Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Chapel DB. Undifferentiated uterine sarcoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/uterussarcoma.html. Accessed April 3rd, 2025.
Definition / general
- Undifferentiated uterine sarcoma is a diagnosis of exclusion applied to malignant mesenchymal tumors of the uterus with no lineage specific morphologic or immunohistochemical features and no entity defining molecular alterations
Essential features
- Rare, aggressive malignant mesenchymal tumor, predominantly affecting postmenopausal women
- Diagnosis of exclusion, requiring thorough morphologic, immunohistochemical and molecular evaluation of a comprehensive differential
- Diagnosis of undifferentiated uterine sarcoma has decreased as novel molecular subtypes of uterine sarcoma are codified
Terminology
- Undifferentiated endometrial sarcoma is discouraged
- More general undifferentiated uterine sarcoma terminology was introduced in the 2014 WHO to acknowledge that these tumors are not necessarily of endometrial origin
ICD coding
Epidemiology
- Diagnosis of undifferentiated uterine sarcoma is decreasing as novel uterine sarcomas with recurrent molecular alterations are identified
- Predominantly affects postmenopausal women (median, seventh decade) (Gynecol Oncol 2012;127:27, Clin Transl Oncol 2021;23:1210)
Sites
- Uterus
Clinical features
- Typically presents with postmenopausal bleeding, pelvic pain or mass symptoms of uterine or extrauterine disease (Int J Gynecol Cancer 2019;29:691, Clin Transl Oncol 2021;23:1210)
