Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Munkhdelger J, Bychkov A. Langerhans cell histiocytosis. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/thyroidlch.html. Accessed November 27th, 2024.
Definition / general
- Langerhans cell hystiocytosis (LCH) is a clonal neoplastic proliferation of langerin / CD1a / S100 positive dendritic cells (Langerhans-like cells)
- LCH cells initially thought to arise from the epidermal or mucosal derived Langerhans cell due to the morphologic, immunophenotypic and ultrastructural similarities; however, gene expression profiling showed that LCH cells are not derived from terminally differentiated Langerhans cells but rather share a closer kinship with dendritic cells of the bone marrow (Blood 2017;130:176)
- Thyroid involvement is rare
- 100+ morphologically verified cases published as case series and case reports
- Can occur as a primary disease or secondary involvement in systemic disease
Essential features
- Rare histiocytic neoplasm with occasional involvement of thyroid, either a part of systemic dissemination or isolated
- Proliferation of LCH cells (CD1a / S100 / langerin positive histiocytes with convoluted nuclei) on inflammatory, typically eosinophil rich background
Terminology
- Not recommended / obsolete terminology:
- Morphological: eosinophilic granuloma; histiocytosis X
- Clinical: Hand-Schüller-Christian disease; Letterer-Siwe disease
ICD coding
Epidemiology
- Isolated thyroid involvement is extremely rare
