Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Kommoss FKF, Tessier-Cloutier B. SMARCA4 deficient uterine sarcoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/uterusSMARCA4sarcoma.html. Accessed December 1st, 2024.
Definition / general
- SMARCA4 deficient uterine sarcoma (SDUS) is a rare and highly aggressive uterine malignancy that occurs in young women and is defined by an inactivating mutation of the SMARCA4 gene
Essential features
- Undifferentiated morphology (sheet-like architecture, monomorphic, discohesive and mitotically active)
- Loss of nuclear SMARCA4 / BRG1 immunohistochemical staining (> 95% of cases)
Terminology
- SMARCA4 deficient uterine tumor
ICD coding
- Not assigned yet
Epidemiology
- Most patients are adolescent and young adult women (median: 36 years) (Mod Pathol 2018;31:1442)
- There is 1 report of an SDUS associated with germline mutations, including a family history of small cell carcinoma of the ovary, hypercalcemic type (SCCOHT) (Mod Pathol 2019;32:1675)
- Rare cases have been reported in postmenopausal women (Int J Surg Pathol 2023;31:104)
Sites
- Uterus
Pathophysiology
- Inactivating mutation of the SMARCA4 gene, a core subunit of the SWI / SNF complex, drives oncogenesis (Am J Surg Pathol 2020;44:263)
- SWI / SNF (SWItch / sucrose nonfermentable) complex is an important regulator of the chromatin remodeling process
Etiology
- Predisposition factor: rhabdoid tumor predisposition syndrome 2 (RTPS2) reported in 1 patient (Mod Pathol 2019;32:1675)
- Cellular origin remains unknown; studies have shown DNA methylation signatures in SDUS that are distinct from other SWI / SNF deficient cancers of the gynecologic tract (J Pathol 2022;257:140)
Clinical features
- Poor clinical outcome, 7 months is median overall survival (Am J Surg Pathol 2020;44:263)
- Most patients present at an advanced stage (Mod Pathol 2018;31:1442)
- Nonspecific symptoms may include vaginal bleeding and uterine mass (Mod Pathol 2018;31:1442, Mod Pathol 2019;32:1675)