- Approximately 50% of patients present with extrauterine disease (Gynecol Oncol 2012;127:27)
Diagnosis
- Diagnosis of exclusion, rendered after comprehensive morphologic, immunohistochemical and molecular testing rule out other defined entities
- Diagnosis best rendered on a well sampled resection specimen, as focal diagnostic findings may be missed in biopsies of uterine sarcoma (Am J Surg Pathol 2017;41:1231)
Prognostic factors
- Older age, extrauterine spread, tumor necrosis, negative ER / PR and mitoses ≥ 25 per 10 high power fields associated with shorter survival (Int J Cancer 2015;136:1608, Am J Surg Pathol 2017;41:1231, Clin Cancer Res 2019;25:2155)
- After excluding high grade endometrial stromal sarcoma with testing for YWHAE fusion, separation into uniform versus pleomorphic subtypes does not appear prognostic (Am J Surg Pathol 2017;41:1231)
- Novel molecular prognostic groups have been proposed but are not in common use (Clin Cancer Res 2019;25:2155)
- Median progression free and overall survival approximately 6 - 8 and 12 - 24 months, respectively (Gynecol Oncol 2012;127:27, Am J Surg Pathol 2017;41:1231, Arch Gynecol Obstet 2021;304:475)
- Most common sites of metastasis / progression are abdominal cavity (~60%) and lung (~50%)
Case reports
- 65 year old woman with undifferentiated uterine sarcoma (J Clin Diagn Res 2017;11:ED03)
Treatment
- Complete surgical excision is first line treatment, if possible (Gynecol Oncol 2012;127:27, BMC Cancer 2018;18:1247)
