Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Microscopic (histologic) description | Virtual slides | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Parra-Herran C. Endometrial carcinoma-general. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/uterusendometrialcarc.html. Accessed March 31st, 2025.
Definition / general
- Malignant epithelial neoplasm originating from the endometrium
Essential features
- Endometrial cancer is the most common gynecologic malignancy in high income countries
- General principles in the macroscopic approach, histologic classification, diagnosis and management of endometrial cancer are outlined in the recent recommendations by the International Society of Gynecological Pathologists (Int J Gynecol Pathol 2019;38:S1)
ICD coding
Epidemiology
- Most common gynecologic malignancy in developed countries
- In the U.S., endometrial cancer is the fourth most prevalent cancer in women (CDC: United States Cancer Statistics [Accessed 24 November 2020])
- It also is the sixth most common cause of cancer mortality in women (CDC: United States Cancer Statistics [Accessed 24 November 2020])
- Incidence of endometrial cancer is increasing
- Globally, there were 382,069 new endometrial cancer cases in 2018
- From 2007 to 2016, the number of new cases in the U.S. increased by 1% each year for white women and 2% each year for black women
- Most cases arise in the postmenopausal period, with a mean age at presentation of 60 years
- 67% of cases present at early stage
- References: International Agency for Research on Cancer: Corpus Uteri Fact Sheet [Accessed 1 May 2020], American Cancer Society: Key Statistics for Endometrial Cancer [Accessed 1 May 2020], American Society of Clinical Oncology: Uterine Cancer - Statistics [Accessed 1 May 2020]
Sites
- Uterine corpus (fundus, corpus or lower uterine segment)
Pathophysiology
- From a pathophysiologic perspective, endometrial carcinomas have been traditionally divided into 2 types:
- Type 1: includes endometrioid and mucinous carcinoma
- These lesions are associated with long term elevated estrogen levels, which lead to persistent proliferative stimulation of the endometrium
- Risk factors leading to hyperestrogenism include obesity, exogenous hormonal therapy (e.g. tamoxifen use for breast cancer), ovarian cortical hyperplasia / hyperthecosis, polycystic ovarian syndrome and hormone producing tumors (e.g. granulosa cell tumor of the ovary), among others
- PTEN, ARID1A, PIK3CA, KRAS gene alterations are common
- Atypical endometrial hyperplasia / endometrioid intraepithelial neoplasia is regarded as the precursor lesion (J Clin Oncol 2010;28:788, Cancer 2005;103:2304)
- Type 2: includes serous, clear cell, undifferentiated carcinoma and carcinosarcoma
- These tumors have a lesser association with unopposed estrogen exposure
- Serous carcinoma is characterized by early alterations in TP53
- Precursor lesion for clear cell carcinoma has not been identified
- Type 1: includes endometrioid and mucinous carcinoma
