Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Erem AS, Turashvili G. Dysplastic melanocytic nevus. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/vulvadysplasticnevi.html. Accessed April 2nd, 2025.
Definition / general
- Acquired nevus in the genital area with architectural and cytologic atypia
Essential features
- Clinical and epidemiologic features are similar to atypical genital nevi
- Most commonly occurs in women of reproductive age
- Morphologically characterized by a significant lentiginous component, well developed shoulder, randomly distributed melanocytic atypia and marked inflammatory infiltrate
Terminology
- Nevus with architectural and cytologic atypia (acceptable by the 5th edition of World Health Organization [WHO] Classification of Female Genital Tumors)
ICD coding
Epidemiology
- Dysplastic melanocytic nevi account for about 5% of genital melanocytic lesions (J Cutan Pathol 2008;35:24)
- Most often diagnosed in reproductive age women (mean: 32 years; median: 28 years) (Obstet Gynecol Clin North Am 2017;44:339, J Am Acad Dermatol 2014;71:1241)
Sites
- Usually affects the cutaneous part of labia majora > > perineum (Hum Pathol 1998;29:S1, J Cutan Pathol 2008;35:24, Arch Pathol Lab Med 2011;135:317)
- Mucosa is typically uninvolved (J Cutan Pathol 2008;35:24)
Pathophysiology
- Dysplastic melanocytic nevi are usually associated with activating mutations in genes such as BRAF (typically BRAF V600E) and NRAS (N Engl J Med 2015;373:1926)
Etiology
- Largely unknown
- May be associated with dysplastic nevus syndrome (Am J Surg Pathol 2008;32:51)
Clinical features
- During obstetrics - gynecologic visit
- Women rarely examine their vulva closely; consequently, duration and change of vulvar melanocytic lesions are often unclear (Dermatol Clin 2010;28:795)