- Adjuvant chemotherapy (gemcitabine / docetaxel or doxorubicin based regimens) is standard
- Additional chemotherapy regimens may be used for recurrent / progressive disease
- Response to chemotherapy is generally short lived
Gross description
- Generally large (median, 10 cm) (Clin Transl Oncol 2021;23:1210)
- Fleshy, pink-tan cut surface
- Gross necrosis and hemorrhage common
- Deep myometrial infiltration common
- Thorough gross sampling (at least 1 section per cm) is critical to confidently excluding differentiated tumor foci, which would preclude diagnosis of undifferentiated uterine sarcoma
Microscopic (histologic) description
- Conceptually subdivided into tumors with uniform morphology and those with pleomorphic morphology (Am J Surg Pathol 2008;32:1228)
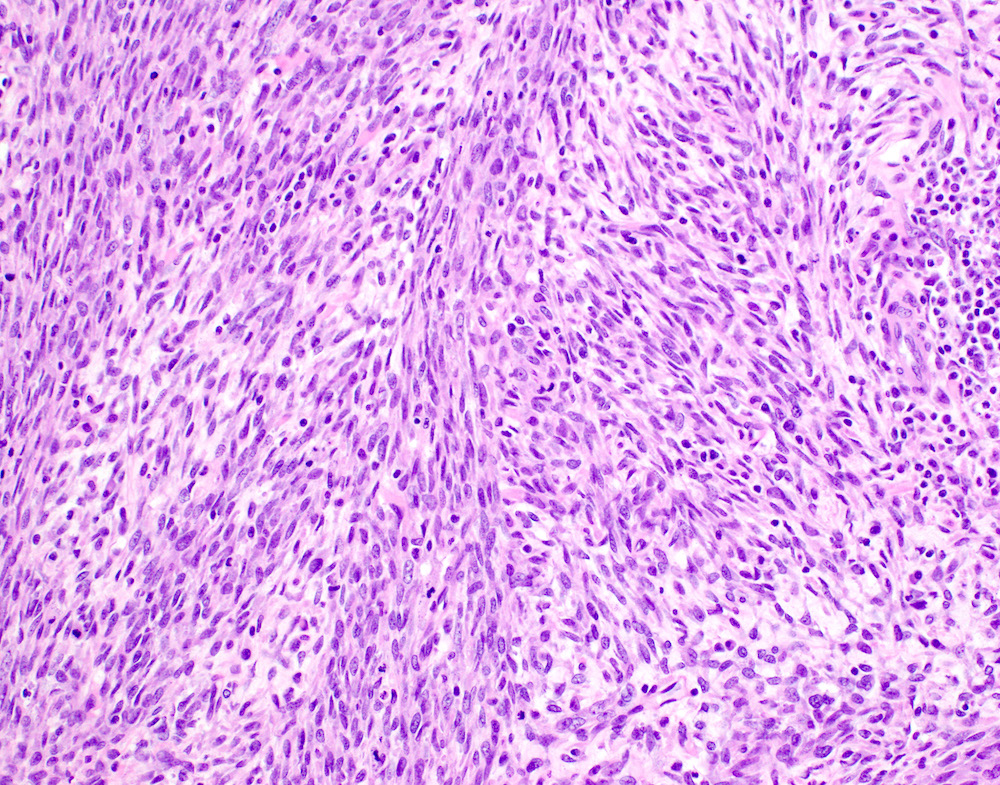
- Undifferentiated uterine sarcoma with uniform morphology
- Many tumors with uniform morphology are more appropriately classified under novel molecular diagnoses:
- Tumors with uniform epithelioid morphology predominantly represent high grade endometrial stromal sarcoma with YWHAE-NUTM2A/B fusion (Am J Surg Pathol 2019;43:662, Histopathology 2014;65:473)
- Tumors with uniform spindled morphology may represent NTRK fusion sarcomas, COL1A1-PDGFB fusion sarcomas or other entities (see Differential diagnosis)
- However, some uterine sarcomas with uniform spindle or epithelioid morphology may remain unclassifiable after comprehensive testing (Am J Surg Pathol 2014;38:1161)
