Table of Contents
Definition / general | Diagrams / tables | Uses by pathologists | Summary on key mutations within tumor type | Treatment | Microscopic (histologic) images | Molecular / cytogenetics images | Videos | Additional referencesCite this page: Bychkov A. Molecular pathology-practical. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/thyroidcancerpracticalmolecpath.html. Accessed April 2nd, 2025.
Definition / general
- Thyroid cancer is a genetically simple disease with a relatively low number of mutations in each tumor
- Driver mutations and gene fusions are identified in over 90% of thyroid cancers, making it one of the best molecular characterized malignancies in humans
- MAPK and PI3K-AKT are 2 main signaling pathways involved in the development of thyroid tumors
- MAPK pathway: activated through point mutations of BRAF or RAS genes and RET / PTC rearrangements; primarily involved in papillary carcinoma
- PI3K-AKT pathway: activated through point mutations in RAS, PIK3CA, AKT1 and PTEN; primarily involved in follicular carcinoma
- Simultaneous activation of both pathways becomes more frequent as the tumor grade increases
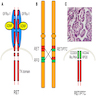
- Collectively, the most common alterations are BRAF and RAS point mutations and RET / PTC and PAX8 / PPARγ chromosomal rearrangements
- Driver gene aberrations in well differentiated thyroid cancer are mutually exclusive (median = 1 mutation per tumor)
- Dedifferentiated cancers accumulate additional genetic alterations, so called late events (median = 6 mutations per tumor)
- Chromosomal rearrangements (and resultant gene fusions) are associated with radiation
- Most somatic mutations are not thyroid specific and are commonly found in various solid cancers
- Molecular techniques are typically applied to cytological smears, formalin fixed paraffin embedded and snap frozen tissue, see details on Molecular pathology basics page
- Mutations are detected with real time PCR and DNA sequencing
- Chromosomal rearrangements are detected with FISH and RT-PCR
- Immunohistochemistry is specific for detecting mutant proteins (BRAF V600E, NRAS Q61R)
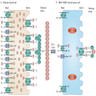
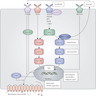
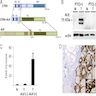
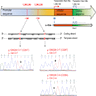
Diagrams / tables
Uses by pathologists
- Preoperative diagnosis of thyroid nodules with indeterminate FNA
- Potential prognostic value to predict aggressive disease
- Targeted therapy
Summary on key mutations within tumor type
- Follicular adenoma (FA)
- RAS 20 - 40%
- PAX8 / PPARγ 5 - 20%
- No RET / PTC translocations, BRAF V600E mutation, PTEN mutations (except germline mutations in Cowden syndrome) or PIK3CA / AKT pathway mutations
- Hyalinizing trabecular tumor
- Although early reports found RET / PTC somatic translocations with similar frequency as PTC, this was not confirmed with more robust techniques
- Absence / extreme rarity of BRAF and RAS mutations
- Follicular thyroid carcinoma (FTC)
- Mutually exclusive RAS point mutations or PAX8 / PPARγ rearrangements in 75%
- RAS 30 - 50%
- PAX8 / PPARγ 30 - 35%
- TERT 10 - 20%
- PTEN < 10%
- PIK3CA 5 - 10%
- Hürthle cell carcinoma (oncocytic variant of FTC)
- Alteration of mitochondrial DNA, including deletions, frameshift and missense point mutations
- Lower prevalence of mutations associated with nononcocytic FTC (RAS, PAX8 / PPARγ)
- GRIM19 mutations 10 - 20%
- RAS 10 - 20%
- PAX8 / PPARγ 5 - 15%, associated with follicular architecture
- TERT 15 - 20%
- TP53 up to 20%
- RET / PTC 35%, all with solid pattern of growth (based on one study)
- Papillary thyroid carcinoma (PTC)
