Table of Contents
Definition / general | Essential features | Diagrams / tables | Metrics | Bethesda categories | Major updates in TBS 2017 | Cytology images | Videos | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Bychkov A, Suzuki A. Bethesda system diagnostic categories. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/thyroiddiagnostic.html. Accessed March 28th, 2025.
Definition / general
- The Bethesda System for Reporting Thyroid Cytopathology (TBS) is an international reporting system for thyroid cytology (Ali: The Bethesda System for Reporting Thyroid Cytopathology, 2nd Edition, 2017)
- Uniform terminology aimed to standardize the reporting of thyroid fine needle aspiration (FNA) cytology
- Understandable by various specialists in different countries
- In conjunction with the International Academy of Cytology, endorsed by the American Thyroid Association and other leading professional communities (Thyroid 2016;26:1)
- Currently widely adopted worldwide
- Similar to reporting systems in other organs (Adv Anat Pathol 2016;23:193, Cancer Cytopathol 2020;128:348)
- FNA is gold standard for the preoperative evaluation of thyroid nodules
- Clinical decision making usually relies on combination of FNA findings, thyroid ultrasound and laboratory / clinical investigation
- Developed and maintained by an international panel of experts, including cytopathologists, thyroid pathologists and clinicians
- Conceived in 2008 at state of the science conference (Diagn Cytopathol 2008;36:425)
- First edition in 2009 (Thyroid 2009;19:1159)
- Second edition in 2017 (Thyroid 2017;27:1341, Ali: The Bethesda System for Reporting Thyroid Cytopathology, 2nd Edition, 2017)
- Next edition is due in 2023
- Structure
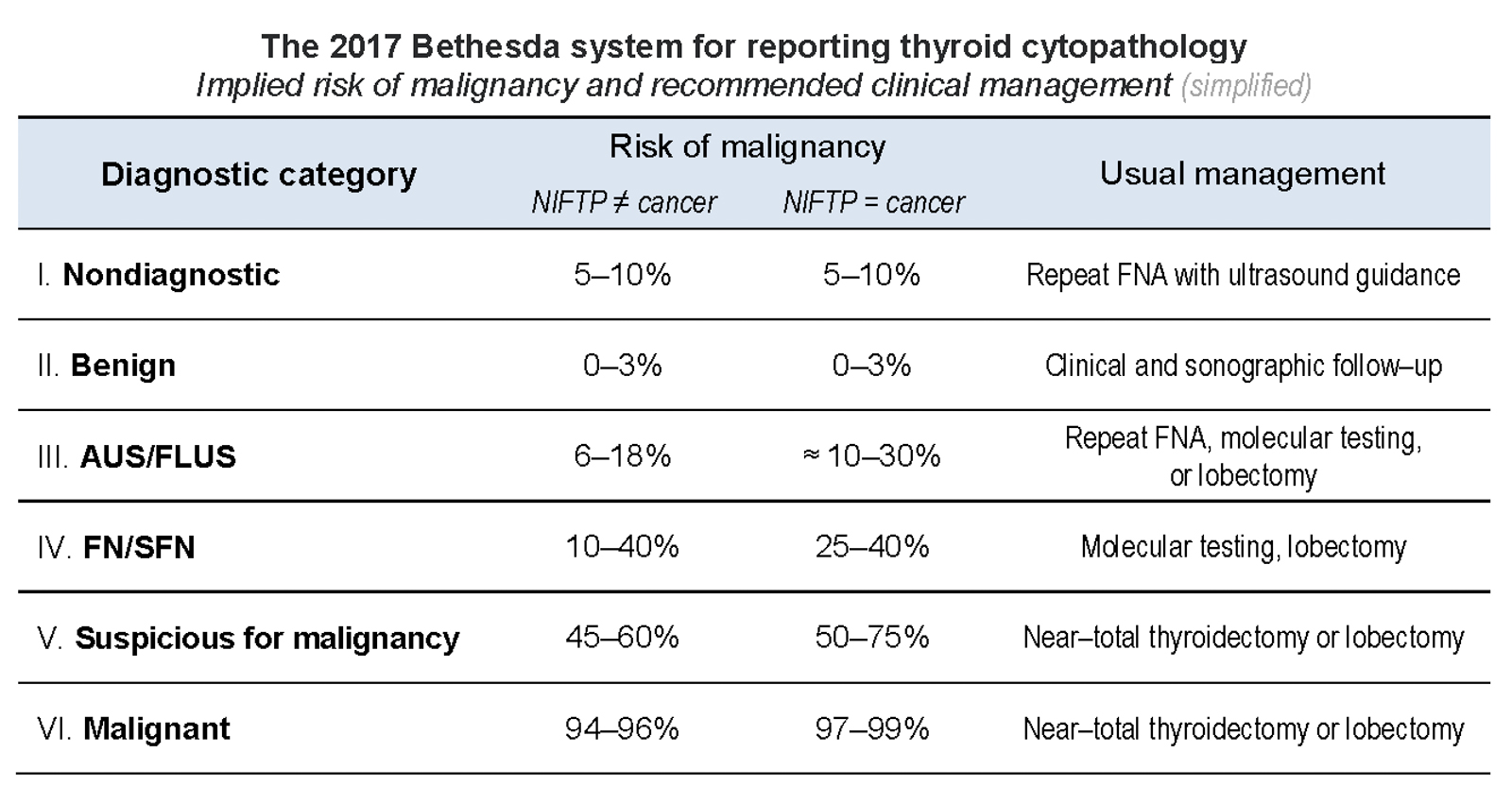
