Table of Contents
Definition / general | Essential features | Terminology | Applications | Implementation | Diagrams / tables | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Cite this page: Onyenekwu CP, George MR, Potochny EM. Lookbacks. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/transfusionmedlookbacks.html. Accessed April 2nd, 2025.
Definition / general
- Traces the source of a relevant transfusion transmissible infection (RTTI) or transfusion transmitted disease (TTD) by a recipient of blood product(s) or notifies a recipient who was transfused with blood product(s) from a donor who is currently confirmed positive for a RTTI, in accordance with Food and Drug Administration (FDA) regulations
- Additionally, recalls / market withdrawals may be issued in situations such as improper donor testing or postdonation information
Essential features
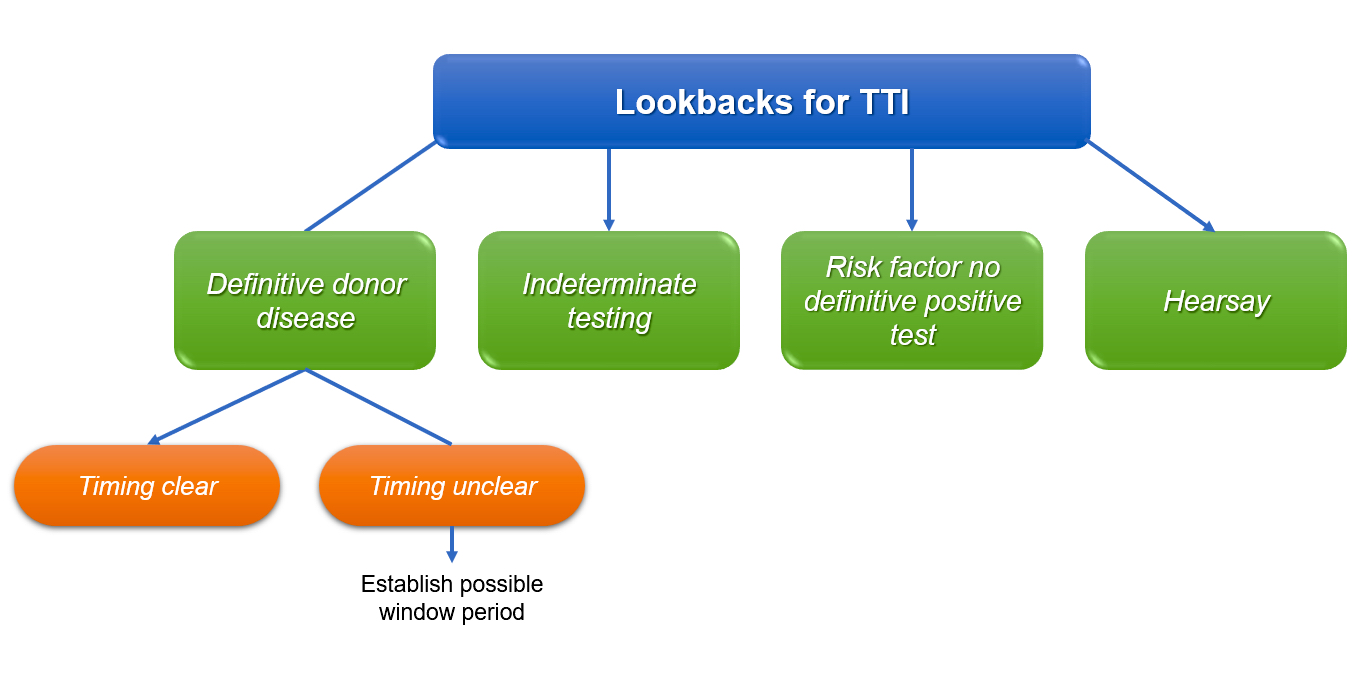
- Performed to find previously distributed units from donors who are considered positive / reactive for a given infectious disease
- Hepatitis C virus (HCV) and human immunodeficiency virus (HIV) require a lookback according to the U.S. FDA
- If blood or a blood product / component implicated in a lookback has been transfused, consignees of the product must have a process to notify a recipient, their physician of record, their legal representative or in the case of HIV, next of kin
Terminology
- Nonconformance
Applications
- Receipt of consignee notification for a product disposition from a collection facility
- Provider notifies transfusion services that a patient has tested positive for a possible TTD after receiving a blood component transfusion at their facility
- TTD or RTTI is identified during a transfusion reaction investigation, RTTIs are defined by the FDA (eCFR: Code of Federal Regulation Title 21 [Accessed 9 November 2023])
Implementation
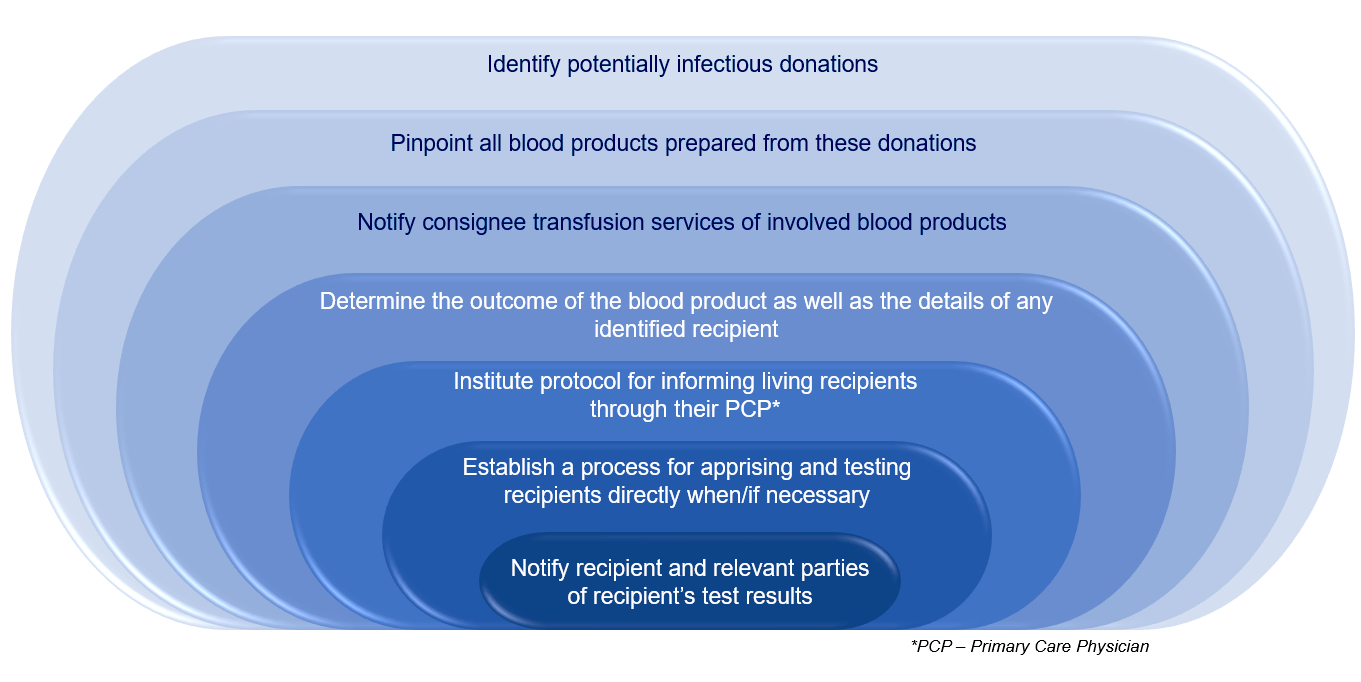
- Blood establishments must
- Quarantine and destroy any products within their control and notify consignees (those in receipt of the blood or blood component, such as a hospital transfusion service)
- Note: for the purpose of this outline, transfusion service and consignee will be used interchangeably
- Test the donor per FDA's code of federal regulations or relevant guidance documents, depending on the infectious disease agent
