Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Diagrams / tables | Diagnosis | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Ma L. POLE ultramutated endometrial carcinoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/uterusPOLEendometrialcarcinoma.html. Accessed April 3rd, 2025.
Definition / general
- Clinically significant, molecularly defined subclass of endometrial carcinoma with favorable prognosis
- Endometrial carcinoma harboring pathogenic mutations in the exonuclease domain of the POLE gene
Essential features
- Carries excellent prognosis compared to the other molecular subgroups, as defined by The Cancer Genome Atlas (TCGA) program
- Detected POLE mutation must be pathogenic, involving the exonuclease domain and correlated with ultramutated phenotype
- Predominantly endometrioid or mixed morphology but is represented across all endometrial carcinoma histotypes
Terminology
- POLE mutant endometrial carcinoma
ICD coding
Epidemiology
- ~5 - 10% of all endometrial carcinomas (Histopathology 2020;76:52)
- Patients are typically younger (median age ~55 - 60) than patients with non-POLEmut endometrial carcinomas (PLoS One 2019;14:e0214318)
Sites
- Uterine corpus
- Also reported in ovarian endometrioid carcinoma and ovarian clear cell carcinoma (Mod Pathol 2017;30:1748, Gynecol Oncol 2021;163:427, Gynecol Oncol 2022;165:577)
Pathophysiology
- TCGA Research Network identified 4 molecularly distinct endometrial carcinoma subgroups with different clinical outcomes (Nature 2013;497:67):
- POLE ultramutated (POLEmut), ultramutated
- Pathogenic POLE exonuclease domain mutations
