Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Diagrams / tables | Clinical features | Treatment | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Hastings H, Cancelas JA. Cryoprecipitate use. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/transfusionmedcryoprecipitate.html. Accessed March 28th, 2025.
Definition / general
- Cryoprecipitate is the insoluble byproduct of fresh frozen plasma (FFP) when thawed at 1 - 6 °C and contains concentrated amounts of fibrinogen (factor I), factor VIII, factor XIII, von Willebrand factor and fibronectin
- Primary usage is treatment of bleeding patients with acquired fibrinogen deficiency
Essential features
- Cryoprecipitate production (see figure 1)
- Cryoprecipitate preparation:
- Whole blood derived fresh frozen plasma is thawed at 1 - 6 °C
- Centrifugation of the thawed fresh frozen plasma produces an insoluble aggregate; the insoluble protein is resuspended in approximately 15 mL of plasma, representing the cryoprecipitate (Br J Anaesth 2014;113:922)
- Remaining supernatant plasma can be transferred to a satellite bag and relabeled as "plasma, cryoprecipitate reduced"
- Primary indication of "plasma, cryoprecipitate reduced" is plasma exchange in thrombotic thrombocytopenic purpura (J Blood Transfus 2016;2016:4860284)
- Cryoprecipitate must be frozen within an hour of production at 18 °C or colder and typically is pooled before freezing for ease of use post thaw, with most pools consisting of 5 or 10 units
- Shelf life = 12 months
- Cryoprecipitate thawing:
- Frozen cryoprecipitate is thawed at 37 °C, then stored at 20 - 24 °C; the expected turnaround time in the blood bank to thaw cryo is approximately 25 - 30 minutes (Methods Mol Biol 2011;728:259)
- In adults, units of cryo are pooled and will expire at either 4 hours in an open system or 6 hours in a closed system
- Cryoprecipitate preparation:
- FDA quality assurance (QA) mandates that each unit tested contains (Br J Anaesth 2014;113:922):
- 150 mg fibrinogen
- 80 IU factor VIII
- Each unit also contains: factor XIII (~170 units/bag), von Willebrand factor (vWF) (~60 units/bag) and fibronectin
- Primary use of cryoprecipitate is treatment of patients with acquired fibrinogen deficiency, with a goal of at least 100 - 150 mg/dL in the bleeding patient
Terminology
- Cryoprecipitated antihaemophilic factor; cryoprecipitated (AHF); cryoprecipitate (cryo)
- Plasma, cryoprecipitate reduced; cryosupernatant; cryo reduced plasma (CRP)
- Fresh frozen plasma (FFP)
- von Willebrand factor (vWF)
- Plasma frozen within 24 hours after phlebotomy (PF24)
- Plasma frozen within 24 hours after phlebotomy held at room temperature up to 24 hours after phlebotomy (PF24RT24)
Pathophysiology
- Fibrinogen is a heterodimeric molecule produced in the liver and the normal concentration in plasma ranges from 2 - 4 mg/mL (Blood Rev 2015;29:17)
- Upon tissue injury, fibrinogen is acted upon by thrombin to produce fibrin monomers
- Individual monomers of fibrin polymerize and are crosslinked to other strands by FXIIIa, forming a stable, mature clot