- Secondary involvement is more common
- LCH incidence: 5 - 9 per million children, 1 - 2 per million adults (Blood 2020;135:1319)
- Age range: 2 months to 55 years
- Childhood onset and adult onset LCH
- Young age (< 20 years) at initial presentation in systemic disease, older age in isolated disease (Endocr Pathol 2002;13:227)
- Slight male predilection (Br J Haematol 2016;174:887)
Sites
- Thyroid involvement can be diffuse (59%) or nodular (25.8%) enlargement (Head Neck Pathol 2012;6:279)
- Main target organs in systemic LCH are bone, skin, lung and pituitary (N Engl J Med 2018;379:856)
Pathophysiology
- Not fully understood
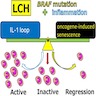
- More than half of LCH cases have MAPK pathway alterations: either BRAF V600E or MAP2K1 (MEK1) mutations (BMC Cancer 2019;19:170, Am J Surg Pathol 2014;38:548, Pediatr Blood Cancer 2015;62:173, Eur Respir J 2020;55:1901190)
- Constitutive activation of MAPK pathway results in continuous stimulation of cell proliferation and promotes cell survival
- Hypothesis of underlying pathogenesis: interleukin 1 loop activation by Merkel cell polyomavirus infection (Cell Commun Signal 2015;13:13)
- Triple risk factor model, including cytogenetic abnormality, stress and reaction (Cell Commun Signal 2018;16:49)
Etiology
- Inflammatory myeloid neoplastic origin (Blood 2015;126:26, Adv Immunol 2013;120:127)
- Frequent BRAF and MAP2K1 mutations in LCH support neoplastic origin (Blood 2014;124:867, Blood 2014;124:3007)