- Mismatch repair deficient (MMRd), hypermutated
- Abnormal expression of mismatch repair proteins by IHC, highly concordant with MSI H status
- p53 abnormal (p53abn), copy number high; also referred to as serous-like
- Aberrant p53 expression by IHC, frequent TP53 mutations
- No specific molecular profile (NSMP), copy number low
- Pathogenic POLE mutation absent, MMR proficient and p53 nonaberrant
- POLE ultramutated (POLEmut), ultramutated
- POLE gene encodes the catalytic subunit of DNA polymerase epsilon
- DNA polymerase epsilon synthesizes DNA of the leading strand and plays a major role in proofreading (e.g., nucleotide and base excision repair) (Nat Rev Cancer 2016;16:71)
- Defects in exonuclease domain of POLE → ultramutational state / high tumor mutational burden → high neoantigen loads (immunogenic) → associated with increased tumor infiltrating lymphocytes / peritumoral lymphocytes and elevated PD-1 / PDL1 expression (JAMA Oncol 2015;1:1319)
- Enhanced T cell antitumor response may play a role in the observed favorable prognosis
Diagrams / tables
Diagnosis
- Detection of pathogenic POLE exonuclease domain mutation
- Molecular diagnostic tests include next generation sequencing, Sanger sequencing, hotspot single nucleotide SNaPshot assay, droplet digital PCR (J Gynecol Oncol 2022;33:e15, Int J Gynecol Pathol 2021 Dec 15 [Epub ahead of print])
- No IHC surrogate marker currently available
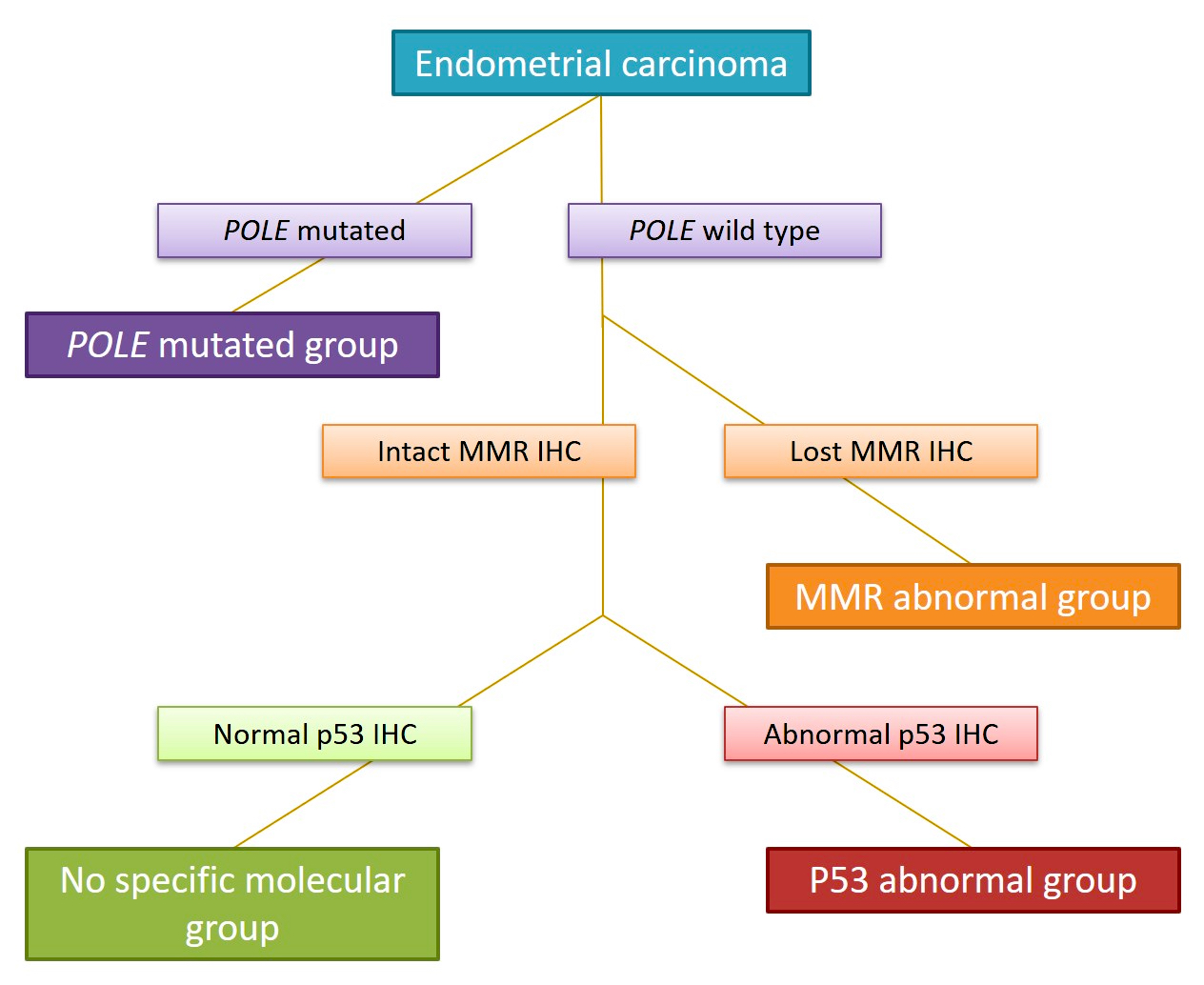
- Accurate molecular classification of endometrial carcinoma requires interpretation of tests according to the recommended algorithm (see Diagram 1) (Int J Gynecol Pathol 2021;40:5)
