Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1Cite this page: Siegmund S, Anderson W, Acosta A. Large cell calcifying Sertoli cell tumor. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/testissertolilargecell.html. Accessed April 2nd, 2025.
Definition / general
- Large cell calcifying Sertoli cell tumor (LCCSCT) was first described by Lange et al. in 1960 (J Urol Medicale Chir 1960;66:259)
- Published by Proppe and Scully in 1980 (Am J Clin Pathol 1980;74:607)
- Approximately 130 cases reported (to date) in English language literature
Essential features
- Generally presents as a benign sex cord stromal tumor found predominantly in patients < 20 years old; a subset presents in older patients and may behave aggressively
- Associated with Carney complex in 20 - 40% of cases due to alterations in the gene PRKAR1A
- Characterized by intratubular and sheet-like growth of large eosinophilic cells with laminated psammomatous, mulberry-like or dystrophic calcifications, a prominent neutrophilic infiltrate and lymphocytic rim
ICD coding
- ICD-10: D29.20 - Sertoli cell tumor, male
Epidemiology
- Male patients, generally < 20 years old
- 20 - 40% of cases associated with Carney complex due to mutations in PRKAR1A gene (part of Carney complex with spotty cutaneous pigmentation, primary pigmented nodular adrenocortical disease, atrial myxomas, superficial angiomyxomas, malignant melanocytic nerve sheath tumors, blue nevus and thyroid carcinoma)