- Other associations include diabetes, dysfunctional uterine bleeding, hypertension, infertility, Muir-Torre syndrome, Turner syndrome (usually well differentiated adenocarcinoma; 2/3 have squamous differentiation) and tamoxifen use for breast cancer (increased risk for endometrioid, serous carcinoma and carcinosarcoma) (Am J Surg Pathol 2001;25:936, OMIM: Muir-Torre Syndrome [Accessed 1 May 2020])
- From a biologic and clinical perspective, the classification of endometrial carcinoma is evolving towards a molecular based grouping (see Molecular / cytogenetics description below)
Etiology
- See Pathophysiology above
Diagrams / tables
Clinical features
- Most patients (~90%) present with abnormal uterine bleeding (hypermenorrhea, menometrorrhagia, postmenopausal bleeding)
- Clear vaginal discharge and constitutional symptoms (weight loss, anemia) can also occur
- Some patients are diagnosed after an abnormal cervicovaginal cytology result (Pap smear)
- As Pap smear abnormalities can be the first presenting sign, the presence of endometrial cells in pap smears of women over the age of 45 needs to be reported (Cytojournal 2017;14:22)
- Likewise, a diagnosis of adenocarcinoma should prompt consideration for endometrial sampling
Diagnosis
- Ultrasound, pelvic or transvaginal, is often used to identify endometrial thickening or masses occupying the endometrial cavity
- Hysteroscopy can be performed to better visualize the cavity and perform directed sampling (for example, intact resection of a polypoid lesion)
- Sampling of the endometrium is the most commonly used test if endometrial cancer is suspected
- Endometrial biopsy can be done at the gynecologist office by inserting a flexible tube in the canal
- Endometrial curettage, also performed as an outpatient procedure, is obtained by dilating the cervix to insert a curette to scrape the uterine lining
- It often results in a more abundant sample compared with endometrial biopsy
Radiology description
- In principle, any anatomic lesion in premenopausal women (e.g. polyp, nodule, lush irregularity) and any worrisome thickening of the endometrium in postmenopausal women requires sampling
- Historically, an endometrial thickness of 10 mm or more has been considered the threshold for endometrial cancer in postmenopausal women
- However, recent meta analyses have shown that lower thresholds of 5 or even 3 mm need to be considered (Gynecol Oncol 2020 Jan 30 [Epub ahead of print], Obstet Gynecol 2010;116:160)
Prognostic factors
- Cancer stage is the strongest predictor of outcome
- Stage requires pathologic evaluation of resection (hysterectomy) material
- 2 main staging systems are used: FIGO (2019) and TNM system from the Union for International Cancer Control and the American Joint Committee on Cancer (2018); both are currently harmonized
- Disease free 5 year survival is > 90% for stage I, ~85% for stage II and ~45% for stage III carcinomas
- 10 - 30% of patients present at advanced stage (FIGO stage III - IV)
- Nodal metastases are most common to pelvic and paraaortic nodes; metastases also occur to bone, brain, liver, lung, skin
- Most recurrences are local (vaginal vault, pelvis)
- Staging depends on several prognostic histologic variables, some of which are discussed below
