Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Diagrams / tables | Blood donor testing | Laboratory | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Cite this page: Fang DC, Pham HP. Pretransfusion testing. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/transfusionmedpretransfusiontesting.html. Accessed April 2nd, 2025.
Definition / general
- Testing performed by the transfusion service before a transfusion
- Current scope excludes donor testing performed by collection facility
Essential features
- Pretransfusion testing is based on serologic testing methods (molecular testing alone is not sufficient)
- Follow up is required for typing discrepancies; the blood bank must adhere to certain restrictions for donor units and patient transfusions until discrepancies are resolved
- Pretransfusion testing criteria are different for neonates compared with nonneonates
- Computer or electronic crossmatches are allowed as an alternative to serologic crossmatch only when particular criteria are met
Terminology
- Type and screen:
- In this context, type refers to ABO typing
- Forward typing for ABO uses anti-A and anti-B reagent to detect A and B antigens on patient red blood cells (RBCs)
- Reverse typing for ABO uses A1 and B reagent red blood cells to detect anti-A and anti-B in patient serum / plasma
- Typing for Rh (D) uses anti-D reagent to detect Rh (D) antigen on RBCs: reverse typing is not performed because anti-D is not naturally occurring (not expected, such as Anti-A or Anti-B)
- In this context, screen (or antibody screen) refers to screening the patient’s serum / plasma for unexpected antibodies to RBC antigens
- Unexpected antibodies: antibodies that we would not expect to see in a nonsensitized patient (e.g. patient with no transfusion / exposure history)
- This is in contrast to naturally occurring antibodies; the best example of these are anti-A and anti-B, which we expect to see in O, A or B patients (but should not expect to see in anti-A or Anti-B in AB patients)
Pathophysiology
- Clinically significant antibodies:
- Frequently associated with adverse clinical consequences, such as:
- Hemolytic transfusion reactions (acute or delayed)
- Hemolytic disease of the fetus and newborn (HDFN)
- Decreased RBC survival
- Clinically significant antibodies are typically:
- IgG antibodies
- Reactive at 37 °C (body temperature) (Harmening: Modern Blood Banking & Transfusion Practices, 5th Edition, 2005, American Association of Blood Banks: Technical Manual, 20th Edition, 2020)
- Most antibodies are IgG; ABO are IgM but clinically significant
- Frequently associated with adverse clinical consequences, such as:
- Crossmatch: assessment of compatibility between patient’s serum / plasma and donor RBCs (in this context); options include:
- Serologic (physical) crossmatch - physically testing patient serum / plasma against donor RBCs
- Immediate spin (IS) crossmatch:
- Primary objective of the IS crossmatch is to detect ABO incompatible RBC unit before it is transfused
- Patient’s serum / plasma is mixed with donor cells (suspended in saline) in a tube which is then immediately spun in a centrifuge; the tube is then assessed for any donor RBC hemolysis or agglutination that would indicate incompatibility between the patient’s serum / plasma and donor RBCs
- Patient’s anti-A and anti-B are typically IgM (pentameric and hence larger) antibodies that can bridge the gap between donor RBCs and thereby cause agglutination
- Other clinically significant antibodies may or may not be caught by the IS crossmatch, depending on the circumstance (Harmening: Modern Blood Banking & Transfusion Practices, 5th Edition, 2005, American Association of Blood Banks: Technical Manual, 20th Edition, 2020)
