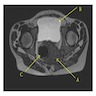
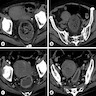
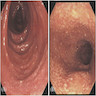
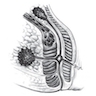
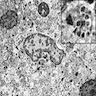
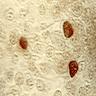
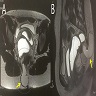
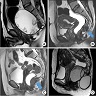
- TX: primary tumor not assessed
- T0: no evidence of primary tumor
- T1: tumor ≤ 2 cm
- T2: tumor > 2 cm but ≤ 5 cm
- T3: tumor > 5 cm
- T4: tumor of any size invading adjacent organ(s), such as the vagina, urethra or bladder
Superpage
Superpage Topics
Adenocarcinoma
Anatomy & histology
Apocrine carcinoma
Apocrine carcinoma (pending)
Basal cell carcinoma
Buschke-Löwenstein tumor
Carcinoma overview
Condyloma acuminatum
Crohn's disease
Cytomegalovirus (CMV)
Fissure
Fistula
Granular cell tumor
Granuloma inguinale
Grossing & features to report
Hemorrhoids
Hypertrophied papillae
Inflammatory cloacogenic polyp
Intradermal nevus (pending)
Lymphogranuloma venereum
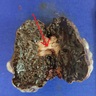
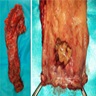
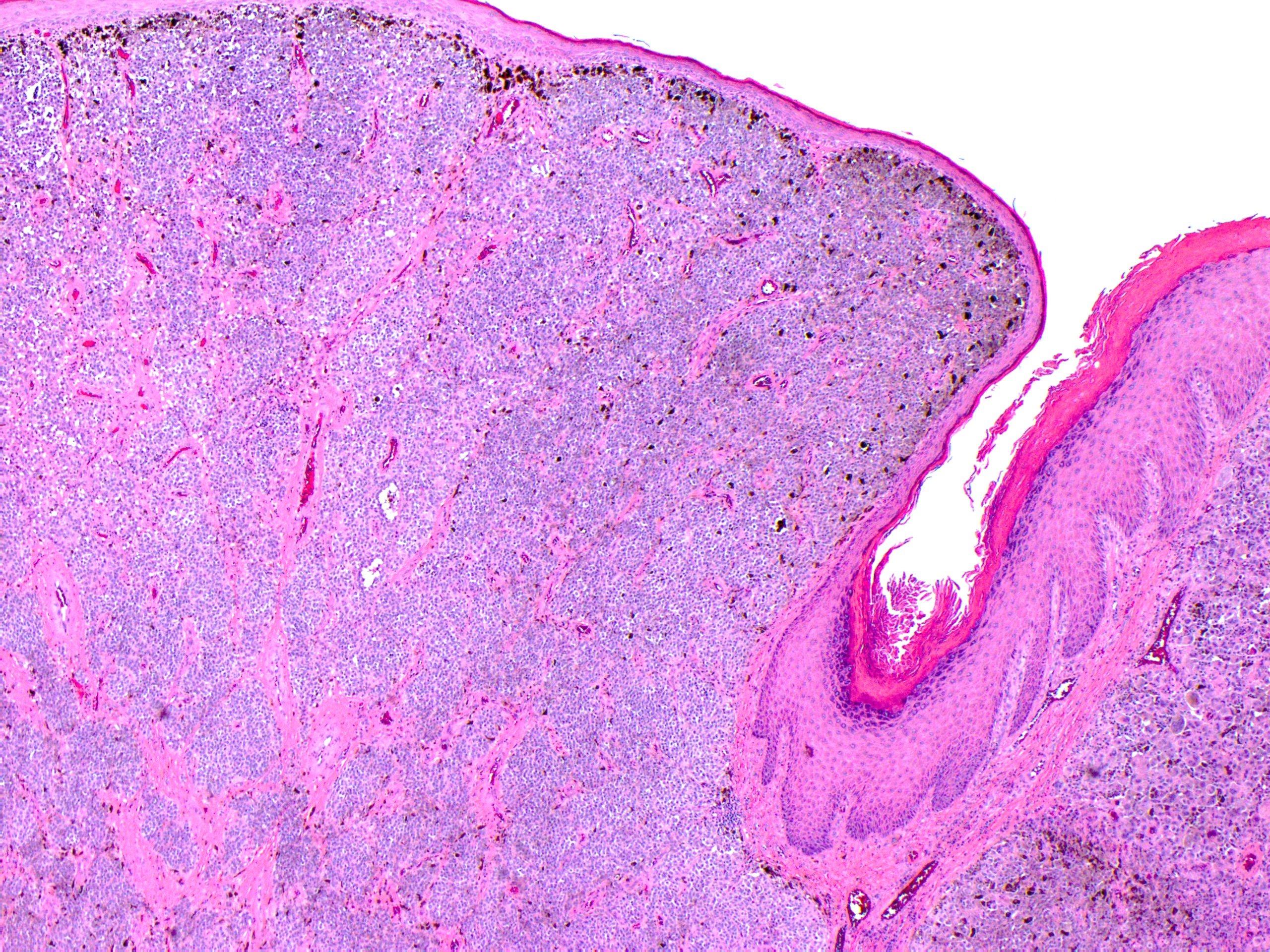
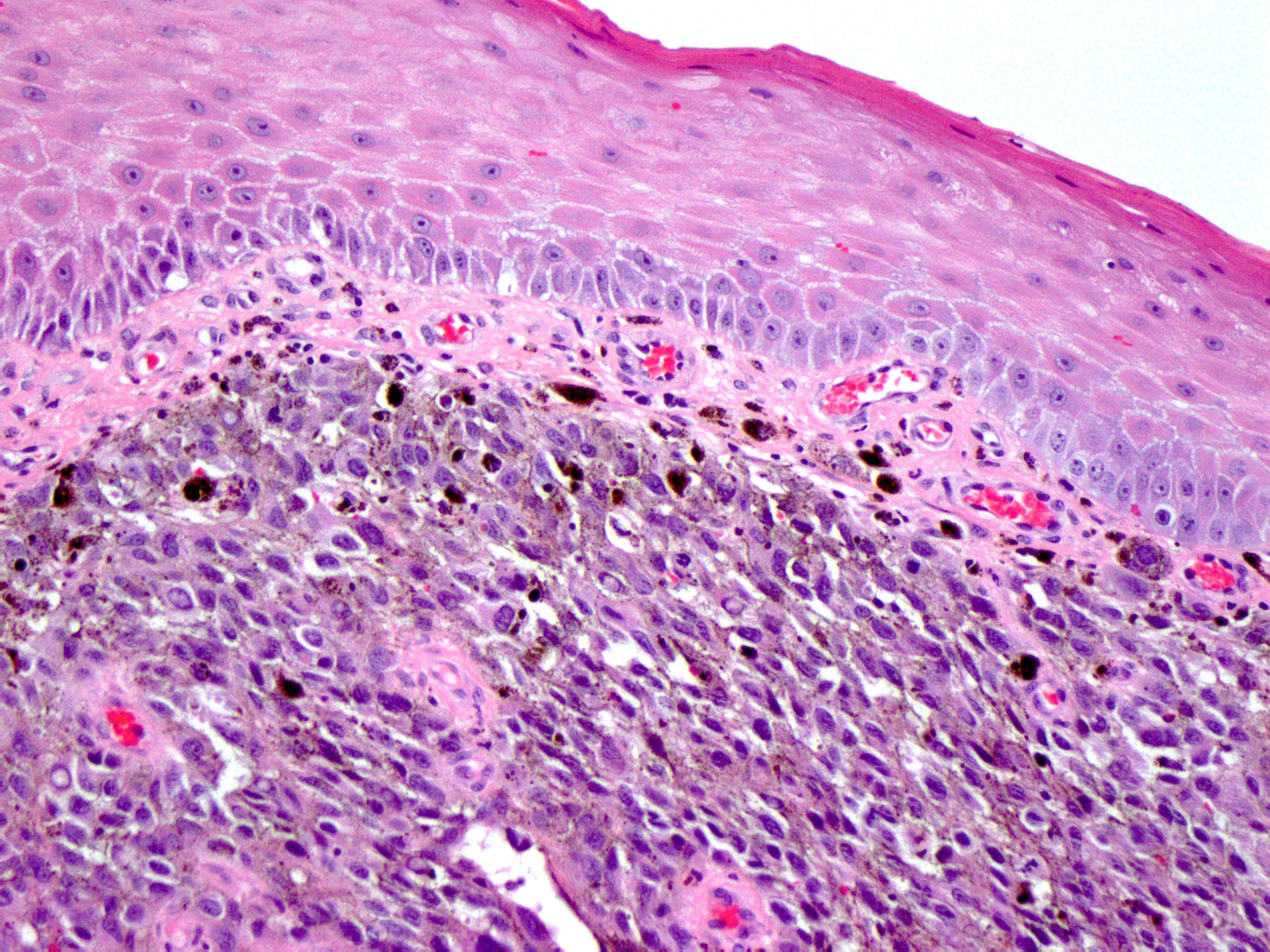
Melanoma
Mixed neuroendocrine nonneuroendocrine neoplasms (pending)
Neuroendocrine carcinoma
Neuroendocrine tumor (pending)
Paget disease
Squamous cell carcinoma
Squamous dysplasia
Staging
Syphilis
Tailgut cyst
Verrucous carcinoma
WHO classificationAdenocarcinoma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
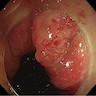
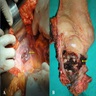
- Rare adenocarcinoma that primarily involves the anal canal (not rectal adenocarcinomas with downward spread)
Essential features
- Rare, primary gland forming malignancy of the anus
- May arise from anal glands, from congenital anorectal duplications or along a fistula tract
- Must be distinguished from adenocarcinoma secondarily involving anus (e.g., rectal carcinoma)
Terminology
- Also known as perianal adenocarcinoma or perianal gland adenocarcinoma
- The term anal duct adenocarcinoma is not recommended
ICD coding
- ICD-10: C21.0 - malignant neoplasm of anus, unspecified
Epidemiology
- 5% of anorectal malignancies (JCO Oncol Pract 2020;16:635)
- Slightly more common in white men (Cancer Med 2019;8:3855)
Sites
- Anus (by definition)
Pathophysiology
- May arise from anal glands, from congenital anorectal duplications or along a fistula tract (Dis Colon Rectum 1998;41:992)
- May demonstrate HPV 18 DNA but not HPV 16 DNA (Mod Pathol 1991;4:58)
- Accordingly, rare cases are linked to HPV infection and are positive for p16 (Mod Pathol 2020;33:944, Int J Surg Pathol 2021;29:672, Br J Cancer 2018;118:1302)
- Rare nonanal gland type, nonfistula associated anal carcinomas have recently been recognized (Pathol Int 2021;71:715)
Etiology
- Proposed etiologic associations include chronic fistula, anal Crohn's disease and anal sexual intercourse (Semin Surg Oncol 1994;10:235)
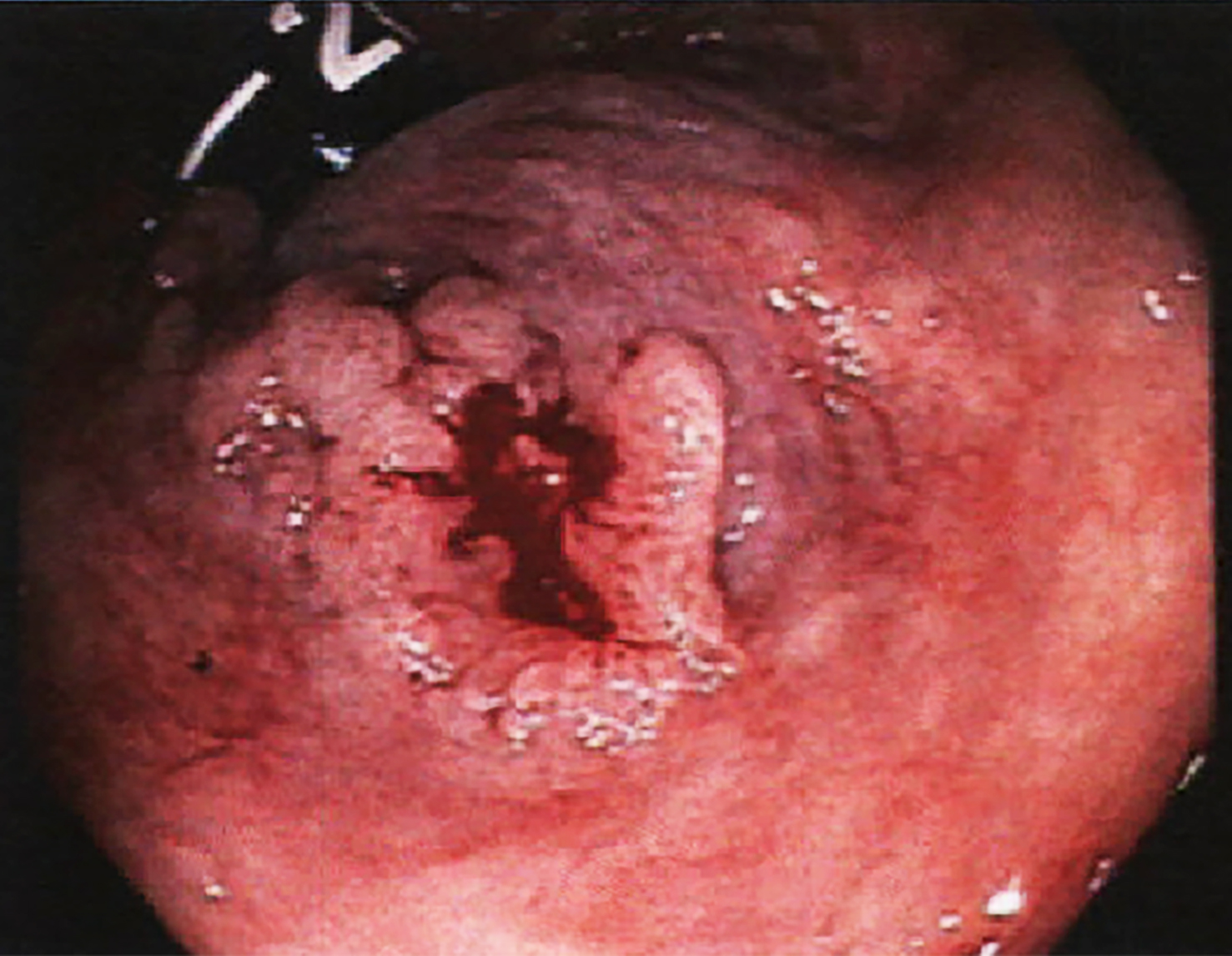
Clinical features
- May present in fistula tract or as a vaginal cyst