- Findings suggesting inflammatory reactive origin
- Features of IL17A related inflammatory disease (Blood 2014;124:867)
- Identification of Merkel cell polyomavirus DNA in peripheral blood and tissues of LCH patients (Hum Pathol 2014;45:119)
- Most cases are sporadic but variant SMAD6 has been associated with susceptibility to LCH (Blood 2020;135:1319)
Clinical features
- Diffuse enlargement or unilateral thyroid nodule
- Commonly associated with Hashimoto thyroiditis
- Presenting features of systemic LCH are variable and depend on the index organ involved: bone pain, fracture, skin rash, lymphadenopathy, diabetes insipidus and more (Blood 2020;135:1319)
Diagnosis
- Fine needle aspiration cytology aided by immunostaining
- If immunocytochemistry is not available, immunostaining in suspicious cases can be performed on core needle biopsy
- Histologic evaluation of surgical specimen, if surgery performed
- History of systemic LCH in multiorgan disease
Laboratory
- 41% euthyroid, 20% hypothyroid (BMJ Case Rep 2014;2014:bcr2014206760)
- Antithyroglobulin or antimicrosomal antibody (AMA) in cases associated with Hashimoto thyroiditis (Head Neck Pathol 2012;6:279)
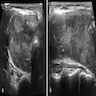
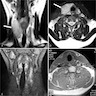
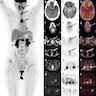
Radiology description
- Cold nodule on thyroid scan
- Ultrasonography: heterogeneous or hypoechoic mixed density nodules (Int J Clin Exp Pathol 2014;7:1229)
- Nonspecific
- Increased uptake on FDG-PET
