Table of Contents
Definition / general | Pathophysiology | Diagrams / tables | Uses by pathologists | Molecular / cytogenetics description | Additional mechanisms and events | Molecular / cytogenetics images | Videos | Additional referencesCite this page: Bychkov A. Molecular pathology basics. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/thyroidcancermolecpath.html. Accessed April 2nd, 2025.
Definition / general
- Thyroid cancer is a genetically simple disease with a relatively low number of mutations in each tumor
- Driver mutations and gene fusions are identified in over 90% of thyroid cancers, making it one of the best molecular characterized malignancies in humans
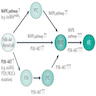
- MAPK and PI3K-AKT are 2 main signaling pathways involved in the development of thyroid tumors
- MAPK pathway: activated through point mutations of BRAF or RAS genes and RET / PTC rearrangements; primarily involved in papillary carcinoma
- PI3K-AKT pathway: activated through point mutations in RAS, PIK3CA, AKT1 and PTEN; primarily involved in follicular carcinoma
- Simultaneous activation of both pathways becomes more frequent as the tumor grade increases
- Collectively, the most common alterations are BRAF and RAS point mutations and RET / PTC and PAX8 / PPARγ chromosomal rearrangements
- Driver gene aberrations in well differentiated thyroid cancer are mutually exclusive (median = 1 mutation per tumor)
- Dedifferentiated cancers accumulate additional genetic alterations, so called late events (median = 6 mutations per tumor)
- Chromosomal rearrangements (and resultant gene fusions) are associated with radiation
- Most somatic mutations are not thyroid specific and are commonly found in various solid cancers
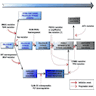
- Molecular techniques are typically applied to cytological smears, formalin fixed paraffin embedded and snap frozen tissue
- Mutations are detected with real time PCR and DNA sequencing
- Chromosomal rearrangements are detected with FISH and RT-PCR
- Immunohistochemistry is specific for detecting mutant proteins (BRAF V600E, NRAS Q61R)
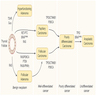
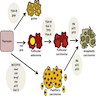
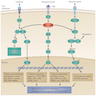
Multistep carcinogenesis
- Thyroid cancer develops through the accumulation of multiple genetic alterations and progressive derangement of signaling pathways, accompanied by numerous secondary molecular alterations in the cell and tumor microenvironment, which amplify and synergize their impacts
- Early (initiating) genetic events are BRAF and RAS mutations and RET / PTC rearrangements, while TP53 mutation is a classic late event
- BRAF mutations occur early in development of papillary thyroid carcinoma / PTC (often found in microcarcinoma); this mutation is sufficient to induce thyroid tumorigenesis
- RAS mutations are found in adenomas and play a role in early thyroid tumorigenesis, but additional genetic alterations are required to transform benign lesions into thyroid cancers, such as PTEN deletion, as shown in mouse models
- Activation of the MAPK pathway, e.g., by BRAF V600E mutation, drives carcinogenesis of the thyroid follicular cell towards papillary thyroid carcinoma (PTC)
- Activation of the PI3K-AKT pathway by mutations in RAS, PTEN and PIK3CA, primarily drives the development of follicular adenoma (FA) and follicular thyroid carcinoma (FTC) from follicular thyroid cells
- Progression from FA to FTC is largely due to increasing activation of the PI3K-AKT pathway
- As genetic alterations accumulate and intensify the signaling of both pathways, PTC and FTC can progress to poorly differentiated (PDTC) and anaplastic thyroid carcinoma (ATC)
- ATC may simultaneously have BRAF V600E, RAS mutations and RET-PTC fusions, which are otherwise mutually exclusive in well differentiated thyroid cancers
- PDTC and ATC may also develop de novo directly from follicular thyroid cells and ATC can develop from PTC or FTC
- C cell hyperplasia is a precursor of hereditary forms of medullary thyroid carcinoma (MTC) driven by activating germline RET mutations; C cell hyperplasia may progress to medullary microcarcinoma and MTC
