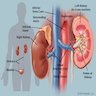
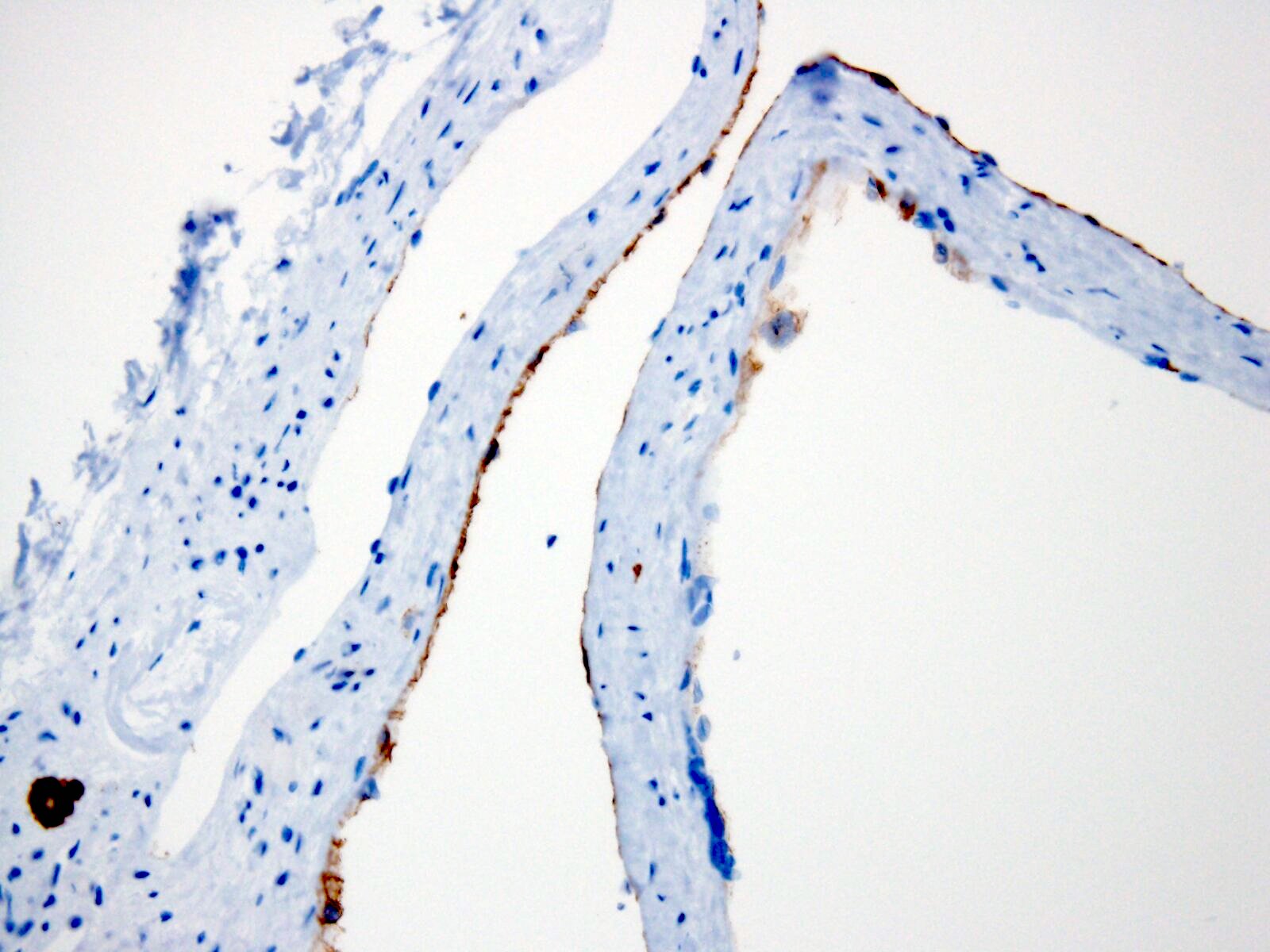
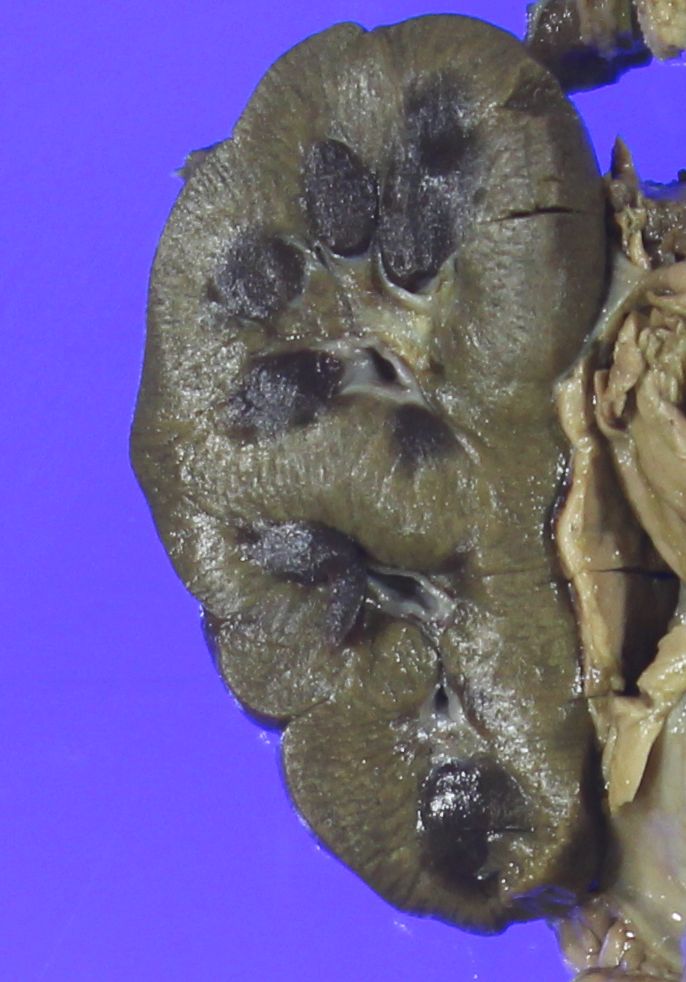
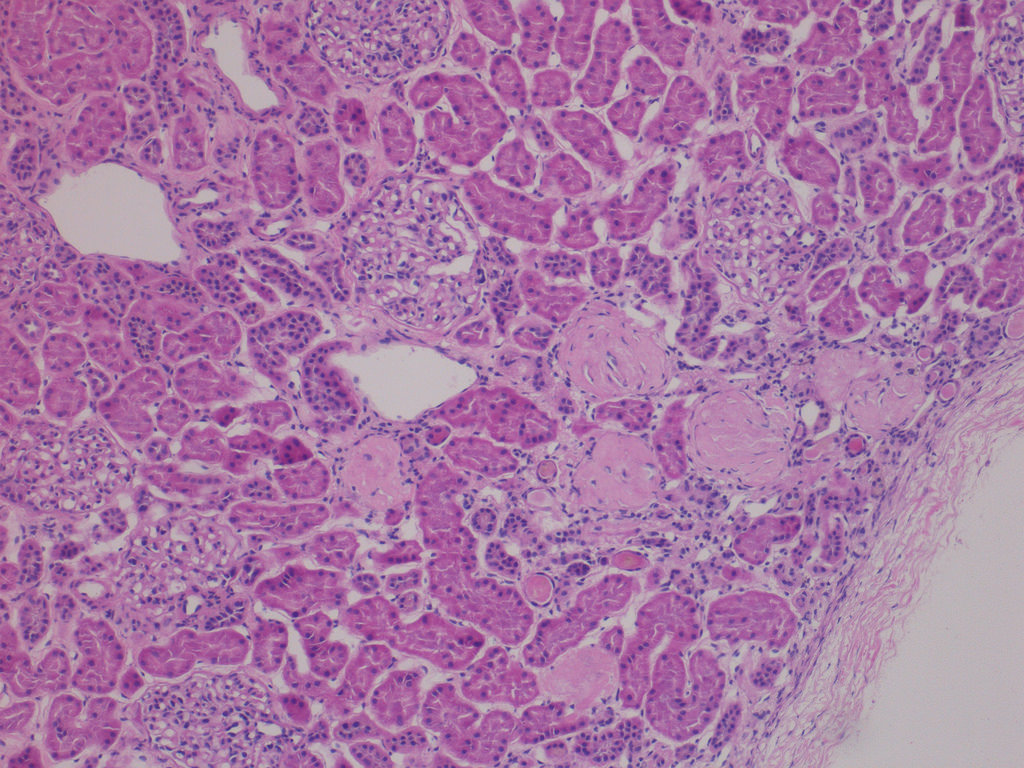
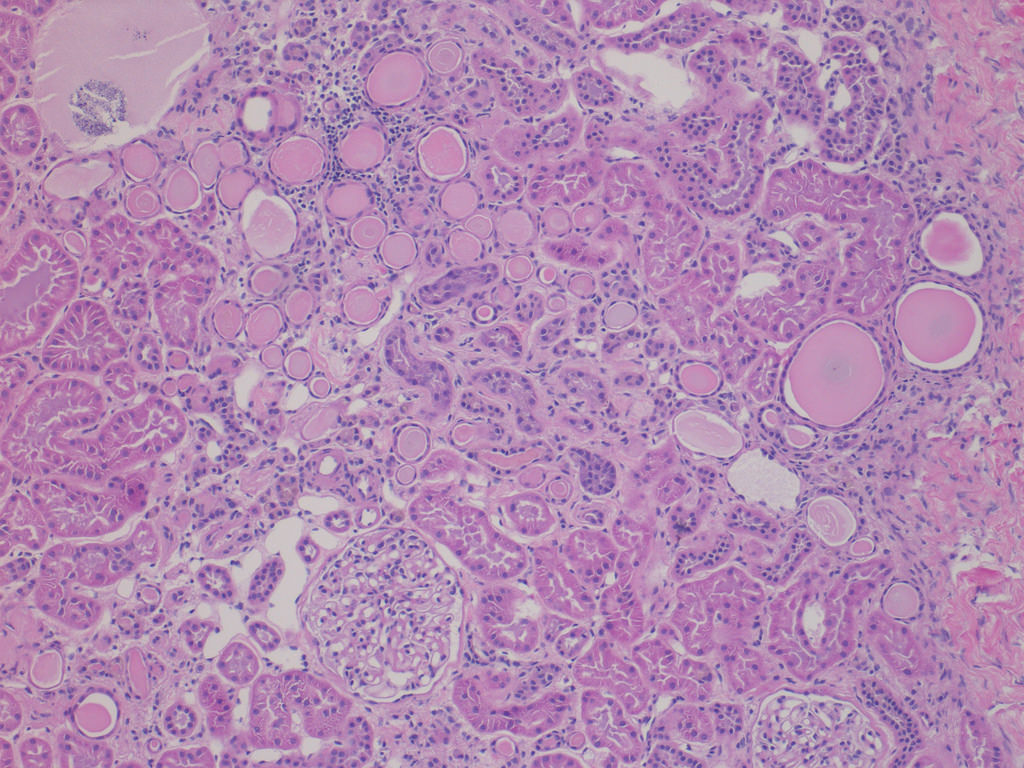
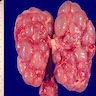
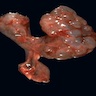
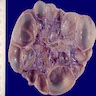
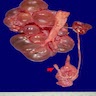
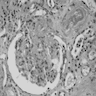
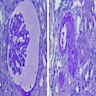
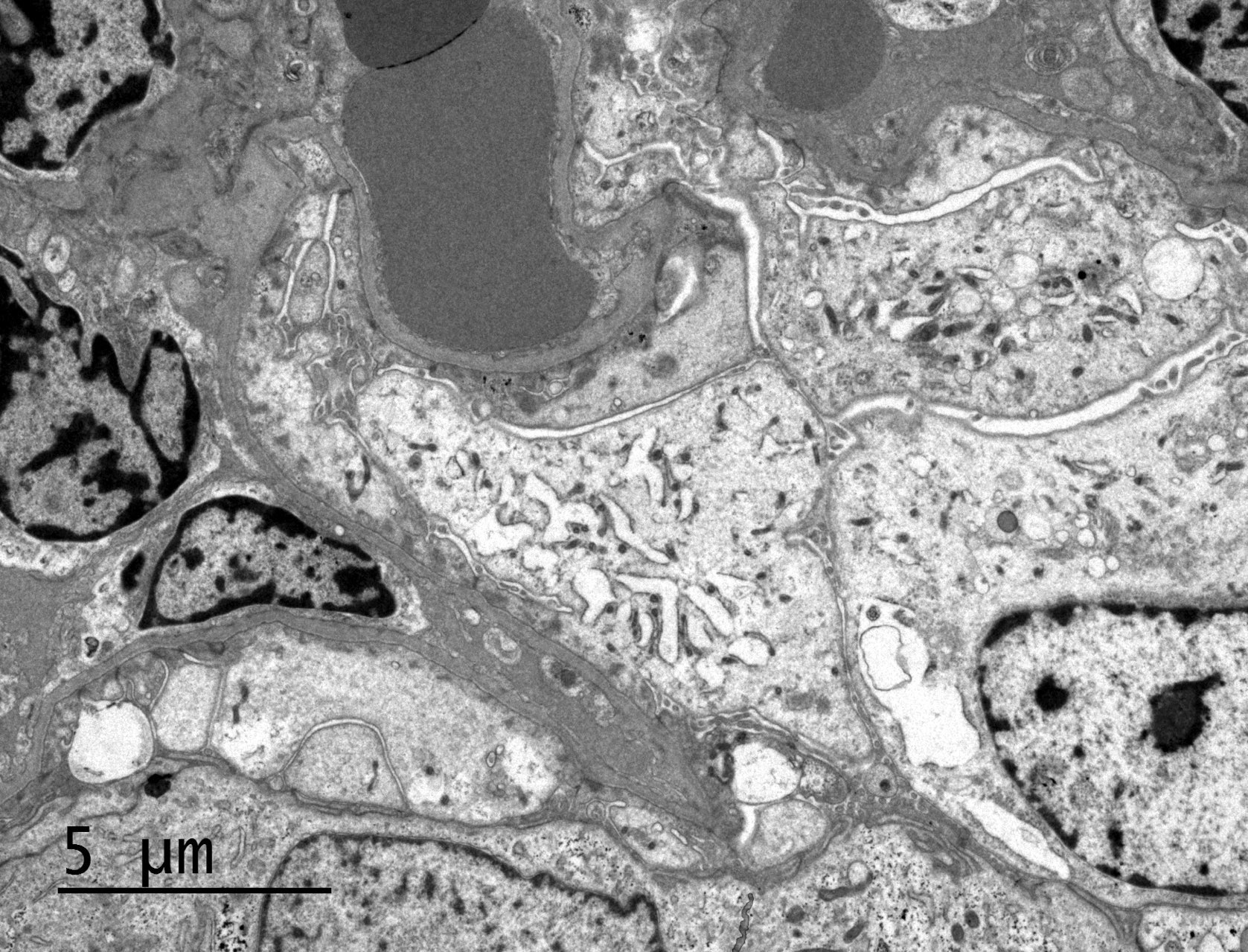
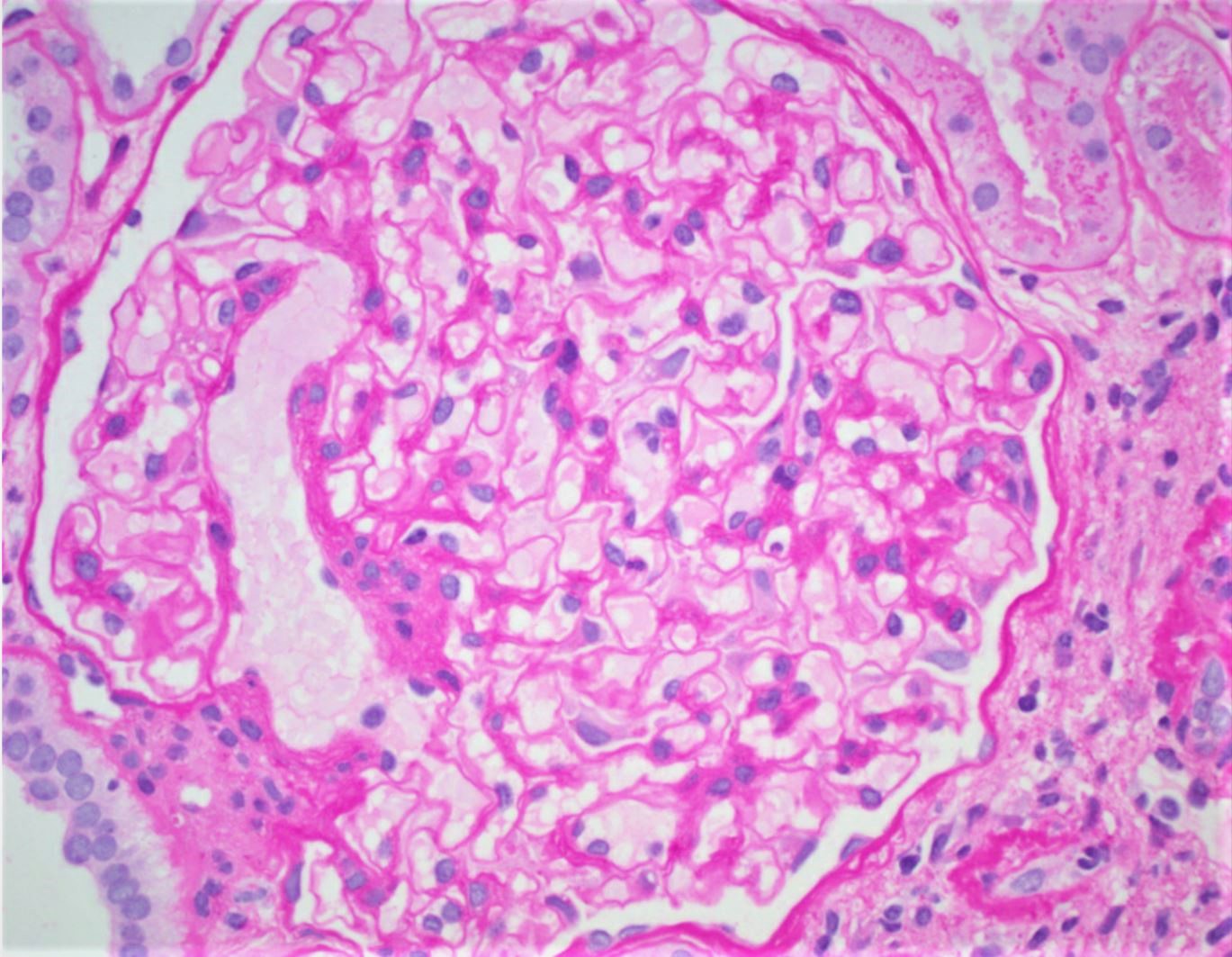
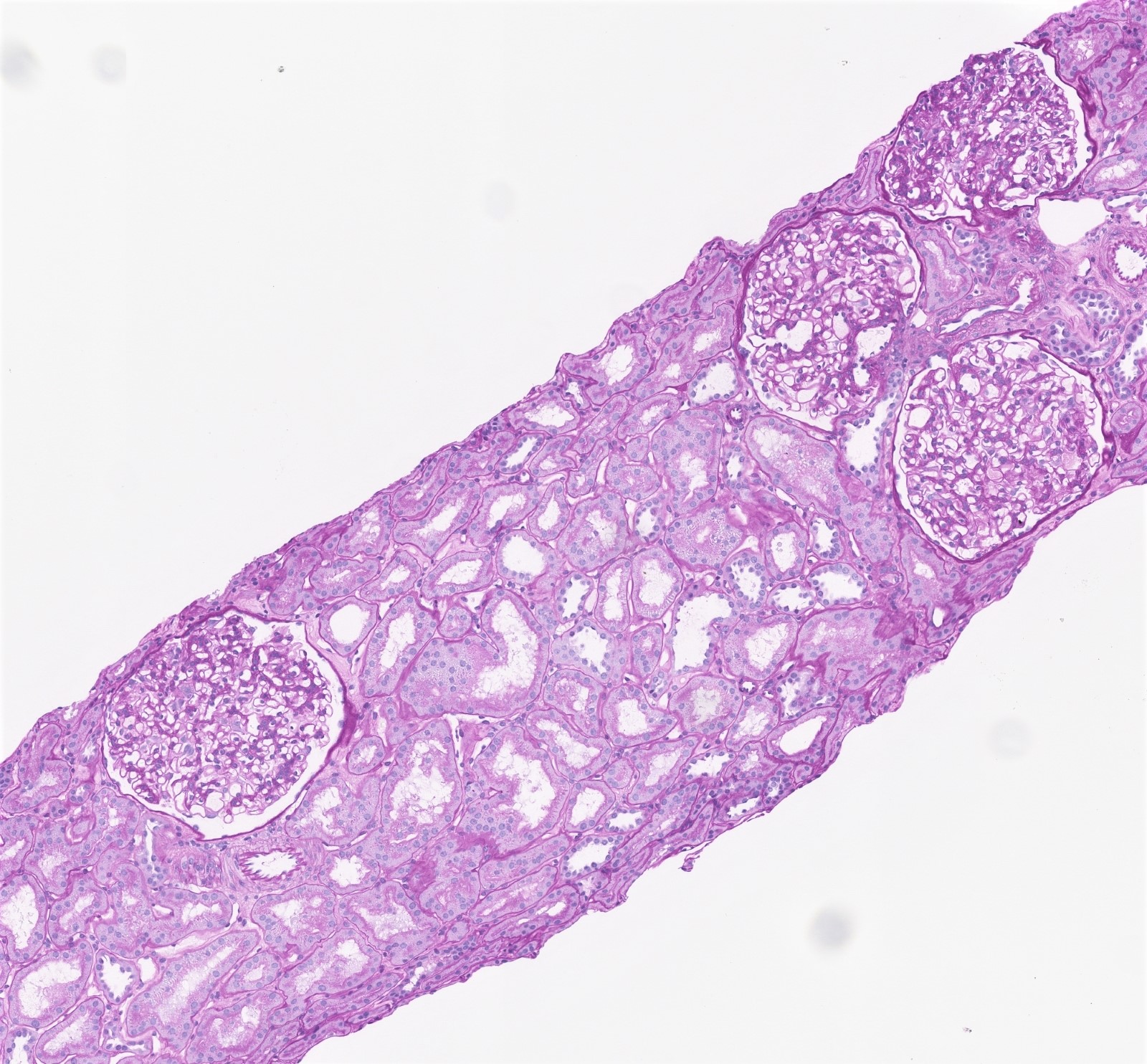
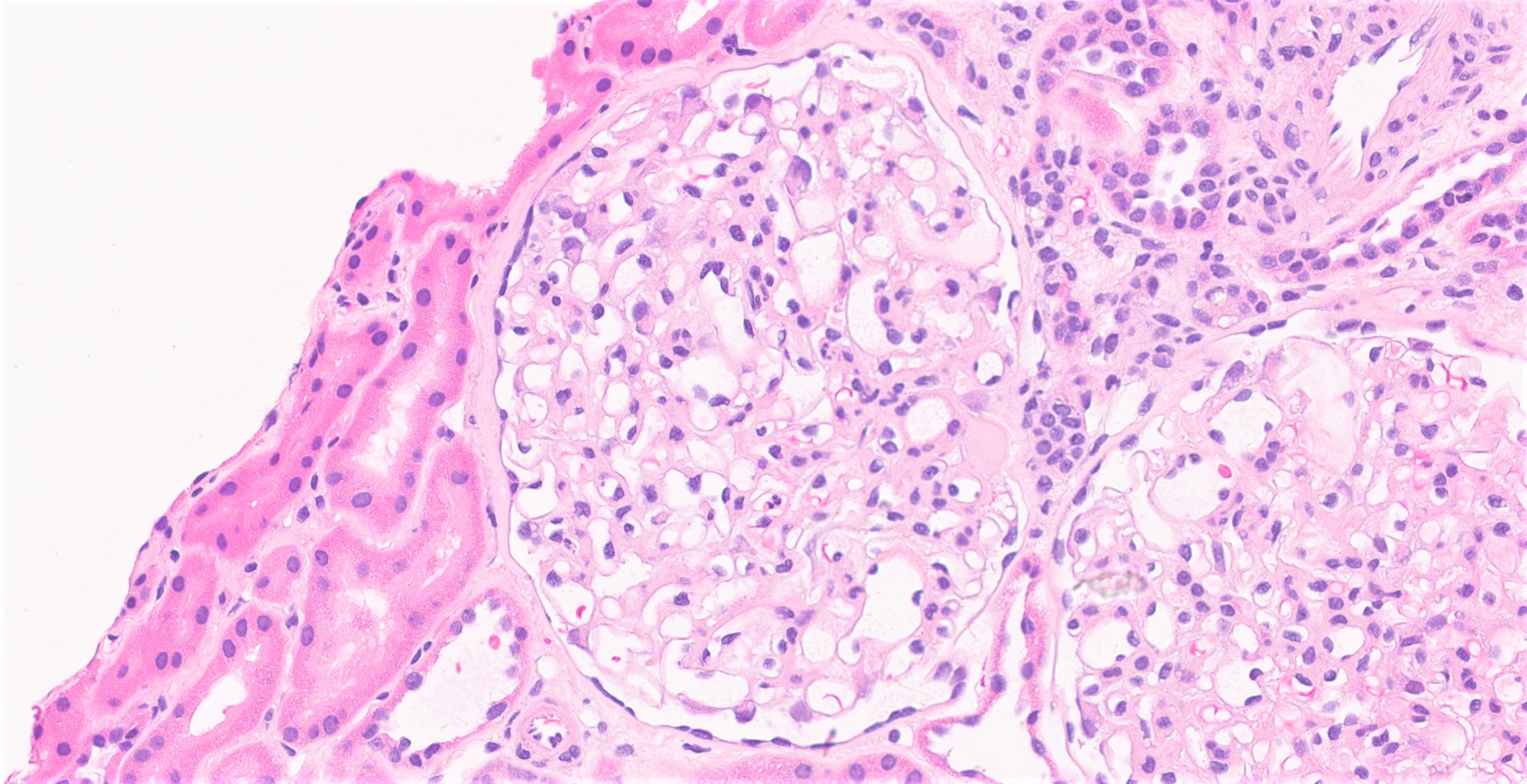
- Three or more cysts per kidney in patients on longstanding hemo- or peritoneal dialysis for end stage renal disease (unrelated to underlying renal pathology)
- Occurs in 10% - 20% of patients within the first three years of dialysis, 50% within the first five years and 90% after ten years
- Also occurs in patients with longterm uremia prior to dialysis
- Males > females during first ten years of dialysis
- Not restricted to adults; occurs in children and young adults on dialysis (Pediatr Nephrol 1997;11:447)
- Frequency and severity not affected by online hemodiafiltration (Ren Fail 2009;31:555)
- May be due to uremia
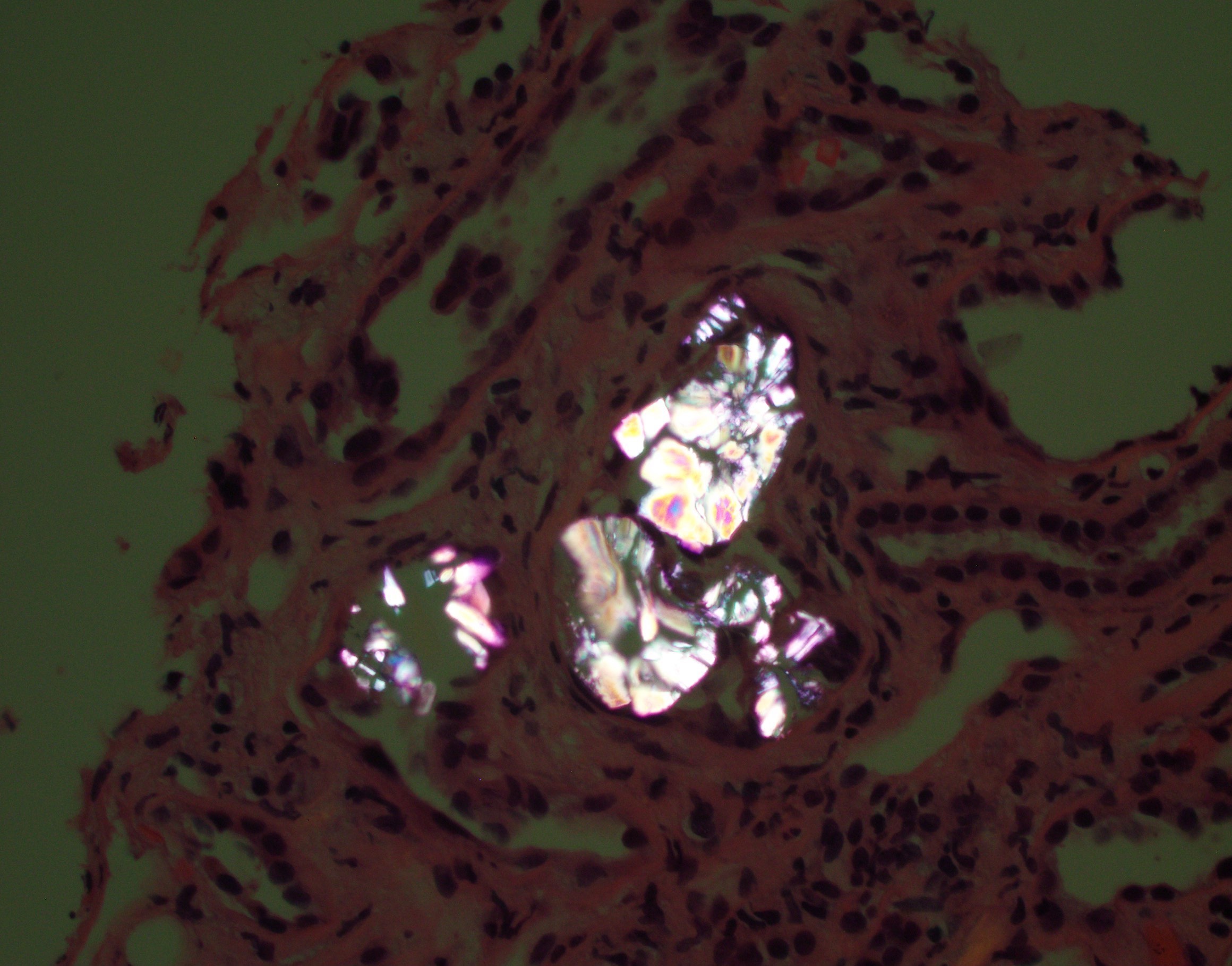
- Cysts may form due to obstruction by oxalate crystals, fibrosis or hyperplasia
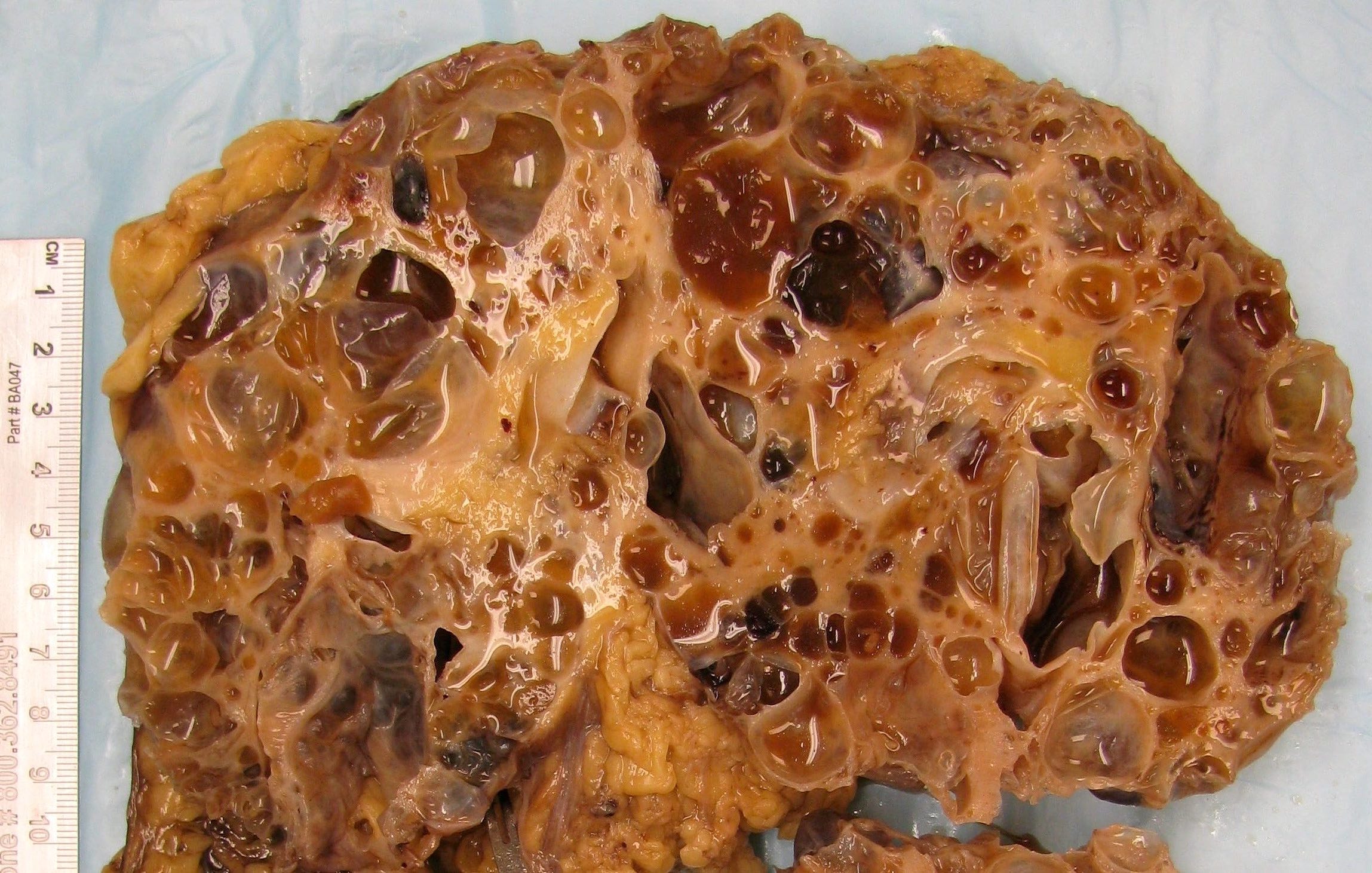
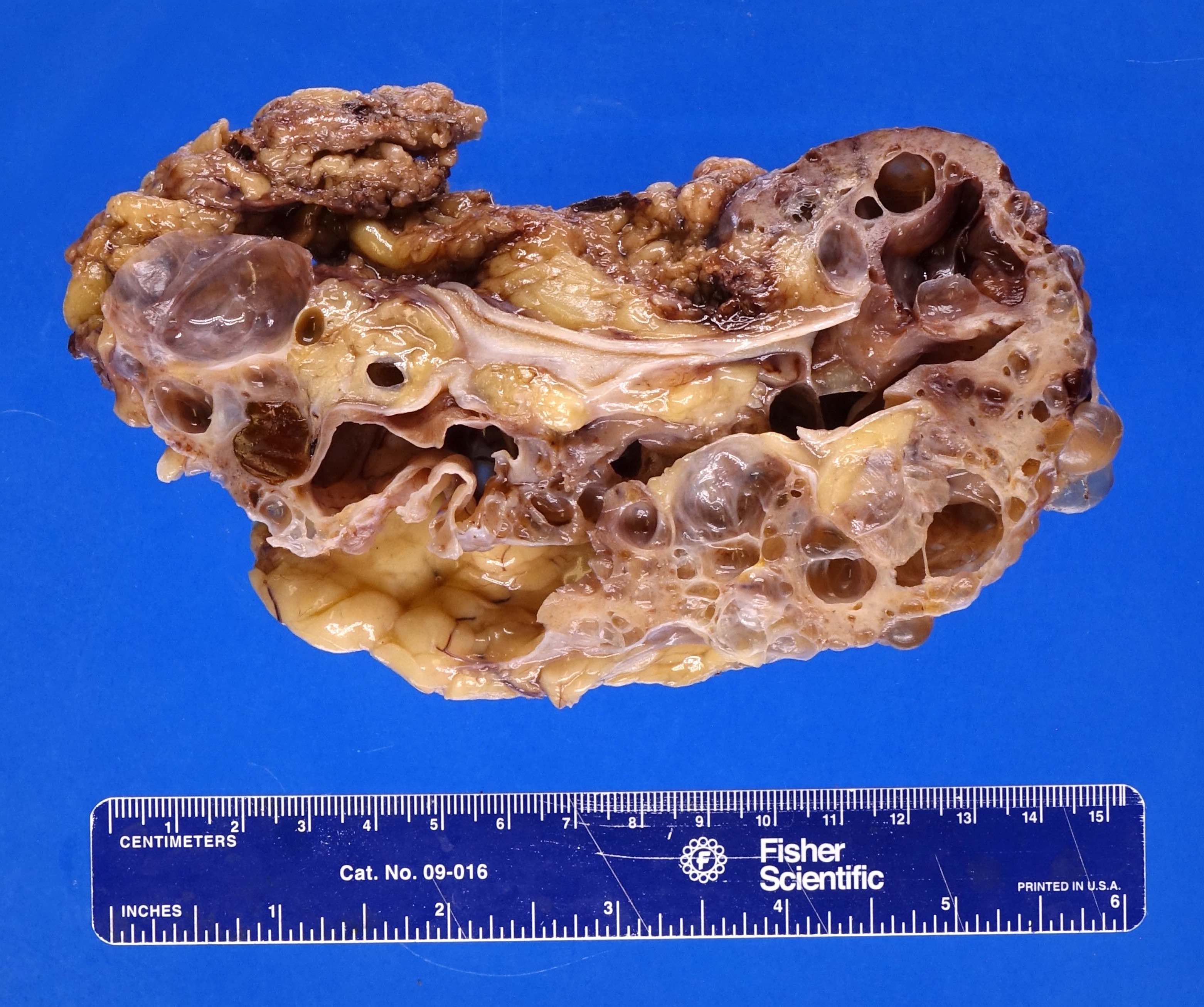
- Increased (7 - 50x) risk of renal cell carcinoma (7% at 10 years), but death is rare (Clin Nephrol 2003;59:153)
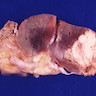
- 36 year old man on CAPD for 6 years (Nephrol Dial Transplant 2002;17:500)
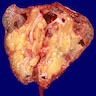
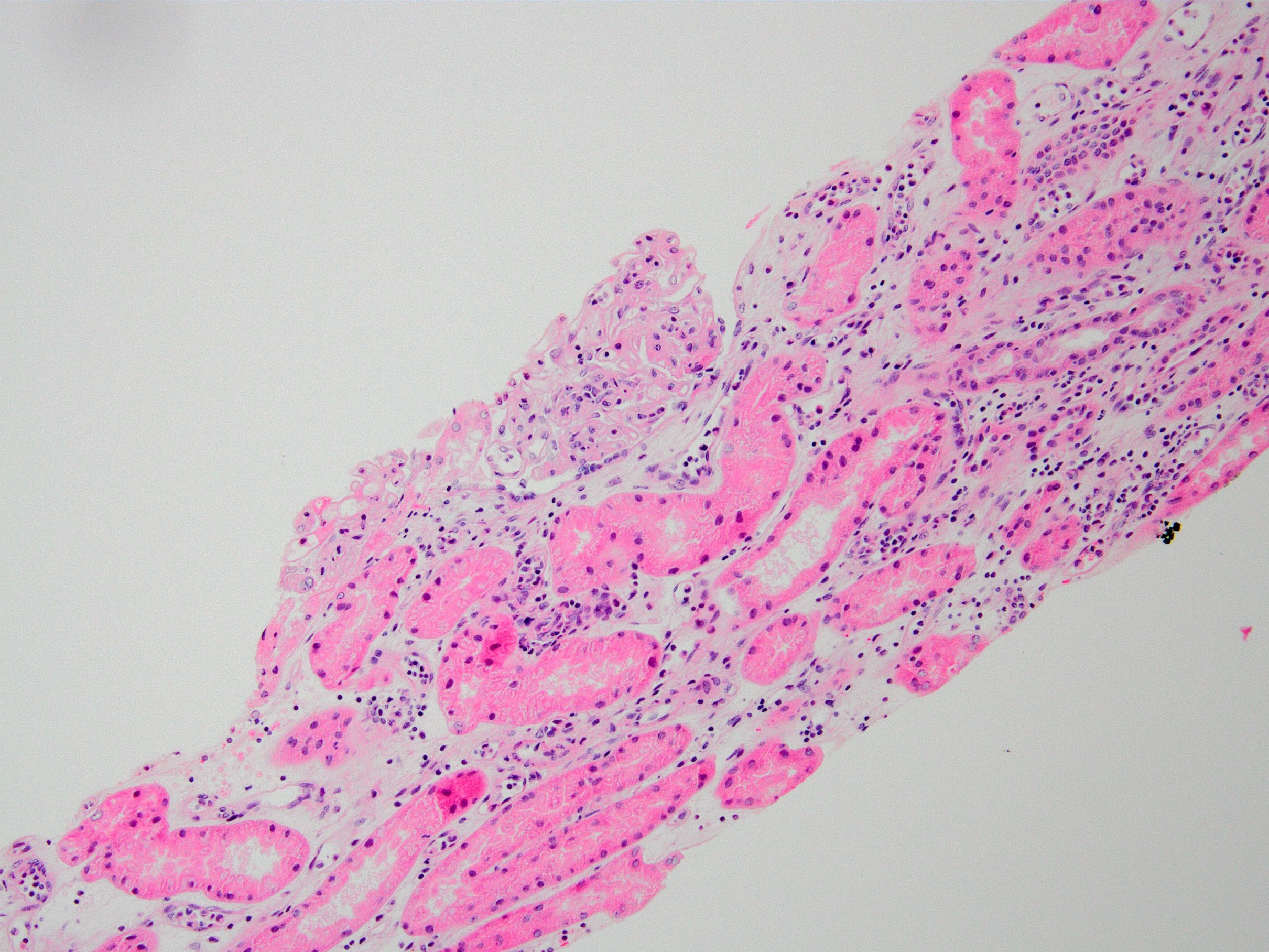
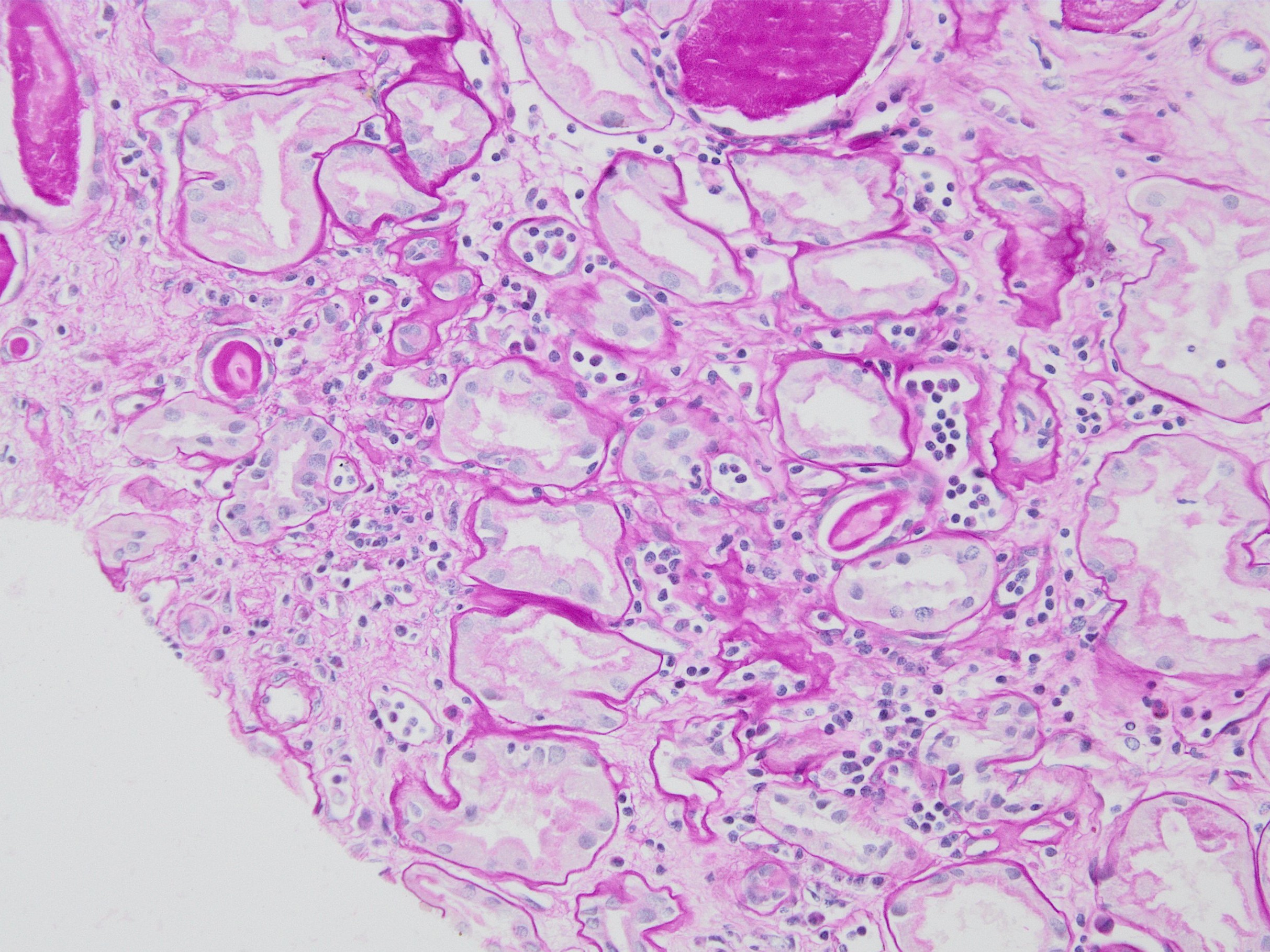
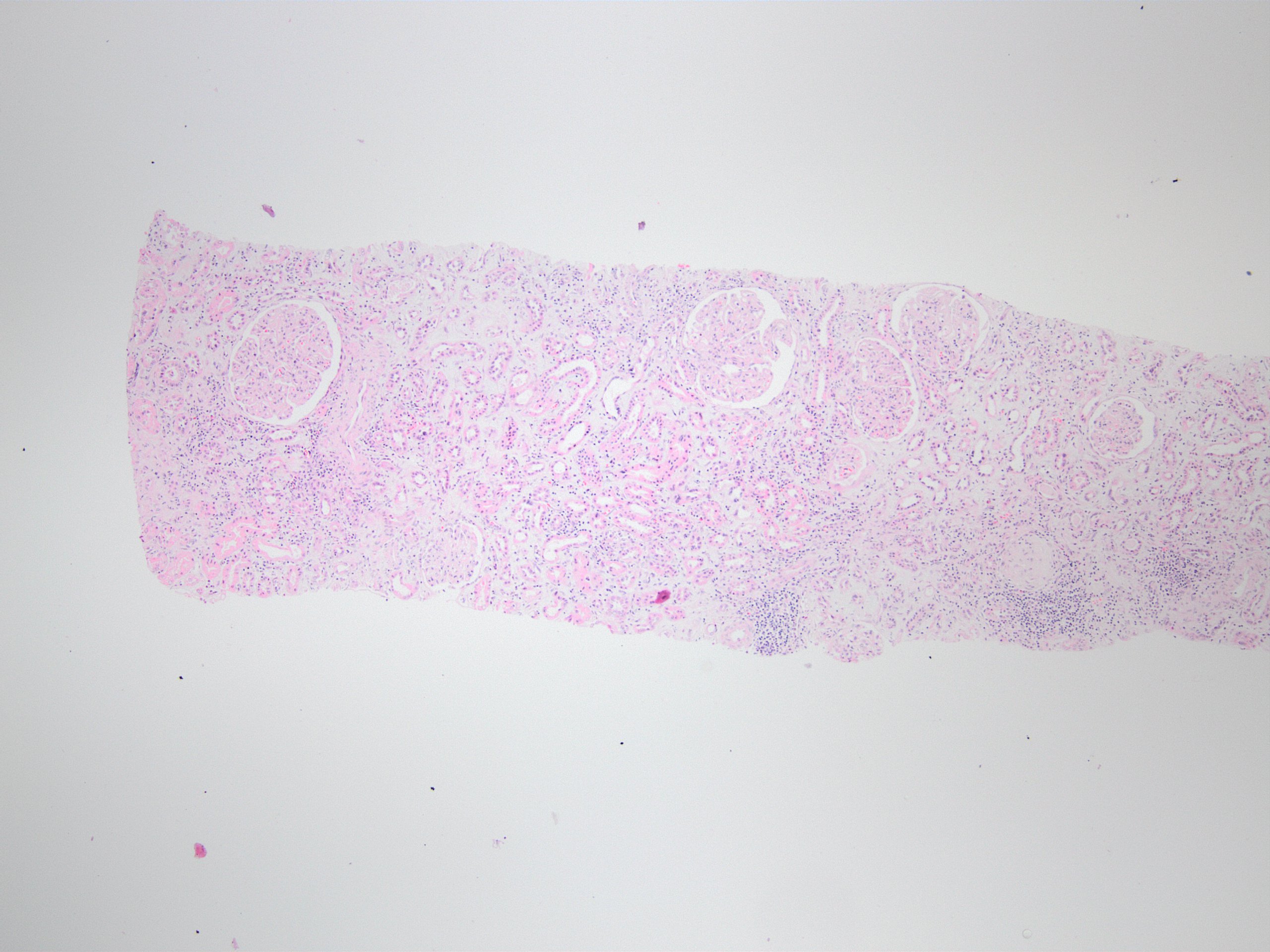
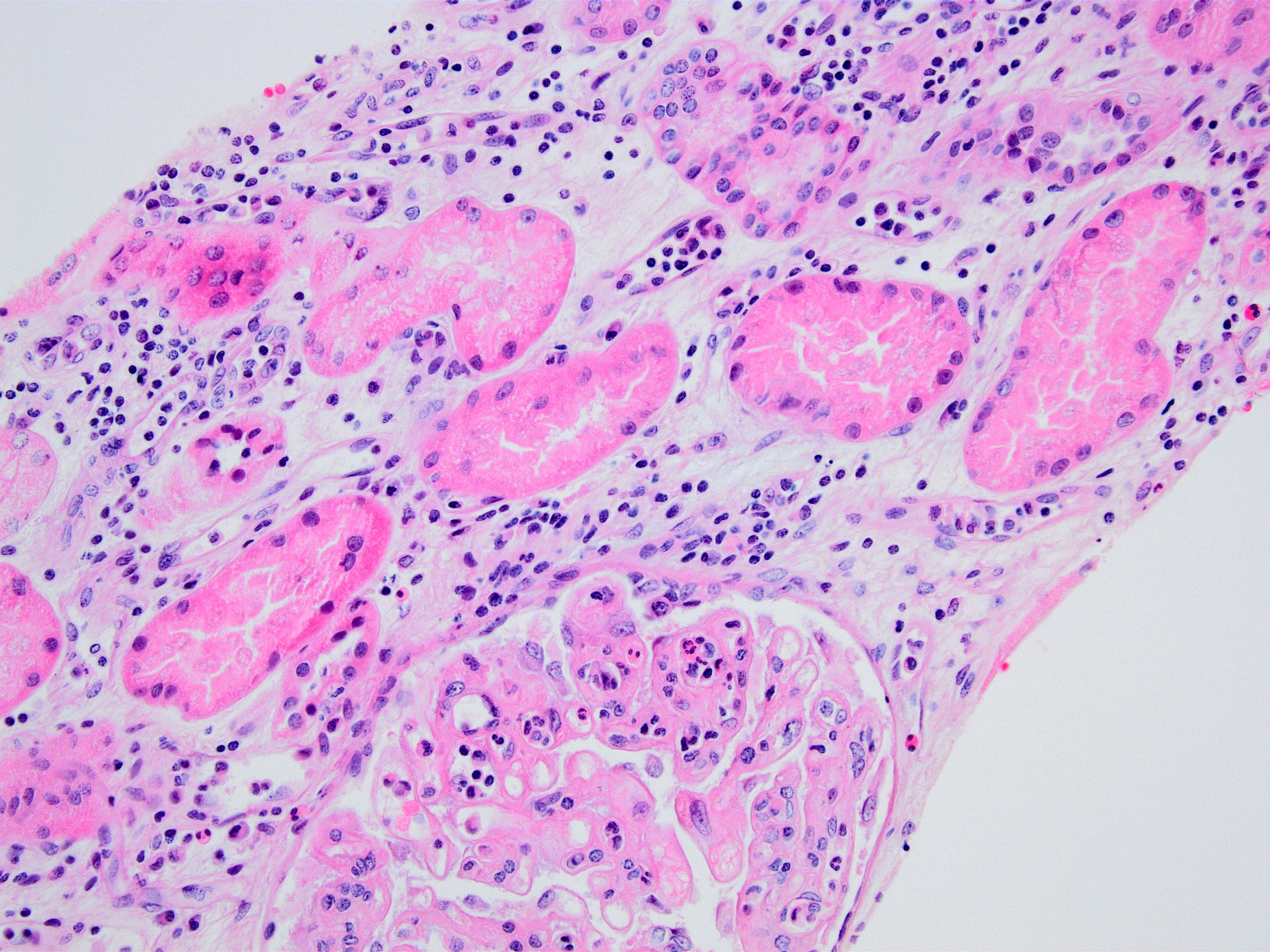
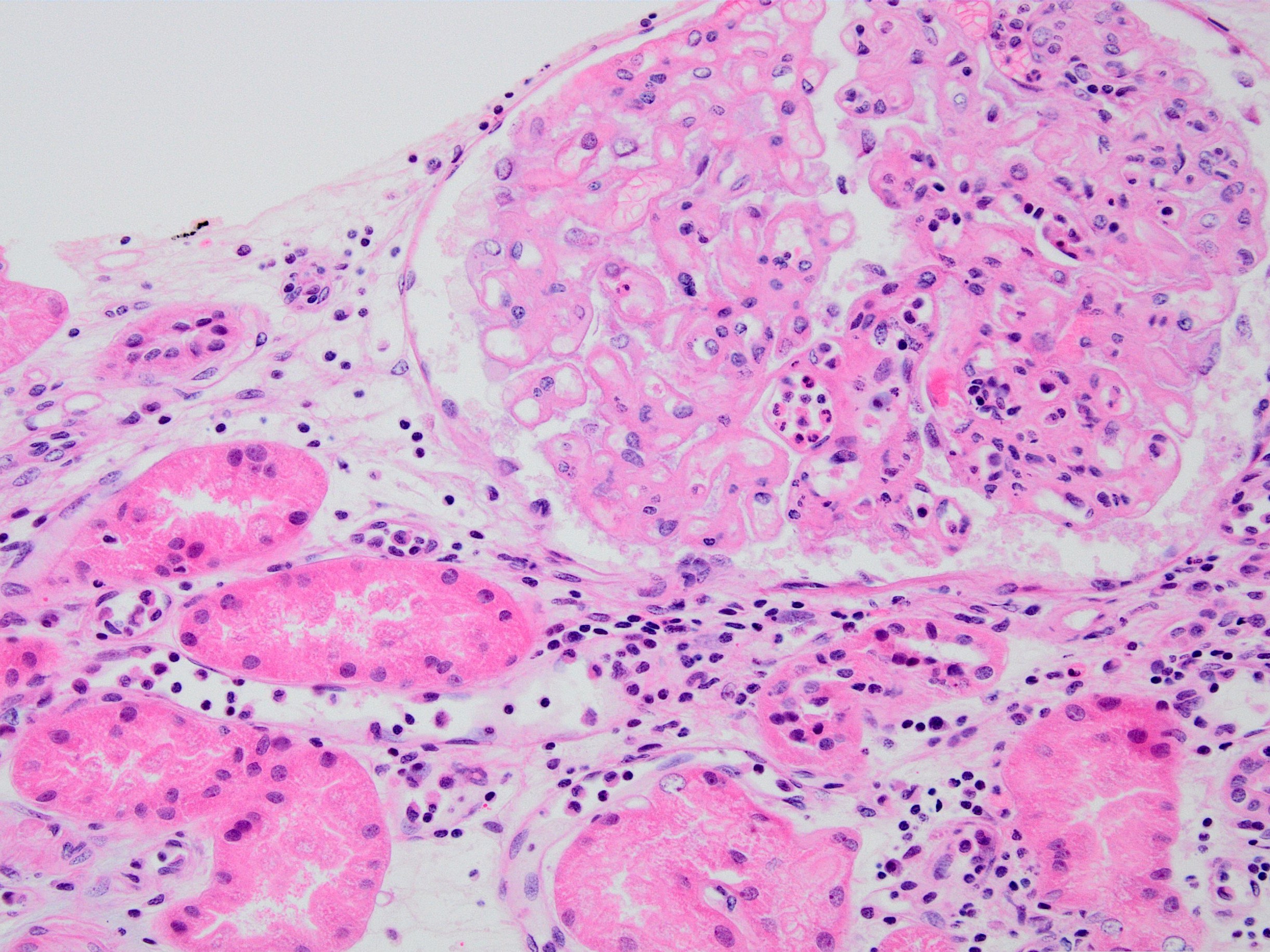
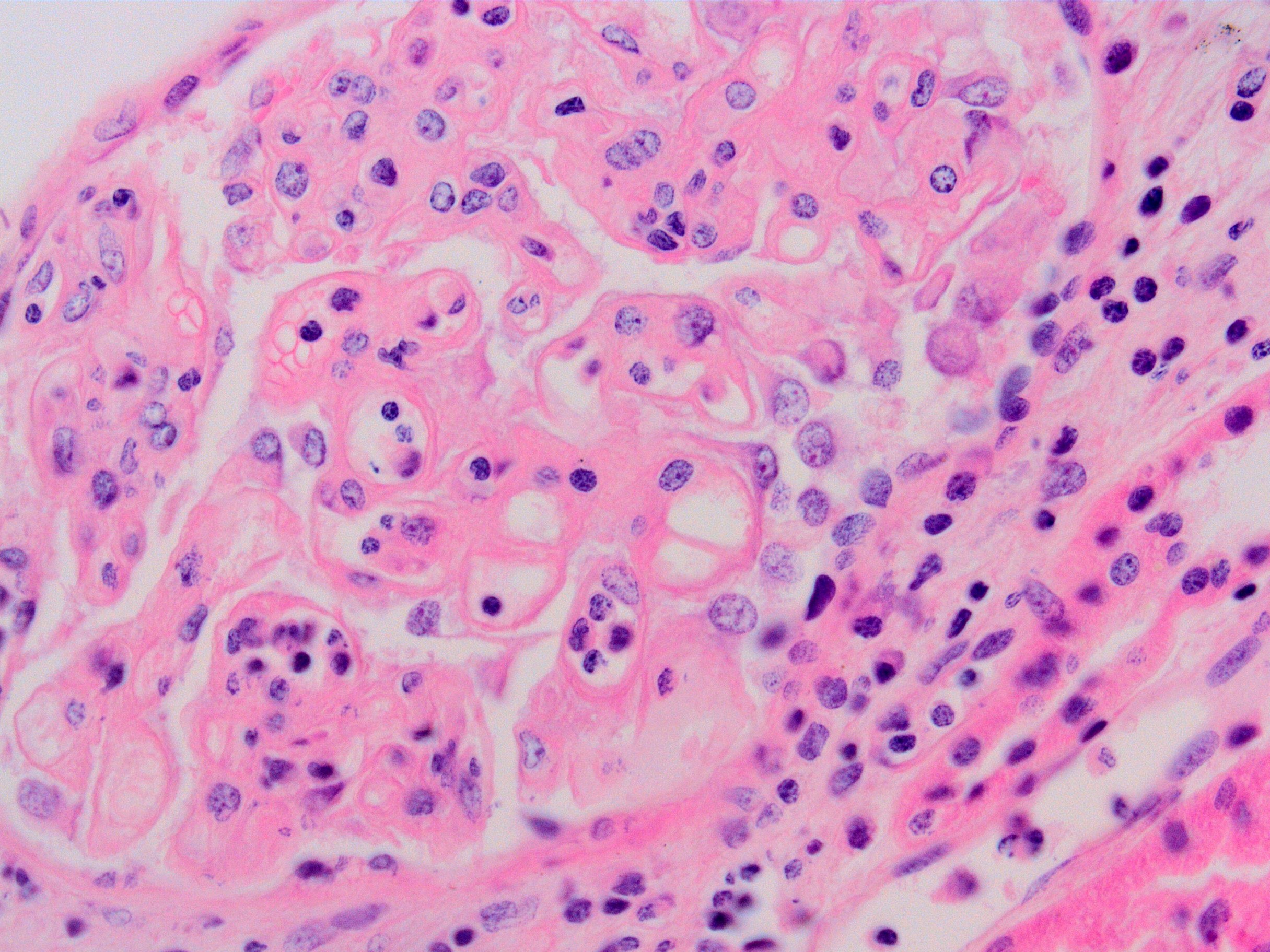
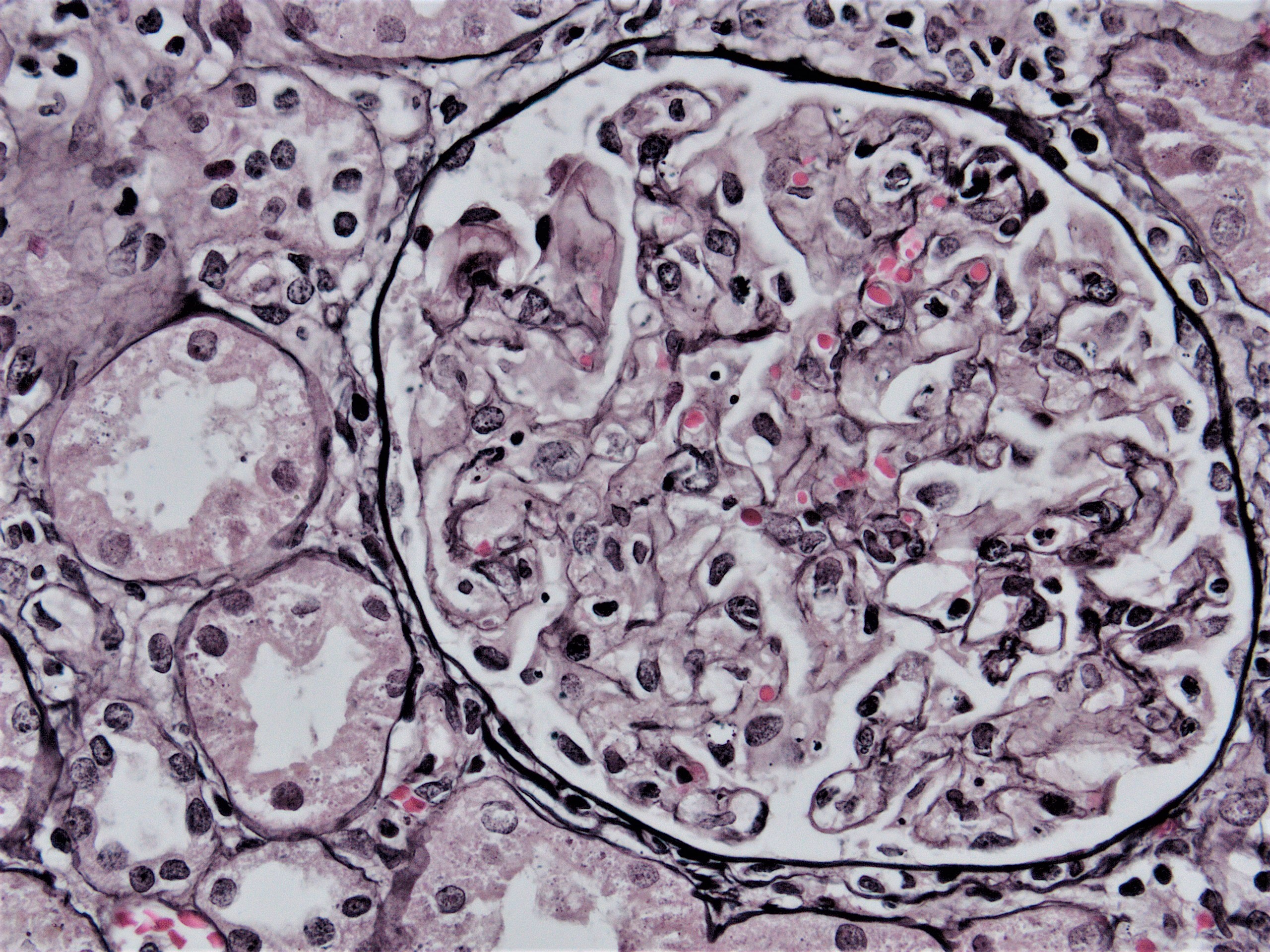
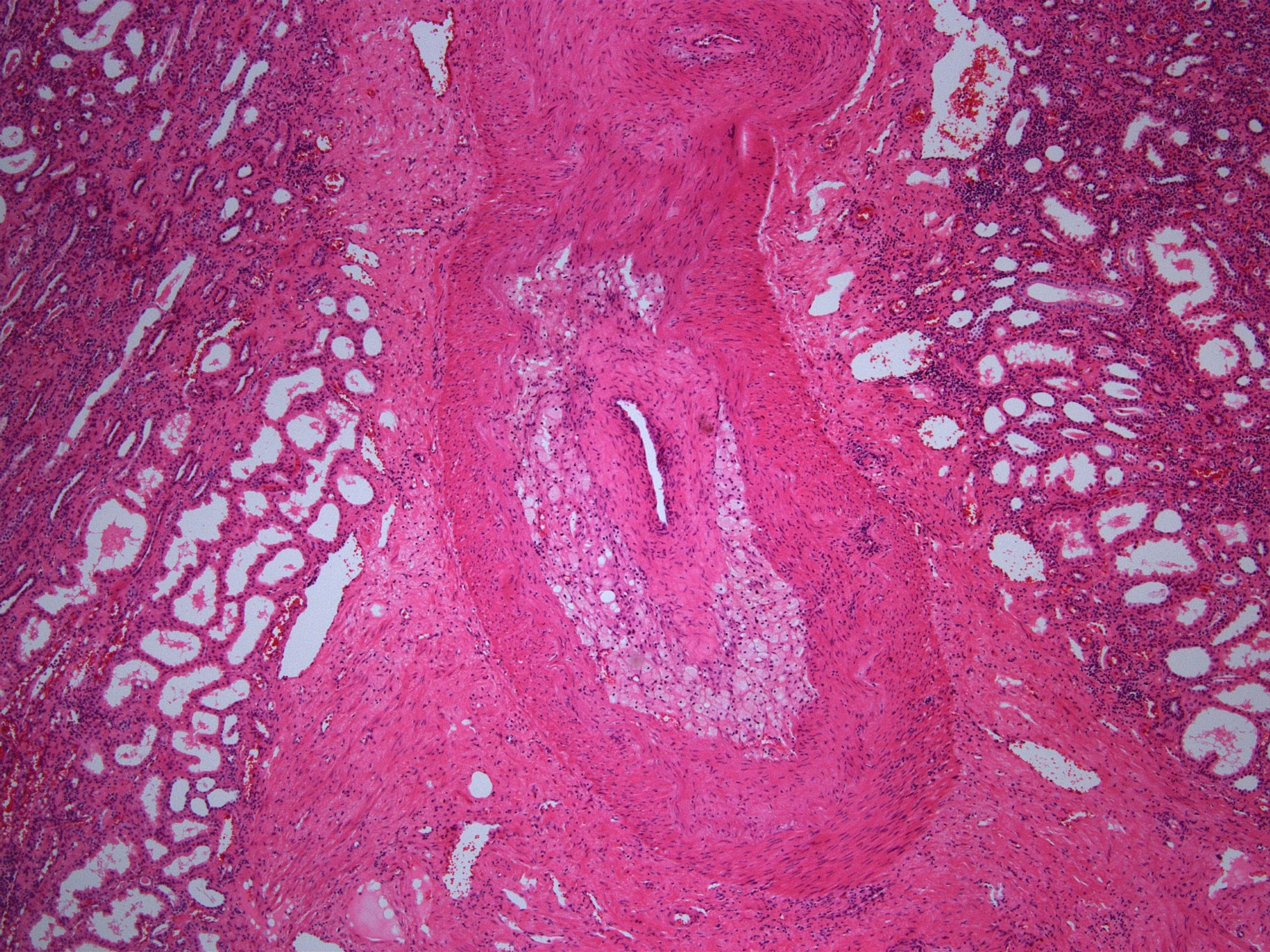
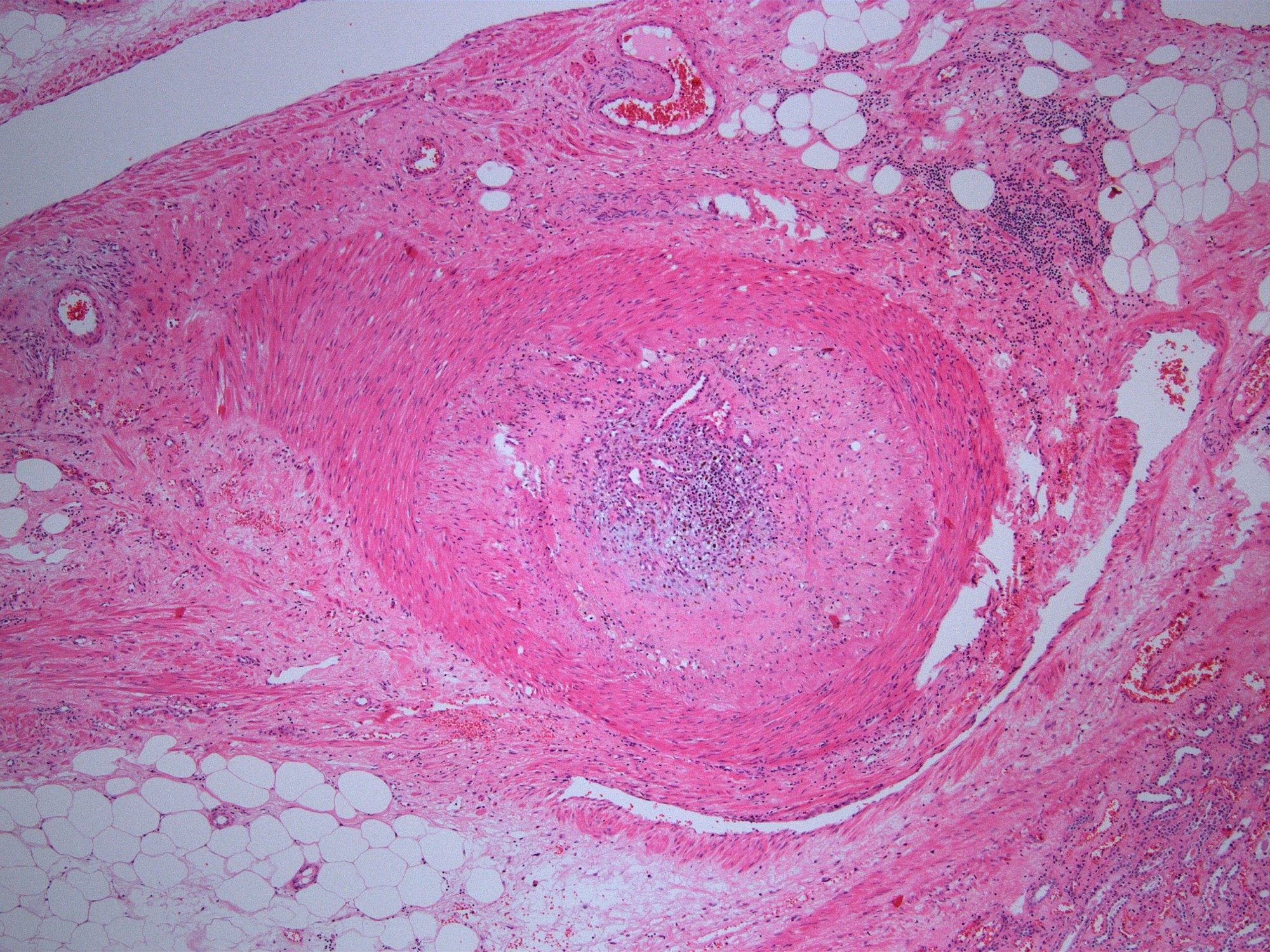
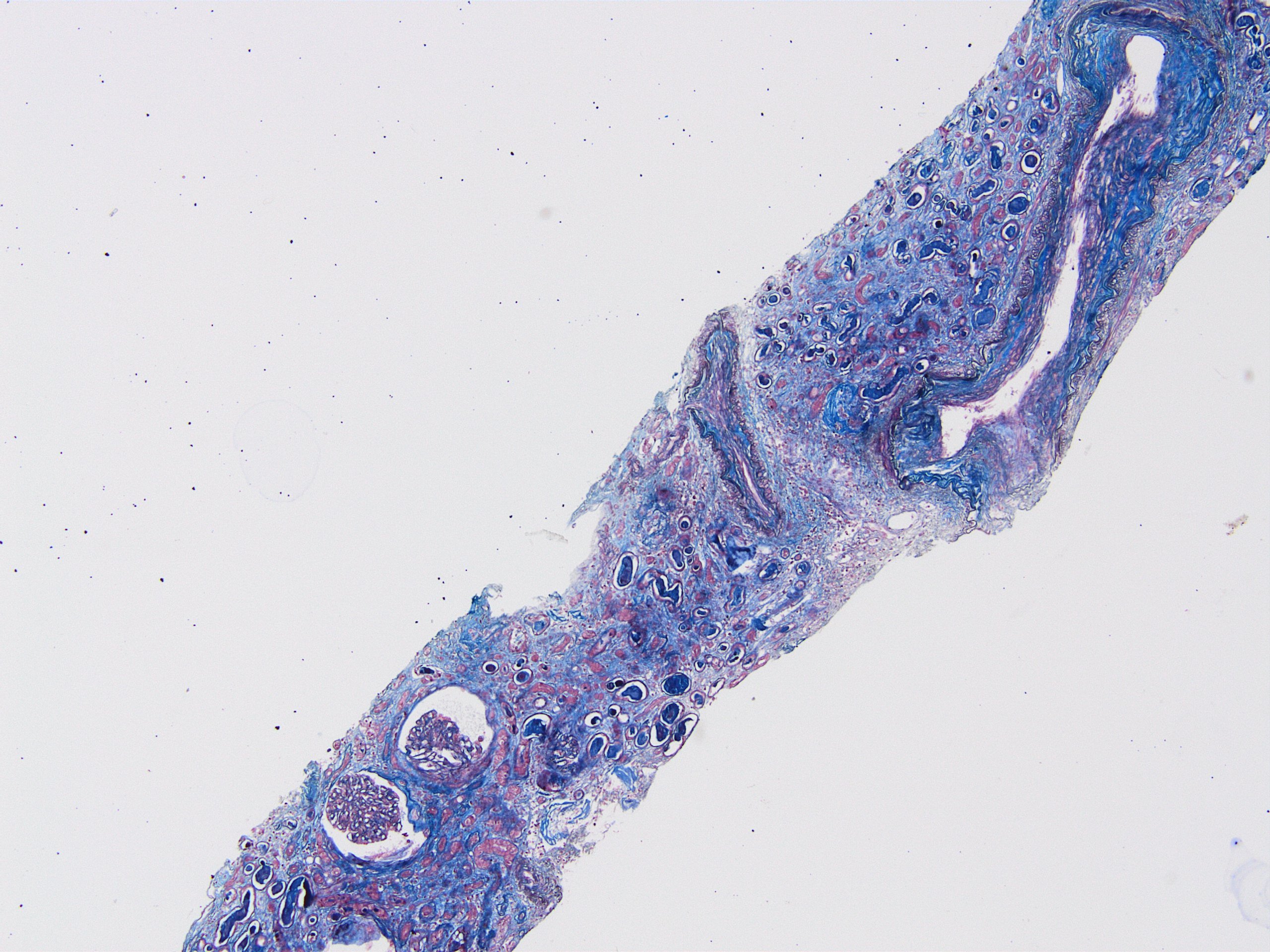
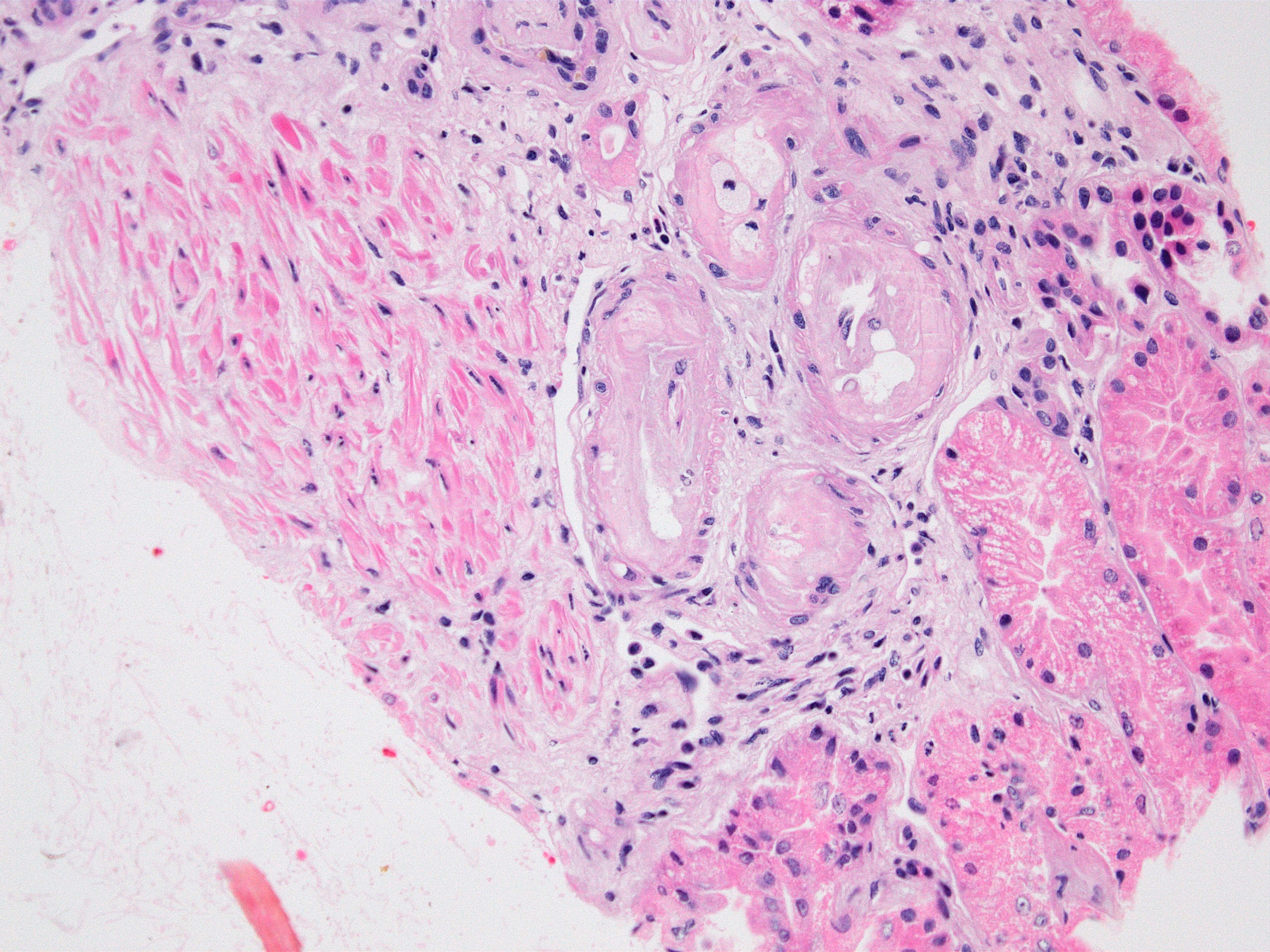
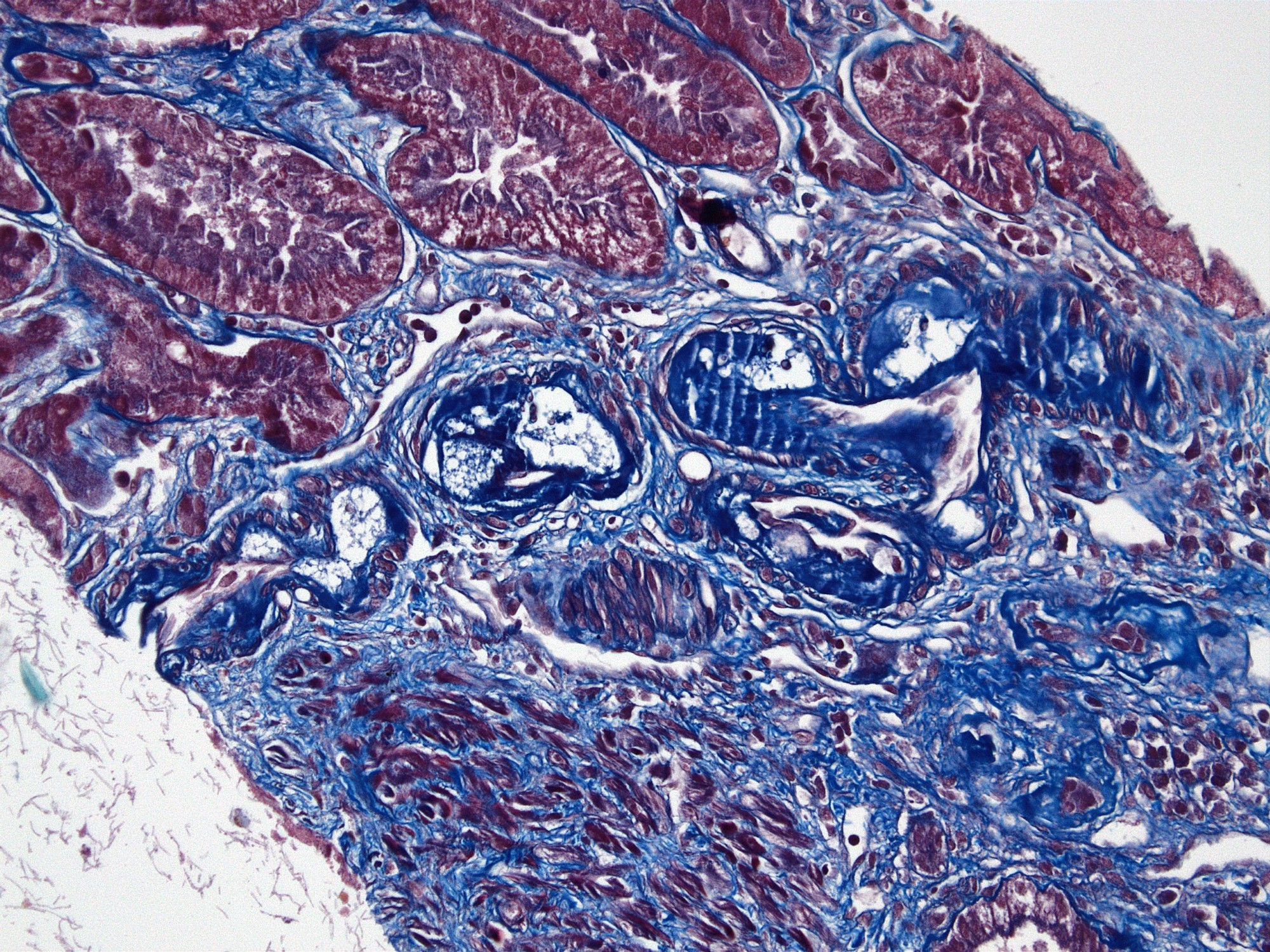
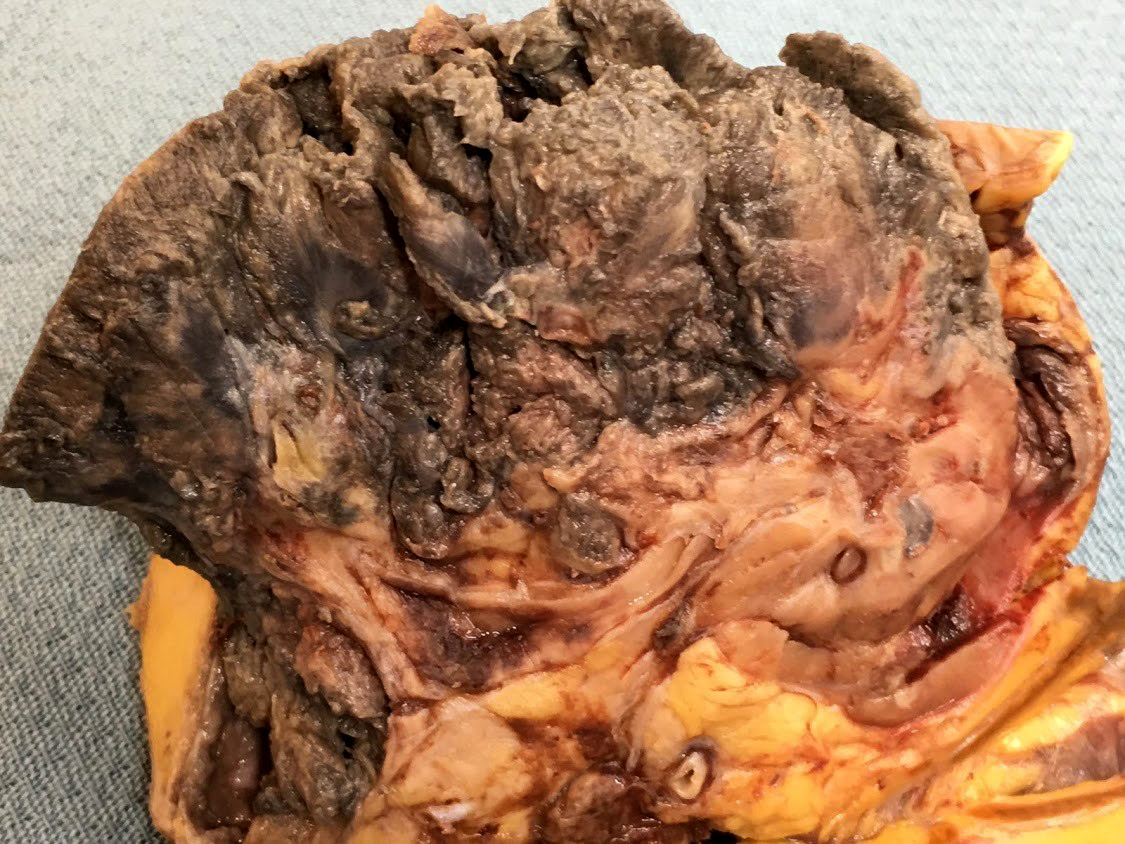
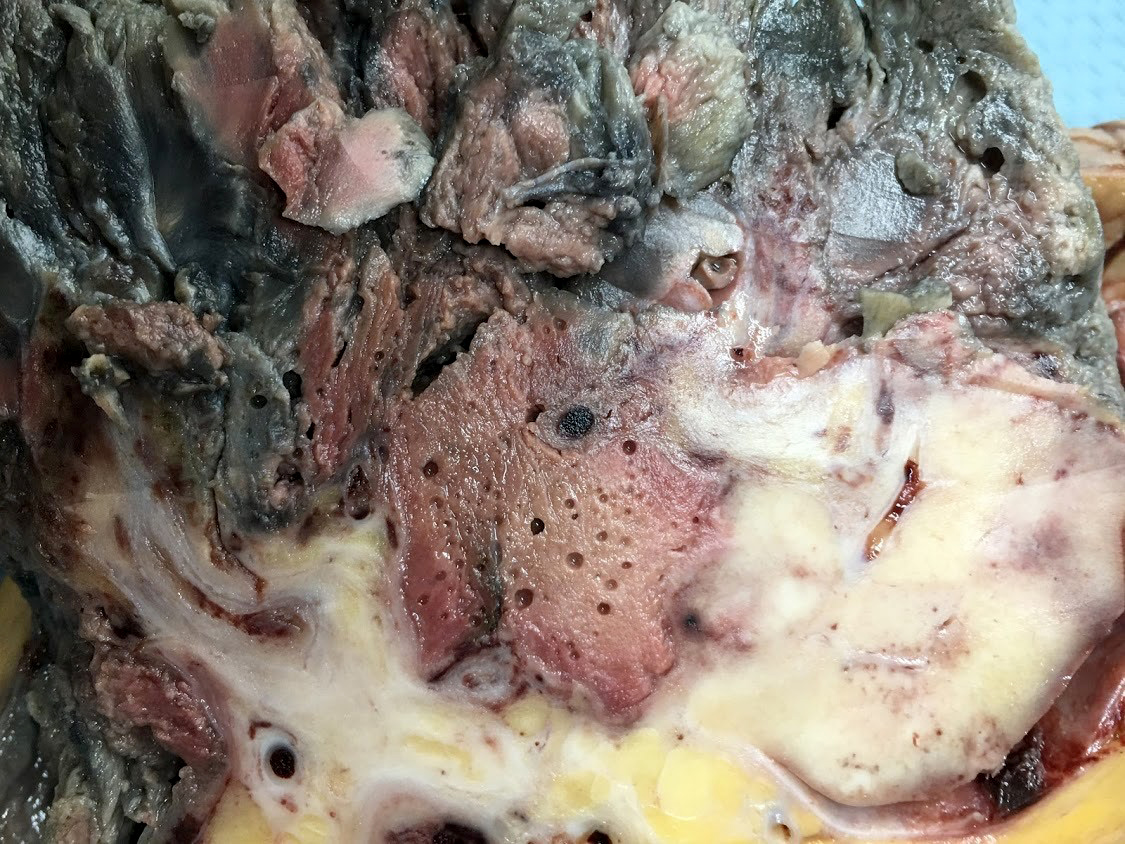
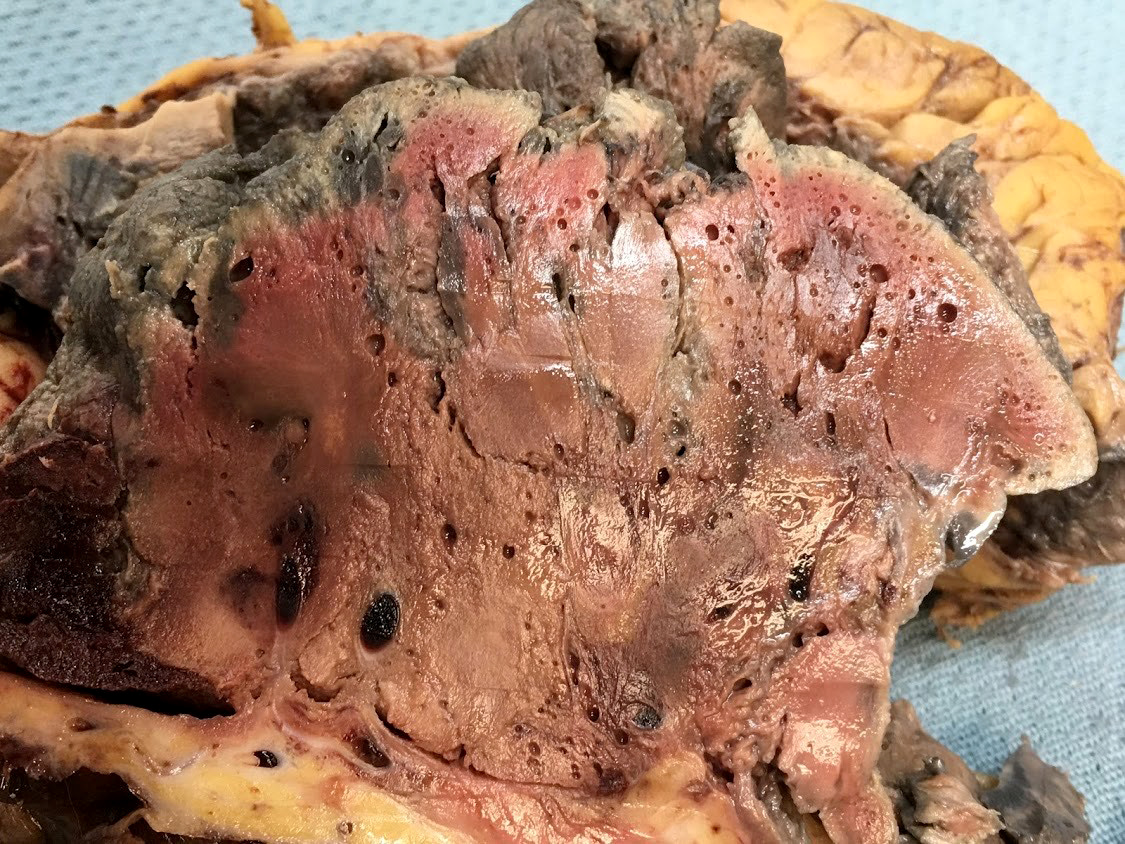
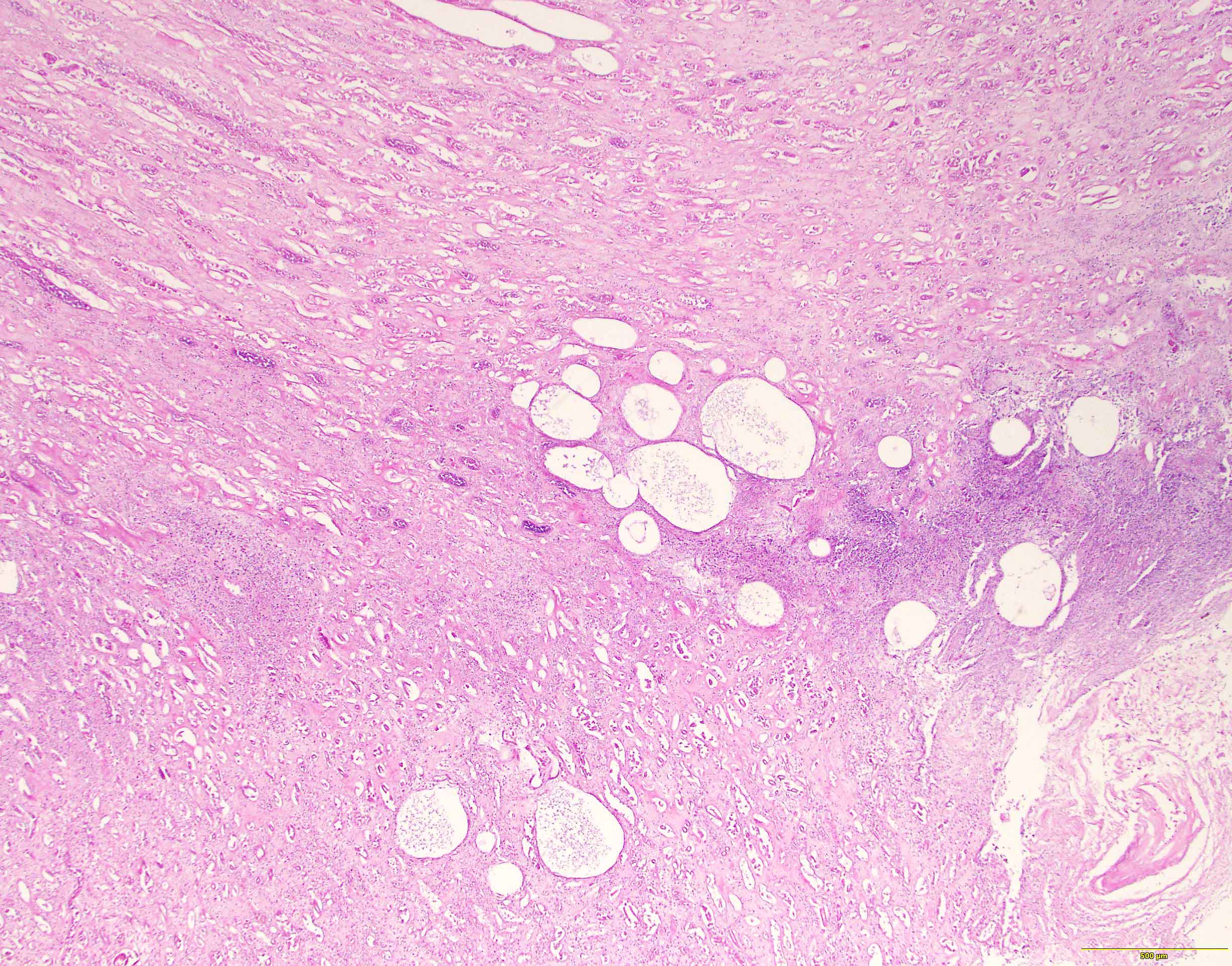
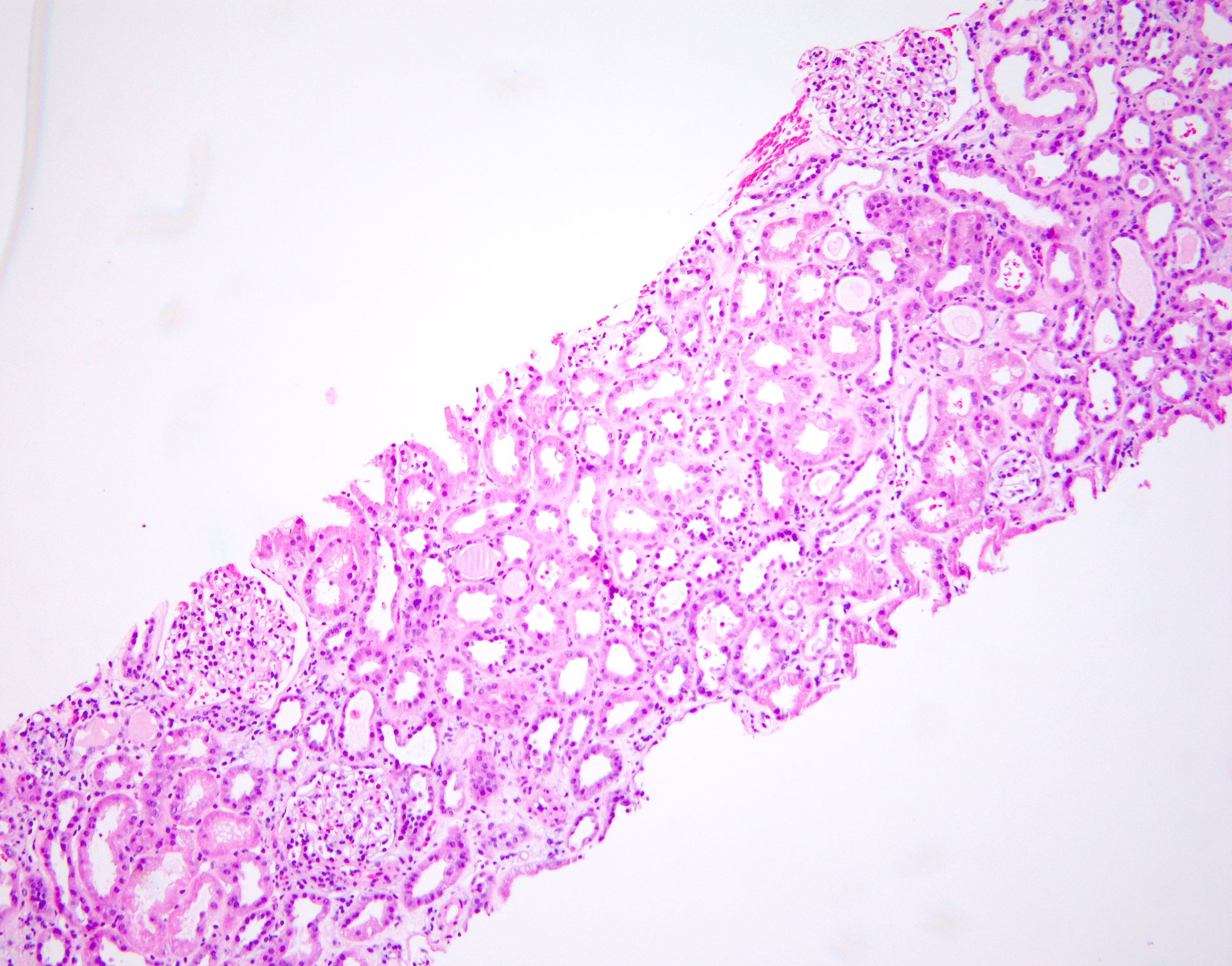
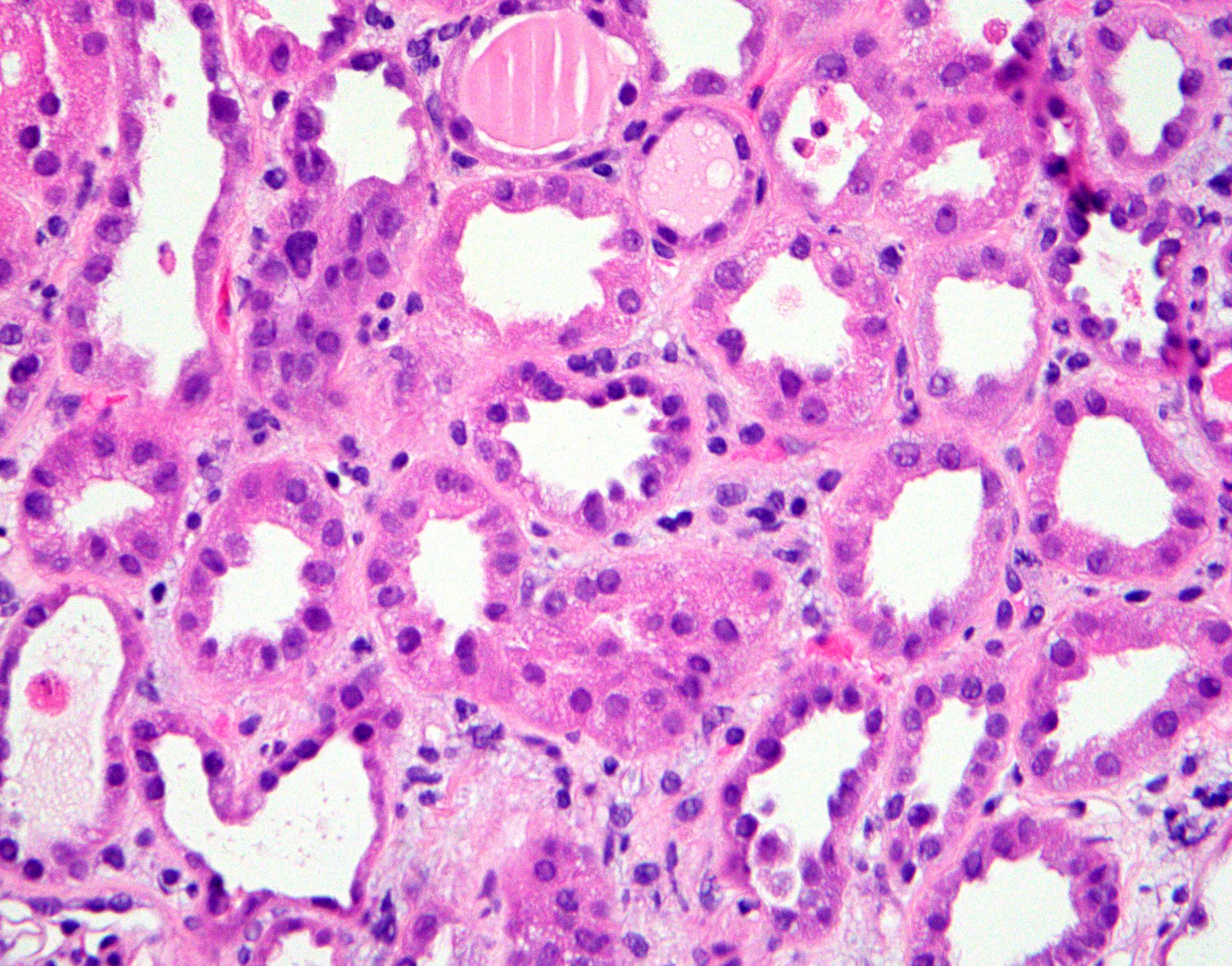
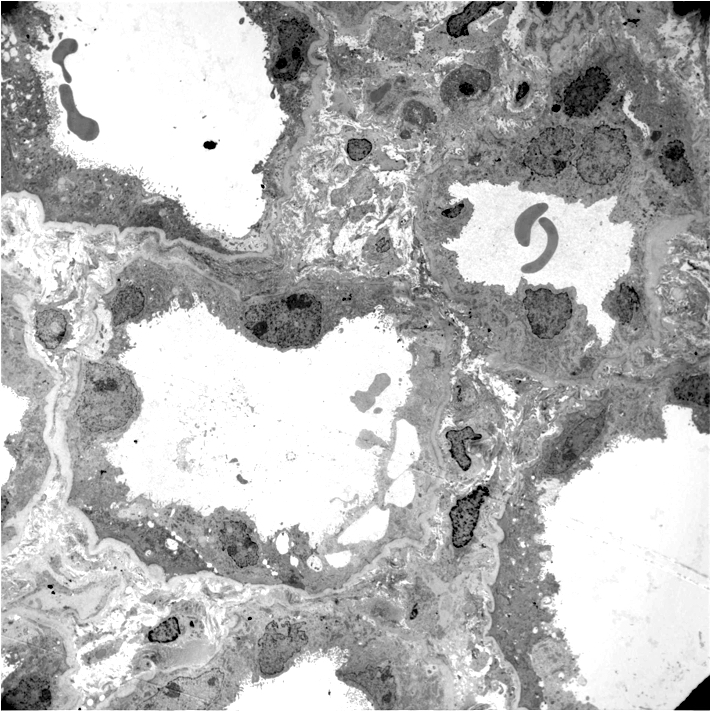
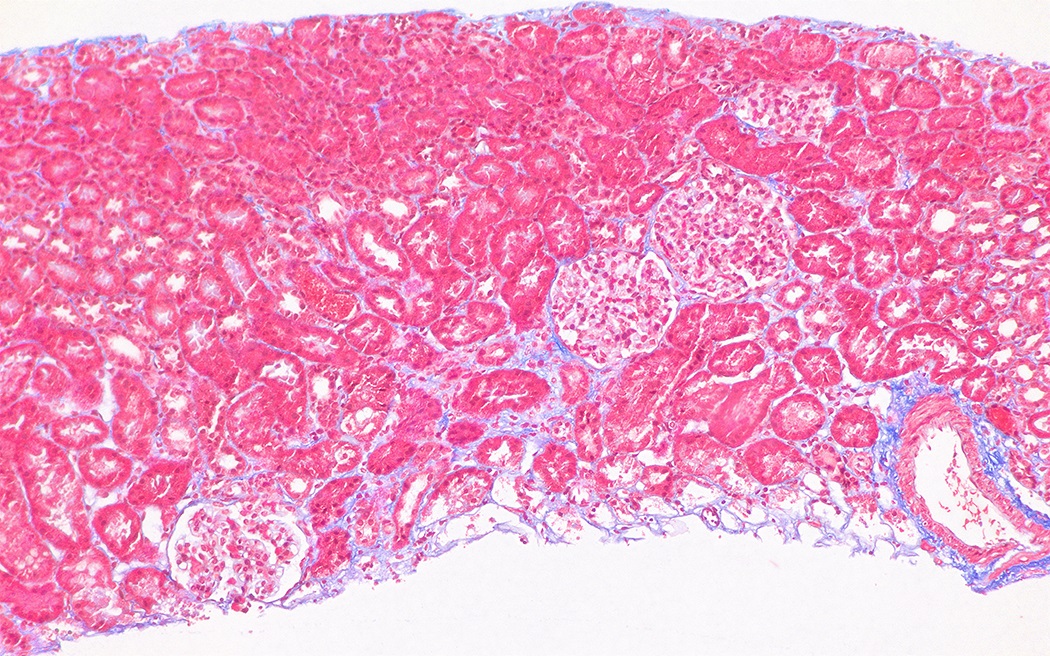
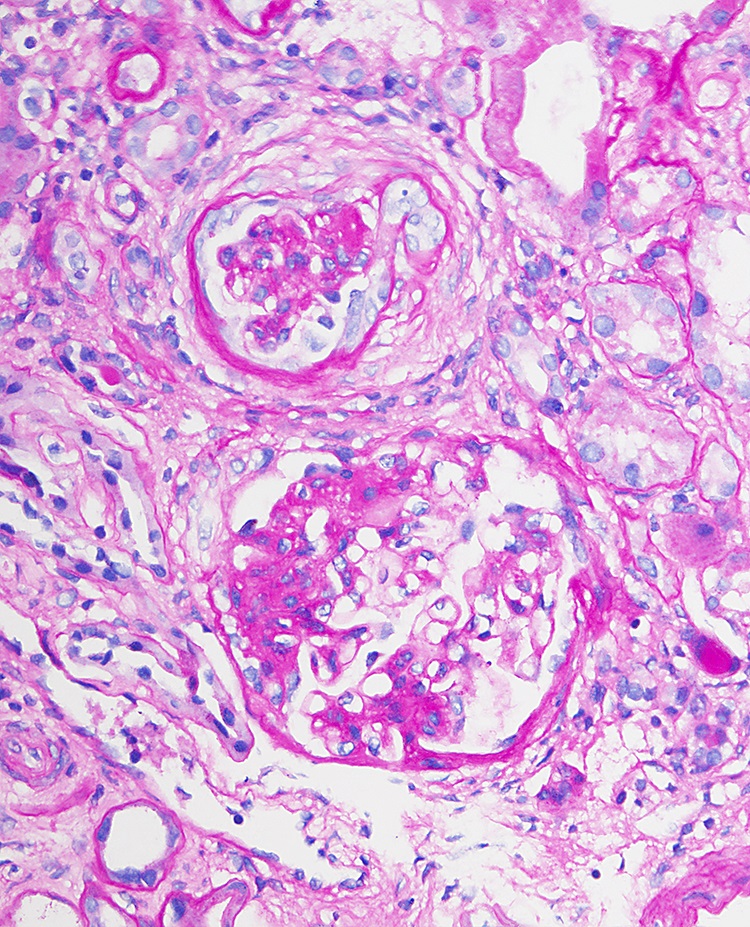
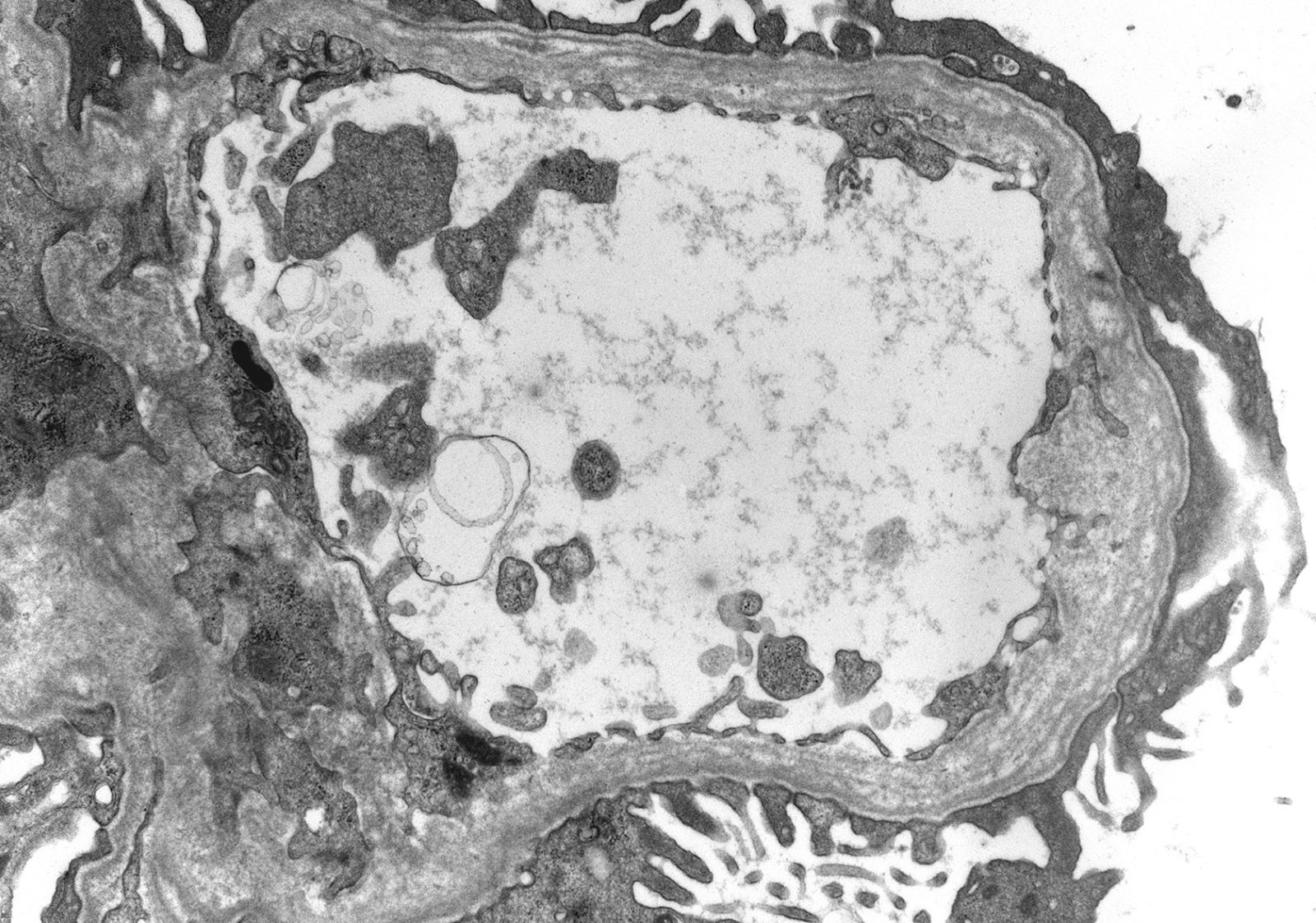
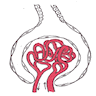
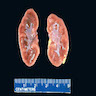
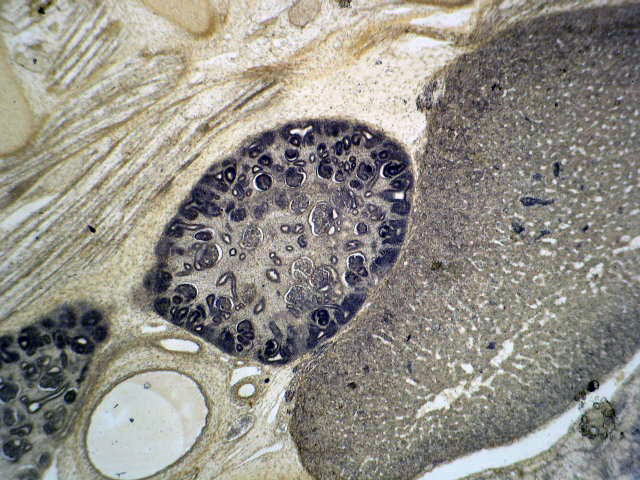
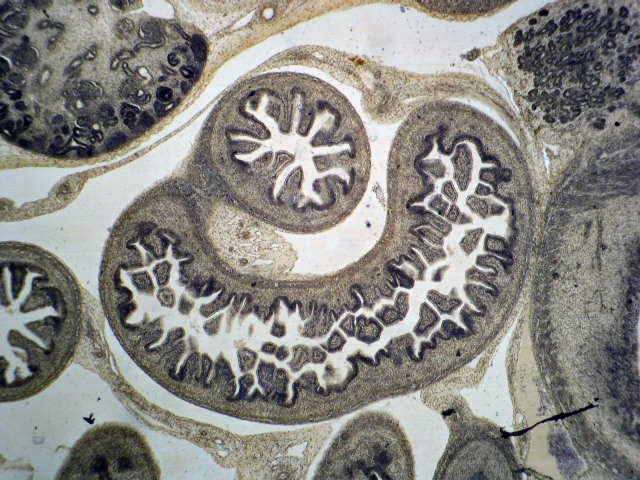
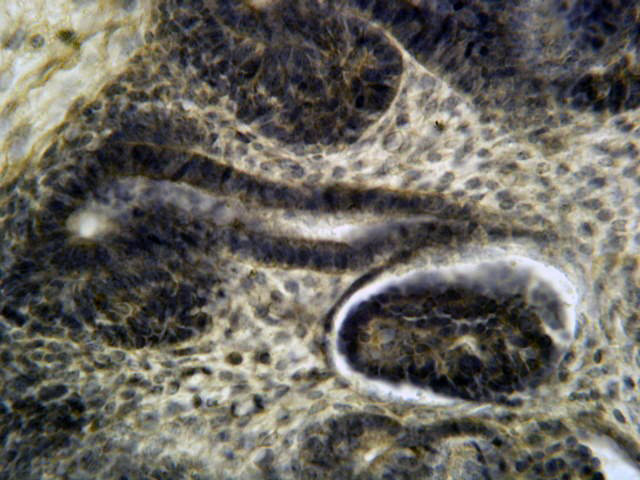
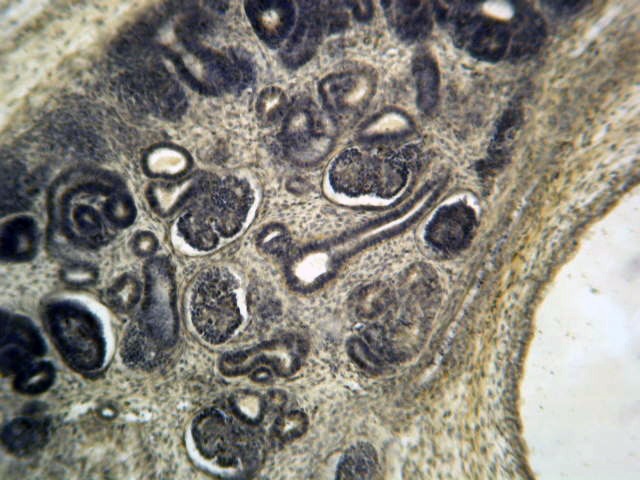
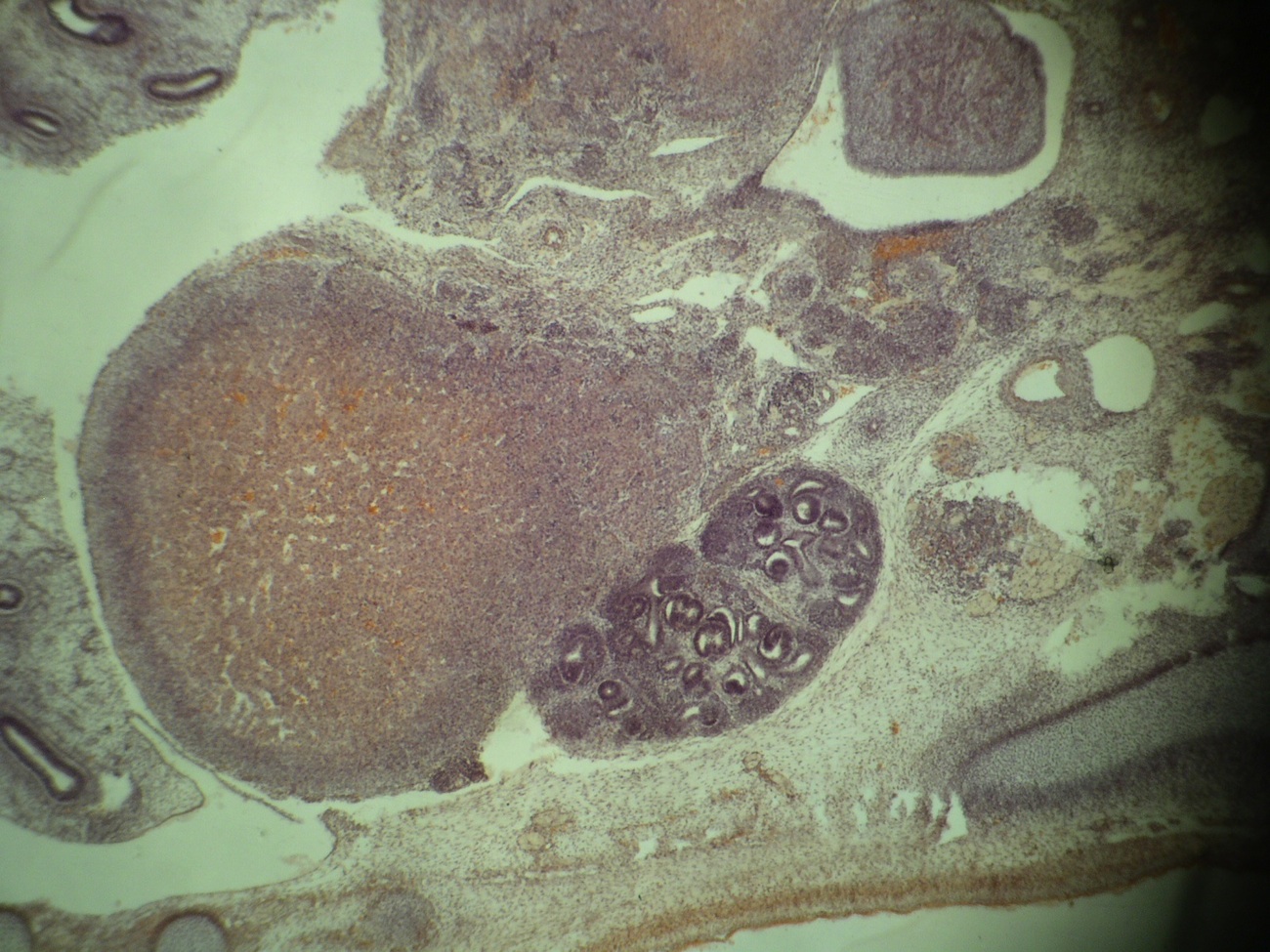
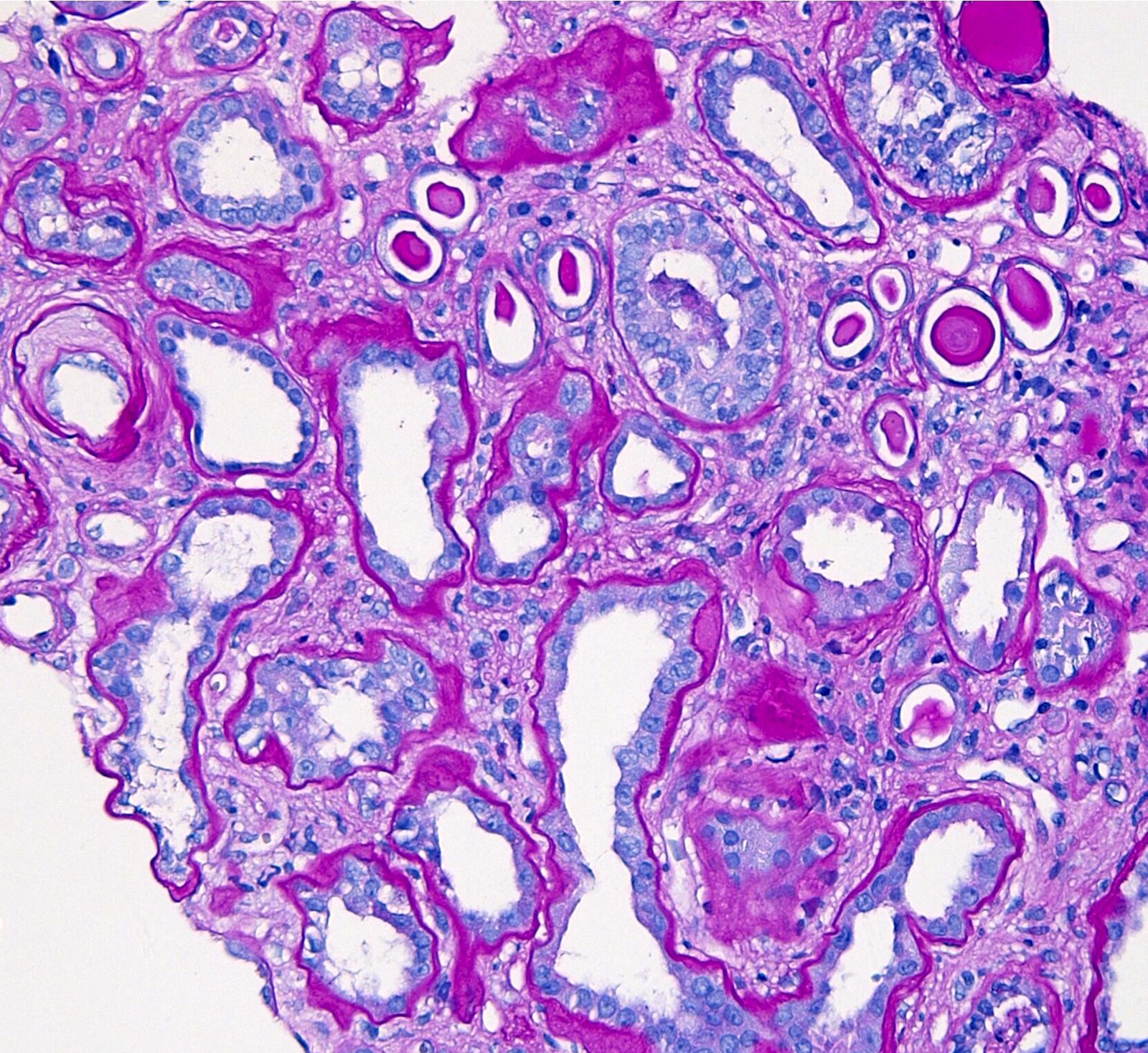
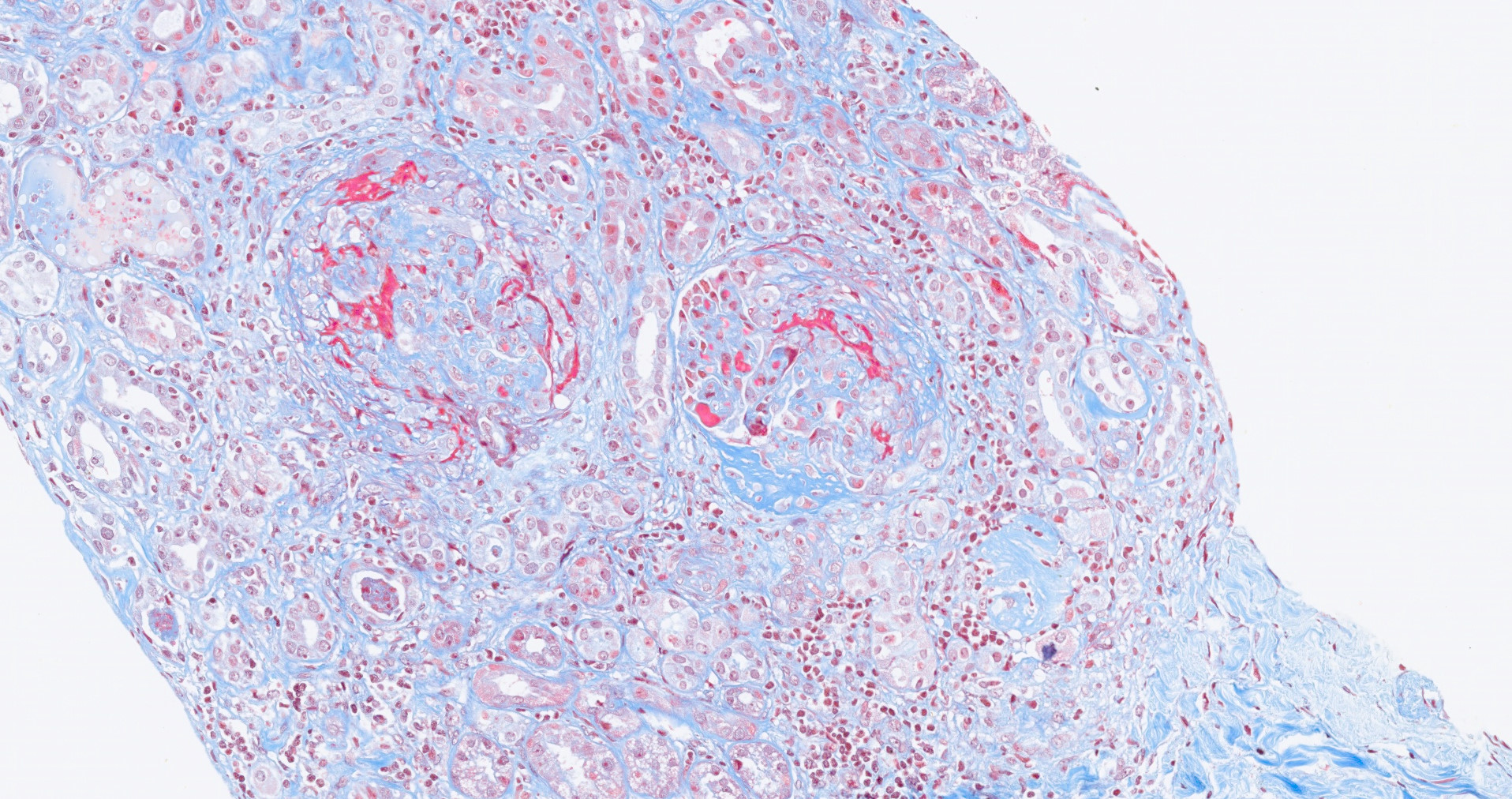
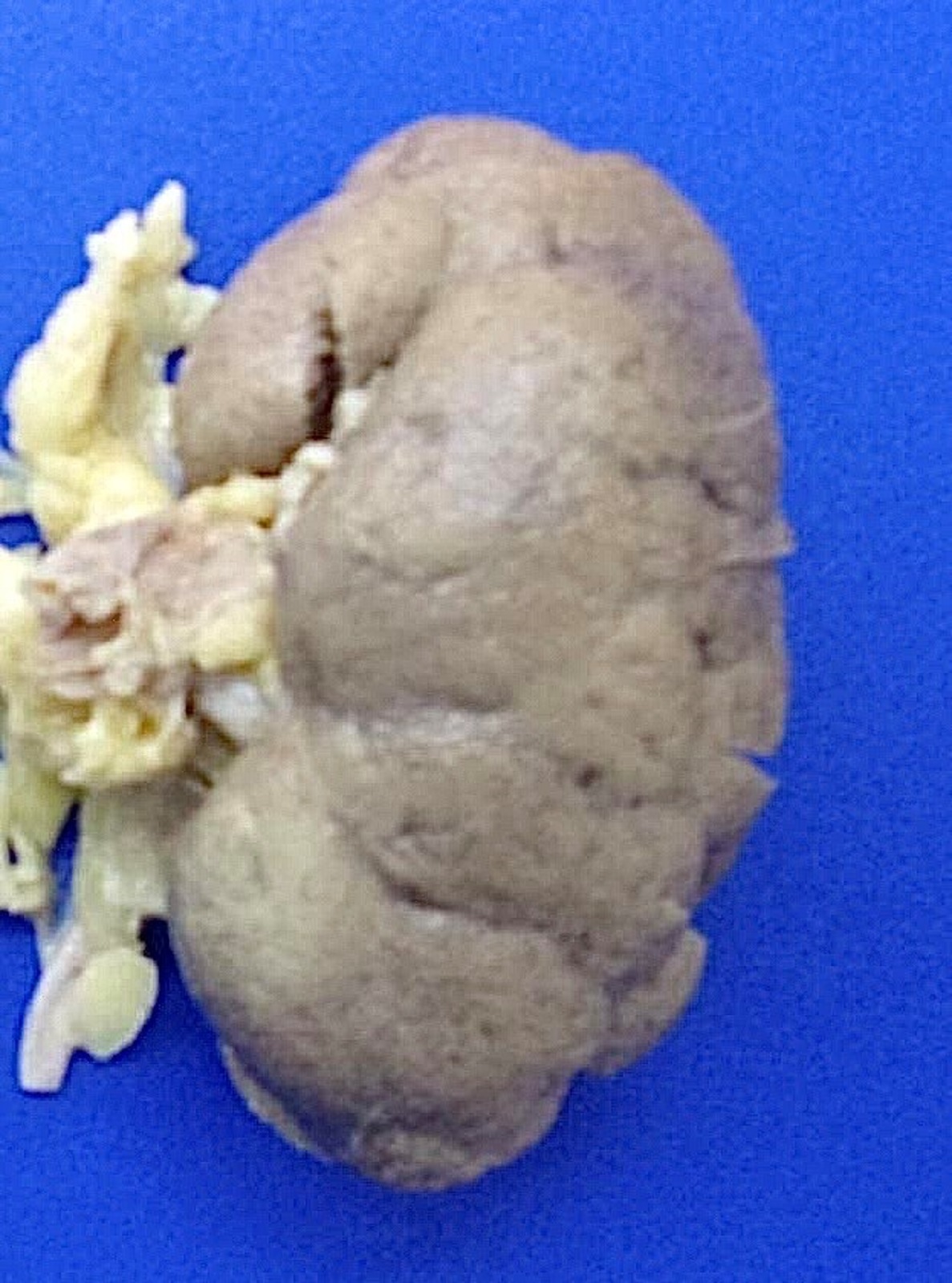
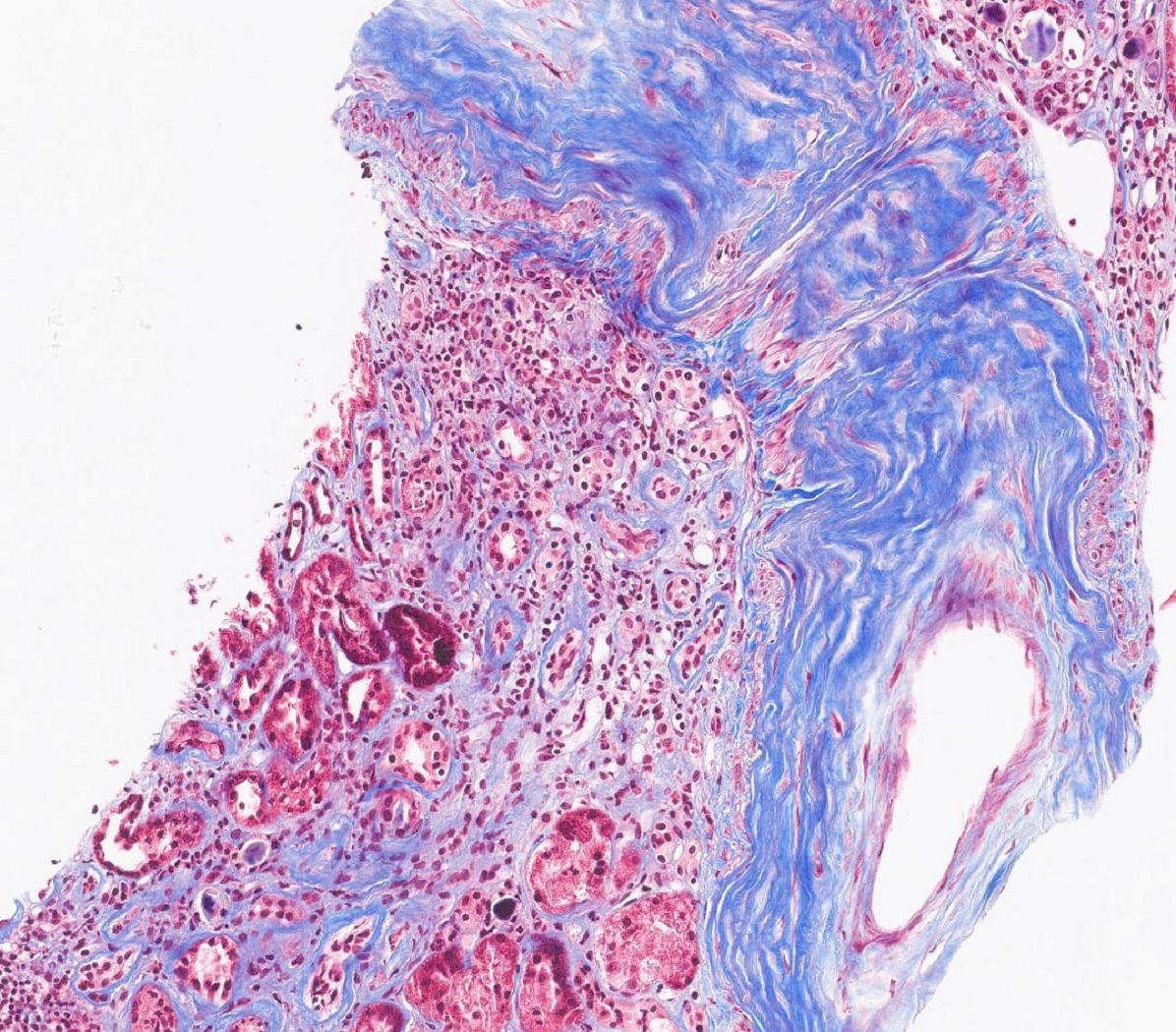
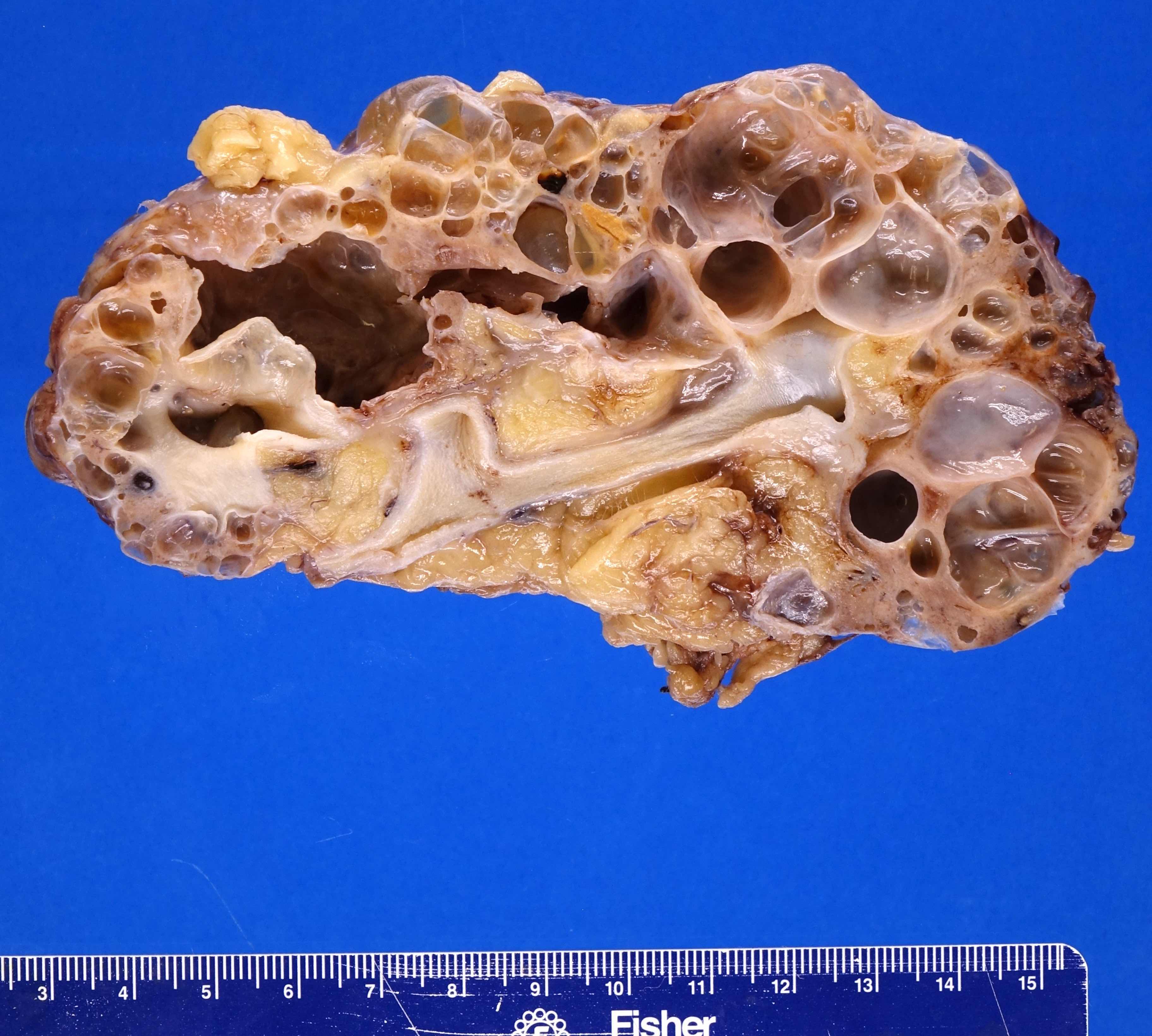
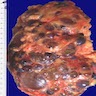
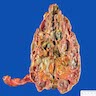
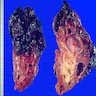
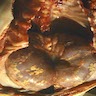
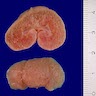
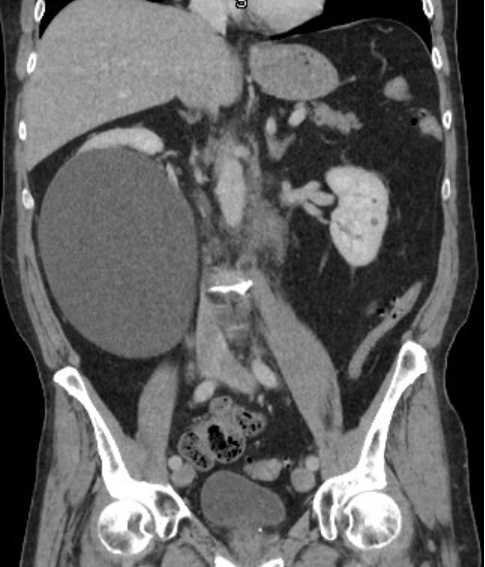
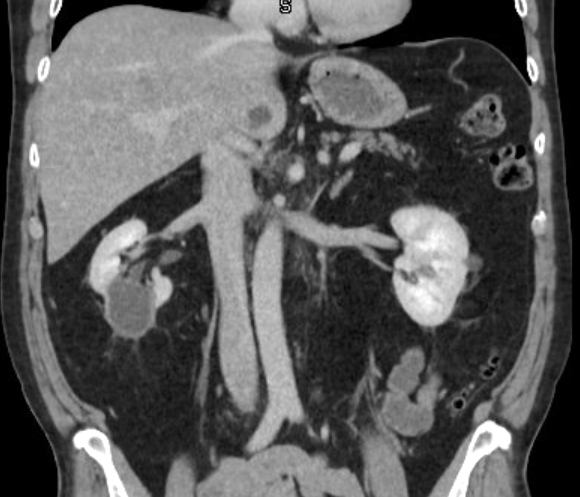
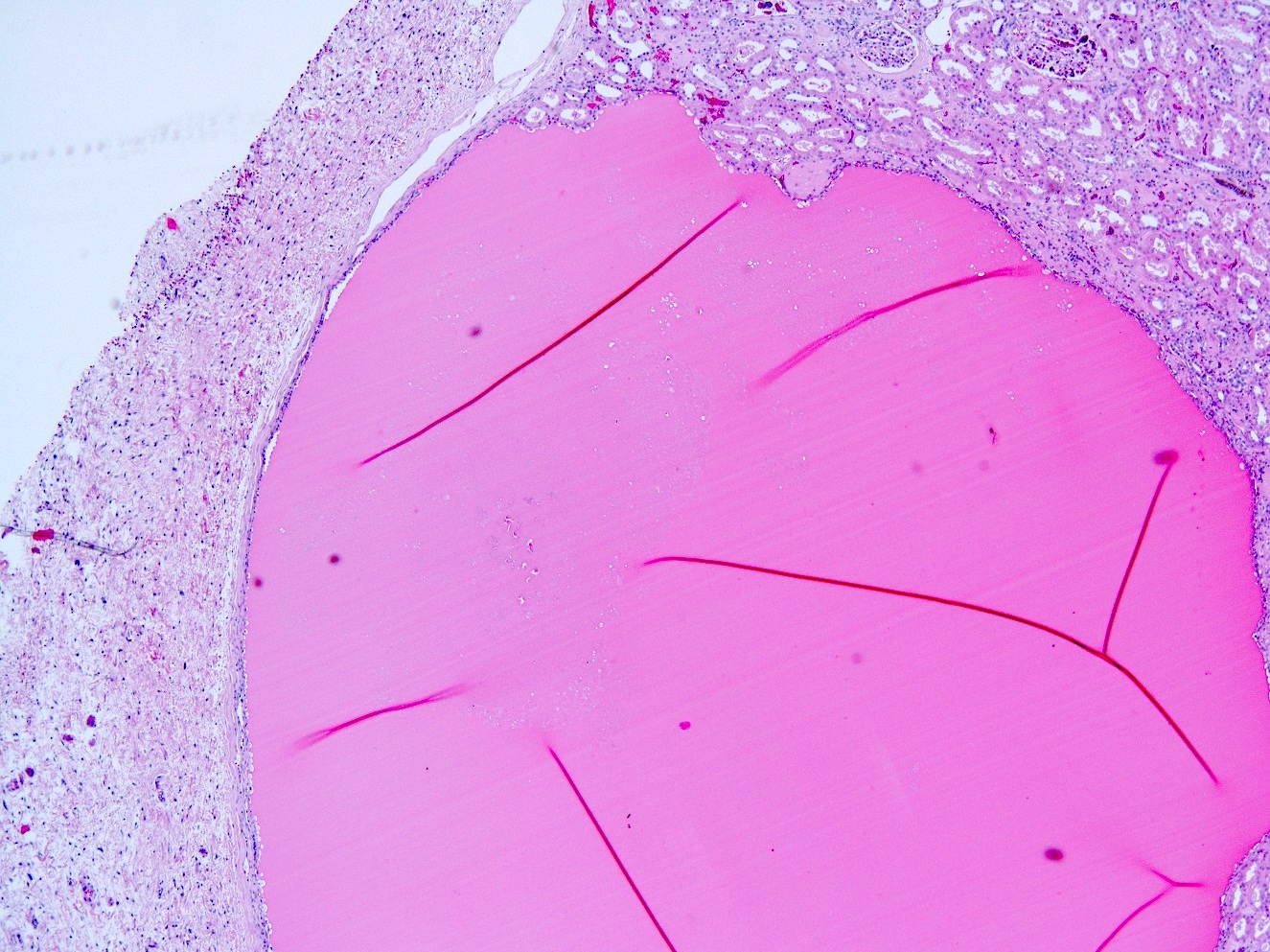
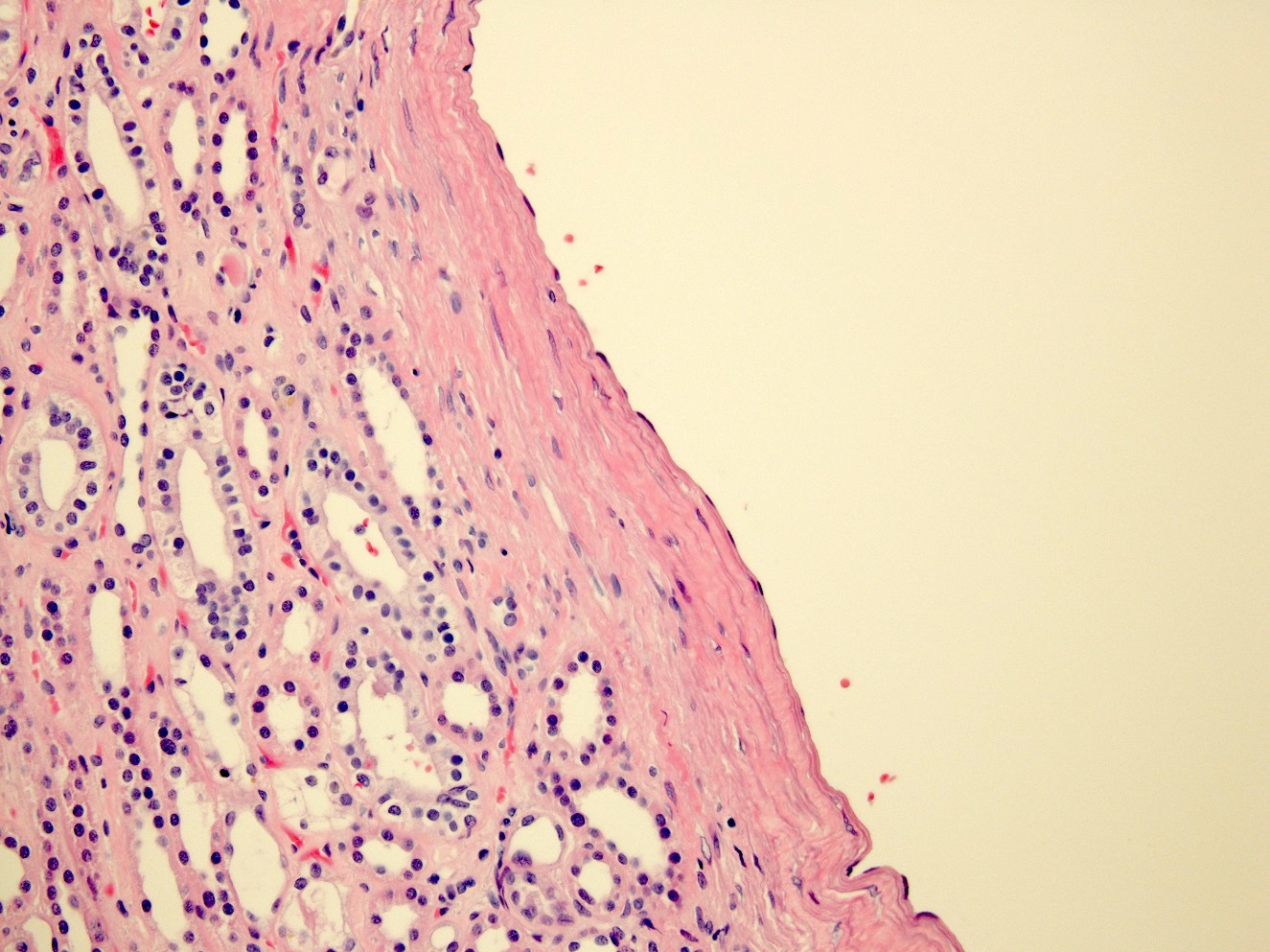
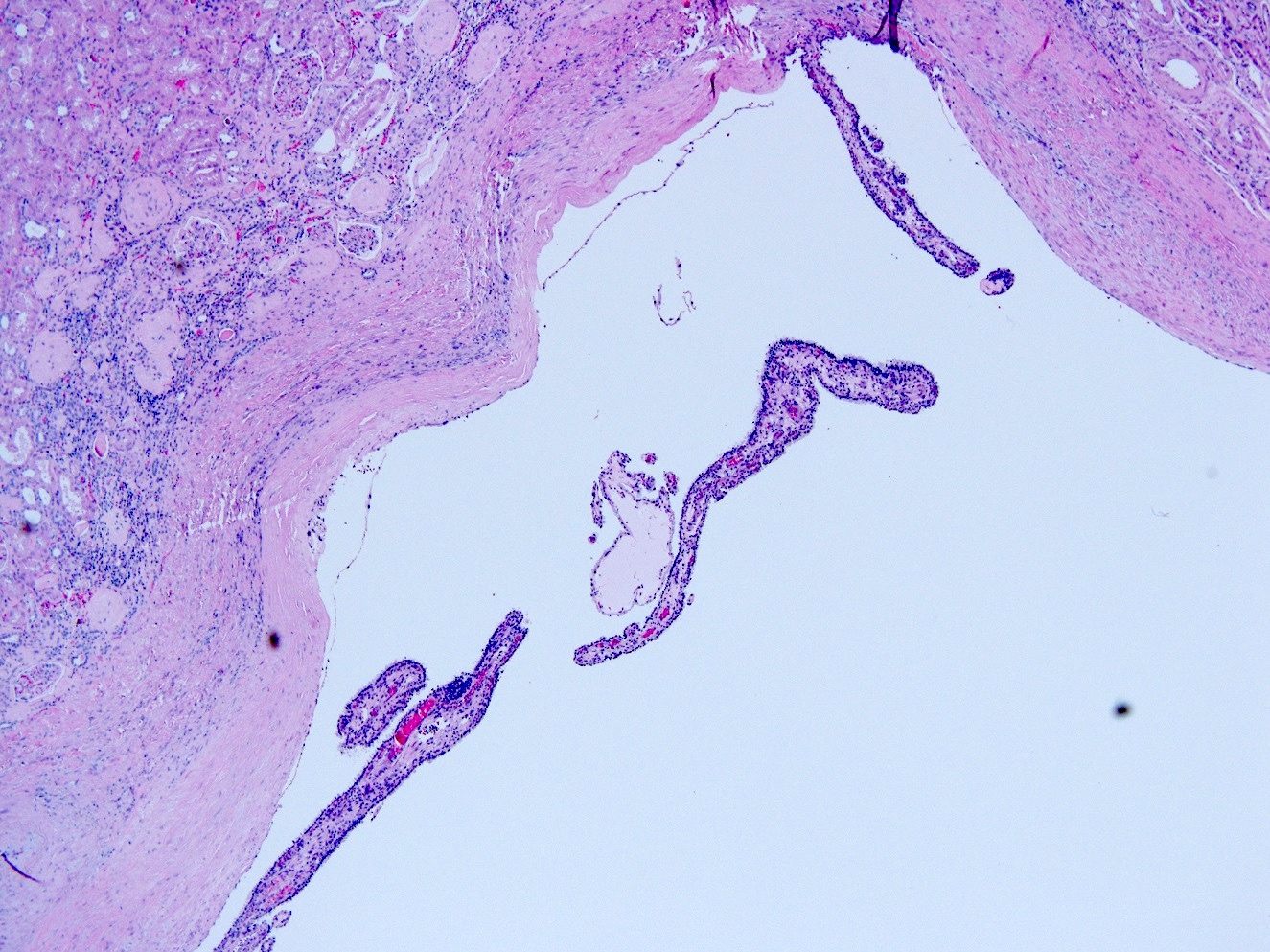
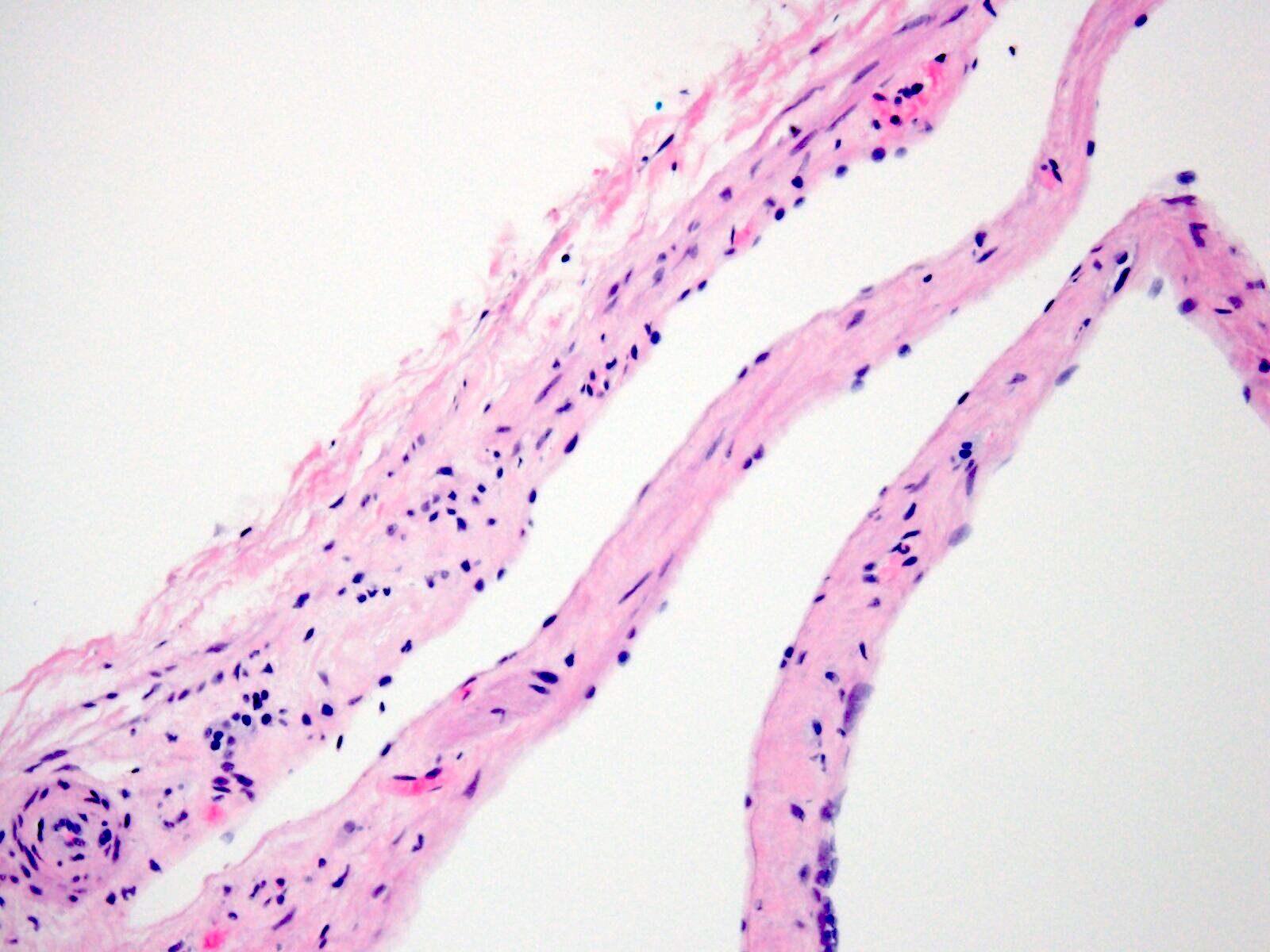
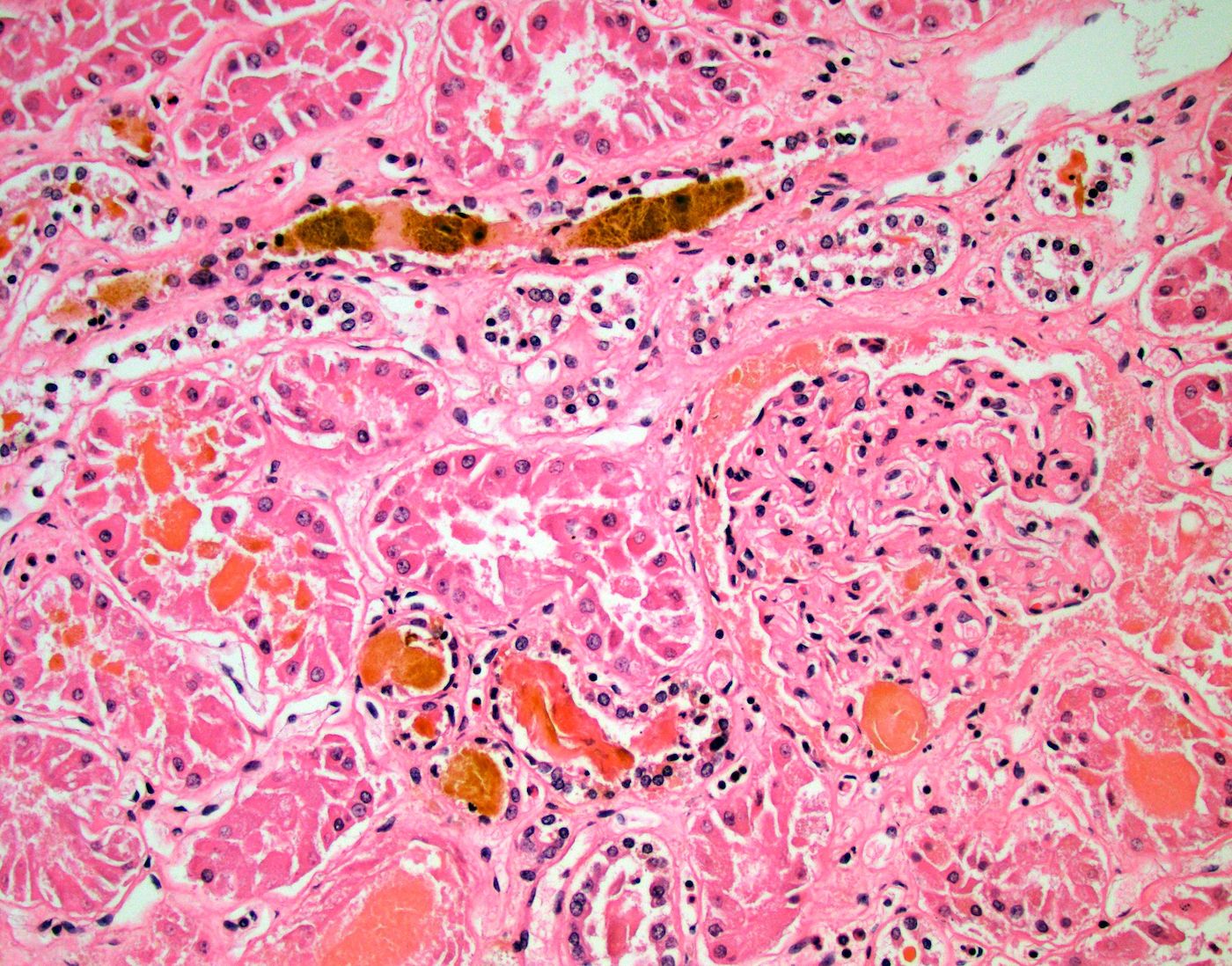
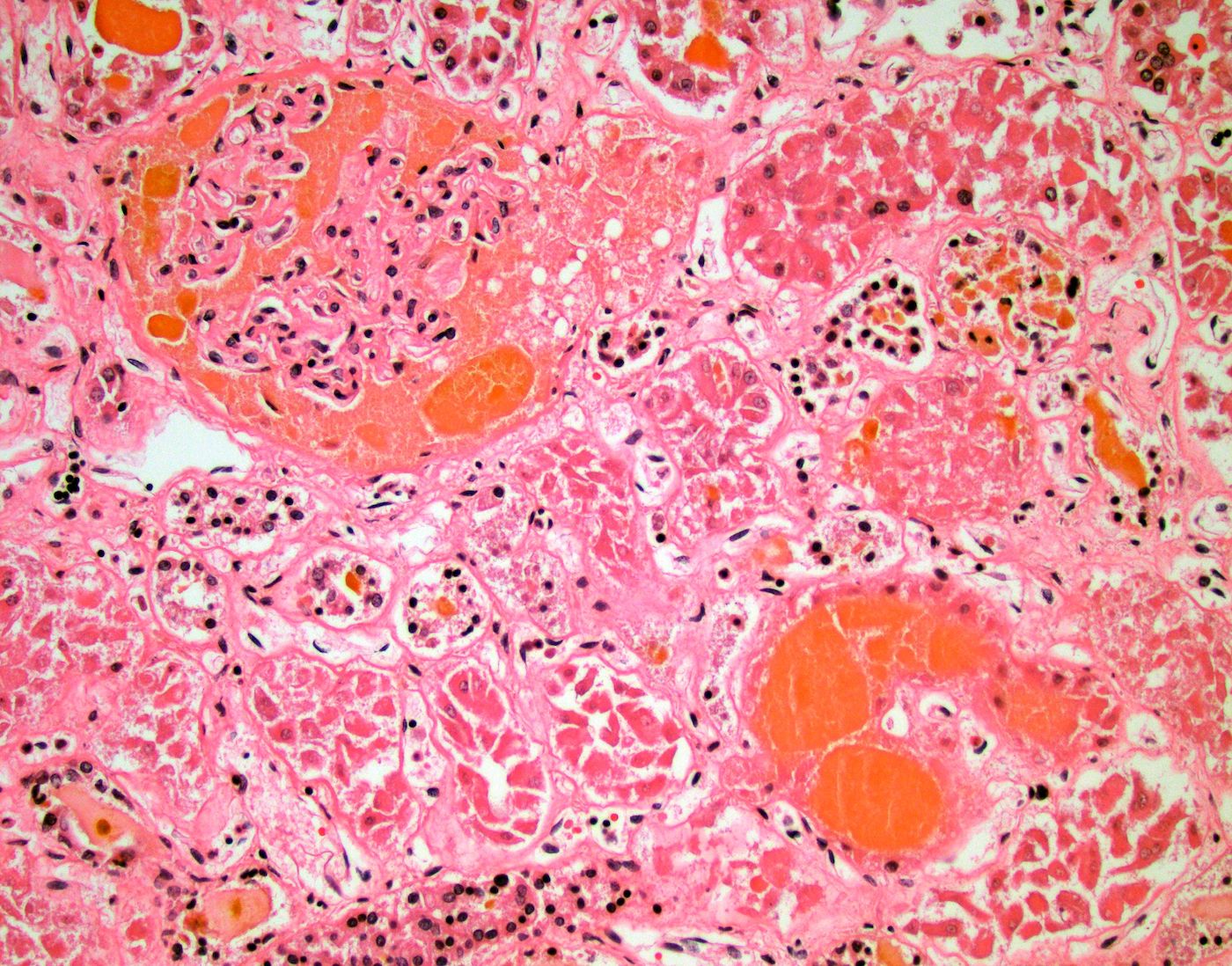
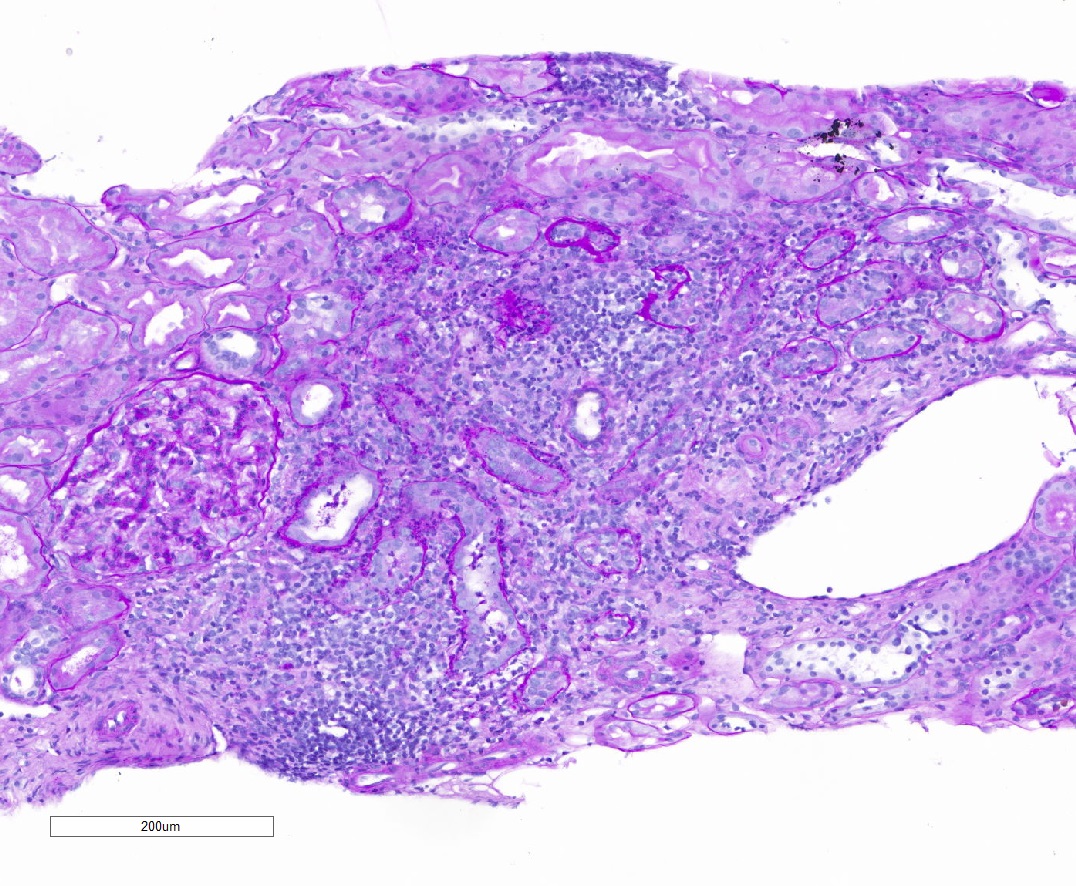
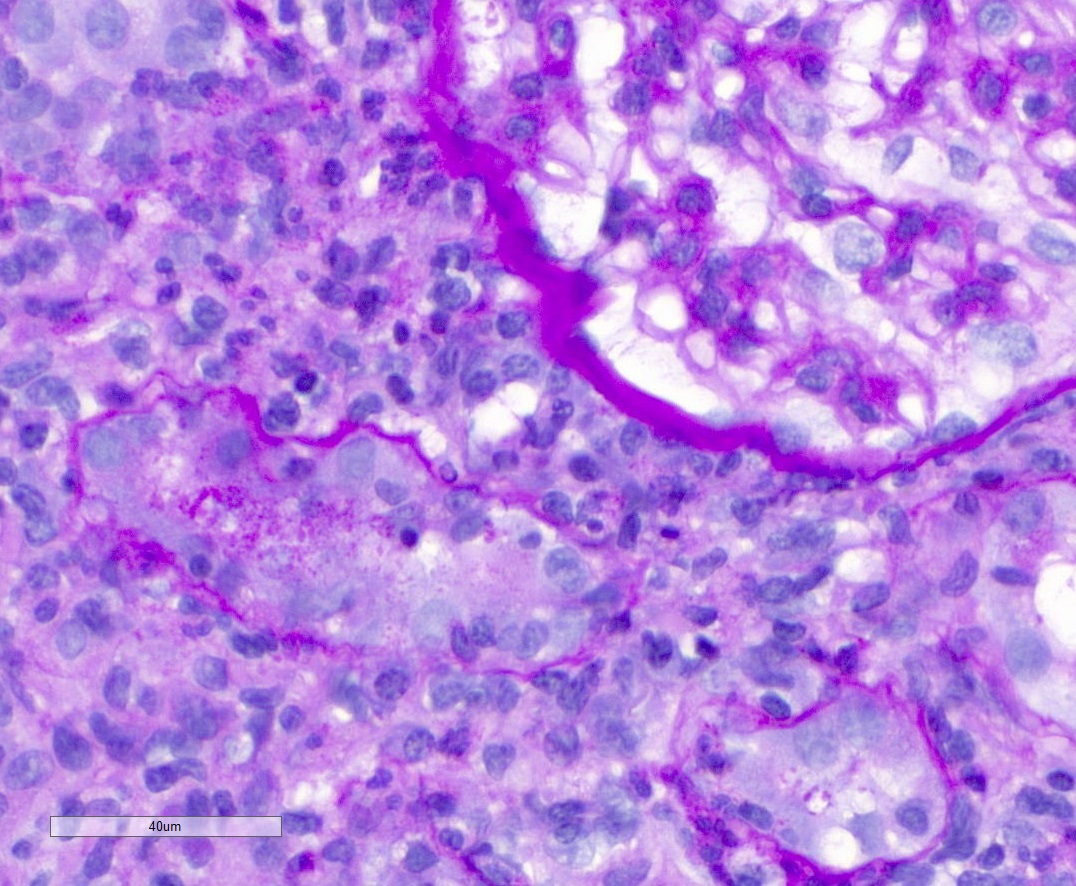
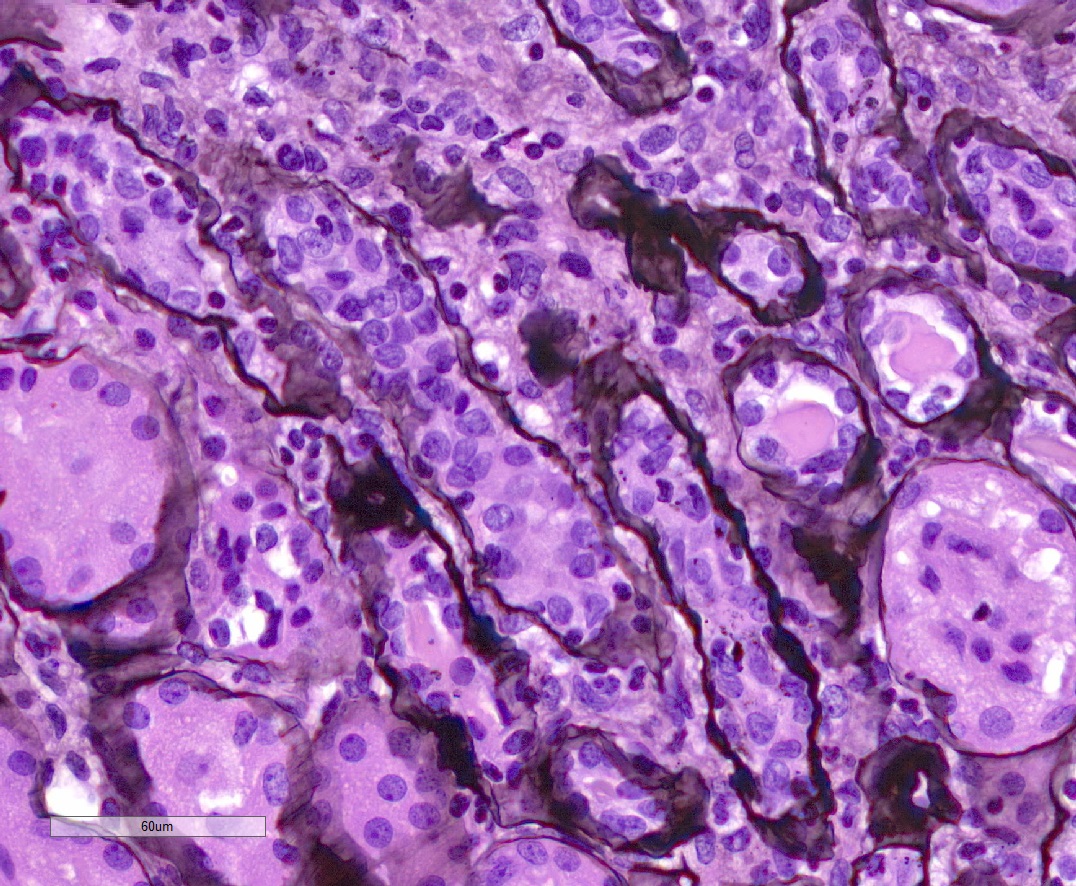
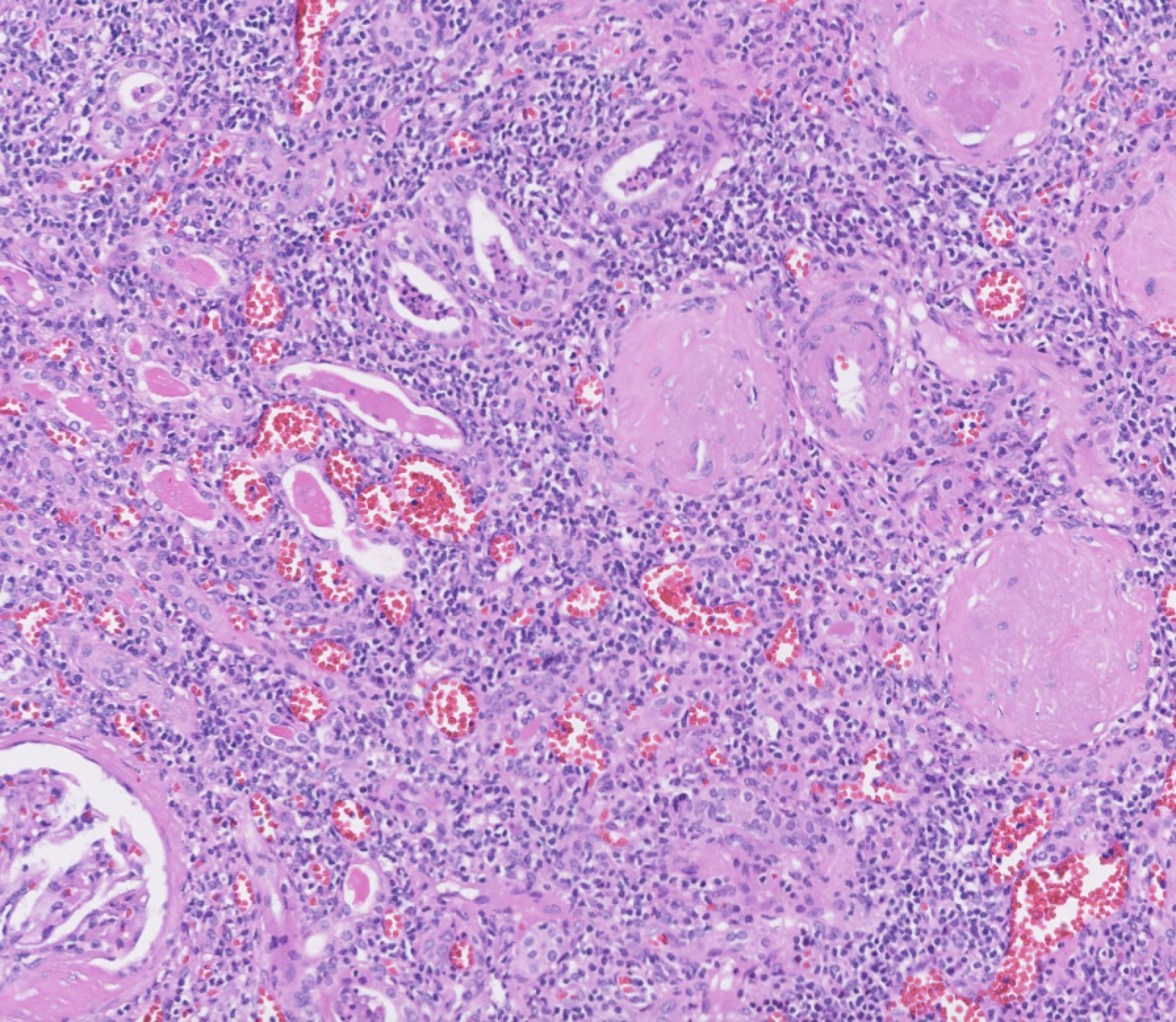
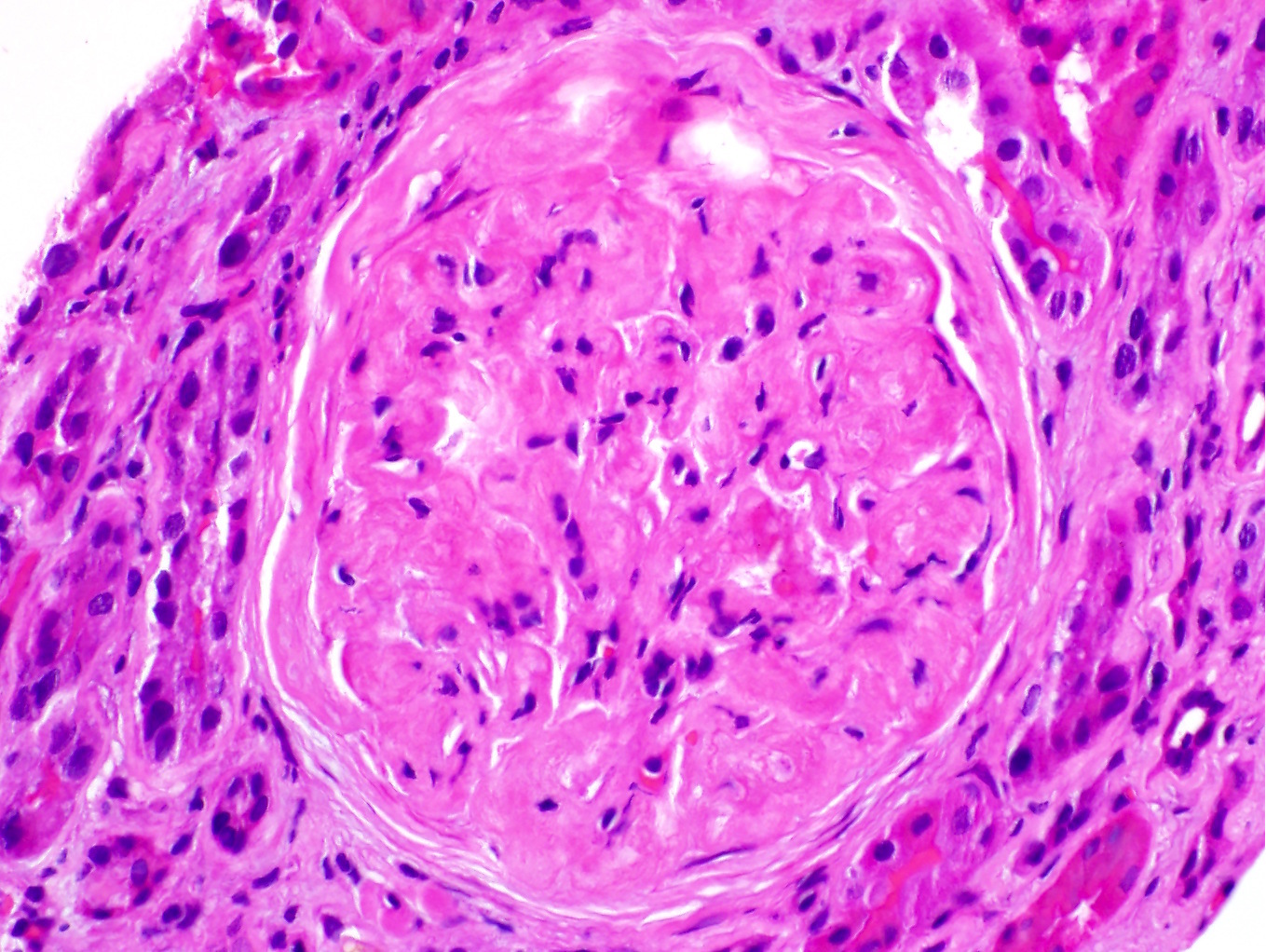
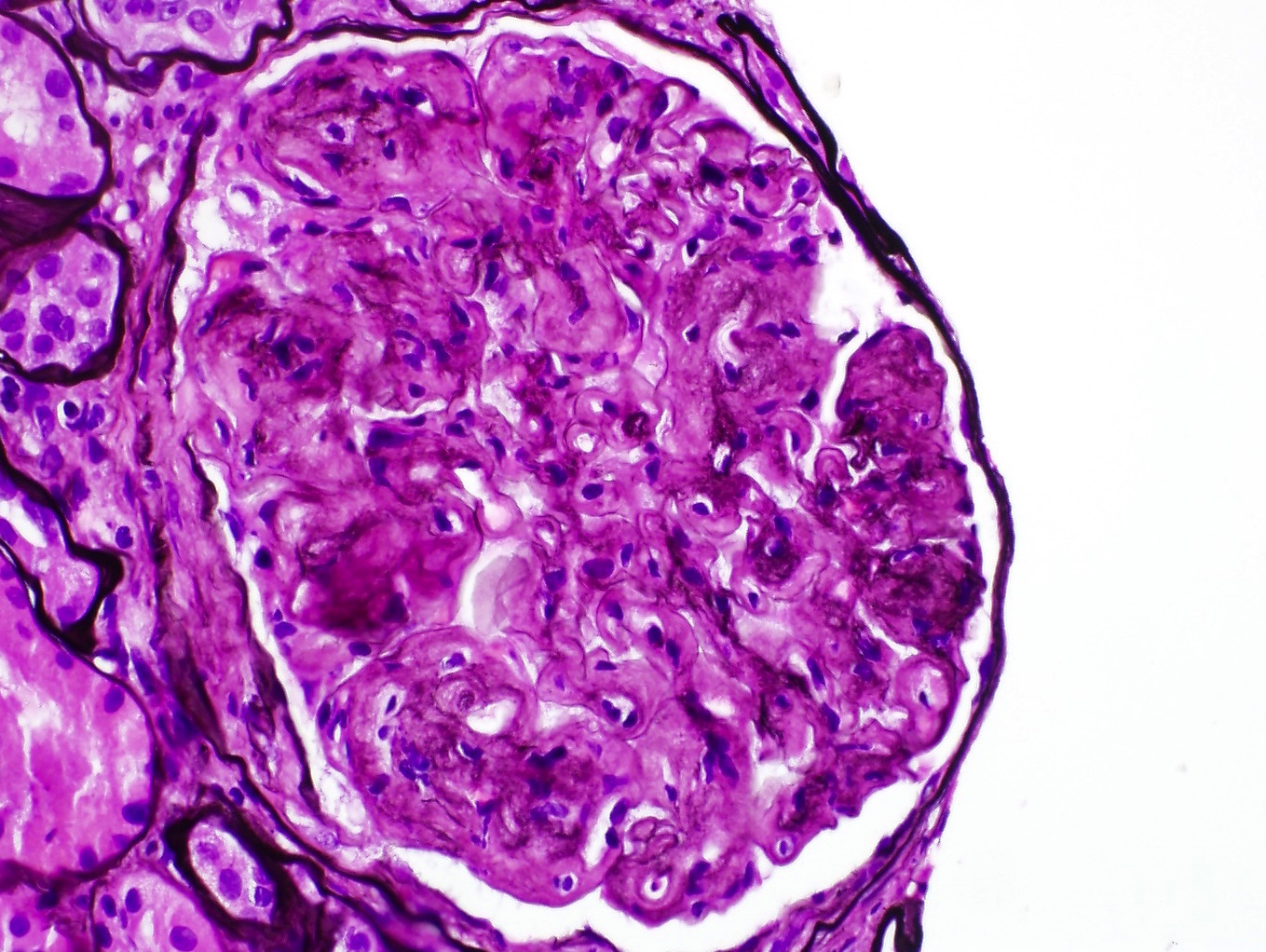
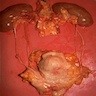
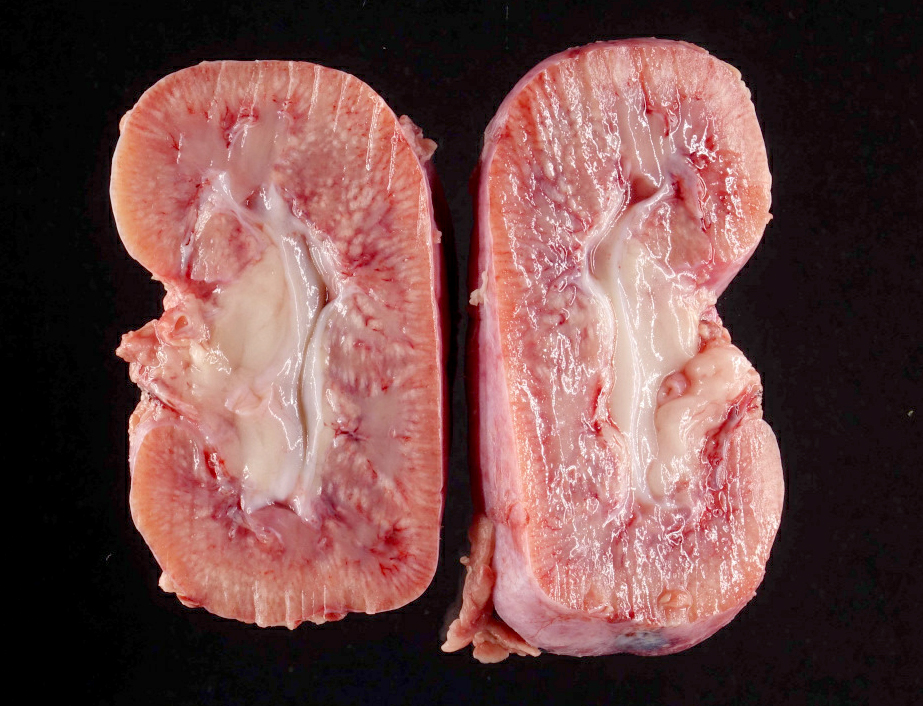
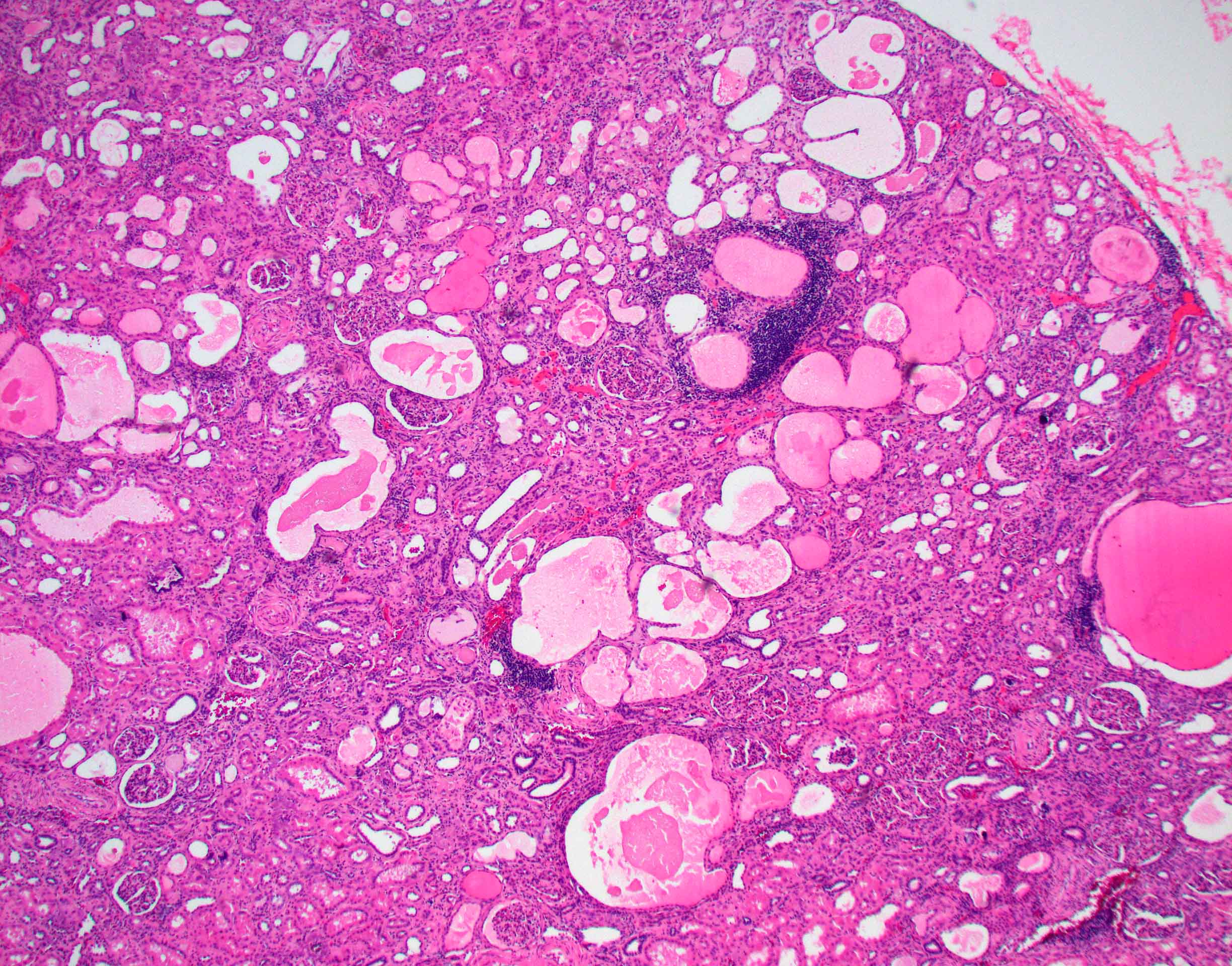
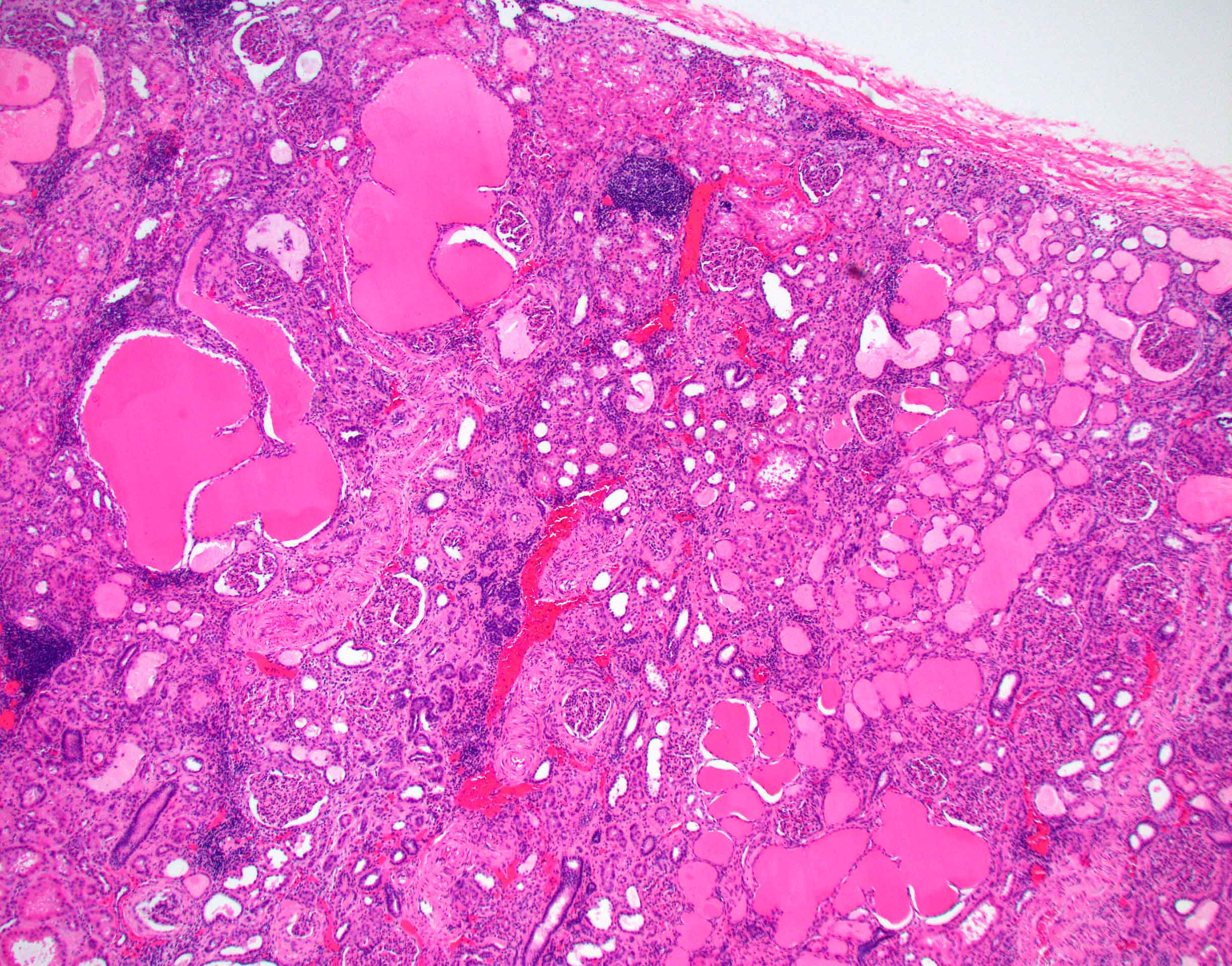
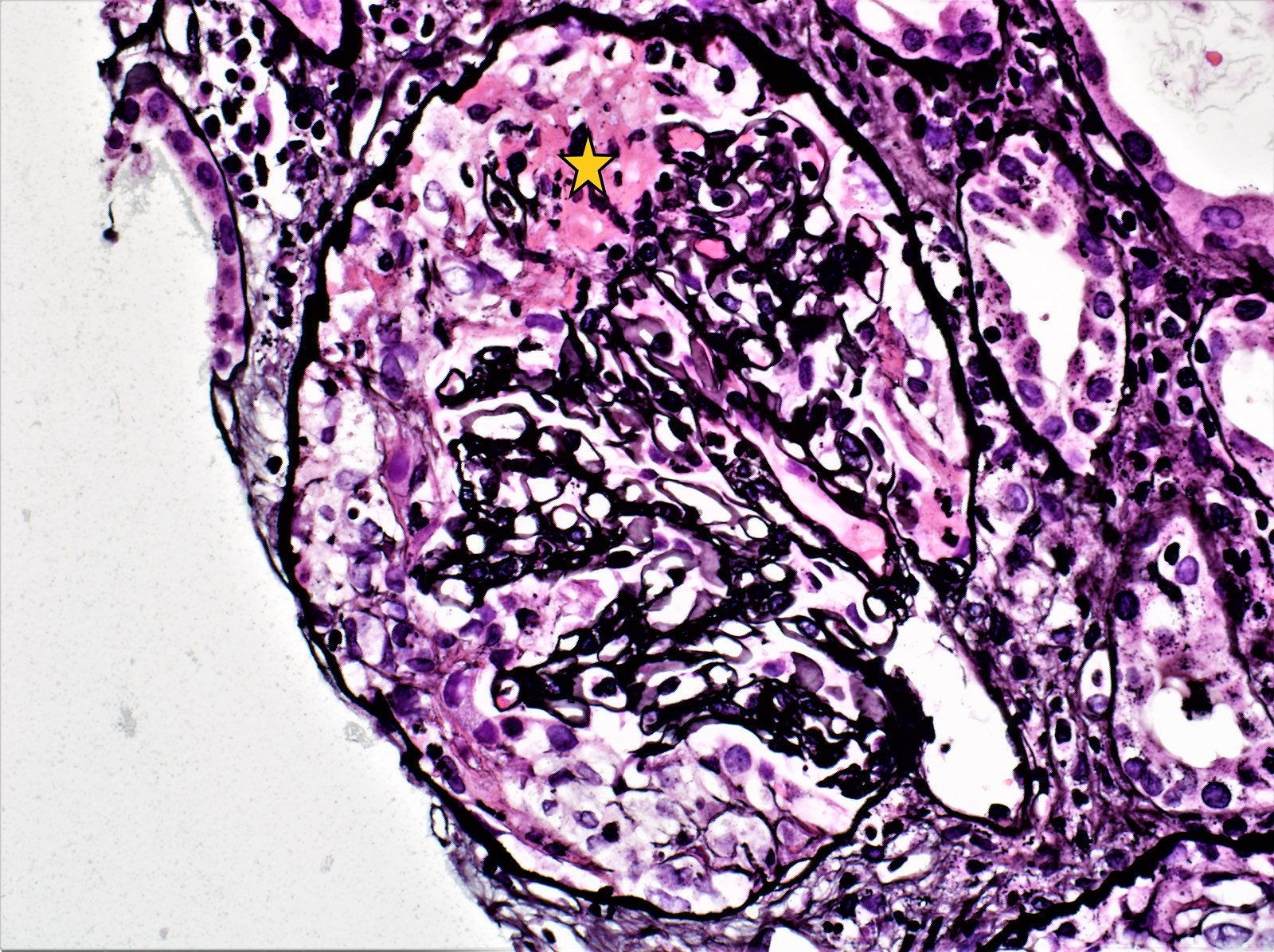
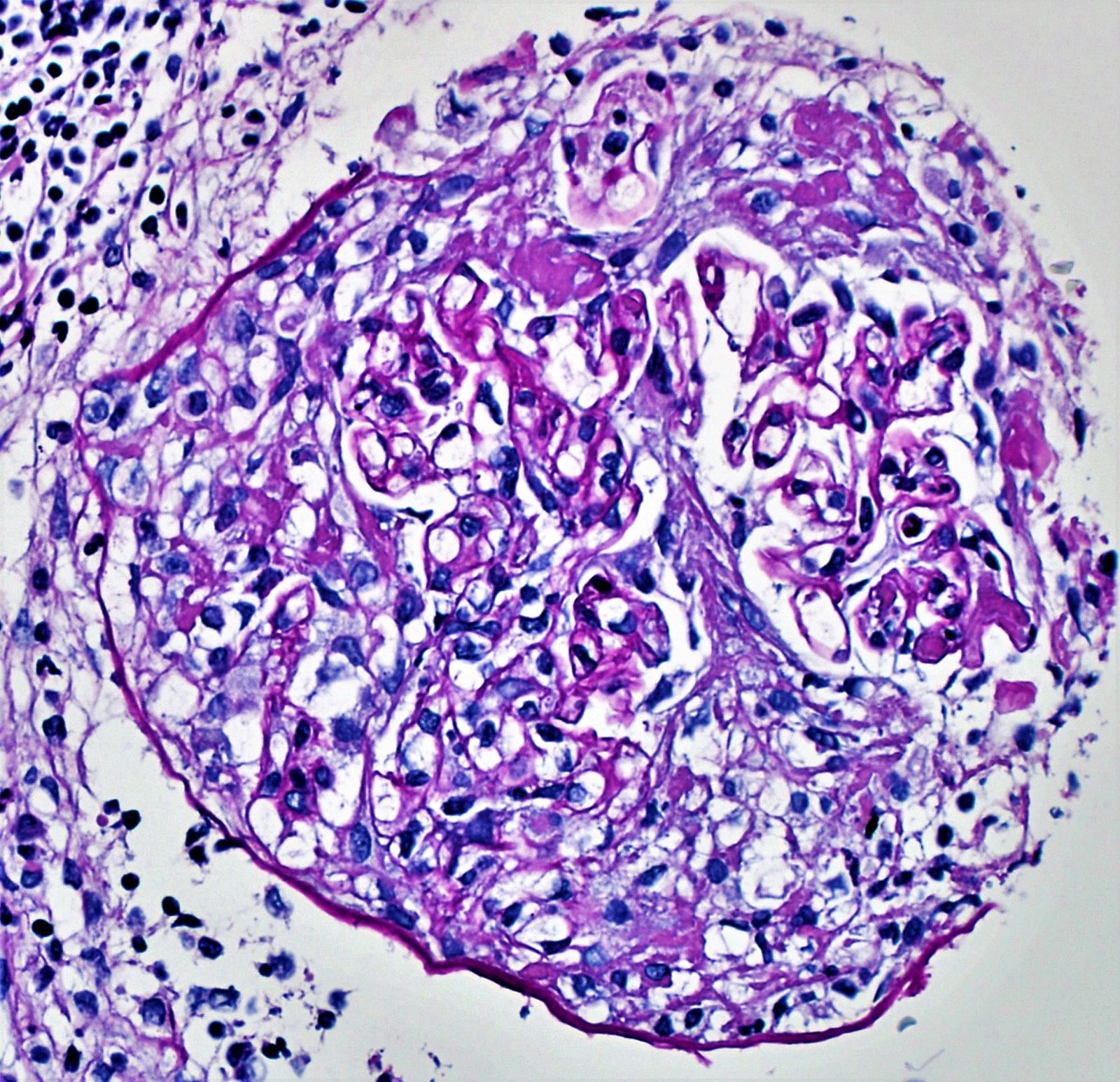
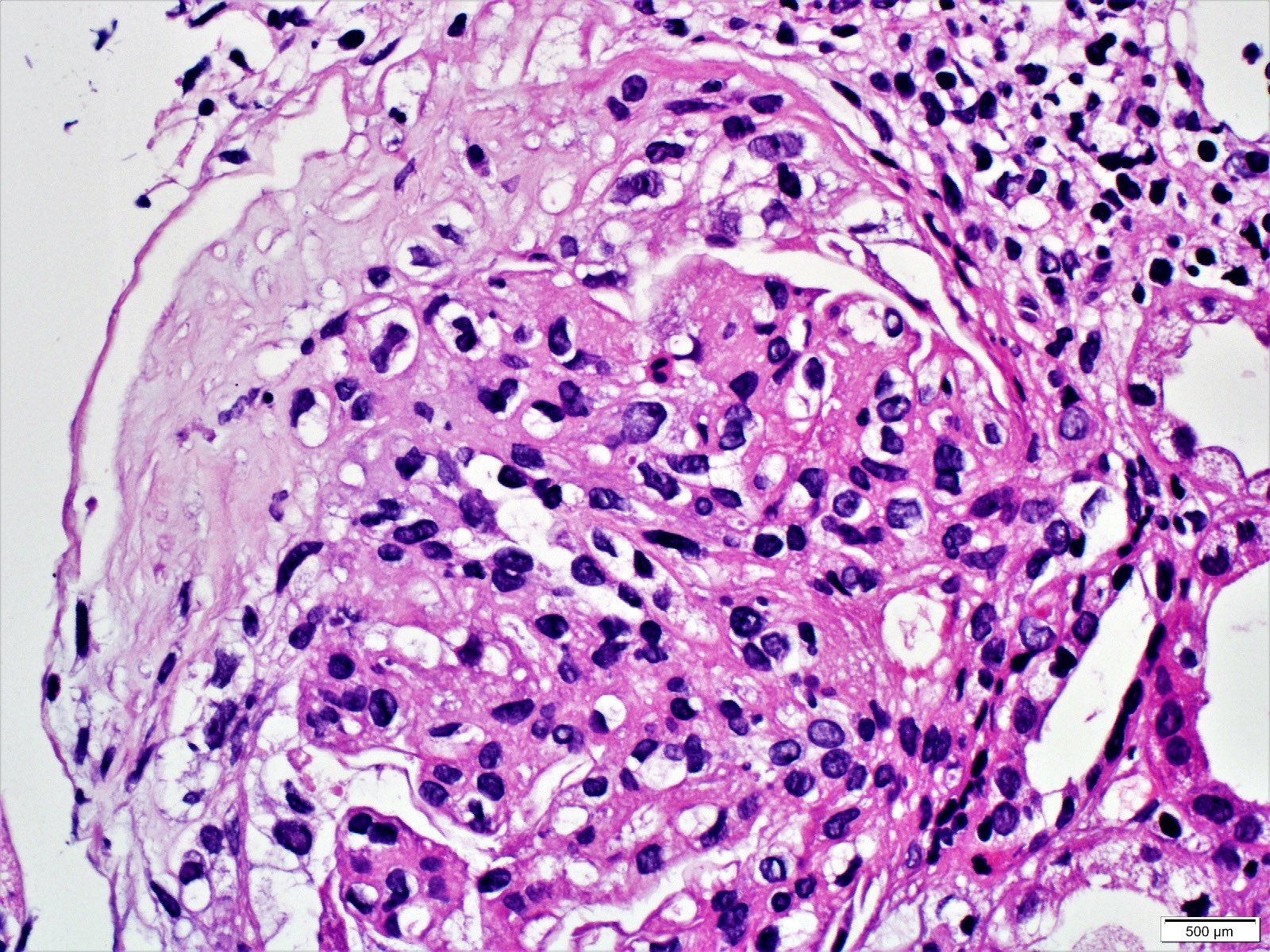
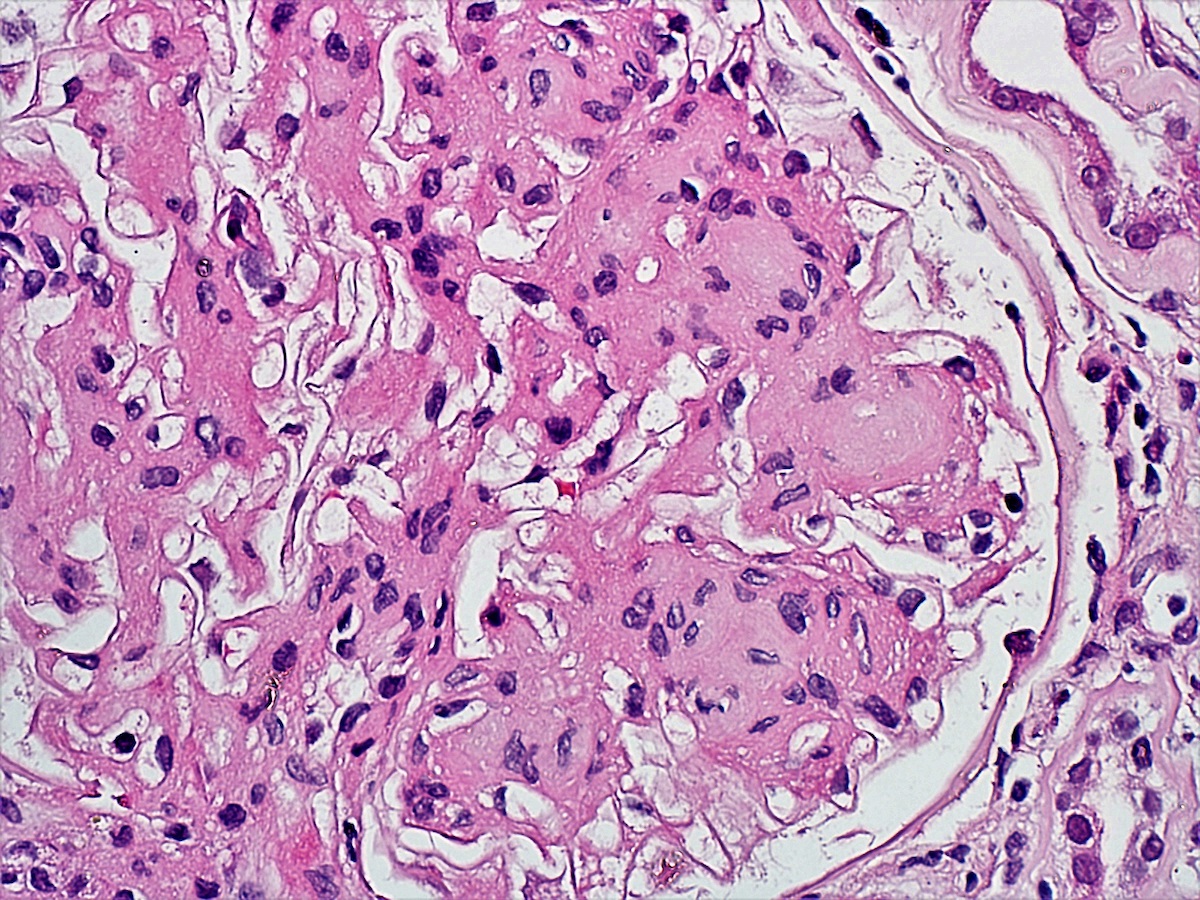
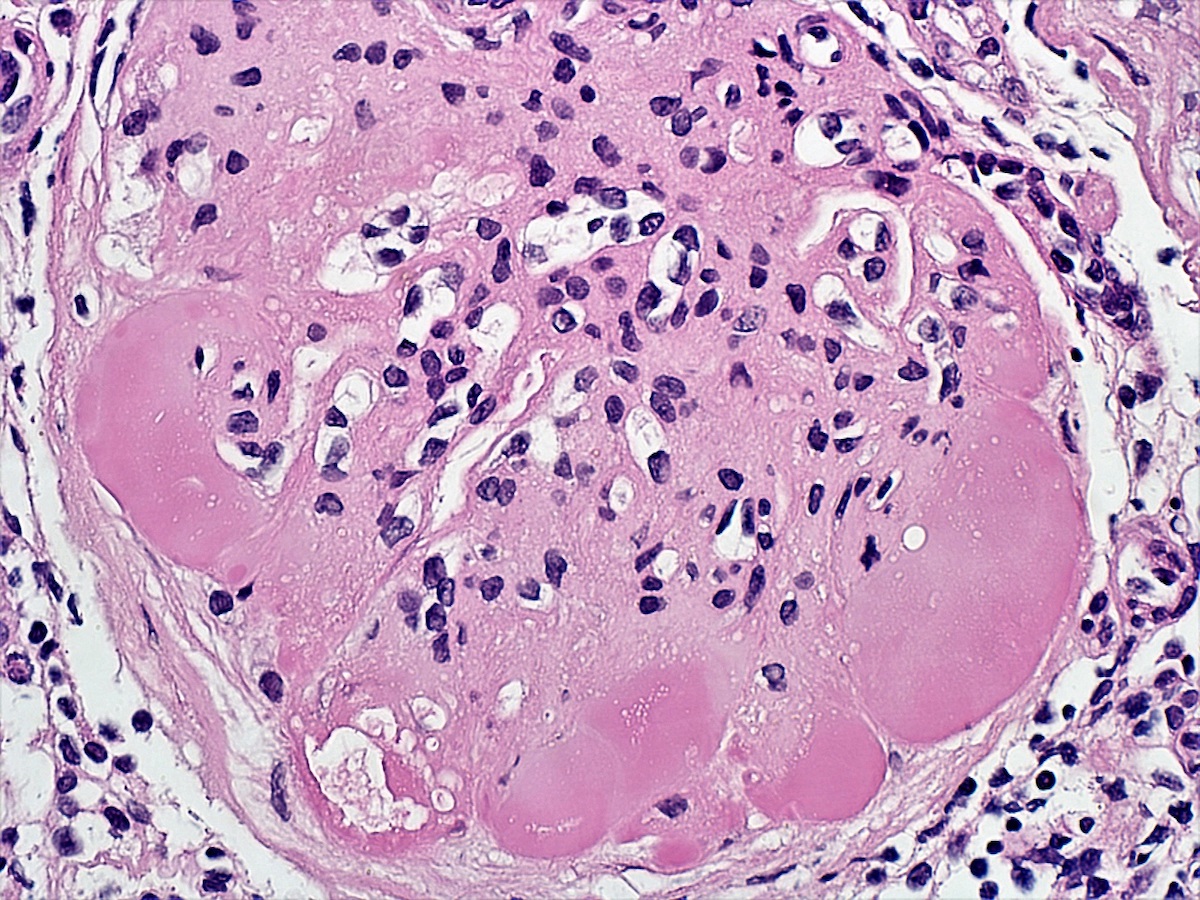
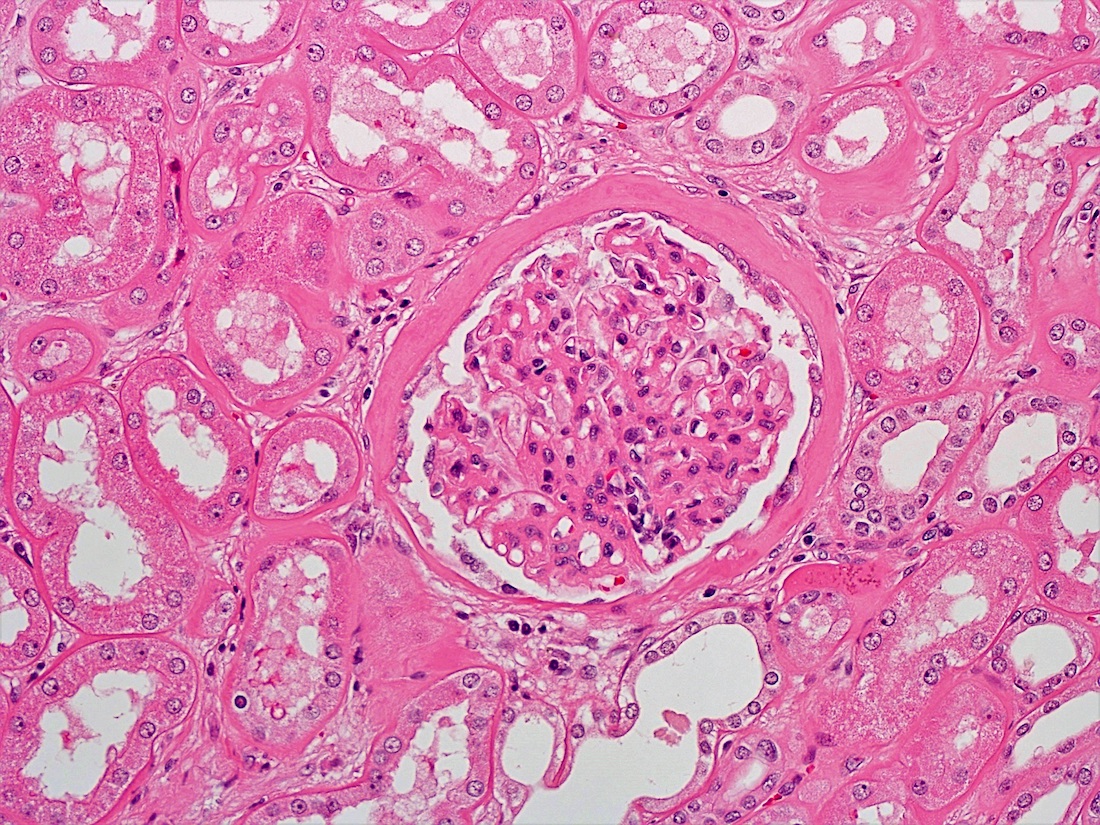
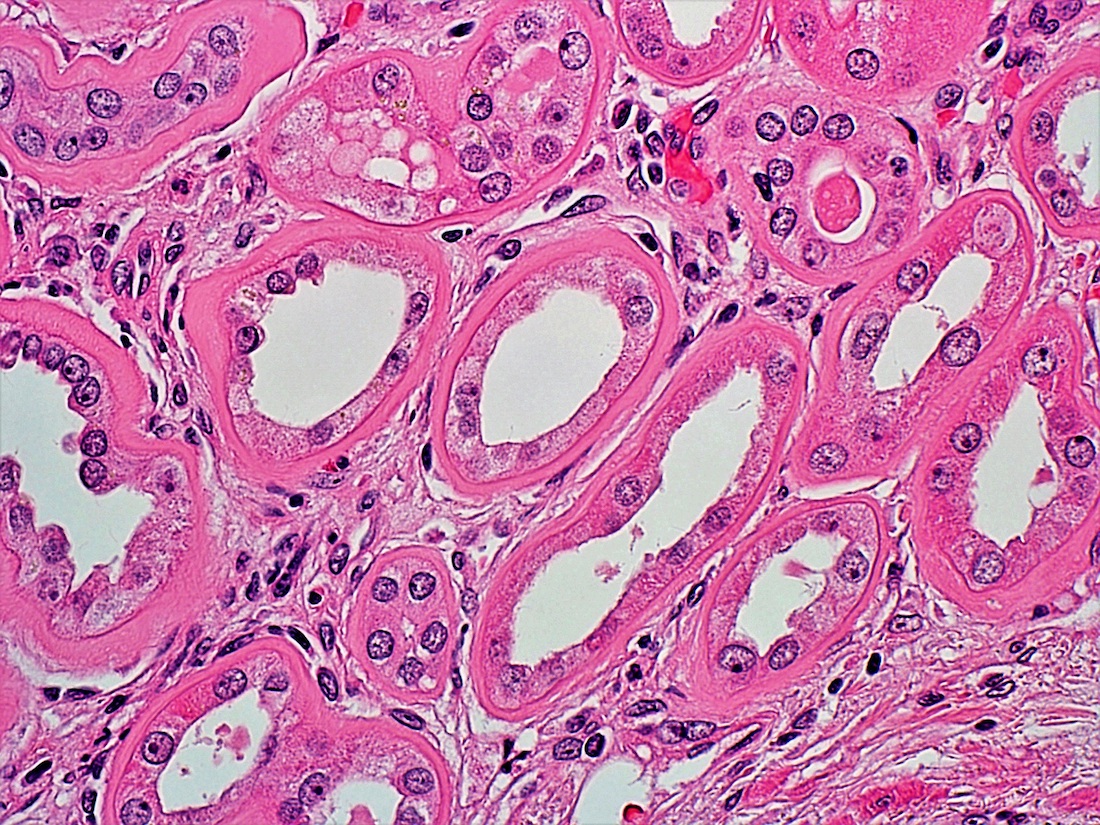
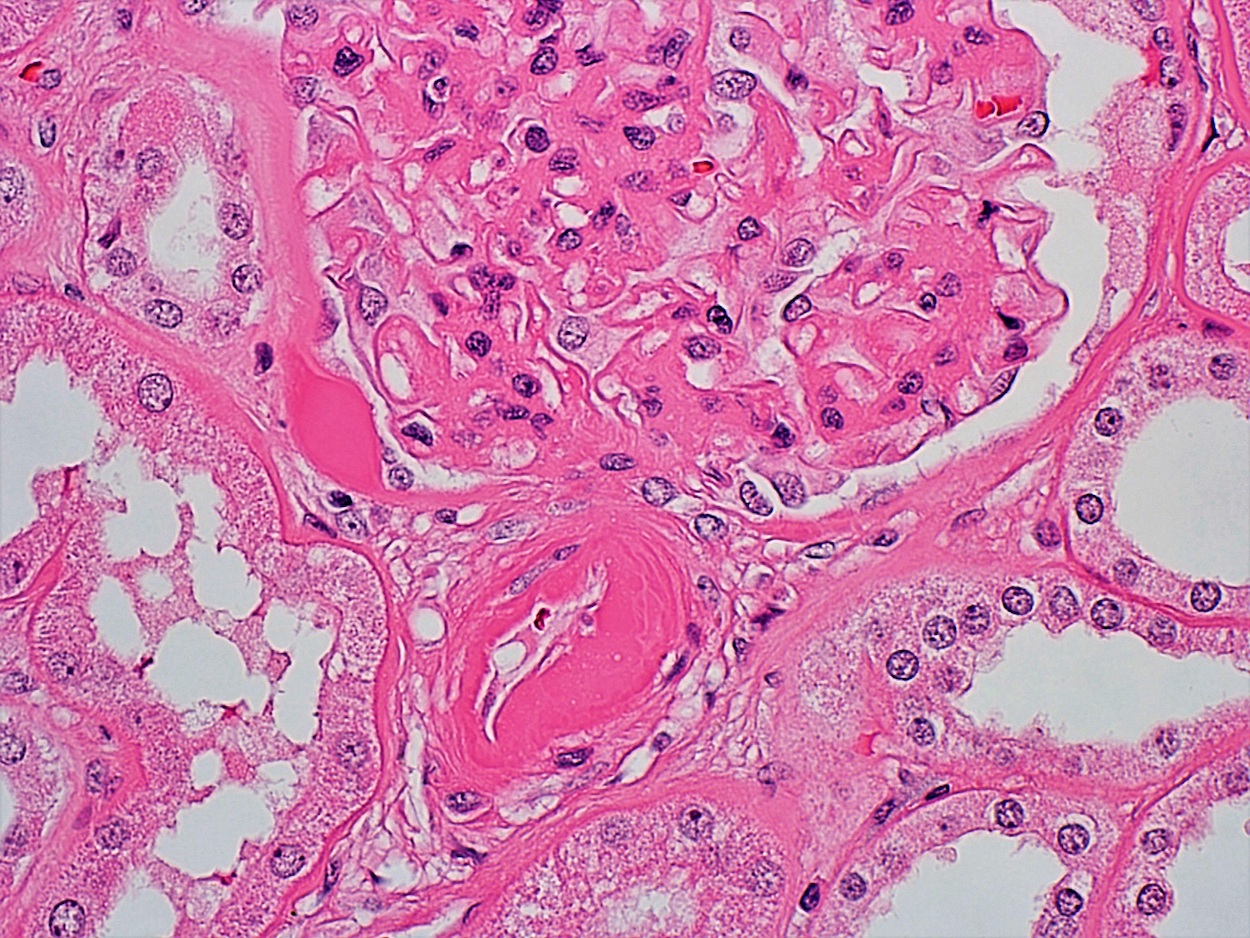
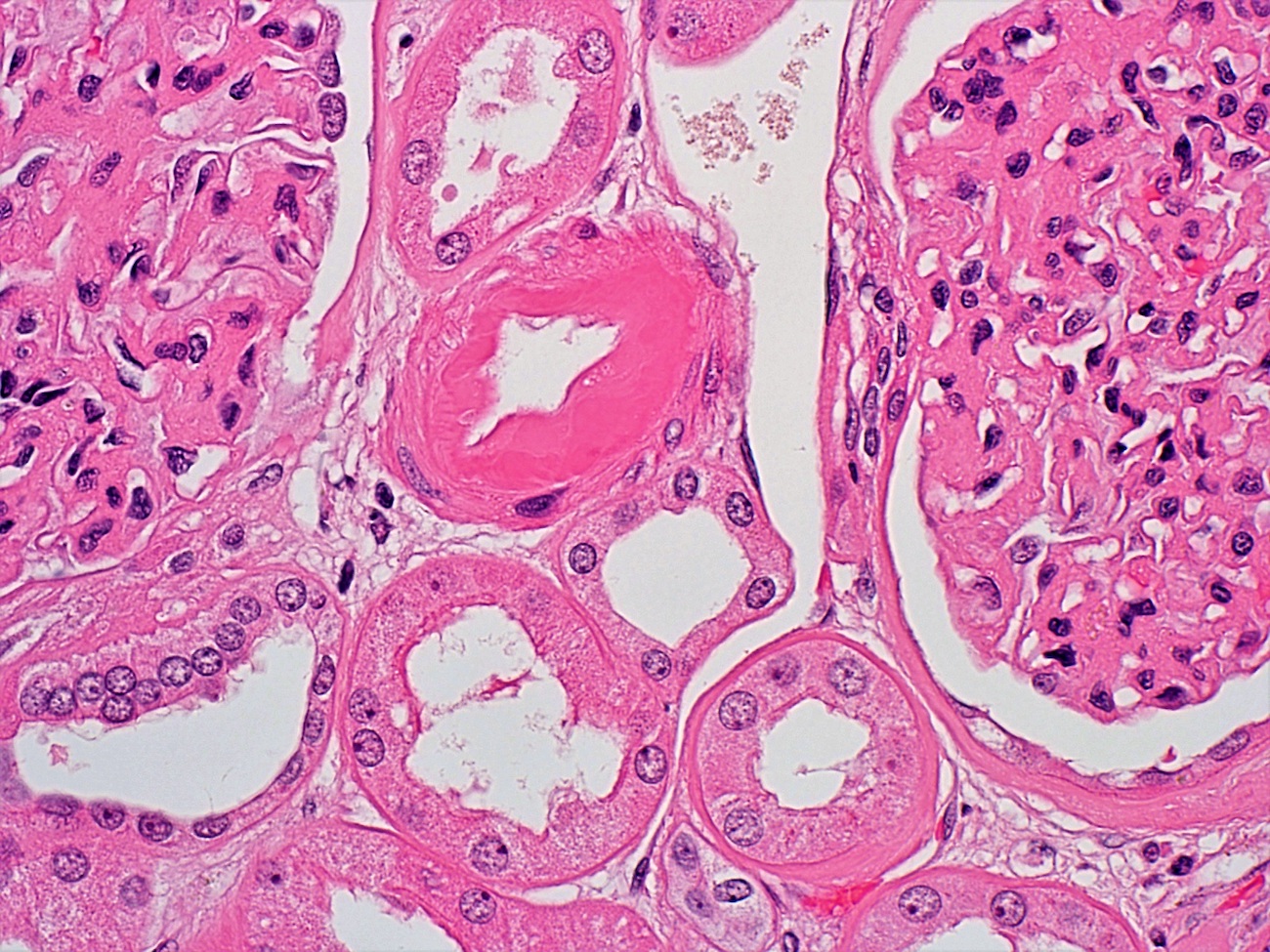
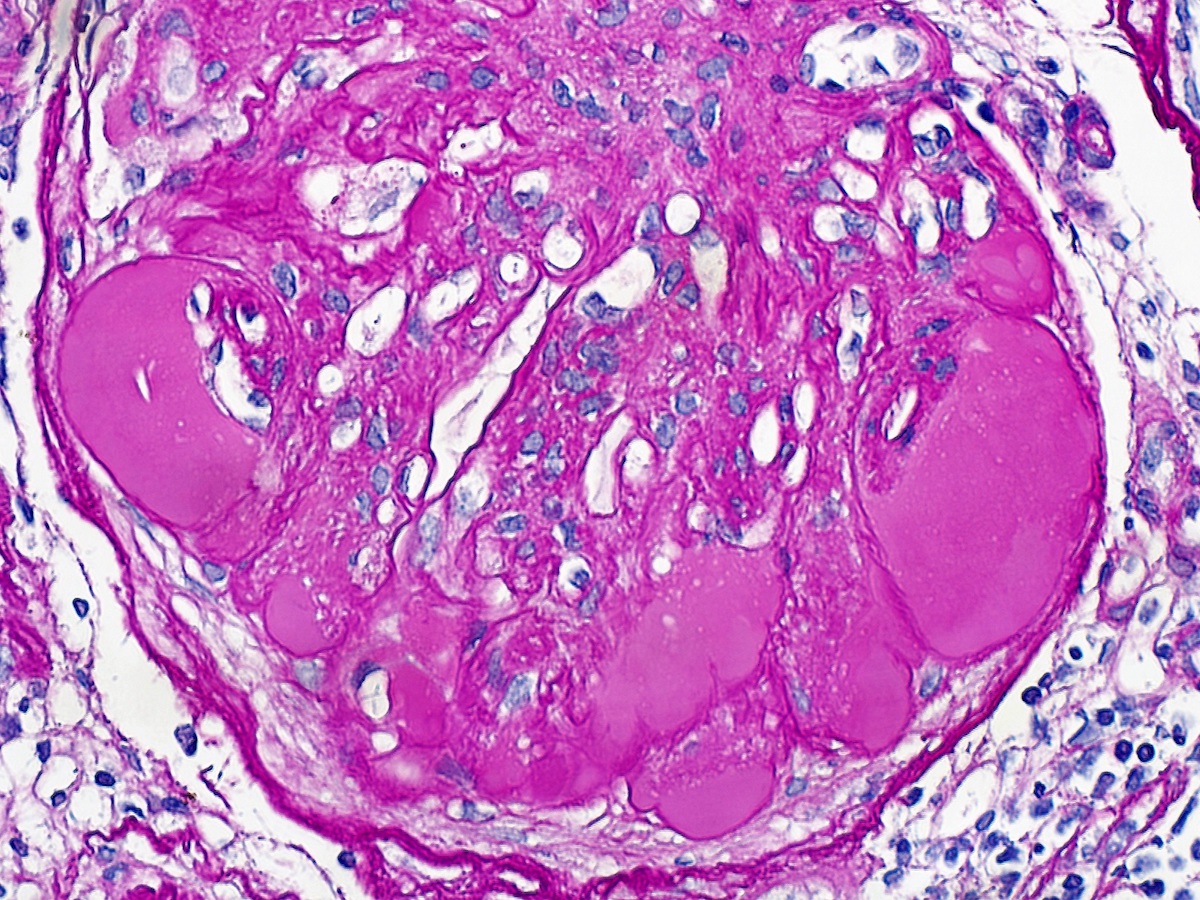
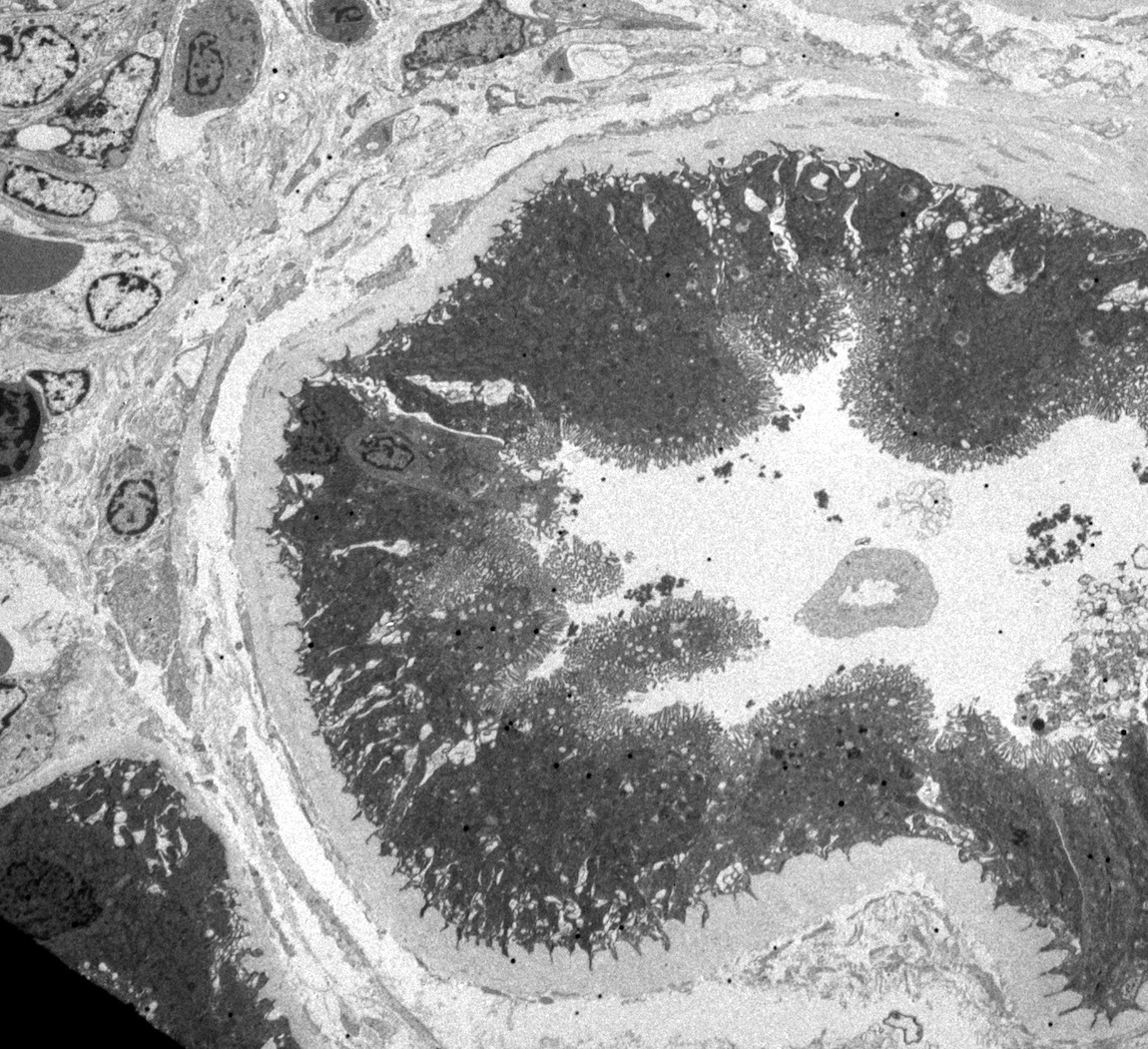
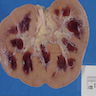
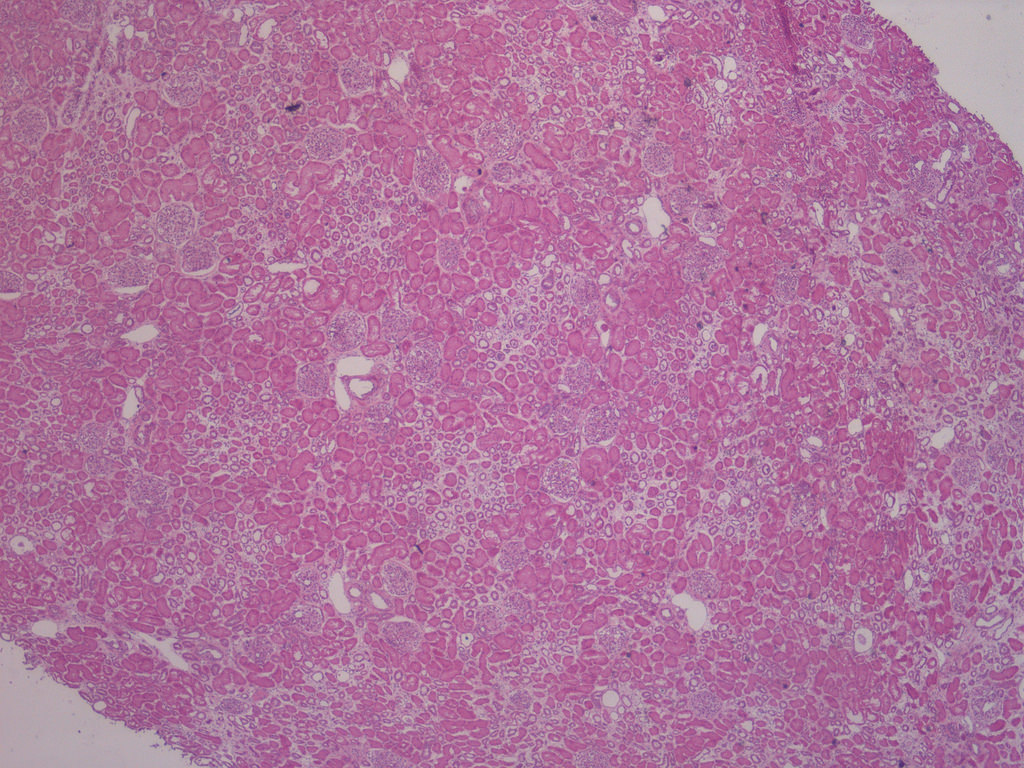
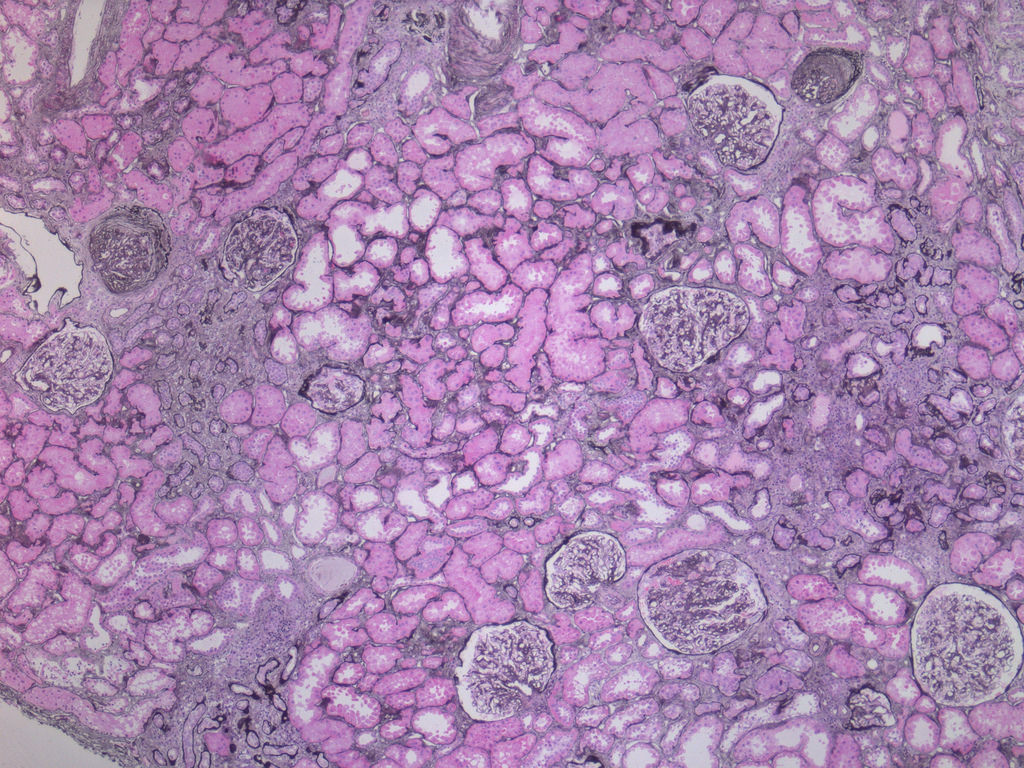
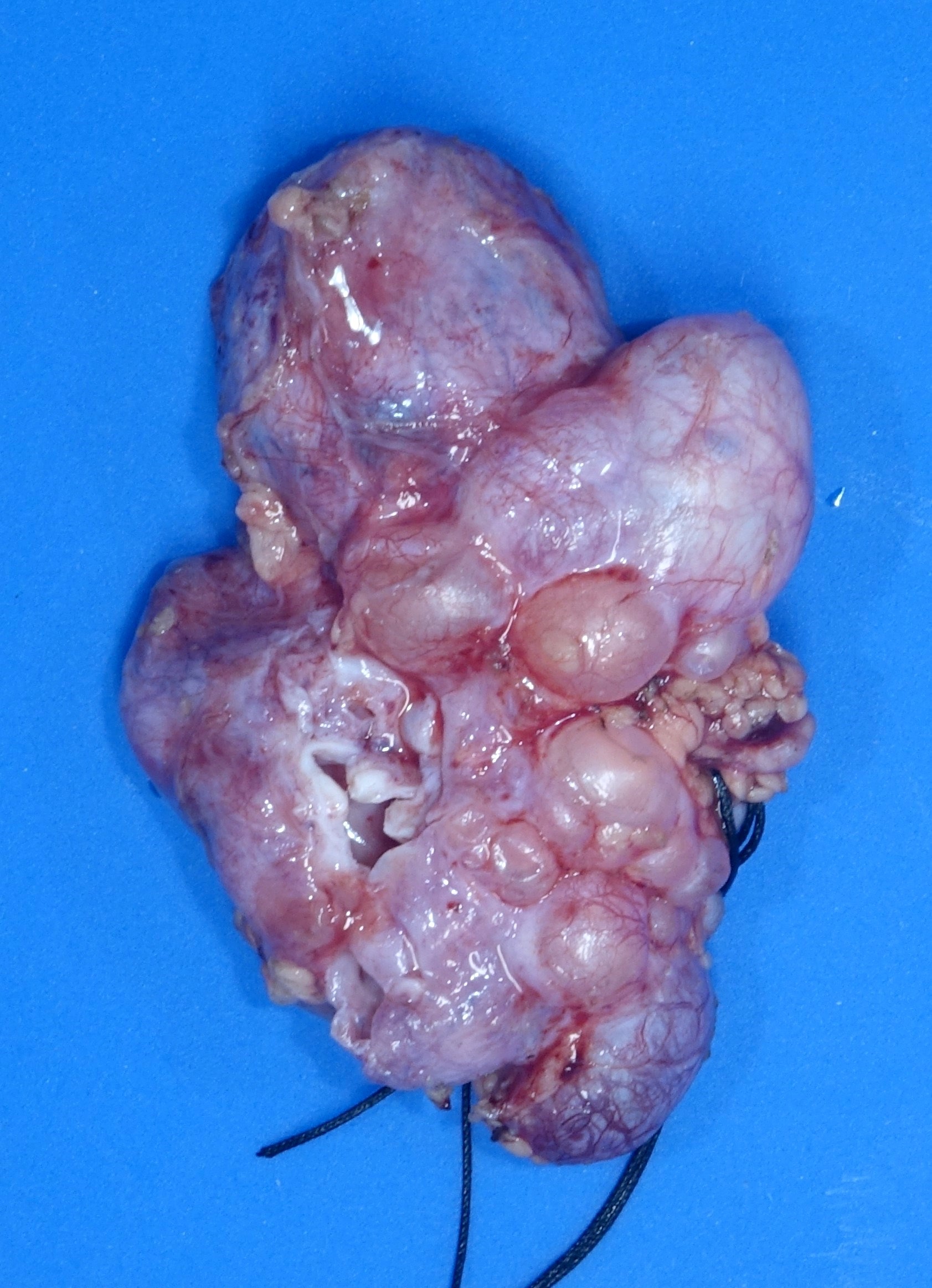
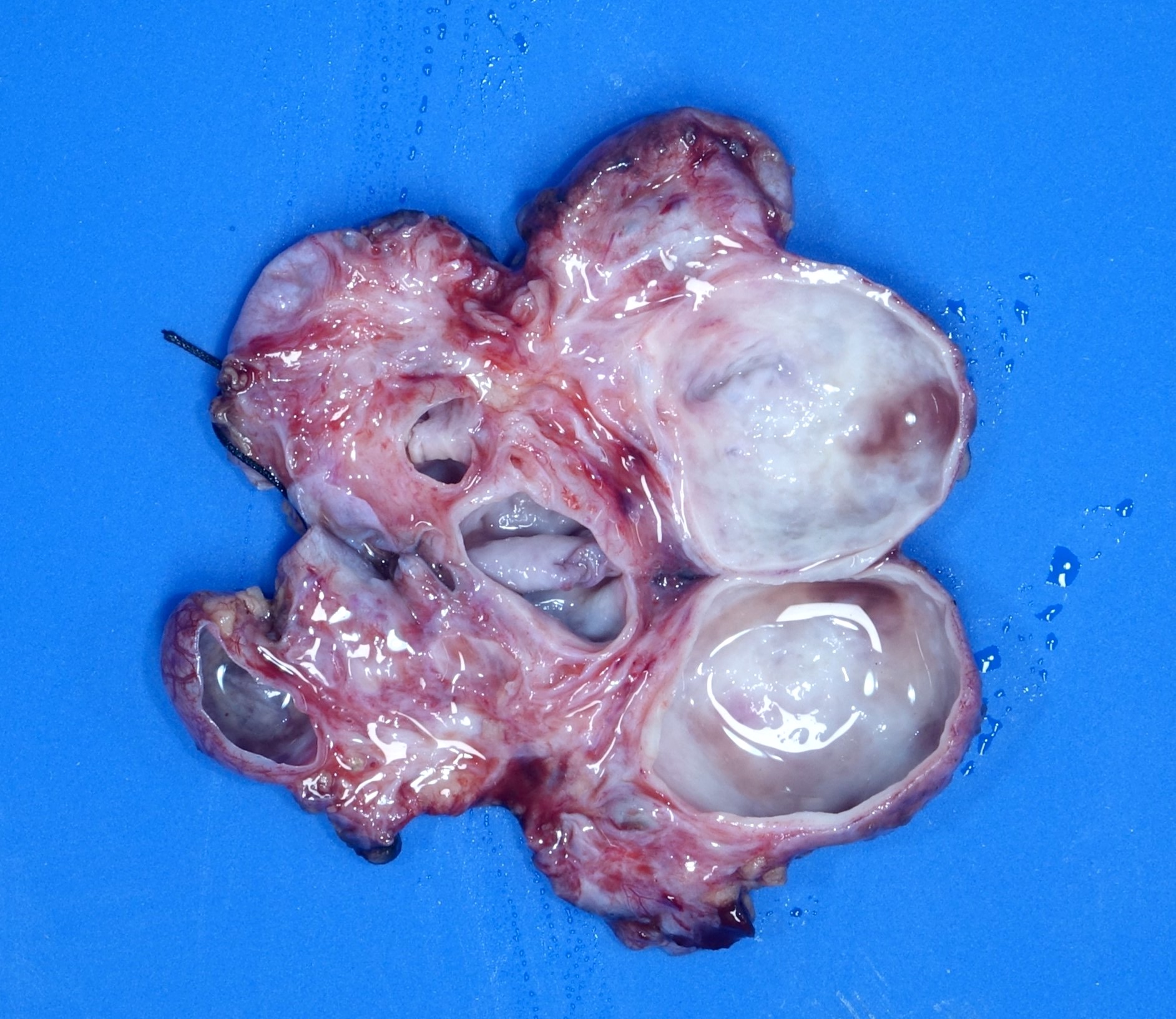
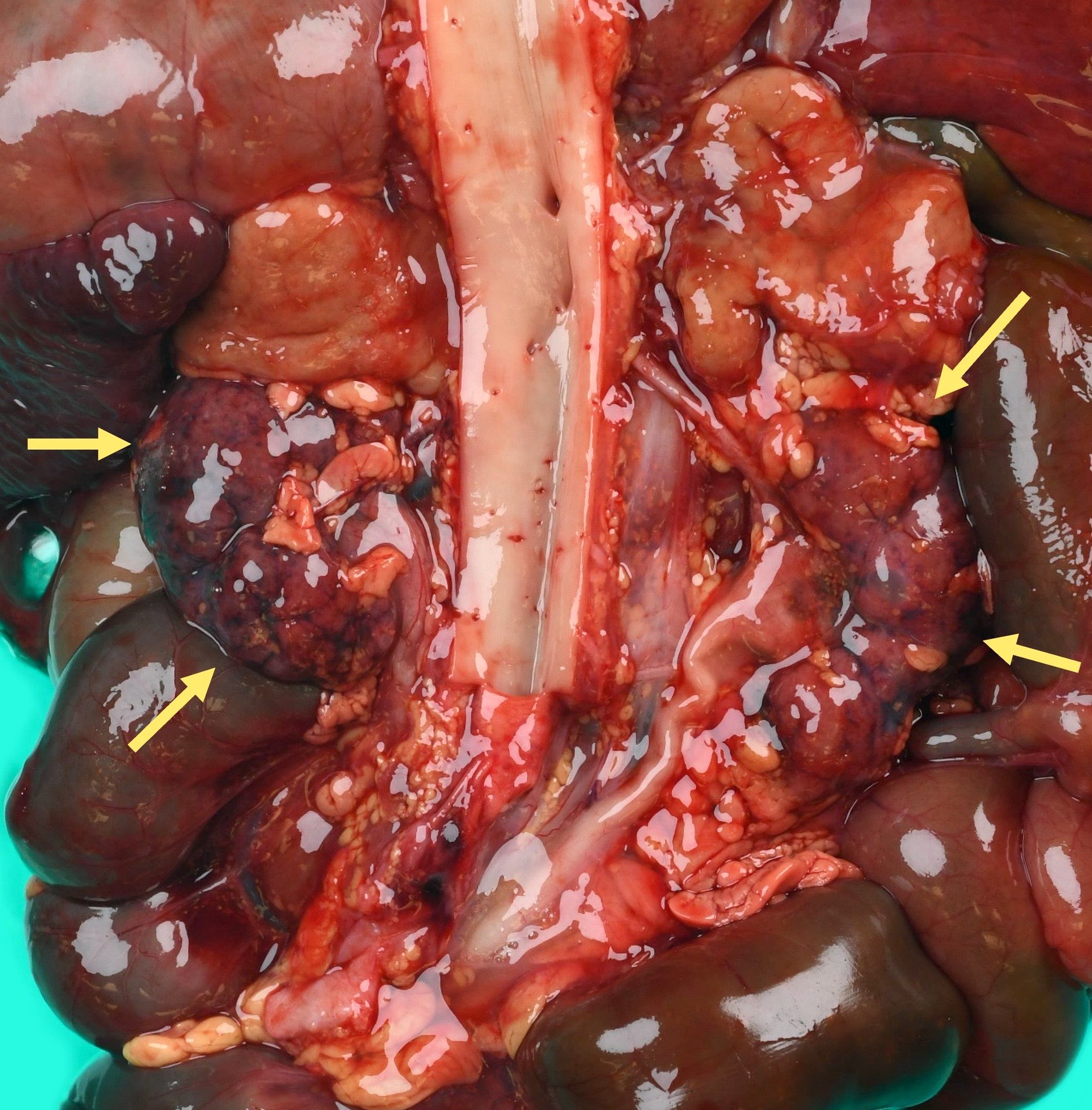
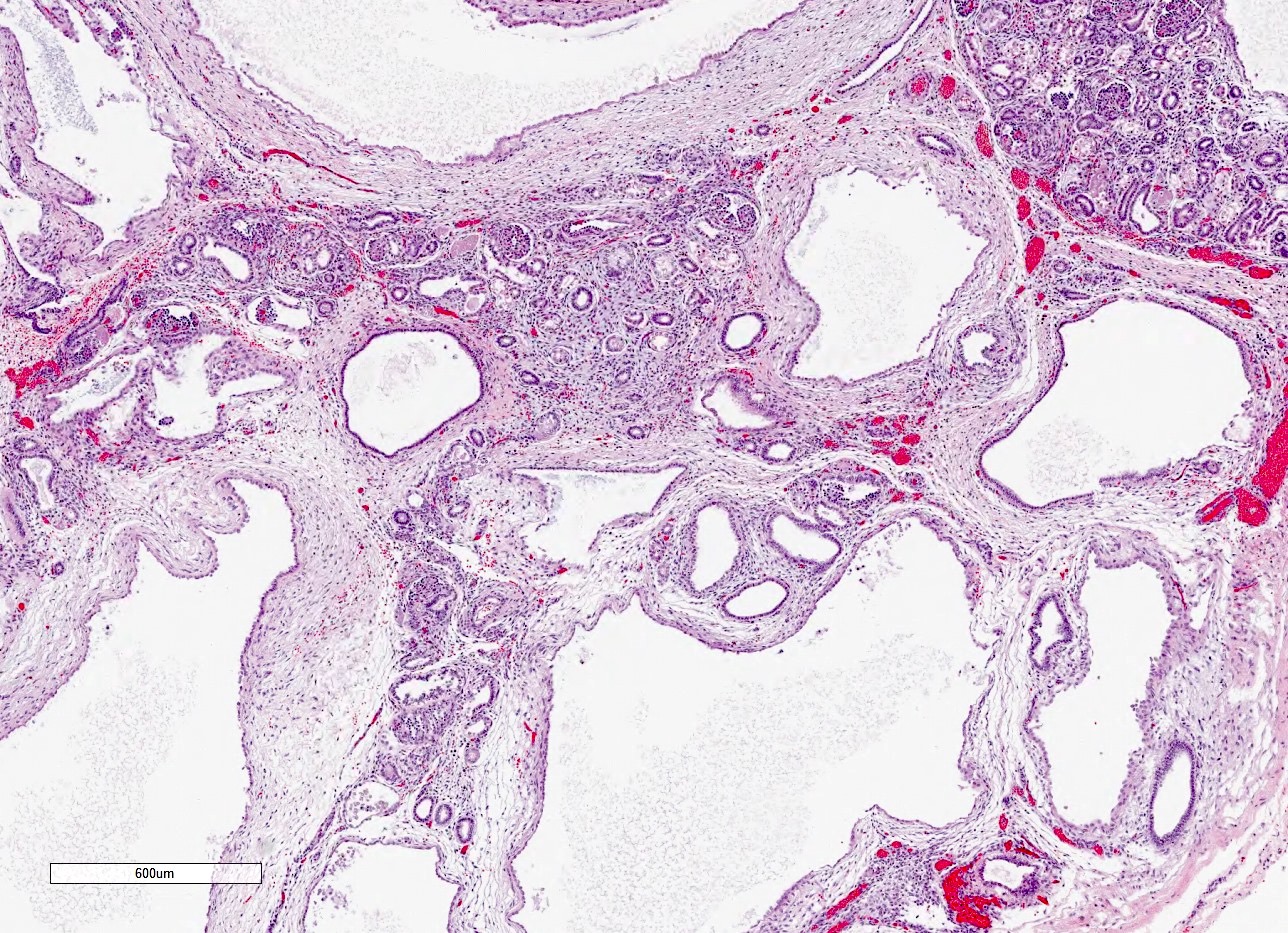
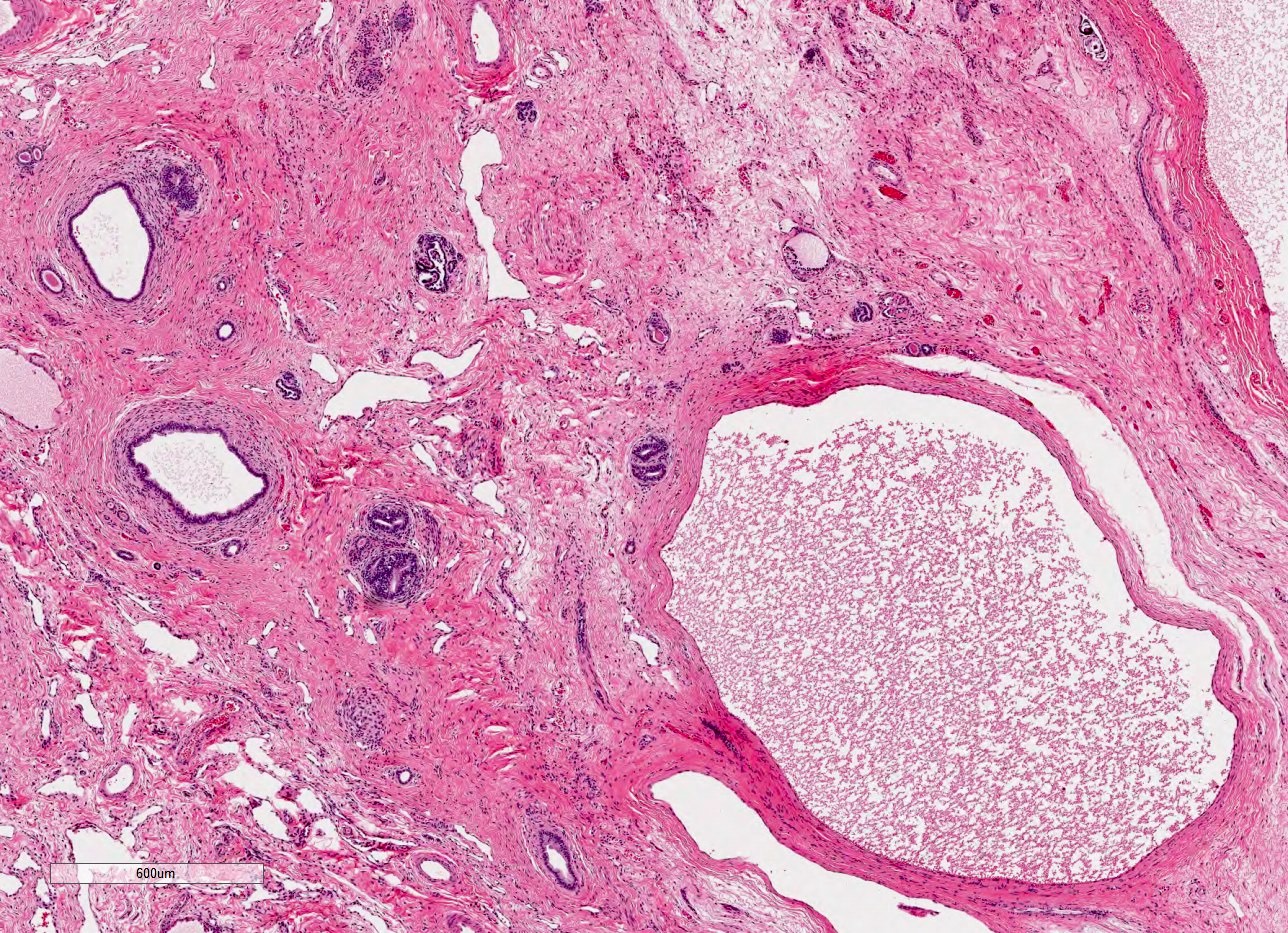
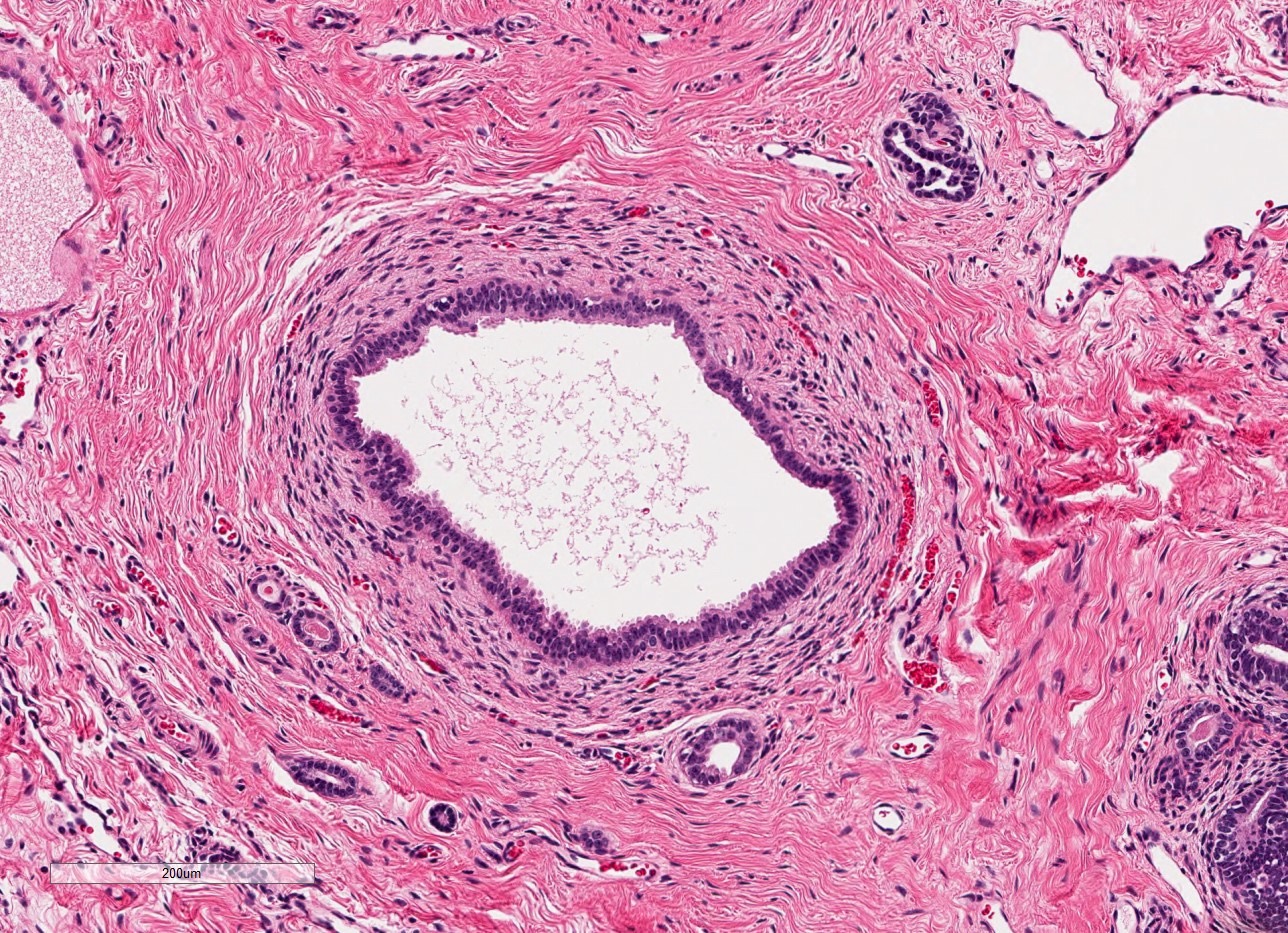
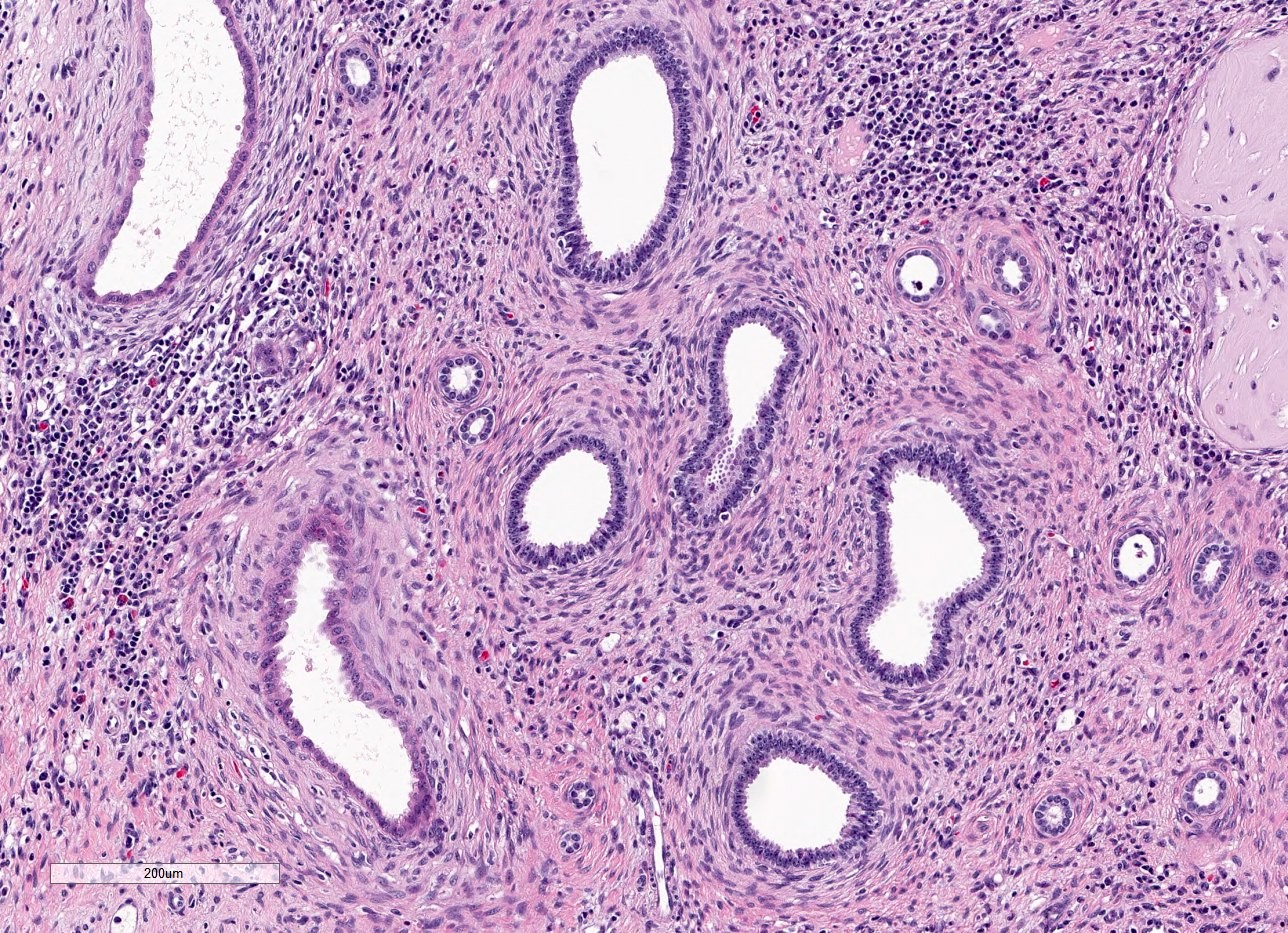
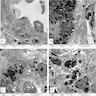
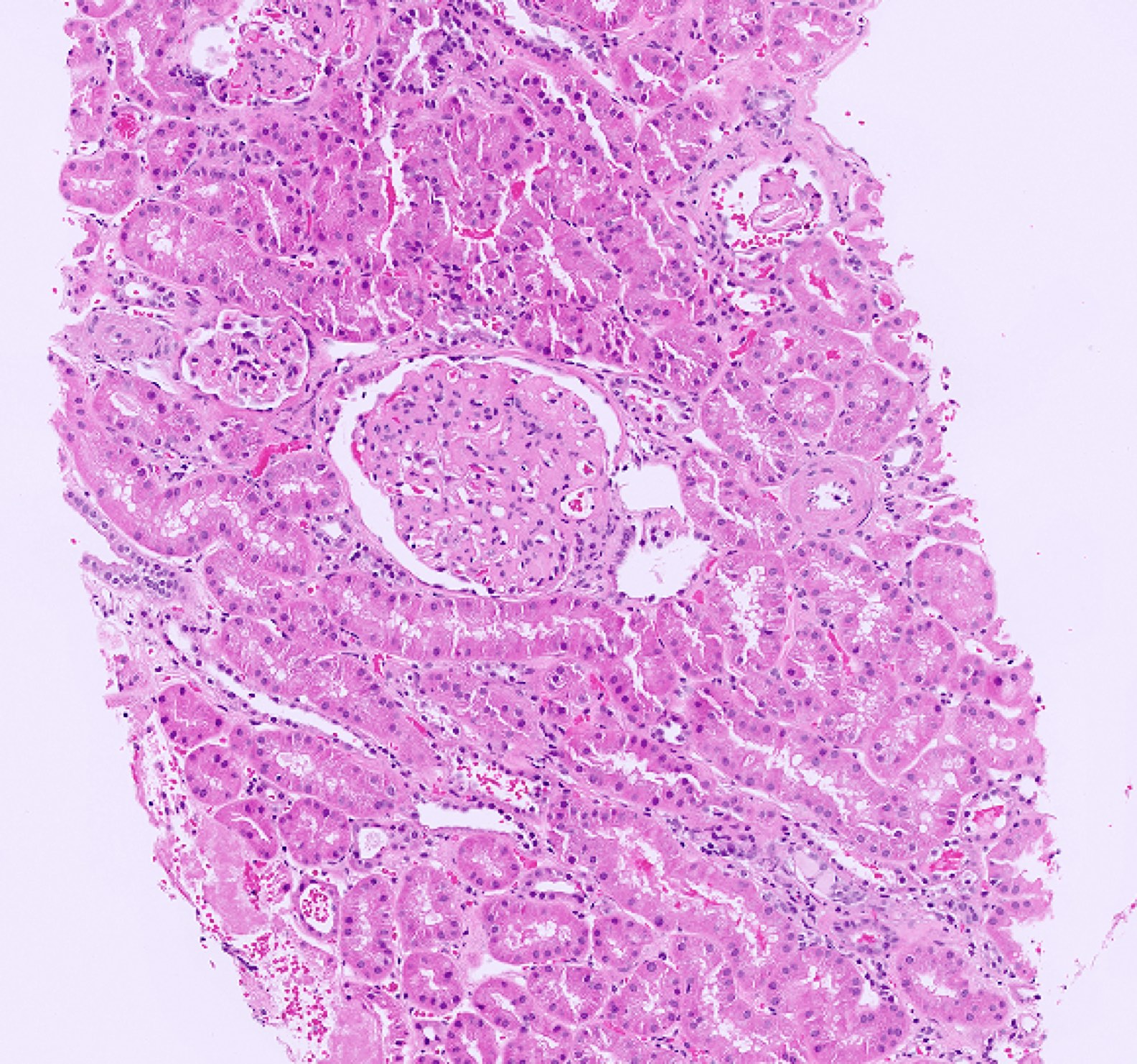
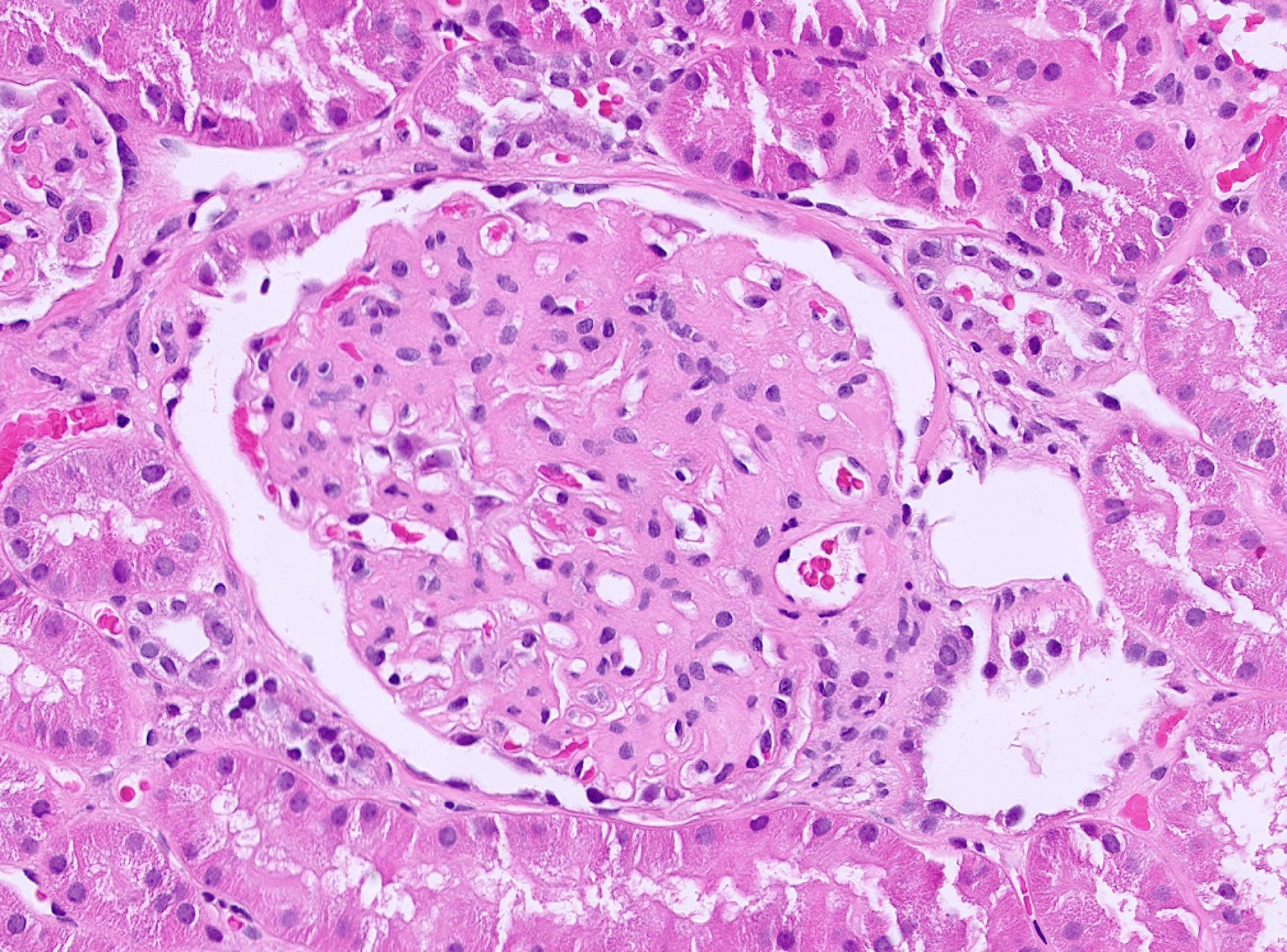
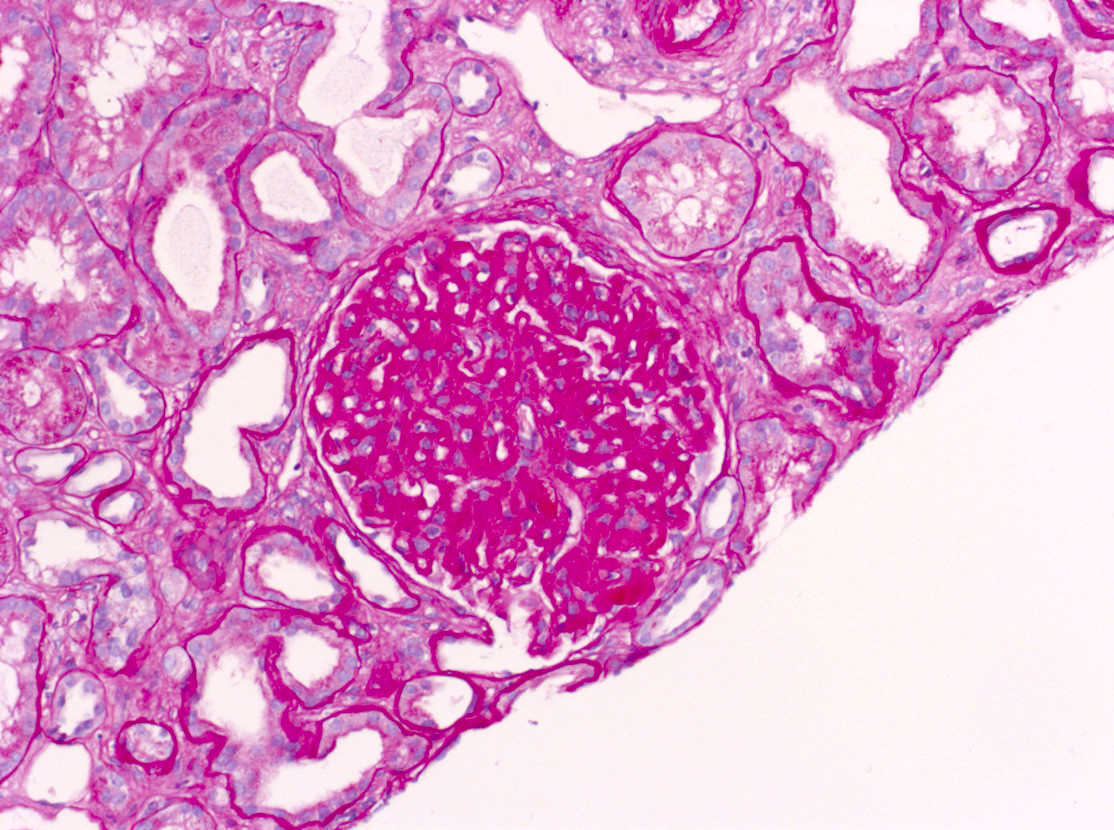
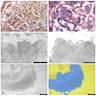
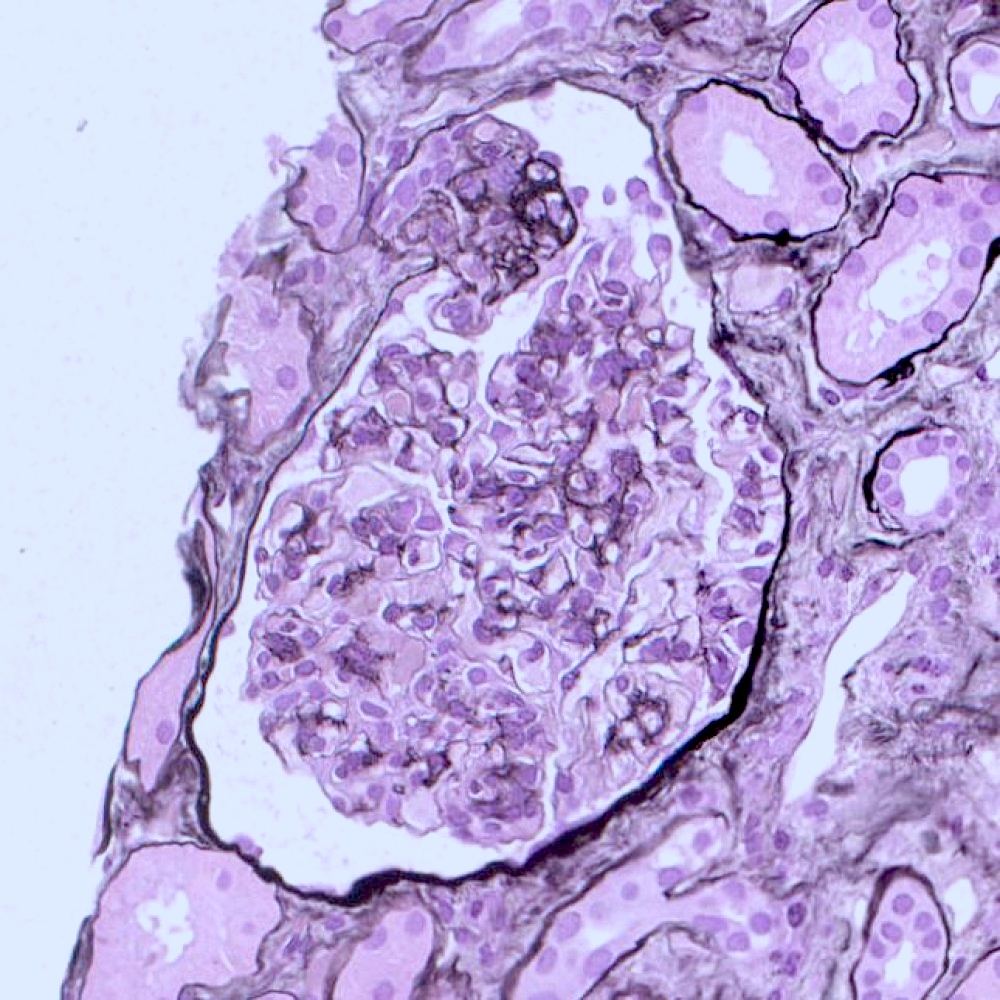
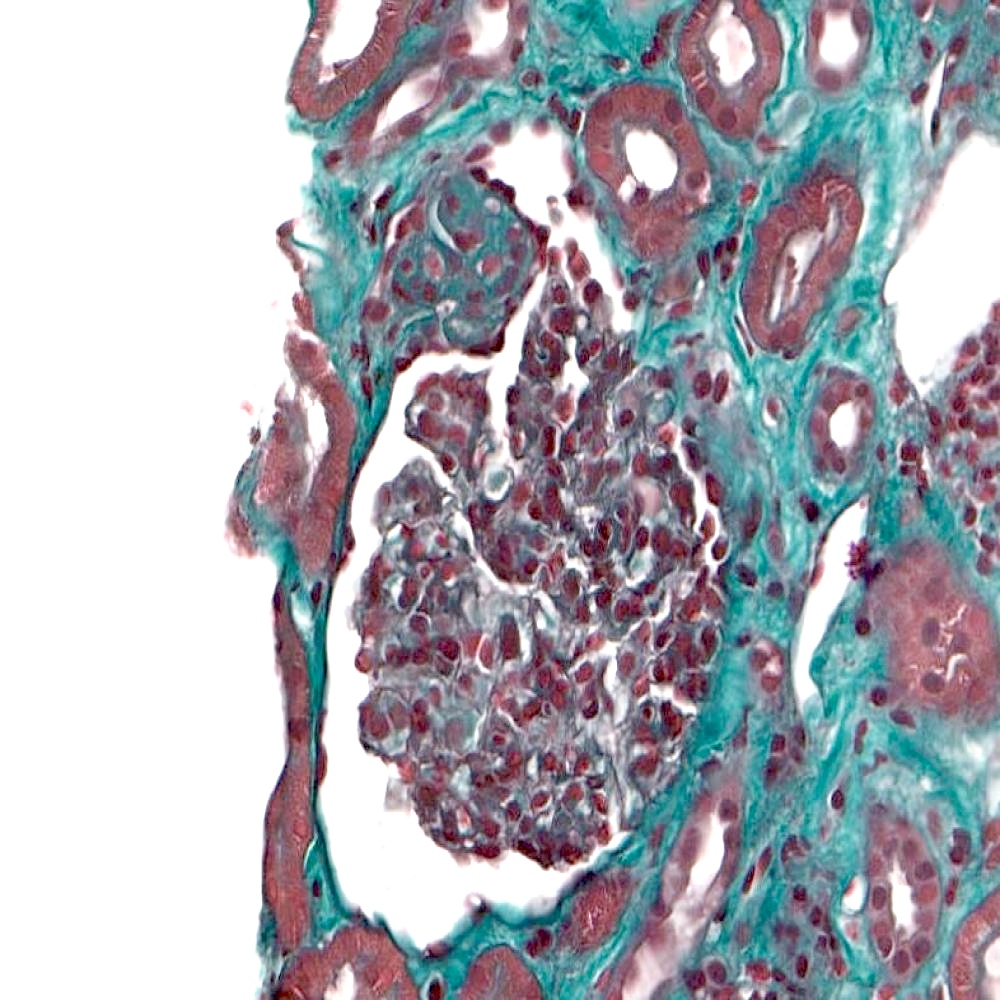
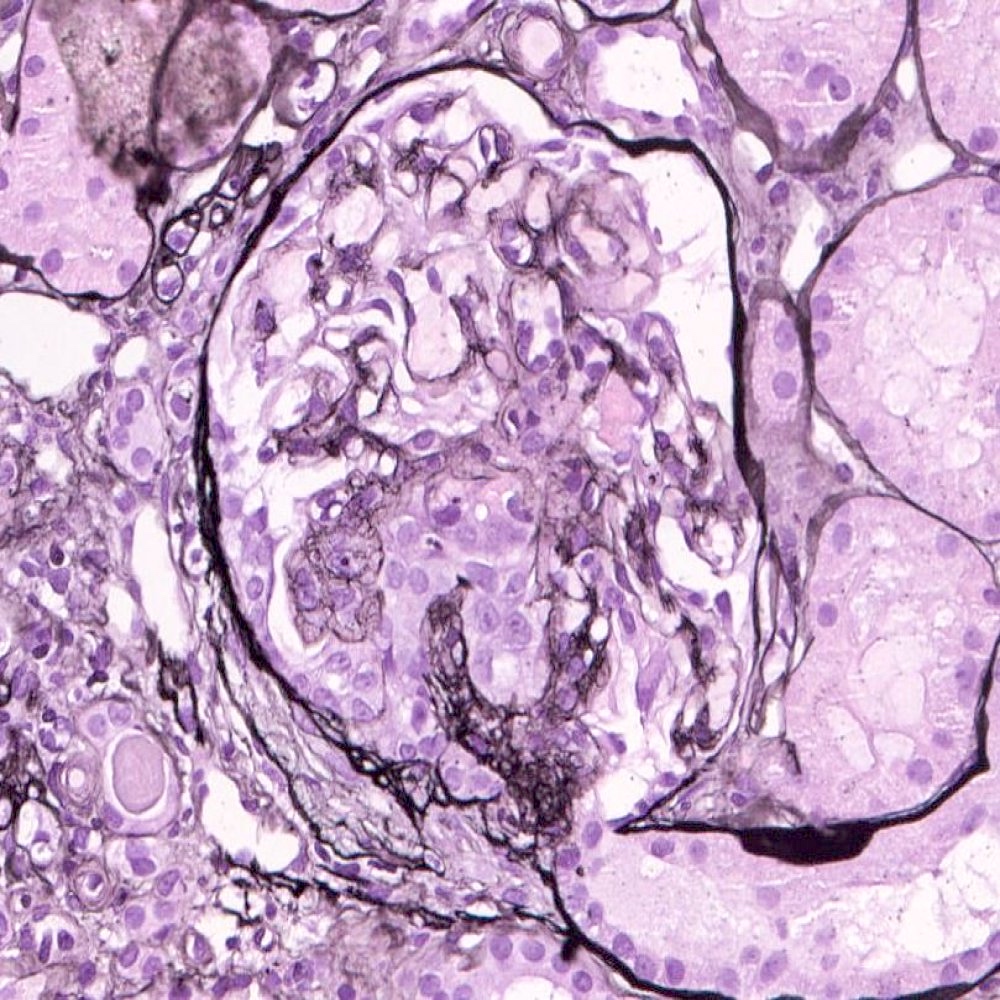
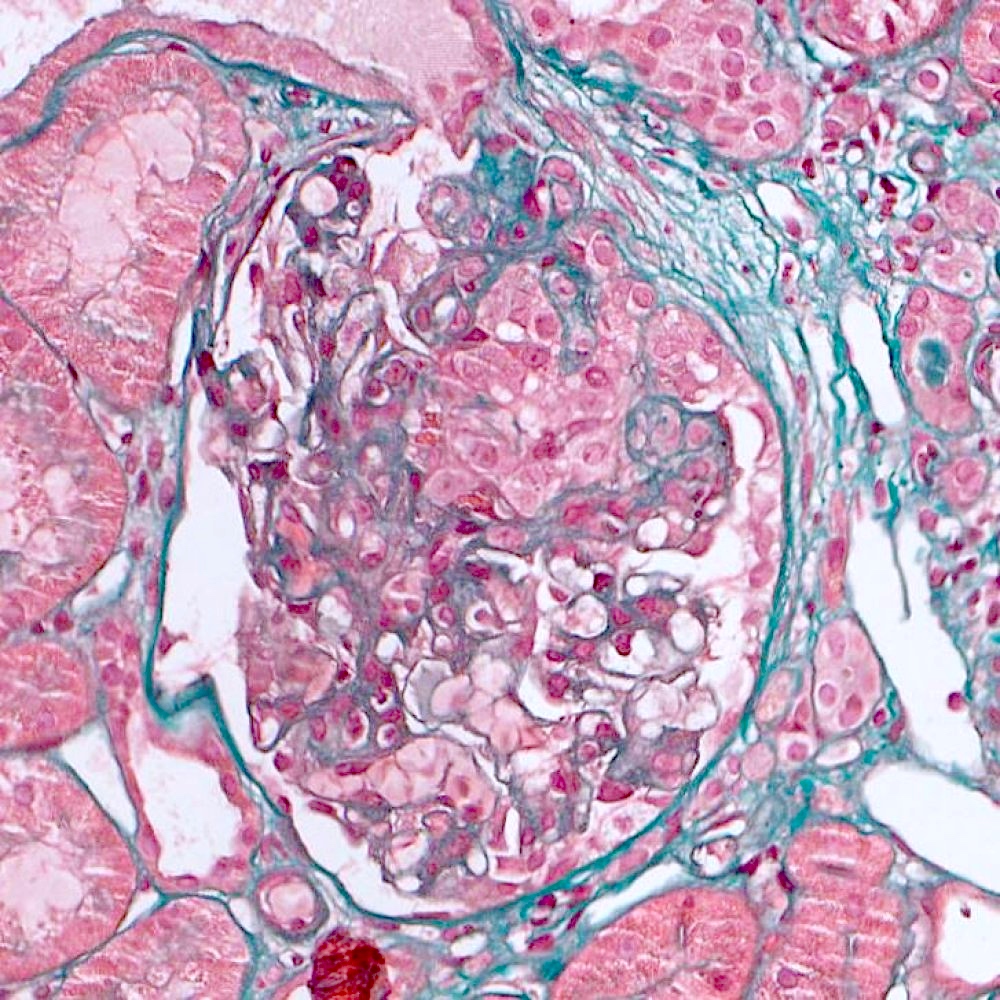
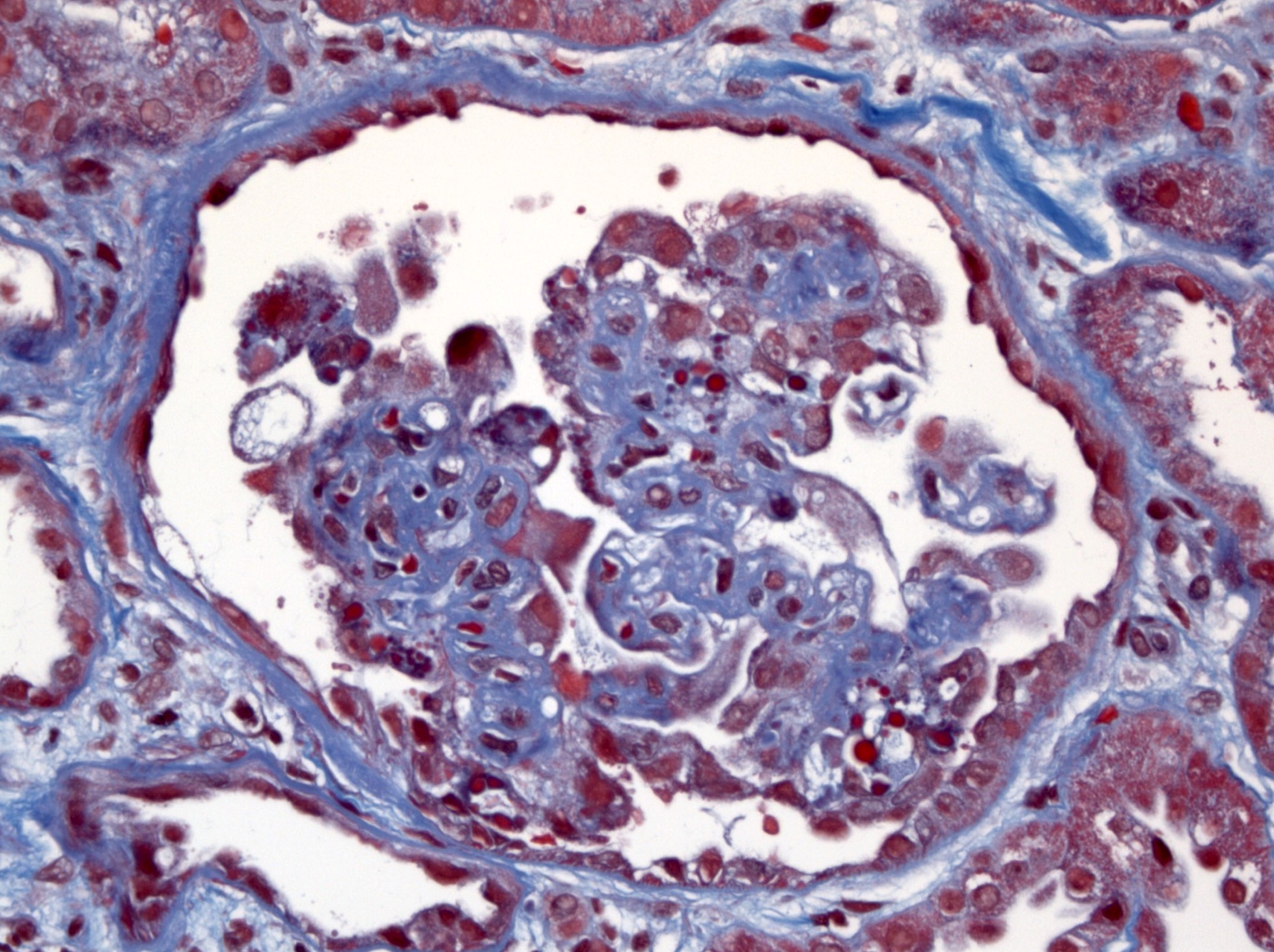
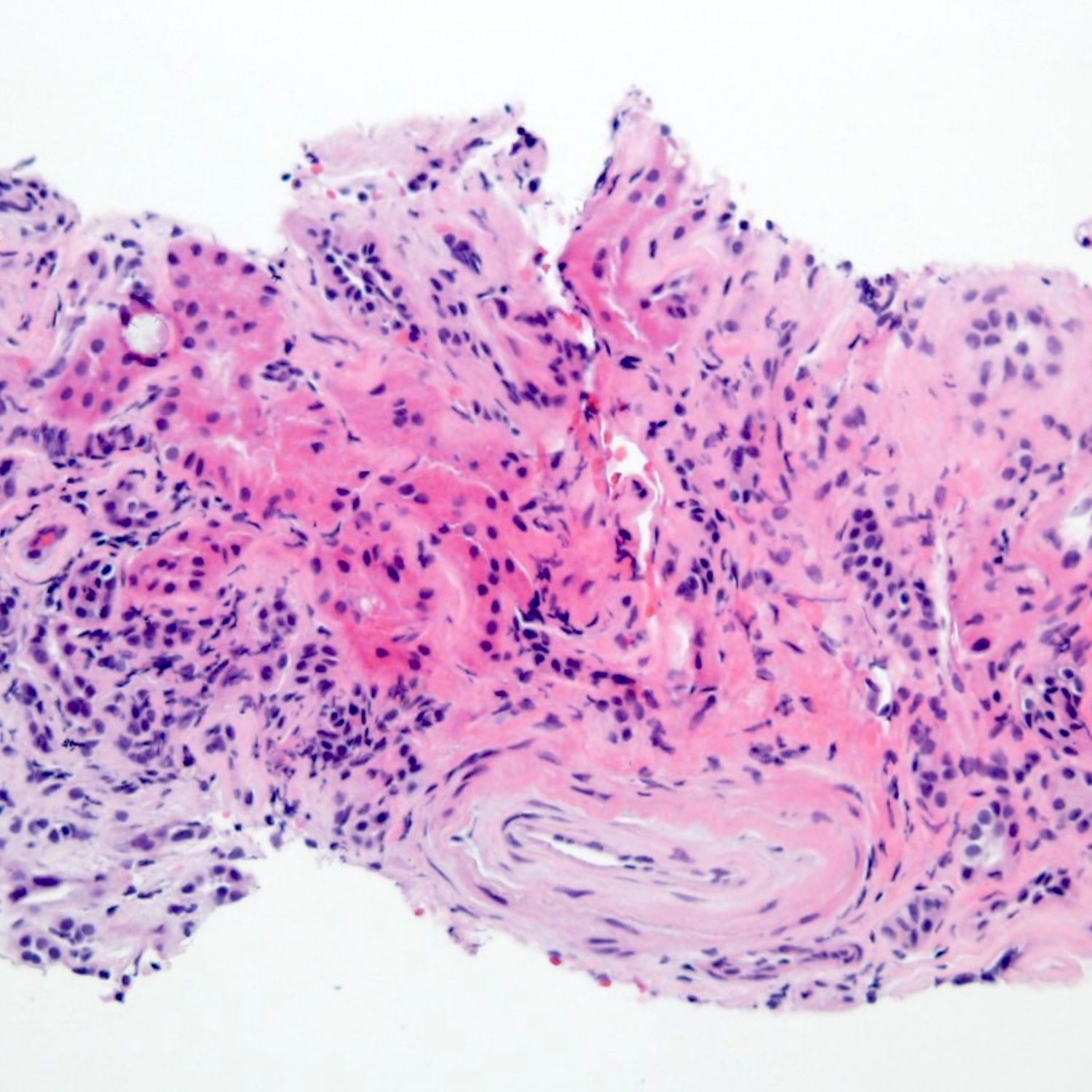
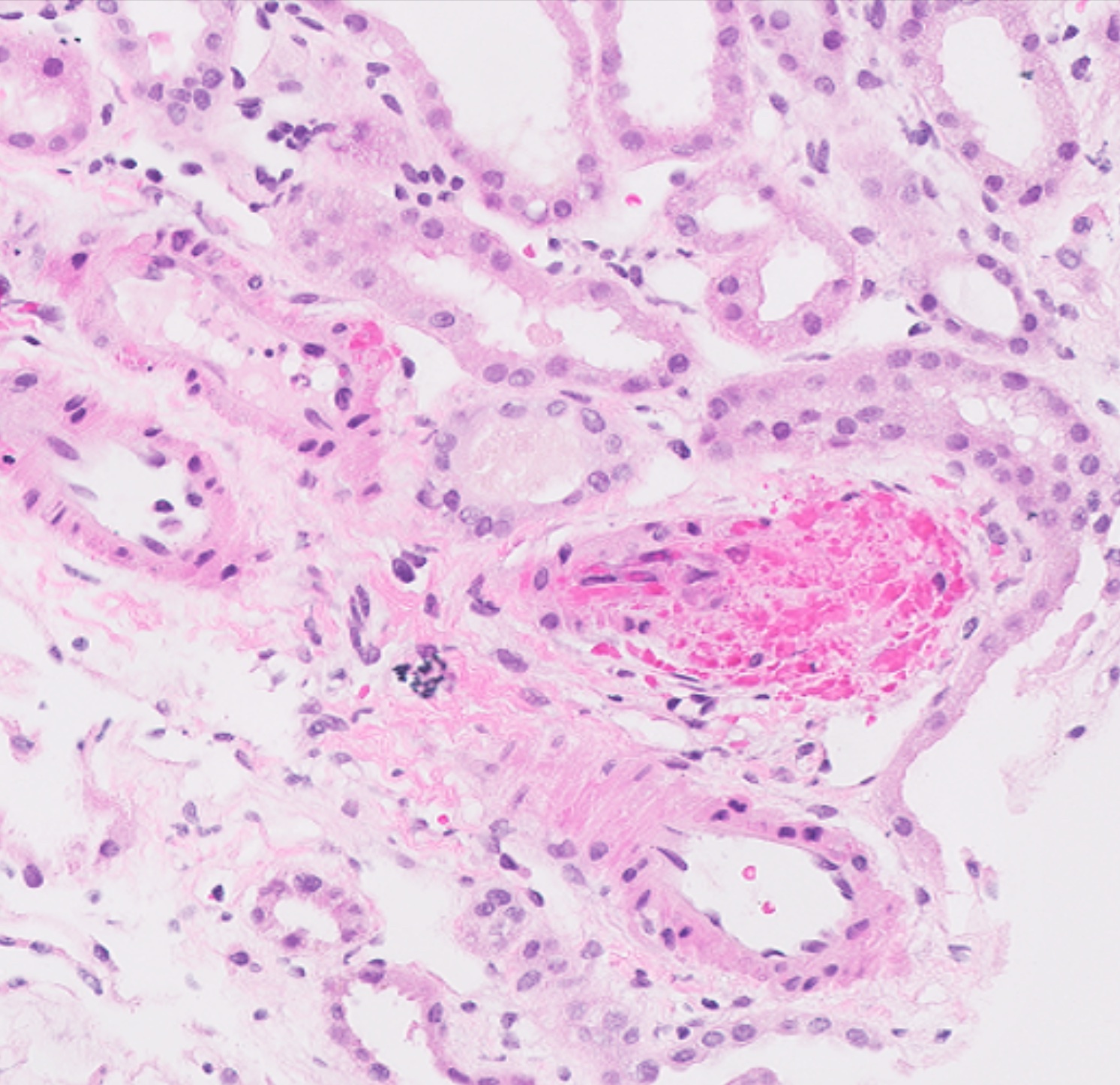
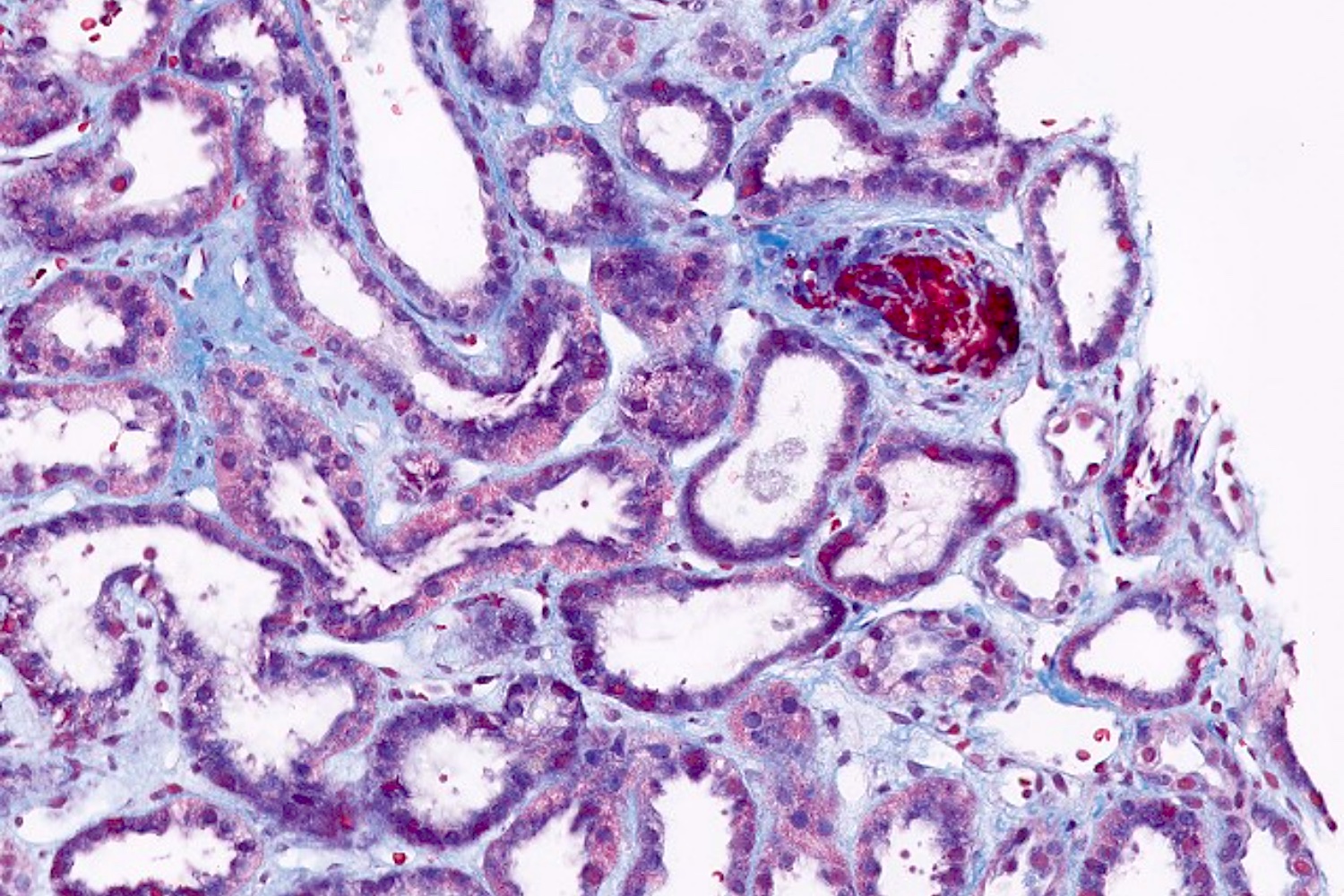
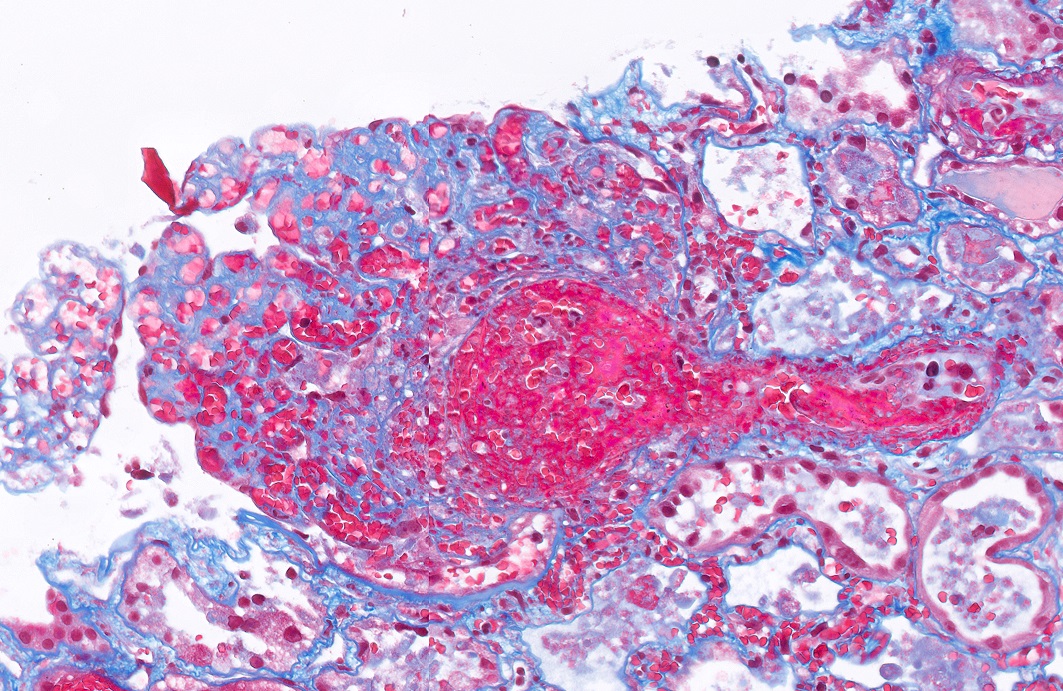
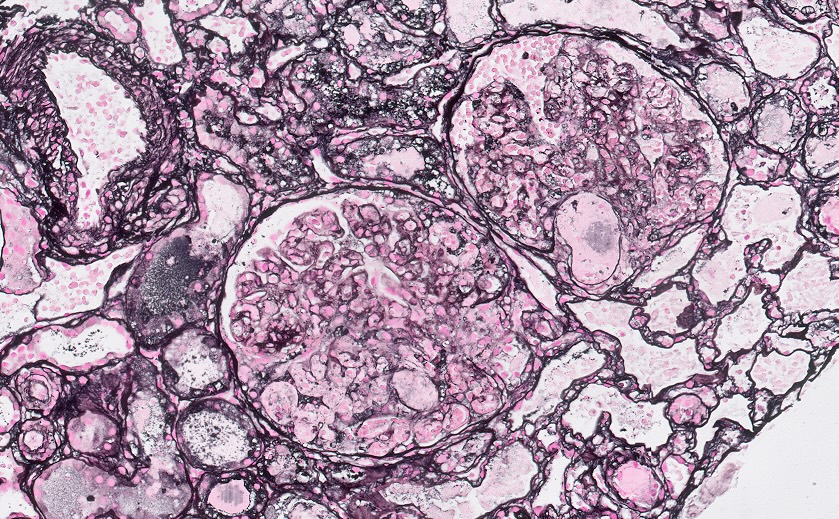
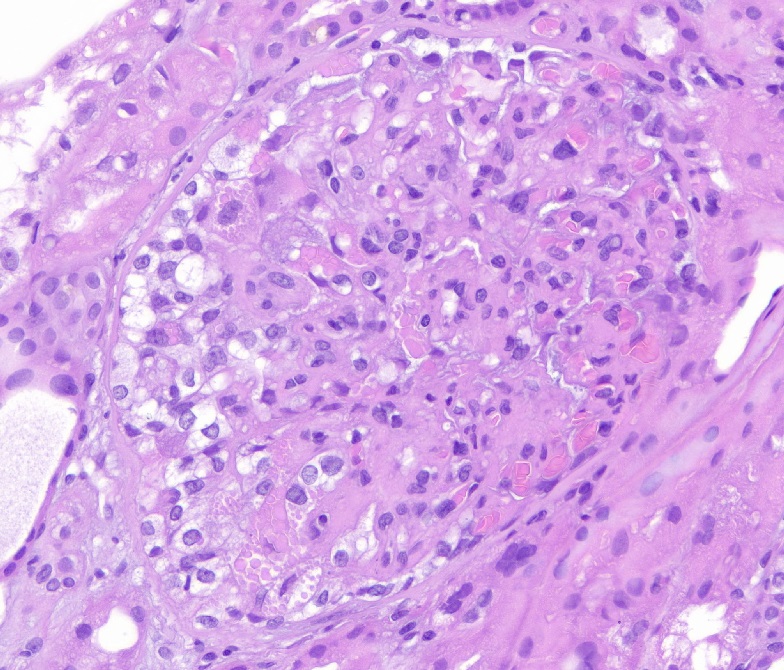
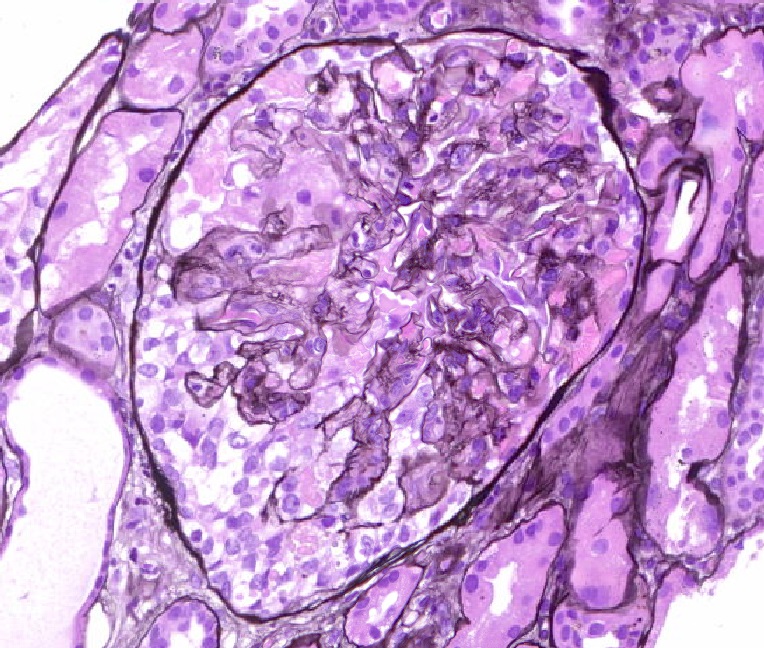
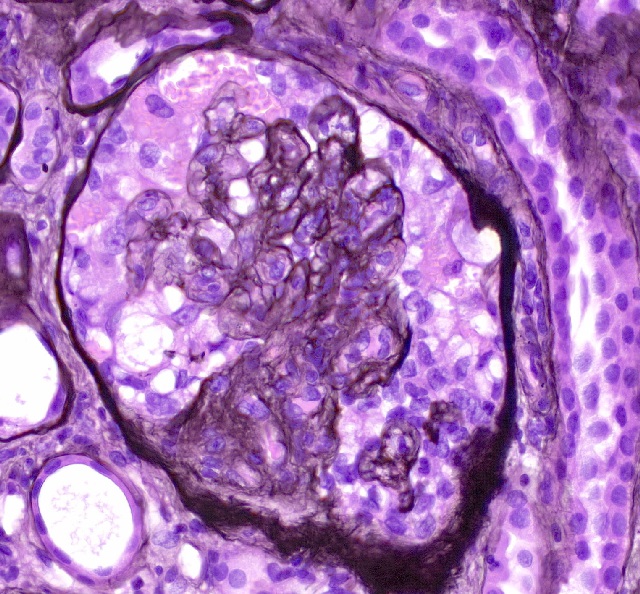
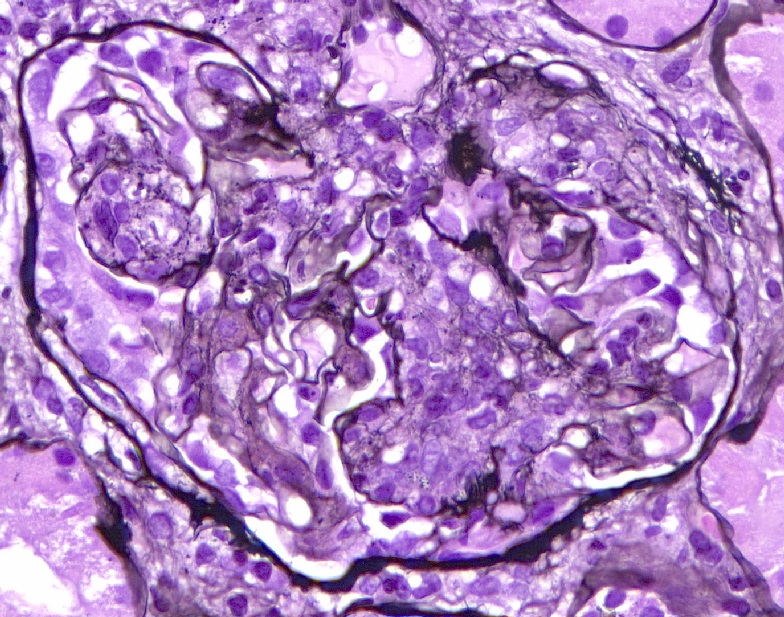
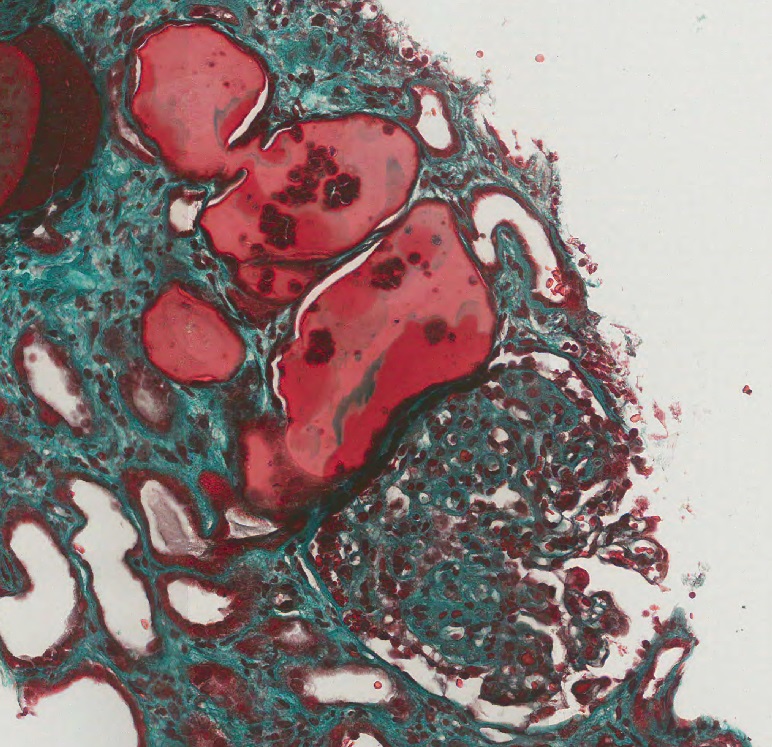
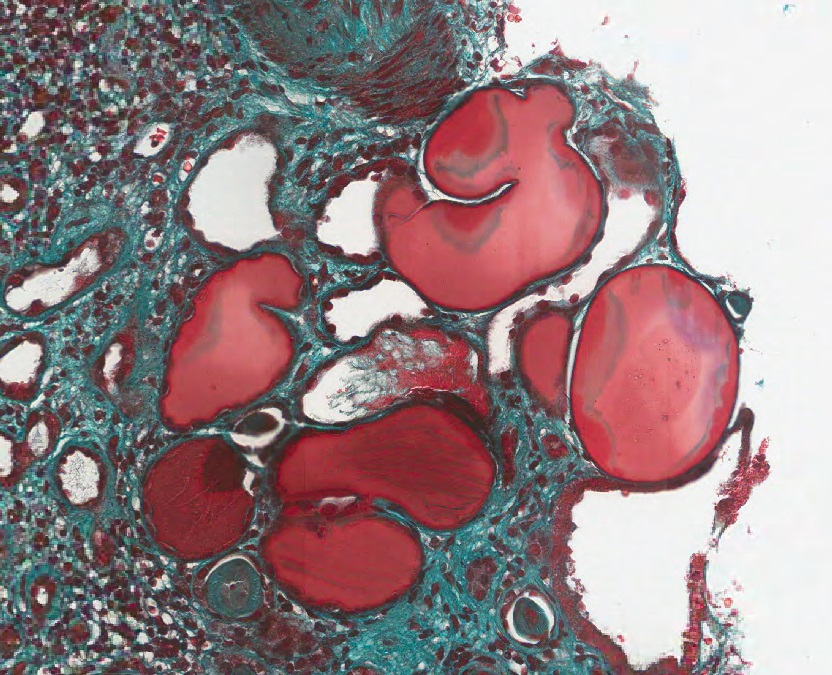
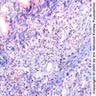
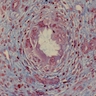
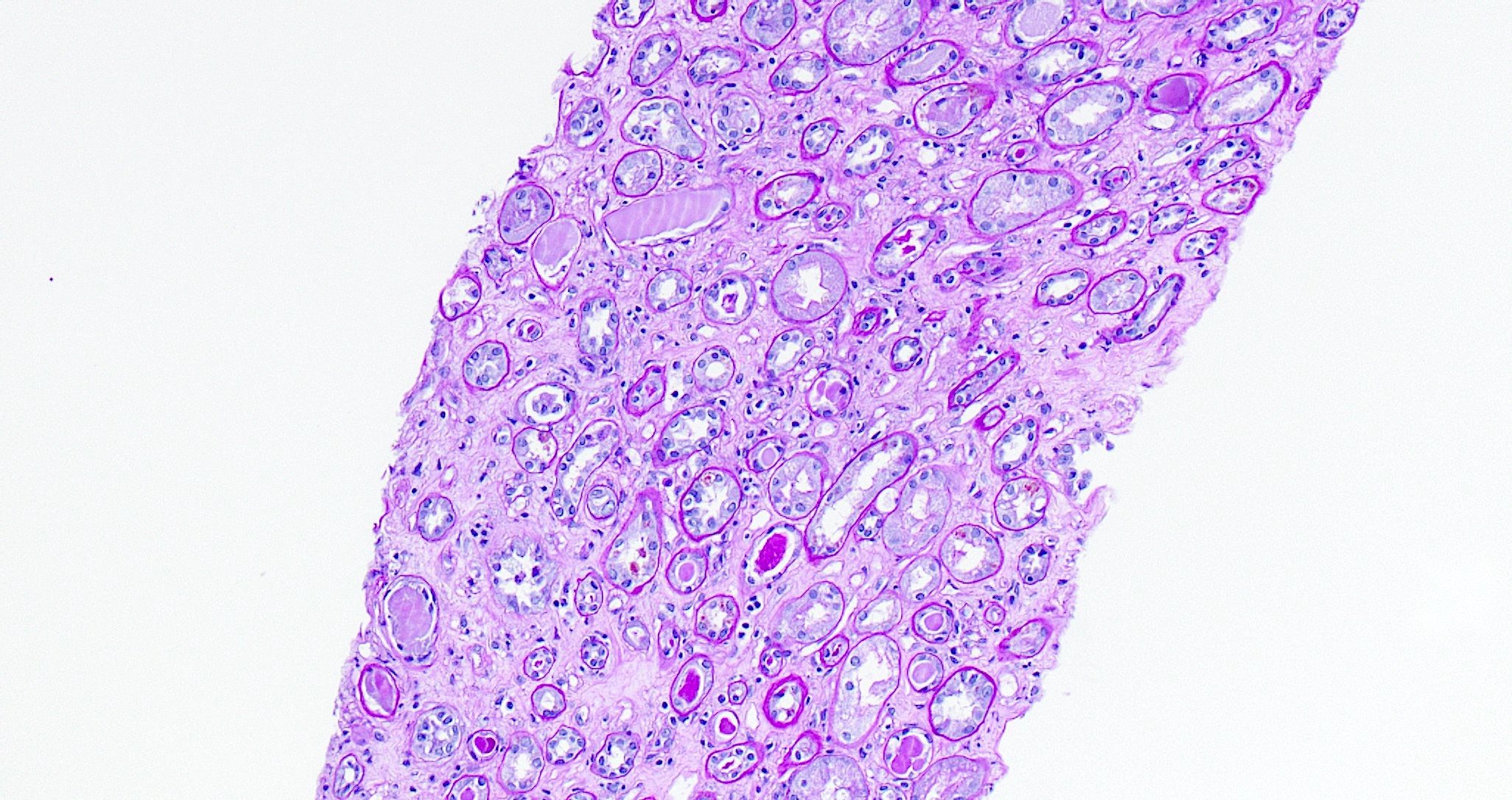
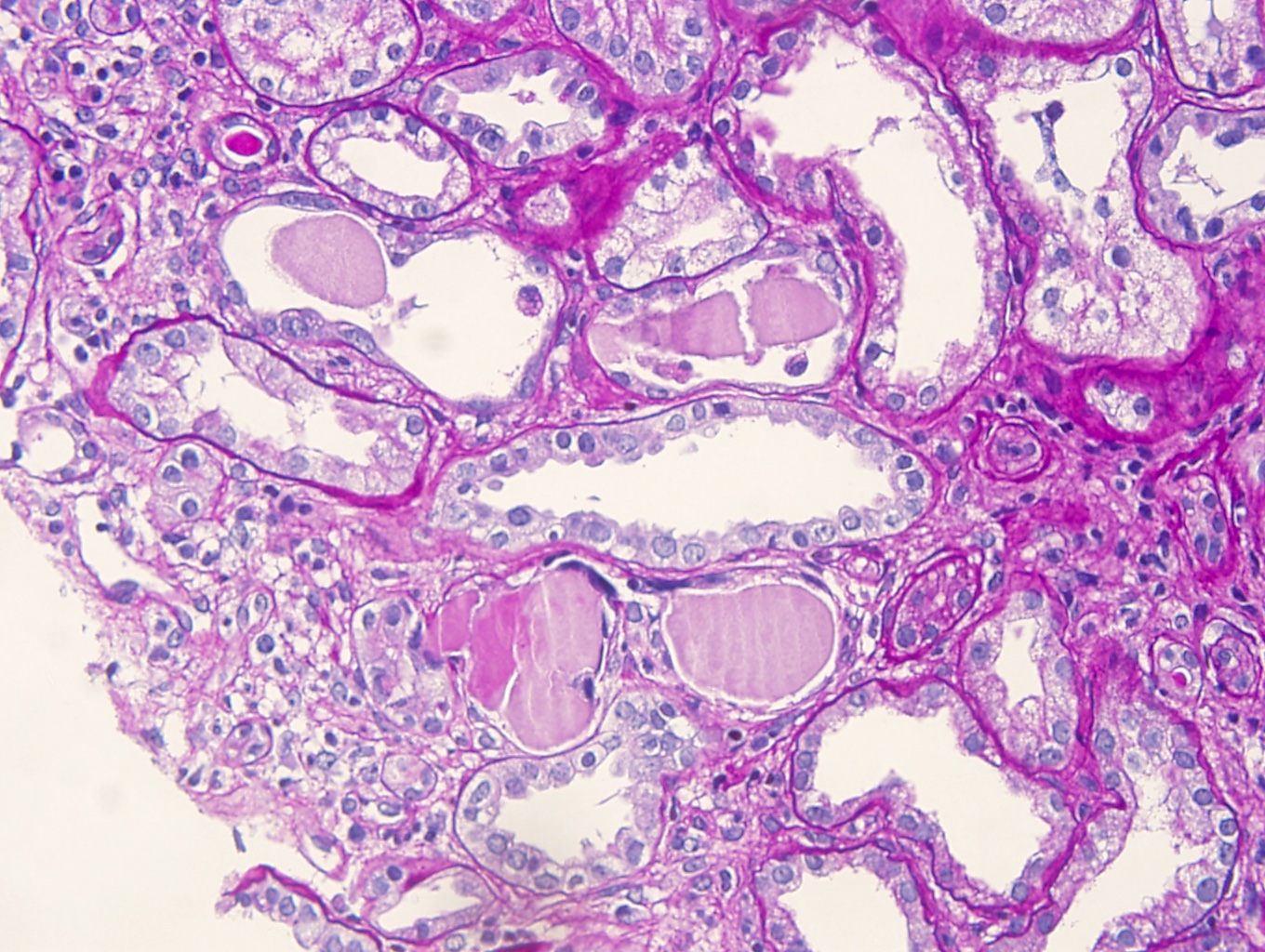
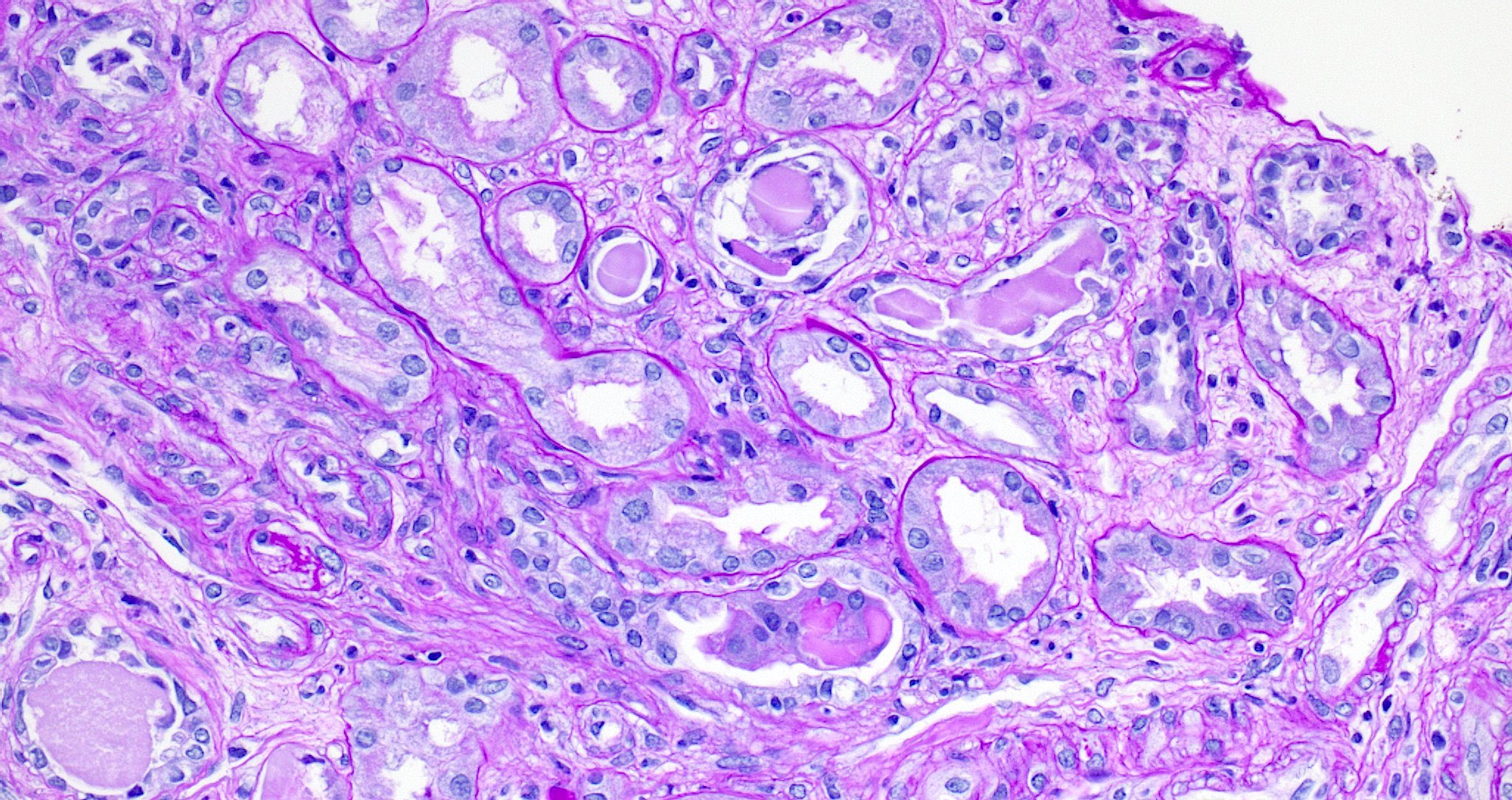
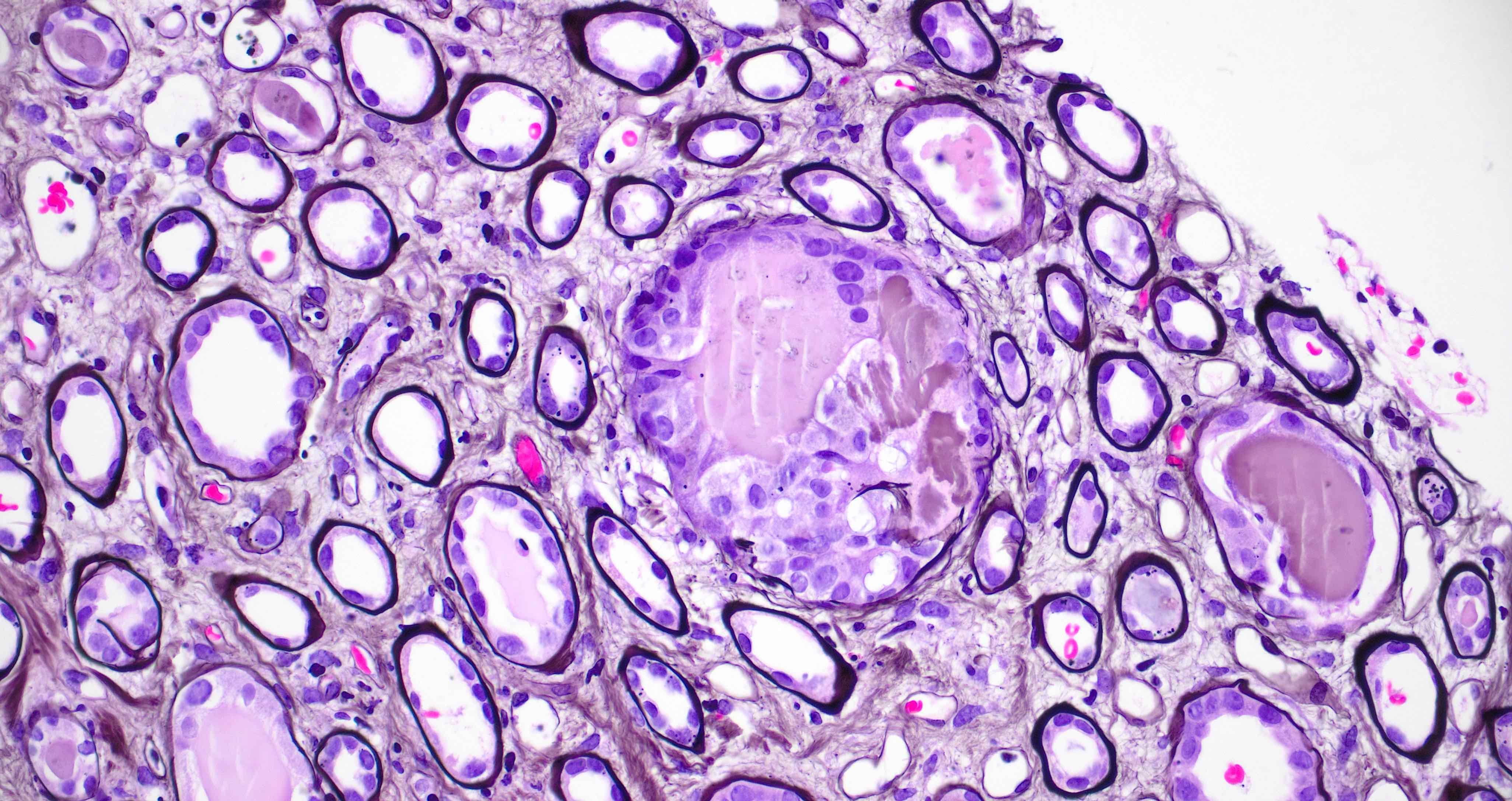
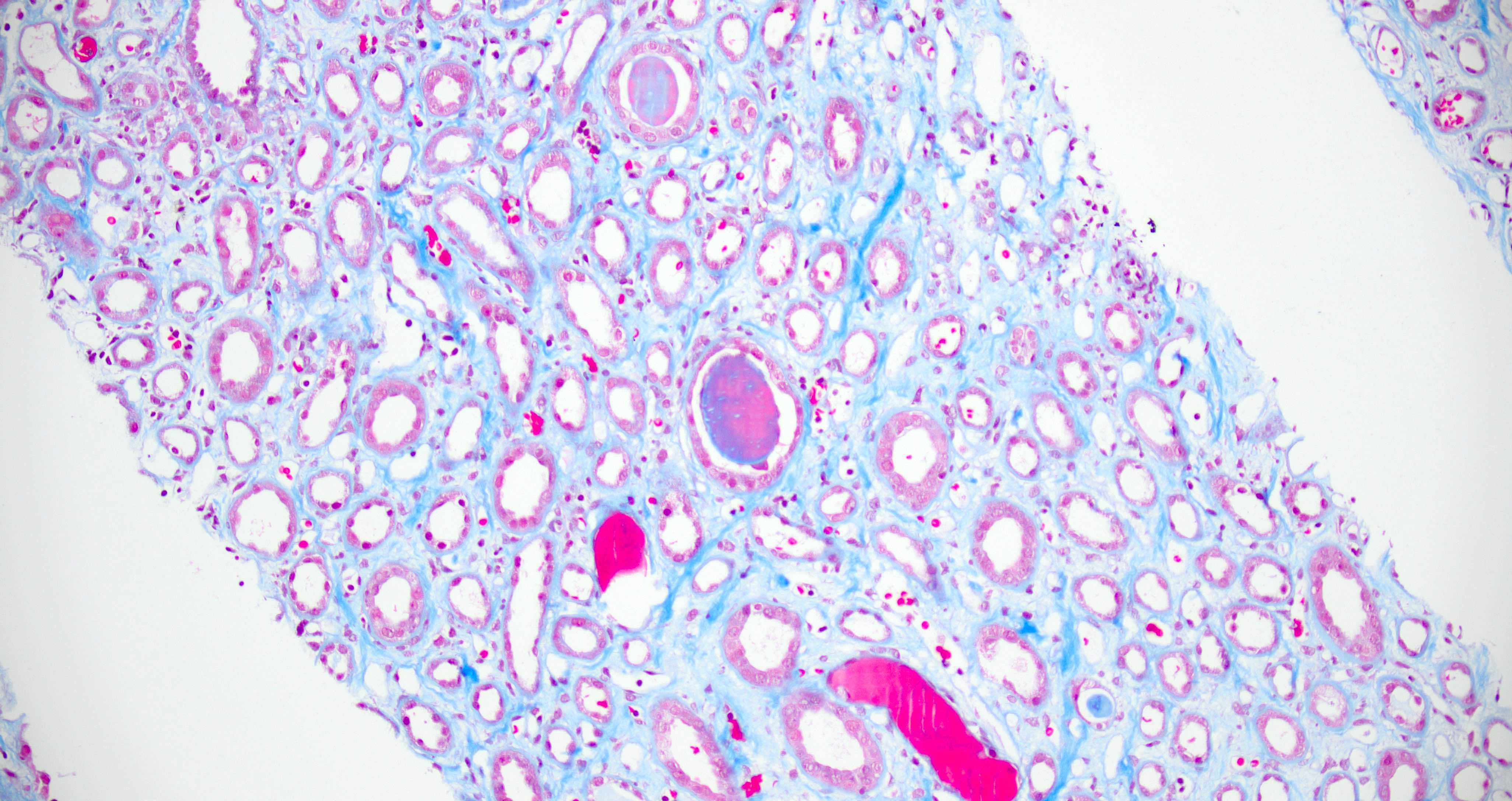
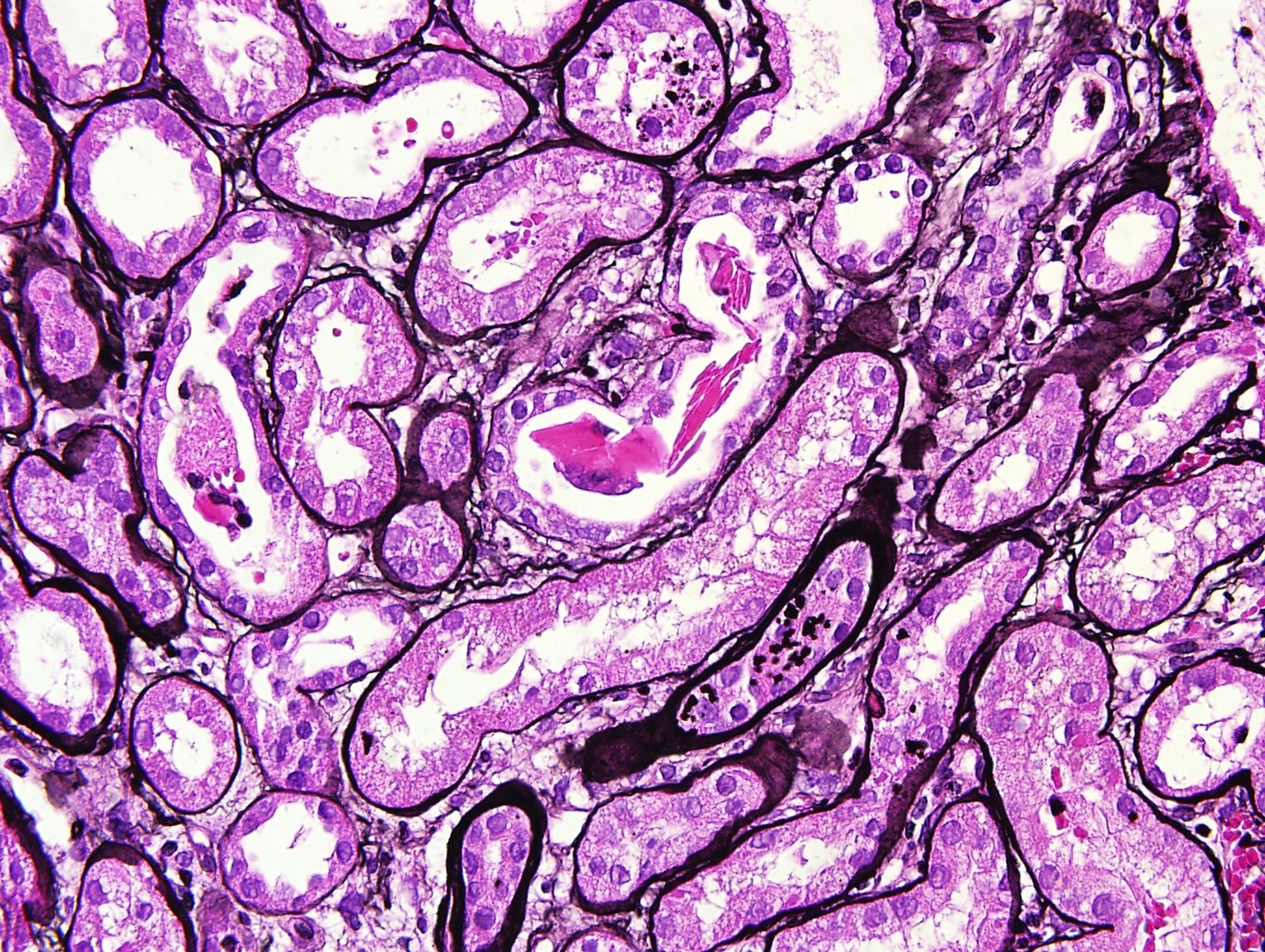
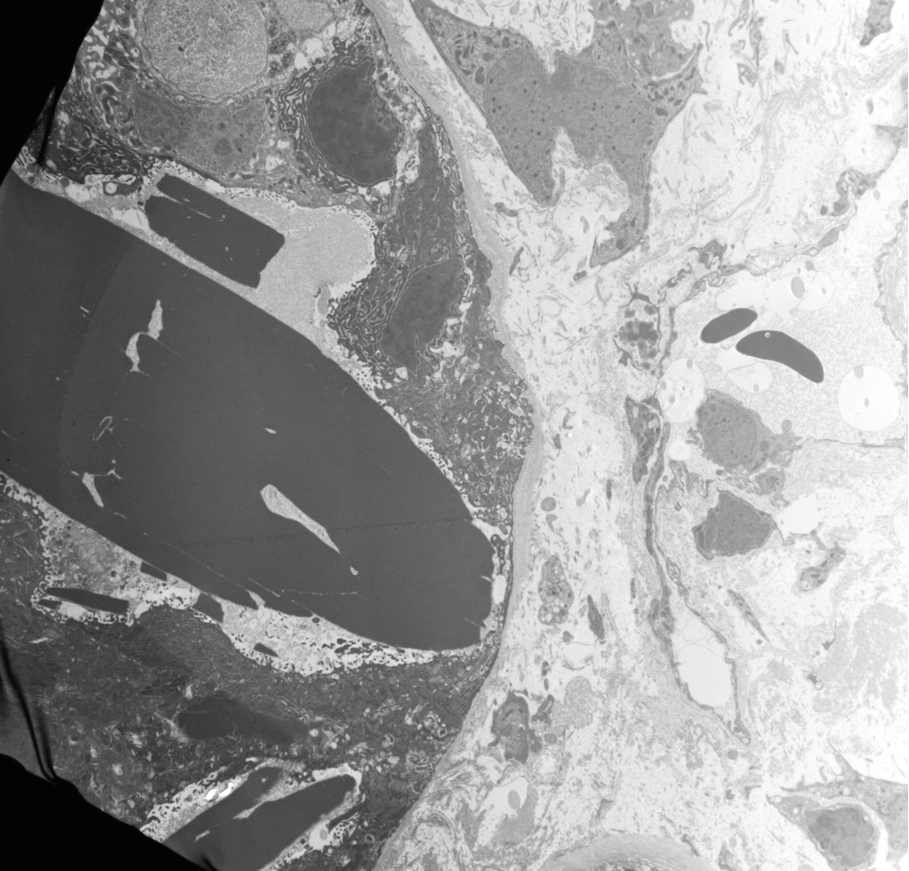
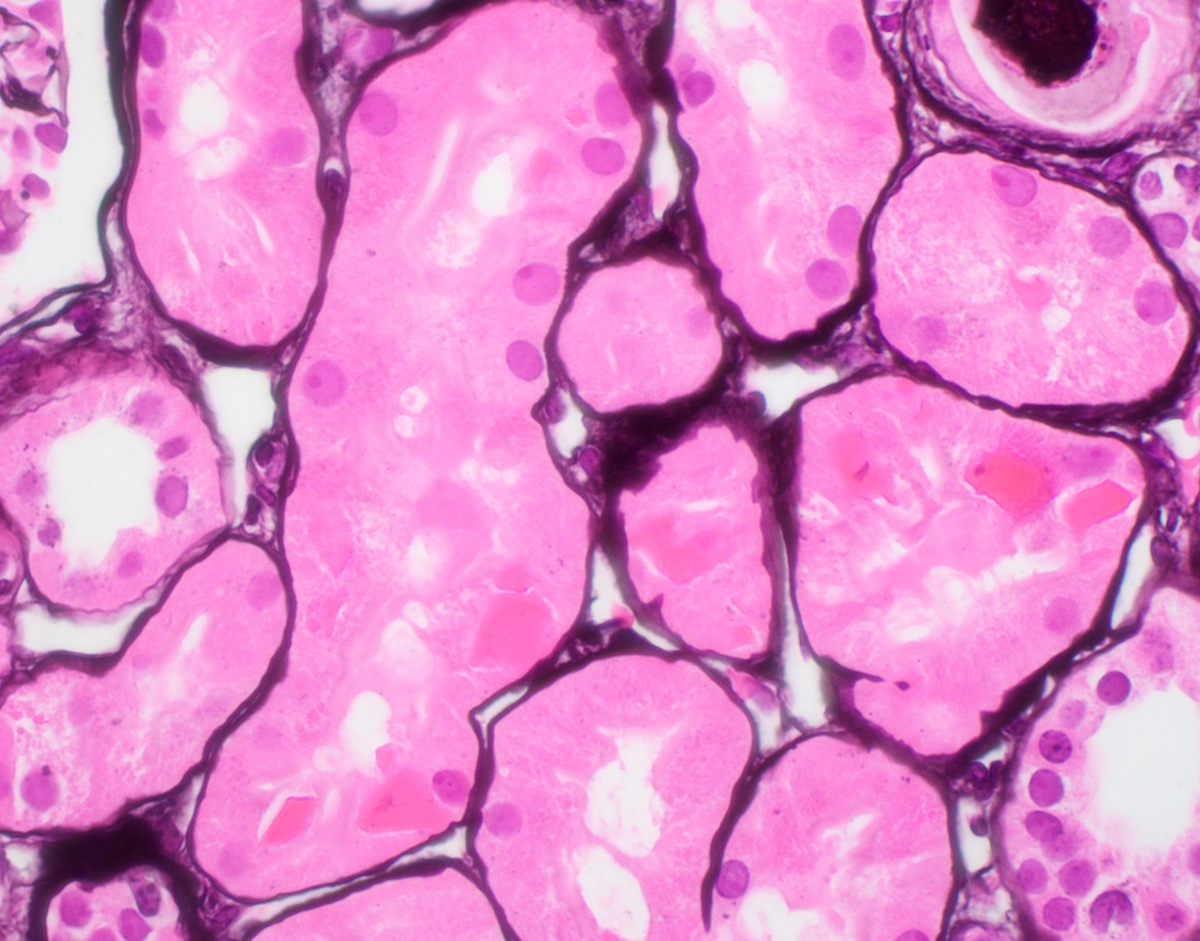
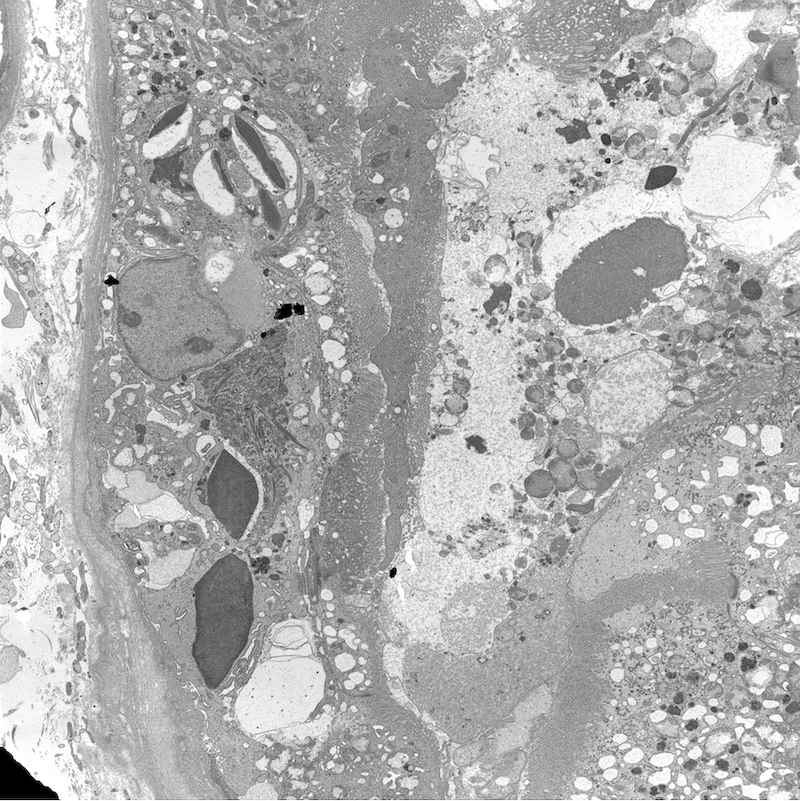
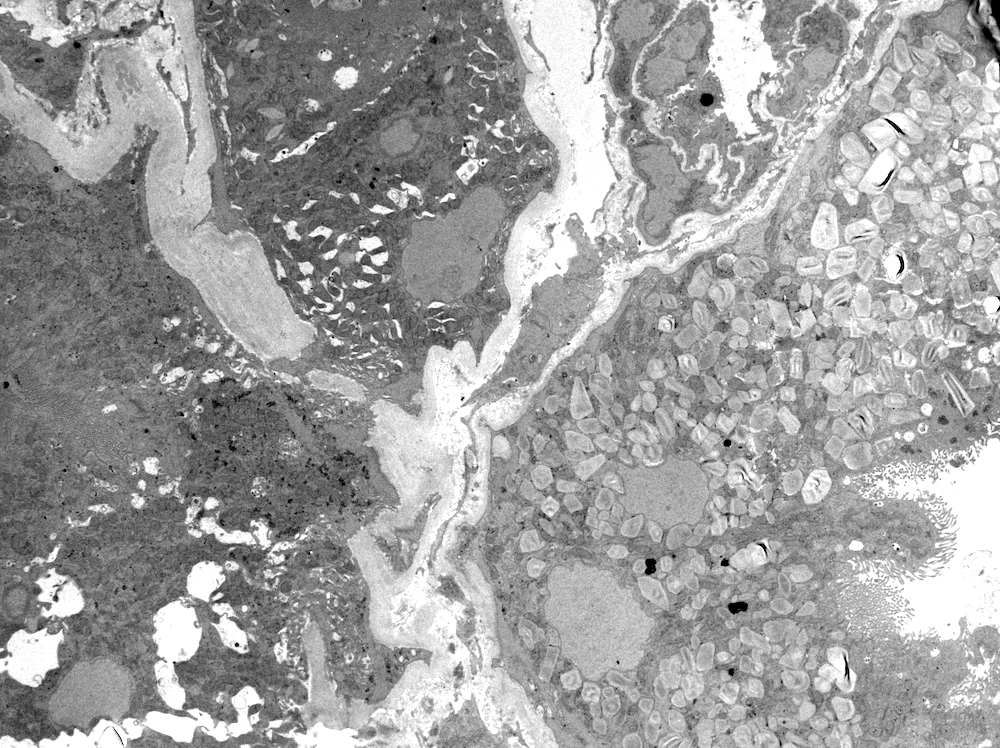
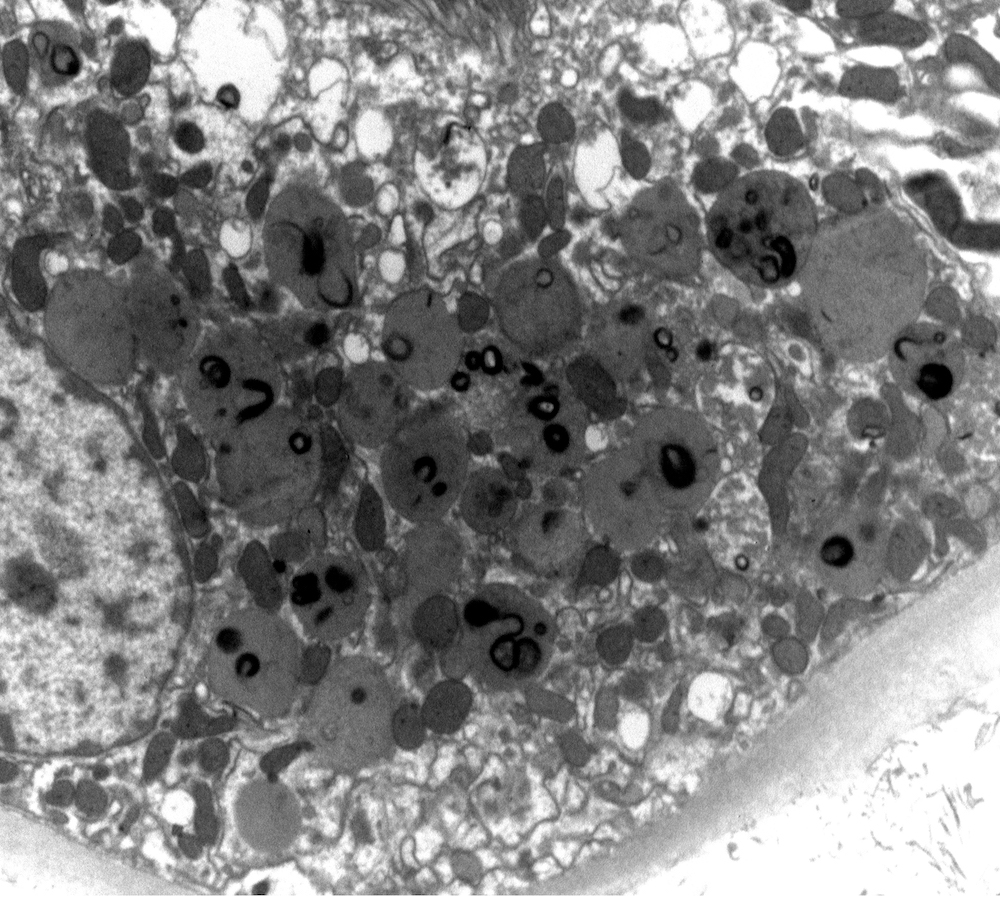
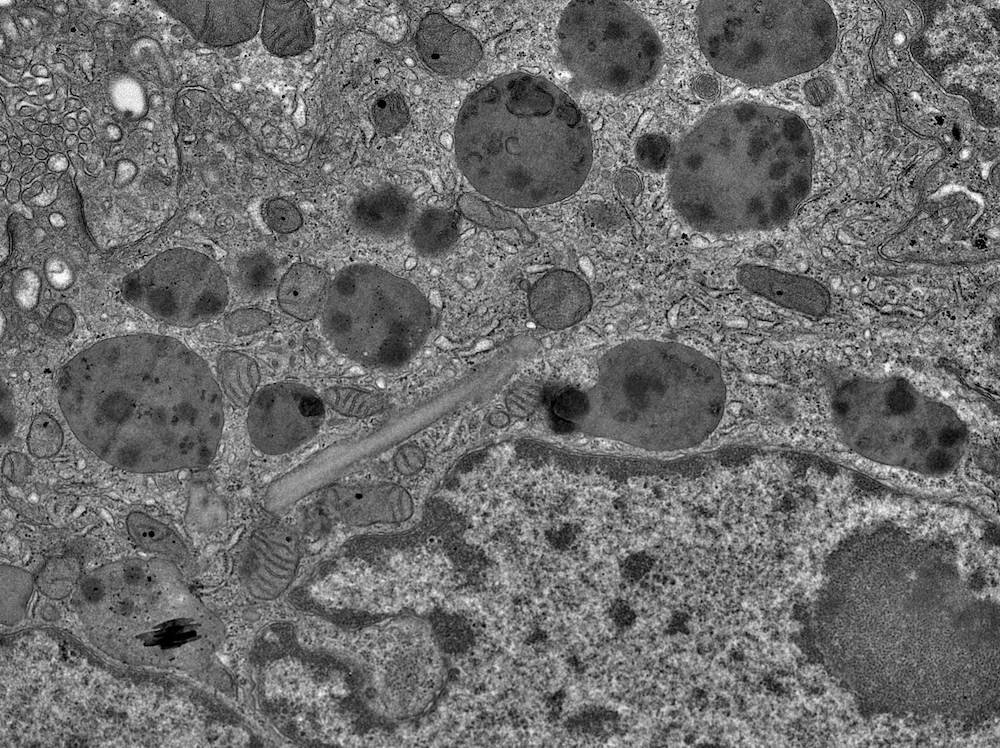
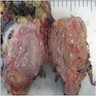
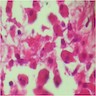
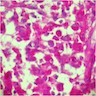
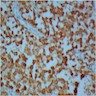
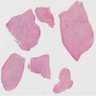
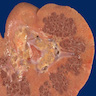
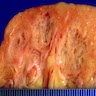
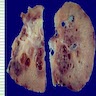
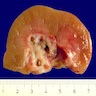
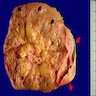
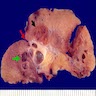
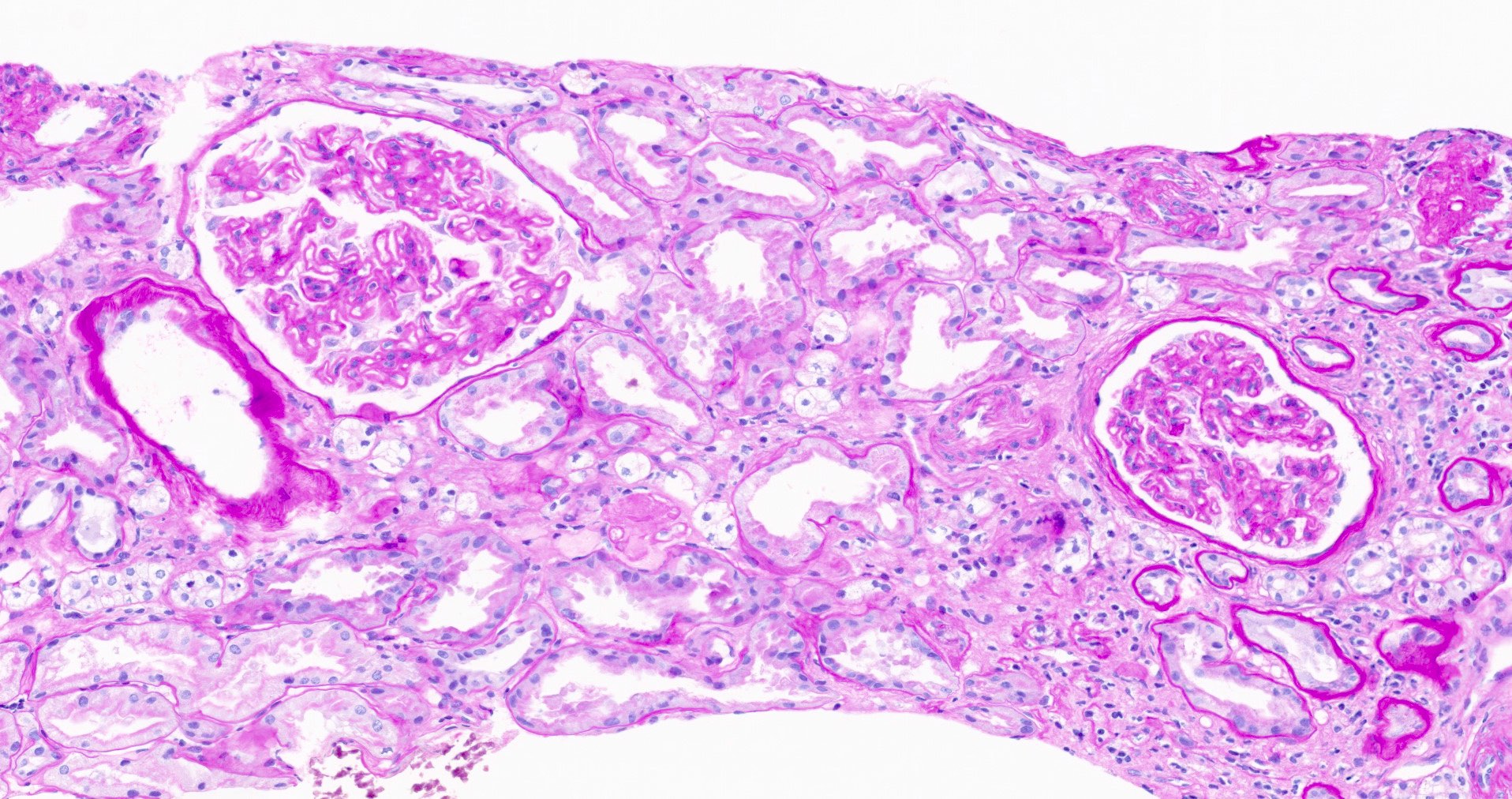
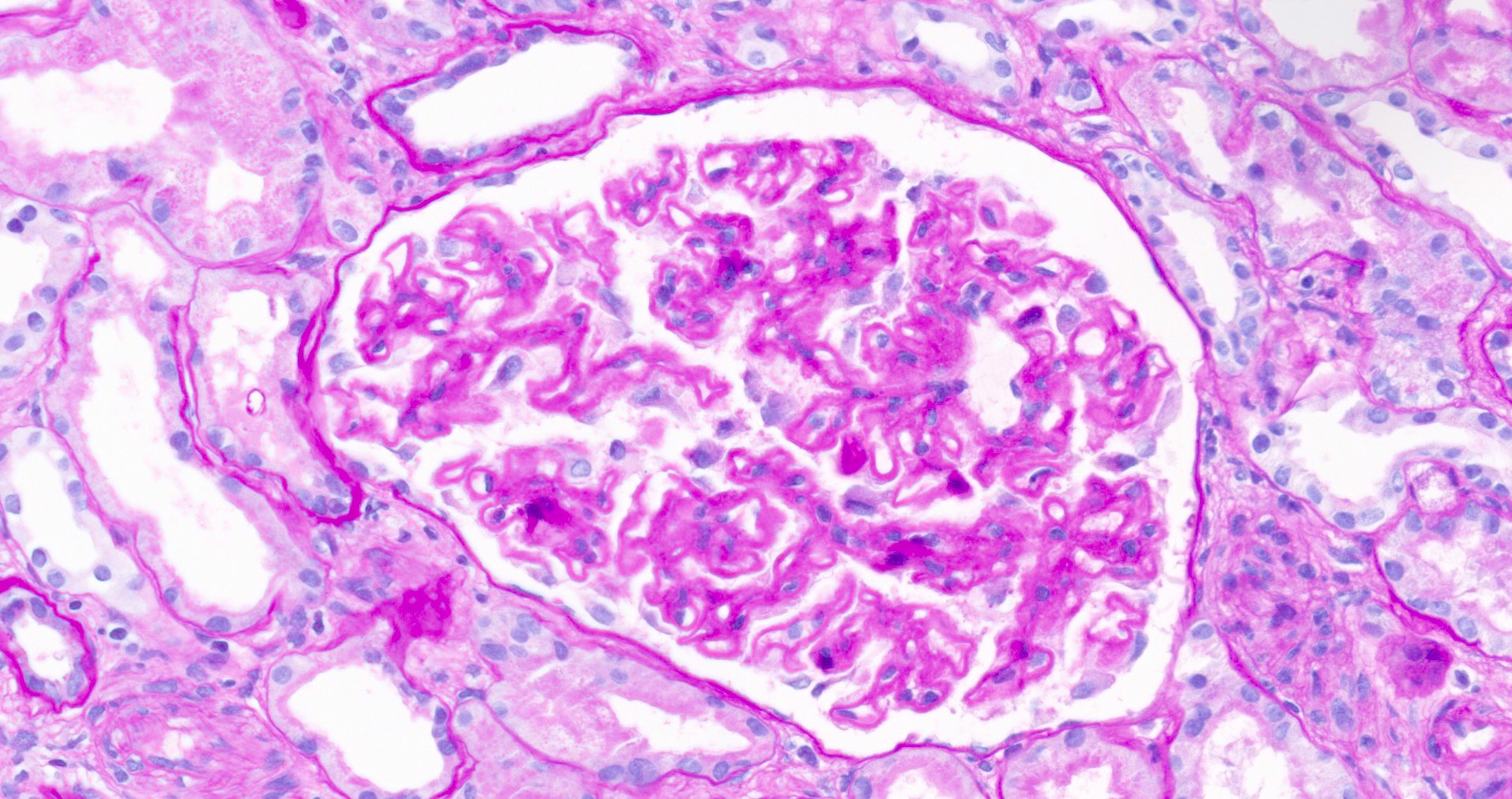
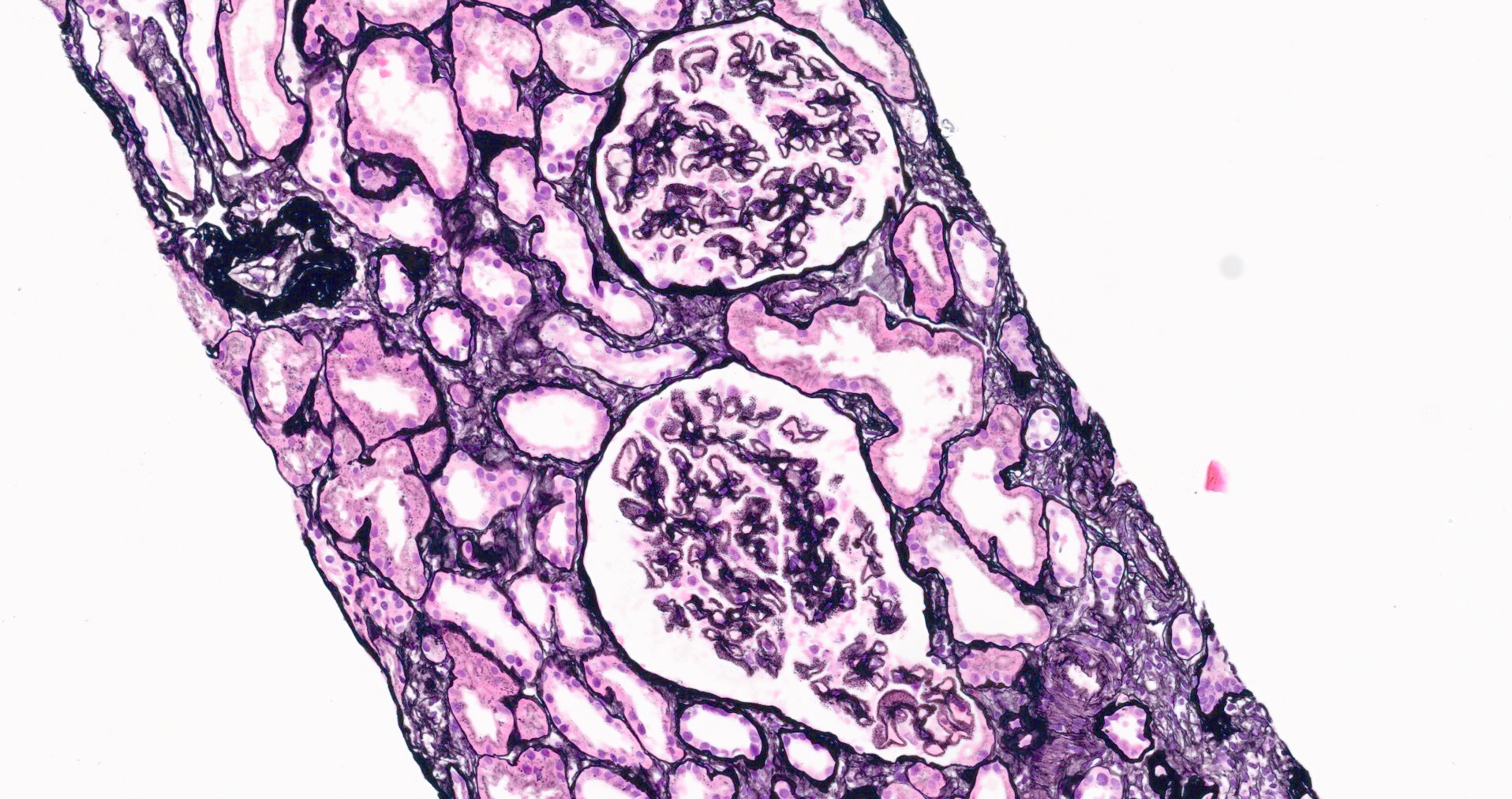
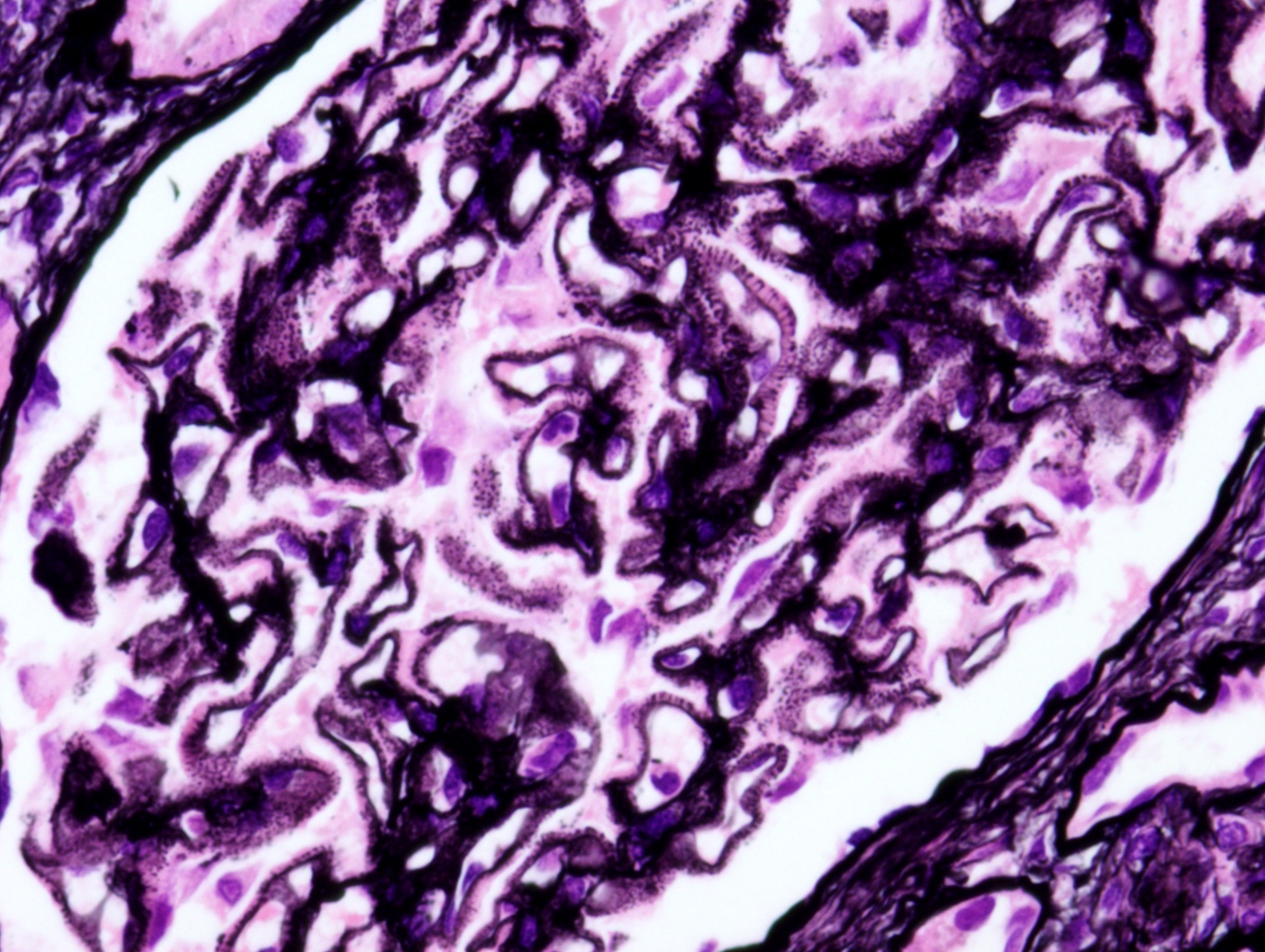
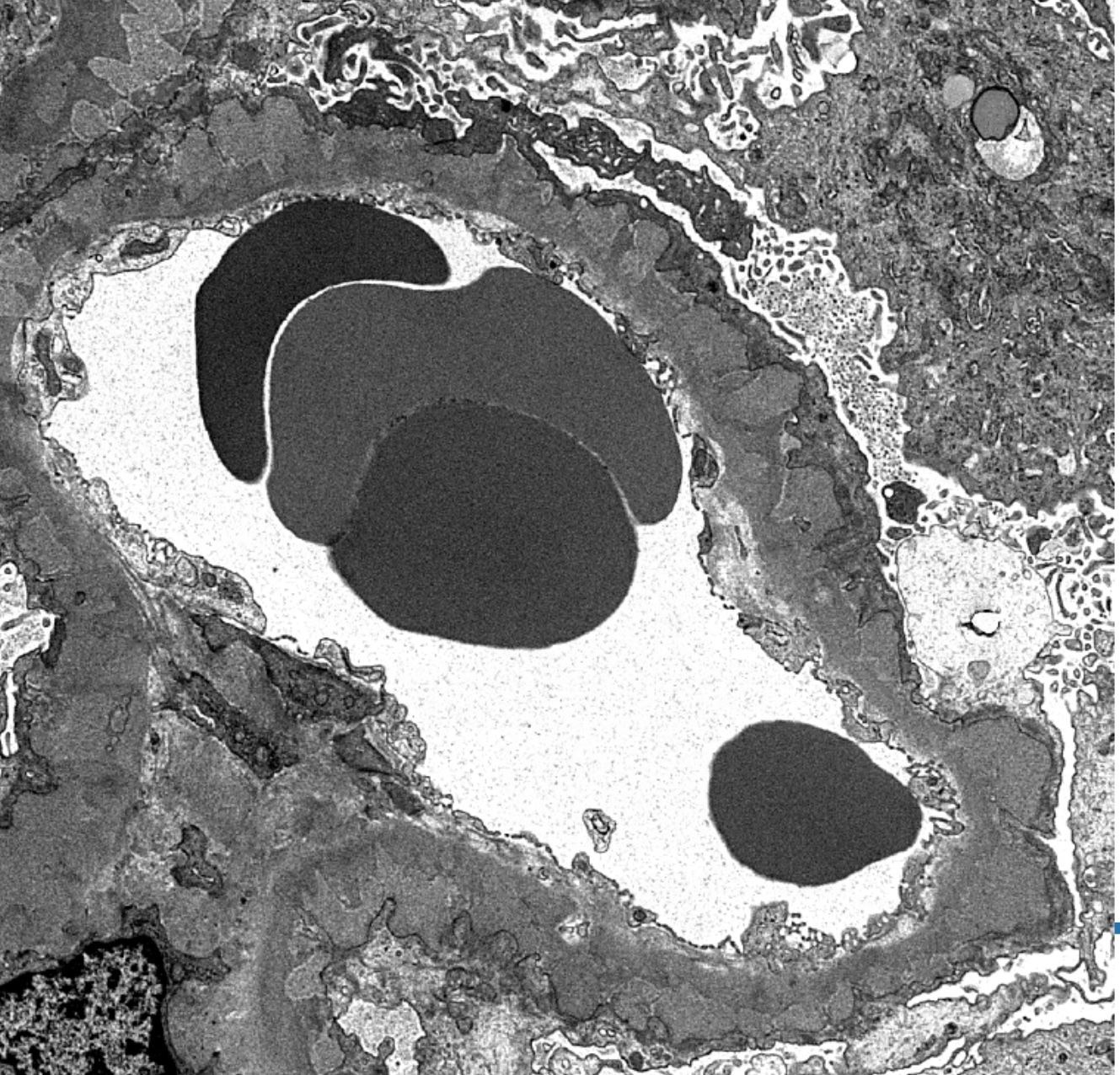
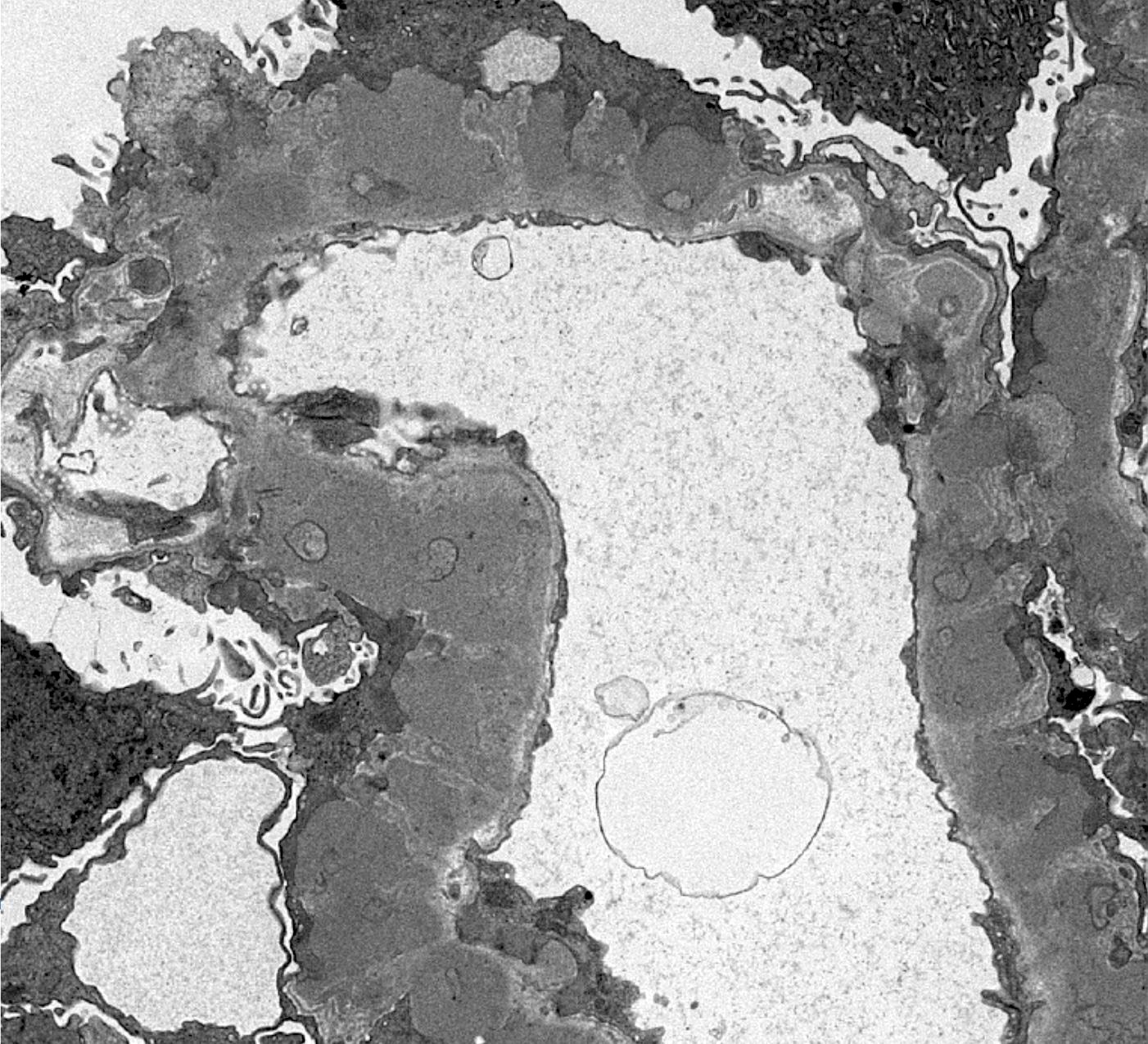
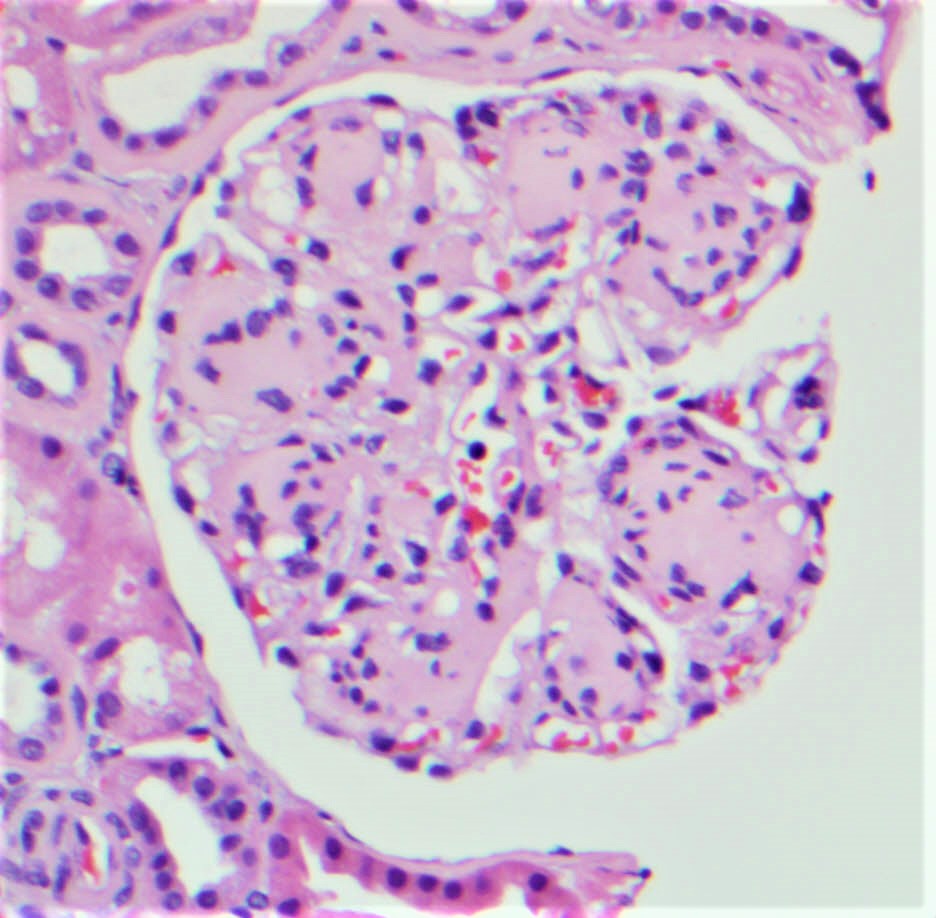
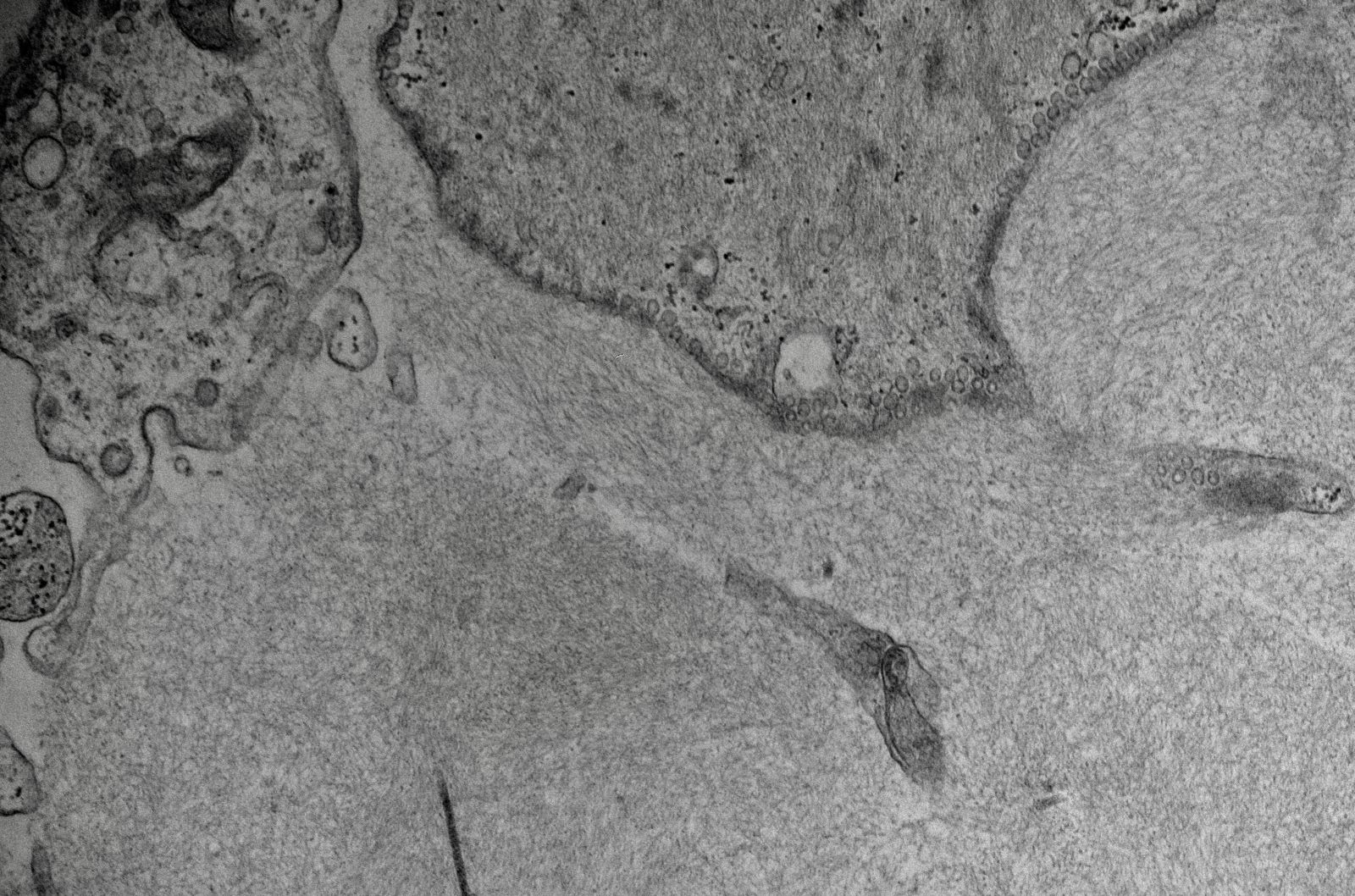
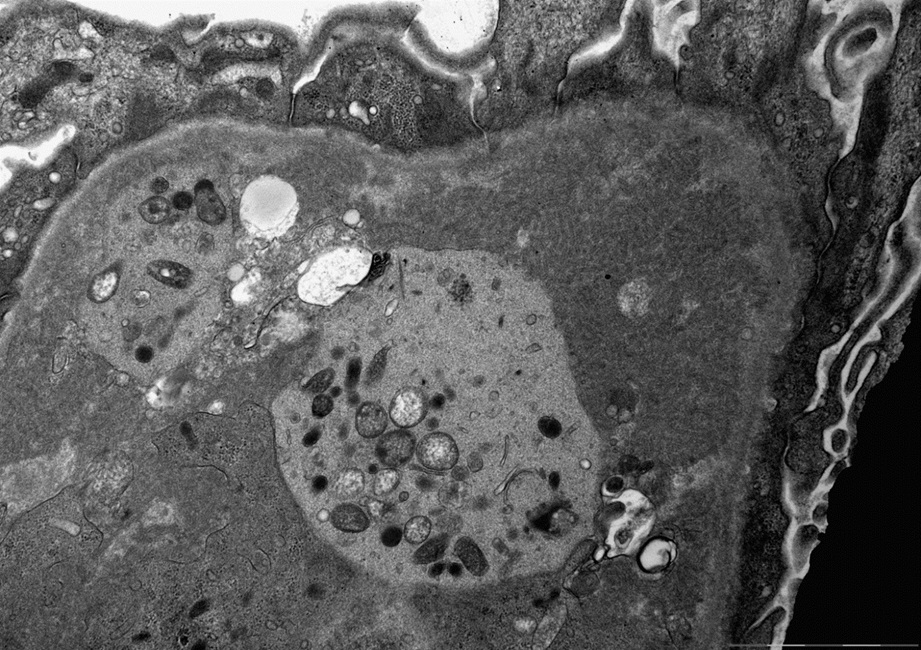
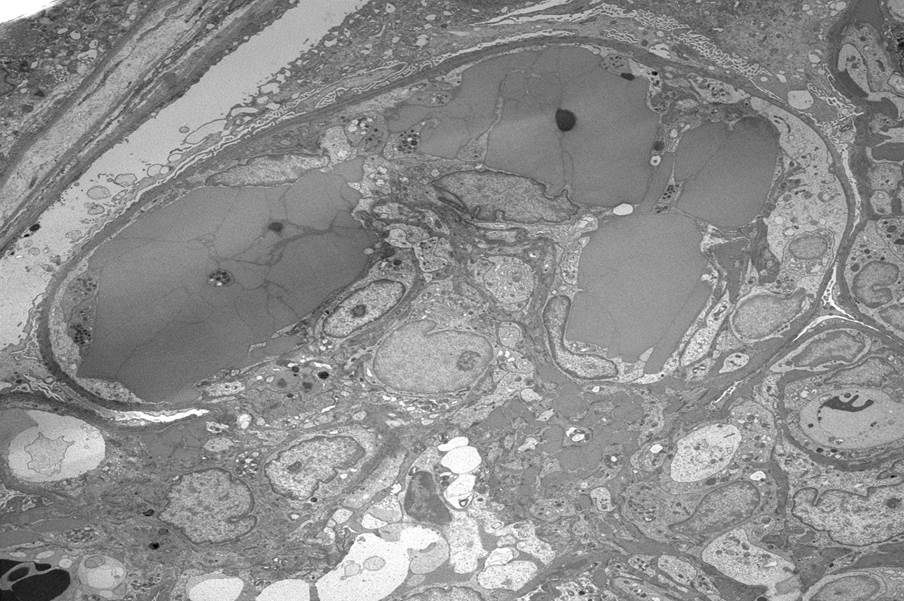
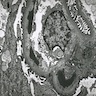
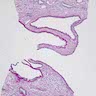
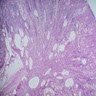
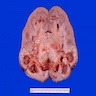
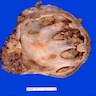
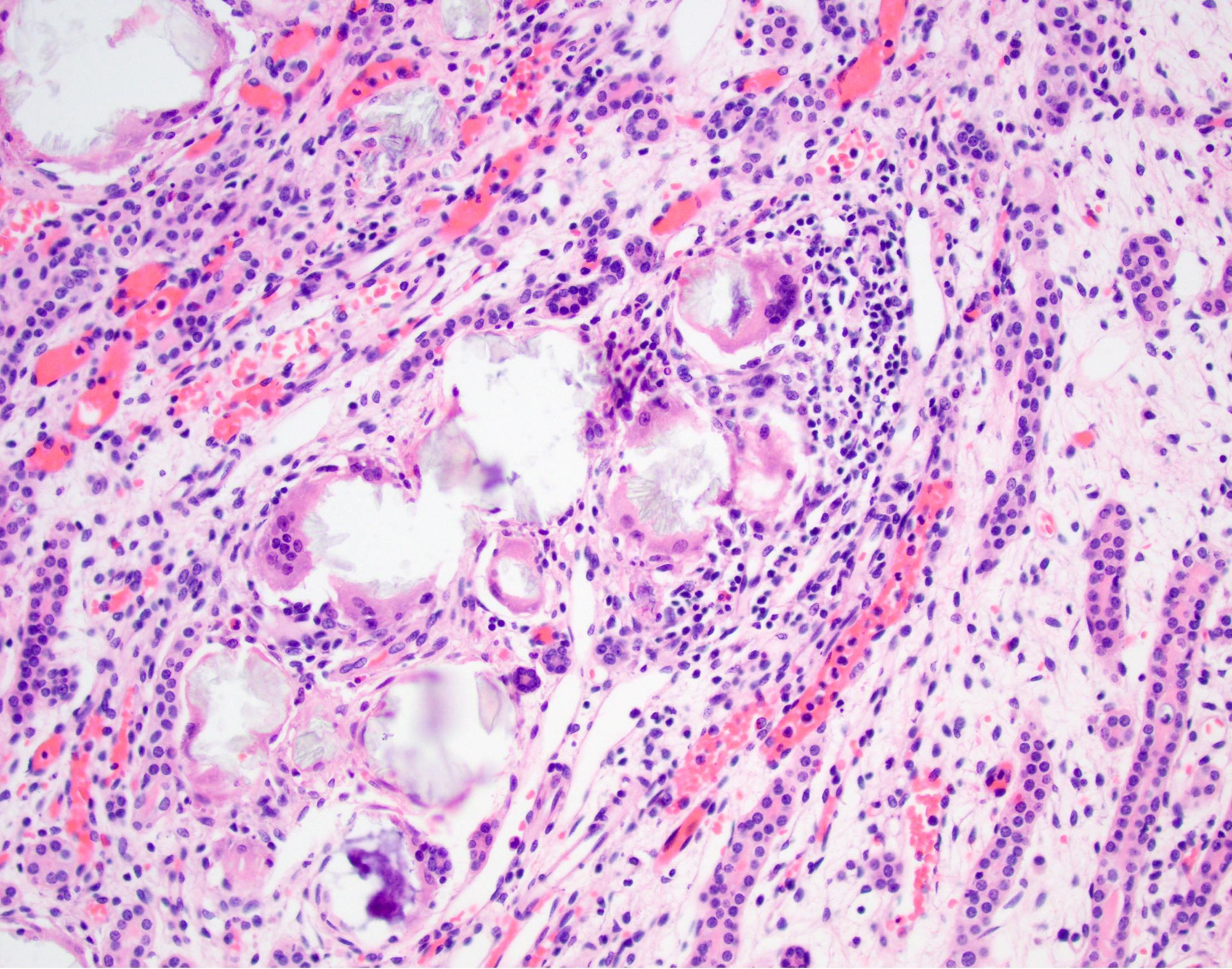
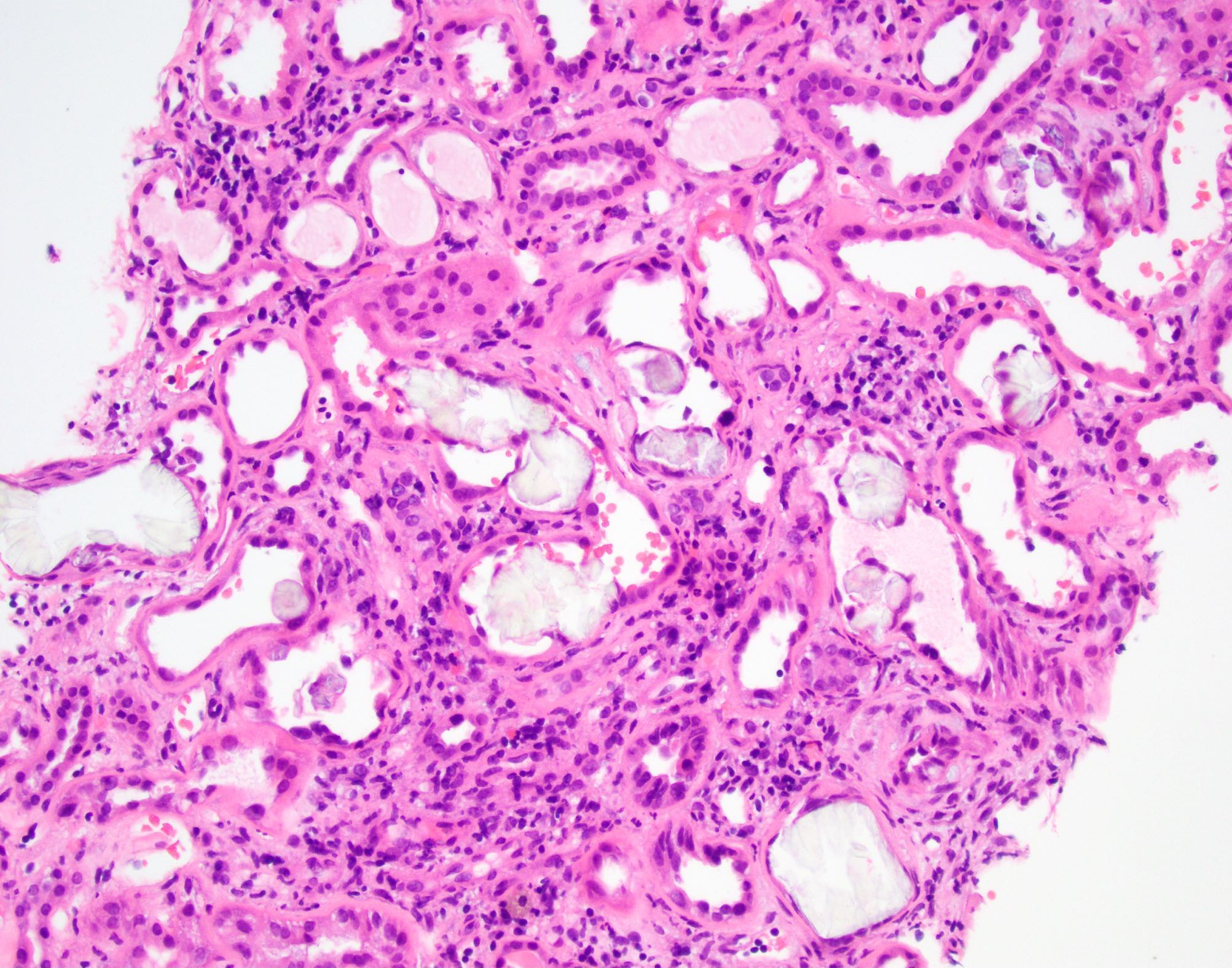
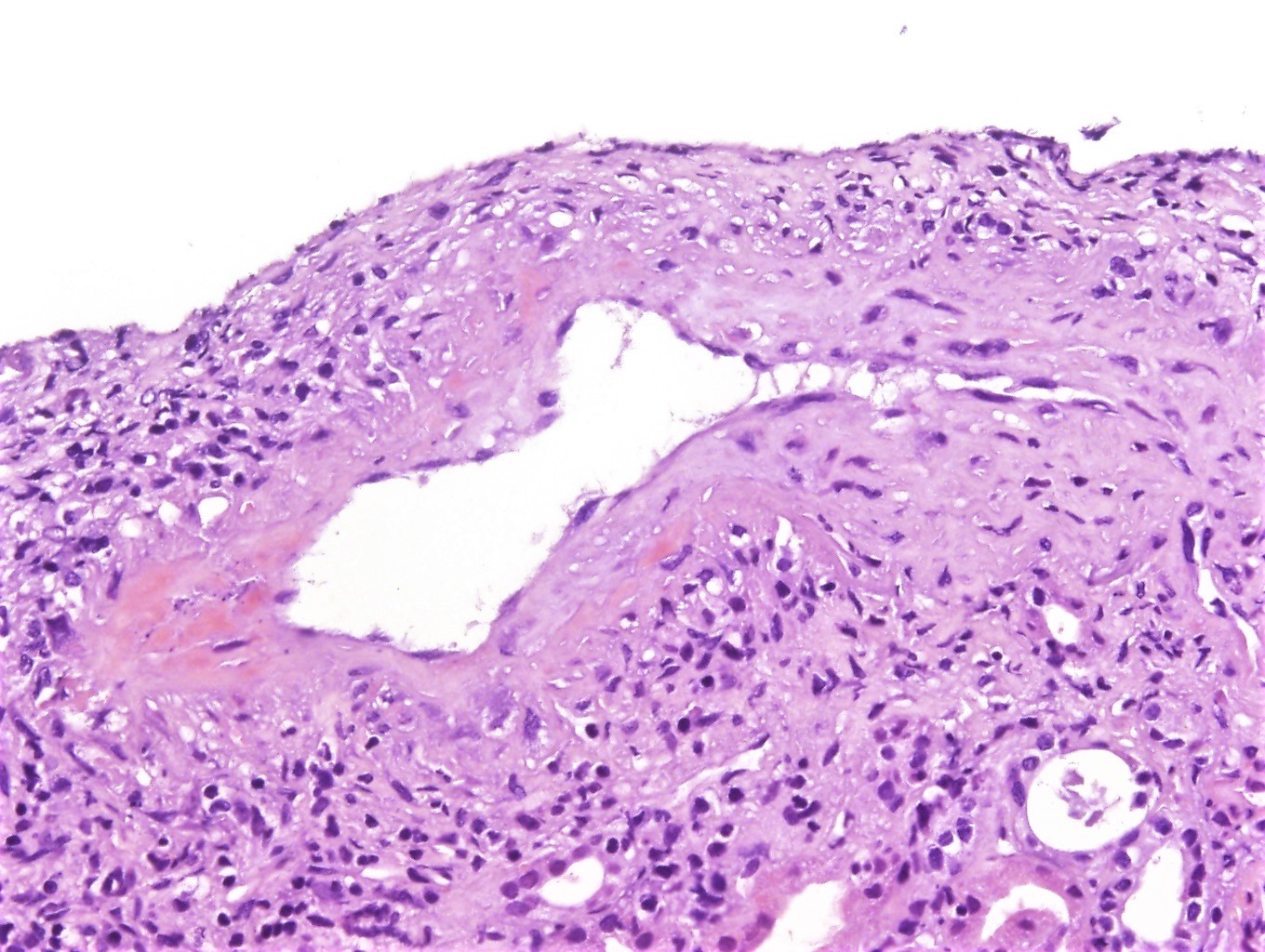
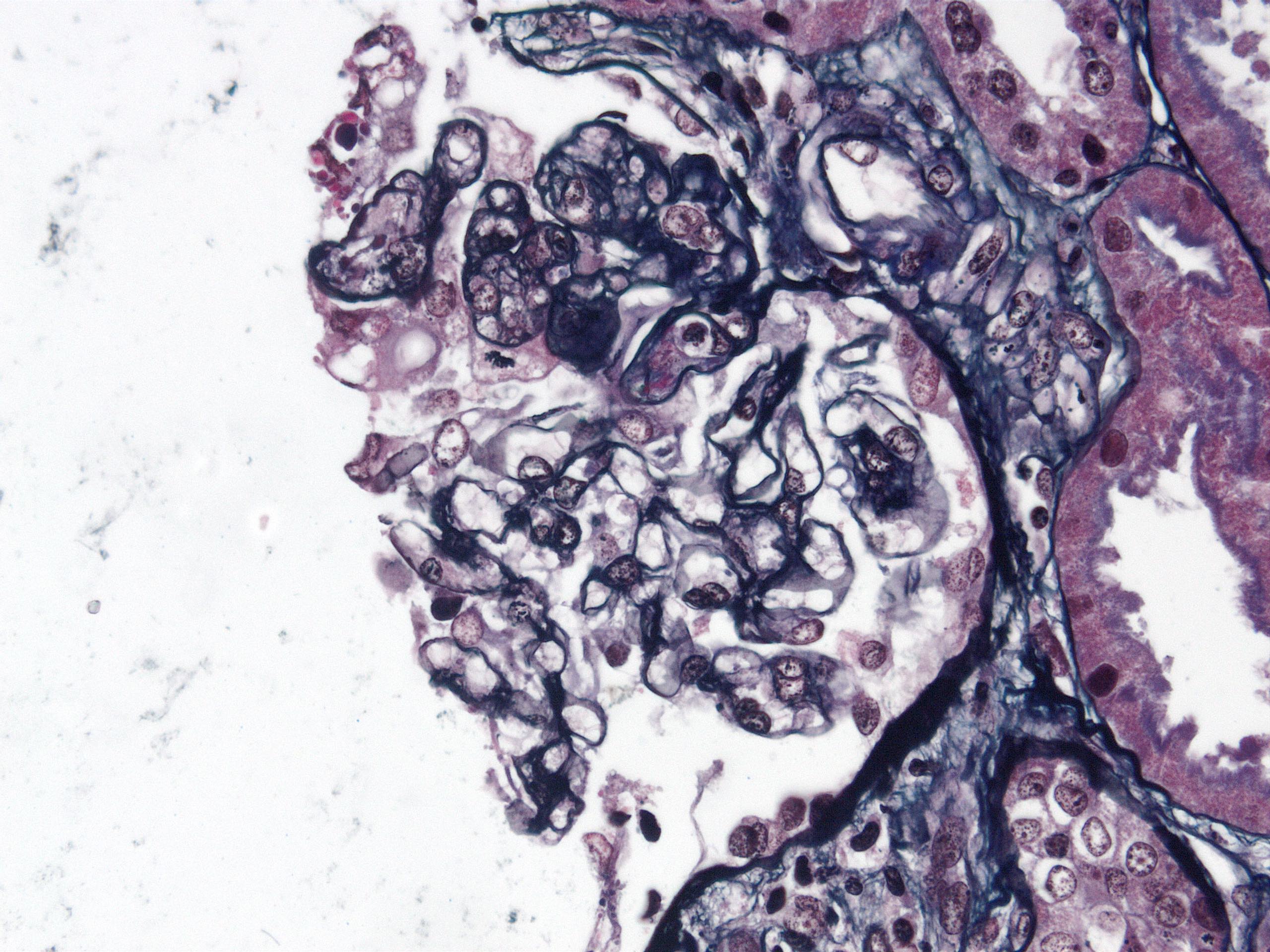
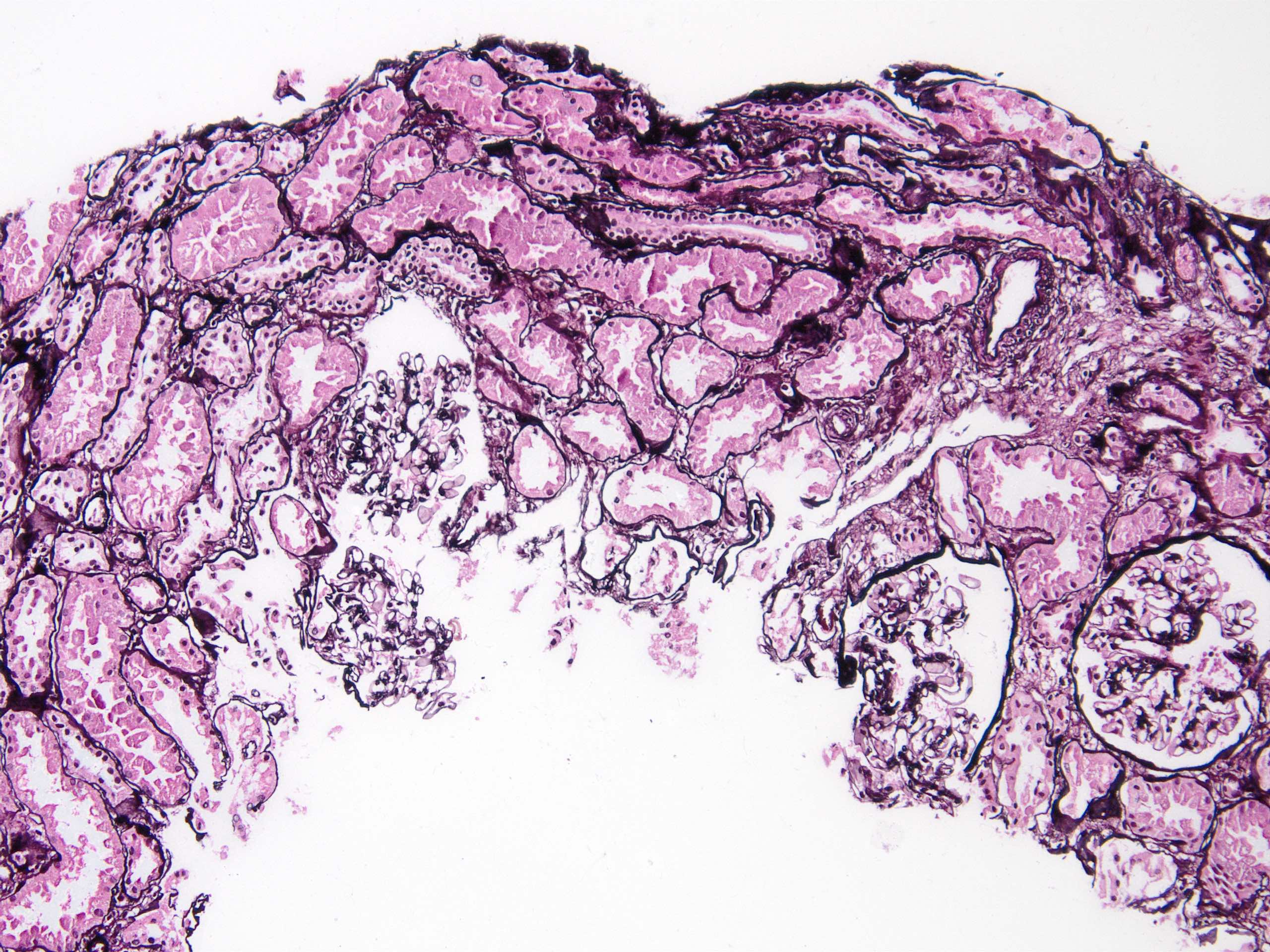
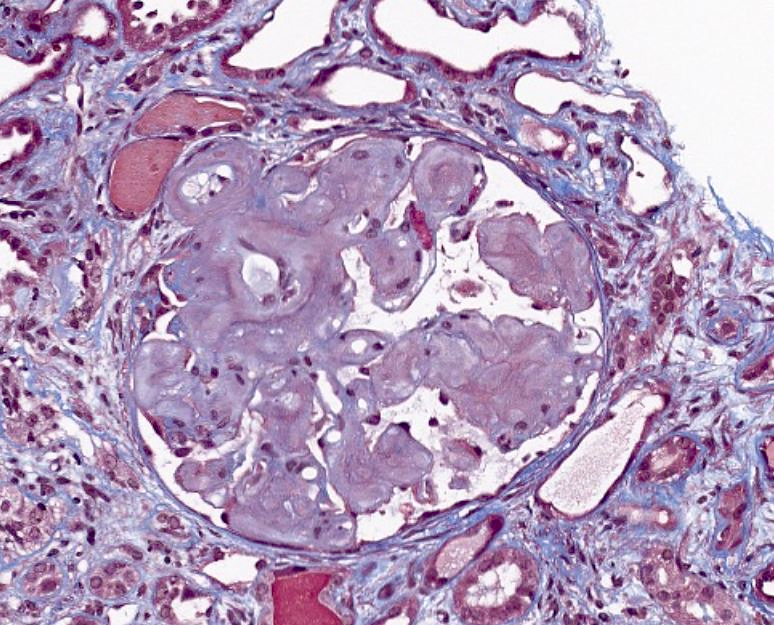
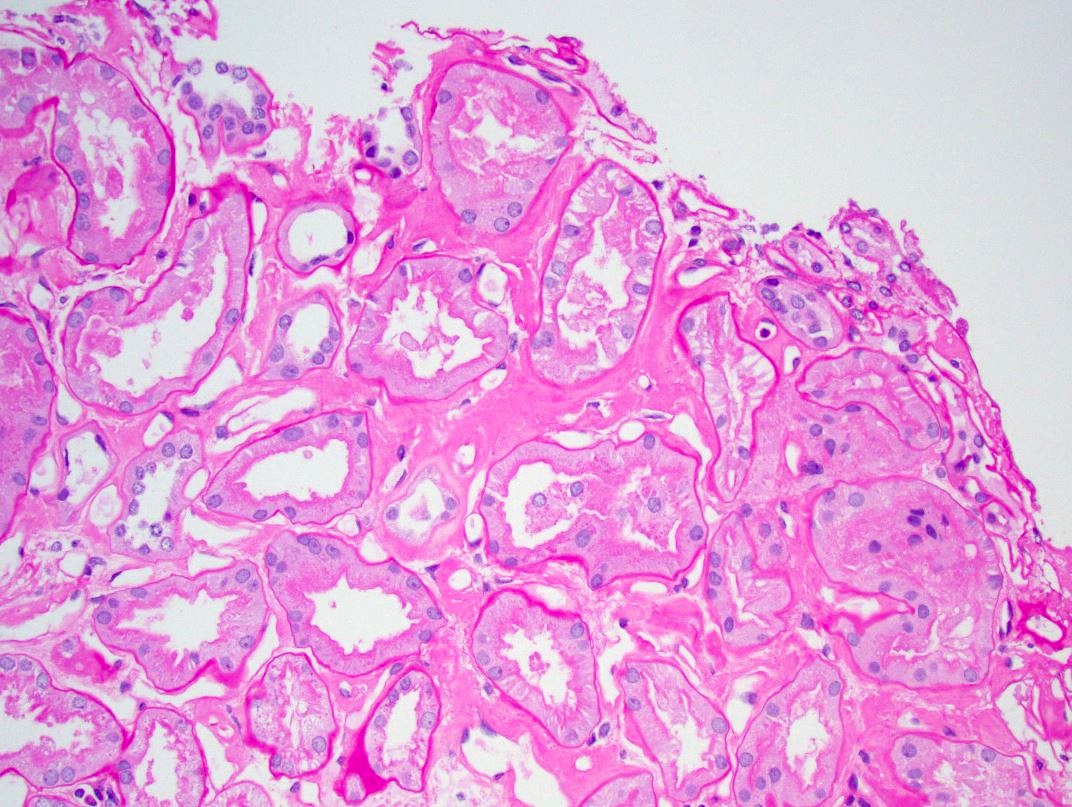
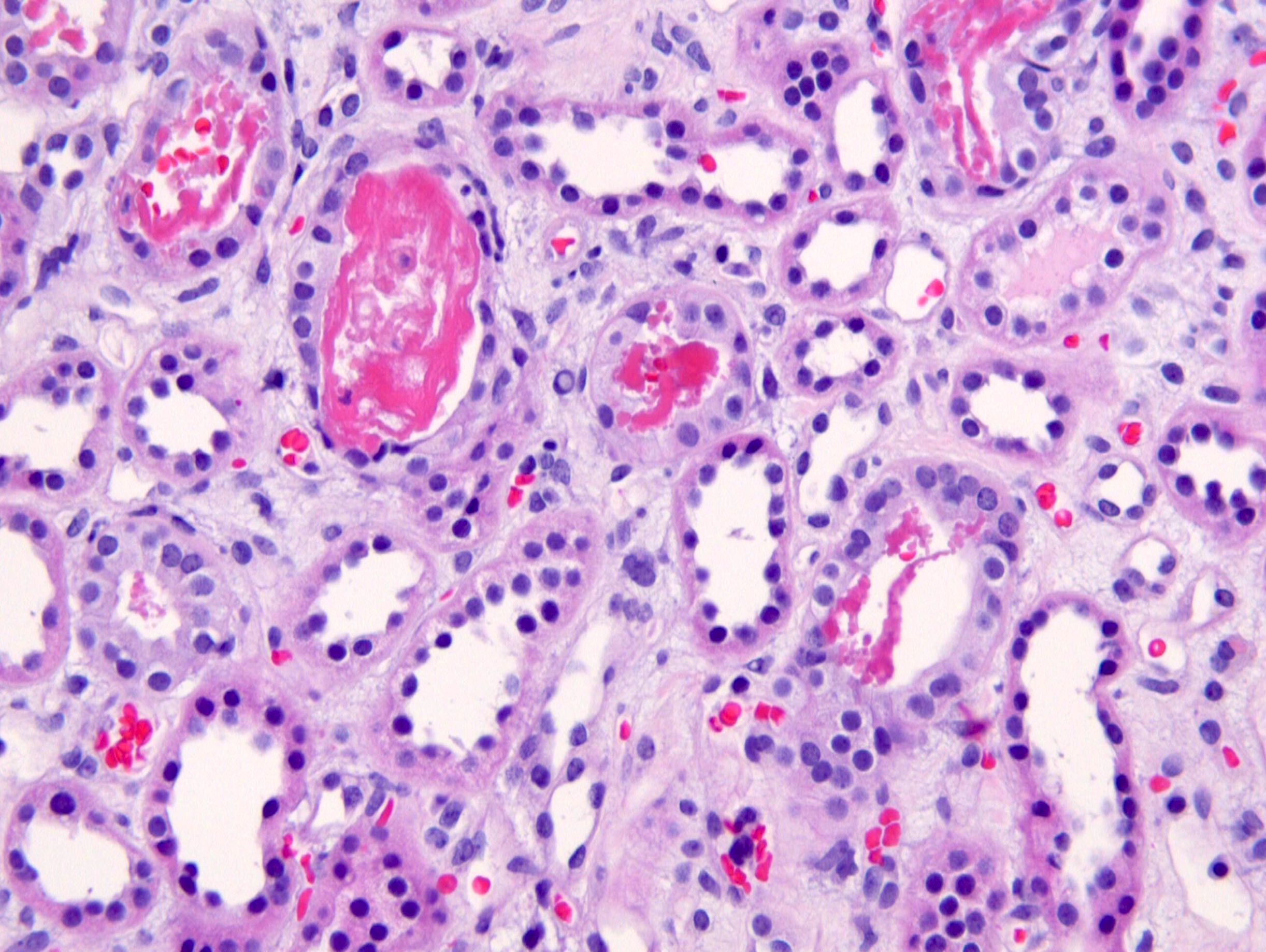
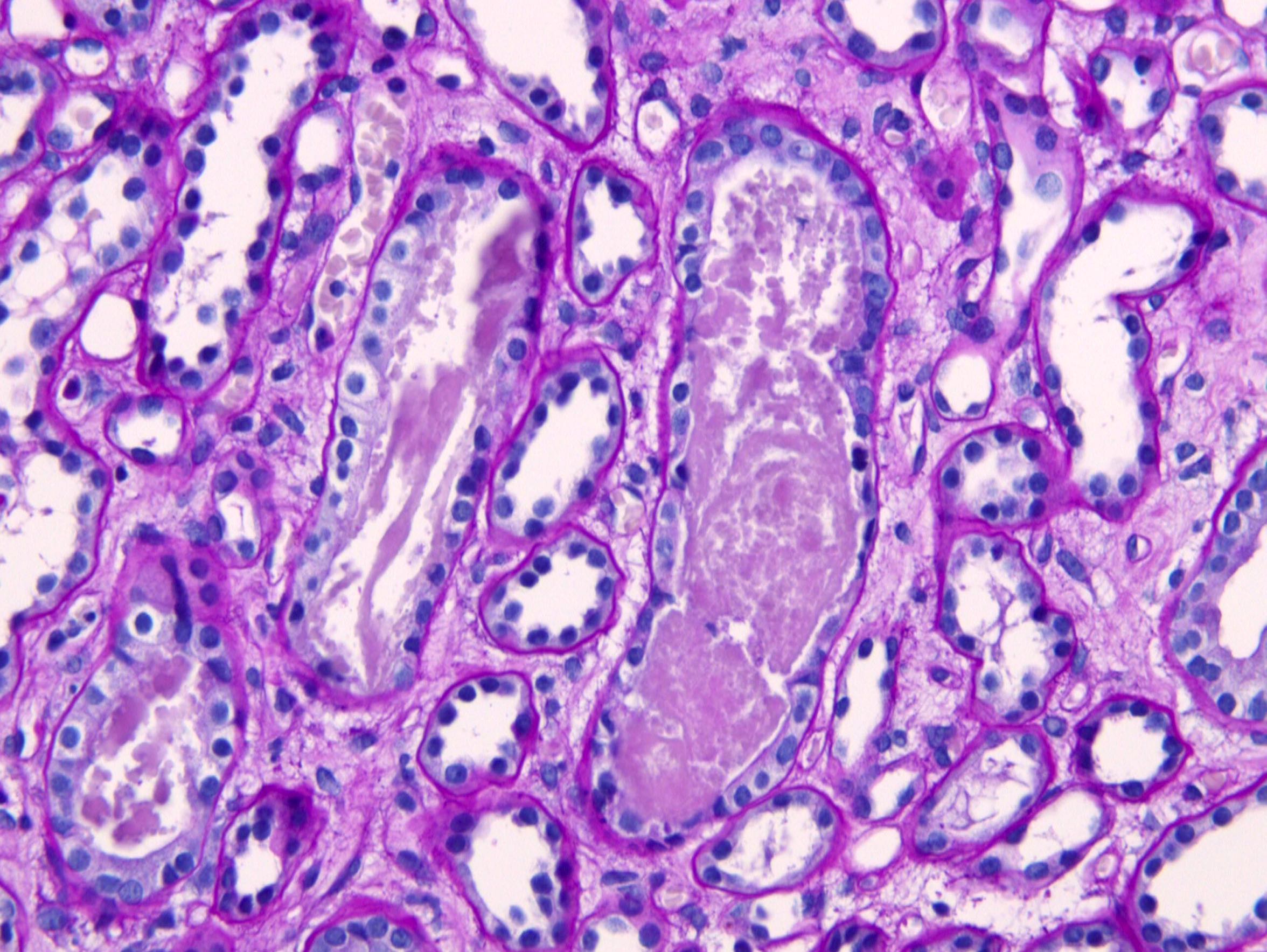
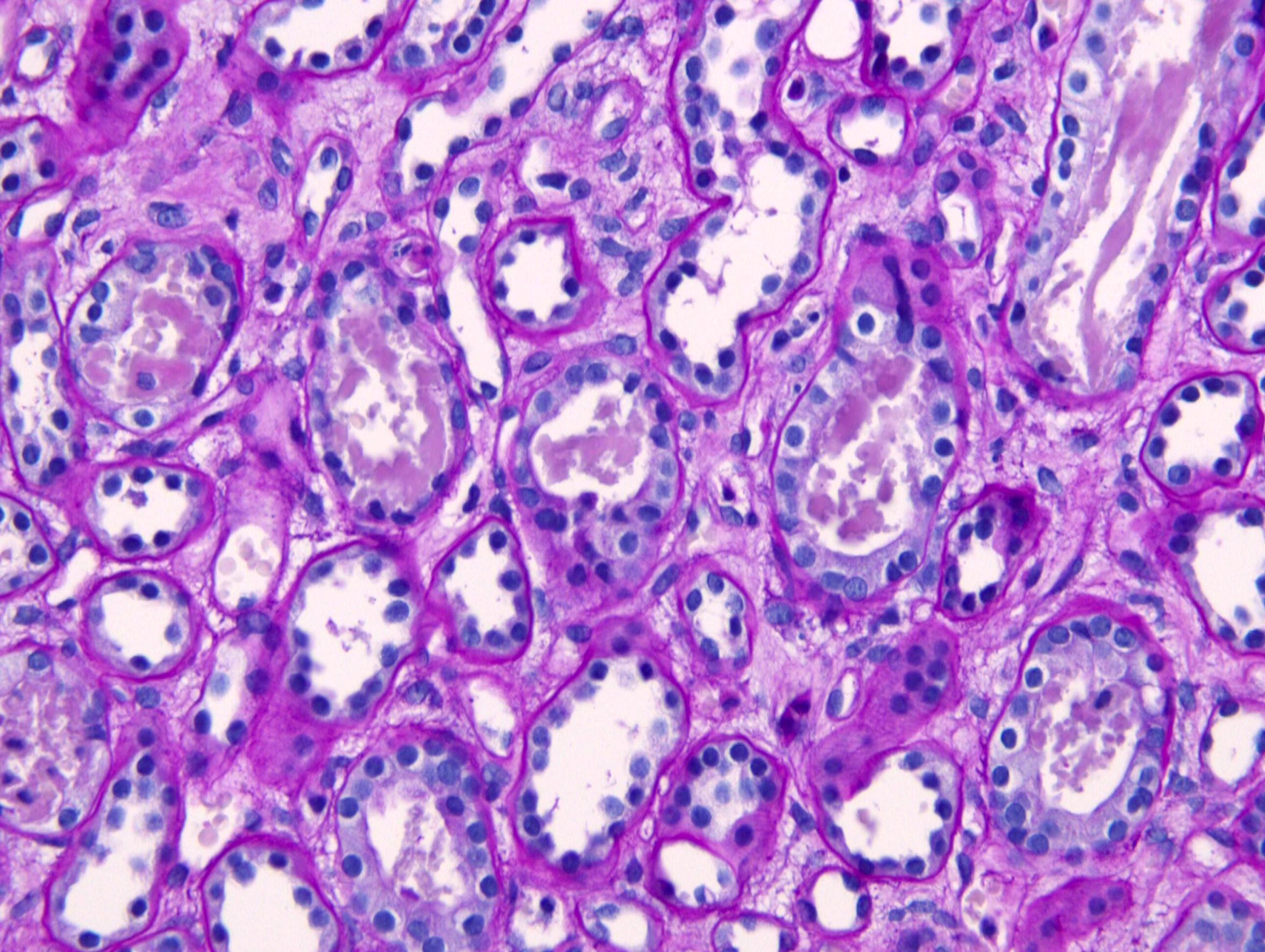
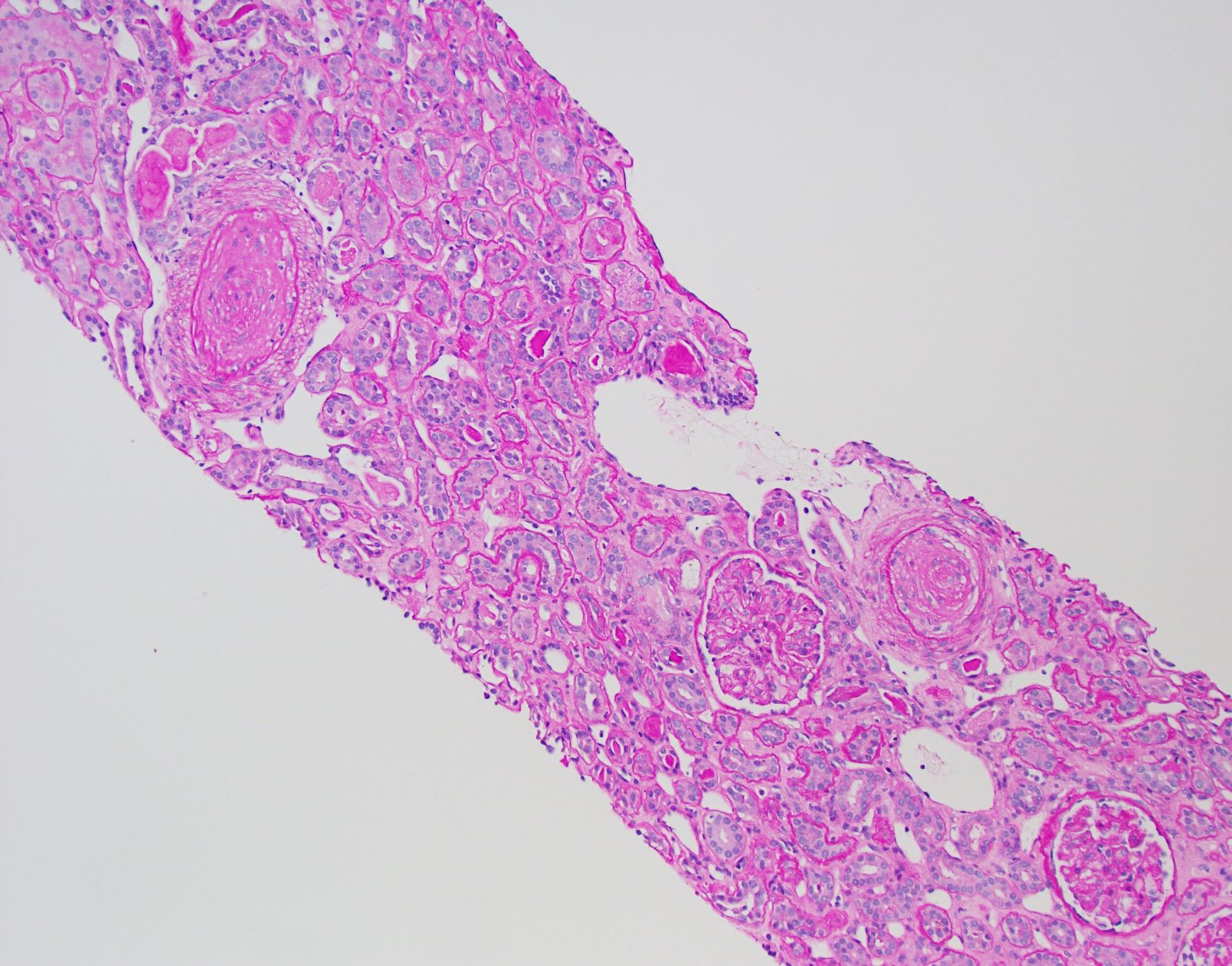
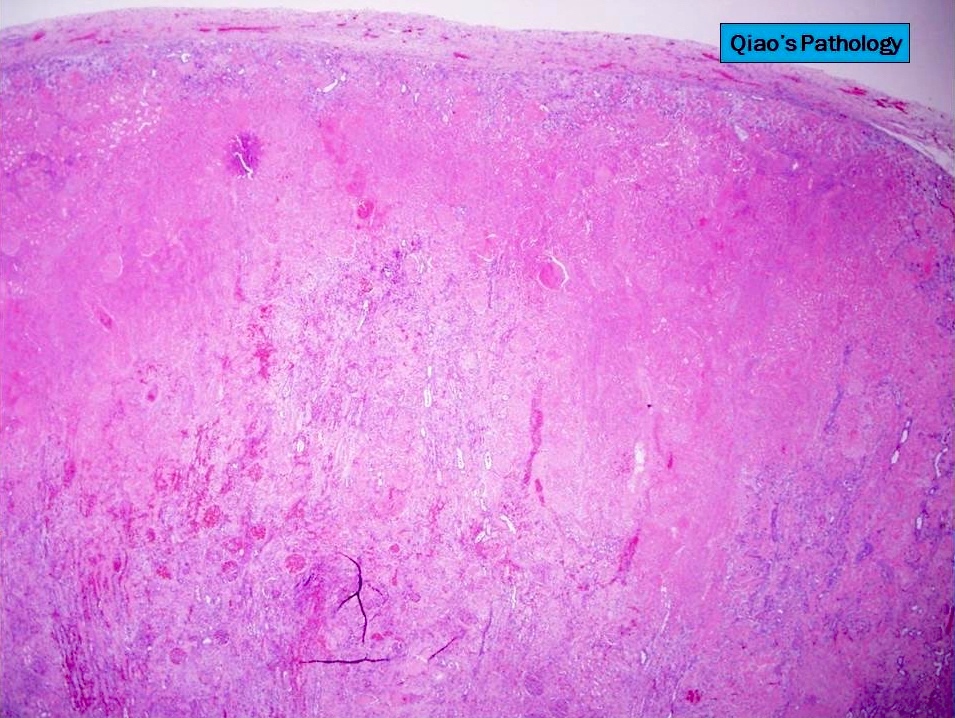
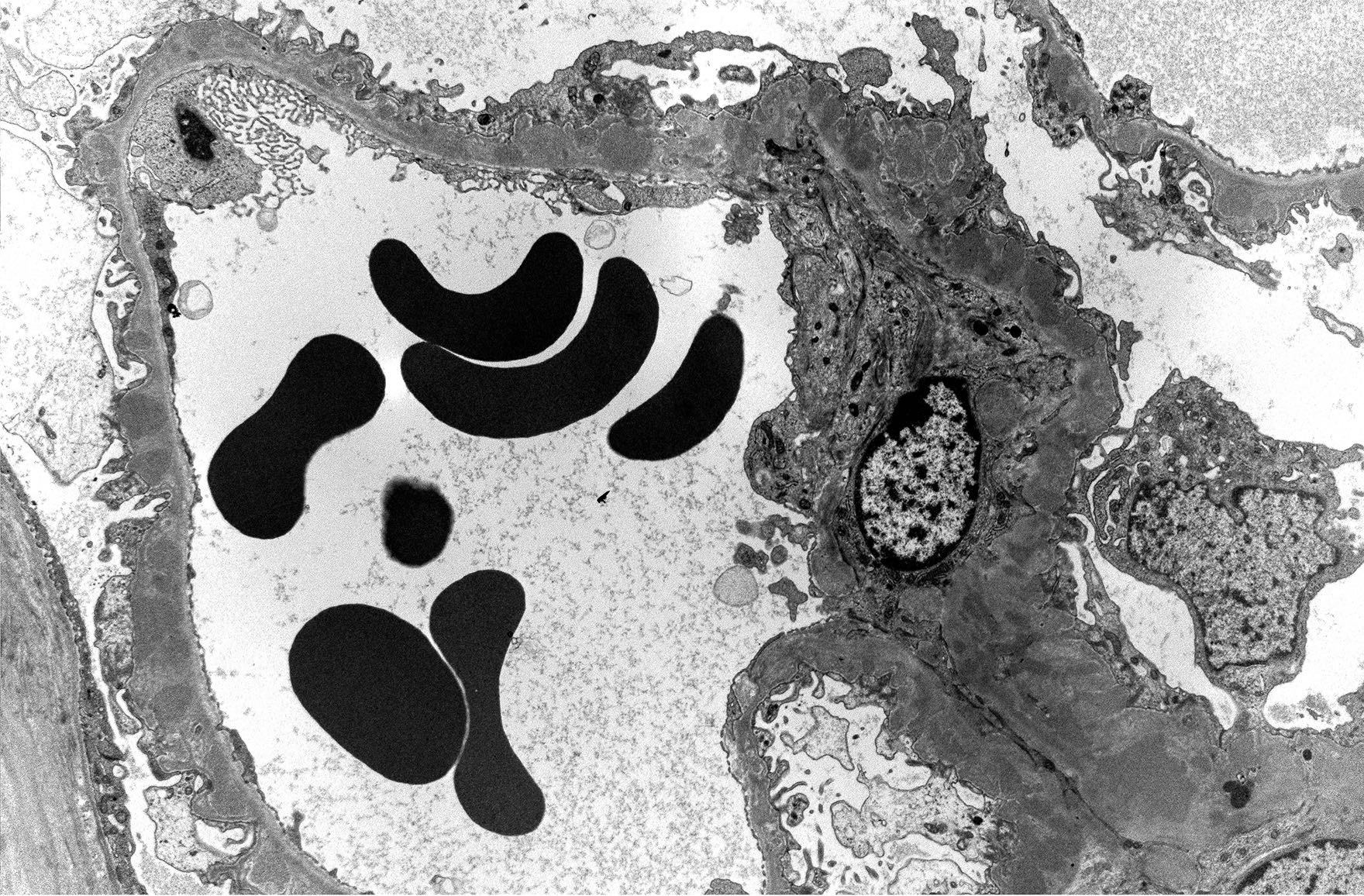
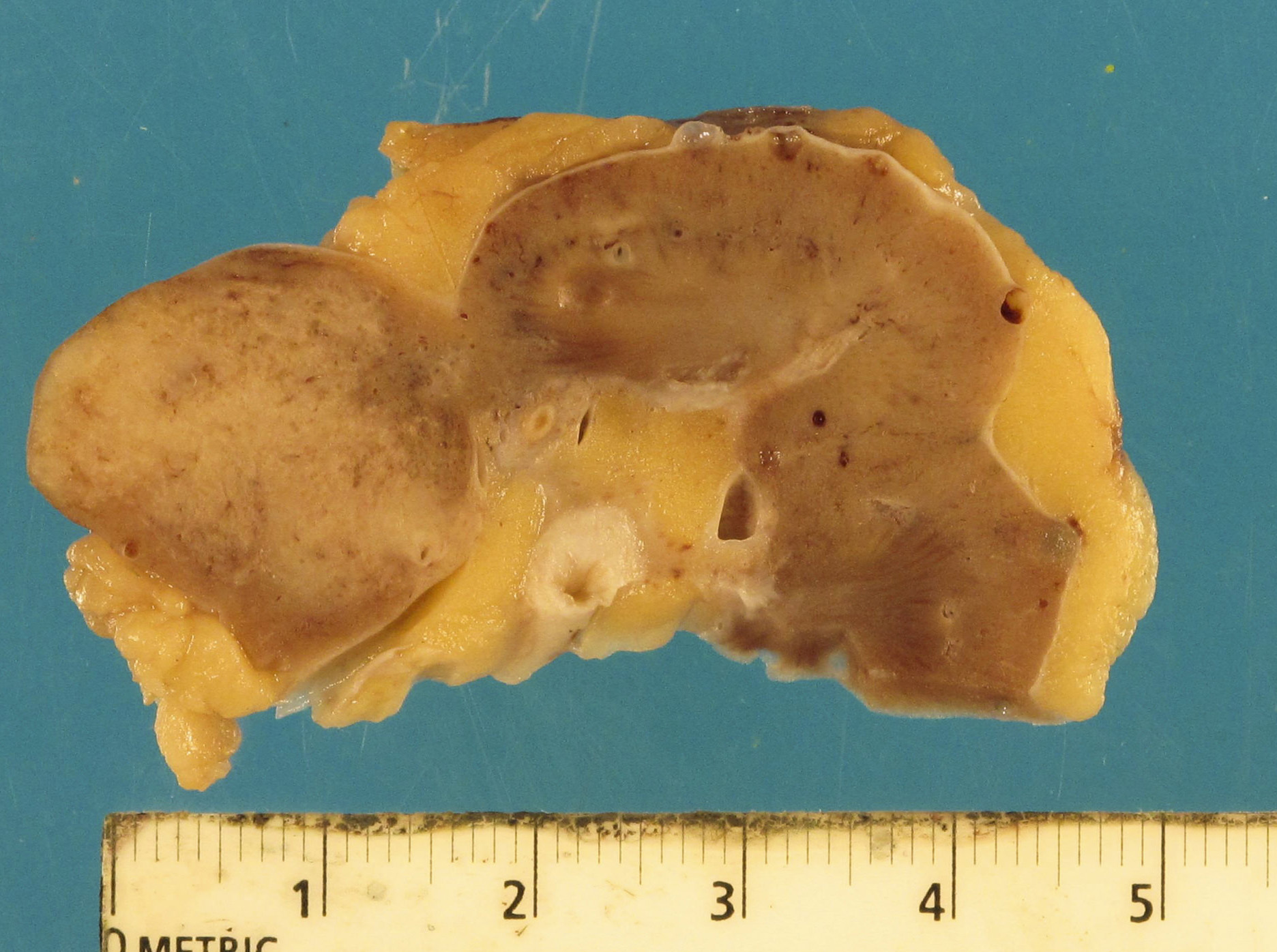
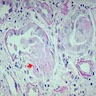
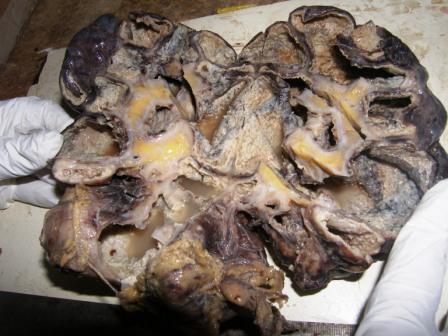
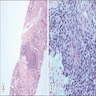
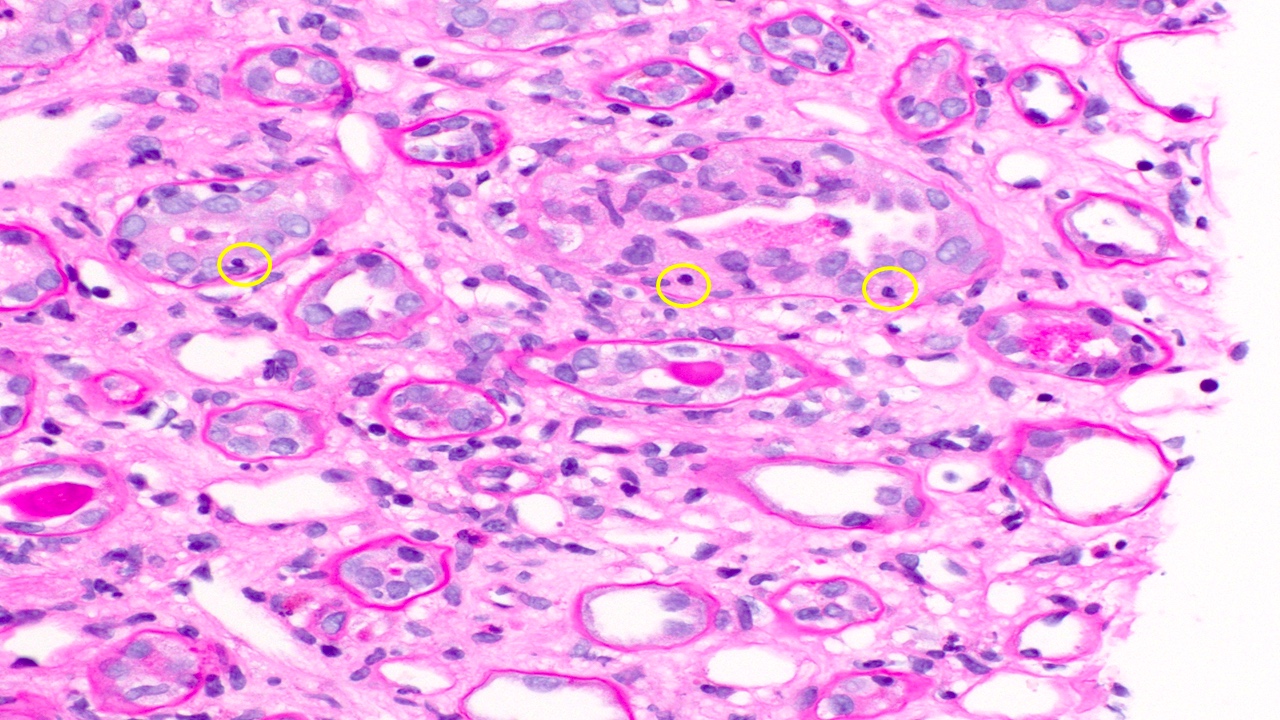
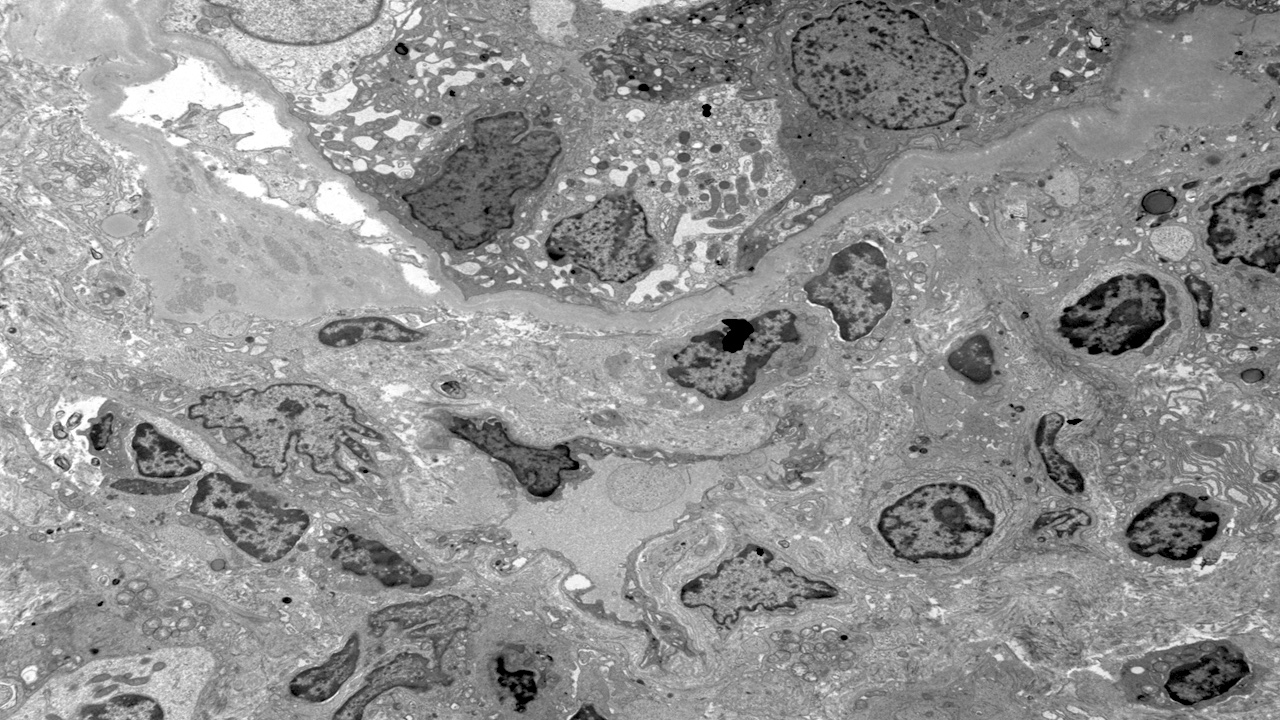
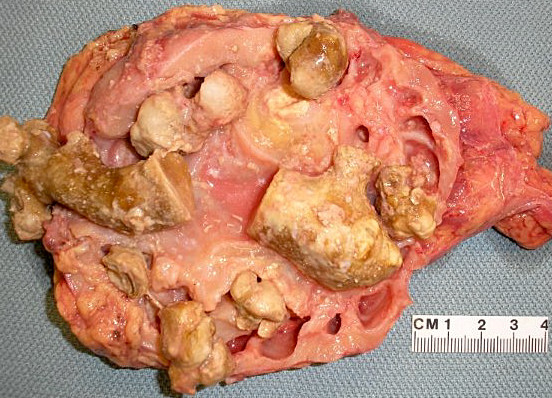
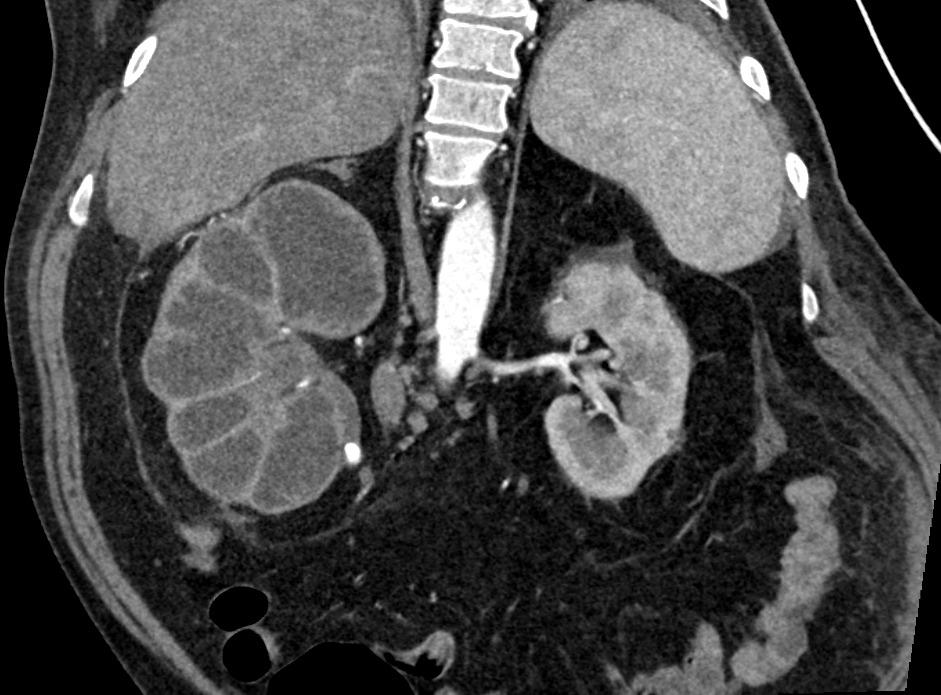
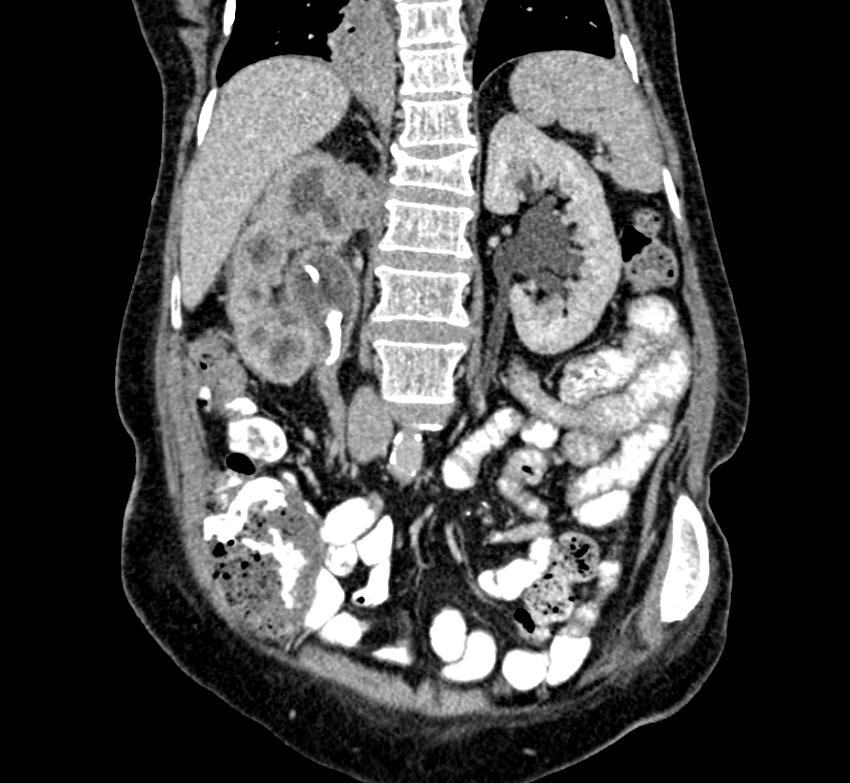
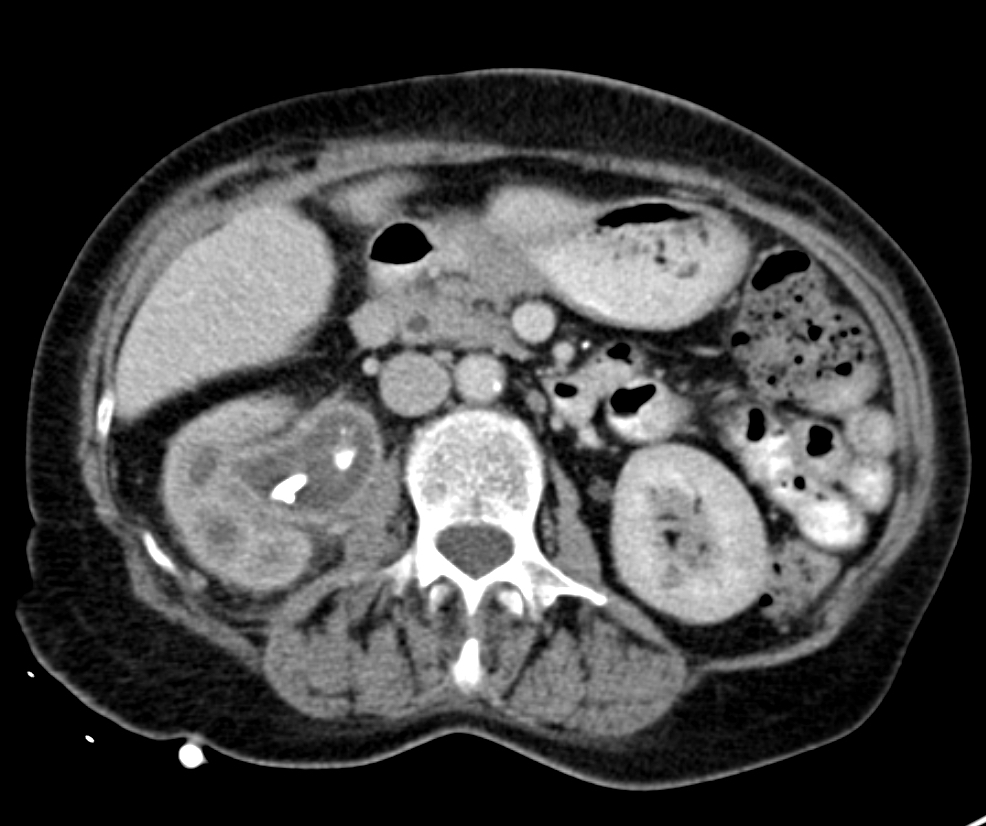
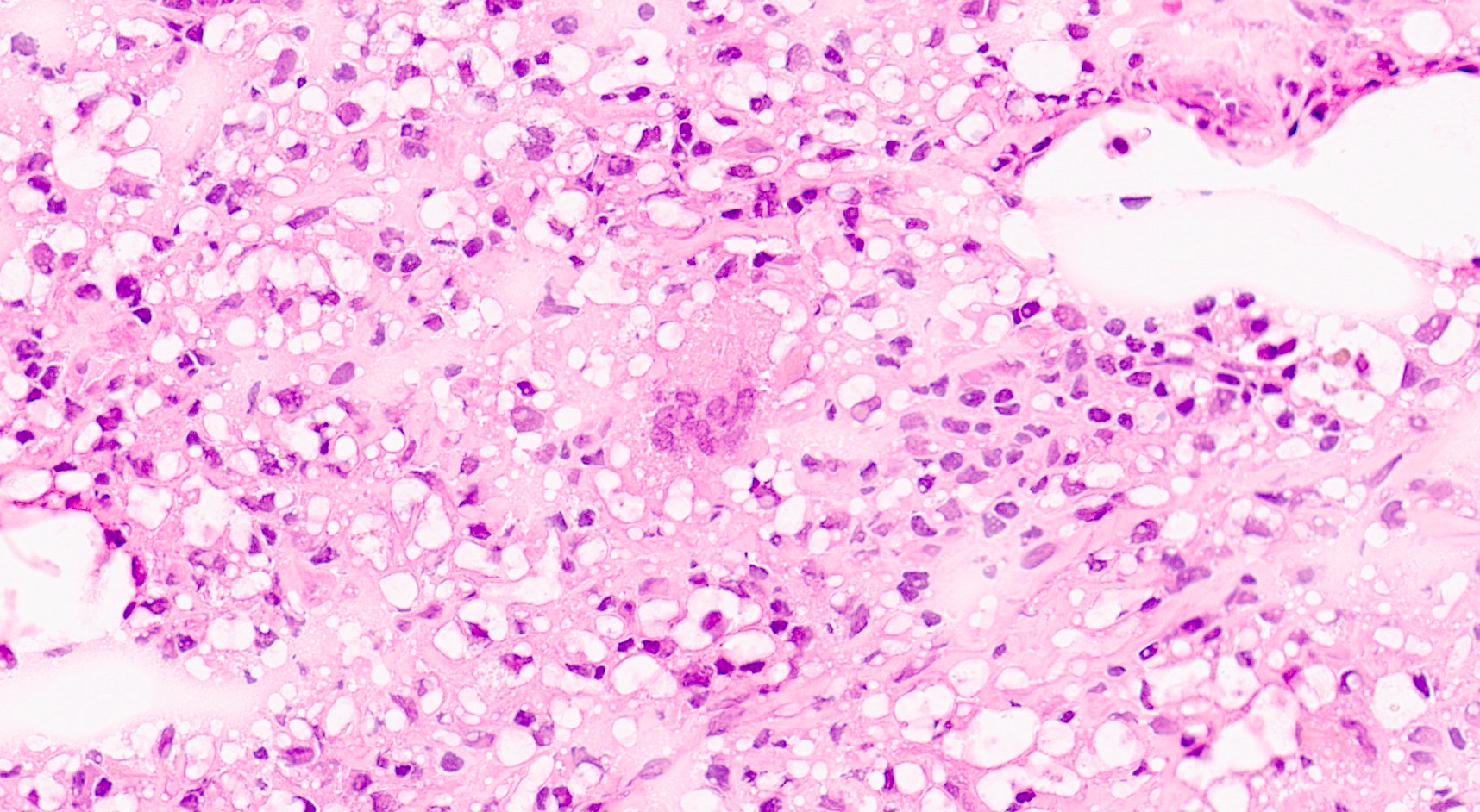
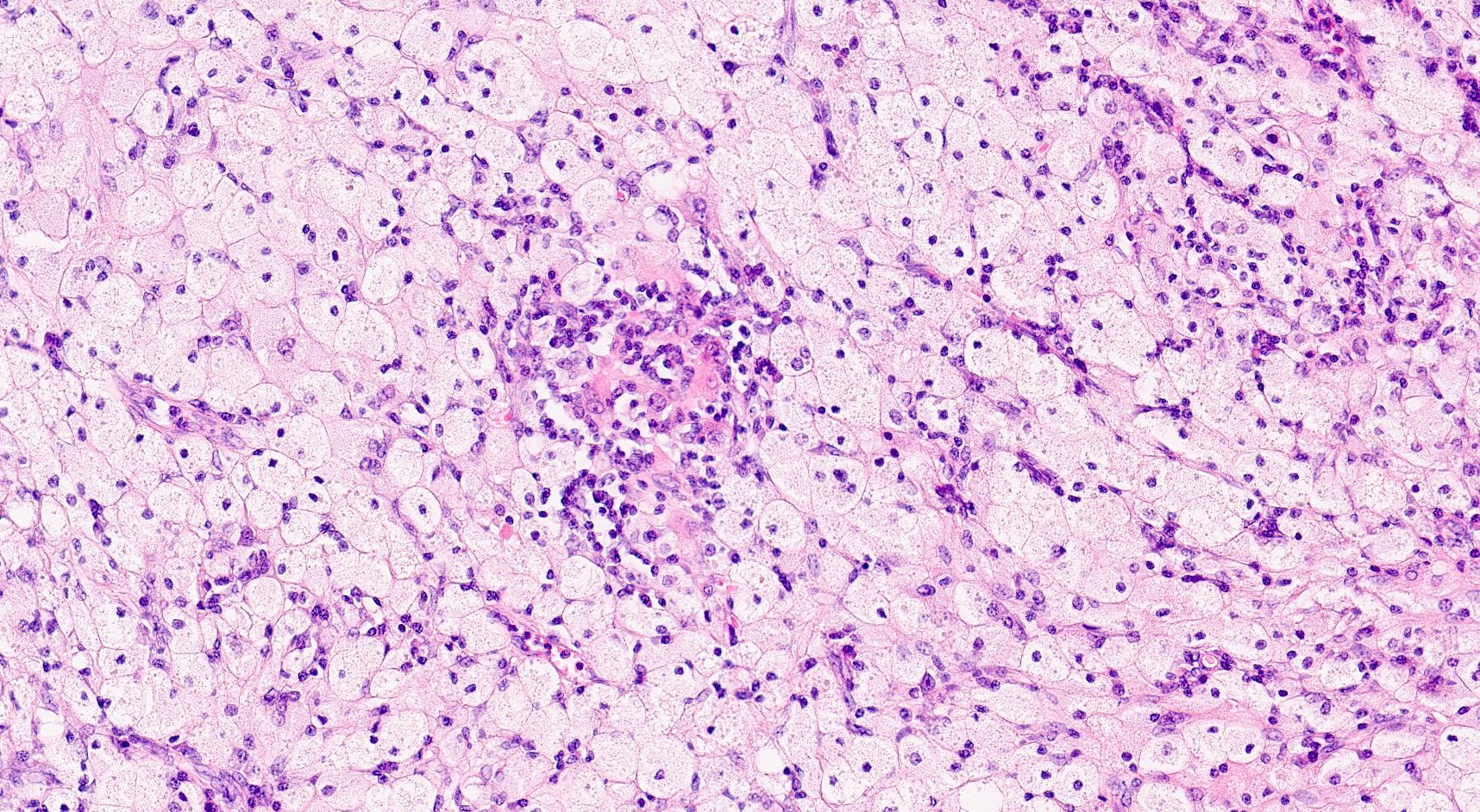
- Moderately enlarged kidneys (usually < 800 g) with cortical and medullary cysts containing clear fluid
- > 40% replacement of kidney with cysts
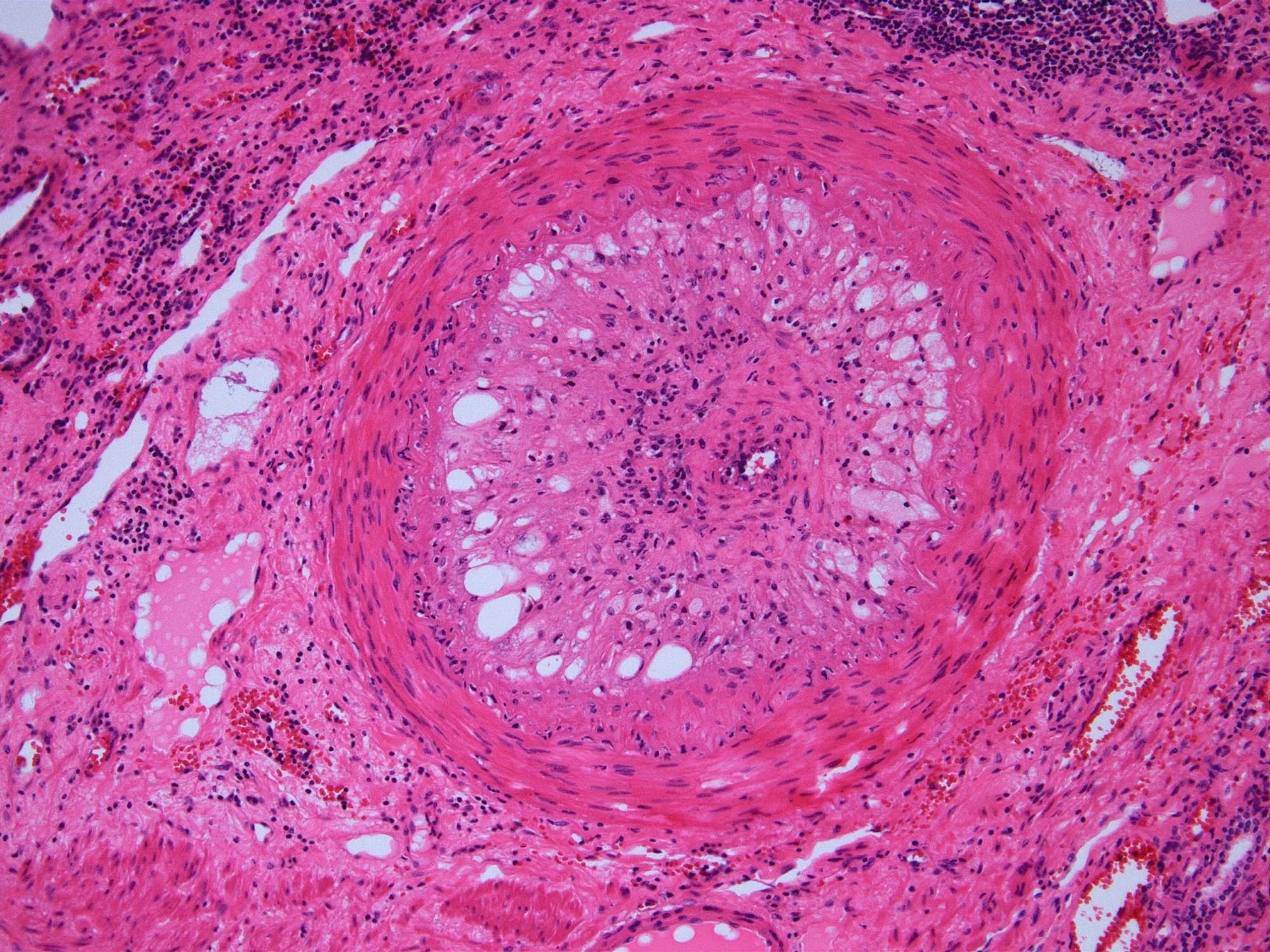
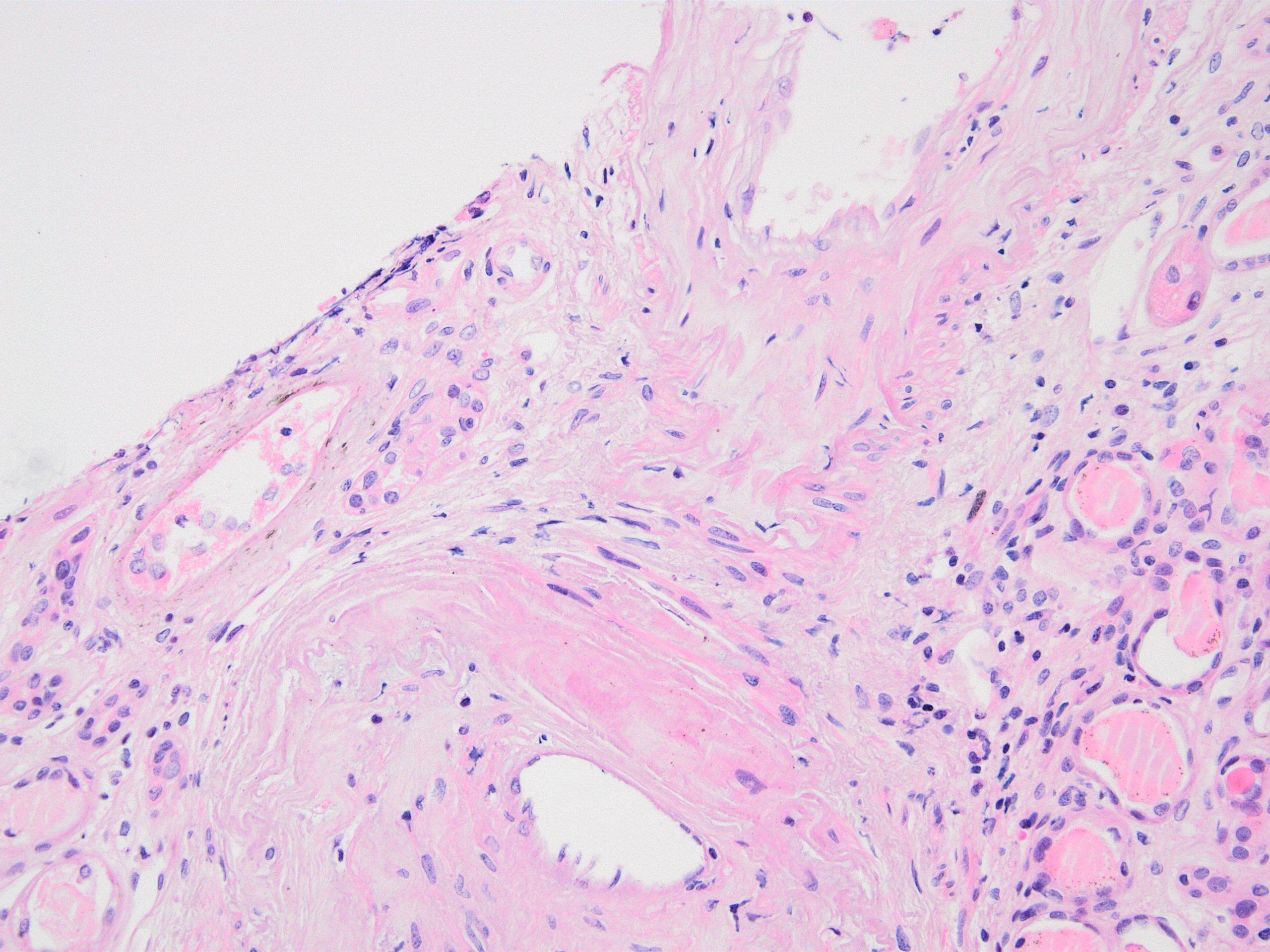
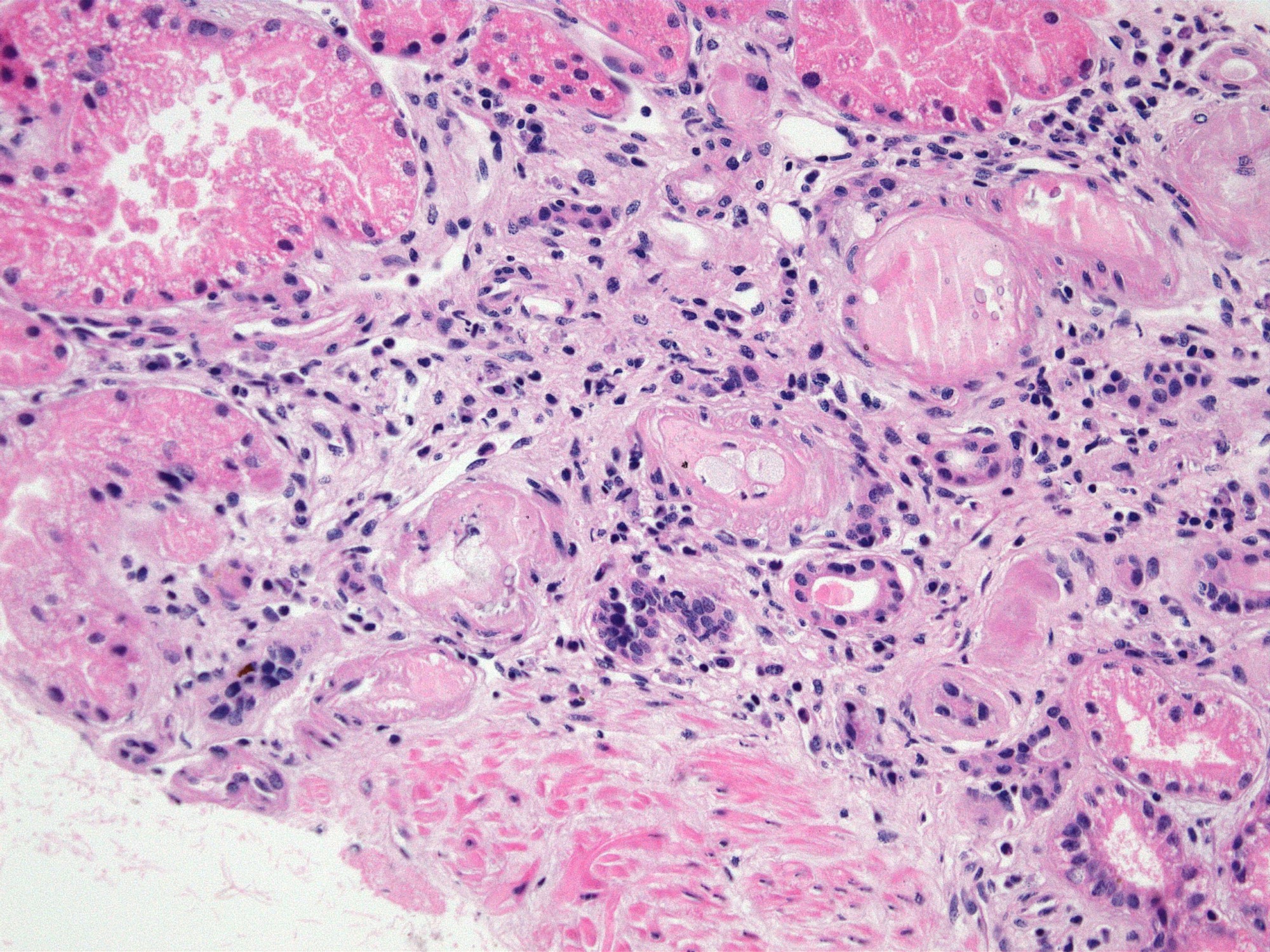
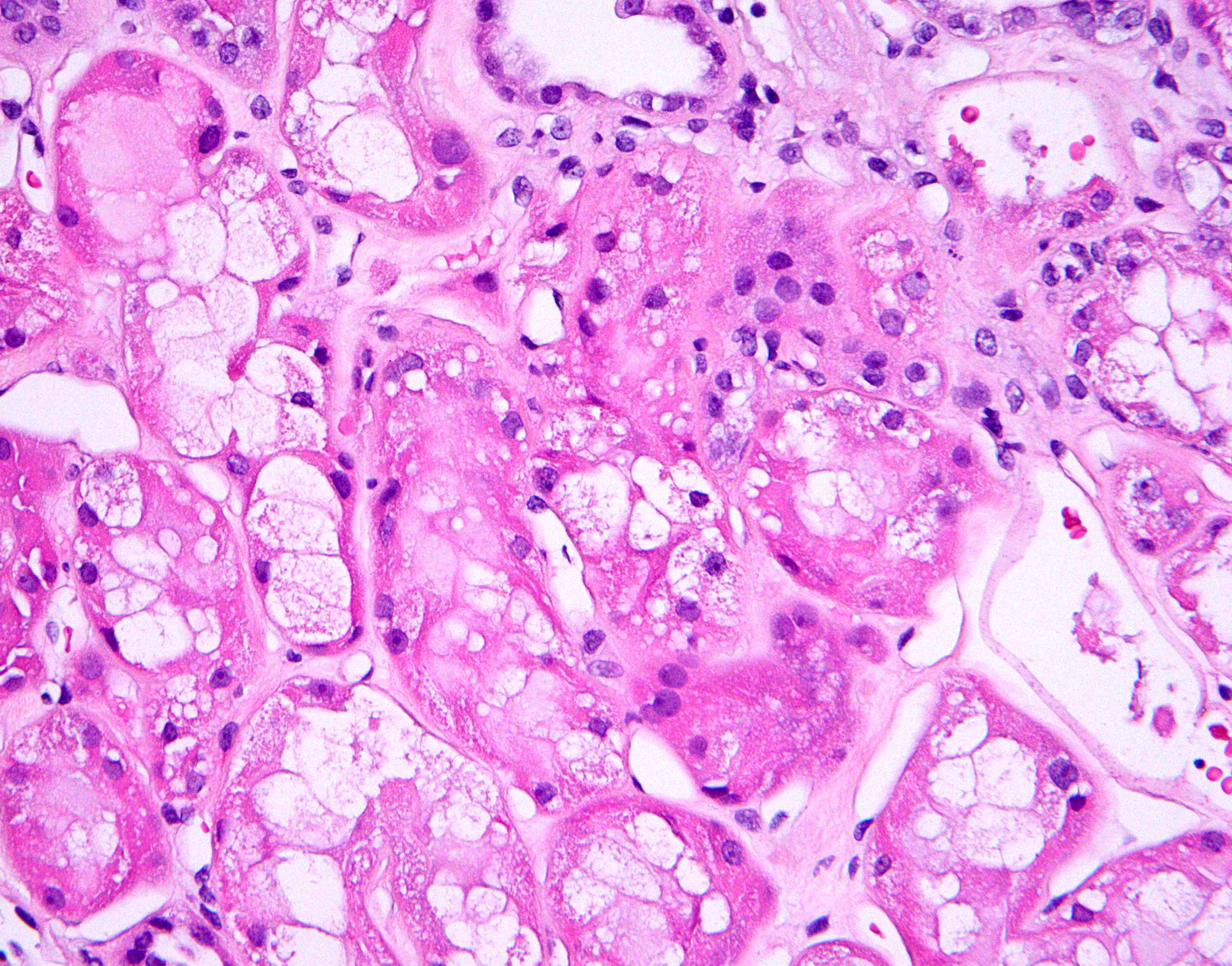
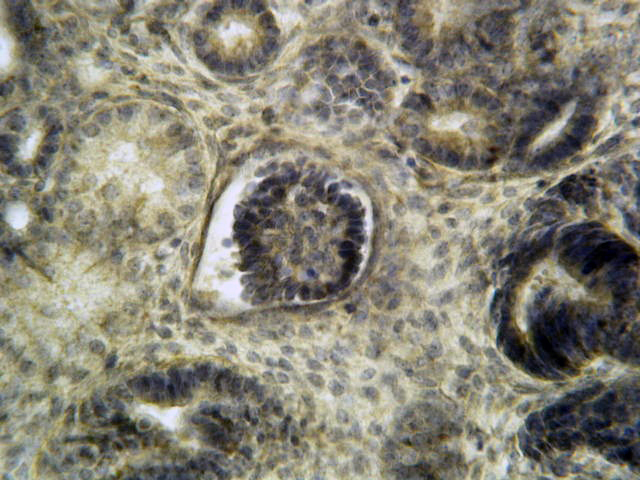
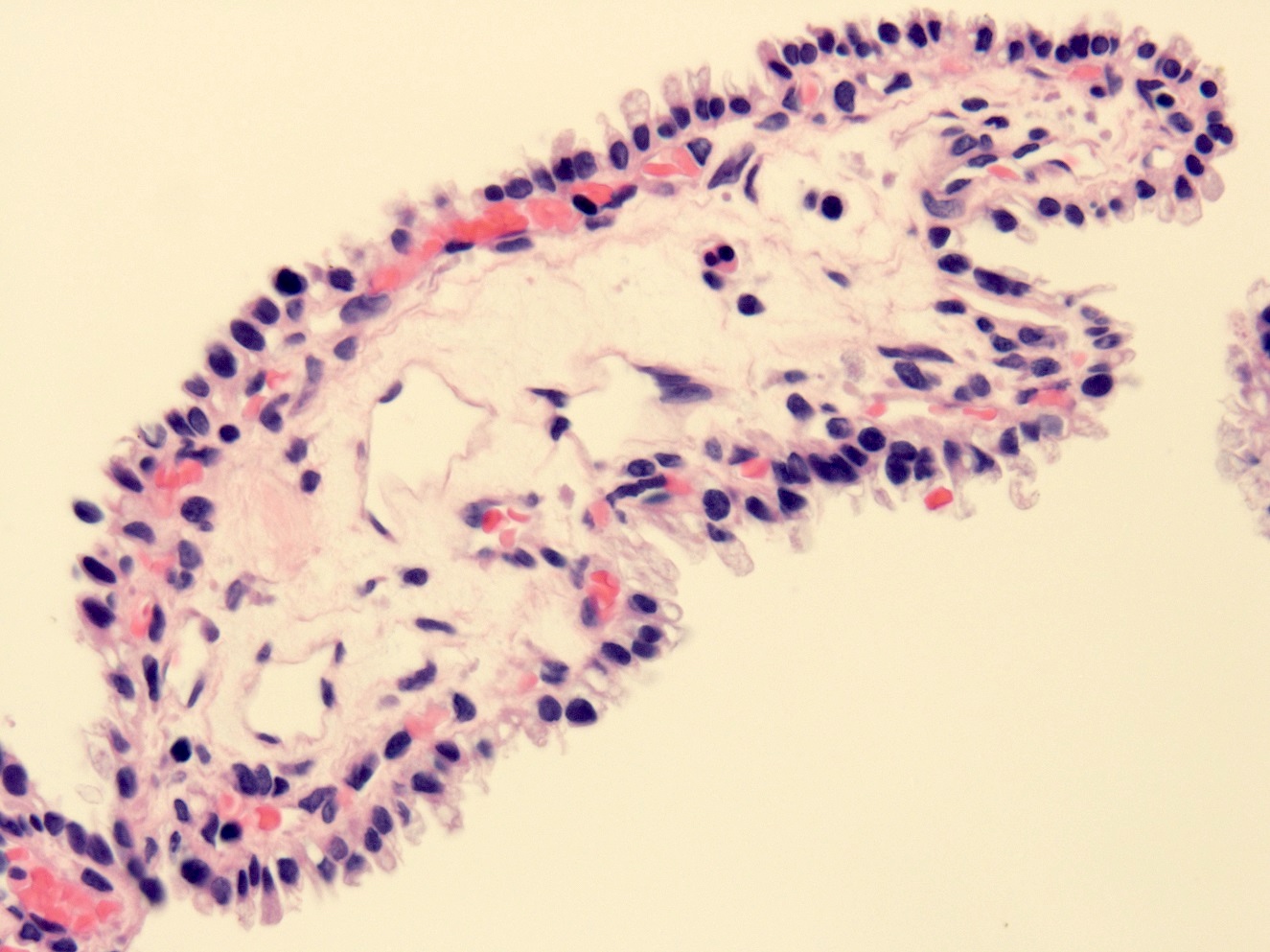
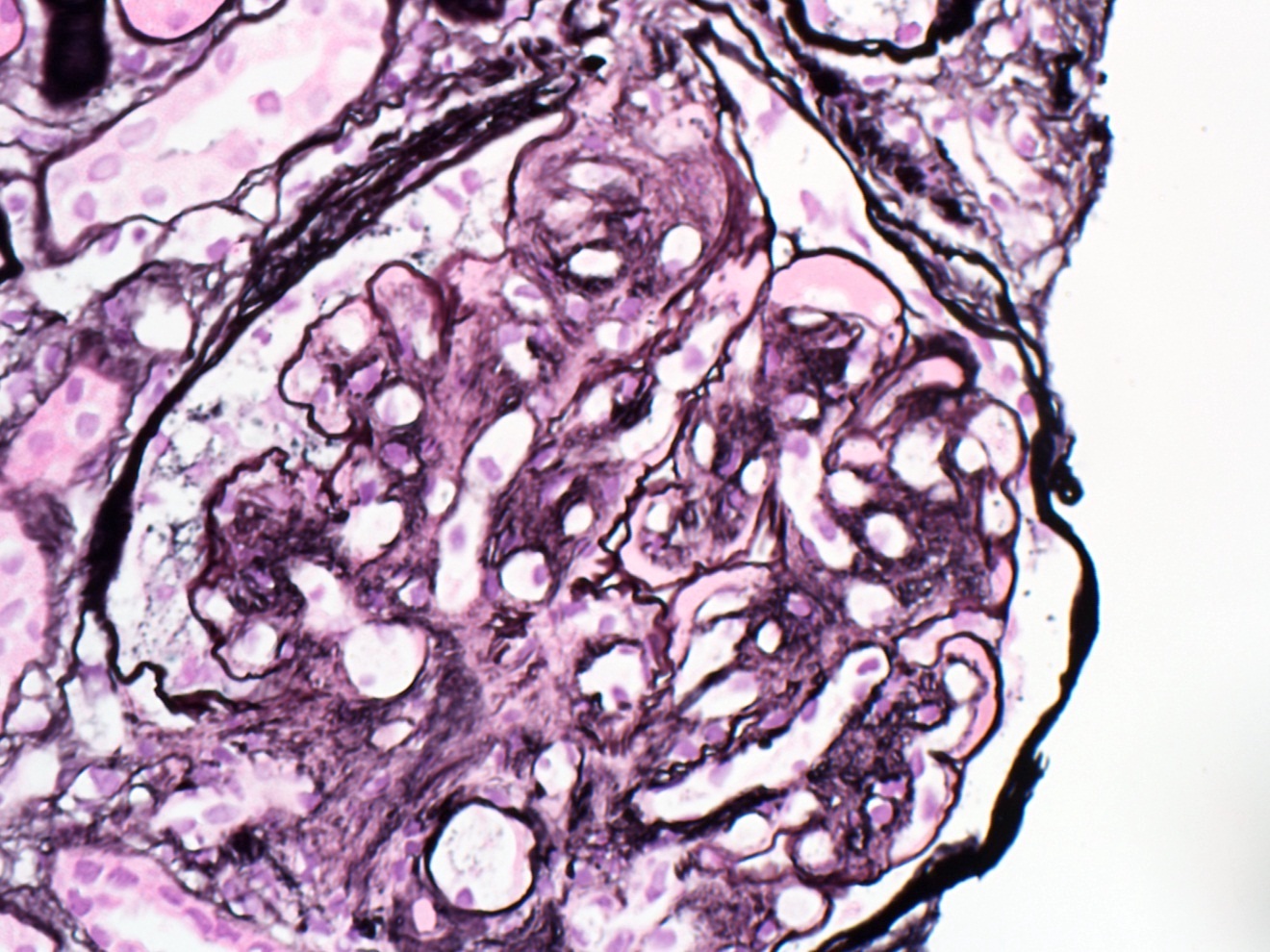
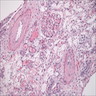
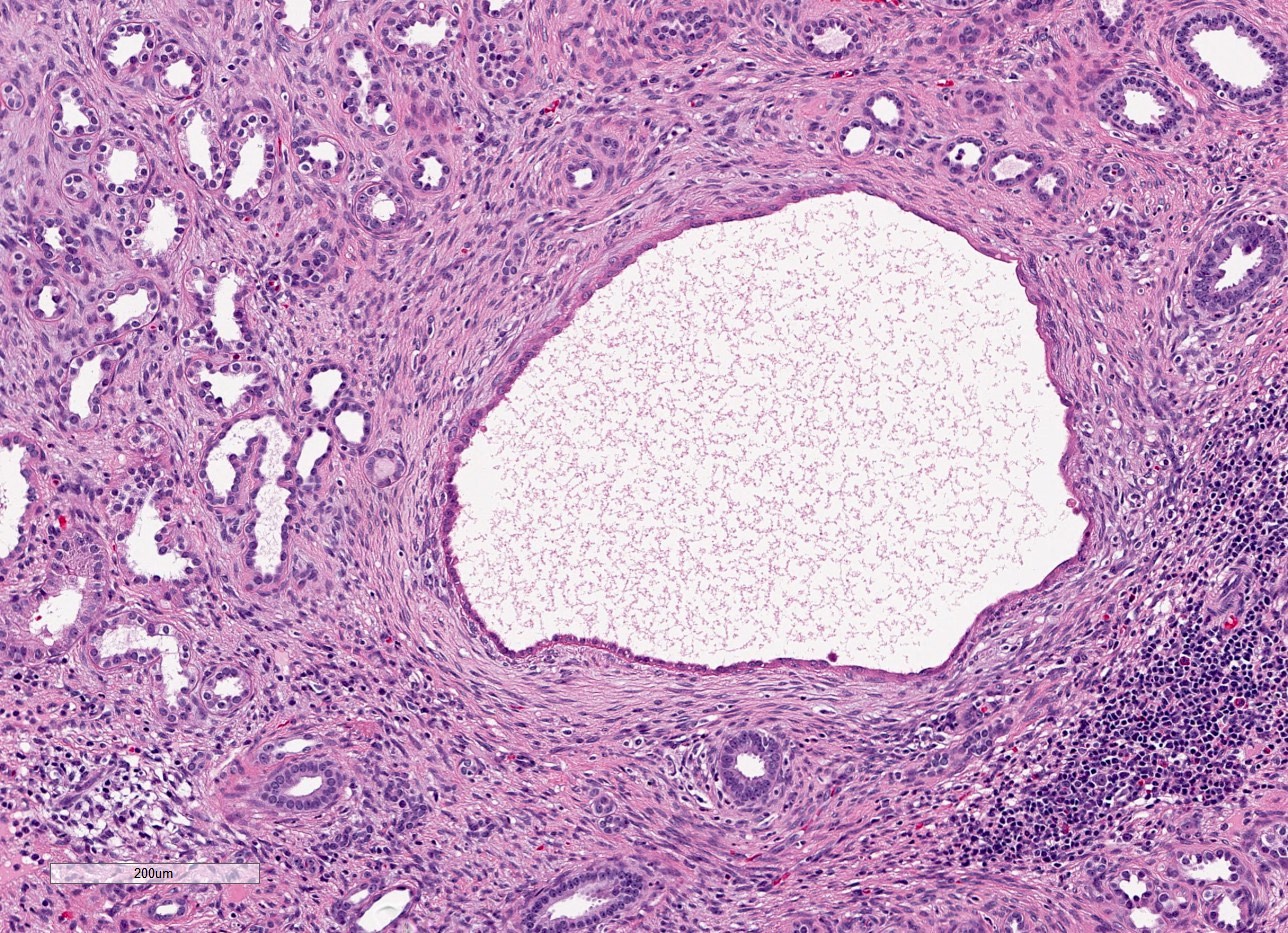
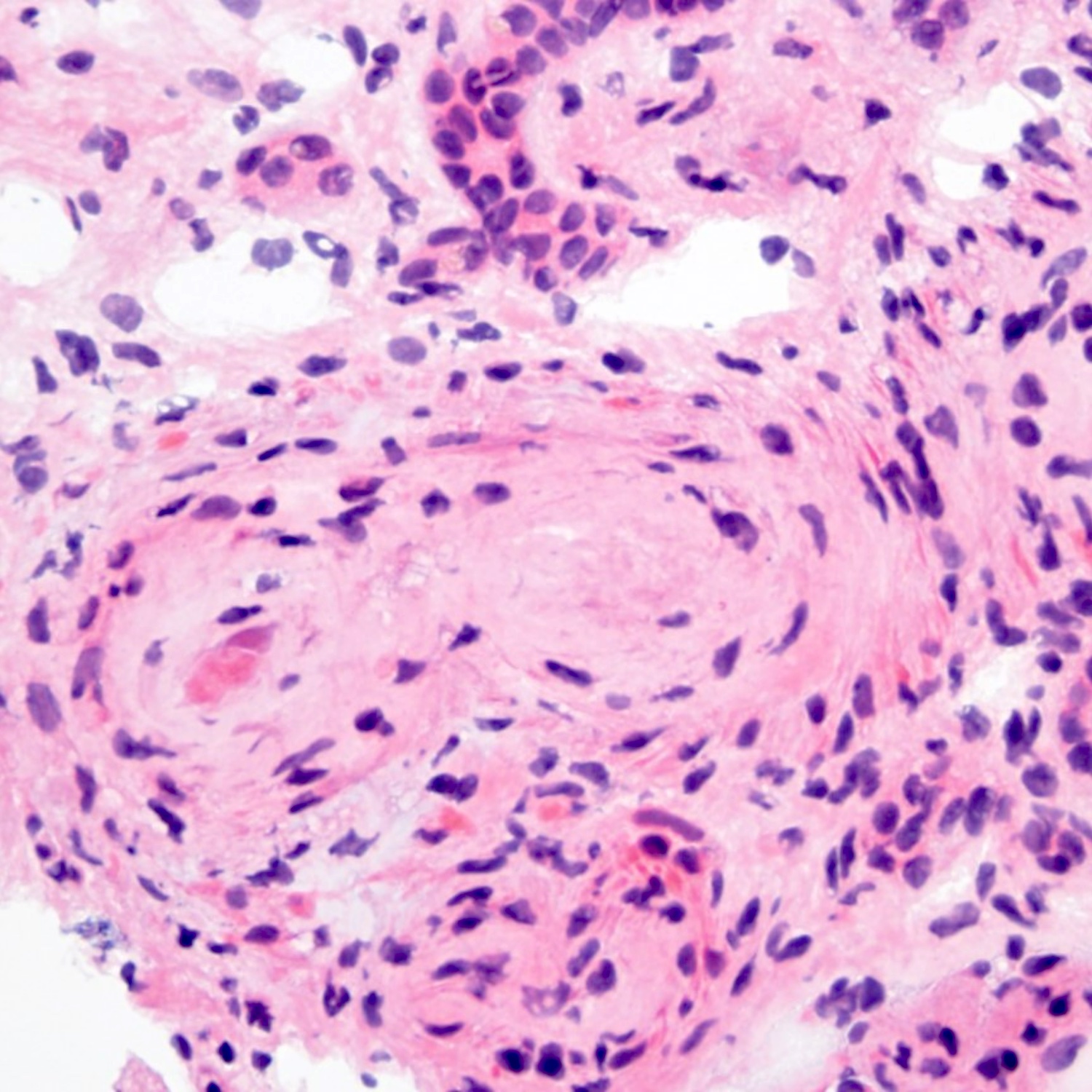
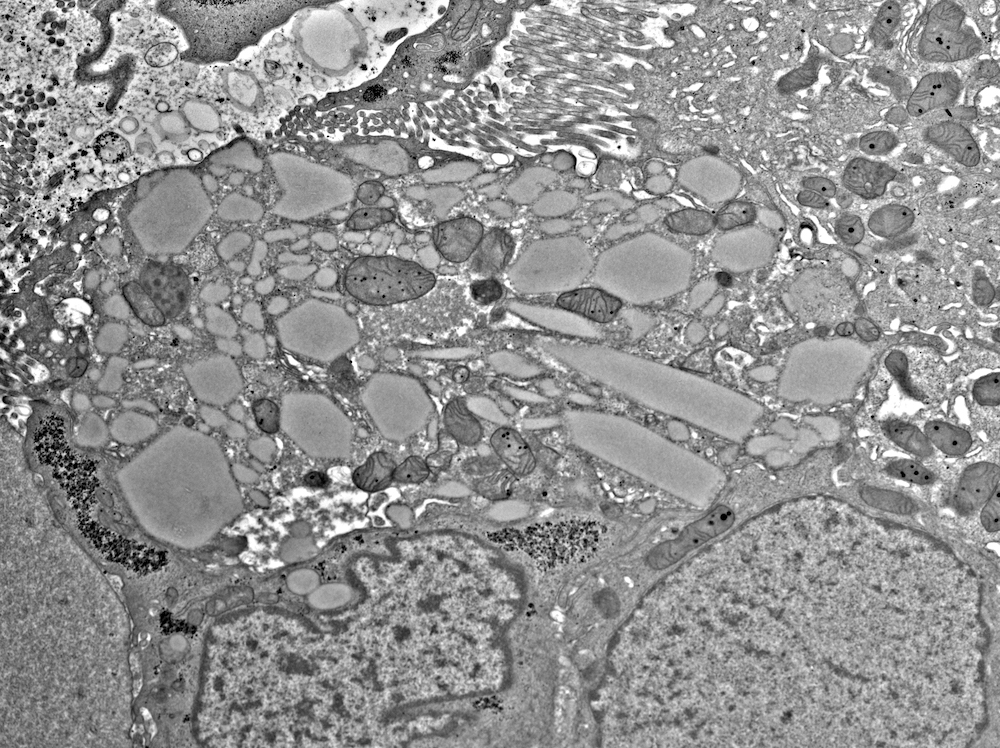
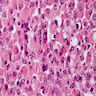
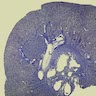
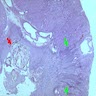
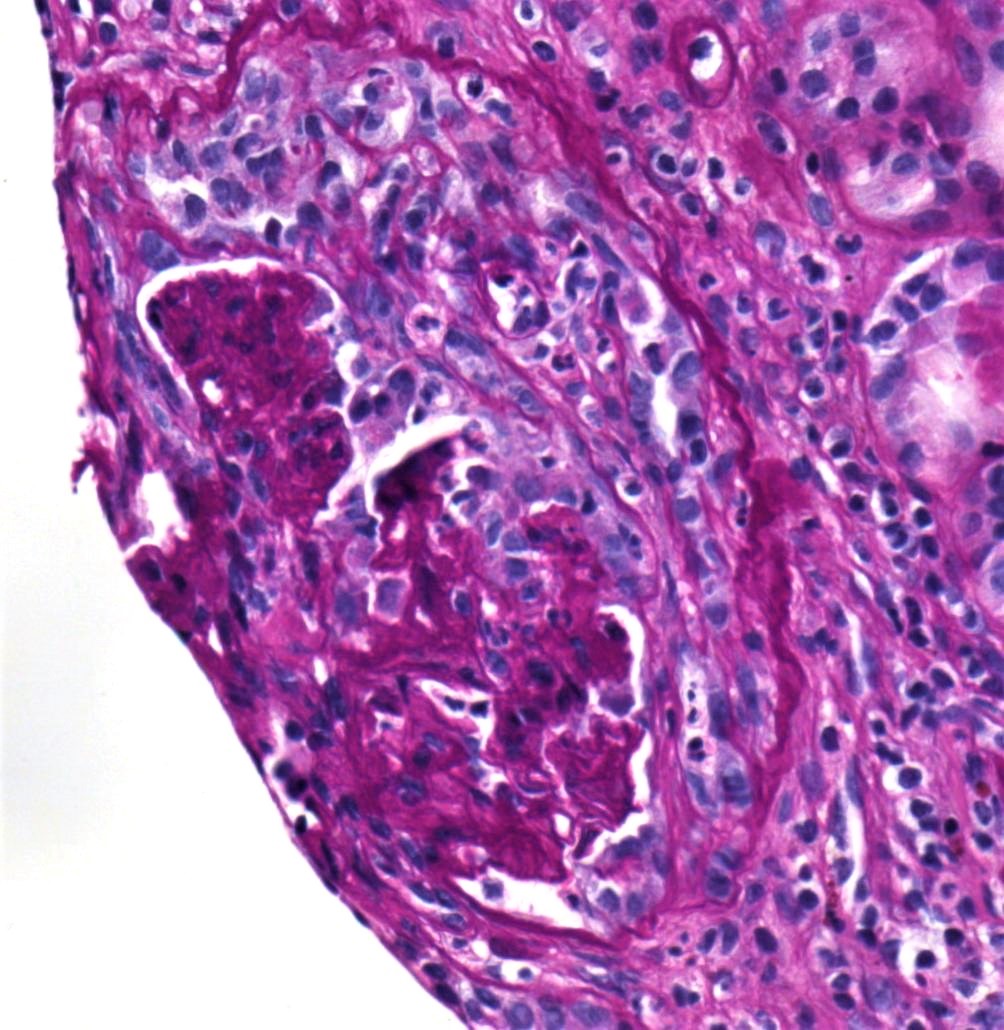
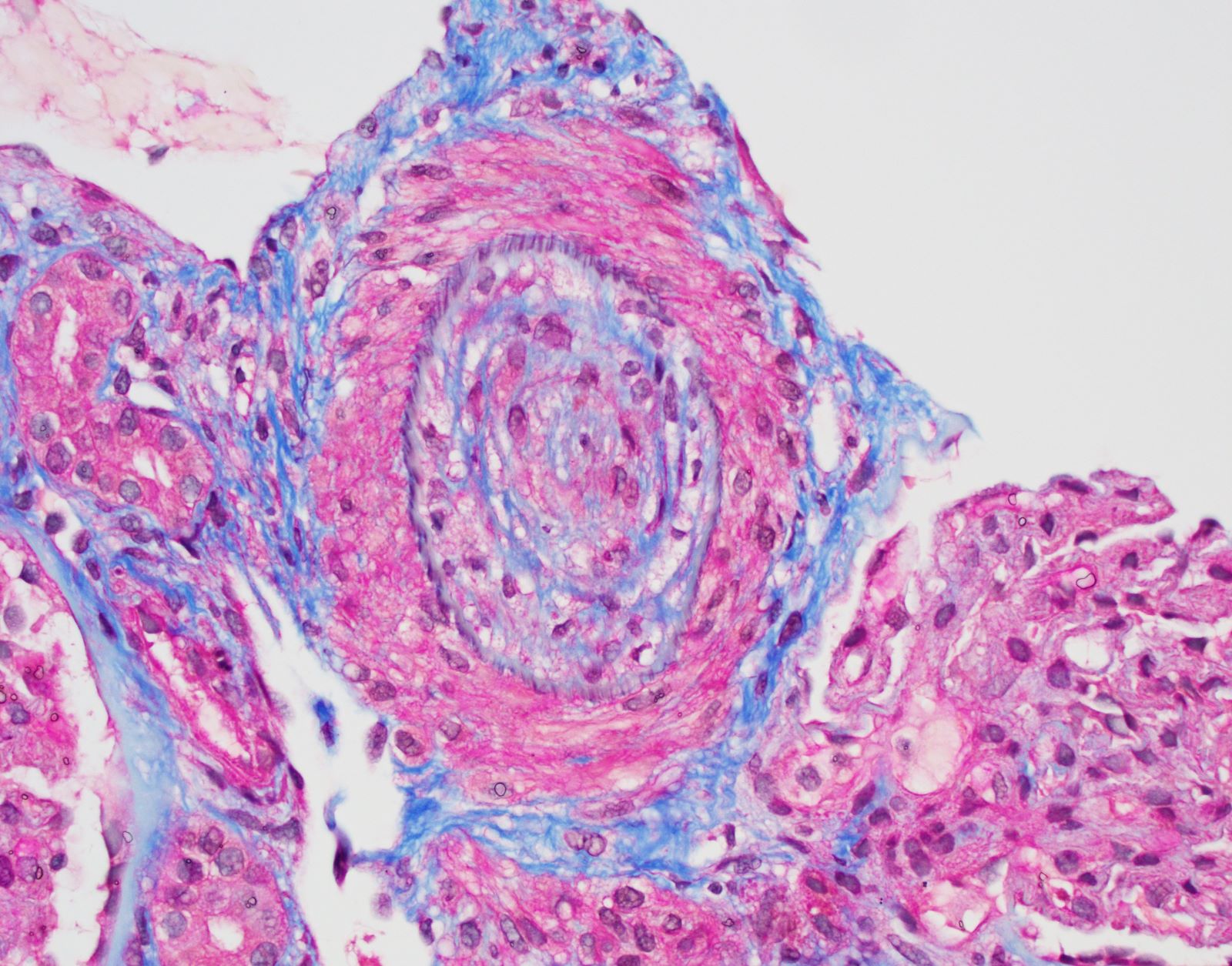
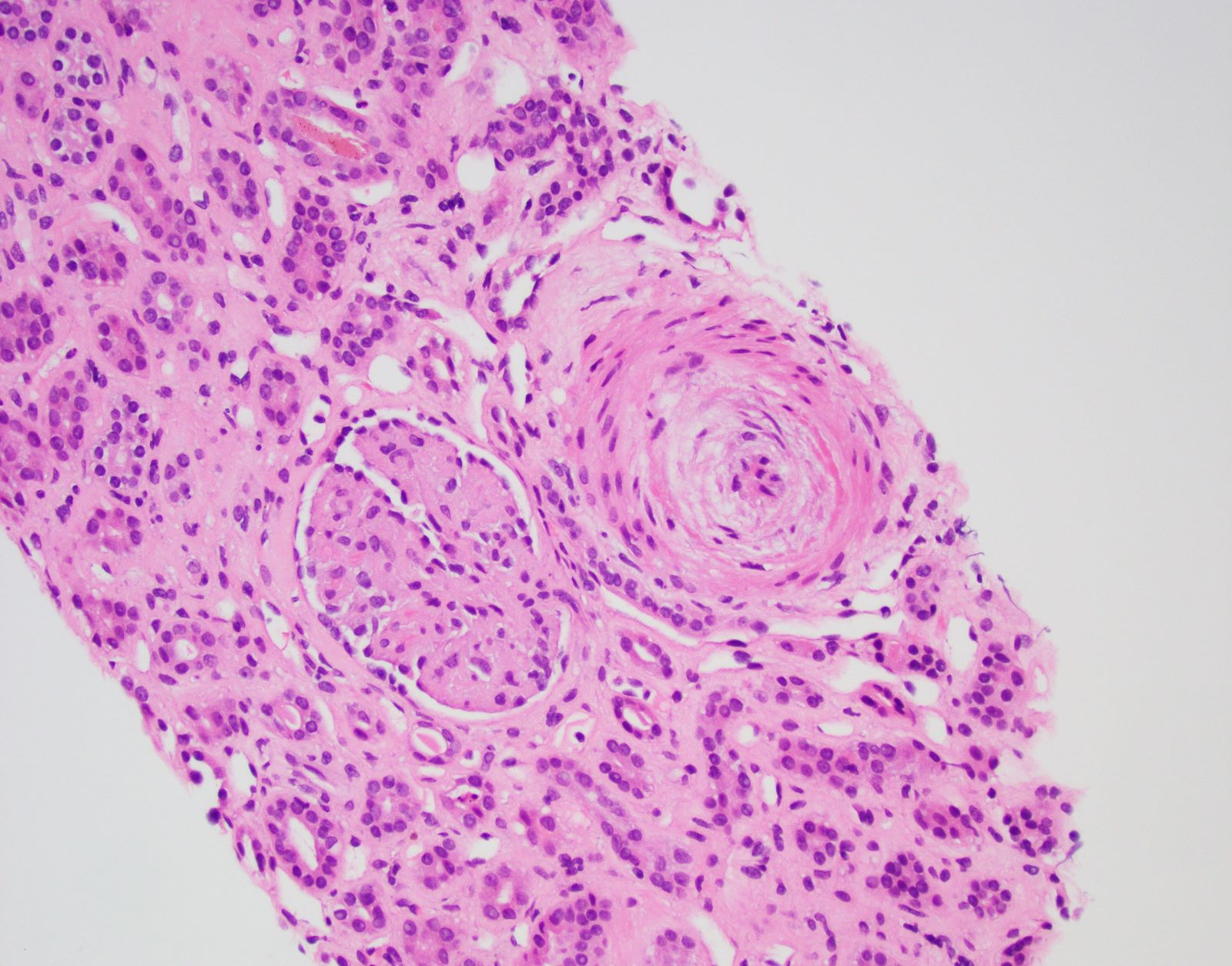
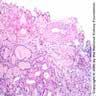
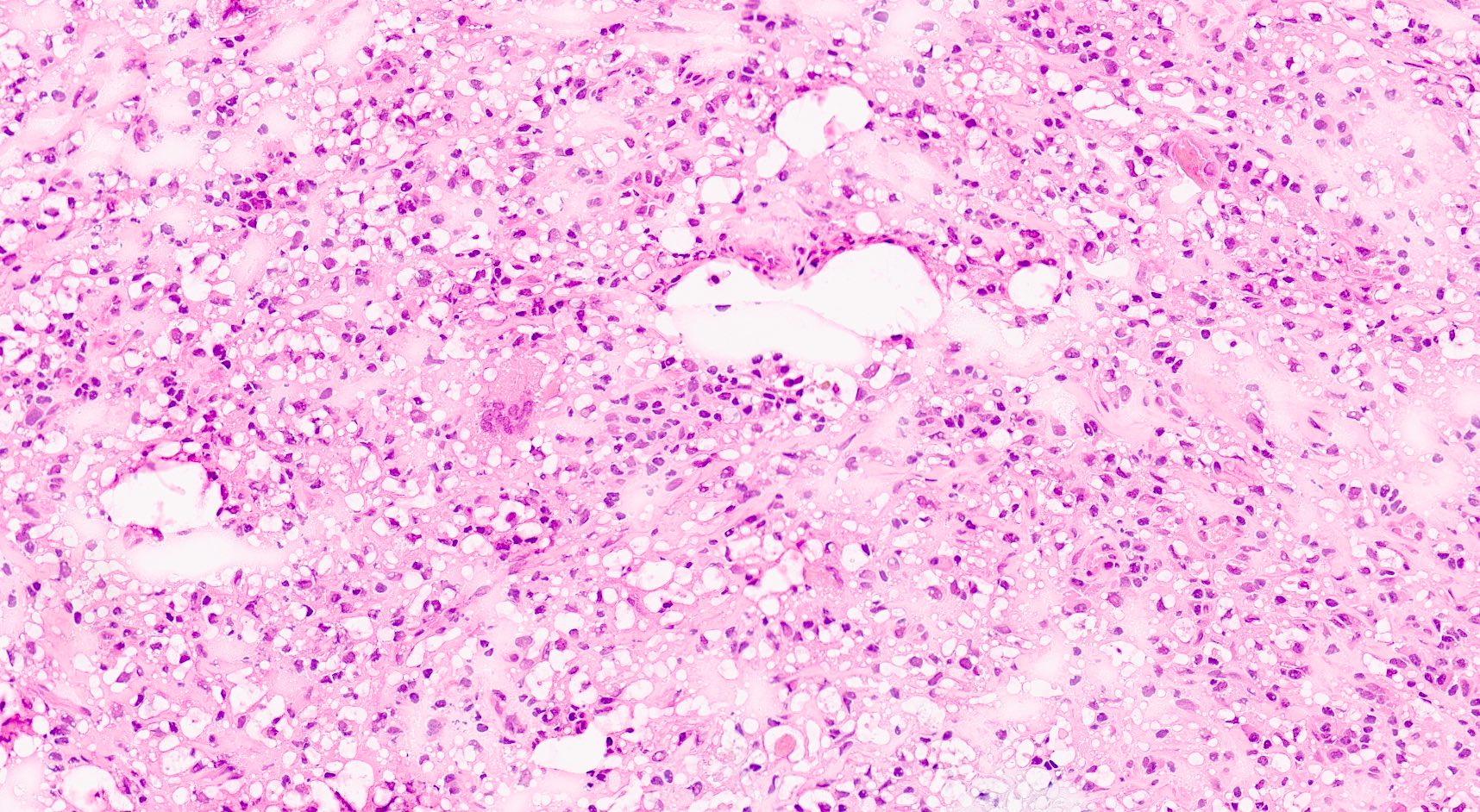
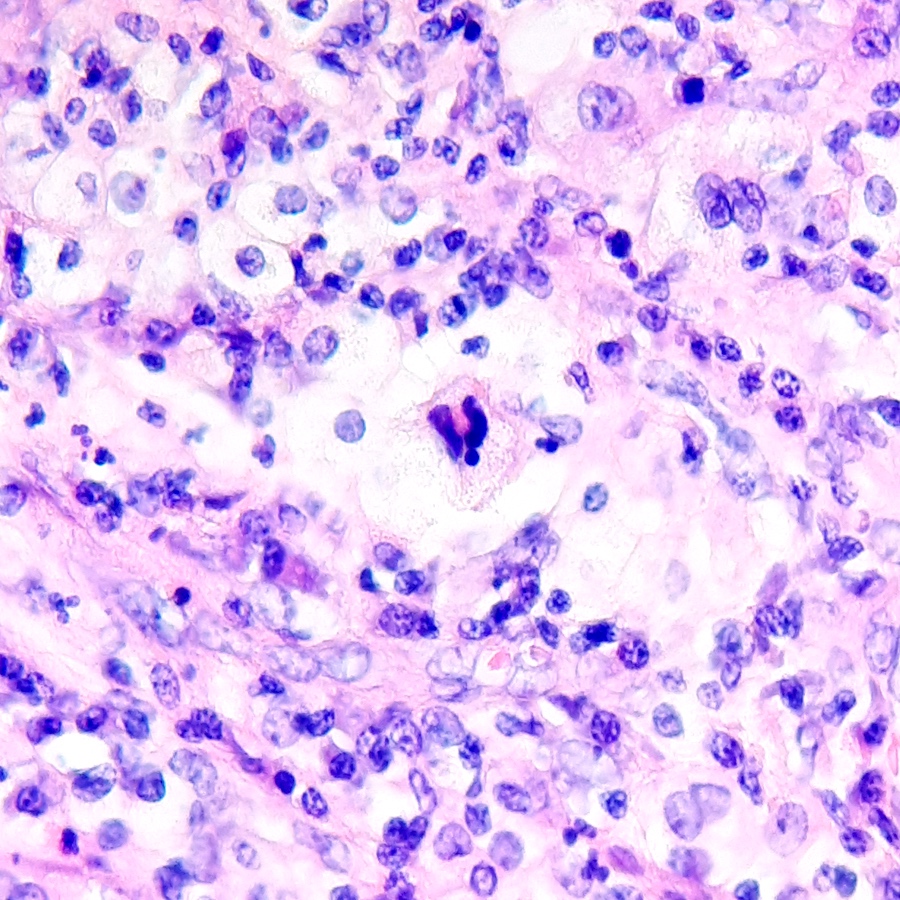
