Table of Contents
Definition / general | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Differential diagnosis | Additional referencesCite this page: Onur I. Adenomyosis / adenomyoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/uterusadenomyosis.html. Accessed December 22nd, 2024.
Definition / general
- A nonneoplastic lesion of myometrial tissue characterized by the presence of endometrial glands and stroma within myometrium (Best Pract Res Clin Obstet Gynaecol 2006;20:511)
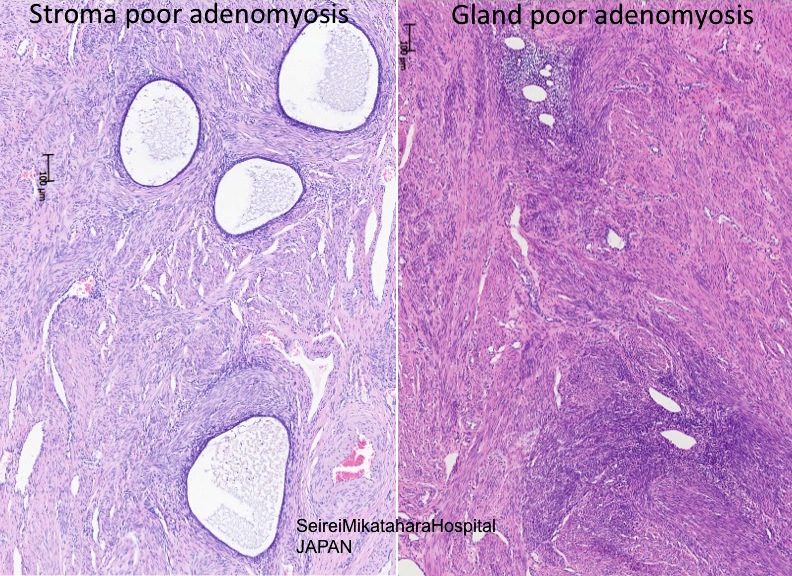
- Synonyms: myometrial endometriosis, superficial adenomyosis (1 - 2.5 mm in myometrium), stromal adenomyosis, incomplete adenomyosis, adenomyosis with sparse glands
- Usually an incidental finding in hysterectomy specimens (Int J Gynecol Pathol 1996;15:217)
- May be diffuse or focal
- May be involved by hyperplasia and carcinoma
Terminology
- Adenomyoma: A circumscribed nodular aggregate of benign endometrial glands surrounded by endometrial stroma, with leiomyomatous smooth muscle bordering the endometrial stromal component
ICD coding
- ICD-10: N80.0 - endometriosis of uterus
Epidemiology
- Diagnosed mostly in late reproductive years
- Involves 5 - 70% of surgically removed uteri (wide range due to imprecision of diagnostic criteria)
- Rare in postmenopausal women (except for tamoxifen - associated cases)
- Am J Obstet Gynecol 1958;76:1044, Am J Obstet Gynecol 1962;83:1541, Journal of Minimally Invasive Gynecology 2011;18:428
Risk factors: - Increasing age up to menopause, multiparity, smoking, increased serum estrogen levels (Hum Reprod 2001;16:2418, Acta Obstet Gynecol Scand 2004; 83:699, N Engl J Med 2009;360:268)
- Previous uterine surgery (Obstet Gynecol 2004;104:1034)
- Tamoxifen (J Mol Biol 1976;106:683) (These cases tend to show stromal fibrosis, glandular dilation and various metaplastic changes (Histopathology 2000;37:340)
- Matrix metalloproteinase (MMP) polymorphisms (J Genet 2016;95:611)
Sites
- Frequently in posterior, less commonly in anterior uterine wall
- Rarely in cornua or by cervical os
Pathophysiology
- May result from (a) chronic uterine autotraumatization by physiological mechanical functions and (b) tissue injury and repair (Arch Gynecol Obstet 2015;291:917)