Prognostic factors
- Excellent prognosis in rare cases with isolated thyroid disease
- ≥ 99% survival for unifocal (single organ) disease
- Up to 20% mortality for patients with organ dysfunction (Blood 2020;135:1319)
- 66% mortality for young children with multisystem involvement who do not respond to therapy (J Pediatr 2001;138:728, Med Pediatr Oncol 2002;39:581, Med Pediatr Oncol 2001;37:108)
- High risk factors: involvement of the bone marrow, liver, lung (Med Pediatr Oncol 2002;39:581, Med Pediatr Oncol 2001;37:108)
- Patient age is less important than extent of disease (Med Pediatr Oncol 2002;39:581, Med Pediatr Oncol 2001;37:108)
Case reports
- 5 month old girl with isolated LCH of thyroid (Eur J Pediatr 2007;166:1151)
- 18 year old man with LCH goiter (Endocr J 2012;59:47)
- 19 year old woman with LCH involving thyroid and parathyroid (Mod Pathol 2001;14:111)
- 27 year old woman with thyroid LCH and papillary thyroid carcinoma involving cervical lymph nodes (Gland Surg 2016;5:537)
- 29 year old woman with thyroid involvement in systemic LCH with EBV infection (Head Neck Pathol 2020 Nov 2 [Epub ahead of print])
- 35 year old woman with thyroid LCH diagnosed on FNA cytology (Head Neck Pathol 2015;9:496)
- 36 year old woman with synchronous papillary thyroid carcinoma and LCH with BRAF mutation (BMC Cancer 2019;19:170)
- 41 year old man with systemic LCH involving endocrine organs (Medicine (Baltimore) 2018;97:e11215)
- 44 year old woman with solitary LCH of the thyroid (Head Neck Pathol 2012;6:279)
