Table of Contents
Definition / general | Essential features | Terminology | History | Procedure to create CAR T product | Pathophysiology | Types of CAR T cells | Diagrams / tables | Challenges | Adverse effects | Clinical features | Laboratory | Case reports | Treatment | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: George MR. CAR T cell therapy. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/transfusionmedcartcelltherapy.html. Accessed July 15th, 2024.
Definition / general
- Chimeric antigen receptor T (CAR T) cells are a form of cellular immunotherapy involving the genetic engineering of T cells to produce surface receptors targeted at specific cell surface receptors
- CAR T cell therapy is primarily used to treat hematologic malignancies, with acute lymphoblastic leukemia (ALL) being the first disease targeted
Essential features
- CAR T cell therapy was originally developed for B cell acute lymphoblastic leukemia
- It is also now Food and Drug Administration (FDA) approved for B cell non-Hodgkin lymphoma (NHL), follicular lymphoma, mantle cell lymphoma and multiple myeloma; its application to solid organ tumors is being studied
- Process starts with collecting autologous T cells via leukocytapheresis, shipping the cells to be engineered / manufactured, expanding the cell population to achieve appropriate dose, cryopreserving CAR T cells and shipping to the treating facility
- Patient is given lymphocyte depleting chemotherapy prior to receiving the treatment
- Key steps of CAR T cell therapy are trafficking, recognition, control, microenvironment, proliferation / persistence
- Safety measures are built in to allow ablation of CAR T cells if severe toxicity develops
- Severe toxicity can include cytokine release syndrome and a CAR T encephalopathy
Terminology
- CART-19: CAR T directed against CD19 antigen on B cells
- BCMA: B cell maturation antigen found on plasma cells in multiple myeloma
FDA approved CAR T cell therapies (NIH: CAR T Cells - Engineering Patients' Immune Cells to Treat Their Cancers [Accessed 9 April 2024])
| Generic name | Brand name | Target antigen | Targeted disease | Patient population |
| Tisagenlecleucel | Kymriah® (Novartis, Basel, Switzerland) | CD19 | B cell acute lymphoblastic leukemia (ALL) | Children and young adults with refractory or relapsed B cell ALL |
| B cell non-Hodgkin lymphoma (NHL) | Adults with relapsed or refractory B cell NHL | |||
| Axicabtagene ciloleucel | Yescarta® (Kite Pharma / Gilead, Los Angeles, CA) | CD19 | B cell NHL | Adults with relapsed or refractory B cell NHL |
| Follicular lymphoma | Adults with relapsed or refractory follicular lymphoma | |||
| Brexucabtagene autoleucel | Tecartus | CD19 | Mantle cell lymphoma (MCL) | Adults with relapsed or refractory MCL |
| B cell ALL | Adults with relapsed or refractory B cell ALL | |||
| Lisocabtagene maraleucel | Breyanzi | CD19 | B cell NHL | Adults with relapsed or refractory B cell NHL |
| Idecabtagene vicleucel | Abecma® (Bristol‐MyersSquibb) | BCMA | Multiple myeloma | Adults with relapsed or refractory multiple myeloma |
| Ciltacabtagene autoleucel | Carvykti® (Janssen Pharmaceutical Companies of Johnson & Johnson) | BCMA | Multiple myeloma | Adults with relapsed or refractory multiple myeloma |
History
- 1989: discovery that it was possible to redirect T cell signaling to an antigen of choice, independent of major histocompatibility complex (MHC) restrictions
- 2006: first results published of human clinical trials using chimeric antigen receptor T cell technology (J Clin Oncol 2006;24:e20, Clin Cancer Res 2006;12:6106)
- 2010: first use of CAR T cell therapy was in a 5 year old girl with relapsed B cell acute lymphoblastic leukemia (ALL); received CAR T cell therapy directed against CD19 antigen on B lymphoblasts (CART-19)
- Developed complications, treated with tocilizumab, an anti-IL6 monoclonal antibody
- Patient survived and became global ambassador for CAR T cell therapy
- 2014: CTL019 was granted breakthrough therapy designation by the United States Food and Drug Administration (Immunol Rev 2015;263:68)
- 2021: ide-cel was FDA approved as a CAR T cell construct with murine BCMA targeting plasma cells in multiple myeloma (CA Cancer J Clin 2023;73:275)
- 2022: cilta-cel became second FDA approved anti-BCMA CAR T cell therapy (CA Cancer J Clin 2023;73:275)
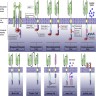
Procedure to create CAR T product
- Collect autologous T cells via leukocytapheresis
- Obtain approximately 109 T cells
- Unknowns
- Is there an optimal time window for collection?
