade 2: nucleoli conspicuous at 400x magnification, visible but not prominent at 100x magnification
- Grade 3: displays sarcomatoid differentiation or rhabdoid morphology
- Grade 4: nucleoli conspicuous at 100x magnification
- Grade 1: nucleoli absent or inconspicuous at 400x magnification
- Grade 2: nucleoli conspicuous at 400x magnification, visible but not prominent at 100x magnification
- Grade 3: nucleoli conspicuous at 100x magnification
- Grade 4: extreme nuclear pleomorphism, anaplastic giant cells, rhabdoid morphology and sarcomatoid differentiation
Comment Here
Reference: Kidney tumor - Grading
- This topic describes how to gross specimens obtained from partial and radical nephrectomy procedures for suspected renal cortical tumors
- It does not describe nephroureterectomy specimens removed for renal pelvic and ureteral tumors
- Essential clinical history: none
- Weigh the specimen
- Measure the overall specimen and renal vein and ureter if present
- Assess the renal vein and margin for presence of tumor
- Measure the distance of tumor to the renal vein margin
- Longitudinally cut open the renal vein and continue cutting the vein branches within the kidney to search for intraluminal tumor
- If tumor is present near or protruding through the renal vein margin, measure the distance of tumor to the renal vein margin
- Partial nephrectomy:
- Examine the parenchymal resection margin and capsular / perirenal margin and note any tumor present
- Ink the entire surface of the renal parenchymal surgical resection margin blue
- Ink the outer surface of the capsule / perinephric adipose margin black
- Make a cut with a long knife perpendicular to the parenchymal margin to bivalve the specimen (i.e. to open like a book)
- Serially section the specimen
- Measure the distance of the tumor to the closest renal parenchymal surgical margin and to the inked outer surface / capsule
- Radical nephrectomy:
- Palpate the specimen to locate the tumor and examine the outer surface for evidence of involvement of Gerota fascia
- Ink the outer surface surrounding the tumor black
- Assess the inked outer surface (Gerota fascia)
- Make a longitudinal cut with a long knife along the long axis of the kidney to bivalve the specimen (i.e. open like a book) either from medial to lateral or vice versa
- Serially section the entire specimen, including perinephric fat, at 1 cm intervals either along the same plane as the first cut creating "pages in a book" (anterior to posterior) or using a "bread loaf" technique (superior to inferior)
- Dictate the pole (upper, mid, lower) in which the tumor is located
- Measure the distance of the nearest tumor to the nearest inked outer surface
- Measure the tumor to 1/10 of a centimeter
- Describe the tumor (color, consistency)
- Assess and document the percentage of necrosis
- For multiple discrete tumors that do not connect to each other and have a similar gross appearance, measure and state the location of at least the largest 3 tumors
- If additional similar tumors are present, provide a range of sizes of these tumors and state which poles of the kidney are involved
- For additional tumors that do not appear grossly similar to the main tumor, measure and state the location
- Assess for areas where the tumor appears to be invading perinephric fat, renal sinus fat or the pelvicaliceal system, as this impacts pT classification, and document this in the gross description
- Measure the distance of the tumor to the nearest perinephric fat, renal sinus fat and pelvicaliceal system if grossly uninvolved
- Assess the nonneoplastic renal parenchyma
- Search the superior perirenal fat for an adrenal gland and serially section if identified
- Examine the adipose tissue for lymph nodes, particularly the perihilar area
- Renal vein with no tumor thrombus, artery and ureter:
- If there is no tumor near the renal vein margin, take shave doughnut margins of ureter, renal vein and artery and place them in 1 cassette
- Renal vein with tumor thrombus, artery and ureter:
- Carefully trim as close as possible to the renal vein margin using scissors and place the trimmings in 1 cassette
- Place the ureter and renal artery in a second cassette
- Take 2 representative cross sections (doughnuts) of the renal vein / branch with thrombus and submit in 2 separate cassettes
- Main tumor, partial nephrectomy:
- Submit 1 block of the tumor per 1 cm of maximum tumor dimension up to 15 blocks maximum
- Include sections that contain tumor to the nearest inked capsule / perirenal adipose tissue, necrosis and adjacent normal parenchyma
- If invasion of perinephric fat, renal sinus fat or pelvicaliceal system is present, submit sections
- If a block of tumor is submitted containing fat, specify if the fat is peripelvic or perirenal in the cassette summary
- Main tumor, radical nephrectomy:
- Representatively submit tumor for a total of 1 block per 1 cm of maximum tumor dimension up to 15 blocks maximum
- About 1/3 of the submitted tumor blocks should be of the tumor to the nearest renal sinus fat
- For areas that are suspicious for gross invasion of peripelvic fat, 1/3 of the tumor blocks should be of tumor to peripelvic fat but at a minimum submit 3 blocks of tumor to peripelvic adipose
- About 1/3 of the submitted tumor blocks should be of the tumor to the nearest perirenal fat
- If the tumor is too far away (greater than 2 cm) from the perirenal or peripelvic fat, submit 1 representative section labeled "perirenal fat closest to tumor" or "peripelvic fat closest to tumor" which will not count as a representative tumor block
- If a block of tumor is submitted containing fat, specify if the fat is peripelvic or perirenal in the cassette summary
- If the inked surface appears to be involved by tumor, submit 2 representative blocks of this area
- If the inked surface is uninvolved, submit 1 block of Gerota fascia with the nearest tumor, which can be counted as part of the representative blocks submitted of the tumor to perirenal adipose
- If the tumor is too far away (greater than 2 cm) from the nearest inked Gerota fascia, submit a representative block labeled "Gerota fascia closest to tumor" which will not count as a representative tumor block
- The remaining 1/3 of the tumor blocks should be of tumor to the nearest pelvicaliceal system (if not present in the peripelvic fat sections), additional sections of tumor (particularly from firm to fleshy gray-white areas suspicious for sarcomatoid differentiation) and representative tumor with necrosis
- Additional tumors:
- For multiple discrete tumors that do not connect to each other and have a similar gross appearance, measure and sample at least the largest 3 tumors
- Additional tumors that do not appear grossly similar to the main tumor should be sampled
- Uninvolved renal parenchyma:
- Take 1 section of normal kidney as far from the tumor as possible and submit in 1 block
- Adrenal gland:
- If the adrenal gland is unremarkable, submit 1 block
- For an adrenal gland that appears to be involved by tumor renal in origin, submit 2 blocks of adrenal gland with tumor, 2 blocks of fat between the adrenal gland and the kidney and 1 block of normal adrenal, in order to differentiate between direct extension of tumor (pT4) versus metastasis (pM1)
- For an adrenal lesion that appears to be adrenal in origin (adrenal cortical adenoma, adrenal cortical hyperplasia), submit 1 block per 1 cm of tumor in addition to 2 blocks of fat between the adrenal gland and the kidney and 1 block of normal adrenal
- Lymph nodes:
- Submit all lymph nodes identified
- Renal vein margin:
- Tumor may protrude several centimeters past the renal vein margin yet the margin may not have adherent tumor and should therefore be described as grossly negative
- Tumor size:
- 4.0, 7.0 and 10.0 cm are size cutoffs for pT classification
- Measurement should include tumor within the perinephric and peripelvic adipose
- Measurement should not include tumor within the renal vein
- Larger tumors frequently have peripelvic fat invasion (more than 90% of clear cell renal cell carcinomas over 7 cm have peripelvic fat invasion)
- Adrenal gland:
- Always note the presence or absence in the dictation
- Tumor submission example:
- If the tumor is 6 cm and does not appear to invade the peripelvic adipose, submit 2 cassettes of tumor with nearest peripelvic fat and pericaval system, 2 cassettes with perirenal fat and 2 additional cassettes of tumor
- Click here for the frozen section procedure topic
Margins:
- Assessment of surgical margins is often performed in partial nephrectomy cases (Urology 2006;67:923, Scand J Urol Nephrol 2005;39:222)
- However, clinical significance may be limited, as only rare patients harbor residual disease in a follow up radical nephrectomy specimen or repeated partial resection of margin
- Recurrence due to positive margin is uncommon (Urology 2011;77:1400, J Urol 2005;173:385)
- Routine use is controversial due to a relatively high false negative rate, controversy over the prognosis of a positive margin and its inconsistent impact on intraoperative management (BJU Int 2015;116:868)
- Other indications (although no consensus exists) are:
- Solid renal mass in a diffusely cystic kidney or other unusual setting
- Synchronous renal and extrarenal masses
- Cystic renal lesions
- Multiple renal masses (Arch Pathol Lab Med 2005;129:1585, J Urol 2008;179:461)
- Cystic and spindle cell tumors are often misdiagnosed at frozen section
- Clear cell renal cell carcinoma can be confused with inflammatory lesions, particularly those containing foamy histiocytes
- Renal cell carcinoma, clear cell type:
- Most common overall and most common to be large with invasion of surrounding adipose
- Yellowish, solid to cystic
- May have hemorrhage and necrosis
- Renal cell carcinoma, papillary type:
- Unencapsulated, tan
- Renal cell carcinoma, clear cell papillary carcinoma:
- Tan, usually cystic
- Renal cell carcinoma, chromophobe type:
- Well circumscribed, tan
- Renal cell carcinoma with sarcomatoid differentiation:
- White, solid
- Oncocytoma:
- Well circumscribed, unencapsulated, mahogany brown
- May have central scar
- Renal medullary interstitial cell tumor:
- Within medulla
- Small (< 2 cm), white
- Partial nephrectomy:
- The specimen is received in a properly labeled container with the patient's name and accession number, designated as "right partial nephrectomy" and consists of a portion of kidney that weighs 150 g and measures 6.2 x 4.3 x 3.1 cm. The renal parenchymal margin is inked blue and the renal capsular margin is inked black. Cut section of the specimen reveals a yellow-white, well circumscribed tumor that measures 4.7 x 3.3 x 2.1 cm. Necrosis is not present. The tumor is 0.3 cm from the renal parenchymal margin. The tumor is 0.5 cm from the inked capsule and perinephric fat. The uninvolved renal parenchyma is unremarkable.
- A1, tumor with nearest renal parenchymal margin (perpendicular); A2, tumor with nearest inked perinephric adipose; A3, firm area of tumor with white discoloration; A4, tumor with hemorrhage; A5, center of tumor; A6, uninvolved renal parenchyma
- Radical nephrectomy:
- The specimen is received in a properly labeled container with the patient's name and accession number, designated as "right nephrectomy" and consists of a kidney and perirenal fat with an attached segment of ureter, renal vein and artery that weighs 350 grams and measures 12.2 x 8.3 x 4.1 cm. The renal vein is 3.2 cm in length by 1.1 cm in diameter. The outer surface surrounding the tumor is inked black. Tumor is not present within the renal vein or its branches. Located in the upper pole is a yellow-tan-white, well circumscribed tumor that measures 6.2 x 5.3 x 4.3 cm. The tumor does not appear to involve the renal sinus adipose or pelvicaliceal system. The tumor does not appear to involve the perinephric adipose. The tumor does not appear to involve the inked outer surface. Nearest tumor is 0.4 cm from the perinephric adipose, 0.3 cm from the renal sinus adipose and 0.6 cm from the inked outer surface. The tumor contains hemorrhage and 10% necrosis. The uninvolved renal parenchyma is unremarkable. The perinephric fat does not contain an adrenal gland. Examination of the renal hilar fat does not reveal lymph nodes.
- A1, margins of ureter, vein and artery (shave); A2-3, tumor with nearest renal sinus fat and pelvicaliceal system; A3, tumor with nearest perinephric fat and inked Gerota fascia; A4-5, tumor with nearest perinephric fat; A6, tumor with necrosis; A7; tumor with firm, white, indurated areas; A8, uninvolved renal parenchyma
Comment Here
Reference: Kidney tumor - Grossing & frozen section
- Very rare, benign tumor in kidney, with fewer than 20 cases reported in English literature (Clin Case Rep 2019;7:2321)
- Renal counterpart of the central nervous system (CNS) hemangioblastoma
- Lipoblast-like epithelioid cells with abundant cytoplasmic microvacuoles
- Complex network of thin walled vessels in arborizing or pericytomatous patterns
- Positive for inhibin, S100, neuron specific enolase (NSE) and vimentin
- Also known as capillary hemangioblastoma
- ICD-O: 9161/1 - hemangioblastoma
- ICD-10: D30.0 - benign neoplasm of kidney
- ICD-11: 2F98 & XH6810 - neoplasms of unknown behavior of urinary organs & hemangioblastoma
- M = F (in contrast to CNS hemangioblastoma, which is male predominant)
- Age range: 16 - 71 (Diagn Pathol 2012;7:49, Am J Surg Pathol 2007;31:1545)
- Sporadic, not associated with von Hippel-Lindau (VHL) disease (different from CNS hemangioblastoma, which is associated with VHL in 25% of cases) (Clin Case Rep 2019;7:2321)
- Extracranial locations include but are not limited to kidney, intestine, orbit, forearm, peritoneum, periadrenal soft tissue, flank and peripheral nerve (Am J Surg Pathol 2014;38:119)
- Unknown at this time
- Mostly asymptomatic (Clin Case Rep 2019;7:2321)
- Occasionally hematuria or abdominal pain
- Radiographic studies showing renal mass
- Histological examination on renal biopsy or resection specimen
- Rare case showing polycythemia (Am J Surg Pathol 2010;34:1695)
- No increase of erythropoietin (EPO)
- Usually unilateral solid renal mass
- Rare case showing multiple renal lesions (Am J Surg Pathol 2014;38:119)
- Rare cases containing cystic spaces (Am J Surg Pathol 2007;31:1545, Am J Surg Pathol 2010;34:1695)
- Benign tumor, no distant metastasis
- Persistent disease after incomplete resection (Am J Surg Pathol 2014;38:119)
- 51 year old woman with right kidney mass (Hum Pathol 2013;44:2247)
- 57 year old woman with right kidney mass (Int J Clin Exp Pathol 2013;6:1953)
- 61 year old man with left kidney mass (Diagn Pathol 2022;17:34)
- 72 year old woman with left kidney mass (Clin Case Rep 2019;7:2321)
- 5 cases of renal hemangioblastomas (J Clin Pathol 2015;68:1020)
- Surgical resection
- Well circumscribed, lobulated, gray to brown, solid
- Sheets, lobulated or nested growth pattern
- Round to oval shaped cells containing microvacuoles in eosinophilic or clear cytoplasm (so called stromal cells), mimicking lipoblasts
- Complex capillary network comprises mainly thin walled vessels
- Some areas show spindle cells surrounding vessels (so called pericytomatous pattern) (Int J Surg Pathol 2012;20:519)
- Focal areas may contain predominant spindle cells with minimal cytoplasm and vague vacuolization (Am J Surg Pathol 2014;38:119)
- Usually mild pleomorphism; however, occasional marked nuclear pleomorphism, rhabdoid features or pseudointranuclear inclusions can be seen (Am J Surg Pathol 2014;38:119, Diagn Pathol 2012;7:39)
- Inhibin
- Vimentin
- S100
- Neuron specific enolase (NSE)
- PAX8: 100% positive (4 out of 4 cases) (Clin Case Rep 2019;7:2321)
- CD10: 67% positive (4 out of 6 cases) (Clin Case Rep 2019;7:2321)
- GLUT1: positive in 70% of extracranial hemangioblastomas (Am J Surg Pathol 2014;38:119)
- CAIX (Int J Clin Exp Pathol 2015;8:2131)
- AE1 / AE3: focal staining (Hum Pathol 2013;44:2247)
- EMA: focal weak staining in about 25% cases (Am J Surg Pathol 2014;38:119)
- Brachyury: positive in CNS hemangioblastoma (cytoplasmic) but not in extracranial tumors
- CD34: highlights the capillary network but negative in the stromal cells
- HMB45
- MelanA
- GFAP
- Myogenin
- Desmin
- Left kidney, partial nephrectomy:
- Hemangioblastoma (see comment)
- Surgical margins negative for tumor
- Comment: Clinically, this patient presented with incidental finding of left kidney mass. No other lesions or history of any malignancies are identified. The histological sections of the renal mass show sheets of round to oval atypical cells with complex capillary network. Some of the atypical cells show abundant microvacuoles within clear cytoplasm. Immunohistochemistry studies show that the atypical cells are positive for inhibin, PAX8, S100 and vimentin and negative for CK8 / 18, HMB45 and MDM2. The overall findings are suggestive of a hemangioblastoma, likely renal primary. Clinical correlation is recommended.
- Renal cell carcinoma, clear cell type:
- Angiomyolipoma / PEComa:
- Atypical lipomatous tumor / well differentiated liposarcoma:
- Adrenal cortical carcinoma:
- Presence of adrenal lesion, involving kidney by direct extension or metastasis
- Lipid microvacuoles in cytoplasm
- Positive for inhibin, MelanA, synaptophysin and calretinin
Which of the following is the most common histological feature of hemangioblastoma?
- Admixture of adipose tissue, smooth muscle and thick walled vessels
- Lobules of mature adipocytes
- Spindle cells with cytoplasmic microvacuoles and complex capillary network
- Storiform fibrosis
- Sustentacular cells wrapped around chief cells, forming zellballen pattern
Comment Here
Reference: Hemangioblastoma
- PAX8+, AE1 / AE3+, inhibin-, HMB45-, S100-
- PAX8+, AE1 / AE3-, inhibin+, HMB45-, S100+
- PAX8-, AE1 / AE3-, inhibin-, HMB45+, S100+
- PAX8-, AE1 / AE3+, inhibin-, HMB45-, S100-
- PAX8-, AE1 / AE3-, inhibin-, HMB45-, S100-
Comment Here
Reference: Hemangioblastoma
- Benign vascular proliferation, rarely diagnosed in kidney (more common in skin or superficial soft tissue)
- Subtypes: capillary, cavernous, mixed, anastomosing
- Benign, circumscribed, unencapsulated proliferation of capillaries with endothelial lining in kidney
- Can appear radiologically similar to renal cell carcinomas, angiosarcomas or other malignancies (Arch Pathol Lab Med 2020;144:240)
- Anastomosing hemangioma: sinusoidal vessels with anastomotic arrangement, hobnail endothelial cells, with or without extramedullary hematopoiesis (Histopathology 2014;65:309)
- Benign, neoplastic vascular proliferation
- M:F = 1.8:1 (Am J Surg Pathol 2010;34:942)
- Pelvis or medullary pyramid, rarely in renal cortex or capsule
- Hemangiomas more commonly found in skin or soft tissues
- Often associated with end stage renal disease (Histopathology 2014;65:309)
- Can be associated with acquired cystic kidney disease, polycythemia or renal cell carcinoma
- Typically asymptomatic but can present with hematuria or flank / abdominal pain
- Often incidentally found on imaging
- May be associated with vascular malformation syndromes, including Klippel-Trenaunay and Sturge-Weber (Virchows Arch 2012;461:669)
- May be diagnosed radiologically but can be difficult to distinguish from renal cell carcinoma or other tumors on imaging
- Diagnosis often made on biopsy or nephrectomy (Radiol Case Rep 2019;14:750)
- Nonspecific echogenicity on ultrasound
- CT: hypoattenuation, arterial enhancement, can be concerning for malignancy
- Difficult to distinguish radiographically from malignant lesions; biopsy or excision often needed for definitive diagnosis (Radiol Case Rep 2019;14:750)
- Excellent prognosis with excision
- 23 year old man with end stage renal disease with isoechoic left kidney mass (Radiol Case Rep 2019;14:750)
- 35 year old woman with end stage renal disease and a renal mass (BMC Nephrol 2021;22:262)
- 40 year old woman with abdominal pain and left kidney mass (Indian J Pathol Microbiol 2020;63:292)
- 47 year old man with end stage renal disease and left kidney mass (Diagn Pathol 2017;12:14)
- 64 year old woman with back pain (Diagn Pathol 2017;12:14)
- May regress spontaneously (J Urol 2002;167:488)
- Variable; can include no treatment, endoscopic ablation or nephrectomy (Am J Case Rep 2017;18:255)
- In healthy patients with minimal symptoms and confirmed diagnosis, observation favored
- Nephrectomy often occurs when difficult to distinguish from carcinoma
- Unilateral, solitary, well circumscribed, red to brown mass
- Most commonly present in papillary tips; can also be found in submucosa, medulla (Am J Surg Pathol 2010;34:942)
- Circumscribed, unencapsulated proliferation of irregular, blood filled vascular spaces lined by a single layer of endothelial cells
- May or may not show lobular growth pattern with large feeding vessels at periphery (Virchows Arch 2012;461:669)
- Multiple subtypes:
- Capillary: more common, characterized by slit-like vascular spaces
- Cavernous: characterized by dilated vessels
- Mixed: features of both capillary and cavernous
- Anastomosing hemangioma (variant of capillary)
- Anastomosing hemangioma (AH):
- Capillary sized sinusoidal vessels with anastomotic arrangement
- Scattered hobnail endothelial cells
- Extramedullary hematopoiesis, fibrin thrombi, extensive perirenal fat entrapment, intravascular growth and mast cells may be seen (Histopathology 2014;65:309)
- Cytologic atypia and mitotic activity rare
- Regressive changes, including hyalinization, cystic changes and fatty overgrowth (Histopathology 2014;65:309)
- Left kidney, radical nephrectomy:
- Anastomosing hemangioma (1.3 cm) (see comment)
- Comment: The tumor is a well circumscribed, low grade vascular neoplasm composed of small capillary channels in an anastomosing pattern. There is no nuclear atypia or significant mitotic activity. In addition, foci of extramedullary hematopoiesis and fibrin thrombi are present. Immunohistochemistry is positive for CD31, supporting the diagnosis of a vascular neoplasm. The morphologic and immunohistochemical profile of the neoplasm is consistent with anastomosing hemangioma.
- Lymphangioma:
- Thin walled cysts filled with homogenous proteinaceous material and delicate fibrous septae
- Positive for D2-40
- Renal cell carcinoma:
- Epithelial proliferation forming acini, tubules and sheets of renal cells within a vascular rich stroma
- Positive for PAX8, cytokeratins and CD10 (Arch Pathol Lab Med 2020;144:240)
- Angiosarcoma:
- Arteriovenous malformation:
- Mixture of benign, variably sized vessels with abundant thrombi
- Thin and thick walled vessels (Arch Pathol Lab Med 2020;144:240, Am J Surg Pathol 2009;33:1364)
- Glomus tumor / glomangioma:
- Very rare in the kidney
- Positive for smooth muscle actin, vimentin, collagen type IV and CD57 (Int J Surg Pathol 2011;19:393)
- Negative for vascular markers such as CD31, CD34, von Willebrand factor and WT1
Comment Here
Reference: Hemangioma
A 42 year old woman with end stage renal disease presents with a renal mass. Excision of the lesion reveals the above vascular lesion. Which of the following is true concerning this lesion?
- Cytologic atypia and mitotic activity is common
- Extramedullary hematopoiesis, fibrin thrombi and mast cells may be present
- Lesion will be positive for cytokeratins and CD10
- Prognosis is poor
Comment Here
Reference: Hemangioma
- Hybrid oncocytic chromophobe tumor (HOCT) is a renal neoplasm with overlapping features of both renal oncocytoma and chromophobe renal cell carcinoma (ChRCC)
- Demonstrates intermediate morphologic and immunophenotypic features between renal oncocytoma and chromophobe RCC (Histol Histopathol 2013;28:1257)
- Classified as a subtype of ChRCC in the 2016 World Health Organization (WHO) Classification but recent molecular data suggest HOCT to be distinct from renal oncocytoma and ChRCC (Mod Pathol 2019;32:1698, Ann Transl Med 2019;7:S356)
- Described in 3 clinical settings: in association with renal oncocytosis, in association with Birt-Hogg-Dubé syndrome and in sporadic cases; recent Genitourinary Pathology Society (GUPS) recommendation for the term hybrid oncocytic tumor to be reserved for hereditary cases (Am J Surg Pathol 1999;23:1094, Am J Surg Pathol 2010;34:620, Am J Surg Pathol 2002;26:1542, Arch Pathol Lab Med 2006;130:1865, Virchows Arch 2010;456:355, Mod Pathol 2021;34:1392)
- Hybrid oncocytic chromophobe tumor - GUPS recommends the term to be reserved for hereditary cases
- Other nonsyndromic tumors with borderlines features could be designated “oncocytic renal neoplasms of low malignant potential, not further classified”
- Typically adults between fourth and eighth decades, slight M > F (Am J Surg Pathol 1999;23:1094, Am J Surg Pathol 2002;26:1542, Am J Surg Pathol 2010;34:620, Virchows Arch 2010;456:355)
- Described in patients with renal oncocytosis, patients with Birt-Hogg-Dubé syndrome and in sporadic cases
- Kidney
- Patients with Birt-Hogg-Dubé syndrome are predisposed to develop fibrofolliculomas, pulmonary cysts leading to increased risk of spontaneous pneumothorax (Am J Surg Pathol 2002;26:1542, Arch Pathol Lab Med 2006;130:1865)
- No specific symptoms in patients with oncocytosis or in sporadic cases (Histol Histopathol 2013;28:1257)
- Sporadic cases - nonspecific symptoms
- Diagnosis by histologic examination of tissue
- CT (J Comput Assist Tomogr 2016;40:513):
- Solid and well circumscribed
- Central stellate hypodensity in 33%, heterogenous appearance 42%
- No calcifications seen
- Mean attenuation values: 25.7 Hounsfield units (HU, noncontrast), 77.4 HU (arterial), 124.8 HU (venous), 76.8 HU (delayed); tumor to cortex ratios for 2 enhanced phases (arterial and venous): 0.56 and 0.79, respectively
- Positive on 99mTc sestamibi SPECT / CT, similar to renal oncocytoma and ChRCC (Eur Urol 2016;69:413)
- Indolent behavior
- Rare reports of distant metastasis and unclassified renal cell carcinoma arising from HOCT (Mod Pathol 2019;32:1698, Int J Surg Pathol 2009;17:158)
- 48 year old woman with multiple renal hybrid oncocytic tumors and Birt-Hogg-Dubé syndrome (Acta Cytol 2006;50:584)
- 48 year old woman with multiple HOCTs and schwannomas (Medicine (Baltimore) 2017;96:e8939)
- 55 year old woman with HOCTs presenting with tumor infection and rupture (Urol Case Rep 2020;33:101304)
- 69 year old woman with 4 cm HOCT (Exp Mol Pathol 2018;105:352)
- Nephrectomy (total or partial) (Am J Surg Pathol 2013;37:1469, Eur Urol 2010;57:661)
- Surveillance may be considered (Eur Urol 2010;57:661)
- Multiple and bilateral tumors in patients with renal oncocytosis or Birt-Hogg-Dubé syndrome, more frequently unifocal / solitary in sporadic cases (Am J Surg Pathol 1999;23:1094, Am J Surg Pathol 2002;26:1542, Virchows Arch 2010;456:355)
- Well circumscribed, nonencapsulated
- Homogeneous tan to brown cut surfaces
- No necrosis
- No gross invasion into adipose tissue or vessels
- 2 general morphologic appearances have been described (Am J Surg Pathol 1999;23:1094, Am J Surg Pathol 2002;26:1542, Eur Urol 2010;57:661, Am J Surg Pathol 2010;34:620, Virchows Arch 2010;456:355, Virchows Arch 2013;462:633, Hum Pathol 2020;104:18)
- Tumors with oncocytic cells with morphologic features between renal oncocytoma and chromophobe renal cell carcinoma
- Nuclei with slight atypia, binucleation, eosinophilic cytoplasm, perinuclear halo / cytoplasmic clearing but no raisinoid nuclei
- More commonly reported in sporadic HOCTs
- Or tumors with more discrete renal oncocytoma-like and chromophobe renal cell carcinoma-like areas (mosaic-like pattern)
- Rare foci with abrupt transition
- More frequently reported in syndromic HOCTs
- Tumors with oncocytic cells with morphologic features between renal oncocytoma and chromophobe renal cell carcinoma
- Arranged in mostly solid alveolar pattern
- Sheets of oncocytic cells with low nuclear / cytoplasmic ratios and uniform round nuclei (Acta Cytol 2006;50:584)
- Tumor cells with clear cytoplasm and slightly larger, irregular nuclei (Acta Cytol 2006;50:584)
- HOCT cells are larger in size with more cytoplasm and less uniform in size and shape than renal oncocytoma (Cancer Cytopathol 2020;128:962)
- Variable amount of oncocytic tumor cells with:
- Hale colloidal iron (apical to diffuse) (Virchows Arch 2013;462:633)
- CK7, AE1 / AE3, parvalbumin, antimitochondrial antigen, EMA, E-cadherin (most), CD117 (Histol Histopathol 2013;28:1257, Virchows Arch 2010;456:355)
- S100A1, CD82 (Pathol Int 2015;65:126)
- Tumor cells with numerous mitochondria of varying sizes (Virchows Arch 2010;456:355)
- Sparse microvesicles with amorphic lamellar content
- Infrequently, small intracytoplasmatic tubuli covered by microvilli may be observed
- Assessment of DNA copy number alterations showed conflicting results:
- Petersson et al.: 14 sporadic HOCTs (Virchows Arch 2010;456:355)
- Monosomy or polysomy in all cases analyzed (most common - chromosome 20 monosomy), distinct from cytogenetic anomalies commonly found in renal oncocytoma and ChRCC
- Poté et al.: 12 HOCTs: 10 sporadic, 2 Birt-Hogg-Dubé cases (Virchows Arch 2013;462:633)
- No chromosome imbalance in 58%
- Chromosome 1 deletions in 33%, similar to renal oncocytoma
- Petersson et al.: 14 sporadic HOCTs (Virchows Arch 2010;456:355)
- Assessment of DNA copy number alterations, as well as mutational and transcriptomic profile compared with renal oncocytoma and ChRCC:
- Ruiz-Cordero et al.: 27 HOCTs: 25 sporadic, 2 Birt-Hogg-Dubé cases (Mod Pathol 2019;32:1698)
- Low mutational frequency and absence of mutations in driver genes that are frequently seen in renal oncocytoma and ChRCC
- Copy number alterations primarily involving losses in chromosomes 1 and X / Y and ~40% lack chromosomal gains or losses - more similar to alteration profile of renal oncocytoma than ChRCC
- Gene expression profile intermediate between renal oncocytoma and ChRCC
- Ruiz-Cordero et al.: 27 HOCTs: 25 sporadic, 2 Birt-Hogg-Dubé cases (Mod Pathol 2019;32:1698)
- Tumor microdissection showed conflicting results:
- Poté et al.: (Virchows Arch 2013;462:633)
- No differences in renal oncocytoma-like and ChRCC-like areas using comparative genomic hybridization
- Pires-Luis et al.: (Exp Mol Pathol 2018;105:352)
- Higher miR-21 expression by microRNA analysis and focal multiple tetrasomies by FISH analysis in renal oncocytoma-like areas and higher relative miR-141 and miR200b expression and no chromosomal alterations in ChRCC-like areas
- Poté et al.: (Virchows Arch 2013;462:633)
- Tumors morphologically predicted to be HOCT were cytogenetically heterogenous and classified into 3 groups: renal oncocytoma (27%) renal oncocytoma variants (46%) and ChRCC (27%) (Hum Pathol 2020;104:18)
- Right kidney, partial nephrectomy:
- Hybrid oncocytic chromophobe tumors, measuring 3.5 and 2.3 cm in greatest dimensions (see comment)
- Surgical margins, negative for tumor
- Comment: The specimens contain a mixture of tumor cells demonstrating morphologic features that overlap with those of renal oncocytoma and chromophobe renal cell carcinoma. Given the patient's clinical history these are consistent with hybrid oncocytic chromophobe tumors associated with Birt-Hogg-Dubé syndrome.
- Chromophobe renal cell carcinoma, eosinophilic variant:
- Oncocytic tumor cells with raisinoid nuclei and koilocytic atypia, including frequent binucleation and perinuclear halos
- Diffuse staining for Hale colloidal iron, CK7 and CD117
- Renal oncocytoma:
- No koilocytic atypia but some degenerative type atypia can be seen
- Negative or only focally positive for Hale colloidal iron and CK7
- Positive for S100A1
- Low grade oncocytic tumors (LOT):
- Provisional entity (Histopathology 2019;75:174, Mod Pathol 2021;34:1167)
- Oncocytic cells with round nuclei, perinuclear halos can be seen
- Archipelagenous cord-like architecture
- Positive for CK7 and negative for CD117
- Clear cell renal cell carcinoma:
- Tumor cells with clear to granular / eosinophilic cytoplasm with intervening vessels
- Diffuse membranous CAIX staining
- MiTF associated renal cell carcinoma:
- Variably morphology; TFEB t6;11 can demonstrate oncocytoma-like areas
- Positive for TFE3 or TFEB and cathepsin K
- Succinate dehydrogenase (SDH) deficient renal cell carcinoma:
- Classic presentation shows flocculent cytoplasmic vacuoles with pale eosinophilic appearance
- Loss of SDHB staining
- Epithelioid angiomyolipoma:
A patient with a history of Birt-Hogg-Dubé syndrome undergoes a partial nephrectomy. Gross examination of the specimen shows multiple well circumscribed tan-brown masses. H&E sections show the above histologic features. What is the likely diagnosis?
- Clear cell renal cell carcinoma
- Epithelioid angiomyolipoma
- Hybrid oncocytic chromophobe tumor
- Succinate dehydrogenase (SDH) deficient renal cell carcinoma
The patient's history of Birt-Hogg-Dubé syndrome and the macroscopic and microscopic findings support the diagnosis of hybrid oncocytic chromophobe tumor. Clear cell renal cell carcinoma has clear to granular tumor cells with intervening blood vessels. Epithelioid angiomyolipoma may contain areas admixed with adipocyte and vessels. Succinate dehydrogenase (SDH) deficient renal cell carcinoma demonstrates flocculent cytoplasmic vacuoles with pale eosinophilic appearance.
Immunohistochemistry can be used to confirm the diagnosis. While hybrid oncocytic chromophobe tumor is positive for CD117, the other choices are negative for CD117. Additionally, clear cell renal cell carcinoma is diffusely positive for CAIX, epithelioid angiomyolipoma is positive for Melan A and HMB45 and SDH deficient renal cell carcinoma shows loss of SDHB staining.
Comment Here
Reference: Hybrid oncocytic chromophobe tumor
- Beckwith-Wiedemann syndrome
- Birt-Hogg-Dubé syndrome
- Tuberous sclerosis
- von Hippel-Lindau
Explanation: A small percentage of kidney tumors are associated with hereditary syndromes. Birt-Hogg-Dubé syndrome is associated with hybrid oncocytic chromophobe tumor. Beckwith-Wiedemann syndrome is associated with Wilms tumor, while von Hippel-Lindau syndrome is associated with renal cell carcinoma. Angiomyolipoma, hereditary papillary renal cell carcinoma and familial renal oncocytoma are seen in patients with tuberous sclerosis.
Comment Here
Reference: Hybrid oncocytic chromophobe tumor
- Rare renin secreting tumor arising from the specialized smooth muscle cells of the glomerular afferent arteriole in the renal juxtaglomerular apparatus
- Occurs mostly in young adults; female predominance
- Frequently associated with severe and poorly controlled hypertension, hypokalemia and hyperaldosteronism secondary to tumor renin secretion
- Almost always benign with single case of metastatic disease
- Sheets of polygonal to spindle cells with well defined cell border and prominent vasculature
- Tumor cells are positive for renin, vimentin, CD34 and CD117
- Reninoma
- ICD-O: 8361/0 - juxtaglomerular tumor
- ~100 cases reported
- Young adults (20 - 35 years) but has been reported in children and older adults (Am J Clin Pathol 2001;116:854)
- F > M (~2:1) (Pediatr Nephrol 1993;7:404, Am J Clin Pathol 2001;116:854)
- Kidney cortex
- 3 clinical categories (Urol Oncol 2010;28:34):
- Typical variant:
- Majority of cases
- Characteristic hypertension, hyperaldosteronism and hypokalemia secondary to hyperreninemia (Clin Sci Mol Med Suppl 1973;45:279s4, J Urol 1973;109:349)
- Hypertension does not respond to medical antihypertensive therapy
- Atypical variant:
- Hypertension with normal potassium levels (Pediatr Nephrol 1993;7:404)
- Nonfunctioning variant:
- Very rare reports of nonfunctioning tumors without hypertension or hypokalemia (Pathol Int 1997;47:393, Case Rep Pathol 2013;2013:973865, Medicine (Baltimore) 2020;99:e22057)
- Typical variant:
- Other symptoms include headache, polyuria, nocturia, dizziness, vomiting (Pediatr Nephrol 1993;7:404, Am J Clin Pathol 2001;116:854)
- Diagnosis is suspected in young patients presenting with severe hypertension and hypokalemia and radiologic findings consistent with renal tumor
- Clinical workup to rule out other causes of hypertension
- Elevated serum renin and aldosterone levels
- Ultrasound: hypoechoic mass (Urology 2003;61:1259, Urol Int 2010;85:121)
- CT (Radiographics 2010;30:1525):
- Hypodense or isodense solid mass
- Dynamic CT shows hypovascular mass in arterial phase despite profuse vascularity
- Moderate enhancement during delayed phase
- MRI (Radiographics 2010;30:1525, Am J Kidney Dis 2019;73:566):
- Variable findings
- Isointensity or hypointensity on T1 weighted images
- Hyperintensity on T2 weighted images
- Renal vein sampling may be useful, especially in small tumors
- Renal arteriography shows no evidence of renal artery stenosis
- 90% of patients will become normotensive following tumor resection
- 10% remain mildly hypertensive despite surgical resection (Am J Clin Pathol 2001;116:854)
- Unlikely to recur after resection
- Almost always benign, with single reported case of metastatic disease to the lung (Am J Surg Pathol 2004;28:1098)
- Single case of death due to massive brain hemorrhage secondary to severe hypertension (Hum Pathol 1974;5:236)
- Single case of juxtaglomerular cell tumor causing fetal demise (Int Urol Nephrol 2011;43:365)
- 16 year old boy with hereditary BRIP1 mutation and spindle cell hemangioma, presenting with juxtaglomerular cell tumor (Genes (Basel) 2021;12:220)
- 22 year old woman with juxtaglomerular cell tumor associated with membranous glomerulonephritis (Ann Diagn Pathol 2003;7:314)
- 29 year old woman with nonfunctioning juxtaglomerular cell tumor mimicking renal cell carcinoma (Medicine (Baltimore) 2020;99:e22057)
- 52 year old man with metastatic juxtaglomerular cell tumor to the lung (Am J Surg Pathol 2004;28:1098)
- 74 year old man with atypical juxtaglomerular cell tumor (Case Rep Pathol 2018;2018:6407360)
- Surgical resection: partial or total nephrectomy
- Unilateral and solitary (Am J Clin Pathol 2001;116:854, Diagn Pathol 2011;6:80)
- Well circumscribed; fully or partially encapsulated
- Gray to yellow-tan, solid cut surface; may have hemorrhage and be partially cystic
- Average: 2 - 3 cm (range: 0.2 - 15 cm)
- Glomoid appearance with sheets of polygonal to spindle cells with well defined cell border (Am J Clin Pathol 2001;116:854, Diagn Pathol 2011;6:80)
- Central, round, uniform nuclei
- Variable amount of clear to eosinophilic cytoplasm
- May demonstrate papillary architecture (Hum Pathol 1993;24:1168)
- Prominent vasculature component consisting of numerous small thin walled vessels and small clusters of thick walled, often hyalinized vessels
- Hemangiopericytoma-like capillaries with branching vessels may be seen
- Scanty, hyalinized or myxoid stroma
- Lymphoplasmacytic infiltrate commonly present
- Entrapped renal tubules in periphery (~50% of cases), sometimes hyperplastic and with papillary pattern
- Mitotic activity, necrosis and pleomorphism are uncommon (Am J Clin Pathol 2001;116:854)
- Rare capsular or vascular invasion (Am J Surg Pathol 2004;28:1098, Nat Clin Pract Nephrol 2008;4:458, Am J Surg Pathol 1994;18:837)
- Sheets of polygonal cells with indistinct cell borders, scant granular cytoplasm and hyperchromatic nuclei, showing mild or no pleomorphism (Int J Surg Pathol 2011;19:93)
- Nuclei with evenly distributed chromatin and occasional noticeable nucleoli
- Background of foamy histiocytes and red blood cells
- Renin: but may also be positive in some cases of Wilms tumor, renal cell carcinoma or renal oncocytoma (Am J Pathol 1987;126:73, Am J Surg Pathol 1994;18:837)
- Vimentin: diffuse (Am J Clin Pathol 2001;116:854, Diagn Pathol 2011;6:80)
- CD34: diffuse (Arch Pathol Lab Med 2006;130:707, Am J Clin Pathol 2001;116:854, Diagn Pathol 2011;6:80)
- CD117: variable (Arch Pathol Lab Med 2006;130:707, Diagn Pathol 2011;6:80)
- SMA, h-caldesmon: variable (Diagn Pathol 2011;6:80, Hum Pathol 2013;44:47)
- PDGFRA: focal to diffuse (Hum Pathol 2013;44:47)
- Beta catenin: nuclear (Hum Pathol 2013;44:47)
- Cytokeratins: AE1 / AE3, CAM 5.2 (highlights entrapped tubules) (Am J Clin Pathol 2001;116:854)
- PAX8 (Int J Surg Pathol 2020;28:87)
- Cathepsin K (Int J Surg Pathol 2020;28:87)
- TFE3 (Int J Surg Pathol 2020;28:87)
- CD31, factor VIII (Am J Clin Pathol 2001;116:854, Diagn Pathol 2011;6:80)
- Neuroendocrine markers: chromogranin, synaptophysin (Am J Surg Pathol 1994;18:837, Am J Clin Pathol 2001;116:854, Diagn Pathol 2011;6:80)
- S100 (Am J Surg Pathol 1994;18:837, Am J Clin Pathol 2001;116:854, Diagn Pathol 2011;6:80)
- HMB45 (Am J Clin Pathol 2001;116:854, Diagn Pathol 2011;6:80)
- Desmin: but can be focally positive (Am J Clin Pathol 2001;116:854, Diagn Pathol 2011;6:80, Hum Pathol 2013;44:47)
- Myogenin (Int J Surg Pathol 2020;28:87)
- CD99, BCL2 (Hum Pathol 2013;44:47)
- Abundant rough endoplasmic reticulum
- Peripherally located rhomboid renin protogranules within the cytoplasm (secretory granules) (Invest Urol 1973;11:65, Pathology 1972;4:193, Ultrastruct Pathol 1997;21:201)
- Loss of chromosomes 9 and 11, as well as aneusomy (Cancer 2005;104:504, Hum Pathol 2008;39:459, Hum Pathol 2013;44:47)
- Whole genome expression analysis (in 2 cases): 415 upregulated genes, including renin and CD117 and 325 downregulated genes (Hum Pathol 2013;44:47)
- Right kidney, mass, partial nephrectomy:
- Juxtaglomerular cell tumor, measuring 2.5 cm in greatest dimension (see comment)
- Surgical margins, negative for tumor
- Comment: The sections show a well circumscribed tumor composed of sheets of uniform round to polygonal cells with clear to slightly eosinophilic cytoplasm and distinct cell borders. Immunohistochemistry was performed and shows diffuse positivity for renin, CD34 and CD117 in the tumor cells. The overall findings support the diagnosis of juxtaglomerular cell tumor.
- Glomus tumor:
- Solitary fibrous tumor / hemangiopericytoma:
- Metanephric adenoma:
- Acinar growth pattern
- Psammoma bodies in the stroma
- PAX8 positive
- Papillary renal cell carcinoma:
- Atypia, pleomorphism
- PAX8 positive and renin negative
- Collecting duct carcinoma:
- Urothelial carcinoma:
- Nuclear hyperchromasia and pleomorphism
- GATA3 positive
- Epithelioid angiomyolipoma:
- Wilms tumor:
- Typically contains blastemal cells with nuclear overlapping
A 25 year old woman presents with uncontrollable hypertension and subsequent workup found a 2 cm left kidney mass. She underwent a partial resection and sections showed the above histologic features. What is the likely diagnosis?
- Glomus tumor
- Juxtaglomerular cell tumor
- Papillary renal cell carcinoma
- Solitary fibrous tumor
Comment Here
Reference: Juxtaglomerular cell tumor
- CD34
- CD99
- PAX8
- STAT6
Comment Here
Reference: Juxtaglomerular cell tumor
- Rare benign mesenchymal tumor of the kidney arising from smooth muscle fibers of the renal capsule, renal pelvis or vascular smooth muscle of renal vessels
- Benign smooth muscle tumor with female predominance
- Arises from smooth muscle fibers of the renal capsule, renal pelvis or vascular smooth muscle of renal vessels
- Bland spindle cells in intersecting fascicles with eosinophilic cytoplasm
- Positive for smooth muscle markers, ER and PR and negative for melanocytic markers, cathepsin K, cytokeratins, CD117, S100 and CD34
- 0.5% in surgically resected renal tumors (Am J Surg Pathol 2016;40:1557)
- 4 - 5.5% in autopsy studies (J Urol 1971;106:503)
- Higher prevalence in middle aged women (Am J Surg Pathol 2016;40:1557)
- Kidney: renal cortex or renal capsule, less commonly in muscularis propria of the renal pelvis and smooth muscle of cortical vessels
- Seen equally in the left and right kidney
- More frequently reported in the lower pole of the kidney (Urol Case Rep 2017;13:3)
- Arises from smooth muscle fibers of the renal capsule, renal pelvis or vascular smooth muscle of renal vessels (J Urol 1990;143:994)
- Unknown
- Most are asymptomatic and found incidentally
- Larger tumors may present as a palpable mass or with flank pain or hematuria, especially if located on the renal pelvis (Pathology 2006;38:454)
- Histologic examination of tissue
- Imaging modalities are not conclusive
- Ultrasound: most are hypoechoic, some with mixed echogenicity (Radiat Med 1993;11:81)
- CT: well circumscribed margins, minimal parenchymal distortion, no evidence of extrarenal invasion (Urol Int 1992;48:219, J Urol 1987;138:853, J Urol 1990;143:994)
- Angiography: hypovascularity or slight vascularity, absence of features indicative of malignancy (e.g., vessel encasement, renal vein invasion, arteriovenous shunting) (Radiat Med 1993;11:81)
- Benign
- Excellent prognosis
- 20 year old woman presenting with large symptomatic renal leiomyoma (Radiol Case Rep 2020;15:515)
- 39 year old man presenting with right flank mass (Urol Case Rep 2017;13:3)
- 79 year old woman with small subcortical renal leiomyoma (Urol Case Rep 2021;39:101792)
- Surveillance or surgical resection depending on the tumor size, location and symptoms
- Solid, well circumscribed, tan to white with whorled and bulging cut surface
- Focal cystic degeneration, hemorrhage or irregular calcifications may be present
- Often solitary but occasionally multiple (Scand J Urol Nephrol 1999;33:138)
- Most are small but the largest reported tumor measured 57.5 cm (Br J Surg 1956;43:497)
- Arranged in intersecting fascicles
- Spindle cells with blunt ended, cigar shaped nuclei and eosinophilic cytoplasm
- Minimal to no nuclear pleomorphism / atypia
- Low mitotic activity
- No necrosis
- Reference: Am J Surg Pathol 2016;40:1557
- Smooth muscle markers: SMA, desmin, h-caldesmon, calponin (Am J Surg Pathol 2016;40:1557)
- ER (87%) and PR (87%) (Am J Surg Pathol 2015;39:349, Am J Surg Pathol 2016;40:1557)
- WT1 (73%) (Am J Surg Pathol 2016;40:1557)
- Cytokeratins (Am J Surg Pathol 2016;40:1557)
- RCC, PAX8, PAX2 (Urol Case Rep 2017;13:3)
- Myogenin, MyoD1 (Am J Surg Pathol 2016;40:1557, Urol Case Rep 2017;13:3)
- CD117 (Am J Surg Pathol 2016;40:1557, Urol Case Rep 2017;13:3)
- S100 (Urol Case Rep 2017;13:3, J Int Med Res 2021;49:3000605211032802, Korean J Urol Oncol 2011;9:130)
- CD34 (Urol Case Rep 2017;13:3, Korean J Urol Oncol 2011;9:130)
- BCL2 (Urol Case Rep 2017;13:3)
- HMB45, although positivity in renal capsular leiomyomas has been reported (Am J Surg Pathol 2015;39:349, Mod Pathol 1996;9:664)
- Cathepsin K (Am J Surg Pathol 2015;39:349)
- Ki67 proliferation rate: low (Am J Surg Pathol 2016;40:1557)
- Breakpoints in the q13-15 region of chromosome 12 (Pediatr Pathol 1993;13:435)
- Single study showed combined losses of chromosomes 4, 6, 12 and 14 (Histol Histopathol 2007;22:883)
- Right kidney, mass, partial nephrectomy:
- Renal leiomyoma, measuring 1.5 cm in greatest dimension (see comment)
- Surgical margins, negative for tumor
- Comment: The sections show a well circumscribed tumor composed of bland spindle cells in intersecting fascicles. Immunohistochemistry was performed to show positivity for SMA, desmin, h-caldesmon, ER and PR. Cytokeratin AE1 / AE3, PAX8, S100, CD117 and CD34 are negative. The overall findings support the above diagnosis of a renal leiomyoma.