- May be caused by disease of junctional zone (Lancet 1995;346:558)
- Prolactin (Am J Obstet Gynecol 1991;165:232) and immune factors may play a role (Hum Reprod Update 1998;4:312)
Etiology
- Adenomyosis and endometriosis are usually regarded as closely related, but
- Microscopic appearance, and probably their pathogenesis, are somewhat different
- They may occur independently of each other
- Adenomyosis mostly is made up of nonfunctional (basal) endometrium and is frequently connected with the mucosa (vs. endometriosis, composed of functional layers)
- Adenomyosis may represents a unique form of endometrial diverticulosis
- Hypothetical mechanisms include (Crum: Diagnostic Gynecologic and Obstetric Pathology, 2nd Edition, 2011)
- Instillation of endometrium within the myometrium
- In situ metaplasia of pluripotent stem cells retained in myometrium or
- Improper partitioning of the endometrium from the myometrium
- Of note, del(7) (q21.2q31.2), a deletion found in typical leiomyoma, has been found in three cases of adenomyosis, suggesting some pathobiologic overlap between leiomyomata and adenomyosis (Cancer Genet Cytogenet 1995;80:118)
- Definitive distinction between these explanations requires further study
Clinical features
- Nonneoplastic condition presenting with palpably enlarged uterus
- Symptoms are nonspecific: dysmenorrhea, menorrhagia, abnormal uterine bleeding, dyspareunia, chronic pelvic pain associated with the menstrual period and infertility (Eur J Obstet Gynecol Reprod Biol 2009;143:103, N Engl J Med 2010;362:2389)
- Associated with deep infiltrating endometriosis, parity, intense dysmenorrhea and increasing age (Eur J Obstet Gynecol Reprod Biol 2014;181:289)
- Tends to regress after menopause (Hum Reprod 2012;27:3432)
- When extensive, it confers a potential risk of infarction and thrombosis and exacerbates menorrhagia via activation of coagulation and fibrinolysis during menstruation (Eur J Obstet Gynecol Reprod Biol 2016;204:99)
Diagnosis
- By histopathologic examination of well oriented hysterectomies
- Essentially should not be diagnosed in curettings or hysteroscopic material
Radiology description
- 2D and 3D transvaginal sonography, sonohysterosalpingography, magnetic resonance imaging and endoscopic techniques (hysteroscopy and laparoscopy) are suggestive (Hum Reprod 2012;27:3432, Eur Rev Med Pharmacol Sci 2015;19:1146)