- Often a long history of perianal fistulas, abscesses, surgery
- Indolent course with gradual progression
Diagnosis
- Gross examination and tissue sampling
Prognostic factors
- Poor prognosis associated with advanced T and N category disease and higher histologic grade (Int J Radiat Oncol Biol Phys 2003;56:1274)
Case reports
- 42 year old man with anal tenderness (Medicine (Baltimore) 2021;100:e27083)
- 70 year old man with firm perianal nodule (BMJ Open Gastroenterol 2021;8:e000661)
- 72 year old man with Crohn's disease and perianal fistula (Cureus 2022;14:e31339)
- 84 year old man with adenocarcinoma arising from an anal gland (Int J Surg Case Rep 2014;5:234)
Treatment
- Standard treatment includes abdominoperineal resection; adding chemotherapy and radiation improves outcome (Int J Radiat Oncol Biol Phys 2003;56:1274)
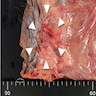
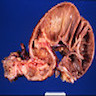
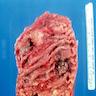
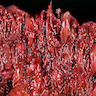
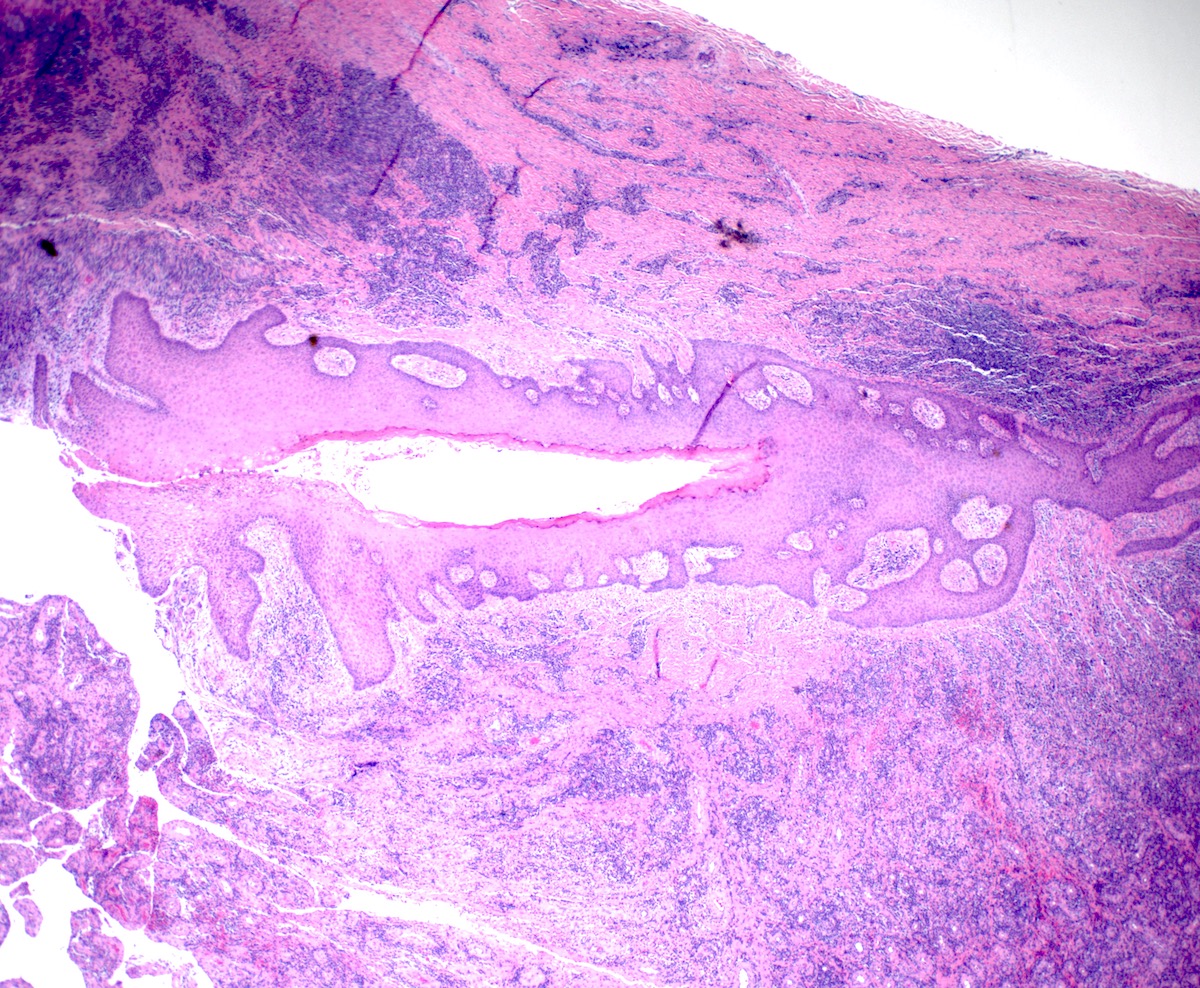
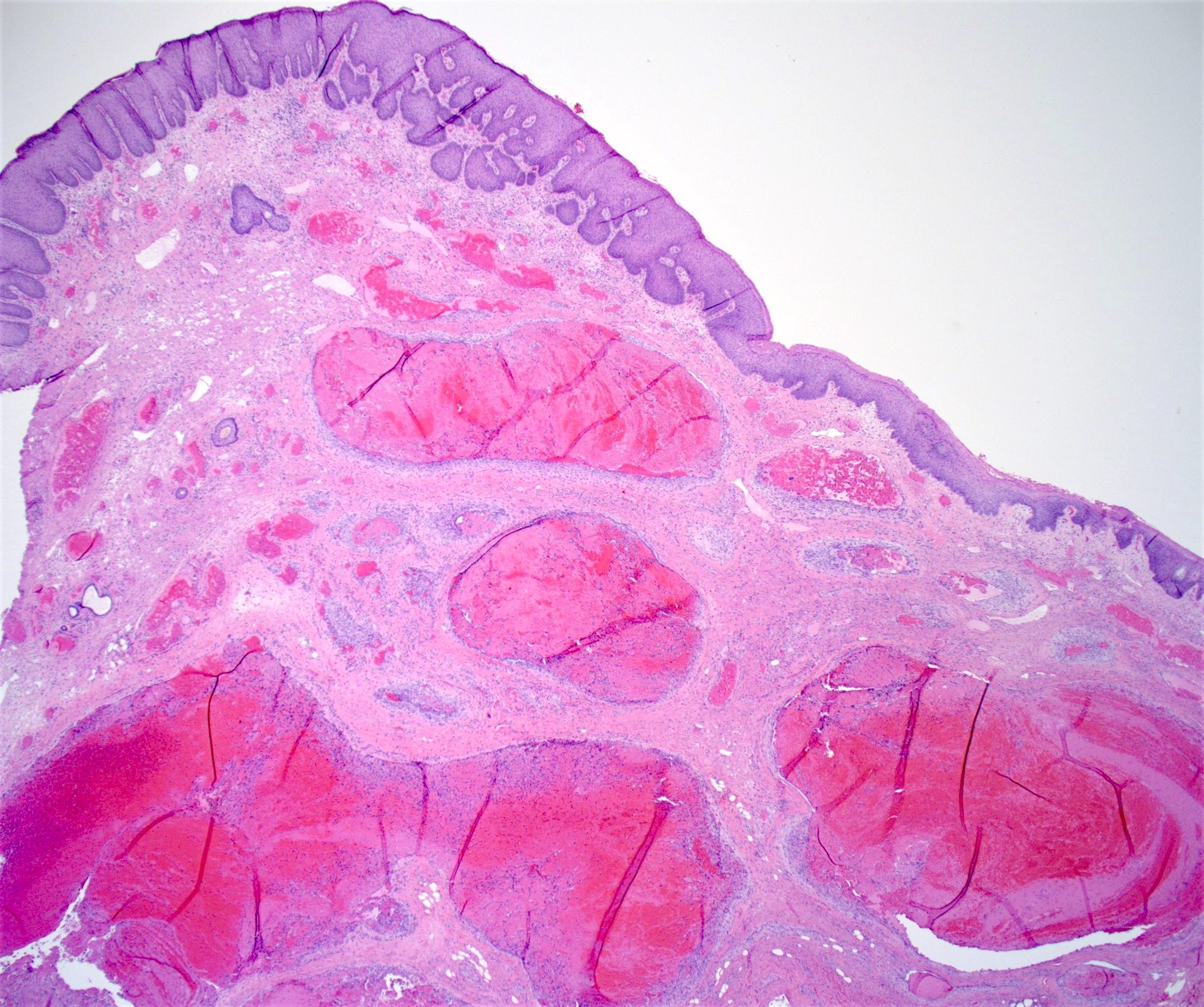
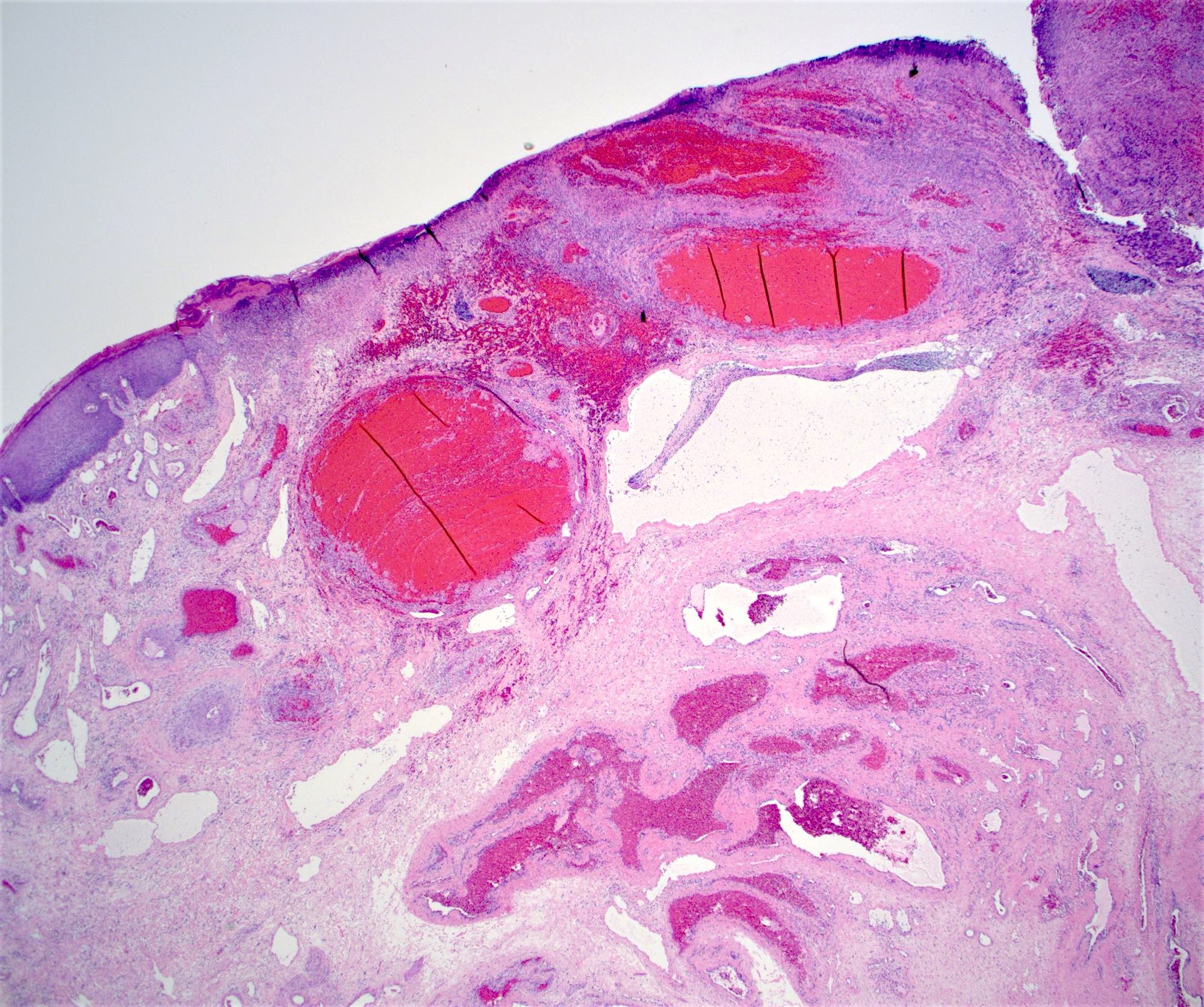
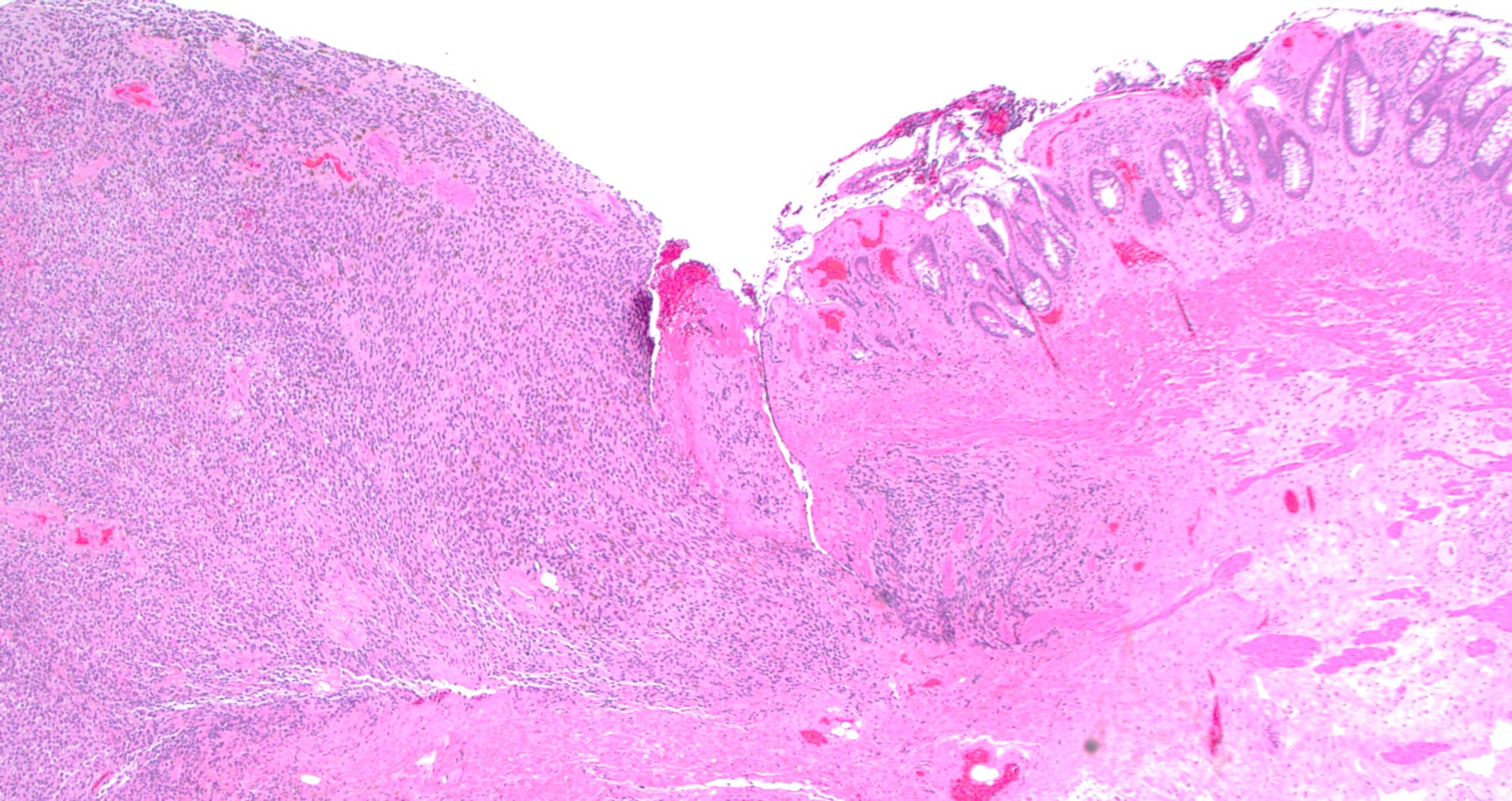
Gross description
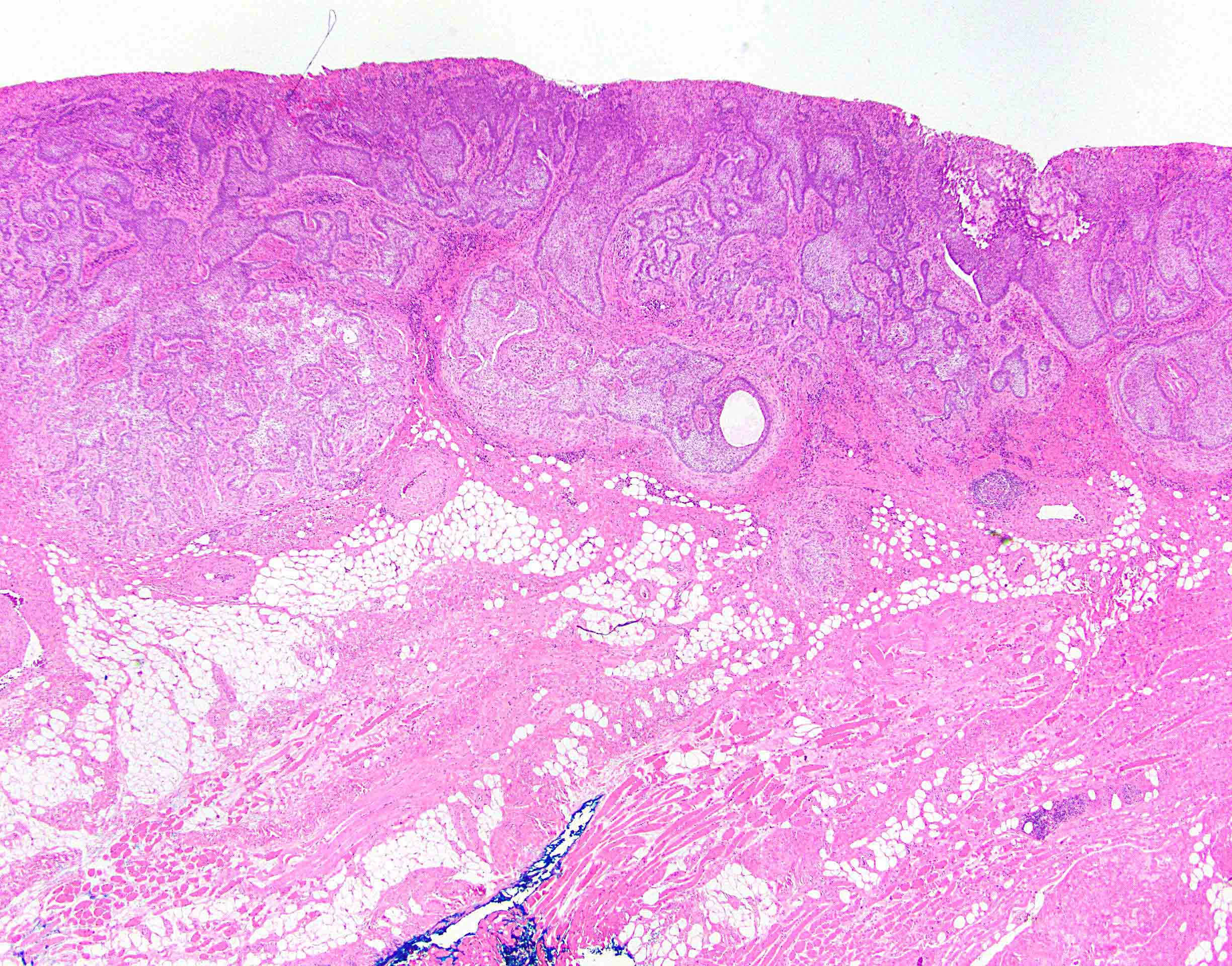
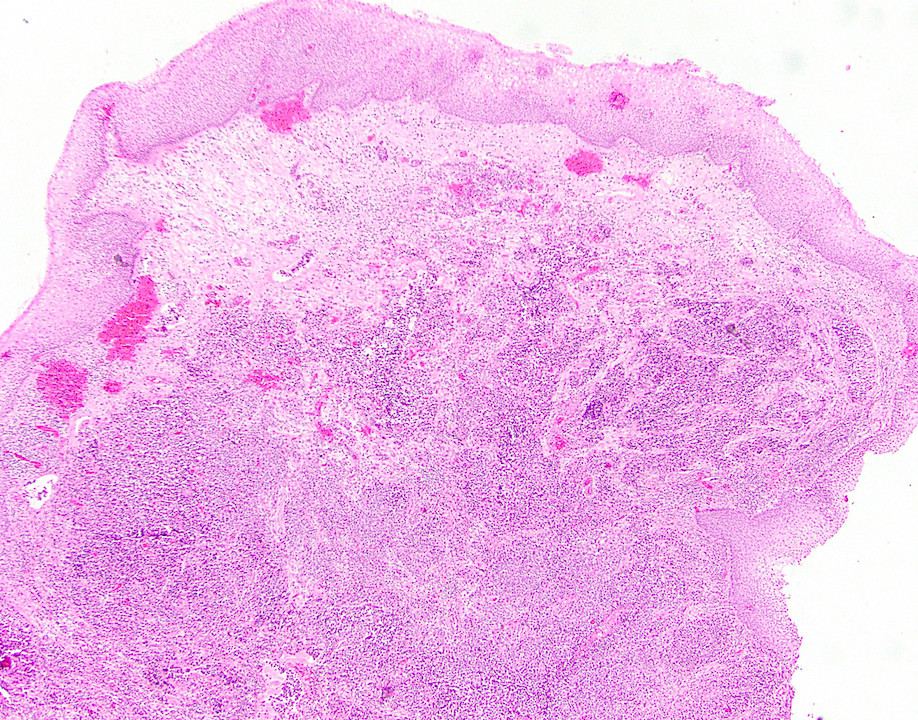
- Anal canal tumors are nodular, ulcerated, 3 - 4 cm or more, invade deeply into wall and spread proximally and distally into submucosa of distal rectum and proximal anus
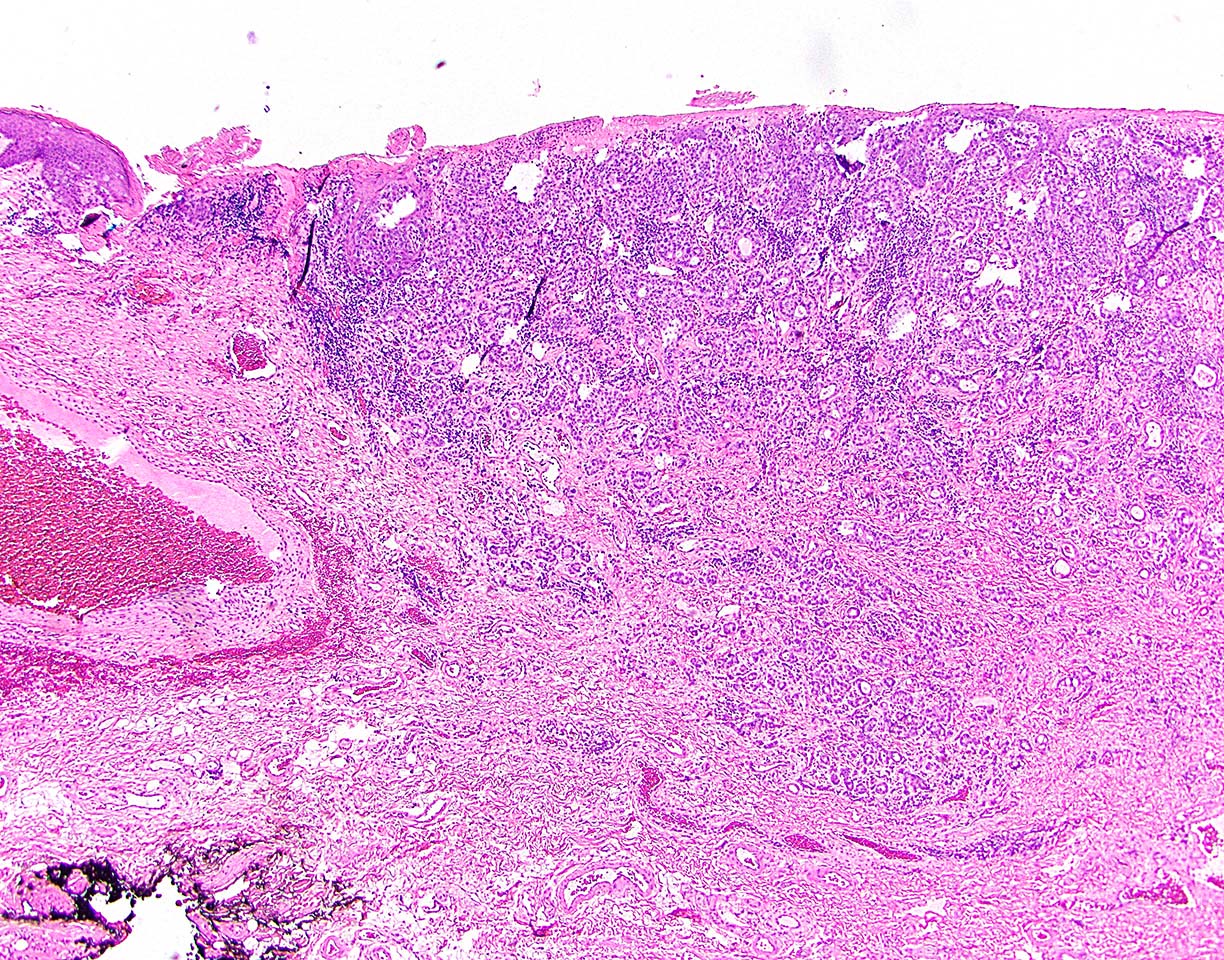
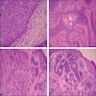
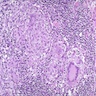
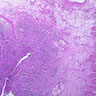
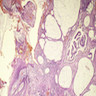
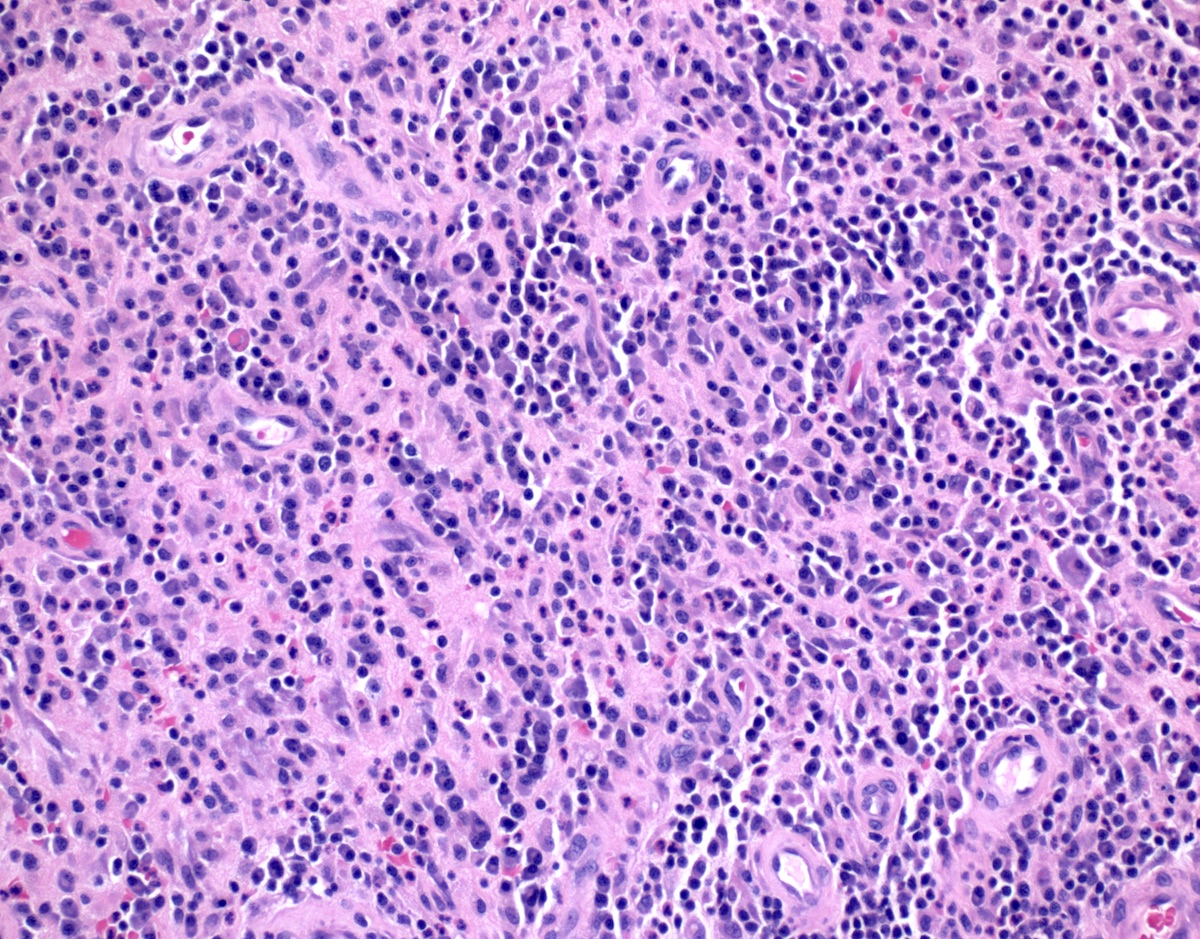
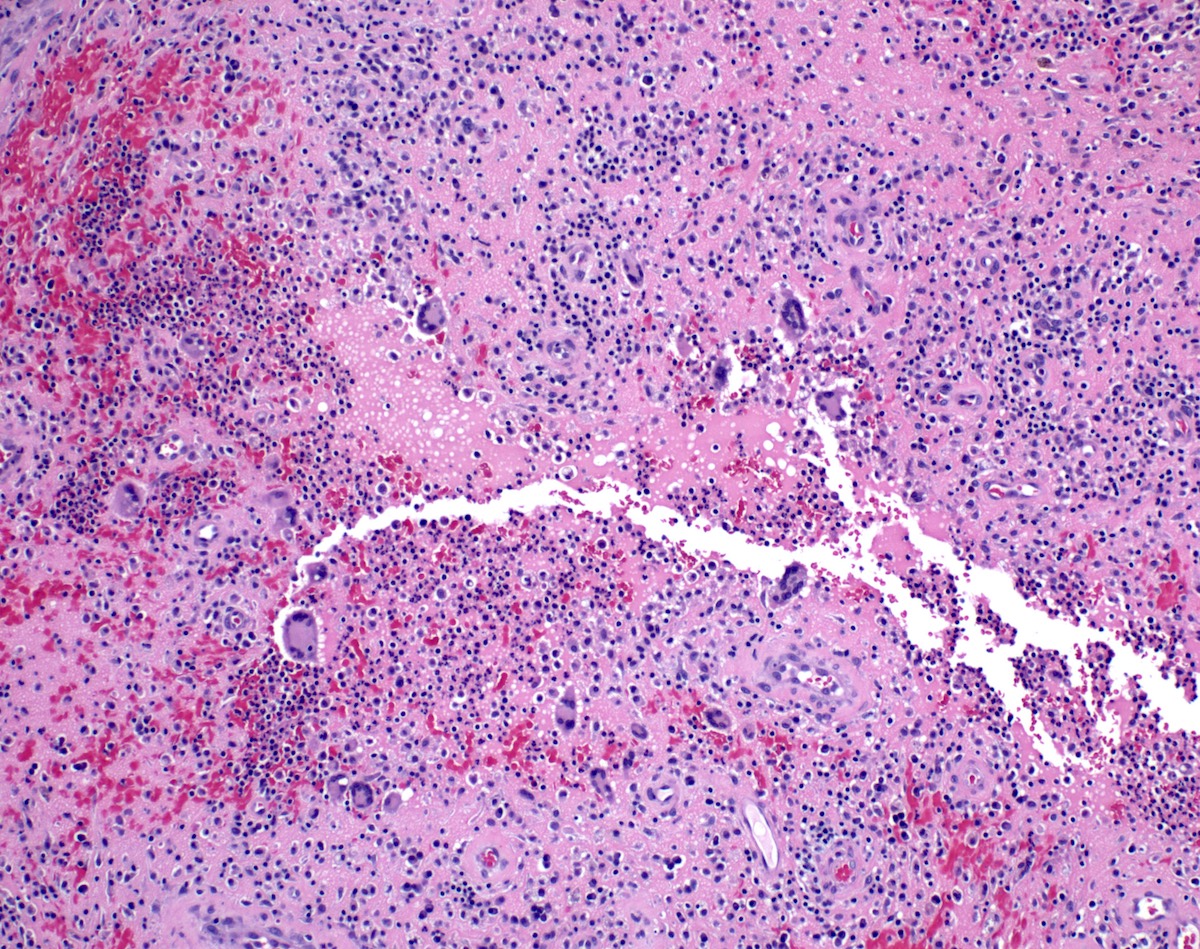
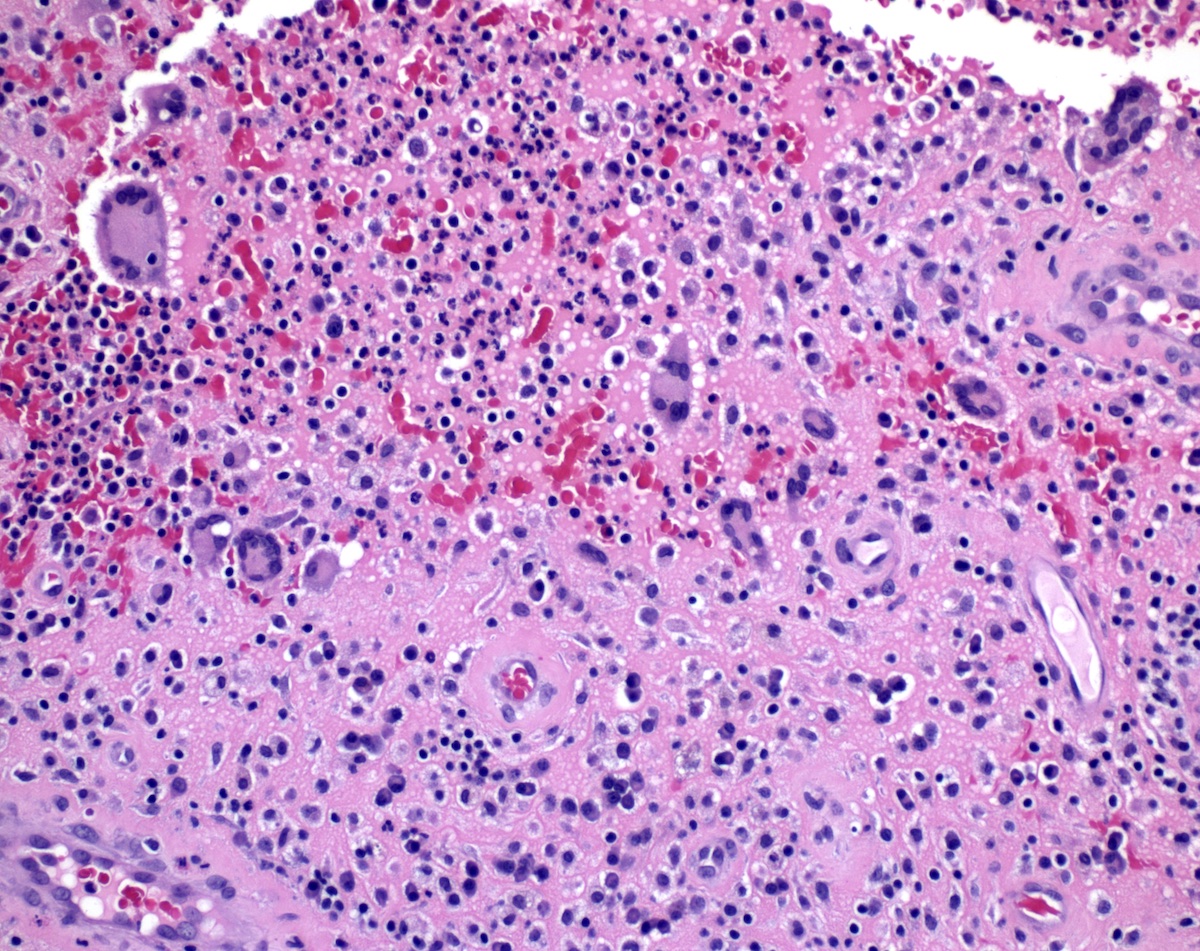
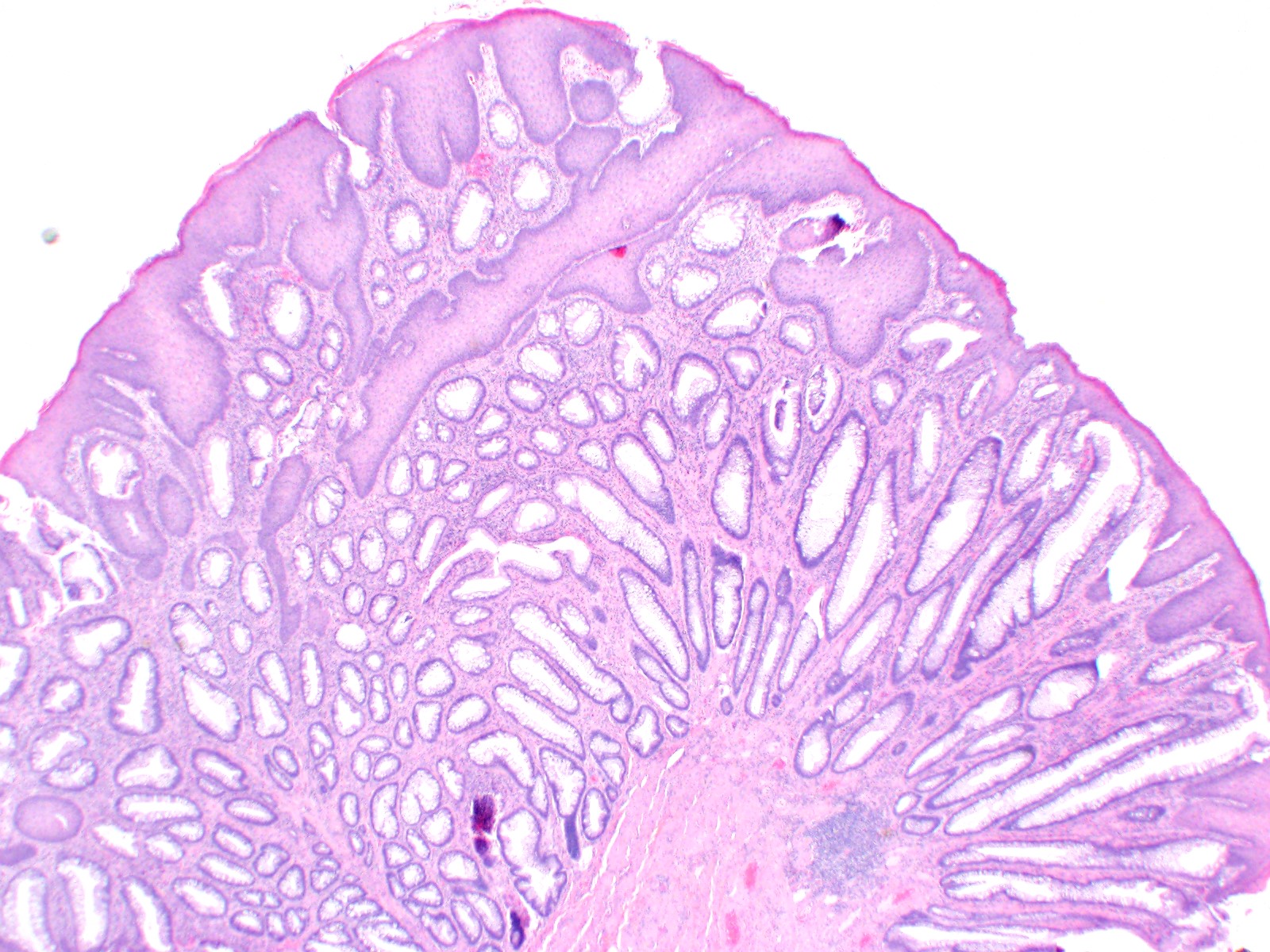
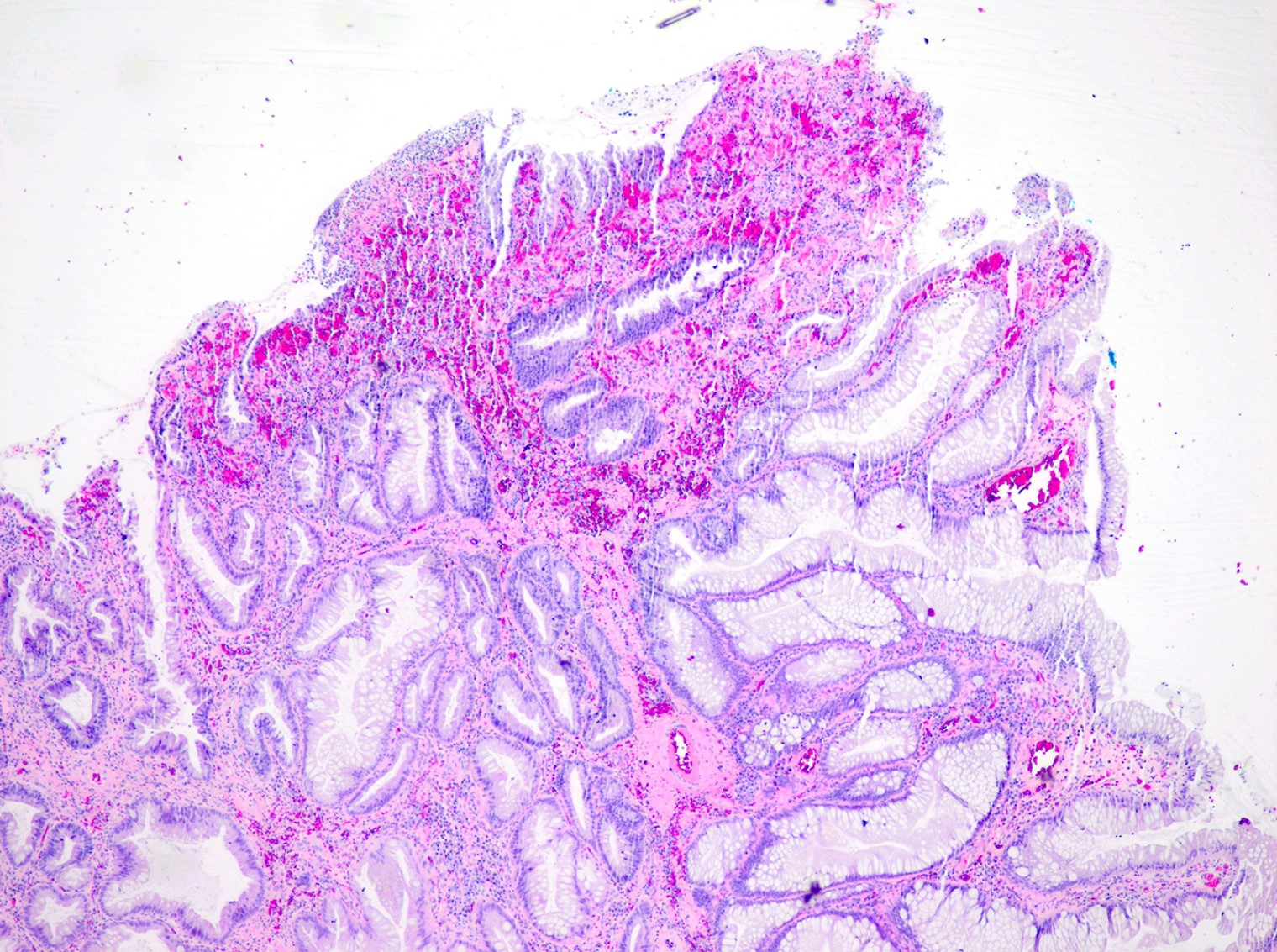
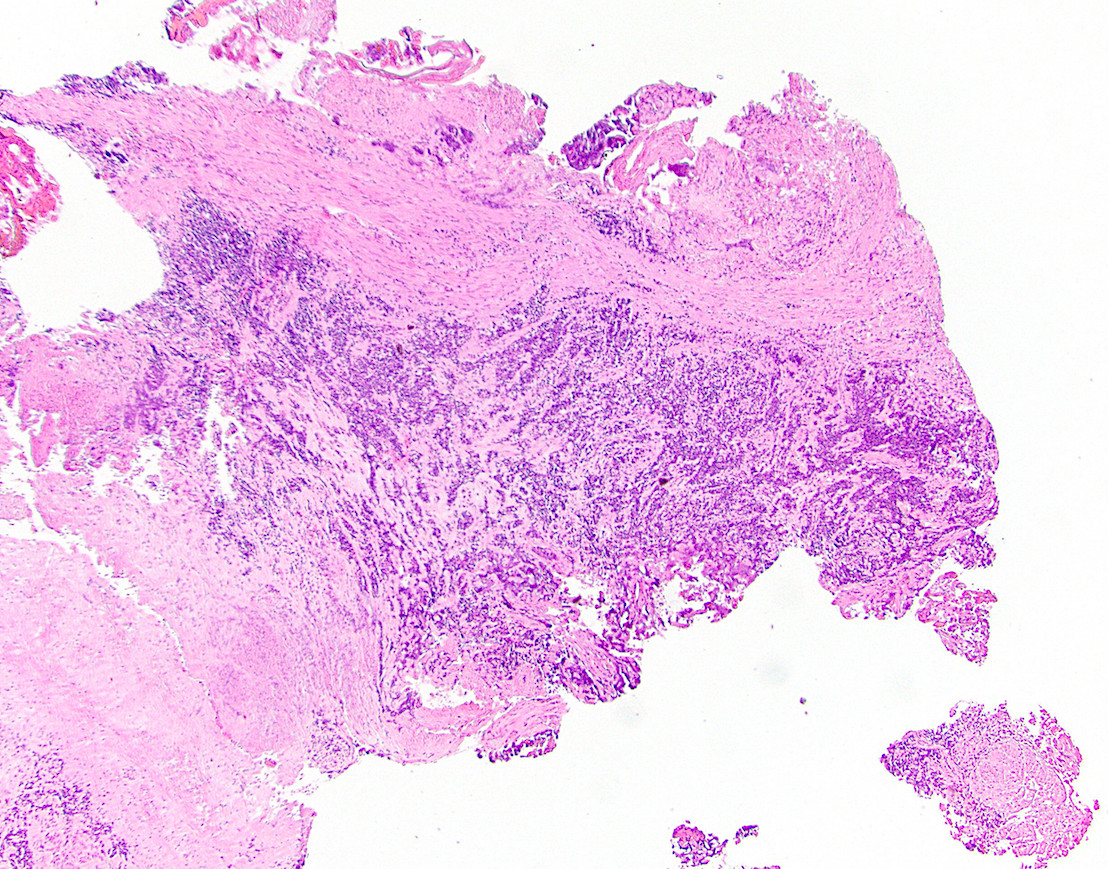
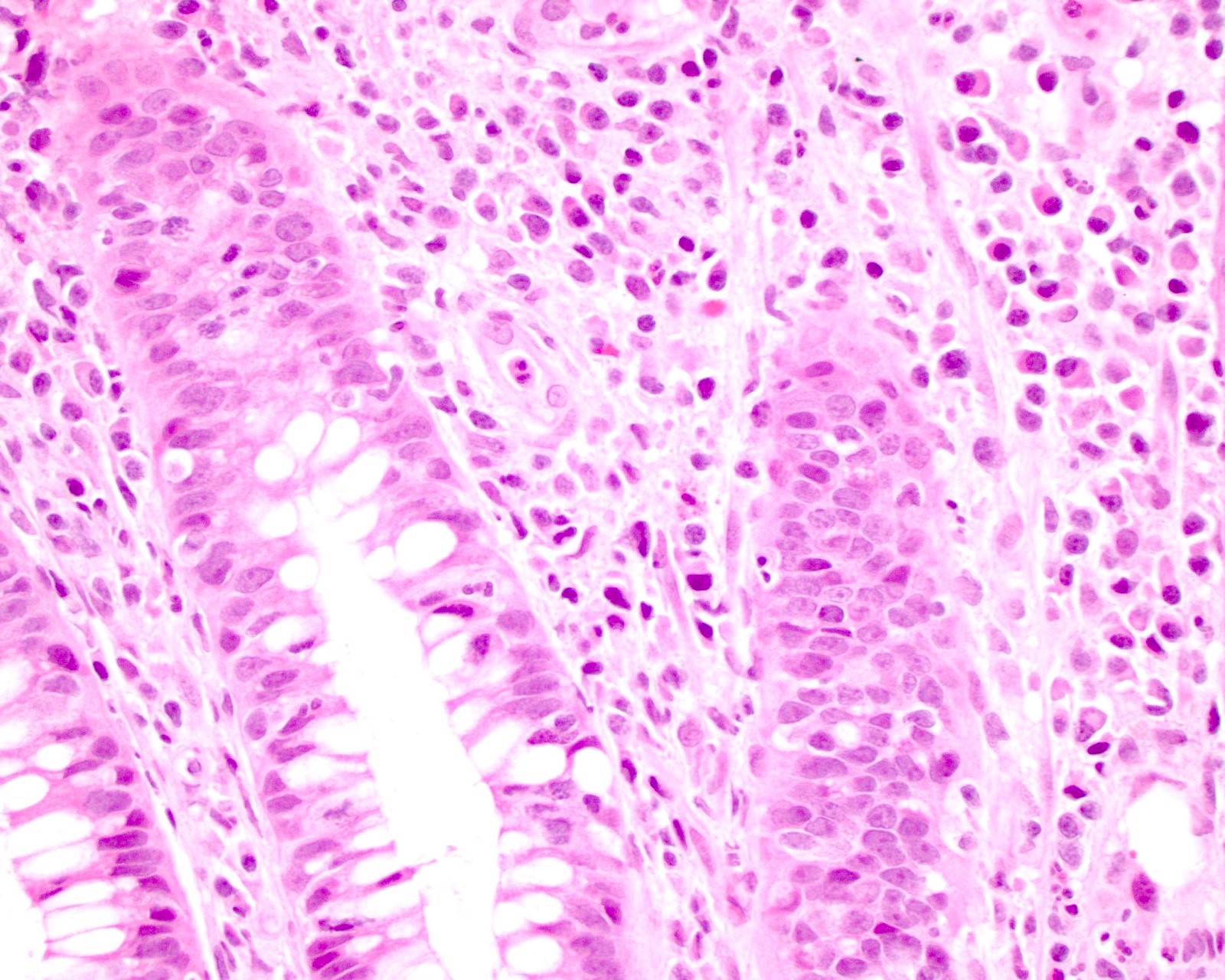
Microscopic (histologic) description
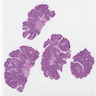
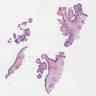
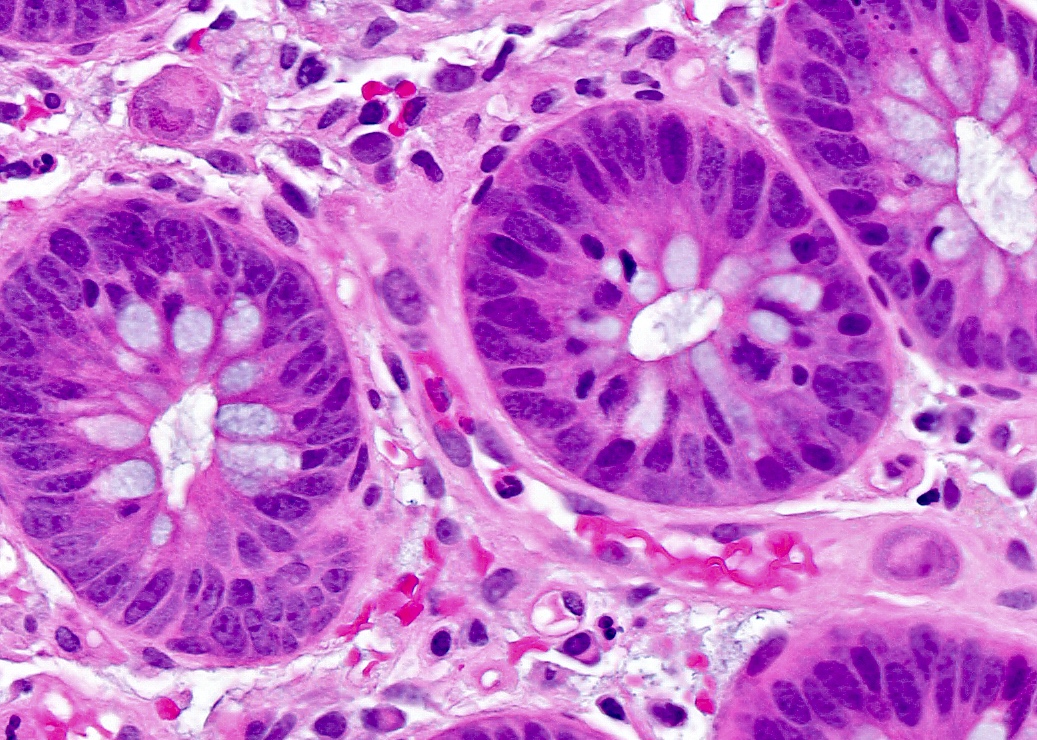
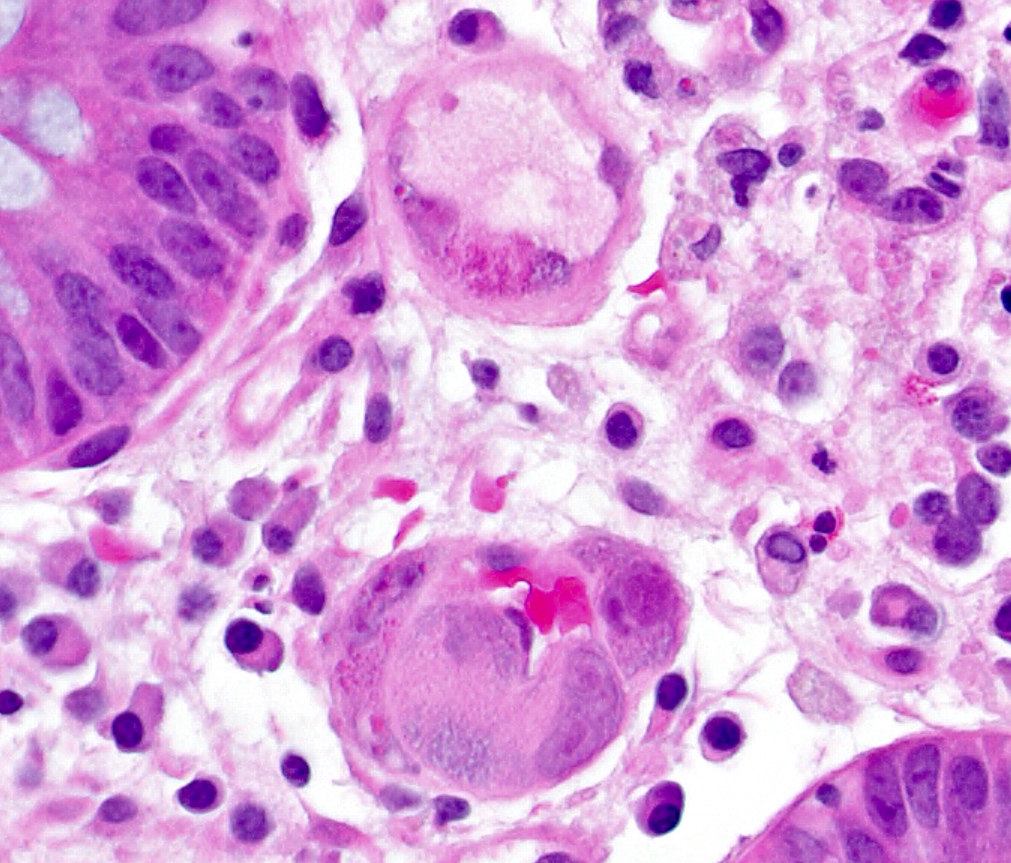
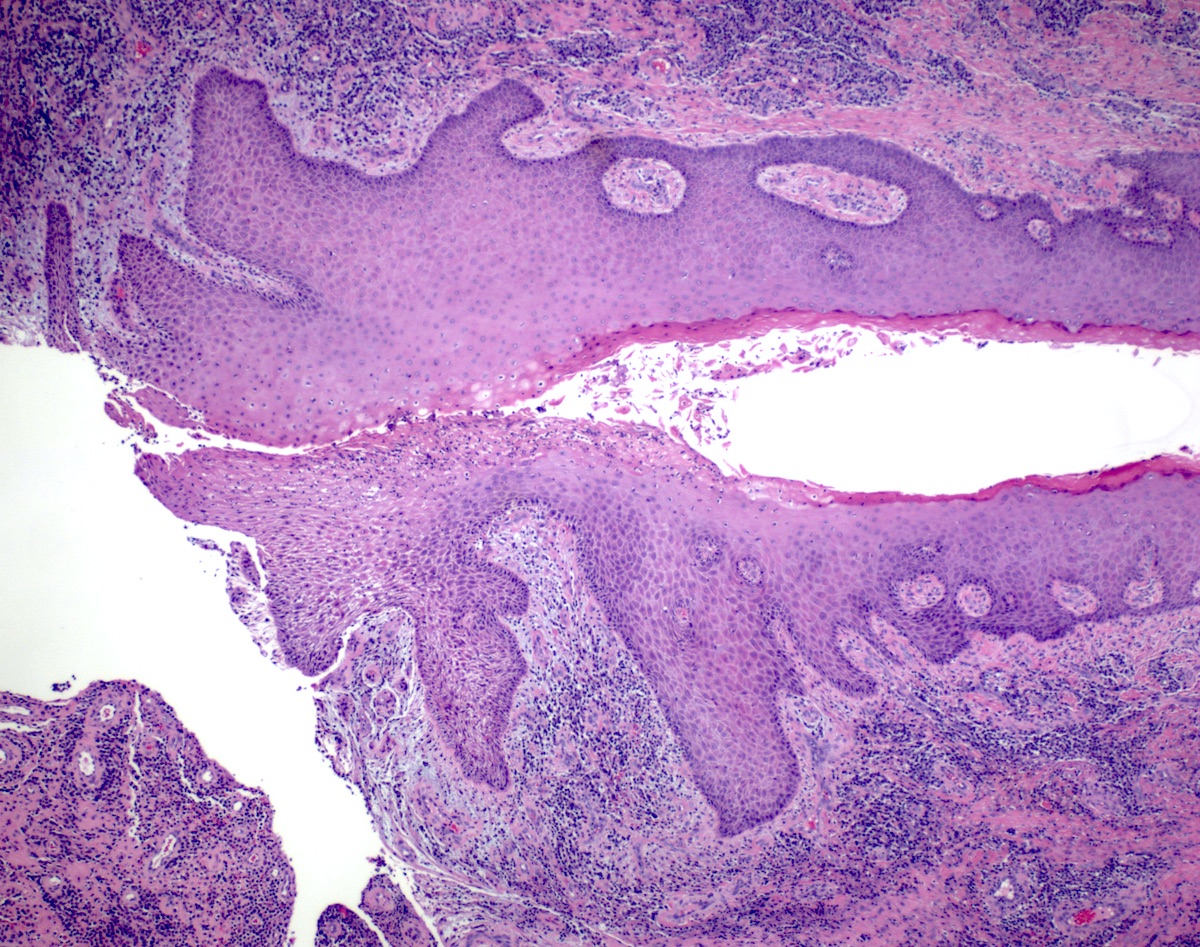
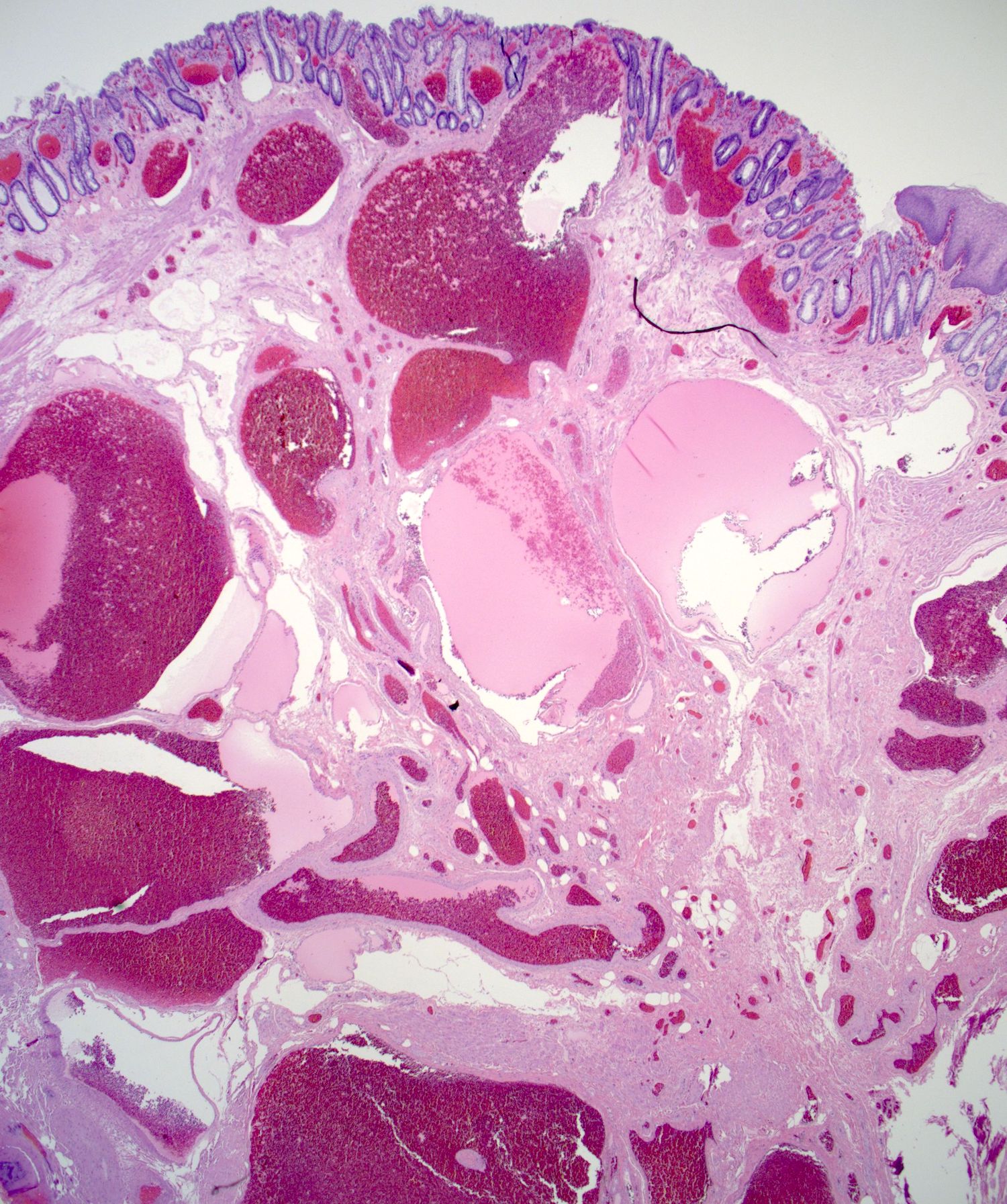
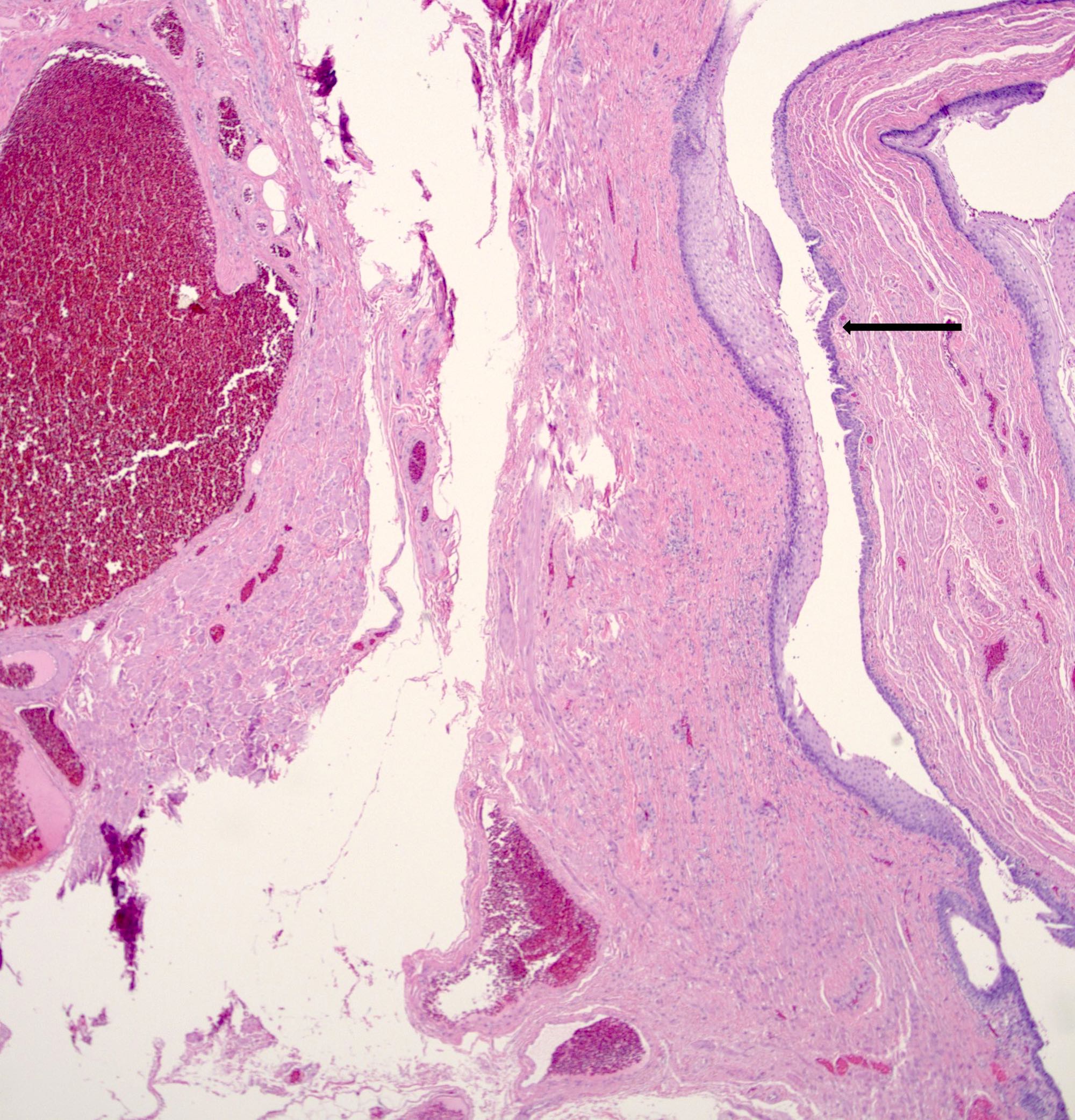
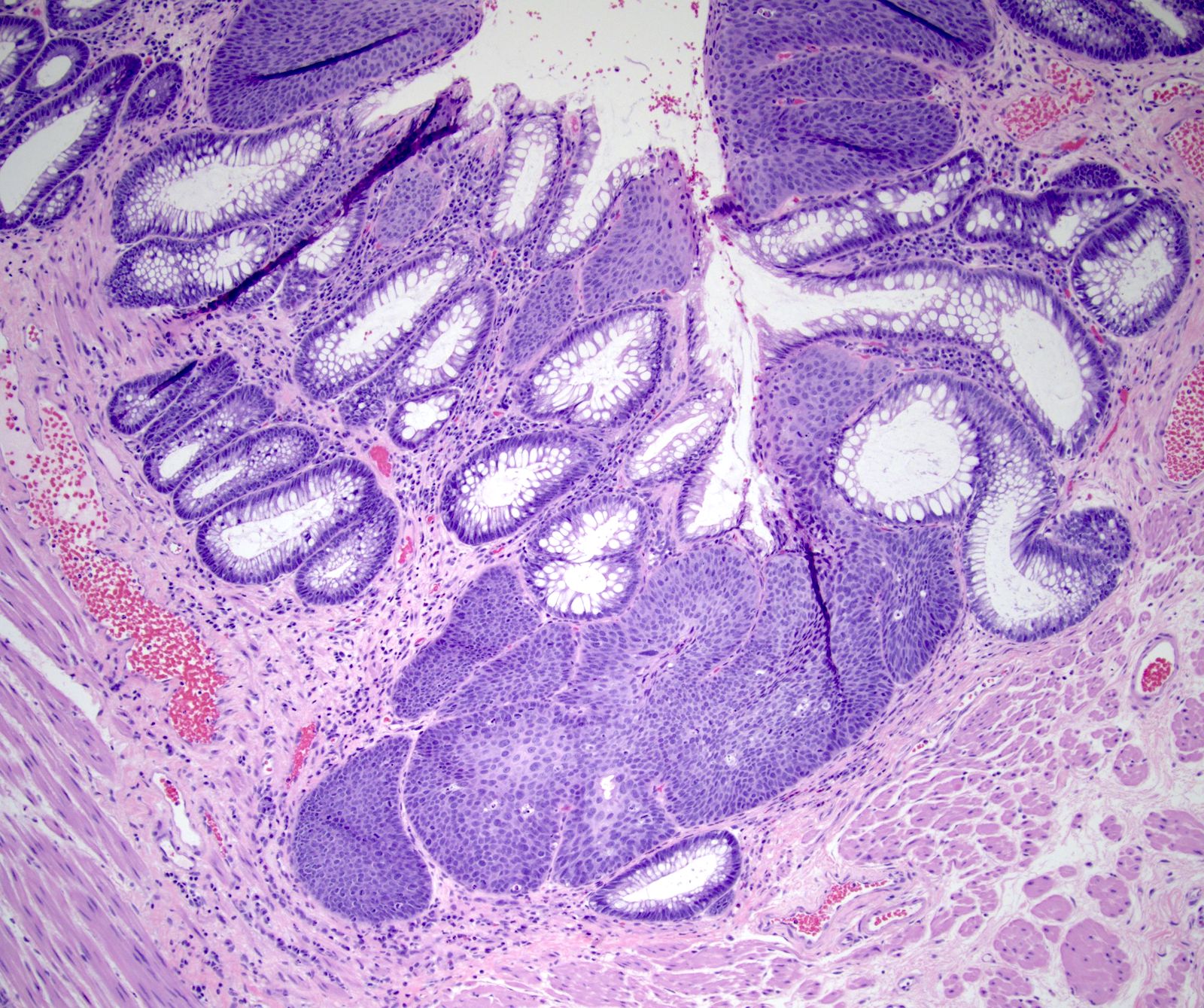
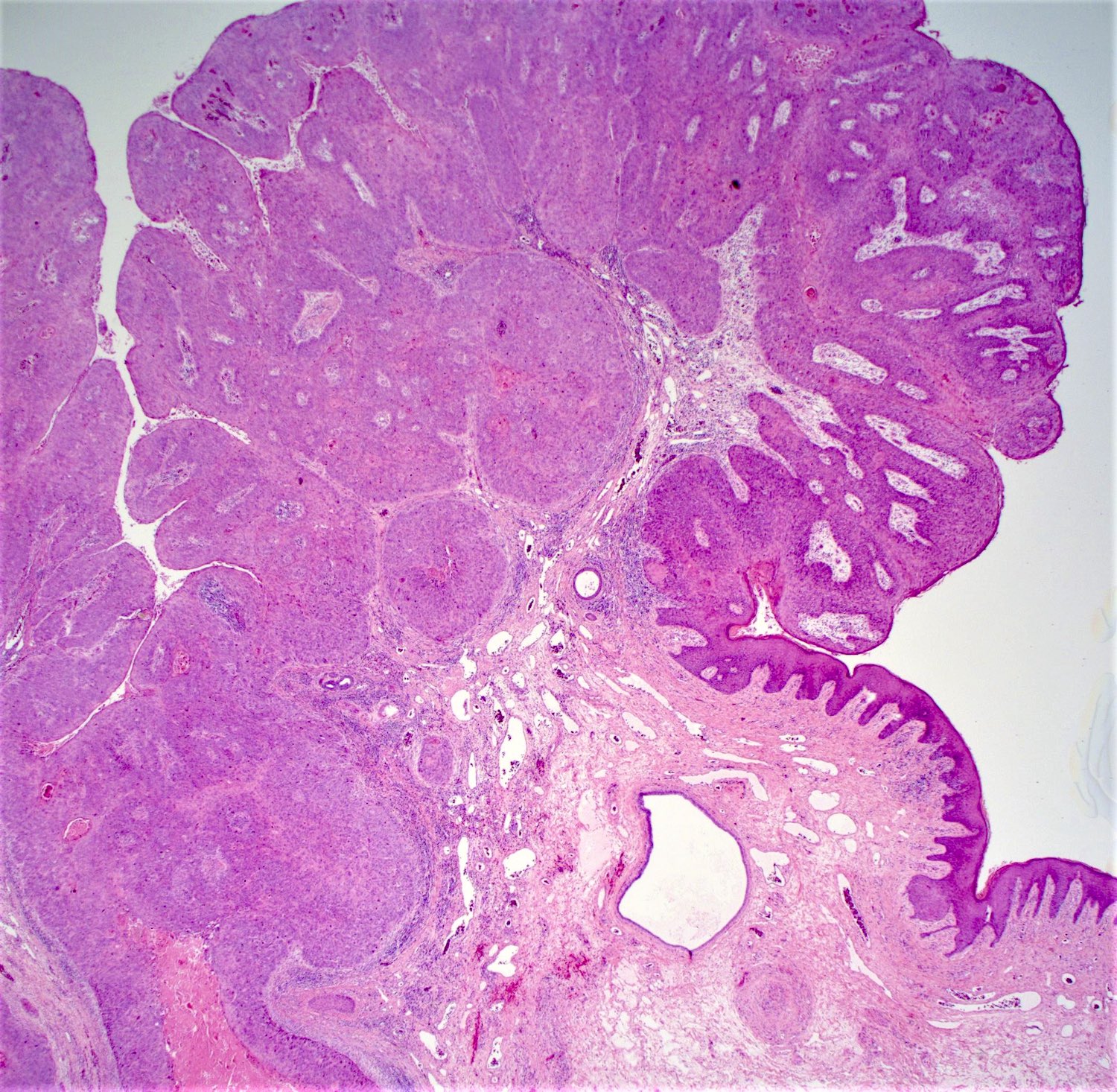
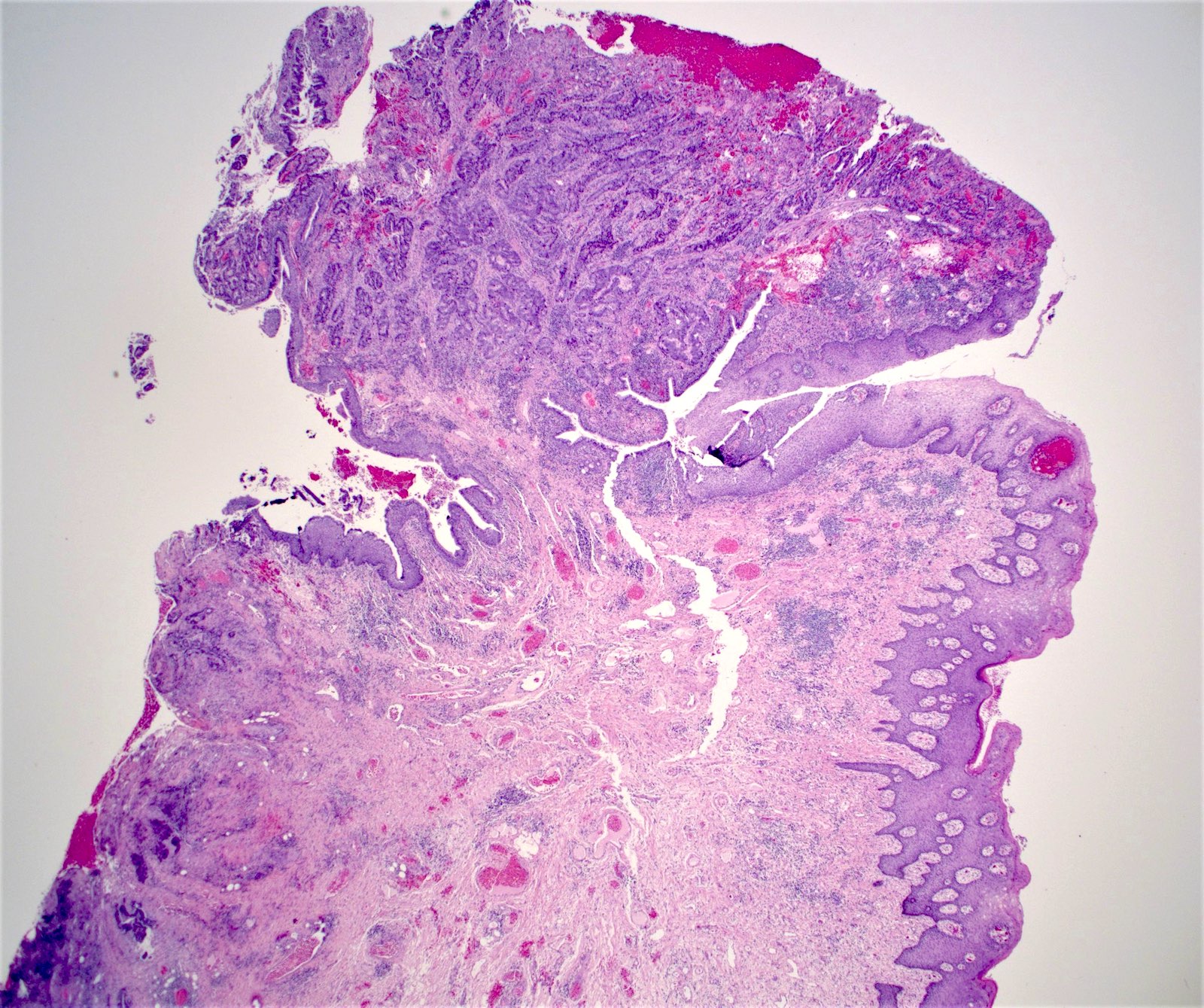
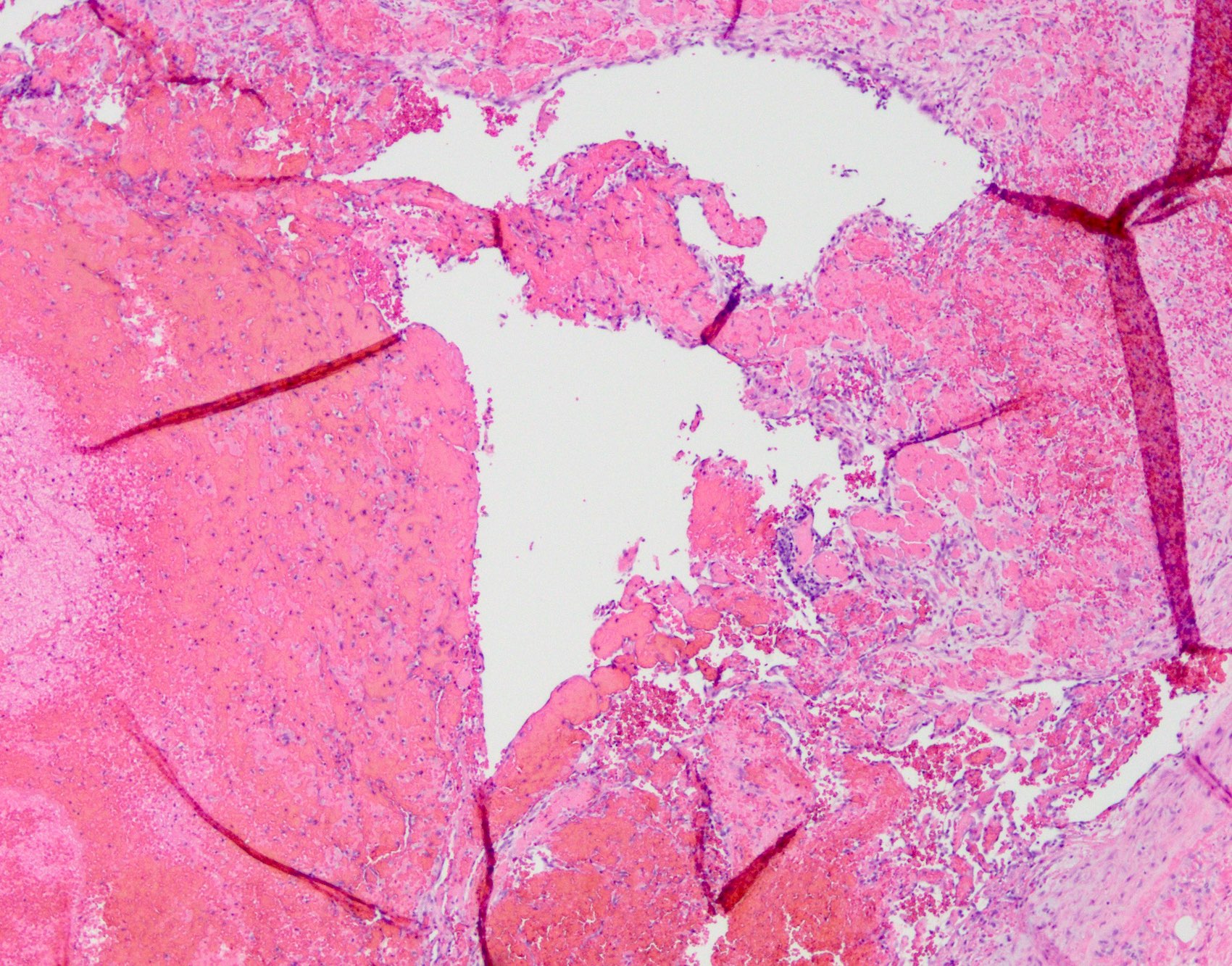
- Adenocarcinomas with an intestinal type appearance involving the anus are likely of rectal origin and should be regarded as such
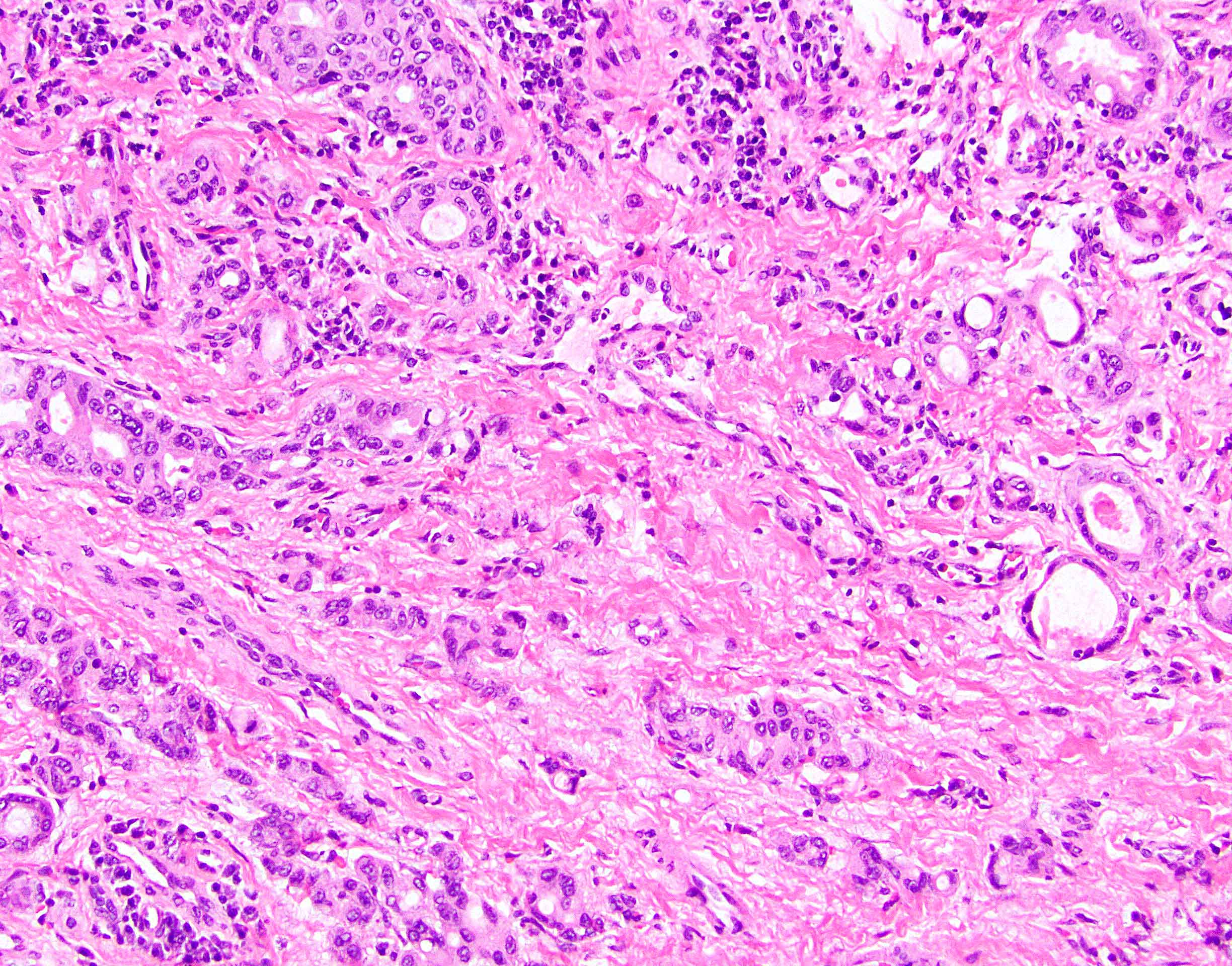
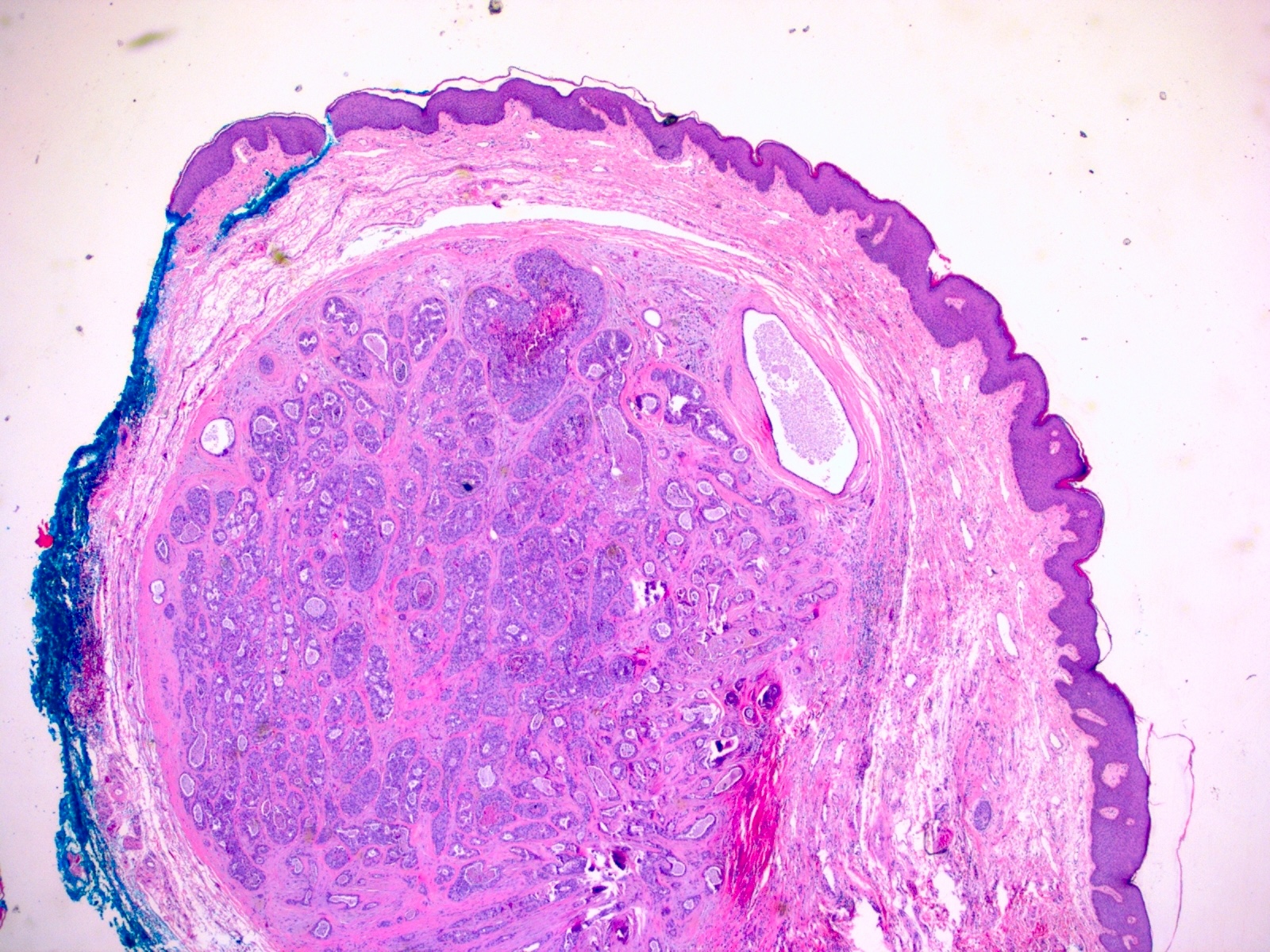
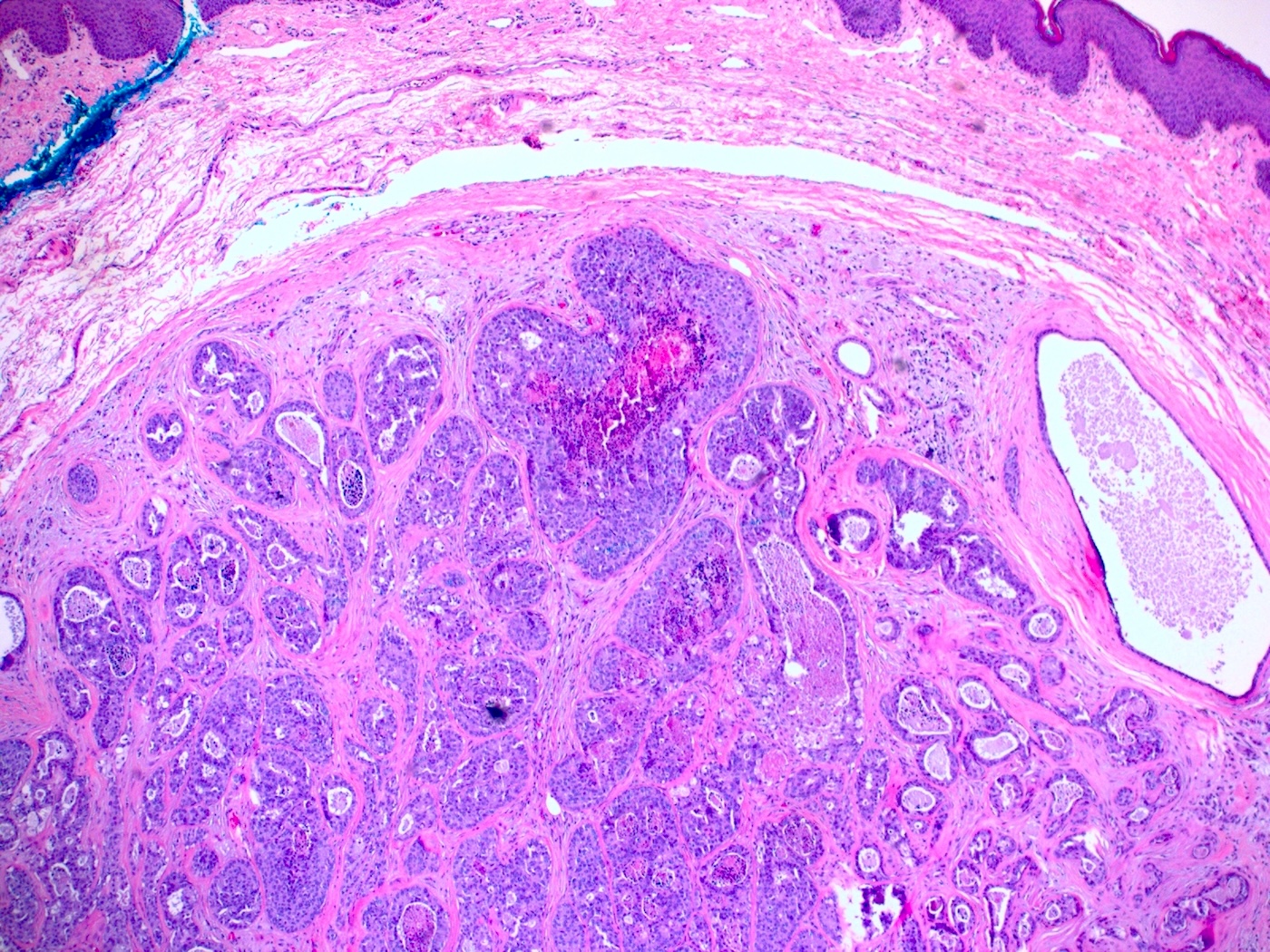
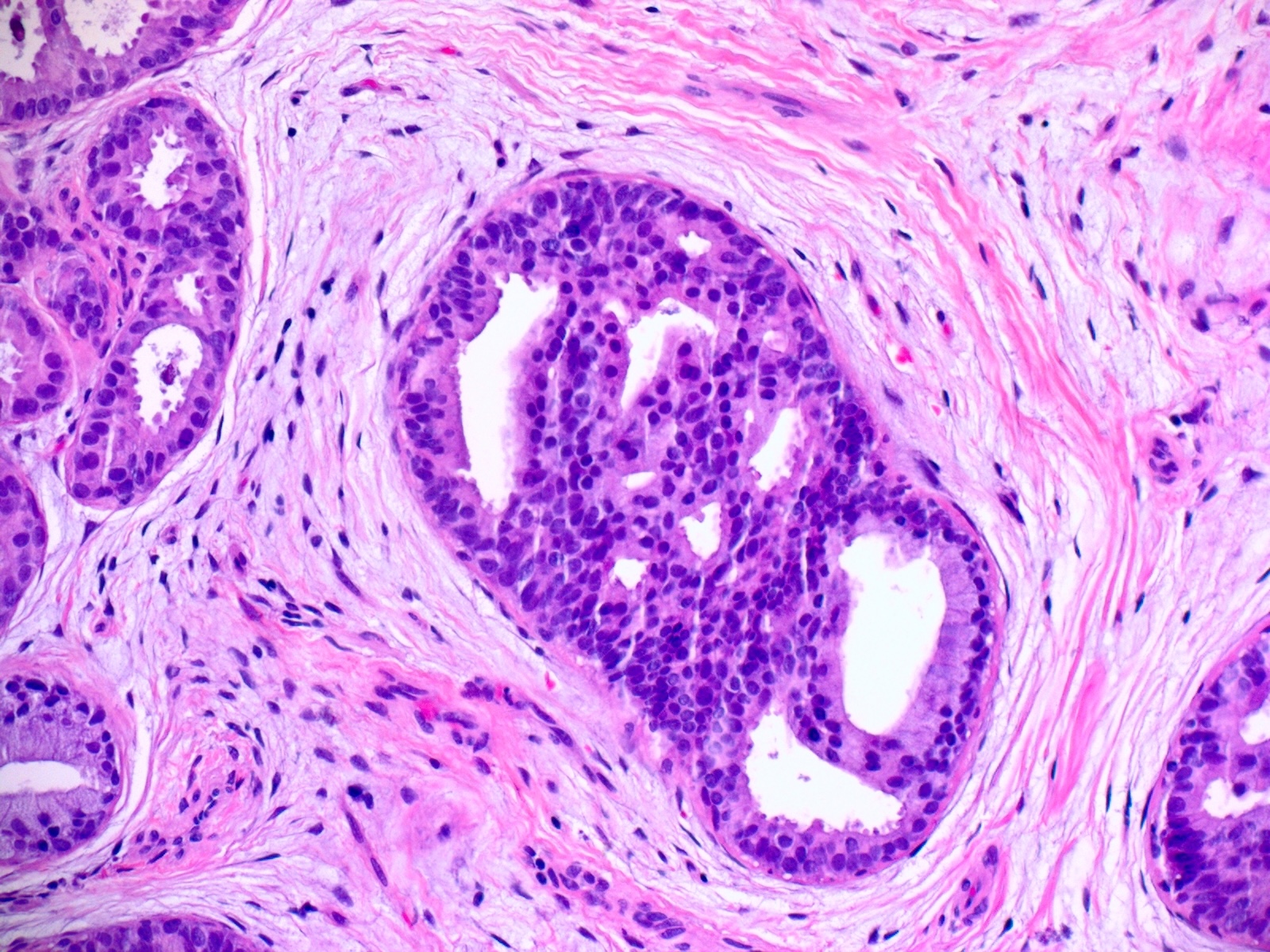
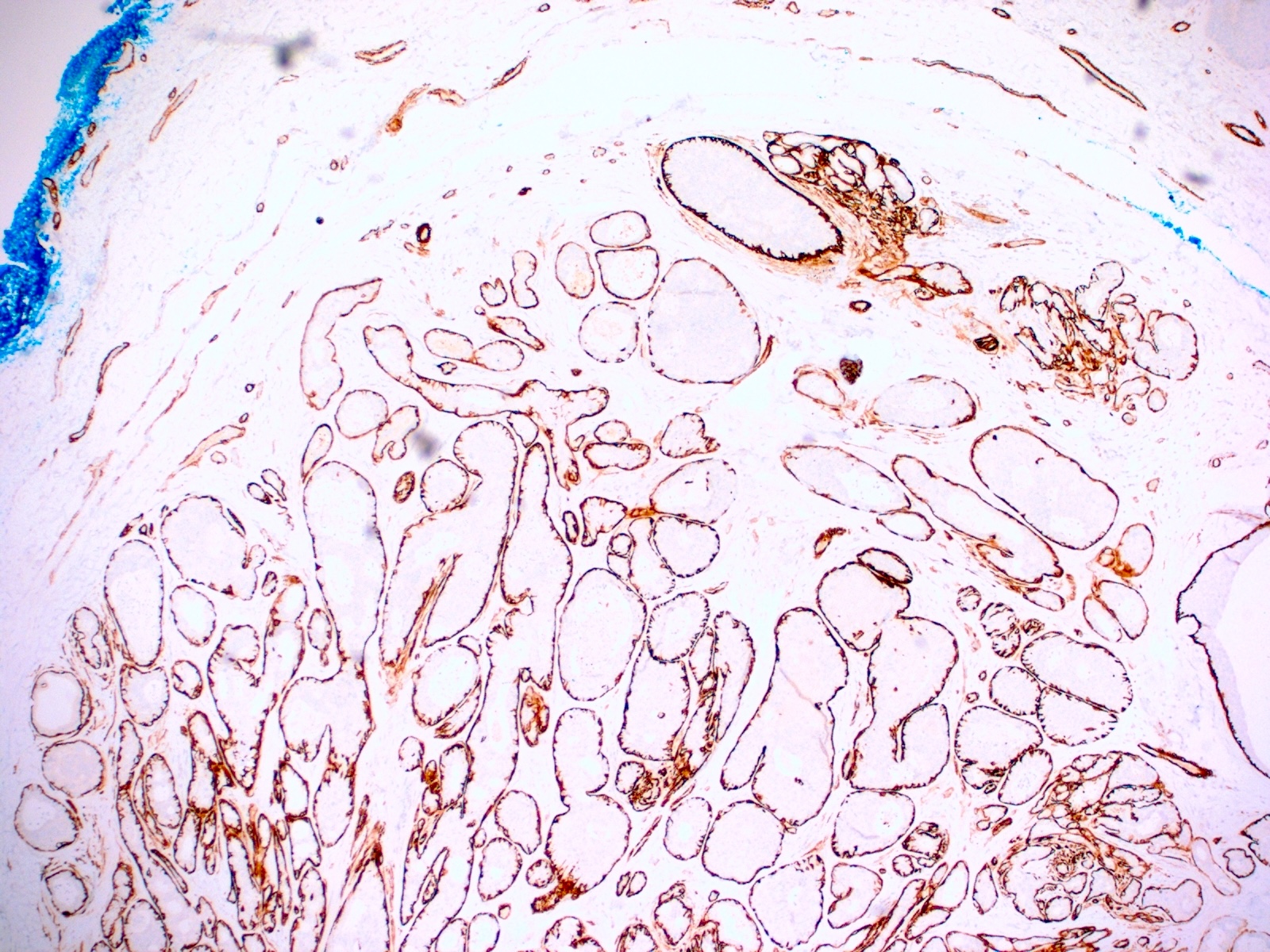
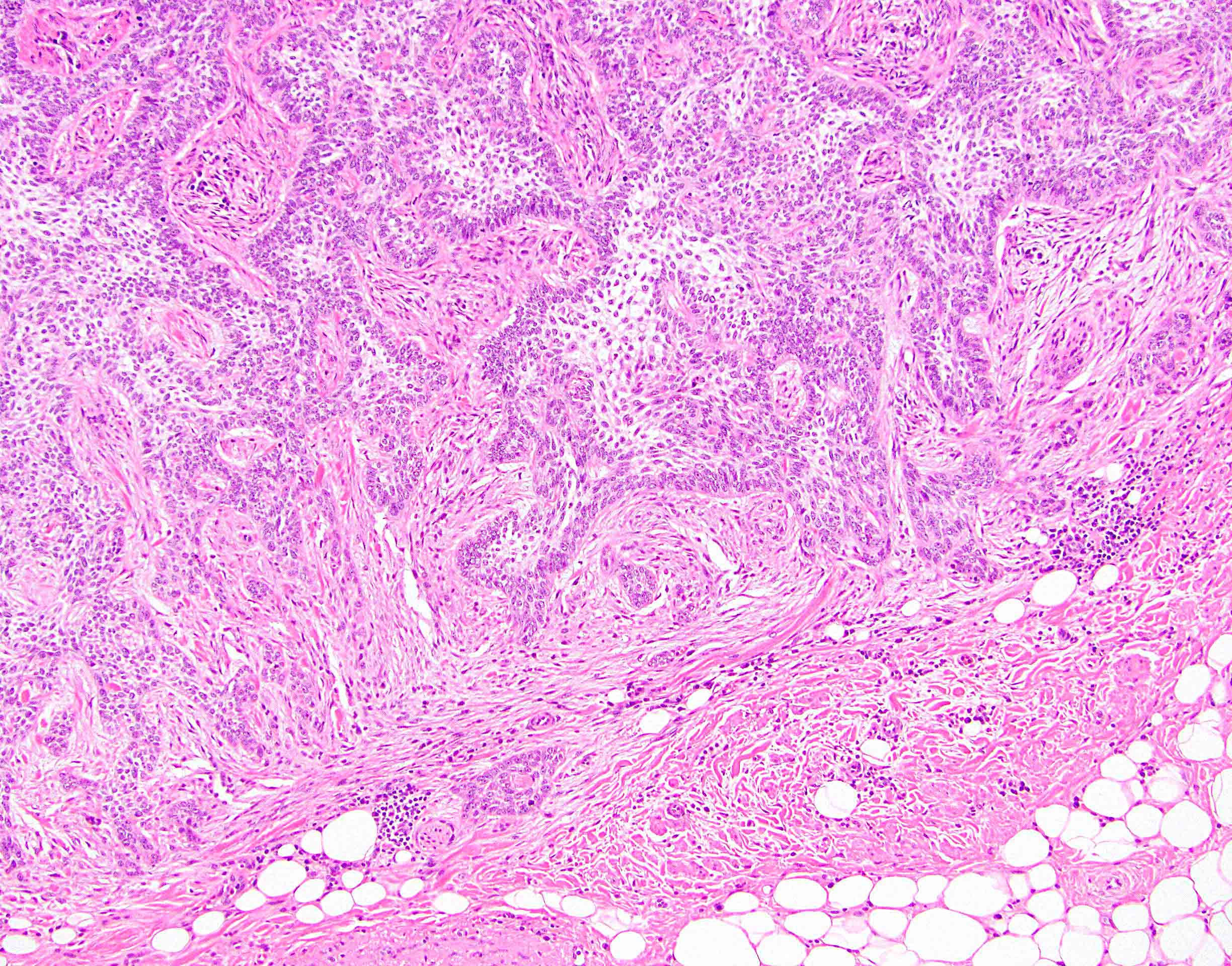
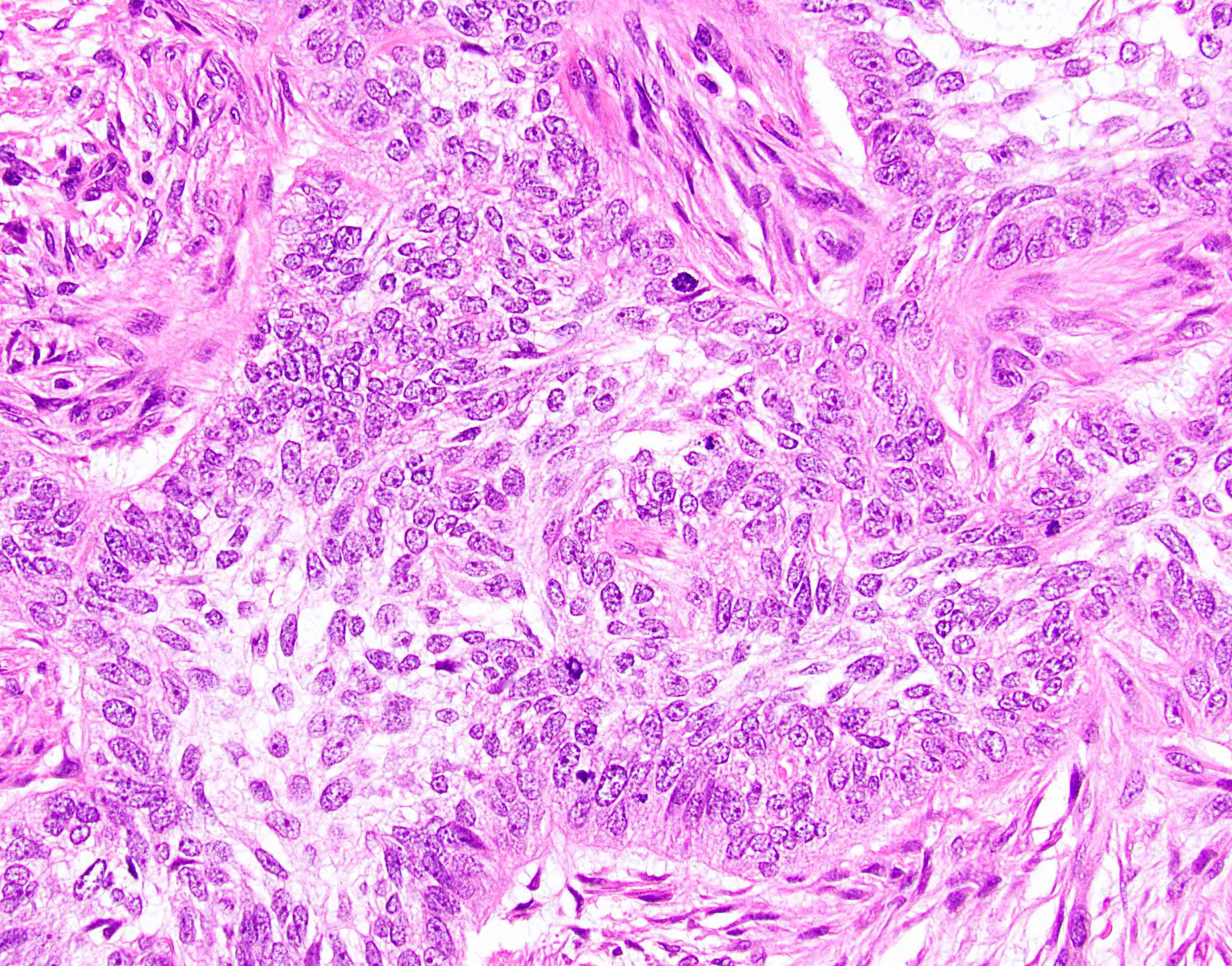
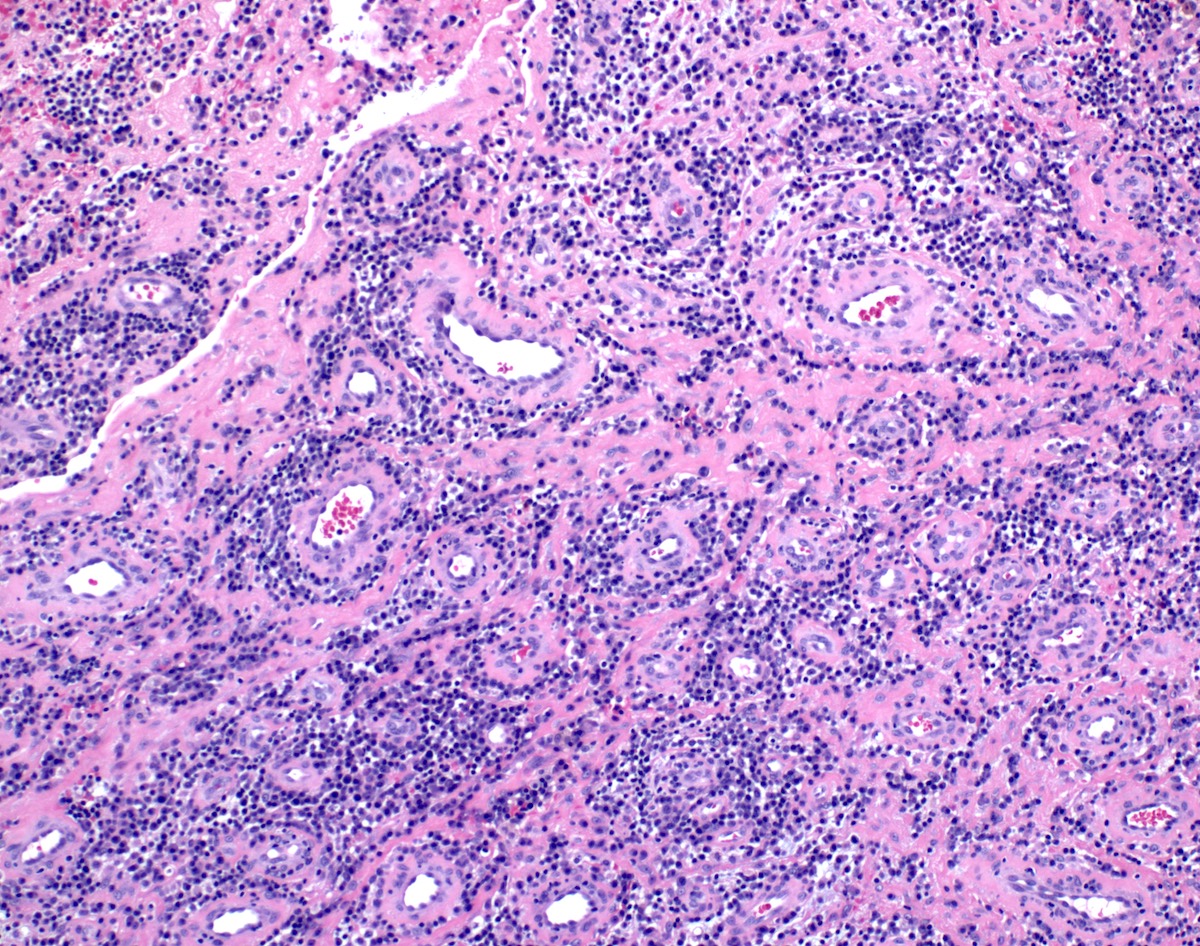
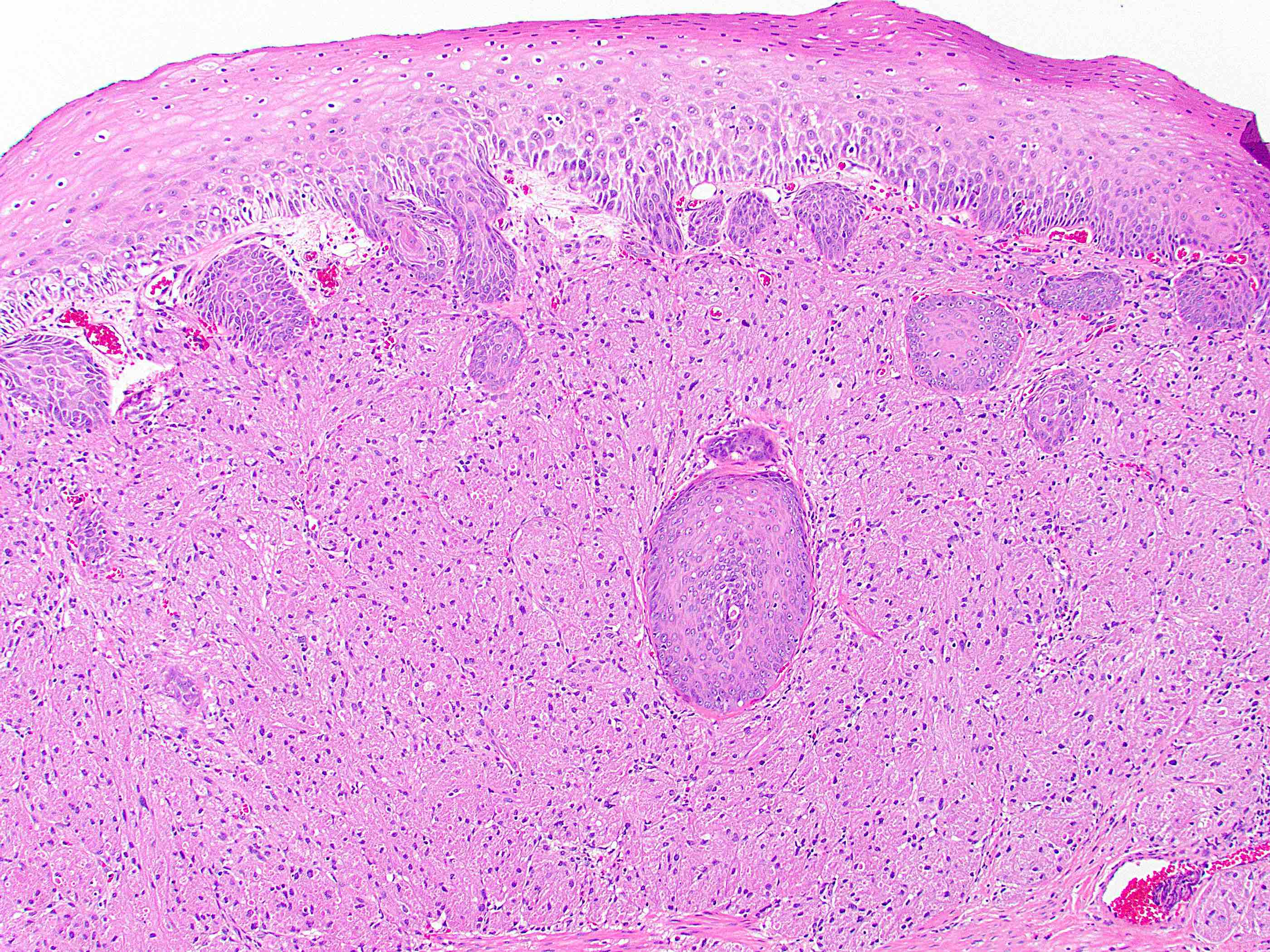
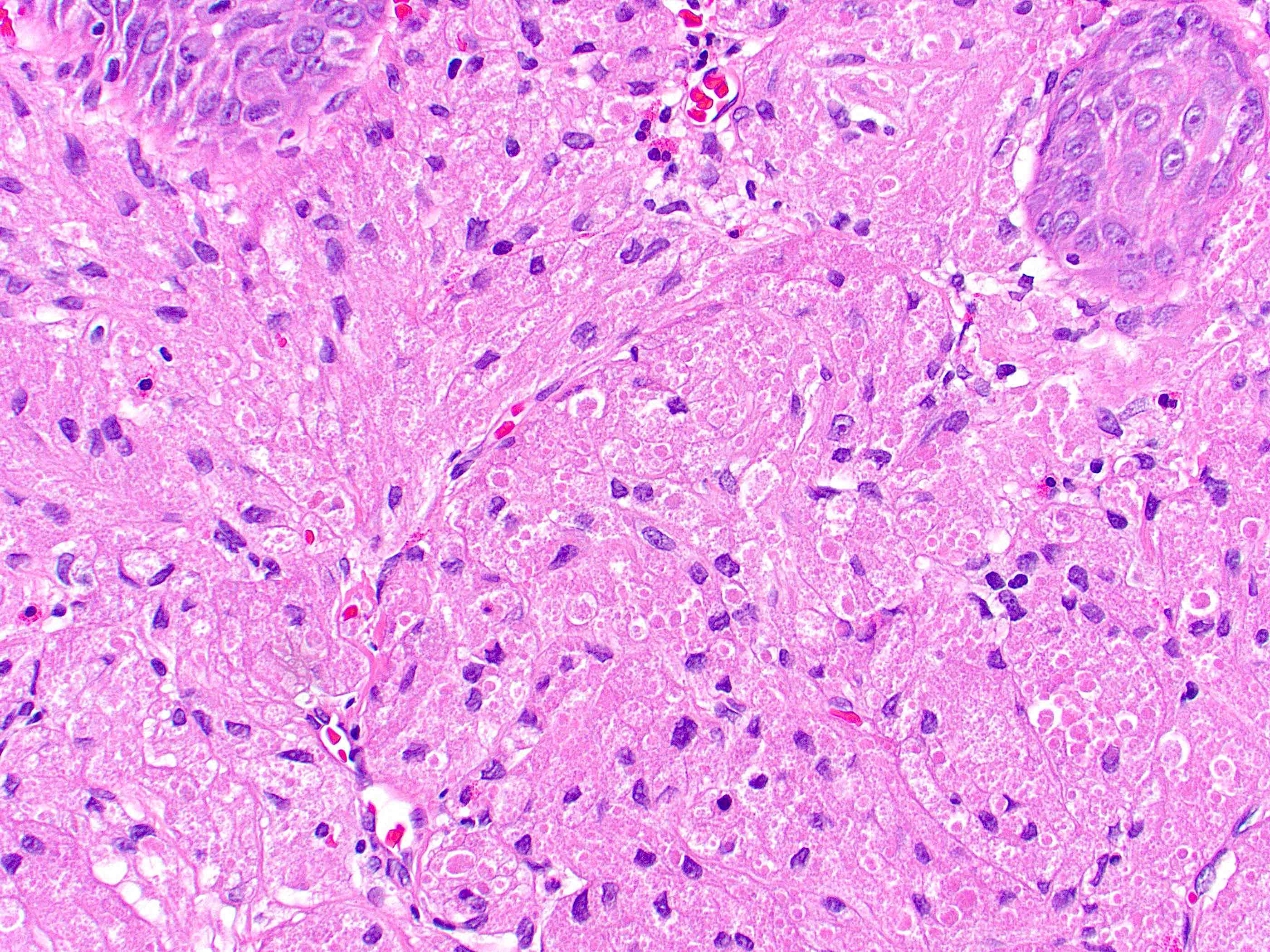
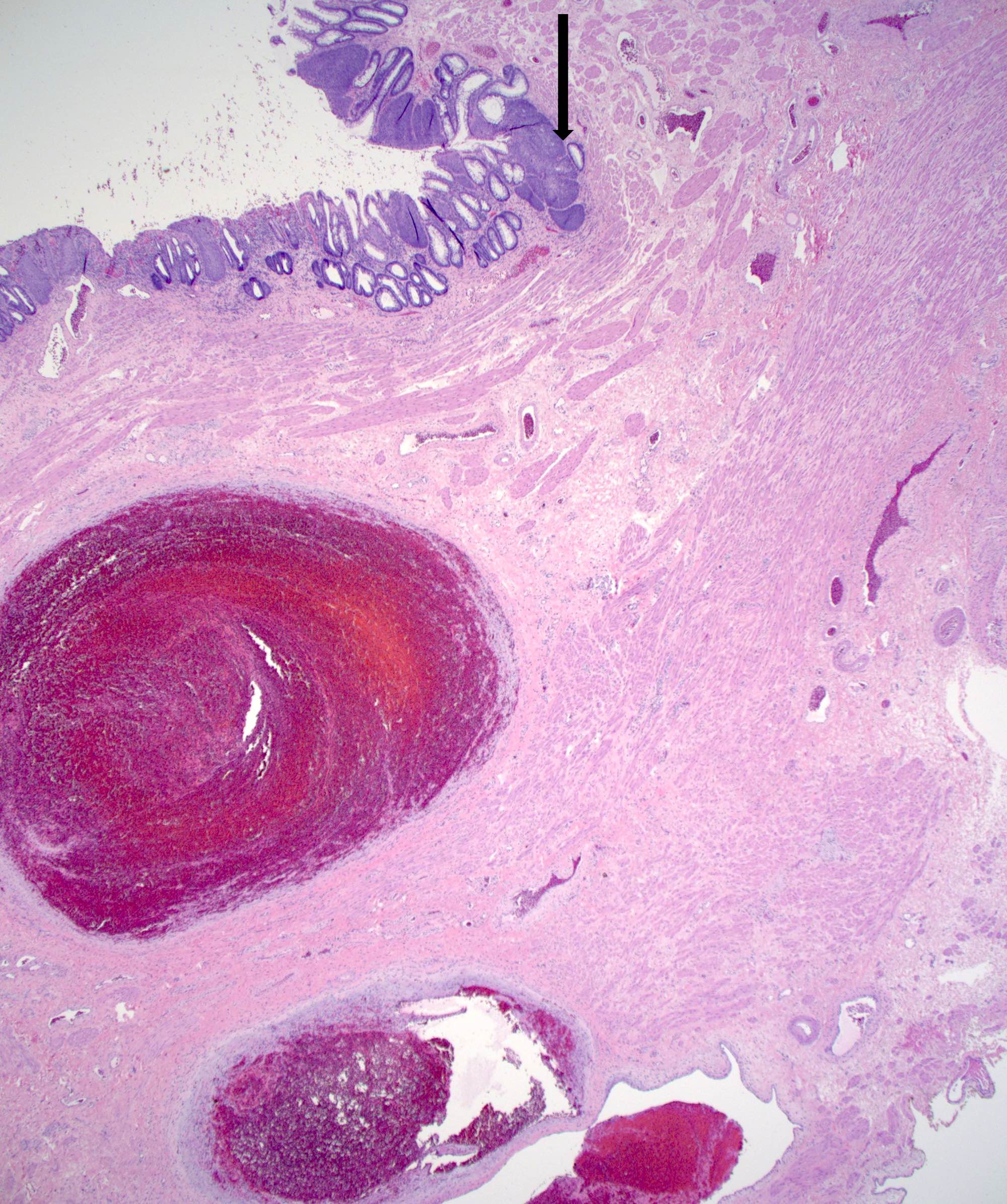
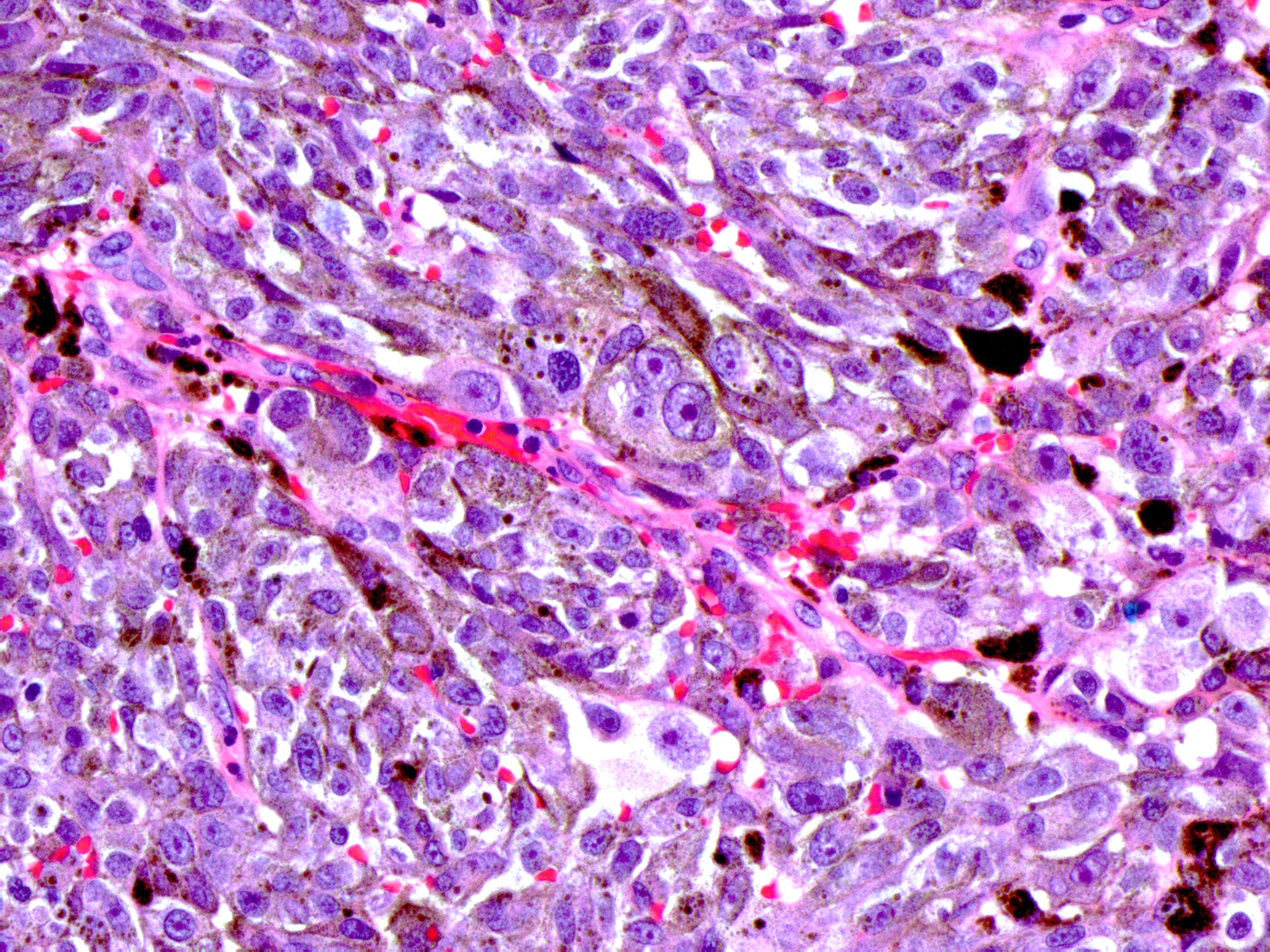