Pathophysiology
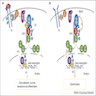
MAPK pathway
PI3K-AKT pathway
Ancillary pathways
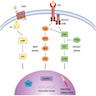
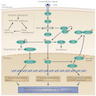
- MAPK signaling pathway mediates differentiation, survival, cell growth and metabolic activities through regulating the expression of various genes
- Also known as RAS-RAF-MEK-MAPK-ERK signaling pathway
- Transmembrane receptor tyrosine kinase RET is the gateway to the MAPK (Mitogen Actvated Protein Kinase)
- RET activation, by binding of growth factors or cytokine ligands to the extracellular domain of the receptor, results in phosphorylation of tyrosine residues in the intracellular domain and initiates a cascade of subsequent phosphorylation events, transducing a downstream signal regulating cell growth
- First targets of the cascade are RAS viral oncogene family (HRAS, NRAS and KRAS); subsequent signaling occurs by activating phosphorylation of the downstream RAF family of proto-oncogen serine / threonine tyrosine kinases (A-RAF, B-RAF and C-RAF), MEK and ERK
- Activated ERK then translocates into the nucleus, where it modulates transcription factors such as PAX8 and hormone receptors such as PPARγ, which are important to thyroid hormone biosynthesis as well as cell growth, survival and apoptosis
- In early thyroid cancer, MAPK pathway is driven by activating mutations in BRAF and RAS or by RET / PTC rearrangements, where all of them are mutually exclusive events
- Plays key role in development and progression of PTC
- Constitutively active MAPK pathway induces secondary molecular alterations that synergize and amplify the oncogenic activity of this pathway
- Upregulation of oncogenic proteins and related pathways, including VEGF, NF-kB, matrix metalloproteinases, HIF1α, TGFβ1 and various chemokines
- Genome wide hypermethylation and hypomethylation
- Collectively, indirect MAPK related events maintain cancer cell proliferation, growth and survival and drive migration, invasion, tumor angiogenesis and metastasis
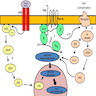
PI3K-AKT pathway
- PI3K-AKT pathway transmits and modulates signals for control of cell growth, survival and migration
- PI3K pathway starts with PI3K (phosphatidylinositol-3 kinase), which initiates downstream PI3K-AKT-mTOR viral oncogene tyrosine kinase cascade
- PI3K is activated both directly by the transmembrane receptor kinase RET and indirectly by RAS
- This pathway is downregulated by PTEN mediated dephosphorylation
- Activating mutations in RAS, PIK3CA and AKT genes or inactivating mutations in PTEN tumor suppressor gene can inappropriately stimulate signaling pathway
- PI3K-AKT pathway has a central role primarily in FTC and its invasion and metastasis
- Secondary molecular alterations, induced by PI3K-AKT signaling, are those of Wnt-β-catenin, HIF1α and NF-kB pathways
Ancillary pathways
- TSHR-cAMP pathway
- TSHR (Thyroid Stimulating Hormone Receptor) is a G protein coupled receptor that triggers cAMP signaling to promote growth and differentiation of thyroid cells
- TSHR partially mediates its effects via RAS-MAPK, PI3K and JAK-STAT kinase pathways
- Overstimulation of TSHR signaling through activating mutations in TSHR or Gsα leads to development of hyperfunctional follicular adenoma
- High serum TSH and increased TSH signaling contributes to development of thyroid cancer
- NF-kB pathway
- Controls proliferative and anti apoptotic signaling in thyroid cancer cells and has important role in the regulation of inflammatory responses linked to tumorigenesis
- Triggered by activated MAPK and PI3K pathways
- Wnt-β-catenin pathway
- Regulates cell growth, proliferation and stem cell differentiation
- β-catenin, when upregulated by upstream Wnt signaling, translocates into nucleus and transcribes various tumor promoting genes
- In thyroid cancer, activation of Wnt-β-catenin signaling is often caused by activating mutations of CTNNB1 (which encodes β-catenin), particularly in PDTC and ATC
- Upregulated PI3K-AKT signaling also causes aberrant activation of this pathway
- HIF1α pathway