Treatment
- Surgical excision for isolated disease
- Combination chemotherapy for systemic disease (Head Neck Pathol 2012;6:279, N Engl J Med 2018;379:856)
Gross description
- Focal of diffuse involvement
- Variable sized nodules
- Similar to other noncystic thyroid nodules
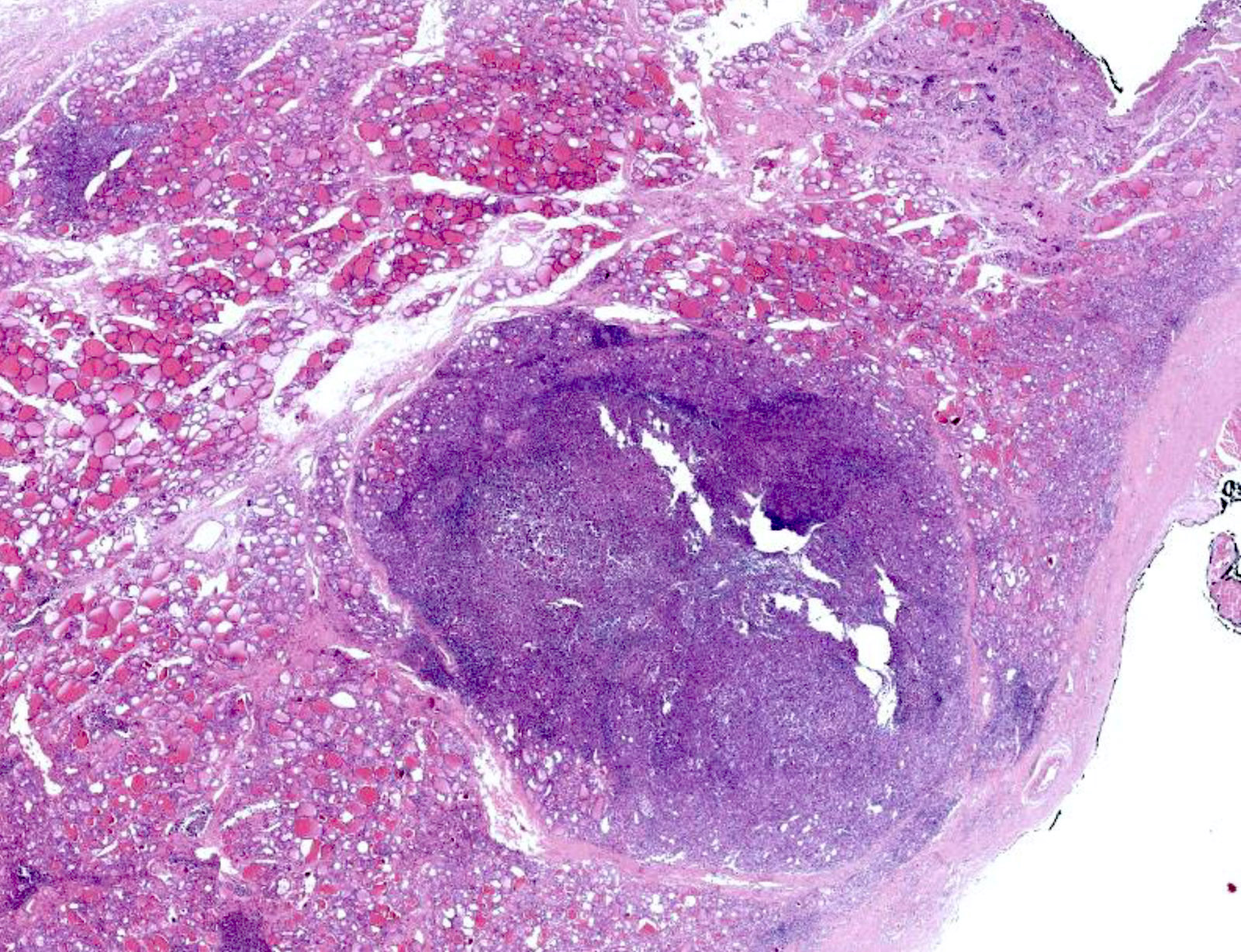
Microscopic (histologic) description
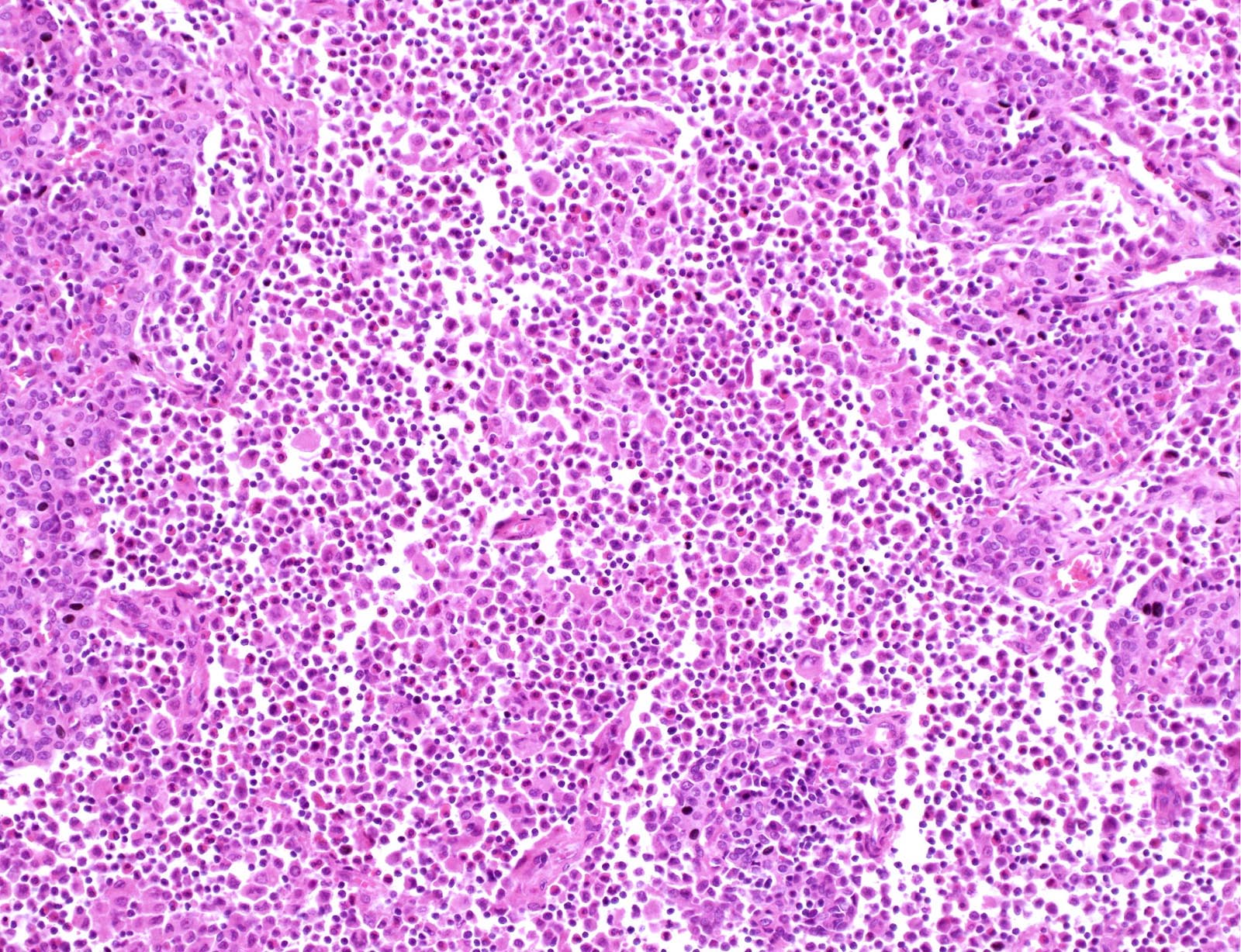
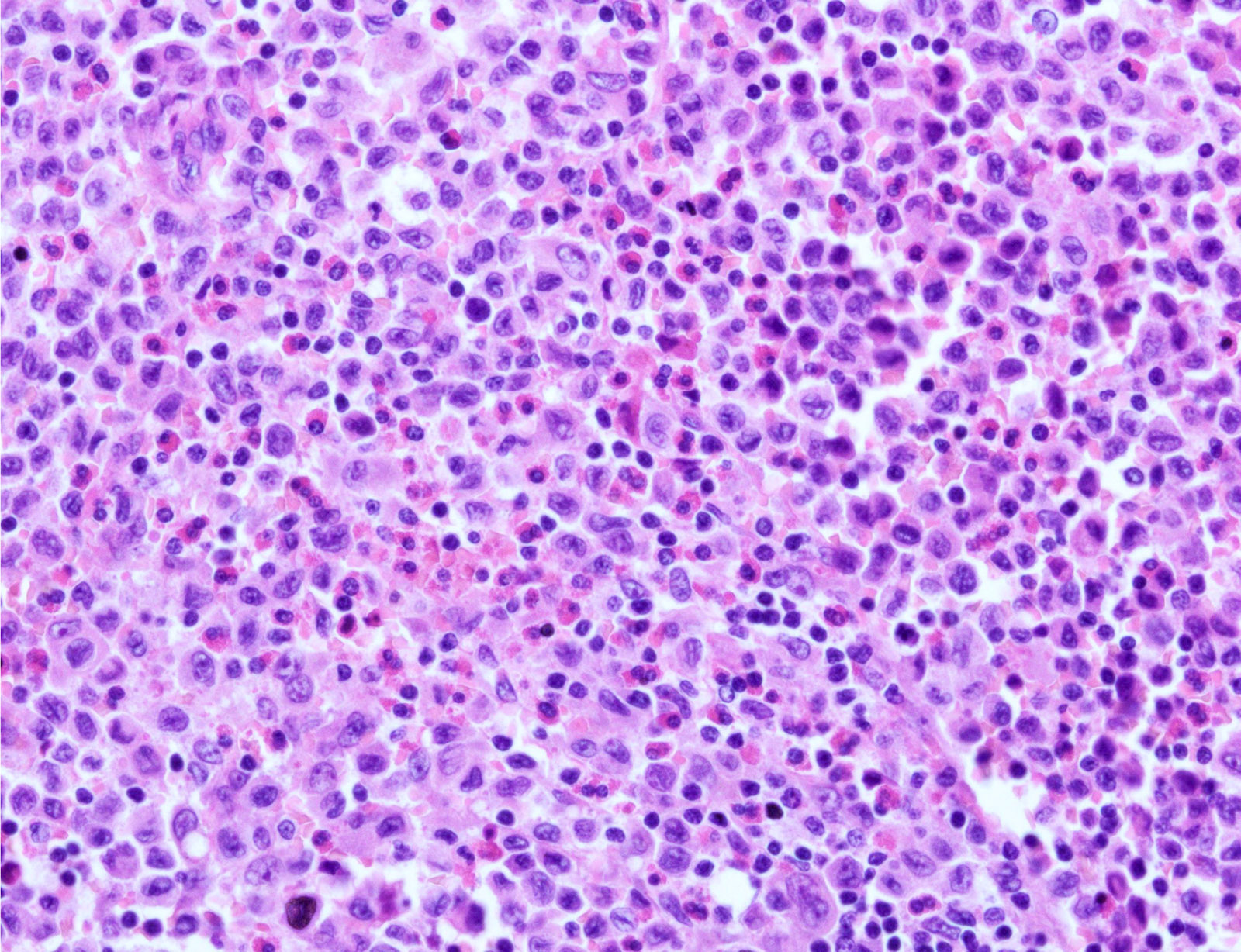
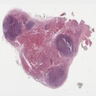
- Nodular or diffuse proliferation of LCH cells
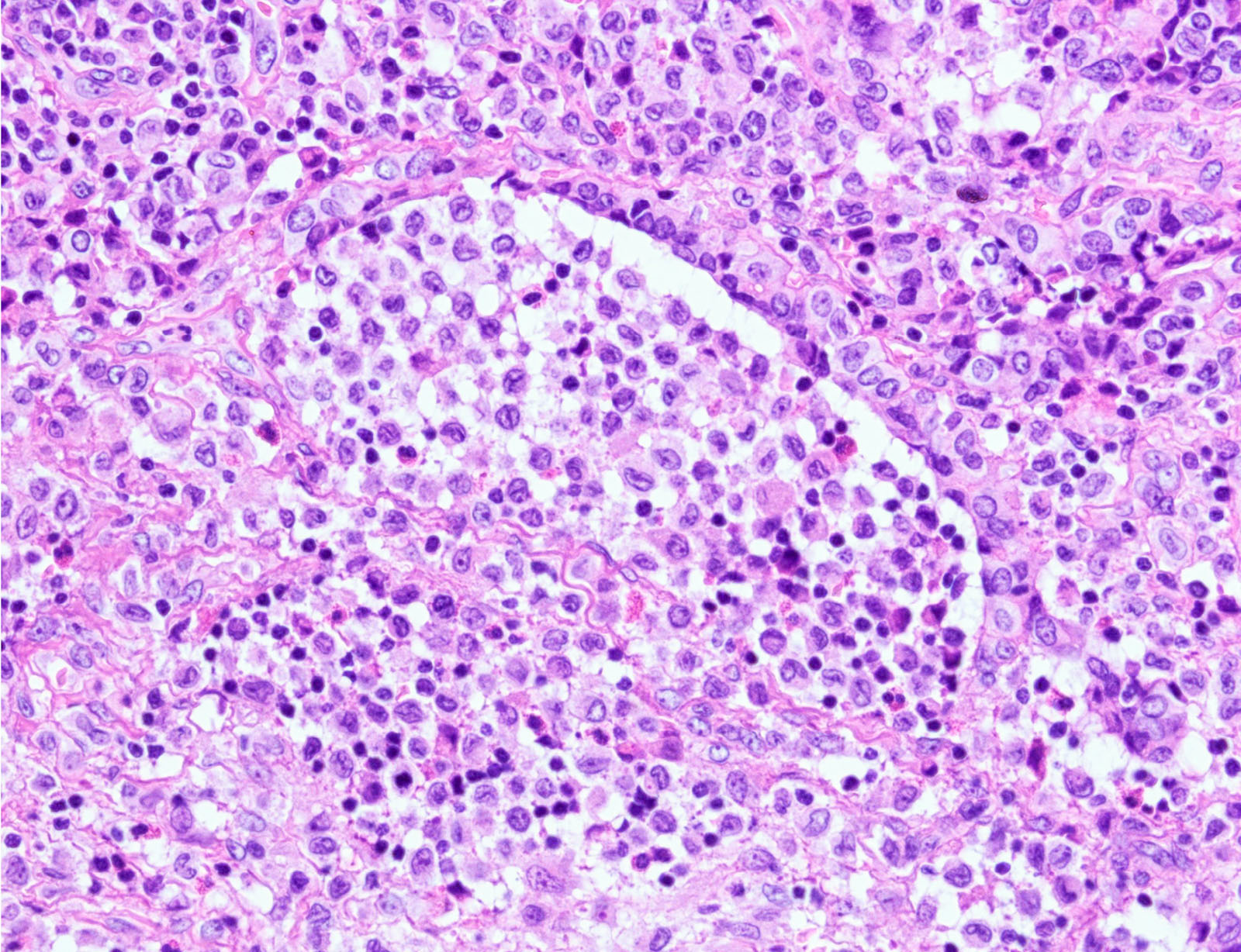
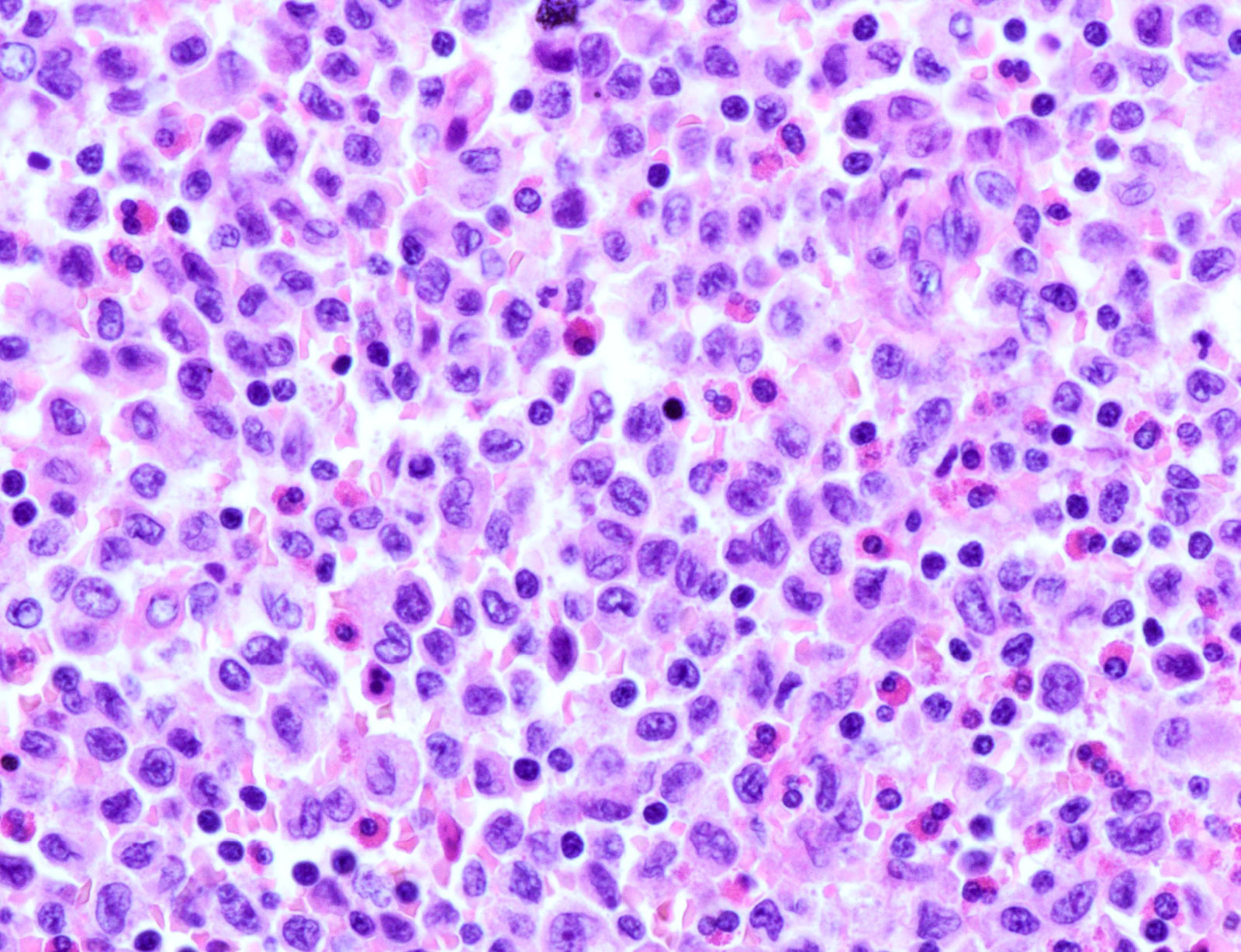
- LCH cells characteristic of disease are histiocytoid cells recognized by their grooved, convoluted, indented or lobed nuclei (Arch Pathol Lab Med 2015;139:1211, Thyroid 2001;11:697, Mod Pathol 1996;9:145)
- Nuclear appearance is often described as resembling coffee beans, horseshoes or kidneys
- Nuclear atypia is absent or minimal
- Mitoses variable, not correlated with aggressiveness
- Cytoplasm is moderately abundant and slightly eosinophilic
- Unlike epidermal Langerhans cells, LCH cells are oval in shape and devoid of dendritic cell processes
- Typical background includes eosinophils with a variable amount of neutrophils, lymphocytes and occasional multinucleated giant cells
- Rarely, eosinophilic abscesses may be formed
- Temporal trend
- In early lesions, LCH cells predominate, along with eosinophils and neutrophils