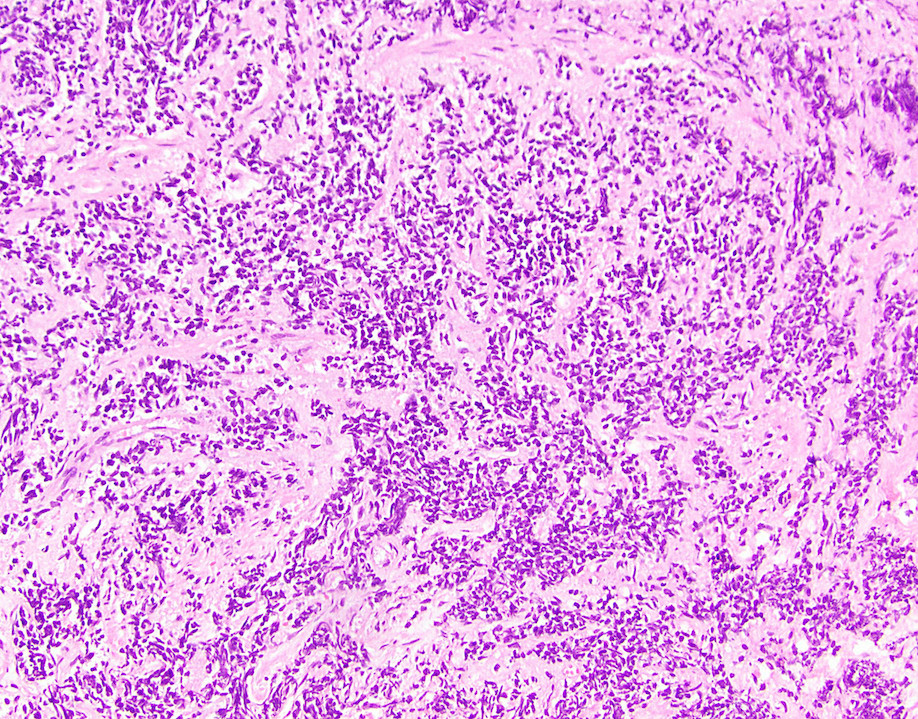
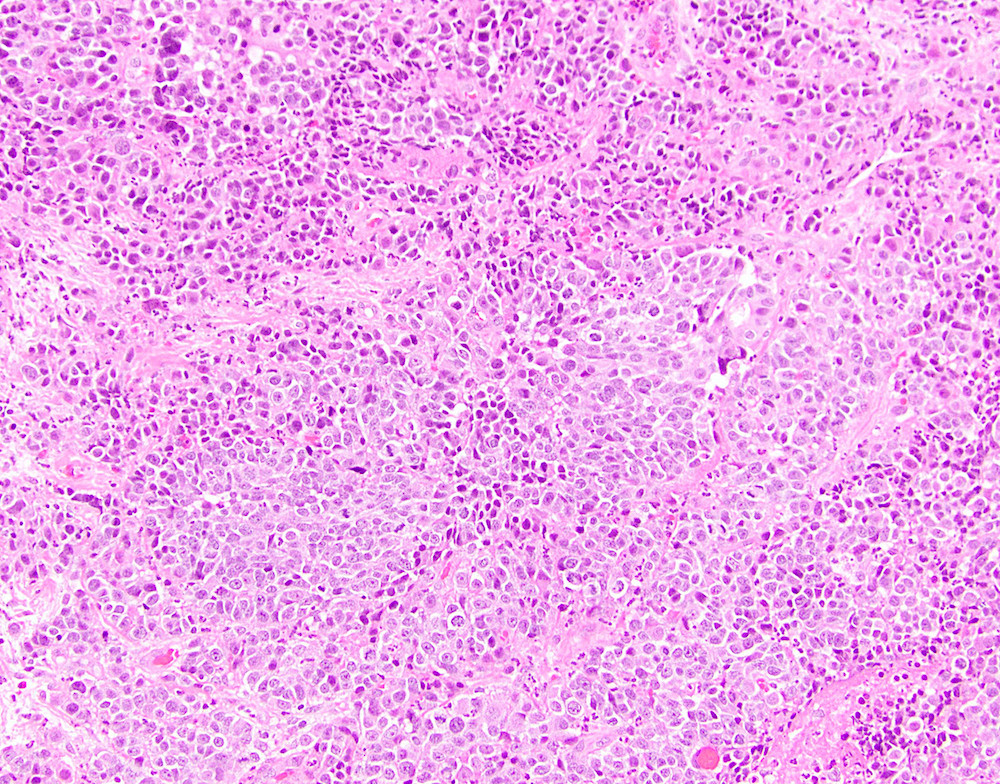
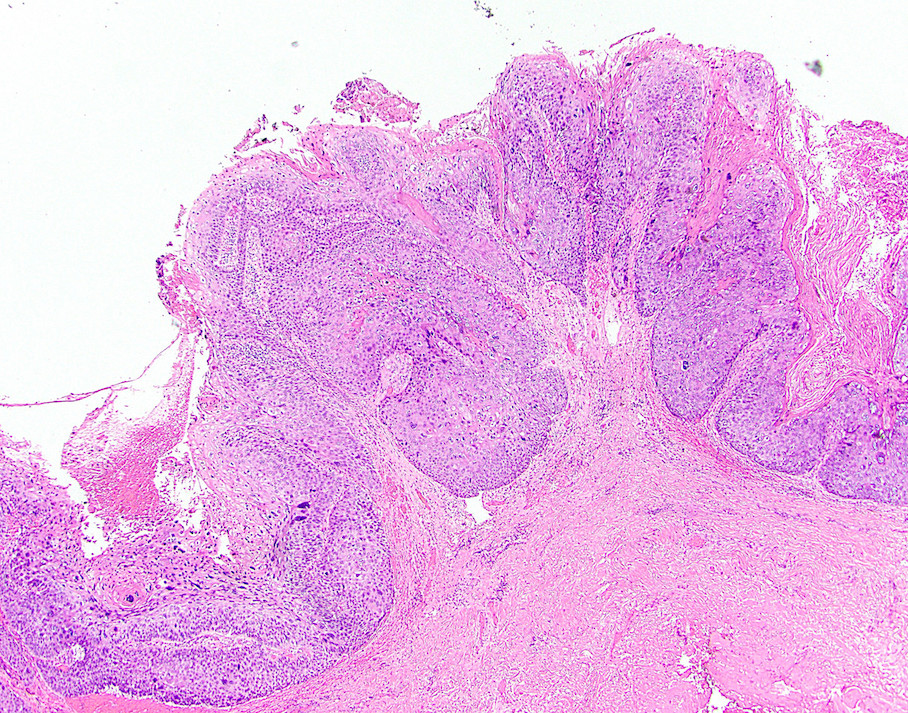
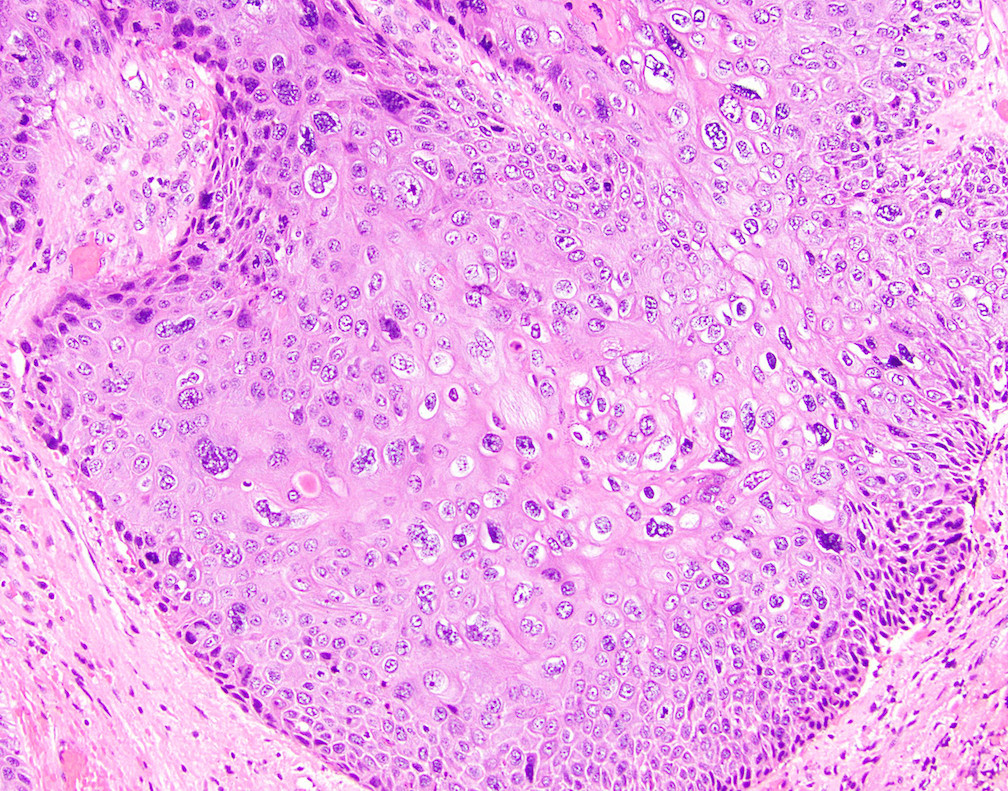
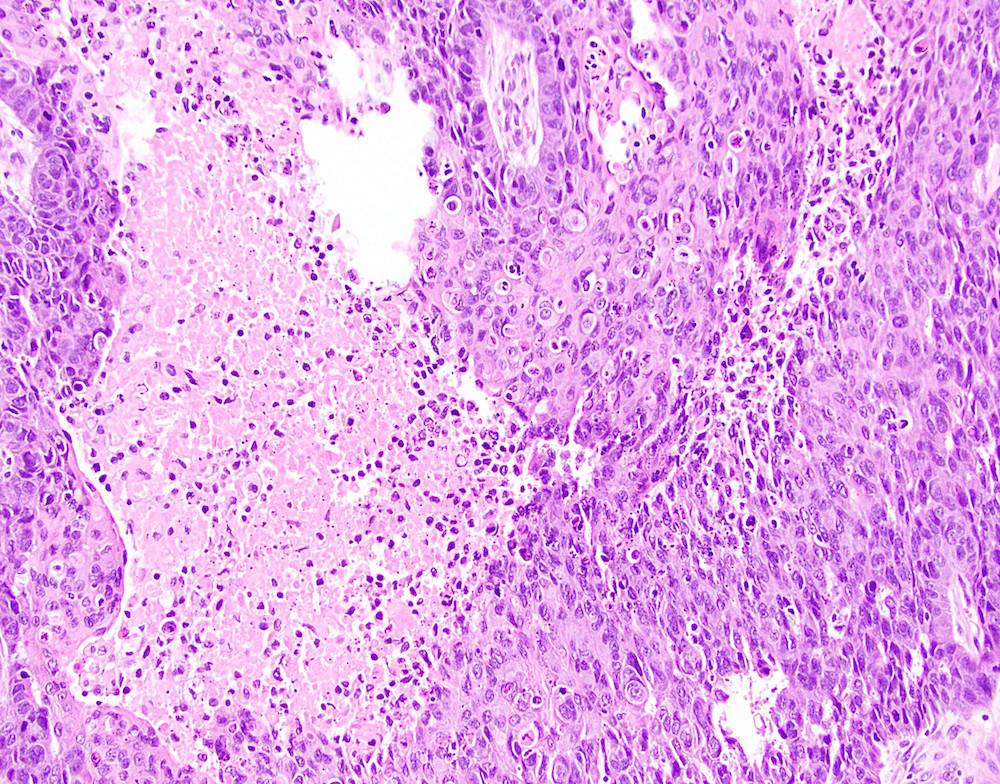
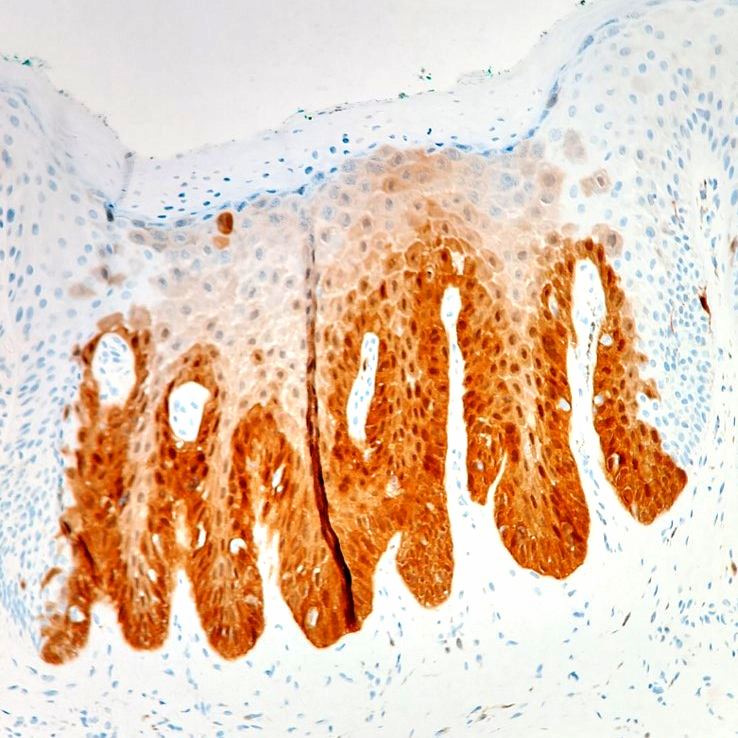
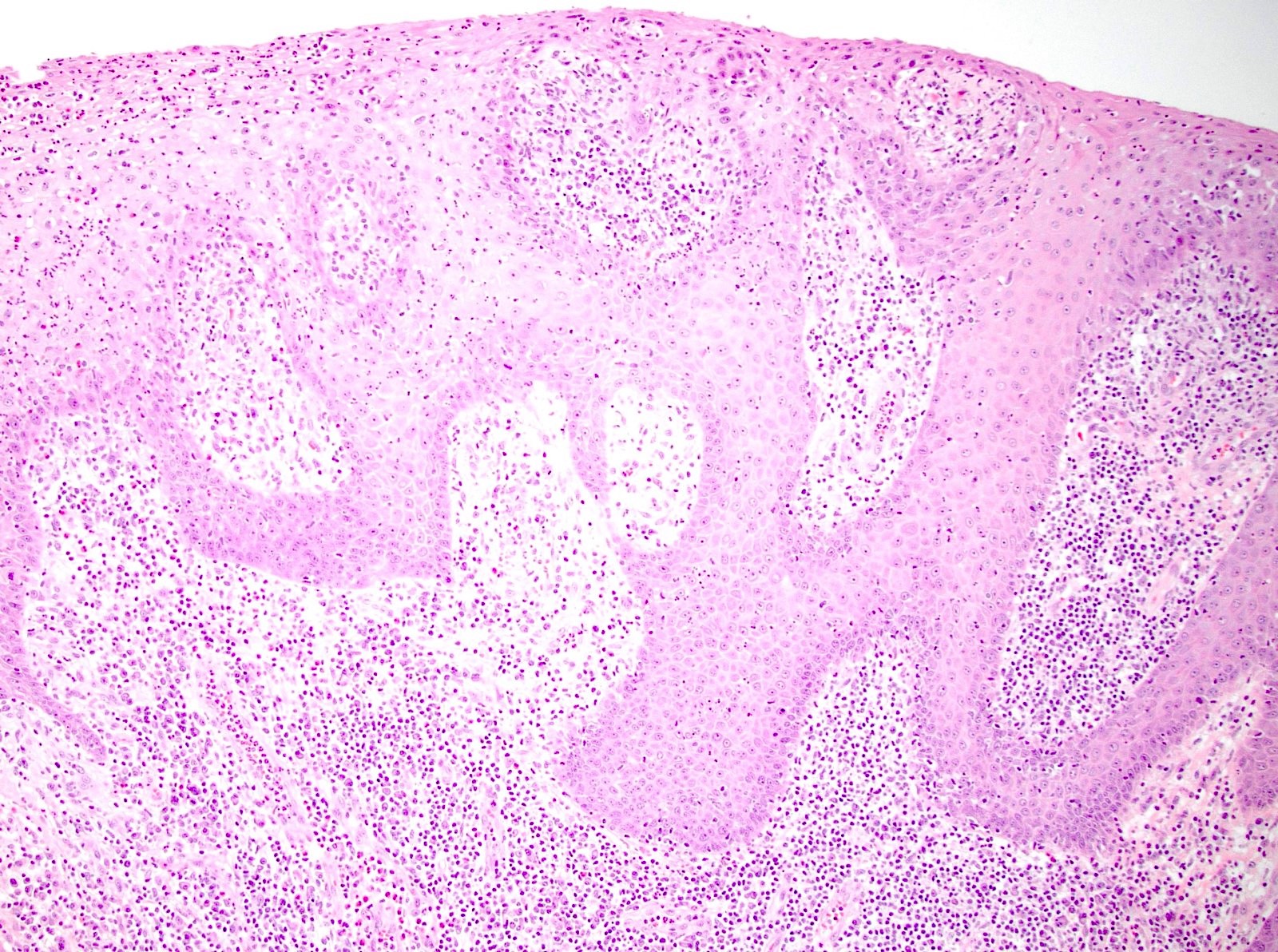
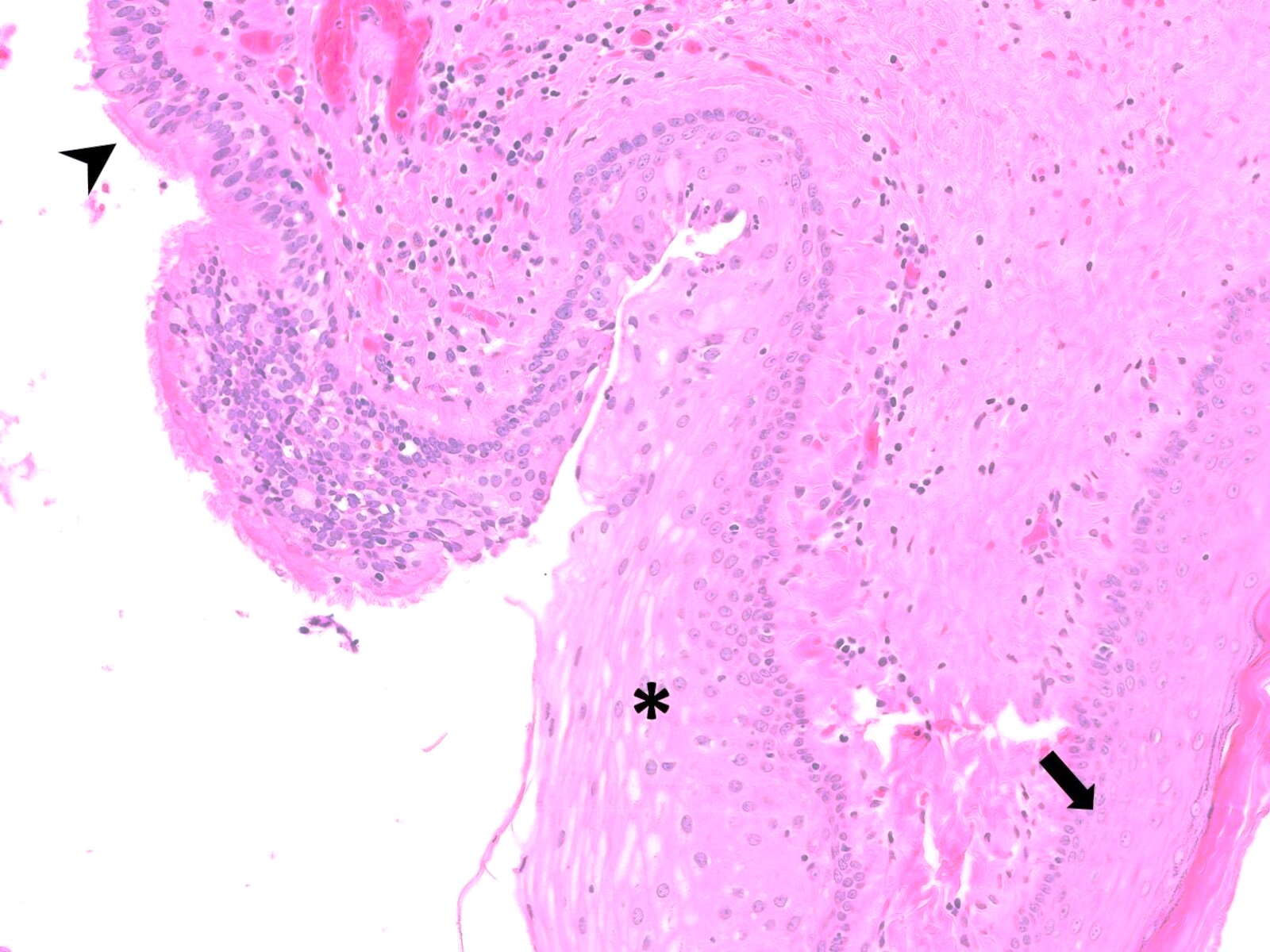
- True anal gland adenocarcinomas form haphazardly dispersed small glands with scant mucin production that invade the wall of the anorectal area without an intraluminal component (Cancer 2001;92:2045)
- Fistula associated adenocarcinomas are often mucinous (> 50% of tumor volume consists of mucin); granulomatous reaction to mucin may be present
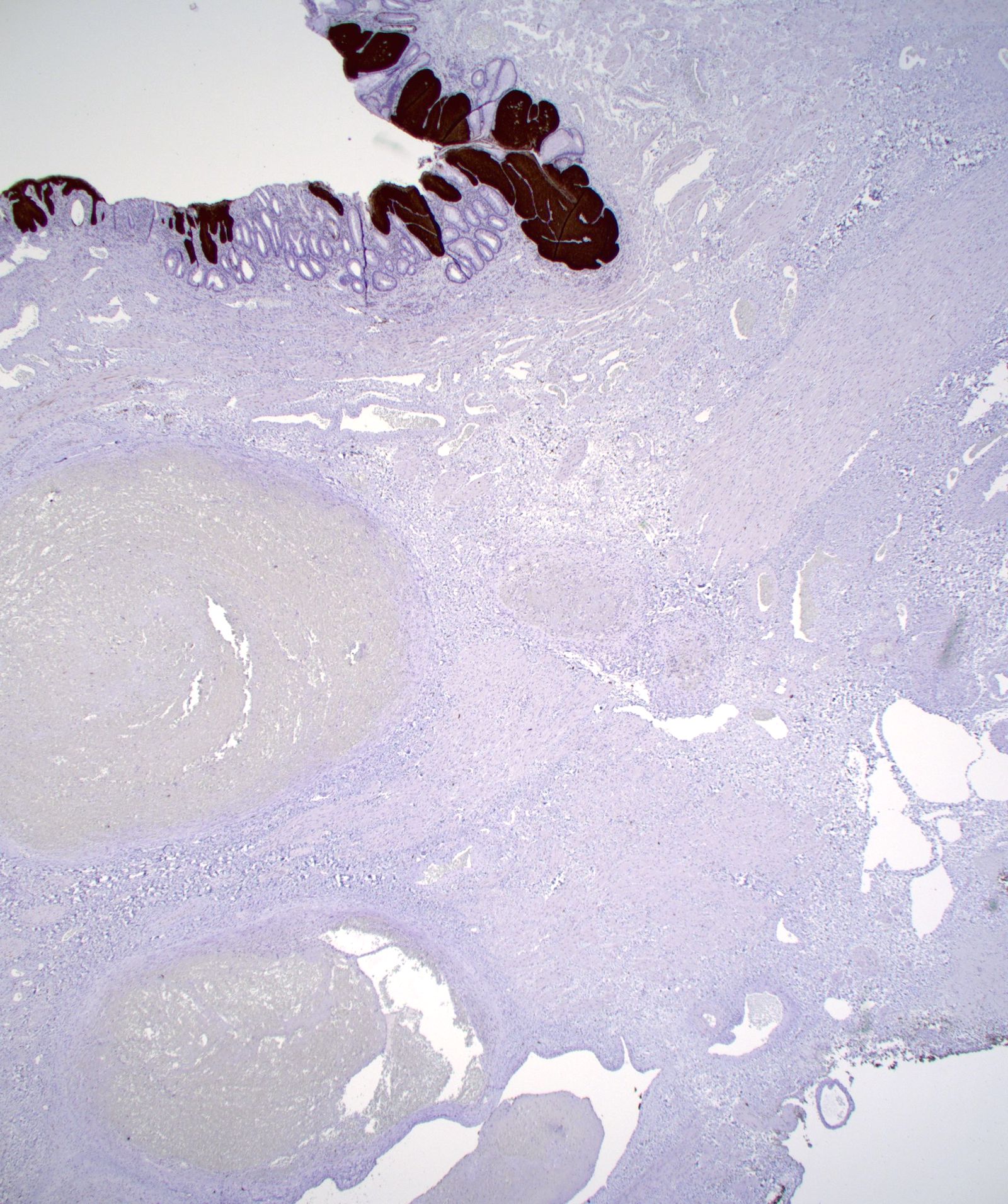
- May contain melanin pigment, perhaps due to tumor cell phagocytosis of melanin from melanocytes (Am J Surg Pathol 1981;5:711)
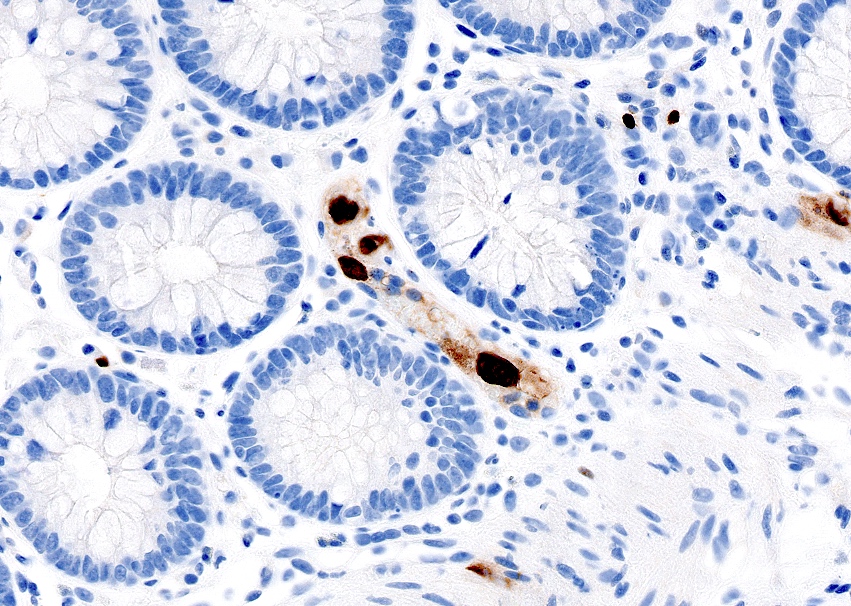
- Single neoplastic cells may colonize the overlying and adjacent squamous mucosa (Paget disease)
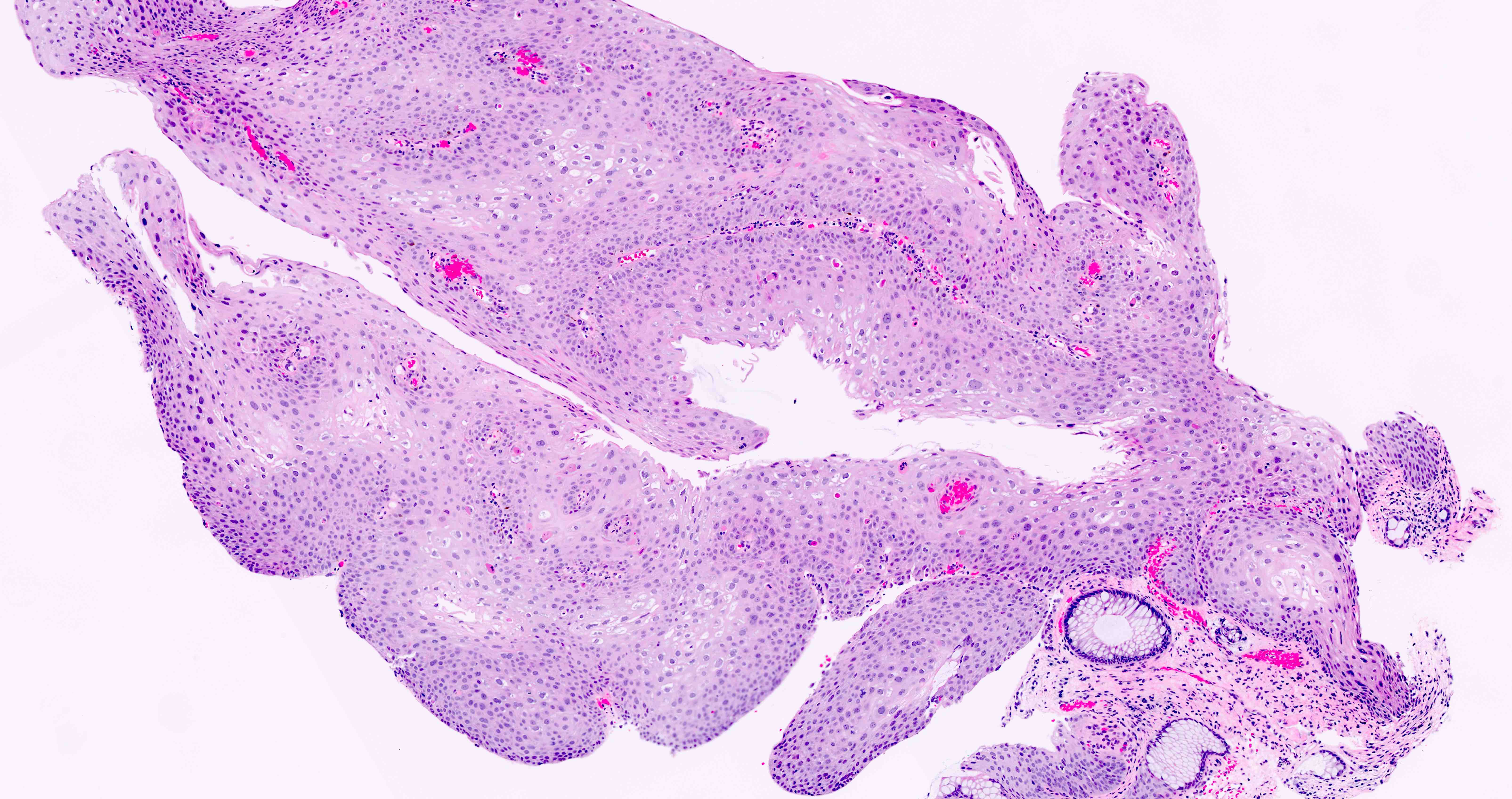
- HPV related cases show papillary or villiform structures lined by columnar cells that often contain abundant mucin (Mod Pathol 2020;33:944)
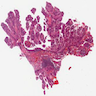
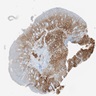
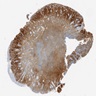
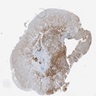
Microscopic (histologic) images
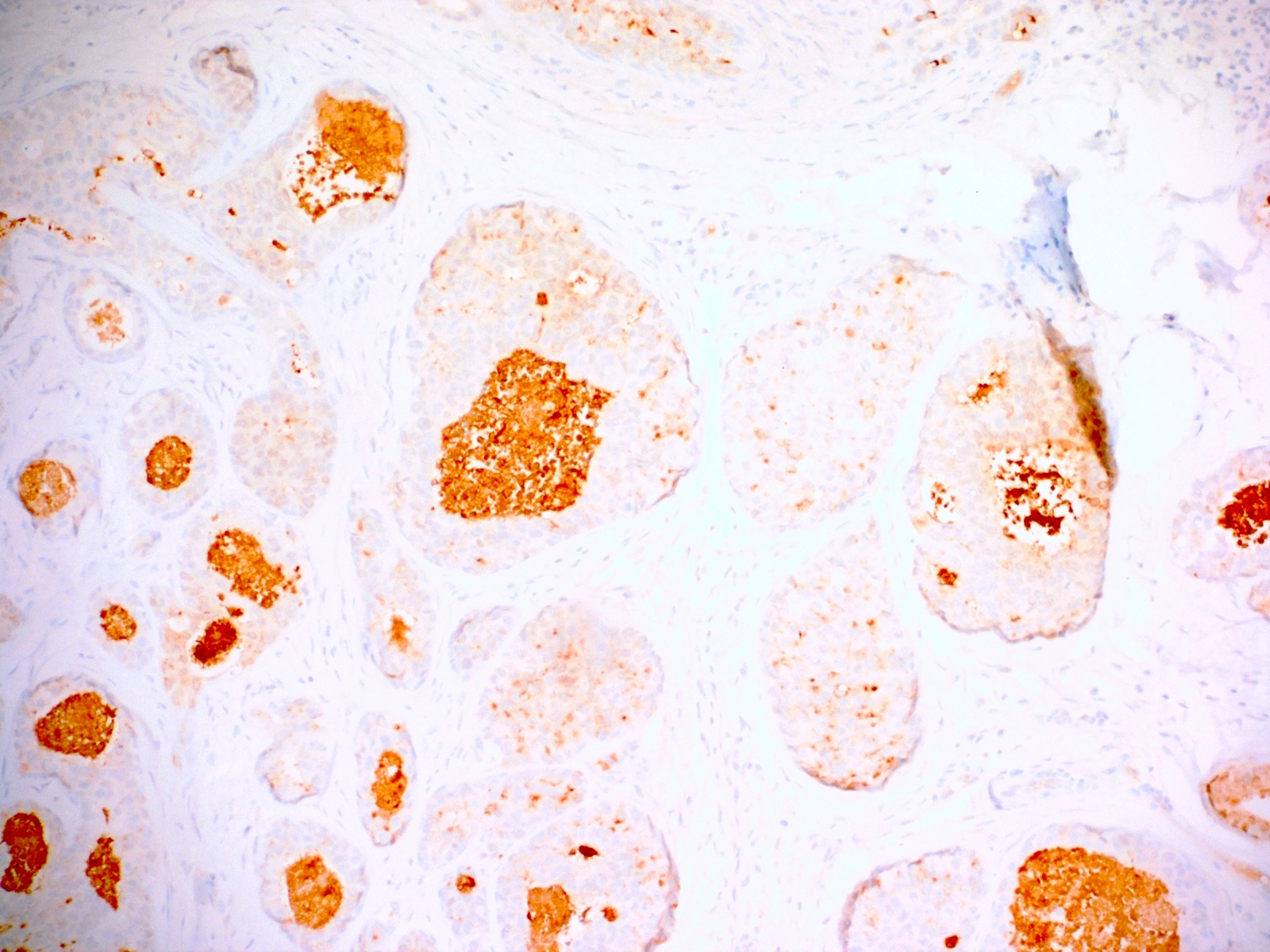
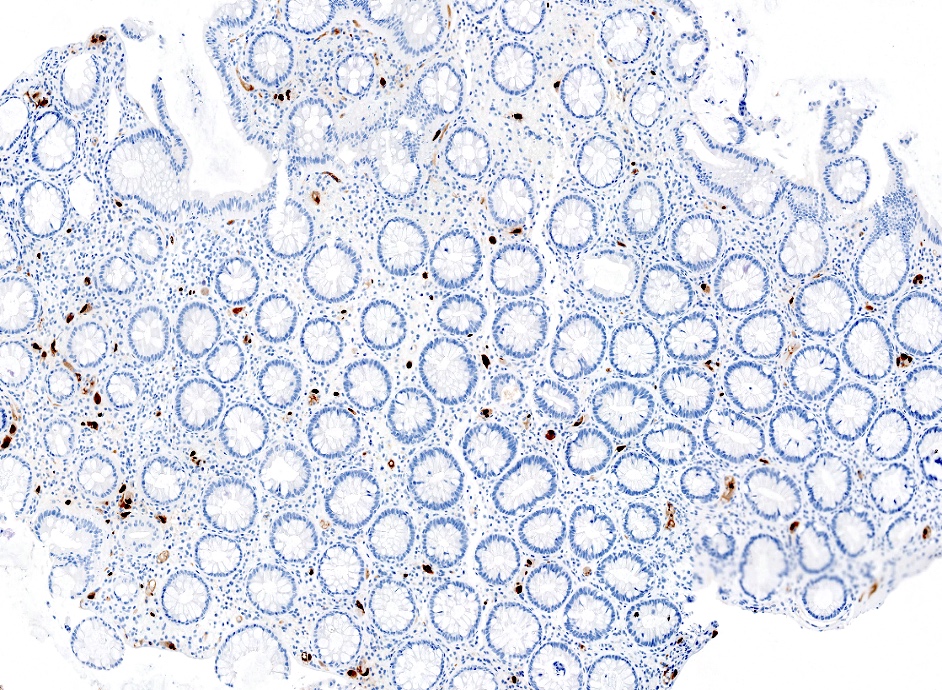
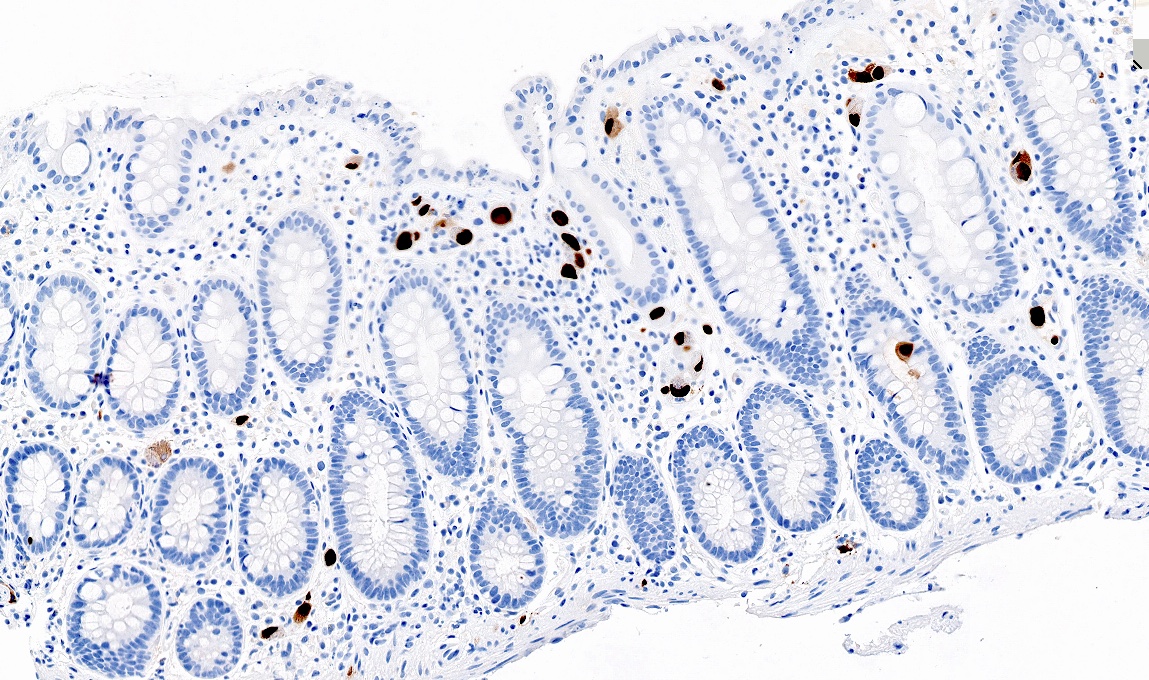
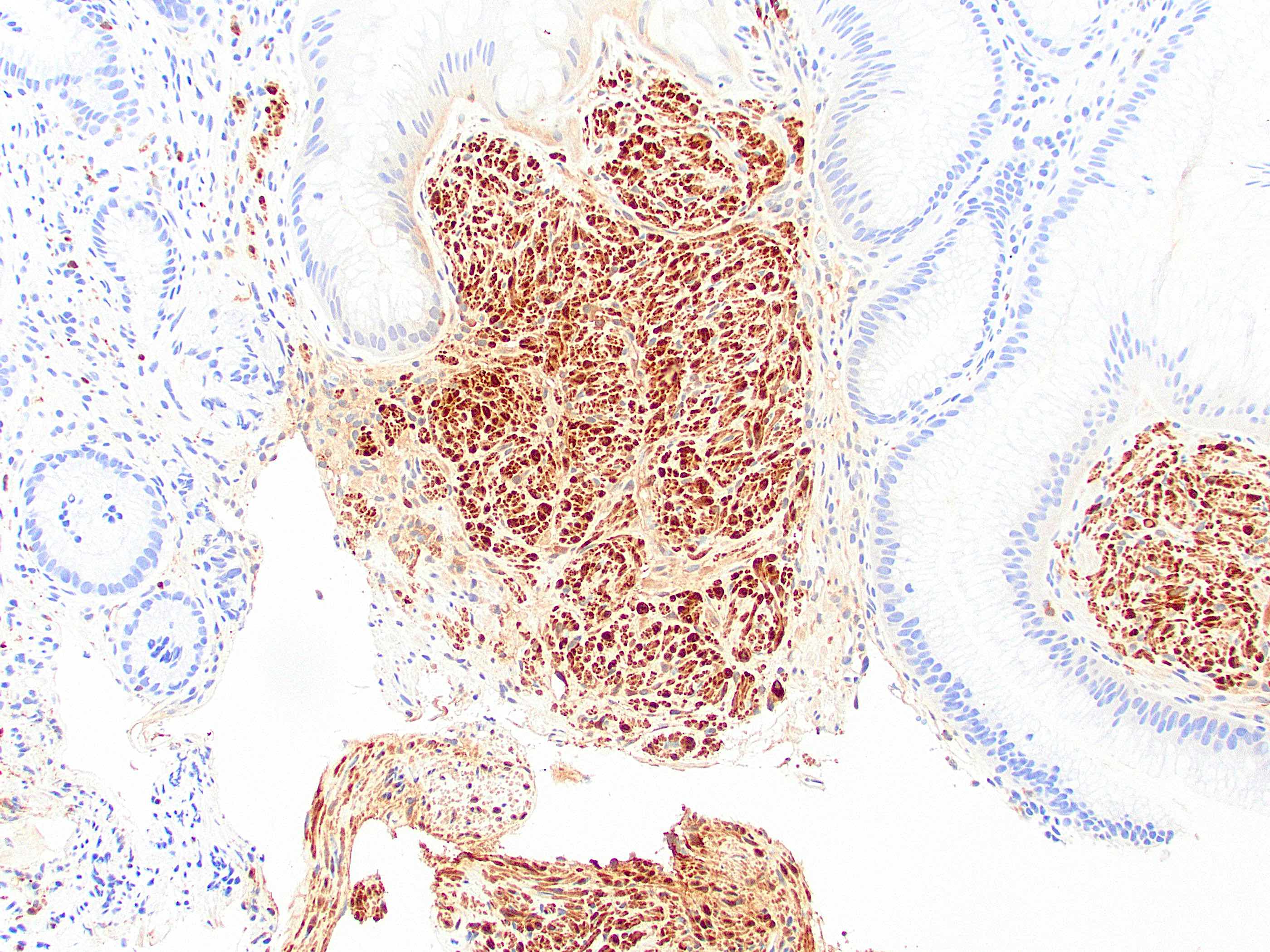
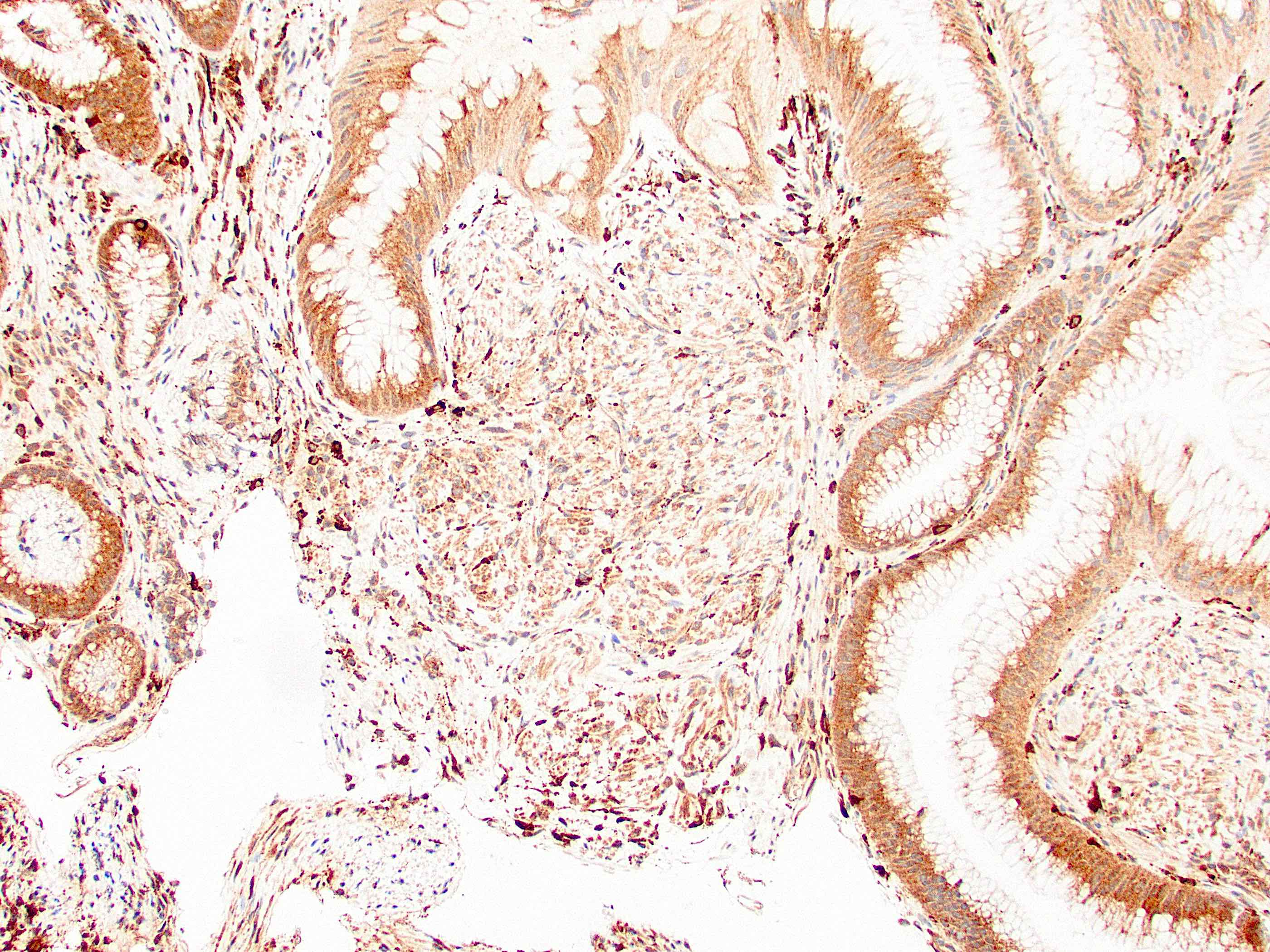
Positive stains
Negative stains
- CK20, CDX2, usually p16 (Arch Pathol Lab Med 2007;131:1304)
Molecular / cytogenetics description
- KRAS mutations in 47%, NRAS mutations in 6% (Br J Cancer 2018;118:1302)
Sample pathology report
- Anus, resection:
- Anal adenocarcinoma, moderately differentiated (see synoptic report)
Differential diagnosis
- Anal mucoepidermoid carcinoma:
- Rare (J Gastroenterol 2001;36:508)
- Secondary involvement by rectal adenocarcinoma:
- More common than primary anal adenocarcinoma
- Usually CK20+
- May require clinical correlation
- Squamous cell carcinoma:
- Far more common at this location
- Distinguishable microscopically
- Secondary involvement by gynecologic tract adenocarcinoma:
Additional references
Board review style question #1
Board review style answer #1
B. It can arise in a fistula tract. This may occur in patients with Crohn's disease. Overall, anal adenocarcinoma is more common in men and advanced stage indicates poor prognosis. It is rare and secondary anal involvement by rectal carcinoma is more common.
Comment Here
Reference: Anal adenocarcinoma
Comment Here
Reference: Anal adenocarcinoma
Board review style question #2
Primary anal adenocarcinoma is usually positive for which of the following immunohistochemical stains?
- CDX2
- CK7
- CK20
- p16
Board review style answer #2
B. CK7. Only rare cases of anal adenocarcinoma are positive for p16. Rectal carcinoma involving the anus is positive for CDX2 and CK20.