- 1 patient has been described to harbor a SMARCA4 germline mutation, which is associated with the rhabdoid tumor predisposition syndrome 2 (RTPS2) and predisposes for other undifferentiated malignancies such as small cell carcinoma of the ovary, hypercalcemic type, atypical teratoid / rhabdoid tumors (ATRT) and other extracranial malignant rhabdoid tumors (Mod Pathol 2019;32:1675, OMIM: Rhabdoid Tumor Predisposition Syndrome 2; RTPS2 [Accessed 31 August 2023])
Diagnosis
- There are no established tests to screen for SDUS
- When clinical suspicion arises, abdominal ultrasound and computed tomography scans are useful adjuncts
- Definitive diagnosis requires biopsy
- Referral to clinical genetics services and testing for germline SMARCA4 pathogenic variants should be considered in patients diagnosed with SDUS
Laboratory
- Although morphologically and molecularly similar to small cell carcinoma of the ovary, hypercalcemic type, which can be associated with hypercalcemia, there are no reports of increased calcium levels in SDUS
Prognostic factors
- Most reported tumors presented at high stage and showed a very aggressive clinical course
- Due to the only small case series reported to date, there are no known prognostic factors
Case reports
- 51 year old woman with SDUS showing response to radiation therapy (Adv Radiat Oncol 2021;6:100728)
- 52 year old woman with uterine mass (Int J Surg Pathol 2023;31:104)
Treatment
- No established standard treatment
- Patients are usually treated by a combination of surgery, chemotherapy and radiation
Gross description
- Solid uterine mass usually with deep myometrial invasion
- Mean size: 14 cm (range: 4 - 25 cm)
- Tan-pink to gray-white soft cut surface
- Hemorrhage and necrosis can be present
- References: Mod Pathol 2019;32:1675, Mod Pathol 2018;31:1442
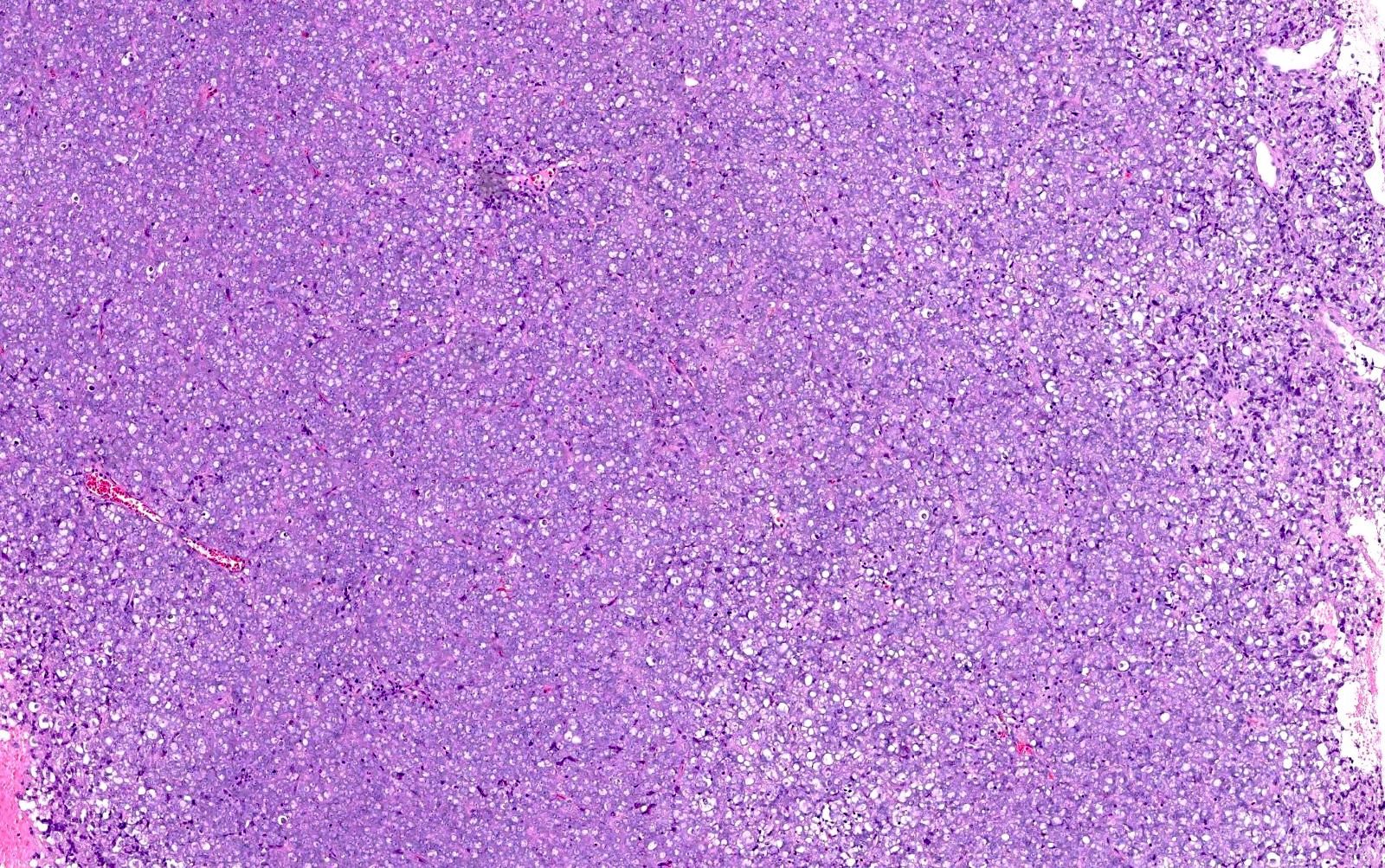
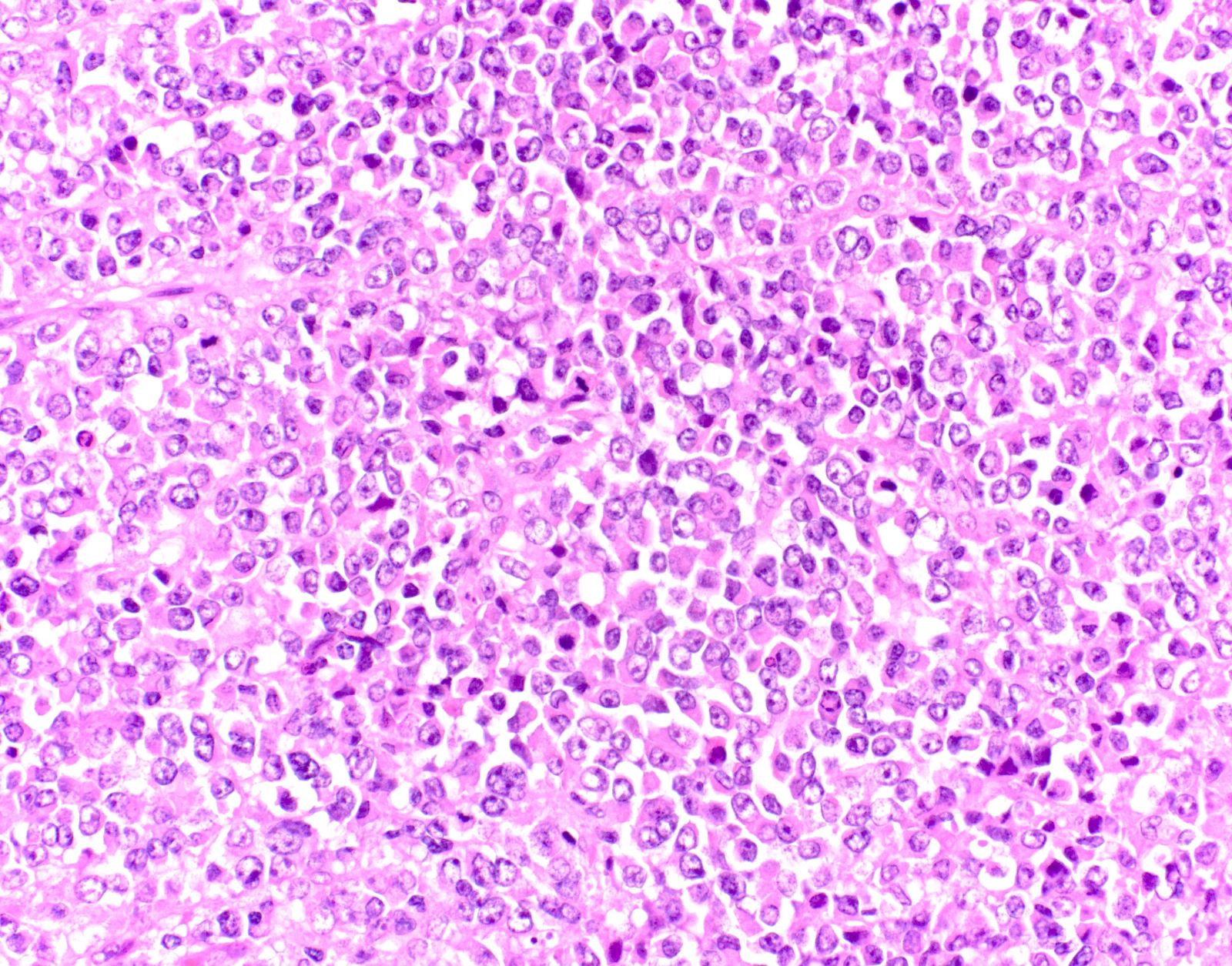
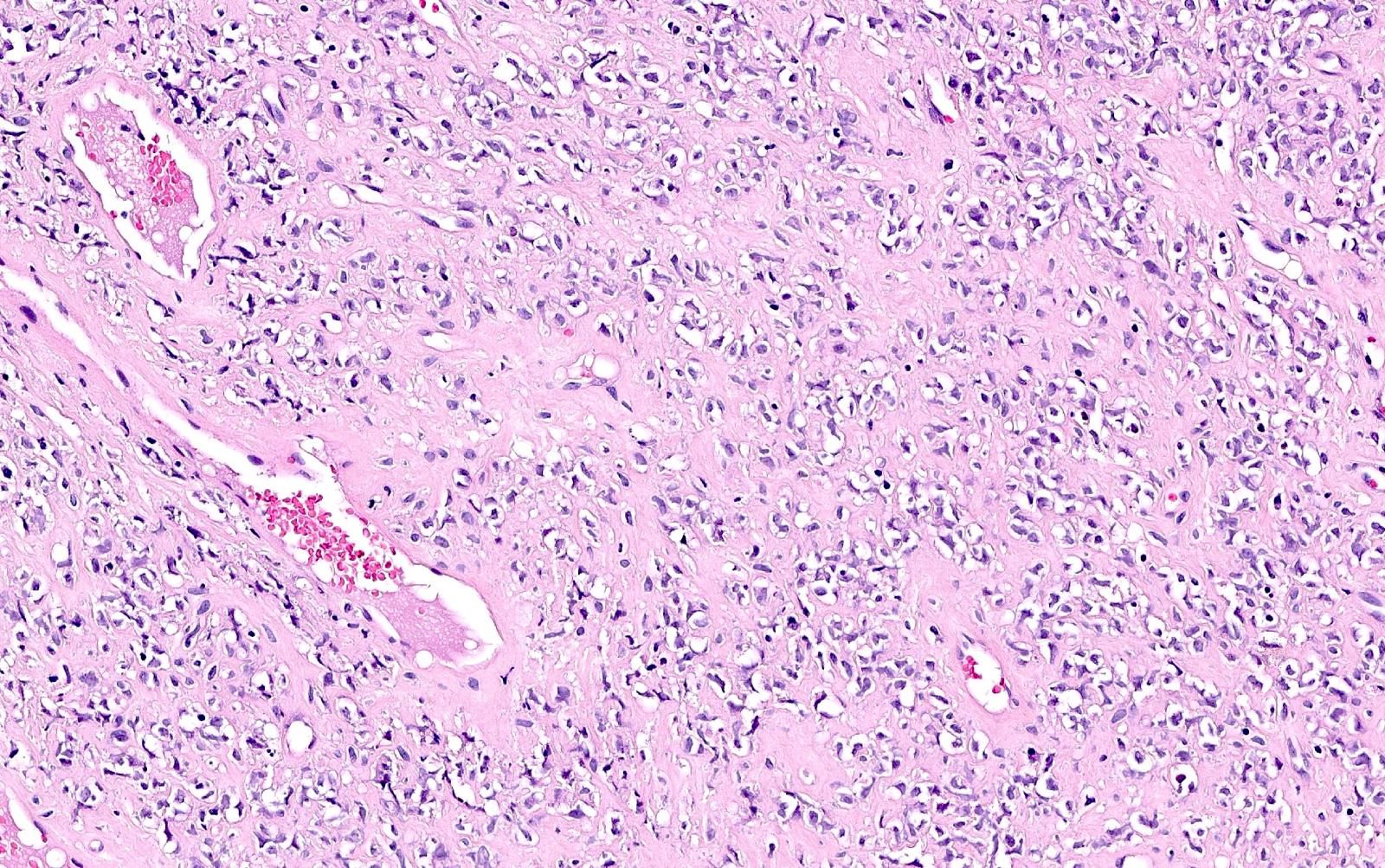