- 6 categories with different propensity to malignancy, from benign to malignant (from < 5% to > 99%), each assigned management approach
- Indeterminate categories constitute categories III - V and may require molecular testing to tailor clinical decision, i.e. surgery versus conservative (Molecular testing in FNA)
- Other national systems exist, including modern British, Japanese, Italian and outdated Papanicolaou, which could be translated into TBS terminology (Cancer Cytopathol 2016;124:457)
Essential features
- TBS is an international standard for reporting thyroid FNA
- Divided into 6 categories that are linked to malignancy risk and recommended clinical management
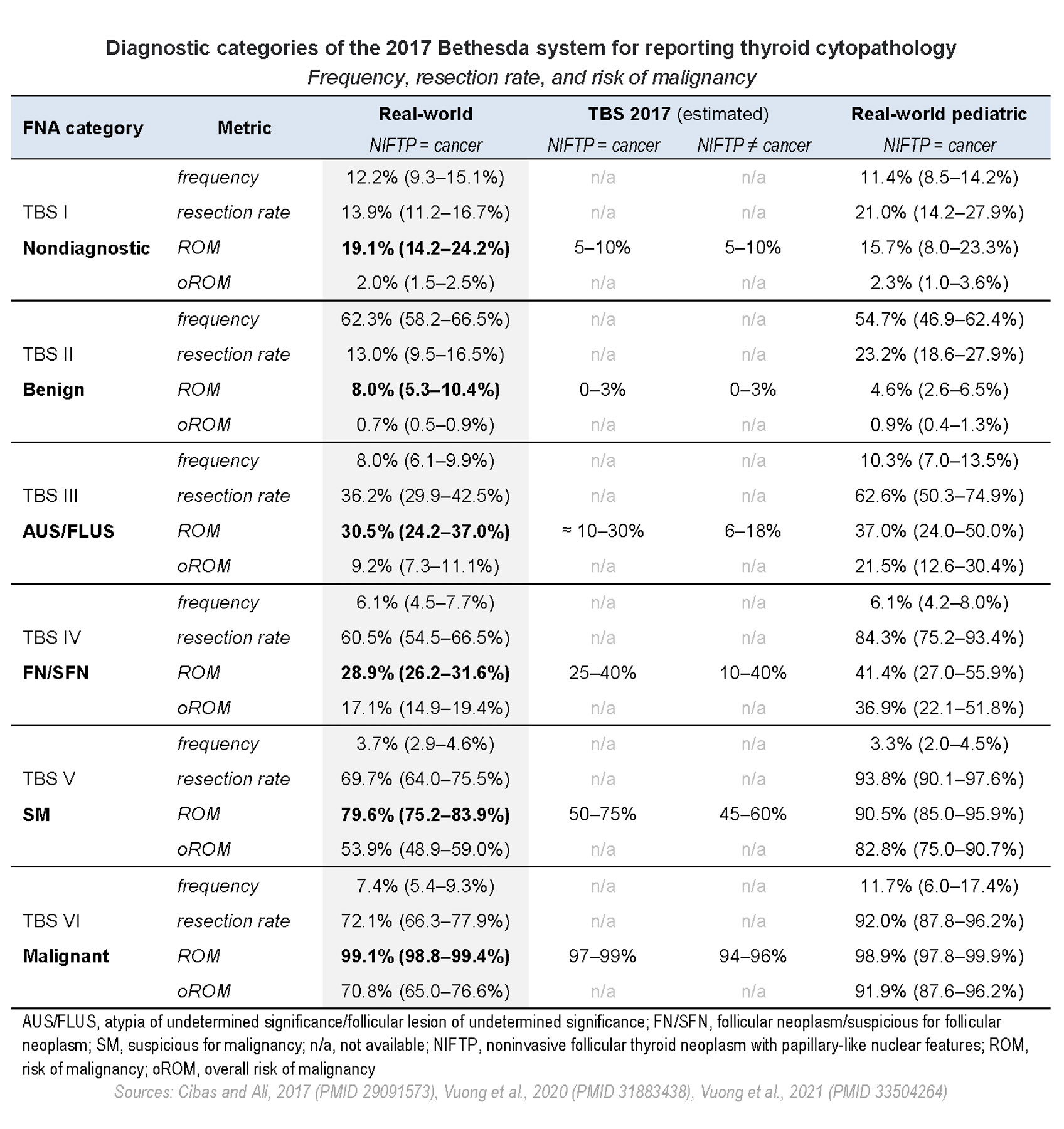
- Clinically significant statistical metrics of each category are frequency, resection rate and risk of malignancy
Metrics
- TBS metrics / outputs include several statistical indicators:
- Frequency: number of nodules in a given category out of all aspirated nodules
- Resection rate (RR): ratio of operated / resected nodules out of all aspirated nodules in a category
- Malignancy risk, also known as risk of malignancy (ROM): number of malignant nodules on surgery out of all resected in a given category
- Indicators not currently included in TBS:
- Overall risk of malignancy (oROM): calculated out of all aspirated nodules, including resected and unresected (Thyroid 2021 May 14 [Epub ahead of print])
- Risk of neoplasia (RON): number of neoplastic nodules (benign plus malignant) on surgery out of all resected in a given category (Cancer Cytopathol 2020;128:232)
- Utility:
- Defines management of thyroid nodules: high ROM suggests surgical resection while low ROM implies conservative strategy, e.g. follow up
- Quality control in lab / hospital:
- High nondiagnostic rate (> 15%) requires revision of FNA sampling procedure and lab workflow, e.g. implementation of rapid onsite evaluation or ultrasound guided sampling