Comment Here
Reference: Anal adenocarcinoma
Comment Here
Reference: Anal adenocarcinoma
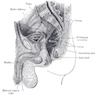
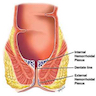
Anatomy & histology
Anatomy
Anal canal:
Classic anatomic definition of anal canal:
Clinical AJCC definition of anal canal:
Histologic definition of anal canal:
Anal valves:
Anal cushions:
Anal verge:
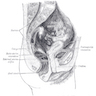
Musculature of anal canal
Internal anal sphincter:
Intersphincteric longitudinal muscle:
External anal sphincter:
Regional lymph node drainage:
- Tubular structure 3 - 4 cm long
- Derived from cloaca (distal hindgut) and arises at level of prostatic apex, is directed downward and backward and ends at anus
- Boundaries are proximal and distal margins of internal sphincter muscle and includes part of rectum
- Embryologically divided by urogenital septum (cloacal membrane) into anterior GU and posterior GI compartments and separated from perianal ectoderm by anal membrane which ruptures at week 7 of gestation
Classic anatomic definition of anal canal:
- Between proximal and distal margins of internal sphincter muscle which includes part of rectum
Clinical AJCC definition of anal canal:
- Begins at puborectalis sling at apex of anal sphincter complex (palpable as anorectal ring but difficult for pathologists to identify)
- Ends at squamous mucocutaneous junction with perianal skin; includes 1 - 2 cm of rectal type glandular mucosa and possibly transitional mucosa at dentate line
Histologic definition of anal canal:
- Anal transitional zone and squamous epithelium down to the perianal skin; cannot be identified by clinicians
- Note: columns, valves and sinuses below are macroscopic landmarks which may not correspond precisely to microscopic structures
- Anal columns of Morgagni: longitudinal folds just distal to dentate line, analogous to lower rectums rectal columns of Morgagni; less pronounced in adults
- Anal papillae: raised toothlike projections on anal columns; extend proximally into rectum
- Anal sinuses of Morgagni: depressions between anal columns
- Anal crypts of Morgagni: minute pockets with anal valves as boundary; site of discharge of anal glands
Anal valves:
- Also called semilunar valves or transverse plicae
- Connect distal ends of anal columns
- Identifiable in children, often obscured in adults
Anal cushions:
- Normal structures of anal canal that contribute to anal closure by close apposition to each other
- Contain blood vessels, connective tissue, smooth muscle; vessels contain abundant smooth muscle
- Resemble erectile tissue due to numerous arteriovenous communications
Anal verge:
- Also called Hiltons line or anal margin
- Junction between anal canal and anal skin
- Mucosa contains cutaneous adnexae
- Corpus cavernosum recti: network formed by peculiar vessels with a complex convoluted appearance
- Dentate (pectinate) line: midpoint of anal canal, formed by anal valves; circumferential musculature of canal
Musculature of anal canal
- Muscularis mucosa: continues from rectum through upper anal transitional zone
- Presence of muscle fibers in lamina propria indicates mucosal prolapse syndrome
- Musculus submucosae ani: fibers from intersphincteric longitudinal muscle which pass through internal sphincter and from the internal sphincter itself form a network around the vascular plexus
Internal anal sphincter:
- Continuation of circular muscle of rectum, but thicker (5-8 mm); ends 5 - 19 mm below dentate line
Intersphincteric longitudinal muscle:
- Between internal and external sphincters
- Contains fibers from longitudinal muscle layer of rectum and levator ani muscles
- Distally breaks up into septa that diverge fan wise through subcutaneous layer of external sphincter and ends in corium which forms characteristic corrugation of perianal skin
External anal sphincter:
- Consists of superficial, subcutaneous and deep parts; provides voluntary control of defecation
Regional lymph node drainage:
- Above dentate line - anorectal, perirectal, paravertebral nodes
- Below dentate line - superficial inguinal nodes
- Arterial supply: superior, middle and inferior rectal arteries
- Venous supply: superior rectal vein
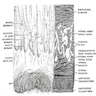
Histology
- Lacks a peritoneal covering
- Three histologic types: glandular (proximal), transitional (also called intermediate, cloacogenic) and keratinized or nonkeratinized squamous (distal)
- Anal glands and transitional zone epithelium are CK7+ / CK20-, different from colorectal carcinoma (CK7- / CK20+, Arch Pathol Lab Med 2001;125:1074)
- Notes: ganglion cells are normally absent 1 - 2 cm above dentate line (important for Hirschsprung's disease biopsies); multinucleated stromal cells are common (may be fibroblasts)
Proximal colorectal zone:
- Top of puborectalis to dentate line
- Glandular and transitional mucosa
- 1 - 2 cm long
- Similar to rectal mucosa but with shorter more irregular crypts, more smooth muscle fibers in lamina propria
Anal transitional zone (ATZ):
- 0.3 cm to 1.1 cm
- Zone between uninterrupted columnar mucosa above and uninterrupted squamous epithelium below
- Wrinkled, glistening appearance
- Transitional epithelium resembles urothelium (small basal cells with nuclei perpendicular to basement membrane, columnar, cuboidal, polygonal or flat) with 4 - 9 cell layers, minimal mucin production
- Not highly specialized and may incorporate features of both urothelium and squamous epithelium (Hum Pathol 1978;9:579)
- Contains anal glands in submucosa, also endocrine cells, rare melanocytes
- Expresses CK7 and CK19 but not CK20
Lower distal zone:
- Dentate line to squamous mucocutaneous junction: nonkeratinizing squamous epithelium without skin appendages, without glands
- Contains melanocytes
- Anal papillae contain squamous mucosa that joins rectal mucosa
- Squamous mucosa merges with perianal skin (with keratin, hair follicles and apocrine glands) at anal verge / anal margin
Diagrams / tables
Apocrine carcinoma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Immunofluorescence description | Immunofluorescence images | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Molecular / cytogenetics description | Molecular / cytogenetics images | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Apocrine adenocarcinoma of the anus is a rare cutaneous adnexal neoplasms with apocrine differentiation
- It originates from anogenital mammary-like glands, which are analogous to apocrine glands found in other areas of the skin
Essential features
- Apocrine adenocarcinoma is a rare cutaneous adnexal neoplasm with apocrine differentiation occurring in the anogenital area
- It may arise de novo or develop from a pre-existing apocrine adenoma
- Histologically, it manifests with unequivocal signs of apocrine secretion, zonal necrosis, cells with pleomorphism and hyperchromatism and increased mitotic activity, including atypical figures
Terminology
- Adnexal carcinoma of skin; apocrine carcinoma; apocrine adenocarcinoma; apocrine gland carcinoma; mammary type tubulocarcinoma of the anogenital area
ICD coding
- ICD-O
- ICD-11: 2C33 & XH9L77 - adnexal carcinoma of skin & apocrine adenocarcinoma
Epidemiology
- Extremely rare, with an incidence estimated to be Eur J Cancer Prev 2024;33:77)
- Predominantly affects adults, with slight female predominance
- Often diagnosed in the fifth to seventh decades of life, with a median age of 67 years
Sites
- Axilla and anogenital region are the most common sites
Pathophysiology
- Originates from anogenital apocrine sweat glands or glands with apocrine differentiation
- Associated with genetic alterations, although molecular mechanisms are still being studied
Etiology
- Exact cause of apocrine carcinoma of the skin is unknown
- In breast, some theories suggest that it may arise from apocrine metaplastic cells, which are often found in breast fibrocystic changes
Diagrams / tables
None
Clinical features
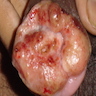
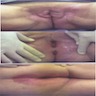
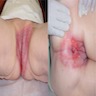
- Tumor may present as a solitary nodule or multinodular mass (J Surg Case Rep 2020 Nov;2020:rjaa463)
- Ulceration and hemorrhage can happen
- Tumor may also present as a perianal mass for several years
Diagnosis
- Diagnosis is based on location and histopathologic findings
Laboratory
None
Radiology description
None
Radiology images
None
Prognostic factors
- Tumor size, depth of invasion and lymph node involvement are key prognostic indicators
- Higher stage at diagnosis is associated with poorer prognosis
- Metastasis can occur in regional lymph nodes in about 40% of cases and less commonly to visceral organs
- In one study including apocrine carcinomas from all sites, 24% of the patients died of metastatic tumor, however, this study included apocrine carcinomas from all sites (Am J Surg Pathol 2008;32:682)
- Small, well circumscribed tumors that are easily excised have a good prognosis (Int J Colorectal Dis 2008;23:121, Am J Surg Pathol 2006;30):1193)
- Analogous tumors in the vulva have been reported to respond to tamoxifen therapy (Future Oncol 2012;8:1199)
- Invasive apocrine carcinomas may be classified according to the modified Bloom-Richardson grading system for breast carcinomas (also called the Nottingham system) (Am J Surg Pathol 2008;32:682)
- This grades tumors based on three criteria
- Mitotic count (number of mitoses/mm2)
- Degree of pleomorphism (mild, moderate or severe)
- Tubule formation
- A score of 1, 2 or 3 is assigned for each criterion and the 3 scores are combined to give an overall tumor grade of 1, 2 or 3
- Statistically significant differences in survival have been found with grade 3 versus grade 1 - 2 tumors
- This grades tumors based on three criteria
Case reports
- 45 year old woman presented with an invasive apocrine adenocarcinoma arising in a benign adenoma in the perianal region (J Clin Pathol 2005;58:217)
- 72 year old woman presented for evaluation of a perianal lesion with persistent drainage that she had noticed for over a year (J Surg Case Rep 2020 Nov;2020:rjaa463)
- 71 year old man presented to clinic with soreness in the anal region for 6 weeks (Am J Dermatopathol 2012;34:438)
Treatment
- Wide local excision
- Chemotherapy or radiotherapy may or may not be needed
Clinical images
None
Gross description
None
Gross images
None
Frozen section description
None
Frozen section images
None
Microscopic (histologic) description
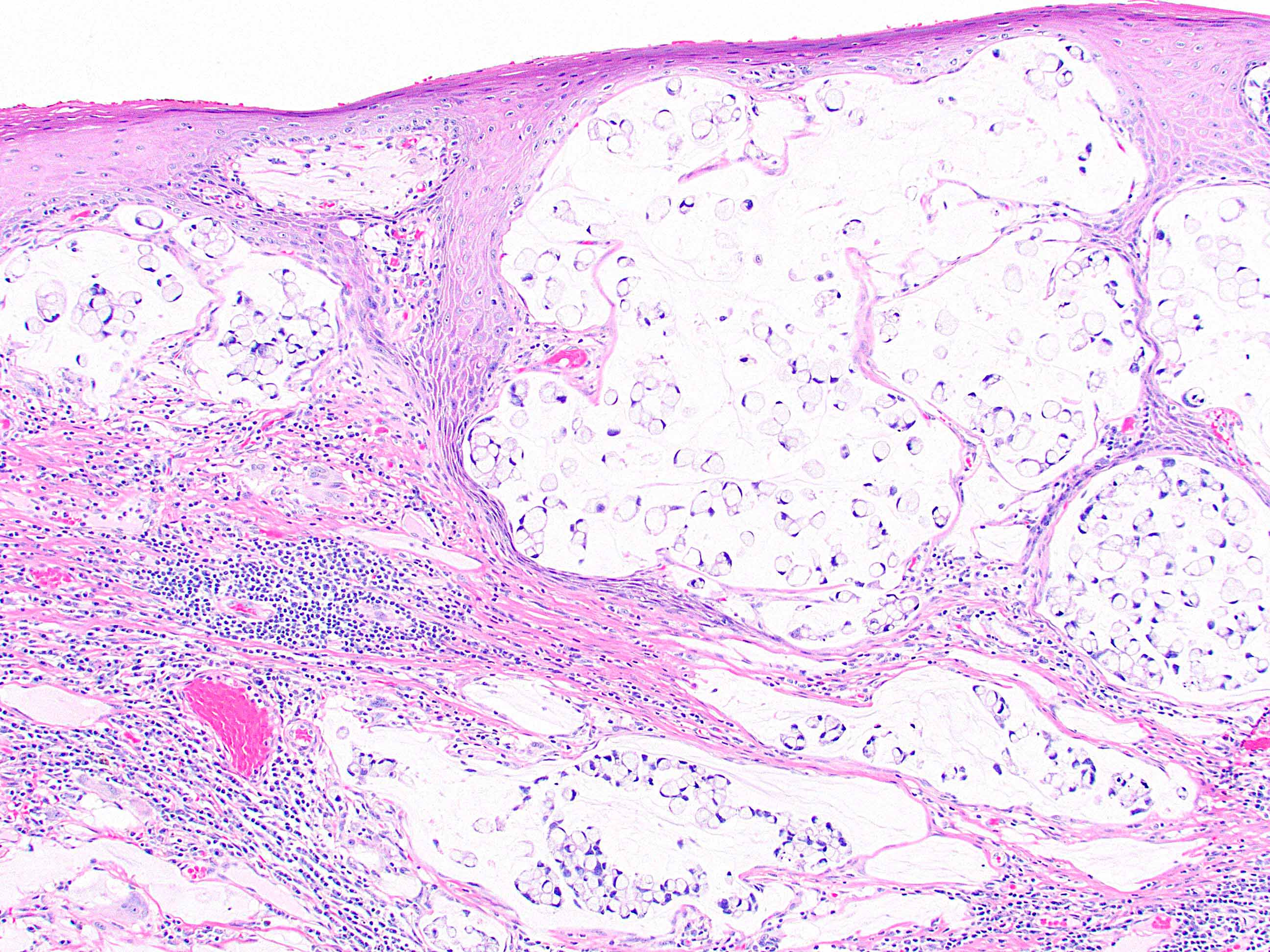
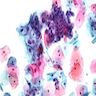
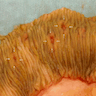
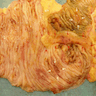
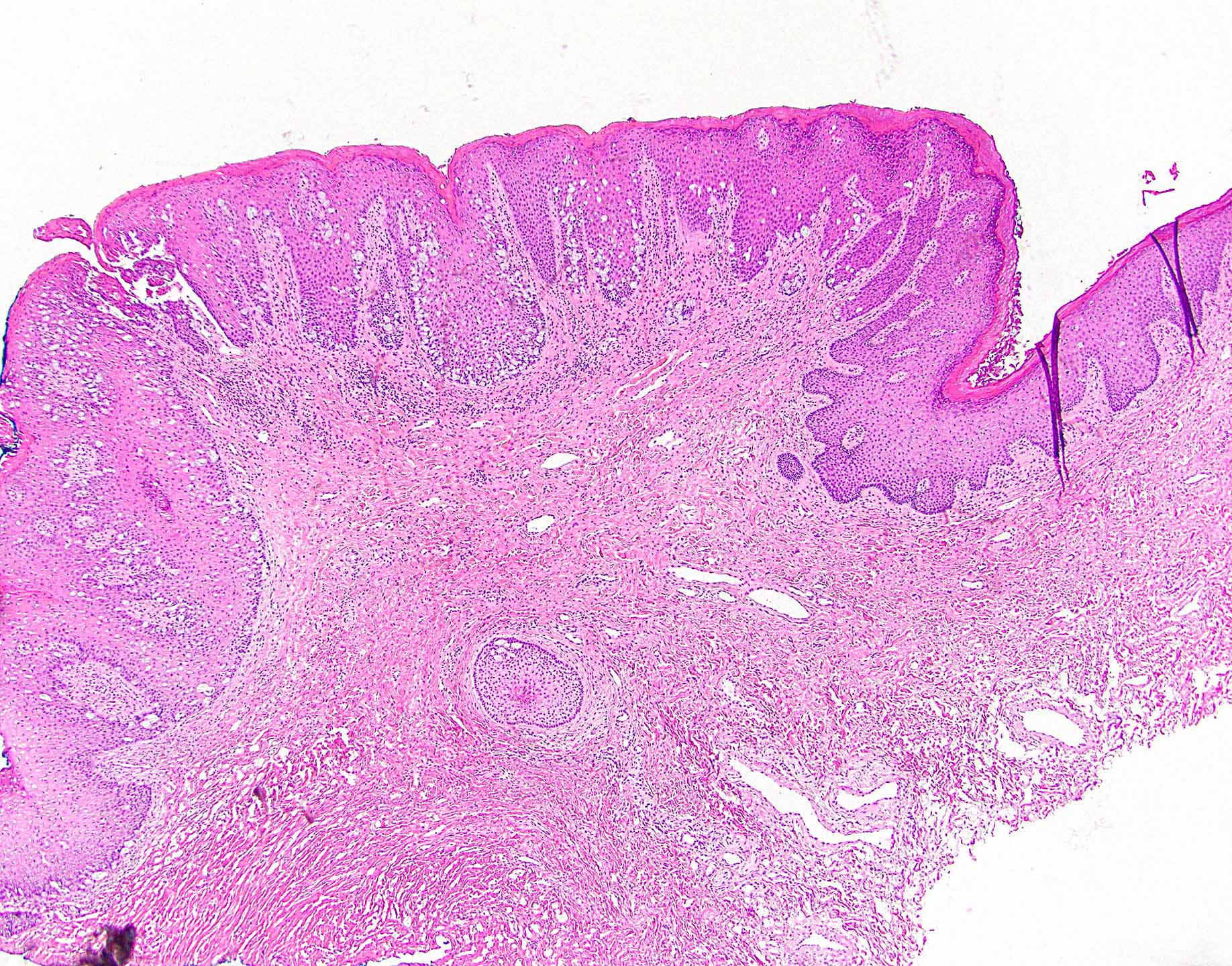
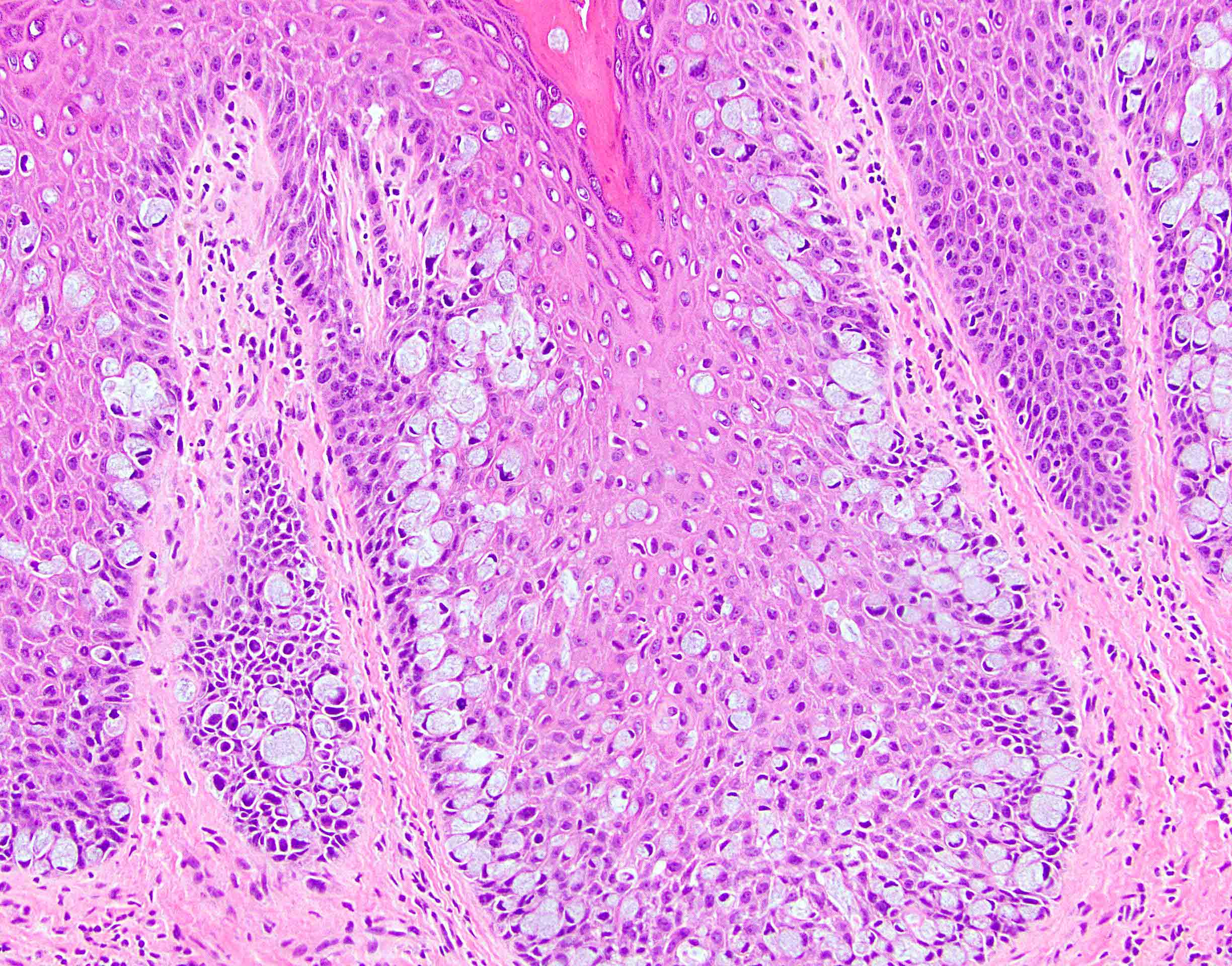
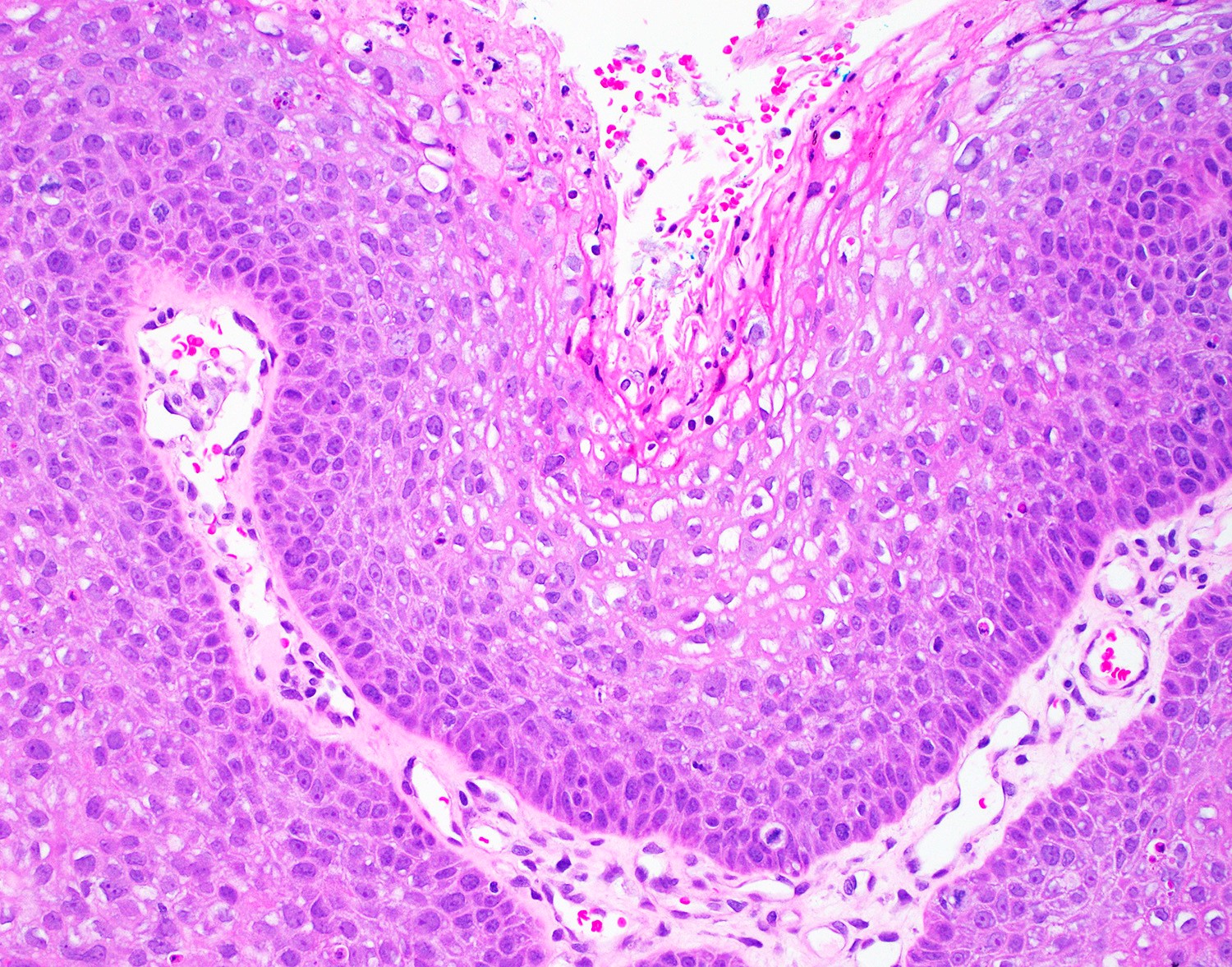
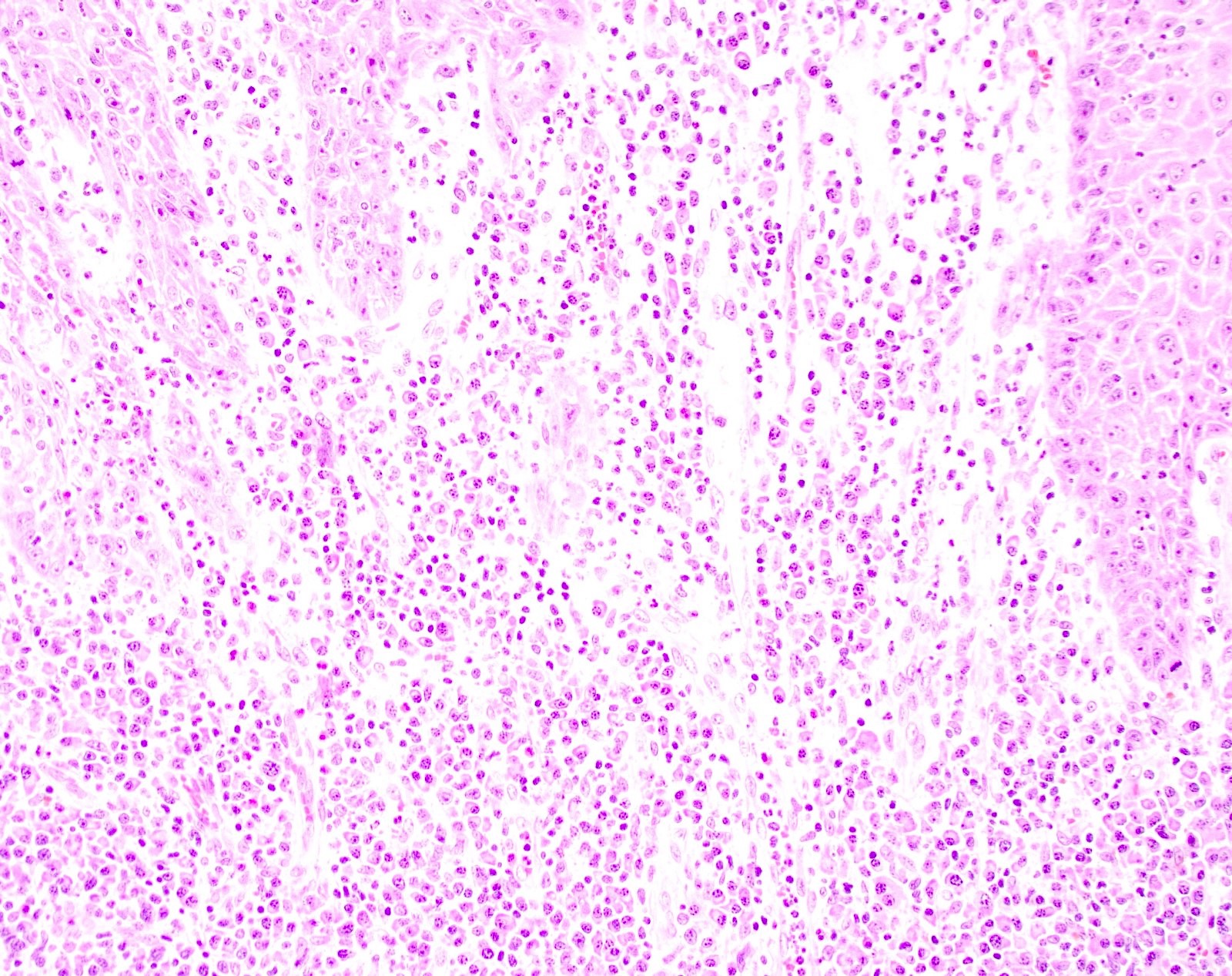
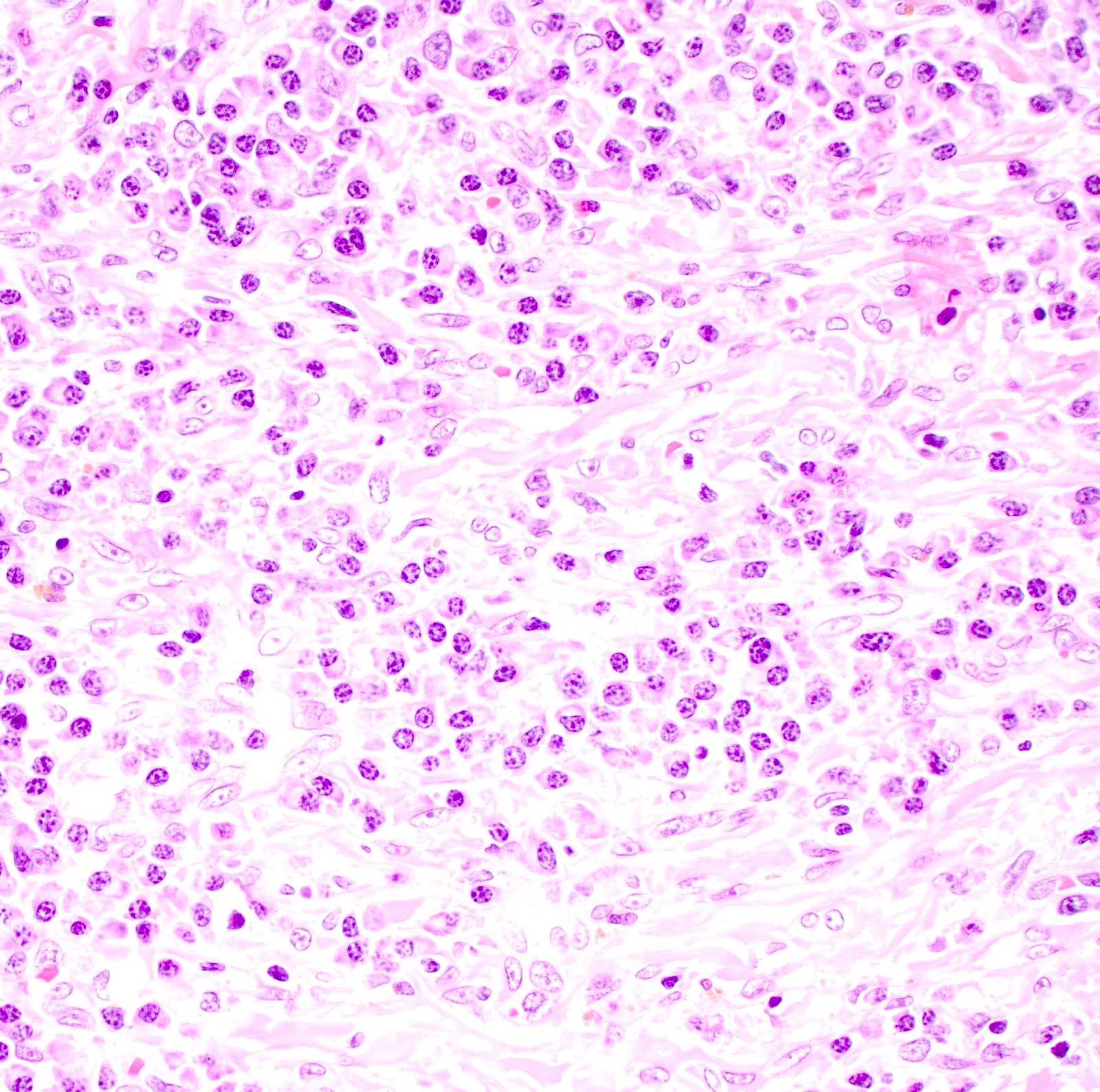
- Tumor is characterized by tubular, ductular and papillary growth patterns, with more solid areas demonstrating significant atypia
- A peripheral myoepithelial cell layer may or may not be present
- Tumor cells demonstrate features of apocrine differentiation with abundant eosinophilic cytoplasm which may appear either granular or have some vacuolization
- Lumina may be present
- Decapitation secretion is variably present, less likely to be seen in poorly differentiated areas of the tumor
- Cytology features variable nuclear pleomorphism and mitotic activity; perineural invasion and intravascular involvement can be seen
- In rare cases of entirely invasive carcinoma, an in situ lesion may have been overgrown by the invasive tumor mass or that the neoplasm developed de novo
- Signet ring and histiocytoid variants have been reported, similar to those reported in periorbital neoplasms
- In situ spread of these tumors into the overlying epithelial structures may lead to extramammary Paget disease in the anogenital region (Am J Surg Pathol 2017;41:1053)
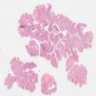
Microscopic (histologic) images
Virtual slides
None
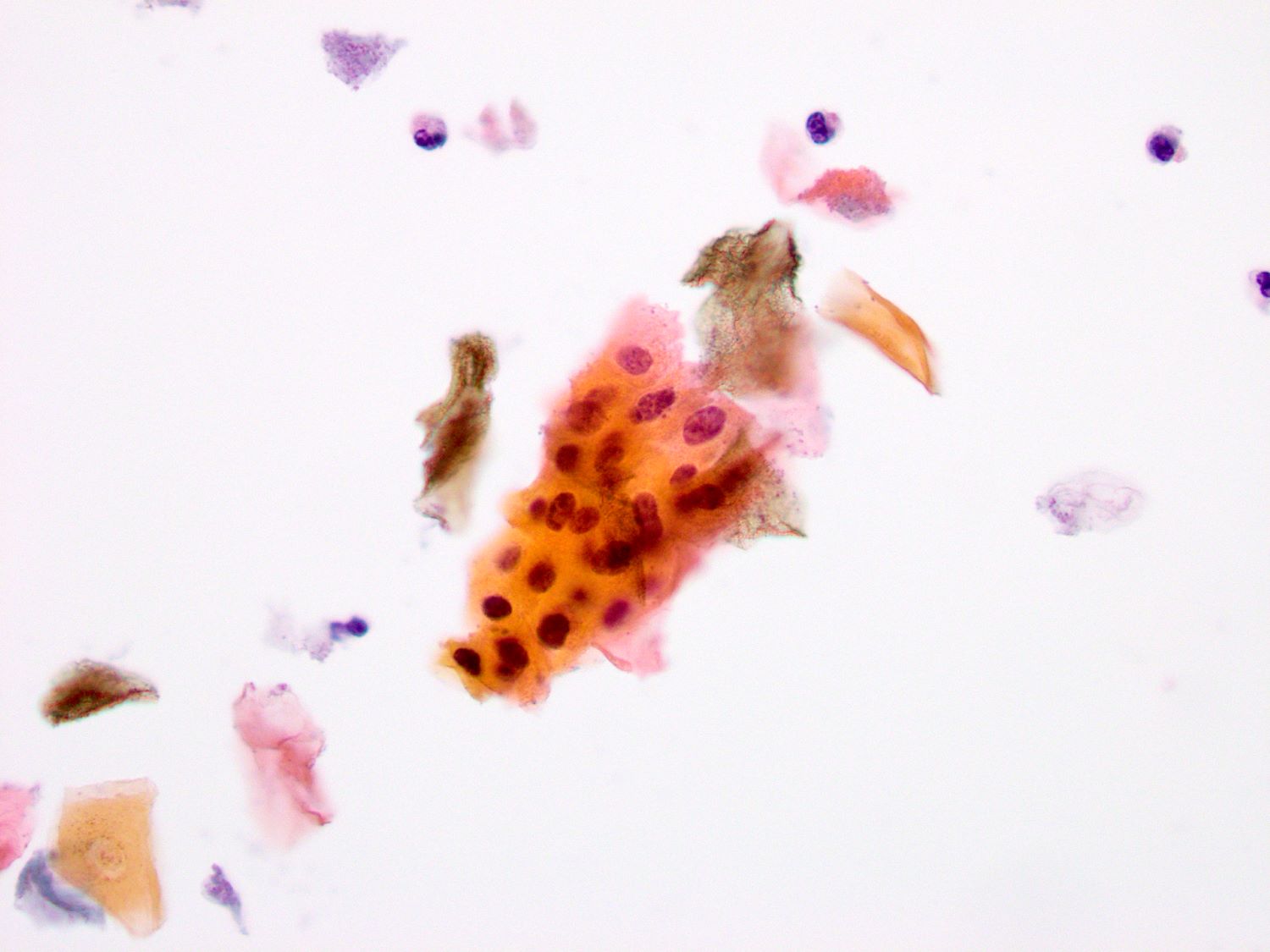
Cytology description
None
Cytology images
None
Immunofluorescence description
None
Immunofluorescence images
None
Positive stains
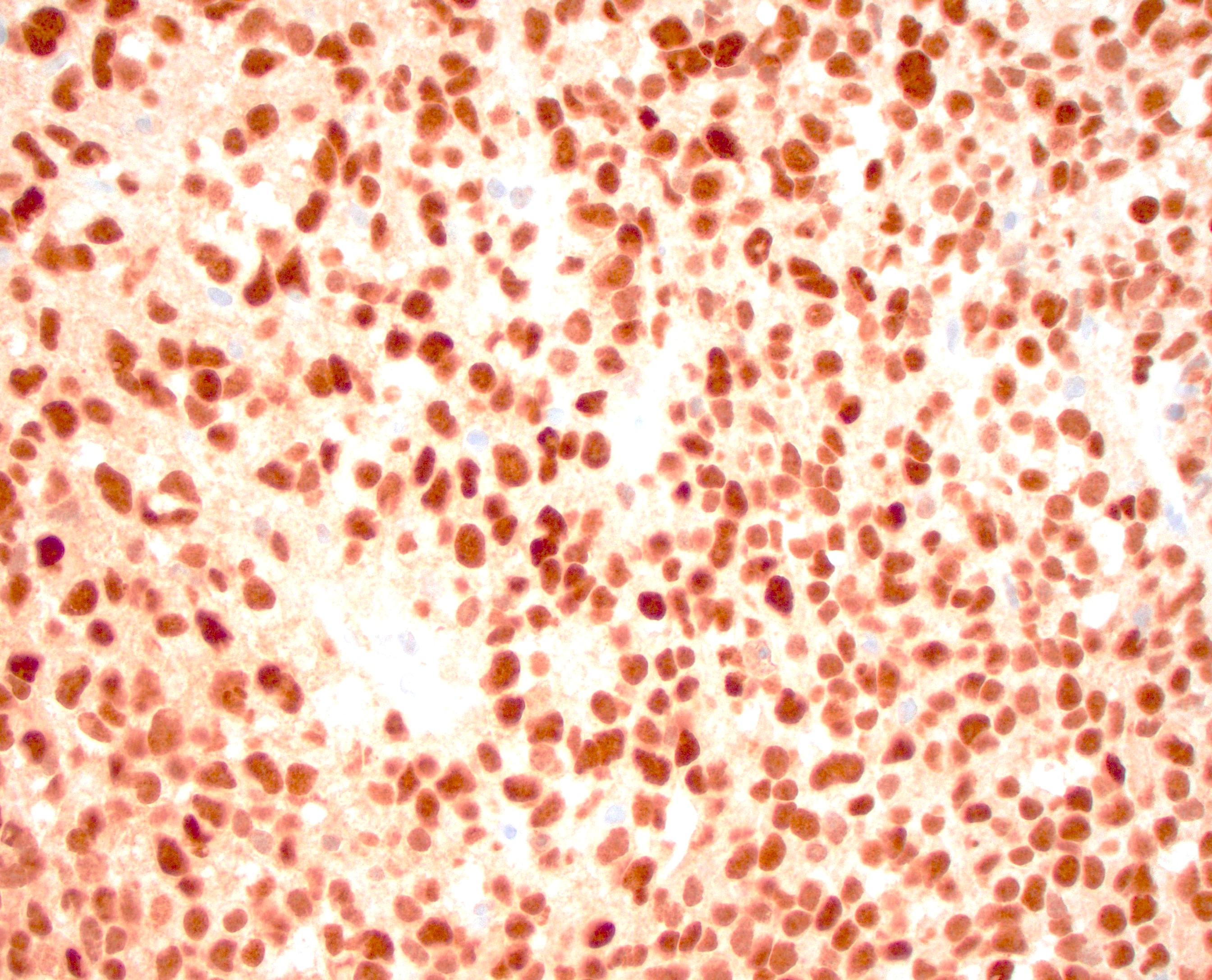
- Gross cystic disease fluid protein-15 (GCDFP-15), GATA3, epithelial membrane antigen (EMA) and CK7 are often positive (J Clin Pathol 2005;58:217, Am J Dermatopathol 2012;34:438)
- Androgen receptor (AR) is positive in most cases (Clin Case Rep 2020;8:3472)
- Myoepithelial markers, including p63, smooth muscle actin (SMA) and calponin may also be positive in the myoepithelial cells (Am J Surg Pathol 2017;41:1053)
Negative stains
- Estrogen receptor (ER) and progesterone receptor (PR) are negative, which helps distinguish apocrine adenocarcinoma from other breast cancers
- CK20 and CDX2 are typically negative, which helps exclude a gastrointestinal origin
Electron microscopy description
None
Electron microscopy images
None
Molecular / cytogenetics description
- PTEN and PIK3CA mutations have been identified in some cases, although specific alterations are variable (Breast Cancer Res 2010;12:R63)
- Androgen receptor gene amplification may play a role in tumorigenesis
Molecular / cytogenetics images
None
Videos
None
Sample pathology report
- Perianal skin, mass, excision:
- Apocrine carcinoma, arising in an apocrine adenoma (see comment)
- Comment: The patient’s history of a perianal skin mass, which has been present for many years with recent increase in size, has been noted. The histology shows co-existence of both high grade and low grade tumor components, suggesting malignant transformation from a pre-existing adenoma.
Differential diagnosis
- Tubular apocrine adenoma:
- Benign premalignant lesion arising from apocrine sweat glands (Am J Surg Pathol 2017;41:1053)
- Tubular lining cells show better decapitation secretion than malignant apocrine carcinoma
- Peripheral myoepithelial cell layer is almost always present
- Simple glands and tubules without crowding, minimal cytologic atypia and mitoses
- Anal gland adenocarcinoma:
- Rare malignant tumor originating from the anal glands (Hum Pathol 2012;43:216)
- Typically arises in the perianal or anorectal region, often infiltrating local tissues
- Composed of irregular glandular structures with pleomorphic cells, prominent nucleoli and frequent mitotic figures; can show mucin production
- Commonly presents with anal pain, bleeding or a palpable mass; symptoms may resemble benign conditions, leading to delayed diagnosis
- Tends to be aggressive with potential for local invasion and metastasis, especially to lymph nodes
- No expression of myoepithelial markers
- Metastatic adenocarcinoma:
- Clinical history of malignant neoplasm is important for diagnosis
- Based upon history, cytomorphologic characteristics, site specific lineage markers, such as prostate specific antigen (PSA), GATA3, thyroid transcription factor 1 (TTF1), should be performed to rule out metastasis of other primary site
- Direct extension or metastatic colorectal adenocarcinoma are usually CK7- and CDX2+
- Metastatic carcinoma of breast also express GCDFP-15 and GATA3 but are usually positive for estrogen receptor (ER) and progesterone receptor (PR)
Additional references
Board review style question #1
Which of the following immunohistochemical markers are commonly positive in apocrine adenocarcinoma?
- CDX2, CK20, EMA
- CK7, CK19, CDX2
- ER, PR, GCDFP-15
- GCDFP-15, androgen receptor, CK7
Board review style answer #1
D. GCDFP-15, androgen receptor, CK7. Answer C is incorrect as apocrine carcinoma is negative for ER and PR. Answer B is incorrect since it refers to gastrointestinal adenocarcinomas. Answer A is incorrect as apocrine carcinoma is negative for CDX2.
Comment Here
Reference: Apocrine carcinoma
Comment Here
Reference: Apocrine carcinoma
Board review style question #2
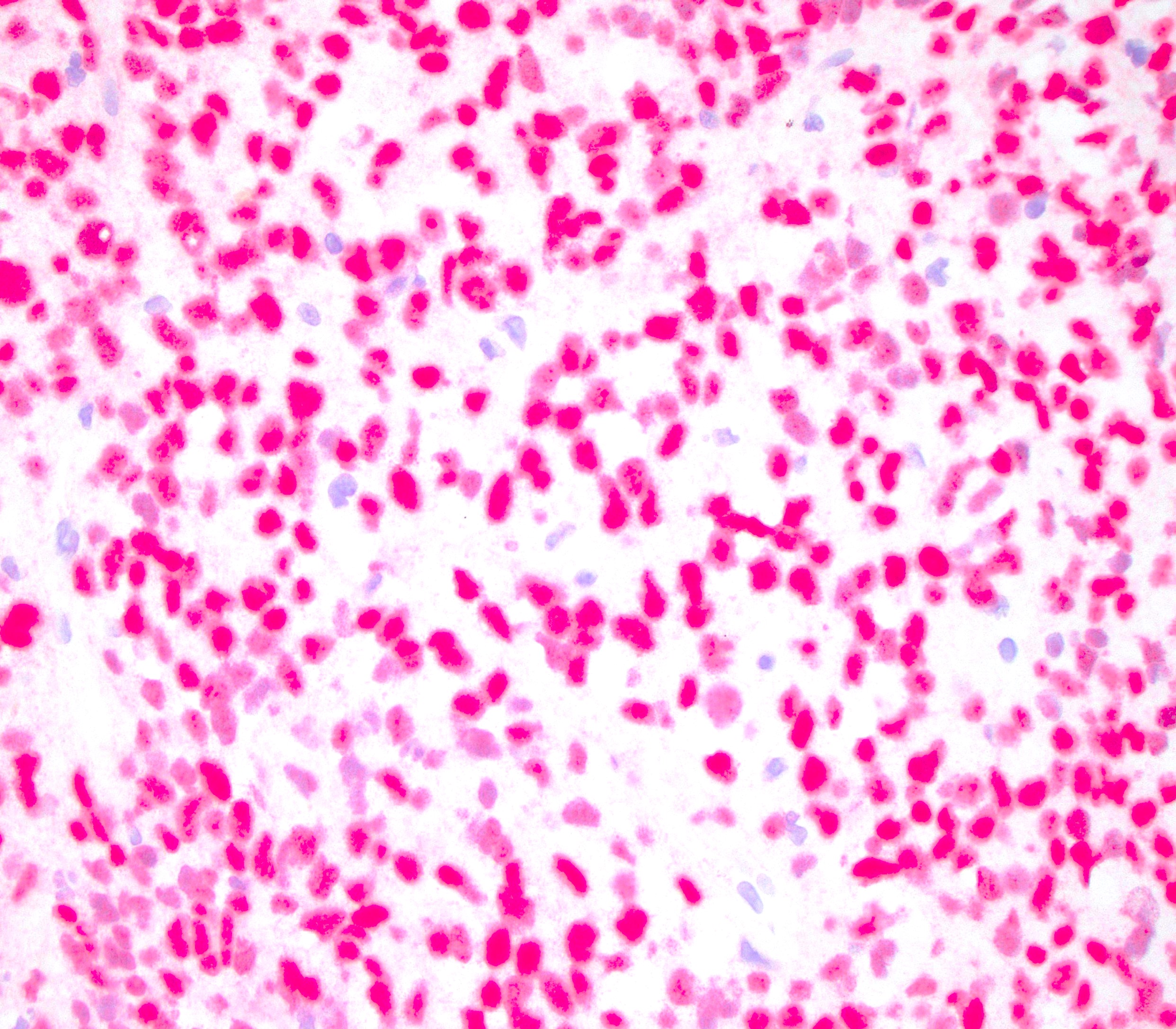
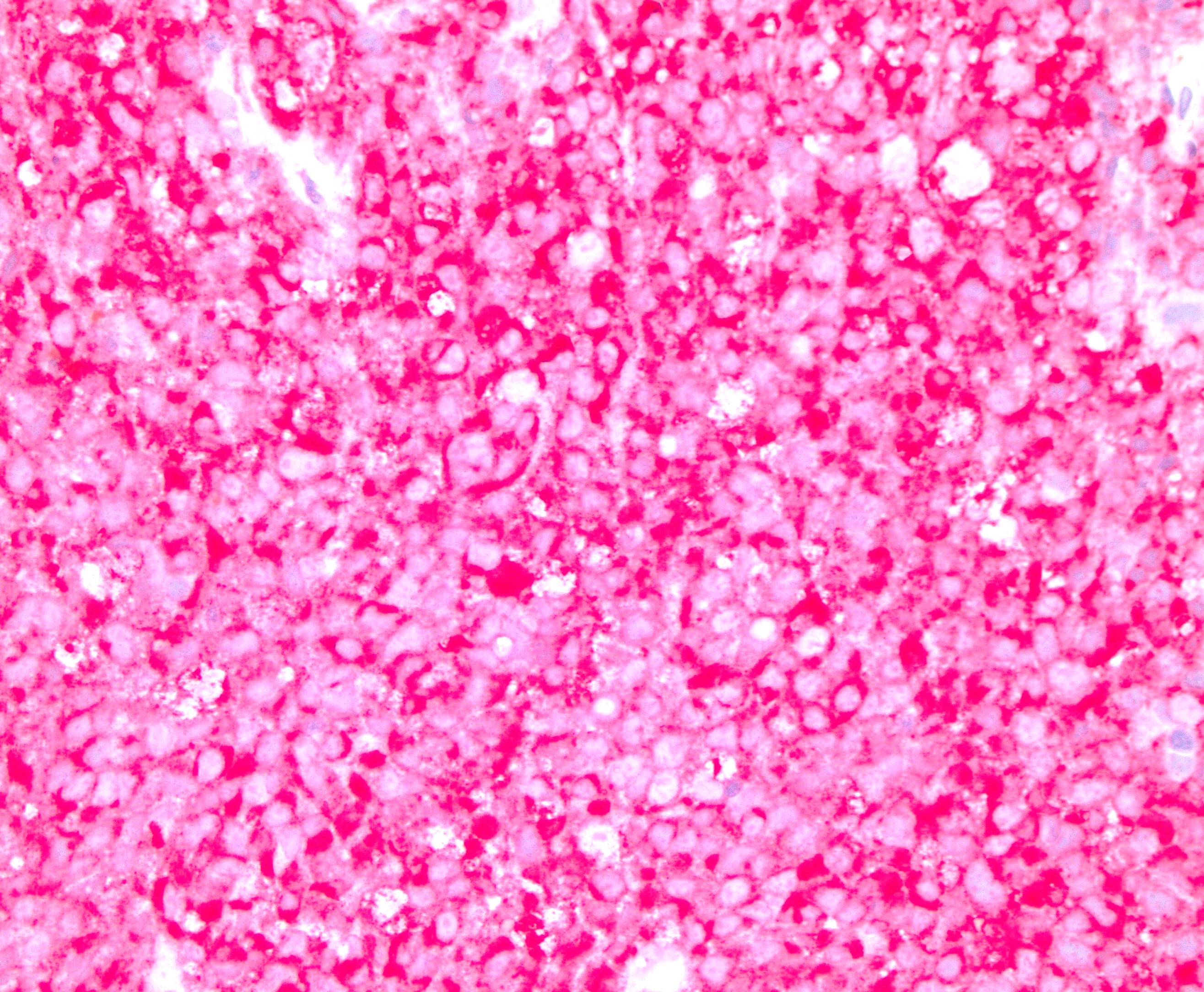
What is a distinguishing histologic feature of apocrine adenocarcinoma (shown above)?
- Discohesive large cells with prominent nucleoli, brown pigments and necrosis
- Large pleomorphic cells with eosinophilic granular cytoplasm and prominent nucleoli
- Signet ring cells with mucin production
- Small blue cells with hyperchromatic nuclei
Board review style answer #2
B. Large pleomorphic cells with eosinophilic granular cytoplasm and prominent nucleoli. Answer C is incorrect because it describes signet ring cell carcinoma of colorectal origin. Answer D is incorrect because it describes basal cell carcinoma or small cell neuroendocrine carcinoma. Answer A is incorrect because it likely represents melanoma.
Comment Here
Reference: Apocrine carcinoma
Comment Here
Reference: Apocrine carcinoma
Apocrine carcinoma (pending)
[Pending]
Basal cell carcinoma
Table of Contents
Definition / general | Essential features | Sites | Pathophysiology | Clinical features | Diagnosis | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Flow cytometry description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1Definition / general
- Very rare tumor of perianal skin, representing 0.2% of anorectal tumors and less than 0.5% of all basal cell carcinomas at all sites (Am J Dermatopathol 1996;18:371, Rev Gastroenterol Mex 2013;78:52)
- Nodular subtype most common in this location
Essential features
- Rare perianal malignancy identical to basal cell carcinoma of skin
Sites
- Perianal skin (not anus proper)
Pathophysiology
- Appears to behave similarly to basal cell carcinoma at other locations
Clinical features
- Usually 1 - 2 cm but can be 10 cm or larger; recurrence uncommon (Br J Surg 1981;68:856)
- Patients may also have basal cell carcinomas elsewhere (Dis Colon Rectum 1999;42:1200)
Diagnosis
- Biopsy
Case reports
- 69 year old man with perianal basal cell carcinoma (Indian J Dermatol 2010;55:178)
- 88 year old woman with polypoid basal cell carcinoma on the perianal region (J Dermatol 2004;31:51)
Treatment
- Wide local excision with negative margins
Gross description
- Indurated, ulcerated lesion with irregular borders
Microscopic (histologic) description
- Resembles basal cell carcinoma at other sites (see Skin nonmelanocytic chapter)
- Retraction artifact helps distinguish from basaloid squamous cell carcinoma
Microscopic (histologic) images
Positive stains
Flow cytometry description
- Lower S phase fraction than anal basaloid squamous cell carcinomas (Am J Dermatopathol 1996;18:371)
Sample pathology report
- Skin, perianal, resection:
- Basal cell carcinoma (1.6 cm), completely excised
Differential diagnosis
- Squamous cell carcinoma of anal canal, basaloid type:
- Much more common
Board review style question #1
Board review style answer #1
C. It histologically resembles basal cell carcinoma at other sites
Comment Here
Reference: Basal cell carcinoma
Comment Here
Reference: Basal cell carcinoma
Buschke-Löwenstein tumor
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Negative stains | Videos | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
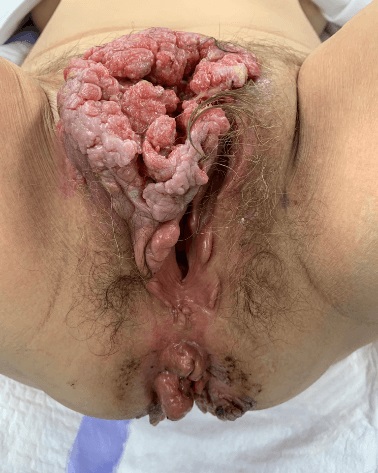
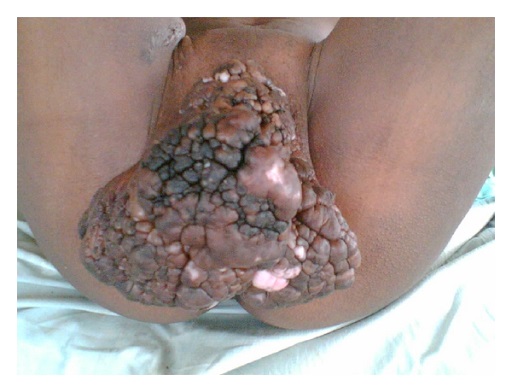
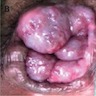
- Buschke-Löwenstein tumor (also known as giant condyloma acuminatum) was first described as a penile neoplasm by Buschke and Löwenstein in 1925
- Previously considered synonymous with verrucous carcinoma
- Recent studies and WHO 2019 have suggested that Buschke-Löwenstein tumors and verrucous carcinoma are 2 separate entities; while Buschke-Löwenstein tumors are associated with low risk HPV types (6,11), verrucous carcinomas usually have a non-HPV etiology (Mod Pathol 2012;25:1354, Histopathology 2017;70:938, WHO Classification of Tumours Editorial Board: Digestive System Tumours, 5th Edition, 2019)
Essential features
- Condylomatous squamous lesion without high grade dysplasia, with only low risk HPV types present, forming an exophytic mass, usually greater than 3 cm
Terminology
- Preferred term: Buschke-Löwenstein tumor (MeSH: Buschke-Lowenstein Tumor [Accessed 18 November 2020])
- Also called giant condyloma acuminatum, giant condyloma of Buschke and Löwenstein
ICD coding
- ICD-O: None
- ICD-11: 1A95.0 - Anal warts
Epidemiology
- Incidence rates: about 0.1% of the population; relatively few cases have been reported (GARD: Buschke-Lowenstein tumor [Accessed 18 November 2020])
- M > F (4.4:1)
- Risk factors:
- HPV 6, 11
- Immunodeficient state (HIV positive, immunosuppression)
- Anal receptive sex (homosexual men)
- Chronic irritation (perianal fistula, ulcerative colitis)
- Poor hygiene
- Cases in children raise the suspicion of sexual abuse, though this association is not well established (Am J Dis Child 1982;136:704, Arch Pediatr 2019;26:473)
Sites
- Anogenital region (involves both inside and outside of anal canal)
- Urinary bladder, genitalia, oropharynx
Pathophysiology
- Similar to the process in other skin and mucosal HPV induced tumors, viral DNA is incorporated into cellular DNA following exposure, redirecting cellular processes into dysregulated cellular proliferation (IARC Monogr Eval Carcinog Risks Hum 2007;90:1)
Etiology
- > 90% of cases associated with low risk HPV 6, 11
- A few cases reported with Netherton syndrome (Pediatr Dermatol 2017;34:e328, J Plast Reconstr Aesthet Surg 2011;64:1533)
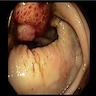
Clinical features
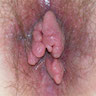
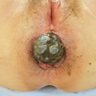