- Cysts lined by flattened or cuboidal epithelium that may show focal pseudopapillae with nuclear enlargement and loss of polarity
- Cysts may contain oxalate crystals
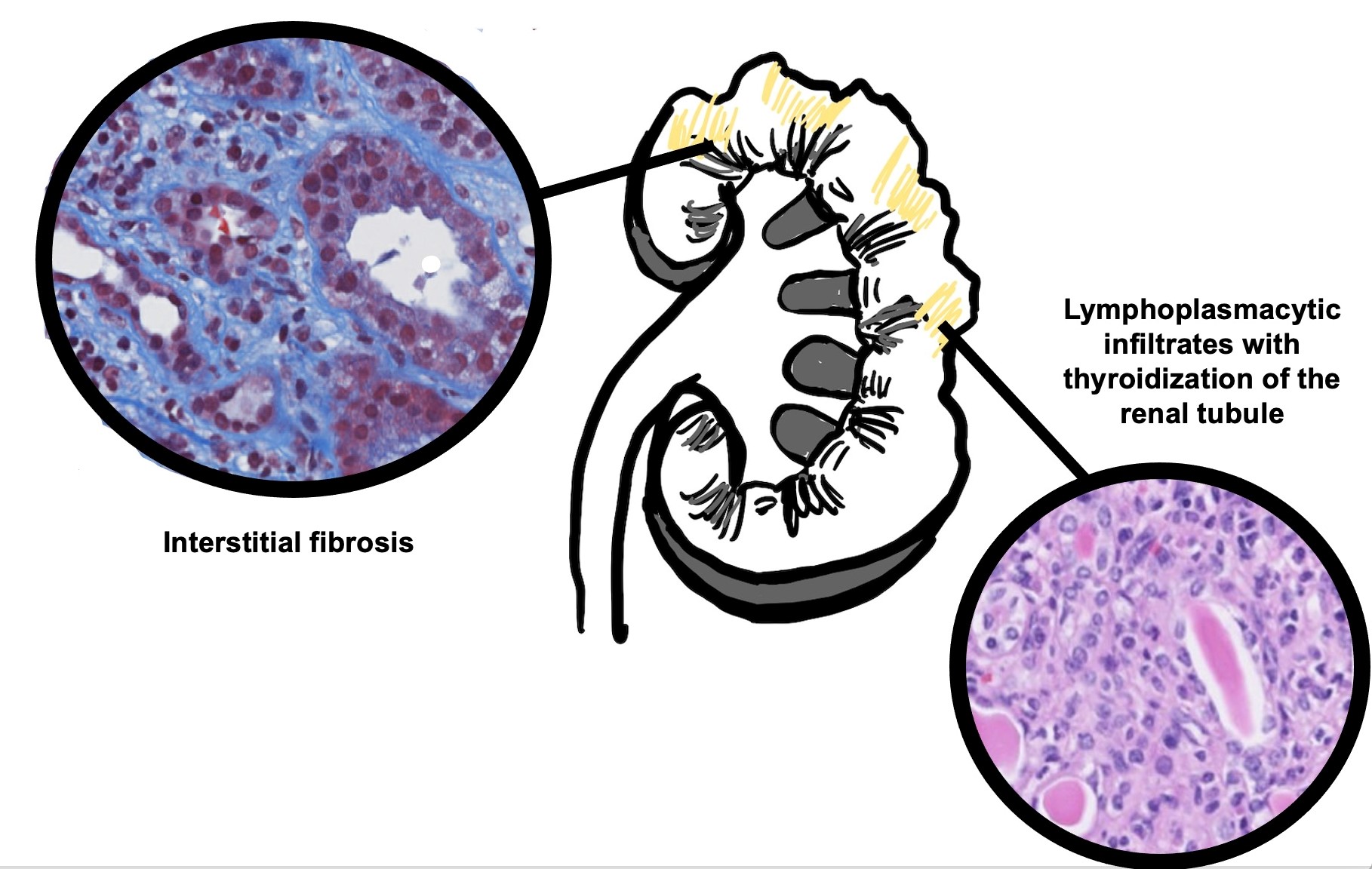
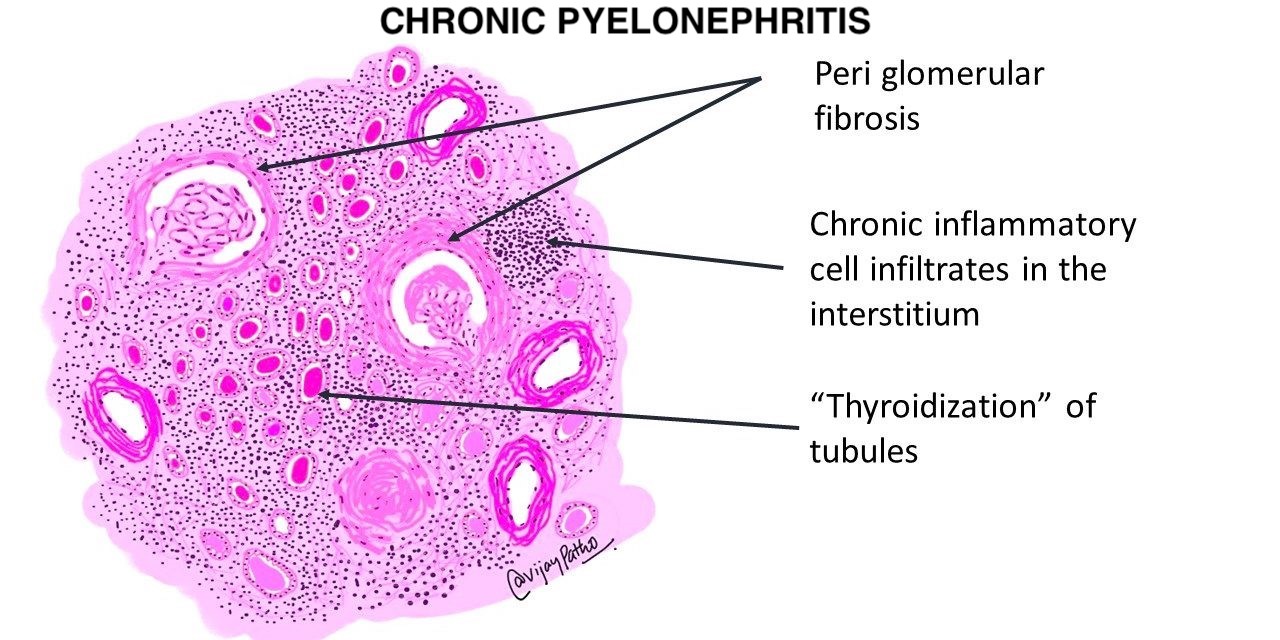
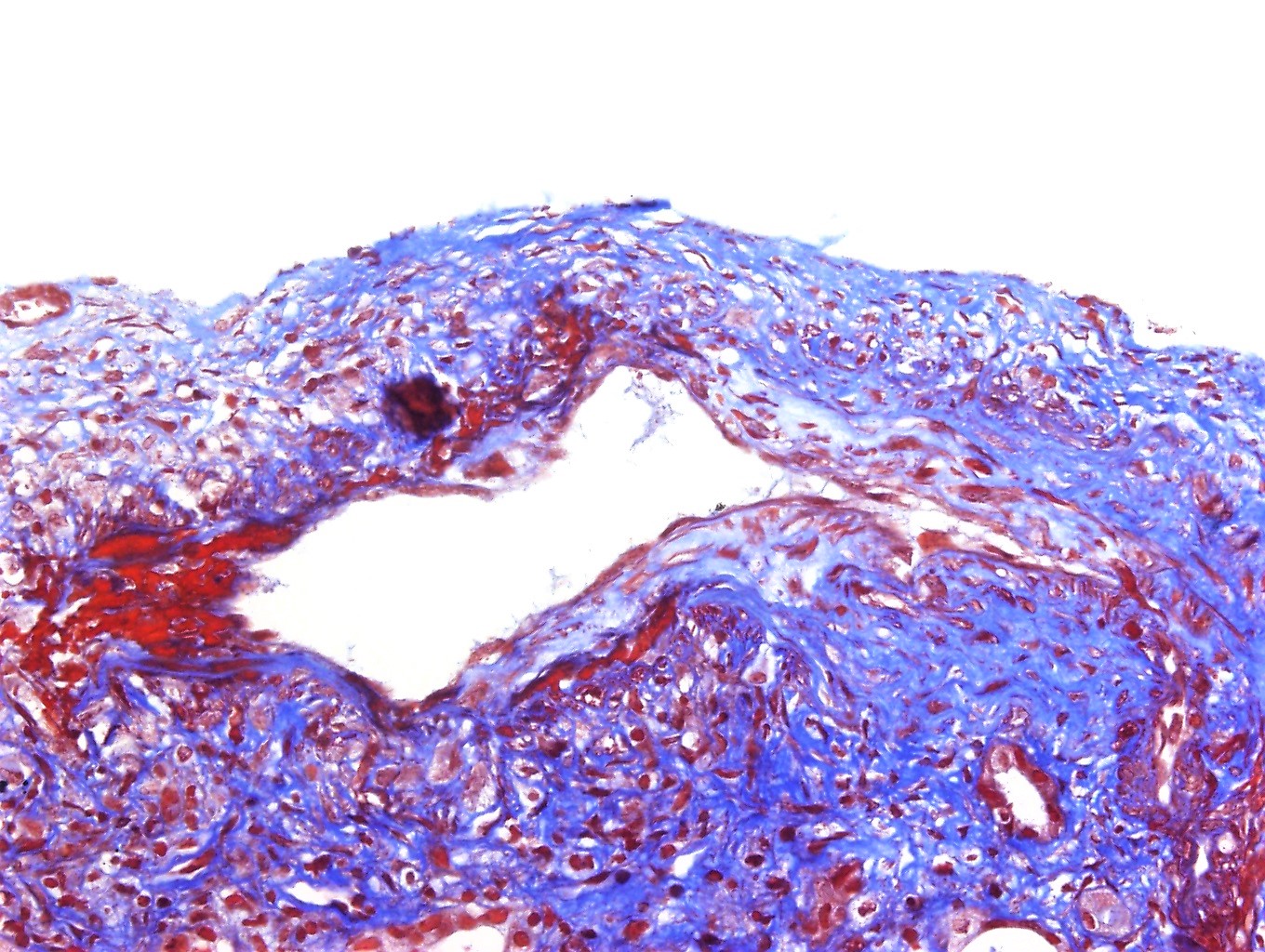
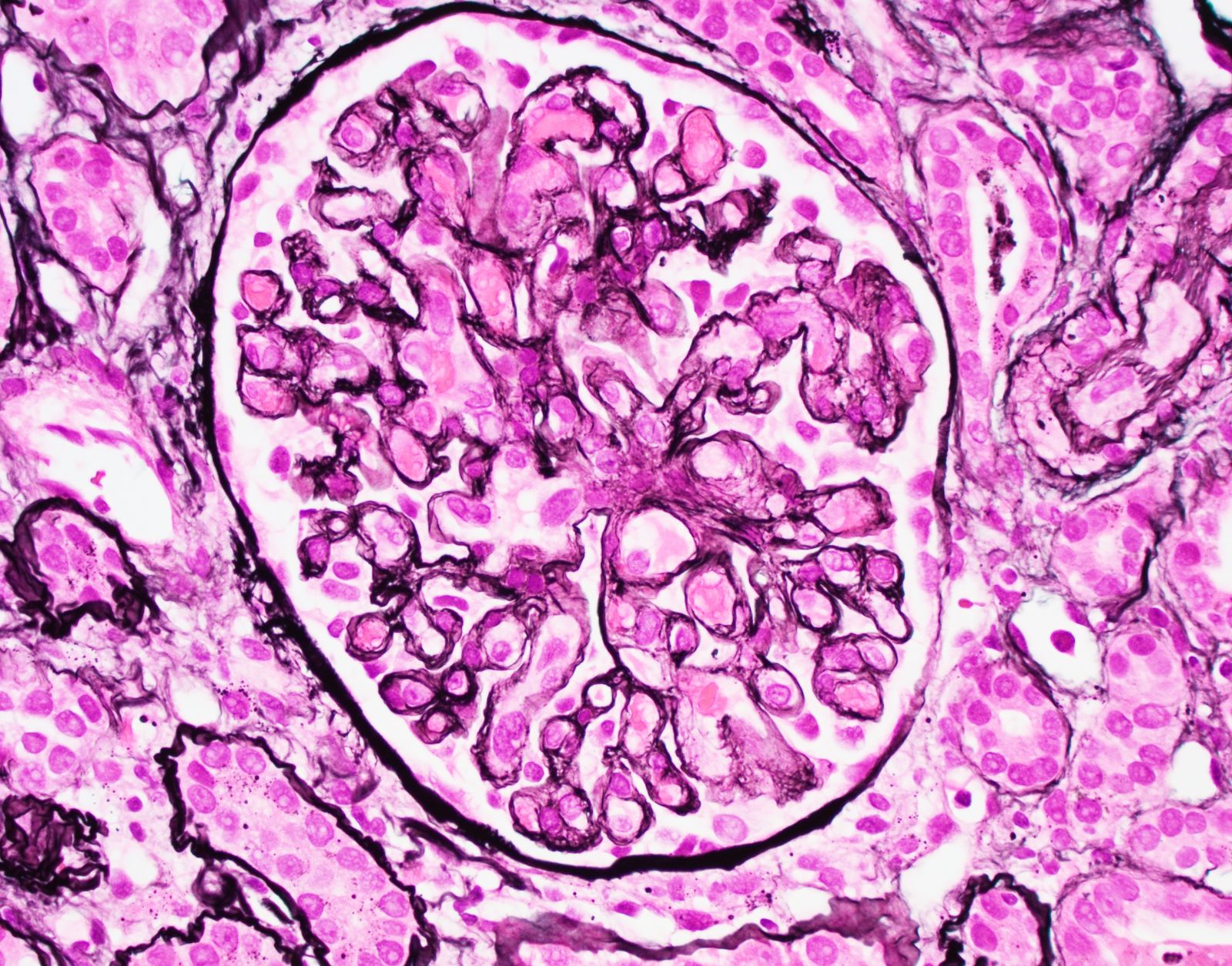
- Surrounding parenchyma shows global glomerulosclerosis, interstitial fibrosis and tubular atrophy
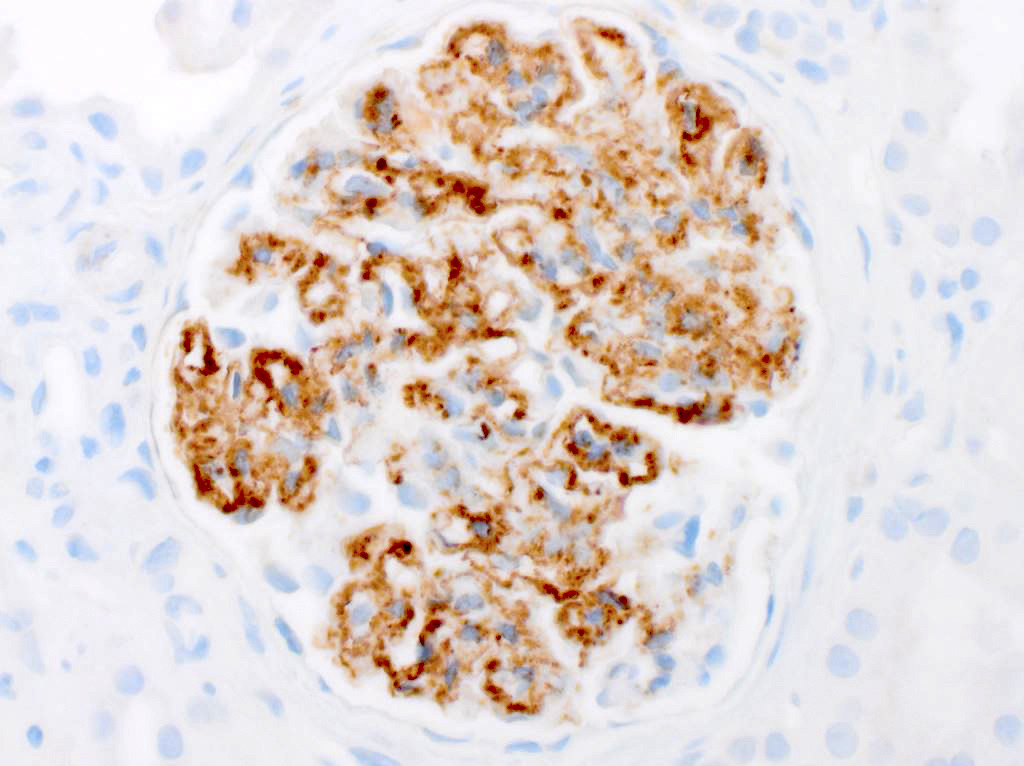
- Atypical epithelial proliferations associated with gains of #7, #12, #17, #20 and Y, suggesting they represent early neoplasms (Hum Pathol 2002;33:761)
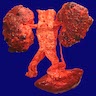
- Autosomal dominant polycystic kidney disease: markedly enlarged kidneys up to 2 - 4 kg and with family history
- Alloimmune reaction to donor specific antigens resulting in damage to the kidney allograft
- Mediated by antibodies produced by B cells and hence referred to as antibody mediated rejection (ABMR) in the Banff 2019 classification (Am J Transplant 2020;20:2318)
- Has 3 major subcategories:
- Active antibody mediated rejection: characterized by an acute immunologic reaction
- Chronic active antibody mediated rejection: chronic renal injury due to persistent / recurrent ABMR
- Chronic (inactive) ABMR: chronic renal injury due to prior active / chronic active ABMR
- Evidence of microvascular / endothelial damage
- Peritubular capillaritis, glomerulitis, microthrombosis and acute tubular injury (active component)
- Transplant glomerulopathy and multilayering of the tubular basement membrane (signs of chronicity)
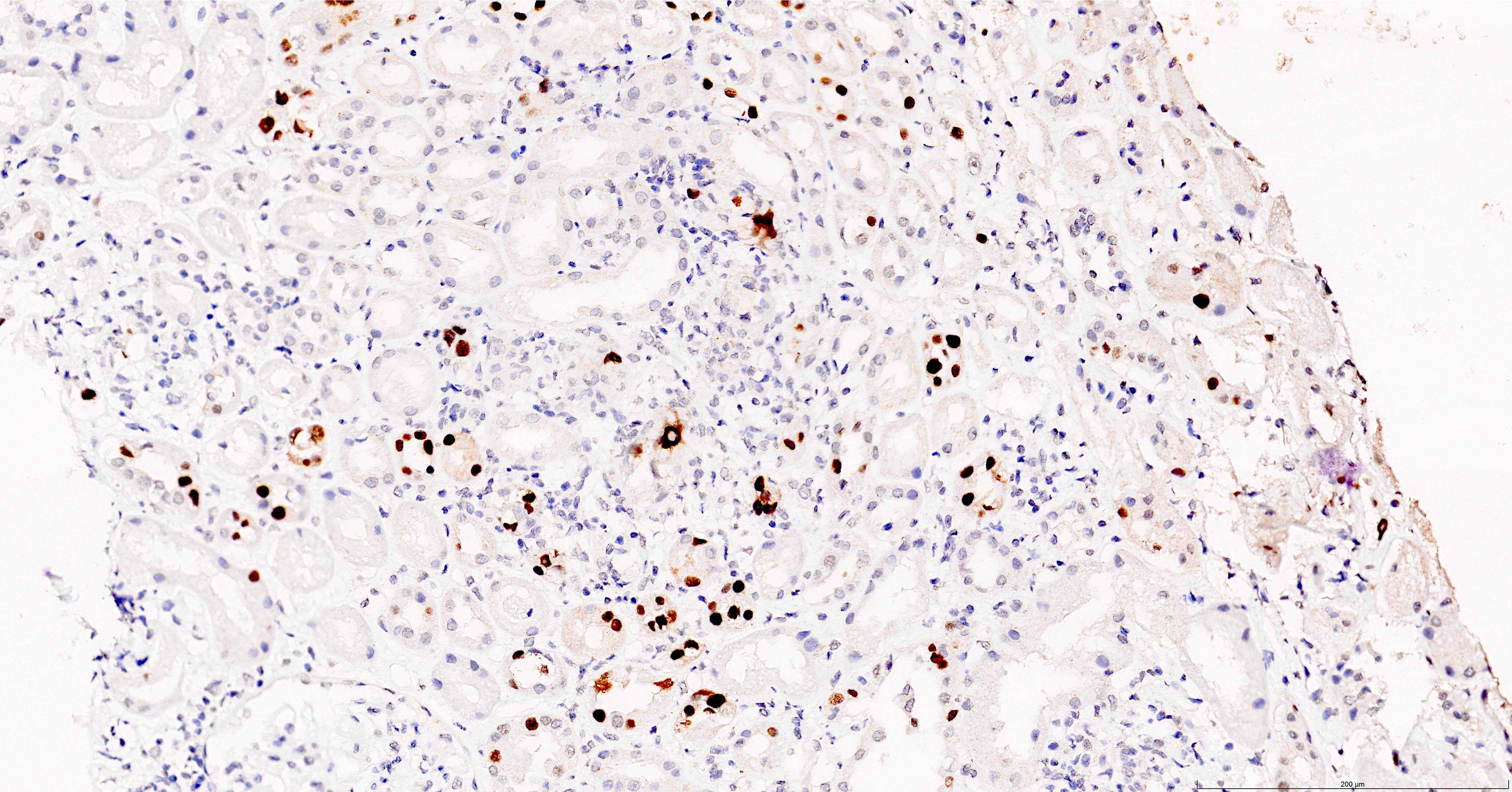
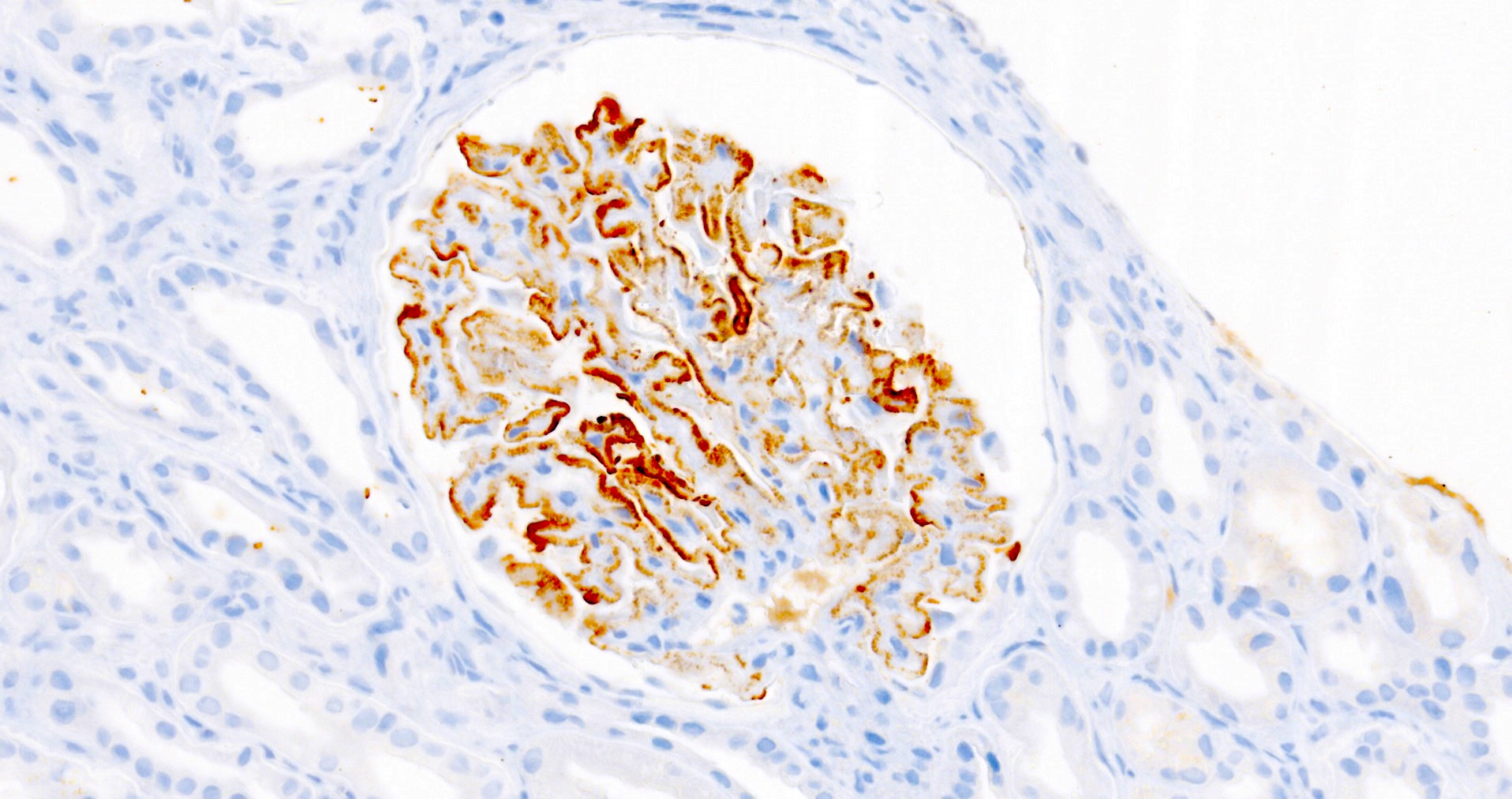
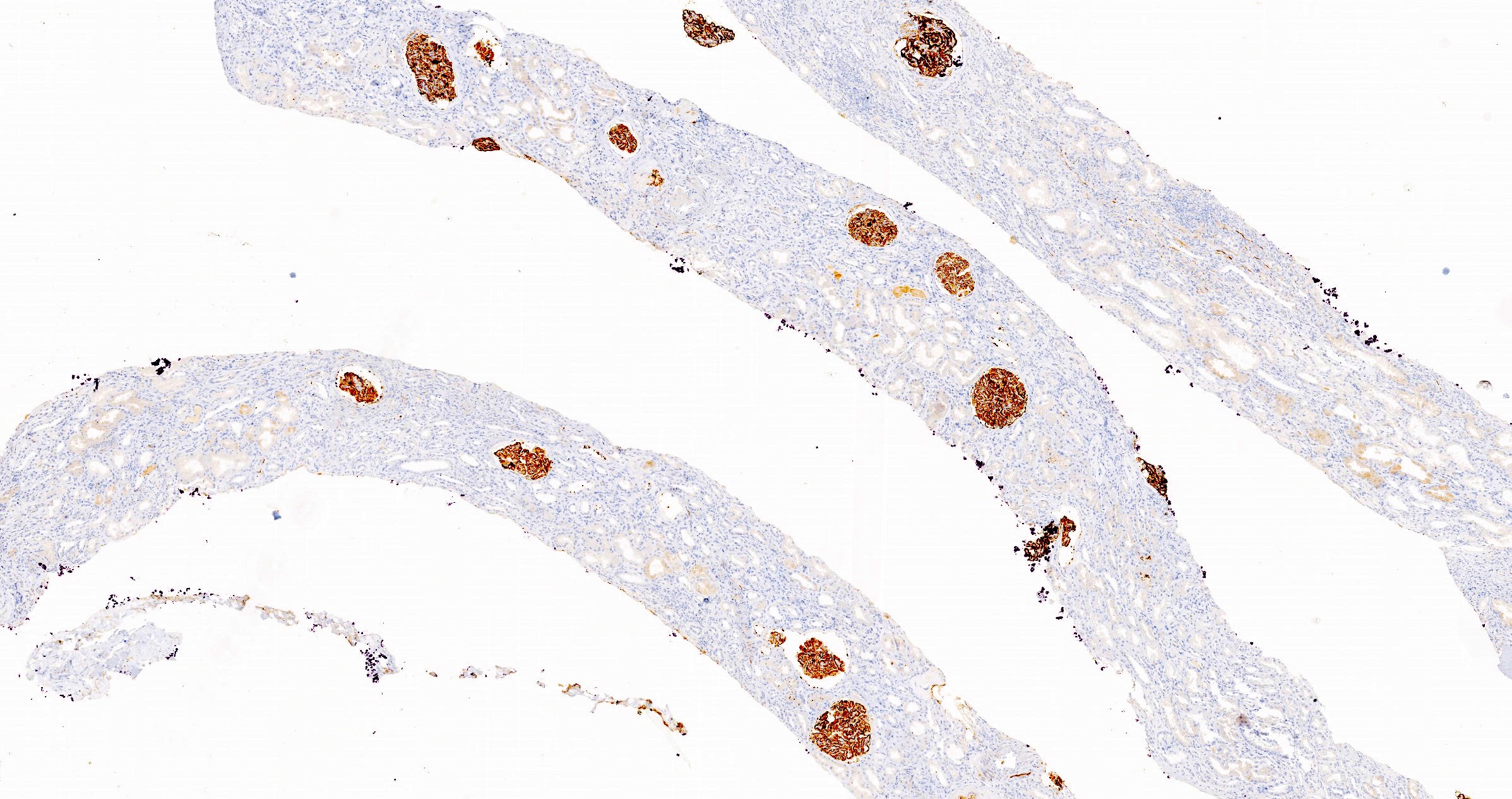
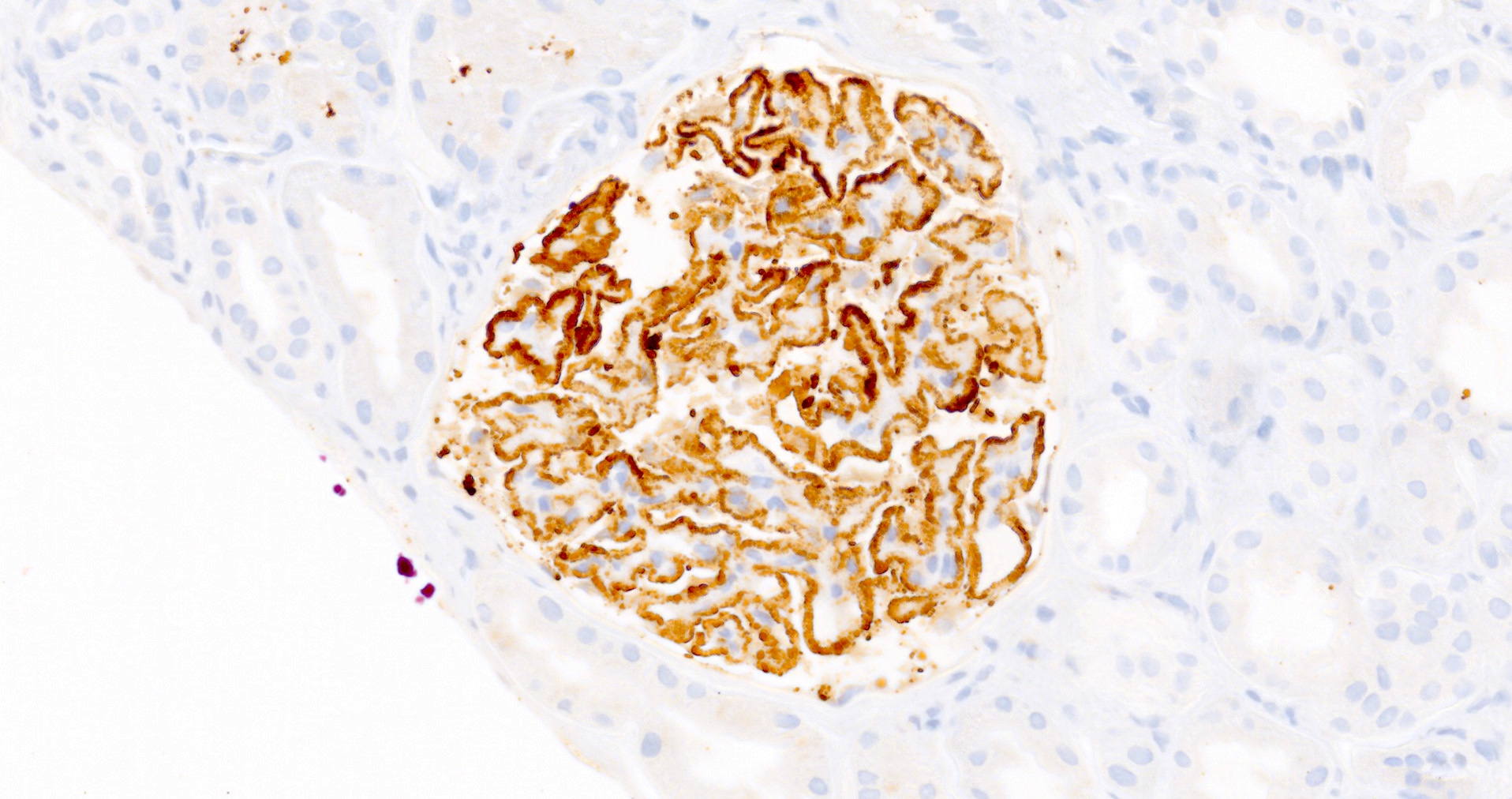
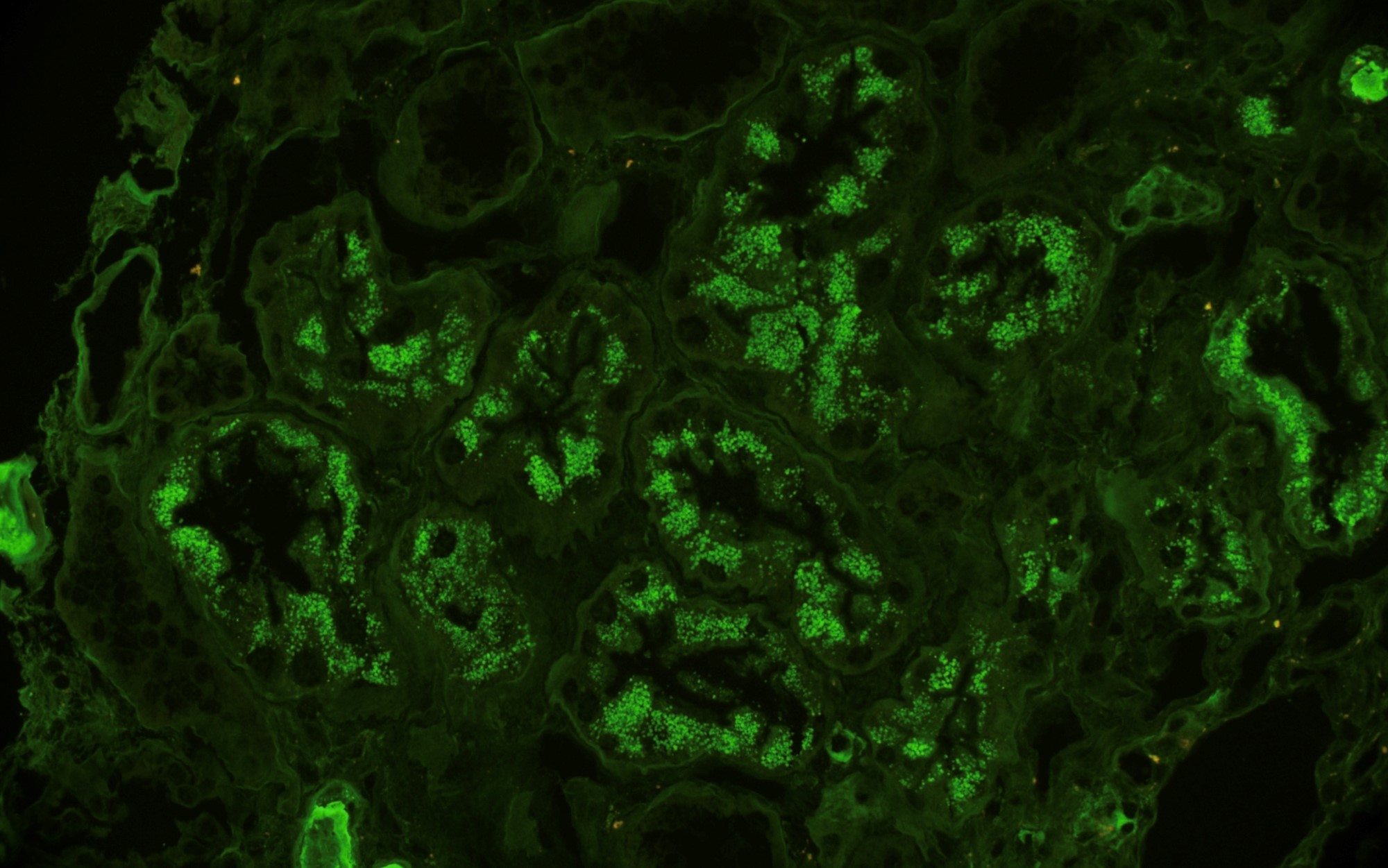
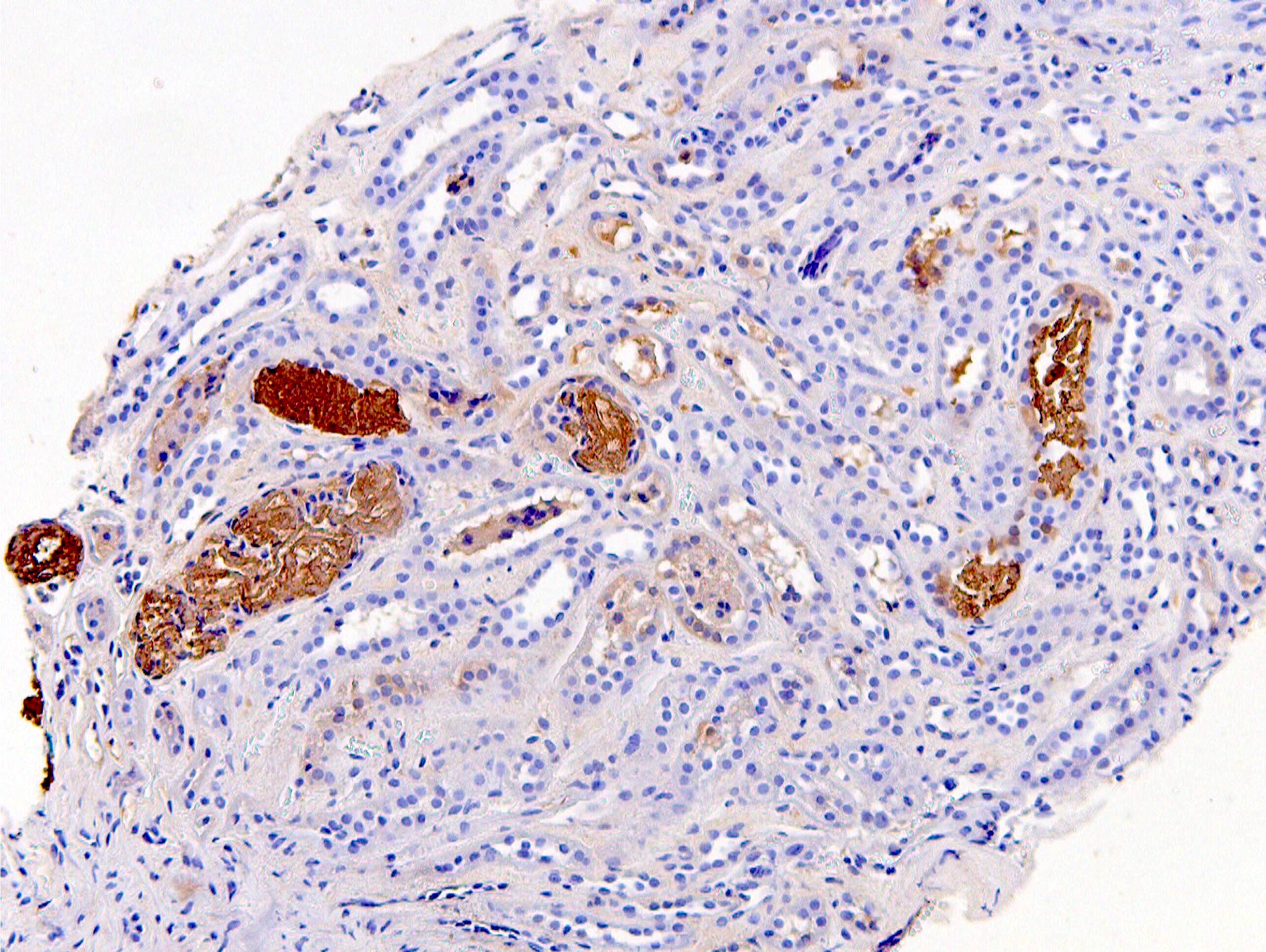
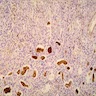
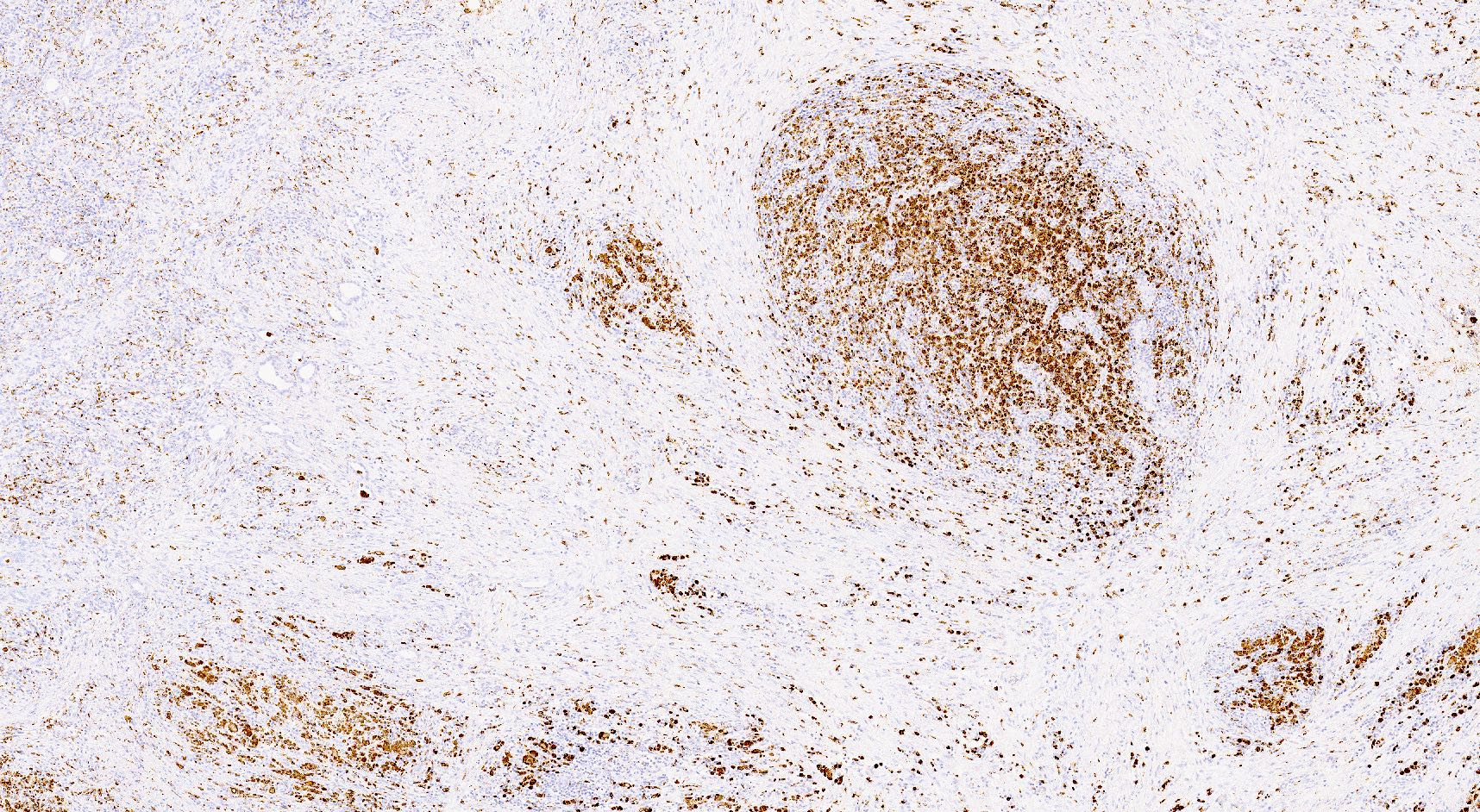
- C4d positivity (a complement degradation product) along peritubular capillaries
- Intimal arteritis or vasculitis; this lesion is not limited to antibody mediated rejection (ABMR) but may also be indicative of acute T cell mediated rejection as well as mixed T cell mediated rejection and ABMR
- Expression of endothelium associated transcripts (ENDAT)
- Coded by the g, cg, ptc, v, cv and C4d Banff scores
- Active antibody mediated rejection: also referred to as acute humoral rejection
- Chronic active antibody mediated rejection: also referred to as chronic humoral rejection
- Chronic antibody mediated rejection: also referred to as chronic humoral rejection
- ICD-10: T86.11 - kidney transplant rejection
- Antibody mediated rejection has been reported to occur in about 5 - 10% of transplant patients (J Transplant 2012;2012:193724)
- Can be as high as 50% in patients with human leukocyte antigen (HLA) incompatible transplant
- Renal disease
- Due to circulating antibodies against donor HLA, non-HLA or ABO antigens, i.e. donor specific antibodies (DSA) (Clin Biochem 2016;49:320)
- DSAs can be preformed (in which case antibody mediated rejection occurs during the very early posttransplant period - hyperacute / accelerated rejection) or may develop de novo after transplantation (usually due to inadequate immunosuppression or nonadherence)
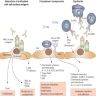
- These antibodies bind to donor specific antigens on the vascular endothelium of the graft and result in complement activation
- This leads to activation of polymorphonuclear inflammatory cells, NK cell and monocyte recruitment and inflammation, as well as activation of the coagulation cascade
- This in turn leads to widespread microvascular injury evident as peritubular capillaritis, glomerulitis and microvascular thrombosis
- Eventually, transplant glomerulopathy develops (chronic phase) due to recurrent injury and repair (manifested as proteinuria) - glomerular basement membrane remodeling, mesangial matrix expansion, capillary obliteration, foot process effacement
- References: Transplant Rev (Orlando) 2017;31:257, Transplant Rev (Orlando) 2017;31:47
- Acute antibody mediated rejection (ABMR):
- Usually seen during the first few weeks after transplantation but can occur later, usually associated with decreased immunosuppression or noncompliance
- Presents with acute renal failure or oliguria, sometimes severe enough to require dialysis
- Chronic / chronic active ABMR: chronic renal failure with proteinuria
- Subclinical ABMR: stable creatinine but histological evidence of ABMR
- Reference: J Transplant 2012;2012:193724
- Established via indication or protocol biopsies (Transplant Rev (Orlando) 2017;31:257, Transplant Rev (Orlando) 2017;31:47)
Diagnostic criteria and Banff classification / grading (modified from the Banff 2019 revision, Am J Transplant 2020;20:2318):
- Active antibody mediated rejection (ABMR): all 3 criteria must be met for diagnosis
- Histologic evidence of acute tissue injury; 1+ of the following should be present:
- Microvascular inflammation (g > 0 or ptc > 0), in the absence of recurrent or de novo glomerulonephritis; ptc ≥ 1 alone is not sufficient and g must be ≥ 1 if:
- Borderline infiltrate is present
- Acute T cell mediated rejection is also present
- Infection is present
- Intimal or transmural arteritis (v > 0)
- Acute thrombotic microangiopathy (in the absence of any other cause)
- Acute tubular injury (in the absence of any other cause)
- Microvascular inflammation (g > 0 or ptc > 0), in the absence of recurrent or de novo glomerulonephritis; ptc ≥ 1 alone is not sufficient and g must be ≥ 1 if:
- Evidence of current / recent antibody interaction with vascular endothelium; 1+ of the following should be present:
- Linear C4d staining in peritubular capillaries (C4d2 or C4d3 by immunoflourescence on frozen sections or C4d > 0 by immunohistochemistry on paraffin sections)
- At least moderate microvascular inflammation ([g + ptc] ≥ 2) in the absence of recurrent or de novo glomerulonephritis; ptc ≥ 2 alone is not sufficient and g must be ≥ 1 if:
- Borderline infiltrate is present
- Acute T cell mediated rejection is also present
- Infection is present
- Increased expression of gene transcripts / classifiers in the biopsy tissue strongly associated with ABMR (endothelial associated transcripts [ENDAT])
- Serologic evidence of donor specific antibodies (DSA) to HLA or other antigens
- C4d staining or expression of validated transcripts / classifiers (criterion 2 above) may substitute for DSA
- However, thorough DSA testing (including testing for non-HLA antibodies if HLA antibody testing is negative) is strongly advised whenever criteria 1 and 2 are met
- Histologic evidence of acute tissue injury; 1+ of the following should be present:
- Chronic active ABMR: all 3 criteria must be met for diagnosis
- Morphologic evidence of chronic tissue injury; 1+ of the following should be present:
- Transplant glomerulopathy (cg > 0) if no evidence of chronic thrombotic microangiopathy or chronic recurrent / de novo glomerulonephritis; includes changes evident by electron microscopy alone (cg1a)
- Severe peritubular capillary basement membrane multilayering (requires electron microscopy)
- Arterial intimal fibrosis of new onset, excluding other causes (leukocytes within the sclerotic intima favor chronic ABMR if there is no prior history of T cell mediated rejection but are not required)
- Same as criterion 2 for active ABMR
- Same as criterion 3 for active ABMR
- Morphologic evidence of chronic tissue injury; 1+ of the following should be present:
- Chronic (inactive) ABMR
- Transplant glomerulopathy (cg > 0) or severe peritubular capillary basement membrane multilayering
- Absence of criterion 2 for active ABMR
- Prior documented active or chronic active ABMR
- C4d staining without evidence of rejection: all 4 features must be present for diagnosis
- Linear C4d staining in peritubular capillaries (C4d2 or C4d3 by immunoflorescence on frozen sections or C4d > 0 by immunohistochemistry on paraffin sections)
- Criterion 1 for active or chronic active ABMR not met
- No molecular evidence for ABMR (criterion 2 for active and chronic active ABMR)
- No acute or chronic active T cell mediated rejection or borderline changes
- Acute antibody mediated rejection (ABMR): acute increase in serum creatinine levels
- Chronic active ABMR: chronic increase in serum creatinine levels along with proteinuria, usually nephrotic range
- Serum donor specific antibodies: anti-HLA or non-HLA antibodies (J Transplant 2012;2012:193724)
- Contrast enhanced ultrasound has been shown to be of diagnostic value in identifying cases of vascular rejection (Clin Hemorheol Microcirc 2018;69:77)
- New technologies for identification of acute rejection are at the experimental stage (Am J Nucl Med Mol Imaging 2019;9:110)
- Imaging seems to rely on analysis of changes in blood flow, which decreases with acute rejection induced inflammation and detection of recruitment of activated leucocytes with 18F-fluoro-deoxy-glucose positron emission tomography (Clin Kidney J 2017;10:97)
- Chronic active antibody mediated rejection is associated with poor graft survival and is today the leading cause of graft scarring and loss (Am J Transplant 2009;9:2520)
- 8 year old boy with development of ABMR shortly after an episode of acute T cell mediated rejection (CEN Case Rep 2018;7:288)
- 22 year old man with severe eosinophilic antibody mediated rejection (Indian J Nephrol 2018;28:389)