- Decision to biopsy is based on atypical appearance of pigmented lesion (Dermatol Clin 2010;28:795)
- Genital pigmented lesions usually appear more atypical than nongenital lesions (Dermatol Clin 2010;28:795)
- Punch or excisional biopsy is preferred over shave biopsy (Dermatol Clin 2010;28:795)
- Dysplastic melanocytic nevus
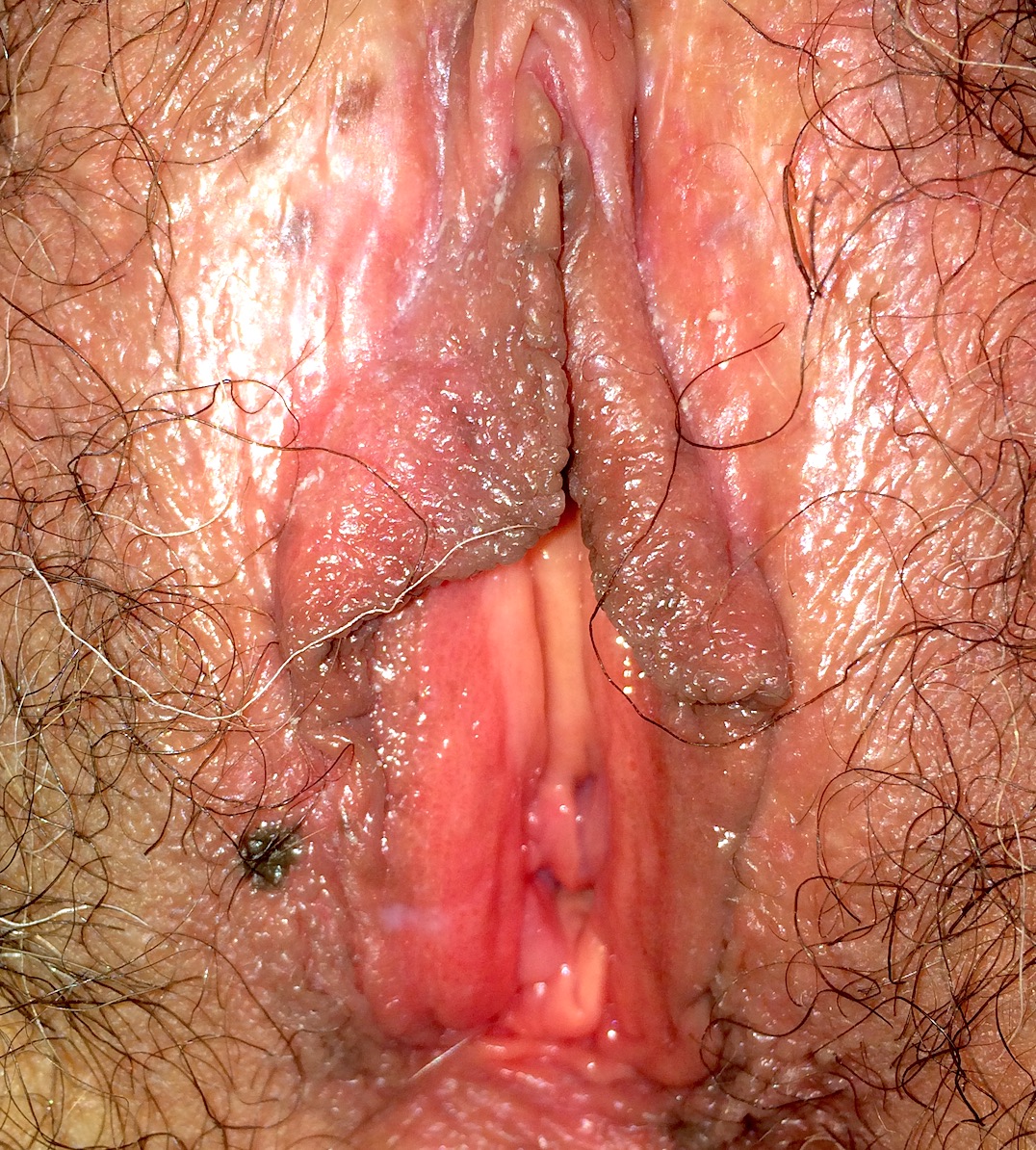
- Commonly presents as macules or papules with irregular borders, pink in the center and tan to brown at the periphery (Dermatol Ther 2010;23:449)
- Usually measures > 0.6 cm in size contrasting with common acquired nevi, which are typically < 0.6 cm
- Difficult to distinguish clinically from atypical melanocytic nevus of genital type (Am J Surg Pathol 2008;32:51, Hum Pathol 1998;29:S1)
Table 1: Clinical - pathologic features of vulvar melanocytic lesions (adapted from Diagn Histopathol 2024;30:P15)
| Vulvar melanocytic nevus | Vulvar melanocytic nevus in association with lichen sclerosus | Atypical genital nevus of special anatomic site | Dysplastic melanocytic nevus | Vulvar melanoma | |
| Age | Premenopausal | Premenopausal | Premenopausal, young adult | Premenopausal | Postmenopausal |
| Size | < 1 cm | < 1 cm | < 1 cm | 0.5 - 1 cm* | > 1 cm |
| Delineation | Well circumscribed | Well circumscribed | Well circumscribed | Well circumscribed | Infiltrative |
| Symmetry | Present | Present | Present | Present | Absent |
| Lateral extension of junctional component | Absent | Absent | Focal | Present | Present |
| Pigmented junctional nests | Absent | Present | Absent | Absent | Absent |
| Junctional nests | Discrete | Coalescent | Mostly discrete | Discrete | Coalescent |
| Retraction artefact | Absent | Absent | Present | Usually absent | Absent |