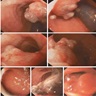
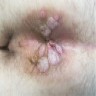
- Presenting symptoms: perianal exophytic and cauliflower-like mass with or without abscess, fistula, pain, pruritus, bleeding and ulceration
- Slow growing, local extension
- Usually no perineural invasion or nodal metastasis at initial diagnosis
- Lymphadenopathy, if present, is usually caused by secondary infection (Clin Colon Rectal Surg 2019;32:386)
- Recurrence rate: from 23.7% up to 66% (Virchows Arch 2020;476:543, Dis Colon Rectum 1994;37:950)
- High risk HPV infection (16, 18) correlated with invasive Buschke-Löwenstein tumors (Virchows Arch 2020;476:543)
- Squamous cell carcinoma transformation: 14 - 56%; more common in HIV positive patients (Virchows Arch 2020;476:543, Dis Colon Rectum 1994;37:950, AIDS Res Hum Retroviruses 2018;34:375)
Diagnosis
- Diagnosis is often based on clinical course, radiology and histopathology
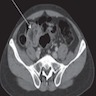
Radiology description
- Preoperative CT / MRI is often required to evaluate local extension
Radiology images
Prognostic factors
- Overall mortality: 10.5 - 20% (Virchows Arch 2020;476:543, Dis Colon Rectum 1994;37:950)
- Prognosis related to:
- Size of tumor
- Local recurrence
- Squamous cell carcinoma transformation
- Immunosuppression and secondary infection (Case Rep Infect Dis 2018;2018:7267213)
Case reports
- 3 year old boy with HIV infection presenting with multiple warts in the anogenital region (Urol Case Rep 2016;7:14)
- 17 year old pregnant girl with giant masses that covered the perineal and perianal region (Dermatol Ther 2019;32:e12972)
- 21 year old woman with severe perineal pain, dysuria and burning micturition (JAAD Case Rep 2018;4:692)
- 31 year old man with anal discomfort due to a protruding mass (J Med Case Rep 2015;9:9)
- 61 year old man with HIV infection presenting with worsening pain and swelling in the anorectal area (Case Rep Infect Dis 2018;2018:7267213)
Treatment
- Preferred initial therapy: complete excision with wide margins
- Other treatment strategies: podofilox cream, topical 5-fluorouracil, regional radiation, CO2 laser or a combined chemoradiation regimen (J Obstet Gynaecol 2020;40:582)
Clinical images
Gross description
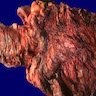
- Size: up to 13 cm (Histopathology 2017;70:938)
- Large, exophytic, cauliflower-like mass
- May show fistula, bleeding, ulceration, superficial infection
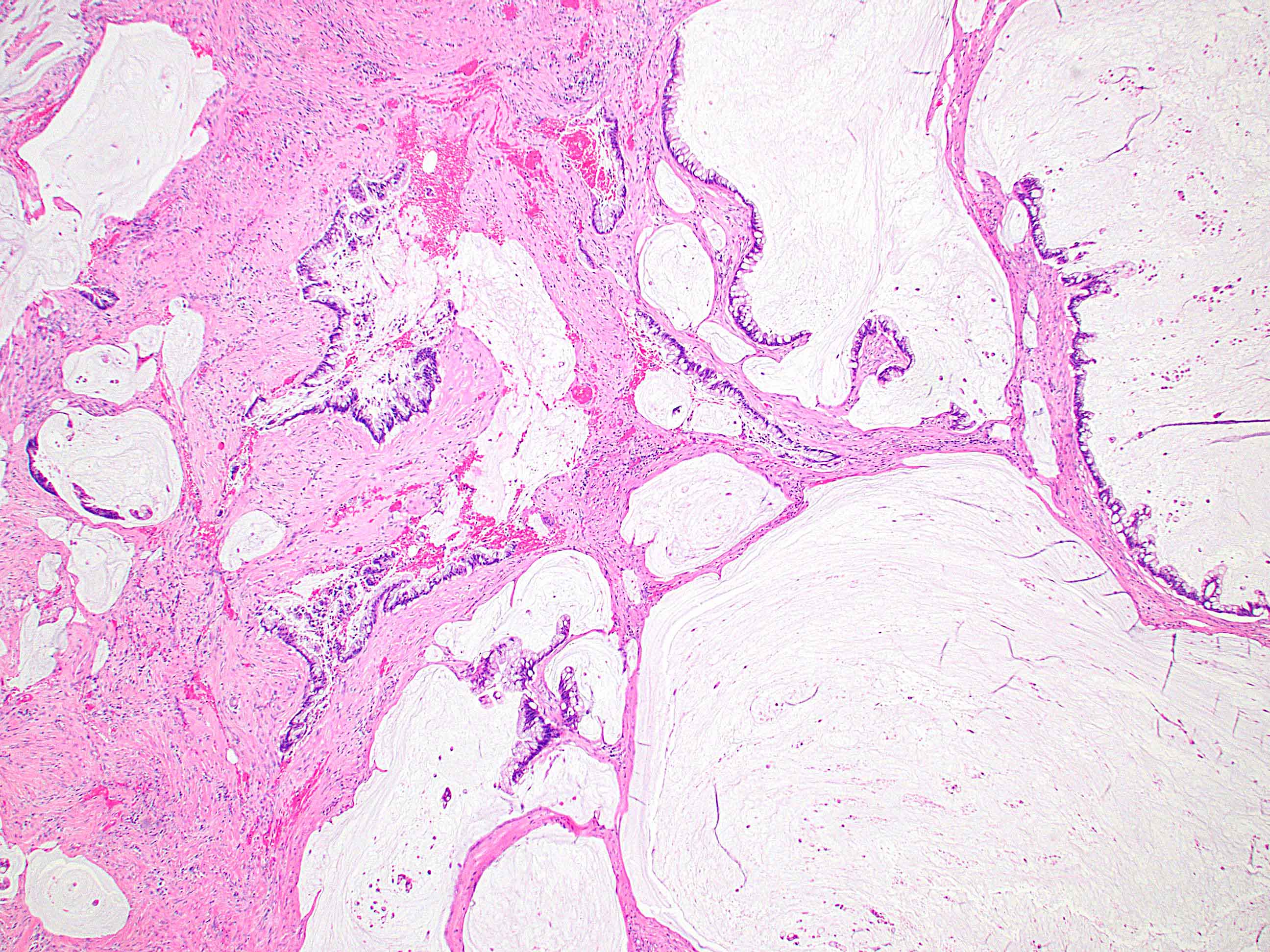
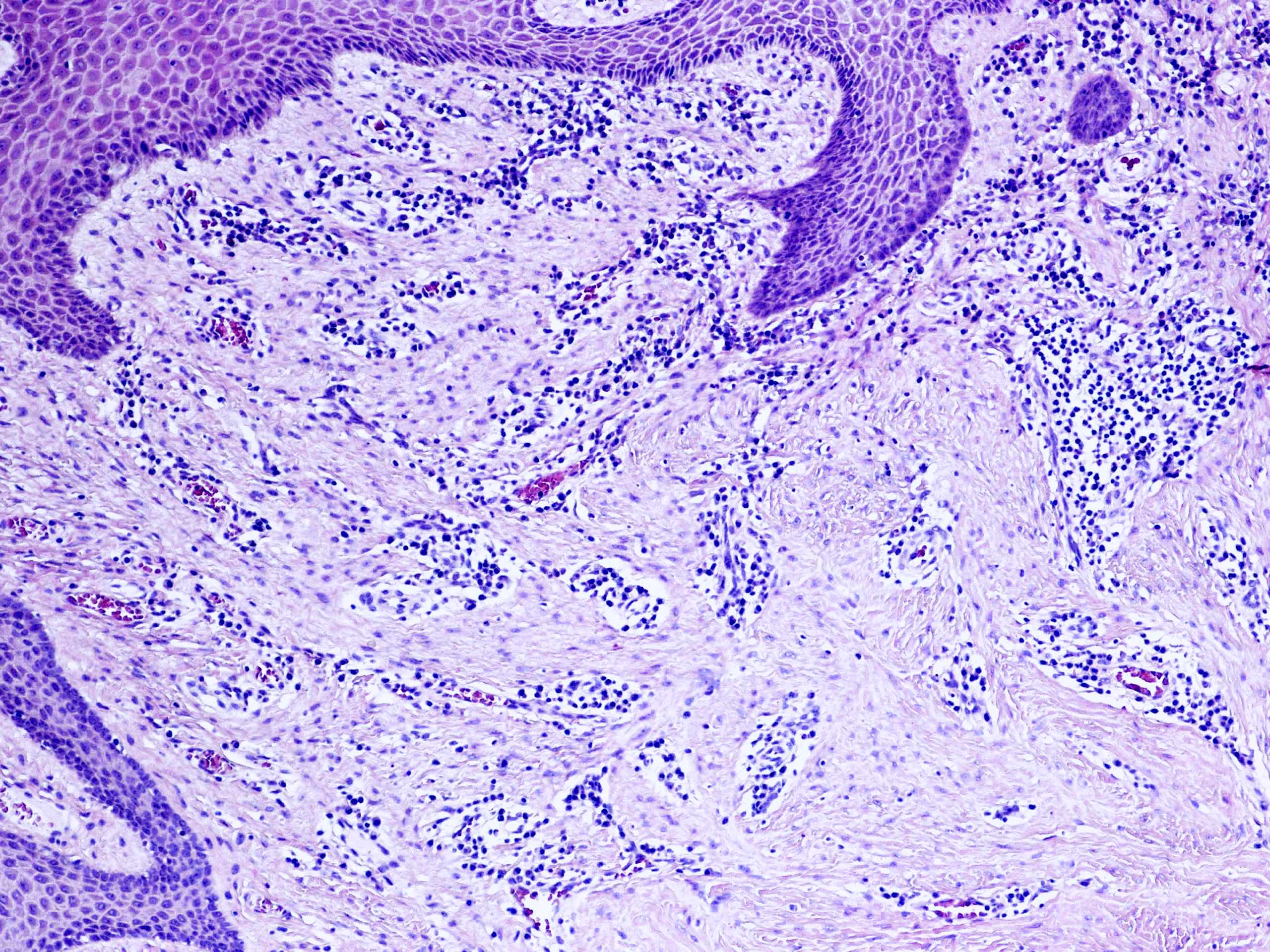
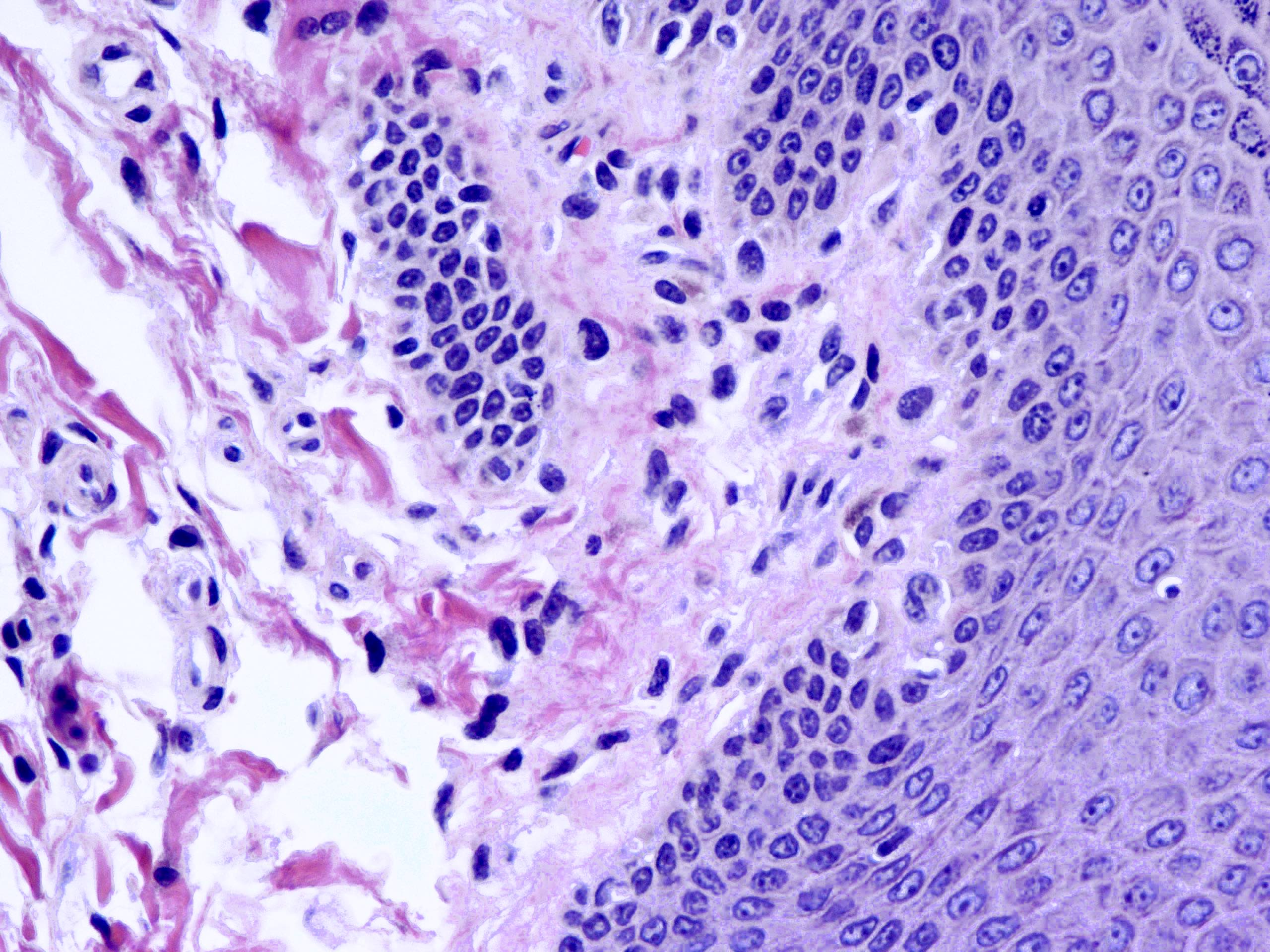
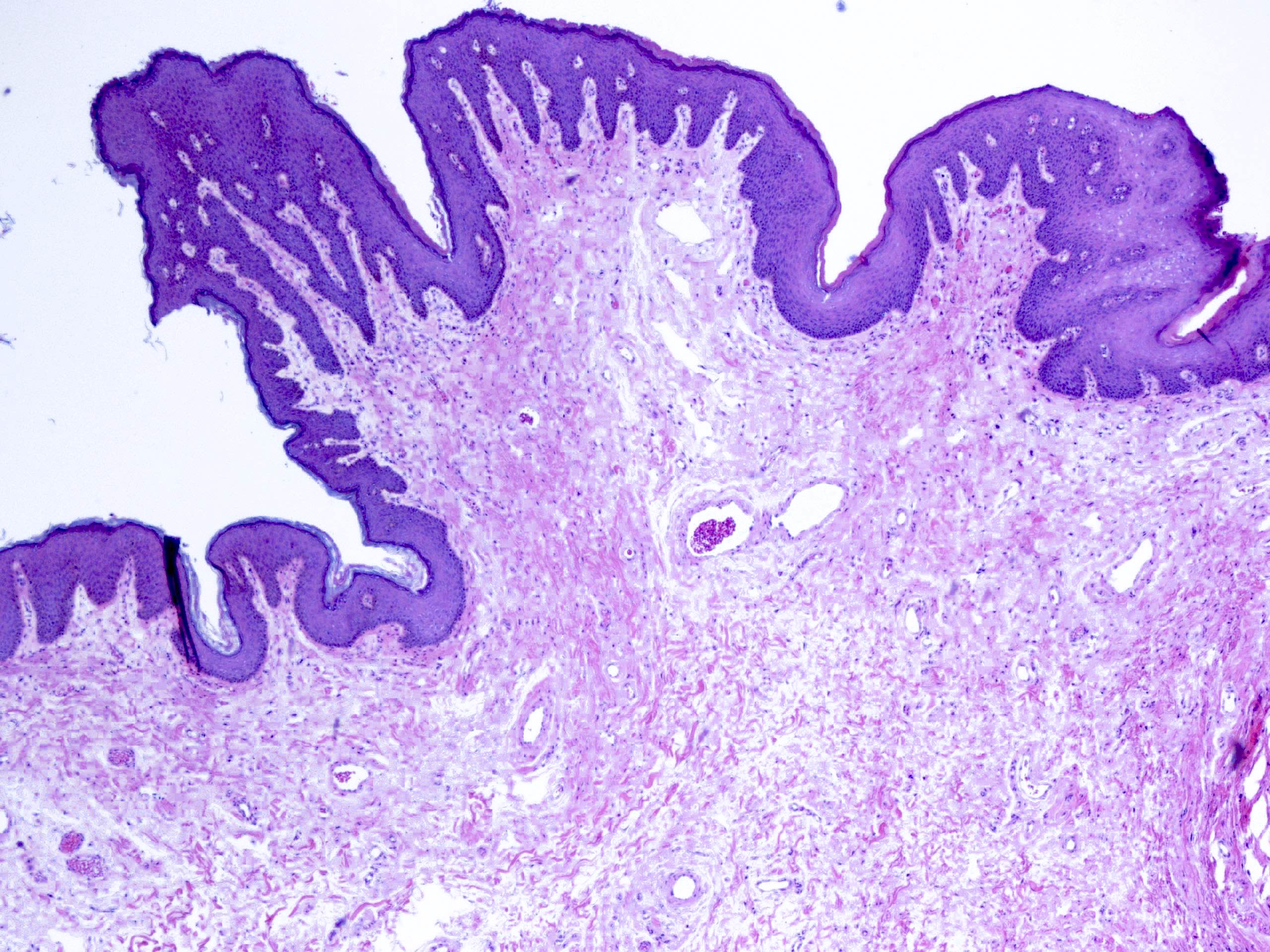
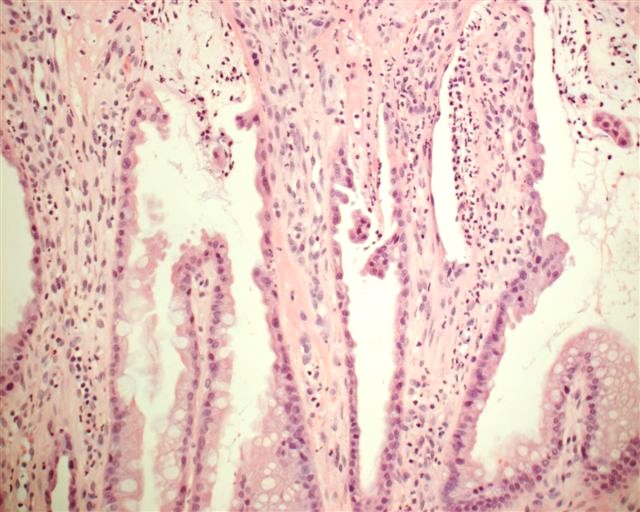
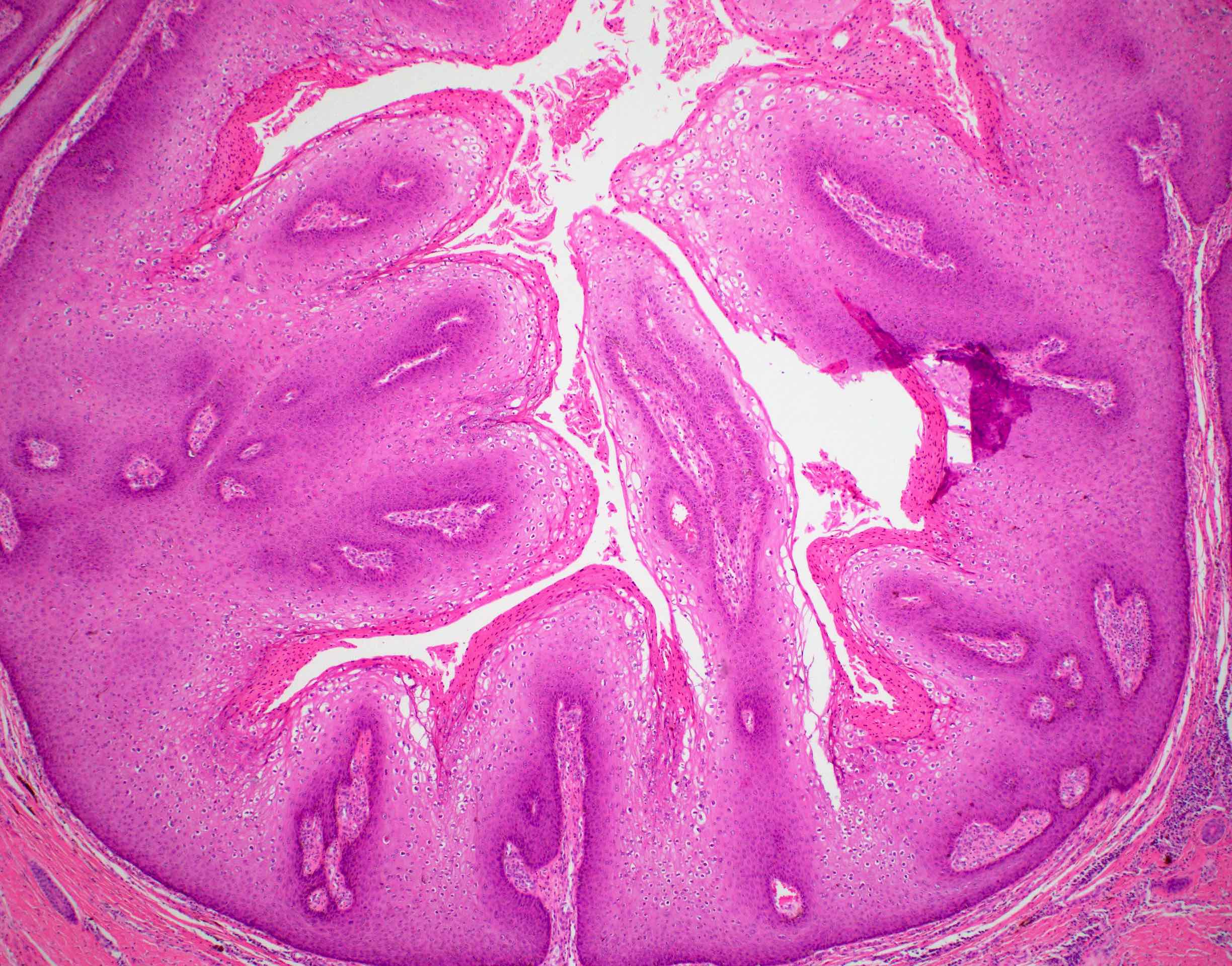
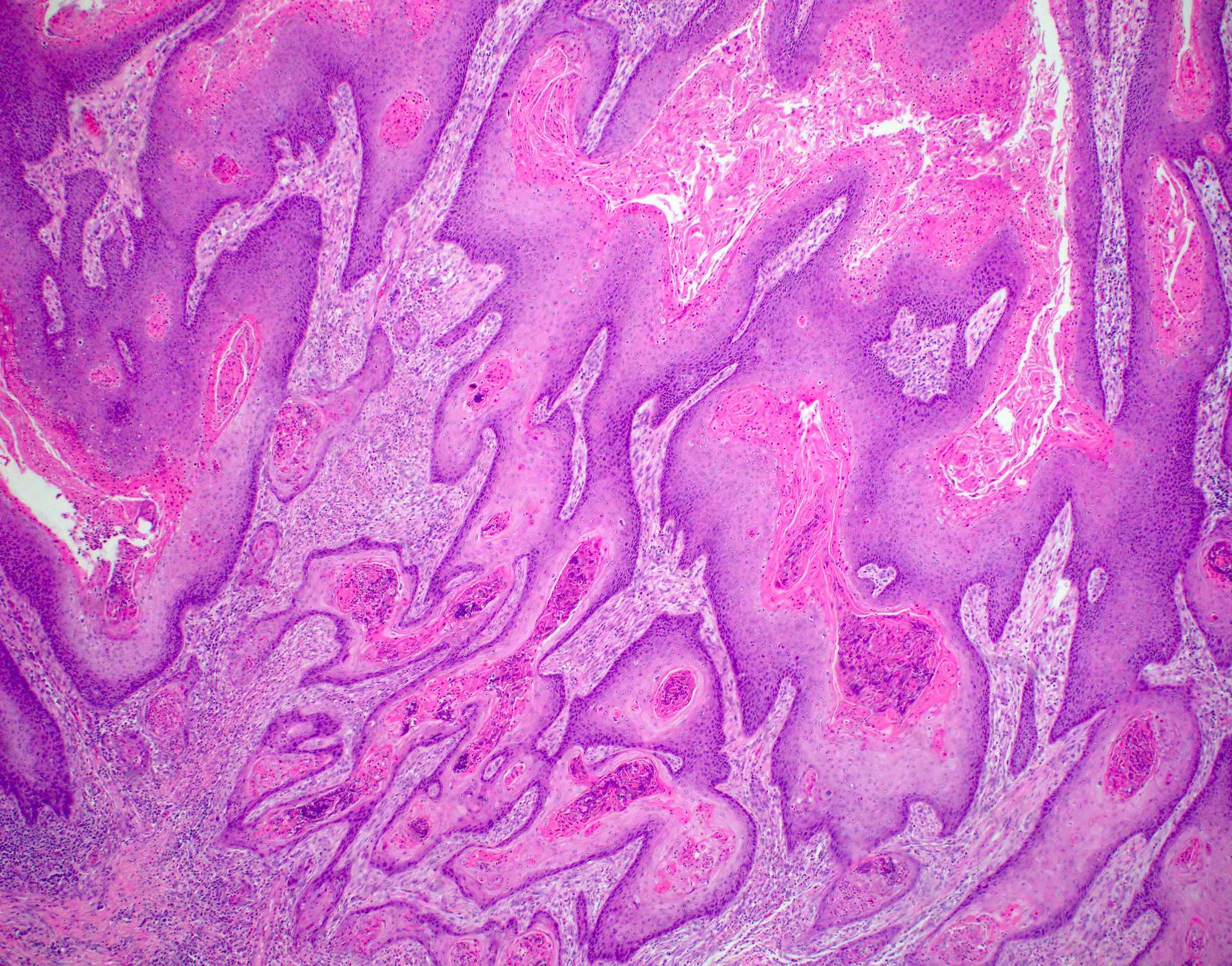
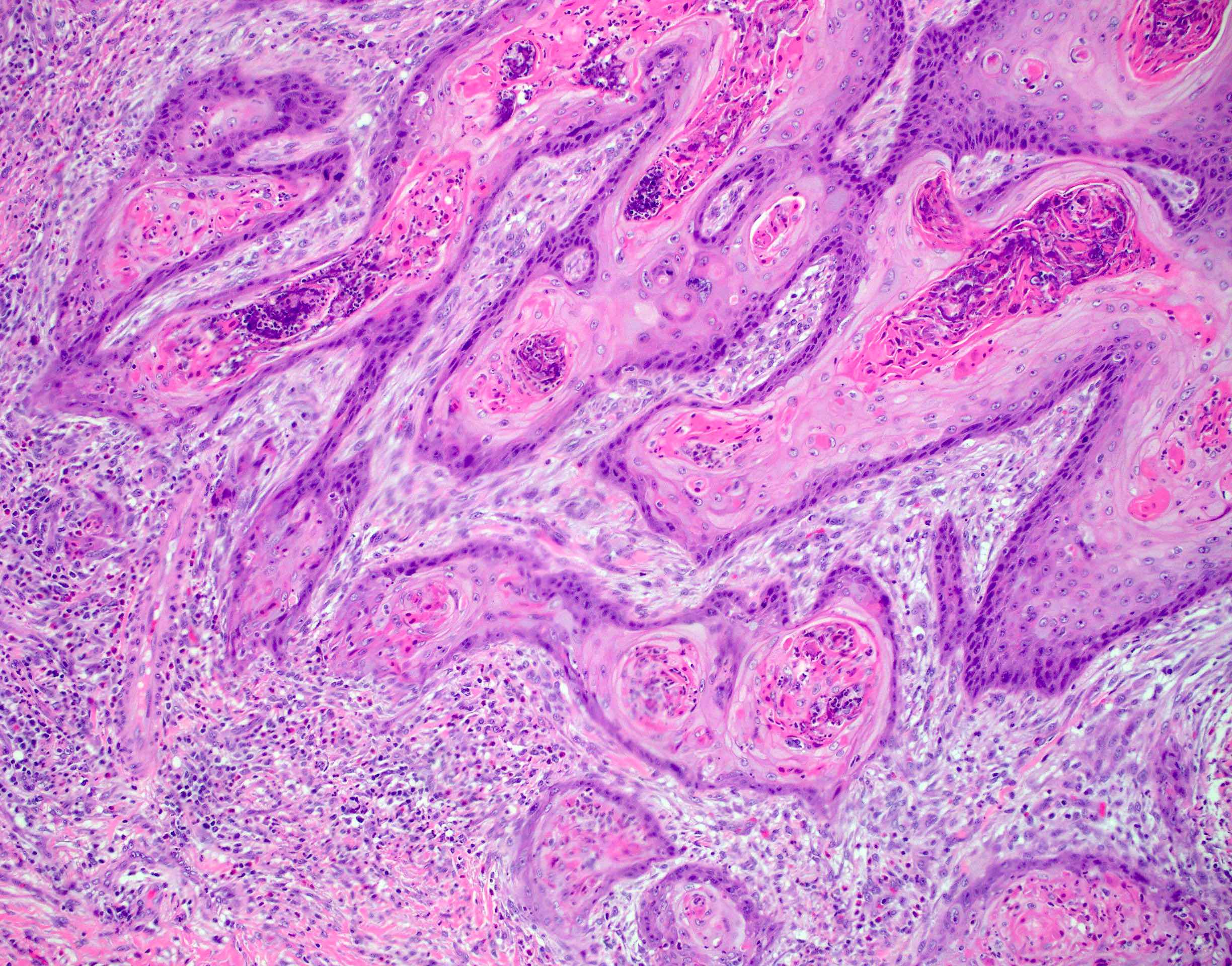
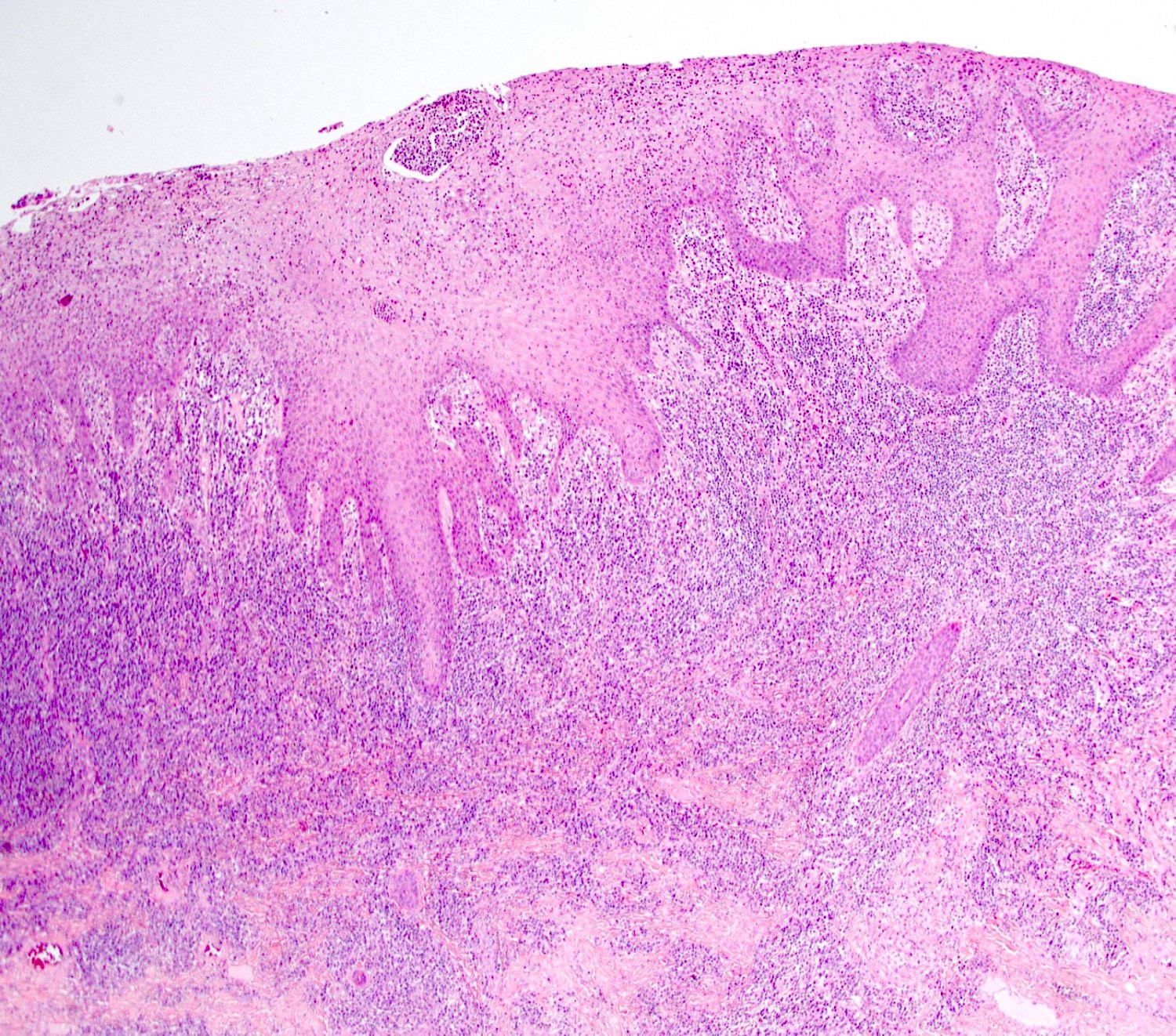
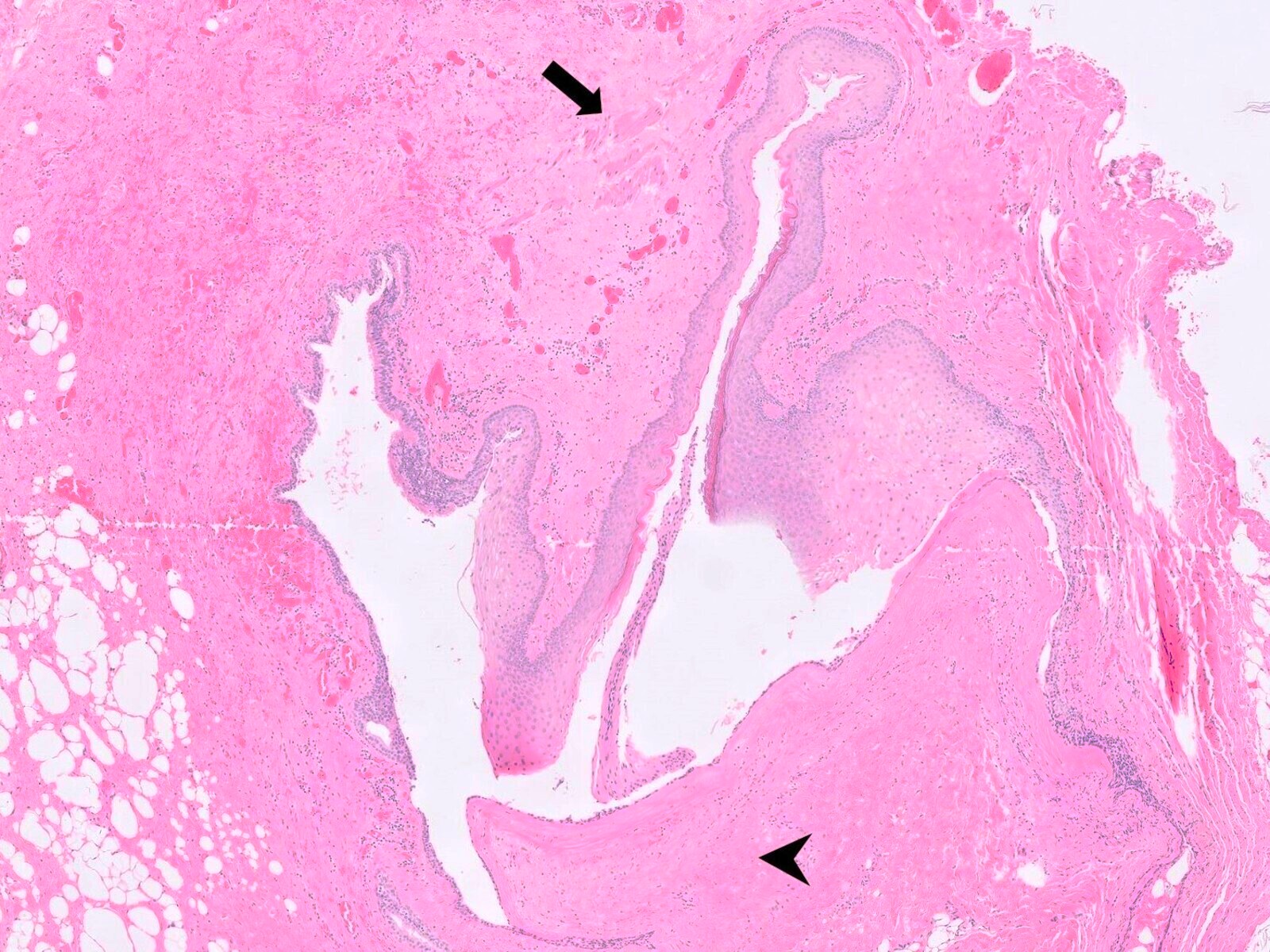
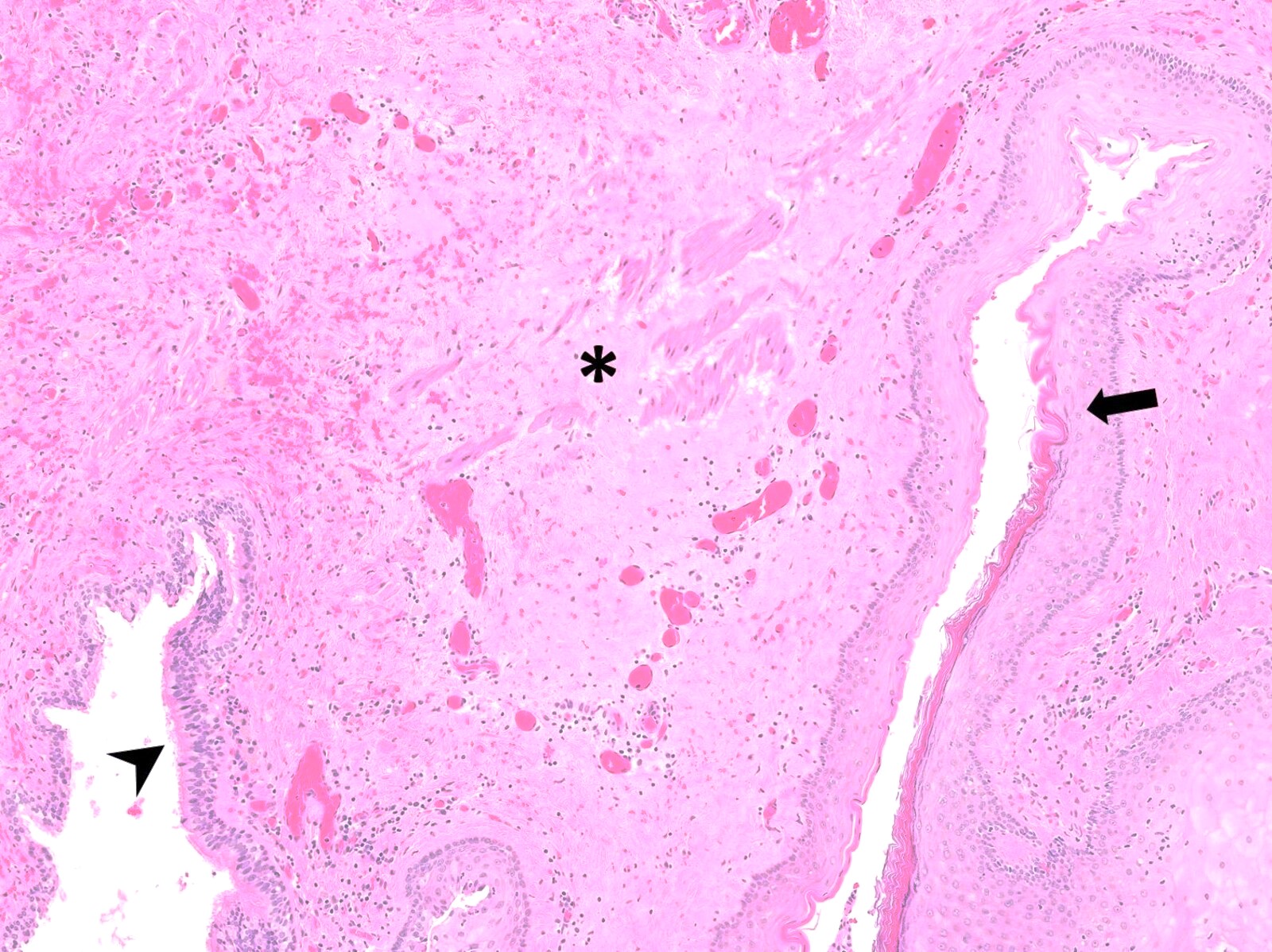
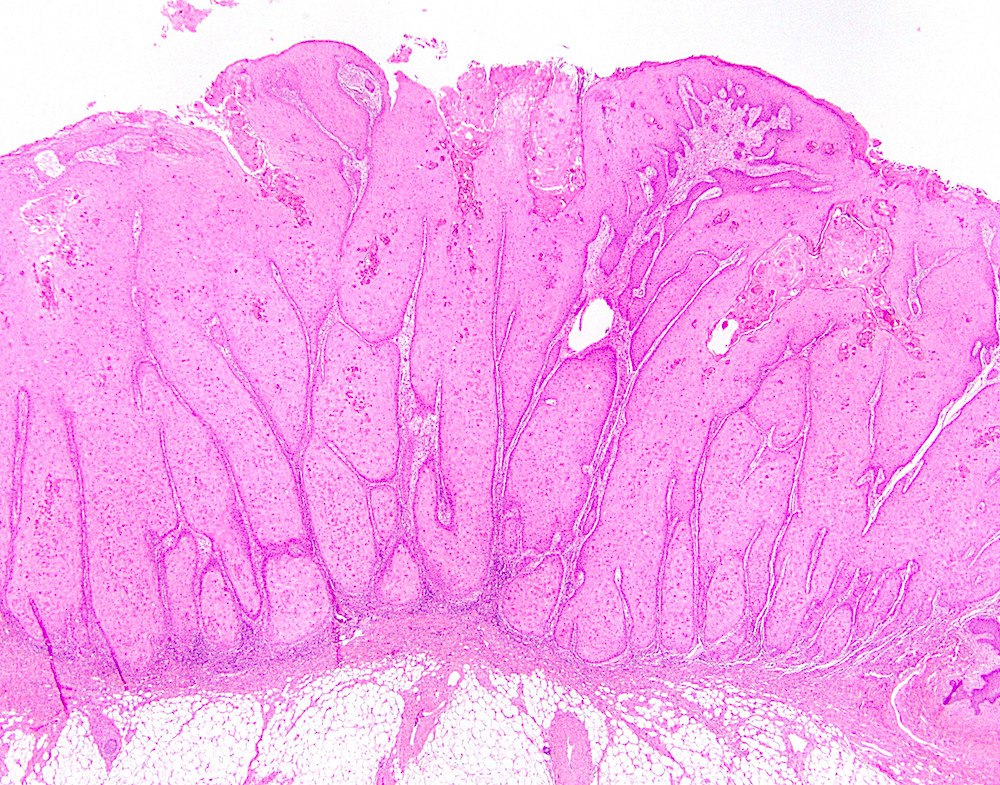
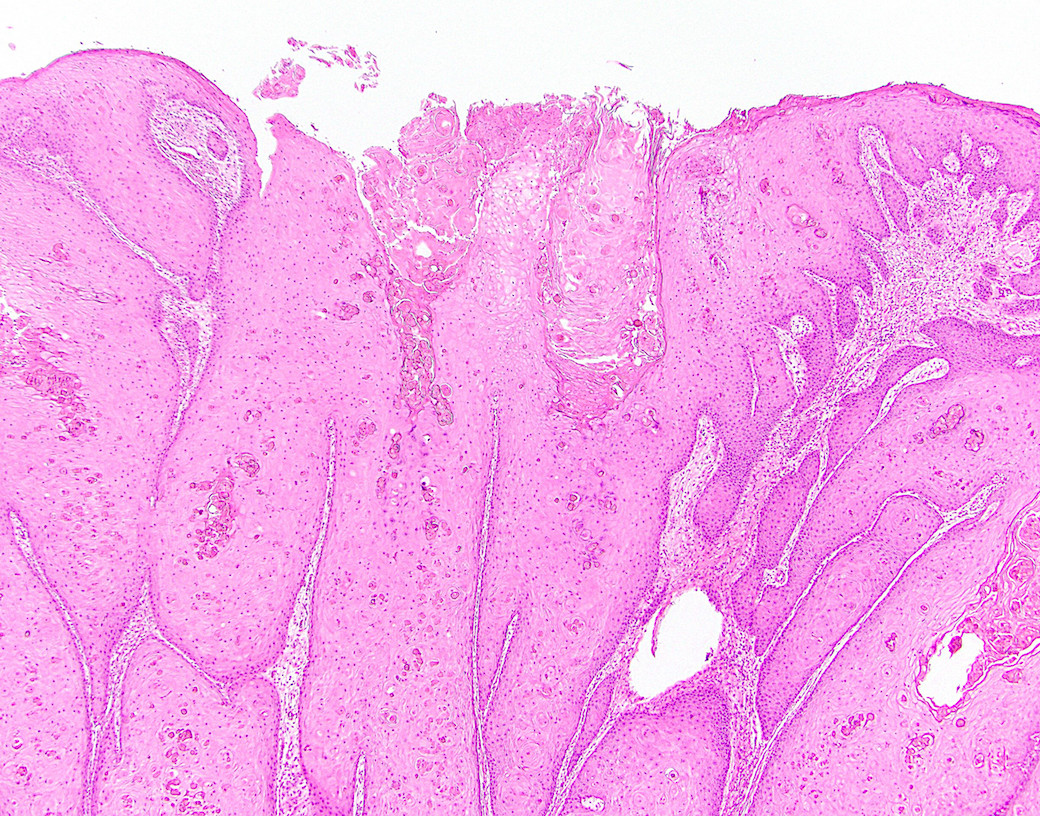
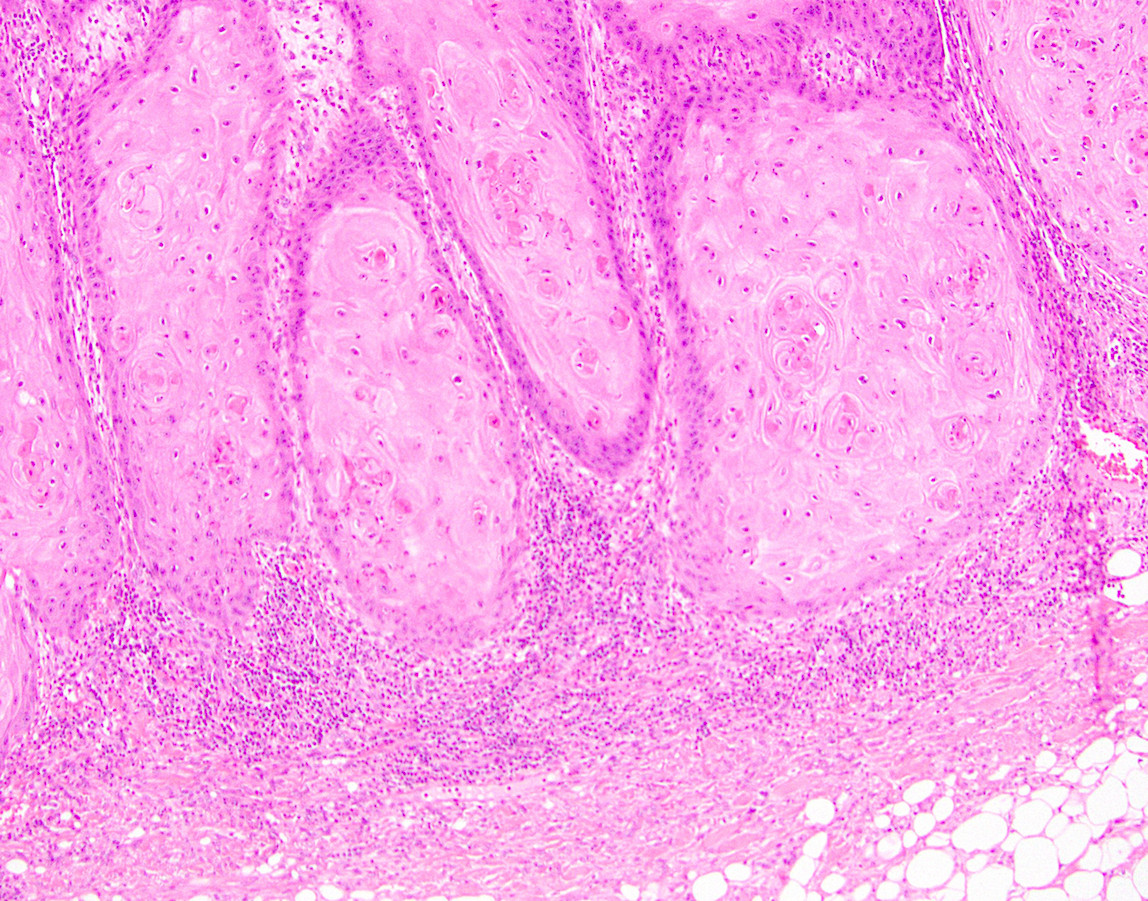
Microscopic (histologic) description
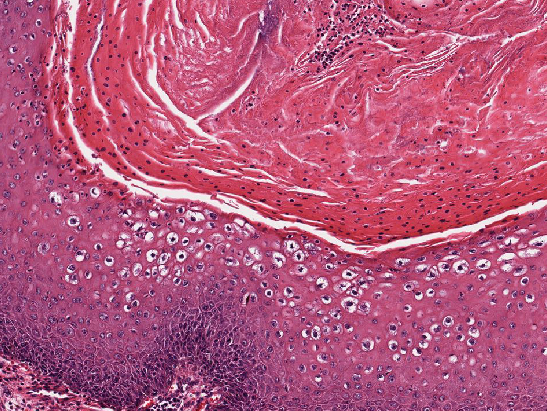
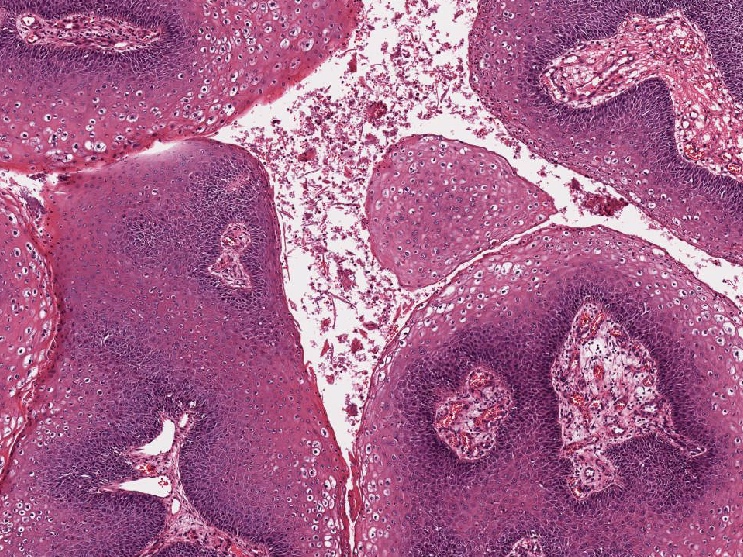
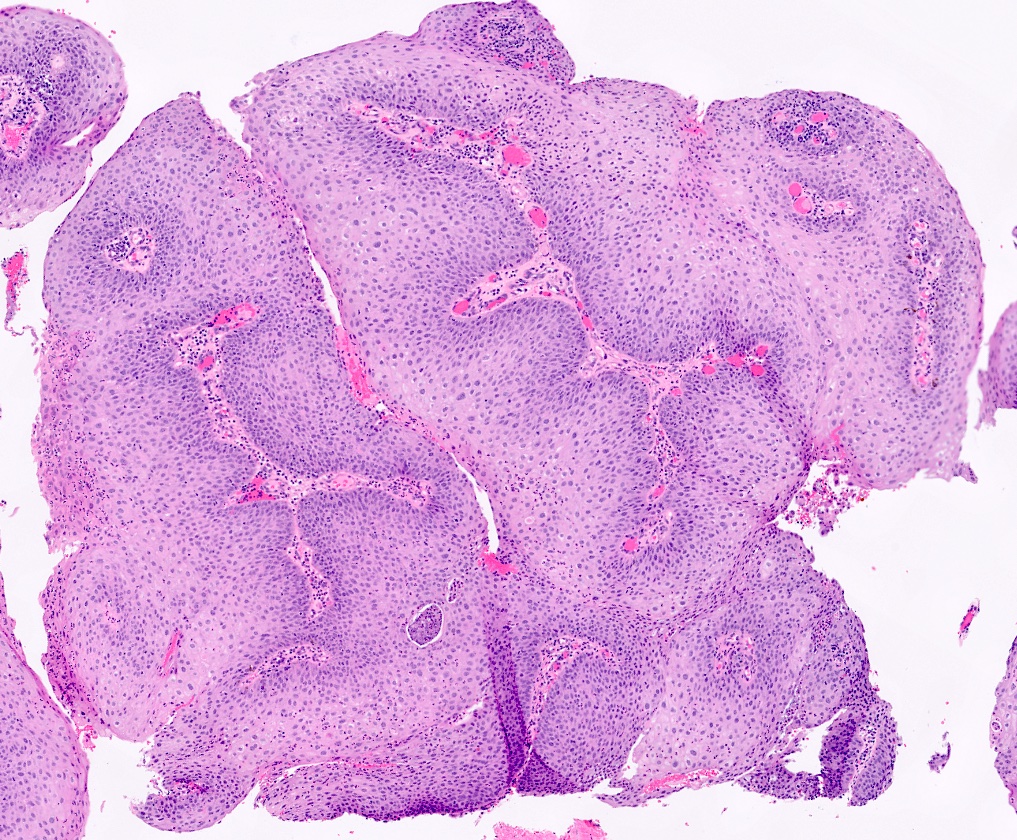
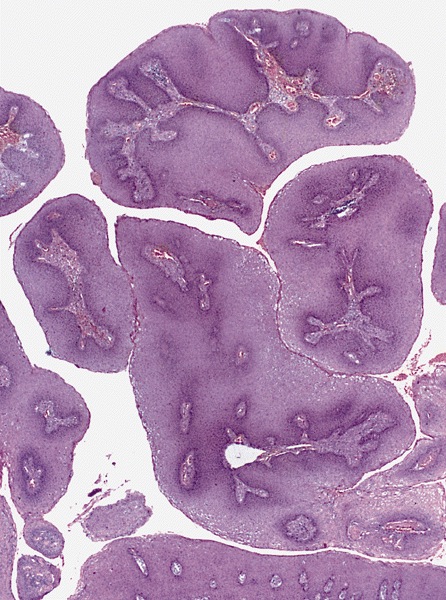
- Resembles condyloma acuminatum
- Diagnostic criteria
- Well formed papillae with a prominent central fibrovascular core
- Hyperkeratosis with parakeratosis and marked acanthosis
- Koilocytes on upper third of the squamous epithelium
- Chronic inflammatory infiltration
- Local extension and displacement of surrounding tissues but no evidence of dysplasia / invasion (WHO Classification of Tumours Editorial Board: Digestive System Tumours, 5th Edition, 2019)
- Low mitotic rate, usually confined to the basal layer with no abnormal mitoses (Histopathology 2017;70:938)
- About 30 - 35% may develop an invasive component; especially in high risk HPV positive cases (Virchows Arch 2020;476:543, Dis Colon Rectum 1989;32:481)
Microscopic (histologic) images
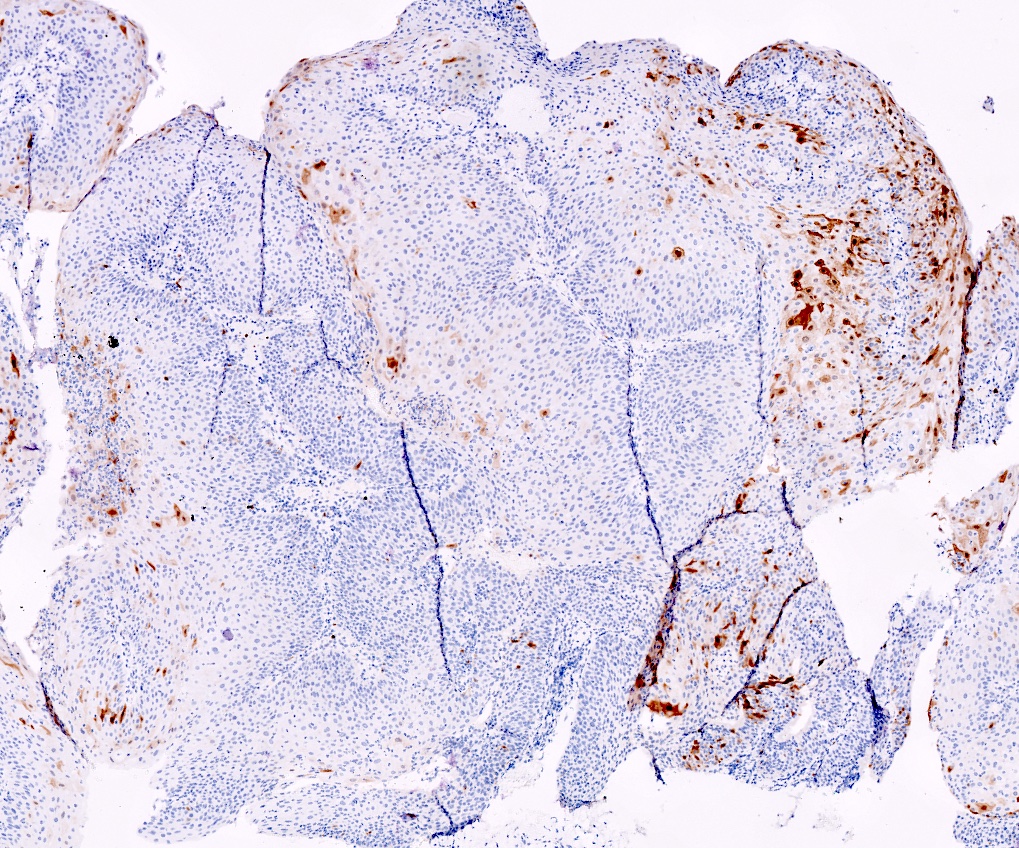
Positive stains
- Squamous markers (p40, p63), cytokeratins
- ISH for low risk HPV (6, 11) (Histopathology 2017;70:938, Virchows Arch 2020;476:543)
Negative stains
Videos
Giant condyloma
Sample pathology report
- Anal canal and perianal skin, complete excision:
- Buschke-Löwenstein tumor / giant condyloma acuminatum (see synoptic report)
| Tumor summary | Anus |
| Procedure | Complete excision |
| Tumor site | Perianal skin |
| Tumor size | 5 cm |
| Histologic type | Giant condyloma acuminatum |
| Margins | Free, 0.5 cm to radial |
| Extent | Anal canal, below the pectinate line |
| Tumor invasion | Not identified |
| Examined sections | 05 blocks |
| Lymph nodes, # sampled | 10 |
| Lymph nodes, # involved | 0 |
Ancillary study result
| Patchy, 5% of cells Restricted to parabasal layers |
Differential diagnosis
- Condyloma acuminatum:
- Histologically, Buschke-Löwenstein tumor resembles condyloma acuminatum
- Clinical findings help differentiate condyloma from Buschke-Löwenstein tumor (large size - usually > 3 cm, locally expansive and displacement of surrounding tissues)
- Verrucous carcinoma:
- No koilocytosis
- Invasion with pushing borders
- Negative for HPV
- Similar p16 staining pattern to Buschke-Löwenstein tumor (patchy / focal or negative) (Arch Pathol Lab Med 2019;143:821)
- Squamous cell carcinoma:
- Marked cytologic atypia with stromal invasion
- p16: diffusely positive
- Squamous papilloma of anus:
- Extremely rare (Endoscopy 2013;45:E42)
Board review style question #1
Which of the following features best distinguishes Buschke-Löwenstein tumor from verrucous squamous carcinoma?
- HPV ISH positivity
- p53 immunostatus
- Patient age less than 50 years
- Pushing invasion
- Size greater than 5 cm
Board review style answer #1
A. HPV ISH positivity. Buschke-Löwenstein tumor is an HPV related neoplasm whereas verrucous carcinoma is not, so HPV ISH staining (positive) would be expected with Buschke-Löwenstein tumor but not with verrucous carcinoma. Buschke-Löwenstein tumor is most often due to a low risk HPV type, such as HPV 6 or 11. Invasive or displacing type growth can be seen in either lesion.
Comment Here
Reference: Buschke-Löwenstein tumor
Comment Here
Reference: Buschke-Löwenstein tumor
Board review style question #2
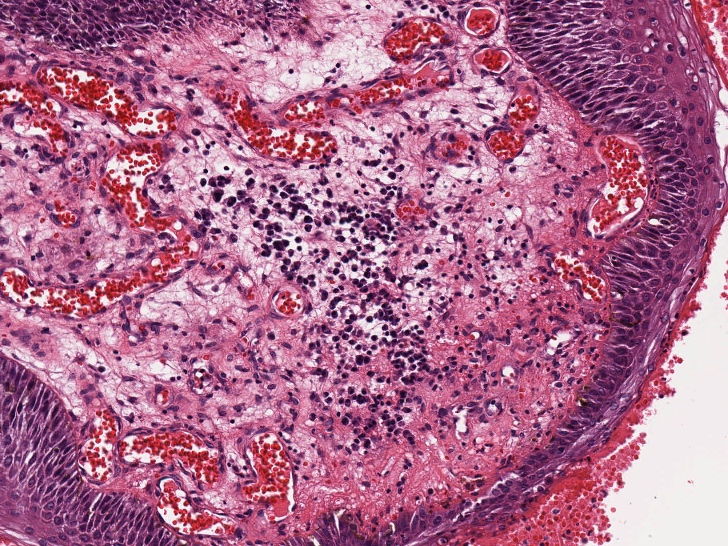
Lymphadenopathy seen in conjunction with a bulky anovaginal tumor characterized by parakeratosis, superficial koilocytosis, HPV ISH positivity and minimal squamous atypia as seen in this image is most likely due to
- Associated lymphoma or other immune deficiency inducing state
- Fatty infiltration of the lymph nodes
- Metastasis from a well differentiated invasive component of the tumor
- Netherton syndrome
- Reactive lymphadenopathy related to tumor associated infection
Board review style answer #2
E. Reactive lymphadenopathy related to tumor associated infection. Buschke-Löwenstein tumor is frequently associated with fistulae and perirectal or peritumoral abscess formation, which can lead to regional lymphadenopathy. Metastasis is very uncommon. While immunocompromise may predispose to development of Buschke-Löwenstein tumor, the cause is infrequently due to a lymphoproliferative disorder that would enlarge the lymph nodes. Netherton syndrome is not primarily associated with adenopathy.
Comment Here
Reference: Buschke-Löwenstein tumor
Comment Here
Reference: Buschke-Löwenstein tumor
Carcinoma overview
Table of Contents
Definition / general | Essential features | Epidemiology | Pathophysiology | Clinical features | Diagnosis | Prognostic factors | Treatment | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Carcinoma arising in anus, usually of squamous origin
- WHO classification includes squamous cell carcinoma, verrucous carcinoma, undifferentiated carcinoma, adenocarcinoma, mucinous adenocarcinoma, neuroendocrine tumor and neuroendocrine carcinoma
- Per WHO: anal canal tumor "cannot be seen in its entirety, or at all, when gentle traction is placed on the buttocks" but perianal tumor "is found within a 5 cm radius of the anus and is seen completely when gentle traction is placed on the anus"
Essential features
- Most anal carcinomas are squamous, with HPV as a risk factor
- Most common in middle aged women
Epidemiology
- Uncommon (1 - 2% of GI tumors)
- More common in women (2 - 4:1); average age at diagnosis is early 60s
- Rising incidence in past 25 years (Oncologist 2007;12:524)
- American Cancer Society estimates for 2015: 7,270 cases and 1,010 deaths in the United States (American Cancer Society: Anal Cancer [Accessed 11 October 2017])
Pathophysiology
- Most anal squamous carcinomas are linked to infection with human papillomavirus 16 or 18 (Mod Pathol 1996;9:614, Mod Pathol 1989;2:439)
- HPV vaccination therefore may lead to decreased incidence (J Low Genit Tract Dis 2013;17:397)
- Other risk factors include HIV infection, smoking, male receptive anal intercourse (Ann Intern Med 2008;148:728, N Engl J Med 1987;317:973)
Clinical features
- Symptoms include rectal / anal bleeding, anal pain or itching, change in bowel habits, feeling of a mass at anal opening
Diagnosis
- Annual screening of at risk populations using anal Pap smear appears effective (JAMA 1999;281:1822)
Prognostic factors
- 5 year survival rate is 80 - 90% for T1 / T2 cancers, compared with 50% for T4 cancers (Int J Health Sci (Qassim) 2012;6:206)
- Lymph node involvement is a poor prognostic factor (Ann Surg Oncol 2007;14:478)
Treatment
- Surgery alone for small lesions; advanced tumors may also require chemoradiation (5-fluorouracil / mitomycin) (Curr Oncol Rep 2009;11:186)
Differential diagnosis
- Rectal carcinoma:
- Depending on subtype, may be difficult to distinguish from anal adenocarcinoma or anal squamous cell carcinoma
Board review style question #1
- What is the most common primary tumor of the anus?
- Adenocarcinoma
- Basal cell carcinoma
- Neuroendocrine carcinoma
- Squamous cell carcinoma
Board review style answer #1
Board review style question #2
- What screening method can be used for patients at high risk of anal carcinoma?
- Annual anal Pap smear
- Annual colonoscopy
- Annual CT scan
- Annual MRI scan
Board review style answer #2
Condyloma acuminatum
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Radiology description | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
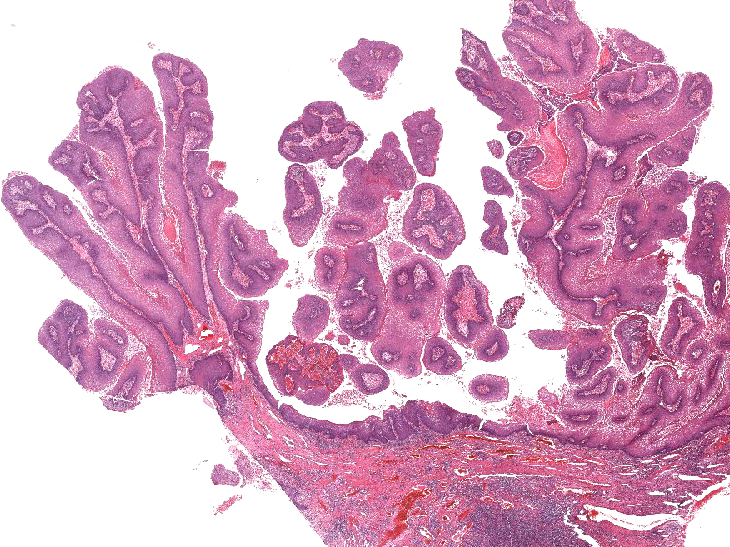
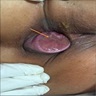
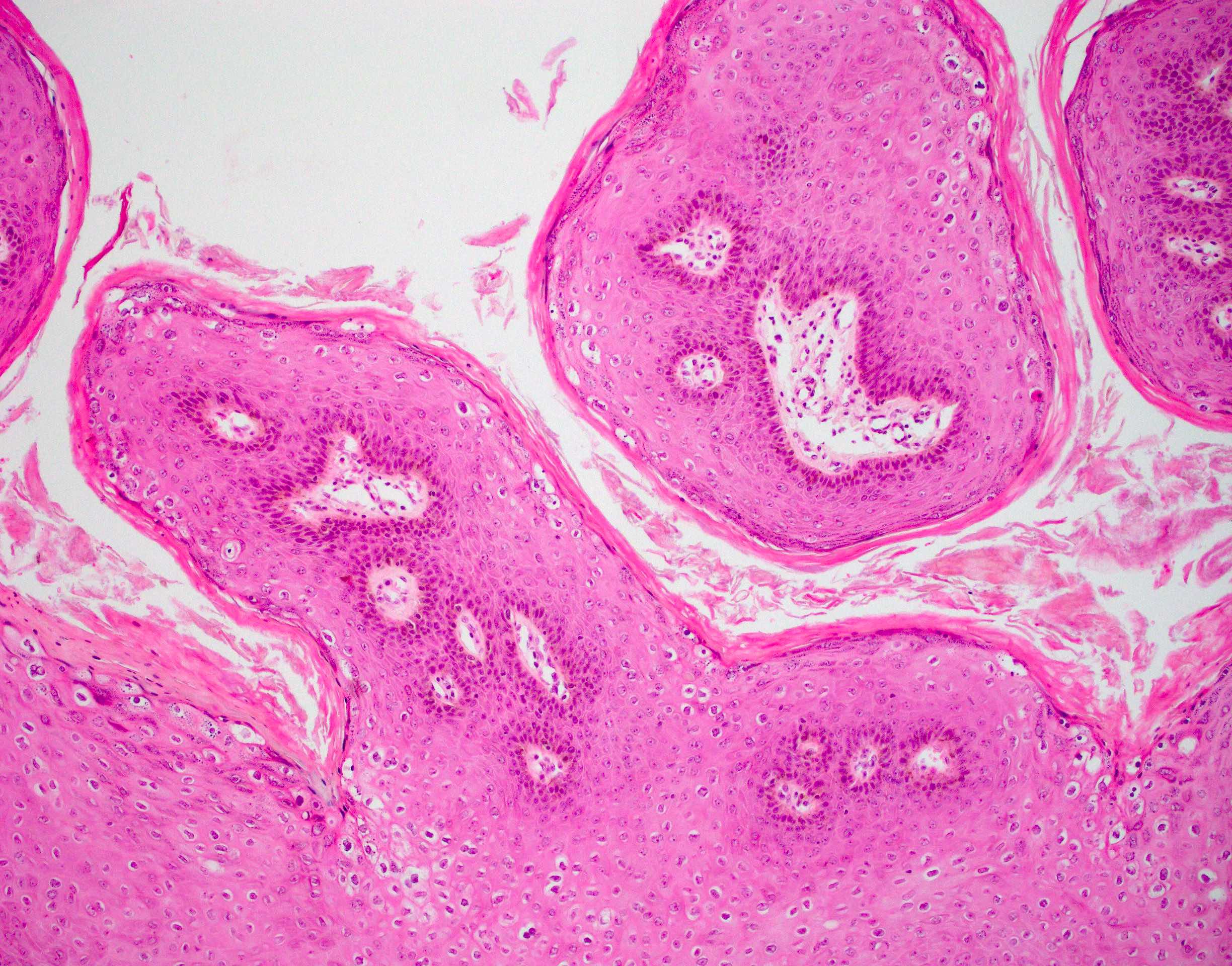
- Benign papillomatous squamous proliferation with a fibrovascular core, caused by human papillomavirus (HPV) infection
- Giant condyloma acuminatum with features of deep growth and local destruction, also known as Buschke-Löwenstein tumor
Essential features
- Benign papillomatous squamous proliferation with a fibrovascular core
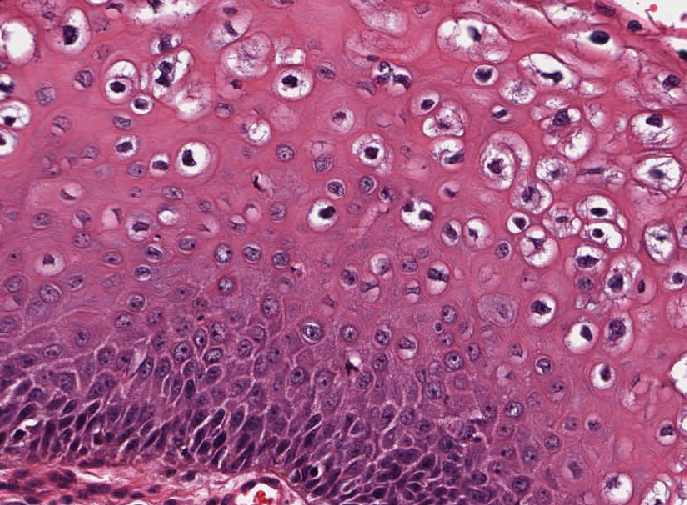
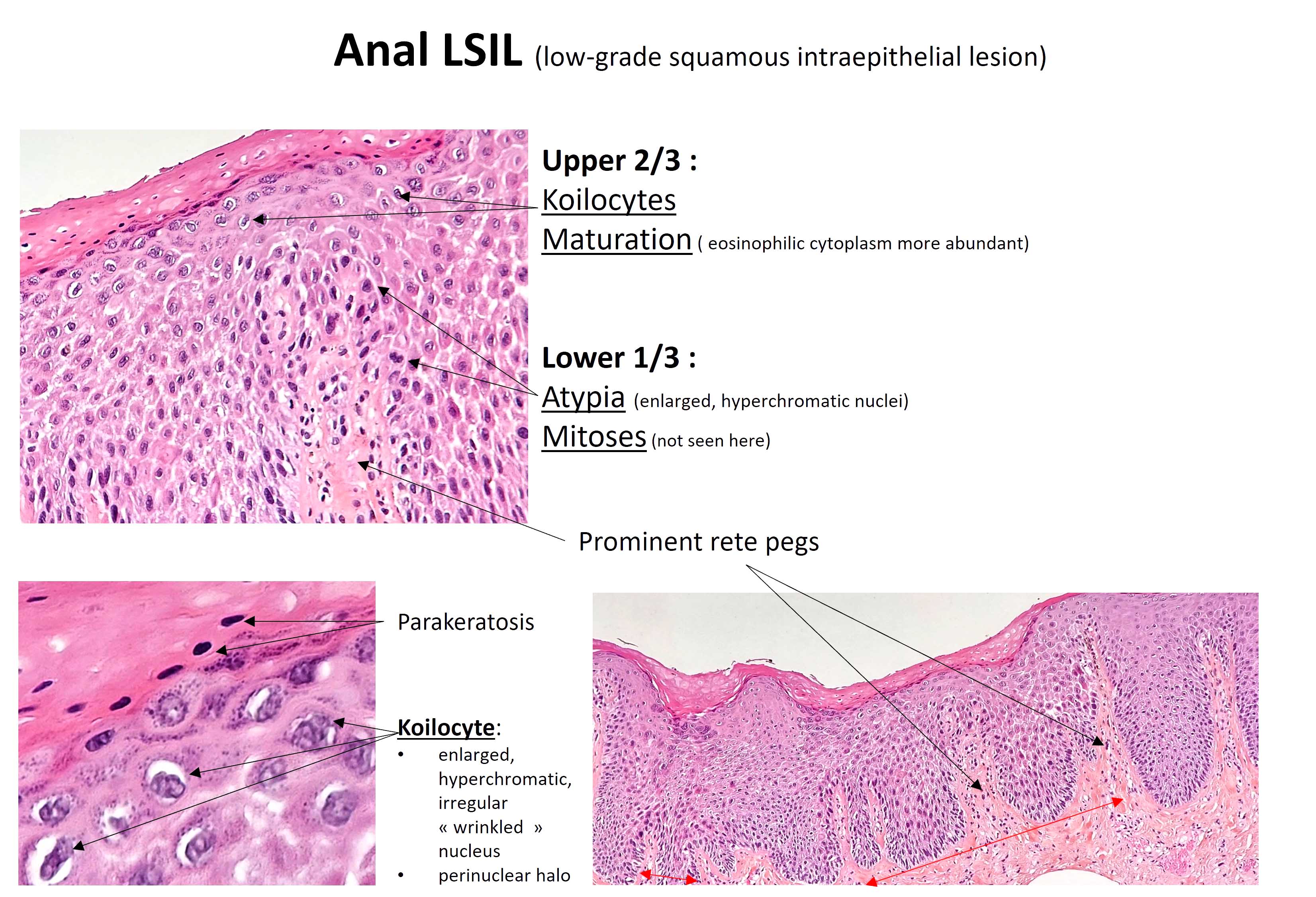
- Koilocytic change in upper 33% of squamous epithelium
- Caused by HPV serotypes 6 and 11
- High grade dysplasia and carcinoma prevalence higher in HIV positive patients
Terminology
- Anal wart
- Anogenital wart
- Anal condyloma
ICD coding
- ICD-11: 1A95.0 - anal warts
Epidemiology
- M = F; third decade of life
- More common in young men who have sex with men, HIV positive
- Risk factors: immunodeficient state, increased contacts (J Med Case Rep 2015;9:9, Acta Gastroenterol Belg 2021;84:343)
- Giant condyloma acuminatum: M > F
Sites
- Most common: upper anal canal and anal transition zone
- Less common: lower anal canal and perianal skin
- May be associated with coinfection in other genital sites (i.e., cervix, vulva, penis)
Pathophysiology
- HPV transient productive infection of basal layer of squamous epithelium
- Low oncogenic risk subtypes: HPV serotypes 6, 11
Clinical features
- Most common: asymptomatic
- Discomfort, pruritis, bleeding, eczematous rash
- Painless mass
Diagnosis
- Anoscopy with biopsy
Radiology description
- CT / MRI used to exclude malignant transformation (Gastrointest Radiol 1991;16:267, Radiology 1984;150:651)
Prognostic factors
- Recurrence ranges from 5% to 70% depending on treatment
- High grade dysplasia and carcinoma prevalence is higher in HIV positive patients (Dis Colon Rectum 2017;60:1078)
- Giant condyloma acuminatum (Buschke-Löwenstein tumor) has aggressive destructive local behavior with propensity for infections and fistulations (Ann Ital Chir 2018;89:291)
Case reports
- 50 year old man with giant condyloma acuminatum with deep infiltration (Dermatology 2009;218:56)
- 53 year old man with perianal giant condyloma acuminatum (Buschke-Löwenstein tumor) (Case Rep Surg 2012;2012:507374)
- 57 year old man with malignant transformation of giant condyloma acuminatum (Dis Colon Rectum 1981;24:462)
- 58 year old woman with coexistence of condylomata acuminata with warty squamous cell carcinoma and squamous cell carcinoma (Med Arch 2017;71:72)
Treatment
- Small condyloma can regress and be treated conservatively with patient applied therapies: imiquimod, podophyllotoxin, sinecatechins
- First line clinician administered therapies: cryotherapy, trichloroacetic acid
- Large condyloma and those in anal canal require wide surgical removal: excision, laser, electrosurgery
- References: UpToDate: Condyloma Acuminata [Accessed 4 March 2022], Dermatol Ther 2020;33:e13193, Clin J Gastroenterol 2021;14:439, Int J STD AIDS 2012;23:362, Am J Obstet Gynecol 1985;153:545
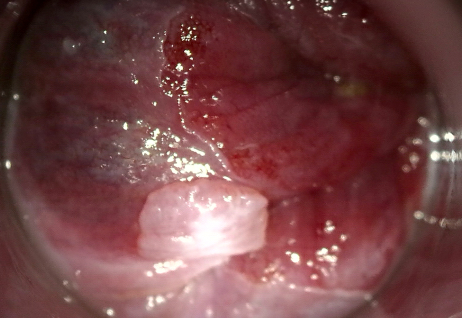
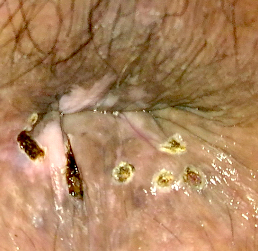
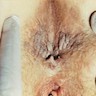
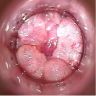
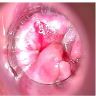
Clinical images
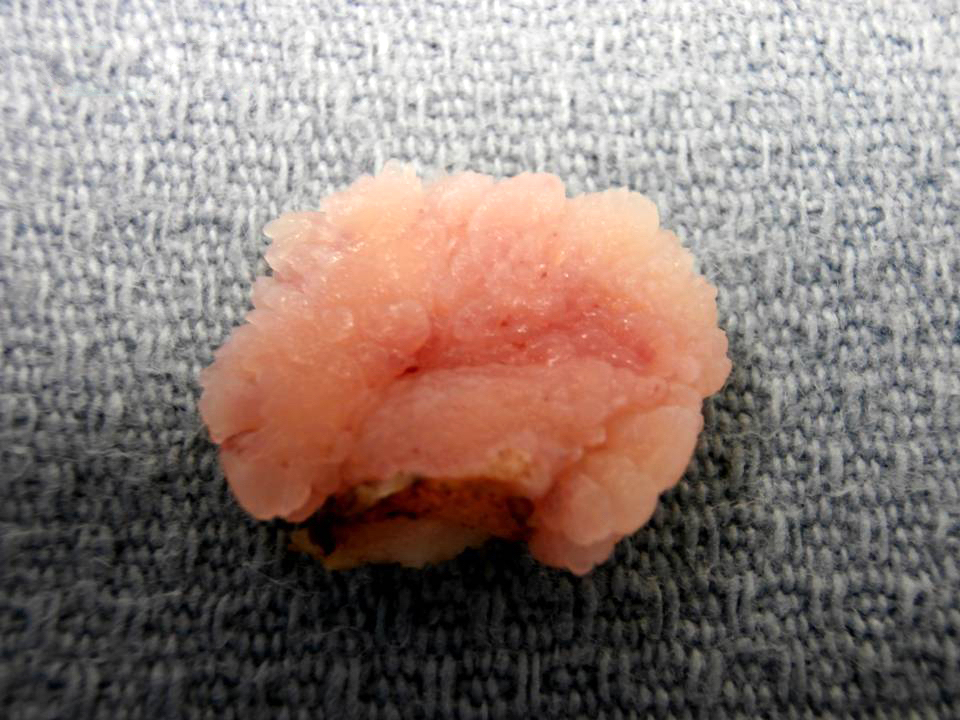
Gross description
- Fleshy, warty, cauliflower-like lesion
- May be flat lesion in anal canal
- Single or multiple (more common)
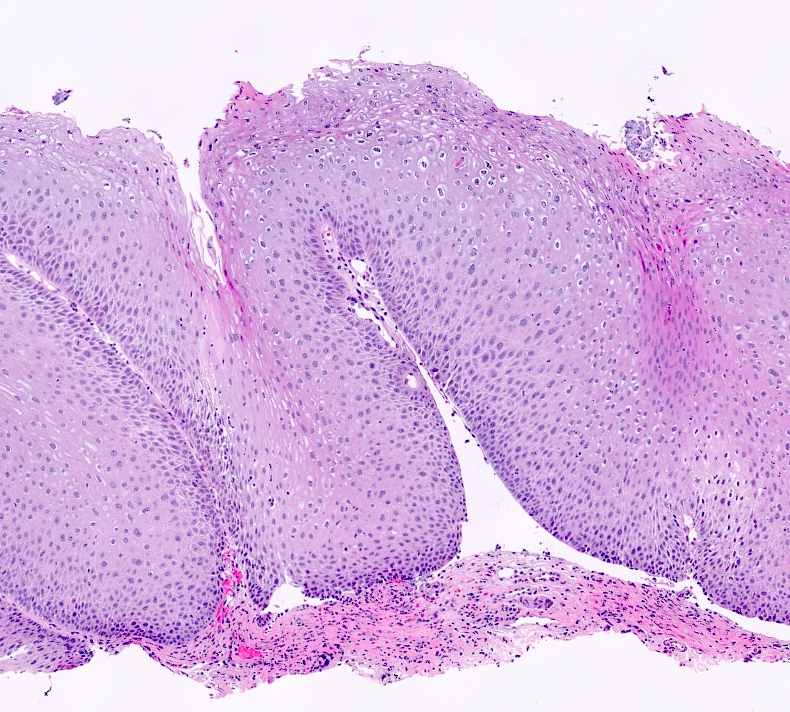
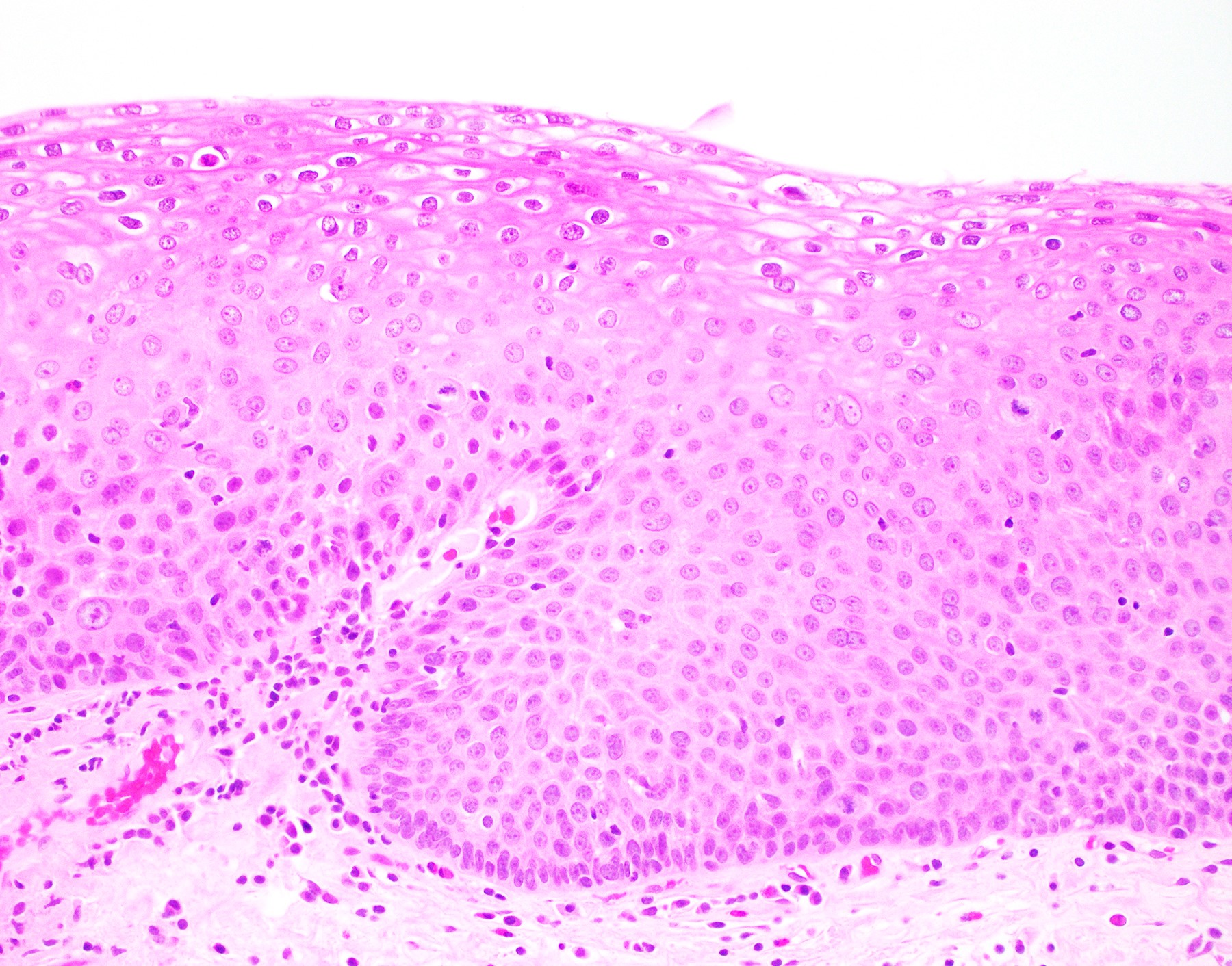
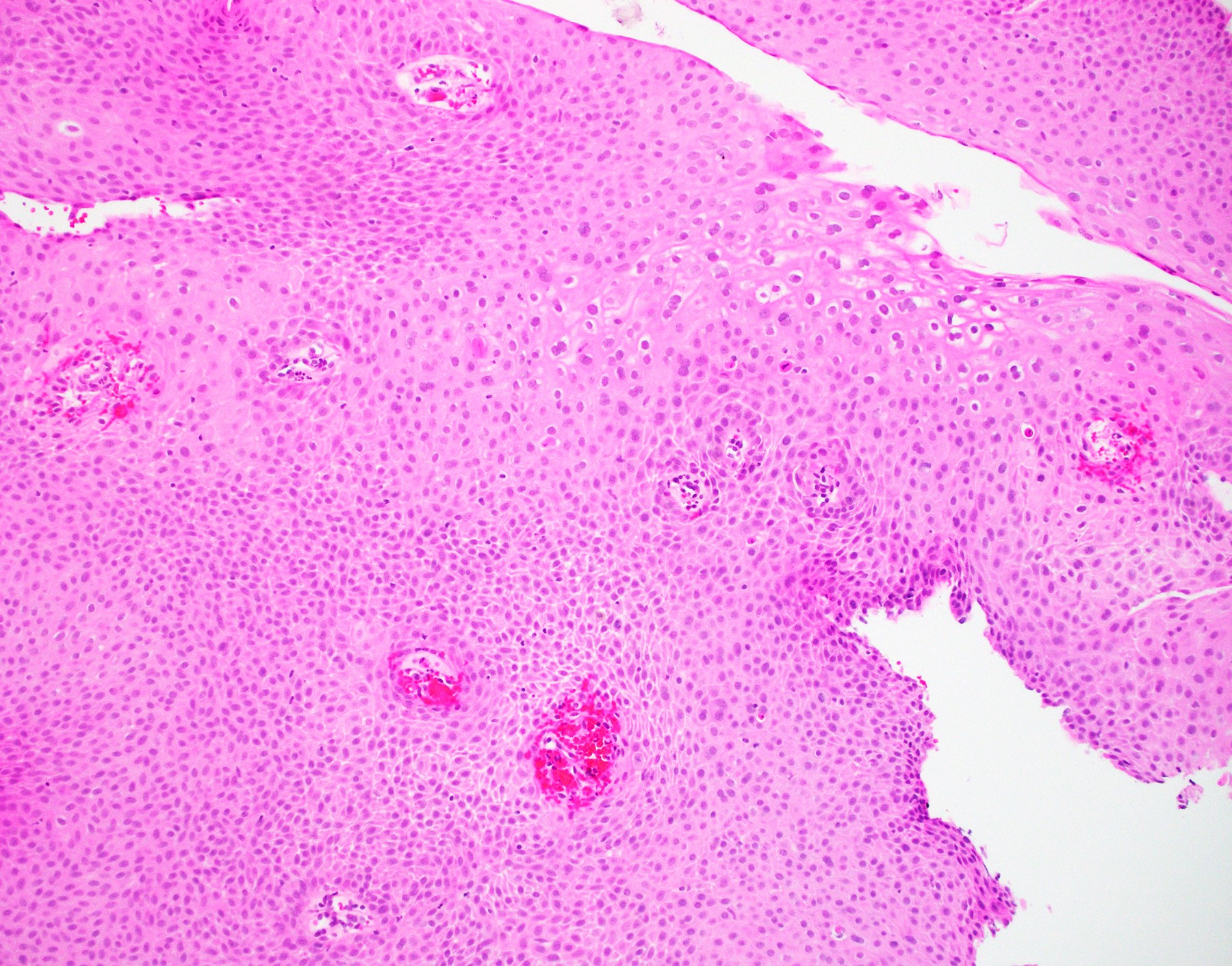
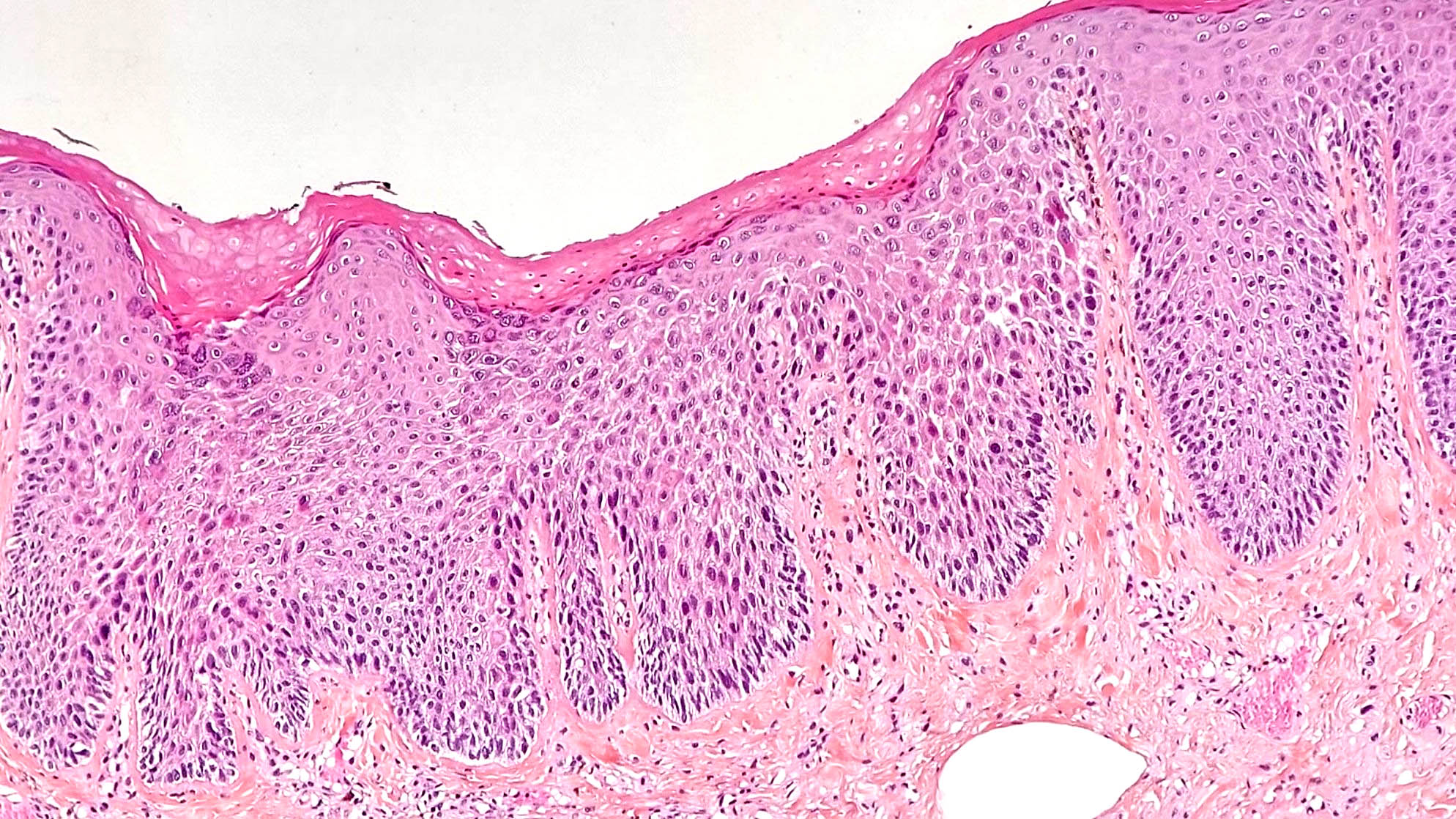
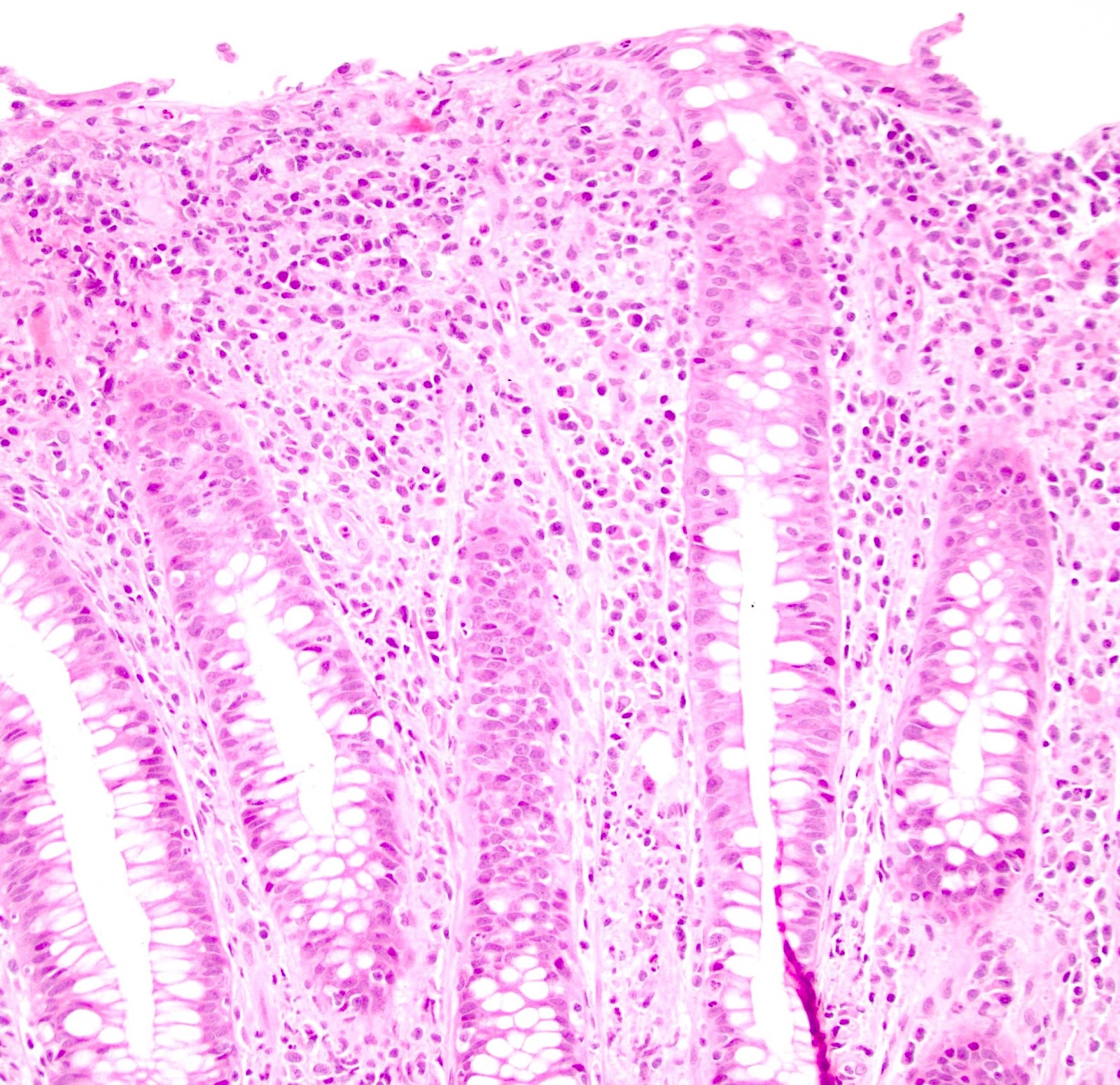
Microscopic (histologic) description
- Hyperplastic papillary exophytic squamous epithelium
- Marked acanthosis
- Parakeratosis
- Fibrovascular core
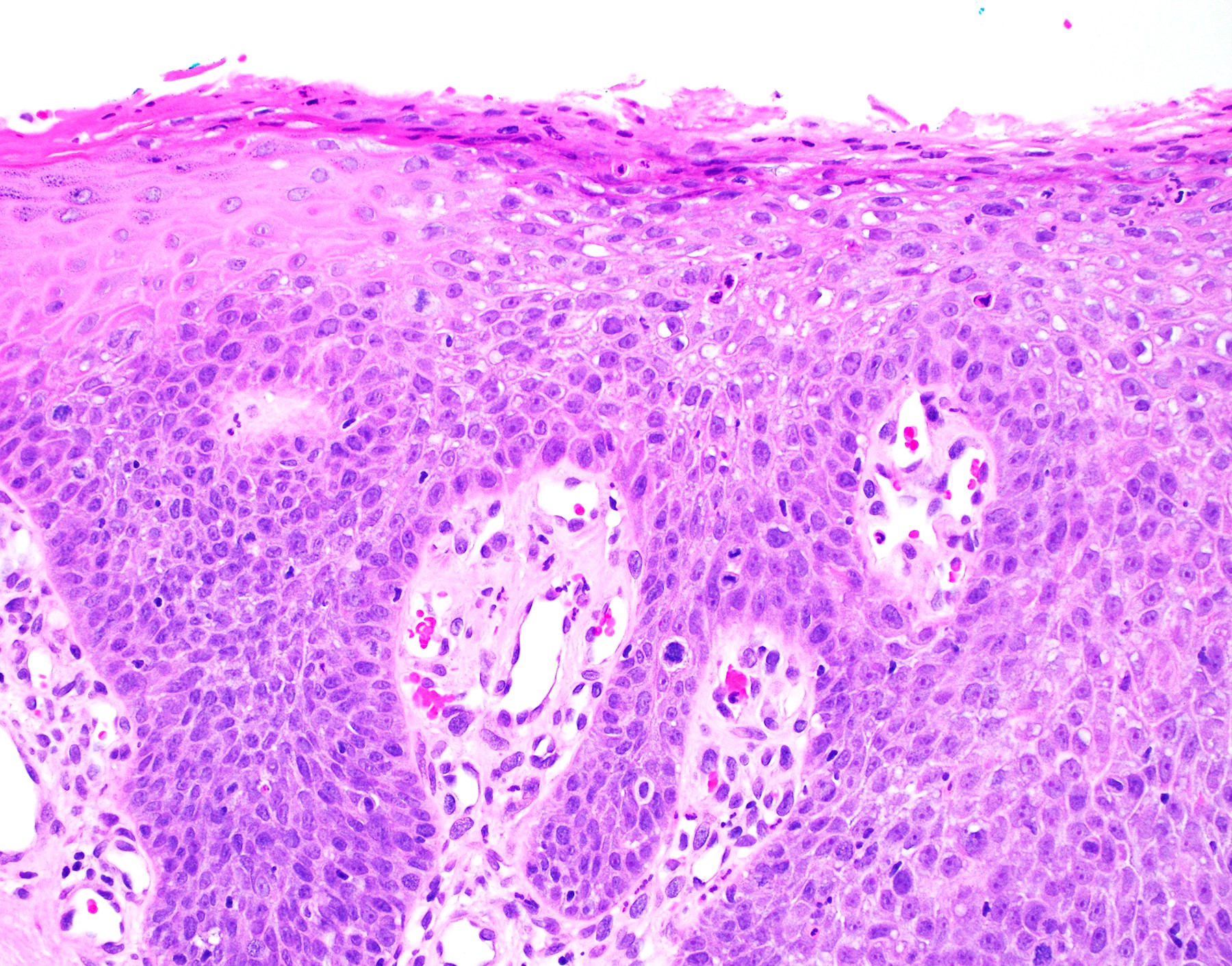
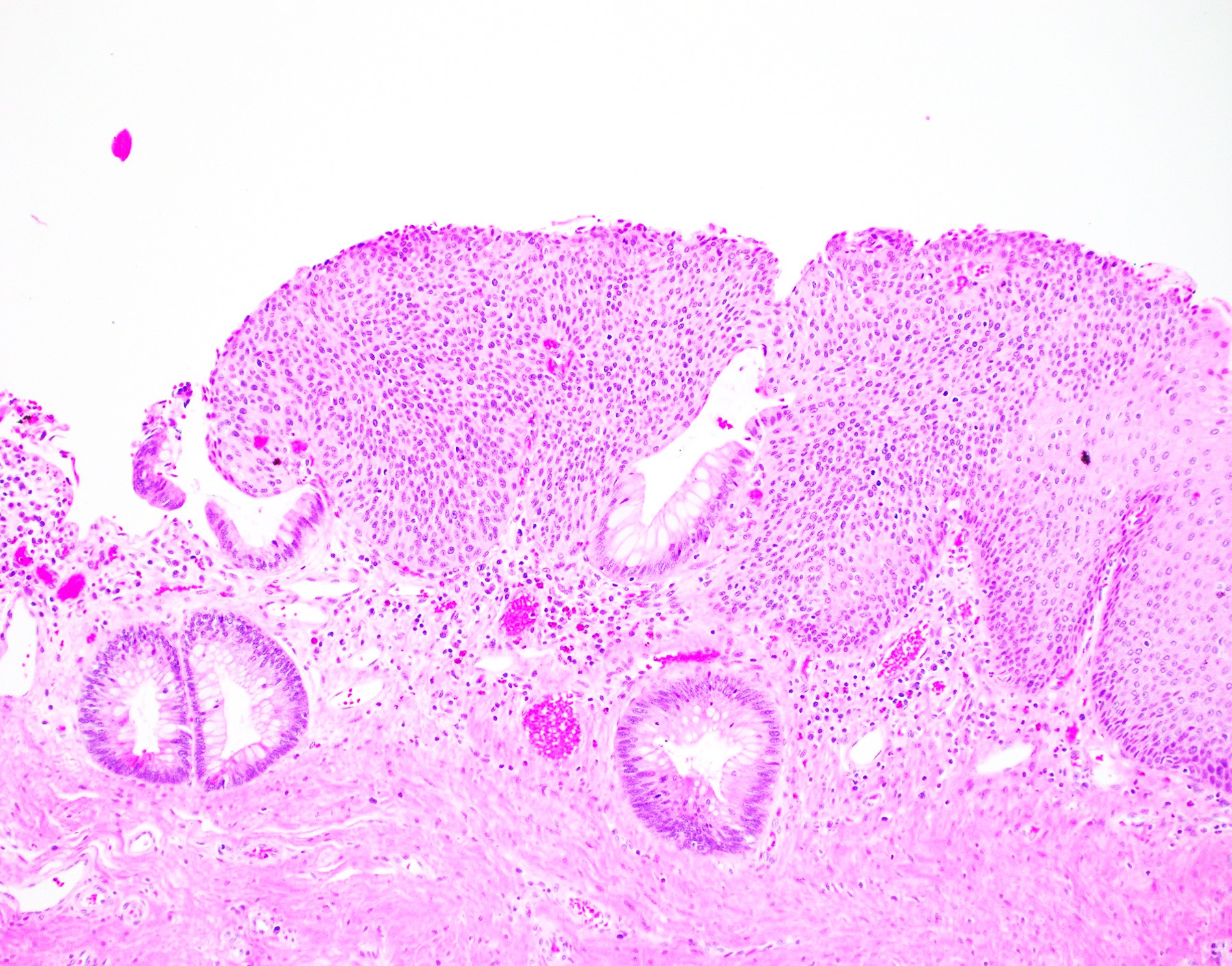
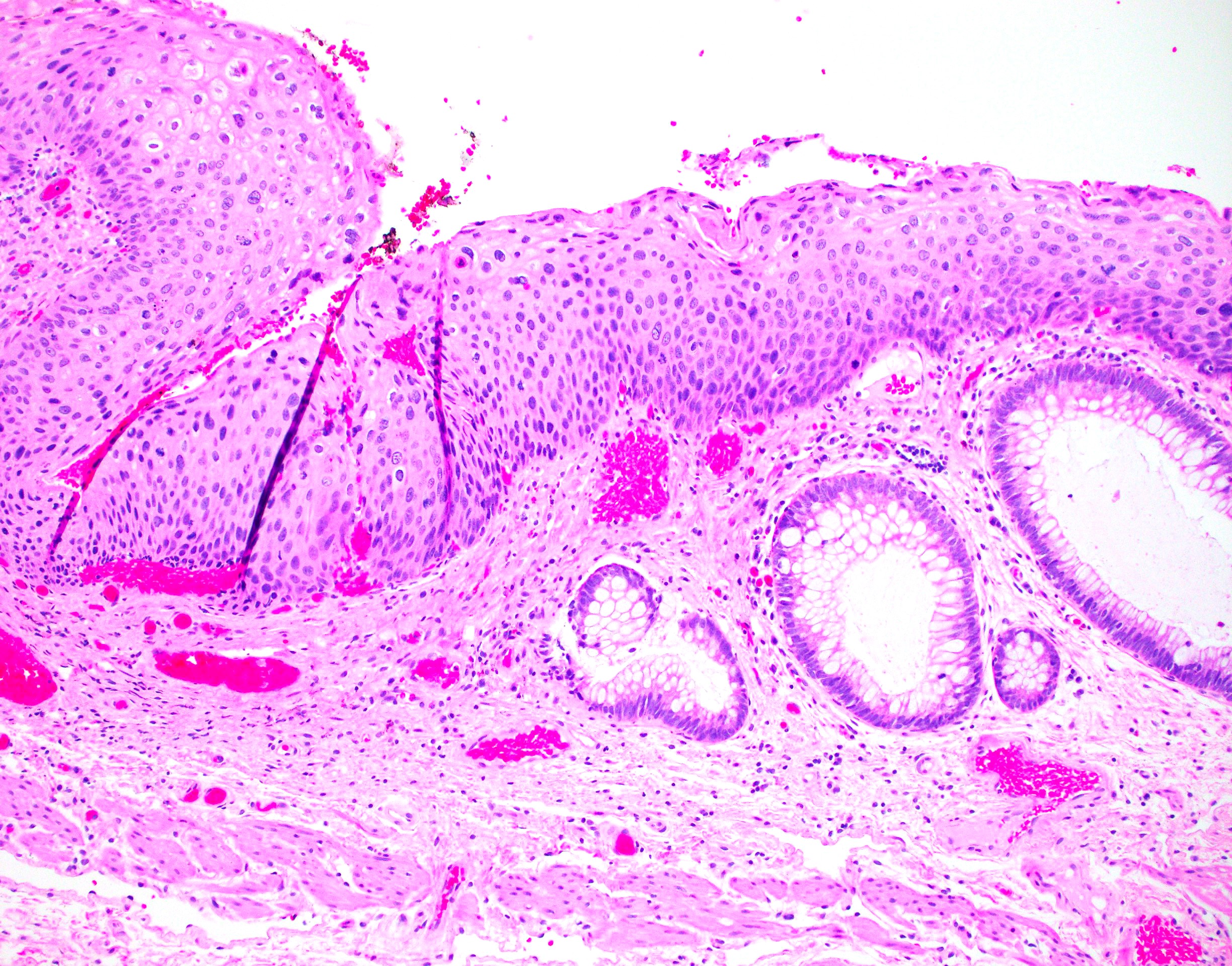
- Koilocytosis (irregular nuclei, bi and multinucleation, perinuclear vacuolization) confined to upper third of squamous epithelium
- No dysplasia or invasive squamous cell carcinoma
- Low grade squamous intraepithelial lesion (LSIL [condyloma acuminatum]) terminology applied according to the Lower Anogenital Squamous Terminology (LAST) project (Arch Pathol Lab Med 2012;136:1266)
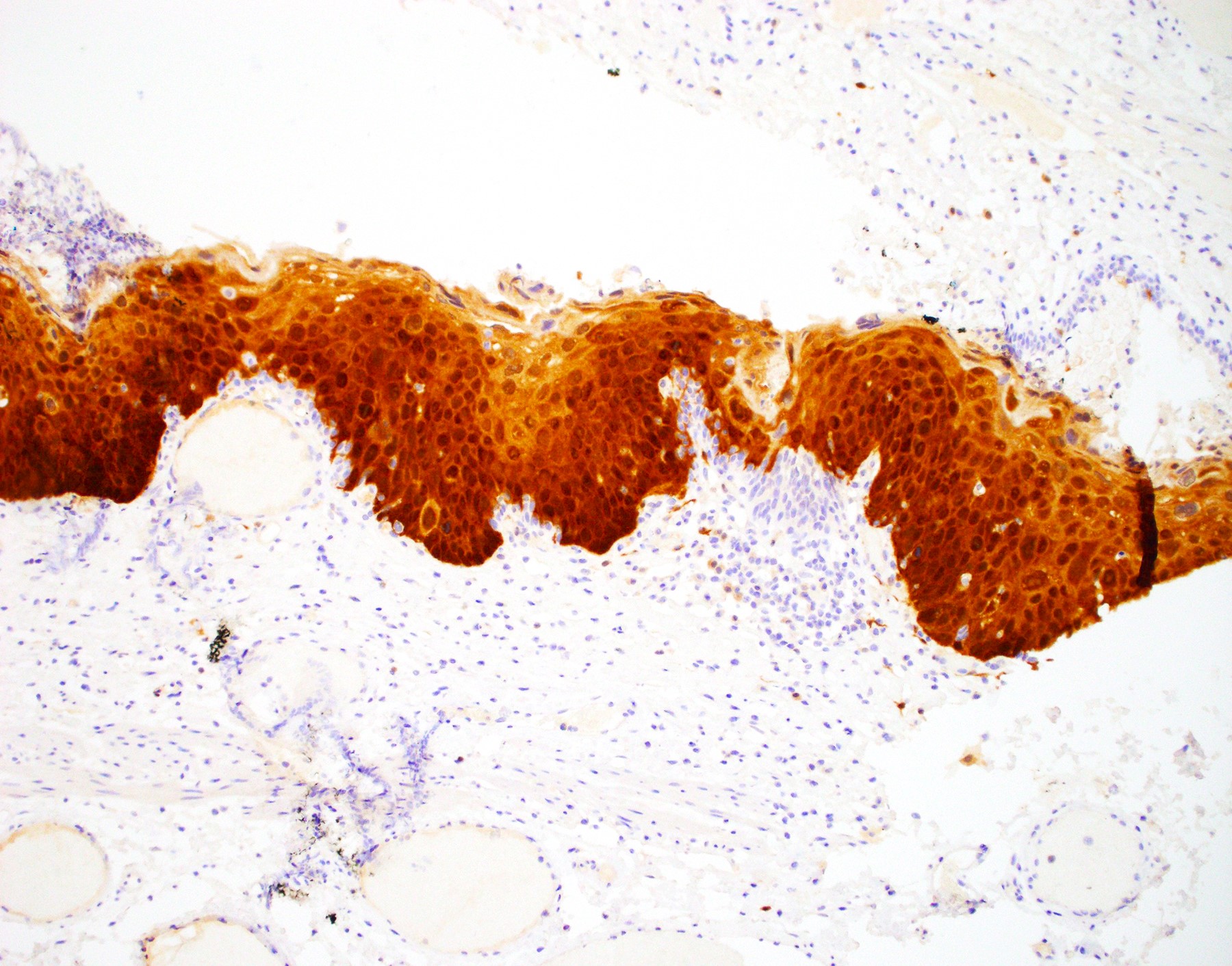
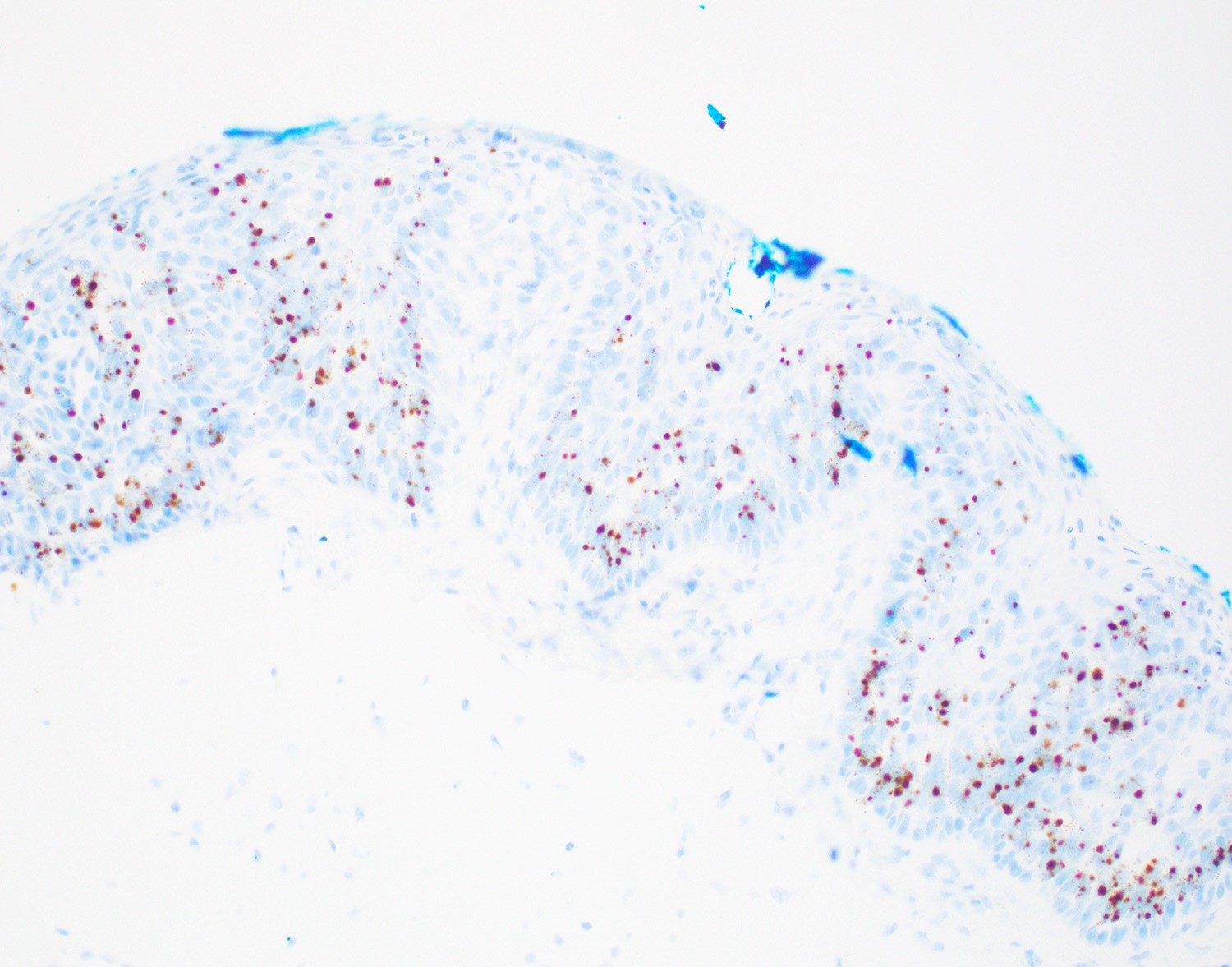
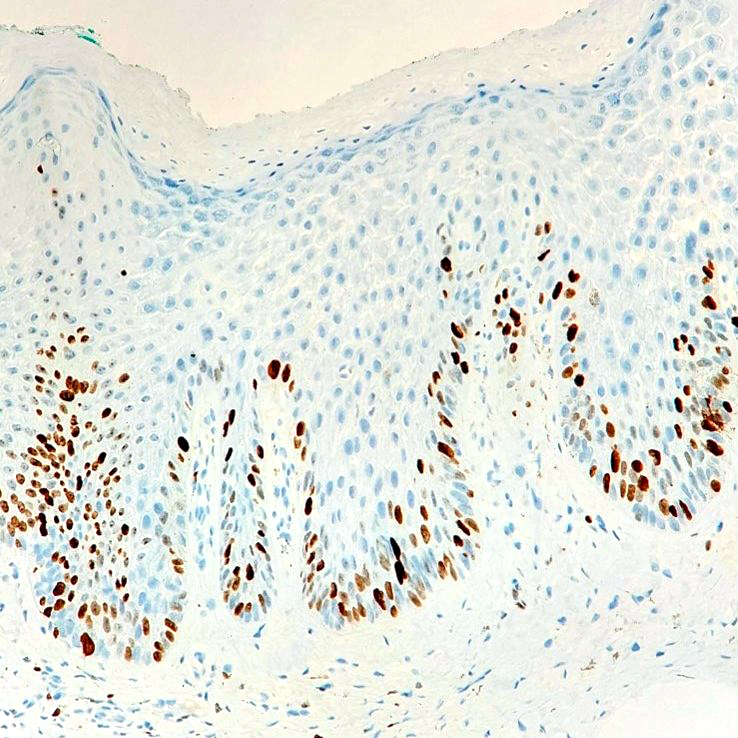
Microscopic (histologic) images
Negative stains
- Negative p16 if without dysplasia (no overexpression / nonblock positivity)
Molecular / cytogenetics description
- In situ hybridization (ISH) and PCR positive for low risk HPV 6 and 11 serotypes
Sample pathology report
- Anus, perianal lesion, biopsy:
- Low grade squamous intraepithelial lesion (AIN 1 / condyloma acuminatum)
Differential diagnosis
- Verrucous carcinoma:
- Pushing invasion of underlying stroma
- Fibroepithelial polyp:
- Lacks papillary proliferation of squamous epithelium, no koilocytes
Additional references
Board review style question #1
A 50 year old HIV positive man is found to have a papillary perianal growth. The H&E section above demonstrates papillary squamous epithelium with koilocytic change in the upper third of the squamous epithelium, without cytologic atypia. p16 immunohistochemical stain is also shown. Which of the following HPV serotypes are associated with this lesion?
- HPV 6 and 11
- HPV 16 and 18
- HPV 31, 33 and 35
- HPV 45
Board review style answer #1
Board review style question #2
According to the Lower Anogenital Squamous Terminology (LAST) project, what is the appropriate terminology for anal condyloma acuminatum?
- AIN 1
- ASIN-L
- Condyloma acuminatum
- LSIL
- LSIL (condyloma acuminatum)
Board review style answer #2
Crohn's disease
Table of Contents
Definition / general | Terminology | Sites | Etiology | Clinical features | Laboratory | Case reports | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosis | Additional referencesDefinition / general
- Anal canal involvement in 25 - 45% of Crohn's patients with small bowel involvement; up to 75% with colonic involvement
Terminology
- Also called granulomatous colitis but this is less specific
Sites
- Anal, anorectal and perianal skin
Etiology
- Etiology of chronic inflammatory bowel disease is unknown - may involve combination of genetic and inflammatory risk factors
- Genetic factors suggested by 10 - 15x increased risk in those with affected first degree relatives, 42 - 58% concordance rate in monozygotic twins
- Environmental factors such as smoking, certain food antigens, NSAID use and infectious agents may be important
- Perianal fistulas may develop from deep fissures or anal gland abscesses
Clinical features
- Symptomatic perianal disease may precede GI symptoms in 5 - 20% of patients (Dis Colon Rectum 1996;39:136, Aust N Z J Surg 1996;66:5, Dis Colon Rectum 1995;38:121)
- The presence of recurrent isolated anal fissures, fistulas or perianal abscesses should raise the suspicion for evolving Crohn's disease; internal fistulas are virtually pathognomic
- Major complications include abscesses, fistulas, anal tags and fissures, which can present as anal pain, purulent discharge, fresh bleeding per rectum or anal incontinence (Am Fam Physician 2010;82:419)
- Disease location and age < 40 years are most common factors associated with perianal complications (Inflamm Bowel Dis 2002;8:244)
- Malignancy (anal canal adenocarcinoma) may be seen in longstanding perianal Crohn's disease (Intern Med 2013;52:445)
- Perianal fistulizing disease is associated with genetic susceptibility involving chromosome 5 with candidate interleukin genes IL3, IL4, IL5, I-13, CSF2 (World J Gastroenterol 2011;17:1939)
Laboratory
- Serum pANCA may be increased in patients with left sided disease with an ulcerative colitis-like clinical phenotype and histological features
Case reports
- 28 year old woman with painful perianal lesions (Am Fam Physician 2010;82:419)
- 50 year old woman with late perianal mucinous adenocarcinoma after Crohn's disease proctectomy (World J Surg Oncol 2005;3:42)
Gross description
- Varies based on location of fistula and associated healing process
- May have firm and fibrotic perirectal areas with adherent perianal skin showing external communication of fistula
- May present as perirectal mass if there has been significant healing
- Some grossing points:
- Recommended to take gross photographs of specimen when fresh
- Communication of fistula is best demonstrated by inserting a blunt metallic probe from the mucosal aspect of unfixed resection specimen - the key is finding the opening of either the sinus or fistula tract at the mucosal aspect
- Formalin fixation of these specimens is best accomplished by opening the luminal aspect of colon in a longitudinal direction and pinning the specimen flat
- This technique may be challenging for large resection specimens with long lengths of colon
- Adhesions or fistulae to other visceral organs or parts of the bowel may also be present, distorting the specimen and making orientation difficult
Microscopic (histologic) description
- Features of acute colitis: cryptitis (neutrophils in crypt epithelium), crypst abscesses (neutrophils within crypt lumens), erosions, ulcers
- Features of chronic colitis: crypt distortion, loss of goblet cells, basal plasmacytosis, crypt shortfall (base of crypts not touching the muscularis mucosae), Paneth cell metaplasia in left colon
- Nonnecrotizing granulomatous inflammation with variable giant cells in mucosa or fibroconnective tissue of fistula tract (may resemble foreign body type granulomas)
- Transmural chronic inflammation (best visualized on resection specimens)
- Patchy mucosal involvement with skip lesions which looks near normal on histology or may have mild reactive epithelial changes
- Acute and chronic inflammatory granulation tissue secondary to ulceration and fistula formation
Microscopic (histologic) images
Differential diagnosis
- Perianal tuberculosis and fungal infections
- Rare ulcerative colitis induced perirectal fistulas: internal fistulas are rare
Additional references
Cytomegalovirus (CMV)
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Cytomegalovirus (CMV) is a double stranded DNA virus and a member of human herpes virus family
- Also known as herpes virus type 5
- 3 patterns of CMV infection:
- Latent infection
- Most common, immunocompetent patients
- Mononucleosis-like syndrome
- Immunocompetent patients
- Tissue invasive disease
- Immunocompromised patients
- Latent infection
- Tissue invasive disease
- Gastrointestinal tract is most commonly involved (30% of tissue invasive cases) (Virol J 2008;5:47)
Essential features
- Double stranded DNA virus and a member of human herpes virus family
- Tissue invasive disease, usually seen in immunocompromised patients
- Most common sites of infection in gastrointestinal tract:
- Colon
- Esophagus
- Symptoms:
- Rectal bleeding (most common)
- Diarrhea
- Abdominal pain
- Fever
- Weight loss
- Microscopy:
- Cytomegalic cells with owl's eye intranuclear viral inclusions