- 32 year old man with acute T cell mediated rejection accompanied by C4d- acute antibody mediated rejection (Nephrology (Carlton) 2015;20:70)
- 44 year old man with probable C4d- accelerated acute antibody mediated rejection due to non-HLA antibodies (Nephrology (Carlton) 2015;20:75)
- 49 year old woman with acute ABMR managed with bortezomid based treatment (Genet Mol Res 2015;14:17951)
- Plasmapheresis (J Transplant 2012;2012:193724)
- Intravenous immunoglobulin
- Rituximab - efficacy unclear
- Bortezomid - efficacy unclear
- Proteosome inhibition - efficacy unclear
- Complement inhibition
- References: Am J Transplant 2018;18 Suppl 3:3, Transplantation 2018;102:557
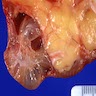
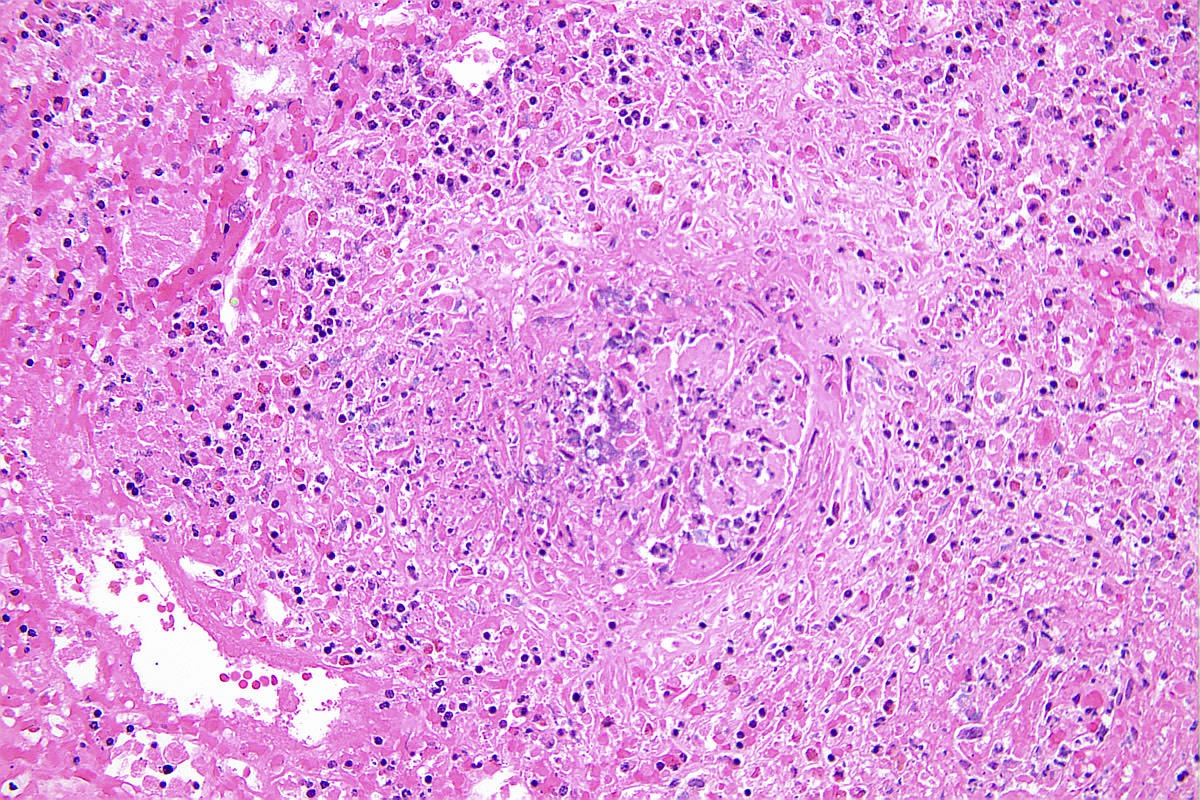
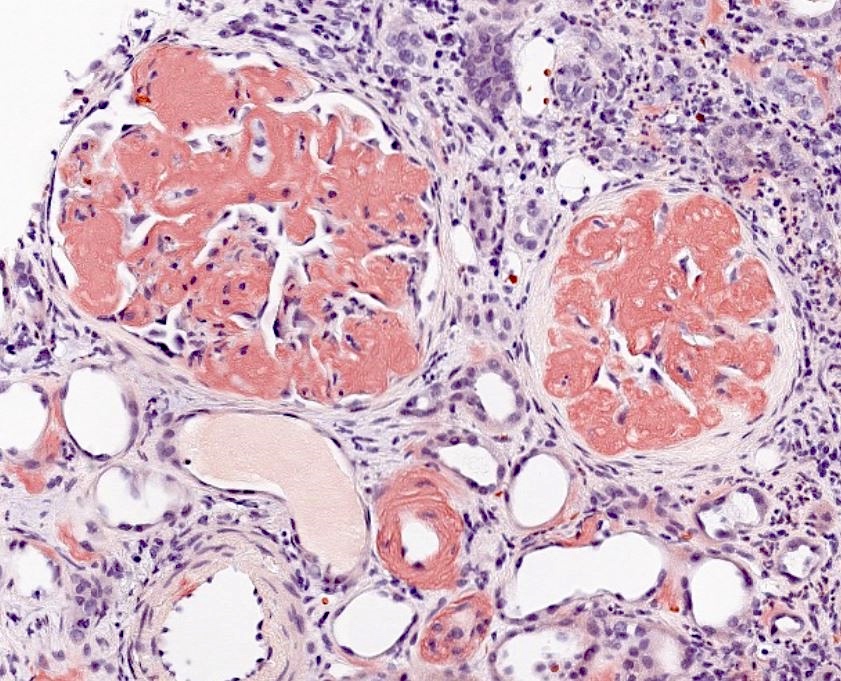
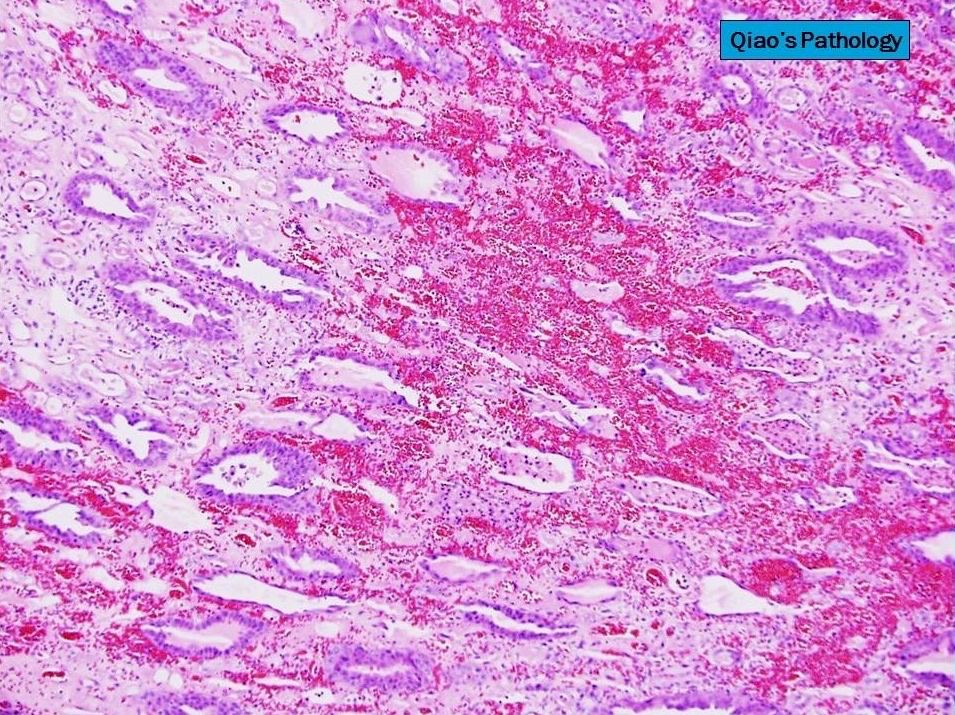
- Active / chronic active ABMR: kidneys are swollen and congested, may have large areas of hemorrhage or infarction
- Hyperacute antibody mediated rejection (ABMR):
- Transmural vasculitis
- Severe cortical necrosis
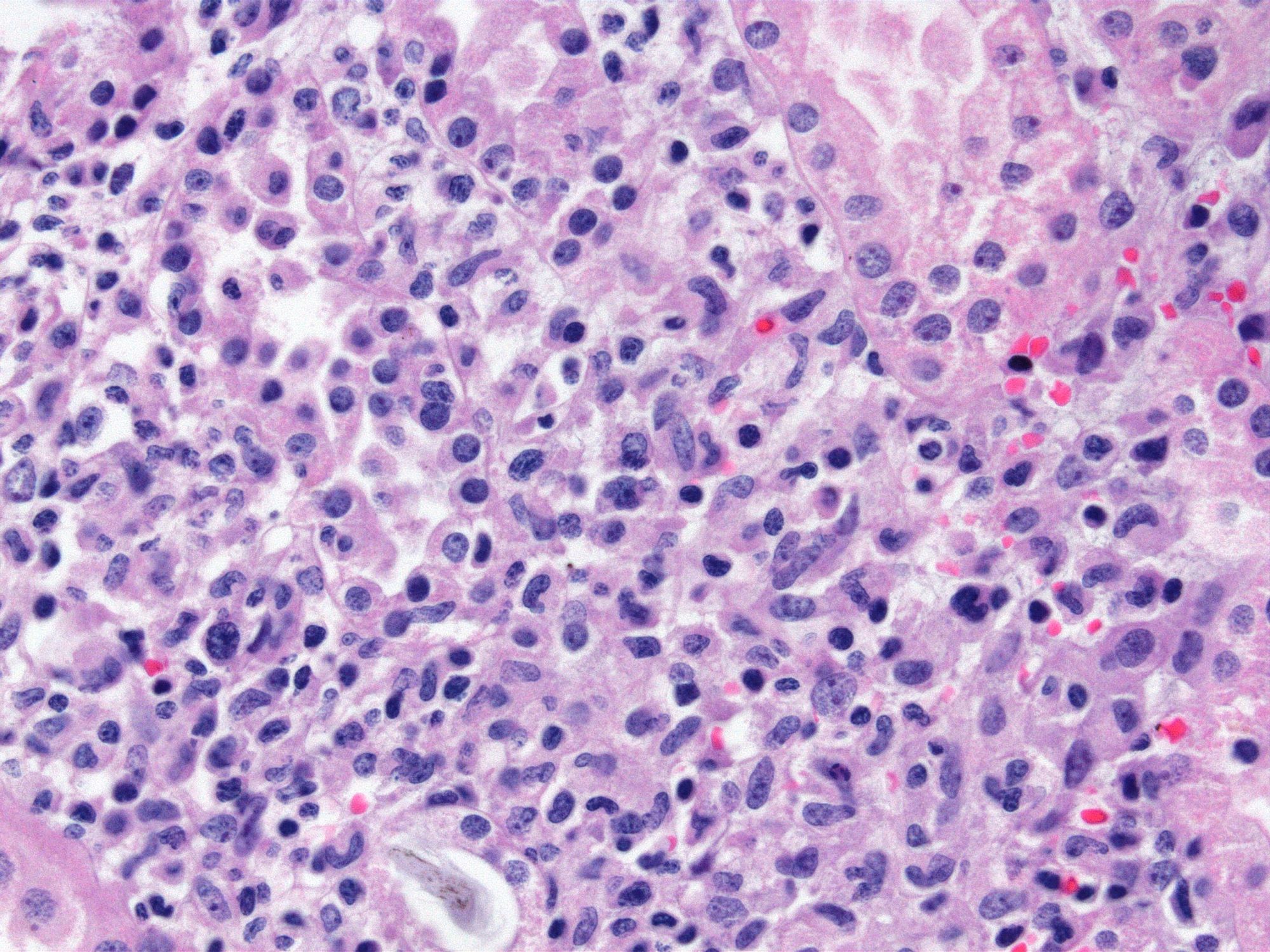
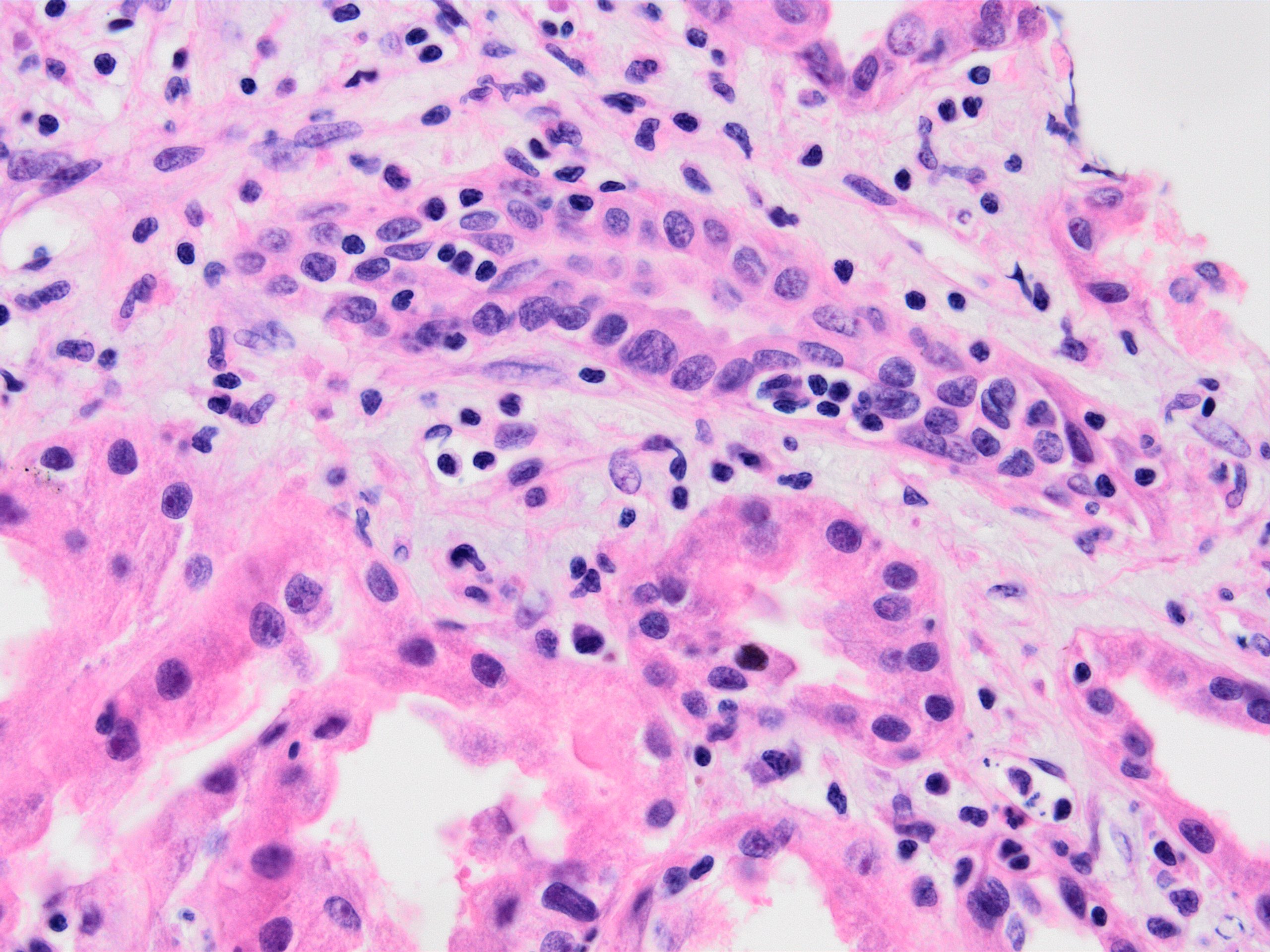
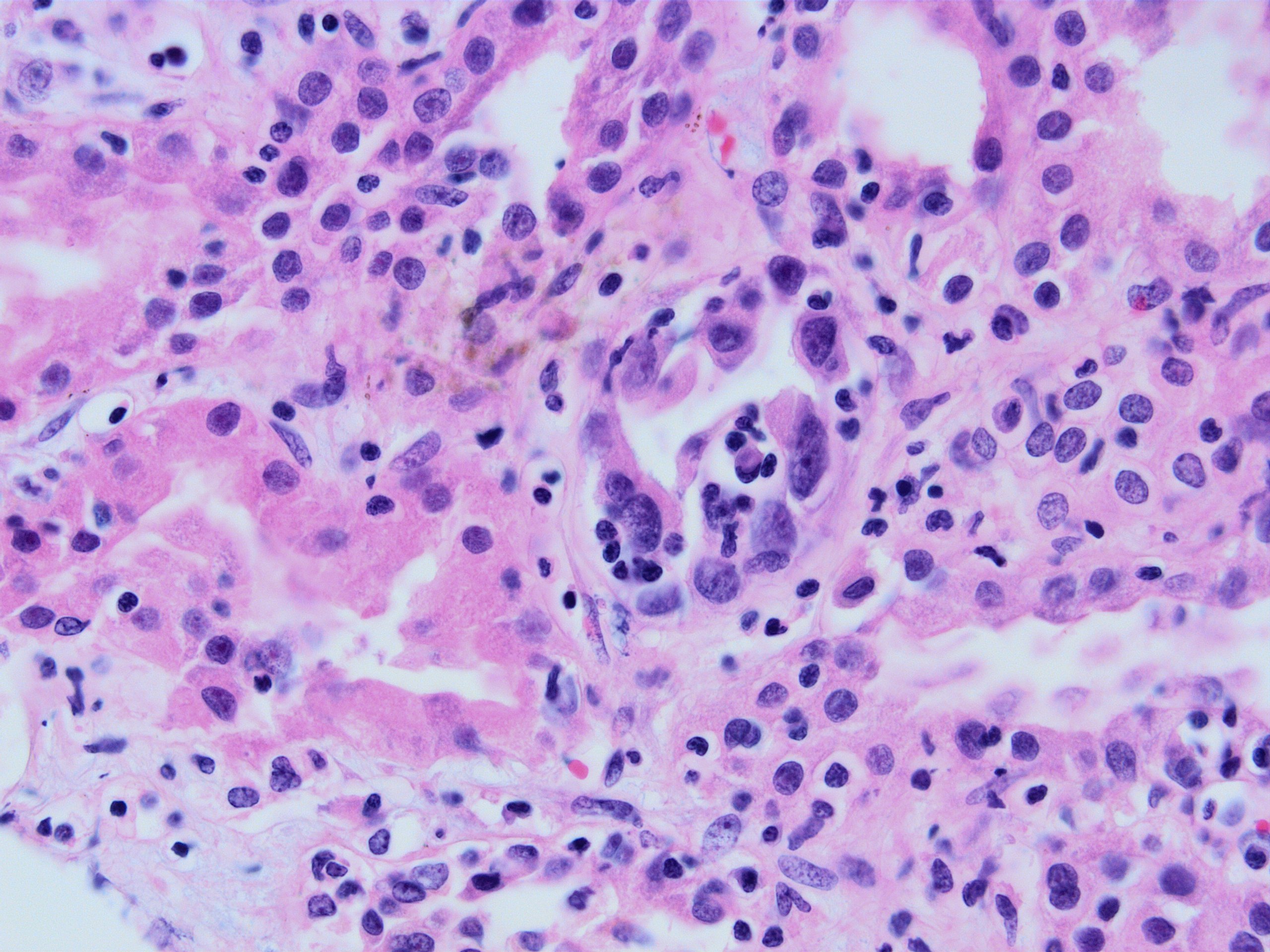
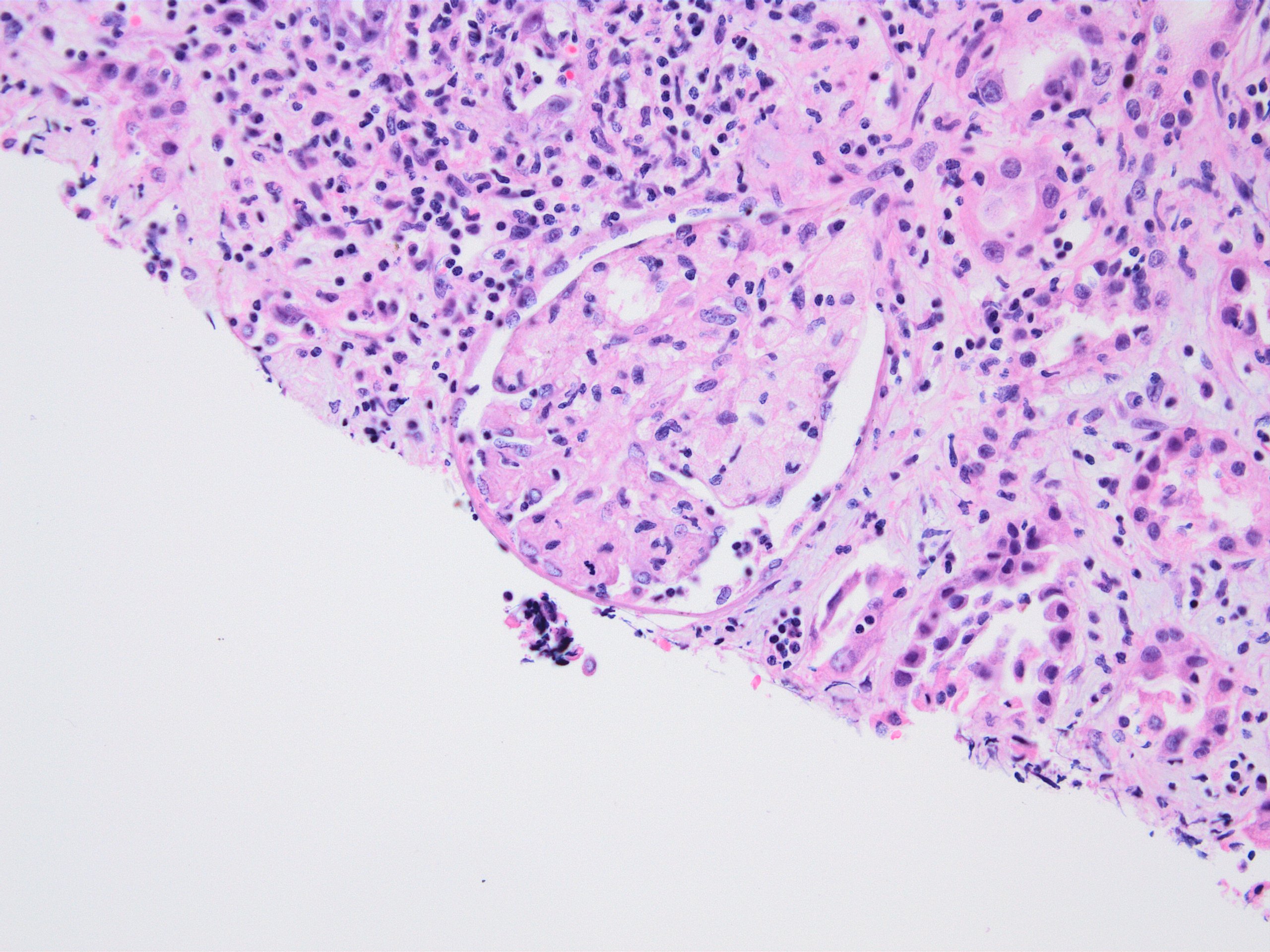
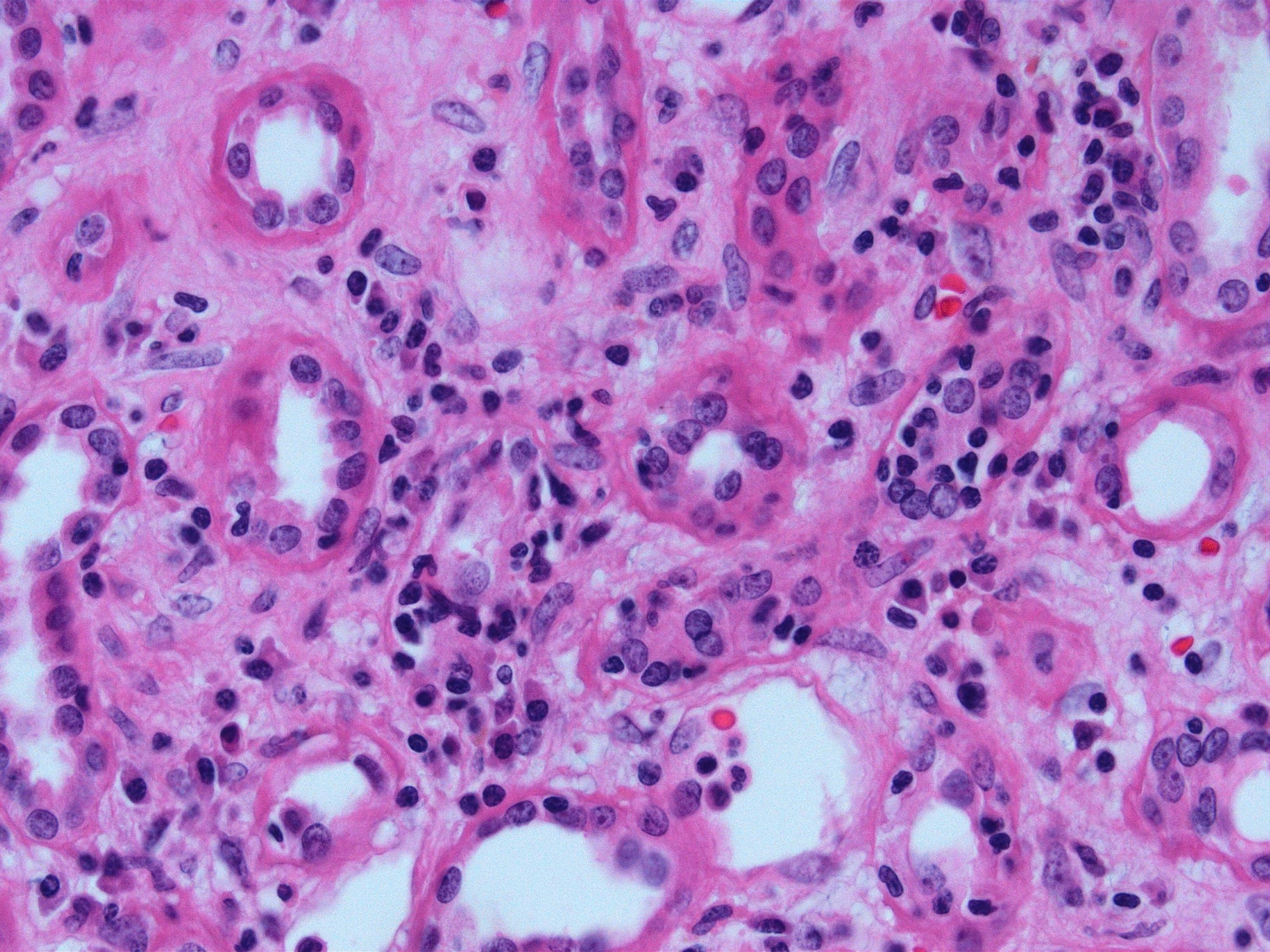
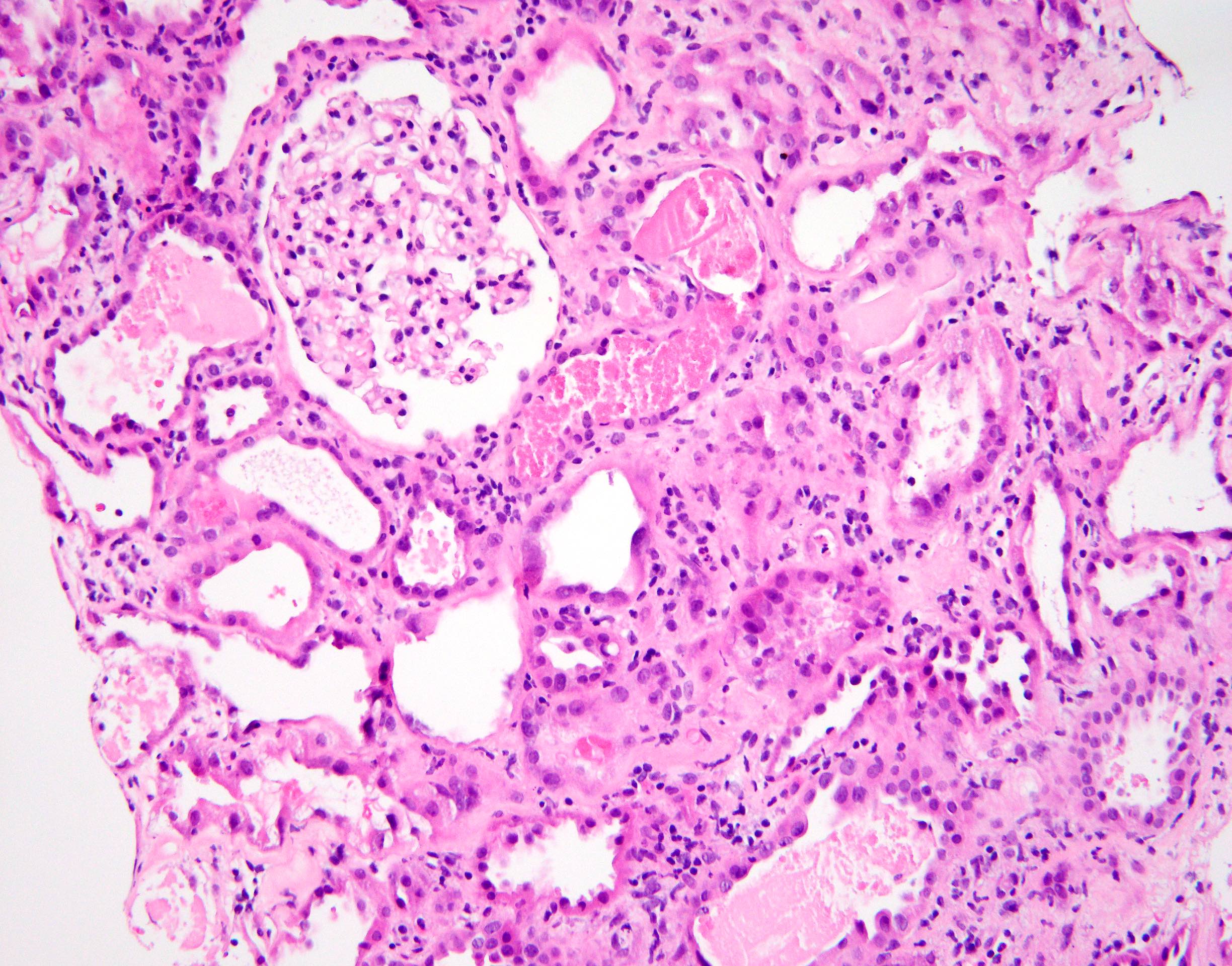
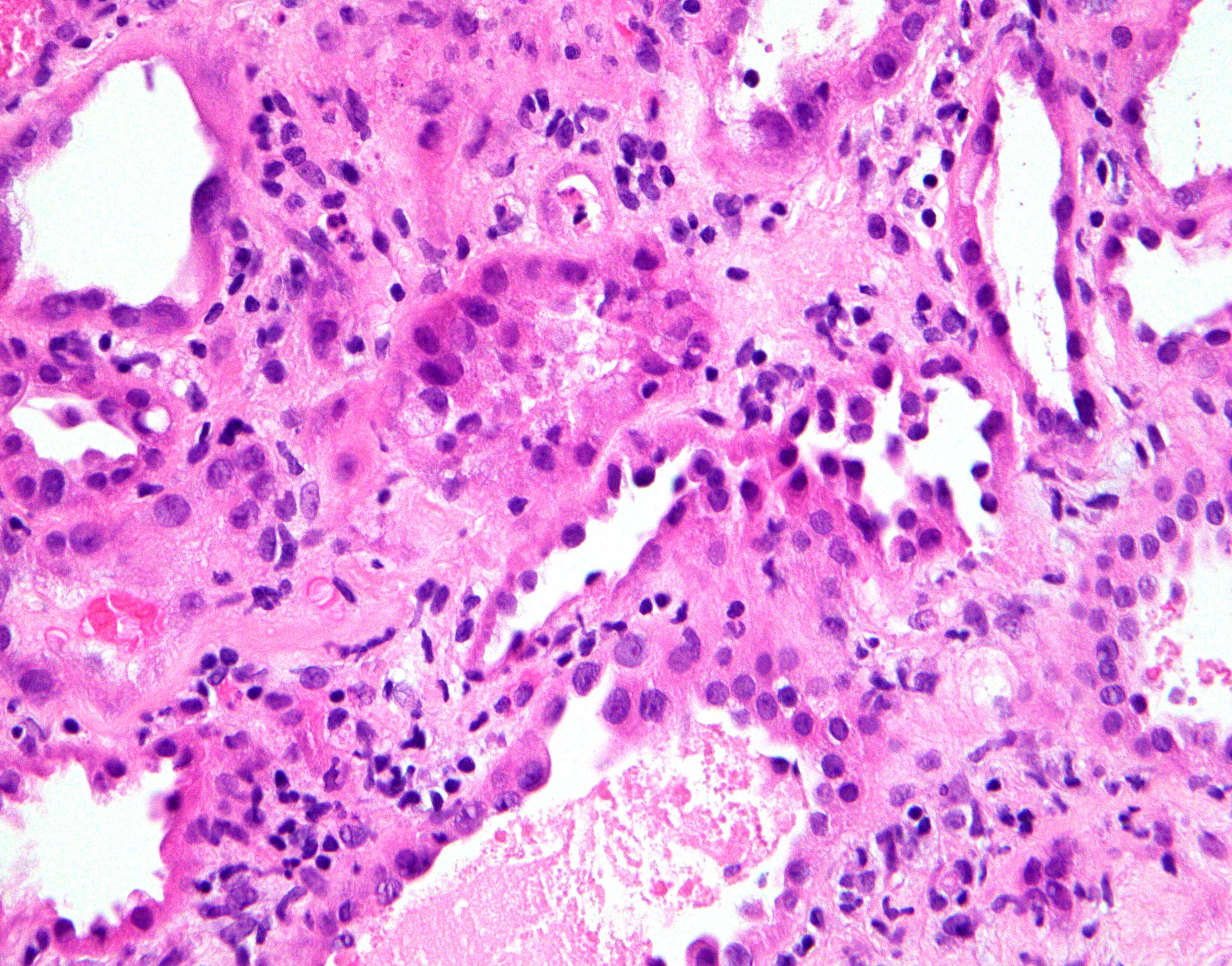
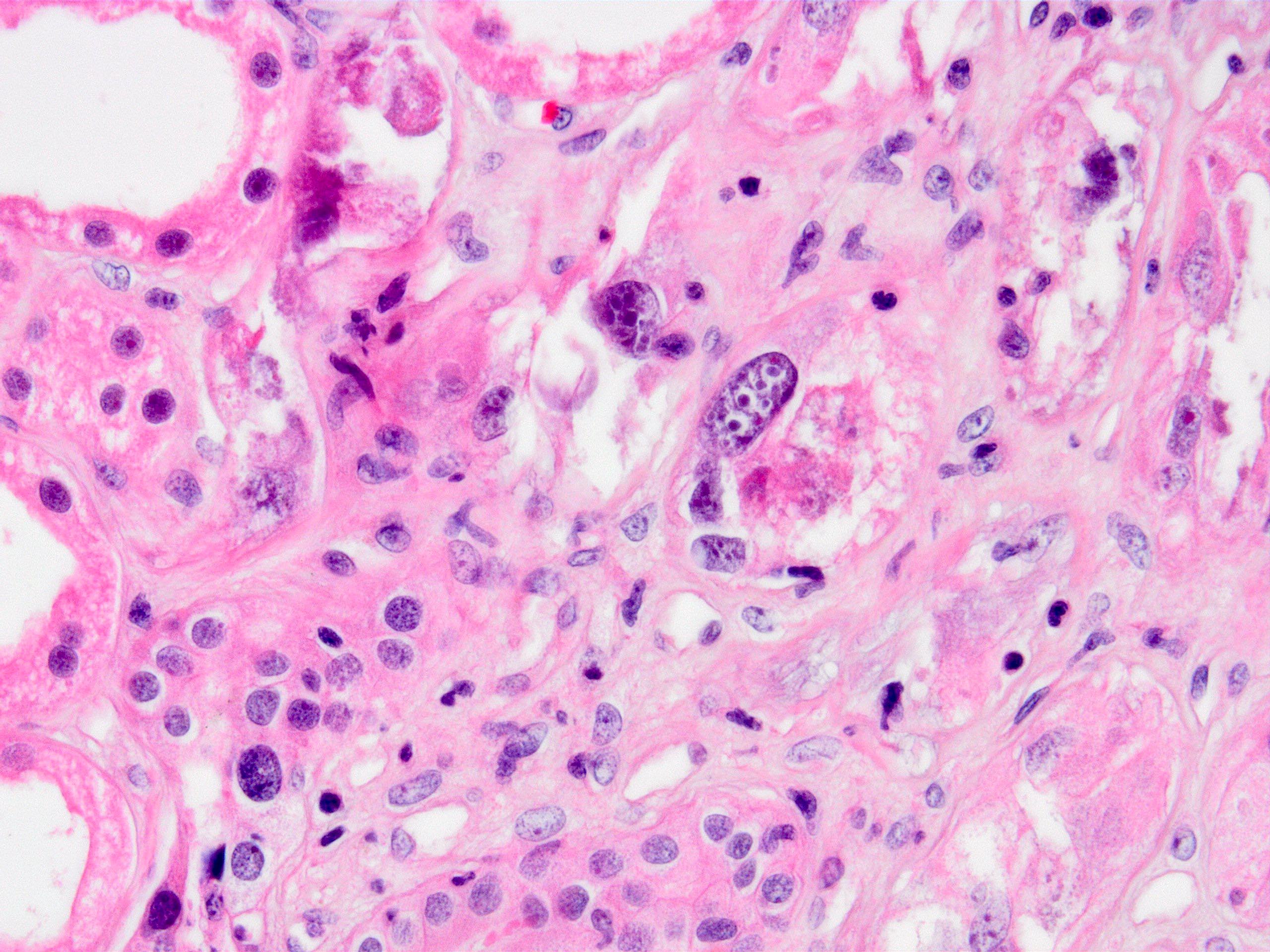
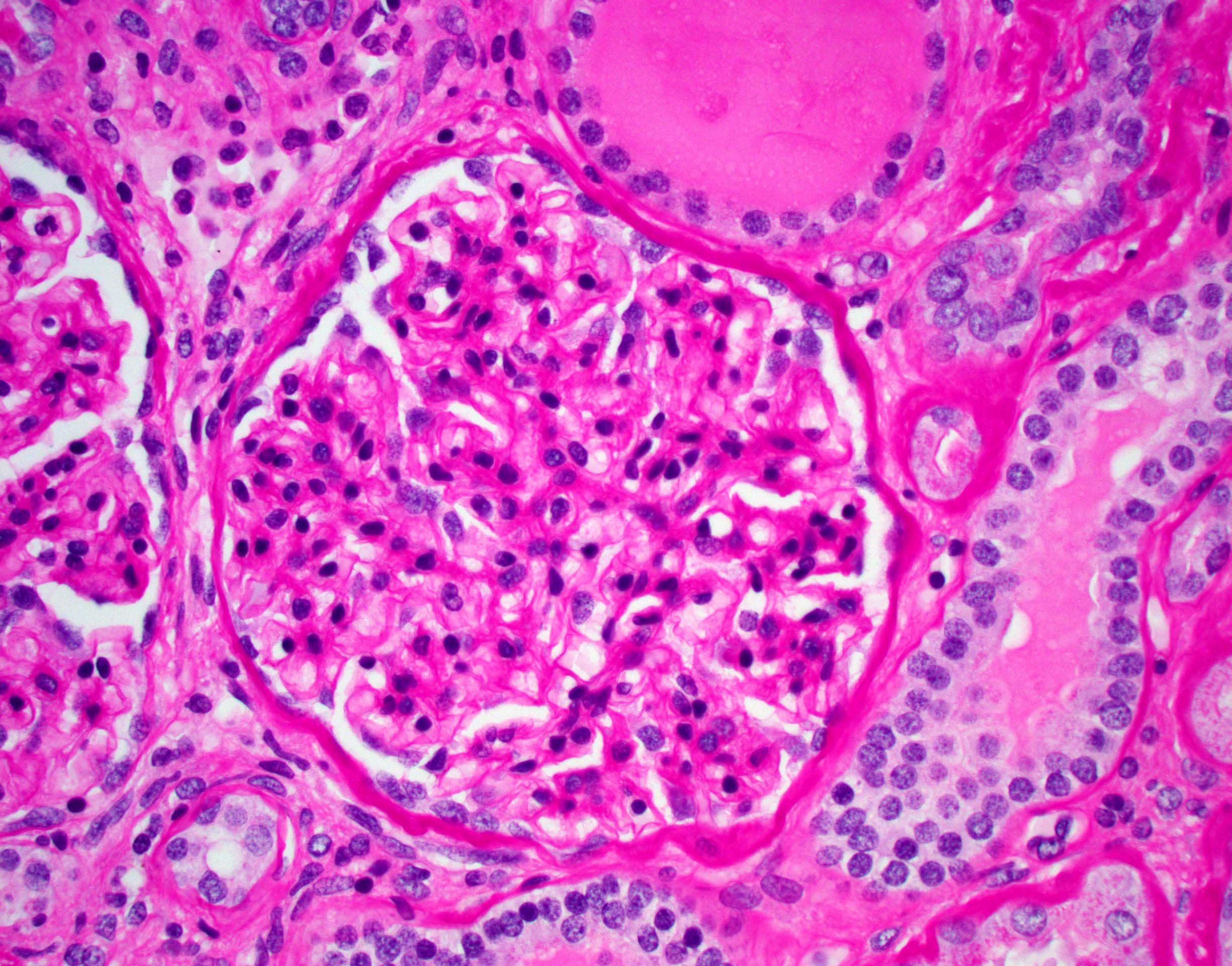
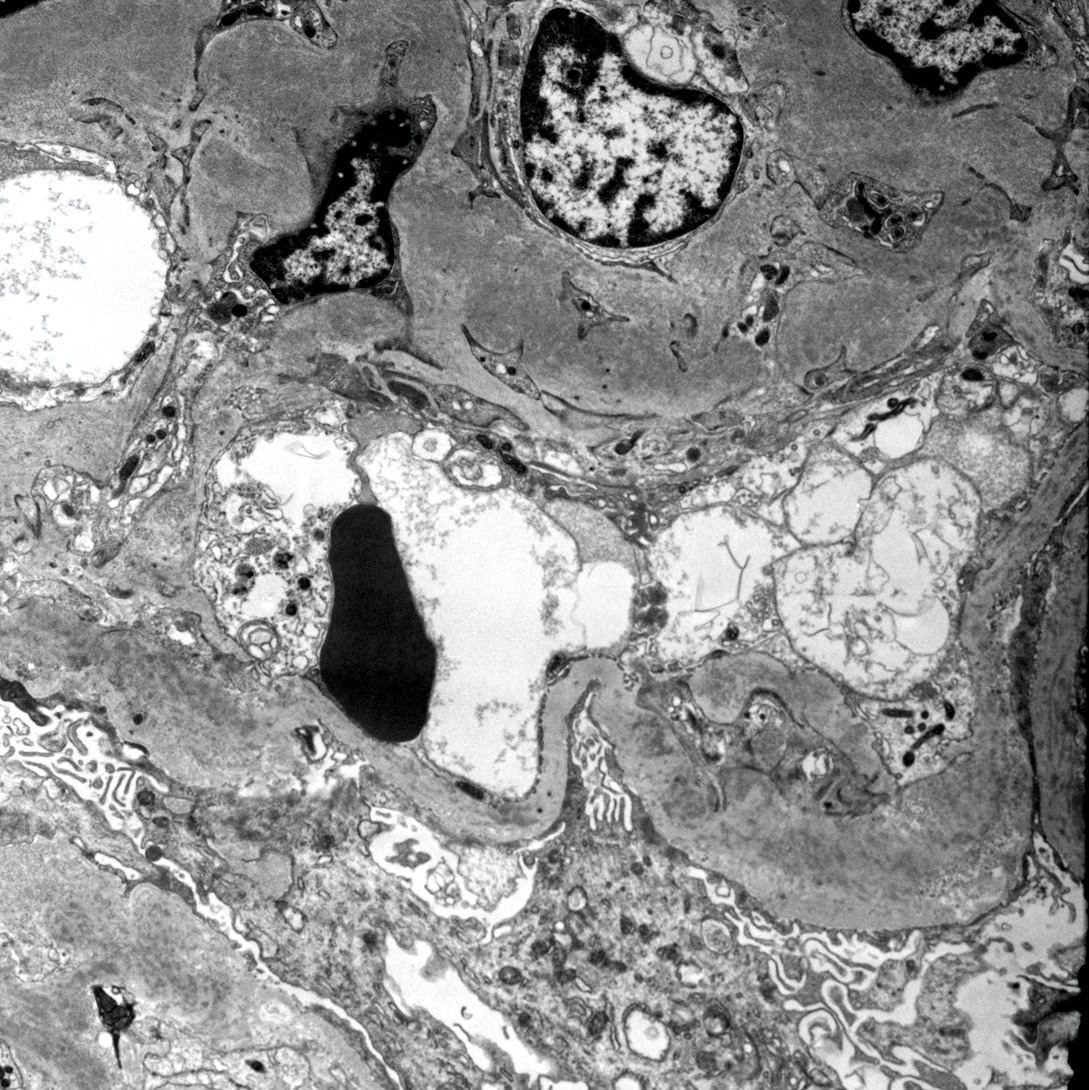
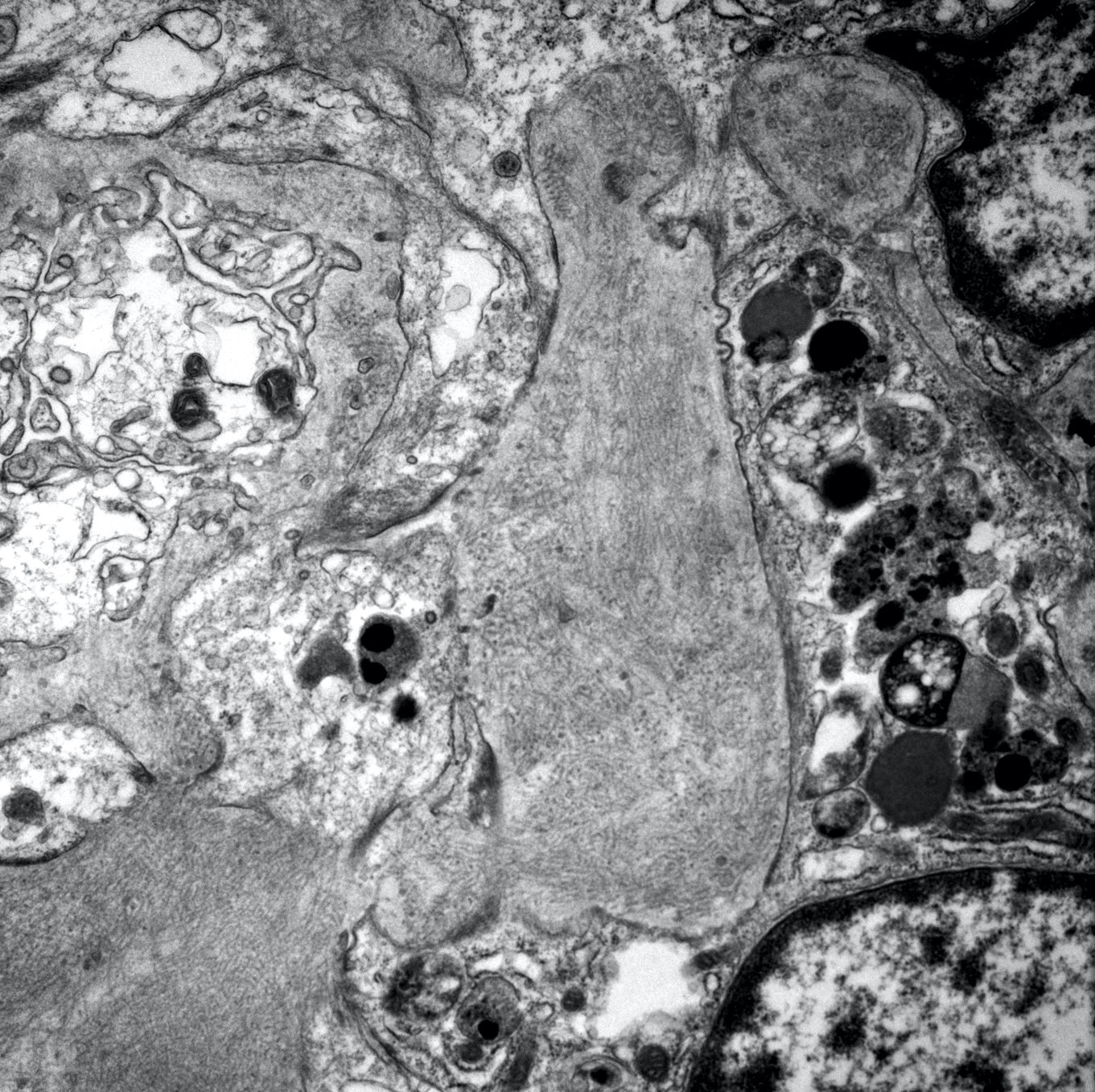
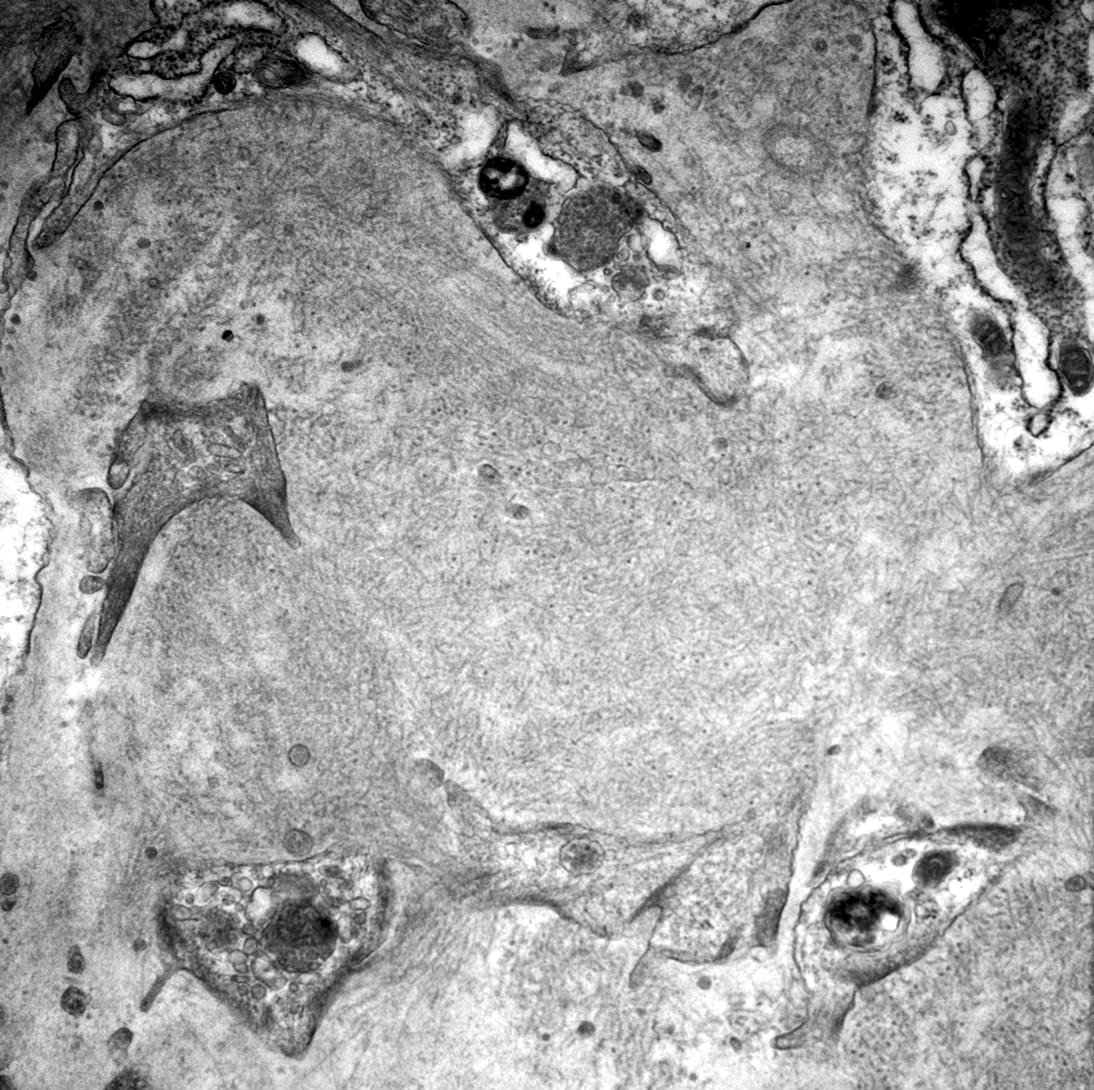
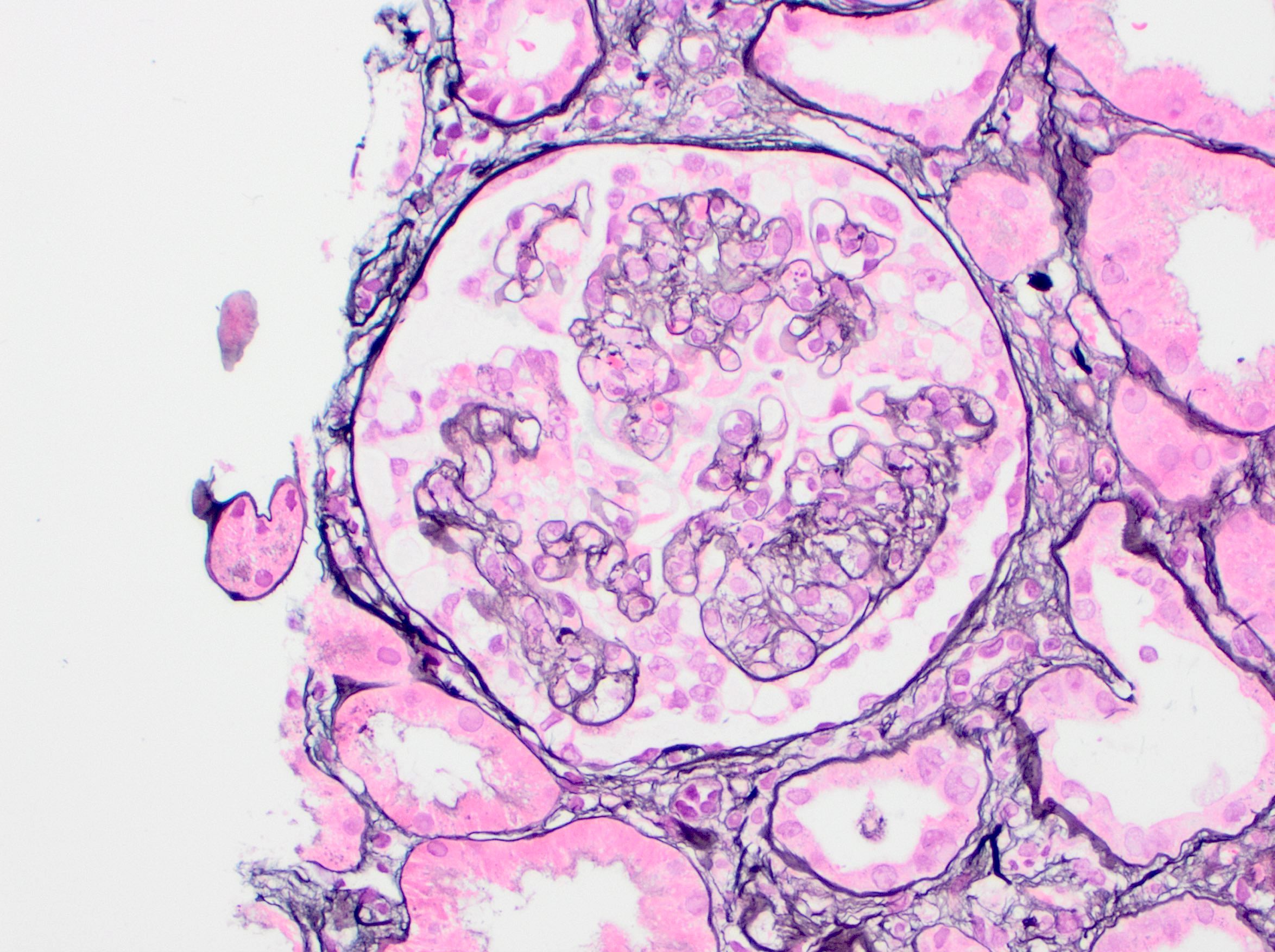
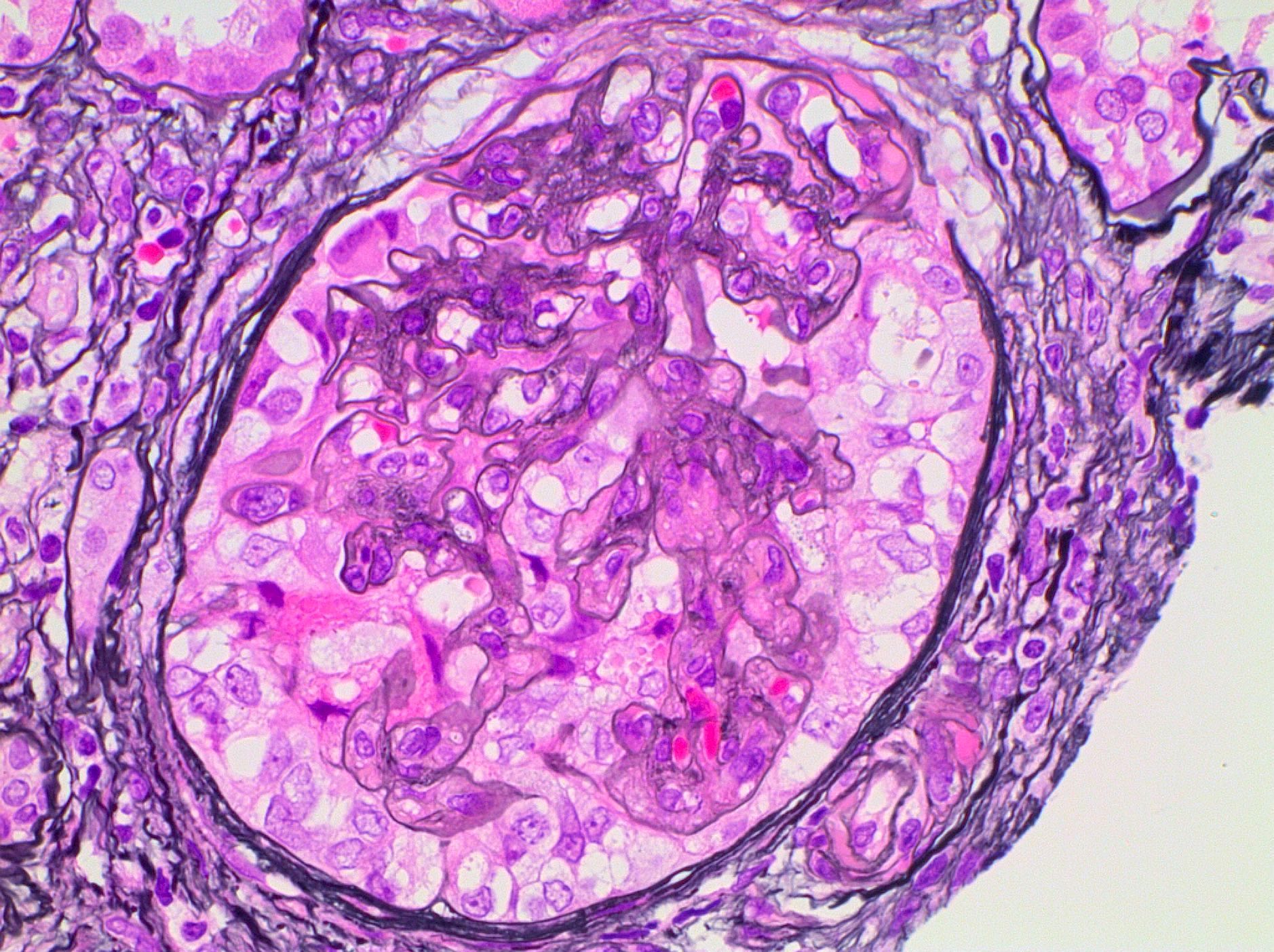
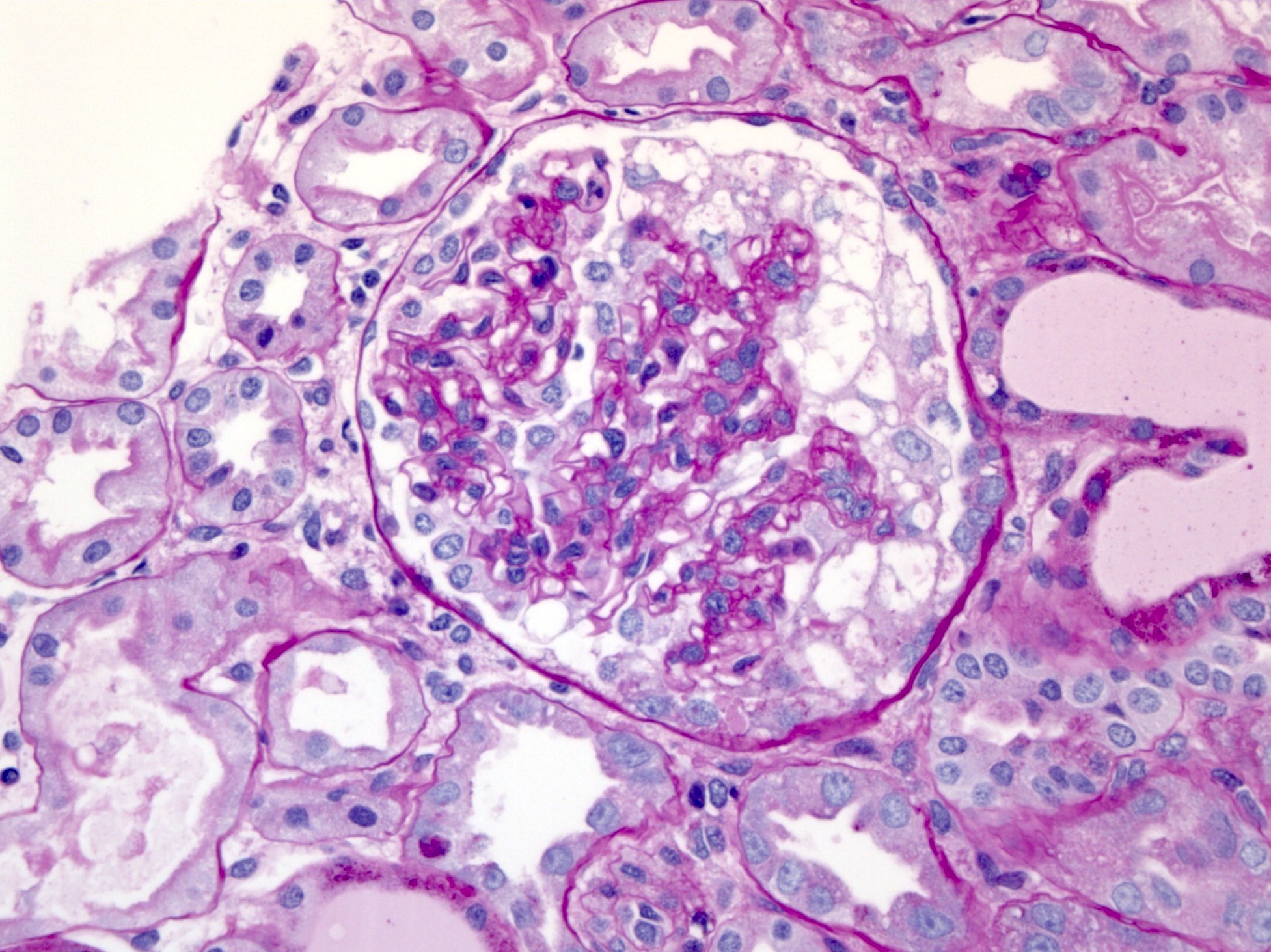
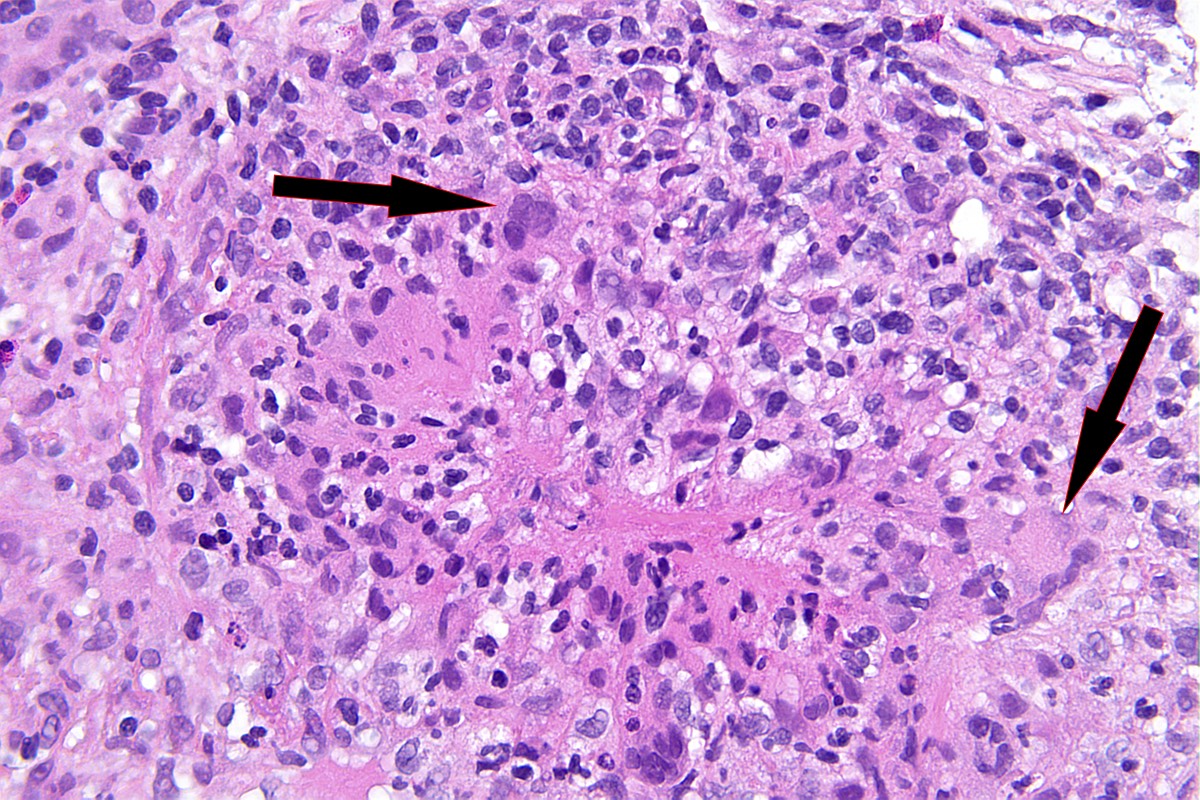
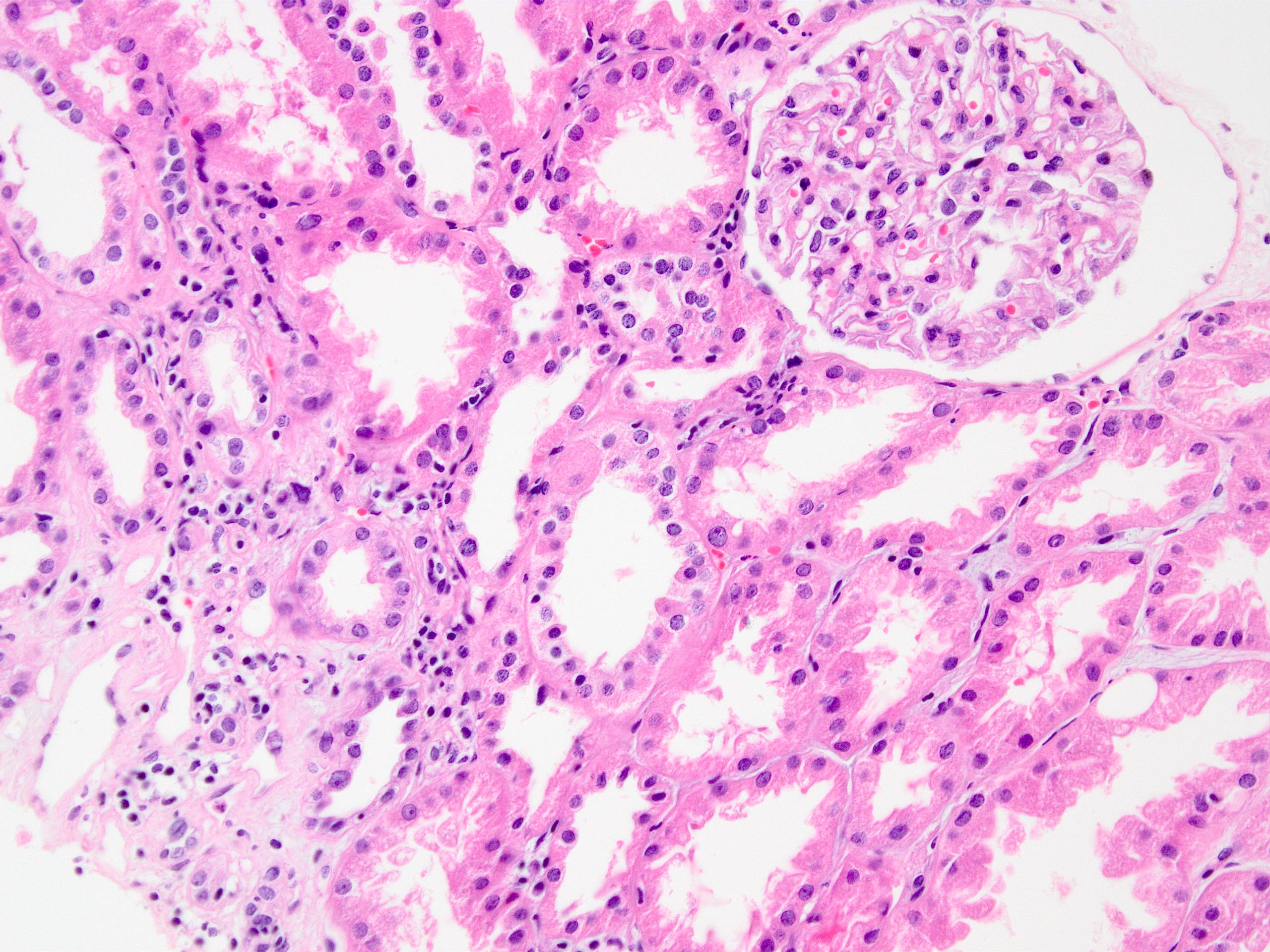
- Active ABMR:
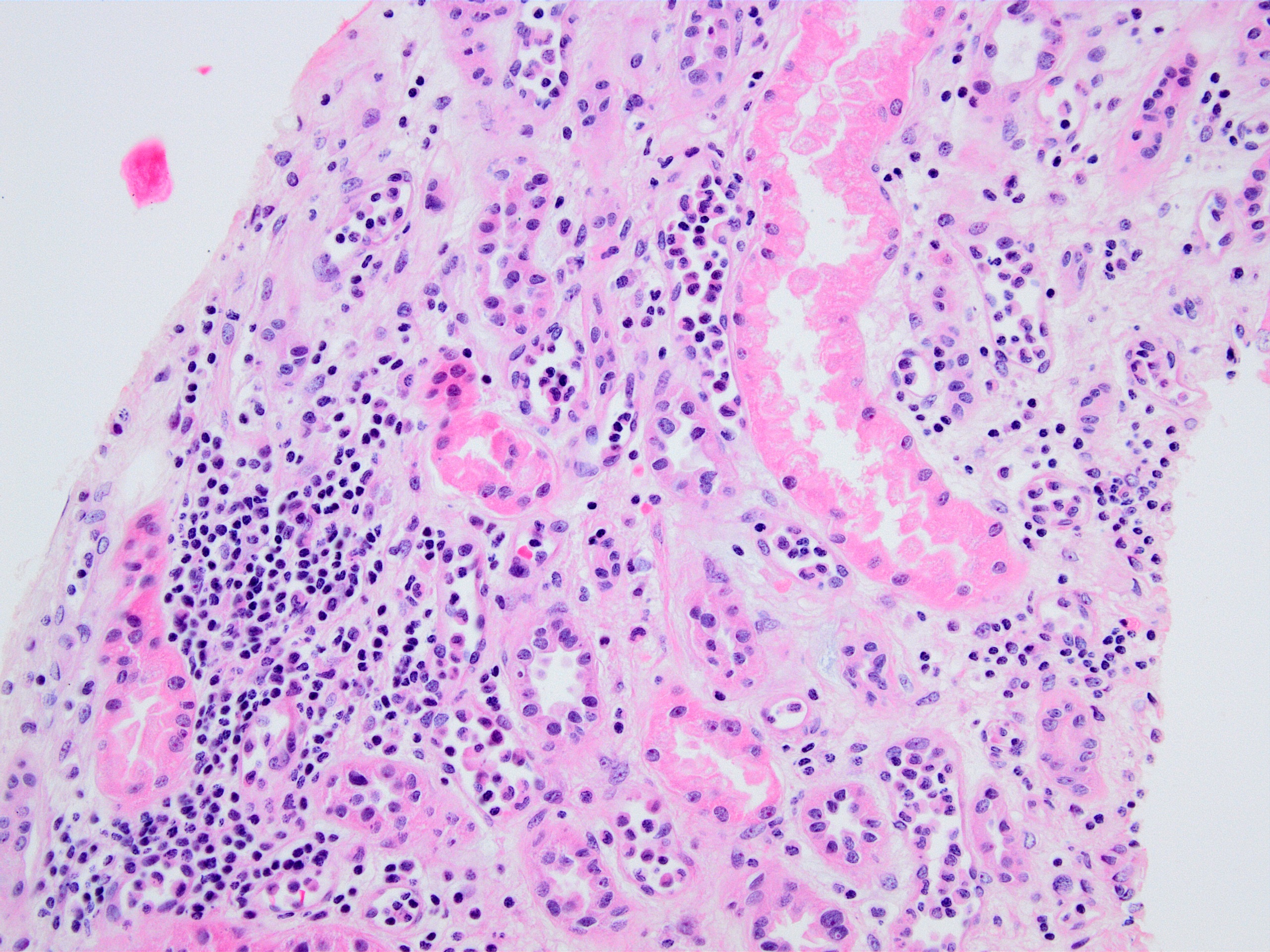
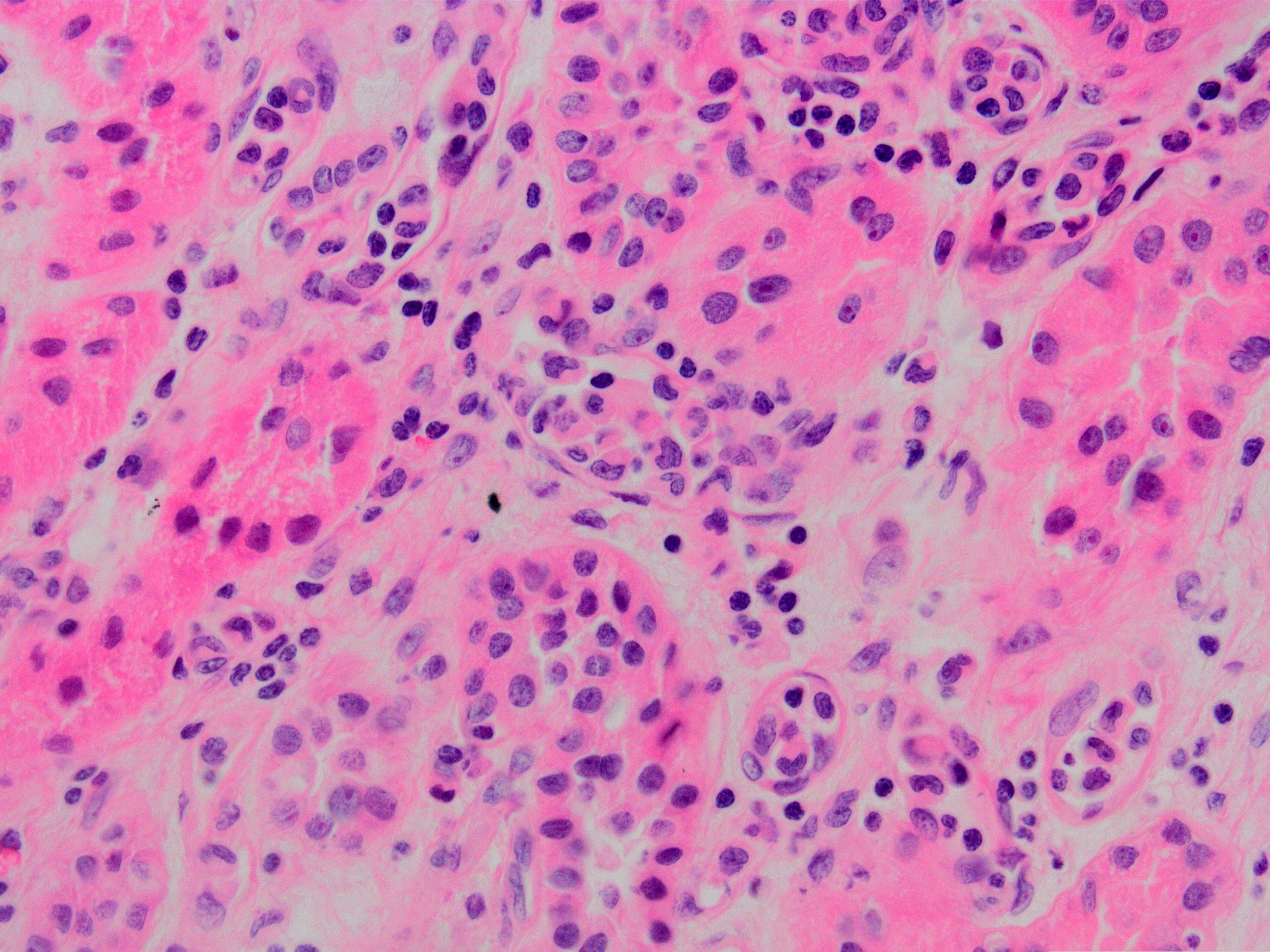
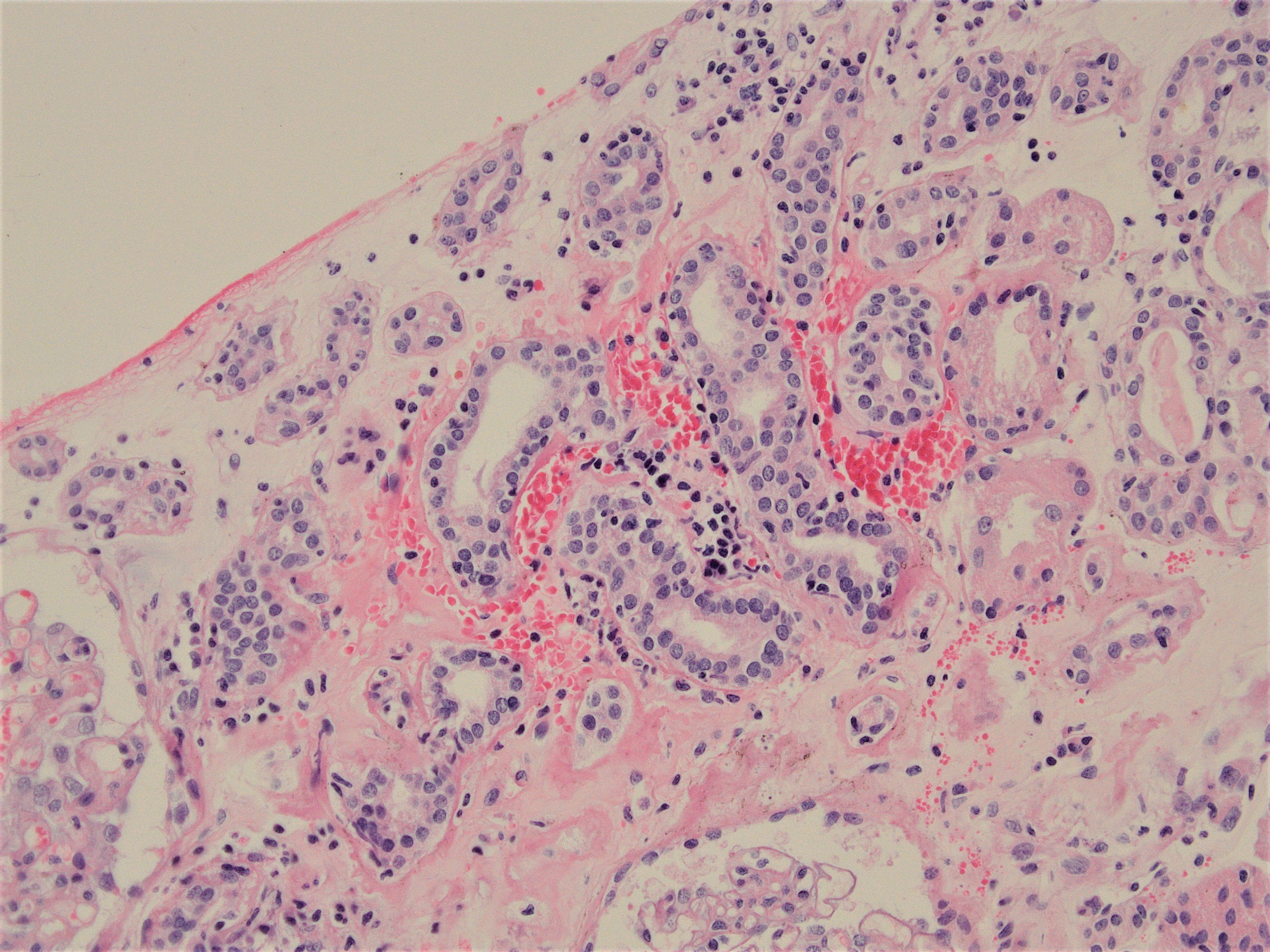
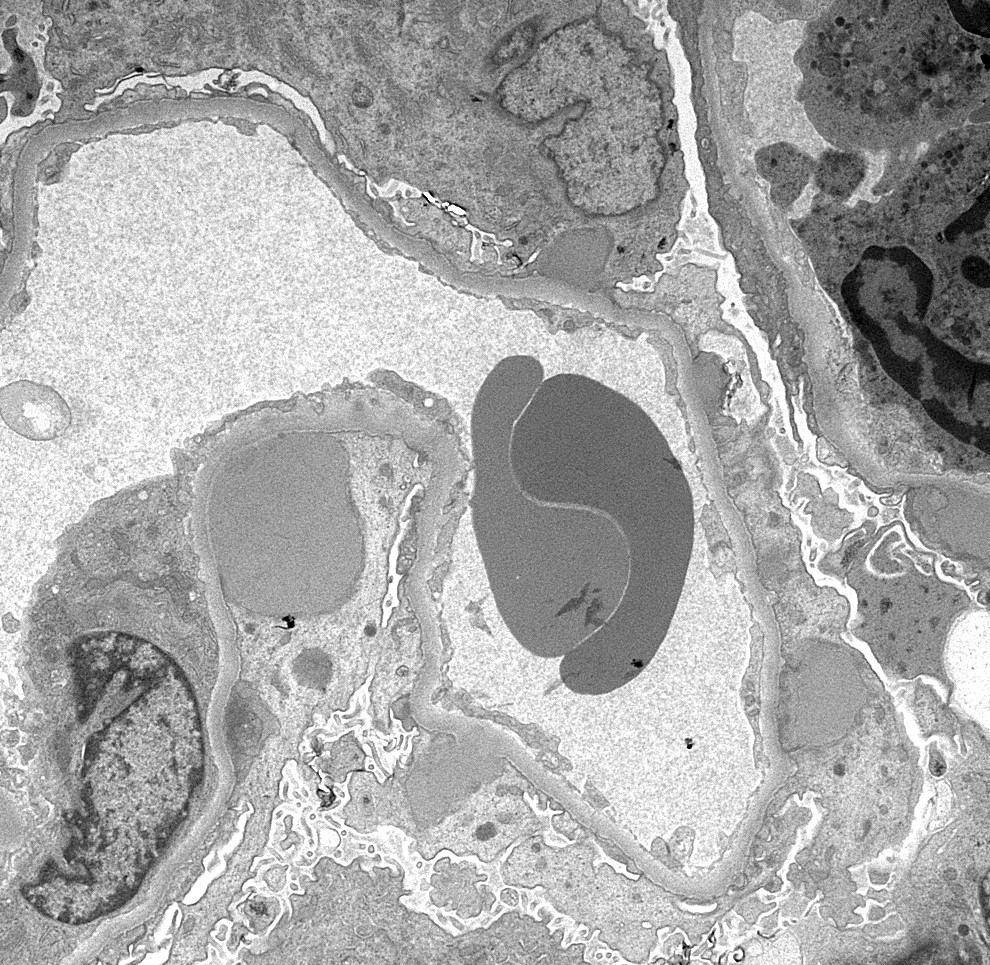
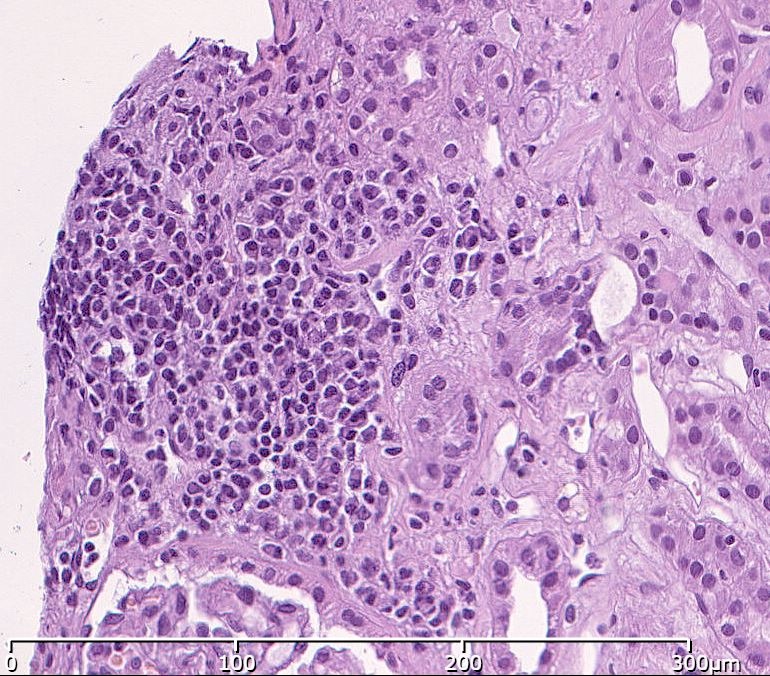
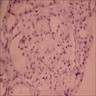
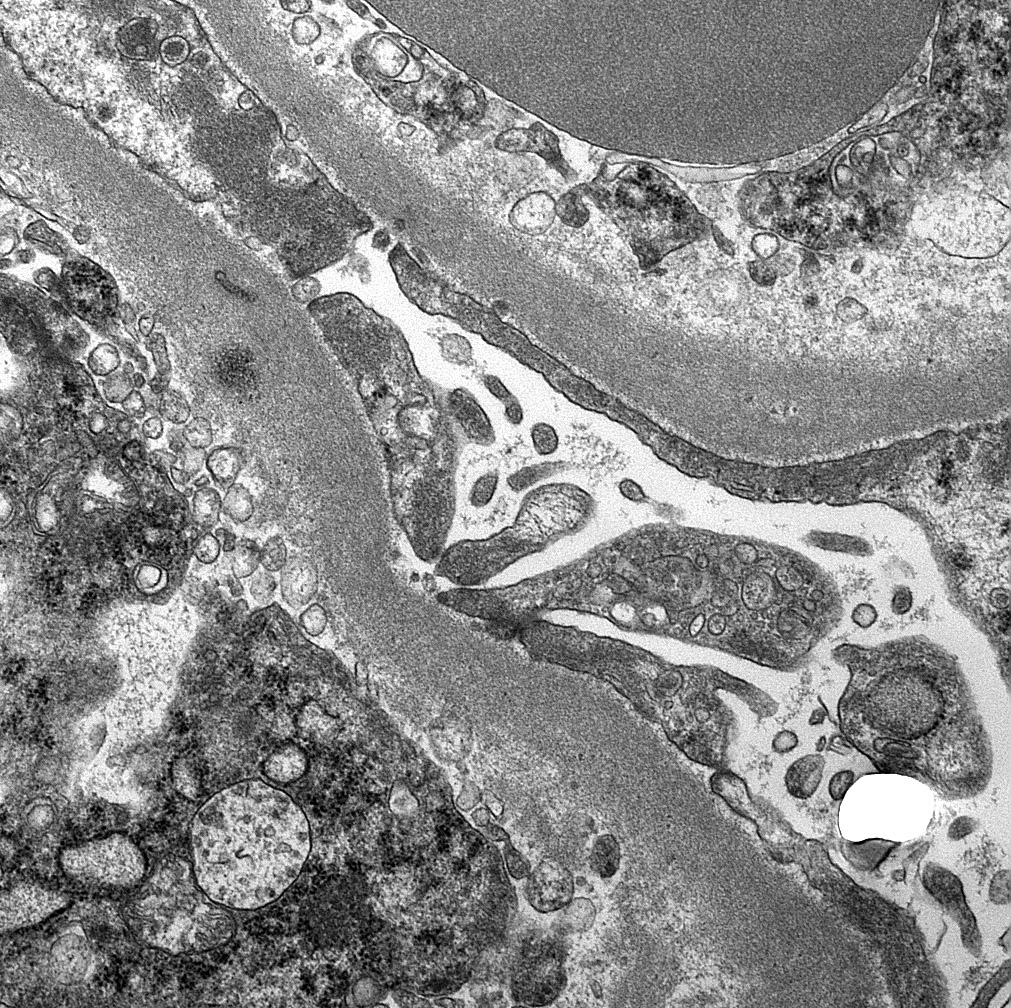
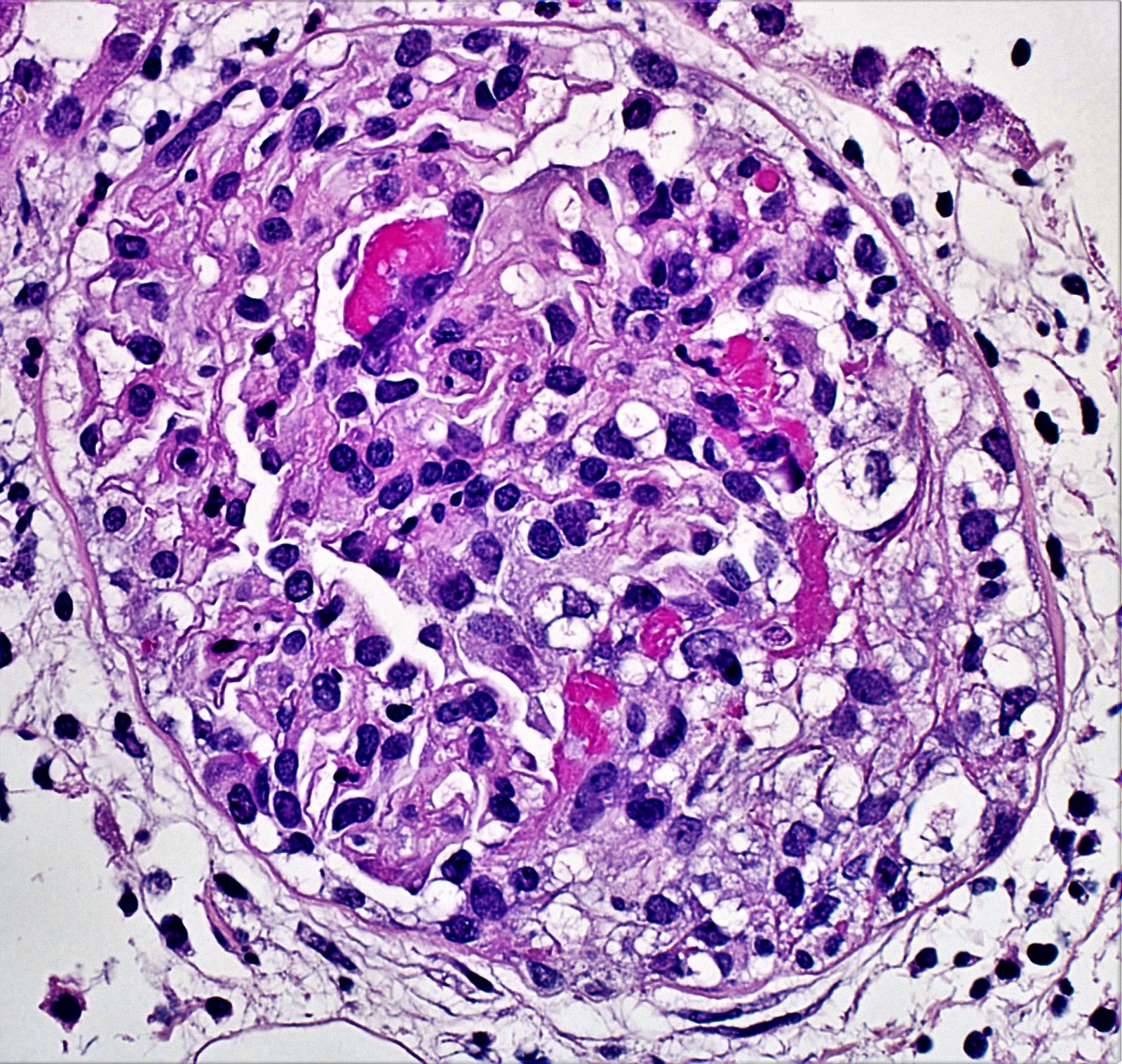
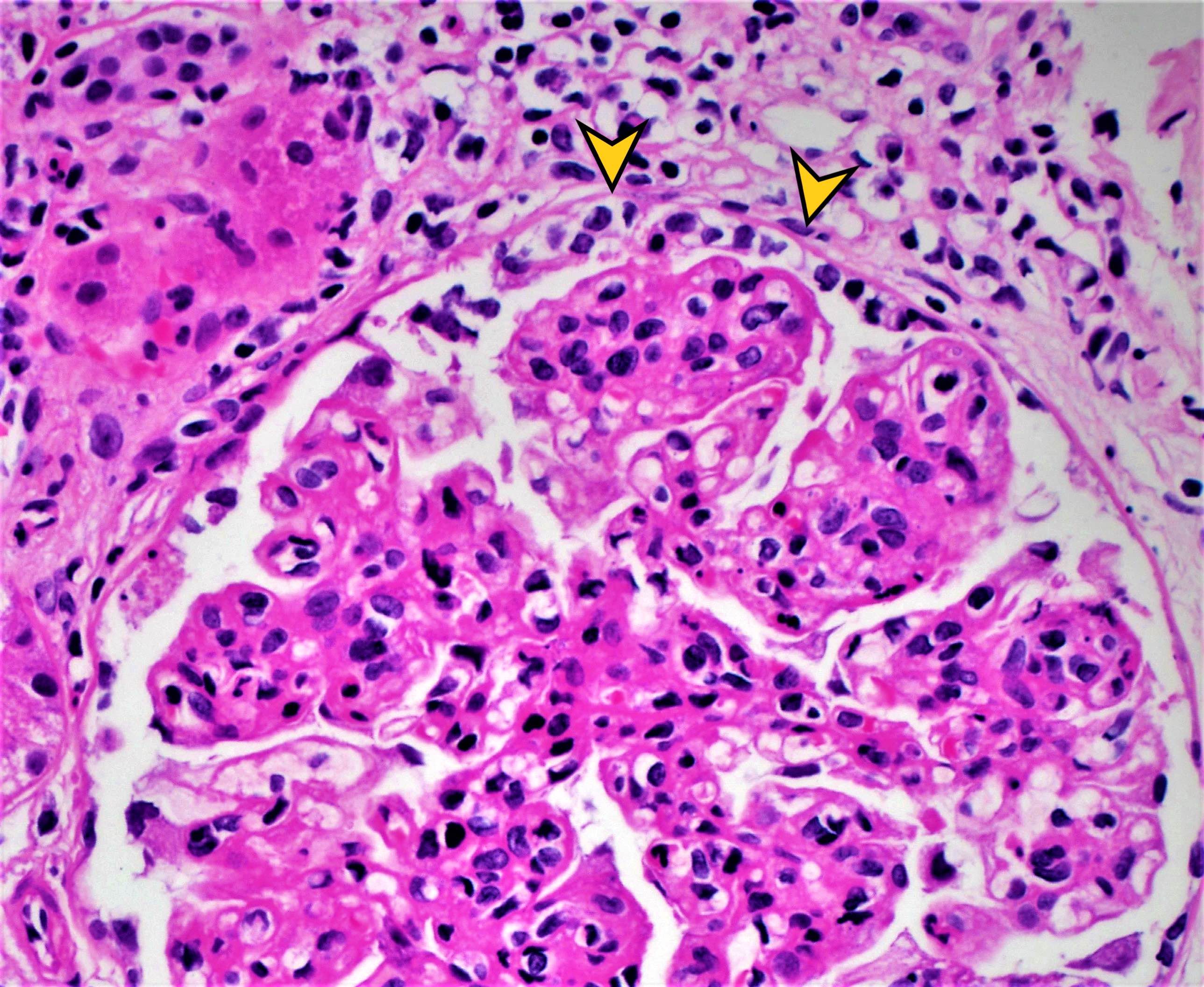
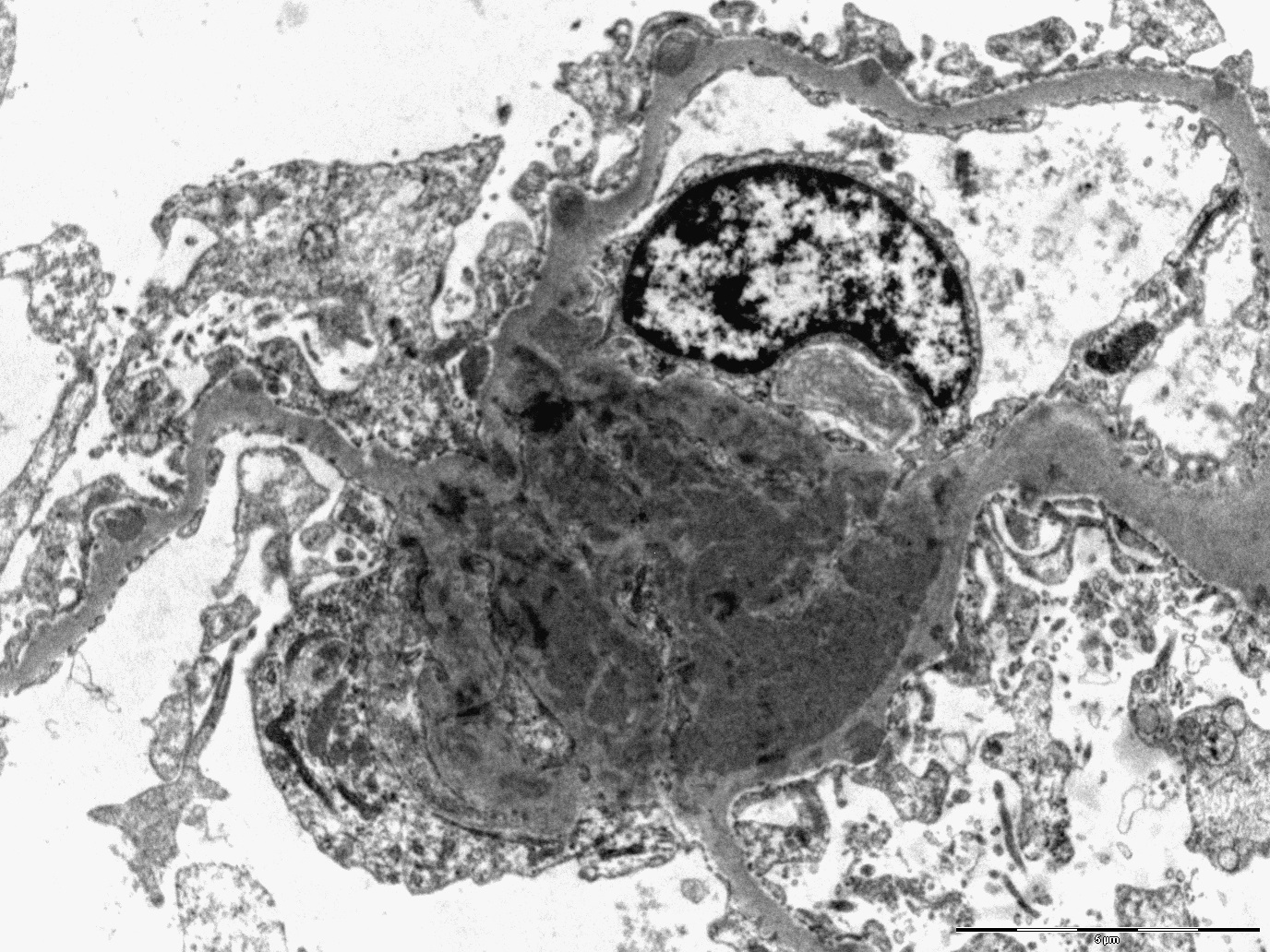
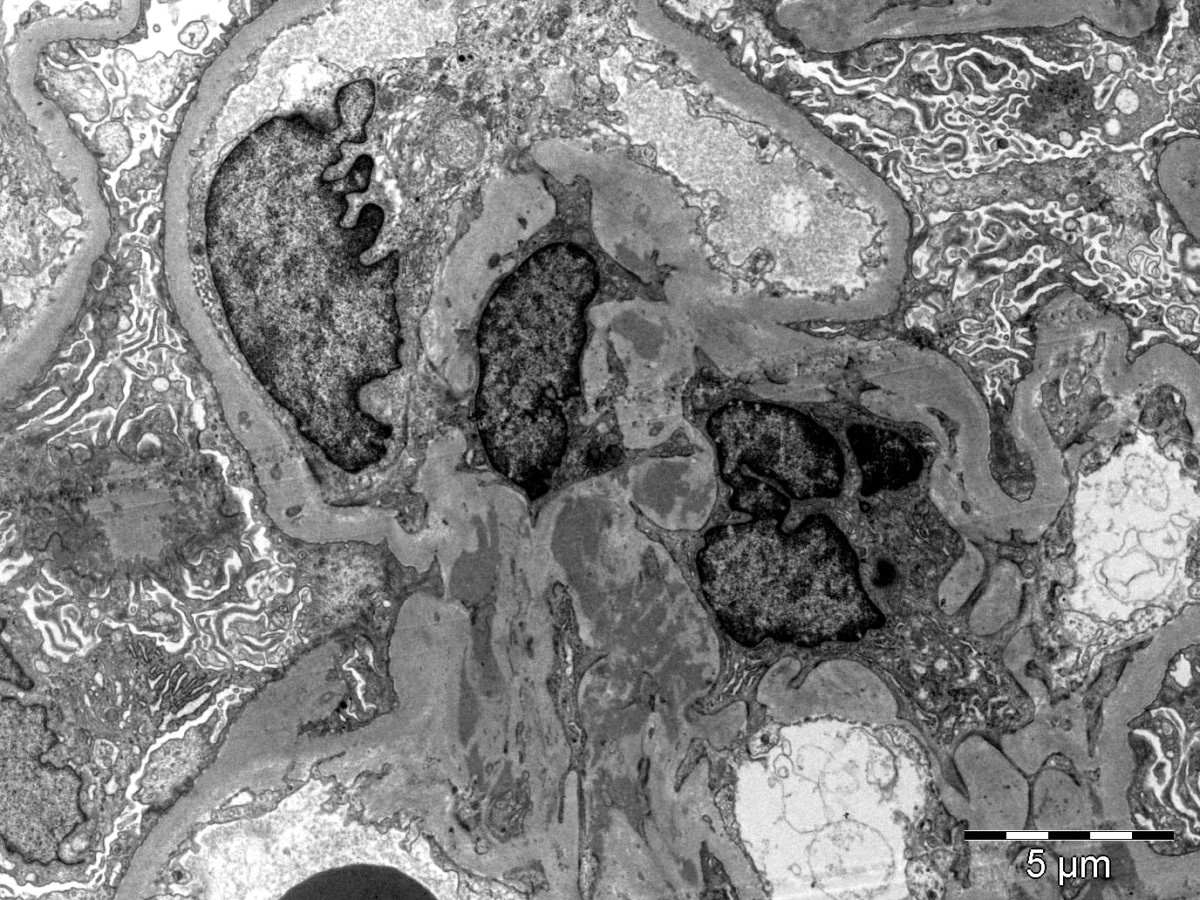
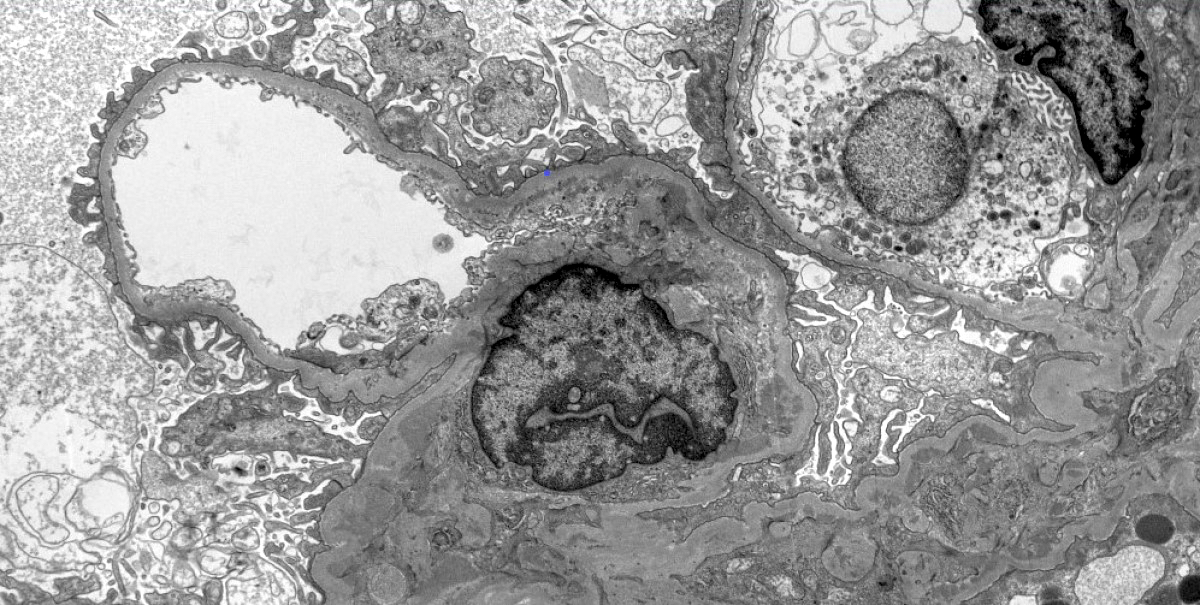
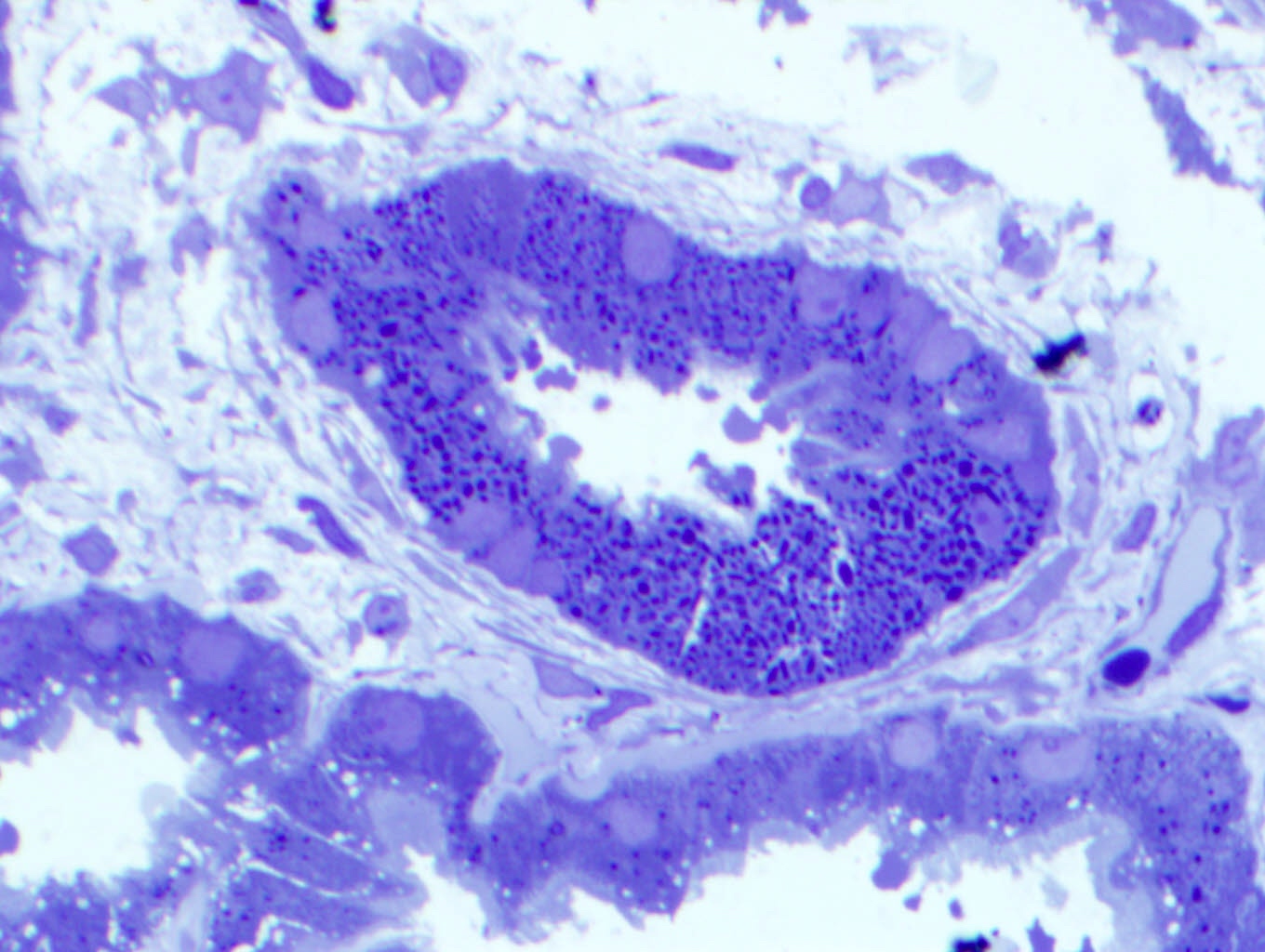
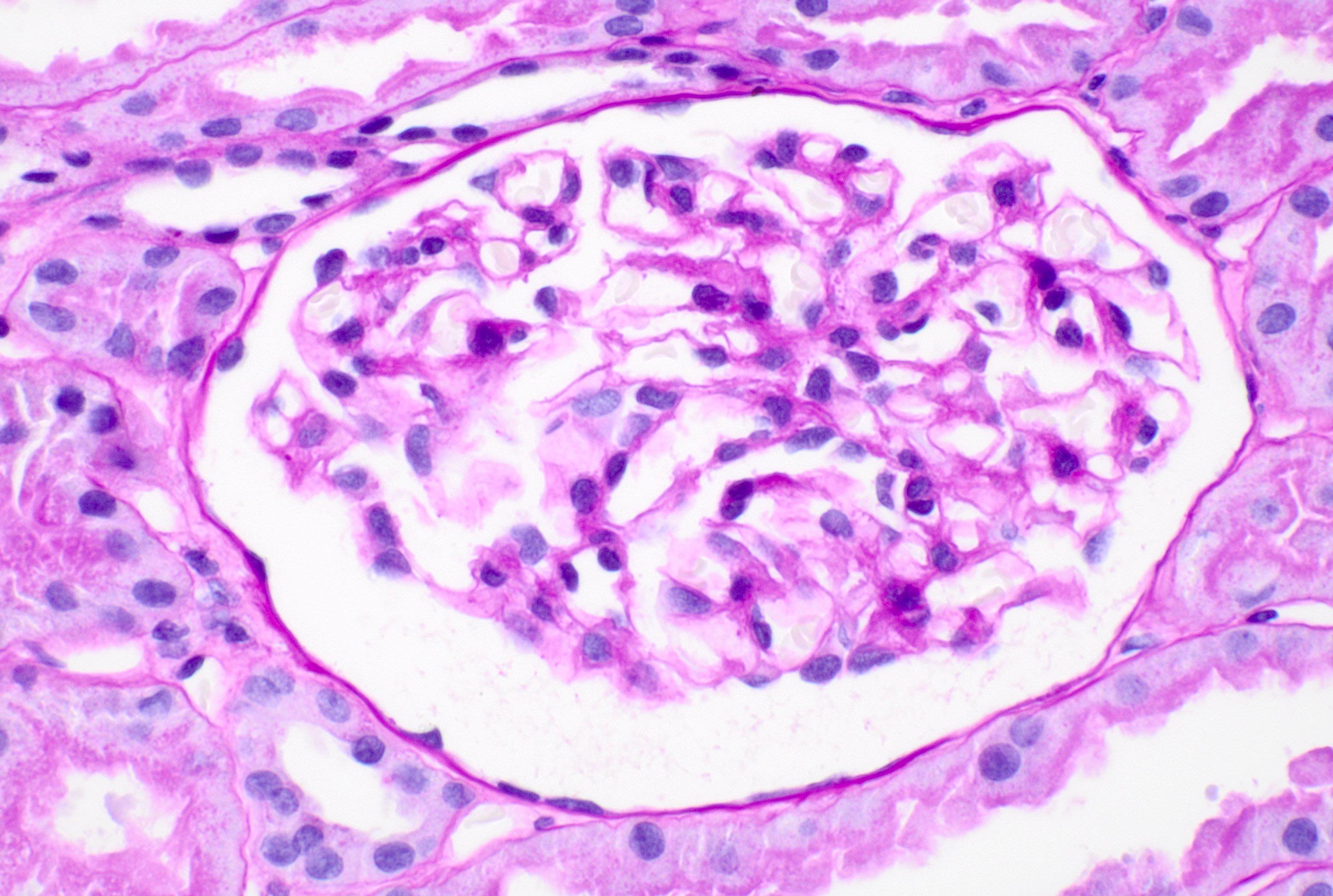
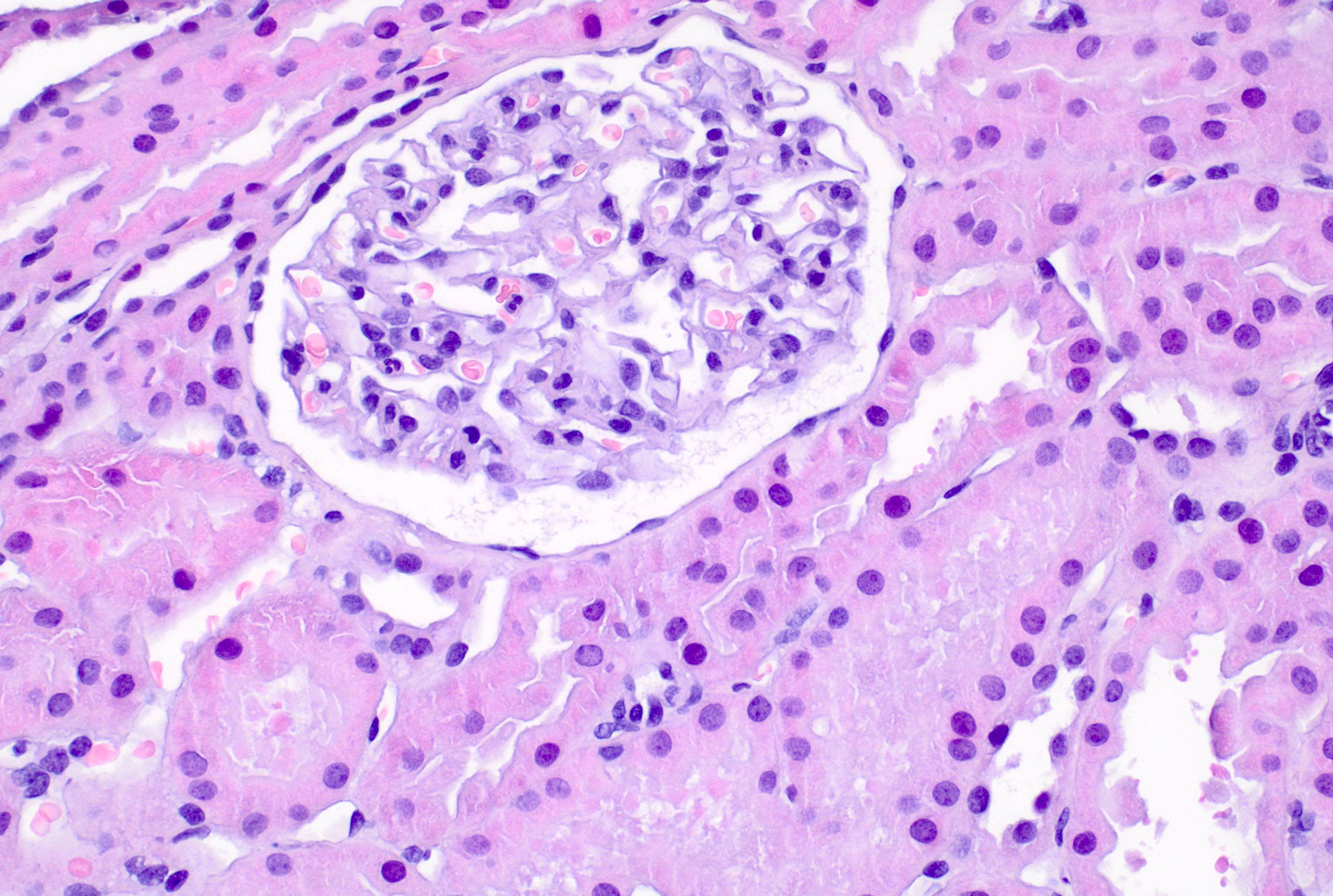
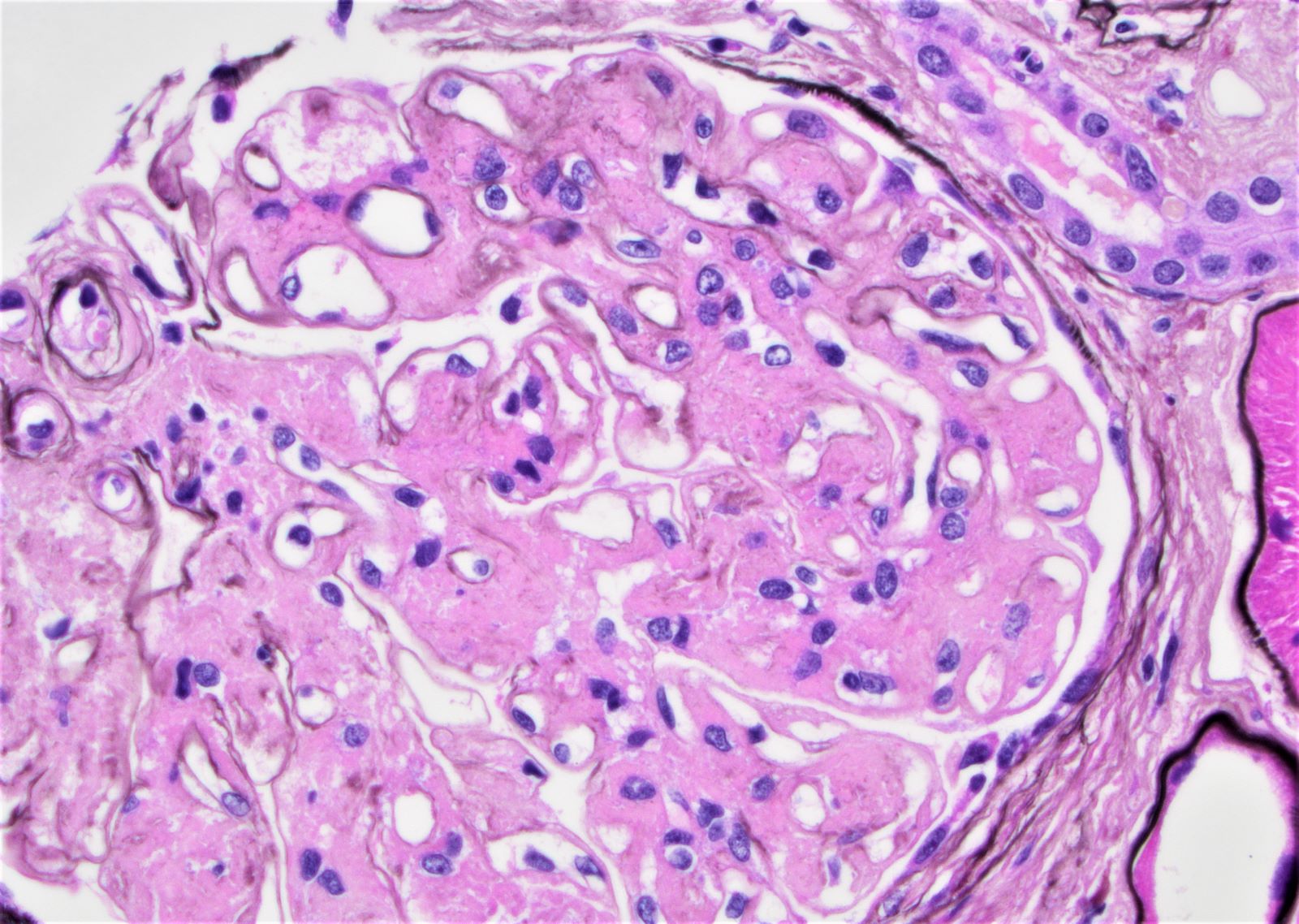
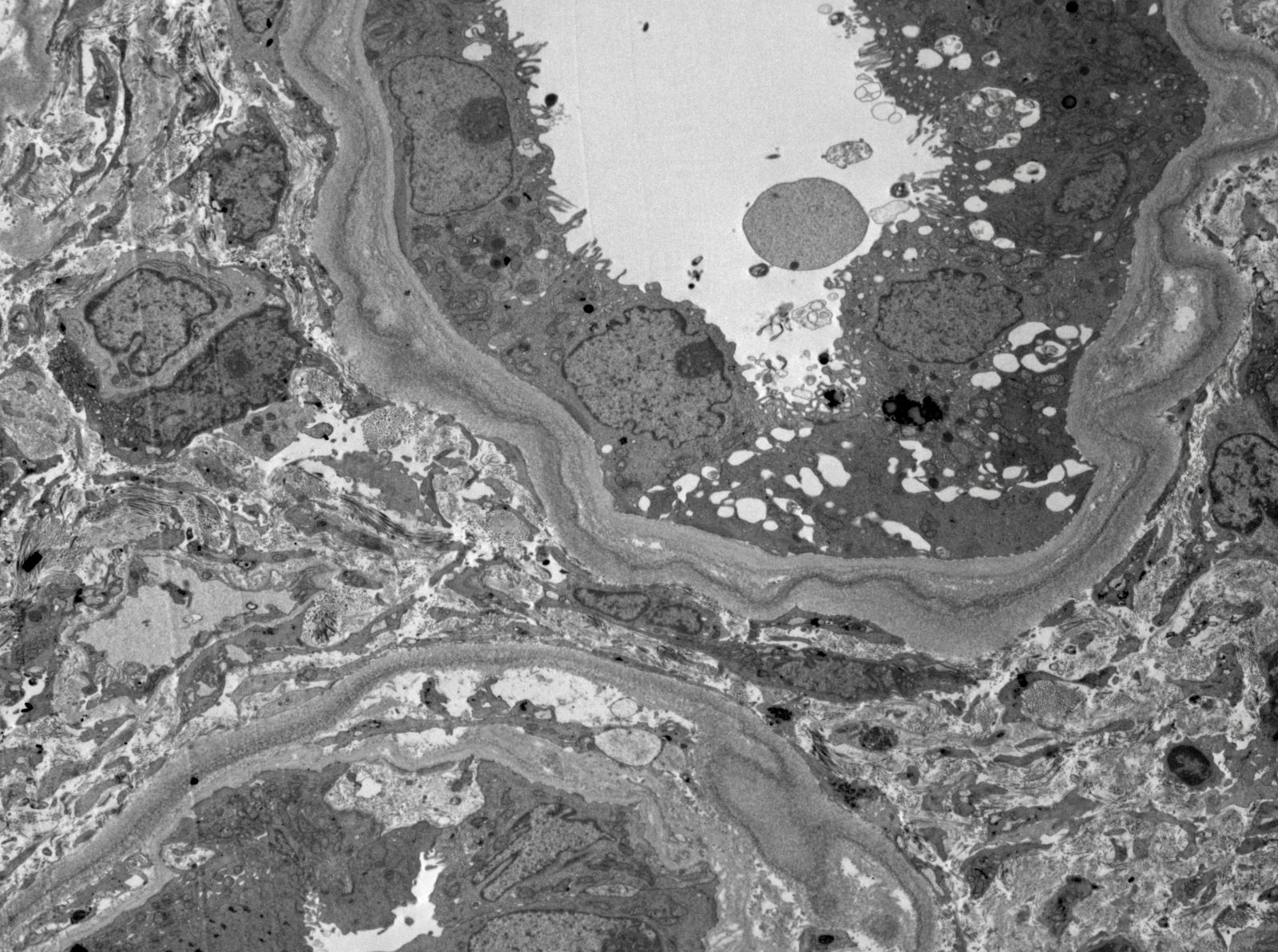
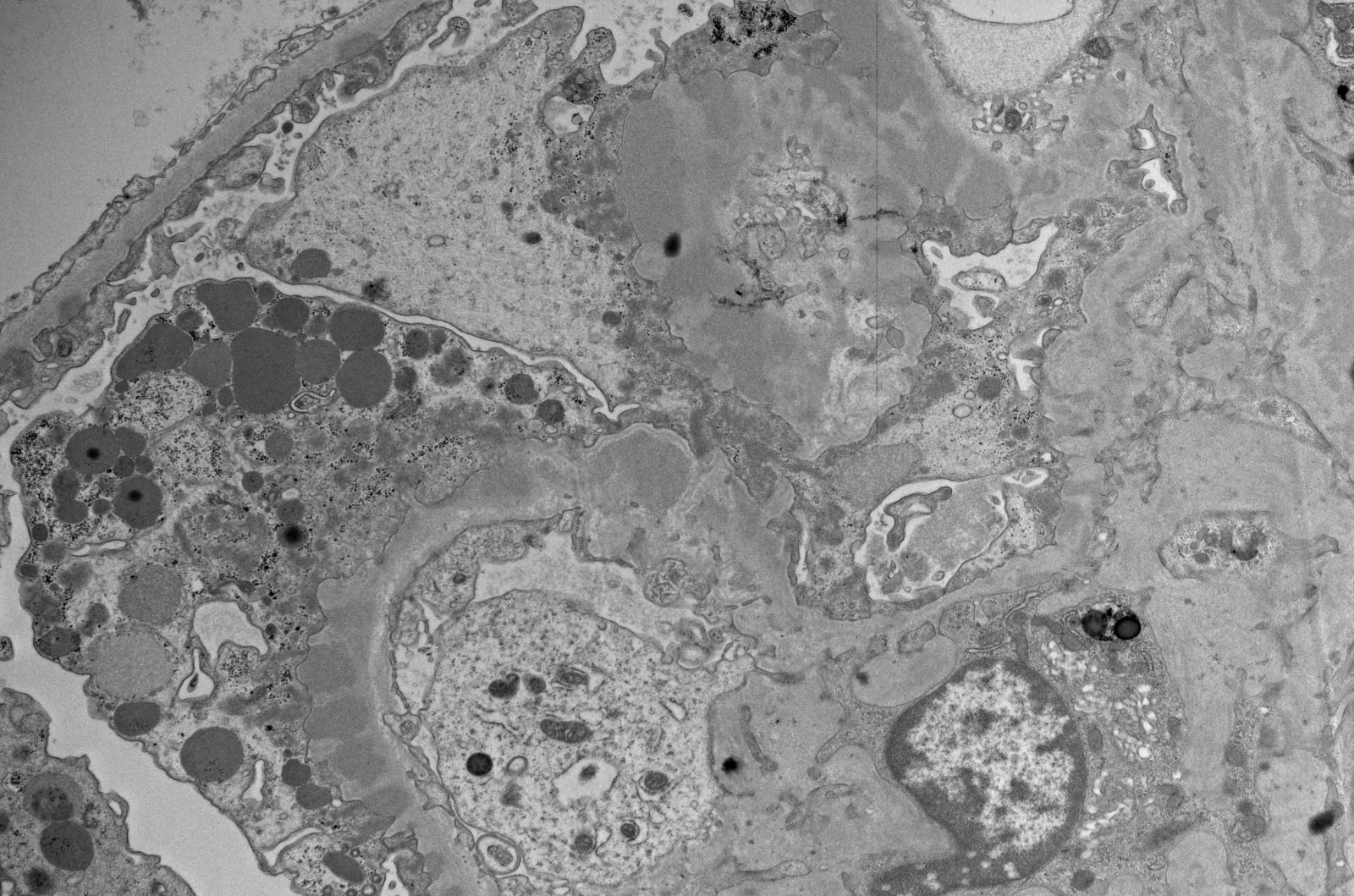
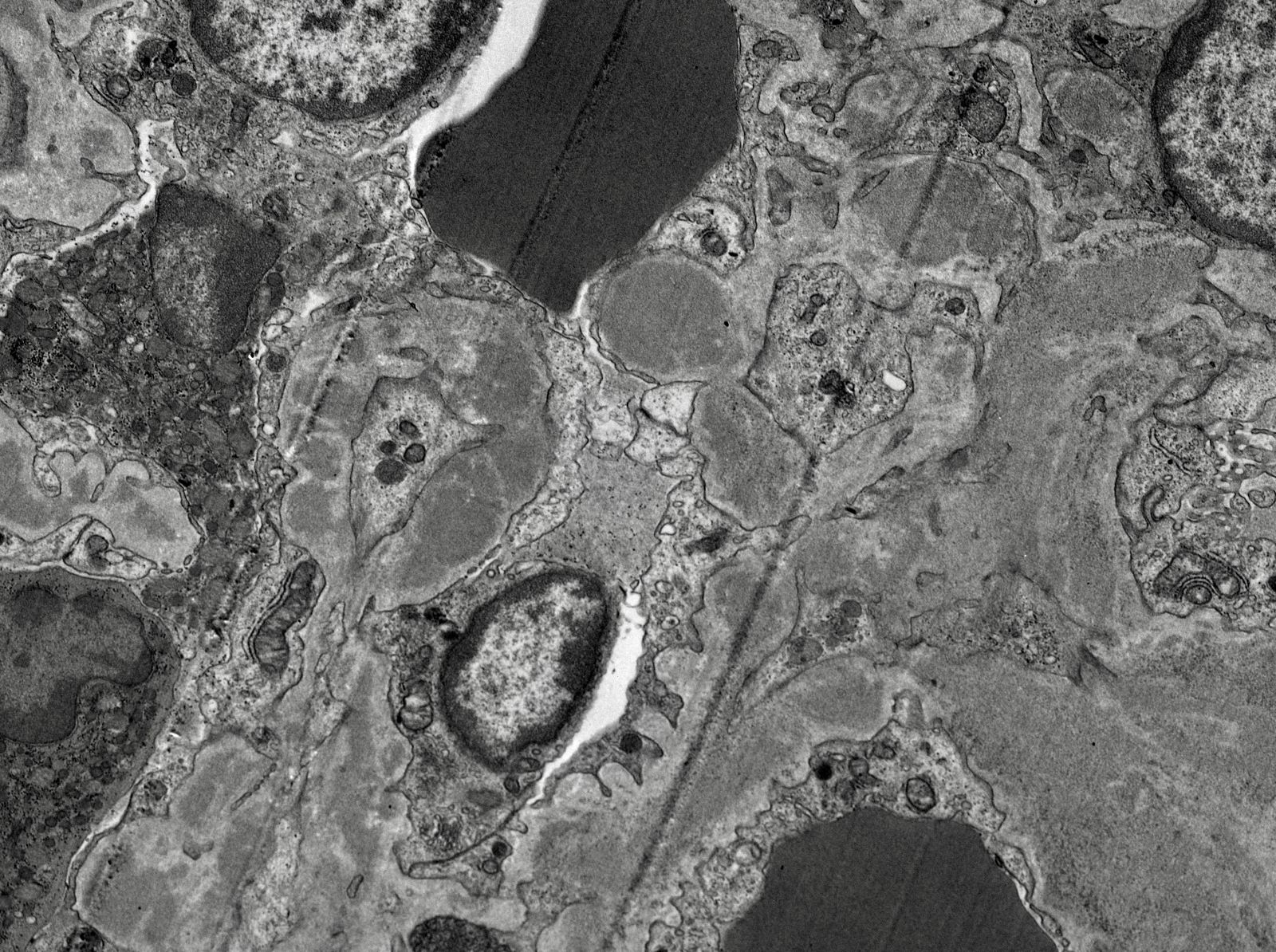
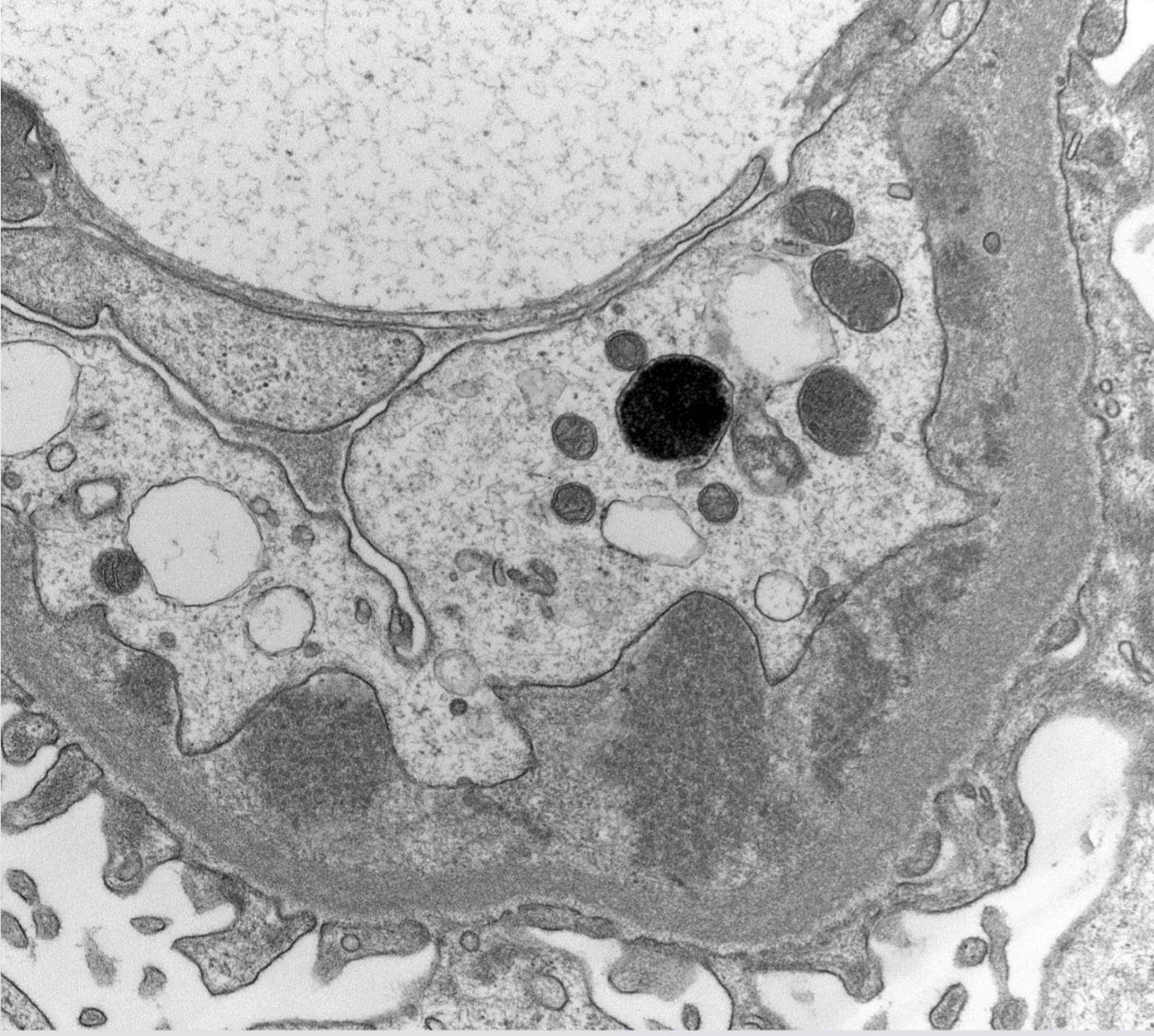
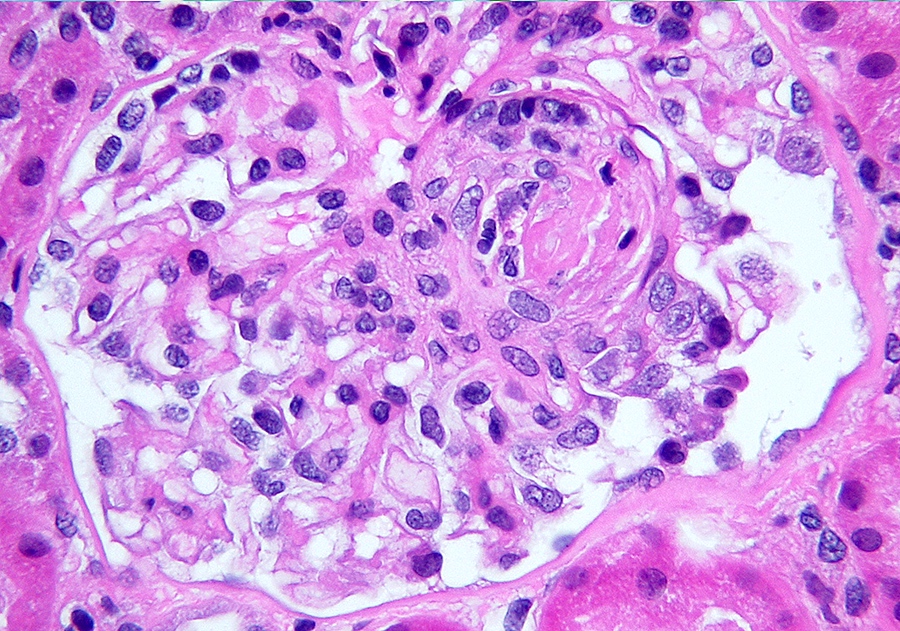
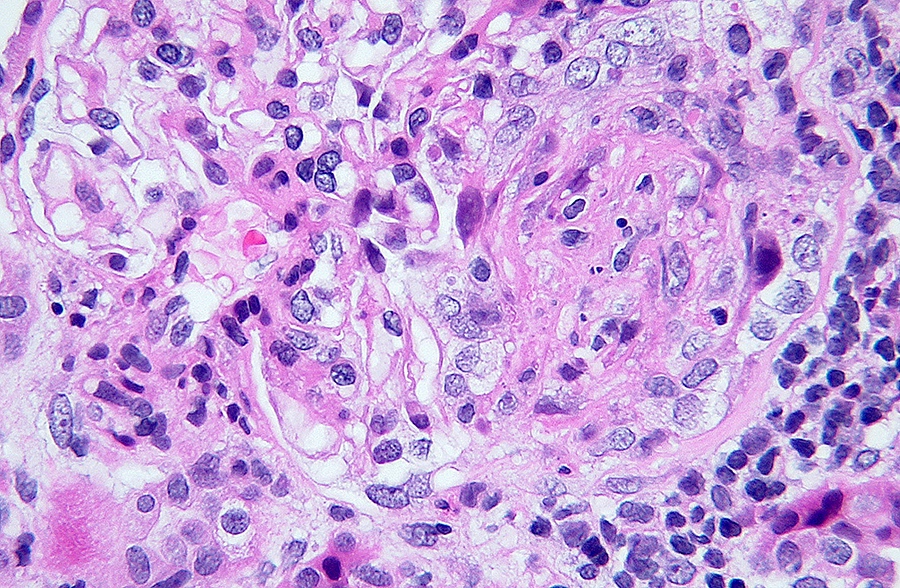
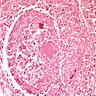
- Peritubular capillaritis: presence of inflammatory cells within the lumens of the capillaries - most prominently neutrophils and monocytes
- Glomerulitis: inflammatory cells within glomerular capillary lumens
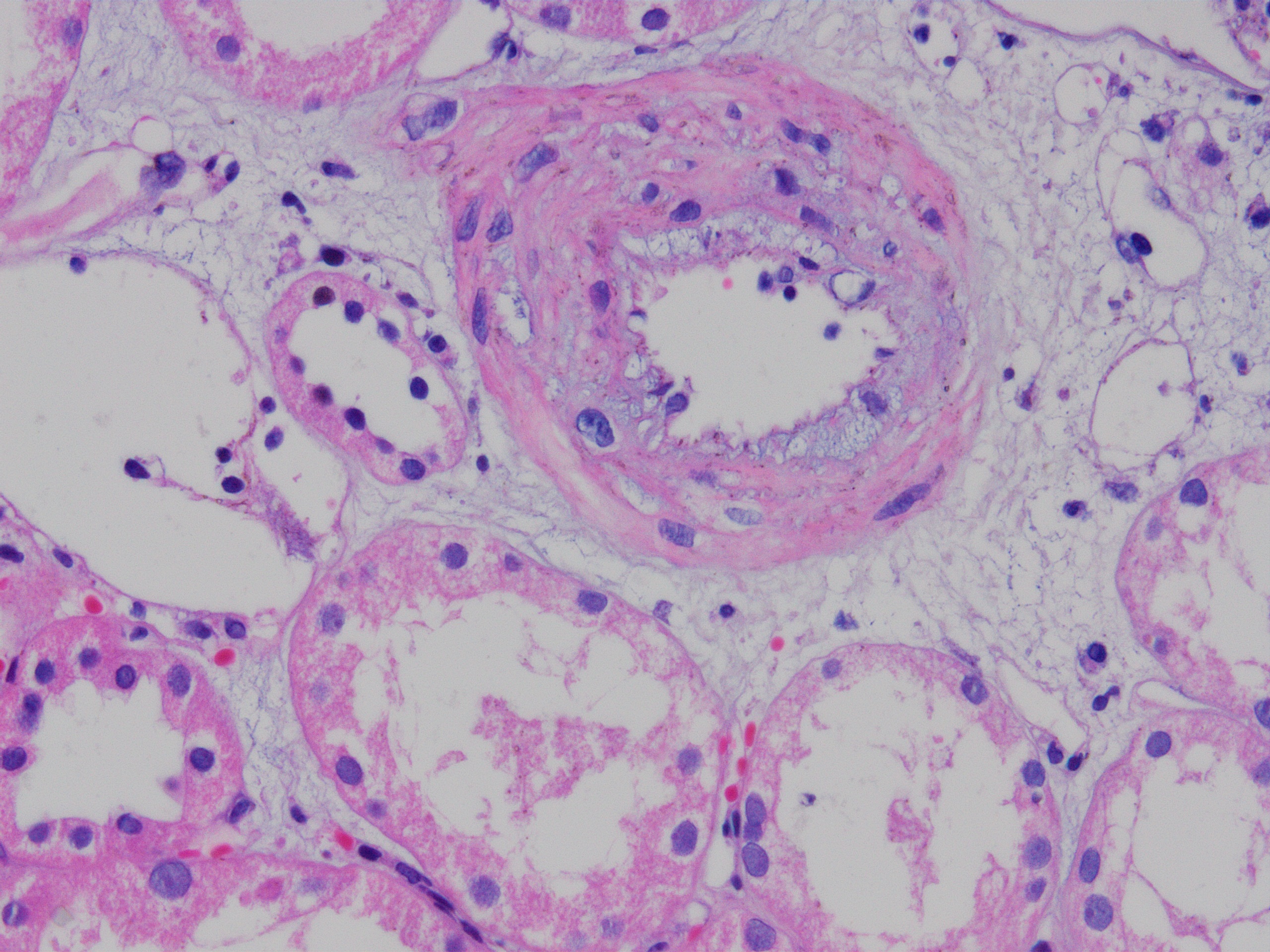
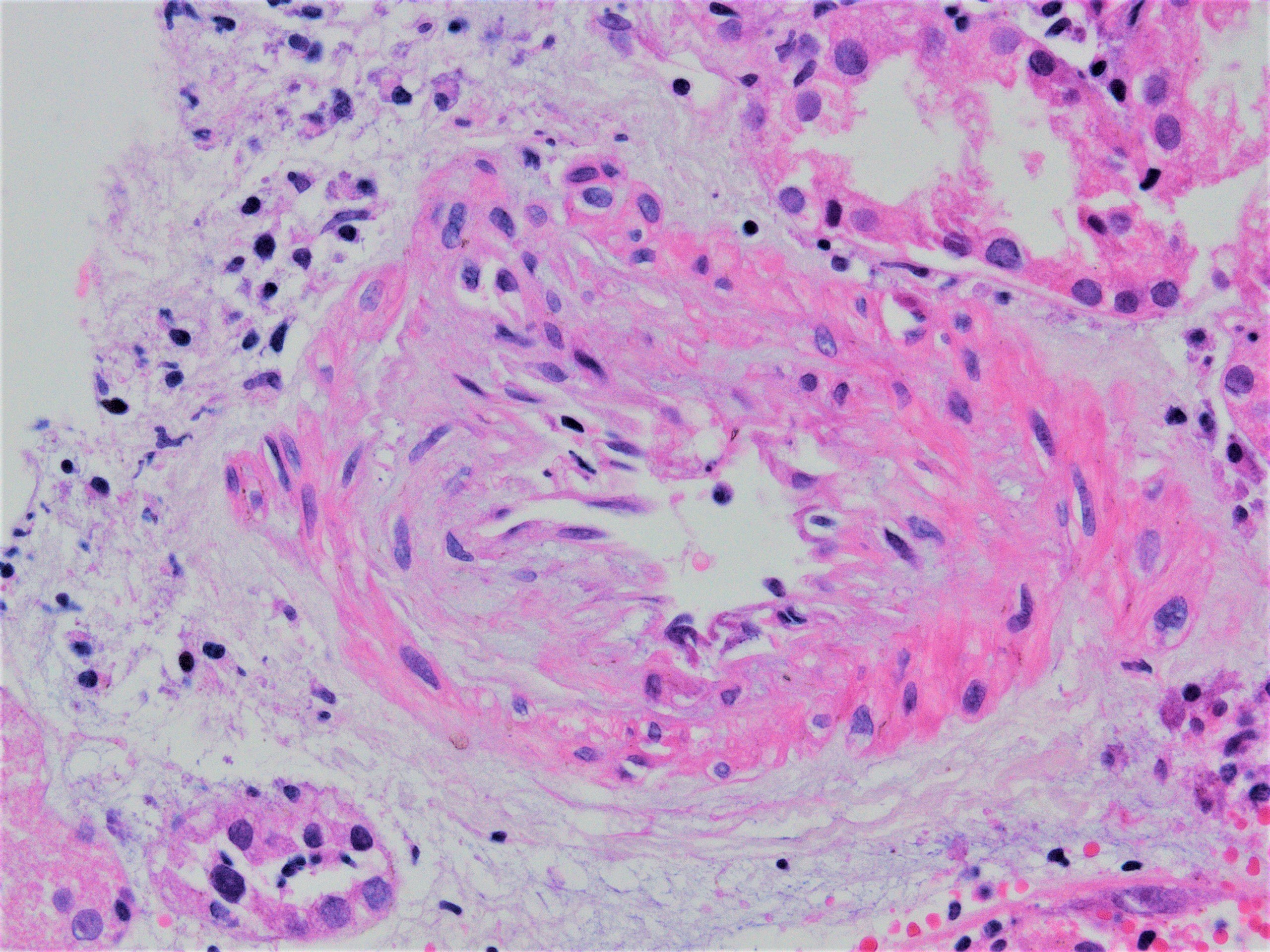
- Intimal or transmural arteritis: inflammatory cells within the intima or walls of vessels
- Thrombotic microangiopathy and fibrinoid necrosis of vessel walls
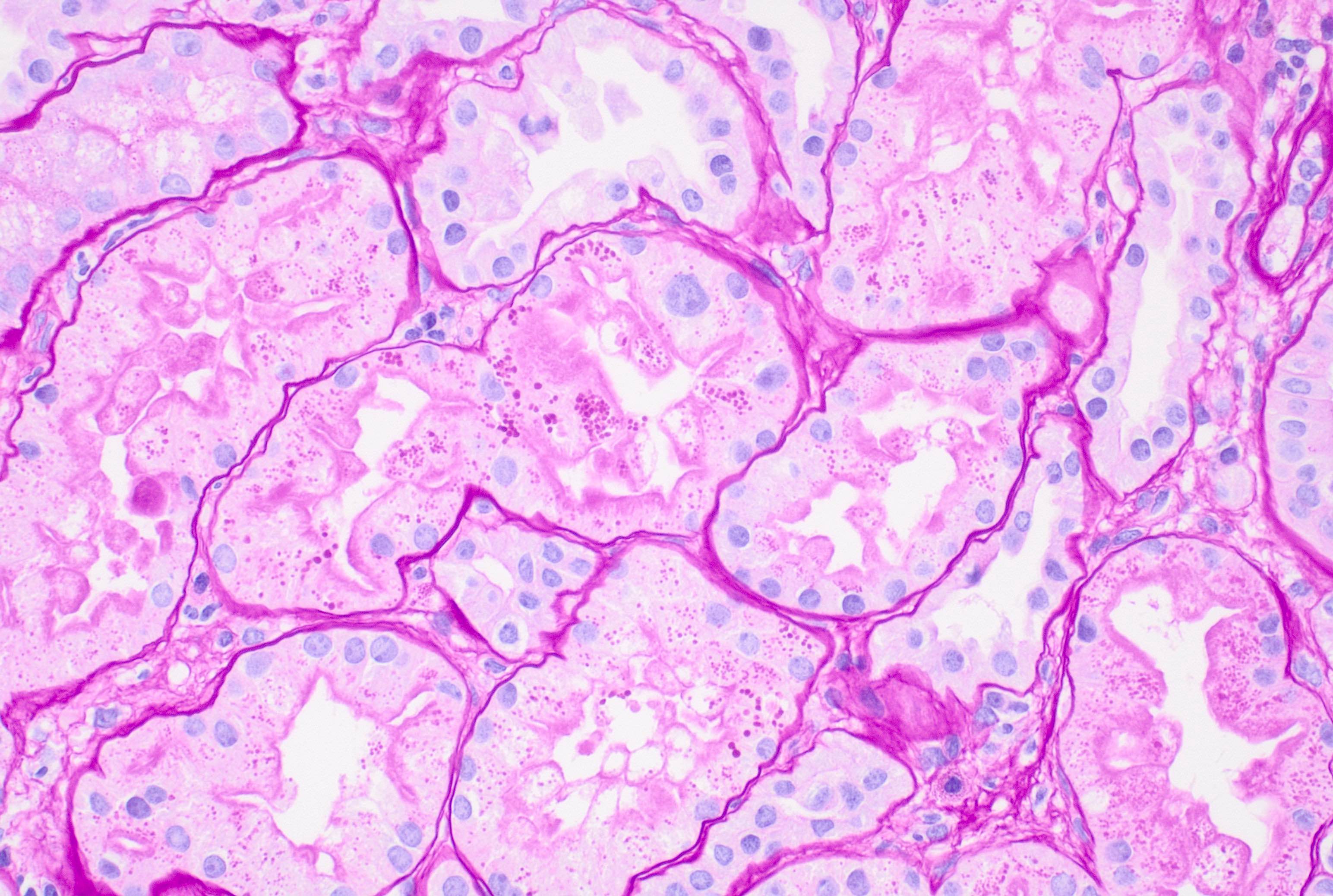
- Acute tubular injury: dilatation of the tubular lumen, flattening of tubular epithelial cells, loss of nuclear staining of tubular epithelial cells, shedding of tubular epithelial cells into the lumen and denudation, regenerative changes in tubular epithelial cells such as nucleolar enlargement and hyperchromasia
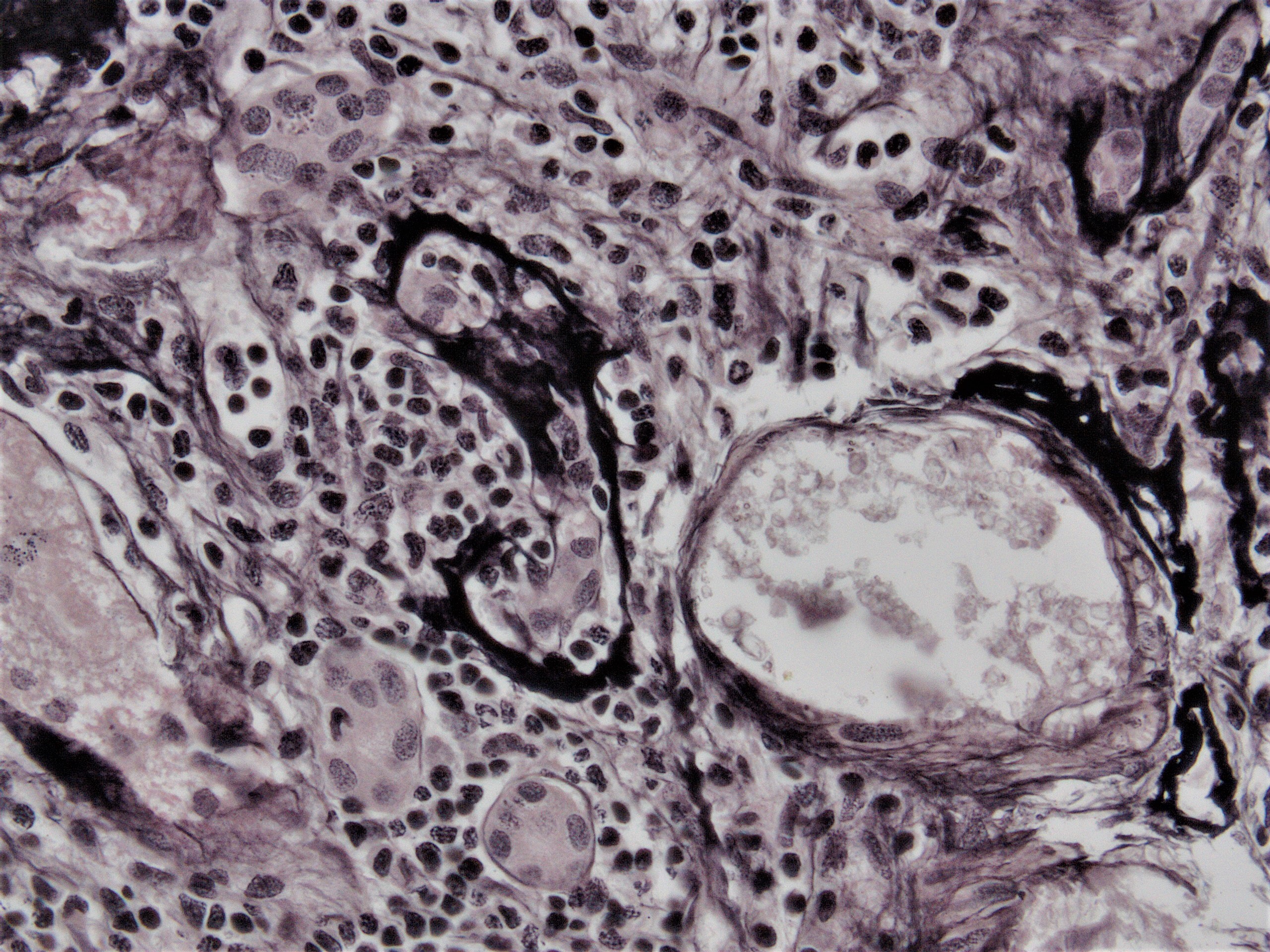
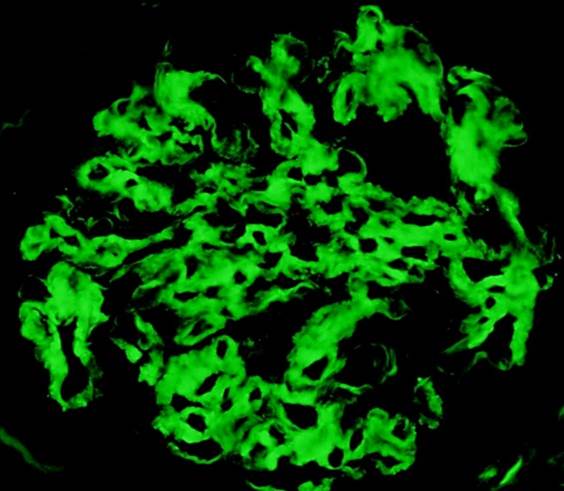
- Linear C4d staining along peritubular capillaries
- Chronic active ABMR: in addition to above mentioned changes for active ABMR, changes associated with chronic injury are present
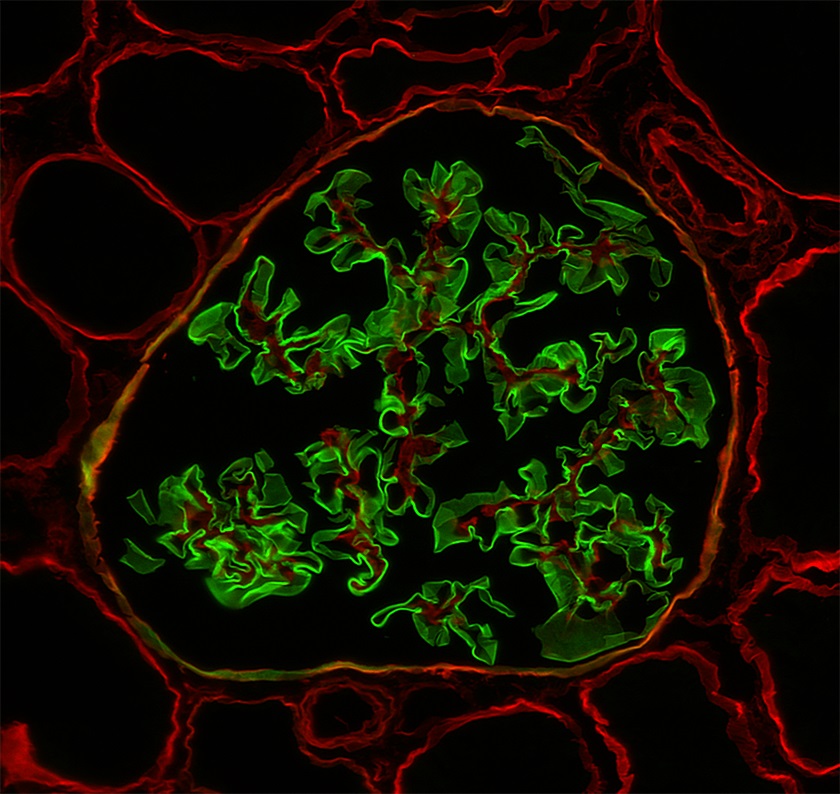
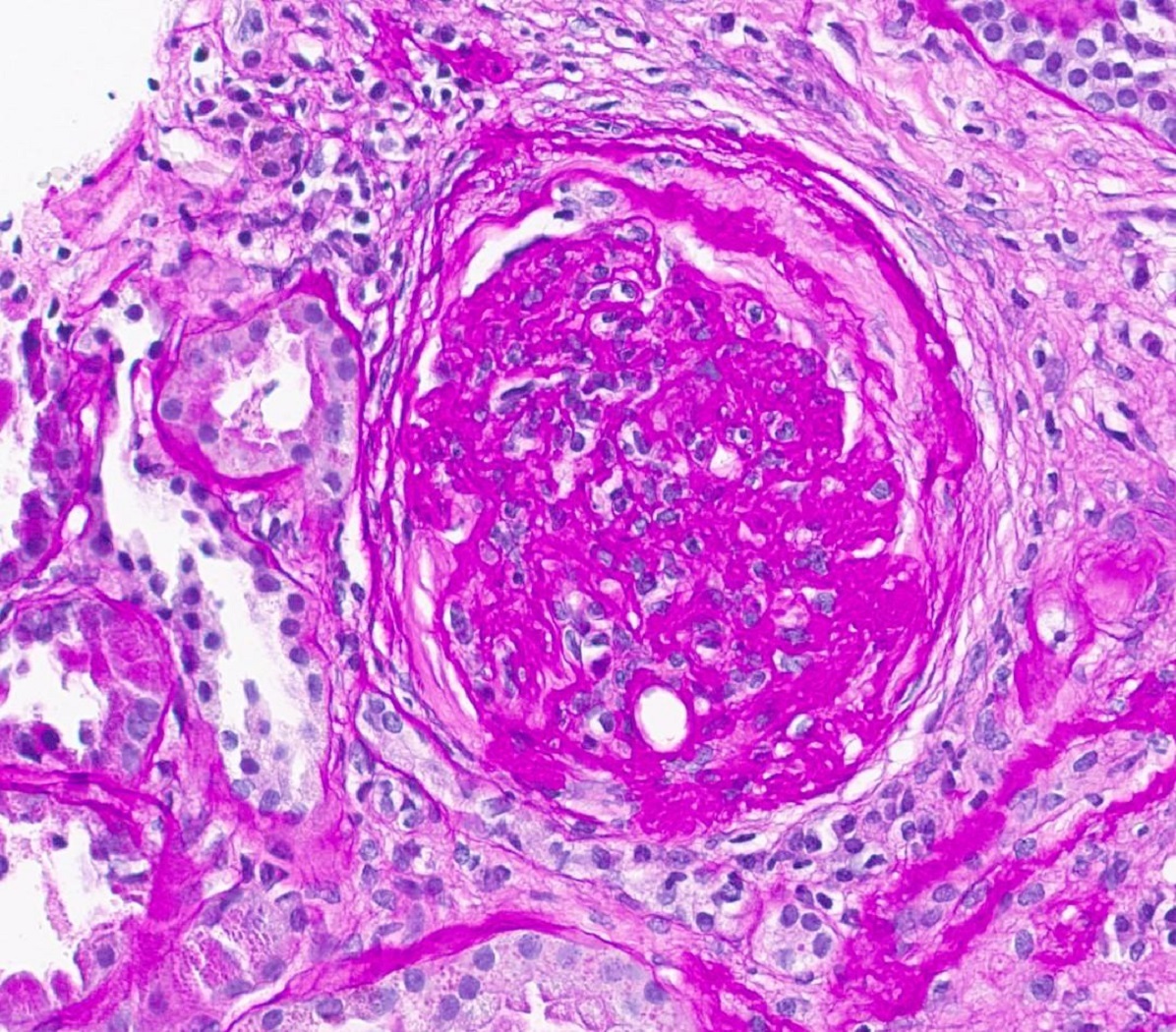
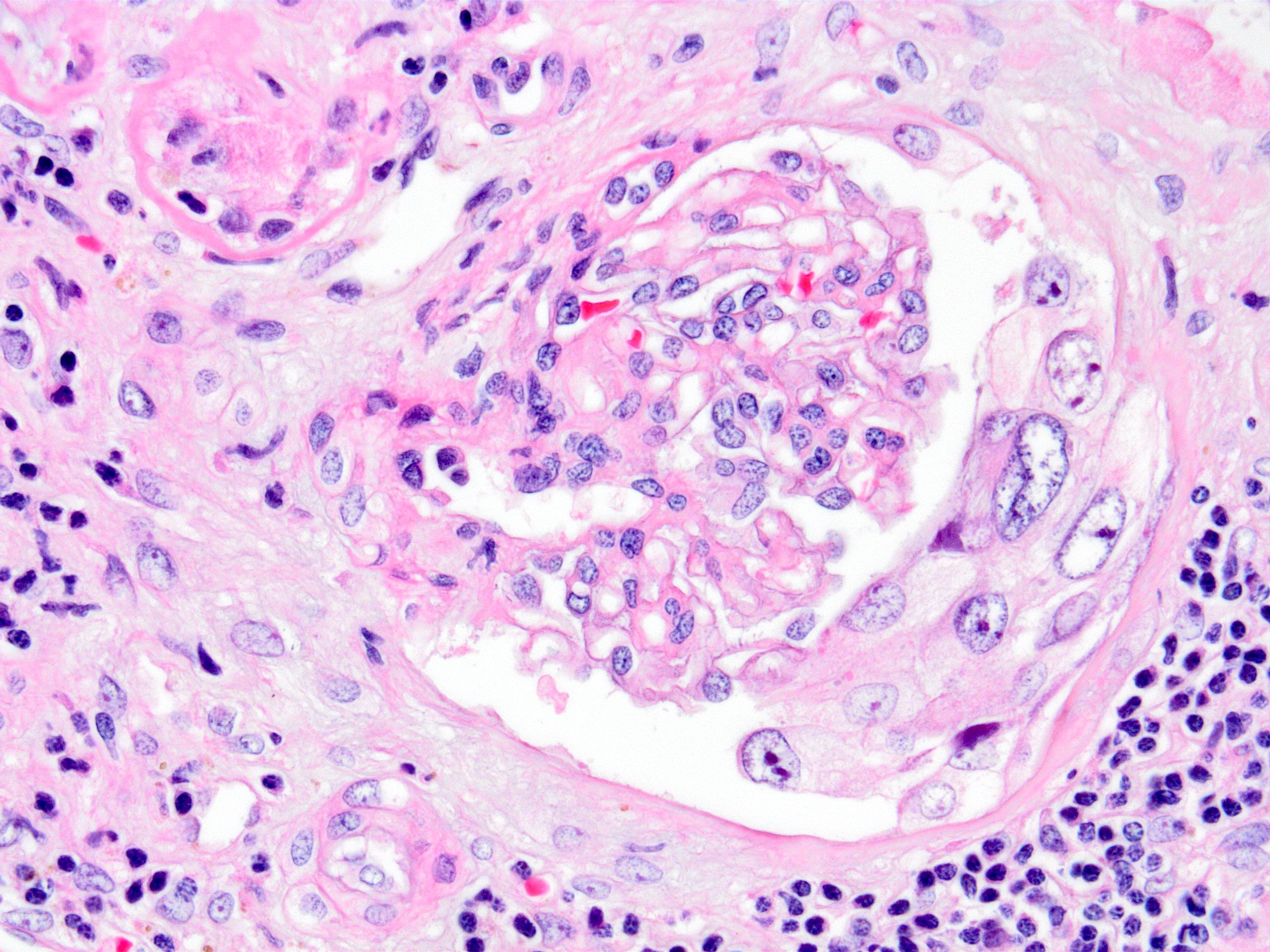
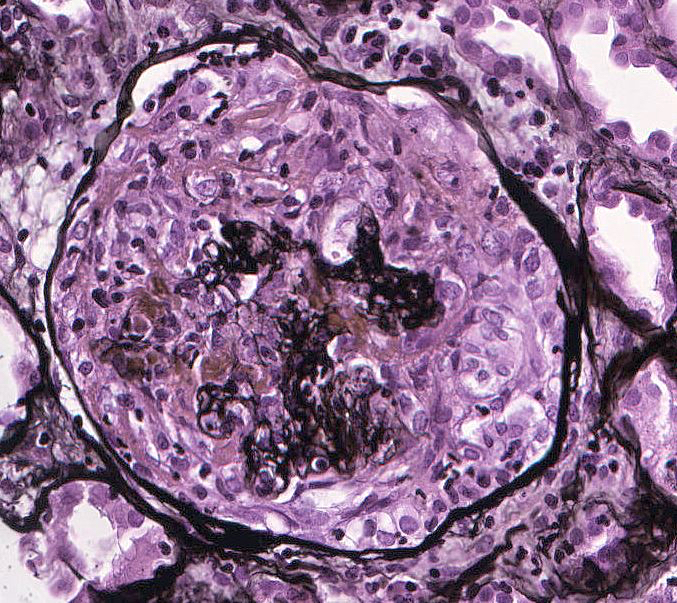
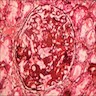
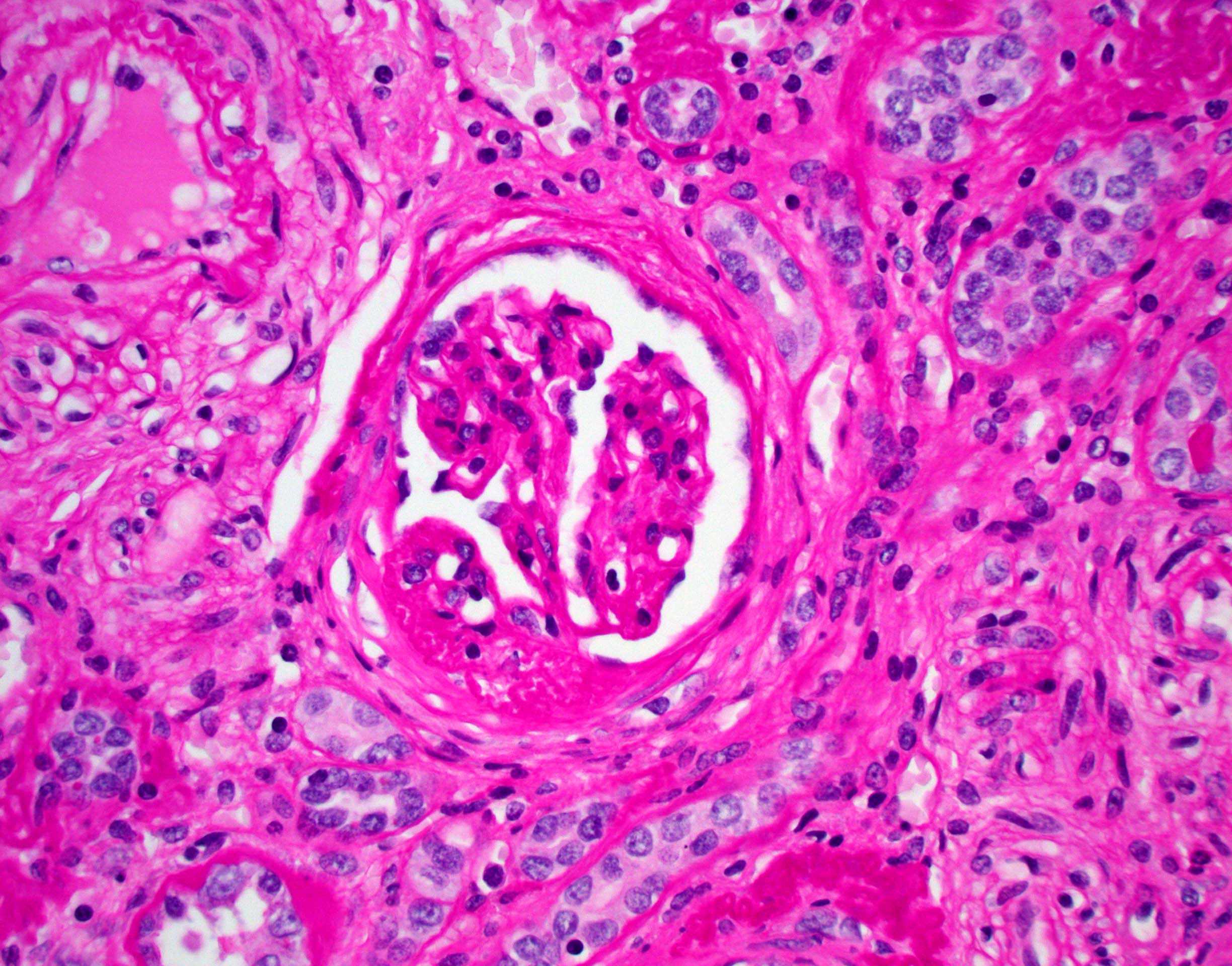
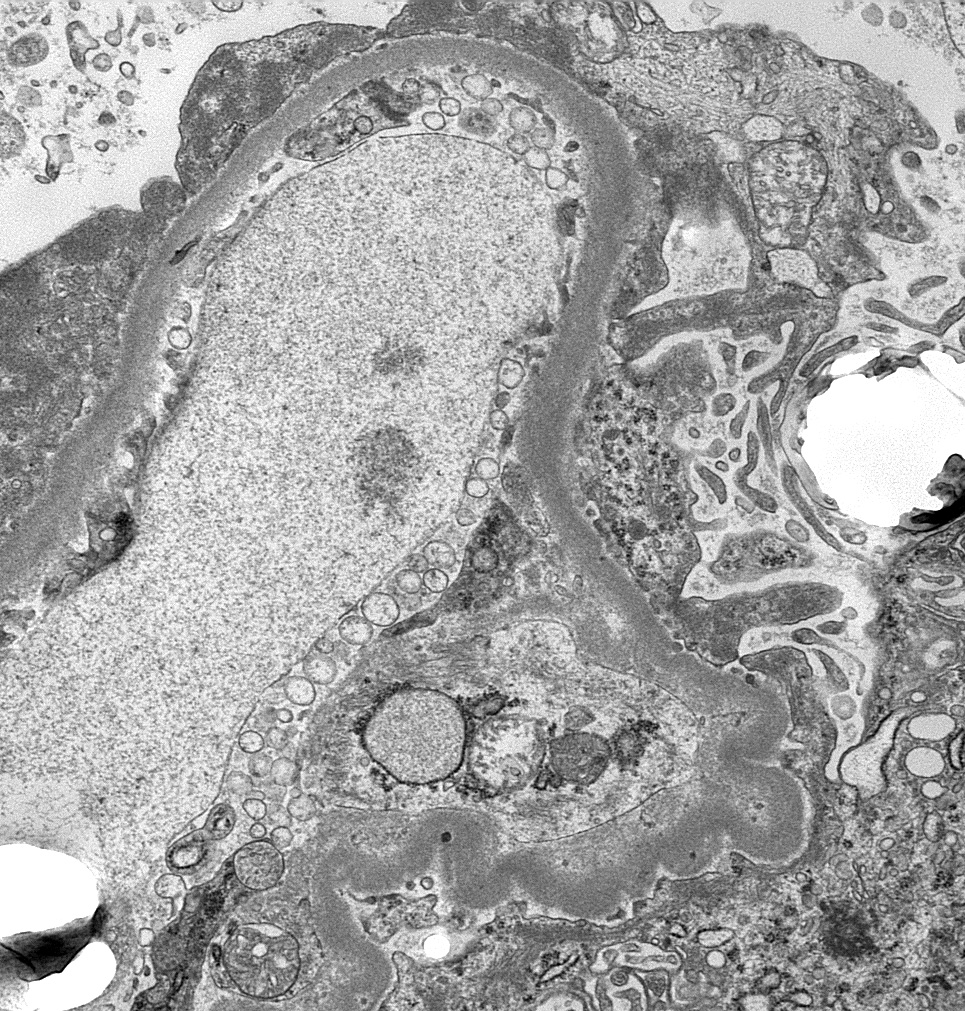
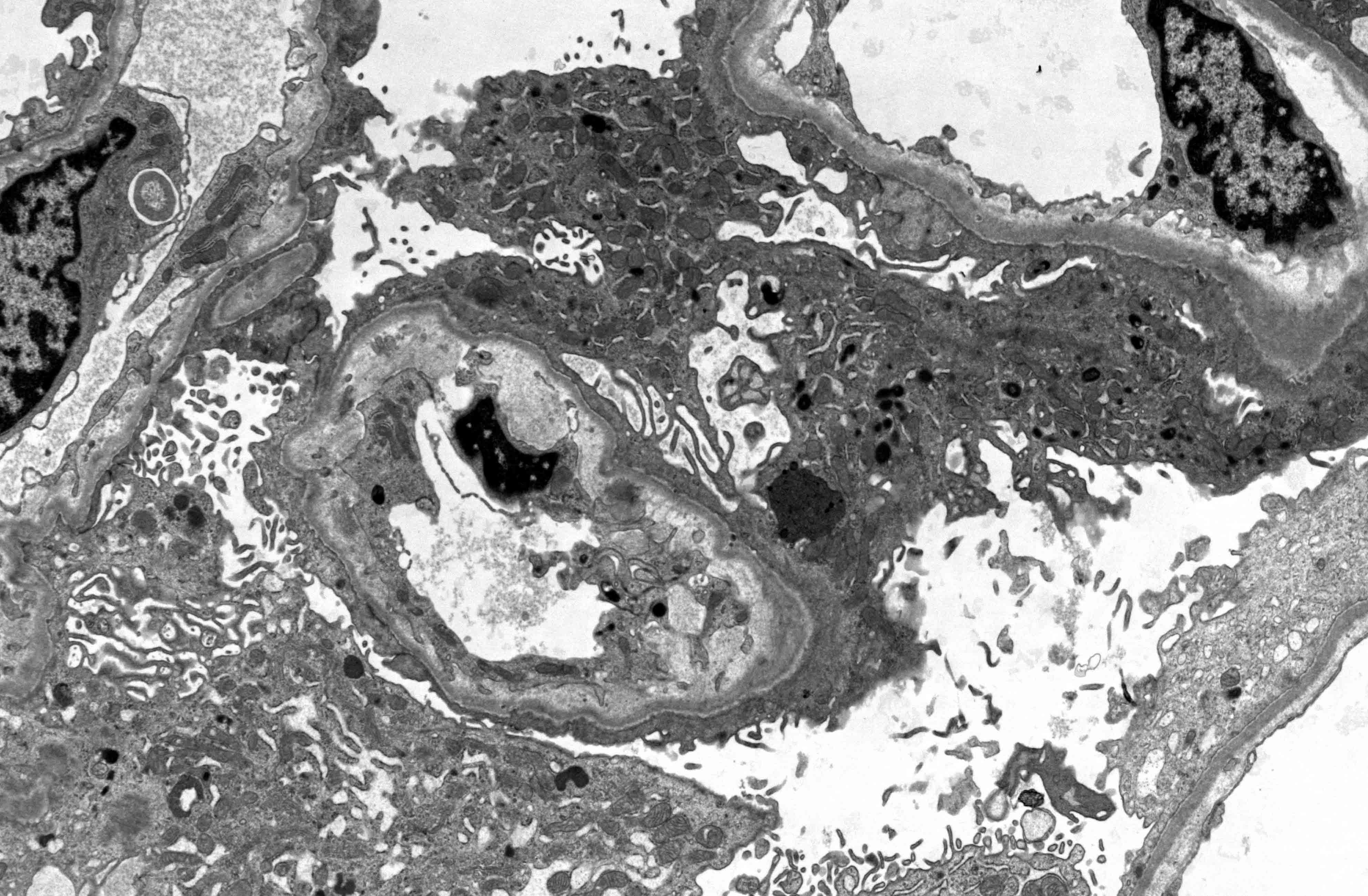
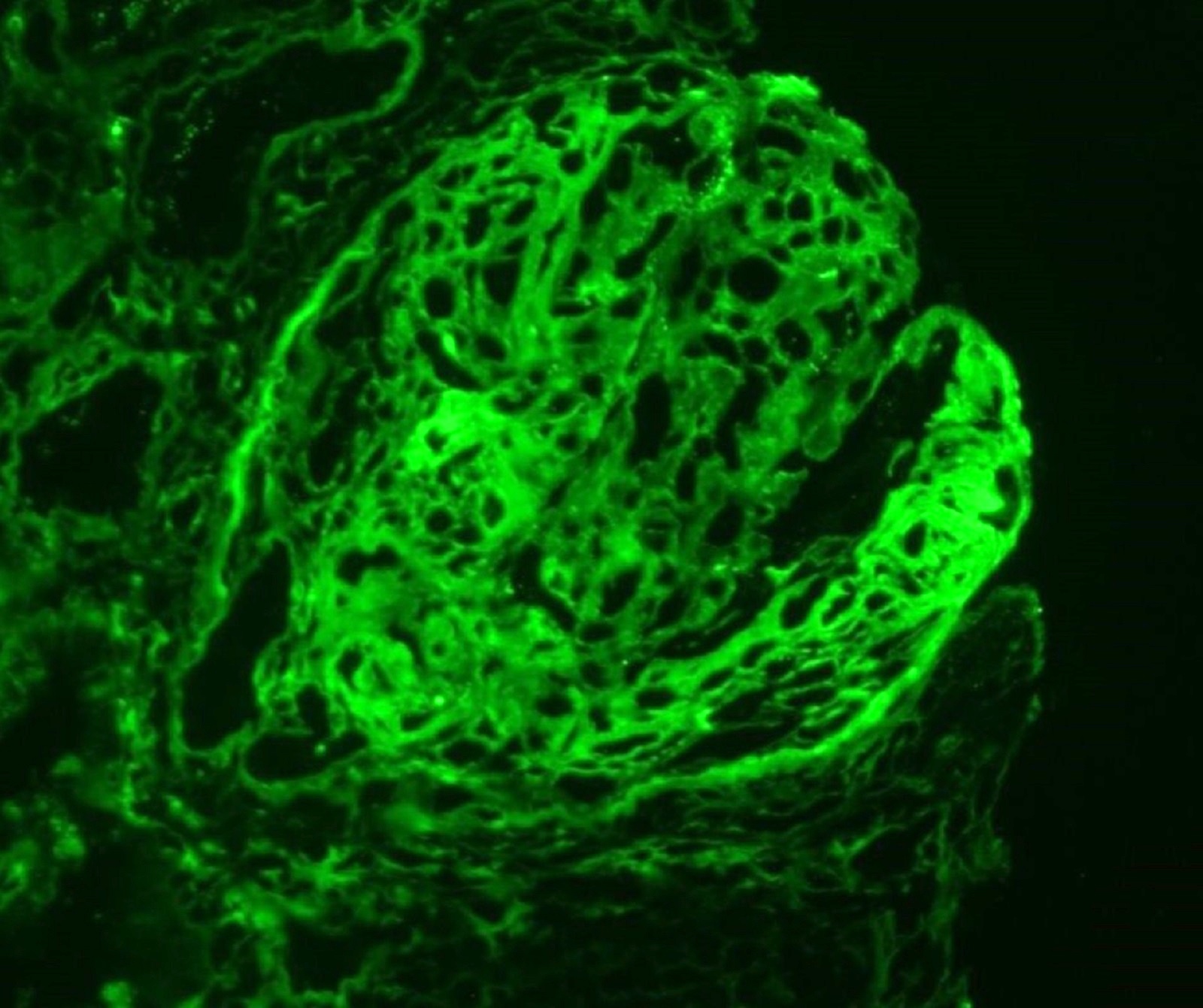
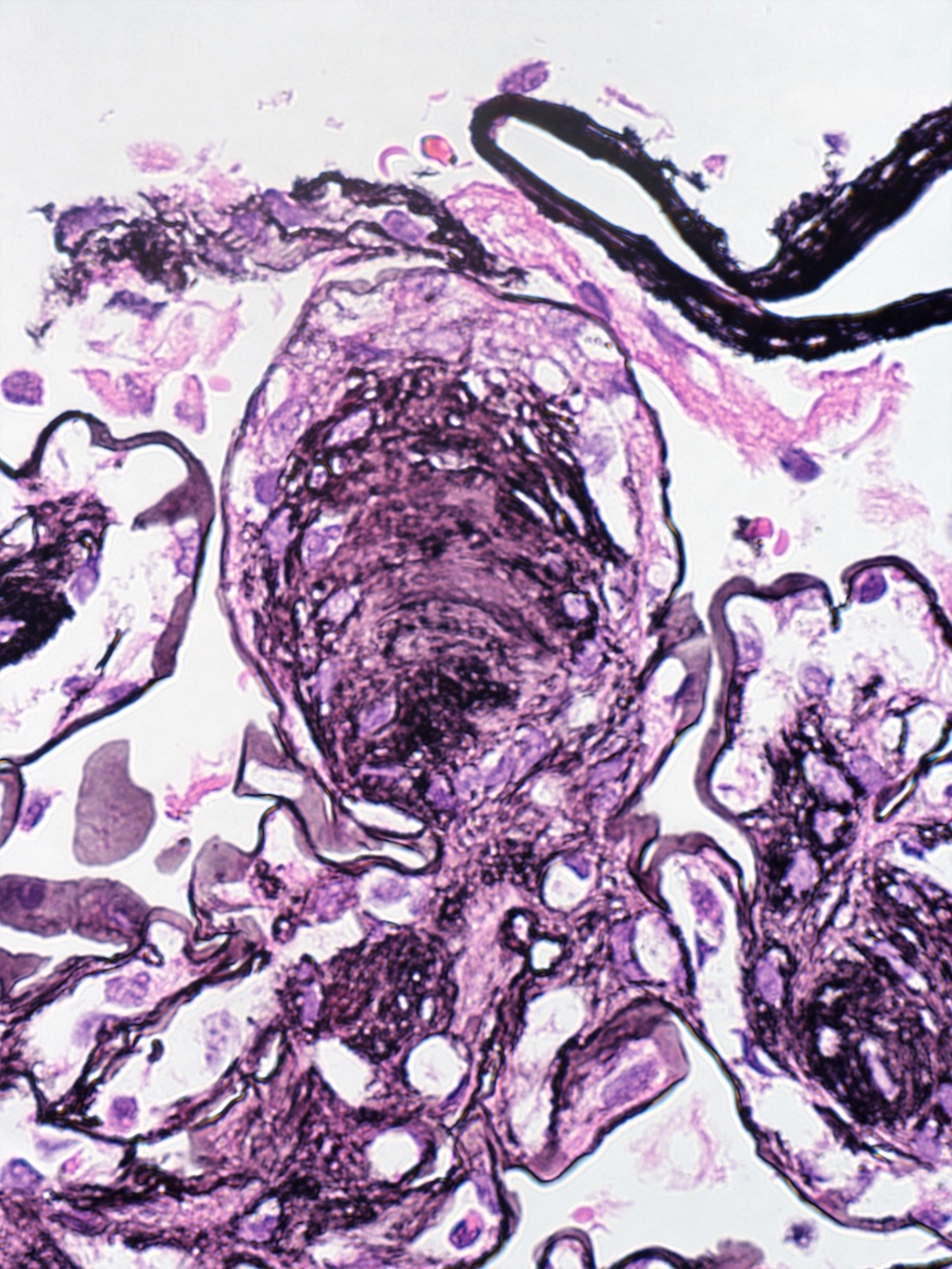
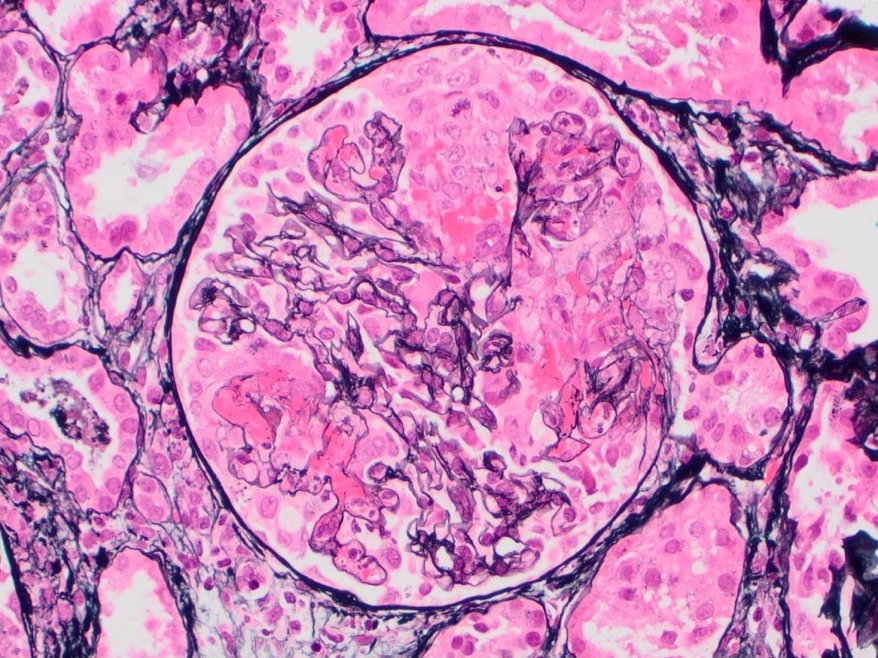
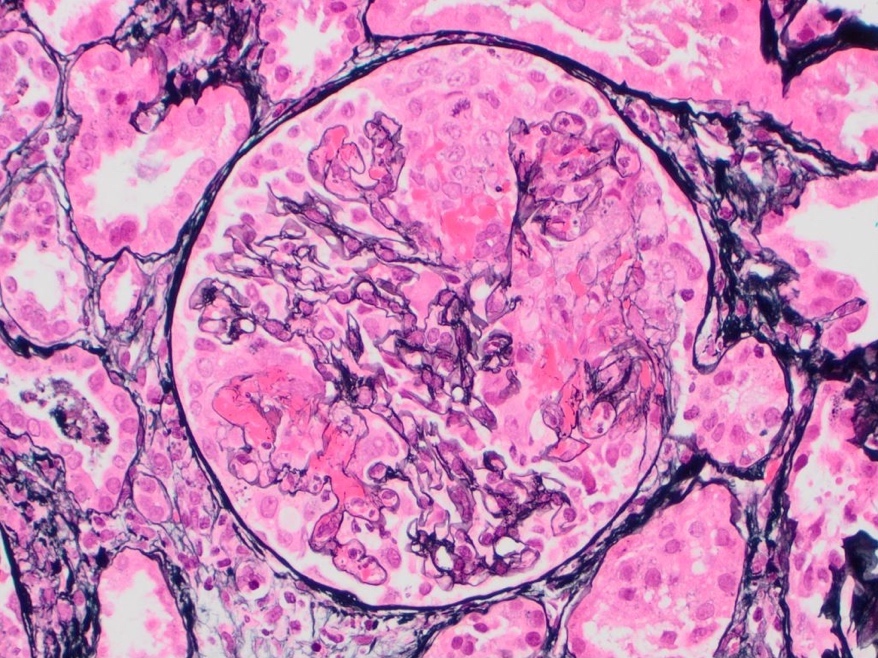
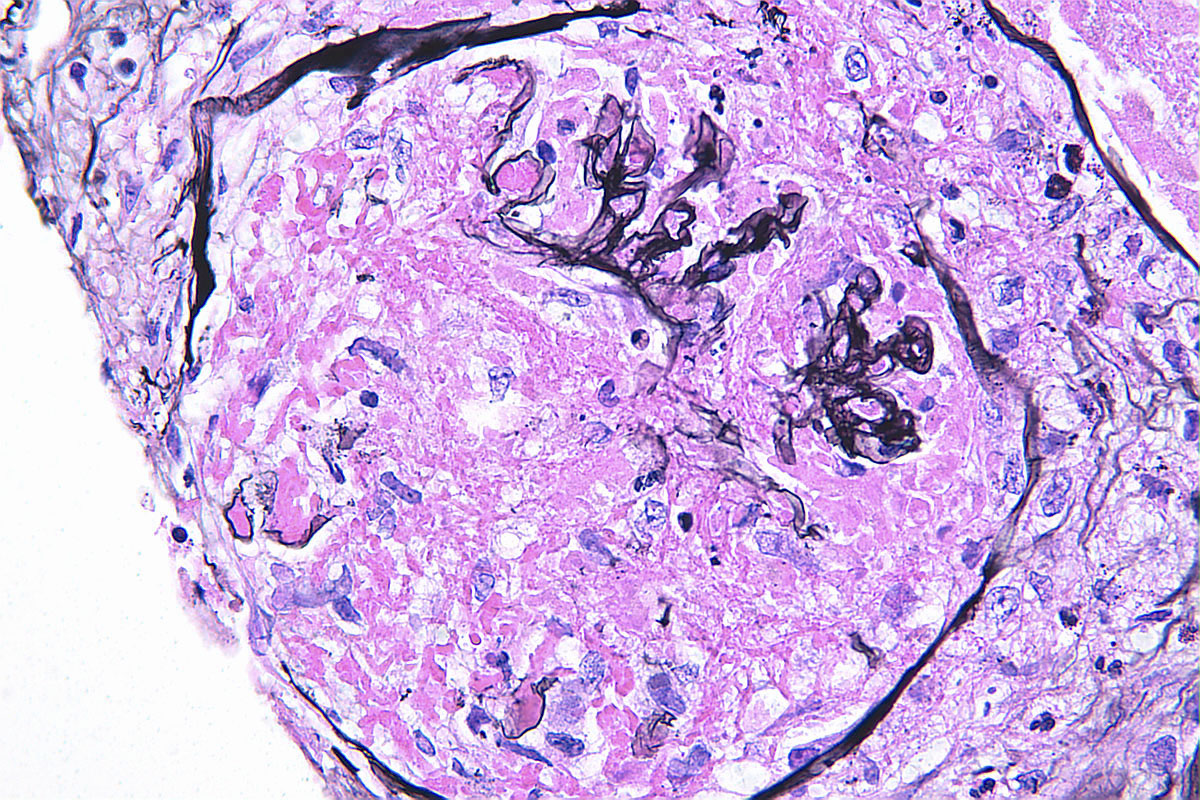
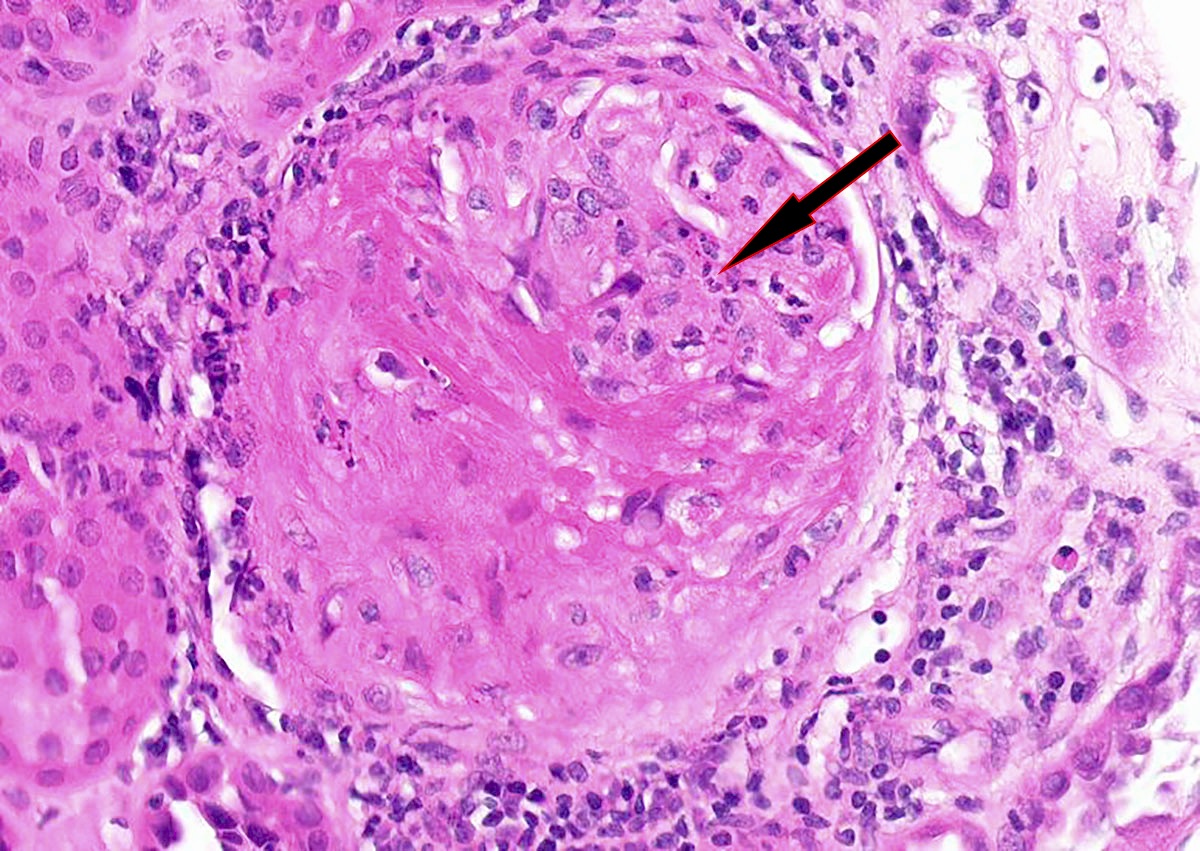
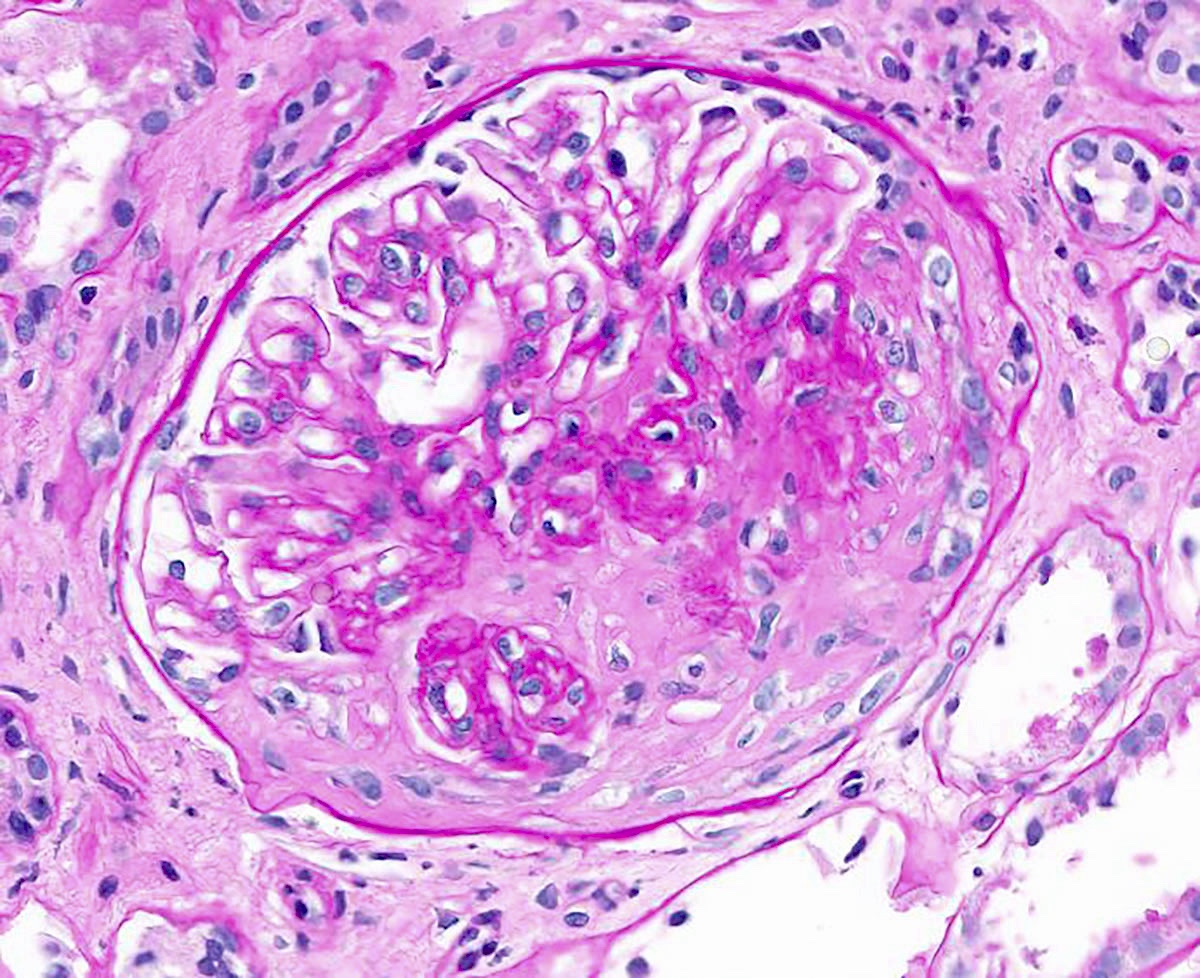
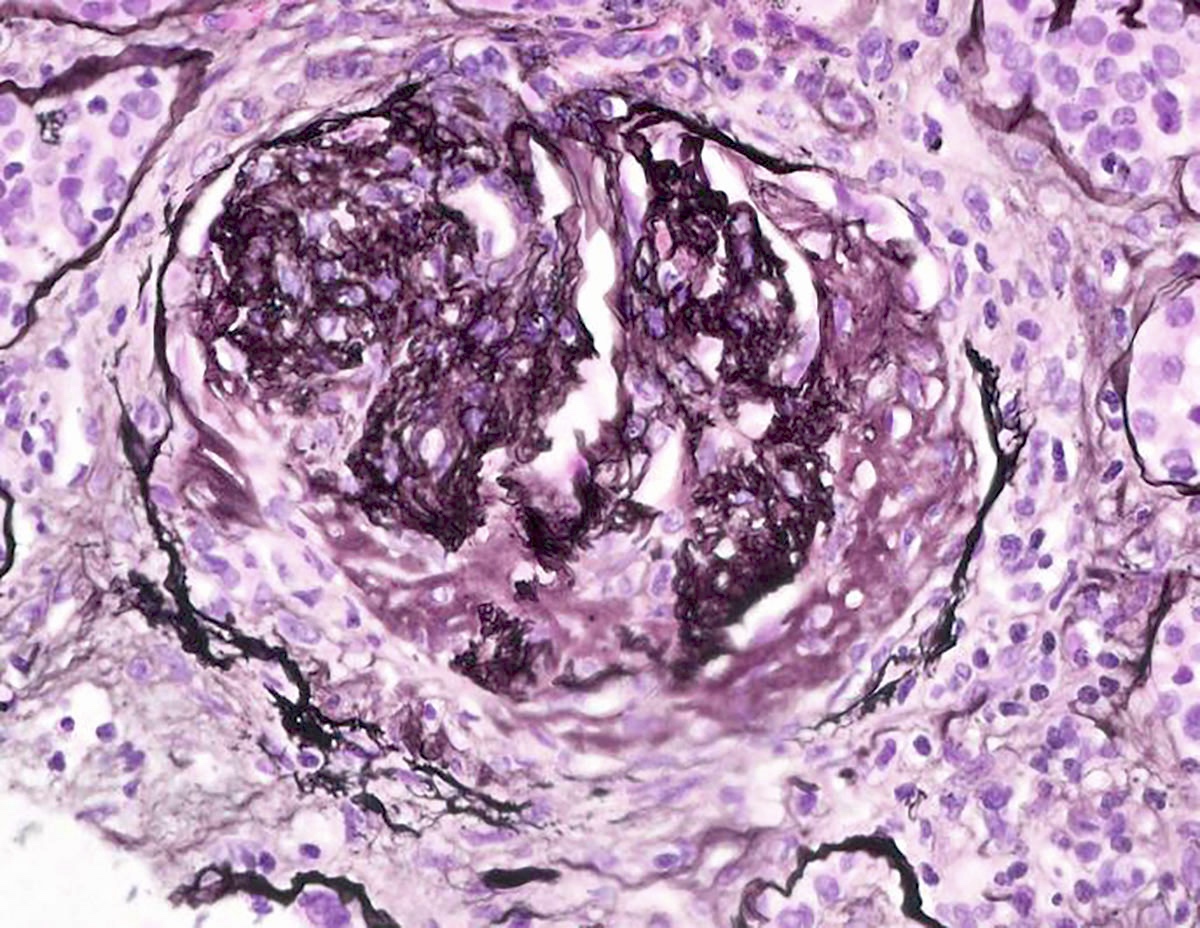
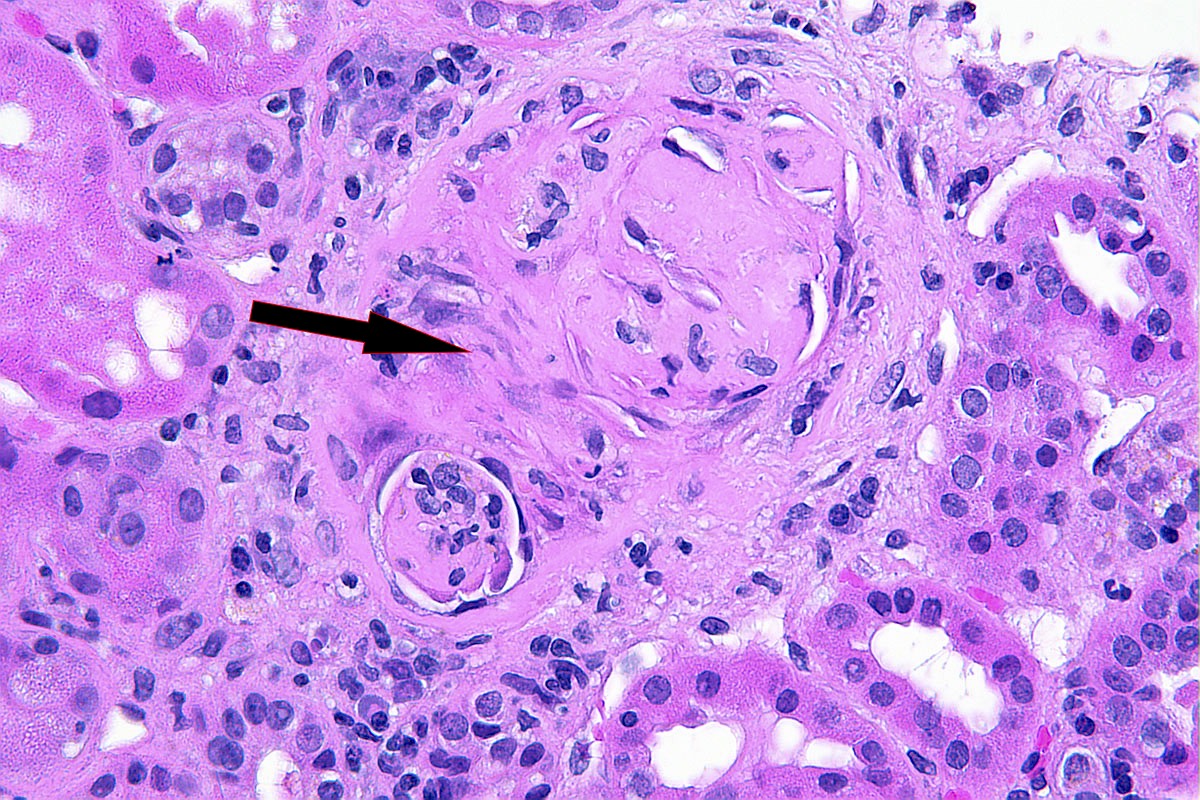
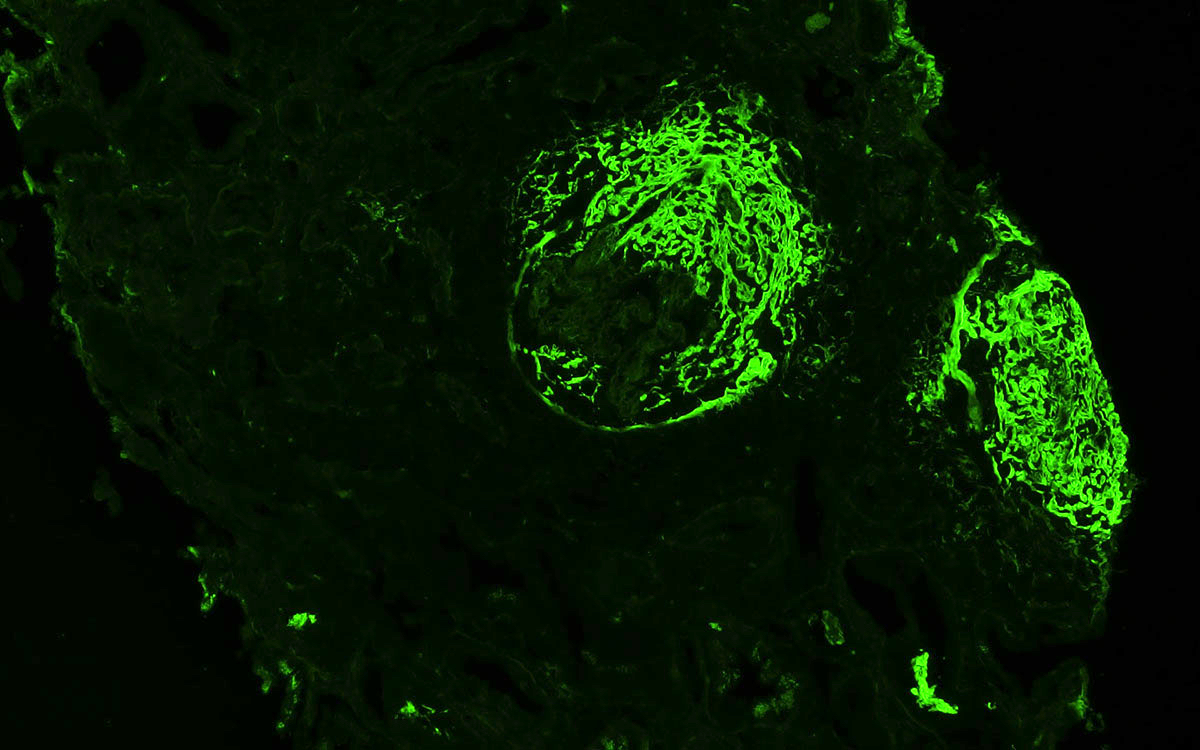
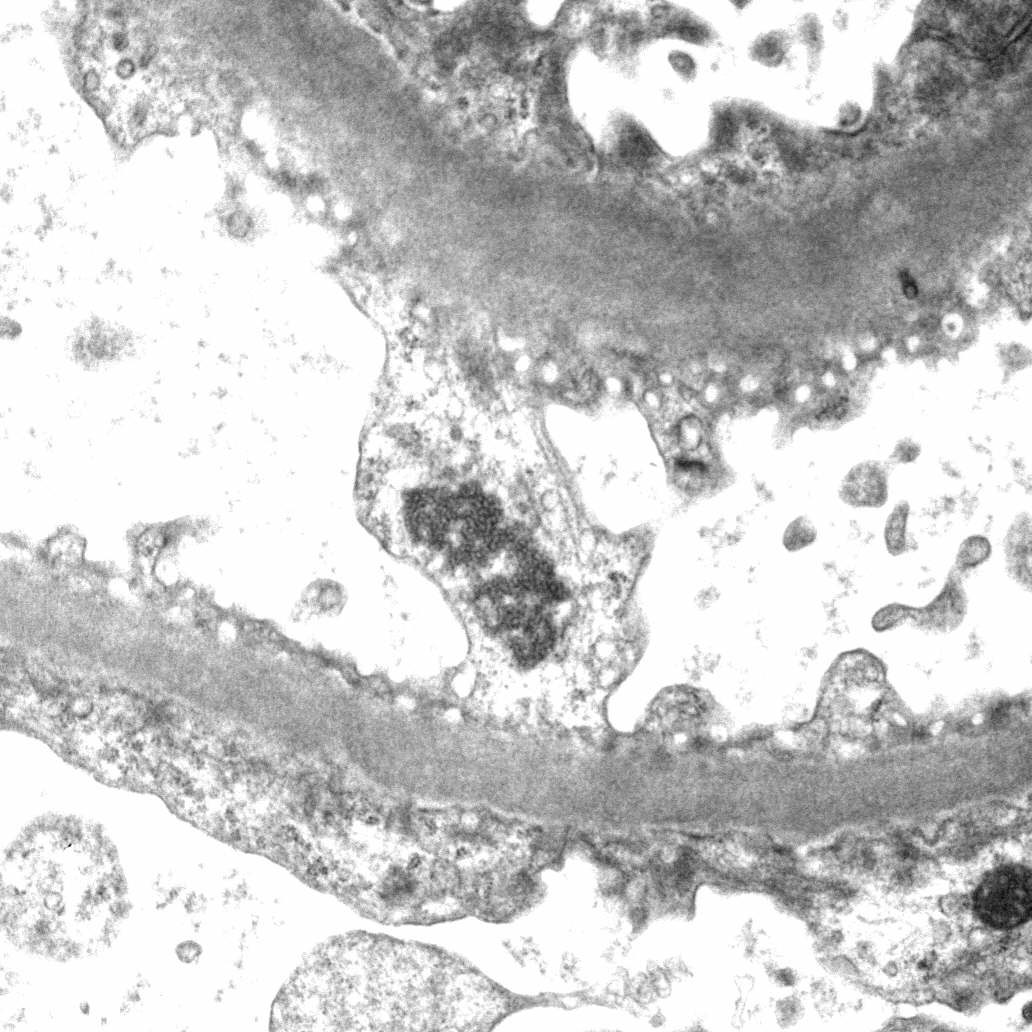
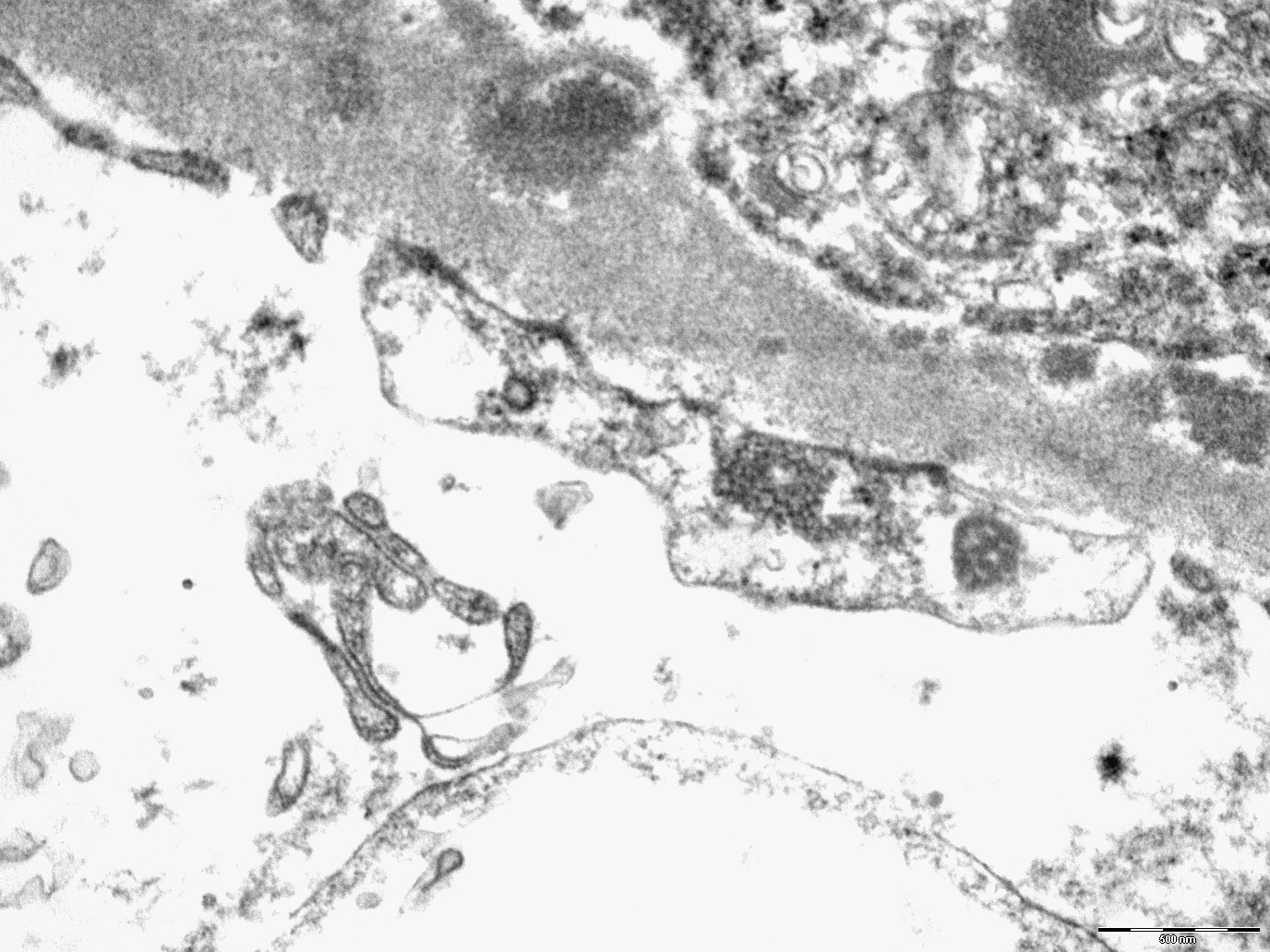
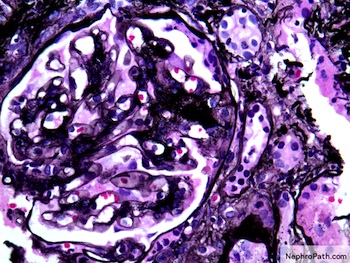
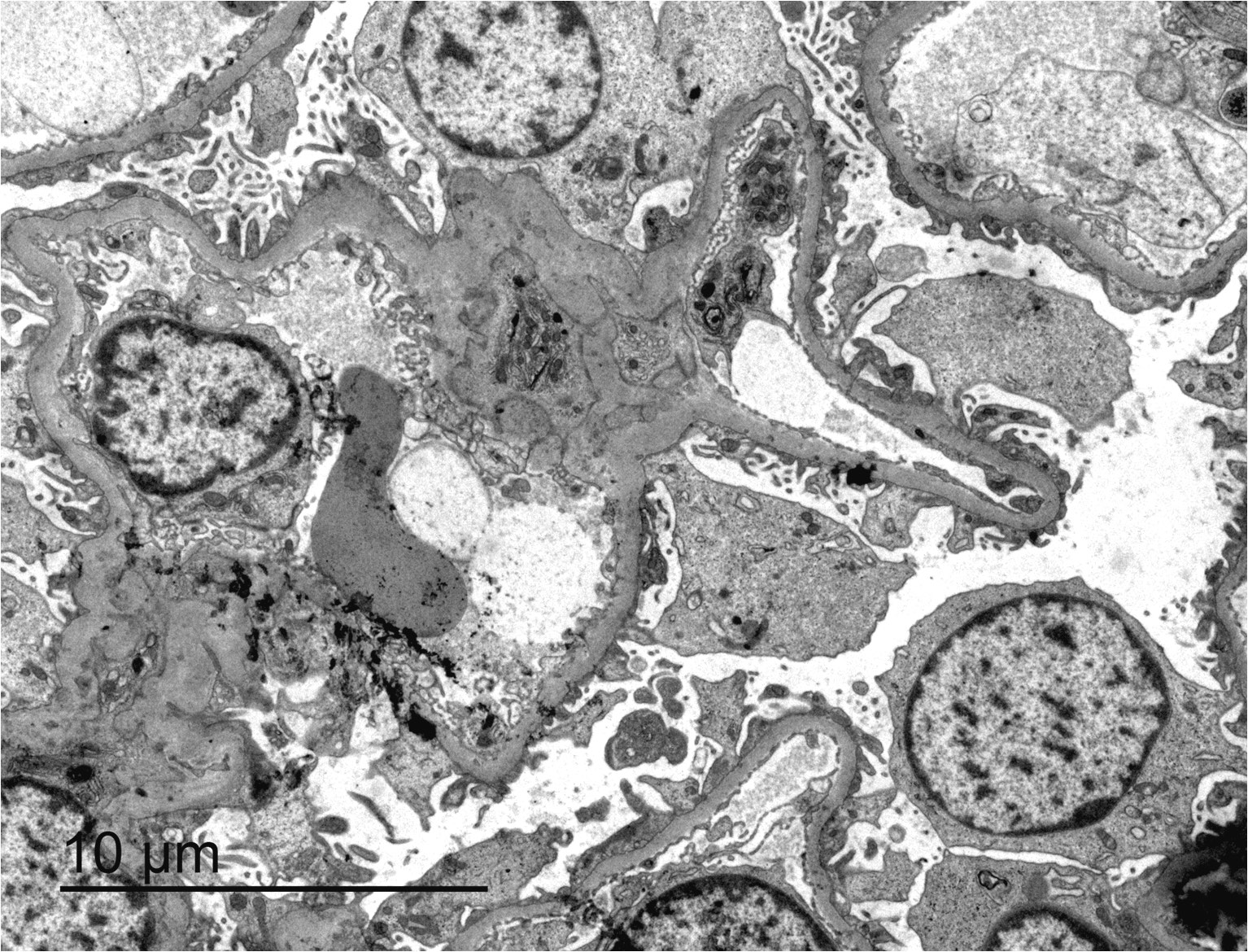
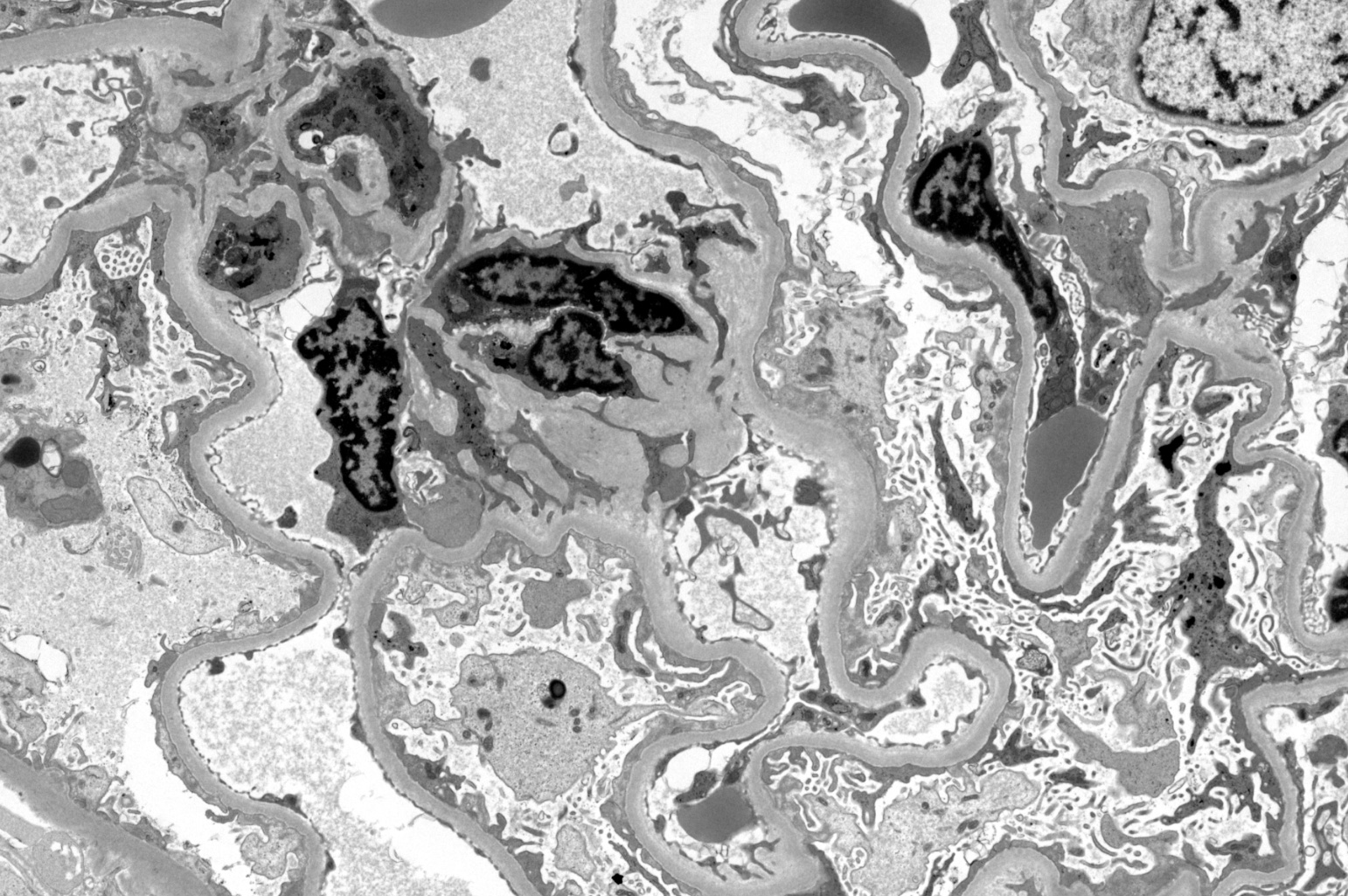
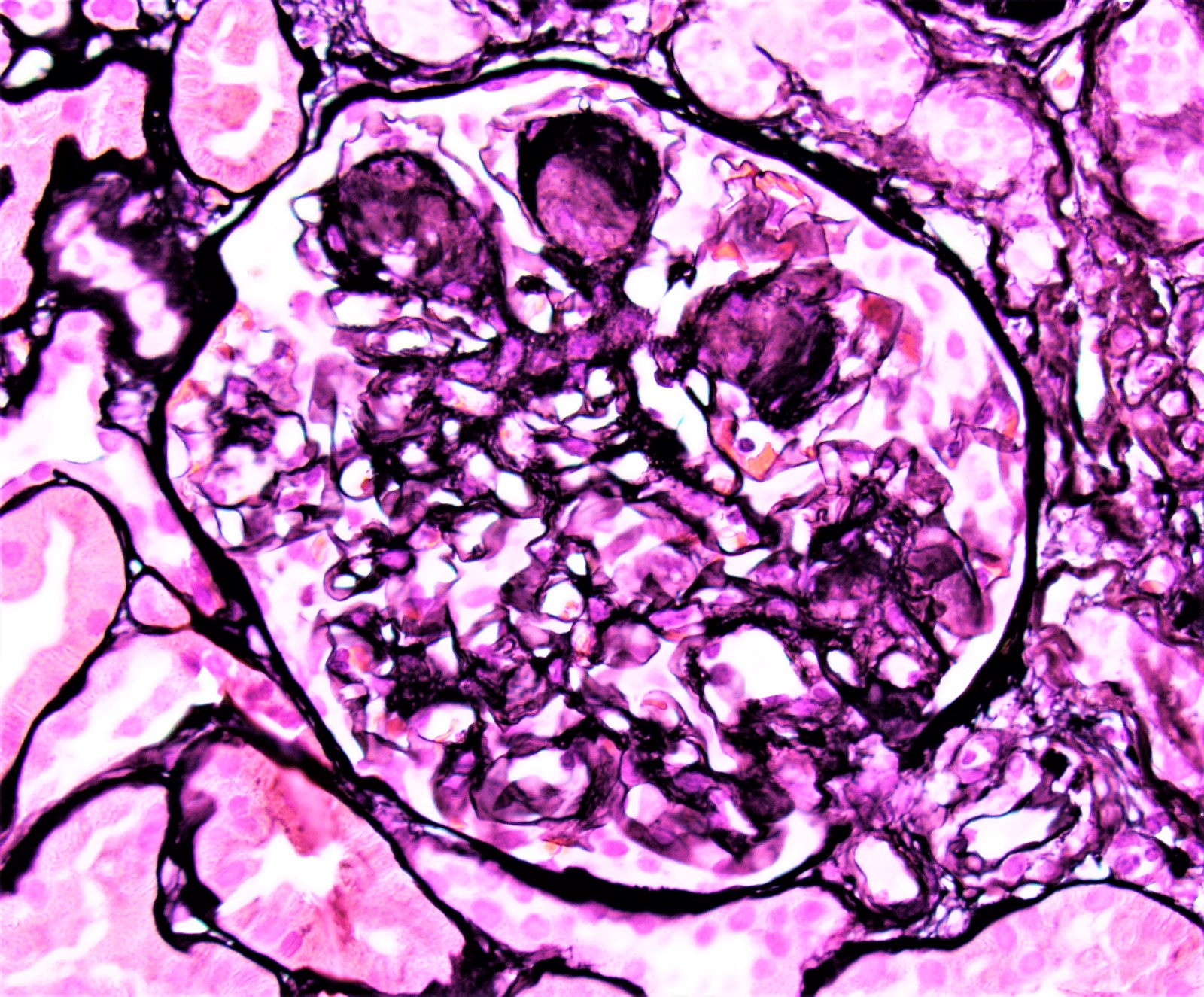
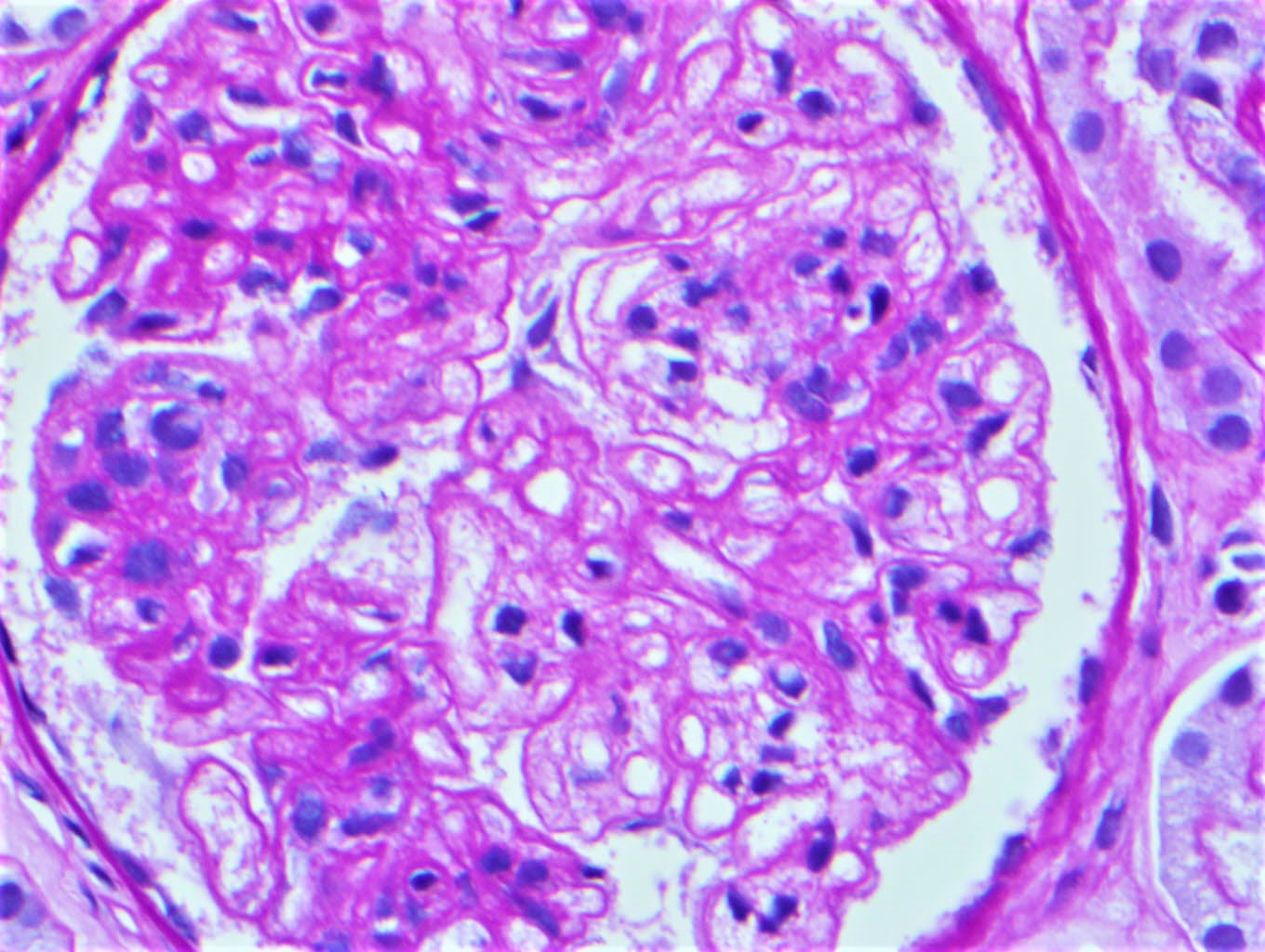
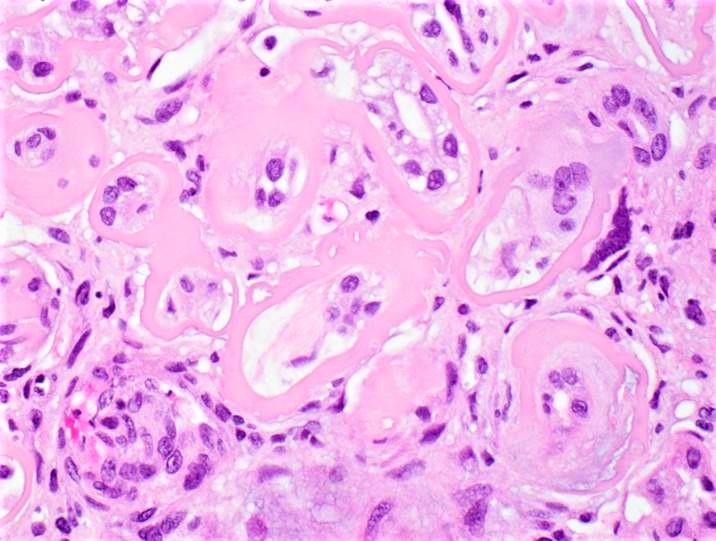
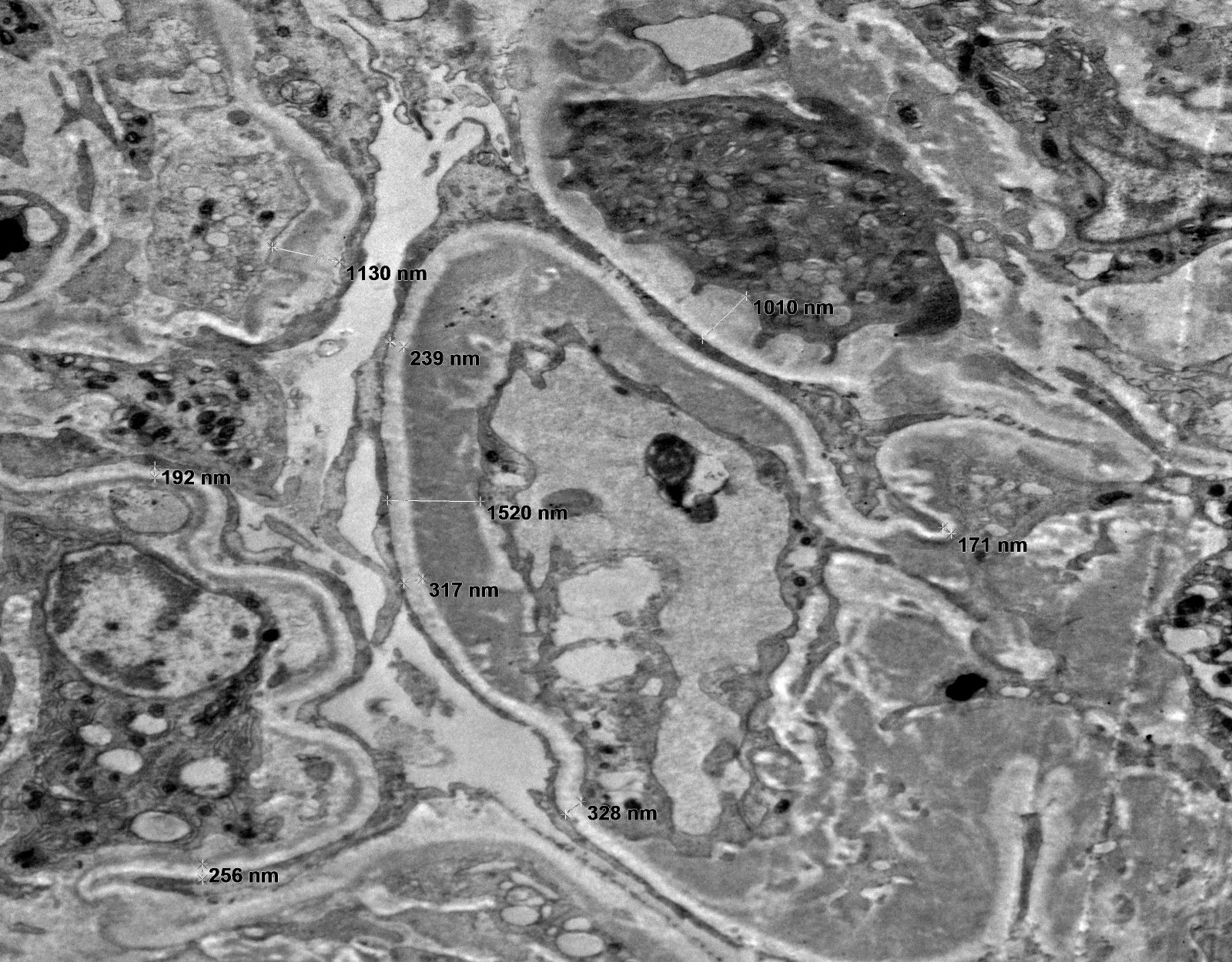
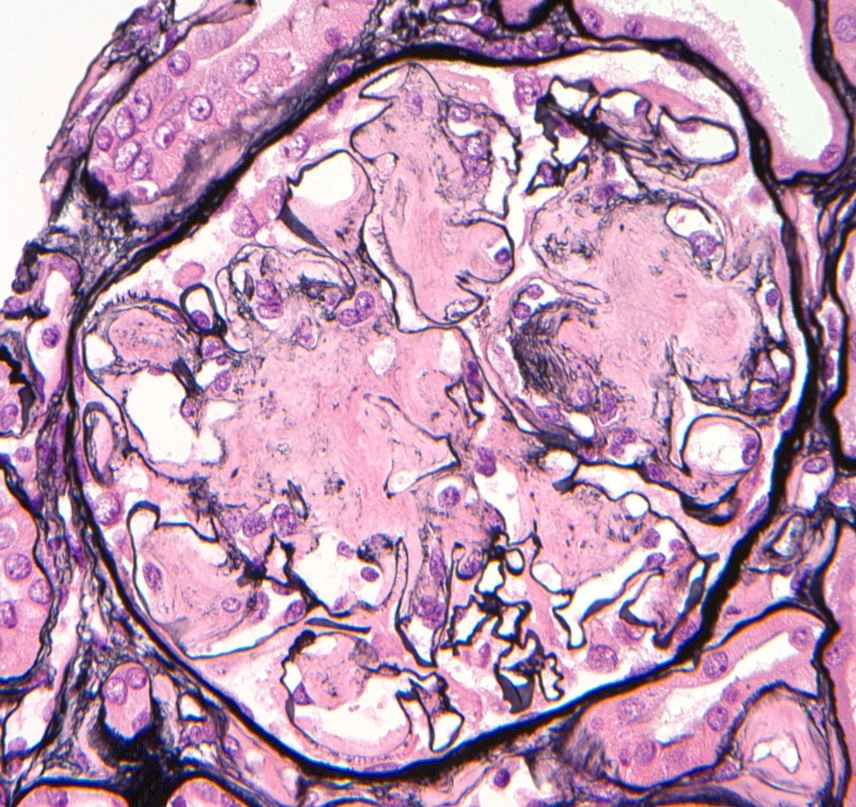
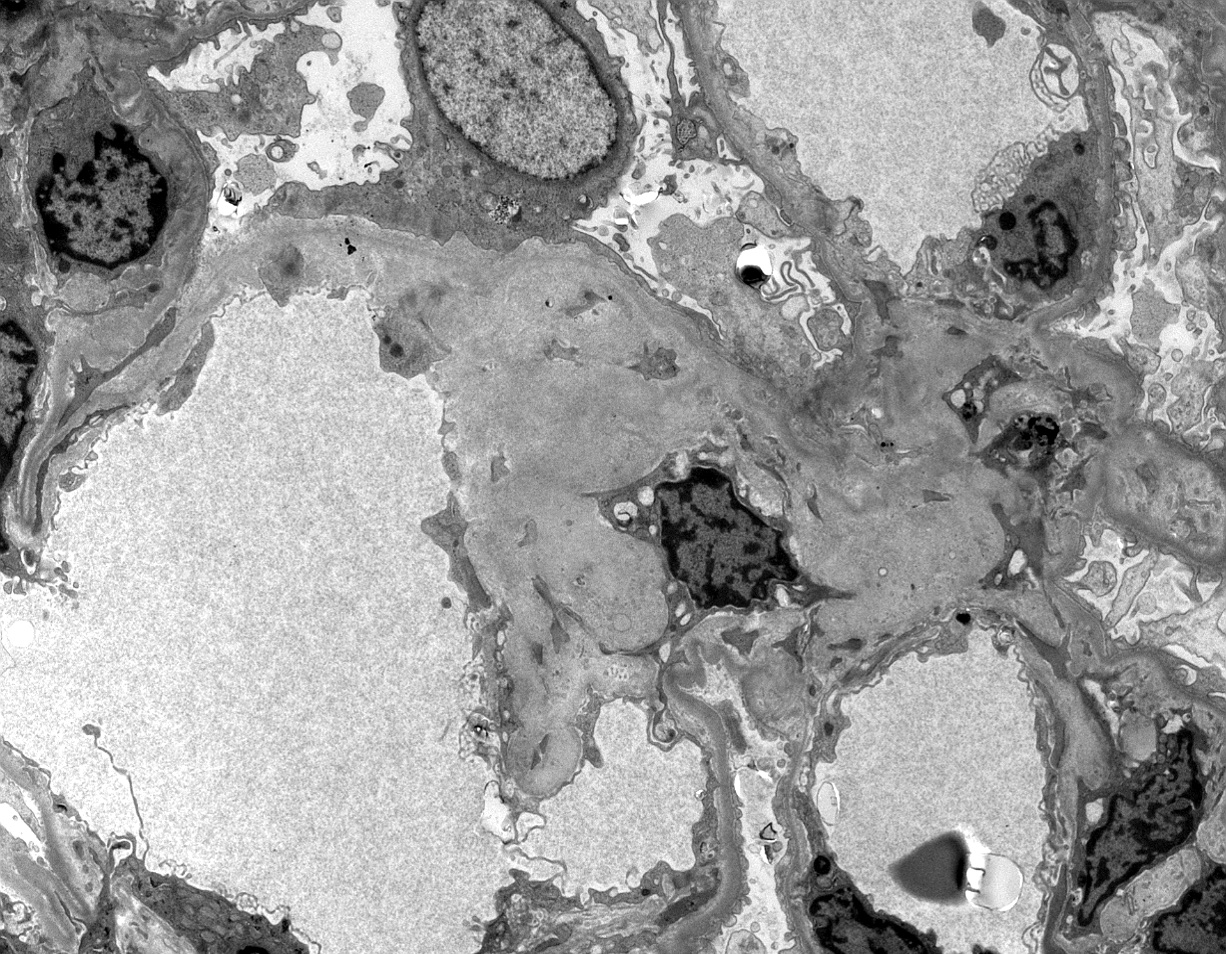
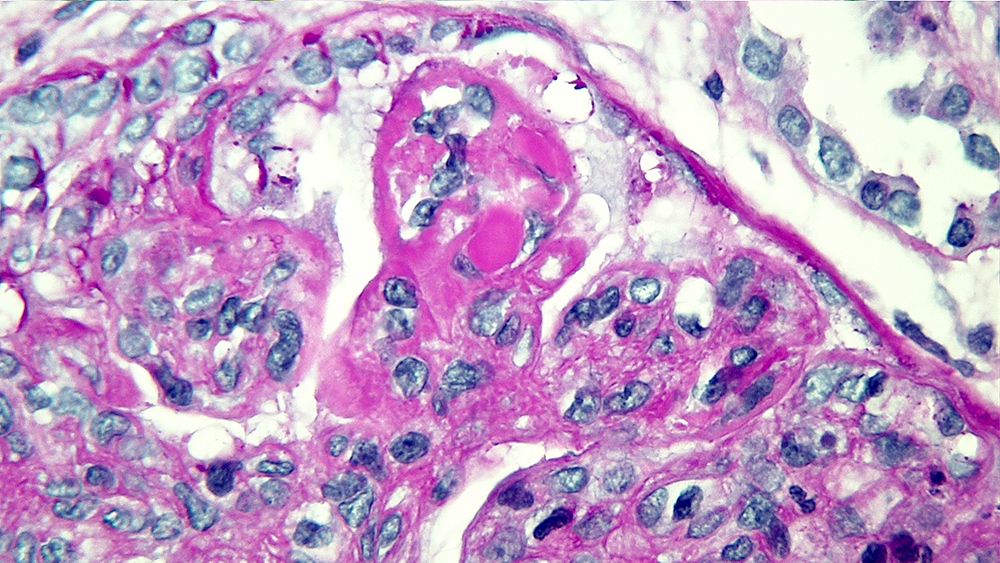
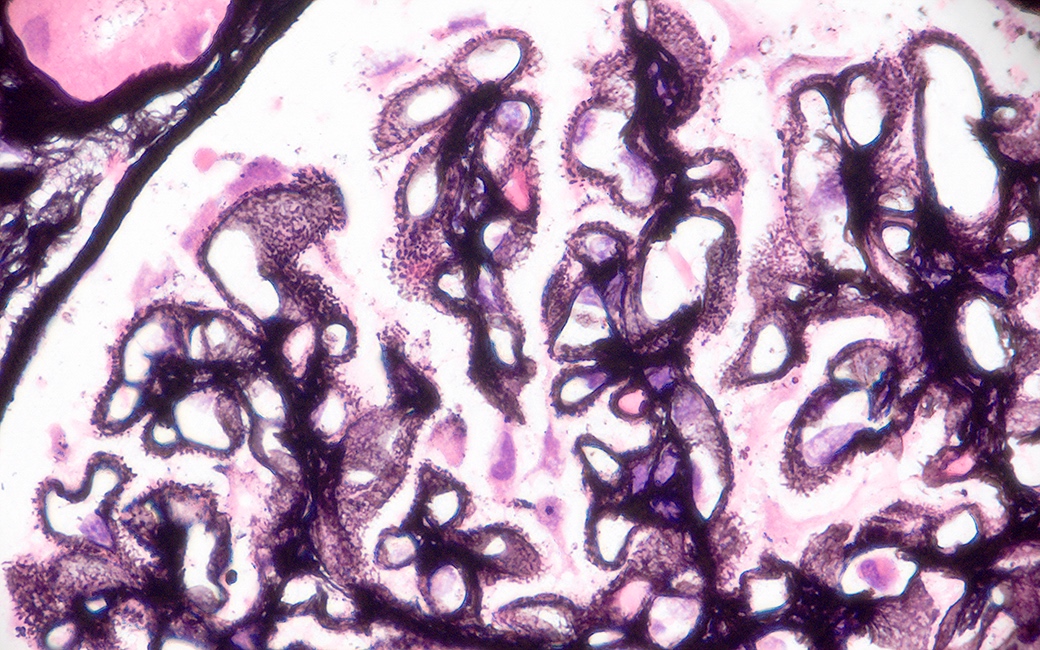
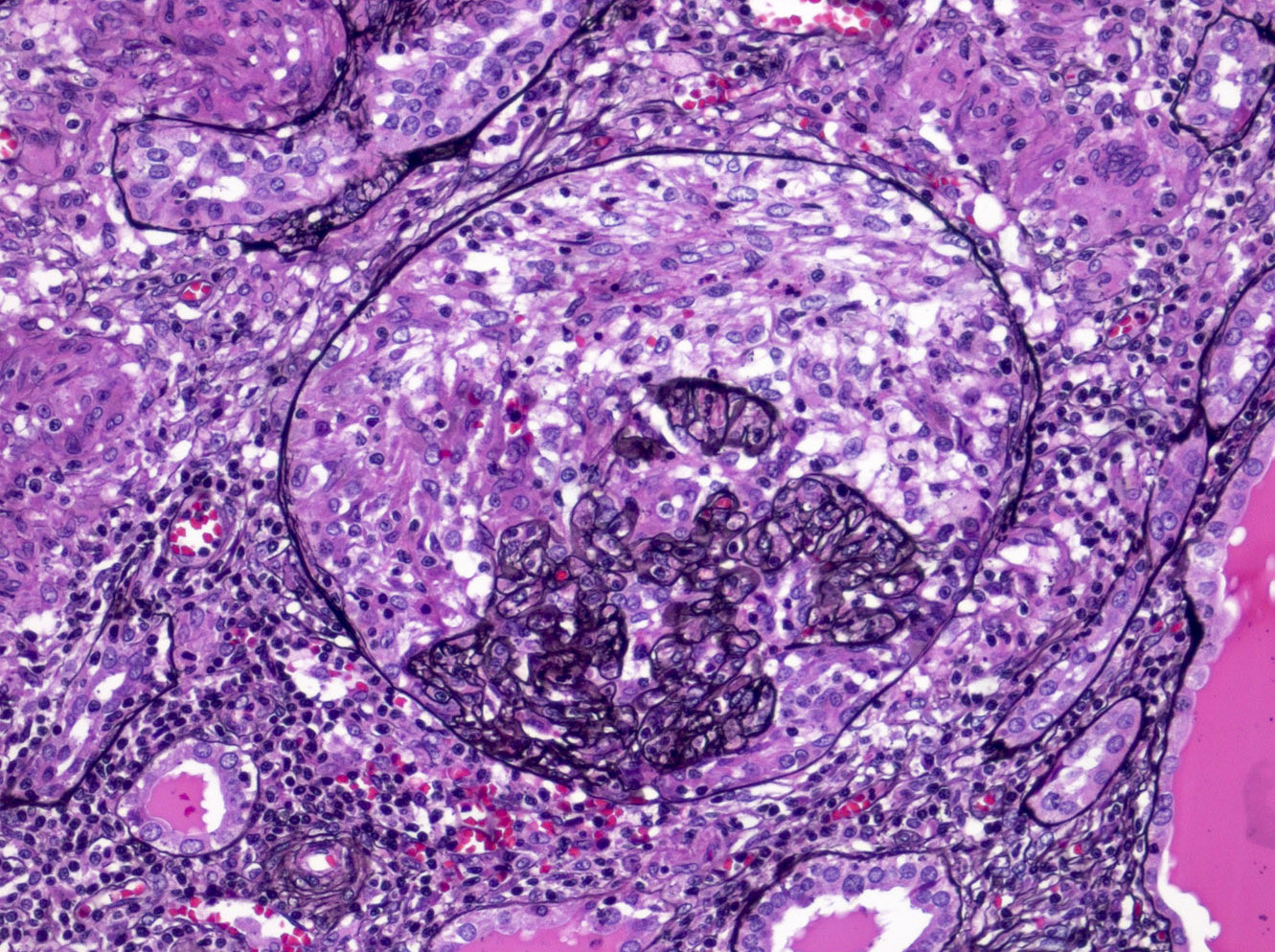
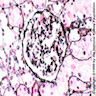
- Transplant glomerulopathy: double contours along the glomerular basement membrane, expansion of mesangium and obliteration of capillary lumina; usually accompanied by linear C4d staining along the glomerular basement membrane
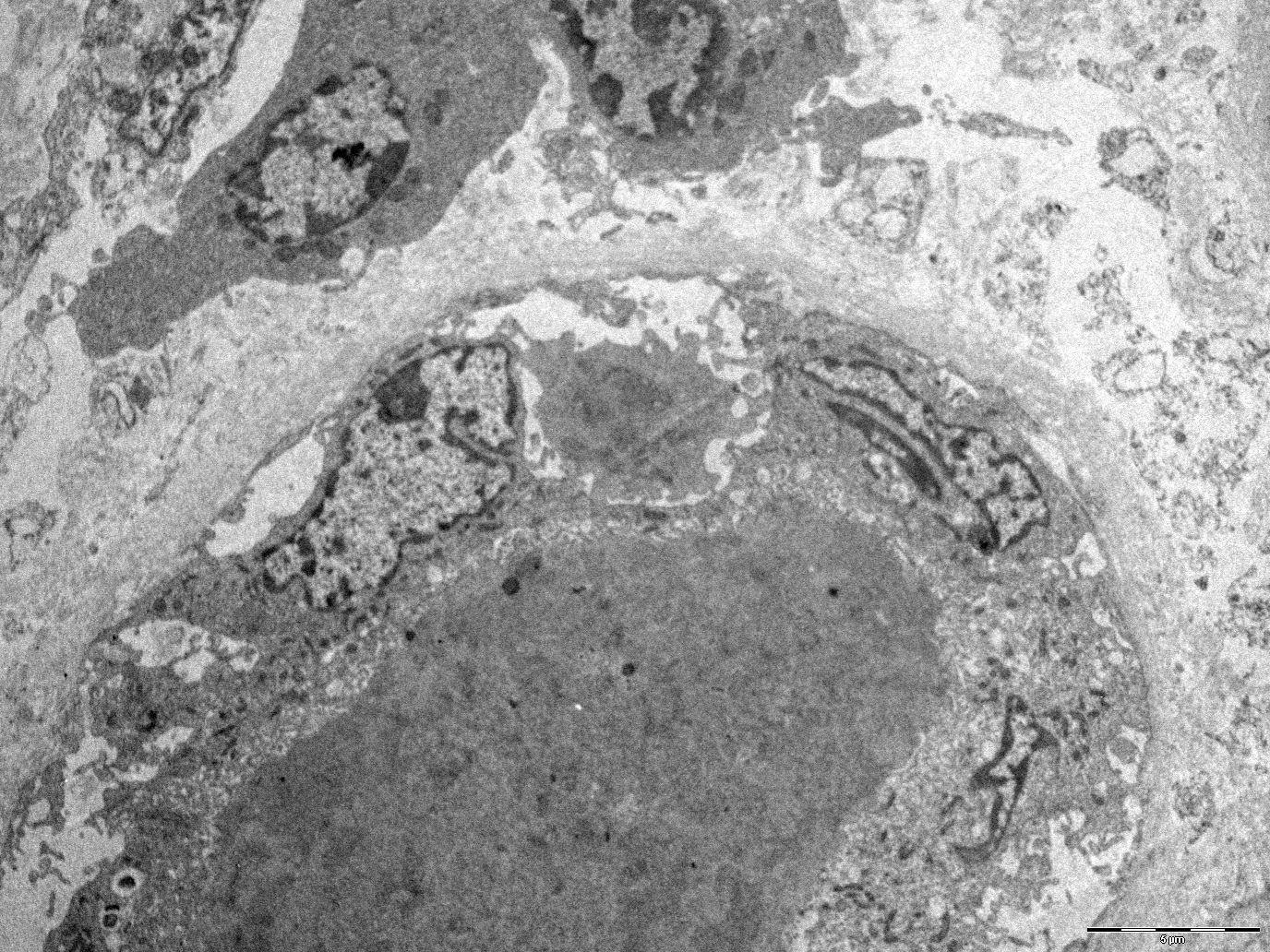
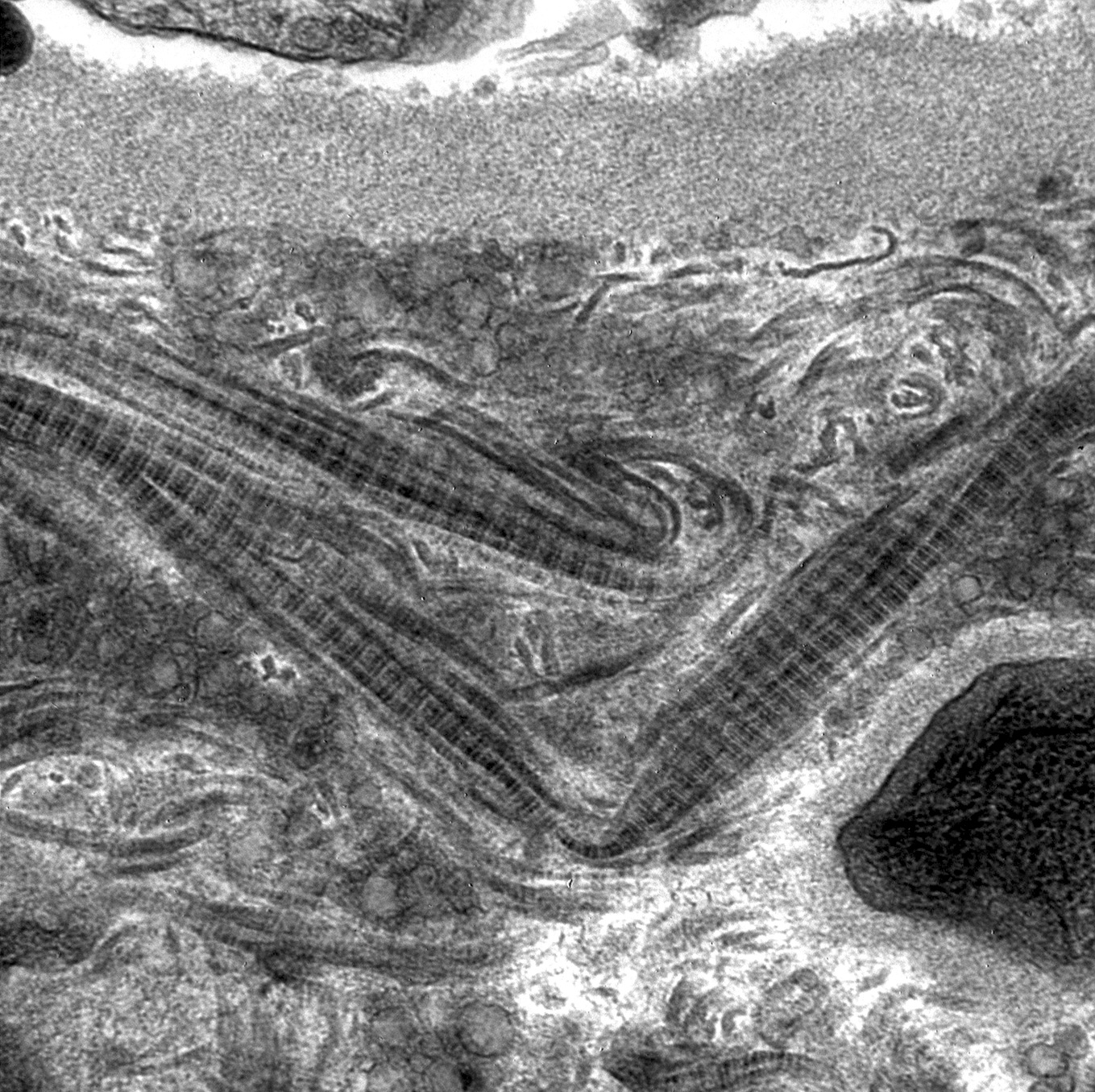
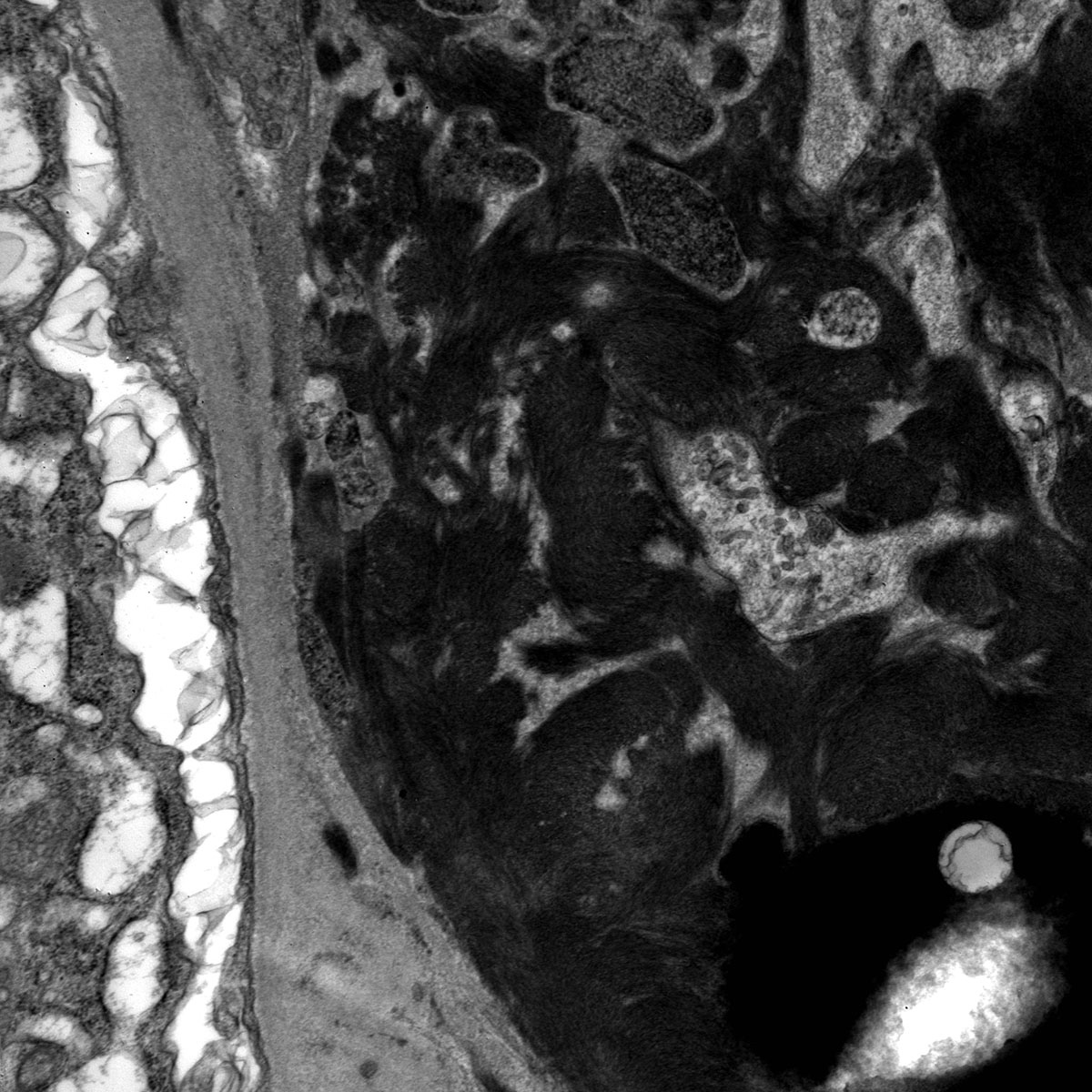
- Peritubular basement membrane multilayering (with electron microscopy)
- Arterial intimal fibrosis with presence of inflammatory cells (transplant arteriopathy)
- Interstitial fibrosis and tubular atrophy
- Chronic ABMR:
- Transplant glomerulopathy: double contours along the glomerular basement membrane, expansion of mesangium and obliteration of capillary lumina; usually accompanied by linear C4d staining along the glomerular basement membrane
- Peritubular basement membrane multilayering (with electron microscopy)
- Arterial intimal fibrosis with presence of inflammatory cells (transplant arteriopathy)
- Interstitial fibrosis and tubular atrophy
- References: Transplant Rev (Orlando) 2017;31:47, Mod Pathol 2018;31:235, Transplantation 2018;102:1795
- v - vascular inflammation: the most severely affected artery dictates the score; an asterisk is added to the v score if interstitial hemorrhage or infarct present
- v0: no arteritis
- v1: intimal arteritis with < 25% luminal area lost (minimum = 1 cell, 1 artery)
- v2: intimal arteritis with ≥ 25% of luminal area lost in 1+ arteries
- v3: transmural arteritis or fibrinoid necrosis (medial smooth muscle necrosis) with lymphocyte infiltrate in vessels
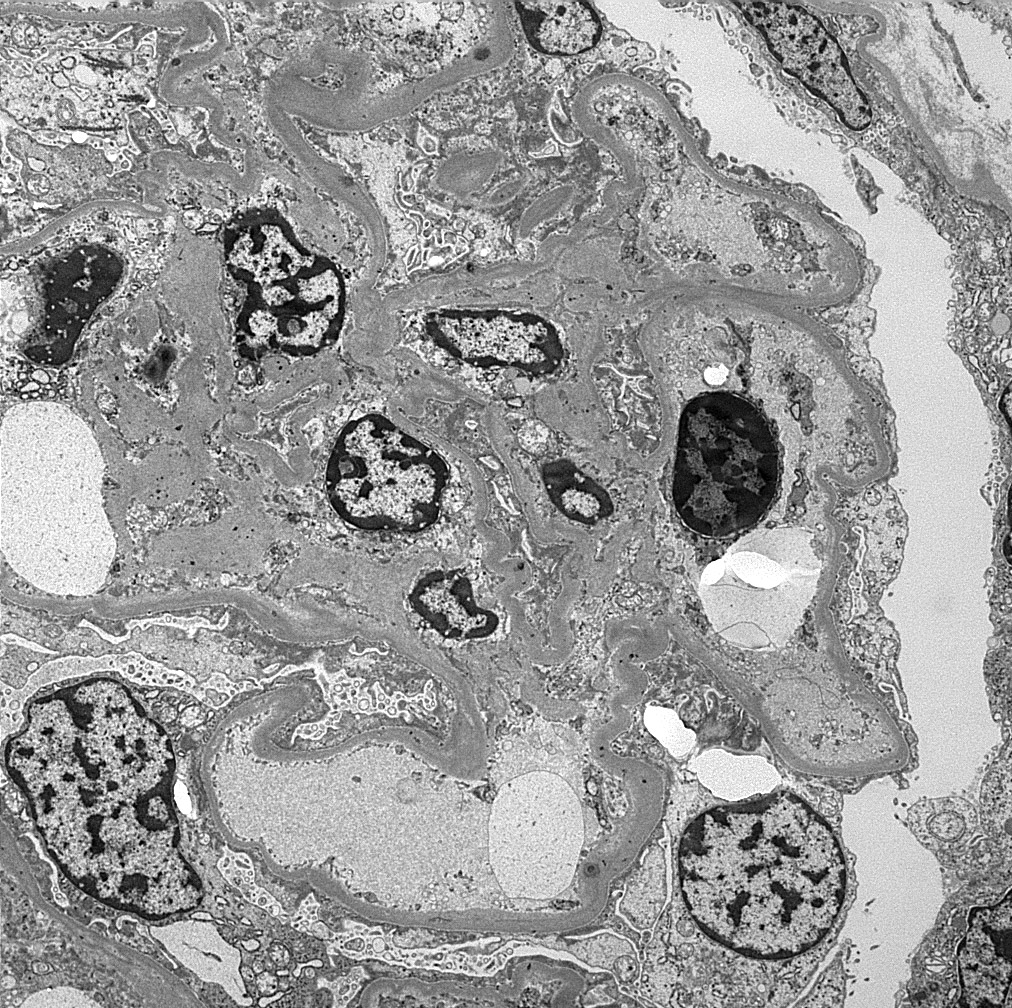
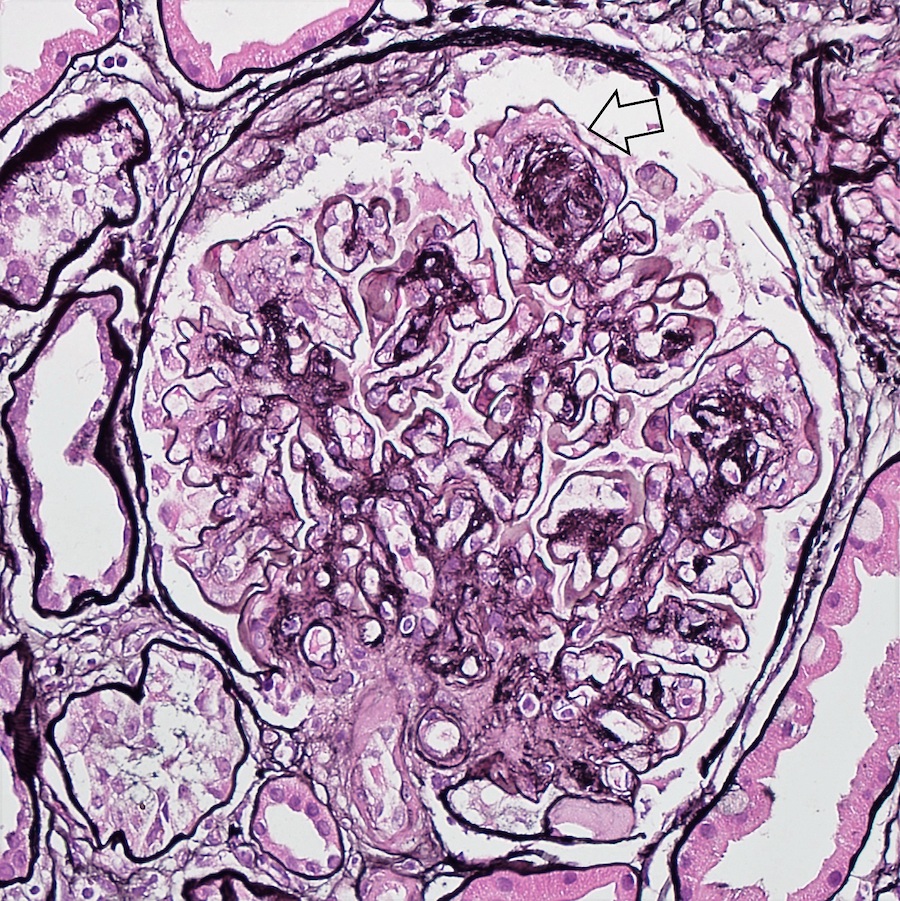
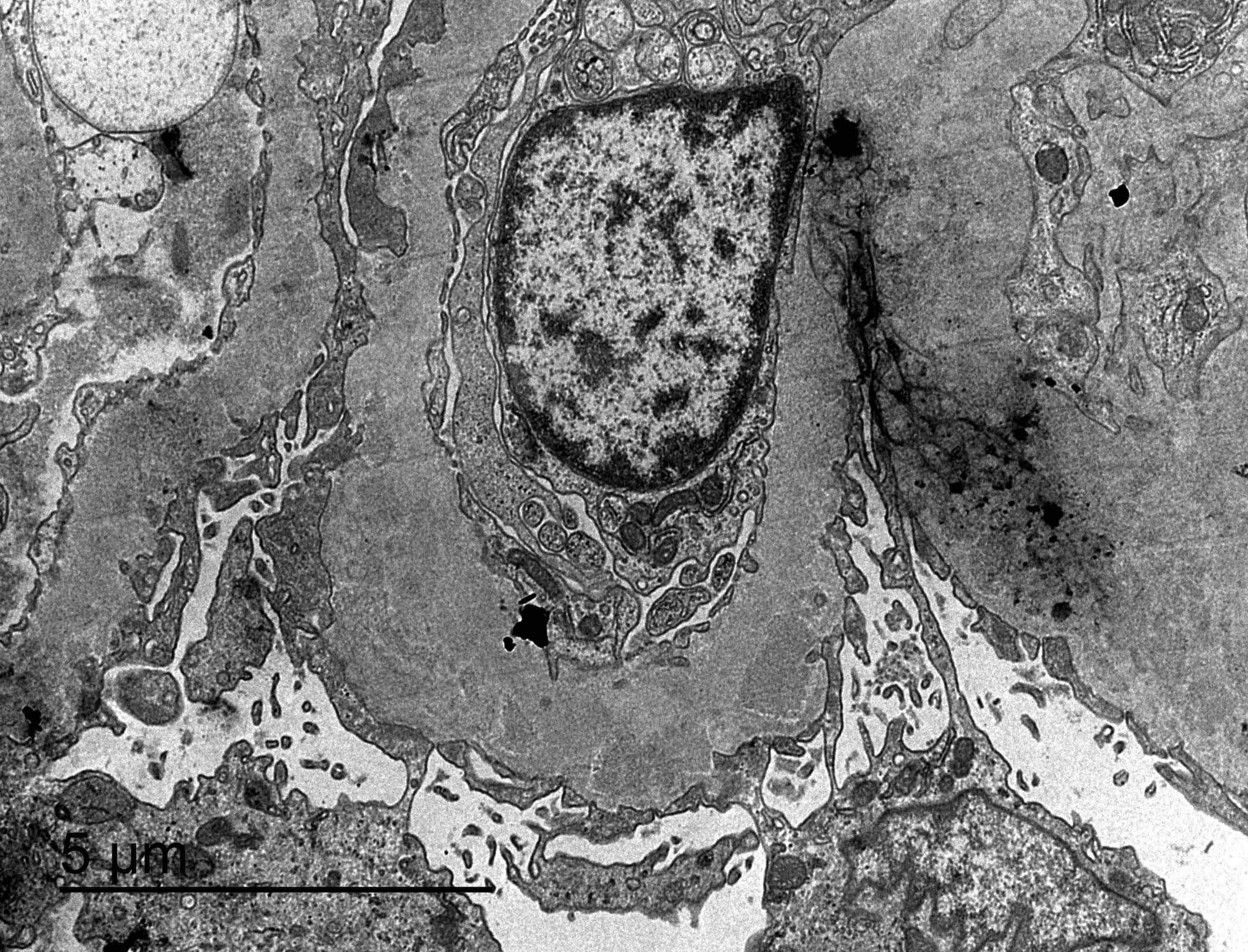
- g - glomerulitis: percentage of glomerular capillaries partially or completely occluded by inflammatory cells (polymorphonuclear leucocytes and mononuclear cells) and endothelial cell enlargement
- g0: no glomerulitis
- g1: < 25% of glomeruli involved (mostly segmental)
- g2: 25 - 75% of glomeruli involved (segmental to global)
- g3: > 75% of glomeruli involved (mostly global)
- ptc - peritubular capillaritis: the most severely affected peritubular capillary (PTC) dictates the score; an asterisk is added to the ptc score if neutrophils are lacking / only mononuclear cells are present
- ptc0: < 3 cells/PTC
- ptc1: 1+ inflammatory cells in > 10% of cortical PTCs with 3 - 4 cells in most severely involved PTC
- ptc2: 1+ inflammatory cells in > 10% of cortical PTCs with 5 - 10 cells in most severely involved PTC
- ptc3: 1+ inflammatory cells in > 10% of cortical PTCs with > 10 cells in most severely involved PTC
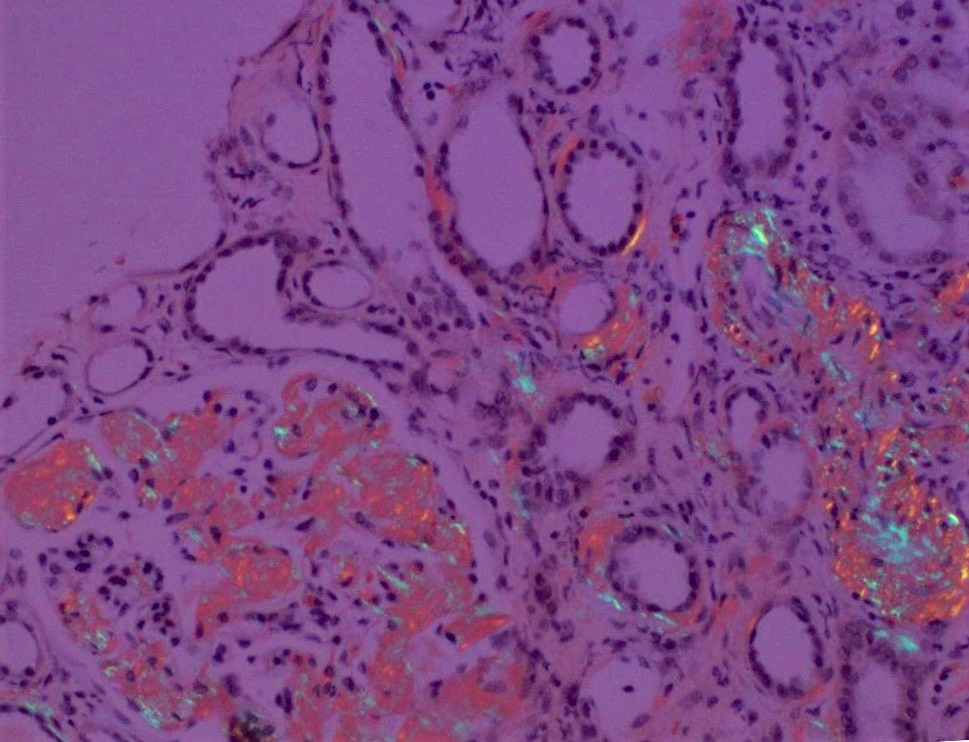
- C4d: percentage of PTC (or vasa recta in the medulla) that has linear circumferential staining, scored in at least 5 high powered fields of cortex or medulla without scarring or infarct
- C4d0: no staining of PTC and medullary vasa recta
- C4d1: < 10% of PTC and medullary vasa recta
- C4d2: 10 - 50% of PTC and medullary vasa recta
- C4d3: > 50% of PTC and medullary vasa recta
- cg - transplant glomerulopathy: percentage of glomerular capillary loops with duplication of glomerular basement membrane in most affected nonsclerotic glomerulus
- cg0: none by light microscopy (LM) and electron microscopy
- cg1a: only by electron microscopy in 3+ glomerular capillaries
- cg1b: ≤ 25% by LM (1+ glomerular capillaries with glomerular basement membrane double contours by LM)
- cg2: 26 - 50% by LM
- cg3: > 50% by LM
- cv - transplant arteriopathy: arterial fibrointimal thickening; percentage of narrowing of lumen of most severely affected artery
- cv0: none
- cv1: ≤ 25% of the luminal area
- cv2: 26 - 50% of the luminal area
- cv3: > 50% of the luminal area
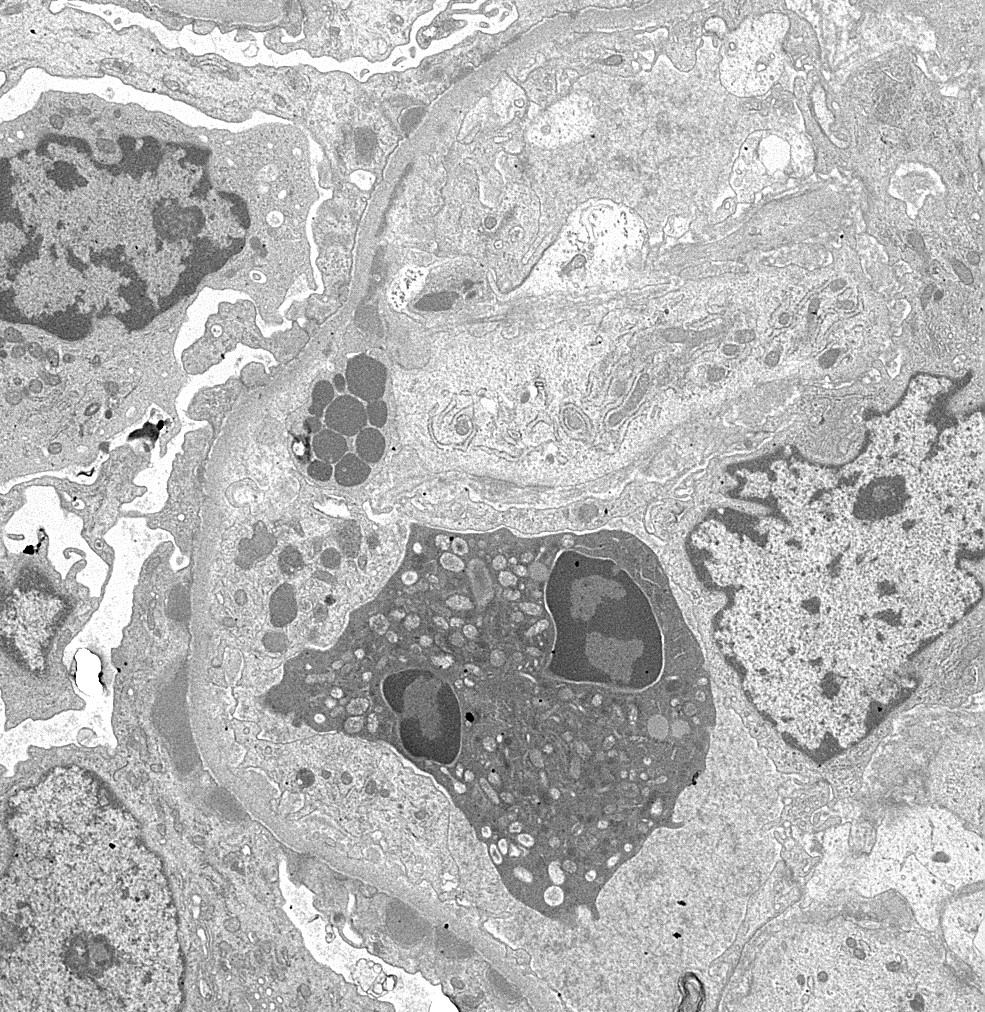
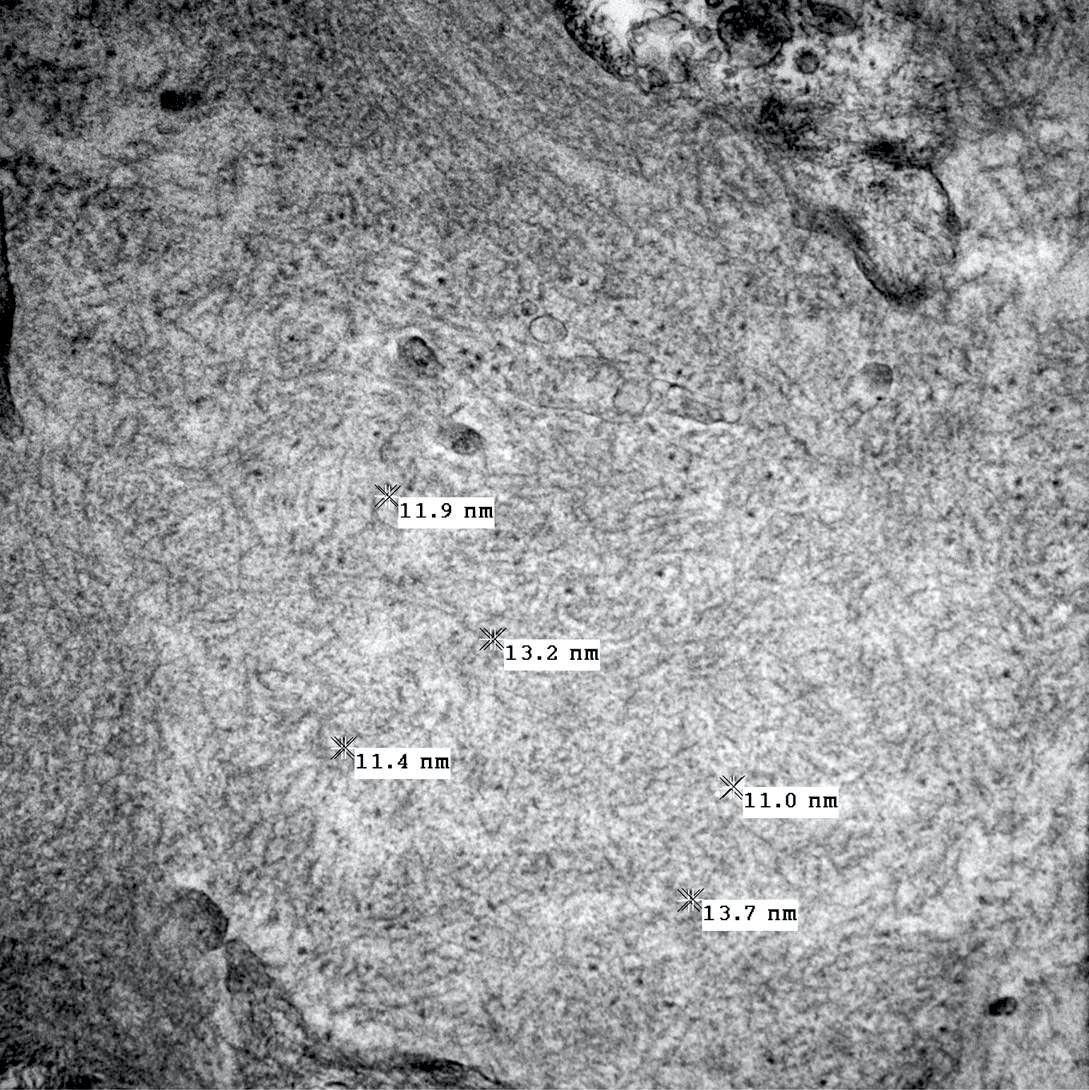
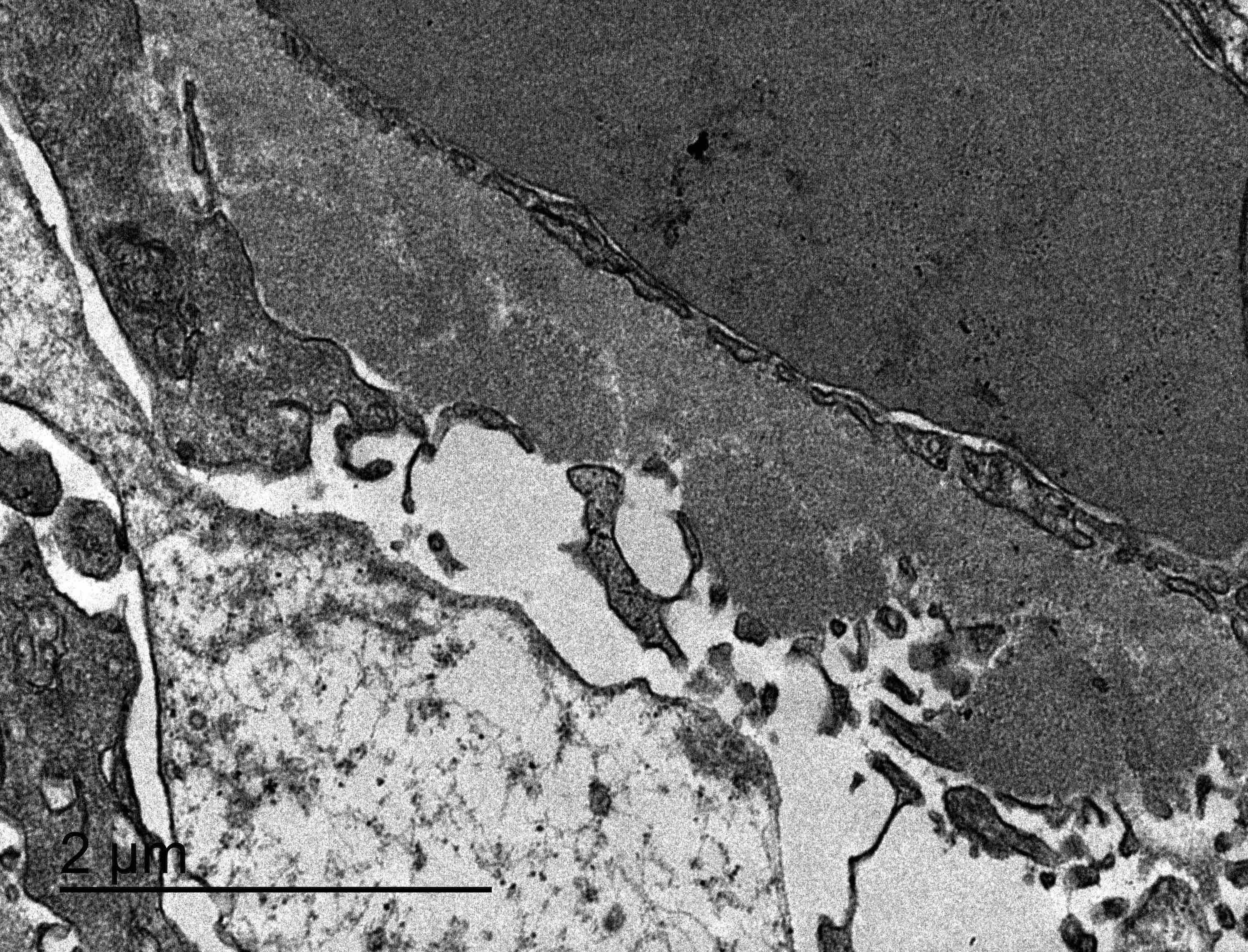
- Peritubular capillary basement membrane multilayering (ptclm) - electron microscopic evaluation of the most affected PTC
- ptclm 1: 1 PTC with ≥ 7 layers +2 PTC with ≥ 5 layers
- Reference: Transplantation 2018;102:1795
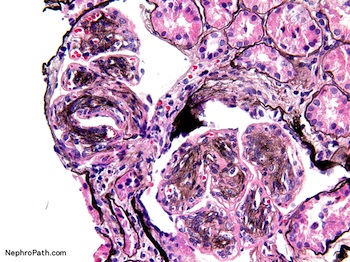
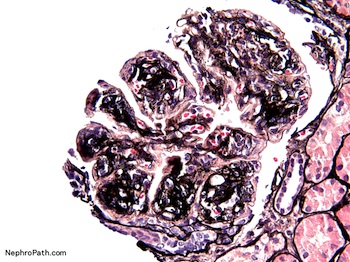
Images hosted on other servers:
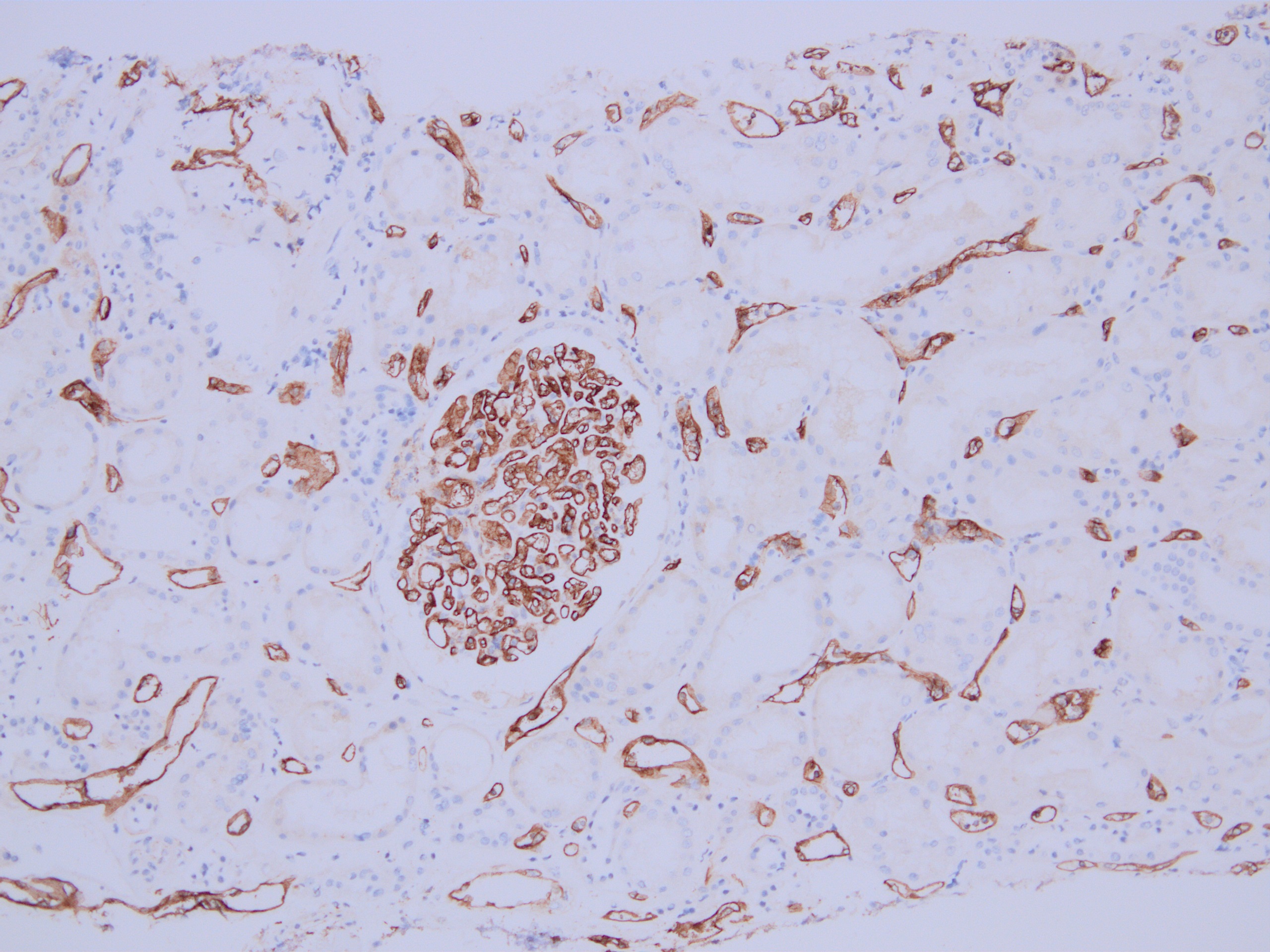
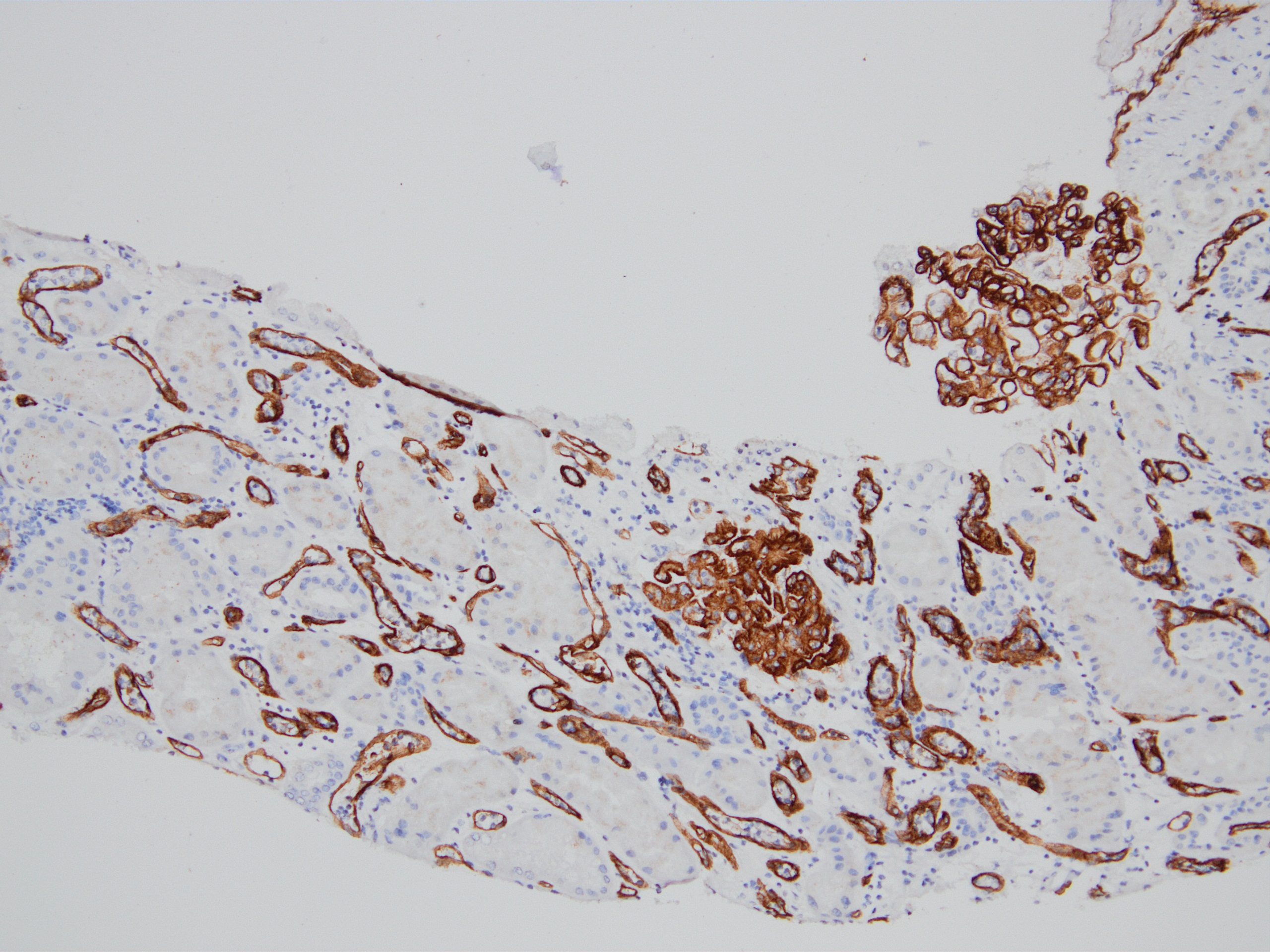
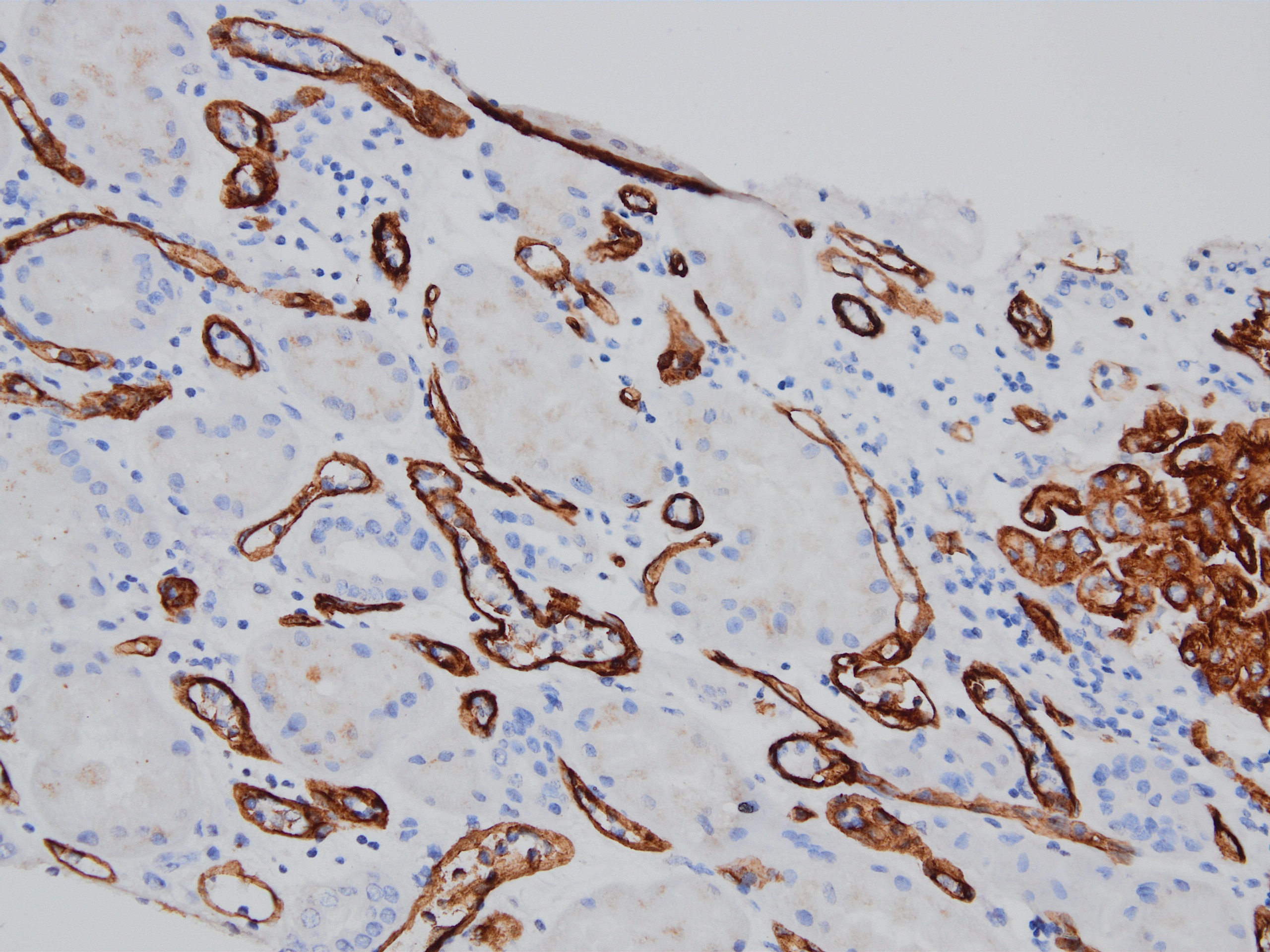
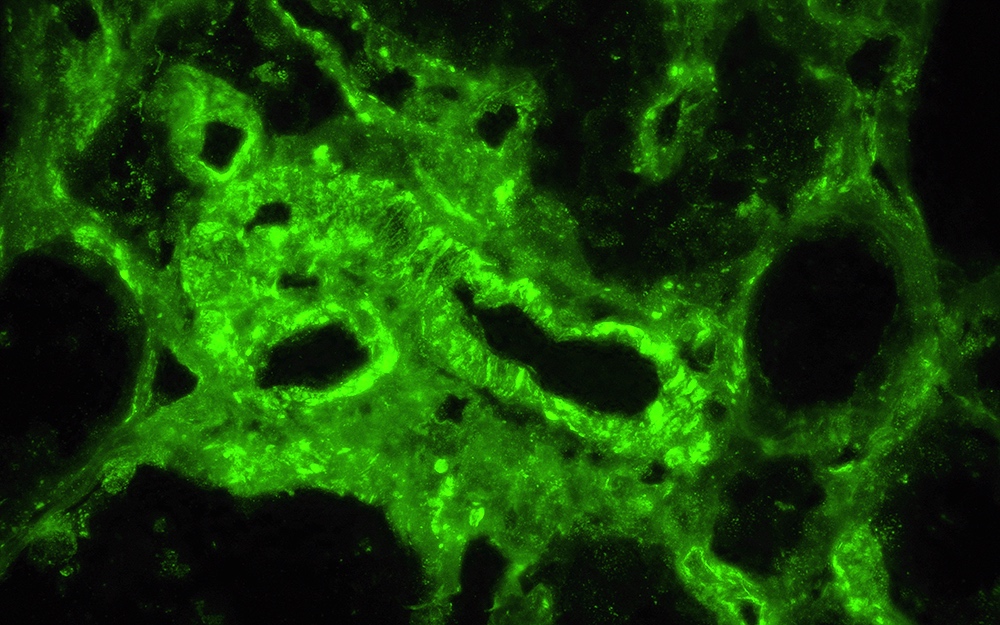
Contributed by Arzu Sağlam, M.D.