- Angiomyolipoma:
- Smooth muscle component with mature adipose tissue and thick walled vessels but could be difficult to distinguish from fat poor (leiomyomatous) variant
- Melanocytic markers (MelanA, HMB45) and cathepsin K positive
- Both smooth muscle markers (SMA) positive
- Leiomyosarcoma:
- Spindle cells with moderate to severe nuclear pleomorphism
- Tumor necrosis and high mitotic activity (with atypical mitoses)
- Infiltrative border
- Sarcomatoid renal cell carcinoma:
- Atypical spindle and giant cells with marked nuclear pleomorphism
- Atypical mitotic figures
- PAX8, cytokeratin positive
- Renomedullary interstitial cell tumor:
- Small, well circumscribed nodules in the renal medulla
- Spindle to stellate cells in basophilic stroma
- Entrapped tubules at the periphery
- Desmin negative
- Mixed epithelial and stromal tumor (MEST):
- Schwannoma:
- Solitary fibrous tumor:
- Synovial sarcoma:
- Monophasic subtype: monotonous spindle cell proliferation with staghorn vessels
- Mast cells commonly seen
- TLE1 positive; desmin, h-caldesmon negative
- Inflammatory myofibroblastic tumor:
- Myofibroblastic proliferation: spindle cells admixed with inflammatory cells, including lymphocytes, plasma cells and eosinophils
- ALK1 (60%) positive
- Gastrointestinal stromal tumor:
A 26 year old woman presents with back pain. CT scan located a 2.8 cm, well circumscribed, subcapsular mass in the right kidney. She underwent partial nephrectomy. Grossly, the mass was tan-white with a whorled and bulging cut surface. Microscopically, it consisted of a low grade, monotonous, spindle cell proliferation with blunt ended, cigar shaped nuclei and eosinophilic cytoplasm. No coagulative necrosis, abnormal vessels or fatty infiltration were noted. What is the most likely diagnosis?
- Angiomyolipoma
- Leiomyoma
- Leiomyosarcoma
- Sarcomatoid renal cell carcinoma
Comment Here
Reference: Leiomyoma
- HMB45+, MelanA+, cathepsin K+, desmin+, caldesmon+
- PAX8+, CK7-, CD117+, CAIX-, vimentin-, AMACR-
- PAX8+, cytokeratins+, vimentin+, CD10+, CK7-, CAIX+ (box-like pattern), S100-
- SMA+, desmin+, ER+, PAX8-, CD34-, cathepsin K-
Comment Here
Reference: Leiomyoma
- Emerging entity currently under the WHO category of other eosinophilic oncocytic tumors with somatic MTOR mutation or TSC1 / TSC2 mutation (Amin: Diagnostic Pathology - Genitourinary, 3rd Edition, 2022)
- Indolent tumor in the category of renal oncocytic neoplasms that also includes renal oncocytoma, eosinophilic chromophobe renal cell carcinoma, oncocytic renal neoplasm of low malignant potential not otherwise classified, hybrid oncocytic tumor and eosinophilic vacuolated tumor (Mod Pathol 2022;35:1140)
- Solid growth of eosinophilic cells with low grade nuclei without prominent nucleoli
- Rule out similar appearing tumors: oncocytoma and eosinophilic chromophobe renal cell carcinoma (RCC)
- Strong CK7 expression, no CD117 / KIT expression
- TSC1, TSC2 or MTOR mutations; deletion 19p, 19q, 1p
- Indolent clinical behavior
- Low grade oncocytic tumor (LOT)
- 4% of cases previously diagnosed as oncocytoma or chromophobe RCC (Mod Pathol 2022;35:1140)
- 6.7% of cases previously diagnosed as unclassified RCC or low grade oncocytic / eosinophilic renal neoplasms (Mod Pathol 2022;35:1140)
- 5.5% of 605 rereviewed oncocytic / eosinophilic renal neoplasms (J Clin Pathol 2024 Jul 20 [Epub ahead of print])
- M:F = 1:1.3 (literature review: Histol Histopathol 2022;37:405)
- Generally older patients but broad age range
- 49 - 78 (mean: 66; n = 28) (Histopathology 2019;75:174)
- 43 - 79 (mean: 63.5; n = 11) (Virchows Arch 2023;483:687)
- 10 - 87 (mean: 63; n = 109) (Histol Histopathol 2022;37:405)
- Single tumor: sporadic
- Rare multiple tumors: associated with end stage renal disease or tuberous sclerosis complex (TSC) (Mod Pathol 2022;35:1140, Hum Pathol 2021;116:1)
- Kidney cortex
- Somatic mutations or biallelic losses in mTOR or TSC1 / TSC2 pathway are considered involved in the pathogenesis of specific eosinophilic tumors, including low grade oncocytic tumor, eosinophilic solid and cystic renal cell carcinoma (ESC RCC), eosinophilic vacuolated tumor (EVT) and RCC with fibromyomatous stroma (FMS)
- Most common molecular alterations in low grade oncocytic tumors
- MTOR > TSC1 > TSC2 in mTOR pathway (Virchows Arch 2023;483:687)
- PIK3CA, NF2, PTEN, outside of mTOR pathway (Virchows Arch 2023;483:687)
- Deletions 19p, 1p, 19q (Histopathology 2019;75:174)
- Incidental discovery on imaging (Amin: Diagnostic Pathology - Genitourinary, 3rd Edition, 2022)
- Indolent clinical behavior, sporadic (Mod Pathol 2022;35:1140, Amin: Diagnostic Pathology - Genitourinary, 3rd Edition, 2022)
- Multifocal and bilateral LOTs could be the first manifestation of clinically silent tuberous sclerosis complex (Hum Pathol 2021;116:1)
- Diagnosis is made readily with histomorphology and immunohistochemistry, in combination with indolent clinical course and well circumscribed mass
- Older patient with small tumor
- Composed of eosinophilic cells with low grade nuclei arranged in solid / nested architecture with demarcated areas of edematous stroma (with boats in a bay oriented tumor cells)
- Characteristic immunoprofile: CK7+, CD117 / KIT- and GATA3+ (Histopathology 2023;82:296)
- Chromophobe RCC ruled out by histomorphology and immunophenotype (CD117 / KIT+, CK7+)
- Oncocytoma ruled out by histomorphology and immunophenotype (CD117 / KIT+, CK7-)
- Additionally important to consider and rule out succinate dehydrogenase (SDH) deficient RCC (Mod Pathol 2022;35:1306)
- Indolent behavior
- 28 cases: 1 - 118 months (mean: 31) patients alive with no disease progression (Histopathology 2019;75:174)
- Indolent, no evidence of progression (42.5 months; range: 0 - 344 months) (Mod Pathol 2022;35:1140)
- 68 year old man with right flank pain and 4.5 cm lesion on CT, CK7 positive and CD117 / KIT negative (abundant mitochondria on EM) (Med J Armed Forces India 2022;78:355)
- 70 year old man with incidental finding of 1.9 cm left kidney nodule on CT, CK7 positive and CD117 / KIT negative (Asian J Surg 2023;46:4580)
- 75 year old woman with 1 cm kidney tumor, CK7 positive and CD117 / KIT negative (Asian J Surg 2024;47:4345)
- Clinical follow up after diagnostic biopsy or resection
- Single, solid, tan-brown, no gross necrosis or cystic change (Histopathology 2019;75:174, Mod Pathol 2022;35:1140)
- 1.1 - 13.5 cm (mean: 3 cm) (Histopathology 2019;75:174)
- 0.9 - 3.1 cm (mean: 2.2 cm) (Virchows Arch 2023;483:687)
- Nonencapsulated, well circumscribed (Histopathology 2019;75:174)
- Solid / compact nested growth with well demarcated areas of edematous stroma featuring loosely arranged tumor cells (Histopathology 2019;75:174)
- Solid / compact nested growth may have transition to tubuloreticular areas (centrally) (Mod Pathol 2022;35:1140)
- Edematous stromal area with boats in a bay arrangement and hemorrhage or cord-like arrangement (Mod Pathol 2022;35:1140, Histol Histopathol 2022;37:405)
- Uniform finely granular oncocytic cytoplasm (Histopathology 2019;75:174)
- Often focal perinuclear halo formation or clearing, which can be prominent in some cases (Histopathology 2019;75:174, Mod Pathol 2022;35:1140)
- Uniform round / oval nuclei without nuclear membrane irregularities
- Nucleoli are typically inconspicuous (usually equivalent to WHO / ISUP grade 2)
- No mitoses, no nuclear pleomorphism or raisinoid nuclei, no coagulative necrosis (Histol Histopathol 2022;37:405)
Contributed by Garrison Pease, M.D. and Maria Tretiakova, M.D., Ph.D.
- CK7, PAX8, AE1 / AE3, E-cadherin, BerEP4, MOC31 (Histopathology 2019;75:174, Virchows Arch 2023;483:687, Mod Pathol 2022;35:1140)
- GATA3 in 94 - 100% cases (Histopathology 2023;82:296, J Clin Pathol 2024 Jul 20 [Epub ahead of print], Virchows Arch 2023;483:687)
- pS6 and p4EBP1 (MTOR pathway activation markers) (Mod Pathol 2022;35:1140)
- CD117 / KIT (rarely focal / weak positivity) (Histol Histopathol 2022;37:405)
- CAIX, CK20, vimentin, CK5/6, p63, HMB45, MelanA, CD15 (Histol Histopathol 2022;37:405)
- CD10, P504S (negative or focally positive) (Histopathology 2019;75:174)
- Cathepsin K, SDH retained, FH retained, FOXI1 negative or low focal (Mod Pathol 2022;35:1140)
- Abundant closely packed cytoplasmic mitochondria (Mod Pathol 2022;35:1140, Med J Armed Forces India 2022;78:355)
- 19p deletion (n = 7), 1p deletion (n = 5), 19q deletion (n = 4) (Histopathology 2019;75:174)
- 17 cases: TSC1 (n = 7), TSC2 (n = 2), MTOR (n = 5), PIK3CA (n = 4) (Histopathology 2023;82:296)
- 11 cases: MTOR (n = 6), TSC1 (n = 2), NOTCH1 and NOTCH4 (n = 1)
- Review of other studies (79 tumors): MTOR (n = 32), TSC1 (n = 21), TSC2 (n = 9), others - PIK3CA, NF2, PTEN (Virchows Arch 2023;483:687)
- Solitary LOT: 19p, 1p and 19q deletions, MTOR, RHEB, TSC1
- Multiple LOTs: TSC1 (Mod Pathol 2022;35:1140)
- Kidney, left, partial nephrectomy:
- Low grade oncocytic renal neoplasm, most consistent with low grade oncocytic tumor (1.9 cm) (see comment)
- Comment: Immunostains demonstrate positive expression of CK7 and no expression of CAIX or CD117 / KIT, in support of the above diagnosis. The margins are negative for tumor.
- Low grade oncocytic tumor is an indolent tumor that can occur in sporadic, tuberous sclerosis complex (TSC) associated or end stage renal disease. Metastases have not been reported. The entity is described in Histopathology 2019;75:174 and Hum Pathol 2021;114:9.
- Approach to work up of low grade oncocytic kidney tumors: Mod Pathol 2022;35:1306
- Series, work up and differential: Histopathology 2019;75:174
- Eosinophilic variant chromophobe renal cell carcinoma:
- Solid growth, prominent cell membranes, perinuclear halos, irregular / raisinoid nuclei
- No loose edematous stromal areas (Histopathology 2019;75:174)
- CD117 / KIT+ / CK7+ usually, sometimes only focal
- Colloidal iron+, FOXI1+ (Mod Pathol 2022;35:1140)
- Oncocytoma:
- Nested, island-like (archipelago) architecture; more tubulocystic growth (Histopathology 2019;75:174)
- Less solid / compact growth, more abundant stroma and central scarring
- No perinuclear halos
- CD117 / KIT+, CK7- usually, sometimes scattered+
- FOXI1+ (Mod Pathol 2022;35:1140)
- SDH deficient RCC:
- Tumor cells with flocculent cytoplasm and cytoplasmic vacuoles
- No perinuclear halos (Histopathology 2019;75:174)
- May also have boats in a bay edematous stromal aspect (Histol Histopathol 2022;37:405)
- CD117 / KIT-, CK7-, SDH- lost expression, AE1 / AE3- usually
- Eosinophilic vacuolated tumor (EVT):
- Tumor cells with voluminous eosinophilic cytoplasm and marked intracytoplasmic vacuoles
- High grade nuclei, prominent nucleoli
- Large vessels at periphery (Mod Pathol 2022;35:1140)
- CD117 / KIT+, CK7- (rare), cathepsin K+
- CK7+, CD117 / KIT+
- CK7+, CD117 / KIT-
- CK7-, CD117 / KIT+
- CK7-, CD117 / KIT-
Comment Here
Reference: Low grade oncocytic tumor (LOT)
The kidney tumor shown above is from an adult patient and is CK7 positive and CD117 / KIT negative. What is the most typical feature associated with this tumor?
- CCDN1 rearrangement, benign clinical behavior
- Frequent MTOR / TSC mutation, indolent clinical behavior
- Multiple tumors suggest association with Birt-Hogg-Dubé syndrome; good prognosis
- Possible history of paragangliomas or succinate dehydrogenase (SDH) deficient gastrointestinal stromal tumor, clinical behavior is related to grade
Comment Here
Reference: Low grade oncocytic tumor (LOT)
- Biphasic neoplasm composed of epithelial and stromal components
- Member of metanephric tumor family including metanephric adenoma (purely epithelial) and metanephric stromal tumor (purely stromal)
- Biphasic neoplasm composed of varying proportions of stromal and epithelial components
- Typically solitary and nonencapsulated
- No nuclear atypia in both components
- Mitotic figures are absent or rare in epithelial component
- Epithelial component is identical to metanephric adenoma
- Stromal / mesenchymal component is identical to metanephric stromal tumor
- Previously known as nephrogenic adenofibroma
- 9013/0 - adenofibroma, NOS
- Rare
- Age range 5 months - 36 years (Am J Surg Pathol 1992;16:325, Am J Surg Pathol 2001;25:433)
- Metanephric adenofibroma occurs in renal cortex and medulla
- Unknown, however, several cases have been reported to harbor BRAF V600E mutations; genomic alterations in BRAF oncogene appear at an early stage in tumorigenesis and are preserved through the tumor progression and therefore are considered as drivers of oncogenesis (Hum Pathol 2015;46:1153, Am J Surg Pathol 2015;39:1301)
- Uncommon renal neoplasm; unknown etiology
- Present with polycythemia, hematuria, hypertension or abdominal pain (Am J Surg Pathol 1992;16:325, Am J Surg Pathol 2001;25:433)
- Associated with papillary renal cell carcinoma and Wilms tumor (Am J Surg Pathol 2001;25:433)
- The diagnosis is made by microscopic examination of resected tissue
- The imaging appearance is nonspecific, with the tumor often resembling Wilms tumor (Radiol Case Rep 2018;13:610)
- Benign
- One case of metanephric adenofibroma in combination with Wilms tumor and renal papillary carcinoma has been reported in literature (Pediatr Dev Pathol 2012;15:65)
- 5 year old boy with incidental finding of an abdominal mass (Radiol Case Rep 2018;13:610)
- 5 year old girl with progressively increasing abdominal swelling for 7 months (APSP J Case Rep 2016;7:37)
- 10 year old girl with a history of recurrent urinary tract infections (Am J Surg Pathol 2015;39:1301)
- 10 year old boy with a history of abdominal pain (Int J Clin Exp Pathol 2015;8:3250)
- 10 year old boy with history of left flank pain following a fall (Can J Urol 2013;20:6737)
- 29 year old woman (Int Braz J Urol 2017;43:563)
- Excision of the tumor is adequate therapy
- Solitary, unencapsulated, vaguely circumscribed / indistinct borders
- Tan / gray / white-yellow cut surface
- Partially cystic
- Reference: Am J Surg Pathol 2001;25:433
- Biphasic tumor composed of varying proportions of stromal and epithelial components
- Epithelial component identical to metanephric adenoma, tightly packed small tubules, papillary structures or glomeruloid structures (blunt short papillae resembling glomeruli)
- Epithelial cells have small round to oval nuclei with delicate chromatin and absent or inconspicuous nucleoli and slight pale cytoplasm; mitotic figures are absent or rare
- Psammoma bodies are common
- Mesenchymal component identical to metanephric stromal tumor, bland spindle and epithelioid / plump cells with thin hyperchromatic nuclei and indistinct cell borders
- Areas of intratumoral angiodysplasia, concentric cuffing of entrapped tubules ("onion skinning") and heterologous differentiation (cartilage, adipose tissue or glia) (Am J Surg Pathol 2001;25:433)
- No vascular invasion
Contributed by Rose Chami, M.D.
- Epithelial component (stains like metanephric adenoma): cytokeratin AE1/AE3, WT1 (nuclear, diffuse), CD57, BRAF V600E
- Stromal component: vimentin, CD34 (commonly), WT1, BRAF V600E (variable)
- References: Hum Pathol 2015;46:1153, Am J Surg Pathol 2015;39:1301
- Epithelial component: EMA, AMACR
- Stromal component: smooth muscle actin, desmin, keratin, S100 protein
- Reference: Am J Surg Pathol 1992;16:325
- BRAF V600E mutation (Hum Pathol 2015;46:1153, Am J Surg Pathol 2015;39:1301)
- Right kidney, partial nephrectomy:
- Metanephric adenofibroma, 3.2 cm, resection margins negative for tumor (see comment)
- Comment: There is a nonencapsulated biphasic renal tumor composed of epithelial and stromal components. There is no nuclear atypia in both components and mitotic figures are rare or absent in the epithelial component. Immunopositive for BRAF V600E.
- Metanephric adenoma:
- Lacks stromal component
- Metanephric stromal tumor:
- Lacks epithelial component
- Congenital mesoblastic nephroma:
- Wilms tumor:
- Particularly epithelial predominant histology, usually encapsulated and mitotically active
- Papillary renal cell carcinoma:
Comment Here
Reference: Metanephric adenofibroma
-
Which of the following is true about the expression of metanephric adenofibroma?
- CD57-
- CK7+
- Desmin+
- WT1+
- Member of the metanephric tumor family, which also includes metanephric adenofibroma and metanephric stromal tumor
- Most tumors have BRAF gene mutation
- Benign tumor composed of cytologically bland embryonic looking epithelium with frequent psammomatous calcifications
- Can be seen in patients from pediatric to older adult age
- Metanephroid renal tumor, nephroblastoma-like adenoma, nephrogenic nephroma
- ICD-O: 8325/0 - metanephric adenoma
- ICD-11: 2F35 & XH0JC7 - benign neoplasm of urinary organs & metanephric adenoma
- Uncommon, usually asymptomatic, incidentally discovered renal neoplasm
- Rare, Age range: 5 - 84 years (mean: ~54) (Oncotarget 2016;8:54096)
- 60% are female
- Renal cortex
- Considered to arise from different forms of maturation arrested embryonal rests, persistent blastema or maturing nephroblastoma due to its resemblance to nephrogenic rests / maturing Wilms tumor (Anticancer Res 2018;38:6663, Am J Surg Pathol 2001;25:1290)
- Recently it has been suggested that at least a subset originates from an adult Wilms tumor (Am J Surg Pathol 2022;46:988)
- Usually discovered incidentally with no presenting symptoms
- If presenting with symptoms, it can demonstrate hematuria, pyrexia, flank pain, abdominal mass and polycythemia (Oncol Lett 2016;11:352)
- Diagnosed by histopathologic examination of excised tissue
- If diagnosis is questionable, immunohistochemistry can differentiate metanephric adenoma from its mimics
- Routine laboratory within normal limits in incidental cases
- Polycythemia might be suggestive of renal tumor
- CT: usually solid and hypovascular and shows prolonged and homogenous mild enhancement (Clin Radiol 2019;74:408.e9)
- Benign course
- Seeding of lymph nodes has been reported (Arch Pathol Lab Med 2005;129:1317)
- 9 year old boy with multifocal tumor (Pathol Int 2009;59:49)
- 15 year old girl with large cystic metanephric adenoma (Urology 2017;101:147)
- 23 year old man with diffuse calcifications in metanephric adenoma (Oncol Lett 2015;10:1816)
- 30 year old woman with passive seeding of hilar lymph node (Arch Pathol Lab Med 2005;129:1317)
- 39 year old woman with partial nephrectomy (Medicine (Baltimore) 2016;95:e3486)
- 54 year old woman with a metanephric adenoma associated with polycythemia (Oncol Lett 2016;11:352)
- Surgical resection is curative
- Most are 3 - 6 cm in diameter although 15 cm tumor has been reported (Urology 2017;101:147)
- Mostly unilateral and unifocal
- Rare cases with cystic change (Urology 2017;101:147)
- Circumscribed, not encapsulated, with pushing border
- Cut surface is tan to grey and can be soft or firm; occasionally, hemorrhage and necrosis can present in larger tumors
- Calcifications are often seen grossly
- Classic appearance is a cellular blue tumor composed of tightly packed tubules, long branching and angulated ducts and abortive glomeruli
- Tumor cells have scant cytoplasm and nuclei are small with no nucleoli
- Mitotic figures are very rare or absent
- Stroma is scant; it can be edematous and occasionally appear scar-like
- Psammomatous calcifications can be abundant (Oncol Lett 2015;10:1816)
- Basophilic cells with cytologically bland nuclei and scant cytoplasm forming tightly packed tubules and occasional psammoma bodies (Diagn Cytopathol 2016;44:263, Acta Cytol 2007;51:468)
- WT1 and CD57 coexpression is a usual finding (Mod Pathol 2015;28:1236)
- BRAF is highly sensitive (Am J Surg Pathol 2015;39:549, Histopathology 2015;66:901)
- PAX8
- Cadherin 17 (Am J Surg Pathol 2015;39:549)
- AMACR (Diagn Pathol 2018;13:54, Mod Pathol 2006;19:218)
- CK7 (can be focal) (Diagn Pathol 2018;13:54)
- CD56, EMA
- Cilia on the luminal side resting on abundant basement membrane (Mod Pathol 1996;9:329)
- BRAF V600E gene mutation is a common finding (Eur Urol 2012;62:917)
- Rare cases with BRAF pV600D or BRAF pK601L gene mutations (Am J Surg Pathol 2015;39:549)
- 1 case of BRAF gene fusions has been reported (Diagn Pathol 2018;13:54)
- 2 cases without BRAF mutations have a t(9;15)(p24;q24) translocation, resulting in a KANK1::NTRK3 gene fusion (Cancer Genet 2017;214:9)
- Right kidney, partial nephrectomy:
- Metanephric adenoma, 3.5 cm in greatest dimension (see comment)
- Surgical margins, negative for tumor
- Comment: The sections show well circumscribed tumor composed of tightly packed tubules. The nuclei are small and devoid of nucleoli. No mitotic activity or necrosis is present. The cytoplasm is scant. Numerous psammomatous calcifications are present. Immunohistochemical stains for WT1 and BRAF are positive.
- Papillary renal cell carcinoma, particularly solid variant:
- Metanephric-like ALK rearranged renal cell carcinoma:
- Morphology is similar to metanephric adenoma with small tubules, some of which are elongated
- Metanephric adenoma-like morphology seen in 1 case only at the periphery, with the rest of the tumor being morphologically heterogeneous
- Vimentin+, PAX8+, CK7+, ALK+, AMACR+, BRAF-, CD57-, WT1- (Mod Pathol 2020;33:2564, Virchows Arch 2020;476:921)
- Nephroblastoma:
- Metastatic thyroid carcinoma, particularly poorly differentiated:
- A 66 year old woman with flank pain is found to have polycythemia. The CT scan shows a 3.8 cm solid, circumscribed and hypovascular renal cortical tumor. Which of the following renal tumors is likely to be associated with this clinical presentation, imaging study and the histomorphology shown above?
- Angiomyolipoma, triphasic
- Clear cell renal cell carcinoma
- Metanephric adenoma
- Nephroblastoma
- Urothelial carcinoma
Comment Here
Reference: Metanephric adenoma
- A renal mass from a 43 year old woman shows a tumor composed of tightly packed basophilic tubules. The cell nuclei are cytologically bland and cytoplasm is scant. Numerous psammomatous calcifications are present. No mitotic activity or necrosis is present. Which of the following is the immunohistochemical profile that should be seen in this tumor?
- PAX8+, WT1+, CK7-, CD57+, BRAF+
- PAX8-, WT1+, CK7-, CD57+, BRAF-
- PAX8+, WT1+, CK7-, CD57+, BRAF-
- PAX8+, WT1-, CK7-, CD57+, BRAF+
- PAX8+, WT1-, CK7+, CD57+, BRAF+
Comment Here
Reference: Metanephric adenoma
- Benign pure mesenchymal renal neoplasm
- Belongs to the group of metanephric tumors
- First described by Argani and Beckwith in 2000 (Am J Surg Pathol 2000;24:917)
- Rare benign purely mesenchymal renal tumor
- Belongs to metanephric tumors
- 67% of patients between 6 months and 10 years (range 2 days to 56 years)
- Multifocal in ~10% of cases; never bilateral in cases reported to date
- Characteristic alternating cellularity resulting in a nodular appearance
- CD34 positive
- Associated with BRAF V600E mutations
- Treated with radical or partial nephrectomy
- Only 1 recurrence and 1 death (related to vascular abnormalities) reported
- Adult mesoblastic nephroma (in the old literature)
- ICD-O: 8935/1 - stromal tumor, NOS
- Rare tumor, < 50 cases reported in the literature (J Pediatr Urol 2020;16:822)
- Age 2 days to 56 years (median 2 years, mean < 4 years)
- 67% between 6 months and 10 years
- 3 adult cases
- M:F = 1.2:1
- Kidney; multifocal in ~10% of cases
- Never bilateral
- Unknown
- Cytogenetic and molecular studies found no evidence that it is linked to Wilms tumor (Cancer Genet 2011;204:340, Am J Surg Pathol 2016;40:719)
- Not known
- Asymptomatic abdominal mass (~53% of patients) (J Pediatr Urol 2020;16:822)
- Always unilateral in reported cases to date
- Abdominal discomfort or pain (~21%)
- Hematuria (17%)
- High blood pressure (~10%)
- No distinguishing imaging features
- Diagnosed as a renal mass, some with cystic and solid components
- Biopsy unhelpful
- No abnormalities
- Benign clinical course
- Only 1 recurrence reported (Urology 2011;78:1411)
- 1 death associated with extrarenal vascular abnormalities (Am J Surg Pathol 2000;24:917)
- 2 year old boy presented with a tumor extending into the bladder (J Urol 2003;169:1095)
- 6 year old boy with a cystic and solid renal mass (J Pediatr Surg 2011;46:e7)
- 15 year old boy with a renal tumor (Urology 2001;58:462)
- 53 year old woman with a renal tumor (J Urol 2002;168:1482)
- Surgery (radical nephrectomy) is regarded as the only mode of treatment necessary
- Some cases treated with partial nephrectomy
- No chemotherapy necessary
- Reference: J Pediatr Urol 2020;16:822
- Centrally located in the renal medulla
- Lobulated, often cystic (~55% of cases)
- Multifocal in ~10% of cases
- 2.5 - 12 cm (median 5 cm)
- Rarely protrudes into renal pelvis (botryoid growth)
- In 5 - 6% of cases, renal artery aneurysms found (Int J Surg Pathol 2011;19:667)
- Well circumscribed and unencapsulated tumor (Am J Surg Pathol 2000;24:917)
- No major infiltration of the renal parenchyma; instead, has a scallop-like border
- Nodular appearance on low power view due to alternating cellularity
- "Onion skin" cuffing around entrapped tubules, which may show:
- Cystic dilatation
- Embryonal hyperplasia
- Intraluminal growth (fibroadenoma-like pattern)
- Vascular changes:
- Angiodysplasia of entrapped arterioles
- Juxtaglomerular cell hyperplasia
- Heterologous elements in ~20% of cases (glial, chondroid but never skeletal muscle)
- CD34 (97% of cases) (J Pediatr Urol 2020;16:822)
- Vimentin (72%)
- Desmin, S100, BCL2, CD117 / KIT, CK, HMB45, PAX8 (J Pediatr Urol 2020;16:822, Appl Immunohistochem Mol Morphol 2018;26:721)
- Rarely positive: SMA, actin, CAM5.2, CD99, EMA, ER / PR, WT1 (Am J Surg Pathol 2016;40:719, Pathol Res Pract 2017;213:863)
- BRAF V600E mutations reported in ~70% of cases (Am J Surg Pathol 2016;40:719, Hum Pathol 2017;60:32, J Pediatr Urol 2020;16:822)
- Kidney, total nephrectomy:
- Metanephric stromal tumor (see comment)
- Comment: Tumor shows a nodular appearance on the low power view due to alternating hypo and hypercellularity of spindle cells. Entrapped renal tubules are surrounded by concentric layers of the same cells ("onion skinning"). There are some blood vessels showing angiodysplasia. The tumor - kidney interface is clearly demarcated with only subtle infiltration. Tumor does not infiltrate the renal sinus or perirenal fat. Tumor cells show strong and diffuse CD34 positivity. Molecular studies show a BRAF V600E mutation. This is a benign tumor, which requires no further treatment.
- Mesoblastic nephroma:
- Up to 3 years of age
- Tumor - kidney border is irregular and infiltrative, with entrapped renal parenchyma
- No nodular appearance
- No vascular changes
- CD34 negative
- No BRAF V600E mutations
- Metanephric adenofibroma:
- Stromal component identical to metanephric stromal tumor
- Epithelial component present (identical to metanephric adenoma)
- Clear cell sarcoma of the kidney:
- Stromal type Wilms tumor:
- Often contains rhabdomyoblasts
- Often associated with nephrogenic rests
- More sampling might reveal epithelial or blastemal elements
A 3.5 year old boy presented with an abdominal mass. Imaging studies revealed a left sided renal mass. Total left nephrectomy was performed. The tumor was composed of hypocellular and hypercellular areas of spindled cells. Which finding is most characteristic for this entity?
- BCOR+
- CD34 positivity of tumor cells
- ETV6-NTRK3 gene fusion
- Infiltrative tumor - kidney border
- It contains a stromal and an epithelial component
- It is associated with BRAF V600E mutations
- It is treated with surgery and chemotherapy
- It never occurs after 3 years of age
- Malignant neoplasm metastatic to kidney, not of primary renal or renal pelvis origin
- Most are carcinomas (80.8%)
- Primary site (BJU Int 2016;117:775)
- Usually lung (43.7%)
- Colorectal (10.6%)
- ENT (6%)
- Breast (5.3%)
- Soft tissue (5.3%)
- Thyroid (5.3%)
- Primary site (BJU Int 2016;117:775)
- Also melanoma, pancreas, ovary, testis
- In surgical pathology based studies, metastasis to kidney is uncommon; usually recognized after the diagnosis of primary tumor and may not be seen until long after that diagnosis
- Epidemiology differs depending on type of cohort data analyzed (radiographic, autopsy or surgical pathology)
- Radiology study showed that the kidney was the 8th most common site of metastases from nonrenal primary malignancies (AJR Am J Roentgenol 2011;197:W680)
- Represents a small proportion of resected / biopsied renal masses (< 1% in one series) (Histopathology 2015;66:587)
- Mean age 56 - 61 years, M:F ratio = 1:1 (BJU Int 2016;117:775, Histopathology 2015;66:587)
- Presents with flank pain (30%), hematuria (16%) and weight loss (12%) (BJU Int 2016;117:775)
- Often solitary (77%), although most later develop more sites of metastasis (BJU Int 2016;117:775)
- 88% diagnosed after primary tumor, 9% concurrent with primary; in 2% the metastasis to kidney preceded diagnosis of primary tumor (Histopathology 2015;66:587)
- In 19%, there is a > 10 year interval between time of diagnosis of primary tumor and diagnosis of kidney metastasis (Histopathology 2015;66:587)
- 37% of patients have no other known metastasis at time of diagnosis (Histopathology 2015;66:587)
- Renal cell carcinoma is the most common recipient of tumor to tumor metastasis in malignant tumors (Urology 1987;30:35)
- Compared with renal primaries, more often solid and endophytic; no difference in tumor size, polar predominance or CT enhancement patterns (AJR Am J Roentgenol 2011;197:W680)
- In a series of 151 patients with tumors metastatic to kidney, median overall survival from time of metastatic diagnosis was 1.1 years and median overall survival from primary tumor diagnosis was 3 years (BJU Int 2016;117:775)
- 21 year old woman with osteosarcoma (Clin Genitourin Cancer 2008;6:124)
- 42 year old man with oral squamous cell carcinoma metastatic to kidney (J Clin Diagn Res 2015;9:TJ01)
- 53 year old woman with cervical metastasis resembling an abscess (Eur J Cancer Care (Engl) 2007;16:526)
- 53 year old and 75 year old men with lung and pancreatic tumors metastatic to angiomyolipoma (Arch Pathol Lab Med 2008;132:1016)
- 65 year old woman with lung adenocarcinoma metastatic to clear cell renal cell carcinoma (Arch Pathol Lab Med 2005;129:e49)
- Patient with breast adenoid cystic carcinoma metastatic to kidney (Hum Pathol 2007;38:1425)
- Parotid gland malignant mixed tumor with initial presentation as solitary kidney tumor (Am J Surg Pathol 2000;24:1159)
- May be treated with ablation or resection
- 30% multiple
- 23% bilateral
- 69% located in cortex (Histopathology 2015;66:587)
- Varies; may mimic urothelial carcinoma with divergent differentiation or unusual primary kidney tumors
- Uncommon to diagnose kidney metastases via cytology (Anal Quant Cytopathol Histpathol 2014;36:345, Nayar: Cytopathology in Oncology, 2013 )
- Other primaries: TTF1, CDX2, GATA3, ER, SF1, calretinin, inhibin, OCT 3 / 4, SALL4, synaptophysin, chromogranin (Adv Anat Pathol 2010;17:377)
- Renal cell carcinoma markers: PAX8 (83% of renal cell carcinomas are positive), EMA (78% of clear cell renal cell carcinomas are positive), CAIX (87% of clear cell renal cell carcinoma are positive), hKIM-1 (human kidney injury molecule-1) (83% of clear cell renal cell carcinoma are positive), PAX2, renal cell carcinoma (Am J Surg Pathol 2011;35:678)
- Molecular testing not currently used consistently; diagnosis is based on history, morphology and IHC
- Depends on metastasis type; H&E and clinical history are best discriminators
- Adrenal cortical carcinoma: SF1 (86% adrenal vs. 0% clear cell renal cell carcinoma (RCC) positive), calretinin (89% adrenal vs. 10% clear cell RCC positive), inhibin (86% adrenal vs. 9% clear cell RCC positive), MelanA (86% adrenal vs. 10% RCC positive) (Am J Surg Pathol 2011;35:678)
- Metastatic carcinoid: nested and insular growth pattern, positive for synaptophysin and chromogranin
- Type 1 papillary renal cell carcinoma: has true fibrovascular cores, foamy macrophages, negative for neuroendocrine markers
- Renal cell carcinoma: usually express PAX8 (83% RCC positive) and CAIX (87% clear cell RCC positive) (Am J Surg Pathol 2011;35:678)
- Urothelial carcinoma: based in renal pelvis, positive for p63, uroplakin, CK20 (variable expression in other metastatic diagnostic considerations)
- Inhibin, calretinin, CD10, PAX2
- MelanA, CK7, RCC, PAX2
- SF1, calretinin, CAIX, PAX8
- SF1, CK7, RCC, CD10
- Uncommon neoplasm composed of variable cystic and solid components with morphologically diverse epithelial and stromal elements
- Classified as part of MEST family, with adult cystic nephroma at opposite end of spectrum, by the 2016 World Health Organization (WHO) classification
- Typically occur in perimenopausal women, often with long term hormonal treatment
- Solitary, well circumscribed tumors, with variable solid and cystic components
- Versus adult cystic nephroma - predominantly cystic tumors with no solid components
- Stroma and epithelium with wide variety of histologic features
- Versus adult cystic nephroma - noncommunicating epithelial-lined cysts separated by fibrous septae
- Mostly benign but rare malignant transformation of epithelial or stromal component
- Stromal cells typically positive for ER / PR
- Renal epithelial and stromal tumor (REST) (Am J Surg Pathol 2007;31:489)
- Cystic hamartoma of the renal pelvis - term not currently recommended (Am J Surg Pathol 1993;17:1169, Semin Diagn Pathol 1998;15:2, Mod Pathol 1999;12:417)
- Leiomyomatous renal hamartoma - term not currently recommended (Eur Urol 1988;14:80)
- Adult mesoblastic nephroma - term not currently recommended (J Urol 1973;110:380, Arch Pathol Lab Med 1990;114:533, Am J Surg Pathol 1993;17:1029, Am J Surg Pathol 1998;22:827)
- ICD-O: 8959/0 - Mixed epithelial and stromal tumor
- F:M = 7:1 (Moch: WHO Classification of Tumours of the Urinary System and Male Genital Organs, 4th Edition, 2016)
- Mean age = 52 years
- Confined to kidney
- Located in renal medulla, with some bulging into renal pelvis, involves both renal cortex and medulla or centered within renal pelvis (Virchows Arch 2004;445:359, Arch Pathol Lab Med 2006;130:80, Am J Surg Pathol 2007;31:489)
- Little is known
- Epithelial and stromal components appear to arise from common cell of origin; same pattern of nonrandom inactivation of X chromosome seen in both components (Am J Surg Pathol 2011;35:1114)
- Possible role of hormones in pathogenesis (Am J Surg Pathol 2000;24:958)
- Often incidentally found
- Others present with abdominal / flank pain, hematuria, urinary tract infection (Am J Surg Pathol 2000;24:958)
- Diagnosis by histologic examination of tissue
- CT (Urology 2008;71:1142, J Comput Assist Tomogr 2010;34:177, Radiographics 2010;30:1541, Abdom Imaging 2012;37:873, J Chin Med Assoc 2016;79:554):
- Cystic with heterogeneous and delayed contrast enhancement of septations and solid components
- Most classified into Bosniak category III or IV
- MRI (BJU Int 2010;105:932):
- Cystic areas: T1 hypointensity and T2 hyperintensity
- Solid areas: T1 hyperintensity and T2 hypointensity
- Mostly benign, with rare local recurrence reported
- Rarely aggressive
- Malignant transformation of either epithelial or stromal component (Hum Pathol 2008;39:463)
- Metastasis of malignant component (Anal Quant Cytopathol Histpathol 2015;37:199)
- 22 year old woman with 25 cm giant tumor (J Clin Diagn Res 2015;9:XD01)
- 27 year old man with tumor extending into inferior vena cava (Case Rep Pathol 2018;2018:8234295)
- 38 year old woman with peritoneal seeding following incomplete resection (Urol Ann 2016;8:114)
- 42 year old woman with thyroid-like follicular renal cell carcinoma arising within MEST (Int J Surg Pathol 2020;28:80)
- 43 year old woman with bilateral and multiple tumors (Mol Clin Oncol 2017;7:1005)
- 61 year old man with malignant tumor and 2 additional malignant epithelial components (Int J Surg Pathol 2018;26:56)
- Nephron sparing surgery (partial nephrectomy), whenever feasible; radical nephrectomy if not possible
- Solitary, rarely bilateral (Urology 2008;71:1142, J Chin Med Assoc 2016;79:554, Mol Clin Oncol 2017;7:1005)
- Unencapsulated but well circumscribed
- Mean size: 9 cm
- Mixture of solid and cystic areas
- Solid areas: firm and white to softer and tan (Am J Surg Pathol 2000;24:958)
- Cystic areas:
- Cysts of different sizes and shape
- Smooth and glistening linings (Virchows Arch 2004;445:359)
- Contain clear serous fluid (Am J Surg Pathol 2007;31:489)
- Combination of diverse stromal and epithelial elements (Am J Surg Pathol 2000;24:958, Virchows Arch 2004;445:359, Am J Surg Pathol 2007;31:489, Arch Pathol Lab Med 2006;130:80, Am J Surg Pathol 2016;40:1538)
- Stroma:
- Paucicellular to hypercellular
- Wide spectrum of morphology:
- Dense and collagenous to edematous and fibrous
- Spindle cells from slender to plump
- Areas may show smooth muscle differentiation or ovarian type stroma with luteinization
- Adipose tissue occasionally seen
- Condensation of spindle cells around epithelial component
- Slit-like to thick walled vessels
- Foamy histiocytes and calcifications
- Epithelium:
- Cysts and glands of varying size and architecture; scattered or clustered
- Lining cells with broad spectrum of morphology: flat, cuboidal, columnar, hobnail, urothelial-like, clear cell, ciliated
- Cytoplasm can be eosinophilic, amphophilic, vacuolated
- Combinations of different epithelial elements commonly seen
- Stroma:
- Versus adult cystic nephroma, which consists of multilocular cysts lined by flat to cuboidal epithelium and separated by fibrous septae
- Minimal cytologic atypia
- Mitoses, necrosis and hemorrhage rare
- Rare cases of malignant transformation reported: undifferentiated sarcoma, synovial sarcoma, sarcoma with rhabdoid differentiation, rhabdomyosarcoma, chondrosarcoma, carcinosarcoma, papillary renal cell carcinoma (Virchows Arch 2001;439:700, Lancet Oncol 2004;5:747, Hum Pathol 2007;38:1432, Hum Pathol 2008;39:463, APMIS 2008;116:1013, Int J Urol 2013;20:448, Int J Surg Pathol 2014;22:266, Int J Surg Pathol 2018;26:56)
- FNA: hypocellular specimen, rare epithelial cells in clusters (Diagn Cytopathol 2014;42:680)
- Stroma:
- SMA: strong and diffuse, 97% (Am J Surg Pathol 2000;24:958, Virchows Arch 2004;445:359, Am J Surg Pathol 2016;40:1538)
- Desmin and caldesmon: more variable, focal to diffuse, 89% and 88%, respectively (Am J Surg Pathol 2000;24:958, Virchows Arch 2004;445:359, Am J Surg Pathol 2016;40:1538)
- ER and PR: 88% and 95%, respectively (Am J Surg Pathol 2000;24:958, Virchows Arch 2004;445:359, Am J Surg Pathol 2016;40:1538)
- CD10: often concentrated around epithelial elements, 90% (Am J Surg Pathol 2016;40:1538)
- Inhibin and calretinin: in ovarian type stroma, particularly in luteinized cells (Am J Surg Pathol 2007;31:489)
- FOXL2: in ovarian type stroma, 90% (Hum Pathol 2014;45:1010)
- Epithelium:
- Cytokeratins
- PAX2, PAX8 (Am J Surg Pathol 2011;35:1264)
- GATA3: coexpression with PAX8, 57% (Am J Surg Pathol 2016;40:1538)
- HMB45
- MelanA
- S100: only positive in adipocytes in stroma when present
- WT1: may show positivity in stromal cells, 26% (Arch Pathol Lab Med 2006;130:80, Am J Surg Pathol 2016;40:1538)
- CD34: may show positivity in pericystic stromal cells, 47% (Am J Surg Pathol 2016;40:1538)
- Spindle cells in stromal component:
- Nonspecific features of fibrocystic cells (Am J Surg Pathol 2000;24:958)
- Myogenic features: cytoplasmic fibrils consistent with myofilaments, subplasmalemmal aggregates of thin filaments
- Characteristics of smooth muscle differentiation: number of pinocytic vesicles and dense plaques, near continuous external lamina and relative paucity of rough endoplasmic reticulum
- Intercellular spaces in stromal component:
- Filled with collagen fibrils
- Similar mRNA expression profile between MEST and adult cystic nephroma may represent opposite ends of same spectrum of tumors (Am J Surg Pathol 2009;33:72)
- Highest differentially expressed gene: insulin-like growth factor 2
- Lowest differentially expressed gene: carbonic anhydrase II
- Lack abnormalities in chromosomes 8, 11 or 17 seen in congenital cellular mesoblastic nephroma (Hum Pathol 2001;32:513)
- CDC73 germ line mutation, associated with hyperparathyroidism jaw tumor syndrome, in family with MESTs (Urology 2019;124:91)
- Right kidney, mass, partial nephrectomy:
- Mixed epithelial and stromal tumor, measuring 2.5 cm in greatest dimension (see comment)
- Surgical margins, negative for tumor
- Comment: The sections show a well circumscribed tumor composed of occasional glands and cysts embedded in a variably cellular stroma. Immunohistochemical stains for ER and PR are positive in the stromal component of the tumor. Overall, the morphologic and immunohistochemical findings support the diagnosis of mixed epithelial and stromal tumor.
- Adult cystic nephroma:
- Part of the MEST family, with MEST at opposite end of spectrum, on basis of comparable clinical features (sex and age distribution), overlapping morphologic findings and similar immunohistochemical profile
- No solid component
- Multilocular cysts lined by flat, hobnail or columnar epithelium
- Fibrous septae with ovarian-like stroma in areas
- Angiomyolipoma with epithelial cysts (Am J Surg Pathol 2006;30:593):
- Renomedullary interstitial cell tumor (Hum Pathol 2018;82:46):
- Metanephric adenofibroma:
- Cystic partially differentiated nephroblastoma:
- Most occur in patients < 24 months old
- Nephroblastematous tissue (e.g. blastema, immature stromal cells, primitive epithelial elements) present
- Mesoblastic nephroma:
- Multilocular cystic neoplasm of low malignant potential:
- Clusters or nests of clear cells present in cystic septa
- No cellular stroma
- Papillary renal cell carcinoma with prominent spindle cell stroma (Ann Diagn Pathol 2020;44:151441):
- Sarcomatoid renal cell carcinoma:
- Infiltrative borders
- Marked cytologic atypia
- Necrosis and mitotic figures frequent
- Synovial sarcoma:
- Most are monophasic type with entrapped renal tubules; biphasic type is rare
- t(X;18)(p11;q11), resulting in SS18-SSX2 gene fusion
A 60 year old woman with hematuria was found to have a 3.5 cm variably cystic and solid renal mass on CT imaging. Partial nephrectomy showed a well circumscribed tumor with the above morphology. The stromal component is positive for ER and PR. What is the likely diagnosis?
- Angiomyolipoma
- Cystic partially differentiated nephroblastoma
- Metanephric adenofibroma
- Mixed epithelial and stromal tumor
- Multilocular cystic neoplasm of low malignant potential
Mixed epithelial and stromal tumor is a well circumscribed, variably solid and cystic mass. Cystic partially differentiated nephroblastoma and metanephric adenofibroma typically occur in children. Multilocular cystic neoplasm of low malignant potential does not have a cellular stroma. None of the above choices, except for mixed epithelial and stromal tumor, demonstrate immunoreactivity for ER and PR.
Comment Here
Reference: Mixed epithelial and stromal tumor
- Commonly bilateral and multiple
- Harbor DICER1 gene mutations
- Most behave in benign fashion
- Typically occur in male patients
Mixed epithelial and stromal tumor is typically seen in perimenopausal women. These tumors are solitary, with very rare exceptions. Most of these tumors have benign behavior following surgical resection. Unlike pediatric cystic nephroma, DICER1 gene mutations are not found in mixed epithelial and stromal tumor.