- Debatable association with Peutz-Jeghers syndrome (rare reports, may represent intratubular large cell hyalinizing Sertoli cell neoplasia) and neurofibromatosis (Am J Surg Pathol 2021 Dec 16 [Epub ahead of print])
Sites
- Testis
- Multifocal and bilateral testicular involvement in > 50% of patients with Carney complex (J Urol 2002;167:1299)
Clinical features
- Majority of cases present as painless testicular mass, and less commonly as testicular pain, gynecomastia or precocious pseudopuberty (Am J Surg Pathol 2021 Dec 16 [Epub ahead of print])
Diagnosis
- Definitive diagnosis by histopathological examination
Prognostic factors
- Kratzer et al. proposed malignant prognostic features that include age > 25 years old and 2 or more of the following adverse features (Am J Surg Pathol 1997;21:1271):
- Size > 4 cm
- Extratesticular extension
- Mitotic index > 3/10 high power fields (HPFs)
- Coagulative tumor necrosis
- Vascular invasion
- High grade cytologic atypia
- Prepubertal status may provide a protective effect (Am J Surg Pathol 2021 Dec 16 [Epub ahead of print])
- Tumors associated with Carney complex may behave in biologically indolent fashion compared with sporadic tumors (Eur Urol 2001;40:699)
Case reports
- 10 year old boy with sporadic bilateral LCCSCT (Ann Clin Lab Res 2015;3:2)