- Presence of pathogenic POLE exonuclease domain mutation, MMR proficient, p53 nonaberrant / wild type
- ~3 - 5% of endometrial carcinomas harbor > 1 molecular classifying alteration and are referred to as multiple classifier (J Pathol 2020;250:312)
- p53 alterations in the presence of POLE pathogenic mutation or MMR deficiency are likely secondary events acquired in tumor progression (passenger mutations) and are typically subclonal
- Hierarchical clustering analyses support classification of multiple classifier endometrial carcinomas as the following single final molecular classification:
- Pathogenic POLE exonuclease domain mutation, p53 abnormal tumors → classified as POLEmut
- MMR deficient, p53 abnormal tumors → classified as MMRd
- Multiple classifier tumors with POLE mutations and MMR deficiency also observed and final classification is dependent on which alteration is primary or secondary event (Mod Pathol 2022;35:688)
- Pathogenic POLE exonuclease domain mutation, MMR deficient (subclonal) tumors → classified as POLEmut
- Nonpathogenic POLE mutation, MMR deficient tumor → classified as MMRd
Prognostic factors
- Based on current literature, shows excellent prognosis as compared to MMRd, p53abn and NSMP endometrial carcinomas (see Diagram 2)
- Order of most favorable to worst prognosis: POLEmut > MMRd > NSMP > p53abn
- Associated with early stage disease
- Favorable prognosis is independent of tumor histotype and grade (Am J Surg Pathol 2018;42:561)
- Unclear whether observed favorable outcome may be due to enhanced response to adjuvant therapy (chemotherapy)