- Abnormal measures, such as high rate of indeterminate diagnoses, low ROM in malignant nodules or high ROM in benign nodules may be due to limited expertise of FNA readers
- Variation in TBS outputs depends on different factors:
- Institutions: reference center versus primary care; expertise of operator and reader
- Geography: North America versus Asia, due to different practice patterns (Cancer Cytopathol 2020;128:238)
- Population: adults versus children (Thyroid 2021;31:1203)
- Classification of noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) either as benign or malignant tumor (Endocr Pract 2019;25:49)
- All of the above explain differences between the model outputs implied by TBS and real world data reported in meta analysis studies
- Ideally, local institutional metrics (especially ROM) should be calculated to adjust clinical decisions in a given hospital (Cancer Cytopathol 2020;128:917)
Bethesda categories
- TBS I: nondiagnostic / unsatisfactory
- Includes inadequate by cellularity, unsatisfactory by quality and cyst fluid only specimens
- TBS criteria for adequacy of thyroid FNA specimens is ≥ 6 groups of well visualized follicular cells (≥ 10 per cluster)
- Frequency 10 - 15%, resection rate 10 - 15%, ROM up to 20% of all nodules and up to 30% of resected nodules
- Management: reaspiration, except for pure cyst
- Includes inadequate by cellularity, unsatisfactory by quality and cyst fluid only specimens
- TBS II: benign
- Cytology sample that is adequate for evaluation and consists of colloid and benign appearing follicular cells
- Frequency 60 - 70%, resection rate 10 - 15%, ROM < 10% (oROM < 1%)
- Usually nodular hyperplasia on resection
- Management: follow up based on ultrasound pattern