Comment Here
Reference: Mixed epithelial and stromal tumor
- Distinct polymorphic, malignant renal cell neoplasm composed of bland anastomosing tubules, spindle cell areas and myxoid stroma / extracellular mucin
- Compact tubules lined by bland cuboidal or flattened cells merging with low grade spindle cell areas
- Myxoid stroma with basophilic extracellular mucin
- Absence of distinct, well formed papillae
- References: Eur Urol 2022;82:458, Mod Pathol 2021;34:1392, Diagn Pathol 2015;10:168, Arch Pathol Lab Med 2020;144:115
- Historic terminology not recommended by WHO 2022
- Low grade tubular mucinous renal neoplasm
- Low grade collecting duct carcinoma
- Low grade myxoid renal epithelial neoplasm with distal nephron differentiation
- Accounts for < 1% of all renal neoplasms
- Median age: 50 - 60 years
- F > M
- References: Am J Surg Pathol 2018;42:767, Clin Genitourin Cancer 2019;17:268, BJU Int 2015;116:85
- Kidney
- These tumors have a phenotypic expression pattern similar to the loop of Henle region of normal nephrons (Am J Surg Pathol 2018;42:1571)
- Hippo pathway dysregulation with increased nuclear YAP1 protein expression (Am J Surg Pathol 2018;42:767)
- CDKN2A or CDKN2B deletion may be present in high grade tumors (Mod Pathol 2021;34:445)
- Unknown
- Most cases are asymptomatic
- Incidental finding on imaging
- Essential morphologic features include anastomosing tubules lined by low grade cells
- Tubules should merge with areas of bland spindle cells
- Areas of myxoid stroma or extracellular mucin should be present
- References: Eur Urol 2022;82:458, Mod Pathol 2021;34:1392, Diagn Pathol 2015;10:168, Arch Pathol Lab Med 2020;144:115
- Majority are homogeneous
- Small subset may show cystic changes, which is more commonly seen in papillary renal cell carcinoma (RCC)
- Typically isodense or hypodense on unweighted CT
- Hyperintense on T2 due to extracellular mucin
- Appears unencapsulated
- References: Br J Radiol 2021;94:20210548, Abdom Radiol 2021;46:5250
- Indolent behavior (Clin Genitourin Cancer 2019;17:268)
- Rarely metastatic (e.g., with epithelial anaplasia or sarcomatoid differentiation)
- 39 year old man with right lower back pain and 8 cm kidney mass (Urol Case Rep 2021:40:101889)
- 40 year old woman with incidental kidney mass (J Kidney Cancer VHL 2022;9:10)
- 45 year old man with incidental kidney mass (Medicine (Baltimore) 2018;97:e12933)
- 69 year old man with kidney mass and osseous lesions (IJU Case Rep 2021;4:333)
- Surgical excision is the mainstay of treatment
- Selected cases received adjuvant therapy with tyrosine kinase inhibitors and PD-1 inhibitors (Clin Genitourin Cancer 2019;17:268)
- Well circumscribed, solid, homogenous (Arch Pathol Lab Med 2020;144:115)
- Capsule may be present (Arch Pathol Lab Med 2020;144:115)
- Tan, gray-pink or pale yellow cut surface (Arch Pathol Lab Med 2020;144:115)
- Typically intraparenchymal or partially exophytic mass (BMC Cancer 2023;23:815)
- Most masses are small (Arch Pathol Lab Med 2020;144:115)
- Areas of hemorrhage and necrosis are rare (Arch Pathol Lab Med 2020;144:115)
- Elongated tubules with low grade epithelial lining merging with areas of low grade spindled cells in a myxoid background
- Extracellular mucin may be present
- Bland tubules merging with bland spindle cells in a myxoid stroma
- Absence of distinct, well formed papillae
- Tightly packed tubules and spindle cell areas with smooth lumina
- Compact arrangements of whorled tubules
- Areas of myxoid stroma
- Presence of a capsule or foamy macrophages may be present
- High nucleolar grade or extensive necrosis have been described as occurring in rare cases (Histopathology 2017;71:719)
- References: Eur Urol 2022;82:458, Mod Pathol 2021;34:1392, Diagn Pathol 2015;10:168, Arch Pathol Lab Med 2020;144:115
Contributed by J. Cody Craig, M.D., Aida Valencia, M.D., Jennifer B. Gordetsky, M.D. and @katcollmd on Twitter
- Tightly packed, often elongated tubules composed of slender attenuated cells, similar to those found in the normal loop of Henle (Int Braz J Urol 2002;28:477)
- Discontinuation of the tubular basement membranes (Int Braz J Urol 2002;28:477)
- Low columnar, cuboidal and spindle cells with a low nuclear to cytoplasmic ratio (Pathol Res Pract 2014;210:454)
- Small nuclei with small nucleoli, dispersed chromatin and smooth nuclear membranes (Pathol Res Pract 2014;210:454)
- Scant cytoplasmic organelles and intermediate filaments (Pathol Res Pract 2014;210:454)
- Short apical microvilli and well developed desmosomes at the site of attachment between the apposing cell membranes (Pathol Res Pract 2014;210:454)
- Foamy macrophages may be present (Pathol Res Pract 2014;210:454)
- Monosomy of chromosomes 1, 6, 9, 14, 15 and 22 (Am J Surg Pathol 2018;42:767)
- Multiple loss of chromosomes 1, 4, 6, 8, 9, 13, 14, 15 and 22 (Am J Surg Pathol 2018;42:767)
- FISH shows no VHL deletions (Am J Surg Pathol 2018;42:767, Int Braz J Urol 2002;28:477)
- Recurrent chromosomal losses and somatic mutations of genes in the Hippo pathway (Am J Surg Pathol 2018;42:1571)
- Moderate to high expression of VSTM2A via RNA in situ hybridization (Am J Surg Pathol 2018;42:1571)
- Biallelic loss of Hippo pathway tumor suppressor genes (PTPN14, NF2, SAV1) (Cancer Discov 2016;6:1258)
- Kidney, right, radical nephrectomy:
- Mucinous tubular and spindle cell carcinoma, 3 cm (see comment)
- Organ confined
- Margins negative for tumor
- No tumor necrosis identified
- No sarcomatoid or rhabdoid features identified
- No metatasis to lymph nodes
- Pathologic stage: pT1a pN0 pMx
- Comment: The specimen shows a kidney composed of low grade tubules merging with areas of spindled cells in a myxoid stroma. No well formed papillary areas are present. Immunohistochemical stains show positivity for PAX8, CK7, racemase and vimentin.
- Papillary renal cell carcinoma (Am J Surg Pathol 2008;32:1353):
- Papillary architecture
- May have necrosis
- Papillary cores show psammoma bodies or foamy macrophages
- Lack of spindled stroma and mucin
- There is no difference in the nuclear expression of YAP / TAZ between mucinous tubular and spindle cell carcinoma and papillary renal cell carcinoma (Am J Surg Pathol 2018;42:767)
- Frequently positive for CD10
- Renal cell carcinoma with sarcomatoid differentiation (Mod Pathol 2021;34:1392):
- High grade cytologic atypia in spindle cells
- Abrupt transition from epithelial to spindle cell component
- Presence of distinct alternative histologic subtype (clear cell, papillary, etc.)
- ALK rearrangement associated renal cell carcinoma (Diagn Pathol 2022;17:52):
Comment Here
Reference: Mucinous tubular and spindle cell carcinoma
Which of the following features would be most helpful in distinguishing a mucinous tubular and spindle cell carcinoma from a renal cell carcinoma with sarcomatoid features?
- PAX8+, CK7+, vimentin+, racemase+
- Presence of foamy macrophages
- Retained FH and SDHB expression on IHC
- VHL mutation on molecular studies
Comment Here
Reference: Mucinous tubular and spindle cell carcinoma
- Indolent neoplasm composed of low grade clear cells with pure multicystic architecture
- Exclusively cystic, multiloculated renal tumor devoid of any expansile solid growth
- Clear cells lining with low grade nuclei (WHO / ISUP grade 1 or 2)
- No progression or metastases when diagnosed on strict criteria
- Distinguish from regressing clear cell renal cell carcinoma (CCRCC) with cystic degeneration or extensive cystic architecture
- Diagnosis should not be made in needle biopsy alone because of limited sampling
- Use of multilocular cystic renal cell carcinoma is considered obsolete (WHO 2016); some literature references in this topic use the older terminology
- ICD-O: 8316/1 - multilocular cystic renal neoplasm of low malignant potential
- ICD-11: 2F35 & XH7PR9 - benign neoplasm of urinary organs & multilocular cystic renal neoplasm of low malignant potential
- Accounts for 0.5 - 2.5% of all renal tumors; 10% of all cystic renal neoplasms (BMC Urol 2014;14:87, Clin Genitourin Cancer 2016;14:e553, Urology 2019;133:145, Diagn Histopathol 2020;26:320)
- Median age: 54 (range: 18 - 84 years), M > F (~2:1) (Virchows Arch 2018;473:85, J Urol 2016;196:1350, BMC Urol 2014;14:87, Am J Clin Pathol 2006;125:217)
- Kidney only, no side preference
- Solitary and unilateral > 95%
- Bilateral or multifocal 2 - 5%
- References: Virchows Arch 2018;473:85, Clin Genitourin Cancer 2016;14:e553, Am J Clin Pathol 2006;125:217, BMC Urol 2014;14:87
- Several mechanisms of pathogenesis described:
- Cyst dependent, VHL associated pathway in which cysts represent a precursor of CCRCC: abnormal microtubule formation and degeneration of primary cilia
- Tumor arising in a preexisting simple renal cyst
- Intrinsic cystic growth as unilocular fluid filled mass
- Intrinsic multilocular cystic growth in proximal convoluted tubules
- Tumor growth causing obstruction of kidney tubules and cyst formation
- Cystic regression
- Most likely represent low grade spectrum in development of CCRCC with ~40% overlapping genes detected both in multilocular cystic renal neoplasm of low malignant potential (MCNLMP) and in cystic CCRCC by next generation sequencing
- References: Diagn Histopathol 2020;26:320, Virchows Arch 2018;473:85, Annu Rev Pathol 2015;10:263, Investig Clin Urol 2019;60:148
- VHL gene alterations or 3p loss in 25 - 77% of cases
- Concurrent CCRCC in ~5% of cases
- Could develop in von Hippel-Lindau (VHL) syndrome
- References: Histol Histopathol 2012;27:969, BMC Urol 2014;14:87, Virchows Arch 2018;473:85
- 90% are discovered incidentally, on radiology for other purposes
- Asymptomatic in > 80% of cases
- Up to 20% of patients present with symptoms such as hypertension, hematuria, a palpable mass, flank or abdominal pain but not the classic renal cell carcinoma (RCC) triad of gross hematuria, pain and a palpable mass
- References: J Cancer Res Clin Oncol 2008;134:433, Histol Histopathol 2012;27:969, Am J Surg Pathol 2013;37:1469, Virchows Arch 2018;473:85, Clin Genitourin Cancer 2016;14:e553
- Radiographic studies: contrast enhanced CT, MRI or ultrasound
- Bosniak IIF - III cysts (World J Urol 2018;36:1643, Diagn Histopathol 2020;26:320, J Urol 2017;198:12, Medicine (Baltimore) 2020;99:e23110)
- There are no current guidelines or definitive evidence regarding the question of whether complete histologic examination is required for diagnosis (Am J Surg Pathol 2013;37:1469)
- Moderately complex, multilocular, cystic
- Well defined margins
- Minimal thickening of wall and smooth septa
- Moderately enhancing fluid equivalent and atypical cysts
- No intracystic enhancement (J Urol 2017;198:12)
- Excellent prognosis with no reports of progression, metastases or cancer related death in multiple case series when diagnosed on strict criteria (Virchows Arch 2018;473:85, Clin Genitourin Cancer 2016;14:e553)
- Recurrence reported in rare cases (J Cancer Res Clin Oncol 2008;134:433, Urology 2019;133:145)
- 30 year old man with 2 year history of intermittent right flank pain and gross hematuria (Can Urol Assoc J 2014;8:E545)
- 38 year old man with concurrent MCNLMP with leiomyoma (Case Rep Oncol 2010;3:218)
- 48 year old man with MCNLMP in a duplex kidney (Int J Surg Pathol 2012;20:613)
- 55 year old man with pain in left lumbar region and hematuria since 2 months (J Lab Physicians 2014;6:50)
- 70 year old man with 5 asynchronous independent tumors, including 3 MCNLMP (APMIS 2016;124:619)
- Resection, preferably nephron sparing surgery (Cancers (Basel) 2022;14:831)
- Usually unilateral and Well circumscribed with fibrous pseudocapsule
- Variably sized cysts with thin septa
- Cysts filled with clear, serous, gelatinous or hemorrhagic fluid
- No mural solid nodules or areas of necrosis
- Reference: Histol Histopathol 2012;27:969
- Thin, fibrous septa lined by clear cells
- Low grade nuclei without prominent nucleoli (WHO / ISUP grade 1 - 2)
- Clear cell clusters may be present within thin septations or fibrous capsule but they should not alter their contours by expansile growth or exceed 1 mm (20x field of view)
- In rare cases, lining of cysts may show focal multilayering, cells with granular cytoplasm and small intracystic papillations
- Septa may contain calcification or ossification
- Presence of necrosis, frequent or atypical mitoses, lymphovascular or fat invasion, rhabdoid or sarcomatoid change are incompatible with diagnosis
- Reference: Virchows Arch 2018;473:85
- AMACR in 80%
- ER, PR, TFE3, GATA3
- Ki67 very low
- References: Histol Histopathol 2018;33:589, Am J Surg Pathol 2012;36:1425
- Genetically related to CCRCC: 74 - 77% of cases demonstrate 3p loss (by FISH) and VHL mutations identified in 25 - 40% of cases
- Next generation sequencing study identified 6 candidate genes (SETD2, GIGYF2, FGFR3, BCR, KMT2C and TSC2) for differentiating MCNLMP from CCRCC with cystic change
- References: Mod Pathol 2011;24:571, Mod Pathol 2010;23:931, Virchows Arch 2018;473:85, Investig Clin Urol 2019;60:148
- Left kidney, partial nephrectomy:
- Multilocular cystic neoplasm of low malignant potential
- Tumor size: 3.7 cm
- Surgical margins: free of tumor
- Benign renal cortical cyst:
- Usually unilocular and lacks clear cell changes
- Cyst lining is CK7 positive and CAIX negative and shows no loss of 3p (Appl Immunohistochem Mol Morphol 2019;27:549)
- Clear cell renal cell carcinoma (CCRCC) with cystic or regressive changes:
- Cysts filled with hemorrhagic or necrotic degenerated cells
- May have extensive hyalinization and has areas of expansile growth of neoplastic cells
- Clear cell papillary renal cell tumor (Hum Pathol 2017;66:152):
- Cystic nephroma (Int J Surg Pathol 2015;23:238):
- TFE3 translocation tumors (especially MED15::TFE3) (Adv Anat Pathol 2022;29:131):
- Usually high grade tumors with (at least) focal solid areas, papillary formation and psammoma bodies
- Diffusely positive for TFE3, variably positive for cathepsin K and melanocytic markers
- Negative for CAIX and CK7
- Tubulocystic renal cell carcinoma (Am J Surg Pathol 2013;37:1469):
Which of the following is true about this encapsulated multilocular cystic renal tumor with clear cell morphology?
- Does not require surgical treatment
- Frequently progresses to conventional clear cell renal cell carcinoma (CCRCC)
- Necrosis, solid growth and invasion are not allowed for this diagnosis
- Often associated with acquired cystic kidney disease
- VHL mutation or 3p loss are highly unlikely
Comment Here
Reference: Multilocular cystic renal neoplasm of low malignant potential
Comment Here
Reference: Multilocular cystic renal neoplasm of low malignant potential
- Nephroblastoma (or Wilms tumor) is a malignant embryonal tumor originating from nephrogenic blastema, which imitates the histology of developing kidney
- Primarily occurs in children
- Named after the German surgeon Max Wilms (who is often wrongly attributed to be the first one describing this entity)
- Tumor consists of blastemal, epithelial and stromal elements (triphasic) which can be present in variable amounts
- Biphasic and monophasic tumors are not infrequent
- Epithelial and stromal elements may show different lines and degrees of differentiation (from poor to well differentiated)
- There are 2 main treatment approaches:
- SIOP (International Society of Paediatric Oncology) which uses preoperative chemotherapy as the first line of treatment
- COG (Children’s Oncology Group) which advocates for primary surgery
- Both followed by postoperative chemotherapy and radiotherapy, if necessary
- Both approaches have their own histologic classification and risk assessment (based on prognostic factors)
- Overall survival rate is approximately 90%
- Wilms tumor
- Nephroblastoma
- Most common: 3 - 4 years of age (Pritchard-Jones: Renal Tumors of Childhood - Biology and Therapy, 2014)
- European and North American rates are about the same (8 per million)
- More common in Africans; least common in East Asian population
- Congenital Wilms tumors very rare (J Pediatr Surg 1995;30:856)
- Rare adult cases (Expert Rev Anticancer Ther 2011;11:1105)
- Slight female preponderance
- Majority of patients with Wilms tumor are nonsyndromic
- 10 - 15% associated with syndromes and congenital anomalies (J Med Genet 2006;43:705)
- 1 - 2% familial (Am J Med Genet C Semin Med Genet 2004;129C:29)
- Relapses occur in ~15% of children, the majority within 2 years of diagnosis
- Kidney
- 5 - 10% are bilateral (Expert Rev Mol Med 2017;19:e8)
- Very rarely extrarenal (J Pediatr Surg 2013;48:E33)
- Nephrogenic rests are precursor lesions (Am J Med Genet 1998;79:268)
- Nephrogenic rests are found in 40% of unilateral and > 90% of bilateral Wilms tumors (Pediatr Blood Cancer 2017;64)
- Not clear how nephrogenic rests progress to tumor
- 2 main types of nephrogenic rests:
- Perilobar nephrogenic rests
- Intralobar nephrogenic rests
- Perilobar nephrogenic rests in children under 1 year of age are associated with a markedly increased risk of developing a contralateral Wilms tumor
- Thought to develop from persistent metanephric tissue or nephrogenic rests
- Believed to be due genetic alterations of embryological development of the genitourinary tract
- WT1 gene at chromosome 11p13 implicated in tumorigenesis
- WT2 gene at chromosome 11p15 (still not isolated) is a second locus
- Other genes, still to be identified, involved (see below)
- Usually presents as abdominal mass with no associated symptoms
- Other signs or symptoms (present in 20 - 30% of cases):
- Abdominal pain
- Hematuria
- Hypertension
- Anemia
- Most common predisposition syndromes and conditions with different risk of Wilms tumor include:
- High risk (> 20%)
- WAGR (Wilms tumor - aniridia - genitourinary anomalies - intellectual disability)
- Denys-Drash syndrome
- Moderate risk (5 - 20%)
- Beckwith Wiedemann syndrome
- Simpson-Golabi-Behmel syndrome
- Frasier syndrome
- Low risk (
- Bloom syndrome
- DICER1 syndrome
- Li-Fraumeni syndrome
- Isolated hemihypertrophy
- High risk (> 20%)
- Imaging techniques can reliably diagnose 80 - 85% of Wilms tumors (Lancet Child Adolesc Health 2020;4:232)
- No specific / diagnostic laboratory findings
- Catecholamine levels to exclude neuroblastoma
- Complete blood count
- Biochemistry profile
- Renal functions
- Coagulation studies
- Abdominal ultrasound
- Chest Xray (to look for metastases)
- Abdominal and chest CT
- Abdominal MRI
- Recently, MRI diffusion studies showed that Wilms tumor can be differentiated from neuroblastoma
- Differ between 2 main approaches; COG (followed mainly in the U.S. and Canada) and SIOP (followed in the rest of the world) (Am Soc Clin Oncol Educ Book 2014;215)
- In both SIOP and COG, tumor histology and stage are important prognostic factors (see Microscopic description)
- In SIOP, which advocates preoperative chemotherapy, followed by surgery and further chemo or radiotherapy; if necessary, prognostic factors also include:
- Risk stratification into three treatment groups: low, intermediate and high risk group
- Tumor volume before and after preoperative chemotherapy in defined cases
- Responsiveness of lung metastases to initial chemotherapy in some groups
- In COG, which advocates primary surgery, followed by chemo or radiotherapy, if necessary, prognostic factors also include:
- Age
- Tumor weight
- Rapidity of lung nodule response
- Molecular markers (LOH at chromosomes 1p, 16q)
- In SIOP, which advocates preoperative chemotherapy, followed by surgery and further chemo or radiotherapy; if necessary, prognostic factors also include:
- 1q gain associated with poor outcome; it will be used in the coming COG trial as a prognostic factor, whereas SIOP will be testing its importance in the current SIOP-RTSG UMBRELLA 2016 study
- Overall survival for Wilms tumor is approximately 90%
- Newborn girl with congenital Wilms tumor (BMJ Case Rep 2019;12:e228651)
- 28 month old girl and 41 month old boy with bilateral Wilms tumor with TP53 related anaplasia (Pediatr Dev Pathol 2013;16:217)
- 2 year old girl with hemihypertrophy underwent partial nephrectomy for multifocal, unilateral Wilms tumor (Urology 2019 Nov;133:243)
- 2 year old boy with extrarenal Wilms tumor (J Pediatr Surg 2013;48:E33)
- Multimodal treatment which includes a combination of surgery, chemotherapy and, in higher stages, radiotherapy, depending on which approach is followed (COG or SIOP) (Am Soc Clin Oncol Educ Book 2014;215)
- In SIOP, preoperative chemotherapy is given to all patients > 6 months of age, followed by surgery and:
- No further treatment is given for low risk Wilms tumors (completely necrotic) stage 1
- Further chemo and radiotherapy, if necessary, for all other Wilms tumors
- No molecular or other markers are used for risk stratification at present
- Focal anaplasia is regarded as an intermediate risk tumor
- Diffuse anaplasia and blastemal type are regarded as a high risk tumors
- In COG:
- Very low risk Wilms tumors (nonanaplastic, stage 1, negative lymph nodes, All other Wilms tumors are treated with surgery, chemo and radiotherapy if necessary, depending on histology, stage and molecular markers
- Usually large, solitary, spherical mass, sharply demarcated from the renal parenchyma, distorting kidneys contours
- In ~10% multinodular
- In primarily operated cases, tumor is gray-white or pink-gray in color, lobulated, soft and friable
- Some tumors may have a prominent cystic appearance
- In pretreated cases, hemorrhage and necrosis are usually extensive
- Gross sampling notes:
- Very important to adequately sample tumor, at least 1 slice of the whole tumor, for assessment of histologic features
- Renal sinus, its vessels and ureter should be included
- Origin of all tissue blocks should recorded, preferably at a gross picture (block identification key) (see video below) (J Clin Pathol 2010;63:102)
- Typically consists of 3 components: blastemal, epithelial and stromal
- Tumors showing 2 or only 1 component not rare
- Blastema
- Least differentiated component
- Consists of small to medium sized undifferentiated cells with relatively small regular nuclei and small nucleoli
- May show different patterns (diffuse, serpentine, nodular and basaloid patterns) which are of no prognostic significance
- Mitotic figures frequent
- Epithelial component includes:
- Poorly differentiated (rosette-like structures)
- Moderately differentiated (tubules and papillary structures)
- Well differentiated (glomerular-like structures and small, mature tubules) elements
- May contain heterologous elements (squamous and mucinous epithelium, glial tissue)
- Stromal component
- From hypo to hypercellular undifferentiated areas to well differentiated areas
- Cells showing no clear cell borders, oval to spindle shaped nuclei with bland nucleoli
- Often shows heterologous elements (rhabdomyoblasts, adipose tissue, cartilage) (Am J Surg Pathol 2019;43:1583)
- The following descriptive terms should not be used as they are not separate entities, do not exist in any classification and have no prognostic significance
- Fetal rhabdomyomatous Wilms tumor
- Botryoid Wilms tumor
- Teratoid Wilms tumor
- Anaplasia
- Present in 5 - 10% of tumors
- Defined as the presence of enlarged, atypical, tripolar or multipolar mitotic figures, marked nuclear enlargement and hyperchromasia
- All 3 features have to be present
- May occur in any of the components
- Focal anaplasia is defined as a clearly defined focus within a primary intrarenal tumor
- In SIOP: up to 2 foci of up to 15 mm in size
- In COG: up to 4 small foci, size not specified
- Diffuse anaplasia is defined as nonlocalized anaplasia; focal anaplasia with marked nuclear unrest in the remaining tumor; anaplasia beyond the tumor capsule; anaplastic cells in intrarenal or extrarenal vessels, renal sinus, extracapsular sites, metastatic deposits or anaplasia in a biopsy
- Histologic classification
- SIOP histologic classification is based on the assessment of percentage of chemotherapy induced changes and viable tumor components (Tables 1 and 2) (Nat Rev Urol 2018;15:693)
- COG histologic classification includes anaplastic and nonanaplastic Wilms tumors (Table 1) (Am Soc Clin Oncol Educ Book 2014;215)
- Staging criteria in the COG and SIOP are similar but there are some differences (for example, any biopsy in COG but only wedge / open biopsy in SIOP is regarded as a criterion for stage III) (Table 3)
Table 1: histologic risk classifications for Wilms tumor
| International Society of Paediatric Oncology (SIOP) | Children's Oncology Group (COG) | |
| Low risk | Cystic partially differentiated nephroblastoma* Completely necrotic Wilms tumor | Cystic partially differentiated nephroblastoma* |
| Intermediate risk | Epithelial, stromal, mixed, regressive types Focal anaplasia | Favorable histology Wilms tumor No evidence of anaplasia |
| High risk | Diffuse anaplasia Blastemal type | Diffuse anaplasia Focal anaplasia |
Table 2: Histological criteria for Wilms tumor types in SIOP for pretreated cases
| Tumor type | |||||
| Blastema | Epithelium | Stroma | |||
| Completely necrotic | 100% | 0% | 0% | 0% | |
| Regressive | > 66% | 0 - 100% | 0 - 100% | 0 - 100% | |
| Mixed | 0 - 65% | 0 - 65% | 0 - 65% | ||
| 11 - 65% | 0 - 89% | 0 - 89% | |||
| Epithelial | 0 - 10% | 66 - 100% | 0 - 33% | ||
| Stromal | 0 - 10% | 0 - 33% | 66 - 100% | ||
| Blastemal | 66 - 100% | 0 - 33% | 0 - 33% | ||
**The percentage of the components of the viable tumor should add up to 100%
***The presence of diffuse anaplasia in any of the above types supersedes the underlying types. Focal anaplasia also needs to be specifically mentioned in the diagnosis (for example, "focal anaplasia in mixed type")
Table 3: staging criteria for Wilms tumor in SIOP and COG
| Stage | SIOP (pre-operative chemotherapy) | COG (primary surgery) |
| I | Tumor is limited to the kidney and completely resected | Tumor is limited to the kidney and is completely resected |
| Tumor is present in the perirenal fat but is surrounded by a fibrous (pseudo)capsule; the (pseudo)capsule might be infiltrated by viable tumor, which does not reach the outer surface | Renal capsule intact; not penetrated by tumor | |
| Tumor might show protruding ('botryoid') growth into the renal pelvis or ureter but does not infiltrate their walls | Tumor not ruptured or biopsied prior to removal | |
| The vessels or the soft tissues of the renal sinus and not involved by tumor; intrarenal vessel involvement might be present | No tumor invasion of veins or lymphatics of renal sinus | |
| * Fine needle or tru-cut biopsy do not upstage tumor | No nodal or hematogenous metastases | |
| No rupture or biopsy prior to removal | ||
| II | Viable tumor is present in the perirenal fat and is not covered by a (pseudo)capsule but is completely resected (resection margins are clear) | Tumor in the perirenal fat but completely resected |
| Viable tumor infiltrates the soft tissues of the renal sinus | Tumor infiltrates the renal sinus or blood and lymphatic vessels outside the renal parenchyma but is completely resected | |
| Viable tumor infiltrates blood or lymphatic vessels of the renal sinus or of the perirenal tissue | Tumor infiltrates adjacent organs or vena cava but is completely resected | |
| Viable tumor infiltrates the wall of the renal pelvis or the ureter | ||
| Viable tumor infiltrates the vena cava or adjacent organs (except the adrenal gland) but is completely resected | ||
| III | Viable tumor present at resection margin(s) | Residual tumor or nonhematogenous metastases confined to abdomen |
| Abdominal lymph nodes contain viable or nonviable tumor | Involved abdominal lymph nodes | |
| Viable or nonviable thrombus present at resection margins of ureter, renal vein or inferior vena cava | Peritoneal tumor implants | |
| Viable or nonviable tumor thrombus in the inferior vena cava removed piecemeal by a surgeon | Tumor spillage before or during surgery | |
| Preoperative or intraoperative tumor rupture, if confirmed by microscopic examination (viable tumor at the surface of the specimen at the area of rupture) | Gross residual tumor in abdomen | |
| Wedge or open biopsy before preoperative chemotherapy or surgery | Biopsy of tumor (including fine needle biopsy) prior to removal of kidney | |
| Tumor implants (viable or nonviable) in the abdomen | Resection margins involved by tumor or transection of tumor during resection (i.e. piecemeal excision of tumor) | |
| Tumor (viable or nonviable) penetrated through the peritoneal surface | ||
| IV | Hematogenous metastases (lung, liver, bone, brain) or lymph node metastases outside the abdominopelvic region | Hematogenous metastases or spreads beyond abdomen |
| V | Bilateral tumors at diagnosis; each side should be substaged according to the above criteria | Bilateral tumors at diagnosis; each side should be substaged according to the above criteria |
Contributed by Ellen D’Hooghe, M.D. and Gordan M. Vujanic, M.D., Ph.D.
- Cytology (FNA) not recommended or routinely done
- WT1
- The most sensitive and specific marker (positive in ~90% of cases) (Cancers (Basel) 2020;12:729)
- Only nuclear staining should be evaluated, seen with antibodies directed against C terminal and N terminal
- Cytoplasmic expression can be seen, especially using antibodies to the N terminal
- Blastema
- Epithelium
- Stroma
- INI1 retained
- Glypican 3: expressed in the majority of Wilms tumors (blastema and epithelium) (Virchows Arch 2015;466:67)
- Electron microscopy not routinely used
- A number of genetic changes with variable prevalence have been described, including (Nat Rev Nephrol 2019;15:240):
- WT1 (11p13), prevalence 10 - 20%, early event, associated with stromal histology
- CTNNB1 (3p22), prevalence ~15%, late event, not associated with nephrogenic rests
- IGF2 (11p15), prevalence ~70%, early event, associated with perilobar nephrogenic rests and with epithelial and blastemal histology
- TP53 (17p13), prevalence ~70%, associated with anaplasia and with reduced event free and overall survival
- MYCN (2p24), prevalence ~15%, associated with anaplasia and with reduced event free and overall survival
- 1q gain, prevalence ~30 - 40%, associated with reduced event free and overall survival
- Left kidney, total nephrectomy:
- If primarily operated:
- Wilms tumor, nonanaplastic type, stage II (due to renal sinus invasion)
- Comment: Tumor comprises blastemal, epithelial and stromal components, present in equal proportion. No evidence of anaplasia is seen. Tumor is infiltrating the renal sinus but is not reaching the resection margins. Lymph nodes are free of tumor.
- If treated with preoperative chemotherapy:
- Wilms tumor, mixed type, intermediate risk tumor, stage III (due to viable and nonviable lymph node metastases)
- Comment: Tumor shows chemotherapy induced changes occupying 40% of the mass. The viable tumor consists of blastemal (20%), epithelial (50%) and stromal (30%) elements, with no evidence of anaplasia. The renal sinus and perirenal fat are free of tumor. Lymph nodes contain viable and nonviable metastases.
- If primarily operated:
- Triphasic Wilms tumors relatively easy to diagnose on H&E slides
- Pure blastemal, epithelial and stromal tumors may pose a diagnostic challenge
- Extensive sampling may be helpful in identifying other diagnostic features
- Immunohistochemistry and molecular biology investigations help in the majority of cases (Cancers (Basel) 2020;12:729)
- Pure blastemal Wilms tumor needs to be distinguished from:
- Rhabdoid tumor:
- Neuroblastoma:
- Synaptophysin+, WT1-
- Ewing sarcoma:
- Desmoplastic small round cell tumor:
- WT1+, diagnosis established by EWS-WT1 gene fusion
- Pure epithelial Wilms tumor may be difficult to distinguish from:
- Hyperplastic perilobar nephrogenic rests:
- No reliable histologic, immunohistochemical or molecular findings to distinguish from Wilms tumor
- Papillary renal cell carcinoma:
- Metanephric adenoma:
- BRAF V600E+, CD57+, WT1+
- Hyperplastic perilobar nephrogenic rests:
- Pure stromal Wilms tumor may be difficult to distinguish from:
- Clear cell sarcoma of the kidney:
- Mesoblastic nephroma:
- WT1-, 70% of cellular mesoblastic nephromas show ETV6-NTRK3 gene fusion
- Metanephric stromal tumor:
- CD34+, BRAF V600E+, WT1 variable
- Other rare tumors (synovial sarcoma, inflammatory myofibroblastic tumors and renal cell carcinoma with sarcomatous differentiation)
A 2 year old girl presents with an abdominal mass noticed by parents. Imagining studies show a left sided renal mass. No obvious lung metastases. Preoperative chemotherapy given as per the SIOP (International Society of Paediatric Oncology) protocol. Total left nephrectomy performed. The tumor is composed of undifferentiated cells with no particular pattern, shown above. The tumor cells are positive for WT1, CD56 and PAX8, negative for synaptophysin and cyclin D1 and INI1 is retained.
If the whole tumor shows the same pattern, the most likely diagnosis is
- Wilms tumor (nephroblastoma), blastemal type
- Desmoplastic small round cell tumor
- Hyperplastic nephrogenic rest
- Neuroblastoma
- PNET / extraosseous Ewing sarcoma
- Anaplasia
- Botryoid growth
- Fetal rhabdomyomatous pattern
- Teratoid elements
- Nephrogenic rests: persistent foci of embryonal cells seen after the period of normal nephrogenesis
- Nephroblastomatosis: multifocal or diffuse nephrogenic rests
- May be intralobar or perilobar
- 1% of neonatal kidneys; florid cases associated with congenital anomalies and hypertension
- Intralobar rests have an increased rate of progression to Wilms tumor, are more commonly associated with WT1 mutations, Denys-Drash syndrome and WAGR syndrome
- Perilobar rests are seen in sporadic tumors and are associated with genetic / epigenetic dysregulation at 11p15 (Clin Cancer Res 2008;14:7635), idiopathic hemihypertrophy and Beckwith-Wiedemann syndrome
- Precursor lesions of Wilms tumor, seen in 30% - 44% of kidneys resected for Wilms tumor (WT)
- 2 week old 'female' infant with nephrotic syndrome and renal failure (Case of the Week #338)
- Male newborn death with bilateral nephroblastomatosis (Arch Pathol Lab Med 1989;113:729)
- Newborn with lumbosacral ectopic rest (Am J Surg Pathol 2004;28:1389)
- Siblings with universal nephroblastomatosis with nephromegaly (Pediatr Dev Pathol 2009;12:47)
- Conservative
- May be inapparent on gross examination or appear as nodules / foci of capsular thickening / scar beneath the capsule or in the deep cortex / medulla
- Tightly packed nests or diffuse sheets of primitive but nonanaplastic blastemal / primitive epithelial cells with scanty stroma
- No cartilage or primitive mesenchyme
- May have a sclerosing or hyperplastic pattern
- Intralobar: randomly distributed throughout cortex and medulla with irregular margins, more stroma than blastema or tubules
- Perilobar: peripheral with sharply demarcated margins, composed of blastema and tubules with scanty or sclerotic stroma, often solitary
- Nonneoplastic kidney (NNK) in tumor nephrectomies
- Medical kidney disease
- Evaluation for medical renal disease should be performed in every tumor nephrectomy and included in the synoptic report (as per College of American Pathologists [CAP] kidney cancer dataset and in the American Urological Association [AUA] renal mass guidelines)
- Medical kidney disease is common and often unrecognized in tumor nephrectomy specimens (Am J Surg Pathol 2007;31:1703, Arch Pathol Lab Med 2009;133:1012, Arch Pathol Lab Med 2009133:189, Adv Anat Pathol 2010;17:235, Ann Diagn Pathol 2013;17:176)
- Medical conditions that commonly affect the kidney, such as diabetes and hypertension, are risk factors for renal neoplasia
- Nephrectomy is a risk factor for subsequent chronic kidney disease (CKD) (Am J Surg Pathol 2006;30:575, Adv Chronic Kidney Dis 2014;21:91)
- Severity of chronic renal parenchymal damage is prognostically important for postoperative risk of progressive renal insufficiency (Mayo Clin Proc 2000;75:1236, Arch Pathol Lab Med 2013;137:531, Am J Clin Pathol 2019;151:108, Semin Nephrol 2020;40:69)
- Medical kidney disease is common and often unrecognized in adult tumor nephrectomy specimens
- Chronic kidney disease stage 3 or above involves up to 26% of renal cell carcinoma (RCC) patients even before nephrectomy (Lancet Oncol 2006;7:735)
- Depending on baseline medical comorbidity, up to 36% of total nephrectomy and 26% of partial nephrectomy patients develop chronic kidney disease (≥ 3) (Mayo Clin Proc 2000;75:1236, J Urol 2004;171:120, Lancet Oncol 2006;7:735, World J Urol 2013;31:1531, World J Urol 2013;31:835, J Urol 2020;203:475)
- Mortality is more strongly associated with estimated glomerular filtration rate (eGFR) < 45 mL/min/1.73 m² (J Urol 2020;203:475)
- Renal function decline due to nephron loss and consequent hyperfiltration injury in remaining nephrons after nephrectomy (J Am Soc Nephrol 2020;31:1107, Nat Rev Nephrol 2014;10:135)
- Progressive diabetic glomerular disease and hypertensive vascular disease (J Am Soc Nephrol 2020;31:1107, Nat Rev Nephrol 2014;10:135)
- Risk factors:
- Diabetes
- Hypertension
- Smoking
- Aging
- Often asymptomatic
- Can have normal creatinine or estimated glomerular filtration rate
- May have chronic kidney disease
- Can have concomitant microhematuria or proteinuria
- Suggested features to routinely include in kidney cancer synoptic report (College of American Pathologists: Protocol for the Examination of Resection Specimens from Patients with Invasive Carcinoma of Renal Tubular Origin [Accessed 14 April 2021])
- Glomerular:
- Estimate percentage of global glomerulosclerosis
- Specific changes, such as nodular glomerulosclerosis and focal segmental glomerulosclerosis
- Presence of Tamm-Horsfall protein in glomerular Bowman spaces suggests obstructive nephropathy
- Tubulointerstitial:
- Estimate percentage of cortical interstitial fibrosis and tubular atrophy and associated interstitial inflammation
- Presence of tubular Tamm-Horsfall protein casts and neutrophilic casts
- Vascular:
- Severity of arteriosclerosis of intrarenal arteries
- Severity of arteriolar nodular hyalinosis
- Glomerular:
- When features suggestive of diabetic nephropathy or hypertensive vascular disease are identified, clinicopathological correlation is indicated
- There may be insufficient tissue to fully evaluate nonneoplastic kidney in partial nephrectomies with minimal surrounding renal parenchyma, due to glomerular and tubulointerstital scarring from adjacent tumor compression; nodular diabetic glomerulosclerosis lesions can still be identified in the compressed scarred nonneoplastic renal parenchyma immediately surrounding the renal neoplasm and chronic vascular disease can still be assessed (World J Urol 2013;31:1531, World J Urol 2013;31:835)
- If significant chronic renal parenchymal damage is present, recommend careful follow up of postoperative renal function and suggest nephrology consultation
- Renal scintigraphy (rare usage outside transplant evaluation)
- Progressive medical kidney disease in nonneoplastic kidney is a major determinant for the longterm survival of patients with renal tumors, especially those with early stage tumors
- Early detection of a nonneoplastic kidney disease provides better opportunities for early medical intervention
- Sample at least 1 block of nonneoplastic renal cortex and medulla from as far distant from the tumor as possible to avoid nonspecific peritumoral compression related chronic changes (College of American Pathologists: Protocol for the Examination of Resection Specimens from Patients with Invasive Carcinoma of Renal Tubular Origin [Accessed 14 April 2021])
- For partial nephrectomy specimens, CAP protocol recommends 5 mm as minimum sufficient nonneoplastic renal parenchyma in order to evaluate nonneoplastic kidney
- If patient has known history of chronic renal insufficiency or significant proteinuria at time of surgery, consider sampling renal cortex for immunofluorescence (fresh frozen tissue) and electron microscopy studies
- Periodic acid-Schiff (PAS) and Masson trichrome stains are recommended as routine for evaluation of the nonneoplastic kidney
- Some histologic findings might be subtle and easily missed by H&E stain alone; special stains become extremely important to help identify the presence and severity of pathological changes
- PAS is very helpful for examining the glomerular mesangium and the basement membranes of the glomerular capillaries and tubules as well as for identifying nodular hyaline arteriolosclerosis
- Trichrome stains show the severity of interstitial fibrosis and vascular intimal fibrosis
- In selected cases, Jones methanamine silver (PAMS) and Congo red amyloid stains may also be helpful
- Consultation with specialist nephropathologist is recommended
- Specific medical renal pathological findings in nonneoplastic kidney
- Among the medical renal diseases, diabetic nephropathy and arterionephrosclerosis / hypertensive nephropathy are 2 of the most common findings, which may be missed when only an H&E stain is reviewed by a pathologist not familiar with medical renal pathology
- Note: listed below are the summarized microscopic findings of common medical renal diseases in nonneoplastic kidney; for detailed histologic descriptions of each disease entity, refer to kidney nontumor chapter, Fogo: Diagnostic Atlas of Renal Pathology, 3rd Edition, 2016 and Jennette: Heptinstall's Pathology of the Kidney, 7th Edition, 2014
- Diabetic nephropathy
- Diffuse and nodular mesangial matrix expansion, large Kimmelstiel-Wilson PAS positive mesangial nodules, glomerular capillary hyaline insudation and glomerular basement membrane (GBM) thickening
- Hyaline capsular drops on Bowman capsules
- Afferent and efferent arteriolar nodular hyaline arteriolosclerosis
- Arterionephrosclerosis / hypertensive nephropathy
- Arterial intimal fibrosis, with reduplications of internal elastic lamina
- Afferent arteriolar nodular hyaline arteriolosclerosis
- Focal segmental glomerulosclerosis
- Segmental mesangial matrix expansion with obliteration of capillary loops, tuft to capsule adhesions and hyalinosis lesions
- Secondary hilar focal segmental glomerulosclerosis most common
- Collapsing glomerulopathy features with prominence of glomerular epithelial cells suggests APOL1 G1 / G2 risk alleles
- Atheroembolic disease
- Intrarenal arteries with luminal cholesterol clefts, macrophage and multinucleated giant cell reaction or embedded within intimal fibrosis
- Obstructive nephropathy and pyelonephritis
- Abundant PAS positive Tamm-Horsfall protein casts in tubules and Tamm-Horsfall protein within glomerular Bowman spaces
- Tubular neutrophilic casts indicate active pyelonephritis
- Periglomerular fibrosis and chronic tubulointerstitial scarring
- Common with obstructing urothelial carcinoma of renal pelvis / ureter
- Glomerulonephritis
- Membranous nephropathy, primary or secondary to neoplasm, with diffuse glomerular capillary wall thickening, epimembranous spikes on PAMS stain
- Proliferative glomerulonephritis with focal or diffuse mesangial or endocapillary proliferation, focal necrotizing or crescentic glomerular injury
- Suspicion of membranous nephropathy or proliferative IgA nephropathy can be further investigated by immunofluorescence and electron microscopy studies
- Thrombotic microangiopathy (TMA)
- Acute TMA with glomerular capillary and arteriolar fibrin thrombi, arterial endothelial swelling, intimal edema and fibrinoid injury
- Chronic TMA with glomerular capillary glomerular basement membrane reduplications and arterial "onion skin" proliferative change
- Possible TMA changes secondary to chemotherapy for neoplasm
- Renal amyloidosis
- Glomerular mesangial and capillary wall, tubulointerstitial and vascular extracellular eosinophilic and congophilic amyloid deposition
- If positive for amyloid, typing of the amyloid requires further immunohistochemical or mass spectroscopy studies
- Lymphoma infiltrates
- Monotonous, dense, small lymphocytic atypical interstitial infiltration may warrant immunophenotyping for possible involvement by small lymphocytic lymphoma / chronic lymphocytic leukemia
- Diabetic nephropathy
- Immunofluorescence staining of paraffin sections with pronase digestion for immunoglobulins and complement components may be helpful for investigation of possible glomerulonephritis (e.g. IgA nephropathy, membranous nephropathy, including PLA2R staining)
- Electron microscopy of reprocessed paraffin block material may be helpful for further investigation of possible glomerular disease
- Left kidney, nonneoplastic kidney (block #):
- Glomerular: approximately 10% global glomerulosclerosis; nonsclerotic glomeruli show nodular glomerulosclerosis with nodular mesangial matrix expansion and scattered focal segmental glomerulosclerosis (see comment)
- Tubulointerstitial: patchy chronic tubulointerstitial damage affecting approximately 20% of cortex
- Vascular: moderate arteriosclerosis of intrarenal arteries; widespread severe nodular and focally circumferential hyaline arteriolosclerosis (see comment)
- Comment: The nodular glomerulosclerosis and severe hyaline arteriolosclerosis suggest diabetic related nephropathy, if clinically appropriate. Clinicopathological correlation and close follow up of postoperative renal function is recommended. Nephrology consultation should be considered.
- Please refer to kidney nontumor chapter for differential diagnosis of each entity
A 75 year old man underwent radical nephrectomy for renal pelvic urothelial carcinoma. Nonneoplastic kidney tissue shows the above changes. Which of the following medical renal diseases should be considered?