- 19 year old man with Carney complex treated with partial orchiectomy (BMJ Case Rep 2017;2017:bcr2017219557)
- 42 year old man with metastatic LCCSCT (J Endocr Soc 2019;3:1375)
- 62 year old man with locally invasive LCCSCT (J Clin Diagn Res 2016;10:ED03)
Treatment
- Partial or radical orchiectomy for organ confined disease
- Retroperitoneal lymph node dissection with possible adjuvant chemotherapy or radiation therapy for metastatic disease (benefit unknown)
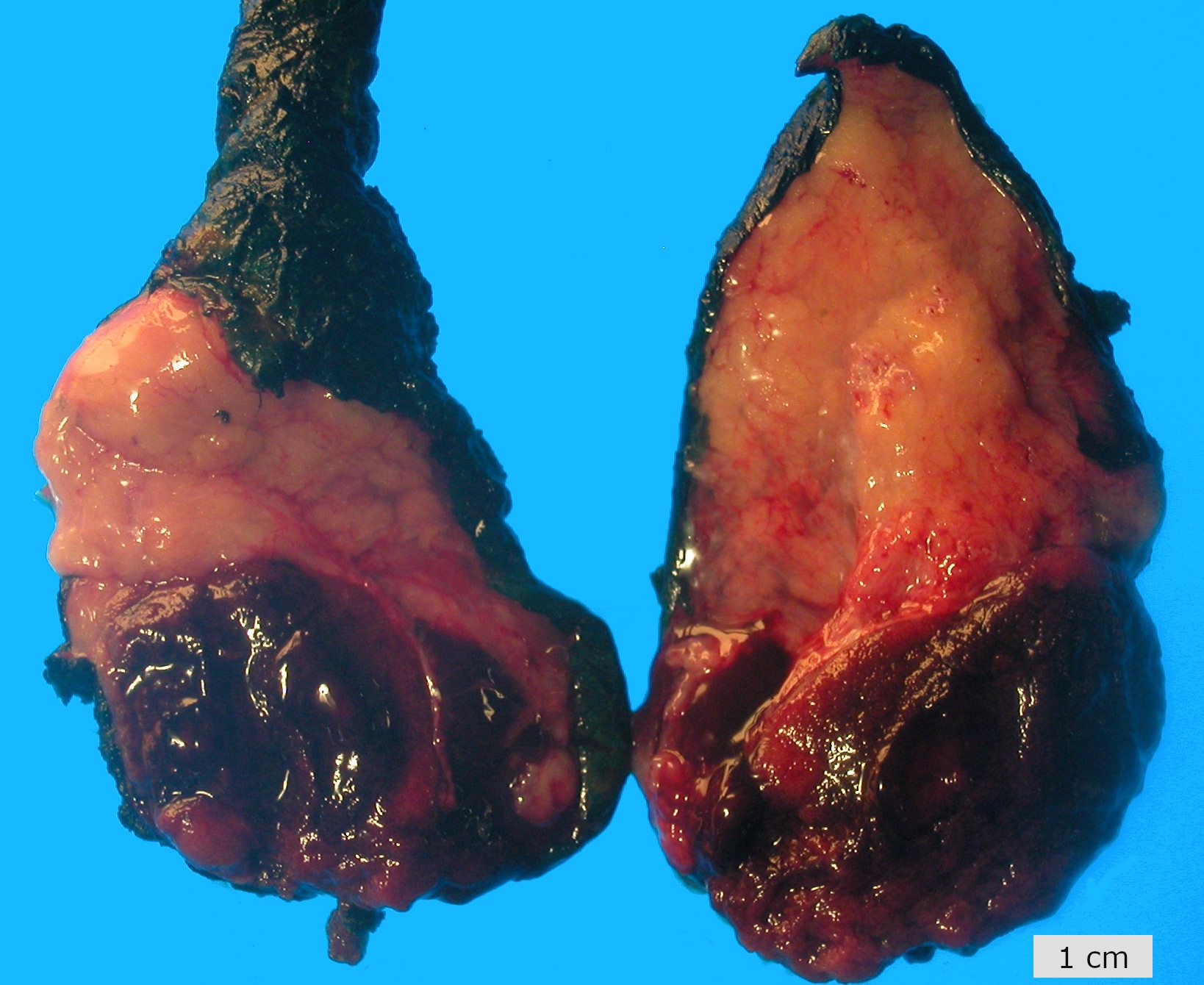
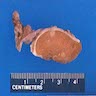
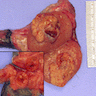
Gross description
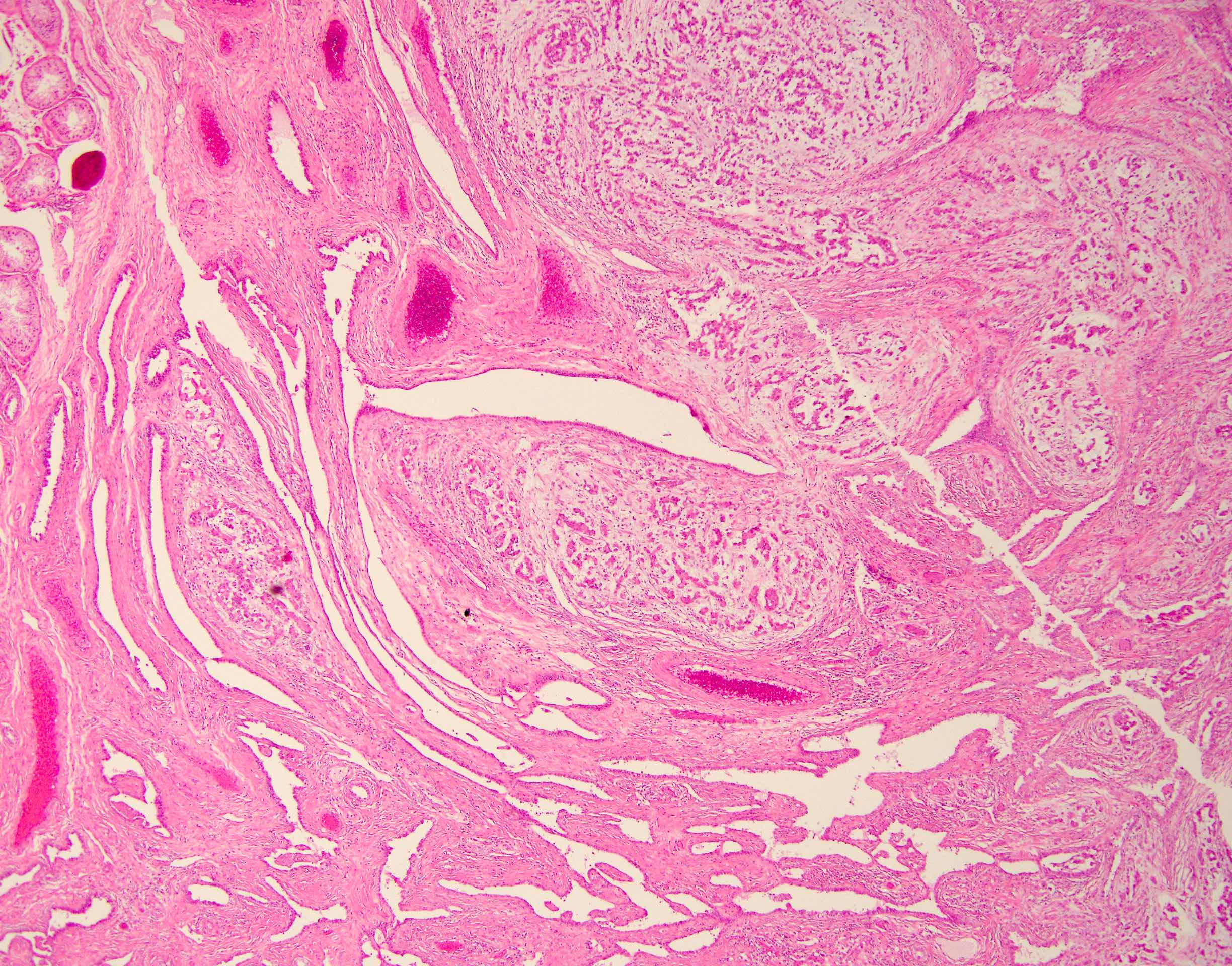