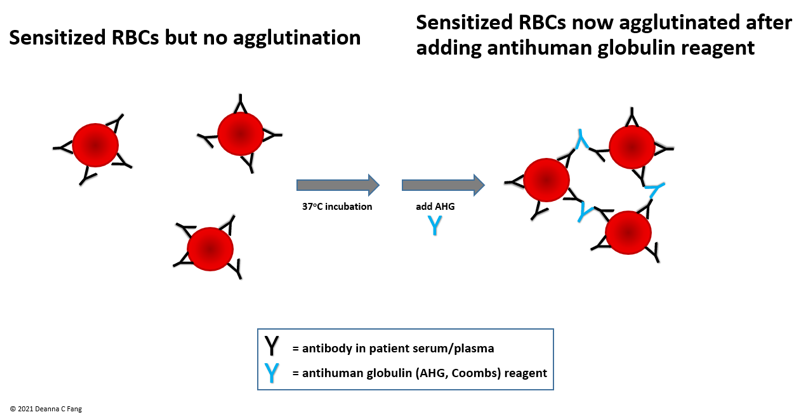
- Antihuman globulin (AHG) crossmatch
- Primary objective of the AHG crossmatch is to detect non-ABO incompatibility (due to clinically significant antibodies) before an RBC unit is transfused
- Antihuman globulin (AHG) is a reagent (historically referred to as Coombs reagent) that increases the sensitivity of the crossmatch
- Because clinically significant antibodies are typically IgG (monomeric and hence smaller) antibodies, even if they bind to donor RBCs (i.e. even if the donor RBCs are sensitized), agglutination may not occur without assistance
- AHG bridges the gap between sensitized RBCs, allowing donor RBCs to more easily agglutinate (see Figure 1)
- AHG reagents can be categorized into the following:
- Polyspecific: AHG reagent contains anti-IgG and anti-complement (C3d or C3b)
- Monospecific: AHG reagent contains either anti-IgG or anti-complement (C3d or C3b)
- Polyclonal
- Monoclonal
- Procedure for the AHG crossmatch typically continues from where the IS crossmatch left off (note: AHG crossmatch is unnecessary if the IS crossmatch is already positive)
- Patient’s serum / plasma is mixed with donor cells (suspended in saline) and incubated at 37 °C (incubation times vary depending on the laboratory procedure used); after washing the RBCs to remove excess (unbound) antibodies, AHG reagent is added and the tube / well is inspected for agglutination
- Positive AHG crossmatch can be caused by:
- Clinically significant antibodies
- Clinically insignificant antibodies (Harmening: Modern Blood Banking & Transfusion Practices, 5th Edition, 2005, American Association of Blood Banks: Technical Manual, 20th Edition, 2020)
- Immediate spin (IS) crossmatch:
- Computer (electronic) crossmatch: where a validated computer software system assesses ABO compatibility based on patient and donor ABO typing (donor sample and intended unit are not physically mixed)
- Serologic (physical) crossmatch - physically testing patient serum / plasma against donor RBCs
Blood donor testing
- See Laboratory section below
Laboratory
- Blood tube requirements for patient testing:
- Most laboratories use tubes with ethylenediaminetetraacetic acid (EDTA) (pink or lavender top) but tubes with acid citrate dextrose (ACD) (yellow top) or without anticoagulant (red top) are also acceptable if they have been validated by the laboratory
- It is the laboratory’s responsibility to validate the acceptable blood tube types for specimen collection for testing
Table A.
Regarding pretransfusion testing of donor: for allogeneic or autologous transfusions
| |||
| |
|||
| Red blood cells or whole blood or granulocytes |
ABO typing | Integrally attached segment |
Any discrepancy must:
|
Rh(D) negative typing
|
|||
| Non-ABO / Rh(D) red blood cell antigens | Not required if already labeled as negative for that antigen | ||
Table B.
| Testing of nonneonatal patient: for allogeneic transfusions | ||||
Any unit |
ABO typing |
Reagents:
|
Must be current sample |
|
| Rh typing | Reagents:
|
Weak D typing optional | ||
Red blood cells or Whole blood or Granulocytes |
Antibody screen |
Procedure must include a 37 °C incubation prior to adding AHG Reagent:
|
Patient sample must be obtained within 3 days of the scheduled transfusion if the patient has:
|
Day 0 = date of sample draw A positive antibody screen requires additional testing
If the patient has a prior history of clinically significant antibody(s), the lab must use testing methods that identify additional clinically significant antibodies |
Table C.
| Testing on nonneonatal patient: for autologous transfusions |
| ABO typing |
| Rh typing |
| Crossmatch (electronic or serologic) |
Table D.