- How to predict an adequate collection?
- How to separate T cells from collected mononuclear cell apheresis product? (Maitta: Immunologic Concepts in Transfusion Medicine, 1st Edition, 2019)
- Ship T cells to manufacturing site
- CAR structure is composed of an extracellular antigen recognition domain fused to intracellular TCR signaling domains (CD3z) and costimulatory domains such as CD28
- Manufacture CAR T cells by CD3 / CD28 bead stimulation and lentiviral transduction (Tisagenlecleucel)
- Manufacture CAR T cells by CD3 antibody / IL2 stimulation and retroviral transduction (Axicabtagene)
- Expand cell population over 6 - 10 days
- Achieve patient dose of 2 - 4 x 106 CAR expressing T cells/kg patient body weight
- Cryopreserve CAR T cells
- Ship to treating facility
- Patient is given lymphodepleting chemotherapy (usually fludarabine and cyclophosphamide)
- Deplete endogenous T cells that might reject the CAR T cells
- Increase likelihood of expanding CAR T population in recipient
- Enhance antigen presentation capabilities
Pathophysiology
- Targets (Front Immunol 2021;12:744823)
- CD19
- CD20, CD22 and BCMA
- Developing targets include CD70, CD7, CD5
- NKG2DL, GD2 and mesothelin for solid tumors potentially
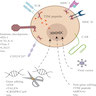
- Structure
- Receptor: combines facets of normal T cell activation into single protein
- Links extracellular antigen recognition domain to an intracellular signaling domain to activate the T cell when an antigen is bound
- 4 parts
- Antigen recognition domain
- Exposed to the outside of the cell in the ectodomain portion of the receptor
- Allows the CAR T cell to attack any cell that expresses the matching molecule
- Derived from variable regions of a monoclonal antibody linked together as a single chain variable fragment (scFv)
- scFv is a chimeric protein made up of the light (VL) and heavy (VH) chains of immunoglobulins connected with a short linker peptide
- VL and VH regions are selected to bind the target antigen like CD19
- Extracellular hinge region
- Structural domain between the antigen recognition region and the cell's outer membrane
- Optimizes flexibility of the scFv receptor head to promote antigen binding between CAR T and target antigen
- Transmembrane domain
- Anchors the CAR to the plasma membrane, links the extracellular hinge and antigen recognition domains with the intracellular signaling region
- Stabilizes the entire structure
- CD28 transmembrane domain is often used and is very stable
- Should not use CD3 zeta transmembrane domain because it can cause incorporation of the artificial TCR into the native T cell receptor
- Intracellular T cell signaling domain
- Receives and perpetuates signal after antigen binds external recognition portion
- Cytoplasmic domain of CD3 zeta is often used to mimic the normal T cell activation dependent on phosphorylation of immunoreceptor tyrosine based activation motifs
- Includes 1 or more chimeric domains from costimulatory proteins
- Antigen recognition domain
- Function of CAR T cells
- Trafficking
- Engineered T cell must be able to get to site of tumor cells
- Target tumor cells to be killed
- Possibility of introducing chemokine receptors into CAR T cells to improve trafficking to tumors that produce cognate chemokines
- Recognition
- Recognize target tumor cells
- Discriminate and ignore bystander tissues
- Control
- T cells are relatively autonomous once they are infused
- New work on regulatory processes to modulate the survival of T cells, timing, strength and location of their activity
- Microenvironment
- Resist immunosuppression
- Prime / mobilize endogenous immunity
- Proliferation / persistence