- Key mediator of the response to hypoxia, when it binds to HIF1β to form the HIF1 transcription factor that induces the expression of various genes associated with cell metabolism and neoangiogenesis (e.g., VEGFA)
- HIF1α is expressed in aggressive thyroid cancers (ATC), which is consistent with a role in thyroid cancer progression
- HIF1 can be upregulated by both MAPK and PI3K-AKT pathways
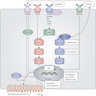
Diagrams / tables
Images hosted on other servers:
Uses by pathologists
- Preoperative diagnosis of thyroid nodules with indeterminate FNA
- Potential prognostic value to predict aggressive disease
- Targeted therapy
Molecular / cytogenetics description
Major (driver) gene mutations
BRAF mutations
RAS mutations
RET mutations
Major gene fusions (chromosomal rearrangements)
RET / PTC rearrangements
PAX8 / PPARγ rearrangement
Other mutations and fusions
BRAF mutations
- Basics
- BRAF is a serine-threonine kinase activated by RAS, which triggers MEK phosphorylation and downstream activation of MAPK-ERK cascade
- Point mutations in BRAF lead to constitutive activation of the kinase and stimulation of MAPK pathway, accompanied by the loss of negative feedback control
- Virtually all point mutations of BRAF involve codon 600 and result in the V600E mutation
- Other BRAF mutations (1 - 2%) are K601E and small in frame insertions / deletions
- BRAF V600E contributes to aggressive behavior of thyroid cancer via multiple mechanisms, e.g., induction of genetic instability, aberrant activation of the TGFβ and NF-kB pathways involved in invasion and angiogenesis, promoting of epithelial-mesenchymal transition, aberrant methylation of tumor suppressor genes, NIS inhibition and others
- Prevalence
- BRAF V600E is the most frequent genetic alteration found in PTC (40 - 60%), usually in classic and tall cell variants, but not in pediatric / solid PTC
- BRAF V600E is found in 20 - 80% of papillary thyroid microcarcinoma (mPTC), comparable to larger PTC
- There is a geographic variation in BRAF mutation prevalence; Japan and South Korea have high rates of BRAF V600E (70 - 90%) compared to Western countries (30 - 50%)
- BRAF mutations found in 20 - 40% of PDTC, 25 - 40% of ATC, but never in FTC or benign thyroid nodules
- BRAF K601E was reported in follicular variant PTC and rarely in FA
- Detection
- DNA based: direct (Sanger) sequencing, realtime PCR, next generation sequencing
- Mutant protein by VE1 immunohistochemistry
- Absent or faint staining correlates with lack of the BRAF V600E mutation, whereas strong and diffuse staining is specific to the mutation
- Equivocal cases should be resolved by molecular testing
- Utility
- Highly sensitive marker of malignancy in preoperative evaluation of indeterminate thyroid nodules (Bethesda III - V)
- Prognosis: associated with aggressive behavior of PTC (and even mPTC), e.g., extrathyroidal extension, lymph node metastasis, radioresistance, recurrence / persistence and increased mortality
RAS mutations
- Basics
- RAS genes are GTPases located at the inner surface of the cell membrane, that transmit signals arising from growth factor bound receptor tyrosine kinases and G protein coupled receptors along the MAPK, PI3K-AKT and other signaling pathways
- Harvey RAt Sarcoma viral oncogene homolog (HRAS), Kirsten rat sarcoma viral oncogene homolog (KRAS) and neuroblastoma RAS viral oncogene homolog (NRAS) are the most common oncogenes in human cancer
- Point mutations in RAS genes typically occur in codons 12, 13 and 61 and lock RAS into a constitutively active state
- Codon 61 mutations of NRAS and HRAS (3:1) are the most common in follicular derived thyroid tumors
- Although RAS is a classic dual activator of the MAPK and PI3K-AKT pathways, RAS mutations preferentially activate PI3K-AKT pathway in thyroid tumorigenesis
- Prevalence
- 30 - 50% of FTC, with lower incidence in oncocytic variant
- 10 - 20% of PTC, virtually all are follicular variant PTC
- PDTC (20 - 40%) and ATC (10 - 50%)
- RAS mutations are also found in 20 - 40% of FA
- HRAS and KRAS mutations occur in MTC (25%)
- Detection
- DNA based: direct (Sanger) sequencing, realtime PCR, next generation sequencing