- In late lesions, the LCH cells are decreased in number, with more foamy macrophages and increased fibrosis
- In most cases, at least some LCH cells can be found
- Effacement of the surrounding thyroid parenchyma due to infiltration of thyroid follicles by LCH cells
- Chronic lymphocytic thyroiditis is common
- Neoplastic cells can extend beyond thyroid capsule and occasionally spread to cervical lymph nodes (Case Rep Pathol 2014;2014:184237)
- Thyroid carcinoma may coexist and even collide with LCH (Endocr Pathol 2010;21:274, Case Rep Pathol 2014;2014:184237)
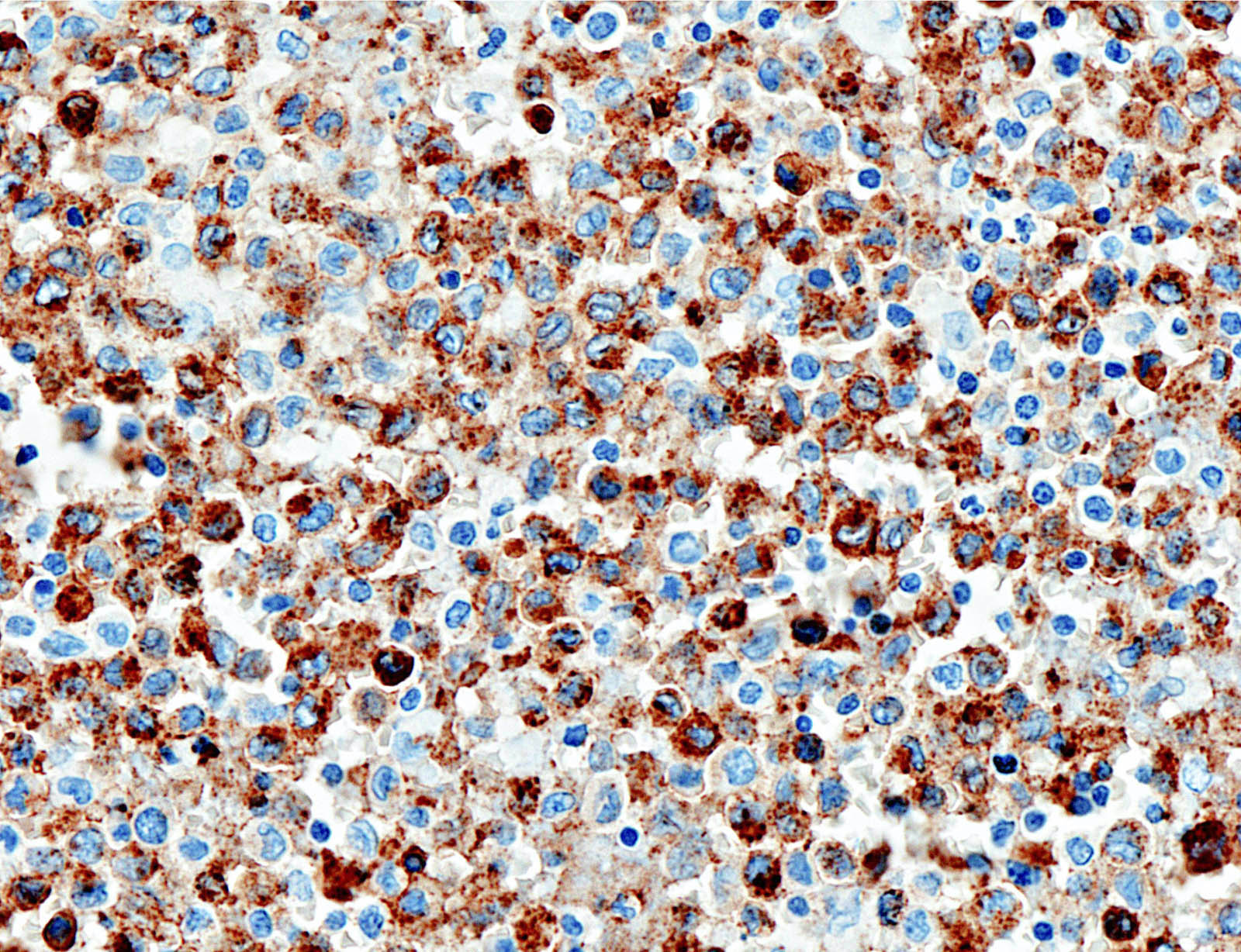
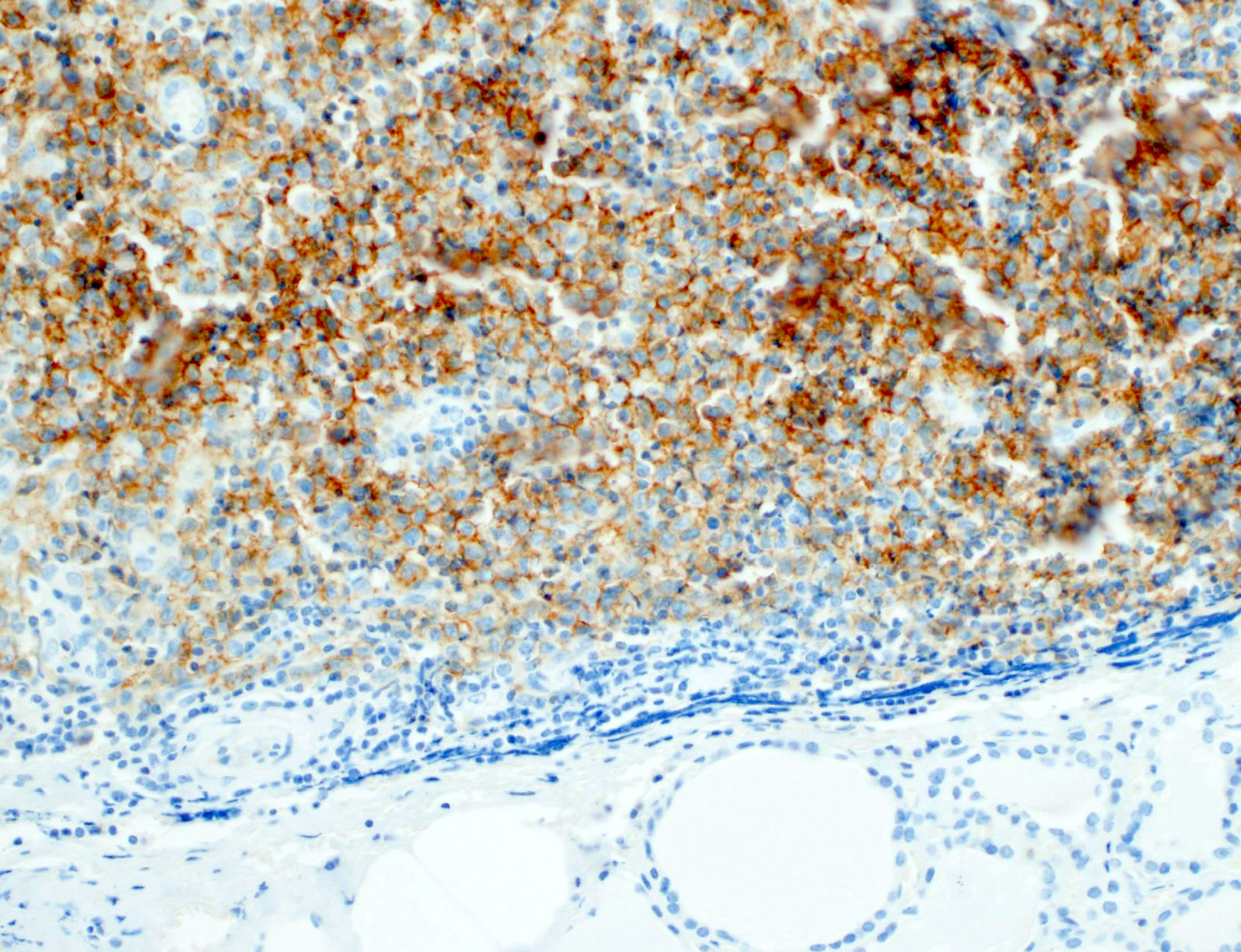
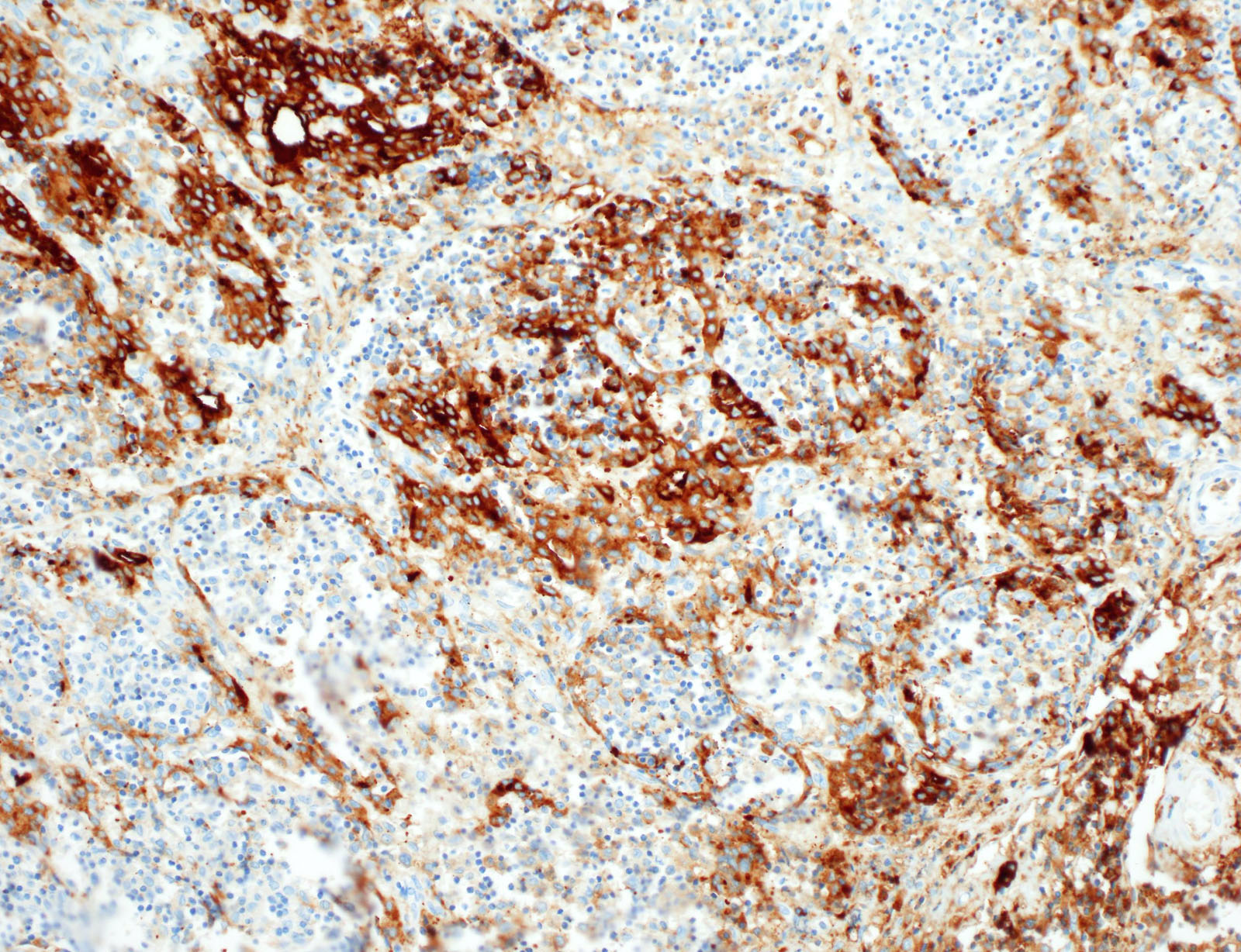
Microscopic (histologic) images
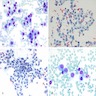
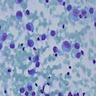
Cytology description
- Variably cellular smears
- Aggregates or isolated histiocytoid LCH cells with grooved / contorted nuclei and moderate amount of pale cytoplasm (Acta Cytol 2015;59:418)
- Inflammatory background with variable amount of eosinophils (occasional Charcot-Leyden crystals), scattered small lymphocytes, foamy histiocytes and multinucleated giant cells (Acta Cytol 2013;57:406)
- FNA samples lack follicular cells and background colloid (Acta Cytol 2017;61:96, Cancer Cytopathol 2021 Jan 25 [Epub ahead of print])
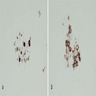
Positive stains
- CD1a, S100, langerin (Blood 2001;97:1241, BMJ Case Rep 2014;2014:bcr2014206760)
- CD1a or combination CD1a / S100 is most commonly used