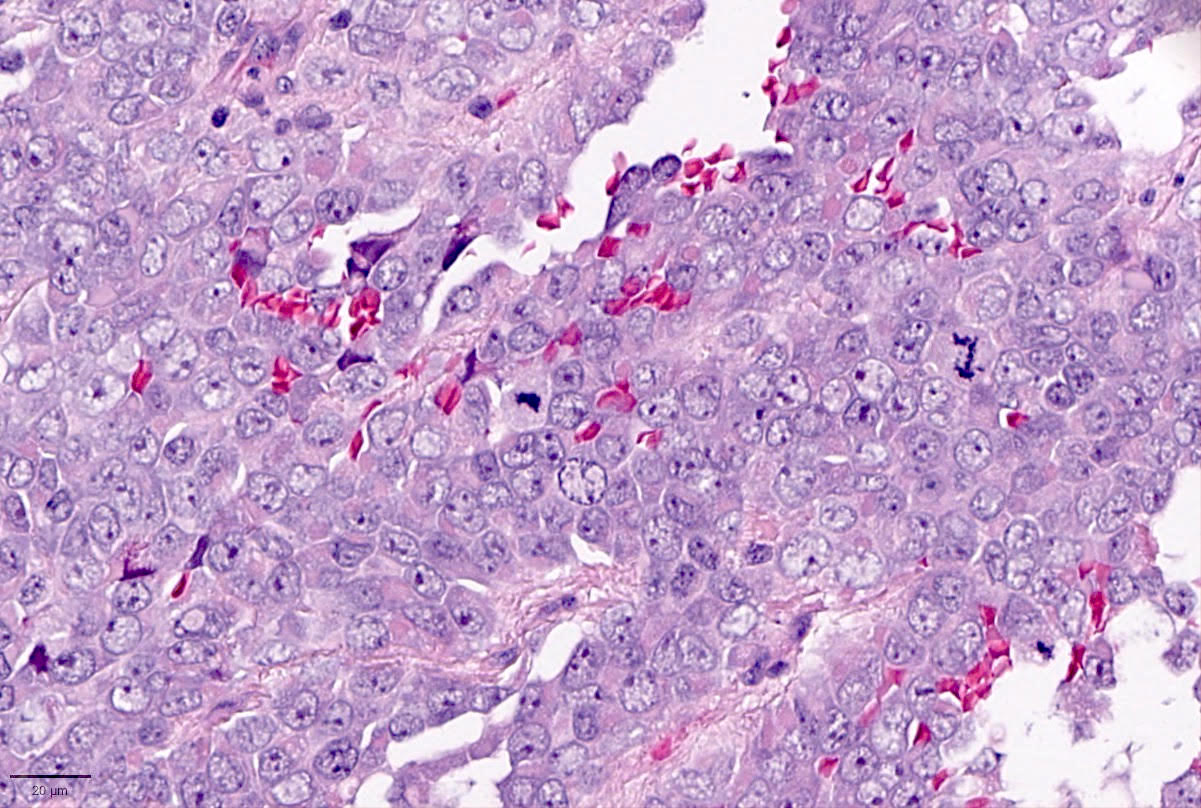
Microscopic (histologic) description
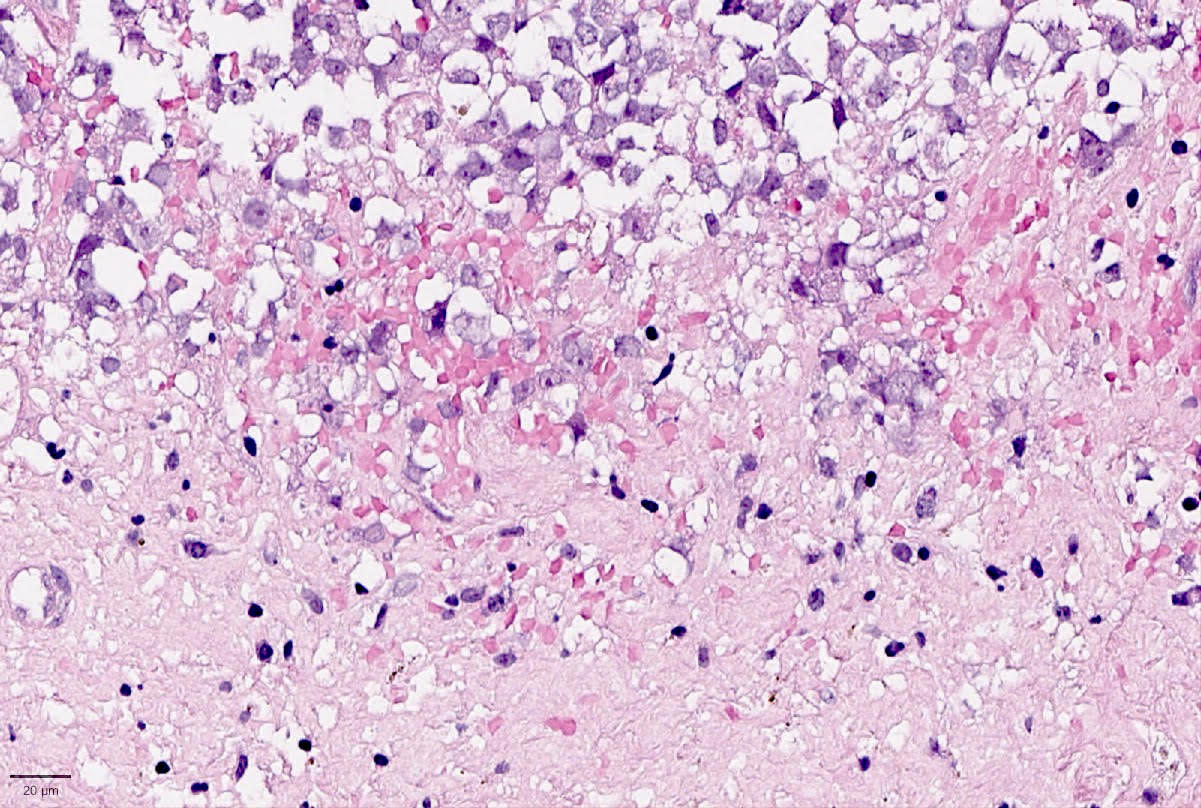
- Small to large epithelioid cells with prominent nucleoli and brisk mitotic activity (Mod Pathol 2018;31:1442, Am J Surg Pathol 2020;44:263)
- Diffuse growth of tightly packed cells, sometimes in a nested / corded architecture with stromal hyalinization (Mod Pathol 2018;31:1442, Am J Surg Pathol 2020;44:263)
- Rhabdoid morphology is common (Mod Pathol 2018;31:1442, Am J Surg Pathol 2020;44:263)
- Necrosis is common (Mod Pathol 2018;31:1442, Am J Surg Pathol 2020;44:263)
- Lymphovascular invasion is common (Mod Pathol 2018;31:1442, Am J Surg Pathol 2020;44:263)
- Phyllodiform-like growth may be present (Am J Surg Pathol 2020;44:263)
Microscopic (histologic) images
Positive stains
- Mismatch repair proteins (intact PMS2, MLH1, MSH6 and MSH2) (Mod Pathol 2018;31:1442, Am J Surg Pathol 2020;44:263)
- p53 wild type pattern (Am J Surg Pathol 2020;44:263)