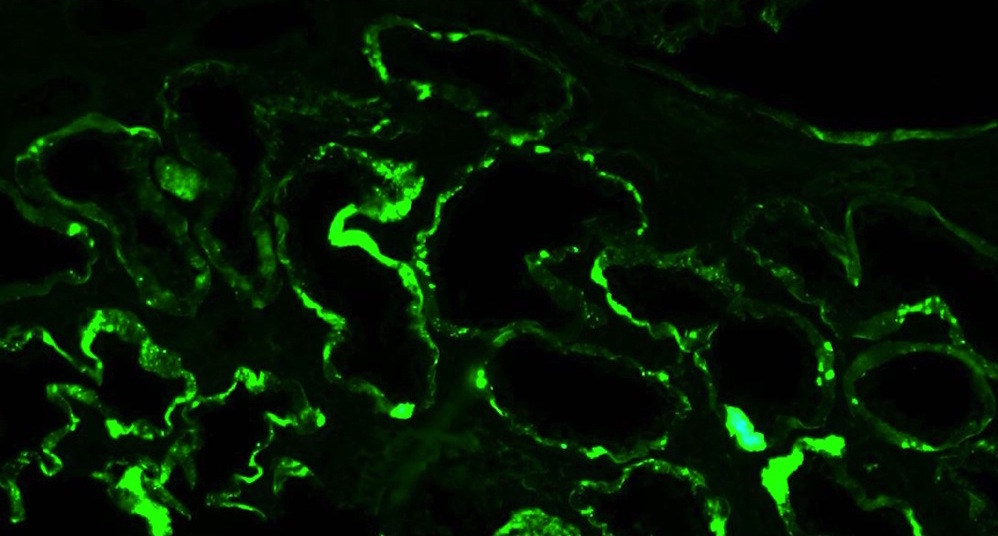
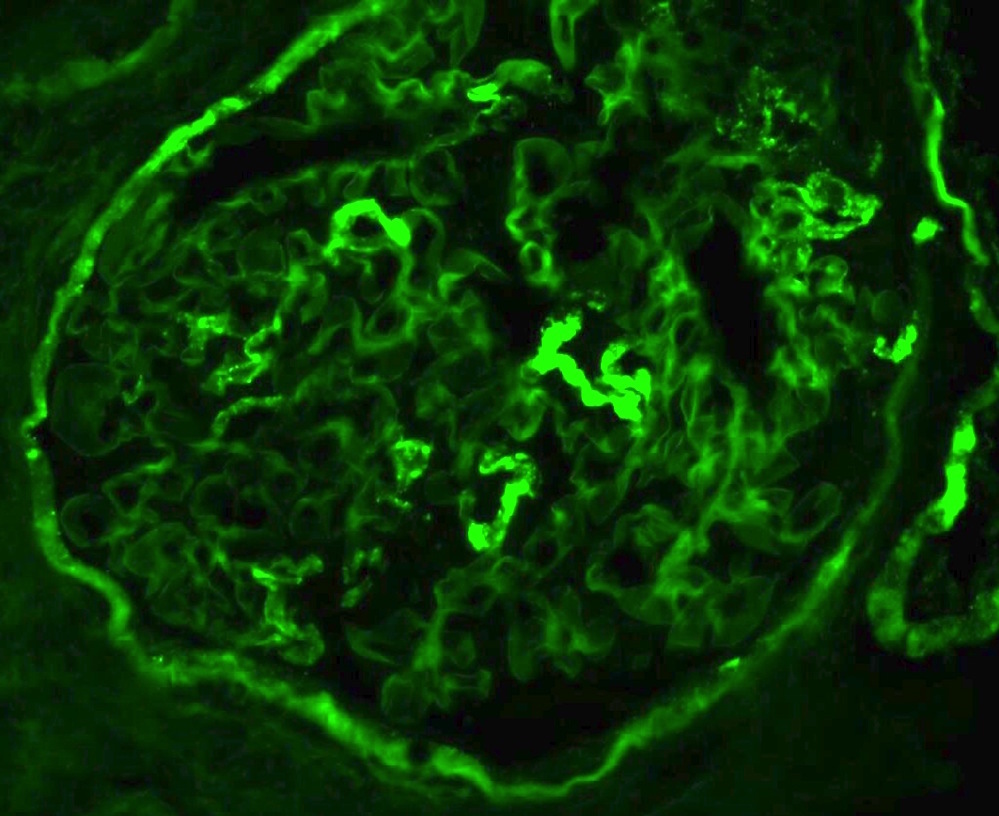
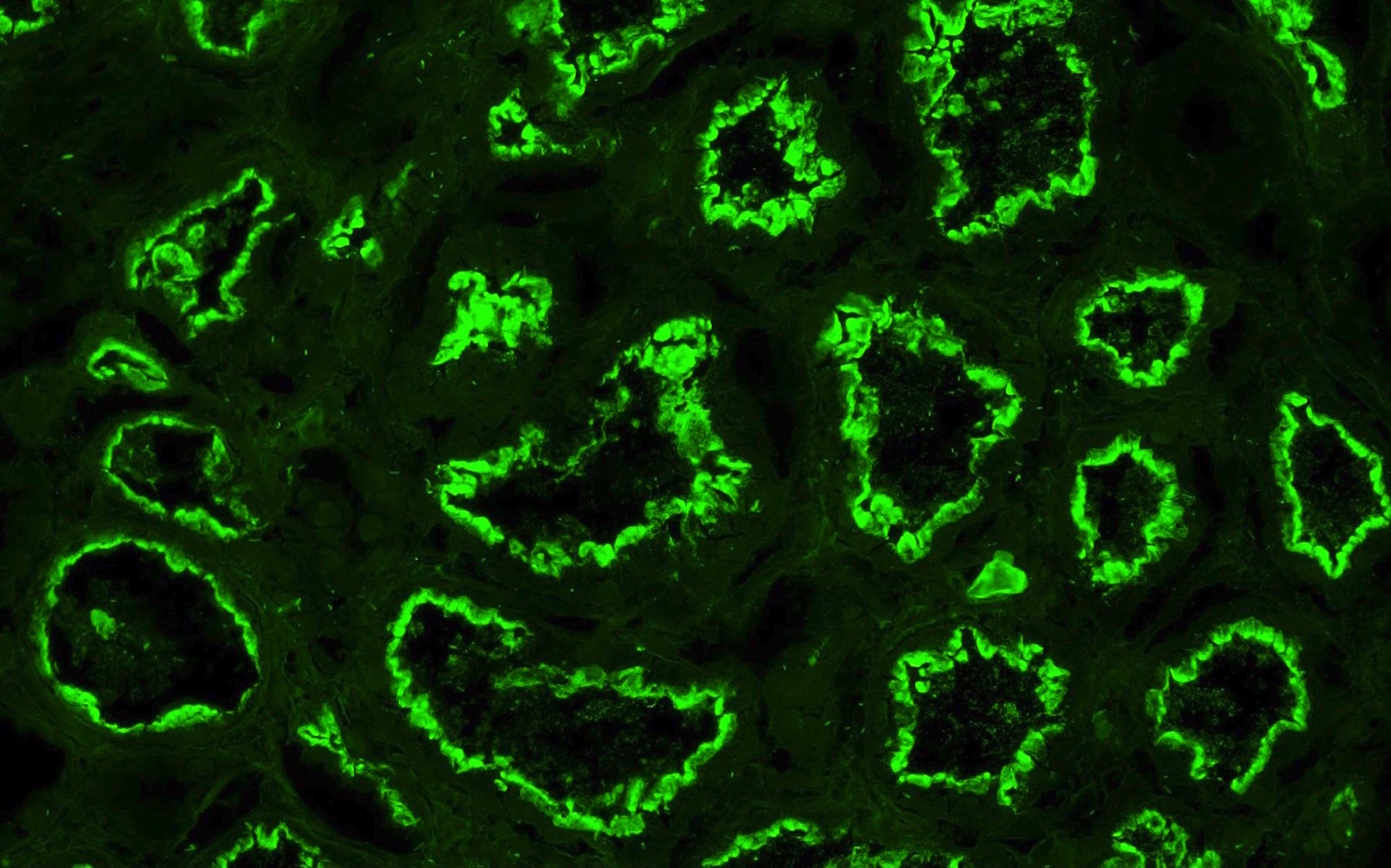
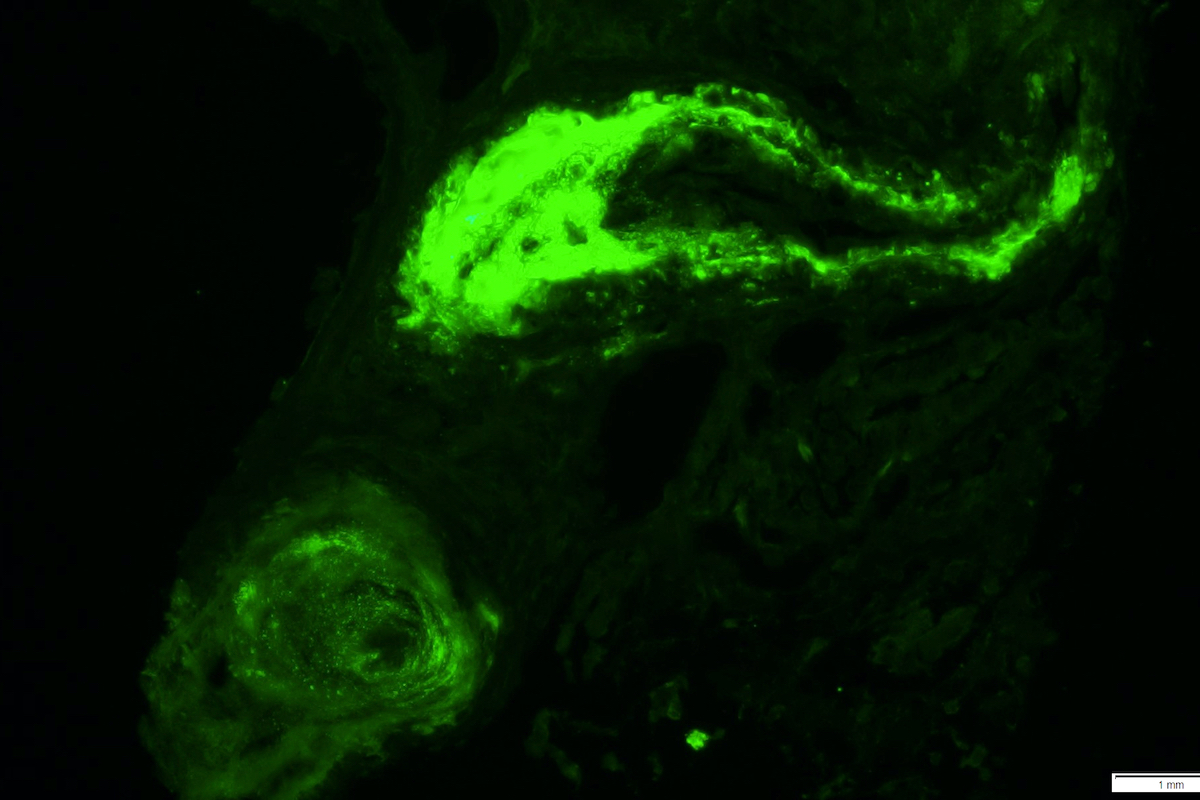
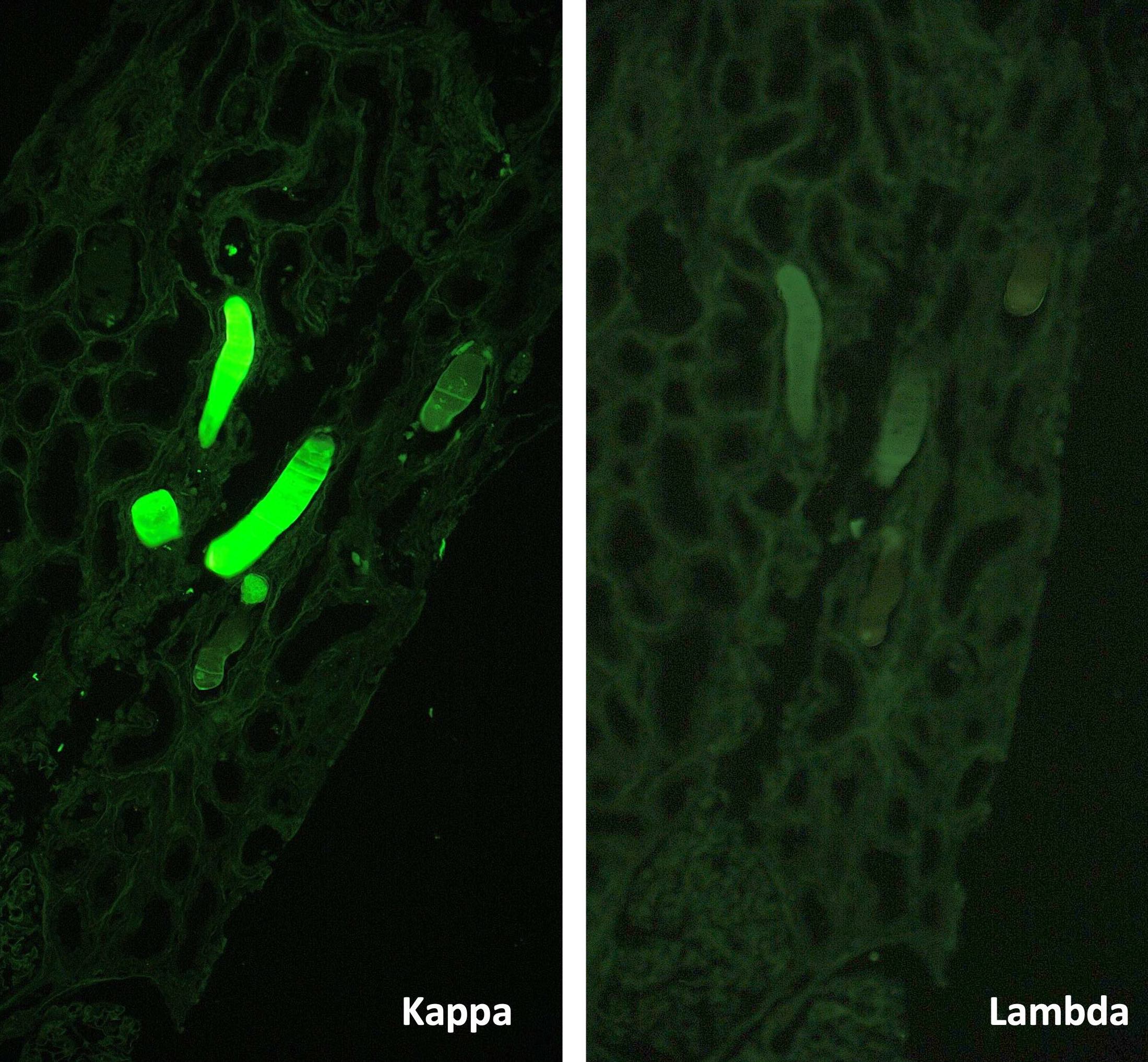
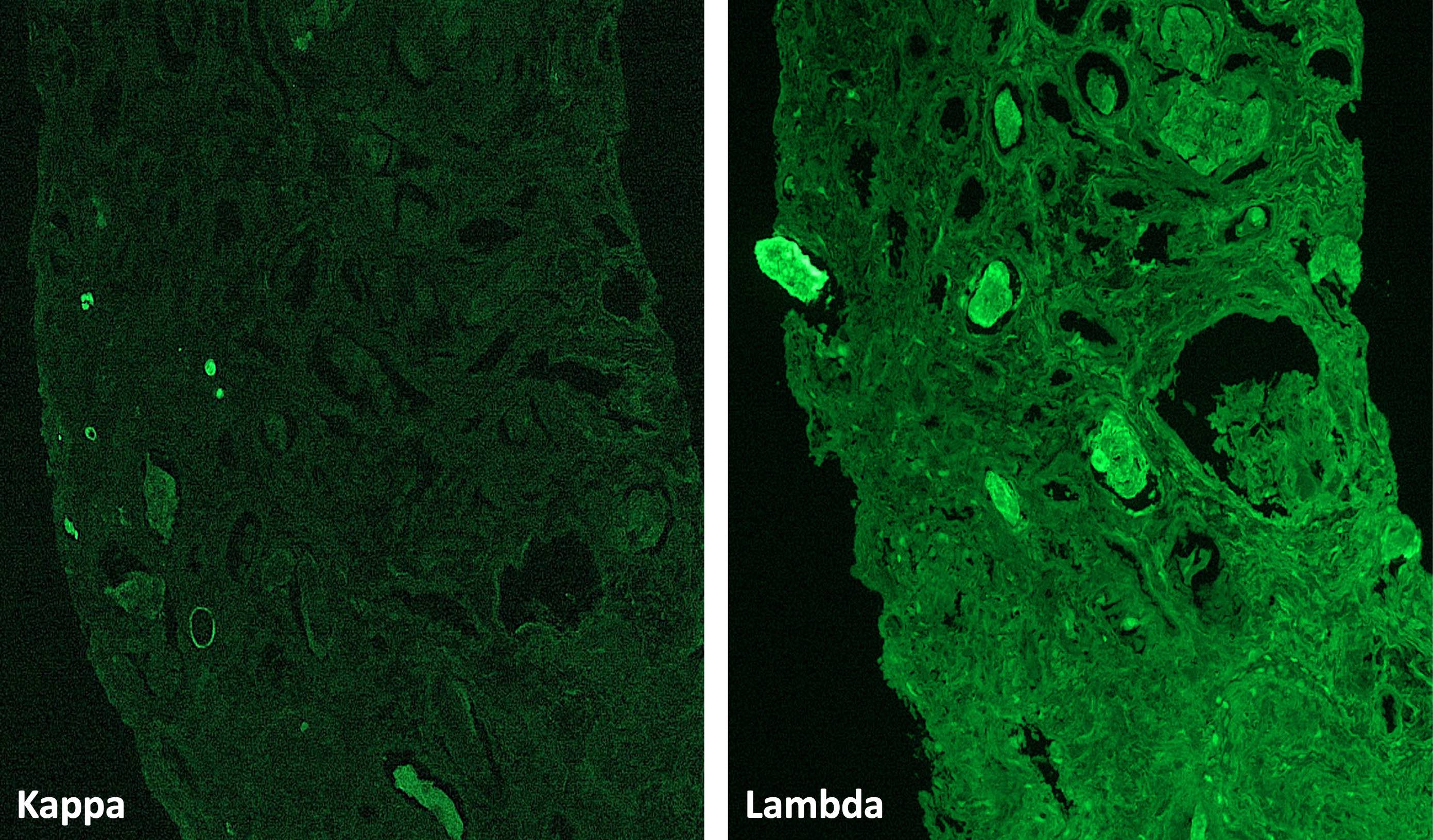
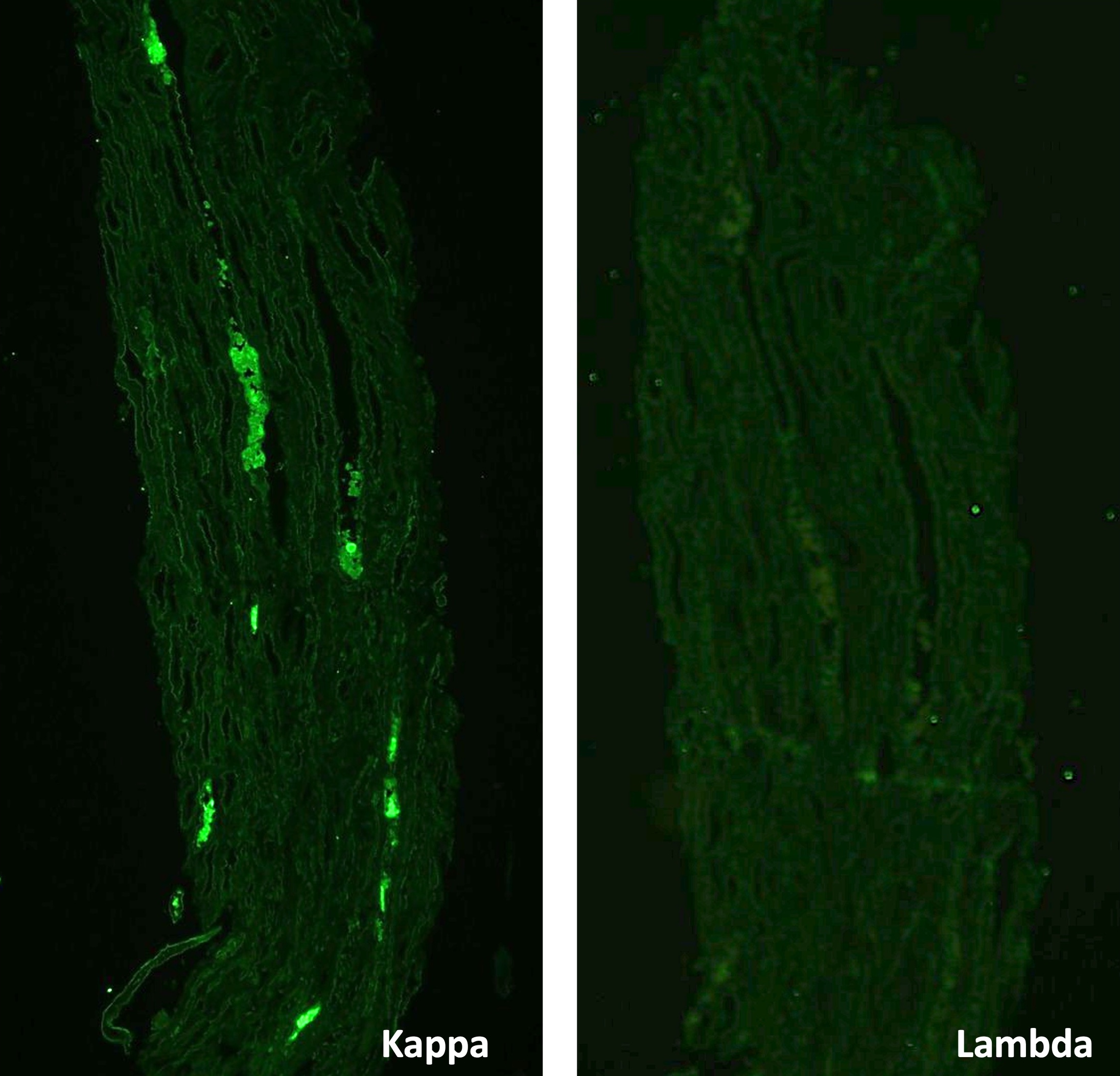
- No immunoglobulin or only nonspecific scarce staining
- C3 may be present along PTC
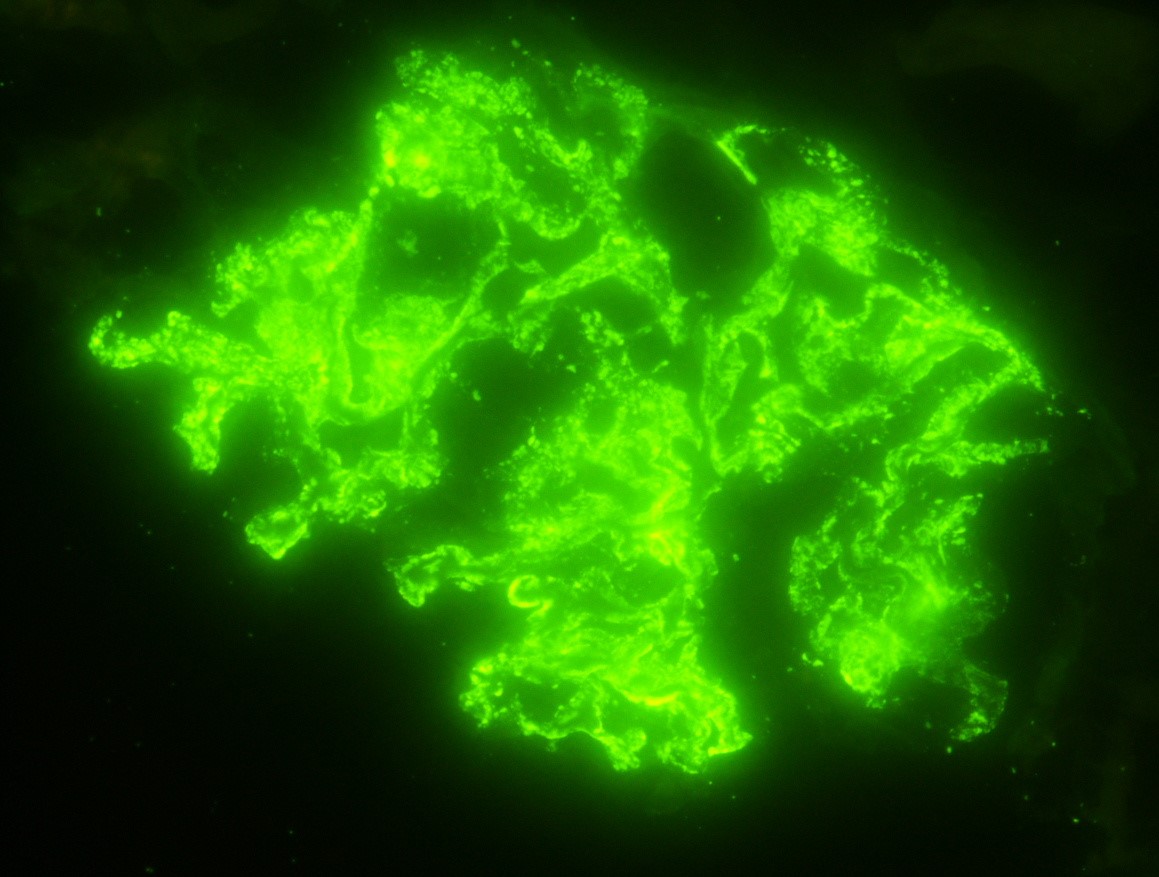
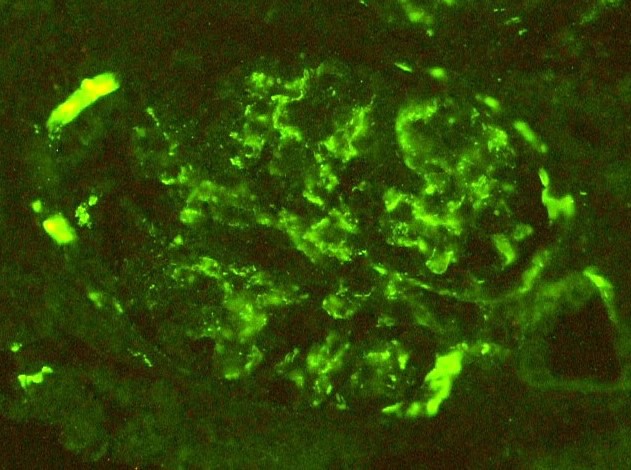
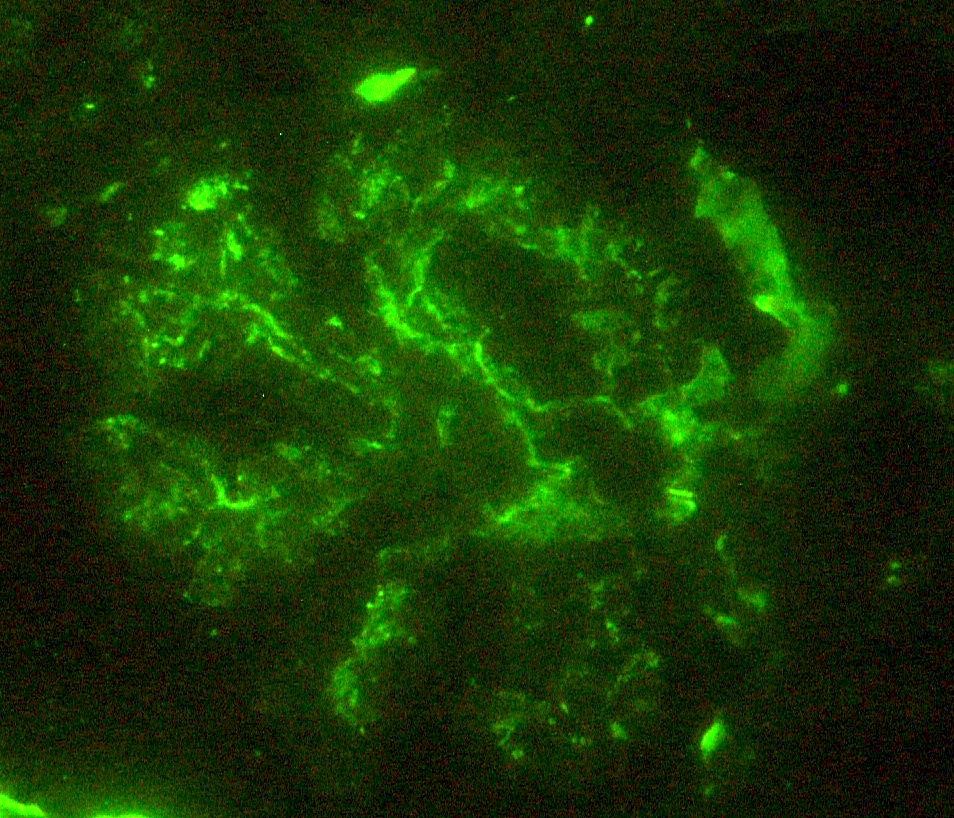
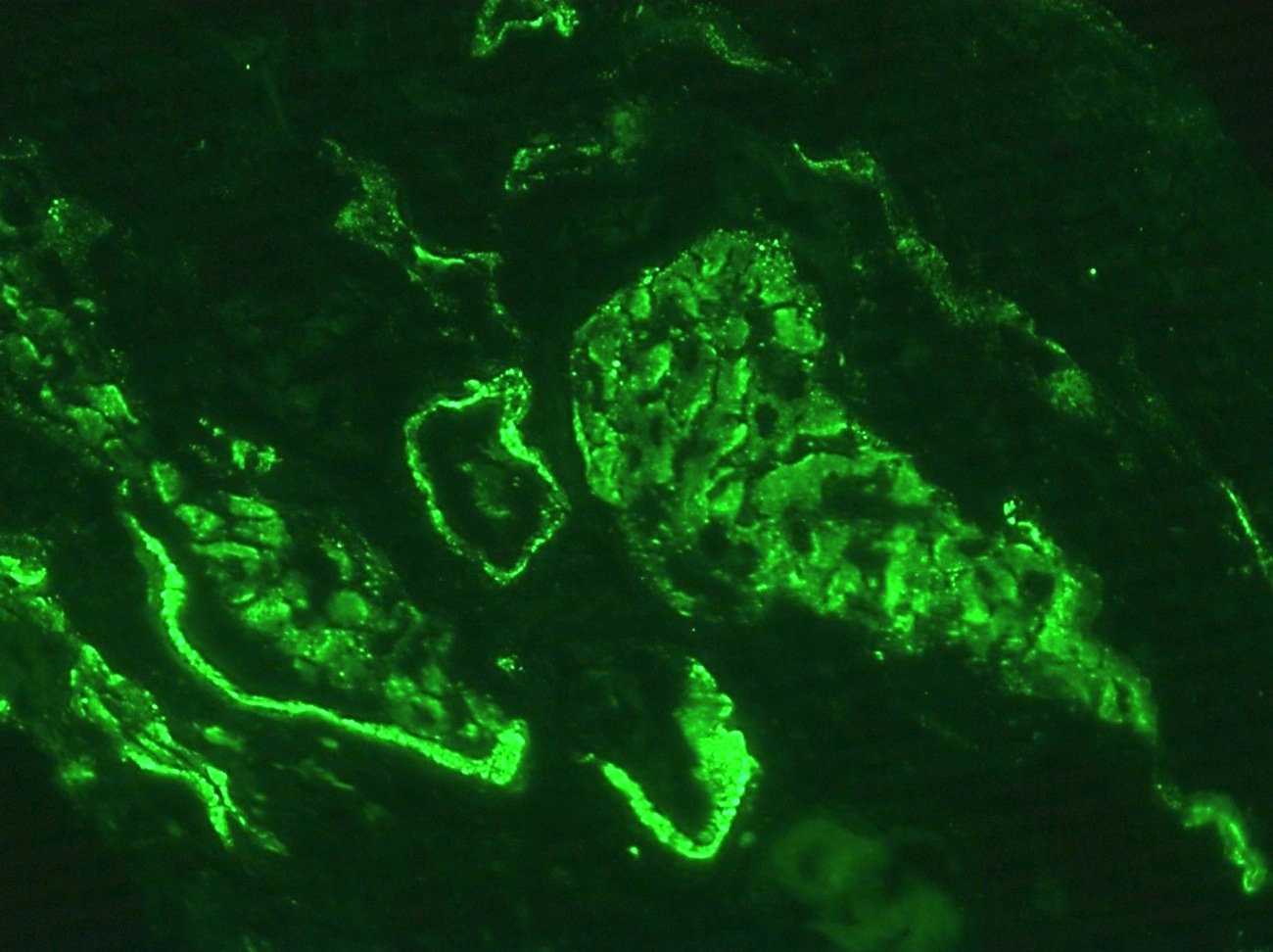
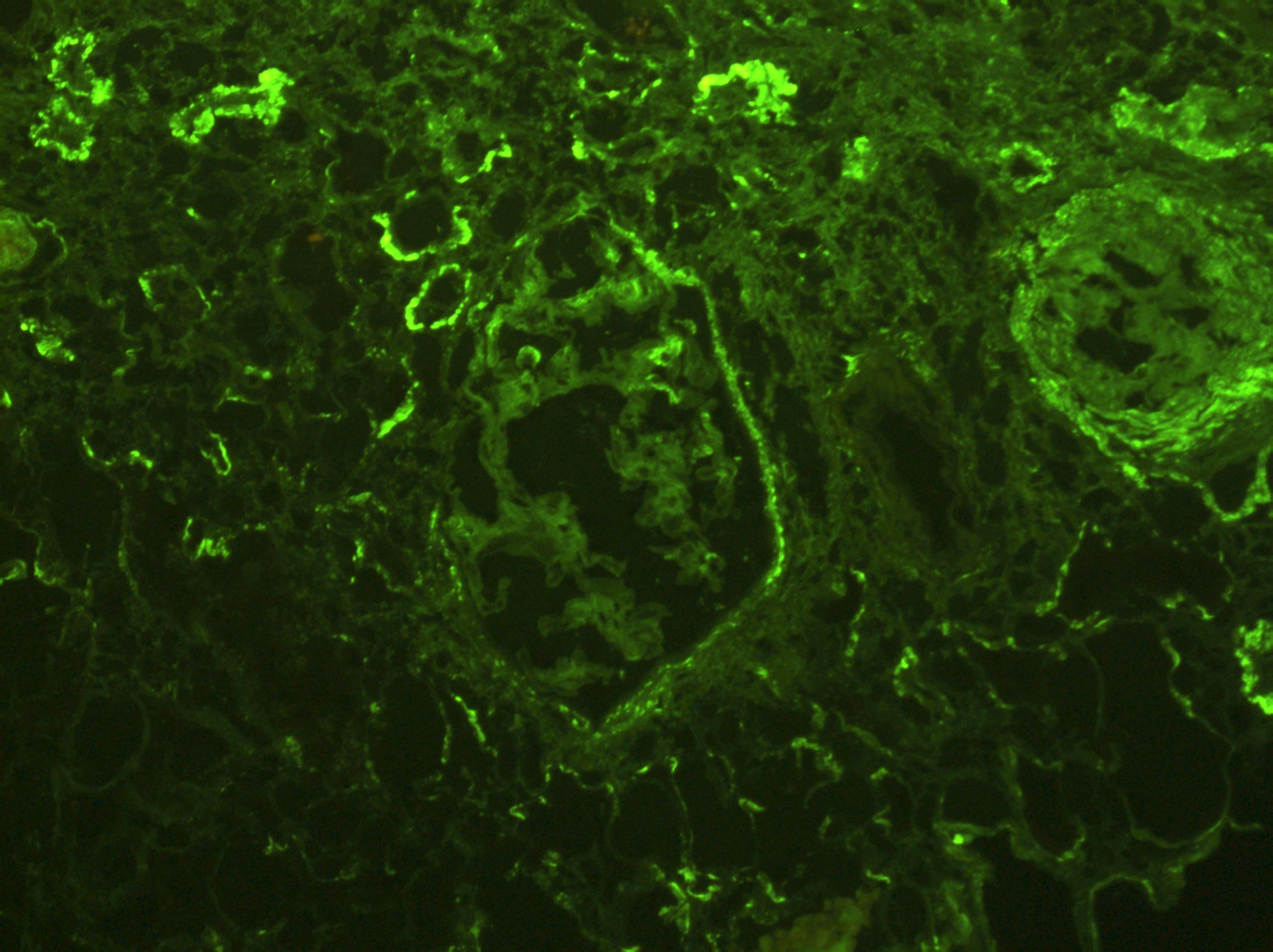
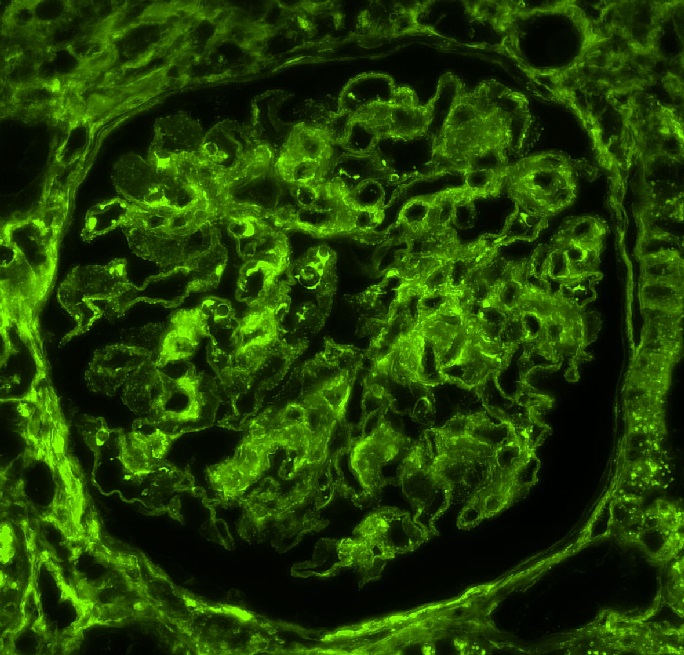
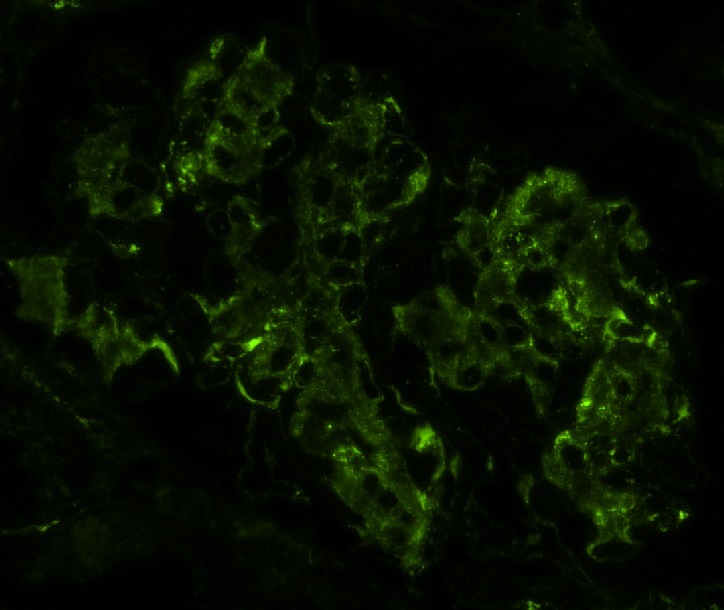
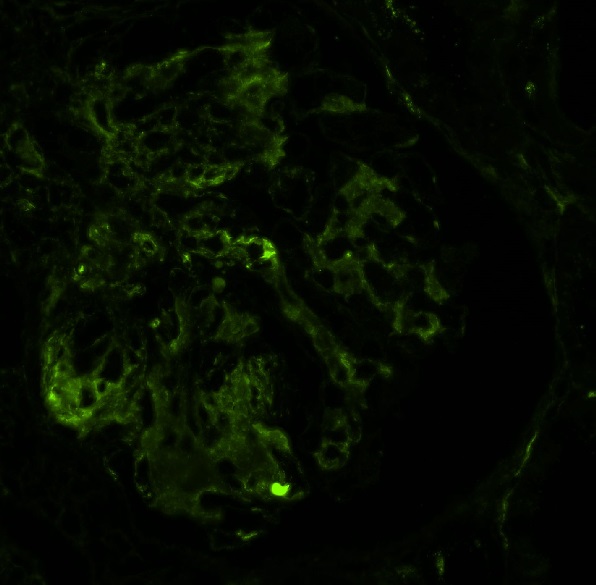
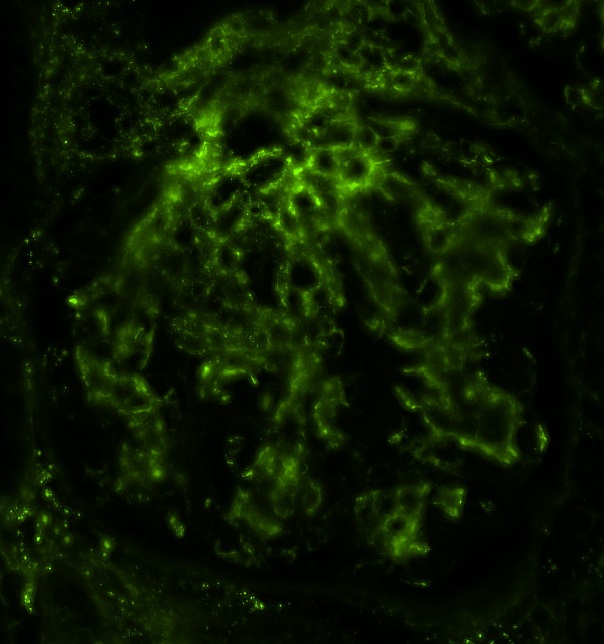
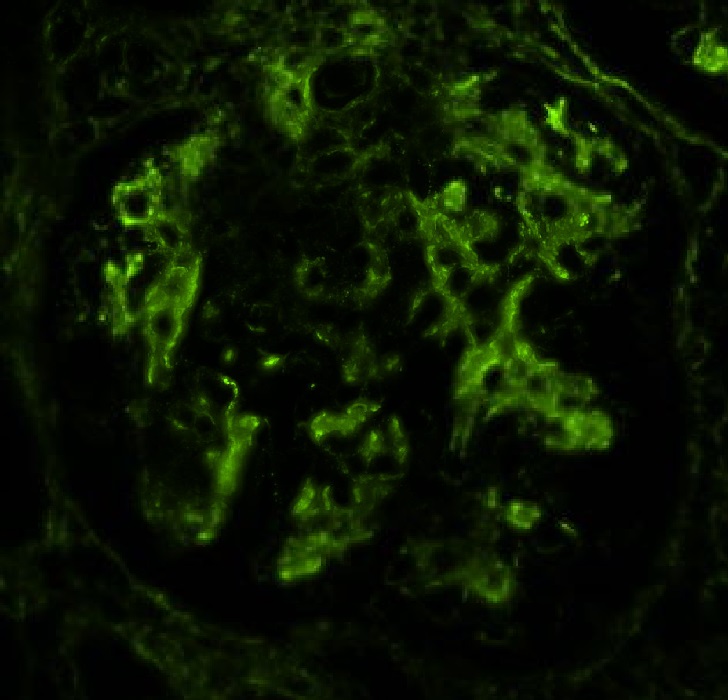
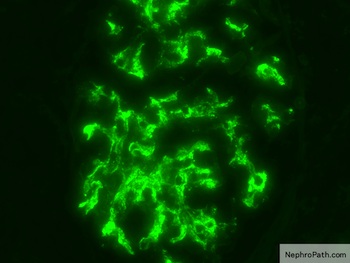
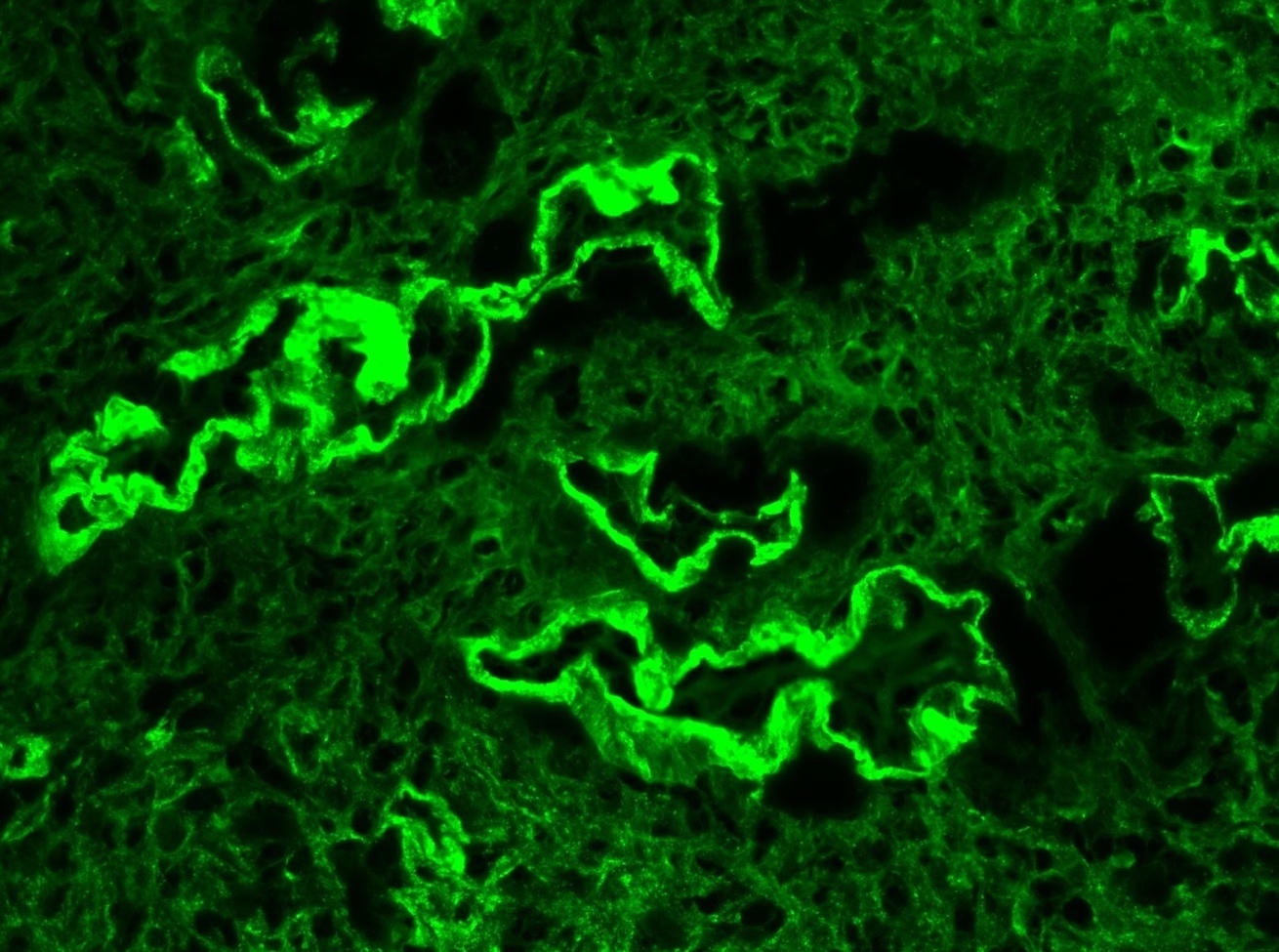
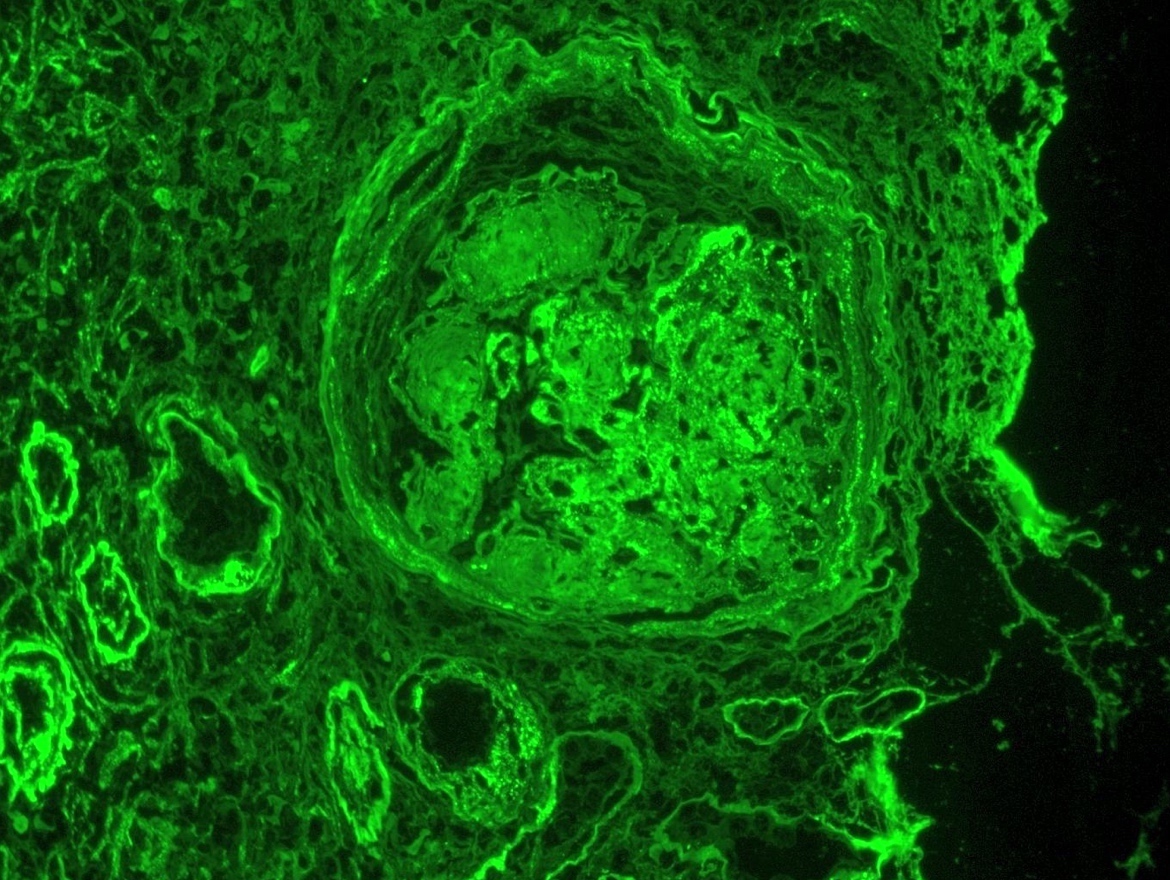
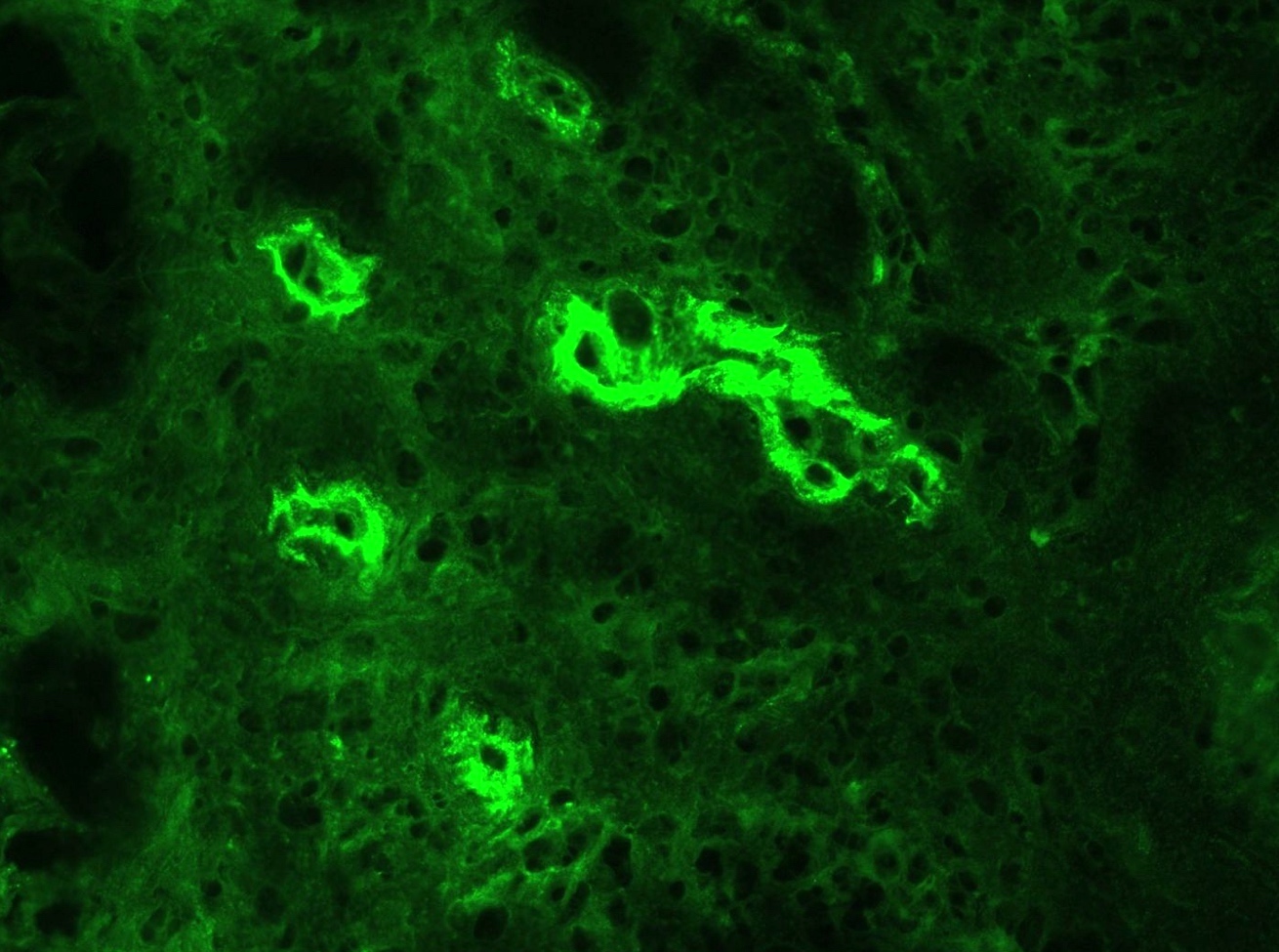
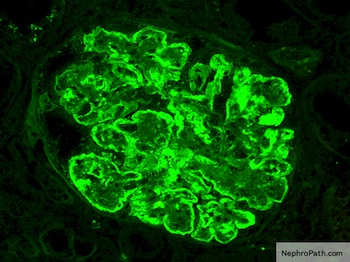
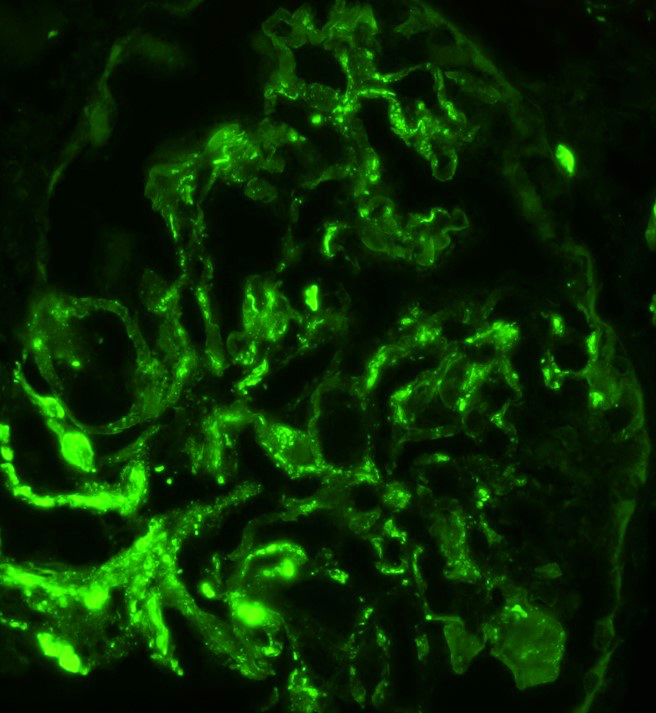
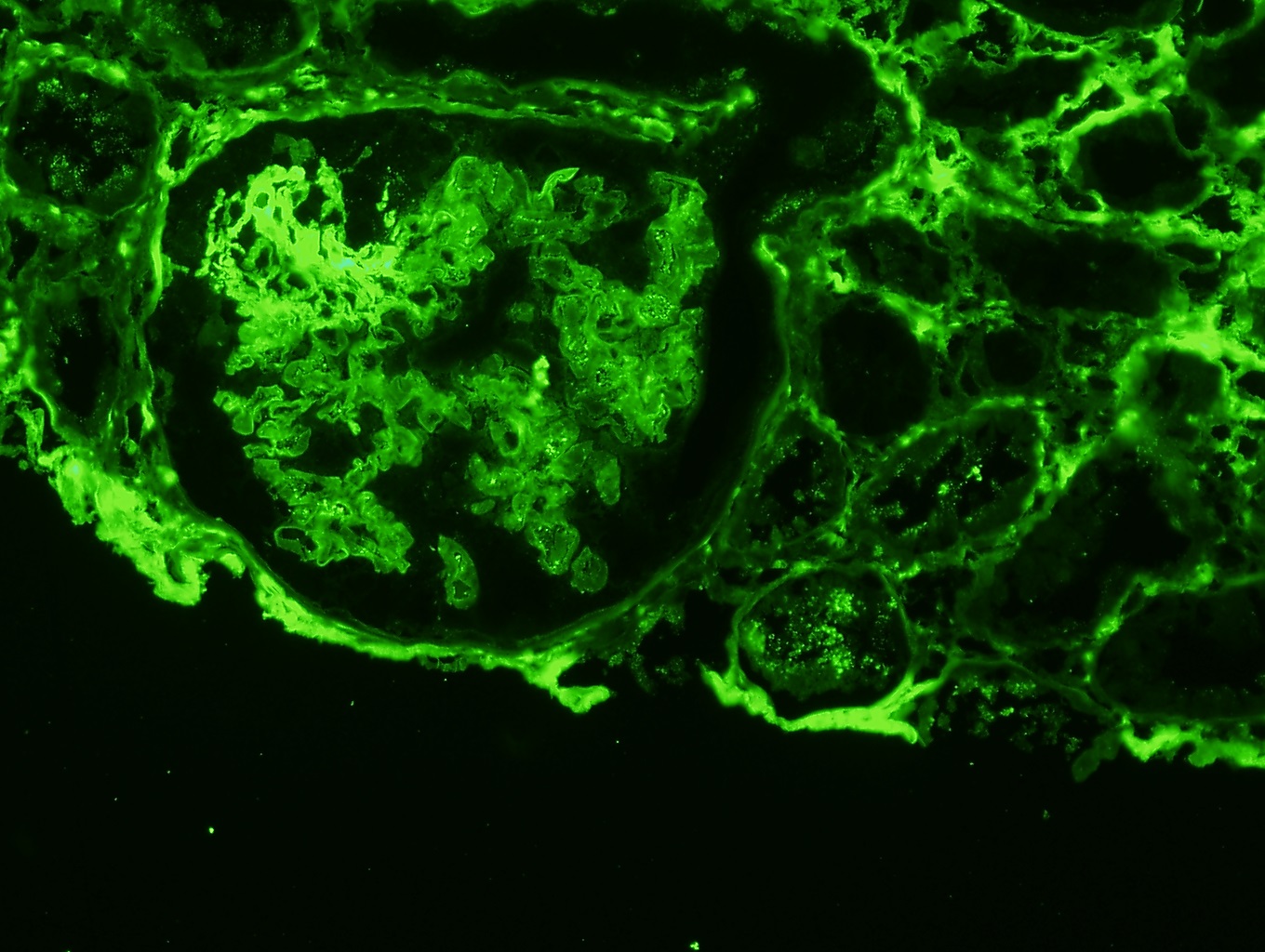
- C4d linear staining in peritubular capillaries (C4d2 or C4d3)
- References: Mod Pathol 2018;31:235, Transplantation 2018;102:1795
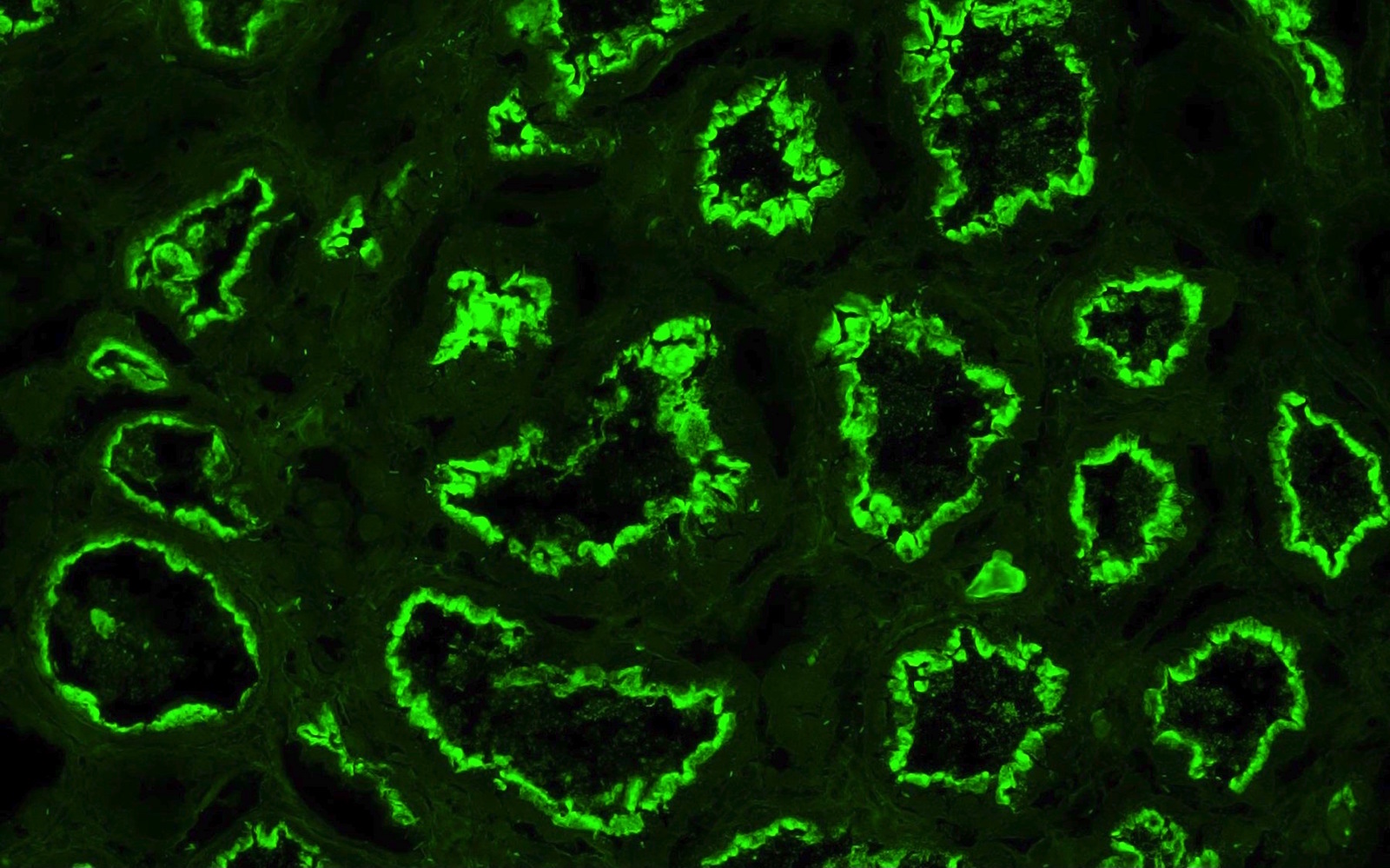
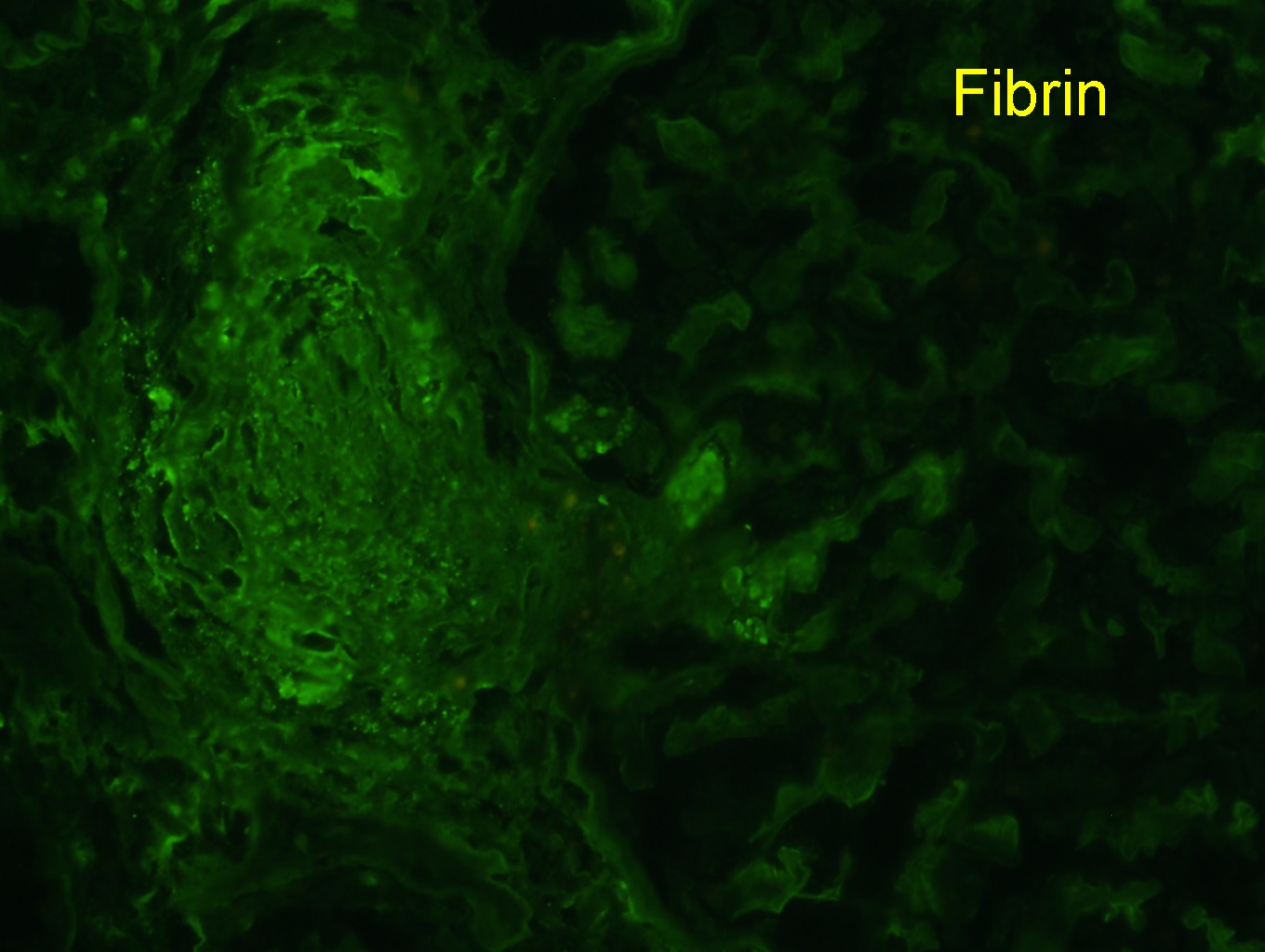
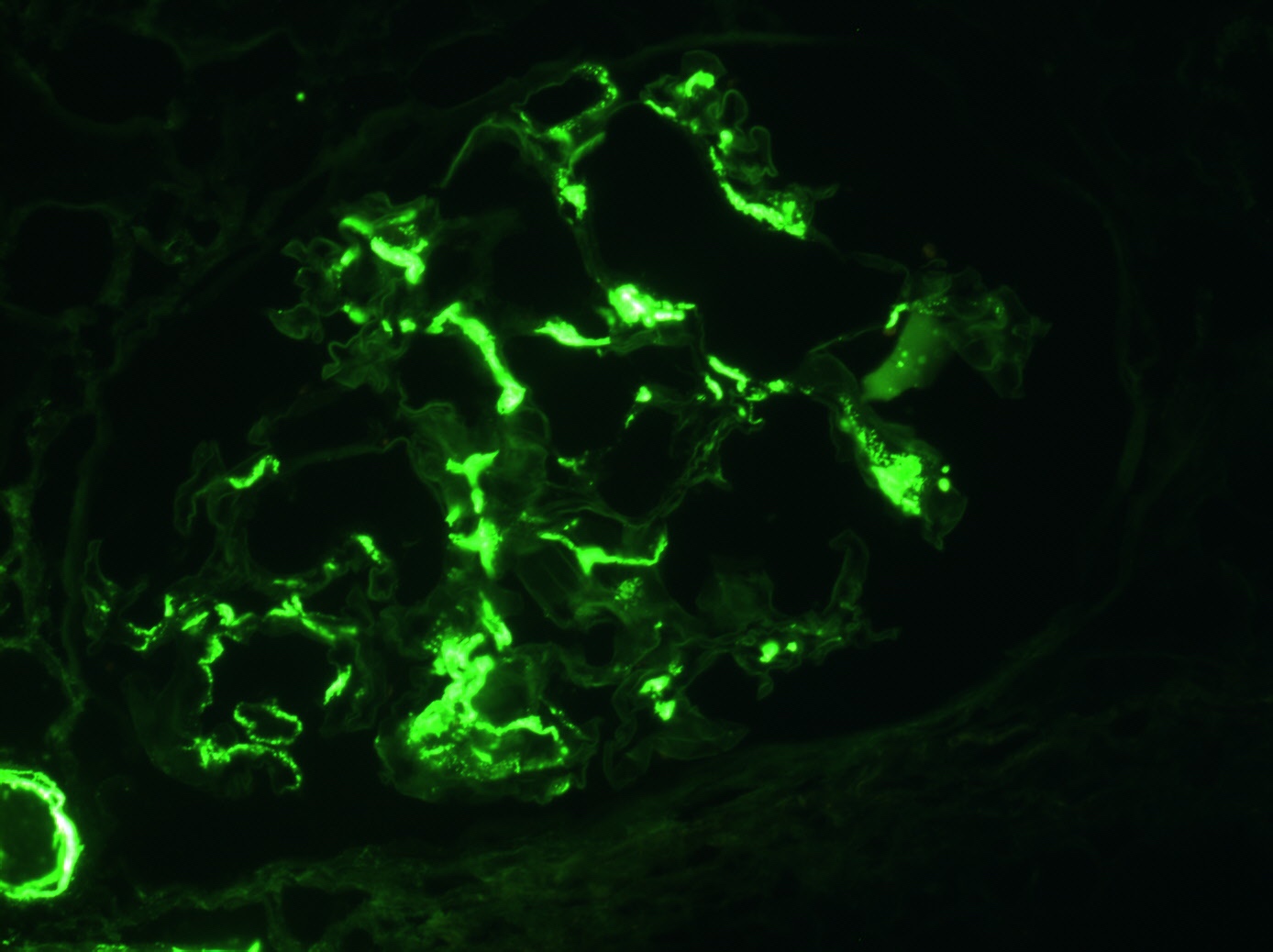
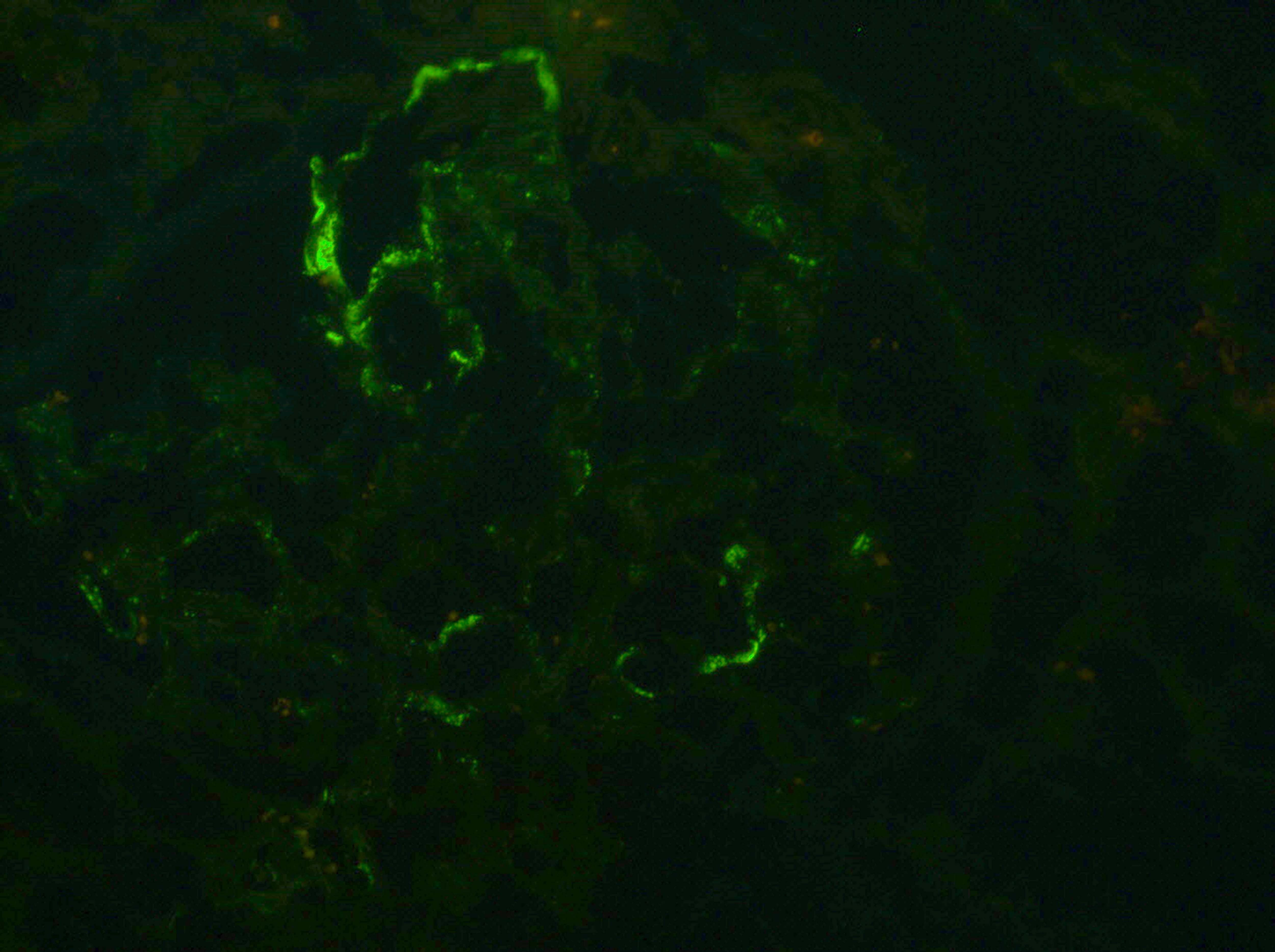
- C4d linear staining in peritubular capillaries (C4d2 or C4d3 by immunofluorescence on frozen sections or C4d > 0 by immunohistochemistry on paraffin sections)
- This is an indirect sign of complement activation - deposition of the complement split product C4d
- May be negative in chronic active antibody mediated rejection (ABMR)
- Can also be present along glomerular capillary basement membrane in addition to peritubular capillaries or sometimes solely (in which case it is supportive of transplant glomerulopathy and chronic active ABMR)
- References: Mod Pathol 2018;31:235, Transplantation 2018;102:1795
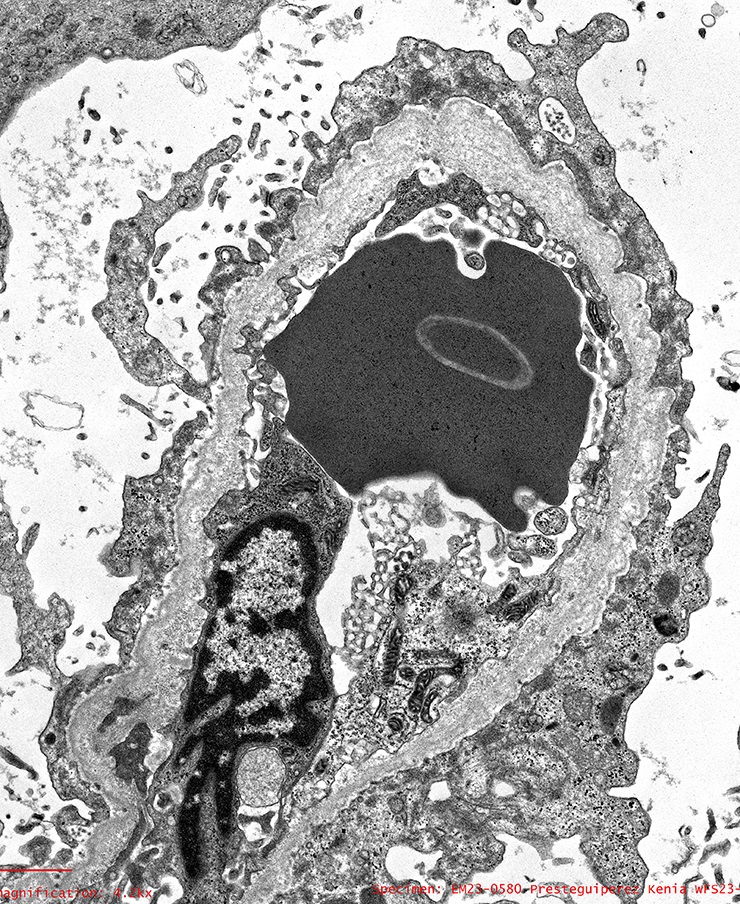
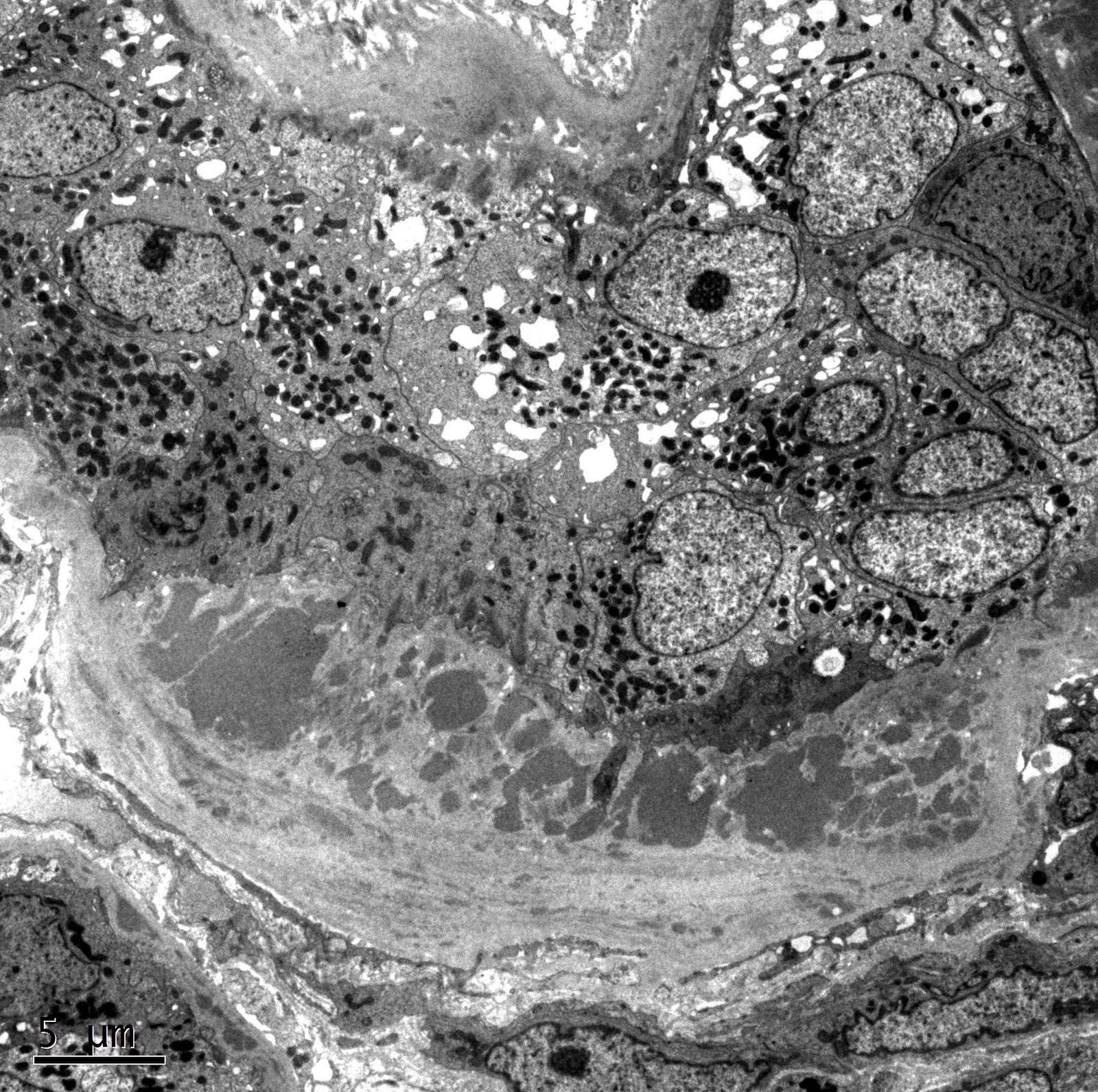
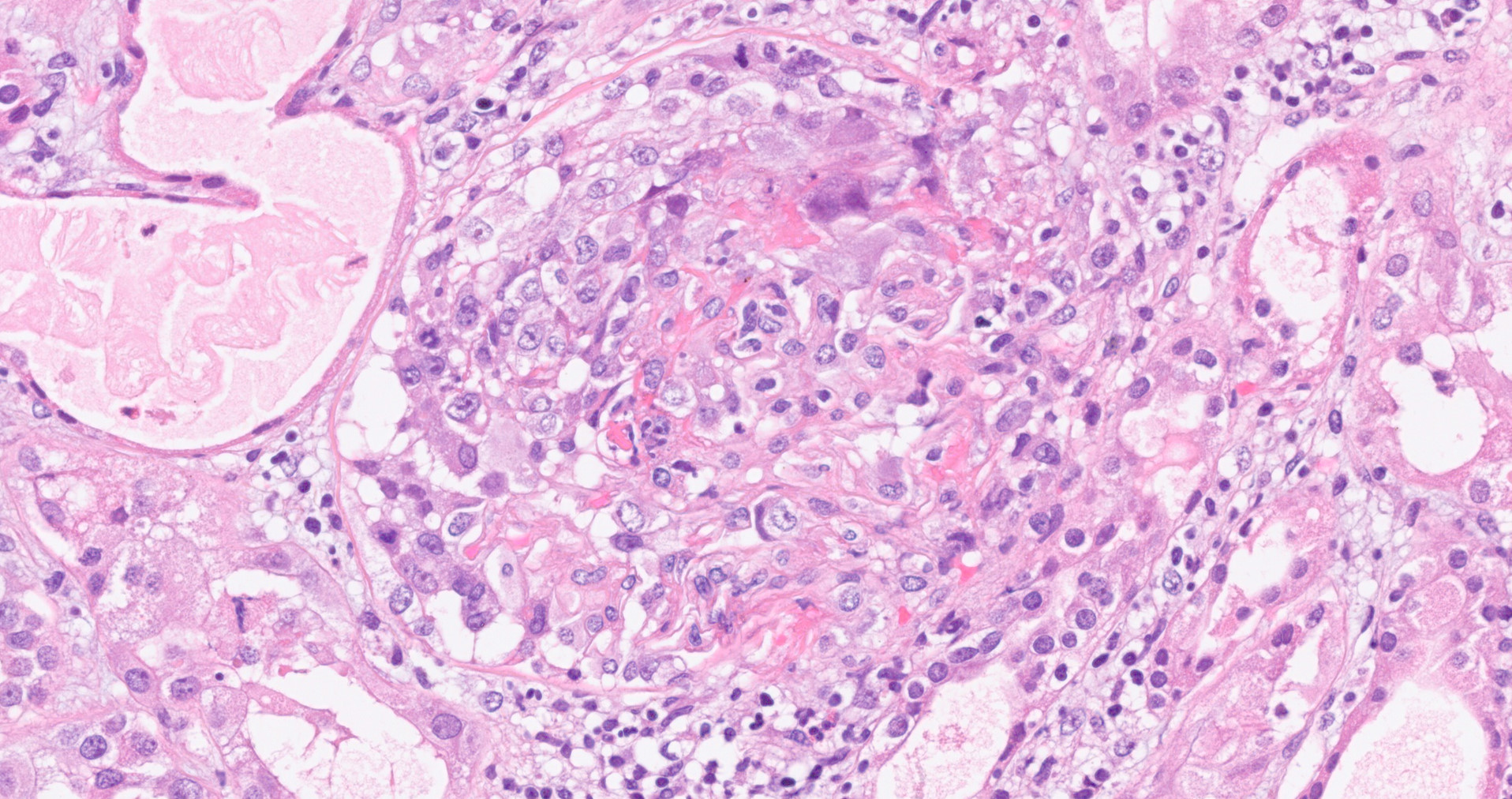
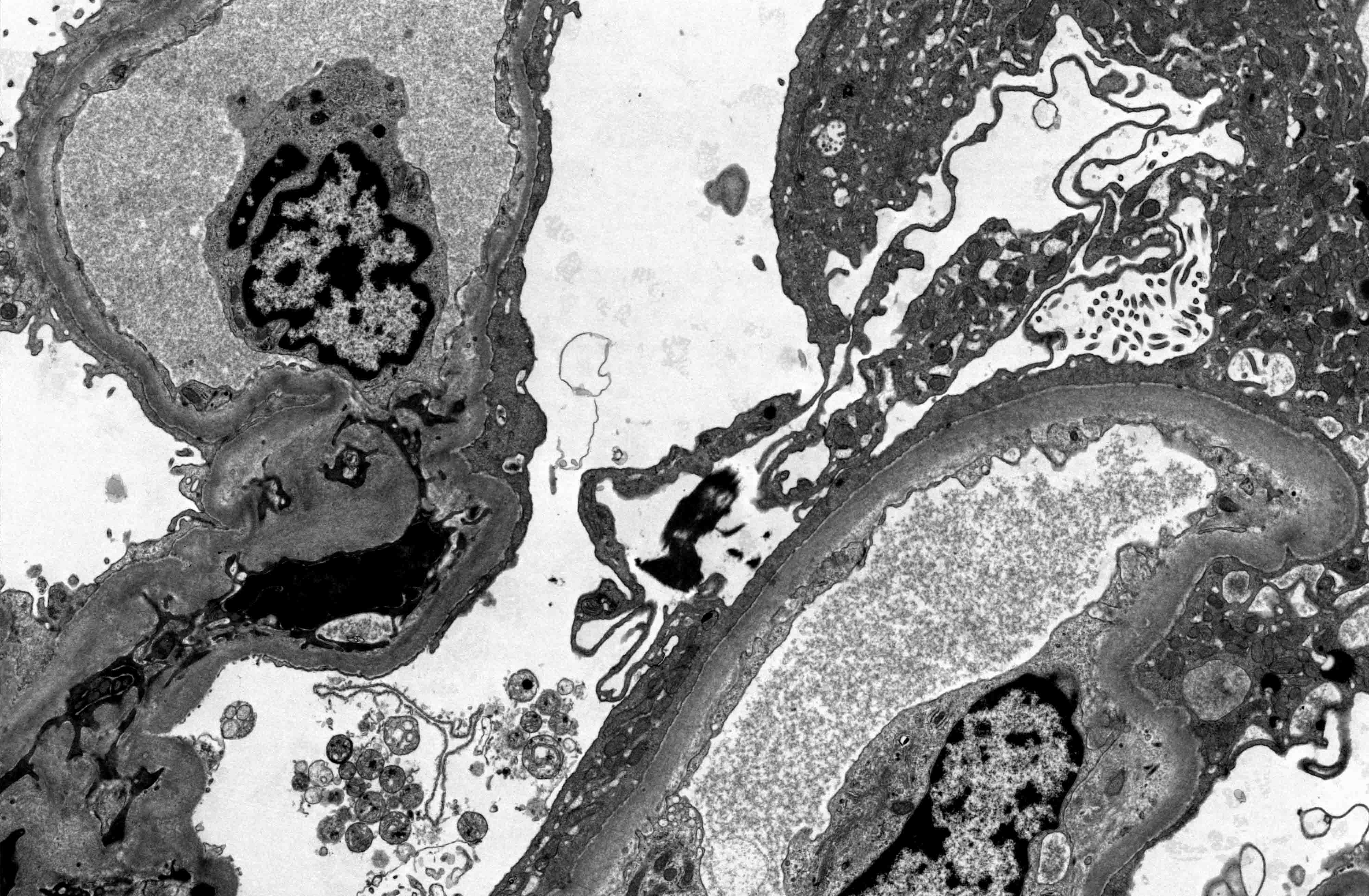
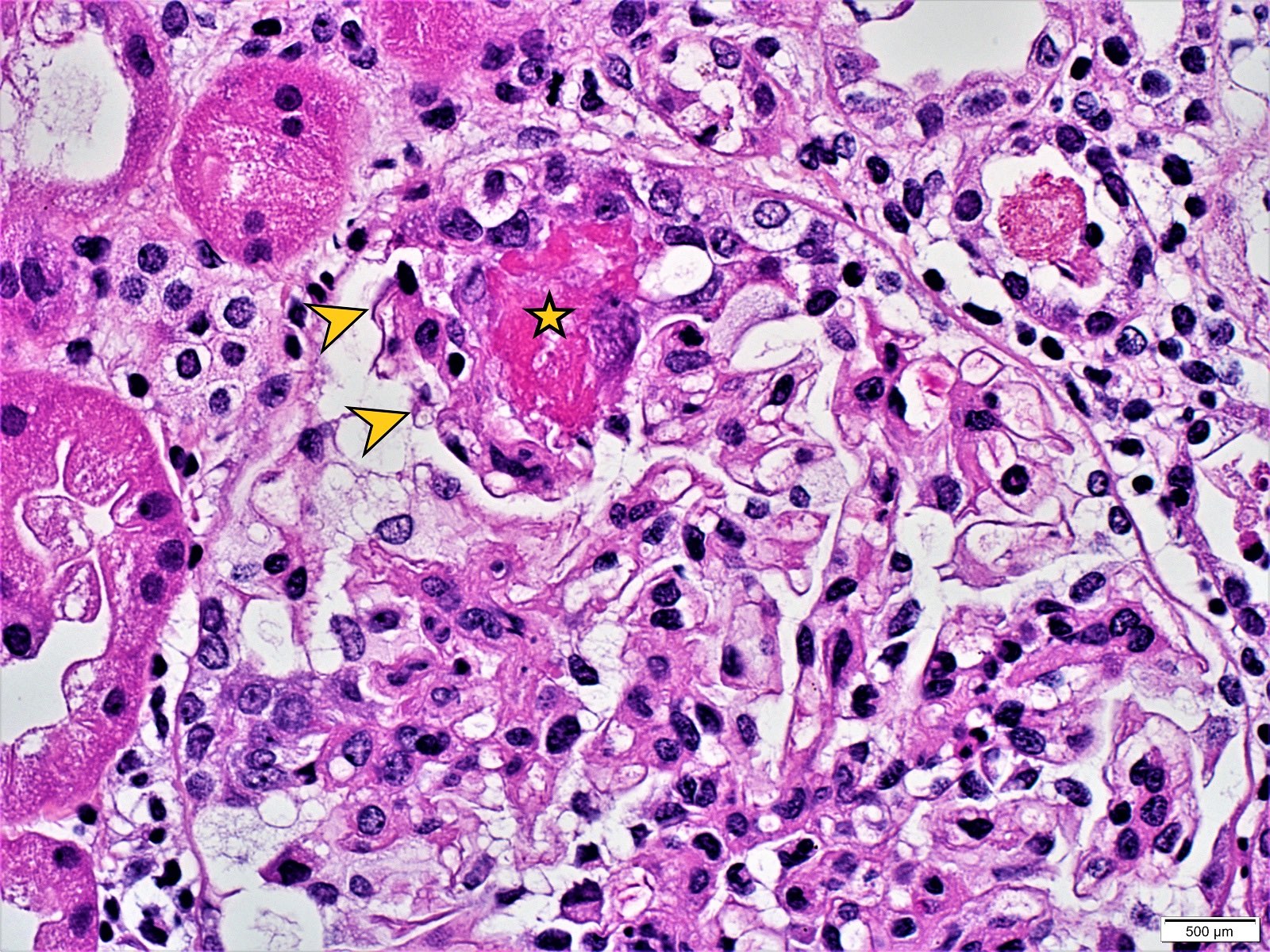
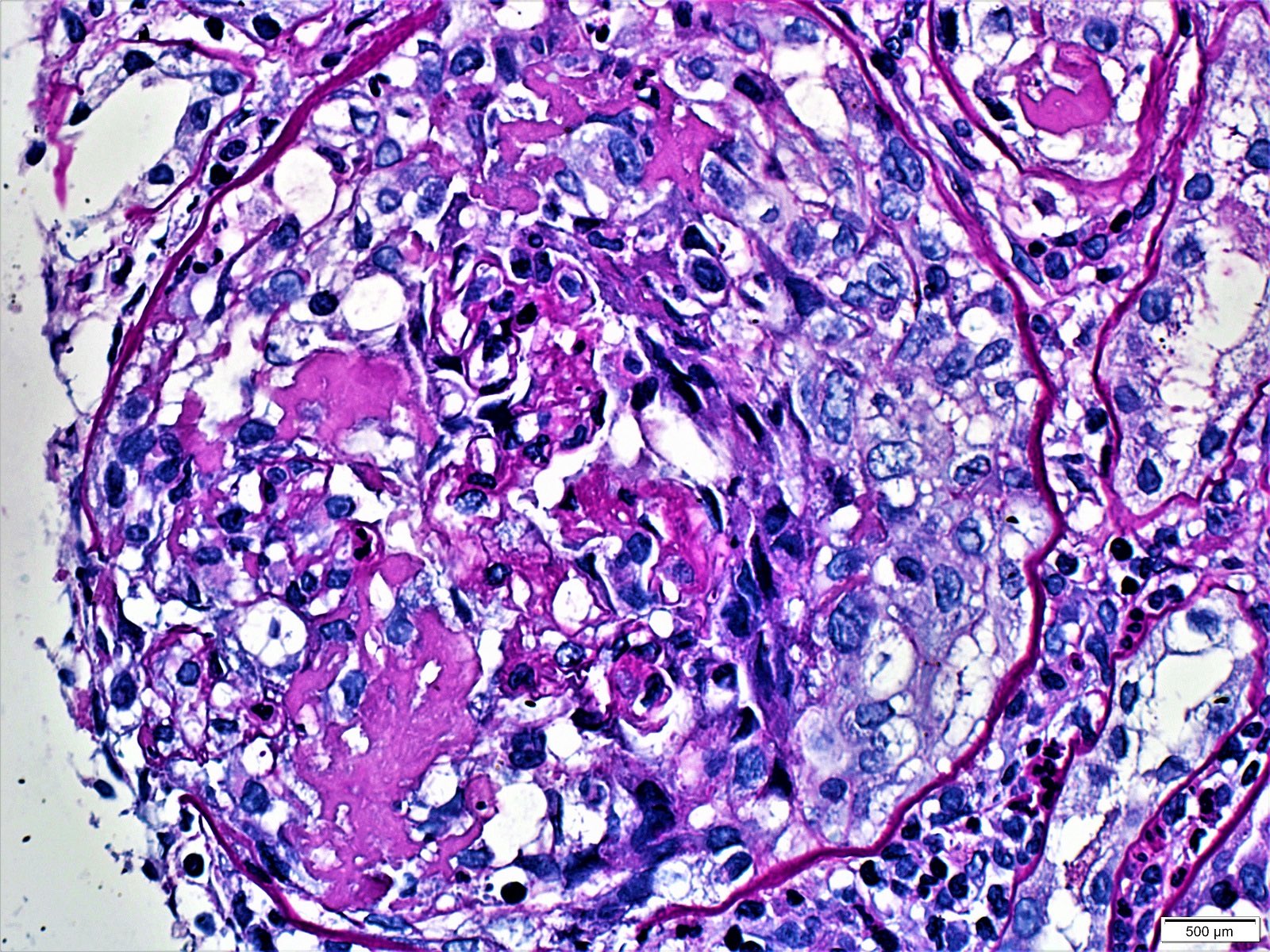
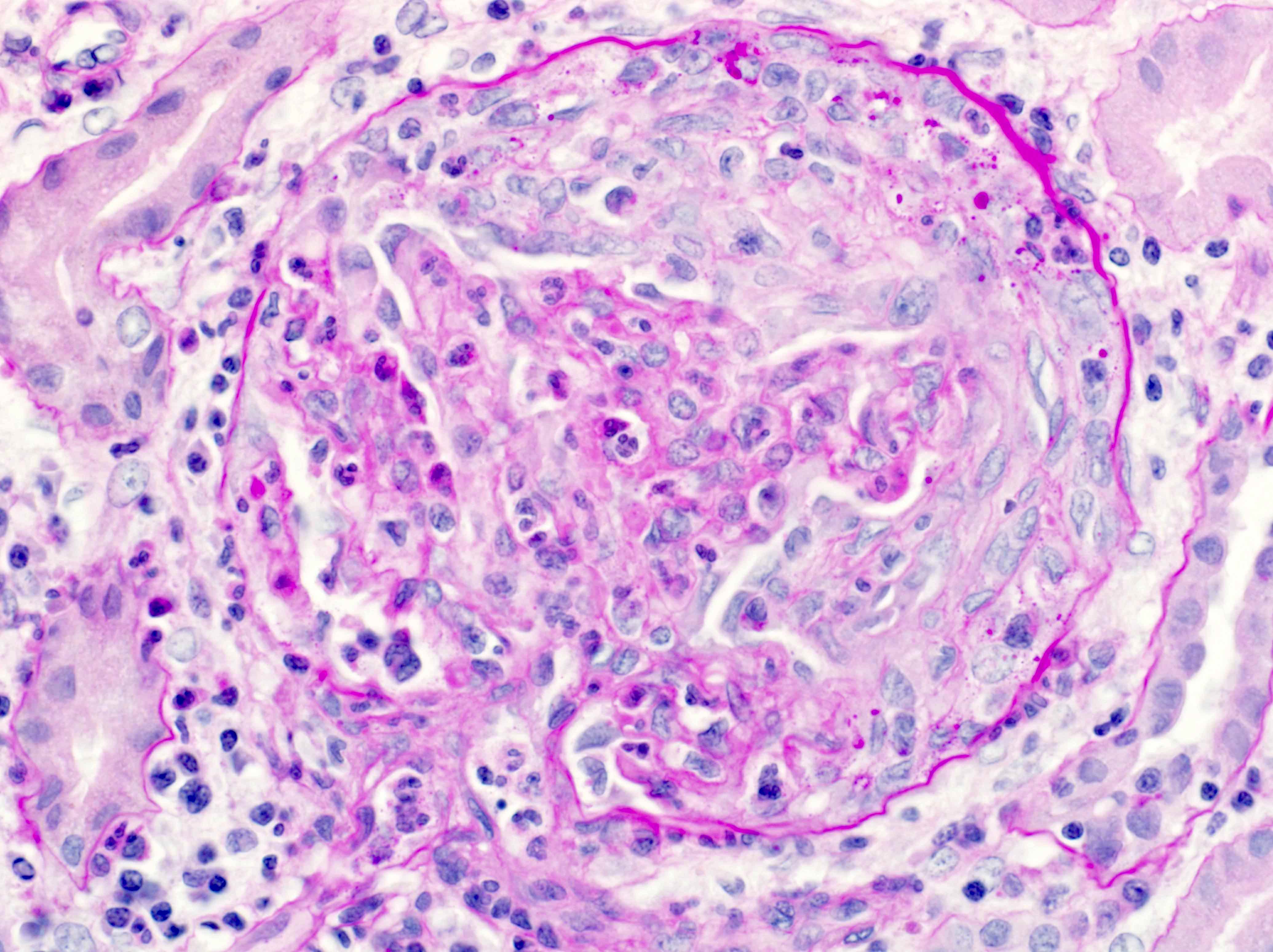
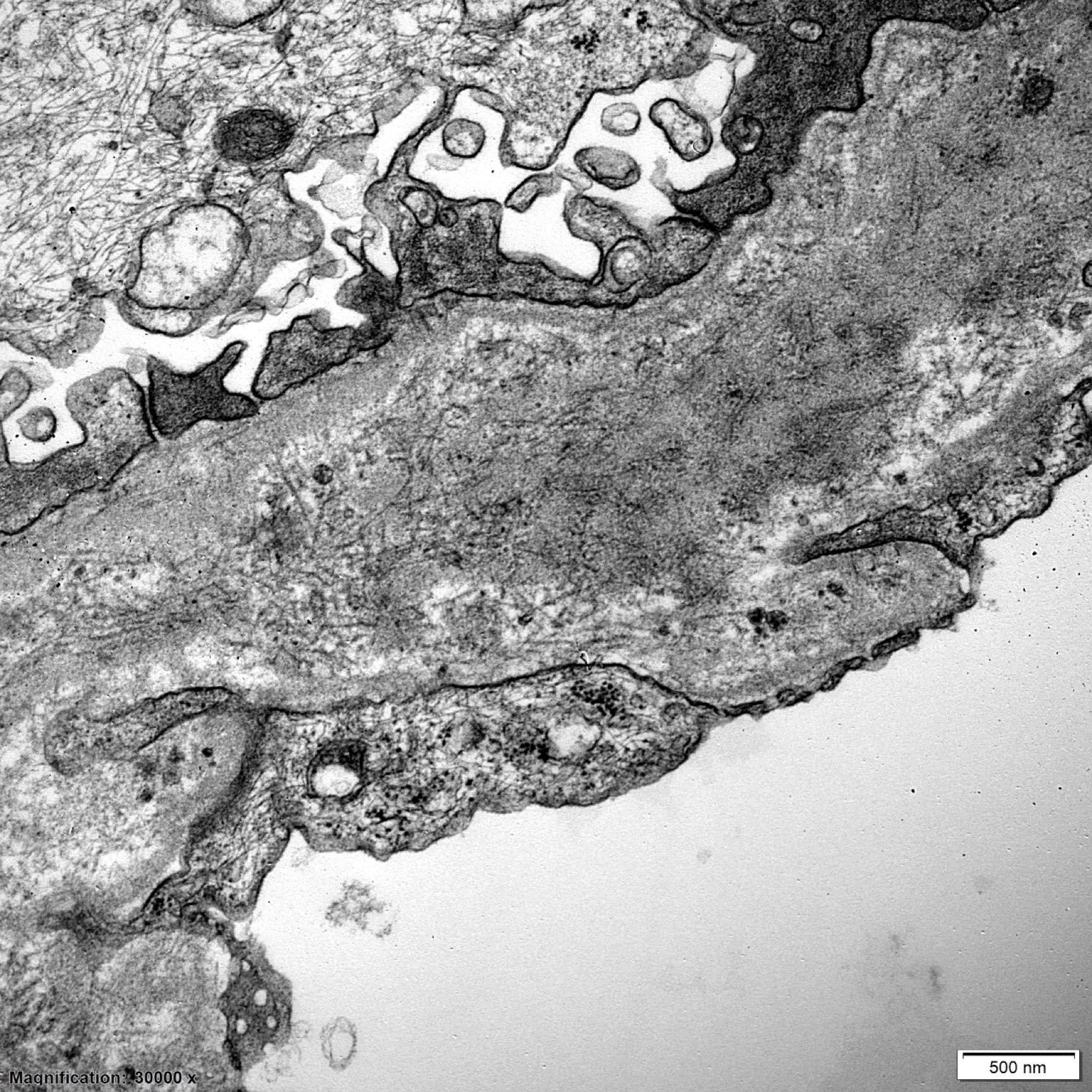
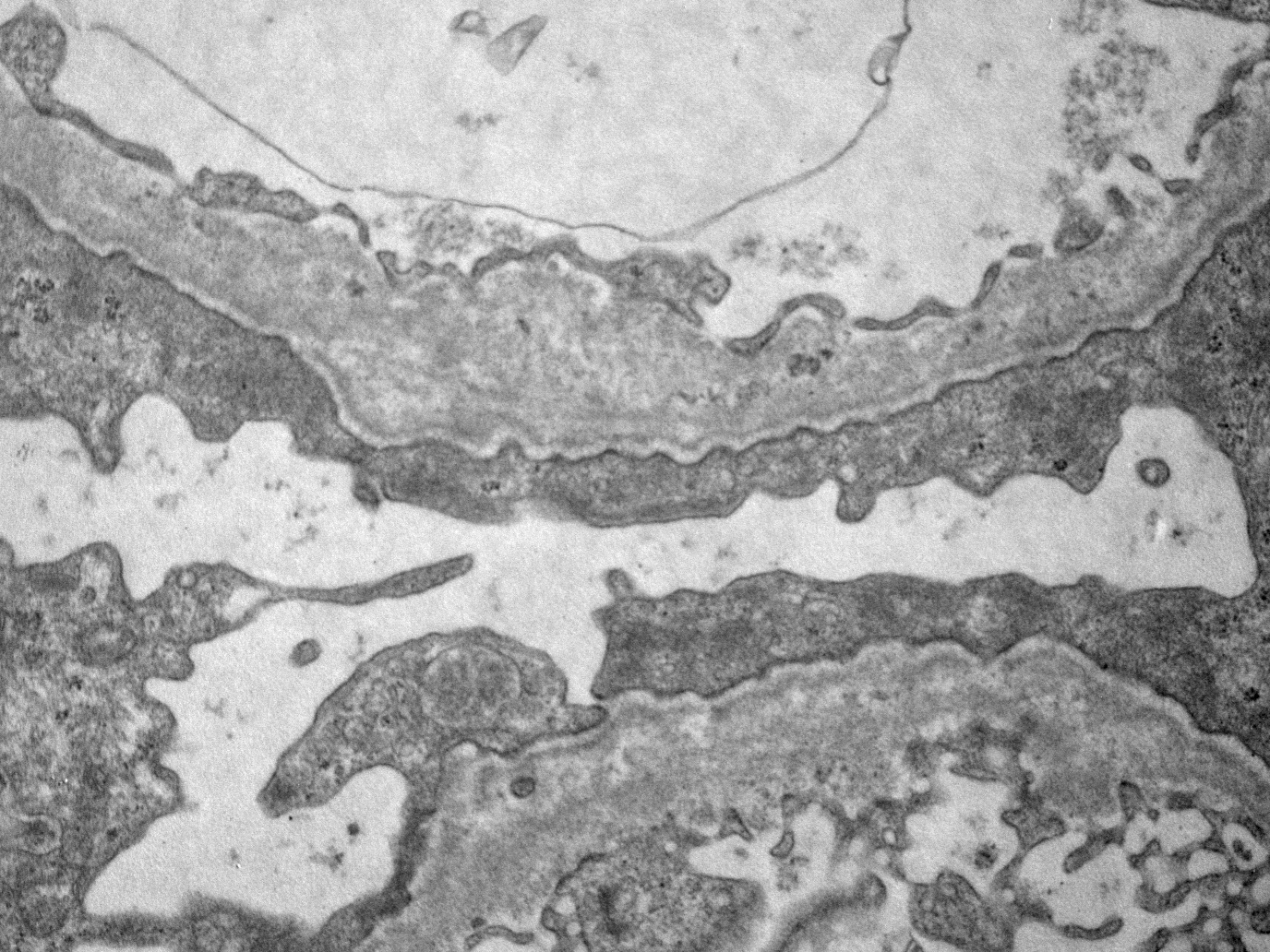
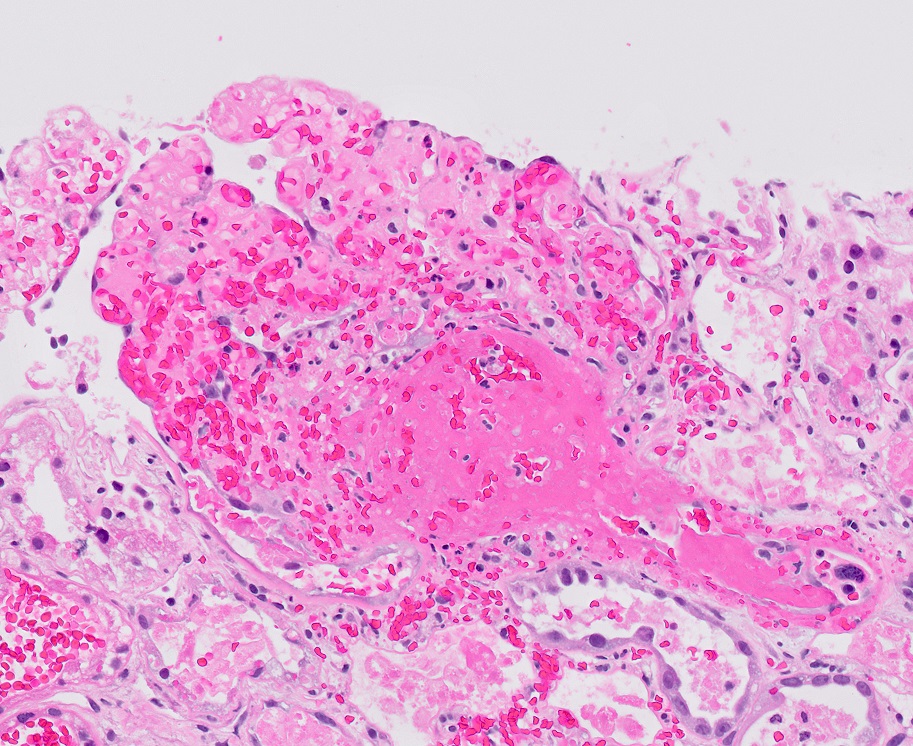
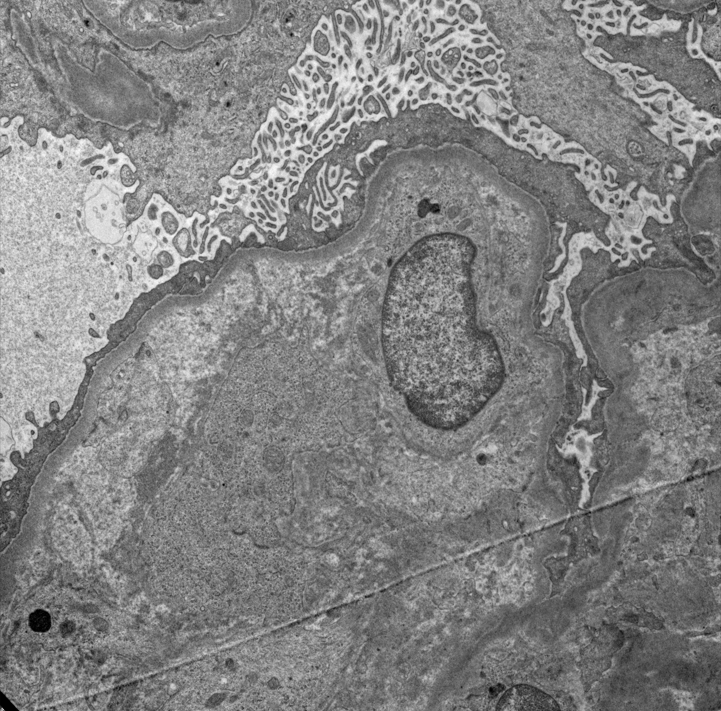
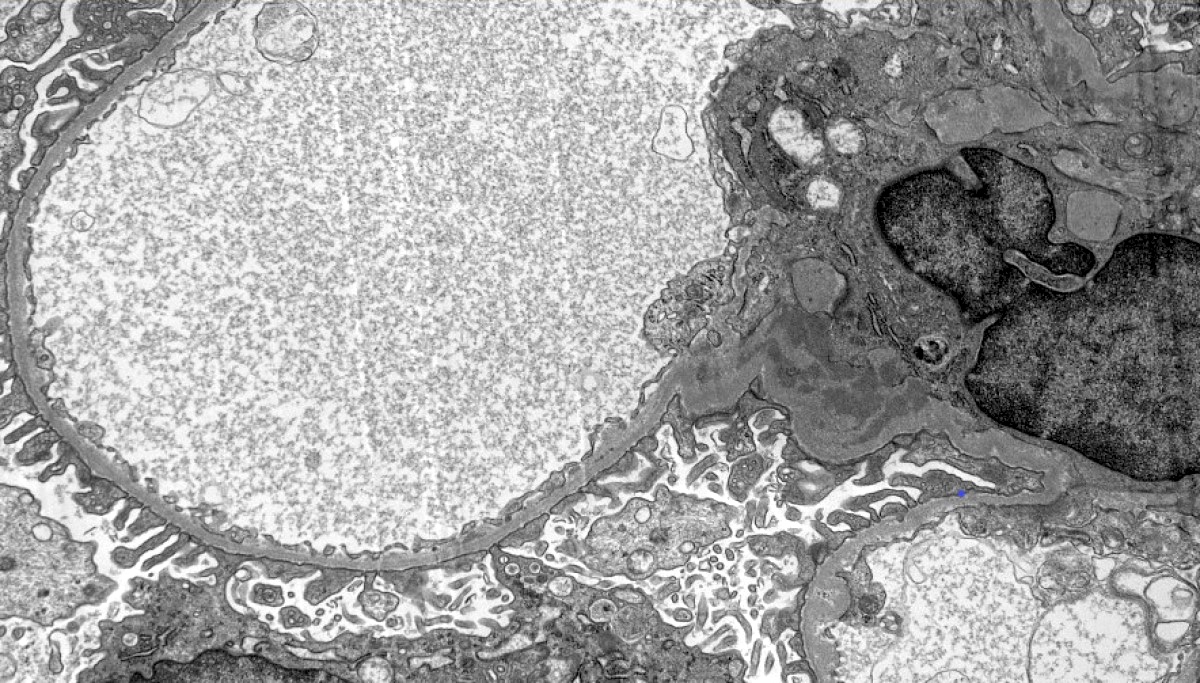
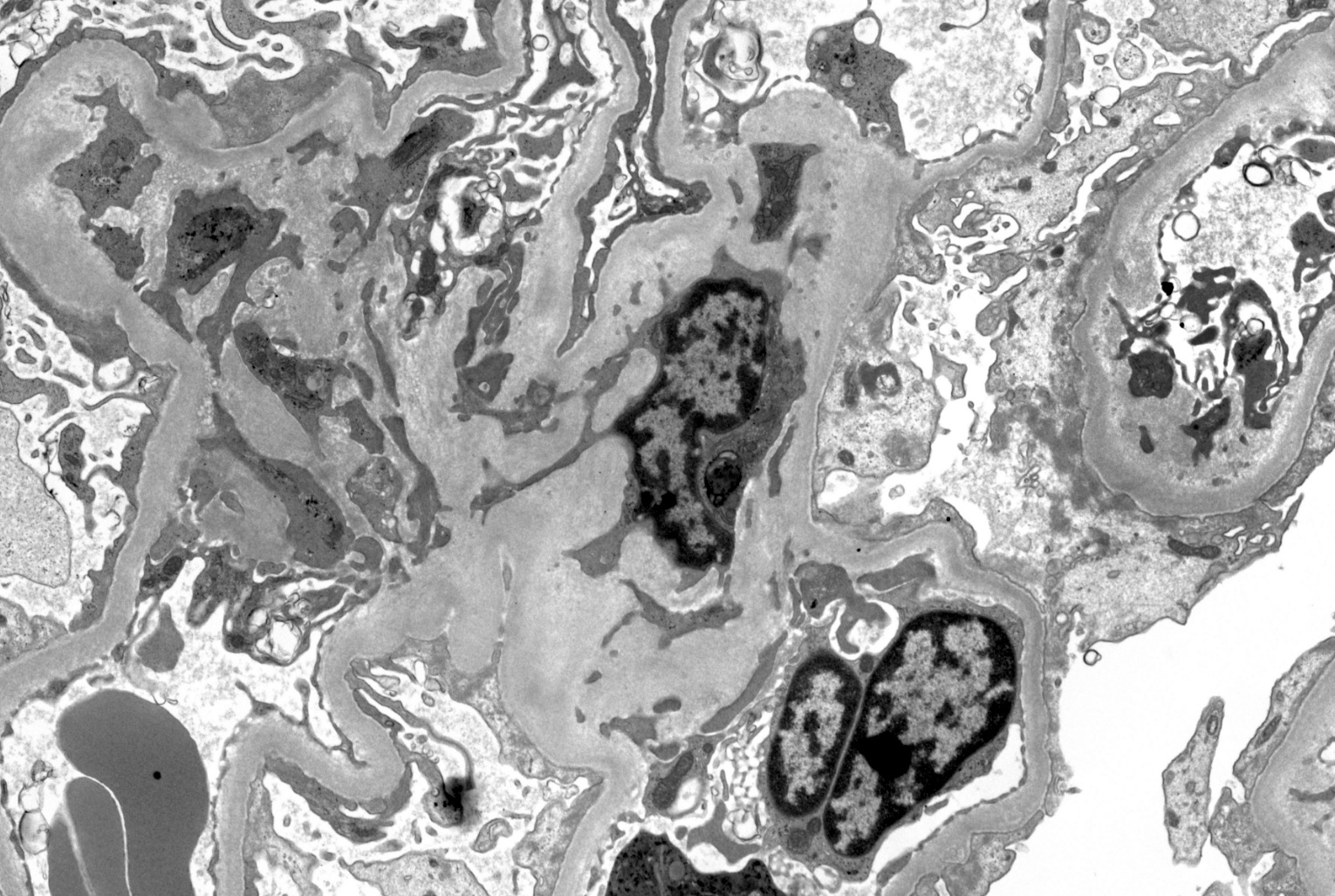
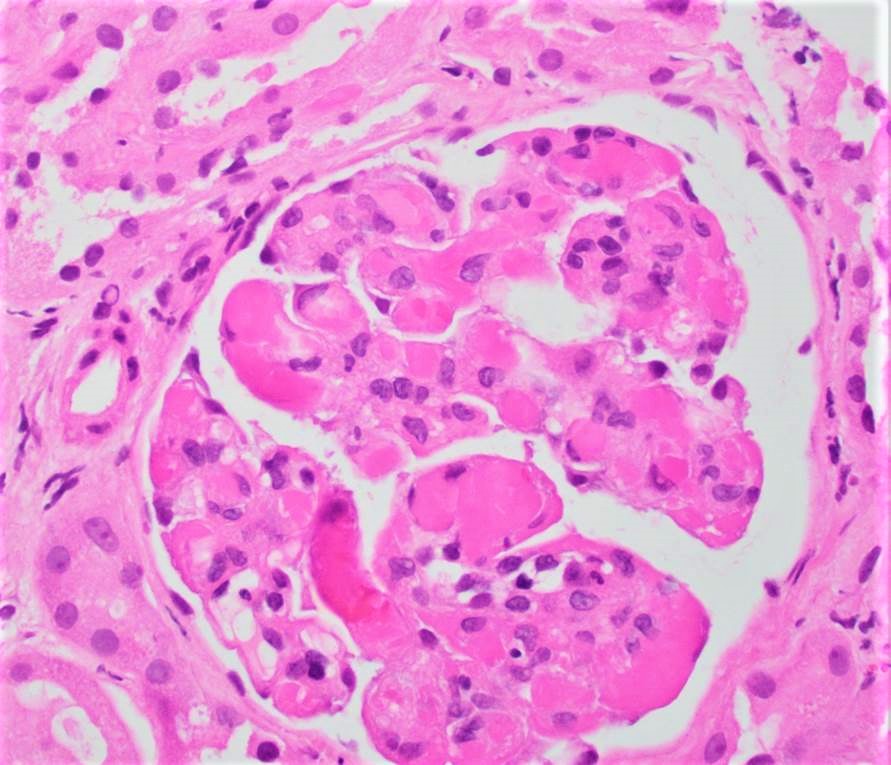
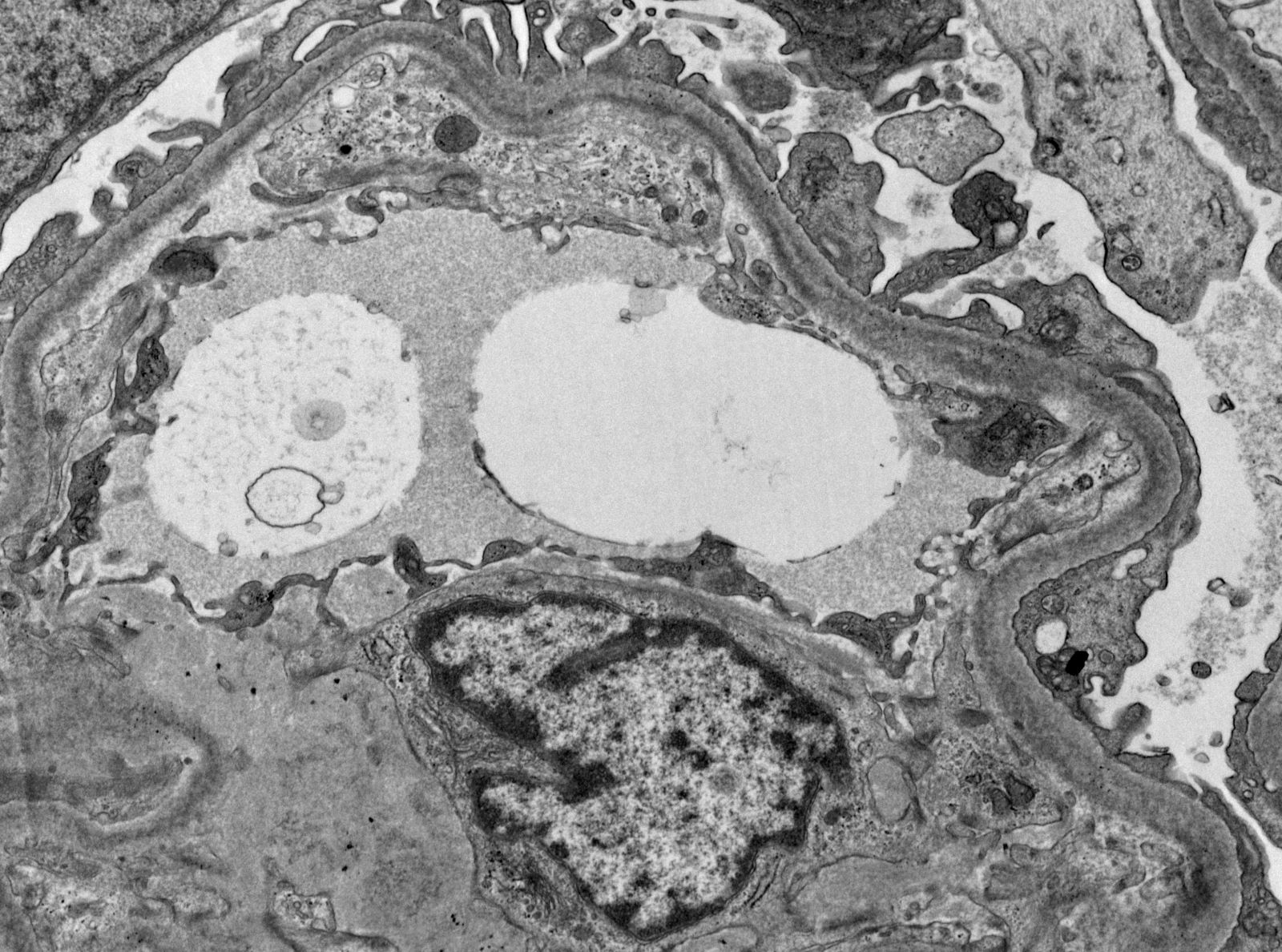
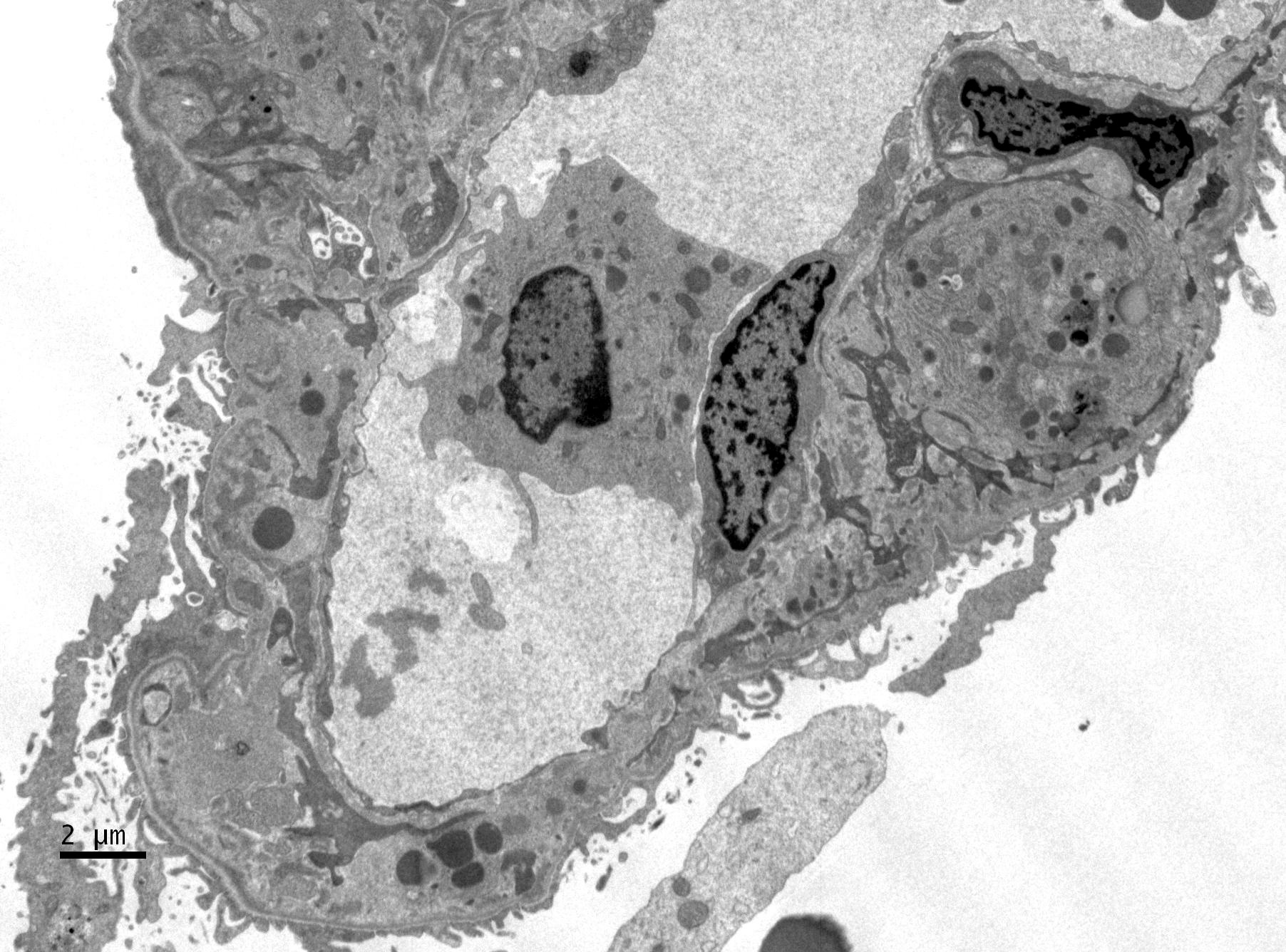
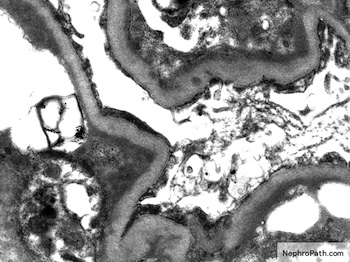
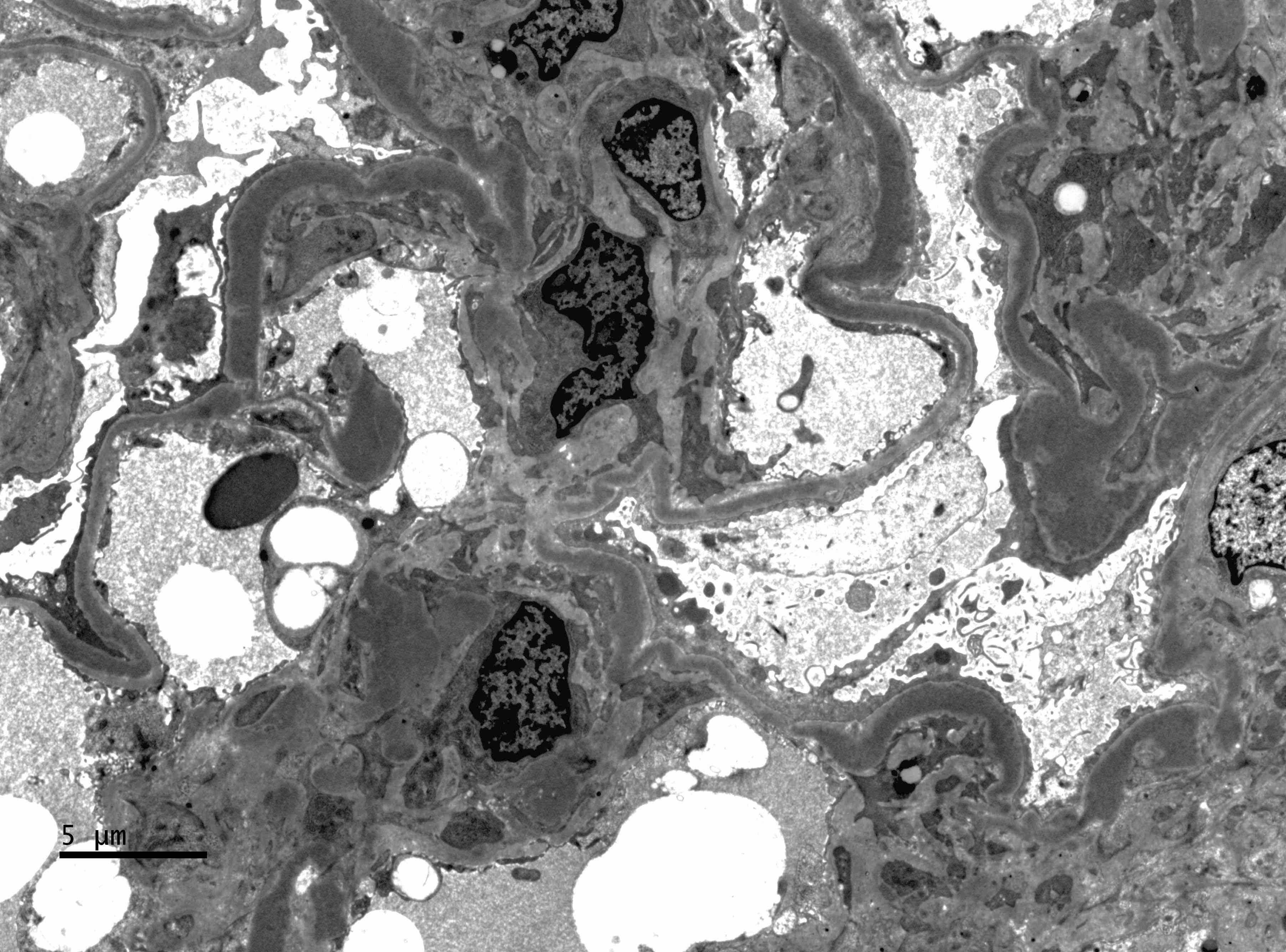
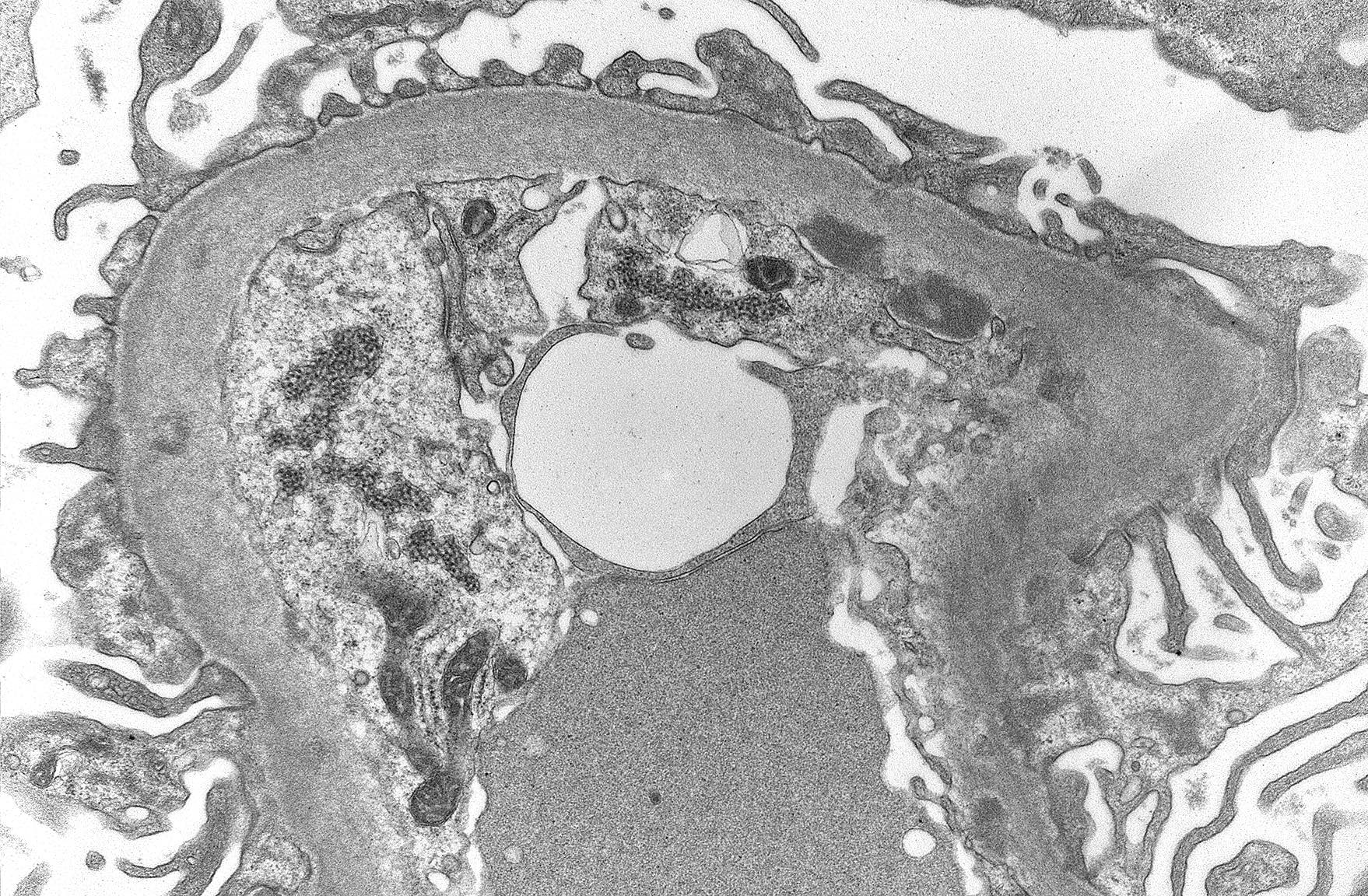
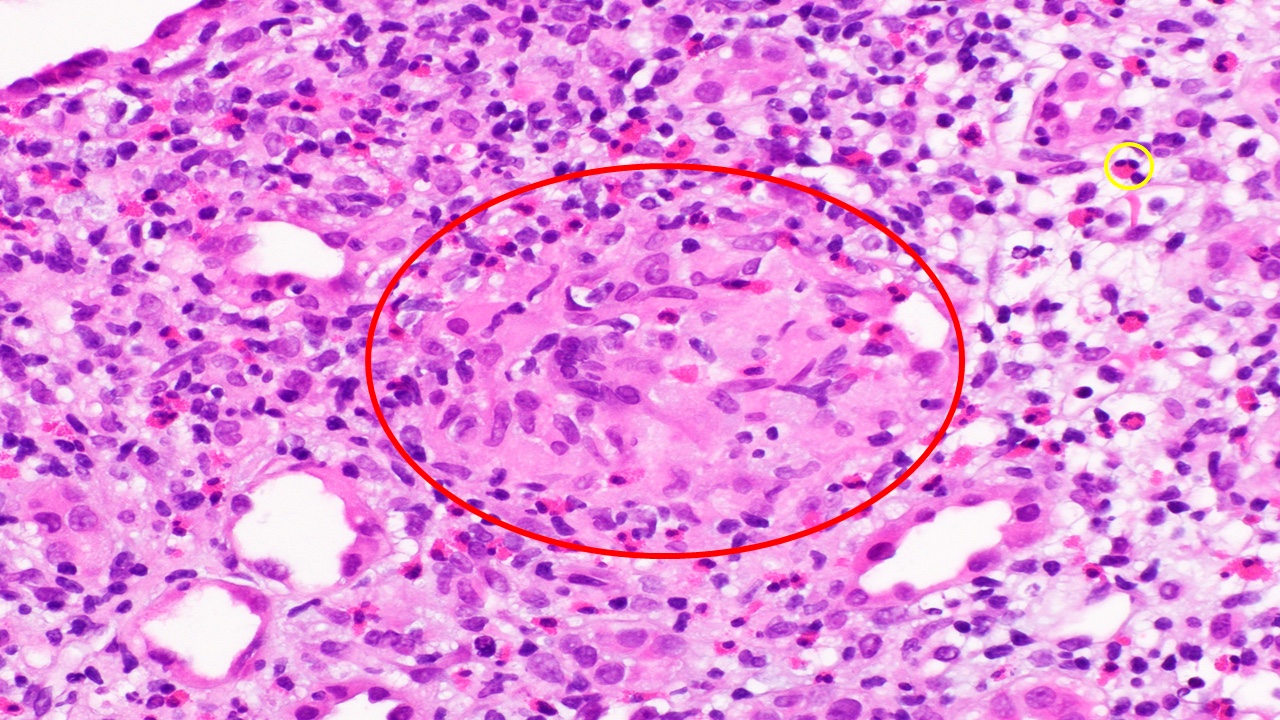
- Active antibody mediated rejection (ABMR):
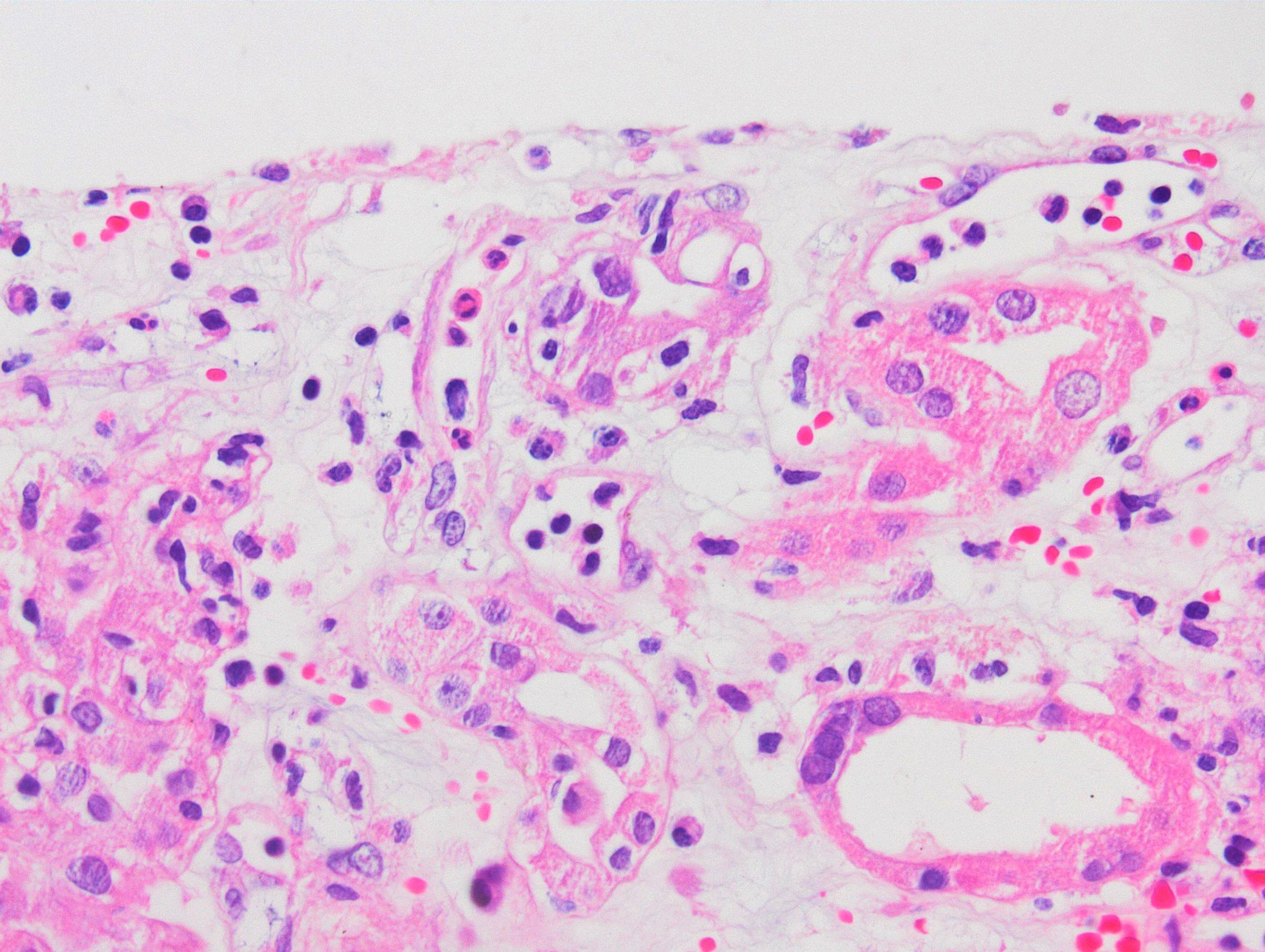
- Glomerular changes resemble those of thrombotic microangiopathy
- Neutrophils, platelets and fibrin within glomerular capillary lumina
- Swelling of endothelial cells and widening of the subendothelial space
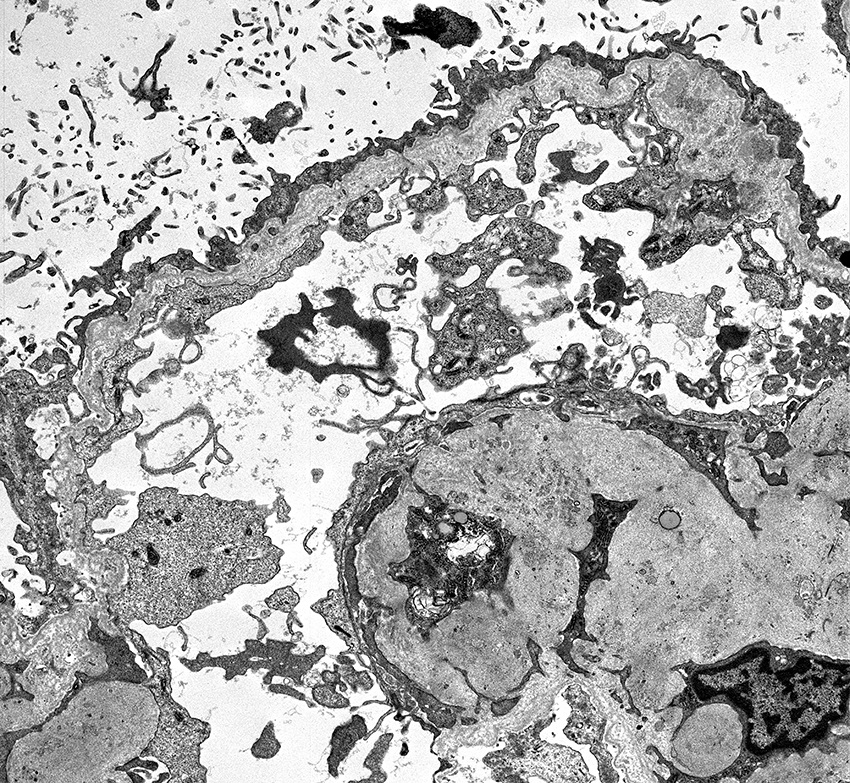
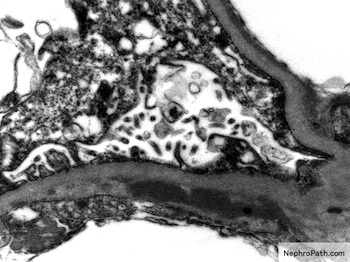
- Peritubular capillaries
- Neutrophils, platelets and fibrin within capillary lumina
- Swelling of endothelial cells and widening of the subendothelial space
- Apoptosis and fragmentation of endothelial cells
- Glomerular changes resemble those of thrombotic microangiopathy
- Most commonly used to diagnose chronic active ABMR:
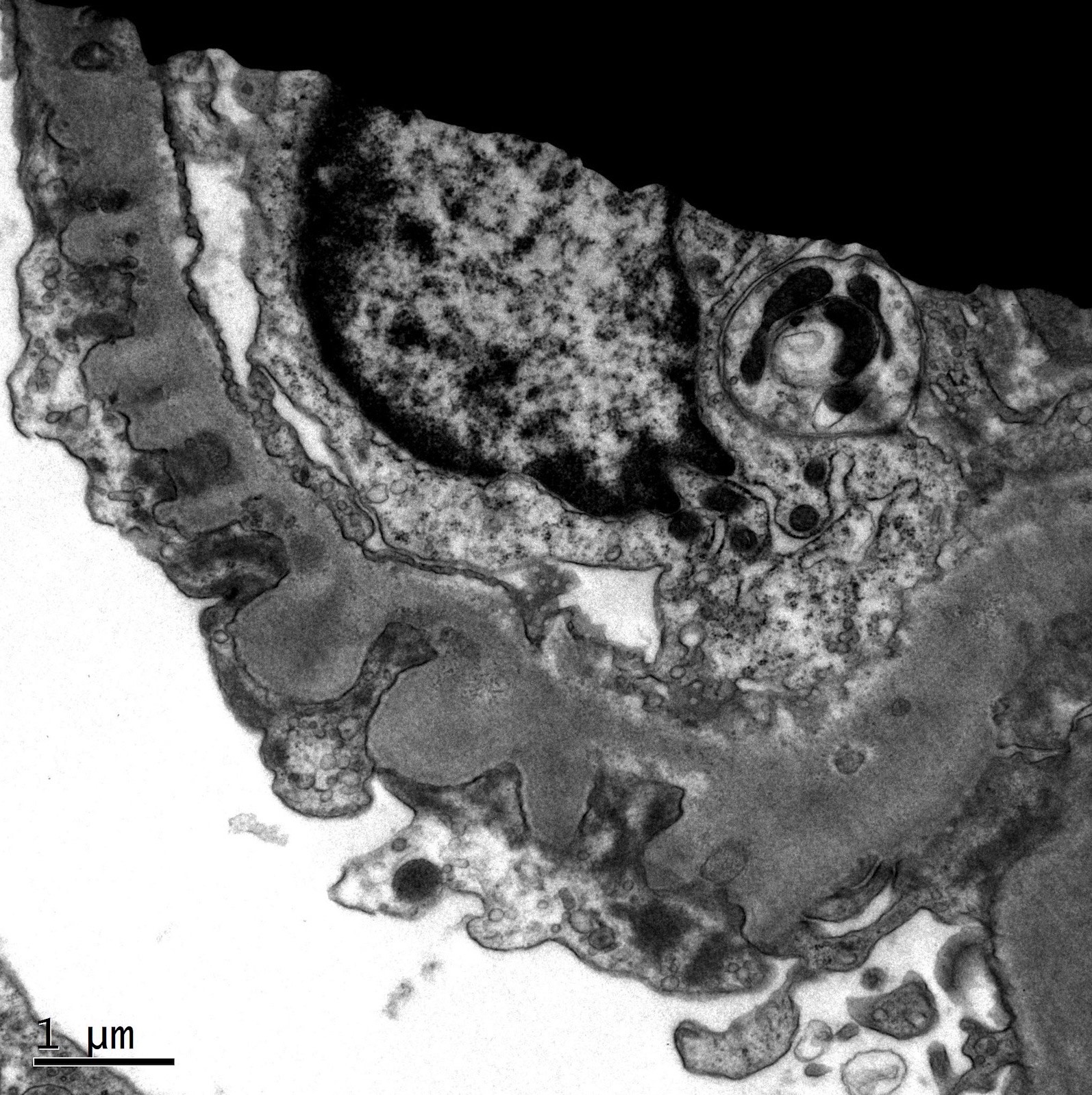
- Early detection of transplant glomerulopathy - formation of new layer of glomerular basement membrane
- Multilayering of tubular basement membrane
- References: Mod Pathol 2018;31:235, Transplantation 2018;102:1795
- Presence of antibody mediated rejection specific gene transcripts - endothelial damage associated transcripts (ENDAT) (Am J Transplant 2018;18:293, BMC Nephrol 2015;16:132)
- Of use in situations in which combination of histologic, immunohistochemical and serologic data remain equivocal for diagnosis of antibody mediated rejection
- Incorporated into the updated Banff classification; however, no specific recommendations are given regarding which molecular classifiers / transcript sets should be tested for or the platform(s) used to assess gene expression
- A 770 gene panel encompassing genes involved in rejection, tolerance, viral infections, innate and adaptive immune responses, the Banff Human Organ Transplant Panel (B-HOT), has been made commercially available and is going to be used on the NanoString platform for research purposes (Am J Transplant 2020;20:2305)
- A simplified molecular panel will hopefully be standardized and integrated into the Banff classification criteria in the near future
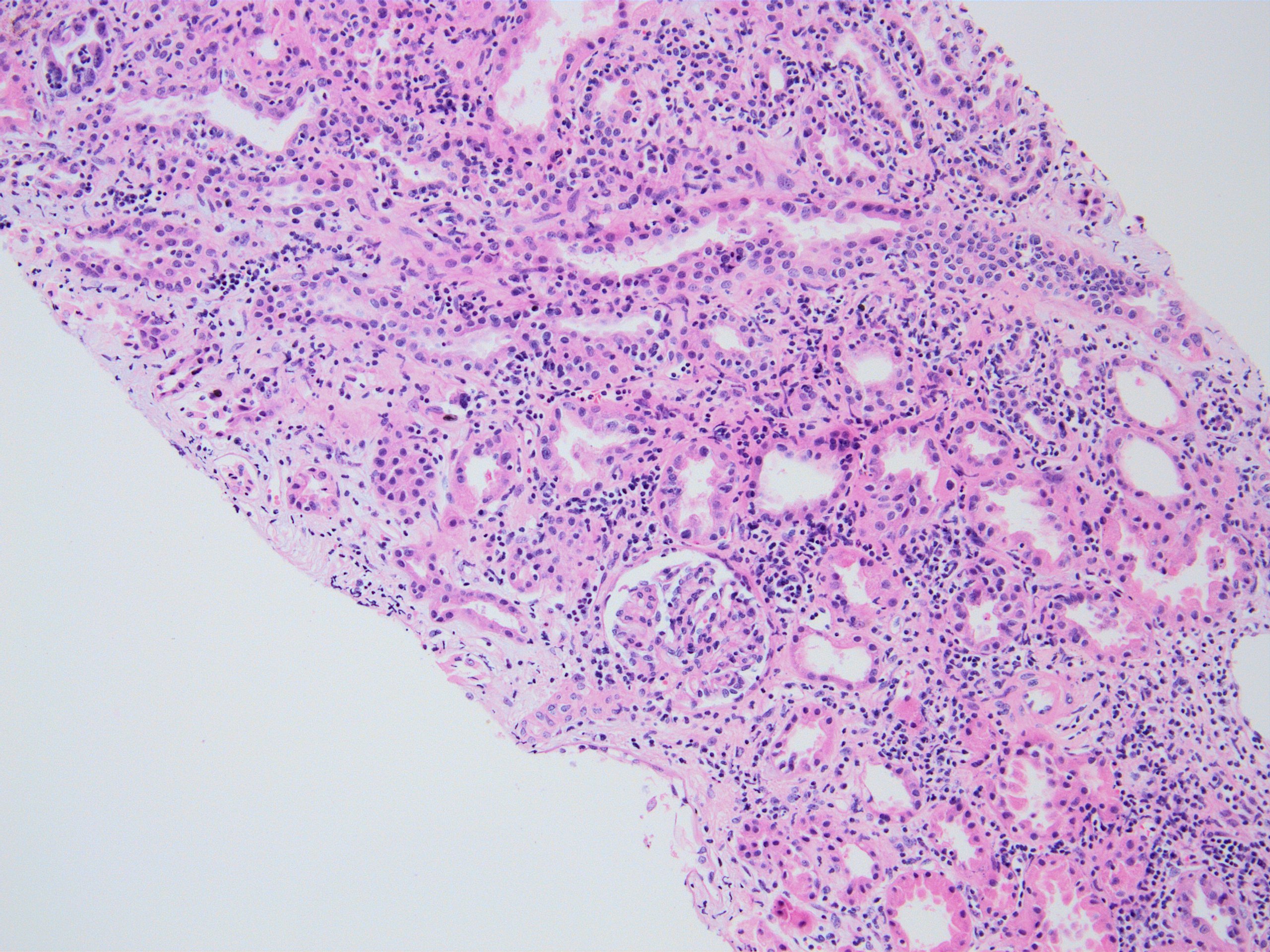
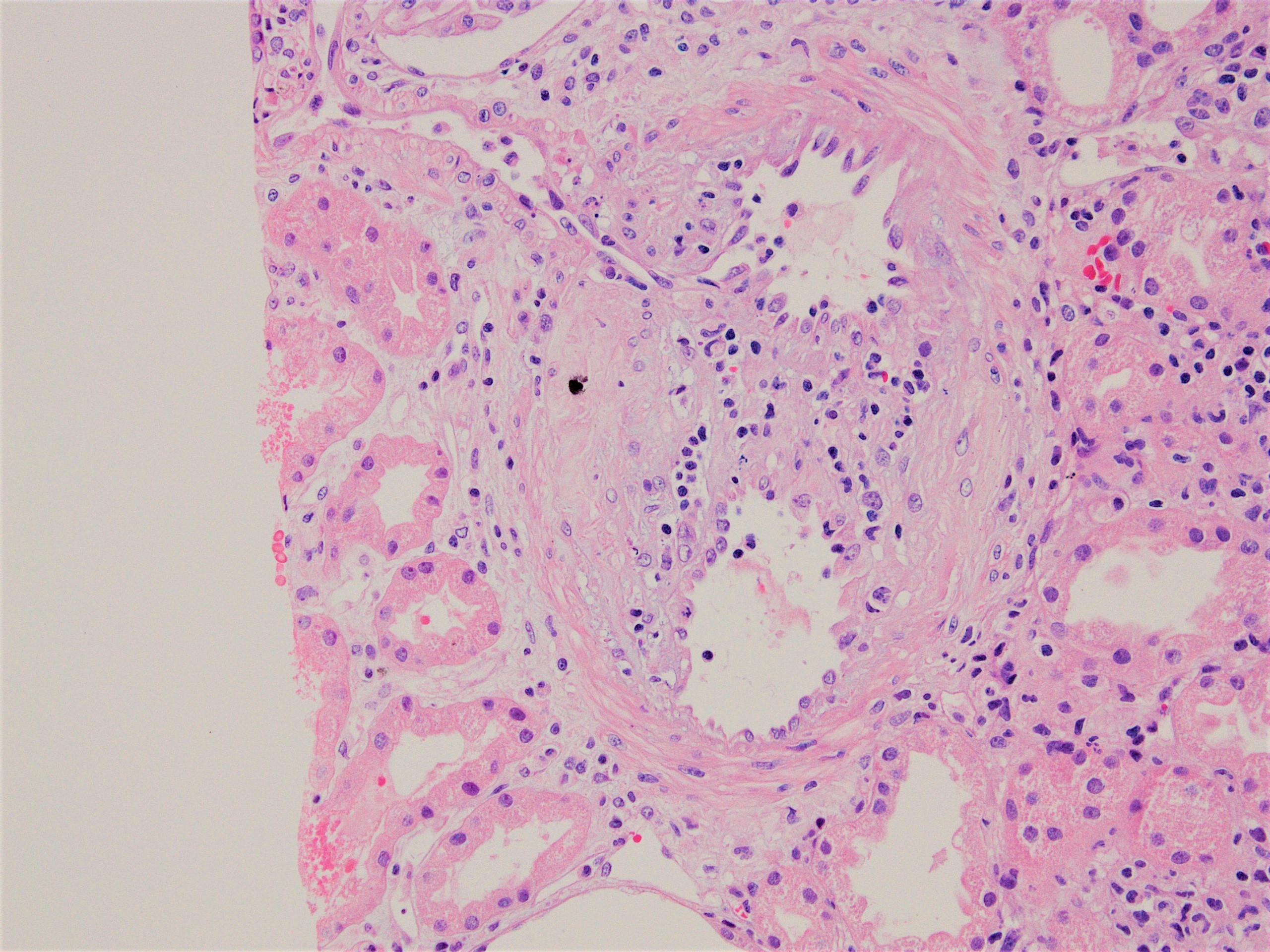
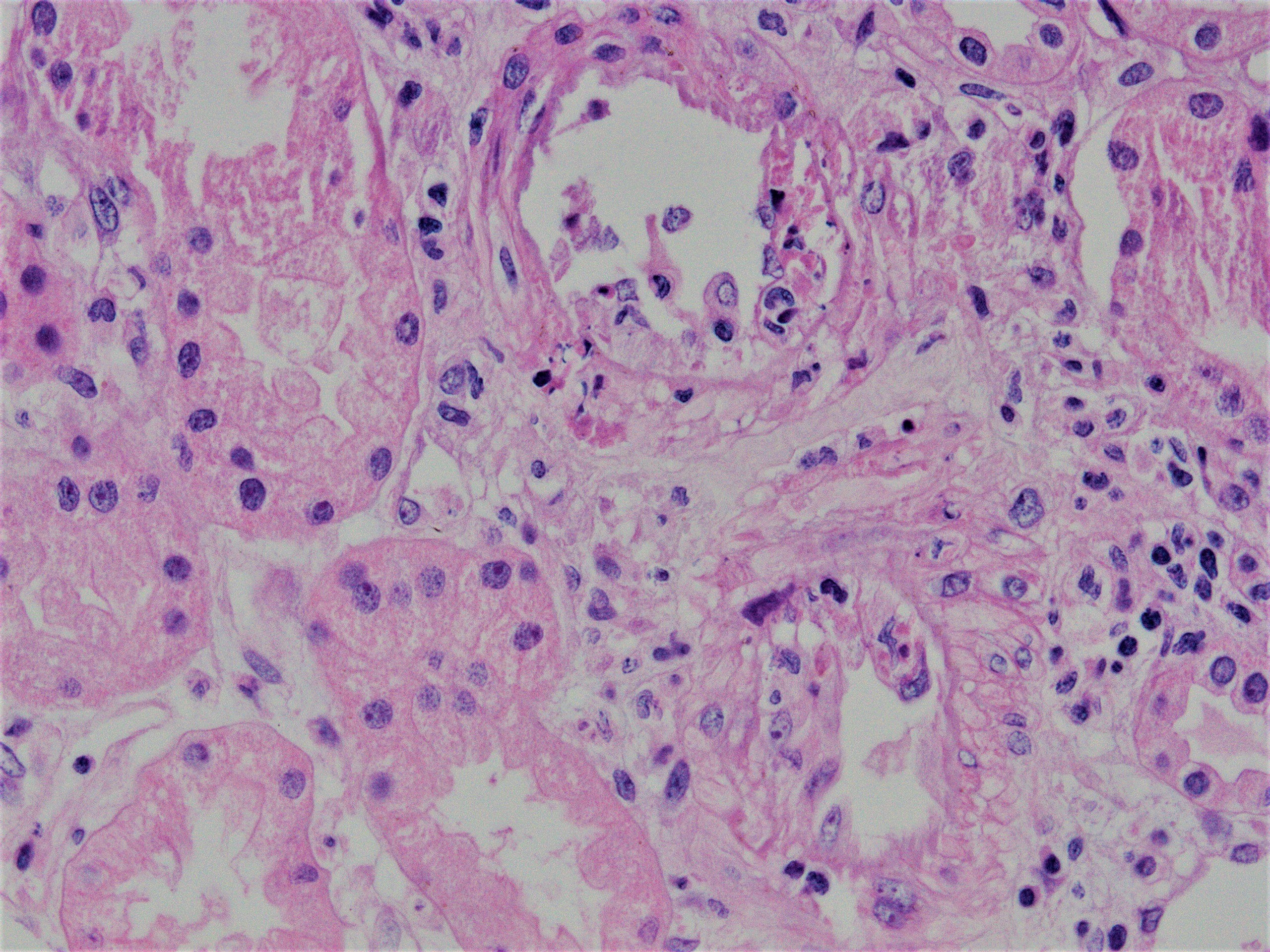
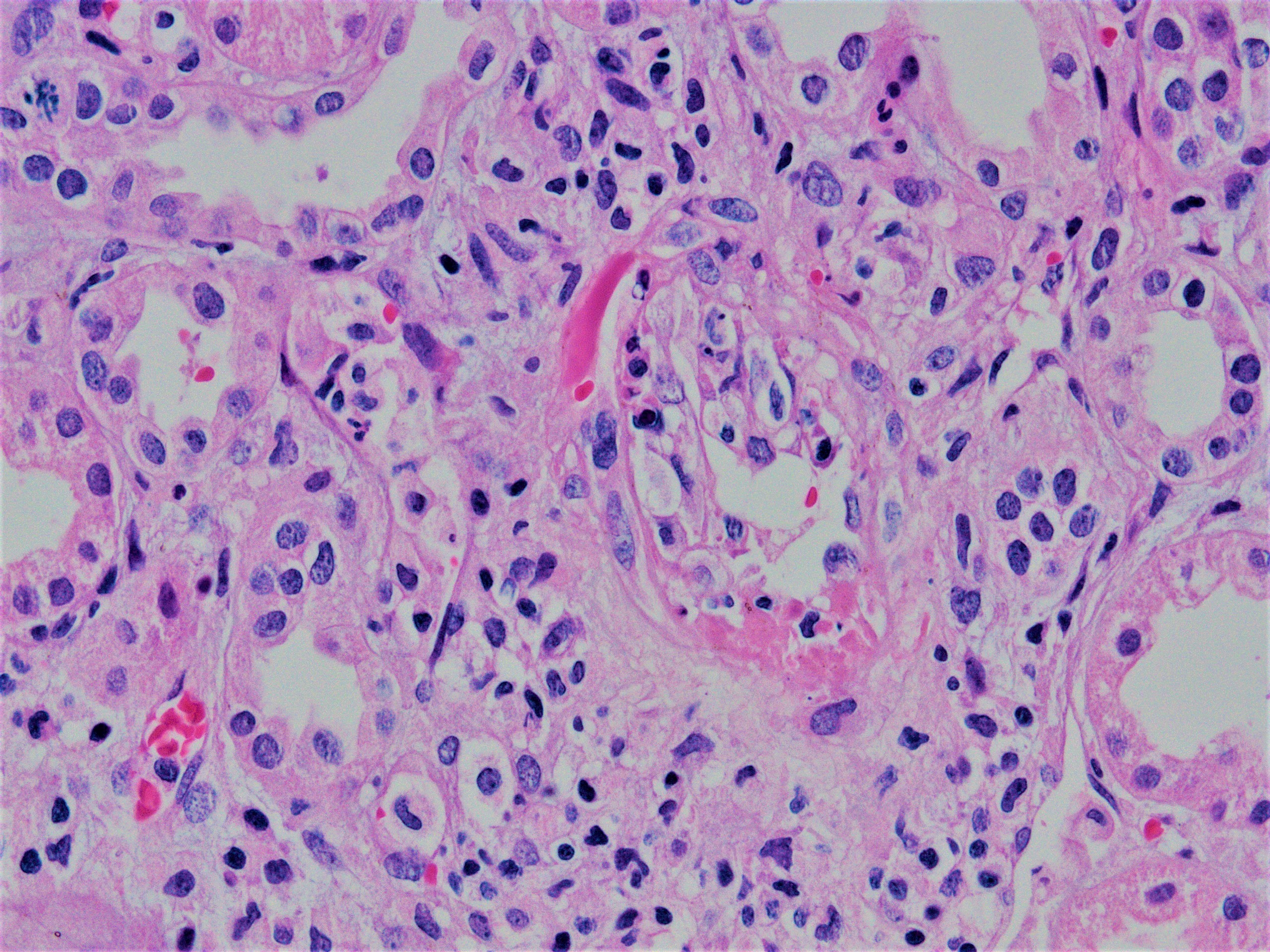
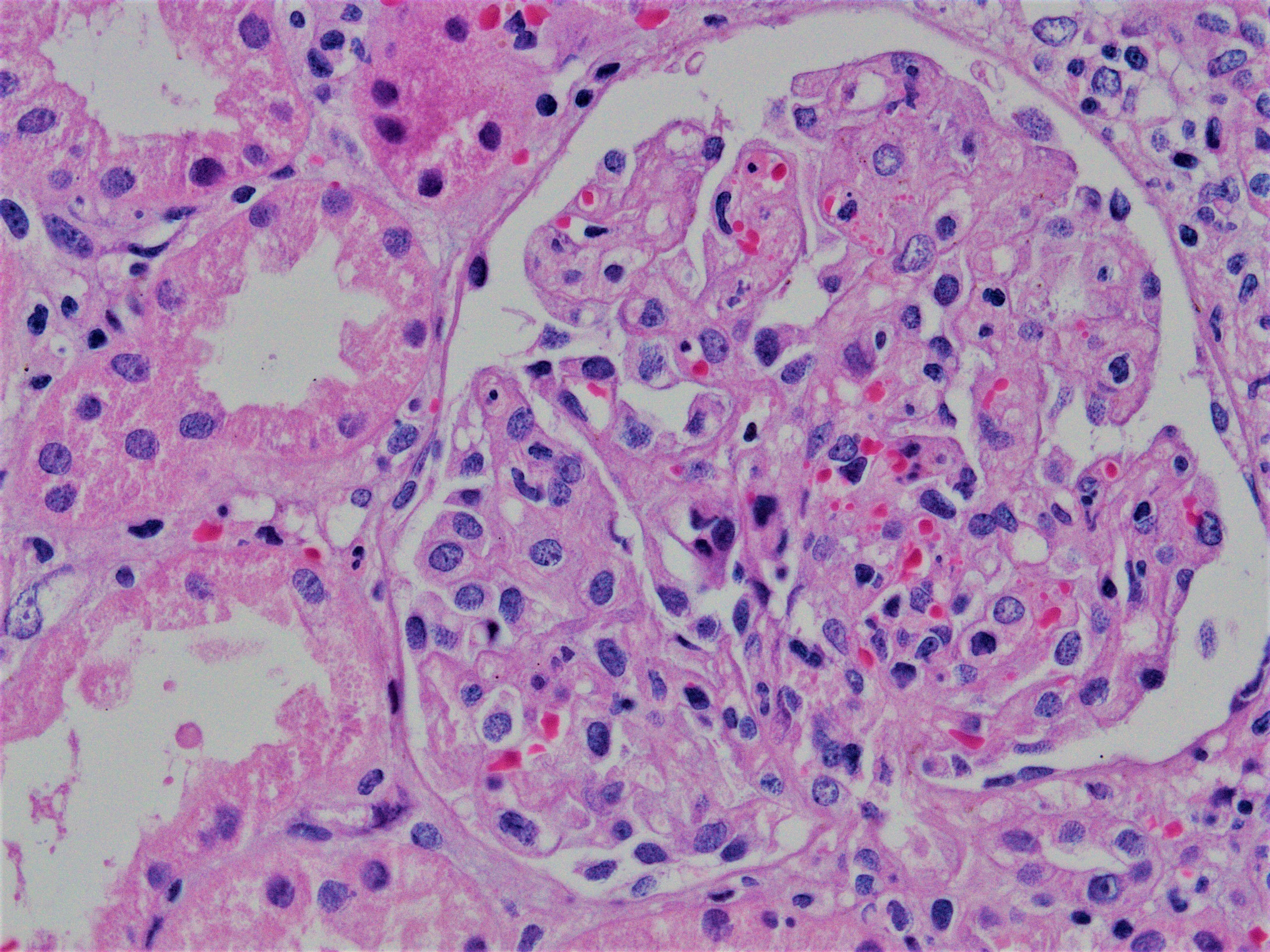
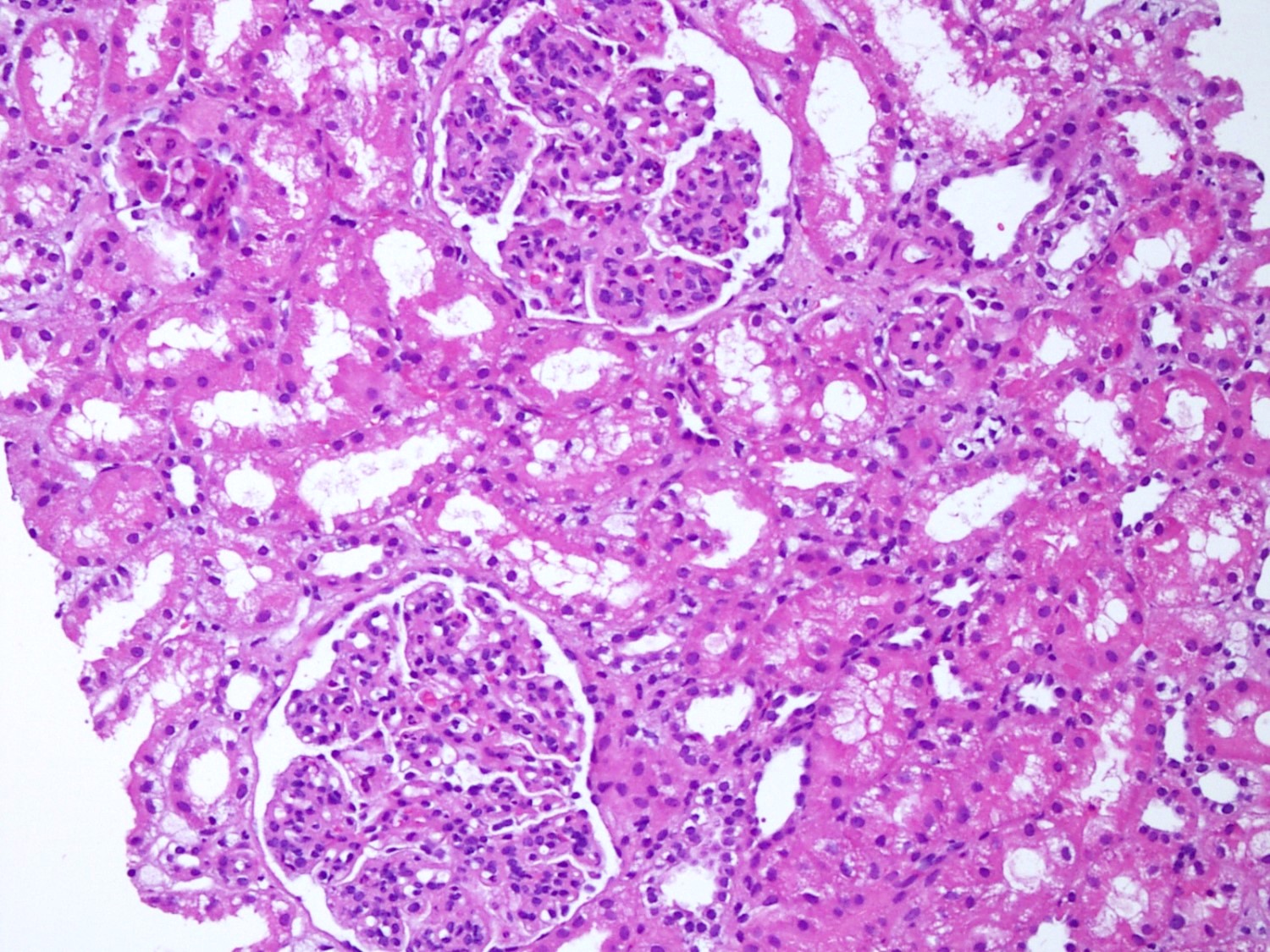
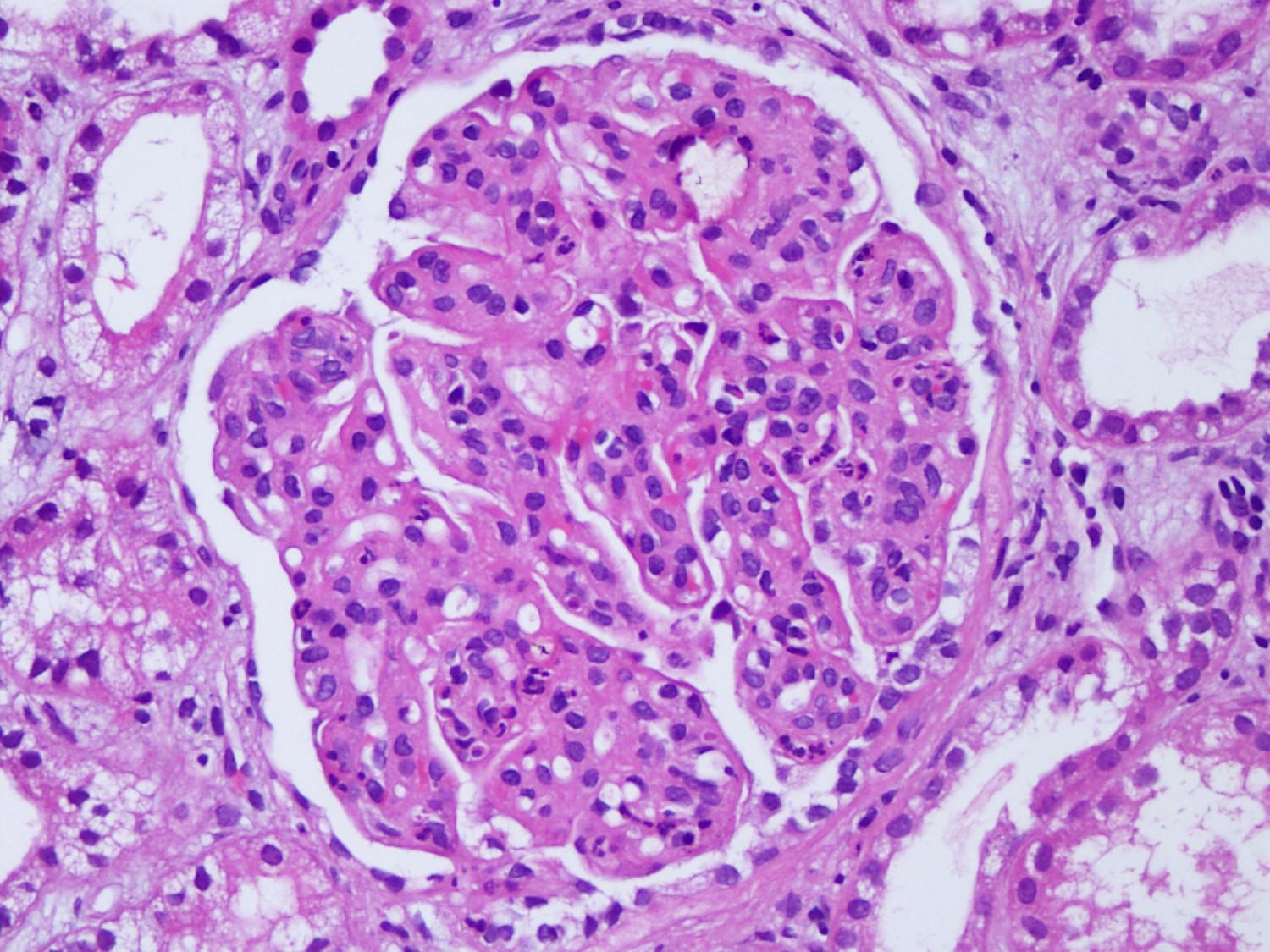
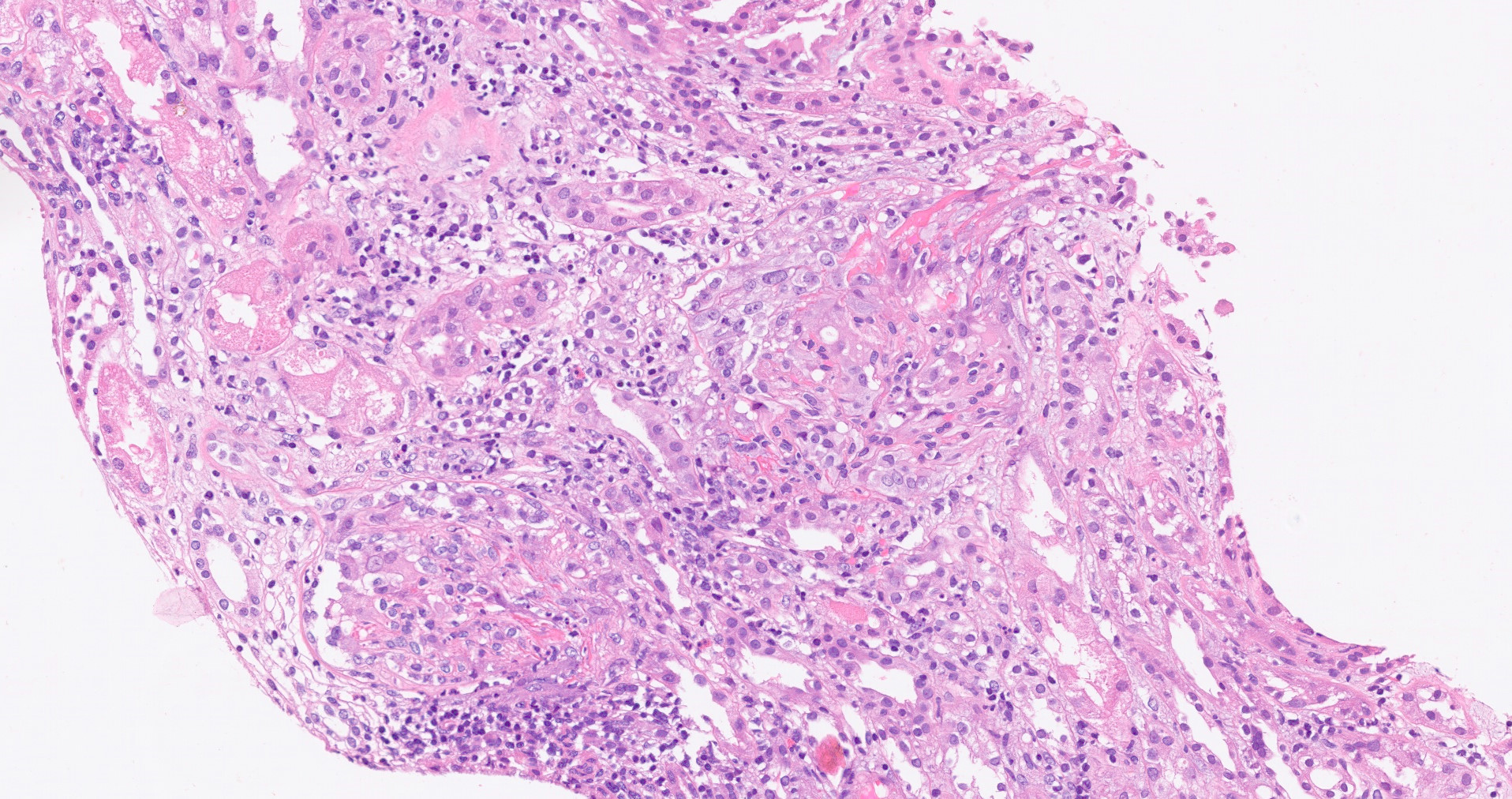
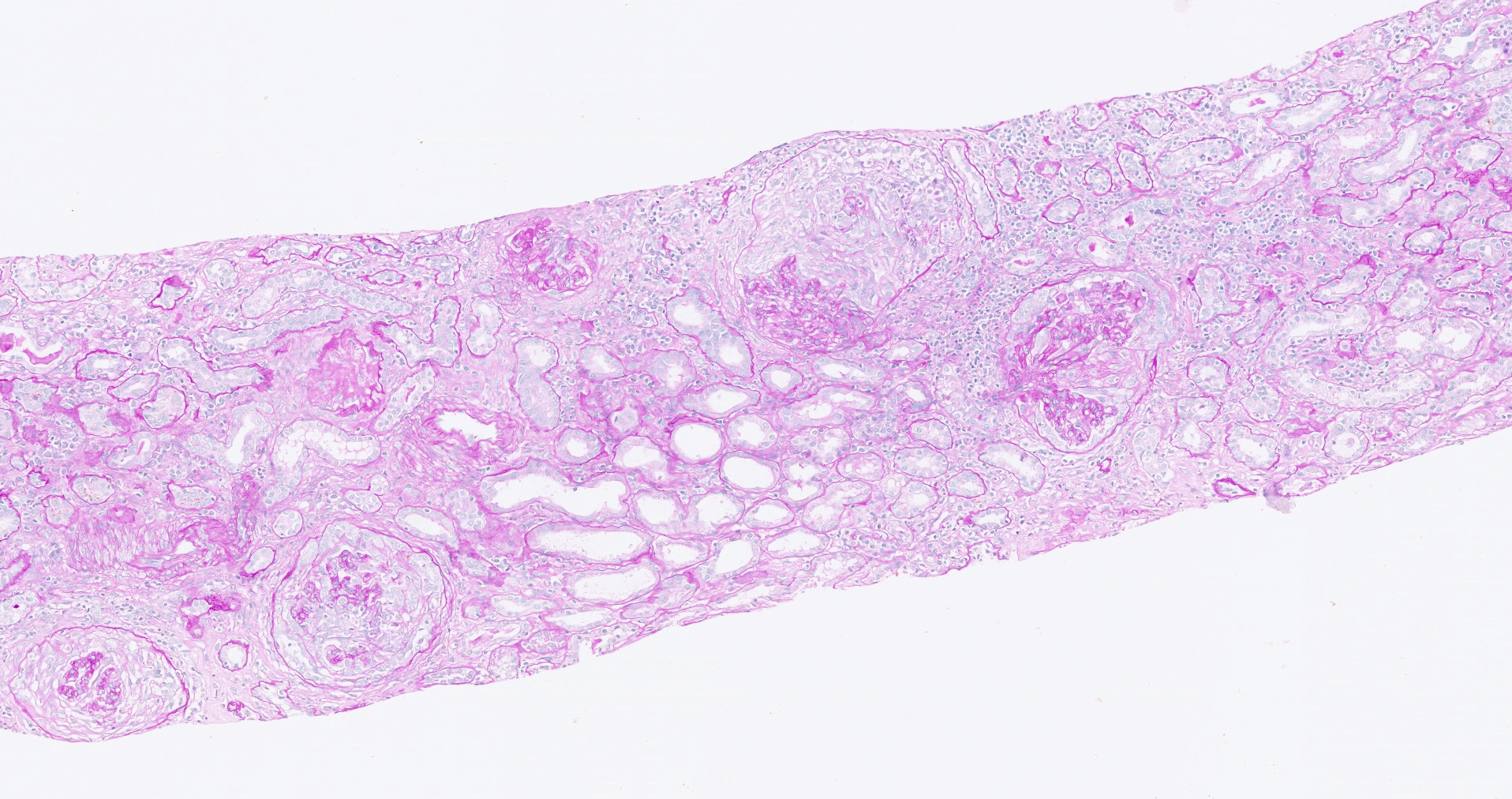
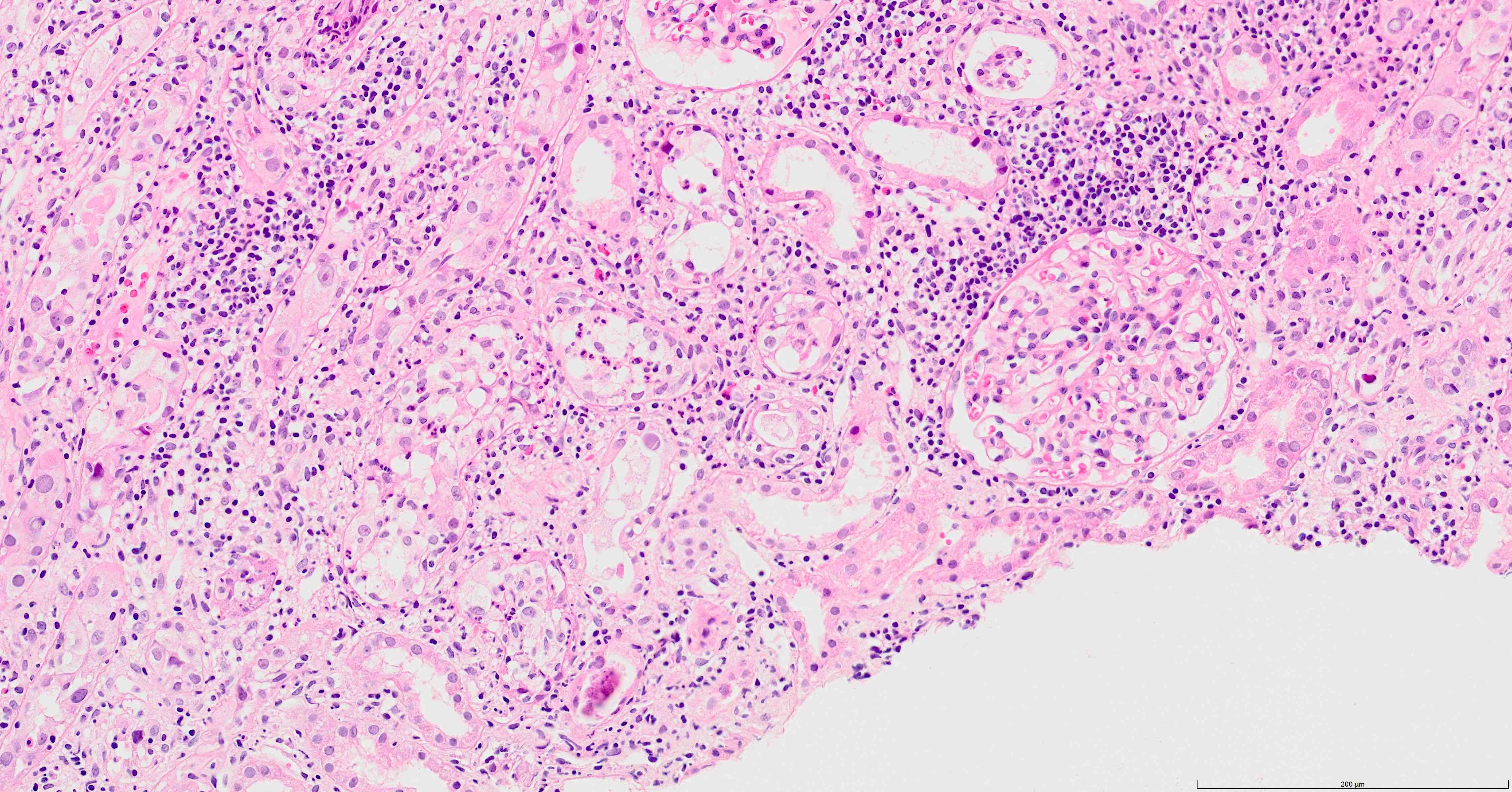
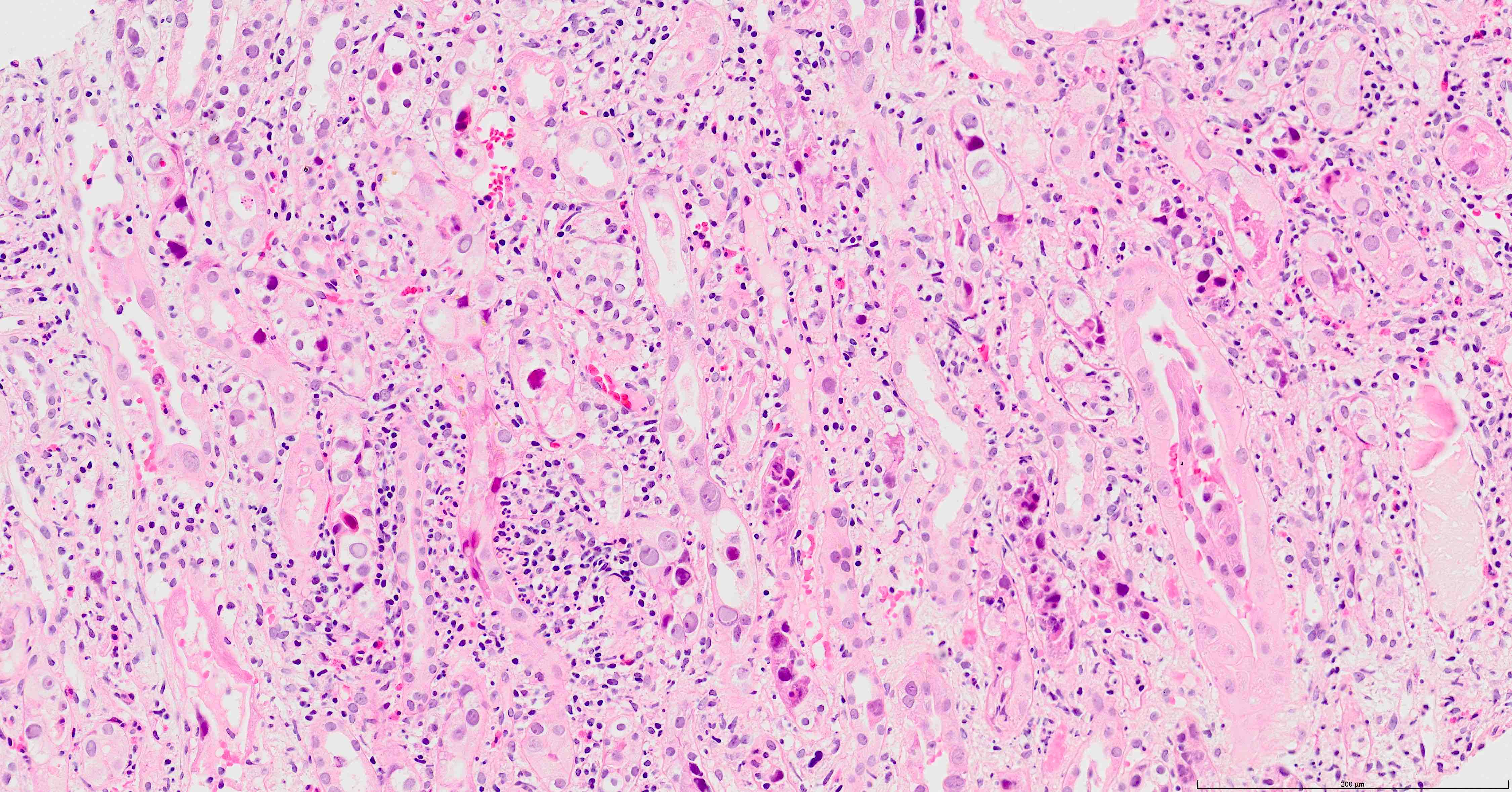
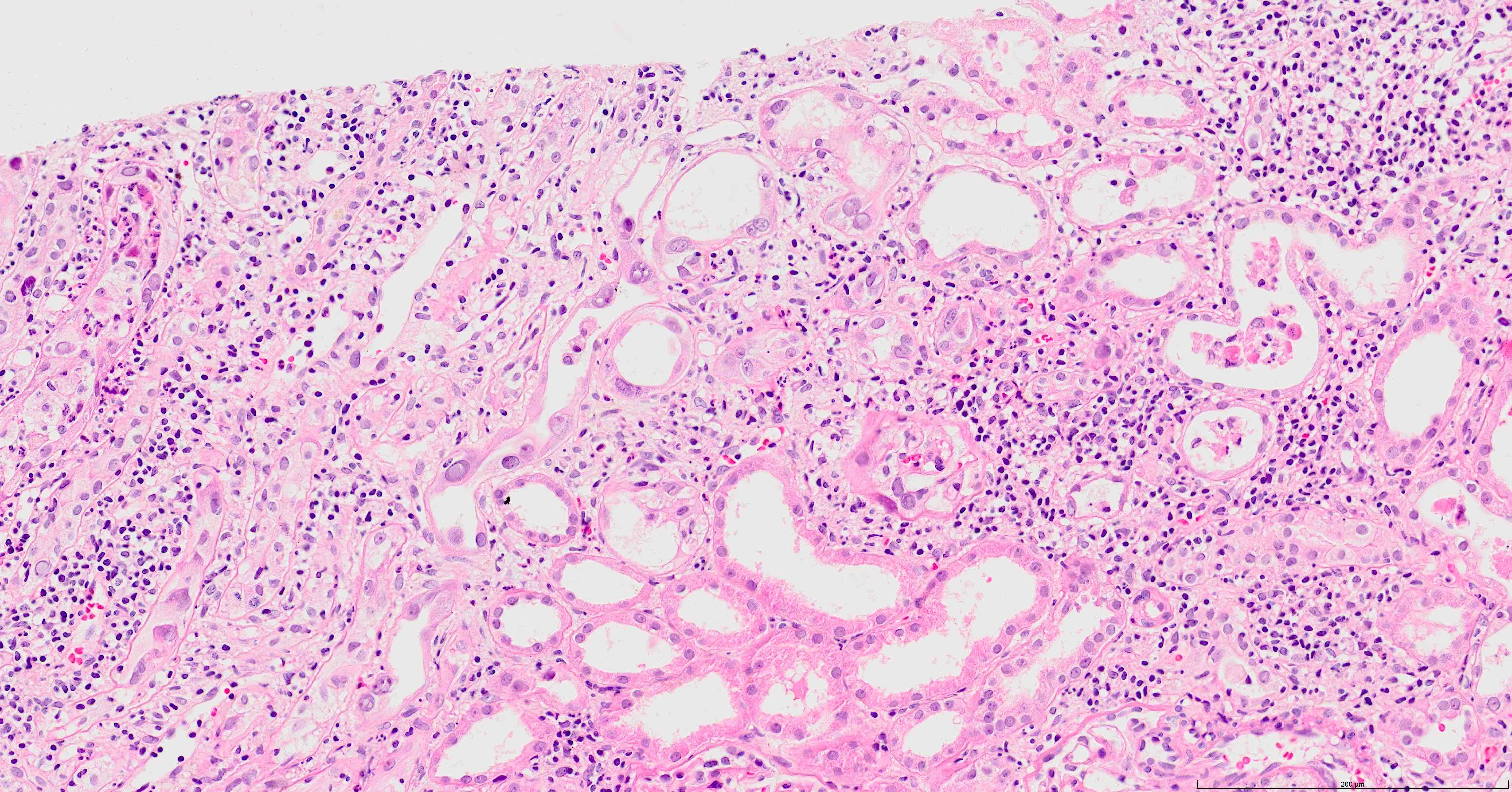
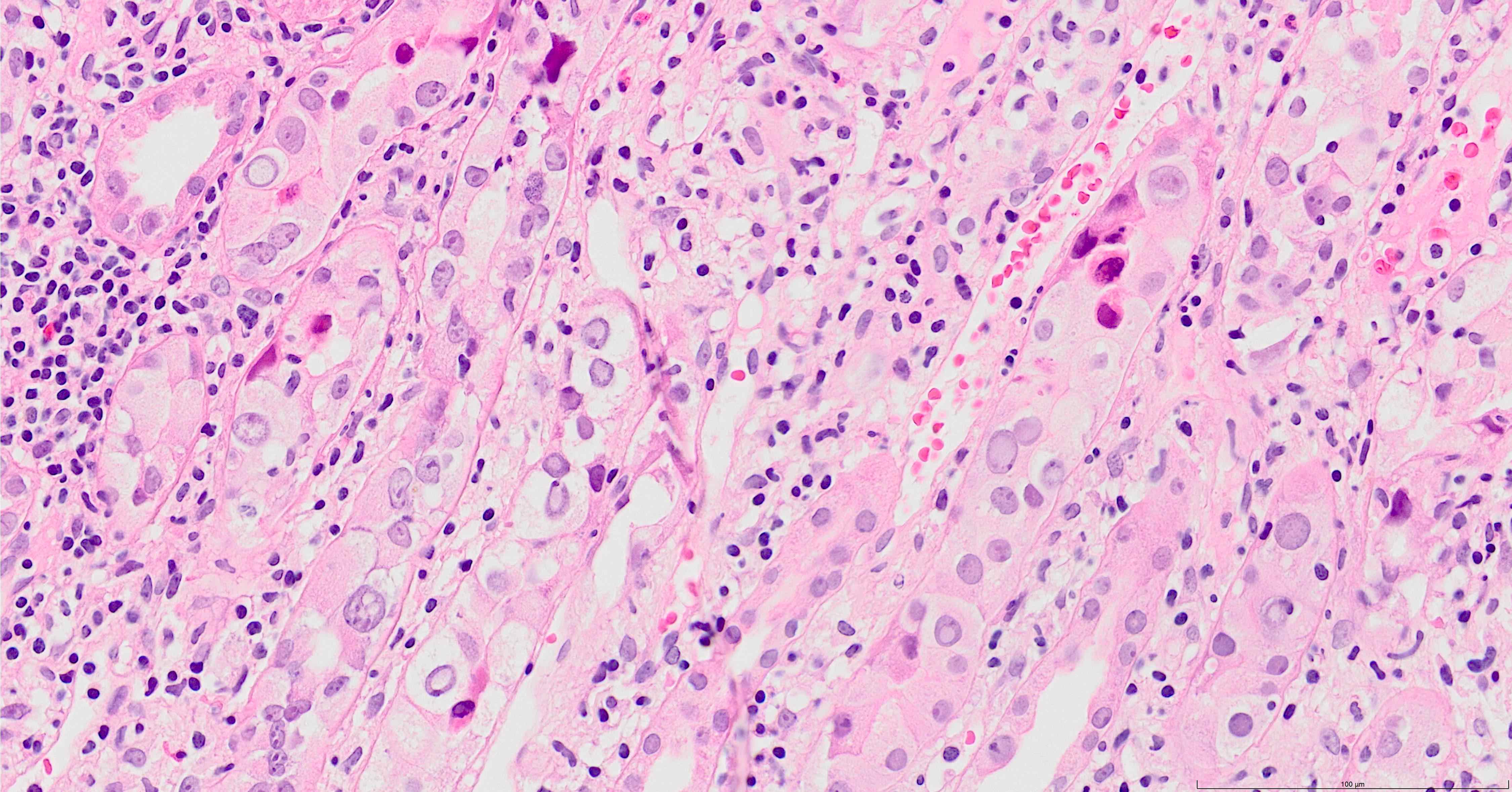
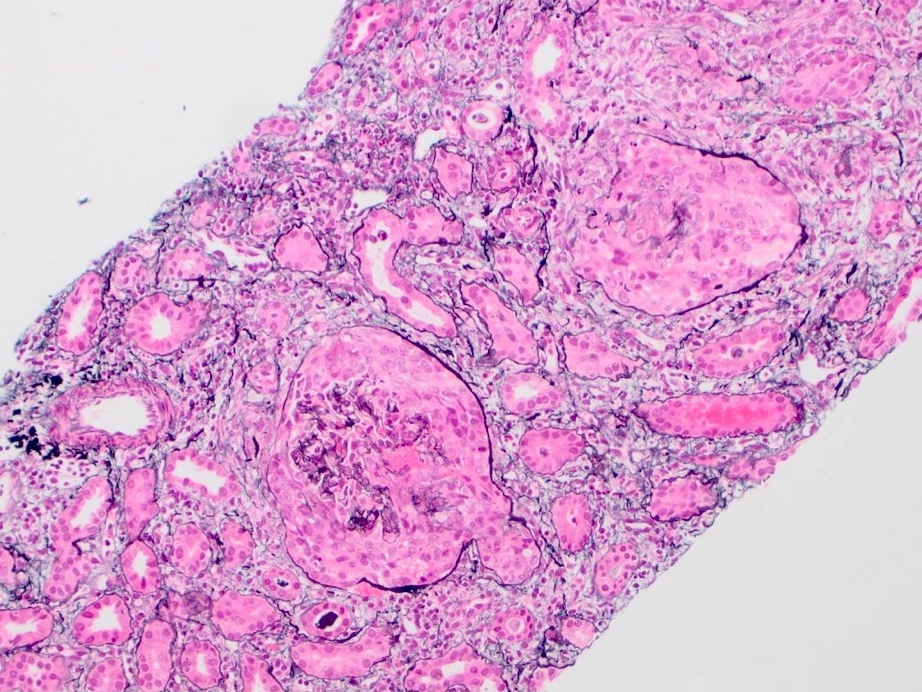
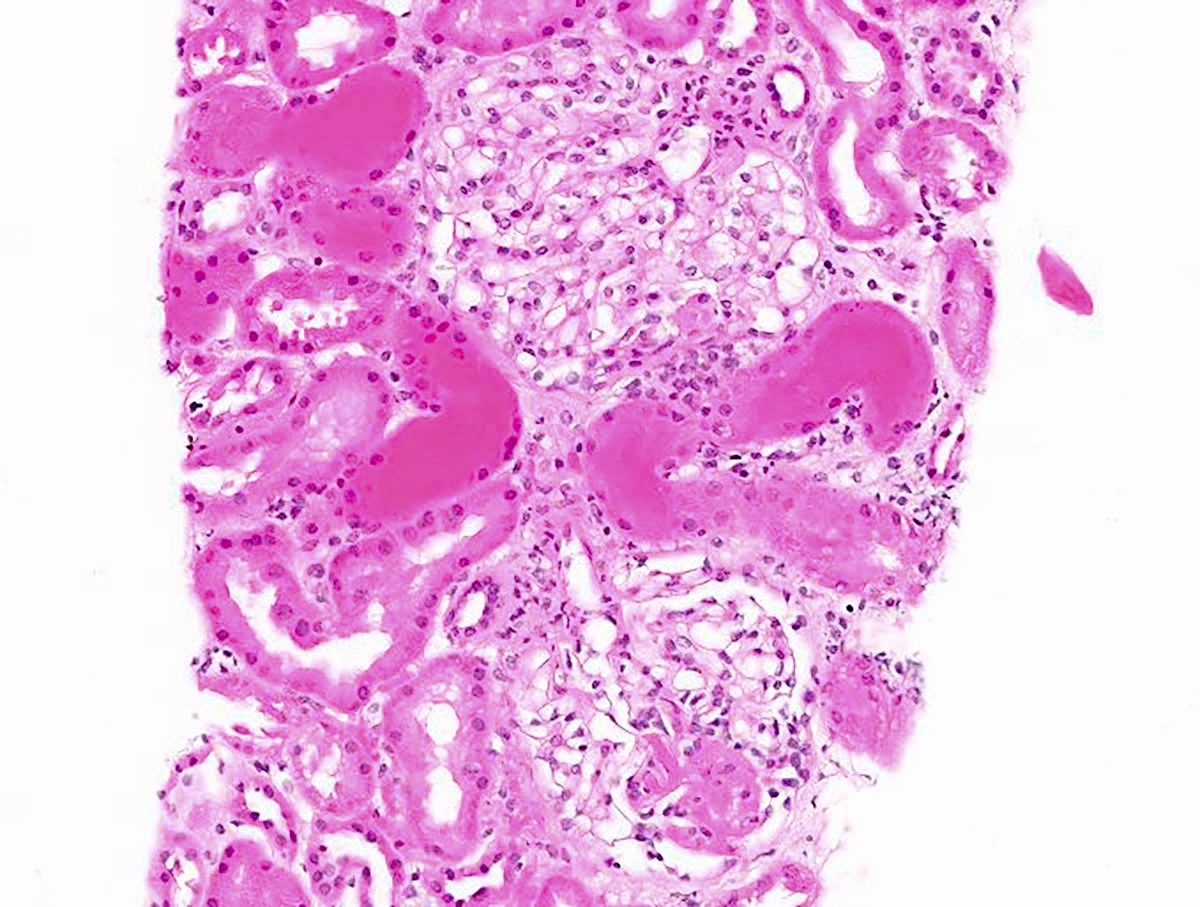
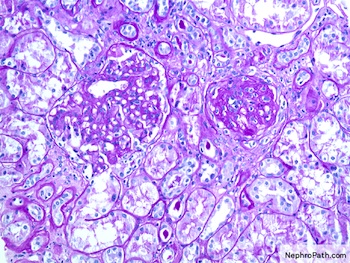
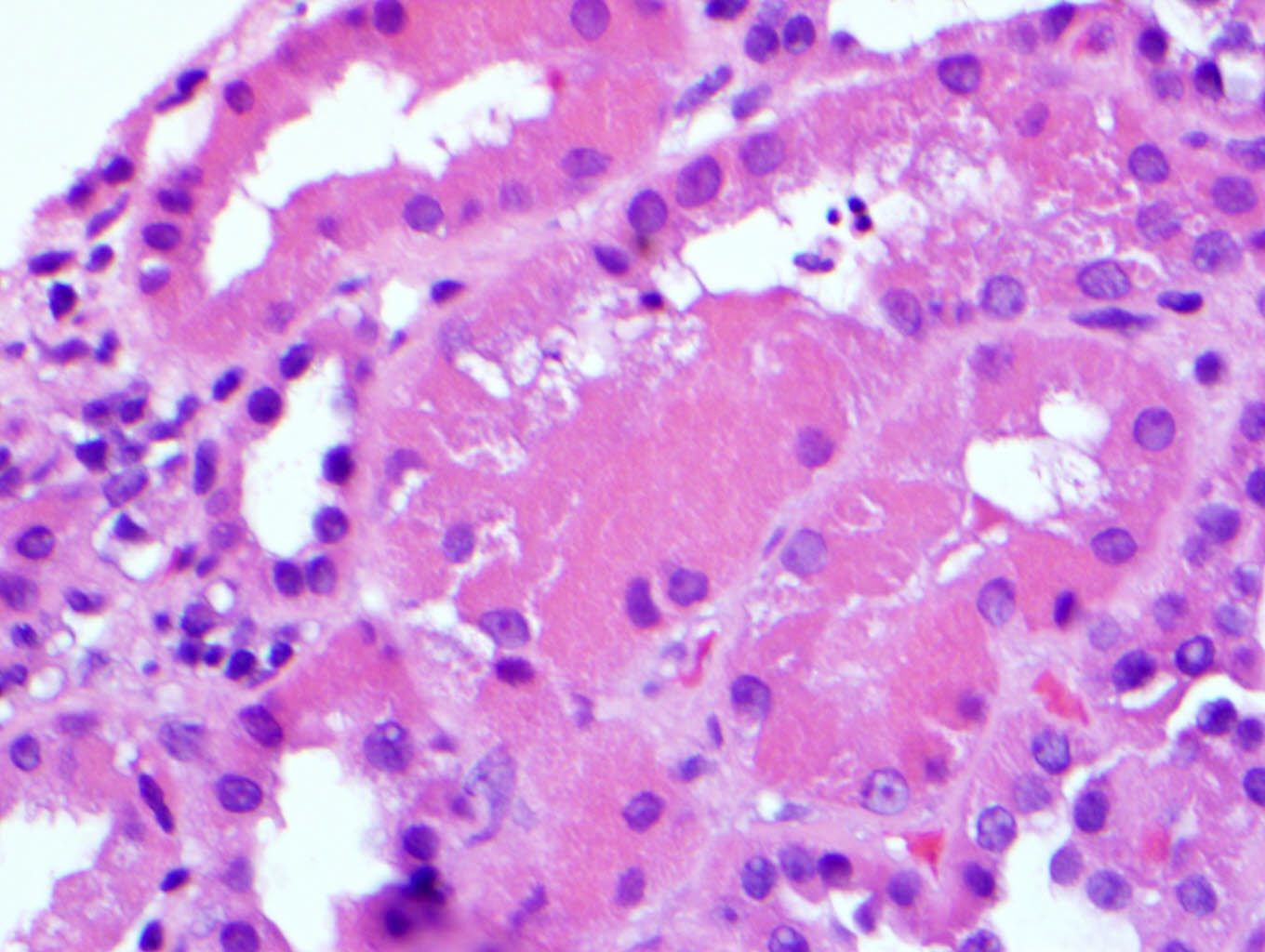
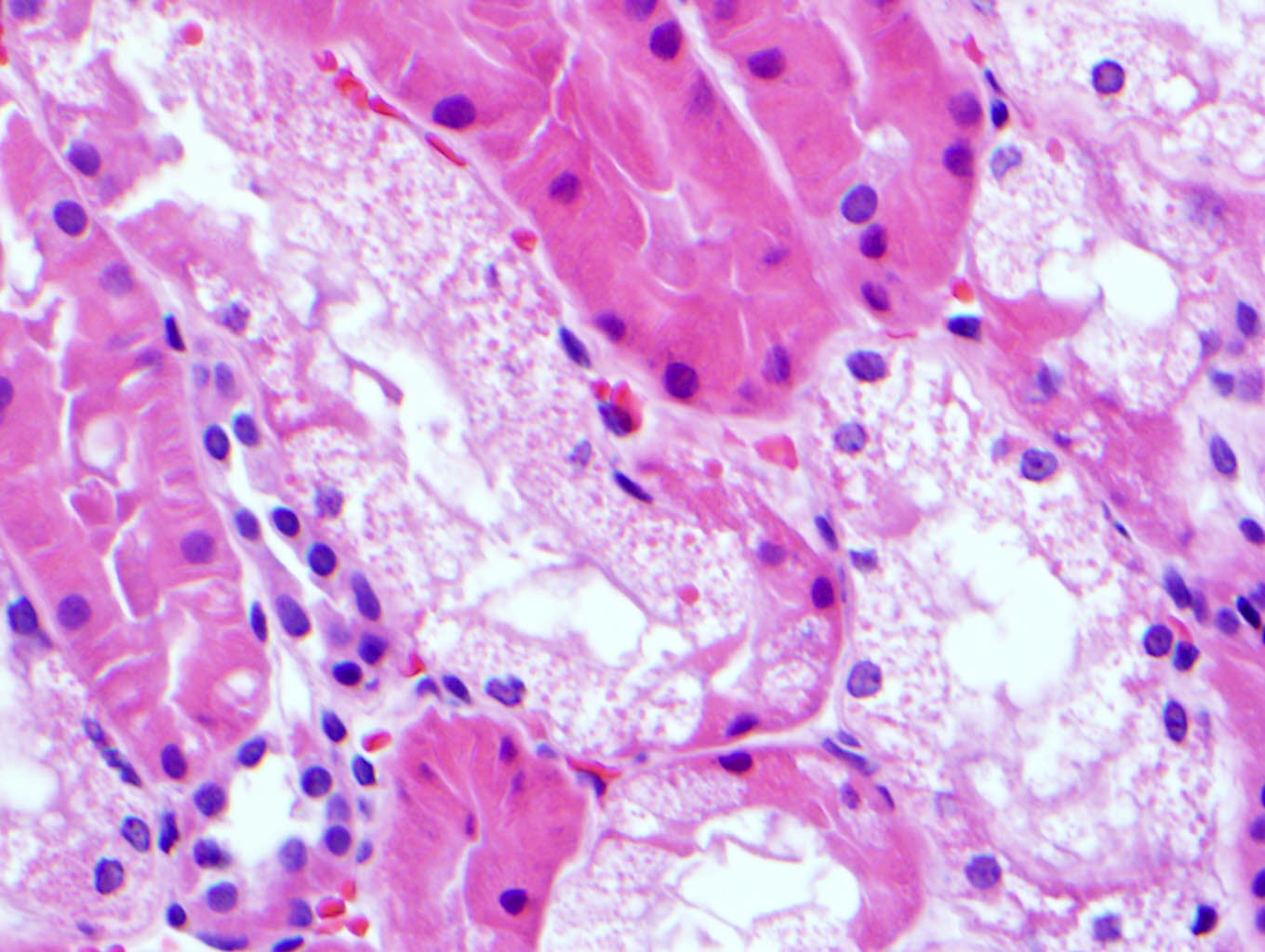
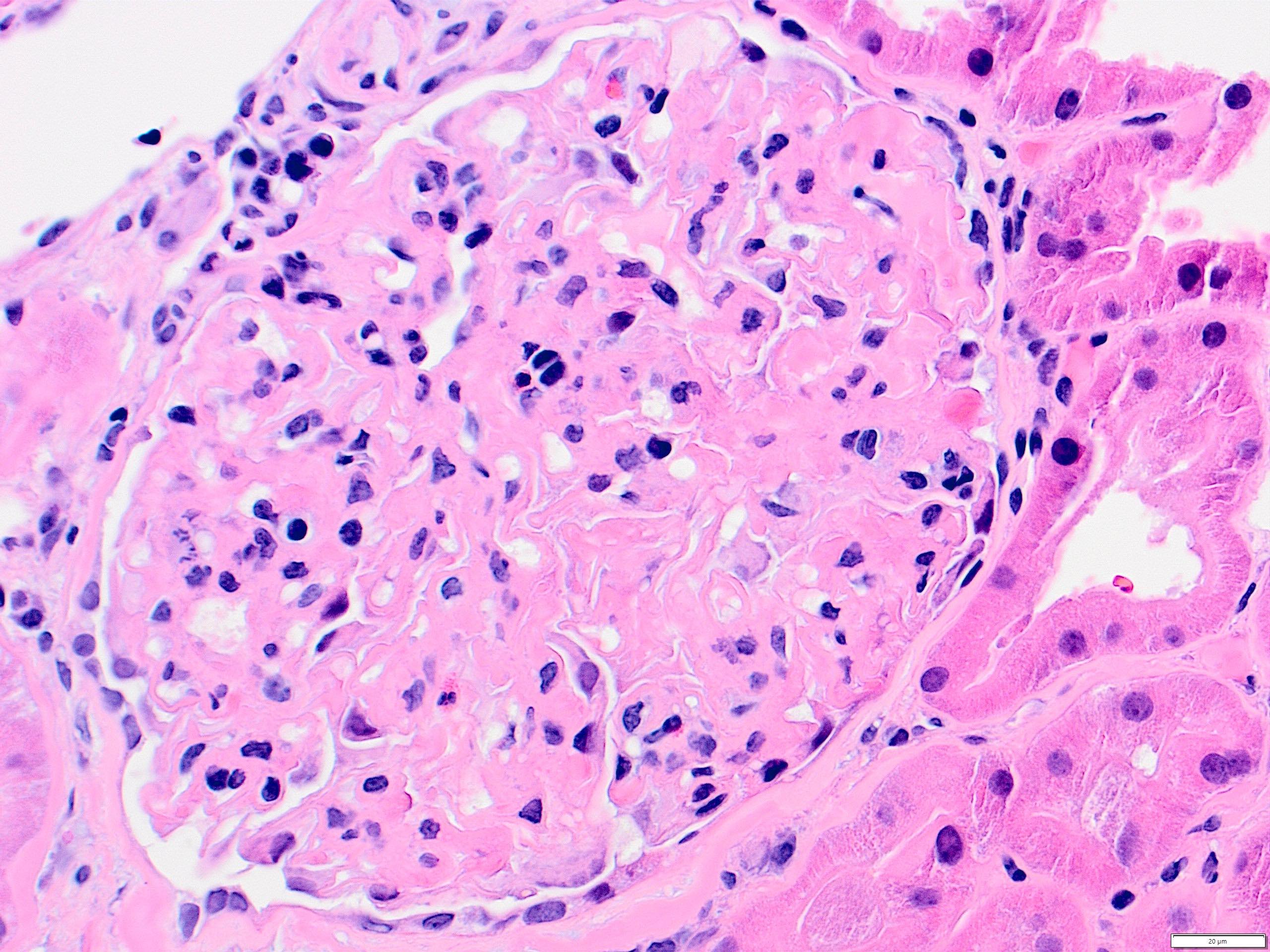
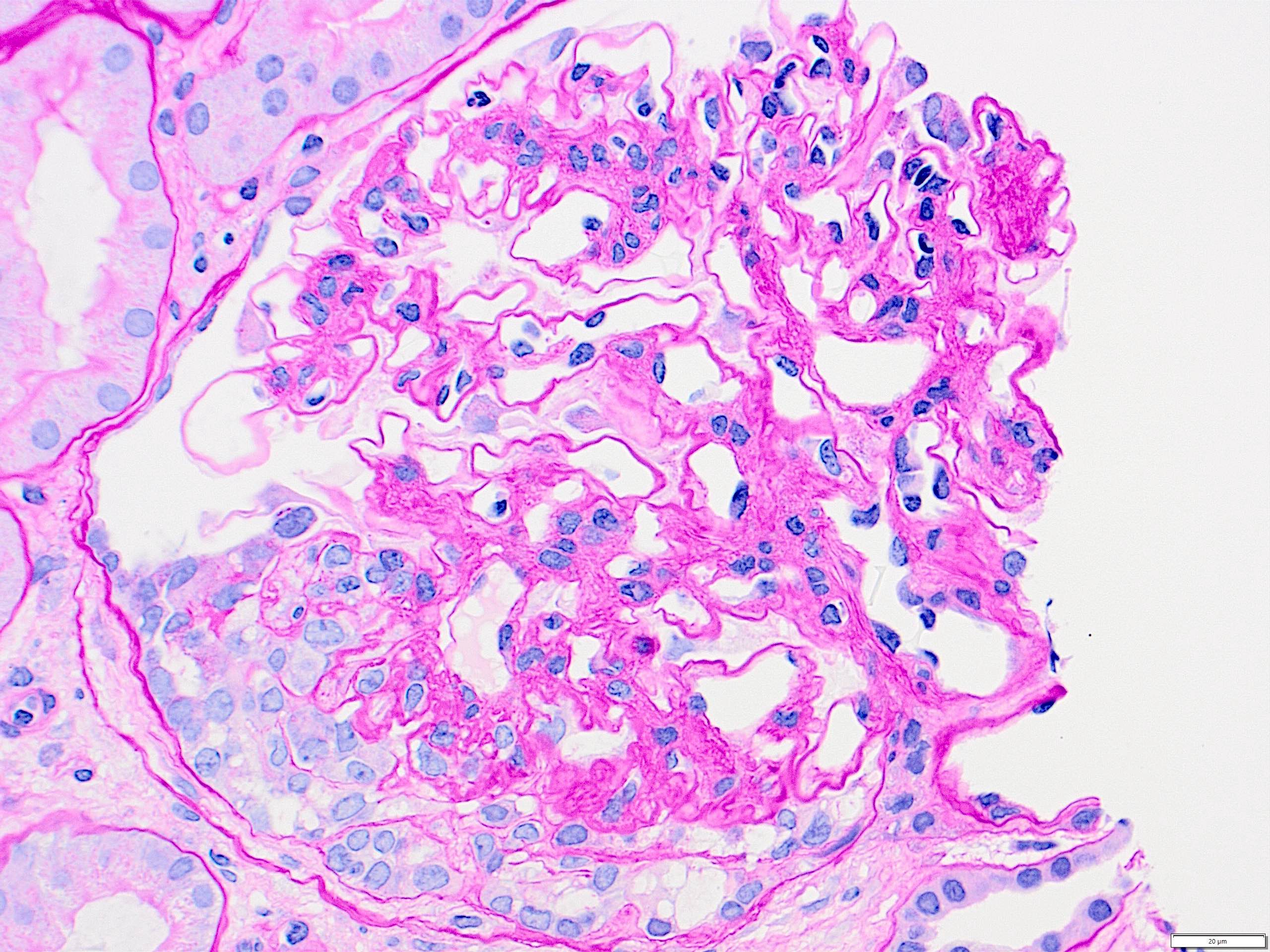
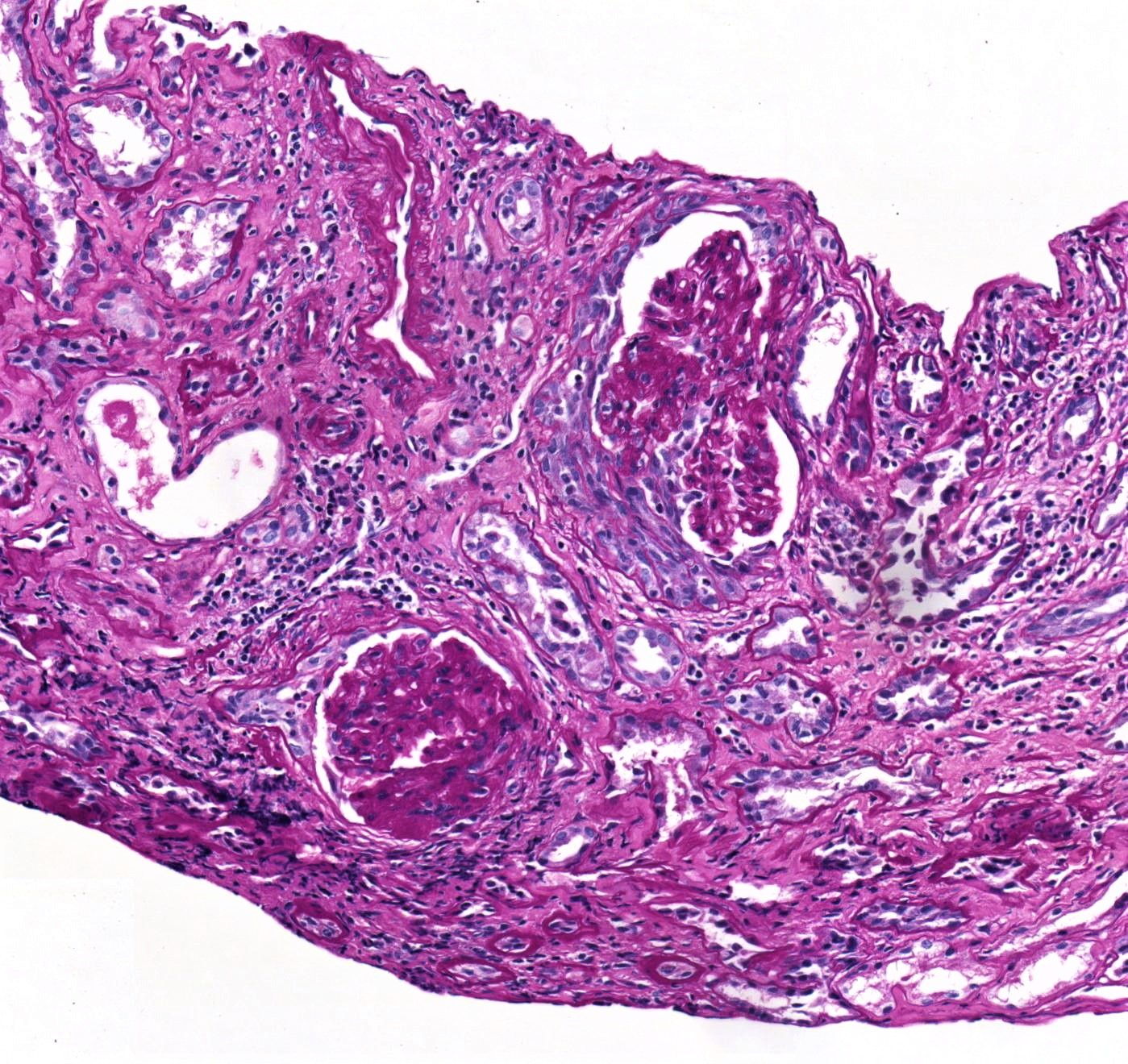
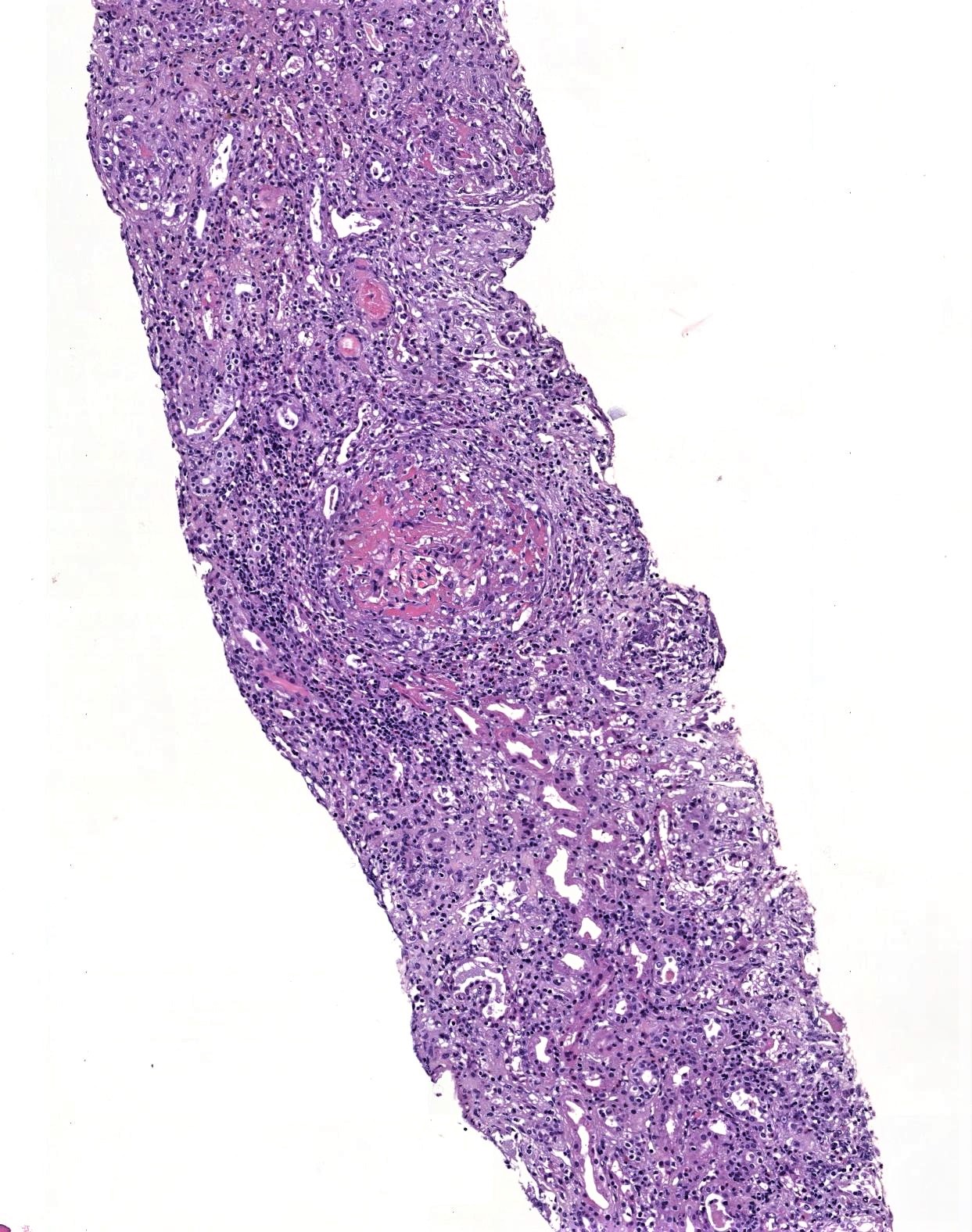
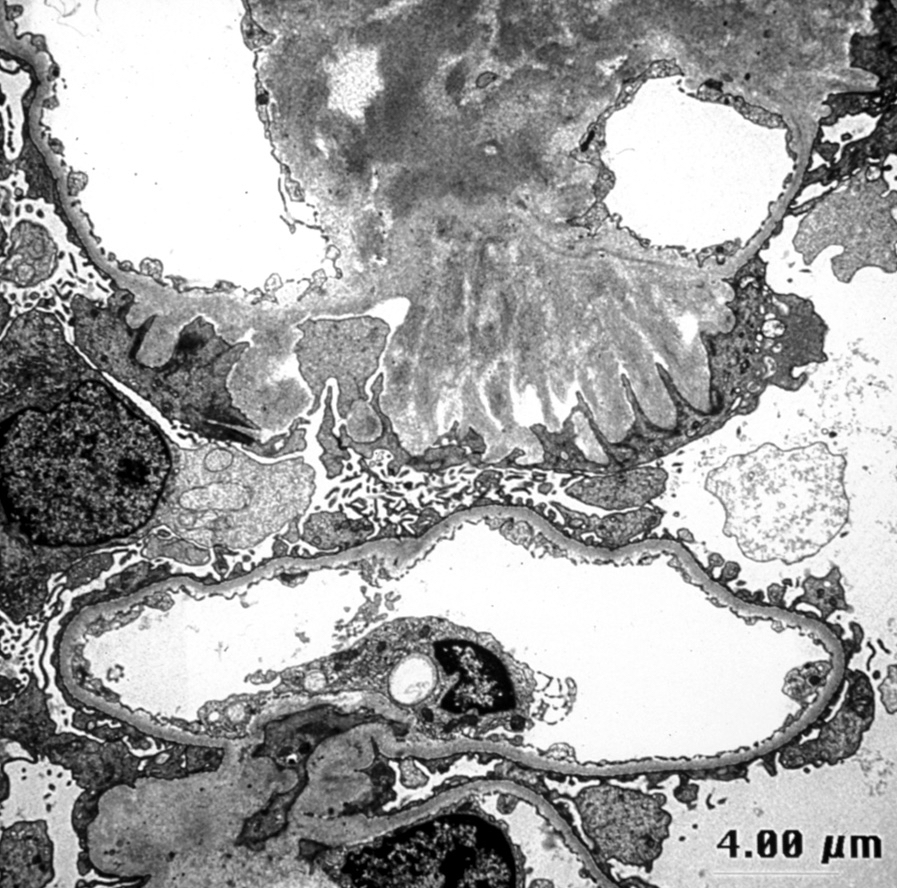
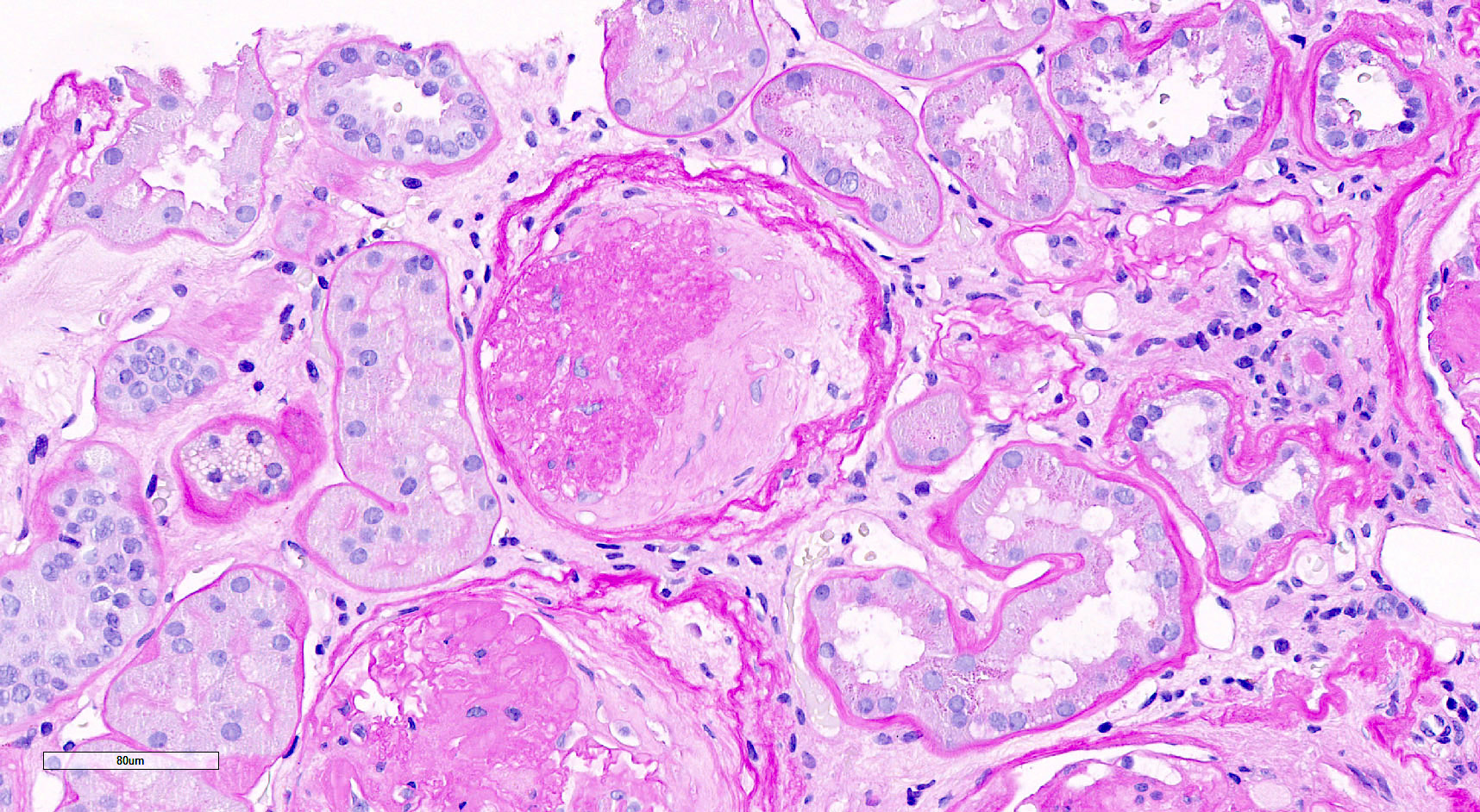
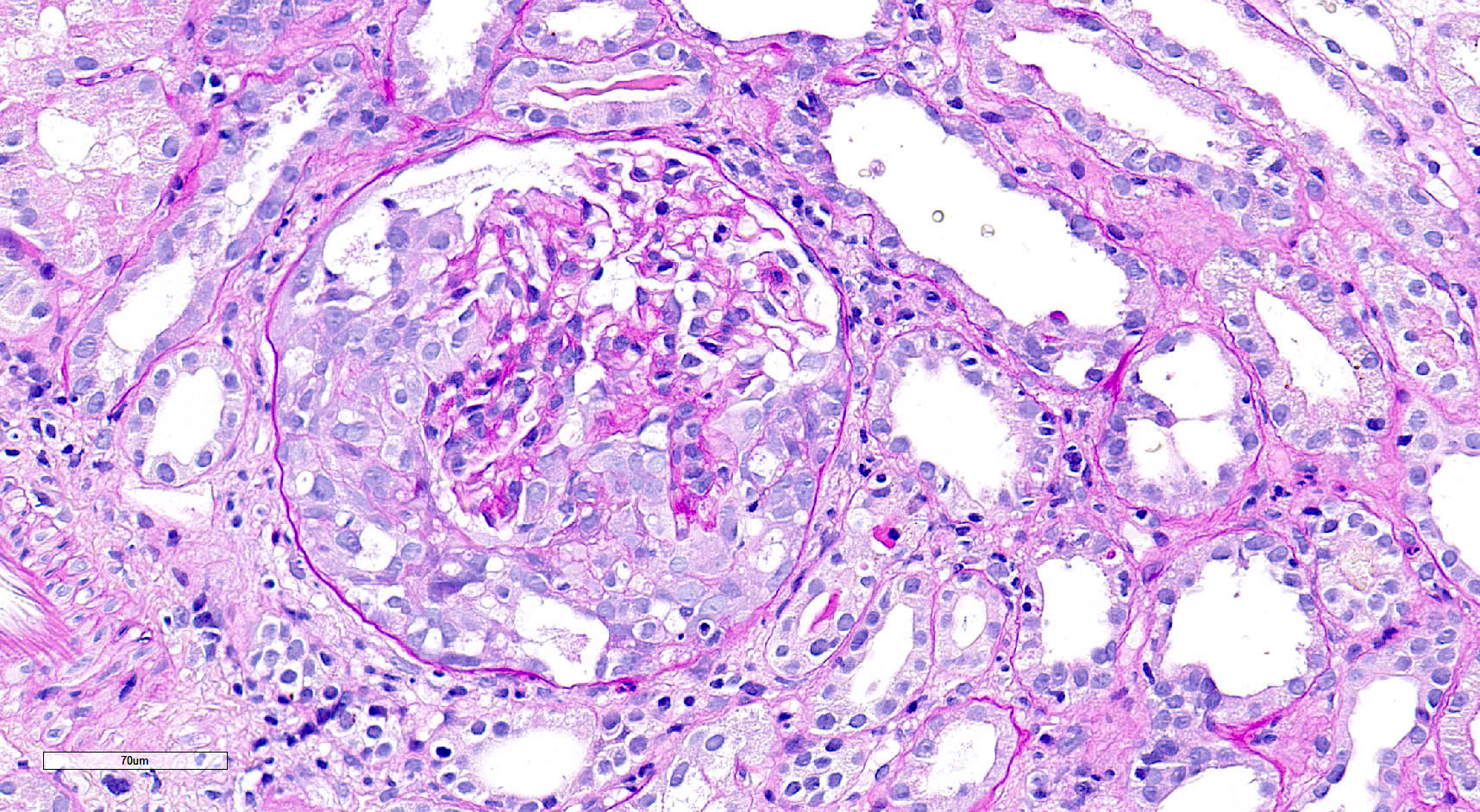
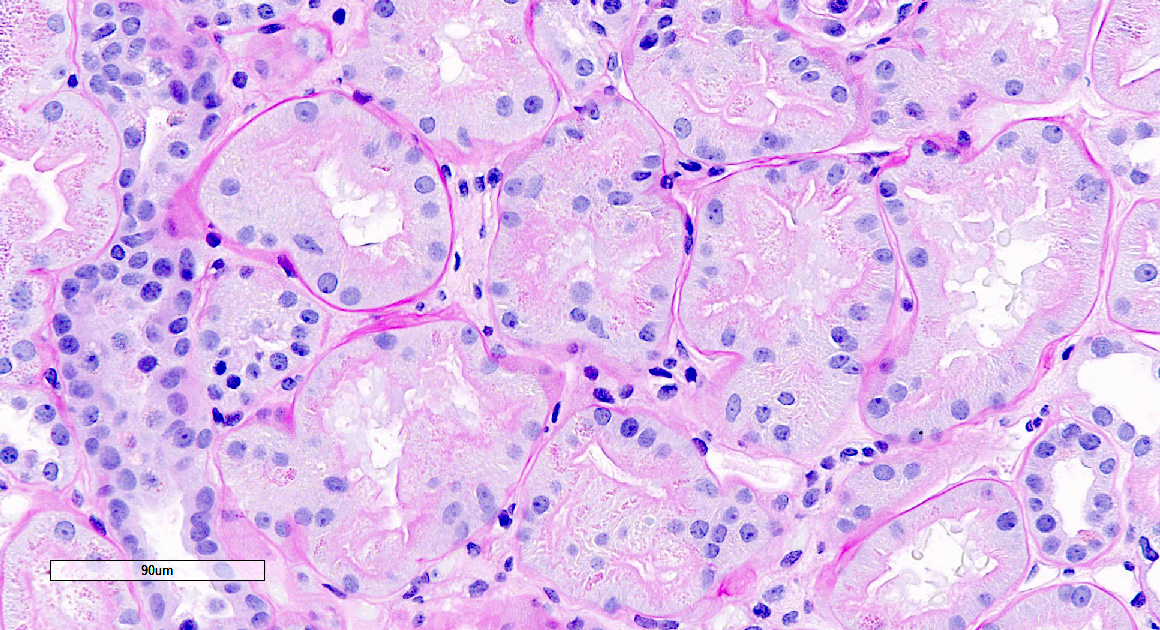
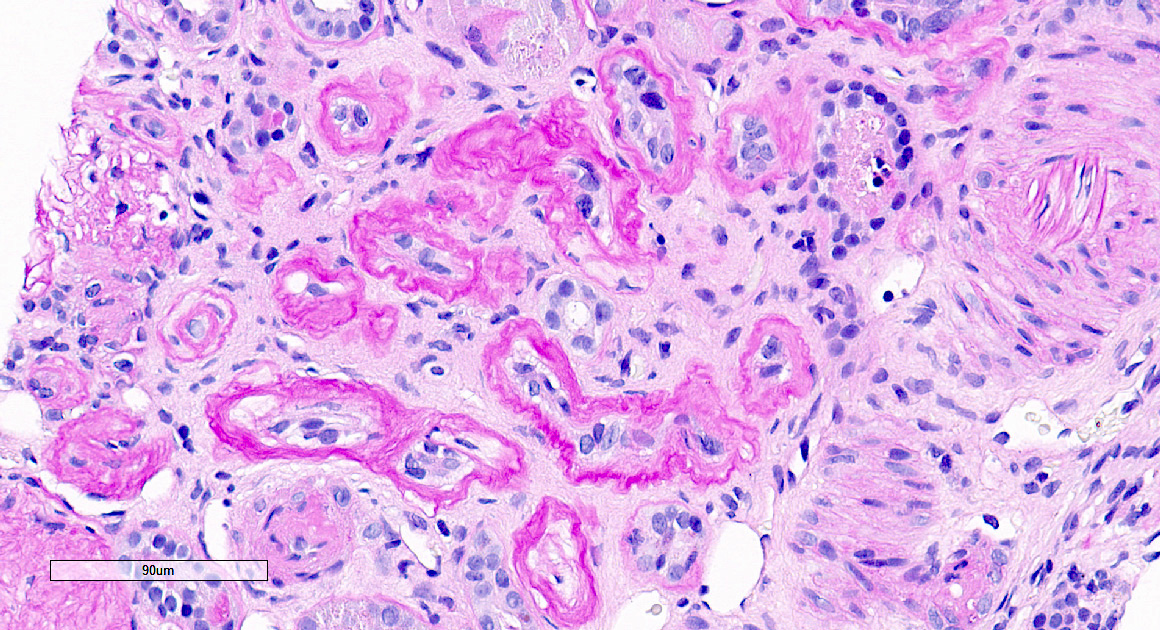
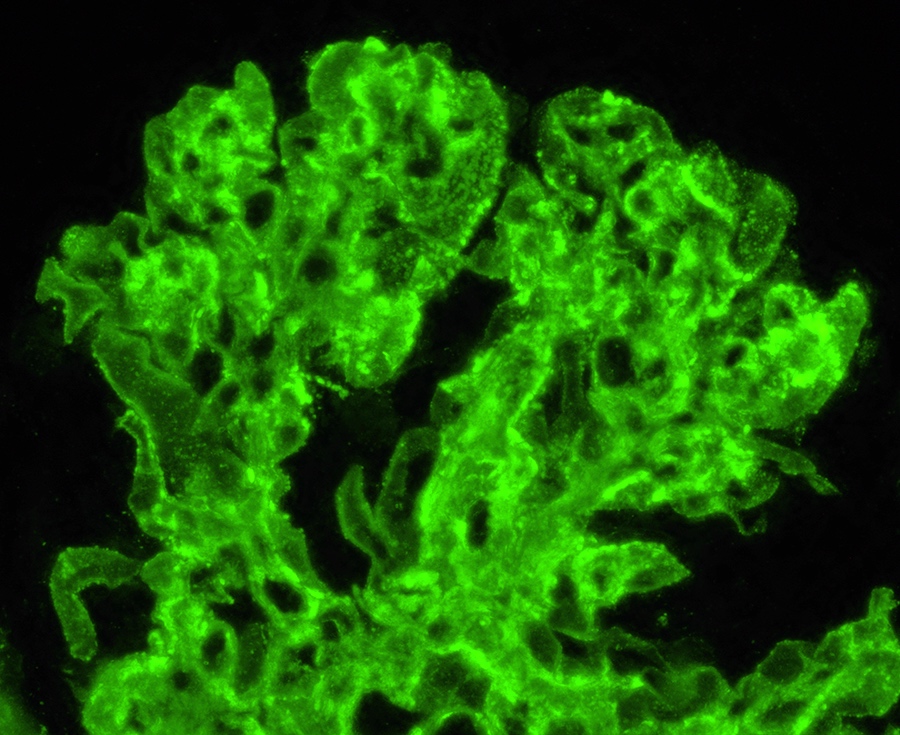
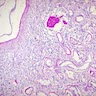
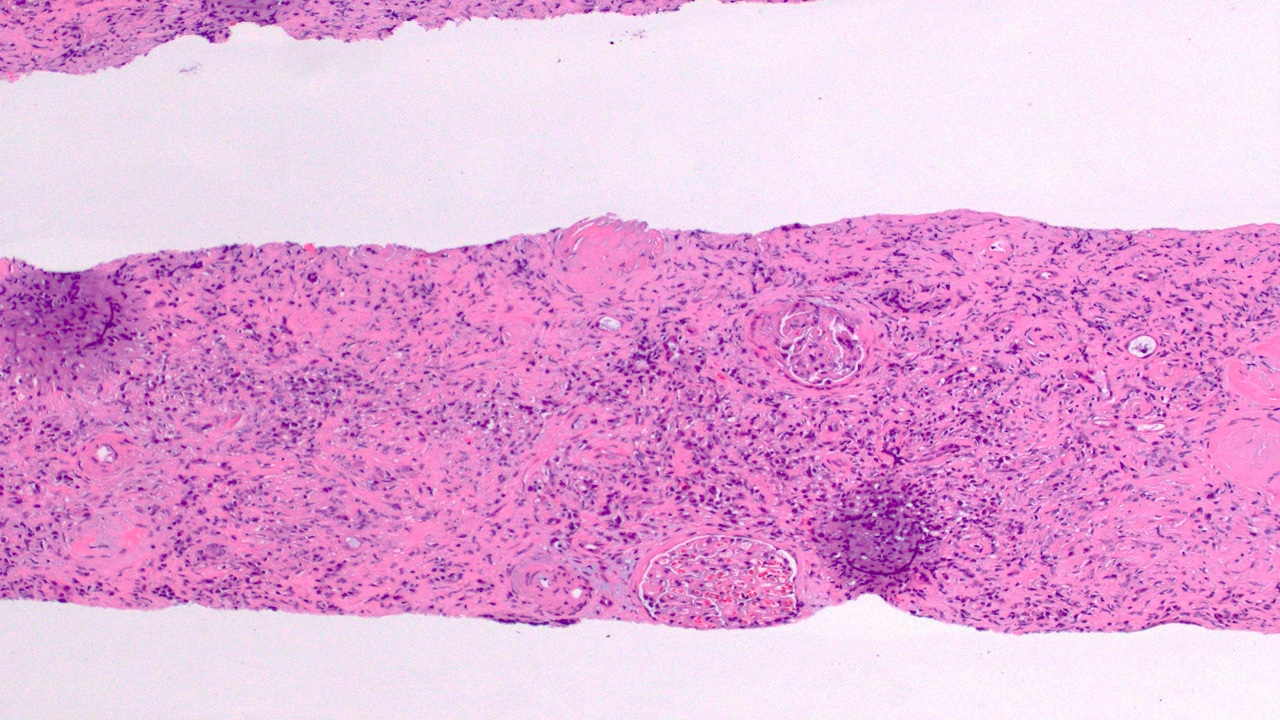
- Kidney, allograft biopsy:
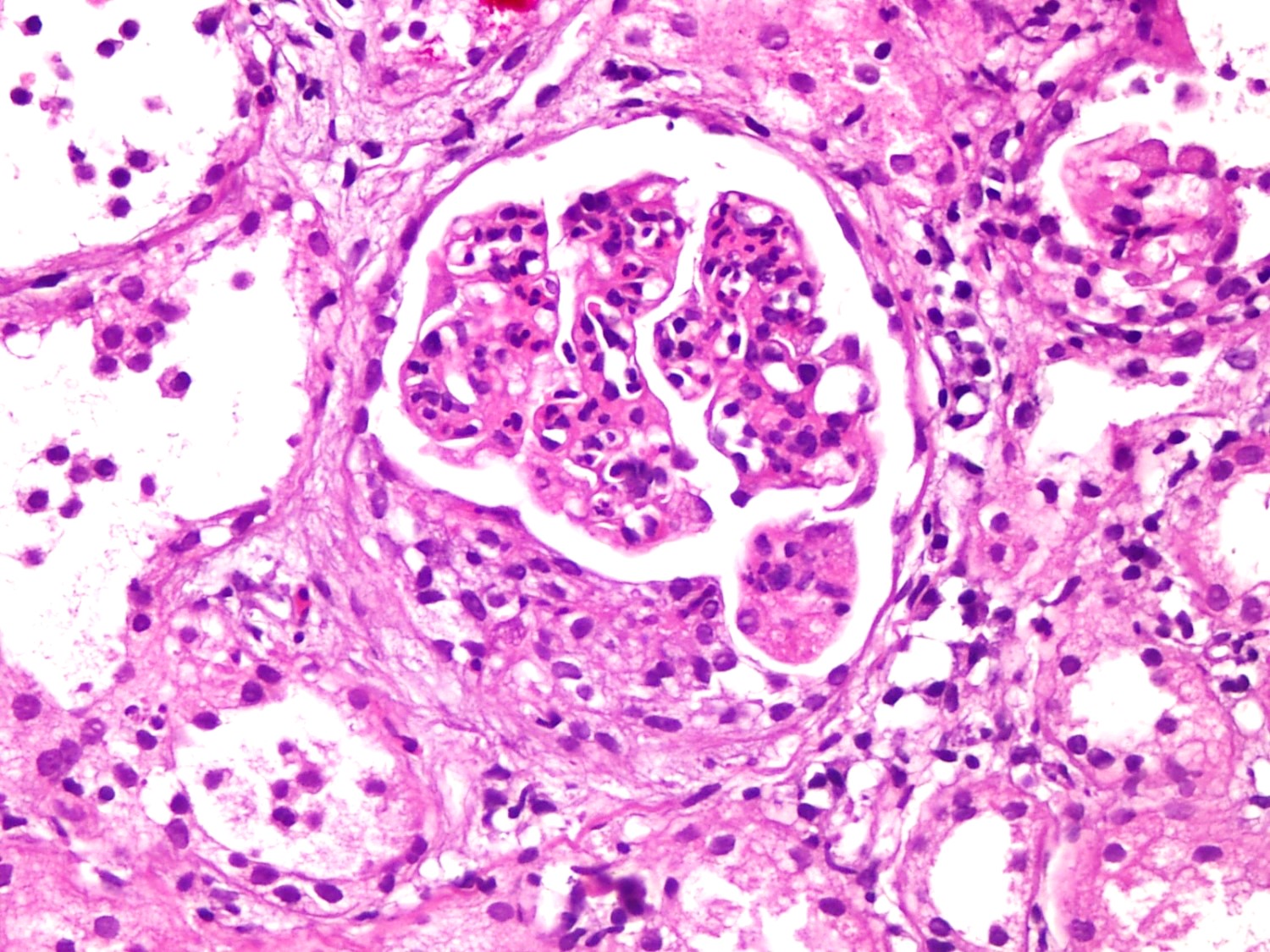
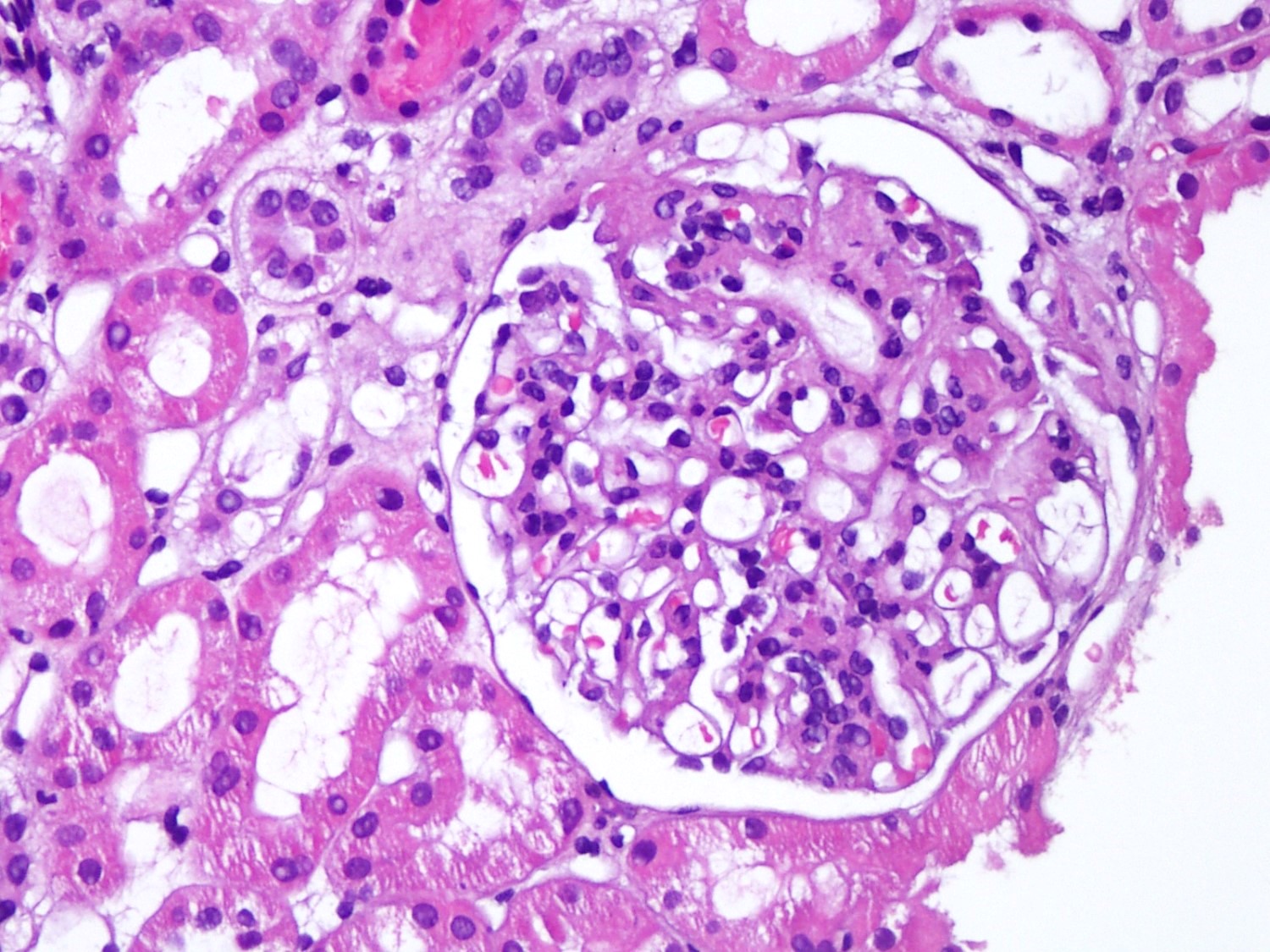
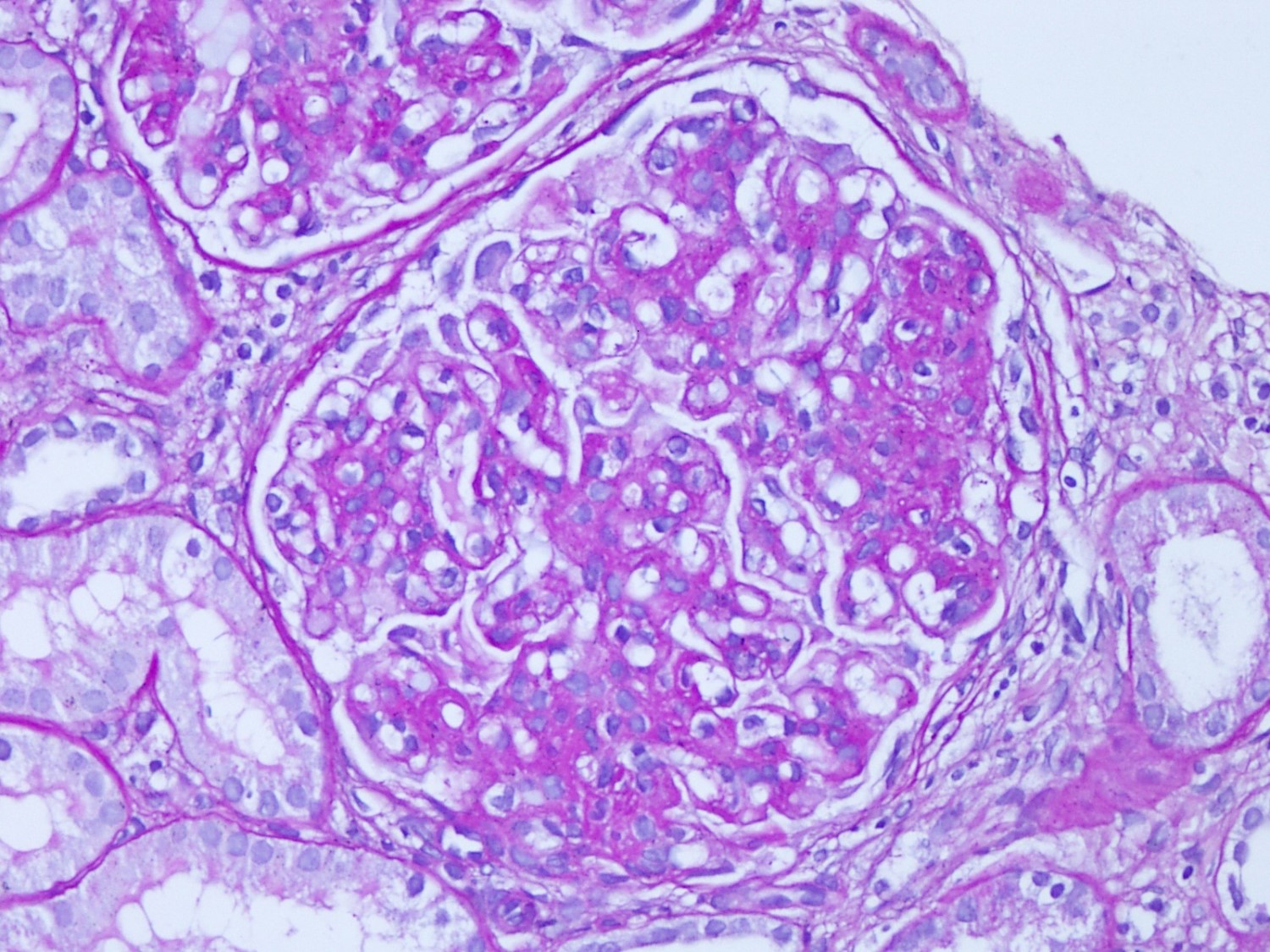
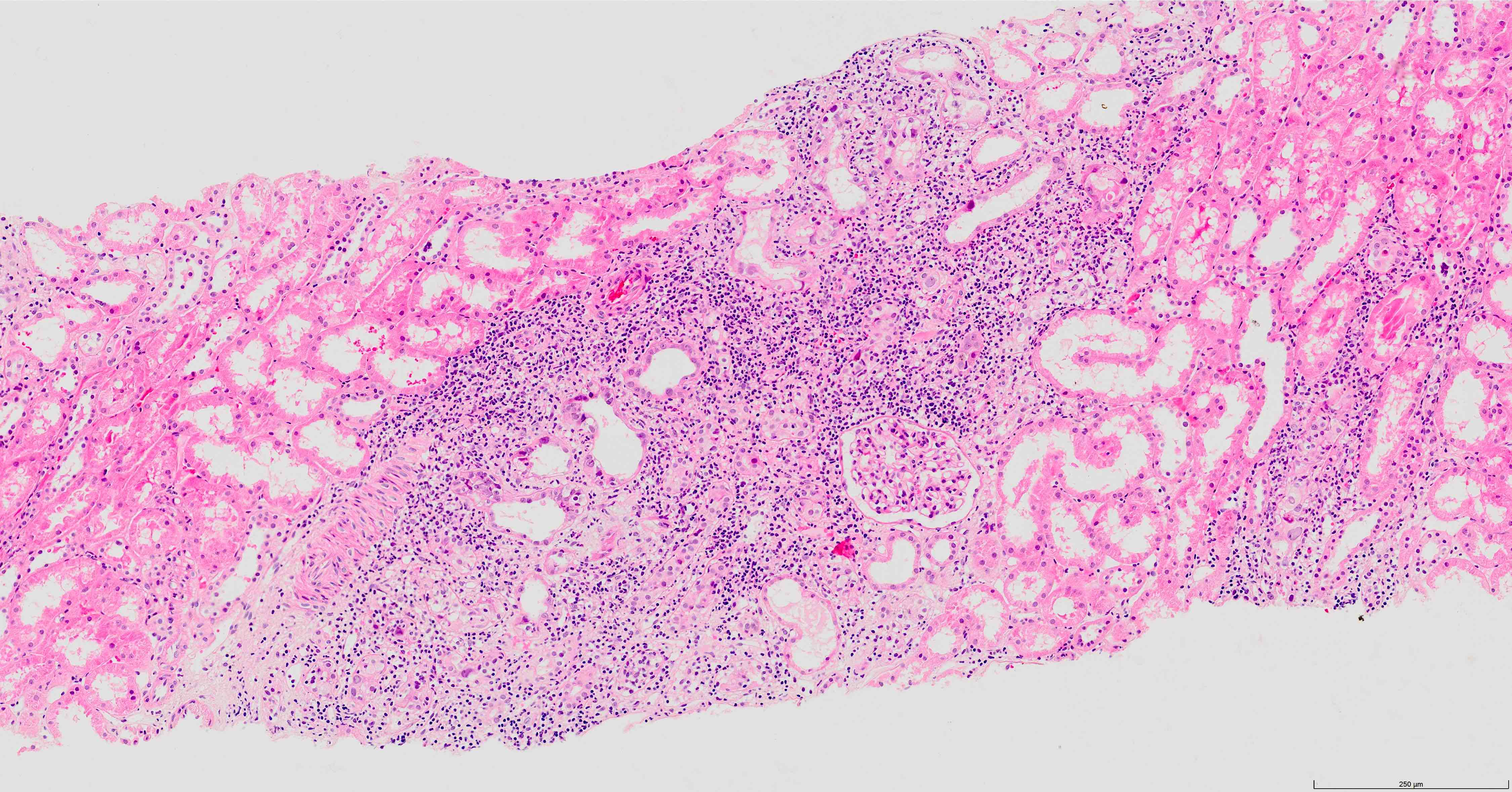
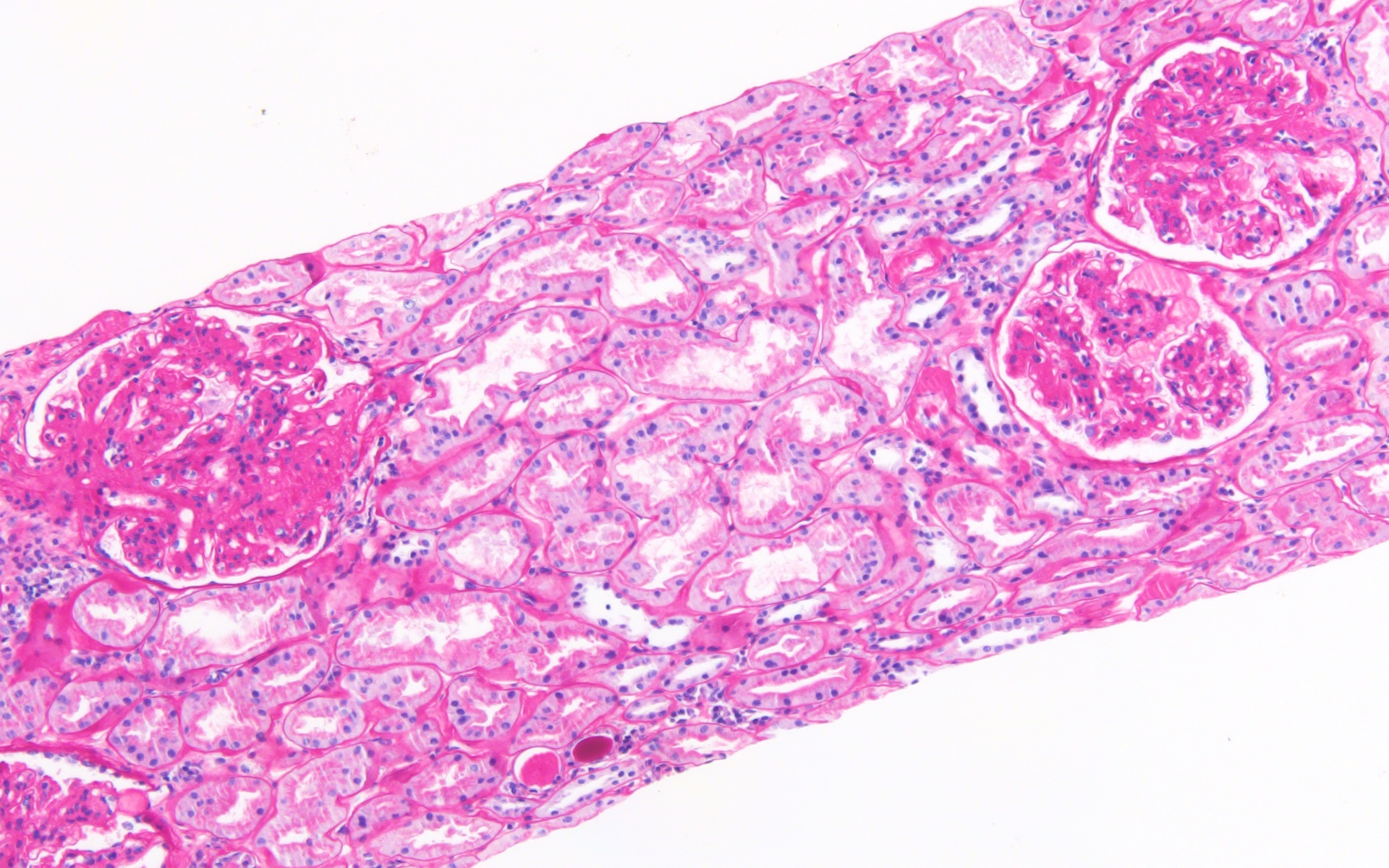
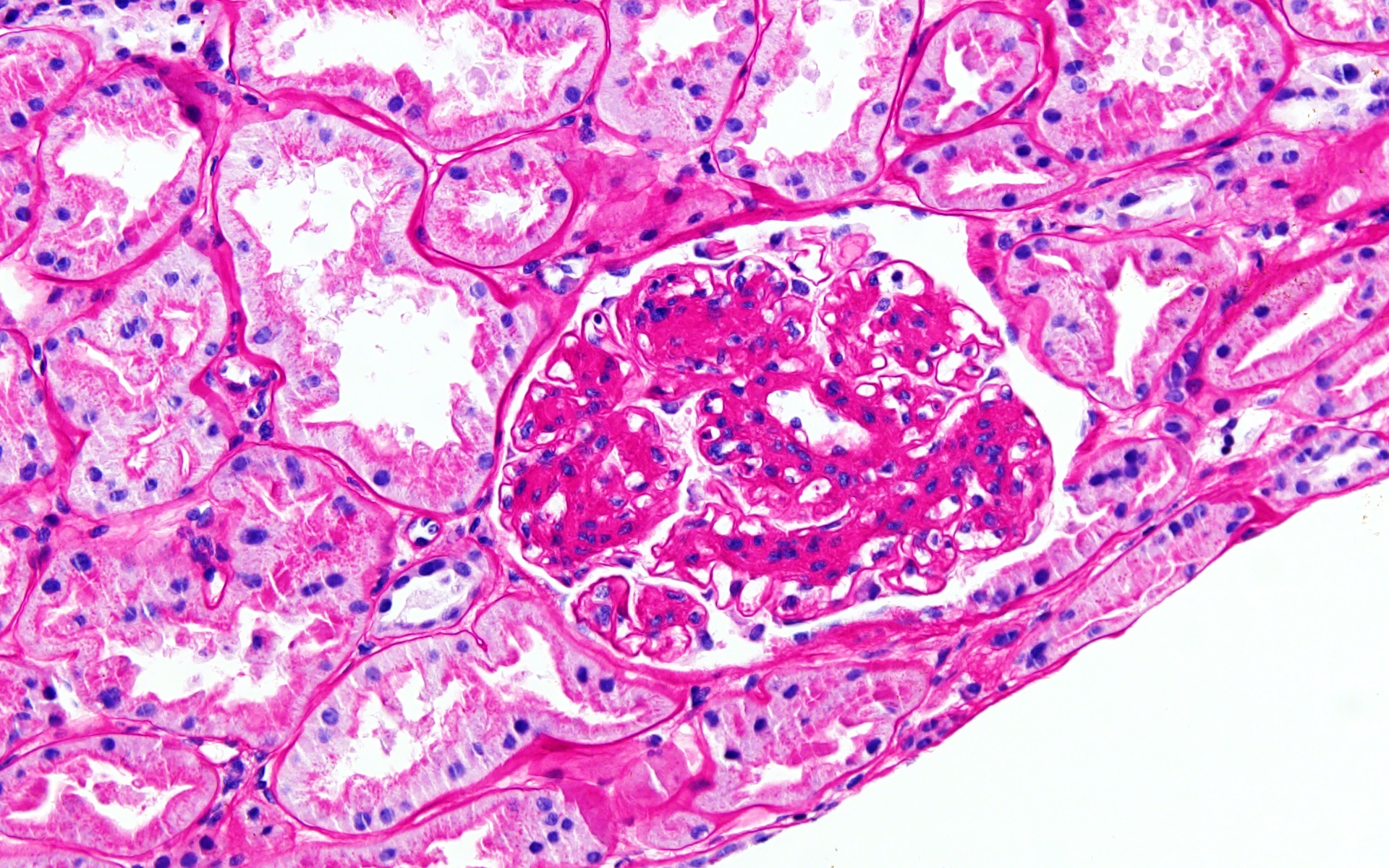
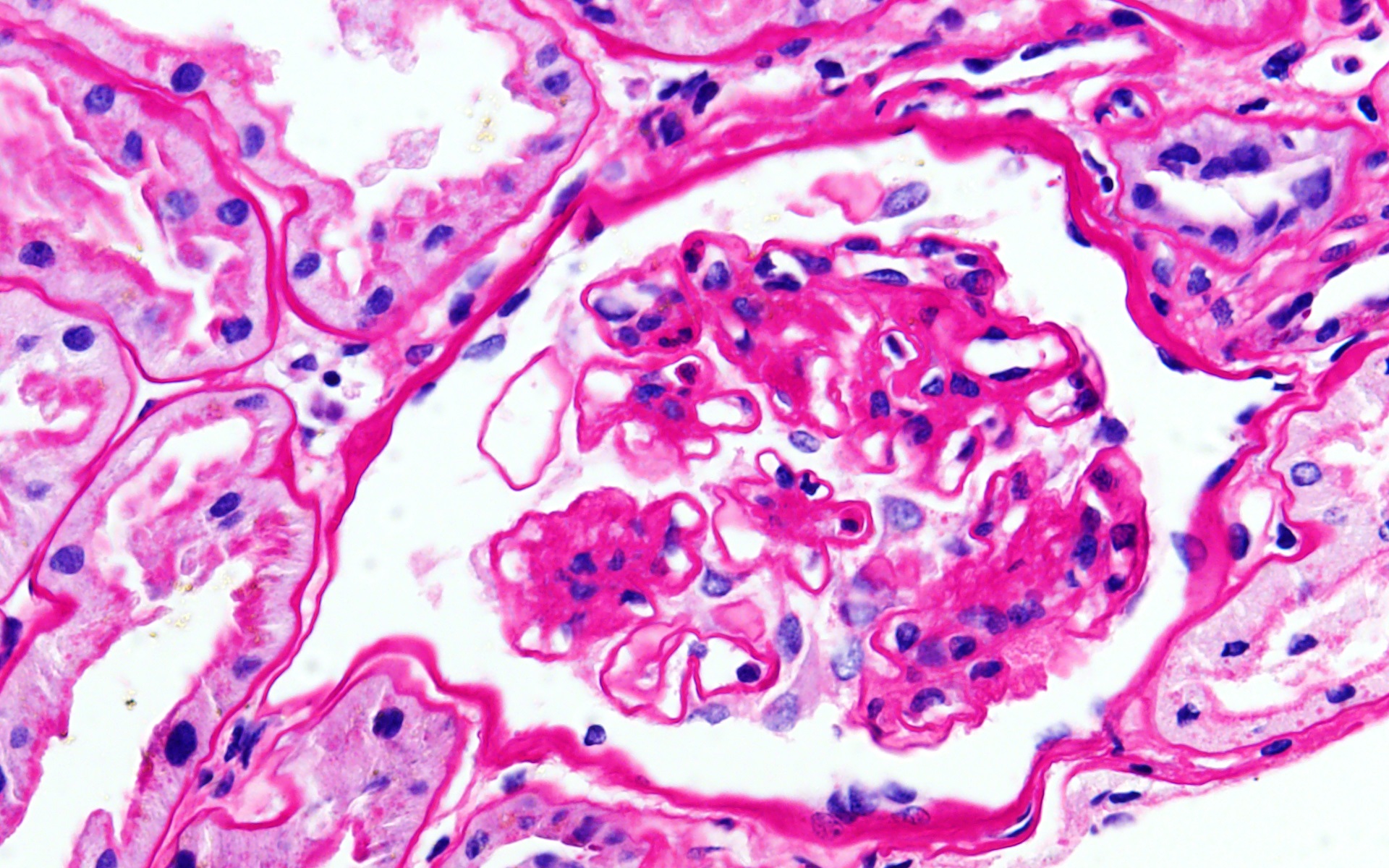
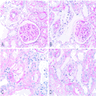
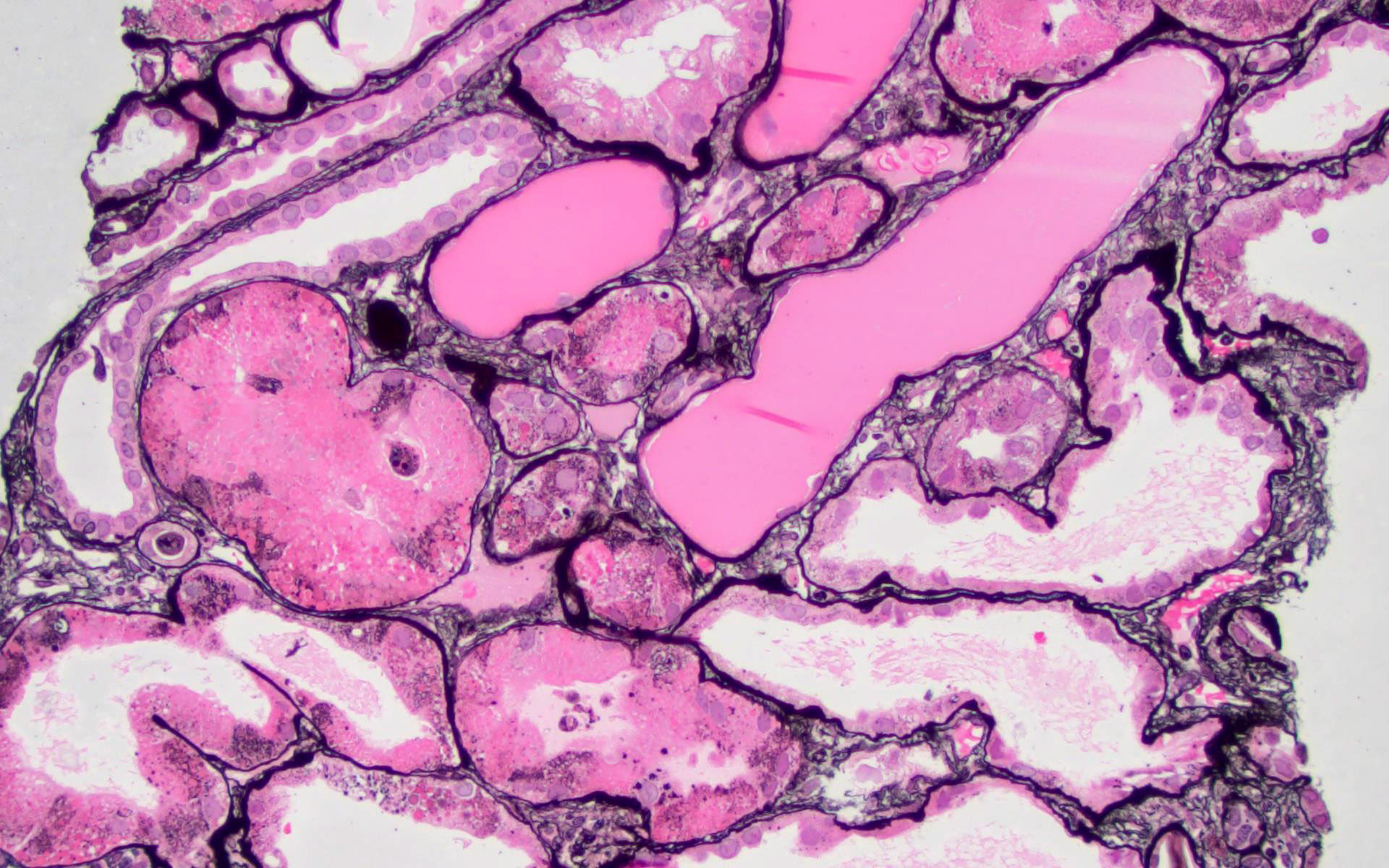
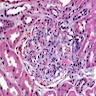
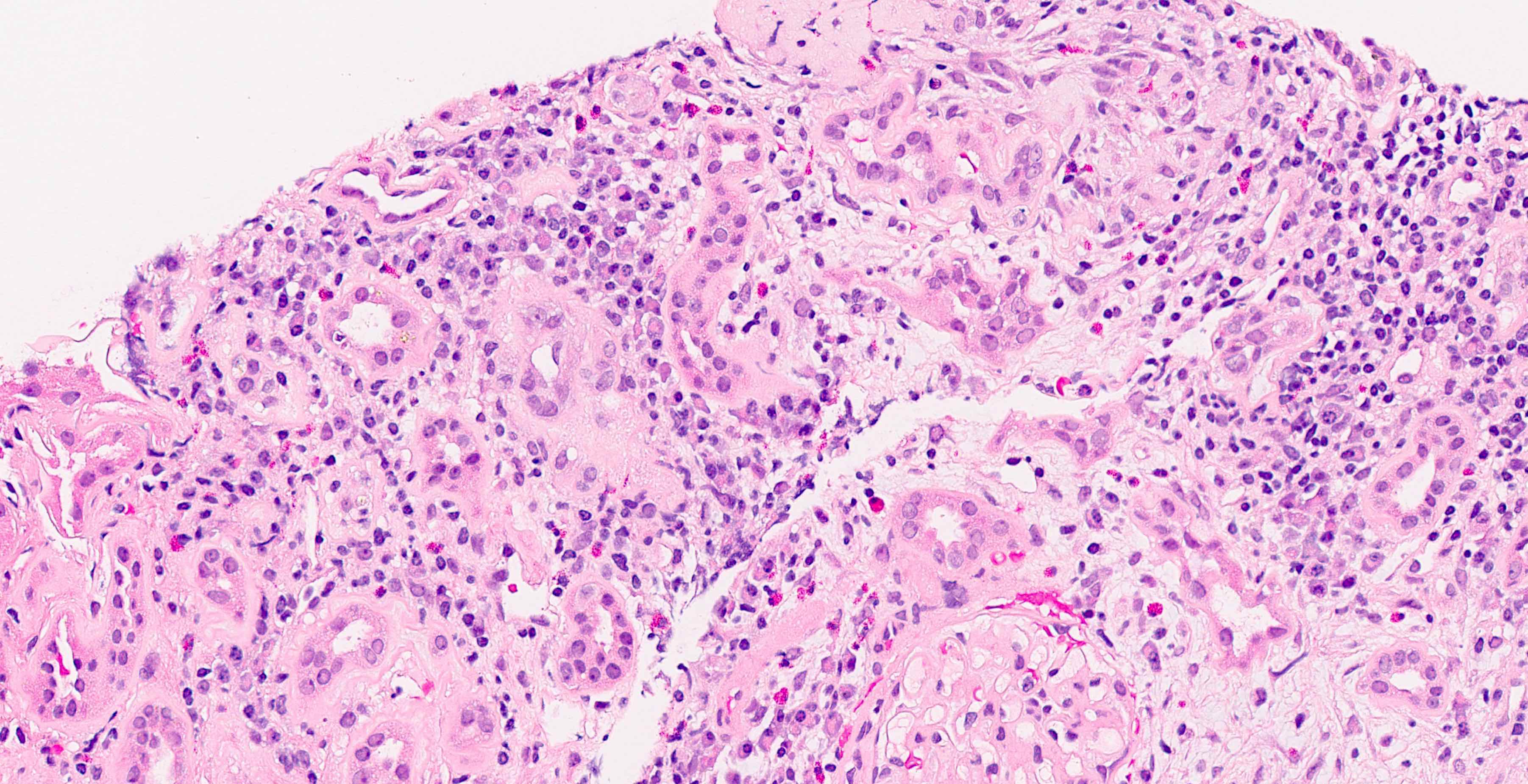
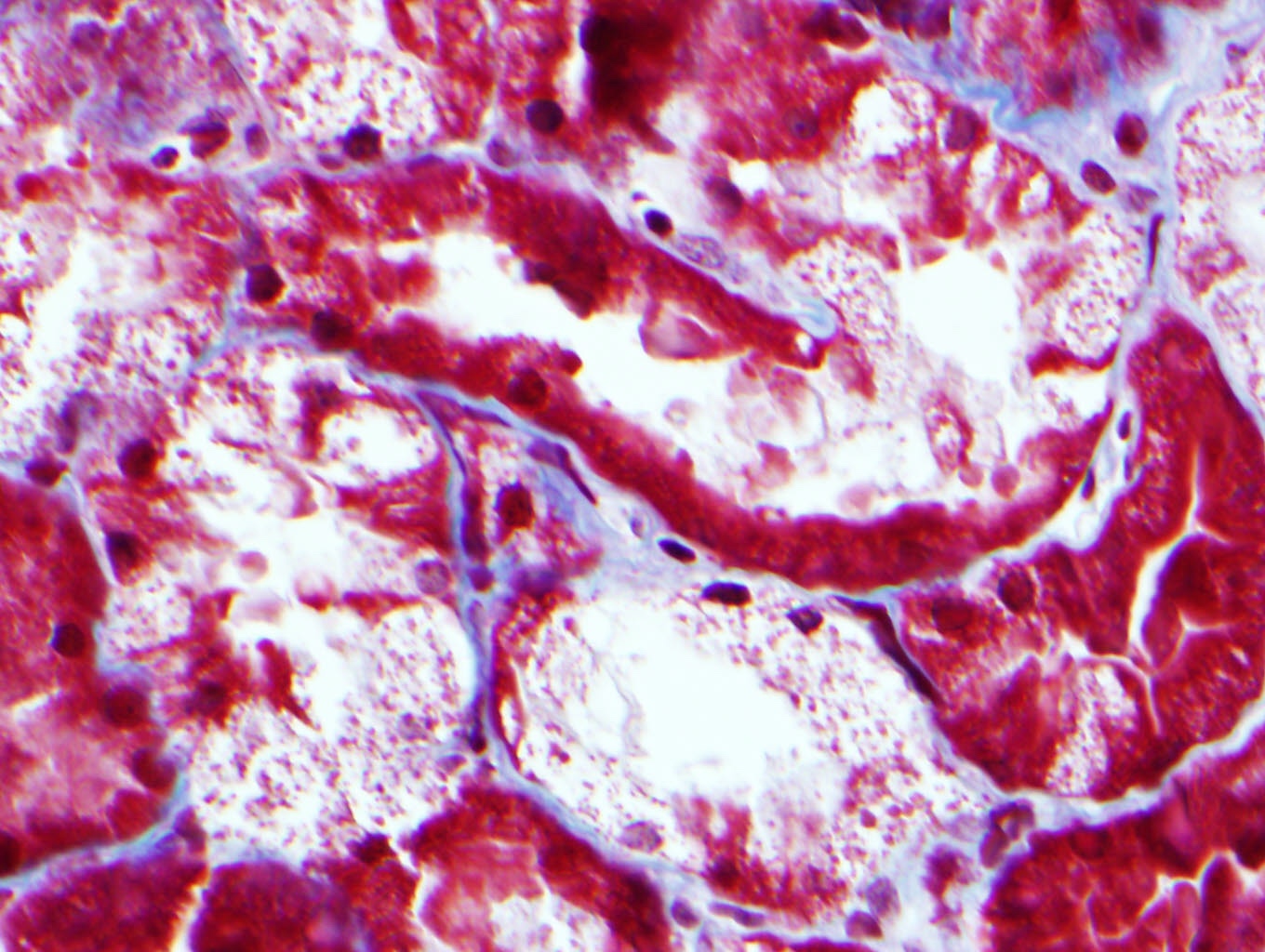
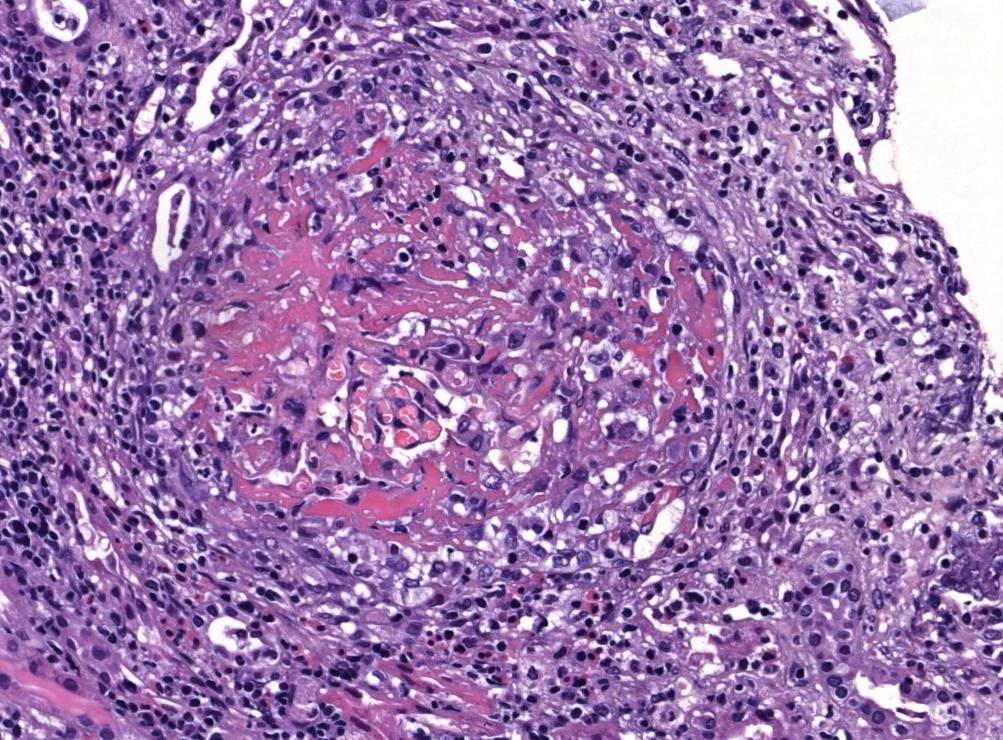
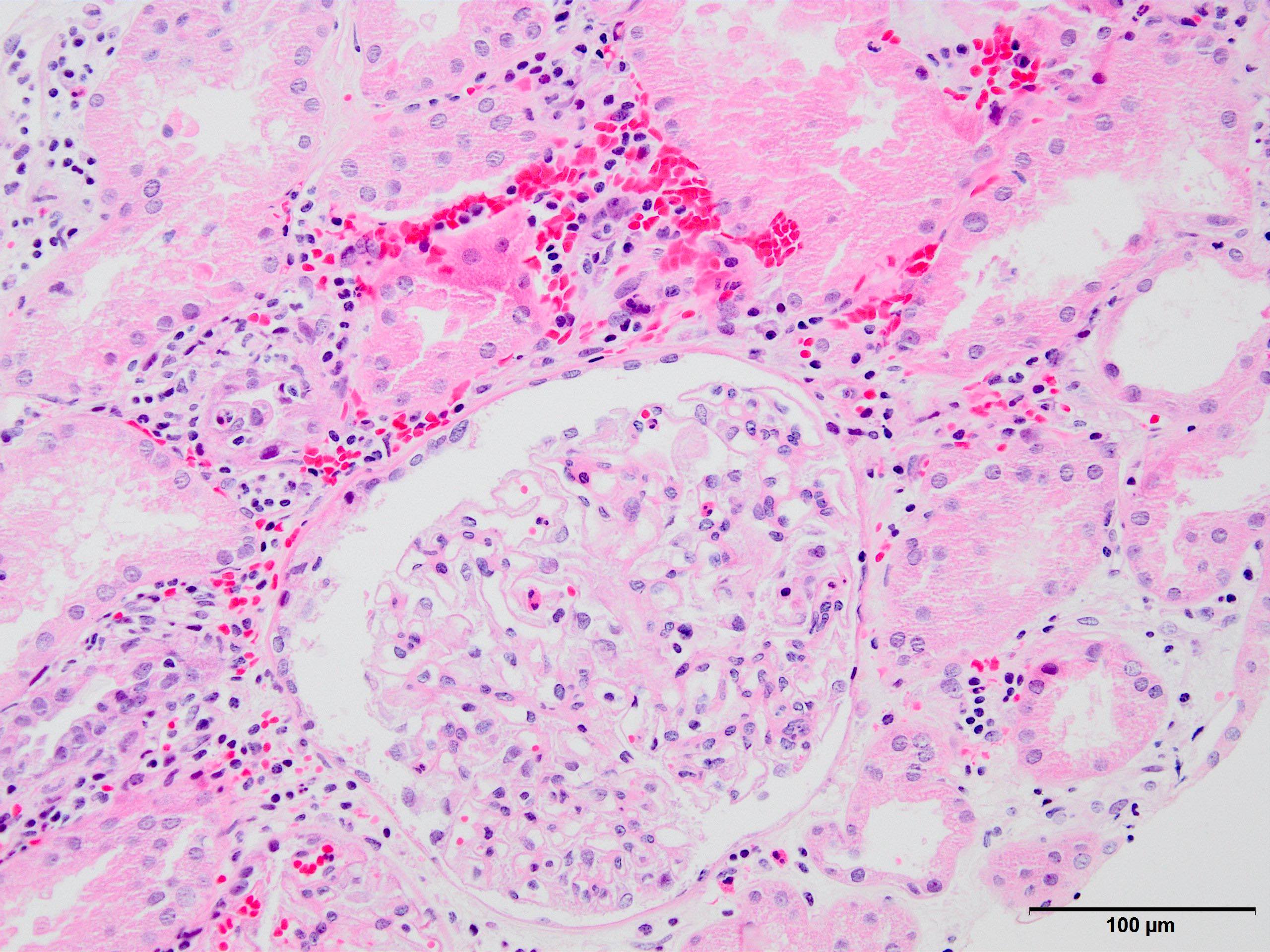
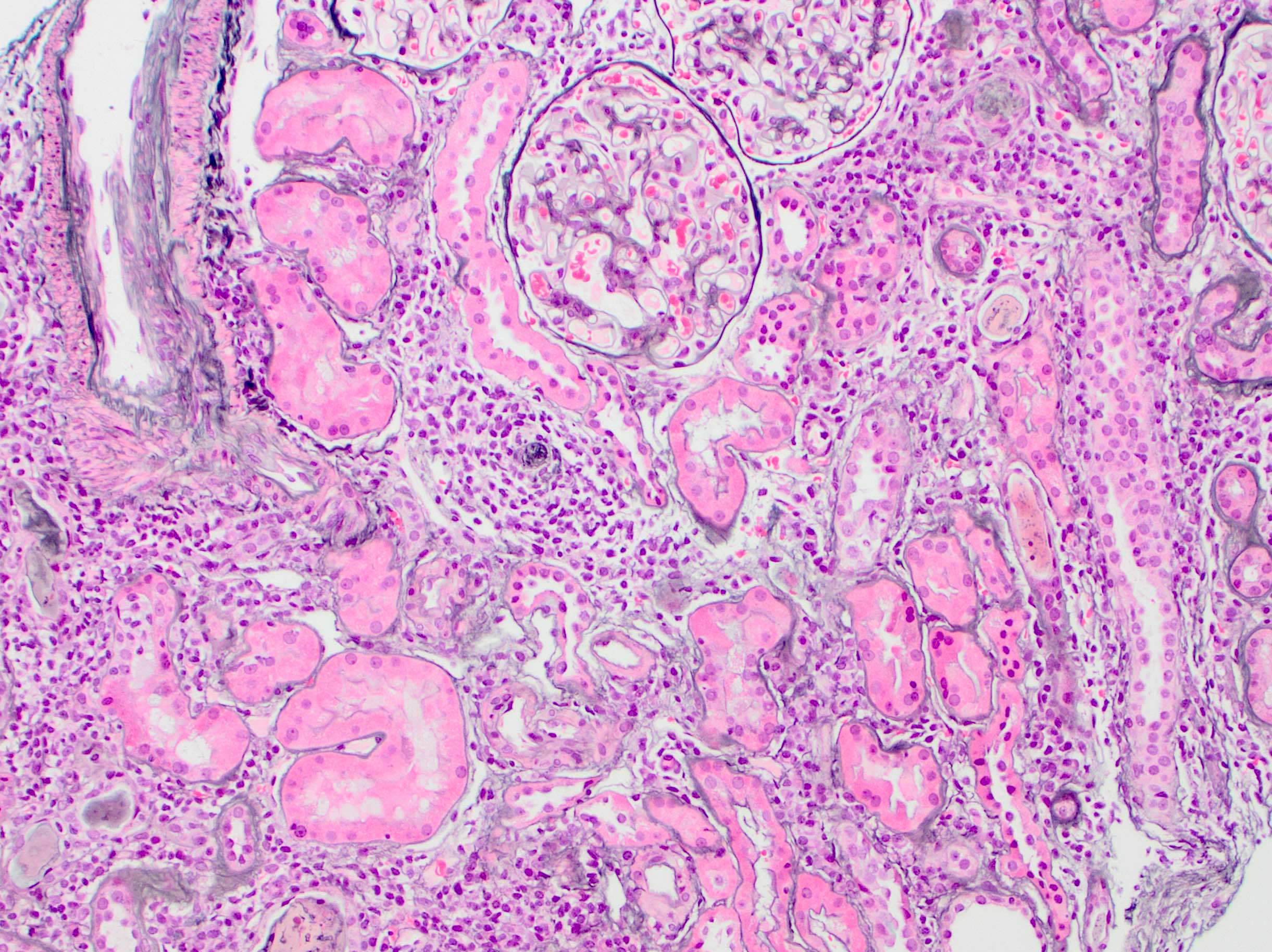
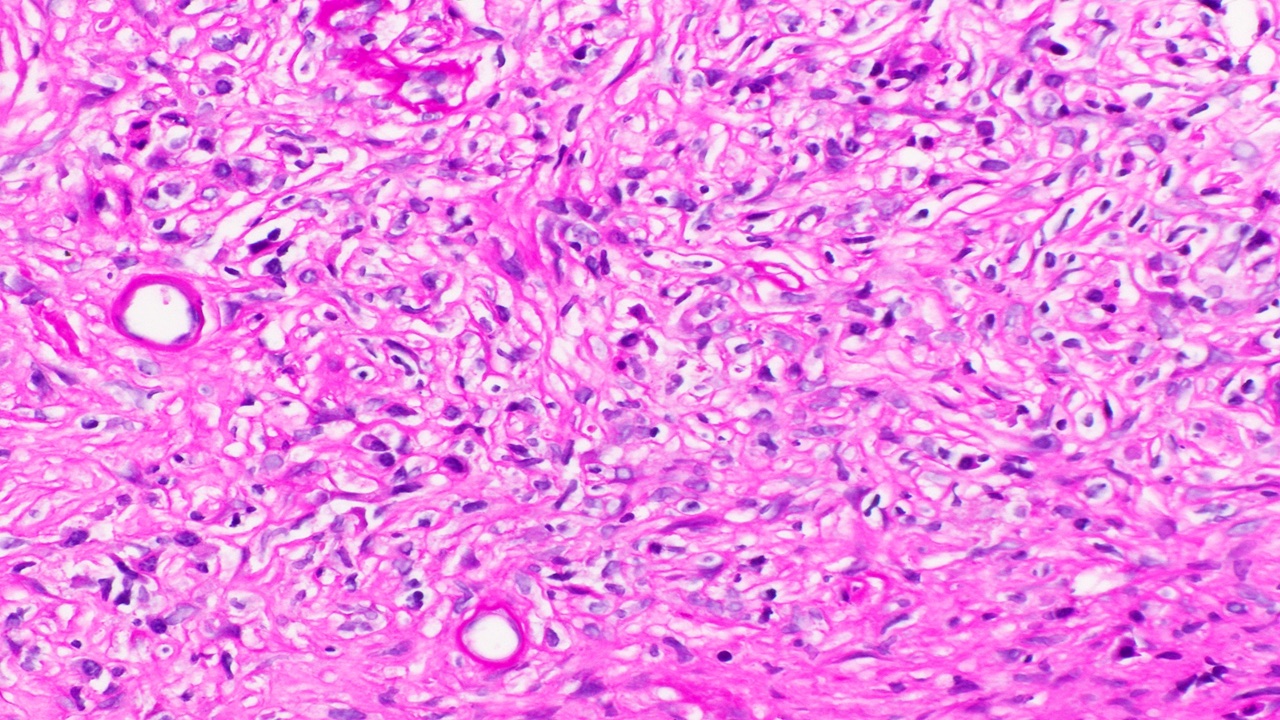
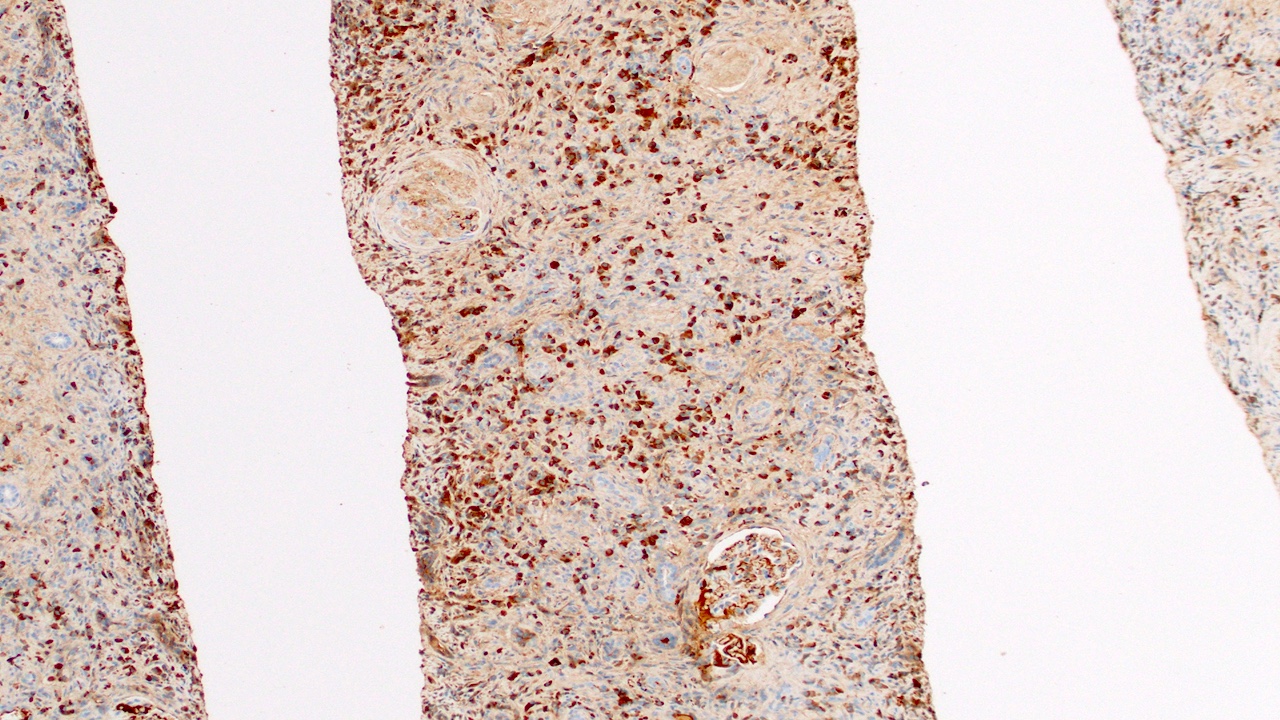
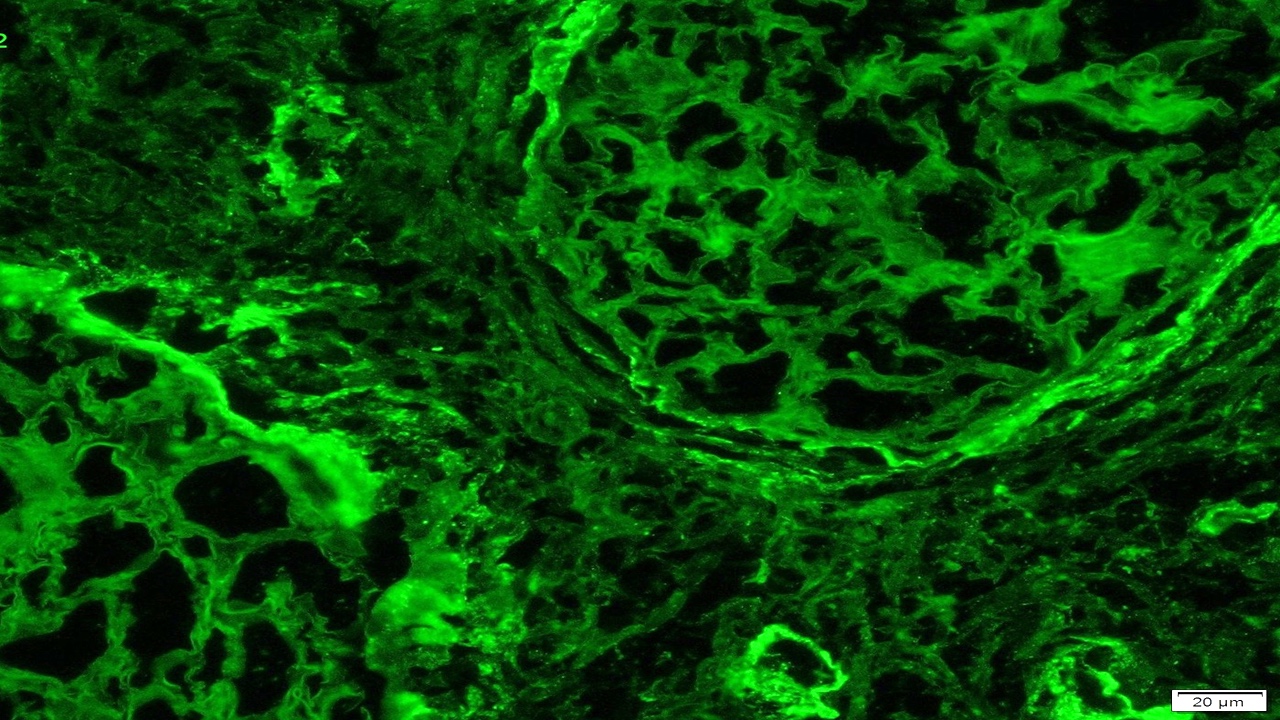
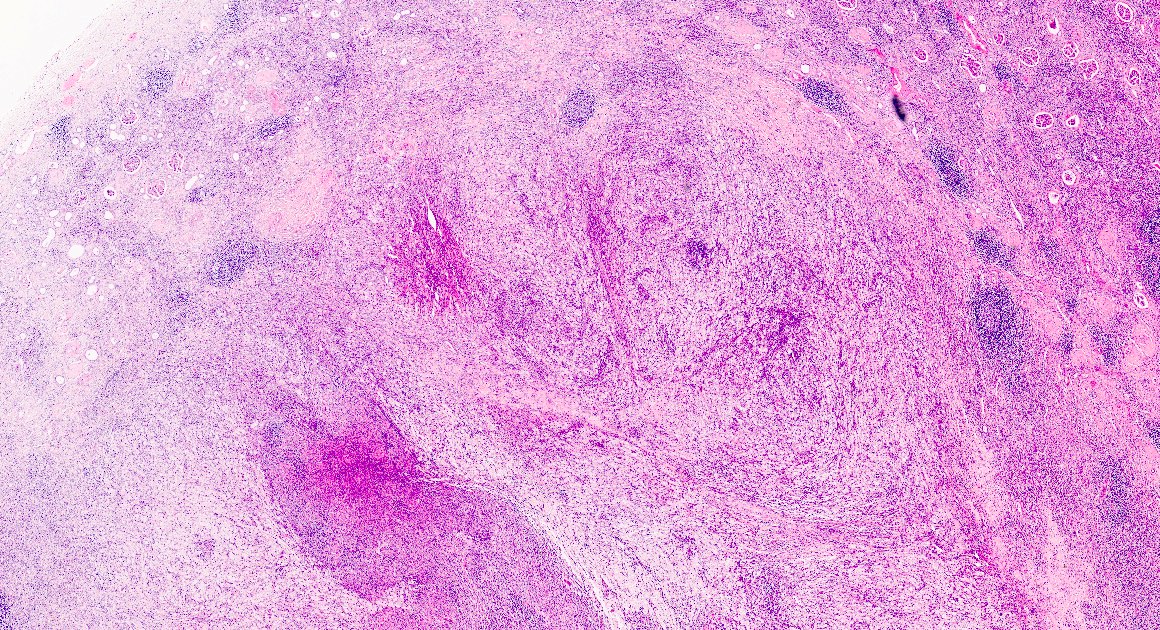
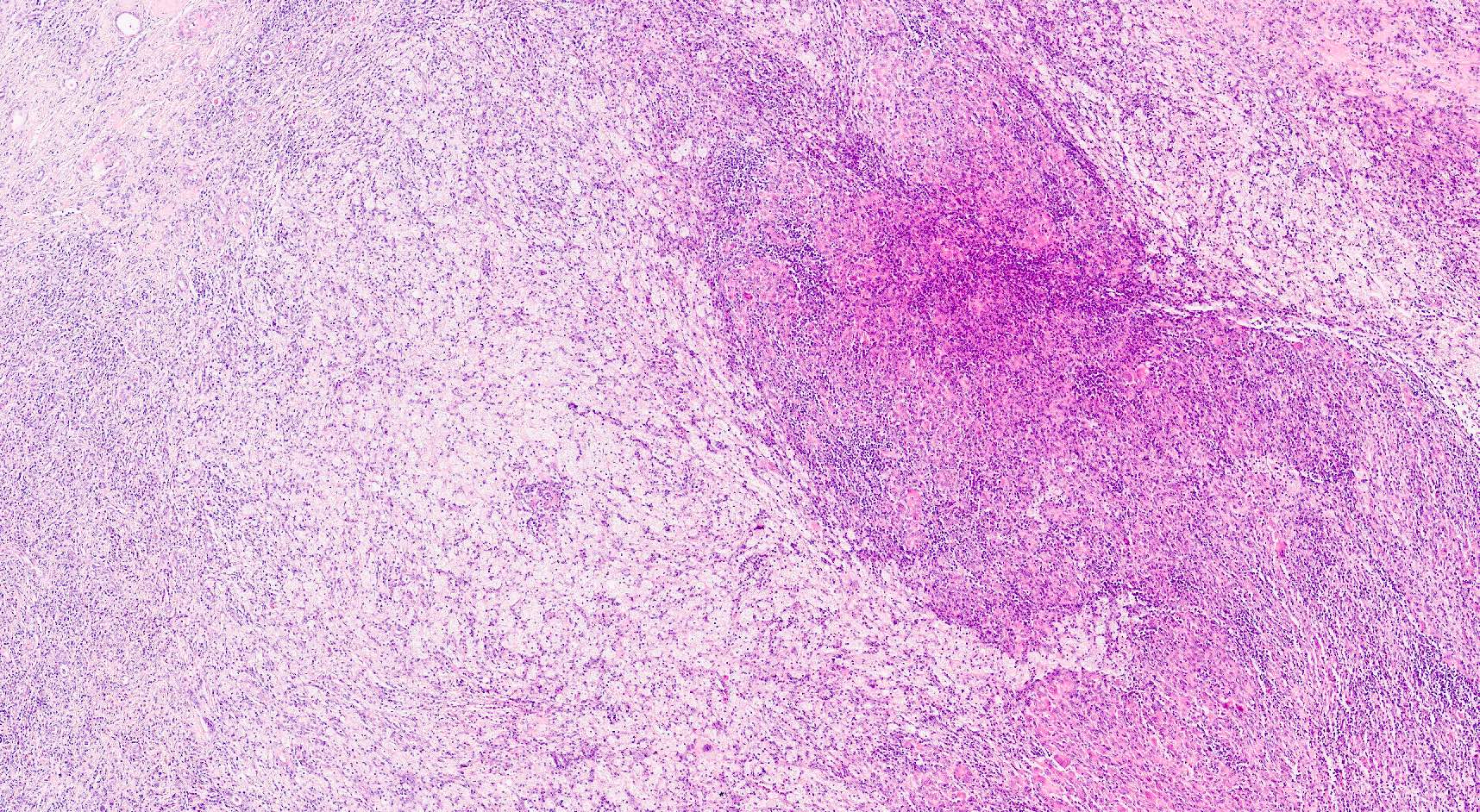
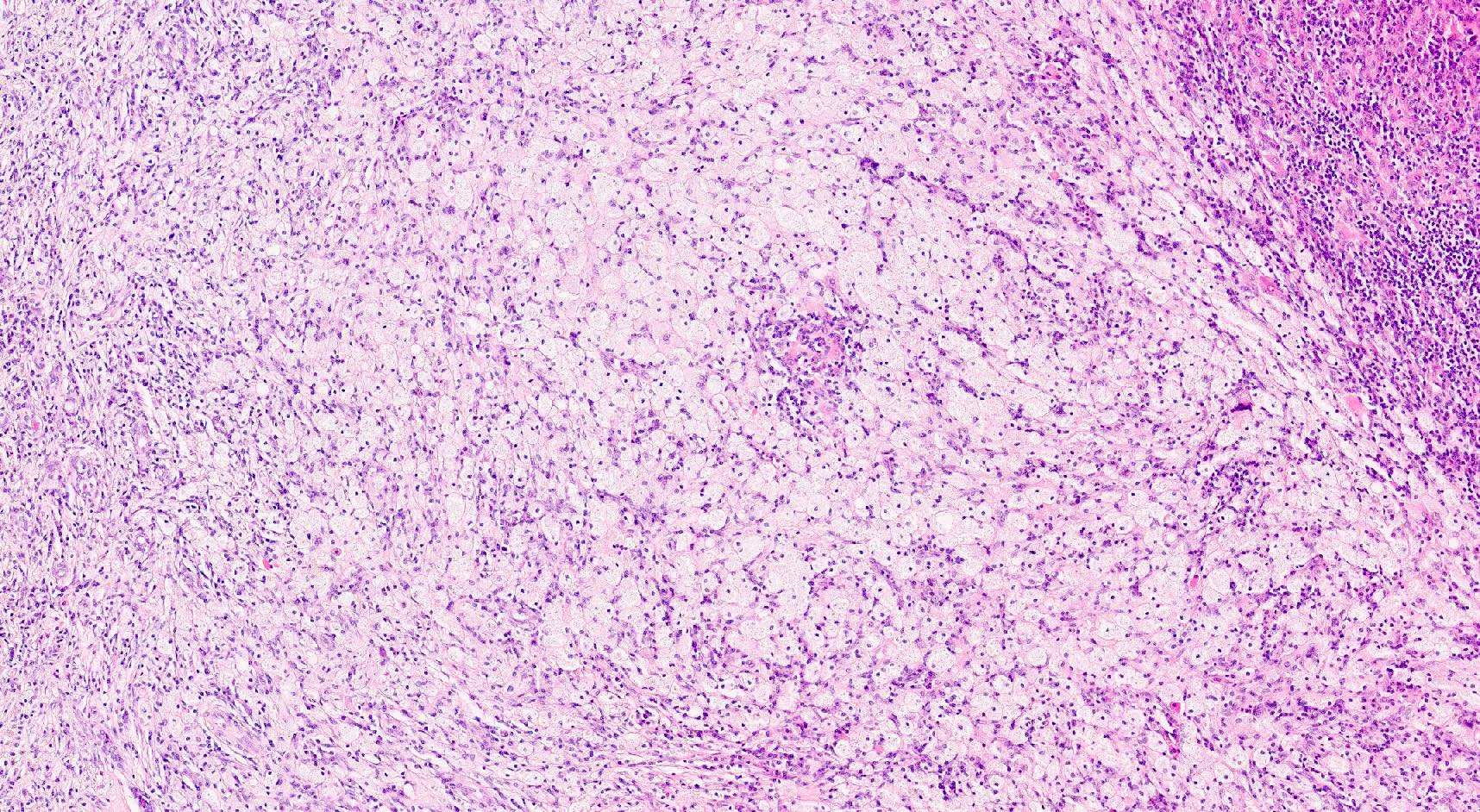
- Microscopic description: Serial sectioning shows 26 glomeruli, 2 of which are globally sclerotic. Most noticeable change in the glomeruli is the presence of intracapillary inflammatory cells (g2) in most, among which neutrophils and monocytes / macrophages can be identified in addition to lymphocytes. Endocapillary or extracapillary proliferation, necrosis, intracapillary thrombi or double contouring of the capillary walls are absent (cg0). İnterstitium shows multifocal edema and inflammatory infiltrate, mostly confined to the lumen of the peritubular capillaries (ptc2, i0). The inflammatory cells are composed predominantly of mononuclear cells with fewer neutrophils. Tubulitis is scarce and when present only mild (t1). Tubular epithelial cells show flattening and reactive nuclear changes suggestive of mild acute tubular injury. Patchy narrow foci of IFTA can be identified, comprising about 5 - 10% of the cortical parenchyma (i-IFTA0, ci0, ct0). Immunohistochemical staining shows presence of diffuse linear C4d along peritubular capillaries and glomerular capillaries (C4d 3). SV40 is negative. Arteries display mild intimal fibrosis (cv1), arterioli show multifocal mild to moderate hyalinosis (ah2). Intimal arteritis not identified (v0).