- In these cases, a diagnosis of undifferentiated uterine sarcoma with uniform morphology is appropriate, with a descriptive comment
- Next generation sequencing based gene fusion testing may be considered to identify novel entities
- Many tumors with uniform morphology are more appropriately classified under novel molecular diagnoses:
- Undifferentiated uterine sarcoma with pleomorphic morphology
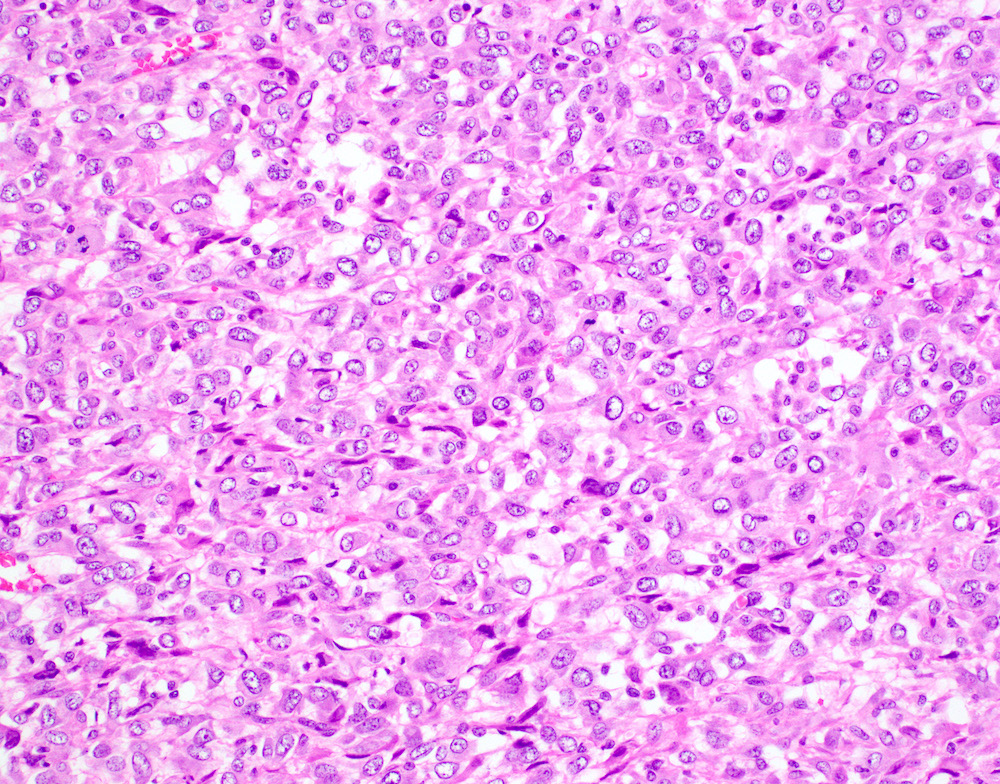
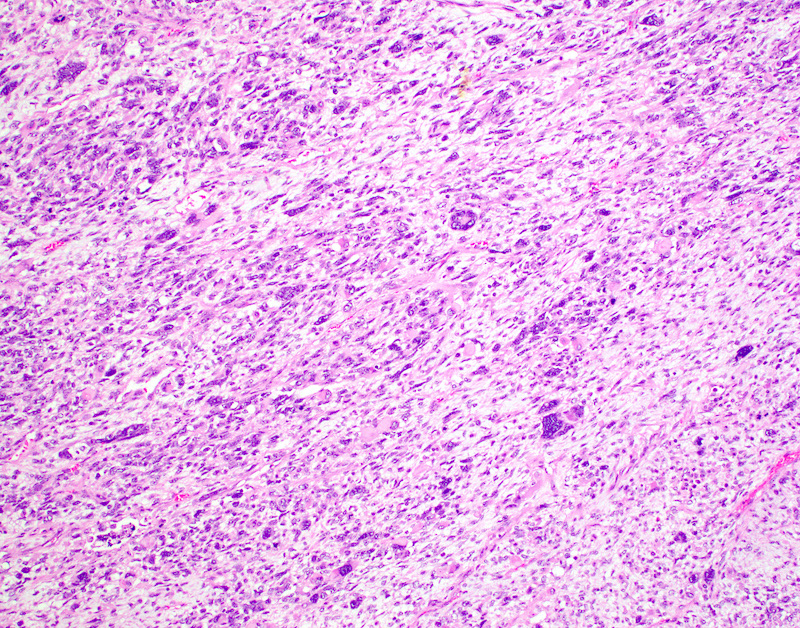
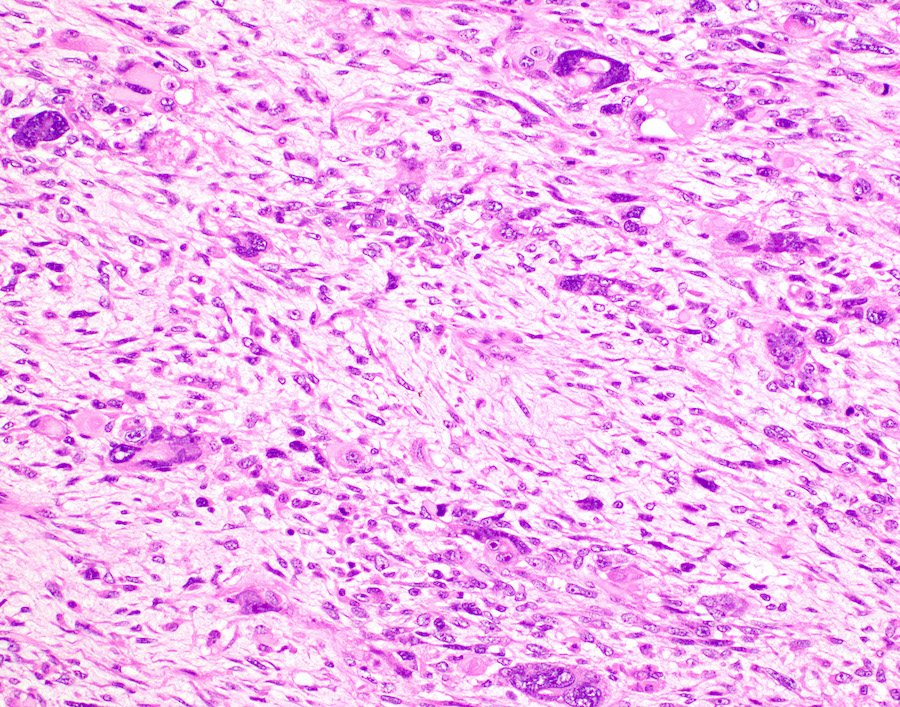
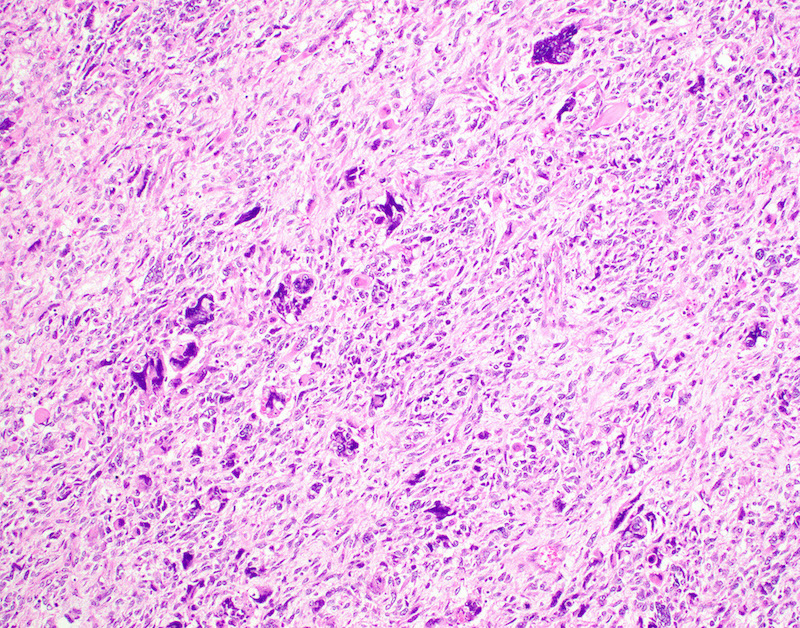
- Spindle, epithelioid or polygonal cells with marked nuclear atypia and pleomorphism, frequent multinucleation and macronucleoli (Am J Surg Pathol 2017;41:1231)
- Tumors with uniform or with pleomorphic morphology characteristically show (Clin Transl Oncol 2021;23:1210):
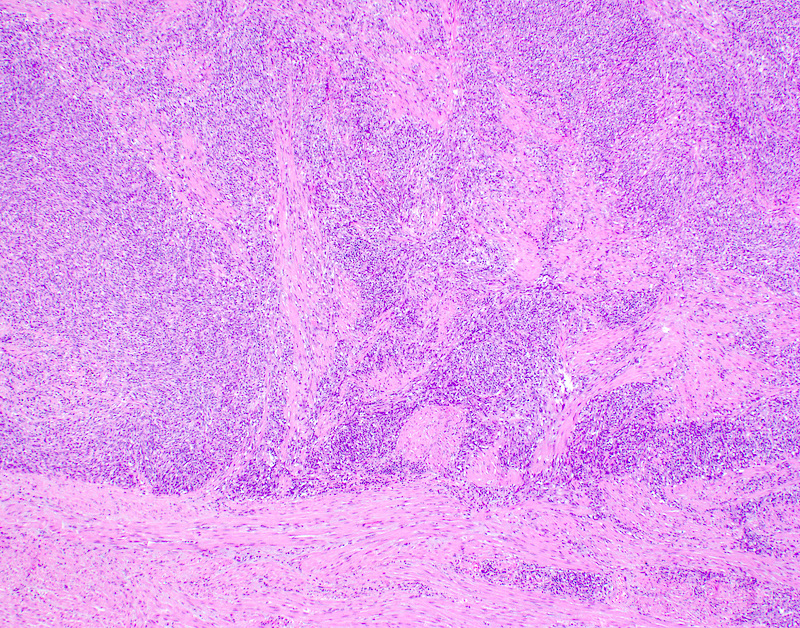
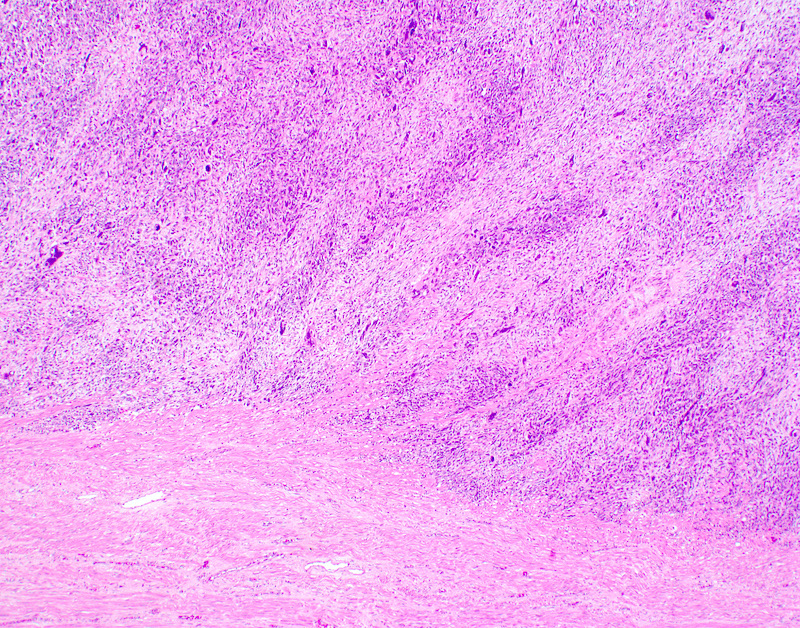
- Destructive myometrial invasion
- Brisk mitotic activity (median, 25 per 10 high power fields)
- Tumor necrosis (~90%)
- Lymphovascular invasion (~50%)
Microscopic (histologic) images
Positive stains
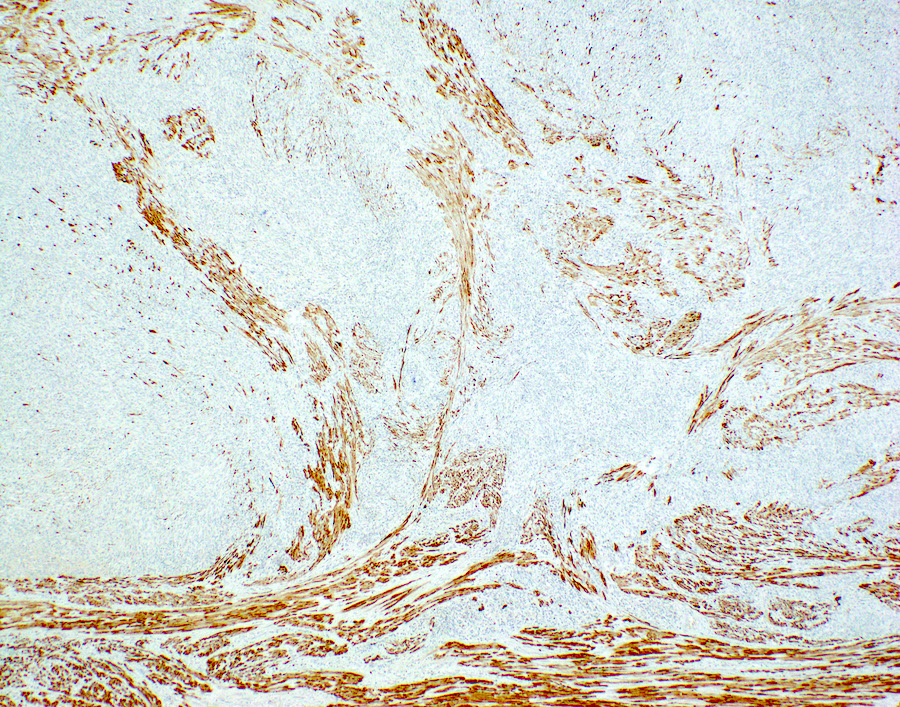
- Focal SMA is permissible (Am J Surg Pathol 2017;41:1231)
- CD10 may be positive but is nonspecific
- Strong diffuse mutant pattern p53 staining in approximately half (Am J Surg Pathol 2008;32:1228, Histopathology 2021;78:805)
- Strong diffuse p16 may be seen