- Nodular, yellow-gray, firm to hard gritty tumors generally < 2 cm in diameter
- Generally well circumscribed but occasionally infiltrative into adjacent paratestis
- Malignant cases more likely to present as fleshy masses with tumor necrosis
- References: Am J Surg Pathol 2021 Dec 16 [Epub ahead of print], Histopathology 2022;80:677
Gross images
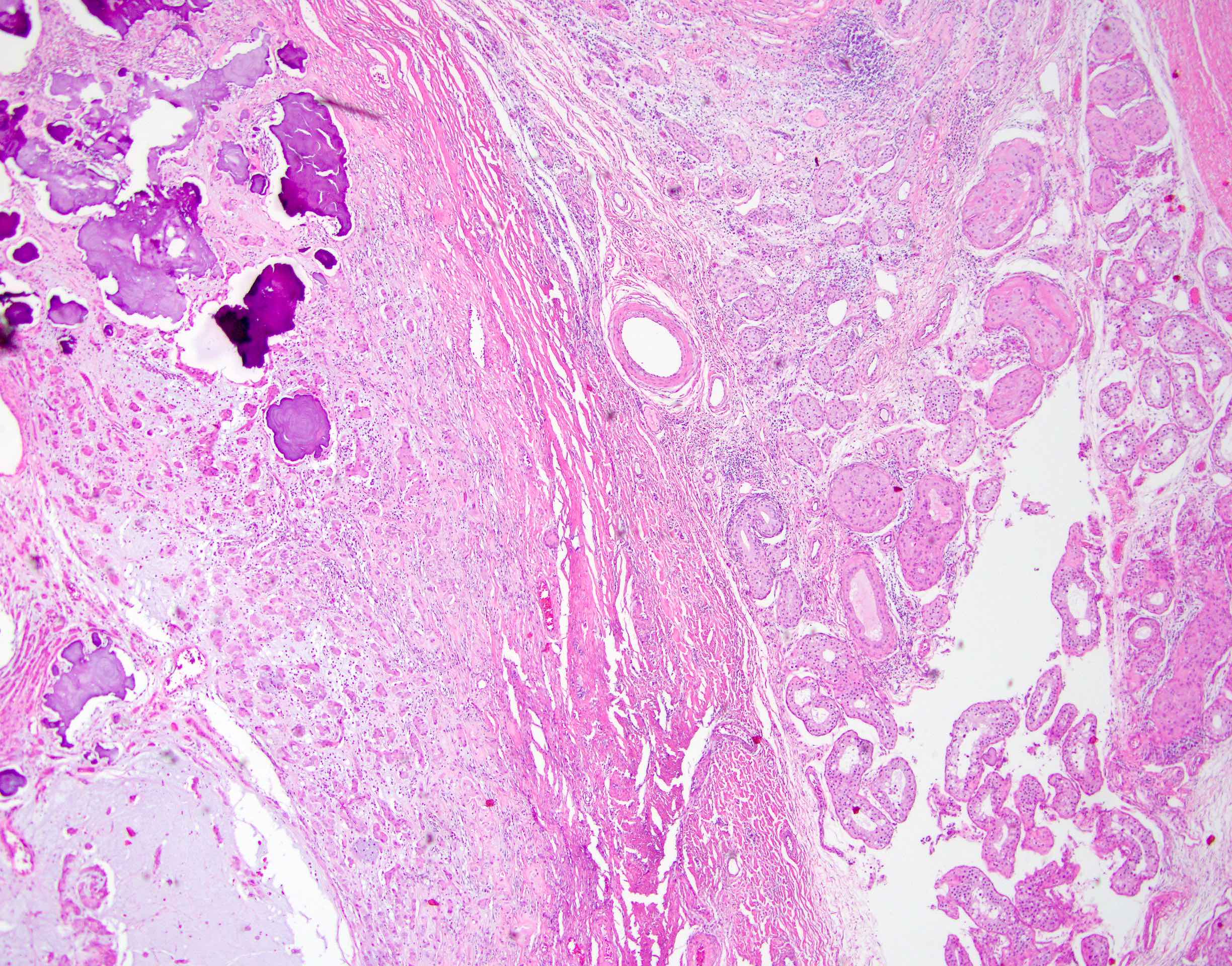
Microscopic (histologic) description
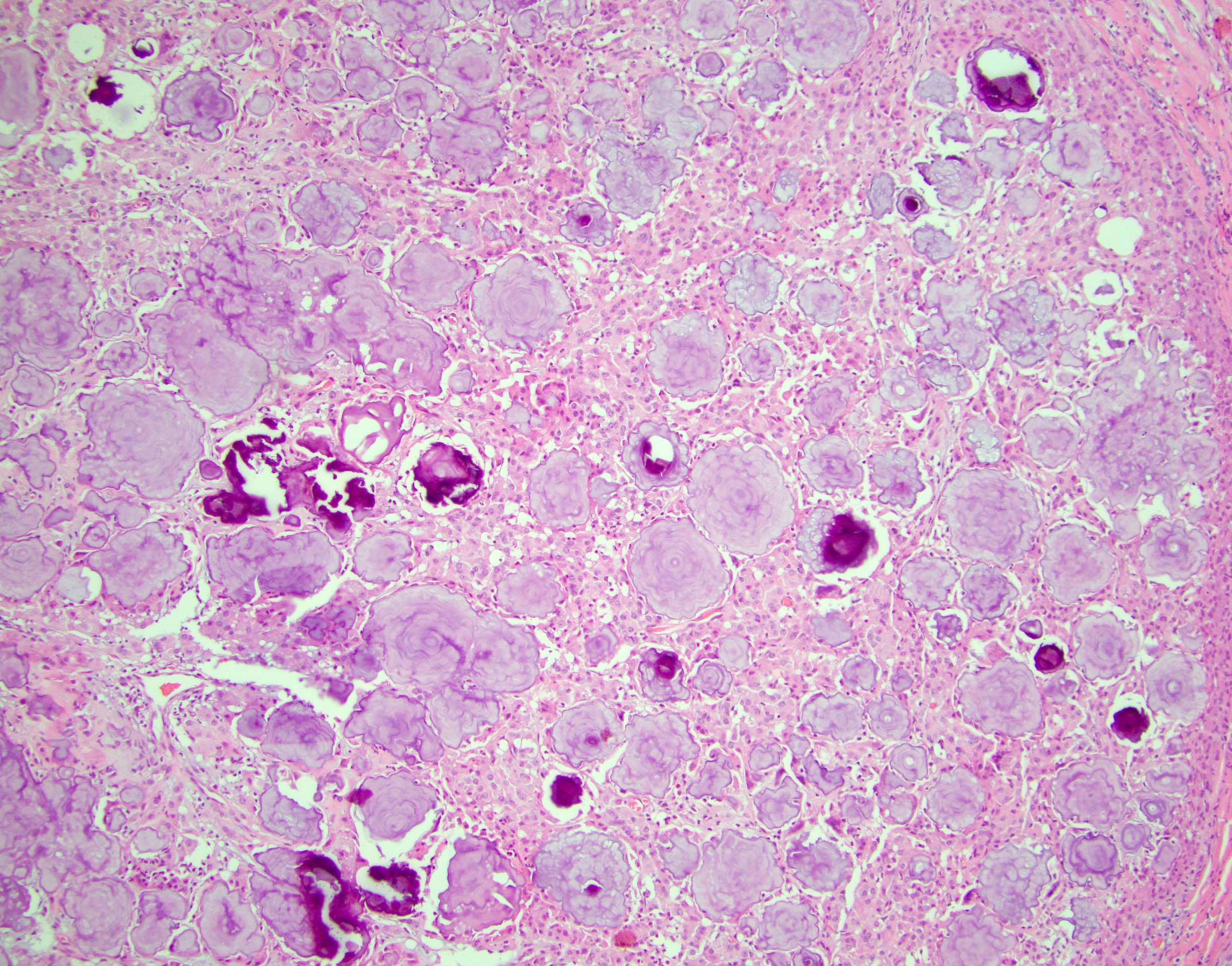
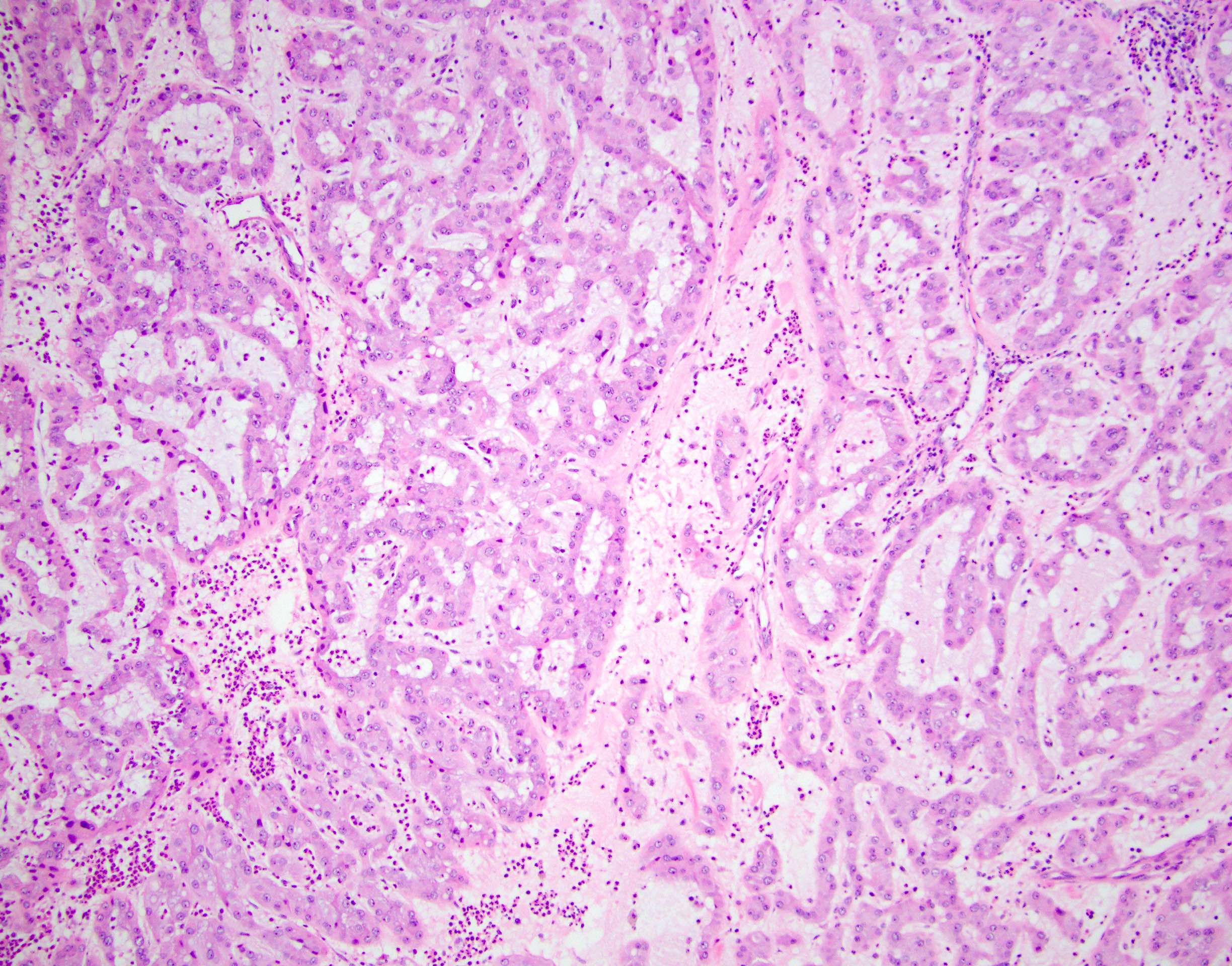
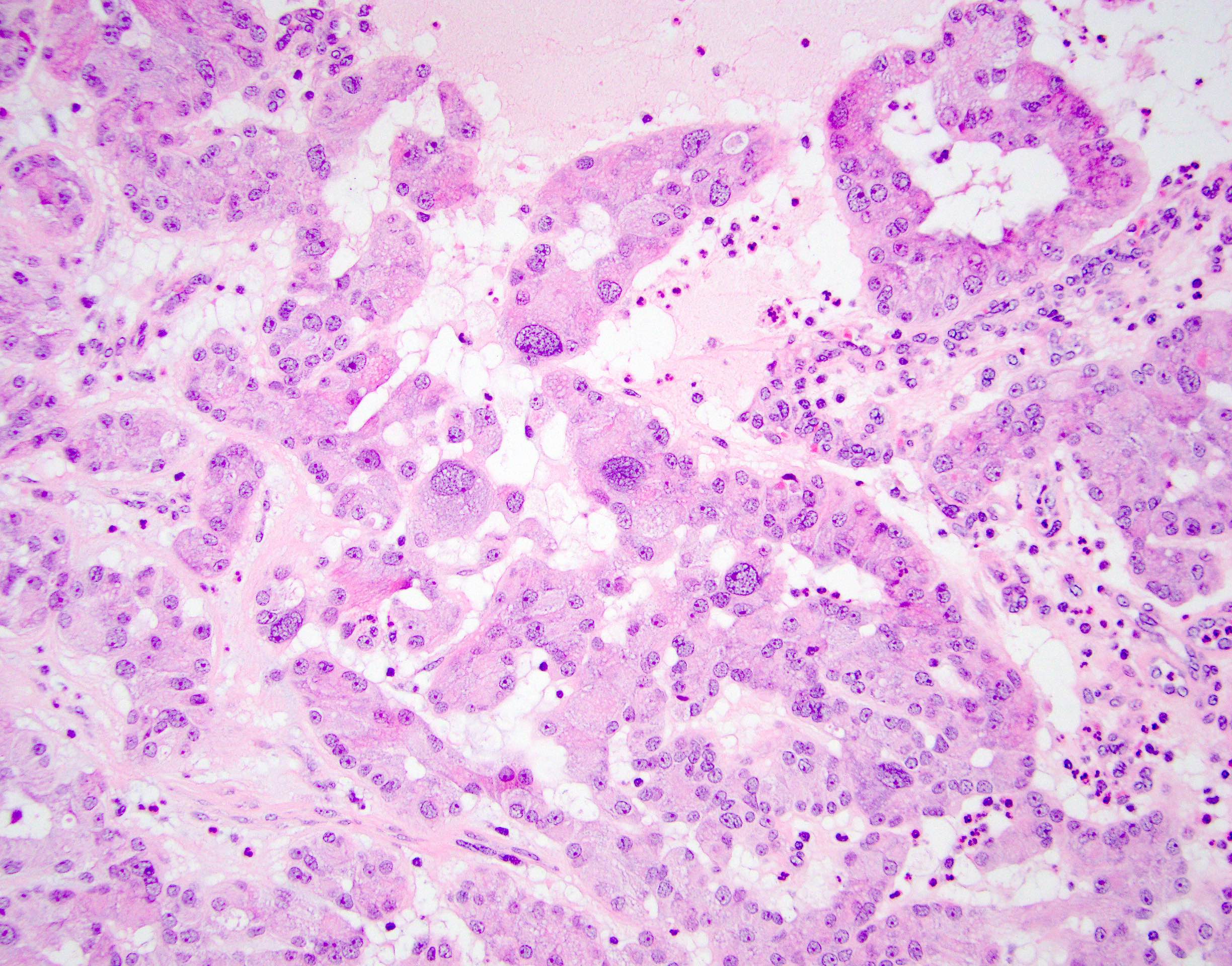
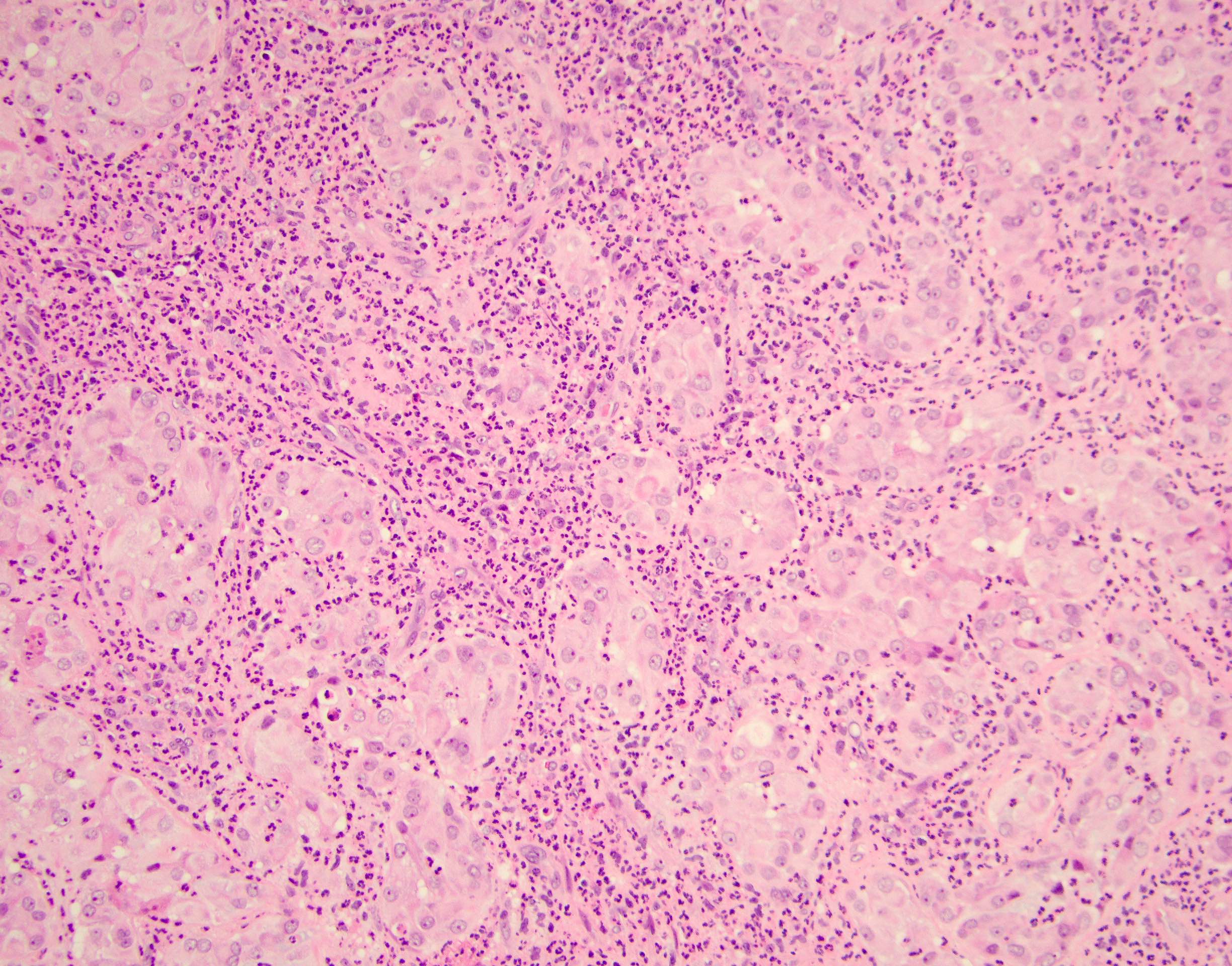
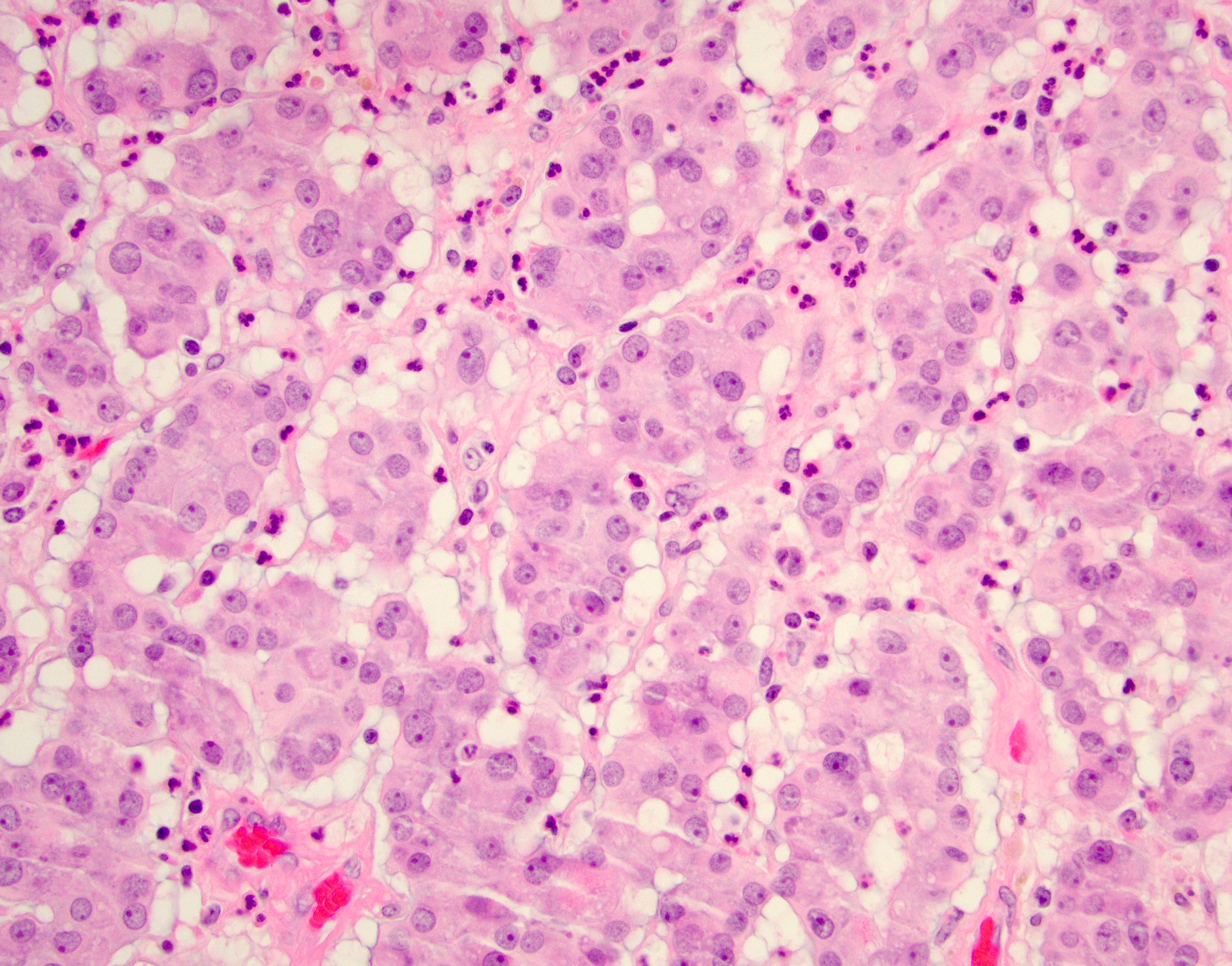
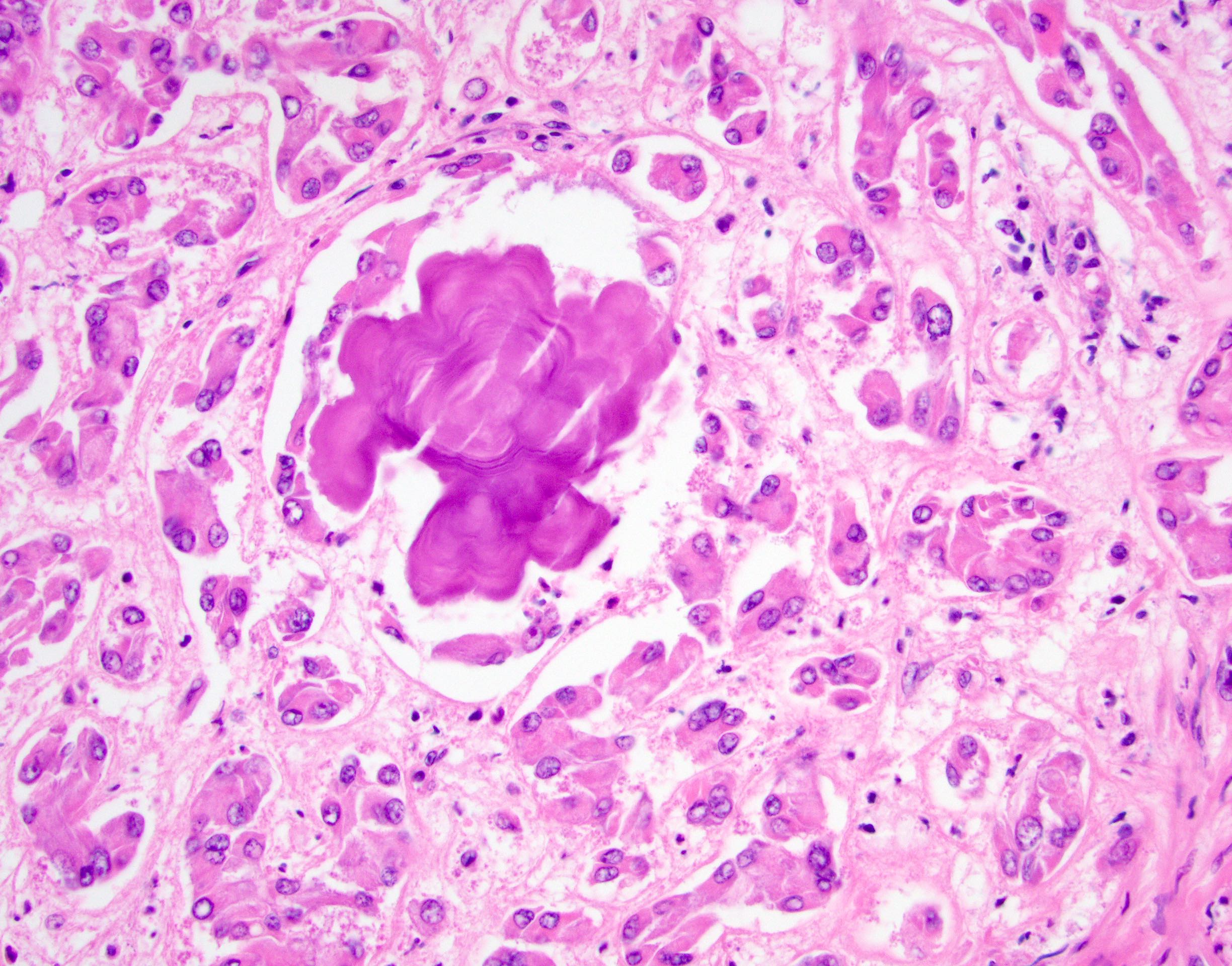
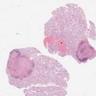
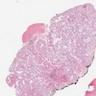
- Laminated psammomatous and mulberry-like calcifications
- Prominent neutrophilic infiltrate and lymphocytic rim
- Large epithelioid cells with eosinophilic cytoplasm, prominent nucleoli and minimal cytologic atypia
- Cords, trabeculae, nests or sheets in a variably myxoid or fibrous stroma
- Generally limited to testis with occasional extension into paratesticular tissue, including rete testis and hilar soft tissue
- Malignant cases more likely to exhibit solid sheet-like growth and frequent nuclear pleomorphism ranging from mild to prominent
Microscopic (histologic) images
Contributed by Stephanie Siegmund, M.D., Ph.D.