- NRAS Q61R immunohistochemistry, evaluation is similar to the VE1 above
- Utility
- Diagnostic value of RAS mutations to predict malignancy in thyroid nodules is lower than that of BRAF, because RAS mutations are also found in benign neoplasms
- Differentiated thyroid cancers harboring RAS mutations alone have excellent prognosis
RET mutations
- Basics
- RET (REarranged during Transfection) proto-oncogene encodes receptor tyrosine kinase that is expressed in thyroid C cells, but not in follicular cells
- RET mutation causes sporadic and hereditary / syndromic MTC (prevalence ratio 3:1), the latter is represented by MEN2A (multiple endocrine neoplasia type 2A), MEN2B (multiple endocrine neoplasia type 2B) and familial MTC
- Prevalence
- RET gene is mutated in MTC, both familial and sporadic (25 - 70%)
- Somatic RET M918T mutation is the most common mutation in sporadic MTC (75 - 95% of all somatic RET mutations)
- Germline RET M918T mutation is the most common mutation in MEN2B; in MEN2A and familial MTC, mutations in RET typically occur in one of five cysteine codons within the cysteine rich extracellular domain
- Detection
- Direct sequencing of the whole RET gene for the detection of germline mutations (DNA is extracted from peripheral blood lymphocytes or buccal swab cells)
- Sequencing or real time PCR for RET M918T detection in sporadic cases
- Utility
- Detection of RET M918T in FNA aspirate predicts MTC with high accuracy
- There are several groups of RET mutations based on aggressiveness of MTC: highest risk (RET M918T), high risk (RET C634 and A883F) and moderate risk (including but not limited to RET C609, C611, C618, C620 and V804 mutations)
Major gene fusions (chromosomal rearrangements)
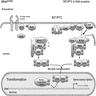
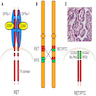
RET / PTC rearrangements
- Basics
- RET gene can fuse with various partners, which lead to the constitutive activation of RET kinase and downstream MAPK and PI3K-AKT pathways
- The most common rearrangement types are RET / PTC1 (formed by fusion of RET with the CCDC6 gene) and RET / PTC3 (formed by fusion of RET with the NCOA4 gene)
- 20+ types of RET / PTC translocation have been described
- Prevalence
- RET / PTC1 and RET / PTC3 rearrangements are found in 10 - 20% of adult sporadic PTC
- Recent studies found lower incidence of RET fusions in PTC (2 - 7%)
- Affected by technical issues (applications and sensitivity cutoffs) and geographic variation
- RET / PTC rearrangements are frequently found in children / young adults (40 - 70%); also in radiation induced PTC (50 - 80%), likely due to the young age of this cohort
- RET / PTC rearrangements usually occur in classic PTC, but are also found in follicular variant PTC and in some FA
- The type of RET rearrangement correlates with tumor morphology: RET / PTC1 is typically found in classic PTC and mPTC, while RET / PTC3 is associated with solid pattern (pediatric or radiation induced)
- Not found in follicular tumors, benign thyroid nodules and Hashimoto thyroiditis (contrary to early findings, which were misinterpreted due to technical limitations)
- Detection
- RT-PCR (fresh or frozen tissue), FISH (FFPE), Southern blot
- These ultrasensitive techniques have inherent risk of false positives, which requires cautious evaluation with pre established cutoffs and rigorous quality control
- NGS (e.g., MassArray based)
- Immunohistochemistry: reliable anti-RET antibody is not currently available
- RT-PCR (fresh or frozen tissue), FISH (FFPE), Southern blot
- Utility
- Diagnostic: presence of RET / PTC rearrangement predicts almost 100% risk of malignancy in thyroid FNA
PAX8 / PPARγ rearrangement
- Basics
- PAX8 / PPARγ rearrangement is a fusion between PAX8 (paired domain transcription factor) and PPARγ (peroxisome proliferator activated receptor) genes
- PAX8 / PPARγ exerts a dominant negative effect on the tumor suppressor PPARγ and also transactivates certain PAX8 responsive genes
- Prevalence
- 30 - 40% of FTC with lower prevalence in oncocytic variant (5 - 15%)
- Follicular variant PTC (5 - 30%) and FA (5 - 20%)
- Detection