- Locations of tumor spread not addressed in FIGO staging system include pelvic serosa (should be classified as stage IIIA), abdominal serosa (should be stages as FIGO IVB (M1)) and omentum (should be staged as FIGO IVB (M1)) (Int J Gynecol Pathol 2019;38:S93)
- Myometrial invasion
- Cervical stromal involvement
- Serosal involvement
- Adnexal involvement
- Lymph node involvement
- Histologic type
- Tumor grade
- Vascular space invasion
- Molecular group
Case reports
- See endometrial carcinoma types
Treatment
- Total abdominal hysterectomy with bilateral salpingo-oophorectomy (TAHBSO) is standard treatment
- Regional nodal dissection is performed in patients with endometrioid carcinoma FIGO 2 or 3, high grade histologic type, tumor size > 2 cm or with myometrial invasion on imaging or suspicious lymph nodes
- Medical treatment with exogenous progestin is a valid option in patients who desire to preserve fertility or are not eligible for surgery, have a FIGO 1 endometrioid carcinoma and no or superficial myometrial invasion on imaging (Obstet Gynecol Int 2010;2010:431950)
- Radiation therapy is usually indicated in patients with FIGO stage Ib or greater or with local (vaginal, pelvic) recurrence
- Chemotherapy is indicated for high risk early stage or advanced stage disease, recurrences and distant metastases (Oncologist 2010;15:1026)
Gross description
- Distinct mass or growth is usually seen upon examination of the cavity
- Lesion is typically exophytic and soft
- It can have homogeneous appearance or contain a heterogeneous cut surface with variable hemorrhage and necrosis (more common in high grade tumors)
- Some cases present as diffuse endometrial thickening; the endometrial tissue is lush, soft and friable
- Carcinomas with extensive squamous differentiation can have a flaky appearance, whereas those with mucinous differentiation are soft and gelatinous (colloid appearance)
- Tumor should be sectioned perpendicular to the anterior and posterior uterine walls in order to identify areas of growth into the wall
- These include myometrial invasion and involvement of adenomyosis, which may be difficult to distinguish grossly
- Area of deepest growth into the wall should always be sampled as a full thickness section (either a single section or a composite section if the uterine wall is thick)
- Inclusion of the adjacent uninvolved endometrium in the section is always preferred (if present)
- If the tumor grossly extends to lower uterine segment, a sagittal section, going from proximal to distal ends to include tumor, lower uterine segment and cervix is recommended
- References: Lester: Manual of Surgical Pathology, 3rd Edition, 2010, Nucci, Parra-Herran: Gynecologic Pathology, 2nd Edition, 2020
Gross images
- See endometrial carcinoma types
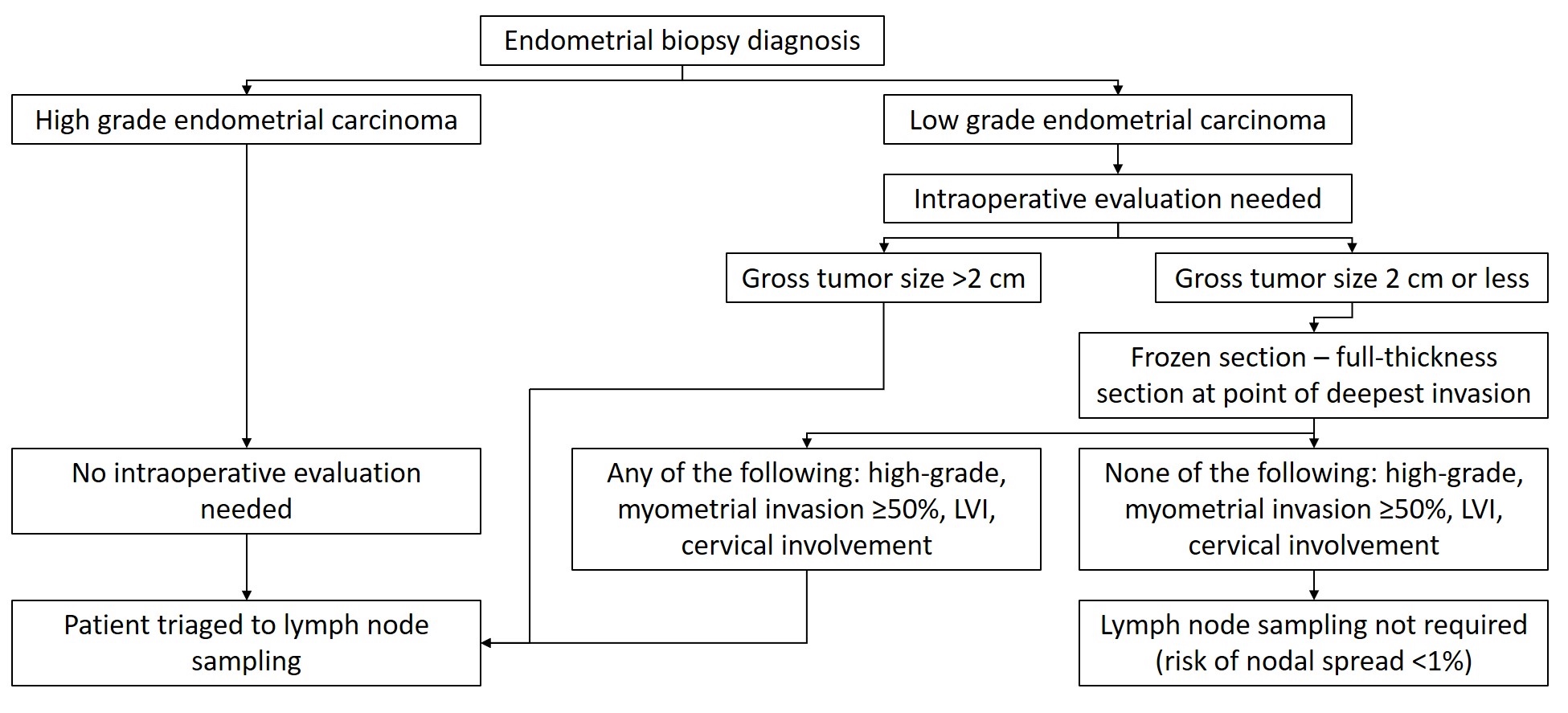
Frozen section description
- Tumor size should be reported routinely and some practices request documentation of tumor size intraoperatively
- Tumor size > 2 cm is a risk factor for lymph node metastases (Int J Gynecol Cancer 2017;27:486)
- Intraoperative consultation and frozen section evaluation of hysterectomy specimens can also be requested to determine tumor type, grade and depth of myometrial invasion, all of which is taken into consideration to determine the need for lymph node sampling