| Ulceration | Absent | Absent | Absent or traumatic | Absent or traumatic | Often present |
| Pagetoid spread | Absent | Focal Central | Focal Central | Focal Central | Prominent |
| Cytologic atypia | None to mild | Moderate to severe | Mild to moderate Superficial | Mild to severe** | Severe Uniform Deep |
| Dermal mitosis | Rare in pregnancy | Rare | Rare Superficial | Rare | Conspicuous Atypical Deep |
| Dermal maturation | Present | Present | Present | Present | Absent |
| Dermal fibrosis | Absent | Dermal sclerosis | Broad zone of superficial coarse dermal fibrosis | Concentric or fibrolamellar fibrosis | Regression type |
**See Table 3 for dysplasia grading per WHO 5th edition
Diagnosis
- Dermoscopy for nonmodified mucous membranes is useful (Dermatol Ther 2010;23:449)
- May show any of the dermoscopic patterns seen in benign nevi
- Globular / cobblestone, homogenous, structureless or mixed patterns are most common (Dermatology 2011;222:157, Dermatology 2010;221:55)
- Histologic diagnosis is confirmatory
- For optimal diagnosis, lichen sclerosus should be controlled before excision of the nevus (J Am Acad Dermatol 2004;50:690, Arch Dermatol 2002;138:77)
Table 2: Differential diagnosis of vulvar melanocytic nevi, melanosis and melanoma (adapted from J Am Acad Dermatol 2014;71:1241)
| Reassuring features suggestive of a benign process | Concerning features for possible malignancy | |
| Clinical |
|
|
| Dermoscopy |
|
|
| Reflectance confocal microscopy |
|
|
Prognostic factors
- Thought to have a benign clinical course (Obstet Gynecol Clin North Am 2017;44:339)
- Grade 2 cytologic atypia (former severe dysplasia) may be a precursor to melanoma (Obstet Gynecol Clin North Am 2017;44:339)