- However, preliminary analyses suggest adjuvant therapy is not associated with outcome in POLEmut cases (Cancer 2021;127:2409, J Clin Oncol 2020;38:3388)
Case reports
- 49 year old premenopausal woman with recurrent high grade mixed endometrial carcinoma (Gynecol Oncol 2019;153:471)
- 53 year old woman with POLEmut pelvic carcinosarcoma (Int J Surg Pathol 2022 Mar 31 [Epub ahead of print])
- 57 year old woman with POLEmut endometrial carcinoma treated with nivolumab (Clin Cancer Res 2016;22:5682)
Treatment
- Surgery with or without adjuvant therapy (vaginal brachytherapy, pelvic radiotherapy and chemotherapy, depending on stage)
- Ongoing clinical trials (e.g., PORTEC-4a) are investigating the potential for treatment de-escalation in high - intermediate risk group patients (Int J Gynecol Cancer 2020;30:2002)
- Immune checkpoint inhibitor therapy in advanced stage or recurrent disease (JAMA Oncol 2015;1:1319)
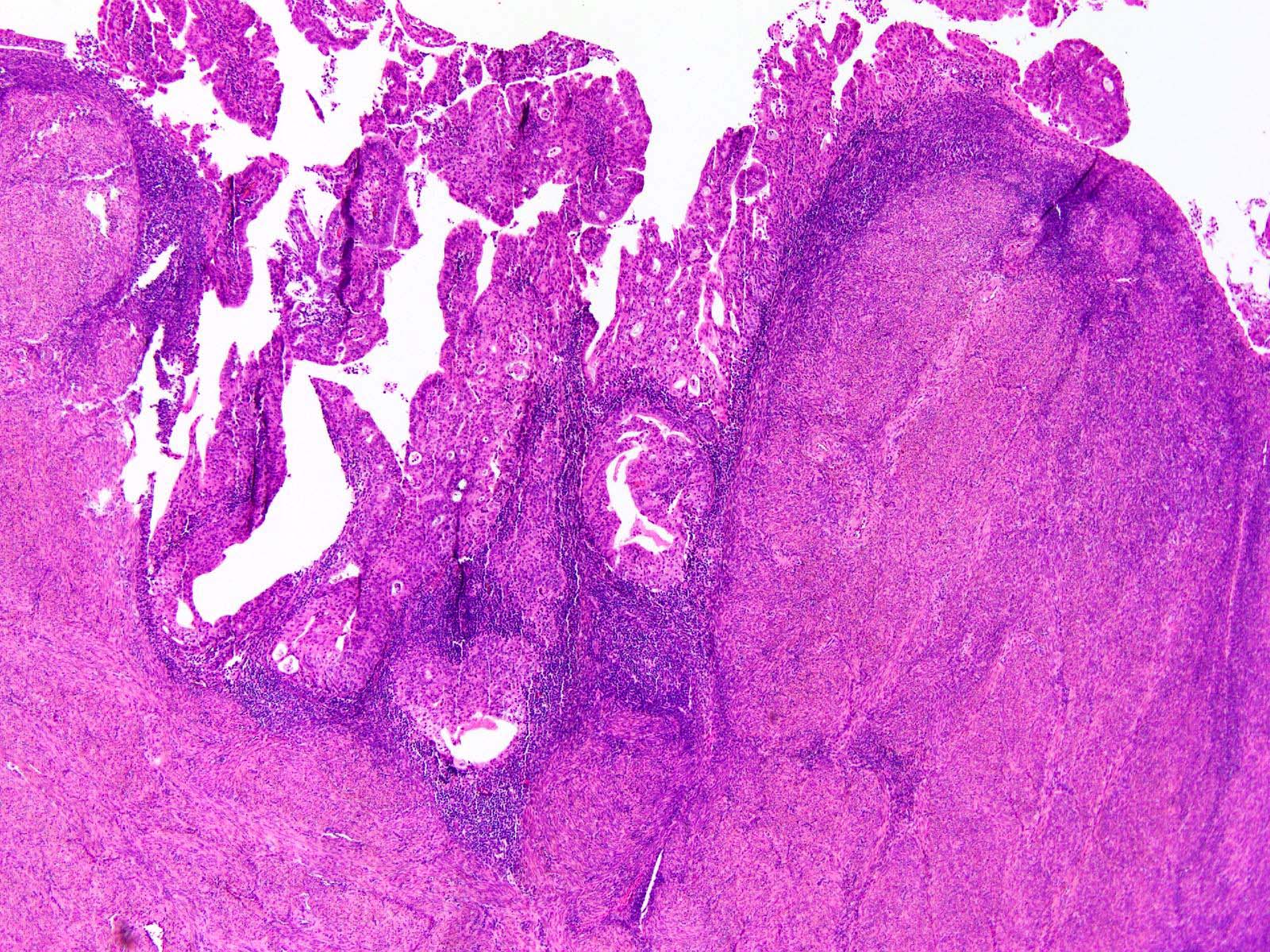
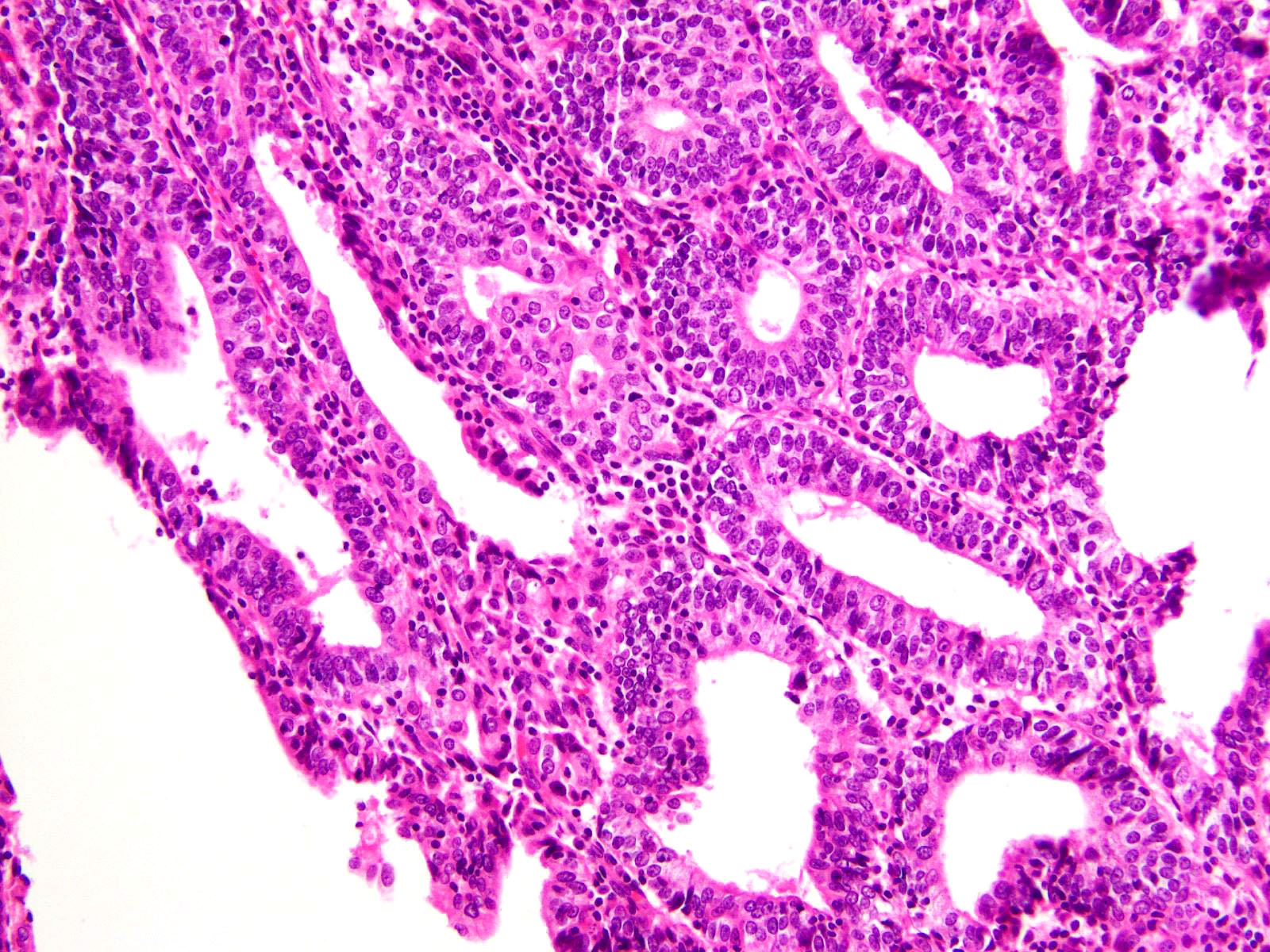
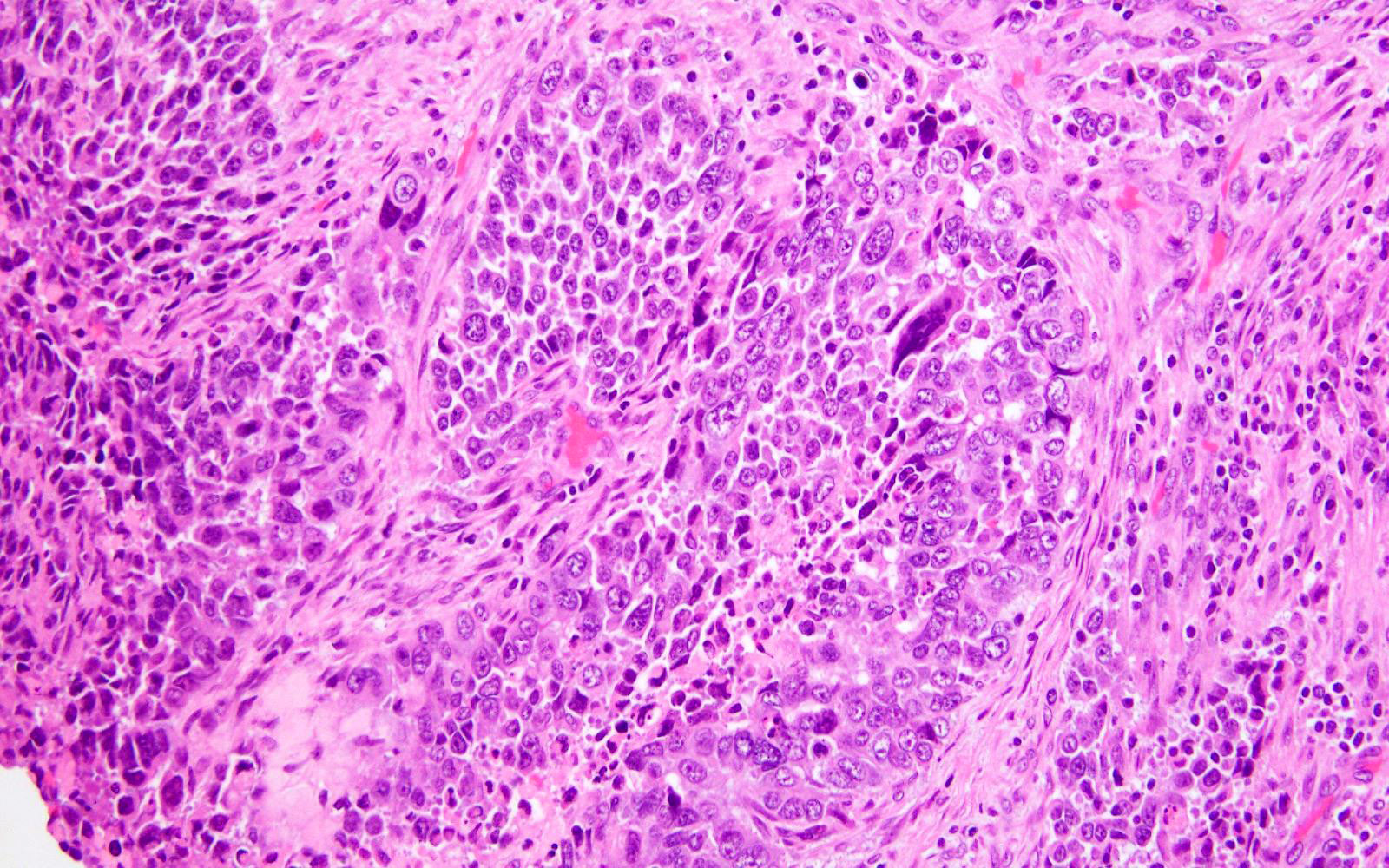
Microscopic (histologic) description
- Represented across all tumor histotypes
- Endometrioid carcinoma, mixed carcinoma, carcinosarcoma, clear cell carcinoma, undifferentiated / dedifferentiated carcinoma, neuroendocrine carcinoma (Nat Commun 2019;10:4965, J Pathol 2017;243:230, Mod Pathol 2016;29:1390, Am J Surg Pathol 2020;44:1541)
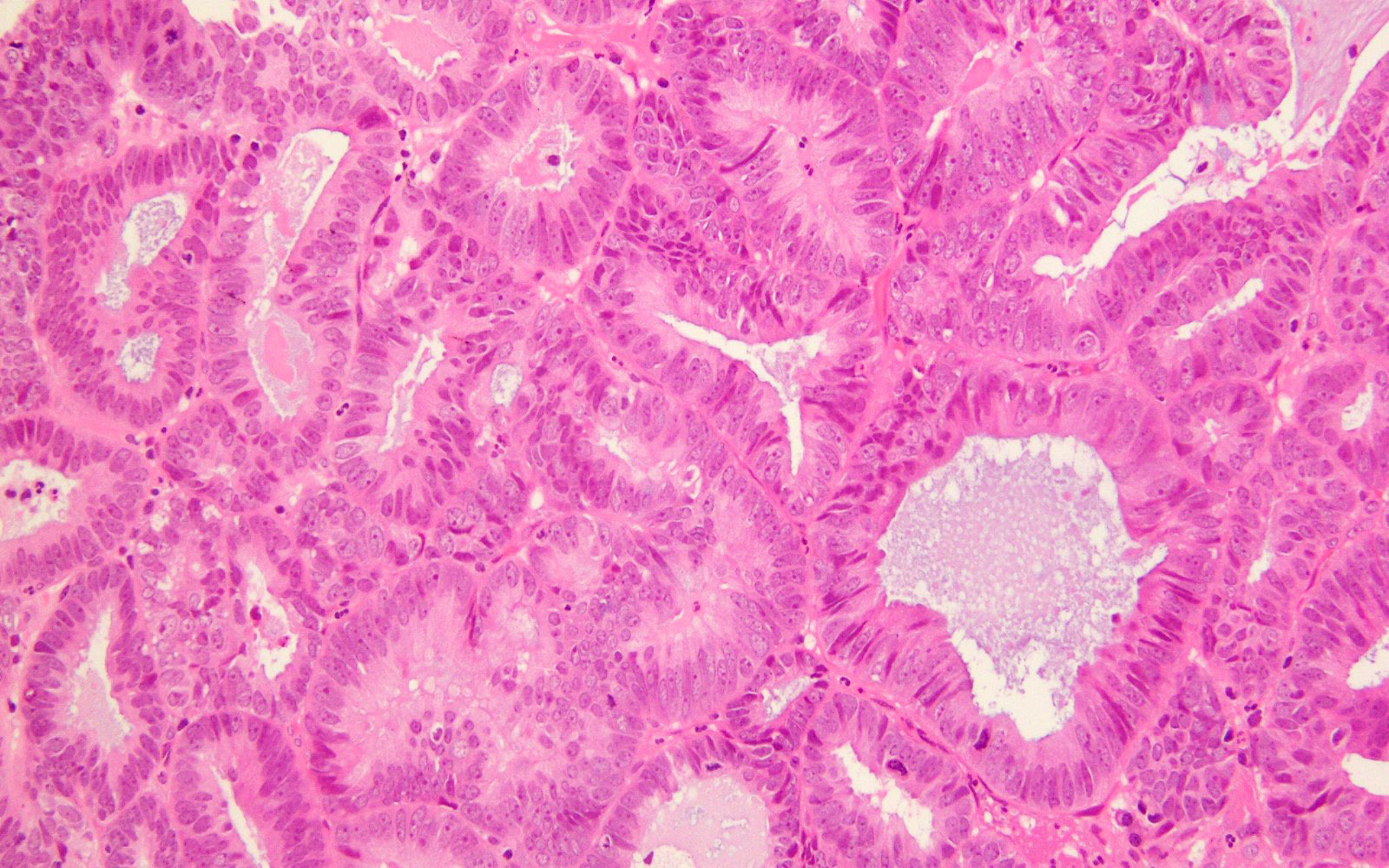
- Majority (> 90%) demonstrate at least a component of endometrioid histology (Mod Pathol 2015;28:505)
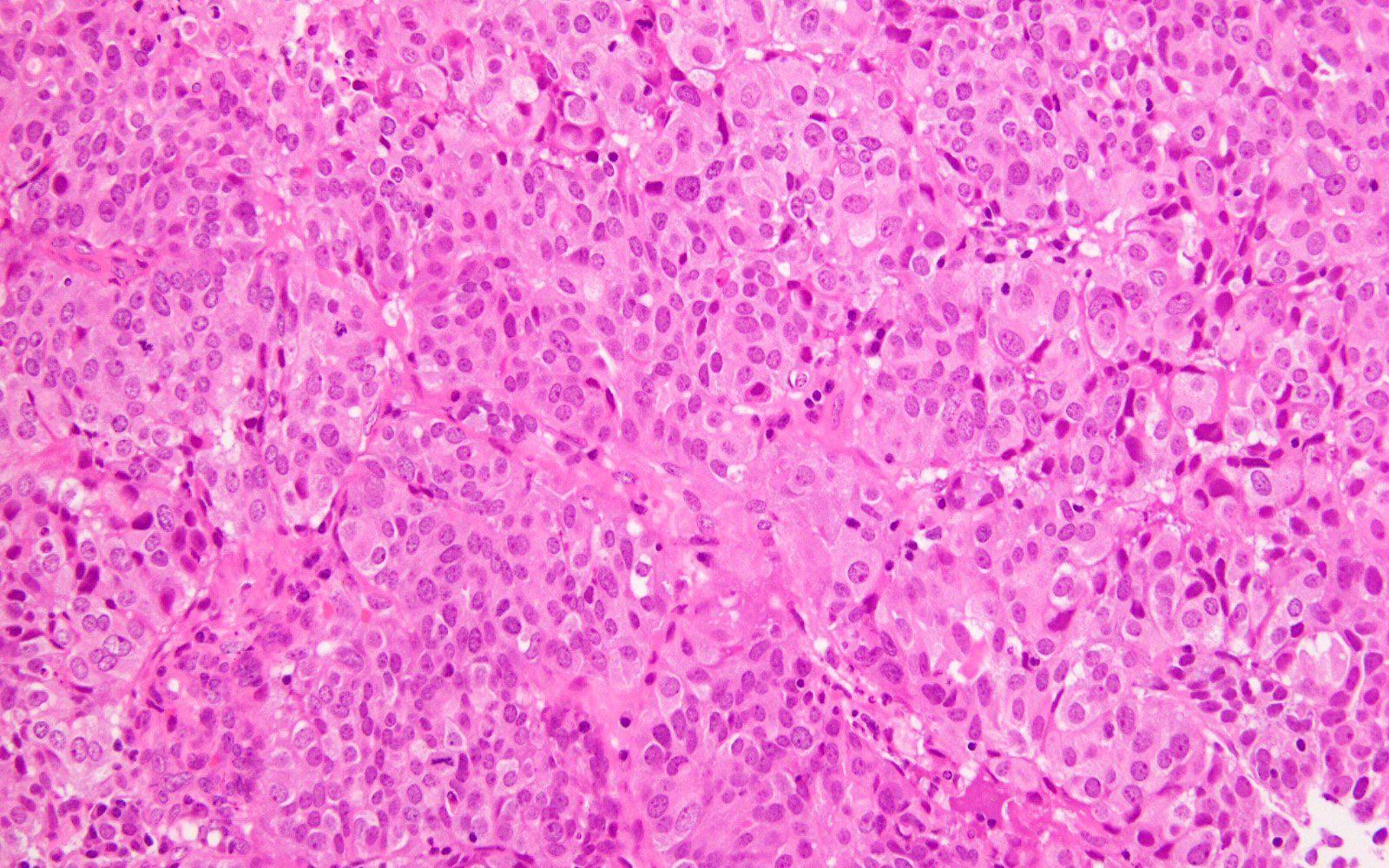
- High or low tumor grade observed