| Pretransfusion testing of neonatal patient: for allogeneic transfusions | ||||
| |
||||
| ABO typing | Reagents:
|
Not required until:
|
ABO reverse typing not required | |
| Rh typing | Reagent:
|
Weak D typing optional | ||
Antibody screen |
Procedure must include a 37 °C incubation prior to adding AHG Reagent:
|
If no history of discharge, use neonatal serum / plasma or maternal serum / plasma If the neonate has been already been discharged and is being readmitted neonate, use neonatal serum / plasma |
Positive screen requires additional antibody testing |
|
Crossmatch |
|
If initial antibody screen is negative:
If initial antibody screen is positive:
|
||
Anti-A, anti-B screening |
|
Serum / plasma of neonate |
Testing is required if:
|
|
Table E. Crossmatch: for autologous or allogeneic transfusions
| Serologic crossmatch | |||
| |
|||
Must:
|
Red blood cells | Integrally attached segment | Serum / plasma |
| Whole blood | |||
| Apheresis granulocytes with > 2 ml red blood cells | Can use donor red blood cells from sample on date of donation |
||
| Apheresis platelet with > 2 ml red blood cells | |||
| Computer crossmatch | |
| |
|
Patient eligibility requires all of the following:
|
|
Board review style question #1
A 51 year old man is admitted for an active gastrointestinal bleed. A single patient sample is sent to the blood bank with orders for ABO and Rh typing and it results as group O Rh+. Review of records shows that the patient received a red blood cell transfusion 2 months ago, at which time the ABO / Rh typing was O Rh+ and screen for unexpected antibodies was negative.
2 STAT red blood cell units are requested. Why, in the interest of time, is the patient currently not eligible for computer crossmatch?
2 STAT red blood cell units are requested. Why, in the interest of time, is the patient currently not eligible for computer crossmatch?
- ABO typing was not confirmed using second ABO testing from the same admission
- Clinical team did not yet order an antibody screen in this admission
- Only patients who type as AB are eligible for computer crossmatch
- This is an irrelevant question; the patient is currently eligible for computer crossmatch
Board review style answer #1
B. The patient is not currently eligible for computer crossmatch. Even though the patient had a prior negative antibody screen 2 months ago, because he was transfused within the past 3 months, he needs a current antibody screen result to confirm his antibody status and this test was not ordered yet by the team (and thus, not yet done by the transfusion service). Historical ABO / Rh typing from a previous admission is one of the allowed methods of confirming the patient's ABO / Rh typing. Blood type is not a factor in computer crossmatch eligibility.
Comment Here
Reference: Pretransfusion testing
Comment Here
Reference: Pretransfusion testing
Board review style question #2
A 48 year old woman has a scheduled cardiac surgery and arranged for 1 autologous red blood cell unit to be collected and held for her use. After testing, the collection facility labels the unit as group A Rh+ and sends it to the blood bank. Which of the following statements is correct regarding which pretesting is required by the transfusion service before issuing and transfusing the autologous red blood cell unit?
- None; no pretransfusion testing is required for issuing autologous units
- Confirmatory ABO and Rh typing of the donor unit only
- ABO and Rh typing of the patient and confirmatory ABO and Rh typing of the donor unit only
- ABO and Rh typing of the patient and confirmatory ABO typing of the donor unit only
- ABO and Rh typing of the patient, confirmatory ABO typing of the donor unit and crossmatch testing only
Board review style answer #2
E. Autologous transfusions require that the patient has ABO typing, Rh typing and crossmatch testing prior to transfusion. Any red blood cell unit (autologous or allogeneic) requires ABO confirmatory typing by the transfusion service before it is issued. Confirmatory Rh typing is not required if the unit is already labeled as Rh+.
Comment Here
Reference: Pretransfusion testing
Comment Here
Reference: Pretransfusion testing
Board review style question #3
Select the correct statement regarding the cutoff requirements for performing a serologic crossmatch:
Unless the patient is eligible for computer crossmatch, a serologic crossmatch is required for a platelet unit that contains
Unless the patient is eligible for computer crossmatch, a serologic crossmatch is required for a platelet unit that contains
- Greater than 0.1 ml red blood cells
- Greater than 0.2 ml red blood cells
- Greater than 0.5 ml red blood cells
- Greater than 1 ml red blood cells
- Greater than 2 ml red blood cells
Board review style answer #3
E. Serologic crossmatch is required if a platelet unit contains greater than 2 ml red blood cells, which rarely happens given that many platelet units were collected by apheresis procedures.
Comment Here
Reference: Pretransfusion testing
Comment Here
Reference: Pretransfusion testing