- CD10 (focal and patchy 55%) (Mod Pathol 2019;32:1675)
- WT1 (1 of 3 reported cases) (Mod Pathol 2018;31:1442)
Negative stains
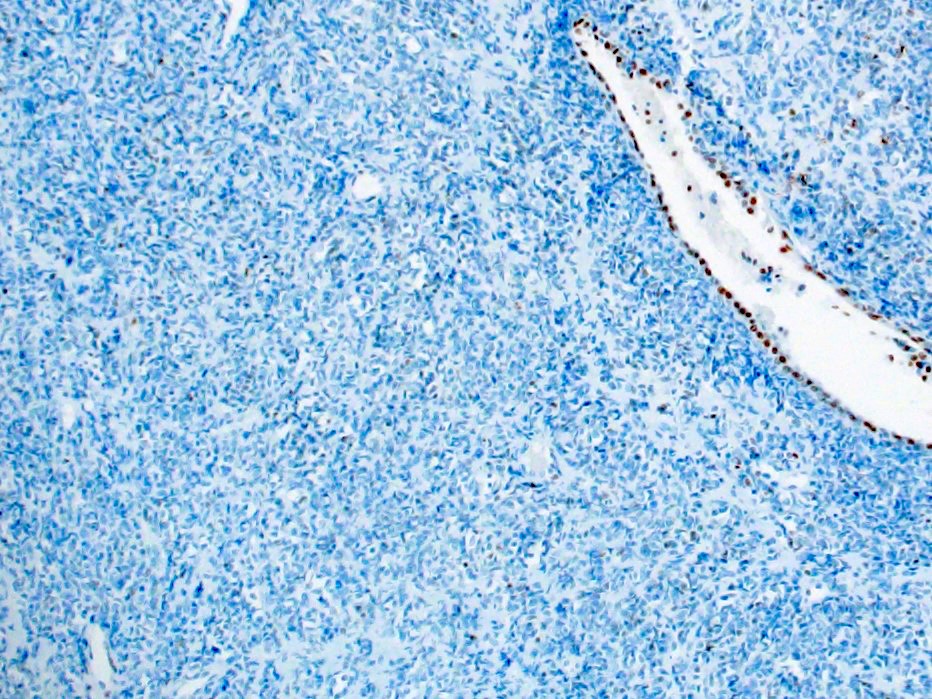
- Loss of nuclear SMARCA4 / BRG1 expression (Mod Pathol 2018;31:1442, Am J Surg Pathol 2020;44:263)
- Loss of nuclear SMARCA2 / BRM expression (Am J Surg Pathol 2020;44:263)
- Claudin4 (Mod Pathol 2018;31:1442, Am J Surg Pathol 2020;44:263)
- Keratins (negative to focal expression in up to 25%) (Am J Surg Pathol 2020;44:263)
- EMA (negative to focal expression in up to 70%) (Am J Surg Pathol 2020;44:263)
- Muscle markers are usually negative (desmin, h-caldesmon, actin, alpha smooth muscle type and myogenin) (Mod Pathol 2019;32:1675)
- Loss of nuclear SMARCB1 / INI1 staining has been reported in 1 SMARCA4 proficient tumor (Am J Surg Pathol 2020;44:263)
Molecular / cytogenetics description
- SDUS is defined by inactivating somatic mutations in SMARCA4, including missense, nonsense, frameshift and splice site mutations or whole gene deletions (Mod Pathol 2018;31:1442, Mod Pathol 2019;32:1675, J Pathol 2022;257:140)
- 1 patient has been described to harbor a SMARCA4 germline mutation (Mod Pathol 2019;32:1675)
- SDUS is a chromosomally stable cancer (Am J Surg Pathol 2020;44:263, Mod Pathol 2019;32:1675, J Pathol 2022;257:140)
- SDUS harbors a distinct DNA methylation profile, which distinguishes it from other SWI / SNF deficient cancers such as small cell carcinoma of the ovary, hypercalcemic type or undifferentiated endometrial carcinoma (J Pathol 2022;257:140)
Sample pathology report
- Uterus with ovaries and fallopian tube, total hysterectomy and bilateral salpingo-oophorectomy:
- SMARCA4 deficient uterine sarcoma (see comment and synoptic report)
- Comment: Immunohistochemical tests for SMARCA4 / BRG1 and SMARCA2 / BRM show loss of expression. SMARCA4 deficient uterine sarcoma (SDUS) can be associated with the rhabdoid tumor predisposition syndrome 2 (RTPS2). Referral to medical genetics is recommended.