- MDM2 IHC positive in rare cases with MDM2 amplification (Int J Gynecol Pathol 2015;34:576)
Negative stains
- ER and PR negative in majority of cases (Gynecol Oncol 2012;127:27, Am J Surg Pathol 2008;32:1228)
- Desmin, caldesmon
- Definitive positive desmin or caldesmon favor a diagnosis of leiomyosarcoma, even if focal (Am J Surg Pathol 2017;41:1231)
Molecular / cytogenetics description
- TP53 mutations and complex copy number alterations are common (JCI Insight 2017;2:e94033)
- Rare cases may harbor MDM2 amplification (Int J Gynecol Pathol 2015;34:576)
- On RNA expression analysis, undifferentiated uterine sarcoma appears to cluster more closely with leiomyosarcoma than with endometrial stromal sarcoma (Mod Pathol 2021;34:1008)
- 4 molecular subtypes identified in 1 study, based on differential activation of (Clin Cancer Res 2019;25:2155):
- Genital tract development pathways
- Extracellular matrix pathways
- Muscle function pathways
- Proliferation pathways
- These subtypes may have prognostic significance but do not currently play a role in routine diagnosis
- By definition, undifferentiated uterine sarcoma lacks disease defining molecular alterations, including:
- Fusions in YWHAE, BCOR, PHF1, JAZF1, NTRK, COL1A1, ESR1, GREB1, TFE3
- Internal tandem duplication of BCOR
- SWI / SNF (SMARCA4, SMARCB1) deficiency
- TSC1/2 alterations
- Recognition of these alterations may permit use of targeted therapies
Sample pathology report
- Uterus, cervix, bilateral tubes and ovaries, total abdominal hysterectomy with bilateral salpingo-oophorectomy:
