Table of Contents
Definition / general | Essential features | Purpose of molecular tests | Diagrams / tables | Background - genetic alterations in thyroid lesions | Overview of molecular tests / platforms | Videos | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Jug R, Jiang X. Molecular testing in FNA. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/thyroidglandmolectestingfna.html. Accessed April 3rd, 2025.
Definition / general
- Ancillary molecular testing is used to further stratify the risk of indeterminate thyroid nodules classified as Bethesda III (atypia of undetermined significance [AUS] / follicular lesion of undetermined significance [FLUS]) or IV (suspicious for follicular neoplasm [SFN] / follicular neoplasm [FN]) for clinical decision making (e.g., close follow up versus repeat FNA versus surgical lobectomy versus thyroidectomy) (Ali: The Bethesda System for Reporting Thyroid Cytopathology, 2nd Edition, 2017)
- Diagnostic performance of molecular tests is determined by sensitivity, specificity, negative predictive value and positive predictive value (Arch Pathol Lab Med 2018;142:446)
- Molecular tests in thyroid FNA evolved from a single gene test (BRAF mutation) and few genes panels to comprehensive molecular classifiers combining next generation sequencing (NGS) detection of multiple mutations / fusions and gene expression with miRNA profiling
- Detection of mutation is considered a rule in approach, i.e., indicative of neoplasia
- Gene expression classifier contributes as a rule out approach, i.e., excluding malignancy
- Combination of both achieves the highest accuracy
Essential features
- Molecular testing in thyroid FNA is used for clinical decision making in indeterminate thyroid nodules (Bethesda III - IV)
- Molecular tests are based on detection of thyroid tumor specific mutations (rule in malignancy), sometimes added by gene expression profiling (rule out)
- Commercially available platforms (ThyroSeq, Afirma and others) are used along with laboratory developed NGS panels
Purpose of molecular tests
- Further characterize lesions diagnosed as indeterminate on FNA and aid in clinical decision making
- Different molecular platforms will categorize noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) differently; for example, the ThyroSeq v3/GC considers NIFTP together with malignant nodules because both tumor types require surgical excision (Cancer 2018;124:1682)
Diagrams / tables
Background - genetic alterations in thyroid lesions
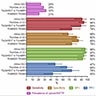
- Mutations in papillary thyroid carcinoma: BRAF mutation (29 - 69%), RET rearrangement (13 - 43%), NTRK1 rearrangement (5 - 13%), RAS mutation (0 - 21%)
- NIFTP and invasive encapsulated follicular variant of papillary thyroid carcinoma (PTC) possess molecular profiles similar to follicular adenomas / carcinomas (higher rates of RAS than BRAF mutations) (Mod Pathol 2010;23:1191)
- Conversely, the infiltrative follicular variant of papillary carcinoma has a molecular profile that is more similar to that of classic papillary thyroid carcinoma (higher rates of BRAF than RAS mutations)
- Hence, FNA indeterminate diagnoses may suggest the need for molecular testing to further prognosticate thyroid lesions into these molecular / behavioral subsets of lesions
- Mutations in follicular thyroid carcinoma: RAS mutation (40 - 53%), PPARG rearrangement (25 - 63%)
- References: Cell 2014;159:676, N Engl J Med 2016;375:1054, Annu Rev Pathol 2018;13:141, Cancers (Basel) 2021;14:204
Overview of molecular tests / platforms
- Commercial thyroid platforms are widely used in North America but not available abroad
- Outside of North America, molecular testing in thyroid FNA is possible via targeted cancer gene commercial panels, custom laboratory developed NGS panels and single gene tests (BRAF, RAS)
- ThyroSeq v3 (UPMC; also known as ThyroSeq GC)
- Sample collection: residual aspirated material collected from routine FNA placed in nucleic acid preservative solution is ideal (J Clin Endocrinol Metab 2011;96:3390)