- No recurrences noted in cases with negative margins (Hum Pathol 1998;29:S1, Am J Surg Pathol 2008;32:51)
Case reports
- 21, 27 and 30 year old women with dysplastic melanocytic nevi of the vulva (Obstet Gynecol 1991;78:968)
Treatment
- Simple excision is usually sufficient
Clinical images
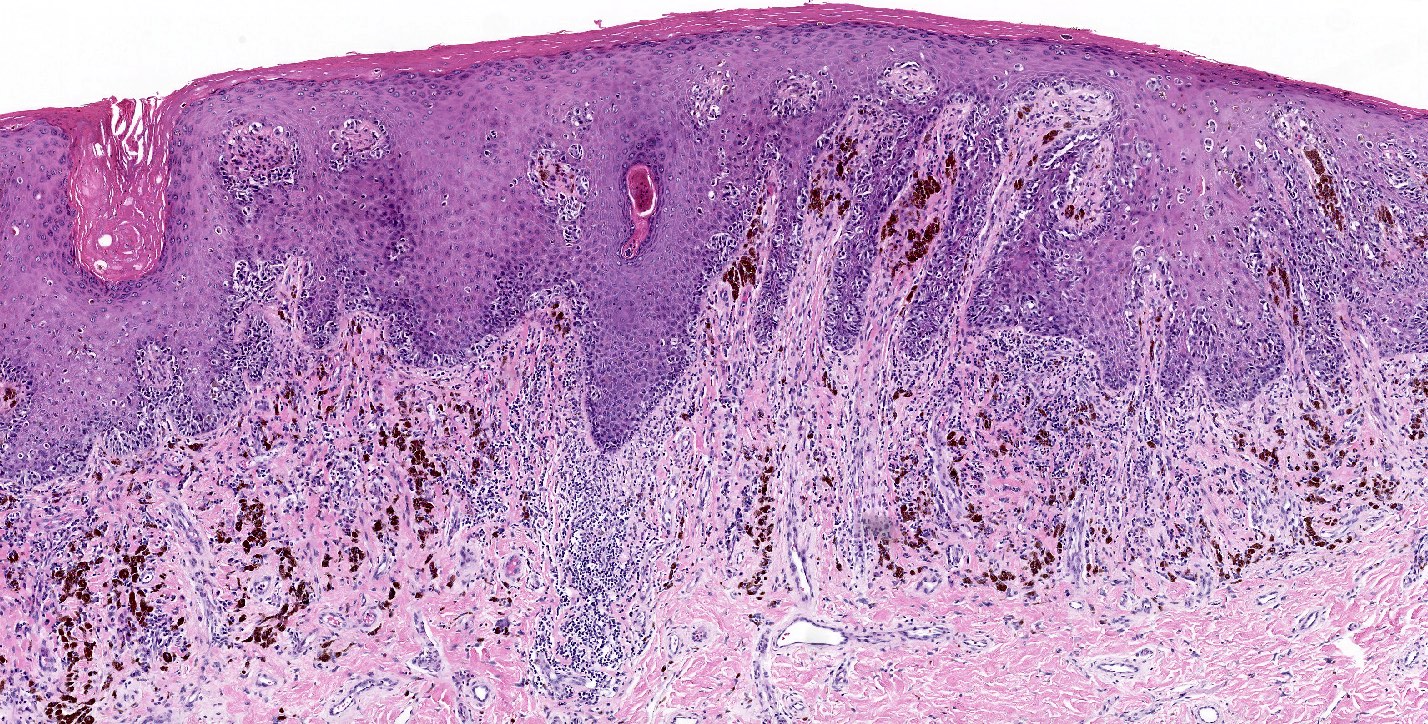
Microscopic (histologic) description
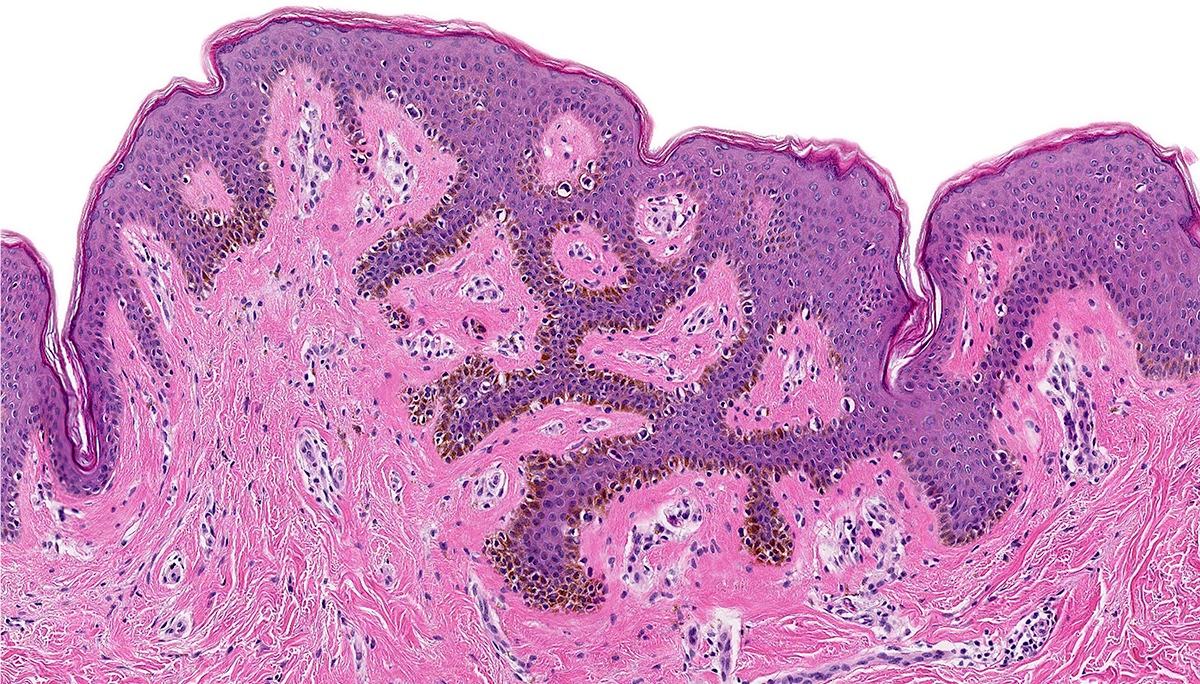
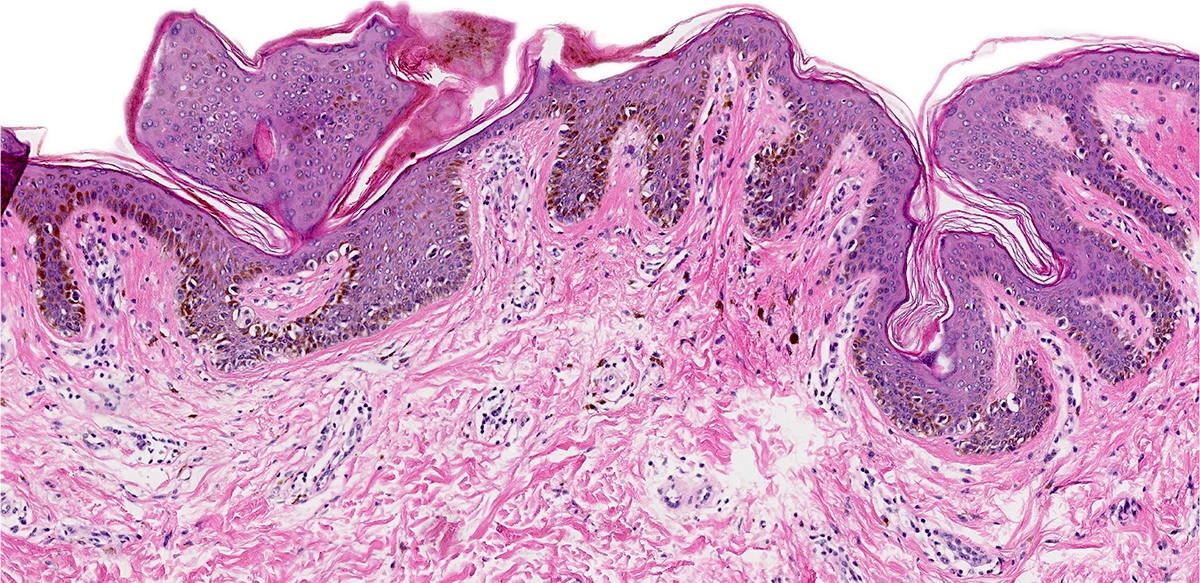
- Dysplastic melanocytic nevus is characterized by architectural and cytologic atypia
- Architectural atypia
- Bridging / fusion of junctional nests between adjacent rete ridges
- Variation in the size, shape or position of junctional nests
- Scattered mild to moderate cytologic atypia in the melanocytes
- Pagetoid spread of melanocytes may be seen, which is typically central and confined to lower epidermal layer
- Bland melanocytes may extend into the papillary dermis
- Concentric, lamellar eosinophilic fibroplasia in the papillary dermis near the dermoepidermal junction
- Presence of shouldering (junctional shoulders): a lentiginous junctional component (basilar proliferation of atypical melanocytes) at the periphery of the lesion, extending beyond the dermal component (> 3 rete ridges)
- Inflammatory infiltrate is usually confined to the superficial dermis
- Usually papillomatous appearance of the epidermis
- Cytologic atypia
- See Table 3 for grading
- Nuclear features are evaluated based on nuclear size, shape and presence of prominent nucleoli
- To estimate the nuclear size, the adjacent keratinocytes are typically referenced
- Chromatin evaluation includes hyperchromasia, coarse versus clumping of chromatin and its condensation within the nucleus
- Typically, absent or low mitotic activity (< 1/mm2)