- Mutually exclusive genetic events found in 75 - 90% cases: point mutations in BRAF and RAS, rearrangements of RET and NTRK1
- BRAF 40 - 50%
- RAS 10 - 20%
- RET / PTC 5 - 20%
- TERT 5 - 10%
- NTRK 5%
- Common PTC variants
- Classic variant: BRAF 40 - 70%, RET / PTC 5 - 40%, RAS 3 - 10%, TERT 10%, NTRK 0 - 5%
- Follicular variant: RAS 25 - 50%, PAX8 / PPARγ 5 - 30%, BRAF V600E up to 25% (invasive type), TERT 1 -10%, RET / PTC 5%, NTRK 0 - 10%, BRAF K601E < 1%
- NIFTP: RAS 30 - 45%, PAX8 / PPARγ up to 20%, THADA fusion up to 20%, EIF1AX 5%, absence of BRAF mutation and RET / PTC translocations
- Microcarcinoma: BRAF 20 - 80%, RET / PTC rearrangements and RAS mutations can be found, TERT < 5%
- Tall cell variant: BRAF 80 - 100%, TERT 20 - 30%, RET / PTC3
- Rare PTC variants (based on small series)
- Columnar variant: BRAF 33%
- Diffuse sclerosing variant: RET / PTC rearrangement frequently found, while BRAF mutation is uncommon
- Hobnail variant: BRAF V600E mutation in most cases (50 - 80%), RET / PTC1 is much rarer (up to 20%)
- Warthin-like variant: BRAF 65%
- Cribriform-morular variant: RET / PTC rearrangements, RAS mutations and BRAF mutations not identified; germline APC or CTNNB1 mutations in familial adenomatous polyposis coli syndrome
- Poorly differentiated thyroid carcinoma (PDTC)
- TERT 30 - 40%
- RAS 20 - 40%
- BRAF 5 - 30%, higher rate if arises from PTC
- EIF1AX 10%
- Rare chromosomal translocations (RET / PTC, PAX8 / PPARγ, ALK1)
- Late genetic events are common: TP53 (10 - 40%), CTNNB1 (0 - 25%) and genes that encode effectors of the PI3K-AKT signaling pathway, including PIK3CA, AKT1 and PTEN (10 - 20% collectively)
- Anaplastic thyroid carcinoma (ATC)
- Coexisting mutations (median is 6 per case)
- TP53 50 - 80%
- TERT 30 - 50%
- RAS 20 - 50%
- BRAF 20 - 45%, especially if progress from PTC
- Less common mutations: CTNNB1 5 - 65%, PIK3CA 5 - 25%, PTEN 5 - 20%, RASAL 15%, EIF1AX 10%
- Fusions, e.g., ALK, are infrequent
- Medullary thyroid carcinoma (MTC), sporadic
- Mutually exclusive RET or RAS mutations
- RET 30 - 65%, mainly RET M918T
- RAS 25% (HRAS > KRAS)
- Medullary thyroid carcinoma (MTC), hereditary
- Germline RET mutations > 95%, with predominant RET C634A in MEN2A, and RET M918T in MEN2B syndromes, respectively
Clinically significant signatures
Diagnostic molecular signatures
- Molecular testing is widely used for preoperative triage of patients with thyroid nodules indeterminate on FNA (Bethesda III - V), see Molecular testing in FNA
- Presence of certain mutations in a sample has high sensitivity and specificity for malignancy, with recommendation for total thyroidectomy instead of diagnostic lobectomy
- BRAF V600E or RET / PTC rearrangement has virtually 100% risk of malignancy, likely to be conventional or tall cell variant PTC
- RAS, PAX8 / PPARγ or BRAF K601E confers 75 - 90% risk of cancer, most likely follicular variant PTC
- TERT, p53 or PIK3CA mutation predicts thyroid cancer (almost 100% risk), particularly advanced disease with propensity for dedifferentiation and distant metastasis
- RET M918T is associated with MTC (very high accuracy)
- Single gene testing (usually BRAF V600E) is inexpensive, and can be performed using in house facilities
- Molecular panels provide the best performance
- 4 genes (BRAF V600E, RAS, RET / PTC and PAX8 / PPARγ) are essential for any thyroid panel
- Commercially available panels include early generation (8 and 15 genes) and extended (60+ genes) panels
- Mutation / fusion panels are highly sensitive for malignancy, often having over 95% positive predictive value ("rule in" cancer), however negative result of mutation test does not always predicts benign thyroid nodule; gene expression classifiers based on mRNA expression signatures provide 95% negative predictive value ("rule out" cancer)