- Langerin (CD207) is a prototypic marker immunolocalized in Birbeck granules but less widely used
- VE1 immunostaining (anti-BRAF V600E) correlates with the mutation status of BRAF (Am J Surg Pathol 2014;38:548)
- Ki67 variable, of no significance
Negative stains
- Thyroid specific: TTF1, PAX8, thyroglobulin
- Cytokeratins
- Most B cell and T cell lineage markers
Electron microscopy description
- Birbeck granules are characteristic cytoplasmic inclusions (Blood 2020;135:1319)
- Rod to flask to tennis racket shaped
- 200 - 400 nm long x 33 nm wide
- Zipper-like appearance due to a median striated line
- Electron microscopy is less often used today
- Replaced by immunostains
- Birbeck granules present in a variable percentage of LCH cells
Molecular / cytogenetics description
- BRAF V600E mutation in about 50% (Am J Surg Pathol 2014;38:548, Hum Pathol (N Y) 2019;17:200302)
- MAP2K1 (MEK1) in about half of remaining cases
Sample pathology report
- Thyroid gland, total thyroidectomy:
- Histiocytic proliferation with eosinophil rich inflammatory background, consistent with Langerhans cell histiocytosis (see comment)
- Comment: The immunohistochemical stains are positive for CD1a / S100 and negative for thyroglobulin in the histiocytes. These findings support the above diagnosis. Clinical correlation (history, systemic involvement) is recommended.
Differential diagnosis
- Other histiocytic disorders / Rosai-Dorfman disease:
- Lymphocytic thyroiditis:
- No LCH cells, no eosinophils