- Notify transfusion services so that they can notify recipients, if required by FDA or per medical director discretion or transfusion service policy when no FDA regulation exists
- Quarantine and destroy any products within their control and notify consignees (those in receipt of the blood or blood component, such as a hospital transfusion service)
- When a lookback notification process by the blood establishment to the transfusion service is required, the transfusion service must disposition the unit and report the status back to the donor center
- If not transfused
- Quarantine recalled products in inventory
- Destroy or return to the collection facility as requested
- Return documentation of product's final disposition to the blood collection facility which issued the withdrawal / lookback
- If the product has been shipped, request final disposition from the receiving facility
- Discarded
- Expired, discarded
- Report back to supplier
- No further action necessary
- If transfused
- Review FDA Code of Federal Regulations
- FDA guidance for industry
- American Association of Blood Banks (AABB) standards / bulletins
- In situations where notification is recommended, it is advisable to contact the recipient's physician first
- If that physician is unavailable or declines to notify the recipient, the medical director of the transfusion medicine service or designee assumes responsibility for notifying the recipient
- In general, the only clearly defined lookbacks by the FDA mandating notification of the recipient or the recipient's physician or a legal representative involve blood components considered positive with HIV or HCV
- Lookbacks require notification of transfusion recipients in receipt of blood or blood components collected during the 12 months before the date of reactive testing
- HIV also requires notification of next of kin, should the recipient be deceased
- For HIV, this would occur when either the nucleic acid test (NAT) for HIV is positive / reactive or HIV antibody testing and confirmatory testing is also positive or indeterminate
- For HCV, this would occur when either nucleic acid testing for HCV is positive / reactive, anti-HCV is repeatedly reactive or another reliable test indicates HCV infection
- Notification of the recipient, their physician, legal representative or next of kin when it applies, should be provided within 12 weeks of receiving the test results (FDA: Code of Federal Regulations Title 21 - Sec. 610.46 [Accessed 9 November 2023], FDA: Code of Federal Regulations Title 21 - Sec. 610.47 [Accessed 9 November 2023], FDA: Lookback for Hepatitis C Virus [Accessed 9 November 2023])