- The utility of this exercise is debated by many (Surg Pathol Clin 2019;12:329)
- If intraoperative consultation is performed:
- Specimen should be carefully examined and opened in the coronal plane resulting in anterior and posterior halves (identical to routine processing)
- If there is no grossly visible lesion, frozen sections are not indicated as sampling is random and identifies malignancy in only 15% of the cases (Am J Clin Pathol 2017;148:345)
- If a tumor is grossly visible, a representative full thickness section at the point of deepest invasion should be obtained
- Of note, depth of invasion on frozen section slides has a 36% risk of underestimation and 3% risk of overestimation (Am J Clin Pathol 2017;148:345)
- Tumor grade has 80% concordance between frozen section and final diagnosis (Am J Clin Pathol 2017;148:345)
- Proposed algorithm is presented in Diagram 1
Microscopic (histologic) description
- Pathologic definition of carcinoma of the endometrium:
- Diagnosis of carcinoma is based on features indicative of invasion into the surrounding mesenchyme (endometrial stroma or myometrium)
- Stromal invasion is typically seen in the form of glandular confluence and complex architecture: loss of individual glandular contours with gland fusion, lack of intervening stroma and back to back architecture
- Invasion usually presents in cribriform, microacinar and solid architectural patterns; other helpful features are stromal desmoplasia (stroma has myofibroblasts, edema, inflammatory cells and myxoid change), stromal necrosis (stroma replaced by necrotic and inflammatory debris) or combinations of these findings between adjacent glands
- So called microcystic, elongated and fragmented (MELF) pattern of growth represents, by definition, invasion
- This pattern can be deceptive and easily missed at low power magnification
- Diagnosis of carcinoma is based on features indicative of invasion into the surrounding mesenchyme (endometrial stroma or myometrium)
- Depth of myometrial invasion, cervical stromal involvement, serosal involvement and adnexal involvement are relevant to pT category (see staging)
- Histologic grading:
- Endometrioid and mucinous carcinomas are graded with a 3 tier system developed by the International Federation of Gynecology and Obstetrics (FIGO):
- FIGO 1: predominant glandular growth and < 5% nonsquamous solid component; glandular architecture is identified by the presence of patent lumina within the gland, relatively preserved polarity of the epithelium and absent to mild epithelial stratification
- FIGO 2: 6 - 50% nonsquamous solid component
- FIGO 3: > 50% nonsquamous solid component
- Architectural grading described above is upgraded by 1 if there is severe nuclear atypia (pleomorphism, nuclear enlargement and nucleoli evident at low power magnification)
- Endometrioid carcinoma FIGO grade 2 purely based on cytologic atypia (that is, with severe atypia but architecturally well differentiated) is extremely rare and must be treated as a diagnosis of exclusion; it is imperative to first exclude serous and clear cell carcinoma