- Combination of rule in (mutation / fusion panel) and rule out (gene expression classifier) tests is potentially the best approach to indeterminate thyroid nodules, however its cost effectiveness is doubtful
Prognostic significance
- BRAF V600E is a marker of higher tumor recurrence and tumor related mortality in PTC patients
- These patients may benefit from more extensive initial surgery with central compartment lymph node dissection to prevent tumor recurrence
- BRAF mutation is a sensitive, but not a specific marker of tumor aggressiveness
- Most patients with BRAF V600E mutation do not have recurrent disease and overall survival remains very high in both groups of patients
- TERT promoter mutations are associated with aggressive phenotype of PTC and FTC, including high persistence / recurrence and increased mortality
- Recent studies have shown that the prognostic value of TERT mutations is significantly stronger than that of BRAF V600E
- Combination of BRAF V600E mutation with TERT, AKT1, PIK3CA or TP53 mutations predicts more aggressive tumor behavior
- Patients with BRAF and TERT mutations alone had recurrence rates of 25% and 50%, respectively, whereas patients with both mutations had a recurrence rate of 70%
Treatment
Therapeutic utility
- With a high rate of targetable ("druggable") molecular abnormalities in thyroid cancer, genotyping has diagnostic and possibly therapeutic relevance
- Patients who may benefit from targeted therapy
- Radioiodine resistant differentiated thyroid cancer (metastatic PTC or FTC)
- PDTC, ATC
- MTC
- The most studied drugs are tyrosine kinase inhibitors (TKI, or MKI, multikinase inhibitors)
- MKIs block various cell surface (growth factor receptor) and intracellular (members of MAPK signaling) kinases
- Inhibition of VEGF (vascular endothelial growth factor) mediated pathways contributes to the antiangiogenic effect of MKI
- Currently, 4 kinase inhibitors are approved for treatment of differentiated thyroid cancer and MTC
- Sorafenib, an inhibitor of VEGFR1, VEGFR2, VEGFR3, RET (including RET / PTC), RAF (including BRAF V600E), and PDGFRβ (platelet derived growth factor receptor β)
- Lenvatinib blocks VEGF receptors 1, 2 and 3, FGF receptors 1 - 4, PDGFRα, RET and KIT
- Vandetanib (VEGFR2, RET, EGFR) and Cabozantinib (VEGFR2, RET, MET) are approved in the USA and EU for treatment of MTC
- MKI treatment is not curative, and patients eventually develop resistance
- Other targeted therapies currently in clinical trials:
- Selective BRAF inhibitors (Vemurafenib, Dabrafenib)
- PPARγ agonists
- ALK inhibitors (Crizotinib)
- Highly selective mTOR inhibitors
- PTEN modulators
- NTRK inhibitors
- Immune checkpoint blockade (anti-CTLA4, anti-PD1, and anti-PDL1)
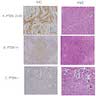
Microscopic (histologic) images
Molecular / cytogenetics images
Images hosted on other servers:
Videos
Molecular influence in thyroid cancer (2014)
Biomarkers in thyroid cancer (2015)
Drugs in development for refractory thyroid cancer (2015)
Molecular targeted therapeutics for medullary thyroid cancer (2015)
Additional references
- Articles: Nat Rev Cancer 2013;13:184, Crit Rev Oncol Hematol 2014;90:233, Endocr Relat Cancer 2014;21:T301, Cell 2014;159:676, Ann Endocrinol (Paris) 2015;76:1S8, Nat Rev Dis Primers 2015;1:15077
- Books: Nikiforov: Diagnostic Pathology and Molecular Genetics of the Thyroid, 2nd ed, 2012, Wenig: Atlas of Head and Neck Pathology, 3rd ed, 2015
- Other: CAP: Protocol for the Examination of Specimens From Patients With Carcinomas of the Thyroid Gland