- Blood establishments issue market withdrawals and recalls for nonlegally binding situations involving positive testing or when postdonation information is disclosed to the blood establishment, leading to deviation from protocol and where there is potential for harm to a transfusion recipient, such as
- Hepatitis B virus (HBV), typically due to positive HBV nucleic acid test or hepatitis B surface antigen (HBsAg) with positive neutralization assay results
- Babesia reactivity in cellular blood products (FDA: Recommendations for Reducing the Risk of Transfusion-Transmitted Babesiosis [Accessed 9 November 2023])
- Malaria, in order to monitor recipient for 3 months posttransfusion of cellular blood product, except source plasma (FDA: Recommendations to Reduce the Risk of Transfusion-Transmitted Malaria [Accessed 9 November 2023])
- Trypanasoma cruzi (Chagas disease), to encourage consignees to notify the recipient's physician (FDA: Use of Serological Tests to Reduce the Risk of Transmission of Trypanosoma cruzi Infection in Blood and Blood Components [Accessed 9 November 2023])
- West Nile virus (FDA: Assessing Donor Suitability and Blood and Blood Product Safety in Cases of Known or Suspected West Nile Virus Infection [Accessed 9 November 2023])
- Ebola virus, where the FDA recommends consignees notify the recipient's physician for notification and monitoring (FDA: Recommendations for Assessment of Blood Donor Eligibility, Donor Deferral and Blood Product Management in Response to Ebola Virus [Accessed 9 November 2023])
- Variant Creutzfeldt-Jakob disease (vCJD), so that the consignee medical director can consider notifying the recipient's physician of record for counseling purposes (FDA: Recommendations to Reduce the Possible Risk of Transmission of Creutzfeldt-Jakob Disease and Variant Creutzfeldt-Jakob Disease by Blood and Blood Components [Accessed 9 November 2023])
- Postdonation information involving certain medications that compromise coagulation or are teratogenic, disclosure of high risk behavior, travel, illness, etc. (Ann Clin Lab Sci 2020;50:538)
Diagrams / tables
Additional references
Board review style question #1
Which of the following includes 3 relevant considerations for the risks / benefits of notification of a recipient for a TTI?