- CMV immunohistochemistry is the gold standard for diagnosis
Terminology
- CMV infection
- CMV antigens or antibodies in blood
- CMV disease
- Clinical symptoms and end organ damage
- CMV colitis
- Identification of characteristic intranuclear / intracytoplasmic inclusions on H&E sections
- Identification of CMV specific antigens by immunohistochemistry (IHC) and clinical symptoms
ICD coding
- ICD-10: B25.8 - other cytomegaloviral diseases
Epidemiology
- Affects 50 - 100% humans worldwide depending on age and race of the population tested
- Infects over half of adults by age 40 (CDC: About Cytomegalovirus (CMV) [Accessed 11 November 2021])
- CMV seroprevalence in the United States is 42 - 93% (Am J Kidney Dis 1991;17:719)
- Immunocompetent patients
- Mean age is 64 - 75 years (Clin Microbiol Infect 2015;21:1121.e1)
- Liver transplant patients
- Cumulative incidence is 4.9% (Transplant Proc 2014;46:832)
- Allogenic hematopoietic stem cell transplant patients
- Incidence is 15 - 25% (Biol Blood Marrow Transplant 2015;21:159)
- Inflammatory bowel disease
- Incidence is 1.5 - 4.5%
- Ulcerative colitis > Crohn’s disease (Eur J Gastroenterol Hepatol 2016;28:1329)
Sites
- Can involve any part of the gastrointestinal tract
- Most common sites:
- Colon
- Esophagus
Pathophysiology
- Spread by saliva, urine, respiratory droplets, sexual contact, breast milk and blood transfusions (Clin Microbiol Rev 1989;2:204, Nihon Rinsho 1998;56:179)
- After initial infection, CMV resides latently in monocytes, fibroblasts, myeloid cells and endothelial cells
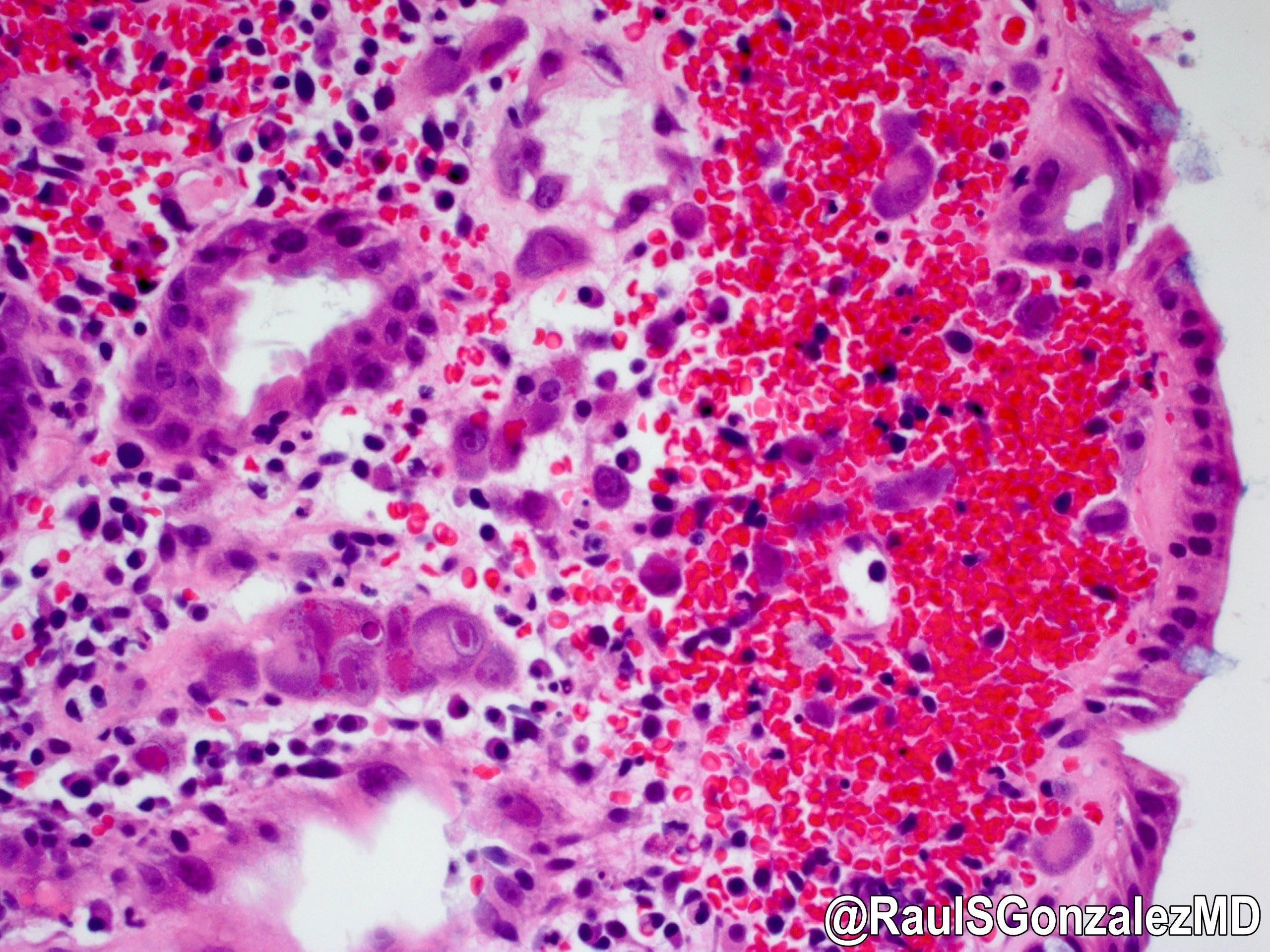
- Tissue invasive disease in colon can lead to ulcerative changes, erosion into blood vessels (causing bloody diarrhea), inflammatory polyps, severe inflammation and vasculitis leading to ischemia and transmural necrosis
Etiology
- Cytomegalovirus (CMV)
- Most commonly in immunocompromised patients
- History of AIDS, organ transplantation, hematologic malignancy, cancer therapy and corticosteroid therapy
- Risk factors in immunocompetent patients:
- Renal disease
- Hemodialysis
- Neurological disease
- Rheumatic disease
- Exposure to antibiotics
- Antacids
- Corticosteroid
- Red blood cell transfusion within 1 month of diagnosis of colitis (Clin Infect Dis 2015;60:e20)
- Severe ulcerative colitis (patients treated with high dose corticosteroids)
Clinical features
- Rectal bleeding (most common)
- Diarrhea, abdominal pain, fever, weight loss (StatPearls: Cytomegalovirus Colitis [Accessed 12 November 2021])
Diagnosis
- Surgical resection specimen or biopsy: histopathologic diagnosis
- Clinical symptoms, endoscopic findings, serologic testing, polymerase chain reaction (PCR) and culture
Laboratory
- Histology:
- Gold standard test
- Immunohistochemistry (IHC) for CMV
- Greater sensitivity than hematoxylin & eosin (H&E)
- H&E can detect CMV infected cells
- Cells larger than normal, containing intranuclear or intracytoplasmic inclusions
- CMV infected cells can be confirmed by IHC staining
- Immunohistochemistry (IHC) for CMV
- Gold standard test
- Serology:
- Acute infection
- CMV IgM antibodies
- 4 times increase in titer of CMV IgG specific antibodies 2 - 4 weeks apart
- CMV antigenemia
- Predictor of clinical outcomes
- Less sensitive for diagnosis of CMV colitis
- Real time polymerase chain reaction (PCR) / CMV DNA quantification
- Positive in only 50% of patients with biopsy proven CMV colitis / enteritis
- CMV culture
- High sensitivity and specificity for diagnosis of CMV colitis
- Takes longer to obtain results (1 - 3 weeks)
- May delay diagnosis and timely treatment (J Clin Microbiol 1993;31:2851)
- Acute infection
Radiology description
- Computed tomography:
- Nonspecific findings
- Bowel wall thickening
- Mural edema
- Pericolonic fat stranding
- Free fluid, free air
- Lymphadenopathy (Radiology 1985;155:585)
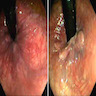
- Endoscopic findings:
- Easy bleeding, loss of vascular pattern, mucosal edema, erythema, mucinous exudate and wide mucosal defect
- Mucosal defects, punched out ulcers (most common), longitudinal ulcers, irregular ulceration or cobblestone appearance (Emerg Radiol 2020;27:277)
Prognostic factors
- Excellent overall prognosis
- Factors associated with poor prognosis and higher mortality (immunocompetent patients)
- M > F
- Age > 55 years
- Patients requiring surgery
- CMV colitis reactivation with ulcerative colitis, tends to have poorer prognosis
- Timely diagnosis and treatment greatly improves clinical outcomes (StatPearls: Cytomegalovirus Colitis [Accessed 12 November 2021])
Case reports
- 42 year old HIV+ man with progressive abdominal pain, palpable right umbilical mass, fever, asthenia and weight loss (Radiol Case Rep 2018;14:273)
- 48 year old woman with severe abdominal pain and constipation (World J Clin Cases 2021;9:5631)
- 60 year old man with history of sarcoidosis and chronic kidney disease with lower abdominal pain and diarrhea (Indian J Nephrol 2021;31:73)
- 71 year old man with history of posttuberculous aspergilloma admitted to ICU with acute respiratory distress syndrome (Clin Case Rep 2020;9:e03600)
Treatment
- Intravenous (IV) ganciclovir (5 mg/kg twice daily)
- After 3 - 5 days of IV ganciclovir, oral valganciclovir (900 mg, twice daily) (Clin Gastroenterol Hepatol 2015;13:949)
- Foscarnet in ganciclovir resistant or tolerant cases
Clinical images
Gross description
- Nonspecific findings
- Inflamed mucosa, hyperemia, mucosal sloughing
- Punched out ulcers, aphthous ulcers, exudate
- Pseudomembrane formation (Arch Pathol Lab Med 2016;140:854)
Gross images
Microscopic (histologic) description
- Most commonly affected cells:
- Endothelial cells
- Mesenchymal cells
- Macrophages
- Larger (cytomegalic) cells:
- Usually 25 - 35 micrometers
- Typically 2 - 4 times larger than normal
- Owl’s eye:
- Large ovoid or pleomorphic nucleus with basophilic intranuclear inclusions (Cowdry bodies) surrounded by a clear halo (Arch Pathol Lab Med 2016;140:854)
- Thickened nuclear membrane
- Coarse red intracytoplasmic granules (Int Med Case Rep J 2011;4:55)
- Increased apoptotic bodies
- Expansion of lamina propria by mixed inflammatory plasma cell rich infiltrate
- Neutrophilic inflammation
- Should raise suspicion for CMV infection in graft versus host disease and mycophenolate injury (Int J Surg Pathol 2018;26:347, J Clin Pathol 2013;66:8)
- Deep fissuring ulcers, cryptitis, crypt abscess, architectural distortion and pseudopolyp formation
- May mimic inflammatory bowel disease (Am Surg 2007;73:58)
- Submucosal vasculitis and thrombosis of microvessels
Microscopic (histologic) images
Positive stains
- Immunohistochemistry for CMV
Negative stains
- Immunohistochemistry for adenovirus, HSV1, HSV2
Molecular / cytogenetics description
- Real time DNA PCR amplification method:
- Rapid results, high negative predictive value
- Contradictory reports regarding sensitivity
- CMV DNA load > 250 copies/mg tissue may predict resistance to steroid treatment in ulcerative colitis (Am J Gastroenterol 2011;106:2001)
Videos
CMV colitis in ulcerative colitis and immunocompromised states
Sample pathology report
- Colon, random, biopsy:
- CMV colitis
- Immunohistochemical stain for CMV is positive
- Colon, colectomy:
- CMV colitis in the background of severely active ulcerative pancolitis
- Immunohistochemical stain for CMV is positive
- Negative for dysplasia
Differential diagnosis
- Infectious colitis:
- Adenovirus:
- Commonly infects surface epithelial cells
- Homogenous glassy amphophilic nuclear inclusions
- HSV1, HSV2:
- Chromatin margination, multinucleation, nuclear molding
- Adenovirus:
- Graft versus host disease:
- Crypt apoptosis
- Crypt dropout
- Ulceration
- No cytomegalic inclusions
- May coexist with CMV infection
- Inflammatory bowel disease:
- No inclusions
- Ulcerative colitis, Crohn's disease
- Mycophenolate mofetyl induced colitis:
- No inclusions
- Increased apoptosis
- Increased lamina propria eosinophils
Additional references
Board review style question #1
A 71 year old man presents with abdominal pain and diarrhea. Colonoscopy showed diffuse mucosal erythema and irregular ulcerations. The patient undergoes biopsy of the lesion. What is the infected cell and organism causing the histopathologic findings?
- Endothelial cell, adenovirus
- Endothelial cell, cytomegalovirus
- Epithelial cell, adenovirus
- Epithelial cell, cytomegalovirus
Board review style answer #1
B. Endothelial cell, cytomegalovirus. The images show cytomegalic endothelial cells with inclusions. Adenovirus typically affects epithelial cells and shows amphophilic nuclear inclusions.
Comment Here
Reference: Cytomegalovirus (CMV)
Comment Here
Reference: Cytomegalovirus (CMV)
Board review style question #2
What is the gold standard method for diagnosing CMV colitis?
- CMV culture
- CMV DNA polymerase chain reaction (PCR) amplification method
- CMV serology
- Immunohistochemistry for CMV
Board review style answer #2
Fissure
Table of Contents
Definition / general | Terminology | Epidemiology | Diagrams / tables | Pathophysiology | Clinical features | Treatment | Microscopic (histologic) description | Positive stainsDefinition / general
- Most common benign lesion resulting from high intraluminal anal pressure
- Painful linear separation or tear of anal canal mucosa distal to dentate line and extending either superficial or deep into anal mucosa
- Fissures are most commonly located at posterior commissure overlying external and internal sphincter bifurcation as it divides to circle the rectum, implying that the sphincter cuff is weakest at posterior anal wall
- Most commonly post traumatic and located in midline; nonmidline anal fissures should raise suspicion of malignancy, inflammatory bowel disease or infection
- Trauma may be due to constipation, instrumentation, childbirth or sexual abuse
- Nontraumatic cases include infections (tuberculosis, cytomegalovirus, herpes, syphilis, gonorrhea, chlamydia), inflammatory bowel disease (4% of Crohn's disease patients have fissures as initial manifestation) or malignancy involving anal mucosa
- Histopathology is generally nonspecific but may have etiology specific features
Terminology
- Acute anal fissure: typically heals after conservative treatment in < 6 weeks
- Chronic anal fissure: resistant to conservative treatment and may require surgical management
Epidemiology
- Most commonly affects infants and young / middle aged individuals
- Equally prevalent in males and females
Diagrams / tables
Pathophysiology
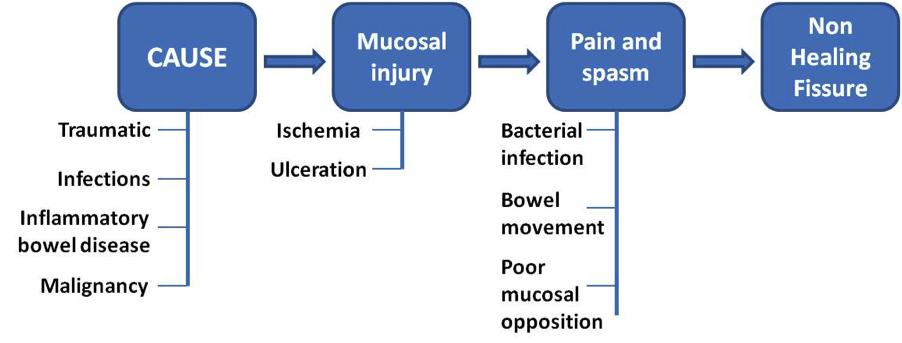
- The basic mechanism of injury is stretching of the anal mucosa beyond its physiological limits, which causes mucosal defects in areas with poor muscular support
- The initiation of a mucosal tear leads to a vicious cycle of healing and repeated injury due to stretching from continuous bowel movements
- The impaired healing and pain leads to persistent spasm of the internal sphincter and increased mean average resting luminal pressure
- The persistent spasm leads to nonopposition of the tear, impaired wound healing, bacterial colonization and progression to chronic anal fissure in up to 40% of patients (World J Gastrointest Pharmacol Ther 2011;2:9)
Clinical features
- 90% of fissures are located in the posterior anal wall
- Midline anterior fissures are more common in females due to anatomical location of vagina and related weak muscular support
- Acute fissures usually have severe tearing pain associated with passage of stools, bright red rectal bleeding evident as streaks of blood, perianal eczema and pruritis
- Chronic fissures are usually less painful
- Physical examination may show a midline defect, superficial tear or laceration of variable depth and size depending on cause and duration
- Chronic fissure may appear as hypertrophied skin tag
Treatment
- Most common treatment is medical and conservative, which includes warm baths and increased fiber intake
- Warm sitz baths may lead to healing of anal fissures via a somatoanal reflex that results in relaxation of internal anal sphincter
- Better healing occurs with topical steroids and local topical anesthetics but caution and medical supervision is advised before using topical agents
- Operative management is indicated after failure of conservative or more extensive medical management (Clin Colon Rectal Surg 2011;24:22)
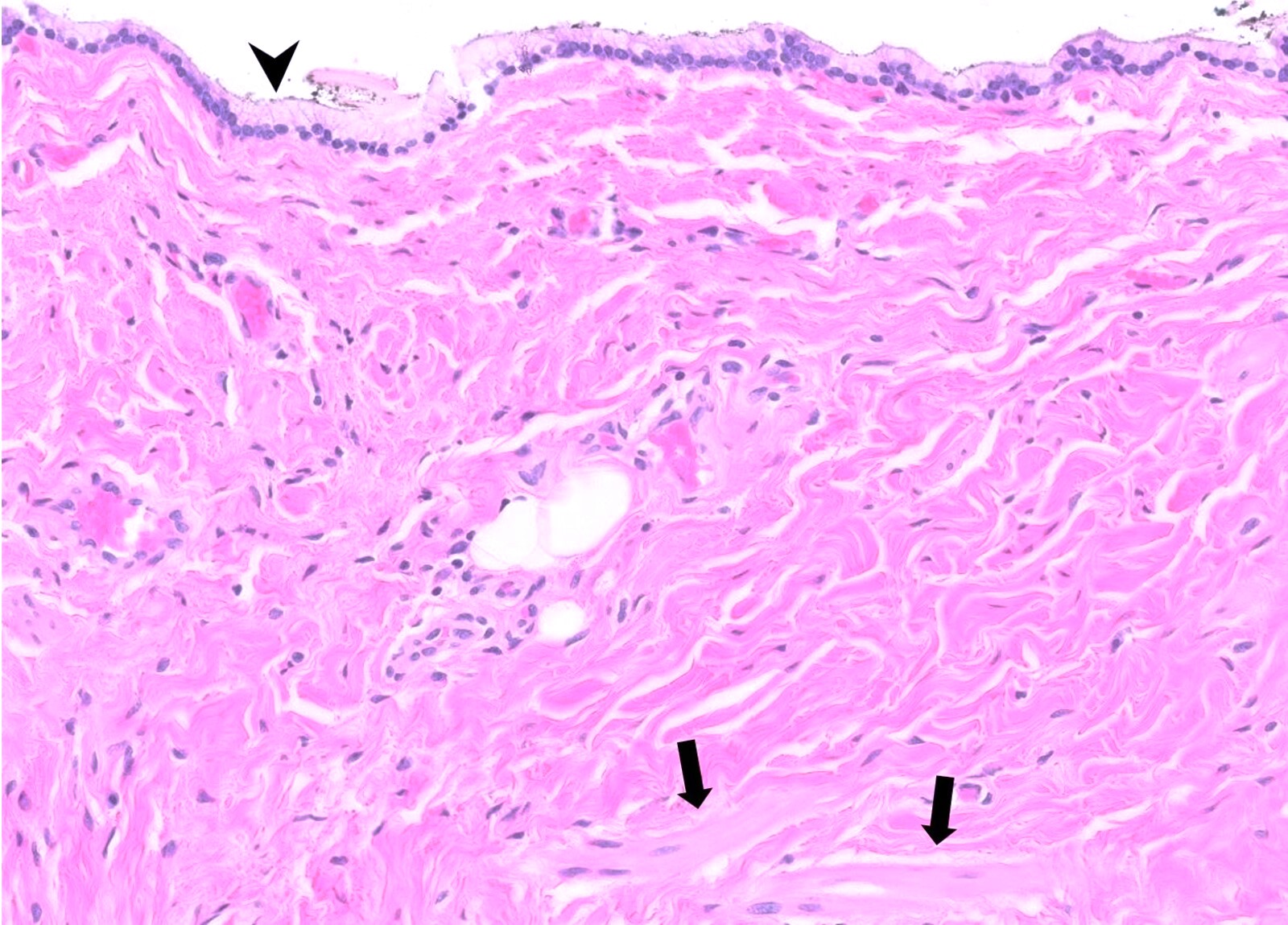
Microscopic (histologic) description
- May be nonspecific; shows ulcerated transitional or columnar mucosa with acute and chronic inflammation, granulation tissue, reactive and regenerative epithelial changes or foreign body giant cell reaction
- Anal fissures related to specific etiology may show epithelioid cell granulomas as in tuberculosis, fungal infection or Crohn's disease (Quizlet: Gastrointestinal Pathology [Accessed 15 October 2021])
- Lymphogranuloma venereum and syphilis induced ulcers may show intense inflammation rich in plasma cells (Am J Surg Pathol 2013;37:38)
Positive stains
- For tuberculosis, acid fast bacilli stains may be positive (Indian J Dermatol Venereol Leprol 2008;74:386)
- Also cultures from anal discharge (Isr Med Assoc J 2013;15:782)
Fistula
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Videos | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
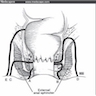
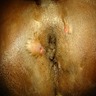
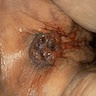
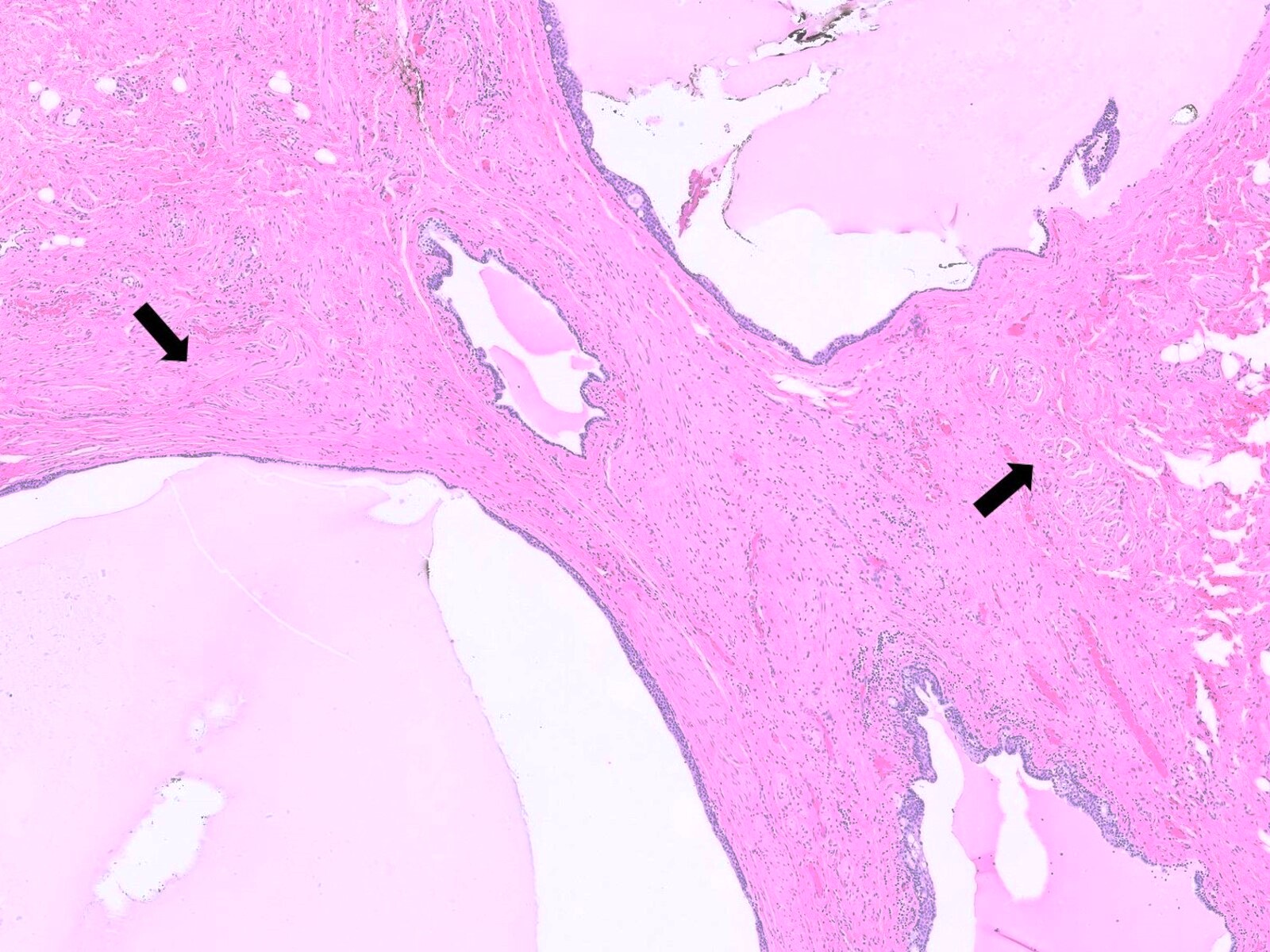
- Anal fistulas are epithelialized tracts created between an external opening in the perianal skin and an internal opening in the anal canal
- Primary opening usually leads to skin or may end blindly in perianal soft tissue (most commonly ischiorectal fossae)
Essential features
- Benign epithelialized communication between anal canal and perianal skin that is most commonly idiopathic
Terminology
- Other names: fistula in ano, cryptoglandular (anal) fistula
- Parks classification of anal fistulae based on anatomical location (Br J Surg 1976;63:1):
- Intersphincteric: fistula located in intersphincteric plane between external and internal anal sphincters
- Tract begins at dentate line and ends at anal verge
- Transsphincteric: tract communicates through external sphincter to ischiorectal fossa
- External opening is located on skin
- Suprasphincteric: tract originates higher in anal gland crypt, extends through all sphincter muscles and ends in ischiorectal fossa
- Extrasphincteric: tract located very high and proximal to dentate line and extends through levator muscles and entire sphincter apparatus
- Intersphincteric: fistula located in intersphincteric plane between external and internal anal sphincters
ICD coding
Epidemiology
- Most anal fistulas are idiopathic
- 15 - 38% of anal fistulas develop from anal abscesses
- Occurs in males at a rate 2 - 4 times higher than females
- Mean age of occurrence is 40 years with a range of 20 - 60 years (Dis Colon Rectum 2009;52:217)
- Recurrent disease is more common in patients Dis Colon Rectum 2009;52:217)
- Recent smoking increases risk of development of anal fistula (Dis Colon Rectum 2005;48:575)
Sites
- Anal canal and external perianal region
Pathophysiology
- Infection and occlusion of the anal glands by gut specific bacteria leads to abscess formation → inflammation and granulation tissue → epithelialized connection between anal canal and external perianal area
Etiology
- Most commonly identified causes of anal fistulas are Crohn's disease (complex fistulae with irregular edges), infections (tuberculosis [lung disease usually present] and lymphogranuloma venereum) and rectal foreign bodies (Nat Rev Gastroenterol Hepatol 2017;14:652)
- Uncommon causes of anal fistula development include:
- Iatrogenic and fulminant ulcerative colitis
- Actinomyces (rare perianal manifestation, mostly in immunocompromised hosts) (Dis Colon Rectum 2005;48:575, Proc R Soc Med 1970;63:108, JAMA 1974;228:1397)
- Diverticulosis
- Obstetric trauma or drainage of abscess
- Pelvic radiation
- Malignancy (anal canal adenocarcinoma rarely develops in background of chronic anal fistula) (Singapore Med J 2012;53:843, Intern Med 2013;52:445)
Diagrams / tables
Clinical features
- Typical presentation includes itching, drainage, discomfort and possible pain with defecation
- Small opening outside the anus, with or without visible drainage, may be seen
- Around the opening, there may be hypertrophied tissue, which is suggestive of a developed tract; this is sometimes palpated on the digital rectal exam
- There may be associated clinical features secondary to the causative agent (inflammatory bowel disease, radiation therapy, etc.) (StatPearls: Anorectal Fistula [Accessed 8 July 2022])
Diagnosis
- Examination of the anal area (fistula probe, anoscopy / proctoscopy, ultrasound, MRI) is essential to evaluate for the location of the primary opening
- Presence of fistula opening in the anal area with the clinical features (listed above) confirms the diagnosis
Laboratory
- No specific laboratory tests
- Serological studies for inflammatory bowel disease, microbial cultures for infectious organisms and metabolic profile for associated comorbidities can be used for causative agents
Radiology description
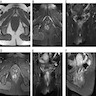
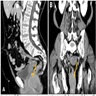
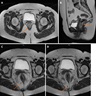
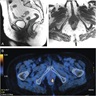
- Radiologic imaging is important in determining the patient's surgical treatment
- MRI clearly shows the relationship of fistulas to the pelvic diaphragm (levator plate) and the ischiorectal fossae; this relationship has important implications for surgical management and outcome and has been classified into 5 MRI based grades:
- If the ischioanal and ischiorectal fossae are unaffected, disease is likely confined to the sphincter complex (simple intersphincteric fistulization, grade 1 or 2); outcome following simple surgical management is favorable
- Involvement of the ischioanal or ischiorectal fossa by a fistulous track or abscess indicates complex disease related to transsphincteric or suprasphincteric disease (grade 3 or 4); correspondingly more complex surgery may be required that may threaten continence or may require colostomy to allow healing
- If the track traverses the levator plate, a translevator fistula (grade 5) is present and a source of pelvic sepsis should be sought (Radiographics 2000;20:623)
Prognostic factors
- Features of complex fistulas or those that are at high risk of failing traditional treatment with a primary fistulotomy (Surg Clin North Am 2010;90:45):
- Fistulas that anatomically are suprasphincteric, high transsphincteric (> 30% of sphincter), extrasphincteric according to the Parks classification
- Recurrent fistulas
- Fistulas with multiple tracts
- Females with anterior fistulas
- Fistulas related to inflammatory bowel disease, infectious diseases, with or without radiation
- Anal incontinence
- Rectovaginal fistulas
Case reports
- 26 year old woman with a complex perianal fistula with actinomyces spp. present in anal cytology (ACG Case Rep J 2017;4:e82)
- 47 year old man with a 20 year history of multiple chronic perianal fistulas with extensive squamous cell carcinoma arising from perianal fistula (J Crohns Colitis 2013;7:e232)
- 53 year old man with a 20 year history of anal fistula with implanted rectal adenocarcinoma (Surg Today 2006;36:747)
Treatment
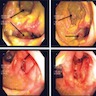
- Exam under anesthesia is the most common first step of a suspected fistula, to determine complexity and characteristics
- Simple fistula is treated with a primary fistulotomy with incision made along fistula tract for curettage and to promote adequate tract drainage and wound healing; this is very curative
- Any complex fistulas must employ approaches that spare the sphincter based on the anatomy of the fistula and clinical features (Surg Clin North Am 2010;90:45)
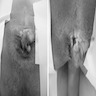
Gross description
- Typically unoriented, irregular shaped soft tissue excision with an inflamed sinus opening on one surface
- When the probe followed from the sinus opening, cut surface shows the sinus tract
- Hemorrhage, abscess with yellow-green pus or scarring may be seen around the sinus tract
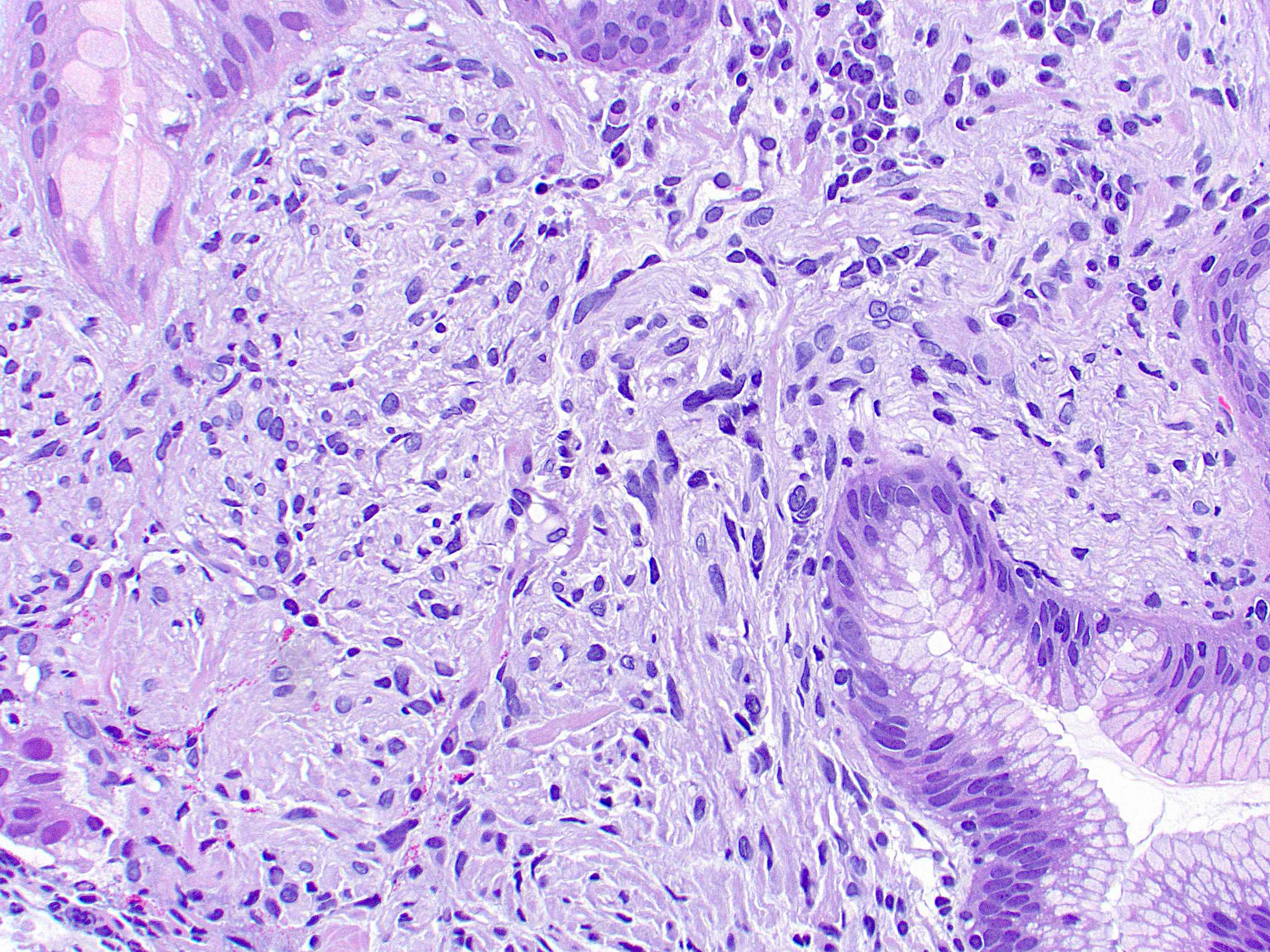
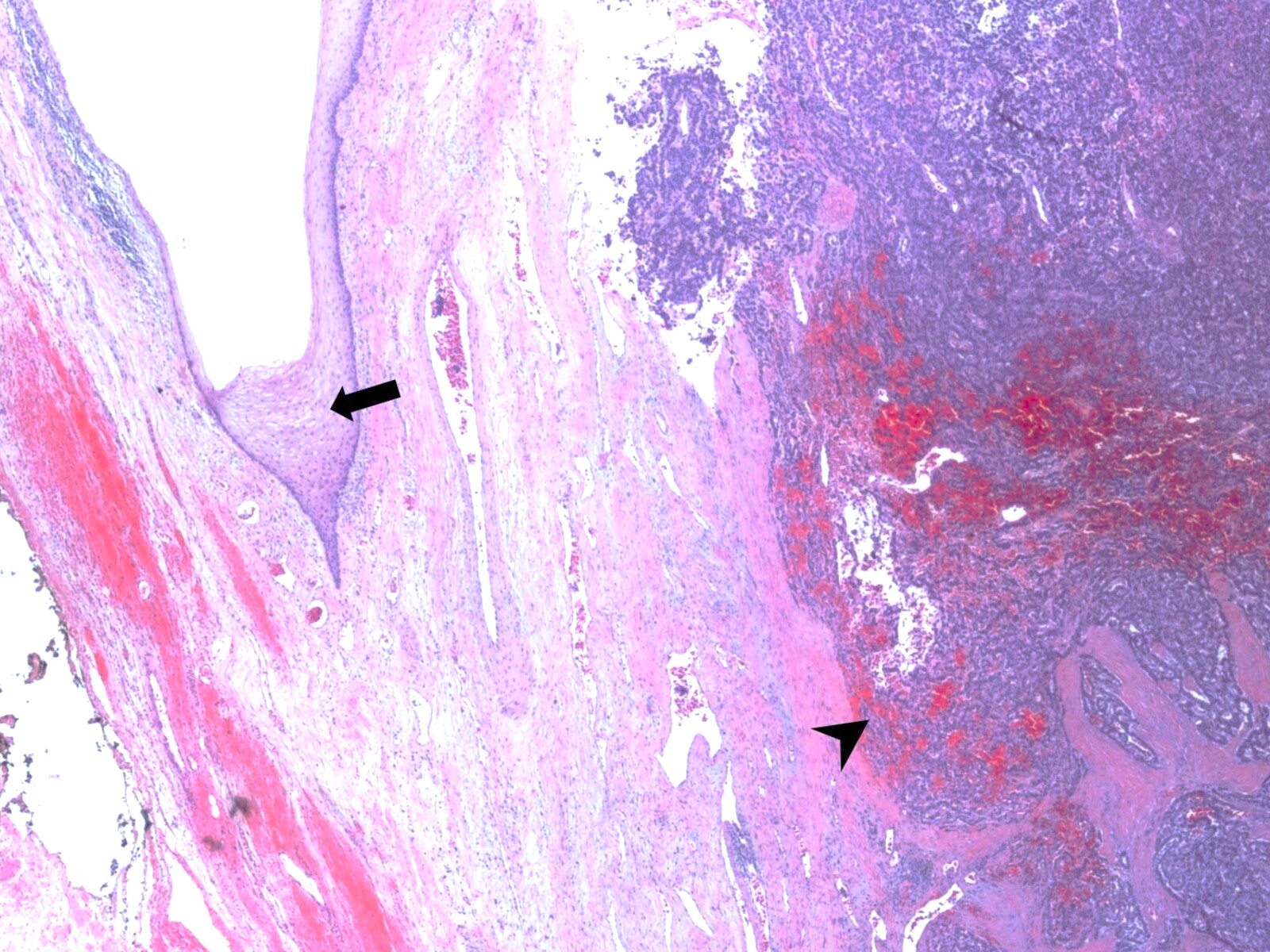
Microscopic (histologic) description
- Histological features vary based on etiology, duration of disease and presence of infection / abscess
- Fibroconnective tissue usually shows variable scarring with mixed acute and chronic inflammation
- If it is inflamed, microabscess, inflammatory granulation tissue with reactive endothelial cells, fibroblastic proliferation, granulomas, histiocytic response and foreign body type giant cells can be seen
- Granulomas and giant cells may require special stains for mycobacteria and fungal organisms
Microscopic (histologic) images
Videos
Perianal fistula
Fistula in ano
Sample pathology report
- Anal fistula, excision:
- Benign anal mucosa with acute inflammation, consistent with anal fistula (see comment)
- Comment: The H&E sections show benign anal mucosa with acute inflammation, fat necrosis and foreign body giant cell reaction. The special stains for AFB and GMS are negative for acid fast bacillus and fungal microorganisms, respectively. Overall, the histological findings are consistent with anal fistula.