- In general, a 2 tier system can be also applied, with FIGO1 and FIGO2 being considered low grade and FIGO 3 being considered high grade
- Other carcinoma types (serous, clear cell, carcinosarcoma, undifferentiated, mixed) are by definition high grade
- Endometrioid and mucinous carcinomas are graded with a 3 tier system developed by the International Federation of Gynecology and Obstetrics (FIGO):
- Lymph node involvement:
- 2018 AJCC staging manual introduces different categories for lymph node metastases in gynecologic malignancies, mostly based on size
- Lymphovascular space invasion:
- Lymphovascular space invasion (LVI) is an independent predictor of nodal metastases and local recurrence (Gynecol Oncol 2012;124:31, Arch Gynecol Obstet 2013;288:1391)
- LVI is defined as tumor cells in a space lined by endothelial cells outside the immediate invasive border
- Extent of LVI, not just the presence, correlates significantly with regional and distal lymph node involvement, locoregional recurrence and survival (Eur J Cancer 2015;51:1742, Histopathology 2019;75:128)
- Extent should be reported as follows:
- Absent: no LVI as defined
- Focal: a single focus of LVI
- Substantial: diffuse or multifocal LVSI
- Extent should be reported as follows:
- Under this system, the term focus is understood as a cluster containing up to 5 individual involved vascular spaces
- It can be inferred that substantial LVI represents either > 1 focus as defined or any focus with > 5 individual involved vascular spaces
- Artificial tumor intrusion into vascular spaces (vascular pseudoinvasion) can be seen in laparoscopic, robotic assisted hysterectomies (Am J Surg Pathol 2008;32:560, Am J Surg Pathol 2009;33:298)
- This phenomenon occurs not only in cancer related surgery: displacement of normal endometrial glands and stroma has been reported in 13% of laparoscopic hysterectomies performed for benign conditions (Am J Surg Pathol 2008;32:560)
- Of note, other studies have shown no association between laparoscopic hysterectomy or the use of a uterine manipulator and the prevalence of LVI (JSLS 2014;18:e2014.00021, Am J Obstet Gynecol 2013;208:71.e1)
- Real vascular invasion is seen in lymphatics and venous vessels, not in arterial vessels; intravascular foci are round and conform to the shape of the vessel; sometimes they are partially adherent to the vessel wall; cells have more eosinophilic cytoplasm and rounder shape compared with the native tumor; the presence of a perivascular lymphocytic infiltrate also supports real LVI (Mod Pathol 2010;23:1073)
- Artificial intrusion should be considered if the intravascular tumor retains a gland shape or stromal elements within it, has a large ("chunky") size and involves arterial vessels or other elements are identified within vascular spaces (benign endometrium, surface necrotic material or exudates) (Mod Pathol 2010;23:1073)
Virtual slides
- See endometrial carcinoma types
Cytology description
- Abnormalities in cervicovaginal cytology can be the first presenting sign of endometrial carcinoma
- Presence of endometrial cells in pap smears of women over the age of 45 needs to be reported (Cytojournal 2017;14:22)
- Diagnosis of adenocarcinoma in cervicovaginal cytology should prompt consideration for endometrial sampling