- FISH, RT-PCR, NGS
- Immunohistochemistry with anti-PPARγ antibody is an ancillary / screening tool, only strong and diffuse staining should be considered as positive (still requires molecular verification)
- Utility
- Diagnostic value to predict malignancy is insufficient for using PAX8 / PPARγ as a sole marker (similar to RAS mutations), can be a part of molecular panel instead
Other mutations and fusions
- AKT1 mutations
- Late event
- Reported in metastatic thyroid cancer, PDTC and ATC (5 - 10%)
- ALK fusions
- ALK (anaplastic lymphoma kinase) fusion proteins activate different signaling pathways, including PI3K and MAPK pathways
- ALK rearrangements are found in radiation induced thyroid cancer (50% in small series)
- 5 - 10% of ATC / PDTC, 1% of sporadic PTC
- Detected by FISH, RT-PCR, NGS
- ALK immunohistochemistry can be a screening tool
- BRAF fusions
- Fusion of BRAF with AKAP9 (A kinase anchor protein 9) is rarely found in sporadic thyroid cancer
- Higher frequencies (up to 11%) in patients with a history of radiation exposure
- CTNNB1 mutations
- CTNNB1 proto-oncogene encodes β-catenin, a member of Wnt signaling pathway
- Point mutations in exon 3 of CTNNB1 stabilize the protein by making it insensitive for adenomatous polyposis coli (APC) induced degradation, leading to the accumulation of β-catenin in the nucleus and constitutive transcription of its target genes
- Somatic CTNBB1 mutation is late event, found in up to 25% of PDTC and 60% of ATC, but very rare in well differentiated thyroid cancer, mainly associated with cribriform-morular variant PTC
- Immunohistochemistry with anti-β-catenin shows translocation of the protein from membranous pattern (typical for normal thyroid cells), to cytoplasmic and nuclear staining in tumor cells, which is highly specific for PTC cribriform-morular variant
- EIF1A mutations
- Point mutations in EIF1AX (eukaryotic translation initiation factor 1A, X linked) gene were recently identified in 1.5% of PTC in a mutually exclusive fashion with other driver events
- Associated with RAS and adverse prognosis in PDTC and ATC
- NTRK rearrangements
- NTRK1 (neurotrophic tyrosine kinase receptor type 1) is a receptor tyrosine kinase, which may activate MAPK signaling when rearranged with one of three potential fusion partners
- NTRK1 rearrangements are found in 1 - 5% of PTC and at higher frequencies in patients with radiation exposure
- ETV6-NTRK3 fusion was found in 2% of sporadic and 15% of radiation associated PTC
- ETV6-NTRK3 rearrangement is an essential feature of the MASC of thyroid
- Detected by FISH, RT-PCR, NGS
- PIK3CA mutations
- Activating mutations in PIK3CA typically occur at hotspots within exons 9 and 20
- Late event
- Found in FTC (5 - 10%), PDTC (10%) and ATC (10 - 20%)
- PTEN mutations
- PTEN is tumor suppressor gene, whose product terminates PI3K-AKT signaling
- Inactivating somatic PTEN mutations are infrequently found in follicular thyroid tumors (PTC 1 - 2%, FTC 10%) and ATC (10 - 20%)
- Germline mutations of PTEN cause Cowden syndrome, characterized by multiple hamartomas and increased risk of certain cancers, including breast cancer and FTC
- Loss of PTEN expression by immunostaining in adenomatous and neoplastic thyroid nodules is sensitive and specific for Cowden syndrome (can be used as a screening tool prior to genotyping)
- RASAL1 mutation
- RASAL1 is a negative modulator of RAS, which suppresses RAS coupled MAPK and PI3K pathways
- RASAL1 can be inactivated via mutations or hypermethylation (mutually exclusive)
- 15% of ATC, rare in PTC and FTC (3 - 5%)
- RASAL1 germline alteration, predicted to be pathogenic, was identified in 1% of patients with Cowden-like syndrome who developed FTC without PTEN germline alteration
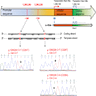
- TERT promoter mutations
- TERT gene encodes telomerase reverse transcriptase, which adds telomeric repeats to the ends of chromosomes and thus contributes to immortalization of stem or cancer cells
- Mutations in TERT promoter (C228T and C250T) enhance transcriptional activity of the promoter
- TERT C228T and C250T (prevalence 3.5:1) are mutually exclusive