- Low levels of fibrinogen may be found in various conditions and may be congenital or acquired (trauma induced injury, obstetric patients and certain invasive procedures)
- Fibrinogen defects may be qualitative (dysfibrinogenemia) or quantitative (hypofibrinogenemia, afibrinogenemia)
Diagrams / tables
Clinical features
- Primary indication for cryoprecipitate is hypofibrinogenemia, either acquired or congenital (Br J Anaesth 2014;113:922)
- Currently, there are 2 FDA approved human fibrinogen concentrates (RiaSTAP, Fibryga) for the treatment of congenital fibrinogen deficiency, including afibrinogenemia and hypofibrinogenemia (Haemophilia 2020;26:25)
- Cryo may also be administered in the following circumstances as second line therapy for uremic bleeding after DDAVP failure:
- Factor VIII, von Willebrand disease, factor XIII deficiency (congenital or acquired) as a second line therapy when factor concentrates are unavailable
- Patients with disseminated intravascular coagulation (DIC) and low fibrinogen are probably best treated with a combination of fresh frozen plasma and cryoprecipitate, to minimize the risk of inducing thrombosis with transfusion of cryoprecipitate alone (Br J Anaesth 2014;113:922)
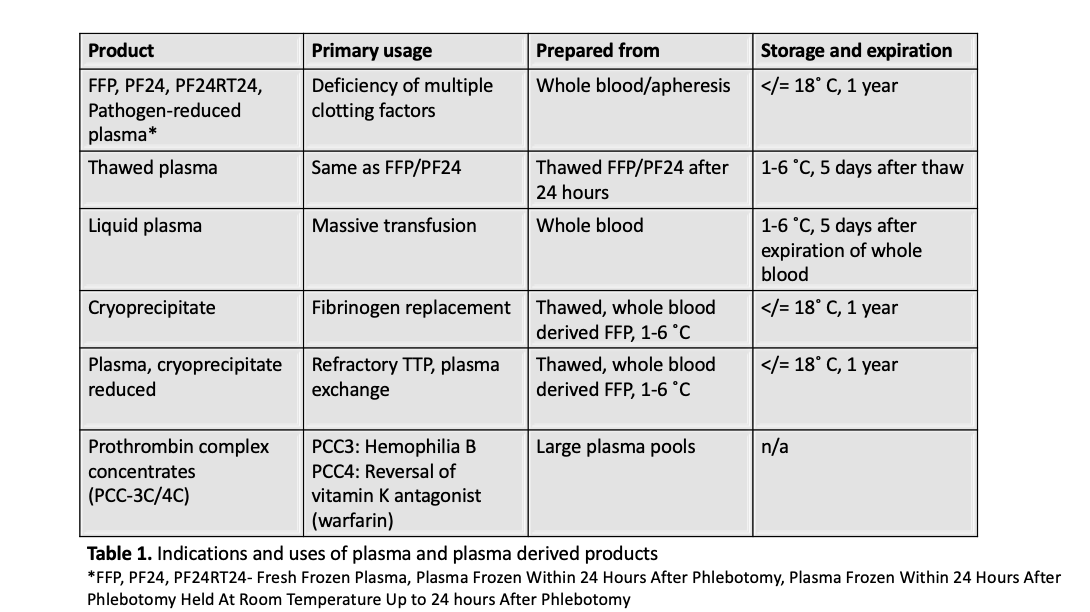
- See table 1 for primary uses of cryoprecipitate compared with fresh frozen plasma and other plasma derived products (Blood Transfus 2010;8:149, J Blood Transfus 2016;2016:4860284)
- Adverse events:
- Similar to fresh frozen plasma including transmission of blood borne pathogens and rare reported cases of cryoprecipitate associated transfusion related acute lung injury (Br J Anaesth 2014;113:922)
- Infrequently, cryoprecipitate may cause a positive direct antiglobulin test or mild hemolysis when ABO incompatible cryo is transfused in large volumes or to pediatric patients (Transfusion 2012;52:635, Vox Sang 2011;101:55)
- Sample assessment & plan
Treatment
- Fibrinogen levels of more than 50 mg/dL are considered sufficient to support physiologic hemostasis; adequate transfusion should be given to maintain the fibrinogen level above 100 mg/dL
- Each unit of cryoprecipitate must contain a minimum of 150 mg fibrinogen and 80 IU of factor VIII, representing 40 - 70% of the original amount of these factors in the original plasma (AABB: Standards for Blood Banks and Transfusion Services, 31st Edition, 2018)
- Cryoprecipitates result in a combination of high molecular weight factors, microparticles containing clotting factors and complexed lipids and lipoproteins, which result in a favorable hemostatic effect higher than the addition of the isolated factors in acquired bleeding coagulopathies
- Currently, there is not enough data to identify whether cryoprecipitates are superior or inferior to fibrinogen concentrates in the treatment of acquired hypofibrinogenemia (Br J Anaesth 2014;113:922, Blood 1986;68:307)
- Cryoprecipitate contains only 10 - 15 mL of plasma and pools of cryoprecipitates have reduced levels of anti-A and anti-B antibody and may be used universally for adult recipients (Shaz: Transfusion Medicine and Hemostasis - Clinical and Laboratory Aspects, 2nd Edition, 2013)
- Standard adult dosing
- Cryoprecipitate, pools of 10