- Existence of a test for donors, moderate risk and existence of a possible intervention
- Existence of a test for recipients, existence of a test for donors and presence of a probable risk
- Presence of small theoretical risk, existence of a test for donors and existence of a possible intervention
- Presence of well established risk, existence of a test for recipients and existence of a possible intervention
Board review style answer #1
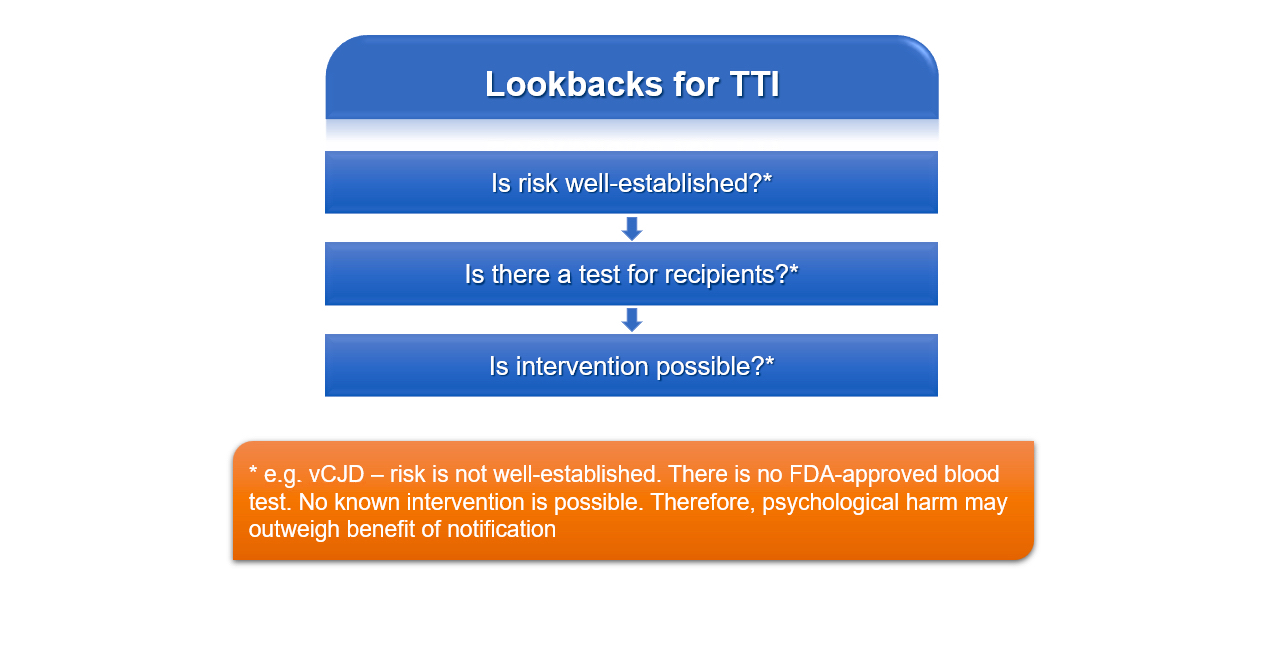
D. Presence of well established risk, existence of a test for recipients and existence of a possible intervention. In recalls without mandatory notification of recipients of a transfused blood component, relevant considerations that may guide the decision to notify include the presence of a well established risk, the existence of a test for recipients and existence of a possible intervention in the case of a positive test result. When these 3 are not present, the risk of the psychological harm associated with notifying a recipient or their relative may outweigh the benefit of such notification. Answers A and C are incorrect as the risk to the donor must be well established. Answer B is incorrect since both the possibility for intervention and the presence of a well established risk to the donor must be present in addition to an available test.
Comment Here
Reference: Lookbacks
Board review style question #2
What transfusion transmitted infections listed in the FDA's code of federal regulations require lookbacks?
- Babesia and HIV
- Ebola and HCV
- HIV and HCV
- Trypanosoma cruzi
Board review style answer #2
C. HIV and HCV. Only HIV and HCV are listed in the FDA's code of federal regulations as having required lookbacks. Answer A is incorrect because Babesia is not addressed in the FDA's code of federal regulations as a lookback. Answer B is incorrect because FDA's code of federal regulations does not require a lookback for Ebola; however the recommendation is to inform the recipient's physician. Answer D is incorrect because a lookback is also not required for Trypanasoma cruzi according to the FDA's code of federal regulations; however it is recommended that consignees of transfused products notify the recipient's physician.
Comment Here
Reference: Lookbacks
Comment Here
Reference: Lookbacks
Board review style question #3
What is the time limit within which notification to the recipient, their physician, legal representative or next of kin should be done when a blood component has been implicated in a lookback mandated by the FDA?
- 72 hours
- 2 weeks
- 12 weeks
- 12 months
Board review style answer #3
C. 12 weeks. Notification to the recipient, their physician, legal representative or next of kin when it applies should be done within 12 weeks. Lookbacks require notification of transfusion recipients in receipt of blood or blood components collected during the 12 months before the date of reactive testing. Answers A and B are incorrect because 12 weeks allows for further testing to confirm the presence of HCV or HIV if screening results are reactive. Answer D is incorrect because 12 months may increase the risk of viral transfer to others.
Comment Here
Reference: Lookbacks
Comment Here
Reference: Lookbacks