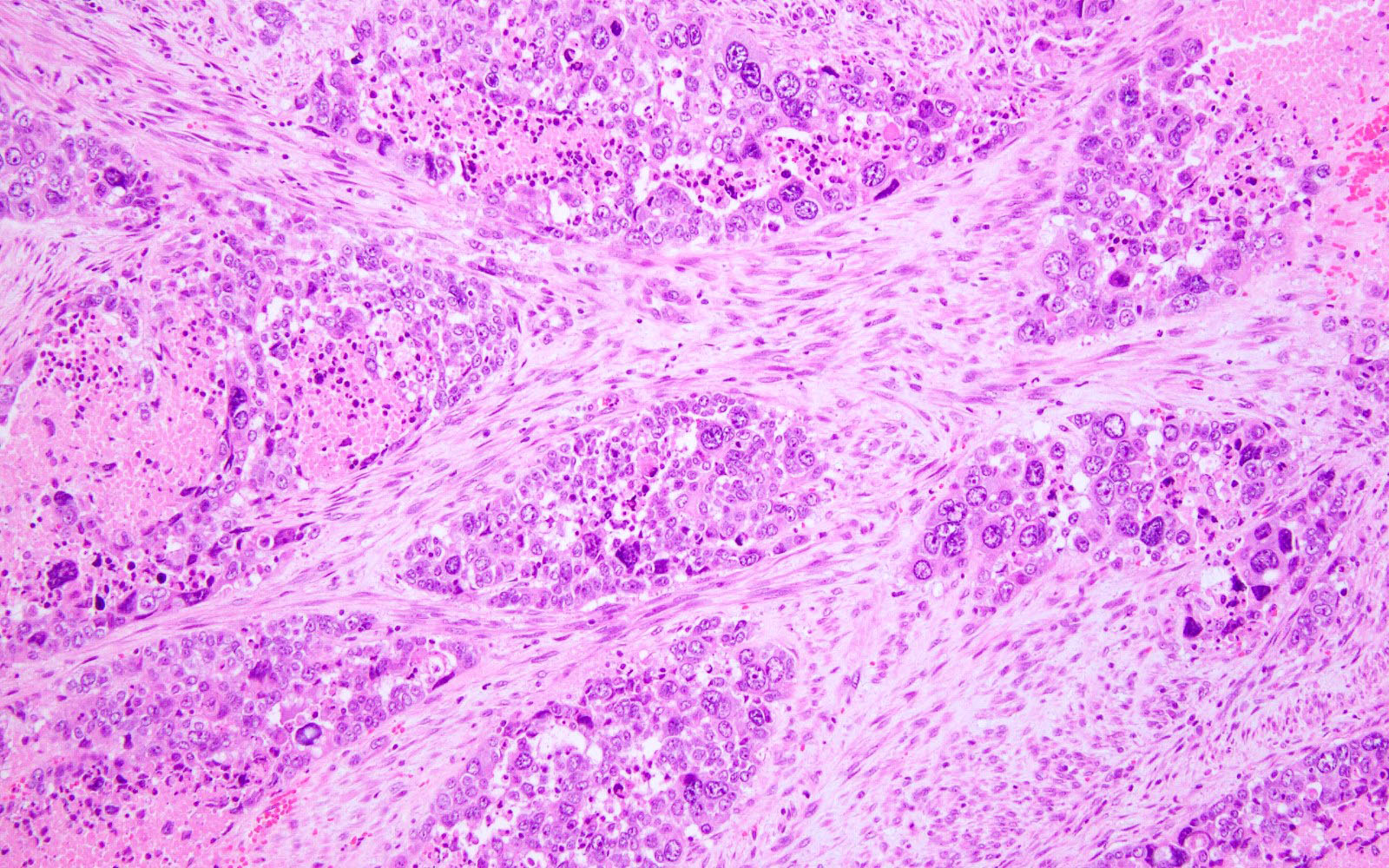
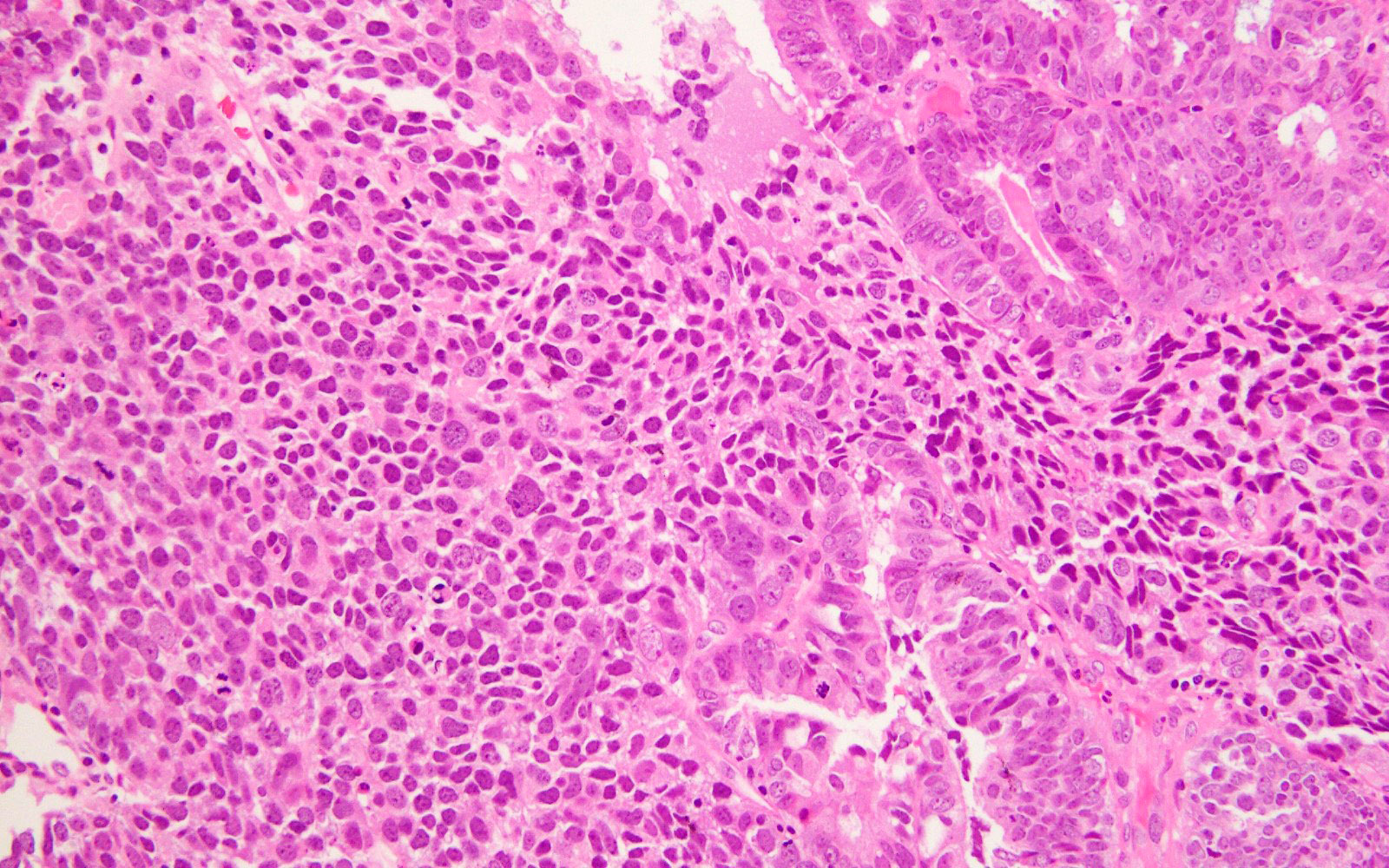
- Prominent tumor infiltrating lymphocytes, peritumoral lymphocytes (Histopathology 2018;72:248)
- Intratumoral heterogeneity, mixed morphology
- Ambiguous tumor morphology
- Patchy bizarre atypia, tumor giant cells (Am J Surg Pathol 2021;45:421)
Microscopic (histologic) images
Positive stains
- Expression of MMR proteins (MLH1, PMS2, MSH2, MSH6) intact but may be lost in multiple classifier tumors (Mod Pathol 2022;35:688)
Negative stains
- p53 nonaberrant / wild type but may be aberrant / mutant in a diffuse or subclonal pattern in multiple classifier tumors (~3 - 5%)
Molecular / cytogenetics description
- Majority of pathogenic POLE mutations are within exons 9, 11, 13 and 14, which encode the proofreading exonculease domain
- 5 recurrent POLE exonuclease domain mutation variants account for the majority of POLEmut cases: P286R, V411L, S297F, A456P and S459F
- Characteristic genomic signatures:
- High prevalence of C → A substitutions
- Low proportion of small insertion and deletion mutations (indels)
- High tumor mutational burden (TMB: > 100 mut/Mb)
- Diagnostic interpretation (i.e., evaluation of pathogenicity of novel POLE mutations may be guided by POLE score) (J Pathol 2020;250:323)
Sample pathology report
- Endometrium, curettage:
- High grade endometrial carcinoma (see comment)