- TBS III: atypia of undermined significance / follicular lesion of undetermined significance (AUS / FLUS)
- Aspirates with few cells that have distinct but mild nuclear atypia or with more extensive but very mild nuclear atypia
- Frequency < 10%, resection rate 30 - 40%, ROM 25 - 40% (NIFTP = malignant) or 6 - 18% (NIFTP ≠ malignant) and up to 40% of resected TBS III nodules
- On resection / histopathology diagnosed as nodular hyperplasia, follicular adenoma and papillary thyroid carcinoma (PTC)
- Management: reaspiration or molecular testing (Thyroid 2016;26:1)
- TBS IV: follicular neoplasm / suspicious for a follicular neoplasm (FN / SFN)
- Cases with most of the follicular cells arranged in cell crowding or microfollicle formation
- Frequency 6%, resection rate 60%, ROM 25 - 30% (NIFTP = malignant) or 10 - 40% (NIFTP ≠ malignant)
- Histopathology: follicular adenoma, adenomatous nodule, follicular variant of papillary thyroid carcinoma and follicular carcinoma
- Management: diagnostic thyroid lobectomy or molecular testing
- TBS IV: follicular neoplasm, Hürthle cell type / suspicious for a follicular neoplasm, Hürthle cell type (FN-H / SFN-H)
- Cases with most of the follicular cells showing abundant fine granular cytoplasm (Hürthle cells)
- Frequency 1.2 - 9%, resection rate 30%, ROM 10 - 40%
- Histopathology: oncocytic (Hürthle cell) adenoma and carcinoma
- Management: diagnostic thyroid lobectomy, molecular testing is not helpful
- TBS V: suspicious for malignancy
- Used when cytology strongly suggests malignancy but is not sufficient for a conclusive diagnosis
- Frequency < 5%, resection rate 70%, ROM 80% (NIFTP = malignant) or 45 - 60% (NIFTP ≠ malignant)
- Histopathology: usually papillary thyroid carcinoma
- Management: surgery (usually)
- TBS VI: malignant
- Used when cytology strongly suggests malignancy