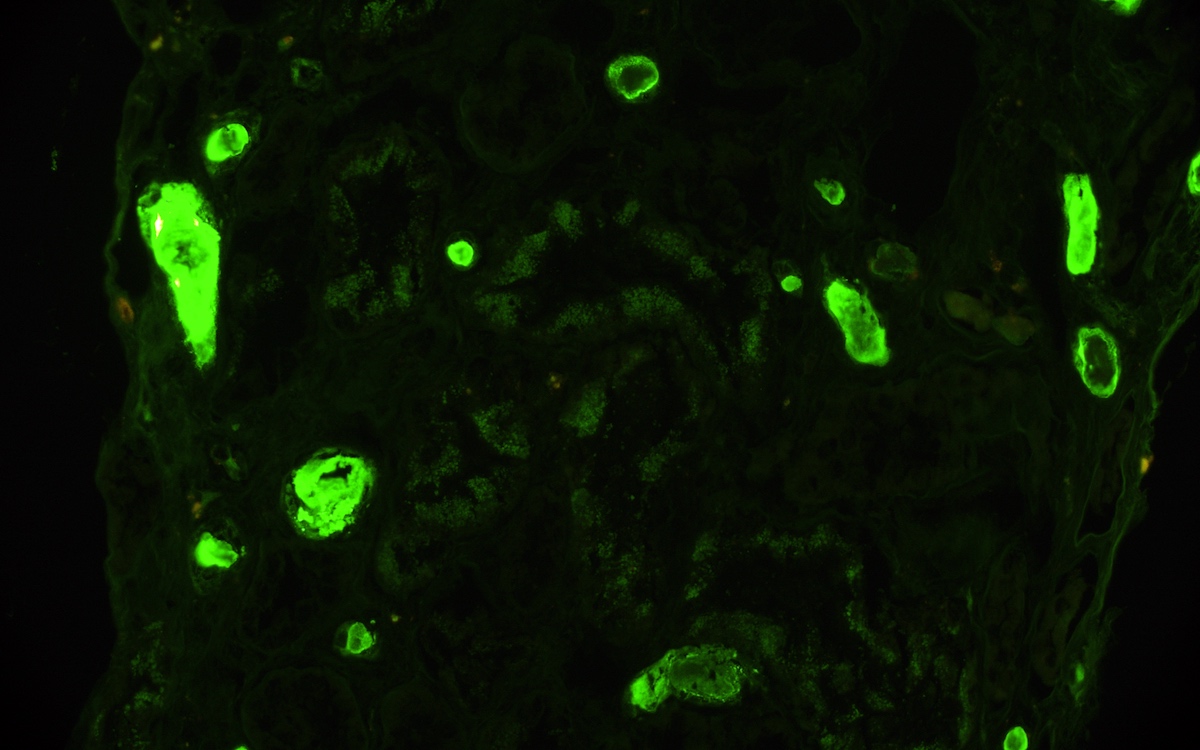
- Immunofluorescence microscopy: 2 glomeruli observed.
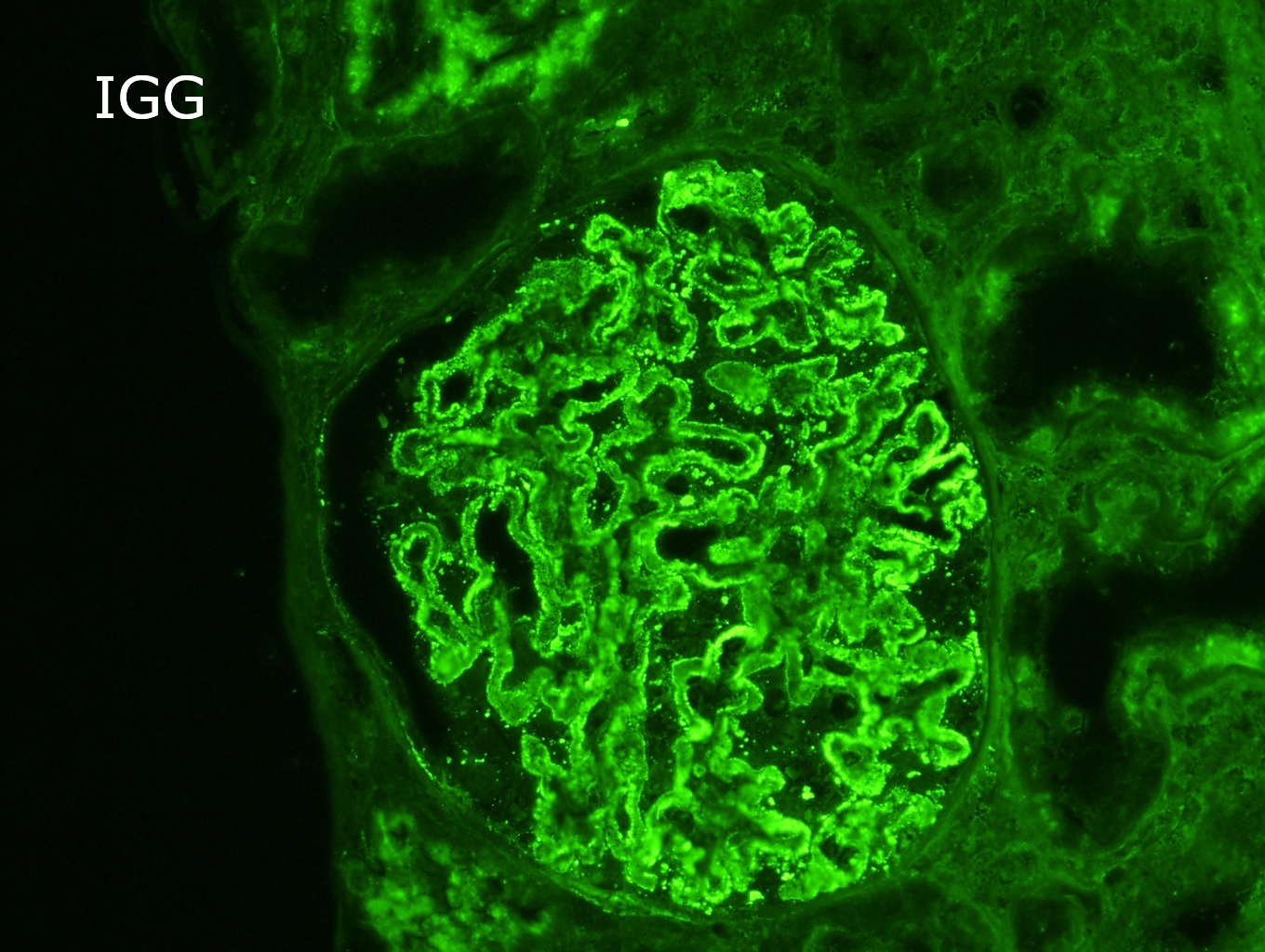
- Anti IgG Ab: no deposits
- Anti IgA Ab: no deposits
- Anti IgM Ab: no glomerular deposits, blush segmental reactivity on walls of arterioli
- Anti C3 Ab: no glomerular deposits, segmental 1+ granular reactivity along ptc, blush segmental reactivity on walls of arterioli
- Anti c1q Ab: no deposits
- Anti kappa Ab: no glomerular deposits, 2+ staining of tubular casts
- Anti lambda Ab: no glomerular deposits, 2+ staining of tubular casts
- Diagnosis
- Kidney, allograft biopsy:
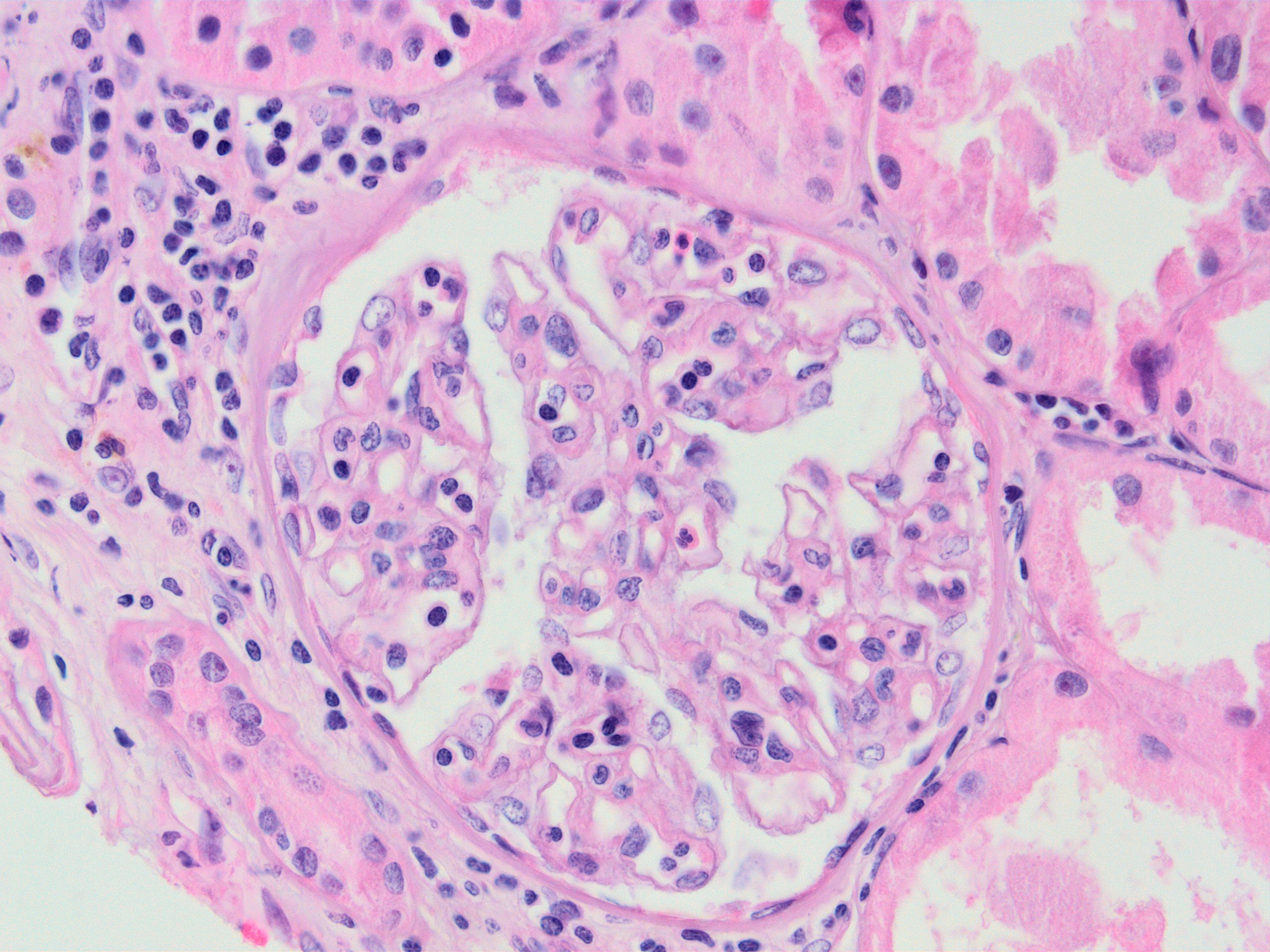
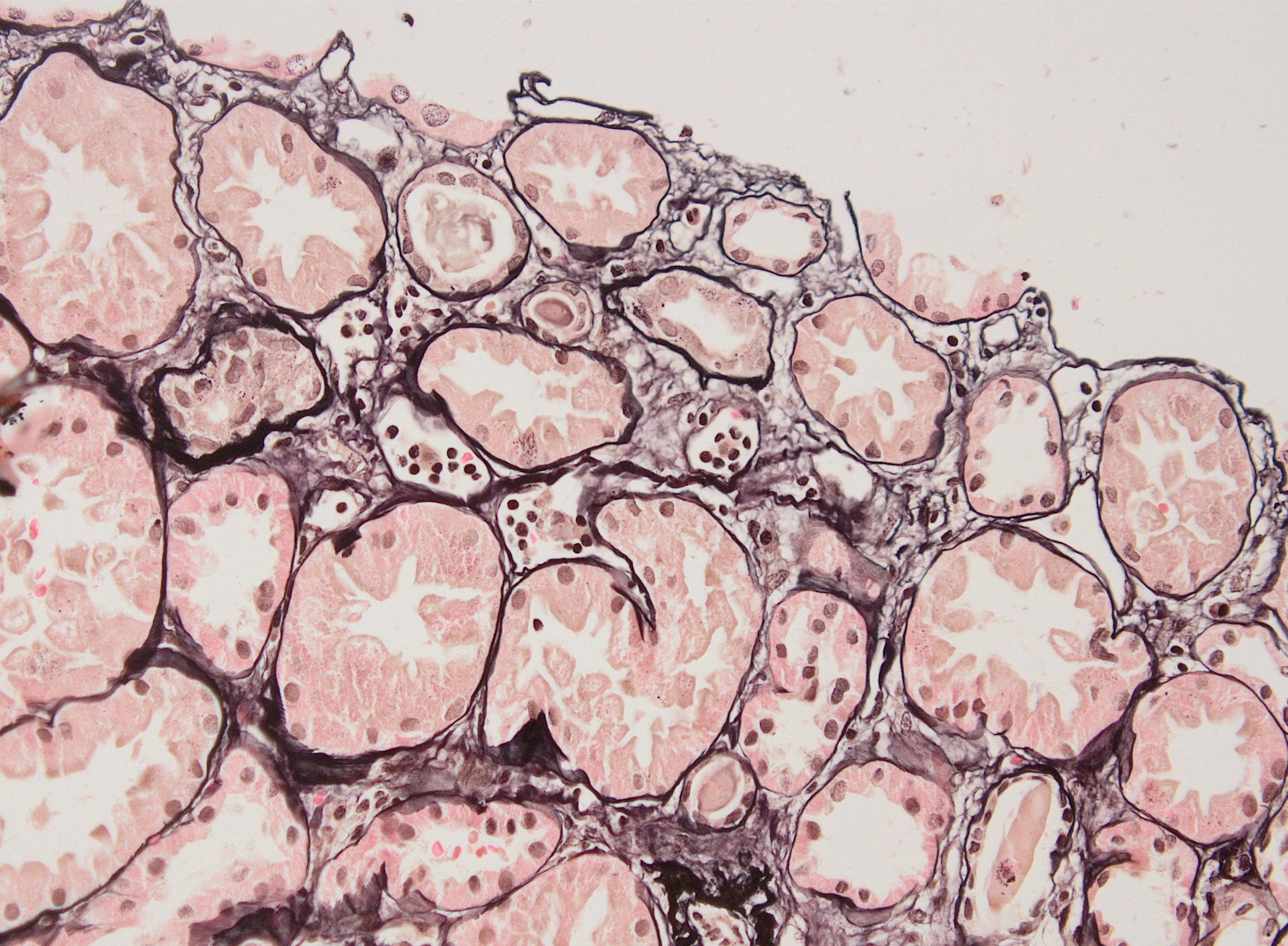
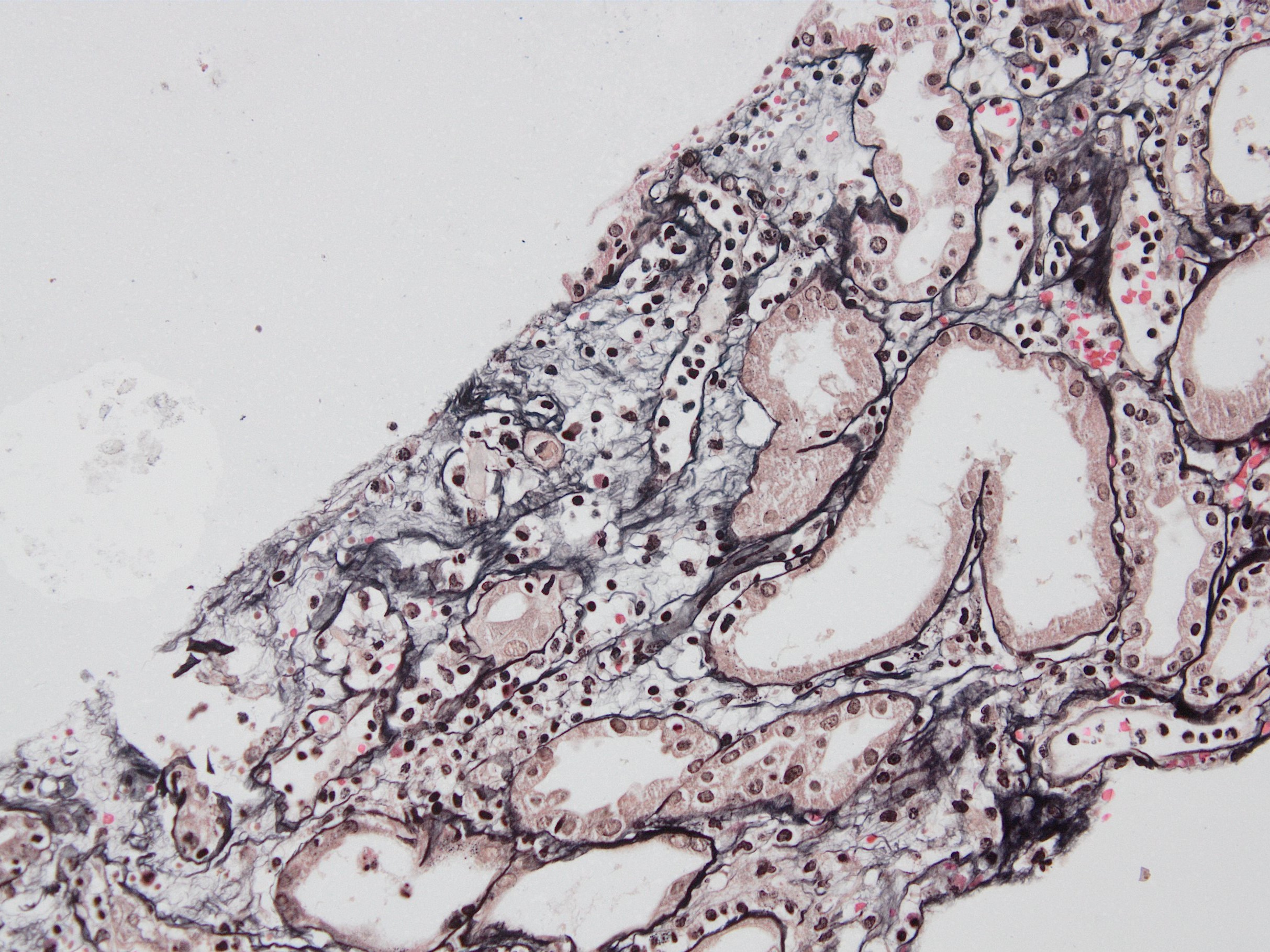
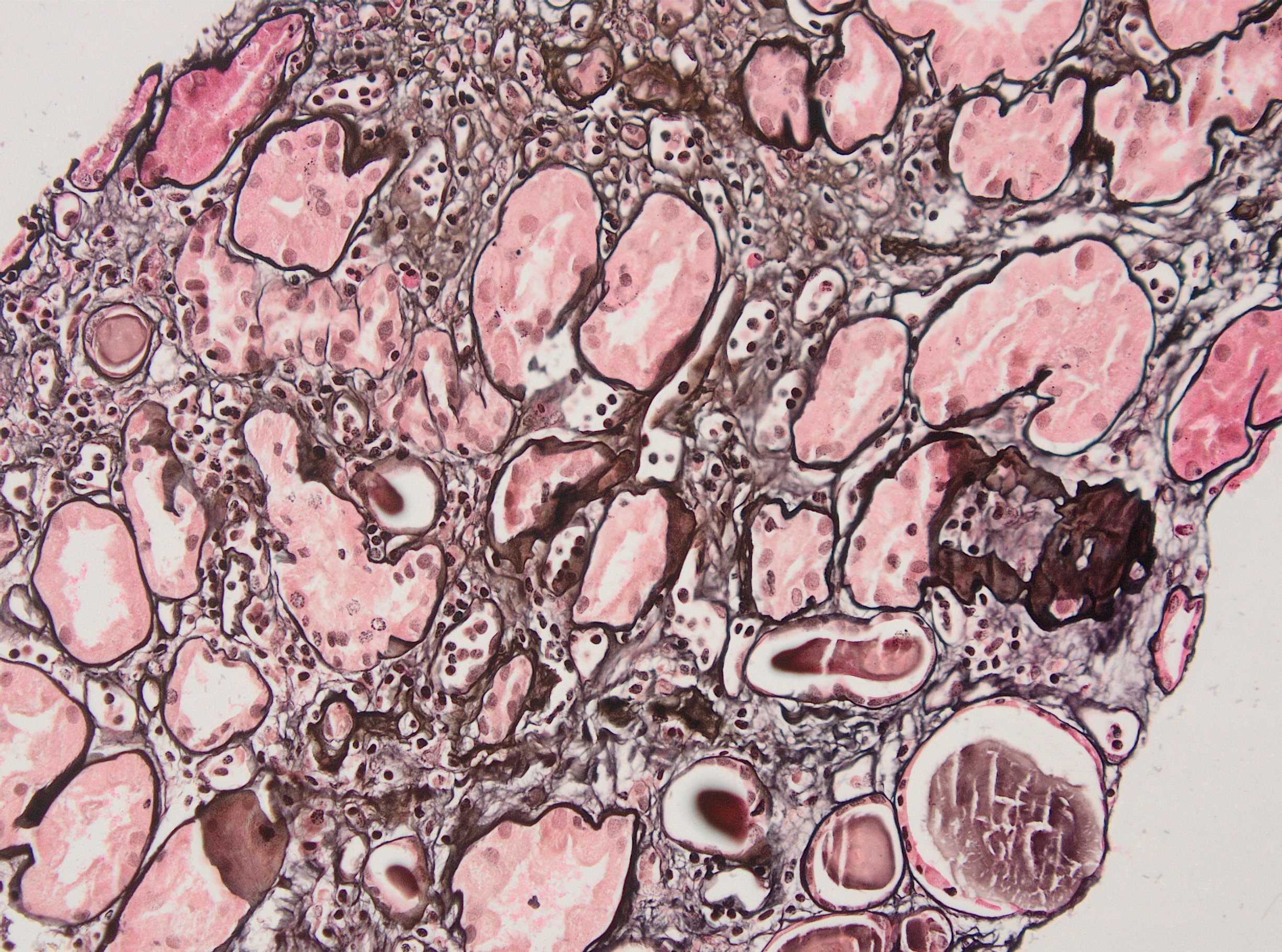
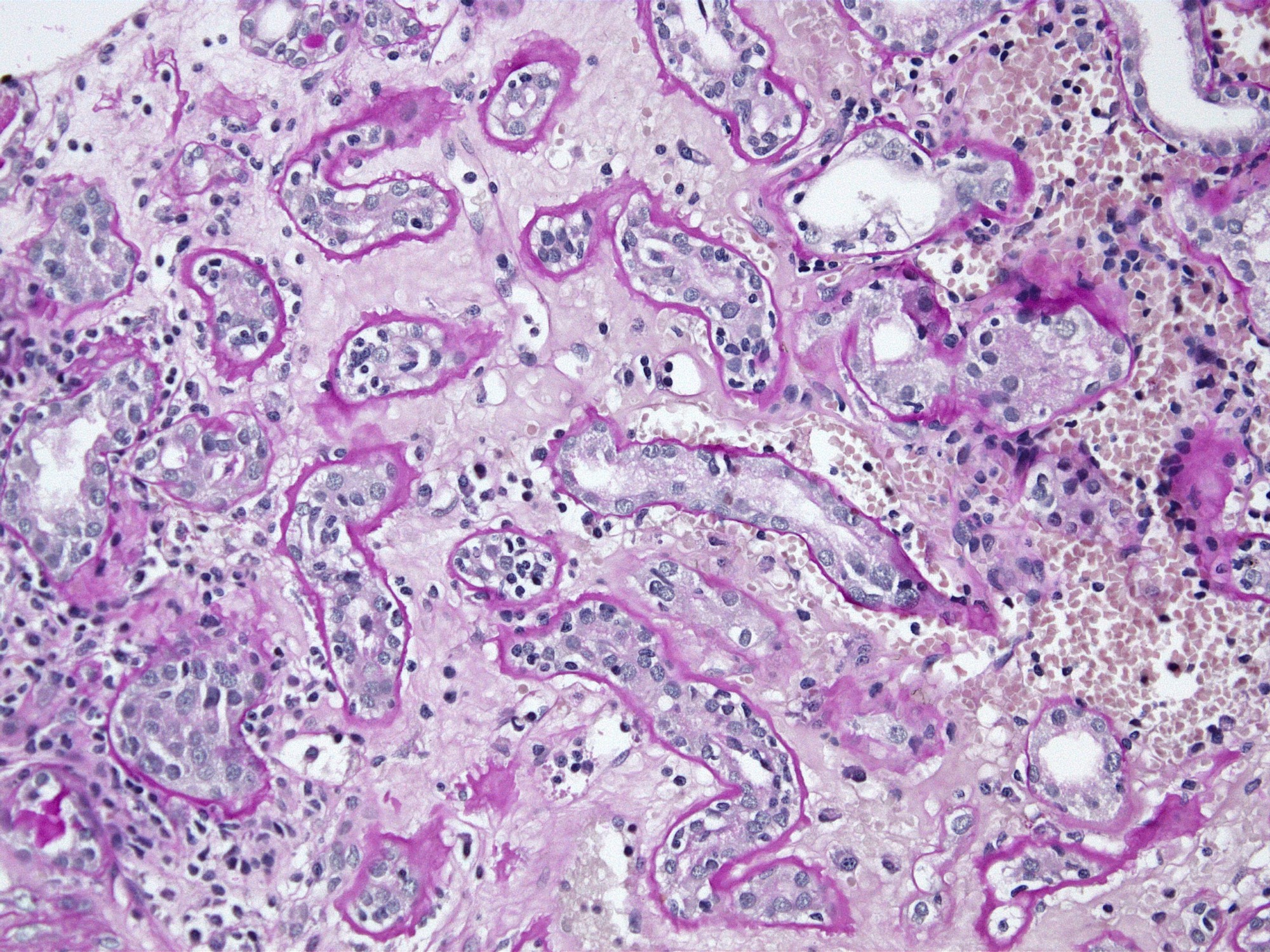
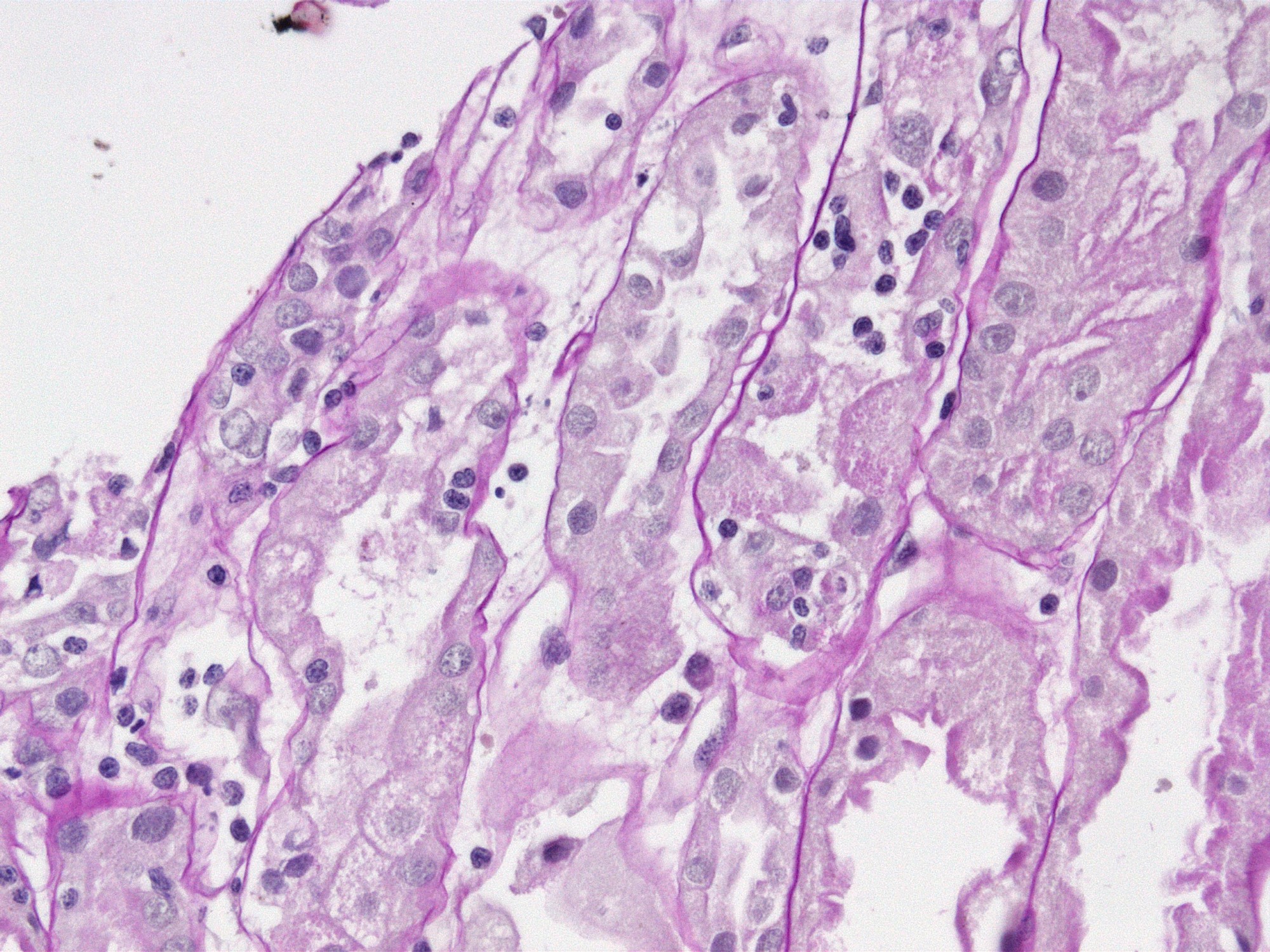
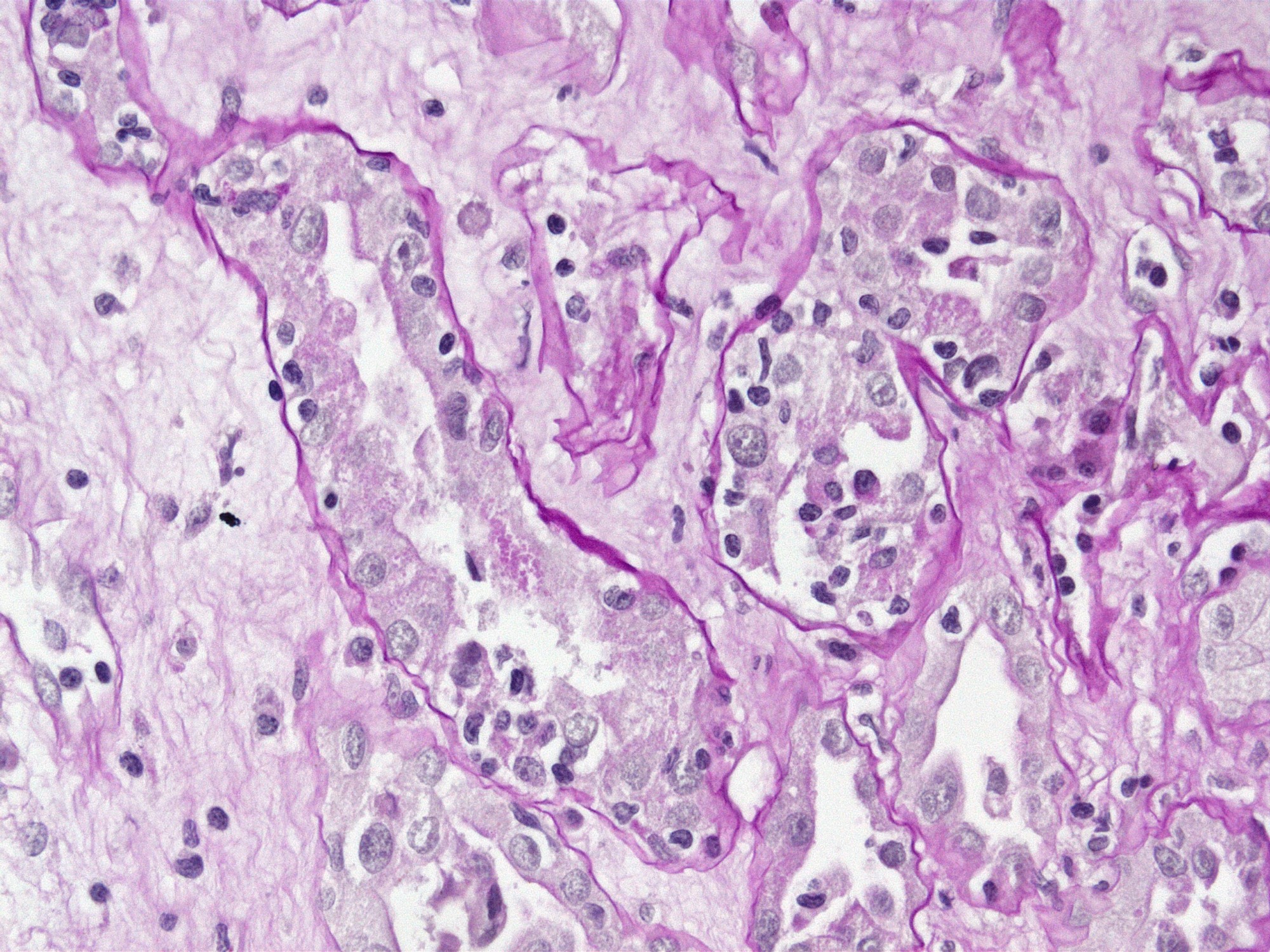
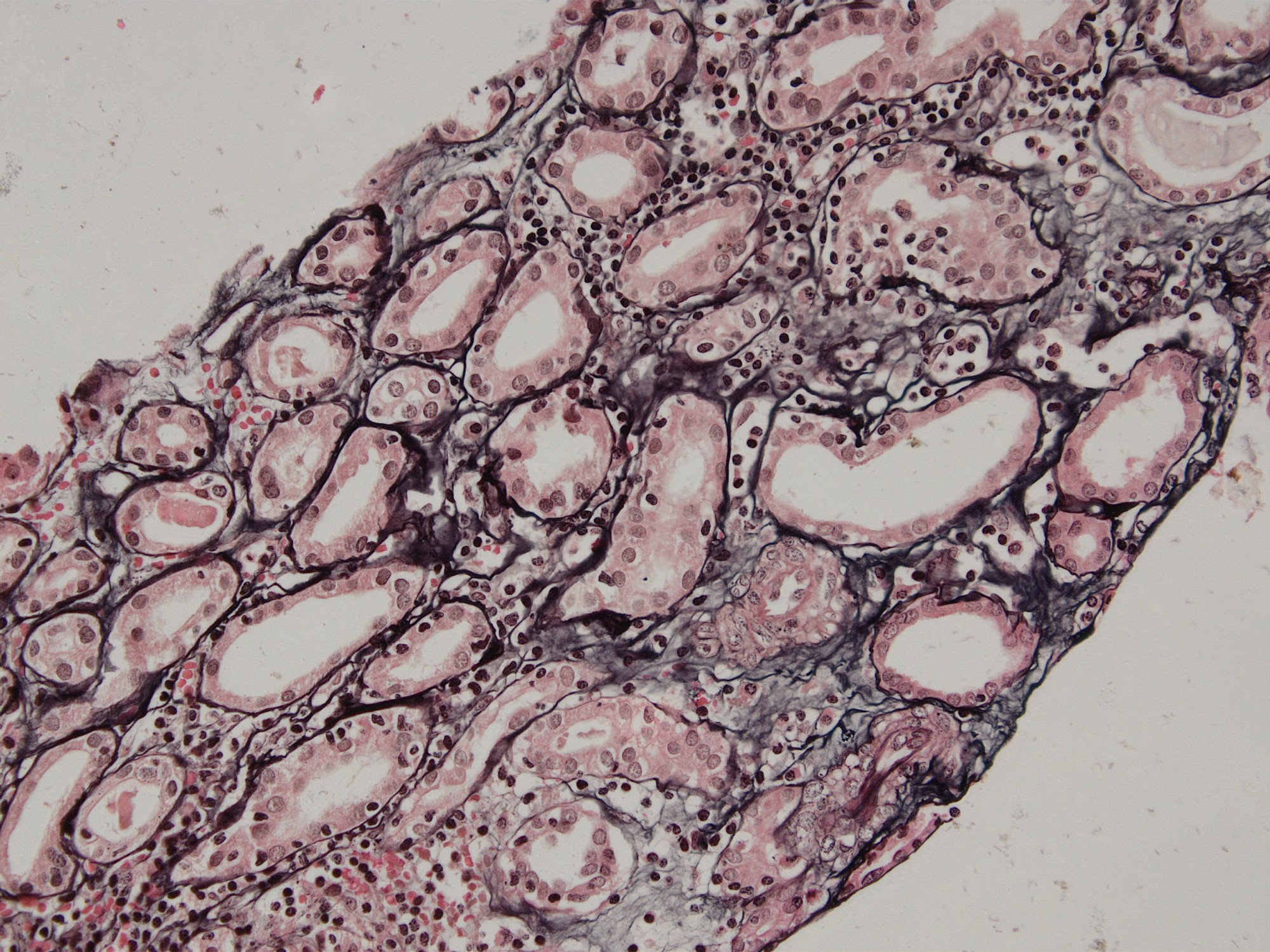
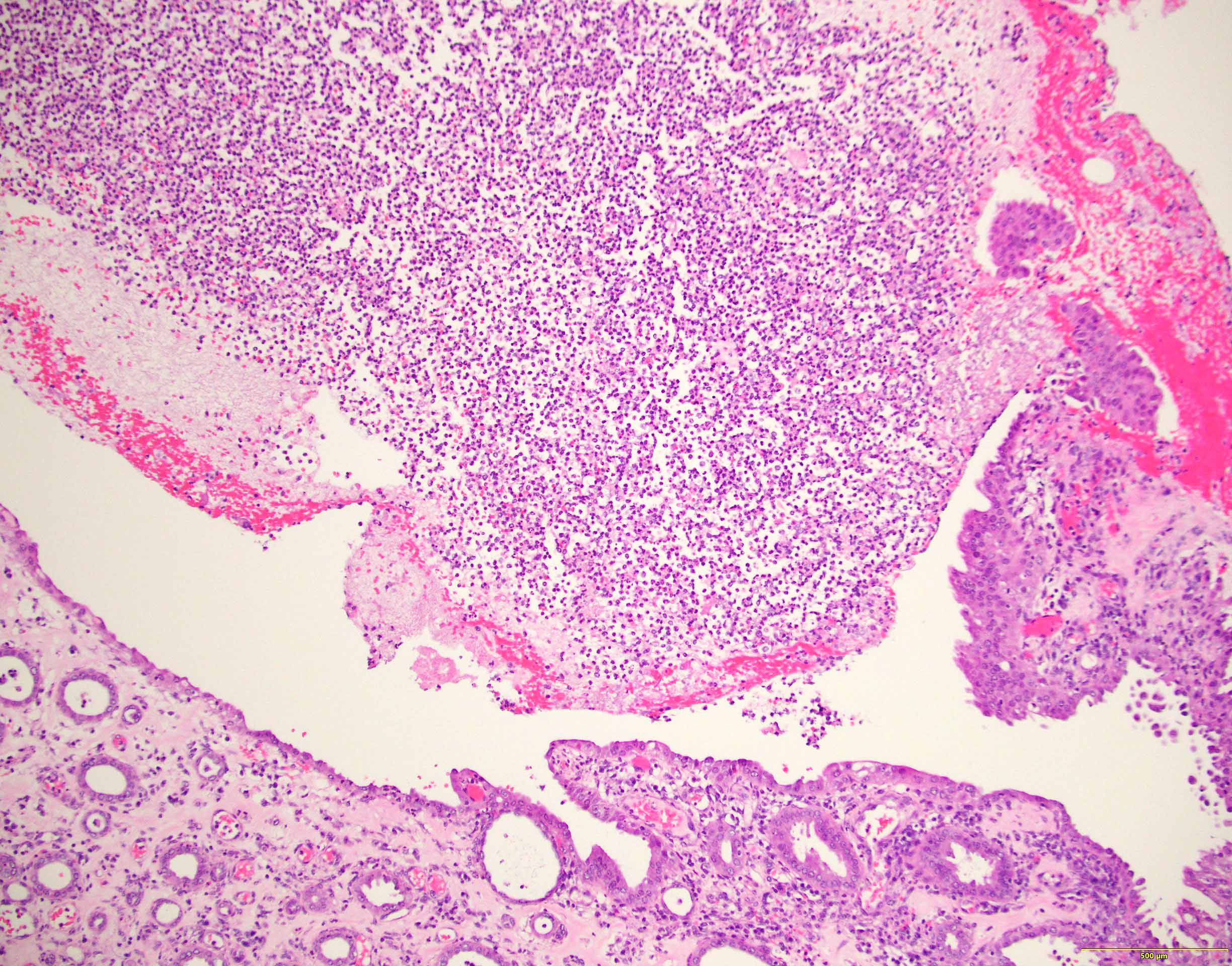
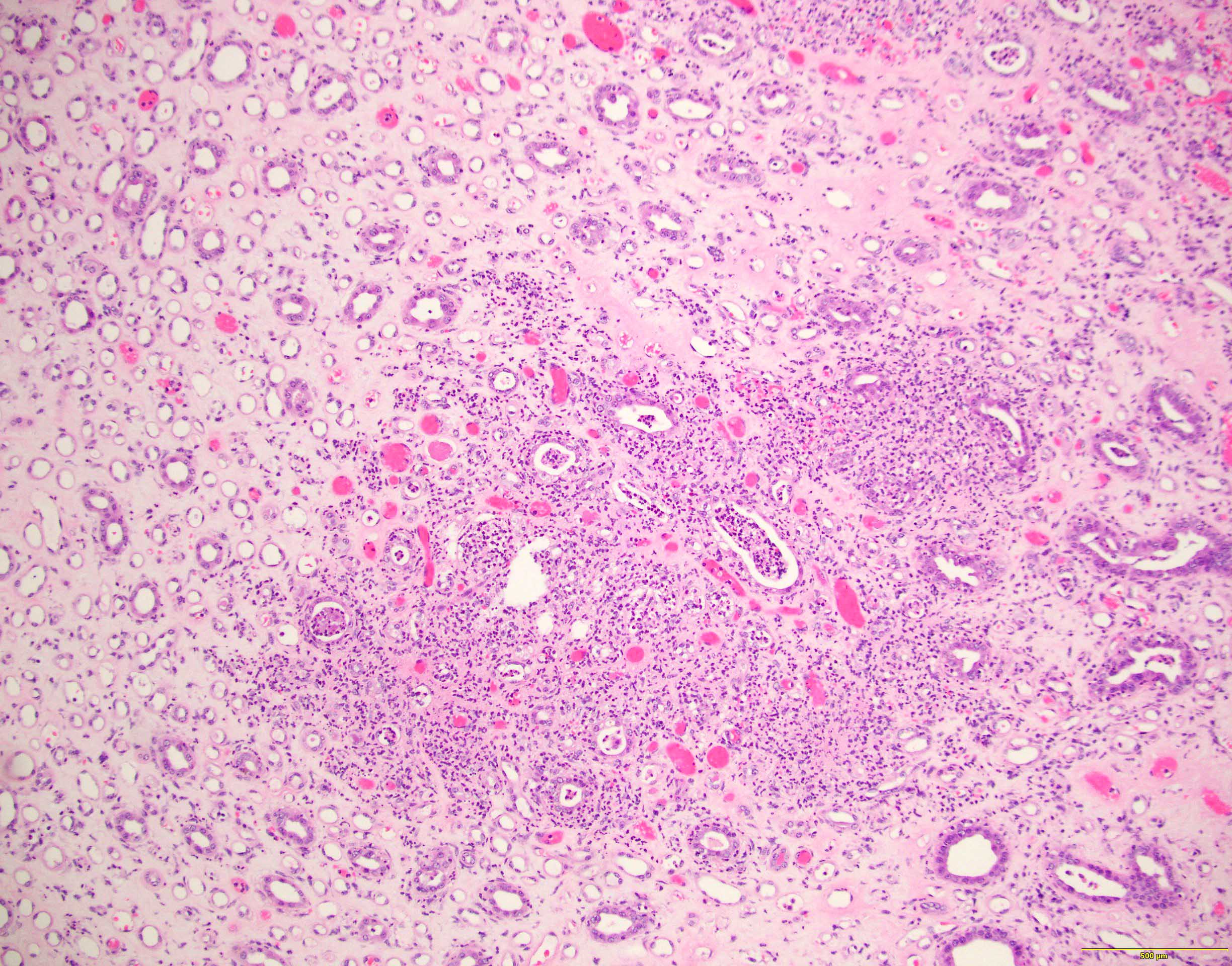
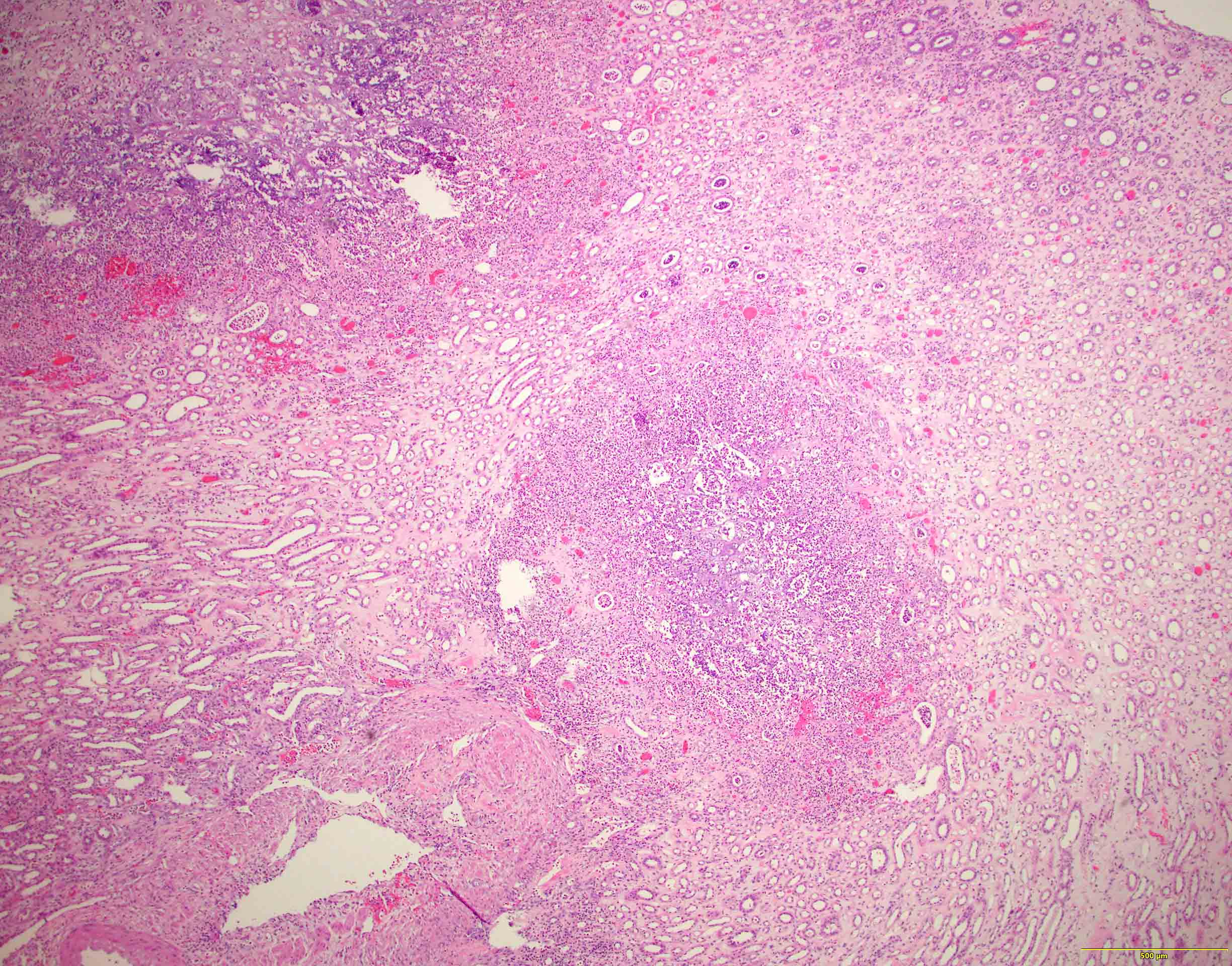
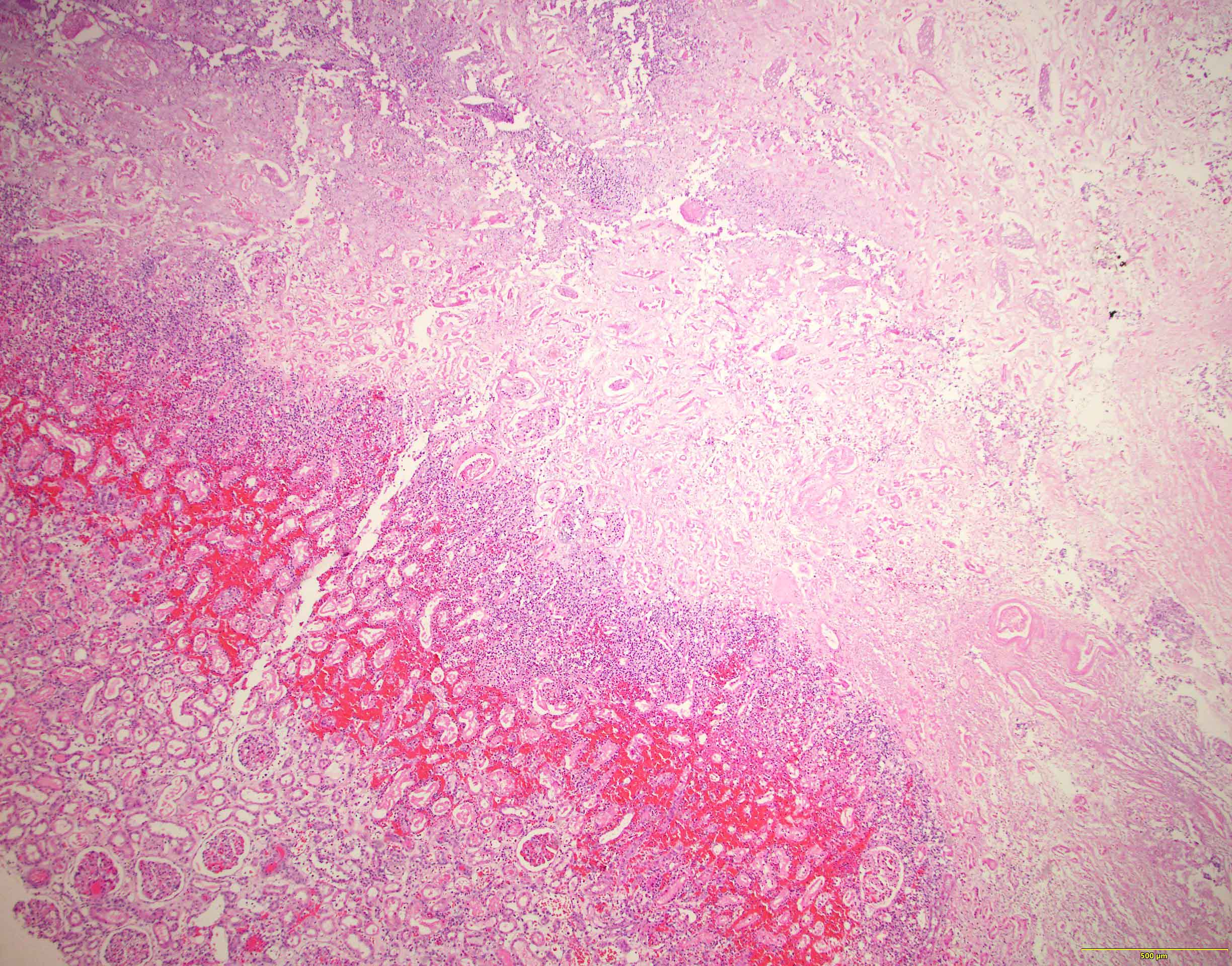
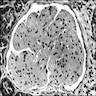
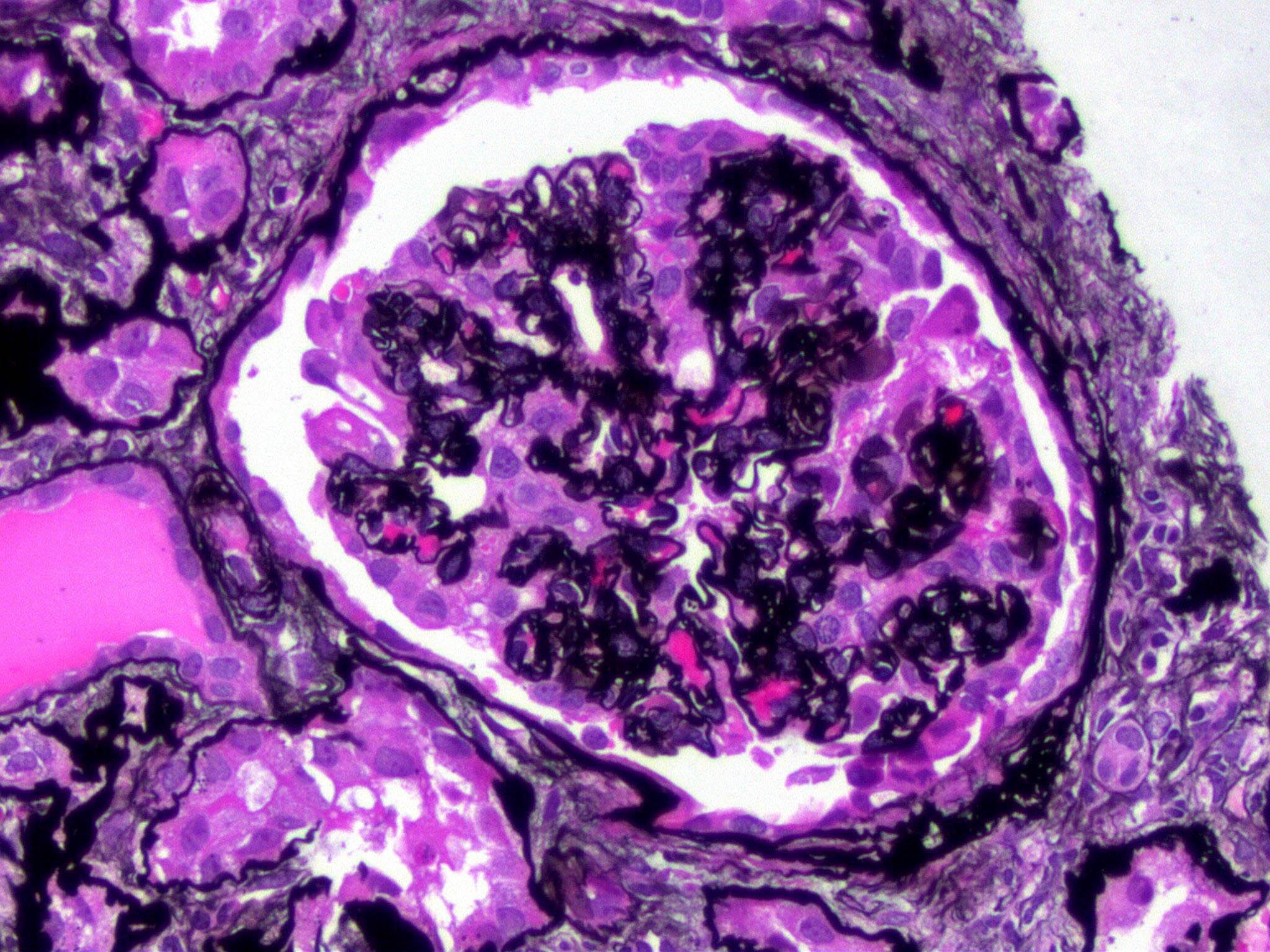
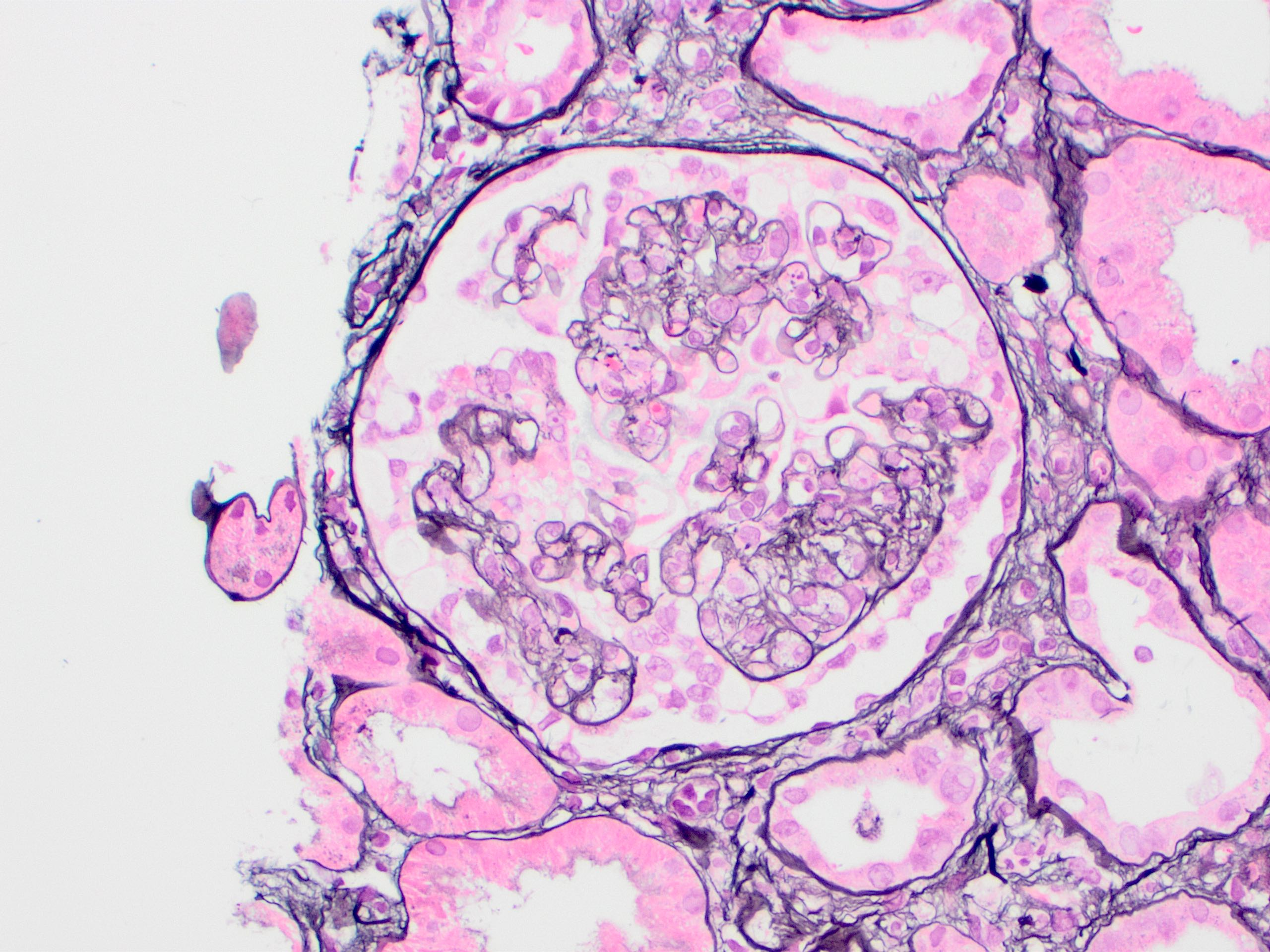
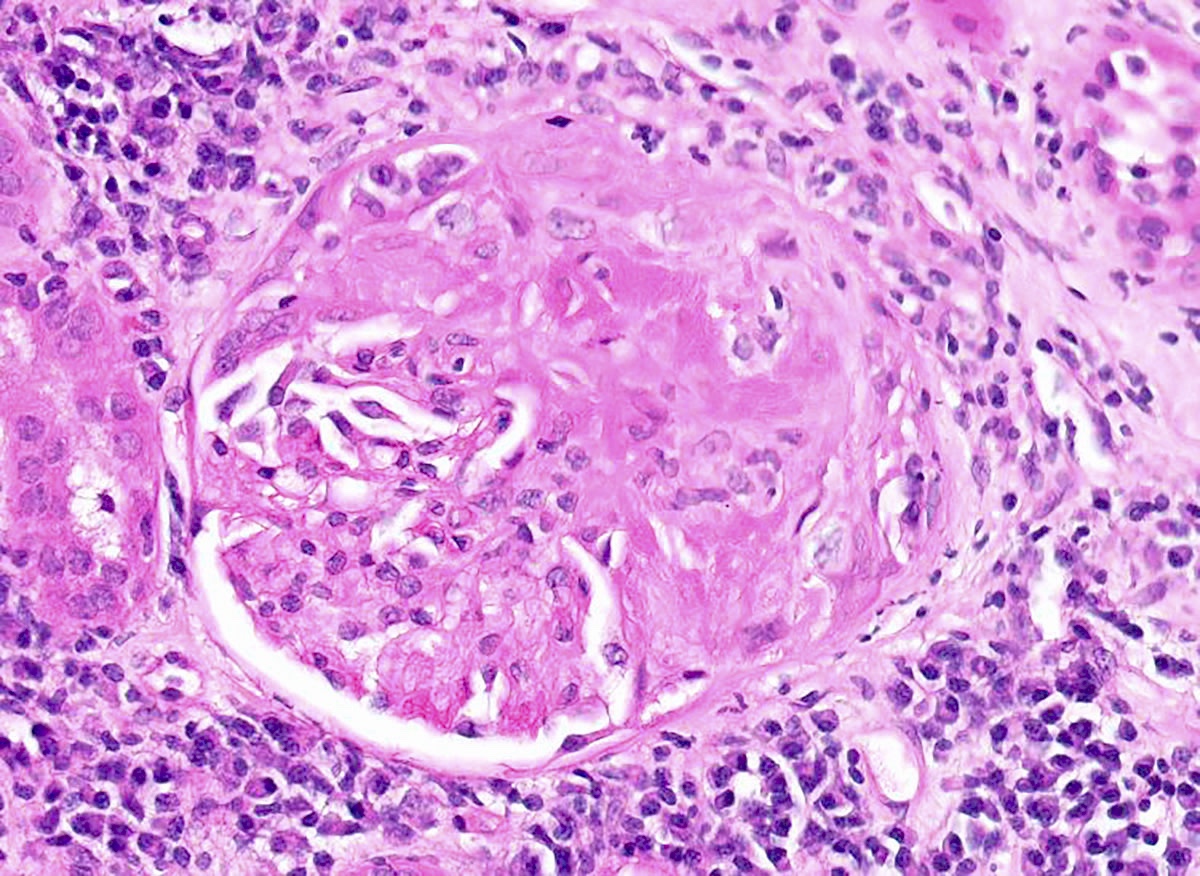
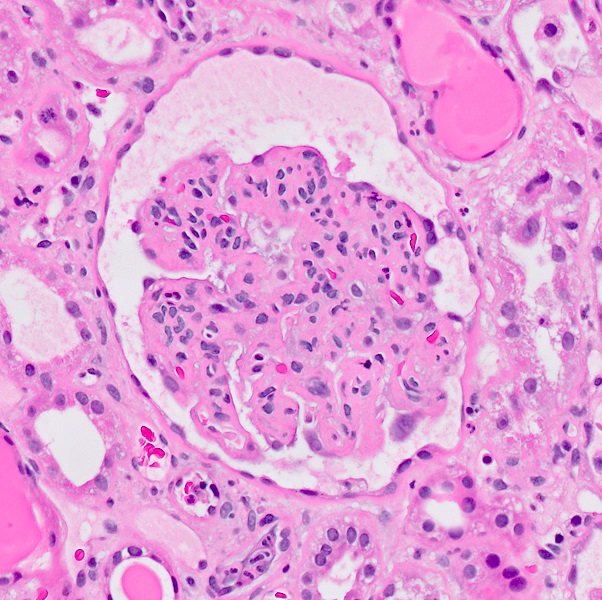
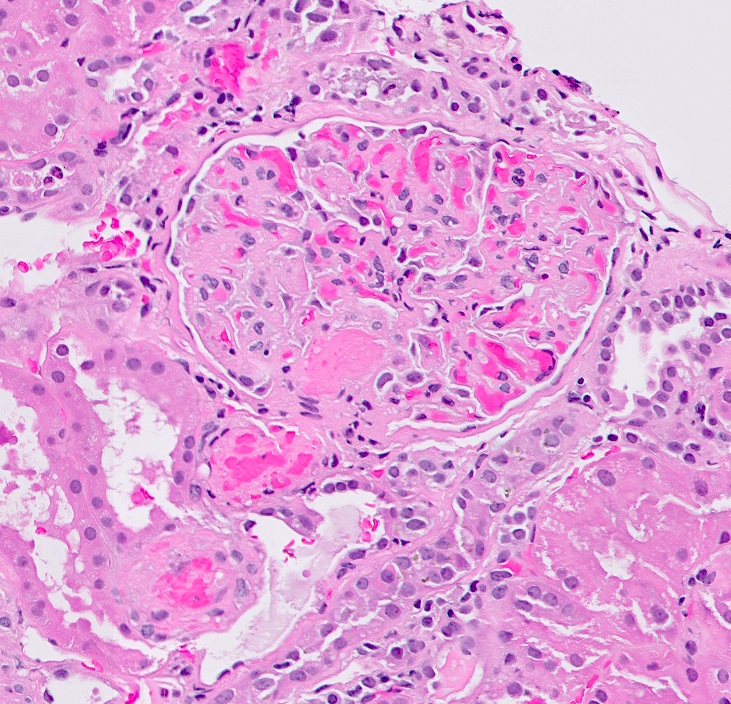
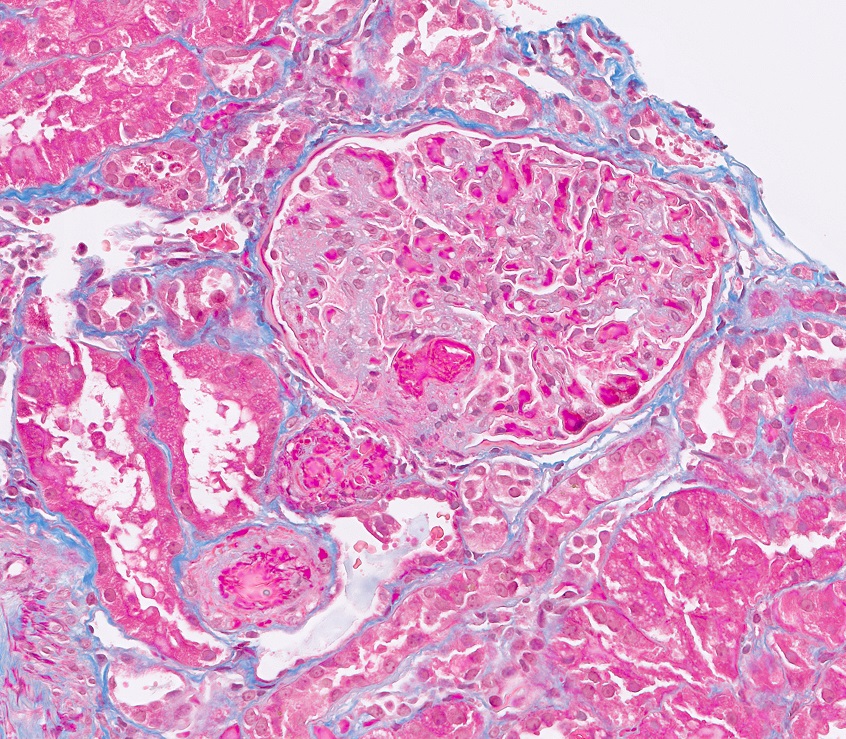
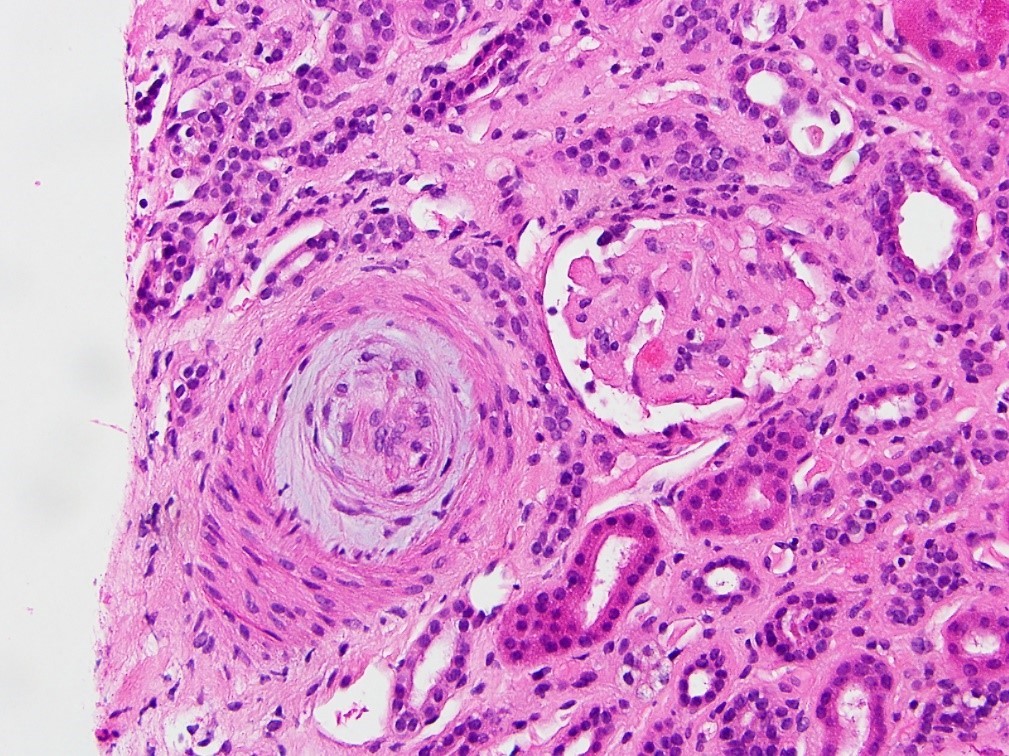
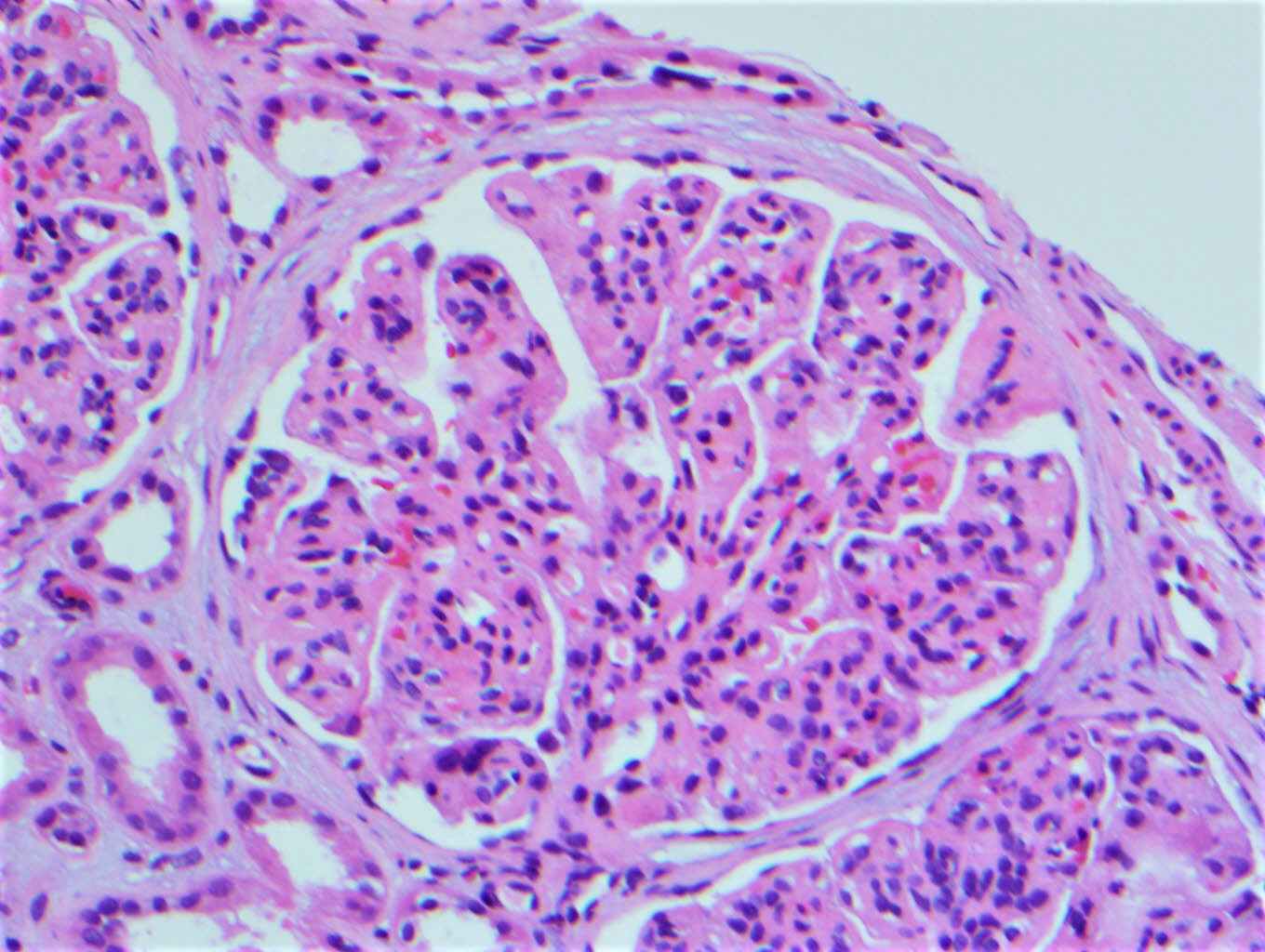
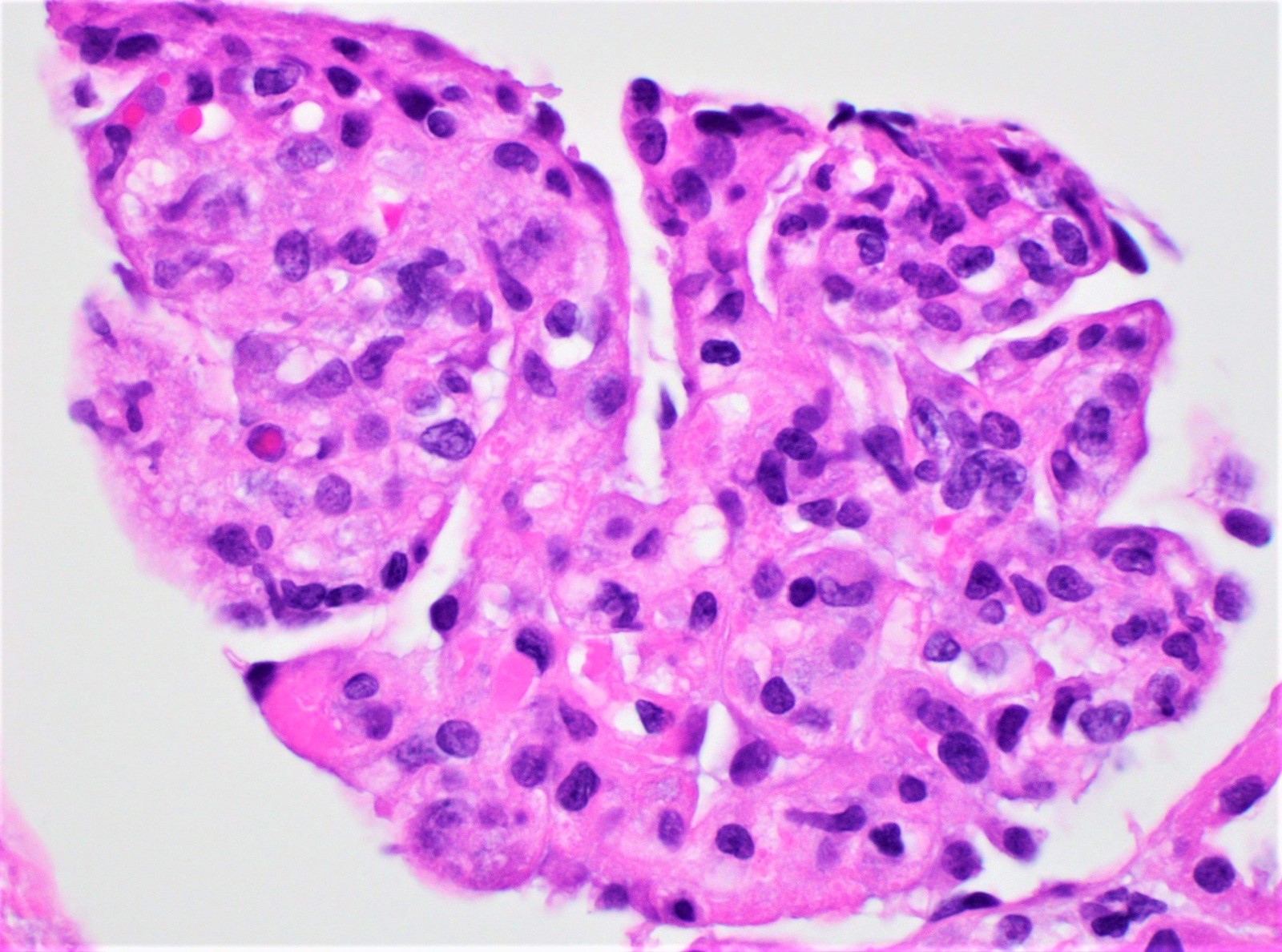
- Acute antibody mediated rejection, moderate peritubullary capillaritis and glomerulitis (ptc2, g2). Arterial intimal fibrosis (mild), arteriolar hyalinosis (ah2) (see comment)
- Comment: Biopsy lacks light microscopic changes suggestive of chronic damage, vascular changes are most probably donor related.
- Kidney, allograft biopsy:
- Peritubular capillaritis (ptc) is also present in the following conditions:
- Acute tubular necrosis / injury (ATN / ATI): morphological distinction is not possible but absence of C4d staining is helpful; possibility of concurrent ATN / ATI and antibody mediated rejection
- Acute pyelonephritis: neutrophilic tubulitis and presence of neutrophil aggregates within tubuli are helpful but their absence does not rule out acute pyelonephritis, especially considering that the patients in question are immunosuppressed; absence of C4d staining is helpful
- BK nephritis: viral cytopathic changes in tubular epithelial cells and positive polyomavirus immunostain (should be performed on every allograft renal biopsy specimen)
- Thrombotic microangiopathy is also present in other conditions such as drugs (most notably acute cyclosporine toxicity), infections and recurrent thrombotic microangiopathy: absence of C4d staining is helpful
- C4d staining is also present in patients with ABO blood group incompatible (and less frequently in HLA incompatible) donors: biopsy specimen lacks morphological findings of microvascular inflammation and clinical evidence of graft dysfunction
- Transplant glomerulopathy is also present in the following conditions:
- Chronic thrombotic microangiopathy: capillaritis and C4d in ptc is lacking
- Recurrent / de novo immunocomplex glomerulonephritis (those with MPGN pattern): Immunofluorescence positivity helps; may also have C4d staining along the glomeruli but ptc staining is missing
- Transplant arteriopathy:
- Arteriosclerosis: no inflammatory cells in the vessel wall
- May also be a sign of T cell mediated rejection or both T cell mediated rejection and AMR
- Acute T cell mediated rejection: may frequently accompany AMR, presence of tubulitis and interstitial inflammatory infiltrate is seen; vascular lesions can be a feature of both entities
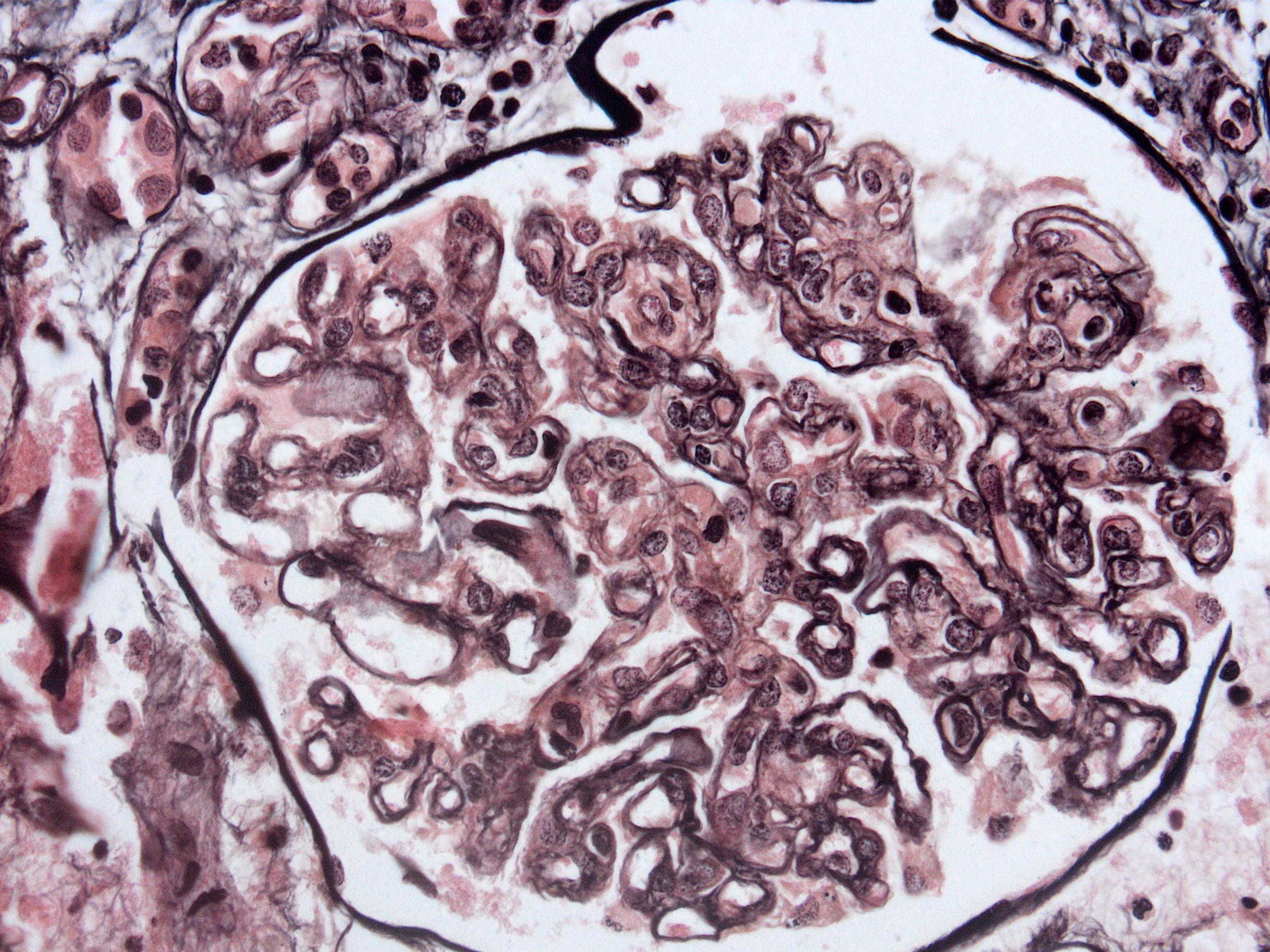
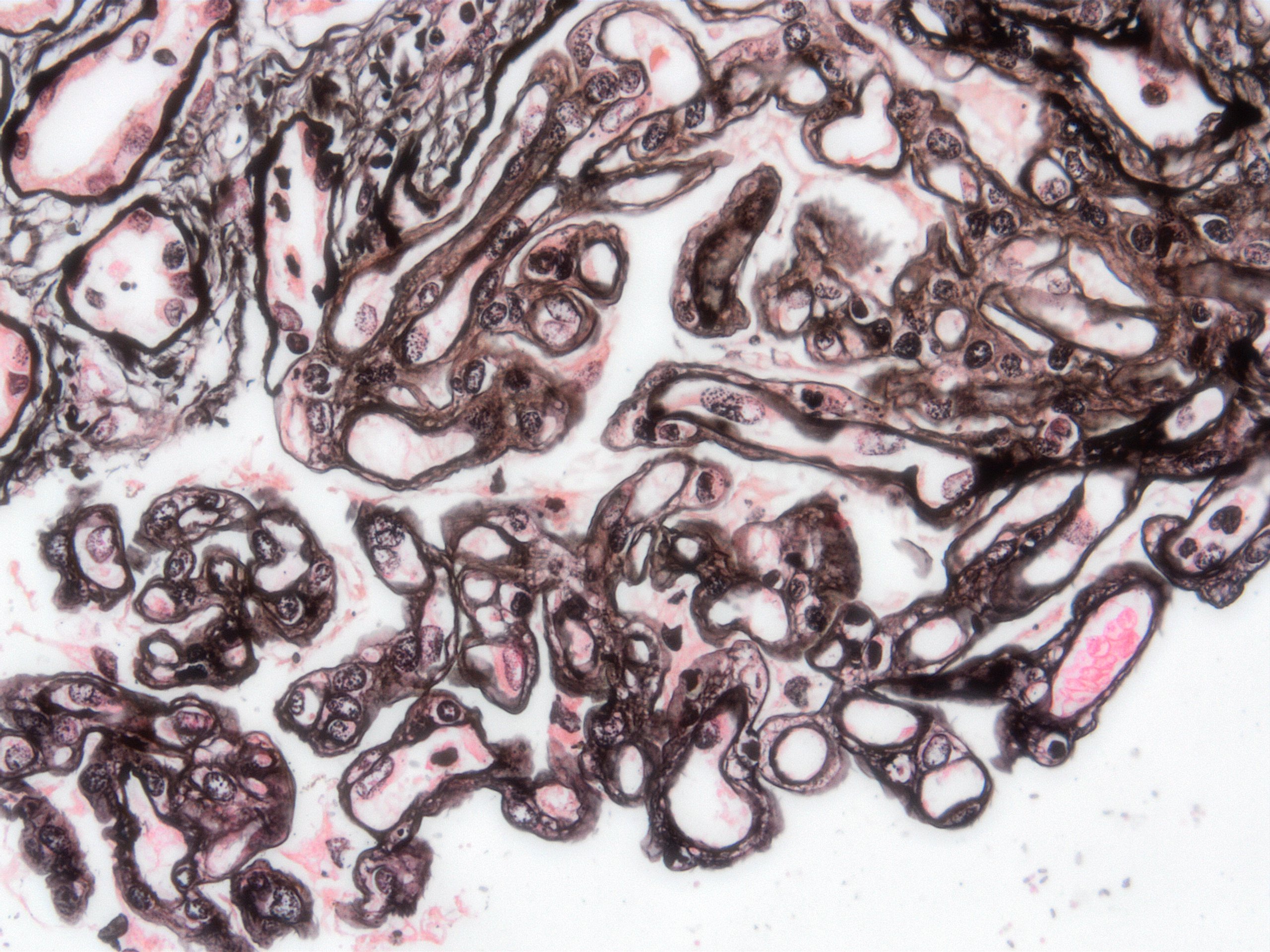
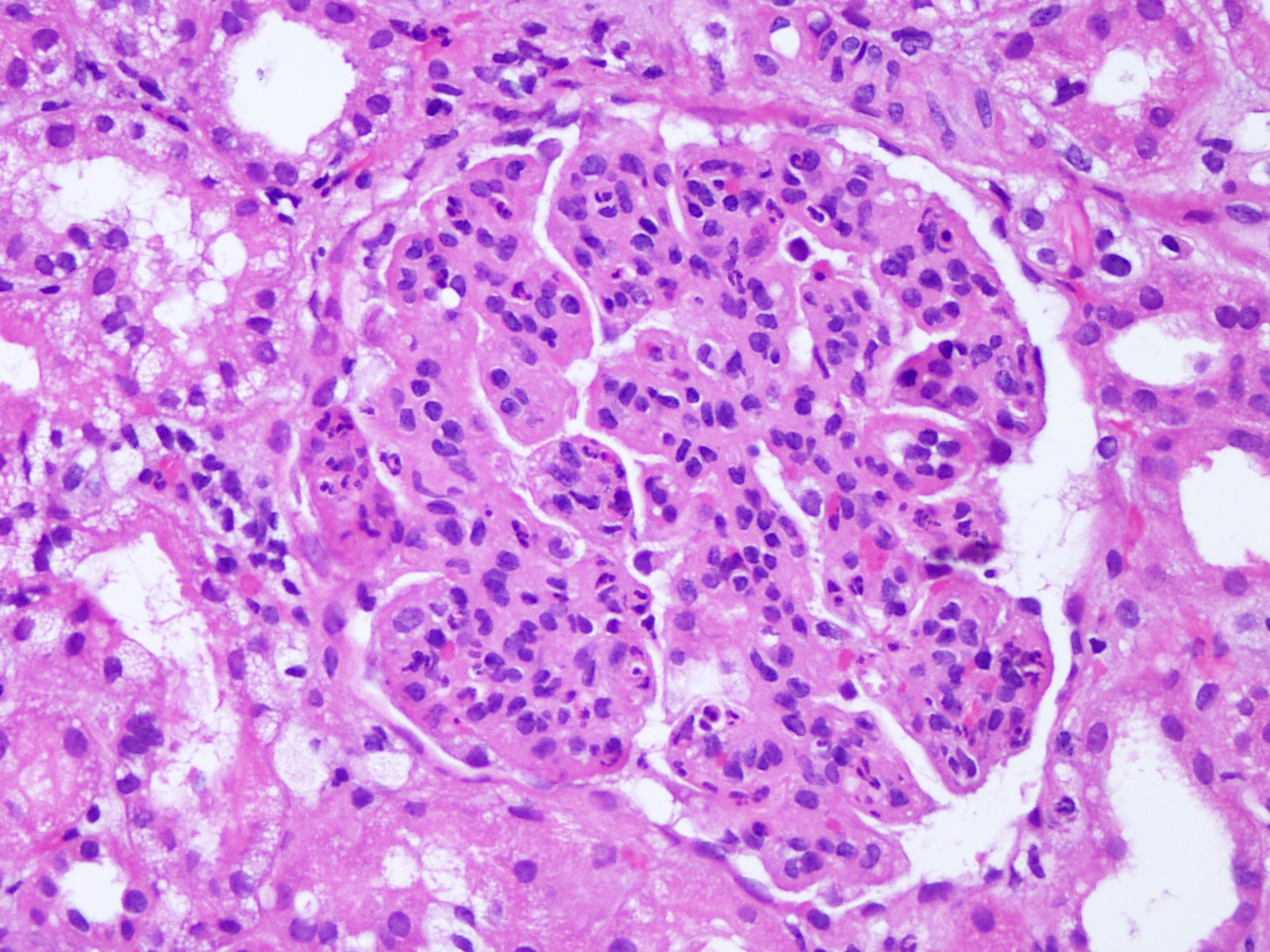
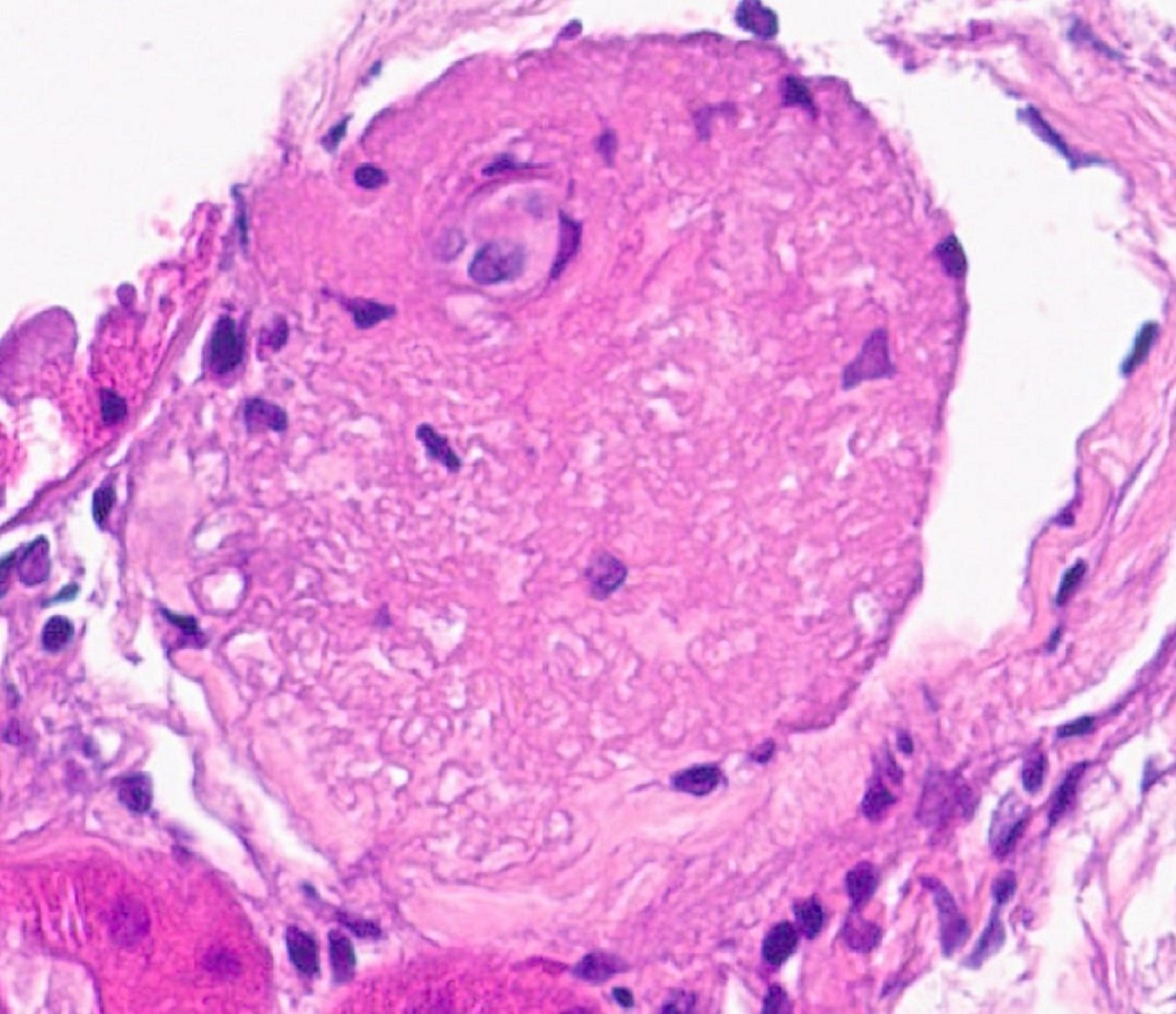
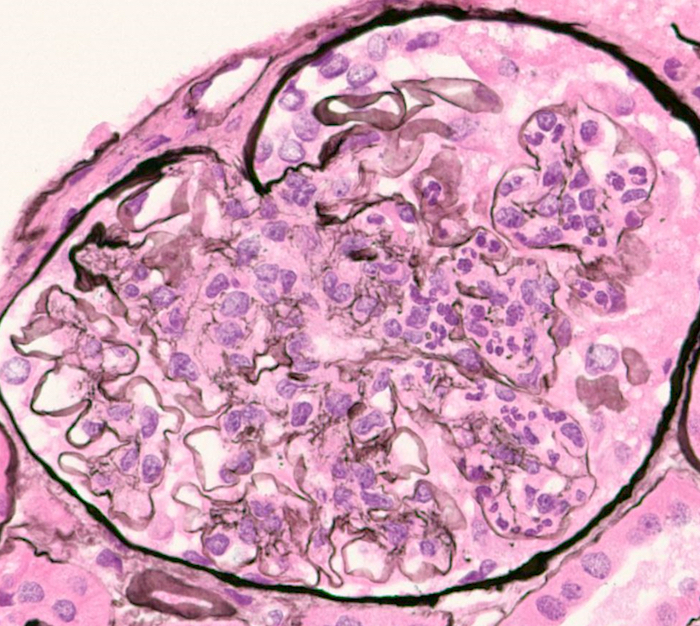
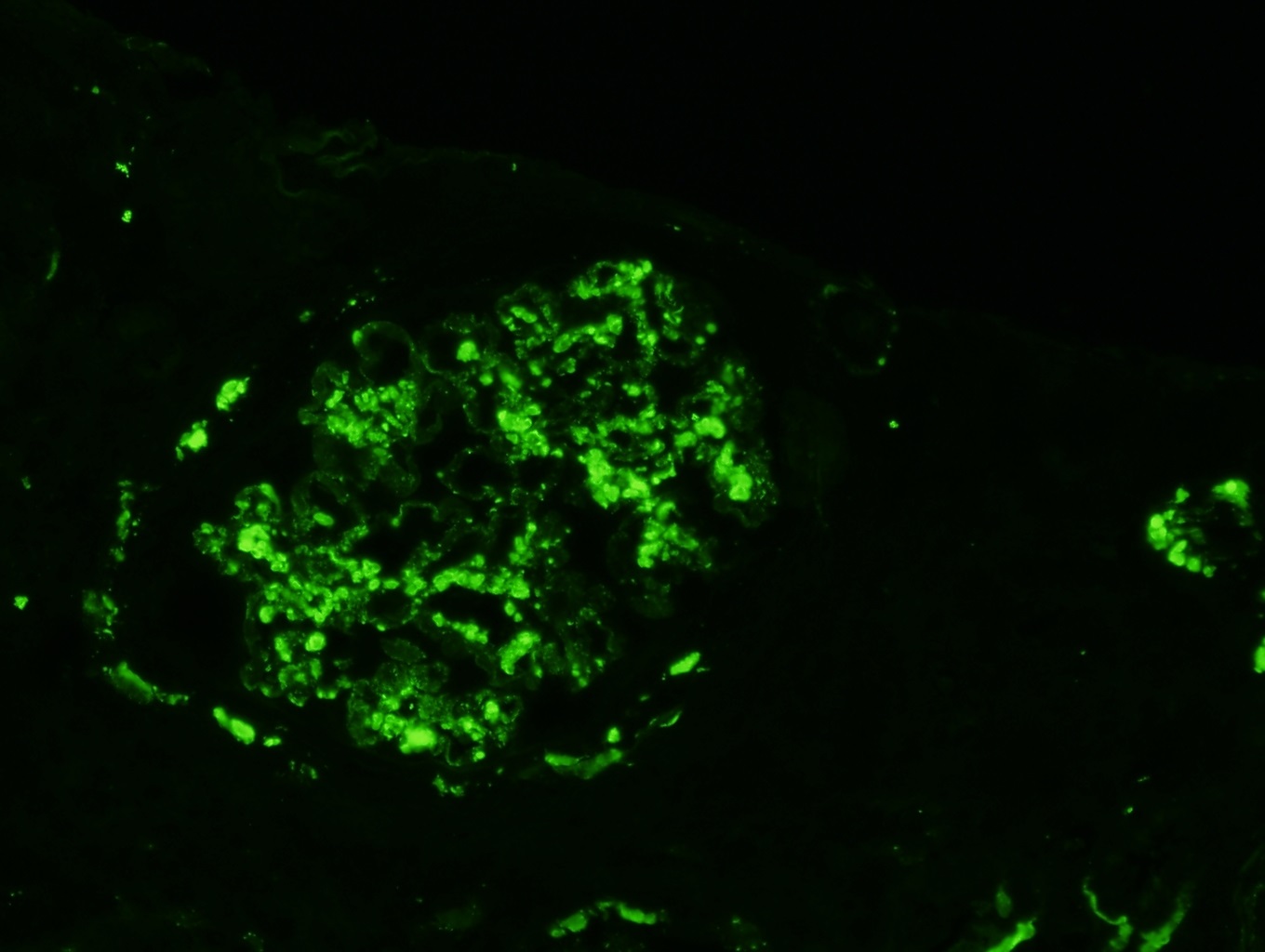
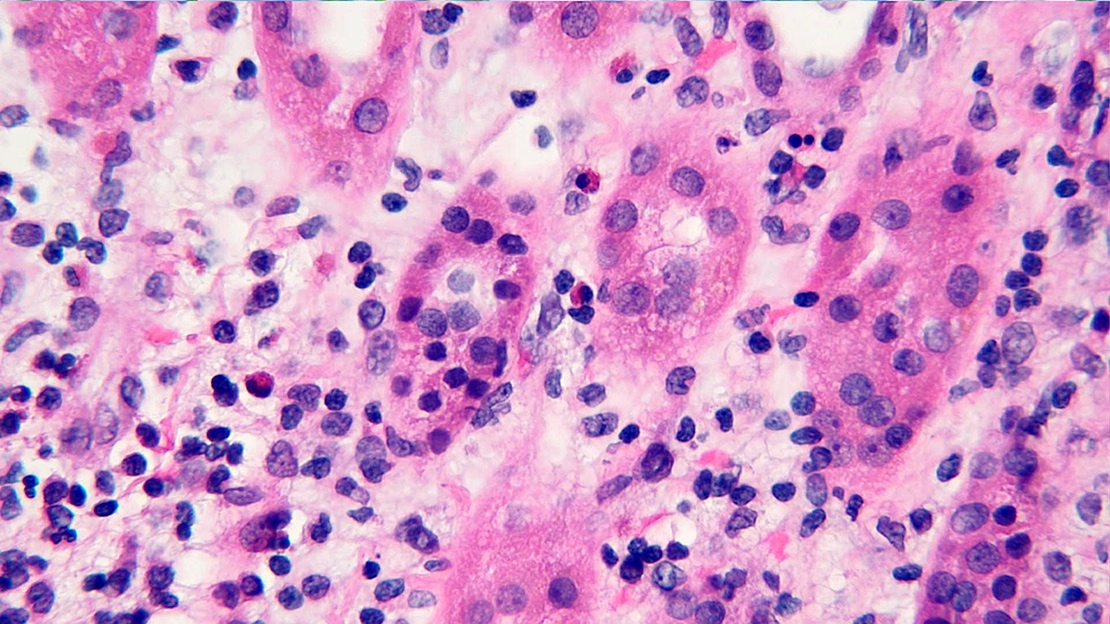
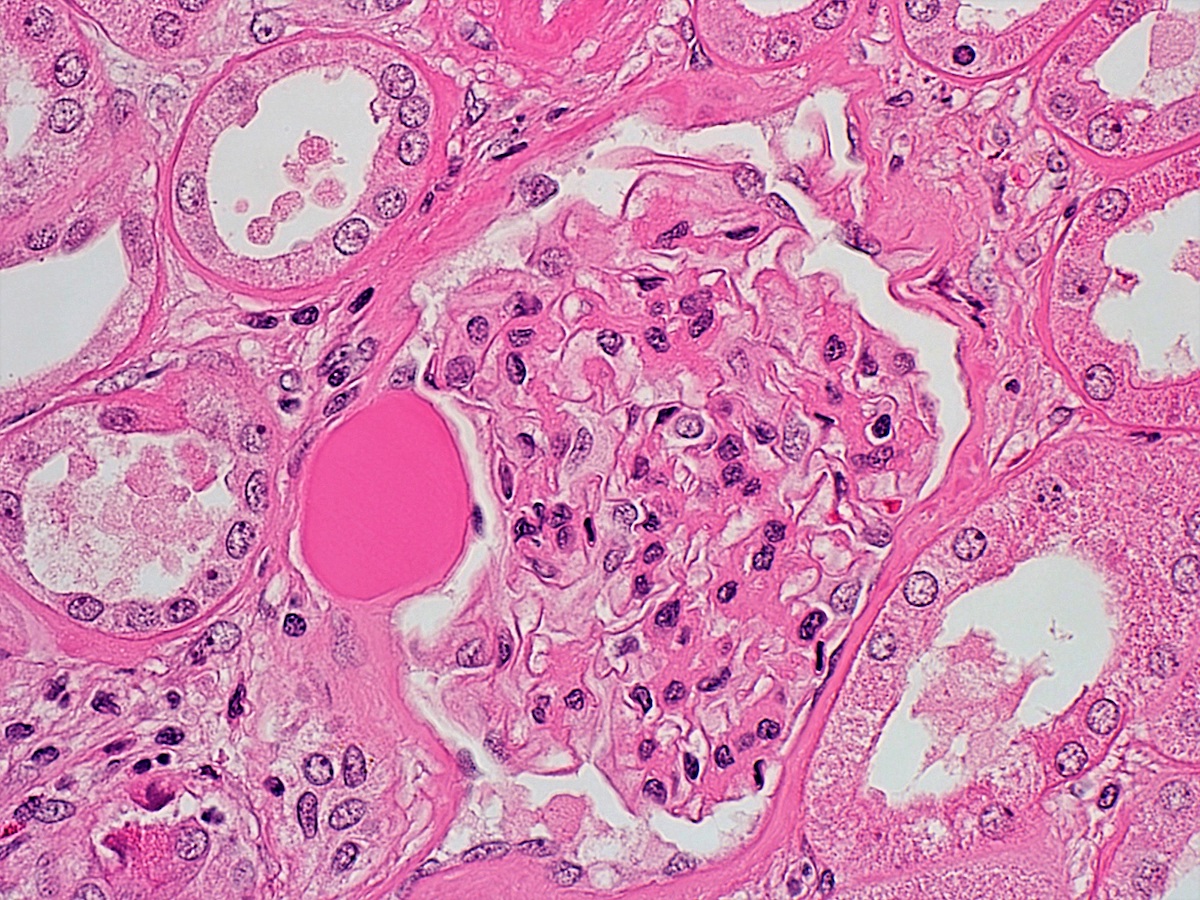
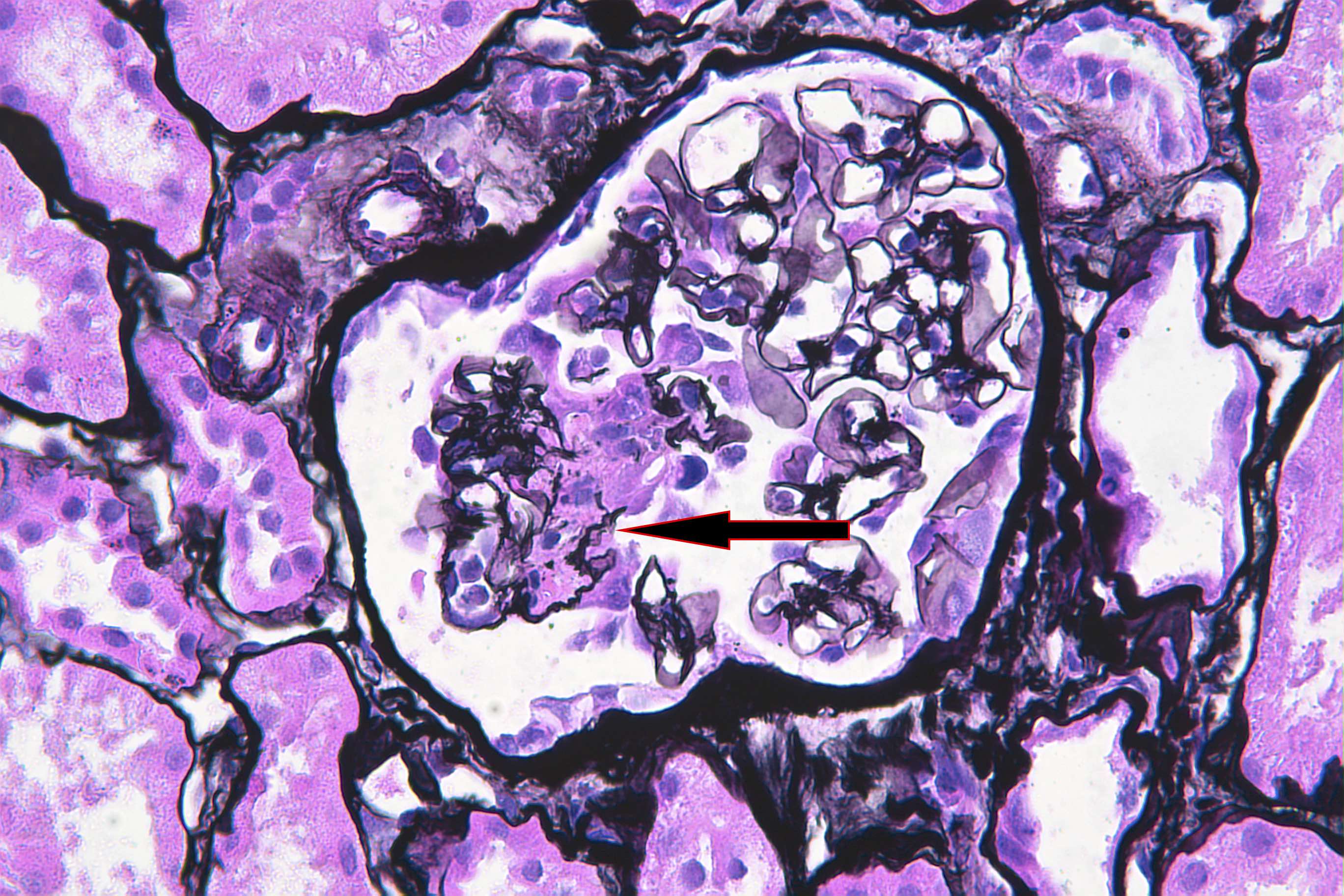
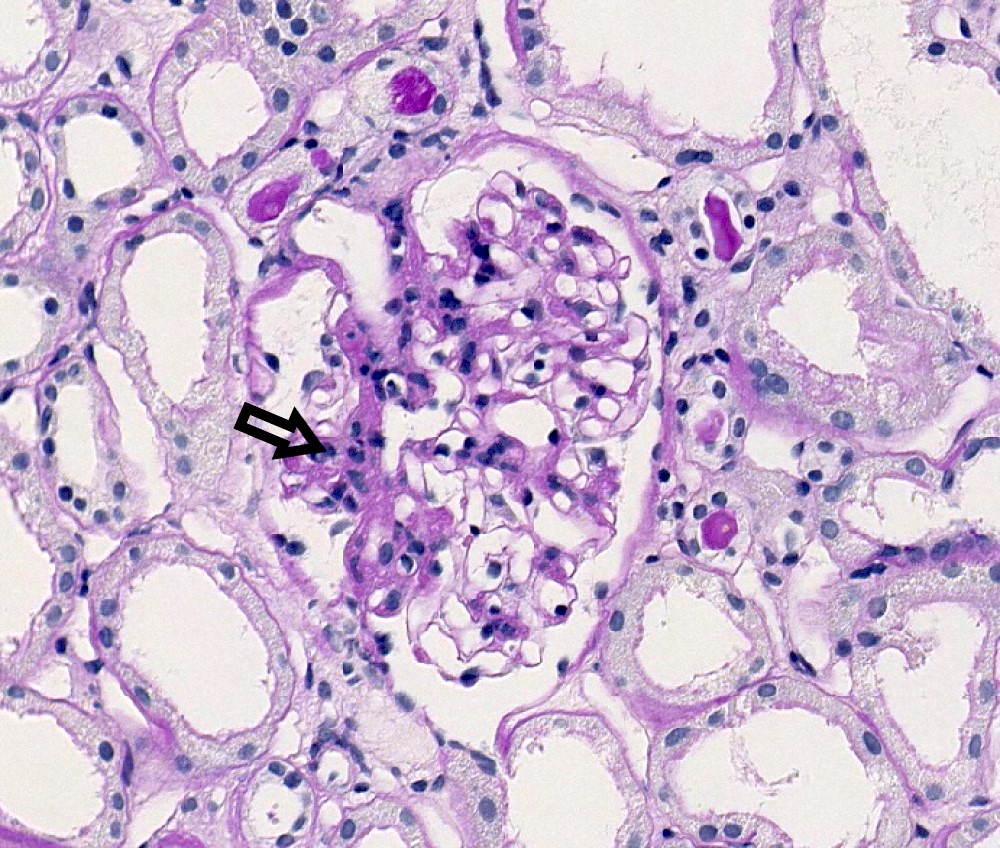
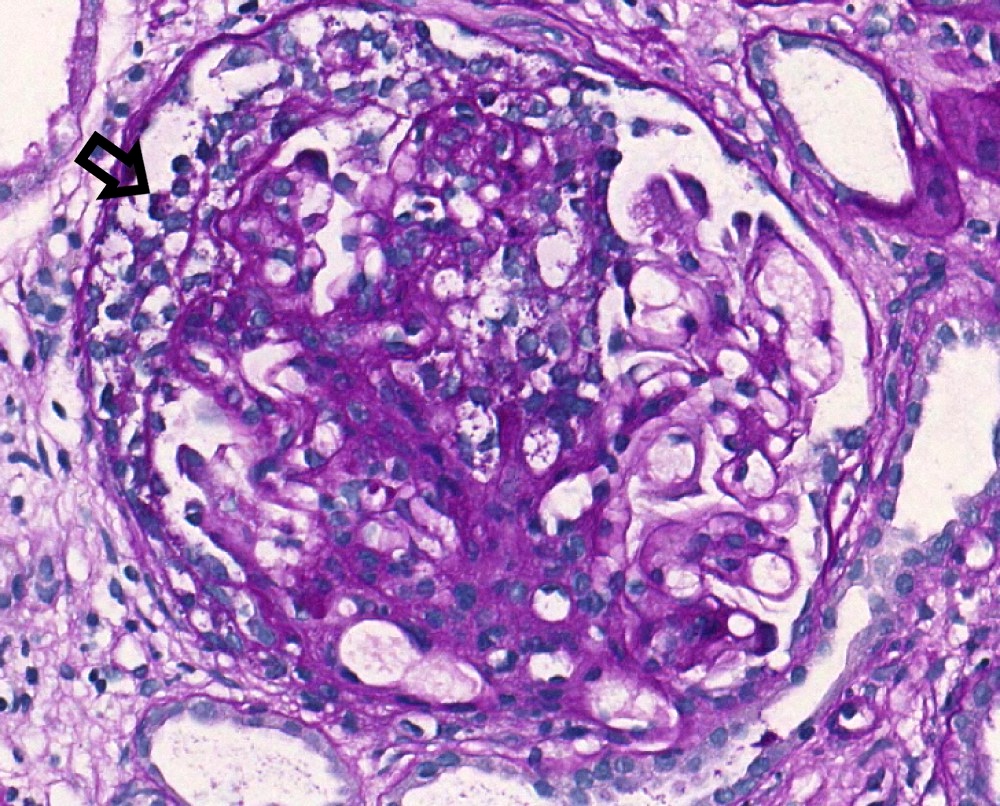
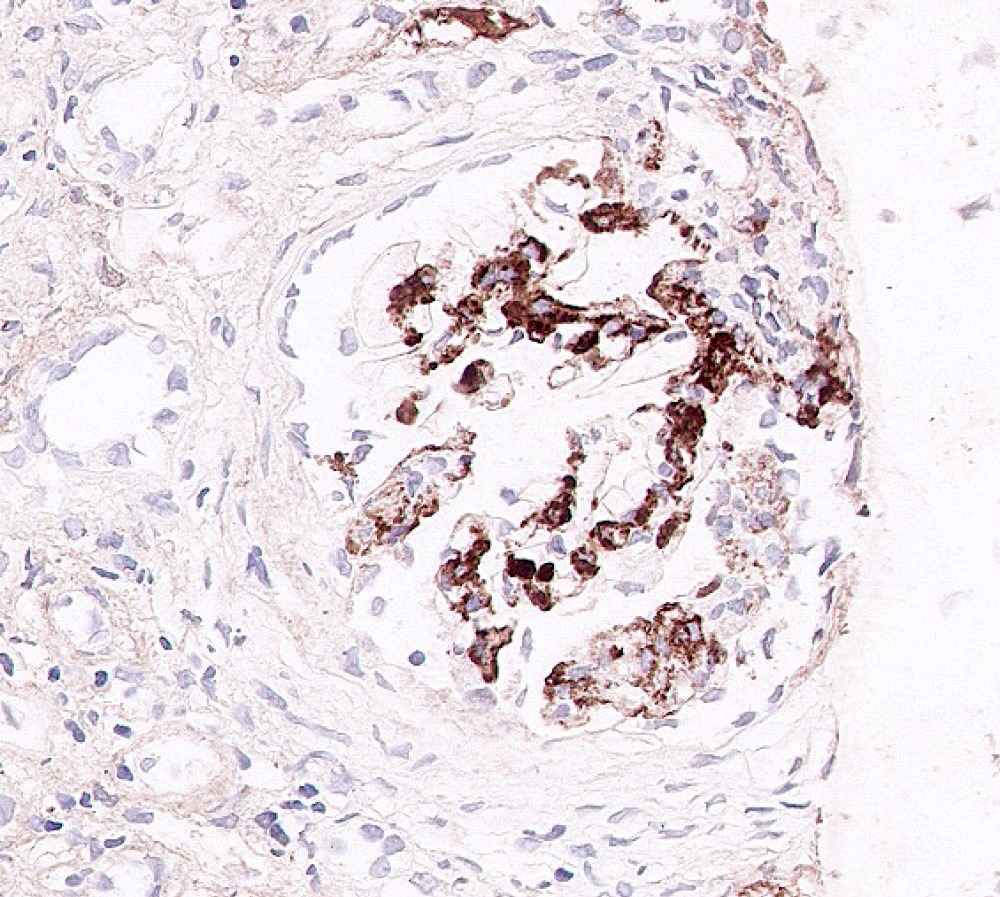
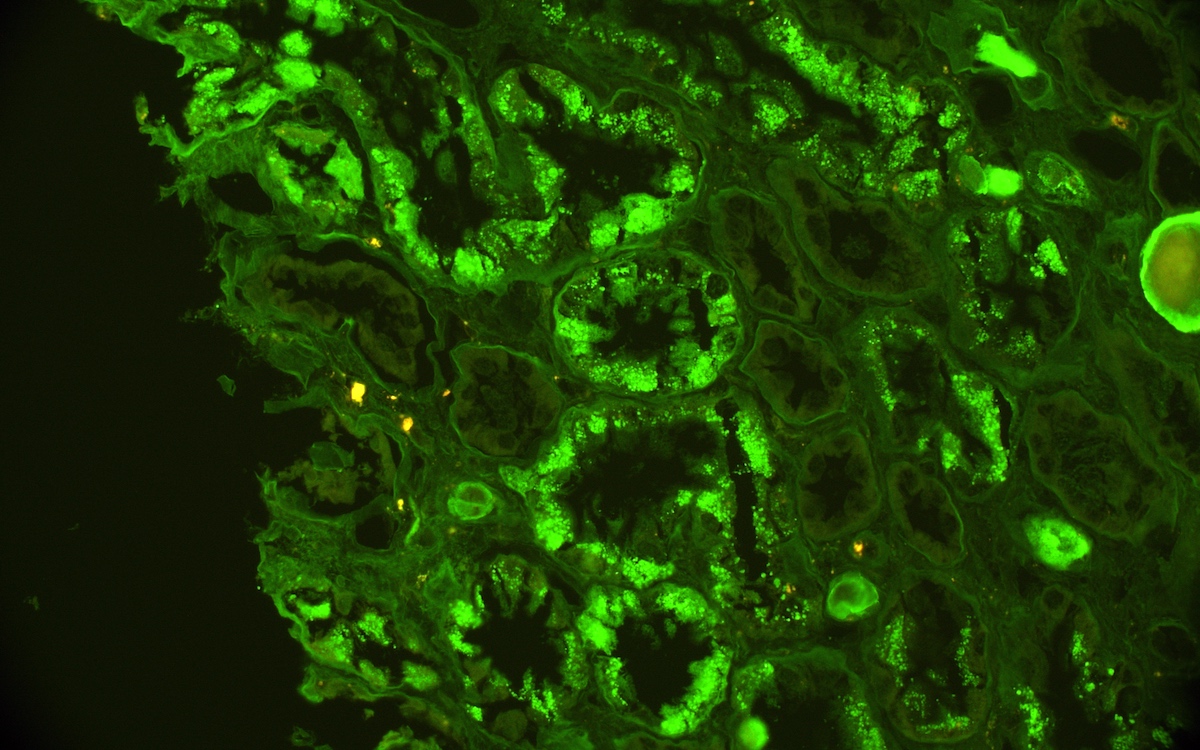
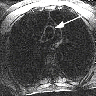
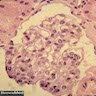
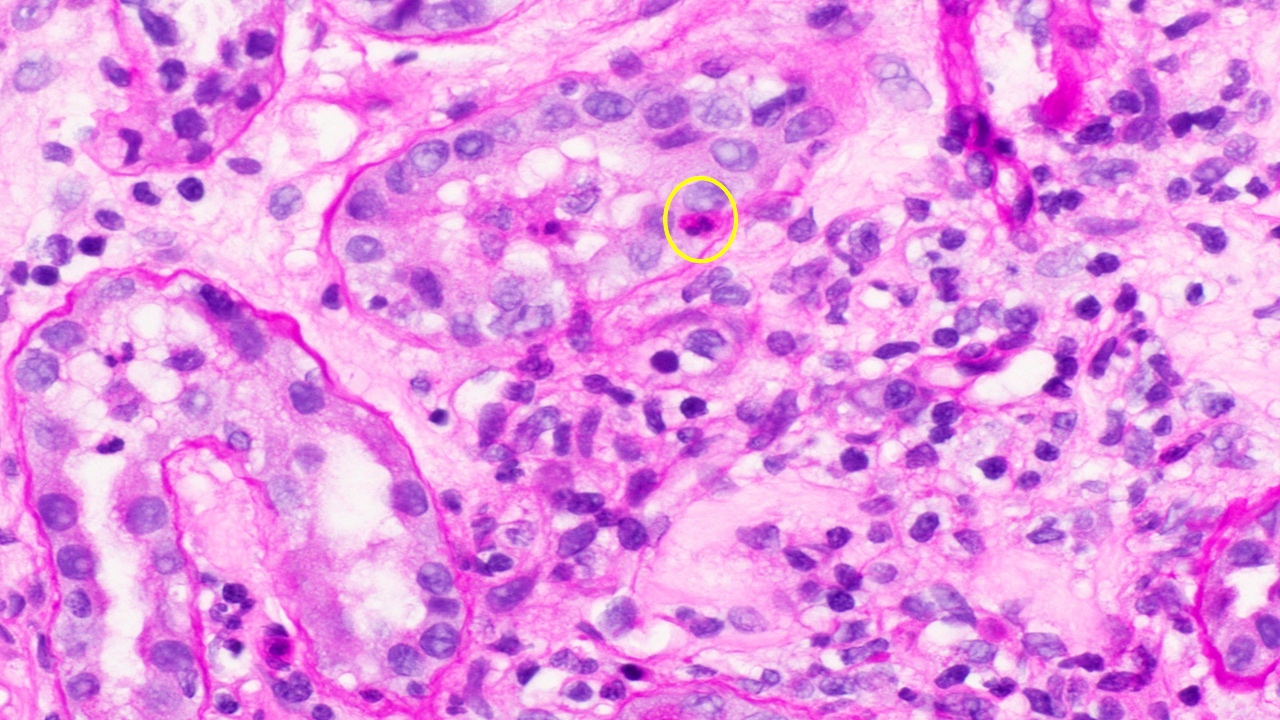
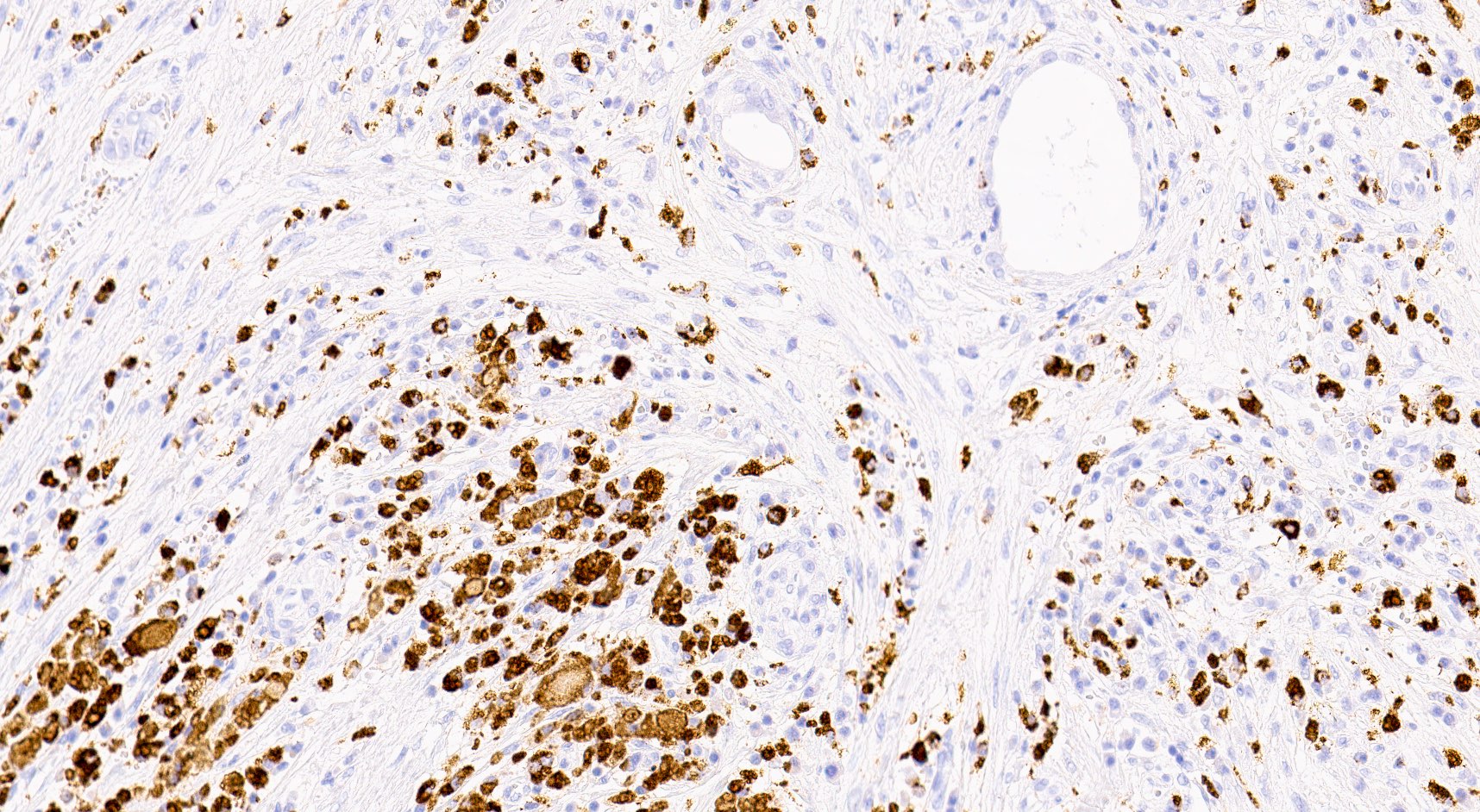
Based on the photomicrograph of C4d immunohistochemistry, which of the following would be expected on the H&E stained sections?
- Intranuclear inclusions within tubular epithelial cells
- Neutrophil leucocytes within tubular lumens
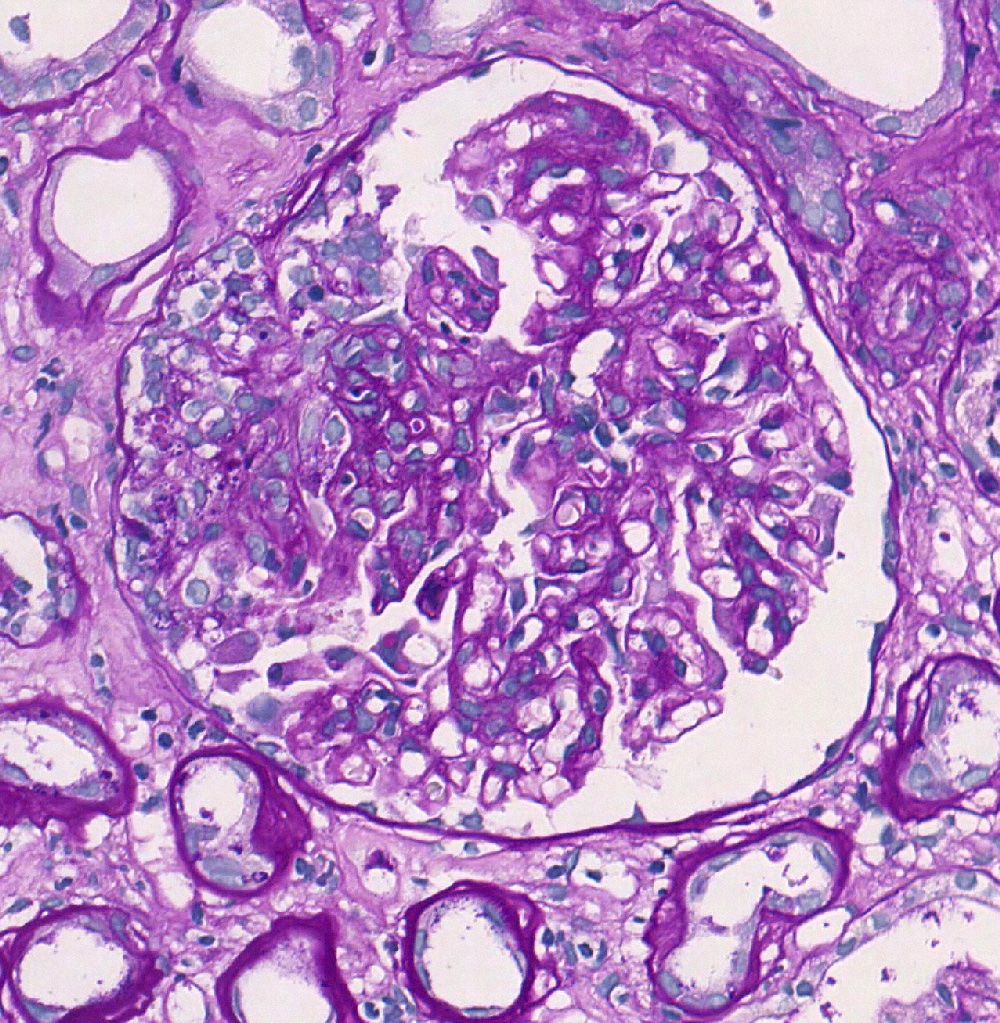
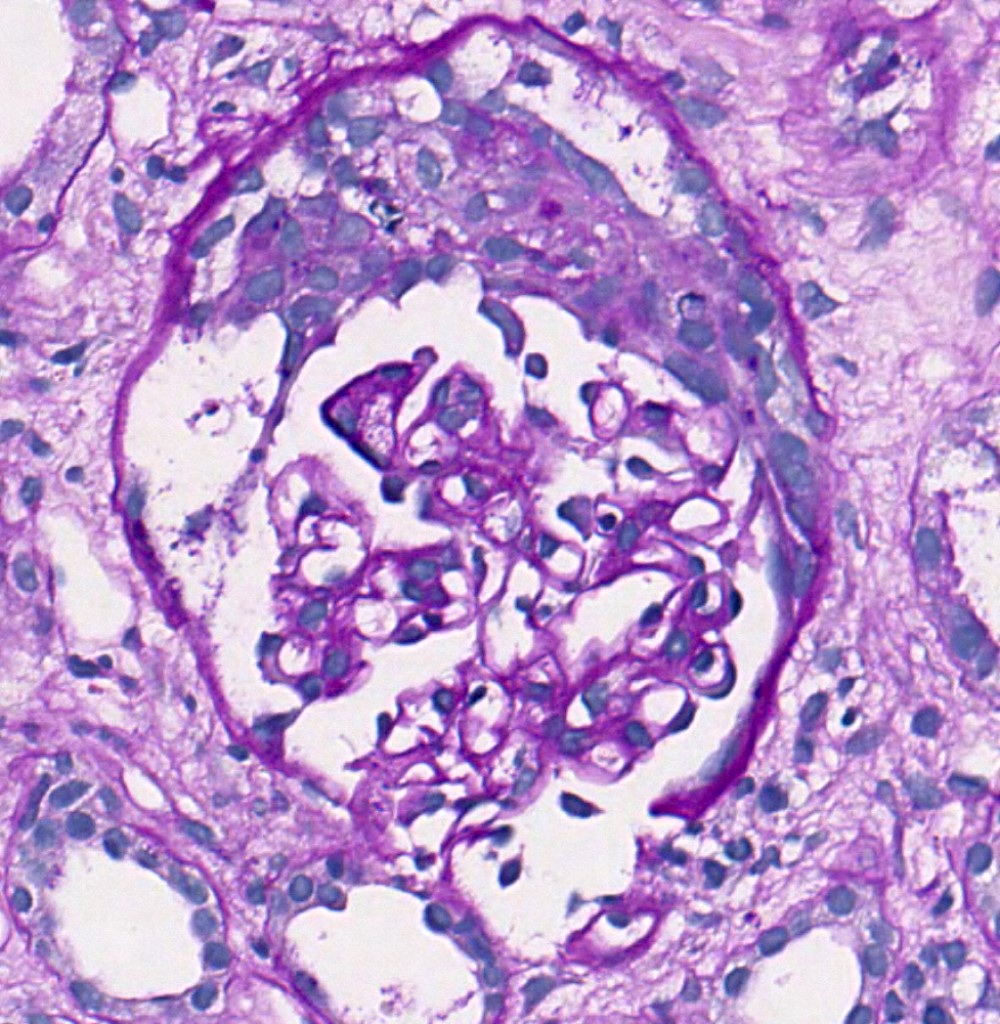
- Normal glomeruli
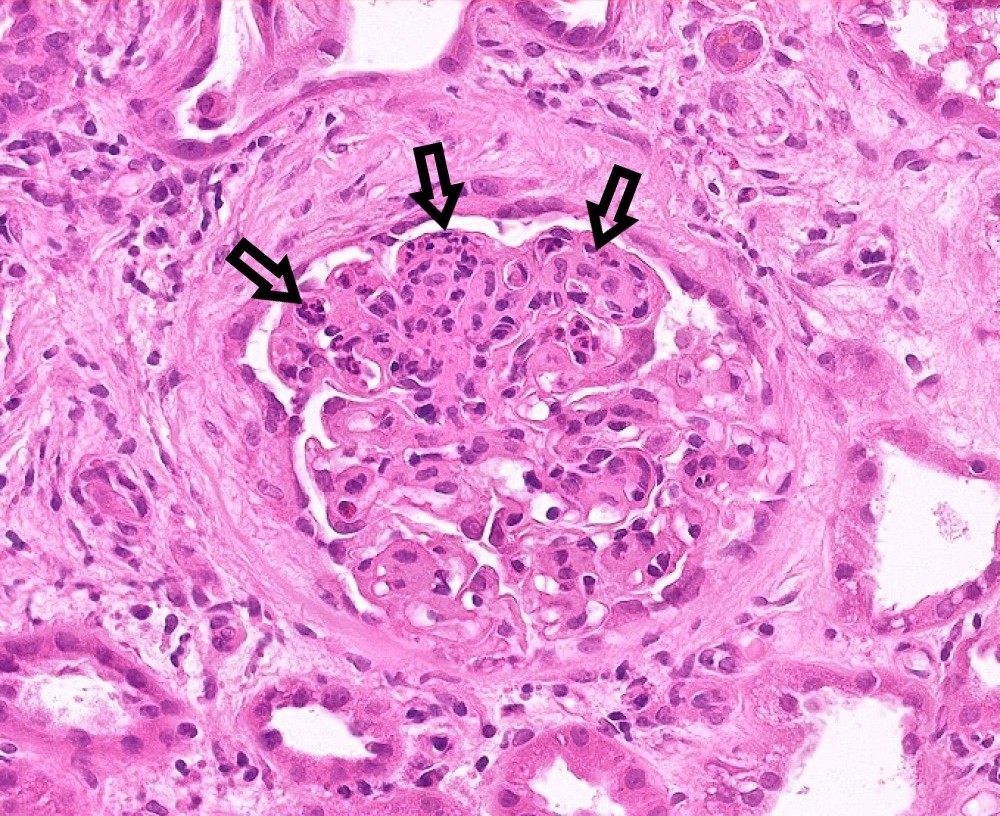
- Presence of inflammatory cells within peritubular capillaries
- Severe tubulitis
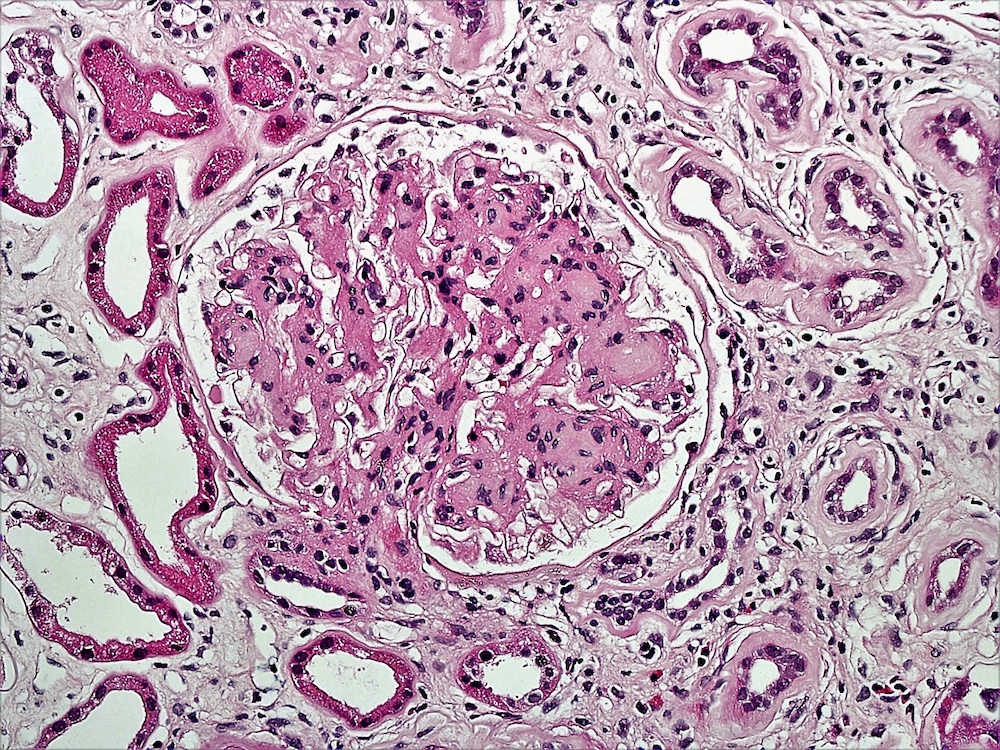
Comment Here
Reference: Active / chronic active antibody mediated rejection
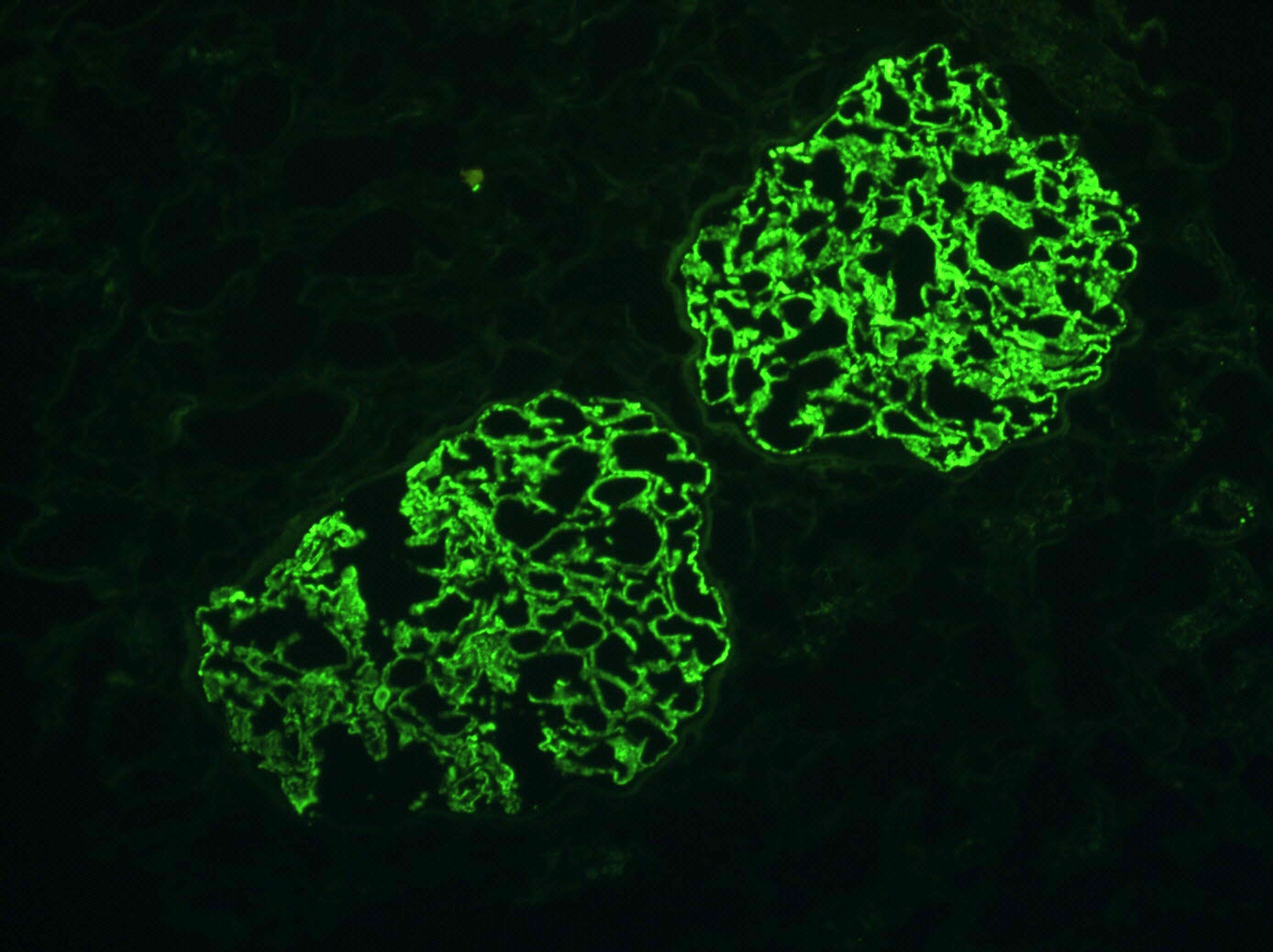
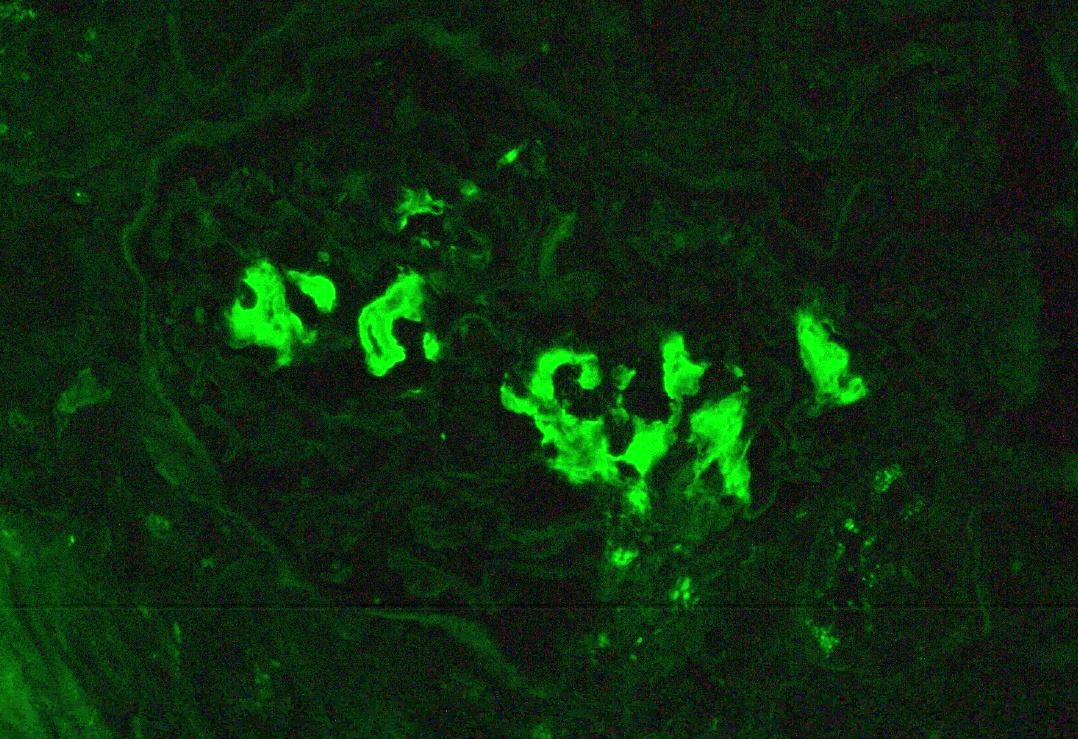
- C4d positivity along peritubular capillaries
- Presence of glomerular basement membrane double contours
- Presence of inflammatory cells within glomerular capillaries
- Presence of inflammatory cells within peritubular capillaries
- Presence of inflammatory cells within the interstitium
Comment Here
Reference: Active / chronic active antibody mediated rejection
- Alloimmune reaction to major histocompatibility complex (MHC) and non-MHC donor alloantigens resulting in damage to the kidney allograft
- Mediated by T cells and hence referred to as T cell mediated rejection (TCMR) in the Banff 2019 classification (Am J Transplant 2020;20:2318)
- 2 major subcategories:
- Acute T cell mediated rejection: characterized by an acute immunologic reaction
- Chronic active T cell mediated rejection: chronic renal injury due to persistent / recurrent TCMR
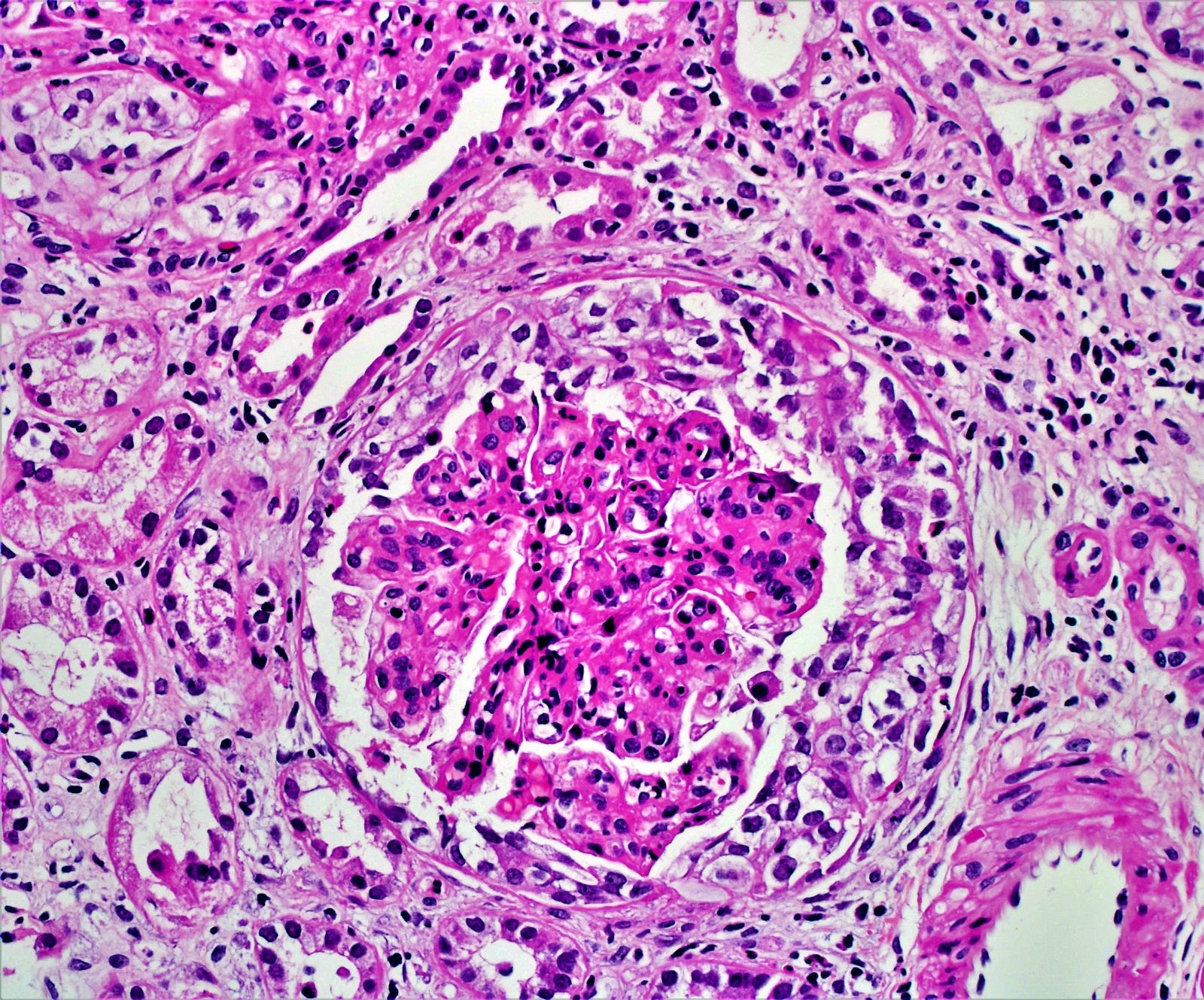
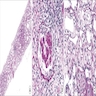
- Tubulitis and active interstitial inflammation
- Coded by the i and t Banff scores (see Banff grading below)
- Intimal arteritis or vasculitis may also be present (can sometimes be the sole component)
- This lesion can be caused by T cell mediated rejection (TCMR), active antibody mediated rejection or both
- Acute T cell mediated rejection: also referred to as acute cellular rejection, acute interstitial rejection, acute tubulointerstitial rejection
- Chronic active T cell mediated rejection: also referred to as chronic cellular rejection
- ICD-10: T86.11 - kidney transplant rejection
- With currently used combination therapies, 1 year acute rejection rates have decreased to 10 - 15%
- Renal disease
- T cells are sensitized and react against donor antigens expressed on antigen presenting cells and donor cells; delayed type hypersensitivity is also involved (Clin Biochem 2016;49:320, Nephron 2016;132:227)
- Macrophages and granulocytes are frequently recruited and also mediate tissue injury
- Tissue injury is manifested predominantly as tubular epithelial injury (tubulitis) and endothelial cell injury (intimal arteritis)
- Due to transplantation of foreign donor kidney allograft into recipient
- Acute T cell mediated rejection (TCMR):
- Usually seen during the first month after transplantation or later as immune suppression decreases due to noncompliance, adjustment of medication by physicians (as complications present), gastrointestinal diseases, metabolic diseases, etc.
- Presents with acute renal failure or oliguria; may also be asymptomatic (diagnosed on protocol biopsies)
- Chronic active TCMR: chronic renal failure with or without proteinuria; may also be asymptomatic (diagnosed on protocol biopsies)
- Established via indication or protocol biopsies
Diagnostic criteria and Banff classification / grading (modified from the Banff 2019 revision, Am J Transplant 2020;20:2318):
- Suspicious / borderline for acute T cell mediated rejection (TCMR):
- Tubulitis (t1, t2, t3) with interstitial inflammation comprising 10 - 25% of the nonscarred cortex (i1)
- Interstitial inflammation comprising > 25% of the nonscarred cortex (i2 or i3) with mild tubulitis (t1, pertaining to 1 - 4 inflammatory cells per tubular cross section)
- No arteritis
- Acute TCMR:
- Grade IA: interstitial inflammation involving > 25% of nonscarred cortex (i2 or i3) with moderate tubulitis (t2) of at least one tubule, excluding atrophic tubules
- Grade IB: interstitial inflammation involving > 25% of nonscarred cortex (i2 or i3) with severe tubulitis (t3) of at least one tubule, excluding atrophic tubules
- Grade IIA: mild to moderate intimal arteritis (v1) with or without interstitial inflammation or tubulitis
- Grade IIB: severe intimal arteritis (v2) with or without interstitial inflammation or tubulitis
- Grade III: transmural arteritis or arterial fibrinoid necrosis of medial smooth muscle with accompanying mononuclear cell intimal arteritis (v3) with or without interstitial inflammation or tubulitis
- Chronic active TCMR:
- Grade IA: interstitial inflammation involving > 25% of the total cortex (ti2 or ti3) and > 25% of the scarred cortex (i-IFTA2 or i-IFTA3) with moderate tubulitis (t2 or t-IFTA2) of at least one tubule, excluding severely atrophic tubules; other known causes of i-IFTA should be ruled out
- Grade IB: interstitial inflammation involving > 25% of the total cortex (ti2 or ti3) and > 25% of the scarred cortex (i-IFTA2 or i-IFTA3) with severe tubulitis (t3 or t-IFTA3) of at least one tubule, excluding severely atrophic tubules; other known causes of i-IFTA should be ruled out
- Grade II: chronic allograft arteriopathy
- Prior to Banff classification, cooperative clinical trials in transplantation (CCTT) criteria set the threshold for acute TCMR as active interstitial inflammation in ≥ 5% of cortex and at least 3 tubules with tubulitis in 10 consecutive high power fields from the most severely affected areas (J Am Soc Nephrol 1997;8:1930)
- Acute T cell mediated rejection (TCMR): acute increase in serum creatinine levels
- Chronic active TCMR: chronic increase in serum creatinine levels, proteinuria may develop eventually
- Contrast enhanced ultrasound has been shown to be of diagnostic value in identifying cases of vascular rejection (Clin Hemorheol Microcirc 2018;69:77)
- New technologies for identification of acute rejection are at the experimental stage (Am J Nucl Med Mol Imaging 2019;9:110)
- Imaging seems to rely on analysis of changes in blood flow, which decreases with acute rejection induced inflammation and detection of recruitment of activated leucocytes with 18F-fluoro-deoxy-glucose positron emission tomography (Clin Kidney J 2017;10:97)
- Vascular rejection is associated with a poor long term outcome and is generally resistant to antirejection therapy (Kidney Int 2002;61:1516)
- Eosinophils and plasma cells within the inflammatory infiltrate is associated with poor long term outcome and resistance to antirejection therapy
- 24 year old woman with severe interstitial lymphoplasmacytic infiltrate (Exp Clin Transplant 2020;18:106)
- 39 year old man with refractory CD20+ cellular rejection (Exp Clin Transplant 2019;17:823)
- 56 year old man with refractory cellular rejection treated with photopheresis (Nefrologia 2016;36:327)
- Pulse steroid therapy for grade I acute T cell mediated rejection (TCMR) (Talanta 2014;130:542)
- Anti-T cell agents for grade II acute TCMR
- Grade III acute TCMR is resistant to therapy
- Newer immunosuppresive agents with more potent activity are emerging (Expert Opin Emerg Drugs 2017;22:123)
- Acute T cell mediated rejection (TCMR): kidney is swollen, cortex is pale and shiny; hemorrhage and necrosis may be present in severe cases with vascular involvement
- Chronic active TCMR: kidney may be shrunken with surface depressions due to scarring of the underlying parenchyma; corticomedullary junction is blurred
- Acute T cell mediated rejection (TCMR):
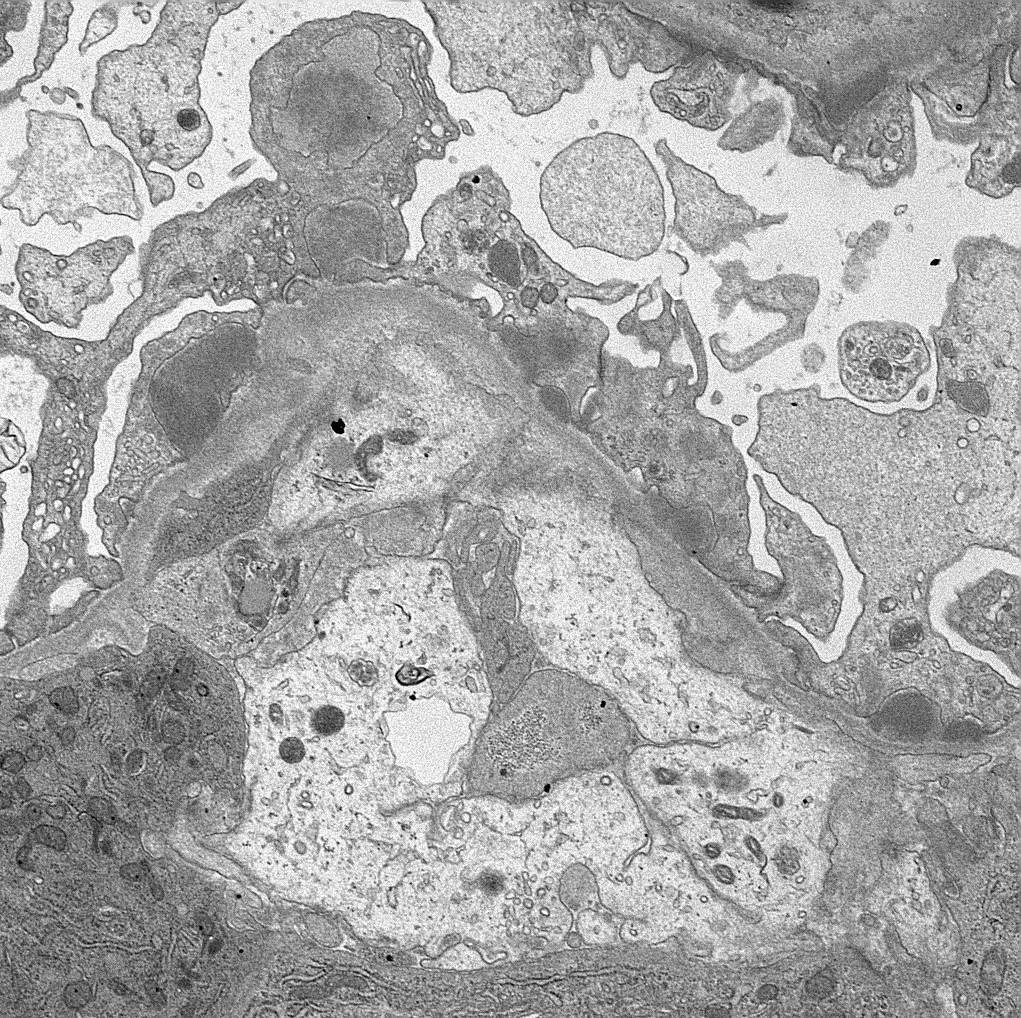
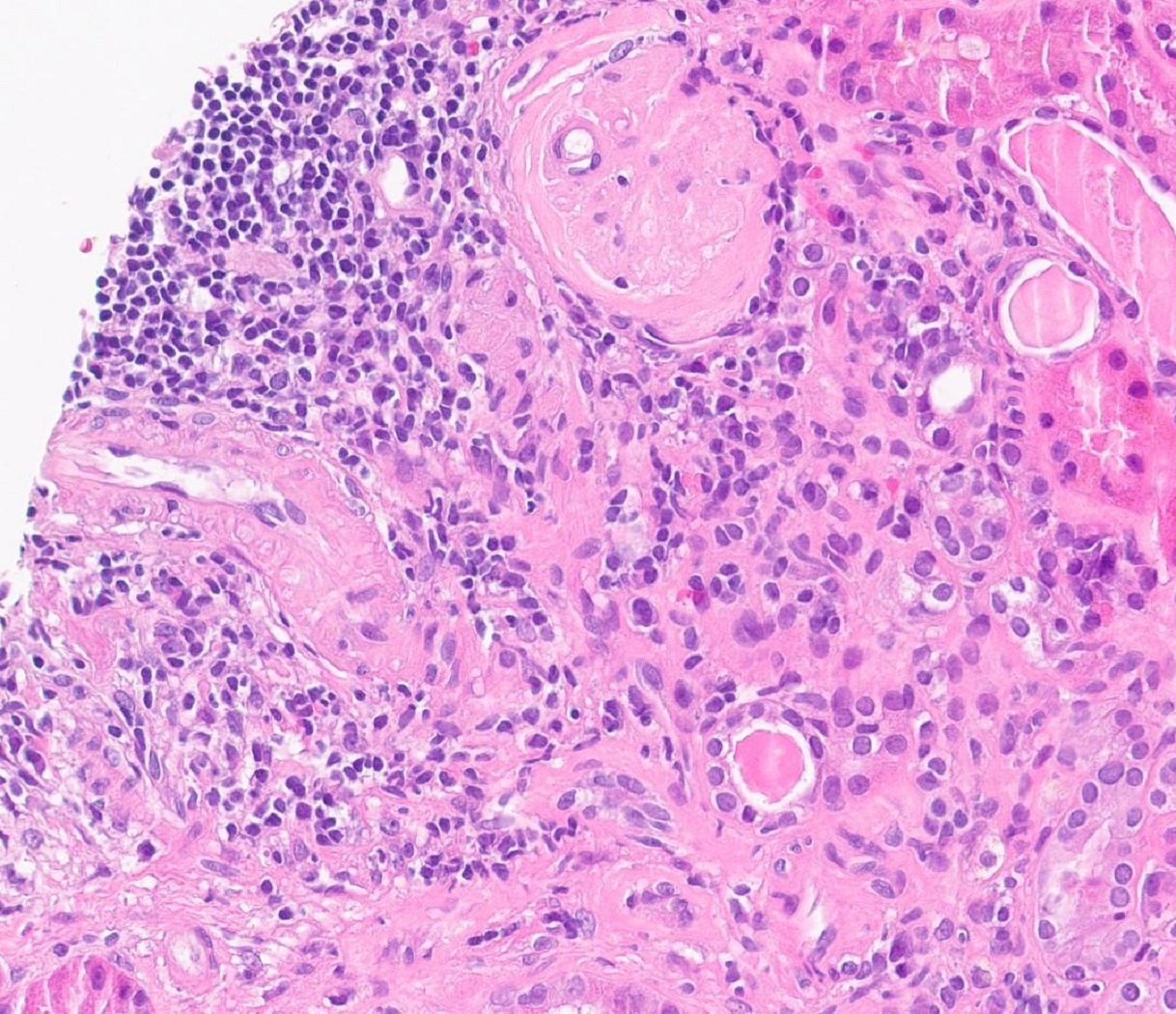
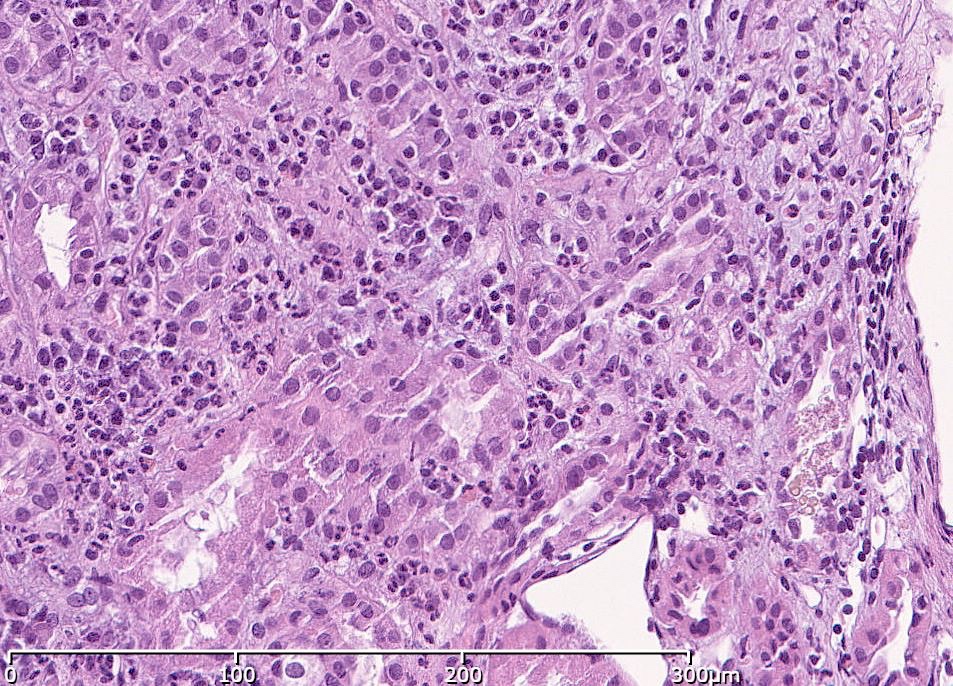
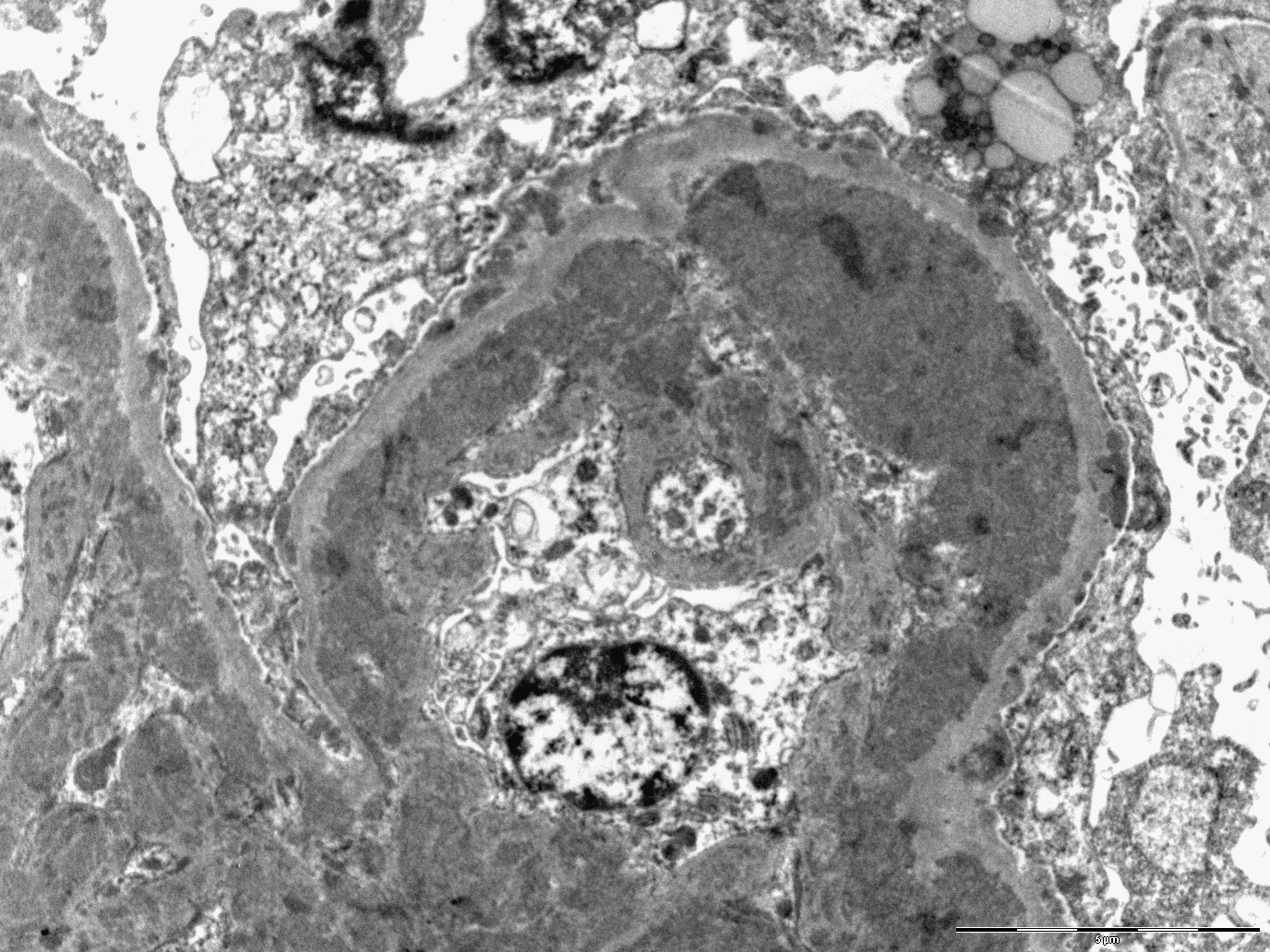
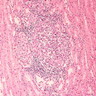
- Interstitial edema and inflammatory infiltrate involving at least 25% of the nonscarred cortex; graded according to severity (i score ≥ 2)
- Presence of tubulitis: inflammatory cells within and among tubular epithelial cells within the confines of the basement membrane; is graded according to severity (t score ≥ 2)
- Lesser degrees of inflammation (10 - 25% of the nonscarred cortex - i1) or inflammation involving ≥ 25% of the nonscarred cortex (i score ≥ 2) but accompanied by only mild tubulitis (t1 - up to 4 inflammatory cells / tubular cross section) is considered suspicious / borderline for acute TCMR
- Some studies suggest a lower threshold for diagnosis of rejection (t1i1) (Transplantation 2018;102:2120)
- Inflammatory population is mainly mononuclear - T cells and macrophages, CD4+ T cells dominate
- Eosinophils and plasma cells may also be present, especially in severe cases and more commonly if acute TCMR develops late (after the first year of transplantation)
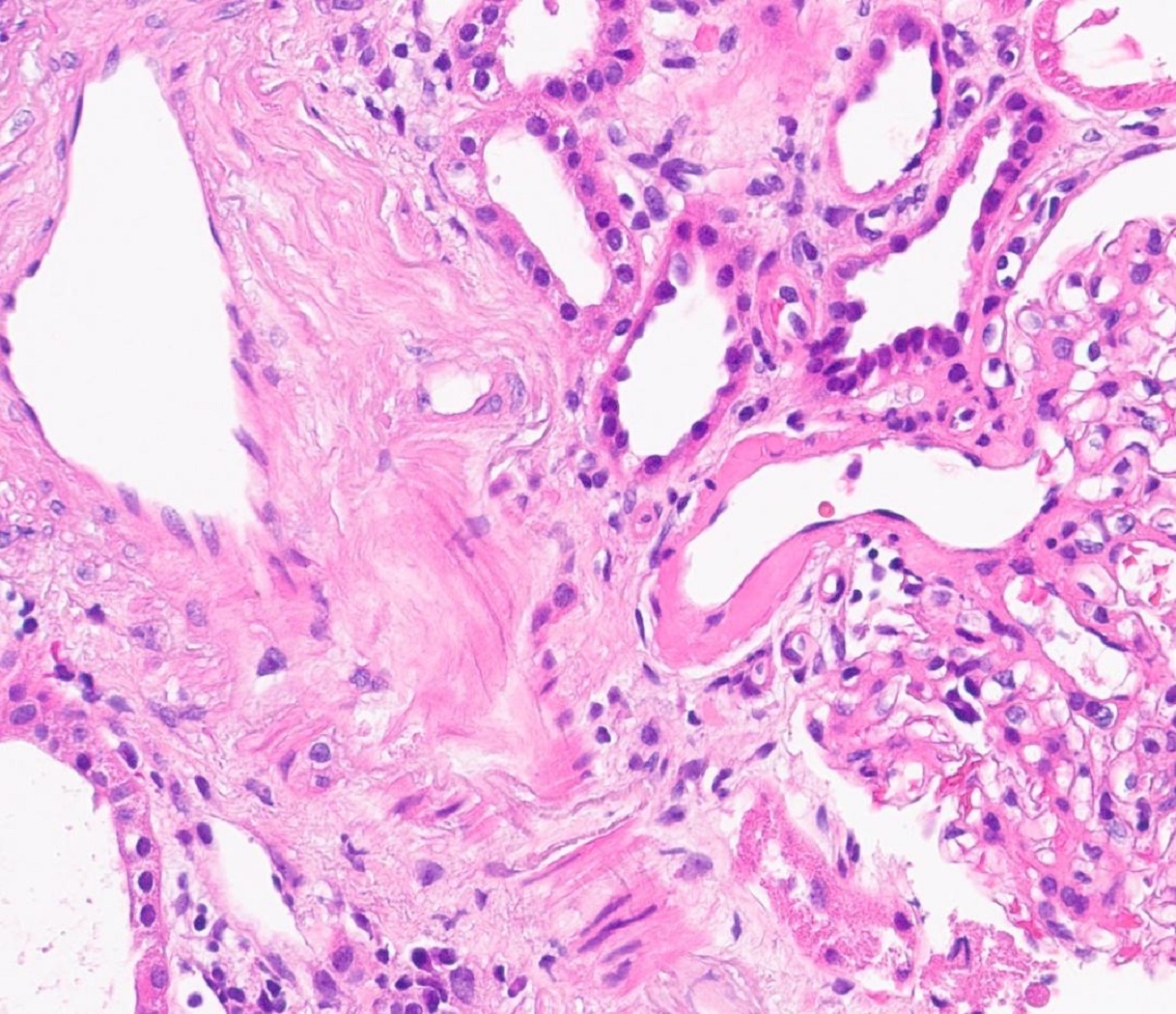
- Glomeruli usually spared; however, in cases with accompanying vascular involvement, endothelial swelling and mesangiolysis may be present - acute allograft glomerulopathy
- Vascular involvement (type II and III): inflammation of the arterial or arteriolar wall, intimal infiltration (intimal arteritis, endarteritis, endothelialitis) or transmural infiltration by lymphocytes and macrophages, with or without accompanying fibrinoid necrosis; note that these vascular lesions can also be a part of active antibody mediated (humoral) rejection and are not a defining feature (unlike tubulitis)
- Chronic active TCMR:
- Areas of tubular atrophy and interstitial fibrosis with mononuclear inflammatory infiltrate and tubulitis; this morphology is referred to as i-IFTA (inflammation in areas of interstitial fibrosis / tubular atrophy) and t-IFTA (tubulitis in areas of interstitial fibrosis / tubular atrophy) (Am J Transplant 2018;18:364, Am J Transplant 2018;18:377)
- Transplant arteriopathy: intimal fibrosis together with mononuclear cells and foam cells in the intima
- Glomeruli may show focal segmental and global sclerosis
- Other causes of i-IFTA (such as BK virus nephropathy, chronic pyelonephritis, antibody mediated rejection, recurrent glomerulonephritis) should be ruled out because i-IFTA is a nonspecific lesion
- References: Transplantation 2018;102:1795, Nephrology (Carlton) 2018;23 Suppl 2:45
- Banff i interstitial inflammation scoring (Am J Transplant 2008;8:753, Zhou: Silva's Diagnostic Renal Pathology, 2nd Edition, 2017):
- i0: < 10% of nonfibrotic cortex
- i1: 10 - 25% of nonfibrotic cortex
- i2: 26 - 50% of nonfibrotic cortex
- i3: > 50% of nonfibrotic cortex
- Banff t tubulitis scoring:
- t0: no mononuclear cells in tubules
- t1: 1 - 4 cells / tubular cross section
- t2: 5 - 10 cells / tubular cross section
- t3: > 10 cells / tubular cross section
- v - vascular inflammation: the most severely affected artery dictates the score
- v0: no arteritis
- v1: intimal arteritis with v2: intimal arteritis with ≥ 25% of luminal area lost in 1+ arteries
- v3: transmural arteritis or fibrinoid necrosis (medial smooth muscle necrosis) with lymphocyte infiltrate in vessels
- cv - transplant arteriopathy: arterial fibrointimal thickening; percentage of narrowing of lumen of most severely affected artery
- cv0: none
- cv1: ≤ 25% of the luminal area
- cv2: 26 - 50% of the luminal area
- cv3: > 50% of the luminal area
- i-IFTA and t-IFTA scored similarly to “i” and “t”, only in areas of interstitial fibrosis / tubular atrophy
- Reference: Transplantation 2018;102:1795
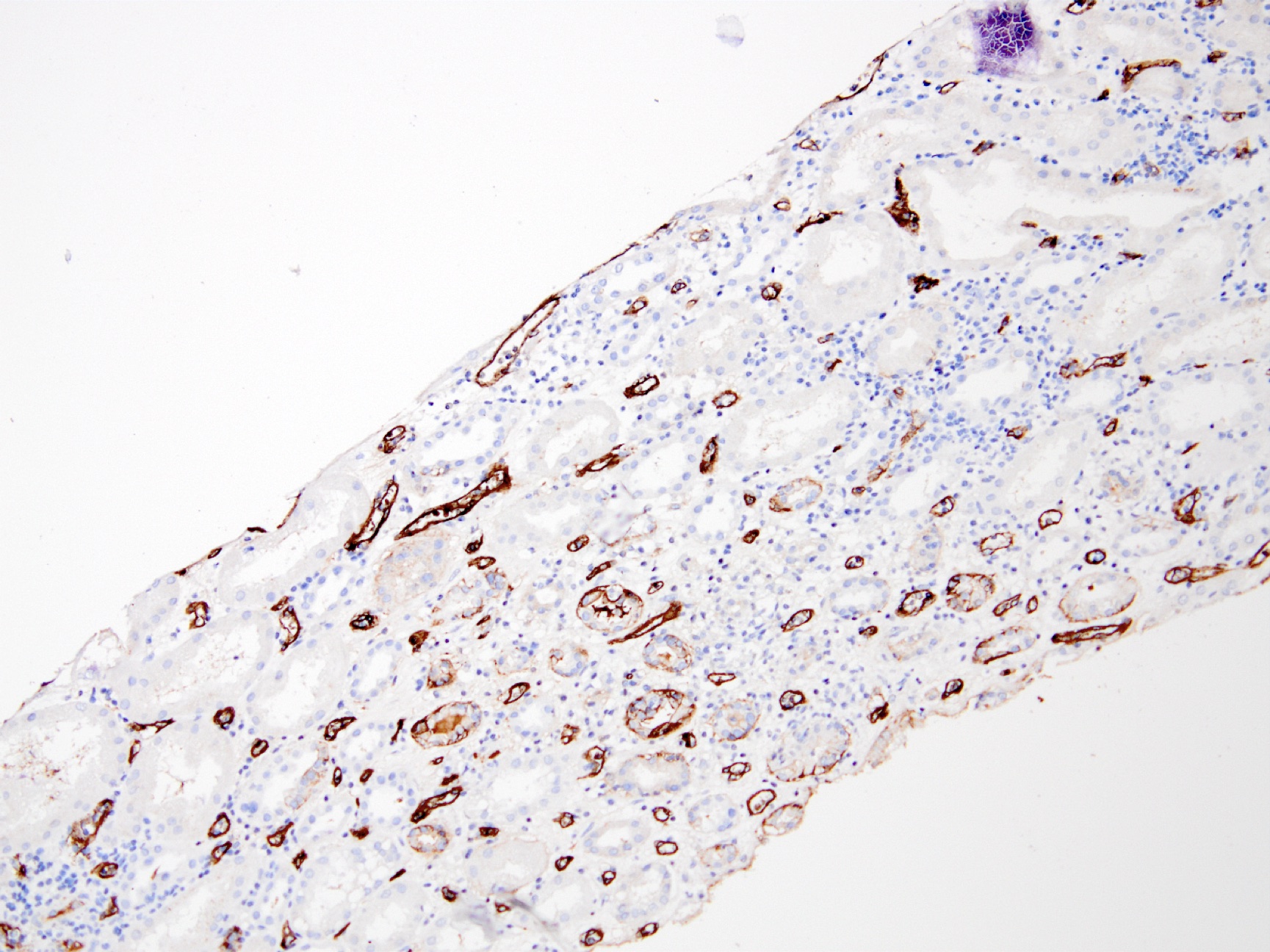
Images hosted on other servers:
Contributed by Arzu Sağlam, M.D.
- No immunoglobulin or only nonspecific scarce staining
- C3 may be present along tubular basement membranes, interstitium and vessel walls
- C4d negative if not accompanied by concurrent antibody mediated rejection
- PAS, Jones methenamine silver and periodic acid silver methenamine stains are very useful for identifying tubulitis by highlighting the basement membrane and stromal epithelial interface
- Silver and trichrome stains are useful for identifying fibrinoid necrosis
- SV40 and C4d immunohistochemistry negative
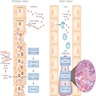
- Genome wide expression studies in kidney transplants have identified certain sets of genes / transcripts, which correlate with T cell mediated rejection (TCMR) (BMC Nephrol 2015;16:132)
- Top molecules involved with T cell effector mechanisms: granzyme, perforin
- Top molecules involved with γ interferon effects: CXCL9, CXCL10, CXCL11
- Top molecules involved with effector / inflammatory cell recruitment: CCL5, PSMB9
- Molecular phenotype for TCMR, however, is not present amongst the criteria for TCMR in the most recent Banff classification
- A 770 gene panel encompassing genes involved in rejection, tolerance, viral infections, innate and adaptive immune responses, the Banff Human Organ Transplant Panel (B-HOT), has been made commercially available and is going to be used on the NanoString platform for research purposes (Am J Transplant 2020;20:2305)
- A simplified molecular panel will hopefully be standardized and integrated into the Banff classification criteria in the near future
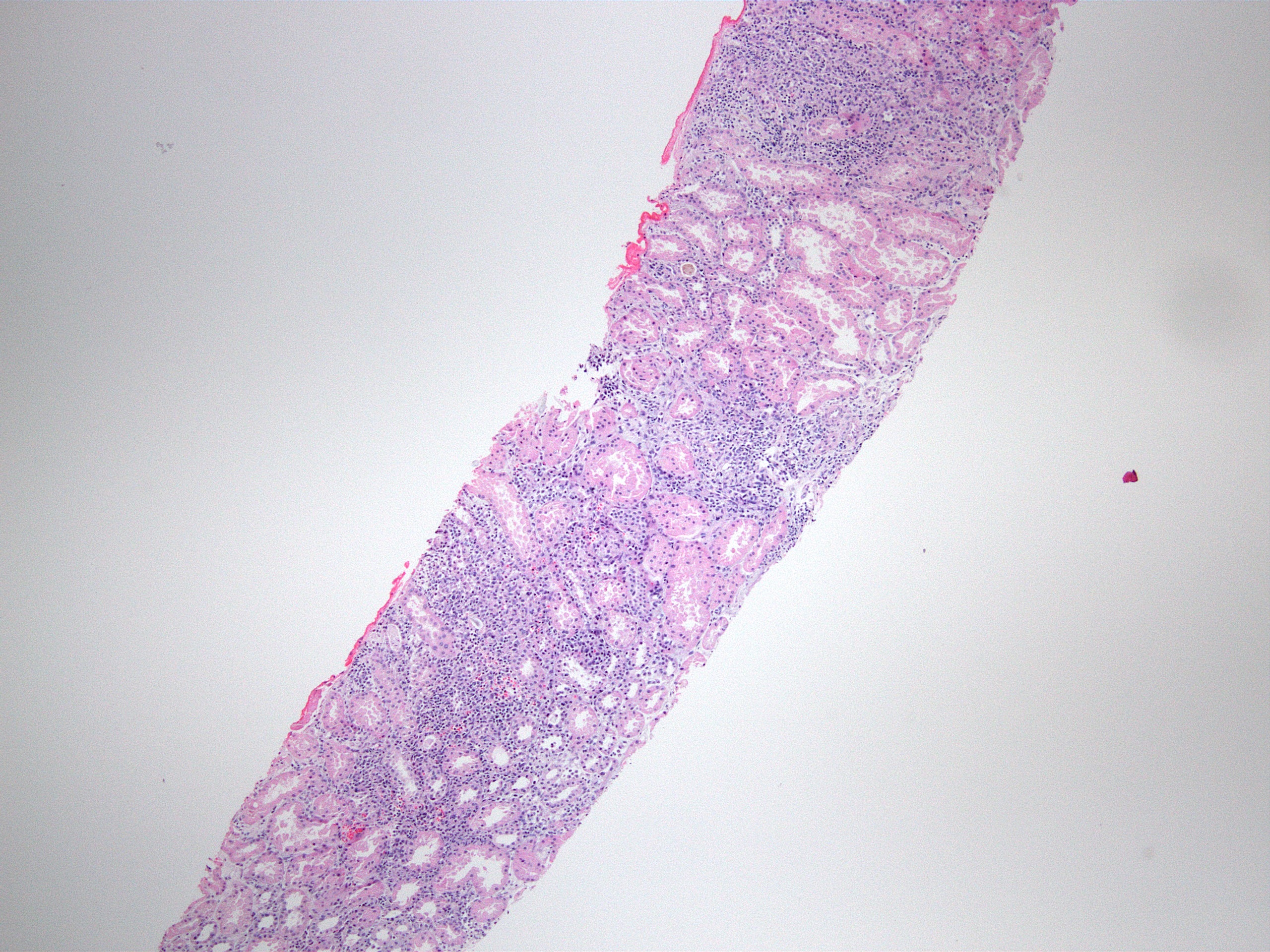
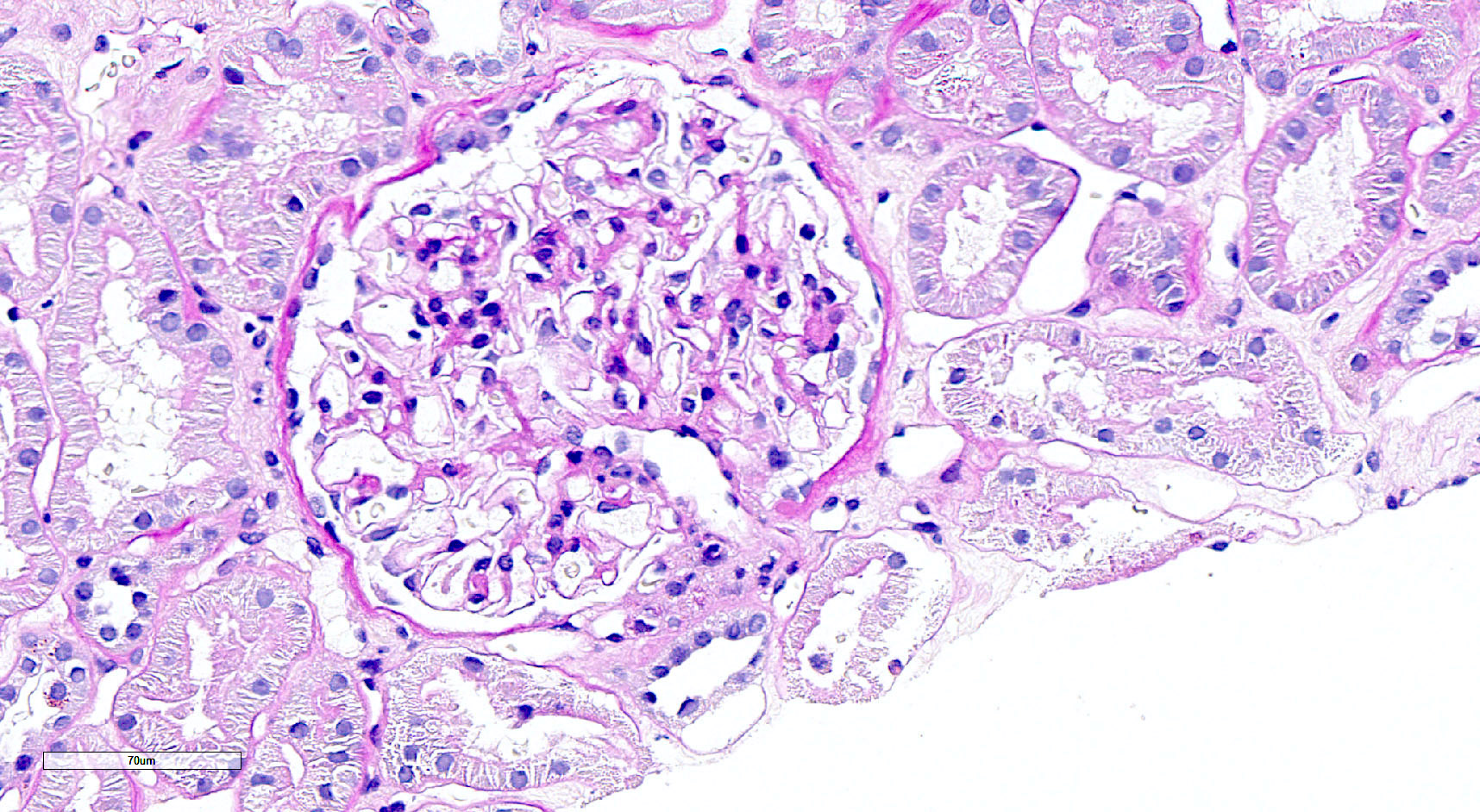
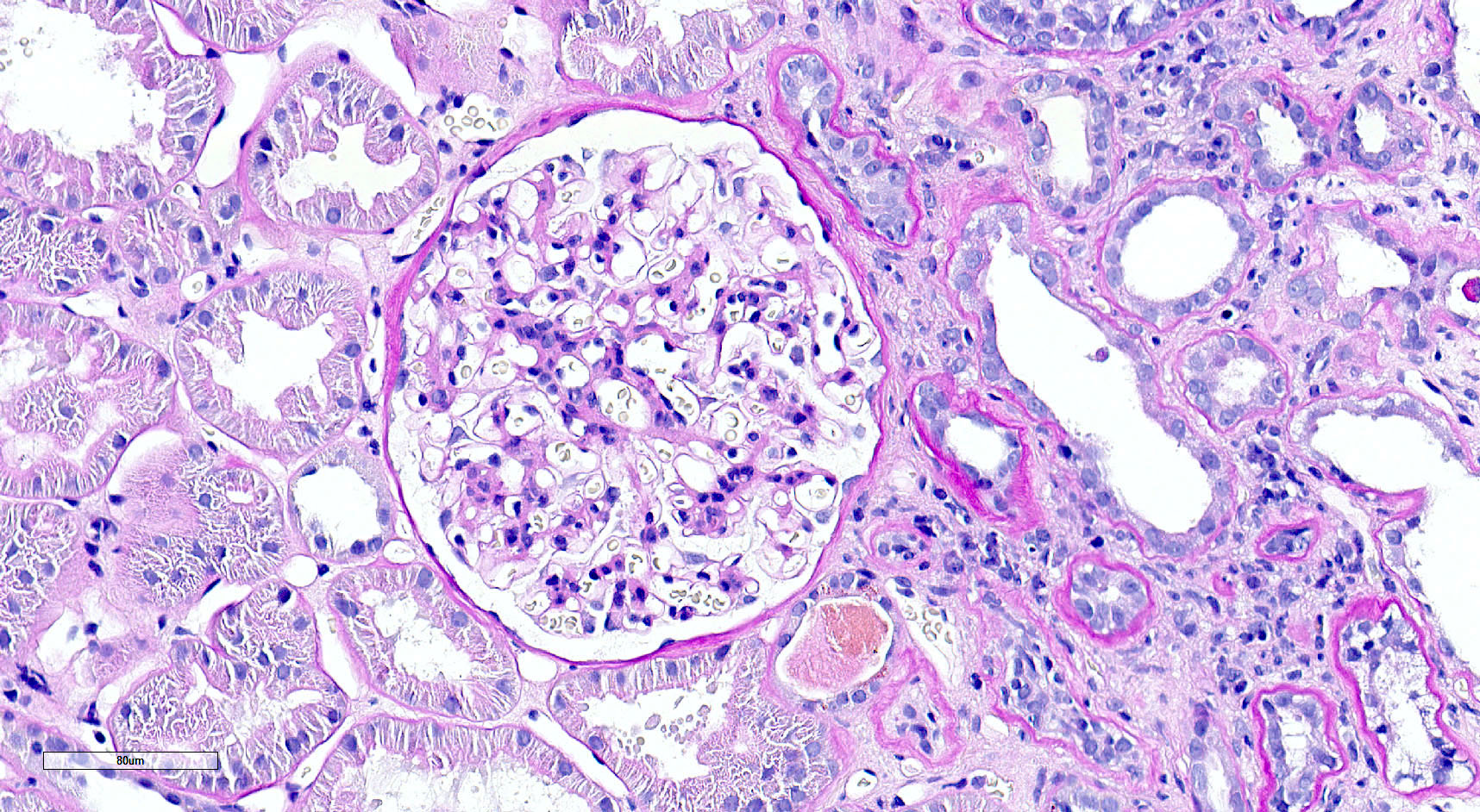
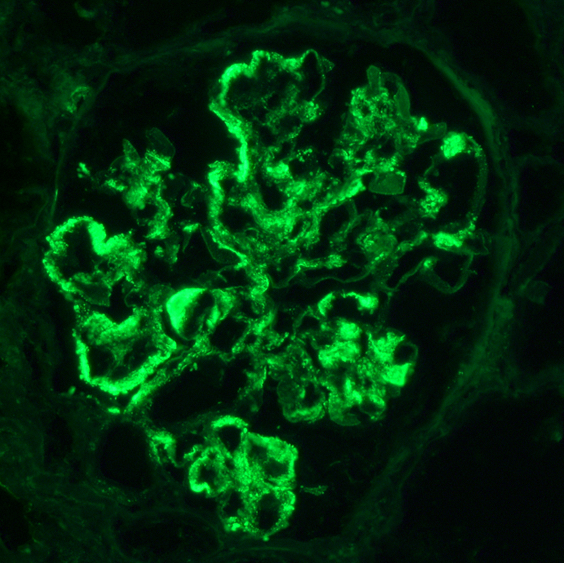
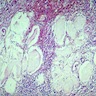
- Kidney, allograft biopsy
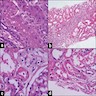
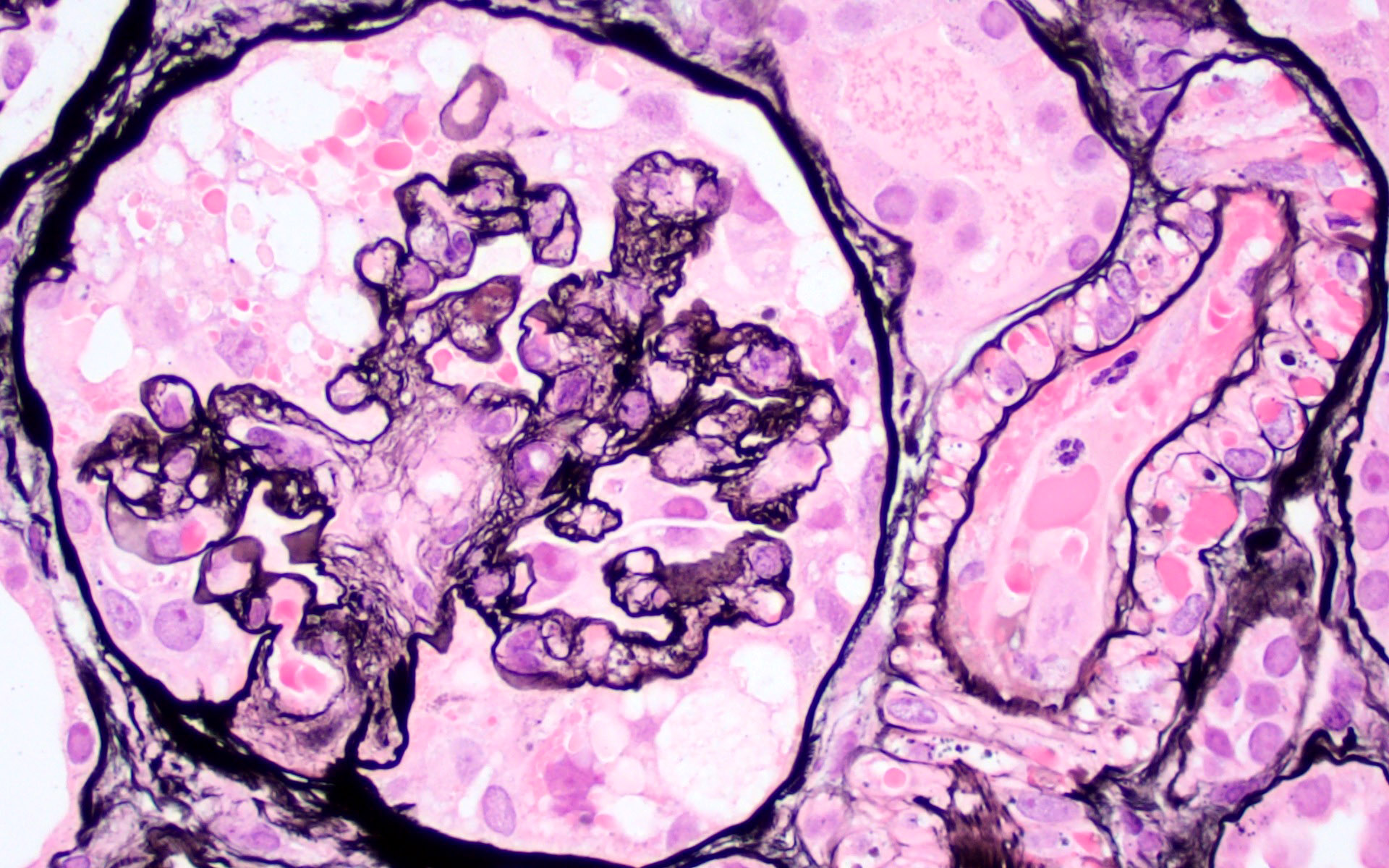
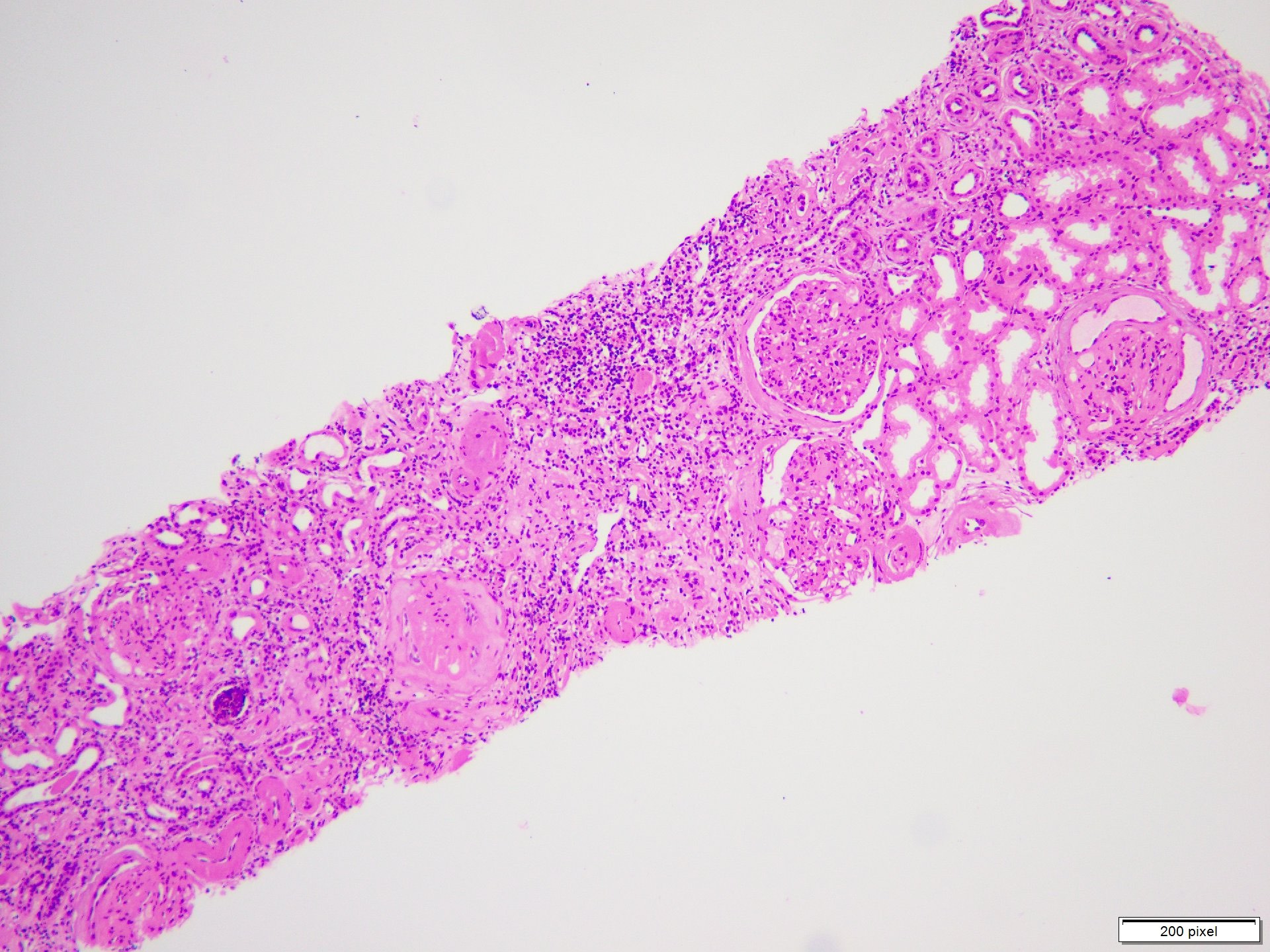
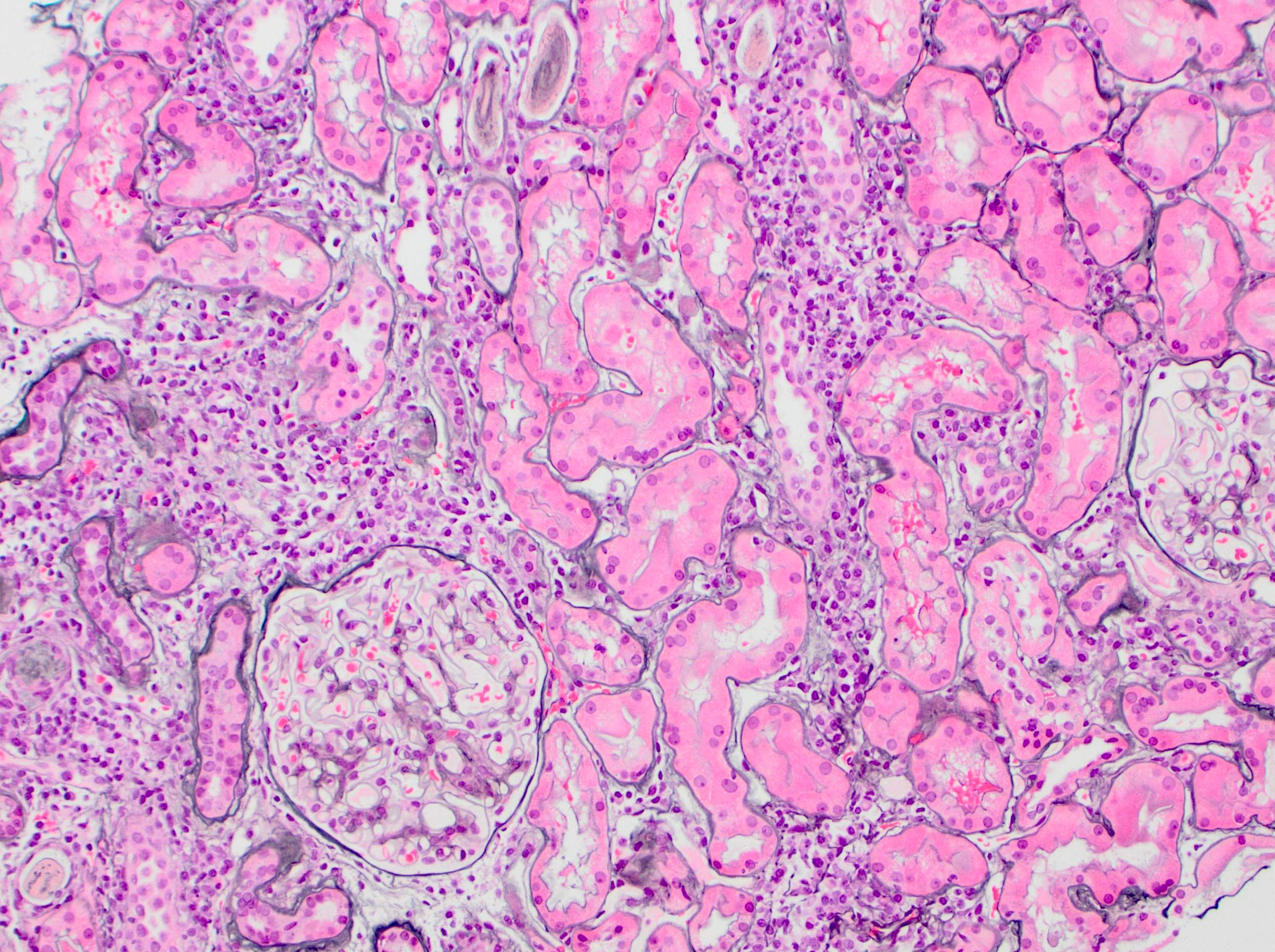
- Microscopic description: Serial sectioning shows 37 glomeruli, 6 of which are globally sclerotic. Glomeruli appear close to normal by light microscopy. Endocapillary or extracapillary proliferation, necrosis, intracapillary inflammatory cells (g0), intracapillary thrombi or double contouring of the capillary walls are absent (cg0). İnterstitium shows multifocal edema and inflammatory infiltrate comprising 26 - 50% of the nonscarred cortical parenchyma (i2). The infiltrate is composed predominantly of mononuclear cells. Tubulitis can easily be identified, ranging from mild to severe (t3). Patchy narrow foci of IFTA can be identified, comprising about 15% of the cortical parenchyma (i-IFTA1, ci1, ct1). No significant peritubullary capillaritis is seen (ptc0). Immunohistochemical staining for C4d and SV40 is negative. Arteries display mild intimal fibrosis, arterioli show multifocal mild to moderate hyalinosis (ah2). Intimal arteritis not identified (v0).
- Immunofluorescence microscopy: 3 glomeruli observed
- Anti IgG Ab: no deposits
- Anti IgA Ab: no deposits
- Anti IgM Ab: no glomerular deposits, blush segmental reactivity on walls of arterioli
- Anti C3 Ab: no glomerular deposits, blush segmental reactivity on walls of arterioli
- Anti c1q Ab: no deposits
- Anti kappa Ab: no glomerular deposits, 2+ staining of tubular casts
- Anti lambda Ab: no glomerular deposits, 2+ staining of tubular casts
- Diagnosis: Acute T cell mediated rejection, Banff classification grade IB. Patchy interstitial fibrosis and tubular atrophy (Banff grade 1). Arterial intimal fibrosis (mild), arteriolar hyalinosis (ah2).
- Comment: Biopsy findings are consistent with mild acute T cell mediated rejection, findings suggestive of chronic damage are minimal. Vascular sclerosis is most probably donor related.
- Acute T cell mediated rejection (TCMR):
- Acute allergic / drug induced tubulointerstitial nephritis:
- Tubulitis may be less prominent, eosinophils may be more prominent but differentiation from acute TCMR is not possible on morphologic grounds
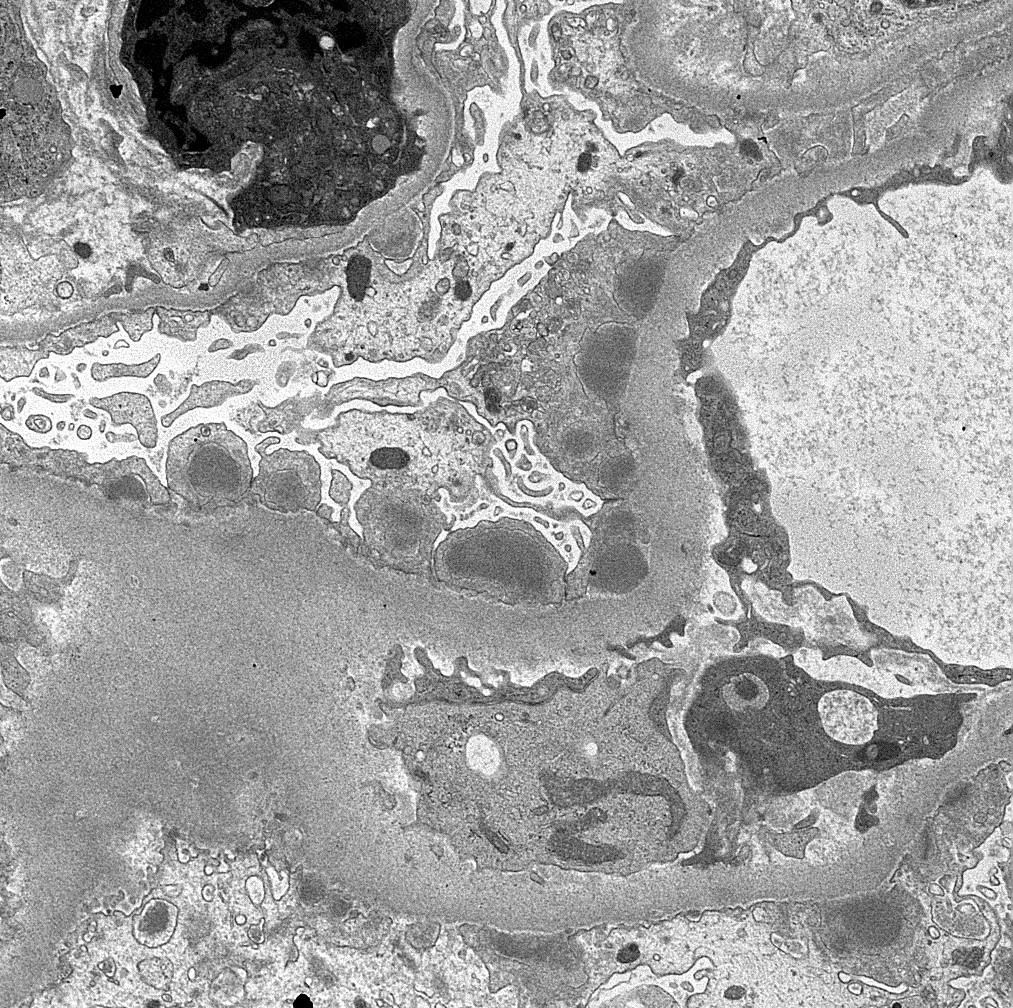
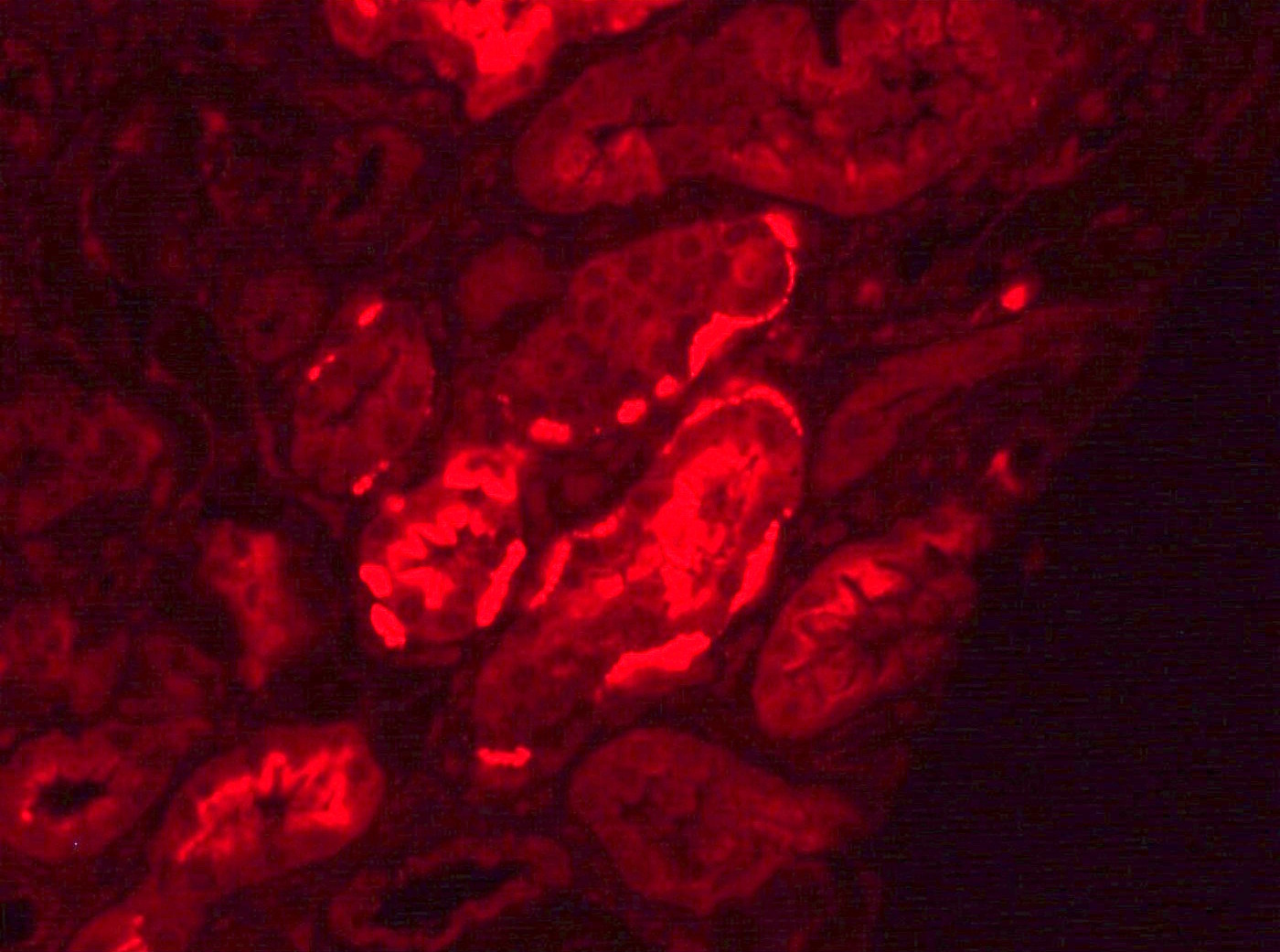
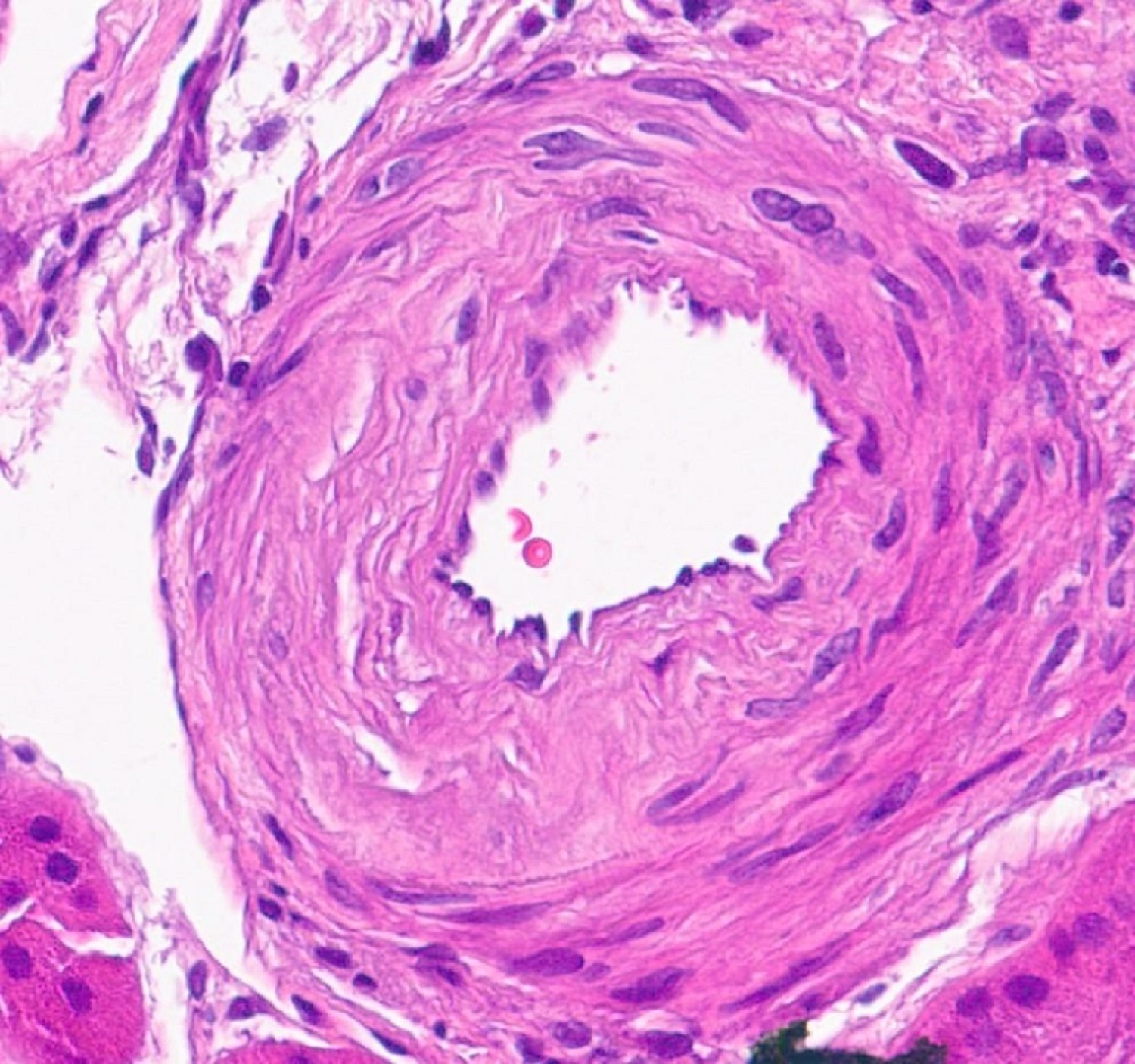
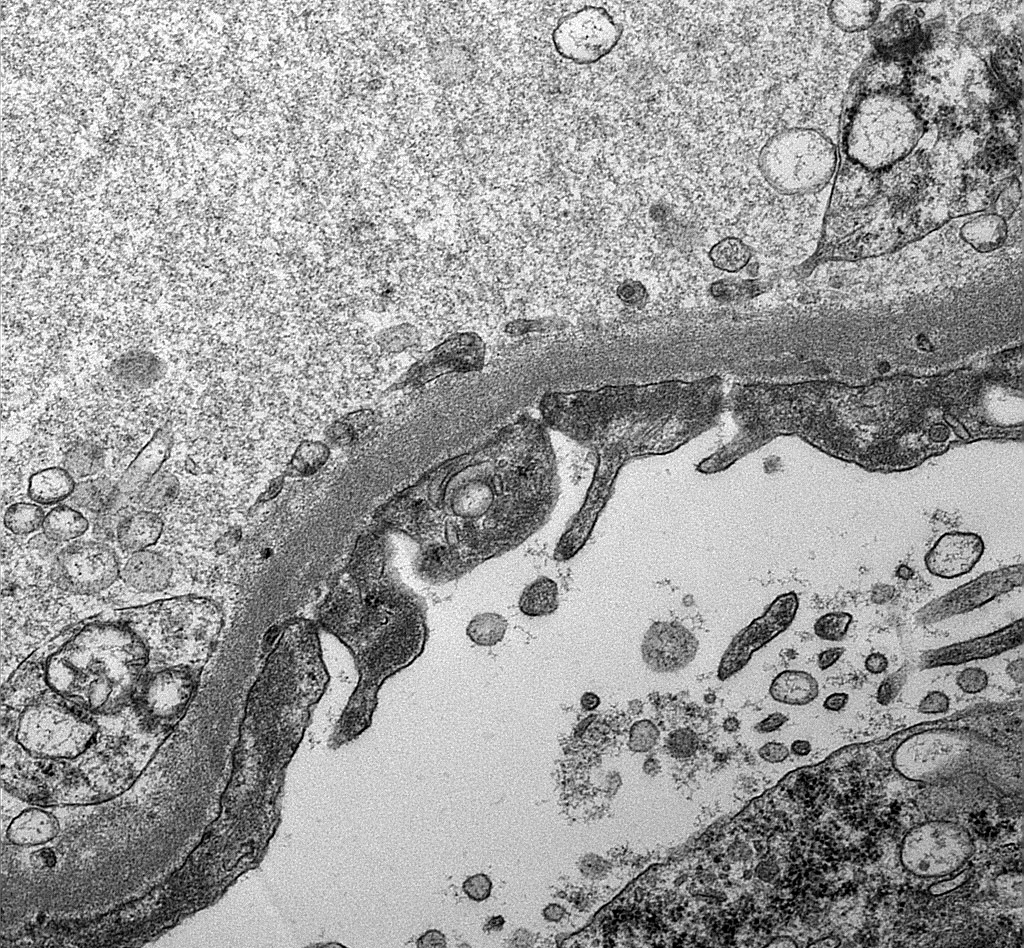
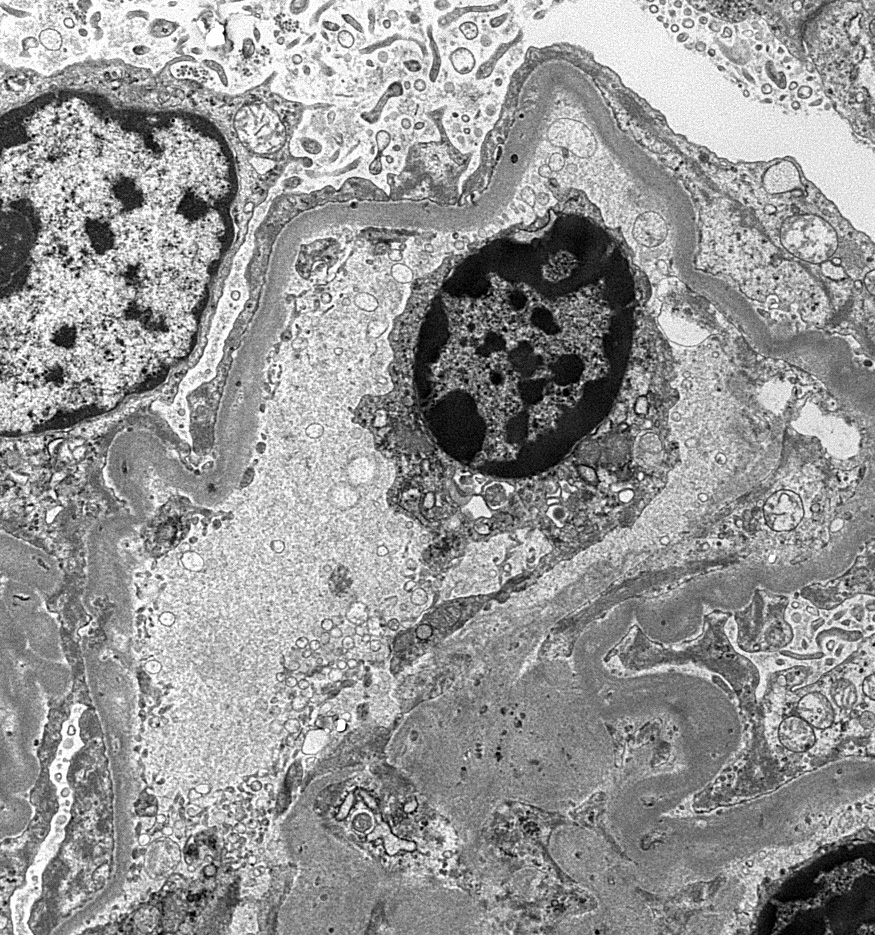
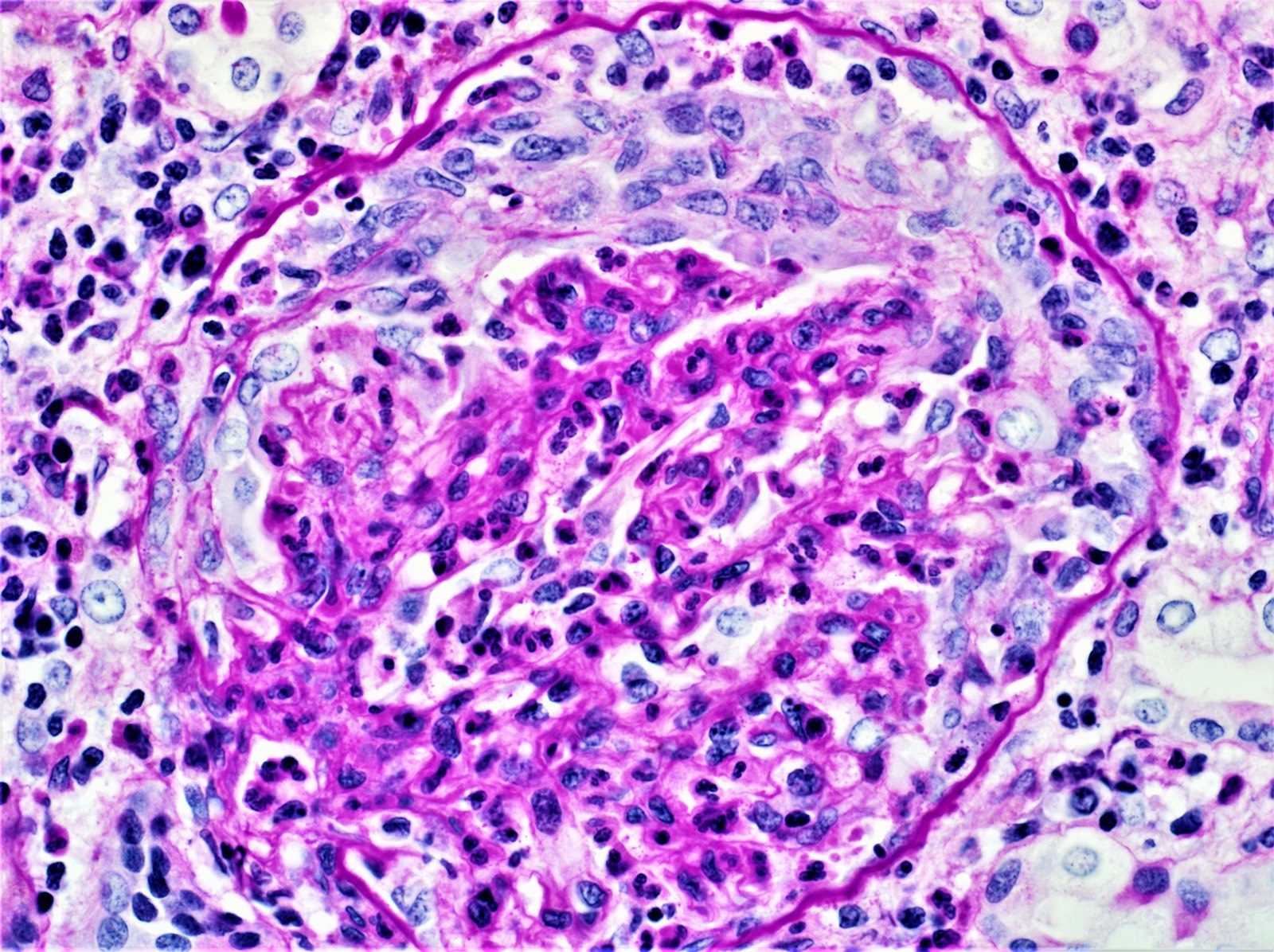
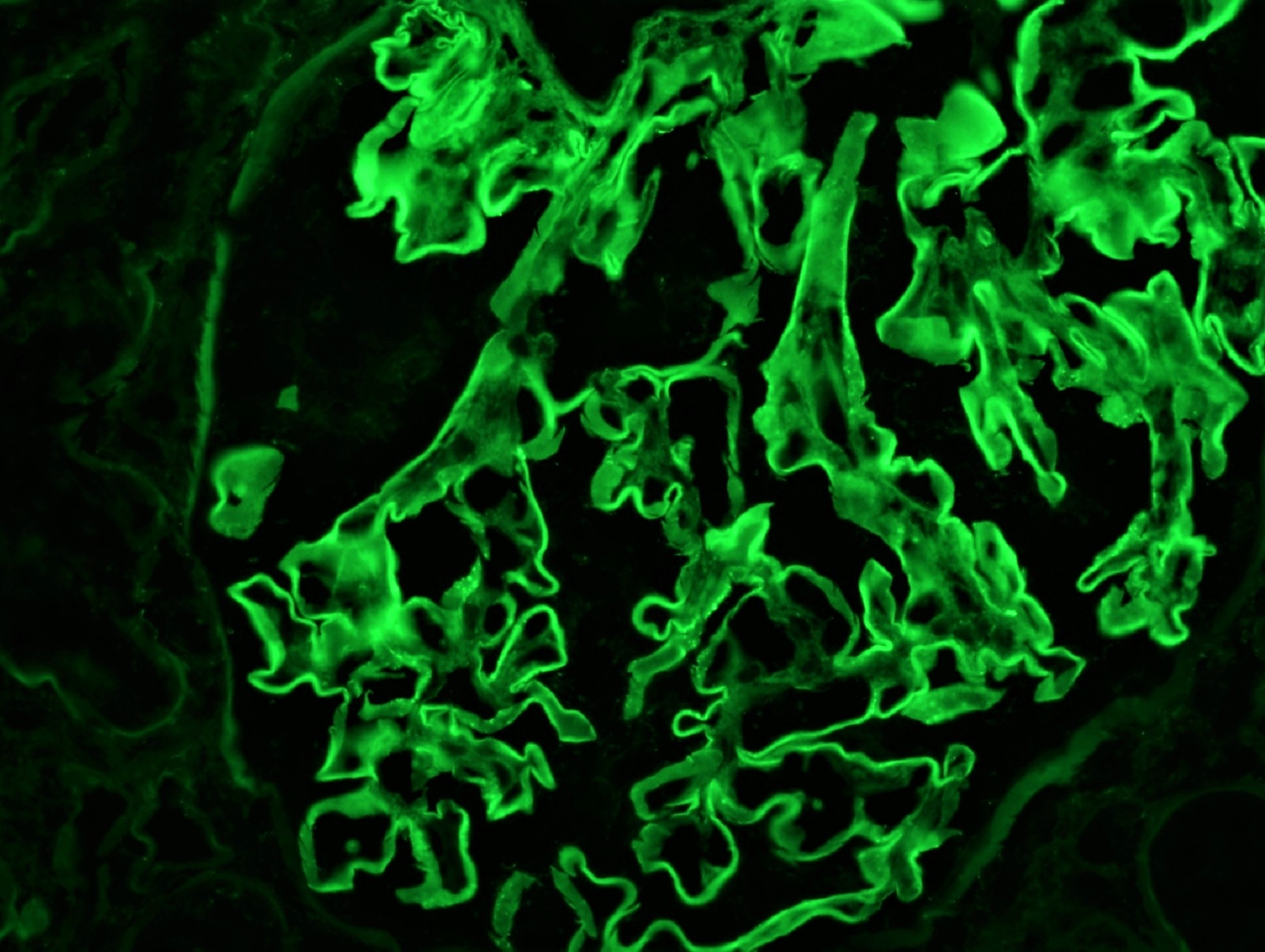
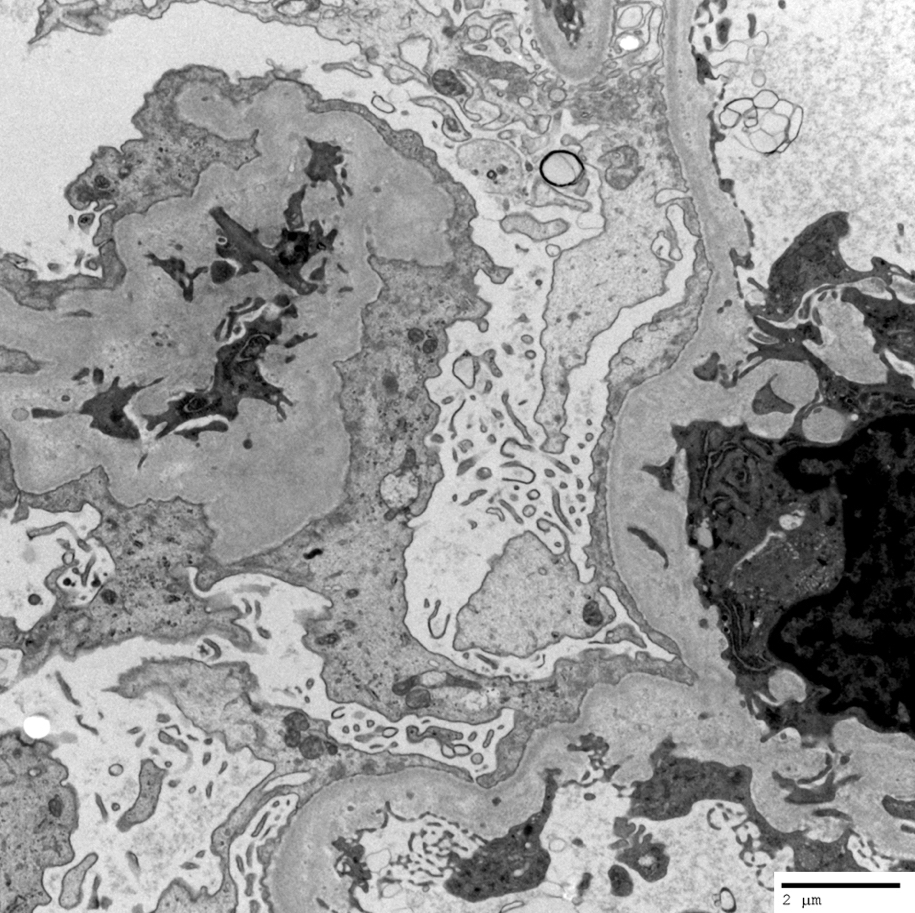
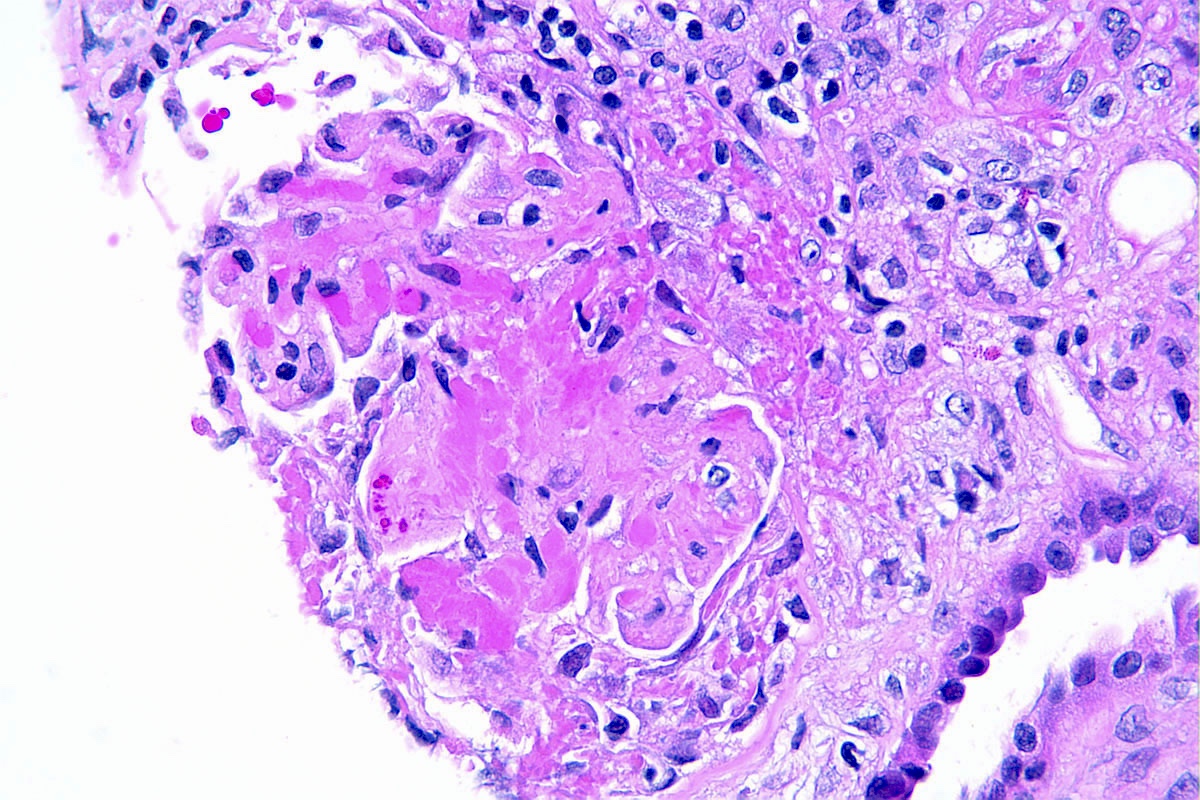
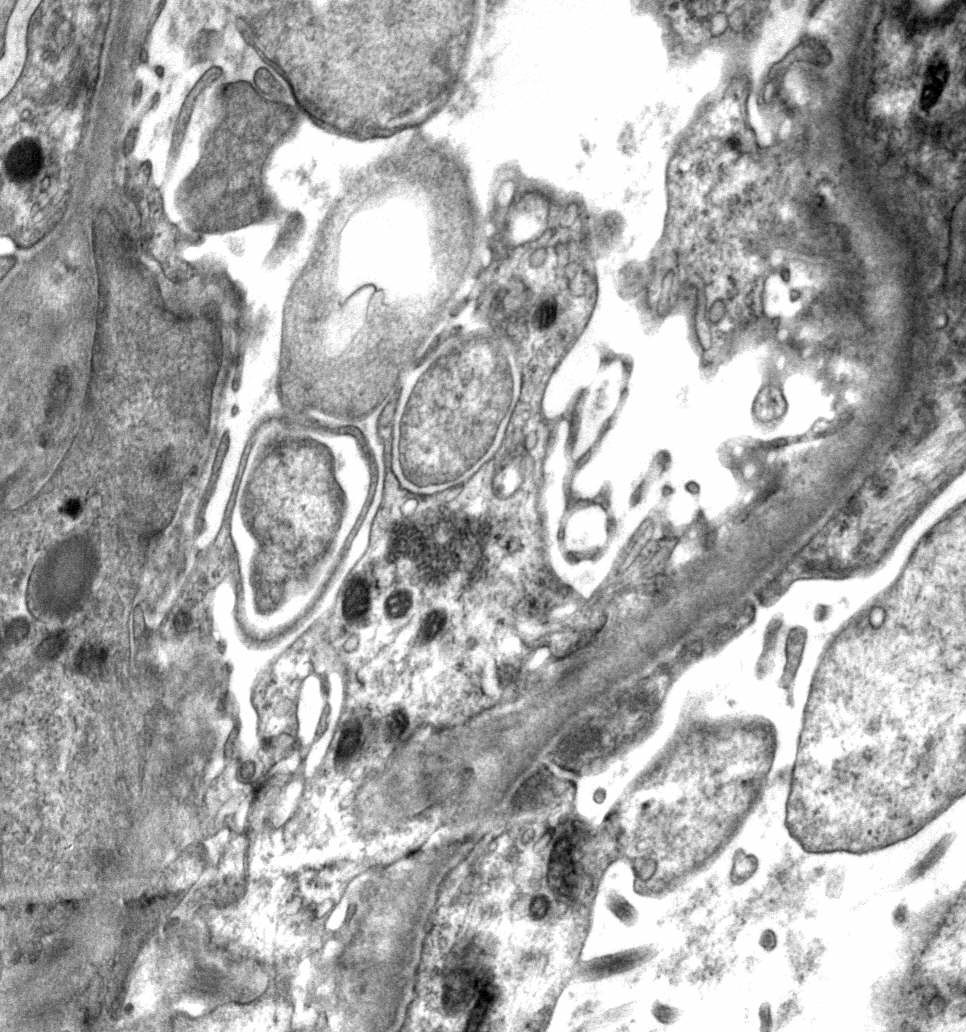
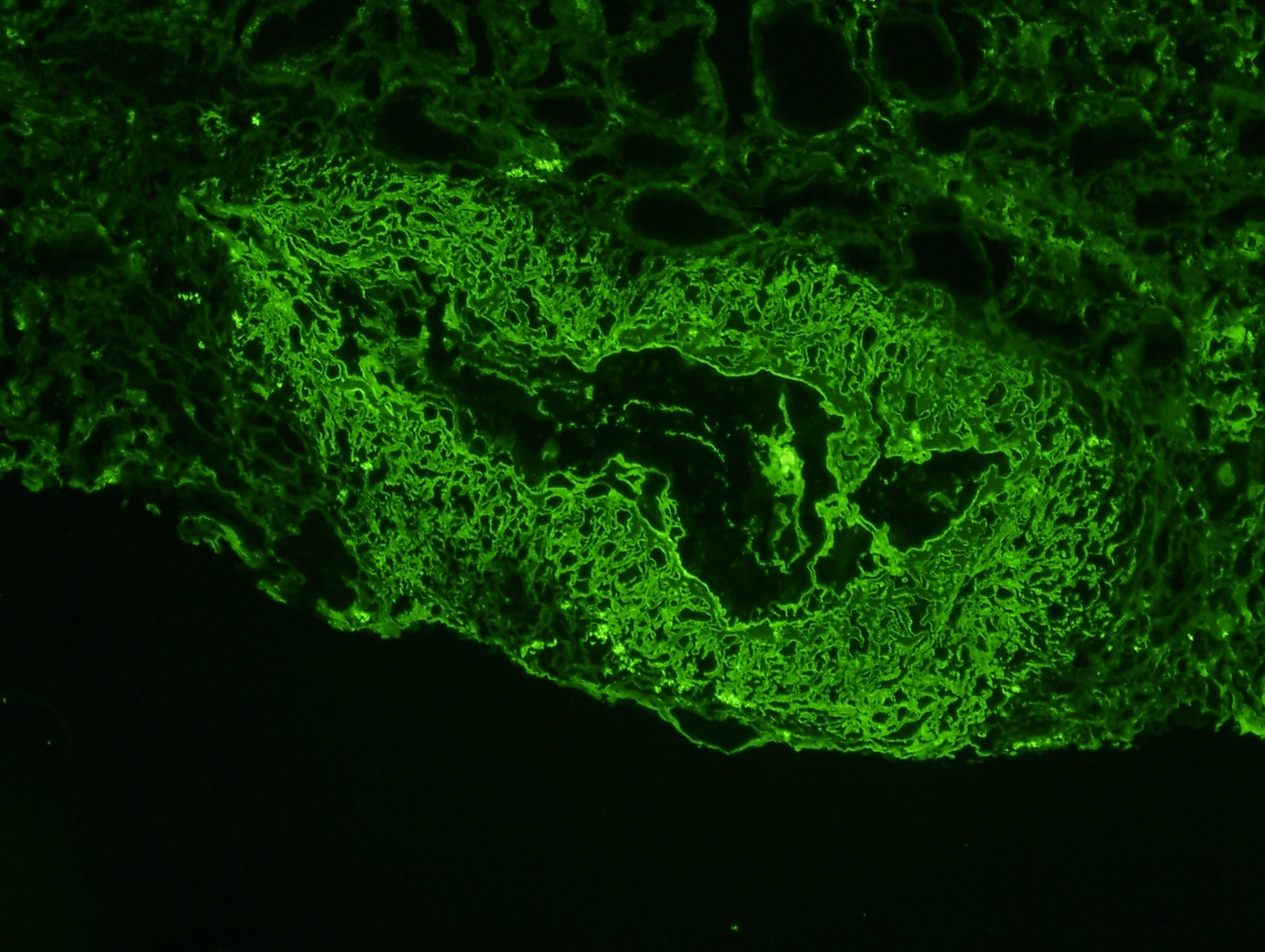
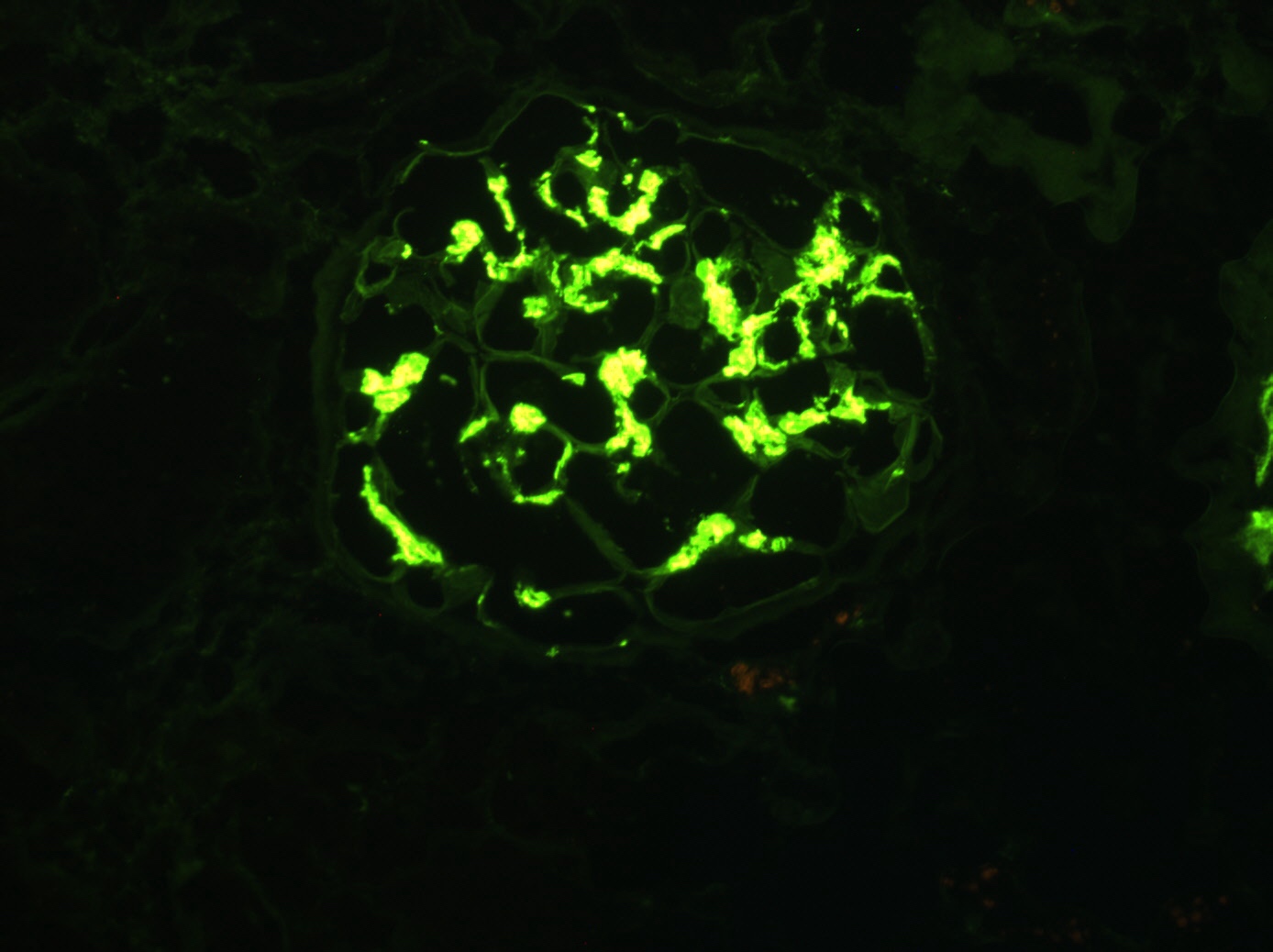
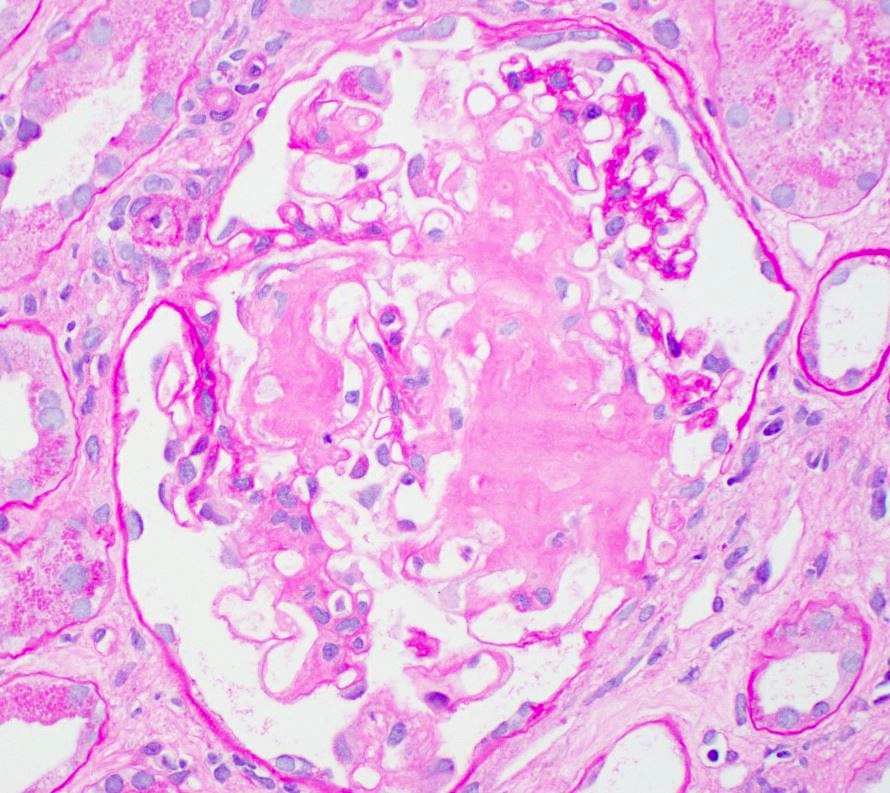
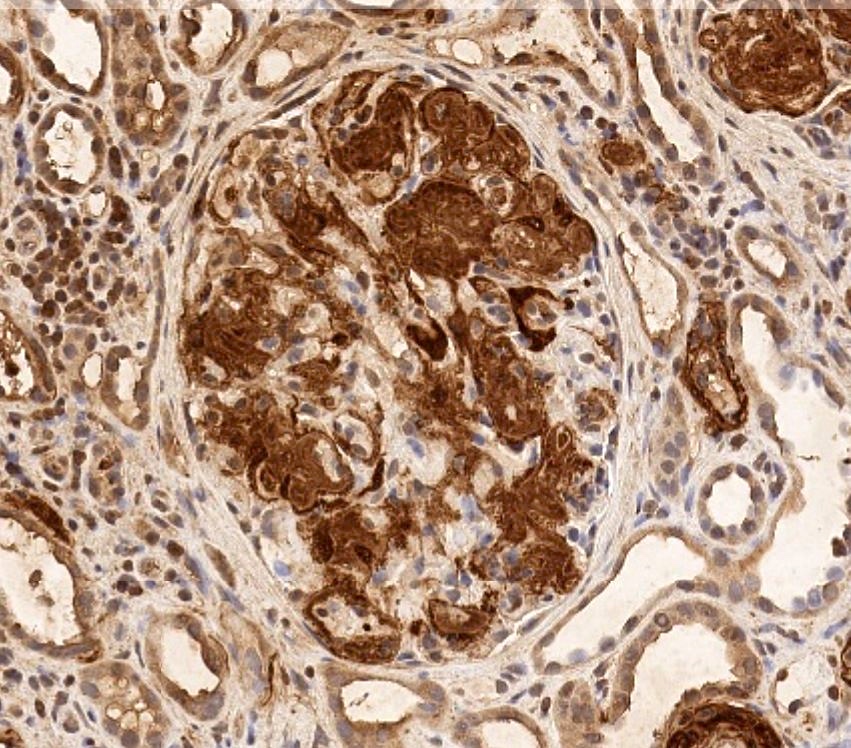
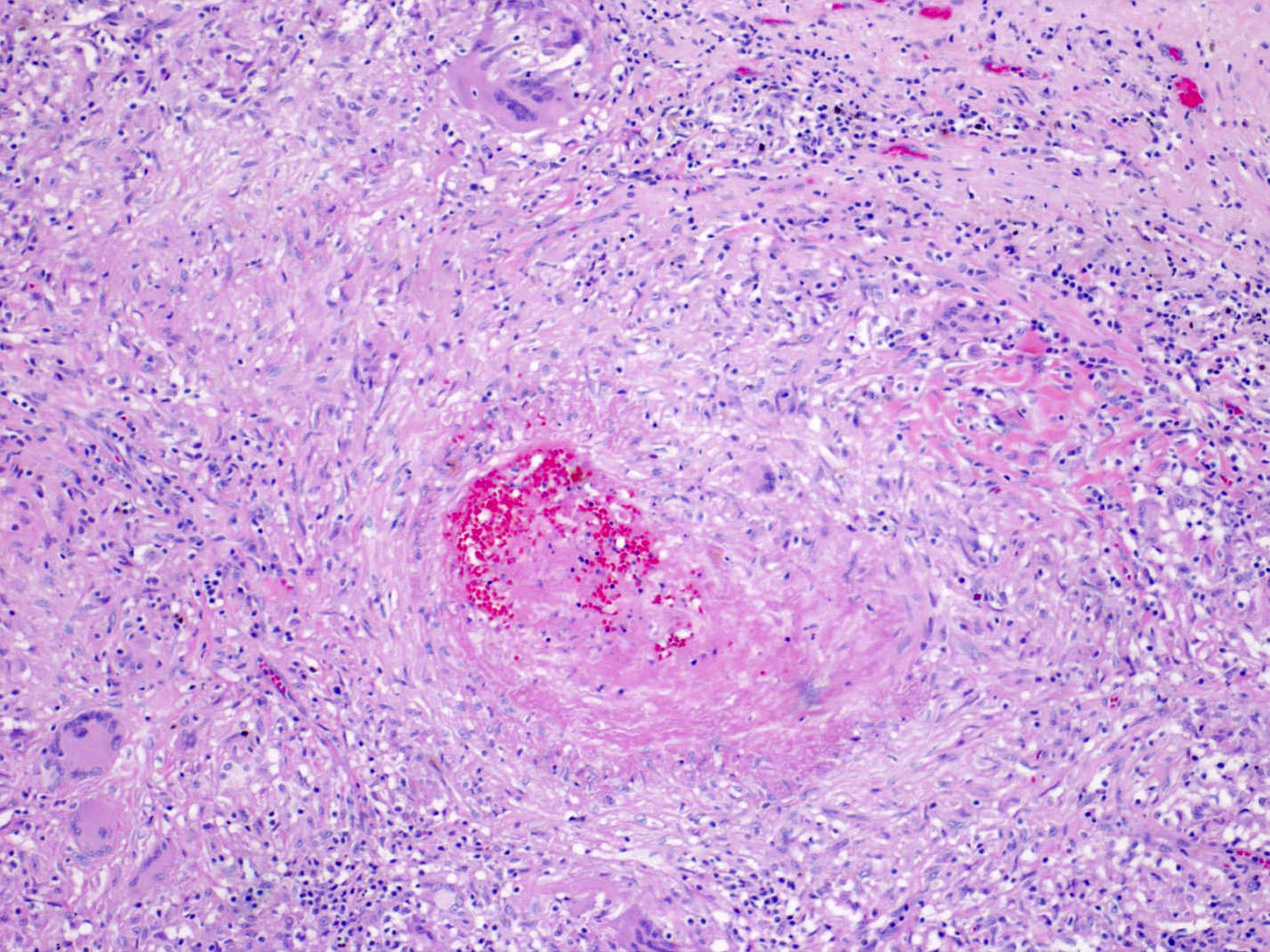
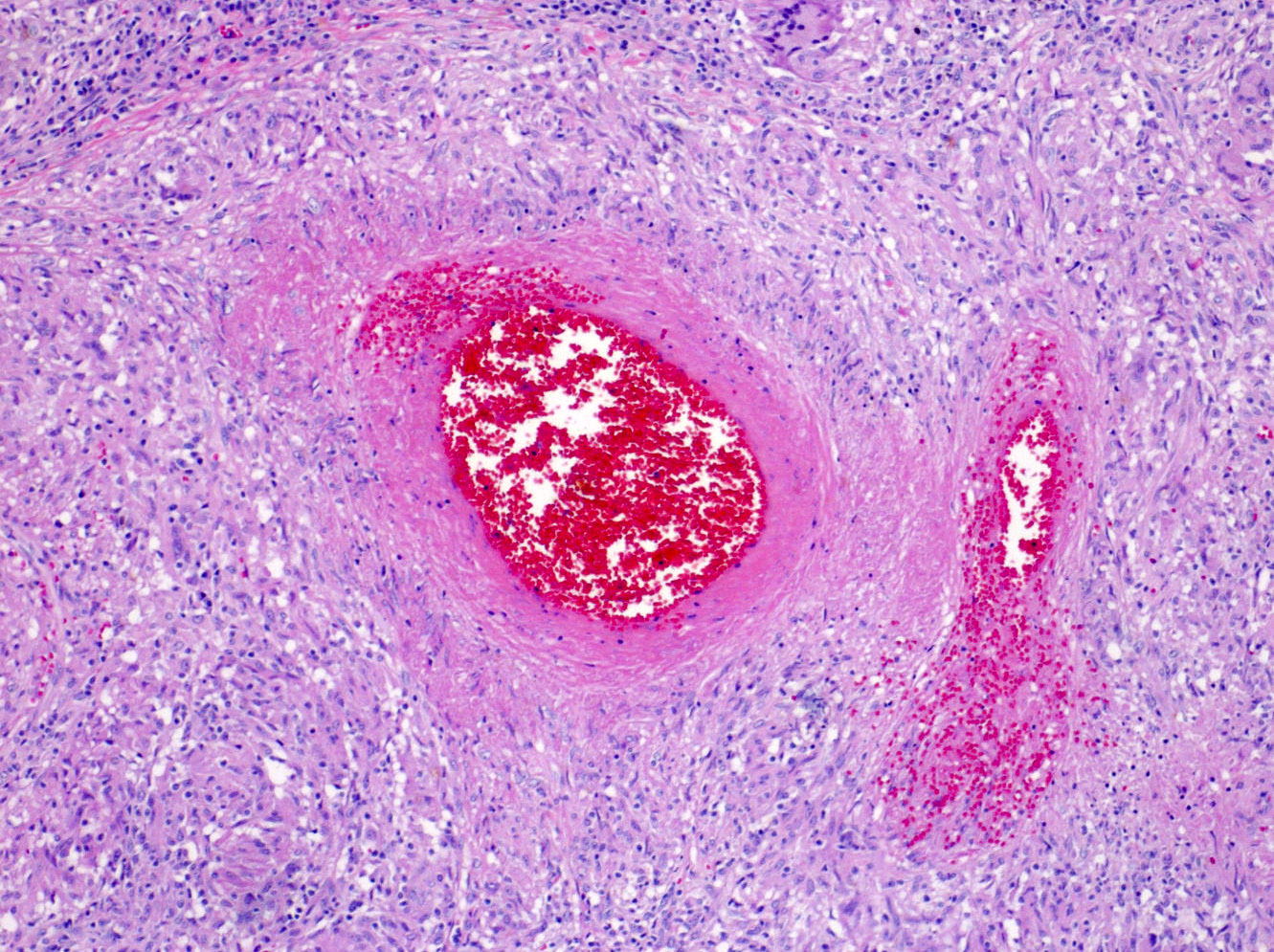
- Active antibody mediated rejection (active ABMR):
- Inflammatory infiltrate mostly confined to lumen of peritubular capillaries, signs of microvascular endothelial cell injury (peritubular capillaritis, glomerulitis, thrombotic microangiopathy) and peritubular C4d positivity (immunofluorescence or immunohistochemistry) are present; active AMR frequently accompanies acute TCMR
- Acute pyelonephritis:
- Usually patchy inflammatory infiltrate with predominance of polymorphonuclear leucocytes, neutrophilic tubulitis and neutrophil casts within tubular lumen
- Polyomavirus infection:
- Usually patchy inflammatory infiltrate, viral cytopathic changes in tubular epithelial cells and positive polyomavirus immunostain (should be performed on every allograft renal biopsy specimen)
- Posttransplant lymphoproliferative disease (PTLD):
- Is mainly of differential diagnostic concern in cases of PTLD where inflammatory infiltrate is polymorphic; however, the presence of transformed atypical large B lymphocytes within the infiltrate is a clue to diagnosis; stains to detect EBV will help in the differential
- Acute allergic / drug induced tubulointerstitial nephritis:
- Chronic active TCMR:
- All entities that can cause chronic renal damage (chronic active antibody mediated rejection, diabetes, hypertension, medications - such as seen with chronic calcineurin inhibitor toxicity, recurrent glomerulonephritis, recurrent pyelonephritis, etc.)
- Differentiation on morphological grounds is very difficult, if not impossible
- Identification of transplant arteriopathy suggests rejection; however, this lesion is usually not represented in small needle biopsies and is a feature of chronic rejection in general (both chronic active TCMR and chronic active AMR)
- Are always characterized by fibrinoid necrosis
- Are associated with a good prognosis
- Are only associated with chronic rejection
- Can be seen in both T cell mediated and antibody mediated rejections
- Indicate mild rejection
Comment Here
Reference: Acute / chronic cellular rejection
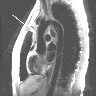
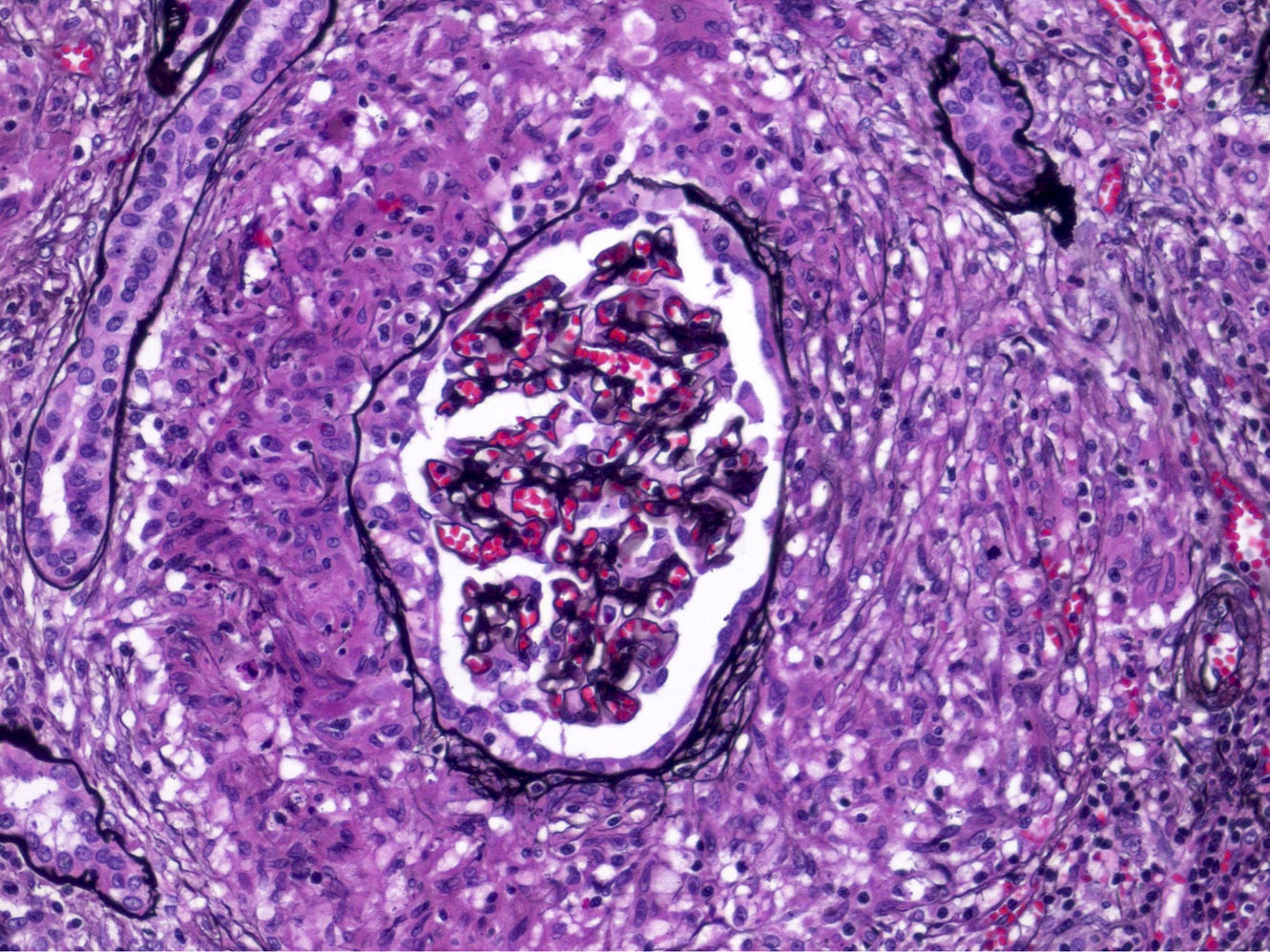
A 32 year old renal transplant patient wished to become pregnant. Her medication was adjusted accordingly. Within 2 months, her creatinine levels increased from 0.67 to 4.28 mg/dl. The biopsy sample displayed the finding shown above. What is the most probable diagnosis?