- Arteriosclerosis
- Diabetic nodular glomerulosclerosis
- Focal segmental glomerulosclerosis
- Membranous nephropathy
- 1 mm
- 3 mm
- 5 mm
- 1 cm
- 2 cm
- Benign renal epithelial neoplasms characterized by large round eosinophilic cells packed with mitochondria
- Renal oncocytoma are benign renal oncocytic neoplasms
- Cells are packed with mitochondria under electron microscopy
- Essential to differentiate from mimicker oncocytic renal cell carcinoma entities
- Multiple tumors can be associated with syndromic conditions
- Some cases have hybrid morphologies: hybrid oncocytic / chromophobe renal cell carcinoma (HOCT); these are described in syndromic settings but can also be encountered as sporadic cases
- 5 - 9% of renal tumors
- M:F = 2:1
- Age range is very broad: 24 - 91 years old; mean age is in the 60s (Am J Surg Pathol 1997;21:1, J Kidney Cancer VHL 2017;4:1)
- Rarely associated with renal oncocytosis; these kidneys develop multiple oncocytic lesions, oncocytomas, chromophobe tumors and tumors with hybrid oncocytic / chromophobe morphology (HOCT); these are believed to be associated with Birt-Hogg-Dubé syndrome (Mod Pathol 2019;32:1698)
- Renal cortex
- Benign neoplasms are believed to arise from renal intercalated cells and are packed with respiration defective mitochondria
- Often possess pathogenic mitochondrial mutations; it is suggested that the defective mitochondria activates a metabolic checkpoint leading to autophagy impairment and mitochondrial accumulation and that this acts as tumor suppressive mechanism limiting tumor progression (Cell Rep 2015;13:1895)
- 2 main molecular variant events associated with oncocytomas: rearrangements of CCDN1 (11q13 translocations) or aneuploidy manifested mostly in losses of chromosome Y and 1
- Also suggested that in some oncocytomas (the ones that harbor aneuploidy losses of chromosome 1 and Y), additional p53 mutations may allow for progression to eosinophilic chromophobe renal cell carcinoma (Cell Rep 2015;13:1895)
- Benign
- Usually asymptomatic; detected incidentally on imaging for unrelated reasons
- Rarely presents with hematuria and dysuria
- Diagnosis is made with the combination tissue histologic morphology and immunohistochemical profile, which should be consistent with indolent clinical behavior and a circumscribed mass lesion
- No specific radiologic findings
- Diagnosis may be suggested by the presence of a central stellate scar on CT, MRI or US; however, this feature is not specific and can be present in any slow growing renal tumor
- Benign tumor
- There are literature suggestions that it can potentially accumulate genetic abnormalities and progress to a chromophobe renal cell carcinoma (Cell Rep 2015;13:1895)
- 25 year old woman presenting with a giant renal oncocytoma (J Med Case Rep 2010;4:52)
- 44 year old man presenting with a collision tumor, papillary renal cell carcinoma type 1 and oncocytoma (Pathologica 2019;111:37)
- 48 year old woman presenting with renal hybrid oncococytic / chromophobe tumor and multiple schwanommas (Medicine (Baltimore) 2017;96:e8939)
- 69 year old man with oncocytoma with invasive histopathological features (Klin Onkol 2014;27:138)
- Clinical follow up / surveillance or excision / ablation
- Well circumscribed mass (J Kidney Cancer VHL 2017;4:1, Mod Pathol 2019;32:1698, Am J Surg Pathol 1997;21:1)
- Mass present in the cortex but large masses can extend into and expand the perinephric fat, sinus fat or even the renal vein (Am J Surg Pathol 1997;21:1, Cesk Patol 2001;37:51)
- Mahogany brown cut surface to tan or yellow
- Central stellate scar commonly present; might be in an eccentric location
- Hemorrhage might be present but should not show necrosis
- Well circumscribed lesion; usually no pseudocapsule, tumor directly abuts the renal parenchyma (Am J Surg Pathol 1997;21:1, J Kidney Cancer VHL 2017;4:1)
- Architecture is variable: typically small solid nests in a loose connective tissue (edematous looking) stroma
- Other architectures include tubular, micro and macrocystic, packed nests projecting a solid appearance (Am J Surg Pathol 1997;21:1, Cesk Patol 2001;37:51)
- Archipelagenous / trabecular architecture has been described; however, the newly described low grade oncocytic tumor comes in the differential with that pattern (Histopathology 2019;75:174)
- Should not have a papillary architecture; more than focal intracystic papillations is not compatible with the diagnosis of oncocytoma
- Composed of large round eosinophilic cells (oncocytes) with dense granular cytoplasm; nuclei are round and regular with even chromatin; small but conspicuous nucleoli are present
- Bizarre pleomorphic hyperchromatic cells are commonly present and are thought to be degenerative in nature
- Population of small cells (oncoblasts) has been occasionally described at the edge of the scar or nests
- Mitotic figures are rare with no atypical mitosis
- Hemorrhage can be present but no coagulative necrosis
- Rarely have foci of dystrophic calcifications
- Cytoplasmic clearing can be identified around scar areas
- Some show perinephric fat or vascular invasion; this does not change the diagnosis of a benign oncocytoma
- Renal oncocytosis
- Rare conditions where kidneys can have numerous oncocytic lesions; the background kidney shows clusters of oncocytic cells lining benign tubules, cysts and can also be present within renal interstitium (Histol Histopathol 2012;27:1407)
- Chromophobe renal cell carcinoma and hybrid oncocytic tumors may also be present in the background of oncocytosis (Histol Histopathol 2003;18:935)
- Believed to be related to Birt-Hogg-Dubé syndrome
Contributed by Rola Saleeb, M.D., Ph.D.
Contributed by Maria Tretiakova, M.D., Ph.D.
- Highly cellular (Br J Urol 1988;61:534, Acta Cytol 1980;24:355, Cancer 2001;93:390)
- Rounded nests (on cell block)
- Cohesive fragments with discohesive cells
- Abundant eosinophilic and uniformly granular cytoplasm
- Round nuclei with medium sized nucleoli (grade 2)
- Mitosis absent and necrosis absent or very infrequent
- Can see occasional hyperchromatic bizarre cells, considered degenerative in nature
- CK7 negative or scattered rare positive cells
- AMACR
- CAIX
- Vimentin negative but can be positive in cells around central scars or in oncoblasts
- HMB45, melanA
- CK20
- Hale colloidal iron
- References: Semin Diagn Pathol 2005;22:51, Cancers (Basel) 2020;12:168, Am J Surg Pathol 2013;37:1469
Contributed by Maria Tretiakova, M.D., Ph.D.
| Hale | KIT | CK7 | S100A1 | VIM | CAIX | AMACR | SDH | TFE3 | |
| Chromophobe RCC | +++ | +++ | +++ | - | - | - | - | +++ | - |
| Clear cell RCC | - | - | - | - | +++ | +++ | - | +++ | - |
| Oncocytoma | - | +++ | rare | +++ | - | - | - | +++ | - |
| Papillary RCC | - | - | +++ | - | +++ | - | +++ | +++ | - |
| Translocation RCC | - | - | - | - | - | - | ++ | +++ | +++ |
| SDH deficient RCC | - | - | - | - | - | - | - | - | - |
References: Pathol Res Pract 2015;211:303, Ann Diagn Pathol 2020;44:151448, Am J Surg Pathol 2014;38:e6, Arch Pathol Lab Med 2019;143:1455, Transl Androl Urol 2019;8:S123, Hum Pathol 2020 Jul 13 [Epub ahead of print]
- Cytoplasm packed with mitochondria with lamellar cristae (Hum Pathol 1984;15:1054, Histol Histopathol 2003;18:935)
- Commonly possess mitochondrial mutations
- Commonly harbor losses of chromosome 1 and Y and also chromosome 14
- Some cases show translocation of 11q13 that involve the CCDN1 gene and overexpress cyclin D1 (gene product)
- Has been suggested that tumors with chromosomal losses (aneuploid) have the potential to develop into eosinophilic chromophobe renal cell carcinomas with more additional molecular events as p53 mutations, while the cases that have CCDN1 rearrangements don't tend to progress in that manner (Cell Rep 2015;13:1895)
- Familial cases of Birt-Hogg-Dubé syndrome have mutations in the FLCN gene; it is inherited in an autosomal dominant pattern
- Right kidney, needle core biopsy:
- Oncocytic renal neoplasm, favors oncocytoma (see comment)
- Comment: Tumor shows an oncocytic neoplasm with morphological features and immunoprofile consistent with a renal oncocytoma. The lesion is formed of oncocytic cells arranged in small nests in surrounding loose edematous stroma. Tumor cells have round regular nuclei with intermediate sized prominent nucleoli. No irregular nuclear contours or perinuclear halos identified. Lesional cells are positive for PAX8 and KIT and are negative for CK7. However, taking into account possible tumor heterogeneity, we cannot rule out a hybrid oncocytic tumor or an eosinophilic variant of chromophobe renal cell carcinoma in this limited specimen.
- Right kidney, robotic partial nephrectomy:
- Oncocytoma, 2.6 cm
- Surgical margins negative for tumor
- Eosinophilic variant of chromophobe renal cell carcinoma (Anticancer Res 2019;39:2785):
- Nuclear membrane irregularities (wrinkling) and perinuclear halos
- CK7 positive (however, sometimes patchy in eosinophilic chromophobe)
- S100A1 usually negative
- Hybrid oncocytic / chromophobe tumors (HOCT) (Mod Pathol 2019;32:1698):
- Term usually applied in syndromic settings as the Birt-Hogg-Dubé associated tumors but occasionally sporadic cases can present as such
- Overlapping features morphologically and immunophenotypically between oncocytoma and chromophobe renal cell carcinoma
- Best characterized currently as low grade oncocytic tumors
- SDH deficient renal cell carcinoma (Histopathology 2019;74:31):
- Oncocytic papillary renal cell carcinoma:
- Eosinophilic clear cell renal cell carcinoma:
- Epithelioid angiomyolipoma:
- Nuclear pleomorphism
- May have areas of smooth muscle and adipose tissue
- Negative keratins and negative PAX8
- Positive for melanocytic markers HMB45 and MelanA / MART1; positive for cathepsin K
- MiTF associated renal cell carcinoma (TFEB t6;11 can present with oncocytoma-like features) (Cancers (Basel) 2019;11:1110):
- Morphology can be variable; includes a described biphasic morphology
- Positive for TFE3 or TFEB and cathepsin K
- Negative or focally positive for keratin
- Can be focally positive for HMB45 and MART1
- Low grade oncocytic tumors (LOT) (newly proposed entity) (Histopathology 2019;75:174):
- Eosinophilic, solid and cystic renal cell carcinoma (ESC) (newly proposed entity) (Cancers (Basel) 2020;12:168):
- Described in both sporadic and tuberous sclerosis syndrome setting
- Coarse basophilic cytoplasmic granules
- Positive for CK20
- Preliminary data but seem to exhibit indolent clinical behavior
A 40 year old man presented with an incidentally discovered renal cortical mass measuring 4.5 cm on imaging. Histological examination revealed an oncocytic lesion. Lesional cells were positive for PAX8, LMWK and CD117 and only rare cells expressed CK7. Which is most likely the correct diagnosis?
- Angiomyolipoma
- Metanephric adenoma
- Oncocytoma
- Papillary adenoma
- Renal cell carcinoma, chromophobe type
- FLCN mutations
- FH gene mutation
- SDH gene mutations
- VHL gene mutations
Comment Here
Reference: Oncocytoma
- Exceptionally rare, benign, renal neoplasm of early life
- Calcified mass in the renal pelvis composed of (partially) calcified osteoid matrix with osteoblast-like cells, mixed with blastema-like cells and sometimes spindle cells
- First described by Chatten et al. in 1980 (Cancer 1980;45:609)
- Rare benign renal tumor
- Not linked with other renal tumors of childhood
- The majority in the first year of life
- Characteristic osteoid areas with osteoblast-like cells and areas with blastema-like cells
- Spindle cells can be present or absent
- Treated with surgery only
- No recurrences reported
- Ossifying renal tumor of infancy (ORTI)
- No other terms used
- ICD-O: 8967/0 - ossifying renal tumor
- ICD-11: 2F35 & XH3SR2 - benign neoplasm of urinary organs & ossifying renal tumor
- Very rare tumor; 23 reported in the literature (Pediatr Dev Pathol 2017;20:511)
- Age at presentation: 6 days to 2.5 years
- Median age: 6.5 months; only 3 patients older than 1 year (Pediatr Dev Pathol 2017;20:511)
- M:F = ~7:1 (20 males; 3 females)
- Kidney (renal pelvis)
- Unifocal
- Never bilateral
- Unknown
- Cytogenetic and molecular studies found no evidence that it is linked to Wilms tumor
- Not known
- Presents as painless, intermittent, macroscopic hematuria
- Rarely as a mass (incidentally found)
- On imaging, a calcified mass in the renal pelvis
- Diagnosis is made histologically using the surgical specimen
- No abnormalities
- Can be diagnosed on US, CT or MRI investigations
- Visible as a calcified mass (Pediatr Radiol 2014;44:625)
- Benign clinical course
- No recurrences or metastases reported (Pediatr Radiol 2014;44:625)
- 8 week and 2 year old boys with gross hematuria (J Pediatr Urol 2007;3:258)
- 6 month old boy underwent laparoscopic treatment for ORTI (Case Rep Urol 2018;2018:1935657)
- 6 month old boy presented with macrohematuria (J Pediatr Surg 2013;48:e37)
- Usually total nephrectomy (16 cases)
- Rare cases are treated with partial nephrectomy (4 cases)
- Enucleation also possible in rare cases (3 cases)
- No other treatment necessary
- Reference: Pediatr Dev Pathol 2017;20:511
- Exophytic / polypoid mass attached to the renal parenchyma protruding into the renal pelvis
- Rarely greater than 2 to 3 cm in size
- Reference: Pediatr Dev Pathol 2017;20:511
- Consists of 2 to 3 components
- Ossifying component with large osteoblast-like cells
- Blastema-like small, undifferentiated cells
- Spindle cells (not always found)
- Mitoses can be seen in the blastema-like component
- Reference: Pediatr Dev Pathol 2017;20:511
- Immunohistochemistry is only reported in a limited number of cases
- EMA (osteoblast-like cells 8/9; blastema-like cells 0/9)
- Vimentin (osteoblast-like cells 8/9; blastema-like cells 4/9)
- S100 (osteoblast-like cells 1/1; blastema-like cells 0/1)
- Cytokeratin (osteoblast-like cells 2/4; blastema-like cells 0/4)
- WT1 (osteoblast-like cells 1/2; blastema-like cells 1/2)
- Desmin (osteoblast-like cells 1/3; blastema-like cells 0/3)
- Reference: Pediatr Dev Pathol 2017;20:511
- Chromosome 4 trisomy: unknown significance (Pathol Res Pract 2016;212:1004)
- Kidney, total nephrectomy:
- Ossifying renal tumor of infancy (see comment)
- Comment: The tumor consists of a well defined mass with calcification and osteoid with osteoblast-like cells, blending with dense, blastema-like areas. The tumor does not infiltrate the renal sinus or perirenal fat. Tumor cells show strong and diffuse vimentin and EMA positivity in the osteoid component. This is a benign tumor, which requires no further treatment.
- Wilms tumor (WT) with prominent heterologous osteoid differentiation:
- Other Wilms tumor components normally present
- WT1 does not differentiate
- Mesoblastic nephroma:
- Does not contain blastema-like cells
- Cartilage but not osteoid may be present
- Renal teratoma:
- Other tissues are present
A 6 month old boy presents with a painless macroscopic hematuria that was noticed by his parents. Imaging studies show a right sided calcified mass in the renal pelvis. Total right nephrectomy is performed. Both histology (microscopic image shown above) and imaging fit with the very rare entity, ossifying renal tumor of infancy. Which is the most characteristic pathologic finding for this tumor?
- Infiltrative intrarenal growth
- Numerous mitoses
- Osteoid with osteoblast-like and blastema-like cells
- Unique immunohistochemical profile
Comment Here
Reference: Ossifying renal tumor of infancy
- Benign
- High grade malignant
- Low grade malignant
- Uncertain / undetermined malignant potential
- Usually circumscribed malignant neoplasm characterized by predominantly papillary or tubulopapillary architecture
- Predominantly papillary or tubulopapillary pattern lined by small, basophilic cells or large, eosinophilic cells
- Size cut off > 15 mm; often contain foamy histiocytes and psammoma bodies
- Must exclude other renal cell carcinomas (RCCs) with papillary architecture
- Alpha methyacyl CoA racemase (AMACR) positivity is a desirable feature
- Histologic subclassification to type 1 and type 2 is no longer recommended
- Due to poor interobserver reproducibility, the presence of overlapping features in a significant proportion of cases, the lack of prognostic significance and the historical inclusion of outliers (newly recognized entities) in the old type 2 category (Mod Pathol 2021;34:1392)
- Not recommended: tubulopapillary renal cell carcinoma, renal papillary adenocarcinoma, chromophil renal cell carcinoma
- ICD-O: 8260/3 - papillary renal cell carcinoma
- ICD-11: 2C90.0 & XH1D07 - renal cell carcinoma of kidney, except renal pelvis & papillary renal cell carcinoma
- Second most common type of kidney tumors in adults; 15 - 20% of renal cell carcinoma (Curr Opin Urol 2022;32:344)
- Male predominance (M:F = 1.5 - 2:1) (Urol Oncol 2021;39:327)
- Renal cortex
- MET alterations are found mainly in low grade tumors
- CDKN2A, MYC pathway and NRF2-ARE pathway aberrations are observed in high grade tumors
- Other pathways or complexes implicated in papillary renal cell carcinoma (PRCC) are the Hippo signaling pathway, SWI / SNF complex and chromatin modifier pathways (N Engl J Med 2016;374:135)
- No definite specific etiology but common in end stage kidney disease (ESRD)
- Could arise from progenitor-like cell population in proximal renal tubules in ESRD (Am J Pathol 2011;178:828, Am J Pathol 2019;189:2046)
- Usually asymptomatic; incidental finding on imaging
- Maybe multifocal or bilateral in chronic kidney disease and hereditary PRCC (germline MET mutation)
- Based on the assessment of both the morphology and immunohistochemical profile
- Essential to exclude outliers particularly in high grade lesions
- No specific radiologic findings
- Homogenous, solid mass with relative hypovascularity compared to nonpapillary RCC; may have cystic change and calcifications (Urol Oncol 2021;39:327)
- Generally thought to have a better prognosis than other clear cell RCC (Am J Surg Pathol 2002;26:281)
- High WHO / ISUP grade, high stage and ATP binding cassette subfamily C member 2 (ABCC2) brush border are associated with worse prognosis (Hum Pathol 2022;120:57, Histopathology 2023 Sep 7 [Epub ahead of print])
- 11 year old renal transplant boy with bilateral PRCC (European J Pediatr Surg Rep 2022;10:e160)
- 59 year old man with collision tumor of papillary and chromophobe RCCs (Oxf Med Case Reports 2022;2022:omac048)
- 63 year old man with PRCC with concurrent primary renal glomus tumor (Int J Clin Exp Pathol 2022;15:459)
- 69 year old man with metastatic PRCC to testicle and penis (Urol Case Rep 2020;33:101362)
- 71 year old man with synchronous ipsilateral PRCC and urothelial carcinoma (Oncol Lett 2023;25:221)
- Resection or ablation for localized disease
- Selective MET kinase inhibitor, MET / VEGF inhibitor, multitargeted tyrosine kinase inhibitors (TKIs) and PD-1 / PDL1 inhibitors for advanced or metastatic disease (Curr Opin Urol 2022;32:344)
- For advanced metastatic PRCC cabozantinib shows higher efficacy over sunitinib and other MET kinase inhibitors (Lancet 2021;397:695)
- Most tumors are circumscribed, surrounded by a pseudocapsule (Adv Anat Pathol 2019;26:124)
- Yellow-tan, red-brown or variegated cut surfaces
- Granular or friable in appearance consistent with the papillary morphology
- Variable areas of necrosis and cystic degeneration (Cancer 1976;38:2469)
Contributed by Nicole K. Andeen, M.D. and Maria Tretiakova, M.D., Ph.D.
Images hosted on other servers:
- Predominantly papillary or tubulopapillary architecture (Mod Pathol 2021;34:1392)
- Lined by cells with variation of morphologies, ranging from small, basophilic cuboidal cells with inconspicuous nucleoli to large, eosinophilic cells with prominent nucleoli; may have clear cytoplasm, known pitfall to have clearing in PRCC
- Cells can be arranged in linear or a pseudostratified pattern
- Heterogeneity and mixed morphology are very common (47 - 48% of cases), hence this is one of the reasons why typing is no longer favored (Mod Pathol 2021;34:1392, Am J Surg Pathol 2014;38:887, Am J Surg Pathol 2017;41:1618)
- Often infiltrated by foamy macrophages
- Psammoma bodies and hemosiderin pigments can be present
- Reported patterns (Mod Pathol 2021;34:1392)
- Biphasic (alveolar / squamoid) PRCC
- Dual cell populations, nests of larger (squamoid) eosinophilic cells surrounded by smaller amphophilic cells forming an alveolar pattern
- Evidence of aggressive behavior has been documented in up to 15% of the cases (Histopathology 2018;72:777)
- Warthin-like PRCC
- Eosinophilic papillae with dense lymphocytic infiltrates resembling Warthin tumor of the salivary gland
- Usually high grade tumors that can exhibit aggressive clinical behavior (Ann Diagn Pathol 2017;27:48)
- Solid (pseudosolid) PRCC
- Solid architecture due to compressed tubular and papillary structures, lined by small cells with low grade nuclei
- Reported to have an indolent clinical behavior (Ann Diagn Pathol 2016;23:51)
- Biphasic (alveolar / squamoid) PRCC
- Histologic patterns associated with worse prognosis: solid, micropapillary, hobnailing, microcystic architecture (Am J Surg Pathol 2020;44:582, Am J Surg Pathol 2022;46:392)
- WHO / ISUP grading system has been validated as a prognostic parameter for PRCC
- Based on nucleolar prominence (grades 1 - 3); nuclear pleomorphism or sarcomatoid / rhabdoid differentiation (grade 4) (Am J Surg Pathol 2013;37:1490, Histopathology 2019;74:4)
Contributed by Rola Saleeb, M.D., Ph.D., Vincent Francis Castillo, M.D., Nicole K. Andeen, M.D.,
Maria Tretiakova, M.D., Ph.D., Semir Vranić, M.D., Ph.D. and @SueEPig on Twitter
- Papillary fragments with fibrovascular cores
- Cohesive clusters of small cells with bland nuclei in low grade tumors
- Intracellular hemosiderin, foamy macrophages (Cancer 1998;84:303)
- AMACR
- Sensitive but not entirely specific (Am J Surg Pathol 2004;28:69)
- CK7
- Tends to be more positive in lower grade than higher grade tumors (Am J Surg Pathol 2014;38:887)
- PAX8, AE1 / AE3, EMA, CAM 5.2, vimentin
| Renal tumors | AMACR | CK7 | Vimentin | CAIX | CD117 | TFE3 | FH | SDH |
| Papillary RCC | + | Variable | + | - | - | - | + (retained) | + (retained) |
| Clear cell RCC | -/+ | - | + | + | - | - | + (retained) | + (retained) |
| Chromophobe RCC | - | + | - | - | + | - | + (retained) | + (retained) |
| Oncocytoma | - | - | - | - | + | - | + (retained) | + (retained) |
| TFE3 rearranged RCC | + | - | - | - | - | + | + (retained) | + (retained) |
| FH deficient RCC | Variable | - | Variable | - | - | - | - (loss) | + (retained) |
| SDH deficient RCC | - | - | - | - | - | - | + (retained) | - (loss) |
- References: Mod Pathol 2021;34:1392, Cancers (Basel) 2020;12:602
- No molecular markers specific for the diagnosis of PRCC
- Chromosome 7 and 17 gains and loss of Y chromosome in low grade tumors (N Engl J Med 2016;374:135)
- MET mutations seen in former type 1 and low grade PRCC; CDKN2A mutation or promoter methylation in aggressive PRCC (N Engl J Med 2016;374:135)
- Exclude FH gene mutation and TFE3 rearrangements for high grade PRCC
- Right kidney (mass), biopsy:
- Papillary renal cell carcinoma
- WHO / ISUP grade 3 in this limited specimen
- Right kidney (mass), partial nephrectomy:
- Papillary renal cell carcinoma, pT1a (see synoptic report)
- WHO / ISUP grade 2
- Tumor size: 2.5 cm
- Tumor is limited to kidney
- Negative for sarcomatoid / rhabdoid features and necrosis
- Negative for vascular and lymphovascular invasion
- All margins are negative for tumor
- Main DDx for low grade PRCC (see Diagrams / tables)
- Clear cell papillary (CCP) renal cell tumor (Mod Pathol 2013;26:697):
- Metanephric adenoma (MA) (Mod Pathol 2015;28:1236):
- Mucinous tubular and spindle cell carcinoma (MTSC) (Diagn Pathol 2015;10:168):
- Anastomosing tubules lined by low grade cuboidal cells with bland spindle cells in a myxoid stroma; multiple chromosomal losses
- Mucin special stains are positive in MTSC and negative in PRCC
- Main DDx for high grade PRCC (see Diagrams / tables)
- FH deficient RCC (Am J Surg Pathol 2016;40:865):
- MiT family translocation RCC (mostly TFE3 rearranged RCC) (Semin Diagn Pathol 2015;32:103, Adv Anat Pathol 2022;29:131):
- Key feature is mixed histologic patterns including large nests, alveolar areas as well as papillary areas
- Commonly mixed areas of clear and eosinophilic cells
- Commonly associated with psammoma bodies, however, can also be observed in PRCC
- Often a panel of IHC stains is required to exclude translocation RCC; positive TFE3 (MRQ37 clone is known to be associated with false positive expression), cathepsin K (positive only in ~30% of the cases), weak / patchy keratins and often negative EMA
- In cases with ambiguous IHC profile, FISH studies are recommended
- Other possible DDx for high grade PRCC
- Acquired cystic disease associated RCC (Am J Surg Pathol 2018;42:1156):
- Cribriform (sieve-like) appearance; high grade eosinophilic cells, oxalate crystals often present
- Occurs in end stage kidneys
- Overlapping immunoprofile with high grade / eosinophilic PRCC
- Morphology and cystic ESRD are key clues to the diagnosis
- ALK rearranged RCC (Mod Pathol 2020;33:2564):
- Rare entity, morphology characterized by vacuoles, mucinous stroma, cribriform architecture and possibly some papillary morphology
- Sickle cell trait
- ALK IHC positive or ALK rearrangement by FISH
- Renal medullary carcinoma (Mod Pathol 2008;21:647):
- Infiltrative (noncircumscribed), high grade adenocarcinoma
- Sickle cell trait
- SMARCB1 (INI1) IHC loss (key feature); possible OCT4 positive staining
- Mixed, high grade infiltrative morphology is key to exclude PRCC
- Collecting duct carcinoma (Am J Surg Pathol 2018;42:279):
- Infiltrative growth (noncircumscribed), adenocarcinoma, desmoplastic stroma
- Diagnosis of exclusion for high grade infiltrative lesions
- Mixed, high grade infiltrative morphology is key to exclude PRCC
- Acquired cystic disease associated RCC (Am J Surg Pathol 2018;42:1156):
- Papillary renal neoplasm with reversed polarity (PRNRP):
- Papillary architecture lined by cells with abundant and eosinophilic cytoplasm and apically located linearly arranged nuclei (Am J Surg Pathol 2019;43:1099)
- WHO / ISUP grade 1 - 2 (key feature)
- Positive for GATA3 nuclear staining (key feature), weak / negative AMACR and vimentin (Am J Surg Pathol 2019;43:1099)
- GATA3 positive in papillary renal neoplasm with reversed polarity (Am J Surg Pathol 2019;43:1099)
- Commonly harbor KRAS mutations (Mod Pathol 2020;33:1157, Mod Pathol 2020;33:690)
- Older terminologies: oncocytic low grade PRCC or type 4 PRCC (Am J Surg Pathol 2017;41:1618)
- Often smaller in size than classic PRCCs (most cases reported were pT1a disease)
- Indolent entity, no cases with disease progression reported to date (Front Oncol 2023;13:1101268)
- Important to recognize from eosinophilic PRCC, which is often a high grade lesion (Lab Investig 2023;103:S697)
A 65 year old man presented with an 8 cm kidney tumor located in the right lower pole extending to the renal sinus. Cut sections showed an encapsulated mass with brown-red surface and necrotic areas. Microscopic examination showed almost exclusively tubulopapillary architecture, which is lined by cells with voluminous, eosinophilic cytoplasm and nuclei with very prominent nucleoli. Calcifications were also noted. Which of the following IHC results are expected in PRCC?
- CAIX negative, CD117 negative, AMACR positive, CK7 negative, FH loss, TFE3 negative
- CAIX negative, CD117 negative, AMACR positive, CK7 negative, FH retained, TFE3 positive
- CAIX negative, CD117 negative, AMACR positive, CK7 patchy, FH retained, TFE3 negative
- CAIX negative, CD117 positive, AMACR negative, CK7 positive, FH retained, TFE3 negative
- CAIX positive, CD117 negative, AMACR negative, CK7 negative, FH retained, TFE3 negative
Comment Here
Reference: Papillary renal cell carcinoma
- CK7 is always positive in PRCC
- MET mutations are usually seen in aggressive PRCC
- Tumors with tubulopapillary, solid and cystic architecture lined by eosinophilic, high grade nuclei is diagnostic of PRCC and does not warrant further IHC studies
- WHO / ISUP nucleolar grading has prognostic significance in PRCC and should be reported
Comment Here
Reference: Papillary renal cell carcinoma
- Benign unencapsulated renal epithelial neoplasm characterized by papillary, tubular or tubulopapillary architecture with low grade nuclei and a diameter of ≤ 15 mm
- Benign papillary or tubulopapillary, low nuclear grade lesions of the kidney, similar in morphology to classic papillary renal cell carcinoma (PRCC); however, they lack fibrous capsules and are ≤ 15 mm
- Prevalent in damaged and injured kidneys
- Could be related to a progenitor / stem cell-like renal tubular cell population that increases upon kidney damage
- Postulated to be precursor lesions to PRCC
- However, the prevalence of adenoma is much higher than of carcinoma, indicating that many of them do not progress
- Tubulopapillary adenoma
- Renal adenoma (not recommended)
- ICD-O: 8260/0 - papillary adenoma, NOS
- ICD-11: 2F35 & XH09B0 - benign neoplasm of urinary organs & papillary adenoma, NOS
- ~20% in autopsy kidneys; 7% in nephrectomy specimens (Am J Surg Pathol 2019;43:277, Hum Pathol 2007;38:239)
- Incidence increases with age
- 8% (20 - 40 years old) versus 25% (> 40 years old) versus 40% in 70 - 90 year olds (Am J Surg Pathol 2019;43:277, Semin Diagn Pathol 1998;15:41)
- M:F = 4.6 - 5:1 (Am J Surg Pathol 2019;43:277, Kidney Int 2002;61:2201)
- More frequent in resected kidneys with PRCC compared with other subtypes (Hum Pathol 2007;38:239)
- More common in those with end stage renal disease, acquired cystic disease, glomerulosclerosis, prolonged hemodialysis and a history of smoking (Am J Surg Pathol 2019;43:277, Kidney Int 2002;61:2201)
- Renal cortex
- Studies hypothesize that the origin is renal tubular progenitor / stem cells (Am J Pathol 2011;178:828)
- Renal tubular progenitor / stem cells proliferate upon renal injury or damage (Int Urol Nephrol 2014;46:2127)
- These cells give rise to papillary adenoma, which in turn could be a precursor to PRCC (Hum Pathol 2007;38:239, Mod Pathol 2003;16:1053)
- Unknown, possibly secondary to renal injury as chronic medical renal diseases, pyelonephritis or nearby mass tumor effect (Am J Pathol 2019;189:2046)
- Asymptomatic; incidentally found in nephrectomies removed for larger tumors or other causes
- Common in end stage kidneys (Virchows Arch 2008;453:313, Transplant Proc 2008;40:3354, Int Urol Nephrol 2014;46:2127)
- Imaging: usually computed tomography (CT) scan, ≤ 15 mm, often not seen
- Biopsy or resection
- Diagnosis on needle biopsy should be made with caution as capsule and nuclear grade heterogeneity may not be visualized
- CT / magnetic resonance imaging (MRI): ≤ 15 mm in size
- No specific imaging findings; may be difficult to distinguish from other renal lesions
- Benign lesions thought to be precursors of PRCC (Hum Pathol 2007;38:239, Mod Pathol 2003;16:1053)
- However, the incidence of PRCC is much lower than papillary adenoma, indicating that not all cases have the potential to progress to PRCC (Am J Pathol 2019;189:2046)
- Size criterion has been changed from 5 mm to 15 mm based on data that these lesions have no capacity to metastasize (Eur Urol 2011;60:983)
- Change allows for increased acceptance of donor kidneys for transplantation, in which kidneys with papillary lesions ≤ 15 mm would not be rejected (Ann Transplant 2014:19:362)
- 34 year old man with autosomal dominant polycystic kidney disease and renal papillary adenomas with unusual clinical presentation (Iran J Kidney Dis 2013;7:439)
- 47 year old man with incidentally detected renal adenomatosis in hydronephrotic kidney (Turk J Urol 2013;39:56)
- 62 year old woman with renal adenomatosis with 10 year imaging follow up (J Med Case Rep 2022;16:168)
- Treatment is based on the presenting tumor or condition
- Considered benign; does not on its own require treatment
- Usually subcapsular
- Spherical or wedge shaped, grayish white to yellow (Am J Surg Pathol 2019;43:277)
- Smaller lesions may not be visible
- May be multiple (renal adenomatosis)
- Papillary, tubular or tubulopapillary architecture
- No fibrous capsule; in direct contact to adjacent renal parenchyma
- Lined by cuboidal cells with scant, pale cytoplasm with low nuclear grade (WHO / International Society of Urological Pathology [ISUP] grade 1 - 2) (similar to classic pattern of PRCC)
- Papillary adenomas are benign lesions and are not graded
- Tumors with high grade nuclei should not be diagnosed as papillary adenoma
- Some lesions show cytoplasmic clearing with fine granularity similar to what is occasionally seen in PRCC
- Lesions diagnostic of clear cell papillary renal cell tumor should not be considered as papillary adenoma
- Absent to rare mitosis
- Foamy macrophages and psammomatous calcifications may be present (Am J Surg Pathol 2019;43:277)
- Cellular, bland cells in tubules spherules and papillae, minimal cytologic atypia (identical to low grade PRCC) (Cancer Cytopathol 2022;130:927)
- CK7, AMACR, vimentin (similar to PRCC) (Hum Pathol 2007;38:239)
- CAIX (positive in CCPRCT)
- GATA3, L1-CAM (positive in papillary renal neoplasm with reversed polarity) (Am J Surg Pathol 2019;43:1099)
- WT1, CD57, BRAF V600E (positive in metanephric adenoma) (Mod Pathol 2015;28:1236)
- Trisomy 7 and 17 and loss of Y chromosome; may have gains of chromosomes 12, 16 and 20
- Chromosomal profile is similar to classic PRCC and end stage kidney disease (Hum Pathol 2007;38:239, Mod Pathol 2003;16:1053, Virchows Arch 2008;453:313, Am J Pathol 2019;189:2046)
- KMT2C mutation
- Could be an early molecular event in papillary adenoma (Am J Pathol 2019;189:2046)
- Kidney, left, partial nephrectomy:
- Papillary adenoma, 1.2 cm
- Kidney, right, needle core biopsy:
- Low grade papillary renal lesion (see comment)
- Comment: The differential diagnosis includes papillary adenoma and papillary renal cell carcinoma. The clinical history is noted regarding the 0.6 cm size of the lesion. Clinical correlation is advised as to the size of the lesion by imaging. A lesion ≤ 1.5 cm would be consistent with a papillary adenoma, while a lesion > 1.5 cm would be classified as a papillary renal cell carcinoma.
- Papillary renal cell carcinoma (PRCC):
- Size > 15 mm
- Capsule is present or with high grade morphology
- See differential diagnosis of low grade PRCC
- Papillary renal neoplasm with reversed polarity (PRNRP):
- Papillary architecture lined by cells with abundant and eosinophilic cytoplasm and apically located linearly arranged nuclei (Am J Surg Pathol 2019;43:1099)
- WHO / ISUP grade 1 - 2 (key feature)
- Positive for GATA3 nuclear staining (key feature), weak / negative AMACR and vimentin (Am J Surg Pathol 2019;43:1099)
- GATA3 positive in papillary renal neoplasm with reversed polarity (Am J Surg Pathol 2019;43:1099)
- Commonly harbor KRAS mutations (Mod Pathol 2020;33:1157, Mod Pathol 2020;33:690)
- Older terminologies: oncocytic low grade PRCC or type 4 PRCC (Am J Surg Pathol 2017;41:1618)
- Often smaller in size than classic PRCCs (most cases reported were pT1a disease)
- Indolent entity, no cases with disease progression reported to date (Front Oncol 2023;13:1101268)
- Important to recognize from eosinophilic PRCC, which is often a high grade lesion (Lab Investig 2023;103:S697)
- Clear cell papillary renal cell tumor (CCPRCT) (Mod Pathol 2013;26:697):
- Metanephric adenoma (MA) (Mod Pathol 2015;28:1236):
- Primitive blue cells forming small, crowded acini; WT1 and CD57 positive in MA while negative in PRCC
- Positive for BRAF V600E mutation
A 38 year old woman with recurrent pyelonephritis was found to have a 1 cm left renal mass on imaging. A biopsy of the lesion was performed and showed the morphology above. Which of the following is the correct diagnosis and WHO / ISUP grade?
- Papillary adenoma, not graded
- Papillary adenoma WHO / ISUP grade 2
- Papillary renal cell carcinoma WHO / ISUP grade 1
- Papillary renal cell carcinoma WHO / ISUP grade 2
Comment Here
Reference: Papillary adenoma
- Multiple chromosomal gains and losses
- Trisomies of 7 and 17 only
- Trisomies of 7, 17 and loss of Y and less frequently gains in chromosomes 12, 16 and 20
- Trisomies of 7, 17 and loss of Y as well as deletions of the short arm of chromosome 3
Comment Here
Reference: Papillary adenoma
- Most experts believe it is a variant of type 1 papillary renal cell carcinoma
- NOT considered a distinct entity by ISUP (Am J Surg Pathol 2013;37:1469) or WHO classification
- Synonymous term: oncocytic papillary renal cell carcinoma (OPRCC) (Ann Diagn Pathol 2006;10:133, Int J Clin Exp Pathol 2013;6:1392)
- May be the same entity as oncocytic papillary renal cell carcinoma with inverted nuclear pattern (Pathol Int 2009;59:137)
- Kidney
- Usually male, median age 71 years (Am J Surg Pathol 2005;29:1576)
- Low stage, indolent clinical behavior
- 81 year old woman with oncocytic papillary renal cell carcinoma (Int J Urol 2009;16:765)
- Resection
- Median 3 cm, well circumscribed, no extrarenal extension
- Thin, nonfibrotic papillae lined by single layer of oncocytic cells
- Low grade, round, regular, nonoverlapping nuclei with central nucleolus (Am J Surg Pathol 2005;29:1576)
- Abundant granular eosinophilic cytoplasm
- Variable foamy macrophages within papillae
- Hemosiderin accumulation, focal necrosis
- May have luminal orientation of tumor nuclei (Pathol Int 2009;59:137) or solid oncocytoma-like areas (Ann Diagn Pathol 2006;10:133)
Contributed by Nicole K. Andeen, M.D. and Maria Tretiakova, M.D., Ph.D.
Contributed by Semir Vranić, M.D., Ph.D., University of Sarajevo (Bosnia)
Images hosted on other servers:
- AMACR, vimentin, mitochondrial antigen, CD10 (79%),
- Variable CK7 (17% - 100%), variable RCC (Int J Clin Exp Pathol 2013;6:1392, Am J Surg Pathol 2014;38:887)
- c-kit / CD117, low Ki67 proliferative rate
- Abundant mitochondria with lamellar cristae (Ann Diagn Pathol 2006;10:133)
- Majority have trisomy 7 and 17, loss of Y (Hum Pathol 2008;39:96, Int J Clin Exp Pathol 2013;6:1392, Ann Diagn Pathol 2006;10:133,, Am J Surg Pathol 2014;38:887)
- Gene expression studies show closer relationship to type 1 than type 2 papillary RCC (Cancer Res 2005;65:5628)
- Oncocytoma: no papillary architecture, no necrosis; negative for vimentin, CD10, AMACR, CK7; positive for c-kit / CD117
- Papillary adenoma with oncocytic cells: 15 mm (1.5 cm) or less (size criteria)
- Papillary type 1 tumors: single layer of cells lining papillae but with pale basophilic cytoplasm, strong diffuse CK7 positivity
- Papillary type 2 tumors: higher nuclear grade, crowded and pseudostratified nuclei
- Clinically indolent papillary renal cell tumor characterized by oncocytic granular tumor cells in papillary or tubulopapillary architecture, apically located nuclei, diffuse and strong GATA3 expression and frequent KRAS mutation
- Predominant thin papillary or tubulopapillary growth
- Tumor cells with eosinophilic, finely granular cytoplasm and low grade, apically located nuclei
- Diffuse and strong GATA3 nuclear staining
- Recurrent KRAS missense mutation at codon 12
- Indolent, no recurrence or metastasis reported
- Papillary renal cell carcinoma with oncocytic cells and nonoverlapping low grade nuclei (Hum Pathol 2008;39:96)
- Oncocytic papillary renal cell carcinoma with inverted nuclear pattern (Pathol Int 2009;59:137)
- Papillary renal cell carcinoma, type 4 (Am J Surg Pathol 2017;41:1618)
- Oncocytic papillary renal neoplasm with inverted nuclei (Histopathology 2020;76:1070)
- Papillary adenoma, type D (Am J Surg Pathol 2019;43:277)
- Accounting for 8.6 - 9.1% of previously diagnosed papillary renal cell carcinoma (Histopathology 2021;78:1019, Hum Pathol 2021;112:48)
- Mean age: 63 years (range: 36 - 82 years) (Am J Surg Pathol 2021 Aug 5 [Epub ahead of print])
- M:F = 56:44 (Am J Surg Pathol 2021 Aug 5 [Epub ahead of print])
- Kidney, renal cortex
- Arising from the epithelial cells of the distal renal tubule, especially the cortical collecting duct (Histopathology 2020;76:1070)
- Recurrent KRAS missense mutation at codon 12 (Mod Pathol 2020;33:1157, Mod Pathol 2020;33:690, Histopathology 2020;76:1070, Histopathology 2021;78:1019, Hum Pathol 2021;112:48, Am J Surg Pathol 2021 Aug 5 [Epub ahead of print])
- Type D papillary adenoma represents the precursor or the small sized analogue (Histopathology 2021;78:1019)
- No specific risk factors reported
- Usually asymptomatic and incidentally found (Histopathology 2021;78:1019, Hum Pathol 2021;112:48)
- 21 - 25% associated with end stage renal disease and hemodialysis (Histopathology 2021;78:1019, Hum Pathol 2021;112:48)
- Mostly incidental mass on the radiologic imaging (Histopathology 2021;78:1019, Hum Pathol 2021;112:48)
- MRI: hyperintense in T1 and hypointense in T2
- Ultrasonography: isoechoic or heterogeneous mass with a sharp margin
- Indolent; no recurrence or metastasis reported (Mod Pathol 2020;33:1157, Mod Pathol 2020;33:690, Histopathology 2020;76:1070, Histopathology 2021;78:1019, Hum Pathol 2021;112:48, Am J Surg Pathol 2021 Aug 5 [Epub ahead of print])
- 61 year old man with concurrent clear cell renal cell carcinoma (Diagn Pathol 2020;15:123)
- 80 year old patient with a 2 cm renal mass (NYU Langone Health: Genitourinary Pathology - Papillary Renal Cell Carcinoma with Reversed Polarity [Accessed 22 September 2021])
- Partial or radical nephrectomy (Histopathology 2021;78:1019, Hum Pathol 2021;112:48, Am J Surg Pathol 2021 Aug 5 [Epub ahead of print])
- Often circumscribed, soft and brown-tan
- May contain cystic component and hemorrhage
- Mean size: 2.1 cm (median: 1.7 cm; range: 0.8 - 8.5 cm) (Am J Surg Pathol 2021 Aug 5 [Epub ahead of print])
Contributed by @katcollmd on Twitter
Images hosted on other servers:
Courtesy of Katrina Collins, M.D.
- Major morphologic features (Am J Surg Pathol 2019;43:277, Hum Pathol 2021;112:48, Am J Surg Pathol 2021 Aug 5 [Epub ahead of print]):
- Predominant thin papillary or tubulopapillary growth
- Focal or diffuse stromal hyalinization
- Eosinophilic, finely granular cytoplasm
- Apically located, low grade (WHO / ISUP grade 1 - 2) nuclei
- Minor morphologic findings: cystic change, edematous papillae, aggregates of foamy histiocytes, mast cell infiltration, hobnail cells, clear cell change, cytoplasmic vacuoles, intracytoplasmic hemosiderin, pseudostratification, multinucleation and metaplastic ossification (Mod Pathol 2020;33:1157, Am J Surg Pathol 2019;43:1099, Histopathology 2020;76:1070, Int J Surg Pathol 2020;28:728, Histopathology 2021;78:1019, Hum Pathol 2021;112:48)
- No mitosis or necrosis
Contributed by Hsin-Yi Chang, M.D., Jen-Fan Hang, M.D., @ahmsab_MD on Twitter and @katcollmd on Twitter
- GATA3, CK7, PAX8: diffuse and strong
- EMA: apical membranous
- L1CAM (CD171): basolateral membranous
- Fumarate hydratase: retained cytoplasmic expression
- Variable: AMACR, CD10, RCC, E-cadherin
- References: Am J Surg Pathol 2017;41:1618, Am J Surg Pathol 2019;43:1099, Mod Pathol 2020;33:1157, Mod Pathol 2020;33:690, Histopathology 2020;76:1070, Int J Surg Pathol 2020;28:728, Hum Pathol 2021;112:48
- CD117, vimentin, except rare cases with weak, focal expression (Am J Surg Pathol 2021 Aug 5 [Epub ahead of print])
- CK20, CAIX, TFE3 (Pathol Int 2009;59:137, Int J Surg Pathol 2020;28:728)
- Recurrent KRAS missense mutation at codon 12 in 75 - 93.3% of cases (Mod Pathol 2020;33:1157, Mod Pathol 2020;33:690, Histopathology 2020;76:1070, Histopathology 2021;78:1019, Hum Pathol 2021;112:48, Am J Surg Pathol 2021 Aug 5 [Epub ahead of print])
- Gain of chromosomes 7 and 17 and loss of chromosome Y noted in some cases by fluorescence in situ hybridization (Am J Surg Pathol 2019;43:1099, Int J Surg Pathol 2020;28:728)
- No copy number abnormalities in chromosomes 7, 17 and Y by whole exome sequencing or microarray analysis (Hum Pathol 2021;112:48, Am J Surg Pathol 2021 Aug 5 [Epub ahead of print])
- Kidney, right, partial nephrectomy:
- Papillary renal neoplasm with reverse polarity (see comment)
- Comment: Papillary renal neoplasm with reverse polarity is currently considered to be a histologic variant of papillary renal cell carcinoma; however, recent studies suggest that it has a very indolent clinical behavior. No recurrence or metastasis has been reported.
- Papillary renal cell carcinoma, type 2:
- Nuclear stratification without reverse polarity
- Frequent high grade nuclei (WHO / ISUP grade 3)
- GATA3 negative
- No KRAS mutation
- Papillary renal cell carcinoma, oncocytic variant:
- Centrally located nuclei without reverse polarity
- WHO / ISUP grade ranging from 2 to 3
- GATA3 negative
- No KRAS mutation
- Fumarate hydratase deficient renal cell carcinoma:
- Mixed architectural patterns of papillary, solid, tubulocystic, cribriform, etc. (Am J Surg Pathol 2016;40:865)
- High grade nuclei with prominent eosinophilic nucleoli and perinucleolar halo (Am J Surg Pathol 2007;31:1578)
- Fumarate hydratase loss of expression; 2SC overexpression
- GATA3 negative
- No KRAS mutation
- Acquired cystic disease associated renal cell carcinoma:
- Mixed architectural patterns of papillary, solid, tubulocystic, cribriform, etc.