- Frequency 5 - 10%, resection rate 65 - 80%, ROM 99% (NIFTP = malignant) or 94 - 96% (NIFTP ≠ malignant)
- Histopathology: wide spectrum of thyroid malignancies, from papillary thyroid carcinoma (most common) to medullary thyroid carcinoma, anaplastic thyroid carcinoma, lymphoma, etc.
- Management: surgery (usually)
Major updates in TBS 2017
- The 2017 revision was influenced by (Thyroid 2017;27:1341):
- Accumulation of knowledge and meta analysis studies of TBS
- American Thyroid Association 2015 guidelines for the management of patients with thyroid nodules
- Introduction of molecular testing as an adjunct to cytopathologic examination
- Introduction of NIFTP to replace noninvasive encapsulated follicular variant of papillary thyroid carcinoma
- Adjustments to the ROM based on the post 2010 data
- NIFTP impact:
- Incorporate criteria to recognize NIFTP to avoid the malignant category for these lesions
- 2 types of ROM, i.e. when NIFTP = malignant and NIFTP ≠ malignant
- NIFTP note for follicular neoplasm / suspicious for a follicular neoplasm, suspicious for malignancy and malignant; papillary thyroid carcinoma
- Updated management recommendations, including molecular testing for AUS / FLUS and FN / SFN
- Subclassified AUS / FLUS
- Diagnostic criteria for papillary thyroid carcinoma subset of the malignant category limited to cases with classical features of papillary thyroid carcinoma
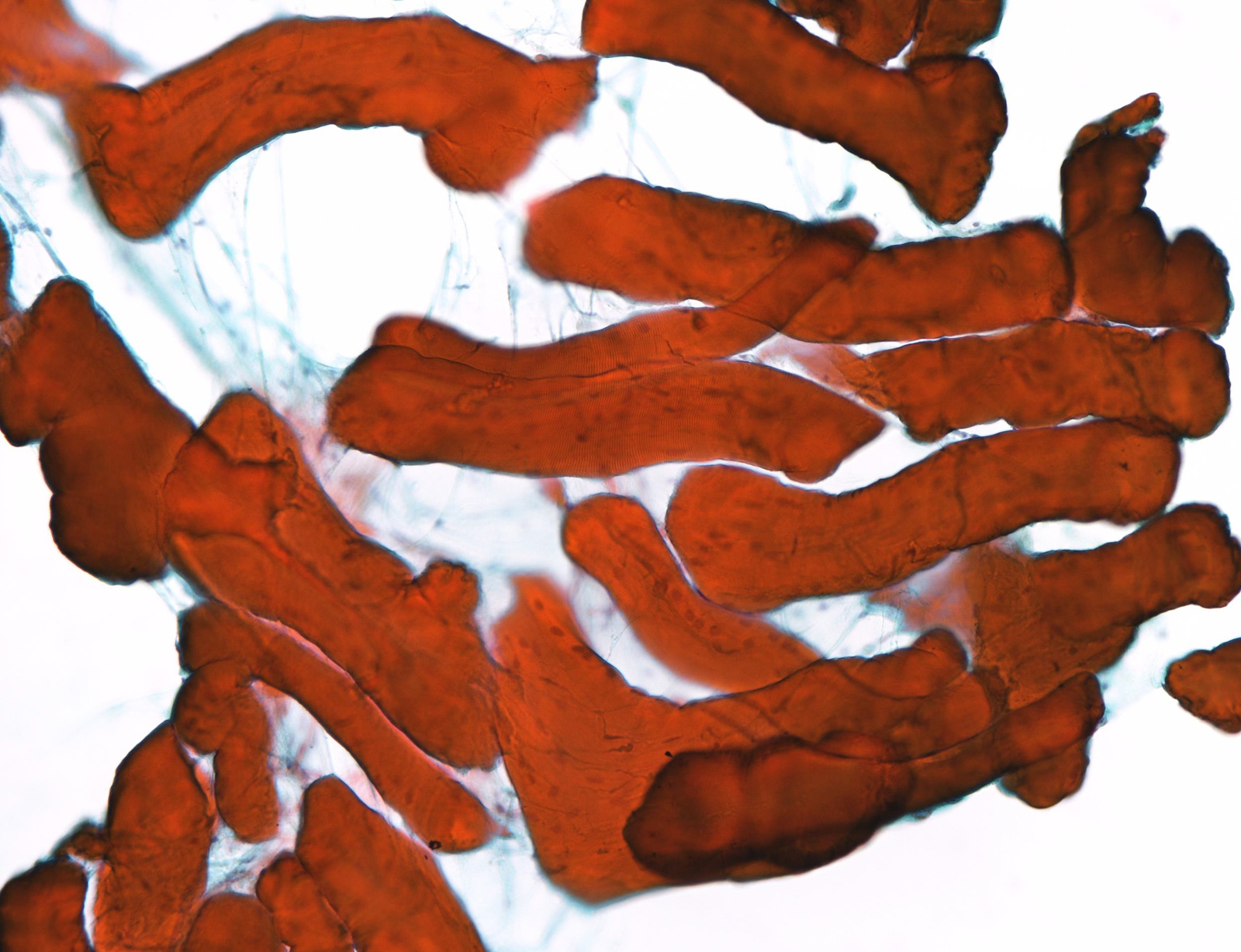
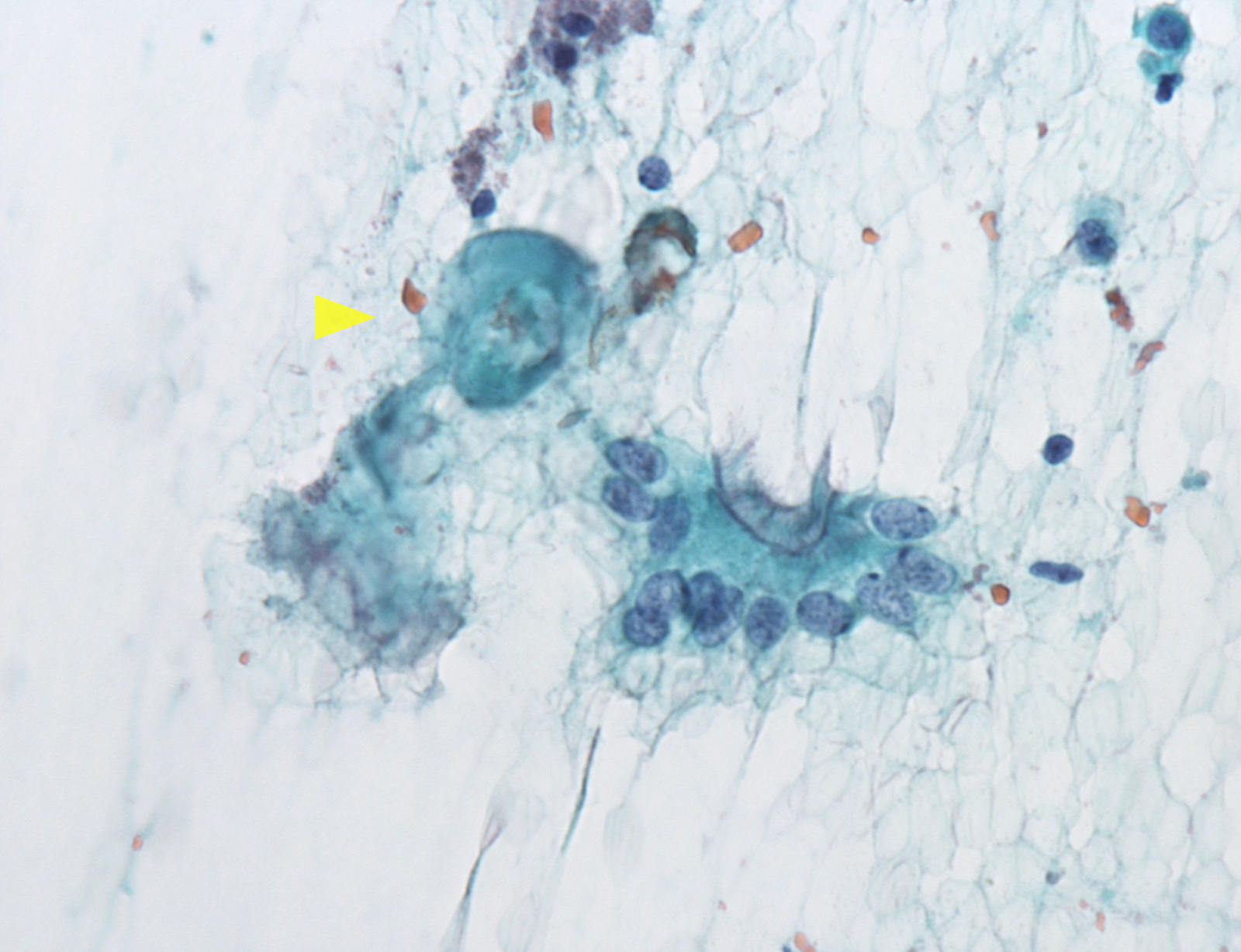
Cytology images
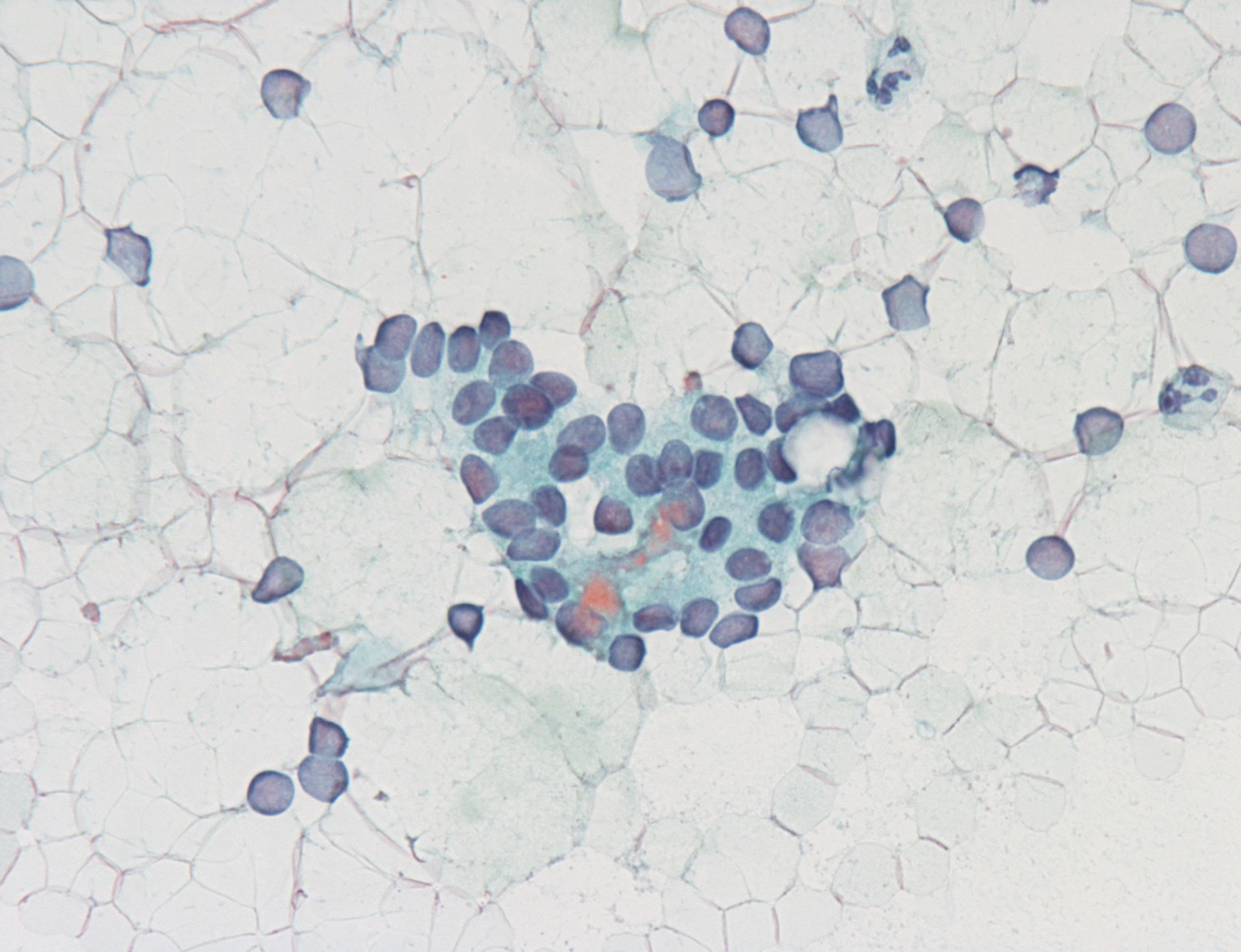
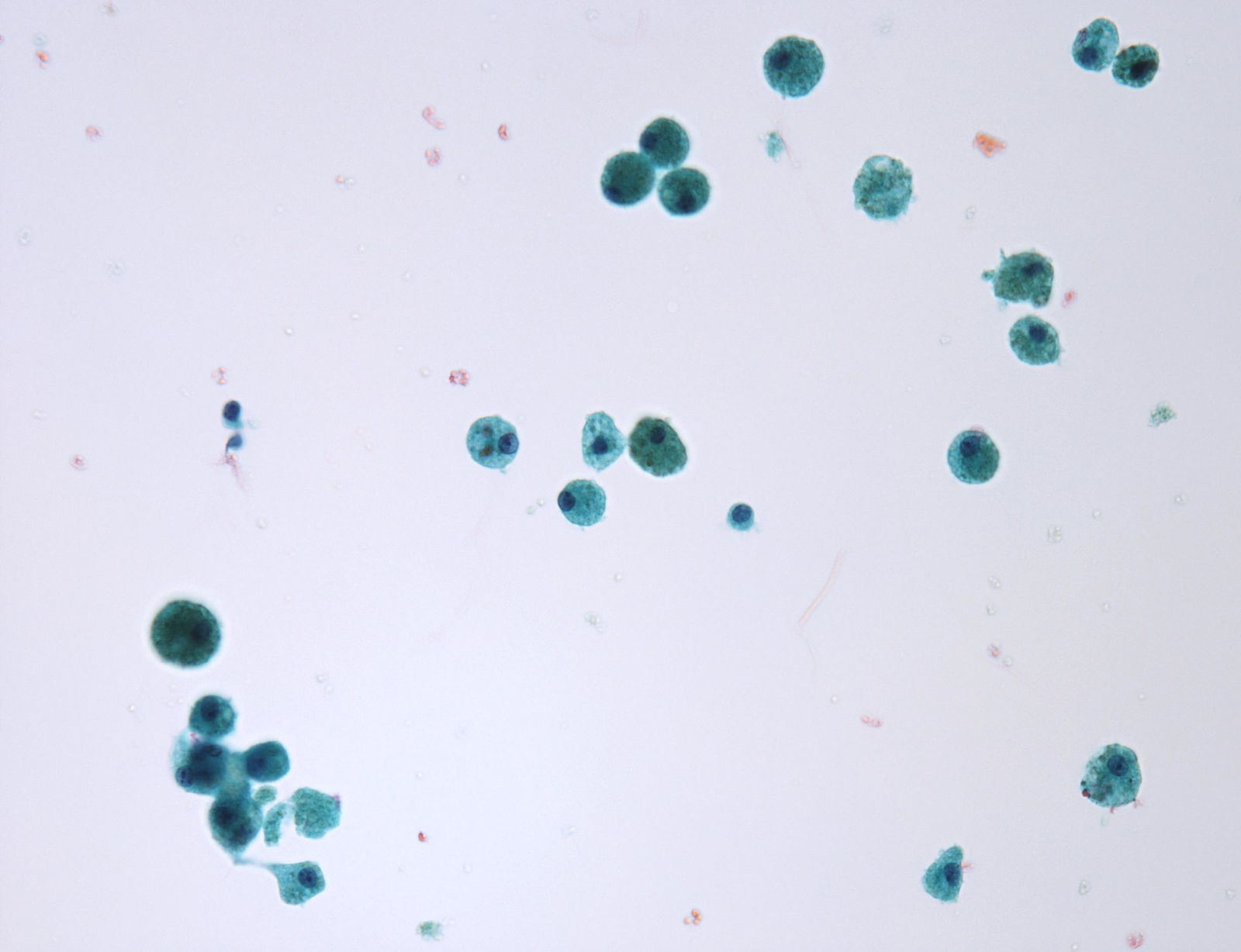
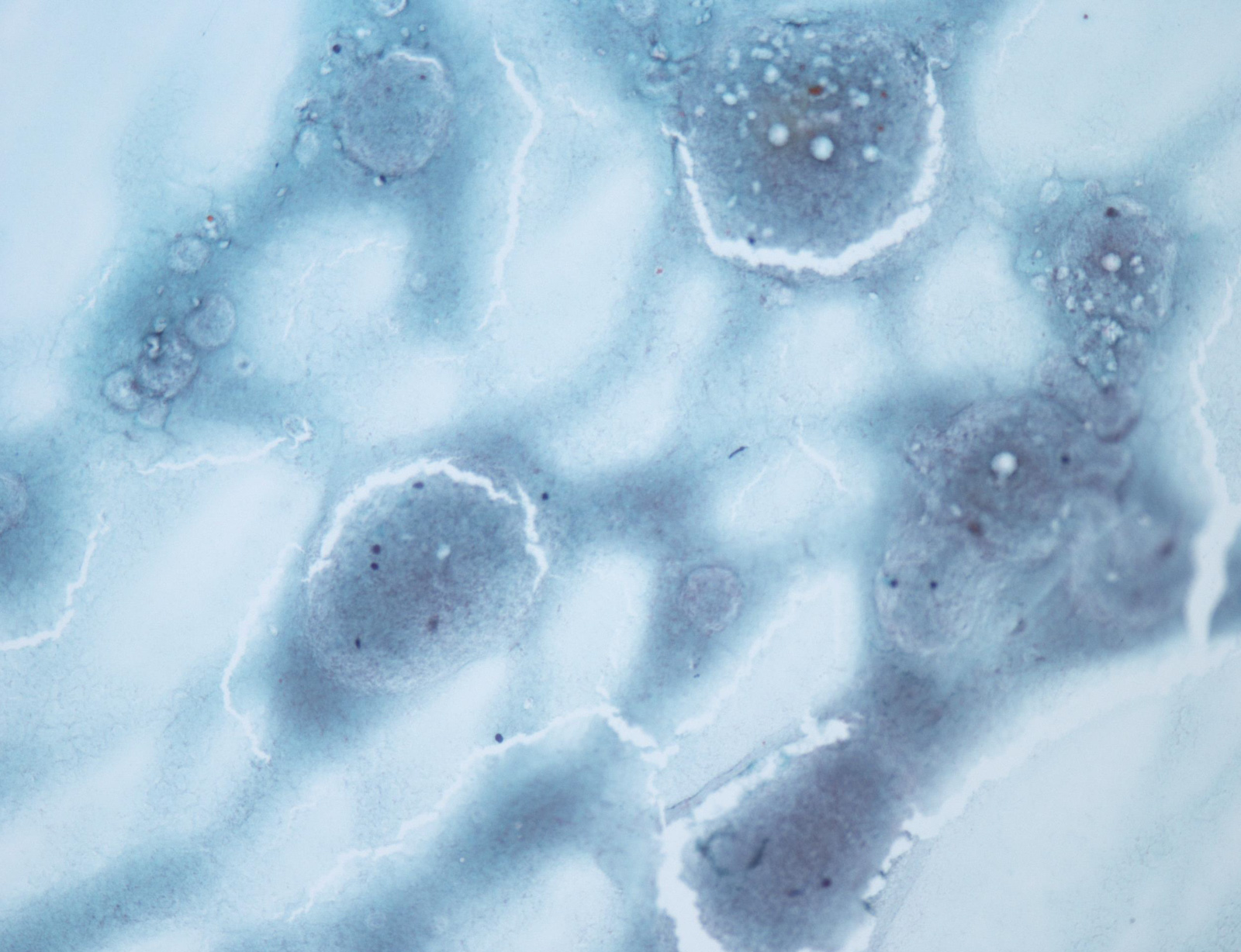
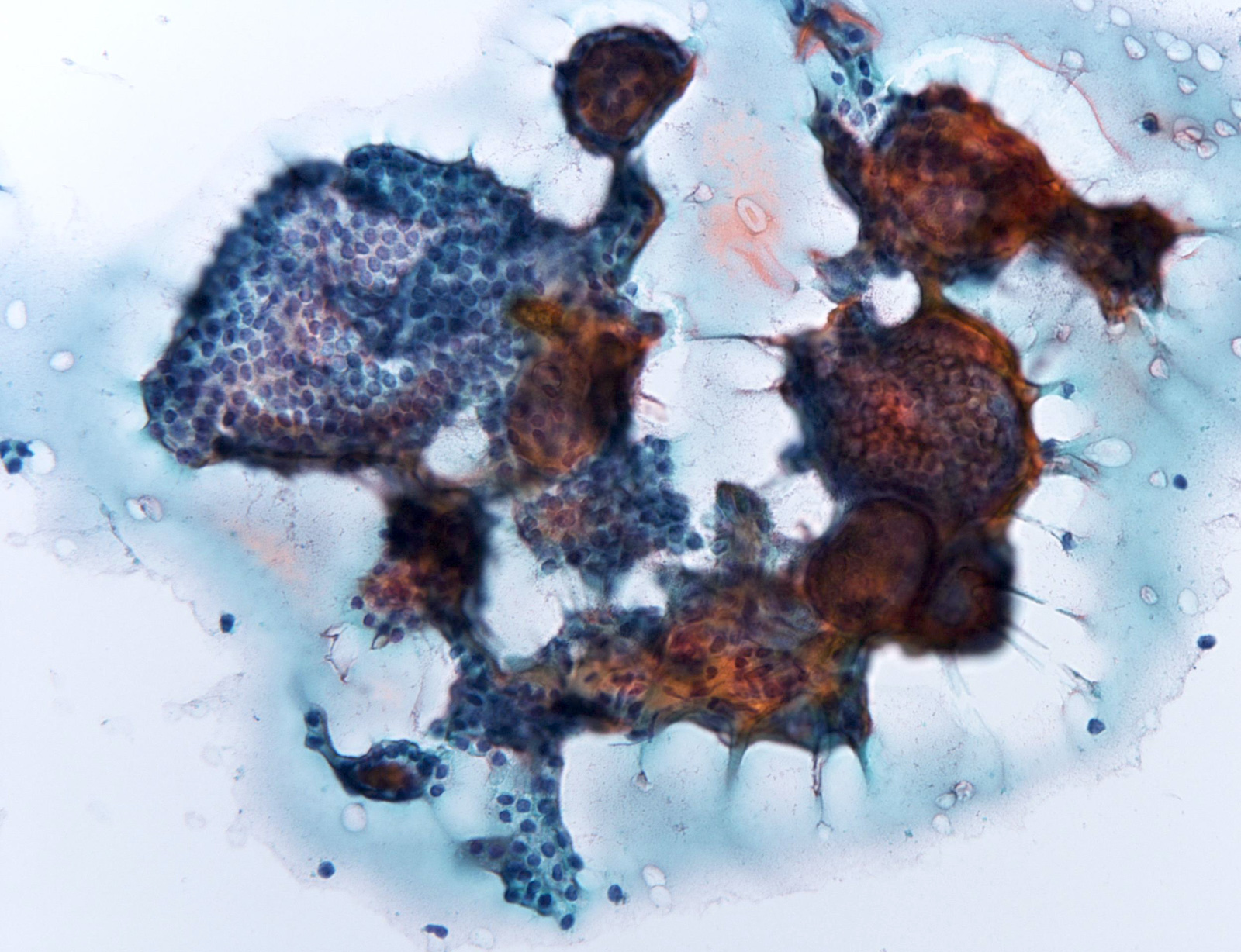
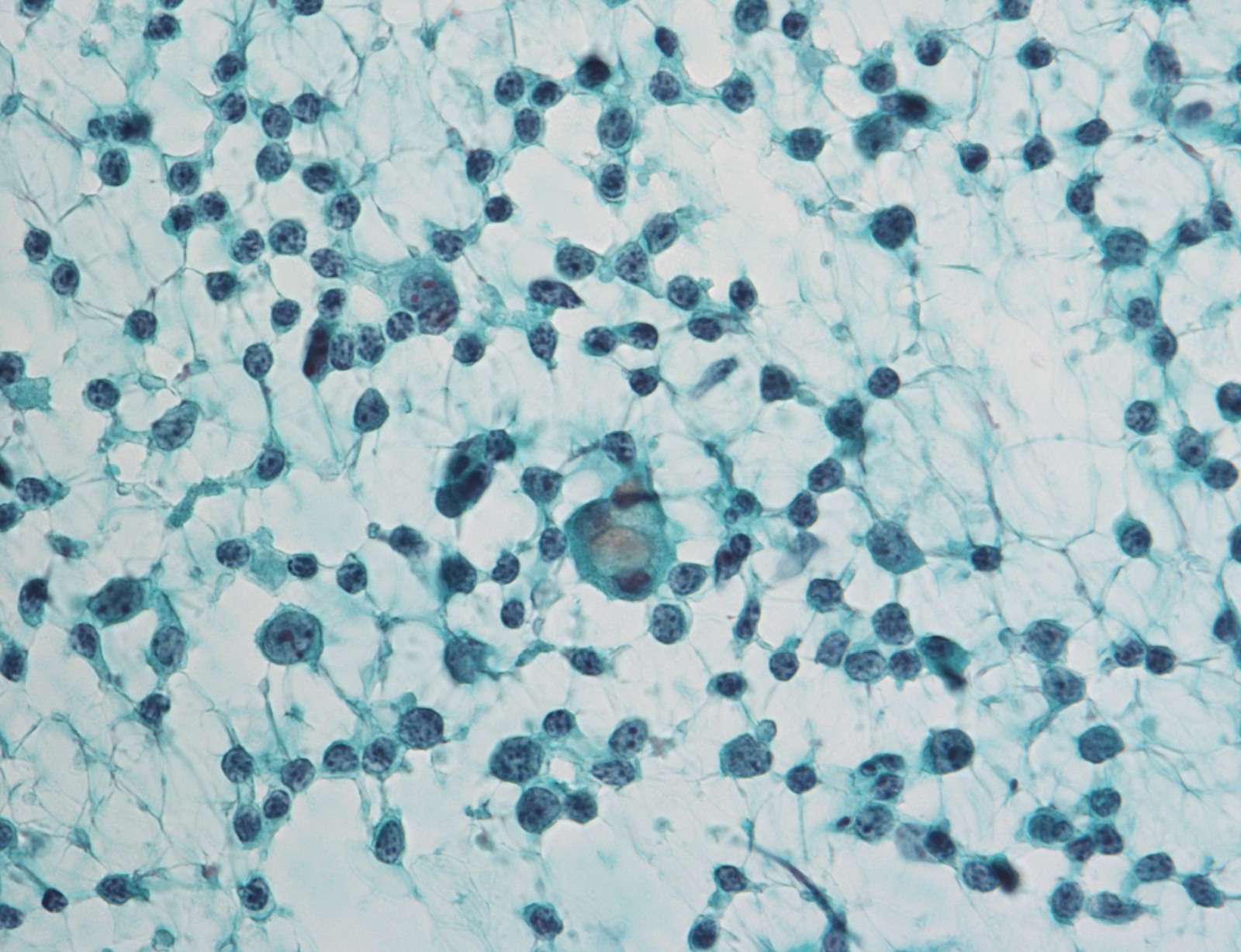
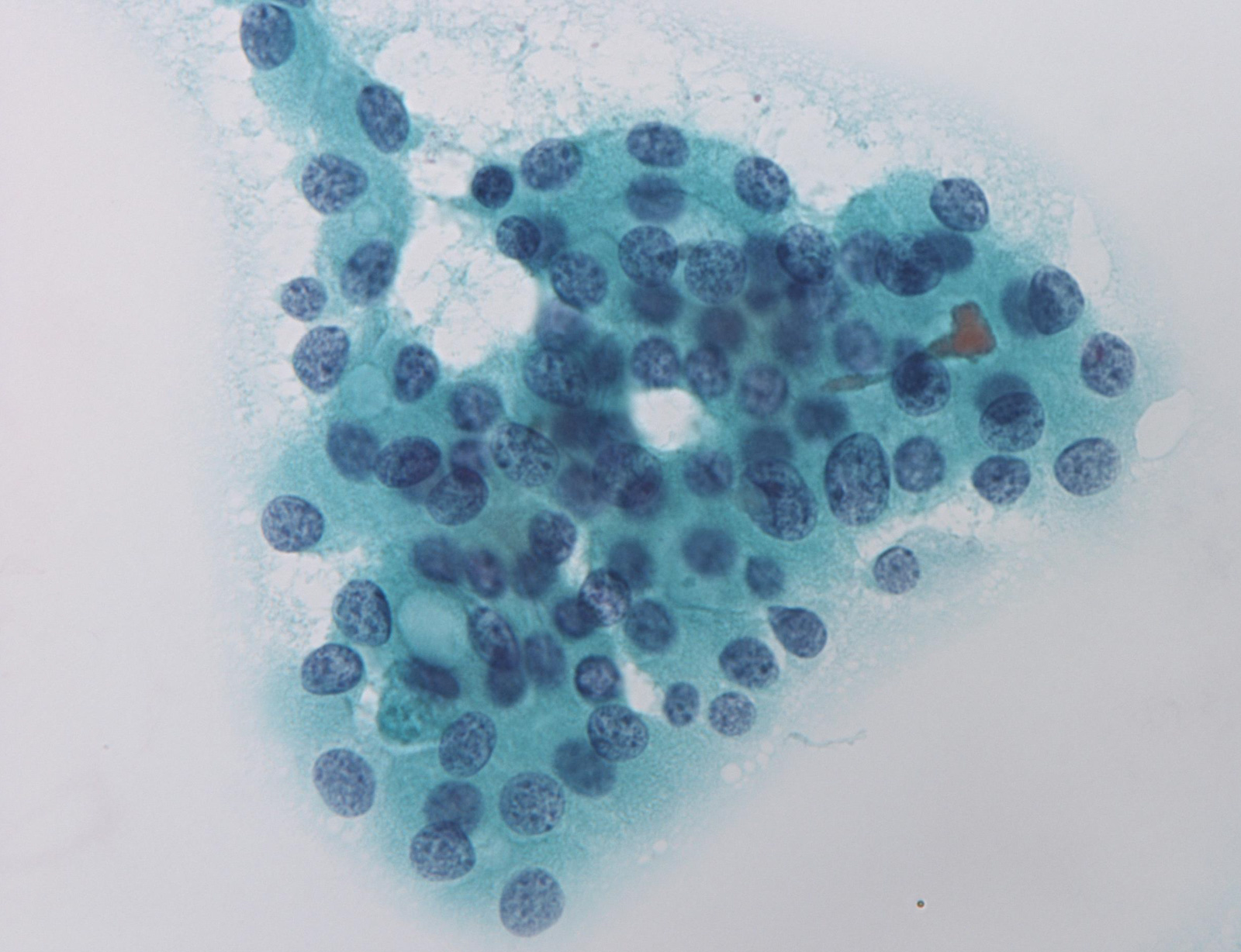
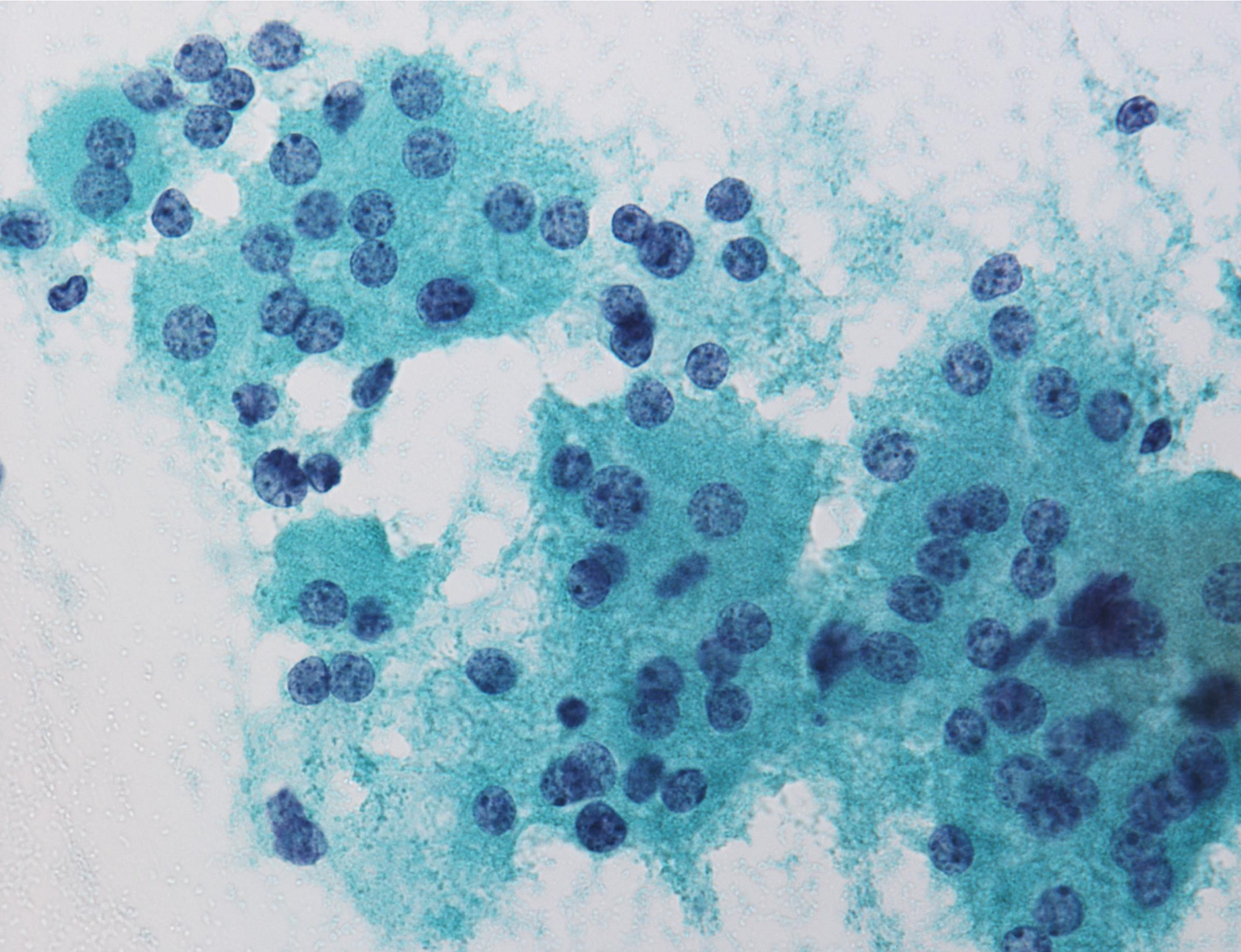
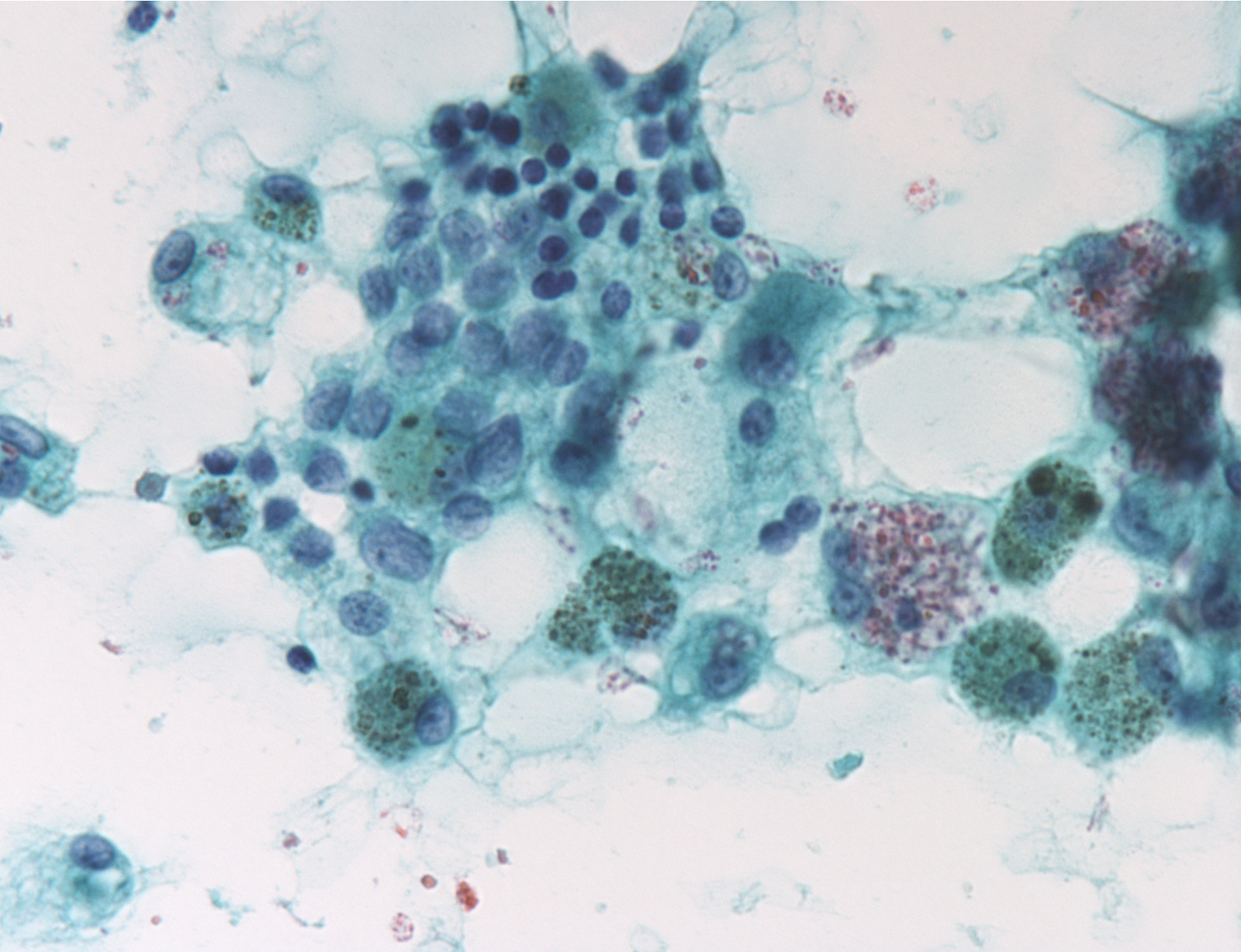
Contributed by Ayana Suzuki, C.T.