- Comment: Sections show fragments of endometrial carcinoma with varied morphology, including areas with endometrioid and focal serous differentiation. The tumor shows complex glandular, solid and focal papillary architecture. The lining cells show predominantly high grade cytologic atypia, characterized by enlarged hyperchromatic nuclei with high nuclear to cytoplasmic ratio, loss of polarity and increased mitotic activity. Scattered markedly atypical bizarre cells are also noted. Immunohistochemical stains show that the tumor cells are positive for ER (80%, variable intensity) and PR (20%, weak). p53 shows predominantly nonaberrant (wild type) expression but a small subset of tumor cells show aberrant p53 overexpression. Mismatch repair protein (MLH1, PMS2, MSH2 and MSH6) expression is intact (retained / normal). POLE mutational analysis by Sanger sequencing has been requested and the result, along with the final molecular classification of this tumor, will be issued in an addendum report.
- Addendum: final molecular classification: POLE ultramutated (pathogenic POLE mutation detected - see molecular report #. Mismatch repair protein expression is intact. p53 shows subclonal aberrant overexpression.)
- Uterus, fallopian tubes and ovaries, total hysterectomy and bilateral salpingo-oophorectomy:
- Endometrial endometrioid carcinoma, FIGO grade 2; final molecular classification: POLE ultramutated (see synoptic report and comment)
- Tumor invades the inner half of the myometrium (< 50%)
- Lymphovascular invasion is absent
- Cervix, uterine serosa, bilateral fallopian tubes and ovaries are uninvolved
- Comment: Pathogenic POLE mutation detected (see molecular report #). DNA mismatch repair protein (MLH1, PMS2, MSH2 and MSH6) expression is intact. p53 expression is nonaberrant (wild type).