- Associated with BRAF V600E mutation, functionally explained by activation of TERT transcription via MAPK pathway
- PTC 10%, FTC 15%, with lower incidence in Asian series enriched with BRAF V600E, i.e. the higher the rate of BRAF, the lower the TERT incidence
- Highly prevalent in aggressive thyroid tumors, e.g., PDTC (30 - 40%), ATC (30 - 50%), also widely invasive oncocytic carcinoma and tall cell variant PTC (20%)
- Not found in benign thyroid lesions and MTC
- Detected by DNA based techniques (direct sequencing, next generation sequencing)
- Diagnostic value: 100% specific for malignancy
- Prognostic significance: associated with disease persistence / recurrence, distant metastasis, radioresistance and higher mortality in well differentiated thyroid cancer (PTC and FTC)
- TP53 mutations
- TP53 / p53 is a tumor suppressor that plays important role in cell cycle regulation and DNA repair
- Most commonly mutated tumor suppressor gene in human cancer
- Wild (nonmutated) gene arrests cell cycle and activates apoptosis in response to DNA damage and diverse cellular stresses
- Virtually all mutations are located in hot spot region between exons 5 and 9, including codon 273, the most commonly affected
- Inactivating mutation in TP53 is a late event in thyroid cancer progression, which determines tumor dedifferentiation
- Occur at high frequency in PDTC (10 - 40%) and ATC (50 - 80%)
- Recently, TP53 mutations were found in some PTC and 22% of oncocytic FTC (small series)
- Detected by DNA based techniques (direct sequencing, realtime PCR, next generation sequencing)
- p53 immunoreactivity represents nuclear accumulation of the mutant TP53 (often evident in PDTC-ATC) and often, but not always, correlates with the molecular detection of TP53 mutation
- Well differentiated thyroid cancers carrying TP53 mutation have a potential for tumor dedifferentiation and more aggressive clinical course
- TSH signaling pathway mutations
- Somatic TSHR mutations frequently occur in autonomously functioning thyroid nodules / adenomas (> 50%)
- Also found in extremely rare cases of "toxic" thyroid carcinomas
- Mutations in GNAS, a gene which encodes α subunit of heterotrimeric G protein complexes, occur predominantly in benign hyperfunctioning nodules, but with much lower rate (5%) than TSHR mutations
- Rarely reported mutations
- IDH1 in FTC, PTC and ATC (10 - 20% collectively)
- EGFR in classic variant PTC (5%)
Additional mechanisms and events
mRNA expression
miRNA
lncRNA
Copy number variations
Epigenetics
Genetic susceptibility
- Transcriptomic studies (mainly based on DNA chip / microarray technology) found altered mRNA expression profiles in various thyroid tumors
- Cancer initiating mutations and subsequent activation of MAPK or PI3K-AKT pathways cause altered expression of hundreds of genes involved in cell differentiation, energy metabolism, cell adhesion, etc.
- mRNA microarrays find genes differentially expressed between benign and malignant thyroid and produce a gene signature (set of genes) for differential diagnosis, used in preoperative diagnosis of indeterminate thyroid nodules (e.g., Afirma gene expression classifier)
miRNA
- Micro-RNAs (miRNAs) are small, noncoding RNA genes composed of 21 - 25 nucleotides
- miRNAs may regulate pathway signaling, cell differentiation, invasion and metastasis by fine tuning gene expression
- Overexpression of oncogenic miRNAs (miR-21 and miR-146b) in BRAF V600E PTC and inhibition of tumor suppressor miRNAs (let-7 family, miR-204 and miR-375) are implicated in thyroid cancer
- OncomiRNAs miR-221 and miR-222 may play a role in PTC aggressiveness and are associated with less differentiated tumors
- High expression levels of miR-146b correlate with lower overall survival in PTC
- miRNA expression profiles differ between neoplastic and nonneoplastic thyroid tissue, which may have potential diagnostic utility
lncRNA
- Long noncoding RNAs refers to nonprotein encoding transcripts 200 nucleotides in length or larger
- Current data on their role in thyroid cancer are scarce, with only a few markers identified (HOTAIR, PTCSC2, PTCSC3)
Copy number variations