- Cervix:
- No significant pathologic change.
- Endomyometrium:
- Undifferentiated uterine sarcoma (12 cm) (see comment)
- Lymphovascular invasion is present.
- Uterine serosa:
- Negative for tumor.
- Fallopian tubes and ovaries:
- No significant pathologic change.
- Comment: Gross examination reveals a poorly circumscribed, fleshy, 12 cm mass, diffusely infiltrating the myometrium. On microscopic examination, the tumor comprises sheets of polygonal cells with abundant eosinophilic cytoplasm and pleomorphic nuclei with prominent macronucleoli. Numerous multinucleated tumor cells are seen. Mitoses number up to 35 per 10 high power fields. There is extensive tumor necrosis (~60% of the tumor volume) and lymphovascular invasion.
- On immunohistochemical stains, the tumor shows patchy variable CD10 positivity, strong diffuse mutant pattern p53 and strong diffuse p16. Tumor cells are negative for SMA, desmin, caldesmon, ER, PR, cyclin D1, BCOR, S100, MelanA, HMB45 and cytokeratin AE1 / AE3 / CAM5.2. SMARCA4 and SMARCB1 / INI1 are retained.
- The morphologic and immunophenotypic findings are mostly in keeping with a diagnosis of undifferentiated uterine sarcoma. I particularly considered the possibility of a pleomorphic leiomyosarcoma or malignant PEComa. However, immunophenotype does not support these diagnoses. The tumor appears confined to the uterine corpus in this specimen (pT1b); however, correlation with surgical and radiographic findings is necessary.