- Granulomatous thyroiditis:
- Well formed granulomas
- No LCH cells, no eosinophils
- Lymphoma:
- Monotonous population of large atypical lymphoid cells
- Lymphoepithelial lesion
- Anaplastic carcinoma:
- Greater degree of pleomorphism
- Necrosis
- No inflammatory background
- Medullary thyroid carcinoma:
- Positive for calcitonin, CEA
- Papillary thyroid carcinoma:
- Positive for cytokeratin, thyroglobulin, TTF1
| Differential diagnosis of thyroid LCH | |||
| Entity | Potential pitfall | Morphological hallmark | IHC profile |
| Rosai-Dorfman disease | Histiocytic origin; S100+ | Histiocytes with emperipolesis | CD1a-, CD207- |
| Lymphoma | Lymphocyte rich neoplasm | Monotonous proliferation of atypical lymphoid cells | B or T lineage markers+; CD1a-, CD207- |
| Papillary thyroid carcinoma | Grooved and indented nuclei; BRAF mutation | Papillary and follicular patterned epithelial tumor | TTF1 / Tg+; LCH immunophenotype- |
| Hashimoto thyroiditis | Lymphocyte rich background obscuring LCH cells | Lymphocytic infiltration | LCH immunophenotype- |
| Anaplastic thyroid carcinoma | Unusual morphology | Marked pleomorphism | LCH immunophenotype- |
Additional references
Board review style question #1
Board review style answer #1
Board review style question #2
What is the most common molecular alteration in Langerhans cell histiocytosis?
- BRAF V600E mutation
- EGFR mutation
- RAS family mutations
- TERT promoter mutation
- TP53 mutation
Board review style answer #2