- Presence of oxalate crystals
- Frequent high grade nuclei (WHO / ISUP grade 3) without reverse polarity
- GATA3 negative
- No KRAS mutation
- Papillary adenoma:
- Size ≤ 1.5 cm
- Type D papillary adenoma is the smaller sized equivalent tumor
Comment Here
Reference: Papillary renal neoplasm with reverse polarity
Comment Here
Reference: Papillary renal neoplasm with reverse polarity
- Uncommon, benign, exclusively cystic neoplasm of the kidney; lacks immature nephroblastic elements or solid growth (Hum Pathol 2016;48:81)
- Multicystic neoplasm, often found in children under 4 years old, composed of fibrous septa and differentiated tubules
- Correlation to DICER1 mutations
- Excellent prognosis
- Pediatric cystic nephroma (PCN) occurs most commonly in children younger than 4 years old, most of whom are boys (Am J Surg Pathol 2016;40:1591)
- Familial cases have been linked to DICER1 germline mutations and familial pleuropulmonary blastoma (PPB), whose main phenotypic spectrum includes PPB, PCN, ovarian Sertoli-Leydig tumors and multinodular goiter and less commonly, pineoblastoma, pituitary blastoma, nasal chondromesenchymal hamartoma and medulloepithelioma (Hum Pathol 2016;48:81)
- Palpable abdominal mass may be incidentally found by parents, caretakers or during routine physical examination
- Most often unilateral but familial forms may present bilaterally (J Med Genet 2010;47:863)
- Imaging modalities (e.g., ultrasound, CT, MRI) show a multilocular cystic encapsulated mass
- Partial or radical nephrectomy will show diagnostic features
- On CT / MRI, appears as a cystic, multilocular mass, often with pseudocapsule defined as a thin rim of tissue demarcating the margin of the lesion from the adjacent renal parenchyma; may abut the renal pelvis or show protrusion / herniation into the renal pelvis (Hum Pathol 2016;48:81, Radiographics 1995;15:653)
- Ultrasonographic findings are multiple anechoic spaces separated by thin septa (Radiographics 1995;15:653)
- Prognosis is excellent: overall survival was 100% over a median followup of 2.4 years in one study (J Urol 2007;177:294)
- Presumed transformation to anaplastic sarcoma in rare cases; however, it is unknown whether all anaplastic sarcomas of the kidney arise from pre-existing cystic nephromas
- Anaplastic sarcomas show presence of cysts with solid areas that may be composed of undifferentiated spindle cells with anaplastic changes, benign or malignant chondroid differentiation; less common features include blastemal-like areas, foci of rhabdomyoblastic differentiation and small islands of osteoid
- Co-occurrence of TP53 mutation may be present with DICER1 mutation
- Longitudinal risks for transformation, responsiveness and prognosis are unknown
- References: Mod Pathol 2018;31:169, Mod Pathol 2014;27:1267
- 5 month old girl with pleuropulmonary blastoma in association with cystic nephroma and DICER1 syndrome (Radiology 2014;273:622)
- 7 month old girl with histologic features predominately of cystic nephroma but with foci containing atypical mitotic figures and anaplastic nuclei thought to be nascent anaplastic sarcoma (Hum Pathol 2016;53:114)
- 16 month old girl with multilocular cystic renal tumor extending into the renal pelvis and ureter (J Urol Surg 2014;1:39)
- 2 year old girl with pediatric cystic nephroma and pleuropulmonary blastoma with mutation analysis and pedigree (BMC Cancer 2017;17:146)
- 8 year old girl with DICER1 mutation, anaplastic sarcoma and septated renal cysts, thought to be cystic nephroma (Pediatr Blood Cancer 2016;63:1272)
- 9 year old boy with bilateral and recurrent pediatric cystic nephroma (Can Urol Assoc J 2021;15:E290)
- Adequately treated by resection with excellent prognosis if completely excised (J Urol 2007;177:294)
- Relatively large (9 cm average diameter) cystic lesion, well circumscribed from the surrounding normal kidney
- Multiple smooth variably sized (mm to cm) cysts with clear fluid
- Thin septa with no expansile nodules altering cyst contours (Sebire: Diagnostic Pediatric Surgical Pathology, 1st Edition, 2009)
- Often adjacent, abutting, protruding or in direct contiguity to pelvicaliceal structures (Hum Pathol 2016;48:81)
- Multicystic architecture with variably sized simple cysts lacking immature nephrogenic elements, solid areas and cytologic atypia
- Cystic septa are usually hypocellular and fibrous with variable amounts of mixed inflammation and rarely differentiated tubules
- Focally increased subepithelial cellularity may be present either due to spindle cells or inflammation
- Wavy / ropy collagen is absent
- Cyst lining epithelium is flattened to cuboidal, with frequent hobnailing
- No epithelial complexity (branching glands, papillary projection, cribriforming)
- Partial or complete fibrous pseudocapsule often containing entrapped tubules and glomeruli; some tumors intermingle with normal parenchyma (Hum Pathol 2016;48:81)
- ER positive in subepithelial stromal cells; PCN usually shows stronger and more diffuse positivity in contrast to adult cystic nephroma (Am J Surg Pathol 2017;41:472)
- DICER1 is a multidomain protein with 2 type III endoribonucleases
- Involved in maturation of several classes of small noncoding RNAs such as miRNAs (Nat Rev Cancer 2014;14:662)
- PCNs usually carry DICER1 mutations, which may be somatic or germline
- Majority of mutations are nonsense and frameshift (truncating) substitutions and missense substitutions
- Somatic mutations often involve RNase IIIb and germline mutations may involve multiple DICER1 domains (Nat Rev Cancer 2014;14:662, Hum Pathol 2016;48:81, Mod Pathol 2014;27:1267)
Pediatric renal tumors: molecular diagnostics and COG updates
- Left kidney, partial nephrectomy:
- Pediatric cystic nephroma; margins are negative (see comment)
- Comment: Pediatric cystic nephroma is characterized by mutations in the DICER1 gene and may occur as a part of DICER1 syndrome. Clinical correlation and correlation with germline DICER1 mutation analysis is recommended.
- Adult cystic nephroma:
- Separate pathologic entity mainly in women who are > 30 years and lack the DICER1 mutation (Hum Pathol 2016;48:81, Am J Surg Pathol 2016;40:1591)
- Usually have thicker septa composed of more cellular ovarian type stroma and ropy / wavy collagen
- Stromal cells are often inhibin positive at least focally while PCN is inhibin negative
- ER positivity is seen in both but typically more diffuse and intense in PCN (Am J Surg Pathol 2017;41:472)
- Segmental polycystic kidney disease:
- Clinical, radiologic correlation and family history may be helpful
- Lack of well defined fibrous wall
- Extension of cysts into the adjacent renal parenchyma with intermingling of nephrons; in contrast, PCN usually pushes aside native nephrons
- Lack of DICER1 mutation
- Cystic partially differentiated nephroblastomas and cystic Wilms tumor:
- Cystic Wilms tumor has immature nephrogenic elements plus solid nodules distorting the septal contours (Hum Pathol 2016;48:81)
- Both lack DICER1 mutations (Mod Pathol 2014;27:1267)
- Cystic renal dysplasia:
- Usually bilateral and diffuse
- Disorganized architecture with cysts, primitive tubules and undifferentiated mesenchyme
- Other cystic lesions of the kidney
A 2 year old boy with metastatic pineoblastoma was noted to have a palpable kidney mass. CT imaging showed a low attenuating cystic mass arising from the left kidney. Partial nephrectomy was performed and showed the above morphology on H&E. Given the findings, what syndromic condition needs to be considered?
- Denys-Drash Syndrome
- DICER1 syndrome
- Hereditary retinoblastoma
- Von Hippel-Lindau (VHL) syndrome
Hereditary retinoblastoma is associated with pineablastoma and retinoblastoma. VHL is associated with hemangioblastomas, pheochromocytomas, renal cell carcinomas and endolymphatic sac tumors. Denys-Drash syndrome is a WT1 related Wilms tumor syndrome and associated with disorders of sexual development and Wilms tumor.
Comment Here
Reference: Pediatric cystic nephroma
- Rare kidney tumor that morphologically mimics well differentiated thyroid follicular neoplasms
- Considered an emerging / provisional entity (Am J Surg Pathol 2018;42:1585, Transl Androl Urol 2021;10:1506, Mod Pathol 2021;34:1167)
- Extremely uncommon (~40 cases reported)
- Resembles well differentiated thyroid follicular neoplasms
- Encapsulated mass with thyroid-like follicles (macro and microfollicles) containing inspissated, eosinophilic colloid-like material
- Negative for TTF1 and thyroglobulin
- Most have low malignant potential
- Thyroid-like follicular renal cell carcinoma
- ICD-10: C64.9 - malignant neoplasm of unspecified kidney, except renal pelvis
- Predominance in women (2:1)
- Age range between 10 - 83 years, with mean age of 41 - 44.5 years (Am J Surg Pathol 2009;33:393, Pathology 2018;50:24, Mod Pathol 2021;34:1921)
- Median age: 35 years (Transl Androl Urol 2021;10:1506)
- ~10% of patients had concurrent or historical hematopoietic neoplasia (Pathology 2018;50:24)
- Renal cortex
- Asymptomatic and found incidentally in most but may be symptomatic (hematuria, flank pain, abdominal pain) in up to 33% of patients (Pathology 2018;50:24)
- Diagnosis is made primarily on H&E (see Essential features and Microscopic description)
- Essentially a diagnosis of exclusion; adequate sampling should be applied and immunohistochemistry could be considered, if clinically indicated, to exclude morphologic mimics (see Differential diagnosis)
- Typically solid, low attenuation mass without measurable postcontrast enhancement, metastatic disease or renal involvement
- May contain calcifications and possible necrosis
- Corresponding lack of thyroid mass suggestive of primary thyroid-like follicular renal cell carcinoma over metastatic thyroid carcinoma
- References: Case Rep Pathol 2013;2013:687427, Mol Clin Oncol 2016;4:143, Medicine (Baltimore) 2016;95:e3314
- Majority appear to have low malignant potential (Am J Surg Pathol 2009;33:393)
- Aggressive cases have been described with ~10% metastases and 13% of cases invading perinephric tissues (Hum Pathol 2011;42:146, Medicine (Baltimore) 2016;95:e3314, Pathology 2018;50:24)
- Rare cases have reported spread to lymph nodes and distant metastasis, including to skull, meninges and lung but all patients survived after surgical resection (Transl Androl Urol 2021;10:1506, Pathology 2018;50:24)
- 10 year old girl with thyroid-like follicular renal cell carcinoma (Int Braz J Urol 2019;45:834)
- 32 year old woman with immunohistochemical and genetic analysis (Am J Surg Pathol 2006;30:411)
- 34 year old woman with metastasis to lungs and retroperitoneal lymph nodes (Hum Pathol 2011;42:146)
- 47 year old man with bilateral renal tumors of different subtypes, including thyroid-like follicular of the right kidney (Front Oncol 2021;11:659706)
- 48 year old woman with primitive small round / spindle cell sarcomatoid differentiation and metastatic disease (Int J Surg Pathol 2019;27:678)
- 68 year old woman with skull and meningeal metastasis (Medicine (Baltimore) 2016;95:e3314)
- Partial or radical nephrectomy
- Solitary mass, 1 - 16.5 cm (mean 4.7 cm) (Mod Pathol 2021;34:1167)
- Well circumscribed with solid to cystic cut surface; may show areas of hemorrhage and necrosis but not typical (Mod Pathol 2021;34:1921, Pathology 2018;50:24)
- Tan to brown in color; some tumors have been described as yellow or gray (Pathology 2018;50:24)
- Follicular-like architecture with variably sized follicles, with micro and macrofollicles, containing inspissated, eosinophilic colloid-like material
- Follicles are lined by a single layer of cuboidal or low columnar epithelium with moderate amphophilic to eosinophilic cytoplasm, round nuclei, mostly inconspicuous to occasionally prominent nucleoli
- Majority of tumors show pure follicular architecture, with some variations including branching, resulting in focal papillary-like pattern (Mod Pathol 2021;34:1167)
- Papillary areas appear to be broken interfollicular septa rather than true papillae (Pathology 2018;50:24)
- May be cystically dilated (Diagn Pathol 2014;9:186)
- May have focal tightly packed follicles without secretions, imparting a somewhat solid appearance
- Few cases demonstrate nuclear grooves or ground glass nuclear appearance (Pathology 2018;50:24)
- Lymphocytic infiltrate may be present intratumorally or around periphery and macrophages may be present in background (Diagn Pathol 2013;8:108)
- Calcifications may be seen (Pathology 2018;50:24)
- Necrosis and lymphovascular invasion are absent in most tumors
- Sarcomatoid differentiation has been reported (Int J Surg Pathol 2019;27:678, Int J Surg Pathol 2021;29:327, Histopathology 2022;80:745)
- May be hypercellular and arranged in sheets (Diagn Cytopathol 2014;42:273)
- Presence of acellular eosinophilic material is associated with the neoplastic epithelial cells in the background of the smear
- Individual tumor cells may be oval, round and plasmacytoid with mild nuclear pleomorphism, finely stippled nuclear chromatin and inconspicuous nucleoli with a moderate amount of eosinophilic cytoplasm and rare nuclear grooves (Diagn Cytopathol 2014;42:273, Diagn Pathol 2013;8:108)
- Thyroglobulin, TTF1
- May be negative but focal to variable reactivity has been reported for RCC (14%), AMACR (17%), CD10 (23%), CK20 (40%) (Mod Pathol 2021;34:1167)
- Typically negative for CD56, WT1 (Zhou: Uropathology - A Volume in the High Yield Pathology Series, 1st Edition, 2012, Mod Pathol 2021;34:1921)
- Other reports claim carcinoembryonic antigen, CK7, CD15, CK19, CK 34 beta E12 and epithelial membrane antigen are typically negative (Am J Surg Pathol 2006;30:411)
- In 3 of 3 tumors tested in one study, an in frame fusion of exon 8 of EWSR1 and intraexonic of PATZ1, both on chromosome 22, were detected by RNA sequencing and confirmed with reverse transcription PCR; per authors, other approaches instead of FISH may be preferable to detect the EWSR1::PATZ1 fusion (Mod Pathol 2021;34:1921, Histopathology 2022;80:745)
- Gene expression profile is distinct from clear cell and chromophobe renal cell carcinoma
- Amin et al. reported overexpression in cell cycle regulatory genes and mixed lineage leukemia (MLL) / trithorax homolog in 3 tumors; however, comparative genetic hybridization failed to reveal cytogenetic alterations in one study (Am J Surg Pathol 2009;33:393)
- Another study showed chromosomal gains of 7q36, 8q24, 12, 16, 17p11-q11, 17q24, 19q, 20q13, 21q22.3 and Xp and losses of 1p36, 3 and 9q21-33 detected by comparative genomic hybridization (Am J Surg Pathol 2006;30:411)
- Right kidney, partial nephrectomy:
- Thyroid-like follicular renal cell carcinoma (2.2 cm) (see synoptic report and comment)
- Comment: The tumor cells are positive for PAX8, CK7 and are negative for TTF1, thyroglobulin and WT1.
- Follicular architecture is not unique to thyroid-like follicular renal cell carcinoma (RCC) and may be seen in other RCC subtypes, such as papillary RCC, clear cell RCC, FH deficient RCC, tubulocystic RCC, SDHB deficient RCC, microcystic chromophobe RCC, atrophic kidney-like tumor / lesion (provisional entity) and mixed epithelial and stromal tumor (Mod Pathol 2021;34:1167, Am J Surg Pathol 2018;42:1585)
- Papillary RCC:
- Will demonstrate ordinary papillary renal cell carcinoma areas
- Atrophic kidney-like tumor / lesion (provisional entity):
- Atrophic / flattened epithelium with occasional hobnailing within follicles, more pronounced microcalcifications, entrapped benign atrophic renal tubular structures and morphologic similarity to glomerulocystic change
- Will demonstrate WT1 positive / PAX8 negative / CK7 negative IHC profile (Mod Pathol 2021;34:1921, Am J Surg Pathol 2018;42:1585)
- Metastatic thyroid carcinoma (Case Rep Pathol 2015;2015:701413, Mol Clin Oncol 2021;15:268):
- Very rare to have metastatic disease to kidney; usually obvious thyroid primary with widely disseminated metastases
- Thyroglobulin+ or TTF1+
- Most follicular and follicular variant of papillary thyroid carcinomas have RAS or BRAF mutations, respectively (Mod Pathol 2021;34:1921)
- Thyroid carcinomas can have a Hürthle (oncocytic) phenotype, which could be a potential pitfall for eosinophilic / oncocytic renal neoplasms; these more commonly metastasis to the lung and bone (Thyroid 2010;20:429, Nucl Med Mol Imaging 2017;51:256)
- Metastatic follicular carcinoma arising in struma ovarii in women:
- If this metastasizes, usually goes to bone (Pathologica 2020;112:224, Int J Gynecol Pathol 2009;28:405, J Clin Neurosci 2010;17:269)
- Chronic pyelonephritis and end stage kidney disease:
- May be considered due to presence of colloid-like hyaline casts in atrophic, microcytic tubules resembling thyroid (thyroidization)
- Differentiated by clinical history, lack of mass formation and histologic differences (inflammation, demonstration of normal constituents, such as glomeruli and tubules, with chronic injury pattern resulting in fibrosis, atrophy, etc.)
- Well differentiated neuroendocrine tumor (carcinoid):
- Rare
- Patterns are insular, cords, nests or ribbons but not follicular
- Neuroendocrine histology
- Positive for neuroendocrine markers synaptophysin and chromogranin
- Urothelial carcinoma with cystitis cystica pattern:
- Arises from the renal pelvis
- GATA3 positivity (Mod Pathol 2021;34:1921)
- Oncocytoma:
- Areas of classical oncocytoma with nests of oncocytic cells embedded in loose myxoid stroma
A 42 year old woman undergoes a left radical nephrectomy for a 4 cm renal mass. Which of the following is most consistent with a diagnosis of thyroid-like follicular renal cell carcinoma?
- Grossly well circumscribed renal tumor with microscopic follicular growth and inspissated colloid-like material
- Immunohistochemistry demonstrates tumor cells to be negative for PAX8 and CK7 and positive for WT1
- Poor clinical prognosis after diagnosis with high mortality rate
- Will demonstrate NRAS and HRAS or BRAF mutations by sequencing
Comment Here
Reference: Primary thyroid-like follicular carcinoma
A 65 year old woman undergoes a right radical nephrectomy for a 3 cm renal mass. The tumor displays follicular architecture with inspissated colloid-like material without papillary-like nuclear features or papillary growth. By immunohistochemistry, the tumor cells are positive for TTF1 and thyroglobulin. This patient will likely have which of the following?
- An intact thyroid with no nodules
- No evidence of other metastatic foci on PET / CT
- PAX8 IHC negativity
- RAS mutation
Comment Here
Reference: Primary thyroid-like follicular carcinoma
- Adult renal cell carcinoma (RCC) is a malignant epithelial neoplasm arising from renal tubular epithelium
- Sixteenth most common cause of death from cancer worldwide
- Clear cell RCC represents ~65 - 70% of adult renal carcinomas
- Second and third most common RCC subtypes are papillary (10 - 15%) and chromophobe RCC (5%)
- See subtypes: Kidney tumor
- Historic synonyms (obsolete): nephrocellular carcinoma, Grawitz tumor, hypernephroma (due to perceived origin from adrenal gland)
- ICD-O: see Kidney tumor subtypes
- ICD-10: C64 - malignant neoplasm of kidney, except renal pelvis
- ICD-11: 2C90 - malignant neoplasms of kidney, except renal pelvis
- Incidence: worldwide as of 2020, it is the seventh most common cancer in men (271,249 new cases) and the tenth most common in women (160,039 new cases)
- M:F = ~2:1; some subtypes are more common in women (e.g., eosinophilic solid and cystic RCC)
- Patients are usually > 50 years old; peak incidence between the ages of 60 and 70 years
- ~70% of new cases occur in countries with high socioeconomic development
- Estimated lifetime prevalence of 2.3% for men and 1.3% for women in the U.S. (JAMA 2024;332:1001)
- RCC incidence varies widely from region to region with the highest rates (as of 2018) in Belarus (16.8 cases per 100.000 population), followed by other Central and Eastern European countries
- High incidence rates of RCC are also found in the U.S., with an estimated ~81,800 new cases in 2023 and over 14,000 deaths from RCC each year (CA Cancer J Clin 2017;67:7, JAMA 2024;332:1001)
- Kidney (based in cortex or medulla, depending on subtype)
- RCC arising in extrarenal locations with no identifiable renal primary is a rare phenomenon that has been described (Histopathology 2022;81:635)
- Various molecular pathways, depending on Kidney tumor subtype
- Risk factors: obesity, smoking, hypertension, acquired cystic kidney disease due to end stage renal disease, occupational exposure to trichloroethylene, heavy metals and industrial solvents, prior chemotherapy (JAMA 2024;332:1001)
- Genetic susceptibility is estimated to account for 2 - 4%
- Von Hippel-Lindau (VHL) syndrome
- Autosomal dominant, due to germline mutation of VHL tumor suppressor gene on chromosome 3p25
- Associated renal lesions: clear cell renal cell carcinoma, benign or atypical renal cysts and numerous microscopic nodules of clear cells (Semin Diagn Pathol 2024;41:20)
- RCC in VHL syndrome can be multifocal or bilateral in 50% (eMedicine: Von Hippel-Lindau Syndrome Imaging [Accessed 7 November 2024])
- Clear cell RCC in VHL syndrome may show branching tubulopapillary growth and apical polarization of nuclei resembling clear cell papillary renal cell tumor but shows characteristic molecular and immunohistochemical profile of clear cell RCC
- Other associated lesions: hemangioblastomas of cerebellum and retina, pancreatic or liver cysts, clear cell tumors of other sites, papillary cystadenoma of epididymis, pheochromocytoma
- Hereditary papillary renal cell carcinoma (PRCC) syndrome
- Autosomal dominant, due to germline activating mutations of MET proto-oncogene on chromosome 7q31 with nearly 100% penetrance
- Patients typically have a positive family history of RCC
- Multifocal and bilateral papillary renal cell carcinoma (formerly type I)
- Papillary adenomas may also be numerous
- No association with other, extrarenal lesions
- Hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome
- Autosomal dominant, due to pathogenic germline mutations of FH gene on chromosome 1q42 encoding fumarate hydratase (enzyme of Krebs cycle)
- Usually multiple cutaneous or uterine leiomyomas at a younger age
- Associated renal tumors are classified as a separate entity in the current WHO 2022, termed FH deficient RCC, with distinctive morphology showing a combination of architectural patterns with at least focal papillary growth and large nuclei with a very prominent, inclusion-like eosinophilic nucleoli surrounded by perinucleolar haloes
- Rare, reported cases of testicular Leydig cell tumors (Med Rep 2024;5:100057)
- Genetic testing is always warranted for these aggressive tumors, as purely somatic biallelic FH mutations may occur (rarely in the setting of FH deficient RCC but commonly in older patients with solitary FH deficient uterine leiomyomas)
- Birt-Hogg-Dubé (BHD) syndrome
- Autosomal dominant with incomplete penetrance
- Due to germline mutations in FLCN (BHD) gene on chromosome 17p12, which codes for folliculin protein
- Skin lesions: fibrofolliculomas, trichodiscomas, acrochordons
- Lung: cysts, spontaneous pneumothorax
- Renal tumors are multiple and bilateral in 15 - 30% of cases (WHO 2022): most commonly hybrid oncocytic / chromophobe tumor, followed by chromophobe RCC and oncocytoma, as well as renal oncocytosis (Semin Diagn Pathol 2024;41:119)
- Clear cell, papillary and RCC, NOS can also be seen
- Renal tumors of BHD syndrome are considered low risk tumors suitable for conservative therapy; however, patients develop chronic renal insufficiency due to tumor multifocality
- Tuberous sclerosis complex (TSC)
- Autosomal dominant syndrome with variable penetrance, due to mutations in TSC1 on chromosome 9q or TSC2 on chromosome 16p (GeneReviews: Tuberous Sclerosis Complex [Accessed 13 January 2025])
- Multisystem disorder with numerous manifestations, usually affecting the CNS
- Renal lesions: multiple, bilateral angiomyolipomas or epithelioid angiomyolipoma, renal cysts and variable, typically indolent renal tumors, including eosinophilic solid and cystic RCC, RCC with fibromyomatous stroma, hybrid oncocytic / chromophobe tumors and RCC NOS (Pediatr Nephrol 2021;36:1427, J Kidney Cancer VHL 2020;7:5)
- Succinate dehydrogenase (SDH) deficient tumor syndromes
- Group of tumor predisposition syndromes due to germline inactivating mutations of SDH genes (SDHA, SDHB, SDHC, SDHD, SDHAF2)
- Endocrine lesions: pheochromocytoma / paraganglioma, pituitary neuroendocrine tumor (PitNET, formerly pituitary adenoma)
- GI tract lesions: SDH deficient gastrointestinal stromal tumor (GIST)
- Pulmonary lesions: chondroma
- Renal lesions (~14% penetrance): RCC subtype classified as a distinct entity in the current WHO 2022, termed SDH deficient RCC, with characteristic morphology showing ground glass cytoplasmic vacuoles with pale eosinophilic, flocculent material (Semin Diagn Pathol 2024;41:32)
- BAP1 tumor predisposition syndrome
- Autosomal dominant, due to heterozygous germline mutations in BAP1 tumor suppressor gene encoding BRCA associated protein 1
- Associated with uveal and cutaneous melanocytic tumors / atypical Spitz tumors, cutaneous melanoma, basal cell carcinoma, malignant mesotheliomas, meningiomas and other tumors
- Renal lesions: usually clear cell RCC with aggressive phenotype; isolated reports of non-clear cell tumors; may be multifocal (JAMA Netw Open 2021;4:e2132615)
- Other familial cancer syndromes associated with renal cell tumors
- Hyperparathyroidism jaw tumor syndrome
- Cowden or PTEN hamartoma syndrome (Am J Surg Pathol 2023;47:1001)
- Familial papillary thyroid carcinoma syndrome
- Constitutional balanced chromosome 3 translocation
- Lynch syndrome
- Most common manifestation is gross or microscopic hematuria
- Classic clinical triad of costovertebral pain, palpable mass and hematuria is present in ~10% of patients
- Incidental RCC detection in 37 - 61% of cases due to increased use of imaging; historically, large (10 cm) or already metastatic at diagnosis (JAMA 2024;332:1001)
- Great mimicker due to associated paraneoplastic syndromes (20% of patients), including Cushing syndrome, gynecomastia, hypercalcemia, hypertension, leukemoid reaction, polycythemia, Stauffer syndrome (hepatomegaly with hepatic dysfunction), systemic amyloidosis, polyneuromyopathy
- Diagnosis is usually established based on clinical findings and contrast enhanced computed tomography (CT) imaging
- Histologic confirmation on renal mass biopsy is indicated in the following situations (AUA: Renal Mass and Localized Renal Cancer Evaluation Management and Follow Up [Accessed 13 January 2025])
- Confirmation of renal primary versus metastasis when there is a known extrarenal primary
- Unresectable solid renal mass before initiation of systemic therapy
- In comorbid patients noneligible for surgery to further guide treatment and prognosis
- Clinically suspected mass forming infectious process that may be managed with antibiotics, such as pyelonephritis or abscess
- Before ablative procedures to ensure histologic diagnosis
- For tumors involving renal sinus to rule out urothelial carcinoma or lymphoma (JAMA 2024;332:1001)
- For reporting elements in the synoptic pathology report, see Features to report
- Kidney function tests may show elevated serum creatinine and decreased glomerular filtration rates (GFR) as a reflection of compromised renal function
- Anemia and hypercalcemia may be seen
- Ultrasound
- Most frequently used for initial diagnosis of a renal mass and to assess the urinary tract
- Lower sensitivity and specificity for assessing malignancy and for staging than CT and magnetic resonance imaging (MRI)
- RCC may appear solid or cystic
- Can be hyper, iso or hypoechoic compared to noninvolved renal parenchyma
- Visualizing the tumor pseudocapsule as a hypoechoic halo is fairly specific but has low sensitivity (20%) (Radiopaedia: Renal Cell Carcinoma [Accessed 8 November 2024])
- CT
- Modality of choice for diagnosis and staging of RCC as well as for posttreatment follow up
- Dedicated renal CT protocols are used
- Can differentiate clear cell RCC from non-RCC subtypes with a specificity of up to 100% (Cureus 2021;13:e13231)
- RCC may appear solid or cystic, with irregular enhancement due to the presence of necrosis and can show peripheral or central calcifications
- Clear cell RCC typically demonstrates hypervascularity and enhancement similar to normal renal cortex as opposed to non-clear cell RCC subtypes (Cureus 2021;13:e13231)
- Bosniak classification (5 categories): helps predict risk of malignancy and guides clinical management for cystic renal masses based on contrast enhanced CT imaging (Cureus 2021;13:e13231)
- MRI
- Similar sensitivity and specificity as CT for evaluating renal masses
- Clear cell RCC is typically iso or hypointense on precontrast T1 weighted imaging and heterogeneously hyperintense on T2 weighted imaging
- Clear cell RCC is hypervascular and shows prominent enhancement
- Grading
- WHO / ISUP grading system is currently validated only for clear cell and papillary RCC
- Grade 1: nucleoli inconspicuous or absent at 400x (objective magnification 40x)
- Grade 2: nucleoli prominent at 400x
- Grade 3: nucleoli prominent at 100x magnification
- Grade 4: extreme nuclear pleomorphism, multinucleated giant cells, sarcomatoid or rhabdoid change
- Sarcomatoid transformation: poor prognostic indicator in other RCC subtypes as well (e.g., chromophobe RCC, SDH deficient RCC); presentation with advanced stage disease and metastases in 45 - 77% of cases (Am J Surg Pathol 2024;48:e65)
- WHO / ISUP grading system is currently validated only for clear cell and papillary RCC
- Staging
- Histologic subtype: see Kidney tumor
- Necrosis: tumor type necrosis (also known as granular necrosis) has been shown to be an independently significant predictor of a less favorable outcome in clear cell RCC (Pathology 2020;52:507, Histopathology 2019;74:284)
- Microscopic coagulative tumor necrosis is also associated with worse outcomes in clear cell and chromophobe RCC (Eur Urol 2021;79:225, Am J Surg Pathol 2013;37:1490)
- Lymphovascular invasion (LVI)
- Conflicting data; according to ISUP 2013 consensus, there is still insufficient evidence to support prognostic significance (Am J Surg Pathol 2013;37:1490)
- Newer studies suggest the detrimental impact of LVI on overall survival in patients with surgically treated renal cell carcinoma (Urol Oncol 2023;41:435.e1)
- 5 year survival: 70% (all histologic types and stages), varies from 94% for stage I versus ~80% for stage II versus ~60% for stage III versus ~5 - 10% for stage IV
- Resection (partial or radical nephrectomy) for localized disease
- Active surveillance may be an option for comorbid patients with low stage, histologically confirmed low risk tumors
- Generally limited use for chemotherapy in RCC management but may be offered to patients with collecting duct or SMARCB1 deficient RCC (formerly medullary carcinoma) (Uroweb: Renal Cell Carcinoma [Accessed 8 November 2024])
- Especially in the metastatic setting, it is important to differentiate between clear cell RCC and non-clear cell RCC subtypes, as several HIF1α pathway targeted therapies (e.g., tyrosine kinase inhibitors, anti-VEGF agents) and immune checkpoint inhibitors have been approved for clear cell RCC management in both the U.S. and Europe
- HLRCC associated renal tumors frequently require aggressive treatment with specific chemotherapeutic considerations and follow up regimens even if small and solitary (J Natl Compr Canc Netw 2019;17:1278)
- Dual immune checkpoint inhibitors combinations (anti-CTLA4 and anti-PDL1) are used in the U.S. as first line treatment for patients with intermediate or poor risk metastatic clear cell RCC (JAMA 2024;332:1001)
- Staging according to UICC / AJCC 8th edition
- Risk of downstaging in RCC is greater than in other cancers
- Refer to Grossing topic for sections to take and grossing tips
- Greatest dimension of tumor should be measured after serial sectioning, including tumor extending into extracapsular soft tissue
- Do not include tumor extending into renal vein / inferior vena cava in the measurement
- Measurement of predominantly cystic lesions with focal solid tumor nodules is controversial; some survey data suggests most urological pathologists would measure the entire cyst if cyst lining and solid nodule are composed of similar cells (Am J Surg Pathol 2018;42:1253)
- Significantly greater likelihood for tumors > 7 cm to show invasion into sinus or perirenal fat
- Renal sinus invasion (pT3a)
- If tumor is in direct contact with the sinus fat or the loose connective tissue of the sinus, clearly beyond the renal parenchyma
- If tumor involves any endothelium lined spaces within the renal sinus
- It is recommended to extensively sample tumor sinus interface (> 3 - 5 blocks) if tumor size > 5 cm
- Perinephric fat invasion (pT3a)
- Tumor directly touching fat
- Protruding nodules or irregular tongues / bands of tumor cells into perinephric soft tissue, with or without desmoplasia
- Beware of tangential sectioning of tumor capsule or invasion into but not beyond the capsule (can obtain deeper levels or additional sections)
- Renal vein / segmental branches invasion (pT3a)
- Requirement of gross identification of vein invasion was removed (Amin: AJCC Cancer Staging Manual, 8th Edition, 2016)
- Requirement that venous segmental branches have muscular walls were removed (Amin: AJCC Cancer Staging Manual, 8th Edition, 2016)
- Presence of tumor multinodularity within renal sinus should always raise suspicion for venous invasion, even if nodules are well circumscribed with apparent pseudocapsule
- Invasion of pelvicalyceal system mucosa recently introduced into pT classification (pT3a) (Amin: AJCC Cancer Staging Manual, 8th Edition, 2016)
- Renal vein margin: margin is positive only when tumor is grossly adherent to vessel wall and is confirmed microscopically at the actual margin; if submitted separately as caval thrombus, take 2 or more sections to look for tumor adherent to caval wall
- Microscopic infiltration of vessel wall at renal vein margin is associated with a worse prognosis
- Uninvolved renal parenchyma: should be sampled, distant from tumor for underlying renal disease
- Hilar lymph nodes: should be sought but are found in < 10% of radical nephrectomy specimens; N1 disease is grossly visible in 80% of cases
- Reporting of multifocal tumors
- Provide size range from largest to smallest
- If same histologic subtype, classify as pT(m)
- If different histologic subtypes, stage each tumor separately
- References: Pathology 2021;53:120, Histopathology 2019;74:18
- Depends on subtype, with classical golden yellow appearance in clear cell RCC, mahogany brown appearance with central scar in oncocytoma and variants of chromophobe RCC or brown hemorrhagic and granular appearance in papillary RCC
- Usually well circumscribed, centered in cortex
- May bulge / distort kidney contour without invading perirenal fat
- Cut surface often shows hemorrhage, necrosis, calcification, cystic change
- Often extends into the renal vein or vena cava
- Renal sinus invasion is common in large tumors
- May have satellite nodules
- Reference: Cureus 2022;14:e32338
- Depends on Kidney tumor subtype
Contributed by Maria Tretiakova, M.D., Ph.D., Miruna Claudia Popescu, M.D. and Katrina Collins, M.D.
- Tumor cells have abundant cytoplasm that is vacuolated, fluffy or granular, usually with indistinct cell borders (chromophobe RCC has distinct borders)
- Tumor nuclei have variable atypia, irregular contours, haphazard orientation with abnormal chromatin, variably prominent nucleoli
- Renal tubular cells have well defined cell borders, homogeneous cytoplasm, round, regular and orderly nuclei
- Important features to distinguish from other neoplasms include heterogeneous cell population, small cytoplasmic vacuoles and hemosiderin deposits
- References: Cancer Cytopathol 2024;132:186, J Am Soc Cytopathol 2023;12:S18
- PAX8 and PAX2: useful to prove renal cell origin
- CAIX
- Circumferential membranous (box shaped) in 75 - 100% of clear cell RCC; useful to support the diagnosis of clear cell RCC
- Also expressed in clear cell papillary renal cell tumor (basolateral, cup shaped) and RCC with fibromyomatous stroma (box or cup shaped)
- Focally expressed in areas of ischemia in non-clear cell RCC (potential pitfall)
- Generally, CD10 (proximal tubular marker), RCC, vimentin and epithelial markers including AE1 / AE3, CAM 5.2, EMA
- Cathepsin K (60% of TFE3 rearranged RCC)
- MelanA and HMB45 (50% of TFEB rearranged RCC)
- KIT (marker of intercalated cells), positive in chromophobe RCC and oncocytoma
- Reference: Semin Diagn Pathol 2022;39:1
- Generally, CK20 (except ESC RCC)
- Inhibin (except rare cases of hemangioblastoma-like clear cell RCC)
- Calretinin, SF1, TTF1, CEA, p63
- Vimentin is consistently negative in almost all oncocytic tumors (pitfall: focal expression in scar areas)
- Reference: Cancers (Basel) 2020;12:602
| CAIX | KIT | CK7 | AMACR | Vimentin | CD10 | HMWCK / GATA3 | FH | SDHB | Other | |
| CCRCC | + (D) (box) | - | - (↑ in cystic areas) | -/+ (P) | + | + | - | + | + | |
| CCPRCT | + (D) (cup) | - | + (D) | - | - | - | + | + | + | |
| RCC FMS | + (D) (box / cup) | - | + (P - D) | -/+ (F) | + | + | - | + | + | MelanA-, HMB45-, cathepsin K- |
| PRCC | - | - | + (D), ↓ when eosinophilic | + (D) | + (D) | + | -, GATA3+ in PRNRP | + | + | |
| MiTF family tRCC | - | - | -, ↓ CK, EMA expression than other RCC types (30 - 50% +) | +/- | -/+ | + | - | + | + | TFE3+ (nuclear), TFEB+ (nuclear and cytoplasmic), cathepsin K+ (60%), MelanA +/-, HMB45 +/- (50% in TFEB), GPNMB+ |
| ChRCC | - | + (D) | + (↓ in eosinophilic variant) | - | - (except scar and sarcomatoid areas) | - | - | + | + | Hale+ |
| Oncocytoma | - | + (D) | - (except rare cells / central scar) | - | - | - | - | + | + | Hale- (or apical bar) |
| LOT | - | - | + (D) | - | - | -/+ (F) | GATA3+ (D) | + | + | Hale- (or apical bar) |
| EVT | - | + (P - D) | -/+ (F) | - | - | + | - | + | + | Cathepsin K +, MelanA-, HMB45- |
| ESC RCC | - | - | -/+ (F) | + | + | -/+ | - | + | + | CK20+ (F - D), CK20 > CK7, cathepsin K+, MelanA variable |
| FH deficient RCC | - | - | - | - | + | - | + | Loss | + | 2SC+ (D) |
| SDH deficient RCC | - | - | - | - | - | - | - | + | Loss | Hale- |
| ALK rearranged RCC | - | - | +/- | +/- | + | -/+ | - | + | + | ALK+, INI1 retained, MelanA-, HMB45-, TFE3+ (10%) |
| SMARCB1 deficient RCC | - | - | + | N/A | N/A | - | + | + | + | INI1 loss, OCT3/4 + (50%), variable p53 |
- F = focal (≤ 25%); P = patchy (25 - 75%); D = diffuse (≥ 75%); +/- = usually positive but can also be negative; -/+ = usually negative but can also be positive
- CCRCC = clear cell renal cell carcinoma; CCPRCT = clear cell papillary renal cell tumor; RCC FMS = renal cell carcinoma with fibromyomatous stroma (to include ELOC mutated RCC); PRCC = papillary renal cell carcinoma; PRNRP = papillary renal neoplasm with reverse polarity; MiTF tRCC = microphthalmia associated transcription factors family translocation associated renal cell carcinoma; ChRCC = chromophobe renal cell carcinoma; LOT = low grade oncocytic tumor; EVT = eosinophilic vacuolated tumor; FH = fumarate hydratase; SDH = succinate dehydrogenase
- References: J Pathol 2022;257:158, Int J Surg Pathol 2023;31:509, Ann Diagn Pathol 2020;44:151448, Arch Pathol Lab Med 2019;143:1455, Transl Androl Urol 2019;8:S123, Hum Pathol 2020;104:18
- Depends on Kidney tumor subtype
- Various molecular findings depending on Kidney tumor subtype
- Diagnosis of some RCC subtypes requires molecular confirmation
- Current WHO 2022 has introduced a designated chapter on molecularly defined RCC (see What's New in Kidney Tumor Pathology)
Pathology resident led live unknown slide session: pink renal tumors (part 1)
A dummies guide to diagnosis of renal tumors using pattern based approach by Dr. Rajal B. Shah
A dummies guide to diagnosis of clear renal tumors by Dr. Rajal B. Shah
- Differential diagnosis with primary renal tumors of nonrenal cell origin
- Epithelioid angiomyolipoma (EAML) / epithelioid PEComa of the kidney:
- May resemble high grade clear cell RCC or other eosinophilic renal tumors
- Frequently discohesive or sheet-like growth, lacking characteristic nested growth pattern and chicken wire capillary network of clear cell RCC
- May contain areas of classical AML with smooth muscle, fat and dysmorphic blood vessels
- Negative for cytokeratins, EMA, PAX8 and CAIX
- Positive for MelanA, HMB45, cathepsin K, SMA
- Subset has TFE3 rearrangements
- Upper urinary tract urothelial carcinoma:
- Hemangioblastoma:
- Closely resembles clear cell RCC
- More common in the brain, where it must be differentiated from metastatic clear cell RCC
- As a renal primary, it is overwhelmingly rarer than clear cell RCC but may pose significant diagnostic difficulty in this location because of overlapping features (Biomedicines 2023;11:1467)
- Usually solid, diffuse growth, less well formed epithelial formations
- Flocculent or vacuolated cell cytoplasm
- Focal to negative staining for cytokeratins and EMA
- Positive for inhibin A, S100, NSE
- In the kidney, PAX8 alone should not be used to differentiate hemangioblastoma from clear cell RCC, as PAX8 expression has been reported in the former (Int J Clin Exp Pathol 2015;8:2131, Am J Surg Pathol 2014;38:119, Clin Case Rep 2019;7:2321)
- Rare clear cell RCCs have hemangioblastoma-like morphology with inhibin expression (Asian J Surg 2024;47:1859, Int J Surg Pathol 2019;27:631)
- Renal anastomosing hemangioma:
- May mimic RCC with regressive changes where there is tumor cell dropout with retained prominent vascularity
- Anastomosing vascular spaces with fibrin thrombi
- Usually contains extramedullary hematopoiesis or hyaline globules
- Negative for cytokeratins, EMA, PAX8, CAIX, CD10
- Metanephric adenoma:
- Epithelioid angiomyolipoma (EAML) / epithelioid PEComa of the kidney:
- Differential diagnosis with renal nonneoplastic lesions
- Malakoplakia:
- Solid sheets of eosinophilic histiocytes
- Rare spindle cell morphology may mimic sarcomatoid RCC
- Von Kossa, Prussian blue, PAS stain highlights characteristic Michaelis-Gutmann bodies
- Negative for cytokeratins, EMA and PAX8
- Positive for histiocytic markers CD68, CD163
- Xanthogranulomatous pyelonephritis:
- Histiocytic granulomatous inflammation with admixed lymphocytes, plasma cells and neutrophils in a diffuse distribution
- May mimic clear cell RCC especially if foamy histiocytes predominate
- Spindle cell morphology may mimic sarcomatoid RCC
- Cholesterol clefts and histiocytic giant cells may be seen
- Negative for cytokeratins, EMA and PAX8
- Positive for histiocytic markers CD68, CD163
- Malakoplakia:
- Differential diagnosis with renal metastatic tumors
- Metastatic tumors to the kidney (< 1%) are mostly carcinomas (> 80%), lung being the most common
- 10% of renal masses included in one study were of metastatic origin (Diagn Cytopathol 1999;21:35)
- In > 90% of cases the primary tumor is already known and rarely diagnosed by evaluating the renal mass
- Adrenal cortical carcinoma:
- Foamy cell cytoplasm
- Negative for EMA, CEA, PAX8, CAIX, cathepsin K, mostly negative AE1 / AE3
- Positive for SF1, calretinin, inhibin A, MelanA, neurofilament, synaptophysin, vimentin
- Metastatic tumors to the kidney (< 1%) are mostly carcinomas (> 80%), lung being the most common
The above image is from a renal tumor. Which of the following statements is true about the immunohistochemical profile of the tumor type shown?
- Consistently negative for CD117
- Diffusely positive for CD117 and CK7 and consistently negative for vimentin
- Diffusely positive for GATA3
- Positive for CAIX, vimentin and CD10
- Positive for CK20 more than CK7
Comment Here
Reference: Renal cell carcinoma overview
- Autosomal dominant syndrome, due to germline mutation of VHL tumor suppressor gene on chromosome 3p25
- Autosomal dominant syndrome, due to heterozygous germline mutations in BAP1 tumor suppressor gene encoding BRCA associated protein 1
- Autosomal dominant syndrome, due to pathogenic germline mutations of FH gene on chromosome 1q42 encoding fumarate hydratase (enzyme of Krebs cycle)
- Autosomal dominant syndrome with incomplete penetrance, due to germline mutations in FLCN (BHD) gene on chromosome 17p12, which codes for folliculin protein
- Autosomal dominant syndrome with variable penetrance, due to mutations in TSC1 on chromosome 9q or TSC2 on chromosome 16p
Comment Here
Reference: Renal cell carcinoma overview
- Benign kidney tumor
- Asymptomatic, commonly found incidentally
- Arise from renomedullary interstitial cells
- Also known as medullary fibroma
- Frequently found at autopsy, incidentally on imaging or resection for other reasons
- Arising from medullary interstitial cells
- Small, well circumscribed, single or multiple gray-white nodules in the renal medulla
- Spindle or stellate cells in basophilic stroma with entrapped tubules
- Prior terms include medullary fibroma and renal hamartoma
- ICD-10: D30.00 - Benign neoplasm of kidney
- Incidence between 16% and 46% according to autopsy studies (Hum Pathol 1972;3:559)
- Mean age: 58 years (18 - 92 years), rarely seen in children (Am J Surg Pathol 2016;40:1693)
- M = F with similar incidence (Am J Surg Pathol 2016;40:1693)
- Predominance: right = left kidney (Am J Surg Pathol 2016;40:1693)
- Bilateral tumors: in 41%, bilateral tumors occurred more in the elderly (Am J Surg Pathol 2016;40:1693)
- Mostly occur in the renal medulla, occasionally found in renal cortex (Urology 2014;83:1104)
- 60% of patients have multiple interstitial cell tumors, ranging from 1 to 23, mean of 3 (Am J Surg Pathol 2016;40:1693)
- Appears to originate as a proliferation of renomedullary interstitial cells between medullary tubules (Am J Surg Pathol 2016;40:1693)
- Renomedullary interstitial cells are involved in release of renin and regulation of sodium excretion (Eur Urol 2016;70:120)
- As their size increases, cellularity decreases, viscous eosinophilic material is deposited and tubules disappear (Am J Surg Pathol 2016;40:1693)
- Viscous eosinophilic material is not associated with amyloid but with collagen type III (Am J Surg Pathol 2016;40:1693)
- No association with hypertension (Am J Surg Pathol 2016;40:1693)
- No statistically significant associated causes (Am J Surg Pathol 2016;40:1693)
- Asymptomatic, found incidentally during surgery (0.2%), autopsy (16 - 46%) or radiologic investigation (Urology 2014;83:1104)
- May be symptomatic in case reports (e.g. urosepsis or hematuria) (Urology 2014;83:1104)
- Diagnosis is made on histology post resection or on core biopsy (Adv Anat Pathol 2000;7:47)
- Imaging modalities are not conclusive and may mimic malignant conditions (Radiographics 2010;30:1525)
- CT: small, nonenhancing noncalcified hypoattenuating solid mass within the renal medulla (Radiographics 2010;30:1525)
- MR imaging: medullary fibromas are hypointense on T1 and T2 weighted images (Radiographics 2010;30:1525)
- Favorable prognostic factors and mostly asymptomatic (Urology 2014;83:1104)
- After resection, no recurrence (Urology 2014;83:1104)
- 20 year old woman with incidental detection at autopsy (Int J Health Sci (Qassim) 2014;8:440)
- 29 year old man found to have a calcium oxalate deposition in a renomedullary interstitial cell tumor at autopsy (Pathol Oncol Res 2003;9:47)
- 32 year old man presented with hematuria, left flank pain and incidental tumor (J Clin Imaging Sci 2013;3:43)
- 50 year old man was found to have renomedullary interstitial cell tumor during a kidney transplant (Indian J Urol 2012;28:202)
- Surgical resection is recommended if imaging is inconclusive (Urology 2014;83:1104)
- Well circumscribed, homogenenous white / gray-white nodules usually in the medulla
- Small (1 - 13 mm), median tumor size 4 mm and up to 5 cm has been reported in case reports (Hum Pathol 2018;82:46)
- Arises from medulla but occasionally from the cortex (Urology 2014;83:1104)
- Surrounding kidney tissue showed that 47% had normal renal parenchyma, 28% showed slight glomerulosclerosis, 19% moderate and 7% severe glomerulosclerosis (Am J Surg Pathol 2016;40:1693)
- Small, spindle or stellate cells in a background of loose basophilic stroma or fibrotic stroma with entrapped tubules (Urology 2014;83:1104, Am J Surg Pathol 2016;40:1693)
- Some cases may contain irregular deposits of amyloid (Eur Urol 2016;70:120)
- Entrapped tubules may be seen diffusely at the periphery and may be cystically dilated (Urology 2014;83:1104, Am J Surg Pathol 2016;40:1693)
- Nuclear features: open chromatin and minimal atypia or hyperchromasia (Urology 2014;83:1104, Am J Surg Pathol 2016;40:1693)
- Absent / rare mitotic activity (Urology 2014;83:1104, Am J Surg Pathol 2016;40:1693)
- Surrounding kidney tissue showed that 47% had normal renal parenchyma, 28% showed slight glomerulosclerosis, 19% moderate and 7% severe glomerulosclerosis (Am J Surg Pathol 2016;40:1693)
- Calponin (weak to moderate)
- Oil Red O and Sudan Black B (Hum Pathol 2018;82:46)
- Resembles renomedullary interstitial cell of kidney with large cytoplasmic lipid droplets (Hum Pathol 1972;3:559)
- Kidney, right, laparascopic complete nephrectomy:
- Renal cell carcinoma, clear cell type (see synoptic report)
- Renomedullary interstitial cell tumor
- Kidney, right, mass, needle core biopsy:
- Renomedullary interstitial cell tumor
- Metanephric stromal tumor:
- Concentric rings or collarettes of stromal cells around entrapped renal tubules and blood vessels
- Unencapsulated infiltrative tumor of spindle and epithelioid cells
- Rare in adults
- Scar:
- History of poor renal function, renal stones or prior procedures
- Not well circumscribed
- Less basophilic
- More likely to be associated with inflammation
An 85 year old man underwent nephroureterectomy for low grade papillary urothelial carcinoma of the renal pelvis. A 3 mm white, solid, medullary based incidental lesion is noted on gross examination. Which of the following is true for this tumor?