- See endometrial carcinoma types for detailed description
Cytology images
- See endometrial carcinoma types
Positive stains
- CK7, CK8 / 18, CK19
- Also vimentin (65%), CEA (areas of squamous metaplasia), CA-125, ER, PR, PTEN (80%), cyclin D1 (40%)
- CD10, as a marker of endometrial stroma, is not useful to distinguish myometrial invasion from adenomyosis involvement (Mod Pathol 2003;16:22, Am J Surg Pathol 2003;27:786)
- IFITM1, another endometrial stroma marker, has superior specificity in this differential (Am J Clin Pathol 2016;145:486)
Negative stains
- See endometrial carcinoma types
Molecular / cytogenetics description
- Determination of the tumor histologic type is critical for patient risk stratification and management
- However, there is poor interobserver reproducibility in tumor type and grade among expert pathologists (Mod Pathol 2013;26:1594, Am J Surg Pathol 2013;37:874, Int J Gynecol Cancer 2011;21:654)
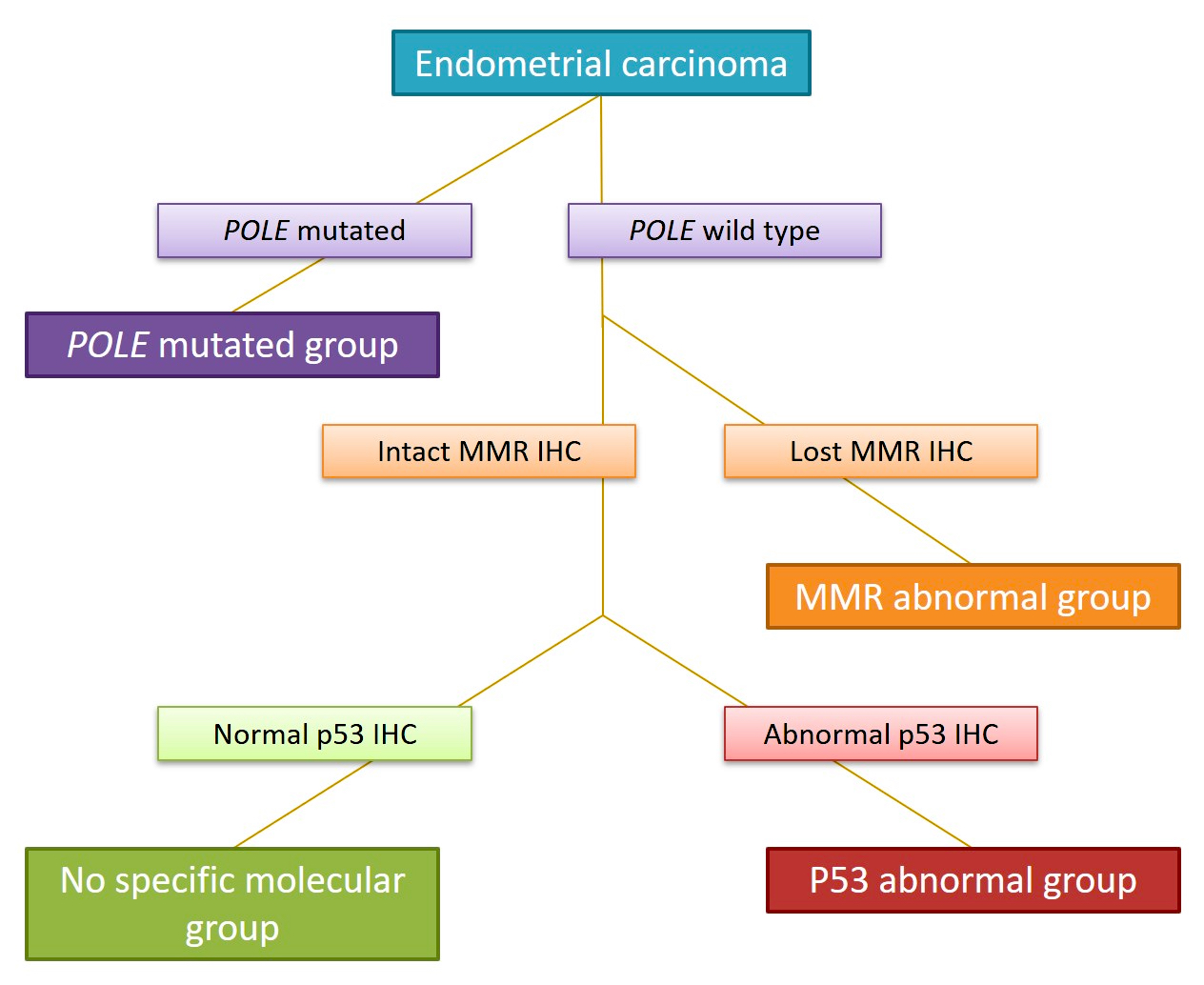
- Recent studies have provided a comprehensive characterization of the genomic profiles of endometrial carcinoma:
- In 2013, The Cancer Genome Atlas (TCGA) Research Network published an integrated genomic characterization of endometrial carcinoma based on genomic data from array and sequencing based technologies (Nature 2013;497:67)
- It proposed a classification that separates endometrial carcinomas in 4 groups:
- Copy number - high (frequently involving mutations of TP53); this group includes the vast majority of serous carcinomas and 25% of high grade endometrioid tumors
- Copy number - low (frequently involving mutations of PTEN, PIK3CA, ARID1A and KRAS); this group is mostly composed of low grade endometrioid carcinomas
- Microsatellite instability hypermutated (frequently involving alterations of mismatch repair protein genes)
- Polymerase ε (POLE) ultramutated; this group is mostly composed of endometrioid cancers, which despite having a dramatically increased transversion mutation frequency and newly identified hotspot mutations in the POLE gene (which encodes the central catalytic subunit of DNA polymerase epsilon), appear to have a better prognosis than other groups (J Natl Cancer Inst 2014;107:402, Cancer 2015;121:386)
- Molecular based classification correlates with clinical outcomes: survival rates are best in POLE mutated tumors, followed by copy number - low, microsatellite instability and copy number - high carcinomas (Nature 20132;497:67)
- Thus, the molecular fingerprint can better assist in patient risk stratification and management
- Ancillary testing using formalin fixed, paraffin embedded tumoral tissue can serve as a surrogate to detect its molecular alterations and determine the molecular group (J Pathol 2012;228:20)
- PROMISE algorithm is based on POLE mutational analysis and immunohistochemistry for p53 and mismatch repair proteins (MLH1, MSH2, MSH6 and PMS2) as a valid surrogate to determine the molecular group (Br J Cancer 2015;113:299, Diagram 2)
- When combined with clinicopathologic features, the molecular classifier is highly correlated with outcome and survival curves