- Cervix:
Differential diagnosis
- Spindle cell leiomyosarcoma:
- Myxoid leiomyosarcoma:
- Subset shows brisk mitotic activity, marked atypia and necrosis
- SMA, desmin or caldesmon at least focally positive (Am J Surg Pathol 2016;40:285, Mod Pathol 2019;32:1688)
- PLAG1 IHC positive in 50% (Am J Surg Pathol 2019;43:382, Mod Pathol 2019;32:1688)
- High grade endometrial stromal sarcoma with BCOR rearrangement:
- High grade endometrial stromal sarcoma with BCOR internal tandem duplication:
- Variably spindle to epithelioid cells with moderate to marked atypia but typically without frank pleomorphism, in myxoid stroma
- Low grade fibromyxoid component often present
- Cyclin D1, BCOR and CD10 IHC positive
- May be focally desmin positive but caldesmon and SMA negative
- BCOR internal tandem duplication (ITD) in exon 15
- High grade endometrial stromal sarcoma with YWHAE-NUTM2A/B fusion:
- Often biphasic morphology
- High grade epithelioid component: uniform population of atypical epithelioid cells, arranged in nests surrounded by delicate capillary vasculature
- Low grade spindle component: uniform population of bland stubby spindle cells, resembling low grade endometrial stromal sarcoma
- Relative frequency:
- Biphasic tumors most common
- Occasional tumors show only the high grade epithelioid component
- Rarely, only the low grade spindle component is present
- Cyclin D1 and BCOR IHC consistently positive in the high grade epithelioid component and negative or focally positive in the low grade spindle component
- CD10 negative in high grade epithelioid component but positive in low grade spindle component
- YWHAE-NUTM2A/B fusion on molecular testing
- Often biphasic morphology
- NTRK fusion uterine sarcoma:
- COL1A1-PDGFB fusion uterine sarcoma:
- Malignant inflammatory myofibroblastic tumor:
- Spindle cells with moderate to marked atypia and inflammatory infiltrate
- Infiltrative borders, brisk mitoses and tumor necrosis may be present
- ALK IHC positive in almost all cases, most often with granular cytoplasmic staining
- ALK fusion present in almost all cases
- Rare uterine IMT may show RET, ETV6-NTRK3 or ROS1 fusions
- Malignant perivascular epithelioid cell tumor:
- Epithelioid leiomyosarcoma:
- Uniform to pleomorphic epithelioid cells with eosinophilic cytoplasm, often with an admixed spindle component
- SMA, desmin or caldesmon positive
- PGR fusions present in a subset
- This subset characteristically shows uniform cytomorphology, with a rhabdoid eosinophilic cytoplasmic inclusion and eccentric nucleus
- Uterine tumor resembling ovarian sex cord tumor with GREB1 fusion:
- Uniform epithelioid cells with vesicular chromatin and prominent nucleoli, predominantly arranged in solid sheets, often with focal sex cord patterns (sertoliform cords, tubules, retiform structures)
- Spindled growth may also be present
- Polyphenotypic: smooth muscle markers, cytokeratin, sex cord markers (calretinin, inhibin, SF1, WT1, CD56, CD99), CD10 and hormone receptors typically positive, though expression is variable
- Defined by GREB1 fusions (usually with NCOA1/2)
- Precise clinical and biologic relationship to classic UTROSCT remains unsettled
- Uniform epithelioid cells with vesicular chromatin and prominent nucleoli, predominantly arranged in solid sheets, often with focal sex cord patterns (sertoliform cords, tubules, retiform structures)
- Pleomorphic rhabdomyosarcoma:
- Primary pleomorphic rhabdomyosarcoma of the uterus is exceptionally rare
- Polygonal to spindle cells with marked nuclear pleomorphism, frequent multinucleation and abundant eosinophilic cytoplasm
- Desmin, myogenin, MyoD1 IHC positive
- Must be differentiated from rhabdomyosarcomatous differentiation in carcinosarcoma or adenosarcoma
- SMARCA4 deficient uterine sarcoma:
- Uniform epithelioid cells with moderate nuclear atypia and eosinophilic cytoplasm, arranged in solid sheets
- Phyllodiform growth may be present
- SMARCA4 (or more rarely, SMARCB1 / INI1) IHC negative (i.e. abnormal loss of expression)
- SMARCA4 loss of function alterations on molecular testing
- Subset associated with germline SMARCA4 mutations
- Undifferentiated / dedifferentiated or poorly differentiated carcinoma:
- Extensive sampling may reveal a component of differentiated carcinoma
- Mismatch repair deficiency in approximately half of cases
- SMARCA4 or SMARCB1 / INI1 deficiency in approximately 20%
- May express cytokeratins, epithelial membrane antigen, claudin 4 or E-cadherin (though these have not been rigorously tested in undifferentiated uterine sarcoma)
- Carcinosarcoma:
- Extensive sampling will reveal a malignant epithelial component
- In case of complete overgrowth by undifferentiated sarcoma, distinction will not be possible
- Adenosarcoma with sarcomatous overgrowth:
- Extensive sampling will reveal a component with conventional adenosarcomatous morphology
- In case of complete overgrowth by undifferentiated sarcoma, distinction will not be possible
- Melanoma:
Additional references
Board review style question #1
A 73 year old woman presents with postmenopausal bleeding. Ultrasound shows a 12 cm, sonographically heterogeneous uterine mass. Gross examination shows extensive necrosis and hemorrhage. A representative photomicrograph from the tumor is shown. Immunohistochemical stains for SMA, desmin, caldesmon, ALK, CD34, S100, cyclin D1, BCOR, ER, PR, SOX10, HMB45, MelanA, MyoD1, myogenin and broad spectrum cytokeratins are negative. SMARCB1 / INI1 and SMARCA4 immunostains show retained / intact expression. A CD10 immunostain shows patchy positivity and a p53 immunostain shows strong diffuse mutant pattern expression. Which of the following is the most appropriate diagnosis?