- They are uncommon tumors seen more frequently in children
- They are typically medullary based tumors composed of stellated interstitial cells immersed in a basophilic matrix with entrapped renal tubules
- Although the majority are benign, rarely metastasis have been described
- Frequently, they present with hematuria and flank pain
Comment Here
Reference: Renomedullary interstitial cell tumor
- Often, these tumors are unifocal, found within the cortex and more than 10 mm in size
- They are asymptomatic and often found incidentally during nephrectomy or at autopsies
- They are CD34 and ER positive and desmin and smooth muscle negative
- Congo red stain is characteristically positive in all tumors
- Specialized myofibroblast is the probable cell of origin
Comment Here
Reference: Renomedullary interstitial cell tumor
- An adult renal cell carcinoma (RCC), usually clear cell type, with a population of neoplastic cells (5% - 90%) which morphologically resemble rhabdomyoblasts but do not have true muscle differentiation by ultrastructure or immunohistochemistry
- Independent poor prognostic indicator (Am J Surg Pathol 2014;38:1260)
- Rhabdoid morphology is classically defined as "sheets and clusters of variably cohesive, large epithelioid cells with vesicular nuclei, prominent nucleoli and large paranuclear intracytoplasmic inclusions" (Am J Surg Pathol 2000;24:1329)
- Rhabdoid tumor of the kidney is a distinct mesenchymal neoplasm in children (see separate topic) but not in adults
- Incidence is 4% - 7% of RCCs, most frequently clear cell RCC type but also other subtypes (Am J Surg Pathol 2000;24:1329)
- Mean age 53 - 62 years; M:F = 2:1 (Am J Surg Pathol 2013;37:1490)
- Likely represents morphologic and genetic clonal evolution of neoplastic epithelial cells (Hum Pathol 2015;46:9)
- Kidney mass; rarely hematuria or flank pain
- Aggressive behavior with metastases in up to 70% of cases (Am J Surg Pathol 2013;37:1490); often into regional lymph nodes or lung (J Urol 2011;186:675)
- Rhabdoid phenotype in maintained in 65% of metastases; RCC should be strongly considered in evaluating an unknown primary with rhabdoid differentiation (Am J Surg Pathol 2014;38:1260)
- Median survival 8 to 31 months (Am J Surg Pathol 2013;37:1490)
- International Society of Urologic Pathologists (ISUP) Consensus recommends:
- Report as underlying histologic subtype (i.e. clear cell RCC) with rhabdoid differentiation
- No need to specify % of rhabdoid area; and
- Tumors are ISUP grade 4 (Am J Surg Pathol 2013;37:1490)
- RCC with rhabdoid differentiation has increased risk of death (hazard ratio 5.25) when compared with RCC without rhabdoid differentiation, independent of nonrhabdoid component grade, tumor stage and presence of necrosis (Am J Surg Pathol 2014;38:1260)
- Usually high stage and high grade nuclei (Fuhrman 3 or 4) in nonrhabdoid component and aggressive behavior similar to sarcomatoid carcinoma (Arch Pathol Lab Med 2007;131:102)
- 53 year old man with extensive peritoneal carcinomatosis secondary to renal cell carcinoma with sarcomatoid and rhabdoid differentiation (BMJ Case Rep 2013 Apr 22;2013)
- 56 year old man with acquired cystic disease associated renal cell carcinoma with sarcomatoid change and rhabdoid features (Ann Diagn Pathol 2011;15:462)
- 84 year old woman with malignant mixed epithelial and stromal tumor of the kidney with rhabdoid features (Hum Pathol 2007;38:1432)
- Resection
- Targeted therapy (tyrosine kinase inhibitors, VEGF inhibitors, mTOR inhibitors), cytokine therapy
- Rhabdoid component is white, firm, homogeneous
- Solid clusters and sheets of variably cohesive large epithelioid cells with central eosinophilic intracytoplasmic inclusions
- Large, eccentric and irregular nuclei and prominent nucleoli
- Tumor necrosis common and sometimes extensive
- Transition areas between RCC and rhabdoid morphology
Contributed by Nicole K. Andeen, M.D.
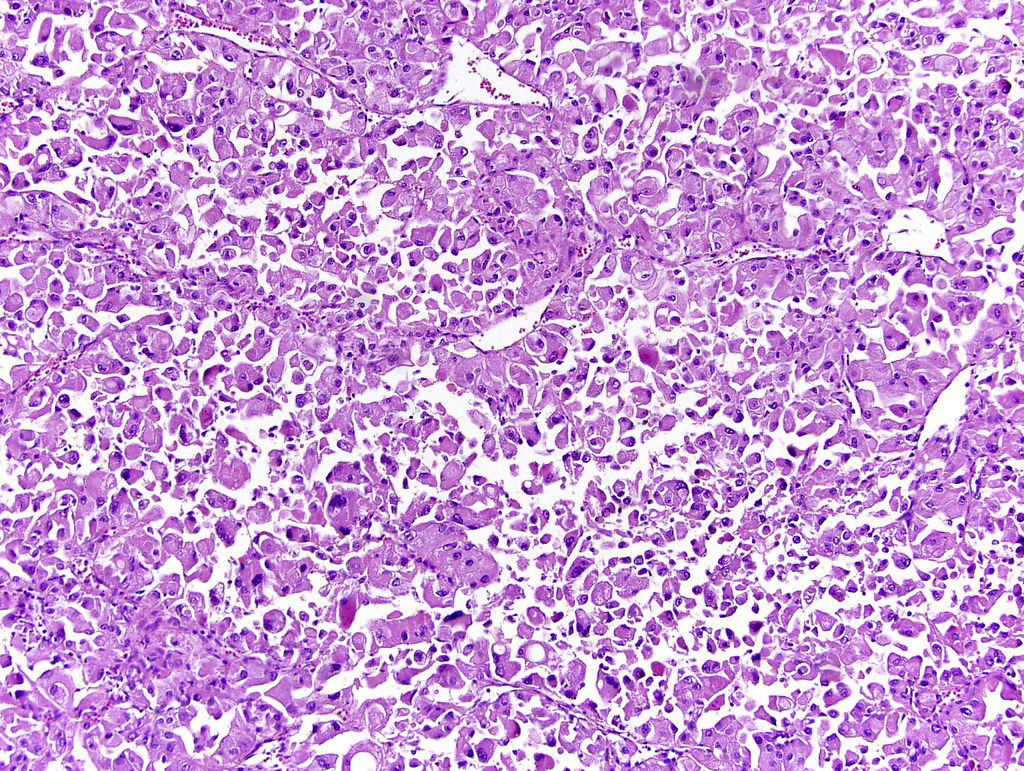
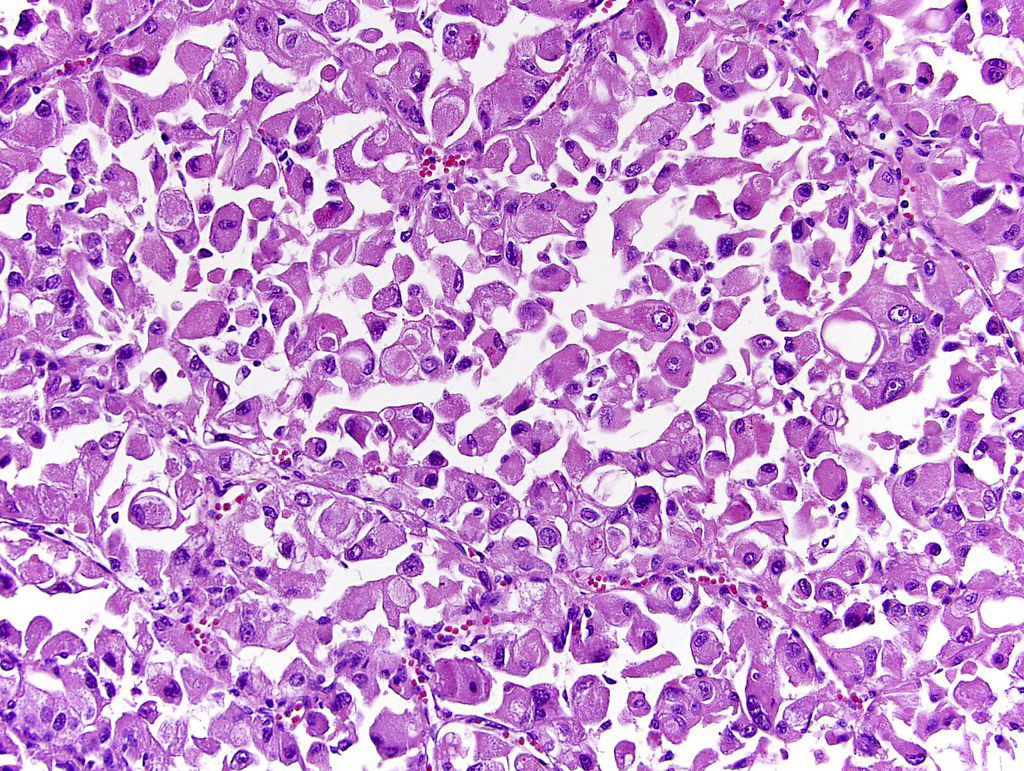
Discohesive rhabdoid cells with eccentrically placed nuclei, prominent nucleoli and densely eosinophilic cytoplasm
Contributed by @SueEPig on Twitter
- Majority of cases positive for vimentin, EMA, pan CK, p53, CAIX, PAX2, PAX8, CD10, NSE, PAS (Am J Surg Pathol 2000;24:1329, Ann Diagn Pathol 2015;19:57)
- Retained INI1 expression, except in cases with renal medullary carcinoma (Am J Surg Pathol 2013;37:1490, Mod Pathol 2008;21:647)
- Immunophenotype is similar to nonrhabdoid component
- Mutations in tumor suppressor genes BAP1 or PBRM1 located on chromosome 3p (Nat Genet 2012;44:751)
- Loss of 11p specific to rhabdoid differentiation in one study (n = 20; 11p loss seen in 29% of rhabdoid areas but not in clear cell areas)
- Rhabdoid cells share many chromosomal aberrations with coexisting clear cell RCCs but have greater number of copy variations (Hum Pathol 2015;46:9)
- Eosinophilic change in usual RCC: no resemblance to rhabdomyoblasts
- Renal rhabdoid tumors: pediatric patients, loss of INI1 gene / protein
- Rhabdomyosarcoma: skeletal muscle differentiation and lack of usual RCC
- Adult renal cell carcinoma (RCC) with a subpopulation of neoplastic cells that have rhabdoid morphological features (resemble striated muscle / rhabdomyoblastic cells) with discohesive cytology containing irregular eccentric nuclei, large nucleoli and abundant eosinophilic cytoplasm
- They are, however, not of a myogenic origin and differ in ultrastructural features and immunophenotype (Am J Surg Pathol 2000;24:1329)
- Not recognized as a distinct histologic type of RCC in the World Health Organization (WHO) 2022 classification but rather a high grade dedifferentiation or transformation that may arise from some RCC subtypes, predominantly clear cell RCC (CCRCC) (Arch Pathol Lab Med 2007;131:102, Ann Diagn Pathol 2015;19:57)
- Considered as WHO / ISUP grade 4 (Am J Surg Pathol 2013;37:1490)
- Associated with aggressive tumor behavior, poor prognosis and low response towards VEGF tyrosine kinase inhibitors (TKIs) however more recent studies show clinical benefits from immune checkpoint inhibitor (ICI) therapy (Clin Genitourin Cancer 2021;19:103, Nat Commun 2021;12:808, Am J Surg Pathol 2024;48:e65)
- Recommended: rhabdoid features or rhabdoid differentiation
- Other terminologies: focal rhabdoid / plasmacytoid appearance, rhabdoid variant, adult rhabdoid renal cell carcinoma, rhabdoid morphology, rhabdoid phenotype, rhabdoid component, rhabdoid changes, rhabdoid pattern, rhabdoid quality
- Not relevant to this topic
- Median age: 60 - 64 years old (Am J Surg Pathol 2014;38:1260, Int Urol Nephrol 2016;48:1253, Eur Urol 2015;68:5)
- M:F = 1 - 2:1 (Am J Surg Pathol 2014;38:1260, Int Urol Nephrol 2016;48:1253, Eur Urol 2015;68:5)
- Overall incidence of rhabdoid differentiation in RCC is 4 - 5%, with CCRCC being the subtype where it is most commonly observed (Am J Surg Pathol 2000;24:1329, Eur Urol 2015;68:5)
- Not relevant to this topic
- Considered a dedifferentiation of RCC with clonal and genetic progression associated with a high grade biological state (Ann Diagn Pathol 2011;15:333, Mod Pathol 2021;34:1392)
- Not relevant to this topic
- Not relevant to this topic
- Symptomatic in ~90% of cases and usually present with renal mass and abdominal mass, hematuria and flank or abdominal pain (Am J Surg Pathol 2024;48:e65, Eur Urol 2015;68:5)
- Frequent lymph node metastasis (up to ~20%) or distant metastasis (up to ~40%) at initial diagnosis (Am J Surg Pathol 2024;48:e65)
- Based on histomorphology of a rhabdoid differentiation in a RCC tumor
- Not relevant to this topic
- Nonspecific
- Complex hypervascular mass on ultrasound (Can Urol Assoc J 2008;2:631)
- Heterogeneous, irregular mass often with necrosis on computed tomography (CT) scan (Can Urol Assoc J 2008;2:631)
- Very aggressive disease; associated with higher stage (pT3 and pT4) and metastasis (up to 70%) (Histopathology 2002;41:538, Arch Pathol Lab Med 2007;131:102, Am J Surg Pathol 2024;48:e65)
- Poor prognosis with overall survival rate of 8 - 46 months (Am J Surg Pathol 2024;48:e65)
- Presence of any rhabdoid cells is diagnostic for RCC with rhabdoid features, indicating a poor prognosis (Am J Surg Pathol 2024;48:e65)
- Designated as WHO / ISUP grade 4 disease because rhabdoid dedifferentiation is associated with increased hazard ratio (HR 2.45) when compared to grade 3 tumors (Eur Urol 2015;68:5)
- 22 year old woman presented with CCRCC exhibiting rhabdoid features (Urol Case Rep 2020;32:101244)
- 40 year old woman presented with CCRCC with rhabdoid features that metastasized to the colon (Int J Surg Case Rep 2021;80:105627)
- 54 year old man has CCRCC with rhabdoid differentiation, showing co-occurring mutations in BAP1 and ATM (Am J Clin Exp Urol 2023;11:429)
- 81 year old man with rhabdoid RCC presented with renal vein thrombosis (Ann Med Surg 2024;86:2194)
- Radical nephrectomy due to large tumor size and invasion into adjacent tissues, typically followed by adjuvant therapy due to high risk of recurrence (Am J Surg Pathol 2024;48:e65)
- Exhibits an immune inflamed phenotype, higher PDL1 expression and is responsive to ICI based regimens therapies (Oncologist 2020;25:252, Nat Commun 2021;12:808)
- Nivolumab as monotherapy or in combination with ipilimumab or VEGFR TKI demonstrated clinical activity in CCRCC with rhabdoid dedifferentiation (Oncologist 2020;25:252)
- Role of ICI is not well established for rhabdoid RCC with non-clear cell component (Clin Genitourin Cancer 2021;19:103)
- Generally, rhabdoid differentiation is less studied than its sarcomatoid counterpart; studies have often lumped the sarcomatoid and rhabdoid RCC as one category (Cancer Treat Res Commun 2022;33:100640)
- Not relevant to this topic
- Homogenous, white and firm cut surfaces with hemorrhage and necrosis (Am J Surg Pathol 2000;24:1329, Ann Saudi Med 2013;33:495)
- See Microscopic (histologic) description section
- Not relevant to this topic
- Solid sheet-like growth pattern with a discohesive morphology (Am J Surg Pathol 2000;24:1329, Am J Surg Pathol 2024;48:e65)
- Large epithelioid cells with large eccentric, atypical vesicular nuclei with prominent nucleoli and abundant eosinophilic cytoplasm and possible paranuclear intracytoplasmic inclusions (Pol J Pathol 2015;66:3)
- Often coexist with sarcomatoid differentiation and high grade bizarre cells (Am J Surg Pathol 2014;38:1260, Pol J Pathol 2015;66:3)
- Necrosis is common; focal to extensive inflammatory cell infiltrates may be seen (Nat Commun 2021;12:808)
- Considered as WHO / ISUP grade 4 (Am J Surg Pathol 2013;37:1490)
- It is recommended to include the percentage of rhabdoid component in the pathology report
- Specific histologic RCC types may be seen adjacent to the rhabdoid areas (Arch Pathol Lab Med 2007;131:102, Ann Diagn Pathol 2015;19:57, Histopathology 2002;41:538)
- Most common histologic subtype of RCC with rhabdoid features is CCRCC but other RCC types including papillary RCC, chromophobe RCC, SMARCB1 deficient renal medullary carcinoma have been described (Histopathology 2002;41:538, Wien Klin Wochenschr 2012;124:419, Am J Surg Pathol 2013;37:368)
- Tumors with pure rhabdoid morphology after extensive sampling are designated as RCC, NOS
- Highly cellular consisting mainly of dissociated tumor cells with some arranged in small clusters (Cytopathology 2004;15:237)
- Cells are enlarged with eccentrically located nuclei and abundant cytoplasm with deeply stained inclusions (Cytopathology 2004;15:237)
- Cells resembling those found in RCC subtypes can also be seen (Cytopathology 2004;15:237)
- Not relevant to this topic
- Not relevant to this topic
- PanCK, EMA (Am J Surg Pathol 2000;24:1329, Histopathology 2002;41:538, Am J Surg Pathol 2024;48:e65)
- Rhabdoid cells typically express epithelial markers but some may lose this immunoreactivity
- PAX8 (to establish renal origin) (Ann Diagn Pathol 2015;19:57)
- Also could be lost in high grade dedifferentiated areas of the tumor
- CAIX, CD10, AMACR, CD117 may be expressed in the adjacent corresponding low grade RCC components
- Vimentin, NSE (Am J Surg Pathol 2000;24:1329, Histopathology 2002;41:538, Am J Surg Pathol 2024;48:e65)
- Retained INI expression, except for SMARCB1 deficient renal medullary carcinoma (Mod Pathol 2008;21:647)
- High Ki67
- p53 overexpression (Arch Pathol Lab Med 2007;131:102)
- Desmin, myoglobin / myogenin, SMA, MSA (Am J Surg Pathol 2000;24:1329, Histopathology 2002;41:538, Am J Surg Pathol 2024;48:e65)
- Lack expression of muscle specific markers
- CK7, CK20, GATA3, HMB45, cathepsin K
- Aggregates of paranuclear intermediate filaments arranged in a concentric whorl-like pattern, corresponding to the intracytoplasmic inclusion bodies observed in light microscopy
- Cells lack features of rhabdomyoblastic differentiation (Am J Surg Pathol 2000;24:1329)
- Not relevant to this topic
- Gains in chromosome 7 and losses in chromosomes 4, 9, 14 and 17p are frequently seen in areas with rhabdoid differentiation (Hum Pathol 2015;46:9)
- Loss of chromosome 11p is specific to rhabdoid differentiation, occurring in ~30% of rhabdoid components but not in clear cell areas (Hum Pathol 2015;46:9)
- Associated with inactivating mutations in BAP1 and PBRM1 and genes in SWI / SNF complex subunit such as SMARCB1 (Nat Genet 2012;44:751, Am J Surg Pathol 2017;41:253)
- Molecular features that may contribute to its aggressive behavior include mutations in the BAP1 gene, deletions of the CDKN2A gene and increased expression of MYC transcriptional programs (Nat Commun 2021;12:808)
- Not relevant to this topic
- Not relevant to this topic
- Kidney (mass), left, biopsy:
- Renal cell carcinoma with rhabdoid features (see comment)
- WHO / ISUP grade 4
- Comment: Favor a CCRCC origin. Lesion is positive for PAX8, LMWK, vimentin and CAIX.
- Kidney, right, radical nephrectomy:
- Clear cell renal cell carcinoma, pT3a (see synoptic report)
- WHO / ISUP grade 4
- Tumor size: 8 cm
- Tumor extends into perinephric fat
- Positive for rhabdoid differentiation (20%)
- Tumor necrosis present
- Negative for vascular and lymphovascular invasion
- All margins are negative for malignancy
- All lymph nodes are negative for tumor (0/3)
- Urothelial carcinoma of the renal pelvis (Int J Clin Exp Pathol 2015;8:9638, Am J Surg Pathol 2014;38:e20):
- Can exhibit rhabdoid differentiation if high grade
- Associated with urothelial carcinoma in situ or a traditional papillary urothelial carcinoma
- Negative for a low grade conventional RCC component
- CK20+, GATA3+, PAX8- IHC
- It can be difficult to differentiate an aggressive RCC as SMARCB1 deficient and collecting duct RCC and fumarate hydratase (FH) deficient RCC from urothelial carcinoma due to overlap in PAX8 and GATA3 staining; hence, extensive sampling is often needed to identify a lower grade morphology of a urothelial carcinoma or a conventional RCC
- Metastatic carcinoma with rhabdoid features:
- PAX8-
- Negative for a low grade conventional RCC component
- Clinical and imaging correlation to assess for the presence of a primary tumor
- Primary rhabdomyosarcoma of kidney (J Kidney Cancer VHL 2022;9:55, Urol Case Rep 2022;45:102183):
- Very rare in adults
- Positive muscle specific markers like myogenin, SMA, desmin
- Negative epithelial markers including panCK, EMA
- It is important to rule out the presence of an epithelial component
- Dedifferentiated part may lose keratin staining and resemble a sarcoma
- Key factor is morphology, specifically the absence of any low grade carcinoma component
- Rhabdoid tumor of the kidney (Pediatr Dev Pathol 2018;21:6):
- While it resembles RCC with rhabdoid features in terms of histomorphology and IHC profile, it predominantly occurs in infants and young children (~80% less than 2 years old)
- Demonstrates SMARCB1 or SMARCA4 biallelic aberrations and SMARCB1 (INI1) IHC loss
- Extrarenal rhabdoid tumors including atypical teratoid / rhabdoid tumor of the brain may be present
- No low grade RCC component in the tumor
- Epithelioid angiomyolipoma of the kidney (Mod Pathol 2013;26:1355):
- Some soft tissue tumors may also present with rhabdoid component:
- Dedifferentiated liposarcoma:
- Retroperitoneal mass
- May show panCK+
- MDM2 and CDK4 amplification (Hum Pathol 2018;77:20)
- Primary synovial sarcoma of the kidney:
- May mimic rhabdoid RCC especially if monophasic or poorly differentiated
- BCL2+, CD99+ and CD56+
- SYT::SSX1 or SYT::SSX2 fusion (Int J Surg Pathol 2007;15:421)
- Epithelioid angiosarcoma of the kidney:
- Primitive vascular lumens containing RBCs may be present
- CD31+, CD34+, ERG+ (Oncol Lett 2014;8:1155, Am J Surg Pathol 2011;35:432)
- Dedifferentiated liposarcoma:
- Others:
- CCRCC with predominant eosinophilic morphology:
- Absence of high grade, bizarre, eccentric nuclei and intracytoplasmic inclusions
- Fixation artifact:
- Can make nuclei appear atypical in tissue samples
- CCRCC with predominant eosinophilic morphology:
- Not relevant to this topic
A 70 year old man presents with a right renal mass measuring 15 cm that extends beyond Gerota fascia. The tumor exhibits white, firm cut surfaces with areas of hemorrhage and necrosis. Please refer to the images above. Which of the following immunohistochemistry (IHC) panels supports the diagnosis of renal cell carcinoma with rhabdoid differentiation?
- CK+, EMA+, CK20+, GATA3+, INI+, desmin-, myogenin-
- CK-, EMA-, HMB45+, cathepsin K+, variable SMA, desmin-, myogenin-
- CK+, EMA+, variable CAIX, variable PAX8, desmin-, myogenin-
- CK-, EMA-, variable vimentin, desmin+, myogenin+
Comment Here
Reference: Renal cell carcinoma with rhabdoid features
- This tumor is associated with favorable clinical outcomes
- This tumor often demonstrates loss of BAP1 and PBRM1
- This tumor should be classified as WHO / ISUP grade 3
- This tumor shows a high response rate to mTOR and VEGF inhibitors
Comment Here
Reference: Renal cell carcinoma with rhabdoid features
- Highly malignant undifferentiated tumor composed of noncohesive cells with large, eccentric nuclei, prominent nucleoli and intracytoplasmic inclusions; characterized by loss of INI1 immunostaining and SMARCB1 genetic abnormalities (in ~95% of cases)
- The most aggressive renal tumor of young age
- Noncohesive cells with large, eccentric nuclei and prominent nucleoli
- Loss of INI1 (~95% of cases) or BRG1 (~5% of cases) immunostaining
- Young age (< 2 years, rarely > 5 years)
- Other terms: malignant rhabdoid tumor, atypical teratoid rhabdoid tumor, rhabdoid tumor predisposition syndrome
- ICD-O: 8963/3 - malignant rhabdoid tumor
- ICD-11: 2C90.Y & XH3RF3 - other specific malignant neoplasms of kidney, except renal pelvis & rhabdoid tumor, NOS
- 1 - 2% of childhood renal tumors
- Median age at presentation is ~1 year (Pediatr Blood Cancer 2018;65:e26746, Pediatr Blood Cancer 2011;56:733)
- 80% diagnosed before the age of 2 years
- Can be congenital
- Exceptionally rare > 5 years of age
- ~15% are associated with atypical teratoid rhabdoid tumor in the brain
- M:F = 1.5:1
- Kidney
- Extrarenal rhabdoid tumors share the same histologic, immunohistochemical and molecular features
- The majority (~95%) of rhabdoid tumors of kidney have genetic changes resulting in the loss of function of SMARCB1, a tumor suppressor gene encoding BAF47 (INI1), which is a core member of the BAF chromatin remodeling complex
- The few remaining cases show a loss of function of the SMARCA4 gene, which encodes BRG1, the helicase / ATPase protein of the BAF complex (Pediatr Dev Pathol 2018;21:6)
- Predisposition factor: rhabdoid tumor predisposition syndromes (RTPS)
- Germline inactivation of one allele of a gene occurs
- If it occurs in the SMARCB1 gene: RTPS1
- If it occurs in the SMARCA4 gene: RTPS2 (Fam Cancer 2021;20:305)
- Children with RTPS develop tumors at a younger age
- Not clear whether germline mutation influences prognosis
- Usually presents as an abdominal mass or hematuria
- In ~20% of patients it is associated with hypercalcemia
- Rarely stage 1 (< 10%); bilateral presentation rare
- Metastases occur in ~80% of patients, mainly in the lungs, liver and brain
- Associated with rhabdoid tumor predisposition syndromes (RTPS1 and RTPS2) in ~30% of patients (Fam Cancer 2021;20:305)
- Imaging features not diagnostic (J Pediatr Surg 2008;43:1301)
- Diagnosed as a renal mass but if distant metastases detected, should be suspected
- Biopsy (with INI1 immunostain) diagnostic
- Very aggressive tumor with poor prognosis
- Widespread, hematogeneous and lymphatic metastases to bone, brain and other sites
- > 75% of patients die within 1 year, ~90% within 2 years of diagnosis
- Age at diagnosis is a highly significant factor: infants < 6 months of age have a very poor prognosis (an 8.8% 4 year survival)
- Survival for children ≥ 2 years of age is 41.1% (J Clin Oncol 2005;23:7641, Pediatr Blood Cancer 2011;56:733)
- 28 week gestation fetus with INI1 negative sarcoma diagnosed as malignant rhabdoid tumor, presented as hydrops fetalis metastatic to the placenta (J Matern Fetal Neonatal Med 2021;34:3790)
- 8 month old girl presented with malignant rhabdoid tumor of the kidney and spine (J Korean Neurosurg Soc 2014;55:57)
- 3 year old boy presented with WT1 positive renal malignant tumor and loss of INI1 expression (Transl Androl Urol 2020;9:2275)
- 5 year old girl presented with malignant rhabdoid tumor of the kidney and multicystic dysplasia (J Korean Med Sci 2010;25:785)
- 11 year old boy presented with rhabdoid tumor predisposition syndrome with renal tumor 10 years after brain tumor (Pathol Int 2021;71:155)
- No established standard treatment
- Most patients are treated with intensive multimodal regimens; surgical resection, intensive multidrug chemotherapy regimens and local radiotherapy or high dose chemotherapy followed by autologous stem cell rescue (Cancer Manag Res 2022;14:479)
- Unilateral and unicentric tumor
- Rare bilateral cases
- Sharply demarcated from the renal parenchyma
- Necrotic areas may be seen (Histopathology 1996;28:333)
- Classical, diffuse pattern: sheets of noncohesive, monotonous looking tumor cells
- Tumor cells have abundant eosinophilic cytoplasm which may contain hyaline inclusions (in ~20% of cases)
- Nuclei are large, vesicular and contain 1 - 2 prominent nucleoli
- High mitotic activity; mild to moderate cellular pleomorphism
- Rhabdoid tumors of kidney may exhibit other patterns too: sclerosing, myxoid, epithelioid, spindled, clear cell sarcoma-like, microcystic, solid lymphomatoid (Am J Surg Pathol 1989;13:439, Histopathology 1996;28:333)
- Different patterns may be present in the same tumor
- Extremely invasive; the lymphatics and blood vessels of the adjacent parenchyma are usually stuffed with tumor cells
- Staging criteria for rhabdoid tumor of the kidney are the same criteria as nephroblastoma (Histopathology 2022;80:1026)
Contributed by Ellen D’Hooghe, M.D. and Gordan M. Vujanic, M.D., Ph.D.
- Highly cellular with clusters and noncohesive tumor cells (Diagn Cytopathol 2021;49:711)
- Sometimes arranged in an alveolar fashion attached to fibrovascular stroma (Acta Cytol 2003;47:1055)
- Large cells with round to oval nuclei and often a single nucleolus
- Scant to moderate pale eccentric cytoplasm, vacuolation or intracytoplasmic inclusions / densities can be seen
- Mostly mononuclear cells but also some bi and multinuclear cells (Cancer Cytopathol 2011;119:49)
- Epithelioid cells but also spindle cells are described
- Atypical mitotic figures and necrosis might be present
- International Society of Paediatric Oncology (SIOP) and Children’s Oncology Group (COG) do not recommend the use of fine needle aspiration biopsy in diagnosis
- Vimentin (almost always)
- CD99
- Cytokeratin
- EMA
- Desmin
- Neurofilaments (frequent and patchy) (Cancers (Basel) 2020;12:729)
- WT1 can be positive (Acta Histochem 2013;115:70)
- PAX8 can be focally positive (Appl Immunohistochem Mol Morphol 2018;26:721)
- Loss of nuclear INI1 / SMARCB1 staining (Pathology 2008;40:664)
- Rarely, loss of nuclear BRG1 / SMARCA4 staining (Am J Hum Genet 2010;86:279)
- MyoD1
- Myogenin
- Hyaline globules composed of whorls of intermediate filaments
- Alterations in SMARCB1 (located on 22q11.2) include:
- Whole gene deletion (~22%)
- Intragenic duplication and deletions (~24%)
- Nonsense mutations (~24%)
- Frameshift mutations (~20%)
- Rare SMARCB1 wild type tumors have SMARCA4 loss instead (Pediatr Dev Pathol 2018;21:6)
- ~33% of patients with a newly diagnosed rhabdoid tumor of kidney have a germline mutation in SMARCB1
- Missense mutations are virtually absent
- Splice mutations are uncommon
- Otherwise, a small number of somatic mutations
- Kidney, total nephrectomy, stage 2:
- Rhabdoid tumor (see comment)
- Comment: Tumor shows diffuse growth of poorly cohesive cells, which have large nuclei with prominent nucleoli. Some cells show eosinophilic, intracytoplasmic inclusions. Tumor is infiltrating the renal sinus and the perirenal fat. Immunohistochemistry for INI1 shows a loss of nuclear staining in the tumor cells.
- Renal medullary carcinoma:
- INI1 not retained (as in rhabdoid tumors of kidney)
- Older age, associated with sickle cell disorder, reticular or cribriform pattern
- Ewing sarcoma:
- Wilms tumor:
- INI1 is retained
- Usually other component present (epithelial and stromal)
- Collecting duct carcinoma:
- Anaplasia
- Cells with large nuclei and prominent nucleoli
- Epithelial structures
- Heterologous muscle differentiation
- Succinate dehydrogenase (SDH) deficient renal cell carcinoma is defined by the WHO as a malignant epithelial tumor composed of vacuolated eosinophilic to clear cells with loss of immunohistochemical expression of SDHB, a marker of dysfunction of mitochondrial complex II (WHO 2016)
- Characteristic flocculent cytoplasmic vacuoles with a pale eosinophilic, wispy or bubbly appearance and low grade nuclei (at least focally)
- May have high grade areas
- Oncogenesis is driven by metabolic derangements due to double hit inactivation of SDH genes, leading to dysfunction of mitochondrial complex II
- Most patients have a germline mutation in an SDH gene, usually SDHB
- Terminology which is sometimes used but not recommended is SDHB negative renal carcinoma or SDH deficient renal oncocytoma
- Rare
- Represents 0.05% to 0.2% of all renal cell carcinomas (WHO 2016)
- Median age 35 years (range 14 to 76); M:F ratio = 1:8:1
- Bilateral in 26% (Am J Surg Pathol 2014;38:1588)
- Kidney
- Most cases occur in setting of germline mutation of an SDH gene; neoplasia occurs with double hit inactivation, leading to dysfunction of mitochondrial complex II, increased reactive oxygen species, DNA damage and HIF1α stabilization (Int Journal of Cell Biology 2012:2012)
- SDHB is most commonly affected in SDH deficient RCC, then SDHC
- Rarely SDHA or SDHD (different for other SDH deficient tumors)
- Often confined to the kidney at presentation
- Presents with flank pain or as incidental finding
- Personal or family history of paragangliomas or SDH deficient gastrointestinal stromal tumor may be present (WHO 2016) (GeneReviews 2008;NBK1548)
- Overall metastasis rate of ~ 33% (based on 27 cases) but metastasis uncommon if only low grade features (Am J Surg Pathol 2014;38:1588)
- Higher metastasis rate if high grade nuclei (70% metastasize) or coagulative necrosis present (4/4 cases metastasized, Am J Surg Pathol 2014;38:1588)
- Loss of SDHB by immunohistochemistry in 3 patients with known SDHB mutations (N Engl J Med 2011;364:885)
- With SDHA mutation (Hum Pathol 2015;46:1951)
- Resection; further treatment is dependent on grade / stage, may include targeted therapy (J Clin Oncol 2014;32;e10)
- All patients with a diagnosis of SDH deficient RCC should be offered genetic testing (WHO 2016)
- Well circumscribed, solid with red / brown cut surface, variable multicystic change, generally no necrosis
- Usually confined to kidney with no involvement of renal sinus, vein or fat
- Well circumscribed or pushing border, commonly entraps tubules
- Solid, nested or tubular growth pattern with scattered cysts containing eosinophilic material
- Neoplastic cells have smooth nuclear contours and fine chromatin with no nucleoli
- Characteristic finding is flocculent cytoplasmic vacuoles with a pale eosinophilic, wispy or bubbly appearance with low grade nuclei
- This morphology is commonly diffuse and should be at least focal (WHO 2016)
- May have areas of higher grade nuclei, necrosis, sarcomatoid change
- Variant morphologies have been reported but are rare in absence of more characteristic low grade regions (WHO 2016)
- Loss of SDHB immunohistochemical staining (be cautious on overinterpretation of negativity in tumors with very clear cytoplasm)
- Loss of SDHB immunohistochemical staining indicates disruption of the mitochondrial complex 2 for any reason, not just SDHB gene mutation (Am J Surg Pathol 2014;38:1588)
- CK7, CAIX, RCC, c-kit (mast cells only), vimentin (Mod Pathol 2015;28:80), neuroendocrine markers
- Minimal AMACR staining
| Hale | KIT | CK7 | S100A1 | VIM | CAIX | AMACR | SDH | TFE3 | |
| Chromophobe RCC | +++ | +++ | +++ | - | - | - | - | +++ | - |
| Clear cell RCC | - | - | - | - | +++ | +++ | - | +++ | - |
| Oncocytoma | - | +++ | rare | +++ | - | - | - | +++ | - |
| Papillary RCC | - | - | +++ | - | +++ | - | +++ | +++ | - |
| Translocation RCC | - | - | - | - | - | - | ++ | +++ | +++ |
| SDH deficient RCC | - | - | - | - | - | - | - | - | - |
References: Pathol Res Pract 2015;211:303, Ann Diagn Pathol 2020;44:151448, Am J Surg Pathol 2014;38:e6, Arch Pathol Lab Med 2019;143:1455, Transl Androl Urol 2019;8:S123, Hum Pathol 2020 Jul 13 [Epub ahead of print]
- No mutations in VHL, PIK3CA, AKT, mTOR, MET or TP53 genes (WHO 2016)
- Genes for succinate dehydrogenase subunits (SDHA, SDHB, SDHC, SDHD) encode proteins which are part of mitochondrial complex II, which links the Krebs cycle and electron transport chain (N Engl J Med 2011;364:885)
- Eosinophilic variant of chromophobe RCC:
- Prominent cell borders and crinkled / raisinoid nuclei with binucleation
- Also CK7+ with no loss of SDHB expression
- Eosinophilic variant of clear cell RCC: CAIX+, vimentin+
- Oncocytoma: often has myxohyaline stroma; CKIT+ with no loss of SDHB expression (Mod Pathol 2015;28:80)
- Papillary renal cell carcinoma (type 2):
- Cytoplasm is eosinophilic but not as pale wispy/bubbly
- Nuclei often pseudostratified
- Loss of SDHB immunohistochemical expression is rare in other carcinomas (Appl Immunohistochem Mol Morphol 2014;22:31)
- Rare, highly aggressive, typically medulla centered carcinoma with SMARCB1 (INI1) deficiency
- Associated with sickle cell trait or other sickle hemoglobinopathies
- Rare carcinoma arising in the renal medulla
- Occurs predominately in young patients with sickle cell trait
- Variety of histologic patterns
- Sickled erythrocytes (drepanocytes) may be pathognomonic
- Shows loss of SMARCB1 / INI1
- Poor prognosis
- SMARCB1 deficient renal medullary carcinoma, renal medullary carcinoma
- ICD-O: 8510/3 - medullary carcinoma, NOS
- ICD-10:
- ICD-11: 2C90.Y & XH2YP5 - other specified malignant neoplasm of kidney, except renal pelvis & medullary carcinoma, NOS
- Rare, < 0.5% of all renal carcinomas; ~200 cases described, first in 1995 (Am J Surg Pathol 1995;19:1, J Oncol Pract 2017;13:414)
- Arises almost exclusively in patients with sickle cell trait (hemoglobin AS), although it has been reported rarely in (J Oncol Pract 2017;13:414):
- Homozygous SS disease (sickle cell anemia) (Arch Pathol Lab Med 2003;127:e135, Pediatr Blood Cancer 2014;61:567)
- HbS / beta thalassemia (BJU Int 2017;120:782)
- HbSC (Am J Surg Pathol 2014;38:871)
- Rare reports of tumors that are morphologically similar to renal medullary carcinoma in the absence of sickle cell trait, sickle cell disease or other hemoglobinopathy (Nat Rev Urol 2010;7:110, Hum Pathol 2017;67:134)
- These tumors have been previously termed unclassified renal cell carcinoma with medullary phenotype
- Mean age: 26 years (wide age range: 5 - 69 years) (J Oncol Pract 2017;13:414, Am J Surg Pathol 2013;37:368)
- M:F = 2:1 (Am J Surg Pathol 2013;37:368, Mod Pathol 2007;20:914)
- 75 - 89% occur in the right kidney (Urology 2007;70:878)
- Involves the kidney, more often the right kidney, in any location of the renal medulla and with or without cortex
- Arises in the renal medulla from the distal segment of the collecting ducts (Mod Pathol 2007;20:914, Hansel: The Kidney, 1st Edition, 2016)
- Believed to arise from the renal papillae or calyceal epithelium and may be triggered by the chronic medullary hypoxia and hypertonic environment resulting from sickled red cells and microvascular occlusion (Urology 2002;60:1083, Clin Cancer Res 2018;24:2044)
- Environment promotes double strand DNA breaks
- Chronic hypoxia also leads to repression of the RAD51 and BRCA1 pathways associated with high fidelity homologous recombination and induces a switch to nonhomologous end joining (NHEJ) repair pathways, which are more likely to produce deletions and translocations
- Translocations and deletions are the most common causes of SMARCB1 inactivation / loss of expression, a chromatin remodeler and tumor suppressor gene
- Right side predominance may be due to differences in vascular anatomy, particularly the greater length of the right renal artery, which results in less blood flow and therefore relative hypoxia
- Considered to be the seventh sickle cell nephropathy (others: unilateral hematuria, papillary necrosis, renal infarct, nephrotic syndrome, pyelonephritis, inability to concentrate urine) (Afr Health Sci 2016;16:490, Am J Surg Pathol 1995;19:1)
- Symptoms including hematuria, flank / abdominal pain, dysuria and weight loss in virtually all patients (J Oncol Pract 2017;13:414)
- Fever, nausea, vomiting and a palpable abdominal mass may be present
- Aggressive; usually advanced disease at presentation with metastases to lung, liver, lymph nodes, adrenal gland, peritoneum, retroperitoneum, inferior vena cava, etc.
- 90% of patients present with metastasis at the time of diagnosis
- Essential: SMARCB1 deficient, high grade, infiltrating adenocarcinoma
- Desirable: clinical / laboratory evidence of sickle hemoglobinopathy
- Consideration of other carcinomas with SMARCB1 deficiency (see Differential diagnosis)
- May show an infiltrative tumor, more often on the right side, with necrosis, caliectasis and regional adenopathy (AJR Am J Roentgenol 2005;185:268)
- Poor prognosis with a median overall survival of 6 - 13 months (J Oncol Pract 2017;13:422)
- Metastatic stage at diagnosis increases the risk of death about threefold (Can Urol Assoc J 2015;9:E172)
- 15 year old black girl with sickle cell trait, lymphadenopathy and both pleural and pericardial effusions (Arch Pathol Lab Med 2003;127:e288)
- 20 year old pregnant black woman with sickle cell trait (Arch Pathol Lab Med 2002;126:627)
- 21 year old black man with sickle cell disease and 40 year old black man with HbSC disease (Arch Pathol Lab Med 2003;127:e135)
- 22 year old man with renal medullary metastasis to the orbit and temporal fossa (Ophthalmic Plast Reconstr Surg 2019;35:e149)
- 29 year old man with renal medullary carcinoma clinically masquerading as renal infection (BMC Nephrol 2020;21:79)
- 29 year old woman with renal medullary carcinoma with an aggressive clinical course (Case Rep Oncol 2017;10:1)
- 32 year old African American man with sickle cell trait with multiple lung nodules and 11 cm right kidney mass (Cytojournal 2018;15:21)
- 33 year old black man with sickle cell trait (Arch Pathol Lab Med 2000;124:1561)
- Radical nephrectomy
- Chemotherapy is usually administered with platinum based regimens (Clin Genitourin Cancer 2021;19:e401)
- Few data regarding the role of other therapies and immunomodulatory drugs (UptoDate: The Treatment of Advanced Non-Clear Cell Renal Carcinoma [Accessed 10 November 2022], Int J Mol Sci 2022;23:7097, Clin Genitourin Cancer 2021;19:103)
- Ongoing clinical trials (ClinicalTrials: Medullary Carcinoma [Accessed 10 November 2022])
- SMARCB1 loss may be target for proteasome inhibition (Elife 2019;8:e44161)
- Poorly circumscribed, lobulated, firm, rubbery, gray-white tumor centered in renal medulla
- Size ranges from 4 to 12 cm, mean is 5.9 cm (Am J Surg Pathol 2013;37:368)
- Hemorrhage and necrosis are common
- Typically extends into the calyces and pelvis
- Satellite nodules in the cortex, extension into the perinephric and sinus fat can be present
- Variety of morphologic patterns:
- Distinctive patterns include reticular / yolk sac tumor-like and sieve-like / cribriform / microcystic / adenoid cystic-like
- Cords, nests, tubular, glandular, tubulopapillary and solid / sheet-like patterns overlaps with collecting duct carcinoma and fumarate hydratase (FH) deficient renal cell carcinoma (Am J Surg Pathol 2018;42:279)
- Tumor cells are pleomorphic with hyperchromatic enlarged nuclei, vesicular chromatin, prominent nucleoli, nuclear grooves, eosinophilic cytoplasm and may have rhabdoid features
- Hemorrhage, necrosis (may be ischemic, geographic or central / comedo type)
- Frequent mitotic figures
- Angiolymphatic invasion, desmoplastic stroma and infiltrative borders
- Abundant intratumoral neutrophils (sometimes abscess forming) and lymphocytes at tumor rim
- Abundant sickled erythrocytes (drepanocytes) may be pathognomonic
- Loosely cohesive groups, sheets or single cells
- High grade / pleomorphic cells with vacuolated fine pale cytoplasm that often displace nuclei
- Nuclei often have irregular membranes, coarse or vesicular chromatin, smooth borders, membrane grooves and prominent nucleoli (Cancer 2005;105:28, J Am Soc Cytopathol 2021;10:187)
- CAM 5.2, AE1 / AE3, CK7 and CK20 (CK7 and CK20 may be variable), vimentin, EMA and CEA (Urology 2002;60:1083, Am J Surg Pathol 2013;37:368)
- p53 and PAX8 (Urology 2002;60:1083, Mod Pathol 2009;22:1218)
- OCT 3/4 in 50% of cases (Am J Surg Pathol 2012;36:583)
- Ulex may be focally positive in a minority of cases (Urology 2002;60:1083)
- May be strongly positive for vascular endothelial growth factor (VEGF) and hypoxia inducible factor (HIF) (Urology 2002;60:1083)
- Loss of expression of SMARCB1 (also known as INI1, SNF5 or BAF47), colloidal iron, PAS, desmin (Mod Pathol 2008;21:647)
- CK 34 beta E12 (Urology 2002;60:1083)
- Cells contain tight junctions and large intracytoplasmic lumina, which contain long slender microvilli
- Condensed fibrillary electron dense filamentous luminal deposits are also seen (Ultrastruct Pathol 2008;32:252)
- Molecular:
- Inactivation of SMARCB1 / INI1 (22q11.2) from chromosomal translocations or deletions is a major driver mutation
- SMARCB1 protein expression loss arises through concurrent hemizygous loss and translocation or by homozygous loss
- Loss of INI1 has been linked to downregulation of p16INK4a and upregulation of cyclin D1, promoting unregulated progression through the cell cycle (J Oncol Pract 2017;13:414, Am J Surg Pathol 2013;37:368, Adv Anat Pathol 2017;24:65)
- Hypoxia inducible factor and VHL abnormalities (Hum Pathol 2011;42:1979)
- DNA topoisomerase II amplification (BJU Int 2010;106:62)
- Chromosome 8q gain, where c-MYC is located, was noted in 46.7% of renal medullary carcinoma tumors and contains recurrent focal copy number alterations (CNA) in regions of genes related to cell proliferation including a distinct CNA pattern that results in Notch pathway activation (Cancer Cell 2020;37:720)
- Inactivation of SMARCB1 / INI1 (22q11.2) from chromosomal translocations or deletions is a major driver mutation
- Cytogenetics (Arch Pathol Lab Med 2019;143:1556, Eur Urol 2016;69:1055, Histopathology 2012;61:428, Am J Surg Pathol 2011;35:e47):
- Rearrangement of chromosome 8 resulting in a loss of 8p and a gain of 8q can be observed
- Gains of chromosomes 7, 8, 10 and 11 can be seen along with losses of chromosomes 9 and 13
- Additional unknown material on 10p, a deletion of 7q and a dicentric chromosome composed of 13q and 21q
- Rare instances of ABL gene amplification or translocation have also been described
Pathology mini tutorial of renal medullary carcinoma
Cytomorphologic study of renal medullary carcinoma involving serous cavity fluids
- Right kidney, radical nephrectomy:
- SMARCB1 deficient renal medullary carcinoma (X cm), extends into the renal sinus, margins are negative (see comment and synoptic report)
- Comment: The patient's history of sickle cell trait is noted. Immunohistochemistry for SMARCB1 (INI1) is performed and shows loss of expression within the tumor cells. The clinical, morphologic and immunohistochemical findings support the diagnosis.