- Acute pyelonephritis
- Acute T cell mediated rejection
- Chronic active antibody mediated rejection
- Chronic active T cell mediated rejection
- Posttransplant lymphoproliferative disorder
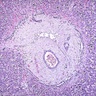
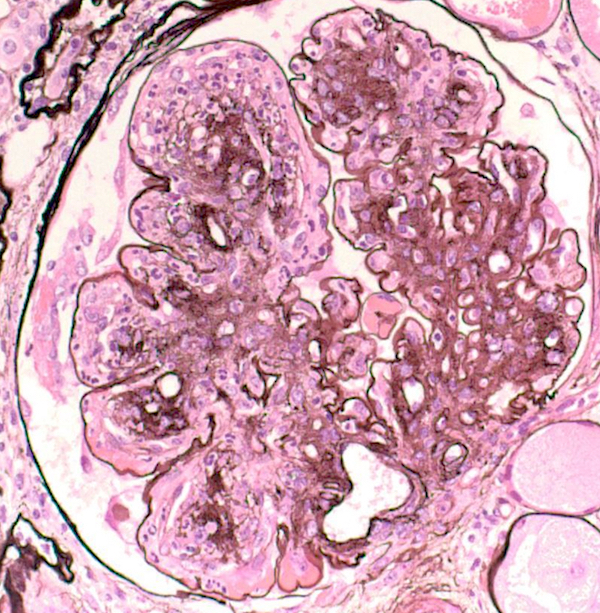
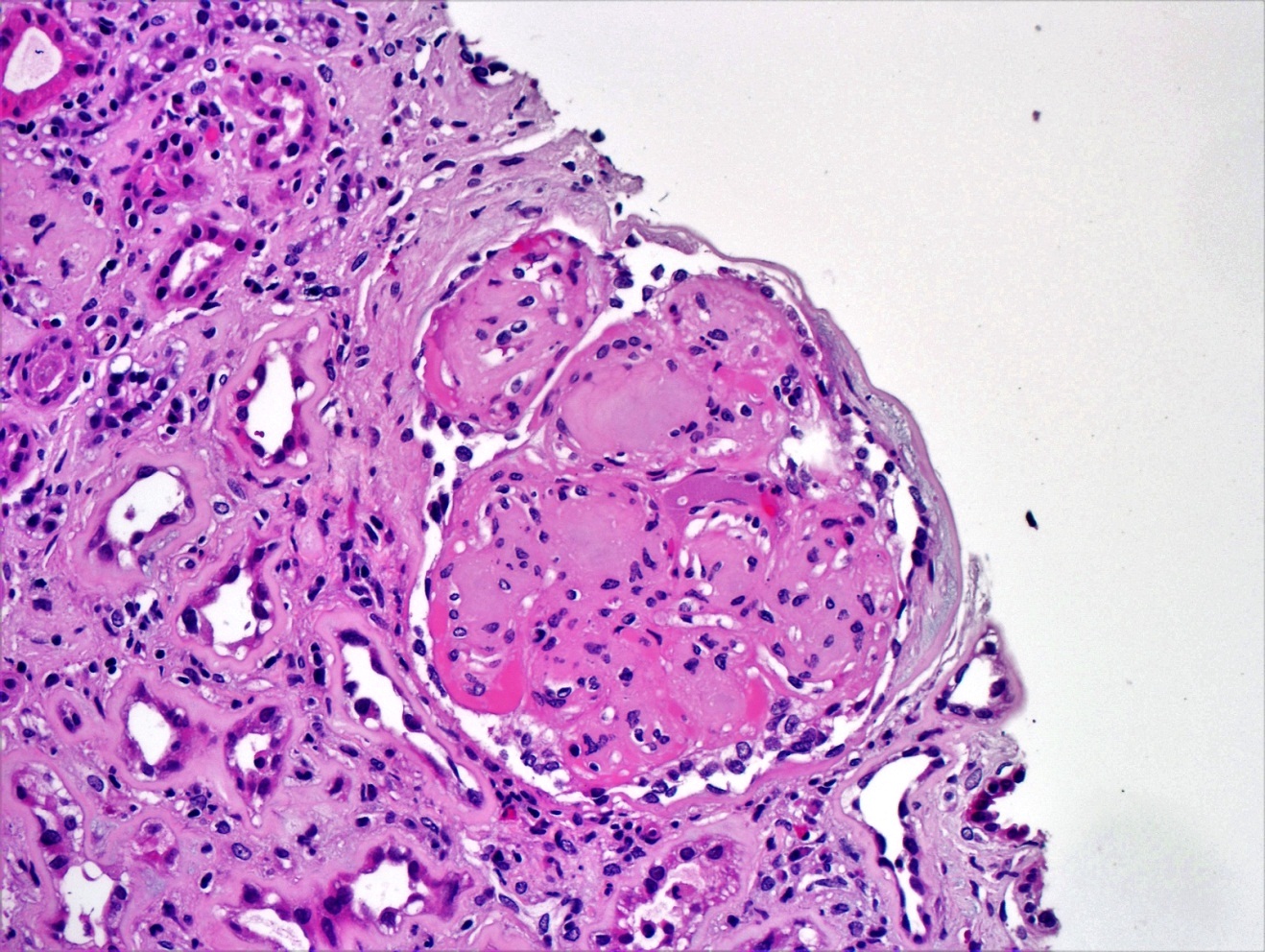
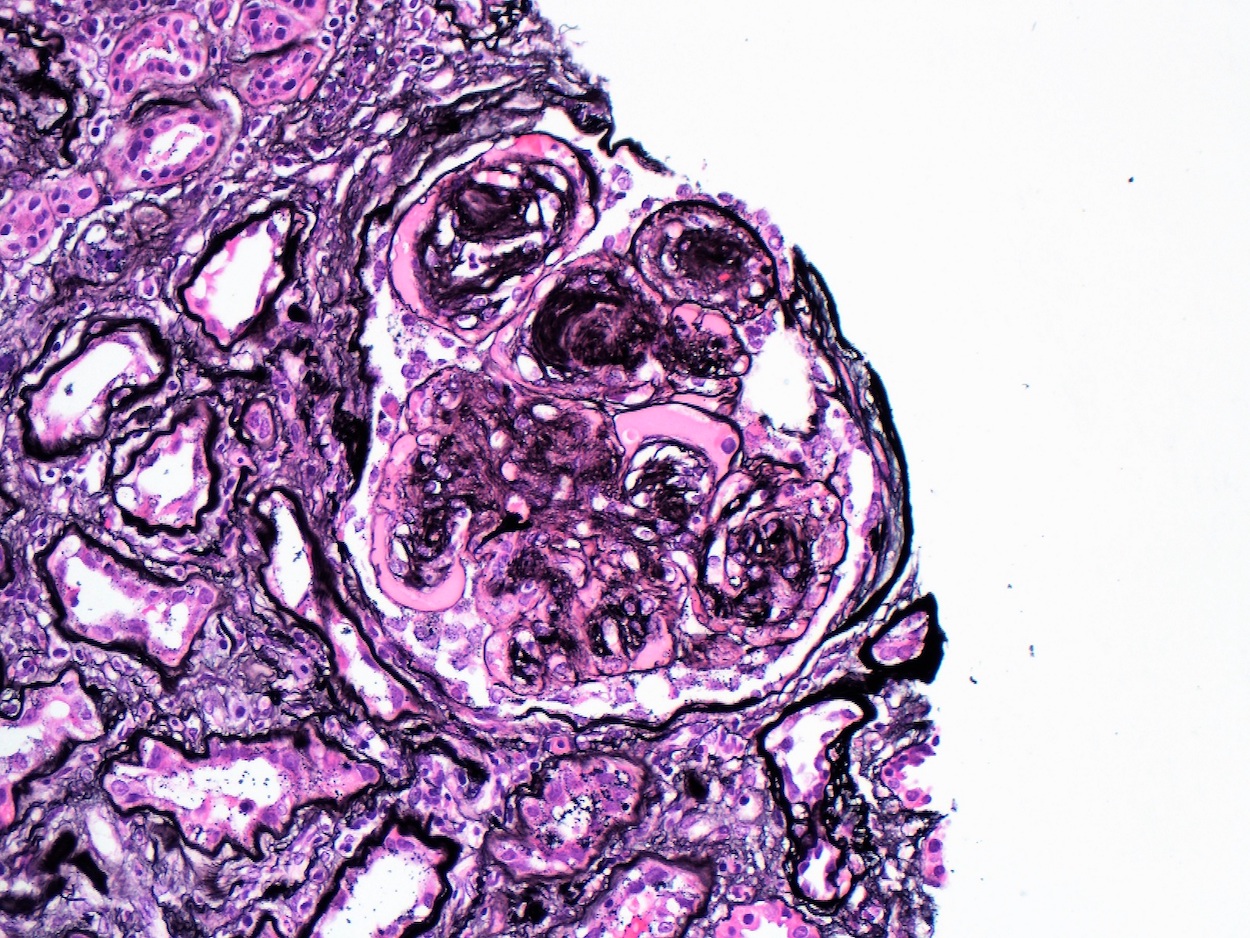
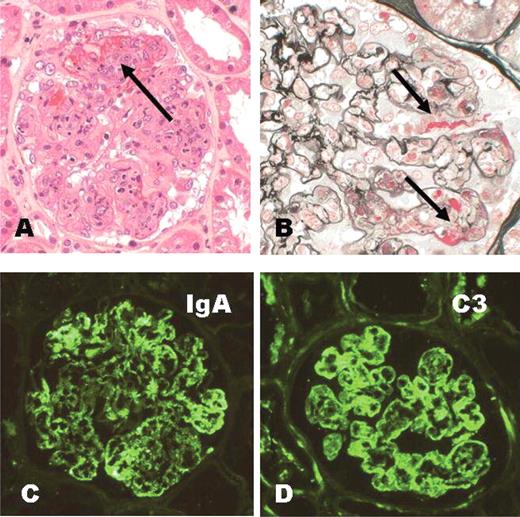
- Infection related glomerulonephritis (GN) encompasses the entities of postinfectious GN, IgA dominant (Staphylococcus associated) infection related glomerulonephritis, endocarditis associated glomerulonephritis and shunt nephritis
- Classic poststrep glomerulonephritis is usually seen in children and has a good prognosis; infection related glomerulonephritis in adults is mostly concurrent with infection and has a poorer outcome; these are more likely to be IgA and C3 codominant and humps are usually not seen on electron microscopy (Glomerular Dis 2021;1:82)
- Postinfectious glomerulonephritis is secondary to bacterial, viral, mycotic or parasitic infections (Dtsch Med Wochenschr 2020;145:240, Pediatr Clin North Am 2022;69:1051)
- Poststreptococcal glomerulonephritis (PSGN) most frequently presents in children 1 - 2 weeks after a sore throat or 6 weeks after a skin infection (impetigo)
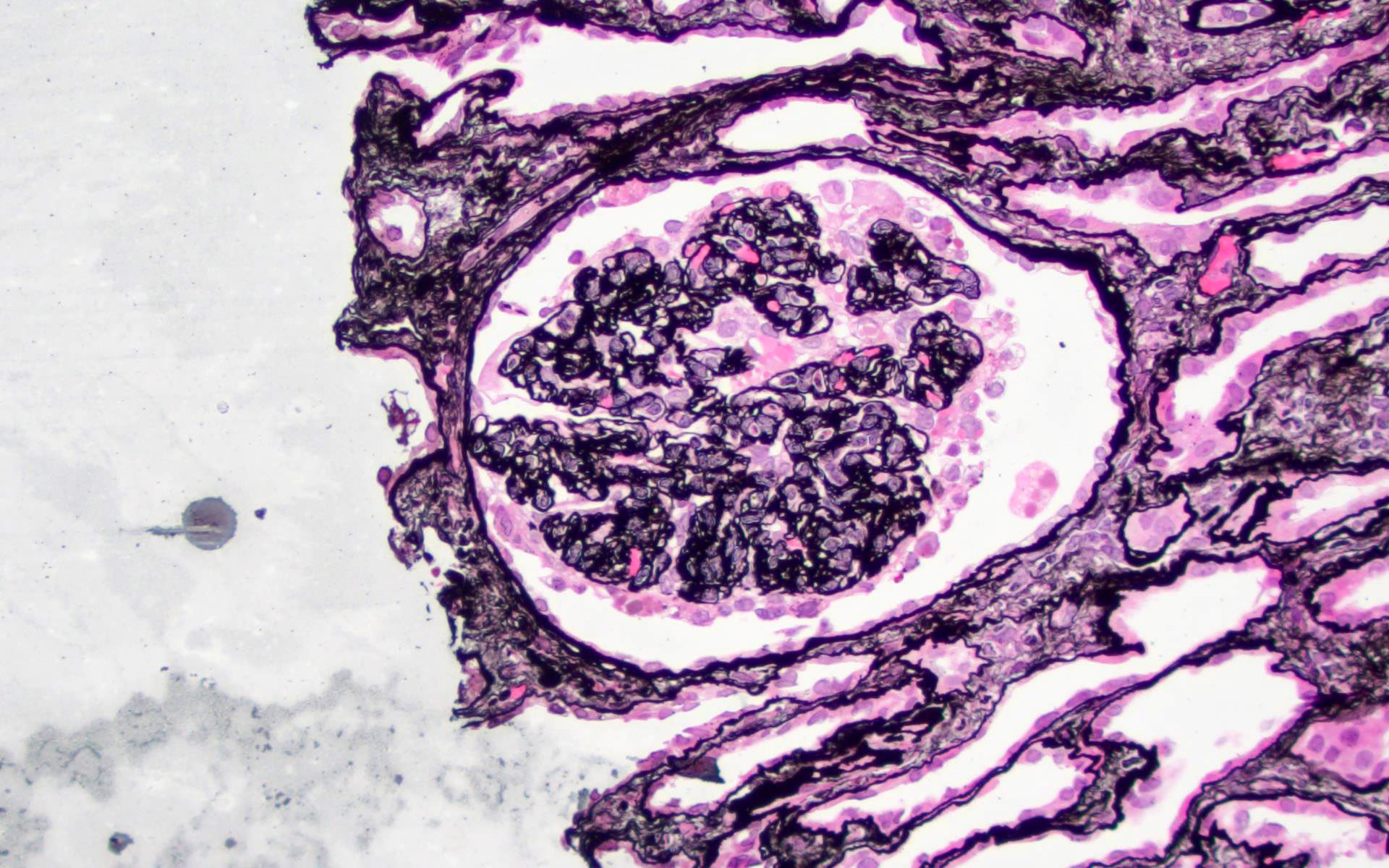
- It is characterized by diffuse exudative, hypercellular glomerulonephritis with prominent endocapillary hypercellularity and numerous neutrophils
- On immunofluorescence, appears as small, irregular, coarse granular (lumpy bumpy) deposits of polyclonal IgG and C3 along the glomerular capillary walls with scattered mesangial deposits
- On electron microscopy, has characteristic hump type large subepithelial deposits, occasional mesangial deposits and rare subendothelial deposits without significant glomerular basement membrane reaction
- It has an excellent prognosis, especially in children, with 95% completely recovering with conservative therapy within 6 - 8 weeks
- ~1% develop rapidly progressive glomerulonephritis and another 1 - 2% develop chronic glomerulonephritis
- Acute postinfectious glomerulonephritis (APIGN)
- Poststreptococcal glomerulonephritis (PSGN)
- Primarily affects children aged 3 - 12 years with a mean age of 6 - 8 years
- Uncommon in children younger than 3 years
- M:F = 2:1 (Pediatr Clin North Am 2022;69:1051)
- Adult cases are well documented (Medicine (Baltimore) 2008;87:21)
- It is rare in industrialized countries
- Incidence is also decreasing in developing countries mainly because of improvement in public health and frequent use of antibiotics (Front Med (Lausanne) 2018;5:327)
- Kidney glomeruli
- Immunological disease; representing a type II or type III hypersensitivity reaction
- Immune complexes containing the streptococcal antigen are formed
- Nephritis associated plasmin receptor (NAPlr) and streptococcal pyrogenic exotoxin B (SPeB) are the 2 common antigens associated with the pathogenesis of PSGN
- Staphylococcal antigen may function as a superantigen
- Immune complex deposition in glomeruli or in situ formation of the antigen antibody complex within the kidney glomeruli
- In situ immune complex formation is either due to a reaction against streptococcal antigens deposited in the glomerular basement membrane or due to an antibody reaction against glomerular components that crossreact with streptococcal antigen due to molecular mimicry (Paediatr Int Child Health 2017;37:240, Pediatr Rev 2015;36:3)
- Activation of the alternate complement pathway, resulting in hypocomplementemia, infiltration of the leukocytes and proliferation of the mesangial cells in the glomerulus, thus impairing the capillary perfusion and glomerular filtration rate (GFR)
- Reduction in GFR can lead to renal failure (oliguria or anuria), acid base imbalance, electrolyte abnormalities, volume overload, edema and hypertension
- Mostly associated with nephritogenic strains of Streptococcus pyogenes (beta hemolytic strep group A)
- Other etiologies include staphylococci, malaria, toxoplasmosis, hepatitis B / C, HIV, varicella, spirochetes, meningococci and other bacteria (Clin J Am Soc Nephrol 2006;1:1179)
- Presentation, clinical course and outcomes of nonstreptococcal cases of APIGN are generally similar to those of PSGN
- A recent identifiable illness is usually present that precedes APIGN, though there is variability in presentation, with the majority of patients having had a preceding upper respiratory tract infection, scarlet fever, impetigo, gastroenteritis, cervical adenitis or no recalled illness (Pediatr Clin North Am 2019;66:59)
- Dark urine (brown, tea or cola colored) is often the first clinical manifestation of PSGN
- Periorbital edema, hypertension or in some cases, generalized edema
- Other features of circulatory congestion, such as dyspnea, may be present
- Nonspecific symptoms including general malaise, weakness and anorexia may also be present
- Clinicopathological diagnosis
- Diagnosis is confirmed on renal biopsy with characteristic light microscopic features, immunofluorescence and electron microscopic findings
- Serological tests: antistreptolysin O (ASO), antihyaluronidase and DNase B together are highly sensitive to detect an immunological response to prior group A streptococcal infection
- Serum complement level (C3) is usually low due to its consumption in the inflammatory reaction
- Urine analysis
- Shows macroscopic or microscopic hematuria, RBC casts, mild proteinuria
- Only 5% of patients with PSGN have massive proteinuria that indicates nephrotic syndrome
- White blood cell casts, hyaline and cellular casts are usually present in the urine analysis
- Renal function tests: blood urea nitrogen (BUN) and serum creatinine typically elevate during the acute phase (Pediatr Nephrol 2011;26:165)
- PSGN has an excellent prognosis especially in children, with 95% completely recovering with conservative therapy within 6 - 8 weeks
- ~1% develop rapidly progressive glomerulonephritis and another 1 - 2% develop chronic glomerulonephritis (Pediatr Int 2001;43:364, Int J Mol Sci 2021;22:905)
- ~50% of the adult patients continue to have reduced renal function, hypertension or persistent proteinuria (J Am Soc Nephrol 2011;22:187)
- 3 year old girl with IgA dominant postinfectious glomerulonephritis (IgA PIGN) presented with acute renal failure, edema, hypertension and heavy proteinuria (BMC Nephrol 2022;23:333)
- 4 year old boy who developed acute glomerulonephritis following pneumococcal bacteremia (Singapore Med J 2014;55:e69)
- 12 year old boy and 18 year old woman with atypical presentation of acute postinfectious glomerulonephritis and sickle cell disease (BMC Nephrol 2020;21:56)
- 32 year old woman diagnosed with postinfectious glomerulonephritis secondary to parvovirus B19 infection (Clin Nephrol 2016;85:238)
- 46 year old man with Granulicatella, a nutritionally variant Streptococcus (NVS) species, that developed infective endocarditis and glomerulonephritis (IDCases 2020;21:e00792)
- 74 year old diabetic woman that developed postinfectious glomerulonephritis with crescents (Cureus 2020;12:e11440)
- Self limiting condition in the majority of cases and thus requires symptomatic treatment only
- Main aim is to control the complications of volume overload such as hypertension and edema, which are prominent during the acute phase of the disease
- Pharmacological therapy
- Antimicrobials
- Patients with evidence of a streptococcal infection should receive a course of antibiotic therapy; however, they may not prevent the development of PSGN
- Diuretics
- Loop diuretics increase urinary output and consequently improve cardiovascular congestion and hypertension
- Antihypertensive medications
- Blood pressure can be managed by restricting salt and fluid intake along with diuretics as needed
- In cases with uncontrolled blood pressure, the use of calcium channel blockers is recommended
- Use of angiotensin converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARBs) is recommended in patients with stable GFR and with near normal potassium levels
- Immunosuppressive therapy
- Steroids, immunosuppressive agents and plasmapheresis are not generally indicated
- However, patients with progressive renal failure or the presence of crescents on the renal biopsy may warrant the use of corticosteroids (Cureus 2018;10:e3150, J Pediatr 1981;98:403)
- Antimicrobials
- Dialysis
- Dialysis is only performed to manage the acid base balance, electrolyte abnormalities (especially hyperkalemia) and fluid management
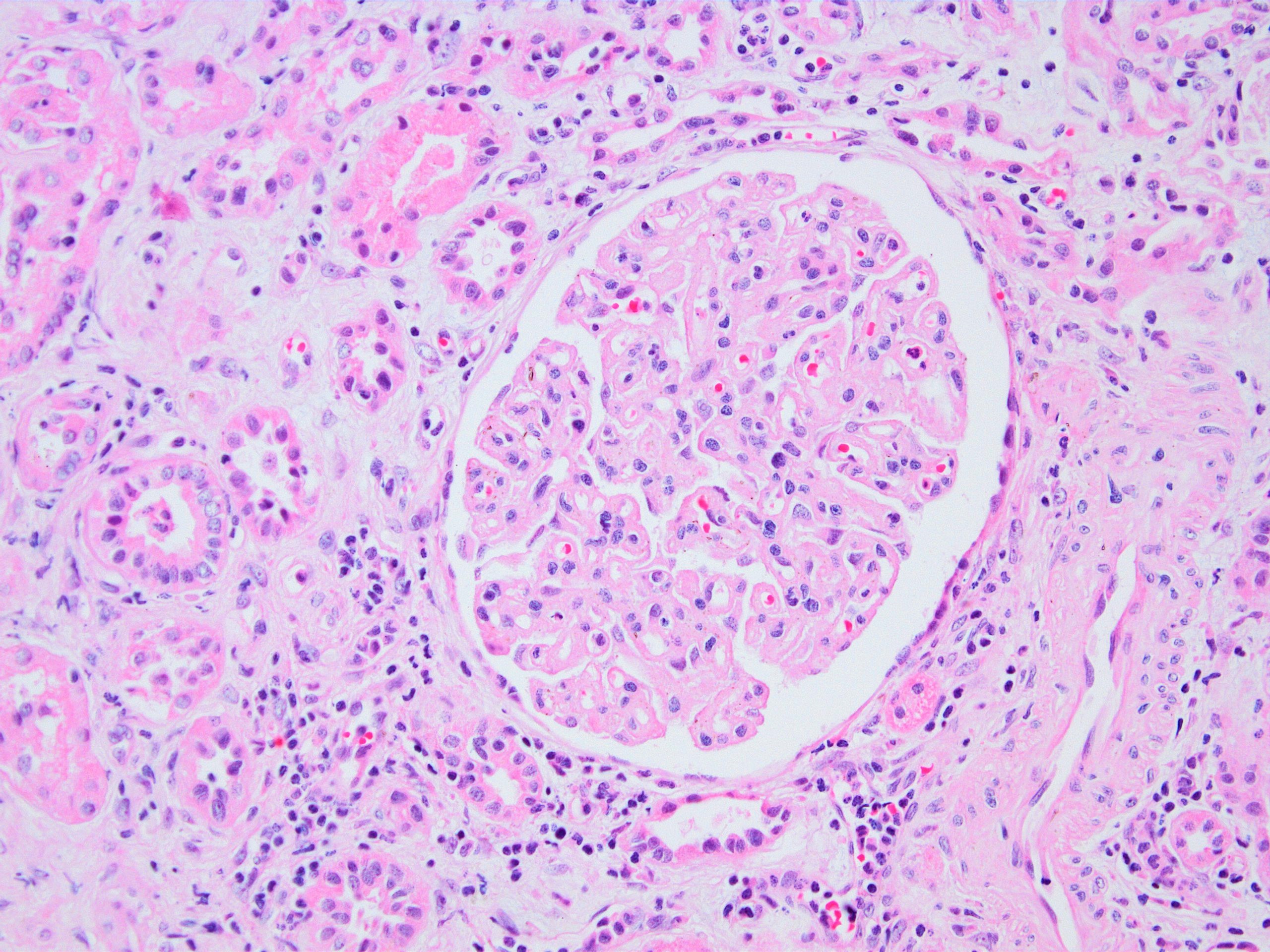
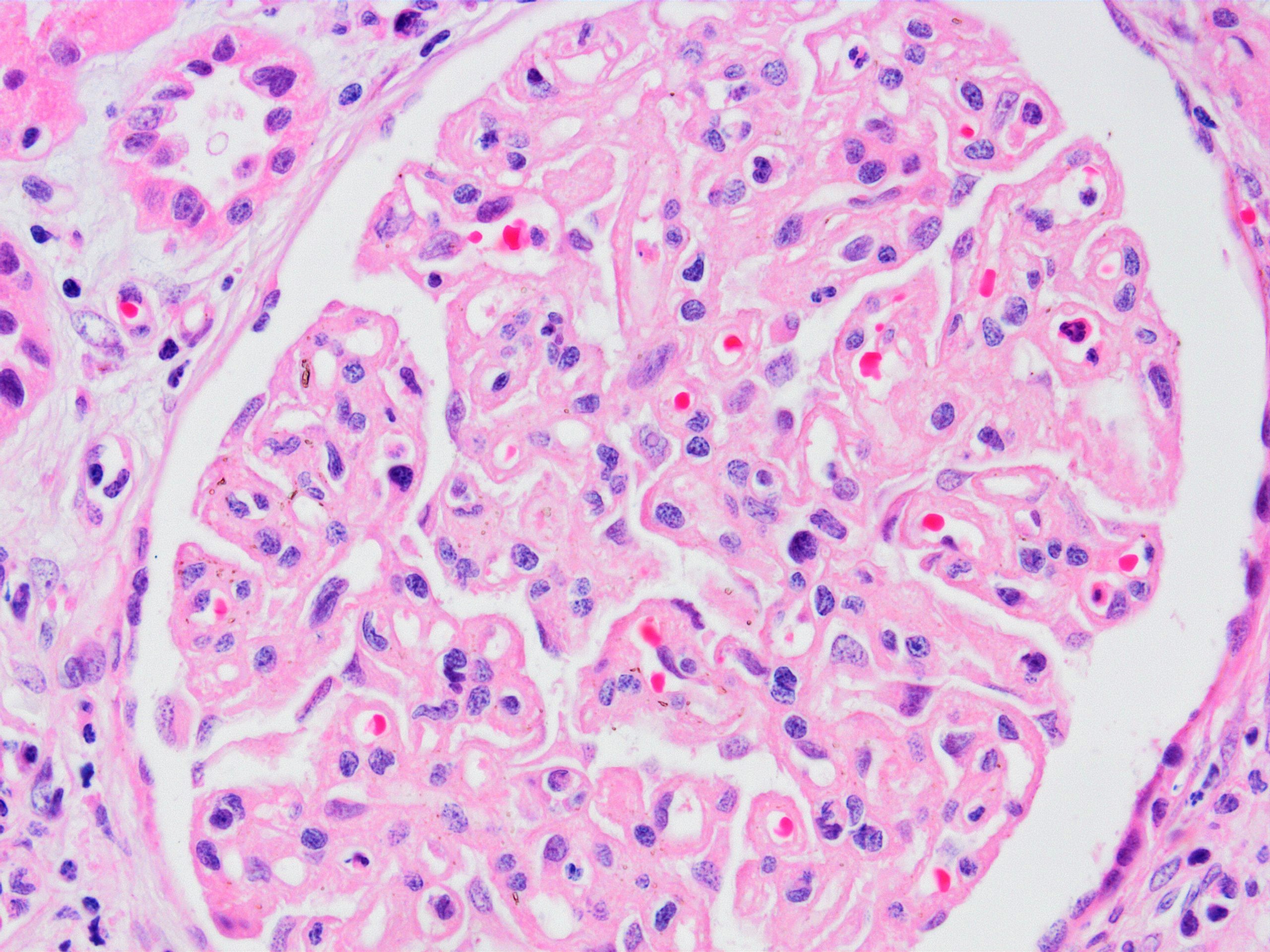
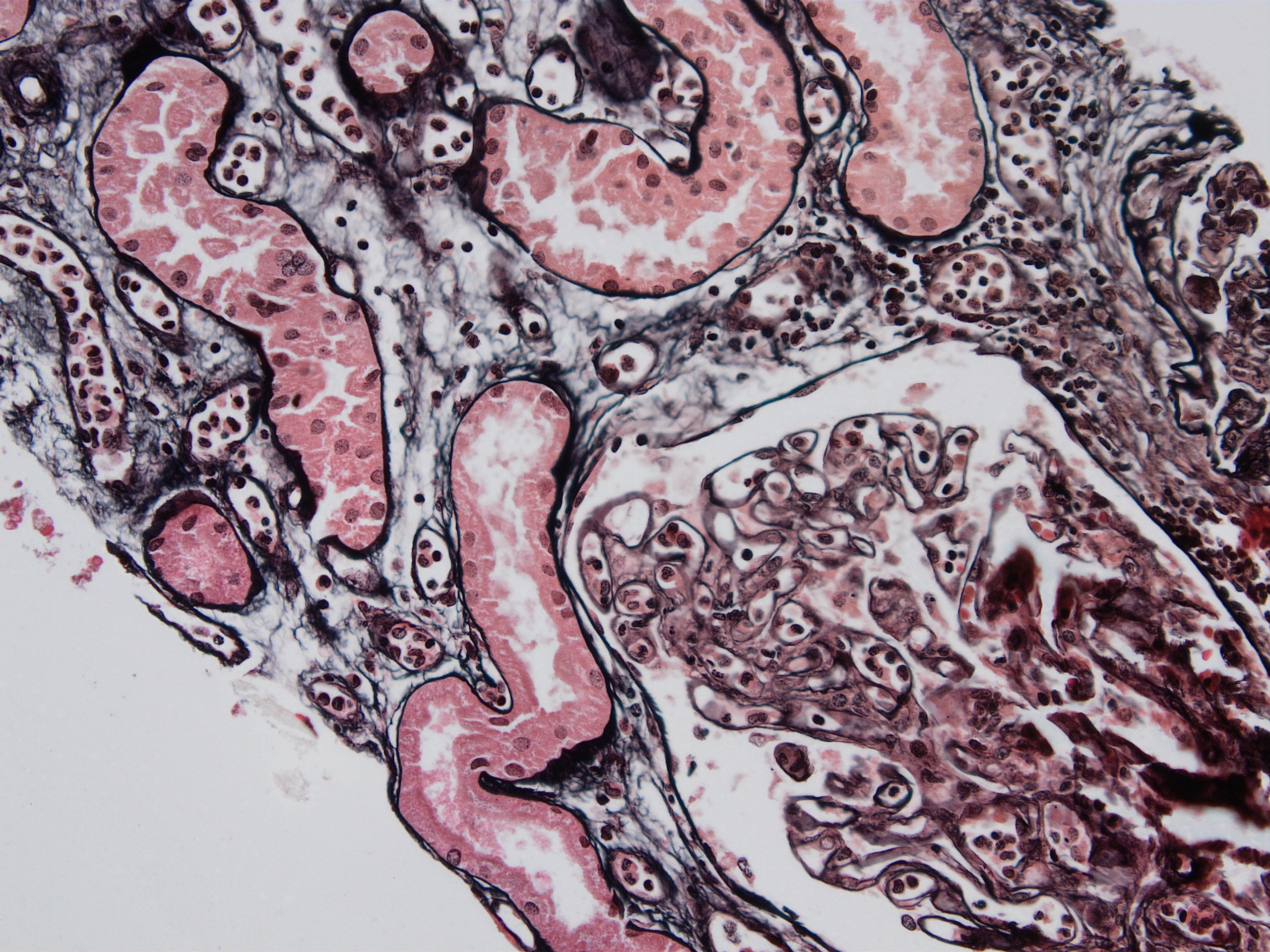
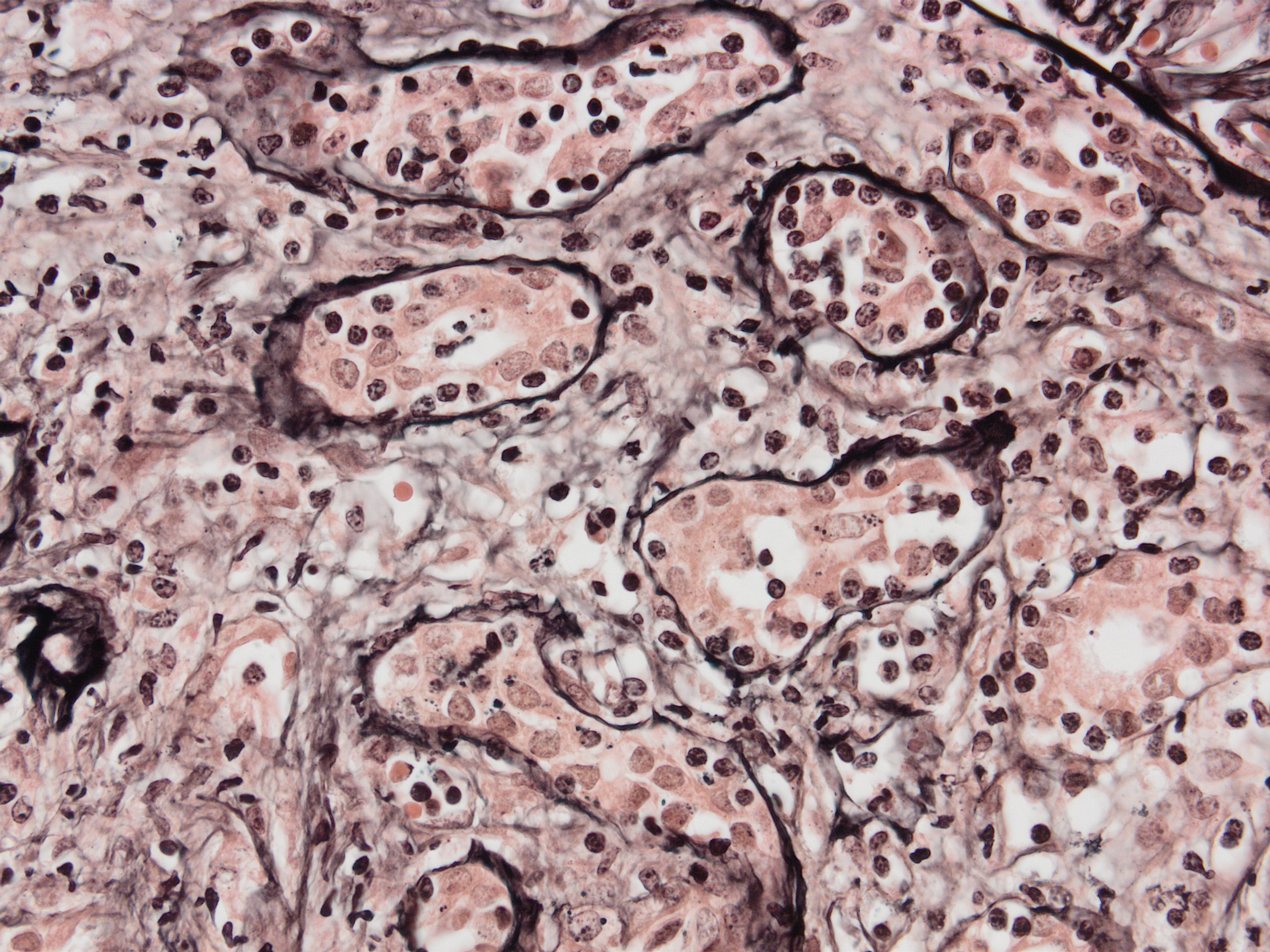
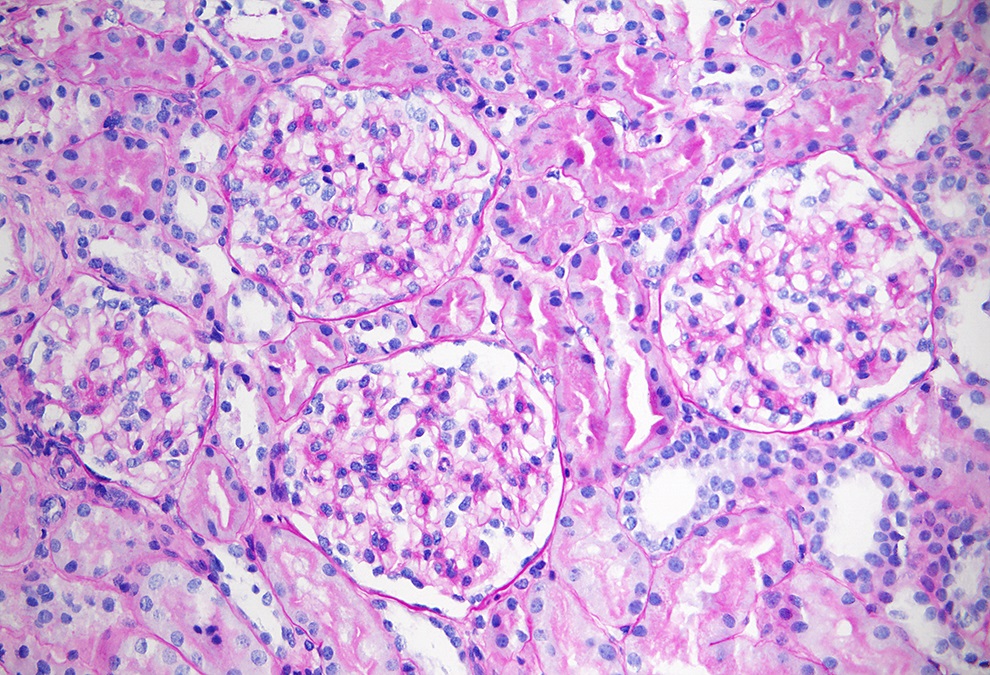
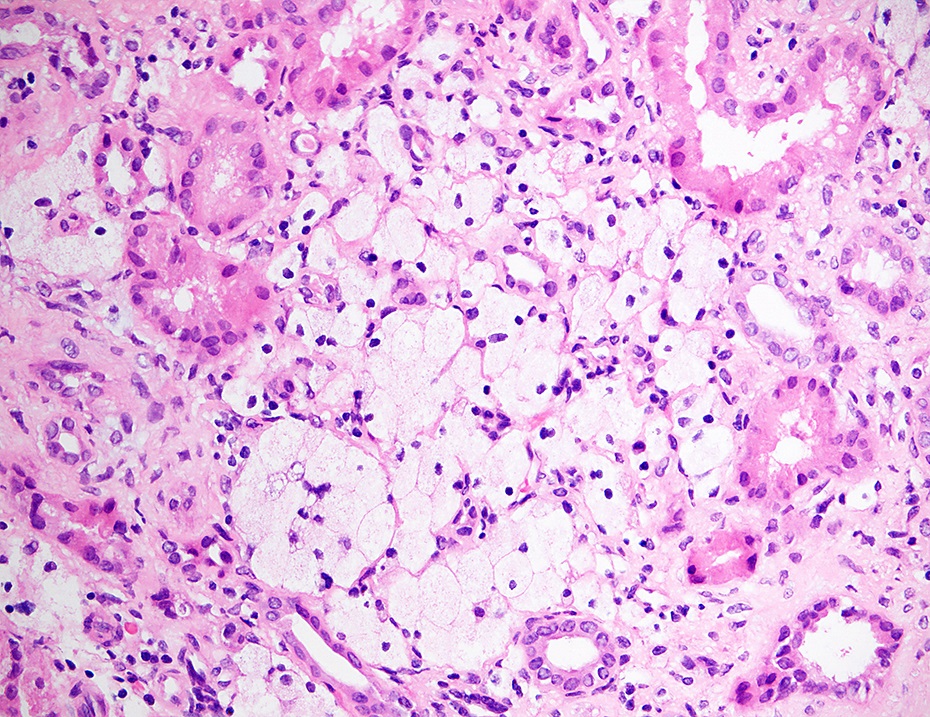
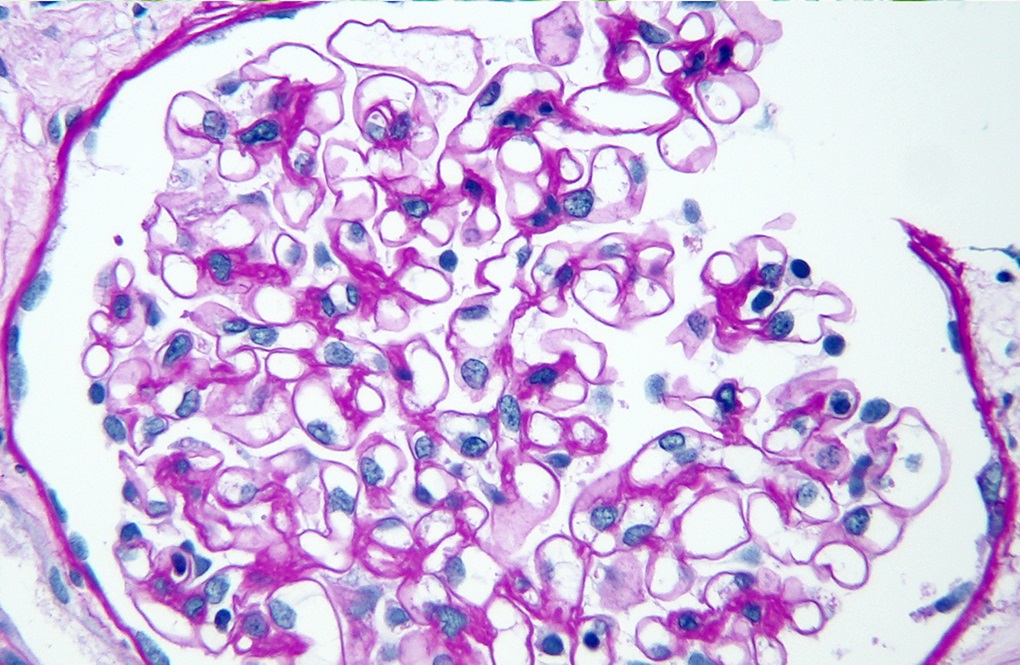
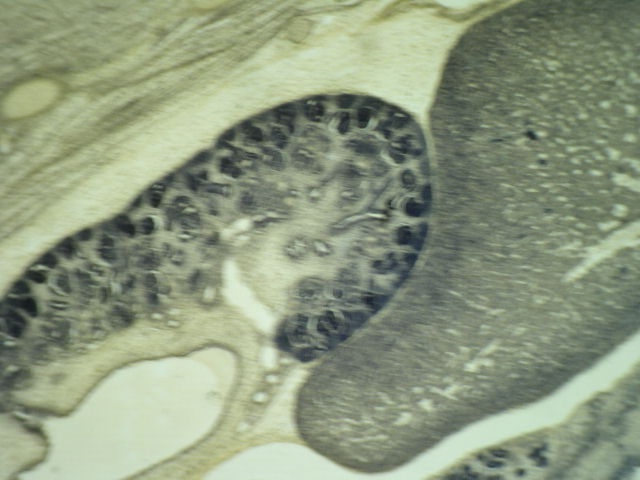
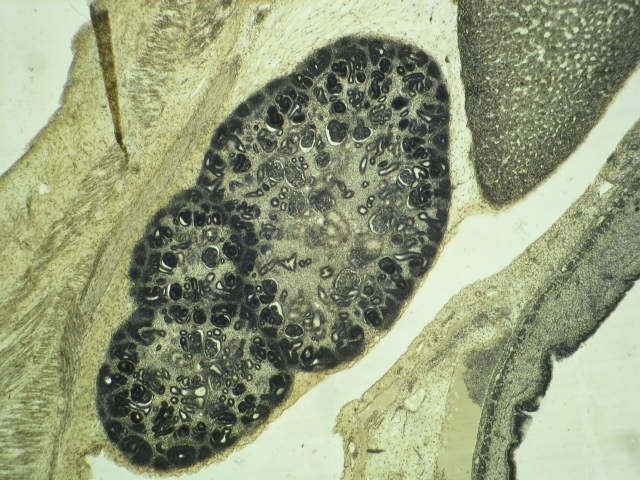
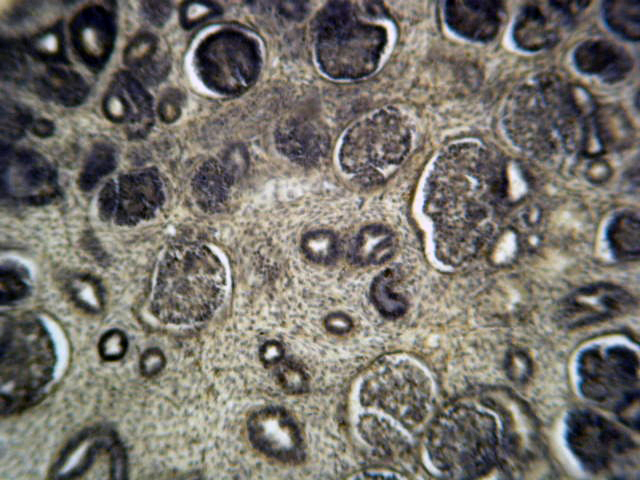
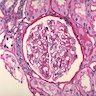
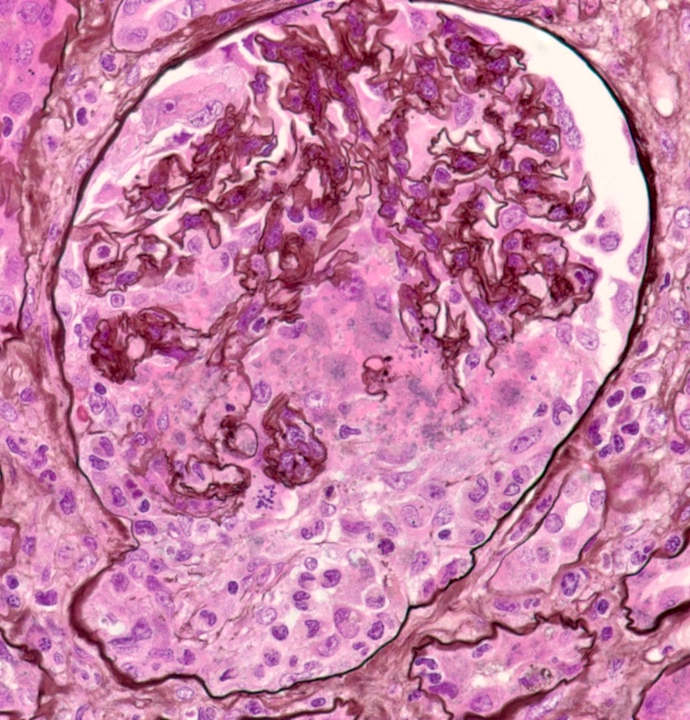
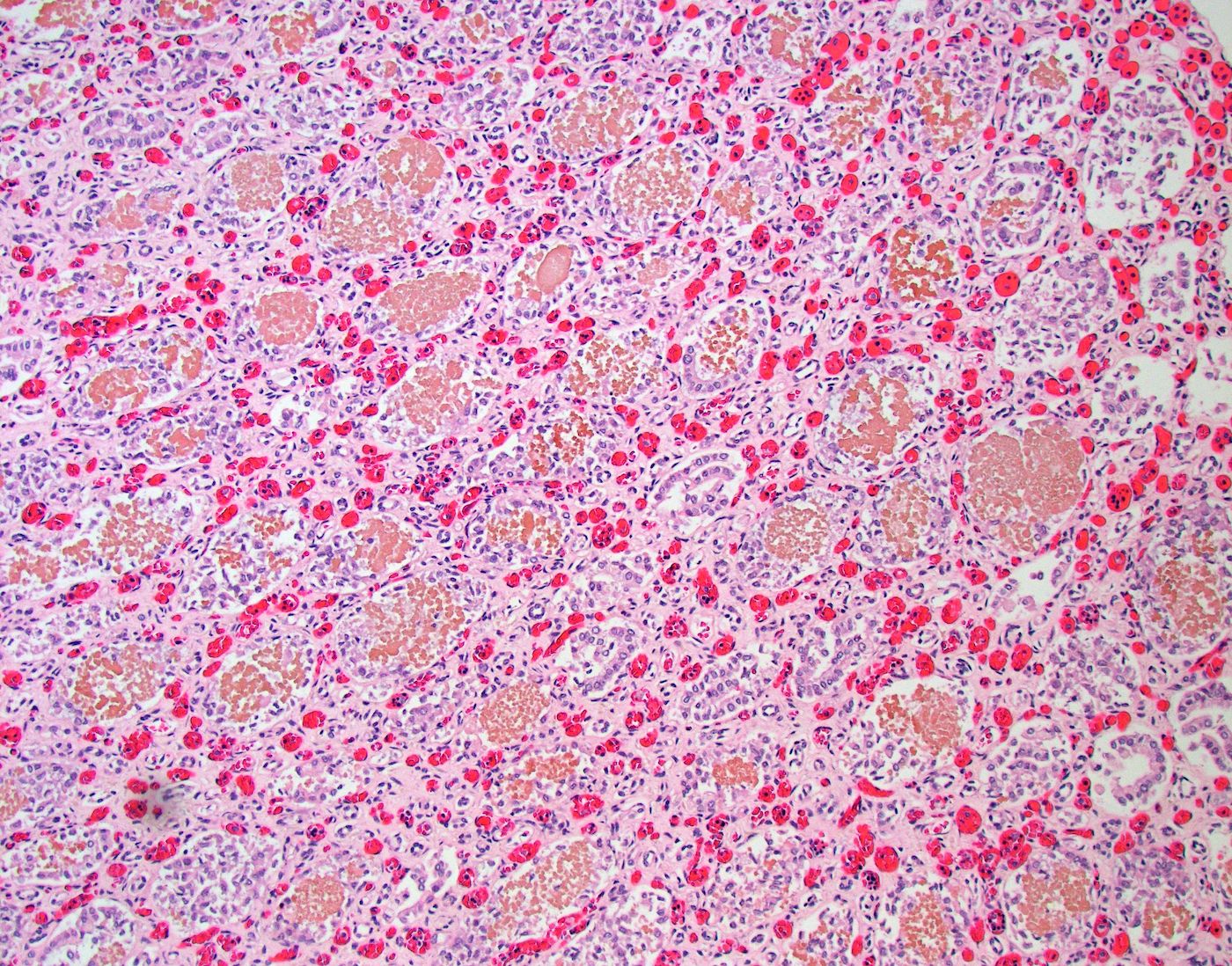
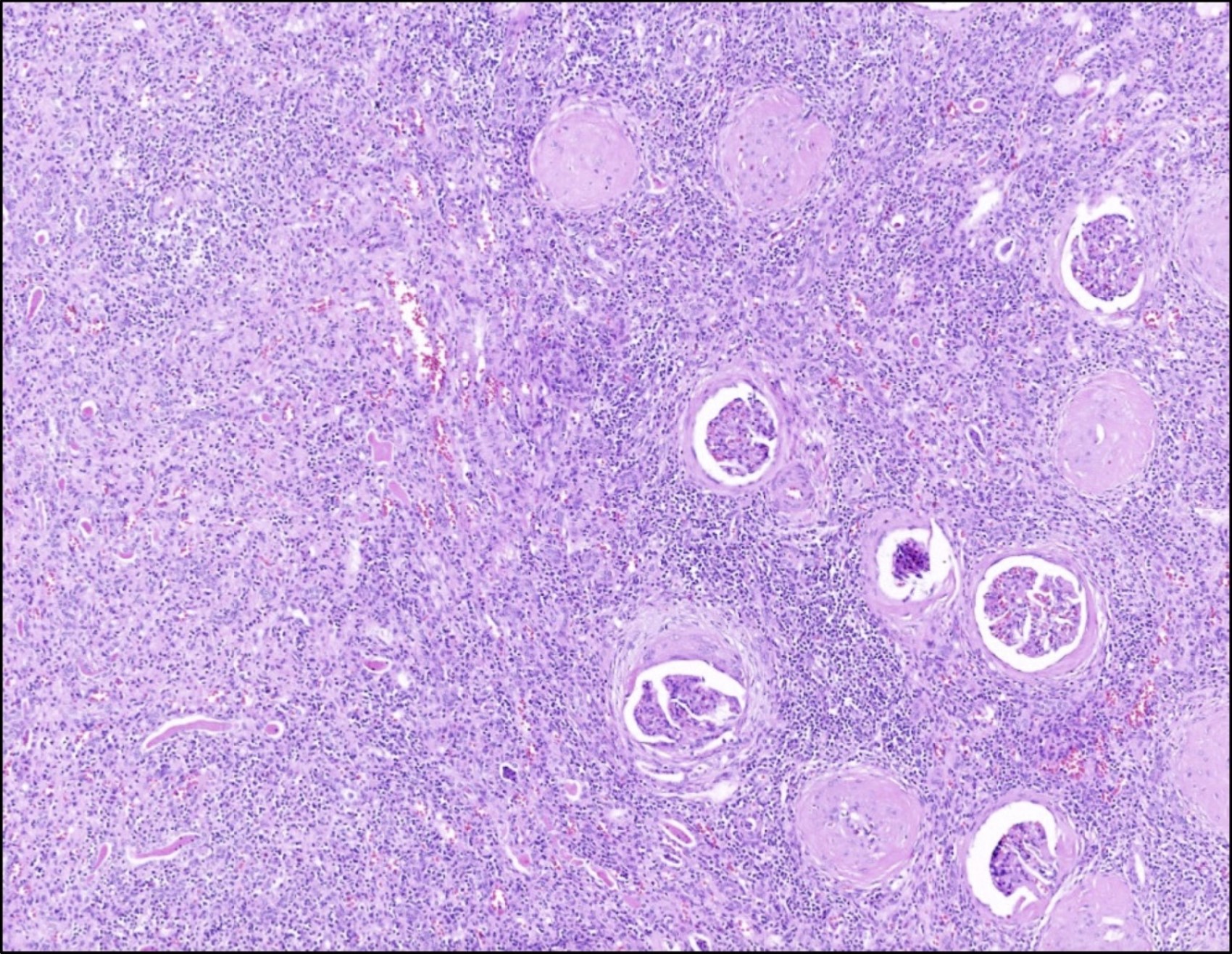
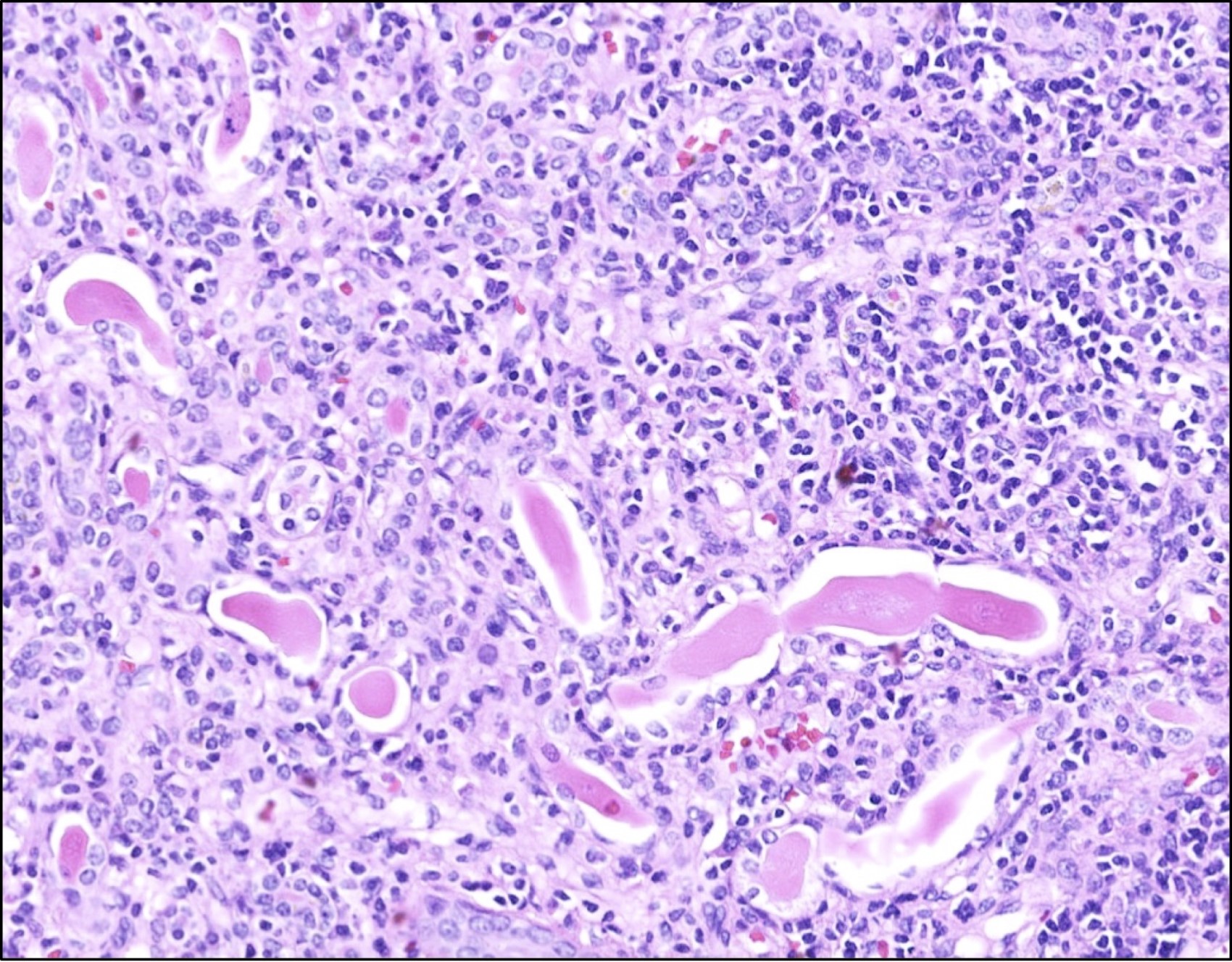
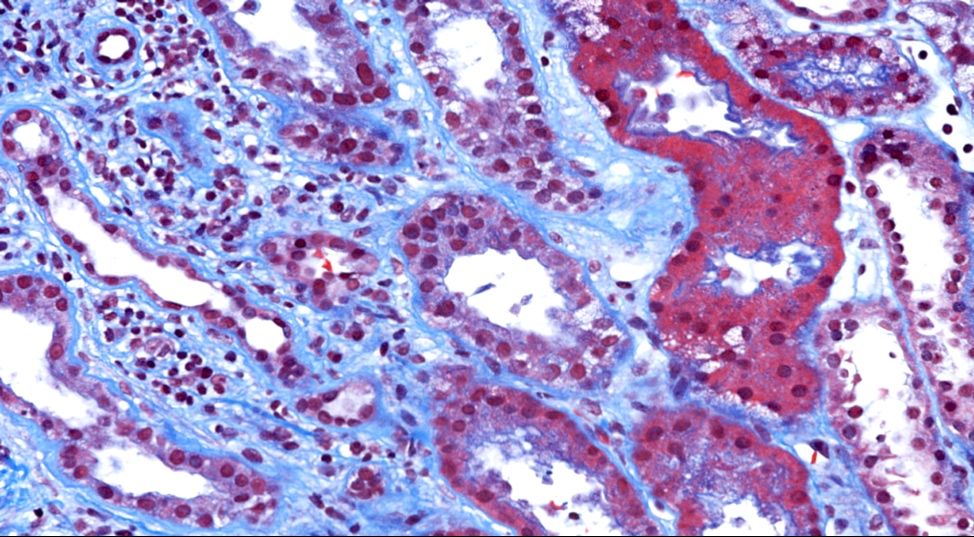
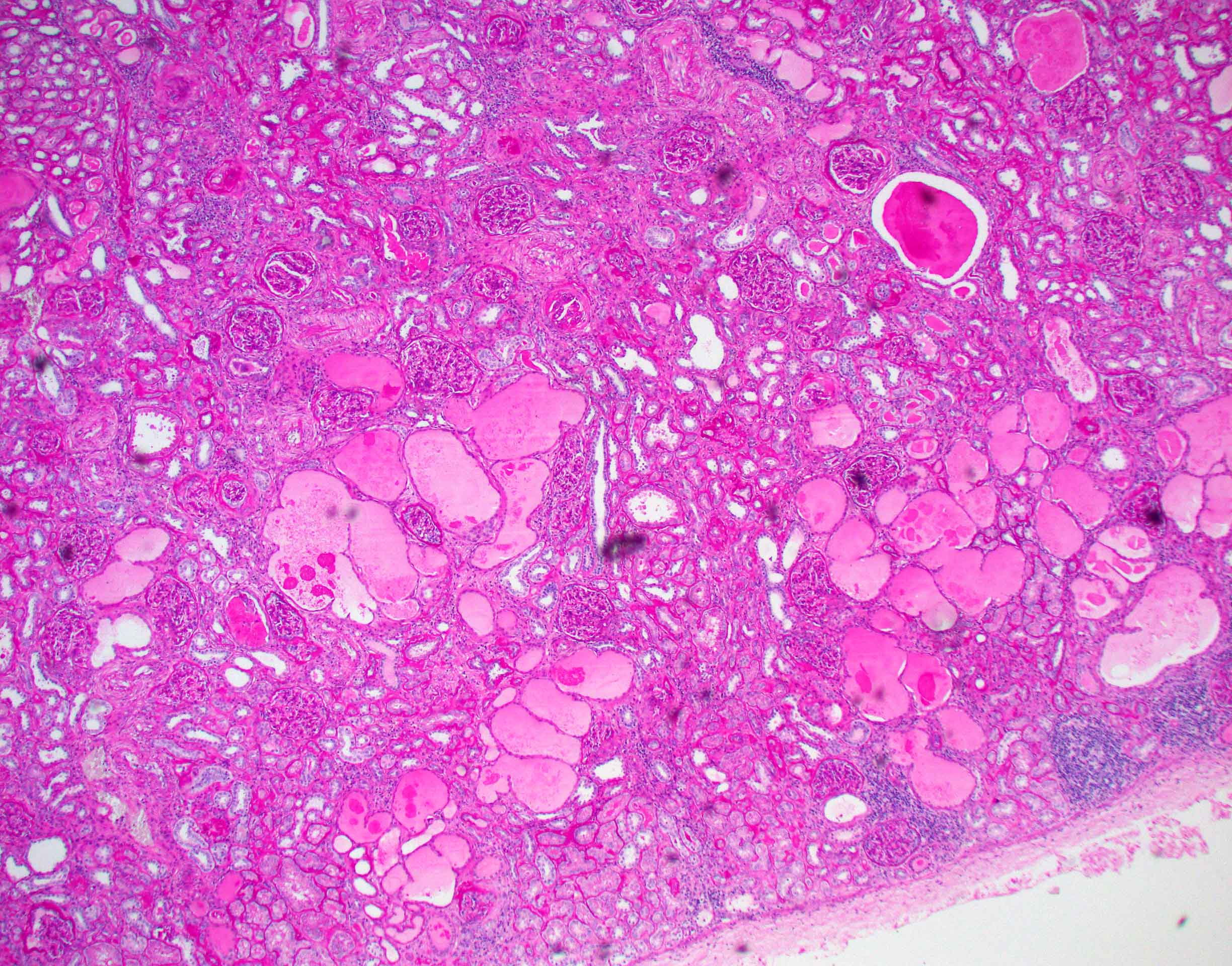
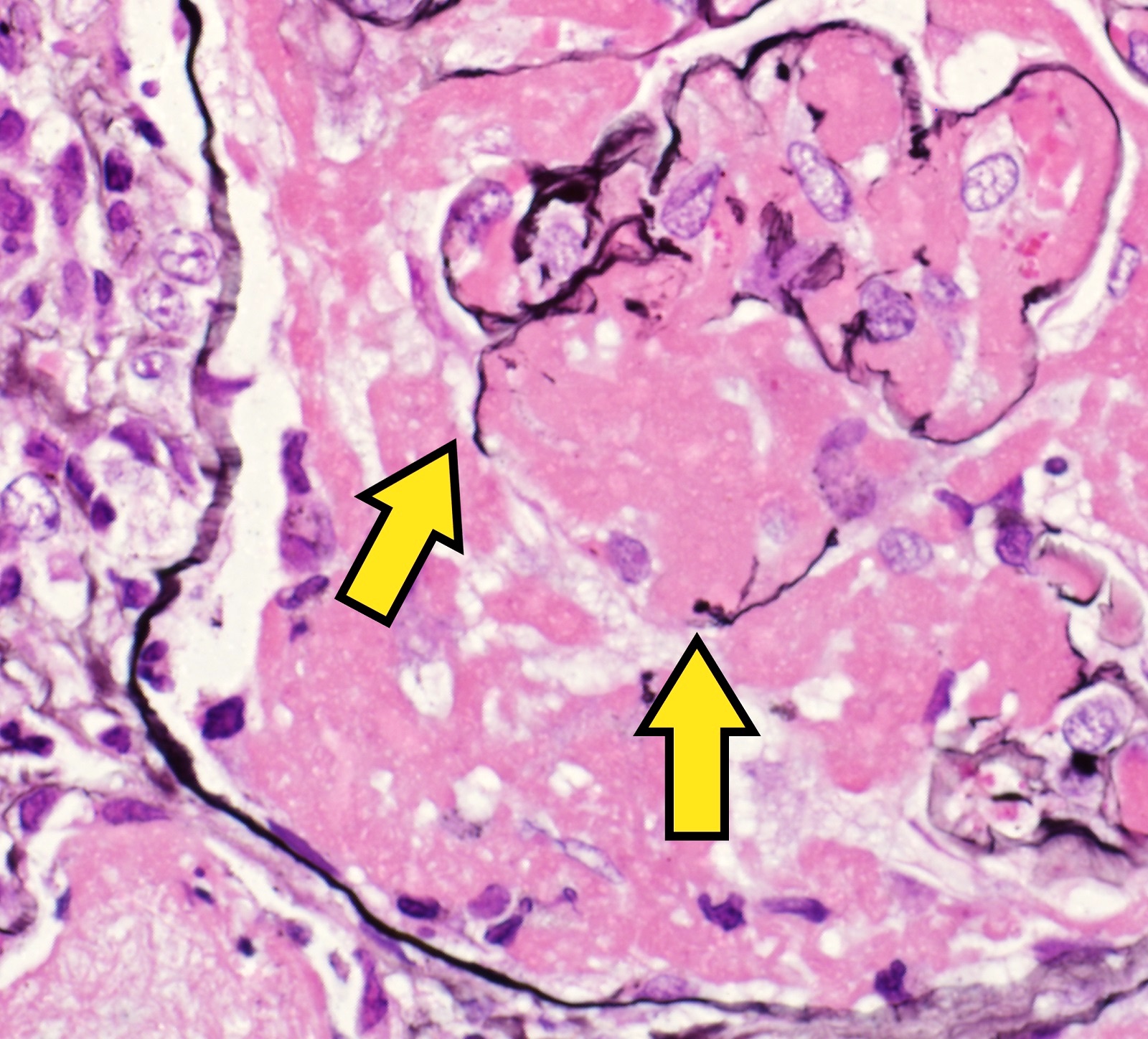
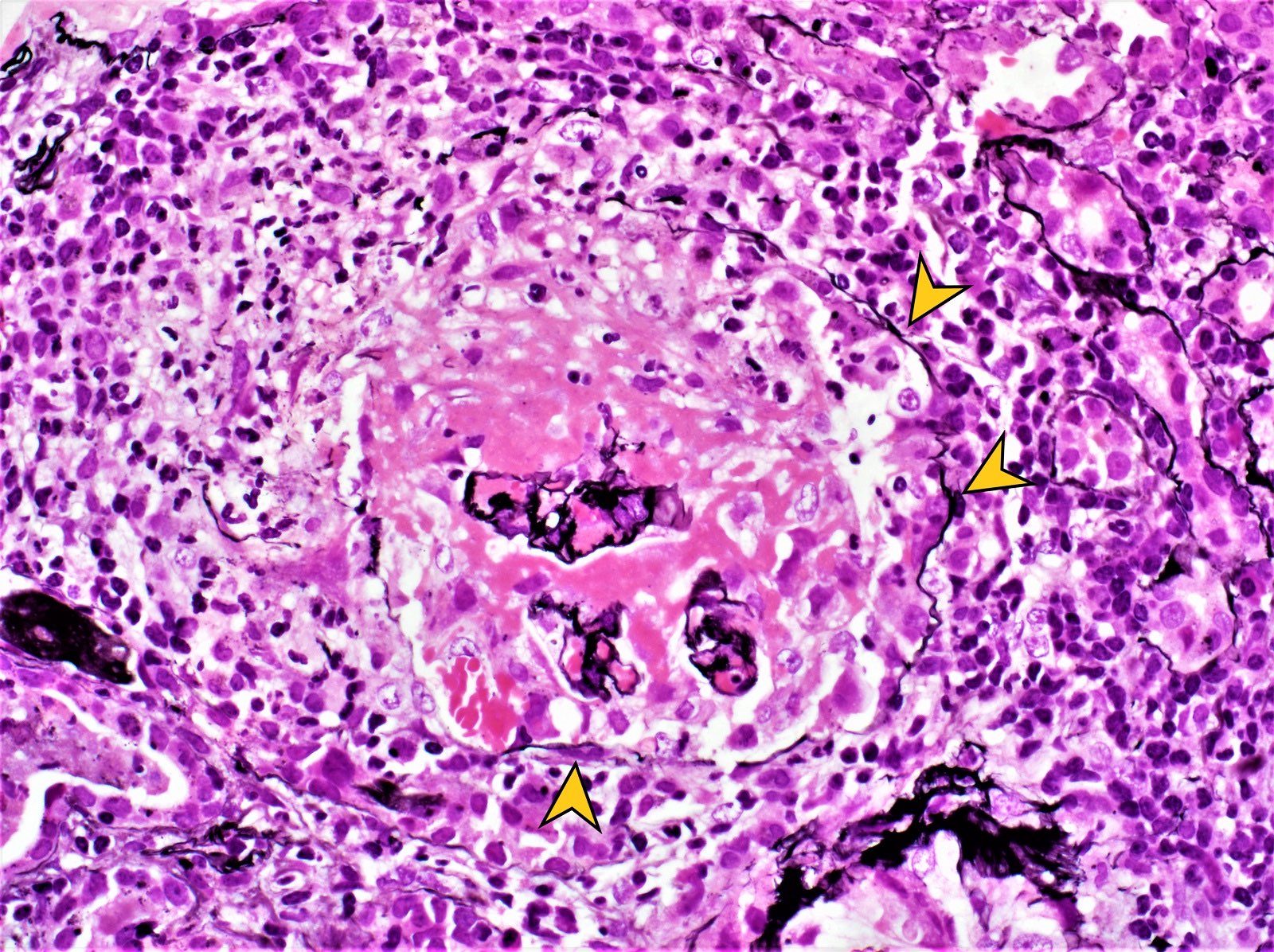
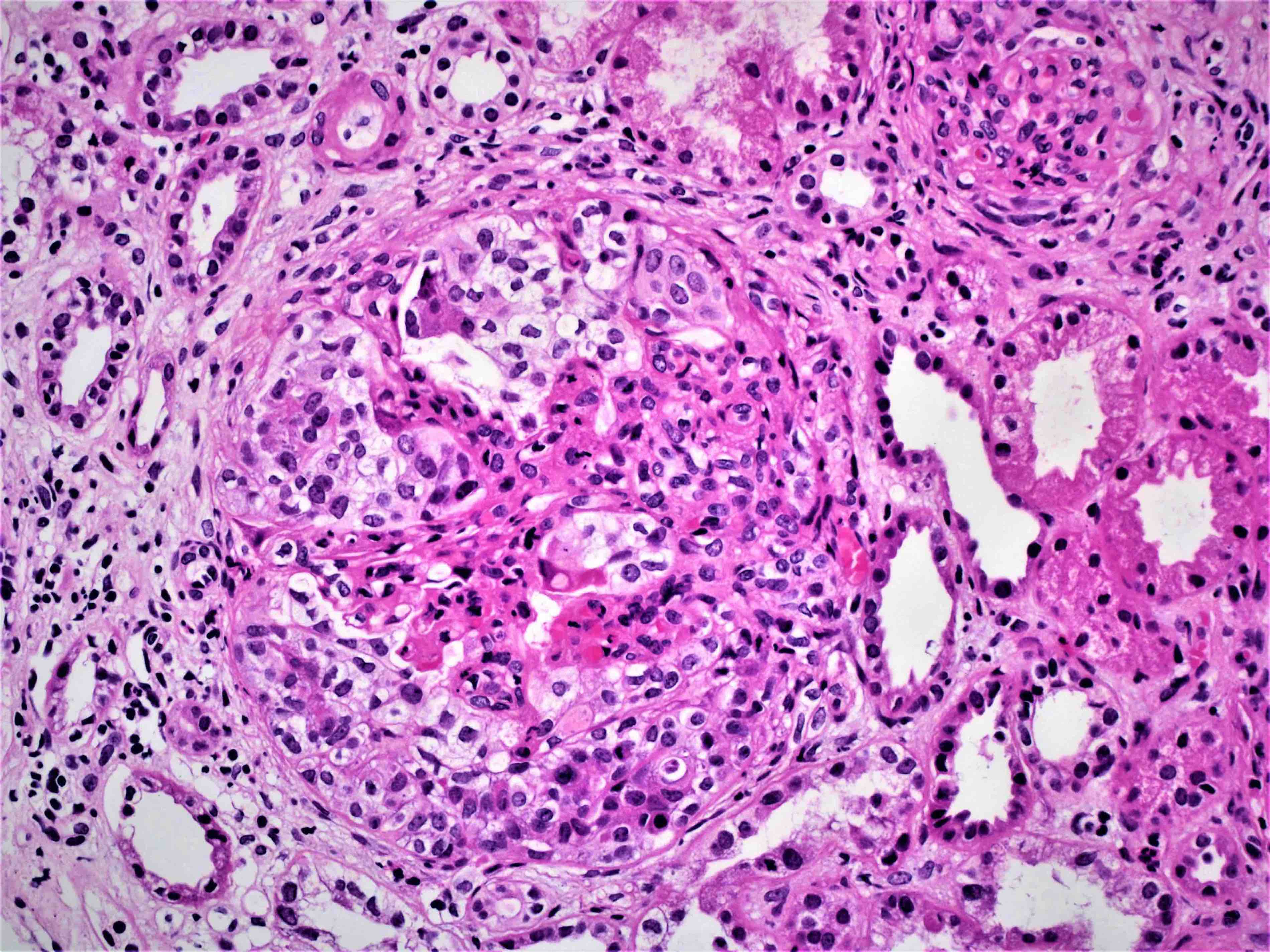
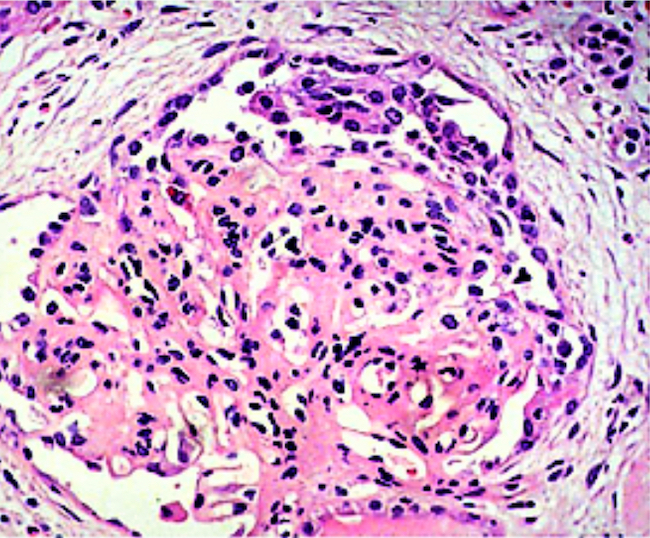
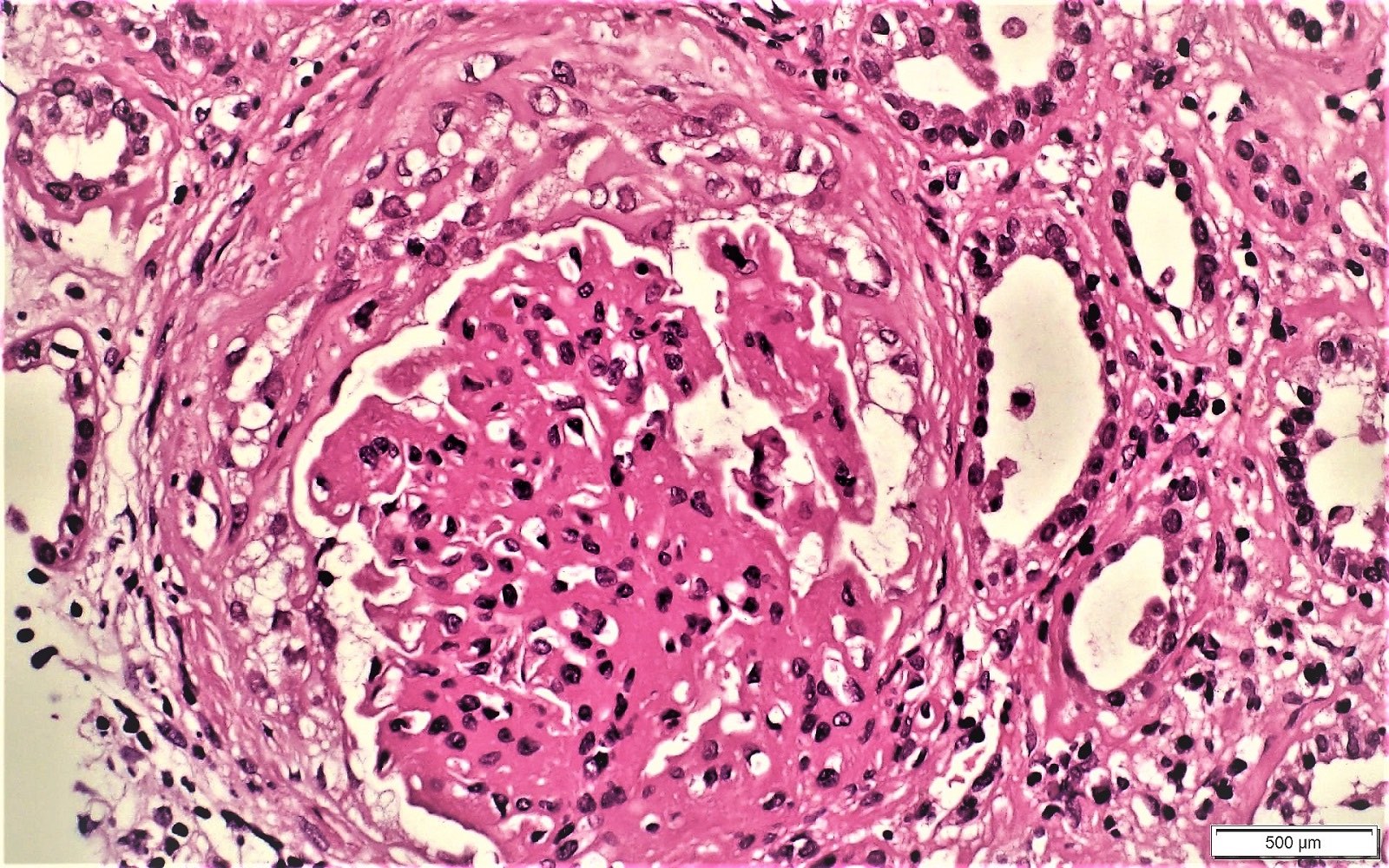
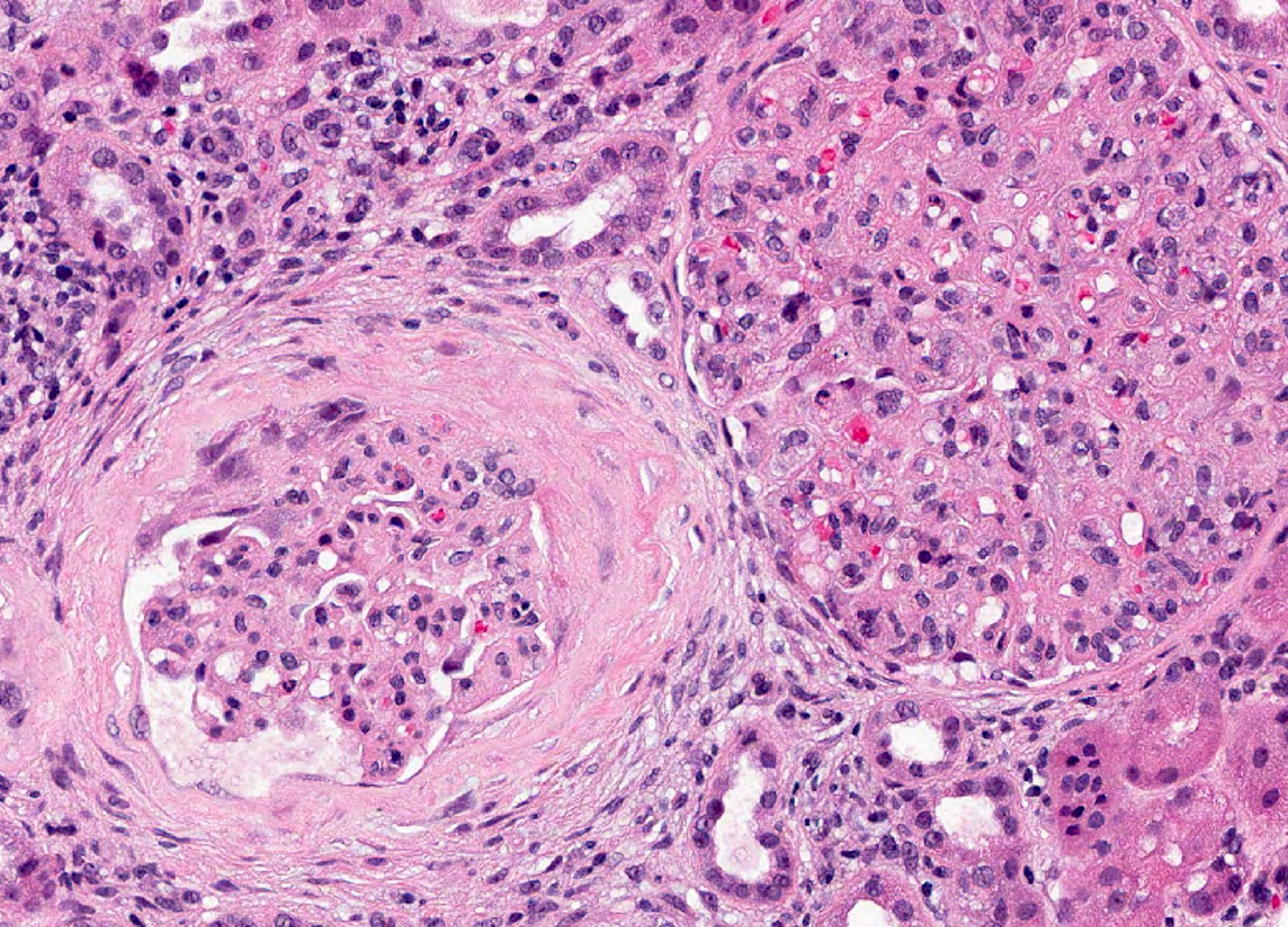
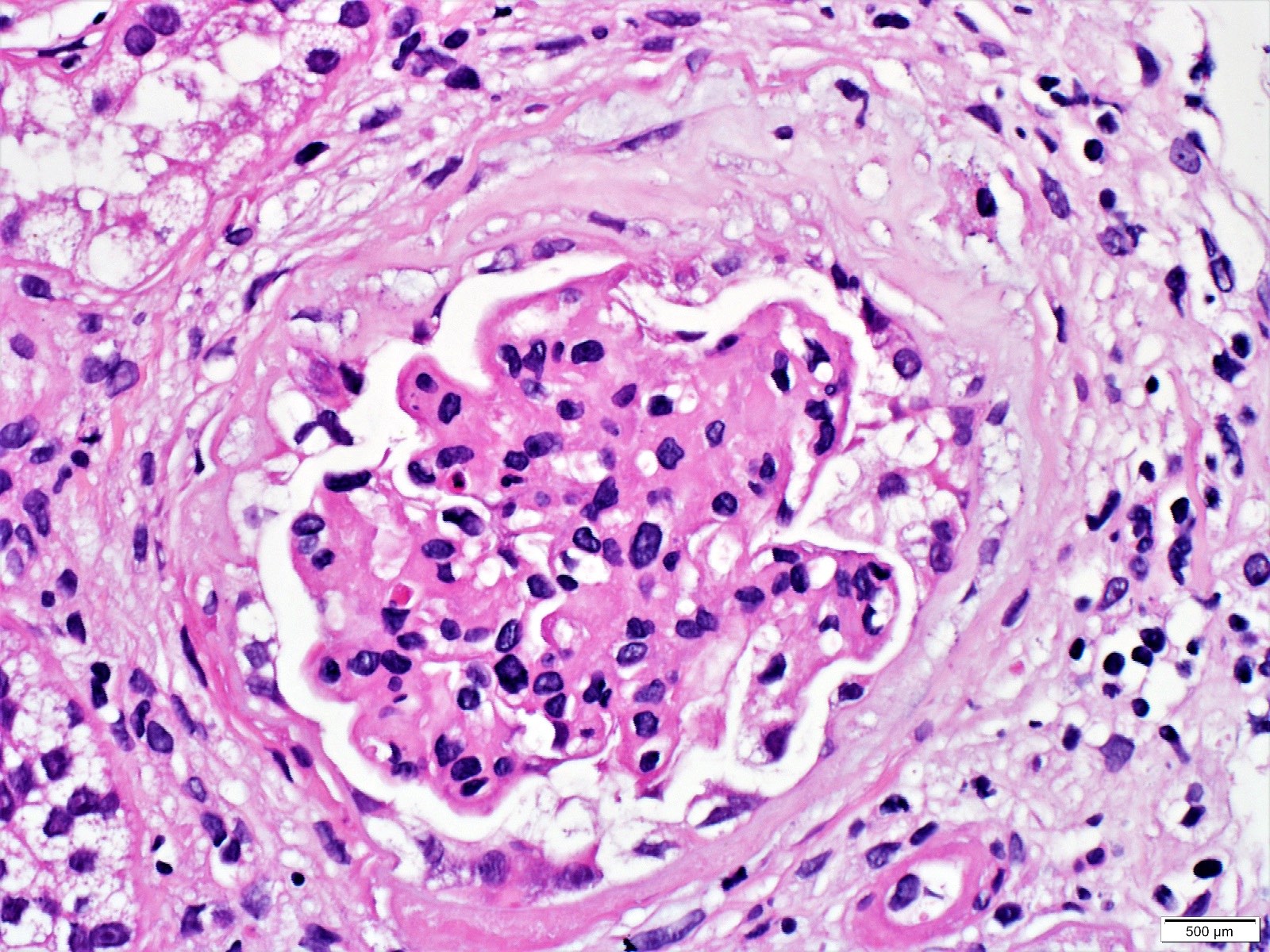
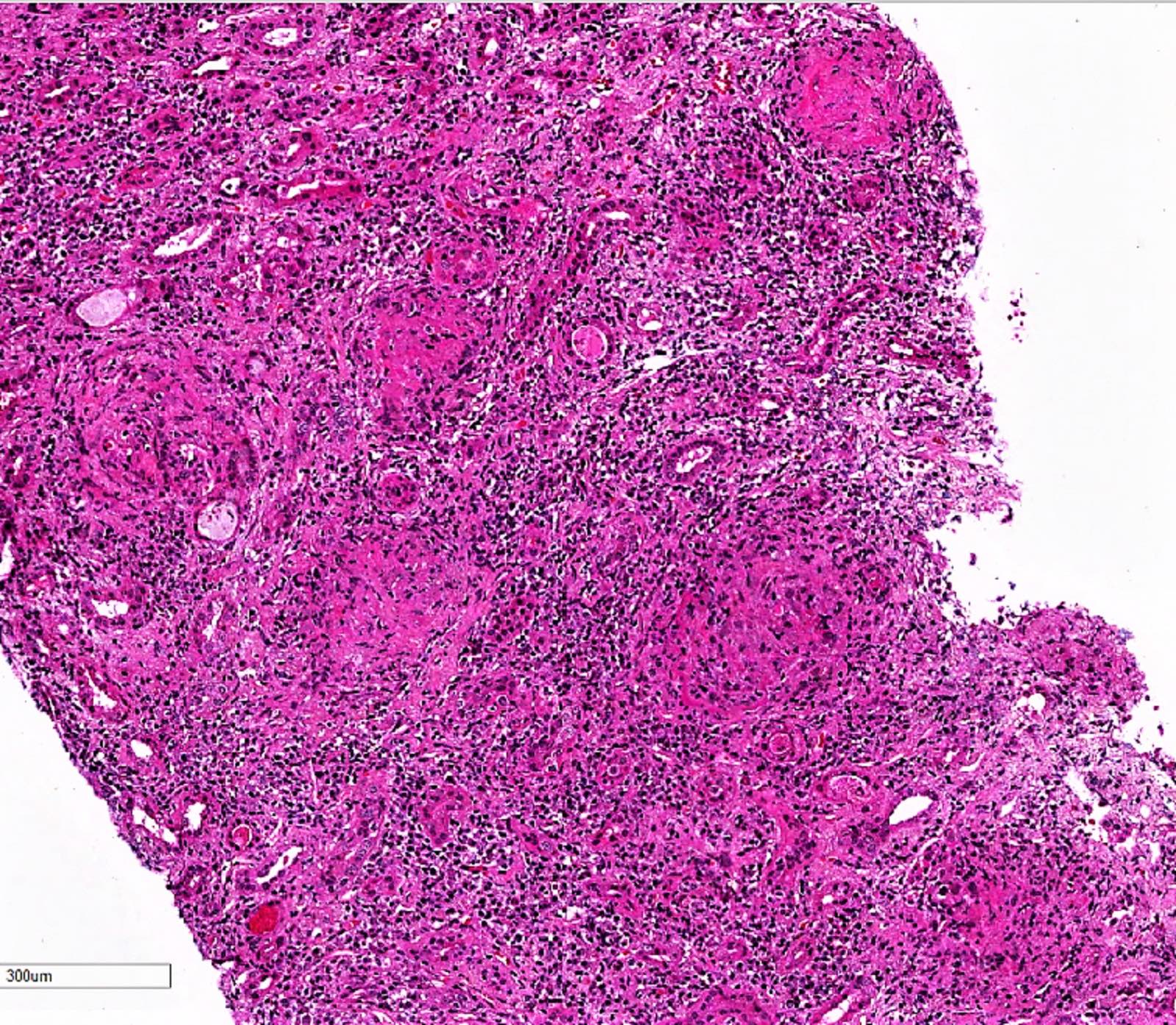
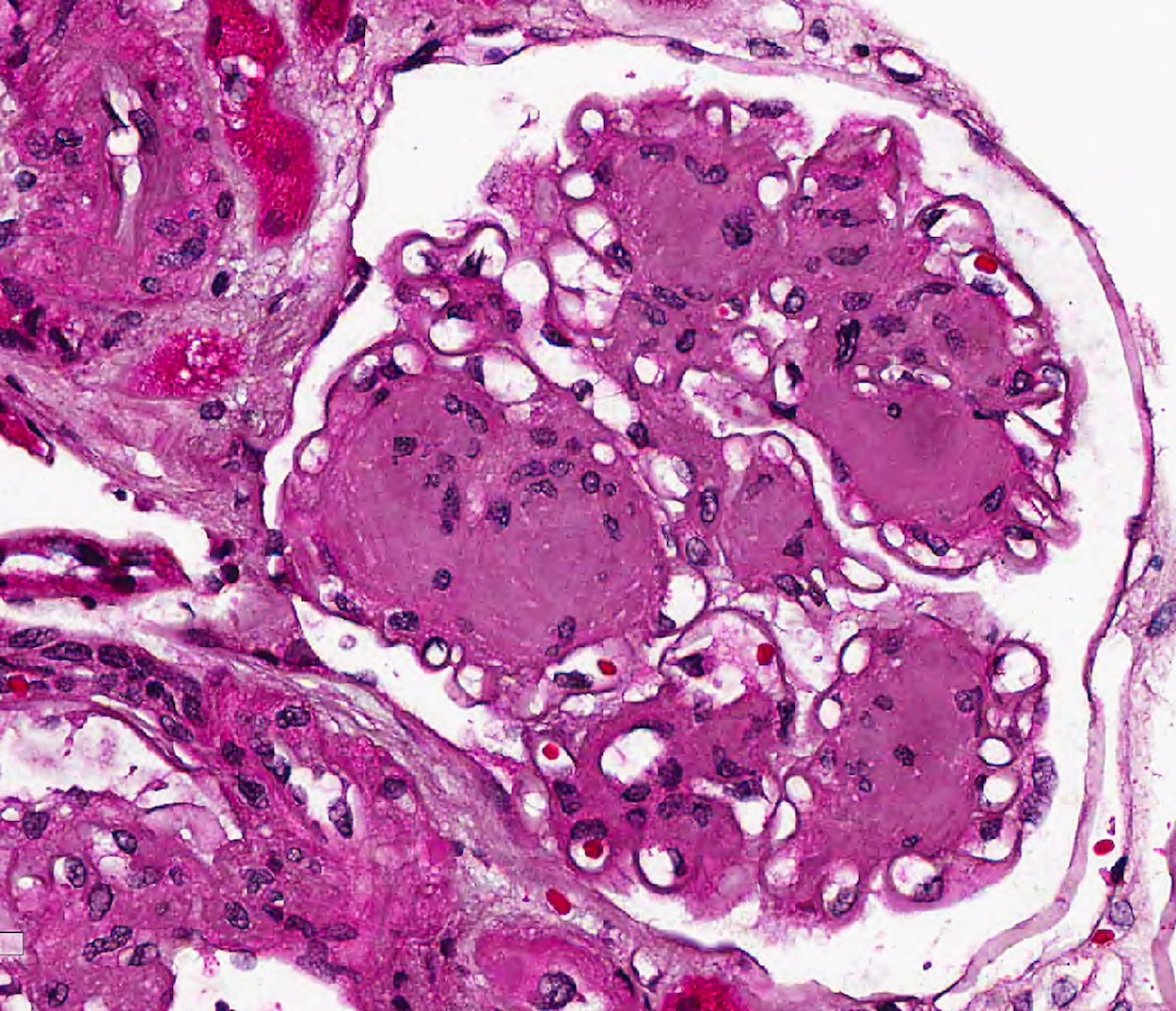
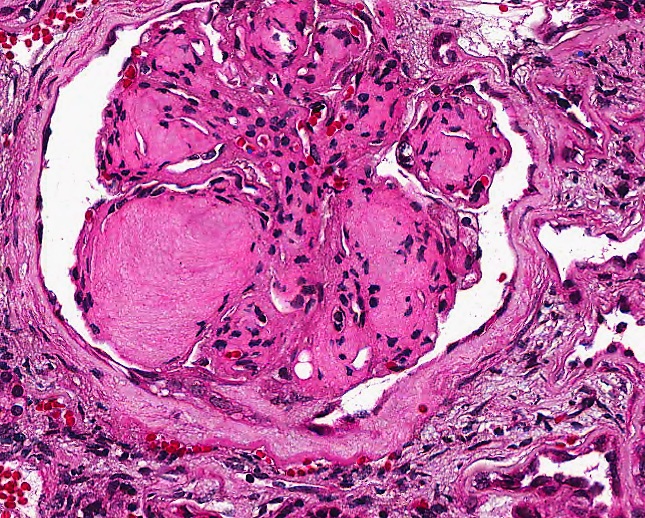
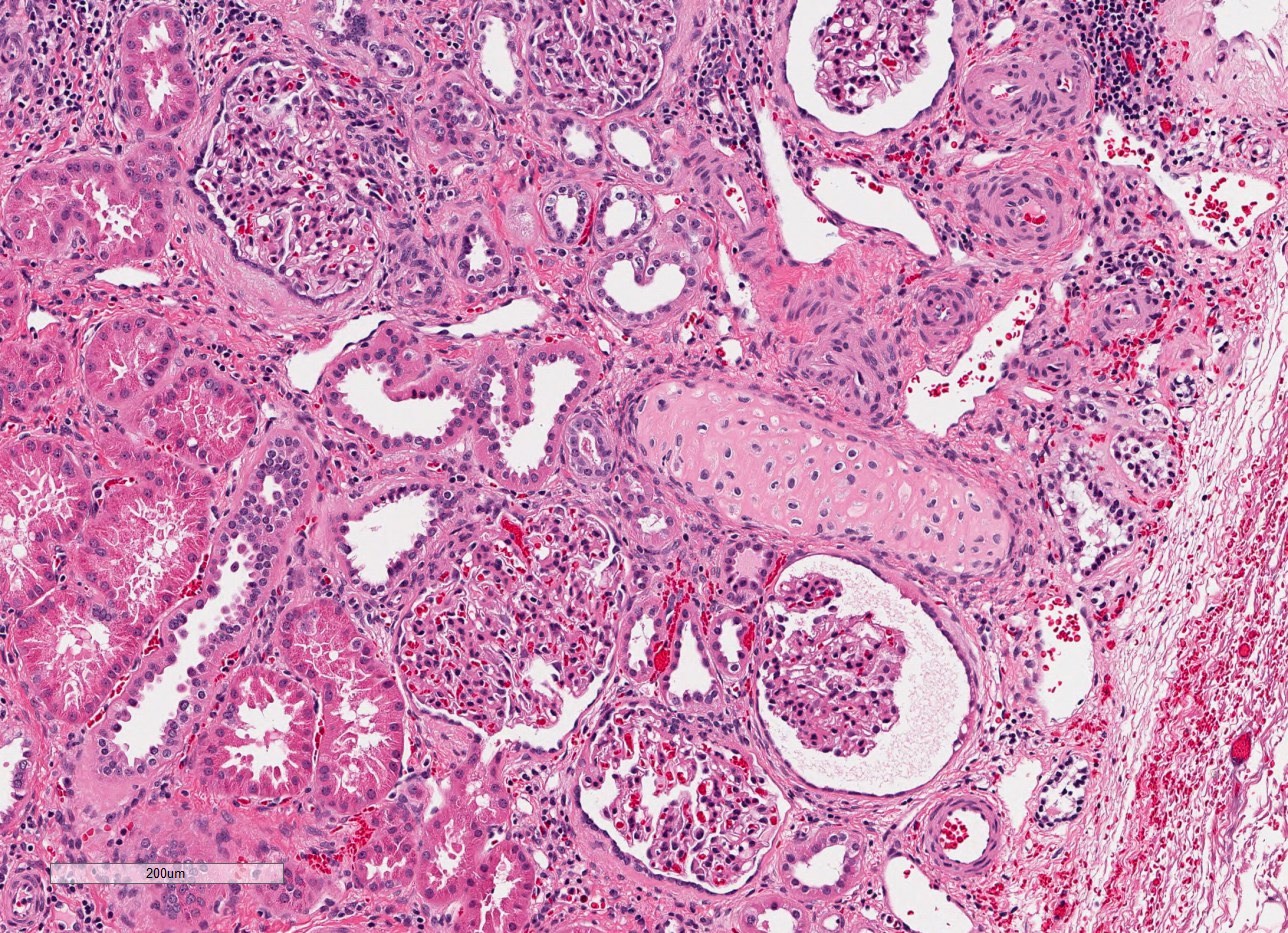
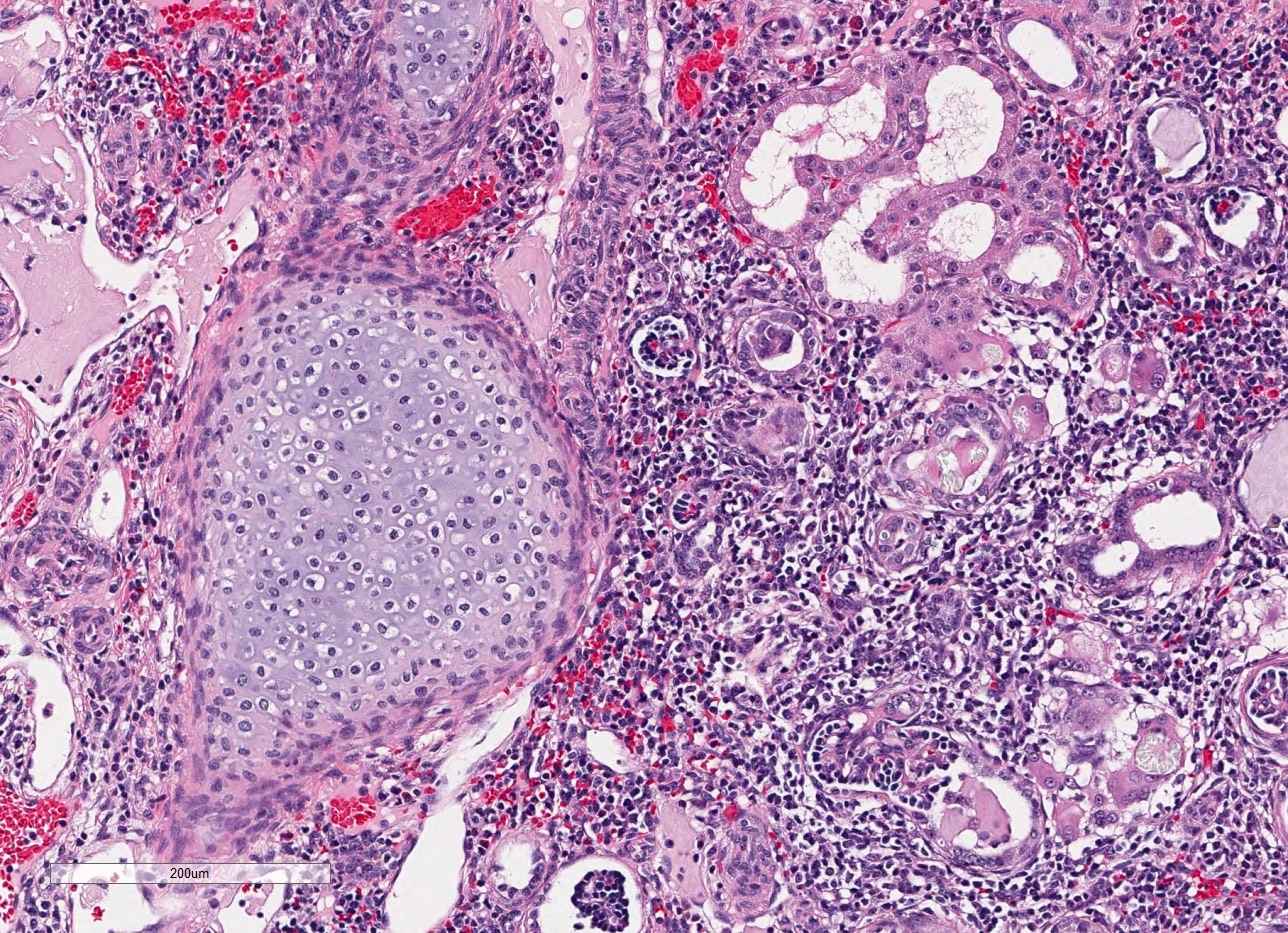
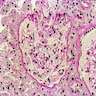
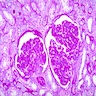
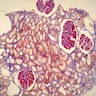
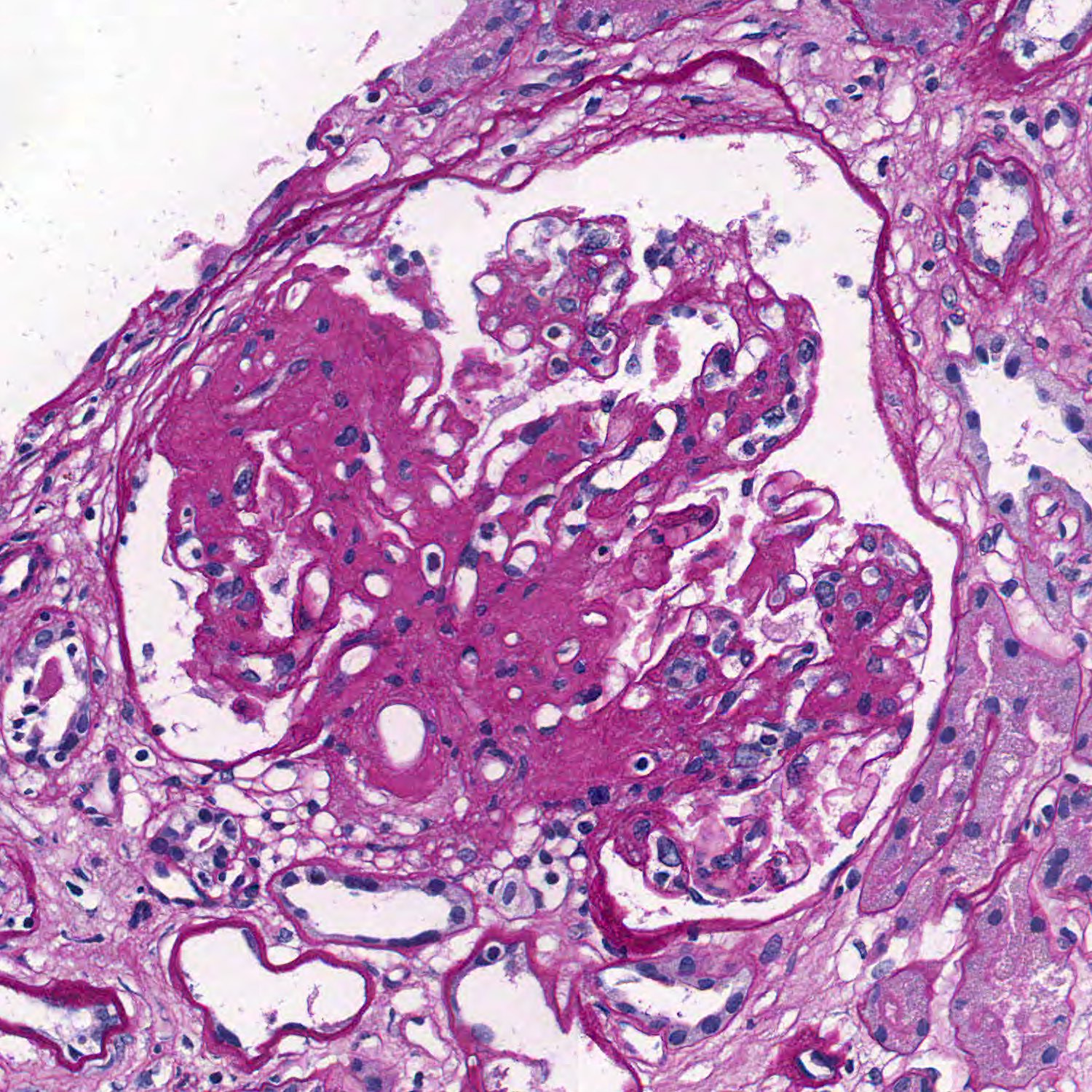
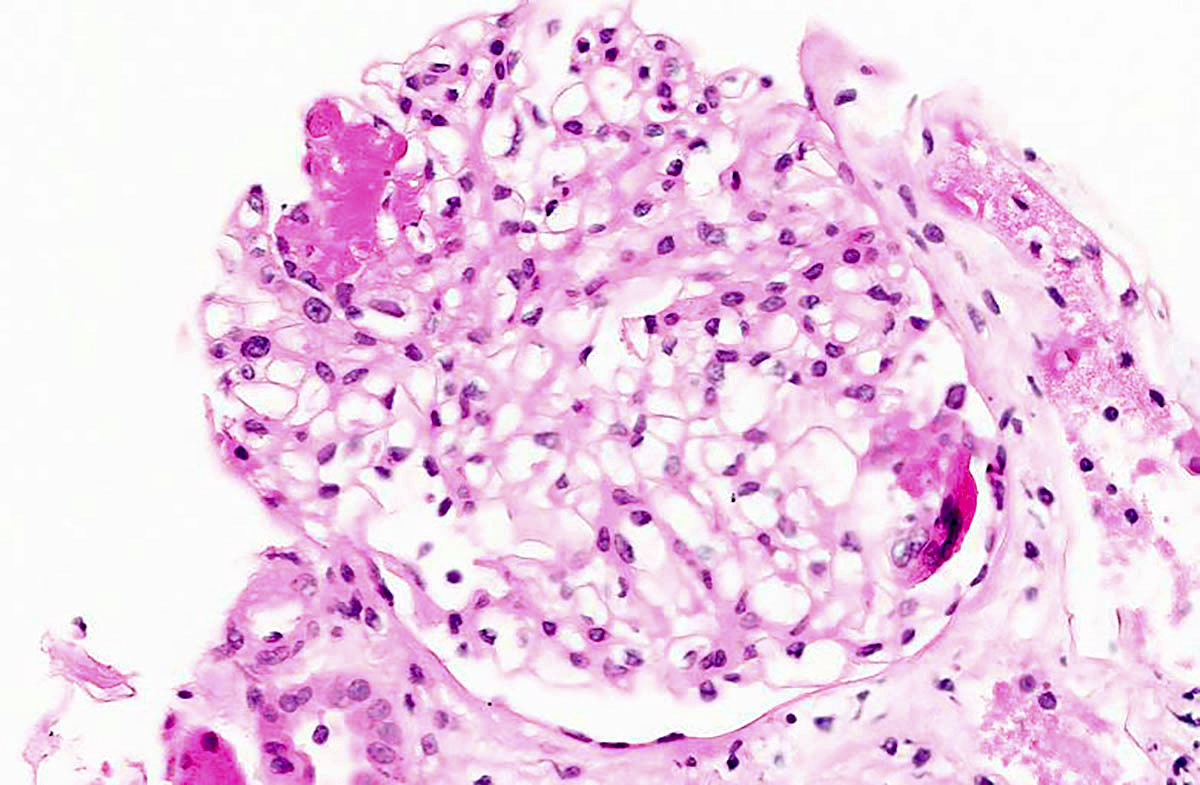
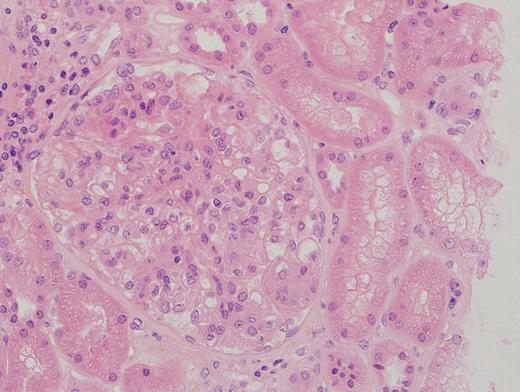
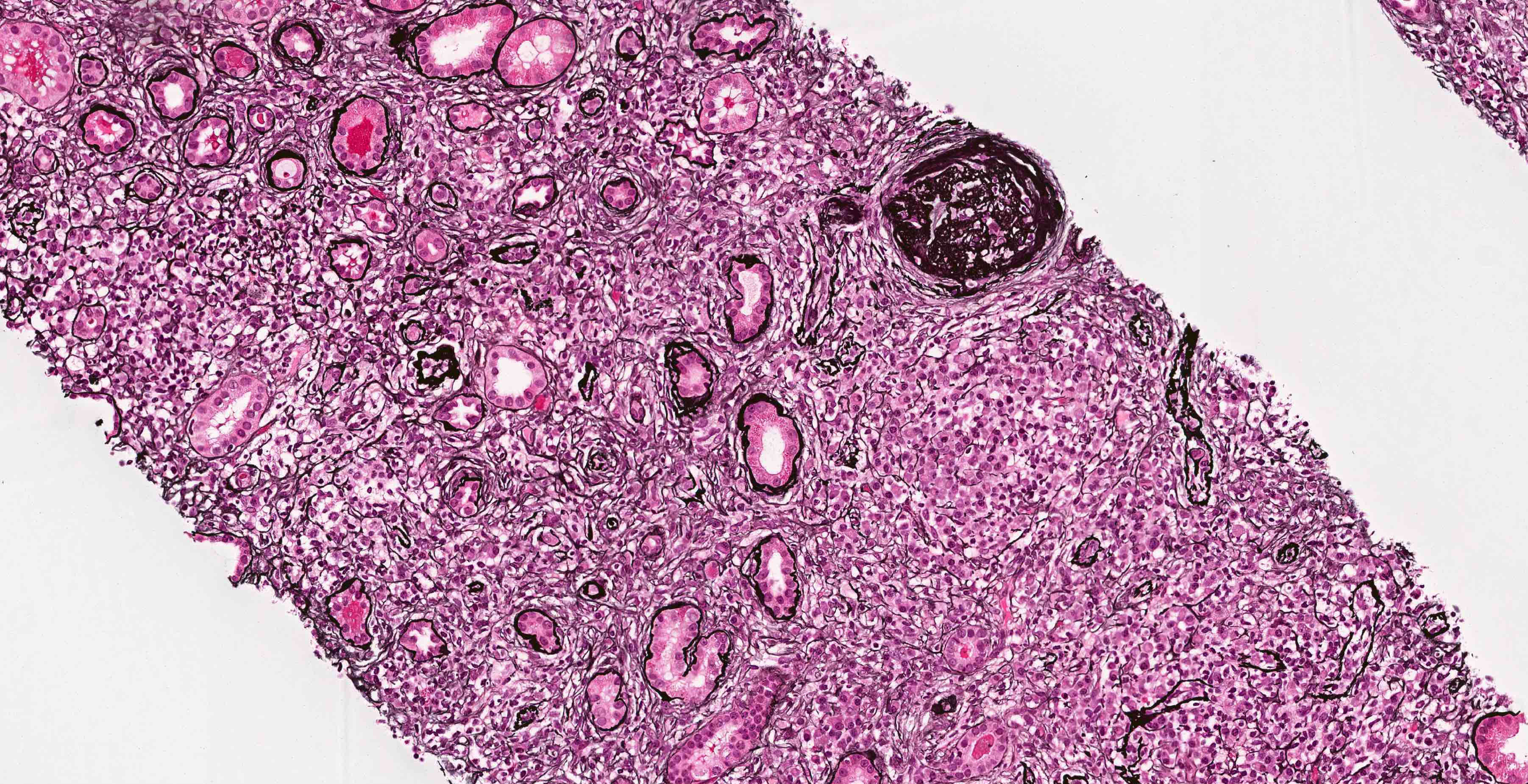
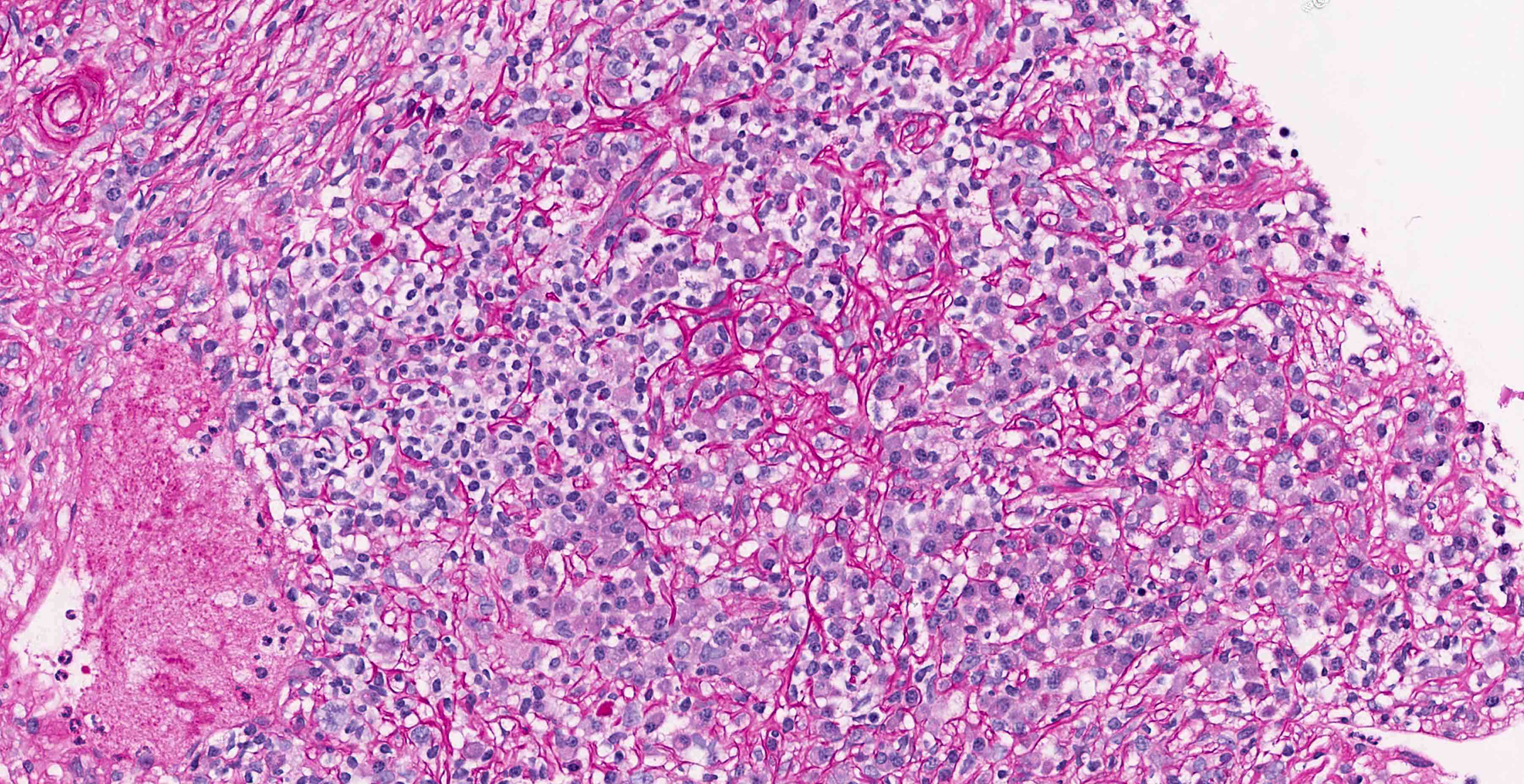
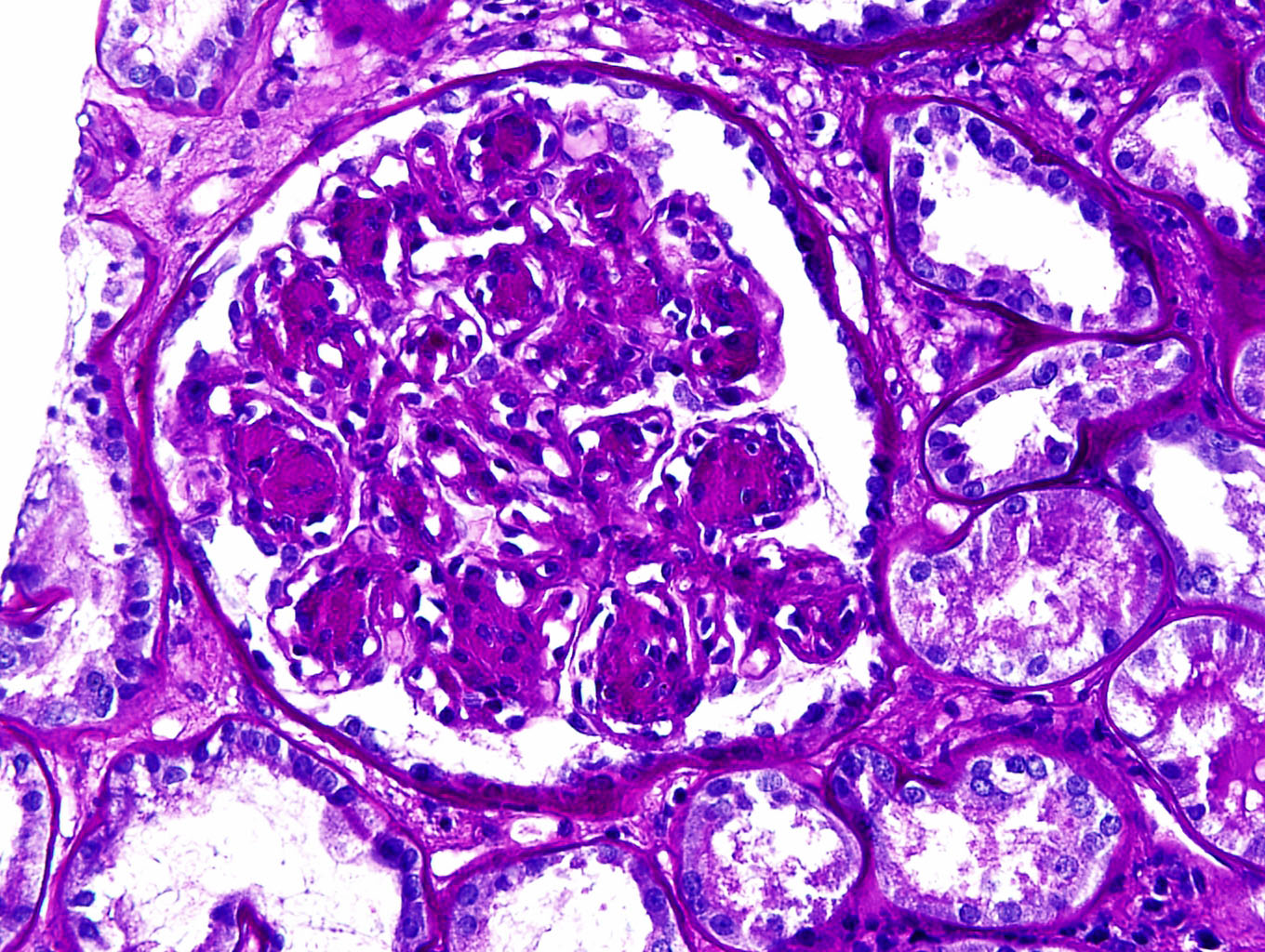
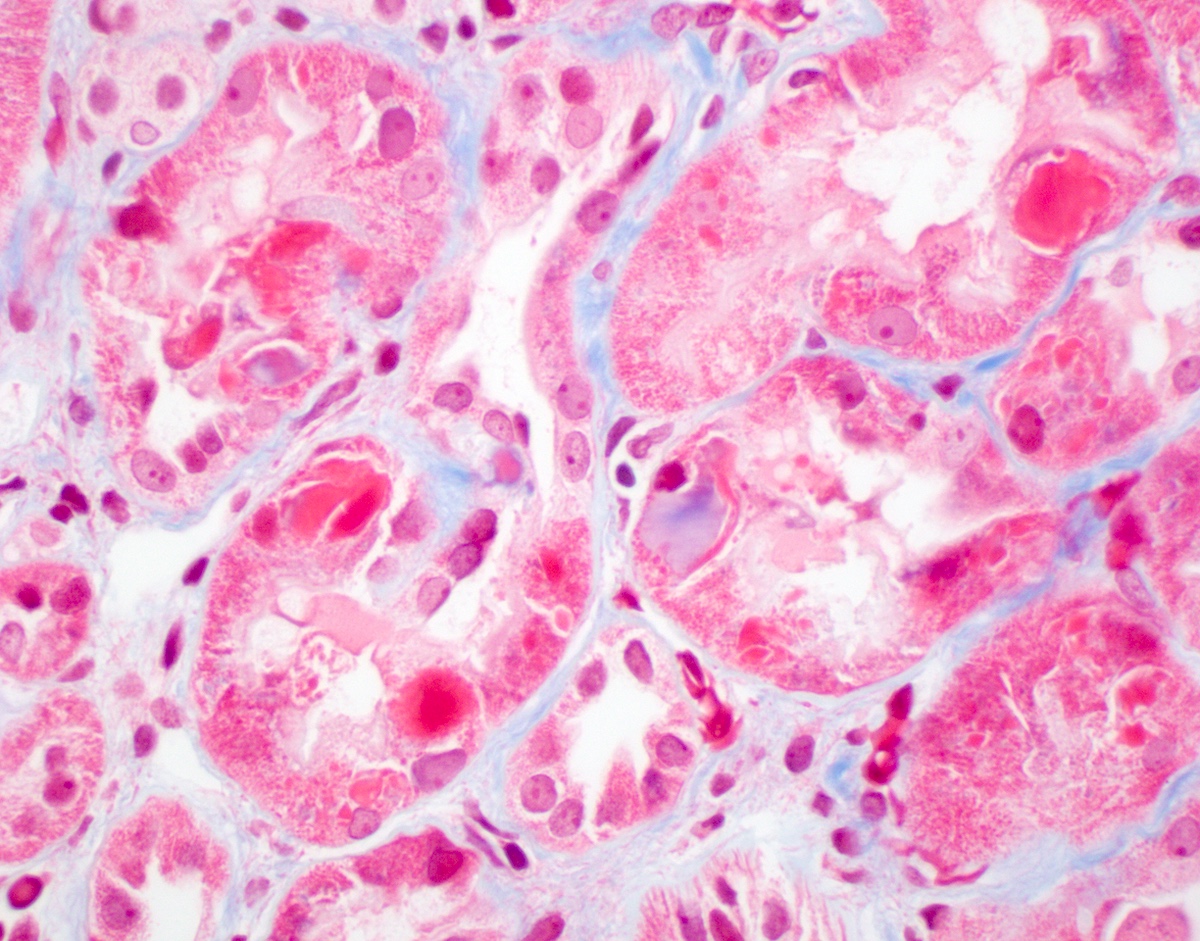
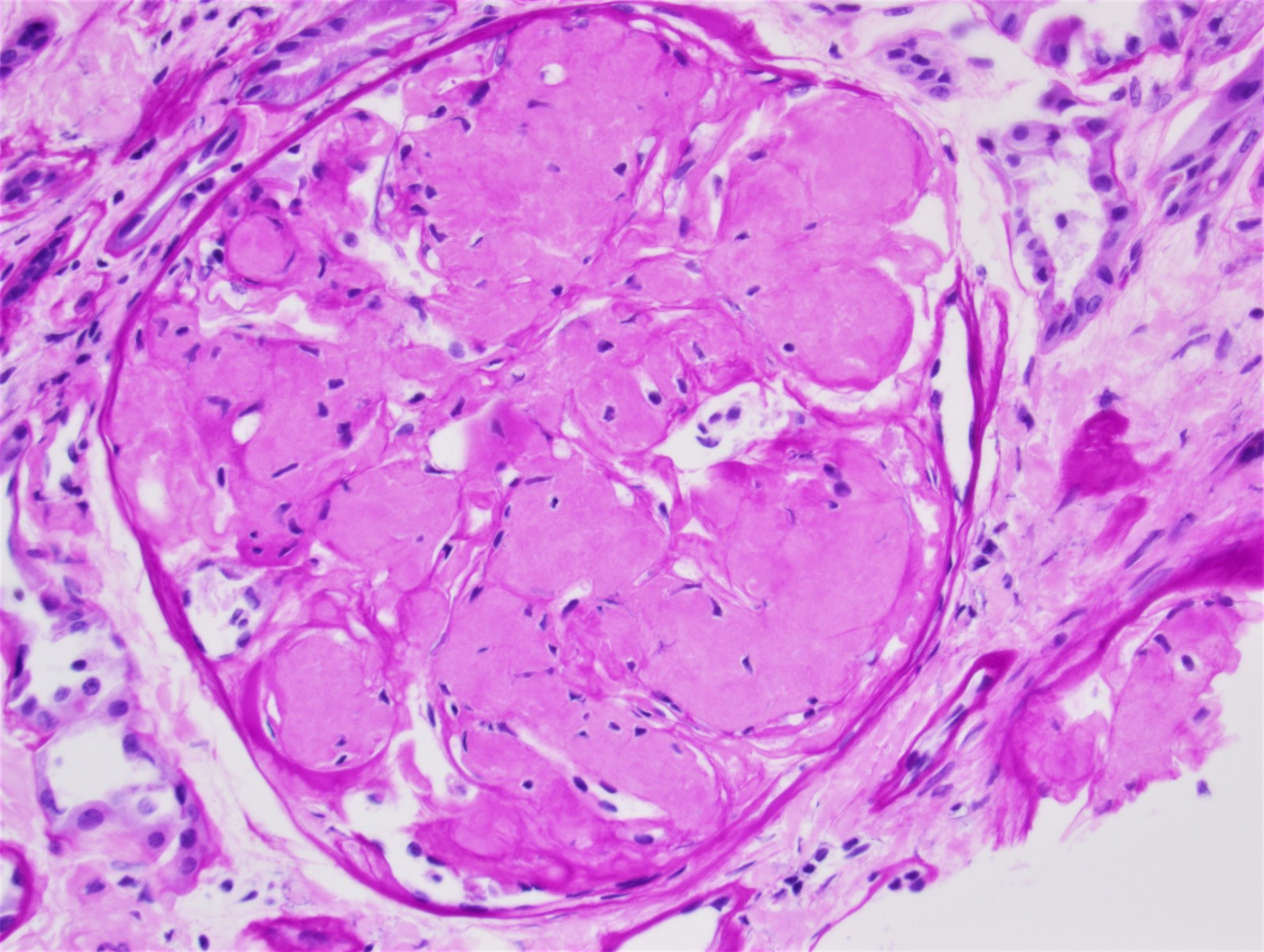
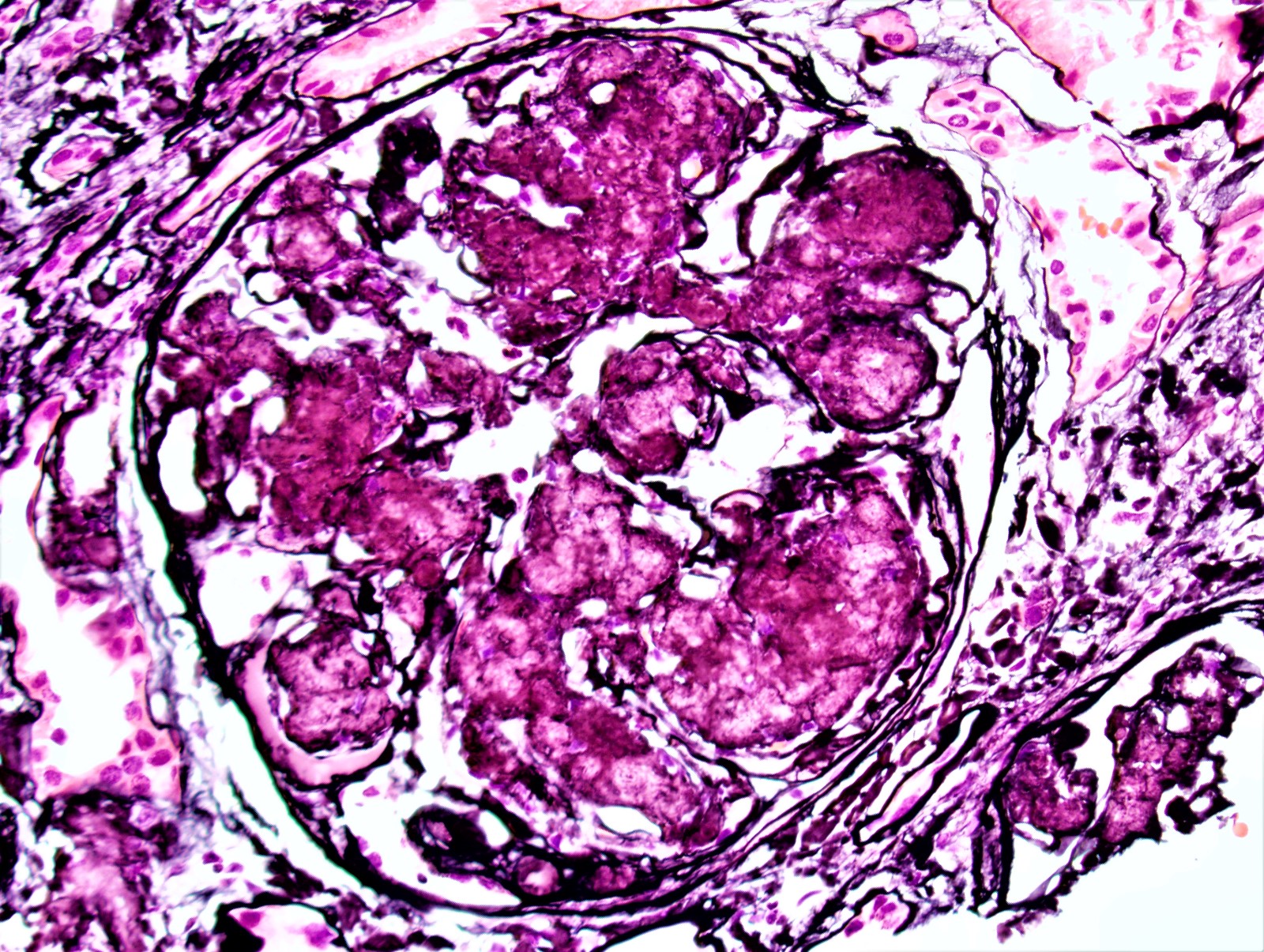
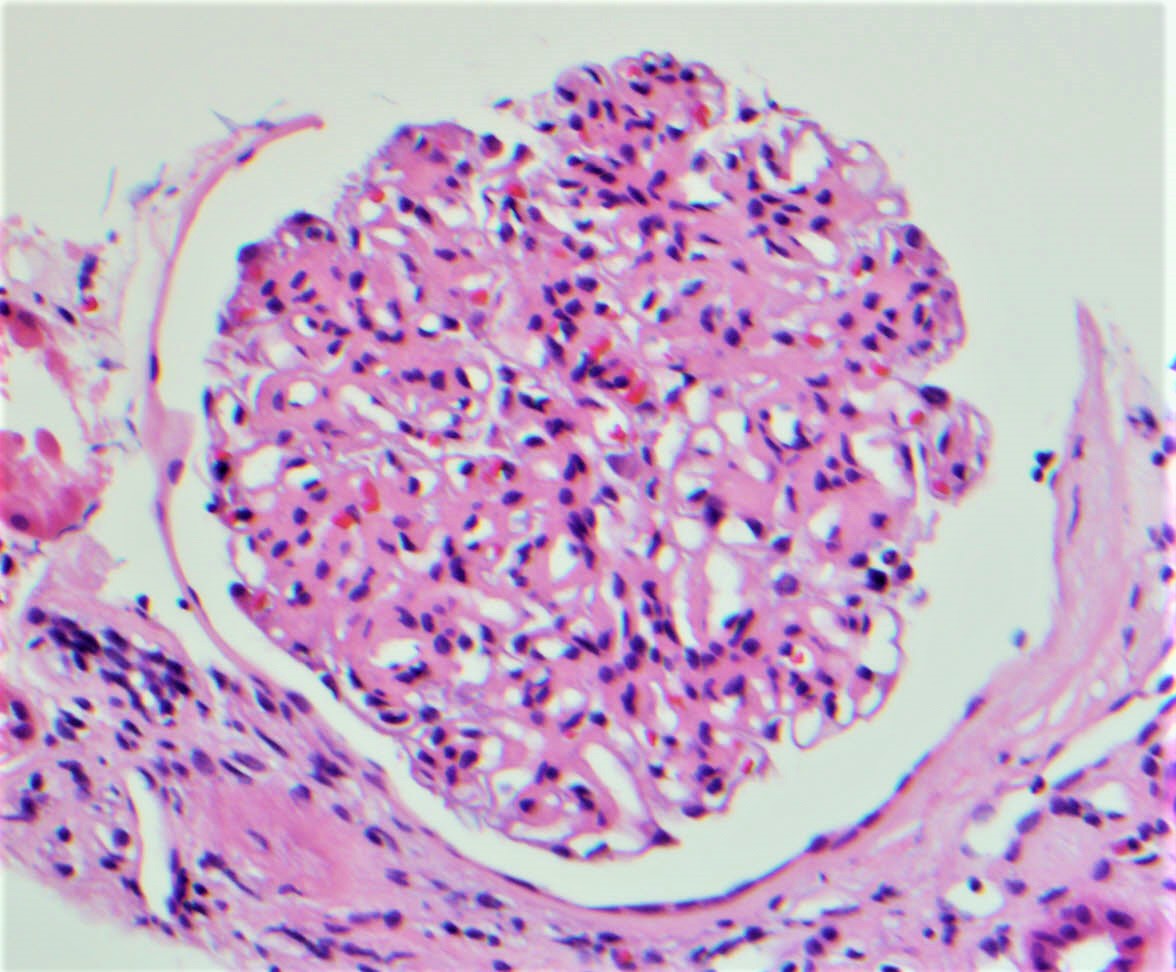
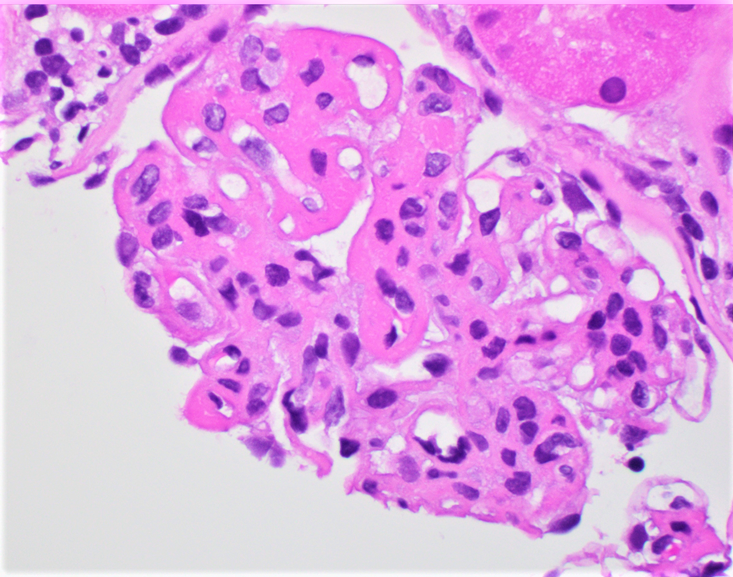
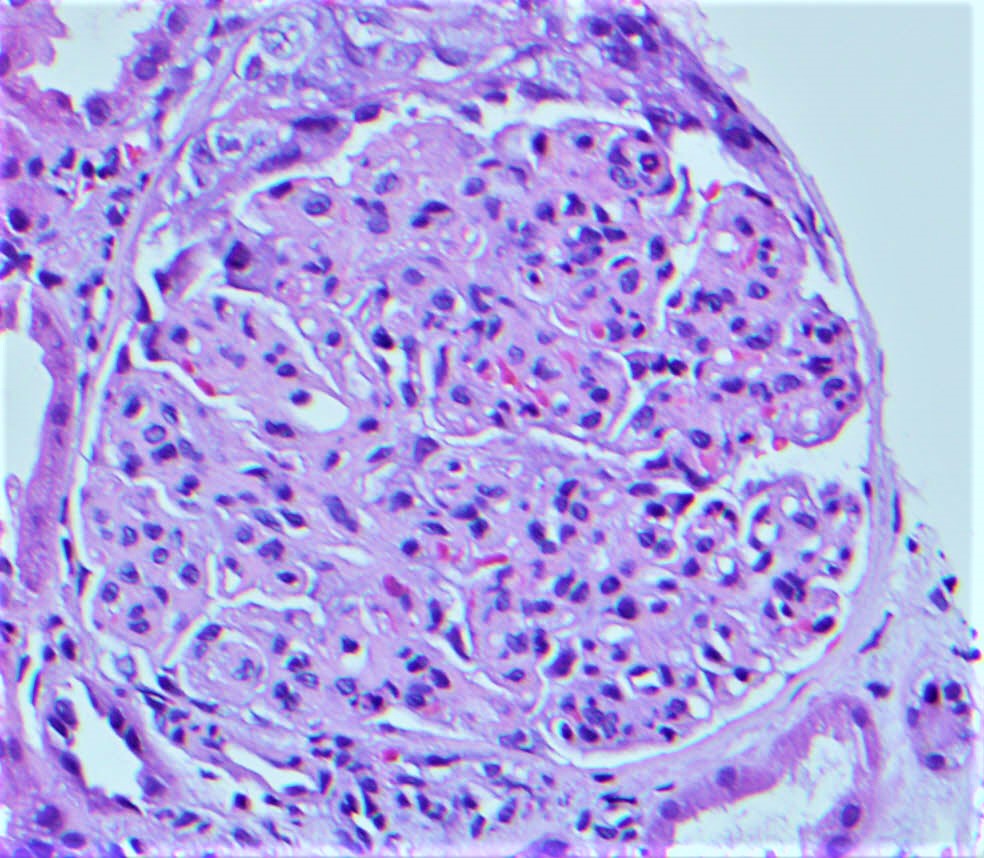
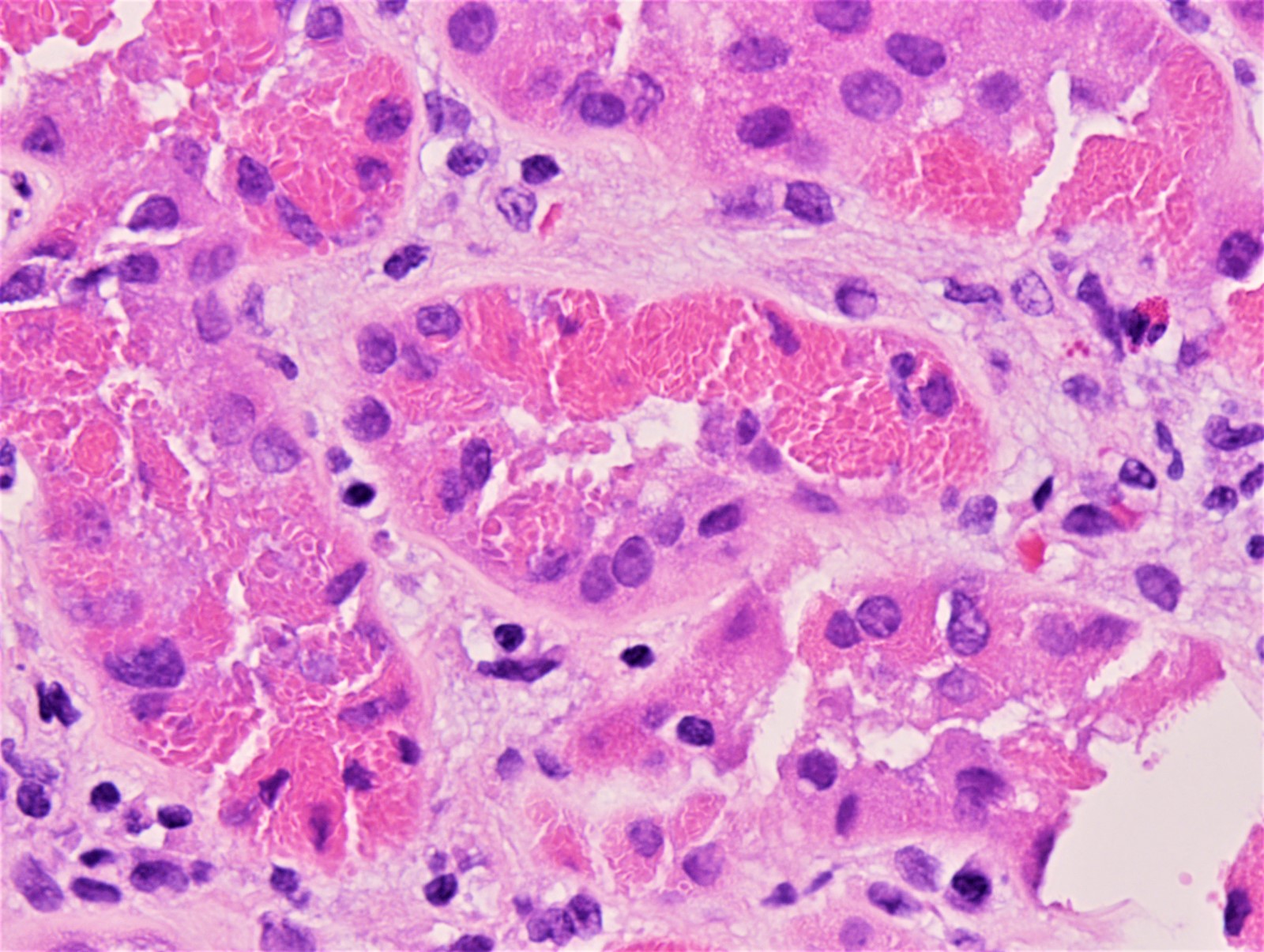
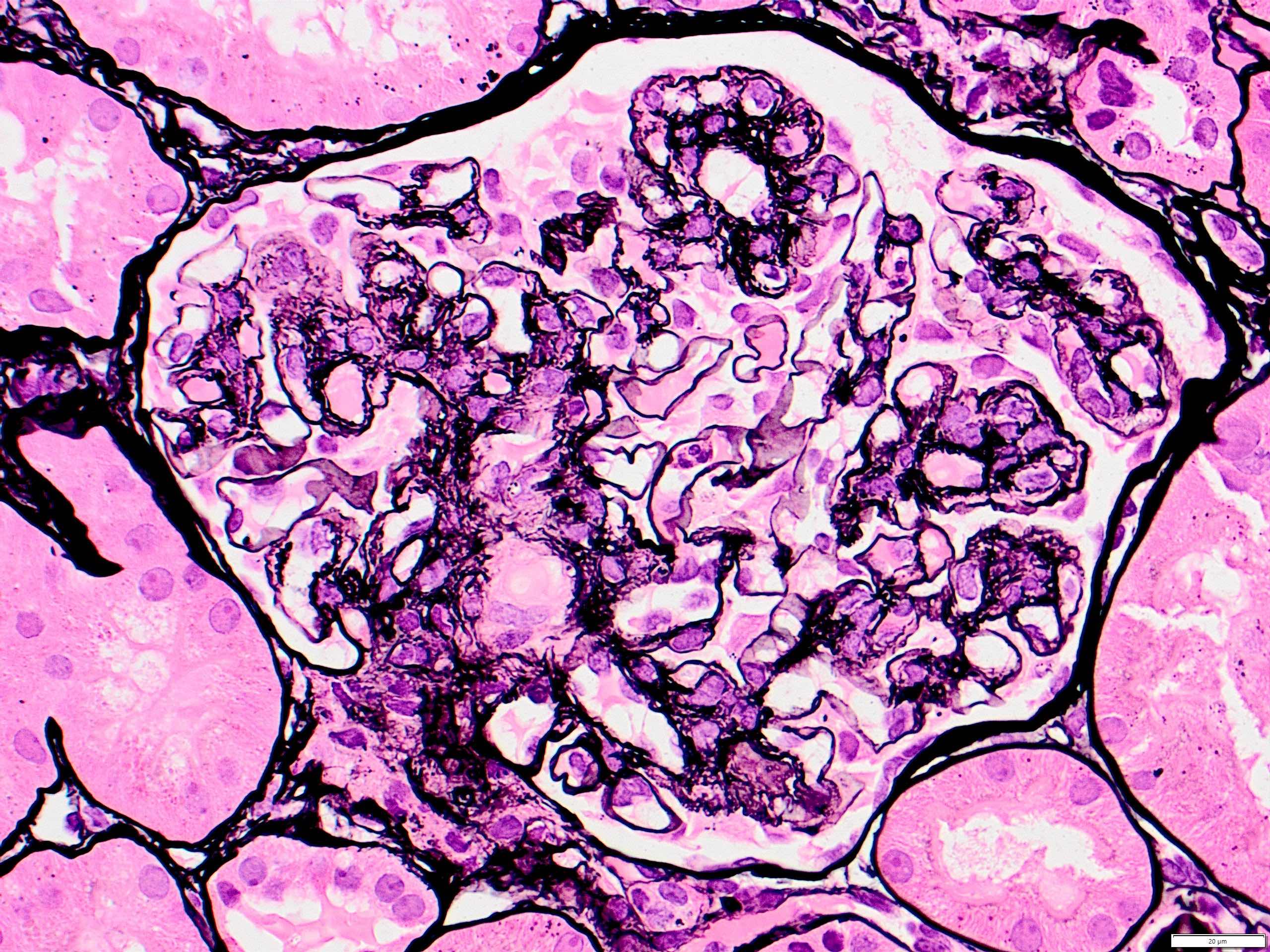
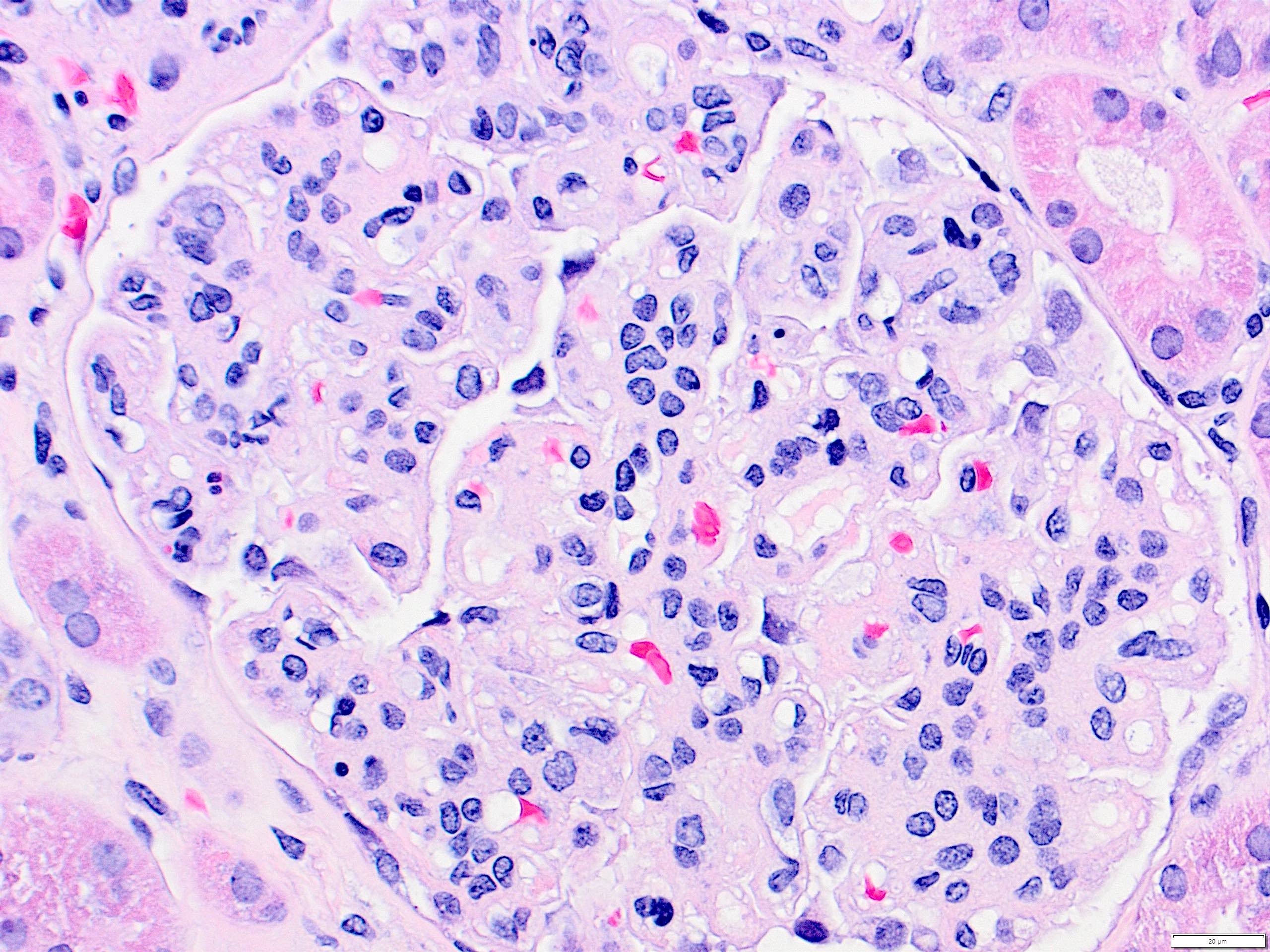
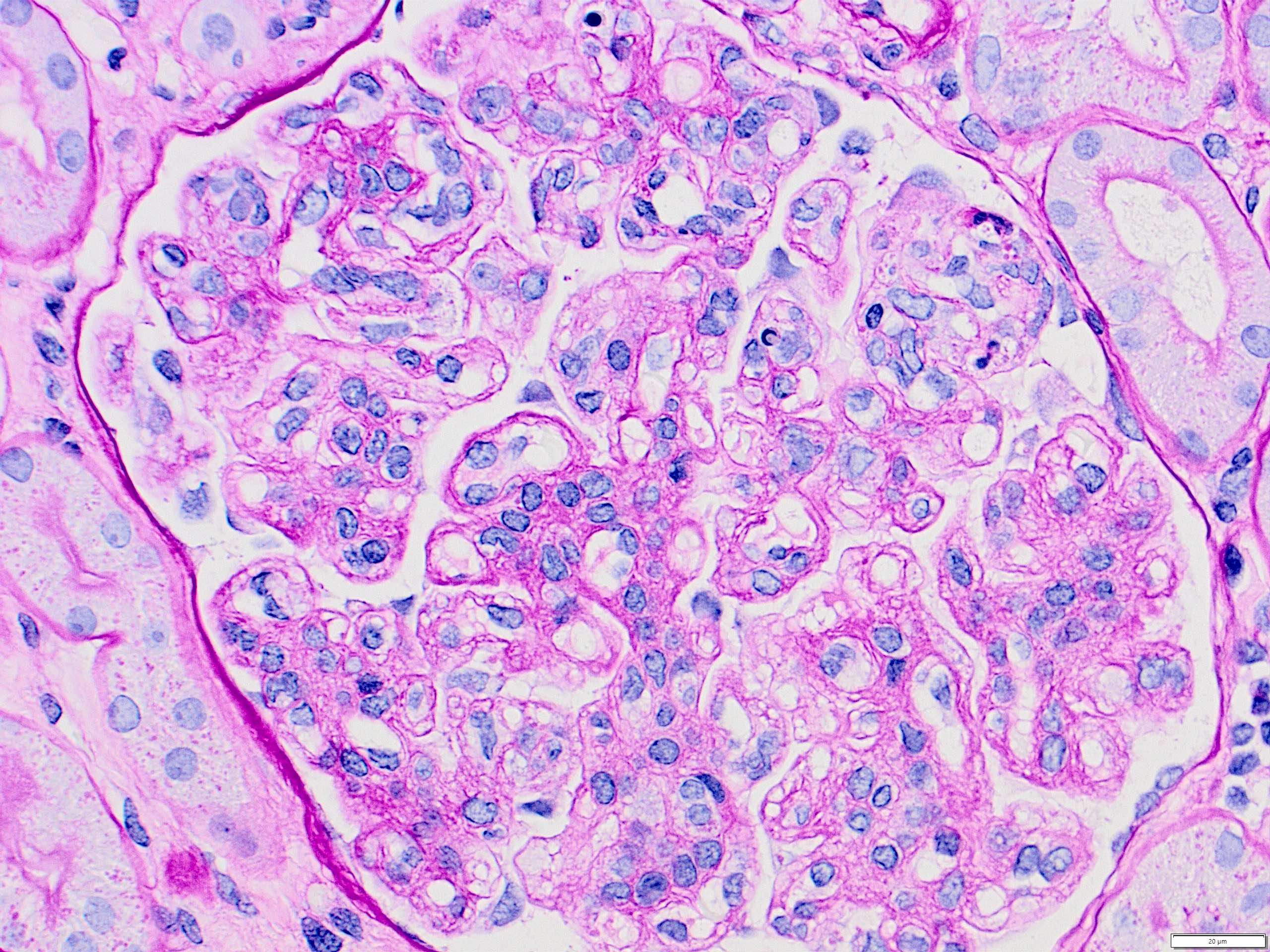
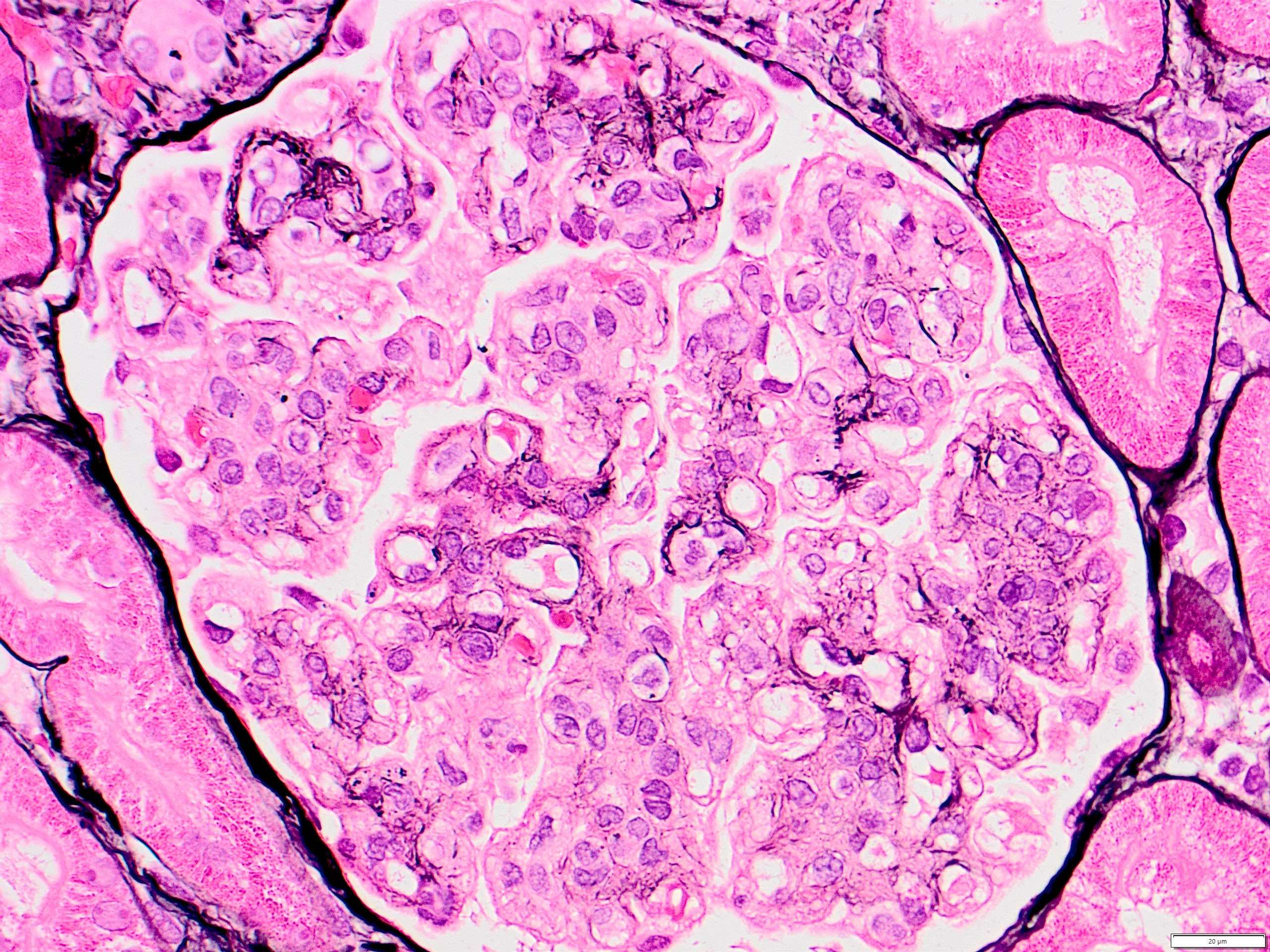
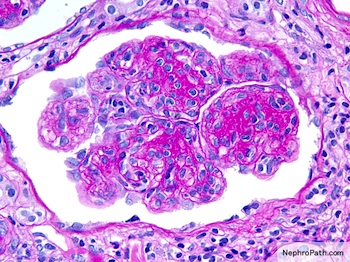
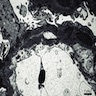
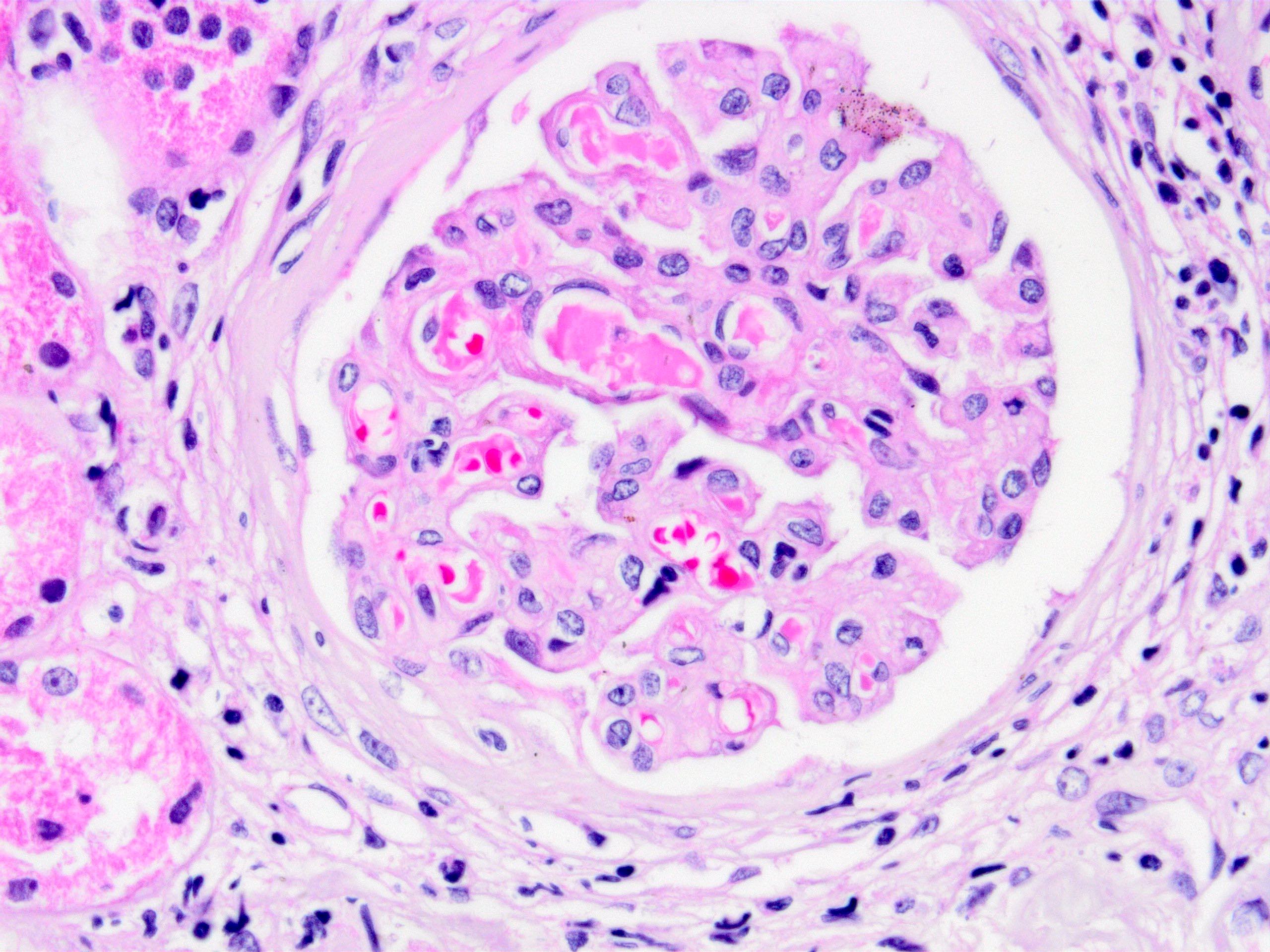
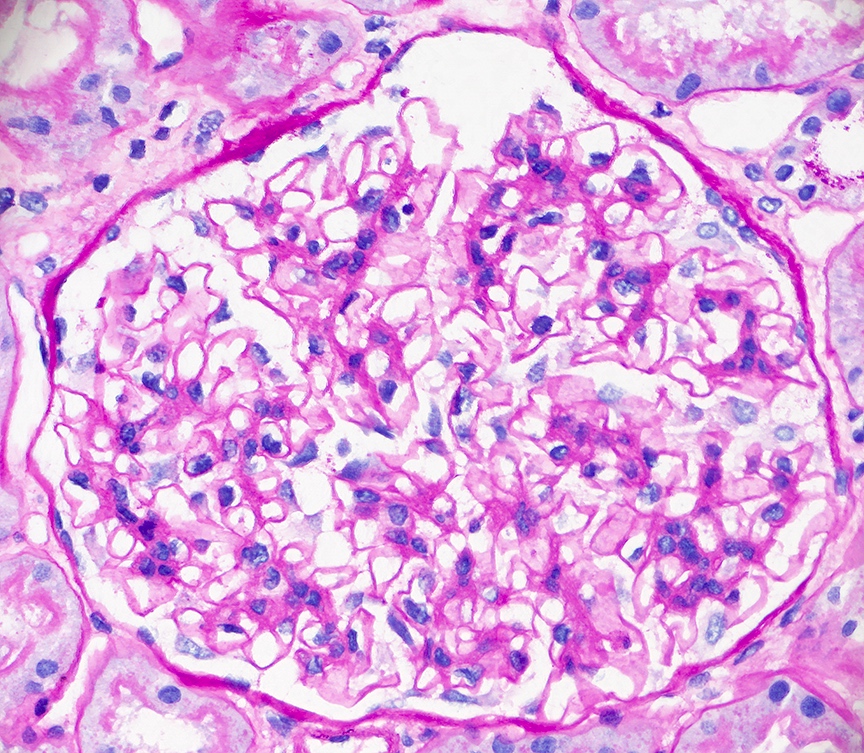
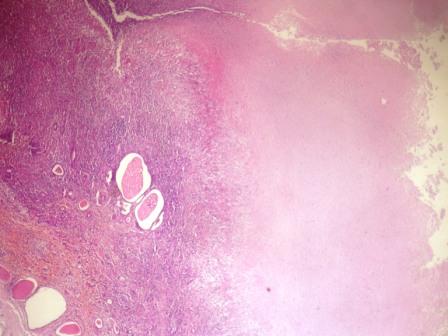
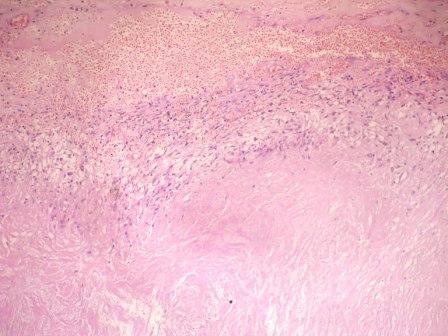
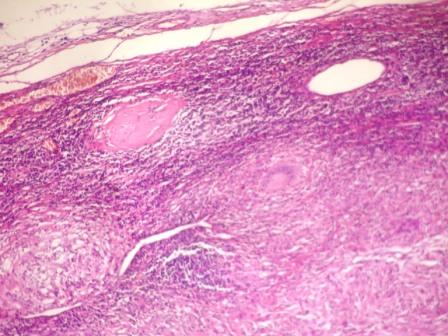
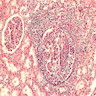
- Glomeruli are diffusely, segmentally or globally enlarged with endocapillary and mesangial hypercellularity resulting in a lobular configuration
- Neutrophilic (hence, exudative glomerulonephritis) and later mononuclear infiltration of glomeruli
- Proliferation of mesangial cells and endothelial cells with mesangial edema and inflammatory cells obstructing the capillary lumina
- Capillary basement membranes are of normal thickness
- Glomerular fibrinoid necrosis, microthrombosis and cellular crescents are rare but can be seen in the setting of rapidly progressive glomerulonephritis (RPGN) (Paediatr Int Child Health 2017;37:240)
- In the later stages of the disease, the glomerular lesions progressively lose neutrophils and the endocapillary hypercellularity evolves into pure mesangial hypercellularity
- Mild mesangial cellularity and matrix expansion may persist for several years, which eventually resolves to leave a normal appearing glomerulus
- Interstitial and tubular leukocyte infiltrate can be prominent
- Tubules contain red blood cells, sometimes mixed with eosinophilic cast-like material
- References: Jennette: Heptinstall's Pathology of the Kidney, 2 Volume Set Edition, 2014, Fogo: Diagnostic Atlas of Renal Pathology, 3rd Edition, 2016
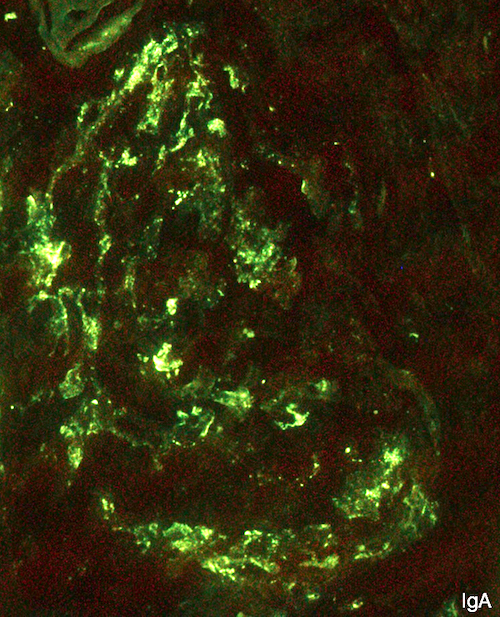
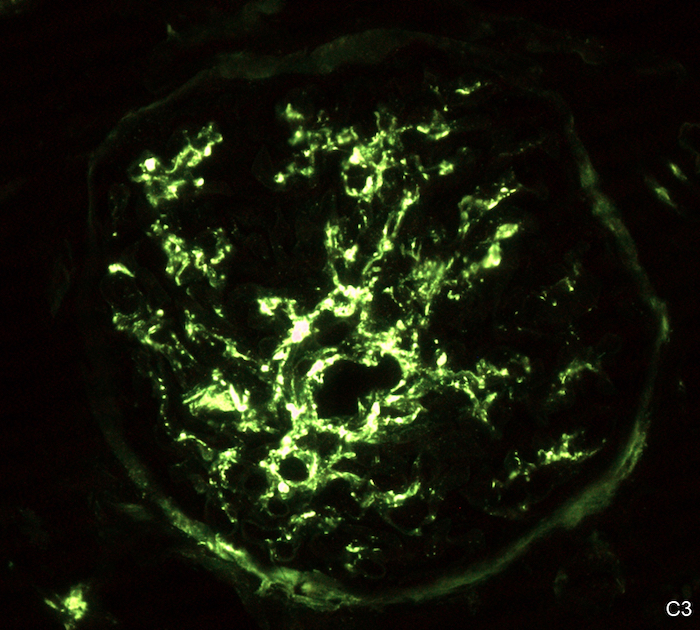
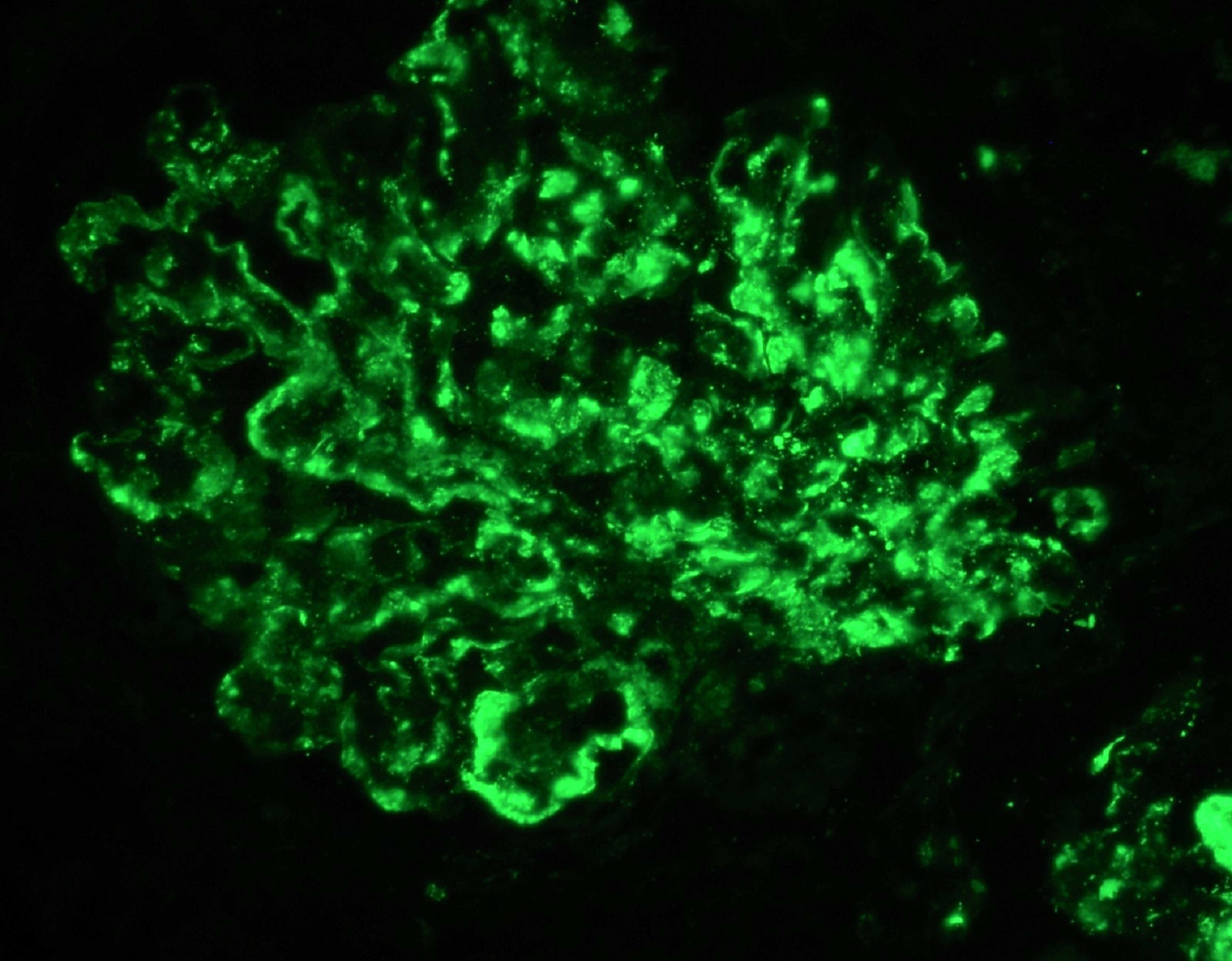
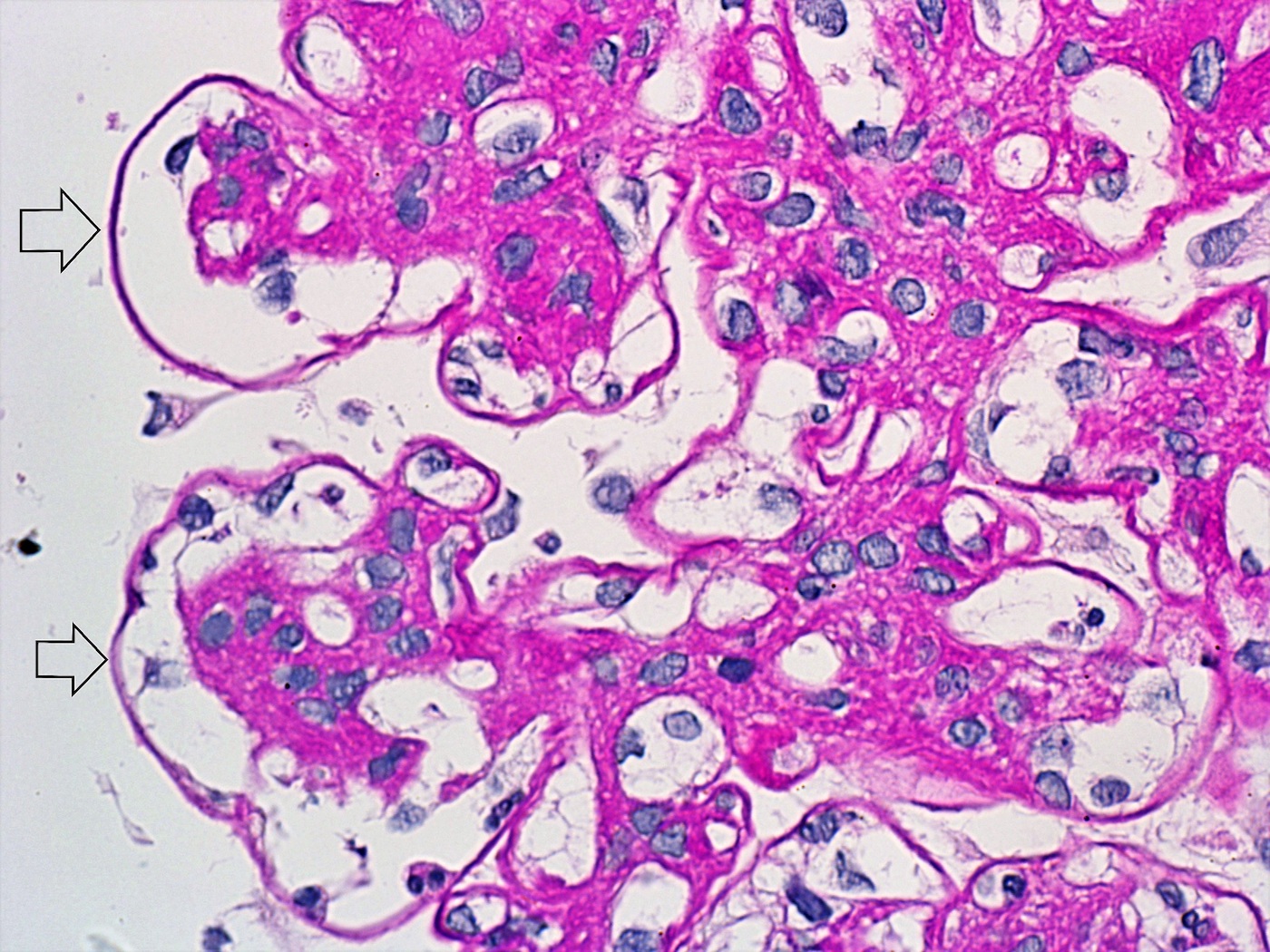
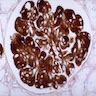
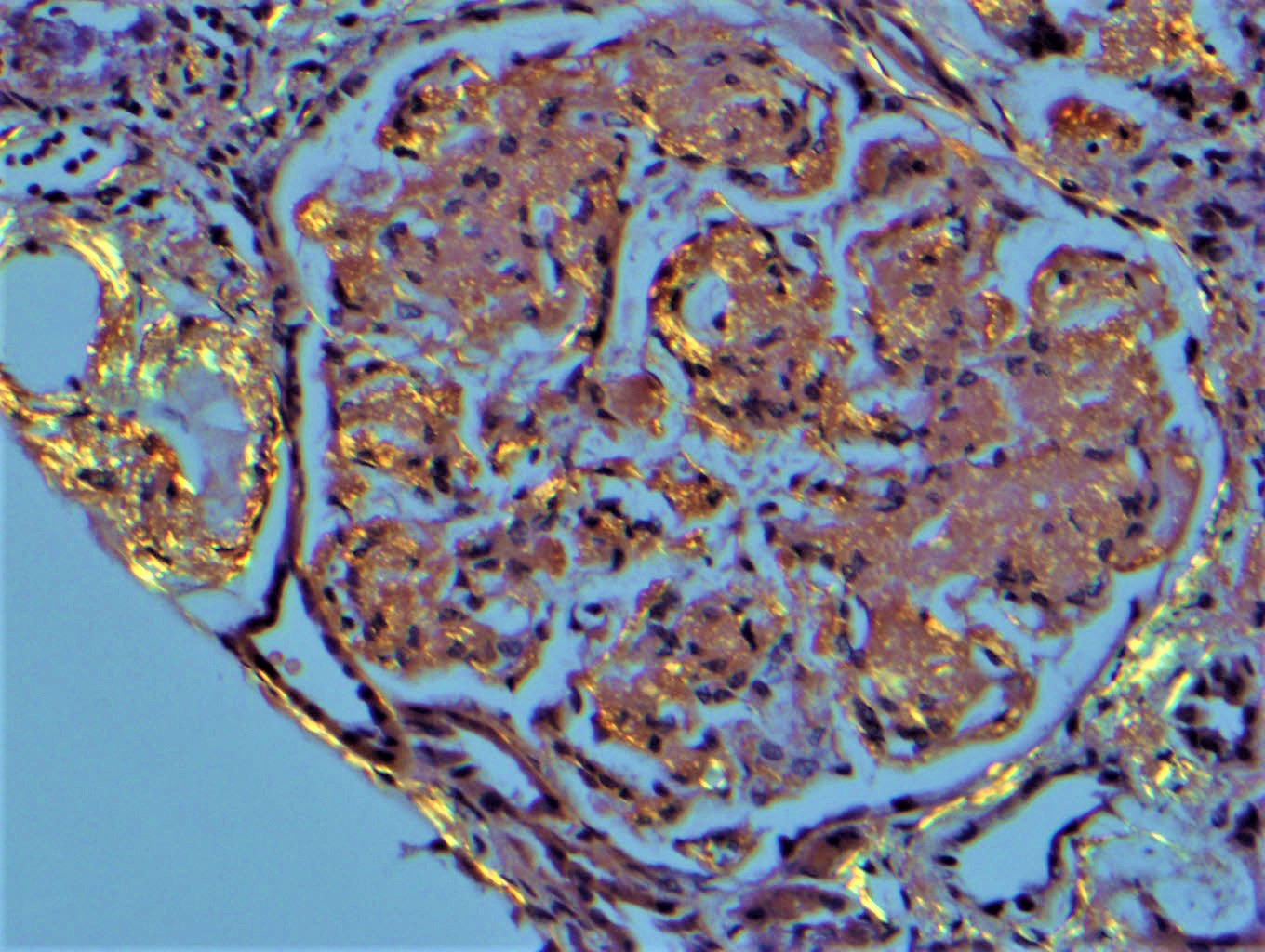
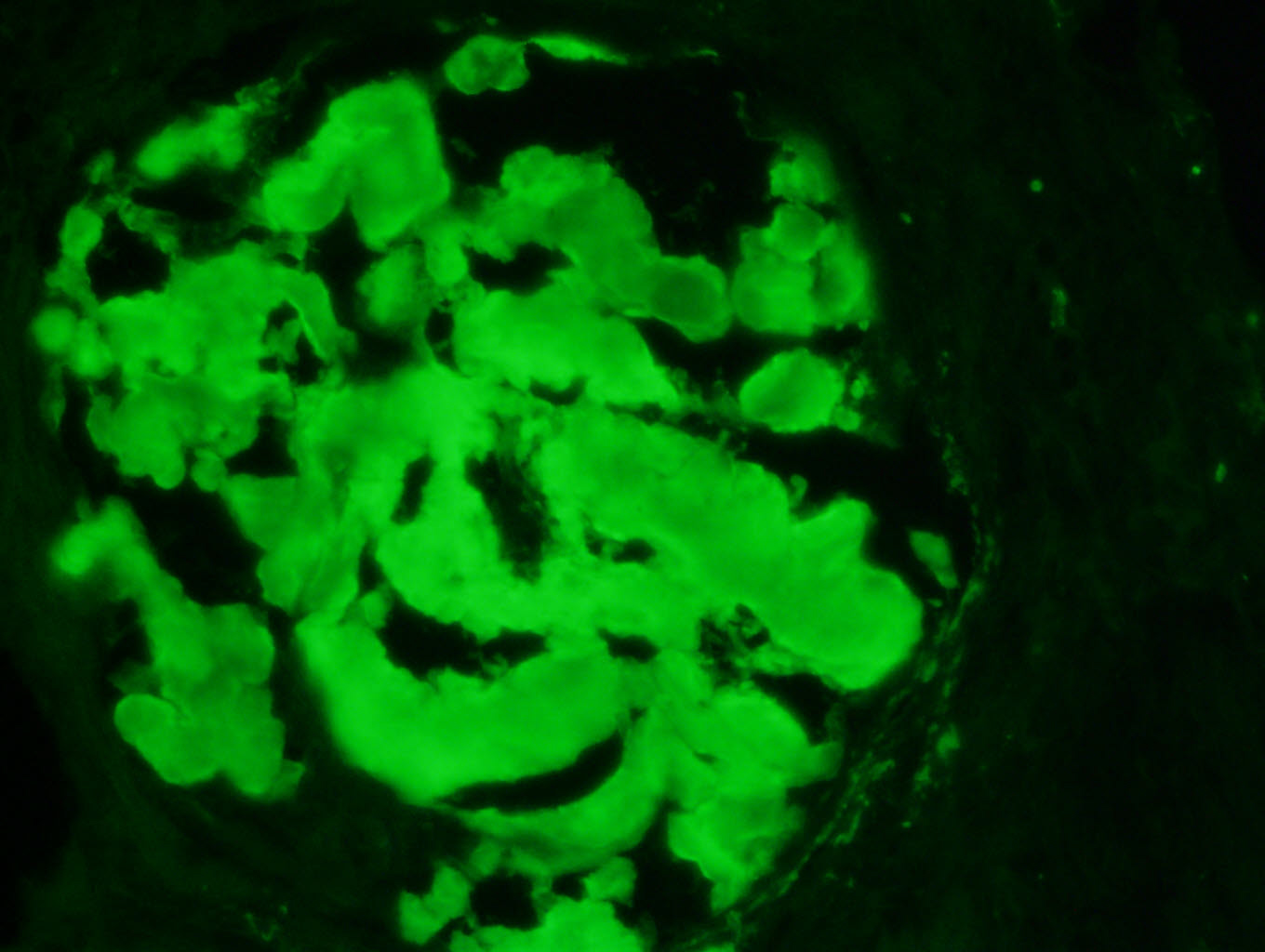
- Small, irregular, coarse granular (lumpy bumpy) deposits of polyclonal IgG and C3 along the glomerular capillary walls with scattered mesangial deposits
- IgM and IgA staining is absent or minimal except in cases caused by Staphylococcus infection where IgA may be predominant
- C1q or C4 are absent
- Properdin may be present
- Starry sky, garland and mesangial patterns are typically described for poststreptococcal glomerulonephritis
- References: Jennette: Heptinstall's Pathology of the Kidney, 2 Volume Set Edition, 2014, Fogo: Diagnostic Atlas of Renal Pathology, 3rd Edition, 2016
- PAS, Jones methenamine silver and trichrome are used to evaluate the morphology
- Endocapillary hypercellularity, frequent neutrophils in the glomerular tuft
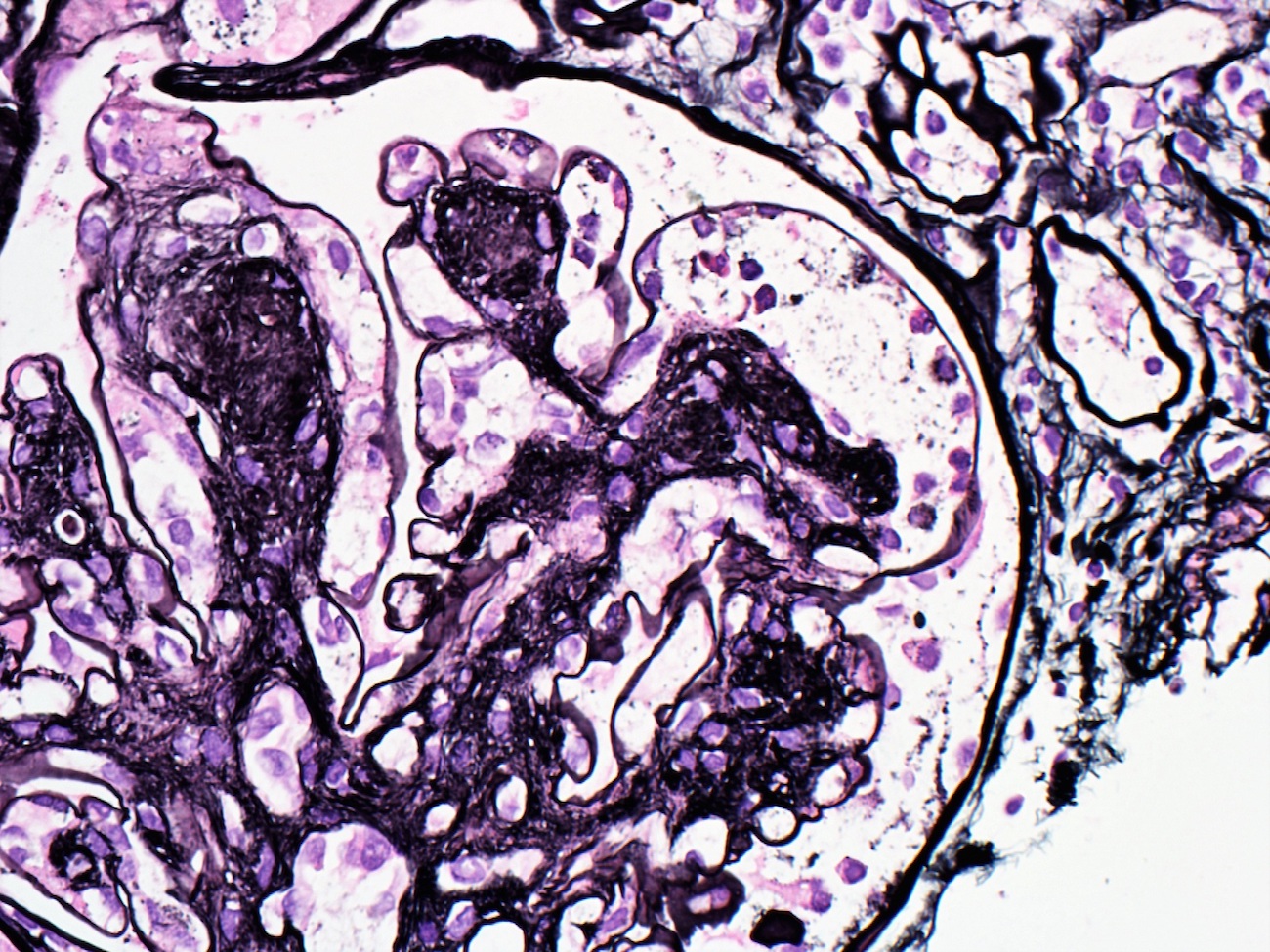
- There may be occasional mesangial and small subendothelial deposits but the most consistent diagnostic change is hump type, large, subepithelial deposits without significant glomerular basement membrane reaction
- In chronic phase of the disease, mesangial deposits predominate, usually with less endocapillary proliferation
- References: Jennette: Heptinstall's Pathology of the Kidney, 2 Volume Set Edition, 2014, Fogo: Diagnostic Atlas of Renal Pathology, 3rd Edition, 2016
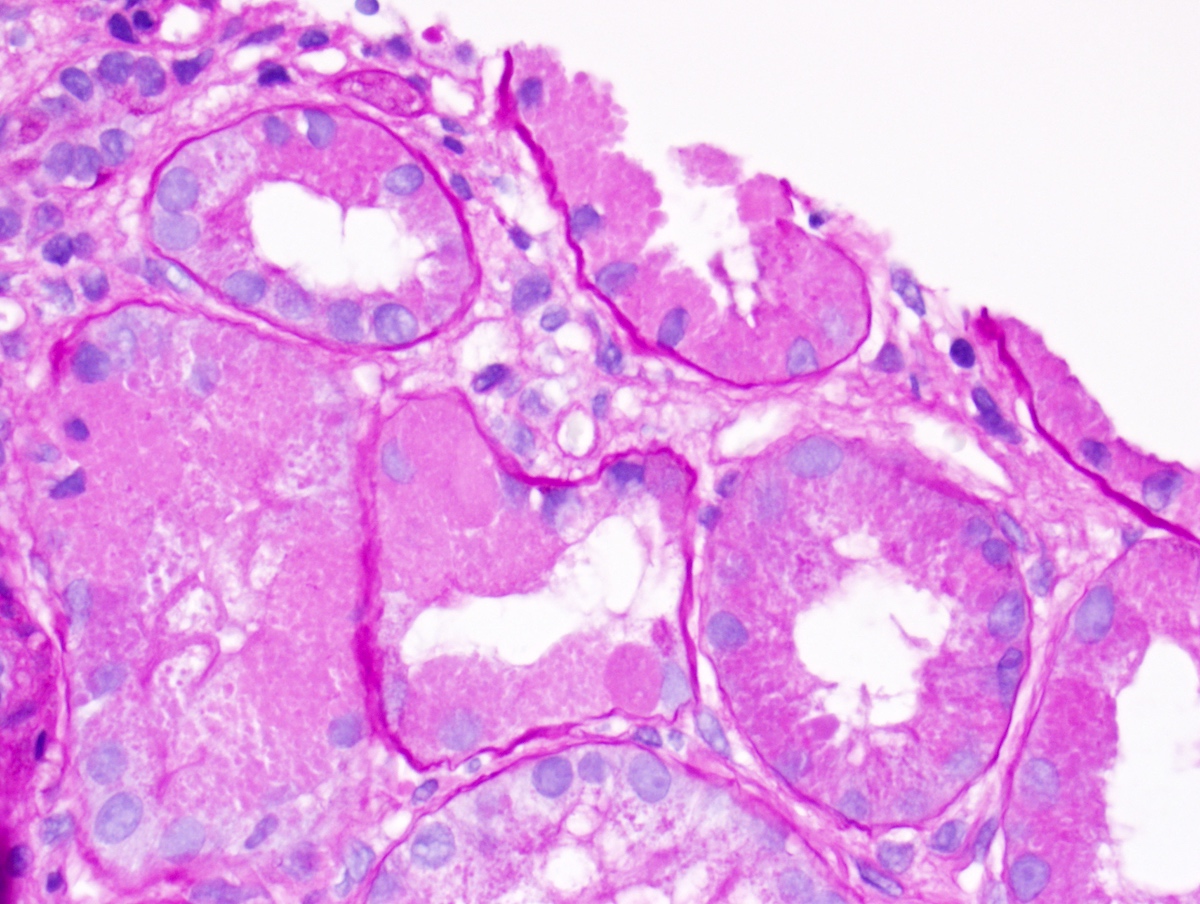
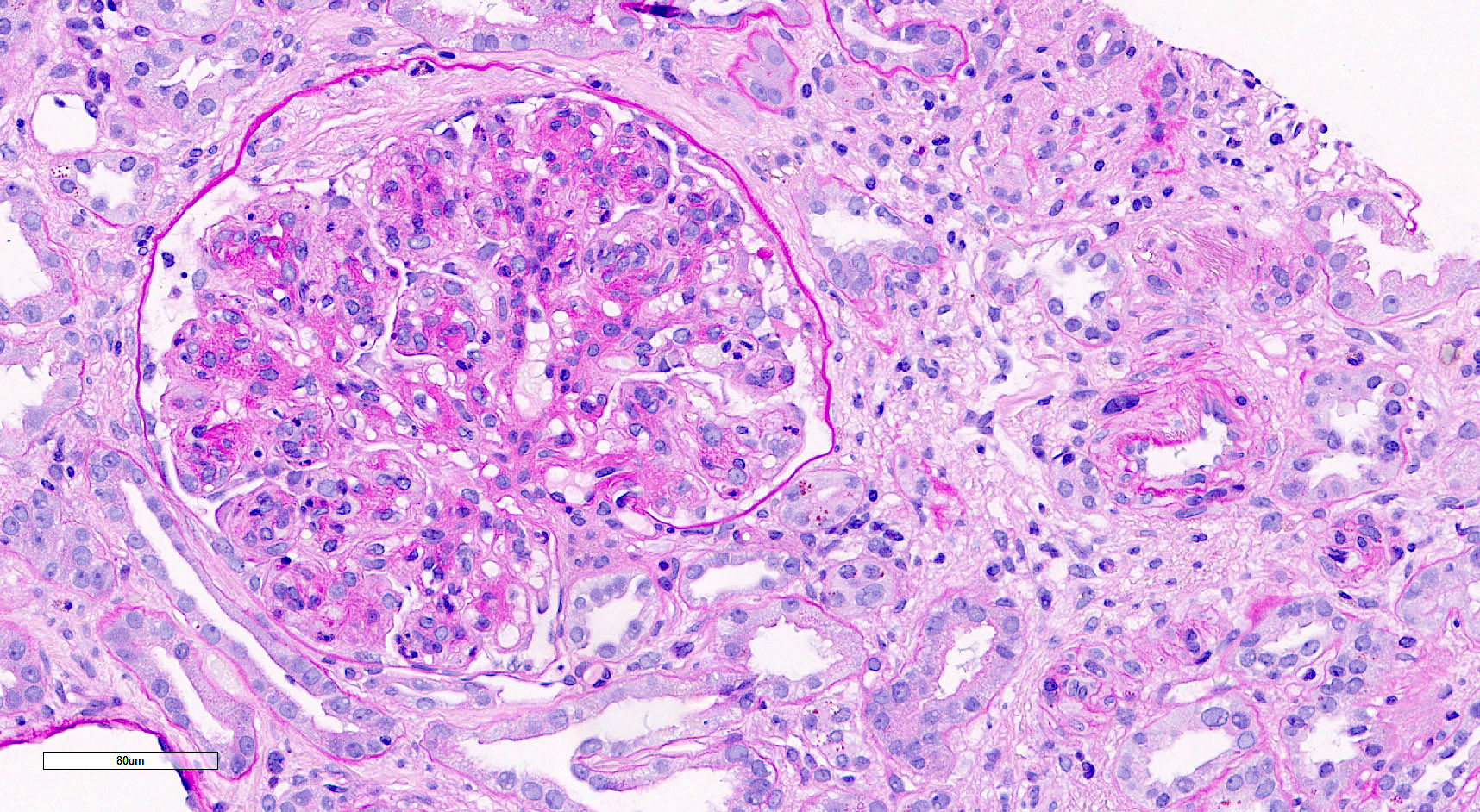
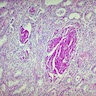
- Right kidney, biopsy:
- Acute postinfectious glomerulonephritis
- Adequacy: adequate (2 cores with 80% cortex and 20% medulla)
- Microscopic description: 16 glomeruli are seen with one exhibiting global sclerosis and the rest showing diffuse, global mesangial and endocapillary hypercellularity with numerous neutrophils. The surrounding tubulointerstitium shows mild inflammation with no significant tubular atrophy or interstitial fibrosis. The vessels are unremarkable with no evidence of vasculitis.
- Immunofluorescence microscopy: IgG and C3 show coarse granular positivity in mesangium and along glomerular basement membrane.
- Electron microscopy: Prominent subepithelial hump type deposits with rare intramembranous and subendothelial deposits.
- Lupus nephritis:
- Postinfectious glomerulonephritis may have dominant C3 positivity on immunofluorescence but there is usually absence of C1q deposits
- Lupus nephritis usually shows full house staining by immunofluorescence
- Tubuloreticular inclusions (reticular aggregates) in endothelial cells on electron microscopy
- C3 glomerulonephritis:
- More likely to have membranoproliferative glomerulonephritis (MPGN) pattern on histology
- Predominant C3 deposition with scant immunoglobulin on immunofluorescence
- Rare or no deposits on electron microscopy
- Not necessarily preceded by infection
- It has a poor outcome
- It is associated with complement alternative pathway dysregulation
- PIGN may be indistinguishable histologically from C3 glomerulonephritis (including exudative glomerulonephritis pattern and subepithelial humps by electron microscopy) and careful clinical correlation is required, especially if renal abnormalities do not resolve as expected
- Dense deposit disease:
- Membranoproliferative or mesangial proliferative features by light microscopy
- C3 only or C3 dominant deposits by immunofluorescence
- Characteristic dense deposits by electron microscopy
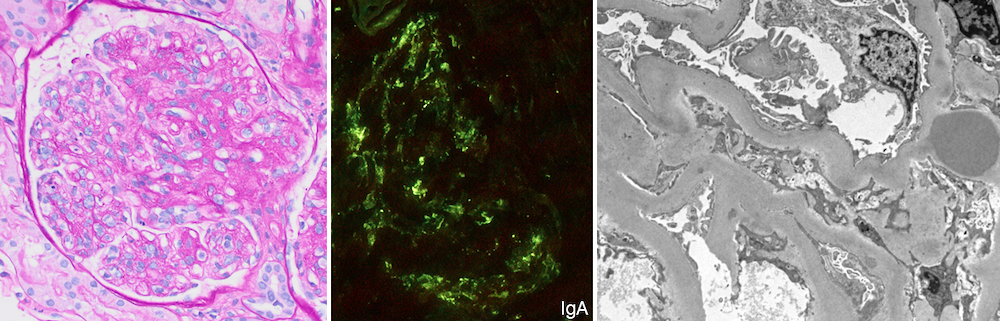
- IgA nephropathy:
- Lack of polymorphonuclear neutrophils, IgA dominant (or codominant) deposits and lambda > kappa on immunofluorescence and presence of mesangial and occasional subendothelial deposits on electron microscopy favors IgA nephropathy (Kidney Int Rep 2022;7:2462)
- In comparison, presence of polymorphonuclear neutrophils, dominant C3 and kappa > lambda on immunofluorescence, hump shaped deposits on electron microscopy favors IgA dominant postinfectious glomerulonephritis
- Staphylococcus infection associated glomerulonephritis (SAGN) often shows C3 > IgA, typically fewer focal segmental glomerulosclerosis (FSGS) lesions (if any) and more endocapillary hypercellularity as compared to IgA nephropathy (Nat Rev Nephrol 2020;16:32)
- IgA deposits in sclerotic glomeruli can be helpful in differentiating IgAN from IgA dominant infection related glomerulonephritis (Kidney Int Rep 2020;5:909)
- Cryoglobulinemic glomerulonephritis:
- Shows PAS+ cryoplugs on light microscopy
- Shows IgM dominance of deposits on immunofluorescence
- May have monoclonal component with either kappa or lambda staining on immunofluorescence
- Effacement of foot processes
- Mesangial deposits
- Reticular aggregates
- Subendothelial deposits
- Subepithelial hump shaped deposits
Comment Here
Reference: Acute postinfectious glomerulonephritis
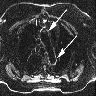
A 12 year old boy presented with history of a sore throat followed by hematuria. Renal biopsy revealed diffuse proliferative pattern and immunofluorescence showed coarse granular deposits in mesangium and along capillary loops for IgG and C3. Which serological test would support the diagnosis?
- Antidouble stranded DNA
- Anti-GBM antibodies
- Antimitochondrial antibodies
- Antinuclear antibodies
- Antistreptolysin O antibodies
Comment Here
Reference: Acute postinfectious glomerulonephritis
- Acute suppurative (pus forming) infection of kidney collecting system as well as renal parenchyma
- Affects infants and young children with congenital lesions, women of childbearing age, men and women age 60+ years (due to nodular hyperplasia of prostate, cystoceles in women, cervical carcinoma, nephrolithiasis)
- Also associated with diabetes or immunocompromise
- Acute suppurative (pus forming) infection of kidney collecting system as well as renal parenchyma
- E. coli is the most common uropathogen isolated from urine or pus cultures in almost 70% of cases (BJU Int 2011;107:1474)
- Early, mild cases treated with antibiotics and percutaneous drainage (PCD) have a good outcome
- Histology shows patchy suppurative inflammation, primarily cortical with edema, neutrophils in interstitium and tubular lumina and tubular necrosis; also cortical abscesses and necrosis
- Acute pyelonephritis, papillary necrosis, perinephric abscess, pyonephrosis, emphysematous pyelonephritis
- ICD-10: N10 - acute pyelonephritis
- More common in women and in patients with diabetes
- Kidney (left, right or bilateral)
- Impaired tissue perfusion and impaired immune response (J Urol 1994;151:125)
- E. coli is the most common uropathogen isolated from urine or pus cultures in almost 70% of cases (BJU Int 2011;107:1474)
- Women, diabetics and immunosuppressed individuals are high risk (Urol Case Rep 2020;33:101390)
- E. coli is the most common pathogen (BJU Int 2011;107:1474)
- Leukocytosis was seen in 70 - 80% of cases (BJU Int 2011;107:1474)
- Causes: urinary tract infection (UTI), instrumentation, obstruction, pregnancy (4 - 6% have bacteriuria, 20% have symptoms if untreated), vesicoureteral reflux
- Bacterial UTI: due to colonization of distal urethra and introitus by coliform bacteria with adhesins on P fimbriae (pili), plus upward spread via instrumentation or catheterization; more common in women than men due to short urethra, no antibacterial prostatic fluid, hormonal changes, sex related trauma
- Usually gram negative rods from fecal flora (E. coli, Enterobacter, Klebsiella, Proteus, Streptococcus faecalis) or staph
- Ascending route most common; also hematogenous spread of bacteria to kidney
- Intrarenal reflux: via open ducts at tips of papillae; most common at poles of kidney where papillae have flat or concave tips; demonstrate via voiding cystourethrogram (seen in 50% of children with UTI)
- Vesicoureteral reflux: due to short intravesical ureter, spinal cord injuries; some UTIs; independent risk factor for renal scar formation after acute pyelonephritis in infants (J Urol 2012;187:1032)
- Signs / symptoms: sudden onset of costovertebral angle pain, symptoms of systemic infection or urinary tract infection, pyuria or white blood cell casts
- Best imaging study for diagnosis is CT of kidneys, ureters and bladder (Arch Intern Med 2000;160:797)
- Bacterial culture of urine and renal pelvis most commonly positive for E. coli
- Papillary necrosis: more common with diabetes and urinary tract obstruction; usually bilateral; variable number of pyramids involved; coagulative necrosis of tubules; usually limited white blood cell response
- Perinephric abscess: extension of pus through renal capsule into adjacent tissue
- Pyonephrosis: total or almost complete obstruction prevents drainage of pus
- Emphysematous pyelonephritis: gas in renal papillae, renal cortex, pararenal space and inferior vena cava; it is a medical emergency
- Early, mild cases treated with antibiotics and percutaneous drainage (PCD) have a good outcome
- In advanced cases, treatment with nephrectomy has the best outcome (Arch Intern Med 2000;160:797)
- Diabetes mellitus is a common risk factor for acute pyelonephritis; it is not associated with increased mortality (J Urol 2007;178:880)
- 36 year old woman with uncontrolled diabetes with emphysematous pyelonephritis (Urol Case Rep 2020;33:101390)
- 56 year old man with diabetes, emphysematous cystitis and bilateral emphysematous pyelonephritis (CEN Case Rep 2020;9:313)
- 64 year old man with diabetes and bilateral emphysematous pyelonephritis (Diabet Med 2001;18:68)
- 65 year old woman with hypertension and fever (Urol Case Rep 2020;31:101172)
- Man with undiagnosed diabetes and symptoms of pyelonephritis (Hippokratia 2013;17:373)
- Antibiotics usually eliminate symptoms in a few days (Am Fam Physician 2011;84:519, Lancet 2012;380:484)
- Persistent bacteriuria is usually associated with obstruction, diabetes and immunocompromise
- In emphysematous pyelonephritis, nephrectomy is usually the choice of treatment
- Focal abscesses or wedge shaped areas of suppuration
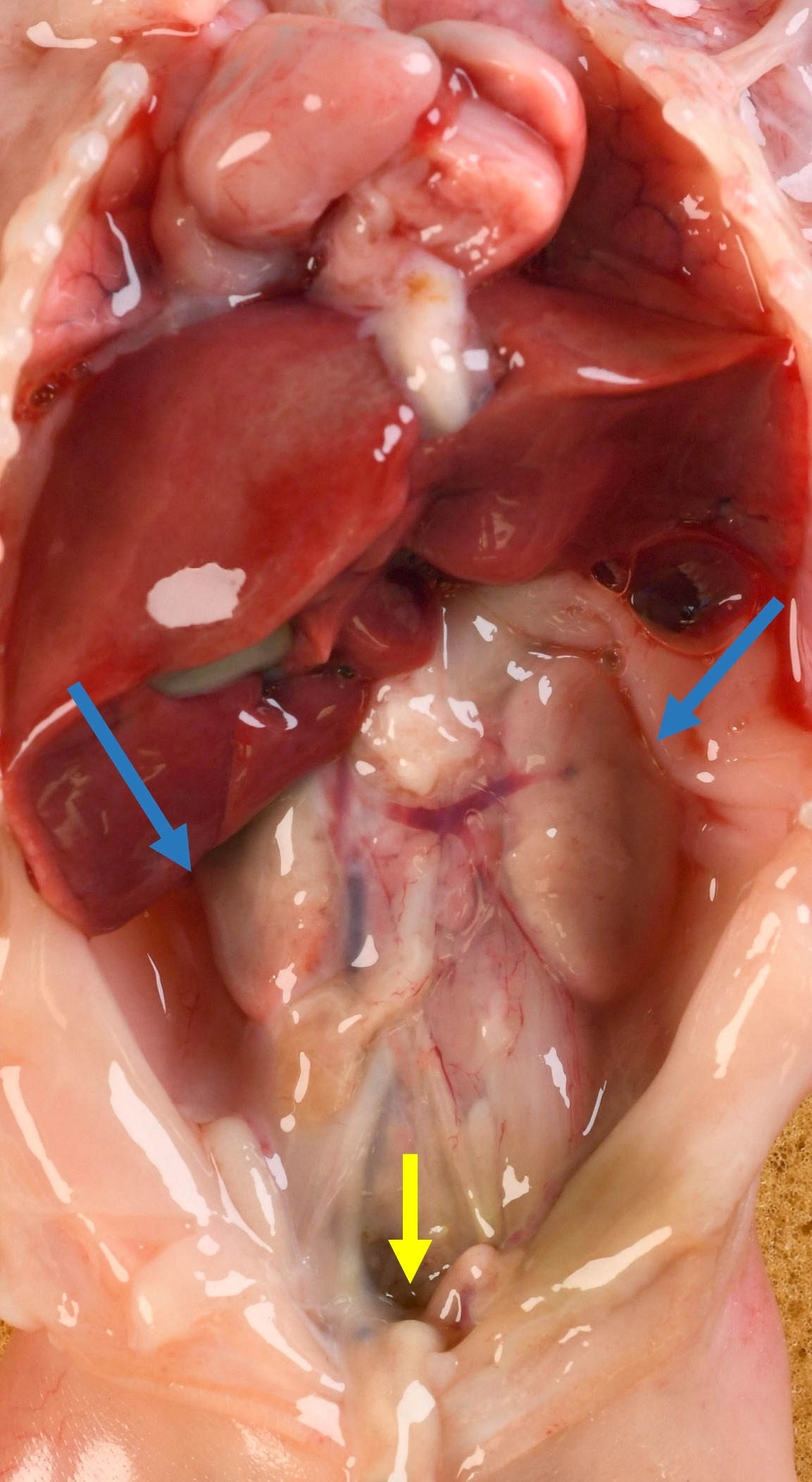
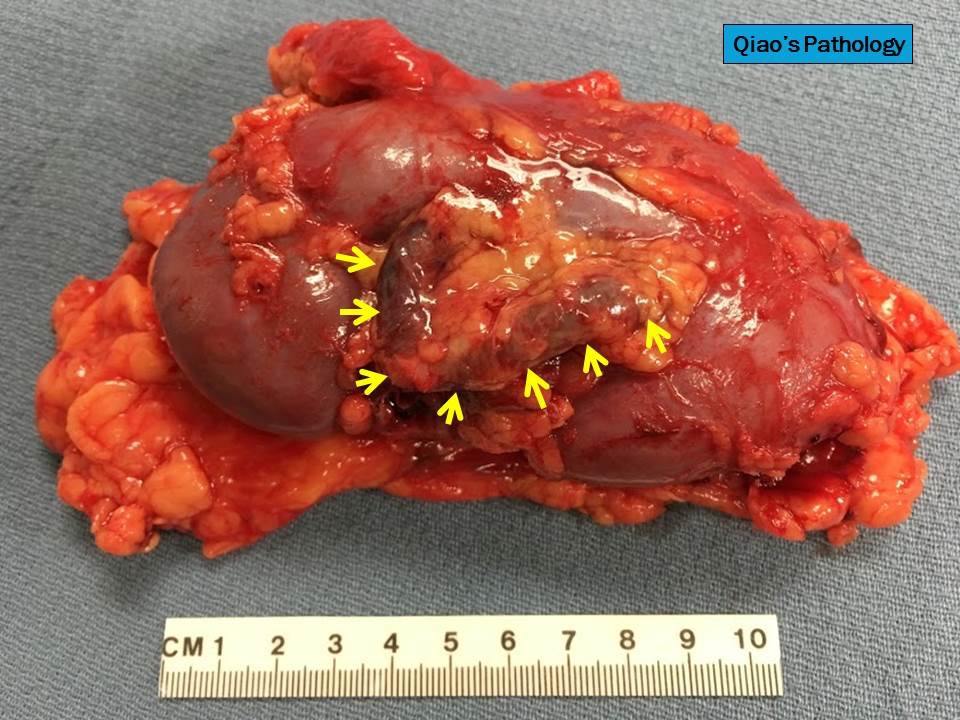
- In emphysematous pyelonephritis, the kidney is smaller than usual with hemorrhage, necrosis and crepitus on palpation; gas filled cysts are present in renal cortex and renal papillae; renal capsule is detached and filled with subcapsular blood / blood clots
- Reference: Medscape: Acute Pyelonephritis [Accessed 22 March 2021]
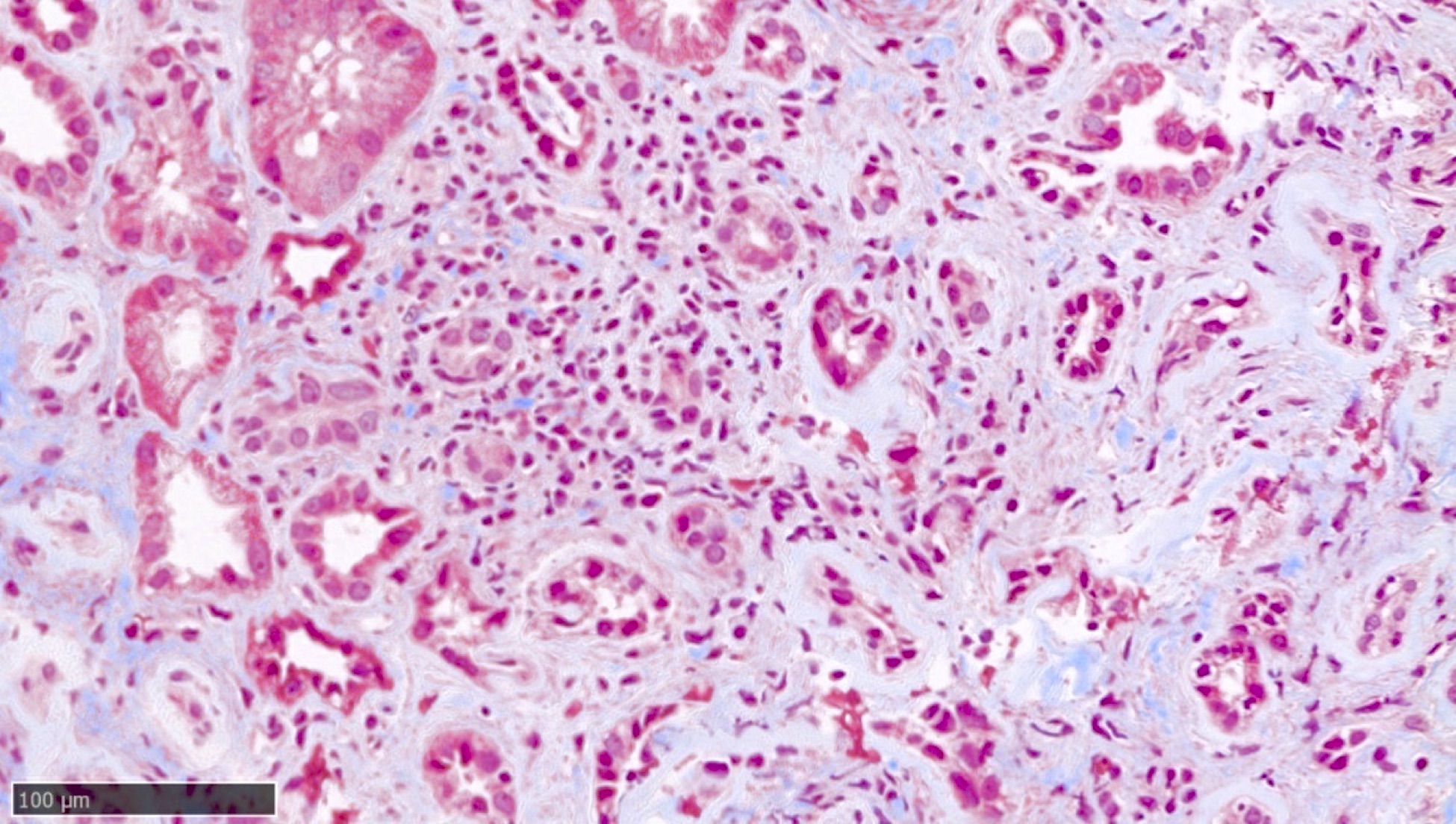
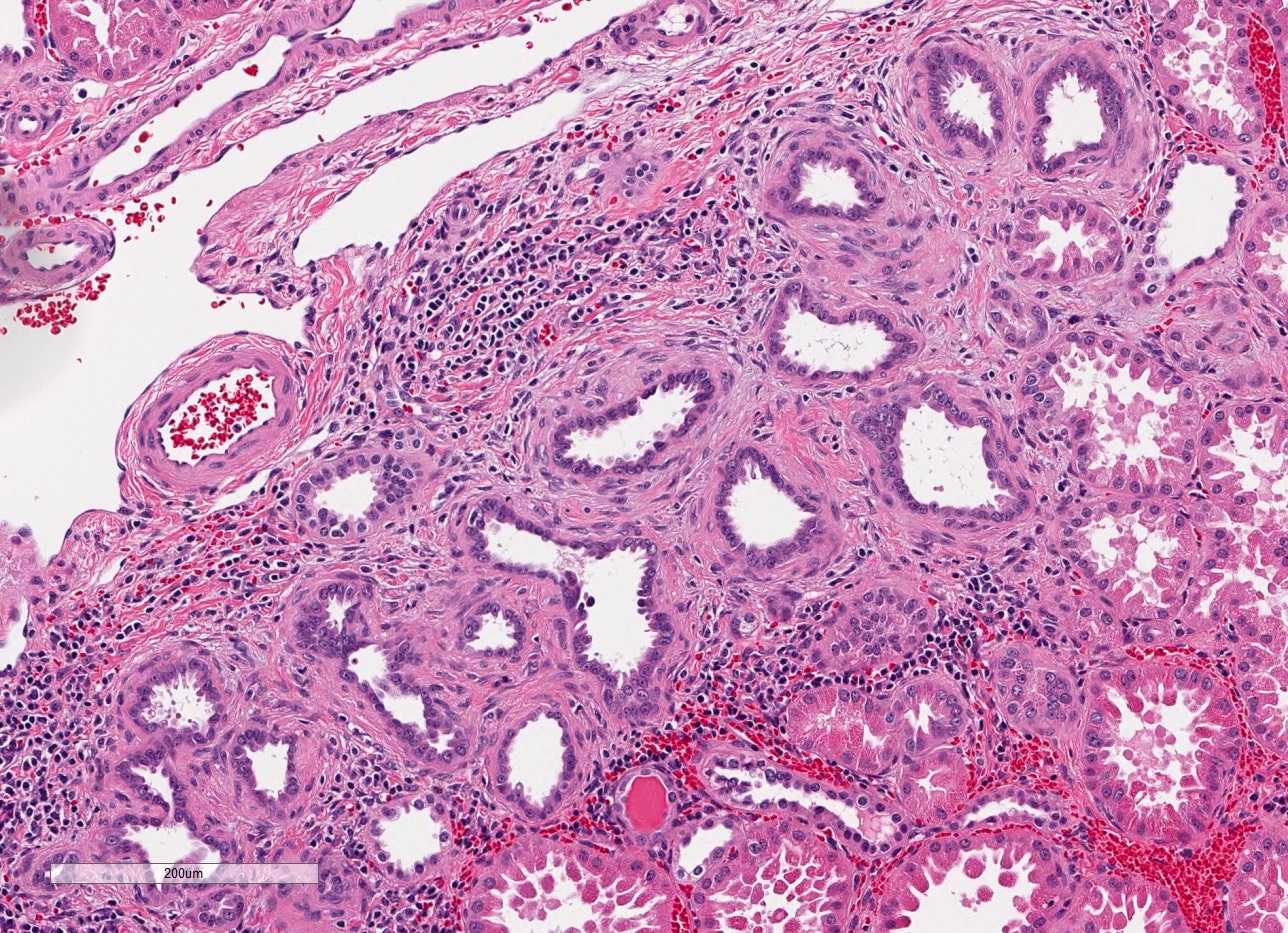
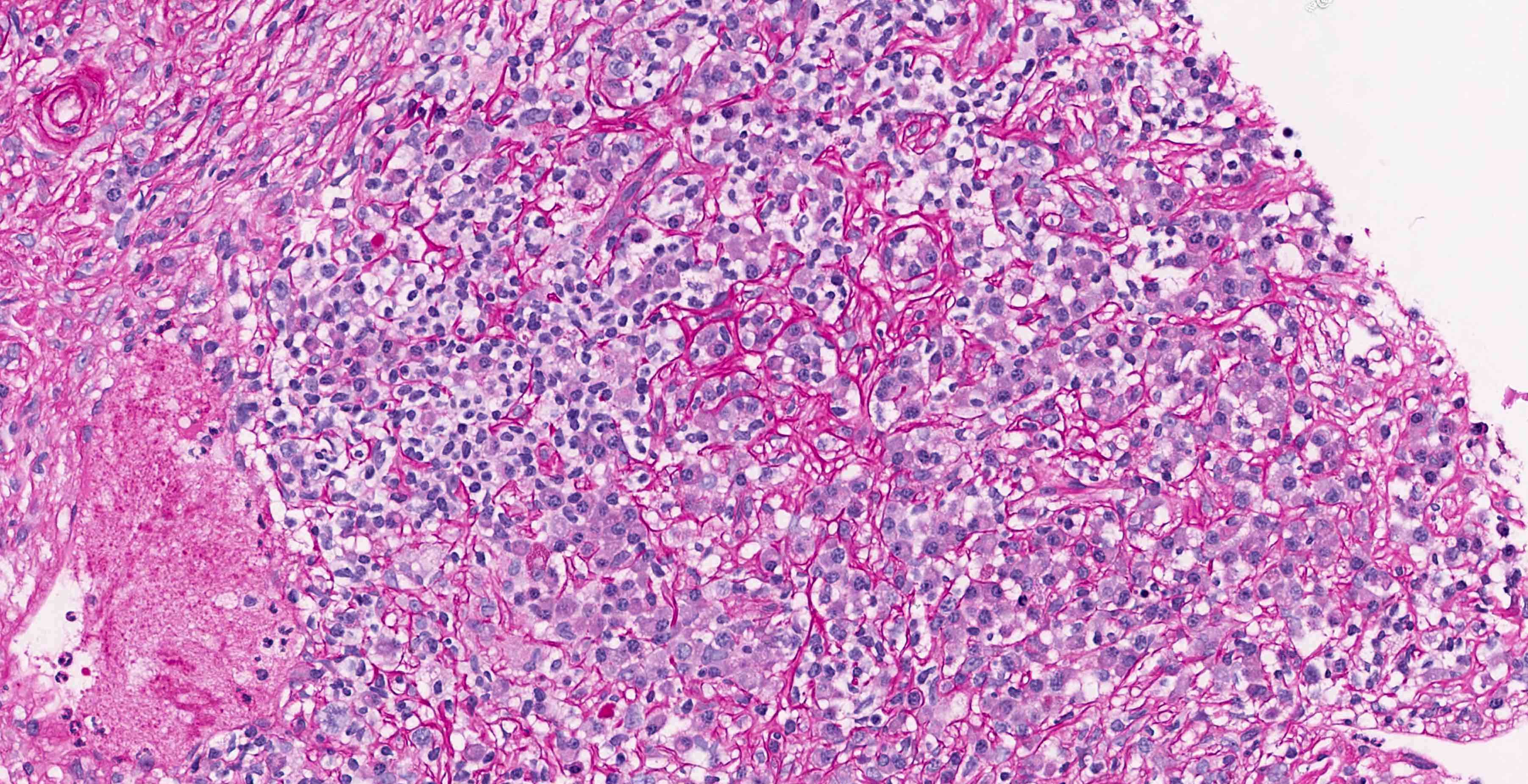
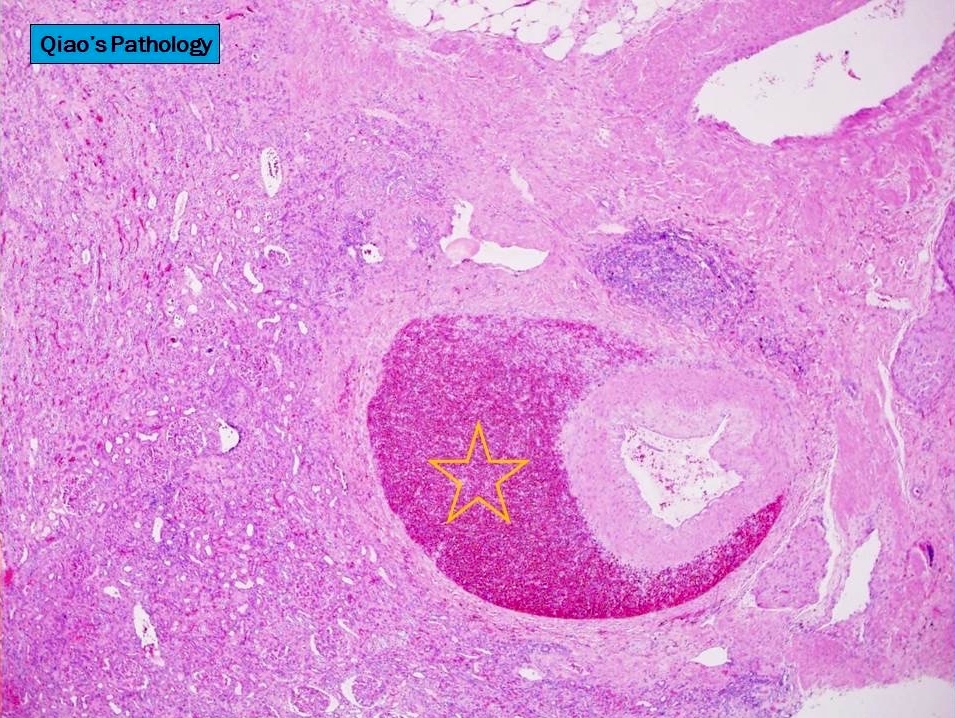
- Patchy suppurative inflammation, primarily cortical with edema, neutrophils in interstitium and tubular lumina and tubular necrosis
- Cortical abscesses and necrosis
- In emphysematous pyelonephritis, hemorrhagic and necrotic renal parenchyma with scattered gas filled cysts
- Reference: Medscape: Acute Pyelonephritis [Accessed 22 March 2021]
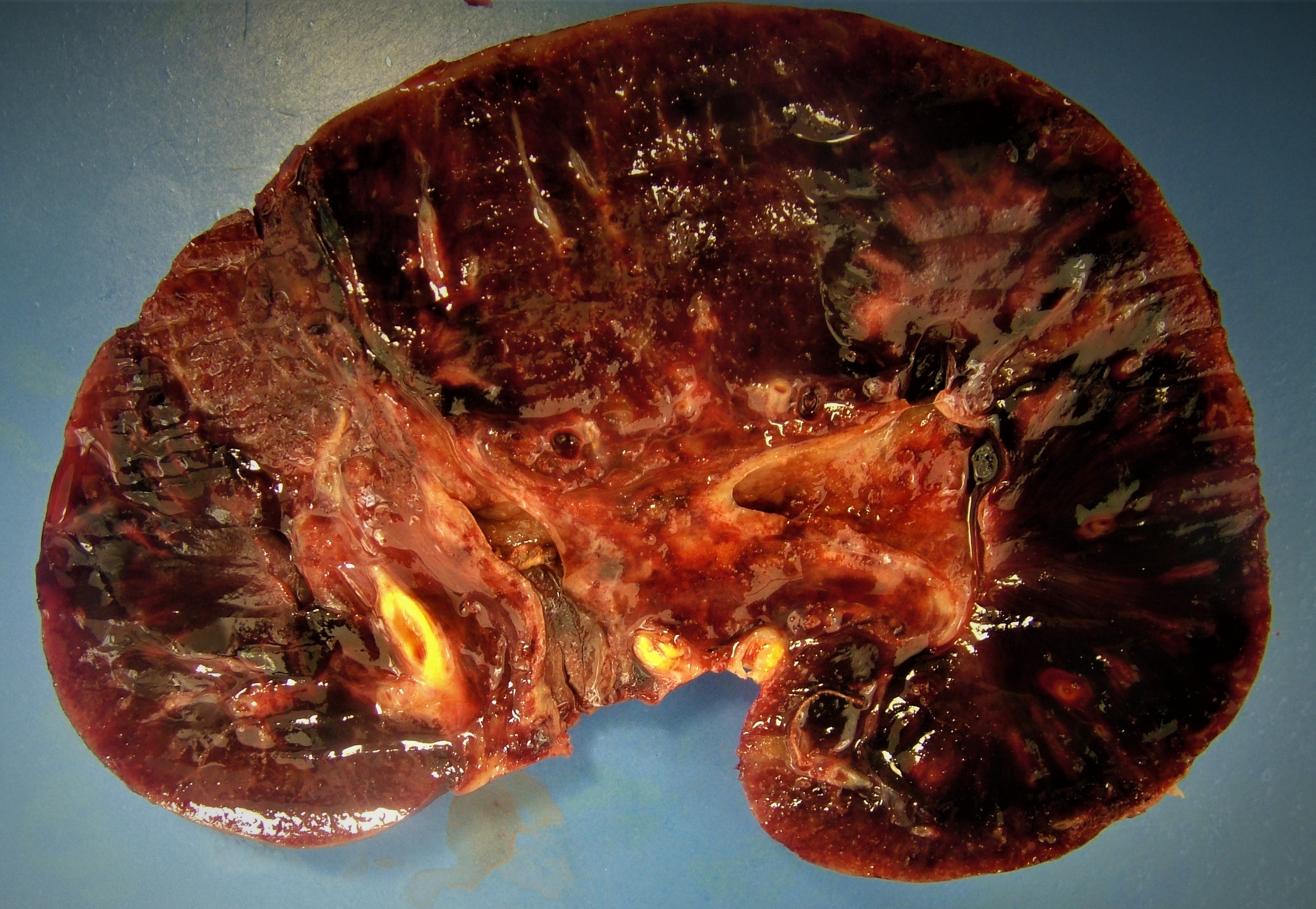
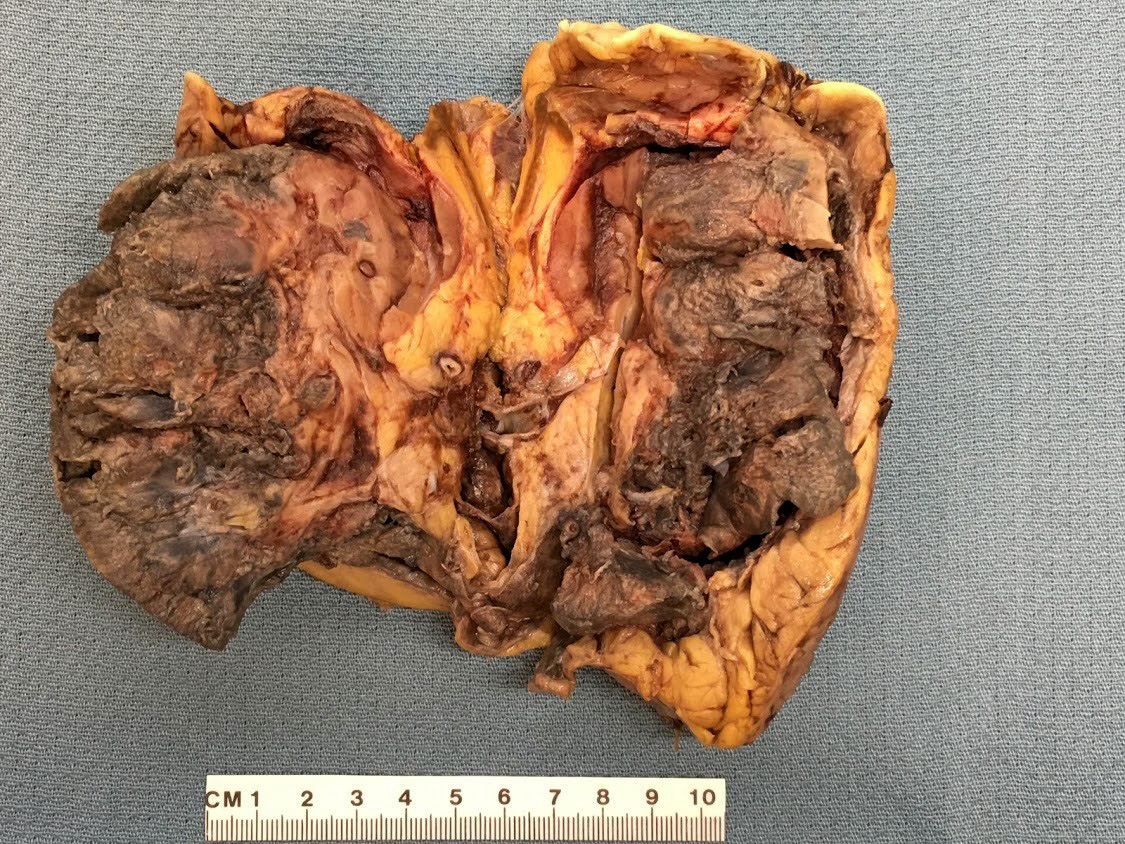
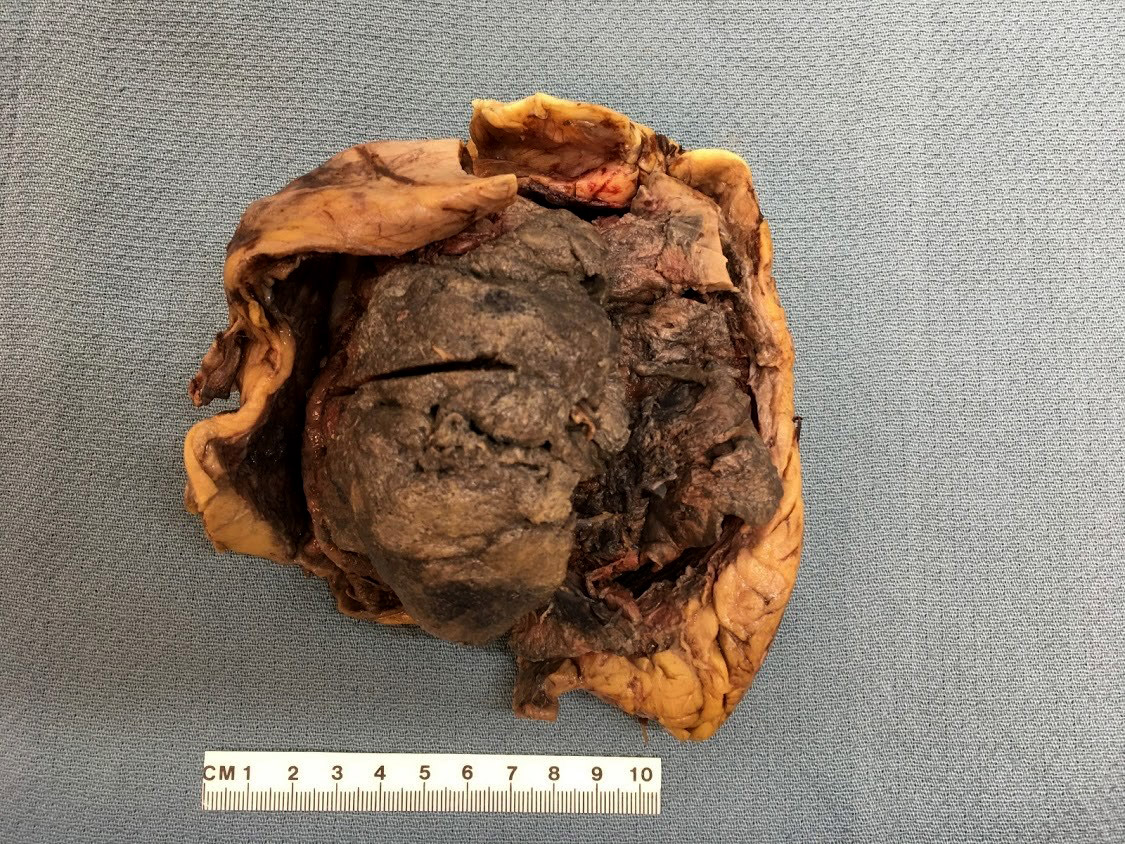
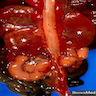
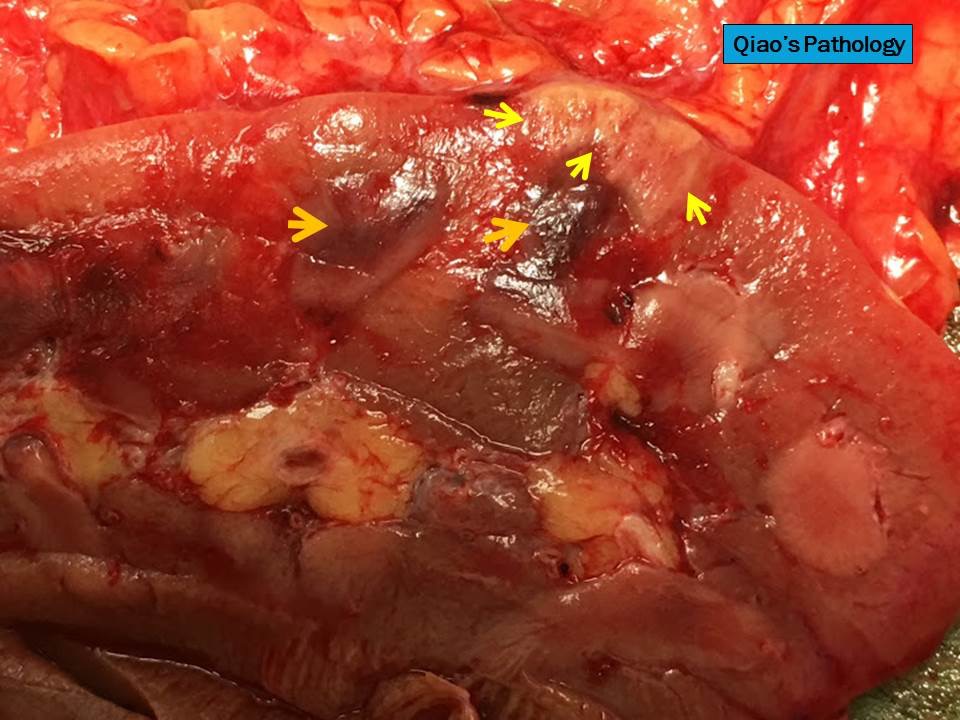
- Left kidney, radical nephrectomy:
- Dissected renal capsule with massive subcapsular hematoma
- Acute pyelonephritis involving renal calyces and renal pelvis with abundant acute inflammatory exudates and abscess formation
- Necrotic renal cortex and renal papillae with formation of numerous gas filled cysts (emphysematous pyelonephritis)
- Culture of renal pelvis pus material positive for E. coli
- Negative for renal stone or malignancy
- One benign hilar lymph node, negative for malignancy (0/1)
- Xanthogranulomatous pyelonephritis:
- In rare cases, emphysematous pyelonephritis may occur as a complication from xanthogranulomatous pyelonephritis (Urol Case Rep 2020;33:101299)
- Acute cystitis
- Viral infections, including BK nephropathy, adenovirus and CMV
- Antibiotics only and fluids only
- Antibiotics, fluids, electrolytes and glucose control
- Bilateral nephrectomy
- Fluids and electrolytes only
- Right nephrectomy
Comment Here
Reference: Acute pyelonephritis
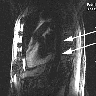
A 44 year old woman presents to the emergency room with fever, nausea, vomiting and dysuria for the past 4 days. Her past medical history is significant for uncontrolled diabetes mellitus. Ultrasound of the right kidney shows intense reflections and prominent acoustic shadows. Nephrectomy of the right kidney is performed. What is the most likely diagnosis?
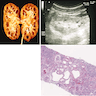
- Autosomal polycystic kidney disease
- Diabetic nephropathy
- Emphysematous pyelonephritis
- Renal cell carcinoma
- Xanthogranulomatous pyelonephritis
- Sudden decline in renal function, secondary to ischemic or toxic damage to renal tubular epithelial cells
- More accurate term is acute tubular injury (ATI), as necrosis is not commonly seen
- Secondary sudden decline in renal function from ischemic (50%) or toxic (25%) causes
- Acute tubular injury (ATI) is more accepted term, as necrosis is rarely seen in ischemic injury
- Acute tubular necrosis (ATN) is a subset of ATI (Kidney Int Rep 2020;5:1993)
- Clinically similar to acute interstitial nephritis (AIN); histopathology needed for diagnosis (Nephron 2019;143:211)
- Acute tubular injury (ATI)
- Acute tubular damage
- Lower nephron nephrosis (Am J Surg Pathol 2018;42:625)
- Most common cause of intrinsic AKI (~85%)
- True ATN presents in 1% of hospitalized patients and ~4/10,000 of the general population
- More likely to affect patients with comorbidities (e.g., diabetes mellitus, heart failure, cancer, atherosclerosis, chronic kidney disease [CKD]), undergoing high risk surgery or presenting with shock (StatPearls: Acute Renal Tubular Necrosis [Accessed 13 June 2022])
- Kidney: tubular disease
- Ischemia: patchy involvement of nephron; S3 of the proximal tubule and more distal tubules, including thick ascending limb, are usually affected
- Toxic: usually more proximal nephron affected, especially convoluted segment of proximal tubules (Semin Nephrol 2018;38:21)
- Ischemia leads to vasoconstriction and decreased renal perfusion, causing damage and dysfunction of renal tubular endothelial cells that in turn leads to damage and dysfunction of renal tubular epithelial cells
- Nephrotoxins are a direct cause of renal tubular epithelial cell damage and dysfunction
- Renal tubular epithelial cell injury can be seen as a loss of brush borders and cell adhesion
- Apoptotic sloughed off tubular cells, other cellular detritus and eosinophilic and granular casts, comprised mainly of Tamm-Horsfall protein, obstruct the lumen leading to a decrease in glomerular filtration rate (GFR) and oliguria
- Loss of adhesion can cause backleak of glomerular filtrate into the interstitium, also leading to a decrease in GFR and oliguria (StatPearls: Acute Renal Tubular Necrosis [Accessed 13 June 2022])
- Most cases are secondary to ischemia (50%), toxins (25%), sepsis (cytokine mediated injury) or obstruction
- Ischemia secondary to hypovolemia (blood loss, fluid loss or third spacing) or decreased perfusion pressure (systemic or local)
- Nephrotoxins include:
- Drugs
- Antibiotics (aminoglycosides, amphotericin, vancomycin)
- Antivirals (acyclovir, indinavir, tenofovir)
- Nonsteroidal anti-inflammatory drugs (NSAIDs)
- Chemotherapy drugs (cisplatin, ifosfamide)
- Checkpoint inhibitors
- Calcineurin inhibitors (cyclosporine, tacrolimus)
- mTOR inhibitors
- Herbs
- IV contrast agents
- Anesthetics
- Heavy metals
- Lead
- Bismuth
- Mercury
- Uranium
- Platinum
- Organic toxins
- Ethylene glycol
- Carbon tetrachloride
- Fuel
- Printer ink
- Toluene
- Endogenous toxins
- Bile casts
- Hemoglobin
- Myoglobin
- Light chains
- Calcium oxalate
- Uric acid
- Drugs
- References: StatPearls: Acute Renal Tubular Necrosis [Accessed 13 June 2022], J Am Soc Nephrol 2020;31:1948
- Events leading to renal hypoperfusion or use of nephrotoxic substances
- Physical exam: signs of volume depletion and hypotension (tachycardia, dry mucous membranes, decreased skin turgor, lethargy, nausea or vomiting), fever if septic, oliguria or anuria initially and possibly polyuria in recovering patients
- Underlying etiology is important for treatment
- References: Contrib Nephrol 2021;199:131, Ren Fail 2019;41:576, Nephron 2019;143:170
- Clinically ATN and AIN are similar
- Acute tubular injury with or without tubular necrosis
- Biopsy needed for definitive diagnosis (Nephron 2019;143:211)
- Decreased GFR, elevated creatinine and blood urea nitrogen (BUN)
- Urinalysis: muddy brown casts, renal tubular epithelial cells
- Fractional excretion of sodium (FENa): usually > 2% while prerenal disease has a value < 1%, though not always the case
- Urine Na: usually > 40 - 50 mEq/L
- Azotemia
- Hyperkalemia
- Metabolic acidosis
- Possibly myoglobinuria or hemoglobinuria (StatPearls: Acute Renal Tubular Necrosis [Accessed 13 June 2022], Nat Rev Nephrol 2019;15:599, Curr Opin Nephrol Hypertens 2019;28:560)
- Favorable unless sustained renal failure or combined with systemic issues or other underlying conditions
- Morbidity is associated with complications secondary to renal failure rather than intrinsic damage from ATI / ATN
- Possible association between biopsy proven ATN and progression to end stage renal disease (ESRD) (J Korean Med Sci 2020;35:e206)
- 31 year old man with rhabdomyolysis and ATN (JBJS Case Connect 2019;9:e0318)
- 33 year old pregnant woman following SARS-CoV-2 infection (Respir Med Case Rep 2020;30:101090)
- 47 year old man with nausea, vomiting and reduced urine output secondary to povidone iodine ingestion (Medicine (Baltimore) 2017;96:e8879)
- 54 year old woman with hypokalemia and ATN secondary to acylovir use (BMC Nephrol 2018;19:324)
- 59 year old man with high serum levels of vancomycin and tobramycin and ATN (Am J Surg Pathol 2018;42:625)
- Underlying cause will determine route of treatment
- Stop toxic insult or medication
- Prevent hypovolemia or hypotension if possible
- Replete volume status
- Avoid angiotensin converting enzyme inhibitors (ACEs), angiotensin receptor blockers (ARBs), NSAIDs, aminoglycosides, IV contrast media and other known renally toxic drugs
- References: Ren Fail 2019;41:576, Nephron 2019;143:170, Am Fam Physician 2019;100:687
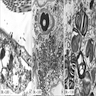
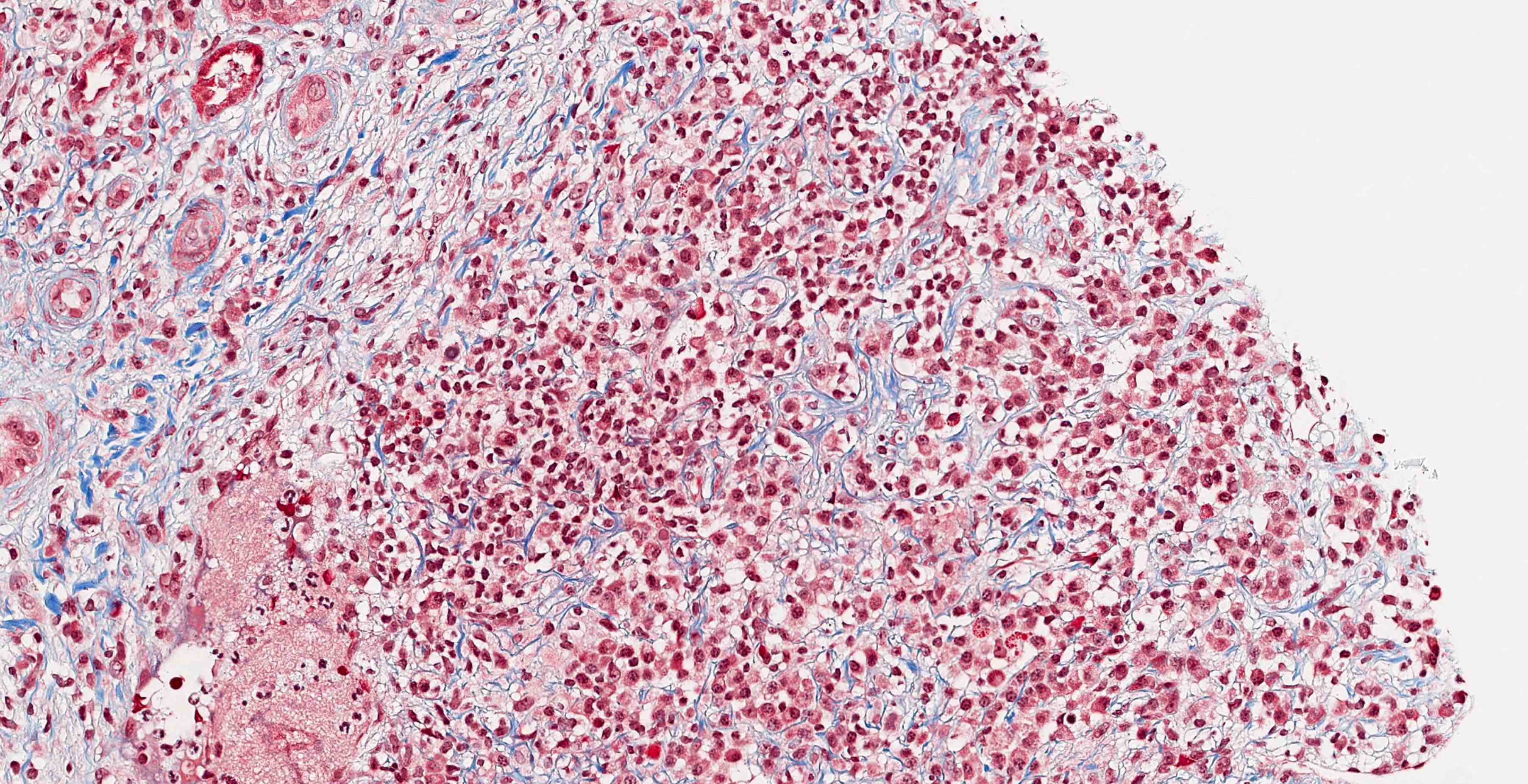
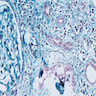
- Attenuation or simplification of tubular epithelium with loss of brush border and blebbing of apical cytoplasm with increased eosinophilic staining
- Tubular epithelial nuclei with condensed chromatin, increased in basophilic staining or loss of distinct nuclear contour
- Tubular lumens filled with sloughed off necrotic tubular epithelial cells, fibrin debris or hyaline casts
- Note: toxic damage more associated with necrosis than ischemic damage (Kidney Int Rep 2020;5:1993, Am J Surg Pathol 2018;42:625)
- Ki67 (low)
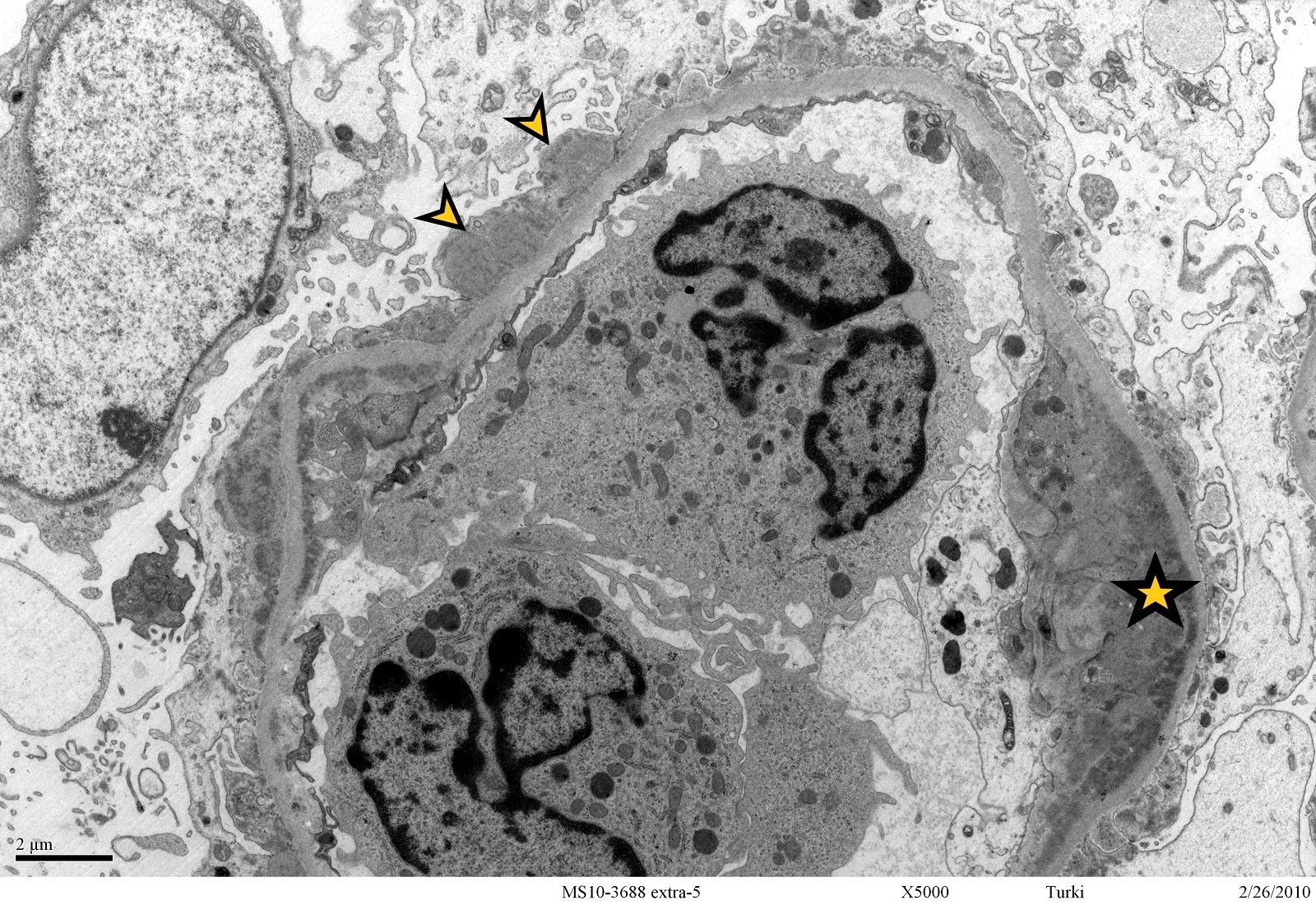
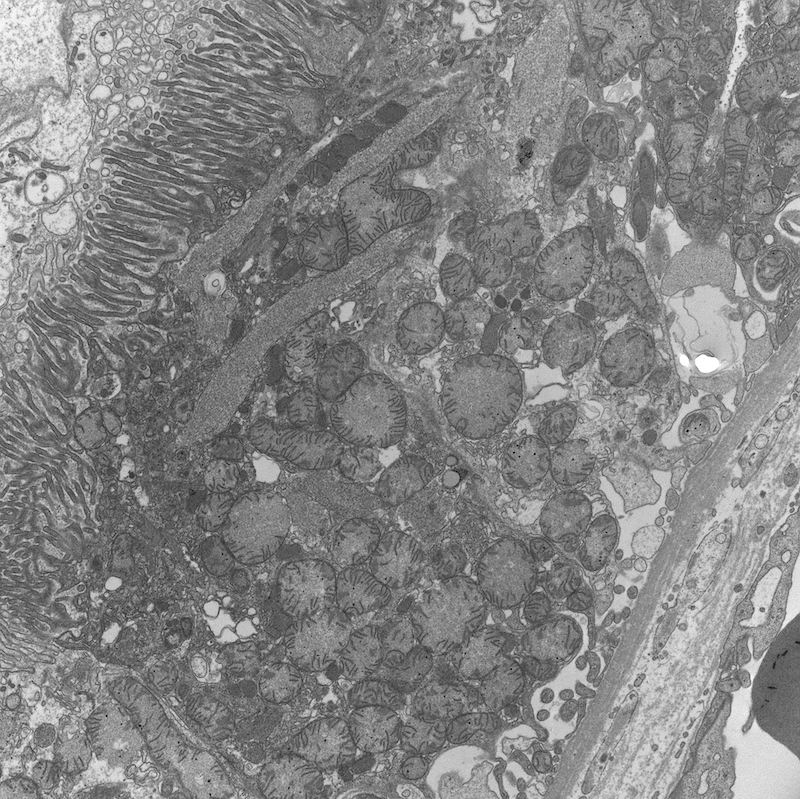
- Proximal tubules show loss of brush border, apical blebs and shedding, cell swelling with mitochondrial condensation, nuclear fragmentation and cell detachment
- Lumen with cellular debris
- Peritubular capillaries with vacuolar degeneration of endothelial cells as well as thickened or multilayered basement membrane
- In ischemic injury, more likely to see autophagy and increased phagolysosomes
- In toxic injury, more likely to see cell contraction and necrosis, dilation of endoplasmic reticulum and mitochondrial swelling with inclusions (Semin Nephrol 2018;38:21)
- IL18 gene, particularly at rs1946518 and rs187238 polymorphisms, may lead to aberrant levels of IL18, which has been linked to pathogenesis of ATN and AKI (J Clin Med 2021;10:3039)
Overview of intrarenal acute kidney injury
- Left kidney, needle core biopsy:
- Acute tubular injury with acute tubular necrosis, widespread and severe, most likely ischemic (see comment)
- There is no evidence of an immune complex mediated disease, a paraprotein deposition disease or a diffuse podocyte disease; there are no signs of active glomerulitis or acute interstitial nephritis
- Minimal chronic changes of the parenchyma, including:
- Focal global glomerulosclerosis (2% of glomeruli)
- Minimal tubular atrophy and interstitial fibrosis (< 5% of the cortex)
- Moderate arterial sclerosis
- Comment: The biopsy reveals widespread tubular injury with severe acute tubular necrosis superimposed on advanced chronic changes of the parenchyma as summarized in the diagnosis above. The process is most likely ischemic.
- Tubulointerstitial nephritis:
- Will show interstitial inflammation and tubulitis
- Cortical necrosis:
- Glomeruli and vessels also affected
- Autolysis:
- Decreased GFR, increased Cr, FENa < 1%, urine Na > 40 mEq/L
- Decreased GFR, increased Cr, FENa > 2%, urine Na > 40 mEq/L
- Decreased GFR, increased Cr, FENa > 2%, urine Na < 40 mEq/L
- Increased GFR, decreased Cr, FENa < 1%, urine Na < 40 mEq/L
Comment Here
Reference: Acute tubular necrosis
- May cause necrotizing tubulointerstitial nephritis with hemorrhagic cystitis or prostate involvement
- Associated with bone marrow or other transplant recipients (Transpl Infect Dis 2011;13:174, Am J Kidney Dis 2012;59:886, Am J Transplant 2011;11:1308) and chemotherapy
- Adenovirus activation after immunosuppression can lead to systemic infection and may trigger rejection or early graft loss (Transpl Infect Dis 2011;13:168)
- Adenovirus is also used as a vector for gene therapy (Transpl Immunol 2011;25:34)
- Hemorrhage and necrosis
- Tubulitis with intranuclear inclusion bodies; either smudge cells, Cowdry A intranuclear inclusions or full type intranuclear containing cells (Hum Pathol 1991;22:1225)
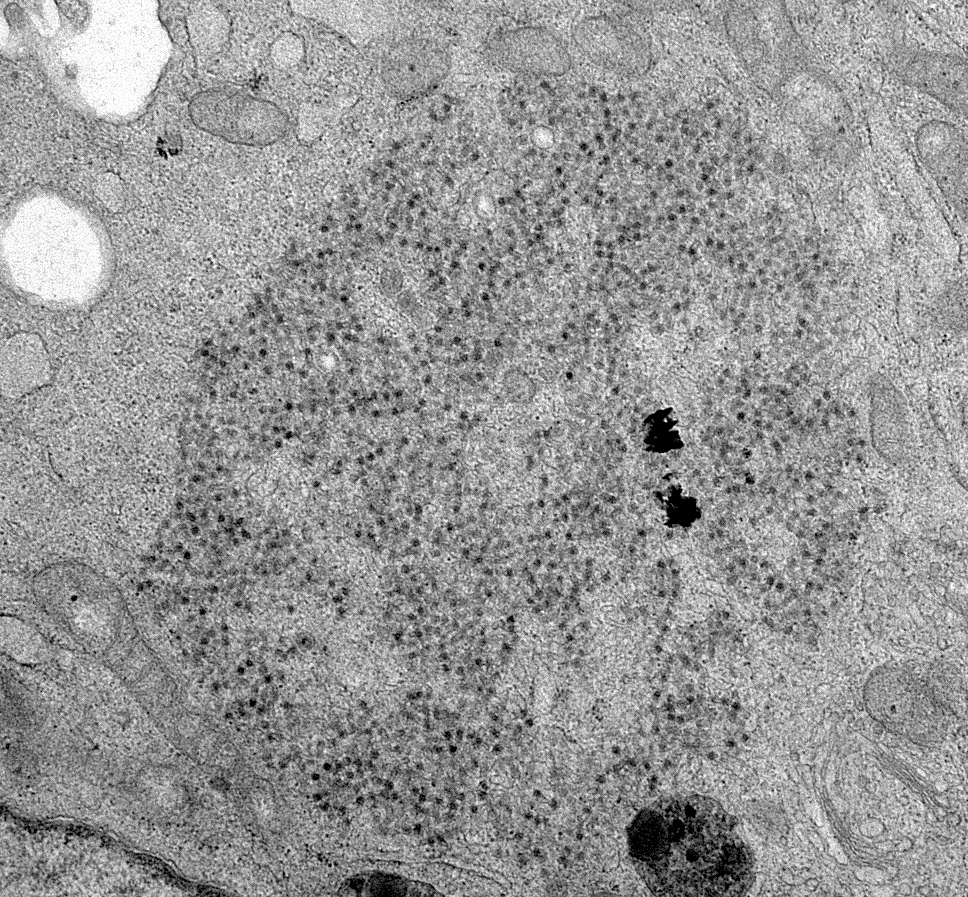
- Intranuclear crystalline arrays of 75 - 80 nm viral particles
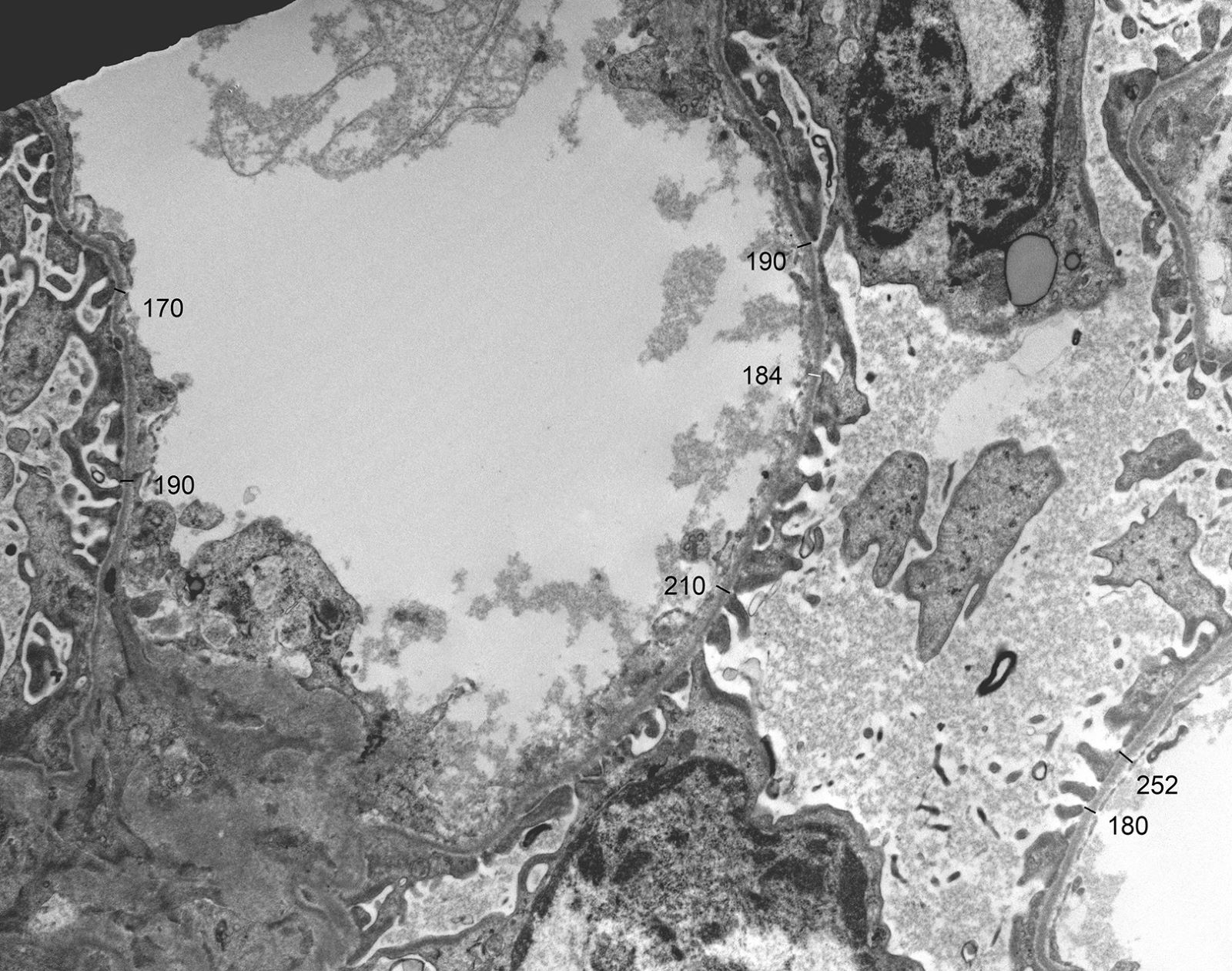
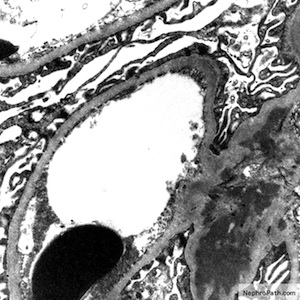
- Alport syndrome (AS) is a progressive renal disease associated with mutations in the genes COL4A3, COL4A4 and COL4A5, affecting the synthesis, assembly, deposition or function of the collagen IV α345 network of the basement membranes; this syndrome has a range of clinical findings from microscopic hematuria to end stage renal disease (ESRD), sensorineural hearing loss and ocular abnormalities
- Thin basement membrane disease is a diffuse and global thinning of the glomerular basement membrane (GBM) not associated with extrarenal features
- Alport syndrome and thin basement membrane disease are genetically heterogeneous conditions associated with abnormalities in glomerular basement membrane collagen
- Alport syndrome and thin basement membrane disease present as structural abnormalities in the glomerular basement membrane
- Alport syndrome and thin basement membrane disease present initially as hematuria
- Alport syndrome often progresses to deterioration of the organ function
- Thin basement membrane disease is largely considered to be a nonprogressive condition
- Some patients with thin basement membrane disease display proteinuria and renal impairment; they may in fact have Alport syndrome but this requires further study (Kidney Int 2004;66:909)
- Alport syndrome, historical term: hereditary nephritis
- Thin basement membrane disease, alternative terms: benign familial hematuria, familial benign essential hematuria, thin membrane nephropathy, thin basement membrane disease, thin basement membrane syndrome, hereditary hematuria
- Exact prevalence of Alport syndrome is unknown, although it is frequently reported as ranging from 1 in 5,000 in the United States to 1 in 50,000 in Europe; predicted pathogenic COL4A3 - COL4A5 variants show a much higher frequency, suggesting that other genetic and environmental factors mitigate the clinical manifestation of the disease (Kidney Med 2023;5:100631, J Am Soc Nephrol 2021;32:2273)
- X linked Alport syndrome accounts for up to 80% of Alport syndrome cases
- Autosomal recessive Alport syndrome is estimated at 15% of cases
- Autosomal dominant Alport syndrome accounts for 20% of cases (Clin Kidney J 2020;13:1025)
- Digenic inheritance is rare
- Thin basement membrane disease is the most common cause of persistent hematuria in children and affects at least 1% of the population
- Kidney (glomerulus), eye (cornea, lens capsule and retina), ear (cochlea)
- Glomerular basement membrane contains 5 isoforms of collagen IV (α1, α2, α3, α4, α5) encoded by genes COL4A1, COL4A2, COL4A3, COL4A4 and COL4A5, providing tensile strength to the glomerular basement membrane and serving as scaffold for assembly of other proteins
- Immature glomerular basement membrane contains isoforms α1 and α2 only, produced by both podocytes and endothelial cells
- At the capillary loop stage of development, the α1α1α2 network is replaced by the α3α4α5 network
- The latter provides greater strength to resist the intracapillary hemodynamic pressure increasing with age
- Collagen IV α3, α4 and α5 isoforms are produced by the podocytes only and form the thicker subepithelial network, whereas collagen IV α1 and α2 forms the subendothelial network (Elife 2013;2:e01149)
- Absence or functional compromise of one of the components of the α3α4α5 network results in reduced ability of the glomerular basement membrane to counter the hemodynamic intracapillary pressure, stressing the filtration barrier and leading to an adaptive reaction of the podocytes, endothelium and mesangial cells; compensatory expansion of the subendothelial α1α2 network provides temporary support (Matrix Biol 2017;57:45)
- An early pathogenetic event in Alport syndrome glomeruli is elevated expression of endothelin 1 by the endothelial cells, leading to subsequent activation of the mesangial cells, deposition of the mesangial matrix proteins in the GBM causing a podocyte reaction with production of proinflammatory cytokines and matrix metalloproteinases (Front Med (Lausanne) 2022;9:846152)
- Slowly progressing structural and biochemical changes in the glomerular basement membrane lead to deterioration of the podocyte architecture and eventually focal and segmental glomerulosclerosis (FSGS)
- Collagen IV is also a major component of the cochlea, cornea, lens capsule and retinal basement membranes, accounting for the sensorineural hearing loss and ocular abnormalities
- A classification system for Alport syndrome and related disorders has been proposed by the Alport Syndrome Working Group and is based on the mode of inheritance and genetic state (see Genetics) (Kidney Int 2018;93:1045)
- X linked Alport syndrome: in addition to the kidney symptoms, there is bilateral sensorineural hearing loss which may be detected in boys during the first decade
- Anterior lenticonus develops progressively in male patients and is very rare in female patients
- Also specific to Alport syndrome is the progressive appearance of asymptomatic, perimacular, yellowish flecks
- Median age to reach end stage renal disease in men is 25 years with large deletion / protein truncating variants, 28 years with splice site variants and 37 years with missense variants (J Am Soc Nephrol 2010;21:876)
- Women with X linked Alport syndrome have a variable phenotype due to X linked inactivation
- 12% of heterozygous X linked women with Alport syndrome reached end stage renal disease by age 40 and 30 - 40% by age 60 (J Am Soc Nephrol 2003;14:2603)
- Hypertension is usually seen with end stage renal disease
- Autosomal recessive Alport syndrome: clinical symptoms are similar to those seen in X linked Alport syndrome; progression to end stage renal disease usually occurs by the age of 30 years in both men and women
- Autosomal dominant Alport syndrome: less severe phenotype than in X linked Alport syndrome, similar presentation in men and women, variable progression to end stage renal disease after the age of 50 years; hearing loss and ocular involvement are rare
- It has been proposed that thin basement membrane disease be classified as a form of collagen IV related renal disease along with Alport syndrome; this approach has not been generally adopted (Kidney Int 2018;93:1045)
- Thin basement membrane disease is suspected clinically if there is persistent microhematuria in the absence of proteinuria, impaired renal function and extrarenal symptoms of Alport syndrome
- Genetic testing is the most sensitive method for detection of COL4A3 - COL4A5 variants; not only should individuals with the typical features of Alport syndrome be tested but also those with proteinuria, FSGS, familial IgA glomerulonephritis or with end stage renal disease without an obvious cause (Eur J Hum Genet 2021;29:1186)
- Given the high prevalence of collagen IV variants in the population (see Epidemiology), it is important that clinical diagnostic features of Alport syndrome are documented as well as histologic features by light, immunofluorescence and electron microscopy
- Since the differential diagnosis for characteristic Alport syndrome ultrastructural lesions is wide (see Differential diagnosis), genetic testing is absolutely necessary for the correct diagnosis
- Anterior lenticonus is diagnostic of Alport syndrome
- Ultrastructural finding of thin glomerular basement membrane does not exclude Alport syndrome nor does the normal renal expression of collagen IV or detection of the COL4A3 / COL4A4 variants in the heterozygous state, as the same may be observed in Alport syndrome; regular follow up examination and reevaluation of patients with thin basement membrane disease is recommended
- In X linked Alport syndrome, microscopic or macroscopic hematuria is often the first sign and can occur as early as infancy (Kidney Med 2023;5:100631)
- Hematuria is the cardinal symptom; it is observed in all male patients and nearly all female patients
- Albuminuria occurs later, followed by decrease in glomerular filtration rate
- Autosomal recessive Alport syndrome: laboratory symptoms similar to those seen in X linked disease (Clin Exp Nephrol 2019;23:158)
- Autosomal dominant Alport syndrome: less severe symptoms (Am J Kidney Dis 2021;78:560)
- Thin basement membrane disease: isolated microhematuria and infrequent episodes of macroscopic hematuria, with no proteinuria and normal kidney function (Nephron 2023;147:383)
- X linked hemizygous Alport syndrome has 100% progression to end stage renal disease; timing of progression depends on genotype
- X linked heterozygous Alport syndrome has up to 25% progression to end stage renal disease; risk factors are gross hematuria, sensorineural hearing loss, proteinuria, glomerular basement membrane thickening or lamellation
- Autosomal recessive Alport syndrome has 100% progression to end stage renal disease; timing depends on genotype
- Autosomal dominant Alport syndrome has 20% progression to end stage renal disease among those with risk factors for progression and < 1% in the absence of risk factors; risk factors are proteinuria, FSGS, glomerular basement membrane thickening and lamellation, sensorineural hearing loss or evidence of progression in patient or family
- Digenic Alport syndrome exhibits COL4A3 and COL4A4 mutations in trans with up to 100% risk of end stage renal disease (Kidney Int 2018;93:1045)
- Digenic Alport syndrome exhibits COL4A3 and COL4A4 mutations in cis with up to 20% risk of end stage renal disease (Kidney Int 2018;93:1045)
- Prognosis for thin basement membrane disease has been considered excellent for many years but development of end stage renal disease at an advanced age is now considered possible (Nephron 2023;147:383)
- 16 year old boy with proteinuria for 15 years and a 31 year old man with hearing loss and end stage renal disease, both with positive family history of renal disease (Biomed Res Int 2021;2021:6664973)
- 20 year old man with Alport leiomyomatosis syndrome (diffuse esophageal leiomyomatosis) (Gen Thorac Cardiovasc Surg 2020;68:199)
- 20 year old man with both antiglomerular basement membrane disease and thin basement membrane disease (Medicine (Baltimore) 2021;100:e26095)
- 50 year old woman presenting with microhematuria and proteinuria (BMC Nephrol 2023;24:97)
- Current clinical practice recommendations (Kidney Int 2018;93:1045)
- Observation for hematuria with normal urine albumin / creatinine ratio
- Consider ACE inhibitors for hematuria and microalbuminuria
- ACE inhibitors for hematuria and overt proteinuria
- Angiotensin receptor blockers, aldosterone antagonists, calcium channel blockers or diuretics for progressive proteinuria despite ACE inhibitors
- Potential therapies currently undergoing clinical trials: sodium glucose cotransporter 2 (SGLT2) inhibitors, aminoglycoside analogs (prevent premature peptide termination), endothelin type A antagonists, lipid modifying drugs, gene replacement therapy
- Early in the course of Alport syndrome
- Glomeruli appear normal (Glomerular Dis 2021;1:135)
- In young children, features of glomerular immaturity may be seen (small glomeruli with indistinct capillaries, circumference of the capillary segments lined with cuboidal epithelial cells); such features have no association with Alport syndrome
- Tubulointerstitial compartment appears normal (Glomerular Dis 2021;1:135)
- Glomeruli appear normal (Glomerular Dis 2021;1:135)
- With progression of the disease
- Segmental thickening of the glomerular capillary walls (more appreciable on the silver stain) and mesangial expansion become visible
- Red blood cell casts may be seen in the tubules
- Foci of interstitial fibrosis and tubular atrophy develop (Glomerular Dis 2021;1:135)
- Lipid laden interstitial foam cells are considered a marker of Alport syndrome; they appear as rows or clusters in the cortex or at the corticomedullary junction (Glomerular Dis 2021;1:135)
- Eventually, with marked thickening of the glomerular basement membranes and expansion of the mesangium, segmental tuft scarring develops in an increasing number of glomeruli; the end point is global glomerulosclerosis
- In patients with thin basement membrane disease, the renal tissue is usually normal by light microscopy (Kidney Int 2003;64:1169)
- Mesangial expansion and mild mesangial proliferation may occur
- Red blood cells may be seen within tubules
- Immunofluorescence for the antibodies and complement is usually negative, although with progression of Alport syndrome, faint deposits of IgM, C3 or C1q may be seen
- Biopsies from most male patients with X linked Alport syndrome are completely negative for collagen IV α5 isoform in the glomerular capillary walls and the Bowman capsule (Glomerular Dis 2021;1:135)
- Female patients with X linked Alport syndrome show a mosaic pattern of collagen IV α5 expression in the glomerular capillary segments (Glomerular Dis 2021;1:135)
- In autosomal recessive Alport syndrome, the glomerular capillary walls are negative for α3, α4 and α5 staining, whereas the Bowman capsule is positive for α5 (Glomerular Dis 2021;1:135)
- Biopsies from patients with thin basement membrane disease show normal distribution of the collagen IV isoforms in the glomeruli (Arch Pathol Lab Med 2001;125:631)
- In younger Alport syndrome patients, a thin glomerular basement membrane may be the only finding (Glomerular Dis 2021;1:135)
- Typical feature in older children is irregular glomerular basement membrane appearance with close alternation of extremely thin and thick split segments
- Breaking of the thin glomerular basement membrane segments is the histological basis of hematuria in such patients; an image of a red blood cell traversing the glomerular basement membrane is provided in N Engl J Med 2007;357:2687
- Characteristic lesion of Alport syndrome seen in many but not all patients consists of marked irregular thickening of the glomerular basement membrane with splitting of the lamina densa into a basket weave pattern, lucent zones containing small electron dense granules (bread crumbs) and sometimes small electron dense deposits
- Fragmentation of the lamina densa may give a laminated appearance to the glomerular basement membrane
- Inner and outer contours are corrugated and the overlying podocytes are hypertrophied and often show foot process effacement (Glomerular Dis 2021;1:135)
- Severity and extent of the Alport syndrome lesions vary very widely depending on the type of mutation and other factors; marked variability in the glomerular basement membrane width within the glomerulus of a patient with persistent hematuria should raise suspicion of Alport syndrome (GeneReviews: Alport Syndrome [Accessed 25 August 2023])
- Mesangium is initially normal but with time, an increase in the mesangial matrix is observed, with focal mesangial hypercellularity and mesangial expansion; the mesangial material appears heterogeneous and contains inclusions (Ren Fail 2000;22:751)
- Thin basement membrane disease manifests ultrastructurally as global and diffuse thinning of the glomerular basement membrane (Kidney Int 1996;50:1445)
- Method of glomerular basement membrane measurement and estimated normal ranges of glomerular basement membrane thickness in children are indicated in Arch Pathol Lab Med 2009;133:224 and Glomerular Dis 2021;1:135
- Total of 30 measurements are made on 10 randomly selected capillaries; the average and at least 50% of the measurements should be below the lower limit of the normal range for the patient's age and sex
- Alport syndrome changes involving > 5% of the glomerular basement membrane length examined as well as evidence of Alport syndrome on immunofluorescence excludes thin basement membrane disease
- Glomerular basement membrane thickness in children increases linearly between 1 and 9 years of age and then plateaus (Arch Pathol Lab Med 2009;133:224)
- Thin basement membrane disease cannot be reliably diagnosed in deparaffinized formalin fixed tissue (Matrix Biol 2018;71:250)
- Measurements of glomerular basement membranes can be reliably interpreted from reclaimed frozen tissue if routine processed EM tissue is unavailable (Kidney Med 2022;4:100562)
- COL4A3, COL4A4 and COL5A5 are the most frequent causative genes of monogenic chronic kidney disease after the polycystic kidney disease genes PKD1 and PKD2 (N Engl J Med 2019;380:142)
- COL4A3 and COL4A4 genes reside on chromosome 2, while COL4A5 gene I is located on the X chromosome
- There are 4 modes of inheritance of Alport syndrome
- X linked Alport syndrome: pathogenic variants in the COL4A5 gene on the X chromosome, genetic state - hemizygous (male subjects) and heterozygous (female subjects)
- Autosomal recessive: pathogenic variants in COL4A3 / COL4A4 genes
- Autosomal dominant: pathogenic variants in COL4A3 / COL4A4 genes due to heterozygous COL4A3 and COL4A4 mutations
- Digenic: combination of 2 mutations in different genes, typically a pathogenic variant in COL4A3 and COL4A4 (Clin Genet 2017;92:34)
- ClinVar database currently documents 680 pathogenic variants of COL4A5, 239 pathogenic variants of COL4A4 and 236 pathogenic variants of COL4A3 (NIH: ClinVar - COL4A5[gene] [Accessed 25 August 2023], NIH: ClinVar - COL4A4[gene] [Accessed 25 August 2023], NIH: ClinVar - COl4A3[gene] [Accessed 25 August 2023])
- Thin basement membrane disease shows autosomal dominant inheritance in 50% of cases with heterozygous COL4A3 or COL4A3 mutations (carrier state for autosomal recessive Alport syndrome) (Pediatr Nephrol 2009;24:877)
- Other genetic causes such as COL4A1 variants have also been proposed (Nephrol Dial Transplant 2016;31:1758)
- Mutations are usually different in each family and there is no hotspot; some sources report that the mutation detection rate in thin basement membrane disease is low (Kidney Int 2003;64:1169)
- Kidney, biopsy:
- Alport syndrome (see comment)
- Focal global glomerulosclerosis (1/12)
- Mild focal interstitial fibrosis and tubular atrophy
- Comment: Genetic study is recommended.
- Clinical history: The patient is an 8 year old boy with a history of microscopic hematuria and proteinuria. Family history is positive for mother developing end stage renal disease at the age of 40 years. Laboratory data: serum creatinine 0.6 mg/dL, BUN 15 mg/dL, serum albumin 4.4 mg/dL, UPC 900 mg/g creatinine, C3 124 mg/dL, C4 25 mg/dL, ANA negative, anti-DNA antibody and ANCA screen negative.
- Light microscopy: The biopsy material consists of tissue from the renal cortex. The tissue is sampled at 10 levels of section with H&E, PAS, trichrome and PAMS stains. Per level of section up to 12 glomeruli are available, of which 1 is globally sclerosed. The nonsclerosed glomeruli show patent capillary lumens, single contoured capillary walls and no segments of sclerosis, adhesion or crescents. A few perihilar capillaries contain hyaline. Tubules show mostly intact epithelium with preserved brush borders. There is mild focal interstitial fibrosis and tubular atrophy; scattered lymphocytes are present in the interstitium. Some distal tubules contain proteinaceous casts. Interlobular arteries of the medium caliber are present and do not show diagnostic arteriosclerotic changes; there is no significant arteriolar hyalinosis.
- Immunofluorescence microscopy: 9 nonsclerosed glomeruli are available for evaluation by immunofluorescence microscopy. No diagnostic immunofluorescence signal is detected with antibodies to IgG-, IgA-, IgM-, C3-, C1q-, kappa light chain-, lambda light chain-. Immunofluorescence staining for the collagen type IV α2 and α5 isoforms was performed at our request and showed normal staining for the α2 isoform with enhanced staining at the glomerular capillary walls and loss of staining for the α5 isoform.
- Electron microscopy: 2 nonsclerosed glomeruli are available for evaluation on semithin sections and do not show significant diagnostic abnormalities. There is no significant interstitial fibrosis and tubular atrophy. Ultrastructural examination of 2 glomeruli shows alternating segments of thick and thin glomerular basement membrane segments with irregular internal and external contours. Thicker glomerular basement membrane segments display lamina densa splitting (basket weave), microgranular inclusions (bread crumbs), large electron lucent areas and contain a few faded electron dense deposits. There is segmental variable mild to marked effacement of the podocyte foot processes and pronounced podocyte microvillus transformation. The mesangial matrix demonstrates mild segmental expansion. Tubular basement membranes are unremarkable.
- Alport syndrome (see comment)
- Alport syndrome associated conditions
- AMME syndrome (Alport syndrome, midface hypoplasia, intellectual deficit and elliptocytosis) (J Med Genet 2017;54:269)
- Associated with contiguous gene deletions in the chromosomal region Xq22.3-Xq23 including the entire COL4A5 gene and its adjacent genes
- Abovementioned dysmorphic, developmental and hematologic abnormalities form the basis for the differential diagnosis
- Diffuse and global Alport-like changes are seen in
- Focal and segmental glomerulosclerosis (FSGS) associated with variants in nonmuscle myosin myosin1e (Pediatr Nephrol 2023;38:439):
- These patients may become proteinuric early in life, with early onset of FSGS and early progression to renal failure
- Fechtner and Epstein syndrome associated with mutations in myosin heavy chain 9 gene (MYH9) (Hum Genet 2002;110:182):
- Platelet macrocytosis, thrombocytopenia, sensorineural hearing loss, cataracts, nephritis and characteristic MYH9 aggregates in the granulocyte cytoplasm (Pediatr Nephrol 2023;38:439)
- Steroid resistant nephrotic syndrome:
- Caused by activation of a Rac1 GTPase as a result of mutation in a regulatory protein ARHGAP24 manifesting ultrastructurally as subepithelial extensions of the glomerular basement membrane and localized areas of glomerular basement membrane lamellation (The Open Urology & Nephrology Journal 2016;9:88)
- Renal coloboma syndrome due to mutated podocyte PAX2 (Pediatr Nephrol 2012;27(:1189)
- Presents as optic disk coloboma and renal insufficiency
- Collagen IV α5 immunostaining is normal
- Frasier syndrome with male pseudohermaphroditism and slowly progressing nephropathy associated with mutation in Wilms tumor suppressor gene WT1 (Am J Kidney Dis 2003;41:1110)
- Focal and segmental glomerulosclerosis (FSGS) associated with variants in nonmuscle myosin myosin1e (Pediatr Nephrol 2023;38:439):
- Conditions that may resemble Alport syndrome
- Nail patella syndrome (hereditary osteo-onychodysplasia) associated with mutations in LIM homeodomain transcription factor LMX1B and showing collagen fibrils in the GBM (Pflugers Arch 2017;469:927)
- Collagen type III glomerulopathy (collagenofibrotic glomerulopathy):
- Seen mostly in Asian patients and manifested by accumulation of collagen type III in the glomerular extracellular matrix (Pflugers Arch 2017;469:927)
- Pierson syndrome:
- Congenital nephrotic syndrome with massive proteinuria, ocular abnormalities and rapid progression to end stage renal disease due to variants in the β2 laminin chain (LAMB2 gene) (Matrix Biol 2018;71:250)
- HANAC syndrome:
- COL4A1 mutations and hereditary angiopathy, nephropathy, aneurysms and muscle cramps
- Renal manifestations include hematuria and bilateral large cysts (N Engl J Med 2007;357:2687)
- Renal perfusion mismatched transplants (Am J Surg Pathol 1999;23:437, Clin Nephrol 2017;88:364):
- Alport-like GBM changes may be seen
- Membranous glomerulopathy and sometimes glomerulonephritis of various etiologies:
- GBM rearrangements resembling Alport syndrome may be seen
- Thin basement membrane disease:
- Alport syndrome
- IgA nephropathy
- Segments of thin glomerular basement membrane may be seen
- AMME syndrome (Alport syndrome, midface hypoplasia, intellectual deficit and elliptocytosis) (J Med Genet 2017;54:269)
A 7 year old girl presents with hematuria and very mild proteinuria; she has not undergone laboratory testing previously. Her mother states that the men in the family develop renal failure. A biopsy is done and a representative glomerulus is displayed in the image shown above. Immunofluorescence shows segmental loss of Collagen IV α5 isoform in the glomerular capillary walls. What is the most likely diagnosis?
- Alport syndrome, autosomal recessive
- Alport syndrome, X linked heterozygous
- Minimal change disease
- Normal kidney
- Thin basement membrane disease
Comment Here
Reference: Alport syndrome and thin basement membrane lesion
- Acute cell mediated rejection
- Antiglomerular basement membrane disease
- De novo immune complex glomerulonephritis
- FSGS relapse
Comment Here
Reference: Alport syndrome and thin basement membrane lesion
- Bilateral chronic renal disease due to excessive intake of analgesics, with papillary necrosis (tips of medullary pyramids) and later chronic tubulointerstitial nephritis
- Disorder appears to be limited to phenacetin containing analgesics (Nephrol Dial Transplant 2009;24:1253)
- High rates in Australia (Clin J Am Soc Nephrol 2008;3:768), southeast USA
- Due to red blood cell damage from phenacetin metabolites in numerous products: phenacetin plus aspirin, caffeine, acetaminophen (a metabolite of phenacetin) or codeine
- 80% women; also people with chronic pain, factory workers
- 50% have co-existing urinary tract infection
- Anemia, renal stones and inability to concentrate urine
- May have gross hematuria or renal colic due to sloughing of necrotic papillae
- Complication: papillary urothelial carcinoma of renal pelvis
-
Other causes of papillary necrosis:
- Diabetes mellitus: 75% women, usually 10+ years of disease, 80% have urinary tract infection, all papillae affected similarly
- Obstruction: 90% male, 90% have infection, frequent calcification
- Sickle cell disease: M=F, few papillae affected
- 55 year old businessman with chronic osteoarthritis and end stage renal disease (Niger J Clin Pract 2012;15:231)
- Depressed cortex due to cortical atrophy overlying necrotic papillae
- Papillae show varying stages of necrosis and sloughing
- Early: papillae have patchy necrosis
- Later: papillae are diffusely necrotic with ghost tubules and dystrophic calcification; renal columns of Berlin are usually spared from tubular atrophy; small vessels have basement membrane thickening
- Posterior abdomen on either side of vertebral column in retroperitoneum
- Surrounded by fat and loose areolar tissue
- Superior border is at T12, inferior border is at L3
- 11 cm long x 5 - 8 cm x 3 cm
- Weighs 125 - 170 g in males, 115 - 155 g in females
- Capsule: covers kidney is surrounded by perirenal fat
- Cortex: outer 1.2 cm of kidney surrounds inner medulla containing pyramids and lacking glomeruli
- Renal sinus: fatty compartment within confines of kidney not delineated from renal cortex by a fibrous capsule
- Gerota fascia: fibromembranous tissue surrounding the kidney that separates it from adjacent musculature
- Ureter ascends into renal pelvis and divides into calyces (2 - 3 major, 12 minor total)
- Related to a calyx are renal pyramids with apices called papillae
- Vasculature: receives 25% of cardiac output, 90% goes to cortex, via interlobar, arcuate, interlobular, afferent arterioles, then into glomeruli, efferent arterioles and peritubular vascular network
- Deeper juxtamedullary glomeruli give rise to vasa recta, which supply outer and inner medulla
- Since arteries are end vessels, their occlusion causes infarction
- Regional lymph nodes: renal hilar, paracaval, aortic and retroperitoneal
- References: Wikipedia: Kidney, eMedicine: Kidney Anatomy, WebMD: Picture of the Kidneys
- Metanephric blastema: forms glomerulus, proximal convoluted tubules, loops of Henle, distal convoluted tubules and connective tissue of renal interstitium
- Ureteric bud: forms cortical and medullary collecting tubules and medullary collecting ducts
- Stages of kidney development:
- Pronephros (based on Wolffian duct, kidneys non-functional)
- Mesonephros (appear at week 4, form nephron-like tubules but degenerate)
- Metanephros (form at week 5, function by week 11)
- References: UNSW Embryology, Wikpedia
- Age related changes occur; are affected by hypertension and heart failure (Kidney Int 1997;51:1196)
- Aldosterone:
- Causes increased reabsorption of NaCl to increase blood volume
- Antidiuretic hormone (vasopressin):
- Stimulates water reabsorption by stimulating insertion of "water channels" or aquaporins (Google: Aquaporins - Water Channels) into the membranes of kidney tubules
- These channels transport solute free water through tubular cells and back into blood, leading to a decrease in plasma osmolarity and an increased osmolarity of urine
- In diabetes insipidus (without ADH), kidney tubules are virtually impermeable to water, which flows out as urine (up to 10 liters of dilute urine/day)
- Erythropoietin:
- Secreted in response to low serum pO2, promotes red blood cell production
- Natriuretic hormones:
- Cause increase in glomerular filtration rate after nephron destruction
- Renin:
- Produced by juxtaglomerular apparatus in response to hypotension, converts angiotensinogen to angiotensin I, which is converted to angiotensin II in the lung by angiotensin converting enzyme (ACE)
- Angiotensin II increases aldosterone production and promotes vasoconstriction
Images hosted on other servers:
- Glomerular filtration: glomeruli are highly permeable to water and solutes through fenestrated endothelium; they are impermeable to large proteins like albumin (proteins are more permeable if smaller and more cationic)
- Glomerular disease causes tubular disease, since efferent arterioles supply tubules
- Glomeruli:
- Tuft-like vascular structure composed of lobules of specialized capillaries that arise from an afferent arteriole and eventually coalesce to drain into an efferent arteriole
- 200 microns in diameter, 20% larger in juxtamedullary area
- Hypercellularity: the presence of more than 3 cells in an individual glomerular mesangial region away from the vascular pole
- References: Mod Pathol 2002;15:988, Wikipedia: Glomerulus
- Juxtaglomerular apparatus:
- Close to glomerulus where afferent arteriole enters it; consists of juxtaglomerular cells (modified smooth muscle cells) plus macula densa (region of distal tubule as it returns to vascular pole of parent glomeruli) plus lacis or Goormaghtigh cells (nongranular cells that reside near afferent arteriole, macula densa and glomerulus and resemble mesangial cells); produces renin; cells of macula densa show reverse polarity (with abluminal nuclei and basal cytoplasmic clearing due to Golgi apparatus) to direct the synthesized molecules towards the glomerulus
- Interstitium: contains fibroblast-like cells and peritubular capillaries; expands due to edema and inflammation
- Medullary rays: in cortex, contain cortical collecting tubules and loops of Henle of superficial nephrons
- Proximal tubules: long microvilli, numerous mitochondria and extensive intercellular interdigitations assist in reabsorption of sodium, water, proteins, glucose, potassium, phosphate and amino acids; vulnerable to toxins and ischemic damage
- Renal columns of Bertin: cortical tissue extending into spaces between pyramids
Contributed by Grigory Demyashkin, M.D., Ph.D.
6 - 8 week embryo:
Images hosted on other servers:
- Layers (inner to outer) are: fenestrated endothelium, then glomerular basement membrane (lamina rare interna, lamina densa and lamina rare externa), then podocytes (visceral epithelium with foot processes); also parietal epithelium which lines Bowman space (which contains the ultrafiltrate of plasma)
- Glomerular basement membrane (GBM): normally 250 - 380 nm, composed of type IV collagen, laminin, polyanionic proteoglycans (mostly heparan sulfate), fibronectin and entactin
- Type IV collagen forms suprastructure to which other glycoproteins attach; composed of 3 alpha chains
- Each alpha chain has amino 7S domain, middle triple helical domain and a carboxyl noncollagenous (NC1) domain; NC1 domain is site of anti-GBM nephritis and dimer formation
- Mesangial cells: type of myofibroblast that supports glomerular tuft, regulates capillary width and blood flow; are phagocytic and can proliferate; the mesangium on the capillary side is covered by endothelial cell; the capillary basement membrane extends to form the paramesangial basement membrane; at ultrastructural level, the mesangium shows cell membrane dense bodies (attachment plaques), which anchors the mesangial cell to the cell membrane
- Podocytes (visceral epithelium): their foot processes embed in lamina rare externa of glomerular basement membrane; the distal diffusion barrier to filtration of proteins is a filtration slit diaphragm between foot processes
- PAS stain allows assessment of glomerular basement membranes, mesangial matrix and tubular basement membranes
- Jones methenamine silver (JMS) highlights these better than PAS
- Masson trichrome stain highlights hyalinosis, scarring, immune deposits and fibrinoid deposits
- Immune complex tubulointerstitial nephritis
- Caused by antibodies against LDL-Receptor Related Protein 2 (LRP2) / megalin, a component of the proximal tubular brush border
- Presence of serum anti-brush border antibodies targeting LRP2 (J Am Soc Nephrol 2018;29:644)
- Acute tubular injury with loss of brush borders, thickened tubular basement inflammation with focal tubulitis
- Diffuse granular IgG and C3 immune complex deposits along tubular basement membranes (J Am Soc Nephrol 2016;27:380)
- Focal glomerular subepithelial immune complex deposition
- Often progresses to end stage kidney disease
- Anti-brush border antibody (ABBA) disease
- ABBA tubulointerstitial nephritis
- Anti-LRP2 nephropathy (more specific name)
- ICD-10: N12 - tubulointerstitial nephritis (not specified as acute or chronic)
- Very rare but may be under recognized
- M > F, mostly reported in older Caucasians (J Am Soc Nephrol 2018;29:644)
- Kidney, tubules and glomeruli
- Not well established
- Circulating antibodies to LRP2 (component of brush border) causes tubular injury and immune complex deposition in tubular basement membranes
- Immune complexes composed of IgG and C3 (mostly IgG4 subtype)
- Scattered glomerular subepithelial immune complexes also present
- Can cause progressive interstitial fibrosis and tubular atrophy, culminating in end stage kidney disease
- May recur after transplant (J Am Soc Nephrol 2016;27:380)
- Idiopathic; no established triggers or associations
- Progressive renal insufficiency
- Presence of serum anti-brush border antibodies targeting LRP2
- Acute tubular injury with loss of brush borders, thickened tubular basement membranes and interstitial inflammation with focal tubulitis
- Diffuse granular IgG and C3 immune complex deposits along tubular basement membranes
- Elevated serum creatinine, slowly progressive decrease in glomerular filtration rate
- Bland urine sediment
- Progressive interstitial fibrosis and tubular atrophy in advanced cases
- 73 year old man with ABBA tubulointerstitial nephritis, recurred in allograft (J Am Soc Nephrol 2016;27:380)
- 90 year old Caucasian woman with anti-LRP2 nephropathy and abundant IgG4+ plasma cells (Am J Kidney Dis 2019;74:132)
- Immunosuppression and plasma exchange have limited utility
- May recur after transplant
- Acute tubular injury with loss of brush borders, thickened tubular basement membranes and interstitial inflammation with focal tubulitis
- Variable but often severe interstitial fibrosis and tubular atrophy
- Presence of abundant IgG4+ plasma cells has been described (Am J Kidney Dis 2019;74:132)
- Diffuse granular IgG and C3 immune complex deposits along tubular basement membranes (mostly IgG4 subclass)
- Scattered glomerular subepithelial immune complexes also present
- Patient serum reacts with normal human brush border (anti-brush border antibodies) (J Am Soc Nephrol 2016;27:380)
- LRP2 co-localizes with tubular basement membrane immune complex deposits (J Am Soc Nephrol 2018;29:644)
- PAS highlights thickened glomerular basement membranes
- IgG/IgG4 immunostains: no increased ratio of IgG4/IgG positive plasma cells (SV40: negative for polyomavirus infection
- Prominent electron dense deposits in the proximal tubular basement membranes
- Scattered glomerular subepithelial electron dense deposits
- Kidney; biopsy:
- Anti-LRP2 nephropathy
- Focal global glomerular sclerosis
- Severe interstitial fibrosis and tubular atrophy
- Microscopic description: 15 glomeruli, 5 globally sclerosed (33%). Remaining glomeruli appear essentially normal. Tubular basement membranes show extensive tubular injury with loss of brush borders, mild interstitial inflammation with tubulitis and thickening of the tubular basement membranes. There is severe interstitial fibrosis and tubular atrophy.
- Immunofluorescence microscopy: Granular to confluent tubular basement membrane staining for IgG and C3. IgG subclass staining demonstrates predominant IgG4 staining. Segmental granular glomerular basement membrane staining for IgG and C3 is also present.
- Electron microscopy: Extensive electron dense deposits in proximal tubular basement membranes. Scattered glomerular subepithelial deposits with glomerular basement membrane spikes and focal podocyte foot process effacement.
- Lupus nephritis
- Clinical diagnosis of lupus and positive lupus serologies
- Usually predominantly glomerular immune complex deposition +/- tubular basement membrane deposits
- "Full house” immunofluorescence (positive for IgG, IgA, IgM, C3, C1q, and kappa and lambda light chains)
- IgG4-related tubulointerstitial nephritis
- Usually multi-organ involvement
- Storiform fibrosis with IgG4+ plasma cells
- ABBA/LRP2 negative
- Idiopathic hypocomplementemic tubulointerstitial nephritis
- Low complement levels
- Giant cell tubulitis with tubular basement membrane deposits
- Associated with aprotinin, a drug used to reduce bleeding during cardiac and liver surgeries
- Multinucleated giant cells cuffing tubular basement membranes
- Polyomavirus nephropathy
- Occasional tubular basement membrane immune complex deposits
- Transplant patients
- Viral cytopathic effects, positive for SV40 immunostain
- Which of the following findings is seen in anti-brush border antibody tubulointerstitial nephritis?
- Diffuse glomerular subepithelial immune complex deposition without tubular basement membrane deposits
- Granular tubular basement membrane immunofluorescence staining for IgG and C3 with sparse glomerular subepithelial immune complex deposition
- Linear glomerular basement membrane and tubular basement membrane immunofluorescence staining for IgG and kappa only, with powdery electron dense deposits by electron microscopy
- Proliferative glomerulonephritis with full house immunofluorescence staining in the glomeruli, tubular basement membranes and arterioles
Comment Here
Reference: Anti-brush border antibody disease/Anti-LRP2 nephropathy
- Which of the following best describes the pathophysiology of anti-brush border antibody tubulointerstitial nephritis?
- Patients develop autoantibodies against components of the alternative pathway of complement
- Patients develop autoantibodies against LRP2 / megalin, a component of the proximal tubular brush border
- Patients develop autoantibodies against podocyte antigen PLA2-R
- Patients develop mutations in the gene encoding LRP2 / megalin, a component of the proximal tubular brush border
Comment Here
Reference: Anti-brush border antibody disease/Anti-LRP2 nephropathy
- Immune complex small vessel vasculitis caused by autoantibody to glomerular basement membrane (GBM) collagen, resulting in rapidly progressive glomerulonephritis and sometimes lung hemorrhage (Clin Exp Nephrol 2013;17:603)
- Anti-GBM disease
- Rapidly progressive glomerulonephritis
- Crescent formation with segmental fibrinoid necrosis
- Bright linear ribbon-like appearance IgG in GBM
- Anti-GBM disease
- Goodpasture syndrome
- Rare, with an incidence of 0.5 - 1.6 cases per 1 million per year (Clin J Am Soc Nephrol 2017;12:1162, Clin J Am Soc Nephrol 2016;11:1392)
- Bimodal age distribution with peaks in second and sixth decades of life; rare in children < 11 years old
- Male predilection in second decade and female in sixth decade
- More common in Caucasians; rare in African Americans
- Kidney
- Lung
- It is likely that environmental triggers act in genetically susceptible individuals to develop pathogenic autoantibodies against the noncollagenous domain 1 of the a3 chain of type IV collagen (a3(IV)NC1)
- These autoantibodies bind to GBM and activate the classical pathway of the complement system, which starts neutrophil dependent inflammation (Nephrol Dial Transplant 2018;33:196)
- The factors that initiate the autoimmune process remain unknown (Clin J Am Soc Nephrol 2017;12:1162)
- Strong HLA gene association, with approximately 80% of patients inheriting an HLA-DR2 haplotype
- Strongly associated with polymorphisms of MHC class II genes, with disease susceptibility correlating with human leucocyte antigen (HLA) DRB1 alleles (Nephrol Dial Transplant 2018;33:196)
- Infectious associations
- Inhalation of hydrocarbons and cigarette smoking (lung hemorrhage)
- Treatment with the anti CD52 mAb, alemtuzumab, a lymphocyte depleting agent that is increasingly used in the treatment of relapsing multiple sclerosis (N Engl J Med 2008;359:768)
- Acute kidney injury (> 90%) with hematuria and proteinuria (rarely in nephrotic range) (J Autoimmun 2014;48-49:108)
- 40 - 60% will have concurrent lung hemorrhage
- Other symptoms are dyspnea, rales, rhonchi, cough, shortness of breath, hemoptysis, chest pain
- A small minority of patients may present with isolated pulmonary disease (5 - 10%)
- Detection of circulating anti-GBM antibodies in conjunction with rapidly progressive glomerulonephritis or alveolitis
- Detection of circulating anti-GBM antibodies by using Western blot and ELISA (Clin J Am Soc Nephrol 2017;12:1162)
- Titers do not correlate with severity
- The pathogenic antibodies are usually of the IgG class, with IgG1 and IgG3 subclasses predominating
- Approximately 10% of patients do not have identifiable circulating antibodies with conventional assays
- Some female patients have high IgG4 titers associated with pulmonary hemorrhage
- Antineutrophil cytoplasmic antibodies (ANCA) present in 1/3 of anti-GBM disease patients
- The proportion of crescents observed in the biopsy sample correlates strongly with the degree of renal impairment at presentation (Nephrol Dial Transplant 2015;30:814, Clin J Am Soc Nephrol 2018;13:63)
- Poor prognostic factors
- Dialysis requirement at presentation or Cr > 5 mg/dL
- Oligoanuria
- Low percentage of normal glomeruli and large extent of interstitial infiltrate on biopsy
- Rarely recurs if anti-GBM alone (no ANCA)
- Poor prognostic factors
- 33 year old man with bizarre posttransplant anti-GBM disease (Cent Eur J Immunol 2019;44:210)
- 37 year old man with recurrent anti-GBM disease and T cell large granular lymphocytic leukemia (Medicine (Baltimore) 2019;98:e16649)
- 53 year old woman with anti-GBM disease treated with rituximab (Medicine (Baltimore) 2019;98:e17801)
- 61 year old woman with double negative anti-GBM disease (Oxf Med Case Reports 2019;2019:omy124)
- 79 year old woman with ANCA associated vasculitis and anti-GBM antibodies (CEN Case Rep 2020;9:42)
- Plasmapheresis along with cyclophosphamide and corticosteroids (Clin J Am Soc Nephrol 2017;12:1162)
- Glomeruli:
- Cellular crescents ± segmental fibrinoid necrosis (hallmark)
- Often involves > 75% of glomeruli
- These crescents will typically be of uniform age, in contrast to other causes of rapidly progressive glomerulonephritis where a mixture of cellular, fibrocellular and fibrous crescents may be seen
- In early or mild disease, segmental proliferative change may be seen, with infiltrating neutrophils or mononuclear lymphocytes
- In severe disease, rupture of Bowman capsule, periglomerular inflammation progressing to granuloma formation with multinucleate giant cells may be observed in a proportion of cases
- Thrombotic microangiopathy involving glomerular capillaries present in subset of anti-GBM glomerulonephritis
- Noncrescentic glomeruli may have segmental fibrinoid change, although often they may appear completely normal
- Atypical, mild forms with lobular mesangial expansion or minimal glomerular lesions and few crescents
- Cellular crescents ± segmental fibrinoid necrosis (hallmark)
- Interstitium:
- Interstitial inflammation consists of lymphocytes, plasma cells, neutrophils and macrophages
- Necrotizing vasculitis rare, may be due to presence of ANCA
- Direct immunofluorescence on frozen kidney tissue
- Bright linear ribbon-like appearance IgG in GBM (99%) (hallmark)
- An important caveat is that fluorescence may be negative or unclear in cases with severe glomerular inflammation, in which the underlying architecture is so disrupted that the linear pattern may not be recognized
- Linear tubular basement membrane IgG staining in 10 - 67%
- Immunohistochemistry not used for diagnosis
- PAS, Jones methenamine silver and trichrome are used to evaluate morphology but are not specific for the type of glomerular disease
- Ultrastructural findings are not specific for anti-GBM disease, showing features of crescentic glomerulonephritis including rupture of the GBM and extracapillary localization of fibrin and proliferating cells
- Electron dense deposits are not seen in isolated anti-GBM disease
GBM, Alport and anti-GBM
- Right kidney, biopsy:
- Anti-glomerular basement membrane glomerulonephritis
- Morphologic pattern:
- Necrotizing and crescentic glomerulonephritis with 85% crescents
- Adequacy:
- Adequate (cortex 80%, medulla 20%)
- Microscopic description:
- 27 glomeruli, 23 of these exhibited cellular crescents, most of them associated with segmental fibrinoid necrosis and/or neutrophilic infiltrate with karyorrhexis. Fibrosis occupies 10% of the interstitium with moderate lymphoplasmacytic infiltrate. Small arteries and arterioles show minimal interstitial fibrosis. There is no vasculitis.
- Immunofluorescence microscopy:
- Number of glomeruli: 3. All glomeruli showed linear IgG staining along the GBM. One glomerulus showed fibrinogen staining.
- Electron microscopy:
- Glomerular capillaries showed neutrophils. Fibrin deposition is present in Bowman space. The glomerular basement membrane showed focal rupture. No electron dense deposits were noted on the glomerular capillary basement membranes or mesangial areas.
- Pauci-immune crescentic glomerulonephritis:
- Lacks strong linear immunoglobulin deposition in GBM
- Presence of fibrocellular to fibrous crescents favors pauciimmune glomerulonephritis
- Immune complex-mediated glomerulonephritis:
- Tends to have more endocapillary cellularity and less necrosis
- Immunofluorescence studies distinguish these types of crescentic glomerulonephritides
- Crescentic glomerulonephritis and anti-GBM glomerulonephritis (concurrent):
- Careful evaluation of immunofluorescence and serologic test for anti-GBM necessary to establish diagnosis
- Diabetic nephropathy:
- Linear immunofluorescence of GBM for albumin and IgG may be noted
- Light microscopy appearances distinguish these types of diseases
- Monoclonal immunoglobulin deposition disease:
- Light chain restricted linear deposits along GBM and tubular basement membrane may be noted
- Cellular crescents rare
- A 28 year old man has had a decreased urine output over the past 6 days. Urinalysis shows 1+ proteinuria, 4+ hematuria and no glucose or ketones. A renal biopsy is done and the immunofluorescence staining with IgG is shown in the image. Which of the following underlying morphological characteristic is most likely to be present on the renal biopsy?
- Cellular crescents
- Hyaline deposits
- Mesangial hypercellularity and nodules
- Spikes in GBM
- Thrombi in glomerulus
Comment Here
Reference: Anti-GBM nephritis
- Which of the following is true about anti-glomerular basement membrane (GBM) nephritis?
- 90% of patients will have concurrent lung hemorrhage
- It is most common in African Americans
- Necrotizing vasculitis is a frequent finding
- The crescents will typically be of uniform age
- Titers of circulating anti-GBM antibodies correlate with severity
- Sclerosis of renal arterioles and small arteries, strongly associated with nonmalignant hypertension
- Hypertension can be both a cause and consequence of nephrosclerosis
- Hypertension associated nephrosclerosis is an important cause of chronic kidney disease (CKD) that affects African Americans disproportionately (Kidney Int Rep 2016;1:10)
- Arterionephrosclerosis is a chronic kidney disease attributed to nonmalignant hypertension
- Histopathological features include hyaline changes involving terminal branches of interlobular arteries, global glomerulosclerosis and proportional tubulointerstitial changes
- Individuals of African descent have strong predisposition to chronic kidney disease (CKD) and end stage renal disease (ESRD)
- APOL1 associated arterionephrosclerosis with risk alleles G1 and G2 tend to progress towards more advanced levels of CKD (Curr Opin Nephrol Hypertens 2013;22:266)
- Also called hypertension associated kidney disease
- The term hypertensive nephrosclerosis is becoming obsolete
- Advanced age
- More common in the African American population, especially at a younger age (as young as 20s) (Kidney Int 2002;62:172)
- M > F
- Strongly associated with hypertension
- Renal arterioles and small arteries
- Glomerulus
- Tubulointerstitium
- Medial and intimal thickening of small arteries and arterioles as a response to hemodynamic changes, aging, genetics or combination of factors
- Hypertension can be associated with vasoregulatory dysfunction at the level of the glomerular microcirculation (Curr Opin Nephrol Hypertens 2013;22:266)
- Arteriolar hyalinosis due to extravasation of plasma proteins through injured endothelium and increased deposition of basement membrane matrix
- Subsequent vascular narrowing and ischemia leading to glomerulosclerosis, chronic tubulointerstitial injury and reduced functional renal mass
- Hypertension
- Aging
- Genetics (G1 and G2 risk allele variants of apolipoprotein L1 [APOL1]) (Curr Opin Nephrol Hypertens 2013;22:266)
- Oxidative stress, chronic inflammation and smoking
- Progressive proteinuria, nephrotic range at times
- Low estimated glomerular filtration rate (eGFR) (Kidney Int Rep 2019;5:339)
- Renal insufficiency when associated with African descent, concomitant diabetes and poorly controlled blood pressure
- End stage kidney disease in 25 - 30% of patients (Am J Kidney Dis 2016;67:e21)
- Arterionephrosclerosis is a diagnosis of exclusion as it can be the cause and consequence of hypertension
- Arterionephrosclerosis is often misdiagnosed and likely includes cases of both focal segmental glomerulosclerosis (FSGS) and non-FSGS glomerular diseases (J Am Soc Nephrol 2008;19:2047)
- Hyperechogenic kidneys on ultrasound
- Deteriorating kidney function in a patient with high blood pressure
- Progressive proteinuria
- Individuals with APOL1 risk variants tend to progress to more advanced levels of CKD (Curr Opin Nephrol Hypertens 2013;22:266)
- Individuals of African descent have strong predisposition to CKD and end stage kidney disease
- Concomitant causes of microvascular pathology such as smoking, obesity, diabetes, aging and oxidative stress
- 31 year old woman with retinitis pigmentosa who had been diagnosed with renal failure due to nephrosclerosis related to hypertensive disorders of pregnancy (BMJ Case Rep 2020;13:e236137)
- 44 year old Hispanic woman, smoker, with hypertension and peripheral arterial disease who presented with nephrotic syndrome for 2 weeks (J Bras Nefrol 2020;42:484)
- 74 year old man, with hypertension and heavy smoking history, who presented with nephrotic proteinuria and chronic kidney disease (Clin Nephrol Case Stud 2022;10:82)
- Strict blood pressure control
- Fluid and salt restriction to manage chronic kidney disease
- Kidneys can be either normal or smaller with fine cortical granularity resembling grain leather; smaller size is due to cortical scarring and shrinkage
- 3 glomerular morphological patterns
- Obsolescent glomerulus, which may be a partially or wholly collapsed glomerular tuft, with accumulation of extracellular material in Bowman space
- Solidified global glomerulosclerosis, in which the glomerular tuft remains expanded but replaced by collagen and possibly other matrix material
- Segmental glomerulosclerosis, possibly due to glomerular hyperfiltration (Curr Opin Nephrol Hypertens 2013;22:266)
- Hyaline arteriolosclerosis predominantly involving the afferent arteriole; this is characterized by thickening of the arteriolar wall due to the accumulation of homogeneous material that stains pink on H&E stained slides
- Arterial medial thickening and subintimal fibrosis
- Proportional tubulointerstitial fibrosis
- Solidified and disappearing glomerulosclerosis, thyroidization type tubular atrophy and microcystic tubular dilatation may be seen more in African Americans with arterionephrosclerosis, with APOL1 risk variants (Mod Pathol 2015;28:95)
- Low intensity staining for IgM, C3 and C1q in areas of hyalinosis
- PAS: highlights hyaline arteriolosclerosis and glomerulosclerosis
- Jones silver: highlights wrinkled glomerular basement membrane, global and segmental glomerulosclerosis (Kidney Int 2002;62:172)
- Trichrome: highlights tubulointerstitial fibrosis (Semin Diagn Pathol 2018;35:360)
- Corrugated and thickened glomerular basement membrane
- Expansion of lamina rara interna
- G1 and G2 risk allele variants of apolipoprotein L1 (APOL1)
- Kidney, biopsy:
- Arterionephrosclerosis (see comment)
- Comment: Marked global glomerulosclerosis is seen in a background of hyaline arteriolosclerosis, medial smooth muscle hyperplasia, subintimal fibrosis and proportional tubulointerstitial fibrosis. Immunofluorescence study results show low intensity mesangial staining for IgM. Electron microscopic evaluation does not show significant podocytopathy. Overall findings are reflective of chronic benign hypertensive related changes.