- Test can be performed on fixed FNA cells from a cytology cell block and on FFPE tissue samples (Cancer 2018;124:1682)
- Test method: DNA and RNA based next generation sequencing assay that detects hundreds of genetic alterations, including point mutations, insertions / deletions, gene fusions, copy number alterations and abnormal gene expression (Cancer 2018;124:1682)
- Genomic classifier (GC) score is calculated as a sum of individual values of detected genomic alterations for each sample to separate malignant lesions from benign lesions (Cancer 2018;124:1682)
- Performance: GC distinguished cancer from benign nodules with a sensitivity of 98.0%, specificity of 81.8% and accuracy of 90.9% in an FNA validation set (Cancer 2018;124:1682)
- Caveats: accurate performance expected with a minimum of 12% tumor cells in a background of nonneoplastic thyroid cells; additionally, a sample is considered adequate with up to 88% blood contamination (Cancer 2018;124:1682)
- Information from UPMC with list of tested mutations: ThyroSeq®
- References: Cancer 2018;124:1682, JAMA Oncol 2019;5:204, Cancer Cytopathol 2019;127:225
- Veracyte's Afirma Gene Expression Classifier (GEC)
- Sample collection: 2 needle passes to collect material for testing in addition to the routine FNA sample for cytology
- Test method: proprietary RNA expression molecular profile analysis
- Performance: 92% sensitivity and 52% specificity for classifying nodules as suspicious; 95% negative predictive value (NPV) for Bethesda III category lesions, 94% NPV for Bethesda IV category lesions (N Engl J Med 2012;367:705)
- Additional tests: Afirma MTC tests for genes associated with medullary thyroid carcinoma and BRAF test available on samples as well
- Caveats: reports nodules as benign or suspicious and does not report specific genetic alterations; while benign result has high NPV, suspicious result has poor positive predictive value (PPV) (16 - 61%, with wide variability in reports) (Cancer Cytopathol 2014;122:737, Diagn Cytopathol 2015;43:966, Endocr Pract 2014;20:364, Ann Surg Oncol 2015;22:3996, J Clin Endocrinol Metab 2014;99:4069, Cancer Cytopathol 2016;124:100, Endocr Pract 2016;22:666)
- ThyGeNEXT and ThyraMIRv2 (Thyroid 2022;32:1362)
- Sample collection: test fresh FNA samples or direct smears / ThinPrep slides; no special shipping or refrigeration requirements
- Test method: the only testing platform that utilizes both mutational (DNA and RNA) and microRNA markers
- Performance: 98% sensitivity, 98% specificity, 99% negative predictive value, 96% positive predictive value
- Additional tests: medullary thyroid carcinoma profile that uses pairwise microRNA expression to detect medullary thyroid carcinoma, particularly helpful in absence of mutations
- Caveats: limited real world clinical experience / follow up
- RosettaGX® Reveal™ thyroid miRNA (Diagn Cytopathol 2018;46:901)
- Sample collection: test material may include ThinPrep prepared slides or a direct smear from a thyroid FNA
- Test method: molecular microRNA analysis
- Performance: overall correct rate of 64.2% and specificity of 60.3% for benign / NIFTP cases and correct rate of 77.8% in malignant cases in a retrospective study
- Caveats: limited real world clinical experience / follow up
Videos
ThyroSeq testing by Y. Nikiforov (2020)
ThyGenX / ThyraMIR panel by Alidad Mireskandari (2018)
Board review style question #1
For which diagnostic category does the American Thyroid Association (ATA) 2015 guideline recommend one can utilize molecular testing results to assess risk of malignancy as an alternative to proceeding directly to surgery?
- Benign (II)
- Follicular neoplasm / suspicious for follicular neoplasm (IV)
- Malignant (VI)
- Nondiagnostic (I)
- Suspicious for malignancy (V)
Board review style answer #1
B. Follicular neoplasm / suspicious for follicular neoplasm (IV)
Comment Here
Reference: Molecular testing in FNA
Comment Here
Reference: Molecular testing in FNA
Board review style question #2
Which genetic alteration is likely to be detected in noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP)?
- BRAF
- NTRK1
- P53
- RAS
- RET
Board review style answer #2