Virtual slides
Positive stains
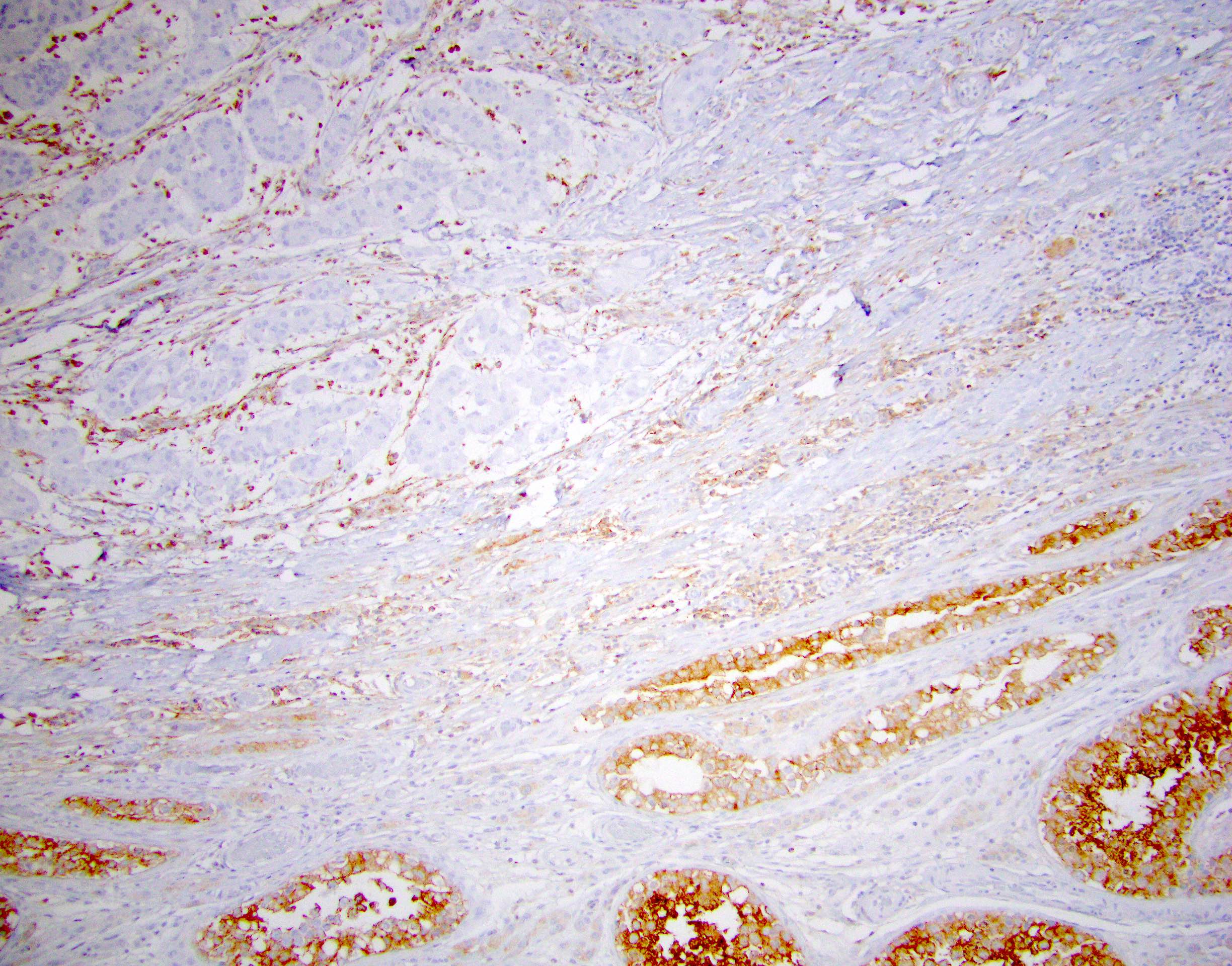
Negative stains
- PRKAR1A loss (93%)
- OCT 3/4, SALL4, CAM 5.2, CK18 (antibody clone DC10)
- Beta catenin (cytoplasmic staining only)
- References: Am J Surg Pathol 2021 Dec 16 [Epub ahead of print], Histopathology 2022;80:677
Molecular / cytogenetics description
- Frequent biallellic inactivation of PPKAR1A gene, secondary to somatic or germline mutations as part of Carney complex (Hum Pathol 2010;41:552, J Endocr Soc 2019;3:1375, Histopathology 2022;80:677)
- No chromosomal abnormalities reported
Sample pathology report
- Testis and spermatic cord, radical orchiectomy:
- Large cell calcifying Sertoli cell tumor (1.0 cm) (see comment)
- Tumor is limited to the testicular parenchyma
- No necrosis is identified
- Mitoses number < 1 per 10 HPFs
- The spermatic cord and cord resection margin are negative for tumor
- No lymphovascular invasion is identified
- Background testis with maturing spermatogenesis
- Comment: The slides demonstrate sheets and cords of large epithelioid cells with eosinophilic cytoplasm and prominent nucleoli. Immunohistochemistry demonstrates the following staining profile in lesional cells: positive staining for inhibin, calretinin, S100, MelanA and beta catenin (cytoplasmic, nonnuclear) and negative staining for PRKAR1A (loss), OCT 3/4 and SALL4. Overall, the findings are consistent with large cell calcifying Sertoli cell tumor. Adverse pathologic features are not identified (e.g., > 3 mitoses per 10 HPF, coagulative necrosis, vascular invasion, size > 4.0 cm, high grade cytologic atypia or extratesticular extension) (Am J Surg Pathol 1997;21:1271). Approximately 20 - 40% of cases are associated with Carney complex and genetic counseling is recommended in the appropriate clinical context.
Differential diagnosis
- Leydig cell tumor (LCT):
- Usually solitary and unilateral
- Frequent Reinke crystals (diagnostic of LCT)
- Lacks calcifications and neutrophilic infiltrate
- Retains IHC expression of PRKAR1A
- Sertoli cell tumor, not otherwise specified (NOS):
- Similar population of large, polygonal Sertoli cells with eosinophilic cytoplasm
- Usually lacks calcifications and neutrophilic infiltrate
- Nuclear localization of beta catenin by IHC
- Retains IHC expression of PRKAR1A
- Intratubular large cell hyalinizing Sertoli cell neoplasia:
- Predominantly intratubular process with expanded tubules and thickened basement membrane
- Tubules filled by globular eosinophilic basement membrane-like material
- Generally lacks calcifications
- Retains IHC expression of PRKAR1A
Board review style question #1
Which of the following features is most consistent with sporadic presentation of large cell calcifying Sertoli cell tumor (LCCSCT), shown in the image, versus a syndromic association (e.g., Carney complex)?
- Bilateral disease
- Loss of PRKAR1A by IHC
- Multifocal disease
- Pediatric presentation
- Sibling(s) with similar presentation
Board review style answer #1
B. Loss of PRKAR1A by IHC. While PRKAR1A alterations are commonly found in patients with Carney complex, the loss of PRKAR1A is seen in most sporadic as well as syndromic LCCSCT and can frequently be demonstrated by as loss of PRKAR1A by IHC in sporadic tumors. All other listed answers (pediatric onset, bilateral and multifocal disease, sibling(s) with similar presentations) are more commonly associated with LCCSCT occurring in Carney complex patients and would suggest a syndromic association.
Comment Here
Reference: Large cell calcifying Sertoli cell tumor
Comment Here
Reference: Large cell calcifying Sertoli cell tumor