- Some carcinomas harbor more than one molecular classifying feature and are referred to as multiple classifier; recent evidence suggests that MMR deficiency and POLE mutations supersede p53 alterations in terms of clinical behavior (J Pathol 2020;250:312)
- MMR deficient, p53 abnormal tumors should be categorized in the MMR deficient / microsatellite instable group
- POLE mutant, p53 abnormal tumors should be classified in the ultramutated POLE mutant group
- POLE mutant, MMR deficient should be classified in the ultramutated POLE mutant group (J Pathol 2020;250:323)
- CTNNB1 mutations have been found to be an adverse prognostic feature in patients with low grade, low risk endometrial carcinoma (Mod Pathol 2017;30:1032)
- Nuclear expression of beta catenin is usually associated with CTNNB1 mutations, although the correlation is not perfect (Mod Pathol 2018;31:1553)
Sample pathology report
- Uterus, cervix, fallopian tubes and ovaries (total hysterectomy and bilateral salpingo-oophorectomy):
- Endometrial carcinoma, ___ type (see synoptic report)
- Tumor grade: Low versus high / FIGO grade 1 (low) versus 2 (low) versus 3 (high).
- Myometrial invasion: Absent versus present (< 50% versus ≥ 50% of the myometrial thickness, __/__ mm, __% of the wall).
- Uterine serosa: Uninvolved / involved.
- Lymphovascular space invasion: Absent versus present (focal - one focus versus substantial - > 1 focus).
- Cervical stromal invasion: Absent versus present (1/3 versus 2/3 versus 3/3 of the cervical wall, __/__ mm, __% of the cervical wall).
- Distal mucosal margin: Negative / positive.
- Parametrial margin: Negative / positive.
- Ovaries and fallopian tubes: Uninvolved / involved.
- POLE mutated MMR deficient versus p53 abnormal versus no specific molecular group.
- Endometrial carcinoma, ___ type (see synoptic report)
Differential diagnosis
- See endometrial carcinoma types
Board review style question #1
Which of the following prognostic variables modifies the FIGO / AJCC stage of endometrial carcinoma?
- Depth of myometrial invasion
- Involvement of adenomyosis
- Lymphovascular space invasion
- Molecular group
- Superficial (glandular) cervical involvement
Board review style answer #1
A. Depth of myometrial invasion. All the options have documented prognostic impact in patients with endometrial carcinoma; however, only depth of myometrial invasion modifies the pathologic stage.
Comment Here
Reference: Endometrial carcinoma - general
Comment Here
Reference: Endometrial carcinoma - general
Board review style question #2
Which of the following variables modifies the FIGO / AJCC stage of endometrial carcinoma?
- Extranodal extension by carcinoma involving a lymph node
- Lower uterine segment involvement
- MELF pattern of invasion
- Number of intravascular tumor foci
- Size of lymph node tumor metastases
Board review style answer #2
E. Size of lymph node tumor metastases. Of all the included features, only the size of the lymph node alters the stage. Tumor metastasis now subclassifies the N (FIGO stage III) category in N0i+ (isolated tumor cells), N1 / N2mi (micrometastases) and N1 / N2a (macrometastases).
Comment Here
Reference: Endometrial carcinoma - general
Comment Here
Reference: Endometrial carcinoma - general