Differential diagnosis
- Undifferentiated and dedifferentiated endometrial carcinoma:
- Older age (median: 55 years)
- May be associated with a differentiated endometrial carcinoma, usually low grade endometrioid adenocarcinoma (dedifferentiated carcinoma)
- Mismatch repair deficiency is common
- Loss of nuclear expression of SMARCA4 / BRG1, SMARCB1 / INI1 or ARID1B
- Associated with other genetic alterations (PTEN, ARID1A, PIK3CA, KRAS, CTNNB1, NRAS, TP53)
- Müllerian adenosarcoma:
- Sarcomatous cells are more spindled and display only mild atypia
- High grade sarcomatous components (sarcomatous overgrowth) usually display marked pleomorphism and may be associated with TP53 alterations
- Embryonal rhabdomyosarcoma:
- High grade endometrial stromal sarcoma:
- Typically, diffuse positivity for cyclin D1
- Typically harbors YWHAE::NUTM2A / B gene fusion, BCOR / BCORL1 rearrangements or BCOR internal tandem duplication (ITD)
- Undifferentiated uterine sarcoma:
- Older age
- Marked pleomorphism
- Associated with aberrant p53 staining pattern and high copy number variations
Board review style question #1
The images above show SMARCA4 / BRG1 IHC for a 28 year old woman with a 10 cm mass of the uterine corpus. What is the most likely diagnosis?
- Dedifferentiated endometrial carcinoma
- High grade endometrial stromal sarcoma
- Low grade endometrial stromal sarcoma
- Metastatic melanoma
- SMARCA4 deficient uterine sarcoma
Board review style answer #1
E. SMARCA4 deficient uterine sarcoma (SDUS). The H&E shows sheets of undifferentiated tumor cells with rhabdoid features. The immunohistochemical test for SMARCA4 / BRG1 shows aberrant expression (loss of nuclear staining). The morphology and immunophenotype are consistent with the diagnosis of SDUS. Answer A is incorrect because although dedifferentiated endometrial carcinoma is morphologically similar to SDUS and may show loss of SMARCA4 expression, this case lacks the presence of a differentiated component. Answers B, C and D are incorrect because these diagnoses may have overlapping morphologic features with SDUS; however, they retain SMARCA4 / BRG1 expression.
Comment Here
Reference: SMARCA4 deficient uterine sarcoma
Comment Here
Reference: SMARCA4 deficient uterine sarcoma
Board review style question #2
Which of the following immunohistochemical tests would most likely show aberrant expression in SMARCA4 deficient uterine sarcoma (SDUS)?
- ARID1B
- MLH1
- MSH6
- p53
- SMARCA2 / BRM
Board review style answer #2
E. SMARCA2 / BRM. SMARCA4 deficient uterine sarcomas (SDUS) are molecularly defined by inactivating SMARCA4 mutations, which are associated with SMARCA4 / BRG1 and SMARCA2 / BRM nuclear loss of expression. Answers A, B, C and D are incorrect because these tests show aberrant expression in other entities such as dedifferentiated and undifferentiated endometrial carcinoma (ARID1B loss of expression and often mismatch repair deficient) and high grade endometrioid and serous carcinoma (aberrant p53 expression).
Comment Here
Reference: SMARCA4 deficient uterine sarcoma
Comment Here
Reference: SMARCA4 deficient uterine sarcoma