Prognostic factors
- Benign; excellent prognosis even if not removed
Case reports
- 43 year old pregnant woman with decidualization of uterine adenomyoma (Arch Gynecol Obstet 2015;291:399)
- 49 year old woman with infiltrating adenomyosis of the cervix with features of a low grade stromal sarcoma (Int J Gynecol Pathol 2014;33:253)
- 57 year old woman with endometrioid adenocarcinoma arising from uterine adenomyosis (Case Rep Obstet Gynecol 2014;2014:569295)
- 63 year old woman with adenomyomatous polyp of uterus (Jpn J Clin Oncol 1997;27:350)
- A postmenopausal woman with endometrioid adenocarcinoma arising from uterine adenomyosis (Eur J Gynaecol Oncol 2010;31:719)
Treatment
- Standard management option for women who have completed childbearing is hysterectomy (J Minim Invasive Gynecol 2016;23:164)
- Newer techniques such as laparoscopic radiofrequency thermal ablation are an effective minimally invasive alternative (JSLS 2015 Sep;19:e2015.00071)
- Medical treatment may be considered (Gynecol Endocrinol 2016 Jul 5 [Epub ahead of print])
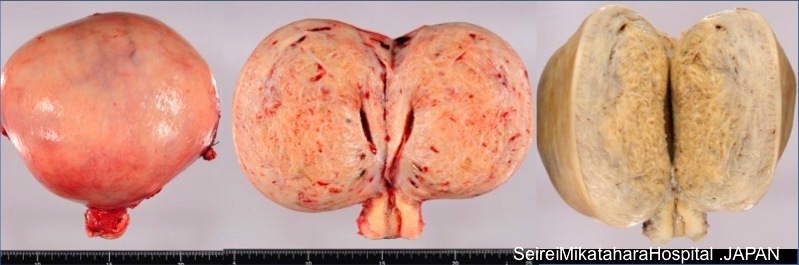
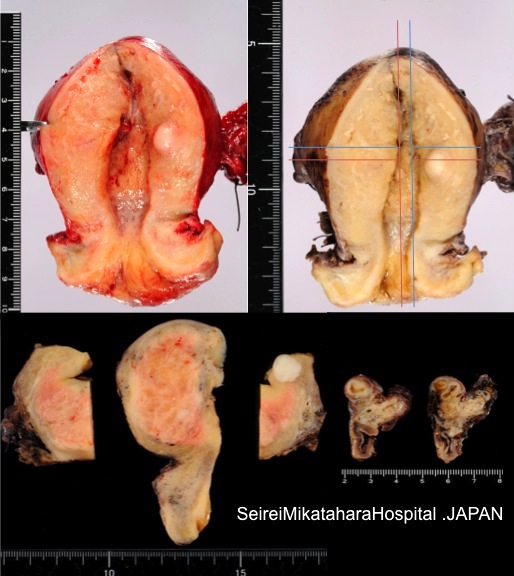
Gross description
- Often asymmetrically enlarged, globular uterus due to associated myometrial hypertrophy reflected by thickened myometrium (Am J Obstet Gynecol 1962;83:1541, Am J Obstet Gynecol 1962;84:1820)
- Trabeculated cut surface with ill defined hypertrophic swirls of smooth muscle and petechia-like gray foci of endometrium (Am J Obstet Gynecol 1962;83:1541)
- Blood filled cystic spaces may be seen
- Cannot be shelled out
- In elderly women, uterus may appear atrophic
- Mutter: Pathology of the Female Reproductive Tract, 3rd Edition, 2014
Gross images
Microscopic (histologic) description
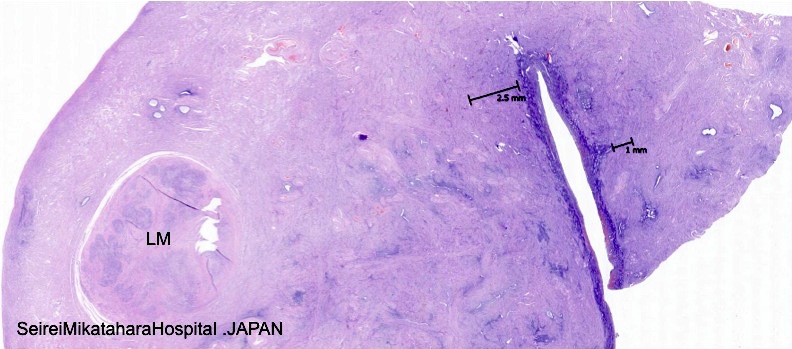
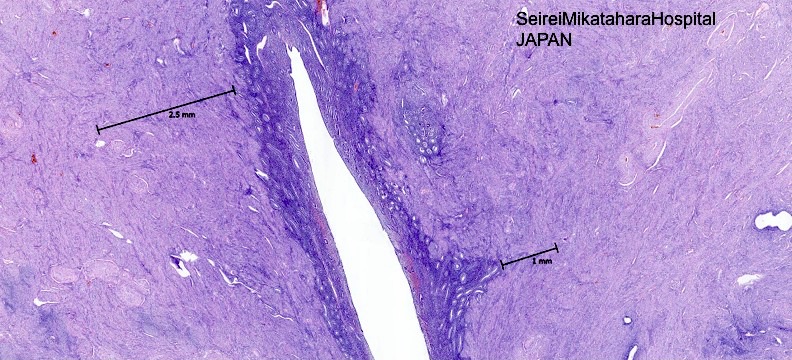
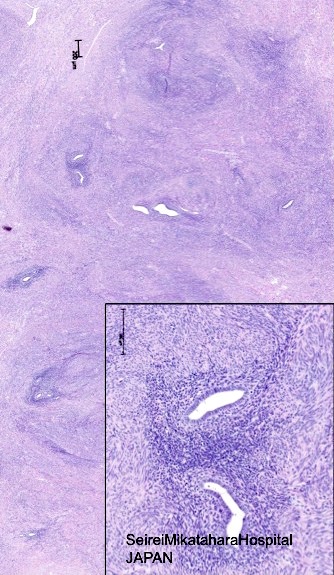
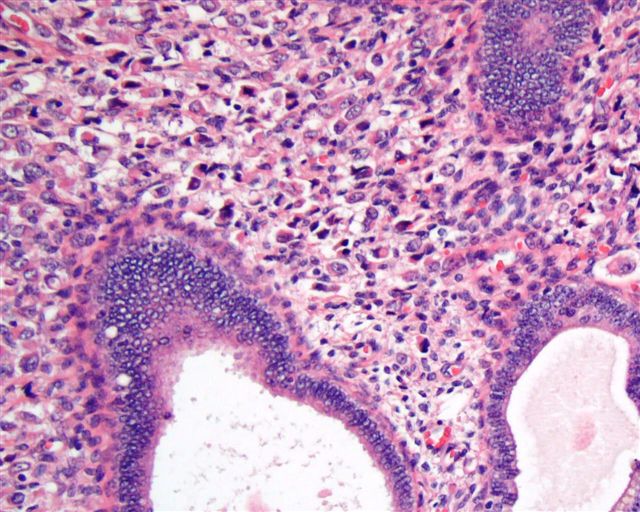
- Endometrial glands and stroma deep in myometrium
- Depth of penetration of endometrial glands to myometrium is arbitrary
- At least one low power field from endomyometrial junction (which is irregular) or
- 1 to 2.5 mm below basal layer of endometrium