- Primary (idiopathic) focal segmental glomerulosclerosis:
- Prominent foot process changes (Am J Kidney Dis 2016;67:e21)
- Fewer hyaline vascular arteriosclerotic changes
- Diabetic glomerulosclerosis:
- Diffusely thickened glomerular basement membrane and nodular mesangial expansion
- Hyaline arteriolosclerosis involving both afferent and efferent arterioles
- Afferent arteriole
- Both afferent and efferent arterioles
- Efferent arteriole
- Interlobar artery
Comment Here
Reference: Arterionephrosclerosis
- Apolipoprotein E (APOE) genotype
- COL4A5 mutations
- G1 and G2 risk allele variants of apolipoprotein L1 (APOL1)
- Phospholipase A2 receptor (PLA2R) antibodies
Comment Here
Reference: Arterionephrosclerosis
The vascular changes depicted in the image above represent which of the following processes or outcomes?
- Atherosclerosis
- Extravasation of plasma proteins through injured endothelium and increased deposition of basement membrane matrix in hypertension associated kidney disease
- Fibrinoid necrosis of small vessel vasculitis
- Misfolded proteinaceous material with a characteristic beta pleated sheet on electron microscopic examination
Comment Here
Reference: Arterionephrosclerosis
- Progressive hereditary cystic kidney disease due to various known mutations, with most present in genes coding for polycystin 1 (PKD1, chromosome 16p, most common) and polycystin 2 (PKD2, chromosome 4q) (Lancet 2019;393:919)
- End stage kidney disease in ~50% of cases by the age of 70, with a minority needing transplant (Can J Kidney Health Dis 2018;5:2054358118778568)
- Progressive hereditary autosomal dominant polycystic kidney disease (ADPKD) with several known associated gene mutations that may predict prognosis
- Associated with PKD1 mutations (85% of cases) and PKD2 mutations (~10% of cases and milder disease)
- Symptoms generally become apparent in the third to fourth decade
- Often progresses to end stage kidney disease, requiring transplant
- ICD-10: Q61.2 - polycystic kidney, autosomal dominant
- Prevalence of 1 - 2:1,000 births
- Occurs in all races
- M = F but tends to be more severe in men (Am J Kidney Dis 2000;35:1072)
- Cystic disease occurrence
- Kidneys
- Liver: most common nonkidney site; higher in females
- Less common sites: pancreas, seminal vesicles, spleen, ovaries
- Noncystic disease sites (Adv Chronic Kidney Dis 2010;17:173)
- Heart: left ventricular hypertrophy, mitral valve prolapse
- Intracranial aneurysms: most severe extrarenal manifestation
- Polycystin 1 and 2 are membrane proteins in renal tubule epithelial cells that help regulate cellular transport
- Mutation in these proteins leads to impaired metabolism of renal tubular epithelial cells, which is thought to cause cellular proliferation (Prim Care 2020;47:673)
- Cystogenesis occurs after a 2 hit process, which is progressive but usually clinically silent for decades (Prim Care 2020;47:673)
- Normal renal function is usually maintained until fifth or sixth decade
- Autosomal dominant mutations mostly present in genes PDK1 or PKD2
- Sporadic cases without known family history are rare
- May be asymptomatic
- Abdominal pain, hypertension, hematuria, renal failure (Kidney Int 2015;88:17)
- Increased risk of urinary tract infections and calculi (GeneReviews: Polycystic Kidney Disease, Autosomal Dominant [Accessed 1 July 2024])
- May present with symptoms due to cysts in other organs (liver, pancreas, spleen) (Kidney Int 2015;88:17)
- Ultrasound is useful for the initial evaluation, due to low cost and availability
- Magnetic resonance imaging (MRI) or computed tomography (CT) detects much smaller cysts and is better for younger people with earlier disease
- Diagnosis can be made with 100% specificity / sensitivity with a family history in combination with sufficient radiological findings (Lancet 2019;393:919)
- Imaging is not as reliable in the absence of family history
- Genetic testing can confirm, particularly without a family history
- Angiogram of adult type cystic kidney shows displaced vessels around numerous cysts
- Major prognostic factors in disease progression include genotype, age, sex, kidney function and total kidney volume (Lancet 2015;385:1993)
- Men have faster progression than women
- Mutation type determines rate of progression (PKD2 slower progression, PKD1 faster progression)
- Severe disease in PKD1 and TSC gene deletions (contiguous gene syndrome)
- Sickle cell trait, early onset of disease and hypertension
- 2 year old girl diagnosed with ADPKD who subsequently developed Wilms tumor (Front Pediatr 2024;12:1322142)
- 48 year old Chinese man who presented with anasarca, hematuria, weight gain with acute kidney injury and acute respiratory distress (Medicine (Baltimore) 2017;96:e8625)
- 53 year old man with ADPKD, flank pain and (difficult to detect) sarcomatoid renal cell carcinoma (CEN Case Rep 2021;10:199)
- Management of blood pressure and dietary restrictions (low sodium) (Prim Care 2020;47:673)
- Tolvaptan (vasopressin V2 receptor antagonist) slows progression of cysts (N Engl J Med 2017;377:1930)
- Laparoscopic nephrectomy for pain management
- Transplant
- Markedly enlarged kidneys with cobbled surfaces containing numerous bulging cysts of variable size, ranging from 0.5 cm to 5 cm in diameter
- Cut surfaces show cysts that contain clear to yellow hemorrhagic fluid and are located within the cortex and medulla
- Reference: D'Agati: Atlas of Nontumor Pathology - Non-neoplastic Kidney Diseases, 1st Edition, 2005
Contributed by Debra L. Zynger, M.D.
Images hosted on other servers:
- Findings within the kidney
- Expansile cysts within the cortex and medulla lined by simple flattened to cuboidal epithelial cells and containing proteinaceous grungy material
- Occasional micropapillary projections present within cysts
- Intervening renal parenchyma, including tubules and glomeruli between cysts
- Extensive tubulointerstitial scar with associated chronic inflammation
- Reference: D'Agati: Atlas of Nontumor Pathology - Non-neoplastic Kidney Diseases, 1st Edition, 2005
- PKD1 gene on 16p13.3 (altered in 85 - 90% of cases) produces polycystin 1; function unknown (OMIM: Polycystin 1; PKD1 [Accessed 1 July 2024])
- PKD2 gene on 4q13-23 (altered in 10% of cases) produces polycystin 2; later onset and development of chronic renal failure than PKD1 (OMIM: Polycystin 2; PKD2 [Accessed 1 July 2024])
- PKD3 gene: minority of cases; gene unmapped (OMIM: Polycystic Kindey Disease 3 With or Without Polycystic Liver Disease; PKD3 [Accessed 1 July 2024])
- Associated with TSC2 and PKD1 contiguous gene syndrome (Am J Surg Pathol 2002;26:198)
- 10% lack a family history and are considered new mutations
- Kidney, left, nephrectomy:
- Markedly enlarged cystic kidney, consistent with autosomal dominant polycystic kidney disease
- Negative for malignancy
- Autosomal recessive polycystic kidney disease:
- Has the PKHD1 mutation
- Very rare childhood condition
- Simple renal cysts:
- Acquired disease
- Usually only a few cortical cysts with normal renal function and size
- Acquired renal cystic disease:
- Normal kidney size
- Associated with chronic kidney disease and patients are usually on dialysis
- Medullary cystic kidney disease:
- Associated with MCKD1 mutations
Comment Here
Reference: Autosomal dominant polycystic kidney disease
- PKD1
- PKD2
- PKHD1
- PKHD2
Comment Here
Reference: Autosomal dominant polycystic kidney disease
- Autosomal recessive cystic kidney disorder, usually presenting with bilateral renal cystic disease at birth
- 1 per 20,000 live births
- Patients present prior to or at birth with frequent complications due to limited urine output including oligohydramnios, Potter sequence, joint deformities and pulmonary hypoplasia
- Early mortality is most common, usually due to pulmonary complications
- Perinatal mortality 30 - 50%; 5 year survival is 80 - 95% if survive first month of life (Pediatrics 2003;111:1072)
- In surviving cases with pulmonary hypoplasia, kidneys must be removed to allow for growth of lungs
- Usually no cysts other than kidney and liver but liver is always affected (every portal triad, every case) with herring duct cysts (ductal plate malformation) and congenital hepatic fibrosis
- Patients later develop hypertension, renal insufficiency, portal hypertension with splenomegaly or cholangitis
- May also include older patients presenting with hepatosplenomegaly, hypersplenism, variceal bleeding and cholangitis (Medicine (Baltimore) 2006;85:1)
- Markedly enlarged kidneys with smooth surface
- Small cysts in cortex and medulla
- Dilated channels are perpendicular to cortical surface
- Cysts are present in medulla (collecting ducts)
- Radially arranged, elongated cysts that form as dilations of all collecting tubules with fluid accumulation
- Cysts lined by cuboidal or flattened cells from collecting tubules
- Normal nephrons without cystic change / interstitial fibrosis are present in between the cysts
- The liver shows portal fibrosis with complex bile ductular profiles
- All portal tracts are involved
- Mutations in PKHD1 gene (Polycystic Kidney and Hepatic Disease 1, produces fibrocystin / polyductin) at 6p12, expressed in kidney, pancreas and liver (Braz J Med Biol Res 2006;39:1537, OMIM 263200)
- Most common bacterial infection related glomerulonephritides in countries with higher human development indices are IgA dominant infection related glomerulonephritis and infectious endocarditis associated glomerulonephritis
- Biopsy findings are variable
- Subepithelial hump shaped deposits in mesangial waist regions are a characteristic ultrastructural finding of IgA dominant infection related glomerulonephritis
- In contrast to acute poststreptoccocal glomerulonephritis, which develops after resolution of infection and is most common in children, both IgA dominant infection related glomerulonephritis and infectious endocarditis associated glomerulonephritis are associated with ongoing infection in adults
- Staphylococcus aureus is the most common infectious trigger of both IgA dominant infection related glomerulonephritis and infectious endocarditis associated glomerulonephritis
- A subset of patients develop positive antineutrophil cytoplasmic antibody (ANCA) serologies
- Treatment of underlying infection is required
- Diabetes and advanced age portend a worse prognosis
- Staphylococcus infection associated glomerulonephritis
- Infection related glomerulonephritis with IgA deposits
- IgA dominant postinfectious glomerulonephritis
- IgA dominant infection related glomerulonephritis (Nat Rev Nephrol 2020;16:32, Diagn Pathol 2020;15:62, Hum Pathol 2003;34:1235, Am J Nephrol 2015;41:98):
- Male predominance
- Older adults with underlying comorbidities, such as diabetes mellitus, alcoholism and hypertension and active bacterial infection
- Most common infectious agent is Staphylococcus aureus
- Infection may be apparent (e.g. skin or soft tissue infections) or occult (e.g. abscess, endocarditis or osteomyelitis)
- Infectious endocarditis associated glomerulonephritis (Kidney Int 2015;87:1241):
- M:F = 3.5:1
- Mean age of 48 years
- Intravenous drug abuse, valvular heart disease, prosthetic heart valves, immunosuppression, malignancy
- Most common infectious agent is Staphyloccocus aureus, followed by Streptococcus species; there are also case reports of Bacillus cereus, Gemella morbillorum, Bartonella species, Cardiobacterium hominis and Coxiella burnetii
- Shunt nephritis (Clin Nephrol 1983;19:48):
- Complication of ventriculoatrial shunts used as palliative therapy for hydrocephalus, hardware infection
- Common pathogens include Staphylococcus epidermidis, Proprionibacterium acnes and Staphylococcus aureus
- As use of ventriculoperitoneal shunts is now favored, shunt nephritis has become very rare
- Kidneys
- Sometimes skin rash, due to ANCA vasculitis or leukocytoclastic vasculitis with IgA staining
- Immune reaction to bacterial proteins, including bacterial superantigens, such as staphylococcal enterotoxins, ultimately result in immune complex deposition in glomeruli, inflammation, complement activation and glomerular injury
- Proposed mechanisms of immune complex deposition in glomeruli:
- Bacterial antigens may be present in glomeruli (Kidney Int 2004;66:121)
- Bacterial antigen specific antibodies may cross react with glomerular antigens
- Neutrophil activation and formation of autoantibodies to neutrophil proteins
- Most patients present with acute kidney injury and nephritic or nephrotic syndrome
- Sites of infection include the upper respiratory tract, skin, lung, heart, urinary tract, teeth / oral mucosa, bone and deep seated abscesses (Kidney Int 2013;83:792)
- Most common bacterial infections are Staphyloccocus aureus and Streptococcus species; many other bacteria also reported (Kidney Int 2013;83:792)
- Infection may not be clinically apparent and is only discovered at the time of glomerulonephritis diagnosis in 16 - 45% of cases (J Am Soc Nephrol 2011;22:187)
- Positive bacterial cultures in 41 - 58% of cases (Medicine (Baltimore) 2008;87:21, Medicine (Baltimore) 1995;74:63)
- Clinical history
- Bacterial culture
- Serum complement levels
- Serologic workup for nephritic and nephrotic syndromes
- Kidney biopsy
- IgA dominant infection related glomerulonephritis (Nat Rev Nephrol 2020;16:32, Diagn Pathol 2020;15:62, Hum Pathol 2003;34:1235, Am J Nephrol 2015;41:98):
- Acute kidney injury (majority with rise in serum creatinine levels to > 3 mg/dL), hematuria and proteinuria (often nephrotic range) common
- Hypocomplementemia reported in up to 60% of cases
- May have circulating cryoglobulins (Clin J Am Soc Nephrol 2006;1:1179, Kidney Int Rep 2018;3:1128)
- Infectious endocarditis associated glomerulonephritis (Kidney Int 2015;87:1241):
- Acute kidney injury and a nephritic urine sediment common
- Nephrotic range proteinuria uncommon
- Hypocomplementemia common: low C3 levels present in 53% of patients, low C4 levels in 19% of patients
- Serologic testing for ANCA positive in 28% of patients
- Diabetes and advanced age portend a worse prognosis (Medicine (Baltimore) 2016;95:e3386)
- IgA dominant infection related glomerulonephritis:
- Progression to end stage renal disease in 20 - 80% of patients (Am J Nephrol 2015;41:98, Hum Pathol 2003;34:1235)
- Infectious endocarditis associated glomerulonephritis:
- Complete recovery in 32% of patients, persistent renal dysfunction in 37%, end stage renal disease in 10% and death in 21% (Kidney Int 2015;87:1241)
- 33 - 75 year old men and women with Staphylococcus auerus and IgA dominant deposits and cryoglobulinemic features on biopsy (Kidney Int Rep 2018;3:1128)
- 56 - 80 year old men and women with Staphylococcus auerus infection, biopsied for acute renal failure with heavy proteinuria and hematuria (Clin J Am Soc Nephrol 2006;1:1179)
- 64 year old man with Staphylococcus auerus infection, presenting with leukocytoclastic vasculitis, oliguric acute kidney injury, microscopic hematuria and nephrotic range proteinuria (Can J Kidney Health Dis 2018;5:2054358118776325)
- Primary treatment is antibiotic therapy targeting underlying infection
- Some treat with immunosuppression in addition to antibiotic therapy in cases with ANCA positivity and crescentic glomerulonephritis (Medicine (Baltimore) 2020;99:e21358)
- IgA dominant infection related glomerulonephritis:
- Most cases with some amount of endocapillary hypercellularity with neutrophils
- May be diffuse and global (acute exudative glomerulonephritis) or focal and segmental
- Focal crescents can be seen
- Rarely may have focal cryoglobulin-like features, including scattered subendothelial wire loop deposits and intracapillary hyaline pseudothrombi (Clin J Am Soc Nephrol 2006;1:1179, Kidney Int Rep 2018;3:1128)
- Infectious endocarditis associated glomerulonephritis:
- Findings variable
- A crescentic glomerulonephritis is often found
- With or without endocapillary or mesangial hypercellularity
- With or without membranoproliferative features
- IgA dominant infection related glomerulonephritis:
- Granular predominantly mesangial and some capillary wall staining for C3 and IgA
- IgA is sole, dominant or codominant immunoglobulin
- Infectious endocarditis associated glomerulonephritis:
- Usually dominant staining for C3 and IgM, followed by IgG and IgA
- May be pauci-immune (< 2+ intensity staining)
- IgA dominant infection related glomerulonephritis:
- Scattered mesangial deposits
- Subepithelial hump shaped deposits particularly at mesangial waist (reported in 31 - 100% of cases) (Hum Pathol 2008;39:1309)
- In rare cases with cryoglobulin-like features, large subendothelial and intraluminal deposits seen
- Infectious endocarditis associated glomerulonephritis:
- Small mesangial and capillary wall deposits
- Subepithelial hump shaped deposits uncommon
- Genome wide association study of individuals of European ancestry identified polymorphisms in genes encoding the MHC class II antigens HLA-DRA and HLA-DRB1 to be associated with susceptibility to Staphylococcus aureus infection (J Infect Dis 2016;213:816)
- Genetic contributions to why only a subset of individuals with bacterial infections develop renal pathology have yet to be determined
- Native kidney, biopsy:
- IgA dominant infection related glomerulonephritis
- IgA nephropathy:
- Chronic disease
- Serum C3 level usually normal
- Light microscopy: exudative glomerulonephritis uncommon
- Immunofluorescence: IgA staining usually stronger than C3
- Electron microscopy: mesangial deposits common, humps absent
- IgA vasculitis:
- Not associated with older age or diabetes
- Light microscopy: exudative glomerulonephritis uncommon
- Immunofluorescence: IgA staining usually stronger than C3
- Electron microscopy: mesangial and subendothelial deposits can be seen, humps absent
- ANCA vasculitis:
- Light microscopy: crescents without mesangial or endocapillary hypercellularity
- Immunofluorescence: pauci-immune
- Electron microscopy: few to absent deposits
What is the most appropriate primary diagnosis on this biopsy from an 80 year old woman with poorly controlled hypertension and diabetes and untreated cellulitis, who presents with new onset hematuria and proteinuria and is found to have low serum C3?
- Acute postinfectious glomerulonephritis
- Diabetic glomerulosclerosis
- IgA dominant infection related glomerulonephritis
- New onset IgA nephropathy
Comment here
Reference: Bacterial infection related glomerulonephritis
- 10 year old boy with acute nephritic syndrome 2 weeks following streptococcal pharyngitis
- 30 year old man with history of intravenous drug abuse, who presents with rash, acute kidney injury, hematuria and heavy proteinuria and is found to have positive MPO and PR3 ANCA serologies
- 40 year old woman with systemic lupus erythematosus, who presents with nephrotic syndrome
- 60 year old man with longstanding, poorly controlled diabetes, who presents with slowly progressive worsening renal function and proteinuria
Comment here
Reference: Bacterial infection related glomerulonephritis
- Discrete fluid filled cystic lesions within the kidney parenchyma or at the capsular surface
- Common benign lesion usually seen incidentally in adults
- Fluid filled cortical or medullary cystic lesions lined by flattened epithelial cells but may have papillary excrescences or clear cell change
- Generally asymptomatic and do not require intervention
- Prevalence in adults: 12 - 25%
- Present in up to 40% of patients undergoing abdominal imaging
- Rare in pediatric populations (< 1%) (J Urol 2002;167:21)
- Male predominance
- Renal cortex and medulla
- Diverticula of the distal collecting duct (DCT) or collecting tubules increase in number with age, probably as a result of weakening of the tubular basement membranes (Urol Res 1977;5:103)
- Predominantly asymptomatic and incidental
- Cyst hemorrhage may present with acute pain or asymptomatic microscopic hematuria
- Solitary or multiple renal cysts
- Unilateral or bilateral
- Risk factors: age, male, hypertension, renal dysfunction (BJU Int 2004;93:1300)
- Complications: hypertension
- Size and quantity increase with age, with more rapid growth under 50 years of age (J Urol 2002;167:21)
- 5.1% in fourth decade
- 36.1% in eighth decade
- Average increase in size and rate of enlargement is 2.82 cm and 6.3% yearly, respectively
- Incidentally found on abdominal CT or at autopsy
- Hairline thin wall with water attenuation (Radiology 2005;236:441)
- Absence of septations or calcifications
- No nodular enhancement
| Proposed Bosniak classification, version 2019* | ||
| Class | CT | MRI |
| I | Well defined, thin (≤ 2 mm) smooth wall; homogeneous simple fluid (29 to 20 Hounsfield units [HU]); no septa or calcifications; the wall may enhance | Well defined, thin (≤ 2 mm) smooth wall; homogeneous simple fluid (signal intensity similar to CSF); no septa or calcifications; the wall may enhance |
| II | 6 types, all well defined with thin (≤ 2 mm) smooth walls | 3 types, all well defined with thin (≤ 2 mm) smooth walls |
| IIF | Cystic masses with a smooth, minimally thickened (3 mm) enhancing wall or smooth minimal thickening (3 mm) of 1 or more enhancing septa or many (≥ 4) smooth thin (≤ 2 mm) enhancing septa | 2 types:
|
| III | 1 or more enhancing thick (≥ 4 mm width) or enhancing irregular (displaying ≤ 3 mm obtusely margined convex protrusion[s]) walls or septa | 1 or more enhancing thick (≥ 4 mm width) or enhancing irregular (displaying ≤ 3 mm obtusely margined convex protrusion[s]) walls or septa |
| IV | 1 or more enhancing nodule(s) (≥ 4 mm convex protrusion with obtuse margins or a convex protrusion of any size that has acute margins) | 1 or more enhancing nodule(s) (≥ 4 mm convex protrusion with obtuse margins or a convex protrusion of any size that has acute margins) |
- 4 year old girl with infected right simple renal cyst (BMC Pediatr 2021;21:265)
- 21 year old man with hypertensive retinopathy and irregular shaped kidney (Balkan J Med Genet 2022;24:83)
- 30 year old woman with back pain (Int J Surg Case Rep 2022;98:107614)
- 75 year old man and woman with asymptomatic renal cystic mass (Medicine (Baltimore) 2019;98:e15249)
- Generally, not indicated
- Percutaneous cyst aspiration if (Int J Urol 2003;10:63):
- Mass effect
- Pelvicalyceal obstruction
- Hypertension secondary to renal segmental ischemia
- Pain
- Hematuria
- Aspiration and sclerotherapy (AJR Am J Roentgenol 2008;190:1193, Abdom Radiol (NY) 2021;46:2875)
- Very rare: partial nephrectomy
- Thin walled round cysts in renal cortex
- Up to 10 cm maximum diameter
- Unilocular or rarely multilocular
- Smooth contour
- Filled with clear or yellow fluid
- References: StatPearls: Simple Renal Cyst [Accessed 6 February 2023], Nephrol Dial Transplant 2014;29:iv106
- Cyst wall is lined by single layer of cuboidal or attenuated epithelium
- Lining epithelium may be absent focally or entirely
- Atypical renal cysts have small papillary infoldings and a lining epithelium composed of cells with clear cytoplasm
- Negative for nests or expansile nodules of clear cells
- Cysts complicated by hemorrhage or infection may have hemosiderin laden macrophages and thicker fibrotic walls with focal calcifications
- Surrounding renal cortex shows effects of compression with chronic tubulointerstitial damage
- References: StatPearls: Simple Renal Cyst [Accessed 6 February 2023], Kidney Int 2006;70:1468, Postgrad Med J 1969;45:767
- CK7 membrane staining of lining epithelial cells
- PAX8 nuclear staining of lining epithelial cells
- Reference: Am J Surg Pathol 2016;40:202
- Right kidney, resection:
- Simple renal cyst
- Multilocular cystic renal neoplasm of low malignant potential:
- Cysts lined by cuboidal clear cells
- Fibrous septa contain small nests of clear cells
- Cystic renal cell carcinoma:
- Extensively cystic neoplasm with solid areas or expansile nodules of renal cell carcinoma
- Most commonly clear cell renal cell carcinoma
- Autosomal dominant polycystic kidney disease:
- Multiple cortical cysts on bilateral kidneys
- Decreased renal function
- Family history
- Genetic predisposition
- Acquired cystic disease:
- Patients undergoing long term renal replacement therapy
- Multiple cortical and medullary cysts in end stage chronic kidney damage
- Adult cystic nephroma / mixed epithelial stromal tumor:
- Female predominance
- Age: 40 - 50s
- Variable sized cysts lined by benign renal tubular epithelium
- Ovarian type stromal component; often estrogen and progesterone receptor positive
- Acquired sporadic simple renal cysts require no further investigation
- Large renal cysts are typically managed with laparoscopic cyst decortication
- Renal cell carcinoma is a common complication of simple renal cysts
- Simple renal cysts frequently result in obstruction of the of the segmental calyces
- The prevalence of sporadic renal cysts remains fairly constant beyond age 40
Comment Here
Reference: Benign renal cysts
Comment Here
Reference: Benign renal cysts
- Acute or chronic renal tubular injury secondary to bile containing casts in the setting of hyperbilirubinemia
- Presence of intratubular bile stained casts, especially in distal tubules / collecting ducts with acute tubular injury
- Associated with various hepatic diseases resulting in cholestasis and hyperbilirubinemia
- Also called cholemic nephrosis, cholemic nephropathy, bile nephrosis, jaundice related renal insufficiency, jaundice associated acute kidney injury, bile acid nephropathy and bile nephropathy
- Bile cast nephropathy is a pathologic term that emphasizes the severe end of the spectrum of the renal injury in this unique clinical situation (Kidney Int 2013;84:192)
- Emphasizes the findings that can be directly observed on histologic examination of the renal tissue (bile casts)
- Bile salts can also cause tubular injury (tubulopathy) in the absence of bile cast (J Nephrol 2006;19:229)
- However, there is no characteristic biopsy finding in this setting
- In the absence of bile casts, the term cholemic nephropathy may be more appropriate
- May be difficult to distinguish from other causes of hepatorenal syndrome
- ICD-10: N28.9 - disorder of kidney and ureter, unspecified
- Bile cast nephropathy is usually found in patients with advanced liver disease concomitant with cholestasis (Biochim Biophys Acta Mol Basis Dis 2018;1864:1356)
- Bile cast formation ranges from 2.6 - 73.5% of examined kidneys
- 61% of patients with cirrhotic jaundice and 100% of patients with alcohol induced cirrhosis (Kidney Int 2013;84:192)
- Renal tubules
- Direct tubular toxicity (J Nephrol 2006;19:229)
- Excess bilirubin believed to cause oxidative damage of the tubular cell membranes and uncoupling of mitochondrial phosphorylation (Hepatology 2013;58:2056)
- Sulfated bile salt inhibit Na-H, Na-K, Na-Cl pumps in proximal tubules and loop of Henle causing pH changes enhance bile cast deposition (Am J Physiol 1990;258:F986)
- Tubular obstruction
- Saturated bilirubin due to limited transportation in the proximal tubules leads to cast formation and tubular obstruction
- Bile acids are poorly water soluble which may also contribute to cast formation within the low pH microenvironment of the distal nephron
- Any insult leading to profound bilirubinemia, usually > 20 mg/dL (Kidney Int 2013;84:192)
- Hepatic or extrahepatic causes including:
- Cirrhosis of any cause (e.g., alcohol, drug)
- Acute hepatitis
- Obstructive jaundice
- However, some etiologies of hyperbilirubinemia including cirrhosis due to hepatitis C virus infection and nonalcoholic steatohepatitis (NASH) show a lower propensity for bile casts (Kidney Int 2013;84:192)
- Hemolytic jaundice generally does not show bile casts formation (Kidney Int 2019;96:1400)
- Symptoms related to hepatic dysfunction including jaundice, pruritus, scleral icterus and abdominal distension (J Nephrol 2006;19:229)
- Acute or chronic renal dysfunction including oliguria, anuria and urine discoloration
- Presence of bile stained tubular casts with acute tubular injury
- Increased serum creatinine, blood urea nitrogen, aspartate transaminase, alanine transaminase and bilirubin (elevated total bilirubin levels, typically > 20 mg/dL) (Kidney Int 2013;84:192)
- Presence of urinary muddy brown granular casts with bile staining and bile casts
- Reversibility of liver failure
- Reduction in serum bilirubin and bile acids might result in cessation of renal injury and renal recovery (World J Gastroenterol 2016;22:6328)
- 41 year old woman with progressive abdominal distension, jaundice and renal failure (Hemodial Int 2015;19:132)
- 54 year old man with chronic kidney disease and chronic osteomyelitis with intractable pruritus, anorexia and jaundice (World J Gastroenterol 2016;22:6328)
- 56 year old man with painless icterus, severe pruritus, acute kidney injury and hyperbilirubinemia (Case Rep Nephrol Dial 2018;8:98)
- 61 year old man with obstructive cholestasis caused by common bile duct stones, elevated serum creatinine and proteinuria (Am J Kidney Dis 2017;69:143)
- 63 year old man with cholangiocarcinoma and chronic kidney disease (Clin Case Rep 2018;6:779)
- Supportive treatment to reduce bilirubin burden and improve renal function including endoscopic retrograde cholangiopancreatography with stent replacement in case of biliary obstruction, plasmapheresis and hemodialysis
- Medical therapies including the use of steroids, cholestyramine, ursodeoxycholic acid and lactulose have shown minimal benefit (J Nephrol 2006;19:229)
- Liver or kidney transplantation
- Yellowish discoloration of the renal cortex and medulla and green discoloration due to conversion of bilirubin to biliverdin after formalin fixation (renal medulla may show deeper green color due to higher concentration of bilirubin in distal nephron segments) (Kidney Int 2013;84:192)
- Pigmented greenish yellow or dark red granular cast material in tubular lumens (Kidney Int 2013;84:192)
- Pigmented casts more prominent in distal nephron segments
- Proximal tubules and Bowman space involvement in severe cases
- Casts associated with variable acute tubular injury
- Pigmented material in cytoplasmic tubular resorption droplets
- Mononuclear inflammatory cells in the vasa recta may be seen
- Bile does not polarize (Nephrol Dial Transplant 2010;25:1909)
- Bile cast should be negative by immunofluorescence
- Hall (Fouchet) stain reveals yellow or grayish green color intratubular casts
- Myoglobin and hemoglobin immunostain
- Iron (Prussian blue) stain
- No specific findings
- Kidney, biopsy:
- Acute tubular injury with increased bile stained casts consistent with bile cast nephropathy
- Other pigmented cast nephropathies:
- Myoglobin casts: positive for myoglobin
- Hemoglobin casts: positive for hemoglobin (Kidney Int 2019;96:1400)
- Acute tubular injury with granular cellular eosinophilic casts (cellular debris):
- Hall stain negative without brown bile stained casts
- 2,8-dihydroxyadeninuria (DHA) crystals:
- Polarizable brown crystals
- Bile does not polarize (Nephrol Dial Transplant 2010;25:1909)
- Light chain cast nephropathy:
- Presence of light chain monoclonality detected from immunofluorescence study
- Light chain casts also show a two tone red blue appearance on trichrome stain and are frequently associated with a cellular reaction
A patient with alcoholic cirrhosis has acute kidney injury. At autopsy their kidneys show numerous red-brown colored tubular casts most notably in distal tubules and collecting ducts. The casts stain positively on Hall stain. What do these renal histology findings represent?
- Myoglobin casts nephropathy
- Hemoglobin casts nephropathy
- Bile cast nephropathy
- Light chain cast nephropathy
- Oxalate nephropathy
- Fulminant hepatic failure in the setting of drug toxicity
- Rhabdomyolysis
- Hemolytic anemia
- Multiple myeloma
Comment Here
Reference: Bile cast nephropathy
- Helps establish diagnosis and determine prognostic factors for renal disorders and transplant recipients
- Needle core or open biopsies are relatively safe and only rarely cause morbidity or mortality; outpatient complication rate is 8% (Clin Nephrol 2011;76:464)
- Also important for appropriate management of elderly and very elderly patients with kidney disease (Adv Chronic Kidney Dis 2012;19:61)
- Percutaneous ultrasound guided renal biopsy is safe, reliable and effective in children; most common indication is steroid resistant, steroid dependent or frequent relapsing idiopathic nephrotic syndrome (Hippokratia 2011;15:258)
- Pathology should correlate complete clinical and laboratory information (using a clinical form is recommended) with light microscopy, immunofluorescence and electron microscopy; cannot diagnose certain diseases without immunofluorescence or EM (Mod Pathol 2004;17:1555)
- Must carefully evaluate glomeruli, tubules, interstitium and vessels
- Specimen must be handled gently
- Don’t: use forceps, pull or stretch tissue, place tissue on dry gauze or water soaked gauze, freeze entire sample or place on ice cold saline
- Do: transport with tissue culture medium on saline moistened gauze; cut with fresh scalpel
- Dissecting microscope helps assess adequacy of glomeruli; place sample on glass slide with saline
- Two cores recommended
- Core #1: take samples 0.5 to 1.0 mm thick from each end with razor / scalpel and put in glutaraldehyde for EM; place remainder in saline, then fixative for light microscopy
- Core #2: take samples for EM, snap freeze the remainder for immunofluorescence
- Wrap light microscopy specimens in lens paper prewetted with fixative (avoid sponges or plastic embedding bags)
- If only one core or a small specimen is obtained, use tissue for EM and immunofluorescence because EM semi thin sections can also provide light microscopic information
- Fixative: mercury fixatives (Zenker, Bouin, other) provide optimal architectural and cytologic detail; ethanol fixation helps find glycogen or crystals of urate / uric acid
- Recommended to section through entire specimen, put 3 - 4 sections on each slide; for every batch of 5 slides, stain 1 with H&E, 1 with PAS and keep 3 unstained slides for possible future use
- Can detect immune complexes with antibodies or using fluorescence microscopy of H&E stained sections, fixed in Hollande fixative (Mod Pathol 2002;15:988)
- Best performed on unfixed, frozen sections
- Examine for IgG, IgM, IgA, IgH, C3, C1q, C4, fibrin, kappa and lambda
- Should include positive and negative controls for each run
- Immunoperoxidase may be a substitute (cheaper, can correlate with H&E, doesn’t fade) but complement antigens are difficult to detect, may have higher background staining
- Minimum glomeruli:
- 5 - 10 in general
- 10 for crescentic disorders
- 1 may be sufficient for diffuse lesions such as membranous glomerulonephritis
- Immunohistochemistry: IgG, IgA, IgM, C1q, C3, C4, fibrinogen and fibrin
- Frozen section: requested to determine adequacy (% sclerotic glomeruli) in donor kidney for transplant
- Transplant biopsies: performed to assess rejection
- Uses osmium tetroxide or glutaraldehyde for fixation (cannot perform if tissue exposed to B5, Zenker or other mercury based fixatives; can reprocess tissue from paraffin block)
- Embed in epoxy resin stain semithin (one micron thick) sections with toluidine blue or methylene blue
- Obtain thin sections for EM stained with uranyl acetate and lead citrate
- Interstitial nephritis caused by invasion of the renal parenchyma by BK polyomavirus; most commonly seen in the posttransplant setting
- Resulting disease is referred to as polyomavirus nephropathy
- Polyomavirus nephropathy is caused by a ubiquitous, opportunistic, nonenveloped double stranded DNA virus belonging to the Polyomaviridae, with a very high seroprevalence rate in adults
- It is characterized by interstitial nephritis and tubular injury and is most commonly encountered in the setting of immunodeficiency, especially in posttransplant patients, where the reactivated dormant virus in the uroepithelium escapes immunosurveillance
- Most common histomorphologic changes encompass patchy interstitial inflammation and cytopathic changes in the tubular epithelial cells
- Minimal and early stages of polyomavirus nephropathy may present with normal renal function and may not show intranuclear viral inclusion bodies, tubular injury or significant renal injury; this is why screening and routine staining of renal allograft biopsies (including surveillance biopsies) with SV40 immunohistochemistry is recommended: unchecked infection leads to renal scarring and allograft loss (BMC Nephrol 2021;22:226, Transplantation 2008;85:1008)
- Reduction of immunosuppression is mainstay therapy
- BK polyomavirus nephropathy
- BK virus associated nephropathy
- Polyomavirus nephropathy
- Definitive BK polyomavirus nephropathy: patients with biopsy proven viral nephropathy
- Presumptive BK polyomavirus nephropathy: patients with high viremia titers but no histologic confirmation of renal disease
- ICD-10: B33.8 - other specified viral diseases
- Encountered in the immunosuppressive settings, most commonly posttransplant and especially in those with overimmunosuppression
- Has become more prevalent with the introduction of new potent immunosuppressive medication
- More common in male patients, Afro-Caribbean ethnicity, pediatric patients and the elderly
- In kidney transplant recipients the incidence of BK polyomavirus viruria is ~30 - 40%, that of viremia is ~13% and that of BK virus nephropathy is ~8% (N Engl J Med 2002;347:488)
- Renal disease
- Virus is acquired by direct person to person contact, through contaminated surfaces, food and water
- Infection, which is most often subclinical, is usually encountered in childhood, where after the virus remains latent in the urothelial and renal tubular epithelial cells lifelong and is kept in check predominantly by T cells
- With immunosuppression (suppression especially of T cell immune surveillance) the virus, either the dormant one in the host or donor derived (in a portion of transplant cases), reactivates and causes viremia by crossing into the peritubular capillaries (J Med Virol 2019;91:1136)
- Lytic replication of the reactivated virus results in lysis of renal tubular epithelial cells, release of daughter virions into tubular lumens, infection of adjacent cells, basement membrane denudation, viruria, influx of inflammatory cells, and eventually leads to renal fibrosis and graft failure if left untreated
- Viremia seldom causes infection of glomerular endothelial and mesangial cells, podocytes and Bowman capsular epithelial cells, potentially resulting in glomerular damage (J Clin Med 2019;8:1477)
- BK virus genome, encodes 2 viral oncoproteins: the large T antigen (TAg) and the small t antigen (tAg), which have been shown to target p63 and pRb, and can potentially modify the normal cell cycle, leading to cell immortalization and neoplastic transformation (Am J Transplant 2016;16:398)
- References: Transplantation 2016;100:2276, Nephrol Dial Transplant 2021;36:587
- BK virus infection is caused by a ubiquitous, opportunistic, nonenveloped double stranded DNA virus belonging to the Polyomaviridae family with a seroprevalence rate that can reach > 90% in adults (J Gen Virol 2003;84:1499, Transplantation 2014;97:451)
- It is nonpathogenic in the immunocompetent
- Infection is most common in renal transplant recipients followed by bone marrow transplant recipients; though it has also been reported following heart, liver and lung transplants (Transplant Rev (Orlando) 2015;29:175, Clin Kidney J 2016;9:310)
- Diminished cellular immune response seems to have a predominant contributory role (Transplantation 2008;85:185)
- Reported risk factors are (Transplantation 2014;97:451, see diagram below)
- Host immune status, pretransplant T cell senescence phenotype and genetic determinants (Transplantation 2017;101:1479, Transplantation 2014;97:451)
- Transplant procedure
- Intensity of immunosuppression
- Male gender
- Extremes of age
- Recipient and donor sero status
- Ureteral stents
- Initial viral load
- Afro-Caribbean ethnicity
- HLA mismatching
- Transplant rejection episodes
- Does not cause significant symptoms in the immunocompetent; causes subclinical infection
- Important cause of chronic kidney disease and early allograft failure following kidney transplantation
- It is also known to cause renal disease with a progressive decline in kidney function in nonrenal solid organ transplants
- In the immunocompromised it is associated with
- Fever
- Asymptomatic / symptomatic increase in serum creatinine levels
- Asymptomatic viruria
- BK virus associated nephropathy
- Hemorrhagic cystitis: gross hematuria
- Ureteric strictures
- Pneumonitis, retinitis, liver disease and meningoencephalitis in HIV patients and those with severe immunosuppression (Clin Infect Dis 2005;41:354, AIDS 2001;15:1196, J Infect 2014;68:S2, Lancet Infect Dis 2003;3:611)
- Possible association with bladder cancer and urogenital cancer (Clin Infect Dis 2005;41:354, Infect Agent Cancer 2018;13:12, Am J Transplant 2016;16:398)
- Reference: Clin Infect Dis 2005;41:354
- DNA or RNA based PCR in blood or urine samples
- Detection of decoy cells in urine cytology
- Urinary polyomavirus Haufen test by electron microscopy
- Molecular sequencing techniques (Front Immunol 2020;11:1763)
- Renal biopsy: gold standard
- References: Semin Nephrol 2016;36:372, Intervirology 2020 Dec 17 [Epub ahead of print], Pathogens 2021;10:150
- Increased serum creatinine, mild proteinuria and hematuria
- Virus positivity in the serum
- Virus positivity in the urine
- References: Semin Nephrol 2016;36:372, Intervirology 2020 Dec 17 [Epub ahead of print], Pathogens 2021;10:150
- Without screening and treatment, the natural course of BK virus nephropathy leads to 50% graft loss (J Am Soc Nephrol 2018;29:680)
- With reduction in suppression, patient and graft outcomes are similar to uninfected patients (Nephrol Dial Transplant 2019;34:1240)
- Graft loss is more common in class 3 polyomavirus nephropathy (J Am Soc Nephrol 2018;29:680)
- Early diagnosis is associated with better outcome
- Risk of graft loss is also associated with (Am J Transplant 2004;4:2082, Am J Transplant 2017;17:2065)
- High level viremia or sustained viremia
- Deceased donor transplantation
- Late acute rejection
- SV40 large T antigen positivity on the follow up biopsy
- 39 year old woman with heart transplant who developed BK virus nephropathy (J Bras Nefrol 2021;43:434)
- 41 year old woman with type I diabetes and pancreas / kidney transplant diagnosed with BK virus associated metastatic renal allograft carcinoma (Transplantation 2021;105:423)
- 58 year old woman with chronic lymphocytic leukemia who developed hematuria due to concurrent BK polyomavirus, adenovirus and cytomegalovirus (BMJ Case Rep 2021;14:e235981)
- 60 year old woman, a liver transplant patient with BK virus nephropathy of the native kidney (Kidney Int Rep 2021;6:1743)
- Screening and reduction of immunosuppression is mainstay therapy but carries the risk of rejection and development of de novo donor specific antibodies
- mTOR inhibitor based regimen may be beneficial
- Novel therapies: on trial
- Immunotherapy: intravenous immunoglobulin, cellular therapy (virus specific T cells)
- Vaccine
- No effective antiviral medication present
- No effective prophylaxis treatment
- Regular screening between 1 - 24 months posttransplant is recommended
- Reference: Viruses 2021;13:487
- Findings are composed of interstitial nephritis and tubular injury; these findings are focal
- Involvement is more prominent in the distal nephron, therefore evaluation of medulla is important, especially in early stage infection; it is recommended that 2 core biopsies be provided, at least 1 where the medulla is sampled (Clin J Am Soc Nephrol 2020;15:1015)
- Interstitial edema and inflammation, composed predominantly of mononuclear cells, accompanied by plasma cells and less frequently eosinophils and neutrophils; these changes may be absent in early stages of infection (Ren Fail 2019;41:855)
- Inflammation is patchy and associated with infected tubules, usually well demarcated from surrounding
- Tubulitis and tubular cell injury; cell necrosis / dropout, desquamation / sloughing, flattening
- Tubular epithelial cell cytopathy; maybe absent in early stages of infection
- Cell enlargement
- Nuclear hyperchromasia and anisonucleosis
- Intranuclear inclusions: basophilic granules, ground glass appearance or clearing, no halo; chromatin changes: smudging, clumping or peripheral margination
- Urine cytology: decoy cells (resemble cells in uroepithelial cancer); BK virus infected tubular epithelial cells that have been shed into the urine
- Rounded basophilic nuclei larger than the average transitional and tubular epithelial cells
- Nuclei have viral inclusions appearing as dense granular basophilia and no halo
- Dirty background: transitional, tubular and inflammatory cells and clumps of amorphous basophilic material (harder to appreciate in slides prepared with thin layer methods)
- Graded as rare, up to 4 per cytospin and > 10 per cytospin; when rare cells are present with lack of inflammation, chances of positive infection in biopsy sample is low
- Cytopathic changes of the glomeruli (Bowman capsular epithelial cells, podocytes, mesangial cells) and crescent formation may also be seen (J Clin Med 2019;8:1477)
- In early stage infection, morphological findings are only present in scattered cells, mostly in the medulla and may even be characterized by only a handful of SV40 positive cells without discernable cytopathy
- Interstitial fibrosis and tubular atrophy: late stage disease
- Histologic classification system: consisting of polyomavirus replication load / level (pvl) score and Banff score for interstitial fibrosis (ci) (J Am Soc Nephrol 2018;29:680)
- PVN class 1: pvl 1; ci ≤ 1
- PVN class 2:
- pvl 1; ci ≥ 2
- pvl 2; any ci score
- pvl 3; ci ≤ 1
- PVN class 3: pvl 3; ci ≥ 2
- Scoring of polyomavirus replication load / level (pvl): percentage of positive tubules / ducts by morphologic assessment of cytopathy or SV40 immunohistochemistry in the entire biopsy (cortex and medulla)
- pvl 1: 1%
- pvl 2: 1 - 10%
- pvl 3: > 10%
- References: Yonsei Med J 2004;45:1065, Nephrol Dial Transplant 2021;36:587
- No immunoglobulin or only nonspecific scarce staining
- Immune deposits (most commonly IgG and C3) may be present along tubular basement membranes (Am J Transplant 2007;7:1552)
- Peritubular C4d negative (positivity indicates accompanying antibody mediated rejection)
- Anti-SV40 antibody, immunohistochemistry, is positive (part of the routine panel used in transplant biopsy assessment)
- Large T antigen (LTAg), one of the proteins encoded by the BK polyomavirus genome shares sequence homology with the LTAg of the simian virus (SV40)
- Anti-SV40 antibody directed against the SV40 T antigen, hence crossreacts with the BK polyomavirus LTAg and is used to identify BK polyomavirus
- This antibody also crossreacts with the JC polyomavirus
- In situ hybridization for BK virus DNA
- Anti-BK polyomavirus VP1 recombinant antibody, immunohistochemistry: a novel monoclonal antibody directed against the viral VP1 capsid protein (Am J Transplant 2007;7:1552)
- C4d immunohistochemistry, routinely used to assess transplant biopsies: peritubular and glomerular capillary wall staining is negative unless accompanying antibody mediated rejection is present
- Urine cytology: Haufen - icosahedral aggregates of polyomavirus particles and Tamm-Horsfall protein visualized using negative staining electron microscopy (Ultrastruct Pathol 2009;33:222)
- These are viral particles that form in virally injured tubular epithelial cells and excreted in the urine and hence represent evidence of active disease
- Presence of BK virions, 30 - 50 nm in diameter nonenveloped particles arranged in paracrystalline arrays, in tubular epithelial cells
- This finding is not diagnostic of BK polyoma nephropathy / interstitial nephritis since dormant virions can persist in the tubular epithelial cells (Ultrastruct Pathol 2005;29:469)
- Presence of necrotic tubular cells, prominent lysosomal inclusions and luminal protein and cellular cylinders should also be sought
- Tubular basement membrane immune deposits: finely granular electron dense deposit with no substructure along the tubular basement membrane (Am J Transplant 2007;7:1552)
- Presence of certain HLA alleles may predispose to (lack of HLA-C7) or protect against BK virus nephropathy
- Killer cell immunoglobulin receptor genes of NK cells are also correlated with risk of infection; genotyping of recipients in the pretransplant or early posttransplant period is being envisioned
- Genotype of the virus and single nucleotide polymorphisms in the genes coding for the major viral capsid protein VP1 and the noncoding control region affects pathogenicity of the virus
- References: Viruses 2020;12:1417, Kidney Int 2013;84:359, Viruses 2021;13:1502
Histomorphological characteristics of BK virus associated nephropathy in a transplant patient
by Dr. Arzu Sağlam
- Kidney, allograft biopsy:
- BK virus associated nephropathy (see comment)
- No significant deposition on immunofluorescence microscopy. No evidence of T cell or antibody mediated rejection. Interstitial fibrosis / tubular atrophy absent. Arterial intimal fibrosis (mild) arteriolar hyalinosis (ah2).
- Microscopic description: Serial sectioning shows 120 glomeruli, 5 of which are globally sclerotic. Glomeruli appear close to normal by light microscopy. Endocapillary or extracapillary proliferation, necrosis, intracapillary inflammatory cells (g0), intracapillary thrombi or double contouring of the capillary walls are absent (cg0). Interstitium shows patchy multifocal edema and inflammatory infiltrate with predominance of lymphomononuclear cells and plasma cells. Tubular epithelial cells in these areas display features of cytopathic changes characterized by nuclear enlargement, coarse granular chromatin and nuclear clearing with peripherally condensed chromatin. Some of the tubuli contain granular casts with sloughed epithelial cells, others show simplification with flattened epithelial cells and dilated lumina. Immunohistochemical staining for SV40 shows positivity affecting 5 - 10% of tubuli represented in the biopsy sample (pvl 2). Narrow foci of IFTA can be identified, comprising less than 10% of the cortical parenchyma with scattered mononuclear cells (i-IFTA0, ci0, ct0). Immunohistochemical staining for C4d is negative. Arteries display mild intimal fibrosis (cv1). Intimal arteritis is not identified (v0).
- Immunofluorescence microscopy: 3 glomeruli observed
- Anti-IgG Ab: no deposits
- Anti-IgA Ab: no deposits
- Anti-IgM Ab: no deposits
- Anti-C3 Ab: no deposits
- Anti-C1q Ab: no deposits
- Anti-kappa Ab: no glomerular deposits, 2+ staining of tubular casts
- Anti-lambda Ab: no glomerular deposits, 2+ staining of tubular casts
- Comment: Biopsy findings are consistent with BK virus associated nephropathy. Tubulitis is restricted to areas of viral cytopathy. There is no evidence of accompanying T cell or antibody mediated rejection or significant chronic tubulointerstitial damage.
- JC virus nephropathy:
- Should be considered in patients with BK viremia loads inconsistent with strong SV40 staining in renal biopsy samples
- High grade urothelial carcinoma:
- Causes confusion especially in urine cytology, presence of Haufen in urine cytology and SV40 immunohistochemistry is diagnostic; other helpful morphologic findings are as follows:
BK virus: High grade urothelial carcinoma: - Hyperchromasia less pronounced
- Smudgy chromatin
- Homogeneous, opaque nucleus
- Absence of cell clusters
- Hyperchromasia more prominent
- Coarse chromatin
- Eccentrically located nucleus
- Presence of cell clusters
- Causes confusion especially in urine cytology, presence of Haufen in urine cytology and SV40 immunohistochemistry is diagnostic; other helpful morphologic findings are as follows:
- Acute T cell mediated rejection:
- SV40 immunohistochemistry is diagnostic (does not resolve diagnostic dilemma in cases with concurrent infection and rejection); other helpful morphologic findings are as follows:
BK virus: Acute T cell mediated rejection: - Cytopathic changes
- Tubulitis less prominent
- Inflammation patchy, well demarcated, more B cells
(Transplantation 2001;71:896) - No edema
- No vasculitis
- Neutrophils may be present
- Cytopathic changes absent but degenerative
regenerative changes may be confused with cytopathy - Tubulitis more prominent
- Inflammation less demarcated, more T cells
(Transplantation 2001;71:896) - Edema positive
- Vasculitis may be present
- Neutrophils rarer
- SV40 immunohistochemistry is diagnostic (does not resolve diagnostic dilemma in cases with concurrent infection and rejection); other helpful morphologic findings are as follows:
- Acute tubular injury:
- Nuclear degenerative / regenerative changes that accompany acute tubular injury may resemble viral cytopathy; the nuclear changes, however, are usually not as exaggerated, are composed of nucleolar enlargement and hyperchromasia and less often show chromatin changes that resemble intranuclear inclusions
- Moreover, inflammatory infiltration is not prominent, as that which accompanies viral infection
- SV40 immunohistochemistry also aids diagnosis
- Other viruses may have similar cytopathic changes, such as cytomegalovirus (presence of both intranuclear and intracytoplasmic inclusions and perinuclear halo may aid histologic diagnosis), adenovirus and human herpes simplex virus (presence of both intranuclear and intracytoplasmic inclusions may aid histologic diagnosis):
- Immunohistochemistry is diagnostic
- It should be kept in mind that concurrent infection with multiple viruses can be present in the immunosuppressed (BMJ Case Rep 2021;14:e235981)
- References: Yonsei Med J 2004;45:1065, Nephrol Dial Transplant 2021;36:587
A 36 year old kidney transplant patient presents with hematuria and laboratory tests show increased serum creatinine. Biopsy of the renal allograft reveals findings demonstrated in the photomicrograph above. Which of the entities below should be first on the differential diagnostic list?
- Acute tubular injury
- Antibody mediated rejection
- BK polyomavirus nephropathy
- High grade urothelial carcinoma
- T cell mediated rejection
- Intranuclear inclusions in tubular epithelial cells in renal biopsy
- Nuclear staining of tubular epithelial cells with SV40 immunohistochemistry in renal biopsy
- Inflammatory infiltrate composed predominantly of lymphomononuclear cells in renal biopsy
- Urinary Haufen in electron microscopic evaluation of urine cytology
What is your diagnosis?
- Acute hypersensitivity type tubulointerstitial nephritis
- Acute tubular injury
- BK virus associated nephropathy
- Graft versus host disease
- Pyelonephritis
- C3 related glomerulopathy manifested by broad, linear, extremely electron dense deposits with C3 within glomerular basement membrane, mesangium, Bowman capsule and tubular basement membrane (Colvin: Diagnostic Pathology - Kidney Diseases, 2nd Edition, 2015)
- Rare C3 related glomerulonephritis affecting mostly children and older people
- Clinical syndrome of glomerulonephritis with low level of serum C3, related to abnormalities of alternative complement pathway
- Varied glomerular pathology by light microscopy with predominant C3 and little or no immunoglobulin deposition by immunofluorescence
- Hyperdense intramembranous deposits in the glomerular basement membrane by electron microscopy (pathognomonic)
- Recurs in almost all renal allografts
- Membranoproliferative glomerulonephritis, type II
- ICD-10: N00-N08 - glomerular diseases
- Rare; estimated at 1 - 3 cases/million (Kidney Int 2018;93:977)
- Mean age at diagnosis 19 years; 45 - 60% in children 5 - 15 years old; 40% > 60 years old (J Am Soc Nephrol 2005;16:1392)
- F:M = 1.5:2 (Kidney Int 2012;82:454)
- Kidney (glomerular disease)
- Chronic activation of alternative complement pathway by precipitant factors / autoantibodies / genetic predisposition, resulting in the accumulation of complement components in the tissue, recruitment of leukocytes and inflammatory damage of glomerulus (Pediatr Nephrol 2017;32:43)
- Autoantibodies: C3 nephritic factor, autoantibodies to complement factor H, factor B or C3
- Genetic predisposition in complement component genes: CFH, CFI, C3, CFHR5 and factor H
- Precipitating factors: infection, post chemotherapy for breast cancer, monoclonal gammopathy
- Reference: Clin Exp Nephrol 2017;21:541
- Hematuria (~90%)
- Proteinuria (~95%) that may be in the nephritic range (~60%)
- Acute nephritic syndrome (~50%)
- Usually some degree of renal insufficiency
- Ocular drusen (yellow deposits) common
- Associated with acquired partial lipodystrophy (3 - 5%) (Nefrologia 2018;38:258)
- References: J Am Soc Nephrol 2005;16:1392, Pediatr Nephrol 2012;27:773
- Clinical syndrome of glomerulonephritis
- Light microscopy: varied glomerular pathology
- Immunofluorescence microcsopy: C3 dominant glomerular staining and little or no immunoglobulin deposition (C3 staining intensity ≥ 2 orders of magnitude more than any other immune reactant on a scale of 0 - 3+) (Kidney Int 2013;84:1079)
- Electron microscopy: hyperdense intramembranous deposits in the glomerular basement membrane (pathognomonic)
- ↓ Serum C3 in ~80% of patients (↓ C3dg and C3d)
- More common in children (100%) than adults (40%)
- C3NeF (Ab to C3bBb) present in > 80%
- Persists in > 50%
- CFH mutations 17%
- Reference: Colvin: Diagnostic Pathology - Kidney Diseases, 2nd Edition, 2015
- Spontaneous remissions are uncommon (J Am Soc Nephrol 2005;16:1392)
- End stage renal disease occurs in 40 - 50% after 10 years (Pediatr Nephrol 2017;32:43)
- Best predictors of outcome are kidney function, degree of proteinuria and arterial hypertension at initial manifestation (Pediatr Nephrol 2017;32:43, Clin J Am Soc Nephrol 2014;9:46)
- In allografts, recurrence is the norm, typically in first 3 years, with a 50% graft failure rate (Clin J Am Soc Nephrol 2014;9:600)
- 10 year old boy with dense deposit disease mimicking a renal small vessel vasculitis (J Am Soc Nephrol 2016;27:59)
- 11 year old girl with dense deposit disease treated with eculizumab (N Engl J Med 2012;366:1161)
- 12 year old boy with dense deposit disease and febrile sore throat (Saudi J Kidney Dis Transpl 2017;28:925)
- 52 year old man with dense deposit disease associated with monoclonal gammopathy (BMC Nephrol 2018;19:108)
- 63 year old woman with dense deposit disease associated with multiple myeloma (Clin Nephrol 2018;89:300)
- At this time, there is no universally effective treatment
- Mycophenolate mofetil (MMF) and steroids (Clin J Am Soc Nephrol 2020;15:1287)
- Optimal blood pressure control (Kidney Int 2017;91:539)
- Steroids, immunosuppression (not yet proven effective) (Semin Nephrol 2013;33:493)
- Complement inhibitors such as eculizumab (anti-CD5Ab) under evaluation (Clin J Am Soc Nephrol 2012;7:748, Clin J Am Soc Nephrol 2015;10:1773)
- In patients with CFH mutations, plasma exchange and recombinant CFH (Colvin: Diagnostic Pathology - Kidney Diseases, 2nd Edition, 2015)
- Varied glomerular pathology (Mod Pathol 2007;20:605):
- Mesangial proliferation (30 - 50%)
- Membranoproliferative glomerulonephritis (25 - 45%)
- Acute exudative glomerulonephritis (10 - 20%)
- Crescentic glomerulonephritis (10 - 20%)
- Predominant C3 staining (ribbon-like [garland] pattern) in glomerular basement membranes with or without railroad track or double contour pattern along glomerular basement membranes; coarse mesangial spherules or ring-like deposits
- Focal Ig deposits in minority (almost always 2 levels of fluorescence intensity less than C3)
- Reference: Colvin: Diagnostic Pathology - Kidney Diseases, 2nd Edition, 2015
- Immunohistochemistry not used for diagnosis
- PAS, Jones silver and trichrome are used to evaluate morphology but are not specific for the type of glomerular disease
- Glomerular basement membranes are thickened, eosinophilic, refractile and brightly PAS+; fuchsinophilic on trichrome
- Reference: Zhou: Silva's Diagnostic Renal Pathology, 2nd Edition, 2017
- Accumulation in the glomerular basement membrane of uniquely electron dense material in a continuous, elongated, ribbon-like pattern or in small nodular aggregates within the irregularly thickened lamina densa; these dense deposits usually involve long segments of basement membrane but occasionally only a few loops are involved (sausage string pattern)
- Same type of deposit is characteristically also present in the mesangium, Bowman capsule, tubular basement membranes and occasionally in the walls of arterioles and peritubular capillaries
- Similar dense deposits have been noted in splenic sinusoidal basement membranes and in the eye involving the choriocapillaris basement membranes and Bruch membrane
- Podocyte injury eventually occurs
- Reference: Dickersin: Diagnostic Electron Microscopy - A Text/Atlas, 2nd Edition, 2000
- Genetic predisposition in complement component genes (CFH, CFI, C3, CFHR5 and factor H) (J Am Soc Nephrol 2007;18:2447)
Membranoproliferative GN
- Right kidney, biopsy:
- Dense deposit disease with C3 membranoproliferative glomerulonephritis pattern
- Adequacy: adequate (cortex 80%, medulla 20%)
- Microscopic description:
- 23 glomeruli, 4 of these with global sclerosis
- Majority of glomeruli showed a diffusely thick glomerular basement membrane and mesangial hypercellularity with increased mesangial matrix
- Some glomerular basement membrane showed double contour
- Fibrosis occupying 15% of the interstitium with minimal lymphoplasmacytic infiltrate
- Immunofluorescence microscopy:
- Number of glomeruli: 4
- All glomeruli showed granular C3 (+++) deposits in mesangium and capillary walls
- There were no deposits of IgA, IgM, IgG, C1q or fibrin
- Ultrastructural study:
- A glomerulus was evaluated that showed a preserved general structure with thickening of the basement membranes due to deposits in the lamina densa of glomerular basement membrane
- These deposits were extensive, of different thickness and showed a very electron dense appearance
- These morphological findings are characteristic of dense deposit disease
- C3 glomerulonephritis:
- C3GN and DDD are a shared spectrum of disease: similar pathogenesis of aberrant activation of alterative complement pathway
- Similar light and immunofluorescence microscopy findings
- Lacks hyperdense deposits in densa lamina
- Monoclonal gammopathy of renal significance:
- C3 glomerulonephritis and dense deposit disease in older adults (≥ 65 years) is often associated with and may be driven by a monoclonal protein (Kidney Int 2018;94:178)
- Distinct from childhood form of C3GN and DDD; clone directed therapy should be considered (Kidney Int 2018;94:178)
- “Masked” monotypic immune deposits
- Paraffin immunofluorescence should be performed in older adults with C3GN / DDD to exclude masked monotypic deposits (Kidney Int 2015;88:867)
- Acute post-streptococcal or acute post-infectious glomerulonephritis:
- Post-infectious GN has prominent exudative features / neutrophilic influx, lacks chronic injury and often has a different clinical scenario
- IgG present and lacks hyperdense deposits in densa lamina
- Immunoglobulin mediated glomerulonephritis:
- Immune complex present, lacks hyperdense deposits in densa lamina
- Monoclonal Ig deposition disease:
- By definition, has Ig deposition (single light chain or heavy chain)
- C4 dense deposit disease:
- C4d present in deposits but little or no C3
- Lectin pathway activated rather than alternative pathway
- Clinical symptoms alone
- Combination of clinical symptoms and light microscopy
- Electron microscopy
- Light microscopy alone
- Low C3 levels alone
- Anticalcineurins (cyclosporine A and tacrolimus) were discovered in late 70's; constitute a basic component of all immunosuppressive protocols to control transplant rejection for solid organ graft recipients
- Nephrotoxicity is major concern (Clin J Am Soc Nephrol 2009;4:481)
- Nephrotoxic, hepatotoxic and neurotoxic
- Also causes gingival hyperplasia, hypertrichosis and lymphoma
- Nephrotoxicity is dose related, occurs in 3%
- Tacrolimus: immunosuppressive drug used frequently in renal transplants, effect may be mediated by binding to FKBP12, a cytosolic protein; FKBP12/FK506 binds to and inactivates calcineurin (a serine / threonine phosphatase), which inhibits calcium and calmodulin dependent B and T cell responses by blocking NFAT-mediated transcription
- Systemic levels of tacrolimus, if kept within a relatively narrow target window, may not be associated with nephrotoxicity (Transplant Proc 2009;41:3393)
- The presence of rejection does not rule out Tacrolimus or Cyclosporine toxicity
- Red cell exchange transfusion may be useful for severe toxicity (Pediatr Nephrol 2011;26:2245)
- Although mechanism of action is similar to cyclosporine A, mechanisms causing nephrotoxicity may differ (J Proteomics 2011;75:677)
- Treatment: reduce dosage
- Mild decrease in renal function and increase in serum creatinine, hypertension in 50%, reversible if dosage lowered and no morphologic changes in kidney
- Toxicity due to alteration in intrarenal hemodynamics (vasoconstrictive phenomenon)
- Similar to functional toxicity but more severe
- Microscopic changes include vacuoles in proximal tubules (due to dilated endoplasmic reticulum with giant mitochondria, large lysosomes) and microcalcifications
- Also arteriolar smooth muscle cell degeneration, endothelial cell swelling, intimal thickening, variable hyaline or mucoid deposits which narrow lumen
- Dose dependent and reversible
- Resembles hemolytic uremic syndrome
- Occurs days to weeks after transplantation
- Glomeruli and vessels show thrombotic microangiopathy with platelet and fibrin thrombi and minimal inflammatory infiltrate
- Poor prognosis
- In one study, most common cause was antibody mediated rejection, not cyclosporine toxicity (Am J Transplant 2010;10:1804)
- Hypertension and slow progression to renal failure
- Arterioles show nodular or diffuse hyalinosis of vessel walls or mucoid thickening of intima, leading to luminal narrowing
- Also diffuse interstitial fibrosis and tubular atrophy
- Early glomerular changes are aggregates of platelets and fibrin
- Late changes are focal and segmental glomerulosclerosis or global scarring
- Changes are irreversible
- Toxicity (in 1%) of tacrolimus is similar to cyclosporin A at level of renal vascular endothelium, leading to fibrin thrombi in glomerular capillaries and afferent arterioles (Am J Surg Pathol 1996;20:306, Am J Surg Pathol 1993;17:60)
- May also cause arteriolar hyalinosis and splitting / reduplication of glomerular basement membrane
- Definite immune checkpoint inhibitor associated acute kidney injury (ICI AKI) is confirmed by histological diagnosis and clinical consideration of risk factors in a patient who has undergone or is undergoing treatment with immune checkpoint inhibitor
- Criteria beyond histology indicate a probable or possible diagnosis (Kidney360 2020;1:130)
- ICI AKI develops at a median of 14 - 16 weeks after ICI initiation (J Immunother Cancer 2021;9:e003467, J Am Soc Nephrol 2020;31:435)
- ICI AKI should be considered in cancer patients with AKI once other common causes, such as volume depletion, hemodynamic fluctuations and sepsis, have been ruled out (Front Med (Lausanne) 2022;9:964335)
- Kidney biopsy is recommended when feasible (J Immunother Cancer 2021;9:e002435)
- Histology shows an interstitial inflammatory infiltrate composed of lymphocytes, plasma cells and eventually neutrophils with frequent tubulitis (Kidney Int 2016;90:638)
- ICI AKI, ICPI AKI: immune checkpoint inhibitor associated acute kidney injury (J Immunother Cancer 2021;9:e003467)
- Immune related adverse events (irAEs) (J Immunother Cancer 2021;9:e003467)
- Antiprogrammed cell death receptor 1 (anti-PD-1) antibodies, antiprogrammed cell death ligand 1 (anti-PDL1) antibodies and anticytotoxic T lymphocyte associated antigen 4 (anti-CTLA4) antibodies associated AKI
- ICD-10: N14.1 - nephropathy induced by other drugs, medicaments and biological substances
- Estimated incidence of AKI in a cohort study with projected AKI rates ranges from 9.9% to 29% (Am J Nephrol 2017;45:160)
- Other studies report lower incidence of the disease (2.2%) (Kidney Int 2016;90:638)
- Patients with ICI AKI are older, have lower baseline estimated glomerular filtration rate (eGFR) and are more likely to have genitourinary cancer, proton pump inhibitor (PPI) exposure and to have received combination ICI therapy compared with patients without ICI AKI (J Immunother Cancer 2021;9:e003467, Clin Kidney J 2022;15:1881)
- Most recently approved class, PDL1 inhibitors (e.g., atezolizumab, durvalumab, avelumab), may have a favorable safety profile with lower rates of organ toxicity, including AKI, compared to other ICI classes (Kidney Med 2021;3:1074)
- Atezolizumab had the strongest association with renal adverse events among all ICI monotherapy regimens, whereas avelumab appeared to show a relatively weaker association with renal adverse effects (Cancer Med 2020;9:6576)
- Kidney
- Anti-CTLA4 and anti-PD-1 / anti-PDL1 reactivate the suppressor immune response through various mechanisms
- Mechanism by which ICIs induce AKI is not well established (Kidney Int Rep 2020;5:1139)
- One mechanism by which ICIs may induce kidney damage is related to the activation of autoreactive T cells normally kept dormant by immune checkpoint mechanisms under physiological conditions
- PD-1 is expressed after activation on T cells, B cells, natural killer T cells, activated monocytes and dendritic cells, whereas its ligand PDL1 is expressed on kidney tubules, especially the proximal tubular segments (Clin Immunol 2005;115:184)
- Another mechanism may involve the reactivation of exhausted drug specific T cells previously primed by nephritogenic drugs (e.g., PPIs, nonsteroidal anti-inflammatory drugs [NSAIDs]) (Cancers (Basel) 2023;15:1891)
- Another potential mechanism is through haptenization, when low molecular weight drug compounds bind tubular antigens, thus creating a hapten that can be trapped in the parenchyma, leading to an immune response and tubular damage (Kidney Int Rep 2020;5:1139)
- Renal damage caused by anti-CTLA4 leads to early lymphocyte infiltration of renal tissue, with rapid onset averaging 6 - 12 weeks (Front Med (Lausanne) 2022;9:1014257)
- Conversely, anti-PD-1 / anti-PDL1 treatment determines loss of tolerance and subsequent stimulation of immune response, resulting in nephrotoxicity onset at 3 - 12 months (Front Med (Lausanne) 2022;9:1014257)
- Combination of CTLA4 and PD-1 inhibitors carries a higher risk of ICI induced AKI (Kidney Med 2021;3:1074)
- Glomerular damage, including podocytopathy, membranous nephropathy and thrombotic microangiopathy, has been reported after administration of ipilimumab alone; ipilimumab has also been associated with systemic lupus erythematosus-like nephritis, characterized by diffuse tissue damage and glomerular sclerosis (Front Med (Lausanne) 2022;9:1014257)
- ICI AKI: developed at a median of 14 - 16 weeks after ICI initiation (J Immunother Cancer 2021;9:e003467, J Am Soc Nephrol 2020;31:435)
- While hematuria (16%), eosinophilia (21%) and worsening renal hypertension (11%) were noted in some patients, rising serum creatinine (100%) and pyuria (68%) may be the only clinical clue in a large majority of the cases
- Only a few patients exhibit the classic symptoms of fever, eosinophilia or rash
- If distal renal tubule injury is involved, manifestations include hypokalemia and metabolic acidosis (Clin Kidney J 2019;13:42)
- 43% had a concomitant history of extrarenal irAEs, such as hypophysitis, thyroiditis, dermatitis, rash or colitis (J Am Soc Nephrol 2020;31:435)
- ICI can reactivate preexisting autoimmune diseases, such as rheumatoid arthritis, lupus and inflammatory bowel disease, in 27 - 50% of patients but adverse kidney outcomes in preexisting glomerulonephritis are less commonly reported (Kidney Med 2021;3:1074)
- ICI AKI should be considered in cancer patients with AKI once other common causes, such as volume depletion, hemodynamic fluctuations and sepsis, have been ruled out (Front Med (Lausanne) 2022;9:964335)
- The Society for Immunotherapy of Cancer (SITC) recommends kidney biopsy when feasible (J Immunother Cancer 2021;9:e002435)
- Identification of non-ICPI induced kidney lesions, such as acute tubular injury, on biopsy may avoid unnecessary corticosteroid exposure and permit continued ICI therapy, especially in cases of suspected ICI related glomerulopathy
- If empiric corticosteroids are started before biopsy and the patient fails to respond, a biopsy should be considered to assess alternative etiologies of AKI (J Immunother Cancer 2021;9:e003467)
- Similar to those observed with AKI caused by other drug induced acute tubulointerstitial injury (ATIN)
- Bland urinalysis with subnephrotic range proteinuria, sterile pyuria, white blood cell casts, eosinophilia, unless a vascular and glomerular lesion is present (Front Med (Lausanne) 2022;9:964335, Kidney Int Rep 2020;5:1139)
- Normal complement levels (C3 and C4) (Kidney Int 2016;90:638)
- Noninvasive markers for the definite diagnosis of ICI AKI are not yet available
- Bilateral increase in kidney size, new / increasing perinephric stranding and bilateral wedge shaped hypoenhancing cortical foci can occur in immunotherapy related nephritis (Eur Radiol 2023;33:2227)
- On PET / CT, a diffuse increase in radiotracer uptake throughout the renal cortex and a decrease in radiotracer activity in the renal pelvis can be seen (Eur Radiol 2023;33:2227)
- During a median follow up of 30 weeks from ICI AKI diagnosis, 39.2% of patients died (J Immunother Cancer 2021;9:e003467)
- Higher baseline eGFR, lung cancer and stage 3 AKI were each associated with lower odds of renal recovery, whereas concomitant treatment with ATIN causing medications was associated with higher odds of renal recovery (J Immunother Cancer 2021;9:e003467)
- Treatment with corticosteroids within 14 days following ICI AKI was also associated with a higher odds of renal recovery (J Immunother Cancer 2021;9:e003467)
- Critical role of the PD-1 pathway both in immune resistance by cancers arising in the context of long term immunosuppression and in the maintenance of partial adaptive tolerance to transplanted organs (N Engl J Med 2016;374:896)
- Outcomes for de novo ICI induced glomerular diseases are poor, with a limited ability to rechallenge with ICI, progression to end stage kidney disease and a significant mortality rate (Kidney Med 2021;3:1074)
- 57 year old man with successfully treated late onset ICI associated membranous nephropathy (Front Immunol 2022;13:898811)
- 60 year old man with sarcoid-like granulomatous reaction after ICI treatment for renal cell carcinoma (Kidney Med 2023;5:100626)
- 62 year old man with renal cell carcinoma and nivolumab associated nephrotic syndrome (J Immunother 2017;40:345)
- 67 year old man with stage IV non-small cell lung cancer who developed kidney injury during treatment with the anti-PD-1 antibody nivolumab (BMC Nephrol 2018;19:48)
- 73 year old man with atezolizumab associated rapidly progressive pauci-immune glomerulonephritis (Antibodies (Basel) 2023;12:10)
- Nephrology oncology approach is mandatory for treatment decisions
- When ICI related renal involvement is strongly suspected, the first step in management includes temporarily discontinuing ICI treatment and starting steroid therapies (Cancers (Basel) 2023;15:1891)
- Corticosteroid taper begins when creatinine improves to grade 1 or below
- Guidelines from the National Comprehensive Cancer Network are similar and specify a starting dose and duration for the corticosteroid taper at 0.5 - 1 mg/kg/day for grade 2 and 1 - 2 mg/kg/day for grade 3 AKI with the dose being tapered over 4 - 6 weeks after creatinine decreases to less than or equal to grade 1
- Compared with nonrechallenged patients, rechallenged patients were less likely to initially have had ICI AKI stage 3 and were more likely to have had renal recovery following the initial ICI AKI episode; survival was similar among patients rechallenged versus not rechallenged in the first 90 days and in the first 180 days following ICI AKI
- Consider additional immunosuppression (cyclophosphamide, azathioprine, cyclosporine, infliximab or mycophenolate) if AKI does not improve to less than grade 2 within 1 week (Kidney Med 2021;3:1074)
- Acute tubulointerstitial nephritis (ATIN) is the most common observation in ICI related AKI, histologically indistinguishable from ATIN secondary to other drugs (Kidney Int 2016;90:638)
- ATIN affects > 90% of patients who undergo biopsy (J Am Soc Nephrol 2020;31:435)
- Infiltrate is predominantly composed of lymphocytes, with varying degrees of plasma cells, eosinophils and neutrophils (Kidney Int 2016;90:638)
- Further characterization of the lymphocyte infiltrate shows a predominance of CD3+ T lymphocytes
- It can occur either alone or in combination with other glomerular or tubular pathologies (Kidney Med 2021;3:1074)
- Granulomatous formations with multinucleated giant cells
- Thrombotic microangiopathy
- Lupus nephritis
- Focal segmental glomerulosclerosis
- Membranous nephropathy
- IgA nephropathy
- Minimal change disease
- Renal tubular acidosis
- Acute tubular necrosis
- Non-ATIN patterns of damage likely cause < 10% of all ICI induced AKI (Kidney Med 2021;3:1074)
- Pauci-immune glomerulonephritis / vasculitis (27%), podocytopathies (20%) and C3 glomerulopathy (11%) are the most common non-ATIN manifestation of ICI toxicity (Kidney Int Rep 2020;6:66)
- ICIs may reactivate preexisting autoimmune diseases (Kidney Med 2021;3:1074)
- Immunostaining for IgA, IgG, IgM, C3, C1q, fibrinogen and kappa and lambda chains is usually negative or unspecific (Br J Cancer 2016;115:1457)
- Immunofluorescence may show a background staining for C3 along vessel walls, with no significant tubular basement membrane or glomerular staining (Kidney Int 2016;90:638)
- In electron microscopy, no specific findings are observed unless a concomitant glomerular pathology, such as focal segmental glomerulosclerosis (FSGS), IgA or membranous nephropathy, develops due to ICI therapy (Kidney Int 2016;90:638)
- Nonspecific inflammatory changes such as tubulitis can be observed
- Left kidney, needle core biopsy:
- Acute tubulointerstitial nephritis with predominant neutrophilic component
- Microscopic description: 7 glomeruli, 2 of which are globally sclerotic. Glomeruli show no evidence of mesangial or endo / extracapillary hypercellularity. No evidence of segmental sclerosis of the tuft. Tubular atrophy and interstitial fibrosis involving ~10% of the cortex. Focal areas of inflammation with lymphocytes and numerous neutrophils, extending to nonfibrotic areas with frequent tubulitis. Mild intimal fibrosis of medium size arteries.
- Immunofluorescence on the biopsy containing up to 2 glomeruli, negative for all antisera.
- Electron microscopy: no significant ultrastructural modifications affecting glomeruli, sparse mononuclear and polymorphonuclear inflammatory cells in the interstitium with occasional tubulitis foci.
- Use of nephritogenic drugs (PPIs, NSAIDs):
- Generally ATIN with prominent eosinophilic infiltrate sometimes associated with the formation of nonnecrotizing interstitial granulomas
- Urinary tract infections:
- Generally neutrophil dominant interstitial infiltrate with frequent intratubular neutrophilic plugs
- Avelumab
- Ipilimumab
- Nivolumab
- Pembrolizumab
Comment Here
Reference: Checkpoint inhibitor associated tubulointerstitial nephritis
Comment Here
Reference: Checkpoint inhibitor associated tubulointerstitial nephritis
- Chronic kidney disease of unknown etiology (CKDu) is likely a multifactorial chronic kidney disease seen among manual laborers (mostly agricultural workers) in low altitude areas of tropical countries; CKDu displays features of acute and chronic tubulointerstitial nephritis
- Early CKDu presents as acute tubulointerstitial nephritis with almost exclusively lymphocytic infiltration
- Many kidney damaging factors are thought to be responsible for CKDu initiation and progression; the environmental, toxicological, clinical and histological data does not support any single factor as the primary cause
- Disease progression is generally slow and inexorable, resulting in kidney failure
- CKDu at chronic kidney disease stages 2, 3 and 4 presents histopathologically as focal or diffuse interstitial fibrosis and tubular atrophy (IFTA) with or without lymphocytic inflammation
- Depending on the geographic location, CKDu is also called Mesoamerican endemic nephropathy (MeN), Sri Lankan nephropathy, Indian or Uddanam nephropathy
- Other CKDu synonyms: chronic interstitial nephritis of unknown etiology, chronic interstitial nephritis in agricultural communities (CINAC), global warming nephropathy
- Chronic kidney disease is defined based on the presence of kidney damage or glomerular filtration rate (GFR) 2 for ≥ 3 months, irrespective of the cause (Kidney Int 2011;80:1000)
- Tropical countries, low lying areas with hot tropical climate
- More common in men, 20 - 60 years of age, performing hard manual labor in agriculture; also (but much less frequently), CKDu is seen among their family members and the city population
- Incidence and prevalence rates for CKDu vary by location but are generally very high; for example, prevalence of CKDu (defined by glomerular filtration rate < 60 mL/min/1.73 m2) in a community in Nicaragua was 9.8% among women and 41.9% among men (MEDICC Rev 2014;16:16)
- CKDu has been reported in the following geographic locations
- Mesoamerican nephropathy: Pacific Coast from Mexico to Panama
- Sri Lankan nephropathy: North Central Province of Sri Lanka (Kidney Int Rep 2017;2:282)
- Uddanam nephropathy or Indian CKDu: central Indian states of Andhra Pradesh, Odisha, Chhattisgarh and Maharashtra (Kidney Int Rep 2017;2:282)
- Egypt (Kidney Int Rep 2017;2:282)
- Tunisia (Kidney Int Rep 2017;2:282)
- France (Kidney Int 2020;97:350)
- United States: CKDu is seen among the immigrant workers from Central America (Am J Emerg Med 2016;34:1323.e5)
- California Central Valley agricultural workers show increased odds of acute kidney injury (AKI) after heat strain but there are only a few unconfirmed reports of CKDu (N Engl J Med 2014;371:58, Workplace Health Saf 2019;67:481)
- Kidney
- Tubulointerstitial compartment
- Glomeruli
- Blood vessels (unclear)
- Acute kidney injury may lead to chronic kidney disease; this is the key pathogenetic event in CKDu development (N Engl J Med 2014;371:58)
- Progressive renal fibrosis requires repetitive damage of previously normal nephrons unless primary interstitial disease is itself the instigating factor for fibrosis (J Am Soc Nephrol 2015;26:1765)
- Progressive tubular atrophy and fibrosis are associated with failed differentiation of regenerating tubular epithelium, failure of capillary endothelial cells to regenerate and loss of vascular endothelial growth factor (VEGF) expression in proximal tubules (J Am Soc Nephrol 2015;26:1765)
- Further research is needed to understand the mechanism of acute kidney injury in CKDu endemic regions and its progression to CKDu
- Etiology of CKDu is unknown; it is likely a multifactorial disease
- Potential causes include
- Recurrent acute tubular injury due to strenuous physical work in a very hot climate leading to heat stress, dehydration and volume depletion
- Toxicity: pesticides (glyphosate), paraquat, heavy metals (Texas A&M Today: Study Reveals the Role of a Common Agricultural Chemical in Chronic Kidney Disease [Accessed 13 December 2023])
- Silica airborne from burned sugarcane during harvesting in Mesoamerican nephropathy or in the groundwater in Uddanam nephropathy (Am J Physiol Renal Physiol 2022;323:F48, Ann Work Expo Health 2023;67:i54)
- High nitrate level in groundwater (N Engl J Med 2014;371:58)
- NSAIDs
- Infections: leptospirosis, hantavirus
- Other toxins (Kidney Int 2020;97:350)
- Genetic (see below)
- Typical presentation of CKDu is a young male from an agricultural community in an endemic area with a family history of chronic kidney disease
- Acute kidney injury may be the first manifestation of CKDu; 8.4% of patients with acute kidney injury progress to chronic kidney disease (Kidney Int 2018;94:1205)
- In the acute kidney injury stage of CKDu, symptoms may include low grade fever, nausea, back pain, vomiting, headache, muscle weakness and paresthesias (Am J Trop Med Hyg 2017;97:1247)
- Anemia and paresthesias at presentation are the strongest predictors of progression to chronic kidney disease, while leukocytosis is associated with renal recovery (Kidney Int 2018;94:1205)
- Most patients are asymptomatic during the early stages of chronic kidney disease transition
- Nocturia, dysuria and foamy urine appear in chronic kidney disease stage 2
- Patients are normotensive; hypertension sets in with kidney failure
- Suspected CKDu
- Estimated glomerular filtration rate (eGFR) 2 or albuminuria > 30 mg/g creatinine or proteinuria > 150 mg/g creatinine
- All or any of the above in the absence of hypertension, diabetes mellitus or acute kidney injury
- Patients living and working in an agricultural community in a CKDu endemic area (Int J Nephrol Renovasc Dis 2020:13:261)
- Probable CKDu: meets the criteria of suspected CKDu + hypokalemia or hyperuricemia in the absence of autoimmune etiology, glomerular disease, congenital kidney disease, obstructive kidney disease (Int J Nephrol Renovasc Dis 2020:13:261)
- Confirmed CKDu: meets the criteria for probable CKDu + histopathological features consistent with CKDu on kidney biopsy in the absence of pyelonephritis and interstitial nephritis due to specific etiology (Int J Nephrol Renovasc Dis 2020:13:261)
- In acute CKDu (acute kidney injury leading to CKDu), most patients display leukocytosis, neutrophilia, lymphopenia, elevated serum creatinine levels, hyperuricemia, hypokalemia, hypomagnesemia, leukocyturia, epithelial cells in urine and low level proteinuria (Am J Trop Med Hyg 2017;97:1247)
- Nocturia, dysuria, urinary hesitancy and foamy urine appear in chronic kidney disease stage 2 and tend to progress (Nephrol Dial Transplant 2017;32:234)
- Persistently increased excretion of magnesium, phosphate, sodium, potassium, uric acid and calcium as well as acid base disorders begin to appear in chronic kidney disease stage 2 (Nephrol Dial Transplant 2017;32:234)
- In CKDu, kidneys are small with irregular contours in chronic kidney disease stage 3 - 4 (Nephrol Dial Transplant 2017;32:234, Nephrol Dial Transplant 2017;32:603)
- CKDu is a primary chronic kidney disease with inexorable progression to kidney failure
- CKDu patients show a mean GFR decline of -1.7 mL/min/1.73 m2 per year, with 15% of patients having a rapid progression, 35% of patients with GFR decline between -5 and -1 mL/min/1.73 m2 per year and 50% of patients with stable or improved GFR (Kidney Med 2021;3:871)
- Risk factors: extreme poverty, agricultural fieldwork, poor hydration, lack of rest breaks, lack of workplace regulation (N Engl J Med 2014;371:58)
- 32 year old Salvadorian male immigrant presented with shortness of breath and leg swelling, small kidneys found on ultrasound (Am J Emerg Med 2016;34:1323.e5)
- 39 year old male immigrant from Nicaragua presented with elevated serum creatinine, elevated uric acid levels, low potassium and magnesium levels, undergoes kidney biopsy (Cureus 2022;14:e24566)
- 50 year old Sri Lankan man presented with loin pain, arthralgia and high serum creatinine (Kidney Int Rep 2022;7:S2)
- Preventive measures, such as hydration with safe water and electrolytes and providing rest and tents for shade (J Occup Environ Med 2019;61:239)
- Restriction from performing heavy manual labor in hot weather
- There is no etiological treatment; treatment is supportive
- If the renal disease has progressed to end stage, dialysis or kidney transplantation are indicated
- In most CKDu endemic regions, kidney biopsies are not performed routinely
- Some patients with a clinical diagnosis of CKDu show histological features of other etiologies and should receive an appropriate pathological diagnosis
- Similar spectrum of glomerular and tubulointerstitial pathological changes is reported in CKDu patients with no arterial changes, in those with early arteriopathy (such as mild intimal fibroelastosis and mild smooth muscle hyperplasia) and those with moderate arteriosclerosis (Am J Kidney Dis 2013;62:908, PLoS One 2018;13:e0193056, Environ Health Prev Med 2012;17:213)
- However, samples displaying significant arteriosclerosis should be treated with caution as there is an overlap between many features of CKDu and vascular pathology
- Atherosclerosis and essential hypertension are only rarely seen in CKDu susceptible populations; whether CKDu can directly affect the blood vessels is not known
- In the acute kidney injury stage preceding CKDu, patients display histological features consistent with acute tubulointerstitial nephritis; however, the inflammatory infiltrate is predominantly lymphocytic
- Eosinophils and plasma cells are rare (Am J Trop Med Hyg 2017;97:1247)
- Both acute and chronic components of the tubulointerstitial nephritis are present (Kidney Int 2018;93:681)
- Most CKDu patients with chronic kidney disease stages 2, 3 and 4 display interstitial fibrosis and tubular atrophy with or without chronic inflammation (Am J Kidney Dis 2013;62:908, MEDICC Rev 2014;16:49, Am J Kidney Dis 2023 Oct 19 [Epub ahead of print])
- Inflammatory infiltrate is predominantly mononuclear and eosinophils are rare (Am J Trop Med Hyg 2017;97:1247, Environ Health Prev Med 2012;17:213)
- Tubulitis is either not seen or is mild and rare (Environ Health Prev Med 2012;17:213, Am J Kidney Dis 2013;62:908)
- More advanced interstitial fibrosis is seen in male patients (MEDICC Rev 2014;16:49)
- Second most frequently observed lesion is significant global glomerulosclerosis, obsolescent glomeruli as well as glomerular deflation with capillary wall wrinkling (Am J Kidney Dis 2013;62:908, MEDICC Rev 2014;16:49, Environ Health Prev Med 2012;17:213)
- Glomerulomegaly is reported in ~30% of patients, likely compensatory due to kidney parenchyma loss (MEDICC Rev 2014;16:49, Environ Health Prev Med 2012;17:213)
- Mild mesangial matrix expansion is often seen (Am J Kidney Dis 2013;62:908, PLoS One 2018;13:e0193056)
- In addition to the features above, a study described silver stained cytoplasmic granules revealed in the proximal tubules by Jones staining and reacting with lysosomal markers; lysosomal lesions were found by electron microscopy (see below) and resemble those seen in toxic tubular lesions, supporting the toxic etiology of CKDu (Kidney Int 2020;97:350)
- Focal segmental glomerulosclerosis (FSGS), specifically perihilar FSGS, is reported in patients with CKDu and is hypothesized to be secondary to nephron loss (FSGS) (Environ Health Prev Med 2012;17:213)
- There are no immunofluorescence features specific for CKDu; some samples show weak segmental mesangial staining for IgA or IgM (Kidney Int 2018;93:681)
- This staining is seen both in snap frozen and reprocessed paraffin tissue samples (Kidney Int 2018;93:681, Am J Kidney Dis 2013;62:908)
- Small amounts of IgG can be identified in the mesangium in some patients (Am J Kidney Dis 2013;62:908)
- CD3 and CD68 immunohistochemical stains highlight numerous T lymphocytes in the interstitial infiltrates and monocytes / macrophages mostly concentrated at the corticomedullary junction (Am J Trop Med Hyg 2017;97:1247)
- CD20 and CD138 immunohistochemical stains reveal rare B cells and plasma cells in the infiltrates (Kidney Int 2018;93:681)
- There are no ultrastructural diagnostic findings specific for CKDu
- Most often seen glomerular abnormalities include podocyte vacuolization and fat droplets (Am J Kidney Dis 2013;62:908, PLoS One 2018;13:e0193056)
- Deflated capillaries with wrinkled and focally thickened glomerular basement membrane are seen in ischemic glomerular segments (Kidney Int 2018;93:681)
- Ultrastructural examination of 34 biopsies from 3 continents demonstrated enlarged dysmorphic lysosomes in the proximal tubules, resembling the dysmorphic lysosomes seen in kidney transplant patients undergoing immunosuppressive therapy with calcineurin inhibitors (Kidney Int 2020;97:350)
- CKDu shows familial clustering, suggesting genetic predisposition; the disease is seen in sharply demarcated geographical regions (Clin Kidney J 2018;11:491)
- Variants of CYP1A1 gene (cytochrome C) are associated with 1.4 - 2 fold increased risk of CKDu in Indian patients (Environ Toxicol Pharmacol 2013;36:164)
- Polymorphisms in SLC13A3 (sodium dicarboxylate transporter 3 in the basolateral membranes of the proximal tubules) and KCNA10 (voltage gated potassium channel in glomerular endothelium and apical plasma membrane of proximal tubular epithelium) are associated with a higher risk of CKDu in Sri Lanka (J Occup Health 2014;56:28, Environ Health Prev Med 2015;20:354)
- Kidney, biopsy:
- 60% global glomerulosclerosis (12/20) (see comment)
- No significant arteriosclerosis
- Focal moderate interstitial fibrosis and tubular atrophy
- No evidence of immune complex or monoclonal etiology
- Comment: Significant chronic tubulointerstitial changes in a young patient working in the sugarcane industry in a CKDu endemic area, with no evidence of known etiologies or comorbidities, strongly suggests chronic kidney disease of unknown etiology (CKDu).
- Clinical history: The patient is a 25 year old man employed as a sugarcane cutter and living in the Chinandega Department of Nicaragua. His father had chronic kidney disease. He presented to the emergency department of the Chinandega Hospital with nausea, vomiting, weakness and headaches that were gradually getting worse over several months. Laboratory data: serum creatinine 2.5 mg/dL, proteinuria 800 mg/g creatinine, urinalysis with mild proteinuria and large numbers of epithelial cells. Renal ultrasound revealed kidney pole to pole length of 97 mm and the Doppler study showed resistance index > 0.7.
- Light microscopy: The biopsy material consists of tissue from the renal cortex and medulla. The tissue is sampled at 4 levels of section with H&E, PAS, trichrome and PAMS stains. Per level of section up to 20 glomeruli are available, of which 12 are globally sclerosed. Globally sclerosed glomeruli are often obsolescent and are mostly located in the areas of chronic tubulointerstitial changes close to the corticomedullary junction. The nonsclerosed glomeruli display patent capillary lumens, single contoured capillary walls and no segments of sclerosis, adhesion or crescents. Some glomeruli show capillary tuft deflation and wrinkled capillary walls. There are large foci of interstitial fibrosis and tubular atrophy of the usual and thyroid type with associated moderate mononuclear infiltration; nonseverely atrophic tubules show rare mild tubulitis. Tubules in the nonatrophic areas display mildly flattened epithelium with focally absent brush borders. Interlobular arteries of medium caliber are present and do not show diagnostic arteriosclerotic changes; there is no significant arteriolar hyalinosis.
- Immunofluorescence microscopy: 6 glomeruli, 2 of them globally sclerosed, are available for evaluation by immunofluorescence microscopy. Mild granular immunofluorescence staining is present in the mesangial areas with antibodies to IgM. No diagnostic immunofluorescence staining is detected with antibodies to IgG, IgA, C3, C1q, kappa light chains and lambda light chains.
- Electron microscopy
- 2 glomeruli, 2 of them globally sclerosed, are available for evaluation on the semithin sections. The nonsclerosed glomeruli appear unremarkable. There is mild interstitial fibrosis and tubular atrophy and mild arteriolar hyalinosis.
- Ultrastructural examination of 2 glomeruli show glomerular basement membranes (GBMs) of normal thickness, except in a few deflated capillary segments where the GBMs are wrinkled and somewhat thickened. Podocytes display vacuolated cytoplasm with a few lipid vacuoles. There is segmental mild podocyte foot process effacement with foci of podocyte microvillous transformation. Capillary lumens are patent. The mesangial matrix does not demonstrate expansion or hypercellularity.
- Chronic kidney disease due to known etiology (e.g., diabetes, hypertensive vascular disease, glomerulopathies, congenital kidney diseases, allergic acute tubulointerstitial nephritis, chronic tubulointerstitial nephritis, chronic pyelonephritis):
- In summary, any kidney disease leading to chronic tubulointerstitial changes
- Clinical history, family history, work history, laboratory studies and kidney biopsy displaying characteristic etiological features help establish the correct diagnosis
- Itai-Itai disease:
- Due to chronic cadmium exposure (Kidney Int Rep 2017;2:282)
- Kidneys are markedly atrophic with widespread proximal tubule atrophy and global glomerulosclerosis (ScienceDirect: Itai-Itai Disease [Accessed 13 December 2023])
- Lead toxicity:
- Prolonged exposure to lead is associated with increase in blood pressure, symptoms of tubular dysfunction and hematuria
- Kidney biopsy shows focal tubular atrophy and interstitial fibrosis (Interdiscip Toxicol 2015;8:55)
- Ultrastructural studies display loss of the tubular brush border, mitochondrial swelling and intranuclear inclusion bodies (Toxicol In Vitro 2009;23:1298)
- Balkan endemic nephropathy:
- Synonyms: Chinese herb nephropathy, aristolochic acid nephropathy
- Due to ingestion of herbs containing aristolochic acid, does not show the interstitial inflammatory reaction frequently seen in CKDu (Kidney Int Rep 2017;2:282, Environ Health Prev Med 2012;17:213)
A 25 year old male agricultural worker living in California Central Valley gets an infected skin wound. He receives a course of vancomycin. Routine laboratory testing a week later revealed serum creatinine 2.2 mg/dL, proteinuria 350 mg/g creatinine and urinalysis displayed red blood cells 10 - 20/high power field, white blood cells 2/high power field. A kidney biopsy is performed and a representative H&E stained section of the main histological finding is shown above. What is the most likely diagnosis?
- Acute pyelonephritis
- Allergic tubulointerstitial nephritis
- Chronic kidney disease of unknown etiology (CKDu)
- Postinfectious glomerulonephritis
Comment Here
Reference: Chronic kidney disease of unknown etiology (CKDu)
- Lead level in the patient's blood should be urgently tested
- The disease is due to ingestion of indigenous herbs containing aristolochic acid
- This is an unknown disease and needs further investigation
- This is likely CKDu
Comment Here
Reference: Chronic kidney disease of unknown etiology (CKDu)
- Diffuse, patchy tubulointerstitial inflammation and scarring accompanied by blunting of calyces and the renal pelvis
- Blunted, deformed, atrophic calyces with scarring due to chronic injury
- Diffuse, patchy lymphoplasmacytic tubulointerstitial inflammation and fibrosis
- Atrophic renal tubules with thyroid type tubular atrophy and intraluminal colloid-like proteinaceous casts
- References: Kumar: Robbins Basic Pathology, 10th Edition, 2017, Urol Case Rep 2018;19:65, Emerg Radiol 2020;27:561
- Chronic pyelonephritis
- F > M
- Common in pediatric population with congenital anomalies and vesicoureteral reflux disease
- Most common site: upper pole of the kidney
- Reference: Porter: The Merck Manual of Diagnosis and Therapy, 20th Edition, 2018
- Renal parenchyma
- Reflux of urine and ascending infection into the renal pelvis
- Obstructive uropathy
- Vesicoureteral reflux
- Chronic inflammation and infection leading to deformed and atrophic calyces with fibrosis and scarring of the renal parenchyma
- Recurrent damage and scarring leads to renal insufficiency and end stage renal disease (ESRD)
- Complicated chronic pyelonephritis is associated with nephrolithiasis
- Most common bacterial organism associated with chronic pyelonephritis: Escherichia coli
- Reference: Am J Physiol Renal Physiol 2017;312:F43, Radiol Med 2021;126:505, Am J Kidney Dis 2016;68:e23, Nephrol Dial Transplant 2006;21:1423
- Urinary tract obstruction
- Reflux nephropathy including congenital abnormalities
- Recurrent, untreated pyelonephritis
- Neurogenic bladder
- Long term urethral catheterization
- References: Kumar: Robbins Basic Pathology, 10th Edition, 2017, J Am Geriatr Soc 1994;42:1286
- Nonspecific, generalized malaise and abdominal pain
- Proteinuria in the nephrotic range; if severe, extensive damage to the renal parenchyma
- Silent clinical course if unilateral; presents as hypertension
- Recurrent acute pyelonephritis with fever, abdominal / flank pain
- Involvement of both kidneys can result in hyperchloremic acidosis
- References: Niger Postgrad Med J 2020;27:37, Porter: The Merck Manual of Diagnosis and Therapy, 20th Edition, 2018
- Helical CT, intravenous urography (IVU)
- CT scan: gold standard for imaging assessment of the severity of pyelonephritis
- Voiding cystourethrogram for neonates, pediatric population with congenital abnormalities
- References: Porter: The Merck Manual of Diagnosis and Therapy, 20th Edition, 2018, Kidney Int 1999;55:1486, Radiographics 2008;28:255
- Elevated blood urea nitrogen (BUN) and elevated serum creatinine
- Urinalysis: proteinuria, renal epithelial cells, granular casts, pyuria (acute on chronic, recurrent acute pyelonephritis)
- Focal, polar, coarse cortical scars, calyceal distortion and renal atrophy
- Hypertrophy of residual renal parenchyma, mimicking infarct on imaging
- Ureteral dilation if severe reflux disease is present
- Obstruction with calculi may be present in obstructive nephropathy
- References: Emerg Radiol 2020;27:561, Radiographics 2008;28:255
- Slow progression, > 2 decades before consequences of damage are observed
- Can lead to end stage renal disease
- Reference: Porter: The Merck Manual of Diagnosis and Therapy, 20th Edition, 2018
- 22 year old woman with xanthogranulomatous pyelonephritis (Urol Case Rep 2018;19:65)
- 68 year old woman with a nonenhancing left renal sinus mass (J Med Case Rep 2009;3:9054)
- 69 year old woman with primary chronic pyelonephritis (Ann Short Reports 2019;2:1039)
- 73 year old woman with chronic pyelonephritis and renal pyelocalyceal squamous cell carcinoma (J Med Case Rep 2019;13:154)
- Surgical intervention: correction of anatomic / congenital abnormalities resulting in reflux
- Medical management: antimicrobial therapy for treatment of recurrent, acute pyelonephritis
- Long term antibiotic prophylaxis with trimethoprim / sulfamethoxazole, trimethoprim, nitrofurantoin, among others
- In pediatric patients who develop end stage renal disease due to reflux, transplant is a treatment modality
- References: Tunis Med 2018;96:495, Porter: The Merck Manual of Diagnosis and Therapy, 20th Edition, 2018
- Patchy interstitial lymphoplasmacytic inflammation with occasional, focal neutrophilic infiltration
- Patchy, well demarcated scarring of the renal pelvis and calyces
- Abundant intraluminal Tamm-Horsfall protein casts
- Tubular atrophy with thyroid type tubular atrophy and interstitial and periglomerular fibrosis
- Secondary segmental glomerulosclerosis
- References: Niger Postgrad Med J 2020;27:37, Yale J Biol Med 1985;58:91, Am J Kidney Dis 2016;68:e23
- No specific findings
- Mutant allele 299Gly TLR4 polymorphism
- References: Wiad Lek 2017;70:47, Pediatr Res 2007;61:371, PLoS One 2010;5:e14223
Overview of chronic pyelonephritis
Histological features of chronic pyelonephritis
- Kidney, radical nephrectomy:
- Renal cortical thinning with blunted calyces and scarring
- Global glomerulosclerosis with marked tubular atrophy and thyroid type tubular atrophy of the renal tubules
- Diffuse interstitial lymphoplasmacytic infiltration
- These findings are consistent with chronic pyelonephritis
- End stage renal disease due to other predisposing causes:
- Cortical interstitial fibrosis, tubular atrophy, global glomerulosclerosis and arterionephrosclerosis
- Additional features vary based on the predisposing cause
- Xanthogranulomatous pyelonephritis:
- Dilated pelvicalyceal system
- Can be associated with multinucleated giant cells
- Sheets of foamy macrophages admixed with inflammatory cell infiltrate
- Hypertensive / vascular injury related renal disease:
- Adherent renal capsule that does not come off easily
- Sclerotic glomeruli with ischemic changes
- Arteriolar hyalinosis, arterial myointimal hyperplasia and arterionephrosclerosis
- References: Indian J Nephrol 2019;29:111, Curr Opin Nephrol Hypertens 2008;17:266, Kumar: Robbins Basic Pathology, 10th Edition, 2017
A 79 year old man presented with benign prostatic hyperplasia, obstructive uropathy and lobular segmental hypertrophy of the left kidney. A nephrectomy is performed revealing the histology above. What is the most likely diagnosis?
- Acute pyelonephritis
- Chronic pyelonephritis
- Diabetic nephropathy
- Emphysematous pyelonephritis
- Xanthogranulomatous pyelonephritis
Comment Here
Reference: Chronic pyelonephritis
- Glomerular cellular crescents with segmental necrotizing change
- Histiocytes with granular eosinophilic cytoplasm and Michaelis-Guttman bodies
- Interstitial dense neutrophilic inflammation and tubular luminal neutrophils
- Tubular atrophy and luminal casts; interstitial lymphoplasmacytic inflammation and fibrosis
Comment Here
Reference: Chronic pyelonephritis
- Also called collagenofibrotic glomerulopathy
- Idiopathic deposition of type III collagen in glomeruli, normally absent in kidneys
- Deposition of abnormally curved and serpentine type III collagen fibers in mesangial and subendothelial regions
- Rare (No sex predilection
- All ages (youngest: 3 months)
- Most cases reported from Japan and China
- Most pediatric cases from Europe
- References: Pediatr Nephrol 1993;7:354, Clin Kidney J 2012;5:7, Histopathology 2015;67:568, Exp Ther Med 2015;10:1445, Clin Kidney J 2015;8:543, Diagn Pathol 2009;4:33, Am J Kidney Dis 1999;33:123
- Renal limited
- Occasional systemic deposition (Am J Kidney Dis 1999;33:123)
- Sporadic in adults
- Autosomal recessive in children
- Unknown mutation, type III collagen gene (COL3A1) normal
- References: Clin Kidney J 2015;8:543, BMC Vet Res 2013;9:218, Adv Chronic Kidney Dis 2012;19:101
- Proteinuria is a cardinal sign, sometimes nephrotic range (30 - 60%)
- Microscopic hematuria, chronic anemia (50 - 75%) and hypertension are common
- Variable progression
- Occasional stable disease but generally protracted course with increasing proteinuria and progressive renal failure
- In children, may be associated with hemolytic uremic syndrome (HUS); precipitous course more likely in children, especially if superimposed HUS
- References: Histopathology 2015;67:568, Clin Kidney J 2012;5:7, Am J Kidney Dis 2007;49:499, Indian J Nephrol 2011;21:52, Pediatr Nephrol 1993;7:354
- 10 - 100x increase in levels of procollagen type III peptide (PIIINP), not specific
- Serum hyaluronan may be increased to > 1000x, more specific (Intern Med 2014;53:1801)
- 6 year old boy with inherited factor H deficiency and collagen type III glomerulopathy (Pediatr Nephrol 1995;9:11)
- 26 year old man with simultaneous Hodgkin lymphoma (Saudi J Kidney Dis Transpl 2011;22:126)
- 49 year old woman with simultaneous hepatic perisinusoidal deposition (Nephron 1993;63:183)
- 38 cases of collagenofibrotic glomerulopathy (Clin Kidney J 2012;5:7)
- Patient with collagenofibrotic glomerulopathy (Am J Kidney Dis 1999;33:123)
- No curative treatment available; one case with reportedly no recurrence after renal transplant (Adv Chronic Kidney Dis 2012;19:101)
- Diffuse increase in mesangial matrix and generalized widening of glomerular capillary walls with pale eosinophilic material; may not be obvious in pediatric cases
- Negative or weakly positive with periodic acid Schiff, negative with methenamine silver, blue with Masson trichrome
Contributed by Joseph Grande, M.D., Ph.D.
Images hosted on other servers:
- Strong collagen type III staining in capillary loops and mesangium (normally absent in kidneys)
- Negative; nonspecific IgM and C3 entrapment
- Large accumulation of collagen fibrils in subendothelial glomerular basement membrane and mesangial matrix (Ultrastruct Pathol 2010;34:68)
- Banded with 60 nm periodicity
- Serpentine, curved, frayed
- More prominent curvilinear structure and better detail on phosphotungstic acid or tannic acid lead staining
- Nail-patella syndrome: has other clinical features (e.g. bone / nail abnormalities), sparser fibers, typically located in lamina densa of glomerular basement membrane versus more subendothelial or mesangial location in collagenofibrotic glomerulopathy
- Onset of collagen type III glomerulopathy in childhood is associated with what manifestation?
- Aplastic anemia
- Hemolytic uremic syndrome
- Warm autoimmune hemolytic anemia
- Idiopathic thrombocytopenic purpura
- 10% of individuals have urinary tract malformations, although many are asymptomatic
- 15% of congenital urogenital anomalies are secondary to an underlying chromosomal disorder
- In children, 20% of chronic renal failure is due to renal dysplasia or hypoplasia
- In adults, 10% of chronic renal failure is due to adult polycystic kidney disease
- Mass ultrasound screening detects congenital renal and urinary tract anomalies in 1% but not recommended since most would ultimately be detected via symptoms; most commonly vesicoureteral reflux, ureteropelvic junction obstruction, ectopic kidney, renal dysplasia (Pediatr Nephrol 2012;27:949)
- Complete absence of renal tissue; unilateral or bilateral; 0.03% of newborns but 0.3% of stillborn
- Bilateral agenesis: incompatible with life; associated with large adrenal glands; leads to Potter (oligohydramnios) sequence; possible causes include maternal insulin dependent diabetes mellitus and male sex of fetus but usually no specific etiology (PLoS One 2010;5:e12375)
- Unilateral agenesis: not fatal
- < 1% of individuals
- Usually asymptomatic; may be associated with obstruction
- Usually at pelvic brim, may have kinking of ureters (Urologia 2010;77:212, Clin Exp Nephrol 2009;13:531)
- Most common congenital kidney anomaly, 0.15 - 0.25% of all newborns
- 90% are fused at lower pole
- Associated with obstruction, anomalous superior vena cava (Circ J 2012;76:1253)
- Complete fusion of the kidneys produces a formless mass in the pelvis (pancake kidney)
- Rare; failure of kidney to develop to normal size without scarring
- Usually unilateral with a reduced number of nephrons and pyramids (6 or less) but otherwise normal architecture
- Associated with PAX2 mutations (J Am Soc Nephrol 2001;12:1769)
- Oligomeganephronia: type of hypoplasia with small kidney but hypertrophied nephrons due to compensatory hypertrophy caused by reduced number of nephrons
- Rare congenital abnormality of renal development characterized by short and poorly developed proximal convoluted tubules without dysplasia or cystic disease (Hum Pathol 1986;17:1259)
- Characterized by oligohydramnios and the Potter sequence, pulmonary hypoplasia, calvarial bone hypoplasia with enlarged fontanels
- Causes: acquired (associated with renal hypoperfusion, Pediatr Dev Pathol 1999;2:25) or autosomal recessive (mutations in genes associated with angiotensinogen, renin, angiotensin converting enzyme or angiotensin II receptor type 1, J Am Soc Nephrol 2006;17:2253, Hum Mutat 2012;33:316)
- Identified in 1% of perinatal autopsies in 1991 (Hum Pathol 1991;22:147)
- 36 week gestational age male with Klinefelter syndrome and bilateral renal agenesis (Arch Pathol Lab Med 2004;128:e44)
- Congenital nephrotic syndrome caused by nephrin (NPHS1) mutations
- Excessive proteinuria starting within the first 3 months of life; patients typically develop end stage renal disease by early childhood
- Dysfunction of glomerular filtration barrier due to absent or mutant nephrin
- No response to immunosuppressive medication
- Finnish nephropathy
- Congenital nephrotic syndrome of the Finnish type (CNF)
- Congenital nephrotic syndrome (CNS) type I
- ICD-10: N04.9 - nephrotic syndrome with unspecified morphologic changes
- M = F
- Affects 1 - 3 per 100,000 live births worldwide
- Estimated to affect 1 in 10,000 live births in Finland (Nephron 1973;11:101, J Pediatr 1959;55:152)
- Glomerular filtration barrier: podocyte layer and slit diaphragms
- Nephrin is coded by NPHS1, which is located at the long arm of chromosome 19 (19q13.1) (Mol Cell 1998;1:575)
- Nephrin is a transmembrane protein of the podocyte foot processes and is a main structural protein of the glomerular slit diaphragm
- Absence or dysfunction of nephrin results in nonfunction or dysfunction of the glomerular slit diaphragm; proteins pass the glomerular filtration barrier easily, massive proteinuria results
- Heavy proteinuria, hypoproteinemia and edema starting soon after birth (Pediatr Nephrol 1995;9:87)
- Increased maternal serum and amniotic fluid alpha fetoprotein in some cases
- Massive fluid retention and generalized swelling, hypertension and signs of malnutrition and failure to thrive
- Large amounts of protein and the presence of fat in the urine
- Kidney biopsy
- Genetic tests to confirm the diagnosis (Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2019;36:1022)
- Elevated plasma alpha fetoprotein (AFP) level in the mother
- Proteinuria, hypoalbuminemia or hypogammaglobulinemia
- Enlarged, hyperechogenic kidneys without normal corticomedullary differentiation
- Microcystic changes
- Unfavorable
- Typically develop end stage renal disease between ages 2 and 8
- Newborn Korean girl and a 2 month old Korean boy with genetically confirmed cases (J Korean Med Sci 2009;24:S210)
- 2 week old boy with a NPHS1 mutation (Med Arch 2016;70:232)
- 33 week old gestational age boy with a novel NPHS1 mutation (Pediatr Int 2016;58:1211)
- 4 month old girl of Eritrean origin presented with severe generalized edema (Pediatr Nephrol 2018;33:1269)
- 5 month old Pakistani boy presented with nephrotic syndrome (Front Pediatr 2020;8:151)
- 15 month old girl presented with a history of generalized body swelling since birth and neurodevelopmental delay (EJIFCC 2017;28:156)
- Supportive therapy: albumin infusion, gamma globulin replacement, high protein low salt diet, vitamin and thyroxine substitution and infections and thrombotic complications prevention
- Diuretic medications
- Angiotensin converting enzyme (ACE) inhibitor medications and nonsteroidal anti-inflammatory drugs
- Dialysis
- Kidney transplant (patient weight should be > 8 kg):
- About 20% of patients develop recurrent nephrotic syndrome in the transplanted kidney due to antinephrin antibodies (Transplantation 2007;83:1316)
- Circulating antinephrin antibodies can be detected
- Plasmapheresis combined with anti-CD20 antibodies and cyclophosphamide may be used to treat recurrent nephrotic syndrome (Pediatr Nephrol 2014;29:2309)
- Enlarged kidneys
- Cystically dilated proximal and distal tubes with an attenuated tubular epithelium, microcytic changes (Int J Pediatr Nephrol 1980;1:10)
- May be minimal glomerular changes in early disease
- With disease progression may see mesangial hypercellularity, increased mesangial matrix
- Rare immature glomeruli often present
- Focal segmental glomerulosclerosis (FSGS) or global glomerulosclerosis (Int J Pediatr Nephrol 1980;1:10)
- Interstitial fibrosis and tubular atrophy with progressive disease
- Generally negative
- Segmental glomerulosclerosis lesions may exhibit segmental smudgy staining for IgM, C1q and C3 due to nonspecific trapping
- Other findings may include increased tubular protein reabsorption droplet staining with albumin
- Podocin
- Nephrin stain negative with frame shift or truncating mutations
- Sometimes detected with point mutations of nephrin
- Complete podocyte foot process effacement (Kidney Int 2000;58:972)
- Microvillus transformation of podocytes
- No slit diaphragms
- Various sized filtration slits
- Increased mesangial cellularity
- Swelling of endothelial cells and endothelial blebs
- Normal fenestrations and normal glomerular basement membrane
- Autosomal recessive disease
- Homozygous mutation in NPHS1
- Located at chromosome 19q13.1 (Am J Hum Genet 1994;54:757, Am J Hum Genet 1995;57:1377)
- 29 exon gene, encodes protein nephrin
- Transmembrane protein, immunoglobulin superfamily containing 1241 amino acids with 8 extracellular C2 type Ig-like domains and 1 fibronectin type III-like module (Mol Cell 1998;1:575)
- Essential component of interpodocyte spanning slit diaphragm (Proc Natl Acad Sci U S A 1999;96:7962)
- 2 most common mutations (Fin-major and Fin-minor) found in the affected Finnsh population:
- Fin-major (nt-121delCT, L41fsX91) (Mol Cell 1998;1:575)
- Frameshift mutation causing a stop codon in exon 2 results in a 90 amino acid truncated nephrin
- Complete loss of nephrin
- Fin-minor (c.3325 C>T, R1109X) (Mol Cell 1998;1:575)
- Nonsense mutations in exon 26
- Leading to a truncated 1109 residual nephrin
- Fin-major (nt-121delCT, L41fsX91) (Mol Cell 1998;1:575)
- > 250 mutations of NPHS1 in non Finnish populations
- Involving entire NPHS1
- Including insertions, deletions, nonsense mutations and missense mutations
- Heterozygous NPHS1 mutations uncommon
- May present as adult onset focal segmental glomerulosclerosis
- Heterozygotes with 1 normal NPHS1 allele
- Transient deficiency of nephrin in utero
- False positive alpha fetoprotein test in amniotic fluid and maternal serum may happen
- Normal after birth
- Kidney, right, total nephrectomy:
- End stage kidney consistent with history of congenital nephrotic syndrome, type 1 (NPHS1 mutation) (see comment)
- Comment: Histologic sections of the renal parenchyma are examined with H&E, periodic acid-Schiff, stained sections. Sections consist of renal cortex, medulla common and collecting system. Cortical structures exhibit normal developmental orientation and generation of glomeruli. Glomeruli are mildly enlarged and display variable mild mesangial hypercellularity. Focal segmental glomerulosclerosis is present. A few glomeruli exhibit embryonal hyperplasia / metaplasia of Bowman capsule epithelium. Prominent cystically dilated proximal and distal tubules with an attenuated epithelium are present. There is extensive interstitial fibrosis associated with patchy and focally heavy interstitial inflammation (mainly lymphocytes) as well as scattered lymphoid follicles.
- Minimal change disease:
- Primary (idiopathic) disease frequently in children, secondary to drugs, allergic reactions, immunologic diseases and neoplasia at all ages
- Minimal or no light microscopic or immunofluorescence abnormalities
- Diffuse podocyte foot process effacement by electron microscopy (J Exp Med 1957;106:649)
- Decreased nephrin staining along glomerular basement membrane due to loss of slit diaphragms; loss of nephrin is seen in advanced stages
- Decreased podocyte α dystroglycan
- Genetic studies negative
- Usually steroid responsive
- Primary focal and segmental glomerulosclerosis:
- Common cause of nephrotic syndrome in children and adults (15 - 20% of biopsies) (Nephrol Dial Transplant 1999;14:68)
- Affects juxtamedullary glomeruli early
- Segmental scars in some glomeruli and adhesion of tuft to Bowman capsule
- Extensive podocyte foot process effacement by electron microscopy
- IgM and C3 in segmental scars by immunofluorescence
- May be steroid responsive
- Genetic studies negative
- NPHS2 related nephrotic syndrome:
- No distinctive feature described by light microscopy: focal segmental glomerulosclerosis (50%) and minimal changes (50%) (Pediatr Nephrol 2009;24:2121)
- Loss of podocin immunoreactivity (podocin deficiency)
- Effacement of podocyte foot processes and segmental adhesion by electron microscopy
- Genetic studies required to differentiation from NPHS1 mutations
- Secondary congenital nephrotic syndrome:
- Infectious causes, such as in utero infection (rubella, pertussis, syphilis, malaria toxoplasmosis and others) (Clin Pediatr (Phila) 2005;44:169)
- Others: drug reaction, toxins and systemic lupus erythematosus
Comment Here
Reference: Congenital nephrotic syndrome
- Allergic reaction
- Heart failure
- Nephritic syndrome
- Nephrotic syndrome
- Weight loss
Comment Here
Reference: Congenital nephrotic syndrome
- Acute kidney injury, nephrotic range proteinuria and acute thrombotic microangiopathy (TMA) that can be seen during or following COVID-19 disease
- Kidney disease may occur even with mild COVID-19 symptoms in susceptible individuals
- Kidney allograft recipients may experience rejection episodes
- Autopsy based studies often have patients who died with COVID-19, while biopsy based studies often include patients with primary presentation of kidney dysfunction, often with only mild COVID-19 symptoms
- Acute tubular injury / acute tubular necrosis is the most common finding in autopsy and biopsy based studies
- In biopsy based studies, collapsing glomerulopathy is seen almost exclusively in Black patients with high risk APOL1 genotypes
- Acute endothelial cell injury / acute thrombotic microangiopathy can also be seen in 15% of patients
- Chronic tubulointerstitial injury, even with microcystic tubular atrophy (which may be driven by COVID-19 or pre-existing in the patient), is variable
- Sometimes called COVID-19 associated nephropathy (COVAN) when collapsing glomerulopathy is present
- Causative agent is the novel coronavirus, SARS-CoV-2
- In autopsy series, 70% are men, mean age of 73 years; 59% have hypertension and 33% have diabetes (Curr Opin Nephrol Hypertens 2021;30:324)
- Almost all cases of collapsing glomerulopathy have been detected in Black patients; of those tested, virtually all have high risk APOL1 genotypes
- Kidney injury is likely an indirect effect of pulmonary failure (severe COVID-19) or cytokine mediated injury (mild COVID-19); direct infection of the kidney does not seem to be a common mechanism of injury
- In severe COVID-19, pulmonary failure and circulating cytokines are thought to injure the kidney (autopsy studies)
- In mild COVID-19, the immune response to viral infection in the lungs / upper airways is thought to injure glomerular podocytes via interferon mediated increase in expression of high risk APOL1 proteins → collapsing glomerulopathy (biopsy studies)
- Indirect cytokine mediated effects are also thought to be responsible for acute tubular injury and acute endothelial injury, though mechanisms have not been completely elucidated (Curr Opin Nephrol Hypertens 2021;30:324)
- Direct or recent infection with SARS-CoV-2
- Urinalysis, blood metabolic panel (BMP), 24 hour urine protein quantification, serum albumin quantification
- Elevated serum creatinine, blood urea nitrogen (acute kidney injury)
- Decreased serum albumin, increased proteinuria (collapsing glomerulopathy)
- Decreased hemoglobin, lowered platelets, increased lactate dehydrogenase (LDH), decreased haptoglobin (thrombotic microangiopathy)
- Reference: Am J Kidney Dis 2021;77:82
- 29 year old man, kidney transplant patient, who developed collapsing glomerulopathy in the allograft in the setting of COVID-19 (Am J Kidney Dis 2020;76:590)
- 49 year old woman, kidney transplant patient, who developed minimal change disease during COVID-19 disease (Transplant Proc 2020;52:2693)
- 54 year old man, kidney transplant patient, with chronic active antibody mediated rejection following COVID-19 disease (Transplant Proc 2021;53:1202)
- 69 year old woman with COVID-19 who developed thrombotic microangiopathy (Kidney Int 2020;98:509)
- Early 70s man who died of COVID-19 pneumonia and was treated with hydroxychloroquine (Ultrastruct Pathol 2020;44:519)
- If kidney injury is severe, kidney replacement therapy (dialysis) may be required, either on a short term or permanent basis
- Acute tubular injury: tubular epithelial cell attenuation / simplification, shedding of epithelial cells, tubular apoptosis, regenerative mitosis, occasionally frank necrosis; some tubules may have eosinophilic cast material and atrophic appearing epithelium (microcystic tubular atrophy)
- Collapsing glomerulopathy: collapse of capillary loops, prominence of overlying epithelial cells, some of which may have eosinophilic cytoplasmic droplets
- Thrombotic microangiopathy (TMA): intimal edema or onion skinned appearance of blood vessels, presence of thrombi and sheared red blood cell fragments in blood vessel lumens or trapped in expanded intima; glomeruli may appear shrunken and bloodless
- May occur together with collapsing glomerulopathy
- Myoglobin cast nephropathy and proliferative glomerulonephritis with monoclonal IgG deposits are enriched in this patient population (Kidney Int 2021 Aug 2 [Epub ahead of print])
- No specific immunofluorescence findings
- May incidentally detect concurrent renal injury processes with immune complexes (e.g. membranous nephropathy, IgA nephropathy, etc.)
- Collapsing glomerulopathy typically exhibits diffuse effacement of podocyte foot processes
- Thrombotic microangiopathy (TMA) shows expansion of the subendothelial space with electron lucent material and swelling of glomerular endothelial cells, which may lose their fenestrae
- Viral particles have not been conclusively detected in kidney biopsy / autopsy tissue; studies purporting to detect viral particles in the kidney have misidentified normal structures as viral particles (J Pathol 2020;252:346, Histopathology 2021;78:358, J Am Soc Nephrol 2020;31:2223)
- Fabry disease-like myelin figures / zebra bodies were seen in some patients treated with hydroxychloroquine before the drug was no longer recommended for treating COVID-19 (Ultrastruct Pathol 2020;44:519)
- Tubuloreticular inclusions may be seen in some cases (Kidney Int 2020;98:241)
- Individuals with high risk APOL1 genotypes (homozygous G1, homozygous G2 or G1 / G2) are most at risk for collapsing glomerulopathy, even with only mild COVID-19 symptoms (Am J Kidney Dis 2021;77:82)
- Kidney, biopsy:
- COVID-19 associated nephropathy (COVAN) with features of collapsing glomerulopathy, acute tubular injury and acute endothelial cell injury (see comment)
- Comment: The finding of collapsing glomerulopathy in patients with ongoing or recent COVID-19 infection is highly associated with high risk APOL1 genotypes. Acute tubular injury is a common but nonspecific finding in patients with COVID-19 disease. Acute endothelial cell injury can also be seen with COVID-19, though the mechanisms driving this injury process are unclear.
- Collapsing glomerulopathy (unrelated to COVID-19):
- Associated with other viral infections (HIV, parvovirus B19), medications (bisphosphonates), drugs (heroin)
- Collapse of capillary loops with hypertrophy of overlying epithelial cells / podocytes
- No specific immunofluorescence findings
- Electron microscopy usually shows diffuse effacement of podocyte foot processes, no immune deposits
- Strongly associated with high risk APOL1 genotypes
- Focal and segmental glomerulosclerosis:
- Segmental occlusion of capillary loops with matrix, hyaline or foam cells with attachment to Bowman capsule
- No specific immunofluorescence findings
- Electron microscopy usually shows diffuse effacement of podocyte foot processes, no immune deposits
- Membranous nephropathy:
- Immune complex mediated glomerulopathy presenting with nephrotic syndrome
- Light microscopy shows thickened capillary loops with epimembranous spikes, best seen on Jones methenamine silver stained sections
- Immunofluorescence microscopy shows granular glomerular capillary wall immune deposits staining for IgG, C3, kappa and lambda light chains
- Electron microscopy shows numerous subepithelial immune deposits and diffuse effacement of podocyte foot processes
- Primary / idiopathic membranous nephropathy is associated with circulating antibodies against PLA2R (~70% of cases); minor antigenic targets include THS7DA, NELL1 and others
- Mesangial, subendothelial and extraglomerular immune deposits suggest a secondary form of membranous nephropathy (e.g. lupus, malignancy, infection, drug induced)
- Diabetic nephropathy:
- Early stages show thickened basement membranes and mesangial matrix accumulation / hypercellularity
- Later stages show large Kimmelstiel-Wilson nodules, mesangiolysis and microaneurysm formation
- Severe arteriolar hyalinosis and arteriosclerosis are also common
- Tubular atrophy and interstitial fibrosis usually concomitant with chronic glomerular injury
- No specific immunofluorescence findings
- Electron microscopy shows thickening of glomerular and tubular basement membranes, mesangial matrix expansion, occasional fibrils in the mesangium (diabetic fibrillosis)
- Minimal change disease:
- Usually no changes in glomeruli by light microscopy; may have mild mesangial expansion
- No specific immunofluorescence findings
- Electron microscopy shows diffuse effacement of podocyte foot processes, no immune deposits
- Acute tubular injury:
- Proximal tubules are most commonly affected
- Characterized by loss of brush borders, epithelial attenuation, luminal ectasia, blebbing of epithelial cell cytoplasm, cytoplasmic vacuolization, widening of basolateral interdigitations
- Severe cases can display epithelial cell apoptosis / frank necrosis
- Tubular cells can also display regenerative mitoses and cellular atypia
- No specific immunofluorescence findings
- Acute interstitial nephritis:
- Inflammation and edema of the tubules and interstitium
- Increased numbers of eosinophils suggests an allergic / hypersensitivity etiology (e.g. drug induced)
- Lymphocyte rich acute interstitial nephritis can still have an allergic trigger
- Acute tubular injury is a rare histologic finding
- If present, collapsing glomerulopathy is associated with low risk APOL1 genotypes
- Immune complexes directed against SARS-CoV-2 are detected by immunofluorescence microscopy
- Viral particles are not routinely detected in tubular epithelial cells by electron microscopy
Comment Here
Reference: COVID-19 associated kidney injury
Comment Here
Reference: COVID-19 associated kidney injury
- Rapidly progressive glomerulonephritis (RPGN) is a clinical syndrome, not a pathologic diagnosis
- Often presents with a rapid decline in kidney function, hematuria, proteinuria, oliguria or hypertenison
- Often crescentic glomerulonephritis (CGN) is characterized by glomerular crescents in > 50% of glomeruli
- Often crescent formation in > 50% of the glomeruli
- Immunofluorescence and serologic findings distinguish etiology:
- Antiglomerular basement membrane (GBM) disease is characterized by circulating anti-GBM antibody, linear staining for immunoglobulin (Ig) G on immunofluorescence microscopy and absent electron dense deposits on electron microscopy
- Immune complex mediated glomerulonephritis (GN) occurs in various glomerulonephritis (lupus, IgAN) and is characterized by granular staining for Ig and complements immunofluorescence with electron dense deposits on electron microscopy
- Pauci-immune complex mediated / vasculitic type glomerulonephritis is frequently associated with circulating antineutrophil cytoplasmic antibody (ANCA) and is characterized by negative or scanty Ig deposits on immunofluorescence (pauci-immune complex pattern) and electron microscopy
- Rapidly progressive nephritic syndrome
- Rapidly progressive nephritic syndrome, diffuse crescentic glomerulonephritis
- Rapidly progressive nephritic syndrome with extracapillary glomerulonephritis
- CGN accounts for < 10% of all patients presenting with primary glomerulopathy
- M:F = 1:1, more common in white population, rare in Africans
- Bimodal age distribution: first peak around age 30 and second after 60
- Pauci-immune complex glomerulonephritis is the most common subtype of CGN in adults (Kidney Int 2003;63:1164)
- Anti-GBM disease is the most aggressive form of CGN (Kidney Int 2003;63:1164)
- Kidney
- Kidney and lung (pulmonary renal syndrome, Goodpasture’s syndrome)
- Systemic, including various organs such as skin, lung, gastrointestinal and nervous system, if associated with vasculitis and connective tissue disease
- For pathophysiology of crescents, endothelial injury is a key driver, which leads to breaks and rupture of GBM (J Pathol 2012;228:482)
- This triggers coagulation cascade within Bowman space, ultimately leading to fibrin deposition
- Fibrin stimulates parietal epithelial cells to proliferate, resulting in formation of cellular crescents
- Cellular crescents lead to increase in counter pressure, collapse of glomerular tuft and tubular outflow obstruction
- This will result in decline in the single nephron glomerular filtration rate
- Multilevel growth of parietal epithelial cells may lead to epithelial - mesenchymal transition in cell phenotype, which leads to the formation of fibrocellular and fibrous crescents (Curr Opin Nephrol Hypertens 2020;29:302)
- Anti-GBM disease: circulating antibodies directed against the noncollagenous domain of alpha 3 chain of type IV collagen
- Immune complex mediated crescentic GN: secondary to immune complex deposition in conditions such as postinfectious GN, lupus nephritis, membranoproliferative GN, IgA nephropathy and cryoglobulinemia
- Pauci-immune complex crescentic GN: 80 - 90% are associated with ANCA
- ANCA associated vasculitis includes granulomatosis with polyangiitis, microscopic polyangiitis, eosinophilic granulomatosis with polyangiitis or renal limited vasculitis (J Am Soc Nephrol 2010;21:1628)
- Variety of drugs may also cause the disease (hydralazine, penicillamine, rifampicin)
- Mixed antibody patterns may also occur (anti-GBM + ANCA, antidouble stranded DNA + ANCA, antidouble stranded DNA + anti-GBM)
- Rapid deterioration of renal function over short period (days to weeks)
- Acute nephritic syndrome: (Clin Exp Nephrol 2016;20:322)
- Oliguria
- Hypertension
- Hematuria, macroscopic and less frequently microscopic
- Proteinuria
- Clinicopathological diagnosis, mainly achieved on renal biopsy
- Elevated serum creatinine and blood urea nitrogen (BUN) (Clin Exp Nephrol 2016;20:322)
- Elevated C reactive protein (CRP) and erythrocyte sedimentation rate (ESR)
- Hematuria
- Red blood cell casts
- Proteinuria (usually nonnephrotic range)
- Complement levels
- Autoantibodies (J Am Soc Nephrol 2016;27:1278)
- Anti-GBM antibody
- ANCA, antimyeloperoxidase (pANCA) and antiproteinase 3 (cANCA)
- Antidouble stranded DNA antibody
- Best predictor of outcome for all types of crescentic glomerulonephritis is the severity of renal failure at time of initiation of therapy (Kidney Int 2003;63:1164)
- Dialysis dependency at presentation is considered a poor prognostic sign
- Cellular crescents are usually reversible with prompt and aggressive treatment or may resolve spontaneously in postinfectious GN
- Fibrocellular and fibrous crescents are irreversible in terms of potential recovery of single nephron glomerular filtration rate (Curr Opin Nephrol Hypertens 2020;29:302)
- 26 year old woman with necrotizing CGN related to sarcoidosis (J Med Case Rep 2015;9:282)
- 59 year old man presented with membranous nephropathy with crescents (J Am Soc Nephrol 2011;22:1804)
- 68 year old woman with CGN due to coexistent anti-GBM disease and fibrillary glomerulonephritis (Clin Kidney J 2016;9:97)
- 74 year old woman with systemic lupus erythematosus presented with RPGN secondary to IgA nephropathy (Case Rep Nephrol 2019;2019:8354823)
- Anti-GBM disease: plasmapheresis and immunosuppressive agents (glucocorticoids and cyclophosphamide)
- Immune complex mediated crescentic GN: depends on the underlying etiology
- Pauci-immune complex crescentic GN: immunosuppressive agents, rituximab (Clin J Am Soc Nephrol 2017;12:1162)
- Extracapillary cellular proliferation (crescents) composed of parietal epithelial cells, macrophages and fibrin
- Segmental fibrinoid necrosis of the glomerular tufts
- Active periglomerular inflammation in cases with rupture of Bowman capsule
- In anti-GBM disease, crescents are uniform and temporally homogeneous, showing the same stage of activity and chronicity (Adv Anat Pathol 2021;28:59)
- In pauci-immune complex crescentic GN, crescents are heterogeneous at variable ages with mixture of cellular, fibrocellular and fibrous crescents; the underlying glomerular architecture is generally normal (Adv Anat Pathol 2021;28:59)
- In immune complex mediated crescentic GN, there are often underlying glomerular architectural changes including esangial or endocapillary proliferation (systemic lupus erythematosus, IgAN)
- Small vessel vasculitis is a feature for pauci-immune complex crescentic glomerulonephritis CGN
- Crescents are classified based on their composition and age into: (J Am Soc Nephrol 2016;27:1278)
- Cellular crescent: extracapillary cell proliferation of more than 2 cell layers, involving at least 10% of glomerular circumference with > 50% of the lesion occupied by cells
- Fibrocellular crescent: extracapillary lesion comprising cells and extracellular matrix with < 50% cells and < 90% matrix
- Fibrous crescent: extracapillary crescents with > 90% matrix
Contributed by Khaled A. Murshed, M.D., Noheir M. Taha, M.B.B.Ch., M.D. and Mohammed Akhtar, M.D.
- Anti-GBM disease: strong linear staining of IgG and frequently C3 along GBM (Adv Anat Pathol 2021;28:59)
- Similar pattern along tubular basement membranes in some cases
- Immune complex mediated crescentic GN: granular deposits of Ig and complement; their type and location (subepithelial, subendothelial, mesangial) depend on the underlying etiology
- Pauci-immune complex crescentic GN: negative or weak (≤ 1+) staining of Ig and complement (pauci-immune pattern)
- Fibrin deposition in cellular crescents and foci of fibrinoid necrosis
- PAS, Jones methenamine silver and trichrome are used to evaluate the morphology
- Anti-GBM disease: no electron dense deposits
- Immune complex mediated crescentic GN: electron dense deposits are present within glomeruli; their location depends on the underlying etiology (J Am Soc Nephrol 2016;27:1278)
- Pauci-immune complex crescentic GN: no electron dense deposits
- Breaks and rupture of GBM
- Left kidney, needle core biopsy:
- Crescentic glomerulonephritis secondary to anti-GBM disease (see comment)
- Cellular crescent (9/13), fibrocellular / fibrous crescents (0/13)
- Global glomerulosclerosis (0/13), segmental glomerulosclerosis (0/13)
- Interstitial inflammation, moderate
- Tubular atrophy and interstitial fibrosis, mild
- Arteriosclerosis, moderate
- Comment: Indirect immunofluorescence studies show diffuse linear staining of IgG (2+) and C3 (2+) along the GBM and tubular basement membrane with negative staining for IgA, IgM and C1q. The findings are consistent with anti-GBM disease.
- RPGN is a clinical diagnosis, not a pathology diagnosis so the differential is most important to nephrologists, not pathologists; the usual pathology correlate is a crescentic glomerulonephritis due to different etiologies
Which of the following is true about the glomerular disease depicted in the image?
- It presents clinically as a slowly progressive disease
- Immune complex mediated crescentic glomerulonephritis (type 2) is associated with linear staining of IgG along glomerular basement membrane
- Anti-GBM disease (type 1) is associated with granular staining of IgG along glomerular basement membrane
- In pauci-immune complex crescentic GN (type 3), electron dense deposits are commonly present
- Pauci-immune complex crescentic GN (type 3) is the most common subtype of crescentic glomerulonephritis
Comment Here
Reference: Crescentic glomerulonephritis overview
- Podocyte injury
- Epithelial mesenchymal transition
- Podocyte effacement
- Endothelial cell injury
- Parietal cell damage
- See also topic in Bone marrow neoplastic chapter
- Usually systemic disease in which deposits of IgG or IgM immune complexes cause glomerulonephritis (focal, diffuse), cutaneous vasculitis (skin rash) and synovitis (arthritis)
- Defined by presence of serum cryoglobulins, which are immunoglobulin complexes that precipitate at 4°C and become soluble again at 30°C
- Type I: cryoglobulin is single monoclonal immunoglobulin class, usually due to myeloma, Waldenström macroglobulinemia or other lymphoma
- Type II: mixture of 2+ immunoglobulins, one a monoclonal antibody against polyclonal IgG; usually IgG-IgM, in which IgM is monoclonal and has rheumatoid factor activity
- Type III: both immunoglobulin components are usually polyclonal IgG and IgM
- Symptoms of fatigue, purpura over lower extremities, arthralgias, hepatosplenomegaly, lymphadenopathy, Raynaud phenomenon, glomerulonephritis in 50% with proteinuria and hypertension, progressing to renal failure in 5%
- Usually women ages 30+ years
- Hepatitis C virus is major cause of mixed cryoglobulinemia
- Renal disease unrelated to Hepatitis C is often related to primary Sjögren Syndrome (Medicine (Baltimore) 2009;88:341)
- Cryoglobulinemia found in 83% of chronic hemodialysis patients, although not necessarily symptomatic (Ren Fail 2011;33:801)
- Types II and III associated with B cell lymphoma, chronic infection, chronic liver disease, SLE and hepatitis C
- 7 year old boy with glomerular rhomboid inclusions (Am J Kidney Dis 2009;53:866)
- 55 year old man with Hepatitis C (J Med Case Rep 2009;3:91)
- 58 year old woman with ultrastructural diagnosis of cryoglobulinemic glomerulonephritis (Arch Pathol Lab Med 1981;105:474)
- 60 year old man with Hepatitis B virus associated type II cryoglobulinemia and severe multisystem disease, treated with Rituximab (J Med Case Rep 2012;6:39)
- 81 year old man with essential type II cryoglobulinemia unrelated to Hepatitis virus (Geriatr Gerontol Int 2009;9:92)
- Double filtration plasmapheresis; cryofiltration (Ther Apher Dial 2012;16:91)
- Possibly interferon for Hepatitis C related cases (Ren Fail 2009;31:149)
- Possibly Rituximab in nonviral patients age 70 years or less (Arthritis Care Res (Hoboken) 2010;62:1787, Autoimmun Rev 2011;11:48)
- Expanded mesangium with thickened capillaries
- Diffuse proliferative glomerulonephritis, often with membranoproliferative pattern
- Also focal and segmental glomerulonephritis and less often crescentic or membranous glomerulonephritis
- Acutely may produce wire loops or thrombi seen in lupus nephritis, vasculitis of interlobular arteries and afferent arterioles
- Immunoglobulins, strong C3; also C1q and C4
- Large amounts of subendothelial immune complex deposits; lesser amounts of subendothelial deposits
- 50% have glomerular deposits with 25 - 35 nm microtubules forming bundles or arranged in fingerprint-like array
- Fibrillary glomerulonephritis: 15 - 30 nm fibrils
- Immunotactoid glomerulopathy
- Diabetic kidney disease is a morphologic structural change in the kidney induced by longstanding hyperglycemia manifested as relentless matrix expansion
- Diabetic kidney disease is a morphological structural change in the glomeruli that occurs due to longstanding hyperglycemia (Contrib Nephrol 2011;170:36)
- Characterized by mesangial matrix expansion and diffuse thickening of glomerular, tubular and Bowman capsule basement membranes
- Mesangial matrix expansion can be diffuse, nodular or both
- Additional insudative lesions due to deposition of lipohyaline involving blood vessels (vascular hyalinosis), glomerular capillaries (hyaline caps) and Bowman capsule (capsular drop)
- Immunofluorescent microscopy usually reveals diffuse linear staining for immunoglobulin IgG along glomerular capillaries and tubular basement membranes; intensity usually 1+
- Diabetic nephropathy
- Diabetic glomerulosclerosis (nodular or diffuse)
- Kimmelstiel-Wilson lesion
- Intercapillary glomerulosclerosis
- Occurrence of diabetes is worldwide; incidence is rising in developing countries (Adv Anat Pathol 2020;27:87)
- Highly susceptible ethnic groups: Black, Native American, Hispanic (Kidney Dial 2022;2:433)
- Renal cortical glomeruli
- Hyperglycemia is the main initiator
- Interaction between high intracellular sugar levels and the free amino groups on proteins, lipids and nucleic acids results in the formation of advanced glycation end products
- These compounds induce excess extracellular matrix production and reduce matrix degradation by matrix metalloproteinases (J Diabetes Investig 2011;2:243)
- Increase in type IV collagen and a decrease in laminin and heparan sulfate proteoglycan resulting in loss of anionic charge leading to increased glomerular filtration of albumin
- Hyperglycemia may also lead to podocyte damage and progressive loss resulting in focal denudation of glomerular basement membrane (GBM)
- Denuded GBM attaches to the parietal epithelial cells and the Bowman capsule, resulting in segmental glomerulosclerosis (Kidney Int Suppl 2007;72:S36)
- Usually presents after 15 years of having diabetes mellitus (Adv Anat Pathol 2020;27:87)
- Type 1 diabetes mellitus
- 10% of cases
- Develops in 30% of patients
- Associated with loss of insulin producing cells
- Generally seen in younger patients (
- Type 2 diabetes mellitus
- 90% of cases
- Develops in 50% of patients
- Due to less than adequate insulin production and insulin resistance
- Generally seen in older patients
- Usually have additional comorbidities (hypertension, cardiovascular disease)
- Severity is related to diabetes duration, degree of glycemic control and genetic factors (Nat Rev Dis Primers 2015;1:15018)
- Begins with microalbuminuria, defined as loss of small amounts of albumin into the urine (30 - 300 mg/d)
- Later, larger amounts of albumin are lost in the urine macroalbuminuria, detectable by dipstick urinalysis (> 300 mg/d)
- With disease progression, gradual decline in kidney function occurs, ultimately leading to end stage renal disease
- Diagnosis is usually established on kidney biopsy
- Proteinuria
- Hyperglycemia
- Glycosuria
- Hemoglobin A1C
- Type 1 diabetes: end stage renal disease in 50% with overt nephropathy within 10 years and in > 75% by 20 years
- Type 2 diabetes: end stage renal disease is faster due to the presence of several comorbidities that affect the kidney, such as hypertension and atherosclerosis (Ren Fail 2022;44:1309)
- 56 year old man with collapsing glomerulopathy superimposed on diabetic nephropathy (Indian J Nephrol 2019;29:207)
- 58 year old nondiabetic man with diabetic nephropathy (Pathol Res Pract 2016;212:1199)
- 59 year old woman with diabetic nephropathy aggravating the progression and prognosis of COVID-19 associated acute limb ischemia (F1000Res 2021;10:584)
- 62 year old Chinese man with advanced diabetic kidney disease complicated by acute interstitial nephritis (World Acad Sci J 2021;3:37)
- 70 year old man with diabetes mellitus for 26 years combined with IgA nephropathy (Front Endocrinol (Lausanne) 2022;13:992933)
- Strict glycemic control is the mainstay of therapy for both type 1 and type 2 diabetes mellitus
- Long term glycemic control may halt progression and possibly reverse some matrix expansion over many years (Nat Rev Dis Primers 2015;1:15018)
- Diffuse uniform thickening of glomerular basement membrane (GBM)
- Matrix expansion encroaching on the capillary lumina may be diffuse, nodular or both
- Nodular lesions are also called Kimmelsteil-Wilson lesions (Contrib Nephrol 2011;170:36)
- Microaneurysms of glomerular capillaries develop due to mesangiolysis
- These later give rise to large solitary nodules, which may reveal a laminated architecture on Jones silver stain
- Vascular hyalinosis is a common finding
- Hyalinosis of afferent and efferent arterioles usually seen but rare in other diseases
- Large subendothelial lipohyaline deposits may be present at the periphery of the glomerular tuft (hyaline caps)
- Similar deposits along the Bowman capsule capsular drops, which are specific
- In later stages, segmental glomerulosclerosis, especially at the tubular outlet tip lesion, is common
- Tubular atrophy, chronic interstitial inflammation and fibrosis, thickening of the tubular basement membranes
- Interstitial eosinophilic inflammatory cell infiltrate can sometimes be seen (Nephrol Dial Transplant 2015;30:1370)
- Papillary necrosis characterized by necrosis and sloughing off of renal papillae
- Renal Pathology Society histologic classification system for diabetic nephropathy proposed in 2010 (J Am Soc Nephrol 2010;21:556)
- Class I: mild or nonspecific changes on light microscopy and confirmed GBM thickening proven by electron microscopy
- > 395 nm in women and > 430 nm in men
- Class II: diffuse mesangial expansion
- IIa: mild mesangial expansion in > 25% of the observed mesangium
- Area of mesangial expansion < area of the capillary cavity
- IIb: severe mesangial expansion in > 25% of the observed mesangium
- Area of mesangial expansion > area of the capillary cavity
- IIa: mild mesangial expansion in > 25% of the observed mesangium
- Class III: nodular sclerosis (Kimmelstiel-Wilson lesions)
- At least 1 Kimmelstiel-Wilson lesion and none of the changes described in class IV, without > 50% globally sclerosed glomeruli on biopsy
- Class IV: advanced diabetic glomerulosclerosis
- > 50% globally sclerosed glomeruli on biopsy with clinical or pathologic evidence indicating that the sclerosis stems from diabetic nephropathy
- Class I: mild or nonspecific changes on light microscopy and confirmed GBM thickening proven by electron microscopy
Contributed by Khaled A. Murshed, M.D., Noheir M. Taha, M.B.B.Ch., M.D. and Mohammed Akhtar, M.D.
- Diffuse linear accentuation of the glomerular basement membrane (GBM) and tubular basement membrane (TBM) with IgG, kappa and lambda light chains and albumin
- 33% of cases may show similar staining for IgM and C3 with lesser intensity
- Faint staining of the mesangial areas and mesangial nodules may be seen
- Lipohyaline caps at the periphery of the glomerulus usually show positivity for IgM, C3 and C1q
- This should not be interpreted as indication of immune complex deposition (Contrib Nephrol 2011;170:36)
- Expanded mesangium is positive for periodic acid-Schiff (PAS), Jones methenamine silver, trichrome
- Diffuse thickening of the glomerular basement membrane is the earliest structural change demonstrable in almost all diabetic patients, with or without nephropathy (Contrib Nephrol 2011;170:36)
- Mesangial regions are expanded due to increased mesangial matrix limiting the capillary luminal space
- No immune complex deposits
- Variable foot process effacement and podocyte loss
Diabetic nephropathy
- Left kidney, ultrasound guided needle biopsy:
- Diabetic kidney disease, diffuse and nodular type (class III) (see comment)
- Global glomerulosclerosis (6/15), focal segmental glomerulosclerosis (1/15)
- Tubular atrophy and interstitial fibrosis, moderate
- Hyaline arteriolosclerosis, moderate to severe
- Comment: Biopsy reveals changes of moderately advanced diabetic kidney disease. There is no evidence of any superimposed immune complex mediated glomerulonephritis. The proteinuria in this patient is probably due to the diabetic kidney disease complicated by secondary focal and segmental glomerulosclerosis (FSGS). Clinicopathologic correlation is suggested.
- Renal amyloidosis:
- Mesangial nodules are Congo red positive with apple green birefringence under polarized light (Adv Anat Pathol 2020;27:87)
- IgA nephropathy:
- IgA dominant immunofluorescent pattern
- Renal monoclonal immunoglobulin light chain deposition disease or monoclonal immunoglobulin heavy chain deposition disease:
- Immunofluorescence reveals diffuse linear staining of the glomerular basement membrane with monoclonal immunoglobulin light or heavy chains
- Electron microscopy shows a continuous band of electron dense granular powdery deposits within the mesangial nodules, subendothelial space and along external side of the tubular basement membranes
- Membranoproliferative glomerulonephritis (MPGN):
- Marked endocapillary proliferation with double contouring of the peripheral capillary loops identified on PAS and Jones methenamine silver stains
- Immunofluorescence shows diffuse granular glomerular capillary loop and mesangial deposition of C3 with variable immunoglobulin deposition
- Electron microscopy shows mesangial and subendothelial electron dense deposits
- Dense deposit disease:
- Light microscopic features may resemble MPGN, immunofluorescence reveals deposition of C3 only
- Ultrastructurally characterized by large intramembranous nonimmune complex type electron dense deposits
- Fibrillar glomerulonephritis:
- Deposition of usually polyclonal IgG by immunofluorescence, corresponding haphazardly arranged fibrils measuring 12 - 24 nm diameter by electron microscopy and DNAJB9 positivity by immunohistochemistry
- Immunotactoid glomerulonephritis:
- Deposition of usually monotypic IgG by immunofluorescence and corresponding haphazard to parallel arranged fibrils with hollow core measuring ≥ 30 nm diameter by electron microscopy
- Fibronectin glomerulopathy:
- Glomerular nodules are PAS positive but silver and Congo red stains are negative
- Electron microscopy shows electron dense deposits in the mesangium that are finely granular or have a fibrillary substructure with randomly arranged 12 - 16 nm fibrils
- Diagnosis confirmed by intense immunohistochemical staining for fibronectin in the mesangium and along the glomerular capillaries
A 57 year old woman was found to have proteinuria. A renal biopsy showed the findings in the image above (PAS). Which of the following conditions is the most important differential diagnosis?
- Lupus nephritis
- Membranous glomerulonephritis
- Minimal change disease
- Postinfectious glomerulonephritis
- Renal amyloidosis
Comment Here
Reference: Diabetic kidney disease
- Diffuse coarse granular staining of C3 along the glomerular capillaries and mesangium
- Diffuse coarse granular staining of IgG along the glomerular capillaries
- Diffuse mesangial staining for IgA
- Dull linear staining of glomerular and tubular basement membranes for IgG
- Immunofluorescent microscopy is typically negative
Comment Here
Reference: Diabetic kidney disease
- Deposits of randomly oriented nonbranching fibrils in the mesangium
- Diffuse effacement of podocyte foot processes
- Diffuse thickening of the glomerular basement membrane
- Electron dense subepithelial deposits
- Expansion of the mesangial regions
Comment Here
Reference: Diabetic kidney disease
- Necrosis sharply limited to cortex and columns of Bertin
- Rare cause of acute renal failure secondary to ischemic necrosis of the renal cortex (eMedicine: Renal Cortical Necrosis [Accessed 29 December 2017])
- May occur after obstetric emergency, due to disseminated intravascular coagulopathy; fatal if bilateral (J Ayub Med Coll Abbottabad 2010;22:74)
Histopathology kidney: diffuse cortical necrosis
- Clinically and genetically heterogeneous group of disorders in non-Finnish patients (J Am Soc Nephrol 2010;21:1209)
- Early onset of severe proteinuria (50% within months 0 - 2, 50% in months 3 - 9 months), with rapid progression to end stage renal disease by age 3 years
- May be associated with WT1 abnormalities and Denys-Drash syndrome (OMIM: Nephrotic Syndrome, Type 4; NPHS4), PLCE1 mutations (Nephrol Dial Transplant 2008;23:1291) or be isolated (Pediatr Nephrol 2009;24:1013)
- Normal placenta, no premature births but is associated with cataracts and corneal clouding, aniridia, microencephaly, developmental disability and hypertelorism
- Does not recur after transplantation
- Two cases of isolated diffuse mesangial sclerosis with WT1 mutations (J Korean Med Sci 2006;21:160)
- Diffuse mesangial sclerosis; tubular atrophy with focal tubular dilatation and interstitial fibrosis
- Nonspecific staining with mesangial deposits of IgM, C3 and C1q
- Obliteration of foot processes, basement membrane thickening and increase in mesangial matrix
- Performed to determine suitability of allograft prior to transplantation
- Findings to report: specimen type (wedge or core biopsy), # glomeruli, # and % of globally sclerosed glomeruli, # arteries, interstitial fibrosis, tubular atrophy, interstitial inflammation, arterial intimal fibrosis, arteriolar hyalinosis, other findings (such as FSGS or suspicious lesions) (Am J Transplant 2017;17:140)
- Biopsy should be of renal cortex, 10 mm long × 5 mm wide × 5 mm deep, and contain at least 25 glomeruli
- Donor glomerulosclerosis, chronic vascular and interstitial damage are prognostic for graft outcome (Kidney Int 2005;67:1595)
- Demand for donor kidneys far exceeds supply
- Frozen section may be performed to determine suitability of allograft prior to transplantation
- Findings to report: specimen type (wedge or core biopsy), # glomeruli, # and % of globally sclerosed glomeruli, tubular atrophy, interstitial fibrosis, interstitial inflammation, # arteries, arterial intimal fibrosis, arteriolar hyalinosis, other findings (such as FSGS, periglomerular fibrosis, etc) (Am J Transplant 2017;17:140)
- Demand for donor kidneys far exceeds supply
- Discard rate of recovered kidneys is > 40%
- Organ Procurement and Transplantation Network (OPTN) recommends preimplantation biopsy for all kidneys with a Kidney Donor Profile Index (KDPI) > 85%
- KDPI is derived from 10 donor factors: age, height, weight, ethnicity, serum creatinine, history of diabetes, hypertension, hepatitis C virus, cause of death, and donor after circulatory death status
- KDPI ability to predict graft failure is 0.6; further assessment and pathologic findings may help improve on this (Am J Transplant 2017;17:140)
- Preimplantation biopsy findings correlate with flow and resistance to machine perfusion (Transplant Proc 2012;44:2197)
- Expanded Criteria Donor: These are at increased risk of graft failure (relative HR 1.7 compared with Standard Criteria Donor; Clin J Am Soc Nephrol 2009;4:1827)
- At time of death, donors age > 60 OR
- Aged 50 - 59 with any 2 of the following:
- Cause of death is cerebral vascular accident
- Pre existing hypertension
- Terminal serum creatinine > 1.5 mg / dL
- 4 cases of living donor renal transplant with incidental renal cell carcinoma from donor allograft (Transplant International 2015;28:1126)
- Donor monoclonal gammopathy and lymphoproliferative disorders in solid organ transplant recipients (Am J Transplant 2016;16:2676)
- Donor kidneys with miliary papillary renal cell neoplasia: the role of the pathologist in determining suitability for transplantation (Ann Transplant 2014;19:362)
- A decision to implant or discard may be based on the frozen section analysis
- Wedge or needle biopsy
-
Recommended microscopic findings to report: (adapted from Am J Transplant 2017;17:140)
- Periglomerular sclerosis and FSGS should be recorded under other glomerular findings
- Artery: vessel with internal elastic lamina OR diameter greater than one third the diameter of a typical glomerulus cut in the median plane OR a vessel with 3 or more layers of smooth muscle
- # glomeruli, # and % of global glomerulosclerosis have good reproducibility
- In most cases, tubular atrophy, interstitial fibrosis, and nonspecific interstitial inflammation are seen together
- Interstitial inflammation and tubular atrophy are often easier to see than fibrosis on frozen sections
- Reproducibility is fair for interstitial fibrosis, tubular atrophy, interstitial inflammation, and arteriosclerosis
- Assessment of interstitial fibrosis and arteriolar hyalinosis is compromised by freeze artifact
| Type of specimen (wedge or core biopsy) | |
| GLOMERULAR FINDINGS | |
| Number of glomeruli | |
| Number of globally sclerosed glomeruli | |
| Percentage of global glomerulosclerosis | |
| Other glomerular findings | |
| TUBULOINTERSTITIAL FINDINGS | |
| Tubular atrophy, interstitial fibrosis, non-specific interstitial inflammation | |
| VASCULAR FINDINGS | |
| Number of arteries | |
| Arterial intimal fibrosis | |
| Arteriolar hyalinosis | |
| OTHER FINDINGS | |
Contributed by Nicole K. Andeen, M.D.
Case 1:
Low power with focal global GS
predominantly in subcapsular
region and patchy tubular atrophy
and IF (H&E perm from FS)
Case 2:
Focal global GS, ischemic glomeruli
with periglomerular fibrosis, adjacent
tubular atrophy, IF and nonspecific
interstitial inflammatory infiltrate (FS)
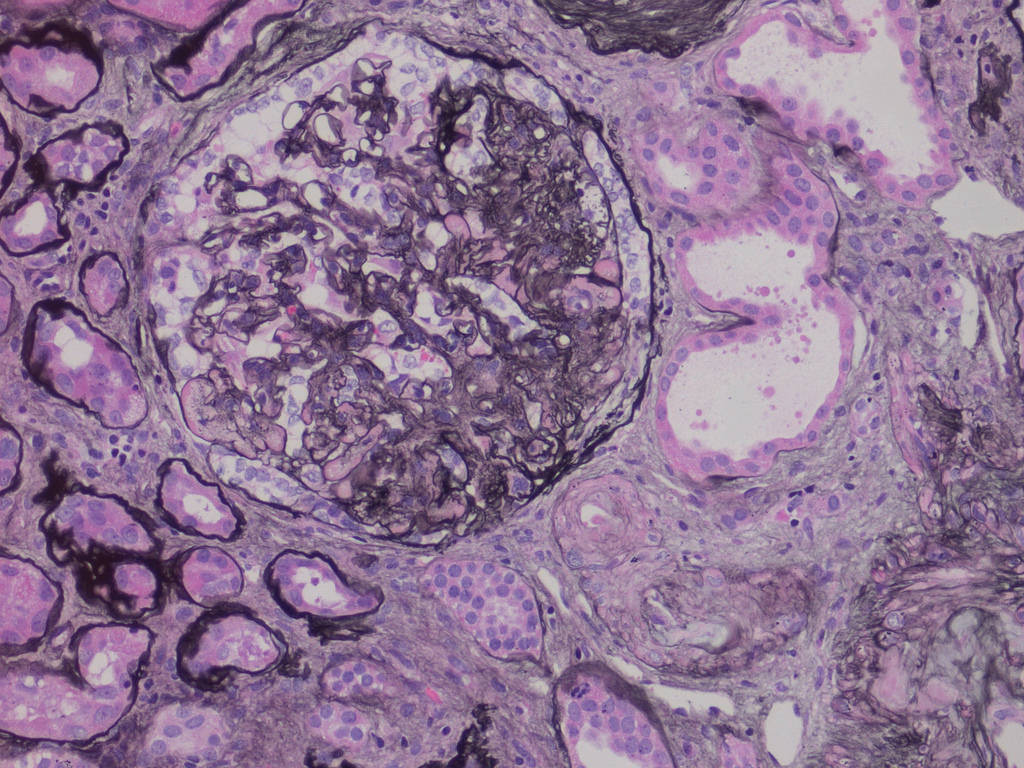
Focal segmental GS, adjacent
arteriolar hyalinosis, tubular atrophy
and IF (perm, Jones silver stain)
Note: FS = frozen section, GS = glomerulosclerosis, IF = interstitial fibrosis, perm = permanent section
- On permanent sections, a basement membrane stain (Jones silver, PAS), and thin sections (2 microns) aid in evaluation of potential underlying donor disease (FSGS, diabetes)
- Dysplasia: abnormal renal development characterized by cysts and immature tubules with collarettes of immature mesenchyme
- Hypoplasia: reduced amounts of normal renal parenchyma; histologically normal kidney(s) with weight > 2 standard deviations below normal for age / body size
- Agenesis: unilateral or bilateral absence of renal tissue
- Renal dysplasia often results from urinary tract obstruction
- Renal dysplasia is frequently associated with Müllerian tract abnormalities
- Cystic renal dysplasia can be distinguished from polycystic kidney disease by the presence of immature tubules, mesenchyme collarettes and cartilage
- Dysplasia, hypoplasia and agenesis are sporadic or associated with a broad range of genetic syndromes, many affecting additional organ systems
- Dysplasia: multicystic renal dysplasia, multicystic dysplastic kidney
- Hypoplasia: simple hypoplasia
- Agenesis: aplasia
- Dysplasia
- Most common pediatric cystic renal disease
- Affects 1 in 1,000 - 4,300 live births; 90% of cases are unilateral
- 90% are associated with ureteropelvic obstruction, ureteral agenesis, atresia or reflux
- Many sporadic cases but also associated with numerous genetic disorders (see Genetics section)
- Hypoplasia
- When rigorously defined, hypoplasia is present in 1 in 400 live births (Adv Anat Pathol 2020;27:311)
- > 90% of cases are bilateral
- Associated with hypertension and extrarenal congenital abnormalities
- Agenesis
- Unilateral renal agenesis affects 1 in 1,000 live births; generally good prognosis if contralateral kidney is normal (Am J Obstet Gynecol 2021;225:B28)
- 33% of unilateral cases are associated with other congenital abnormalities of the kidney and urinary tract, most commonly vesicoureteral reflux
- 30% of unilateral renal agenesis cases in females are associated with unicornuate or bicornuate uterus or other Müllerian abnormality
- If contralateral kidney is involved by hypoplasia or dysplasia, high morbidity and mortality due to oligohydramnios sequence and pulmonary hypoplasia
- Bilateral renal agenesis affects 1 in 3,000 - 4,000 pregnancies; high morbidity and mortality due to oligohydramnios sequence and pulmonary hypoplasia
- For bilateral renal agenesis, M:F = 2:1
- Kidneys
- Dysplasia: abnormal differentiation of renal parenchyma results from genetic abnormality or urinary tract obstruction or reflux (Arch Pathol Lab Med 2015;139:547)
- WT1 expression stimulates ureteric bud development and early induction of renal mesenchyme
- PAX2 and BCL2 expression induces differentiation of mesenchyme to nephron epithelium
- In dysplastic kidneys, WT1 expression is decreased and there is persistent PAX2 and BCL2 expression, driving continued expression of immature epithelium
- Hypoplasia: inadequate branching of ureteric ducts results in a decreased number of reniculi (renal lobes) (Pediatr Res 2010;68:91)
- Agenesis: failure of the ureteric bud to develop or failure of the ureteric bud to induce metanephric mesenchyme differentiation (Am J Obstet Gynecol 2021;225:B28)
- See Pathophysiology
- Common genetic etiologies of renal dysplasia, hypoplasia and agenesis are covered in Genetics section; nonclinical associations include some maternal conditions, sporadic conditions and toxic exposures (Clin J Am Soc Nephrol 2020;15:723, Husain: Stocker and Dehner's Pediatric Pathology, 5th Edition, 2021)
- VACTERL association
- Prune belly sequence
- Maternal alcohol use (fetal alcohol spectrum disorder)
- Maternal diabetes (diabetic embryopathy)
- Severe renal abnormalities are usually diagnosed prenatally via ultrasound (Clin J Am Soc Nephrol 2020;15:723)
- Oligohydramnios / anhydramnios is a common finding
- Less severe abnormalities may be found during workup for other congenital abnormalities, urinary tract infection or renal insufficiency
- Less severe abnormalities are sometimes discovered incidentally
- Evidence of renal failure: elevated BUN and creatinine
- Dysplasia (Semin Fetal Neonatal Med 2008;13:142)
- Ultrasound: large bright kidneys
- Cystic spaces in cortex or uniformly echogenic parenchyma
- Hypoplasia
- Ultrasound shows reduced renal volume but cannot distinguish between true hypoplasia and dysplasia, scarring, atrophy or other etiologies
- Agenesis (Am J Obstet Gynecol 2021;225:B28)
- Ultrasound: empty renal fossa
- Lying down adrenal sign
- Oligohydramnios / anhydramnios after 16 - 18 weeks gestation
- Absent renal artery by Doppler
- Absent (nonvisualized) bladder
- Favorable: unilateral involvement with normal contralateral kidney
- Unfavorable: bilateral involvement or abnormalities of both kidneys
- Bilateral involvement results in oligohydramnios sequence and pulmonary hypoplasia in utero or renal insufficiency and end stage kidney disease following birth
- Recurrence risk in first degree relatives (including siblings): 4 - 20% (Int J Mol Sci 2017;18:796)
- 19 week fetus with unilateral multicystic renal dysplasia (Cureus 2023;15:e37786)
- Term newborn boy with renal hypoplasia, biopsy demonstrated oligomeganephronia (Kobe J Med Sci 2021;67:E34)
- 31 year old woman with unilateral renal agenesis, uterus didelphys and obstructed hemivagina with hematohemicolpos (BJR Case Rep 2020;7:20200132)
- Conservative management if renal function is adequate
- Nephrectomy of involved kidney to manage hypertension, abdominal mass or recurrent infection
- Renal replacement therapy (dialysis or transplant) for renal insufficiency / end stage kidney disease
- For fetuses with oligohydramnios / anhydramnios, clinical trial of amnioinfusion to promote lung development (Clin Perinatol 2022;49:849)
- Dysplasia
- Kidney may be small, normal in size or enlarged; shape is distorted by cysts and sometimes by pelvicalyceal dilation
- Ureter may be stenotic or dilated
- Small areas of remnant renal parenchyma are interspersed with cysts of varying sizes
- Hypoplasia
- Weight > 2 standard deviations below median weight for patient age / size (Pediatr Res 2010;68:91)
- Reniform (kidney shaped) kidney with reduced numbers of renal lobes and calyces
- Normal number is ~10; in hypoplasia there are 5 or fewer
- Gross appearance is otherwise normal
- Ipsilateral adrenal gland may be disc shaped rather than triangular, because the kidney is not large enough to indent it
- Agenesis
- Absent kidney, ureter and renal artery and vein
- Ipsilateral adrenal gland is disc shaped rather than triangular, because the kidney is not there to indent it
Contributed by Robyn C. Reed, M.D., Ph.D.
Images hosted on other servers:
- Dysplasia
- Normal or scarred parenchyma alternating with cysts of varying sizes, lined by flat to cuboidal epithelium
- Collections of tubules lined by immature epithelium and surrounded by collarettes of immature mesenchyme
- Metaplastic cartilage
- Nodules of blastema (immature renal parenchyma) in 3 - 5% of cases
- Hypoplasia
- By definition, normal histology
- If dysplastic changes are present it is classified as dysplasia
- If glomeruli are markedly enlarged, consider oligomeganephronia (Adv Anat Pathol 2020;27:311)
- At least 40 genes have been implicated in congenital abnormalities of the kidney and urinary tract, accounting for ~18% of cases (J Am Soc Nephrol 2018;29:36)
- Many genetic alterations have a phenotypic spectrum that encompasses dysplasia, hypoplasia, agenesis and other structural abnormalities
- Dysplasia is associated with numerous genetic disorders; this is a partial list (Fetal Pediatr Pathol 2014;33:293, Husain: Stocker and Dehner's Pediatric Pathology, 5th Edition, 2021)
- Meckel-Gruber syndrome (autosomal recessive, at least 16 genes) (GeneReviews: Nephronophthisis-Related Ciliopathies [Accessed 26 July 2023])
- Joubert syndrome (autosomal recessive or X linked, at least 34 genes) (GeneReviews: Joubert Syndrome [Accessed 26 July 2023])
- Zellweger syndrome (autosomal recessive, at least 13 genes) (GeneReviews: Zellweger Spectrum Disorder [Accessed 26 July 2023])
- HNF1B deficiency syndrome (autosomal dominant, HNF1B) (Cells 2023;12:307)
- Renal - hepatic - pancreatic dysplasia (autosomal recessive, NPHP3 or NEK8) (OMIM: Renal-Hepatic-Pancreatic Dysplasia 1; RHPD1 [Accessed 26 July 2023], OMIM: Renal-Hepatic-Pancreatic Dysplasia 2; RHPD2 [Accessed 26 July 2023])
- Short rib polydactyly syndromes, including Jeune syndrome (autosomal recessive, at least 23 genes) (Hum Mutat 2018;39:152)
- Carnitine palmitoyltransferase II deficiency (autosomal recessive, CPT2) (GeneReviews: Carnitine Palmitoyltransferase II Deficiency [Accessed 26 July 2023])
- Hereditary renal adysplasia (autosomal dominant, ITGA8 or FGF20) (Fetal Pediatr Pathol 2014;33:293)
- Nail patella syndrome (autosomal dominant, LMX1B) (GeneReviews: Nail-Patella Syndrome [Accessed 26 July 2023])
- Genitopatellar syndrome (autosomal dominant, KAT6B) (GeneReviews: KAT6B Disorders [Accessed 26 July 2023])
- Bardet-Biedl syndrome (autosomal recessive, at least 25 genes) (GeneReviews: Bardet-Biedl Syndrome Overview [Accessed 26 July 2023])
- Beckwith-Wiedemann syndrome (usually sporadic, CDKN1C or 11p15.5 alterations) (GeneReviews: Beckwith-Wiedemann Syndrome [Accessed 26 July 2023])
- 22q11.2 deletion syndrome (autosomal dominant, 22q11.2 alterations) (GeneReviews: 22q11.2 Deletion Syndrome [Accessed 26 July 2023])
- Trisomies 13, 18, 21 and numerous chromosomal deletions and duplications (sporadic) (Int J Pediatr Nephrol 1987;8:215)
- Dysplasia and other congenital abnormalities of the kidney and urinary tract have also been linked with alterations in numerous additional genes (Curr Opin Pediatr 2016;28:209, J Am Soc Nephrol 2018;29:36)
- Renal agenesis and hypoplasia are associated with numerous genetic disorders, including some of the disorders listed above for renal dysplasia as well as the following syndromes (Am J Obstet Gynecol 2021;225:B28)
- Acro renal ocular syndrome (autosomal dominant, SALL4) (GeneReviews: SALL4-Related Disorders [Accessed 26 July 2023])
- Branchiootorenal syndrome (autosomal dominant, EYA1, SIX1 or SIX5) (GeneReviews: Branchiootorenal Spectrum Disorder [Accessed 26 July 2023])
- Pallister-Hall syndrome (autosomal dominant, GLI3) (GeneReviews: GLI3-Related Pallister-Hall Syndrome [Accessed 26 July 2023])
- Fraser syndrome (autosomal recessive, FRAS1, FREM2 and GRIP1) (OMIM: Fraser Syndrome 1; FRASRS1 [Accessed 26 July 2023])
- Renal agenesis has also been linked with alterations in GREB1L, GFRA1, ITA8 and FGF20 (Am J Obstet Gynecol 2021;225:B28)
Development of renal dysplasia
Development of renal agenesis; explanation of oligohydramnios sequence
- Kidney, left (32 g), nephrectomy:
- Multicystic renal dysplasia (see comment)
- Comment: Sections show frequently scarred renal parenchyma interspersed with multiple cysts, some lined by attenuated simple epithelium and some unlined. There are scattered collections of primitive tubules surrounded by collarettes of immature mesenchyme and a few foci of metaplastic cartilage. The features are typical of multicystic renal dysplasia.
- Dysplasia:
- Autosomal recessive polycystic kidney disease:
- Familial, bilateral; presents at birth
- Uniform, radially arranged fusiform cysts arising in collecting ducts
- No immature mesenchyme or cartilage
- Alterations in PKHD1
- Autosomal dominant polycystic kidney disease:
- Familial, bilateral, usually presents in adulthood but does rarely present at birth
- No immature mesenchyme or cartilage
- Alterations in PKD1, PKD2 or PKD3
- Nephronophthisis:
- Familial, bilateral; presents in infancy or childhood
- Corticomedullary cysts with interstitial fibrosis and tubular atrophy
- No immature mesenchyme or cartilage
- Alterations in NPHP1 or at least 24 other genes (Husain: Stocker and Dehner's Pediatric Pathology, 5th Edition, 2021)
- Medullary sponge kidney:
- Usually sporadic, usually bilateral, usually presents in adulthood but rarely in children and infants
- Ectatic and cystic collecting ducts in the renal medulla
- No immature mesenchyme or cartilage
- Cystic nephroma:
- Sharply demarcated from adjacent normal kidney by thick fibrous capsule, no renal tissue between cysts, no immature mesenchyme or cartilage
- Autosomal recessive polycystic kidney disease:
- Hypoplasia:
- Segmental hypoplasia (Ask-Upmark kidney):
- Exterior has one or more deep cortical grooves
- On cut section, normal parenchyma alternates with areas of marked cortical thinning and scar (Adv Anat Pathol 2020;27:311)
- Oligomeganephronia:
- Bilateral small kidneys with markedly enlarged glomeruli, juxtaglomerular apparatus and tubules (Adv Anat Pathol 2020;27:311)
- Acquired atrophy secondary to infarction of infection:
- Normal parenchyma alternates with areas of cortical scar or inflammation
- Segmental hypoplasia (Ask-Upmark kidney):
- Agenesis:
- Ectopic kidney (e.g., pelvic kidney, horseshoe kidney):
- Kidney is located elsewhere in body (usually pelvis or contralateral abdomen; rarely thorax)
- Severe acquired atrophy secondary to infarction or infection:
- Small scarred remnant kidney is present
- Ectopic kidney (e.g., pelvic kidney, horseshoe kidney):
An infant died of respiratory insufficiency shortly after birth. At autopsy, both kidneys had the appearance shown in the image above. Which of the following is most suggestive of bilateral renal dysplasia as the correct diagnosis?
- Ectatic and cystic collecting ducts in the medulla
- Family history of congenital renal cysts in 2 of 5 siblings
- Foci of primitive tubules with collarettes of mesenchyme
- Known homozygous mutation in PKHD1
- Markedly small bilateral kidneys with normal histology
Answer A is incorrect because medullary collecting ducts are dilated in autosomal recessive polycystic kidney disease and nephronophthisis but are not specifically affected in renal dysplasia. Answer B is incorrect because this strong family history is more suggestive of autosomal recessive polycystic kidney disease or autosomal dominant polycystic kidney disease. Although renal dysplasia can recur within families, inheritance is rarely Mendelian. Answer D is incorrect because mutations in PKHD1 are associated with autosomal recessive polycystic kidney disease, not renal dysplasia. Answer E is incorrect because small bilateral kidneys with normal histology is the definition of renal hypoplasia, not renal dysplasia.
Comment Here
Reference: Dysplasia / hypoplasia / agenesis
- Agenesis of the left kidney, dysplastic right kidney
- Agenesis of the left kidney, normal appearing right kidney
- Horseshoe kidney with fused lower poles
- Normal appearing left kidney, hypoplastic right kidney
Answer B is incorrect because a single, normally functioning kidney is sufficient to support renal function and amniotic fluid production. Answer C is incorrect because, despite its aberrant shape and location, a horseshoe kidney maintains normal renal function in utero. Answer D is incorrect because a normal kidney and a hypoplastic kidney together are sufficient to support renal function and amniotic fluid production.
Comment Here
Reference: Dysplasia / hypoplasia / agenesis
- Systemic, small to medium vessel, eosinophil rich granulomatous vasculitis
- Rare, systemic, small to medium vessel granulomatous vasculitis (Allergy 2013;68:261)
- Diagnosed clinically by the presence of
- Asthma
- Blood eosinophilia exceeding 1,500/mm3
- Evidence of vasculitis involving 2 or more organs
- Renal manifestations occur in 45% of those with the diagnosis and include
- Pauci-immune crescentic glomerulonephritis
- Renal arteritis, arteriolitis and medullary angiitis
- Microscopic features include
- Glomerular crescents
- Fibrinoid necrosis
- Necrotizing vasculitis
- Extravascular eosinophil rich granulomatous inflammation
- Associated with HLA-DRB4 and antineutrophil cytoplasmic antibodies (ANCA)
- Eosinophilic granulomatosis with polyangiitis (EGPA), Churg-Strauss syndrome
- ICD-10: M30.1 - polyarteritis with lung involvement (Churg-Strauss)
- Annual incidence of 0.5 - 4.2 cases per 1 million people (Best Pract Res Clin Rheumatol 2005;19:191)
- Prevalence of 11 - 14 cases per 1 million adults (Arthritis Rheum 2004;51:92)
- Age 40 - 60 years, mean age at diagnosis is ~49 years (Ann Rheum Dis 2013;72:1011)
- Pediatric cases have been described (Semin Arthritis Rheum 2009;39:108)
- No identified gender predominance, ethnic predisposition or familial pattern
- Renal manifestations occur in 45% of patients with the diagnosis (N Engl J Med 1997;337:1512)
- Upper airway tract and lungs
- Kidneys
- Peripheral nervous system
- Gastrointestinal tract
- Heart
- Skin
- Immune dysregulation
- Eosinophil infiltration (Ann N Y Acad Sci 2005;1051:121, Rheumatology (Oxford) 2008;47:804)
- Activated Th2 cells release cytokines IL4, IL13 and IL5
- IL4, IL13 and IL5 promote eotaxin3 release from endothelial and epithelial cells
- Eotaxin3 brings eosinophils from the blood stream into tissue
- Activated eosinophils secrete granules containing eosinophil basic protein and eosinophil derived neurotoxin, damaging tissue
- Activated eosinophils secrete IL25, which activates Th2
- Dysregulation of the following immune cells are also implicated: Th1, Th17, IL17A, B cells
- ANCA induced endothelial damage: ANCA leads to neutrophil degranulation and tissue damage (Ann N Y Acad Sci 2005;1051:121, Rheumatology (Oxford) 2008;47:804)
- Considered an idiopathic condition (Ann N Y Acad Sci 2005;1051:121, Rheumatology (Oxford) 2008;47:804)
- Exposure to allergens or drugs (Autoimmun Rev 2012;12:235)
- Propylthiouracil, hydralazine, minocycline, penicillamine (Int J Rheumatol 2009;2009:504105)
- Strongly associated with asthma
- Associated with HLA-DRB4
- 40 - 70% of patients have antimyeloperoxidase ANCA positivity (Arthritis Rheum 2005;52:2926, N Engl J Med 1997;337:1512)
- ANCA positivity is associated with renal and peripheral nervous system disease (Arthritis Care Res (Hoboken) 2016;68:374)
- ANCA positivity is less common in eosinophilic granulomatosis with polyangiitis than in granulomatosis with polyangiitis (Arthritis Rheum 2005;52:2926)
-
- Symptomatology
- Prodromal phase (months to years) (Allergy 2013;68:261)
- Asthma, rhinitis arthralgia, fever, malaise, weight loss, nasal polyps, chronic sinusitis
- Eosinophilic phase (Allergy 2013;68:261)
- Peripheral eosinophilia and eosinophilic infiltrative disease involving lung, heart or gastrointestinal tract
- Vasculitic phase (Allergy 2013;68:261)
- Vasculitis with peripheral neuropathy, renal and skin involvement
- Usually within 3 years of onset of asthma, though may be delayed for decades (N Engl J Med 1997;337:1512)
- Prodromal phase (months to years) (Allergy 2013;68:261)
- Clinical diagnosis
- Biopsy of an affected organ with microscopic confirmation: characteristic pathological changes are helpful but not essential to make the diagnosis
- In 1984, new clinical diagnostic criteria were proposed (Medicine (Baltimore) 1984;63:65)
- 3 diagnostic criteria, all must be present
- Asthma
- Blood eosinophilia exceeding 1,500/mm3 or > 10%
- Evidence of vasculitis involving 2 or more organs
- 3 diagnostic criteria, all must be present
- Peripheral eosinophilia (usually > 1,500 cells/μl or > 10%) is present in active disease
- Perinuclear / antimyeloperoxidase ANCA
- Renal involvement: hematuria, proteinuria, elevated serum creatinine
- Emerging biomarkers: eotaxin3 (at cutoff level of 80 pg/ml, the sensitivity and specificity of eotaxin3 for the diagnosis was 87.5% and 98.6%, respectively) (Rheumatology (Oxford) 2011;50:1737)
- Overall 5 year survival rate is 97% (Medicine (Baltimore) 2011;90:19)
- The following criteria are associated with a higher 5 year mortality (Medicine (Baltimore) 2011;90:19)
- Age > 65
- Cardiac symptoms
- Gastrointestinal involvement
- Renal insufficiency (serum creatinine > 150 μmol/l)
- Lack of ear, nose and throat manifestations
- 51 year old man presented with a stomach perforation (Case of the month #541)
- 54 year old man with pauci-immune necrotizing and crescentic glomerulonephritis, accompanied by acute coronary syndrome due to vasospasm (Am J Kidney Dis 2010;56:e5)
- 58 year old man with polyneuropathy, peripheral eosinophilia, pauci-immune necrotizing and crescentic glomerulonephritis, small bowel eosinophilic vasculitis, without asthma (Saudi J Kidney Dis Transpl 2014;25:402)
- 59 year old woman with Churg-Strauss syndrome and eosinophilic tubulointerstitial nephritis (Intern Med 2012;51:1555)
- 67 year old woman with pauci-immune necrotizing and crescentic glomerulonephritis, tubulointerstitial nephritis with eosinophils and peripheral blood eosinophilia without asthma (Am J Kidney Dis 1998;31:1032)
- Immunosuppressants (e.g. cyclophosphamide, glucocorticoids, azathioprine) (Postepy Hig Med Dosw (Online) 2016;70:654, Eur J Rheumatol 2016;3:122)
- B cell depleting agent rituximab (several case series) (Ann Rheum Dis 2016;75:396)
- Anti-IL5 antibody mepolizumab (efficacy has been described in small clinical trials) (J Allergy Clin Immunol 2010;125:1336)
- Systemic (Jennette: Heptinstall's Pathology of the Kidney, 7th Edition, 2015):
- Necrotizing vasculitis and extravascular eosinophil rich granulomatous inflammation
- Kidney (Jennette: Heptinstall's Pathology of the Kidney, 7th Edition, 2015):
- Necrotizing and crescentic glomerulonephritis, without significant immune complex deposition
- Fibrinoid necrosis of arteries
- Medullary angiitis
- Tubulointerstitial nephritis with eosinophils
Contributed by Nicole K. Andeen, M.D., Patrick A. Maher, M.D., Juhi D. Mahadik, M.B.B.S., M.D. and Naziheh Assarzadegan, M.D. (Case #541)
- Variable staining for immunoglobulin (< 2+ on a scale from 0 - 4+) (Jennette: Heptinstall's Pathology of the Kidney, 7th Edition, 2015)
- Variable staining for complement components
- Immunoglobulin or complement staining often focally and segmentally present in areas of necrosis / crescents (nonspecific)
- Fibrinoid necrosis is fuchsinophilic with trichrome staining (Jennette: Heptinstall's Pathology of the Kidney, 7th Edition, 2015)
- Disruption of the glomerular basement membrane in areas of fibrinoid necrosis highlighted with Jones methenamine silver (JMS) stain
- Scant or absent immune complex deposits (Jennette: Heptinstall's Pathology of the Kidney, 7th Edition, 2015)
- Elongated aggregations of fibrin (fibrin tactoids)
- May have breaks in glomerular basement membrane
- May have capillaries with endothelial swelling, necrosis, marginating neutrophils
- Kidney, biopsy:
- Focally necrotizing and crescentic glomerulonephritis, pauci-immune complex type (see comment)
- Comment: Consistent with this patient's positive ANCA, the acute renal insufficiency and hematuria are due to a focally necrotizing and crescentic glomerulonephritis, pauci-immune complex (vasculitic) type. Active injury is manifest as segmental necrosis or cellular crescents in 36% of glomeruli. Chronic injury is manifest as segmental glomerulosclerosis / scarring in 9% of glomeruli, global glomerulosclerosis in 9% and approximately 10% tubular atrophy and interstitial fibrosis. Extraglomerular vasculitis is not identified.
- Etiologies of necrotizing and crescentic glomerulonephritis, pauci-immune complex (vasculitic) type are generally best distinguished clinically, not histologically (J Autoimmun 2014;48:99)
- Granulomatosis with polyangiitis:
- No asthma, eosinophilia or nondestructive sinus involvement
- Has anti-PR3 (versus antimyeloperoxidase antineutrophil cytoplasmic antibodies for eosinophilic granulomatosis with polyangiitis)
- Polyarteritis nodosa:
- No asthma, eosinophilia or lung infiltrates
- Hypereosinophilic syndrome:
- No vasculitis, no antineutrophil cytoplasmic antibodies, lower eosinophil count
- Helminth infection:
- No systemic vasculitis
A 56 year old man presents with acute kidney injury and positive antineutrophil cytoplasmic antibodies (ANCA). Kidney biopsy demonstrates the above finding in 30% of glomeruli, with trace granular mesangial staining for IgA by immunofluorescence microscopy and no deposits by electron microscopy. What is the best diagnosis?
- ANCA associated glomerulonephritis superimposed on IgA nephropathy
- Antiglomerular basement membrane antibody (anti-GBM) disease
- Crescentic IgA nephropathy
- Focally necrotizing and crescentic glomerulonephritis, pauci-immune complex type, consistent with ANCA associated disease
Comment Here
Reference: Churg-Strauss syndrome
- Asthma, peripheral eosinophilia, vasculitis
- Elevated eotaxin levels
- Kidney biopsy demonstrating pauci-immune complex mediated necrotizing and crescentic glomerulonephritis
- Positive antimyeloperoxidase antineutrophil cytoplasmic antibodies (MPO ANCA)
- Antimitochondrial antibody (AMA)
- Antinuclear antibody (ANA)
- MPO-Anti neutrophil cytoplasmic antibody (P-ANCA)
- PR3-Anti neutrophil cytoplasmic antibody (C-ANCA)
Comment Here
Reference: Churg-Strauss syndrome
- Fabry disease is a lysosomal storage disorder caused by genetic deficiency of lysosomal enzyme α-galactosidase A (α-Gal), leading to accumulation of globotriaosylceramide (GL3) in various organs and resulting in dysfunction
- It is inherited in an X linked recessive pattern
- Lysosomal storage disorder with an X linked recessive mode of inheritance due to deficiency of enzyme α-galactosidase A
- Multiorgan involvement including skin, nervous system, heart and kidneys
- Light microscopy shows foamy and vacuolated podocytes and tubular epithelial cells with corresponding lamellated inclusions by electron microscopy
- α-galactosidase A deficiency
- Anderson-Fabry disease
- Angiokeratoma corporis diffusum
- Ceramide trihexosidase deficiency
- Sweeley-Klionsky disease
- Ruiter-Pompen-Wyers syndrome
- ICD-10: E75.21 - Fabry (-Anderson) disease
- Rare inherited disease, although it is the second most prevalent inherited lysosomal storage disorder after Gaucher disease (Oxford PharmaGenesis: Fabry Disease - Perspectives From 5 Years of FOS [Accessed 29 November 2024])
- It is inherited in an X linked recessive pattern and primarily affects males
- It is a panethnic disease with an estimated birth incidence of 1 per 117,000 in the general population and 1 per 40,000 male live births (Adv Rheumatol 2024;64:22)
- Actual prevalence of this condition may be higher because of challenges in diagnosing this disease which has a wide range of clinical presentations and ages at presentation (Int Urol Nephrol 2024;56:3161)
- Multisystem disease
- Kidney (glomeruli)
- Heart
- Sweat glands
- Nerves
- Deficiency in lysosomal enzyme exoglycohydrolase, ceramide trihexosidase commonly referred to as α-galactosidase A (α-GAL)
- The enzyme catalyzes the cleavage of glycosphingolipids, especially globotriaosylceramide present in cell membranes and deficiency of enzyme leads to accumulation of globotriaosylceramide in lysosomes of endothelial cells of kidney, heart, skin, brain and nerves (Orphanet J Rare Dis 2010;5:30)
- X linked inheritance of deficiency of lysosomal enzyme α-galactosidase A
- Symptoms and signs vary with mutation and functional level of α-GAL diagnosis (Int Urol Nephrol 2024;56:3161)
- Hemizygous males present with multisystem involvement starting in infancy or childhood with acroparesthesias, hypohidrosis and angiokeratomas
- Early renal manifestations result from defects in the ability to concentrate urine, with proteinuria in adulthood and renal failure by 40 years of age
- Younger males can have albuminuria and show lesions on kidney biopsy
- Patients with residual enzyme activity and heterozygous females vary in symptomatology from asymptomatic to renal failure or cardiomyopathy
- Other systemic manifestations include stroke, autonomic dysfunction, heart attack, deafness and cardiomyopathy
- Newborn screening using fluorometric enzyme assay for α-GAL activity
- Genetic testing for disease causing variant in GLA gene that encodes α-GAL (Adv Rheumatol 2024;64:22)
- For males, a very low leukocyte α-GAL activity (A genetic test for mutations in GLA gene is required for females and for confirmation in males (Ther Clin Risk Manag 2020;16:551)
- Nonspecific findings of medical renal disease including increased echogenicity, thinned out renal cortex and multiple renal cysts seen on imaging (AJR Am J Roentgenol 2006;186:1184, J Comput Assist Tomogr 2004;28:158)
- Prognosis is linked to the extent of organ involvement and timeliness of diagnosis (Int Urol Nephrol 2024;56:3161)
- Urine protein creatinine ratio is the most important indicator of renal disease progression in adult Fabry patients (Clin J Am Soc Nephrol 2010;5:2220)
- Patients may end up with end stage kidney disease requiring transplant
- Although little is known about recurrence of Fabry after kidney transplant, in general it is thought to not recur in patients on enzyme replacement therapy; although biopsies may show inclusions, graft function is not usually compromised (J Am Soc Nephrol 2002;13:S134, Medicina (Kaunas) 2020;56:284)
- 21 year old woman with maternal history of Fabry disease (Case Rep Nephrol Dial 2021;11:355)
- 24 year old woman with nocturia and left ventricular hypertrophy (Cureus 2024;16:e63661)
- 37 year old man with recurrent proteinuria and ventricular septal thickening (BMC Nephrol 2024;25:61)
- 45 year old man with hematochezia and proteinuria (Korean J Intern Med 2015;30:925)
- Case series of 5 patients with Fabry disease and end stage renal disease (Ann Med Health Sci Res 2016;6:193)
- Enzyme replacement therapy
- Chaperone drugs
- Transplant in patients developing end stage kidney disease
- Reference: Mol Genet Metab 2018;123:416
- Standard light microscopy processing may extract the accumulated lipids due to processing in xylene
- Glomeruli show hypertrophic glomerular visceral epithelial cells (podocytes) with vacuolated, foamy or pale and lacy cytoplasm, because of accumulated lipids (J Am Soc Nephrol 2002;13:S134)
- With progressive disease, mesangial expansion, global and segmental glomerulosclerosis and interstitial fibrosis and tubular atrophy develops
- Heterozygotes in females may have patchy and irregular distribution of the above findings (Kidney Int 1978;13:223)
- 1 micron (semi thin) sections of glutaraldehyde or formaldehyde fixed plastic embedded tissue that are stained with toluidine blue are ideal for detecting clusters of lipid inclusions within lysosomes as the processing for electron microscopy studies does not extract the lipids
- Inclusions vary in size and shape and have a lamellated appearance with alternating dark and light layers that are known as myelin bodies or zebra bodies
- These inclusions are most prominent in podocytes but can also be seen in tubular epithelial cells, mesangial cells, endothelial cells and interstitial cells
- Generally negative
- May have nonspecific trapping of IgM and C3 in mesangial areas
- Lamellated electron dense lipid inclusions within lysosomes, seen predominantly in podocytes and tubular epithelial cells, are diagnostic
- Percentage of podocytes with lipid inclusions decreases with age
- X linked disorder with deficient activity of α-galactosidase A enzyme encoded by GLA gene located on X chromosome (GeneReviews: Fabry Disease [Accessed 29 November 2024])
Washington University in St. Louis renal pathology teaching series: Fabry disease
- Kidney, percutaneous needle biopsy:
- Findings consistent with Fabry disease (see comment)
- Comment: The biopsy shows 22 glomeruli by light microscopy, 7 of which are globally sclerosed, with ~40% interstitial fibrosis. Glomeruli show foamy appearing podocytes but are otherwise unremarkable. Focal tubular epithelial cells show foamy vacuolated cytoplasm. Immunofluorescence performed on frozen tissue does not reveal any positive staining. Electron microscopy shows dense lamellated inclusions within podocytes and proximal tubular epithelial cells. Taken together, these findings are consistent with Fabry disease.
- Iatrogenic phospholipidosis:
- Secondary to drug toxicity of amphophilic drugs such as chloroquine, hydroxychloroquine and aminoglycosides that inhibit lysosomal enzyme activity
- Indistinguishable on light microscopy
- Extent and distribution of inclusions by electron microscopy is sparse compared to that of Fabry disease
- Laboratory testing will not reveal any deficiency of lysosomal enzyme α-galactosidase A (α-Gal)
- Longstanding proteinuria:
- Lipid deposition may be an incidental finding in patients with longstanding proteinuria
- Extent and intensity of inclusions by electron microscopy is usually not as extensive as in Fabry
A 15 year old boy with a history of paresthesia, reddish-purple rash on trunk and recent history of shortness of breath was noted to have proteinuria and ECHO showed ventricular septal thickening. There was a family history of unknown kidney disease in his maternal uncles. After multiple serologic tests, a kidney biopsy was performed. Light microscopy revealed vacuolization and foamy changes in podocytes but no segmental sclerotic lesions and no endocapillary or mesangial hypercellularity. Immunofluorescence microscopy did not reveal any deposits or staining. Electron microscopy showed the findings above and no significant foot process effacement. What is the most probable diagnosis?
- Fabry disease / nephropathy
- Focal and segmental glomerulosclerosis
- IgA nephropathy
- Lupus nephritis
Comment Here
Reference: Fabry disease
- Autosomal dominant
- Autosomal recessive
- Mitochondrial
- X linked recessive
Comment Here
Reference: Fabry disease
- Also called alpha-galactosidase A deficiency, angiokeratoma corporis diffusum universale
- X linked (Xq22.1) recessive lysosomal storage disease which causes deficiency in lysosomal alpha-galactosidase A, which catabolizes neutral glycosphingolipids
- Deficiency causes intracellular accumulation of galabiosylceramide (ceramide trihexoside) and digalactosyl ceramide within skin, renal glomeruli, renal tubular epithelium, blood vessels, corneal epithelium, myocardium and ganglion cells
- Affects 1 per 40,000
- Highly penetrant in hemizygous males with symptoms at infancy or childhood
- Later presentation in heterozygous females, who have more variable severity due to variable lyonization of X chromosome and may have normal leukocyte alpha-galactosidase A activity
- Clinical symptoms include angiokeratomas on skin of abdomen, buttocks, lips, genitalia and upper thighs
- Also hematuria and proteinuria progressing to renal failure, corneal dystrophy and recurrent shooting pains in legs
- Death due to renal, cardiac or cerebrovascular disease at age 40+ years
- Low blood or urine levels of alpha-galactosidase by enzymatic assay (may be normal in female heterozygotes)
- Elevated ceramide trihexoside in urine by thin layer chromatography
- Immunostains for ceramide trihexoside
- In women, must perform DNA mutation analysis of alpha-galactosidase A gene to exclude carrier state
- Patients may present with advanced disease identifiable only by ultrastructural studies (Ultrastruct Pathol 2010;34:307)
- 23 year old man with congenital agammaglobulinemia (J Korean Med Sci 2011;26:966)
- 34 year old man with atypical variant (Arch Pathol Lab Med 1996;120:86)
- 42 year old woman with persistent proteinuria (Arch Pathol Lab Med 1985;109:89)
- Cases with accumulation in heart, not kidney or liver (Hum Pathol 1990;21:1067)
- Recombinant human alpha-galactosidase A replacement therapy
-
Kidney:
- Enlarged and bubbly, clear vacuoles in visceral epithelium (demonstrated by trichrome stain), parietal epithelium, mesangial cells, endothelial cells, vascular smooth muscle and distal tubular cells
- Narrowing and thrombosis of arteries and arterioles
- Patchy tubular atrophy and interstitial fibrosis
- Progression to focal segmental and global glomerulosclerosis
- Negative
- PAS, Oil red O, Sudan black and Luxol fast blue (stain glycolipid and phospholipid-like material)
- Characteristic single membrane bound intracellular inclusions (myelin-like figures, zebra bodies), that are 0.1 to 10 microns in diameter, round and lamellated with concentric electron dense layers, found in endothelial and smooth muscle cells, myocardium, fibroblasts and glomerular epithelium; deposits reduced after enzyme therapy (Clin Nephrol 2009;71:550)
- Changes also present in urine sediment (Arch Pathol Lab Med 1981;105:361)
- Wide molecular heterogeneity (Rev Med Interne 2010;31 Suppl 2:S275)
- Foam cell change of Gaucher’s disease, gangliosidoses, fucosidosis, mucopolysaccharidoses (all have different intracellular distribution and ultrastructural features of inclusions, lack electron dense myeloid bodies and can detect by laboratory assays)
- Treatment with chloroquine, amiodarone or aminoglycosides (have similar myelin-like figures, Hum Pathol 2003;34:285)
- Glomerular disease caused by the deposition of randomly oriented, nonperiodic noncongophilic fibrils with a mean diameter of 20 nm
- Rare form of glomerulonephritis characterized by organized, nonbranching fibrils in random orientation with a diameter of 15 - 25 nm and without a hollow core
- Most deposits are positive for IgG, C3 and kappa and lambda light chains and are negative for Congo red
- DNAJB9 heat shock protein comprises a major component of the fibrils and IHC for DNAJB9 is sensitive and specific for fibrillary glomerulonephritis
- Treatment options are limited, prognosis is poor and transplant recurrence is frequent
- Most patients present with a variable degree of proteinuria
- Fibrillary glomerulopathy
- ICD-10: N03.8 - chronic nephritic syndrome with specified morphological changes
- Middle aged to older adults, most studies show a mean age close to 55 years (range of 20 - 80 years)
- Slight female predominance (M:F = 1.0:1.3 - 1.8)
- Most frequently seen in White patients (up to 87%)
- Can be seen in association with malignancy (more frequently solid organ neoplasms, up to 23% of patients), hepatitis C virus infection (up to 23%) and autoimmune diseases (13 - 30%) (Am J Nephrol 2017;45:248)
- Kidneys
- Rare cases of splenic and pulmonary involvement have been reported (Ultrastruct Pathol 2008;32:113, N Engl J Med 1992;326:36)
- In fibrillary glomerulonephritis (FGN), there is an abnormal deposition of haphazardly arranged fibrils with a diameter between 15 and 25 nm in most cases (Kidney Int Rep 2019;4:917)
- These fibrils are composed of DNAJB9 (a heat shock protein that is a chaperone in the endoplasmic reticulum), IgG (mainly IgG1 and IgG4) and components of the classical complement pathway
- Exact pathophysiology of fibrillary glomerulonephritis is not yet completely understood but studies indicate that DNAJB9 may act as an autoantigen that elicits an autoimmune response with abnormal deposition of DNAJB9, IgG and complement components within the kidney
- Etiology is poorly understood
- DNAJB9 may act as an autoantigen that elicits an abnormal autoimmune response with DNAJB9, IgG and complement components abnormally deposited within the kidney in a fibrillary structure (Kidney Int Rep 2019;4:917)
- Most patients present with a variable degree of proteinuria, which is commonly within the nephrotic range; the disease is usually insidious and the diagnosis is frequently made when patients are already in an advanced stage with chronic renal failure (Clin J Am Soc Nephrol 2019;14:1741)
- Can be seen in association with malignancy (more frequently solid organ neoplasms, up to 23% of patients), hepatitis C virus infection (up to 23%) and autoimmune diseases (13 - 30%)
- Renal biopsy with the characteristic light microscopic, immunofluorescence, immunohistochemical and ultrastructural findings is currently the only means of definitive diagnosis (Kidney Int Rep 2019;4:917)
- Patients usually present with proteinuria (Am J Kidney Dis 2013;62:679, Am J Kidney Dis 2015;66:e27)
- Proteinuria seen in most patients (mean urine protein is between 4.1 and 7.3 g/d)
- Full nephrotic syndrome (nephrotic range proteinuria, low serum albumin, edema) is seen in 25 - 69% of patients
- Microscopic hematuria is seen in 25 - 95% of patients
- Decreased renal function is seen in ~67% of patients (mean serum creatinine between 2.1 and 3.7 mg/dL)
- Acute decline in renal function can be seen in patients whose fibrillary glomerulopathy is associated with crescent formation
- Patients usually (> 90%) have normal serum complement levels
- Monoclonal serum or urine protein is rarely present
- Majority of which are thought to be a coincidental finding due to the age of the patient population and frequent lack of concordance with the staining seen in the glomeruli
- Monoclonal fibrillary glomerulonephritis is rare (3 - 11% of cases) and the monoclonality of the deposits must match what is seen in the serum or urine (see Immunofluorescence description for additional information)
- References: Kidney Int 2003;63:1450, Clin J Am Soc Nephrol 2011;6:775
- Overall prognosis is poor, with 33 - 50% of patients advancing to end stage renal disease (ESRD) within 4 years of diagnosis (Kidney Int 2019;96:581)
- Poor prognostic factors include advanced age, elevated serum creatinine levels and extent of glomerulosclerosis, tubular atrophy and interstitial fibrosis
- Black patients with concomitant hepatitis C virus infection and fibrillary glomerulonephritis have an exceptionally poor renal prognosis (Am J Nephrol 2017;45:248)
- Patients with ESRD due to fibrillary glomerulonephritis show no difference in mortality compared to those with ESRD from other causes (Am J Nephrol 2015;42:177)
- 63 year old man with hypertension, edema, proteinuria, monoclonal gammopathy of undetermined significance (MGUS) and fibrillary glomerulonephritis (Cureus 2023;15:e47862)
- 63 year old man with cirrhosis secondary to hepatitis C virus, multiple myeloma and fibrillary glomerulonephritis (Cureus 2022;14:e28250)
- 66 year old woman with vulvar squamous cell carcinoma and fibrillary glomerulonephritis (Cureus 2023;15:e35068)
- Unfortunately, no therapies have been shown to clearly improve outcomes in patients with fibrillary glomerulonephritis
- Some studies indicate that almost 50% of patients with fibrillary glomerulonephritis will progress to ESRD within 5 years of diagnosis (Clin J Am Soc Nephrol 2011;6:775)
- Fibrillary glomerulonephritis can recur in the renal allograft, with recurrence rates of up to 50% reported
- Recurrence usually occurs after several years with one study reporting a median time to recurrence of 10 years (Am J Kidney Dis 2020;76:500)
- References: Am J Kidney Dis 2015;66:e27, Kidney Int Rep 2020;6:239
- Fibrillary glomerulonephritis can cause several histologic patterns (Clin J Am Soc Nephrol 2011;6:775)
- Most common glomerular finding is mesangial expansion by eosinophilic material that is PAS positive, silver negative or weak and Congo red negative; this characteristic finding gives a moth eaten appearance similar to that described in amyloidosis (Case Rep Med 2013;2013:935172)
- In more advanced cases, there is glomerular capillary wall thickening owing to fibrillary deposits in the glomerular basement membrane (GBM)
- Glomerular hypercellularity is usually mild
- Occasionally, the fibrils can be seen in the tubular basement membranes and vessel walls, although usually only appreciated on immunohistochemistry or immunofluorescence staining
- Other glomerular patterns include
- Membranoproliferative pattern with focal glomerular basement membrane duplication (Cureus 2023;15:e47862)
- Crescentic glomerulonephritis
- May occur in up to 25% of cases
- Usually a focal process but has rarely been reported as diffuse (over 50% glomerular involvement by crescents)
- Crescentic disease portends a worse prognosis (Am J Kidney Dis 2023;81:368)
- Secondary segmental sclerosis can be seen
- Tubular atrophy and interstitial fibrosis are common findings but the extent can vary depending on the severity and chronicity of the disease
- Congo red staining is negative
- Rare Congo red positive cases have been reported but these may represent concomitant amyloid deposition
- If the Congo red stain is positive, mass spectrometry testing is recommended to exclude renal amyloidosis
- DNAJB9 immunohistochemical stain is very sensitive and specific (up to 99% reported for both) (Kidney Int Rep 2017;3:56)
- References: Cureus 2022;14:e26001, Am J Kidney Dis 2015;66:e27
- Bright mesangial and glomerular capillary wall staining for IgG
- Staining can appear smudgy to granular
- Most cases are IgG4 dominant (90%) with colocalization of IgG1 (80%)
- Rare cases are positive for IgG1 only (10%)
- Most cases show equal kappa and lambda light chain staining (85%)
- Rare cases appear monotypic, usually kappa light chain
- Staining for C3 (92%), C1q (60%), IgM (47%) or IgA (28%) can also co-occur; however, staining for these antigens is usually less intense than that seen with IgG
- Staining within the arteries or tubular basement membranes can also occur
- References: Kidney Int Rep 2019;4:917, Kidney Int 2019;96:581, Kidney Int Rep 2022;8:202
- DNAJB9
- Immunohistochemical stain for DNAJB9, a member of the heat shock protein family
- Positive staining within the mesangium and capillary walls (Biomedicines 2022;10:2102)
- Has a sensitivity of 88 - 98% and specificity of > 99%
- Reference: Kidney Int Rep 2017;3:56
- Congo red
- Should be negative but congophilic fibrillary glomerulonephritis has been reported (Am J Kidney Dis 2018;72:325)
- If it is positive, consider a diagnosis of renal amyloidosis
- Staining for DNAJB9 and mass spectrometry testing should be considered to differentiate between fibrillary glomerulonephritis and renal amyloidosis (Kidney Int Rep 2017;3:56)
- Nonbranching, haphazardly arranged fibrils with an average diameter between 15 and 25 nm are found within the mesangium and glomerular basement membranes (GBM) (Kidney Int Rep 2019;4:917, Kidney Int Rep 2020;6:239)
- These fibrils should lack a hollow core (they are not microtubules)
- Fibrillary deposits can occasionally be seen within the tubular basement membranes
- Kidney, native, biopsy:
- Fibrillary glomerulonephritis (see comment)
- Comment: The biopsy shows enlarged glomeruli with mesangial expansion by material that is silver and Congo red negative. Immunofluorescence shows polyclonal IgG (IgG1 and IgG4) and C3 staining within the mesangium and capillary walls. Electron microscopy confirms the presence of haphazardly arranged fibrils with a mean diameter of 15 nm within the mesangium and glomerular basement membrane. An immunohistochemical stain for DNAJB9 is positive in these areas as well. Overall, these findings are consistent with a diagnosis of fibrillary glomerulonephritis. The background cortex shows focal global glomerulosclerosis (20%) and mild tubular atrophy and interstitial fibrosis.
- Clinical history: The patient is a 65 year old man who presents with a history of proteinuria and decreased renal function. Medical history includes diabetes, hypertension and obesity. Serum creatinine has gradually increased from 1.0 mg/dL to 1.5 mg/dL over the past 2 years. Urinalysis shows proteinuria (3.7 g/24 h) without hematuria. Autoimmune, infectious and monoclonal testing are negative.
- Light microscopy: Sections stained with H&E, PAS, Jones and trichrome contain 3 cores of the renal cortex (60%) and medulla (40%). Up to 15 glomeruli are present per section, 3 of which are globally sclerosed. The patent glomeruli are slightly enlarged but normocellular. There is mesangial expansion by eosinophilic material that is PAS positive but appears negative on silver and Congo red stains. A DNAJB9 immunohistochemical stain is positive within the mesangium and segmentally along glomerular capillary loops. No crescents, necrosis or thrombi are identified. There is mild tubular atrophy and interstitial fibrosis, estimated to involve 20% of the cortical sample. The preserved proximal tubules are unremarkable. There is focal mononuclear interstitial inflammation primarily in areas of atrophy. Up to interlobular size arteries are sampled and show moderate fibrointimal thickening. The arterioles show mild hyaline deposition.
- Immunofluorescence microscopy: 4 glomeruli are available for evaluation by immunofluorescence microscopy. Granular staining is seen within the mesangium and segmental glomerular capillary walls for IgG (3+), IgG1 (1+), IgG4 (3+), C3 (2+) and kappa (3+) and lambda (3+) light chains.
- Electron microscopy: Semithin sections contain renal cortex with 2 glomeruli, both of which are examined under the electron microscope. Ultrastructurally, there are innumerable haphazardly arranged fibrils within the mesangium and glomerular basement membranes. These fibrils have a mean diameter of 15.2 nm (+/- 1.2 nm). Podocyte foot process effacement is mild and segmental. No tubuloreticular inclusions are seen. Tubular basement membranes are unremarkable.
- Immunotactoid glomerulopathy:
- Material deposited in the glomeruli is also silver and Congo red negative
- Ultrastructurally, in most cases the immuntactoid organized substructure is localized to otherwise electron dense discrete deposits (the substructure may not be evidence on low magnification under the electron microscope)
- Usually monoclonal staining is seen on immunofluorescence (mostly IgG1 kappa) (Kidney Int 2003;63:1450)
- Substructure consists of hollow tubules arranged in parallel arrays with a larger diameter (usually 20 - 90 nm)
- Renal amyloidosis:
- Diabetic nephropathy:
- Diffuse linear accentuation of the GBM and tubular basement membrane (TBM) with IgG, kappa and lambda light chains and albumin
- Expanded mesangium positive for PAS, silver and trichrome
- Glomerular fibrillary deposits are noncongophilic and DNAJB9 negative (Lab Invest 2024;104:100322)
- Membranous glomerulopathy:
- Fibrillary GN may resemble membranous glomerulopathy immunofluorescent staining
- Fibrillary glomerulonephritis
- Immunotactoid glomerulopathy
- Proliferative glomerulonephritis with monoclonal IgG deposits
- Renal amyloidosis
Comment Here
Reference: Fibrillary glomerulonephritis
A renal biopsy from an elderly woman shows mesangial expansion by eosinophilic material that is silver and Congo red negative. Immunofluorescence shows polyclonal IgG deposition within the mesangium and glomerular basement membranes. The image above is seen on electron microscopy. Fibril diameter ranges from 15 to 25 nm. What is the best diagnosis?
- Fibrillary glomerulonephritis
- Immunotactoid glomerulopathy
- Lupus nephritis
- Renal amyloidosis
Comment Here
Reference: Fibrillary glomerulonephritis
- Massive fibronectin deposition in glomeruli due to autosomal dominant, non sex-linked disorder with 1q32 abnormality (Am J Hum Genet 1998;63:1724, OMIM #601894)
- Proteinuria, often nephrotic syndrome, microhematuria, hypertension and progressive loss of renal function
- May recur after renal transplant
- 3 year old boy with microhematuria and hypertension (Pediatr Nephrol 2002;17:363)
- 34 year old man with microhematuria and hypertension (Ultrastruct Pathol 2010;34:240)
- 41 year old man with nephrotic syndrome (Int J Clin Exp Pathol 2009;3:210)
- Nephrol Dial Transplant 1998;13:2417
- Lobular accentuation of glomeruli with minimal hypercellularity (Nihon Jinzo Gakkai Shi 1999;41:49)
- Marked enlargement of mesangium and subendothelial space due to massive deposition of fibronectin and fibulin (PAS+, Congo red negative homogenous substance, Mod Pathol 2012;25:709), causing obliteration of capillary lumens
- Strongly positive for plasma fibronectin (not cell-derived fibronectin), scanty immunoglobulin or complement deposition
- Fibronectin has dense granular appearance with 12 - 16 nm fibrils
- Group of podocytopathies with different etiologies presenting with focal and segmental glomerulosclerosis (FSGS) along with nephrotic or subnephrotic proteinuria (Front Med (Lausanne) 2020;7:604961)
- Podocytes are the principal target of the lesions, leading to the characteristic sclerotic pattern involving part (segmental) of some (focal) glomeruli (Nephron 2020;144:413)
- FSGS is a glomerular disease characterized by segmental and focal sclerosis of the tuft in the absence of underlying immune complex mediated disease
- Patients generally present with nephrotic or subnephrotic proteinuria
- Primary, genetic and secondary / maladaptive forms are recognized
- Distinction should be made between the nonimmune complex mediated disease (FSGS) and the pattern of injury (segmental and focal sclerosis) due to other underlying glomerular conditions (e.g., immune complexes)
- Often abbreviated as focal sclerosis or FSGS (Nephrol Dial Transplant 2015;30:375)
- ICD-10: N04.1 - nephrotic syndrome with focal and segmental glomerular lesions
- Difficult to establish due to marked geographical and racial differences (Am J Kidney Dis 2016;68:533)
- FSGS is one of the leading cause of end stage renal disease (ESRD), with an increasing incidence rate, likely due to improved recognition and detection of the entity (Clin J Am Soc Nephrol 2017;12:502)
- Incidence of FSGS is ~7 per 1 million, with a prevalence of 4% (Am J Kidney Dis 2004;44:815)
- Typically, idiopathic FSGS is seen in patients aged 18 - 45; however, no age group is exempt (StatPearls: Focal Segmental Glomerulosclerosis [Accessed 25 April 2023])
- Incidence of FSGS is 3 - 7 times higher in young African American men than in European Americans (Kidney Int 2009;75:736)
- Kidney - glomerular disease
- FSGS is considered a podocytopathy, which is characterized by damage directed against the podocyte causing foot process effacement of different extension (J Pathol Transl Med 2016;50:405)
- Different mechanisms have been advocated for its development, starting from inherited alterations to structural podocyte genes, to circulating factors (e.g., cytokines) and mechanical stressful conditions (e.g., hypertension and obesity) (Biomed Res Int 2016;2016:1630365)
- In genetic forms, mutation to genes encoding for proteins with structural and signaling function either of the podocyte or glomerular basement membranes (GBM) causes disruption of the glomerular filtration barrier (Clin Kidney J 2018;11:179)
- Autoimmunity is linked to the observation of several antibodies in the serum of patients with minimal change disease (MCD) / primary FSGS, such as antinephrin, antiannexin A2 and anti-UCHL1 antibodies, suggesting a link / progression between MCD and FSGS (Front Immunol 2022;13:985925)
- Maladaptive forms of secondary FSGS ensue from a reduction in the number of functioning nephrons or from a normal nephron population subjected to abnormal stress (J Am Soc Nephrol 2017;28:2825)
- Other forms of secondary FSGS include virus associated FSGS and drug induced FSGS, which typically improve on resolution of the infection or cessation of the drug (Expert Opin Drug Saf 2006;5:95, Clin Kidney J 2013;6:1)
- These mechanisms lead to progressive podocyte loss and podocytopenia, increased nonselective filtration flow through bare areas of GBM, eventual collapse and occlusion of capillary loops on bare areas of GBM, disruption of glomerular tuft and adhesion to Bowman capsule (Biomed Res Int 2016;2016:1630365)
- Recently proposed clinicopathological classification divided FSGS based on etiology into 3 categories
- Primary FSGS
- Secondary FSGS
- Genetic FSGS
- In primary FSGS, circulating permeability factors such as soluble urokinase receptor (suPAR), cardiotrophin-like cytokine 1 and angiopoietin-like 4 (Angptl4) have been proposed as the initiating factors of podocyte injury in primary FSGS (Nat Rev Nephrol 2016;12:768)
- Recent description of autoantibodies (e.g., antinephrin) in patients with nephrotic syndrome, especially in MCD forms, suggested an autoimmune etiology for these forms, adding evidences to the link of MCD and primary FSGS (J Am Soc Nephrol 2022;33:238)
- In secondary FSGS, different etiologies are recognized that either cause an excessive stress to the glomerular filtration barrier (maladaptive) or cause podocyte damage
- Maladaptive forms can be due to systemic hypertension, obesity, increased lean body mass, renal vaso-occlusive disease, cyanotic congenital heart disease, sickle cell anemia, reduced number of functioning nephrons (e.g., unilateral renal agenesis, renal dysplasia, oligomeganephronia, glycogen storage disease, low birth weight) (Biomed Res Int 2016;2016:1630365, J Am Soc Nephrol 2018;29:759)
- Infection associated forms have been described in association with HIV (established), CMV (probably), parvovirus B19 (possibly), EBV (possibly), HCV (possibly), hemophagocytic syndrome (possibly), COVID-19 (established) (Biomed Res Int 2016;2016:7351964)
- Drug induced forms are secondary to pamidronate, direct acting antiviral therapy (ledipasvir, sofosbuvir), mTOR inhibitors, calcineurin inhibitors, anthracyclines, heroin (adulterants), lithium, interferon, anabolic steroids (Clin J Am Soc Nephrol 2015;10:1291)
- In genetic FSGS, > 50 genes have been described as potential sites of mutation responsible for monogenic forms of FSGS
- > 90% of childhood and adolescence onset cases have mutations involving a limited number of genes: NPHS1, NPHS2, WT1, LAMB2, PLCE1, COL4A5 and SCARB2 (Clin Kidney J 2018;11:179)
- Genetic predisposing factor for the development of FSGS, especially in African American ethnicity, is recognized in APOL1 polymorphisms, for which the trigger of environmental factors (e.g., viral infection) most frequently leads to collapsing forms (J Am Soc Nephrol 2011;22:2129)
- Proteinuria is the most common presenting feature of FSGS (Biomed Res Int 2016;2016:4632768)
- In patients with primary FSGS, full nephrotic syndrome is very common and the degree of renal insufficiency depends on the chronic changes of the renal parenchyma (Biomed Res Int 2016;2016:4632768)
- Tip variant FSGS typically presents with sudden onset nephrotic syndrome with anasarca and weight gain (Am J Kidney Dis 2020;75:955)
- In secondary FSGS, patients tend to have subnephrotic range proteinuria at presentation (although nephrotic range proteinuria will develop in the majority over time) in the absence of edema, hypoproteinemia or hypoalbuminemia (Biomed Res Int 2016;2016:4632768)
- Extensive history collection and large panel of laboratory test (including autoimmune and viral serology, serum and urine protein electrophoresis and immunofixation) should be done to exclude other causes of proteinuria (Nephron 2020;144:413)
- Presentation (sudden onset of nephrotic syndrome or more subtle gradual changes) and associated medical conditions (infections, obesity, hypertension, etc.) help to make a distinction between primary and secondary FSGS (Nephron 2020;144:413)
- Biopsy is necessary to establish the diagnosis of FSGS and determine the subtype of FSGS (Nephron 2020;144:413)
- There is consensus on performing genetic tests in steroid resistant FSGS in patients with positive family history or extrarenal physical signs suggestive of a genetic disorder (Fabry disease, nail patella syndrome or Alport syndrome) (Nephron 2020;144:413)
- New markers such as suPAR have been proposed to diagnose and predict FSGS recurrence after transplantation and monitor treatment response in patients with primary FSGS (Biomed Res Int 2016;2016:4632768)
- Full nephrotic syndrome: proteinuria (> 3.5 g per 1.73 m² body surface area per day or > 40 mg per square meter body surface area per hour in children), hypoalbuminemia (< 2.5 g/dL), hyperlipidemia and edema (Kidney Int 2021;100:S1)
- Imaging studies are generally not helpful in assessing persons with nephrotic syndrome (Am Fam Physician 2009;80:1129)
- ~30 - 60% of patients will inexorably progress to end stage kidney disease (ESKD) over a 5 - 10 year period of observation (Adv Chronic Kidney Dis 2011;18:332)
- It can recur in 20 - 25% of patients who receive a kidney transplant (Adv Chronic Kidney Dis 2011;18:332)
- Tip variant exhibits a better outcome in terms of achieving remission, the collapsing variant is the worst one (BMC Nephrol 2014;15:52)
- Prognosis of collapsing FSGS is much worse in the Black population compared to the White population (StatPearls: Focal Segmental Glomerulosclerosis [Accessed 25 April 2023])
- 23 year old man with rapid recurrence of FSGS after transplantation with graft reused in 78 year old man (Am J Kidney Dis 2021;78:897)
- 25 year old woman with INF2 mutation developing unusual FSGS (Kidney Int Rep 2022;7:2741)
- 46 year old woman with pembrolizumab induced FSGS (Medicine (Baltimore) 2021;100:e27546)
- High dose oral glucocorticoids is the first line immunosuppressive treatment for primary FSGS (Kidney Int Rep 2022;7:2081)
- Immunosuppression should not be used in adults with FSGS of undetermined cause (FSGS-UC) or in those with secondary FSGS; renin angiotensin aldosterone system (RAAS) blockade may have a role (Kidney Int 2021;100:S1)
- For adults with steroid resistant primary FSGS, cyclosporine or tacrolimus may be given for ≥ 6 months rather than continuing with glucocorticoid monotherapy or not treating (Kidney Int 2021;100:S1)
- Limited role for plasmapheresis (Nephron 2020;144:413)
- FSGS is characterized by focal (involving some but not all glomeruli) and segmental (< 100% glomerular tuft involvement with some residual patent glomerular capillaries) sclerosis of glomeruli without underlying immune complex disease (Nephrol Dial Transplant 2020;35:1219)
- Based on differences in the sclerotic lesion, a 5 tiered classification system (Columbia classification) was proposed in 2004 (Am J Kidney Dis 2004;43:368)
- Collapsing variant: at least 1 glomerulus with segmental or global collapse and overlying podocyte hypertrophy and hyperplasia
- Tip variant
- At least 1 segmental lesion involving the tip domain (outer 25% of tuft next to origin of proximal tubule)
- Tubular pole must be identified in the defining lesion
- Lesion must have either an adhesion or confluence of podocytes with parietal or tubular cells at the tubular lumen or neck
- Tip lesion may be cellular or sclerosing
- Cellular variant: at least 1 glomerulus with segmental endocapillary hypercellularity occluding lumina, with or without foam cells and karyorrhexis
- Perihilar variant: at least 1 glomerulus with perihilar hyalinosis, with or without sclerosis in > 50% of glomeruli
- FSGS, not otherwise specified (NOS): at least 1 glomerulus with segmental increase in matrix obliterating the capillary lumina; there may be segmental glomerular capillary wall collapse without overlying podocyte hyperplasia
- Glomerulosclerosis is accompanied by tubular atrophy and interstitial fibrosis with interstitial lymphocytes, proportional to the degree of scarring in the glomerulus (Am J Kidney Dis 2015;66:e1)
- In collapsing glomerulopathy, tubular lesions are disproportionately severe (Am J Kidney Dis 2015;66:e1)
- Cases of FSGS associated with severe tubulointerstitial lesions were also reported in patients taking cocaine, heroin, calcineurin inhibitors or lithium (Front Med (Lausanne) 2020;7:604961)
- Perihilar variant is generally associated with secondary maladaptive FSGS (Nat Rev Nephrol 2015;11:76)
- Cellular variant might be an early abnormality revealed by light microscopy for recurrent FSGS in a kidney transplant (Am J Kidney Dis 2015;66:e7)
- Etiology and pathogenesis of tip lesions has not yet been defined
- Injury to podocytes by turbulent flow at the tubular pole has been proposed (Am J Kidney Dis 2015;66:e5)
- Collapsing variant is commonly present in HIV associated nephropathy (HIVAN), COVID-19 associated nephropathy (COVAN) and APOL1 FSGS
- Other etiologies include infection, autoimmune disease, malignancy, drugs (bisphosphonates, interferon, anabolic steroids, calcineurin inhibitors and mTOR inhibitors) and posttransplantation (J Nephropathol 2012;1:87)
- Immunofluorescence typically negative or shows nonspecific IgM and C3 trace staining in sclerotic areas (Am J Kidney Dis 2015;66:e1)
- Jones methenamine silver (JMS), periodic acid-Schiff (PAS): highlight glomerular architecture and sclerosis
- Further stains are not required and often not helpful in differential diagnosis
- Due to its focal and segmental nature, diagnosis of FSGS could also be challenging on electron microscopy (Kidney Int 2008;74:1568)
- Podocyte injury including foot process effacement, microvillous transformation, detachment from the underlying GBM and cytoplasmic vacuolization (Kidney Int 2008;74:1568)
- Podocyte effacement in patients with FSGS secondary to maladaptive responses is less extended than in idiopathic FSGS (Kidney Int 2008;74:1568)
- Thick GBM in cases of maladaptive FSGS, thin GBM or lamellated appearance in cases of genetically determined FSGS correlated with Alport syndrome (Kidney Int 2008;74:1568)
- Multiple foci of increased mesangial matrix volume, exceeding the width of 2 mesangial cell nuclei (Kidney Int 2008;74:1568)
- Mild increase in the number of podocytes (Ultrastruct Pathol 2019;43:6)
- See Etiology
- Left kidney, needle core biopsy:
- Focal and segmental glomerulosclerosis, NOS (see comment)
- Comment:
- Microscopic description: Expansion of the mesangial matrix resulting in 2 glomeruli in segmental sclerosis of the tuft with adhesion to Bowman capsule and satellite podocyte hyperplasia. Interstitial fibrosis and tubular atrophy involving ~10% of the cortex with slight interstitial lymphocytic infiltrate. No significant changes in the vascular structures.
- Immunofluorescence: Trace focal and segmental staining for IgM. Negative IgG, IgA, C3, C1q, kappa and lambda light chains.
- Electron microscopy: Extensive foot process effacement, microvillous transformation of the cytoplasm of podocytes. No electron dense deposits.
- Minimal change disease (MCD):
- No remarkable modifications to the glomerular tuft, no detectable glomerular sclerosis
- Biopsies containing up to 10 glomeruli have a 35% chance of missing a segmental sclerosis involving 10% of the nephrons; this probability decreases to 12% in biopsies with 20 glomeruli (Clin J Am Soc Nephrol 2017;12:502)
- Immune complex glomerulonephritis with sclerosis:
- Immunofluorescence shows glomerular deposits with distribution and composition that depends on the specific underlying immune complex glomerulonephritis
- FSGS, cellular variant
- FSGS, collapsing variant
- FSGS, perihilar variant
- FSGS, tip variant
Comment Here
Reference: Focal segmental glomerulosclerosis - general
Comment Here
Reference: Focal segmental glomerulosclerosis - general
Comment Here
Reference: Focal segmental glomerulosclerosis - general
- Morphologic variant of focal segmental glomerulosclerosis (FSGS) characterized by capillary loop collapse and prominence of overlying epithelial cells (Semin Nephrol 2003;23:209)
- At least 1 glomerulus with capillary loop collapse and prominence of overlying podocytes or parietal epithelial cells
- Worse prognosis than other variants of focal segmental glomerulosclerosis; supersedes other variants if others present in biopsy
- May be associated with viruses, genetics, drugs, vascular injury and autoimmune diseases
- Collapsing glomerulopathy (CG), collapsing focal segmental glomerulosclerosis (FSGS), FSGS collapsing variant
- Early descriptions as a component of HIV associated nephropathy
- ICD-10: N04.1
- APOL1 high risk alleles common in individuals with African ancestry are associated with increased rates of kidney injury including collapsing focal segmental glomerulosclerosis (Kidney Int 2015;87:332)
- No single pathogenic trigger has been identified
- Multiple associations described (J Am Soc Nephrol 2006;17:2854, Clin Kidney J 2017;10:443):
- Infection
- Human immunodeficiency virus (HIV)
- Parvovirus B19 (Kidney Int 2001;59:2126)
- Cytomegalovirus
- Hepatitis C
- Drugs
- Bisphosphonates (pamidronate) (J Am Soc Nephrol 2001;12:1164)
- Interferons alpha and beta (Clin J Am Soc Nephrol 2010;5:607)
- Anabolic steroids
- Heroin
- Vascular and metabolic disease (Lancet 1996;347:231)
- Thrombotic microangiopathy (Kidney Int 2016;90:1321)
- Severe diabetic nephropathy (Nephrol Dial Transplant 2014;29:392)
- Severe vascular and microvascular disease (Mod Pathol 2018 Oct 16 [Epub ahead of print])
- Genetic conditions
- APOL1 high risk alleles
- Mitochondrial abnormalities
- Autoimmune disorders
- Posttransplant, recurrent and de novo
- Donor APOL1 high risk genotypes associated with poorer allograft outcome (Kidney Int 2018 Oct 2 [Epub ahead of print])
- Infection
- High incidence of nephrotic syndrome
- Renal insufficiency
- Renal biopsy is required for diagnosis
- Nephrotic range proteinuria
- Elevated serum creatinine at presentation (Am J Kidney Dis 1999;33:652)
- Worse 1 year (74%) and 3 year (33%) renal survival compared with other variants of focal segmental glomerulosclerosis (Kidney Int 2006;69:920)
- Worse prognosis if steroid unresponsive (J Am Soc Nephrol 2004;15:2169)
- Renal insufficiency at time of presentation is a poor prognostic factor (Saudi J Kidney Dis Transpl 2015;26:173)
- 6 year old boy with hepatitis B (Indian J Nephrol 2016;26:291)
- 21 year old African American woman with sickle cell disease (J Med Case Rep 2011;5:71)
- 38 year old Chinese man with coxsackievirus infection (Exp Ther Med 2016;11:1871)
- 50 year old man with lupus and diffuse alveolar hemorrhage (Transplant Proc 2017;49:913)
- 2 patients with lambda light chain amyloidosis (Am J Kidney Dis 2018;72:612)
- Treatment of underlying etiology, if identifiable
- In patients with HIV, highly active antiretroviral therapy (HAART) is the first line treatment and reduces rates of progression to end stage renal disease (J Am Soc Nephrol 2005;16:2412)
- In virus negative patients, steroid therapy and other immunosuppression is frequently used, although often refractory (J Am Soc Nephrol 2006;17:2854)
- At least 1 glomerulus with segmental collapse of the tuft, obliteration of the capillary loops and overlying podocyte or parietal cell hypertrophy or hyperplasia (Am J Kidney Dis 2004;43:368)
- May appear as pseudocrescents filling Bowman space
- Podocytes frequently form a radial corona around the glomerular tuft and have protein resorption droplets
- Lacks necrosis
- Particularly in HIV associated collapsing focal segmental glomerulosclerosis, may have significant chronic tubulointerstitial scarring and tubular microcysts
- Jones methenamine silver, periodic acid Schiff (PAS): highlight glomerular architecture with basement membrane collapse
- Further stains not required and often not helpful in differential diagnosis
- Podocyte can have a dysregulated immunophenotype and lose expression of WT1, synaptopodin and p57 (J Am Soc Nephrol 2006;17:2854)
- Negative or nonspecific
- Collapsed segments with nonspecific IgM or C3 staining
- Visceral epithelial resorption droplets: IgG, IgA, light chains or albumin
- Tubular resorption droplets: IgG, IgA, light chains or albumin
- Podocyte foot process effacement
- Endothelial tubuloreticular inclusions may be present in high interferon states, including HIV associated, lupus associated and interferon mediated collapsing glomerulopathy
- Focal segmental glomerulosclerosis (FSGS), cellular variant:
- Cellular variant has segmental to global endocapillary hypercellularity (occlusion of capillary loops due to influx of leukocytes, foam cells or endothelial cell swelling) and prominence of overlying epithelial cells in Bowman space
- Lacks collapse of glomerular tuft
- Crescentic glomerulonephritis:
- Segmental breaks in glomerular basement membrane and necrosis, influx of leukocytes in glomeruli and layered crescent orientation of cells in Bowman space
- May occasionally be difficult to distinguish in isolation; perform additional levels and evaluate surrounding parenchyma and clinical scenario
- Focal segmental glomerulosclerosis (FSGS), NOS:
- Lacks collapse of glomerular tuft
- Cellular variant
- Collapsing variant
- Hilar variant
- Tip variant
Comment Here
Reference: Focal segmental glomerulosclerosis (FSGS), collapsing variant
Comment Here
Reference: Focal segmental glomerulosclerosis (FSGS), collapsing variant
- Necrotizing vasculitis of small to medium sized arteries with concomitant necrotizing granulomatous tissue inflammation that is frequently associated with positive antineutrophil cytoplasmic autoantibodies (ANCA)
- This disease can be systemic but usually involves the respiratory tract and kidney (90%) (Clin Exp Nephrol 2013;17:603, Colvin: Diagnostic Pathology - Kidney Diseases, 3rd Edition, 2019)
- Crescentic and necrotizing glomerular lesions, usually with concomitant fibrocellular and fibrous crescents
- With or without fibrinoid necrosis of intrarenal arteries
- Absent to few immune type deposits, mild IgA staining in some cases
- Important to rule out underlying bacterial infection, since histologic features can overlap and ANCA serology is positive in up to 20% cases of Staphylococcus infection associated glomerulonephritis (GN)
- Reference: Jennette: Heptinstall's Pathology of the Kidney, 7th Edition, 2014
- Granulomatosis with polyangiitis (GPA) is categorized as an ANCA associated vasculitis along with microscopic polyangiitis and renal limited vasculitis; due to their overlapping histologic findings on renal biopsy, the term ANCA associated glomerulonephritis may be encountered for these entities
- Since little or no evidence of immunoglobulin or complement deposition is seen on biopsy, the term pauci-immune glomerulonephritis may also be seen
- GPA was previously known as Wegener granulomatosis but this eponym was discarded in 2011
- Reference: Jennette: Heptinstall's Pathology of the Kidney, 7th Edition, 2014
- Incidence of 2.3 - 146 per 1,000,000 persons with higher frequencies seen in people of Northern European (white) ancestry (Nat Rev Dis Primers 2020;6:71)
- Usually seen in adults (50 - 70 years), although pediatric cases can occur
- M = F
- Respiratory and renal systems are the most frequently impacted
- GPA can affect the upper (nasopharynx) and the lower (lungs and trachea) respiratory systems
- Other organ systems, such as the eyes, skin and nervous system, can also be affected
- Reference: Am J Med 2004;117:39
- Although the trigger is unknown, autoantibodies to neutrophilic surface and cytoplasmic antigens are characteristically present and are pathogenic (antiproteinase 3 [PR3], myeloperoxidase [MPO], LAMP2, etc.)
- These antibodies interact with their respective receptors, usually on neutrophils or monocytes, causing further activation of inflammatory cell degranulation, formation of neutrophilic extracellular traps, autoantibodies to sequestered antigens and activation of complement pathway
- This leukocyte activation also leads to increased endothelial adhesion, which culminates in extensive microvascular injury
- Reference: Jennette: Heptinstall's Pathology of the Kidney, 7th Edition, 2014
- Unknown
- May present insidiously with nonspecific systemic symptoms (weight loss, malaise, fatigue, arthralgias)
- Respiratory symptoms vary and can include chronic rhinitis, epistaxis, nonproductive cough and hemoptysis
- Renal involvement usually presents acutely with hematuria, proteinuria and rising serum creatinine
- However, renal involvement can also be insidious with a slow decrease in renal function with mild proteinuria or hematuria
- Strongly associated with PR3 ANCA (c-ANCA, 75%) but MPO ANCA (p-ANCA, < 25%) is also seen
- Some cases are ANCA negative or associated with other autoantibodies (LAMP2, plasminogen and tissue plasminogen activator)
- Negative ANCA serology does not exclude a diagnosis of GPA (Colvin: Diagnostic Pathology - Kidney Diseases, 3rd Edition, 2019)
- Positive ANCA serologies can be seen in infections
- Exclusion of an underlying infection is paramount if clinical concern or worrisome biopsy findings (immune complex or complement deposition) are present
- Clinical presentation
- Rapidly progressive glomerulonephritis with nephritic urine sediment
- Recent worsening of renal function, active urine sediment, subnephrotic proteinuria
- Subacute rise in serum creatinine over several months, sinus symptoms, worsening blood pressure control
- Renal involvement usually presents acutely with hematuria, proteinuria and rising serum creatinine
- However, renal involvement can also be insidious with a slow decrease in renal function with mild proteinuria or hematuria
- Strongly associated with PR3 ANCA (c-ANCA, 75%) but MPO ANCA (p-ANCA, < 25%) is also seen
- Some cases are ANCA negative or associated with other autoantibodies (LAMP2, plasminogen and tissue plasminogen activator)
- Negative ANCA serology does not exclude a diagnosis of GPA (Colvin: Diagnostic Pathology - Kidney Diseases, 3rd Edition, 2019)
- 12 year old girl presented with cutaneous and renal features of IgA vasculitis subsequently diagnosed as GPA (Clin Med Insights Arthritis Musculoskelet Disord 2020;13:1179544120967371)
- 26 year old man presented with cough, epistaxis, weight loss and dark colored urine (Cureus 2021;13:e19814)
- 60 year old woman presented with a renal mass subsequently diagnosed as GPA (Indian J Nephrol 2021;31:406)
- Immunosuppression is warranted in active cases of GPA (Arthritis Rheumatol 2021;73:1366)
- Induction therapy usually includes glucocorticoids in conjunction with rituximab or cyclophosphamide
- Both regimens appear to be similarly efficacious with remission rates varying between 50 - 90%
- Avacopan (C5a receptor inhibitor) can be used as an adjunctive induction agent when the standard dosage of glucocorticoids is contraindicated (J Am Soc Nephrol 2017;28:2756)
- After remission, maintenance therapy can begin using a variety of medications based on the organs involved and the tolerance of the patient
- Patients should be monitored for relapse through physical exam and laboratory values (serum creatinine, urinalysis)
- ANCA titers can be followed to assess for relapse, especially in patients with renal involvement, though they may not consistently reflect disease severity (J Am Soc Nephrol 2015;26:537)
- If end stage renal disease occurs, renal transplant can be performed with standard outcomes (Clin Transplant 2011;25:380)
- In the active phase, glomeruli are primarily normocellular with fibrinoid necrosis or cellular crescents, which can range from segmental and focal to global and diffuse (Jennette: Heptinstall's Pathology of the Kidney, 7th Edition, 2014)
- Silver and PAS stains can highlight the breaks in the glomerular basement membrane (GBM) in these areas
- Trichrome stain can highlight the fuchsinophilic fibrinoid necrosis
- In areas of GBM rupture, segmental to severe capillary tuft thrombosis can occur with extension into the afferent arteriole
- Patients can have recurrent episodes of glomerular injury with fibrocellular or fibrous crescents frequently encountered in cases with concomitant features of active injury
- Destruction of Bowman capsule by crescent formation may result in conspicuous periglomerular inflammation with occasional granuloma formation
- In active cases, features of acute tubular injury (epithelial simplification, flattening) and lymphocytic tubulitis can be seen
- Red blood cells and red blood cell casts can be identified within tubular lumens
- Interstitial edema and inflammation can be seen, occasionally with a prominent eosinophilic or neutrophilic component
- In rare cases, necrotizing neutrophilic granulomatous interstitial inflammation is present, representing a component of the systemic necrotizing granulomatosis
- Review of the special stains is recommended to ensure these are not foci of exuberant cellular crescent formation
- Vasculitis may affect vessels of any size within the biopsy but interlobular arteries are the most frequently involved
- Features of vasculitis include segmental to circumferential fibrinoid necrosis with associated mural and perivascular mononuclear and neutrophilic inflammation
- Occasional granuloma formation can also be seen
- In the chronic phase, disruption of the internal elastic lamina with extension of asymmetrical scarring into the muscularis suggests a prior vasculitic injury
Contributed by Anjali A. Satoskar, M.D.
- GPA is a pauci-immune glomerulonephritis
- Specimens usually show no immunofluorescence staining for immunoglobulins or complement
- However, mild staining (up to 2+ on a 0 - 4+ scale) may be seen
- In areas of fibrinoid necrosis, bright staining for fibrin can be seen
- These areas can also show staining for immunoglobulins and complement and should not be over interpreted as true immunoglobulin or complement deposition
- In areas of glomerular scarring, irregular staining for IgM, C3 and C1q can occur
- This finding should also not be over interpreted as true immunoglobulin or complement deposition
- If strong staining (3 - 4+) for immunoglobulin or complement is seen in areas without sclerosis or necrosis, the diagnosis of pauci-immune glomerulonephritis should be reconsidered
- Reference: Jennette: Heptinstall's Pathology of the Kidney, 7th Edition, 2014
- Smudgy fibrinogen staining around disrupted glomerular capillary tuft and Bowman capsule on immunofluorescence workup can help to confirm presence of crescents
- Similar to the immunofluorescence findings, patients with GPA should have no to very little immune complex type electron dense deposits
- Fibrin tactoids and inflammatory cells may be seen in areas of fibrinoid necrosis or crescent formation
- Kidney, native, biopsy:
- Active ANCA associated pauci-immune crescentic and necrotizing glomerulonephritis (see comment)
- Comment: Active cellular crescents and necrotizing lesions with absent or only a few electron dense immune type deposits, in the absence of underlying bacterial infection with positive ANCA serology are together supportive of ANCA associated glomerulonephritis. Concomitant focal fibrocellular and fibrous crescents can be present. Necrotizing vasculitis involving interlobular or arcuate sized intrarenal arteries can be present but can be subject to sampling bias and therefore may not be seen in every biopsy. In the presence of peripheral eosinophilia, the possibility of eosinophilic granulomatosis with polyangiitis emerges; however, histologically, it may not be possible to distinguish from granulomatosis with polyangiitis or microscopic polyangiitis. 10 - 20% of the patients can have negative ANCA serology (ANCA negative small vessel vasculitis). A few small electron dense deposits may be seen and mild IgA staining also can occasionally be present.
- Infection associated glomerulonephritis:
- Anti-glomerular basement membrane disease:
- Crescents all are similar age
- Bright linear staining of the GBM for IgG
- Anti-GBM titers positive
- Drug induced ANCA vasculitis:
- Clinical history should include drugs associated with ANCA vasculitis, such as hydralazine, propylthiouracil, minocycline, anti-TNFα agents, levamisole and cocaine
- Anti-MPO antibodies are more likely to be elevated
- Other pauci-immune vasculitis:
- Can be impossible to distinguish between these entities
- Requires correlation with lab findings and clinical history to suggest the appropriate differential diagnoses
In a patient with renal involvement by granulomatosis with polyangiitis, what immunofluorescence staining pattern would be seen in their glomeruli?
- Bright (2 - 3+) fibrinogen staining in active crescents with no or little immunoreactivity in uninvolved glomeruli
- Bright (3+) linear staining of the basement membrane by IgG
- Bright (3+) mesangial C3 staining
- Bright (3+) mesangial IgA staining
Comment Here
Reference: Granulomatosis with polyangiitis
- Lymphoplasmacytic neoplasm that produces an abnormally truncated immunoglobulin heavy chain protein that lacks associated light chains (eMedicine: Gamma Heavy Chain Disease [Accessed 17 January 2018])
- Less common than light chain deposition disease
- May have milder hematological disorders than in light chain deposition disease (Clin Nephrol 2009;71:9)
- Associated with various lymphoproliferative disorders, but usually has features overlapping with cases previously reported as "vaguely nodular, polymorphous" lymphoplasmacytic lymphoma (Am J Surg Pathol 2012;36:534)
- 51 year old man with linear deposits of gamma 4 heavy chains along glomerular, tubular and vascular basement membrane and in mesangial regions (Mod Pathol 1994;7:874)
- 68 year old woman with gamma 1 heavy chain disease (Intern Med 2010;49:1411)
- Monotypic mu heavy chain mesangial deposits causing nodular glomerulosclerosis in woman without evident hematopoietic malignancy (Hum Pathol 2000;31:122)
- Follow up biopsy post chemotherapy showed remarkable diminution of both nodular glomerular lesions and IgG heavy chain deposits (Clin Nephrol 2008;69:383)
- IgG heavy chains along glomerular, tubular and vascular basement membranes and in mesangium
- No light chain deposition
- Definition: renal injury after hematopoietic stem cell transplantation (HSCT)
- It can be multifactorial; risk factors include radiotherapy, chemotherapy, nephrotoxic medications, infections and graft versus host disease
- History of hematopoietic stem cell transplant
- Endothelial cell injury
- Bloodless glomerulus
- Arterial mucoid intimal thickening
- Subendothelial widening on electron microcopy
- Bone marrow transplantation nephropathy
- Bone marrow transplantation associated thrombotic microangiopathy
- Radiation nephropathy / nephritis (old terminology)
- ICD-10
- Z94.81 - bone marrow stem cell transplantation status
- N17 - acute kidney failure
- N18.1 - chronic kidney disease, stage 1
- N18.2 - chronic kidney disease, stage 2 (mild)
- N18.3 - chronic kidney disease, stage 3 (moderate)
- N18.4 - chronic kidney disease, stage 4 (severe)
- N18.5 - chronic kidney disease, stage 5
- N18.6 - end stage renal disease
- N18.9 - chronic kidney disease, unspecified
- 25 - 40% of patients received total body high dose radiation
- Pediatric patients have higher susceptibility (Int J Radiat Oncol Biol Phys 2017;99:486)
- Other risk factors include older age, female gender, unrelated donor transplant, high dose conditioning regimen, calcineurin inhibitor use and human leukocyte antigen (HLA) mismatch (Am J Kidney Dis 2013;61:809)
- Kidney
- Endothelial cell injury is the central and defining feature of hematopoietic stem cell transplantation associated thrombotic microangiopathy (HSCT-TMA) (Mediterr J Hematol Infect Dis 2010;2:e2010033)
- Endothelial cell injury may result from multiple factors (Bone Marrow Transplant 2021;56:1805)
- Chemotherapy
- Infection
- Cyclosporine
- Conditioning regimen for HSCT
- Endothelial cell injury leads to endothelial cell activation and subsequent activation of platelets and the coagulation system
- Complement activation plays a significant role in HSCT-TMA (J Blood Med 2016:7:181)
- Allogeneic HSCT is associated with a higher risk of acute kidney injury than autologous HSCT
- Acute kidney injury can be associated with medications such as myeloablative conditioning regimens and immunosuppressive agents, infections, acute graft versus host disease (GVHD), renal ischemia or total body irradiation (Am J Kidney Dis 2013;61:809)
- Chronic kidney injury has been associated with chronic GVHD, medications, acute injury, exposure to high dose radiation and total body irradiation
- Radiation dose thought to be implicated is 20 - 25 Gy to the kidneys for more than 1 month
- Renal dysfunction, hypertension, pedal edema, proteinuria, hematuria (Radiat Oncol 2021;16:43)
- Imaging studies
- Computed tomography (CT)
- Magnetic resonance imaging (MRI)
- Renal ultrasound
- Percutaneous renal biopsy
- Laboratory workup includes (Nephron 1995;70:217)
- Serum creatinine
- Blood urea nitrogen (BUN)
- Urinalysis
- 24 hour urine
- Complete blood count (CBC)
- Peripheral blood smear
- Serologies (antinuclear antibody, antineutrophil cytoplasmic antibodies [ANCA], antiglomerular basement membrane, complement C3, C4)
- Can be normal (Kidney Int 2014;86:1063)
- Renal atrophy
- Renal scarring
- Cortical thinning (Wiad Lek 2022;75:128)
- Variable
- May lead to chronic kidney disease
- 38 year old man presented with proteinuria and renal failure after allogeneic bone marrow transplantation from HLA identical sibling (Intern Med 1996;35:489)
- 51 year old woman presented with hematuria and low urine output 2 months after autologous bone marrow transplantation for acute lymphoblastic leukemia (Transplant Proc 2006;38:295)
- 54 year old man with IgG kappa multiple myeloma developed acute kidney injury after allogeneic hematopoietic stem cell transplant (Exp Hematol Oncol 2018:7:14)
- Treatment modalities include (J Blood Med 2016:7:181)
- Supportive treatment
- Prednisone
- Plasma exchange
- Eculizumab (complement blocking therapy)
- Light microscopy examination (Mod Pathol 2008;21:396)
- Glomeruli
- Mesangiolysis
- Capillary thrombi
- Duplication of glomerular basement membrane (GBM), if chronic
- Global or segmental glomerular sclerosis
- Interstitium and tubules
- Interstitial edema
- Interstitial fibrosis and tubular atrophy
- Arteries and arterioles
- Mucoid intimal change
- Thrombi
- Hyalinosis
- Glomeruli
- Fibrinogen highlights thrombi
- IgM and C3 may show nonspecific trapping along GBMs and arterioles
- Special stains: Masson trichrome stains fibrin thrombi strongly red
- CD61 highlights the platelets in thrombi
- Separation of endothelial cells from GBM with widening of subendothelial space by electron lucent material
- Duplication of GBM, if chronic
- Kidney, left, core biopsy:
- Acute thrombotic microangiopathy (see comment)
- Comment: The arteries show mucoid intimal thickening with focal fibrinoid necrosis of the wall and luminal thrombi. These findings are consistent with thrombotic microangiopathy. Due to the patient's history of allogenic hematopoietic stem cell transplant and exposure to radiation, this is most likely the underlying cause.
- Thrombotic thrombocytopenic purpura:
- Associated with low ADAMTS13 levels or activity
- Hemolytic uremic syndrome:
- Enterohemorrhagic E. coli (O157:H7)
- Thrombotic microangiopathy from various causes such as drugs:
- Clinical correlation is necessary to identify the possible offending drug
- Examples include gemcitabine and cyclosporine
- Atypical hemolytic uremic syndrome (complement mediated TMA):
- Genetic or acquired abnormalities in alternative complement pathway
- Graft versus host glomerulopathies:
- Include membranous glomerulonephritis and minimal change disease
- Can also present similarly with thrombotic microangiopathy
A 65 year old woman with a history of hematopoietic stem cell transplant presented with acute renal failure. She underwent a percutaneous renal biopsy. The H&E of the biopsy is shown in the image above. Immunofluorescence was negative. What is the most likely diagnosis?
- Bone marrow transplant nephropathy
- Glomerulus looks normal
- Membranoproliferative pattern of injury
- Membranous nephropathy
Comment Here
Reference: Hematopoietic stem cell transplantation associated thrombotic microangiopathy (HSCT-TMA)
- Focal segmental glomerulosclerosis (FSGS)
- IgA glomerulonephritis
- Minimal change disease
- Thrombotic microangiopathy
Comment Here
Reference: Hematopoietic stem cell transplantation associated thrombotic microangiopathy (HSCT-TMA)
- Thrombotic microangiopathy (TMA) is a pathologic term used to describe thrombi within microvasculature; TMA is clinically characterized by ischemic organ injury, particularly acute kidney injury, thrombocytopenia and microangiopathic hemolytic anemia (MAHA)
- TMA is a heterogeneous disease that is classically seen in hemolytic uremic syndrome and thrombotic thrombocytopenic purpura but can also be seen in a variety of clinical settings including severe hypertension, scleroderma renal crisis and preeclampsia / eclampsia, among others (Semin Diagn Pathol 2020;37:121)
- Classic / typical hemolytic uremic syndrome (HUS) is an infection mediated process caused by Shiga toxin producing bacteria most commonly Escherichia coli O157:H7 or Shigella dysenteriae; numerous other infections have also been associated with TMA
- Atypical HUS is due to an inherited or acquired abnormality in complement proteins causing dysregulation of the alternative pathway
- Thrombotic thrombocytopenic purpura (TTP) is an inherited or acquired deficiency of ADAMTS13 enzyme which normally cleaves large multimers of von Willebrand factor (vWF)
- Endothelial injury with microvascular thrombi
- Correlation with clinical and laboratory data necessary to determine etiology
- Often due to infection, complement disorders or ADAMTS13 deficiency
- Many secondary causes including severe hypertension, sclerodermal renal crisis, preeclampsia / eclampsia
- Infection associated TMA, Shiga toxin producing Escherichia coli (STEC HUS), postdiarrheal HUS
- Complement mediated atypical hemolytic uremic syndrome (aHUS), complement mediated TMA (CM TMA), diarrhea negative HUS
- ADAMTS13 mediated TMA
- ICD-10
- D50 - D89 - diseases of the blood and blood forming organs and certain disorders involving the immune mechanism
- D59.3 - hemolytic uremic syndrome
- D59.30 - hemolytic uremic syndrome, unspecified
- D59.31 - infection associated hemolytic uremic syndrome
- D59.32 - hereditary hemolytic uremic syndrome
- D59.39 - other hemolytic uremic syndrome
- HUS is most common in infants and young children, peak age 3 - 5 years
- Streptococcus pneumoniae HUS (SP HUS) usually affects younger children than STEC HUS (Pediatr Clin North Am 2019;66:235)
- aHUS and TTP are more common in adults
- ~10% of TTP occurs in children
- Most patients with aHUS are Am J Kidney Dis 2023;81:591)
- HUS affects men and women equally
- TTP: F > M
- HUS / aHUS: kidney, followed by central nervous system
- TTP: strongly associated with central nervous system and cardiac involvement, less commonly kidney involvement; kidney involvement does not exclude TTP (Expert Rev Hematol 2019;12:383)
- Other organ systems can be involved including cardiovascular, respiratory, gastrointestinal
- Endothelial injury is fundamental feature of all causes of TMA
- Endothelial cell injury activates platelet aggregation within the microvasculature and forms thrombi
- Results in microangiopathic hemolytic anemia and tissue ischemia / end organ damage
- TTP: von Willebrand factor ultralarge multimers accumulate due to deficiency of ADAMTS13 and promotes platelet adhesion and aggregation (Expert Rev Hematol 2019;12:383)
- aHUS: dysregulation of the alternative pathway with C3b deposition leading to endothelial injury
- STEC: Shiga toxin (Stx), from the intestines, enters circulation via binding with globotriaosylceramide (Gb3) receptors on endothelial cells and inhibits protein synthesis leading to endothelial cell injury
- Kidney has high expression of Gb3 receptors
- Reference: Am J Kidney Dis 2023;81:591
- Highly variable, can be primary or secondary
- Primary TMA
- Hereditary: ADAMTS13 mutation, complement gene mutation, cobalamin C (cblC) deficiency, diacylglycerol kinase epsilon (DGKE)
- Acquired: factor H (FH) autoantibody, ADAMTS13 autoantibody
- Secondary TMA
- Pregnancy associated
- Hemolysis, elevated liver enzymes, low platelet count (HELLP)
- Solid organ / bone marrow transplantation
- Severe hypertension
- Drug induced
- Autoimmune (systemic lupus erythematosus [SLE], scleroderma renal crisis [SRC], cryopyrin associated periodic syndromes [CAPS])
- Malignancy associated
- Glomerular diseases (antineutrophilic cytoplasmic antibody [ANCA] associated, immunoglobulin A nephropathy [IgAN], membranous nephropathy [MN], focal segmental glomerulosclerosis [FSGS], complement 3 glomerulopathy [C3G] / membranoproliferative glomerulonephritis [MPGN])
- Infection associated TMA
- STEC HUS
- Undercooked, contaminated beef, unpasteurized milk or contaminated fruits and vegetables
- E. coli OH157:H7 in United States and Europe
- Shigella dysenteriae in other countries
- Pneumococcal
- Human immunodeficiency virus (HIV) associated
- STEC HUS
- Other infections
- Reference: Clin J Am Soc Nephrol 2018;13:300
- HUS: classic clinical triad includes acute kidney injury, anemia and thrombocytopenia
- STEC HUS typically presents with gastrointestinal symptoms followed by thrombocytopenia and acute kidney injury
- aHUS may present in a fulminant manner following a triggering event, such as upper respiratory or gastrointestinal infection; in a smaller number of patients, aHUS presents in an insidious, indolent manner
- TTP: classic pentad includes fever, thrombocytopenia, MAHA, neurological disorders, acute kidney injury (occurs in Expert Rev Hematol 2019;12:383)
- Extrarenal manifestations dependent upon organ(s) involved but can include petechiae, purpura, neurologic symptoms including seizures and coma, pancreatitis, myocardial infarction, hepatitis (Clin J Am Soc Nephrol 2018;13:300)
- Recognize laboratory findings and investigate underlying disease cause (Am J Kidney Dis 2023;81:591)
- Includes ADAMTS13 activity study
- Screening for Shiga toxin or other infections
- Complement studies
- Evaluation for secondary causes: autoimmune screen, metabolic (vitamin B12, methylmalonic acid [MMA] and homocysteine) pregnancy test, transplant rejection markers (Am J Kidney Dis 2023;81:591)
- Because decreased platelet counts and elevated blood pressures are common at presentation, kidney biopsy is not often performed at onset; it may be performed in more severe cases of acute kidney injury (AKI) but is not required for diagnosis (Clin J Am Soc Nephrol 2018;13:300)
- Anemia
- Thrombocytopenia
- Impaired renal function: oligouria, hematuria or low grade proteinuria; elevated creatinine in more severe cases
- Elevated bilirubin, lactate dehydrogenase
- Reticulocytosis
- Schistocytes
- Low haptoglobin
- Negative Coombs test
- Reference: Clin J Am Soc Nephrol 2018;13:300
- STEC HUS has 3 - 5% acute mortality with neurologic, cardiovascular and intrabdominal issues as primary cause of death
- SP HUS is a generally more severe disease than STEC HUS and has worse outcomes
- Long term renal sequelae reported in 25% of patients (Pediatr Clin North Am 2019;66:235)
- Poorer prognosis
- Prolonged anuria
- Increased length of dialysis requirement
- TTP: older age, high lactate dehydrogenase (LDH) and cardiac troponin levels (Expert Rev Hematol 2019;12:383)
- Progression to end stage renal disease (ESRD) in patients with aHUS has declined; 79% patients with aHUS progressed to ESRD or death prior to use of escluzumab (Pediatr Clin North Am 2019;66:235)
- 21 year old woman developing aHUS after vaccination with mRNA vaccine against SARS-CoV-2 (Front Immunol 2022;13:1001366)
- 27 year old post partum woman with TTP (Ann Med Surg (Lond) 2022;84:104828)
- 42 year old woman developing aHUS after myomectomy (Case Rep Womens Health 2022;35:e00424)
- 62 year old woman developing TTP after receiving the first dose of the Ad26.COV.S vaccine (Am J Emerg Med 2021;49:441)
- 62 year old man presenting with immune mediated TTP (iTTP) 1 week after diagnosis of COVID-19 (IDCases 2021;26:e01256)
- 65 year old man with gemcitabine induced HUS (Cureus 2022;14:e20926)
- Highly dependent upon the underlying etiology of the TMA
- Supportive care, primarily
- Eculizumab (anti-C5 monoclonal antibody) for complement mediated and pregnancy associated aHUS
- Recommended to start within first 24 hours in patients with aHUS, with time to treatment being a significant factor in renal function recovery (Pediatr Clin North Am 2019;66:235)
- Ravulizumab (longer acting anti-C5 antibody)
- Immunosuppression (steroids, rituximab, cyclophosphamide)
- Systemic antibiotic administration for infections
- Therapeutic plasma exchange (TPE) is first line in the setting of TTP (Expert Rev Hematol 2019;12:383)
- Caplacizumab prevents interaction of platelets and vWF and has been approved for acute TTP treatment (Am J Kidney Dis 2023;81:591)
- Acute
- Glomerular and arteriolar fibrin thrombi, red blood cell fragmentation
- Other glomerular changes include endothelial cell swelling, giving the glomerulus a bloodless appearance and mesangiolysis
- Arterial endothelial swelling and intimal mucoid / myxoid change of the intima
- Chronic
- Glomerular basement membrane double contouring (duplication)
- Multilayering of the peritubular capillary basement membrane
- Arterial myointimal hyperplasia (onion skinning)
- Reference: Kidney Res Clin Pract 2022;41:524
- Fibrin / fibrinogen within glomeruli capillaries and arterioles; otherwise, negative aside from nonspecific trapping of immunoglobulins and complement
- Fibrin thrombi are best identified with H&E, trichrome and Jones silver stains
- Subendothelial widening may be seen in early stage with acellular subendothelial fibrillary material (J Clin Med 2022;11:3124)
- Glomerular capillary microthrombi composed of fibrin tactoids, platelets
- Red blood cell (RBC) fragmentation
- Reduplication of the glomerular basement membrane in chronic TMA
- > 50 - 60% of aHUS cases are due to genetic variants in genes such as CFH, CFI, MCP / CD46, C3, CFB, CFH::CFHR, THBD, DGKE; incomplete penetrance (Am J Kidney Dis 2023;81:591)
- Variants in the ADAMTS13 gene can cause congenital TTP (Am J Kidney Dis 2023;81:591)
- Kidney, core biopsy:
- Thrombotic microangiopathy (see comment)
- Comment: The biopsy findings show fibrin thrombi within the microvasculature compatible with thrombotic microangiopathy. Determination of the underlying etiology requires clinical and laboratory correlation.
- Malignant hypertension:
- Clinically severe hypertension, usually a pre-existing condition
- Mucoid intimal thickening / onion skinning of arteries, fibrinoid necrosis
- Systemic sclerosis:
- Systemic disease
- Mucoid intimal thickening / onion skinning of arteries
- Antinuclear antibody (ANA)
- Antiphospholipid antibody syndrome:
- Positive lupus anticoagulant or anticardiolipin antibody
- Drug induced TMA:
- Immune and nonimmune mediated
- Careful medication history
- Pregnancy associated TMA:
- Typically in the setting of preeclampsia, HELLP syndrome
A 50 year old patient presents with acute kidney injury and a biopsy is performed. A representative glomerulus is shown in the H&E image above. What is the most important laboratory test in the initial workup of this patient?
- ADAMTS13 activity
- Antinuclear antibody (ANA)
- Bacterial culture
- Full complement evaluation
- Imaging workup for malignancy
Comment Here
Reference: Hemolytic uremic syndrome / thrombotic thrombocytopenic purpura
- Basketweave appearance of the glomerular basement membrane
- Diffuse foot process effacement
- Mesangial electron dense deposits
- Reduplication of the glomerular basement membrane
- Tubuloreticular inclusions
Comment Here
Reference: Hemolytic uremic syndrome / thrombotic thrombocytopenic purpura
- Need clinical history to distinguish between renal limited IgA nephropathy and systemic HSP
- Purpuric skin lesions on extensor arms and legs and buttocks
- Also abdominal pain, vomiting, GI bleeding, arthralgias, hematuria, proteinuria and nephrotic syndrome
- Due to systemic small vessel leukocytoclastic vasculitis
- Most common systemic vasculitis in children
- Also called anaphylactoid purpura
- Significant clinicopathological differences with IgA nephropathy (Zhongguo Dang Dai Er Ke Za Zhi 2012;14:506)
- Renal symptoms in 30 - 70%; some adults develop rapidly progressive glomerulonephritis
- 70% are ages 2 - 11 years; rare in adults or infants 1 year or less
- Higher rate of renal involvement in children ages 10 - 18 (Iran J Kidney Dis 2012;6:269)
- Associated with atopy in 1/3; may follow respiratory infection
- Related to IgA nephropathy, due to elevated serum IgA, circulating immune complexes with IgA, similar kidney lesions (Arch Pathol Lab Med 1982;106:192), high serum galactose deficient immunoglobulin A1 levels (Kidney Int 2011;80:79)
- Hypertension, serum creatinine, proteinuria, cellular crescents, glomerular necrotizing lesions and chronic renal lesions are associated with renal failure (Mod Pathol 2001;14:635)
- Variable recurrence rates (12 - 69%) after renal transplant, but usually not clinically important (Transplantation 2011;92:907, Clin J Am Soc Nephrol 2011;6:1768, Clin J Am Soc Nephrol 2011;6:2034)
- Prognosis: excellent in children (50% have spontaneous remission); poorer in adults (Clin Nephrol 2011;76:49) or with nephrotic syndrome; often difficult to predict (Clin J Am Soc Nephrol 2011;6:679)
- 15 year old girl with prior onset of IgA nephropathy (Fukushima J Med Sci 2010;56:157)
- 72 year old man with coexisting IgG4 related tubulointerstitial nephritis (Allergy Asthma Clin Immunol 2011;7:5)
- 75 year old man with rectal bleeding and acute renal injury (J Med Case Rep 2011;5:364)
- Recommended to administer intensive therapy initially (Indian J Pediatr 2012;79:207)
- Corticosteroids, cytotoxic drugs
- Mycophenolate mofetil for children with nephrotic-range proteinuria (Pediatr Nephrol 2012;27:765)
-
Acute:
- Leukocytoclastic vasculitis of small vessels due to deposition of IgA immune complexes
- Diffuse proliferation of mesangial cells and matrix without significant involvement of capillary walls or lumina
- Also segmental necrotizing lesions (50%), endocapillary proliferation (13%), cellular crescents, glomerular acute and chronic inflammatory infiltrate
-
Chronic:
- Glomerular sclerosis, tubular loss, interstitial fibrosis and hyaline arteriolosclerosis
-
Skin:
- Hemorrhage and necrotizing vasculitis in dermal small vessels, which contain IgA
- Vasculitis is present in other organs but usually NOT kidney
- IgA deposition in mesangium, resembling IgA nephropathy
- Variable IgG, IgM, C3 and properdin
- Mesangial deposits, may extend into subendothelial areas
- May have subepithelial deposits
- HIV associated renal disease refers to the group of kidney conditions that develop in patients with HIV infection
- The term includes cases with HIV associated nephropathy (HIVAN), HIV associated immune complex kidney disease (HIVICK) and kidney injury resulting from prolonged exposure to antiretroviral therapy or from opportunistic infections (Kidney Int 2018;93:545)
- Lack of certainty of HIV causality in presentation other than HIVAN
- HIVAN: nephrotic range proteinuria with collapsing glomerulopathy (less frequently focal segmental glomerulosclerosis [NOS], minimal change disease, thrombotic microangiopathy), eventually associated with microcystic tubular dilatation and active tubulointerstitial nephritis (Am J Kidney Dis 2016;68:e13)
- HIVICK: lupus-like lesions in HIV infected patients without serologic evidence of lupus; other immune complex diseases (e.g., IgA nephropathy and postinfectious glomerulonephritis) should be better diagnosed as those specific entities (Am J Kidney Dis 2016;68:e9)
- HIV associated nephropathy (HIVAN)
- HIV associated immune complex kidney disease (HIVICK)
- Average age at presentation: 41 years (Expert Rev Anti Infect Ther 2008;6:365)
- Marked reduction with antiretroviral therapies (peak incidence of 4 - 10% of end stage renal disease in U.S., now 0.97% with 800 - 900 cases) (Drugs 2008;68:963, J Nephrol 2001;14:377)
- Strongest association with African American ethnicity compared to other causes of renal failure except sickle cell anemia (J Nephrol 2001;14:377)
- HIVAN accounted for 76% in 1987 and only 14% in 2018 of biopsy diagnoses in HIV infected patients, largely due to highly active antiretroviral therapy (HAART) (Kidney Int 2020;97:1006)
- Non-HIV related immune complex glomerulonephritis (17%) and diabetic nephropathy (16%) most frequent diagnosis in HIV patients (Kidney Int 2020;97:1006)
- Glomeruli are most often involved in the form of podocytopathy, less frequently with immune complex disease; tubulointerstitial compartment can show microcystic tubular dilatation and active tubulointerstitial nephritis
- Infectious agent (HIV)
- Direct HIV1 infection of renal tubular epithelial cells (RTEc), podocytes and parietal epithelial cells (J Am Soc Nephrol 2017;28:719)
- Use of other receptors than the canonical CD4 receptor mediated entry mechanism (J Am Soc Nephrol 2017;28:719)
- Viral replication is not necessary for the HIVAN phenotype (Front Med (Lausanne) 2018;5:177)
- Stimulation of cytokines, including interferon γ (IFNγ)
- Cell cycle dysregulation and aberrant podocyte cell cycle reentry are involved in glomerular injury
- Increase of profibrotic factors and cytokine expression are involved in tubular microcyst and interstitial fibrosis
- Genetic factors
- Mechanism of APOL1 mediated kidney disease is currently unknown but HIV infection seems to act as a second hit for disease expression (J Am Soc Nephrol 2015;26:2882)
- Direct HIV infection of the renal parenchyma, hyperproduction of IFNγ, cell cycle dysregulation (J Am Soc Nephrol 2017;28:719)
- APOL1 polymorphisms predispose to HIVAN in African Americans (J Am Soc Nephrol 2015;26:2882)
- HIVAN
- Nephrotic syndrome, increased serum creatinine, rapid progression, about half reaching end stage renal disease after 3 years (Am J Kidney Dis 2016;68:e13)
- Most cases with classic HIVAN have advanced HIV disease / AIDS with CD4 counts 3 (Front Med (Lausanne) 2018;5:177)
- HIVICK
- Varying kidney manifestation: proteinuria, may be nephrotic, hematuria, reduced glomerular filtration rate (GFR) and low levels of complement are common (Am J Kidney Dis 2016;68:e9)
- HIVAN suspected when typical clinical symptoms (nephrotic syndrome with acute kidney injury) are associated with renal enlargement at imaging in HIV infected patients (Nephrol Dial Transplant 2012;27:3969)
- Final diagnosis is based on histology
- Low estimated glomerular filtration rate (eGFR) (Nephrol Dial Transplant 2012;27:2349)
- High proteinuria (Nephrol Dial Transplant 2012;27:1114)
- High HIV load (Nephrol Dial Transplant 2012;27:1114)
- Low CD4+ count (Nephrol Dial Transplant 2012;27:2349)
- A nonnegligible proportion of patients do not present with these typical features
- Normal sized or enlarged kidneys, increased cortical echogenicity, renal pelvicalyceal thickening and loss of the renal sinus fat appearance (Radiographics 2008;28:1339)
- Prognosis is poor in patients with HIVAN, even among those treated with HAART (Nephrol Dial Transplant 2012;27:1114)
- Prognosis of HIVICK appears better than for HIVAN (Am J Kidney Dis 2016;68:e9)
- 13 year old boy with noncollapsing HIVAN (Pediatr Nephrol 2011;26:973)
- 25 year old woman with relapse of HIVICK with lupus-like features after transplantation (Am J Kidney Dis 2013;62:335)
- 41 year old man with HIVAN before seroconversion (Am J Kidney Dis 2001;37:E39)
- HAART: in patients diagnosed with HIVAN who are not already receiving HAART
- Angiotensin converting enzyme (ACE) inhibitor or angiotensin II receptor blockers (ARB) in proteinuric or hypertensive patients with HIVAN (Kidney Int 2018;93:545)
- Glomerular injury:
- HIVAN
- Segmental or global glomerular tuft collapse with overlying visceral epithelial hyperplasia and hypertrophy, frequent protein droplets into the podocytes seen in collapsing cases (Kidney Int 2018;93:545)
- Noncollapsing focal segmental glomerulosclerosis, NOS is more commonly encountered at biopsy in antiretroviral therapy (ART) treated HIV infected patients (Kidney Int 2018;93:545)
- HIVICK
- Variable mesangial and focal or diffuse endocapillary hypercellularity
- Pinpoint hole appearance and spikes of the glomerular basement membrane on silver stain in cases with dominant membranous pattern (Am J Kidney Dis 2016;68:e9)
- HIVAN
- Tubulointerstitial injury:
- HIVAN: tubulointerstitial disease, including tubular microcyst formation, interstitial inflammation and tubular injury
- Proximal tubulopathy due to antiretroviral agent tenofovir disoproxil fumarate
- Prominent CD8 T cell infiltrates in diffuse infiltrative lymphocytosis syndrome (DILS) and immune restoration inflammatory syndrome (IRIS)
- Vascular injury:
- Thrombotic microangiopathy (rare in the HAART era)
- Usually negative or nonspecific in HIVAN (Kidney Int 2018;93:545)
- Protein droplets in visceral epithelial cells may stain for immunoglobulins and albumin (Kidney Int 2018;93:545)
- Full house staining with IgG, IgA, IgM, C3 and C1q granular staining in the mesangium and capillary loops in cases of HIVICK (Am J Kidney Dis 2016;68:e9)
- Immunofluorescence for fibrinogen can highlight thrombi in cases of thrombotic microangiopathy
- Jones methenamine silver (JMS), periodic acid-Schiff (PAS): highlight glomerular architecture with basement membrane collapse in HIVAN
- Further stains not required and often not helpful in differential diagnosis
- HIVAN is accompanied by downregulation of the podocyte maturation markers such as Wilms tumor 1 (WT1), synaptopodin and podocalyxin (J Am Soc Nephrol 2001;12:1677)
- HIVAN (Kidney Int 2018;93:545)
- Extensive foot process effacement
- Hypertrophy and hyperplasia of overlying visceral epithelial cells
- Wrinkled and collapsed glomerular basement membranes
- Tubuloreticular aggregates / inclusions (TRI) in endothelial cells
- Tubuloreticular aggregates may be rare or even absent in patients with low viral loads who are receiving antiretroviral therapy
- HIVICK (Am J Kidney Dis 2016;68:e9)
- Variable deposits are seen, including mesangial, subendothelial and subepithelial deposits
- Tubuloreticular aggregates are present in endothelial cells
- Tubuloreticular aggregates may be decreased or even absent in patients with low viral loads being treated with combination antiretroviral therapy
- Risk variants in the SRA domain of APOL1 act by a gain of injury or a toxicity mechanism on podocytes or vascular cells (J Am Soc Nephrol 2015;26:2882)
- Left kidney, needle core biopsy:
- HIV associated nephropathy (HIVAN) (see comment)
- Comment:
- Microscopic description: Segmental collapse of the capillary loops with occlusion of the lumen and associated podocyte satellite hyperplasia (collapsing lesion). Interstitial fibrosis and tubular atrophy extend to about 20% of the cortex with moderate interstitial lymphocytic infiltrate. Diffuse tubular microcyst formation. No significant changes in the vascular structures.
- Immunofluorescence: No deposits of IgG, IgA, IgM, C3, C1q, kappa or lambda light chains.
- Electron microscopy: Extensive foot process effacement, hypertrophy and hyperplasia of overlying visceral epithelial cells with intracytoplasmic protein droplets, wrinkled and collapsed glomerular basement membranes, tubuloreticular aggregates / inclusions (TRI) in endothelial cells, no deposits.
- Focal segmental glomerulosclerosis collapsing variant, non-HIV related (Kidney Int 2018;93:545):
- Infections (e.g., parvovirus)
- Drugs (e.g., bisphosphonates and calcineurin inhibitors)
- Severe vascular disease (e.g., thrombotic microangiopathy and cocaine use)
- Autoimmune disease (e.g., systemic lupus erythematosus)
- Clinical history of HIV infection and the presence of tubuloreticular aggregates indicate HIVAN as the etiology of the collapsing lesions
- Crescentic glomerulonephritis:
- Antineutrophil cytoplasmic antibody (ANCA) associated glomerulonephritis can show parietal epithelial proliferation but generally does not have protein reabsorption droplets in the epithelial cells
- Crescents are also often accompanied by segmental necrosis and glomerular basement membrane breaks with fibrinous exudates, features that distinguish them from collapsing lesions
- Lupus nephritis (Am J Kidney Dis 2016;68:e9):
- Distinction among lupus nephritis and lupus-like HIVICK must be made on clinical grounds
- ADAMST7
- APOL1
- COL4A5
- PLA2R1
Comment Here
Reference: HIV associated renal disease
Comment Here
Reference: HIV associated renal disease
- Occurs within minutes to hours after revascularization, causing abrupt cessation of urine production
- Due to preformed circulating antibodies in recipient to donor endothelial cells, caused by prior pregnancies, blood transfusions or transplants
- Can perform ABO incompatible transplants if low titers (Nephrol Dial Transplant 2010;25:3794)
- Rare currently due to routine screening and cross matches
- With negative pretransplantation T and B cell flow cytometric crossmatches and blood group identity (Transplant Proc 2008;40:2422)
- Mottled cyanosis and diminished turgor in renal graft
- Fibrin thrombi in glomerular capillaries, peritubular venules and other vessels leading to infarction and tubular necrosis
- Variable white blood cells within glomeruli, peritubular capillaries and interstitium
- Linear staining of IgM or IgG, as well as C3 and C4d along glomerular and peritubular capillaries
- At later stages Ig may not be detectable
- Capillary occlusion by degranulated platelets and endothelial denudation of glomerular basement membrane
- Also fibrin, sludged red blood cells
-
Other conditions that can cause primary graft failure include:
- Acute tubular necrosis
- Major vascular occlusion
- Perfusion injury
- Idiopathic nodular glomerulosclerosis (ING) is characterized by a vasculopathic glomerular histological pattern presenting in individuals with a history of smoking or hypertension in the absence of diabetes mellitus (J Bras Nefrol 2020;42:484, CEN Case Rep 2023;12:311)
- Defining characteristic of this disease is the identification of nodular mesangial sclerosis on histological examination in the absence of any clinical indications of diabetes mellitus (Nephrol Dial Transplant 2021;37:72)
- Idiopathic nodular glomerulosclerosis is distinguished by the presence of diffuse nodular glomerulosclerotic lesions, bearing a striking resemblance to Kimmelstiel-Wilson lesions yet occurring independently of diabetic mellitus (Clin Nephrol 2007;67:32, CEN Case Rep 2023;12:311)
- Documented in patients with hypertension, smokers, chronic obstructive pulmonary disease, obesity, metabolic syndrome and other relevant factors rendering diagnosis of exclusion (Indian J Nephrol 2016;26:145)
- Idiopathic nodular glomerulopathy (ING) / glomerulosclerosis (GS)
- Smoking associated nodular glomerulosclerosis
- Nodular glomerulosclerosis associated with smoking or hypertension without diabetes mellitus
- Smoking modified hypertension associated nodular glomerulosclerosis (SHaNGS) (J Bras Nefrol 2020;42:484)
- ICD-10: N26.9 - renal sclerosis, unspecified
- Most common in elderly white men with mean age of 68.2 years (J Bras Nefrol 2020;42:484, Hum Pathol 2008;39:1771, Nephrol Dial Transplant 2021;37:72, Clin Nephrol Case Stud 2022;10:82)
- M:F = 4:1 (J Bras Nefrol 2020;42:484, Clin Nephrol Case Stud 2022;10:82)
- Involves the renal cortical glomeruli; however, the systemic effect on other organs depends on the underlying cause of disease
- There are limited data that support the underlying specific mechanism involved in the pathophysiology of ING; however, it remains incompletely understood (Front Med (Lausanne) 2024;11:1379547)
- Hypertension (Clin Nephrol Case Stud 2022;10:82)
- Hypertension stands as not only one of the most prevalent causes of end stage renal disease but also is present in > 90% of patients diagnosed with nondiabetes mellitus associated nodular glomerulosclerosis at the time of biopsy
- Environmental exposure (Clin Nephrol Case Stud 2022;10:82, CEN Case Rep 2018;7:48)
- Smoking is a well known risk factor for chronic kidney disease and even in absence of diabetes or hypertension, an association with microalbuminuria is observed
- Suggested mechanism by which smoking may induce proteinuria and renal damage involves the interaction of smoking derived advanced glycation end products (AGE) and their receptor (RAGE); AGE alters extracellular matrix (ECM) by protein cross linking and interaction with the receptor to AGE
- Additionally, cigarette smoking generates free radicals that trigger oxidative stress and stimulate the sympathetic nervous system, leading to the activation of the renin angiotensin aldosterone system and altered intrarenal hemodynamics through vasoconstriction and reduction of blood flow
- These various mechanisms culminate in a nephrotoxic effect, potentially contributing to the development of nodular glomerular lesions
- Obesity (Clin Nephrol Case Stud 2022;10:82)
- In addition to smoking and hypertension, nodular glomerulosclerosis has been linked to several cardiovascular risk factors, including obesity and hypercholesterolemia, both recognized as promoters of vasculopathy
- In the presence of diverse combinations of cardiovascular risk factors, ING indicates a severe manifestation of metabolic microvasculopathy
- ING is also expected to overlap with obesity related glomerulopathy
- Commonly occurs in patients with a history of
- Hypertension (mean of 15 years; 95.8%)
- Active smoking with cumulative intake of 52.9 packs per year (91%)
- Obesity (body mass index, > 30 kg/m2) and hyperlipidemia (90%)
- References: Hum Pathol 2008;39:1771, Nephrol Dial Transplant 2021;37:72, Clin Nephrol Case Stud 2022;10:82, BMJ Case Rep 2018;2018:bcr2018224407, J Bras Nefrol 2020;42:484
- Renal dysfunction (80% of patients) (Hum Pathol 2008;39:1771, J Bras Nefrol 2020;42:484)
- Nephrotic range proteinuria (70% of patients) and 53% presented with nephrotic syndrome (Hum Pathol 2008;39:1771, J Bras Nefrol 2020;42:484)
- Normal blood glucose and hemoglobin A1C (HbA1c) levels (CEN Case Rep 2023;12:311)
- Diagnosis of exclusion (Front Med (Lausanne) 2024;11:1379547)
- Given its associations with various other conditions, nodular glomerulosclerosis necessitates a comprehensive diagnostic approach, thorough laboratory investigation and meticulous histological assessment of renal biopsy, incorporating techniques, such as immunohistochemistry, immunofluorescence and electron microscopy (Clin Nephrol Case Stud 2022;10:82, J Bras Nefrol 2020;42:484)
- Proteinuria
- Blood glucose to rule out diabetes
- Hemoglobin A1C to rule out diabetes
- Reference: CEN Case Rep 2023;12:311
- ING is a rare ailment characterized by a poor renal prognosis with median time from biopsy to end stage renal disease (ESRD) of 26 months with 23.5% of patients requiring renal replacement therapy at a mean of 8.7 months (J Bras Nefrol 2020;42:484, Hum Pathol 2002;33:826)
- Factors that promote ESRD are persistent smoking (p = 0.0165), angiotensin II blocker noncompliance (p = 0.0007), advanced renal changes that include tubular atrophy and interstitial fibrosis (p = 0.0517) and advanced arteriosclerosis (p = 0.0096) (J Bras Nefrol 2020;42:484, Hum Pathol 2002;33:826)
- On the other hand, patients who had an initial serum creatinine < 3.0 mg/dL showed slower progression rate to ESRD with side by side usage of angiotensin II blockers (p = 0.0126) and smoking cessation (J Bras Nefrol 2020;42:484, Hum Pathol 2002;33:826)
- 53 year old nondiabetic man who developed atypical antiglomerular basement membrane (anti-GBM) disease and idiopathic nodular glomerulosclerosis (Clin Nephrol 2023;99:98)
- 66 year old Chinese woman who received a kidney transplant and developed nodular glomerulosclerosis (CEN Case Rep 2021;10:273)
- 68 year old Black man who was nonobese, nondiabetic, hepatitis C positive and diagnosed with idiopathic nodular glomerulosclerosis (BMJ Case Rep 2018;2018:bcr2018224407)
- 81 year old man who was hypertensive and a smoker developed renal enlargement and progressive renal failure, which was diagnosed as sporadic medullary cystic kidney disease complicated by idiopathic nodular glomerulosclerosis (Intern Med 2024 Feb 1 [Epub ahead of print])
- Treatment depends on the underlying cause of disease; there is mostly conservative management (Front Med (Lausanne) 2024;11:1379547)
- Aggressive management of blood pressure with angiotensin II blockers is required for treatment of SHaNGS, in addition to treatment of hyperlipidemia with statins and cessation of smoking (J Bras Nefrol 2020;42:484)
- Interference against the glycation end products and their receptor could also be a therapeutic strategy to prevent progression (J Bras Nefrol 2020;42:484)
- Histological findings of ING are very similar to those of nodular diabetic glomerulosclerosis, including degree of glomerular obsolescence and interstitial fibrosis (Front Med (Lausanne) 2024;11:1379547)
- In glomeruli, there is diffuse hyaline expansion of the mesangial matrix along with partial nodular configuration and thickening of glomerular basement membrane and neovascularization of glomeruli (Front Med (Lausanne) 2024;11:1379547, Clin Nephrol Case Stud 2022;10:82)
- Neovascularization is usually seen at the vascular pole of glomeruli with an increased number of efferent arterioles than usual (Nephrol Dial Transplant 2021;37:72)
- Tubular atrophy and interstitial fibrosis are also present (J Bras Nefrol 2020;42:484, Clin Nephrol Case Stud 2022;10:82)
- In blood vessels, there is moderate to exuberant hyalinosis of the wall of arterioles with almost complete occlusion of the lumen (Congo red negative) and mild media thickening of interlobular arteries (J Bras Nefrol 2020;42:484, Clin Nephrol Case Stud 2022;10:82)
- Compared to diabetic nephropathy, ING at the time of diagnosis had less hyaline arteriolosclerosis and less proteinuria (Nephrol Dial Transplant 2021;37:72)
- Global sclerosis of glomeruli is not uncommon and slight segmental mesangial proliferation and mesangiolysis can also be present (Clin Nephrol Case Stud 2022;10:82, J Bras Nefrol 2020;42:484)
- On special stains, these mesangial nodules are periodic acid-Schiff stain and silver positive, stained blue with Masson trichrome and are negative for Congo red (J Bras Nefrol 2020;42:484)
- Immunoglobulin G and albumin reveal diffuse, linear staining accentuation of glomerular basement membrane (GBM) and tubular basement membranes (TBM) (J Bras Nefrol 2020;42:484)
- IgM and C3 can be seen in nonspecific patterns (Clin Nephrol Case Stud 2022;10:82)
- There is no immune deposit (J Bras Nefrol 2020;42:484)
- Expanded mesangium is positive for PAS, Jones methenamine silver, trichrome (J Bras Nefrol 2020;42:484)
- CD34 highlights increased number of vascular channels within glomeruli compared with normal controls (Zhou: Silva's Diagnostic Renal Pathology, 2nd Edition, 2017)
- There is diffuse thickening of glomerular basement membranes, with laminated and healed areas up to 1,000 nm, without aspects of GBM lamellation or basket weave (J Bras Nefrol 2020;42:484)
- Extensive foot process effacement of podocytes is also present but is variable (Clin Nephrol Case Stud 2022;10:82)
- Moderate to severe segmental expansion of mesangial matrix, duplication of the glomerular basement membrane with widening of lamina rara interna and segmental interposition of cells, with no increase of cellularity (Clin Nephrol Case Stud 2022;10:82)
- Electron dense deposits or fibrils are not often seen (Clin Nephrol Case Stud 2022;10:82)
- TBM thickening is also seen
- In cases of ING, there are different expressions of known genes of renal significance
- Downregulation of podocyte markers like NPHS1 (nephrin) and PLCE1 are observed in ING, which may be related to podocyte cell loss in glomeruli (Nephrol Dial Transplant 2021;37:72)
- Left kidney, needle core biopsy:
- Idiopathic nodular glomerulosclerosis (see comment)
- Comment:
- Microscopic description: Global and diffuse severe expansion of the mesangial matrix determining the formation of sclerotic nodules with focal and segmental remodeling of the mesangial matrix with Jones stain. Focal and segmental double contours of the glomerular basement membranes. Minimal focal and segmental mesangial hypercellularity. No endocapillary nor extracapillary hypercellularity. Interstitial fibrosis and tubular atrophy involving ~10% of the cortex with slight interstitial lymphocytic infiltrate. Moderate hyalinosis of the arterioles, mild intimal fibrosis of medium size arteries.
- Immunofluorescence: Diffuse linear staining for IgG of glomerular basement membrane and tubular basement membranes and trace focal and segmental staining for IgM. Negative IgA, C3, C1q, kappa and lambda light chains.
- Electron microscopy: Moderate / severe expansion of the mesangial matrix, duplication of the glomerular basement membrane with widening of lamina rara interna and segmental interposition of cells. Foot process effacement involving ~40% of the glomerular capillary surface. Significant and diffuse thickening of the glomerular basement membrane, up to 1,000 nm, without aspects of GBM lamellation or basket weave. No electron dense deposits. In a nondiabetic, hypertensive and smoker patient, these findings are consistent with idiopathic nodular glomerulosclerosis.
- Diabetic nephropathy (J Bras Nefrol 2020;42:484):
- Indistinguishable except that at laboratory levels, it always has abnormal blood glucose levels and glycated hemoglobin (HbA1c) levels
- On light microscopy, there is more diffuse and dramatic thickening of glomerular basement membrane (GBM) as well as tubular basement membrane (TBM) in addition to more severe vascular disease and hyalinosis of afferent and efferent arterioles
- In addition, there are nonisometric Kimmelstiel-Wilson nodules in class III diabetic nephropathy, while in ING there are typically more nodules of the same size (Colvin: Diagnostic Pathology - Kidney Diseases, 3rd Edition, 2019)
- On immunofluorescence, IgG and albumin reveal linear accentuation of GBM and TBM, while IgA, IgM, C1q and C3 are negative
- On electron microscopy, there is increased mesangial matrix and cellularity, diffuse thickening of TBM and GBM thickening and diffuse effacement of the foot process
- Membranoproliferative glomerulonephritis (J Bras Nefrol 2020;42:484):
- Dependent on underlying etiology
- On light microscopy, there is lobular accentuation of glomerulus structure, endocapillary proliferation and diffuse thickening of GBM with double contour highlighted on Jones methenamine silver
- On immunofluorescence, there is diffuse granular staining of GBM and mesangium by IgG (polyclonal or monoclonal); dominant or only C3
- On electron microscopy, there are granular deposits in mesangial and subendothelial area while immune type deposits are absent in ING (Colvin: Diagnostic Pathology - Kidney Diseases, 3rd Edition, 2019)
- Amyloidosis (J Bras Nefrol 2020;42:484):
- Dependent on underlying etiology
- On light microscopy, there is acellular pale pink amorphous material present in GBM, mesangium, arteries and in the interstitium; this material is negative on Jones methenamine silver and trichome, while positive under polarizer as apple green birefringence
- On immunofluorescence, there is restricted monoclonal light chain staining
- On electron microscopy, there are randomly oriented, nonbranching, straight fibrils (8 - 12 nm in diameter) and there is effacement of the foot process
- Fibrillary glomerulonephritis (J Bras Nefrol 2020;42:484):
- Unknown etiology
- On light microscopy, the glomeruli are nodular and membranoproliferative in appearance but are Congo red negative
- On immunofluorescence, polyclonal IgG (IgG4) and C3 staining is positive
- Under electron microscope, randomly oriented, nonbranching, straight fibrils (12 - 24 nm in diameter) are present in mesangium and along GBM
- Immunotactoid glomerulonephritis (J Bras Nefrol 2020;42:484):
- On light microscopy, the glomeruli are nodular and membranoproliferative in appearance but are Congo red negative
- On immunofluorescence, monoclonal IgG with kappa or lambda light chain staining
- Under electron microscope, microtubular deposits arranged in parallel arrays (> 30 nm in diameter) are present in mesangium and along GBM
- Collagen type 3 glomerulopathy (J Bras Nefrol 2020;42:484):
- Clinically, there is blood and urine N terminal procollagen type III peptide
- On light microscopy, glomeruli can be hypercellular
- Immunofluorescence is negative
- On electron microscopy, there are curved fibers with 60 nm periodicity
- Fibronectin glomerulopathy (J Bras Nefrol 2020;42:484):
- There is always a positive family history
- On light microscopy, there are PAS positive mesangial deposits
- Immunofluorescence is negative except for fibronectin staining
- On electron microscopy, there are massive electron dense deposits in mesangial matrix
- Chronic cyanotic or ischemic conditions (J Bras Nefrol 2020;42:484, Colvin: Diagnostic Pathology - Kidney Diseases, 3rd Edition, 2019):
- Dependent on underlying etiology
- On light microscopy, nodules are not prominent but rather there is centrilobular mesangial thickening along with mesangiolytic lesions, microaneurysms of glomeruli and hyaline material deposition in a mosaic pattern in glomeruli and arterioles
- Immunofluorescence is negative
- On electron microscopy, there are intramembranous and mesangial electron dense deposits
- Monoclonal immunoglobulin deposition diseases (MIDD) (Iran J Kidney Dis 2021;15:457):
- Various morphologic patterns may be observed, including nodular glomerulopathy, mesangioproliferative patterns, membranoproliferative patterns or crescentic patterns of which nodular pattern mimics diabetic nephropathy or ING
- Monotypic light chain deposits, either kappa or lambda, can be observed in a linear pattern along the glomerular capillary basement membranes, mesangium, tubular basement membranes, vessels and occasionally in the interstitium
- Atypical anti-GBM disease (Colvin: Diagnostic Pathology - Kidney Diseases, 3rd Edition, 2019):
- At laboratory level, there are anti-GBM antibodies positive in serum
- On light microscopy, small crescents might be present in glomeruli
- On immunofluorescence, IgG reveals linear accentuation of GBM and not of TBM
Biopsy is done on a young man with a history of smoking and hypertension who presented with nephrotic range proteinuria but with HbA1c of normal range. Electron microscopy reveals the findings shown above. Which characteristic finding is seen in this patient diagnosed as idiopathic nodular glomerulosclerosis (ING)?