- Each unit will raise fibrinogen by 5 to 10 mg/dL
- Cryoprecipitate dosing for fibrinogen replacement = (fibrinogendesired (mg/dL ) - fibrinogencurrent (mg/dL )) × plasma volume × (1.0 dL/100 mL) × (1 unit cryoprecipitate/150 mg)
- Plasma volume = weight (kg) × 70 mL/kg × (1.0 - hematocrit) (Br J Anaesth 2014;113:922)
- Pediatric dosing
- 1 - 2 units/10 kg, ~100 mg/dL rise in fibrinogen
- Fibrinogen levels should be reassessed after cryoprecipitate administration to ensure a high enough dose was administered
- In adults, ABO compatible cryoprecipitate is not required
- Cryoprecipitate, pools of 10
Additional references
Board review style question #1
A 76 kg male patient is admitted to the intensive care unit for workup of a consumptive coagulopathy. Laboratory evaluation reveals a hematocrit of 35% and a fibrinogen level of 35 mg/dL (reference range: 200 - 400 mg/dL). How many units of cryoprecipitate would be necessary to raise fibrinogen to 100 mg/dL?
- 5 units
- 15 units
- 24 units
- 32 units
Board review style answer #1
B. 15 units. Calculation of cryoprecipitate dosing requires knowledge of the formula:
Comment Here
Reference: Cryoprecipitate use
- Cryoprecipitate dosing for fibrinogen replacement = (fibrinogendesired (mg/dL ) - fibrinogencurrent (mg/dL )) × plasma volume × (1.0 dL/100 mL) × (1 unit cryoprecipitate/150 mg)
- Plasma volume = weight (kg) × 70 mL/kg × (1.0 - hematocrit)
- Cryoprecipitate dosing for fibrinogen replacement = (100 mg/dL - 35 mg/dL) × (76 kg × 70 mL/kg × (1.0 - .35)) × (1.0 dL/100 mL) × (1 unit cryoprecipitate / 150 mg) = 15 units of cryoprecipitate
Comment Here
Reference: Cryoprecipitate use
Board review style question #2
Which of the following is true?
- ABO compatible cryoprecipitate is required in adult patients
- Cryoprecipitate contains concentrated amounts of factors VIII and XIII
- Cryoprecipitate when thawed is stored at 1 - 6 °C with a 24 hour shelf life
- "Plasma, cryoprecipitate reduced" contains high levels of von Willebrand factor
- There are currently no FDA approved human fibrinogen concentrates in the United States
Board review style answer #2
B. Cryoprecipitate contains concentrated amounts of factors VIII and XIII. Cryoprecipitate is produced when whole blood derived fresh frozen plasma is thawed at 1 - 6 °C, followed by centrifugation. The resulting insoluble protein is collected with ~15 mL of plasma. Cryo contains concentrated levels of fibrinogen, factor VIII, FXIII, von Willebrand factor and fibronectin. The remaining supernatant is collected and labeled as "plasma, cryoprecipitate reduced," which is deficient in von Willebrand factor as well as the other proteins present when the insoluble protein is collected from the thawed fresh frozen plasma. When cryoprecipitate is thawed, it is stored at 20 - 24 °C and expires at 4 hours or 6 hours if it was pooled in an open or closed system respectively. ABO compatibility is typically not necessary in adult patients as the amount of plasma present is minimal. There are currently 2 approved human fibrinogen concentrates in the United States.
Comment Here
Reference: Cryoprecipitate use
Comment Here
Reference: Cryoprecipitate use