- Epithelioid leiomyosarcoma
- Malignant inflammatory myofibroblastic tumor
- Melanoma
- Pleomorphic rhabdomyosarcoma
- Undifferentiated uterine sarcoma
Board review style answer #1
E. Undifferentiated uterine sarcoma. The clinical history and gross findings are suggestive of a uterine malignancy but they are not specific to a particular diagnosis. Microscopic examination shows a malignant neoplasm with a loose, vaguely spindled background and numerous superimposed pleomorphic tumor giant cells. An extensive immunohistochemical workup shows no identifiable line of differentiation, ruling out epithelioid leiomyosarcoma (negative SMA, desmin and caldesmon), malignant inflammatory myofibroblastic tumor (negative SMA, desmin, caldesmon, ALK), melanoma (negative S100, SOX10, HMB45, MelanA) and pleomorphic rhabdomyosarcoma (negative desmin, myogenin, myoD1). The other stains also rule out malignant PEComa (negative smooth muscle and melanocytic markers) and high grade endometrial stromal sarcoma (negative BCOR and cyclin D1). A novel fusion related uterine sarcoma (e.g. NTRK fusion sarcoma) is unlikely, given the pleomorphic cytomorphology and mutant pattern p53 immunostaining. Having excluded all items on this lengthy differential diagnosis, a diagnosis of undifferentiated uterine sarcoma is most appropriate.
Comment Here
Reference: Undifferentiated uterine sarcoma
Comment Here
Reference: Undifferentiated uterine sarcoma
Board review style question #2
Which of the following statements regarding the diagnosis of undifferentiated uterine sarcoma is true?
- Patchy CD10 staining rules out a diagnosis of undifferentiated uterine sarcoma
- Undifferentiated uterine sarcoma characteristically shows a myomelanocytic immunoprofile
- Undifferentiated uterine sarcoma is a diagnosis of exclusion applied to malignant uterine mesenchymal tumors with no lineage specific morphologic or immunohistochemical and no entity defining molecular alterations
- Undifferentiated uterine sarcoma is defined by YWHAE-NUTM2A/B fusion
- Undifferentiated uterine sarcoma most commonly occurs in the cervix of young women (< 40 years old)
Board review style answer #2
C. Undifferentiated uterine sarcoma is a diagnosis of exclusion applied to malignant uterine mesenchymal tumors with no lineage specific morphologic or immunohistochemical and no entity defining molecular alterations. Patchy CD10 staining may be seen in undifferentiated uterine sarcoma (answer A) and is considered nonspecific. PEComa characteristically shows a myomelanocytic immunprofile (answer B), which should not be seen in undifferentiated uterine sarcoma. YWHAE-NUTM2A/B fusions define a subset of high grade endometrial stromal sarcomas (answer D); this fusion is not compatible with a diagnosis of undifferentiated uterine sarcoma. Undifferentiated uterine sarcoma principally affects postmenopausal patients (answer E); in contrast, NTRK fusion uterine sarcomas most commonly occur in the cervix of young women (< 40 years old).
Comment Here
Reference: Undifferentiated uterine sarcoma
Comment Here
Reference: Undifferentiated uterine sarcoma