- Or deeper than 25% of overall myometrial thickness
- Often round masses of myometrial smooth muscle proliferation present around endometrial islands
- Glandular tissue usually inactive and of basalis or proliferative type endometrium, but one fourth is functional; hemosiderin is generally absent (Am J Obstet Gynecol 1971;110:275)
- These foci are subject to any disease affecting ortothopic endometrium:
- May show atrophy, metaplasia or decidual change
- May exhibit hyperplasia, atypical hyperplasia / EIN, endometrial carcinoma, endometrial stromal sarcoma, carcinosarcoma (Gynecol Obstet Invest 1999;48:141)
- Microscopic foci may be in vascular spaces resembling endometrial stromal sarcoma (Int J Gynecol Pathol 2010;29:117, Am J Surg Pathol 2013;37:1395, Am J Obstet Gynecol 2012;207:417)
- Stromal adenomyosis (incomplete adenomyosis, adenomyosis with sparse glands) is characterized by lack of glands; it is rare and difficult to diagnose
- References: Robboy: Pathology of the Female Reproductive Tract, 1st Edition, 2001, Reichert: Diagnostic Gynecologic and Obstetric Pathology, 2011, Soslow: Uterine Pathology (Cambridge Illustrated Surgical Pathology), 1st Edition, 2012
Microscopic (histologic) images
Contributed by Ayse Ayhan, M.D., Ph.D.
Images hosted on other servers:
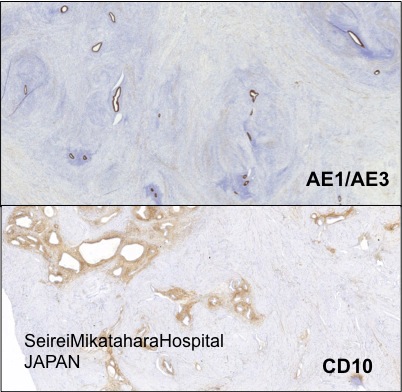
Positive stains
- CD10 (in endometrial stroma rather than smooth muscle around glands)
- In atrophic adenomyosis CD10 may be weak or focal
- Interferon-inducible transmembrane protein 1 (IFITM1): has superior performance distinguishing endometrial stroma of adenomyosis from mesenchyme surrounding invasive endometrial adenocarcinoma (Am J Clin Pathol 2016;145:486)
Differential diagnosis
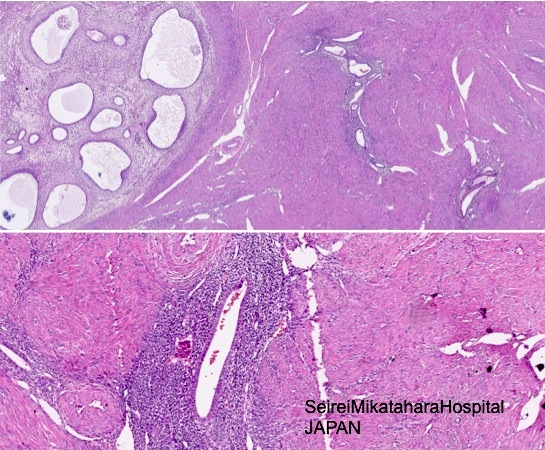
- Normal endometrium extension into myometrium (tangential section):
- The diagnosis of adenomyosis depends on the thresholds used by the individual pathologist (Int J Gynecol Pathol 1996;15:217)
- No smooth muscle hypertrophy around islands of endometrium when normal (Robboy: Pathology of the Female Reproductive Tract, 1st Edition, 2001), glands continuous with endometrium (Gattuso: Differential Diagnosis in Surgical Pathology, 3rd Edition, 2014)