- Copy number variation / alteration is a genetic aberration in which the copy number of a chromosomal region or gene is different from the normal two copies, due to gene gain or loss (through chromosomal instability, aneuploidy, amplification or deletion)
- Detected by array based comparative genomic hybridization (aCGH)
- Copy number gains in genes encoding members of the PI3K-AKT pathway may activate downstream signaling
- In PTC, copy number alterations are detected with a relatively low frequency (25%) and are more common in PTC follicular variant and NIFTP; gene losses / deletions are more prevalent than gains / amplifications
Epigenetics
- Aberrant methylation occurs via promoter hypermethylation (gene silencing) and hypomethylation (gene overexpression)
- In PTC, the BRAF V600E mutation drives hypermethylation / silencing of important tumor suppressor genes (TIMP3, SLC5A8, DAPK1, RARB) and genes of iodide handling machinery (e.g., NIS, TSHR)
- In FTC and ATC, activation of the PI3K-AKT pathway causes hypermethylation and silencing of PTEN, which in turn leads to failure to terminate the PI3K-AKT signaling and creates a self amplifying loop
- Epigenetic events are important drivers of aggressive thyroid cancers, e.g., ATC
- Certain methylation markers are useful for early detection of thyroid cancer and its subtypes
- Histone modification (aberrant acetylation, methylation) is another epigenetic mechanism implicated in progression of PDTC and ATC, which can be targeted by drugs
Genetic susceptibility
- Genome Wide Associated (GWAS) and candidate gene studies found a strong association / inherited predisposition of some single nucleotide polymorphisms (SNPs) with thyroid tumors
- These polymorphic sites may affect the enhancer activity of genes or gene regulation
- Risk of inherited susceptibility to the cancer increases with the presence of several risk alleles in an individual
- SNPs inside (rs1867277) and upstream (rs965513) of FOXE1 gene on chromosome 9q22.33, which encodes thyroid transcription factor TTF2, are strongly associated with sporadic and radiation induced thyroid cancer; the potential mechanism is activation of FOXE1 transcriptional activity by the risk allele
- SNPs rs944289 (area of the NKX2-1 gene, which encodes TTF1) and rs116909374 on chromosome 14q13.3 predispose to thyroid cancer, the former locus is also associated with FA risk
- More susceptibility SNPs were found at 2q35 (rs966423 in DIRC3), 8p12 (rs2439302 in NRG1) and 8q24 (multicancer SNP rs6983267 in a gene poor region)
- Several SNPs in RET gene are implicated in the increase of MTC risk
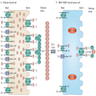
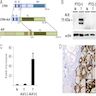
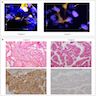
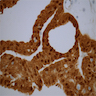
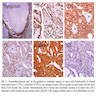
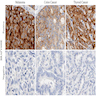
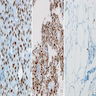
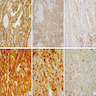
Molecular / cytogenetics images
Images hosted on other servers:
Videos
Genetics and genomics of thyroid neoplasms (2013) by Dr. Electron Kebebew, NCI
Integrated genomic characterization of papillary thyroid carcinoma (2014) by Prof. Tom Giordano, University of Michigan
Advances in molecular pathogenesis of thyroid cancer (2014) by Prof. Pilar Santisteban, Universidad Autónoma de Madrid
Molecular influence in thyroid cancer (2014) by The American Head and Neck Society
Understanding the biology of medullary thyroid cancer (2015) by Prof. Gilbert Cote, MD Anderson Cancer Center
Molecular targeted therapeutics for medullary thyroid cancer (2015) by Dr. Ann Gramza, NCI
MAPK pathway
MAPK pathway
PI3K-AKT pathway
PI3K-AKT pathway
PI3K-AKT pathway
Additional references
- Articles: Nat Rev Cancer 2013;13:184, Crit Rev Oncol Hematol 2014;90:233, Endocr Relat Cancer 2014;21:T301, Cell 2014;159:676, Ann Endocrinol (Paris) 2015;76:1S8, Nat Rev Dis Primers 2015;1:15077
- Books: Nikiforov: Diagnostic Pathology and Molecular Genetics of the Thyroid, 2nd ed, 2012, Wenig: Atlas of Head and Neck Pathology, 3rd ed, 2015
- Other: CAP: Protocol for the Examination of Specimens From Patients With Carcinomas of the Thyroid Gland