- Expand the population of CAR T cells to optimize activity (Cell 2017;168:724)
- Trafficking
- Safety measures: allow ablation of CAR T cells if severe toxicity develops
- Inclusion of suicide gene, iCaspase9
- Surface tag such as epidermal growth factor receptor
Types of CAR T cells
- First generation: contained only TCR complex CD3ξ chain domain with additional costimulatory domains
- Second generation: incorporated costimulatory domains like CD28 or CD137 to boost survival, proliferation and antitumor activity
- Third generation: combined CD3ξ chain domain with additional costimulatory domains (Cell 2016;164:780)
- Fourth generation: T cells redirected for universal cytokine killings (TRUCKS)
- Improve tumor microenvironment
- May have potential for solid tumors
- Newer generation: additional gene editing, such as CRISPR-Cas9
- Armored CARs: CAR T cells modified to express cytokines, ligands or scFv that help turn suppressive tumor environment into proinflammatory to better fight tumor
- Tandem CARs: CAR molecule engineered to recognize multiple antigens via 2 binders on a single molecule
- Designer CARs: modified by CRISPR-Cas9 to edit CAR T genomes for advantageous features, such as being less susceptible to immunosuppressive effects
- Smart CARs: logic gated, regulated
- Have new receptors that function independently of CAR / TCR pathways but interfere with CAR activity in controlled way
- Use of modular receptors called synthetic Notch (synNotch) receptors
- Use extracellular domain like scFv to recognize target antigen, without triggering T cell activation
- Ligand engagement results in cleavage of receptor and subsequent release of transcriptional activator domain, which enters nucleus and drives expression of user specified target genes (Maitta: Immunologic Concepts in Transfusion Medicine, 1st Edition, 2019, Cell 2016;164:780)
- Split, universal and programmable (SUPRA) CARs
- Universal receptor found on T cells and tumor targeting scFv adaptor molecule
- 2 component receptor system with universal receptor (zipCAR) expressed on T cells and tumor targeting scFv adaptor (zipFv)
- Fusion of intracellular signaling domains and leucine zipper as extracellular domain
- scFv of zipFv binds tumor antigen
- Leucine zipper binds and activates zipCAR on T cells
- Fusion of intracellular signaling domains and leucine zipper as extracellular domain
- Regulate activity to limit over activation, decrease cytokine secretion and improve tumor targeting (Maitta: Immunologic Concepts in Transfusion Medicine, 1st Edition, 2019, Cell 2018;173:1426)
- Universal CAR T (UCAR T) (Front Immunol 2021;12:744823)
- Potential advantages
- Could be taken from healthy allogeneic donors
- Could have batched rather than customized manufacturing
- More immediate availability
- Lower cost
- Possible application in T cell malignancies
- Potential disadvantages
- Need for additional gene editing to avoid graft versus host disease and rejection
- Lower amplification and shorter persistence in vivo
- Potential advantages
Diagrams / tables
Challenges
- Tumor specific antigens
- CD19 is a great target, since a loss of normal B cells can be compensated for by replacement antibody therapy (IVIG)
- How specific must they be?
- Tumor cells that do not express the target antigen may evade therapy
- Tumor cells with splice variants, lacking a specific epitope may also escape targeting (Blood 2018;131:2621)
- Can we pinpoint antigens expressed by tumors versus normal cells reliably?
- Do all tumor cells in a given tumor express the same antigens?
- Can a tumor be effectively treated even if only some of the cells are susceptible to targeting?