- Viniculin (VCL)::ALK fusion renal cell carcinoma:
- Also occurs in young patients with sickle cell trait, usually younger (mean age of 9 years)
- Shows VCL::ALK fusion, retained (positive) INI1 and much lower proliferative activity (Am J Surg Pathol 2014;38:858)
- Rhabdoid tumor of the kidney:
- Occurs in very young patients (mean / median patient age of 1 year), nearly all cases occurring in patients Shows loss of SMARCB1 (INI1)
- Diverse pattern of immunoreactivity may include cytokeratins, vimentin, SMA, synaptophysin, GFAP and CD99
- Many show eosinophilic intracytoplasmic inclusions
- High grade urothelial carcinoma:
- Collecting duct carcinoma:
- Older patients (mean age of 55 years, whereas renal medullary carcinoma is uncommon in patients older than 40 years of age)
- Not associated with sickle cell trait
- Retained SMARCB1 (collecting duct carcinomas have been reported to have secondary loss of SMARCB1 / INI1 in up to 15% of cases)
- Negative for OCT 3/4
- Multinodular, infiltrating papillary morphologic patterns may be favored (Am J Surg Pathol 2018;42:279)
- Metastatic germ cell neoplasm (e.g., embyronal carcinoma) (Am J Surg Pathol 2012;36:583):
- Fumarate hydratase (FH) deficient renal cell carcinoma:
- Often viral inclusion-like (CMV-like) macronucleoli with perinucleolar halos
- Tubulocystic and intracystic papillary with or without hyalinization patterns may favor FH deficient RCC (Am J Surg Pathol 2018;42:279)
- Loss of fumarate hydratase, nuclear and cytoplasmic expression of 2SC
- Negative for OCT 3/4 (Am J Surg Pathol 2018;42:279)
- Rare secondary SMARCB1 loss has been reported (Hum Pathol 2018;77:139)
- SMARCB1 deficient renal cell carcinoma with medullary-like features or phenotype, also known as renal cell carcinoma, unclassified, with medullary phenotype (RCCU MP) (Hum Pathol 2017;67:134):
- Morphologically similar to renal medullary carcinoma, lacking SMARCB1 expression by immunohistochemistry but without evidence of a hemoglobinopathy (Hum Pathol 2017;67:134)
- Other renal cell carcinoma subtype with secondary SMARCB1 (INI1) deficiency
- If the size of the tumor obscures the site of origin, infiltrative growth that extends between normal structures, such as glomeruli and tubules, can be seen as a clue that the tumor is medullary based
- This pattern can be seen in collecting duct carcinoma, medullary carcinoma and invasive urothelial carcinoma; similar patterns can also be seen in metastatic tumors and lymphoma (Surg Pathol Clin 2015;8:587)
A 24 year old man with sickle cell trait presents with hematuria and flank pain. Imaging shows a 6 cm medulla centered renal mass. A radical nephrectomy is performed. What will the pathologic workup likely show?
- Inactivation of 12q11.2 due to deletion or translocation
- Papillary growth with pale to eosinophilic, flocculent cytoplasm
- Reticular or cribriform growth pattern with high grade cells
- TFE3 (on Xp11) translocation with ASPSCR1 (ASPL)
Comment here
Reference: SMARCB1 deficient renal medullary carcinoma
- Both tumors have an indolent prognosis
- CD20 positivity
- May show positive staining for GFAP, SMA, synaptophysin, CD99
- Will show SMARCB1 (INI1) loss / inactivation by immunohistochemistry
Comment here
Reference: SMARCB1 deficient renal medullary carcinoma
- Sarcoma-like histology is not a distinct entity but a pathway of transformation (dedifferentiation) in subtypes of renal cell carcinoma (RCC) (Cancer 2005;104:1195)
- An estimated 4% to 5% of RCCs have a sarcomatoid component but it varies depending on subtype (Am J Surg Pathol 1997;21:1188, Ann Diagn Pathol 2003;7:296, Nat Rev Urol 2020;17:659)
- Presence of sarcomatoid component is associated with a worse prognosis (Am J Surg Pathol 2001;25:275, Am J Surg Pathol 2004;28:435)
- Represents RCC loss of epithelial phenotype and gain of mesenchymal characteristics, epithelial mesenchymal transition, a more aggressive phenotype with increased risk for local spread and high metastatic potential (Cancer 2005;104:1195)
- May be diffuse or focal; even focal sarcomatoid component is important and associated with a poorer prognosis
- Sarcomatoid morphology is categorized as WHO / ISUP grade 4 (CAP: Protocol for the Examination of Specimens from Patients with Invasive Carcinoma of Renal Tubular Origin [Accessed 13 January 2023])
- Previously called carcinosarcoma of the kidney, sarcomatoid RCC and spindle cell carcinoma (J Pathol Bacteriol 1963;85:139, Cancer 1968;22:556, Jpn J Clin Oncol 1997;27:58)
- Currently not considered its own variant
- When the background carcinoma subtype is recognized, the primary histologic diagnosis should be the RCC subtype with sarcomatoid dedifferentiation mentioned in the report (e.g., papillary RCC with sarcomatoid features)
- Sarcomatoid elements, sarcomatoid dedifferentiation, sarcomatoid differentiation, sarcomatoid features and sarcomatoid component are terms that are used interchangeably
- If pure sarcomatoid with no recognizable epithelial component upon extensive sampling, it should be considered as an unclassified RCC once primary or metastatic sarcoma is excluded
- ICD-10: C64.9 - malignant neoplasm of kidney, unspecified laterality
- Mean age: 60 years
- M:F = 1.3:1 - 2:1
- Usually presents with locally advanced or metastatic disease at presentation (stage III or IV)
- Aggressive with median survival of 4 to 19 months (J Urol 2002;167:65, Oncologist 2012;17:46, Urol Oncol 2015;33:427.e17)
- Patients with clear cell RCC (ccRCC) with a sarcomatoid component have a worse prognosis than those without at every stage of disease (World J Urol 2016;34:1429)
- 5 year disease specific survival for stage I, II, III and IV: 77.7%, 67.8%, 35.4% and 3.5%, respectively (Clin Genitourin Cancer 2019;17:e447)
- For patients with no evidence of disease following nephrectomy, 77% of patients experienced a recurrence; median time to recurrence is 26.2 months (Urol Oncol 2015;33:166.e21)
- Metastases in decreasing order of prevalence: lung, bones, lymph nodes, liver and brain
- Kidney or metastatic sites
- Not clearly understood
- May arise from common cell of origin epithelial cell, which undergoes epithelial mesenchymal transition
- Beta catenin translocates to nucleus and acts as transcription factor for Snail, a transcriptional repressor for E-cadherin, loses epithelial phenotype and gains mesenchymal characteristics (upregulation of Snail and cytoplasmic N-cadherin) (J Clin Pathol 2011;64:1088, Cancer Sci 2010;101:293)
- 90% symptomatic with pain, gross hematuria, weight loss, fatigue, fever, nights sweats
- Presentation with advanced / late stage disease and metastasis is common
- 60.9% had stage IV disease at presentation (Clin Genitourin Cancer 2019;17:e447)
- Sarcomatoid growth represents a pathway of transformation (dedifferentiation) in subtypes of RCC
- It is associated with poor prognosis and is therefore important to identify and document its presence and percentage
- It constitutes ISUP / WHO grade 4 in applicable RCC
- Extensive sampling should be performed in order to identify the primary RCC subtype and rule out involvement by other carcinomas (urothelial, adrenocortical) and sarcomas
- Immunohistochemistry may be helpful to subtype the neoplasm based on the histologic findings present in one's case and is generally best done in more well differentiated areas
- No specific methods to identify sarcomatoid differentiation (J Urol 2009;182:2164, BJU Int 2012;110:1742)
- Sarcomatoid carcinoma component is more often associated with neovascularity, larger peritumoral vessels and a more heterogenous appearance on CT (AJR Am J Roentgenol 2015;204:1013)
- MRI with T2 weighted low signal intensity (Clin Imaging 2013;37:908, Abdom Imaging 2015;40:112, Quant Imaging Med Surg 2018;8:373, Abdom Radiol (NY) 2022;47:2168)
- Percentage of sarcomatoid component may be prognostic
- Higher percentage of sarcomatoid dedifferentiation may confer a worse prognosis; recommended to report percentage by ISUP consensus conference (Am J Surg Pathol 2004;28:435, Am J Surg Pathol 2013;37:1490)
- 6% increase in risk of death for every 10% increase in the amount of sarcomatoid differentiation
- 52% more likely to die from RCC if ≥ 30% sarcomatoid differentiation (BJU Int 2015;115:405)
- > 40% less likely to be alive at 1 year (Urol Oncol 2015;33:427.e17)
- > 10% sarcomatoid component had higher risk of death (Urol Oncol 2015;33:427.e17)
- In ≥ T3, M0 disease, patients with ≥ 25% sarcomatoid features had ~30% increased overall risk of dying of any cause over those without sarcomatoid features
- Overall survival was not affected by % sarcomatoid in patients with cM1 disease (Clin Genitourin Cancer 2015;13:225)
- Older age, higher tumor stage and nephrectomy found to independently affect disease specific survival (Clin Genitourin Cancer 2019;17:e447)
- 45 year old woman with sarcomatoid chromophobe RCC with squamous differentiation (Arch Pathol Lab Med 2008;132:1672)
- 53 year old man with autosomal dominant polycystic kidney disease on hemodialysis with pure sarcomatoid RCC (CEN Case Rep 2021;10:199)
- 56 year old Japanese man with acquired cystic disease associated RCC, sarcomatoid change and rhabdoid features (Ann Diagn Pathol 2011;15:462)
- 62 year old man with sarcomatoid RCC with wide metastasis (J Kidney Cancer VHL 2018;5:1)
- 71 year old woman with divergent growth pattern (Arch Pathol Lab Med 2005;129:1057)
- No clear treatment guidelines
- Radical nephrectomy with or without extended lymph node dissection (Quant Imaging Med Surg 2018;8:373, J Urol 2004;172:465, Clin Genitourin Cancer 2019;17:e447, Curr Oncol 2022;29:5475, Cureus 2022;14:e25395, J Urol 2009;182:2164)
- For localized disease, surgical resection is standard of care (Urol Oncol 2015;33:166.e21)
- Combination chemotherapy with cytotoxic regimens (Eur Urol 1995;27:138, J Urol 2002;168:959, Cancer 2004;101:1545, Med Oncol 2012;29:761, Clin Genitourin Cancer 2017 Aug 10 [Epub ahead of print], Cancer 2015;121:3435, J Clin Oncol 2016;34:833, J Clin Oncol 2016;34:4511)
- Targeted therapies are investigational but response remains poor
- VEGF inhibitors (J Clin Oncol 2009;27:235, Eur J Med Res 2010;15:287, Clin Genitourin Cancer 2015;13:e79, BJU Int 2011;107:741)
- mTOR inhibitors (Ann Oncol 2014;25:663, Clin Genitourin Cancer 2015;13:e79)
- Antiangiogenesis targeted therapies (Am J Clin Oncol 2011;34:454)
- Cytokine therapy (J Urol 2017;198:530, Am J Clin Oncol 2011;34:454, J Urol 2002;167:65, PLoS One 2017;12:e0190084)
- PDL1 expression is increased in sarcomatoid dedifferentiated renal tumors, suggesting potential benefit from PD-1, PDL1 immune checkpoint blockade or anti-CTLA4 therapy (Nat Rev Urol 2020;17:659, Clin Genitourin Cancer 2017;15:e1127, Eur Urol 2015;68:912, J Oncol Pract 2018;14:511, Mod Pathol 2019;32:1344, J Clin Oncol 2016;34:833, Clin Cancer Res 2021;27:78, J Clin Oncol 2019;37:4500, Annals of Oncol 2019;30:361, Eur Urol 2021;79:659, Lancet 2019;393:2404, Curr Oncol 2022;29:5475, J Med Case Rep 2022;16:193, Front Genet 2022;13:985641, J Kidney Cancer VHL 2021;8:38, Cancer Rep (Hoboken) 2021;4:e1356, Eur Urol 2021;79:663)
- Combination immunotherapy recommended by Society of Immunotherapy of Cancer as first line treatment option (J Immunother Cancer 2019;7:354)
- No survival benefit from postnephrectomy metastasectomy (J Urol 2009;182:2164)
- Radiotherapy does not seem to increase overall survival (Int Urol Nephrol 2015;47:1653)
- Grayish white bulging mass with infiltrative margins and fleshy to fibrous cut surface; must sample these areas
- Mean size: 9 cm (Am J Surg Pathol 2001;25:275)
- May have heterogeneous patchy appearance: yellow, hemorrhagic, necrotic
- Areas of sarcomatoid dedifferentiation may be heterogenous or uniform and may display fibrosarcoma-like, pleomorphic undifferentiated sarcoma (malignant fibrous histiocytoma-like) or unclassified morphology
- Heterologous differentiation such as chondrosarcoma, osteosarcoma or rhabdomyosarcoma is rare
- Usually fibrosarcoma-like with haphazard, infiltrative growth without well formed fascicles
- Atypical, spindle cells or tumor giant cells with marked nuclear pleomorphism
- Frequent mitosis, abnormal mitotic figures
- Necrosis common
- Sarcomatoid pattern is due to dedifferentiation from subtype of RCC
- Sarcomatoid and carcinomatous areas may be intermingled or may show sharp demarcation (Am J Surg Pathol 2013;37:1490)
- Most will have an epithelial component with generous sampling; pure sarcomatoid is extremely rare (4%)
- Sarcomatoid change occurs in 5.2% to 8% ccRCC, 1.9% to 5.4% papillary RCC, 2% to 9% chromophobe RCC, 25% to 29% collecting duct RCC, 11% unclassified RCC (Am J Surg Pathol 2001;25:275, Am J Surg Pathol 2013;37:1490)
- Carcinomatous subtype does not appear to impact prognosis
- Additionally reports of sarcomatoid dedifferentiation in hereditary leiomyomatosis RCC syndrome associated RCC / fumarate hydratase deficient RCC and acquired cystic disease associated RCC (Am J Surg Pathol 2014;38:567, Am J Surg Pathol 2014;38:627, Ann Diagn Pathol 2011;15:462)
- Metastases of sarcomatoid RCC can exhibit only sarcomatoid (58%), only carcinoma pattern (38%) or both components; > 30% sarcomatoid pattern in primary tumor frequently associated with metastatic sarcomatoid histology (Cancer 2010;116:616)
- Sarcomatoid morphology by definition is WHO / ISUP grade 4
- Rhabdoid regions that maintain epithelial features should not be considered sarcomatoid but reported separately (Am J Surg Pathol 2004;28:435, Am J Surg Pathol 2013;37:1490)
- Biopsy's ability to detect sarcomatoid differentiation low on primary biopsy is ~7.5% (J Urol 2017;198:530)
- Presence of any amount of sarcomatoid dedifferentiation sufficient for diagnosis of sarcomatoid differentiation (Am J Surg Pathol 2013;37:1490)
- ISUP consensus conference addressed definition and issues surrounding sarcomatoid RCC (Am J Surg Pathol 2013;37:1490)
- 41% of participants felt that a tumor is sarcomatoid if it consists of atypical spindle cells and resembles any form of sarcoma
- 34% voted that a spindle cell morphology need not be present as long as the tumor is very atypical and resembles any form of sarcoma
- 22% considered a tumor sarcomatoid if it had a spindle cell pattern
- 3% considered a tumor to be sarcomatoid if it showed early sarcomatoid change characterized by elongation of epithelial tumor cells
- Consensus among participants (71%) that a minimum proportion of sarcomatoid tumor was not required to make a diagnosis of sarcomatoid carcinoma
Images hosted on other servers:
- AE1 / AE3, vimentin (56%), EMA (50%), CAM 5.2 (40%)
- N-cadherin (cytoplasmic) (Anticancer Res 2013;33:4875)
- CAIX in sarcomatoid ccRCC
- PAX8; however, lack of staining does not exclude renal origin
- p53 overexpression (Cancer Res 1995;55:658)
- PDL1 overexpression (Mod Pathol 2019;32:1344, Cancer 2017;123:4823, Cancer Immunol Res 2015;3:1303, CEN Case Rep 2021;10:199)
- Actin (33%) and LeuM1 (22%) may be expressed in spindled areas
- High molecular weight keratin 34 beta E12, S100 (usually) (Arch Pathol Lab Med 1993;117:636)
- Focal desmin is uncommonly seen
- Shows epithelial differentiation, including desmosomal cell junctions with sarcomatoid differentiation such as rhabdomyosarcomatous differentiation (Hum Pathol 2002;33:68)
- Complex set of chromosomal gains and losses, -13q (75%) and -4q (50%)
- Similar X chromosome inactivation patterns in both sarcomatoid and parent tumor suggesting common cell origin (Cancer 2005;104:1195)
- Significant heterogeneity in the tumor components, suggesting genetic divergence (Mod Pathol 2007;20:303, Proc Natl Acad Sci U S A 2016;113:2170, Clin Cancer Res 2017;23:6686, CEN Case Rep 2021;10:199)
- Sarcomatoid component in comparison to epithelioid component have
- Higher mutational burden / hypermutation status
- More frequent mutations of p53, RELN, SETD2, ARID1A, BAP1 and PTEN which were associated with decreased survival but fewer deletions of genes VHL (3p21-25) and PBRM1 (Clin Cancer Res 2017;23:6686)
- Reelin (RELN) inhibits TGFB1 induced cell migration
- Loss of heterozygosity on chromosomes 1p, 9, 10, 14, 17p, 18 and 22 (Proc Natl Acad Sci U S A 2016;113:2170)
- Upregulation of TGFβ signaling
- In a single patient with autosomal dominant polycystic kidney disease and sarcomatoid RCC, there were mutations in ATM (c.9156G>C), CTNNB1 (c.1187A>T) and NF2 (c.1021C>T); gene upregulation in Wnt, PI3K-mTOR, MAPK and interleukin 6 and downregulation of CDKN2A in the sRCC component (Mol Genet Genomic Med 2022;10:e1853)
- Some had increased expression of BAP1 (10.5%), ARIDA1A (15.7%) and novel somatic single nucleotide variants in FAT1, FAT2, FAT3, TSG101, LRIF1, RQCD1 and PTK7 (Proc Natl Acad Sci U S A 2016;113:2170)
- Most frequently mutated genes were TP53 (42.3%), CDKN2A (26.9%) and NF2 (19.2%) (Eur Urol 2016;70:348)
- NF2 mutations may be associated with sarcomatoid papillary RCCs (CEN Case Rep 2021;10:199)
- Frequency of Hippo-YAP pathway mutations in sRCCs was significantly higher than in nonsarcomatoid RCCs (Sci Rep 2020;10:701)
- Increased prevalence of JAK2 amplification with coamplification of PDL1 and PDL2 on 9p24.1 locus; finding correlates with higher PDL1 IHC expression than grade 4 ccRCC (Mod Pathol 2019;32:1344, Cancer 2017;123:4823, Cancer Immunol Res 2015;3:1303, CEN Case Rep 2021;10:199)
- mRNA expression of POLR2A and RUNX2 increased and miR-378 suppressed in sRCC (Anticancer Res 2022;42:811)
- FOXO3A induced epithelial to mesenchymal transition of tumor cells by up regulating Snail
- Snail protein expression significantly associated with tumor stage, histological grade and presence of sarcomatoid differentiation
- Epithelial to mesenchymal transition orchestrates the sarcomatoid conversion of ccRCC, characterized by E-cadherin to N-cadherin switching, dissociation of beta catenin from the membrane and increased expression of Snail and Sparc (Mol Diagn Ther 2016;20:111)
- Study on sarcomatoid transformation in chromophobe RCC shows that TP53 variants followed by widespread loss of heterozygosity, likely whole genome duplication / imbalanced chromosomal duplication events, seem to underlie sarcomatoid transformation (Mod Pathol 2024;37:100396)
- Recurrent single nucleotide variants were found in RB1 (37.5% of cases) and PTEN (25% of cases)
- Copy number variant analysis showed the characteristic pattern of chromosomal losses associated with chRCC (1, 2, 6, 10, 13, 17, and 21) in the conventional histologic components only
- Right kidney, radical nephrectomy:
- RCC, unclassified (15 cm), WHO / ISUP grade 4 of 4, with sarcomatoid and rhabdoid features (see comment and synoptic report)
- Unifocal
- Tumor necrosis: present, 25%
- Sarcomatoid: present, 60%
- Tumor extension: tumor extends into the renal sinus and into major veins (renal vein or segmental branches), pelvicalyceal system and extends into the perinephric adipose tissue
- Margins: carcinoma is present in soft tissue at renal vein margin.
- Lymphovascular invasion: present, extensive
- Adrenal gland without significant diagnostic alteration; negative for neoplasm
- Comment: Immunohistochemistry is performed. The carcinoma cells are positive for AMACR, CD10, RCC, CAIX (focal) and TFE3 (patchy). CK7 and HMB45 are negative. Per report, TFE3 FISH is negative. On H&E stained slides, there are areas of tumor with clear cell features and areas of papillary growth with more oncocytic cytoplasm. CAIX displays staining that is not seen in ccRCC. Therefore, the tumor is best categorized as RCC, unclassified, due to the heterogeneity of morphological findings and unclear immunohistochemical pattern.
- Adequate sampling is the most useful step in making the correct diagnosis
- Clear cell renal cell carcinoma with early spindle cell change:
- Stroma is not malignant (Hum Pathol 2007;38:1372)
- Sarcomatoid urothelial carcinoma:
- Usually associated with prior history of lower tract urothelial carcinoma
- Filling defect on imaging, positive urine cytology
- If located in renal calyces or pelvis, urothelial carcinoma is favored
- Carcinoma in situ (CIS) or residual papillary component may be present in renal pelvis
- Careful sampling of renal pelvis is recommended
- Usually, lower stage disease at presentation
- Urothelial carcinoma typically p63+ / GATA3+ / uroplakin II+ and negative for PAX8 (Am J Surg Pathol 2010;34:965, Hum Pathol 2013;44:2651)
- Sarcomatoid adrenal cortical carcinoma:
- Clinical, radiologic and gross assessment to determine epicenter
- SF1, calretinin, inhibin, MelanA / MART1, synaptophysin+; PAX8-
- Mesenchymal neoplasms / sarcomas:
- Either primary or metastatic
- Primary renal mesenchymal tumor most often leiomyosarcoma (J Cancer Res Ther 2022;18:1186)
- Primary renal sarcomas have no epithelial component after careful sampling
- Leiomyosarcoma more often has well formed fascicles
- Nuclei may range from low grade tumors to prominent atypia
- Nuclei are often cigar shaped with perinuclear vacuoles
- Often relatively well circumscribed without infiltration around pre-existing glomeruli and renal tubules
- Desmin typically diffuse with negative PAX8, focal to negative cytokeratins
- DDx includes solitary fibrous tumor, synovial sarcoma, others
- Direct involvement by primary retroperitoneal soft tissue sarcoma
- Usually liposarcoma; will not have an epithelial component after careful sampling
- Angiomyolipoma:
- Mucinous tubular and spindle cell carcinoma:
- These neoplasms typically have characteristic areas of anastomosing tubules and bland spindle cells with myxoid / mucinous stroma without infiltrative growth, cellular atypia, necrosis and increased cellular density usually seen in sarcomatoid carcinoma
- However, high grade / sarcomatoid transformation has been rarely reported in this entity (Diagn Pathol 2015:10:168)
Comment Here
Reference: Sarcomatoid renal cell carcinoma
Comment Here
Reference: Sarcomatoid renal cell carcinoma
- Fibroblastic mesenchymal tumor with haphazard spindle cells, staghorn vasculature and NAB2::STAT6 gene fusion
- Fibroblastic tumor characterized by patternless architecture and staghorn vasculature
- STAT6 expression by immunohistochemistry; NAB2::STAT6 gene fusion
- About 16% of renal solitary fibrous tumors (SFTs) show histological features that may indicate aggressive behavior: hypercellularity, cytological atypia, increased mitosis and necrosis
- Hemangiopericytoma (not recommended)
- ICD-O
- ICD-10: D49.519 - neoplasm of unspecified behavior of unspecified kidney
- ICD-11
- 2F7C & XH7E62 - neoplasms of uncertain behavior of connective or other soft tissue & solitary fibrous tumor, NOS
- 2B5Y & XH1HP3 - other specified malignant mesenchymal neoplasms & solitary fibrous tumor, malignant
- M:F = 1:1.8 (Int J Surg Pathol 2015;23:34)
- Mean age: 49 (range: 3 - 85) (Int J Surg Pathol 2015;23:34)
- Any anatomic site; majority are pleural or abdominopelvic (Mod Pathol 2012;25:1298, Ann Surg Oncol 2017;24:3865)
- Rare in kidney; ~105 cases reported (Int J Surg Case Rep 2019;62:112)
- Metachronous SFTs of kidney and synchronous pleurorenal SFTs can happen (Urol Case Rep 2015;4:45, Minerva Chir 2009;64:669)
- Intrachromosomal inversion of chromosome 12q results in NAB2::STAT6 fusion (Nat Genet 2013;45:131)
- NAB2 is a transcriptional repressor of early growth response 1 (EGR1), while NAB2::STAT6 is a transcriptional activator of ERG1 (Nat Genet 2013;45:180)
- Overactivation of EGR1 leads to increased expression of its target genes, such as IGF2, FGF2, PDGFD, FGFR1, NTRK1, etc. (Nat Genet 2013;45:180)
- Most asymptomatic, can present with hematuria and flank pain (Int J Surg Pathol 2015;23:34)
- Hypoglycemia (Doege-Potter syndrome): in IGF2 (Arch Ital Urol Androl 2020;92:392)
- Histological examination
- NAB2::STAT6 gene fusion by immunohistochemical or molecular studies
- Rare cases showed hypoglycemia and hypokalemia (Asian J Surg 2023;46:1260)
- Ultrasound: heterogeneous, mainly hyperechoic (Cureus 2016;8:e490)
- Computed tomography (CT): heterogeneous enhanced lesion (Mol Clin Oncol 2020;13:39)
- Magnetic resonance imaging (MRI): highly variable, may show isointense in T1 and hyperintense in T2 (Int J Surg Case Rep 2019;62:112)
- Histological features cannot precisely predict aggressive behavior; only 36% of histologically malignant kidney SFT patients presented with recurrent / metastatic disease (Int J Surg Pathol 2015;23:34)
- Multivariable risk stratification model has shown to more accurately predict prognosis
- Variables in risk stratification model of SFTs from all anatomic sites are: age ≥ 55, tumor size ≥ 10 cm, mitotic count ≥ 4/10 high power fields, tumor necrosis ≥ 10% (Mod Pathol 2017;30:1433)
- 39 year old woman with left kidney mass (Cureus 2023;15:e46475)
- 52 year old woman with right kidney tumor (Mol Clin Oncol 2020;13:39)
- 55 year old man with left kidney mass (Int J Surg Case Rep 2019;62:112)
- 66 year old woman with new onset hematuria (Cureus 2016;8:e490)
- 83 year old man with kidney mass and Doege-Potter syndrome (Arch Ital Urol Androl 2020;92:392)
- Complete surgical resection
- Ablation or systemic chemotherapy for unresectable tumors
- Average tumor size of benign kidney SFTs: 8 cm (Int J Surg Pathol 2016;24:281)
- Firm, rubbery, usually well circumscribed
- White-tan or yellow-tan cut surface
- Myxoid changes, hemorrhage and necrosis can be seen
- Spindle cell neoplasm with patternless architecture and staghorn vessels
- Haphazard arrangement of spindle to oval shaped cells (patternless architecture)
- Both hypo and hypercellular areas
- Staghorn branching vasculature
- Intertwined collagen bands
- Myxoid areas and pseudocystic areas can be present (J Clin Pathol 2017;70:508)
- Features with increased risk for malignancy: high cellularity, cytological atypia, increased mitosis, necrosis (Am J Surg Pathol 1989;13:640, J Clin Pathol 2017;70:508)
- Round cell histological subtype (Genitourinary Pathology Society: Case of the Week [Accessed 25 October 2024])
- Fat forming histological subtype (In Vivo 2018;32:649)
- SFTs with low risk of aggressive behavior: low to moderate cellularity of clusters and single cells, spindle, oval or stellate cells with monotonous nuclei, wispy cytoplasm, collagenous intercellular stroma (Cancer Cytopathol 2018;126:36)
- SFTs with high risk of aggressive behavior: hypercellular, crowded clusters, focal nuclear pleomorphism, mitoses, necrosis (Cancer Cytopathol 2010;118:83)
- STAT6: 99 - 100% in benign SFTs of soft tissue, 93% in retroperitoneal SFTs (Iran J Pathol 2016;11:195, Appl Immunohistochem Mol Morphol 2019;27:195)
- CD34: 96.1% in benign SFTs of kidney, 76.5% in malignant SFTs (Int J Surg Pathol 2015;23:34)
- BCL2: 68% in benign SFTs of kidney, 53.8% in malignant SFTs (Int J Surg Pathol 2015;23:34)
- CD99: 83.3% in benign SFTs of kidney, 75% in malignant SFTs (Int J Surg Pathol 2015;23:34)
- Vimentin: 98.3% in benign SFTs of kidney, 100% in malignant SFTs (Int J Surg Pathol 2015;23:34)
- PAX8: positive in 43% of cases (Appl Immunohistochem Mol Morphol 2019;27:195)
- S100: positive in 1.8% of cases (Int J Surg Pathol 2015;23:34)
- MDM2 (Appl Immunohistochem Mol Morphol 2019;27:195)
- Pancytokeratin (Int J Surg Pathol 2015;23:34)
- CD31 (Int J Surg Pathol 2015;23:34)
- Cells with fibroblastic features, well developed rough endoplasmic reticulum, surrounded with collagen fibers (Med Mol Morphol 2009;42:239)
- Multiple NAB2::STAT6 gene fusion variants detected by whole exome sequencing (Nat Genet 2013;45:131, Nat Genet 2013;45:180)
- Reverse transcriptase polymerase chain reaction (RT-PCR) based tests are more sensitive than STAT6 FISH due to close proximity of the 2 genes (J Clin Pathol 2017;70:508)
- STAT6 FISH is more sensitive than STAT6 immunostain (J Clin Pathol 2017;70:508)
- Left kidney, nephrectomy:
- Solitary fibrous tumor, 3.5 cm, margins of resection free of involvement (see comment)
- Comment: Clinically, this patient presented with hematuria and flank pain. CT showed a left renal mass. No other lesions or history of any malignancies are identified. The histological sections of the renal mass show both hypo and hypercellular areas composed of monotonous spindle cells in haphazard pattern with staghorn vascular network. No mitosis or necrosis is seen. Immunohistochemistry studies show that the spindle cells are positive for STAT6, CD34, BCL2 and vimentin and negative for PAX8, CK8/18, MDM2 and S100. The overall findings favor a solitary fibrous tumor, likely renal primary. According to the modified risk stratification criteria, this tumor is classified as low risk. Clinical correlation is recommended (Mod Pathol 2017;30:1433).
- Angiomyolipoma / PEComa:
- Sarcomatoid renal cell carcinoma:
- High grade cytological atypia
- Positive for PAX8 and cytokeratin
- Negative for STAT6
- Mixed epithelial and stromal tumor (MEST):
- Most are benign, well circumscribed, with solid and cystic areas
- Stromal and epithelial components showing diverse morphology and minimal cytological atypia
- Stroma positive for ER, PR and smooth muscle markers
- Epithelium positive for cytokeratin and PAX8
- Renomedullary interstitial cell tumor (RMICT):
- Well differentiated liposarcoma (WDL):
- Contains adipose tissue and mimics fat forming SFTs but usually no staghorn vessels
- Positive for MDM2 and CDK4
- Dedifferentiated liposarcoma may be positive for STAT6, when amplification of CDK4 and MDM2 genes involves contiguous STAT6 gene (Appl Immunohistochem Mol Morphol 2019;27:195)
- Synovial sarcoma:
- Schwannoma:
- Inflammatory myofibroblastic tumor (IMT):
- Myofibroblastic proliferation with mixed inflammation
- Most show spindle cells with haphazard arrangement but no staghorn vessels
- Positive for vimentin, SMA and ~60% are positive for ALK1 (cytoplasmic staining in spindle cells, nuclear membrane or perinuclear staining in epithelioid variant)
- Negative for STAT6 and CD34
Which of the following are the most commonly seen histological features of solitary fibrous tumor of kidney?
- Admixture of adipose tissue, smooth muscle and thick wall vessels
- Haphazard arrangement of spindle cells and staghorn vasculatures
- Spindle cells with cytoplasmic microvacuoles and complex capillary network
- Storiform fibrosis
- Sustentacular cells wrap around chief cells and form zellballen pattern
Comment Here
Reference: Solitary fibrous tumor
- BCL2
- CD34
- CD99
- MDM2
- STAT6
Comment Here
Reference: Solitary fibrous tumor
- All carcinomas of the renal cortex are covered by this staging system
- These topics are not covered: urothelial carcinoma, lymphoma, sarcoma and Wilms tumor
- AJCC 7th edition staging was sunset on December 31, 2017; as of January 1, 2018, use of the 8th edition is mandatory
- pNX: cannot be assessed
- pN0: no regional lymph node metastasis
- pN1: regional lymph node metastasis
- Regional lymph nodes=hilar, caval, aortic
- pM1: distant metastasis, including noncontiguous adrenal involvement
- Stage group I: T1a-1b N0 M0
- Stage group II: T2a-2b N0 M0
- Stage group III: T1a-3c N1 M0, T3a-3c NX-0 M0
- Stage group IV: T4 any N M0, any T any N M1
- Histologic subtype
- WHO / ISUP grade
- Tumor size
- Perinephric fat invasion, renal sinus tissue invasion
- Venous involvement (specify renal vein, branches of renal vein, inferior vena cava)
- Lymphovascular invasion
- Adrenal gland involvement (specify direct extension pT4 or metastasis pM1)
- Presence and percentage of sarcomatoid features
- Presence of rhabdoid features
- Histologic tumoral necrosis
- GX: cannot be assessed
- G1: nucleoli inconspicuous or absent and basophilic at 40x objective
- G2: nucleoli conspicuous and eosinophilic at 40x but not prominent at 10x objective
- G3: nucleoli conspicuous and eosinophilic at 10x objective
- G4: marked nuclear pleomorphism, multinucleated tumor giant cells, rhabdoid differentiation or sarcomatoid differentiation
- Clear cell renal cell carcinoma
- Multilocular cystic renal neoplasm of low malignant potential
- Papillary renal cell carcinoma
- Hereditary leiomyomatosis renal cell carcinoma associated renal cell carcinoma/fumarate hydratase deficient renal cell carcinoma
- Clear cell papillary renal cell carcinoma
- Chromophobe renal cell carcinoma
- Collecting duct renal cell carcinoma
- Renal medullary carcinoma
- MiT family translocation renal cell carcinoma
- Succinate dehydrogenase deficient renal cell carcinoma
- Mucinous and tubular spindle cell carcinoma
- Tubulocystic renal cell carcinoma
- Acquired cystic disease associated renal cell carcinoma
- Renal cell carcinoma, unclassified
Contributed by Debra Zynger, M.D.
Comment Here
Reference: Pathologic TNM staging of renal cell carcinoma (AJCC 8th edition)
Comment Here
Reference: Pathologic TNM staging of renal cell carcinoma (AJCC 8th edition)
- MiT family translocation renal cell carcinomas (MiT RCC) comprise a distinct group of kidney tumors characterized by genetic alterations involving the TFE3 or TFEB genes
- They are more common in younger patients and may present with various architectural patterns, necessitating specific molecular and immunohistochemical techniques for accurate diagnosis
- TFE3 rearranged renal cell carcinoma (TFE3 rRCC) is the most common subtype of renal cell carcinoma within the MiT translocation family
- Characterized by various translocations on chromosome Xp11.2, leading to gene fusions involving the TFE3 gene, which can be detected by immunohistochemistry showing diffuse strong nuclear expression of TFE3 protein (by break apart fluorescence in situ hybridization [FISH] or by RNA sequencing)
- Although the majority of TFE3 rRCC cases have a unique morphology of predominantly clear cells, papillary architecture and psammoma bodies, they often show substantial morphologic overlap with clear cell renal cell carcinoma (ccRCC) and papillary renal cell carcinoma (pRCC)
- MiT family comprises a group of transcription factors (including TFE3 and TFEB, among others) that are associated with TFE3 rRCC, TFEB rearranged RCC (TFEB rRCC) and TFEB amplified RCC (Histol Histopathol 2022;37:311)
- TFE3 rRCC was previously referred to as Xp11 translocation renal cell carcinoma or renal cell carcinoma associated with Xp11.2 translocations / TFE3 gene fusions
- TFE3 rRCC constitutes up to 50% of pediatric renal cancers, ∼15% of RCC cases in adults under the age of 45 and 1 - 2% of all RCC cases (Int J Mol Sci 2022;23:7649, Adv Anat Pathol 2022;29:131)
- Since RCC overall is much more common in adults, TFE3 rRCC in adults vastly outnumbers pediatric cases (Histol Histopathol 2022;37:311, Genes Chromosomes Cancer 2022;61:219)
- Higher occurrence of this condition in female patients (F:M = 2:1) can be partly explained by oncogenic fusions involving the inactive X chromosome; this process also partially reactivates some silenced genes on the rearranged X chromosome (Int J Mol Sci 2022;23:7649, bioRxiv 2023 Aug 6 [Preprint])
- Average age of onset is 40, with the most frequent decade age group being 20 - 29 (Int J Mol Sci 2022;23:7649)
- Kidney
- Prior cytotoxic therapy is the only known risk factor predisposing to the development of TFE3 rRCC cases and accounts for 15% of patients (J Clin Oncol 2006;24:1529)
- As with other RCCs, ~33% of all tumors are asymptomatic at the time of diagnosis and are often incidentally discovered (Int J Mol Sci 2022;23:7649)
- ~33% of patients present with nodal involvement at presentation (Int J Mol Sci 2022;23:7649)
- Of the symptomatic patients, the most common clinical features include abdominal pain and gross hematuria (Front Oncol 2023;13:1252282, Front Pediatr 2023;11:1141223)
- Usually asymptomatic and incidentally discovered by imaging
- Favorable prognostic factors
- Patients with lymph node involvement (N+) but without metastatic spread (M0) maintained a favorable prognosis following surgery (Int J Mol Sci 2022;23:7649)
- Poor prognostic factors
- Distant metastasis and older age at diagnosis independently predicted death (Genes Chromosomes Cancer 2022;61:219)
- Large tumor size and the presence of necrosis (but not nucleolar grade) are associated with more aggressive tumor behavior (Pathology 2020;52:297)
- Large series of consecutively treated RCC patients showed that cancer specific survival for patients with TFE3 rRCC was significantly worse than for patients with TFE3 rearranged negative pRCC (P TFE3 rearranged negative ccRCC (Am J Surg Pathol 2012;36:663)
- 16 year old girl presented with hematuria lasting for 5 days (Open Med (Wars) 2024;19:20240985)
- 28 year old woman presented with hematuria for 1 day at 29 weeks of gestation (Front Oncol 2024;14:1388880)
- 48 year old man who developed papillary RCC in transplanted kidney and TFE3 rearranged RCC in native kidney (J Korean Soc Radiol 2024;85:437)
- 71 year old woman with end stage kidney disease who was found to have a multiloculated cystic renal neoplasm on imaging (Adv Anat Pathol 2024;31:34)
- For localized tumors, including patients with positive regional lymph nodes, surgery is the treatment of choice
- Therapies targeting vascular endothelial growth factor (VEGF) receptor, immunotherapy, mTOR inhibitors and target therapies for the MET signaling pathway are possible options for hematogenous metastases (J Immunother Cancer 2018;6:159, Int J Mol Sci 2022;23:7649)
- No specific gross appearance; can closely resemble ccRCC (Semin Diagn Pathol 2015;32:103)
- Tumor mass located at the renal cortex
- Usually well circumscribed with a pseudocapsule
- Can have infiltrative edges that may extend beyond the kidney
- Yellow or tan cut surfaces
- May have papillary excrescences
- Classic triad is present in ~66% of cases (Adv Anat Pathol 2022;29:131)
- Papillary architecture
- Large pale or clear cells with prominent nucleoli
- Psammoma bodies
- Cells are characteristically large with voluminous cytoplasm, discrete cell border and discohesion resulting in free floating cell clusters
- Other architectures can be appreciated: nested, alveolar, solid, cystic, tubular, hyalinized stroma
- Touch preparations show tumor cells arranged in follicular, papillary or solid clusters with abundant vacuolated to granular cytoplasm and variably conspicuous nucleoli
- Psammoma bodies and hyaline globules are characteristic but nonspecific
- Reference: Diagn Cytopathol 2022;50:E382
- PAX8 (100%)
- TFE3 (95%)
- Very high sensitivity; positive in 95% of cases
- Should be used with cautious interpretation given that it can be overexpressed in other tumor types that do not harbor a TFE3 rearrangement, including epithelioid angiomyolipoma (AML), paragangliomas, adrenocortical carcinomas, melanomas and others (Adv Anat Pathol 2022;29:131)
- CD10 (89%)
- RCC (87%)
- AMACR / P504 (82%)
- GPNMB (glycoprotein nonmetastatic B) (J Pathol 2022;257:158)
- Sensitive screening tool for translocation RCC and TSC1 / TSC2 / MTOR alteration associated renal tumors (examples include eosinophilic solid and cystic carcinoma, eosinophilic vacuolated tumor and low grade oncocytic tumor)
- Helpful in distinguishing these tumors from more common RCC subtypes (ccRCC, pRCC, chromophobe RCC, oncocytoma)
- TRIM63 in situ hybridization (ISH): a positive result is highly specific to MiT RCC and results are highly concordant with TFE3 / TFEB FISH results (Mod Pathol 2021;34:1596)
- PDL1: positive in ~50% of rearranged renal cell carcinomas (Mod Pathol 2024;37:100404)
- Vimentin (40%)
- Cathepsin K (35%)
- Limited sensitivity
- Its expression is highly dependent on fusion subtype (ranging from 16 - 60%); highest rates of positivity are in PRCC::TFE3 and lowest in SPSCR1::TFE3 (Adv Anat Pathol 2022;29:131, Genes Chromosomes Cancer 2022;61:219)
- EMA (32%)
- AE1 / AE3 (28%)
- CAM 5.2 (23%)
- CAIX (3%)
- CK7 (10%)
- Melanocytic markers (HMB45 [17%], MelanA [19%])
- Largely negative, in contrast to TFEB rRCC
- Reference: Adv Anat Pathol 2022;29:131
| Hale | KIT | CK7 | S100A1 | VIM | CAIX | AMACR | SDH | TFE3 | |
| Chromophobe RCC | +++ | +++ | +++ | - | - | - | - | +++ | - |
| Clear cell RCC | - | - | - | - | +++ | +++ | - | +++ | - |
| Oncocytoma | - | +++ | Rare | +++ | - | - | - | +++ | - |
| Papillary RCC | - | - | +++ | - | +++ | - | +++ | +++ | - |
| Translocation RCC | - | - | - | - | - | - | ++ | +++ | +++ |
| SDH deficient RCC | - | - | - | - | - | - | - | - | - |
References: Pathol Res Pract 2015;211:303, Ann Diagn Pathol 2020;44:151448, Am J Surg Pathol 2014;38:e6, Arch Pathol Lab Med 2019;143:1455, Transl Androl Urol 2019;8:S123, Hum Pathol 2020;104:18
- Background
- TFE3 rRCC occurs when transcription factor binding to IGHM enhancer 3 (TFE3) gene located on chromosome Xp11.2 and a partner gene on either chromosome X or an autosome fuse (Adv Anat Pathol 2022;29:131, bioRxiv 2023 Aug 6 [Preprint])
- Translocation of TFE3 forms oncogenic fusions with over 20 different partner genes in RCC, leading to the upregulation of over 300 genes involved in cell growth and invasion
- Fusion partners thus far include ASPL (ASPSCR1), EWSR1, CLTC, DVL2, FUBP1, GRIPAP1, KAT6A, KHDRBS2, KHSRP, LUC7L3, MED15, NEAT1, NONO, PARP14, PRCC, RBM10, SETD1B, SFPQ, ZC3H4, SRRM2 (Mod Pathol 2024;37:100404)
- Loss of specific chromosomes, such as 9p and 22q, is associated with poor survival and higher tumor stages; ASPSCR1::TFE3 fusions are particularly aggressive and often show a loss of 22q
- Most common TFE3 gene fusion subtypes include ASPSCR1::TFE3 and PRCC::TFE3
- ASPSCR1::TFE3 (Nat Commun 2021;12:5262)
- 23.7% frequency
- Poor outcomes with overall survival of 5 years
- Aggressive nature supported by a molecular signature that increases angiogenesis and proliferation, low levels of cytotoxic T cells and frequent copy number alterations with 22q loss
- PRCC::TFE3 (Nat Commun 2021;12:5262)
- 23.7% frequency
- Lower incidence of copy number alterations with a more favorable molecular signature
- ASPSCR1::TFE3 (Nat Commun 2021;12:5262)
- Notably, 90% ccRCC cases exhibit mutations or methylation of the VHL gene; other frequently mutated genes in ccRCC include PBRM1 (50%), SETD2 (20%) and BAP1 (15%)
- In contrast, renal cell carcinomas resulting from rearrangements rarely harbor these mutations, underscoring that these are distinct entities (Mod Pathol 2024;37:100404)
- Conversely, TERT promoter mutations are present in both ccRCC and rRCC but are more prevalent in rRCC (ccRCC: 9.2%, rRCC: 18.2%) (Mod Pathol 2024;37:100404)
- Detection techniques
- TFE3 break apart probe for FISH is currently the gold standard for diagnosing TFE3 rRCC
- Separate signals indicate that a rearrangement has occurred; however, this probe does not provide information about the specific gene fusion partner
- While fusion probes targeting the TFE3 gene and its fusion partners are available, their clinical utility is limited due to the rarity of these tumors and the maintenance required to keep these probes in a reference lab
- RNA sequencing is a confirmatory molecular test due to possible false negative FISH results in case of cryptic fusions and intrachromosomal inversions, which can be seen in up to 10% of tumors, especially with low grade morphology (Genes Chromosomes Cancer 2022;61:219)
MiT family translocation renal cell carcinoma by Rajal Shah, M.D.
- Left kidney, core biopsy:
- Renal cell carcinoma, favor TFE3 rearranged subtype (see comment)
- Comment: Molecular studies for TFE3 translocation (RNA sequencing or FISH) will be attempted to confirm diagnosis and reported in an addendum. RNA sequencing is a preferred molecular test due to possible false negative FISH results in case of cryptic fusions and intrachromosomal inversions, which can be seen in up to 10% of tumors, especially with low grade morphology.