- Architectural atypia
- Stereotypical features of junctional nevi are usually seen, including a well circumscribed, symmetrical lesion with intradermal component demonstrating maturation (J Cutan Pathol 2008:35:24)
- Nevi in the background of lichen sclerosus may appear more atypical (Arch Dermatol 2002;138:77)
- See Table 1 for clinicopathologic correlations
Table 3: Grading of cytologic atypia (adapted from WHO Classification of Skin Tumors, 5th edition)
| Grade | Previously | WHO grade, 4th edition | Nucleus | Presence of nucleoli | Chromatin | |
| Size | Shape variation | |||||
| 0 | Mild | Not dysplastic nevus | 1x | Minimal | Absent or small | Can be hyperchromatic |
| 1 | Moderate | Low grade dysplasia | 1 - 1.5x | Random atypia* | Hyperchromatic or dispersed | Hyperchromatic or dispersed |
| 2 | Severe | High grade dysplasia | 1.5x or more | More than random atypia but still in minority of cells** | Prominent, often lavender | Hyperchromatic Coarse granular Peripheral condensation |
*Random atypia is referred to prominent shape variation in the minority of cells
**Shape variation would be more than random atypia or in a larger minority of cells, hence, not diagnostic for melanoma
Microscopic (histologic) images
Positive stains
- Melanocytic markers
- MelanA (MART1) may help to highlight fused nests (J Cutan Pathol 2020;47:446)
- HMB45 (Ann Diagn Pathol 2023;67:152211, Biomed Rep 2016;5:327, J Am Acad Dermatol 2012;67:446)
- In common junctional and compound nevi, expressed in melanocytes at dermoepidermal junction
- Highlights a gradient or maturation (zonation) pattern (diminishing or loss of expression from top to bottom)
- Highly variable in Spitz and congenital nevi