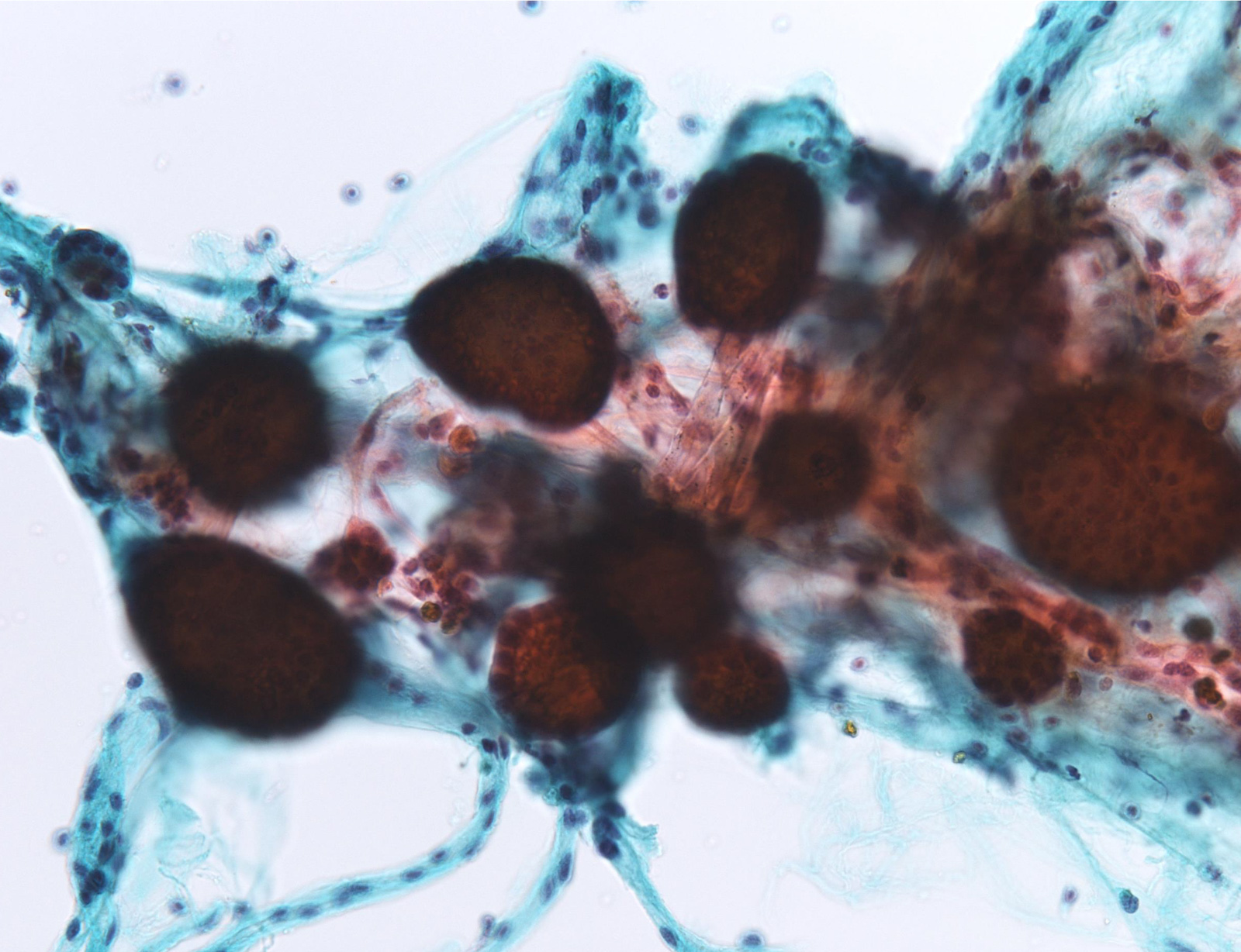
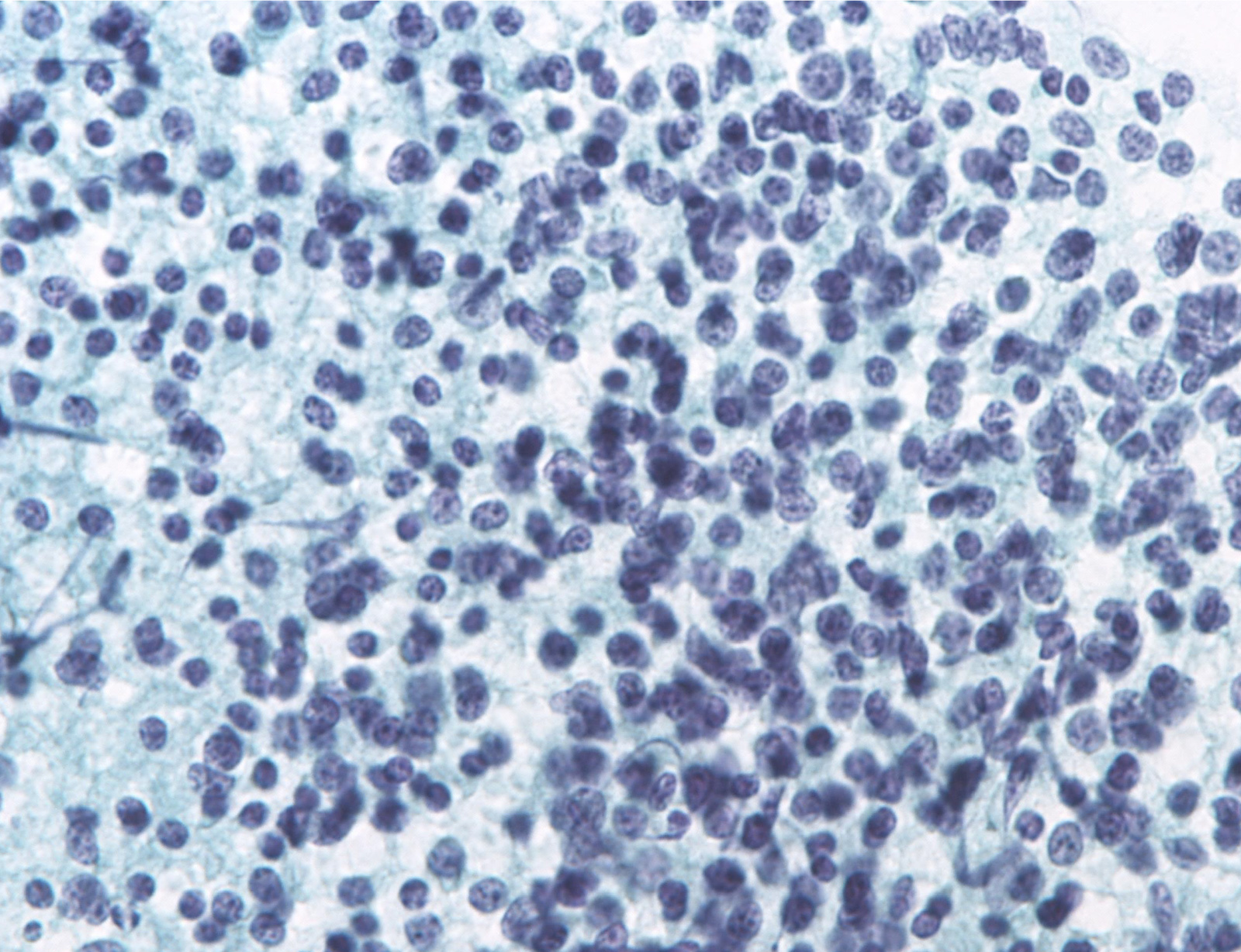
Unsatisfactory:
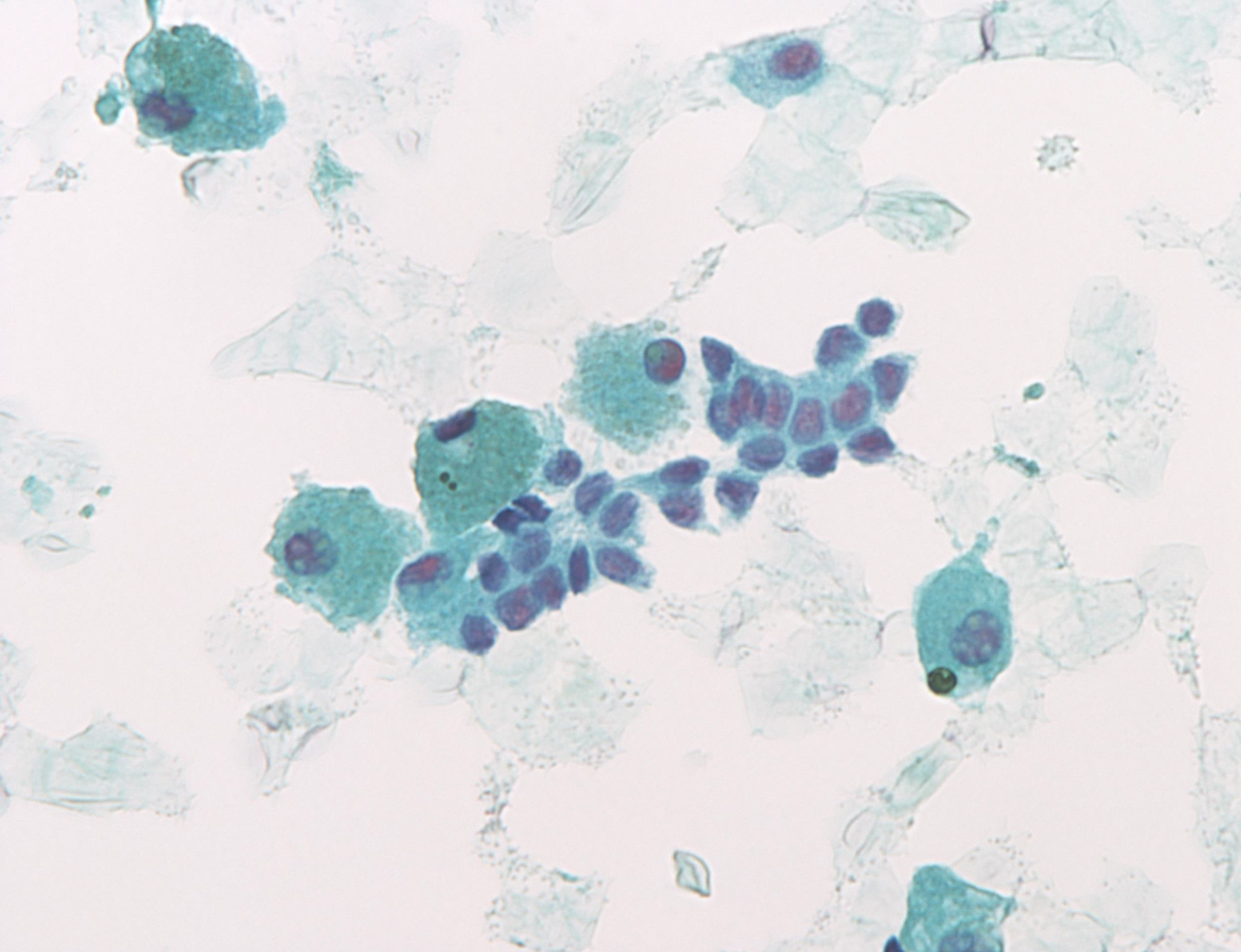
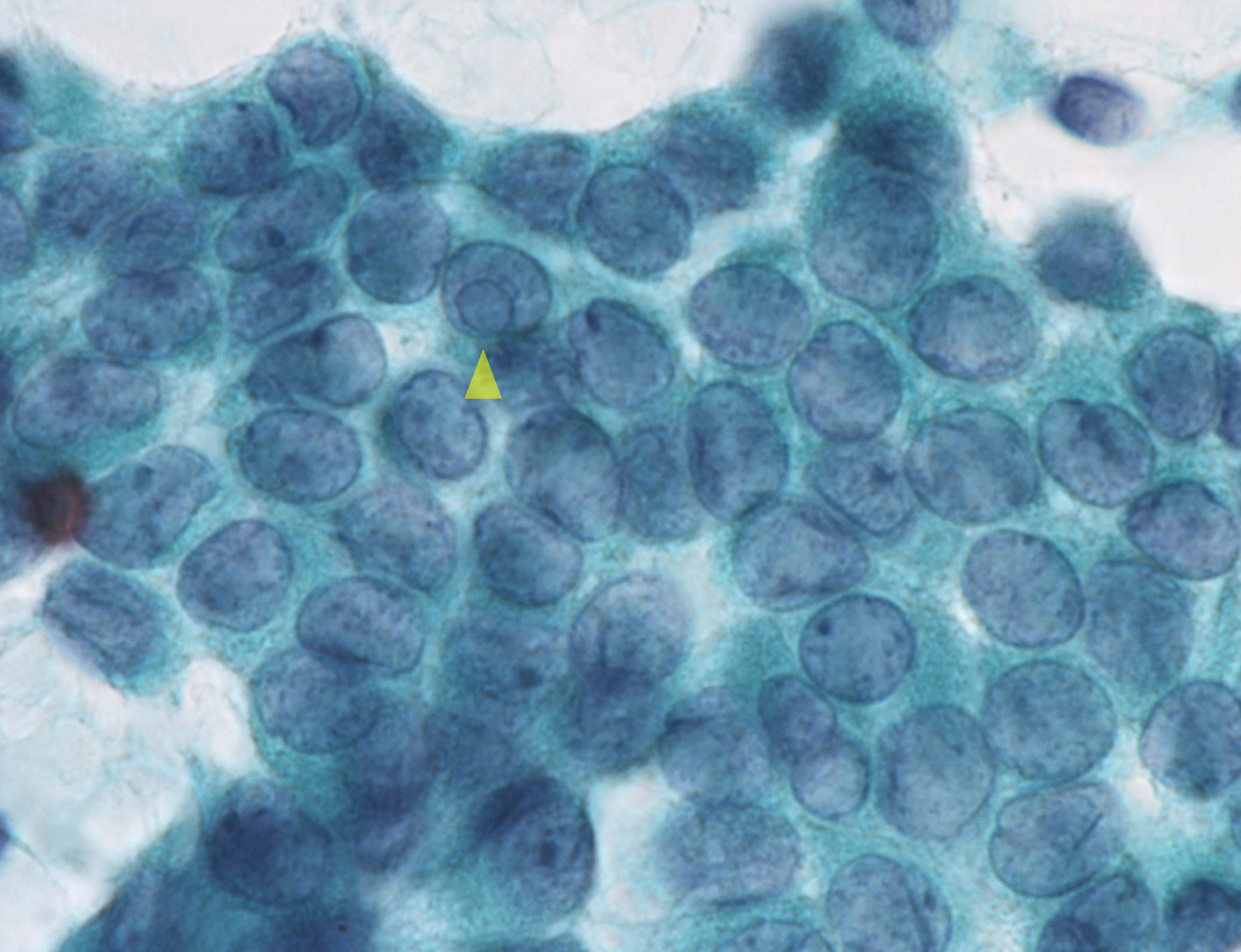
Benign:
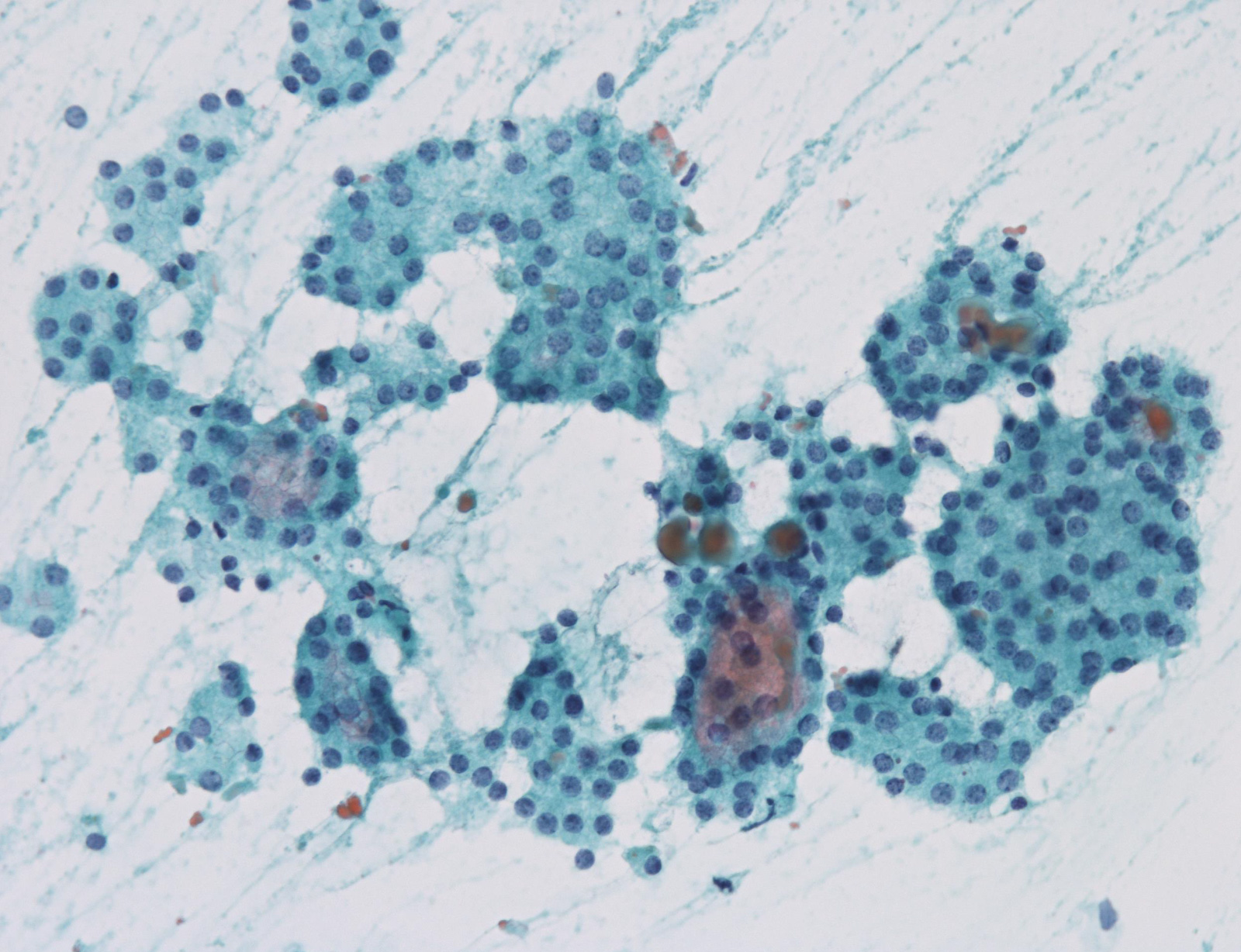
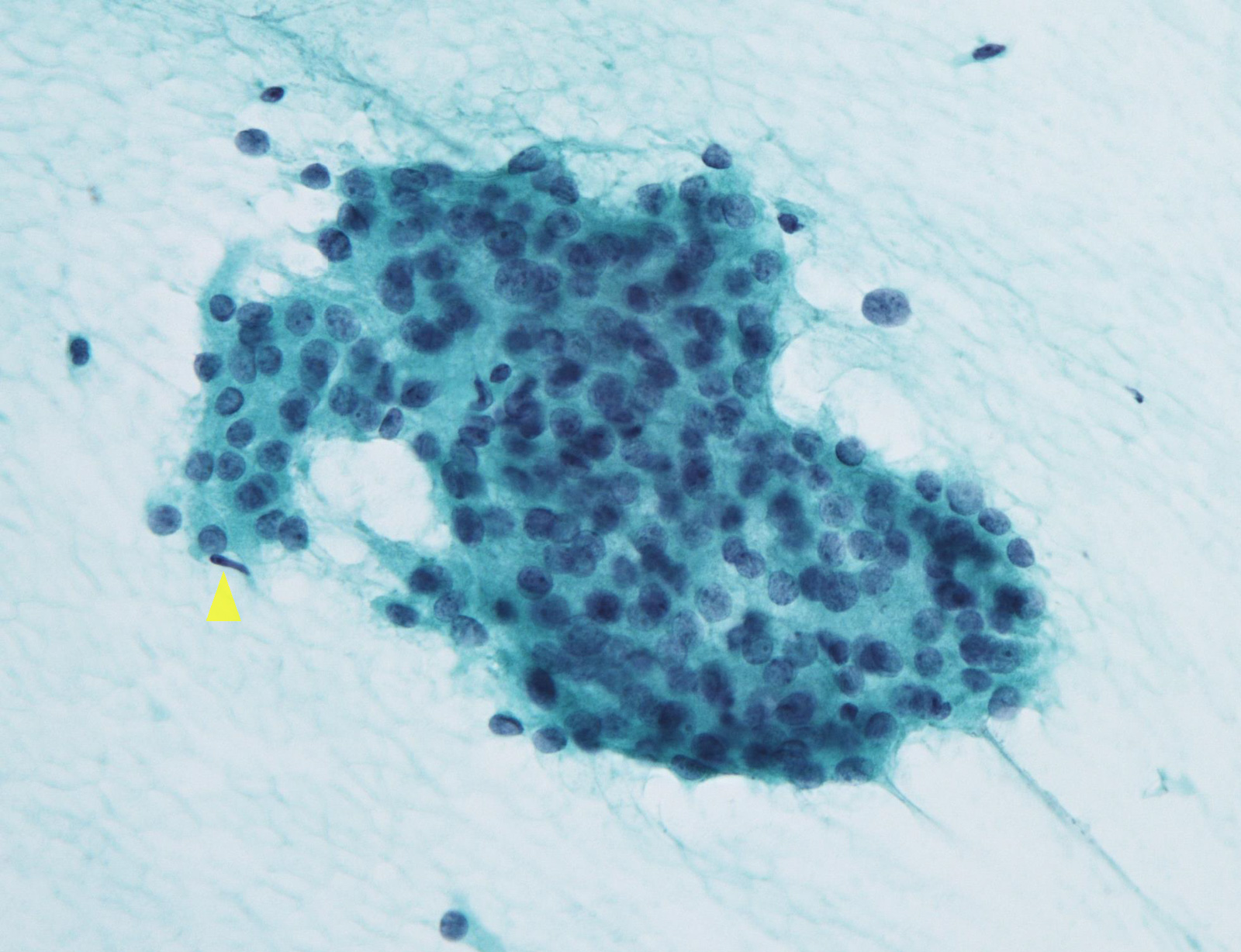
Atypia of undermined significance /
follicular lesion of undetermined significance:
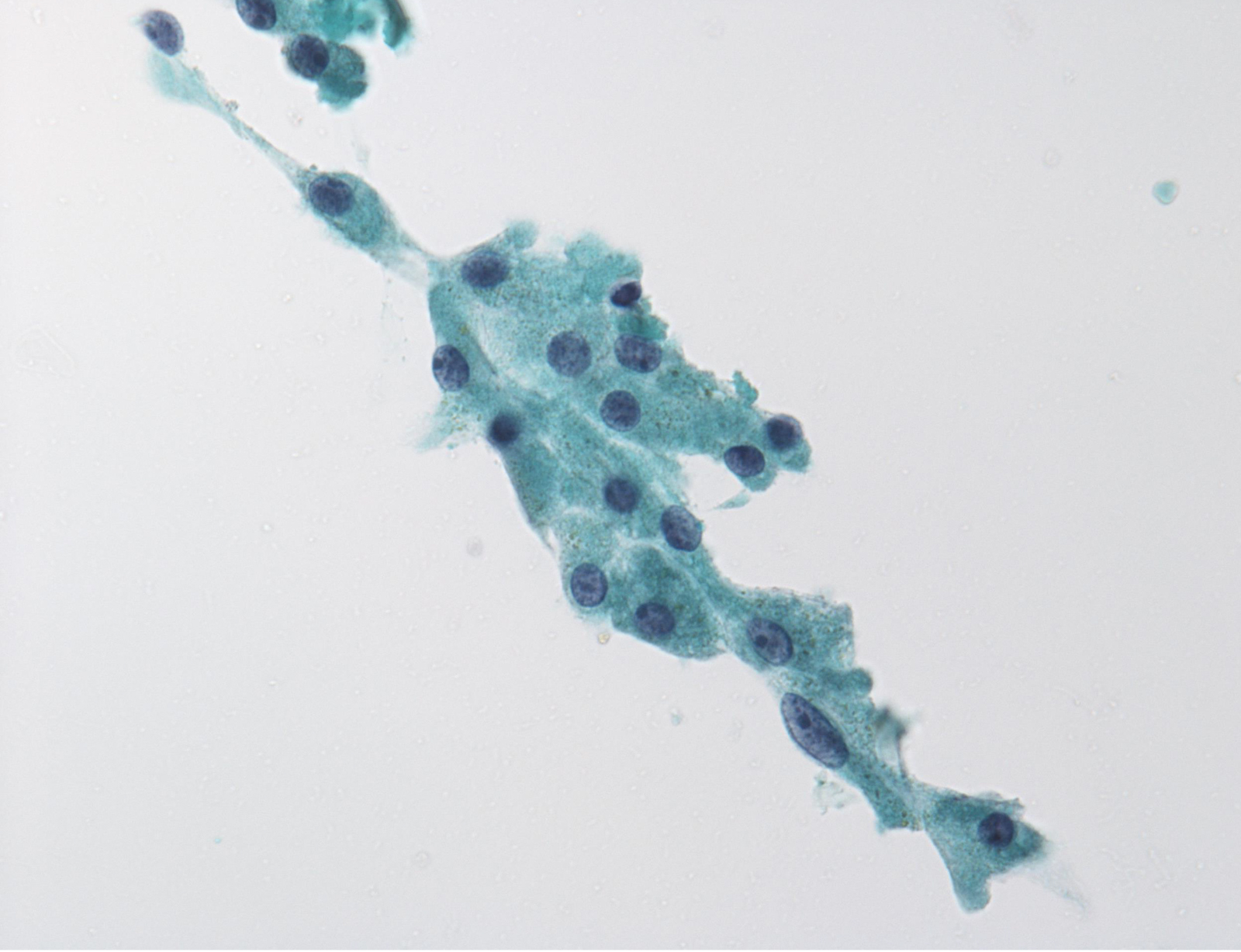
follicular lesion of undetermined significance:
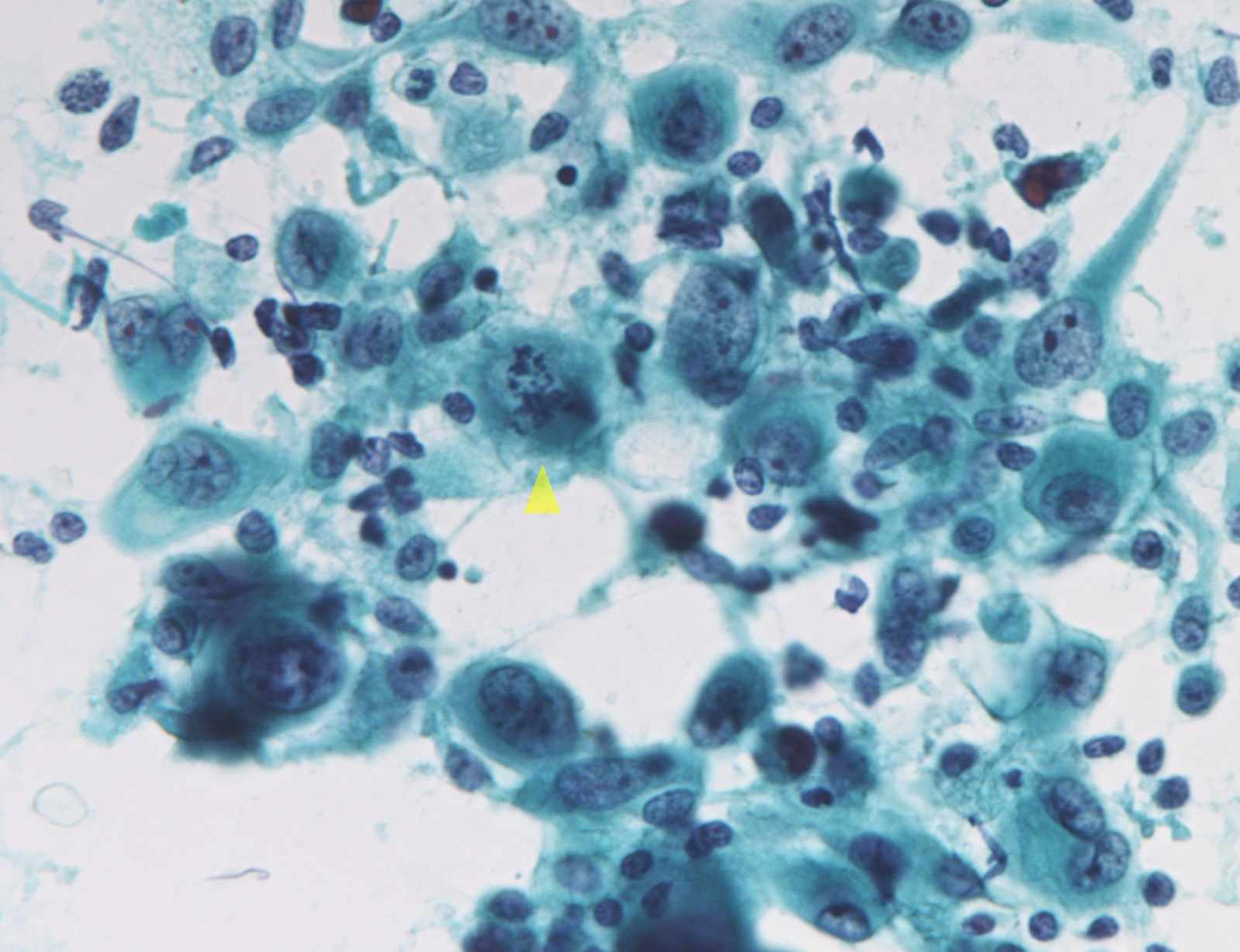
Suspicious for malignancy:
Malignant:
Videos
Algorithmic approach to thyroid FNA
Head & tail of the Bethesda system
Thyroid cytology: approach
Thyroid cytology: cases
Thyroid cytology: ND/UNS, benign and FN/SFN
Thyroid cytology: malignant, SUS and AUS/FLUS