- Adenomyoma: not diffuse, has distinct borders (Fletcher: Diagnostic Histopathology of Tumors, 4th Edition, 2013); smooth muscle is not neoplastic (Nucci: Diagnostic Pathology: Gynecological, 1st Edition, 2014)
- Endometrial stromal sarcoma vs adenomyosis with gland-poor or sparse glandular component: no glandular tissue, invades myometrium in tongues, no muscular hypertrophy, unusual to contain diffuse small regular glands (Am J Clin Pathol 1995;103:218), widespread vascular involvement, forms a definable tumor mass (Robboy: Pathology of the Female Reproductive Tract, 1st Edition, 2001; diagnostic gynecologic and obstetric pathology, Soslow: Uterine Pathology (Cambridge Illustrated Surgical Pathology), 2012)
- Low grade endometrial stromal sarcoma with endometrioid glandular differentiation: endometrium usually involved by neoplastic process, has a dominant stromal component with widely spaced glands, lacks associated myometrial hypertrophy, increased stromal mitotic activity and widespread vascular involvement, periglandular cuffing (Soslow: Uterine Pathology (Cambridge Illustrated Surgical Pathology), 2012, Oncol Lett 2016;11:1213)
- Adenomyosis with atrophic and fibrotic stromal component (scattered glands within myometrium) vs. invasion by a well differentiated endometrioid adenocarcinoma: atrophic glands, absence of a low magnification infiltrative pattern, endometrial stroma strands around some glands, typical adenomyosis elsewhere in uterus, lack of a host reaction to the glands and absence of an associated endometrial adenocarcinoma (Soslow: Uterine Pathology (Cambridge Illustrated Surgical Pathology), 2012)
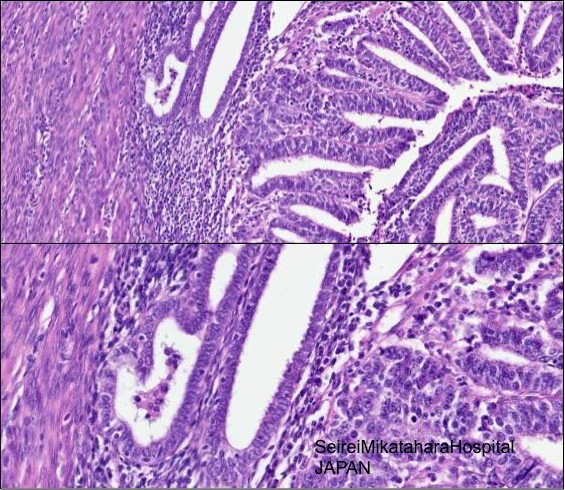
- Carcinoma involving adenomyosis vs endometrioid carcinoma invading myometrium: lobulated, rounded contours, residual nonneoplastic endometrial glands and / or stroma of adjacent uninvolved adenomyosis, typically compressed at periphery (Nucci: Diagnostic Pathology: Gynecological, 1st Edition, 2014)
- Please note that “adenomyosis like infiltration of endometrial cancer” is an entity describing the pattern of infiltration (Adv Anat Pathol 2013;20:141)
- Adenomatoid tumor: no endometrial stroma, glandular spaces lined by flat cuboidal epithelium, mesothelial origin (Gattuso: Differential Diagnosis in Surgical Pathology, 3rd Edition, 2014)