- SOX10 highlights junctional melanocytes to help differentiate melanoma in situ from benign counterparts (Appl Immunohistochem Mol Morphol 2014;22:142)
- Ki67 proliferation rate should be low in the dermal component of genital nevi (J Am Acad Dermatol 2012;67:446)
Negative stains
- HMB45 is often negative in intradermal and compound nevi
- PRAME (preferentially expressed antigen in melanoma) differentiates dysplastic melanocytic nevi with grade 2 cytologic atypia (see Table 3) from melanoma with 85% specificity and 80% sensitivity (Virchows Arch 2023 Dec 19 [Epub ahead of print])
Molecular / cytogenetics description
- Dysplastic melanocytic nevi typically have a higher mutational burden than benign lesions with broader spectrum of initiating oncogenes, including BRAF V600K or BRAF K601E and NRAS (N Engl J Med 2015;373:1926)
Videos
Dysplastic nevus: 5 minute pathology pearls by Dr. Jerad Gardner
Dysplastic nevi by Prof. Naseem Ahmed
Melanoma arising in a dysplastic nevus by Drs. Philip H. Mckee and Antonina Kalmykova
Histopathology and dermoscopy of severely dysplastic nevus / in situ melanoma by Dr. Sasi Kiran Attili
Dysplastic nevus: fact or fiction by Prof. Cliff Rosendahl
Junctional dysplastic lentiginous nevus by Dr. Ian McColl
Dysplastic nevus by Dr. Sonam Kumar Pruthi
Dysplastic nevus by Audiopedia
Sample pathology report
- Vulva, labium majus, biopsy:
- Dysplastic melanocytic nevus with moderate to focally severe cytologic atypia and moderate architectural atypia; the lesion extends to 1 peripheral section edge (see comment)
- Comment: Multiple levels have been examined. Given the presence of the focally severe atypia present in this melanocytic proliferation and extension to a peripheral section edge, re-excision to ensure complete removal and minimize the risk of recurrence is recommended.