- Endometrial endometrioid carcinoma, FIGO grade 2; final molecular classification: POLE ultramutated (see synoptic report and comment)
Differential diagnosis
- Mismatch repair deficient (hypermutated) endometrial carcinoma:
- Abnormal expression of MMR proteins by IHC
- Microsatellite instability high
- Frequent hypermethylation of MLH1 promoter
- Pathogenic POLE exonuclease domain mutation absent
- p53 abnormal (copy number high) endometrial carcinoma:
- Comprises vast majority of serous carcinoma
- p53 aberrant / mutant by IHC
- TP53 mutations
- Pathogenic POLE exonuclease domain mutation absent; MMR proteins intact
- No specific molecular profile (copy number low) endometrial carcinoma:
- Diagnosis of exclusion; largest molecular subgroup of endometrial carcinoma
- Pathogenic POLE exonuclease domain mutation absent, MMR proteins intact, p53 nonaberrant / wild type
- High frequency of PI3K / AKT alterations, presence of CTNNB1 exon 3 mutations
Board review style question #1
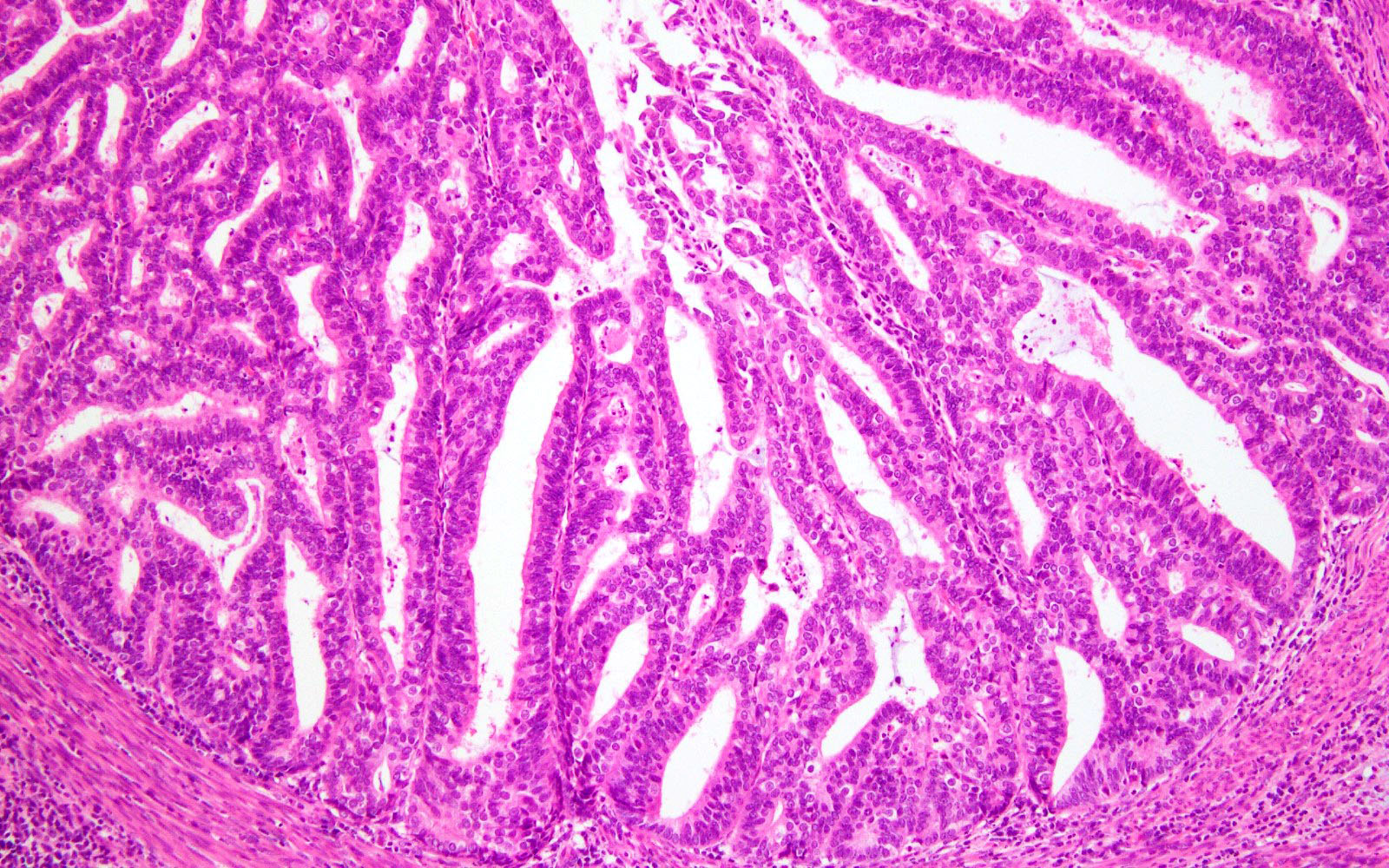
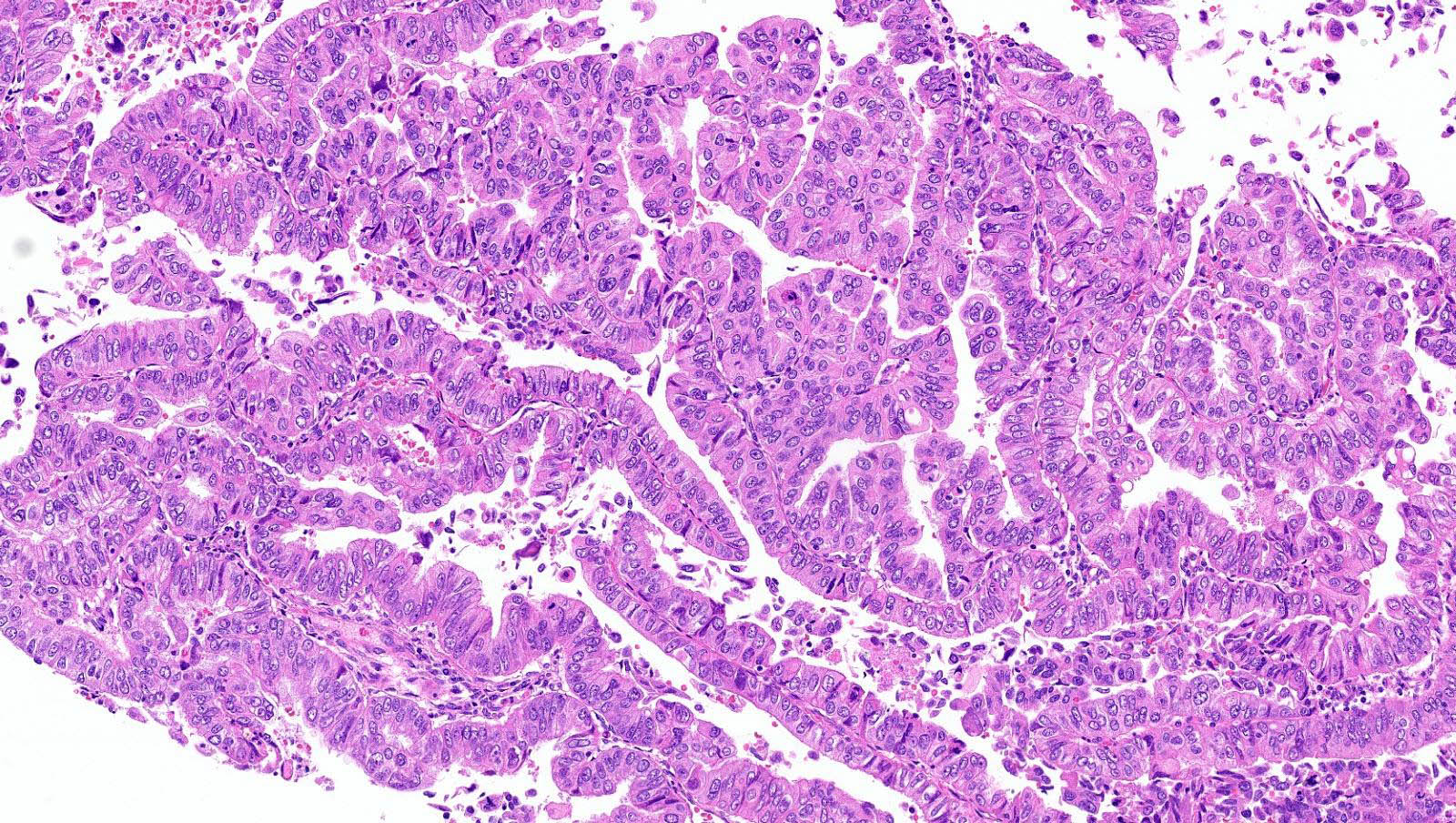
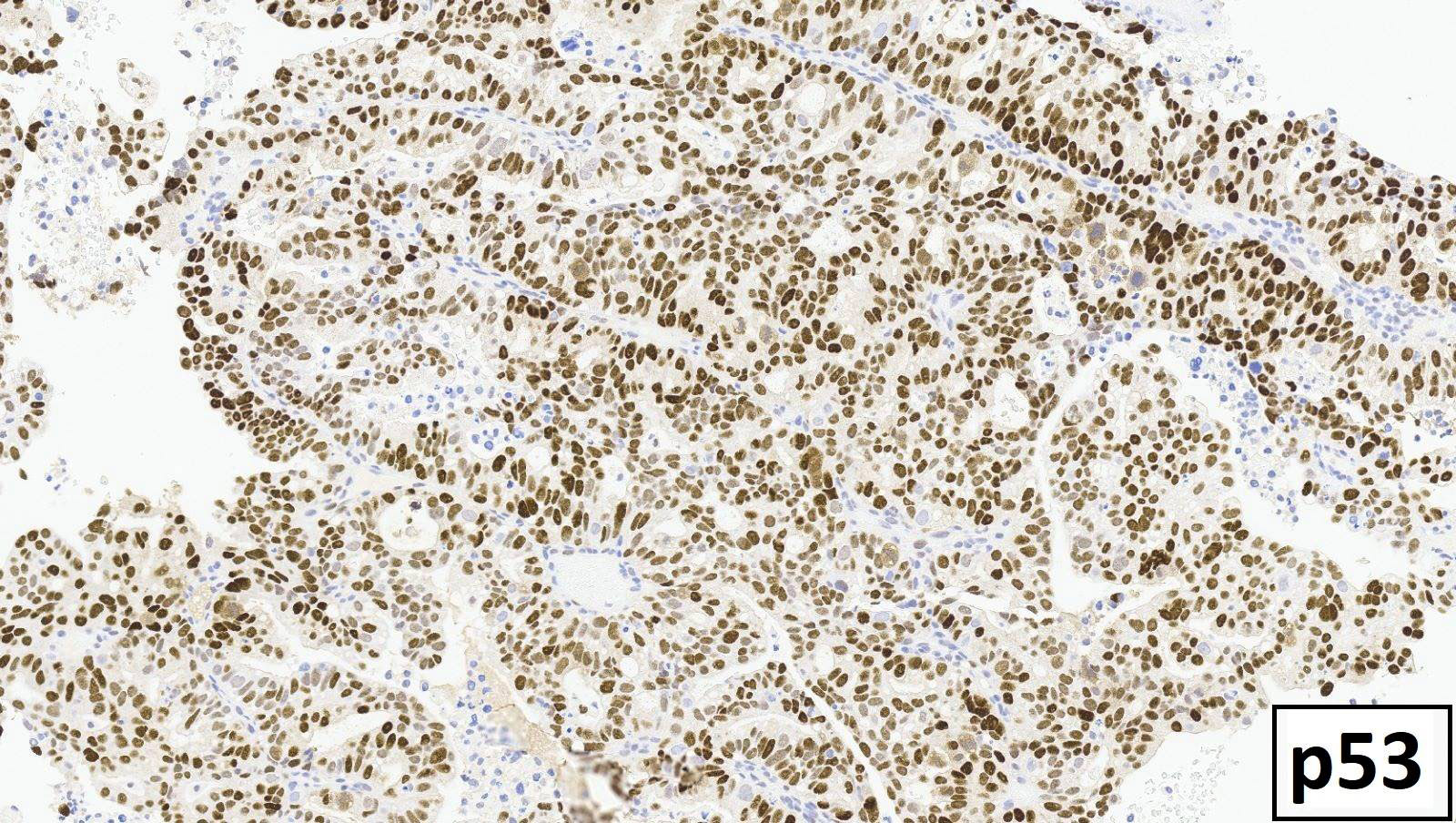
Shown above are representative images from an endometrial tumor in a 60 year woman. The tumor shows intact nuclear PMS2 and MSH6 expression, as well as p53 mutant overexpression by immunohistochemistry (seen in image 3). What additional test is required for accurate molecular classification of this endometrial cancer?
- ARID1A immunohistochemistry
- HER2 gene amplification by FISH
- p16 immunohistochemistry
- POLE exonuclease domain mutation analysis
- Promoter hypermethylation testing of MLH1
Board review style answer #1
D. POLE exonuclease domain mutation analysis. TCGA defined 4 molecular and prognostic subgroups of endometrial carcinoma: POLEmut, MMRd, p53abn and NSMP. Accurate molecular classification relies on correct interpretation of tests following the recommended algorithm (see Diagram 1).
Comment Here
Reference: POLE ultramutated endometrial carcinoma
Comment Here
Reference: POLE ultramutated endometrial carcinoma
Board review style question #2
Which of the following molecular subgroups of endometrial carcinoma confers the most favorable prognosis?
- MMR deficient
- No specific molecular profile
- p53 abnormal
- POLE ultramutated
Board review style answer #2