- Deposits in mesangial and subendothelial area
- Intramembranous and mesangial electron dense deposits
- Mesangial expansion but no electron dense deposits
- Subepithelial humps
Comment Here
Reference: Idiopathic nodular glomerulosclerosis
- Electron dense deposits are usually absent
- Microtubular deposits arranged in parallel arrays (> 30 nm in diameter)
- Nonbranching, straight fibrils (12 - 24 nm in diameter)
- Randomly oriented, nonbranching fibrils (8 - 12 nm in diameter)
Comment Here
Reference: Idiopathic nodular glomerulosclerosis
- Immunoglobulin A (IgA) nephropathy: dominant or codominant mesangial IgA deposits in the absence of systemic diseases
- Variable light microscopic and clinical features
- Most common primary glomerulonephritis worldwide
- Macro / microscopic hematuria with or without proteinuria
- 80% renal survival in 10 years; 25 - 50% progression to end stage renal disease over 20 years; 30% recurrence in transplants
- Etiology: abnormally glycosylated IgA1
- Dominant or codominant IgA deposits in mesangium; variable light microscopic features; electron microscopy most often with mesangial electron dense deposits
- Histopathological grading: Oxford classification - mesangial hypercellularity (M), endocapillary hypercellularity (E), segmental glomerulosclerosis (S), tubular atrophy / interstitial fibrosis (T) and crescents (C) are independent prognostic factors
- Immunoglobulin A nephropathy
- Berger disease
- ICD-10: N02.8 - Recurrent and persistent hematuria with morphologic changes
- SNOMED CT: 68779003 - Primary immunoglobulin A nephropathy
- SNOMED II: D67300 - Mesangial IgA / IgG disease
- SNOMED II is no longer supported but is still in use in some histopathology databases (SNOMED International 2019: A Brief History of SNOMED Code Systems [Accessed 17 April 2020])
- Most common primary glomerulonephritis worldwide (UptoDate: Clinical Presentation and Diagnosis of IgA Nephropathy [Accessed 6 April 2020])
- More common in Southern Europe, Asia and Native Americans; less common in individuals of African lineage
- First to seventh decade of life; peak 20 - 30 years
- M:F = 2:1 in Europe / U.S. and 1:1 in Asia
- Renal cortex
- Increased serum levels of poorly galactosylated IgA1 (galactose deficient IgA1; GD-IgA1)
- Overproduction of GD-IgA1 in response to immune challenges in mucosal sites (upper respiratory and GI tract infections)
- GD-IgA1 binds to CD71 on mesangial cells; it also triggers autoimmune response with autoantibody production against GD-IgA1 GalNAc epitopes (Colvin: Diagnostic Pathology - Kidney Diseases, 3rd Edition, 2019)
- Disease progression (Colvin: Diagnostic Pathology - Kidney Diseases, 3rd Edition, 2019):
- Mesangial cell proliferation
- IgA / anti-IgA complex deposition in mesangium
- Cytokine release by mesangial cells leading to podocyte injury
- 25 - 50% of patients will progress to renal failure at 20 years; recurs in 20 - 60% of allografts (average 30%)
- Heightened IgA response to mucosal antigens
- Secretion of poorly galactosylated IgA1
- Autoantibodies to poorly galactosylated IgA1
- 40 - 50%: at least one episode of visible hematuria associated to upper respiratory tract infection (synpharyngitic hematuria) (UptoDate: Clinical Presentation and Diagnosis of IgA Nephropathy [Accessed 6 April 2020])
- 30 - 50%: microscopic hematuria
- < 10%: nephrotic syndrome or rapidly progressive glomerulonephritis
- Rare: acute kidney injury (due to either rapidly progressive glomerulonephritis or red cell cast nephropathy)
- Secondary IgA nephropathy: associated with chronic liver disease, celiac disease and HIV
- Renal biopsy with immunofluorescence / immunohistochemistry is the cornerstone of the diagnosis (UptoDate: Clinical Presentation and Diagnosis of IgA Nephropathy [Accessed 6 April 2020])
- Serum polymeric IgA1: elevated in 30 - 50% of cases (UptoDate: Clinical Presentation and Diagnosis of IgA Nephropathy [Accessed 6 April 2020])
- Urine dip: hematuria with or without proteinuria
- Oxford classification MEST-C scores: mesangial hypercellularity (M), endocapillary hypercellularity (E), segmental glomerulosclerosis (S), tubular atrophy / interstitial fibrosis (T) and crescents (C) are independent prognostic factors (Curr Opin Nephrol Hypertens 2017;26:165):
- MEST-C scores and eGFR can predict outcome as accurately as 2 year follow up eGFR and proteinuria (JAMA Intern Med 2019;179:942)
- Limitations: original cohort excluded patients with proteinuria < 0.5 g/day, those with eGFR < 30 ml/min and follow up < 1 year; angiotensin blockade and immunosuppressive regimens were uncontrolled, resulting in treatment bias to outcome data
- Online risk calculator (Calculate by QxMD: International IgAN Prediction Tool [Accessed 6 April 2020])
- In patients with proteinuria < 0.5 g/24 h: M1 and E1 predict proteinuria > 1 g/24 h over 5 year follow up; M1 independently predicts rapid loss of renal function
- E1: independently predicts rapid loss of renal function in patients not treated with steroids (J Nephrol 2016;29:367, Kidney Int 2017;91:235)
- S1 with podocyte hypertrophy and tip lesions (S1p): associated to higher proteinuria/24 h and worse renal survival in patients not treated with steroids (Kidney Int 2017;91:235)
- Endocapillary hypercellularity, crescents and necrosis seem to be responsive to immunosuppression (J Nephrol 2015;28:441)
- Extent of interstitial fibrosis / tubular atrophy: reflects chronic damage; associated with long term outcome
- Immunohistochemistry:
- Evidence of lectin pathway activation (deposition of mannose binding lectin, L ficolin, MASP1/3 and C4d): seen in 25 - 30% of cases and is associated with more severe histologic damage and proteinuria (Kidney Int Rep 2017;3:426)
- Mesangial C4d: predicts worse long term renal outcome (J Nephrol 2016;29:1)
- Glomerular deposition of complement factor H related protein 5 (CFHR5): associated to disease progression (Kidney Int Rep 2017;3:426)
- Clinical predictors (UptoDate: Treatment and Prognosis of IgA Nephropathy [Accessed 6 April 2020]):
- High serum creatinine / reduced eGFR
- Hypertension (> 140/90 mmHg)
- Proteinuria > 1 g/24 h for over 6 months
- 24 year old man with atypical celiac disease presenting with nephrotic syndrome (Cent Eur J Immunol 2019;44:106)
- 36 year old man with recurrent crescentic disease in renal allograft (Clin Case Rep 2019;7:1773)
- 50 year old woman with concomitant primary membranous glomerulonephritis (Saudi J Kidney Dis Transpl 2019;30:531)
- 74 year old woman with systemic lupus erythematosus and acute kidney injury due to crescentic disease (Case Rep Nephrol 2019;2019:8354823)
- 81 year old man with differential diagnosis of IgA dominant infection associated glomerulonephritis (Am J Case Rep 2019;20:508)
- Aims at slowing progression of renal damage (nonimmunosuppresive measures: control of blood pressure and proteinuria) and dampening inflammatory activity (immunosuppression: steroids) (UptoDate: Treatment and Prognosis of IgA Nephropathy [Accessed 6 April 2020])
- If hematuria, normal eGFR with or without proteinuria < 0.5 g/day: watchful monitoring at 6 to 12 month intervals
- If proteinuria > 500 mg/day over 6 months and or mildly reduced eGFR and mild to moderate histologic findings on renal biopsy: nonimmunosuppressive therapies (ACE-I / ARB)
- If rising creatinine, active lesions on renal biopsy (E1, C1/2) or nephrotic range proteinuria or proteinuria after 3 - 6 months of nonimmunosuppressive: consider association of immunosuppressive therapy in addition
- There is some evidence that patients with E1, podocytopathic S1 lesions and C1 and C2 have a markedly lower rate of decline in eGFR when immunosuppressed (J Am Soc Nephrol 2017;28:691)
- Fish oil: low quality evidence; tonsillectomy: evidence from randomized controlled trials missing
- Oxford classification: first evidence based glomerular disease classification (Semin Nephrol 2018;38:477)
- Variable light microscopic patterns; in the original classification 4 lesions were reproducible and predictive of outcome (Kidney Int 2017;91:1014):
- Mesangial hypercellularity (M0 ≤ 50% of glomeruli; M1 > 50% of glomeruli): > 3 mesangial cell nuclei / peripheral mesangial area, not adjacent to vascular pole, in 2 - 3 μm thick PAS stained section
- Endocapillary hypercellularity (E0 absent; E1 present): CD68 count > 6 in most affected glomerulus of the sample is associated with E1
- Segmental glomerulosclerosis / adhesions / synechiae (S0 absent; S1 present): due to hyperfiltration, healed necrotizing lesions or podocytopathy
- Tubular atrophy / interstitial fibrosis (T0 absent to involving ≤ 25% of the cortex; T1 26 - 50% of the cortex; T2 > 50% of the cortex)
- Crescents: added to the classification in 2016 (C0 absent; C1 1 - 24% of glomeruli with cellular / fibrocellular crescents; C2 ≥ 25% of glomeruli with cellular / fibrocellular crescents)
- Other lesions: fibrinoid necrosis (10% of biopsies), glomerular basement membrane duplication (< 10% of biopsies), thrombotic microangiopathy changes (5 - 50% of biopsies; usually associated with malignant hypertension) (Colvin: Diagnostic Pathology - Kidney Diseases, 3rd Edition, 2019)
Contributed by Maria Fernanda Soares, M.D., Ph.D.
Contributed by Arkana
- Dominant IgA mesangial deposits (100%) - only defining and consistent feature of the disease
- Predominance of J chain containing IgA1 and lambda light chains
- Other mesangial deposits: C3 (90%), IgG (20 - 30%), IgM (50%), C1q (< 10% - almost always absent, indicating lack of activation of classical pathway) (Colvin: Diagnostic Pathology - Kidney Diseases, 3rd Edition, 2019)
- Occasional capillary loop subendothelial deposits
- CD68: maximum count of glomerular CD68 positive shows relationship with E score (Histopathology 2019;74:629)
- C4d and mannan binding lectin (MBL): mesangial staining associated with worse prognosis (J Nephrol 2016;29:1)
- Electron dense deposits without substructure (Colvin: Diagnostic Pathology - Kidney Diseases, 3rd Edition, 2019)
- Most commonly mesangial
- Subendothelial deposits associated to more active lesions on light microscopy
- Subepithelial deposits exceedingly rare
- Family history in 10 - 15% of cases (Colvin: Diagnostic Pathology - Kidney Diseases, 3rd Edition, 2019)
- Autosomal dominant IgA nephropathy identified in a Sicilian family with SPRY2 mutation that causes inhibition of inhibition of MAPK / ERK1/2
- Genome wide association study studies: susceptibility loci at 1q32 (CFH and CFHR1-5 genes), 16p11 (ITGAM / ITGAX), 17p13 (α defensin gene cluster), 8q23 (TNFSF13), 1p13 (VAV3) and 9q34 (CARD9)
- Left kidney, core biopsy:
- IgA nephropathy (see comment)
- Comment: There is an IgA nephropathy with diffuse mesangial hypercellularity and focal segmental glomerulosclerosis with podocytopathic features. There is severe chronic tubulointerstitial damage. Oxford classification: M1 E0 S1p T2 C0.
- Specimen: A core of renal cortex containing up to 28 glomeruli in the planes of section.
- Glomeruli: 15/28 are globally sclerosed. 6 show segmental sclerosis, 4 of which with podocytopathic features. There is diffuse mesangial hypercellularity but no endocapillary or extracapillary hypercellularity. Basement membranes appear normal on silver stain. Congo red stain is negative for amyloid.
- Tubules and interstitium: There is severe chronic damage with 60% of the cortex showing interstitial fibrosis and tubular atrophy. There is a mild mononuclear infiltrate, which is confined to the areas of fibrosis. There are uromodulin casts but no red cell casts.
- Vessels: Arteries show moderate fibroelastosis. There is focal arteriolar hyalinosis.
- Immunofluorescence microscopy: There is diffuse mesangial and focal capillary wall positivity for IgA (3+), C3 (1+), kappa (2+) and lambda (3+). IgG, IgM and C1q are negative.
- Henoch-Schönlein purpura nephritis:
- Morphologically indistinguishable
- Clinical information is important for differential diagnosis (e.g. history of purpuric rash, abdominal pain, arthritis)
- IgA dominant infection associated glomerulonephritis (in general, Staphylococcus associated):
- Electron microscopy contains hump shaped deposits
- Mesangial IgA deposits in chronic liver disease:
- Present in 60% of autopsies of cirrhotic patients
- Rarely associated with active glomerular disease (UptoDate: Clinical Presentation and Diagnosis of IgA Nephropathy [Accessed 6 April 2020])
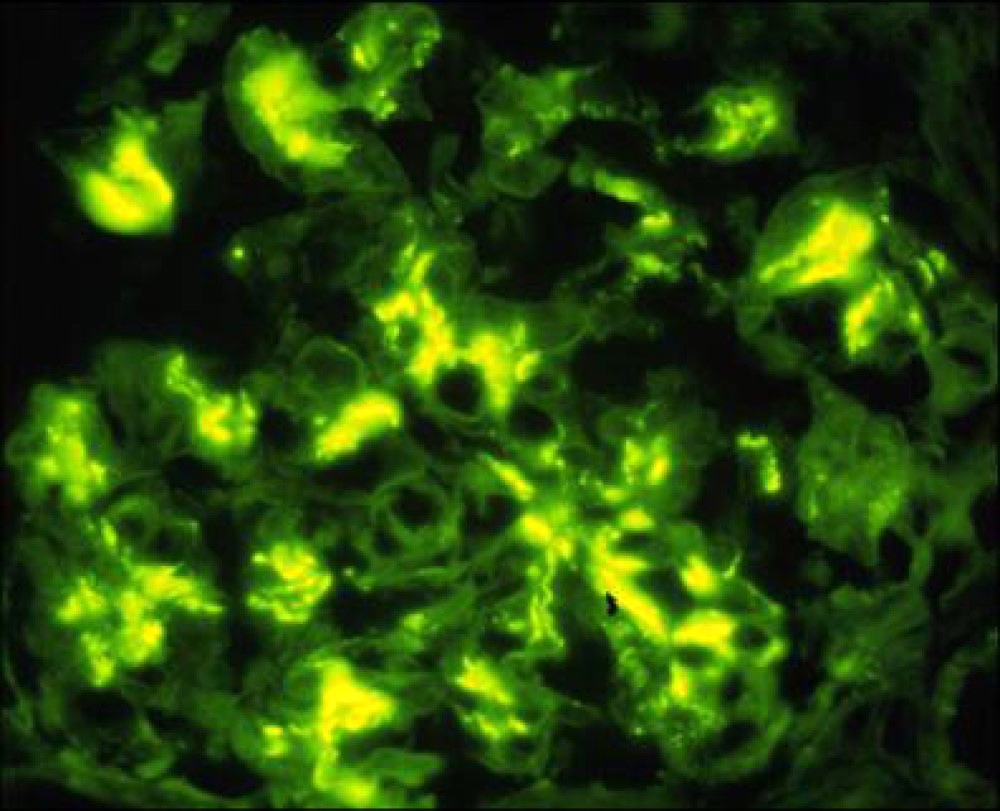
A 32 year old man presents with mild reduction in eGFR, proteinuria and microscopic hematuria. Urine protein / creatinine ratio in nephrotic range. Immunofluorescence microscopy of a renal biopsy core showed mesangial deposition of IgA (strong - see image), C3, kappa and lambda (moderate) and IgG (weak and focal). IgM and C1q were negative. Based on these findings, what is the best diagnosis?
- Accurate diagnosis of a renal biopsy cannot be assigned on the basis of the immunofluorescence findings only
- Alport syndrome
- Immunoglobulin A nephropathy
- Lupus nephritis
- Membranous glomerulonephritis
- Absence of necrosis / karyorrhexis - NO
- Crescents in 10% of glomeruli - C2
- Interstitial fibrosis / tubular atrophy in 25 - 50% of the cortex - T2
- Presence of endocapillary hypercellularity - E1
- Presence of segmental sclerosis in > 50% of glomeruli - S2
- A component of a systemic immune mediated fibroinflammatory condition which generally manifests in the kidney as an acute or chronic interstitial nephritis with lymphoplasmacytic inflammation containing increased IgG4+ plasma cells, tubulointerstitial immune complex deposits and frequently storiform fibrosis, which may be mass forming / tumor-like
- Varying degrees of glomerular involvement, predominantly membranous nephropathy, can also be seen
- Usually chronic interstitial nephritis with dense lymphoplasmacytic inflammation with a predominance of IgG4+ plasma cells (hallmark)
- Some degree of fibrosis, usually storiform in character
- Generally associated with other systemic manifestations of IgG4 disease
- Good response to glucocorticoids
- Antineutrophil cytoplasmic antibody (ANCA) related disease has been excluded
- Also has been called
- IgG4 related systemic disease
- IgG4 syndrome
- IgG4 associated disease
- IgG4 related sclerosing disease
- IgG4 related systemic sclerosing disease
- IgG4 related autoimmune disease
- IgG4+ multiorgan lymphoproliferative syndrome
- Hyper-IgG4 disease
- Systemic IgG4 related plasmacytic syndrome
- Systemic IgG4 related sclerosing syndrome
- Multifocal fibrosclerosis
- Multifocal idiopathic fibrosclerosis
- Average ~65 years (range 20 - 80 years) (Am J Surg Pathol 2010;34:1812, Adv Chronic Kidney Dis 2017;24:94)
- Male predominance (3:1 - 7:1)
- Likely underestimated; 0.28 - 6 individuals/100,000 people (Japan) (Int J Rheumatol 2012;2012:358371)
- Affects almost any organ, including the pancreas, bile duct, lacrimal glands, salivary glands, central nervous system, thyroid, lungs, liver, gastrointestinal tract, kidney, prostate, retroperitoneum, arteries, lymph nodes, skin, breast (N Engl J Med 2012;366:539)
- Within the kidney / ureter
- Predominantly tubulointerstitial compartment
- Less frequently glomerulus (membranous nephropathy)
- Ureter (strictures / pseudotumor)
- Pathogenesis poorly understood
- Findings consistent with both an autoimmune disorder and an allergic disorder are present (Adv Anat Pathol 2010;17:303, Am J Surg Pathol 2006;30:1537)
- Follicular helper T cells and CD4+ cytotoxic T cell appears to be involved in the disease (J Allergy Clin Immunol 2016;138:825, Adv Chronic Kidney Dis 2017;24:94)
- Unclear
- May be related to genetic background, bacterial infection and molecular mimicry, and autoimmune disease (World J Nephrol 2018;7:29)
- Acute or progressive kidney insufficiency in most patients (Nat Rev Nephrol 2015;11:599)
- May present as kidney mass lesion(s) resembling renal cell carcinoma
- May be multiple lesions
- Diffuse renal enlargement, sometimes bilateral, can occur
- Renal pelvis thickening alone can occur
- Ureteral stricture
- Multiple organs are affected in 60 - 90% of patients
- Lymphadenopathy is common
- Substantial weight loss (~9 - 14 kg) over months in patients with multiorgan disease
- Up to 90% with elevated serum IgG4 levels (Mod Pathol 2012;25:1181)
- ~50% have hypocomplementemia (low C3, C4 or CH50)
- Peripheral eosinophilia can be seen
- 3 tiered diagnostic terminology (Mod Pathol 2012;25:1181)
- Histologically highly suggestive of IgG4 related disease
- Requires at least 2 of 3 histologic features:
- Dense lymphoplasmacytic infiltrate
- Fibrosis, usually storiform in character
- Obliterative phlebitis
- > 40% IgG4/IgG plasma cell ratio
- IgG4+ plasma cells/high power field > 30 in surgical specimen or > 10 in biopsy specimen
- Requires at least 2 of 3 histologic features:
- Probable histological features of IgG4 related disease
- Lacks the full histological spectrum or immunohistochemical profile associated with IgG4 related disease
- Usually only a single histopathological feature, typically a dense lymphoplasmacytic infiltrate and required numbers of IgG4+ cells
- Requires additional clinical, serological or radiological evidence to confirm the diagnosis of IgG4 related disease:
- Serum IgG4 > 135 mg/dl
- Other organ involvement, as demonstrated by radiological or pathological examination
- Or other evidence of IgG4 related disease
- Lacks the full histological spectrum or immunohistochemical profile associated with IgG4 related disease
- Insufficient histopathological evidence of IgG4 related disease
- Outside of 2 categories above
- Does not necessarily exclude the diagnosis of IgG4 related disease entirely
- May reflect sampling artifact, prior treatment or advanced fibrotic stage
- Histologically highly suggestive of IgG4 related disease
- Elevated total serum IgG or IgG4 levels (79 - 90% of patients) (Clin Exp Nephrol 2011;15:615, J Am Soc Nephrol 2011;22:1343)
- ~50% have hypocomplementemia with decreased C3 (42%) or C4 (46%) (J Am Soc Nephrol 2011;22:1343)
- Hypergammaglobulinemia
- Eosinophilia (33%)
- Single or multiple mass lesion(s) within the kidney (N Engl J Med 2012;366:539)
- Masses may extend into perinephric tissues
- Masses in other organs may be clue to diagnosis
- > 80% response rate with ~10% relapse rate has been achieved in a Japanese cohort (Arthritis Rheumatol 2015;67:1688)
- Elevation in serum IgG4 and IgE levels and an increased number of circulating eosinophils could predict IgG4 related disease relapses independently
- No consensus has been reached regarding patients at high risk of relapse
- Can recur in kidney allografts (Am J Transplant 2018;18:1799)
- 28 year old man with recurrent IgG4 related tubulointerstitial nephritis concurrent with chronic active antibody mediated rejection (Am J Transplant 2018;18:1799)
- 40 year old man with IgG4 related membranous glomerulonephritis and generalized lymphadenopathy without pancreatitis (BMC Nephrol 2017;18:139)
- 56 year old man with a right renal mass suspected of being malignant (Case Rep Surg 2017;2017:9690218)
- 80 year old woman with IgG4 related inflammatory pseudotumor mimicking renal cell carcinoma (Oncol Lett 2016;11:3438)
- 81 year old man with IgG4 related tubulointerstitial nephritis and membranous glomerulonephritis during the clinical course of gastric cancer (Mod Rheumatol 2019;29:542)
- Glucocorticoids (Arthritis Rheumatol 2015;67:1688)
- Rituximab may be appropriate if relapse or glucocorticoid treatment is contraindicated (Arthritis Rheumatol 2015;67:1688)
- Resection specimens of the kidney may show masses / tumor-like lesions
- Most cases show chronic interstitial nephritis with dense lymphoplasmacytic interstitial inflammation with prominent plasma cells (J Am Soc Nephrol 2011;22:1343)
- Increased IgG4+ plasma cells
- Storiform fibrosis
- Often marked effacement of tubulointerstitial compartment
- Tubulitis may be present
- Tubular basement membrane deposits may be seen on trichrome stain
- Obliterative phlebitis usually not seen in kidney biopsies
- Rarely, injury pattern may resemble acute interstitial nephritis
- Glomeruli may show membranous pattern of injury (Nat Rev Nephrol 2015;11:599)
- True degree of interstitial fibrosis is difficult to estimate on biopsy alone
- Granulomatous inflammation or necrosis should not be present and excludes diagnosis of IgG4 disease
- Tubulointerstitium
- Granular tubular basement membrane deposits
- Majority show IgG (predominantly IgG4) (J Am Soc Nephrol 2011;22:1343)
- Both light chains
- C1q and IgM less common
- Tend to be concentrated in areas of fibrosis and may be absent in uninvolved areas
- Plasma cells may show staining with IgG and both light chains
- Quantification of IgG4 plasma cells is not recommended on immunofluorescence tissue
- Granular tubular basement membrane deposits
- Glomerulus
- If membranous glomerulopathy present: granular capillary loop staining
- IgG, C3 and light chain staining
- Some cases without membranous pattern may show granular mesangial staining
- Glomeruli may show negative staining if no membranous component
- Bowman capsule deposits may be seen (Adv Chronic Kidney Dis 2017;24:94)
- If membranous glomerulopathy present: granular capillary loop staining
- CD138 can highlight increased plasma cells
- IgG4 shows increased positivity in plasma cells (J Am Soc Nephrol 2011;22:1343)
- Jones methenamine silver (JMS), PAS and trichrome stains can highlight storiform fibrosis
- Jones methenamine silver (JMS) stain can highlight membrane capillary spike / pinhole pattern
- Trichrome can highlight tubular basement membrane and capillary deposits
- Electron dense deposits, without substructure, along tubular basement membranes
- Subepithelial deposits associated with glomerular basement membrane remodeling if membranous component present
- Identical to any other cause of membranous nephropathy pattern of injury
- May see increased interstitial type collagen bundles
- Some have proposed classification of fibrosis based on pattern of collagen bundles (Hum Pathol 2012;43:536)
- Kidney, needle core biopsy:
- Chronic interstitial nephritis with increased IgG4 positive plasma cells and storiform fibrosis
- Membranous glomerulopathy
- Comment: The morphologic features in this biopsy including > 10 IgG4 positive plasma cells/high powered field and an IgG/IgG4 plasma cell ratio of > 40% are highly suggestive of IgG4 related tubulointerstitial nephritis. Correlation with serum IgG4 levels is recommended. ANCA related disease can also show increased IgG4 positive plasma cells and thus correlation with ANCA serologies is recommended. Membranous glomerulopathy can be a manifestation of IgG4 related renal disease; however, serological / clinical correlation is recommended to completely exclude other etiologies for membranous nephropathy (e.g. other systemic rheumatologic disease, chronic infections, malignancy and idiopathic / primary membranous nephropathy).
- Sjögren syndrome related tubulointerstitial nephritis:
- Usually no tubular basement membrane deposits, no increased IgG4 plasma cells, no storiform fibrosis
- Interstitial inflammation associated with pauci-immune (ANCA associated) glomerulonephritis:
- May show increased IgG4 plasma cells
- Usually will show other features such as necrotizing / crescentic glomerulonephritis, vasculitis, medullary angiitis; no tubular basement membrane deposits
- Allergic / drug related acute or chronic interstitial nephritis:
- Usually no tubular basement membrane deposits; plasma cells usually not prominent
- No increased IgG4 plasma cells; no storiform fibrosis, may show increased eosinophils
- Chronic pyelonephritis:
- May show increased plasma cells
- No tubular basement membrane deposits, no increased IgG4+ plasma cells
- Lupus nephritis:
- May show active and chronic interstitial nephritis with tubular basement membrane deposits
- Usually glomerulonephritis is present
- Full house immunofluorescence staining (staining with all 3 Ig classes [IgG, IgA, IgM] and both complement proteins [C1q and C3])
- No increased IgG4+ plasma cells
- Clinical history and serologic findings can be characteristic
- Small B cell lymphomas (e.g. extranodal marginal zone):
- May show a chronic interstitial nephritis-like pattern with increased plasma cells
- May form mass lesion
- Should show numerous B cells with light chain restriction
- Diabetic nephropathy:
- May show increased interstitial IgG4+ plasma cells
- May be difficult to differentiate from true IgG4 tubulointerstitial nephritis
- IgG4 patients may have diabetes from autoimmune pancreatitis
- Multicentric Castleman and Erdheim-Chester disease:
- May show dense lymphoplasmacytic inflammation sometimes with increased IgG4+ plasma cells (Am J Kidney Dis 2017;69:e19)
- Other etiologies of membranous glomerulopathy:
- Clinical history for secondary causes and other markers for membranous etiologies can help differentiate (e.g. phospholipase A2 receptor, thrombospondin containing domain 7A (THSD7A), exostosin 1/2)
- Anti-brush border antibody disease / Anti-LRP2 nephropathy:
- Also has granular tubular basement membrane deposits and membranous pattern glomerulopathy
- Has less tubulointerstitial inflammation, fewer IgG4+ plasma cells, no storiform fibrosis and positive staining of TBM deposits for LRP2 / megalin
- Lacks systemic features of IgG4 related disease
- Chronic tubulointerstitial nephritis
- Glomerular crescent formation
- Membranous glomerulonephritis
- Mesangial immune complex deposition
- Rare ( < 1% of renal biopsies) disorder with extracellular glomerular deposition of nonamyloid fibrils
- Patients have monoclonal immunoglobulin deposition in glomeruli and may have circulating paraproteins
- More common in whites and females
- Related to fibrillary glomerulonephritis but different fibril size and arrangement
- May overlap with hepatitis C virus induced cryoglobulinemic glomerulonephritis
- Presents with nephrotic syndrome
- Patients with circulating or urinary paraproteins are more likely to have lymphoproliferative disorders
- Poor long term survival
- Diagnosis based on EM findings and exclusion of other possible causes of fibrillary deposits, such as amyloidosis, cryoglobulinemia, systemic lupus erythematosus or paraproteinemia
- Hypertensive control, possibly steroids, possibly rituximab, kidney transplant (Clin Exp Nephrol 2009;13:378, Transplant Proc 2009;41:3953)
- 43 year old woman with spontaneous remission (Neth J Med 2011;69:341)
- 59 year old woman with proteinuria, immunotactoid glomerulopathy, heavy chain disease and follicular lymphoma (Arch Pathol Lab Med 2004;128:689)
- 69 year old man with lobular glomerulonephritis (Clin Nephrol 2005;63:368)
- Progression to end stage renal disease within 1 week of initial presentation (ScientificWorldJournal 2009;9:1348)
- Mesangial widening and occasional hypercellularity, capillary wall thickening; 25% have crescents
- Deposition of usually monoclonal IgG and C3 in glomeruli
- Extracellular, nonamyloid deposits 30 - 50 nm wide, focally arranged in parallel arrays and with a visible lumen (microtubules) usually within mesangium but also involving basement membrane
- In comparison, fibrillary glomerulonephritis has smaller fibrils, 10 - 30 nm diameter with only focal parallel arrangement
- Protease inhibitor used to treat HIV, strongly associated with direct nephrotoxicity (Adv Chronic Kidney Dis 2010;17:72)
- May cause tubulointerstitial nephritis due to intratubular precipitation of indinavir crystals, with damage to tubular epithelial cells and histiocytic reaction
- Tolerance may be higher in those from sub Saharan Africa versus US / Europe (AIDS Res Hum Retroviruses 2007;23:62)
- May resemble tuberculosis (Braz J Infect Dis 2008;12:99)
- 49 year old man with HIV and indinavir induced nephrolithiasis 3 years after discontinuing indinavir (Int Urol Nephrol 2011;43:571)
- Dose reduction (Eur J Clin Pharmacol 2007;63:901), discontinuation, urine acidification, early insertion of bilateral double J ureteral stents (Int Urol Nephrol 2007;39:743)
- Usually due to emboli from left atrial mural thrombi or left ventricular myocardial infarction
- Also endocarditis, abdominal aortic aneurysm and atherosclerotic emboli
- Infarcts are "white" anemic type; ringed by intense hyperemia; wedge shaped with apex towards medulla
- Generically included under plasma cell dyscrasias or dysproteinemias affecting the kidney
- Myeloma defining event that results from precipitation of monoclonal light chains in distal, nephron forming casts
- May coexist with other manifestations of the monoclonal light chains, such as amyloidosis, light chain proximal tubulopathy or light chain deposition disease
- Pathognomic appearance of casts with angulated and fractured appearance, associated with syncytial giant cell reaction and chronic interstitial nephritis (Am J Kidney Dis 2016;67:e17)
- Clonal staining of casts by immunofluorescence or immunohistochemistry for either kappa or lambda
- Also called myeloma cast nephropathy, myeloma kidney or Bence Jones cast nephropathy
- ~90% of patients with light chain cast nephropathy have overt myeloma
- Age: median age of 66 years
- Gender: M:F = 3:2
- Kidney, tubulointerstitial disease
- Mechanism by which urinary light chains (Bence Jones proteins) lead to kidney failure is incompletely understood
- It can be because of direct toxicity of the light chains or secondary to obstruction (J Clin Med 2021;10:3871)
- When light chains excessed the threshold of reabsorption in the proximal tubules, they are delivered to the distal tubules and collecting ducts
- Here they form casts as a result of coaggregation of light chains and Tamm-Horsfall proteins (uromodulin) produced by the thick ascending limb of the loop of Henle
- These casts lead to kidney injury and obstruction by incompletely understood mechanisms
- Plasma cell dyscrasia leading to overproduction of mutated monoclonal light chains
- Precipitating factors include dehydration, hypercalcemia, use of nephrotoxic drugs such as nonsteroidal anti-inflammatory drugs (NSAIDs), contrast media or infections (Int J Nephrol Renovasc Dis 2022;15:173)
- Patients present with acute kidney function deterioration / injury or frank kidney failure or nephrotic range proteinuria
- Most common cause of acute kidney injury (AKI) in patients with myeloma
- It is seen in approximately half of patients with multiple myeloma who have renal disease (Int J Nephrol Renovasc Dis 2022;15:173)
- On the other hand, ~90% of patients with light chain cast nephropathy have overt myeloma (Colvin: Diagnostic Pathology - Kidney Diseases, 4th Edition, 2023)
- It can also be seen in patients with monoclonal protein, who do not meet hematologic criteria for multiple myeloma
- Patients frequently have proteinuria that is predominantly composed of light chains (Bence Jones proteins) and can be nephrotic range
- Routine urinalysis using a dipstick fails to pick up monoclonal protein as it detects albuminuria rather than light chains
- May show a monoclonal protein on serum and urine protein electrophoresis
- Increased serum or urine free light chains (Clin J Am Soc Nephrol 2016;11:2273)
- Renal function improves in ~50% associated with reduction of free light chains
- 5 year survival rate 20 - 25%
- End stage kidney disease has major impact on survival: median survival with renal recovery is 43 months and is around 8 months without renal recovery (Blood 2020;135:1833)
- 57 year old man with new onset acute kidney injury, anemia and new diagnosis of multiple myeloma (Case Rep Hematol 2022;2022:7531142)
- 57 and 72 year old men with multiple myeloma and acute kidney injury (BMC Nephrol 2021;22:42)
- 64 year old man with diastolic heart failure and chronic kidney disease of unknown etiology (J Community Hosp Intern Med Perspect 2019;9:319)
- 65 year old woman with generalized weakness and low back ache (Indian J Nephrol 2019;29:204)
- 73 year old man with 2 week history of oliguria (Korean J Intern Med 2021;36:1025)
- Reduction of circulating light chains by targeting plasma cell dyscrasia
- Plasmapheresis to decrease the concentration of circulating light chains
- Supportive care with hydration and reduction of hypercalcemia
- Glomeruli
- Nonspecific changes that can be related to preexisting conditions or other monoclonal protein related findings
- Tubules
- Distal convoluted tubules and collecting ducts show presence of casts
- Casts show a characteristic appearance with irregular, angulated and geometric shapes and fracture planes and sometimes with a peculiar jigsaw puzzle type of arrangement
- Occasionally, they have lamellated appearance or crystalline appearance
- Casts appear strongly eosinophilic on H&E, pale or weak positive on PAS and red-violet (metachromatic) on trichrome stained sections
- Casts can appear metachromatic because of the varying composition of light chains (pale on PAS stain) and Tamm-Horsfall proteins (eosinophilic on PAS stain)
- Casts may be lined with flattened to reactive tubular epithelium
- Casts can be associated with polymorphonuclear cell or syncytial giant cell or granulomatous reaction
- Some of the casts are congophilic and elicit apple green birefringence on Congo red stain (amyloidogenic activity)
- This finding may be a risk factor for systemic amyloidosis (Mod Pathol 2018;31:452)
- Some casts contain crystals
- Proximal convoluted tubules may show acute tubular injury
- Interstitium
- Varying degrees of inflammation with mononuclear cells, plasma cells, polymorphonuclear cells and occasional eosinophils that is sometimes similar to chronic interstitial nephritis often can be seen
- When casts break through tubular basement membranes, multinucleated giant cell reaction or peritubular granulomas are seen
- Rarely neoplastic plasma cells may infiltrate kidney
- Varying degree of interstitial fibrosis and tubular atrophy can be seen
- Vasculature
- Nonspecific changes that can be related to preexisting conditions or other monoclonal protein related findings
- Light chain restricted staining of the casts for either kappa or lambda helps to confirm the diagnosis
- Only seen when casts are acutely formed but not when they are present for a prolonged period
- Only about half of the casts in proven light chain cast nephropathy stain in a monoclonal pattern; therefore, absence of monoclonal staining cannot be taken as definitive evidence to rule out light chain cast nephropathy (Fogo: Diagnostic Renal Pathology, 4th Edition, 2022)
- Clonal staining may be absent when casts are present in few numbers focally only or secondary to mutated light chains not recognized by commercial antibodies
- Kappa and lambda staining on paraffin embedded sections after pronase digestion may help
- Glomeruli and vasculature reveal no specific findings
- Kappa or lambda IHC stains can be done to demonstrate monoclonal staining in biopsies without any casts in the tissue submitted for immunofluorescence studies
- Casts can be Congo red positive (amyloidogenic activity) in 28% of cases
- Monoclonal staining of the casts can be absent when casts are few and focal and when the mutated proteins are not recognized by commercially available antibodies
- Glomeruli and vasculature show no specific findings
- Tubular casts composed of finely granular material of moderate electron density are seen
- Spectrum of appearances including fibrillar, microvesicular or crystalline substructure can be seen
- Ultrastructural immunogold labeling to demonstrate monoclonality can be performed in cases where immunofluorescence is not diagnostic
Washington University in St. Louis: renal pathology teaching series
- Kidney, percutaneous needle biopsy:
- Light chain cast nephropathy (see comment)
- Comment: There are scattered granular to globular PAS pale and metachromatic casts with fractured appearance with rare syncytial giant cell reaction and associated acute tubular injury. These casts demonstrate monoclonal lambda staining by immunofluorescence without corresponding staining for kappa. In this patient with a history of lambda light chain myeloma, these findings are diagnostic of light chain cast nephropathy.
- Myoglobin cast nephropathy:
- Pigmented globular casts that stain positive for myoglobin
- Absence of light chain restriction by immunofluorescence (IF)
- Vancomycin cast nephropathy:
- Casts stain for vancomycin
- Absence of light chain restriction by IF
- Electron microscopy shows vancomycin nanospheres entangled with uromodulin
- Monoclonal immunoglobulin deposition disease:
- Deposition of monoclonal light or heavy chains along glomerular basement membrane (GBM) and tubular basement membranes (TBM)
- Monoclonal linear / ribbon staining along GBMs and TBMs by IF
- Electron microscopy shows powdery deposits along GBMs and TBMs
- Can occur concurrently with light chain cast nephropathy in 10% of cases
- Light chain proximal tubulopathy:
- Intracytoplasmic crystalline or noncrystalline deposits that are PAS and trichrome positive within proximal tubular epithelial cells
- Granular cytoplasmic positivity for either kappa or lambda on IF
- Electron microscopy shows intracytoplasmic electron dense crystals with sharp edges
- Other drug related cast nephropathies:
- Chemotherapy drugs such as ripamycin, etc.
- End stage kidney with hyaline casts:
- PAS positive casts by light microscopy
- Absence of syncytial giant cell reaction
- No monoclonal staining by IF
A 65 year old man with history of chronic kidney disease now presents with oliguria, fatigue and nausea for the past 2 weeks. Laboratory studies show anemia with a hemoglobin of 8.6 g/dL, serum creatinine of 5.2 mg/dL compared to 1.7 mg/dL a few months ago. Urine studies show 2+ proteinuria without hematuria. Spot urine protein creatinine ratio is 4.8. Serum and urine free light chains show elevated lambda levels. Renal biopsy was performed and is shown in the images above. A Congo red stain is negative. Electron microscopy does not show any fibrillar deposits, no powdery deposits along tubular basement membranes and glomerular basement membranes and no crystals within the tubules. What is the diagnosis?
- AL amyloidosis
- Light chain cast nephropathy
- Light chain deposition disease
- Light chain proximal tubulopathy
Comment Here
Reference: Light chain cast nephropathy
- AL amyloidosis
- Light chain cast nephropathy
- Light chain deposition disease
- Light chain proximal tubulopathy
Comment Here
Reference: Light chain cast nephropathy
- Monoclonal gammopathy of renal significance (MGRS) is defined by the 2019 consensus statement as any B cell or plasma cell clonal lymphoproliferation with both of the following characteristics (Nat Rev Nephrol 2019;15:45)
- One or more kidney lesions that are related to the produced monoclonal immunoglobulin
- Underlying B cell or plasma cell clone does not cause tumor complications or meet any current hematological criteria for specific therapy
- Light chain deposition disease is generically included under plasma cell dyscrasias or dysproteinemias affecting the kidney
- May occur with malignant plasma cell or lymphoplasmacytic neoplasms or may be in the spectrum of MGRS
- Part of a broad spectrum of diseases known as monoclonal immunoglobulin deposition diseases (MIDD), which include heavy chain deposition disease (HCDD), light chain deposition disease (LCDD) and light and heavy chain deposition disease (LHCD)
- MIDD, including LCDD, HCDD, etc. are considered a subtype of MRGS
- Systemic disease that primarily involves the kidneys and is characterized by light chain deposits that may be identified in any or all renal compartments (glomeruli, tubules, interstitium and vessels)
- Monotypic staining of glomerular basement membrane (GBM) or tubular basement membrane (TBM) with or without mesangium, for either kappa or lambda light chain, a single heavy chain or single light and heavy chain by immunofluorescence microscopy
- Powdery deposits along same locations by electron microscopy
- May coexist with other manifestations of the monoclonal light chains, such as amyloidosis, light chain proximal tubulopathy or light chain cast nephropathy
- Included under a broad spectrum of monoclonal gammopathies or monoclonal immunoglobulin deposition diseases (MIDD)
- Incidence of LCDD in autopsy series of patients with myeloma has been reported to be 3 - 5% (Arch Pathol Lab Med 1990;114:986, Arch Pathol Lab Med 2004;128:875)
- Light chain deposits can frequently be seen in other organs (in up to 75% of cases), most notably liver, heart and lungs (J Am Soc Nephrol 2001;12:1482)
- MIDD predominantly affects patients older than 50 years; the largest cohort of patients studied with renal MIDD, including 64 cases (51 LCDD, 7 HCDD and 6 LHCD), revealed a mean age at diagnosis of 56 years with 36% of the patients being less than 50 years of age (Clin J Am Soc Nephrol 2012;7:231)
- Kidney, glomeruli and tubulointerstitium
- Commonly composed of kappa chain deposits (80%), specifically the VκIV subgroup, which has a longer complementarity determining region 1 loop, which is part of an antigen binding site
- These immunoglobulins display peculiar physicochemical properties of the variable domain, including unusual hydrophobic residues that are abnormally glycosylated and positive charge
- This promotes aggregation and increases precipitation of the light chains as amorphous deposits along the negatively charged glomerular and tubular basement membranes (N Engl J Med 2021;384:1931)
- Light chains that precipitate can also stimulate synthesis of extracellular matrix proteins by mesangial cells, contributing to the advanced lesion of nodular sclerosis
- Interactions of glomerulopathic light chains with peripheral glomerular capillary walls result in alterations of the glomerular permeability, leading to proteinuria
- Clonal proliferation of B cells / plasma cells that lead to deposition of abnormal light chains in various regions in the kidney
- Clinical presentation includes proteinuria (more than 90% and usually nephrotic range), kidney insufficiency / acute kidney injury (about 30%), hematuria, hypertension and select cases with tubular dysfunction (Adv Anat Pathol 2004;11:49, Arch Pathol Lab Med 1989;113:781, N Engl J Med 1976;294:71)
- Overall, the most common clinical presentation is proteinuria (usually nephrotic range but can also be nonnephrotic), hypertension and renal insufficiency (Adv Anat Pathol 2004;11:49, Arch Pathol Lab Med 1989;113:781, N Engl J Med 1976;294:71)
- Clinical evidence of dysproteinemia is present in a majority of the patients with plasma cell dyscarasias (myeloma); additionally, classic features of multiple myeloma have been noted in 59% of patients (Arch Pathol Lab Med 2009;133:249)
- Minority of patients with LCDD have no detectable monoclonal proteins in urine or serum (Clin J Am Soc Nephrol 2012;7:231)
- LCDD may coexist with light chain cast nephropathy in up to 21% of the affected patients and may also be associated, though much less commonly, with amyloid light chain amyloidosis (J Am Soc Nephrol 2001;12:1482, Ann Intern Med 1990;112:455)
- In cases without malignant hematopoietic neoplasm, considered to be within the spectrum of MGRS
- Acute or chronic kidney injury, with proteinuria (may or may not be nephrotic range)
- Monoclonal proteins (M spike) in serum or urine protein electrophoresis or immunofixation
- Serum or urine free light chain assays
- 5 year survival of about 67% in the largest series
- Associated myeloma cast nephropathy is a poor prognostic factor
- Diabetes is a risk factor for progression
- Recurs in 75% of transplant patients
- 54 year old woman with new onset of nephrotic syndrome and preserved kidney function (Acta Haematol 2024 Jan 16 [Epub ahead of print])
- 54 year old woman status post-living related kidney transplantation (from sister) followed by a diagnosis of proteinuria 6 months posttransplant (Nephron 2023;147:96)
- 72 year old woman with nephrotic syndrome (Cureus 2022;14:e26357)
- 83 year old woman with a history of a monoclonal gammopathy of undetermined significance (MGUS) with shortness of breath and anasarca (Diseases 2023;11:24)
- Targeting the underlying plasma cell clone using steroids, cyclophosphamide, melphalan / autologous stem cell transplant, bortezomib
- Supportive therapy
- Variable morphologic patterns seen, including nodular glomerulopathy, mesangioproliferative, membranoproliferative or crescentic patterns
- Most characteristic finding is nodular glomerulopathy / glomerulosclerosis that mimics diabetic nephropathy
- Mesangial nodules are argyrophilic (Jones silver positive) with or without lamellations and composed of matrix proteins admixed with monotypic light chains
- Some cases show associated mesangial hypercellularity
- Glomerular capillary basement membranes appear thickened because of deposits
- Light chain deposits are Congo red negative
- Extraglomerular changes include ribbon-like thickening of tubular basement membranes and thickened vascular walls; sometimes interstitial deposits in the form of PAS positive aggregates can be seen
- Can have associated light chain cast nephropathy
- Can coexist with other paraprotein related diseases including amyloidosis and light chain cast nephropathy (Kidney Int 2022;101:152)
- Monotypic light chain deposits for either kappa or lambda can be seen in a linear pattern along the glomerular capillary basement membranes, mesangium, tubular basement membranes, vessels and rarely interstitium
- Alternatively, monotypic heavy chain staining can be seen with or without monotypic light chain staining (heavy chain deposition disease or heavy and light chain deposition disease)
- If IgG heavy chain, restriction to single IgG heavy chain subclass
- Pattern and intensity depend on the amount of light chain deposits
- In most cases of LCDD, the pathogenic light chain is kappa isotype, with a kappa to lambda ratio of 9:1
- Immunoglobulin heavy chains and complements are usually negative (in light chain deposition disease)
- Immunofluorescence studies on paraffin embedded tissue after pronase digestion can be performed in suspicious cases with negative immunofluorescence on frozen tissue
- References: J Am Soc Nephrol 2018;29:1810, Curr Opin Nephrol Hypertens 2016;25:127
- Kappa or lambda IHC stains can be done to demonstrate monoclonal staining in biopsies without any tissue submitted for immunofluorescence studies
- Paraffin immunofluorescence can be performed to demonstrate monoclonal staining or masked monoclonal staining, even on biopsies with tissue submitted for immunofluorescence
- Granular to powdery electron dense deposits are seen in all renal compartments
- In glomerular capillary walls, the powdery deposits form a thin band along the lamina rara interna and larger deposits may pool along subendothelial region
- Deposits are present along the outer aspect of tubular basement membranes
- References: J Am Soc Nephrol 2018;29:1810, Curr Opin Nephrol Hypertens 2016;25:127
Washington University in St. Louis Nephrology Web Series - multiple myeloma and the kidney
- Kidney, percutaneous needle biopsy:
- Light chain deposition disease (see comment)
- Comment: The biopsy shows 22 glomeruli by light microscopy, 7 of which are globally sclerosed, with ~60% interstitial fibrosis. Glomeruli show segmental to global nodular expansion of mesangium with segmental hypercellularity. Tubular basement membranes appear prominent with mild interstitial lymphocytic infiltrate in areas of scarring with rare tubulitis. Immunofluorescence performed on frozen tissue shows diffuse, ribbon-like staining of glomerular basement membranes and tubular basement membranes for kappa (2 - 3+) without any lambda and focal, segmental, chunky mesangial staining for kappa without lambda staining. Immunofluorescence performed on paraffin embedded tissue by pronase digestion shows a similar pattern of staining for kappa without immunoglobulin or lambda staining. There are powdery deposits along the inner aspect of the glomerular basement membranes and the outer aspect of the tubular basement membranes as well as amorphous deposits in the mesangium by electron microscopy. Taken together, the findings are diagnostic of kappa restricted light chain deposition disease.
- Diabetic nephropathy:
- Nodular expansion of mesangium and thickened tubular basement membranes
- Absence of monoclonal staining by immunofluorescence and no powdery deposits by electron microscopy
- Proliferative glomerulonephritis with monoclonal IgG deposits:
- Proliferative pattern by light microscopy
- Monoclonal staining by immunofluorescence
- Amorphous deposits along glomerular basement membranes by electron microscopy but without any deposits along tubular basement membranes
- Atypical anti-GBM nephritis:
- Linear glomerular basement membrane staining for heavy and light chains by immunofluorescence but without any deposits by electron microscopy
- ~38% can have monotypic staining by immunofluorescence (Am J Kidney Dis 2024;83:713)
- Amyloidosis:
- Nodular glomerulosclerosis by light microscopy
- But Congo red positive and randomly arranged fibrils by electron microscopy
A 65 year old man with history of chronic kidney disease now presents with oliguria and edema. Clinically, he is found to have nephrotic syndrome with urinalysis showing 3+ proteinuria without hematuria and urine protein of 4.2 gm/day; laboratory studies show anemia with a hemoglobin of 8.6 g/dL and serum creatinine of 1.7 mg/dL. Serum and urine free light chain assays show elevated kappa levels. Renal biopsy was performed and shows the following findings: casts do not have a fractured appearance or reaction around them and no monoclonal staining of casts by immunofluorescence; Congo red stain is negative; electron microscopy shows powdery deposits along the glomerular basement membranes (GBMs) and tubular basement membranes (TBMs) and no crystals within the tubules. What is the diagnosis?
- Amyloid light chain amyloidosis
- Light chain cast nephropathy
- Light chain deposition disease
- Light chain proximal tubulopathy
Comment Here
Reference: Light chain deposition disease
- Amyloid light chain amyloidosis
- Light chain cast nephropathy
- Light chain deposition disease
- Light chain proximal tubulopathy
Comment Here
Reference: Light chain deposition disease
- Acute or chronic involvement of proximal tubules of kidney in monoclonal light chain disease
- Characterized by intracytoplasmic accumulation of nephrotoxic light chains within proximal tubular epithelial cells in crystalline or noncrystalline form, causing tubular injury
- Light chain proximal tubulopathy (LCPT) is a rare variant of monoclonal light chain associated renal disease
- May be associated with acute or chronic renal failure, proteinuria or Fanconi syndrome
- Characterized by intracytoplasmic deposition of light chains in the cytoplasms of proximal tubular epithelial cells and may be crystalline or noncrystalline variant
- Kappa or lambda (never both) staining of proximal tubular epithelial cells on routine or pronase digested immunofluorescence is pathognomonic
- Electron microscopy shows crystals of various shapes in crystalline variant and numerous, large or abnormal looking (mottled) lysosomes with or without mitochondrial swelling in noncrystalline variant
- References: J Am Soc Nephrol 2016;27:1555, Mod Pathol 2011;24:1462
- Proximal tubulopathies, monoclonal light chain related
- LCPT, crystalline variant
- LCPT, noncrystalline variant
- Real incidence and prevalence are still unknown
- Age: Mostly adults, median age: 60 years (range: 39 - 87 years) (J Am Soc Nephrol 2016;27:1555)
- Gender: M > F (63% men) (J Am Soc Nephrol 2016;27:1555)
- Race: can affect all races; more common in Caucasian population as per few studies (J Am Soc Nephrol 2016;27:1555)
- Strong association with some form of overt or clinically silent hematolymphoid neoplasm
- Kidney, proximal tubular (PT) epithelium
- Main pathogenesis occurs as a consequence of imbalance in interaction of tubulopathic light chains and lysosomes within the proximal tubular cells due to excess light chain production
- In monoclonal renal disease, the excess light chains (particularly their variable domains) resist degradation and start to deposit locally, either in crystalline or noncrystalline form
- These pathologic light chains are more commonly of kappa type and specifically belong to the VK1 subgroup
- References: Mod Pathol 2011;24:1462, Arch Pathol Lab Med 2014;138:1365
- Monoclonal gammopathy from a hematolymphoid neoplasm, causing excess light chain production of one type, either kappa (K) or lambda (L), with K > L
- Hematolymphoid neoplasm may be some form of overt plasma cell dyscrasia or B cell lymphoma or covert disease (elevated monoclonal light chain in blood or urine in absence of symptoms)
- Abnormal light chain gets reabsorbed and likely inadequately excreted by proximal tubules, causing tubular injury
- Reabsorbed light chains may or may not form crystals
- References: Mod Pathol 2011;24:1462, Arch Pathol Lab Med 2014;138:1365, J Am Soc Nephrol 2016;27:1555
- Plasma cell dyscrasia
- B cell lymphoma
- Monoclonal gammopathy of undetermined significance (MGUS)
- Monoclonal gammopathy of renal significance (MGRS)
- Fanconi syndrome
- Acute renal failure
- Chronic renal failure
- Proteinuria
- Osteomalacia
- References: Mod Pathol 2011;24:1462, Arch Pathol Lab Med 2014;138:1365, J Am Soc Nephrol 2016;27:1555
- Diagnosis is by renal biopsy using different modalities - light microscopy (LM), immunofluorescence microscopy (IF) and electron microscopy (EM)
- Immunofluorescence of paraffin sections by pronase digestion is recommended when routine IF is negative
- In challenging cases, immunoelectron microscopy may be helpful
- Increased urinary protein on routine urinalysis
- Monoclonal M spike detected on serum or urine electrophoresis
- Elevated free light chain assay in serum or urine (kappa or lambda)
- Detection of monoclonal B cells or plasma cells by flow cytometry, fluorescent in situ hybridization or bone marrow biopsy
- Increased serum creatinine and blood urea nitrogen
- Renal tubular acidosis in Fanconi syndrome
- References: Arch Pathol Lab Med 2014;138:1365, J Am Soc Nephrol 2016;27:1555, Nat Rev Nephrol 2019;15:45
- Good control (complete or partial remission) of the hematological disease often results in recovery of renal and tubular function
- Many patients develop chronic kidney disease if early diagnosis is not made and sometimes in spite of treatment (J Am Soc Nephrol 2016;27:1555)
- Patients with Fanconi syndrome often have an indolent course
- In treated patients, those who receive stem cell treatment had the best prognosis
- 53 year old woman with moderate proteinuria and renal failure (BMC Nephrol 2018;19:322)
- 64 year old man who presented with acute renal failure and Fanconi syndrome (BMJ Case Rep 2020;13:e234361)
- 66 year old woman with known history of K-restricted multiple myeloma, acute kidney injury and crystalline structures in urine sediment (Clin Nephrol 2020;93:203)
- Usually directed towards the underlying hematolymphoid neoplasm
- Conventional chemotherapy
- Monoclonal antibodies (rituximab, daratumumab)
- Immunomodulatory drugs (thalidomide)
- Glucocorticoids
- Autologous stem cell transplant
- Reference: J Am Soc Nephrol 2016;27:1555
- Main light microscopic finding is some form of tubular pathology seen on kidney biopsy
- Intracytoplasmic crystals of various shapes within tubular epithelial cells, which are PAS and trichrome positive (fuchsinophilic), mainly in crystalline variant
- PAS negative and trichrome negative eosinophilic finely granular cytoplasmic inclusions within proximal tubular epithelial cells, particularly in noncrystalline variant
- Acute tubular injury / necrosis, which may include tubular luminal dilatation, loss of brush border, epithelial cytoplasmic blebbing, epithelial cell desquamation or cytoplasmic vacuolization
- Tubulointerstitial inflammation, characterized by chronic inflammatory infiltrate (lymphocytes and plasma cells) in the interstitium, often with tubulitis
- Usually unremarkable glomeruli
- References: Mod Pathol 2011;24:1462, Arch Pathol Lab Med 2014;138:1365, J Am Soc Nephrol 2016;27:1555, BMC Nephrol 2020;21:146
- Strong granular cytoplasmic positivity for either kappa or lambda light chain (never both) within proximal tubules on frozen section (routine) immunofluorescence
- Immunofluorescence (IF) after pronase digestion of paraffin sections is critically important in cases where routine immunofluorescence is nondiagnostic or negative (for both light chains)
- Kappa only staining is overall more common, particularly in crystalline variant of LCPT (Arch Pathol Lab Med 2014;138:1365)
- Lambda only staining has been seen to be more common in noncrystalline variant of LCPT (Mod Pathol 2011;24:1462)
- Kappa light chain positive by routine or pronase immunofluorescence or lambda light chain positive by routine or pronase immunofluorescence; never both (J Am Soc Nephrol 2016;27:1555, Mod Pathol 2011;24:1462, BMC Nephrol 2020;21:146)
- Congo red
- Thioflavin T
- Reference: Kidney Int 2020;98:310
- All findings restricted to tubular epithelial cells
- Intracytoplasmic electron dense or electron lucent crystals, usually with sharp edges (needle-like, rectangular, rhomboidal, polygonal or any other shape)
- Abnormal size, shape or number in intracytoplasmic lysosomes - increased number, large size, abnormal shape (including mottled lysosomes) and rarely, lysosomal packing, also known as lysosomal indigestion or constipation
- Mitochondrial swelling
- Tubular epithelial cell injury changes - segmental loss of brush border, cytoplasmic blebbing and vacuolization
- References: Mod Pathol 2011;24:1462, Arch Pathol Lab Med 2014;138:1365, BMC Nephrol 2020;21:146
- Kidney, needle biopsy:
- Light chain proximal tubulopathy (see comment)
- Comment: Cytoplasmic accumulation of monoclonal kappa or lambda light chain (as the case may be) in the proximal tubules as confirmed by immunofluorescence fulfils criteria for LCPT diagnosis. Light microscopy and EM support the diagnosis. Congo red stain is negative. The findings are consistent with the patient’s documented history of X (if patient has a known diagnosis of any hematolymphoid neoplasm) OR clinical correlation with hematologic studies is recommended (if there is no known hematolymphoid diagnosis).
- Monoclonal light or heavy chain deposition disease:
- Monoclonal light chain (LC) or rarely heavy chain (HC) deposition in powdery form along the tubular basement membrane, best visualized by immunofluorescence and electron microscopy
- Concurrent monoclonal LC or HC deposit in the glomerular basement membrane usually present
- Light chain cast nephropathy:
- Angulated sharp, often fractured LC casts filling tubular lumen
- Usually associated with tubular epithelial injury (acute tubular injury / acute tubular necrosis) and tubulointerstitial nephritis
- More commonly involves distal tubules and collecting ducts
- Amyloid cast proximal tubulopathy:
- Monoclonal light chain sharp looking casts filling tubular lumina and staining for Congo red
- Casts show characteristic amyloid type fibrillary structure (with diameter ranging from 8 - 12 nm) on electron microscopy
- Acute tubular injury with or without tubulointerstitial inflammation:
- Only tubular injury or interstitial inflammation without accumulation of monoclonal LC; for example, in allergic type tubulointerstitial nephritis
A 54 year old woman presented with features of phosphaturia, glucosuria, proteinuria and proximal renal tubular acidosis. No significant medical history or family history is present. Her kidney biopsy showed kappa restricted staining pattern in proximal tubules on immunofluorescence. A microscopic image of the proximal tubules seen on kidney biopsy is shown above. No other histopathological changes are noted. What is the diagnosis?
- Interstitial nephritis
- Light chain proximal tubulopathy
- Light chain cast nephropathy
- Minimal change disease
- Ankylosing spondylosis
- Multiple myeloma
- Osteosarcoma
- Sarcoidosis
- Also spelled malacoplakia
- Foamy macrophages with PAS+ granular cytoplasm and Michaelis-Gutmann bodies (may be due to deposition of amorphous salts, Am J Surg Pathol 1984;8:151)
- Associated with transplants
- May be due to defective macrophage function that blocks lysosomal degradation of engulfed bacteria, filling up cytoplasm with undigested debris
- 65 year old woman with malakoplakia of kidney extending to descending colon (Korean J Fam Med 2011;32:367)
- Soft, yellow mucosal plaques
- Foamy macrophages with PAS+ granular cytoplasm due to phagosomes stuffed with bacterial debris and Michaelis-Gutmann bodies (laminated mineralized concretions)
- May have rare Michaelis-Gutmann bodies, late fibrous stage (Am J Surg Pathol 1989;13:225)
- Intracellular calcific inclusions
- Xanthogranulomatous pyelonephritis: no Michaelis-Gutmann bodies
- Also called hypertensive emergency, malignant nephrosclerosis
- Severe hypertension with acute impairment of one or more organ systems (especially CNS, cardiovascular, renal) that may cause irreversible organ damage
- Vascular damage (due to chronic hypertension, arteritis, coagulopathy) increases permeability of small vessels to fibrinogen and other plasma proteins
- This causes endothelial injury and platelet deposition, which causes fibrinoid necrosis
- Kidneys become ischemic, which stimulates renin-angiotensin system to produce angiotensin II, which causes renal vasoconstriction, as well as increased aldosterone secretion and salt retention, which elevate blood pressure even further
- May be related to increased TRPC3 expression in vascular endothelium (Dtsch Med Wochenschr 2009;134:2224)
- Usually associated with pre-existing hypertension, glomerulonephritis or reflux nephropathy
- Rarely caused by juxtaglomerular cell tumor (J Med Assoc Thai 2012;95 Suppl 2:S251, J Hypertens 2012;30:974)
- 1 - 5% of patients with hypertension; higher frequency in young men, African-Americans
- Symptoms: diastolic blood pressure 130 mm or more, cardiac symptoms, encephalopathy, headache, nausea, vomiting, loss of consciousness, proteinuria and renal failure
- Treatment: antihypertensive therapy before irreversible renal lesions develop
- Renal survival has improved; mean proteinuria is important prognostic factor (Nephrol Dial Transplant 2010;25:3266)
- "Flea bitten" appearance of kidney due to pinpoint petechiae on cortical surface
- Fibrinoid necrosis of arterioles, hyperplastic arteriolitis (onion skinning) due to concentric layering of collagen
- May see necrotizing glomerulitis, wrinkling and collapse of capillary walls and small crescents
- Myointimal hyperplasia and hypertropy is associated with acute or persistent severe high blood pressure
- Necrotizing arteriolitis: fibrinoid necrosis of afferent arteriole, often superimposed on hyperplastic or hyaline lesions; media has deposits of deeply eosinophilic and fibrillar material containing fibrin and fibrinogen
- Early changes: profound intimal thickening by myxoid connective tissue, reducing lumen
- Late changes: scarring and concentric thickening of vessel wall by myointimal cells and deposition of basement membrane type material (onion skinning)
- Occasional fibrin, fibrinogen, IgM and complement
- Swollen endothelium
- May be focally disrupted from glomerular basement membrane by accumulation of electron-dense material
- Sporadic cystic disease characterized by bilateral cystic dilations of medullary collecting ducts; normal cortex
- 1 per 5000 births; no gender preference; not familial
- Associated with hemihypertrophy of body (25% of cases), Marfan's, Caroli's and Ehlers-Danlos
- Usually presents in adulthood
- Patients are usually asymptomatic with normal renal function
- Calcifications on Xray, stones, hematuria and infection at age 30+ years
- Does not progress to end stage renal disease
- Diagnosed with intravenous pyelography
- Cranberry juice to maintain urinary acidity
- Nephrectomy is NOT recommended
- Normal sized kidneys with multiple, small cysts in medullary pyramids and papillae, giving medulla a sponge-like appearance
- Most often bilateral
Images hosted on other servers:
- Medullary cysts lined by cuboidal epithelium or urothelium
- May have concretions adherent to cyst wall
- Often severe inflammation and scarring in interstitium, often with tubular atrophy near papillary tips
- Autoimmune glomerular disease characterized by diffuse subepithelial immune complex deposition with nephrotic range proteinuria, without known systemic cause (Colvin: Diagnostic Pathology - Kidney Diseases, 2nd Edition, 2015)
- Idiopathic autoimmune glomerular disease
- Nephrotic syndrome
- Thickening of glomerular basement membrane and subepithelial deposition of immune complexes (silver stain, spike)
- Anti-PLA2R autoantibodies
- Membranous glomerulopathy
- Membranous glomerulonephritis
- Most common cause of nephrotic syndrome in nondiabetic adults
- Incidence: ~8 - 10 cases per 1 million
- Mean age: 50 - 60 years; rare in children (Nephrology (Carlton) 2015;20:572)
- Caucasian predilection (Postgrad Med J 2019;95:23, Clin J Am Soc Nephrol 2017;12:983)
- Kidney (glomerular disease)
- Circulating autoantibodies bind to an autoantigen on the surface of the podocytes resulting in in situ immune complex formation that activates the lectin complement pathway and causes podocyte injury and proteinuria
- 2 major target antigens are now firmly recognized: the M type phospholipase A2 receptor 1 (PLA2R) (~70%) and the thrombospondin type 1 domain containing 7A (THSD7A) (2 - 5%) (J Am Soc Nephrol 2017;28:421)
- Idiopathic autoimmune disease with a predisposition to autoimmunity conferred by HLA genes and an environmental trigger (J Am Soc Nephrol 2013;24:525)
- Nephrotic syndrome
- 20% with nonnephrotic proteinuria
- Less frequent: hematuria (usually microscopic), hypertension, thrombotic and thromboembolic events (Clin J Am Soc Nephrol 2017;12:983)
- Nephrotic syndrome with serum anti-PLA2R antibody (Clin J Am Soc Nephrol 2017;12:983)
- Demonstration of subepithelial deposition of immune complexes by renal biopsy and immunofluorescence
- Positive PLA2R staining in glomeruli by immunohistochemistry
- Positive serum anti-PLA2R antibody
- High antibody levels (before and after treatment) correlate with proteinuria, response to therapy and (after therapy) long term outcomes (Clin J Am Soc Nephrol 2017;12:983)
- 33% spontaneously remit
- 33% remain stable with proteinuria and little progression
- 33% progress to end stage renal disease (ESRD)
- 10 - 30% recur after kidney transplantation
- Adverse clinical prognostic features: high PLA2R antibody titer, nephrotic syndrome, higher levels of proteinuria, persistence of proteinuria, renal insuffiency, male sex and advanced age (J Am Soc Nephrol 2017;28:421, Colvin: Diagnostic Pathology - Kidney Diseases, 2nd Edition, 2015)
- 38 year old man with nephrotic syndrome (Nephrology (Carlton) 2018;23:94)
- 39 year old woman with severe nephrotic syndrome (Am J Kidney Dis 2016;67:775)
- 50 year old woman with heavy proteinuria (Saudi J Kidney Dis Transpl 2019;30:531)
- 63 year old man with PLAR2 positive membranous nephropathy whose kidney was donated after cardiac death (Am J Transplant 2022;22:299)
- Supportive care with or without immunosuppressive therapy (Clin J Am Soc Nephrol 2017;12:983)
- Glomeruli may appear entirely normal in early disease (stage 1)
- Thickening of glomerular basement membrane
- Subepithelial spike formation or vacuolated appearance on PAS or Jones silver stain (Colvin: Diagnostic Pathology - Kidney Diseases, 2nd Edition, 2015, Zhou: Silva's Diagnostic Renal Pathology, 2nd Edition, 2017)
- Finely granular staining for IgG, predominately IgG4, presents uniformly in a subepithelial distribution in all glomeruli (Colvin: Diagnostic Pathology - Kidney Diseases, 2nd Edition, 2015, Zhou: Silva's Diagnostic Renal Pathology, 2nd Edition, 2017)
- PLA2R and IgG4: intense global and granular staining of glomerular basement membrane (Appl Immunohistochem Mol Morphol 2021;29:414)
- Electron microscopy confirms the subepithelial localization of electron dense deposits
- 4 stages:
- Scattered electron dense deposits on the epithelial side of the glomerular basement membrane
- Subepithelial deposits with basement membrane material (spikes) between deposits
- Subepithelial (or intramembranous) deposits with basement membrane material between and surrounding deposits
- Electron lucent areas represent probable resorption of prior subepithelial immune complexes
- References: Colvin: Diagnostic Pathology - Kidney Diseases, 2nd Edition, 2015, Zhou: Silva's Diagnostic Renal Pathology, 2nd Edition, 2017
Contributed by Ana Belén Larqué, M.D., Ph.D. and Jonathan E. Zuckerman, M.D., Ph.D.
Images hosted on other servers:
- 2 strongly linked loci in genome wide association studies: HLA DQA1 allele and PLA2R allele (Colvin: Diagnostic Pathology - Kidney Diseases, 2nd Edition, 2015)
- Right kidney, biopsy:
- Membranous nephropathy; minimal chronic changes (see comment)
- Adequacy: adequate (cortex 85%, medulla 15%)
- Microscopic description: 18 glomeruli, 1 of these with global sclerosis. Thickening of the glomerular capillary wall. Presence of spikes and internal vacuolizations of the glomerular basement membrane evaluated by silver stain. No evidence of hypercellularity, crescent or necrosis. Fibrosis occupying < 10% of the interstitium.
- Immunofluorescence microscopy:
- Number of glomeruli: 4
- Diffuse granular staining of the glomerular basement membrane for IgG (+++) and C3 (++); there were no deposits of IgA, IgM, C1q or fibrin.
- Immunohistochemistry: diffuse and strong granular positivity of glomerular basement membrane for PLA2R and IgG4
- Comment: positivity for anti-PLA2R and IgG4 favor the diagnosis of primary membranous glomerulopathy
- Membranous nephropathy; minimal chronic changes (see comment)
- Secondary form of membranous nephropathy:
- Requires clinical correlation
- Mesangial or endocapillary hypercellularity
- Mesangial or subendothelial deposits
- PLA2R+ deposits in < 10% (depends on cause)
- Staining for all subclasses of IgG
- Membranous lupus nephritis:
- Postinfectious glomerulonephritis:
- Generally has a history of hypocomplementemia and presents with nephritic features
- Subepithelial deposits have segmental distribution, a hump-like configuration, lack of spikes around humps and stain predominantly for C3
- Deposits in mesangium and subendothelial space
- Intraglomerular inflammation and hypercellularity
- Membranoproliferative glomerulonephritis:
- Presence of mesangial and subendothelial deposits in addition to intramembranous deposits (Colvin: Diagnostic Pathology - Kidney Diseases, 2nd Edition, 2015, Zhou: Silva's Diagnostic Renal Pathology, 2nd Edition, 2017)
Which of the following is true about primary membranous nephropathy?
- Active periglomerular inflammation and rupture of Bowman capsule
- Little or no immunoglobulin or complement deposits by immunofluorescence
- Most common cause of idiopathic nephrotic syndrome in nondiabetic adults worldwide
- Significant mesangial or endocapillary hypercellularity
Comment Here
Reference: Primary membranous nephropathy
Which of the following findings favors primary membranous nephropathy over secondary forms?
- Glomerular basement membrane spikes by PAS and silver stains
- Intense global and granular PLA2R and IgG4 staining of glomerular basement membrane
- Nephrotic range proteinuria
- Thickening of the glomerular capillary wall
Comment Here
Reference: Primary membranous nephropathy
- Idiopathic glomerular disease that causes nephrotic syndrome
- Little or no light or immunofluorescent microscopic abnormalities
- Diffuse podocyte foot process effacement on electron microscopy (BMC Nephrol 2018;19:207)
- Renal glomeruli appear normal by light and immunofluorescence microscopy
- Extensive effacement of podocyte foot processes by electron microscopy (hallmark)
- Highly selective proteinuria, mainly albuminuria
- Responds well to treatment with corticosteroids (90 - 95%)
- Also called minimal change disease, idiopathic nephrotic syndrome, nil disease, lipoid nephrosis and foot process disease
- Most common type of nephrotic syndrome in children: 70 - 90%; in adults: 11 - 16%
- Peak incidence in children: 2 - 3 years; in adults: 80 - 91 years
- In children, M:F = 2:1; in adults, M = F
- More common in white than in black and Asian patients (Clin J Am Soc Nephrol 2007;2:445)
- Glomerular disease
- Extensive foot process effacement appears to be due to epithelial injury with loss of glomerular anionic charge (Kidney Int 1984;25:696)
- Abnormal T cell and B cell function and immune dysregulation (Pediatr Nephrol 2018;33:573, Clin J Am Soc Nephrol 2016;11:710)
- Unknown circulating factor that triggers podocyte dysfunction has been postulated (Pediatr Nephrol 2018;33:1101)
- Nephrin autoantibodies have been discovered in a subset of adults and children with minimal change disease; this aligns with published animal studies and provides further support for an autoimmune etiology (J Am Soc Nephrol 2022;33:238)
- Minimal change disease, diffuse mesangial hypercellularity and focal segmental glomerulosclerosis may be a continuum of the same disease (Kidney Int 2004;65:1690)
- Usually idiopathic, especially in children (Clin J Am Soc Nephrol 2017;12:332)
- Secondary forms due to virus, drugs, allergic reactions, immunologic diseases and neoplasia at all ages (BMC Nephrol 2018;19:162, Intern Med 2014;53:1131, Case Rep Nephrol 2015;2015:987212, J Surg Oncol 2010;102:704)
- Rarely familial (gene mutations: NPHS2, dysferlin) (Semin Nephrol 2003;23:141)
- Nephrotic syndrome
- Proteinuria selective for albumin, causing hypoalbuminemia leading to severe edema
- Lipiduria, hypercholesterolemia but usually no hypertension, no hematuria and no azotemia
- Microhematuria (10 - 30%)
- Can present as acute renal insufficiency in adults (Clin J Am Soc Nephrol 2017;12:332)
- Can present with IgA nephropathy (Clin J Am Soc Nephrol 2014;9:1033)
- Nephrotic syndrome with little or no light or immunofluorescent microscopic abnormalities
- Diffuse foot process effacement on electron microscopy
- CD80 in urine may be a marker (Pediatr Nephrol 2015;30:309)
- Rarely, if ever, leads to end stage renal disease without development of focal segmental glomerulosclerosis
- Adults with minimal change disease and acute renal failure also usually fully recover (Medicine (Baltimore) 2014;93:e300)
- Relapses common and often lead to steroid dependence
- 2 and 15 year old boys with steroid responsive nephrotic syndrome complicated by cerebral venous sinus thrombosis (Nefrologia 2015;35:497)
- 29 year old woman with type I neurofibromatosis and complete remission after neurofibroma resection (Korean J Intern Med 2017;32:186)
- 33 year old man with severe chronic sinusitis (Intern Med 2015;54:2373)
- 43 year old man with nephrotic syndrome following the COVID-19 vaccine (BMC Nephrol 2021;22:376)
- 50 year old woman with minimal change disease after a type B influenza infection (BMC Nephrol 2018;19:162)
- 63 year old man with nephrotic syndrome (JNMA J Nepal Med Assoc 2022;60:399)
- 68 year old man with marginal zone MALT lymphoma manifesting as nephrotic range proteinuria (Clin Nephrol 2016;85:184)
- Corticosteroids
- Immunosuppressive agents in steroid resistant cases (cyclophosphamide, rituximab, abatacept) (Kidney Int 2013;83:511, Pediatr Nephrol 2015;30:469)
- Enlarged, waxy, yellow cortex due to lipid accumulation in proximal tubules
- Glomerulus normal by light microscopy except for variable podocyte hypertrophy
- Protein reabsorption droplets in tubules (Zhou: Silva's Diagnostic Renal Pathology, 2nd Edition, 2017)
- Negative except for variable focal IgM with or without C3
- Punctate IgG podocyte staining in a subset of cases associated with antinephrin antibodies (J Am Soc Nephrol 2022;33:238)
- Extensive foot process effacement (foot processes retract into cell bodies, not actually fusion) (Nephrology (Carlton) 2014;19:392)
- Microvillous transformation of epithelial cells, cyst formation
- Rarely familial (Semin Nephrol 2003;23:141, Hum Mol Genet 1995;4:2155, J Am Soc Nephrol 2002;13:388)
- Histologic findings of focal segmental glomerulosclerosis or more rarely minimal change glomerulopathy are a part of the autosomal recessive steroid resistant nephrotic syndrome
- Mutations in NPHS2 have been detected in both familial and sporadic steroid resistant nephrotic syndrome
- NPHS2 gene, formerly SRN1, encodes podocin, a protein that is almost exclusively expressed in glomerular podocytes
- More than 24 genes are currently known to be pathogenic in steroid resistant nephrotic syndrome (Clin J Am Soc Nephrol 2013;8:637)
- Left kidney, biopsy:
- Minimal change glomerulopathy
- Adequacy: adequate (cortex 85%, medulla 15%)
- Microscopic description: 19 glomeruli showed normal size and cellularity. There is no interstitial inflammation and no significant interstitial fibrosis or tubular atrophy. Tubules are preserved, without significant evidence of injury. Small arteries and arterioles show minimal interstitial fibrosis. There is no vasculitis.
- Immunofluorescence microscopy:
- Number of glomeruli: 4
- There were no deposits of IgA, IgM, IgG, C3, C1q or fibrin
- Electron microscopy: Extensive effacement of the foot processes covering over 80% of the glomerular capillary surface. The glomerular basement membrane showed normal thickness and a generally smooth contour. No electron dense deposits were noted on the glomerular capillary basement membranes or mesangial areas. The mesangial area contained normal amounts of matrix and cells.
- Focal segmental glomerulosclerosis:
- Segmental hyalinosis or synechiae to Bowman capsule, interstitial fibrosis or tubular atrophy
- Cases with unsampled glomerulosclerosis may be misdiagnosed as minimal change disease
- Secondary causes of minimal change disease (Nephrol Dial Transplant 2003;18:vi52):
- Secondary to drugs, especially nonsteroidal anti-inflammatory drugs
- Features of acute interstitial nephritis support the diagnosis
- May precede or occur simultaneously with the diagnosis of Hodgkin disease or other diseases (hematologic malignancies, thymoma, food allergies, infectious, etc.)
- Genetic disease (Front Med (Lausanne) 2021;8:761600):
- Should be considered in patients with family history of nephrotic syndrome, disease onset within the first 6 months of life or steroid resistant nephrotic syndrome
- Mesangial glomerulonephritis:
- Early mesangial glomerulonephritis can be indistinguishable on light microscopy
- Positive immunofluorescence and electron dense subepithelial deposits on electron microscopy
- Chyluria:
- Normal podocytes with heavy proteinuria
- IgA nephropathy:
- Minimal change disease could be superimposed on IgA nephropathy
- IgM nephropathy:
- Possibly a variant of minimal change disease / focal segmental glomerulosclerosis
- Diffuse, global mesangial staining for IgM (≥ 2+) in cases that otherwise resemble minimal change disease; codeposits of C3 in 30+%
- C1q nephropathy:
- Dominant or codominant mesangial staining for C1q (≥ 2+) by immunofluorescence; other deposits are C3, IgG and IgM
- Light microscopic findings range from normal appearing glomeruli to mesangial proliferation to focal or diffuse endocapillary proliferation and frequently reveal focal segmental glomerulosclerosis
- Interstitial inflammation and fibrosis are usually absent
- It is the most common type of nephrotic syndrome in adults
- Monoclonal antibody therapy should be the first line therapy
- Pretreatment biopsy is always done
Comment Here
Reference: Minimal change glomerulopathy
- Azotemia
- Hypertension
- Macrohematuria
- Selective proteinuria
Comment Here
Reference: Minimal change glomerulopathy
Comment Here
Reference: Minimal change glomerulopathy
- Monoclonal gammopathy of renal significance (MGRS) is a broad term that includes all kidney diseases caused by a nephrotoxic monoclonal immunoglobulin (mostly immunoglobulin light chains, also known as M protein) secreted by any clonal low grade / smoldering plasma cell disease or low grade B cell lymphoproliferative disease
- Underlying hematologic disorder typically does not cause tumor complications or meet any specific criteria for any immediate specific therapy
- References: Nat Rev Nephrol 2019;15:45, Neth J Med 2019;77:243, N Engl J Med 2021;384:1931
- MGRS includes all kidney diseases caused by any plasma cell or B cell clonal disorder (leukemia / lymphoma) that does not meet criteria for neoplasm but produces monoclonal immunoglobulins or light chains
- Kidney biopsy is the gold standard for diagnosis of MGRS, with the light chain restriction exhibited by the clonal deposit in the kidney being the same as the paraprotein present in blood, bone marrow or lymph node tissue
- Paraprotein deposits either in a single compartment (glomeruli, tubules, vessels or interstitium) or synchronously in multiple compartments resulting in MGRS associated kidney symptoms
- Some cases, due to indirect mechanism, do not show deposition of the paraprotein in the kidney
- Clone directed therapy (bortezomib for plasma cell clones and rituximab for B cell clones that express CD20 receptors) is the current standard of care
- References: N Engl J Med 2021;384:1931, Blood 2012;120:4292
- Kidney disease associated with monoclonal gammopathy of undetermined significance (MGUS)
- MGRS has an overall prevalence of 6% in individuals diagnosed with MGUS in the U.S. (Neth J Med 2019;77:243)
- MGRS related diseases are present in 40 - 45% of patients with monoclonal gammopathy who undergo kidney biopsy (N Engl J Med 2021;384:1931, J Am Soc Nephrol 2020;31:2400)
- Age: mostly adults (50 and beyond); frequency is 3% in people aged > 50 years, 5% in > 70 years and up to 8% in > 80 years (Nat Rev Nephrol 2019;15:45)
- M:F exact ratio not known but incidence likely higher in men
- 2 - 3 times higher incidence of MGUS in African Americans than in Caucasians (Nat Rev Nephrol 2019;15:45)
- No geographic bias known
- Associated in 100% of cases with a covert or overt B cell or a plasma cell clonal disease that does not meet current criteria for immediate treatment and simultaneously causes symptomatic renal disease (N Engl J Med 2021;384:1931, Nat Rev Nephrol 2019;15:45)
- Kidney, involvement of one or more compartments (glomerulus, tubule, blood vessel and interstitium)
- Excess of the monoclonal protein (entire immunoglobulin or only the light chain, rarely heavy chain) is present within the kidney or systemic microcirculation and may form misfolded proteins and deposit as fibrils, may resist normal proteolytic degradation due to unusual variable domains (kappa light chain mostly) and deposit as crystals or may activate complement pathways (classical or alternative pathway)
- Immunoglobulins or complement may be deposited in the glomerulus; this is in addition to the monoclonal light chain or sometimes even without the light chain (as in C3 glomerulopathy)
- Glomerulus with monoclonal light chain or sole complement deposition often shows influx of inflammatory cells in addition to the proliferation of resident glomerular cells or visceral epithelial cells (forming crescents)
- Kappa light chain clone is more common in MGRS lesions, except in amyloid light chain (AL) amyloidosis wherein lambda is more common
- Some cases result from paraprotein disruption of complement pathway without direct kidney paraprotein deposition
- References: Nat Rev Nephrol 2019;15:45, Neth J Med 2019;77:243, Mod Pathol 2011;24:1462
- Low grade plasma cell disorder (MGUS) and smoldering plasma cell disease (smoldering multiple myeloma)
- Smoldering Waldenström macroglobulinemia
- Low grade or early stage chronic lymphocytic leukemia (CLL)
- Monoclonal B cell lymphocytosis (MBL), a recently recognized B cell clonal equivalent of MGUS
- Low grade B cell non-Hodgkin lymphomas such as marginal zone lymphoma, mantle cell lymphoma and mucosa associated lymphoid tissue (MALT) lymphoma
- All lesions listed above result in a nephrotoxic monoclonal protein (mostly a light chain, rarely a heavy chain) that deposits in single or multiple compartments of the kidney (glomeruli, tubules, vessels or interstitium)
- Small dangerous B cell or plasma cell clones that are below the detection threshold for conventional hematopathologic work up
- Reference: Nat Rev Nephrol 2019;15:45
- Acute renal failure
- Acute on chronic renal failure
- Proteinuria
- Edema
- Anemia
- Fanconi syndrome
- Arthralgia
- Dizziness
- Nausea and vomiting
- Peripheral neuropathy
- Lymphadenopathy (for certain low grade B cell lymphomas)
- References: Clin J Am Soc Nephrol 2018;13:128, Clin J Am Soc Nephrol 2016;11:2260
- Urinalysis
- 24 hour urine collection
- Detection of monoclonal free light chains (kappa or lambda, never both) in blood or urine
- Kidney biopsy examination by light microscopy, immunofluorescence and electron microscopy
- Bone marrow examination
- Flow cytometry of blood or bone marrow
- Cytogenetics and FISH studies
- Immunoelectron microscopy (where available)
- Laser dissection tandem mass spectrometry (LDTMS), where available
- References: Neth J Med 2019;77:243, Blood 2012;120:4292, Nat Rev Nephrol 2019;15:45
- Increased urinary protein (subnephrotic or nephrotic)
- Microscopic hematuria
- Increased serum creatinine
- Abnormal serum calcium, bicarbonate, chloride, phosphate and uric acid levels
- Renal tubular acidosis
- Bence-Jones protein in urine
- Serum / urine protein electrophoresis (SPEP / UPEP) showing an abnormal M spike protein
- Serum or urine immunofixation showing an abnormal kappa or lambda light chain (never both) with or without a complete monoclonal immunoglobulin (IgG, IgM, IgA)
- Elevated free kappa (K) or lambda (L) light chain with elevated K/L or L/K ratio
- Low serum complement levels (not always)
- Positive serum cryoglobulin level
- Blood or bone marrow flow cytometry showing light chain restriction, either for K or L
- Cytogenetics and FISH studies may reveal MYD88 mutation, cyclin D1 mutation, IGHV mutation, del(17p) mutation, hyperdiploidy, trisomy 21 mutation, etc.
- LDTMS shows K or L restriction in biopsy tissue
- References: Neth J Med 2019;77:243, N Engl J Med 2021;384:1931
- In response to treatment, a greater hematologic response of the patient's primary hematolymphoid disease translates to a greater probability of recovery of kidney function
- Favorable factors for renal recovery include no minimal residual disease at all (reduction of involved light chain level at In patients with MGRS who did not receive appropriate hematologic treatment prior to kidney transplantation, recurrence in transplant kidney is common
- References: N Engl J Med 2021;384:1931, Am J Kidney Dis 2022;79:202
- 44 year old woman with nephrotic syndrome and decreased renal function (Rom J Morphol Embryol 2017;58:1065)
- 54 year old man with progressive bone pain and renal dysfunction (Medicine (Baltimore) 2018;97:e12027)
- 55 year old hypertensive woman presenting with generalized edema, proteinuria and recent history of seizure (Indian J Nephrol 2014;24:376)
- 57 year old man with recent diagnosis of hypercalcemia and presenting with lethargy, anorexia and right flank pain (Pathology 2020;52:283)
- 75 year old man with known chronic kidney disease who presents with rapid decline in renal function and proteinuria (BMC Nephrol 2018;19:129)
- Aim of MGRS treatment is to preserve or improve kidney function by targeting the B cell or plasma cell clone
- Current consensus supports clone directed therapy over general immunosuppressive therapy
- Patients with plasma cell clones and MGRS: treatment with bortezomib is indicated
- Patients with B cell clones (expressing CD20) and MGRS: rituximab based therapy is indicated
- Supportive care for hypertension, proteinuria and impairment of mineral metabolism
- References: N Engl J Med 2021;384:1931, Neth J Med 2019;77:243
- Various morphologic subtypes, depending on location within kidney and physical structure
- Deposits are mostly monoclonal and composed of light chains (in most MGRS subtypes)
- Deposits may occasionally be nonmonoclonal (C3 glomerulopathy with monoclonal gammopathy)
- Deposits may rarely be absent (thrombotic microangiopathy, monoclonal gammopathy associated)
- Deposits may be organized (deposits with substructure - fibrils, microtubules, crystals) or nonorganized (solid amorphous or powder-like)
- Common morphologic subtypes include
- AL or AH or AHL amyloidosis (L for light chain, H for heavy chain)
- Fibrillary glomerulonephritis (FGN), monoclonal type
- Immunotactoid glomerulonephritis (ITGN), monoclonal subtype
- Cryoglobulinemic glomerulonephritis, type I and type II (type 1 and 2 CryoGN)
- Light chain proximal tubulopathy (LCPT), crystalline and noncrystalline type
- Monoclonal immunogloubulin deposition disease (MIDD), presenting either as light chain deposition disease (LCDD, common) or heavy chain deposition disease (HCDD, rare)
- Proliferative glomerulonephritis with monoclonal immunoglobulin deposits (PGNMID)
- C3 glomerulopathy with monoclonal gammopathy (C3G)
- Crystal storing histiocytosis (CSH)
- Crystalglobulin induced nephropathy (CIN) / crystalglobulinemic glomerulonephritis
- Thrombotic microangiopathy, monoclonal gammopathy associated (TMA)
- Patterns of glomerular pathology (glomerulonephritis / glomerulopathy) include
- Membranoproliferative glomerulonephritis (PGNMID, ITGN, C3G, CryoGN, FGN)
- Mesangioproliferative glomerulonephritis (PGNMID, FGN, ITGN)
- Endocapillary proliferative glomerulonephritis (PGNMID)
- Membranous glomerulonephritis (rare, PGNMID)
- Nodular glomerulosclerosis pattern (AL or AH amyloid, MIDD)
- Variable nonnodular mesangial or paramesangial expansion (AL amyloid, FGN)
- Mesangiolysis, capillary double contours and intracapillary microthrombi (TMA)
- Crescentic glomerulonephritis (AH amyloid, PGNMID, FGN)
- Capillary wall spikes or eyelash sign (AL amyloid)
- Crystals occluding glomerular capillary loops (CIN)
- Cryoplugs occluding glomerular capillary loops (CryoGN)
- Crystal laden histiocytes occluding glomerular capillary loops (CSH, glomerular type, rare)
- Patterns of tubular pathology include
- Acute tubular injury (LCPT)
- Crystals within proximal tubular epithelium (LCPT)
- Pale material in tubular basement membranes (AL or AH amyloid)
- Amyloid cast within lumina (rare)
- Patterns of vascular pathology include
- Pale waxy material in vessel wall (AL or AH amyloid)
- Thrombus in vessel lumina (TMA)
- Crystal within vessel lumina (CIN)
- Patterns of interstitial pathology include
- Interstitial amyloid (AL or AH amyloid)
- Monoclonal plasma cell or B cell infiltrate (as in chronic lymphocytic leukemia, for example)
- Infiltrate by crystal laden histiocytes (CSH, interstitial type, more common)
- Reference: Nat Rev Nephrol 2019;15:45
Contributed by Rajib K. Gupta, M.D., Ashley Flowers, M.D. and Lois J. Arend, M.D., Ph.D.
- Frozen section immunofluorescence (IF) confirms light chain restriction (K or L, never both)
- Immunofluorescence after pronase digestion (pronase IF) should also be performed if available to confirm light or heavy chain restriction
- Light chain restriction on IF may be seen in the form of granular or smudgy staining for K or L in the glomerular mesangium or capillary walls, linear staining of K or L in the tubular basement membranes, granular staining for K or L in tubular cytoplasmic inclusions or intraluminal K or L staining of cryoplug or crystal
- In C3G, there will be solitary IF staining for C3 only or dominant staining for C3 but no light chain staining
- IgG subclass (IgG1, IgG2, IgG3 and IgG4) staining should be performed in all cases with IgG dominant deposits (e.g., PGNMID; HCDD; HLCDD, Type 1 Cryogobulenemic GN)
- Heavy light chain antibody staining may be useful but still in validation phase
- References: Nat Rev Nephrol 2019;15:45, Kidney Int 2021;100:155
Contributed by Rajib K. Gupta, M.D. and Ashley Flowers, M.D.
- Kappa & lambda light chains
- Positivity by routine (frozen) or pronase IF of glomeruli or tubules
- IHC staining of interstitial histiocytes in CSH
- IHC staining of tubular basement membranes in MIDD (not very sensitive)
- In situ hybridization (ISH) staining of interstitial clonal plasma or B cells when present
- Weak PAS positive (AL amyloid) or strong PAS positive (FGN)
- May be silver positive (AL amyloid, FGN)
- Congo red positive staining of glomeruli, tubules, vessel walls and interstitium in AL or AH amyloid
- Thioflavin T positive staining (similar to Congo red stain) observed under fluorescent microscope
- DNAJB9 staining in FGN
- References: Clin J Am Soc Nephrol 2018;13:128, J Am Soc Nephrol 2016;27:1555, Arch Pathol Lab Med 2010;134:512, Ann Diagn Pathol 2019;43:151403
- Congo red and thioflavin T stains negative in all nonamyloid fibrillary MGRS cases (ITGN and most cases of FGN)
- Nonfuchsinophilic (trichrome negative) - both AL amyloid and FGN
- May be silver negative (AL amyloid, FGN)
- References: Clin J Am Soc Nephrol 2018;13:128, Arch Pathol Lab Med 2010;134:512
- Electron dense amorphous deposits in mesangium and capillary loops, subepithelial or subendothelial (PGNMID, C3G)
- Extremely dense contiguous intramembranous deposits in the capillary glomerular basement membrane (dense deposit variant of C3G)
- Randomly arranged fine electron dense fibrils (7 - 12 nm diameter) in mesangium and within capillary loop glomerular basement membranes, usually in subendothelial or intramembranous locations or rarely in subepithelial locations as spikes (AL / AH amyloid)
- Randomly arranged electron dense fibrils (15 - 30 nm) in mesangium and within capillary loop glomerular basement membranes, subendothelial or intramembranous locations (FGN)
- Microtubules (hollow cylinders) arranged in stacks in mesangium and capillary loops with fibrillary diameter of 10 - 90 nm (immunotactoid GN)
- Microtubules arranged in mesangium, capillary loop glomerular basement membranes (subendothelial) or as occlusive deposits within capillary lumina (CryoGN)
- Powdery amorphous punctuate deposits along glomerular and tubular basement membranes (MIDD / LCDD)
- Crystals (electron dense or electron lucent) within tubular epithelial cells (crystalline variant of LCPT)
- Abnormal shaped lysosomes or mitochondria within tubular epithelial cells (noncrystalline variant of LCPT)
- Electron dense crystals with lattice substructure occluding glomerular capillary loops and small interstitial vessels (CIN)
- Subendothelial electron lucent widening without any deposits and loose mesangial matrix (TMA)
- Reference: Nat Rev Nephrol 2019;15:45, Arch Pathol Lab Med 2010;134:512, Kidney Int 2004;65:85, J Am Soc Nephrol 2016;27:1555, Kidney Int 2018;94:178, J Am Soc Nephrol 2015;26:525
Contributed by Rajib K. Gupta, M.D., Ashley Flowers, M.D., Judy King, M.D., Ph.D. and Xin Gu, M.D.
- Kidney, needle biopsy:
- Primary diagnosis (AL amyloid / PGNMID / LCPT, etc.), associated with kappa or lambda light chain restriction (as the case may be) (see comment)
- Comment: Light microscopy, immunofluorescence (frozen or pronase) and electron microscopy support the above findings. Congo red stain is positive (AL or AH amyloid diagnosis) or negative (nonamyloid MGRS diagnosis). The findings are consistent with the patient's documented history of X (if patient has a known diagnosis of hematolymphoid neoplasm) or clinical correlation with appropriate hematologic studies is recommended (if there is no known hematolymphoid diagnosis).
- Diabetic (nodular) glomerulosclerosis:
- Strong PAS and silver positive mesangial nodules seen on light microscopy, although has an overall nodular glomerulosclerosis pattern
- Mesangial nodules are Congo red negative (positive in AL amyloid) and DNAJB9 negative (positive in FGN, monoclonal type)
- Often these mesangial nodules show peripheral microaneurysms which are not seen in any of the MGRS entities
- Immunofluorescence does not show any light chain restriction for either kappa or lambda
- More than 90% of the time, electron microscopy shows only diffuse nodular expansion of mesangial matrix without any fibrillary substructure
- Rarely, fibrillary substructure may be seen in mesangium (diabetic fibrillosis) but the fibrillary substructure is never seen within capillary loop glomerular basement membranes (intramembranous or subendothelial)
- Membranoproliferative glomerulonephritis (MPGN) due to infection:
- Patient has preceding history of recent infection
- No serologic evidence of monoclonal gammopathy
- No light chain restriction seen on immunofluorescence
- Cryoglobulinemic GN in hepatitis C:
- Patient usually has history of hepatitis C
- No serologic evidence of monoclonal gammopathy
- On serology, cryoglobulin type is usually type 2 or type 3 but typically never type 1
- No light chain restriction seen on immunofluorescence
- Hyaline in blood vessel wall in hypertensive nephrosclerosis:
- Hyaline in blood vessel wall is Congo red negative on light microscopy
- Vessel wall does not show any light chain restriction on immunofluorescence
- On electron microscopy, the vessel wall does not show AL amyloid type fibrillary substructure
- Fibronectin glomerulopathy:
- No serologic evidence of monoclonal gammopathy
- Mutation in fibronectin FN1 gene may be present
- No light chain restriction seen on immunofluorescence
- Mesangial lobules or nodules intensely fuchsinophilic (trichrome positive) and silver negative
- Electron microscopy shows amorphous or granular deposits in mesangium and subendothelial space but no fibrillary deposits akin to AL amyloid, FGN or ITGN
A 70 year old man with known hypertension presents with slow rising creatinine, nephrotic range proteinuria and microscopic proteinuria. Serology is negative for ANA, ANCA, viral hepatitis markers and anti-GBM antibody. Serum protein electrophoresis shows an abnormal M spike. A kidney biopsy is done. Light microscopy shows pale nodular expansion of the mesangium of the glomeruli which is weak PAS positive and silver and trichrome negative and Congo red stain positive, while immunofluorescence shows positive staining of glomeruli and interstitial vessel walls for lambda light chain and negative staining for all other immunoreactants, including for kappa light chain. An electron microscopy image of the glomerulus is given above. What is the likely diagnosis here?
- Amyloid light chain (AL) amyloidosis, lambda restricted
- Light chain deposition disease, lambda restricted
- Light chain proximal tubulopathy, lambda restricted
- Type 1 cryoglobulinemic glomerulonephritis, lambda restricted
Comment Here
Reference: Monoclonal gammopathy of renal significance (MGRS) / paraprotein related kidney disease
- Prominent granular staining of glomerulus for IgG and C3 on routine immunofluorescence
- Prominent granular staining of proximal tubular cytoplasms for C1q on routine immunofluorescence
- Prominent granular staining of proximal tubular cytoplasms for kappa on pronase immunofluorescence
- Prominent granular staining of proximal tubular cytoplasms for lambda on routine (frozen) immunofluorescence
Comment Here
Reference: Monoclonal gammopathy of renal significance (MGRS) / paraprotein related kidney disease
- Also called hypocomplementemic, lobular or mesangiocapillary glomerulonephritis
- Histologic lesion, not a specific disease entity
- MPGN should be diagnosed with specific etiology or underlying cause (such as C3GN, immune complex mediated, monoclonal), not as types I - III (Kidney Int 2012;81:434, N Engl J Med 2012;366:1119)
- A morphologic pattern of glomerular injury, characterized by endocapillary and mesangial hypercellularity, mesangial and subendothelial deposits and duplicating of glomerular basement membrane
- Idiopathic membranoproliferative glomerulonephritis (MPGN) thought to be a diagnosis of exclusion
- Either immunoglobulin (polyclonal or monoclonal) mediated or complement mediated
- Immunoglobulin mediated: due to infections, autoimmune diseases, paraproteinemias
- Complement mediated: dysregulation of alternative pathway due to genetic or acquired abnormalities in regulatory factors
- Traditional classification based on electron microscopy findings: MPGN type I (subendothelial and mesangial deposits), MPGN II (intramembranous dense ribbon-like deposits) and MPGN III (subendothelial and subepithelial deposits)
- Newer classification based on immunofluorescence emphasizing pathophysiology: immune complex / monoclonal immunoglobulin mediated MPGN (activation of classic complement pathway) and C3 glomerulopathies (including dense deposit disease, C3 glomerulonephritis and CFHR5 nephropathy, activation of alternate complement pathway)
- MPGN lesion due to immune complex now referred to simply as MPGN
- Poor prognostic signs including nephrotic syndrome, an elevated serum creatinine and hypertension at presentation, crescents and tubulointerstitial disease on biopsy
- 28 year old woman with fever, weight gain of 13 kilograms, lower extremity edema, hepatosplenomegaly and multicentric peripheral lymphadenopathy (Front Immunol 2019;10:1489)
- 46 year old Chinese man presented with bilateral tumor-like nodules (Ren Fail 2019;41:126)
- Left kidney, native, needle core biopsy:
- Immune complex mediated membranoproliferative glomerulonephritis (type I) (see comment)
- Cellular / fibrocellular crescent (4/15), fibrous crescents (0/15)
- Global glomerulosclerosis (1/15), segmental glomerulosclerosis (0/15)
- Interstitial fibrosis with tubular atrophy and interstitial fibrosis, mild
- Arteriosclerosis, mild
- Comment: Light microscopy shows glomeruli with diffuse endocapillary hypercellularity and focal cellular crescents. Indirect immunofluorescence studies show diffuse global capillary granular staining of IgG (3+) and C3 (2+) with polyclonal light chain expression and negative staining for IgA, IgM and C1q. Electronic microscopic examination shows subendothelial, mesangial electron dense deposits and mesangial interpositioning. The findings are consistent with type I MPGN. The patient is a young male with no history of infections, autoimmune diseases, paraproteinemias or other known causes of MPGN. An idiopathic MPGN is favored at this point.
- C3 glomerulopathy:
- Abnormal control of complement activation, deposition or degradation and characterized by predominant glomerular C3 fragment deposition (C3c intensity ≥ 2 orders of magnitude more than any other immune reactant) with electron dense deposits on electron microscopy
- Overlapping with type I and III MPGN (Kidney Int 2013;84:1079)
- Thrombotic microangiopathy (TMA):
- MPGN pattern injury with no immunoglobulins or complements by immunofluorescence or electron microscopy, commonly seen in the chronic phase
- MPGN pattern of injury with discrete subendothelial and mesangial electron dense deposits
- Mostly immune complex deposition indicating aviation of classic complement pathway and some alternative complement pathway (overlaps with C3 glomerulonephritis)
- Distinguished from new category of C3 glomerulopathies by prominent Ig or C1q
- Primary MPGN mostly affecting adolescents and young adults
- > 50% with nephrotic syndrome
- 10 - 20% with acute nephritis syndrome
- ~50% with low C3
- May be secondary to chronic infections (e.g. hepatitis C), autoimmune diseases (e.g. SLE), paraproteinemias, alpha-1-antitrypsin deficiency and malignancies
- ~50% renal survival at 10 years
- Recurs in ~30% of children 6 - 12 months after transplantation
- MPGN due to a monoclonal gammopathy or complement mediated disease with a higher risk of graft recurrence than immune complex mediated MPGN secondary to infection or autoimmune disease
Case reports
- 5 year old girl with selective IgA deficiency and MPGN (BMC Nephrol 2020;21:68)
- 43 year old woman with recurrent membranoproliferative glomerulonephritis after kidney transplantation due to ventriculoatrial shunt infection (BMC Nephrol 2019;20:296)
Treatment
- Most patients with immune complex mediated MPGN have an identifiable underlying cause, such as a chronic infection, autoimmune disease or monoclonal gammopathy
- Such patients should receive therapy directed against the underlying cause of the MPGN, since resolution of the MPGN usually occurs after successful treatment of the primary disease
- For idiopathic MPGN, patients with mild disease may need only conservative therapy with antihypertensive or antiproteinuric regimens
- Patients with heavy proteinuria (> 3 grams/day) or nephrotic syndrome, immunosuppressive therapy in addition to conservative therapy is suggested, such as glucocorticoids and calcineurin inhibitors
- Patients presenting with a rapidly progressive crescentic MPGN should receive immunosuppressive therapy without delay, since they are at high risk for progression to end stage kidney disease if untreated
- We treat such patients with pulse IV methylprednisolone followed by daily oral prednisone and cyclophosphamide (Kidney Int 2020;98:1135)
Microscopic (histologic) description
- Mesangial and endocapillary hypercellularity with lobular accentuation
- Irregular thickening of glomerular basement membrane by interposition of mesangial cells between endothelium and basement membrane
- Forming double contour / tram track appearance (PAS or silver stain)
- Crescents in ~20% cases
- Neutrophils (exudate) may present
- May have immune complex aggregates forming hyaline thrombi in capillary lumina
- Reference: Nat Rev Nephrol 2015;11:14
Microscopic (histologic) images
Contributed by Haiyan Zhang, M.D., Ph.D.
Contributed by Nephropath
Immunofluorescence description
- Lumpy bumpy (granular) for C3, IgG, early complement (C1q, C4) at subendothelial and mesangial areas
Immunofluorescence images
Contributed by Haiyan Zhang, M.D., Ph.D. and Nephropath
Electron microscopy description
- Subendothelial and mesangial electron dense deposits, mesangial interpositioning with splitting and duplication of basement membrane, possible increased mesangial matrix, variable degree of foot process effacement
Electron microscopy images
Contributed by Haiyan Zhang, M.D., Ph.D. and Nephropath
- Essential diagnostic feature based on the presence of highly electron dense ribbon-like deposits of the glomerular basement membrane
- Now categorized under C3 glomerulopathies
- Could be familial or associated with partial lipodystrophy (~5%)
- Due to uncontrolled activation of alternative complement pathway: autoantibodies, e.g. C3 nephritic factor detected in 80 - 85% cases (stabilizes C3 convertase; promotes C3 degradation); gene mutation complement component, e.g. factor H
- Can be acquired by infections or monoclonal paraprotein
- Average age at diagnosis: 14 years
- Typically present with renal insufficiency, nearly all with hematuria and 33% with nephrotic syndrome
- Also deposits in basement membranes of spleen, choroid and Bruch membrane of retina
- Poorer prognosis than type I; 50% have renal failure in 10 years; 80 - 100% recur after renal transplant
Case reports
- 9 year old girl presented with macrohematuria and persistent proteinuria (Clin Nephrol Case Stud 2020;8:96)
- 35 year old man with partial lipoatrophy presented with leg edema and oliguria (Kidney Int 2020;98:1355)
Treatment
- Currently, no targeted therapies are available in clinical settings for the treatment of C3 glomerulopathy, although several new molecules are under investigation
- Treatment with corticosteroids plus mycophenolate mofetil has been shown to be associated with improved renal outcomes, as compared with other immunosuppressive regimens
- Yet, the main determinants of treatment response with this regimen and the influence of the underlying pathogenic drivers have not been extensively studied
- Therapeutic response to eculizumab, an anti-C5 monoclonal antibody, has been shown to be highly heterogeneous (Nephron 2020;144:272)
Microscopic (histologic) description
- Similar to type I but less prominent cellular proliferation
- Eosinophilic, refractile and ribbon-like thickening of glomerular basement membrane and basement membrane of Bowman capsule and tubules
- Strongly PAS+, stains red with trichrome but dense deposits are pale with silver stain
- Reference: Am J Kidney Dis 2015;66:e21
Microscopic (histologic) images
Contributed by Haiyan Zhang, M.D., Ph.D.
Contributed by Nephropath
Immunofluorescence description
- C3 only or C3 dominant
- C3 irregularly along capillary wall in ribbon-like, granular or discontinuous pattern
- C3 in mesangium with characteristic globular pattern
- Broad linear deposits of TBM (~60%) or Bowman capsule (~30%)
- Reference: Am J Kidney Dis 2015;66:e21
Immunofluorescence images
Contributed by Haiyan Zhang, M.D., Ph.D.
Electron microscopy description
- Highly dense deposits in lamina densa of glomerular basement membrane with ribbon / sausage string appearance
- Nodular deposits of similar material in mesangium
- Similar deposits in TBM (~50%) or Bowman capsule (~45%)
- Reference: Am J Kidney Dis 2015;66:e21
Electron microscopy images
Contributed by Haiyan Zhang, M.D., Ph.D.
Images hosted on other servers:
- MPGN pattern with subendothelial and subepithelial deposits
- Some overlapping with new category of C3 glomerulopathies
- 2 rare morphologic subtypes (Anders and Burkholder variants) that are clinically similar to each other and to type I membranous glomerulonephritis
- Reference: Case Rep Nephrol Dial 2019;9:15
- Hybrid of type I and II MPGN
Electron microscopy description
- Intramembranous deposits in addition to subendothelial and mesangial deposits
- Deposits extending from subendothelial to subepithelial, GBM lamina densa disruption with laminated / woven appearance
Electron microscopy images
Images hosted on other servers:
- Combined features of type I MPGN and membranous glomerulonephritis
- Associated with hepatitis C and HIV coinfection
Electron microscopy description
- Subepithelial deposits similar to membranous glomerulonephritis in addition to subendothelial and mesangial deposits
Electron microscopy images
Images hosted on other servers:
A 15 year old boy presents with nephritis syndrome with rising creatine. Urine protein 3 g/24 h, urine red blood cells 5 - 10/high power field, hepatitis B and C negative, C3 decreased, normal C4, ANCA negative, anti-GBM negative, no M spike by serum protein electrophoresis or urine protein electrophoresis. Renal biopsy is performed. Light microscopy shows diffuse global endocapillary hypercellularity with rare cellular crescent. Immunofluorescence studies show IgG weak-1+, IgM weak, IgA negative, C3+ (granular, capillary and mesangial), C1q negative, kappa weak and lambda weak. Electron microscopy shows intramembranous and mesangial deposits as in the picture. What is the most acute diagnosis?
- C3 glomerulonephritis
- Dense deposit disease
- Immune complex mediated membranoproliferative glomerulonephritis
- Thrombotic microangiopathy
- Endothelial cell
- Mesangial cell
- Parietal epithelial cell
- Podocyte / visceral epithelial cell
- Technically, disease of increased renal calcium levels (eMedicine: Nephrocalcinosis [Accessed 02 January 2018]), but usually refers to chronic tubulointerstitial nephropathy of tubular calcium phosphate deposition causing slowly progressive renal insufficiency
- Less common than nephrolithiasis
- Hypercalcemia can also cause glomerular calcium deposition and glomerulosclerosis (Arch Pathol Lab Med 2003;127:E80)
- Hyperphosphaturia and hypercalciuria types differ histologically (Nephrol Dial Transplant 2012;27:1122)
- Endoscopy may be required to distinguish nephrolithiasis (Urol Res 2010;38:421)
- Conditions associated with chronic hypercalcemia, including primary hyperparathyroidism, myeloma or other malignancy; also distal renal tubular acidosis, hypercalciuria due to chronic furosemide use in premature infants (Hum Pathol 2000;31:1363), milk alkali syndrome, oral sodium phosphate as bowel preparation (Hum Pathol 2004;35:675), sarcoidosis, vitamin D intoxication
- Reduce urinary saturation of calcium by increasing fluid intake, crystallization inhibitors, disease-specific medication, (Kidney Int 2011;80:1278)
- Diffuse tubular injury with atrophy, interstitial fibrosis and abundant tubular deposition of calcium phosphate
- Over time, glomerulosclerosis and vascular disease
- Group of autosomal recessive disorders characterized by corticomedullary cysts, atrophy and interstitial fibrosis
- "Phthisis" (Greek): dwindling or wasting away
- Also referred to as juvenile nephronophthisis medullary cystic kidney disease complex
- 1:50,000
- Most common genetic cause of pediatric end stage renal disease (ESRD)
- Mutations in NPHP genes (produce nephrocystins in cilia / basal body structures, (Eur J Hum Genet 2009;17:406, J Am Soc Nephrol 2007;18:1855), which cause defects in signaling mechanisms that result in defects of planar cell polarity and tissue maintenance (J Am Soc Nephrol 2009;20:23)
- Juvenile form: most common; presents in childhood with polyuria / polydipsia due to cortical and tubulointerstitial damage that progresses to chronic renal failure in 5 - 10 years; no gender preference; also anemia and growth retardation; 12% associated with retinitis pigmentosa
- Adolescent form: similar to juvenile with later (mean, 19 years) presentation
- Infantile: bilateral disease due to cortical cysts with renal failure by 3 years of age; may have extrarenal presentation including retinitis pigmentosa, hepatic fibrosis, skeletal / CNS malformations, situs inversus, etc.
- 4 year old girl with basal ganglia calcification and pancreatic lipomatosis (Arch Pathol Lab Med 1988;112:630)
- Renal transplant for ESRD (Pediatr Transplant 2008;12:878)
- Normal or small kidneys with firm, granular cortices and corticomedullary cysts
- Infantile forms have enlarged kidneys with cortical cysts
- Severe tubular atrophy with thick basement membranes, interstitial fibrosis and chronic inflammation
- Minimal to advanced glomerulosclerosis
- Cysts at corticomedullary border lined by flattened or cuboidal epithelium
- Liver biopsy may show congenital hepatic fibrosis
- Mutations in NPHP2 and NPHP3 genes (Kidney Int 2009;75:839), also associated with retinitis pigmentosa, intellectual disability, cerebellar ataxia, bone anomalies and liver fibrosis
- Juvenile subtype associated with mutations in NPHP1 (OMIM 256100) (chromosome 2q), 4 (chromosome 1p)
- Infantile subtype associated with NPHP2 (chromosome 9q)
- Adolescent subtype associated with NPHP3 (chromosome 3q)
- Homogenously thickened tubular (NOT glomerular) basement membrane, split into thin lamellae, reticulated or disintegrated
Images hosted on other servers:
Tubules:
Glomeruli:
- Glomerulomegaly with or without accompanying focal segmental glomerulosclerosis occurring in the setting of obesity with or without the metabolic syndrome
- Glomerulomegaly is the pathological hallmark (Kidney Int 2001;59:1498)
- Hypertrophied glomeruli that fill the microscopic visual field at 40x objective lens
- Often secondary / maladaptive focal segmental glomerulosclerosis present (Nephron 2017;136:273)
- Segmental patchy podocyte foot process effacement (Nephron 2017;136:273)
- Proteinuric presentation, most often subnephrotic
- Obesity related glomerulopathy
- Metabolic syndrome related glomerulopathy
- ICD-10: N08 - glomerular disorders in diseases classified elsewhere
- Associated with obesity and preterm birth (Nat Rev Nephrol 2016;12:453)
- 46% of patients have class 1 or class 2 obesity and 54% have class 3 obesity (Kidney Int 2001;59:1498)
- Kidney
- Congenital or functional low nephron endowment (Kidney Int 2000;58:770)
- Glomerular filtration rate (GFR) is increased in order to maintain adequate natriuresis in obesity
- Leads to adaptive hemodynamic modulations and glomerular hypertension
- Chronic glomerular hypertension causes nephron injury, which manifests as glomerular hypertrophy, basement membrane thickening and podocyte damage
- Subsequent podocyte loss from continued mechanical injury eventually leads to podocyte loss and segmental sclerosis (Kidney Int 1992;42:148, Am J Hypertens 1988;1:335)
- May also be contributions from endocrinopathies of metabolic syndrome
- Obstructive sleep apnea may also be a contributing factor
- Obesity (Nat Rev Nephrol 2016;12:453)
- Metabolic syndrome
- Possibly congenital reduced nephron mass (e.g. preterm birth) (Nephrology (Carlton) 2013;18:180)
- Middle aged adults (35 - 45 years) (Am J Kidney Dis 2008;52:58)
- Slowly progressive proteinuria (Kidney Int 2001;59:1498)
- Proteinuria often subnephrotic but may reach nephrotic range
- Nephrotic syndrome is rare
- Slow decline in renal function
- Sometimes present without overt clinical disease
- Diagnosis is made on renal biopsy (Kidney Int 2008;73:947)
- Can be suspected clinically in obese patients with proteinuria
- Patients commonly present with proteinuria, sometimes in nephrotic range
- Median estimated glomerular filtration rate (eGFR) of 30 ml/min/1.73 m² (Kidney Int 2019;95:647)
- Body mass index (BMI) is directly proportional to risk of end stage renal disease
- 37 year old Caucasian man with a BMI of 38 kg/m² presented to general nephrology clinic with a 3+ proteinuria on urinalysis (Oxf Med Case Reports 2020;2020:omz148)
- 49 year old morbidly obese Caucasian man was found to have obstructive sleep apnea, proteinuria and renal insufficiency (Am J Kidney Dis 1987;10:470)
- 69 year old Caucasian man, with previous normal renal function, suffering from diabetes mellitus, obesity (BMI = 38 kg/m²), hypercholesterolemia, hypertension and coronary artery disease started hemodialysis because of severe acute renal failure (Bioimpacts 2015;5:155)
- Weight loss
- Renin angiotensin system blockade
- Bariatric surgery
- Glomerular hypertrophy in 100% of cases (Kidney Int 2001;59:1498)
- Cross sectional glomerular diameter of > ½ 40x high power field
- Often perihilar segmental sclerosis (Nephron 2017;136:273)
- Reduced glomerular density (Clin J Am Soc Nephrol 2012;7:735)
- Focal global glomerulosclerosis
- Mild thickening of glomerular basement membrane (Kidney Int 2001;59:1498)
- Mild mesangial sclerosis may be present
- Variable interstitial fibrosis and tubular atrophy
- Variable arteriosclerosis and arteriolosclerosis
- Focal afferent arteriole dilation (Am J Physiol Renal Physiol 2000;278:F817)
- Tubular hypertrophy may also be present
- Glomeruli negative for immune complex deposits
- Nonspecific accumulation of IgM and complement C3 in sclerotic and hyalinized lesion (Kidney Int 2001;59:1498)
- Podocytes may contain intracytoplasmic protein resorption droplets that stain for IgG, IgA and albumin
- Podocytes exhibit segmental foot process effacement (25 - 40%)
- Mild thickening of glomerular basement membrane
- Mild mesangial sclerosis
- Podocytes and mesangial cells will contain lipid droplets (Lancet Diabetes Endocrinol 2014;2:417)
- Lipid vacuoles can be seen in the tubular epithelial cells of the proximal tubule
- No immune complex deposits
- Unknown at this time
- Left kidney, native, needle core biopsy:
- Glomerulomegaly with secondary focal segmental glomerulosclerosis (FSGS), perihilar variant consistent with obesity related glomerulopathy (see comment)
- Comment: In addition to obesity, other clinical risk factors for these biopsy findings may include obstructive sleep apnea, chronic hypertension and diabetes. These findings may also reflect situations of congenital or acquired states of low nephron number (e.g. low birth weight) or congenital susceptibility to maladaptive glomerular injury.
- Primary focal segmental glomerulosclerosis (FSGS) (Kidney Int 2001;59:1498):
- Sudden onset of proteinuria
- Hypoalbuminemia and edema
- > 75% podocyte foot process effacement
- Diabetic nephropathy:
- Glomerulomegaly in early diabetic nephropathy
- Hyperglycemia and elevated A1C
- Hyalinization of afferent and efferent arterioles
- Difficult to distinguish on morphologic grounds alone
- Sickle cell anemia (N Engl J Med 1992;326:910):
- Glomerulomegaly may be present
- Sickled red blood cells may be present in capillaries
- Requires clinical correlation
- Congenital cyanotic heart disease (Nephrol Dial Transplant 2002;17:144):
- Glomerulomegaly may be present
- Requires clinical correlation
- Congenital renal hypoplasia / oligomeganephronia (Nephrol Dial Transplant 2011;26:2202):
- Glomerulomegaly may be present
- Tubular hypertrophy may also be present
- Kidneys may be small
- Absence of obesity
- Difficult to exclude in obese patients
- Other etiologies of secondary forms of FSGS:
- Due to arteriosclerosis / ischemia / chronic glomerulonephritis
- Perihilar segmental sclerosis
- Glomerulomegaly may not be present
- Often more severe interstitial fibrosis / tubular atrophy and vascular sclerosis than obesity related glomerulopathy
- Some genetic forms of FSGS:
- ACTN4 mutations associated FSGS often exhibit perihilar FSGS (J Am Soc Nephrol 2009;20:961)
- Irregularly aggregated electron dense material in the podocyte cytoplasm
- Autosomal dominant, correlation with family history and genetic testing may be required
- Other etiologies of glomerular hypertrophy:
- Preterm birth
- Sickle cell disease (N Engl J Med 1992;326:910, Kidney Int 1985;27:711)
- Congenital cyanotic heart disease (Nephrol Dial Transplant 2002;17:144)
A morbidly obese patient has a renal biopsy for subnephrotic range proteinuria that has been gradually worsening. Her serologic workup is negative. A renal biopsy is performed and is significant for the histologic finding shown above. Immunofluorescence is negative and there are no electron dense deposits or glomerular basement membrane abnormalities by light microscopy. What is the most likely diagnosis?
- IgA nephropathy
- Lupus nephritis
- Minimal change disease
- Obesity related glomerulopathy
A 50 year old Caucasian woman is found to have mild proteinuria during routine urinary screening. Her serologic workup is negative. A renal biopsy is performed and is significant for the finding shown above. Immunofluorescence is negative and there are no electron dense deposits or glomerular basement membrane abnormalities by light microscopy. In which clinical settings is this biopsy finding typically seen?
- Advanced diabetic disease
- Elevated BMI
- Lupus
- Mild essential hypertension
- Obstructive uropathy is structural or functional hindrance of normal urine flow, sometimes leading to renal dysfunction (obstructive nephropathy)
- Acute obstruction increases susceptibility to infection; chronic obstruction causes hydronephrosis
- Causes: bladder neck obstruction, congenital (meatal stenosis, anterior / posterior urethral valves [Pediatr Surg Int 2009;25:613], ureteropelvic junction narrowing, urethral strictures, vesicoureteral reflux); cystocoele, inflammation, neurogenic ureter / bladder, pregnancy, prostatic hypertrophy, sloughed papillae or blood clots, stones, tumors, uterine prolapse
- Symptoms: none if unaffected kidney maintains adequate function; pain due to distention of collecting system or renal capsule; early - nocturia, polyuria; after relief of obstruction, may get massive diuresis with sodium wasting
- Hydronephrosis: cystic dilation of renal pelvis and calyces associated with progressive atrophy of kidney, due to obstructive uropathy
- Thin cortical rim due to atrophy
- More calyceal dilation if incomplete obstruction, since glomerular filtration is not suppressed
- Initial functional alterations are tubular, cause interstitial inflammatory infiltrate
- Chronic changes are cortical atrophy, diffuse interstitial fibrosis and blunting of calyces
- Excess oxalate accumulation in the kidney resulting in acute and chronic tubulointerstitial injury
- May be primary genetic (primary hyperoxaluria) resulting in oxalate overproduction or secondary due to excess intake or decreased clearance of oxalate
- Detection of excess calcium oxalate crystals in the kidney
- Crystals are rhomboid / fan shaped and polarizable
- Primary: hyperoxaluria, hyperoxalosis, primary hyperoxaluria
- Secondary: oxalate nephropathy, oxalosis
- Primary:
- Autosomal recessive
- Prevalence is < 3:1,000,000 individuals worldwide (J Am Soc Nephrol 2015;26:2559)
- 1 - 2% of all children with end stage renal disease (N Engl J Med 2013;369:649, Clin J Am Soc Nephrol 2012;7:458)
- 3 types (Nat Rev Nephrol 2012;8:467)
- Type 1 is the most common (~80%) and most severe form
- Type 2 (~10%)
- Type 3 (~10%)
- Secondary:
- 5 - 24% of all patients with gastrointestinal diseases associated with malabsorption including those with a history of (Nephrol Dial Transplant 2016;31:375)
- Bariatric surgery
- Celiac
- Crohn's
- Pancreatic insufficiency
- Cystic fibrosis
- 5 - 24% of all patients with gastrointestinal diseases associated with malabsorption including those with a history of (Nephrol Dial Transplant 2016;31:375)
- Primary:
- Insoluble calcium oxalate salts deposit primarily in the kidneys
- Other sites include retina, myocardium, vascular walls, skin, bone and central nervous system (Kidney Int 2009;75:1264)
- Secondary:
- Kidney, both native and allograft
- Primary:
- Defective glyoxylate metabolism in hepatocytes resulting in excessive oxalate production
- Oxalate is renally cleared as calcium salts
- Excess oxalate results in oversaturation leading to crystallization in the renal tubules
- Crystals cause direct renal tubular toxicity and obstruction resulting in acute tubular injury and chronic renal failure
- Urolithiasis may also occur
- Secondary:
- Mechanism of renal injury is similar to primary
- Excess intake of oxalate results in increased deposition within the kidney, usually mild
- Enteric hyperoxaluria (Nephrol Dial Transplant 2016;31:375):
- Fat malabsorption results in increased calcium sequestration in the gut resulting in increased oxalate absorption from the small intestine and, to some degree, colon
- Free fat and bile acids may also contribute to colonic permeability of oxalate
- Oxalate degrading bacteria such as Oxalobacter formigenes may be a factor (Kidney Int 2013;83:1144)
- Primary:
- 3 most common subtypes are caused by mutations in the AGXT (type 1), GRHPR (type 2) and HOGA1 (type 3) genes
- Secondary:
- Increased oxalate intake of foods or supplements rich in oxalate (Curr Rheumatol Rep 2013;15:340)
- Rhubarb
- Parsley
- Spinach
- Black tea
- Vitamin C (an oxalate precursor)
- Ethylene glycol toxicity
- Increased gut absorption of oxalate in malabsorptive states (enteric hyperoxaluria)
- Bariatric surgery
- Inflammatory bowel disease (Crohn's disease)
- Celiac disease
- Pancreatic insufficiency
- Short gut syndrome
- Increased oxalate intake of foods or supplements rich in oxalate (Curr Rheumatol Rep 2013;15:340)
- Acute renal failure
- Chronic renal failure
- Recurrent nephrolithiasis
- In primary disease, clinical presentation and disease course is quite variable (Am J Nephrol 2005;25:290)
- Median age at symptom onset is ~6 years
- Onset reported to range from birth to > 50 years
- Renal biopsy demonstrating excess oxalate accumulation with compatible clinical history
- Presence of characteristic molecular findings in hereditary forms
- Urinalysis / stone analysis with oxalate stones
- Molecular testing
- Primary:
- Type 1 is most severe form
- Patients with the Gly170Arg or Phe152Ile mutation in AGXT gene have better overall outcome due to sensitivity to pyridoxine (Kidney Int 2005;67:1704)
- Type 2 has less severe course
- Type 3 is least severe; nephrocalcinosis and chronic kidney failure are uncommon (N Engl J Med 2013;369:649)
- Type 1 is most severe form
- Secondary (Kidney Int Rep 2018;3:1363):
- Prognosis is guarded with up to 58% progressing to end stage renal disease
- Complete recovery of renal function is rare
- 3 month old boy with hyperoxaluria type 1 and autosomal dominant polycystic kidney diesease (Pediatr Radiol 2011;41:107)
- 4 year old girl with end stage renal disease and primary hyperoxaluria type 1 (Pediatr Dev Pathol 2009;12:229)
- 51 year old man with hyperoxaluria nephropathy after Roux-en-Y bypass induced by vitamin C (Oxf Med Case Reports 2016;2016:omw054)
- 57 year old woman with oxalate nephropathy following vitamin C intake (Clin Nephrol 2017;88:354)
- 65 year old woman with oxalate rich green smoothie juice cleanse causing acute oxalate nephropathy (Am J Kidney Dis 2018;71:281)
- 67 year old man with calcium oxalate crystal related kidney injury after Roux-en-Y hepaticojejunostomy (BMC Nephrol 2017;18:106)
- 70 year old woman with calcium oxalate nephropathy due to short bowel syndrome and cholecystectomy (Case Rep Nephrol Dial 2018;8:147)
- Acute kidney injury due to oxalate nephropathy after chelating therapy with oral ethylenediamine tetra acetic acid (Am J Kidney Dis 2017;70:722)
- Acute oxalate nephropathy due to pancreatic atrophy in newly diagnosed pancreatic carcinoma (Hum Pathol 2016;48:163)
- Primary:
- Hydration
- Pyridoxine supplementation for type 1 (N Engl J Med 2013;369:649)
- Combined liver / kidney transplant (inevitable recurrence without liver transplantation in type 1 disease)
- Secondary:
- Elimination of sources of excess oxalate
- Enteric oxalosis (Nephrol Dial Transplant 2016;31:375):
- Oxalate binding agents, calcium
- Calcium supplementation
- Citrate supplements
- Hemodialysis
- Otherwise supportive care
- Specific findings:
- Translucent polyhedral, rhomboid and fan-like calcium oxalate crystals found within cortical and medullary tubular lumens and interstitial spaces
- Crystals are birefringent under polarized light
- Crystal deposition can be associated with giant cell reaction
- Occasionally arterial / arteriolar deposits (usually in cases of primary hyperoxaluria)
- Advanced primary hyperoxaluria generally shows massive oxalate deposition with extensive interstitial fibrosis / tubular atrophy
- Nonspecific findings:
- Acute tubular injury
- Interstitial fibrosis and tubular atrophy
- Generally mild tubulointerstitial inflammation
- Early primary hyperoxaluria difficult to distinguish from secondary causes
- No immunofluorescence staining of oxalate depositions
- Usual nonspecific background IgA and light chain staining of adjacent tubular casts
- von Kossa stain: oxalate crystals will be negative (calcium phosphate crystals will be positive)
- Oxalate crystals or outlines of crystals (may not survive processing) may be seen in tubule lumens or interstitial spaces
- Primary:
- All forms autosomal recessive
- AGXT gene mutations (type 1), which encodes the hepatic peroxisomal enzyme alanine:glyoxylate aminotransferase (AGT)
- GRHPR gene (type 2), which encodes the GRHPR enzyme
- HOGA1 gene (type 3), which encodes the mitochondrial 4-hydroxy-2-oxoglutarate (HOG) aldolase enzyme
- ~5% do not have demonstrable mutations in any of these 3 genes (N Engl J Med 2013;369:649, J Am Soc Nephrol 2015;26:2559)
- Left kidney, biopsy:
- Acute tubular injury (acute tubular necrosis) with marked calcium oxalate deposition suggestive of oxalate nephropathy
- Comment: The differential diagnosis for these findings includes both secondary and primary causes. Secondary hyperoxaluria / oxalate nephropathy can be seen in the setting of gastrointestinal malabsorption (enteric hyperoxaluria) or exogenous / dietary oxalate ingestion (e.g. excess vitamin C, rhubarb, parsley, spinach, beet greens, star fruit, nuts, black tea) usually in a background of underlying chronic kidney disease. Of note, oxalate deposition can also be seen in the setting of ethylene glycol ingestion. Primary oxaluria can present in adulthood; however, this possibility should be investigated after all secondary causes are excluded.
- Calcium phosphate:
- Purple crystals, not polarizable
- 2,8-Dihydroxyadenine (DHA) crystals / adenine phosphoribosyltransferase deficiency:
- Crystals polarizable, brown in color (Clin J Am Soc Nephrol 2012;7:1521)
- Urate crystals:
- Needle shaped birefringent crystal
- Positive for uric acid stain (Arch Pathol Lab Med 2000;124:774)
- Drug crystals:
- E.g. triamterene (Am J Kidney Dis 2014;63:148)
- Acute tubular injury:
- May show nonspecific calcium oxalate crystals in tubular lumens
- Usually less extensive deposition than in cases of true oxalosis
- May be difficult to distinguish from secondary oxalosis; requires clinical correlation
- Nonspecific accumulation can be seen in end stage kidney disease due to any cause:
- Usually more focal than in true oxalosis; clinical correlation
- Acute interstitial nephritis
- Obesity related glomerulopathy
- Oxalate nephropathy
- Phosphate nephropathy
- Urate nephropathy
You receive a liver and bilateral kidneys from an 8 year old boy undergoing combined liver / kidney transplantation. The liver shows no significant gross or histologic findings. The kidneys appear atrophic with a gritty cut surface. Microscopic examination reveals extensive rhomboid and fan shaped translucent crystals that are polarizable and shows extensive cortical scarring. This boy most likely suffers from which of the following conditions?
- Adenine phosphoribosyltransferase deficiency
- Congenital nephrocalcinosis
- Primary hyperoxaluria type 1
- Primary hyperoxaluria type 2
- Uromodulin kidney disease (ADTKD-UMOD)
- Pauci-immune necrotizing crescentic glomerulonephritis related to or caused by antineutrophil cytoplasmic antibody (ANCA)
- Pauci-immune necrotizing crescentic glomerulonephritis (renal biopsy: gold standard)
- Rapidly progressive glomerulonephritis with hematuria and proteinuria
- Small vessel necrotizing vasculitis associated with ANCAs that can be renal limited or with systemic vasculitis: granulomatous with polyangiitis, microscopic polyangiitis, eosinophlic granulomatous with polyangiitis or renal limited vasculitis (J Am Soc Nephrol 2010;21:1628)
- Pauci-immune glomerulonephritis
- Pauci-immune crescentic glomerulonephritis
- ANCA associated vasculitis
- ICD10: NO1.7 - rapidly progressive nephritic syndrome with diffuse crescentic glomerulonephritis
- By far the most common cause of rapidly progressive glomerulonephritis in adults (Kidney Int 2003;63:1164)
- 80% of pauci-immune crescentic glomerulonephritis is associated with ANCA (Kidney Int 2003;63:1164)
- Crescentic glomerulonephritis occurs in 75 - 80% of patients with granulomatosis with polyangiitis, 80 - 100% of microscopic polyangiitis and 25 - 40% of eosinophilic granulomatosis with polyangiitis (Semin Arthritis Rheum 2005;35:95, Clin Rheumatol 2017;36:1949)
- Age: > 60 years (53%); 21 - 60 years (39%); Kidney Int 2003;63:1164)
- No gender predilection
- Geographic variation of antimyeloperoxidase and proteinase 3 (PR3) ANCA (Mod Rheumatol 2010;20:54)
- Kidney glomeruli
- ANCA is a primary pathogenic factor, mainly by augmenting leukocyte endothelial interactions (Mod Rheumatol 2010;20:54)
- In vitro evidence:
- ANCA IgG can activate cytokine primed neutrophils by interacting with myeloperoxidase (MPO) or PR3 at the surface of the cells
- Endothelial injury by ANCA activated neutrophils and disruption of glomerular capillary walls
- Alternative complement pathway activation by ANCA activated neutrophils (Nat Rev Rheumatol 2014;10:463)
- Trigger for ANCA production generally unknown
- Presumed immune dysregulation (Th17 / Treg) (Eur J Immunol 2017;47:724)
- Microbial factors and molecular mimicry (J Immunol Res 2015;2015:858027, Nat Med 2008;14:1088)
- Can be precipitated by drugs: propylthiouracil, hydralazine, minocycline, levamisole adulterated cocaine (Curr Opin Rheumatol 2014;26:42, Curr Opin Rheumatol 2013;25:50)
- Suggested risk factors
- Distinct HLA class II associations for MPO and PR3 ANCA (Am J Kidney Dis 2013;62:1176)
- Leptin receptor gene (LEPR) polymorphism (Rheumatology (Oxford) 2010;49:907)
- CTLA4 and PTPN22 (single nucleotide polymorphisms) (BMC Med Genet 2009;10:121)
- Epigenetic control of MPO and PR3 expression, both neutrophil cytoplasmic enzymes (J Clin Invest 2010;120:3209, Presse Med 2015;44:e223)
- Rapid deterioration of renal function
- Oliguria, hematuria and proteinuria (usually nonnephrotic range)
- Flu-like syndrome common at onset (fever, arthralgia, myalgia)
- Signs of extrarenal vasculitis in ~ 75% (Mod Rheumatol 2010;20:54, Semin Arthritis Rheum 2005;35:95)
- Microscopic polyangiitis: renal involvement (90%), lung usually affected, also skin, ear, nose, throat, musculoskeletal, nervous system, gastrointestinal
- Granulomatosis with polyangiitis: kidney, upper airway and lung involvement in 90% of cases
- Eosinophilic granulomatosis with polyangiitis: four phases - allergic, eosinophilic, vasculitic, postvasculitic (Colvin: Diagnostic Pathology - Kidney Diseases, 2nd Edition, 2015)
- Rapidly progressive glomerulonephritis clinically
- Pauci-immune crescentic glomerulonephritis pathologically
- Positivity for ANCA (Clin Rheumatol 2017;36:1949)
- Extrarenal clinical manifestations in systemic vasculitis
- Positive ANCA test by indirect immunofluorescence plus enzyme linked immunosorbent assays in serum
- Sensitivity of 80% and specificity of 96% for pauci-immune glomerulonephritis
- Negative ANCA in ~ 20% of pauci-immune glomerulonephritis
- Type of ANCA does not permit specific diagnosis
- MPO ANCA most common in pauci-immune glomerulonephritis, microscopic polyangiitis and eosinophilic granulomatosis with polyangiitis (50 - 60%)
- PR3 ANCA most common in granulomatosis with polyangiitis (~ 75%)
- Other (atypical) ANCA specificities described
- React with lactoferrin, elastase and P-cathepsin-G in serum
- Found in variety of conditions with chronic inflammation or infection
- Negative ANCA associated with absence of disease activity
- Normal complement levels in serum
- Peripheral eosinophilia in 10 - 20% of microscopic polyangiitis
- References: Kidney Int 2000;57:846, Clin Rev Allergy Immunol 2013;45:109
- ~ 75% achieve remission
- ~ 30% relapse, frequently with PR3 ANCA
- 60 - 75% 5 year patient and kidney survival
- Risk factors for early death
- Higher serum creatinine or dialysis dependence at onset
- Female gender
- Age > 65 years (J Am Soc Nephrol 2006;17:1224, Clin Exp Rheumatol 2004;22:S94, J Am Soc Nephrol 2010;21:1628)
- Pathological prognostic features
- Number of normal glomeruli (strong predictor of renal function)
- Active lesions are associated with renal recovery: active glomerular necrosis and crescents, higher in granulomatosis with polyangiitis
- Chronic lesions are associated with poor renal prognosis: glomerulosclerosis higher in microscopic polyangiitis or MPO ANCA than in granulomatosis with polyangiitis or PR3 ANCA patients (Clin Rheumatol 2017;36:1949, Nephrol Dial Transplant 2005;20:96)
- 19 year old woman with PR3 vasculitis presenting with symptomatic renal wedge infarction (BMC Nephrol 2019;20:84)
- 55 year old man with MPO ANCA associated necrotizing glomerulonephritis in rheumatoid arthritis (J Nephropathol 2017;6:58)
- 59 year old man with myeloperoxidase ANCA negative microscopic polyangiitis with pulmonary hemorrhage and IgA nephropathy (Case Rep Dermatol 2011;3:22)
- 66 year old woman with de novo ANCA associated vasculitis after renal transplantation (BMC Nephrol 2018;19:270)
- 67 year old woman with giant cell arteritis and granulomatosis with polyangiitis (Am J Case Rep 2018;19:651)
- 78 year old woman with ANCA associated necrotizing glomerulonephritis overlapping with mesangial proliferative lupus nephritis (Case Rep Rheumatol 2018;2018:3076806)
- Cyclophosphamide and prednisolone to induce remission
- Maintenance therapy with less toxic drugs such as mycophenolate mofetil, azathioprine
- Plasmapheresis or plasma exchange in refractory cases
- Rituximab (anti-CD20) for induction or relapses (Nat Rev Rheumatol 2014;10:484)
- Pathological classification (J Am Soc Nephrol 2010;21:1628)
- Focal (> 50% normal glomeruli)
- Crescentic (> 50% cellular or fibrocellular crescents):
- Crescents containing > 10% cellularity included
- Fibrous crescents not counted
- Mixed (heterogeneous glomerular lesions, none predominating as > 50%)
- Sclerotic (> 50% global glomerulosclerosis, defined as > 80% of capillary tuft sclerosed)
- Focal segmental fibrinoid necrosis (glomerular basement membrane is disrupted in areas of necrosis)
- Extracapillary proliferation with the accumulation of macrophages and epithelial cells in Bowman space (age of crescents: cellular, fibrocellular, fibrous)
- Karyorrhectic debris and fibrin thrombi are frequently seen within the affected glomerular capillary lumens
- Active periglomerular inflammation and rupture of Bowman capsule
- Sometimes periglomerular granulomatous inflammation
- Normal glomeruli usually present
- Endocapillary hypercellularity, typical of immune complex mediated glomerulonephritides, is lacking
- Variable inflammation, predominantly composed of lymphocytes, histiocytes, plasma cells and sometimes brisk number of eosinophils
- Granulomas suggest the possibility of underlying granulomatous with polyangiitis or eosinophilic granulomatous with polyangiitis
- Note that an apparent interstitial granuloma adjacent to a disrupted Bowman capsule does not carry the same connotation
- Vasculitis 5 - 35%; involves small arteries, arterioles, capillaries, venules
- Interlobular arteries usually site affected in systemic vasculitis
- Leukocytoclastic vasculitis pattern (neutrophils, fibrinoid necrosis)
- Necrotizing, leukocytoclastic angiitis of the medullary vasa recta (frequently associated with interstitial hemorrhage and the presence of neutrophilic tubulitis and neutrophils within tubular lumens) (Colvin: Diagnostic Pathology: Kidney Diseases, 2nd Edition, 2015, Zhou: Silva's Diagnostic Renal Pathology, 2nd Edition, 2017)
- Immunohistochemistry not used for diagnosis
- PAS, Jones silver and trichrome are used to evaluate morphology but are not specific for the type of glomerular disease
- Pauci-immune glomerular pattern (weak ≤ 1+) granular staining for IgG, IgM, IgA, C3 and C1q
- There is no evidence of glomerular immunodeposits
- Active crescents and fibrinoid necrosis stain for fibrin
- Reference: Am J Pathol 1989;135:921
- Electron microscopy generally contributes little, mainly recapitulating the changes seen on light microscopy
- Subendothelial edema, microthrombosis and degranulation of neutrophils are present but immune deposits are absent (J Am Soc Nephrol 2010;21:1628)
- Left kidney, biopsy:
- Focal necrotizing and crescentic ANCA glomerulonephritis
- Adequacy: adequate (cortex 80%, medulla 20%)
- Microscopic description: 13 glomeruli, 5 of these exhibited crescents (one with fibrinoid necrosis), including 2 cellular crescents and 3 fibrocellular crescents. Fibrosis occupying 30% of the interstitium with minimal lymphoplasmacytic infiltrate. There was no evidence of extraglomerular arteritis.
- Immunofluorescence microscopy: Number of glomeruli: 3. There were no deposits of IgA, IgM, IgG, C3, C1q or fibrin.
- Focal necrotizing and crescentic ANCA glomerulonephritis
- ANCA negative crescentic glomerulonephritis:
- Serologic testing for ANCA negative
- Antiglomerular basement membrane disease (anti-GBM):
- Bright, linear staining of glomerular basement membranes for IgG
- Vasculitis absent
- Immune complex mediated crescentic glomerulonephritis:
- 2+ or greater of Ig and complement
- Some due to specific diseases such as lupus, cryoglobulinemia, IgA nephropathy, membranoproliferative glomerulonephritis
- ANCA superimposed on other diseases:
- Membranous glomerulonephritis, IgA nephropathy, lupus nephritis may have crescents and necrosis due to superimposed ANCA glomerulonephritis
- Drug induced pauci-immune crescentic glomerulonephritis (CEN Case Rep 2016;5:188)
- Thrombotic microangiopathies:
- Subendothelial widening along the glomerular capillaries with endothelial cell swelling and loss of fenestration by ultrastructural examination
Comment Here
Reference: Pauci-immune complex crescentic glomerulonephritis / ANCA associated vasculitis
- Which of the following signs and symptoms are common in ANCA related glomerulonephritis?
- Edema
- Lipiduria
- Nephrotic range proteinuria
- Rapidly progressive renal failure
Comment Here
Reference: Pauci-immune complex crescentic glomerulonephritis / ANCA associated vasculitis
- Diseases that recur or develop de novo in the renal allograft
- Recurrent or de novo diseases can injure the renal allograft
- Most native kidney diseases can recur or develop de novo in the allograft, including cardiovascular diseases / hypertension, diabetes, metabolic diseases, infectious diseases, drug toxicities, perfusion problems and cancer, in addition to de novo / recurrent primary glomerular diseases
- Cause of end stage renal disease has to be known in order to differentiate recurrence from de novo disease
- Morphology is similar to those in the native kidney but frequently has additional features related to allograft rejection or other complications of transplantation
- Clinical course of the disease usually differs from those of the native kidney due to presence of iatrogenic immunosuppression
- Recurrent "name of disease entity" of the renal allograft: original disease that damaged the native kidney recurring in the allograft
- De novo "name of disease entity" of the renal allograft: development of a new disease in the allograft, different than that which led to end stage renal disease
- Unknown: when native kidney disease is not known
- ICD-10: T86.19 - other complication of kidney transplant
- With improvement in treatment of rejection related complications, recurrent and de novo diseases have become a significant contributing factor to graft dysfunction (Transplantation 1999;68:635)
- Today recurrent / de novo cardiovascular diseases and cancer are the most common cause of morbidity and mortality
- Focal segmental glomerulosclerosis, IgA nephropathy, membranous glomerulonephritis and membranoproliferative glomerulonephritis are among the most common recurrent glomerular diseases that lead to graft loss (Nat Clin Pract Nephrol 2008;4:446)
- True incidence of recurrent or de novo diseases has not been clearly established, predominantly due to complexity in initial classification of the primary disease leading to end stage kidney
- Figures vary widely between studies and with regard to disease subtype; exact figures are hard to determine due to
- Anonymity of primary cause of end stage renal disease
- Possibility of subclinical disease going underdiagnosed where protocol biopsies are not performed (Nephrology (Carlton) 2014;19:6)
- Possibility of newly developed disease being donor derived
- Coexistence or morphological overlap of certain primary renal diseases and forms of rejection (World J Transplant 2017;7:285)
- Differences in morphology of early recurrent / de novo disease (especially those observed in protocol biopsies of asymptomatic patients) from those of clinically symptomatic diseases in the native kidney (Kidney Int 2017;91:304)
- Lower utilization of immunofluorescence and electron microscopy in transplant biopsies
- Risk of recurrence of glomerular disease is higher in patients with living related transplantation (BMC Nephrol 2017;18:25)
- Better HLA compatibility is also associated with increased risk of recurrence of glomerular disease but longterm outcome is better despite this drawback (Kidney Int 2018;93:482)
- Estimated rates given, in a comprehensive review article, in relation to glomerular diseases can be seen in table 1 below (Nephrology (Carlton) 2014;19:6)
- Individual disease entities
- Membranous nephropathy
- Recurrence rates vary (10 - 50%); recurrence usually takes place within the first year
- Most cases of de novo membranous nephropathy develop after the first year
- Detectable serum phospholipase A2 receptor before or at transplantation or reemergence after transplantation increases risk of recurrence
- Focal segmental glomerulosclerosis
- Recurrence rates vary: 30 - 80%, higher in pediatric patients and in patients with primary / idiopathic focal segmental glomerulosclerosis; lower in those with secondary or genetic focal segmental glomerulosclerosis in the native kidney (Pediatr Transplant 2010;14:314, Front Immunol 2019;10:1944)
- Usually develops within the first 2 years posttransplant
- Risk factors for recurrence are (Nephrol Dial Transplant 2006;21:1053, Transplant Proc 2007;39:737)
- Childhood onset disease
- Age < 15 years
- Rapid progression from diagnosis to end stage renal disease
- Presence of mesangial hypercellularity
- Recurrence of focal segmental glomerulosclerosis in a previous allograft (risk of recurrence increases to 80 - 100%)
- Presence or absence of nephrotic syndrome in presentation of native focal segmental glomerulosclerosis (Clin J Am Soc Nephrol 2021;16:1730)
- Subclass of focal segmental glomerulosclerosis: primary / idiopathic, genetic and secondary; risk of recurrence being highest in primary / idiopathic focal segmental glomerulosclerosis
- Focal segmental glomerulosclerosis may also develop / progress over time, in which case separation of recurrent versus de novo disease can be problematic
- IgA nephropathy
- IgA deposition is frequently encountered in the renal allograft and recurrence rates vary between 10 - 53% depending on whether protocol or indication biopsies are involved and the follow up period (Nephrology (Carlton) 2018;23:4, Front Immunol 2019;10:1944)
- Risk of recurrence increases with time after transplant
- Latent IgA deposition should be differentiated from IgA nephropathy
- Latent IgA deposition: mesangial IgA deposition in an asymptomatic patient (no hematuria or proteinuria) established by protocol biopsies
- IgA nephropathy: IgA deposition in a patient with urinary abnormalities
- Membranoproliferative glomerulonephritis - immune complex mediated (BMC Nephrol 2016;17:7)
- Polyclonal immunoglobulins
- Recurrence risk is lower (30 - 35%)
- Presents later, during the first 5 years
- Monoclonal immunoglobulins
- Recurrent disease is more common, 66%
- Usually occurs within the first year
- Risk of recurrence is correlated with
- Living related donation: possible genetic background
- Preemptive transplants
- Low complement levels
- Presence of monoclonal gammopathy
- Polyclonal immunoglobulins
- C3 glomerulopathy (World J Transplant 2016;6:632)
- Genetic C3 glomerulopathy has high rates of recurrence (up to 66.7%) (J Am Soc Nephrol 2014;25:1110)
- Thrombotic microangiopathy (Curr Opin Organ Transplant 2014;19:283, Transplant Rev (Orlando) 2018;32:58)
- Most cases are due to development of de novo thrombotic microangiopathy
- Risk of recurrence depends on etiology
- Typical hemolytic uremic syndrome due to infections rarely recurs (Pediatr Nephrol 2002;17:809)
- Recurrence is most common in atypical hemolytic uremic syndrome; overall recurrence is 60% and usually occurs during the first year posttransplant (Clin J Am Soc Nephrol 2006;1:88)
- Risk of atypical hemolytic uremic syndrome changes in relation to genetic abnormality; i.e., risk of recurrence is 4 times higher in patients with mutations in complement genes (Am J Transplant 2013;13:663)
- ADAMTS13 deficiency: if deficiency is genetic, disease can recur (BMC Nephrol 2013;14:156)
- De novo immune complex glomerulonephritis, unclassified (Hum Pathol 2015;46:1521, Hum Pathol 2018;71:109)
- Various immune complexes and complement components can be identified in the renal allograft with immunofluorescence
- Although the majority of immune complex glomerulonephritis in the allograft have a correlate in the native kidney, some do not and are not readily classifiable
- Cardiovascular diseases and posttransplant diabetes mellitus: a common cause of mortality and morbidity in kidney transplant recipients
- Infectious diseases: especially opportunistic microorganisms
- Cancer (Nat Rev Nephrol 2018;14:508)
- Is the second most common cause of mortality and morbidity in kidney transplant recipients
- Kidney transplant recipients have at least a twofold higher risk of developing or dying from cancer than the general population
- Types with greatest risk increase are: Kaposi sarcoma, nonmelanoma skin cancers, lip cancer (> 10 times) and cancers with viral oncogenesis (posttransplant lymphoproliferative disorder and anogenital cancers)
- Membranous nephropathy
- Renal disease
- Persistence of original inciting agents related to glomerular diseases increases the chances of recurrence of glomerular disease; examples are the presence of
- Putative circulating factors that are toxic to the podocyte in primary / idiopathic focal segmental glomerulosclerosis (Am J Transplant 2013;13:266)
- Resulting podocyte injury is thought to be reversible early in the disease course
- Antiphospholipase A2 receptor antibody in membranous glomerulonephritis (Transplantation 2015;99:1709, Nephrol Dial Transplant 2014;29:2334, Clin Transplant 2016;30:1394)
- High titer of antiglomerular basement membrane antibody in antiglomerular basement membrane antibody glomerulonephritis (J Am Soc Nephrol 2001;12:394)
- Paraproteins / free light chains and abnormal kappa/lambda ratio in AL amyloidosis and other glomerulopathies due to paraproteins (Nephrology (Carlton) 2014;19:6)
- Persistence of high level acute phase reactants in AA amyloidosis (Transplantation 2020;104:1703)
- Low ADAMTS13 activity in atypical hemolytic uremia syndrome (Nephrology (Carlton) 2014;19:6)
- Mutations of or autoantibodies to complement regulatory factors in atypical hemolytic uremia syndrome and C3 nephropathies (Clin J Am Soc Nephrol 2006;1:88)
- Genetic deficiency of liver peroxisomal enzyme alanine:glyoxylate aminotransferase in primary hyperoxaluria (World J Nephrol 2015;4:235)
- Autoantibodies directed against phospholipid binding proteins in antiphospholipid syndrome (Nat Rev Nephrol 2014;10:279)
- Poor diabetic control in diabetic nephropathy (Diabetes 1983;32:948)
- Poor blood pressure control in hypertensive nephropathy
- Putative circulating factors that are toxic to the podocyte in primary / idiopathic focal segmental glomerulosclerosis (Am J Transplant 2013;13:266)
- Certain factors are associated with risk of de novo disease
- Hyperfiltration (chronic allograft injury with graft loss, transplantation of pediatric kidney into adult), vascular diseases (allografts from diseased donors) and medications (calcineurin inhibitors, mTOR inhibitors) for focal segmental glomerulosclerosis (World J Transplant 2017;7:285, Clin Transplant 2011;25:6)
- Antiglomerular basement membrane antibody disease in transplants for Alport disease due to development of antibodies against α3, α4 or α5 chains of collagen IV of the allograft (Clin J Am Soc Nephrol 2017;12:1162)
- Risk is higher in patients with large COL4A5 gene deletions and genetic testing is recommended to detect risk pretransplant (J Am Soc Nephrol 2013;24:364)
- Steroid / immunosuppression use: diabetic nephropathy (Diabetes Metab Syndr Obes 2011;4:175)
- Thrombotic microangiopathy due to use of calcineurin inhibitors and antibody mediated transplant rejection (Transplant Rev (Orlando) 2018;32:58, Exp Clin Transplant 2018;16:131)
- Individual disease entities
- Membranous nephropathy (Clin Transplant 2016;30:1394)
- IgG4 is dominant in recurrent disease (similar to primary membranous nephropathy), whereas IgG1, IgG2 and IgG3 are dominant in de novo disease (similar to secondary membranous nephropathy) (Transplant Proc 2011;43:3743)
- There is lack of phospholipase A2 receptor in de novo disease (Transplantation 2013;95:1259)
- De novo disease can be associated with Alport syndrome, ureteral obstruction, newly acquired hepatitis B virus, hepatitis C virus and rejection
- It is hypothesized that renal injury (including alloimmune injury due to transplant reactions) may result in exposure of hidden epitopes and autoantibody production (Transpl Int 2012;25:1205)
- Focal segmental glomerulosclerosis
- Recurrent primary / idiopathic focal segmental glomerulosclerosis is thought to be associated with continued presence of circulating factors and can take place within hours after transplantation, presenting as heavy proteinuria (Am J Transplant 2013;13:266, Clin J Am Soc Nephrol 2010;5:2115)
- De novo focal segmental glomerulosclerosis is similar to secondary focal segmental glomerulosclerosis and is associated with multiple pathogenetic factors that can be seen in the allograft much like those in the native kidney
- These factors include decreased nephron mass and hyperfiltration injury, obesity, hypertension, diabetes, urinary reflux, infections and medications (World J Transplant 2017;7:285, Clin Transplant 2011;25:6, Indian J Nephrol 2015;25:82)
- Membranoproliferative glomerulonephritis (BMC Nephrol 2016;17:7)
- Classical or lectin complement system is involved in the pathogenesis of immune complex mediated type whereas the alternative complement system is involved in the pathogenesis of C3 glomerulopathy
- Type of membranoproliferative glomerulonephritis (immune complex mediated versus alternative complement mediated / C3 glomerulopathy) may change after transplant
- Thrombotic microangiopathy (Curr Opin Organ Transplant 2014;19:283, Transplant Rev (Orlando) 2018;32:58)
- De novo thrombotic microangiopathy development is associated with
- Medications
- Antibody mediated rejection
- Infections
- Acquired deficiencies in complement regulatory proteins or ADAMTS13 (rare) (Am J Transplant 2008;8:1694)
- De novo thrombotic microangiopathy development is associated with
- C3 glomerulopathy and atypical hemolytic uremic syndrome / thrombotic microangiopathy (World J Transplant 2016;6:632)
- Associated with genetic or acquired abnormalities of the complement system
- Posttransplant diabetes mellitus and diabetic nephropathy
- De novo disease development is frequently associated with the use of immunosupression (Diabetes Metab Syndr Obes 2011;4:175)
- De novo immune complex glomerulonephritis, unclassified (Hum Pathol 2015;46:1521, Hum Pathol 2018;71:109)
- These cases are sometimes associated with infections and alloimmune injury
- Cancer: risk of developing de novo or recurrent cancer is multifactorial and associated with (Nat Rev Nephrol 2018;14:508)
- Oncogenic viruses
- Immunosuppression and altered T cell immunity
- Membranous nephropathy (Clin Transplant 2016;30:1394)
- References: Kidney Int 2017;91:304, Transplant Proc 2013;45:3
- Diseases may recur / present subclinically and be diagnosed on protocol biopsies - histological and immunophenotypic recurrence / presentation
- Clinical picture may be complicated due to accompanying rejection
- Individual disease entities
- Membranous nephropathy: proteinuria and antiphospholipase A2 receptor (PLA2R) can be used to monitor recurrence (Clin Transplant 2016;30:1394)
- Thrombotic microangiopathy: clinical triad of findings (hemolytic anemia, thrombocytopenia and acute kidney injury) may be incomplete or absent and require biopsy for diagnosis (Curr Opin Organ Transplant 2014;19:283, Transplant Rev (Orlando) 2018;32:58)
- De novo immune complex glomerulonephritis, unclassified: these patients usually have nonnephrotic proteinuria and are biopsied due to allograft dysfunction (Hum Pathol 2015;46:1521, Hum Pathol 2018;71:109)
- Similar to that of individual native kidney diseases; however, take into consideration indication of biopsy (protocol biopsy versus biopsy due to clinical symptoms) and accompanying alterations due to the effects of
- Immunosuppressive agents (most notably calcineurin inhibitors)
- Immunological injury
- Posttransplant infections (especially viral)
- Ischemia reperfusion injury
- Hyperfiltration injury
- Abnormalities in urine analysis: proteinuria, hematuria, leukocyturia
- Abnormalities in serum creatinine, albumin and protein levels
- No abnormal lab findings in early disease recurrence / de novo development (established via protocol biopsies)
- Recurrent glomerulonephritis is associated with increased risk of graft failure
- Allograft glomerulopathy in general, both de novo and recurrent is responsible for about 25% of death censored kidney allograft failures and is among the top 3 leading causes of graft failure
- Early age at transplantation is a major risk factor for recurrence / de novo disease development
- Persistence of original inciting agents related to glomerular diseases adversely effects prognosis
- Lupus has a very good prognosis (Biomed Res Int 2014;2014:746192)
- Membranous nephropathy has a good prognosis, possibly due to maintenance immunosuppression (Clin Transplant 2016;30:1394)
- Focal segmental glomerulosclerosis, membranoproliferative glomerulonephritis and atypical hemolytic uremia syndrome have the worst prognosis (Transplantation 1999;68:635)
- Development of posttransplant antiglomerular basement membrane disease in patients with Alport disease has a high risk of graft loss (Pediatr Transplant 2006;10:651)
- IgA nephropathy
- Latent IgA deposition (asymptomatic, absence of hematuria and proteinuria) can be observed in the donated kidney and is not associated with worse prognosis even if mesangial expansion is present; most of these depositions disappear in about a year, which may be due to use of immunosuppression (Kidney Int 2003;63:2286, Clin Transplant 2013;27:14, Nephrology (Carlton) 2018;23:4)
- Presence of crescents is associated with worse graft outcome (Am J Transplant 2019;19:145)
- Membranoproliferative glomerulonephritis - immune complex mediated
- Polyclonal immunoglobulins: progresses slowly, risk of graft loss low (10%)
- Monoclonal immunoglobulins: aggressive course, graft loss due to disease is common (50%) (Kidney Int 2017;91:304)
- Alternative complement mediated membranoproliferative disease / C3 glomerulopathy and atypical hemolytic uremic syndrome / thrombotic microangiopathy (World J Transplant 2016;6:632)
- Genetic C3 glomerulonephritis that cannot be effectively controlled by immunosuppression, has a high rate of graft loss (50%) (J Am Soc Nephrol 2014;25:1110)
- Graft loss is more common in atypical hemolytic uremic syndrome in patients with mutations in the CFH gene or carriers of the hybrid gene CFH / CFHR1 (Am J Transplant 2013;13:663)
- Also see table 3 above (Front Immunol 2019;10:1944)
- 12 year old boy with de novo lupus nephritis (Transplant Proc 2014;46:648)
- 17 year old boy with de novo amyloidosis (Pediatr Transplant 2014;18:E259)
- 22 year old man with granulomatous interstitial nephritis (Transplant Proc 2016;48:946)
- 22 year old man with de novo C3 glomerulonephritis in a renal allograft with morphologic transformation to dense deposit disease (Ultrastruct Pathol 2016;40:112)
- 40 year old man, 42 year old woman and 53 year old man with successful renal transplantation for antiphospholipid syndrome (Medicine (Baltimore) 2016;95:e5419)
- 41 year old woman who had a late diagnosis of 2,8-dihydroxyadenine nephropathy induced end stage renal disease, made on the native nephrectomy that accompanied the renal transplant (Exp Clin Transplant 2017;15:574)
- 43 year old man with nephropathy of unknown etiology developed a nephrotic syndrome with kidney failure at 2 years followup (J Med Case Rep 2014;8:205)
- 47 and 53 year old women, transplant recipients treated with long term plasmapheresis therapy for management of recurrent focal segmental glomerulosclerosis (Transfus Apher Sci 2021;60:103046)
- 52 year old woman receiving retransplant of an allograft due to focal segmental glomerulosclerosis recurrence in the initial recipient (Am J Transplant 2018;18:2818)
- 55 year old man presented with rapid deterioration of renal function (Saudi J Kidney Dis Transpl 2016;27:381)
- 6 cases of kidney transplantation in patients with monoclonal immunoglobulin deposition disease (Am J Kidney Dis 2021;78:755)
- Maintenance immunosuppression to prevent rejection generally alters disease course in immune mediated glomerulonephritis in comparison to native kidneys
- Due to reduction of antibody levels, recurrence of some diseases, particularly systemic lupus erythematosus, antineutrophil cytoplasmic antibody associated vasculitis, IgA and membranous nephropathy, is lower and disease progression slower
- Concomitant or subsequent pancreas transplantation may be considered in diabetic nephropathy (N Engl J Med 1998;339:69)
- Concomitant or subsequent liver transplantation may be considered in oxalosis (Curr Med Res Opin 2014;30:2109)
- Membranous nephropathy (Clin Transplant 2016;30:1394, Clin J Am Soc Nephrol 2021;16:1730)
- Treatment at early stages is best to prevent progression (unlike observation due to possibility of spontaneous remission in primary membranous nephropathy); with effective treatment, recurrence does not portend worse graft survival
- Renin-angiotensin-aldosterone blockade recommended for all patients
- Rituximab is treatment of choice given allograft related immunosuppression (Clin J Am Soc Nephrol 2009;4:1083, Clin J Am Soc Nephrol 2021;16:1730)
- Recurrent focal segmental glomerulosclerosis
- Treatment involves plasmapheresis (especially for early aggressive disease) / immunoabsorption and anti-CD20 monoclonal antibodies (rituximab, ofatumumab); however, these therapies do not seem to be effective in prevention of disease in high risk patients (Artif Organs 2011;35:420, Transplantation 2018;102:e115, World J Transplant 2021;11:303)
- Thrombotic microangiopathy
- Address underlying etiology, including management of calcineurin or mTOR inhibitors; plasma exchange, intravenous immunoglobulin, complement inhibition (Curr Opin Organ Transplant 2014;19:283, Transplant Rev (Orlando) 2018;32:58)
- C3 glomerulopathy
- Plasma exchange and rituximab
- Eculizumab for patients with worsening or high grade proteinuria and decline in kidney function
- De novo immune complex glomerulonephritis, unclassified (Hum Pathol 2015;46:1521, Hum Pathol 2018;71:109)
- These cases are usually self limited and do not require additional therapy
- Also see table 4 above (Front Immunol 2019;10:1944)
- References: Transplant Proc 2013;45:3, Semin Dial 2000;13:195, Clin J Am Soc Nephrol 2021;16:1730
- Histology of early disease may be very subtle and different from that of typical findings one is used to seeing in native biopsies; strict adherence to diagnostic criteria can lead to delays in diagnosis
- Membranous nephropathy (Clin Transplant 2016;30:1394)
- Light microscopy: normal initially; development of basement membrane thickening, vacuolization and spikes later on
- Immunofluorescence: peripheral granular IgG, C4d, kappa and lambda staining
- Lack of phospholipase A2 receptor staining in de novo disease (Transplantation 2013;95:1259)
- Electron microscopy: only small subepithelial deposits initially, may even be absent; formation of well-formed subepithelial deposits later during the disease course
- De novo disease is more likely to have segmental distribution of immune deposits and accompanying mesangial hyperplasia / expansion
- Changes in relation to accompanying allograft related complications may also be present
- Focal segmental glomerulosclerosis (Adv Chronic Kidney Dis 2014;21:448, Clin Transplant 2011;25:6)
- Light microscopy: may resemble minimal change disease initially, with development of segmental sclerosis later on
- Recurrence is usually in the same morphological variant as that of the disease in the native kidney (J Am Soc Nephrol 2008;19:2219)
- Perihilar and not otherwise specified focal segmental glomerulosclerosis variants are more common in de novo disease (Clin Transplant 2011;25:6)
- Electron microscopy: ultrastructural findings of podocyte effacement can be seen very early (Transplantation 2012;93:1238)
- In general, proteinuria precedes the histologic findings of focal segmental glomerulosclerosis seen by light microscopy
- Changes in relation to accompanying allograft related complications may also be present (J Clin Diagn Res 2017;11:EC39)
- IgA nephropathy
- Light microscopy: near normal glomeruli to mesangial and endocapillary hypercellularity
- Immunofluorescence: dominant IgA in the mesangium
- Changes in relation to accompanying allograft related complications may also be present
- Membranoproliferative glomerulonephritis - immune complex mediated
- Early findings show sole mesangial proliferation without characteristic double contours of the glomerular capillary basement membrane
- Typical findings can evolve rapidly as disease progresses
- Changes in relation to accompanying allograft related complications may also be present
- Alternative complement mediated membranoproliferative disease / C3 glomerulopathy (World J Transplant 2016;6:632)
- Light microscopy: membranoproliferative pattern is common in C3 glomerulonephritis and chronic thrombotic microangiopathy
- Immunofluorescence: isolated C3 deposition in C3 glomerulonephritis
- Changes in relation to accompanying allograft related complications may also be present
- Thrombotic microangiopathy (Curr Opin Organ Transplant 2014;19:283, Transplant Rev (Orlando) 2018;32:58)
- Biopsy usually cannot identify the underlying cause (Curr Opin Organ Transplant 2014;19:283)
- Light microscopy: thrombi within glomerular capillaries, arteries / arterioles
- Bloodless glomeruli: glomerular capillary lumina occluded by endothelial swelling and amorphous material
- Fragmented red blood cells within arterial / arteriolar walls or glomeruli
- Severe mucoid intimal thickening of small arteries and arterioles
- Concentric thickening of the small arteries / arterioles
- Focal and segmental glomerular capillary thickening due to endothelial swelling (causing double contours)
- Immunofluorescence: negative
- Electron microscopy: subendothelial space expansion due to electron lucent material, new matrix formation and duplication of glomerular basement membranes
- Chronic thrombotic microangiopathy has a membranoproliferative pattern of glomerular injury and resembles transplant glomerulopathy
- Presence of donor specific antibodies, C4d staining in peritubular capillaries or microvascular inflammation represents chronic and active antibody mediated rejection, not chronic thrombotic microangiopathy
- Diabetic nephropathy
- Light microscopy: vascular changes predominate in recurrence; fewer mesangial nodules than in de novo disease (Transplantation 1996;62:632)
- Immunofluorescence: negative
- Changes in relation to accompanying allograft related complications may also be present
- De novo immune complex glomerulonephritis, unclassified (Hum Pathol 2015;46:1521, Hum Pathol 2018;71:109)
- Light microscopy: minimal mesangial expansion
- Immunofluorescence: mesangial deposits of IgM or IgG
Contributed by Arzu Sağlam, M.D.
- See disease specific descriptions above
- See disease specific descriptions above
- See disease specific descriptions above
- Genetic forms of focal segmental glomerulosclerosis have very low risk of recurrence, therefore, genetic testing may be considered in children and those adult onset focal segmental glomerulosclerosis patients where cause of focal segmental glomerulosclerosis is uncertain, especially if there is a family history
- Screening of the donors for patients undergoing live related donor transplantation may also be considered in these circumstances (Transplant Proc 2019;51:3077)
- Some studies suggest a genetic link between HLA antigens and risk of membranous nephropathy recurrence (N Engl J Med 2011;364:616, Kidney Int 2021;99:671, Kidney Int 2021;100:243)
- Increased risk of recurrence of atypical hemolytic uremic syndrome (HUS) in patients with mutations in complement factor genes and complement regulatory factor genes, therefore genetic complement testing is recommended for all atypical HUS patients undergoing transplant (Am J Transplant 2013;13:663)
Case #34 - HacettepePathology
- Kidney, allograft biopsy:
- Recurrent focal segmental glomerulosclerosis (see comment)
- No significant deposition on immunofluorescence microscopy. No evidence of T cell or antibody mediated rejection. Interstitial fibrosis / tubular atrophy absent. Arterial intimal fibrosis (mild).
- Microscopic description: Serial sectioning shows 26 glomeruli, one of which is globally sclerotic. 2 glomeruli with segmental sclerosis are identified; these segments display obliteration of the capillary tuft and adhesion to the Bowman membrane, accompanied by hyalinosis, lipid vacuoles and hypertrophy of the parietal epithelial cells. Other glomeruli appear close to normal by light microscopy. Endocapillary or extracapillary proliferation, necrosis, intracapillary inflammatory cells (g0), intracapillary thrombi or double contouring of the capillary walls are absent (cg0). Interstitium shows multifocal edema and minimal inflammatory infiltrate comprising < 10% of the nonscarred cortical parenchyma (i0). No significant tubulitis or peritubullary capillaritis is seen (t0, ptc0). Narrow foci of IFTA can be identified, comprising less than 10% of the cortical parenchyma with scattered mononuclear cells (i-IFTA0, ci0, ct0). Immunohistochemical staining for C4d and SV40 is negative. Arteries display mild intimal fibrosis (cv1). Intimal arteritis is not identified (v0).
- Immunofluorescence microscopy: 3 glomeruli observed
- Anti IgG Ab: No deposits
- Anti IgA Ab: No deposits
- Anti IgM Ab: Segmental peripheral 1+ granular staining (interpreted as nonspecific)
- Anti C3 Ab: No glomerular deposits, blush segmental reactivity on walls of arterioles
- Anti C1q Ab: No deposits
- Anti kappa Ab: No glomerular deposits, 2+ staining of tubular casts
- Anti lambda Ab: No glomerular deposits, 2+ staining of tubular casts
- Comment: Biopsy findings are consistent with recurrent focal glomerulosclerosis in this patient biopsied due to proteinuria during the early posttransplant period. There is no evidence of accompanying T cell or antibody mediated rejection or significant chronic tubulointerstitial damage. Vascular sclerosis is most probably donor related.
- De novo versus recurrence:
- Differentiation of late recurrence versus development of de novo disease may be challenging
- In glomerular diseases early onset (< 1 - 2 years after transplant) favors recurrent disease
- Antibody mediated rejection (ABMR) may have similar morphology with:
- Immune complex mediated glomerular disease with membranoproliferative pattern of injury:
- Can resemble chronic active ABMR
- Immunofluorescence findings of immunoglobulins or complement deposition provides evidence for immune complex or complement mediated disease
- Acute and chronic thrombotic microangiopathy:
- Can resemble active and chronic active ABMR, respectively
- Negative immunofluorescence
- Absence of microvascular inflammation, C4d staining in peritubular capillaries and donor specific antibodies
- Presence of microvascular inflammation (transplant glomerulitis, peritubular capillaritis and transplant glomerulopathy), C4d staining in peritubular capillaries, presence of donor specific antibodies in the serum and lack of glomerular immune complex deposition by immunofluorescence are characteristics of antibody mediated rejection
- Immune complex mediated glomerular disease with membranoproliferative pattern of injury:
- Donor transmitted glomerular diseases:
- Can be diagnosed with time zero biopsies
- IgA nephropathy:
- IgA deposits usually disappear following the first 3 months of transplantation (Kidney Int 2003;63:2286)
- Membranous nephropathy:
- Deposits may be asymptomatic and resolve over time (Nephrol Dial Transplant 2014;29:2343)
- Patients with Alport syndrome
- Patients with diabetic nephropathy
- Patients with IgA nephropathy
- Patients with membranous glomerulonephritis
- Patients with primary oxalosis
Comment Here
Reference: Recurrent and de novo diseases
Above is a photomicrograph of the renal allograph biopsy of a patient who has been transplanted 3 weeks ago. The patient has a clinical history of hemolytic uremic syndrome due to infection. Immunohistochemistry reveals presence of peritubular and glomerular C4d staining. Which of the following differential diagnostic entities is the most likely diagnosis?
- Active antibody mediated rejection
- Acute calcineurin inhibitor toxicity
- Recurrent C3 glomerulopathy
- Recurrent hemolytic uremic syndrome
- Recurrent membranoproliferative glomerulonephritis
Comment Here
Reference: Recurrent and de novo diseases
- A group of insoluble proteins organized into beta pleated sheets, which deposit within extracellular spaces of a variety of tissues leading to organ dysfunction and disease
- Renal involvement largely consists of AL, AA, ALECT2 and rare hereditary types such as ATTR (transthyretin) amyloidosis (BMC Nephrol 2022;23:144)
- Amyloid deposits show similar morphology (irrespective of type) and display orangeophilic staining with Congo red and apple green birefringence under polarization
- Deposition of acellular, eosinophilic material within glomeruli, tubulointerstitium and vessel walls; this is negative on Jones silver, pale on PAS / H&E and blue-gray on trichrome
- Immunofluorescence and immunohistochemistry findings can be helpful in determining amyloid type
- Laser dissection of amyloid, followed by tandem mass spectrometry analysis, is the gold standard for definitive amyloid typing
- Treatment options rely on accurate typing of amyloid (Kidney Int 2015;87:516)
- Dependent on the amyloid type
- AL type:
- Primary amyloidosis is most commonly seen in people over the age of 50
- Most common type in the United States
- Caused by a monoclonal gammopathy of light chains (AH - heavy chains and mixed AH / AL types are much rarer)
- Most frequently associated with lambda light chains
- Related to both monoclonal gammopathy of undetermined significance (MGUS) and multiple myeloma
- AA type:
- Secondary amyloidosis characterized by serum amyloid A protein
- More common in developing countries
- Associated with prolonged inflammatory states including rheumatoid arthritis and other connective tissue disorders, infections, IV drug use, inflammatory bowel disease and paraneoplastic syndromes
- ALECT2 type:
- Leukocyte cell derived chemotaxin 2 associated amyloidosis
- Kidney is the most common organ involved, followed by the liver
- Initial reports indicated that Mexican Americans are preferentially affected but new reports document different ethnicities including Middle Eastern, Indian / Punjabi and Chinese populations (Nephrol Dial Transplant 2018;33:241, J Investig Med 2022;70:348)
- Hereditary types:
- Rare group of diseases involving mutations in a number of genes
- Autosomal dominant with variable penetrance
- Most common type is transthyretin (prealbumin) amyloidosis (ATTR), may be wild type but renal involvement is nearly always hereditary due to Val30Met mutations (Nephron 2022 Mar 18 [Epub ahead of print])
- Fibrinogen alpha chain (AFIB) followed by apolipoprotein A1 (AapoA1) are the next 2 (rarely seen) types (Amyloid 2022 May 3 [Epub ahead of print])
- Growing number of amyloid protein types, involving various organ systems and tissues, with relatively few involving the kidney specifically
- All components of the kidney may be involved (glomerular, tubular basement membranes and casts, interstitium, vessels)
- Specific causes are dependent on amyloid type
- Polypeptides become misfolded, usually into beta sheet secondary structures, where beta strands are oriented perpendicular to the long axis of the fiber
- These misfolded proteins have a propensity for aggregation and deposition within various organs and tissues (including vessels and nerves), causing damage (StatPearls: Amyloidosis [Accessed 8 June 2022])
- Dependent on amyloid type
- Monoclonal protein (AL), chronic infection / inflammation (AA), specific populations with unknown cause (ALECT2), hereditary (ATTR, AApol1)
- Varies with type of amyloidosis and component of kidney affected
- Glomerular involvement presents with nephrotic range proteinuria and nephrotic syndrome, which may present suddenly
- Often with AL and AA type amyloidosis
- Tubulointerstitial predominant involvement will present with slow progressive renal failure and less proteinuria
- Often seen with ALECT2 amyloidosis
- Rarely, rapidly progressive renal failure and nephrotic syndrome in the setting of amyloid induced crescentic glomerulonephritis
- Most often with AA type amyloidosis (Indian J Nephrol 2020;30:352)
- Acute renal failure may be seen in the setting of amyloid cast nephropathy
- Glomerular involvement presents with nephrotic range proteinuria and nephrotic syndrome, which may present suddenly
- May be suspected clinically
- Visualization of the characteristic amyloid deposits, as seen on special stains
- Confirmation with Congo red staining and polarization of amyloid showing typical apple green birefringence
- Thicker tissue sections (8 - 10 microns) are helpful in visualizing the polarized deposits
- Immunohistochemistry stains are available including serum amyloid A, transthyretin and antiserum amyloid P (SAP)
- Immunofluorescence microscopy to determine light or heavy chain restriction
- Electron microscopy findings with characteristic fibrillary deposits
- Amyloid type can be confirmed with mass spectrometry based proteomics
- Serum and urine protein electrophoresis with immunofixation
- Serum free light chain elevation
- Unfavorable factors (J Res Med Sci 2014;19:644, Blood 2003;101:827, J Clin Oncol 2016;34:2037):
- Dialysis requirement
- Massive proteinuria
- Renal failure at presentation
- Severe hypoalbuminemia
- Coexisting multiple myeloma
- Heavy organ involvement
- IgM related monoclonal protein
- 31 year old woman presents with proteinuria during her second pregnancy (Clin Res Cardiol 2020;109:1438)
- 56 year old man with longstanding Crohn's disease presents with nephrotic syndrome (Ochsner J 2021;21:291)
- 60 year old Caucasian woman with 8 weeks of lower extremity edema, renal failure and a 10 cm kidney mass (Kidney Int Rep 2019;4:882)
- Treatment based on amyloid type, underlying cause of disease and symptoms
- Dialysis and kidney transplant in end stage kidney disease
- Pale eosinophilic, amorphous and extracellular material on H&E and PAS stains, typically silver negative and gray-blue on trichrome
- Amyloid involving glomerular basement membranes may show long perpendicular spikes
- Light chain and heavy chain restriction may be seen within amyloid
- Jones silver: does not typically stain amyloid
- Randomly arranged, nonbranching fibrils with a variable thickness, close to 10 nm
- Mostly acquired with rare inherited forms
- Hereditary forms
- All show an autosomal dominant inheritance
- Transthyretin gene mutation most common - Val30Met seen with renal involvement (Nephron 2022 Mar 18 [Epub ahead of print])
- Other gene mutations include fibrinogen alpha chain, apolipoprotein A1 and A2
- Kidney, native, core biopsy:
- Amyloidosis, lambda restricted (AL type), predominantly glomerular
- No significant interstitial fibrosis / tubular atrophy
- Congo red stain confirms amyloid deposits
- Fibrillary glomerulonephritis:
- Immunotactoid glomerulonephritis:
- Extremely rare, microtubules of 20 - 50 nm
- Diabetic nephropathy:
- Strong mesangial silver stain, no well formed fibrils
- Microscopic hematuria
- Nephrotic syndrome and renal failure
- Slow decline in renal function
- Sudden nephrotic range proteinuria
- Hypertension that responds to ACE inhibitors, with stenosis by intravenous pyelogram or renal scans, bruit, elevated renin
- 2 - 5% of cases of hypertension, surgery curative in 70%
- Atheromatous plaque: more common in older men with diabetes, often at orifice of renal artery and associated with aneurysmal dilatation of aorta distal to renal arteries
- Fibromuscular dysplasia: more common in younger women; may involve other vessels; not responsive to hypertensive drugs; 50% bilateral, often involve distal 2/3 of renal artery; due to intimal, medial, perimedial or periarterial fibroplasia, medial hyperplasia or medial dissection
- Radiation injury: loss of muscle, intense fibrosis in all wall layers; usually after radiation treatment, including renal artery field years previous
- Takayasu’s aortitis: also called pulseless disease; chronic sclerosing aortitis of unknown etiology
- 4 year old boy with segmental renal artery stenosis (Am J Surg Pathol 1994;18:512)
- 35 year old woman with aortic coarctation and renal artery stenosis due to fibromuscular dysplasia (Arch Pathol Lab Med 1989;113:809)
- 53 year old woman with renal artery stenosis (BMJ Case Rep 2012;2012: bcr2012006499)
- Anti-hypertensive medications
- Angioplasty with stent placement may be effective, even in transplanted kidney (Nefrologia 2012;32:455)
- Revascularization is usually not effective for atherosclerotic renovascular disease (Nephrol Dial Transplant 2012;27:3843, Cardiol Rev 2012;20:189)
- Shrunken kidney
- Diffuse atrophy with crowded and smaller glomeruli, atrophic tubules and interstitial fibrosis (Lab Invest 1991;65:558)
- Arterioles usually normal
- Usually no hypertensive changes in small vessels, although opposite kidney may show hypertensive changes
- Medial fibroplasia: multiple foci of stenosis alternate with microaneurysms to produce “string of beads”
- Perimedial fibroplasia: normal outer half of media but hyperplasia of muscle causes uniform circumferential thickening of vessel wall with luminal narrowing
- Intimal fibroplasia: hyperplasia of intima, resembling atherosclerosis, but without lipid deposition
- Periarterial fibroplasia: rare, fibrosis of adventitia extends into adjacent adipose and connective tissue, causing constriction from without
- Renal disease: basic terminology in nontumor kidney pathology and kidney diseases
- Each of the 3 anatomical kidney compartments should be assessed and reported (Am J Kidney Dis 2022;80:119)
- Glomeruli (count overall number of glomeruli and number of globally sclerotic glomeruli)
- Tubulointerstitium (semiquantitatively estimate the extent of interstitial fibrosis and tubular atrophy)
- Vasculature (focusing on arteries and arterioles, estimate degree of narrowing of the vascular lumen)
- Injuries should be categorized as active versus chronic lesions (Am J Kidney Dis 2022;80:119)
- Lesions and nomenclature should be reported according to an international standards (Kidney Int 2020;98:1120)
- Focal (< 50% of the glomeruli or the tubulointerstitium)
- Diffuse (> 50% of the glomeruli or the tubulointerstitium)
- Segmental (< 50% of a glomerulus)
- Global (> 50% of a glomerulus, special case: global glomerulosclerosis denotes almost fully sclerotic glomeruli)
- Congenital abnormalities of the kidney and urinary tract (CAKUT) occur in ~1 in 500 live births and are responsible for 40 - 50% of childhood end stage renal disease (StatPearls: Embryology, Kidney, Bladder, and Ureter [Accessed 7 August 2023])
- Acute kidney injury (AKI) can be seen in up to 7% of hospital admissions and 30% of intensive care unit (ICU) admissions (StatPearls: Acute Kidney Injury [Accessed 7 August 2023])
- Glomerulonephritis constitutes 25 - 30% of all end stage renal disease cases (StatPearls: Glomerulonephritis [Accessed 7 August 2023])
- Chronic kidney disease (CKD) affects ~13% of the world's population and is the 16th leading cause of years of life lost worldwide (Adv Ther 2022;39:33, JAMA 2019;322:1294)
- Kidney
- Causes of CAKUT (StatPearls: Embryology, Kidney, Bladder, and Ureter [Accessed 7 August 2023])
- Disruptions to the signaling pathways of LIM1, PAX2 / PAX8 and ODD1, GDNF-RET, bone morphogenic protein 4 (BMP4) and gremlin
- Genes associated with bilateral renal agenesis, including ANOS1, EYA1 and RET
- Multicystic dysplastic kidney disease (MCDK) is thought to be caused by a defect in the genes involved in the GDNF-RET signaling pathway between the ureteric bud and the metanephric blastema
- Autosomal dominant polycystic kidney disease (PKD) caused by heterogeneous mutations in PKD1 in 85% of cases, with the remaining 15% due to PKD2 mutations; autosomal recessive PKD caused by mutations in PKHD1
- Causes of AKI (StatPearls: Acute Kidney Injury [Accessed 7 August 2023])
- Prerenal
- Reduced blood flow to the kidney (caused by hypotension, hypovolemia, renal vasoconstriction or glomerular efferent arteriolar vasodilation)
- Renal
- Acute tubular necrosis, acute interstitial nephritis, glomerulonephritis, intratubular obstruction
- Postrenal
- Obstruction of the urinary flow by renal / ureteral calculi, tumors, blood clots or any urethral obstruction
- Prerenal
- Causes of glomerulonephritis (GN) (StatPearls: Glomerulonephritis [Accessed 7 August 2023])
- Immune complex GN
- Pauci-immune GN
- Antiglomerular basement membrane (GBM) GN
- Monoclonal Ig GN
- C3 glomerulopathy
- Causes of CKD (StatPearls: Chronic Renal Failure [Accessed 7 August 2023], Lancet 2017;389:1238)
- Diabetes mellitus type 2 (30 - 50%)
- Diabetes mellitus type 1 (3.9%)
- Hypertension (27.2%)
- Primary glomerulonephritis (8.2%)
- Chronic tubulointerstitial nephritis (3.6%)
- Hereditary or cystic diseases (3.1%)
- Secondary glomerulonephritis or vasculitis (2.1%)
- Plasma cell dyscrasias or neoplasm (2.1)
- Sickle cell nephropathy (SCN)
- Clinical indications for renal biopsy (typical presentations) (adapted from BMJ Open 2019;9:e023479)
- Microscopic or macroscopic hematuria (StatPearls: Hematuria [Accessed 7 August 2023])
- Alport syndrome
- Immune complex GN: IgA nephropathy, IgA vasculitis, infection related GN, lupus nephritis and fibrillary GN with polyclonal Ig deposits
- Membranoproliferative glomerulonephritis
- Antiglomerular basement membrane (GBM) GN (Goodpasture syndrome)
- Pauci-immune GN: PR3 antineutrophil cytoplasmic antibodies (ANCA) GN, myeloperoxidase (MPO) ANCA GN and ANCA negative GN with systemic or renal vasculitis
- C3 glomerulopathy: C3 glomerulonephritis, dense deposit disease
- Nephrotic syndrome (proteinuria > 3.5 g/24 h or 40 mg/m2 per hour, edema, hypoalbuminemia StatPearls: Glomerulonephritis [Accessed 7 August 2023])
- Minimal change disease
- Focal segmental glomerulosclerosis
- Membranoproliferative glomerulonephritis
- Membranous nephropathy
- Human immunodeficiency virus (HIV) associated nephropathy
- Diabetic nephropathy
- Amyloidosis
- Nephritic syndrome (proteinuria 2 per hour, rapid decrease in glomerular filtration rate [GFR], oliguria, hematuria, systemic hypertension, decrease in serum complement levels) (StatPearls: Nephritic Syndome [Accessed 7 August 2023])
- Antiglomerular basement membrane (GBM) GN (Goodpasture syndrome)
- Immune complex GN: IgA nephropathy, IgA vasculitis, infection related GN, lupus nephritis and fibrillary GN with polyclonal Ig deposits
- Pauci-immune GN: PR3 ANCA GN, MPO ANCA GN and ANCA negative GN with systemic or renal vasculitis
- Impaired kidney function (acute or chronic decrease of estimated GFR [eGFR])
- Acute decrease of eGFR / acute kidney injury (AKI) (StatPearls: Acute Kidney Injury [Accessed 7 August 2023])
- Hypovolemia: hemorrhage, severe burns and gastrointestinal fluid losses, such as diarrhea, vomiting, high ostomy output
- Hypotension from decreased cardiac output: cardiogenic shock, massive pulmonary embolism, acute coronary syndrome
- Hypotension from systemic vasodilation: septic shock, anaphylaxis, anesthetics, hepatorenal syndrome
- Renal vasoconstriction: NSAIDs, iodinated contrast, amphotericin B, calcineurin inhibitors, hepatorenal syndrome
- Glomerular efferent arteriolar vasodilation: ACE inhibitors, angiotensin receptor blockers
- Chronic decrease of eGFR (StatPearls: Chronic Renal Failure [Accessed 7 August 2023], Lancet 2017;389:1238)
- Possible renal involvement in systemic disease in
- Diabetes mellitus type 2 and type 1
- Hypertension
- Hereditary or cystic diseases
- Plasma cell dyscrasias or neoplasm
- Secondary glomerulonephritis or vasculitis
- Sickle cell nephropathy (SCN)
- Possible renal involvement in systemic disease in
- Acute decrease of eGFR / acute kidney injury (AKI) (StatPearls: Acute Kidney Injury [Accessed 7 August 2023])
- Microscopic or macroscopic hematuria (StatPearls: Hematuria [Accessed 7 August 2023])
- Clinical, laboratory, imaging and biopsy findings
- Chronic kidney disease (CKD)
- Glomerular filtration rate: GFR 2 (GFR categories G3a - G5) (Kidney Int Suppl 2013;3:19)
- Decreased GFR can be detected by current estimating equations for GFR based on serum creatinine or cystatin C but not by serum creatinine or cystatin C alone
- Albuminuria as a marker of kidney damage (increased glomerular permeability), urine albumin excretion rate (AER) ≥ 30 mg/24 hours, approximately equivalent to urine albumin to creatinine ratio (ACR) ≥ 30 mg/g (≥ 3 mg/mmol)
GFR categories in CKD GFR category GFR (mL/min/1.73 m2) Terms G1 ≥ 90 Normal or high G2 60 - 89 Mildly decreased* G3a 45 - 59 Mildly to moderately decreased G3b 30 - 44 Moderately to severely decreased G4 15 - 29 Severely decreased G5 Kidney failure Abbreviations: chronic kidney disease (CKD), glomerular filtration rate (GFR)
*Relative to young adult level
In the absence of evidence of kidney damage, neither GFR category G1 nor G2 fulfill the criteria for CKD
Albuminuria categories in CKD
(KDIGO: Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease [Accessed 28 July 2023])ACR (approximate equivalent) Category AER (mg/24 hours) (mg/mmol) (mg/g) Terms A1 Normal to mildly increased A2 30 - 100 3 - 30 30 - 300 Moderately increased* A3 > 300 > 30 > 300 Severely increased** Abbreviations: albumin excretion rate (AER), albumin to creatinine ratio (ACR), chronic kidney disease (CKD)
*Relative to young adult level
**Including nephrotic syndrome (albumin excretion usually 42200 mg/24 hours [ACR 42220 mg/g; 4220 mg/mmol])
- Urinary sediment abnormalities as markers of kidney damage (Kidney Int Suppl 2013;3:19)
- Isolated, nonvisible (microscopic) hematuria with abnormal red blood cell (RBC) morphology (anisocytosis) in GBM disorders
- RBC casts in proliferative glomerulonephritis
- White blood cell (WBC) casts in pyelonephritis or interstitial nephritis
- Oval fat bodies or fatty casts in diseases with proteinuria
- Granular casts and renal tubular epithelial cells in many parenchymal diseases (nonspecific)
- Renal tubular disorders (Kidney Int Suppl 2013;3:19)
- Renal tubular acidosis
- Nephrogenic diabetes insipidus
- Renal potassium wasting
- Renal magnesium wasting
- Fanconi syndrome
- Nonalbumin proteinuria
- Cystinuria
- Glomerular filtration rate: GFR 2 (GFR categories G3a - G5) (Kidney Int Suppl 2013;3:19)
- Acute kidney injury (AKI) (PLoS One 2014;9:e114369)
- Increased serum creatinine
Diagnosis and staging criteria for AKI of RIFLE, AKIN, KDIGO definitions based on serum creatinine Classification Definition for AKI Stage Serum creatinine criteria for AKI staging* RIFLE Increase in SCr ≥ 50% within 7 days Risk To ≥ 1.5 times baseline Injury To ≥ 2 times baseline Failure To ≥ 3 times baseline or ≥ 44 μmol/L increase to at least 354 μmol/L AKIN Increase in SCr ≥ 26.5 μmol/L or ≥ 50% within 48 hours 1 Increase of ≥ 26.5 μmol/L or to 1.5 - 2 times baseline 2 To 2 - 3 times baseline 3 To ≥ 3 times baseline or ≥ 26.5 μmol/L increase to at least 354 μmol/L or initiation of RRT KDIGO Increase in SCr ≥ 26.5 μmol/L within 48 hours
or ≥ 50% within 7 days1 Increase in SCr ≥ 26.5 μmol/L within 48 hours or to 1.5 - 2 times baseline 2 To 2 - 3 times baseline 3 To ≥ 3 times baseline or to at least 354 μmol/L or initiation of RRT For patients meeting diagnosis criteria for AKI according to RIFLE, AKIN or KDIGO, the stage based on percentage increase were determined by the ratio of peak SCr value obtained during hospitalization to baseline
Abbreviations: acute kidney injury (AKI); risk, injury, failure, loss, end stage renal failure (RIFLE); Acute Kidney Injury Network (AKIN); Kidney Disease: Improving Global Outcomes (KDIGO); serum creatinine (SCr); renal replacement therapy (RRT)
*Urine output was not used because records of hourly urine output were not available in the majority of patients
- Markers such as NGAL, KIM1 and IL18 are surrogates for renal damage; their role as biomarkers to diagnose AKI, predict risk for AKI or predict progression of AKI to CKD is still under exploration
- Increased serum creatinine
- Developmental and cystic diseases
- Large group of hereditary and nonhereditary kidney diseases of variable etiology (Adv Anat Pathol 2006;13:26)
- Glomerular disease (including monoclonal gammopathy of renal significance [MGRS] / paraprotein related kidney disease)
| Glomerular lesion | PAS / silver stain | IF / IHC | EM | Differential diagnoses | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thick appearance of GBM | Relatively acellular mesangial expansion / no double contour, feathery spikes on silver stain, Congo red positive | Negative for IgG, IgA, IgM, C3 and C1q positive for 1 light chain, with smudgy mesangial and capillary wall pattern, often also in vessels (most often λ but κ can also form amyloid; rare cases of heavy chain amyloid exist) | Randomly arranged, nonbranching fibrils of 9 - 11 nm diameter |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Double contour | Negative |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Irregular or undetectable by LM | Negative | Basketweaving, thin GBM |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Membranous (thick appearance of GBM), spikes by silver stain | Granular capillary loop IgG polyclonal and C3 staining; in specific etiology positive for PLA2R, THSD7A, EXT1 / EXT2, NELL1 and others (Kidney Int 2023;103:469) | Subepithelial deposits |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Varying double contours by silver stain, negative Congo red | Polyclonal IgG with lesser C3; positive DNAJB9 | Randomly arranged fibrillary substructure 11 - 24 nm |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mesangial hypercellularity / mesangial expansion | Varying mesangial expansion with nodules, thick GBM, arteriolar hyalinosis / no double contour | Negative | No deposits |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Varying mesangial hypercellularity with nodules, thick GBM / no double contour | Monoclonal light chain staining of GBM and tubular basement membrane | Corresponding amorphous powdery deposits |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Varying mesangial hypercellularity without nodules, thick GBM / no double contour | Polyclonal IgG with full house staining (i.e., all immunoglobulins [IgG, IgA, IgM], C3 and C1q) | Subendothelial deposits; may show fingerprint-like substructure; reticular aggregates in endothelial cells |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IgA mesangial deposits | Mesangial deposits |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IgG (starry sky appearance by IF) or IgM predominant deposits | Hump shaped deposits |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| C3 positive deposits | Hump shaped deposits |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
- Tubulointerstitial disease (including infectious disease)
| Tubulointerstitial lesion | PAS / silver stain | Differential diagnoses | ||||||||||||||||||||||||||||||||||
| Tubular injury | Cell detachment, individual cell injury / blebbing / degeneration / apoptosis |
| ||||||||||||||||||||||||||||||||||
| Cell detachment in isolation or with associated glomerular necrosis |
| |||||||||||||||||||||||||||||||||||
| Cell detachment in zones of injury |
| |||||||||||||||||||||||||||||||||||
| With immunglobulin deposition | Tubulitis, TBMs stain strongly for IgG, variable C3 staining |
| ||||||||||||||||||||||||||||||||||
| Interstitial fibrosis and tubular atrophy | Diffuse pattern | Nonspecific | ||||||||||||||||||||||||||||||||||
| Striped pattern along medullary rays |
| |||||||||||||||||||||||||||||||||||
| Patchy / geographic, jigsaw puzzle pattern |
| |||||||||||||||||||||||||||||||||||
| Interstitial edema | Increased interstitial space with loose appearance and normal thickness TBMs |
| ||||||||||||||||||||||||||||||||||
| Interstitial inflammation | Medullary angiitis with increased neutrophils in vasa recta |
| ||||||||||||||||||||||||||||||||||
| Intratubular neutrophils forming plugs |
| |||||||||||||||||||||||||||||||||||
| Interstitial lymphocytic infiltrate |
| |||||||||||||||||||||||||||||||||||
| Lymphocytes and interstitial fibrosis |
| |||||||||||||||||||||||||||||||||||
- Vascular disease
| Vascular lesion | PAS / silver stain | Differential diagnoses |
| Sclerosis | Intimal fibrosis / medial hypertrophy |
|
| Thrombosis, necrosis and red blood cell fragments within vascular wall, mucoid intima expansion | Arteriolar / glomerular predominance |
|
| Arteriolar / interlobular artery predominance, fibrinoid necrosis |
| |
| Emboli | Clear, needle shaped spaces with surrounding foreign body reaction in interlobular arteries, sometimes in smaller vessels, rarely in glomeruli |
|
| Vasculitis | Inflammation with lymphocytes / PMNs; may be transmural or intimal |
|
| Endotheliosis | Swollen endothelial cells |
|
| Abbreviations: antineutrophil cytoplasmic antibodies (ANCA), electron microscopy (EM), hematoxylin eosin staining (H&E), immunohistochemistry (IHC), International Society of Nephrology / Renal Pathology Society (ISN / RPS), tubular basement membrane (TBM), thrombotic microangiopathy (TMA) | ||
| Adapted from Am J Kidney Dis 2022;80:119 | ||
- Renal allograft (Transplantation 2018;102:1795, Am J Transplant 2020;20:2318)
- 2019 Banff classification for antibody mediated rejection (ABMR), borderline changes, T cell mediated rejection (TCMR) and polyomavirus nephropathy (Am J Transplant 2020;20:2318)
- Category 1: normal biopsy or nonspecific changes
- Category 2: antibody mediated changes
- Active ABMR
- Chronic active ABMR; all 3 criteria must be met for diagnosis
- Chronic (inactive) ABMR
- Category 3: borderline (suspicious) for acute TCMR
- Category 4: TCMR
- Acute TCMR
- Chronic active TCMR
- Category 5: polyomavirus nephropathy
- Infectious
- Cytomegalovirus infection (Am J Kidney Dis 2016;68:e35)
- Polyomavirus nephropathy (Am J Kidney Dis 2016;68:e37)
- Calcineurin inhibitor nephrotoxicity (Am J Kidney Dis 2017;69:e21)
- 2019 Banff classification for antibody mediated rejection (ABMR), borderline changes, T cell mediated rejection (TCMR) and polyomavirus nephropathy (Am J Transplant 2020;20:2318)
Contributed by Saskia von Stillfried, M.D.
Comment Here
Reference: Renal disease-general
Comment Here
Reference: Renal disease-general
- Acute tubular injury with myoglobin+ tubular casts seen in setting of rhabdomyolysis
- Acute tubular injury with myoglobin+ tubular casts
- Seen in setting of rhabdomyolysis; also has elevated serum creatine kinase
- Also called pigment nephropathy
- ICD-10: M62.82 - rhabdomyolysis
- Acute kidney injury develops in 10 - 40% of patients with severe rhabdomyolysis (Am J Kidney Dis 2018;71:A12)
- Myoglobin may damage tubules via multiple mechanisms:
- Vasoconstriction with subsequent tubular epithelial ischemia (Free Radic Biol Med 2011;51:1062)
- Direct cytotoxic and oxidative damage (Biochim Biophys Acta 2009;1792:796)
- Tubular obstruction by pigmented casts
- Rhabdomyolysis results from muscle injury (Crit Care 2014;18:224):
- Traumatic: crush injury, exercise, seizure
- Drug related: cocaine, heroin, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) inhibitors, rapamycin, oseltamivir (Transplantation 2006;82:645)
- Toxic: clostridial toxin, snake venom
- Malignant hyperthermia
- Electrical current
- Oliguria
- Dark (cola colored) urine
- Muscle swelling, stiffness
- Reference: Crit Care 2014;18:224
- Urinalysis: dipstick positive for heme protein, no erythrocytes, dark pigmented casts on urine microscopy
- Elevated creatine kinase, often > 100,000 U/L
- Elevated creatinine
- Electrolyte abnormalities: hyperkalemia, hyperphosphatemia, hyperuricemia, hypocalcemia
- Reference: Crit Care 2014;18:224
- Time to diagnosis and treatment
- Age (younger patients have better recovery)
- Reference: Crit Care 2014;18:224
- 21 year old man with dengue fever (BMJ Case Rep 2015 Jul 14;2015)
- 53 year old man with suspected influenza and oseltamivir administration (Am J Case Rep 2018;19:673)
- 66 year old man with carnitine palmitoyltransferase II deficiency (Am J Kidney Dis 2018;71:A12)
- 82 year old man taking simvastatin and gemfibrozil, successfully managed with plasmapheresis (Cases J 2009;2:8138)
- 11 patients with indoor cycling (spinning) induced rhabdomyolysis (Electrolyte Blood Press 2015;13:58)
- Supportive care
- Correction of inciting factors or disease
- Depends on degree of acute kidney injury (see diagram above, Crit Care 2014;18:224)
- Potentially acetaminophen by decreasing oxidant injury (Proc Natl Acad Sci USA 2010;107:2699)
- Potentially dialysis (Ren Fail 2001;23:183)
- Acute tubular injury: loss of brush borders, attenuation and sloughing of epithelium
- Granular or pigmented casts in tubules
- Interstitial edema
- Glomeruli spared
- Negative immunofluorescence
- Myoglobin immunohistochemistry highlights casts
- Casts have electron dense granules
- Native kidney biopsy:
- Myoglobin cast nephropathy
- Comment: consistent with the clinical history of acute kidney injury and rhabdomyolysis in this patient, kidney biopsy demonstrates acute tubular injury / acute tubular necrosis with associated myoglobin positive casts, characteristic of myoglobin cast nephropathy
- Hemoglobinuria:
- May have similar appearing pigmented casts
- Casts are negative for myoglobin and positive for hemoglobin by IHC
- Light chain cast nephropathy:
- Other acute tubular injury, including toxic or ischemic:
- Also has acute tubular injury with granular debris in tubular lumen
- Normal serum creatine kinase
- Casts are negative for myoglobin by IHC
- Creatine kinase; rhabdomyolysis
- Creatinine; multiple myeloma
- Lactate dehydrogenase; paroxysmal nocturnal hemoglobinuria
- Troponin; myocardial infarction
Comment Here
Reference: Rhabdomyolysis
- Sarcoidosis is a multisystem granulomatous disease of unknown cause histologically characterized by epithelioid noncaseating granulomas
- Classic pathological lesion of renal sarcoidosis is granulomatous interstitial nephritis but there are other forms of renal involvement
- Sarcoidosis is also known as Besnier-Boeck-Schaumann disease
- Renal manifestations of sarcoidosis can present in patients with a diagnosis of sarcoidosis of other systems (notably, hilar adenopathy or pulmonary infiltrates on chest radiographs) or be an initial or sole presentation of the disease
- Best known renal manifestation of sarcoidosis, granulomatous interstitial nephritis, is observed in 7 - 23% or 30 - 79% of native kidney biopsies from patients with sarcoidosis (Sarcoidosis Vasc Diffuse Lung Dis 2021;38:e2021017, Nephrol PoC 2019;5)
- In contrast to the female predominance in patients with pulmonary sarcoidosis, 63.8% of patients with granulomatous interstitial nephritis are men (Am J Kidney Dis 2006;48:856)
- Mean age at presentation of granulomatous interstitial nephritis is 46.9 years (range, 11 - 80 years) or is reported at 59, plus or minus 18 years (Am J Kidney Dis 2006;48:856, Sarcoidosis Vasc Diffuse Lung Dis 2018;35:252)
- 74% of patients with granulomatous interstitial nephritis are White individuals (Am J Kidney Dis 2006;48:856)
- Nongranulomatous interstitial nephritis is observed in 14 - 44% of native kidney biopsies from patients with sarcoidosis (Nephrol PoC 2019;5)
- In certain studies, glomerular disease is found in 4 - 26% or up to 42% of native kidney biopsies in patients with sarcoidosis but is generally thought to be a rare occurrence (Nephrol PoC 2019;5, Orphanet J Rare Dis 2013;8:65)
- Sarcoidosis associated glomerular disorders may precede the diagnosis of sarcoidosis (Orphanet J Rare Dis 2013;8:65)
- Dysfunctional vitamin D and calcium metabolism leading to hypercalcemia, hypercalciuria, nephrolithiasis and nephrocalcinosis are common problems in patients with sarcoidosis (Am J Kidney Dis 2006;48:856)
- Nephrocalcinosis is observed in 10 - 11% of sarcoidosis patients presenting with renal disease (Nephrol PoC 2019;5)
- Nephrolithiasis is found in about 10% of patients with sarcoidosis (Int Braz J Urol 2020;46:15)
- Blau syndrome (early sarcoidosis, a familial granulomatous inflammatory disease) presents in early childhood, usually in the first year of life (Pediatr Rheumatol Online J 2014;12:33)
- Sarcoid granulomatous lesions preferentially involve the lower respiratory tract but may affect any extrapulmonary site, such as the heart, thyroid and parathyroid, skin and eye
- This topic is dedicated to the kidney involvement in sarcoidosis
- Sarcoidosis is associated with chronic hypercalcemia and increased levels of 1,25 dihydroxy vitamin D (calcitriol) (Int Braz J Urol 2020;46:15)
- Altered level of vitamin D and hypercalcemia suppress the parathyroid hormone (Int Braz J Urol 2020;46:15)
- Increased calcium filtration at the glomerulus and suppression of parathyroid hormone secretion by calcitriol (diminished tubular reabsorption of calcium) lead to hypercalciuria and thus to nephrocalcinosis and nephrolithiasis (Int Braz J Urol 2020;46:15)
- Hypercalcemia causes afferent arteriole vasoconstriction, decreasing blood flow and the glomerular filtration rate (Int Braz J Urol 2020;46:15)
- Glomerular diseases found in patients with sarcoidosis are thought to be caused by functional disorders of the immune system
- Cytokines produced locally by granulomas may contribute to alterations in the glomerular filtration barrier (Orphanet J Rare Dis 2013;8:65)
- Incompletely understood (see Lung - Sarcoidosis) (Respir Med 2020;173:106161)
- Granulomatous interstitial nephritis in sarcoidosis is usually clinically silent but in Sarcoidosis Vasc Diffuse Lung Dis 2021;38:e2021017)
- According to other studies, granulomatous interstitial nephritis accounts for 7 - 27% of clinically significant renal failure in patients with sarcoidosis (Am J Kidney Dis 2006;48:856, Nephrol PoC 2019;5)
- Acute kidney injury, when it develops, appears to be more severe in patients with granulomatous interstitial nephritis versus patients with nongranulomatous interstitial nephritis (Sarcoidosis Vasc Diffuse Lung Dis 2021;38:e2021017)
- Acute kidney injury in patients with hypercalcemia is milder than in patients without hypercalcemia (Sarcoidosis Vasc Diffuse Lung Dis 2021;38:e2021017)
- Granulomatous renal masses (so called pseudotumoral renal sarcoidosis) are usually associated with normal renal function (QJM 2013;106:947)
- Blau syndrome presents as a triad of granulomatous dermatitis, arthritis and uveitis (Pediatr Rheumatol Online J 2014;12:33)
- Diagnosis of sarcoidosis is based on 3 major criteria: a compatible clinical presentation, the finding of nonnecrotizing granulomatous inflammation in 1 or more tissue samples and the exclusion of alternative causes of granulomatous disease (Am J Respir Crit Care Med 2020;201:e26)
- Renal biopsy is the key instrument in diagnosis of kidney involvement in sarcoidosis
- For patients with sarcoidosis who have neither renal symptoms nor established renal sarcoidosis, baseline serum creatinine testing is suggested for screening
- Diagnosis and detection of sarcoidosis
- Official American Thoracic Society Clinical Practice Guideline
- Disordered calcium metabolism is found in a significant number of patients (Am J Kidney Dis 2006;48:856, Kidney Int 2008;74:817)
- Levels of serum angiotensin converting enzyme, interleukin 2 receptor (IL2R), C reactive protein (CRP), serum amyloid A (SAA) and chitotriosidase may be helpful in establishing the diagnosis (Sarcoidosis Vasc Diffuse Lung Dis 2021;38:e2021017)
- Contrast enhanced computer tomography scan may show signs of interstitial nephritis or less frequently multiple hypoattenuating nodules that resemble lymphoma or metastases (Radiopaedia: Sarcoidosis (Abdominal Manifestations) [Accessed 25 July 2024])
- On MRI, granulomatous renal masses are poorly circumscribed and infiltrative and slightly hypointense to surrounding renal cortex; the zone of transition between the mass and normal renal parenchyma is ill defined and consistent with interstitial infiltration (AJR Am J Roentgenol 2005;185:697)
- Attenuation corrected whole body positron emission tomography (PET) scan shows intense radiotracer uptake in the kidney and lymph nodes; this can be misconstrued as indicative of malignancy (AJR Am J Roentgenol 2005;185:697)
- Severe interstitial infiltrates tend to predict a worse prognosis (Sarcoidosis Vasc Diffuse Lung Dis 2021;38:e2021017)
- Interstitial fibrosis, severe interstitial infiltration and the presence of giant cells are associated with a worse outcome (Sarcoidosis Vasc Diffuse Lung Dis 2018;35:252, Sarcoidosis Vasc Diffuse Lung Dis 2021;38:e2021017)
- 16 year old girl with multiorgan sarcoidosis and renal involvement (Front Pediatr 2021;9:724728)
- 20 year old African American man presented with acute renal failure (a rare sarcoidosis presentation) (Cureus 2023;15:e49512)
- 30 year old man with renal sarcoidosis without coexisting pulmonary findings (Cureus 2021;13:e15494)
- 45 year old woman with severe acute kidney injury and uveitis; sarcoidosis with renal, hepatic and ocular involvement diagnosed (BMJ Case Rep 2018;11:e227023)
- 56 year old woman with sarcoidosis and acute renal failure (Kidney Int 2008;74:817)
- Corticosteroids are the standard of care in renal sarcoidosis
- Majority of cases respond to steroid therapy (Sarcoidosis Vasc Diffuse Lung Dis 2018;35:252)
- In steroid resistant patients, other immunosuppressive reagents (azathioprine, mycophenolate mofetil or TNF inhibitors) are used (Nephrol Dial Transplant 2014;29:1841, Int Braz J Urol 2020;46:15)
- Steroid responsiveness of sarcoidosis associated glomerular diseases does not correlate with that of the main disease, suggesting that both diseases require a more aggressive therapy (Orphanet J Rare Dis 2013;8:65)
- Granulomas are well formed concentrically arranged layers of immune cells with a central core of macrophages, epithelioid cells and multinucleated giant cells and an outer layer of loosely organized lymphocytes (mostly T cells) and interposed dendritic cells, sometimes surrounded by collections of B lymphocytes
- Epithelioid cells (epithelioid histiocytes) are derivatives of activated macrophages resembling epithelial cells (Wikipedia: Epithelioid Cell [Accessed 25 July 2024])
- Caseous necrosis is the grossly visible, cheese-like appearance of the granulomas; this term has no microscopy counterpart and thus, the term necrotizing is preferable (Arch Pathol Lab Med 2022;146:233)
- Sarcoidosis granulomas are most often nonnecrotic (Am J Respir Crit Care Med 2020;201:e26)
- Epithelioid cells are elongated, with pale eosinophilic cytoplasm and central, ovoid nuclei, which are less dense than that of a lymphocyte (Wikipedia: Epithelioid Cell [Accessed 25 July 2024])
- Presence of numerous noncaseating (nonnecrotizing) granulomas favors sarcoidosis (Am J Respir Crit Care Med 2020;201:e26)
- Sarcoid granulomas feature compact, tightly formed collections of large epithelioid histiocytes and multinucleated giant cells (Am J Respir Crit Care Med 2020;201:e26)
- These granulomas are confined to the renal cortex and stay discrete; interstitial inflammatory infiltration is observed in all cases (Am J Respir Crit Care Med 2020;201:e26)
- Blau syndrome
- Nongranulomatous interstitial nephritis may show numerous eosinophils in the interstitial infiltrate (Am J Respir Crit Care Med 2020;201:e26)
- Nephrocalcinosis manifests as calcium phosphate or oxalate tubulointerstitial deposits
- Such deposits are also found in patients with granulomatous and nongranulomatous interstitial nephritis (Nephrol PoC 2019;5)
- Most frequent glomerular disease occurring in sarcoidosis is membranous glomerulopathy (Orphanet J Rare Dis 2013;8:65)
- Other glomerular diseases observed in patients with sarcoidosis include IgA nephropathy, focal segmental glomerulosclerosis, minimal change disease and lupus nephritis (Orphanet J Rare Dis 2013;8:65)
- Focal segmental glomerulosclerosis and hypertensive nephrosclerosis may be found in sarcoidosis patients with interstitial nephritis (Sarcoidosis Vasc Diffuse Lung Dis 2018;35:252)
- In renal sarcoidosis, immunofluorescence staining for IgG, IgA, IgM, C3, C1q, kappa and lambda light chains is usually negative in all kidney compartments
- Rare cases of nonspecific deposits of IgM or deposits of C3 are reported (Sarcoidosis Vasc Diffuse Lung Dis 2021;38:e2021017)
- Sarcoidosis associated glomerular diseases are diagnosed using the appropriate immunofluorescence features (see Microscopic description section)
- CD68 immunohistochemical staining is used to highlight histiocytes and multinucleated giant cells to facilitate findings of granulomas (Intern Med 2019;58:679)
- Bacterial and fungal stains, such as
- Acid fast (Ziehl-Neelsen) stain (Wikipedia: Ziehl-Neelsen Stain [Accessed 14 August 2024])
- GMS (Grocott-Gomori methenamine silver) stain (Wikipedia: Grocott's Methenamine Silver Stain [Accessed 14 August 2024])
- PAS (periodic acid-Schiff) stain (Wikipedia: Periodic Acid-Schiff Stain [Accessed 14 August 2024])
- No specific electron microscopy findings are associated with granulomatous or nongranulomatous interstitial nephritis, nephrolithiasis or nephrocalcinosis
- Electron microscopy is used to diagnose sarcoidosis associated glomerular pathology and to exclude other etiologies (see Microscopic description section)
- Genetic studies confirm the very strong genetic heterogeneity in sarcoidosis (J Clin Med 2020;9:2633)
- Familial and sporadic forms of sarcoidosis exist (J Clin Med 2020;9:2633)
- Familial sarcoidosis may be associated with variants in mTOR gene and its regulator, a GTPase Rac 1 (J Clin Med 2020;9:2633)
- mTOR pathway regulates autophagy and may be involved in the clearance of pathogens (J Clin Med 2020;9:2633)
- Genomic associations of sarcoidosis renal lesions have not been explored
- Blau syndrome, a monogenic autoinflammatory disease, is due to NOD2 (nucleotide binding oligomerization domain containing 2) gene gain of function mutations (Pediatr Rheumatol Online J 2014;12:33)
- NOD2 protein is primarily expressed in the peripheral blood leukocytes and plays a role in the immune response to intracellular bacterial lipopolysaccharides (NIH: NOD2 Nucleotide Binding Oligomerization Domain Containing 2 [Accessed 31 July 2024])
- Kidney, biopsy:
- Granulomatous acute interstitial nephritis (see comment)
- 0% global glomerulosclerosis (0/9)
- Mild arteriosclerosis and mild arteriolar hyalinosis
- Mild interstitial fibrosis and tubular atrophy
- Comment: The biopsy shows renal cortex extensively involved by granulomatous inflammation. The differential diagnosis includes infections, granulomatous vasculitis and sarcoidosis. No organisms were identified on acid fast bacteria (AFB) and fungal stains. Given the patient's clinical diagnosis of sarcoidosis, these findings are compatible with renal sarcoidosis.
- Light microscopy: The biopsy consists of 1 tissue core containing the renal cortex and medulla. The tissue is sampled at 10 section levels with H&E, PAS, trichrome and periodic acid methamine silver (PAMS) stains. Per level of section up to 9 glomeruli are available, of which none are globally sclerosed. The glomeruli show patent capillary lumens, single contoured capillary walls and no segments of sclerosis, adhesion or crescents. Tubules show mostly intact epithelium with preserved brush borders. The tubulointerstitial compartment is extensively involved by multiple well organized nonnecrotizing granulomas with multinucleated giant cells. Significant lymphocytic infiltrates are seen outside of granulomas. There is mild interstitial fibrosis and tubular atrophy. Interlobular arteries of the medium and small caliber are present and show minimal to mild intimal fibroelastosis; there is mild arteriolar hyalinosis. The AFB and GMS special stains are negative.
- Immunofluorescence: Up to 8 nonsclerosed glomeruli were available for evaluation by immunofluorescence microscopy. No diagnostic immunofluorescence staining is identified with antibodies to IgG-, IgA-, IgM-, C3-, C1q-, kappa light chain-, lambda light chain-.
- Electron microscopy: 3 nonsclerosed glomeruli are available for evaluation on semithin sections. The glomeruli do not show significant diagnostic abnormalities. The tubulointerstitial compartment shows granulomatous inflammation. There is mild interstitial fibrosis and tubular atrophy. Ultrastructural examination of 2 glomeruli shows segmentally wrinkled glomerular basement membranes of normal thickness. There is no significant foot process effacement. Capillary lumens are patent. The mesangial matrix demonstrates a mild expansion. There are no electron dense (immune complex) deposits in any location. The tubular basement membranes are unremarkable.
- Mycobacterial infections:
- History of exposure
- Diagnosis of extrarenal mycobacterial infection
- Mycobacterial DNA fragments may be identified by polymerase chain reaction (PCR)
- Present with necrotizing granulomas
- Positive acid fast stain
- Fungal infections:
- History of exposure
- Positive GMS stain and fungal culture
- Adenoviral infection:
- Seen in renal allografts
- Tubulocentric polymorphonuclear neutrophil (PMN) rich inflammation and acute tubular necrosis
- Ground glass tubular epithelial cells with viral inclusions
- Serum, urine and kidney tissue PCR positive for adenovirus
- Positive immunohistochemical stain for adenovirus
- Intravesical Bacillus Calmette-Guérin (BCG) therapy:
- History of bladder carcinoma and BCG treatment
- Granulomatous inflammation associated with extratubal Tamm-Horsfall protein:
- Glassy PAS+ acellular material
- Associated with interstitial kidney disease
- Positive stain with anti-Tamm-Horsfall protein antibody
- Allergic acute interstitial nephritis, medication related:
- History of medications known to cause granulomatous interstitial nephritis
- Predilection for the corticomedullary junction involvement
- Poorly formed granulomas (Clin Kidney J 2015;8:516)
- Berylliosis (occupational exposure):
- History of inhalation of beryllium dust
- Granulomas are poorly formed (Arch Pathol Lab Med 2022;146:233)
- Tubulointerstitial nephritis with uveitis:
- Affects mainly children and young women
- Uveitis
- Rare nonnecrotizing granulomas
- Crohn's disease:
- History of inflammatory bowel disease
- Granulomatosis with polyangiitis:
- Antineutrophilic cytoplasmic antibody (ANCA) positivity
- Presents as necrotizing and crescentic glomerulonephritis
- In very rare cases, may present with granulomatous acute interstitial nephritis in the absence of glomerulonephritis (Am J Med 2020;133:e679)
- Vessel associated granuloma may be seen
A 46 year old woman, who has a history of pulmonary sarcoidosis diagnosed at the age of 25 years and is a frequent user of aspirin, underwent a CT with contrast due to a suspicion for a relapse of the disease. A week later, she developed a Clostridium difficile associated colitis, for which she received vancomycin treatment. She presents with acute kidney injury with serum creatinine of 3 mg/dL (baseline 1 mg/dL) and her urine contains eosinophils. A kidney biopsy is performed. It shows extensive focally accentuated tubulointerstitial inflammation involving the cortex and the corticomedullary junction; a representative image is shown above. You will report that, based on the clinicopathological data, the most likely etiology of the acute tubulointerstitial nephritis is
- Aspirin
- Computed tomography (CT) contrast material
- Sarcoidosis
- Vancomycin
Comment Here
Reference: Sarcoidosis
A 55 year old man with multiorgan sarcoidosis affecting the lower respiratory tract, the skin and the eyes and a history of longstanding hypertension presents with mild elevation of serum creatinine. Kidney sarcoidosis is suspected and a kidney biopsy is done. The biopsy shows moderate arteriosclerosis and mild to moderate interstitial fibrosis and tubular atrophy with associated mononuclear infiltration. One of the biopsy cores shows an interesting structure. You examine this structure and your conclusion is
- Artifact of tissue processing and staining; of no diagnostic consequence
- Extratubal Tamm-Horsfall protein
- Granulomatous interstitial nephritis, consistent with sarcoidosis kidney involvement
- Necrotizing granuloma; need to order acid fast and GMS stains
Comment Here
Reference: Sarcoidosis
- Scleroderma is a multi-organ disease distinguished by vasculopathy and excess fibrosis (Rheumatology (Oxford) 2019;58:2099)
- Scleroderma associated renal vasculopathy is often clinically insignificant but rarely manifests as scleroderma renal crisis, a thrombotic microangiopathic process which leads to renal ischemia, acute hypertension and rapidly deteriorating renal function (Lancet 2017;390:1685)
- Scleroderma associated renal disease is a spectrum of small arterial and arteriolar vasculopathy ranging from mild fibrointimal thickening to thrombotic microangiopathy (Am J Kidney Dis 2016;67:e19)
- Scleroderma related thrombotic microangiopathy is characterized by mucoid intimal edema, onion skin-like intimal proliferation, fibrinoid necrosis and thrombosis
- Glomerular changes include ischemic collapse and endothelial swelling and thrombosis, acutely and basement membrane duplication, chronically
- Ischemic injury to tubules results in acute tubular injury or necrosis and eventually, interstitial fibrosis and tubular atrophy
- Scleroderma is also called systemic sclerosis
- Scleroderma renal crisis is a clinical diagnosis that is encompassed by the pathological diagnosis scleroderma associated renal disease or scleroderma renal disease
- Scleroderma
- Affects 1:10,000 people globally (Semin Immunopathol 2015;37:443)
- Prevalence varies by geographic location (J Rheumatol 2014;41:1040):
- 200 - 260 cases per million in the U.S. and Australia (Arthritis Rheum 2003;48:2246)
- 20 - 50 cases per million in Asia (Arch Dermatol Res 1991;283:366)
- 100 - 200 cases per million in Europe (Rheumatology (Oxford) 2004;43:1129)
- Women 30 - 50 years of age are most commonly affected (Am J Kidney Dis 2016;67:e19)
- Renal disease is observed clinically in up to 50% of scleroderma patients, usually those with diffuse cutaneous scleroderma (Medicine (Baltimore) 1974;53:1, Am J Kidney Dis 2016;67:e19)
- Scleroderma renal crisis (Nat Rev Nephrol 2016;12:678):
- A rare complication occurring in 2.4 - 15% of diffuse cutaneous scleroderma patients and 2% of limited cutaneous scleroderma patients (Ann Rheum Dis 2015;74:1124, QJM 2007;100:485)
- More commonly seen in the early stages (Ann Rheum Dis 2008;67:110)
- > 80% of patients in scleroderma renal crisis have diffuse cutaneous scleroderma (Ann Rheum Dis 2008;67:110)
- Incidence is decreasing as a result of effective monitoring
- Diffuse versus limited cutaneous systemic sclerosis (Lancet 2017;390:1685)
- Determined by extent of skin involvement
- Diffuse cutaneous systemic sclerosis affects proximal sites
- Limited cutaneous systemic sclerosis affects skin distal to elbows or knees with or without face and neck involvement
- Scleroderma involves multiple organ systems (Lancet 2017;390:1685)
- Pulmonary fibrosis, pulmonary arterial hypertension
- Gastrointestinal symptoms, intestinal pseudo-obstruction, gastric antral vascular ectasia
- Cardiac fibrosis
- Renal vasculopathy, scleroderma renal crisis
- Digital vasculopathy
- Scleroderma renal disease (J Rheumatol 2014;41:1040)
- Small arteries and arterioles (interlobular and arcuate arteries) are primarily and most significantly affected
- Glomeruli may be affected by thrombotic microangiopathy
- Glomeruli and tubules may also be secondarily affected by ischemic sequelae of severe vascular injury
- Scleroderma
- Disease mediated by vasculopathy, immune activation and inflammation and excess fibrosis in response to injury (Rheumatology (Oxford) 2019;58:2099)
- Scleroderma renal crisis
- Endothelial injury or activation by unclear mechanism leads to intimal proliferation, increased permeability and edema (Rheumatology (Oxford) 2019;58:2099)
- Vascular narrowing and hyperreactivity contribute to decreased renal perfusion and ischemic injury (J Rheumatol 2014;41:1040)
- Decreased renal perfusion activates the renin-angiotensin-aldosterone system which can result in accelerated hypertension and progressive renal injury (Int J Rheumatol 2010;2010:543704)
- Increased vascular permeability allows blood components to interact with extravascular connective tissue which can turn on the coagulation cascade and lead to vascular thrombosis as well as injury to adjacent connective tissue and stromal proliferation (Int J Rheumatol 2010;2010:543704)
- Other precipitating factors (J Rheumatol 2014;41:1040)
- Decreased intravascular volume (sepsis, dehydration, congestive heart failure)
- Nephrotoxic drugs (nonsteroidal antiinflammatory drugs, cocaine, cyclosporine, corticosteroids)
- Etiology of scleroderma renal vasculopathy and specifically, scleroderma renal crisis is unknown (Nat Rev Nephrol 2016;12:678)
- Pathogenesis of the vasculopathy may be immune mediated (Int J Rheumatol 2010;2010:543704)
- Cellular and humoral immune activation is described in scleroderma
- Specific autoantibodies are associated with scleroderma renal crisis
- Anti-RNA polymerase III is strongly associated with diffuse systemic sclerosis and scleroderma renal crisis (Arthritis Rheumatol 2016;68:2778)
- Scleroderma renal crisis develops in one third of patients with anti-RNA polymerase III autoantibody and scleroderma (Ann Intern Med 1993;119:1005)
- 85% of patients with scleroderma renal crisis are found to have anti-endothelial cell antibodies, which can cause endothelial cell apoptosis (Ann Rheum Dis 2010;69:319)
- Endothelin and its receptors (endothelin receptors A and B) are overexpressed in the glomeruli, tubules and vessels of patients in scleroderma renal crisis (Hum Pathol 2011;42:95)
- Isolated reduced glomerular filtration rate, reduced renal functional reserve, microalbuminuria and proteinuria (Int J Rheumatol 2010;2010:538589)
- Scleroderma renal crisis
- Acute onset of moderate to marked hypertension
- At least one sign of acute decline in renal function (increase in serum creatinine, proteinuria or hematuria) (Rheumatology (Oxford) 2019;58:2099)
- Sometimes hypertensive encephalopathy and retinopathy
- 90% of scleroderma renal crisis cases present with a blood pressure > 150/90 mm Hg (J Rheumatol 2014;41:1040)
- Scleroderma renal crisis can be the presenting manifestation (Nat Rev Nephrol 2016;12:678)
- 10% of scleroderma renal crisis cases occur without significant hypertension (Ann Rheum Dis 2008;67:110)
- Several diseases associated with scleroderma (Kidney Blood Press Res 2018;43:682, Int J Rheumatol 2010;2010:543704):
- Renal artery stenosis
- Antiphospholipid associated nephropathy
- Myeloperoxidase antineutrophil cytoplasmic antibody (MPO-ANCA) associated glomerulonephritis and vasculitis
- Acute onset of hypertension with evidence of renal failure in a patient with scleroderma
- Thrombotic microangiopathy of small renal arteries and arterioles, including interlobular and arcuate vessels and glomerular capillaries
- No laboratory parameter is predictive of scleroderma renal crisis (Nat Rev Nephrol 2016;12:678)
- Antinuclear antibody is positive in 90% of scleroderma renal crisis cases (Am J Kidney Dis 2016;67:e19)
- Anti-RNA polymerase III is present in 33% of cases (Medicine (Baltimore) 2013;92:1)
- Anticentromere antibodies are usually not detected (J Rheumatol 2014;41:1040)
- Elevated serum creatinine (J Rheumatol 2014;41:1040)
- Urinalysis frequently shows proteinuria and hematuria (J Rheumatol 2014;41:1040)
- If present, thrombocytopenia is usually moderate with a platelet count > 50,000/mm3 (J Rheumatol 2014;41:1040)
- Evidence of intravascular hemolysis may be present, such as increased schistocytes on peripheral blood smear and decreased haptoglobin (J Rheumatol 2014;41:1040)
- Scleroderma renal crisis has high mortality (30 - 40% at 5 years) and low survival (65% 5 year survival) despite monitoring and appropriate therapy (QJM 2007;100:485)
- Normotensive scleroderma renal crisis has a particularly unfavorable prognosis (Lancet 2017;390:1685, Hum Pathol 2009;40:332)
- Clinical factors of poor prognosis (J Rheumatol 2014;41:1040)
- Male gender
- Older age
- Congestive heart failure
- Serum creatinine level > 3 mg/dl at the initiation of treatment
- Difficulty controlling blood pressure beyond 3 days
- ACE inhibitor use prior to the onset of scleroderma renal crisis is associated with poor prognosis
- Patients who survive the first year of scleroderma renal crisis without requiring dialysis have a good long term outcome (J Rheumatol 2014;41:1040)
- Features of severe vascular injury, vascular thrombosis and fibrinoid change, as well as subsequent ischemic changes (glomerular ischemic collapse and acute tubular necrosis) are predictive of poor outcome (Int J Rheumatol 2010;2010:543704, Hum Pathol 2009;40:332)
- Peritubular C4d deposits may indicate a worse prognosis (Hum Pathol 2009;40:332)
- Predictive risk factors for development of scleroderma renal crisis in scleroderma patients include (Nat Rev Nephrol 2016;12:678, J Rheumatol 2014;41:1040):
- Diffuse and rapid skin involvement
- Disease duration of New onset anemia
- Cardiac events
- Systemic inflammatory features
- Anti-RNA polymerase III antibodies
- Moderate to high doses of gluocorticoids (> 15 mg/day) with or without other nephrotoxic drugs (calcineurin inhibitors, antithymocyte globulin, etc.) can increase risk (Nat Rev Nephrol 2016;12:678)
- 21 year old man with history of mixed connective tissue disease presented with acute renal failure and accelerated hypertension (Saudi J Kidney Dis Transpl 2014;25:844)
- 40 year old woman with renal failure, hypertension and microangiopathic hemolytic anemia treated with eculizumab (Case Rep Nephrol 2018;2018:6051083)
- 60 year old woman with Sjögren syndrome and cutaneous vasculitis presented with hypertension, elevated serum creatinine and mild thrombocytopenia (Clin Exp Rheumatol 2015;33:S171)
- 69 year old man with limited cutaneous systemic sclerosis presented in hypertensive crisis with anti-RNA polymerase III autoantibodies (Mod Rheumatol 2018;28:369)
- 70 year old woman with hypertension, elevated serum creatinine and serologies positive for antinuclear antibodies and myeloperoxidase antibodies (J Investig Med High Impact Case Rep 2018;6:2324709618785188)
- High dose ACE inhibitors are first line therapy (Nat Rev Nephrol 2016;12:678, Ann Rheum Dis 2017;76:1327)
- Additional antihypertensives that can be used in case of refractory hypertension include dihydropyridine calcium channel blockers, diuretics, clonidine, nitrates or other vasodilators, prazosin (Nat Rev Nephrol 2016;12:678, J Rheumatol 2014;41:1040)
- Beta blockers should not be used as they can provoke Raynaud phenomenon
- ET1 receptor antagonists (ERAs) as a standard therapy in addition to antihypertensive drugs are being evaluated (Nat Rev Nephrol 2016;12:678)
- Scleroderma related end stage renal disease can be managed with either dialysis or kidney transplantation (Nat Rev Nephrol 2016;12:678)
- Plasma exchange as a therapy for scleroderma associated microangiopathic hemolytic anemia is controversial (Rheumatology (Oxford) 2019;58:2099, J Rheumatol 2014;41:1040)
- Renal pathology findings in scleroderma patients without scleroderma renal crisis:
- Fibrointimal thickening of arteries (arteriosclerosis) (Int J Rheumatol 2010;2010:543704)
- Scleroderma renal crisis (Int J Rheumatol 2010;2010:543704, Am J Kidney Dis 2016;67:e19):
- Arterial / arteriolar injury
- Acute / early
- Endothelial swelling
- Intimal mucoid change
- Fibrin thrombi within arteries / arterioles or glomeruli
- Subacute to chronic
- Concentric intimal proliferation (onion skinning) and narrowing of the lumen
- Fibrointimal sclerosis
- Acute / early
- Glomeruli
- Acute
- Ischemic corrugation
- Endothelial swelling
- Mesangiolysis
- Glomerular capillary thrombosis
- Chronic
- Glomerular basement membrane duplication and glomerulosclerosis
- Acute
- Tubulointerstitium
- Ischemic effect on tubules is acutely seen as acute tubular injury or necrosis
- Ongoing injury can result in tubular atrophy and interstitial fibrosis
- Mild interstitial lymphohistiocytic inflammatory infiltrate may be present
- Arterial / arteriolar injury
- Fibrinogen staining is in areas of vascular or glomerular thrombosis (Int J Rheumatol 2010;2010:543704, Am J Kidney Dis 2016;67:e19)
- Peritubular C4d deposits may suggest antibody mediated injury and indicate a worse prognosis as staining appears to be increased in patients who died or had irreversible loss of renal function (Hum Pathol 2009;40:332)
- Negative staining for immunoglobulins within glomeruli
- Trichrome can highlight fibrin thrombi and interstitial fibrosis
- Jones silver highlights onion skin change and glomerular double contours
- Endothelial cell injury (Int J Rheumatol 2010;2010:543704, Am J Kidney Dis 2016;67:e19)
- Swollen endothelial cells
- Expansion of subendothelial space within glomeruli with interporision of lucent material and cell processes
- Platelet aggregates and fibrin tactoids in areas of thrombosis
- Glomerular basement membrane duplication can be seen in cases of chronic injury
- Swollen endothelial cells within vessels
- Subendothelial edema and basement membrane remodeling within vessels
- Left kidney, native, needle core biopsy:
- Diffuse active chronic thrombotic microangiopathy (TMA), arterial predominant, consistent with scleroderma associated renal disease (see comment)
- Comment: While the marked arterial changes are highly consistent with scleroderma associated renal disease, the differential diagnosis for these findings includes any potential cause of thrombotic microangiopathy (e.g. TTP, HUS, complement associated TMA, antiphospholipid antibody syndrome, drug toxicity, infection, etc.).
- The main differential diagnoses include other renal diseases that cause thrombotic microangiopathy:
- Scleroderma renal crisis generally shows vascular > glomerular involvement
- No specific morphologic features can reliably distinguish underlying etiologies for thrombotic microangiopathy on renal biopsy alone
- Differential diagnosis for thrombotic microangiopathy on renal biopsy includes:
- Thrombotic thrombocytopenic purpura:
- Low ADAMTS13 levels (Int J Rheumatol 2010;2010:543704, Nat Rev Nephrol 2016;12:678, J Rheumatol 2014;41:1040)
- Complement mediated thrombotic microangiopathy (atypical hemolytic uremic syndrome):
- Genetic or acquired abnormalities in alternative complement cascade may be present
- Hemolytic uremic syndrome associated with E. coli enterocolitis
- Viral infections (e.g. HIV)
- Antiphospholipid antibody syndrome
- Many medications (e.g. quinine, gemcitabine and cyclosporin) (Rheumatology (Oxford) 2019;58:2099)
- Malignancy
- Illicit drugs (e.g. cocaine)
- Metabolism mediated thrombotic microangiopathy, such as disorders of intracellular vitamin B12 metabolism (Rheumatology (Oxford) 2019;58:2099)
- Thrombotic thrombocytopenic purpura:
- The differential diagnosis in a renal allograft would include other entities:
- Antibody mediated rejection
- Calcineurin inhibitor toxicity
- Infection and other less common allograft related abnormalities (Int J Rheumatol 2010;2010:543704)
Comment Here
Reference: Scleroderma renal disease
- Associated with low ADAMTS13 levels
- Can present with normal or only mildly elevated blood pressure
- Evidence of acute renal failure is not required for diagnosis
- Microangiopathic hemolytic anemia is seen in all cases
- More commonly seen in patients with limited rather than diffuse cutaneous systemic sclerosis
Comment Here
Reference: Scleroderma renal disease
- Autoimmune disease characterized by lymphoplasmacytic inflammation in exocrine glands, leading to dry eyes and mouth (sicca syndrome) (Nat Rev Nephrol 2016;12:82)
- Renal disease is present in < 10% of patients with Sjögren syndrome (Nat Rev Nephrol 2016;12:82)
- Usually manifests as plasma cell rich tubulointerstitial nephritis
- ICD-10: N16.4 - Renal tubulointerstitial disorders in systemic connective tissue disorders
- Primary Sjögren syndrome affects 0.01 - 0.1% of population (Ann Rheum Dis 2015;74:1983)
- F:M = 9:1
- Peak incidence at age 50 (N Engl J Med 2018;378:931)
- Renal involvement in 5 - 14% of Sjögren patients in European studies and 30% in a Chinese study (Nat Rev Nephrol 2016;12:82)
- Kidney, mouth, eyes
- Genetic predisposition plus environmental factors (Nat Rev Nephrol 2016;12:82)
- Injury to salivary gland and local presentation of autoantigens with T and B cell activation
- Release of type 1 and type 2 interferons promoting T cells, continuous B cell activation and antibody production
- Renal disease may be caused by tubulointerstitial infiltration by lymphocytes and plasma cells or by autoantibodies to tubular epitopes, some with associated distal renal tubular acidosis (RTA) (Nat Rev Nephrol 2016;12:82)
- Renal disease may be caused by immune complexes (commonly cryoglobulins) in the glomerulus, leading to a membranoproliferative glomerulonephritis (Nat Rev Nephrol 2016;12:82)
- Likely multifactorial with genetic component and viral or exogenous instigator (Nat Rev Nephrol 2016;12:82)
- Renal involvement occurs 2 - 7 years after primary Sjögren syndrome diagnosis (Nat Rev Nephrol 2016;12:82)
- Tubulointerstitial nephritis:
- Present in 67% of Sjögren patients undergoing renal biopsy
- Lymphoplasmacytic infiltrate with chronic and active damage of tubules and interstitium
- Damage may lead to electrolyte disturbances associated with distal renal tubular acidosis and diabetes insipidus and more rarely Fanconi / proximal tubular acidosis or acquired Gitelman or Bartter syndrome (renal hypokalemia with secondary hyperaldosteronism) (Nat Rev Nephrol 2016;12:82, Rheumatology (Oxford) 2015;54:1541)
- Cryoglobulinemic membranoproliferative glomerulonephritis (MPGN):
- 5 - 30% of patients of Sjögren patients undergoing renal biopsy
- Due to continuous B cell activation and subsequent immune complex formation with already circulating autoantigens (Nat Rev Nephrol 2016;12:82, Rheumatology (Oxford) 2015;54:1541)
- Often a mixed (type 2 or 3) cryoglobulin with an IgM (rheumatoid factor) directed against the Fc portion of IgG but may be monoclonal (type 1 cryoglobulin) if associated with lymphoproliferative disorder (Arthritis Rheum 2013;65:2945)
- Sjögren syndrome has classic triad of sicca syndrome (dry eyes and mouth), fatigue and pain (N Engl J Med 2018;378:931, Nat Rev Nephrol 2016;12:82)
- If symptomatic, kidney biopsy (Rheumatology (Oxford) 2017;56:362)
- Tubulointerstitial nephritis
- Immune complex mediated glomerulonephritis
- Rule out other autoimmune diseases
- Anti-Ro (SSA) antibodies in 66% of patients
- Anti-La (SSB) antibodies relatively specific
- Rheumatoid factor positive in 50% of patients
- dsDNA usually negative (N Engl J Med 2018;378:931)
- Tubulointerstitial nephritis is generally associated with less severe manifestations and better prognosis than membranoproliferative glomerulonephritis but more likely to remain undetected and can lead to chronic kidney disease (Arthritis Rheum 2013;65:2945, Nat Rev Nephrol 2016;12:82)
- Glomerular disease is more likely to be symptomatic and treated earlier (Nat Rev Nephrol 2016;12:82)
- 24 year old woman with diabetes insipidus and renal tubular acidosis (Am J Case Rep 2017;18:622)
- 37 year old woman with acute interstitial nephritis, renal tubular acidosis and thin basement membrane nephropathy (Medicine (Baltimore) 2020;99:e21644)
- 44 year old woman with distal renal tubular acidosis (BMJ Case Rep 2019;12:e230402)
- 44 year old man with cryoglobulinemic glomerulonephritis and spontaneous kidney rupture (Int Med Case Rep J 2016;9:77)
- 47 year old woman presenting with hypophosphatemic osteomalacia (Medicine (Baltimore) 2017;96:e6493)
- Tubulointerstitial nephritis:
- Immunosuppression or immunomodulation (Nat Rev Nephrol 2016;12:82, Int J Med Sci 2017;14:191, BMC Musculoskelet Disord 2016;17:2)
- Membranoproliferative glomerulonephritis due to cryoglobulinemia:
- Immunosuppression or immunomodulation (Nat Rev Nephrol 2016;12:82)
- Tubulointerstitial nephritis:
- Plasma cell rich interstitial inflammation with tubulitis, mixed B and T cells (Am J Kidney Dis 2017;69:e29)
- May have tubular basement membrane or interstitial immune deposits detectable by immunofluorescence microscopy
- Membranoproliferative glomerulonephritis:
- Mesangial expansion due to increased matrix and cellularity
- Endocapillary hypercellularity with occlusion of capillary lumina due to endothelial cell swelling and influx of leukocytes
- Duplication of glomerular basement membranes (tram tracking)
- Immune complex deposition, often of IgG and IgM, in mesangial and subendothelial regions of peripheral capillary loops
- Cryoglobulin plugs of immune complexes in capillary microvasculature
- References: Nephrol Dial Transplant 2015;30:1363, Nat Rev Nephrol 2016;12:82, Rheumatology (Oxford) 2017;56:362
- Jones silver stain, periodic acid Schiff and trichrome highlight basement membranes and renal architecture
- T, B and plasma cell markers dependent upon makeup of lymphocytic infiltrate (Rom J Morphol Embryol 2017;58:409)
- IgG4 low
- Left kidney, biopsy:
- Plasma cell rich tubulointerstitial nephritis (see comment)
- Comment: This patient’s renal insufficiency is due to a chronic and active, plasma cell rich tubulointerstial nephritis. Although not specific to etiology, in the provided clinical context of Sjögren syndrome with anti-Ro / SSA and anti-La / SSB antibodies, the findings are consistent with Sjögren associated tubulointerstitial nephritis. There is no significant eosinophilic infiltrate to suggest a hypersensitivity reaction, nor IgG4 positive plasma cells to suggest IgG4 related tubulointerstitial nephritis. There is no evidence of a glomerulonephritis nor immune complex deposition process. Chronic injury is mild. (Microscopic description, Immunofluorescence microscopy and Electron microscopy descriptions should also be reported.)
- IgG4 related disease (J Am Soc Nephrol 2011;22:1343):
- Lacks female predominance
- Has expansile storiform fibrosis
- Abundant IgG4+ plasma cells by immunohistochemistry
- Different criteria for systemic disease, including elevated serum levels of IgG4 or IgG
- Drug induced allergic interstitial nephritis:
- Eosinophils more common
- Tubular basement membrane or interstitial immune deposits less common
- Correlate with clinical history
- Sarcoidosis:
- Granulomas more common
- Correlate with clinical history
- Hepatitis C and other causes of cryoglobulinemic glomerulonephritis:
- Determine whether the glomerular immune deposits are monoclonal (type 1 cryoglobulin, associated with a lymphoproliferative disorder) or polyclonal (type 2 or 3 cryoglobulin)
- Correlate with clinical and laboratory findings, rheumatoid factor and circulating cryoglobulins
- Reference: Nat Rev Nephrol 2016;12:82
- Increase in IgG4+ plasma cells
- Lymphoplasmacytic tubulointerstitial nephritis
- Membranous nephropathy
- Pseudotumoral infiltrate
- Spontaneous renal artery dissection is a rare entity with fewer than 200 cases reported in the literature
- SRAD is a rare condition and accurate clinical diagnosis is often delayed
- Clinical presentation of SRAD is nonspecific, including hypertension, hematuria, flank pain or lower back pain as in renal colic
- SRAD is more common in male patients
- SRAD usually is unilateral but 10 - 15% of cases involve both renal arteries
- The consequence of SRAD is renal hypoperfusion, severe hypertension, renal infarction and decreased or lost renal function
- Treatment includes anticoagulation, revascularization and nephrectomy
- Healthy men in fourth to sixth decade of life with 4:1 male to female ratio
- Mostly involves one kidney; about 10 - 15% of cases have bilateral involvement
- Renal vascular hypertension, renal insufficiency and renal infarction
- Flank pain, severe hypertension and renal insufficiency
- Abdominal CT with contrast and angiogram
- Decreased renal function tests
- CT scan often shows renal infarction, angiogram remains the definitive study (Rev Urol 2007;9:156)
- Six cases in 33 - 55 year old men complicated with renal infarction (Clin Kidney K 2012;5:261)
- Anticoagulation, endovascular management (stenting or coiling) and nephrectomy
- Dilated renal artery at the hilar region of the affected kidney and variable sized renal infarction
- Renal artery dissection with elongated hematoma in renal artery wall, renal cortical ischemic infarction and renal papillae hemorrhagic infarction
- Spontaneous renal artery dissection is a rare clinical condition
- The entity is more commonly seen in male patients
- Clinical presentation includes frank pain and hypertension
- Spontaneous renal artery dissection usually involves both renal arteries
Comment Here
Reference: Spontaneous Renal Artery Dissection (SRAD)
- Lupus nephritis (LN) is an immune complex mediated renal injury encountered in systemic lupus erythematosus (SLE)
- Associated with activation of the classical complement pathway and presents as glomerular, tubulointerstitial and vascular disease, significantly increasing the risk of death in systemic lupus erythematosus patients
- Diagnosis is made in patients with a clinical diagnosis of systemic lupus erythematosus
- Light, immunofluorescence and electron microscopy are required for the correct classification of lupus nephritis
- Lupus nephritis class is determined by the histological type of glomerular changes (mesangial, endocapillary / extracapillary, membranous or global sclerosing) and the localization and abundance of the immune complexes
- Full house immunofluorescence staining, significant C1q positivity, extraglomerular immunostaining and the finding of tubuloreticular inclusions by electron microscopy establish the lupus etiology of nephritis
- Mesangial hypercellularity: > 3 nuclei fully surrounded by matrix in ≥ 1 mesangial areas, not including perihilar region, on a 3 um section (Kidney Int 2020;98:1120)
- Crescent: a lesion consisting of extracapillary hypercellularity forming more than 2 cell layers, composed of a variable mixture of cells
- Fibrin and fibrous matrix may be present
- 10% or more of Bowman capsule should be involved (Kidney Int 2018;93:789)
- Cellular crescent: > 75% cells and fibrin and Kidney Int 2018;93:789)
- Fibrous crescent: > 75% fibrous matrix and Kidney Int 2018;93:789)
- Fibrocellular crescent: 25 - 75% cells and fibrin and the remainder fibrous matrix (Kidney Int 2018;93:789)
- Fibrinoid necrosis: fibrin associated with glomerular basement membrane disruption or lysis of the mesangial matrix (Kidney Int 2018;93:789)
- Karyorrhexis: presence of apoptotic, pyknotic and fragmented nuclei (Kidney Int 2020;98:1120)
- Commonly seen in women in the third decade
- More common in African, Asian and Hispanic populations than in Europeans
- Lupus nephritis most often occurs during the first 3 years after the diagnosis of systemic lupus erythematosus
- Males, patients Reference: UpToDate: Lupus Nephritis - Diagnosis and Classification [Accessed 21 April 2023]
- Kidney glomeruli, tubulointerstitial compartment and less frequently, renal arteries
- Anti-double stranded DNA (dsDNA) antibody binds to dsDNA, forming an immune complex
- Immune complexes deposit in the glomerular compartments according to their charge
- Inflammatory response with activation of the classical complement pathway leads to an influx of inflammatory cells
- Complement activation damages the glomerular endothelium and dying neutrophils release nuclear antigens, enhancing the inflammatory response (Kidney Int 2016;90:493)
- Lupus nephritis is primarily caused by type 3 (immune complex) hypersensitivity reaction
- Hydralazine can unmask and aggravate incipient lupus; other most common medications implicated in drug induced lupus are procainamide, isoniazid, minocycline and TNF inhibitors (Cureus 2019;11:e4996)
- Lupus nephritis is typically detected by an abnormal urinalysis (see Laboratory)
- Classification of LN, International Society of Nephrology (ISN) / Renal Pathology Society (RPS), 2004 (J Am Soc Nephrol 2004;15:241)
- Class I, minimal mesangial LN: glomeruli are normal by light microscopy (LM), mesangial immune deposits by immunofluorescence (IF) or electron microscopy (EM)
- Class II, mesangial proliferative LN: pure mesangial hypercellularity of any degree or mesangial expansion by light microscopy, mesangial immune deposits with none or rare isolated subepithelial or subendothelial deposits by immunofluorescence or electron microscopy
- Class III, focal LN (involving Class III (A), purely active lesions: focal proliferative LN
- Class III (A / C), active and chronic lesions: focal proliferative and sclerosing LN
- Class III (C), chronic inactive with glomerular scars: focal sclerosing LN
- Class IV, diffuse LN (involving > 50% of glomeruli): active or inactive diffuse endocapillary or extracapillary glomerulonephritis with diffuse subendothelial immune deposits; mesangial alterations may or may not be present
- Class IV-S: segmental lesions
- Class IV-G: global lesions
- Class IV-S (A) or IV-G (A), purely active lesions: diffuse segmental or global proliferative LN
- Class IV-S (A / C) or IV-G (A / C), active and chronic lesions: diffuse segmental or global proliferative and sclerosing LN
- Class IV-S (C) or IV-G (A / C), chronic inactive with glomerular scars: diffuse segmental or global sclerosing LN
- Class V, membranous LN: global or segmental subepithelial immune deposits at any stage () by light microscopy or immunofluorescence / electron microscopy, with or without mesangial alterations
- Class VI, advanced sclerosing LN: no activity, 90% or more of glomeruli globally sclerosed
- Combined class III / IV and V (focal or diffuse and membranous LN) may be diagnosed if the subepithelial immune deposits involve at least 50% of the glomerular capillary surface area in at least 50% of glomeruli in the presence of class III / IV lesions
- Lupus nephritis can transform from one class to another
- 2017 proposal for revision of the ISN / RPS Classification includes the elimination of the S and G subdivisions in class IV, elimination of the term endocapillary proliferation and modified activity / chronicity indices that should be applied to all classes (see Microscopic description) (Kidney Int 2018;93:789)
- Patients with known lupus who develop persistent hematuria, proteinuria, active urinary sediment, elevated serum creatinine / glomerular filtration rate decrease, low complement, elevated anti-dsDNA
- Kidney biopsy establishes the diagnosis and guides treatment
- Class I LN: normal urinalysis, no or minimal proteinuria, normal serum creatinine
- Class II LN: microhematuria or mild proteinuria
- Class III LN: hematuria and proteinuria, which may be accompanied by nephrotic syndrome, active urinary sediment, decreased glomerular filtration rate, less frequently hypertension, rarely renal insufficiency; reduced complements and positive anti-dsDNA antibody
- Class IV LN: hematuria, proteinuria with nephrotic syndrome, red blood cell casts, hypertension, decreased glomerular filtration rate and renal failure are frequent; low complements, elevated anti-dsDNA antibody levels
- Class V LN: nephrotic range proteinuria, reduced complements, positive anti-dsDNA antibody
- Silent LN: kidney biopsy with class III, IV or V without any clinical evidence of kidney disease (UpToDate: Lupus Nephritis - Diagnosis and Classification [Accessed 21 April 2023])
- Ultrasound, CT, MRI and nuclear medicine have only a minor role in the diagnosis of lupus nephritis (F1000Res 2015;4:153)
- Iodine contract dyes are not indicated in patients with lupus nephritis
- MRI can detect interstitial edema in patients with lupus nephritis
- LN classes I and II: excellent renal prognosis
- LN class III: favorable prognosis with no histological progression and renal function deterioration; a small percent progressing to class IV and severe renal damage
- LN class IV: worst renal prognosis, with class IV-S possibly faring worse than IV-G (controversial)
- LN class V: predisposed to thrombotic complications, such as renal vein thrombosis and pulmonary emboli
- Serological markers of worse renal outcome are low complement levels, high titer of anti-C1q and anti-dsDNA antibodies (Lupus 2021;30:2256)
- Histological markers of worse renal outcome are significant interstitial fibrosis and tubular atrophy, vascular involvement and thrombotic microangiopathy
- African American patients, Hispanic patients and those living in poverty have a worse prognosis (UpToDate: Lupus Nephritis - Diagnosis and Classification [Accessed 21 April 2023])
- 10 - 30% of patients with lupus nephritis progress to end stage renal disease (Adv Chronic Kidney Dis 2019;26:313)
- 24 year old woman with lupus podocytopathy superimposed on diabetic glomerulosclerosis (Medicine (Baltimore) 2021;100:e27077)
- 34 year old woman with membranous glomerulopathy diagnosed as lupus nephritis 11 years after onset (CEN Case Rep 2019;8:301)
- 51 year old woman with lupus vasculopathy presenting with favorable renal outcome (CEN Case Rep 2020;9:74)
- Treatment is guided by the LN class, activity and chronicity indices
- LN classes I and II require blockade of the renin-angiotensin-aldosterone system (RAAS) in all patients with hypertension or proteinuria
- LN class III, class IV and class V require immunosuppressive therapy
- Goal of immunosuppressive therapy is a complete response (a substantial reduction in protein excretion, improvement or stabilization of the serum creatinine, improvement of the urinary sediment) (Ann Rheum Dis 2020;79:713)
- Immunosuppressive therapy consists of initial and subsequent phases
- Initial (induction) therapy typically consists of cyclophosphamide or mycophenolate (MMF) and glucocorticoid
- Subsequent (maintenance) therapy consists of MMF or azathioprine
- Outcomes of kidney transplantation in systemic lupus erythematosus are comparable to nonlupus patients (Adv Chronic Kidney Dis 2019;26:313)
- LN class I: normal glomeruli by light microscopy
- LN class II: mesangial hypercellularity or mesangial matrix expansion
- LN classes III - IV: lesions may be active (endocapillary or extracapillary proliferation) or chronic (sclerosing)
- Wire loop deposits, hyaline microthrombi, membranoproliferative lesions
- Fibrinoid necrosis and karyorrhexis may be present
- LN class V: thickening of the glomerular capillary walls, microspikes and domes may be associated with any mesangial alterations
- LN class VI: extensive glomerular sclerosis
- Lupus podocytopathy: diffuse and severe foot process effacement in the absence of subendothelial or subepithelial immune deposits
- Tubulointerstitial disease: observed in all LN classes, active in LN classes III and IV consisting of lymphocytic interstitial inflammation with plasma cells, monocytes / macrophages and edema
- Vascular lesions: uncomplicated vascular immune deposits, noninflammatory necrotizing vasculopathy, vasculitis, thrombotic microangiopathy, vascular thrombi with recanalization in lupus anticoagulant syndrome
- LN activity and chronicity scoring system modified in 2018 and is based on scoring of 1 ( 50% of glomeruli) (Kidney Int 2018;93:789)
- LN activity index
- Endocapillary proliferation
- Neutrophils / karyorrhexis
- Fibrinoid necrosis (score x 2)
- Hyaline deposits
- Cellular / fibrocellular crescents (score x 2)
- Interstitial inflammation (total score 0 - 24)
- LN chronicity index
- Total glomerulosclerosis score (global or segmental sclerosis; exclude sclerosis due to hypertension and normal aging)
- Fibrous crescents
- Tubular atrophy
- Interstitial fibrosis (total score 0 - 12)
- Reference: Clin Rev Allergy Immunol 2011;40:170
Contributed by Alexei Mikhailov, M.D., Ph.D.
- Diagnostic criteria: IgG dominant full house immunofluorescence (positive IgG, IgA, IgM, C3, C1q, kappa and lambda light chains)
- Especially important are C1q positivity and extraglomerular immunostaining on the Bowman capsules, arteries, tubular basement membranes and in the interstitium
- Immunofluorescence staining is granular and may be subepithelial, intramembranous, subendothelial or mesangial
- In some cases, antinuclear antibody (ANA) test is seen with antibodies to IgG and the light chains
- Reference: Clin J Am Soc Nephrol 2019;14:1605
- PAS special stain is the most useful staining method in every LN class to evaluate mesangial and endocapillary changes
- Masson trichrome special stain is most useful in LN classes III and IV and helps to detect foci of fibrinoid necrosis, glomerular and interstitial sclerosing changes
- PAMS (Jones Silver) special stain is most useful in LN class V and emphasizes capillary wall thickening and neomembrane formation (microspikes and domes)
- IgG1, IgG2 and IgG3 subclasses are highly expressed in lupus nephritis (Intern Med 2002;41:936)
- CD68 immunostaining identifies cells of macrophage lineage, especially useful in evaluating hypercellular glomeruli (Kidney Int Rep 2022;7:841)
- PLA2R
- Finalizes the classification of lupus nephritis for a given case
- Granular electron dense deposits (smaller in LN classes I - II and larger in classes III - IV) are seen in subepithelial, intramembranous, subendothelial and mesangial locations; locations are important in determining the LN class
- Subepithelial immune deposits show adjacent neomembrane formation
- Immune deposit substructure may be present
- Tubuloreticular inclusions: reticular aggregates of branching membranous tubule within the cisternae of endoplasmic reticulum found within the endothelial cells are useful in establishing the diagnosis of lupus erythematosus; are also found in other conditions with elevated levels of interferon (Pathology 2019;51:727)
- HLA-DR3, HLA-DR15 and HLA-DR3 are risk alleles for lupus nephritis (Clin Immunol 2017;185:32)
- Variants in ITGAM (CD11b integrin), FCGR (Fc gamma receptor), IRF5 (interferon regulatory factor 5), TNIP1 (inhibitor of NFκB 1), TNFSF4 (TNF super family 4), APOL1 (apolipoprotein L1), PDGFRA (platelet derived growth factor receptor alpha) are associated with lupus nephritis (Clin Immunol 2017;185:32)
- Kidney, biopsy:
- Diffuse glomerulonephritis with 20% cellular crescents (4/20) and fibrinoid necrosis and focal and segmental glomerular scarring, consistent with lupus nephritis ISN / RPS class IV-G (A / C) (see comment)
- 10% focal global glomerulosclerosis (2/20)
- Mild arteriosclerosis
- Mild interstitial fibrosis and tubular atrophy
- Comment: activity - 8/24, chronicity - 3/12
- Clinical information: The patient is a 47 year old woman with a history of systemic lupus erythematosus and hypertension. Recent lupus flare, proteinuria and acute kidney injury.
- Laboratory data
- Serum creatinine: 1.5 mg/dL
- UPC: 1800 mg/g creatinine
- C3: 47 mg/dL
- C4: 48 mg/dL
- Urinalysis: 150 RBC/HPF, 40 WBC/HPF
- Light microscopy: The biopsy consists of tissue from the renal cortex. The tissue is sampled at 10 levels of section with H&E, PAS, trichrome and PAMS stains. Per level of section up to 20 glomeruli are available, of which 2 are globally sclerosed and a few are segmentally sclerosed. 4 glomeruli display cellular crescents, fibrinoid necrosis and karyorrhexis in the capillary tuft and in the crescent. The nonsclerosed glomeruli display marked endocapillary hypercellularity. Tubules show focally flattened epithelium and absent brush borders; small foci of interstitial lymphocytic infiltration are seen. There is mild interstitial fibrosis and tubular atrophy. Arteries of the medium caliber are present and show mild intimal fibroelastosis; there is no significant arteriolar hyalinosis.
- Immunofluorescence microscopy: Up to 5 nonsclerosed glomeruli are available for evaluation. Mild to high intensity granular immunofluorescence staining was detected in the mesangial areas and less in the capillary walls with antibodies to IgG (4+), IgA (3+), IgM (2+), C3 (4+), C1q (3+), kappa light chains (4+) and lambda light chains (4+). Extraglomerular staining is seen on the arterial walls with antibodies to IgG.
- Semithin sections: 4 nonsclerosed glomeruli are available and 1 of them shows a fibrocellular crescent. All glomeruli show endocapillary hypercellularity. Foci of mild lymphocytic infiltration are seen in the interstitium.
- Electron microscopy: Glomerular basement membranes are of normal thickness. Numerous subendothelial electron dense (immune complex) deposits are seen. There is mild segmental podocyte foot process effacement. Capillary lumens are patent. The mesangium shows a moderately expanded mesangial matrix and contains large electron dense deposits.
- IgA nephropathy:
- IgA dominant
- Postinfectious glomerulonephritis:
- Hump-like subepithelial immune deposits do not show adjacent microspikes
- C1q nephropathy:
- No clinical or serological evidence of lupus
- Primary (idiopathic) membranous glomerulopathy:
- Membranoproliferative glomerulonephritis:
- No C1q positivity
- Fibrillary glomerulonephritis:
- DNAJB9 positive
- Drug induced lupus nephritis:
- No dsDNA antibodies
- Acute tubular injury
- ANA reaction
- Mesangial electron dense deposits
- Microspikes and domes (neomembrane formation) seen with the Jones stain
Comment Here
Reference: Systemic lupus erythematosus
- Hyaline microthrombi
- Mesangial proliferation
- Positive immunostaining on the arterioles and tubular basement membranes with antibodies to IgG
- Subendothelial electron dense deposits
Comment Here
Reference: Systemic lupus erythematosus
Biopsy is done on a patient with a history of systemic lupus erythematosus and nephrotic syndrome. Electron microscopy reveals the findings shown above. Which complication is the patient predisposed to?
- Fibromuscular dysplasia of the renal artery
- Oxalate nephropathy
- Papillary necrosis
- Renal vein thrombosis
Comment Here
Reference: Systemic lupus erythematosus
- Rare large vessel vasculitis
- Usually occurs in patients younger than 50 years old
- May present with renovascular hypertension, anecdotal association with wide range of renal glomerulopathies
- Takayasu arteritis usually involves aorta and its major branches
- Inflammation is granulomatous with lymphoplasmacytic infiltrate and varying numbers of giant cells
- Renal disease is present in most cases, usually as nonspecific changes due to chronic hypertension and renal artery stenosis and other vascular changes (Clin Exp Rheumatol 2007;25:S10)
- Pulseless disease
- ICD-10: M31.4 - aortic arch syndrome [Takayasu]
- Incidence varies: 0.9/million (U.S.); 4.7 - 33/million (Europe); 40/million (Japan) (Int J Nephrol Renovasc Dis 2018;11:225)
- F:M also varies; overall is 8.2 - 9.7:1 but is 1.6 - 2:1 in Middle East and India (J Autoimmun 2014;48:79)
- Women frequently have involvement of the thoracic aorta and its branches
- Men are more likely to have involvement of abdominal aorta and its branches
- Patients commonly 20 - 40 years old (Best Pract Res Clin Rheumatol 2010;24:871)
- Renal artery involvement in Takayasu arteritis is higher in Asian populations and overall ranges from 11.5% to 62%
- Aorta and its major branches
- Kidney
- Genetic predisposition plus environmental factors
- Virus or bacteria via molecular mimicry mechanism leads to autoimmune response (Pathogens 2018;7:E73)
- Inflammatory cells implicated include gamma delta, CD4 and CD8 T cells, natural killer cells, macrophages, dendritic cells and B cells (Am J Med Sci 2013;346:314)
- Autoantibodies include antiaortic, antiendothelial and circulating immune complexes (Autoimmun Rev 2017;16:146)
- Associated with HLA-B genes, especially HLA-B*52 and HLA-B*67 in certain populations and TNFα genes (Int J Nephrol Renovasc Dis 2018;11:225)
- 2 stages of disease:
- Early inflammatory stage with nonspecific symptoms and transmural inflammation
- Later pulseless phase with hypertension, ischemia and occlusive changes including intimal fibrosis
- Precise etiology unknown; likely multifactorial with genetic component and potentially viral or bacterial instigator (Pathogens 2018;7:E73)
- Usually subclinical or nonspecific symptoms at disease onset
- Constitutional symptoms (night sweats, weight loss, fever)
- Limb fatigue and pain, different or absent pulses in extremities, blood pressure difference or unobtainable blood pressure (J Autoimmun 2014;48:79)
- Chapel Hill Consensus Conference 2012: large vessel vasculitis of patients < 50 years old
- 1990 American College of Rheumatology Classification Criteria for Takayasu arteritis: 3 or more of the following criteria yields a sensitivity of 90.5% and a specificity of 97.8% (Arthritis Rheum 1990;33:1129):
- Age at disease onset < 40 years
- Claudication of extremities
- Decreased brachial artery pulse
- Blood pressure difference > 10 mm Hg
- Bruit over subclavian arteries or aorta
- Arteriogram abnormality
- Nonspecific
- Elevated acute phase reactants seen, including erythrocyte sedimentation rate, C reactive protein and IL6
- IL6 best correlates with degree of disease (Am J Med Sci 2013;346:314, Int J Nephrol Renovasc Dis 2018;11:225)
- Early: arterial wall thickening and enhancement
- Late: arterial stenosis, occlusions, aneurysms (AJR Am J Roentgenol 2005;184:1427)
- Advances in imaging leading to early detection has improved outcomes (Int J Cardiol 2013;168:3)
- Disease course generally indolent with 10 year survival ranging from 67.4% to 100%, depending on patient ethnicity (Int J Nephrol Renovasc Dis 2018;11:225)
- 3 toddlers with constitutional symptoms and malignant hypertension (Pediatr Nephrol 2009;24:1227)
- 15 year old girl with oliguria and acute kidney injury (J Bras Nefrol 2019;41:564)
- 31 year old woman with proteinuria (Clin Res Cardiol 2020;109:1438)
- 54 year old man with progressive edema and nephrotic proteinuria (Case Rep Nephrol Urol 2014;4:60)
- 6 patients with glomerulopathy (Chin Med Sci J 2009;24:69)
- Glucocorticoids and other immunosuppressive therapy including methotrexate, azathioprine and cyclophosphamide
- New studies show success with biologic therapy: anti-TNFα, anti-IL6R and monoclonal antibodies (Curr Opin Rheumatol 2014;26:7)
- Surgical repair: stent or bypass, depending on length of segment (Circulation 2012;125:813, Rheumatology (Oxford) 2006;45:600)
- Vascular sclerosis, nonspecific scarring, ill defined white mass-like lesions
- Granulomatous vasculitis with mixed inflammatory infiltrate, including giant cells, with necrosis, disruption of the elastic lamina and features of recanalization (Int J Nephrol Renovasc Dis 2018;11:225)
- Varying renal vascular and parenchymal changes associated with the degree of chronic hypertension and renal ischemia: atherosclerosis, ischemic change, atrophy, infiltrating lymphocytes, tubular dilation and narrowing and glomerulosclerosis
- Specific glomerular and tubulointerstitial changes are rare and often manifestations of chronic disease (Chin Med Sci J 2009;24:69)
- Reported glomerular disease includes: mesangial proliferative glomerulonephritis, IgA nephropathy, membranous glomerulonephropathy, membranoproliferative glomerulonephropathy, crescentic glomerulonephropathy, renal amyloidosis and focal glomerulosclerosis (Clin Exp Rheumatol 2007;25:S10)
- Elastin will show disruption of the elastic lamina
- Use immune cell markers as needed
- Fungal, viral, spirochete, acid fast stains to rule out infectious etiologies for granulomatous inflammation
- Right kidney, nephrectomy:
- Arteritis with granulomatous inflammation and multinucleated giant cells, active and chronic (see comment)
- Comment: Differential diagnosis for the large vessel vasculitis includes Takayasu arteritis and giant cell arteritis. Other etiologies for the vasculitis include antineutrophil cytoplasmic antibody associated vasculitis, necrotizing sarcoid vasculitis, polyarteritis nodosa or possibly infection. No acid fast or fungal organisms are identified on special stains. Takayasu arteritis usually affects the aorta and its major branches and is more common in women under the age of 50.
- Giant cell arteritis:
- Patient population usually > 50 years, M:F = 1:2
- More common in Caucasian populations
- Temporal artery most frequently involved
- Usually presents with polymyalgia rheumatica and localized headache
- Giant cell arteritis seen in medial layer rather than transmural (Ann Rheum Dis 2012;71:1329, Medicine (Baltimore) 2009;88:221, Int J Rheum Dis 2019;22:41)
- Fibromuscular dysplasia:
- Younger female patients
- Multiple arteries are involved including renal and carotid
- Fibromuscular thickening of media with irregular thinning and loss of internal elastic lamina
- Not a large vessel vasculitis
- Aorta is usually not involved (J Am Soc Hypertens 2018;12:506)
- Atherosclerosis:
- Usually older patients
- Primarily intimal sclerosis, eccentric (Int J Cardiol 2013;168:3)
- Not a large vessel vasculitis
- Infectious vasculitis:
- Rickettsial, bacterial, fungal, viral organisms
- Not typically a large vessel vasculitis
- Granulomatous vasculitis due to sarcoidosis:
- Sarcoid may cause a large vessel vasculitis mimicking Takayasu arteritis
- Distinguish clinically (Clin Exp Rheumatol 2000;18:401)
- Isolated granulomatous arteritis:
- Associated with Marfan syndrome (J Clin Pathol 2012;65:362)
- Medial and cystic necrosis that is not usually transmural
- Granulomatous transmural vascular inflammation with giant cells
- Granulomatous vascular inflammation with giant cells of arterial media
- Positive for antineutrophilic cytoplasmic antibody
- Vascular changes limited to arterial intima
Comment here
Reference: Takayasu arteritis
Comment here
Reference: Takayasu arteritis
- Tamm-Horsfall protein (THP) is a high molecular weight glycoprotein discovered by Tamm and Horsfall (J Exp Med 1952;95:71)
- Normally synthesized by thick ascending limb of loop of Henle, possibly distal convoluted tubules
- Most abundant protein in urine of healthy individuals
- May accumulate in renal parenchyma, perirenal soft tissue, renal hilar lymph nodes or bladder with pathologic conditions
- Also known as uromodulin, from UMOD gene on #16 (Wikipedia)
- THP deposits are present in 60% of cystectomy specimens, 4% of bladder biopsies
- In bladder specimens with THP deposits, mean age is 61 years, range 45-78 years, 85% are men (Am J Surg Pathol 1994;18:615)
- Inserted into luminal cell surface of renal tubules by glycosyl-phosphatidylinositol (GPI)-anchor, then excreted in urine at a rate of 50 - 100 mg/day
- Stones may form due to defective urinary Tamm Horsfall protein, due to lack of sialic acid (Int J Biol Sci 2008;4:215)
- Has a strong tendency to form macroaggregates of several million Daltons, particularly in highly tonic solutions
- Forms the matrix of urinary casts and stones
- Deposited in areas of necrosis, inflammation, fibrinous exudates, ulcer, crystalline material
- May be host defense factor against Proteus mirabilis urinary tract infections (J Urol 2009;181:2332)
- May have role in pathophysiology of interstitial cystitis (BJU Int 2009;103:1085)
- Comprises matrix of urinary casts formed during acute kidney injury (Webpath tutorial) and matrix of urinary stones
- Also present in deposits associated with interstitial kidney disease
- Also associated with urothelial carcinoma, nephrogenic adenoma
- Large, waxy, pale or weakly eosinophilic mass
- Glassy, PAS+ acellular material
- May also appear as strands of eosinophilic material obscured by fibrinous exudates or necrotic tissue
- PAS, trichrome (pale blue)
- Anti Tamm-Horsfall protein antibody
- Nonbranching 4 nm wide parallel fibrils
- Hereditary, often autosomal dominant disorder of thinning of lamina dense of glomerular basement membrane; normal renal function initially, but possibly late development of renal insufficiency or hypertension
- Also called benign familiar hematuria
- 1 - 2% of general population (Arch Pathol Lab Med 2006;130:699), rare after age 50; 20 - 25% have isolated hematuria
- Asymptomatic, hematuria discovered on routine urinalysis; rarely gross hematuria; mild proteinuria in 60% (Pol J Pathol 2009;60:35)
- Normal renal function, excellent prognosis, but up to 30% develop late onset renal insufficiency or hypertension
- Investigation of families and long term follow up recommended (Kidney Int 2010;78:1041, Nephrol Dial Transplant 2005;20:545)
- A diagnosis of exclusion
- Associated with abnormalities in alpha 3 and alpha 4 genes for type IV collagen
- Rarely due to mutations in COL4A5 (Pediatr Nephrol 2010;25:545)
- Most patients are heterozygous; in homozygotes, resembles Alport disease and progresses to renal failure, even in women (Arch Pathol Lab Med 2009;133:224)
- Red blood cells in Bowman space and renal tubules, but otherwise normal (Arch Pathol Lab Med 1988;112:794)
- Strong linear glomerular basement membrane staining for alpha 3 and 4 protein (similar to normals, Arch Pathol Lab Med 2001;125:631), positive antibody staining to NC1 domain of glomerular basement membrane from patients with thin basement membrane disease
- Occasional IgM and IgG deposits
- Uniform thinning of lamina dense of glomerular basement membrane to 200 nm in > 50% of glomerular capillaries
- Some authors believe segmental thinning is sufficient for diagnosis (Arch Pathol Lab Med 2006;130:1533)
- Glomerular membrane occasionally ruptures
- No thickening, lamellation or inclusions of glomerular basement membrane
- Alport syndrome in women without typical clinical findings: thick glomerular basement membrane, lamellation, granular inclusions and loss of staining for alpha 3 and alpha 4 protein (Hum Pathol 2002;33:836)
- IgA nephropathy
- Nonspecific GBM thinning is also seen in lupus nephritis and postinfectious glomerulonephritis
- Each year, 9 million new cases of TB, causing 1.5 million annual deaths (WHO: Global tuberculosis report 2017 [Accessed 19 January 2018])
- GU tract is #2 most common site of infection after lungs
- Renal involvement may be indolent, may not become apparent until 20+ years from detection of primary infection
- Urogenital TB is associated with unilateral nonfunctioning kidney in 27% of cases, with renal failure present in 7% (Int J Urol 2008;15:827)
- In chronic kidney disease of all causes, one study from India demonstrated a 4% incidence of TB, which was usually tuberculin skin test negative (Clin Nephrol 2007;67:217)
- In immunocompromised patients, urogenital TB usually has systemic symptoms, dissemination, multiple renal foci (Int Urol Nephrol 2009;41:327)
- 20 year old man with tuberculosis associated chronic kidney disease (Am J Trop Med Hyg 2011;84:843)
- 29 year old man with renal hydronephrosis (Case #128)
- 33 year old man presenting with end stage renal disease secondary to renal TB (Rev Inst Med Trop Sao Paulo 2012;54:57)
- 38 year old man with negative PPD and repeatedly negative AFB tests (Proc (Bayl Univ Med Cent) 2012;25:236)
- Multiple cavities filled with yellow friable necrotic material
- Extensive caseous necrosis, with occasional granulomas composed of epithelioid cells and Langhans giant cells with surrounding lymphocytes
- Very early granulomas might not show caseation
- The interface of viable cells and caseous necrosis is where acid fast bacilli (AFB) are most found
- Occasionally fungal infections and sarcoidosis can cause noncaseating epithelioid cell granulomas
- Xanthogranulomatous pyelonephritis: different clinical picture, AFB negative, although AFB may be difficult to detect even in TB patients; TB PCR and AcuProbe are more sensitive (Int J Urol 2006;13:67)
- Acute interstitial inflammation is commonly associated with involvement of the tubules due to a variety of causes
- Acute inflammation involving tubulointerstitial compartments
- Might be associated with acute tubular injury or tubulointerstitial scarring
- Common etiologies include drug, autoimmune, infection, hereditary and idiopathic
- Acute interstitial nephritis (AIN)
- ICD-10:
- N10 - acute pyelonephritis
- N11 - chronic tubulointerstitial nephritis
- N12 - tubulointerstitial nephritis, not specified as acute or chronic
- N13 - obstructive and reflux uropathy
- N14 - drug and heavy metal induced tubulointerstitial and tubular conditions
- N15 - other renal tubulointerstitial diseases
- N16 - renal tubulointerstitial disorders in diseases classified elsewhere
- Incidence: ~15 - 27% of renal biopsies for acute kidney failure (Am J Kidney Dis 2000;35:433)
- Kidney, interstitium and tubules
- High exposure to nephrotoxic drugs
- Injury of the tubulointerstitial compartment due to decreased blood supply and increase in metabolic demand
- Escalating the inflammatory processes by production and activation of cytokines, tissue necrosis factor (TNF) alpha
- Type IV hypersensitivity reactions in drug induced tubulointerstitial nephritis (Adv Chronic Kidney Dis 2017;24:107)
- Drugs (J Nephrol 2016;29:611, Am J Kidney Dis 1998;31:108):
- Accounts for about 60 - 70% of all tubulointerstitial nephritis
- Allergic (nonsteroidal anti-inflammatory [NSAIDs], antibiotics, proton pump inhibitors [PPI])
- Toxic (lithium)
- Autoimmune:
- Primary (antibrush border antibody [ABBA])
- Secondary to systemic diseases (Sjögren syndrome, IgG4 related disease, tubulointerstitial nephritis with uveitis) or immune upregulation (check point inhibitor therapy)
- Infection:
- Primary kidney (pyelonephritis, BK polyomavirus tubulointerstitial nephritis)
- Secondary to infection associated glomerulonephritis
- Hereditary / toxic / metabolic:
- Autosomal dominant interstitial kidney disease (Semin Nephrol 2010;30:366)
- Idiopathic: 8%
- Acute or subacute renal failure
- Subnephrotic range proteinuria
- Sometimes microscopic hematuria
- Drug induced tubulointerstitial nephritis: fever, skin rash, arthralgia and eosinophilia (Pediatr Clin North Am 2019;66:111)
- Clinical history of newly exposure to new drug (allergic etiology) or presence / absence of systemic disease
- Physical examination; skin rashes (morbilliform, maculopapular, erythroderma and epidermal necrolysis) (Nat Rev Nephrol 2010;6:461)
- Bilateral normal to increased size of the kidney and diffusely increased cortical hyperechogenicity by renal ultrasound (Nat Rev Nephrol 2010;6:461)
- Elevation of serum creatinine and blood urea (Clin J Am Soc Nephrol 2017;12:2046)
- Hyperkalemic
- Hyperchloremic metabolic acidosis
- Elevation of eosinophils level (nonspecific)
- Elevation the level of serum erythrocyte sedimentation rate (ESR) or C reactive proteins
- Positive proteinuria in dipstick test and microscopic or macroscopic hematuria
- Good prognosis for drug induced tubulointerstitial nephritis (Nat Rev Nephrol 2010;6:461)
- Poor prognosis for granulomatous tubulointerstitial nephritis (Clin Kidney J 2015;8:516)
- 14 year old girl with one sided uveitis and renal involvement (J Med Case Rep 2021;15:443)
- 28 year old man with a history of ulcerative colitis and sacroiliitis with progressive kidney dysfunction (Intern Med 2019;58:79)
- 59 year old man with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and acute kidney injury (AKI) (Clin Kidney J 2021;14:2151)
- 70 year old man with renal dysfunction, a medical history of bile duct stenosis, an inflammatory pancreatic mass and elevation of serum creatinine and IgG4 (Ren Fail 2019;41:657)
- Eliminate the causative agents (drugs / toxins)
- Treat associated systemic disorders
- Corticosteroid therapy
- In steroid resistant cases and steroid contraindicated cases, mycophenolate mofetil could be considered (Adv Chronic Kidney Dis 2017;24:94)
- Azathioprine could be used in cases associated with systemic inflammatory and autoimmune diseases, such as Sjögren syndrome
- Interstitial inflammatory cell infiltrate composed of lymphocytes, macrophages, plasma cells and eosinophils (in different proportion) (Adv Chronic Kidney Dis 2017;24:72, Pediatr Clin North Am 2019;66:111, Clin Rheumatol 2018;37:257)
- Predominance of eosinophils suggests allergic etiology (nonspecific)
- Predominance of plasma cells suggest autoimmune etiology (nonspecific)
- Granulomatous inflammatory interstitial nephritis (Indian J Nephrol 2020;30:26):
- Necrotizing granulomatous inflammation in infection etiology, such as tuberculosis and fungal infection
- Nonnecrotizing granulomatous inflammation in drug induced tubulointerstitial nephritis and sarcoidosis
- Tubulitis (mild, moderate and severe) (Adv Chronic Kidney Dis 2017;24:72, Pediatr Clin North Am 2019;66:111)
- Acute tubular injury (luminal ectasia, simplification of the epithelium, loss of brush border or intraluminal necrosis) (Adv Chronic Kidney Dis 2017;24:72, Pediatr Clin North Am 2019;66:111)
- In chronic cases, tubular atrophy and interstitial fibrosis with evidence of active tubulitis in nonseverely atrophic tubules (Pediatr Nephrol 2017;32:577)
- Granular tubular basement membranes deposits with IgG (IgG4 related tubulointerstitial nephritis)
- Linear tubular basement membranes deposits (antitubular basement membrane disease) (Curr Opin Nephrol Hypertens 2012;21:279)
- AFB: positive in acid fast bacilli infection
- GMS highlights fungal elements
- IgG and IgG4 positive plasma cells (increased IgG4/IgG ratio) in IgG4 related disease (Am J Kidney Dis 2017;69:e19)
- Nonspecific; evidence of inflammatory cells in the interstitium and tubules
Histopathology kidney - interstitial nephritis
- Kidney, core needle biopsy:
- Acute and chronic interstitial nephritis, moderate (see comment)
- Comment: There is no evidence of a glomerular disease of the immune complex type seen on immunofluorescence. Light microscopy exhibits mild acute and chronic interstitial nephritis. Given the positive ANA and SSA and the plasma cell rich infiltrate, this could be due to autoimmune disease, such as Sjögren syndrome. Other possibilities include tubulointerstitial nephritis and uveitis (TINU) syndrome and drug allergies. Clinical correlation is recommended.
- Interstitial inflammation secondary to glomerulonephritis:
- Neoplastic processes:
- Infiltrating lymphoid neoplasm or cast nephropathy
- Acute cellular rejection in renal transplant
- Medullary capillaritis:
- IgA vasculitis, pauci-immune necrotizing and crescentic glomerulonephritis
- Urinary tract infection:
- White blood cell casts
- Leukocytosis
- Positive dipstick test for leukocyte esterase
- Crescentic lesions
- Glomerulitis
- Inflammation of the interstitium with active tubulitis
- Segmental sclerosis of the glomeruli
Comment Here
Reference: Tubulointerstitial nephritis
-
Three types:
- Acute uric acid nephropathy due to precipitation of uric acid crystals in tubules, causing acute renal failure; associated with chemotherapy for leukemia / lymphoma
- Chronic urate nephropathy, associated with gout and lead exposure from drinking “moonshine”
- Uric acid stones, in 22% with gout and 42% with secondary hyperuricemia
- Gout causes deposition of monosodium urate crystals with tophus formation that obstructs tubules, leading to cortical atrophy and scarring (eMedicine: Gout and Pseudogout [Accessed 02 January 2018])
- Kidney injury molecule-1 expression identifies proximal tubular injury in urate nephropathy (Ann Clin Lab Sci 2008;38:210)
- Stones within collecting system of kidney are present in 5 - 10% of Americans; most commonly in men ages 20 - 49
- Due to supersaturation of stone constituents, decreased urine volume or deficiency of crystal inhibitors in urine
- 80% unilateral, usually in calyces, pelvis or bladder
- Usually only 2 - 3 mm, but with severe, abrupt flank pain and hematuria
- All stones contain an organic matrix of mucoprotein
- Calcium oxalate / phosphate (75%): due to hypercalciuria (idiopathic, 50%), hypercalcemia (infants may have high Vitamin D levels, Iran J Kidney Dis 2012;6:186), hyperoxaluria (in vegetarians with oxalate rich diet), hyperuricosuria (hyperparathyroidism, bone disease, sarcoidosis) and rarely primary hyperoxaluria (Arch Pathol Lab Med 2002;126:1250); oxalate crystals are highlighted by polarized light; are accompanied by foreign body giant cells and macrophages
- Struvite (triple stones, magnesium ammonium phosphate, 15%): due to urea splitting bacteria (Proteus, Staphylococcus); produce staghorn calculi
- Uric acid (6%): due to hyperuricemia, chemotherapy for leukemia, uricosuric drugs or excess dietary proteins; 50% lack elevated uric acid in blood / urine; may be due to acidic pH; radiolucent; may become staghorn calculi if in renal pelvis; elongated and rectangular crystals in collecting tubules or doubly refractile crystals in interstitium with giant cell reaction
- Cysteine (1%): due to genetic defects in cystine transport (Orphanet J Rare Dis 2012;7:19); autosomal recessive, affects 1 per 20,000; yellow-brown and radioopaque stones form at low urinary pH; crystals are flat hexagons in urine
- Ammonium acid urate: rare, but more common in Asia (Kaohsiung J Med Sci 2012;28:259)
- Stone granuloma: complication of ureteral stone fragmentation and instrumentation
- Xanthinuria (rare): autosomal recessive, due to deficient xanthine oxidase, causing excessive xanthine levels and stones in 1/3 with this disorder
- 48 year old man with nephrectomy for renal mass due to urolithiasis caused by 2,8-dihydroxyadenine crystals (Hum Pathol 1992;23:1081)
- Rare subtype of pyelonephritis, often occurring as a chronic consequence of nephrolithiasis or infections (Indian J Nephrol 2019;29:111)
- Pathognomonic features are lipid laden foamy CD68+ macrophages (xanthoma cells) that give the tissue a yellow-orange color on macroscopy (StatPearls: Pyelonephritis Xanthogranulomatous [Accessed 5 June 2023])
- Rarely associated with renal cell carcinoma or urothelial carcinoma (Indian J Nephrol 2019;29:111)
- Lipid laden foamy CD68+ macrophages (xanthoma cells) give the tissue a yellow-orange color on macroscopy (hallmark)
- Extrarenal extension may be present and is suggestive of malignancy
- May mimic renal cell carcinoma clinically but also macroscopically and microscopically
- Usually occurs unilaterally in all ages, more often in women and elderly patients
- Most often due to nephrolithiasis complicated with infections
- Kidney, almost always unilateral; left kidney more commonly affected (60%) (BJU Int 2023;131:395)
- May extend to perirenal / perinephric fat, perirenal and pararenal spaces or diffusely into the retroperitoneum
- Urinary obstruction occurs as a result of calculus, most commonly staghorn calculus (in almost 80% of patients), which serves as a nidus for infection, resulting in the destruction of the renal parenchyma (J Pediatr Genet 2020;9:114)
- In children, congenital ureteropelvic abnormalities may result in chronic urinary obstruction (J Pediatr Genet 2020;9:114)
- Chronic pyelonephritis
- Nephrolithiasis
- Risk factors include diabetes mellitus, hypertension, immunocompromised individuals, abnormal lipid metabolism, renal transplantation (may be difficult to distinguish from rejection) and brachydactyly mental retardation syndrome in children (J Pediatr Genet 2020;9:114)
- Nonfunctional kidney
- Variable clinical presentation, including flank pain (92.5%), fever (62.5%), dysuria (47.5%), renal angle tenderness (40%) and a palpable lump on per abdomen examination (30%) (Indian J Nephrol 2019;29:111)
- Can raise suspicion for renal cell carcinoma clinically and macroscopically
- Can be associated with septic metastases, which may be clinically difficult to distinguish from neoplastic metastases (Aktuelle Radiol 1997;7:317)
- Intraabdominal or nephrobronchial fistulation is a rare complication of xanthogranulomatous pyelonephritis (Br J Urol 1986;58:488, Int J Surg Case Rep 2022;98:107551)
- In prolonged disease course, secondary amyloidosis (amyloid AA) can occur, presenting as nephrotic syndrome (J Urol 1978;119:589)
- Fever and raised inflammatory markers (C reactive protein [CRP] and erythrocyte sedimentation rate [ESR]) are most commonly associated with xanthogranulomatous pyelonephritis (J Pediatr Genet 2020;9:114)
- If present, the bear paw sign on CT imaging is a pathognomonic feature of xanthogranulomatous pyelonephritis (J Pediatr Genet 2020;9:114)
- Complete blood count may show anemia and leukocytosis
- Elevated ESR and CRP
- Elevated blood urea nitrogen (BUN) and creatinine levels
- Urinalysis may show pyuria, bacteriuria and hematuria
- Urine culture may be positive (Escherichia coli, Proteus mirabilis, Klebsiella pneumoniae, Enterococcus faecalis and others), urine culture sterile in 25% of cases (J Urol 1978;119:589)
- Bear paw sign (i.e., dark dilated calyces surrounded by brighter renal parenchyma on CT imaging)
- Staghorn calculus on radiography in case of nephrolithiasis
- Excellent overall prognosis for xanthogranulomatous pyelonephritis
- Prognosis is better in unilateral cases, while bilateral cases are usually fatal
- Treatment of choice in diffuse cases is nephrectomy without any incidence of recurrence (StatPearls: Pyelonephritis Xanthogranulomatous [Accessed 5 June 2023])
- 51 year old woman with a nodular tumor-like lesion on the upper left pole of the kidney (Patholog Res Int 2010;2010:602523)
- 55 year old man with palpable mass in his right lumbar region (J Clin Endocrinol Metab 1986;63:246)
- 65 year old man with ureteral granulomatous inflammation involving the inferior vena cava (Cureus 2023;15:e34388)
- 66 year old man with renal calculi, squamous cell carcinoma and concomitant xanthogranulomatous pyelonephritis (Ochsner J 2023;23:72)
- 72 year old woman with xanthogranulomatous pyelonephritis and concomitant renal cell carcinoma (BMJ Case Rep 2019;12:e232097)
- In focal or segmental cases, treatment with antibiotics and percutaneous drainage can be attempted, followed by partial (or total) nephrectomy if not successful
- In diffuse and advanced stages, nephrectomy is the treatment of choice (BJU Int 2023;131:395)
- Zonal structure of xanthogranulomatous pyelonephritis may be visible upon macroscopy (Cureus 2021;13:e19133)
- Inner zone: dilated pelvicocalyceal system with necrotic debris
- Middle zone: granulation tissues surrounded by lipid laden macrophages (xanthoma cells) giving a yellow-orange appearance
- Outer zone: fibrosis may extend into renal fat capsule and beyond; decapsulation of kidney may not be feasible
- Dense, sheet-like infiltrate of round cells with macro and microvesicular clear cytoplasm, inflammatory cells and multinucleated giant cells but no cytologic atypia or nucleoli
- Zonal distribution of inflammation may be seen
- Inner zone: bacteria, inflammatory cells (neutrophils, lymphocytes, plasma cells), foreign body giant cells, foreign body granulomas, Liesegang rings, calcifications
- Middle zone (hallmark for the diagnosis): granulation tissues surrounded by lipid laden foamy CD68+ macrophages (xanthoma cells), Touton giant cells, cholesterol crystal clefts
- Outer zone: consists of giant cells, cholesterol clefts and fibrous tissues with lymph follicles
- Zonal distribution may not be visible or zones may be admixed (e.g., xanthoma cells might be intermingled with inflammatory cells)
- Reference: StatPearls: Pyelonephritis Xanthogranulomatous [Accessed 21 June 2023]
- Pankeratin
- PAX8
- Melanocytic markers
- Acid fast bacteria
- Reference: Cancer Cytopathol 2018;126:711
- Malakoplakia:
- Michaelis-Gutmann bodies
- Renal clear cell carcinoma:
- Cells with clear cytoplasm may resemble histiocytes but are keratin+, PAX8+, CD68-
- Arranged in compact, tubulocystic, alveolar or rarely papillary patterns
- Often glassy hyaline globules
- Usually nuclear grade 2 or higher
- Chicken wire / delicate vasculature is common (sinusoids near each packet of cells)
- Renal replacement lipomatosis:
- Atrophic renal parenchyma is replaced by adipose tissue, not xanthoma cells (Cureus 2021;13:e16596)
- Renal tuberculosis:
- Granulomas with caseating necrosis surrounded by histiocytes and Langhans type giant cells
- Acid fast bacteria stain (Ziehl-Neelsen) demonstrates red, rod shaped bacilli at periphery of necrosis
- Caseating granulomas
- Chicken wire vasculature
- Foam cells
- Michaelis-Gutmann bodies
- Tubular casts
Comment Here
Reference: Xanthogranulomatous pyelonephritis
Comment Here
Reference: Xanthogranulomatous pyelonephritis
Find related Pathology books: renal, cardiovascular, hematopathology, immunology / transplant, liver, lung, cytopathology, pediatric