Differential diagnosis
- Hidradenitis suppurativa (HS):
- Chronic, autoinflammatory skin disease in the intertriginous body areas, such as the axillae and inguinal areas but also the buttocks and perianal area
- Presents with recurring abscesses, inflammatory nodules and sinus tracts
- When located in the perianal area, it may be difficult to distinguish from the anal fistula (Int J Colorectal Dis 2019;34:1337)
- Histologic findings are similar with the perianal fistula (chronic inflammation, granulation tissue and epithelized tract)
- Transperineal ultrasound and anal ultrasound may be helpful to show the internal and external orifices of the perianal fistula and to distinguish it from hidradenitis suppurativa (Postepy Hig Med Dosw (Online) 2012;66:838)
- Infected cysts (e.g., sebaceous, Bartholin) with draining tracts:
- Location of the cysts and the absence of a sinus tract helps to differentiate the diagnosis
- Sinus tracts from trauma or foreign body:
- Obtaining a clinical history, detailed examination and careful observation allows for detection of a foreign body or trauma history
- Specific infections (tuberculosis, actinomycosis, lymphogranuloma venereum, gonococcal infection, etc.):
- Blood cultures are helpful if there is any suspicion of a specific infection
- Granulomatous inflammations (necrotizing or nonnecrotizing) should be evaluated carefully for infectious causes
Board review style question #1
Board review style answer #1
A. Anal fistula. An anal fistula is an epithelialized connection between the anal canal and perianal skin.
Comment Here
Reference: Fistula
Comment Here
Reference: Fistula
Board review style question #2
What is the most common cause of anal fistula?
- Adenocarcinoma
- Fungal infection
- Idiopathic
- Pelvic radiation
Board review style answer #2
C. Idiopathic. The most common etiology of anal fistulas is idiopathic. Less common causes include inflammatory bowel disease, infections, radiation and trauma, among others.
Comment Here
Reference: Fistula
Comment Here
Reference: Fistula
Granular cell tumor
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Rare, benign anal neoplasm with neuroectodermal differentiation derived from Schwann cells and composed of epithelioid cells with abundant granular cytoplasm
Essential features
- Benign tumor with neuroectodermal differentiation
- Composed of oval to polyhedral cells with abundant eosinophilic granular cytoplasm
- Positive for S100 and CD68
Terminology
- Older terms, not recommended: Abrikossoff tumor, granular cell myoblastoma, granular cell schwannoma, granular cell nerve sheath tumor
ICD coding
- ICD-O
- ICD-10: D12.9 - benign neoplasm of anus and anal canal
- ICD-11
- 2E8Y & XH09A9 - other specified benign mesenchymal neoplasm & granular cell tumor, NOS
- XH90D3 - granular cell tumor, malignant
Epidemiology
- Granular cell tumors can occur at any age but are more common in adults in the fourth to sixth decades of life (StatPearls: Granular Cell Tumor [Accessed 29 November 2023])
- Strong predilection for women and Black patients (StatPearls: Granular Cell Tumor [Accessed 29 November 2023])
Sites
- Granular cell tumor can arise in various sites, including skin, head and neck, soft tissue, breast, gastrointestinal tract and peripheral nerves (Gastroenterol Hepatol (N Y) 2009;5:798)
- ~5 - 10% arise in the gastrointestinal tract, with the most common site being esophagus (StatPearls: Granular Cell Tumor [Accessed 29 November 2023])
- Very rarely occurs in anus and perianal area (scattered case reports only)
Pathophysiology
- Inactivating somatic mutations in the endosomal pH regulators ATP6AP1 and ATP6AP2 are seen in 72% of granular cell tumors (Nat Commun 2018;9:3533)
- Abnormal RAS-MAPK pathway cell signaling may be linked in granular cell tumors associated with syndromes (Clin Genet 2009;75:185)
Etiology
- Unclear for sporadic tumors
- Patients with multiple tumors may have Noonan syndrome, LEOPARD syndrome or neurofibromatosis type 1 (StatPearls: Granular Cell Tumor [Accessed 29 November 2023])
Clinical features
- Mostly discovered incidentally during endoscopy for other reasons, though may cause symptoms such as perianal discomfort, ulceration and bleeding
- Slow growing, painless anal lesion presenting as a polyp
Diagnosis
- Tissue sampling
Radiology description
- Ultrasound: hypoechoic mass (J Int Med Res 2021;49:300060520982689)
- Magnetic resonance imaging: ovoid and solid mass with low signal intensity on T1 weighted image and high intensity on T2 images (Radiol Case Rep 2019;14:1047)
Prognostic factors
- Most granular cell tumors are benign and do not recur or spread
- Size > 5 cm and metastatic behavior indicate poor prognosis (J Surg Oncol 2018;118:891)
- Metastatic behavior is the only diagnostic criterion for malignancy (Pathol Res Pract 2011;207:164)
Case reports
- 36 year old woman with slow growing painless lump in perianal region (Curr Probl Diagn Radiol 2017;46:452)
- 46 year old woman with perianal nodular lesion (Tumori 2009;95:538)
- 56 year old man with papule of the anus (Presse Med 2003;32:221)
- 66 year old woman with polypoid lesion in the anus with hemorrhoids (Int Surg 2014;99:45)
- 75 year old woman with granular cell tumor of the internal anal sphincter (Dis Colon Rectum 2000;43:1444)
Treatment
- Local excision
Gross description
- Poorly circumscribed, firm, tan-yellow to tan-white nodule
Microscopic (histologic) description
- Poorly circumscribed sheets, nests, lobules or ribbons of cells within collagenous stroma
- Longstanding lesions can show desmoplastic stroma (StatPearls: Granular Cell Tumor [Accessed 29 November 2023])
- Monotonous oval to polyhedral to spindled cells with distinct cell borders, abundant eosinophilic granular cytoplasm and rare mitotic figures
- Large eosinophilic dense granules called phagolysosomes are frequently seen (StatPearls: Granular Cell Tumor [Accessed 29 November 2023])
- Nuclei are eccentrically located, with regular nuclear borders and inconspicuous nucleoli (StatPearls: Granular Cell Tumor [Accessed 29 November 2023])
- Perineural invasion can be seen (Am J Dermatopathol 2012;34:800)
- Pseudoepitheliomatous hyperplasia can be seen in the overlying skin (Dig Dis Sci 1981;26:807)
- Atypical cases may show vesicular nucleoli with prominent nucleoli, high N:C ratio, increased mitotic activity and geographic necrosis (Virchows Arch 2016;468:527)
Microscopic (histologic) images
Positive stains
Negative stains
Electron microscopy description
- Cytoplasmic lysosomes with prominent myelin figures (J Surg Oncol 1980;13:301)
Molecular / cytogenetics description
- Soft tissue cases often have loss of function mutations in ATP6AP1 and ATP6AP2; anal cases have not been tested
Sample pathology report
- Anal, mass, resection:
- Granular cell tumor, 1.1 cm (see comment)
- Surgical margins negative for tumor
- Comment: Immunohistochemical stains show the tumor is positive for S100 and CD68. Granular cell tumors are rare in the anus but have been reported.
Differential diagnosis
- Adult rhabdomyoma:
- Benign tumor with skeletal muscle differentiation
- Also shows round cells with eosinophilic cytoplasm
- Cells show multiple nuclei and peripheral cross striations
- Positive for desmin, muscle specific actin, myoD1, myoglobin
- Alveolar soft part sarcoma:
- Squamous cell carcinoma:
- Most common malignancy of anus
- Architecture of pseudoepitheliomatous hyperplasia overlying granular cell tumor may mimic malignancy
- Squamous cell carcinoma shows clear cytologic atypia
Board review style question #1
Board review style answer #1
C. Granular cell tumor. The histologic appearance and immunohistochemical profile are consistent with granular cell tumor. Answers A, B and D are incorrect because the other lesions are negative for S100 and CD68. Alveolar soft part sarcoma and squamous cell carcinoma would show more cytologic atypia and gastrointestinal stromal tumor would likely be spindled.
Comment Here
Reference: Granular cell tumor
Comment Here
Reference: Granular cell tumor
Board review style question #2
Which of the following statements is true for granular cell tumor of the anus?
- It can never metastasize
- It is a fast growing painful lesion that infiltrates into the surrounding structures
- It is the most common site for granular cell tumors in the digestive tract
- It may arise in syndromic patients
Board review style answer #2
D. It may arise in syndromic patients. Multiple granular cell tumors can occur in patients with Noonan syndrome, LEOPARD syndrome and neurofibromatosis 1. The anus is a potential site. Answer B is incorrect because granular cell tumors are slow growing and painless. Answer A is incorrect because they very rarely metastasize. Answer C is incorrect because the esophagus is the most common site for digestive tract granular cell tumors.
Comment Here
Reference: Granular cell tumor
Comment Here
Reference: Granular cell tumor
Granuloma inguinale
Table of Contents
Definition / general | Epidemiology | Sites | Clinical features | Diagnosis | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Differential diagnosis | Additional referencesDefinition / general
- Synonym "donovanosis"
- Causative organism was previously classified as Calymmatobacterium granulomatosis; subsequent molecular characterization reclassified the causative organism as Klebsiella granulomatis
- Gram negative, nonmotile and encapsulated organism
- May extend to perianal region
Epidemiology
- Most common in tropical regions, no gender preference
- Bacterial infection associated with sexually transmitted disease or via childbirth with infected genital tract
- Development of lesion requires repeated exposure because of low pathogenicity of organism
- Fewer than 100 cases reported annually in United States
- Untreated lesions may have superadded infections, progression to extensive fibrosis, obstruction of lymphatic vessels and massive lymphedema
Sites
- Penile ulcers (sulcocoronal and balanopreputial)
- Labia minora
- Fourchette
- Cervix (uncommon)
Clinical features
- Median incubation period is ~50 days but exact incubation period is unknown
- There are four predominant types of clinical presentations:
- Nodular
- Ulcerovegetative
- Cicatricial
- Verrucous
- The lesion starts as an elevated nodular or papular area which then progresses to anulcerated lesion with communicating satellite nodules and ulcerations
- Anal involvement is more common in men
- Extragenital involvement occurs in minority of cases (6%) and results from direct or local spread
Diagnosis
- Made by swabbing the lesion and Giemsa staining of the air dried smear
- Other stains that may be used are Warthin-Starry, Gram stain, Toluidine blue and Leishman stain
- Recommended to acquire specimen at base or edge of ulceration or by aspirating enlarged regional lymph node
- Culture of the organism is difficult and needs specialized methods using human peripheral blood mononuclear cells or Hep - 2 cells
- Polymerase chain reaction and indirect immunofluorescence are available but not commonly used
Case reports
- 46 year old man with cutaneous metastases of rectal mucinous adenocarcinoma mimicking granuloma inguinale (Intern Med 2012;51:2479)
- HIV+ patient with malignant transformation (Dermatol Online J 2008;14:8)
Treatment
- Either trimethoprim / sulfamethoxazole or tetracyclines (doxycycline) for at least 3 weeks (choice is based on allergies, pregnancy or other clinical conditions)
- Resistant lesions are treated longer
Gross description
- Varies based on age of lesion and immunosuppression
- Ulceration with ragged beefy red edges and indurated base
- Ulcer size ranges from 0.5 cm to 5 cm
- Usually multiple ulcers
Microscopic (histologic) description
- Marked reactive changes in surface epithelium secondary to inflammation and ulceration; marked pseudoepitheliomatous hyperplasia adjacent to ulcer
- Numerous histiocytes and plasma cells, with fewer neutrophils and lymphocytes
- Granulomatous inflammation, neutrophilic microabscesses (particularly in ulcer bed)
- Large vacuolated / foamy macrophages / histiocytes with multiple intracytoplasmic organisms (bacteria are called Donovan bodies, not the histiocytes; have bacillary or coccobacillary appearance); Donovan bodies may be extracellular
- Acute and chronic granulation tissue, fibrosis (dermal and subcutaneous cicatricial) in late stages
- Prominent lymphatic channels
Microscopic (histologic) images
Positive stains
Differential diagnosis
- Infectious causes may have overlapping histology as well as clinical presentations:
- Chancroid
- Herpes simplex
- Lymphogranuloma venereum
- Syphilis
- Tuberculosis (can case massive lymphedema-like donovanosis) (J Med Case Rep 2010;4:369)
- Squamous cell carcinoma: may grossly as well as clinically resemble the ulcerative and verrucous type of granulomas inguinale lesions
Additional references
Grossing & features to report
Table of Contents
Grossing (pending) | Features to report - General | Features to report - Polypectomy (excisional biopsy) - applies to all invasive carcinomas | Features to report - Local excision (transanal disk excision) - applies to all invasive carcinomas | Features to report - Anus resection - applies to all invasive carcinomasGrossing (pending)
Features to report - General
- Mandatory / optional are for accreditation purposes by the American College of Surgeons Committee on Cancer
- Features to report by organization:
Features to report - Polypectomy (excisional biopsy) - applies to all invasive carcinomas
- Mandatory
- Polyp size: at least 1 dimension
- Histologic type: squamous cell carcinoma, adenocarcinoma, mucinous adenocarcinoma, small cell carcinoma, undifferentiated carcinoma, other, carcinoma - cannot determine
- Histologic grade: well, moderate or poorly differentiated, undifferentiated, cannot determine or not applicable; for adenocarcinoma, is based on percentage of tumor that forms glands: well: > 95%, moderate: 50 - 95%, poor: 5 - 49%, undifferentiated: < 5%
- Depth / extent of invasion: no invasion, cannot determine, into lamina propria, into muscularis mucosa, into submucosa
- Resection margin: cannot assess, positive / negative for invasive carcinoma; if negative, closest tumor to mucosal margin is __ mm, carcinoma in situ absent / present at mucosal margin
- Angiolymphatic invasion: absent, present for large / small vessels, indeterminate
- Recommended if known but not required for accreditation purposes
- HPV status
- Tumor site
- Polyp configuration: pedunculated, sessile, unknown
- Additional findings: none, colitis, other
Features to report - Local excision (transanal disk excision) - applies to all invasive carcinomas
- Mandatory
- Specimen type: intact, fragmented, other
- Tumor size: at least 1 dimension
- Histologic type: squamous cell carcinoma, adenocarcinoma, mucinous adenocarcinoma, small cell carcinoma, undifferentiated carcinoma, Paget disease, other, carcinoma - cannot determine
- Histologic grade: well, moderate or poorly differentiated, undifferentiated, cannot determine or not applicable; for adenocarcinoma, is based on percentage of tumor that forms glands: well: > 95%, moderate: 50 - 95%, poor: 5 - 49%, undifferentiated: < 5%
- Depth / extent of invasion: no invasion, cannot determine, into lamina propria, into muscularis mucosa, into submucosa
- For each resection margin: cannot assess, positive / negative for invasive carcinoma; if negative, closest tumor is __ mm from margin, carcinoma in situ absent / present at margin
- Angiolymphatic invasion: absent, present for large / small vessels, indeterminate
- pTNM and stage
- Recommended if known but not required for accreditation purposes
- HPV status
- Tumor site
- Tumor configuration: polypoid, infiltrative, ulcerating, other
- Perineural invasion: absent, present
- Additional findings: none, Crohn’s disease, condyloma, dysplasia, Paget disease, other
Features to report - Anus resection - applies to all invasive carcinomas
- Mandatory
- Specimen type: abdominoperineal resection, other, not specified
- Tumor site: anterior wall, anal margin, not specified
- Tumor size: at least 1 dimension
- Histologic type: squamous cell carcinoma, adenocarcinoma, mucinous adenocarcinoma, small cell carcinoma, undifferentiated carcinoma, Paget disease, other, carcinoma - cannot determine
- Histologic grade: well, moderate or poorly differentiated, undifferentiated, cannot determine or not applicable; for adenocarcinoma, is based on percentage of tumor that forms glands: well: > 95%, moderate: 50 - 95%, poor: 5 - 49%, undifferentiated: < 5%
- Depth / extent of invasion: no invasion, cannot determine, into lamina propria, into muscularis mucosa, into submucosa, into muscularis propria, into subserosa
- For each resection margin (proximal, distal, radial [soft tissue closest to deepest tumor penetration]): cannot assess, positive / negative for invasive carcinoma; if negative, closest tumor is __ mm from margin, carcinoma in situ absent / present at margin
- Angiolymphatic invasion: absent, present for large / small vessels, indeterminate
- Invasion of other structures
- Nodal involvement (# identified, # involved)
- pTNM and stage
- Optional
- HPV status
- Tumor configuration: polypoid, infiltrative, ulcerating, other
- Perineural invasion: absent, present
- Additional findings: none, Crohn’s disease, condyloma, dysplasia, Paget disease, fistula, active colitis, polyps, other
- Protocol for the examination of specimens from patients with carcinomas of the anus and anal canal (Arch Pathol Lab Med 2000;124:21)
Hemorrhoids
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Hemorrhoids are normal vascular structures present as cushions that help to maintain continence (Clin Gastroenterol Hepatol 2013;11:593)
- Although hemorrhoids are normal structures, the term hemorrhoid has been referred to as a pathologic or symptomatic process (Clin Gastroenterol Hepatol 2019;17:8)
- Typically defined as symptomatic enlargement and distal displacement of the normal anal cushions (World J Gastroenterol 2012;18:2009)
Essential features
- Guidelines for routine histological examination of hemorrhoids vary from institution to institution
- IHC stain is not required to make a diagnosis
- Incidental findings in hemorrhoidectomy specimens, including squamous dysplasia, adenocarcinoma, squamous cell carcinoma, melanoma and Kaposi sarcoma, are not uncommon
- Florid papillary endothelial hyperplasia can mimic angiosarcoma and should be differentiated from the latter
ICD coding
Epidemiology
- True epidemiology is unknown because of the use of self medication and low rate of healthcare seeking behavior (World J Gastroenterol 2012;18:2009)
- Approximate prevalence in the United States is 4.4% (Gastroenterology 1990;98:380)
- No gender predilection
- Peak prevalence between 45 - 65 years old; unusual before the age of 20 years
- More frequent in White people than in Black people and more common in individuals of higher socioeconomic status (World J Gastroenterol 2012;18:2009)
Sites
- Usually located at left lateral, right anterior and right posterior positions; multiple sites can be involved at the same time
- Right anterior is the most involved position (Ann Afr Med 2019;18:12)
- Hemorrhoids are generally classified based on their location
- Internal hemorrhoids: originate from the inferior hemorrhoidal venous plexus above the dentate line and are covered by mucosa
- External hemorrhoids: located below the dentate line and are covered with squamous epithelium
- Mixed (interno - external) hemorrhoids: arise both above and below the dentate line
Pathophysiology
- Exact pathophysiology for internal hemorrhoids is not clearly understood but the following hypotheses have been documented in literature
- Deterioration of the connective tissue that anchors hemorrhoids (Dis Colon Rectum 1984;27:442)
- Hypertrophy or increased tone of the internal anal sphincter
- Abnormal distension of the arteriovenous anastomoses within the hemorrhoidal cushions (Br J Surg 1975;62:542)
- Abnormal dilatation of the veins of the internal hemorrhoidal venous plexus
- Hemorrhoids and anorectal varices are proven to be distinct entities
- Patients with portal hypertension and varices do not have an increased incidence of hemorrhoids (Am J Gastroenterol 1991;86:1185)
Etiology
- Constipation and prolonged straining (Br J Surg 1994;81:946)
- Pregnancy
- Dietary factors including low fiber diet, spicy foods and alcohol intake
- Other risk factors: advancing age, diarrhea, pelvic tumors, prolonged sitting and patients on anticoagulation and antiplatelet therapy (Gastroenterology 1990;98:380)
Diagrams / tables
Clinical features
- External hemorrhoids are supplied by somatic nerves, thus producing pain while internal hemorrhoids are innervated by visceral nerve fibers so do not cause pain
- Approximately 40% cases are asymptomatic (Int J Colorectal Dis 2012;27:215)
- Most common presentation is painless rectal bleeding during defecation with or without prolapsing anal tissue (Ann Afr Med 2019;18:12)
- Perineal irritation or anal itching, feeling of incomplete evacuation or rectal fullness
- Anal pain in cases of thrombosed or strangulated hemorrhoids or associated anal fissure and perianal abscess
- Positive fecal occult blood or anemia is not common; if present, the patient should be evaluated for other causes (World J Gastroenterol 2012;18:2009)
- See Diagrams / tables for tabular representation for grading and types of hemorrhoids
- Internal hemorrhoids are further graded based on their appearance and degree of prolapse (Goligher’s classification)
- First degree hemorrhoids (grade I): the anal cushions bleed but do not prolapse
- Second degree hemorrhoids (grade II): the anal cushions prolapse through the anus on straining but reduce spontaneously
- Third degree hemorrhoids (grade III): the anal cushions prolapse through the anus on straining or exertion and require manual replacement into the anal canal
- Fourth degree hemorrhoids (grade IV): the prolapse stays out at all times and is irreducible
Diagnosis
- Precise patient history and careful clinical examination
- Physical examination including digital rectal examination
- Anoscopy
- Flexible sigmoidoscopy and colonoscopy (Clin Gastroenterol Hepatol 2013;11:593)
Prognostic factors
- Excellent prognosis with conservative treatments in mild cases
- Recurrence rate after conservative management is more than 50%, whereas after surgery it is However, postsurgical complications such as pain and urinary retention can happen
- Reference: StatPearls: External Hemorrhoid [Accessed 3 July 2023]
Case reports
- 35 year old Japanese woman with in situ diffuse large B cell lymphoma in hemorrhoidectomy tissue (EJHaem 2022;3:1050)
- 37 year old woman with endometriosis in the rectum accompanied by hemorrhoids leading to diagnostic pitfalls (BMC Womens Health 2018;18:120)
- 42 year old man with traumatic neuroma of anus 5 years after Milligan-Morgan hemorrhoidectomy (Ann Clin Lab Sci 2014;44:324)
- 45 year old man with leukoplakia of the anal verge with bleeding hemorrhoid (Rev Med Inst Mex Seguro Soc 2009;47:101)
- 60 year old man with anal neuroendocrine tumor masquerading as external hemorrhoids (Gastroenterology Res 2017;10:56)
- 69 year old man with perianal melanoma disguised as hemorrhoids (Dig Dis Sci 2007;52:1745)
Treatment
- Conservative (medical)
- Dietary and behavioral modifications (Clin Gastroenterol Hepatol 2013;11:593)
- Nonsurgical / office based treatment
- Rubber band ligation
- Sclerotherapy
- Infrared coagulation
- Bipolar diathermy, direct current electrotherapy, heater probe coagulation
- Cryosurgery
- Surgical
- Hemorrhoidectomy or hemorrhoidopexy in cases with higher grade and recurrent hemorrhoids despite conservative therapy (Hum Pathol 2021;109:12)
Clinical images
Microscopic (histologic) description
- Routine pathological examination of hemorrhoid specimens has been a topic of debate and the guidelines may vary from institution to institution (Hum Pathol 2021;109:12)
- Some incidental benign or malignant findings like adenocarcinoma, squamous cell carcinoma, melanoma and Kaposi sarcoma can be found during routine histopathological examination
- Typical hemorrhoid histology includes (Am J Surg Pathol 1988;12:41)
- Dilated, thin walled vascular plexus that vary in size and configuration within the stroma
- Recent or past thrombosis can be present
- Fibrosis and scattered smooth muscle fibers may be present in the stroma
- Overlying surface epithelium depends on the degree and descent of hemorrhoid, ranging from colorectal, squamous, perianal or transitional epithelium
- Erosion, inflammatory, metaplastic or keratotic changes can happen in the surface epithelium
- Pagetoid dyskeratosis should be distinguished from HPV cytopathic changes
Microscopic (histologic) images
Contributed by James Mueller, M.D., Ph.D. and Vikram Deshpande, M.D.
Positive stains
Negative stains
- p16 is negative in pagetoid dyskeratosis
Videos
Histopathology of hemorrhoids
Overview of hemorrhoids
Sample pathology report
- Anal tissue, right anterior, hemorrhoidectomy:
- Anal squamous and colonic mucosa with dilated and congested submucosal veins consistent with hemorrhoids
Differential diagnosis
- Histologically a straightforward diagnosis; however, associated pathologies (benign or malignant) are important to find and report
- Diverse clinical differentials (i.e., anal fissure, anorectal abscess, proctitis, rectal prolapse, malignancy, polyps, anorectal varices)
- Anal fibroepithelial polyp or hypertrophied papillae:
- Clinically it may appear as a hemorrhoid when giant and protruding from the anal verge
- Fibroepithelial polyp will have varying amount of stroma covered by papillomatous epidermis and loose fibrovascular connective tissue core
- No evidence of dilated vessels, hemorrhage or organizing thrombi
- Inflammatory cloacogenic polyp:
- Located at the anorectal junction / anal transitional zone
- Morphologically mimic hemorrhoids during examination
- Histologically characterized by tubulovillous growth pattern and irregular shaped crypts displaced into the submucosa, surrounded by a prominent fibromuscular stroma with superficial ulceration
- Arteriovenous malformation:
- Rarely occur as polypoidal shape
- Histologically characterized by widespread full-thickness vasodilation from the submucosa to the serosa and are composed of an inflow artery (feeder), an abnormal blood vessel assembly (nidus) and an outflow vein (drainer)
- Solitary rectal ulcer syndrome:
- Crypt and surface epithelial hyperplasia with erosion and ulceration
- Thickened and hyperplastic muscularis mucosae
- Angiosarcoma:
- Organization and recanalization of thrombosed hemorrhoid can lead to florid papillary endothelial hyperplasia, mimicking angiosarcoma
- Angiosarcoma will have invasive growth pattern with extensive anastomosing vessels, marked atypia, necrosis and increased mitosis as opposed to florid papillary endothelial hyperplasia, which is confined to pre-existing vascular spaces only
Additional references
Board review style question #1
A 56 year old woman came to the clinic complaining of a lump protruding from her anal opening. It was initially reducible but it is now irreducible. There is associated pain and itching. She also noticed bright red blood on her stool when she defecates. There is an associated history of chronic constipation. Examination of the perianal area revealed skin tags and a tender perianal mass covered with mucosa. Inspection of the anal mucosa showed no fissure. Histologic examination of the perianal mass revealed the image above. What is the most likely diagnosis?
- External hemorrhoid
- Fourth degree internal hemorrhoid
- Proctitis
- Third degree internal hemorrhoid
Board review style answer #1
B. Fourth degree internal hemorrhoid. The image shows thin walled dilated blood vessels with overlying glandular mucosa. Answer A is incorrect because external hemorrhoids are covered with squamous mucosa. Answer D is incorrect because third degree hemorrhoids are reducible with manual replacement. Answer C is incorrect because proctitis does not protrude from the anal opening.
Comment Here
Reference: Hemorrhoids
Comment Here
Reference: Hemorrhoids
Board review style question #2
Which of the following is most accurate about the etiology and epidemiology of hemorrhoids?
- Hemorrhoids have a male to female predilection of nearly 4:1
- Internal hemorrhoids often cause cutaneous pain because they are innervated by cutaneous nerves
- Pregnancy is not a risk factor to develop hemorrhoids
- Recognized risk factors associated with the development of hemorrhoids include lack of fiber rich diet, prolonged sitting and pelvic tumors
Board review style answer #2
D. Recognized risk factors associated with the development of hemorrhoids include lack of fiber rich diet, prolonged sitting and pelvic tumors. Answer A is incorrect because hemorrhoids have no known sex predilection, although men are more likely to seek treatment. Answer B is incorrect because internal hemorrhoids cannot cause cutaneous pain since they are above the dentate line and are not innervated by cutaneous nerves. Answer C is incorrect because pregnancy is a known risk factor to develop hemorrhoids.
Comment Here
Reference: Hemorrhoids
Comment Here
Reference: Hemorrhoids
Hypertrophied papillae
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Electron microscopy description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Benign acquired polypoid projections of anal squamous epithelium and subepithelial connective tissue at the base of the anal columns
Essential features
- Benign acquired polypoid projections at the base of the anal columns
- Results from enlargement of the anal papillae, with reactive hyperplastic nature of the subepithelial connective tissue due to irritation, infection or injury (Am J Surg Pathol 1998;22:70)
- Loose fibrovascular connective tissue core surfaced by mature squamous epithelium
- Large, multinucleated, reactive stromal cells commonly seen
Terminology
- Anal tags, anal fibroepithelial polyps, anal skin tags, sentinel tag (refers to hypertrophied papillae at proximal end of an anal fissure or ulcer)
ICD coding
- ICD-10: K62.0 - anal polyp
Epidemiology
- Common: in ~45% patients undergoing proctoscopy (World J Gastroenterol 2009;15:3687)
- M > F (2:1)
- Usually middle to late adulthood but rare cases have been reported in adolescent patients (Fetal Pediatr Pathol 2020 Nov 9 [Epub ahead of print])
Sites
- Anal canal, at the base of the anal columns (columns of Morgagni)
Pathophysiology
- Results from enlargement of anal papillae
- Reactive hyperplastic nature of the subepithelial connective tissue of the anal mucosa due to irritation, infection or injury (BMJ Case Rep 2010;2010:bcr08.2009.2169)
- Mast cells may play an important role in the pathogenesis, by means of their fibrogenic, fibrolytic and angiogenic activities (Am J Surg Pathol 1998;22:70)
Etiology
- Reactive hyperplastic process of stromal connective tissue, either in isolation or secondary to nearby inflammatory process or mass lesion
- Analogous to cutaneous skin tags / acrochordons
Clinical features
- Usually asymptomatic, found in isolation as subtle swelling or a solitary firm, palpable mass on digital examination
- Tends to enlarge over time (Fetal Pediatr Pathol 2020 Nov 9 [Epub ahead of print])
- Rarely presents as large and prolapsed lesions associated with irritation, infection or chronic fistula or fissure in the anal canal (BMJ Case Rep 2010;2010:bcr08.2009.2169)
- May coexist with hemorrhoids and are confused with hemorrhoids clinically; often submitted to pathologist as hemorrhoid
Diagnosis
- Hemorrhoids may have similar appearance
- Usually requires a histopathological evaluation to establish the diagnosis and to exclude accompanying lesions, such as low or high grade squamous intraepithelial lesions
Radiology description
- Imaging is rarely done and occurs only when it becomes large enough to prolapse into the rectum
- Typical findings include polypoid stromal proliferation, no focal or diffuse restriction and no pelvic lymph node enlargement
Prognostic factors
- Tends to enlarge over time and may convert from an asymptomatic to a symptomatic mass
- No malignant potential
Case reports
- 15 year old boy, otherwise healthy, presented with a giant pedunculated mass projecting out of the anal verge that enlarged over 2 years (Fetal Pediatr Pathol 2020 Nov 9 [Epub ahead of print])
- 38 year old woman presented at the emergency department after a week of anal pain and 1.5 years of vague anal discomfort (BMJ Case Rep 2010;2010:bcr08.2009.2169)
- 67 year old woman was admitted to the emergency room with diffuse, cramping abdominal pain of progressive onset (World J Gastroenterol 2009;15:3687)
- 67 year old man presented with abdominal pain and hematochezia and was referred for colonscopy after a CT scan demonstrated a 3 cm rectal mass with adjacent lymphadenopathy and numerous lesions in the liver, pancreas and bone (Gastrointest Endosc 2015;82:763)
Treatment
- Endoscopic polypectomy or surgical resection in symptomatic cases
- When accompanying an underlying chronic process, correction of the primary etiology
Gross description
- Polypoid, flesh colored projections at the mucocutaneous junction of the upper anal canal that give a serrated appearance
- Usually small in size (2 - 5 mm) but rarely they become enlarged
- Extremely rare case of a giant hypertrophied anal papilla complicated by obstructive ileus was reported (World J Gastroenterol 2009;15:3687)
Microscopic (histologic) description
- Myxoid or collagenous stroma surfaced by mature squamous epithelium
- Squamous epithelium may be slightly hyperplastic and shows focal hyperkeratosis and parakeratosis
- Loose fibrovascular connective tissue core, morphologically similar to that of the normal anal mucosa
- Large, multinucleated, reactive stromal cells commonly seen (80% of hypertrophied papillae); typically scattered singly in the stroma but occasionally show small areas of aggregation (Am J Surg Pathol 1998;22:70)
- Atypical, bizarre stromal cells may be seen in a polyp of large size
- No mitotic figures present
- Scattered smooth muscle fibers seen in approximately half of polyps
- Mast cells frequently present
- Absent thick walled, thrombosed vessels (features of hemorrhoids)
- Large, multinucleated, reactive stromal cells commonly seen (80% of hypertrophied papillae); typically scattered singly in the stroma but occasionally show small areas of aggregation (Am J Surg Pathol 1998;22:70)
- Chronic inflammation, mostly in the subepithelial region
- Subepithelial zone appears to be more cellular and less collagenous than the center
- Conspicuous lymphatic dilatation
- Epithelial endovascular displacement (vascular pseudoinvasion) is uncommonly seen, associated with morphological signs of traumatism (Int J Surg Pathol 2020;28:764)
Microscopic (histologic) images
Positive stains
- Vimentin, CD34 (stromal cells) (Am J Surg Pathol 1998;22:70)
- Desmin (stromal cells in 30% of cases) (Am J Surg Pathol 1998;22:70)
- Giemsa stain, CD117 (mast cells)
- Masson trichrome (collagen)
Negative stains
- Alpha smooth muscle actin (stromal cells)
Electron microscopy description
- Stromal cells demonstrate fibroblastic and myofibroblastic features (Am J Surg Pathol 1998;22:70)
Sample pathology report
- Anal polyp, excision:
- Hypertrophied anal papillae (anal fibroepithelial polyp)
Differential diagnosis
- Hemorrhoids:
- Dilated thick walled submucosal vessels and sinusoidal spaces
- Often with thrombosis and hemorrhaging into the surrounding connective tissues
- Condyloma accuminatum:
- Acanthosis with papillomatosis
- Variable dysplastic changes, including koilocytic atypia (nuclear wrinkling with perinuclear clearing), increased nuclear atypia / size, increased / atypical mitosis
- Infection / abscess:
- Nonspecific acute and chronic inflammation and granulation tissue
- Anorectal tumors (carcinoma or anal melanoma, neuroendocrine tumor, lymphoma) (Clin Dermatol 1987;5:87)
Board review style question #1
A 65 year old man visited his gastroenterologist for anal bleeding. He had experienced intermittent anal bleeding due to longstanding hemorrhoidal disease over the past 10 years. On physical examination, digital examination revealed a medium sized elastic mass with convoluted grooves on a smooth surface in the proximal anus. This lesion was excised and sent to pathology for histological evaluation. The H&E image is shown. Which of the following is the most likely diagnosis?
- External hemorrhoids
- Hypertrophied anal papillae
- Internal hemorrhoids
- Prolapsed associated inflammatory polyp
Board review style answer #1
Board review style question #2
Which underlying condition(s) is associated with hypertrophied anal papillae?
- Chronic fistula
- Degenerative change of perianal structure
- Elevated intra-abdominal pressure
- HPV infection
Board review style answer #2
Inflammatory cloacogenic polyp
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Benign polyp with colorectal, squamous and transitional epithelium with thickened and prolapsed muscularis mucosa associated with rectal prolapse
Essential features
- Benign polyp with colorectal, squamous and transitional epithelium and prolapsed muscularis mucosae
- Thought to be associated with mucosal prolapse or chronic inflammatory diseases
- Located at anorectal transition zone
Epidemiology
- Wide age range from pediatric to adult population
Sites
- Anorectal transition zone (Eur J Gastroenterol Hepatol 2000;12:247)
Pathophysiology
- Rectal mucosal prolapse due to repeated mucosal ischemia and regeneration are thought to contribute to the development (Histopathology 2008;53:91)
Clinical features
- Symptoms range from asymptomatic to rectal bleeding to constipation, tenesmus, anal swelling or anal itching (Cureus 2022;14:e22014)
- On endoscopy, these polyps can mimic hemorrhoids, solitary rectal ulcer, villous adenoma or anorectal carcinoma (Cureus 2022;14:e22014)
- Associated with solitary rectal ulcer syndrome, mucosal prolapse or chronic inflammatory conditions such as Crohn's disease and colorectal tumors (Cureus 2022;14:e22014, Eur J Gastroenterol Hepatol 2000;12:247)
Diagnosis
- Colonoscopy or sigmoidoscopy typically identifies a sessile or pedunculated polyp (Eur J Gastroenterol Hepatol 2000;12:247)
Laboratory
- Patients sometimes present with a positive fecal immunochemical test (e.g., Cologuard)
Prognostic factors
- Benign entity with no increased risk for malignancy (Eur J Gastroenterol Hepatol 2000;12:247)
Case reports
- 15 year old boy with Marshall-Smith syndrome and perianal subcutaneous mass (Pediatr Pathol 1993;13:409)
- 34 year old woman with anal intraepithelial neoplasia in an inflammatory cloacogenic polyp (J Clin Pathol 1999;52:393)
- 38 year old woman and 41 year old woman with squamous cell carcinoma in situ arising in inflammatory cloacogenic polyp (Histopathology 2008;53:91)
Treatment
- Endoscopic polypectomy or mucosal resection (Am J Gastroenterol 1994;89:438)
- High fiber diet (Cureus 2022;14:e22014)
Microscopic (histologic) description
- Tubulovillous architecture with elongated crypts stretching into the submucosa
- Areas of stratified squamous, colorectal or transitional epithelium that are often eroded and contain mixed inflammation and granulation tissue (Hum Pathol 1987;18:1120, Am J Surg Pathol 1981;5:761)
- Abundant regenerative epithelial changes can be seen
- Thickened muscularis mucosa with extension into the lamina propria
- Fibromuscular obliteration of the lamina propria can be seen
Microscopic (histologic) images
Sample pathology report
- Anus, polypectomy:
- Inflammatory cloacogenic polyp. Negative for dysplasia.
Differential diagnosis
- Solitary rectal ulcer syndrome:
- Should be at anterior rectum, proximal to anorectal junction and is usually flat and ulcerated
- Inflammatory cloacogenic polyp with adenomatous dysplasia:
- Often not necessary but p53 and Ki67 overexpression can assist in identifying areas of dysplasia (Histopathology 2008;53:91)
Additional references
Board review style question #1
Board review style answer #1
A. Inflammatory cloacogenic polyp is located in the anorectal transition zone.
Comment Here
Reference: Inflammatory cloacogenic polyp
Comment Here
Reference: Inflammatory cloacogenic polyp
Board review style question #2
What is the preferred treatment for an inflammatory cloacogenic polyp?
- Chemotherapy
- Colectomy
- Endoscopic / surgical resection
- Fecal immunohistochemical test
- Routine endoscopic surveillance
Board review style answer #2
C. Endoscopic / surgical resection is the preferred treatment for an inflammatory cloacogenic polyp. Routine endoscopic surveillance is necessary after resection because of the risk of recurrence but it is not the treatment modality.
Comment Here
Reference: Inflammatory cloacogenic polyp
Comment Here
Reference: Inflammatory cloacogenic polyp
Intradermal nevus (pending)
[Pending]
Lymphogranuloma venereum
Table of Contents
Definition / general | Terminology | Epidemiology | Sites | Pathophysiology | Diagnosis | Case reports | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosisDefinition / general
- Sexually transmitted chronic ulcerative disease
- Causative organism Chlamydia trachomatis L1, L2, and L3 serovars
- Complications: strictures, perirectal abscess, fistulas and sinuses, squamous cell carcinoma
- Rectal strictures are more common in women
Terminology
- Three stages of infection:
- Primary infection:
- Small often unnoticed ulceration, erosion or erythematic area
- Usually self healing
- Incubation period of 3 - 12 days following an exposure to the causative organism
- Secondary infection:
- Due to the extension of infection to the draining lymph nodes
- LGV causing serovars produce severe disease with systemic symptoms as compared to serovars A to K
- Manifests 2 - 6 weeks after primary infection
- Characterized by enlarged and painful lymph nodes
- Lymph nodes may rupture and form cutaneous sinuses
- Anorectal disease presents as GI bleed, discharge, rectal ulcer, anal pain or constipation
- Clinically and histologically mimics inflammatory bowel disease
- Late infection:
- Complications of longstanding disease in the form of strictures, fistulas, genital lymphedema and infertility
- Primary infection:
Epidemiology
- Endemic disease in the tropical and subtropical regions of Asia, Africa, South America and Africa
- Increased incidence in western population, especially men who have sex with men (MSM)
- Associated coinfections:
- Up to 76% also infected with HIV (Clin Infect Dis 2007;44:26)
- Other sexually transmitted disease in 39%
- Hepatitis C infection in 20%
Sites
- Rectum (LGV proctitis)
- Perianal skin
- Draining lymph nodes
Pathophysiology
- Mainly involves lymphatic system causing lymphangitis, necrosis and abscess formation
- Produces more generalized lymphoproliferative response
- Binds through heparan sulfate receptors
- Recombination of serovars had been reported between serovars L2 and D in cases with hemorrhagic proctitis (MBio 2011;2:e00045)
Diagnosis
- Culture of the organism
- Sampling by direct aspirate from the infected lymph node, perianal swab or rectal swab
- Positive culture is confirmatory but the yield is usually low
- Culture is positive in 20 - 30% of cases and depends on the stage of the illness
- Most common positive culture is during the secondary stage of illness
- Primary stage is usually missed for sampling because of subtle clinical feature
- Chlamydia cultures are not widely available and are done only at referral centers
- Serology
- Complement fixation test and immunofluorescence test
- Complement fixation test measures antibody against group specific lipopolysaccharide antigen
- Serological tests are supportive only in the presence of appropriate clinical findings
- Rising titer is significant for the presence of the disease
- Titer of greater than 1:64 is considered supportive
- Limitation:
- Cannot distinguish recent from past infection
- Cannot distinguish individual serotypes
- Limited diagnostic potential in an isolated site infection
- Nucleic acid amplification test (NAAT)
- A polymerase chain reaction (PCR) based test
- Sensitivity and specificity greater than 95%
- Ease of sampling (swab) and transport of the specimen
- Routine additional testing following a positive NAAT screening test for C. trachomatis no longer recommended by CDC
- See details of CDC recommendation for laboratory diagnosis at MMWR Recomm Rep 2014;63:1
Case reports
- 17 year old boy with LGV and non-Hodgkin lymphoma (Rev Soc Bras Med Trop 2012;45:412)
- 28, 30 and 42 year old men with LGV proctosigmoiditis mimicking inflammatory bowel disease (World J Gastroenterol 2012;18:3317)
- 60 year old man with squamous cell carcinoma of penis with bullous pemphigoid masquerading as LGV (Indian J Sex Transm Dis 2013;34:41)
- May present as proctitis (Rev Esp Enferm Dig 2014;106:59)
Microscopic (histologic) description
- Granulomatous proctitis resembling Crohn's disease
- Follicular infiltrates of lymphocytes, histiocytes and plasma cells, neural hyperplasia, extensive fibrosis
- See also Lymph nodes - Lymphogranuloma venereum
Microscopic (histologic) images
Differential diagnosis
- Crohn's disease
- Other infections causing stricture and fistulas: tuberculosis, fungal infections
- Squamous cell carcinoma
Melanoma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Rare primary malignant melanocytic neoplasm of the anal mucosa
Essential features
- Rare primary malignant melanocytic neoplasm of the anal mucosa, most common in White and female patients in the sixth decade of life
- Biopsy is necessary for diagnosis with positive melanocytic markers (SOX10, S100, HMB45, MelanA, etc.)
- Metastasis from another location must be ruled out before diagnosing primary anal melanoma
- There is currently no consensus on how to pathologically stage anal melanoma
- Patients have a poor prognosis due to advanced disease on presentation and aggressiveness of the tumor
Terminology
- Primary anal melanoma
- Anorectal melanoma
- Mucosal lentiginous melanoma
ICD coding
Epidemiology
- Comprises Int J Cancer 2014;134:2961, Surg Oncol Clin N Am 2017;26:143, Surg Clin North Am 2020;100:629, Hum Pathol 2018;79:77)
- More common in White and female patients, with the average age of diagnosis of 60 years (Surg Oncol Clin N Am 2017;26:143, Surg Clin North Am 2020;100:629)
Sites
- Arises in the anus and can extend into the distal rectum (Clin Colon Rectal Surg 2006;19:78)
- Sometimes the primary site cannot be determined between the anus and the rectum (Surg Oncol Clin N Am 2017;26:143)
Pathophysiology
- See Etiology
Etiology
- Melanocytes are normally found at the anal transition zone and squamous zone (Clin Colon Rectal Surg 2011;24:171)
- Mucosal melanomas, including anal melanoma, are not associated with ultraviolet (UV) radiation exposure, unlike cutaneous melanomas (Nature 2017;545:175)
- Otherwise, the etiology of anal melanoma is not well established
Clinical features
- Symptoms are typically vague, including anorectal bleeding, pain, change in bowel habits and sometimes a mass, which can be confused with other conditions (e.g., hemorrhoids) and may lead to delayed diagnosis (Surg Clin North Am 2020;100:629)
- May be discovered as an incidental finding in a hemorrhoidectomy or anal polyp resection specimen (Surg Oncol Clin N Am 2017;26:143)
- Patients often have delayed presentation or are misdiagnosed, which results in advanced disease, often with local lymph node metastasis (Surg Clin North Am 2020;100:629)
- Some association with human immunodeficiency virus (HIV) (Surg Oncol Clin N Am 2017;26:143)
Diagnosis
- Biopsy is necessary to confirm the diagnosis (Surg Clin North Am 2020;100:629)
- Colonoscopy is advisable to characterize the lesion and rule out the presence of a second malignancy of the colon (Dis Colon Rectum 2020;63:573)
- Additional imaging is needed for staging and to confirm location of primary disease or to rule out metastatic disease (Dis Colon Rectum 2020;63:573)
- Staged clinically
- Stage I - local disease only
- Stage II - local disease with regional lymph nodes
- Stage III - distant metastatic disease (Clin Colon Rectal Surg 2006;19:78)
- There is no current consensus on how to pathologically stage these lesions (Hum Pathol 2018;79:77)
Laboratory
- No specific findings for this entity
Radiology description
- MRI is preferred due to higher soft tissue resolution
- Melanin pigmented lesions typically demonstrate high signal intensity on T1 weighted imaging and mixed signal intensity on T2 weighted imaging
- It is common for amelanotic lesions to not display these characteristic findings on MRI
- Reference: BMJ Case Rep 2021;14:e247421
Radiology images
Prognostic factors
- 5 year survival is based on stage at diagnosis but is generally poor with 32% for local disease, 17% for regional spread and 0% for distant spread (Surg Clin North Am 2020;100:629)
- Gross and histologic features associated with poor prognosis include tumor size and thickness, regional lymph node involvement, perineural invasion and amelanotic subtype (Surg Clin North Am 2020;100:629, Surg Oncol Clin N Am 2017;26:143)
Case reports
- 63 year old woman with bleeding per rectum and painful defecation (Cureus 2021;13:e15474)
- 66 year old woman with anal bleeding, pain and tenesmus (World J Clin Cases 2021;9:11369)
- 81 year old woman with hematochezia and perianal pain (Am J Case Rep 2021;22:e933032)
- 84 year old woman with anal mass (Middle East J Dig Dis 2019;11:166)
- 85 year old woman with recurrent anal mass (BMC Surg 2020;20:68)
Treatment
- Managed with excision
- Adjuvant chemotherapy and radiation are recommended based on patient stage
- Reference: Surg Clin North Am 2020;100:629
Clinical images
Gross description
- Lesions can be varied in their presentation, including size, shape and color
- Usually present as large, expansive, nodular masses
- Majority of lesions are pigmented but can be nonpigmented
- Ulceration is common
- Reference: Surg Clin North Am 2020;100:629
Frozen section description
- Frozen section is not recommended
Microscopic (histologic) description
- Histomorphology can vary greatly but solid sheets of atypical epithelioid cells originating from the epithelium is the most common presentation; however, the cytomorphology may be composed of spindled, pleomorphic or small round blue cells while the architectural patterns may be nested, pseudopapillary or pseudoalveolar (Hum Pathol 2018;79:77)
- Pigmentation is variable and can be absent in up to 40% of cases, which is much more common than in cutaneous melanoma (< 5% amelanotic) (Hum Pathol 2018;79:77, Surg Oncol Clin N Am 2017;26:143)
- Ulceration can be present
- Identification of a junctional component adjacent to the invasive tumor can support the diagnosis of primary anal melanoma (Surg Oncol Clin N Am 2017;26:143)
- Melanoma, including anorectal examples, may undergo dedifferentiation which is defined as a tumor that has lost evidence of melanocytic differentiation (morphologic and immunophenotypic) (Int J Surg Pathol 2019;27:923)
- These dedifferentiated examples may aberrantly express various nonmelanocytic markers
- Dedifferentiation is rare but is seen more commonly in metastases of cutaneous origin
Microscopic (histologic) images
Contributed by Aaron R. Huber, D.O. and @RaulSGonzalezMD on Twitter
https://pathologyoutlines.com/topic/anusmelanoma.html #pathology #gipath #pathtwitter"
Contributed by @RaulSGonzalezMD on Twitter (see original post here)">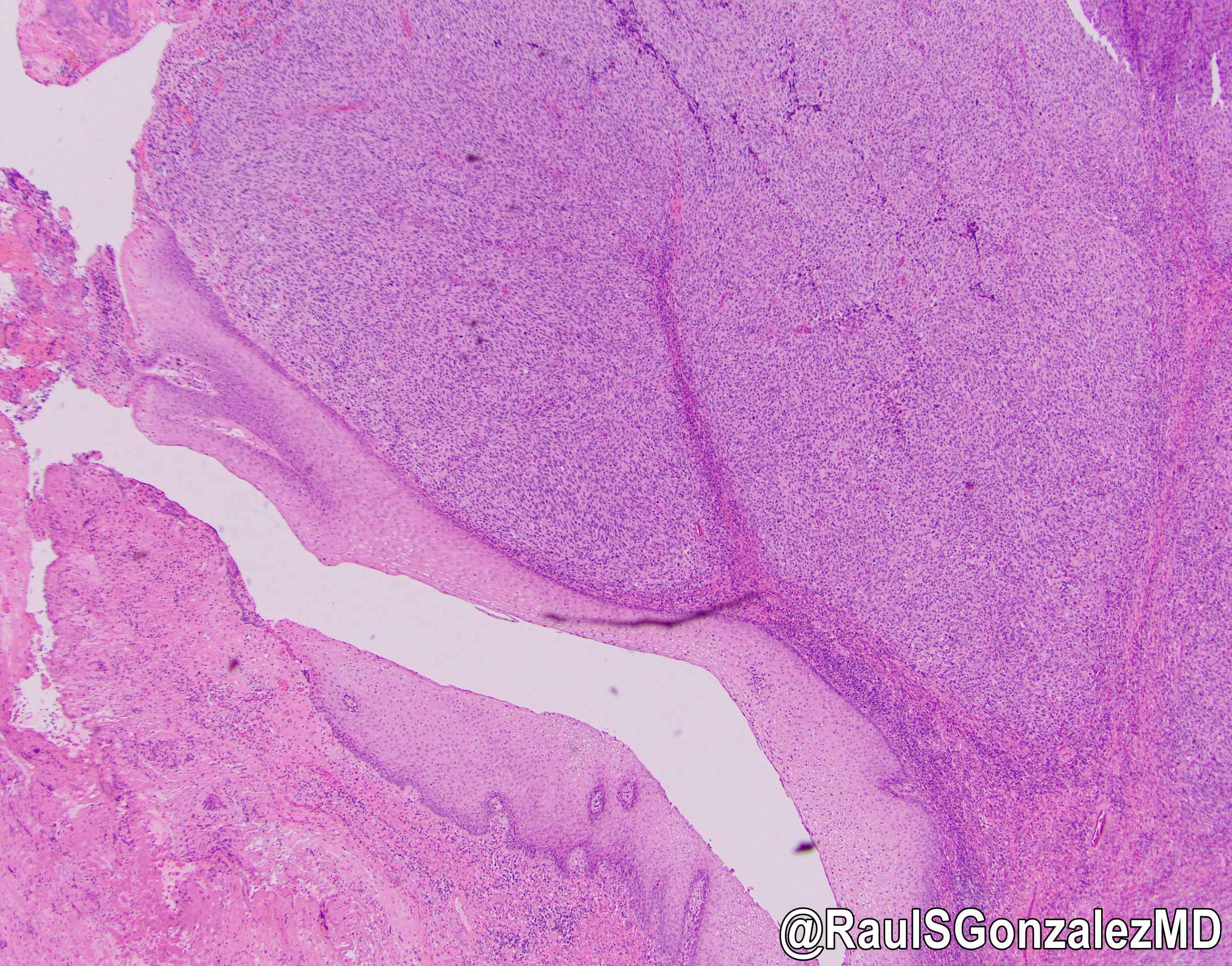 https://pathologyoutlines.com/topic/anusmelanoma.html #pathology #gipath #pathtwitter"
https://pathologyoutlines.com/topic/anusmelanoma.html #pathology #gipath #pathtwitter"
Contributed by @RaulSGonzalezMD on Twitter (see original post here)">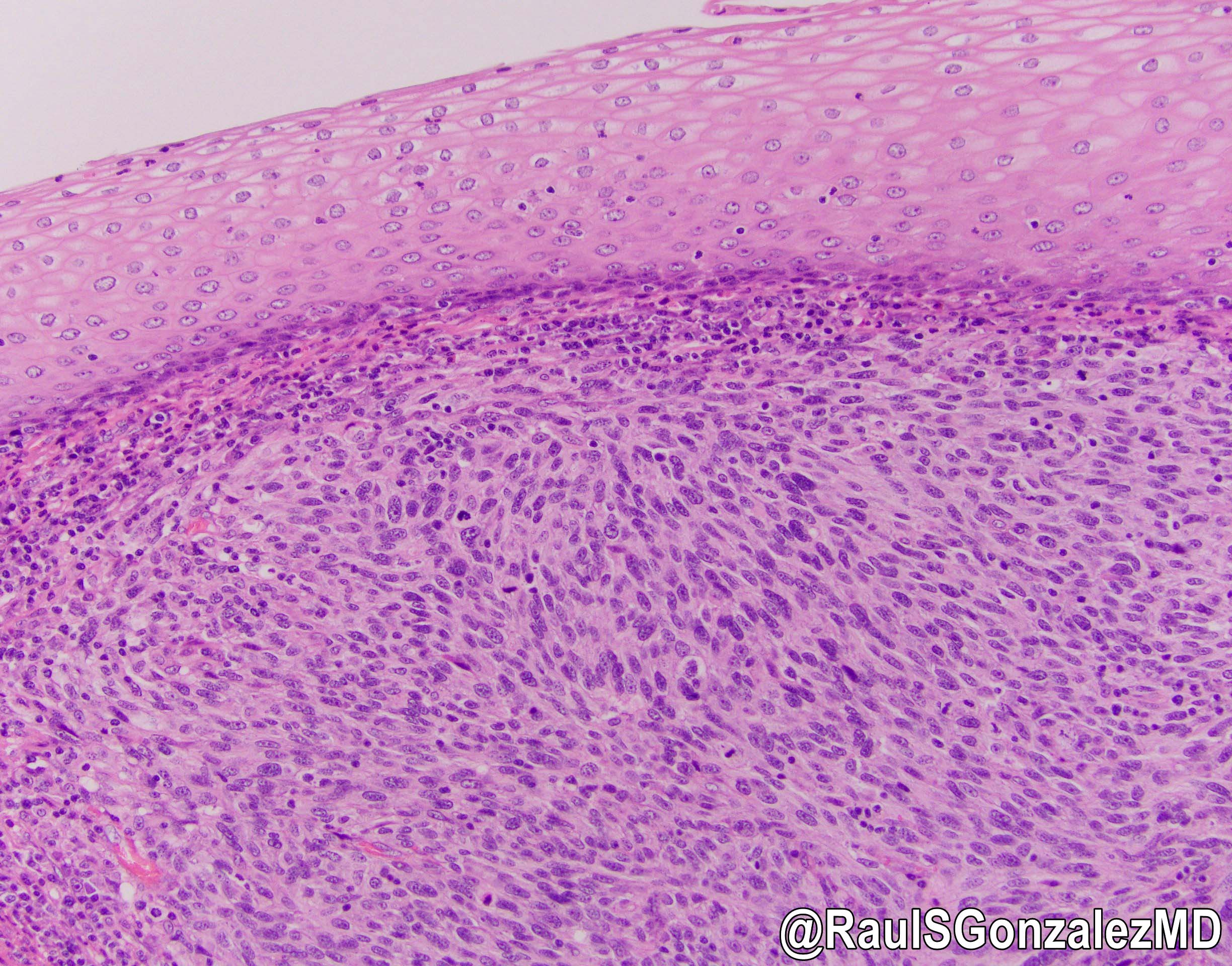 https://pathologyoutlines.com/topic/anusmelanoma.html #pathology #gipath #pathtwitter"
https://pathologyoutlines.com/topic/anusmelanoma.html #pathology #gipath #pathtwitter"
Contributed by @RaulSGonzalezMD on Twitter (see original post here)">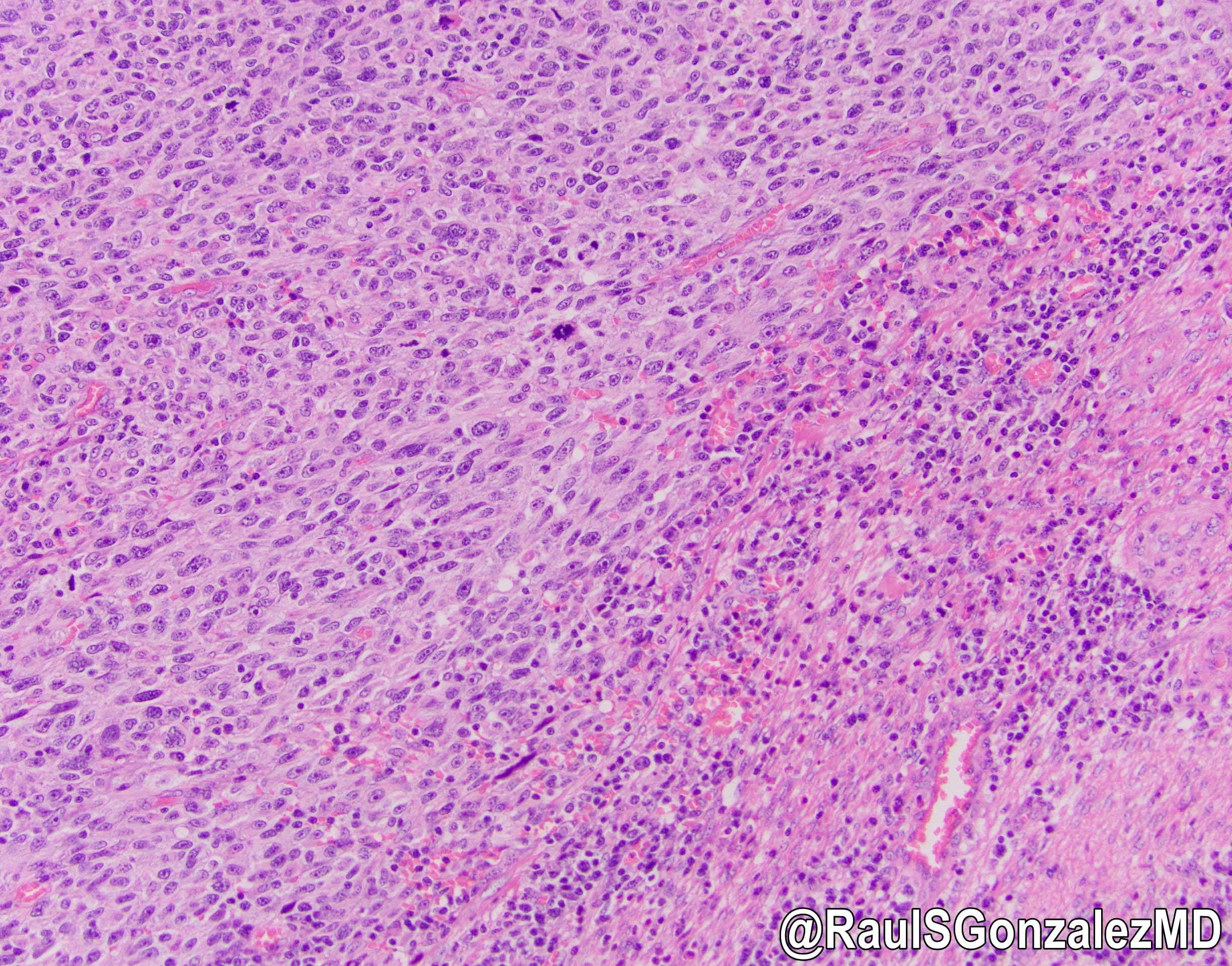
Contributed by @RaulSGonzalezMD on Twitter (see original post here)">
 https://pathologyoutlines.com/topic/anusmelanoma.html #pathology #gipath #pathtwitter"
https://pathologyoutlines.com/topic/anusmelanoma.html #pathology #gipath #pathtwitter"Contributed by @RaulSGonzalezMD on Twitter (see original post here)">
 https://pathologyoutlines.com/topic/anusmelanoma.html #pathology #gipath #pathtwitter"
https://pathologyoutlines.com/topic/anusmelanoma.html #pathology #gipath #pathtwitter"Contributed by @RaulSGonzalezMD on Twitter (see original post here)">

Primary anal melanoma
Virtual slides
Cytology description
- FNA specimens are usually obtained from metastases
- Largely dispersed population of pleomorphic epithelioid cells or spindle cells
- Nuclear pleomorphism and prominent nucleoli
- Eccentric nuclei with pseudoinclusions can be seen
- Cytoplasmic melanin is seen as fine yellow-brown or blue granules, depending on the slide preparation
- May have cells with double image mirror nuclei (demons)
- References: Am J Clin Pathol 2007;127:385, Int J Clin Exp Pathol 2010;3:367, Cancer Cytopathol 2022;130:18
Positive stains
Negative stains
- Keratin, CD45, CDX2 and SATB2
- It is important to note that melanoma can aberrantly express many antigens and demonstrate divergent differentiation, which can be a potential diagnostic pitfall (Histopathology 2008;52:119, Int J Surg Pathol 2015;23:329)
Molecular / cytogenetics description
- Generally, mucosal melanomas are characterized by a relatively low mutational burden (Gastroenterology 2004;127:1815)
- C-KIT mutations can be seen more frequently in mucosal melanomas than in cutaneous melanoma (Clin Cancer Res 2017;23:6120)
- BRAF mutations can be present but not as commonly as in cutaneous melanoma (Gastroenterology 2004;127:1815)
- Mutations of SF3B1 can also be seen (Clin Cancer Res 2017;23:6120)
Sample pathology report
- Anus, pigmented lesion, excision:
- Malignant melanoma (see comment)
- Margins are free of involvement
- Comment: Immunohistochemical staining was performed and the neoplastic cells showed positivity for SOX10 and PRAME, while they were negative for pancytokeratin. The immunophenotypic findings support the diagnosis of primary anal melanoma.