- How significant is bystander toxicity? (Blood 2018;131:2621)
- Rare chance of accidentally introducing CAR gene into a tumor cell during manufacturing
- Proliferation of tumor cells
- Tumor cells escape detection by CAR T cells
- Response in non-Hodgkin lymphoma (NHL) is worse than in ALL
- CD19 loss variants
- Microenvironment factors that limit proliferation and the effect of CAR T cells
- Remission rates 70 - 80%
- Solid tumors
- Challenges identifying suitable cell surface molecules for the CAR T cells to target
- Attempts to engineer T cells with T cell receptors capable of recognizing tumor specific antigens from intracellular proteins
- Modify tumor microenvironment to be more hospitable to CAR T cells
Adverse effects
- Toxicity
- On target effects: reversible when target cells are eliminated or CAR T cell engraftment is terminated
- B cell aplasia
- More severe than that caused by anti-CD20 monoclonal antibody rituximab
- Rapidly reversed after ablation of CAR T cells
- May require immunoglobulin therapy
- Cytokine release syndrome
- Initial flu-like presentation
- Fevers, hypotension, hypoxia, neurologic changes
- Can progress to capillary leak
- T cell activation and high levels of cytokines, IL6 and interferon γ
- Neurotoxicity: CAR T related encephalopathy syndrome (Blood 2014;123:2625, Maitta: Immunologic Concepts in Transfusion Medicine, 1st Edition, 2019)
Clinical features
- Patient selection considerations may differ from autologous stem cell transplant (ASCT) and take into account previous therapies, upper age limit, severity of comorbidities and resistance to chemotherapy
- Some clinical trials may include transplant ineligible patients
- Other clinical trials are studying CAR T cell therapy as second line treatment and for both transplant eligible and ineligible patients
- Other factors that may be considered in therapy include
- Performance status
- Organ function
- T cell count
- B acute lymphoblastic leukemia (ALL); target antigen is CD19
- CART-19: CAR T directed against CD19 antigen on B lymphoblasts
- Tisagenlecleucel, CTL019, also known as Kymriah® (Novartis, Basel, Switzerland)
- Axicabtagene ciloleucel, also known as axi-cel, Yescarta® (Kite Pharma / Gilead, Los Angeles, CA)
- Diffuse large B cell lymphoma; target antigen is CD19, no specific age limit
- Tisagenlecleucel, CTL019, also known as Kymriah® (Novartis, Basel, Switzerland)
- Axicabtagene ciloleucel, also known as axi-cel, Yescarta® (Kite Pharma / Gilead, Los Angeles, CA)
- Other diagnoses under investigation for possible CAR T cell therapies include
- Hodgkin lymphoma (HL); target antigen is CD30
- Anaplastic large cell lymphoma; target antigen is CD30
- Myeloma; target antigens are SLAMF7, B cell maturation antigen (BCMA)
- Acute myeloid leukemia (AML); target antigens are CD123, CD33, Lewis Y and FOLR2
- Other potential costimulatory domains are PD1 / CD28 and CD200R / CD28
- T cell malignancies are a challenge since candidate target antigens are found on normal T cells
- CARs posttransplant: maximize graft versus tumor effect while minimizing graft versus host disease (GVHD)
- CARs in donor leukocyte infusions (DLI): may help improve survival in relapses of hematologic malignancy
- CARS in virus specific T cells
- Chimeric autoantibody receptor T cells (CAARs): autoimmune disease such as pemphigus vulgaris (Immunol Rev 2015;263:68, Clin Cancer Res 2016;22:1875)
Laboratory
- Polymerase chain reaction (PCR) molecular assays are available for all CARs produced; however, such testing might not accurately reflect if the CAR is actually expressed on the cell surface and is generally performed retrospectively
- Flow cytometry may be a useful modality to monitor real time expansion and response
- CD19 CARs are known to expand rapidly, clear target tumor cells, then contract
- CARs have single chain variable fragments (scFv) for specificity against a target antigen and have little else on the cell surface
- There are 3 potential ways to detect the scFv using flow cytometry
- Develop an anti-idiotype antibody to the scFv
- Use Fc conjugated soluble antigen, such as Fc-CD22, that interacts with CD22 CAR T cells followed by a secondary detection antibody (anti-Fc)
- Use biotinylated protein L followed by a streptavidin conjugated fluorophore
- There are benefits and deficits to each method and the process is labor intensive