Thyroid cytopathology
Additional references
Board review style question #1
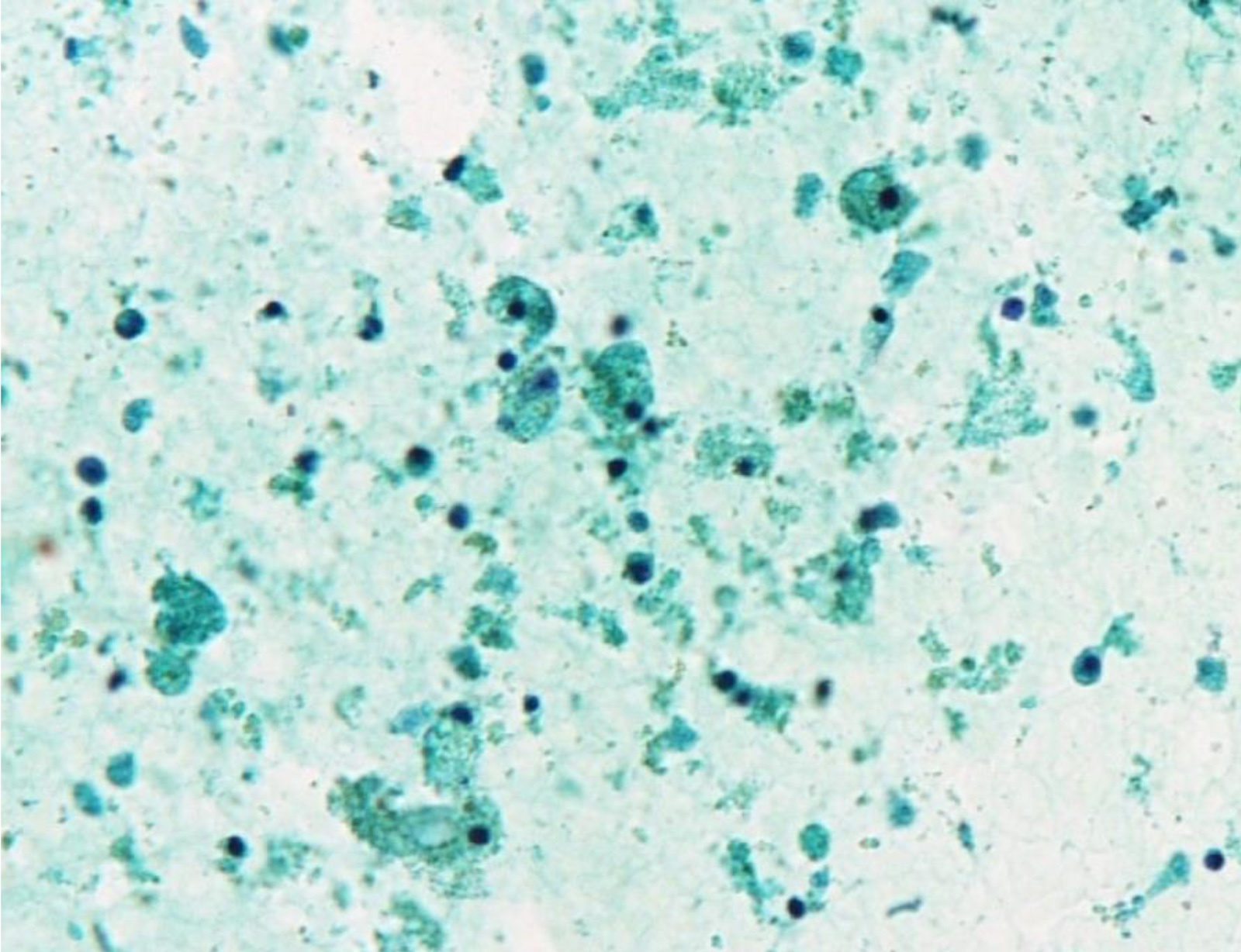
What is most likely The Bethesda System for Reporting Thyroid Cytopathology category of this thyroid aspirate?
- Atypia of undermined significance / follicular lesion of undetermined significance
- Benign
- Follicular neoplasm / suspicious for a follicular neoplasm
- Malignant
- Nondiagnostic / unsatisfactory
Board review style answer #1
E. Nondiagnostic / unsatisfactory. When the aspirated material contains only foamy histiocytes and no follicular epithelium or colloid it is qualified as nondiagnostic. However, in some local reporting systems (e.g. Japanese), these cases are reported as adequate, cyst fluid only, because their malignancy risk is almost the same as that of the benign category and lower than that in the nondiagnostic category.
Comment Here
Reference: Overview / diagnostic categories
Comment Here
Reference: Overview / diagnostic categories
Board review style question #2
Which is the most recommended management for the benign category of The Bethesda System for Reporting Thyroid Cytopathology?
- Follow up
- Molecular testing
- Reaspiration
- Thyroid lobectomy
- Total thyroidectomy
Board review style answer #2
A. Follow up. The American Thyroid Association recommends follow up based on the ultrasound pattern for a benign lesion.
Comment Here
Reference: Overview / diagnostic categories
Comment Here
Reference: Overview / diagnostic categories