Differential diagnosis
- Secondary involvement by rectal adenocarcinoma:
- More common than anal adenocarcinoma
- Usually well or moderately differentiated gland forming carcinoma with marked desmoplasia
- CK20, CDX2 and SATB2 positive
- Anal adenocarcinoma:
- Squamous cell carcinoma:
- Most common carcinoma of the anal tract
- Resembles squamous cell carcinoma as seen elsewhere in body
- Positive for pankeratins and squamous markers (i.e., p40)
- Metastatic melanoma:
- History of melanoma or image findings consistent with a metastatic lesion
- Lack of intraepidermal component does not confirm metastasis
- Lymphoma:
- Atypical population of lymphocytes
- CD45 positive
- Neuroendocrine carcinoma:
- Small and large cell subtypes resemble morphology seen in other locations
- Solid nests of poorly differentiated cells
- Positive for keratin, p16, p63, HPV ISH and neuroendocrine markers (synaptophysin, chromogranin, INSM1)
- Usually high Ki67
- Hemorrhoids:
- Dilated thin walled vascular plexus within stroma
- Can display metaplastic changes in the surface epithelium
- Gastrointestinal stromal tumor (GIST):
- May be spindled or epithelioid but usually lacks the overt cytologic atypia seen in melanoma
- DOG1+
- Malignant peripheral nerve sheath tumor (MPNST):
Additional references
Board review style question #1
Board review style answer #1
C. HMB45. The H&E image demonstrates anal melanoma. HMB45 is a melanocytic marker that would confirm the diagnosis of melanoma, along with the malignant features seen on H&E. Answer A is incorrect because CD45 is a lymphocytic marker. CD45 should be ordered to rule out lymphoma but a negative result would not confirm the diagnosis in this case. Answer D is incorrect because melanoma is typically pancytokeratin negative. This would be a good confirmatory stain but would not be diagnostic of melanoma by itself. Answer B is incorrect because CDX2 is a common marker for colonic adenocarcinomas. CDX2 should be used to rule out a primary rectal adenocarcinoma that involves the anus but will be negative for any anal malignancies, including melanoma.
Comment Here
Reference: Anal melanoma
Comment Here
Reference: Anal melanoma
Board review style question #2
Which of the following characteristics is similar for both cutaneous and anal melanoma?
- Pathologic staging
- Presentation
- Prognosis
- Staining / IHC
Board review style answer #2
D. Staining / IHC. Cutaneous and anal melanoma will stain with similar melanocytic markers (SOX10, S100, HMB45, MelanA, etc.). Answer C is incorrect because anal melanoma has a worse prognosis when compared to cutaneous melanoma, especially because patients with anal melanoma often have advanced disease on presentation due to the vague clinical symptoms and difficulty in diagnosis. Answer B is incorrect because anal melanoma typically presents with vague symptoms and not the typical ABCDE (asymmetry, border, color, diameter, evolving) criteria for cutaneous melanoma. Answer A is incorrect because anal melanoma does not have a standardized pathologic staging system. Anal melanomas can be staged similarly to cutaneous melanoma but this is not universal.
Comment Here
Reference: Anal melanoma
Comment Here
Reference: Anal melanoma
Mixed neuroendocrine nonneuroendocrine neoplasms (pending)
[Pending]
Neuroendocrine carcinoma
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Pathophysiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Poorly differentiated neuroendocrine carcinoma arising in the anus
Essential features
- Rare, primary neuroendocrine carcinoma of the anus
- Very aggressive, with early metastatic spread; 5 year survival is ~10%
- Similar histology to small cell or large cell neuroendocrine carcinoma at other sites
ICD coding
- ICD-10: C21.0 - malignant neoplasm of anus, unspecified
Epidemiology
- Represents ~7% of anal carcinomas (Cancer 1984;54:114)
Pathophysiology
- Associated with high risk HPV infection, though with low viral copy numbers (Am J Surg Pathol 2012;36:1087)
- Rb-E2F pathway dysregulation often occurs (Mod Pathol 2019;32:290)
Clinical features
- May cause rectal bleeding or present as an anal mass (Clin Colorectal Cancer 2021;20:e139)
Diagnosis
- Tissue sampling
Radiology description
- Destructive lesion; no features specific to neuroendocrine nature
Prognostic factors
- Higher stage confers poor prognosis
Case reports
- 37 year old man with painful anal fissure (BMC Gastroenterol 2020;20:290)
- 60 year old woman with small cell carcinoma of the anus (Cases J 2009;2:9396)
- 63 year old woman with extrapulmonary small cell carcinoma of the anal canal (Case Rep Med 2012;2012:341432)
Treatment
- Chemotherapy (often platinum based) and radiation
Microscopic (histologic) description
- Small cell neuroendocrine carcinoma: poorly differentiated malignancy composed of solid nests of small cells with minimal cytoplasm, hyperchromatic nuclei with molding, central necrosis and high mitotic activity; resembles pulmonary counterpart
- Large cell neuroendocrine carcinoma: poorly differentiated malignancy composed of solid nests of large cells with abundant cytoplasm, vesicular nuclei with prominent nucleoli, central necrosis and high mitotic activity
- May have overlying squamous cell carcinoma in situ or a squamous cell carcinoma component (namely, mixed neuroendocrine - nonneuroendocrine neoplasm) (Pathol Int 2012;62:356)
- No longer graded using WHO criteria, as it is essentially always high grade (mitotic rate > 20/2 square mm; Ki67 index > 20%)
Microscopic (histologic) images
Positive stains
- Pankeratin, neuroendocrine markers (synaptophysin, chromogranin, CD56, INSM1), p16, p63, HPV ISH
- Ki67 index is high (almost always > 20% and usually > 55%)
Negative stains
Molecular / cytogenetics description
- Molecular abnormalities often seen in TP53, Rb-E2F pathway (Mod Pathol 2019;32:290)
Sample pathology report
- Anus, mass, biopsy:
- Small cell neuroendocrine carcinoma (see comment)
- Comment: The carcinoma is positive for synaptophysin and chromogranin by immunohistochemistry. Neuroendocrine carcinomas are high grade by definition.
Differential diagnosis
- Basaloid squamous cell carcinoma:
- Negative for neuroendocrine markers
- Colorectal small cell carcinoma:
- May extend into anus
- Usually CDX2 positive
- Basal cell carcinoma:
- No necrosis, lower mitotic activity
- Arises in perianal skin rather than anus
- Well differentiated neuroendocrine tumor:
- Rare in anus
- Has carcinoid-like appearance, with no necrosis and few mitotic figures
- Poorly differentiated carcinoma, NOS:
- May resemble neuroendocrine carcinoma histologically but is negative for neuroendocrine markers
- Also no compelling evidence of glandular or squamous differentiation
Board review style question #1
Board review style answer #1
D. Synaptophysin. Anal small cell carcinoma, like all neuroendocrine carcinomas, is positive for synaptophysin. CD45 is positive in hematolymphoid neoplasms, CDX2 is positive in glandular rectal lesions and napsin A is positive in lung adenocarcinoma.
Comment Here
Reference: Anus & perianal area - Neuroendocrine carcinoma
Comment Here
Reference: Anus & perianal area - Neuroendocrine carcinoma
Board review style question #2
Which of the following is true about neuroendocrine carcinoma of the anus?
- It has a good prognosis
- It is associated with HPV infection
- It is the most common anal malignancy
- It shows morphologic features unique to this site
Board review style answer #2
B. It is associated with HPV infection. High risk HPV is usually detectable in anal neuroendocrine carcinoma, though at low copy numbers. This malignancy is rare and aggressive. Morphologically, it is indistinguishable from neuroendocrine carcinoma arising at other sites.
Comment Here
Reference: Anus & perianal area - Neuroendocrine carcinoma
Comment Here
Reference: Anus & perianal area - Neuroendocrine carcinoma
Neuroendocrine tumor (pending)
[Pending]
Paget disease
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Etiology | Diagnosis | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Intraepithelial tumor usually of apocrine or possibly eccrine gland origin
Essential features
- Intraepidermal neoplastic cells, sometimes linked to anal malignancy (secondary) but may also be primary disease
- More common in older patients
- Most associated malignancies are adenocarcinoma but pagetoid spread of other carcinoma types can occur
Terminology
- Primary Paget disease: no association with underlying invasive malignancy
- Secondary Paget disease: related to intraepithelial spread of invasive malignancy
ICD coding
- ICD-10: C21.0 - malignant neoplasm of anus, unspecified
Epidemiology
- Primary extramammary anal Paget disease is rare
- Men and women are equally affected
- Most often occurs in the fifth to seventh decade
- Typically indolent but often recurs
- Up to 40% of patients with anal Paget disease have secondary disease (associated with an underlying malignancy); usually adenocarcinoma but sometimes other forms, such as neuroendocrine carcinoma (Colorectal Dis 2023 Mar 21 [Epub ahead of print], Pathol Int 2004;54:630)
Sites
- Arises anywhere between the dentate line and the perianal skin
Etiology
- May be related to epithelial - mesenchymal transition (Pathol Res Pract 2023;242:15434)
Diagnosis
- Tissue sampling
Case reports
- 64 year old man with perianal irritation (Int J Surg Case Rep 2012;3:483)
- 65 year old woman with perianal itching (Indian J Surg Oncol 2017;8:619)
- 72 year old woman with perianal bleeding (Dis Colon Rectum 2008;51:1842)
Treatment
- Wide local excision or radiotherapy (Surg Oncol 2011;20:e61, Curr Oncol 2012;19:e496)
Clinical images
Gross description
- Erythematous, ulcerated or eczematous plaques or patches
Microscopic (histologic) description
- Large, pale staining to clear intraepidermal neoplastic cells containing abundant mucin, arranged singly or occasionally in nests or gland-like formations
- Cells are more numerous basally
- Microscopic disease may extend beyond grossly visible changes
- Associated changes include squamous hyperplasia, fibroepithelioma-like hyperplasia and papillomatous hyperplasia (Am J Surg Pathol 2000;24:543)
- Pagetoid cells arising in association with a nonadenocarcinoma malignancy should have an appearance related to the causative malignancy (e.g., neuroendocrine carcinoma) (Pathol Int 2004;54:630)
Microscopic (histologic) images
Positive stains
- Mucicarmine, PAS, MUC5AC, GCDFP-15, CK7, CEA, EMA
- GCDFP-15 in primary disease; CDX2 in secondary disease (Diagn Pathol 2020;15:29)
- Immunophenotype may be different in unusual scenarios with secondary disease of nonanal origin (e.g., CK7 focally positive if related to rectal neoplasm) (Gastroenterology Res 2016;9:99)
Sample pathology report
- Anus, biopsy:
- Paget disease (see comment)
- Comment: The cells are positive for CK7 by immunohistochemistry, confirming the diagnosis. Anal Paget disease sometimes arises in the setting of an invasive anal malignancy. Further clinical workup may be indicated.
Differential diagnosis
- Atypical regenerative basal keratinocytes:
- Intercellular bridges
- Melanoma:
- Pagetoid spread by rectal adenocarcinoma:
- GCDFP-15 negative (Arch Pathol Lab Med 1998;122:1077)
- Squamous anal intraepithelial neoplasia:
- Therapy effect:
- Abnormal reactive cells are negative for CK7 and mucicarmine (Am J Surg Pathol 2018;42:1472)
Board review style question #1
Board review style answer #1
C. Anal Paget disease is positive for CK7 and mucicarmine. It is often but not always secondary, occurring alongside an invasive malignancy. It arises equally in men and women. Treatment related reactive changes may mimic Paget disease.
Comment Here
Reference: Anus & perianal area - Paget disease
Comment Here
Reference: Anus & perianal area - Paget disease
Board review style question #2
What is the typical behavior of anal Paget disease?
- It can metastasize even in the absence of adenocarcinoma
- It is generally indolent but may recur
- It spontaneously regresses
- It will always progress to invasive adenocarcinoma
Board review style answer #2
B. Anal Paget disease is generally indolent but may recur. Fewer than half of anal Paget disease cases are associated with invasive malignancy. It does not itself metastasize but it does not spontaneously regress either.
Comment Here
Reference: Anus & perianal area - Paget disease
Comment Here
Reference: Anus & perianal area - Paget disease
Squamous cell carcinoma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Pathophysiology | Clinical features | Diagnosis | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Primary squamous cell carcinoma of the anus
Essential features
- Most common carcinoma of the anal tract
- Increasing in incidence, although still relatively rare (incidence of Can arise either above or below the dentate line
Terminology
- WHO officially only recognizes squamous cell carcinoma
- Basaloid variant has also been termed cloacogenic or transitional
- Mucoepidermoid carcinoma may be used for tumors with prominent mucinous features, although the biology may be different (J Gastroenterol 2001;36:508)
ICD coding
- ICD-10: C44.520 - squamous cell carcinoma of anal skin
Epidemiology
- Tumors above dentate line: more common in women; usually diagnosed in sixth decade
- Tumors below dentate line: more common in men; usually diagnosed in third decade
Pathophysiology
- Tumors below the dentate line usually have an associated finding (condyloma, fistula, radiation, etc.)
- Can be caused by high risk HPV (Cancer Res 1999;59:753)
- EGFR overexpression is common (Mod Pathol 2006;19:942)
Clinical features
- Presenting symptoms include rectal bleeding, pain, mass
Diagnosis
- Tissue sampling
Radiology description
- MRI is the preferred imaging modality for staging purposes (Korean J Radiol 2017;18:946)
- On T2 weighted images: higher signal intensity than skeletal muscles but lower than ischioanal fat
- Markedly enhanced after injection of gadolinum contrast
Prognostic factors
- Prognosis depends on AJCC stage of tumor
- Distal tumors have a better prognosis (slower growth, earlier detection)
- Better prognosis with higher radiation dose and no / shorter treatment interruptions (Gastrointest Cancer Res 2008;2:10)
- Worse prognosis if older, male or with HIV (Dis Colon Rectum 2009;52:624, Dis Colon Rectum 2001;44:1496)
- HPV and p53 status can stratify prognosis (worst prognosis: HPV negative, p53 aberrant staining) (Mod Pathol 2021;34:1017)
Case reports
- 45 year old man with perianal mass (Int J Surg Case Rep 2021;81:105739)
- 62 year old woman with firm anal mass (Surg Case Rep 2022;8:119)
- 63 year old woman with brain metastases (J Neurooncol 2011;101:141)
Treatment
- Surgery with chemoradiation
Gross description
- Anal canal tumors are nodular, ulcerated, ≥ 3 - 4 cm
- Invade deeply into wall and spread proximally and distally into submucosa of the distal rectum and proximal anus
Microscopic (histologic) description
- Resembles squamous cell carcinoma as seen elsewhere in body
- Tumors often display multiple morphologic patterns, calling into question the utility of subdividing the entity
- May be keratinizing (usually below the dentate line) or nonkeratinizing (anywhere, although tumors above the dentate line are usually nonkeratinizing)
- Uncommon basaloid subtype shows plexiform pattern and palisading of small undifferentiated cells around the border, with central necrosis of tumor nodules (also mitotic figures, invasion, desmoplastic stroma) (Am J Surg Pathol 2016;40:354)
- May have massive eosinophilic infiltration, mucoepidermoid features (with mucinous microcysts) or poorly differentiated morphology
- Can show small cell anaplastic features (but without evidence of neuroendocrine differentiation)
- Often replaces crypts of adjacent rectal mucosa
- May show overlying / adjacent dysplastic changes (anal intraepithelial neoplasia)
Microscopic (histologic) images
Positive stains
Molecular / cytogenetics description
- May have mutations in PIK3CA, FBXW7, TP53, CDKN2A (Mod Pathol 2021;34:1017)
Sample pathology report
- Anus, mass, biopsy:
- Focal invasive squamous cell carcinoma, arising in a background of high grade squamous intraepithelial lesion (AIN 3)
- Anus, resection:
- Squamous cell carcinoma, moderately differentiated (see synoptic report)
Differential diagnosis
- Basal cell carcinoma:
- Fewer mitotic figures, smaller cells, retraction artifact
- Small cell carcinoma:
- Can resemble basaloid squamous cell carcinoma but expresses neuroendocrine markers
- Verrucous carcinoma:
- Grows in an exophytic, not infiltrative, pattern
Additional references
Board review style question #1
Board review style answer #1
C. Patients with HIV have a worse prognosis. Poor prognostic factors in anal squamous cell carcinoma include older age, male sex and HIV positivity. Cases above the dentate line are more common in older women and cases below the dentate line are more common in younger men. Most cases are squamous cell carcinoma, NOS; basaloid examples are uncommon.
Comment Here
Reference: Squamous cell carcinoma
Comment Here
Reference: Squamous cell carcinoma
Board review style question #2
Which of the following etiologic subtypes of anal squamous cell carcinoma has the worst prognosis?
- HPV negative, p53 aberrant staining
- HPV negative, p53 wild type staining
- HPV positive, p53 aberrant staining
- HPV positive, p53 wild type staining
Board review style answer #2
A. HPV negative, p53 aberrant staining. Patients with this subtype have the worst prognosis. HPV positive cancers with p53 wild type staining confer the best prognosis and the other choices have an intermediate prognosis.
Comment Here
Reference: Squamous cell carcinoma
Comment Here
Reference: Squamous cell carcinoma
Squamous dysplasia
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Noninvasive neoplastic proliferation of the anal squamous epithelium with cytologic and architectural abnormalities
- Associated with human papillomavirus (HPV) infection
Essential features
- Often associated with human papillomavirus genotypes
- Low risk HPV mediates the development of low grade lesions
- High risk HPV mediates the development of high grade lesions
- Most cases are incidental findings in asymptomatic patients
- Immunocompromised patients are at increased risk
- Progression to squamous cell carcinoma is influenced by immune status and smoking
Terminology
- Squamous intraepithelial lesion (SIL)
- Anal intraepithelial neoplasia (anal IEN / AIN)
- Anal squamous intraepithelial neoplasia (ASIN)
ICD coding
- ICD-O:
- ICD-11:
- 2E92.5 & XH3Y37 - benign neoplasm of anus or anal canal & esophageal squamous intraepithelial neoplasia (dysplasia), low grade
- 2E61.2 & XH9ND8 - carcinoma in situ of anal canal & esophageal squamous intraepithelial neoplasia (dysplasia), high grade
Epidemiology
- Rising incidence due to evolving sexual behavior and an increase in HPV infection rates
- Immunodeficient (HIV and non-HIV immunosuppression) patients are at increased risk and present at a younger age
- Additional risk factors: anoreceptive intercourse, coinfection with other sexually transmitted diseases and cigarette smoking (Papillomavirus Res 2017;4:90, Br J Cancer 2015;112:1568)
- Incidence is higher in women and is unrelated to the prevalence of HIV, unlike in men
Sites
- Anus: anal canal, usually the transitional zone
- Also occurs in the perianal skin (
Pathophysiology
- HPV associated oncoproteins:
- E6
- Inactivates p53, allowing survival of cells with genotoxic damage
- Inhibits p21, leading to loss of cyclin E / CDK2 inhibition and progression through the cell cycle
- E7
- Inhibits RB1, leading to a continuous proliferation of mutation bearing cells
- Loss of RB1 leads to overexpression of p16, which is used as a diagnostic surrogate marker
- Inhibits RB1, leading to a continuous proliferation of mutation bearing cells
- E6
- Reference: World J Gastrointest Oncol 2022;14:369
Etiology
- Human papillomavirus (HPV) (Tumour Virus Res 2022;14:200239)
- Double stranded DNA virus of Papillomaviridae family
- Infects humans only
- More than 200 types
- Low risk genotypes (6, 11, 42, 43, 44) associated with low grade lesions
- HPV 6 and 11 are most common in > 90% of condyloma acuminatum
- High risk genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68) associated with high grade lesions and invasive squamous cell carcinoma
- HPV 16 is most common in 28% of low grade and 68% of high grade lesions
- > 1 serotype may be found in lesions
- Low risk genotypes (6, 11, 42, 43, 44) associated with low grade lesions
- Subdivided based on tissue tropism (Cancer Epidemiol Biomarkers Prev 2008;17:1611):
- Anogenital
- Cutaneous
- Other mucosal sites
- Multicentricity is common; > 33% of women with anal HPV also have cervical HPV
- Most are asymptomatic or resolve within 12 months (J Natl Cancer Inst 2008;100:513)
- A small fraction persists or progresses to squamous dysplasia or squamous cell carcinoma
Clinical features
- Often asymptomatic, some present with bleeding or pruritis
- Most cases are incidental findings in benign minor surgical specimens such as hemorrhoids
- May present as papules, plaques, polyps or mass lesions
Diagnosis
- Anal exfoliative cytology
- Sensitivity and specificity for detecting high grade squamous dysplasia are 71% and 73%, respectively (Clin Infect Dis 2018;67:1262)
- High resolution anoscopy with tissue biopsy is the gold standard (J Low Genit Tract Dis 2016;20:283)
Laboratory
Prognostic factors
- Most LSIL will regress in immunocompetent patients (Arch Pathol Lab Med 2012;136:1266)
- Recurrence rate of HSIL after wide local excision ranges between 9 - 63% (Dis Colon Rectum 2012;55:735)
- Recurrence rate is much higher in HIV patients
- 53 - 67% recurrence rate over 18 - 24 months in HIV+ women and 24 - 53% in HIV+ men (J Acquir Immune Defic Syndr 2020;84:66, AIDS 2017;31:1245)
- May progress to squamous cell carcinoma
- Recurrence rate is much higher in HIV patients
Case reports
- 43 year old man with HIV presented with persistent bloody stool (Int J Clin Exp Pathol 2014;7:3456)
- 55 year old woman was referred to colonoscopy because of constipation and hematochezia (ACG Case Rep J 2022;9:e00792)
- 65 year old woman was found to have a white flat lesion in contact with the dentate line on screening colonoscopy (Acta Gastroenterol Belg 2022;85:108)
Treatment
- Treatment modalities vary by geographic location
- Fulguration with electrocautery during anoscopy
- For lesions that are too large for the office based procedure
- Office based ablation techniques
- Via infrared coagulation, hyfrecation, argon plasma coagulation or radiofrequency
- Topical therapy (Lancet Oncol 2013;14:346)
- 5-fluorouracil (5FU)
- Imiquimod
- Trichloroacetic acid (TCA)
- HPV vaccination (N Engl J Med 2011;365:1576, Hum Vaccin Immunother 2016;12:1348)
- Decreases the risk of recurrence in high grade lesions
- Effective in anal cancer prevention when administered early
- Fulguration with electrocautery during anoscopy
- Posttreatment surveillance is required given the high rate of local recurrence
- Reference: J Anus Rectum Colon 2022;6:92
Clinical images
Gross description
- May present as papules, plaques or mass lesions
- When present at the anal canal or dentate line, may be mistaken as rectal polyp
Microscopic (histologic) description
- Diagnosis and grading of dysplasia is based on cellular atypia, degree of maturation and mitotic rate (Hum Pathol 2022 Jul 16 [Epub ahead of print])
- Mild dysplasia (AIN1, condyloma acuminatum)
- Cytologic and architectural atypia involving the lower 33% of the epithelial thickness
- Often shows koilocytic changes characterized as perinuclear cavitation and nuclear features of low grade squamous intraepithelial lesion, to include nuclear enlargement, coarse chromatin and irregular nuclear membranes (StatPearls: Koilocytosis [Accessed 4 November 2022])
- Pathognomonic but not required for diagnosis
- Often shows koilocytic changes characterized as perinuclear cavitation and nuclear features of low grade squamous intraepithelial lesion, to include nuclear enlargement, coarse chromatin and irregular nuclear membranes (StatPearls: Koilocytosis [Accessed 4 November 2022])
- Condyloma acuminatum is exophytic, papillary growth of squamous lesion with parakeratosis and koilocytosis
- Cytologic and architectural atypia involving the lower 33% of the epithelial thickness
- Moderate dysplasia (AIN2): cytologic and architectural atypia involving > 33% but
- Increased nuclear enlargement and coarse chromatin, compared to AIN1
- Increased mitosis
- Mild dysplasia (AIN1, condyloma acuminatum)
- Severe dysplasia (AIN3): cytologic and architectural atypia involving > 66% of the epithelial thickness
- Significant nuclear enlargement and atypia, high N:C ratios, increased mitosis with often atypical mitotic figures
- Sometimes interchangeable with carcinoma in situ, which is full thickness squamous dysplasia without any maturation
- Low grade squamous intraepithelial lesion (LSIL)
- Cytologic and architectural alteration involving Includes lesions that were previously classified as mild dysplasia, anal IEN 1 (AIN1) and condyloma acuminatum
- High grade squamous intraepithelial lesion (HSIL)
- Cytologic and architectural alteration involving > 33% of the epithelium
- Includes lesions that were previously classified as moderate dysplasia, severe dysplasia, carcinoma in situ, Bowen disease and bowenoid papulosis
Microscopic (histologic) images
Cytology description
- Can be categorized as LSIL, HSIL or atypical squamous cells of uncertain significance (ASCUS)
- LSIL: hyperchromasia, nuclear irregularity, koilocytotic atypia and > 3 fold nuclear enlargement compared to superficial squamous cells
- HSIL: small, immature appearing cells with markedly increased N:C ratio, coarse chromatin and irregular nuclear contour
- ASCUS: typical cells that do not meet the criteria for LSIL or HSIL
- Reference: Diagn Cytopathol 2010;38:538
Cytology images
Positive stains
- p16:
- Positive is defined as diffuse, strong, nuclear and cytoplasmic block-like staining
- Surrogate marker for high risk HPV infection
- Distinguish HSIL from reactive change (91% sensitivity) (Am J Surg Pathol 2021;45:1573)
- Distinguish AIN2 from AIN1 (89% sensitivity) (Am J Surg Pathol 2021;45:1573)
- Can be positive in LSIL / AIN1 (37%) (Am J Surg Pathol 2021;45:1573)
- Morphology is still the key for diagnosis
- p16 is not recommended when histologic diagnosis is clear cut
- HPV immunostain: positive nuclear staining highlights virally infected cells
- HPV RNA in situ hybridization
- Detect HPV E6 / E7 mRNA transcripts in both transcriptionally active state and formalin fixed paraffin embedded (FFPE)
- Positive staining with dot-like cytoplasmic and nuclear staining
- High sensitivity and specificity (Am J Surg Pathol 2017;41:607)
- More sensitive than HPV immunostain
- HPV DNA in situ hybridization
- High specificity (89%) but lower sensitivity (83%)
- Ki67: proliferation beyond the basal layer
Negative stains
- p16: negative or weak and patchy in low grade lesions
Molecular / cytogenetics description
- Polymerase chain reaction
- Quantitative reverse transcriptase PCR to detect HPV E6 / E7 mRNA
- Considered the gold standard by the FDA
- Limited utility due to exclusiveness to fresh frozen tissue
- Quantitative PCR to detect HPV DNA
- Cannot distinguish transcriptionally active infection versus passenger
- Quantitative reverse transcriptase PCR to detect HPV E6 / E7 mRNA
- Reference: Infect Agent Cancer 2020;15:46
Sample pathology report
- Anal canal, right lateral, biopsy:
- High grade squamous intraepithelial lesion (moderate dysplasia, AIN2)
Differential diagnosis
- Inflammatory changes / repair:
- Superficial maturation and cellular polarity are maintained
- Round nuclei and may have prominent nucleoli
- No hyperchromasia
- Radiation atypia:
- Cytomegaly and karyomegaly with the maintenance of a low N:C ratio
- Smudgy chromatin with prominent nucleoli
- Squamous cell carcinoma:
- Foci of invasion
- Mass forming
- Adjacent area may show AIN as a precursor lesion
Additional references
Board review style question #1
An anal lesion is excised from a 33 year old woman (see image above). No area of invasion is identified on histologic examination. Which of the following statements is true?
- Immunocompromised patients are at increased risk and present at a younger age
- Most cases are incidental findings in asymptomatic patients and do not recur after excision
- Often associated with human papillomavirus genotype 6 and 11
- p16 is often patchy in high grade lesions
Board review style answer #1
A. Immunocompromised patients (particularly those HIV+) are at increased risk and present at a younger age
Comment Here
Reference: Squamous dysplasia
Comment Here
Reference: Squamous dysplasia
Board review style question #2
Regarding human papillomavirus (HPV), which of the following is correct?
- Diffuse p16 immunostaining positivity is a surrogate marker for both low and high risk HPV infection
- High risk HPVs can be associated with low grade lesions
- It is a single stranded RNA virus that does not insert into the human genome
- It only infects the squamous epithelium
Board review style answer #2
B. High risk HPVs can be associated with low grade lesions. While high risk HPVs are usually associated with high grade dysplasia, they can be associated with low grade lesions.
Comment Here
Reference: Squamous dysplasia
Comment Here
Reference: Squamous dysplasia
Staging
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Primary tumor (pT) | Regional lymph nodes (pN) | Distant metastasis (pM) | Prefixes | Primary tumor suffix | Regional lymph nodes suffix | AJCC prognostic stage groups | Prognostic tumor characteristics | Registry data collection guidance | Emerging factors for collection | Histologic grade (G) | Histopathologic type | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- All carcinomas of the anus, including poorly differentiated neuroendocrine carcinomas, are covered by this staging system
- AJCC staging guidelines do not exist for anal mucosal melanomas, well differentiated neuroendocrine tumors or premalignant lesions
Essential features
- AJCC 7th edition staging was discontinued on December 31, 2017; as of January 1, 2018, use of the 8th edition is mandatory
- AJCC 9th edition was released in 2023, with minor changes from the 8th edition, mostly to pN category staging and overall stage groupings
Terminology
- Anal cancer: tumors that develop from mucosa that cannot be visualized entirely when gentle traction is placed on the buttocks
- Perianal cancer: tumors that arise within the skin at or distal to the squamous mucocutaneous junction, can be seen entirely with gentle traction on the buttocks and are within 5 cm of the anus
- Older terms for high grade squamous intraepithelial lesion (pTis disease) include carcinoma in situ, Bowen disease, anal intraepithelial neoplasia II - III and high grade anal intraepithelial neoplasia
ICD coding
- ICD-10: C21.0 - malignant neoplasm of anus, unspecified
Primary tumor (pT)
Regional lymph nodes (pN)
- NX: regional lymph nodes cannot be assessed
- N0: no tumor involvement of regional lymph node(s)
- N1: tumor involvement of regional lymph node(s)
- N1a: tumor involvement of inguinal, mesorectal, superior rectal, internal iliac or obturator lymph node(s)
- N1b: tumor involvement of external iliac lymph node(s)
- N1c: tumor involvement of N1b (external iliac) with any N1a node(s)
Distant metastasis (pM)
- M0: no distant metastasis
- M1: microscopic confirmation of distant metastasis
Prefixes
- c: clinical
- p: pathological
- yc: posttherapy clinical
- yp: posttherapy pathological
- rc: recurrence / retreatment clinical
- rp: recurrence / retreatment pathological
- a: autopsy
Primary tumor suffix
- (m): multiple synchronous primary tumors
Regional lymph nodes suffix
- (sn): sentinel node procedure
- (f): fine needle aspiration (FNA) or core needle biopsy
AJCC prognostic stage groups
| Stage group I: | T1 | N0 | M0 |
| Stage group IIA: | T2 | N0 | M0 |
| Stage group IIB: | T1 - 2 | N1 | M0 |
| Stage group IIIA: | T3 | N0 - N1 | M0 |
| Stage group IIIB: | T4 | N0 | M0 |
| Stage group IIIC: | T4 | N1 | M0 |
| Stage group IV: | any T | any N | M1 |
Prognostic tumor characteristics
- Human papillomavirus (HPV) status or p16 expression status (almost always positive)
- Histologic type (adenocarcinoma and neuroendocrine carcinoma have worse prognosis than squamous cell carcinoma)
- Human immunodeficiency virus (HIV) status (HIV positivity may indicate worse prognosis)
- Gender (male sex negatively impacts prognosis)
- Tumor location: anal, perianal or perineal (location influences surgery type and radiation options)
Registry data collection guidance
- Grade
Emerging factors for collection
- HPV p16 expression status
Histologic grade (G)
- GX: grade cannot be assessed
- G1: well differentiated (low grade)
- G2: moderately differentiated (low grade)
- G3: poorly differentiated (high grade)
- G4: undifferentiated (high grade)
Histopathologic type
- Squamous cell carcinoma
- Verrucous carcinoma
- High grade neuroendocrine carcinoma
- Large cell neuroendocrine carcinoma
- Small cell neuroendocrine carcinoma
- Adenocarcinoma
- Carcinoma, undifferentiated
- Basal cell carcinoma
Board review style question #1
Which of the following criteria characterizes a malignancy as an anal, rather than a perianal, tumor?
- Arises distal to the squamous mucocutaneous junction
- Cannot be entirely visualized after gentle traction on the buttocks
- Squamous cell or basal cell carcinoma histologically
- Visible only on colonoscopy
Board review style answer #1
B. Cannot be entirely visualized after gentle traction on the buttocks. Such cases are defined as anal tumors. Answer A is incorrect because cases that arise distal to the squamous mucocutaneous junction are considered perianal tumors. Answers C and D are incorrect because they are not used as criteria for determining the site of a tumor.
Comment Here
Reference: Anus - Staging
Comment Here
Reference: Anus - Staging
Board review style question #2
Which of the following criteria characterizes an anal squamous cell carcinoma as pT3?
- Invasion of adjacent organs
- Size ≤ 2 cm
- Size > 2 cm but ≤ 5 cm
- Size > 5 cm
Board review style answer #2
D. Size > 5 cm. This is the TNM criterion for pT3 disease.
Answer A is incorrect because it indicates pT4 disease. Answer B is incorrect because it indicates pT1 disease. Answer C is incorrect because it indicates pT2 disease.
Comment Here
Reference: Anus - Staging
Comment Here
Reference: Anus - Staging
Syphilis
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Sexually transmitted infection caused by Treponema pallidum, a gram negative spirochete
- Can affect multiple sites, including the anus, with multiple stages of disease and nonspecific presentations (the great imitator, which is believed to have been coined by Sir William Osler) (Can Med Assoc J 1976;114:503)
Essential features
- Syphilis infections are caused by T. pallidum and are generally regarded as sexually transmitted infections that disproportionally affect men who have sex with men and non-Hispanic persons
- Most cases demonstrate a dense plasma cell inflammatory infiltrate on histology
- T. pallidum IHC provides increased sensitivity and specificity over silver stains (Warthin-Starry or Steiner)
- Clinical and histopathologic features of all disease stages can be general and nonspecific, so appropriate clinical and pathologic work up should be done in all cases in which syphilis is suspected
ICD coding
Epidemiology
- World Health Organization (WHO) estimated 7.1 million new adult cases of syphilis worldwide in 2020 with increasing incidence in the United States (WHO: Syphilis [Accessed 22 September 2023], CDC: Sexually Transmitted Disease Surveillance 2021 [Accessed 22 September 2023])
- Men who have sex with men and non-Hispanic persons are disproportionately affected (CDC: Sexually Transmitted Disease Surveillance 2021 [Accessed 22 September 2023])
- Dual diagnosis with HIV is common and syphilis increases the risk of acquiring HIV as well as other sexually transmitted infections
- Majority of cases are transmitted through vaginal, anogenital and orogenital contact with an infectious lesion during the primary or secondary stages of the disease
- Rarely can be transmitted through nonsexual contact including skin to skin and blood transfusion
- Congenital syphilis can be acquired, at any stage, via transplacental transmission or contact with an infectious lesion during birth (N Engl J Med 2020;382:845)
Sites
Pathophysiology
- Syphilis is primarily transmitted through contact with infectious lesions and disseminates through the blood stream and lymphatics within days, ultimately invading distant tissues (N Engl J Med 2020;382:845)
Etiology
- Sexually transmitted infection caused by Treponema pallidum, a gram negative spirochete
Clinical features
- Primary syphilis
- Single firm, round, painless sore called a chancre at the site of original infection
- Persists for 3 - 6 weeks before spontaneously resolving
- Secondary syphilis
- Maculopapular, nonpruritic rash, typically on the palms and soles
- Large white or gray raised lesions (condyloma lata), commonly on the perineum, which can ulcerate
- Fever, lymphadenopathy, patchy alopecia, weight loss, muscle aches and fatigue are also common
- Latent phase
- No clinical symptoms
- Can last for years
- Tertiary syphilis
- Can affect multiple organ systems, commonly causing destructive visceral, cardiovascular or neurological disorders as well as severe skin lesions (gummas)
- Can appear 10 - 30 years after initial infection
- Can be fatal
- References: CDC: Sexually Transmitted Diseases (STDs) - Syphilis - CDC Detailed Fact Sheet [Accessed 22 September 2023], WHO: Syphilis [Accessed 22 September 2023], Front Cell Infect Microbiol 2021;10:574806, N Engl J Med 2020;382:845
Diagnosis
- Diagnosis relies on history, physical examination and interpretation of laboratory tests (Front Cell Infect Microbiol 2021;10:574806)
- Definitive diagnosis methods for early stages of syphilis include (CDC: Sexually Transmitted Infections Treatment Guidelines, 2021 - Syphilis [Accessed 22 September 2023])
- Direct visualization of T. pallidum through darkfield microscopy
- Molecular tests detecting T. pallidum from lesion exudate or tissue, including PCR
- Darkfield microscopy is not a recommended technique for identification of T. pallidum in the gastrointestinal tract due to not being able to exclude commensal gut treponemes (Open Forum Infect Dis 2021;8:ofab157)
- Presumptive diagnosis of syphilis requires use of 2 laboratory serologic tests: both a nontreponemal test and treponemal test (CDC: Sexually Transmitted Infections Treatment Guidelines, 2021 - Syphilis [Accessed 22 September 2023])
Laboratory
- Nontreponemal tests include
- Venereal Disease Research Laboratory (VDRL)
- Rapid plasma reagin (RPR) test
- Generally used as screening tests
- Treponemal tests include
- T. pallidum passive particle agglutination (TPPA) assay
- Enzyme immunoassays (EIAs)
- Chemiluminescence immunoassays (CIAs) and immunoblots
- Rapid treponemal assays
- Treponemal antibodies appear earlier than nontreponemal antibodies and usually remain detectable for life, even after successful treatment
- Reference: CDC: Sexually Transmitted Infections Treatment Guidelines, 2021 - Syphilis [Accessed 22 September 2023]
Prognostic factors
- Favorable prognosis with early detection and appropriate treatment
- High risk sexual activity is the strongest risk factor for repeat infection (Open Forum Infect Dis 2020;7:ofaa019)
Case reports
- Man in his early 20s with perianal lesions (Aust Fam Physician 2016;45:209)
- 31, 44, 51 and 52 year old men with anal ulcers (Arch Pathol Lab Med 2015;139:1156)
- 35 year old woman with HIV and exophytic vulvar and perianal lesions (Gynecol Oncol Rep 2023;46:101158)
- 37 year old man with HIV and anal ulcer (Perm J 2018;22:17)
- 47 year old man with ulcerated mass at anorectal junction (BMJ Case Rep 2019;12:e226595)
Treatment
- Penicillin is the gold standard of treatment
- Type, dose and duration depends on disease stage
- Pregnant patients allergic to penicillin should be desensitized before receiving penicillin treatment
- Doxycycline, tetracycline and potentially ceftriaxone can be used in nonpregnant patients allergic to penicillin
- Condoms and other types of barrier protection can help prevent disease
- Reference: CDC: Sexually Transmitted Diseases (STDs) - Syphilis - CDC Detailed Fact Sheet [Accessed 22 September 2023]
Clinical images
Microscopic (histologic) description
- Largely nonspecific on H&E sections
- While plasma cells can be present in all stages of the disease, plasma cells may be absent in some cases (Am J Surg Pathol 2018;42:472)
- Primary / chancre
- Ulcer with intense plasma cell infiltrate with scattered macrophages and lymphocytes
- Obliterative endarteritis with endothelial cell proliferation that progresses to intimal fibrosis
- Regional lymph nodes are enlarged due to nonspecific acute or chronic lymphadenitis, plasma cell rich infiltrates or granulomas
- Secondary / condyloma lata
- Epithelial hyperplasia
- Plasma cell infiltrate with scattered macrophages and lymphocytes that can be less intense than primary syphilis
- Obliterative endarteritis with endothelial cell proliferation that progresses to intimal fibrosis
- Ulceration may be present
- Tertiary / gummas
- Centers of coagulative necrosis
- Edges of the lesion comprise of palisading macrophages and fibroblasts surrounded by a large amount of inflammatory cells that are mostly plasma cells
- T. pallidum is hard to visualize in these lesions, even with stains and IHC
- Reference: Int J Surg Case Rep 2015;17:69
Microscopic (histologic) images
Positive stains
- Silver stains (Warthin-Starry or Steiner) were historically used
- T. pallidum IHC provides increased sensitivity and specificity (Front Cell Infect Microbiol 2021;10:574806)
Videos
Perianal condyloma lata
Syphilis stains and IHC
Sample pathology report
- Skin, perianal, biopsy:
- Spirochetes present on silver stain and T. pallidum immunostain, consistent with syphilis (see comment)
- Comment: Serologic and clinical correlation is recommended.
Differential diagnosis
- Anal squamous cell carcinoma:
- Condyloma accuminata:
- Caused by HPV serotypes 6 and 11
- Hyperplastic papillary exophytic squamous epithelium
- Koilocytes in upper third of epithelium
- No plasmacytic inflammation
- p63 and low risk HPV ISH positive
- Negative T. pallidum testing
- Chancroid:
- Caused by Haemophilus ducreyi
- Painful genital ulcer associated with tender suppurative inguinal adenopathy
- Negative T. pallidum testing
- Granuloma inguinale:
- Caused by Klebsiella granulomatis
- Multiple ulcers
- Granulomatous inflammation
- Large vacuolated, foamy macrophages and histiocytes with multiple intracytoplasmic organisms (Donovan bodies)
- Negative T. pallidum testing
- Lymphogranuloma venereum:
- Caused by Chlamydia trachomatis
- Nonspecific features of ulceration and granulation tissue in dermis
- Suppurative inflammation of inguinal lymph nodes
- Negative T. pallidum testing
- Ulcerative colitis:
- Continuous pattern of ulceration progressing proximally from the rectum
- Cryptitis or crypt abscesses if active
- Crohn's disease:
- Transmural inflammation
- Cryptitis or crypt abscesses if active
- Nonnecrotizing granulomas
- Herpes simplex virus (HSV):
- Ulceration with multinucleated giant cells and nuclear inclusions
- Positive HSV DNA PCR
- Negative T. pallidum testing
- IgG4 related disease:
- Elevated total serum IgG or IgG4 levels
- Can have multiple masses in multiple different organs
- Negative T. pallidum testing
- Anal tuberculosis (TB):
- Very rare manifestation of TB
- Variety of morphologies with ulcerative being the most common (IDCases 2018;12:25)
- Chronic granulomatous inflammation
- AFB positive
- Negative T. pallidum testing
- Lymphoma:
- Atypical population of lymphocytes
- Syphilis lesions will show T cell predominate infiltrate with admixed polytypic B cells, typical of a reactive process (Am J Surg Pathol 2018;42:472)
Additional references
Board review style question #1
A 27 year old man presented with a diffuse maculopapular rash, including on his palms and soles and a 4 cm white-gray raised perianal lesion. A biopsy was done of the perianal lesions and the histology is depicted in the H&E image. Which further testing should be ordered on the biopsy to best help determine the diagnosis?
- AFB stain
- IgG4 IHC
- p63 IHC
- T. pallidum IHC
- Warthin-Starry silver stain
Board review style answer #1
D. T. pallidum IHC. The H&E image demonstrates epithelial hyperplasia with a dense subepithelial plasmacytic infiltrate indicative of condyloma lata. T. pallidum IHC is the most sensitive and specific method to highlight the spirochetes within the tissue.
Answer E is incorrect because while the Warthin-Starry silver stain may also be used to highlight T. pallidum, it is not as sensitive or specific as IHC for T. pallidum.
Answer A is incorrect because an AFB stain should be done when chronic granulomatous inflammation is seen to rule out anal tuberculosis.
Answer C is incorrect because p63 IHC is positive in condyloma accuminata, which will demonstrate koilocytes and should not have any plasmacytic inflammation.
Answer B is incorrect because IgG4 related disease presents with masses and does not present with a rash. The plasma cells in condyloma lata should also be negative for IgG4 IHC.
Comment Here
Reference: Anus & perianal area - Syphilis
Comment Here
Reference: Anus & perianal area - Syphilis
Board review style question #2
What histologic feature separates primary syphilis (chancre) from secondary syphilis (condyloma lata)?
- Coagulative necrosis
- Epithelial hyperplasia
- Granulomas
- Obliterative endarteritis
Board review style answer #2
B. Epithelial hyperplasia is seen with secondary syphilis lesions (condyloma lata) but not with primary syphilis lesions (chancre), in which ulceration is more common.
Answer C is incorrect because granulomas are not specific for any stage of syphilis nor specific for syphilis.
Answer A is incorrect because coagulative necrosis is seen in tertiary syphilis lesions (gummas) but not in chancres or condyloma lata.
Answer D is incorrect because obliterative endarteritis can be seen in both primary and secondary syphilis.
Comment Here
Reference: Anus & perianal area - Syphilis
Comment Here
Reference: Anus & perianal area - Syphilis
Tailgut cyst
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Diagrams / tables | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Cystic developmental anomaly of the retrorectal / presacral space that is thought to arise from remnants of the embryonic tailgut
Essential features
- Congenital, well demarcated, usually multiloculated, cystic lesion of the retrorectal / presacral space that is thought to arise from remnants of the embryonic tailgut
- Preoperative diagnosis relies on imaging, as fine needle biopsy is not recommended due to risk of infection or seeding of tumor cells
- Multiple types of epithelial linings have been described; stratified squamous epithelium is most common (~75%)
- Cyst wall shows disorganized / irregular smooth muscle bundles without neural plexi
- Prognosis depends upon presence or absence of malignant transformation, which can be seen in up to ~25% of cases (usually as either adenocarcinoma or well differentiated neuroendocrine tumor)
Terminology
- Retrorectal / perianal cystic hamartoma (misnomer, as there is no good evidence that this lesion is hamartomatous) (Histopathology 2023;82:232)
- Retrorectal space cyst
- Cyst of postanal intestine
- Myoepithelial hamartoma of rectum (Dis Colon Rectum 1961;4:409)
ICD coding
- ICD-10: K62.89 - other specified diseases of anus and rectum
Epidemiology
- Rare; incidence is estimated at 1 per 40,000 hospital admissions based on Mayo Clinic data (Dis Colon Rectum 1985;28:644)
- Can occur at any age
- Most commonly develops in the fifth decade of life (Ann Coloproctol 2019;35:268)
- Uncommon in children
- Female predominance (F:M = 3 - 9:1) (Ann Coloproctol 2019;35:268)
Sites
- Retrorectal / presacral space
- Retrorectal / presacral space is a potential space defined by the following boundaries (AJR Am J Roentgenol 2017;209:790)
- Anterior: rectum
- Superior: pelvic peritoneal reflection
- Inferior: levator ani muscles
- Posterior: presacral fascia, sacrum and coccyx
- Lateral: iliac vessels and ureters
Pathophysiology
- Considered a developmental anomaly
- Precise embryological origin is uncertain but likely is a remnant of the distal hindgut that extends into the vestigial / embryonic tail (Histopathology 2023;82:232)
- Involution of the embryonic tailgut occurs by the eighth week of gestation; failure of regression is thought to give rise to tailgut cyst (Arch Pathol Lab Med 2000;124:725)
Clinical features
- Clinical presentation
- ~50% asymptomatic
- Symptoms are usually nonspecific and due to local mass effect
- Constipation, decreased stool caliber, rectal pain, rectal fullness, obstruction, tenesmus, rectal bleeding (usually painless), discomfort while sitting, dysuria, urinary retention, lower limb neurological symptoms due to sacral plexus compression (Ann Coloproctol 2019;35:268)
- Rarely presents as prolapsing cyst through the anus (Eur J Pediatr Surg 2013;23:e3)
- Physical exam findings
- Palpable mass (usually nontender) on digital rectal exam
- Funnel shaped dimple in the postanal midline (European J Pediatr Surg Rep 2013;1:51)
- Fissures and fistulas, often recurrent
- Secondary infection may lead to sepsis or abscess formation
Diagnosis
- Pelvic magnetic resonance imaging (MRI)
- Ultrasound guided needle biopsy is not recommended due to risk of infection or seeding of tumor cells (Int J Colorectal Dis 2007;22:1283)
Radiology description
- Ultrasound
- Cystic lesion with internal echoes from inflammatory debris or mucus
- Computed tomography (CT)
- Well defined cystic mass in retrorectal space
- Water or soft tissue density
- Loss of well defined margins and bone destruction may be seen with malignant transformation
- Pelvic MRI (Ann Coloproctol 2019;35:268)
- Well defined cystic mass in retrorectal space
- Hypointense T1 weighted
- Hyperintense on T2 weighted
- Loss of well defined margins and bone destruction may be seen with malignant transformation
- Well defined cystic mass in retrorectal space
Prognostic factors
- Prognosis depends upon presence or absence of malignant transformation
- Excision is curative in cases without malignant transformation
- Overall metastatic potential of cases with malignant transformation is unclear, although distant metastasis has been reported (Medicine (Baltimore) 2020;99:e20941, Clin Case Rep 2023;11:e6893)
Case reports
- 50 year old woman with defecation difficulty (Medicine (Baltimore) 2020;99:e20941)
- 54 year old woman with pelvic and perineal pain (Autops Case Rep 2019;10:e2019115)
- 57 year old man with thin stool and lower pelvic heaviness (Ann Coloproctol 2020;36:54)
Treatment
- Complete surgical resection with adequate margins
- Confirmation of diagnosis
- Symptom relief
- Prevention of malignant transformation
- Surgical approach may be anterior (transabdominal with laparoscopy), posterior (paracoccygeal) or combined, depending on tumor size and position (Ann Coloproctol 2019;35:268)
- Adjuvant chemotherapy and radiotherapy in cases with carcinoma transformation
Gross description
- Well circumscribed cystic mass
- Usually multiloculated (~80%) (Am J Clin Pathol 1988;89:139)
- Variable solid areas
- Cysts may contain clear serous fluid, green fluid, amorphous debris, mucinous material or mud-like (opaque brown pasty) contents
- Size
- Range: 1 - 22 cm (Diagn Cytopathol 2000;22:376)
- Average: 3.9 cm (Am J Clin Pathol 1988;89:139)
Gross images
Microscopic (histologic) description
- Multiple types of cyst lining have been described
- Stratified squamous epithelium (most common, ~75%), likely representing a metaplastic response to inflammation (Am J Clin Pathol 1988;89:139)
- Keratinizing or nonkeratinizing
- Columnar epithelium
- Pseudostratified, stratified, goblet cell containing, ciliated
- Transitional epithelium
- Mucinous epithelium
- Cuboidal epithelium
- Stratified squamous epithelium (most common, ~75%), likely representing a metaplastic response to inflammation (Am J Clin Pathol 1988;89:139)
- More than 1 type of epithelial lining may be seen within a single cyst
- Surrounding dense fibroconnective tissue stroma with disorganized / irregular smooth muscle bundles that lack neural plexi
- Histologic evidence of rupture may be seen
- Acute and chronic inflammation
- Xanthogranulomatous reaction
- Neoplastic transformation in ~25% of cases as per comprehensive 2019 meta analysis (Colorectal Dis 2019;21:869)
- Adenocarcinoma (most common, ~43%)
- Well differentiated neuroendocrine tumors (~39%)
- Limited to case reports: squamous cell carcinoma, adenosquamous carcinoma, transitional cell carcinoma and sarcoma (An Bras Dermatol 2018;93:733, J Surg Case Rep 2015;2015:rjv085)
Microscopic (histologic) images
Sample pathology report
- Retrorectal space, excision:
- Tailgut cyst (see comment)
- Comment: Sections show a benign cyst lined by stratified squamous, transitional and ciliated columnar epithelium. Disorganized bundles of smooth muscle are present in the cyst wall, without neural plexi. The findings are consistent with a diagnosis of tailgut cyst (synonym: retrorectal cystic hamartoma). There is focal epithelial disruption with associated xanthogranulomatous inflammation, suggestive of rupture.
Differential diagnosis
- Rectal duplication cyst:
- Lined by rectal mucosa with crypts
- May show heterotopic elements (e.g., gastric, bronchial)
- Cyst lumen contains mucin
- Well developed smooth muscle layer resembling muscularis propria (2 layers, often with nerve plexi)
- Shows continuity with rectum, grossly or microscopically
- Usually unilocular
- Lined by rectal mucosa with crypts
- Epidermoid cyst:
- Lined by keratinizing squamous epithelium
- Cyst lumen contains keratinous debris
- No associated smooth muscle or adnexal structures within cyst wall
- Usually unilocular
- Dermoid cyst:
- Lined by keratinizing squamous epithelium
- Cyst wall shows associated skin adnexal structures (hair follicles, sebaceous glands, sweat glands)
- Cyst lumen contains keratinous debris and oily material
- No associated smooth muscle within cyst wall
- Usually unilocular
- Neurenteric cyst:
- Lined by columnar or cuboidal cells with or without cilia
- Diagnosis is established by presence of glial tissue or connection to spinal cord
- Anterior sacral meningocele:
- Lined by attenuated arachnoid cells
- Communicates with subarachnoid space
- Usually unilocular
- Radiographic scimitar sign is pathognomonic (Dis Colon Rectum 1988;31:806)
- Anal gland cyst:
- Communicates with anal duct or crypt
- Located near anal sphincter
- Cyst lumen contains mucin
- Sacrococcygeal teratoma:
- Contains derivatives of 2 or more germ layers (ectoderm, mesoderm, endoderm)
- Mature tissue elements, immature tissue elements or both may be seen
- Usually solid but cystic areas may develop (Dis Colon Rectum 1988;31:806)
- Most present in infancy or childhood
- Sacrococcygeal chordoma:
Board review style question #1
A 54 year old woman presented with rectal fullness and was found to have a cystic tumor in the retrorectal space on pelvic magnetic resonance imaging (MRI). The lesion was surgically resected and is shown above. Which of the following, if found on histologic examination, would be most suggestive of a tailgut cyst?
- Cyst lining comprised of attenuated arachnoid cells
- Cyst lining of rectal mucosa with associated well developed smooth muscle layer within the cyst wall
- Fibroconnective tissue containing disorganized smooth muscle bundles within the cyst wall
- Keratinizing stratified squamous epithelial lining with associated skin adnexal structures
- Physaliphorous cells showing immunoreactivity for brachyury
Board review style answer #1
C. Fibroconnective tissue cyst wall containing disorganized smooth muscle bundles is characteristic of tailgut cysts. Answer B is incorrect because an associated well developed smooth muscle layer that is muscularis propria-like and often with nerve plexi is seen in rectal duplication cysts. Answer A is incorrect because a cyst lining comprised of attenuated arachnoid cells is featured in anterior sacral meningoceles. Answer D is incorrect because keratinizing stratified squamous epithelial lining with associated skin adnexal structures is seen in dermoid cysts. Answer E is incorrect because physaliphorous cells showing immunoreactivity for brachyury is found in cystic sacrococcygeal chordomas.
Comment Here
Reference: Tailgut cyst
Comment Here
Reference: Tailgut cyst
Board review style question #2
A 59 year old woman presented with constipation and was found to have a cystic tumor in the presacral space. The lesion was surgically resected and submitted for histopathological evaluation. A diagnosis of tailgut cyst was rendered. Which of the following is true regarding the diagnosis?
- Fine needle biopsy is recommended prior to surgical excision
- Prognosis depends upon the presence or absence of malignant transformation
- Squamous cell carcinoma is invariably present on microscopic examination when the lesion is completely submitted for histopathological evaluation
- Tends to be unilocular
- The cyst wall usually contains a well developed smooth muscle layer with nerve plexi
Board review style answer #2
B. The prognosis of tailgut cysts depends upon the presence or absence of malignant transformation. It is reported that up to ~25% of tailgut cysts show malignant transformation, most commonly as adenocarcinoma or well differentiated neuroendocrine tumors, although squamous cell carcinoma, adenosquamous carcinoma, transitional cell carcinoma and sarcoma have also been reported. Answer A is incorrect because fine needle biopsy is not recommended prior to surgical excision due to risk of infection or seeding of tumor cells. Answer C is incorrect because squamous cell carcinoma arising in association with tailgut cyst is extremely rare and limited to case reports. Answer D is incorrect because tailgut cysts are typically multilocular (~80%). Answer E is incorrect because tailgut cysts show disorganized / irregular smooth muscle bundles without nerve plexi within the cyst wall, as opposed to rectal duplication cysts, which show a well developed smooth muscle layer (muscularis propria-like) with nerve plexi.
Comment Here
Reference: Tailgut cyst
Comment Here
Reference: Tailgut cyst
Verrucous carcinoma
Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Pathophysiology | Clinical features | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Uncommon lower grade variant of squamous cell carcinoma
- Considered intermediate between condyloma acuminatum and squamous cell carcinoma
Essential features
- Uncommon very well differentiated form of squamous cell carcinoma, generally negative for HPV (WHO Classification of Tumours Editorial Board: Digestive System Tumours, 5th Edition, 2019)
- Does not metastasize; decent prognosis overall
- May transform into conventional squamous cell carcinoma
Terminology
- Giant condyloma acuminatum / Buschke-Löwenstein tumor: sometimes postulated to represent the same entity or spectrum of disease (Histopathology 1985;9:1155)
- Large condyloma acuminatum term has been proposed for tumors 2 - 10 cm, with giant referring to those > 10 cm (Mod Pathol 2008;21:112A)
Epidemiology
- More common in men
Pathophysiology
- Per reports, can occasionally be caused by HPV 16 (Am J Clin Pathol 1991;96:318)
Clinical features
- Large anal tumor (often > 10 cm) that may be accompanied by abscess or fistula
- Often will recur but does not metastasize; mortality is approximately 20% (Dis Colon Rectum 1994;37:950)
Case reports
- 50 year old man with anal verrucous carcinoma and penile condylomata acuminata (Dermatology 2000;200:320)
Treatment
- Surgery is mainstay; use of neoadjuvant chemoradiation or imiquimod and CO2 laser ablation may be effective (World J Surg Oncol 2013;11:231, Dermatology 2003;207:119)
Gross description
- Polypoid; resembles a condyloma but is larger
Microscopic (histologic) description
- Very well differentiated squamous malignancy with minimal atypia and pushing borders
- Locally destructive, without obvious invasive features, lymphovascular invasion, etc.
- Can exhibit surface maturation and extensive keratinization
- May transform into conventional squamous cell carcinoma; rate of such transformation may be dependent on tumor size
- Similar to verrucous carcinoma at other sites
Sample pathology report
- Anus, resection:
- Verrucous carcinoma (see synoptic report and comment)
- Comment: Verrucous carcinoma is effectively considered a subtype of well differentiated squamous cell carcinoma, with a good prognosis overall.
Differential diagnosis
- Condyloma acuminatum:
- Smaller and more superficial
- No destructive growth
- Squamous cell carcinoma:
- Obviously invasive, with cytologic atypia and frequent mitotic figures
- Buschke-Löwenstein tumor:
- No obvious invasive component, has koilocytes
Additional references
Board review style question #1
Board review style answer #1
C. It may transform to traditional squamous cell carcinoma
Comment Here
Reference: Verrucous carcinoma
Comment Here
Reference: Verrucous carcinoma
WHO classification
Definition / general
- WHO classification of tumors of the anal canal
- Currently on 5th edition, published in 2019
- Based on histologic appearance, not molecular characteristics
WHO (2019)
-
Benign epithelial tumors and precursors ICD-O codes
- Squamous intraepithelial neoplasia, low grade 8077/0
- Squamous intraepithelial neoplasia, high grade 8077/2
-
Malignant epithelial tumors ICD-O codes
- Squamous cell carcinoma, NOS 8070/3
- Adenocarcinoma, NOS 8140/3
- Neuroendocrine tumor, NOS 8240/3
- Neuroendocrine tumor, grade 1 8240/3
- Neuroendocrine tumor, grade 2 8249/3
- Neuroendocrine tumor, grade 3 8249/3
- Neuroendocrine carcinoma, NOS 8246/3
- Large cell neuroendocrine carcinoma 8013/3
- Small cell neuroendocrine carcinoma 8041/3
- Mixed neuroendocrine - nonneuroendocrine neoplasm (MiNEN) 8154/3
Recent Anus & perianal area Pathology books
Find related Pathology books: GI, liver