- Well constructed assay provides meaningful information for individual patient care and a better understanding of CARs as a whole
- Additionally, there is need for standardized methods to profile memory phenotype of CAR T cells to evaluate quality and promote manufacturing improvements
- Use of a standardized memory T cell panel can help evaluate how T cell phenotype impact the efficacy and longevity of response in patients receiving CAR T cell therapies
- One attempt at this includes a dried memory T cell panel containing a prevalidated mixture of 7 antibodies for the identification of naïve, stem cell memory, central memory and effector memory CD4+ and CD8+ T cell subsets (BD Biosciences)
- One study used this prevalidated mix and additional drop in antibodies can complement the panel and enable more in depth evaluation of the T cell phenotype and monitor changes in expression of PD-1, TIM3, LAG3, HLA-DR, CD45RO and CXCR3 on T cells transduced to express a novel anti-CD37 CAR (Transfus Med Hemother 2019;46:15, Blood 2019;134:5626)
Case reports
- 18 month old girl and 52 year old woman treated with CAR T cell therapy for B ALL with mixed lineage leukemia gene (MLL) mutations developed AML clonally related to their B ALL, suggesting CD19 negative immune escape (Blood 2016;127:2406)
- 20 year old man relapsing 9 months after CD19 targeted CAR T cell (CTL019) therapy (Nat Med 2018;24:1499)
- 38 year old woman with CD19 directed CAR T cell therapy combined with BTK inhibitor and PD-1 antibody against secondary central nervous system lymphoma (Front Immunol 2022;13:983934)
- Brief review of control mechanisms for future CAR T cell products (Front Immunol 2020;11:326)
- Advancements in safety for new CAR T cell therapy models (Mol Cancer 2019;18:125)
- Applications of CAR T cell therapy for community oncology physicians (Oncologist 2016;21:608)
Treatment
- Cytokine release syndrome: treated with anti-IL6 monoclonal antibody, tocilizumab
Board review style question #1
A 10 year old boy diagnosed with acute lymphoblastic leukemia with several relapses is participating in a clinical trial for CD19 CAR T cell therapy. He develops rapid onset of flu-like symptoms (including fever) and progresses with mental status change and clinical concern for cytokine release syndrome. What would be an appropriate treatment for suspected cytokine release syndrome?
- Anti-CD20 (rituximab)
- Anti-CD38 (daratumumab)
- Anti-IL6 (tocilizumab)
- Azathioprine
- Interferon γ
Board review style answer #1
C. Anti-IL6 (tocilizumab). Cytokine release syndrome is likely due to high levels of IL6 and interferon γ. Anti-IL6 (tocilizumab) has mainly been used in inflammatory conditions such as juvenile rheumatoid arthritis and has been effective in decreasing the IL6 levels involved in cytokine release syndrome. Answer A is incorrect because anti-CD20 (rituximab) is used in the treatment of B cell lymphomas. Answer B is incorrect because anti-CD38 (daratumumab) is used in the treatment of multiple myeloma. Answer D is incorrect because azathioprine can be used to treat immune disorders such as Crohn's disease, renal transplant rejection and rheumatoid arthritis. Answer E is incorrect because interferon γ is used to treat various autoimmune diseases.
Comment Here
Reference: CAR T cell therapy
Comment Here
Reference: CAR T cell therapy
Board review style question #2
Which of the following functions of CAR T cells refers to the movement of the engineered T cells to the site of tumor cells targeted for destruction?
- Control
- Microenvironment
- Proliferation / persistence
- Recognition
- Trafficking
Board review style answer #2
E. Trafficking is the first function of a CAR T cell. The engineered cell must be able to travel to the site of the target tumor cells. Answer A is incorrect because control refers to the relative autonomy of T cells once they are infused. Answer B is incorrect because microenvironment refers to background immunity that impacts the effect of CAR T cells. Answer C is incorrect because proliferation / persistence refers to the ability of the engineered cells to circulate. Answer D is incorrect because recognition is the ability of the cells to recognize their target.
Comment Here
Reference: CAR T cell therapy
Comment Here
Reference: CAR T cell therapy