Differential diagnosis
- Atypical melanocytic nevus of genital type:
- Symmetrical lesion with dermal component maturation
- A few atypical melanocytes in the epidermis as single cells with rare pagetoid spread, predominantly in the center of the lesion
- S100, SOX10 and nerve growth factor receptor (NGFR) fail to demonstrate invasive growth
- HMB45 highlights a gradient pattern (diminishing or loss of expression from top to bottom)
- PRAME is lost or weakly expressed (J Cutan Pathol 2020;47:1123)
- BRAF is the most common alteration (N Engl J Med 2015;373:1926, J Invest Dermatol 2016;136:1858)
- Melanoma:
- Atypical melanocytes in the epidermis as single cells or forming nests with pagetoid spread
- Dermal component shows no maturation with cells forming sheets, nests, cords, single cells and rarely fascicles
- S100, SOX10 and NGFR are the most sensitive markers in visualization of invasive growth
- PRAME shows diffuse staining
- Usually lacks BRAF mutation (Br J Dermatol 2010;162:677)
- Pigmented epithelioid melanocytoma of the vulva:
- Consists of multiple, usually slow growing, distinct melanocytic entities with the potential to metastasize
- Infiltrative deep dermal tumor that may involve subcutis
- Hypercellular tumor with cells ranging from medium sized epithelioid cells to large epithelioid and spindled cells with low mitotic activity
- PRKAR1A loss in ~66% of cases (Am J Surg Pathol 2017;41:1333, Am J Surg Pathol 2019;43:480)
Additional references
- Am J Surg Pathol 1995;19:792, J Am Acad Dermatol 2023;88:1, J Am Acad Dermatol 2023;88:13, J Cutan Pathol 2008;35:889, Ackerman: Pathology of Malignant Melanoma, 1981, McKee: Pathology of the Skin - With Clinical Correlations, 3rd Edition, 2005, Busam: Pathology of Melanocytic Tumors, 1st Edition, 2018, WHO Classification of Tumours Editorial Board: Female Genital Tumours, 5th Edition, 2020, WHO Classification of Tumours Editorial Board: Skin Tumours, 5th Edition, 2024
Board review style question #1
A 42 year old woman presented to the clinic with a 0.8 cm flat, dark brown lesion with an irregular border on the mons pubis. The histologic findings of the shave biopsy demonstrated a broad junctional atypical melanocytic proliferation with central dermal component, focal bridging of adjacent junctional nests, focal pagetoid spread, moderate cytologic atypia, rare dermal mitoses (2 mitoses/2 mm2) and dense lymphocytic infiltrate. The lesion is transected at the deep dermis. What is the most important histological finding that questions the diagnosis of a dysplastic nevus?
- Cytologic atypia, grade 1
- Dermal mitotic activity (2 mitoses/2 mm2)
- Focal fused junctional nests
- Focal pagetoid spread
Board review style answer #1
B. Dermal mitotic activity (2 mitoses/2 mm2). When considering a dysplastic melanocytic nevus, any dermal mitotic figures, especially atypical mitosis, should warrant additional work up. Answer C is incorrect because unless there is confluent architectural atypia, the focal fusion of junctional nests is not a concerning feature by itself and part of the diagnostic criteria for dysplastic nevus. Answer D is incorrect because it is permissible for dysplastic nevus to demonstrate focal pagetoid spread that does not exceed the lower part of epidermis. The concerning features include extensive wide pagetoid spread of melanocytes, especially if it extends past the epidermis and laterally beyond the underlying junctional component. Answer A is incorrect because cytologic atypia, grade 1 (see Table 3) is the least concerning feature in this lesion and without the presence of architectural atypia would not be enough for diagnosis of dysplastic nevus.
Comment Here
Reference: Dysplastic nevi
Comment Here
Reference: Dysplastic nevi
Board review style question #2
A 38 year old woman presented to the OBGYN clinic with a 0.9 cm, irregular, dome shaped, dark pigmented papule on the right labium majus. A biopsy demonstrated symmetric melanocytic proliferation, with maturation, adjacent bridges of rete ridges and focal pagetoid spread mostly confined to the lower level of the epidermis. What is the most likely molecular alteration in this lesion?
- ALK fusion
- BRAF V600E mutation
- Homozygous deletion of CDKN2A
- NRAS mutation
Board review style answer #2
B. BRAF V600E mutation. Most dysplastic melanocytic nevi have a driver mutation in BRAF V600E. Answer A is incorrect as ALK fusion is more characteristic of Spitz nevus. Answers C and D are incorrect as those alterations are specific to superficial spreading melanoma.
Comment Here
Reference: Dysplastic nevi
Comment Here
Reference: Dysplastic nevi