- Clear cell renal cell carcinoma:
- TFE3 rRCC can have substantial overlap with clear cell RCC due to solid nested architecture and clear cytoplasm (Genes Chromosomes Cancer 2022;61:219)
- ccRCC would be immunopositive for CAIX and epithelial markers while immunonegative for TFE3, TRIM63 ISH and cathepsin K
- Clues to TFE3 rRCC would be younger patient age, hyalinized blood vessels (contrast to endothelial cell rich capillary network of clear cell RCC) and presence of psammoma bodies
- Clear cell papillary renal cell tumor (CCPRCT):
- TFE3 rRCC can have substantial overlap with CCPRCT due to branching tubular architecture and prominent subnuclear vacuolization (Genes Chromosomes Cancer 2022;61:219)
- Diffuse CK7 and CAIX labeling of CCPRCT distinguish it from TFE3 rRCC
- TFEB rearranged renal cell carcinoma:
- Unique histologic features include a biphasic morphology with large clear cells and smaller cells with condensed chromatin arranged around basement membrane material
- However, these features can be focal, making this tumor diagnostically challenging due to morphologic overlap
- Papillary renal cell carcinoma:
- TFE3 rRCC and pRCC both can have foamy histiocytes in the papillary cores
- Diffuse CK7 IHC favors pRCC and is usually negative in TFE3 rRCC
- Multilocular cystic renal neoplasm of low malignant potential (MCNLMP):
- MED15::TFE3 RCC is extensively cystic and can mimic MCNLMP
- MCNLMP is less likely to have psammoma bodies
- Clear cell renal cell carcinoma (ccRCC) is more aggressive than TFE3 rearranged RCC
- Immunohistochemistry for TFE3 is not helpful in the diagnosis of TFE3 rearranged RCC due to its low sensitivity
- MiT RCC primarily affects older adults, with a mean age of onset of over 60 years
- Presence of psammoma bodies in clear cell tumors is a characteristic feature of TFE3 rearranged RCC
- TFE3 rearranged RCC is typically negative for PAX8 immunohistochemistry
Comment Here
Reference: TFE3 rearranged renal cell carcinoma
Comment Here
Reference: TFE3 rearranged renal cell carcinoma
- MiT family translocation renal cell carcinomas (MiT RCC) comprise a distinct group of kidney tumors characterized by genetic alterations involving the TFE3 or TFEB genes
- They are more common in younger patients and may present with various architectural patterns, necessitating specific molecular and immunohistochemical techniques for accurate diagnosis
- TFE3 rearranged renal cell carcinoma (TFE3 rRCC) is the most common subtype of renal cell carcinoma within the MiT translocation family
- Characterized by various translocations on chromosome Xp11.2, leading to gene fusions involving the TFE3 gene, which can be detected by immunohistochemistry showing diffuse strong nuclear expression of TFE3 protein (by break apart fluorescence in situ hybridization [FISH] or by RNA sequencing)
- Although the majority of TFE3 rRCC cases have a unique morphology of predominantly clear cells, papillary architecture and psammoma bodies, they often show substantial morphologic overlap with clear cell renal cell carcinoma (ccRCC) and papillary renal cell carcinoma (pRCC)
- MiT family comprises a group of transcription factors (including TFE3 and TFEB, among others) that are associated with TFE3 rRCC, TFEB rearranged RCC (TFEB rRCC) and TFEB amplified RCC (Histol Histopathol 2022;37:311)
- TFE3 rRCC was previously referred to as Xp11 translocation renal cell carcinoma or renal cell carcinoma associated with Xp11.2 translocations / TFE3 gene fusions
- TFE3 rRCC constitutes up to 50% of pediatric renal cancers, ∼15% of RCC cases in adults under the age of 45 and 1 - 2% of all RCC cases (Int J Mol Sci 2022;23:7649, Adv Anat Pathol 2022;29:131)
- Since RCC overall is much more common in adults, TFE3 rRCC in adults vastly outnumbers pediatric cases (Histol Histopathol 2022;37:311, Genes Chromosomes Cancer 2022;61:219)
- Higher occurrence of this condition in female patients (F:M = 2:1) can be partly explained by oncogenic fusions involving the inactive X chromosome; this process also partially reactivates some silenced genes on the rearranged X chromosome (Int J Mol Sci 2022;23:7649, bioRxiv 2023 Aug 6 [Preprint])
- Average age of onset is 40, with the most frequent decade age group being 20 - 29 (Int J Mol Sci 2022;23:7649)
- Kidney
- Prior cytotoxic therapy is the only known risk factor predisposing to the development of TFE3 rRCC cases and accounts for 15% of patients (J Clin Oncol 2006;24:1529)
- As with other RCCs, ~33% of all tumors are asymptomatic at the time of diagnosis and are often incidentally discovered (Int J Mol Sci 2022;23:7649)
- ~33% of patients present with nodal involvement at presentation (Int J Mol Sci 2022;23:7649)
- Of the symptomatic patients, the most common clinical features include abdominal pain and gross hematuria (Front Oncol 2023;13:1252282, Front Pediatr 2023;11:1141223)
- Usually asymptomatic and incidentally discovered by imaging
- Favorable prognostic factors
- Patients with lymph node involvement (N+) but without metastatic spread (M0) maintained a favorable prognosis following surgery (Int J Mol Sci 2022;23:7649)
- Poor prognostic factors
- Distant metastasis and older age at diagnosis independently predicted death (Genes Chromosomes Cancer 2022;61:219)
- Large tumor size and the presence of necrosis (but not nucleolar grade) are associated with more aggressive tumor behavior (Pathology 2020;52:297)
- Large series of consecutively treated RCC patients showed that cancer specific survival for patients with TFE3 rRCC was significantly worse than for patients with TFE3 rearranged negative pRCC (P TFE3 rearranged negative ccRCC (Am J Surg Pathol 2012;36:663)
- 16 year old girl presented with hematuria lasting for 5 days (Open Med (Wars) 2024;19:20240985)
- 28 year old woman presented with hematuria for 1 day at 29 weeks of gestation (Front Oncol 2024;14:1388880)
- 48 year old man who developed papillary RCC in transplanted kidney and TFE3 rearranged RCC in native kidney (J Korean Soc Radiol 2024;85:437)
- 71 year old woman with end stage kidney disease who was found to have a multiloculated cystic renal neoplasm on imaging (Adv Anat Pathol 2024;31:34)
- For localized tumors, including patients with positive regional lymph nodes, surgery is the treatment of choice
- Therapies targeting vascular endothelial growth factor (VEGF) receptor, immunotherapy, mTOR inhibitors and target therapies for the MET signaling pathway are possible options for hematogenous metastases (J Immunother Cancer 2018;6:159, Int J Mol Sci 2022;23:7649)
- No specific gross appearance; can closely resemble ccRCC (Semin Diagn Pathol 2015;32:103)
- Tumor mass located at the renal cortex
- Usually well circumscribed with a pseudocapsule
- Can have infiltrative edges that may extend beyond the kidney
- Yellow or tan cut surfaces
- May have papillary excrescences
- Classic triad is present in ~66% of cases (Adv Anat Pathol 2022;29:131)
- Papillary architecture
- Large pale or clear cells with prominent nucleoli
- Psammoma bodies
- Cells are characteristically large with voluminous cytoplasm, discrete cell border and discohesion resulting in free floating cell clusters
- Other architectures can be appreciated: nested, alveolar, solid, cystic, tubular, hyalinized stroma
- Touch preparations show tumor cells arranged in follicular, papillary or solid clusters with abundant vacuolated to granular cytoplasm and variably conspicuous nucleoli
- Psammoma bodies and hyaline globules are characteristic but nonspecific
- Reference: Diagn Cytopathol 2022;50:E382
- PAX8 (100%)
- TFE3 (95%)
- Very high sensitivity; positive in 95% of cases
- Should be used with cautious interpretation given that it can be overexpressed in other tumor types that do not harbor a TFE3 rearrangement, including epithelioid angiomyolipoma (AML), paragangliomas, adrenocortical carcinomas, melanomas and others (Adv Anat Pathol 2022;29:131)
- CD10 (89%)
- RCC (87%)
- AMACR / P504 (82%)
- GPNMB (glycoprotein nonmetastatic B) (J Pathol 2022;257:158)
- Sensitive screening tool for translocation RCC and TSC1 / TSC2 / MTOR alteration associated renal tumors (examples include eosinophilic solid and cystic carcinoma, eosinophilic vacuolated tumor and low grade oncocytic tumor)
- Helpful in distinguishing these tumors from more common RCC subtypes (ccRCC, pRCC, chromophobe RCC, oncocytoma)
- TRIM63 in situ hybridization (ISH): a positive result is highly specific to MiT RCC and results are highly concordant with TFE3 / TFEB FISH results (Mod Pathol 2021;34:1596)
- PDL1: positive in ~50% of rearranged renal cell carcinomas (Mod Pathol 2024;37:100404)
- Vimentin (40%)
- Cathepsin K (35%)
- Limited sensitivity
- Its expression is highly dependent on fusion subtype (ranging from 16 - 60%); highest rates of positivity are in PRCC::TFE3 and lowest in SPSCR1::TFE3 (Adv Anat Pathol 2022;29:131, Genes Chromosomes Cancer 2022;61:219)
- EMA (32%)
- AE1 / AE3 (28%)
- CAM 5.2 (23%)
- CAIX (3%)
- CK7 (10%)
- Melanocytic markers (HMB45 [17%], MelanA [19%])
- Largely negative, in contrast to TFEB rRCC
- Reference: Adv Anat Pathol 2022;29:131
| Hale | KIT | CK7 | S100A1 | VIM | CAIX | AMACR | SDH | TFE3 | |
| Chromophobe RCC | +++ | +++ | +++ | - | - | - | - | +++ | - |
| Clear cell RCC | - | - | - | - | +++ | +++ | - | +++ | - |
| Oncocytoma | - | +++ | Rare | +++ | - | - | - | +++ | - |
| Papillary RCC | - | - | +++ | - | +++ | - | +++ | +++ | - |
| Translocation RCC | - | - | - | - | - | - | ++ | +++ | +++ |
| SDH deficient RCC | - | - | - | - | - | - | - | - | - |
References: Pathol Res Pract 2015;211:303, Ann Diagn Pathol 2020;44:151448, Am J Surg Pathol 2014;38:e6, Arch Pathol Lab Med 2019;143:1455, Transl Androl Urol 2019;8:S123, Hum Pathol 2020;104:18
- Background
- TFE3 rRCC occurs when transcription factor binding to IGHM enhancer 3 (TFE3) gene located on chromosome Xp11.2 and a partner gene on either chromosome X or an autosome fuse (Adv Anat Pathol 2022;29:131, bioRxiv 2023 Aug 6 [Preprint])
- Translocation of TFE3 forms oncogenic fusions with over 20 different partner genes in RCC, leading to the upregulation of over 300 genes involved in cell growth and invasion
- Fusion partners thus far include ASPL (ASPSCR1), EWSR1, CLTC, DVL2, FUBP1, GRIPAP1, KAT6A, KHDRBS2, KHSRP, LUC7L3, MED15, NEAT1, NONO, PARP14, PRCC, RBM10, SETD1B, SFPQ, ZC3H4, SRRM2 (Mod Pathol 2024;37:100404)
- Loss of specific chromosomes, such as 9p and 22q, is associated with poor survival and higher tumor stages; ASPSCR1::TFE3 fusions are particularly aggressive and often show a loss of 22q
- Most common TFE3 gene fusion subtypes include ASPSCR1::TFE3 and PRCC::TFE3
- ASPSCR1::TFE3 (Nat Commun 2021;12:5262)
- 23.7% frequency
- Poor outcomes with overall survival of 5 years
- Aggressive nature supported by a molecular signature that increases angiogenesis and proliferation, low levels of cytotoxic T cells and frequent copy number alterations with 22q loss
- PRCC::TFE3 (Nat Commun 2021;12:5262)
- 23.7% frequency
- Lower incidence of copy number alterations with a more favorable molecular signature
- ASPSCR1::TFE3 (Nat Commun 2021;12:5262)
- Notably, 90% ccRCC cases exhibit mutations or methylation of the VHL gene; other frequently mutated genes in ccRCC include PBRM1 (50%), SETD2 (20%) and BAP1 (15%)
- In contrast, renal cell carcinomas resulting from rearrangements rarely harbor these mutations, underscoring that these are distinct entities (Mod Pathol 2024;37:100404)
- Conversely, TERT promoter mutations are present in both ccRCC and rRCC but are more prevalent in rRCC (ccRCC: 9.2%, rRCC: 18.2%) (Mod Pathol 2024;37:100404)
- Detection techniques
- TFE3 break apart probe for FISH is currently the gold standard for diagnosing TFE3 rRCC
- Separate signals indicate that a rearrangement has occurred; however, this probe does not provide information about the specific gene fusion partner
- While fusion probes targeting the TFE3 gene and its fusion partners are available, their clinical utility is limited due to the rarity of these tumors and the maintenance required to keep these probes in a reference lab
- RNA sequencing is a confirmatory molecular test due to possible false negative FISH results in case of cryptic fusions and intrachromosomal inversions, which can be seen in up to 10% of tumors, especially with low grade morphology (Genes Chromosomes Cancer 2022;61:219)
MiT family translocation renal cell carcinoma by Rajal Shah, M.D.
- Left kidney, core biopsy:
- Renal cell carcinoma, favor TFE3 rearranged subtype (see comment)
- Comment: Molecular studies for TFE3 translocation (RNA sequencing or FISH) will be attempted to confirm diagnosis and reported in an addendum. RNA sequencing is a preferred molecular test due to possible false negative FISH results in case of cryptic fusions and intrachromosomal inversions, which can be seen in up to 10% of tumors, especially with low grade morphology.
- Clear cell renal cell carcinoma:
- TFE3 rRCC can have substantial overlap with clear cell RCC due to solid nested architecture and clear cytoplasm (Genes Chromosomes Cancer 2022;61:219)
- ccRCC would be immunopositive for CAIX and epithelial markers while immunonegative for TFE3, TRIM63 ISH and cathepsin K
- Clues to TFE3 rRCC would be younger patient age, hyalinized blood vessels (contrast to endothelial cell rich capillary network of clear cell RCC) and presence of psammoma bodies
- Clear cell papillary renal cell tumor (CCPRCT):
- TFE3 rRCC can have substantial overlap with CCPRCT due to branching tubular architecture and prominent subnuclear vacuolization (Genes Chromosomes Cancer 2022;61:219)
- Diffuse CK7 and CAIX labeling of CCPRCT distinguish it from TFE3 rRCC
- TFEB rearranged renal cell carcinoma:
- Unique histologic features include a biphasic morphology with large clear cells and smaller cells with condensed chromatin arranged around basement membrane material
- However, these features can be focal, making this tumor diagnostically challenging due to morphologic overlap
- Papillary renal cell carcinoma:
- TFE3 rRCC and pRCC both can have foamy histiocytes in the papillary cores
- Diffuse CK7 IHC favors pRCC and is usually negative in TFE3 rRCC
- Multilocular cystic renal neoplasm of low malignant potential (MCNLMP):
- MED15::TFE3 RCC is extensively cystic and can mimic MCNLMP
- MCNLMP is less likely to have psammoma bodies
- Clear cell renal cell carcinoma (ccRCC) is more aggressive than TFE3 rearranged RCC
- Immunohistochemistry for TFE3 is not helpful in the diagnosis of TFE3 rearranged RCC due to its low sensitivity
- MiT RCC primarily affects older adults, with a mean age of onset of over 60 years
- Presence of psammoma bodies in clear cell tumors is a characteristic feature of TFE3 rearranged RCC
- TFE3 rearranged RCC is typically negative for PAX8 immunohistochemistry
Comment Here
Reference: TFE3 rearranged renal cell carcinoma
Comment Here
Reference: TFE3 rearranged renal cell carcinoma
- Renal epithelial neoplasm composed exclusively of tubules and cysts lined by a single layer of cells with prominent nucleoli (equivalent to ISUP / WHO grade 3) and abundant eosinophilic cytoplasm (Eur Urol 2016;70:93)
- Relatively indolent with < 10% cases showing disease progression, local recurrence or metastasis to bone, liver and lymph nodes (Eur Urol 2016;70:93)
- Well circumscribed tumor with spongy bubble wrap appearance
- Male predominance
- Cystic and tubular architecture; no solid areas, hemorrhage or necrosis
- Single layer of flat, cuboidal or hobnailed eosinophilic cells with large nucleoli and lining fibrotic septa
- According to the ISUP, tubulocystic renal cell carcinoma terminology should not be used when there is a tubulocystic pattern admixed with the usual elements of papillary renal cell carcinoma or collecting duct carcinoma (Mod Pathol 2019;32:701)
- Originally named Bellinien epithelioma or collecting duct carcinoma, by Pierre Masson in the 1950s (Am J Surg Pathol 2016;40:1457)
- Low grade collecting duct carcinoma (McLennan) (Urology 1997;50:679)
- Termed tubulocystic renal cell carcinoma and considered a distinct entity since 2009 (Am J Surg Pathol 2008;32:177)
- Recognized by the 2016 WHO classification
- ICD-O: 8316/3 - cyst associated renal cell carcinoma
- ICD-10: C64 - malignant neoplasm of kidney, except renal pelvis
- ICD-11: 2C90.0 & XH7K79 - malignant neoplasms of kidney, except renal pelvis & tubulocystic renal cell carcinoma
- Rare (< 1% of renal tumors)
- Mean age: 58.4 years (range: 30 - 74 years)
- M:F = 7:1 (Am J Surg Pathol 2009;33:384)
- Left kidney predominance with involvement of the cortex and corticomedullary junction
- Histogenesis remains unclear; cells have some features of proximal nephron differentiation and others related to distal nephron (Can Urol Assoc J 2015;9:E654)
- Often an incidental finding
- Less commonly presents with abdominal pain, distention, hematuria, weight loss
- Prognosis is typically excellent with only rare reports of clinical progression and aggressive behavior (Am J Surg Pathol 2016;40:1457)
- References: Am J Surg Pathol 2009;33:384, Mod Pathol 2019;32:701, Am J Surg Pathol 2016;40:1457
- Diagnosis is made on gross and H&E with attention paid to strict criteria of tubular and cystic growth
- Adequate sampling should be applied in order to exclude possible mimics
- Use of immunohistochemistry is limited but fumarate hydratase (FH) and S-(2-succino)-cysteine (2SC) could be considered to exclude FH deficient RCC, a lookalike
- MRI superior to CT; characterized by multilocular cystic lesions, Bosniak classification II - IV, with hypovascular septa (Abdom Radiol (NY) 2018;43:1540)
- Ultrasonography (US) pattern may exhibit high echogenicity and posterior acoustic enhancement (Eur Radiol 2016;26:1108)
- 34 year old man with a 1.4 cm mass (Case #484)
- 40 year old man with end stage renal disease and incidental mass of the native left kidney (GUPS: Case of the Week [Accessed 18 August 2022])
- 45 year old man initially misdiagnosed with a Bosniak type II renal cyst (Rev Urol 2016;18:118)
- 59 year old man with features of cystic renal oncocytoma (Case Rep Urol 2019;2019:2919686)
- 62 year old man with bilateral tumors in diabetic end stage renal disease (Rare Tumors 2013;5:e57)
- 65 year old man with a large cystic tumor (Can Urol Assoc J 2015;9:E654)
- 65 year old man with tubulocystic renal cell carcinoma of the native kidney (Case Rep Nephrol 2020;2020:7145652)
- Radical or partial nephrectomy depending on tumor size and location in the kidney
- Well circumscribed but not encapsulated
- Cut surface that is gray-white with multilocular cystic spaces resembling Swiss cheese, sponge or bubble wrap-like appearance, usually containing clear fluid
- Mean size 4 cm (range 0.2 - 17 cm)
- No hemorrhage or necrosis
- References: Am J Surg Pathol 2009;33:384, Mod Pathol 2019;32:701, Am J Surg Pathol 2016;40:1457
- Mixture of closely packed small to intermediate tubules and variably sized cysts (Urology 1997;50:679)
- Cysts are separated by fibrous septa; no desmoplasia or cellular stroma
- Tubules and cysts are lined by a single layer of flattened, cuboidal or columnar cells with enlarged, irregular nuclei with modest to abundant amounts of eosinophilic cytoplasm; hobnailing may be present
- Distinct nucleoli (equivalent to ISUP / WHO grade 3)
- Cysts are separated by hypocellular, fibrous septa
- Minimal mitotic activity
- Strict morphologic criteria should be used; cases with papillary growth, poor differentiation or sarcomatoid component usually exclude the diagnosis (Mod Pathol 2019;32:701)
- Histologic grading not necessary
Contributed by Maria Tretiakova, M.D., Ph.D., Sabrina Sopha, M.D. and Debra Zynger, M.D. (Case #484)
- Sheets of cells with granular cytoplasm, distinct borders, intracellular windows between cells, distinct nucleoli, rare cytoplasmic vacuoles and rare nuclear grooves (Diagn Cytopathol 2018;46:707)
- Keratins (AE1 / AE3, CAM 5.2, CK8 / 18, CK19, EMA) (Am J Surg Pathol 2005;29:747)
- PAX8, vimentin
- AMACR (racemase), fumarate hydratase (retained)
- CK7 and CD10 may be patchy (Mod Pathol 2019;32:701)
- Variable 34 beta E12 (usually less diffuse than CK7 and CD10) (Mod Pathol 2019;32:701)
- Ultrastructural features may resemble cells of the proximal tubules and collecting ducts
- Type I cells:
- Resemble proximal convoluted tubules and are seen in tubular component
- Cuboidal cells with luminal surface tight junction, short microvilli with brush border
- Type II cells:
- Ultrastructural features resemble distal nephrons
- Found in the cystic component
- Single layer of cells with desmosomal junction and luminal surfaces bound by tight junctions
- Contain rough endoplasmic reticulum, mitochondria and lipid droplets
- No microvilli
- Base bound by basement membrane
- Type I cells:
- Reference: Rare Tumors 2013;5:e57
- Has a distinct genetic signature from other carcinomas (J Mol Diagn 2018;20:34, Mod Pathol 2019;32:701)
- Initially a close relationship to papillary renal cell carcinoma postulated based on gains of chromosome 7, 17, loss of Y but other studies lacked gains of chromosomes 7, 17 by FISH (Am J Surg Pathol 2009;33:1840, Am J Surg Pathol 2008;32:177, Histopathology 2016;68:850)
- Recent study showed chromosome 9 loss and chromosome 17 gain were found in all tumors by next generation sequencing (NGS) (Mod Pathol 2019;32:701)
- Consistent loss of Y chromosome was observed in all tumor cells tested by FISH
- Mutations were found in lysine N methyltransferase (KMT2C), a histone methyltransferase and lysine specific demethylase 5 C (KDM5C) in 2 of 9 (22%) cases
- Other known or likely oncogenic mutations detected in PMS2, RAD21, TP53, FBXW7 and KEAP1 were also detected (Mod Pathol 2019;32:701)
- Noncoding RNA sequencing has identified distinct patterns of small nucleolar RNA and mature and pre-miRNAs as well as mutations (> 60%) of ABL1 and PDFGRA genes; recent study did not identify somatic mutations in ABL1 and PDGGRA genes in 9 cases (J Mol Diagn 2018;20:34, Mod Pathol 2019;32:701)
- Lack of overlap in results is possibly indicative of genetic diversity and requires further study (Mod Pathol 2021;34:1392)
Tubulocystic renal cell carcinoma
by Dr. Rajal Shah
- Kidney, partial nephrectomy:
- Tubulocystic renal cell carcinoma (x cm); margins are negative (see comment) (see synoptic report)
- Comment: Immunohistochemistry shows the tumor cells to be negative for 2SC with positive (retained) staining for fumarate hydratase.
- Care should be taken when making the diagnosis, as tubulocystic architecture may be variably present in many renal tumors including those listed below
- Fumarate hydratase deficient renal cell carcinoma / hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome associated renal cancer:
- High grade tumors with solid, papillary and glandular architecture
- Characteristic large cytomegalovirus-like nucleoli with perinuclear halos
- FH gene mutations
- Will show loss of fumarate hydratase staining (highly specific but not sensitive) with aberrant positive staining for 2SC (sensitive but less specific) (Am J Surg Pathol 2016;40:1457)
- Prior cases described as as tubulocystic RCC with poorly differentiated foci may actually represent FH deficient RCC (Am J Surg Pathol 2016;40:1457)
- Adult cystic nephroma (mixed epithelial and stromal tumor):
- In adults, predominance in women
- Biphasic tumors with epithelial and stromal components
- Multilocular cysts are lined by attenuated cells with inconspicuous nucleoli
- Nonepithelial component could be very prominent with hyalinization, fibrosis and ovarian type cellular stroma
- Overlapping expression profile
- Multilocular cystic clear cell renal neoplasm of low malignant potential:
- Cysts lined by clear cells with low grade nuclei (ISUP grade 1 - 2), nests of clear cells in stroma
- CAIX positive
- Mucinous tubular and spindle cell carcinoma:
- Predominantly women
- Typically long tubular profiles or cord-like growth pattern of uniform, low cuboidal cells with eosinophilic, focally vacuolated cytoplasm and spindling
- Stroma is myxoid and bubbly with abundant extracellular mucin
- Oncocytoma:
- Cut surfaces with dark brown appearance and central scar in 33% of cases
- Occasionally shows extrarenal extension into fat rather than organ confined
- Background has fibromyxoid / hyalinized appearance
- Uncommonly can have tubules with cystic dilation which can be a histologic mimic
- Large, round polygonal cells with eosinophilic cytoplasm
- Usually do not see prominent nucleoli or nuclear variability
- CK7 negative or scattered; positive KIT / CD117
- Chromophobe renal cell carcinoma:
- Usually solid / nested growth with distinct cell borders and raisinoid nuclei
- CK7 diffuse and KIT / CD117 positive
- Collecting duct carcinoma:
- Usually symptomatic (pain, hematuria)
- Grossly solid appearance with irregular, infiltrative borders, involves medulla and sometimes into perirenal adipose tissue
- Tubulopapillary structures, cords, glandular or solid sheets; lacks cyst formation; may have dysplastic medullary collecting duct structures
- Stroma is inflamed and desmoplastic
- Often obvious mitotic activity
- Acquired cystic disease associated renal cell carcinoma:
- Associated with end stage renal disease
- Cribriform appearance with calcium oxalate crystals
- Papillary renal cell carcinoma:
- Papillary growth with strong CK7 positivity, may contain foamy macrophages
- Overlapping expression profile
A 50 year old man undergoes a nephrectomy for a 3 cm cystic mass of the left kidney. A representative photomicrograph of the neoplasm is shown. Which statement is true about this entity?
- Can have prominent papillary growth
- Can show fumarate hydratase loss by immunohistochemistry
- Demonstrates an increased metastatic potential
- May show gain of chromosome 17 and losses of chromosomes 9 and Y
- Shows solid growth often
Comment Here
Reference: Tubulocystic renal cell carcinoma
Comment Here
Reference: Tubulocystic renal cell carcinoma
- Not a distinct subtype of renal cell carcinoma (RCC) but a diagnostic category for tumors that do not readily fit into any of the RCC subtypes or have pure sarcomatoid / rhabdoid histology (IARC: WHO Classification of Tumours of the Urinary System and Male Genital Organs, 4th Edition, 2016)
- Histologically heterogeneous, most commonly high grade
- Heterogeneous group of RCC tumors that are not readily classifiable
- Immunohistochemistry, fluorescent in situ hybridization and other molecular techniques may aid in classification
- Important category enabling separation of unusual tumors from common subtypes and their further grouping / reclassification as distinct entities using advanced techniques
- ICD-10: C64.9 - malignant neoplasm of unspecified kidney, except renal pelvis
- Age range 21 - 91 years, median ~60 (Histopathology 2018;72:305)
- Male predominance (Histopathology 2018;72:305)
- Comprise 0.7 - 7% of RCCs, 3 - 5% in specialized centers (J Urol 2002;168:950, BJU Int 2007;100:802, BJU Int 2012;110:786)
- Kidney
- Clinical presentation similar to other RCC subtypes (IARC: WHO Classification of Tumours of the Urinary System and Male Genital Organs, 4th Edition, 2016)
- Most present at advanced stage and 60 - 80% are grade 3 - 4 (J Urol 2002;168:950, BJU Int 2007;100:802, BJU Int 2012;110:786, BJU Int 2007;100:802, Histopathology 2018;72:305)
- Compared to clear cell RCC, unclassified RCC present as larger tumors with frequent necrosis, higher incidence of nodal metastases and direct invasion into adjacent organs, higher risk of distant metastases and shorter median survival (J Urol 2002;168:950, BJU Int 2007;100:802, BJU Int 2012;110:786, Urology 2010;76:580)
- Radiographic features similar to other RCCs
- Outcome is often associated with grade, stage and clinicopathologic features known to be relevant in other forms of RCC (BJU Int 2012;110:786)
- The majority of studies show that unclassified RCC is more aggressive with 1.6 - 1.7X the mortality of clear cell RCC (J Urol 2002;168:950, BJU Int 2007;100:802, BJU Int 2012;110:786), however one study showed that overall cancer specific survival is similar in matched cohorts after adjusting for grade, stage and other parameters (Urology 2010;76:580)
- Despite that discrepancy, > 50% of high grade unclassified RCC are dead of disease within 2 years
- For low grade tumors there may be an indolent course
- 32 year old man with prominent morphologic heterogeneity (J RPCA 2016;48:S127)
- 51 year old patient with grade 4 unclassified renal cell carcinoma (Cancer Biol Ther 2014;15:1439)
- 55 year old man with initial presentation of tongue metastasis (J Med Case Rep 2017;11:314)
- 56 year old man with response to PD-1 inhibitor (Clin Genitourin Cancer 2017;15:e517)
- 56 year old woman with spontaneous regression of pulmonary nodules after nephrectomy (Mol Clin Oncol 2016;5:49)
- 76 year old woman with unclassified mucinous renal cell carcinoma (Korean J Urol 2014;55:690)
- No standardized treatment but whenever feasible immunotherapy with nephrectomy is warranted (J Urol 2002;168:950)
- Median 5 cm with extensive involvement of kidney and frequent necrosis (Histopathology 2018;72:305)
- Histologic features which do not resemble any classified subtype or have features of multiple subtypes, pure sarcomatoid or rhabdoid morphology
- High grade tumors often extremely heterogeneous with papillary, solid, sheet-like, nested, alveolar, tubular, glandular, rhabdoid or sarcomatoid architecture
- Cytoplasm often variable from eosinophilic to clear to amphophilic to spindled
- Majority are high nuclear grade, ISUP grade 3 or 4
- Necrosis, mitoses and vascular invasion are common
- May be purely sarcomatoid or rhabdoid, must exclude possibility of a distinct subtype RCC
- Low grade tumors often resemble oncocytoma but with more solid architecture, increased pleomorphism, more than occasional mitoses and focal necrosis
- A study of 62 high grade unclassified RCCs revealed mutations in NF2 (18%), SETD2 (18%), BAP1 (13%), KMT2C (10%) and MTOR (8%) (Nat Commun 2016;7:13131)
- Chromosome genomic array testing (array CGH) and single nucleotide polymorphism (SNP) arrays are both useful tools in resolving 80 - 94% of unclassified RCCs with ~50% cases reclassified as clear cell RCC
- Reclassified tumors show characteristic cytogenetic changes of certain subtype plus numerous additional copy number aberrations (Am J Surg Pathol 2009;33:1276)
- Diagnosis of exclusion requiring extensive work up
- Additional sampling of tumor: identify areas with classic morphologic and immunophenotypic features (often clear cell RCC) (AJSP Rvw and Rep 2017;22:301)
- IHC: PAX8, PAX2, CAIX, CD10, CK7, CK20, AMACR, CD117, HMB45, MelanA, CathepsinK, EMA, 34βE12, GATA3, p63, vimentin, TFE3, 2SC, SDHB, INI1 (AJSP Rvw and Rep 2017;22:301)
- FISH: TFE3, TFEB, 3p
- Evidence of genetic conditions: oncocytic neoplasms associated with tuberous sclerosis or Birt-Hogg-Dubé syndrome (Am J Surg Pathol 2014;38:1457, Histol Histopathol 2013;28:1257)
- Subtypes of RCC
- Clear cell: + CAIX, CD10, vimentin; loss of 3p by FISH
- Papillary: + AMACR, CD10 (apical); +/- CK7
- Chromophobe: + CK7
- MiTF family translocation associated: + TFE3 or TFEB; translocation of TFE3 or TFEB by FISH
- Hereditary leiomyomatosis and renal cell carcinoma (fumarate hydratase deficient): +2SC; - FH
- SDH deficient: - SDHB
- Non-RCC:
- Angiomyolipoma, including epithelioid type: + HMB45, MelanA; - AE1 / AE3, PAX8
- Urothelial carcinoma of renal pelvis: based in renal pelvis; often + CK7, CK20, p63; - PAX8
- Metastasis
- Nonepithelial neoplasms infrequently seen in kidney (sarcoma, lymphoma)
- Which of the following is true about unclassified RCC?
- Any degree of staining for an immunohistochemical marker of interest (including TFE3, 2SC, CAIX) can establish the underlying diagnosis
- Areas with classic morphologic and immunophenotypic features of clear cell RCC or chromophobe RCC indicate the likely underlying etiology
- Unclassified RCC is a specific morphologic diagnosis
- Unclassified RCC is always high grade
Comment Here
Reference: Unclassified renal cell carcinoma
ISUP presents: reporting of MRI guided prostate biopsies
by Dr. Jennifer Gordetsky
ISUP presents: maximizing the impact of renal mass biopsy
by Dr. Sara Wobker
ISUP presents: emerging WHO entities in renal neoplasia
by Dr. Ondrej Hess
- The new World Health Organization (WHO) 2022 classification of urinary and male genital tumors (5th edition) replaces the previous WHO 2016 classification
- Diagnostic criteria, molecular correlates and nomenclature have been updated
- Grouping of renal tumors into broader categories
- Clear cell renal tumors
- Papillary renal tumors
- Papillary renal cell carcinoma (PRCC)
- Subclassification into type 1 and type 2 PRCC is no longer recommended
- Previously known type 1 PRCC is regarded as classic PRCC
- Previously known type 2 PRCC has now been recognized to be other entities (e.g., sporadic fumarate hydratase deficient renal cell carcinoma [FH deficient RCC], acquired cystic disease associated RCC, TFE3 altered RCC, etc.) (Mod Pathol 2021;34:1167, Pathologica 2022;115:8)
- Morphologic spectrum is expanded to include biphasic PRCC and Warthin-like PRCC; papillary renal neoplasm with reverse polarity recognized as separate provisional entity (Pathologica 2022;115:8)
- Subclassification into type 1 and type 2 PRCC is no longer recommended
- Renal papillary adenoma
- Papillary renal cell carcinoma (PRCC)
- Oncocytic and chromophobe renal tumors
- New category: other oncocytic tumors of the kidney
- Includes emerging entities such as low grade oncocytic tumor (LOT) and eosinophilic vacuolated tumor (EVT)
- Includes the entity oncocytic renal neoplasms of low malignant potential not otherwise specified (NOS) for tumors with features in between an oncocytoma and chromophobe RCC (Mod Pathol 2021;34:1167)
- Includes the entity hybrid oncocytic tumors (HOT) for oncocytic tumors with borderline features that occur in a hereditary setting (Mod Pathol 2021;34:1392)
- New category: other oncocytic tumors of the kidney
- Collecting duct tumors
- Other renal tumors
- Includes a diverse set of renal tumors that do not fit in any of the other categories
- Clear cell papillary renal cell tumor replaces previously named clear cell papillary renal cell carcinoma due to benign behavior (Pathologica 2022;115:8)
- Molecularly defined renal carcinomas
- Reflects recent discoveries in renal tumor genomics (Mod Pathol 2021;34:1167)
- TFE3 rearranged RCC and TFEB altered RCC are now separated into separate diagnostic entities, rather than both being considered as microphthalmia transcription factor (MiT) family of RCC (Pathologica 2022;115:8)
- New entities (Mod Pathol 2021;34:1167)
- Eosinophilic solid and cystic renal cell carcinoma (ESC RCC)
- ELOC (formerly TCEB1) mutated renal cell carcinoma
- Anaplastic lymphoma kinase (ALK) rearranged RCC
- Grading of renal carcinomas is based on the single high power field showing the greatest degree of pleomorphism, rather than the predominant grade (Histopathology 2019;74:4)
- World Health Organization / International Society of Urological Pathology (WHO / ISUP) grading is not equally significant for all RCC subtypes; the WHO divides them into several categories based on grading value
| Renal cell tumors | ICD-O | ICD-11 |
| ||
| 8310/3 | 2C90.0 & XH46F1 | |
| 8316/1 | 2F35 & XH7PR9 | |
| ||
| 8260/0 | 2F35 & XH09B0 | |
| ||
| 8290/0 | 2F35 & XH9Z86 | |
| 8317/3 | 2C90.0 & XH6153 | |
| 2F98 | ||
| ||
| 8319/3 | 2C90.0 & XH4SQ4 | |
| ||
| 8323/1 | 2C90.0 & XH9T60 | |
| 8480/3 | 2C90.0 & XH5EQ2 | |
| 8316/3 | 2C90.0 & XH7K79 | |
| 8316/3 | 2C90.0 & XH0RU3 | |
| 8311/3 | 2C90.0 | |
| 8312/3 | 2C90.0 & XH05V6 | |
| ||
| 8311/3 | 2C90.0 | |
| 8311/3 | 2C90.0 | |
| 8311/3 | 2C90.0 | |
| 8311/3 | 2C90.0 | |
| 8311/3 | 2C90.0 & XH8EN1 | |
| 8311/3 | 2C90.0 | |
| 8510/3 | 2C90.Y & XH2YP5 | |
| Metanephric tumors | ICD-O | ICD-11 |
| 8325/0 | 2F35 & XH0JC7 | |
| 9013/0 | 2F35 & XH7ZU2 | |
| 8935/1 | 2F35 & XH4N88 | |
| Mixed epithelial and stromal renal tumors | ICD-O | ICD-11 |
| 8959/0 | 2C90.Y & XH0533 | |
| 8959/0 | 2F35 & XH7TJ0 | |
| Renal mesenchymal tumors | ICD-O | ICD-11 |
| ||
| 8860/0 | 2F35 & XH4CC6, 2C90.Y & XH4CC6 | |
| 8860/1 | 2F35 & XH0QR3, 2B5F.2 & XH0QR3 | |
| 9161/1 | 2F98 & XH6810 | |
| 8361/0 | 2F35 & XH6M13 | |
| 8966/0 | 2F35 & XH3470 | |
| ||
| 8967/0 | 2F35 & XH3SR2 | |
| 8960/1 | 2C90.Y & XH10F1 | |
| 8963/3 | 2C90.Y & XH3RF3 | |
| 8964/3 | 2C90.Y & XH0765 | |
| Embryonal neoplasms of the kidney | ICD-O | ICD-11 |
| ||
| 8959/1 | 2C90.Y & XH1JB4 | |
| 8960/3 | 2C90.Y & XH5QN3 | |
| Miscellaneous renal tumors | ICD-O | ICD-11 |
| 9084/0, 9084/3, 9071/3, 9085/3 | 2C90.Y & XH83G5, 2C90.Y & XH09W7 |
Comment Here
Reference: Kidney tumor - WHO classification
The microscopic image above shows a needle core biopsy from a patient with multiple bilateral renal masses. Immunohistochemical stains were performed and are positive for CK7, KIT and succinate dehydrogenase B (SDHB). Which syndrome does this patient likely have?
- Birt-Hogg-Dubé syndrome
- Cowden syndrome
- Fumarate hydratase deficiency / hereditary leiomyomatosis
- Succinate dehydrogenase deficient tumor syndrome
Answer B is incorrect because Cowden syndrome is associated with a PTEN mutation. Patients can present with a wide clinical spectrum: breast cancer, endometrial cancer, hamartomas, trichilemmomas. Renal cell carcinomas have not been described as part of this syndrome. Answer C is incorrect because hereditary leiomyomatosis is a syndrome defined by multiple cutaneous or uterine leiomyomata and a renal cell carcinoma with a characteristic loss of fumarate dehydrogenase. These tumors were previously misclassified under PRCC type 2 due to their high grade cytology and papillary architecture, however, now they are recognized as a separate entity. A key diagnostic feature is prominent eosinophilic inclusion-like nucleoli with perinucleolar clearing, which is not present in these images. Answer D is incorrect because succinate dehydrogenase deficient tumor syndrome is characterized by a loss of succinate dehydrogenase B (SDHB); patients can present with SDH deficient RCC, paraganglioma or GISTs. Although these tumors are eosinophilic, the question stem indicated positive SDHB staining.
Comment Here
Reference: Kidney tumor - WHO classification
- Well differentiated epithelial neoplasm that arises from renal parenchyma and shows neuroendocrine differentiation
- Rare tumor that arises from renal parenchyma; associated with horseshoe kidney and mature teratoma of kidney
- Expresses neuroendocrine markers histologically but most are clinically nonfunctional
- Distant metastasis is common at diagnosis and Dual Tracer positron emission tomography (PET) imaging (DOTATATE scan) can help identify metastases
- Antiquated names: carcinoid tumor, atypical carcinoid tumor
- Well differentiated neuroendocrine carcinoma is not a recommended term
- Rare primary tumor in kidney; 58 cases reported in Surveillance, Epidemiology and End Results (SEER) database (Front Endocrinol (Lausanne) 2021;11:624251)
- 227 cases reported in the English literature between 1966 - 2023 (Virchows Arch 2023;483:465)
- Median age of 51; F:M = 125:93 (Virchows Arch 2023;483:465)
- 20 - 30% of cases are associated with horseshoe kidney (Clin Genitourin Cancer 2020;18:e343)
- Can arise within mature teratoma of the kidney (Diagn Pathol 2007;2:15)
- Arises from renal parenchyma; can extend into renal pelvis and inferior vena cava (Urology 2020;135:e2)
- Indeterminate but may originate from renal stem cells or misplaced neural crest cells since no neuroendocrine cells have been identified in adult renal parenchyma (Arch Pathol Lab Med 2006;130:1693, Front Endocrinol (Lausanne) 2021;11:624251)
- Uncertain
- Symptoms include abdominal / back / flank pain, hematuria, palpable mass, etc. (Front Endocrinol (Lausanne) 2021;11:624251)
- 33% of patients are asymptomatic (J Urol 2006;176:2359)
- Metastasis presented at diagnosis in 12/21 (57%) patients (Am J Surg Pathol 2007;31:1539)
- Tumors are usually nonfunctional but rarely show ectopic adrenocorticotropic hormone (ACTH) production (Cushing syndrome) (Endocrinol Diabetes Metab Case Rep 2021;2021:20)
- Imaging study followed by histological examination of biopsy or resection specimen
- May show increased serum chromogranin A and urine 5-HIAA (Front Endocrinol (Lausanne) 2021;11:624251)
- Computed tomography (CT): solid hypodense (55%) or hyperdense (29%) mass with calcifications (33%), mimicking renal cell carcinoma (Radiol Case Rep 2015;9:923)
- Gallium 68 DOTATATE PET / CT: hypermetabolic / increased uptake (Perm J 2020;24:19.147, Pathol Oncol Res 2020;26:341)
- Unfavorable prognostic factors
- Age: > 40 years
- Size: > 4 cm
- > 1 mitotic figure/10 high power fields (HPF)
- Metastasis at diagnosis
- Extension throughout renal capsule
- Reference: J Urol 2006;176:2359
- Late 40s woman presented with a 14.9 cm mass arising from horseshoe kidney (Mayo Clin Proc 2021;96:1687)
- 51 year old man presented with left renal mass and ectopic ACTH syndrome (Endocrinol Diabetes Metab Case Rep 2021;2021:20)
- 55 year old man with renal well differentiated neuroendocrine tumor and bilateral multiple clear cell papillary renal cell carcinomas (Case Rep Pathol 2017;2017:9672368)
- 64 year old woman with hematuria and right kidney mass (Radiol Case Rep 2023;19:586)
- 5 cases of primary renal well differentiated neuroendocrine tumor in 1 institution (Pathol Oncol Res 2020;26:341)
- Nephrectomy with lymph node dissection
- Somatostatin analogs, tyrosine kinase inhibitors and peptide receptor radionuclide therapy (PRRT) for metastasis (Front Endocrinol (Lausanne) 2021;11:624251)
- Well circumscribed, soft, brown-tan mass with focal hemorrhage, necrosis or cystic degeneration
- Variable architecture: tightly packed cords with minimal stroma (most common, 81%), sheet-like / solid pattern, nests / organoid pattern, trabeculae, ribbons, pseudo glands, etc. (Am J Surg Pathol 2007;31:1539)
- Round to oval / elongated nuclei with stippled / speckled chromatin, inconspicuous nucleoli and moderate eosinophilic / granular cytoplasm
- Occasional calcifications (5/21, 24%) of variable sizes (Am J Surg Pathol 2007;31:1539)
- Usually lack of necrosis and low mitotic activity ( Grading of neuroendocrine neoplasms in nonendocrine organs by measuring proliferation either by mitotic count or Ki67 index assessment
- WHO grade 1: 2 or Ki67 index WHO grade 2: 2 - 20 mitoses/2 mm2 or Ki67 index 3 - 20%
- WHO grade 3: > 20 mitoses/2 mm2 or Ki67 > 20%
Contributed by Maria Tretiakova, M.D., Ph.D., Leandro Freitas, M.D., Ana Grizotto, M.D., Athanase Billis, M.D.,
the Genitourinary Pathology Society (Case #521) and Sleiman Khalil, M.D.
- Tumor cells present singly; in clusters, may show pseudo glandular differentiation
- Monotonous cells with plasmacytoid appearance, oval to round nuclei, fine speckled chromatin (Diagn Cytopathol 2007;35:306, Radiol Case Rep 2015;1:108)
- Cytokeratin markers: CK AE1 / AE3, CK8/18
- Neuroendocrine markers: synaptophysin (90%), chromogranin (65%), CD56 (3/6, 50%) (Am J Surg Pathol 2007;31:1539, Diagn Pathol 2019;14:12)
- Strong membranous CD99 immunoreactivity (8/9) (Hum Pathol 2011;42:1554)
- Renal transcription factors: PAX8 (9/9), PAX2 (9/9) (Hum Pathol 2011;42:1554)
- CK7 (17% positive), CK20 (6% positive) (Am J Surg Pathol 2007;31:1539)
- CDX2 (9/9), TTF1 (9/9) (Hum Pathol 2011;42:1554)
- GATA3 (Case Rep Pathol 2017;2017:9672368)
- Presence of dense core neurosecretory granules
- Genetic alterations are variable and none of them are specific
- Most frequently observed genetic changes are mutations in CDH1 and TET2
- Reference: Histopathology 2019;75:104
- Right kidney, core biopsy:
- Well differentiated neuroendocrine tumor, likely renal primary (see comment)
- Comment: The tumor cells show nested growth pattern, with round to oval nuclei, stippled chromatin and eosinophilic granular cytoplasm. No mitosis or necrosis is seen. Immunohistochemistry studies show that the tumor cells are positive for CK AE1 / AE3, synaptophysin and chromogranin and negative for PAX8, TTF1, CDX2 and GATA3. The overall findings are consistent with a well differentiated neuroendocrine tumor. Given the radiographic finding of solitary renal mass and lack of clinical history of neuroendocrine tumors elsewhere, this tumor is most likely a renal primary.
- Metastatic well differentiated neuroendocrine tumor:
- Paraganglioma:
- Zellballen / organoid growth pattern
- Sustentacular cells are positive for S100 and chief cells are positive for neuroendocrine markers
- Negative for cytokeratin and positive for GATA3 (Mod Pathol 2013;26:1365)
- Metanephric adenoma:
- Nested variants of urothelial carcinoma:
- Variable sized anastomosing nests with irregular projection into stroma
- Positive for CK7, CK20, GATA3 and uroplakin II
- Poorly differentiated neuroendocrine carcinomas of kidney:
- High grade cytological atypia, necrosis is common; brisk mitotic activity and high Ki67 proliferative index
- Positive for cytokeratin and neuroendocrine markers
- Small cell carcinoma: high N:C ratios, hyperchromatic nuclei, nuclear molding
- Large cell carcinoma: large nuclei, abundant cytoplasm, prominent nucleoli
- Ewing sarcoma:
- Usually in children or young adults Small round blue cell tumor, CD99 positive
- Gene rearrangements involving FET and ETS families of genes (most commonly EWSR1::FLI1 gene rearrangement)
A 45 year old man presented with hematuria. Computed tomography (CT) showed a well demarcated 3 cm left renal mass in the lower pole. Core biopsy was performed and the H&E slide is shown above. Immunostains show that the tumor cells are positive for CK8/18, synaptophysin and chromogranin and negative for PAX8, GATA3 and S100. What is the diagnosis of this tumor?
- Metanephric adenoma
- Nested variant of urothelial carcinoma
- Papillary renal cell carcinoma
- Paraganglioma
- Well differentiated neuroendocrine tumor
Comment Here
Reference: Well differentiated neuroendocrine tumor of kidney (carcinoid)
- CD56
- CD99
- Chromogranin
- GATA3
- Synaptophysin
Comment Here
Reference: Well differentiated neuroendocrine tumor of kidney (carcinoid)
Find related Pathology books: GU/adrenal, renal, cytopathology