Table of Contents
Definition / general | Terminology | Epidemiology | Sites | Etiology and Pathophysiology: | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Staging / staging classifications | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Immunohistochemistry & special stains | Electron microscopy description | Differential diagnosisCite this page: Rane SU. Testicular adrenal rest tumor / tumor of adrenogenital syndrome. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/testisadrenogenital.html. Accessed December 23rd, 2024.
Definition / general
- Testicular tumor associated with congenital adrenal hyperplasia and chararacterised by benign behavior and typical hormone sensitivity
Terminology
- Testicular tumors of Adrenogenital syndrome (TTAGS)
- Also know as Testicular adrenal rest tumors (TARTs)
- Biologically, the lesions represent hyperplasia of aberrant rests rather than a true neoplastic tumor
Epidemiology
- Can present at any age but commonly present in pubertal and postpubertal males, mean age ~15 years
- Incidence and prevalence vary based on patient age (prevalence increases with age), geography:
- Often clinically undetected (Radiology 1992;183:425)
- Detected at autopsy in 3 of 7 patients with CAH who were < 8 weeks old (Am J Dis Child 1963;106:243)
- 24% prevalence in 34 children with CAH (age range 2 - 18 years, Eur J Endocrinol 2007;157:339)
- Associated with congenital adrenal hyperplasia - 21 hydroxylase deficiency (Urol Clin North Am 2000;27:519, Indian J Endocrinol Metab 2013;17:S14); 27 - 47% develop testicular adrenal rest tumors (J Clin Endocrinol Metab 2001;86:3070, J Clin Endocrinol Metab 2001;86:5721, J Clin Endocrinol Metab 2012;97:4429)
- Other conditions associated with testicular adrenal rest tumors include Nelson syndrome, Cushing disease, Addison disease
Sites
- Normaly descended testis and adjacent tissues
Etiology and Pathophysiology:
- Development and pathogenesis
- Development of TTAGS is dependent on two factors:
- Presence of adrenal rests in and around the testis
- Abnormal growth stimulated by excess ACTH or Angiotensin II levels
- Duration and concentration of exposure to growth stimulating hormones
- Aberrant adrenal rests in and around the testis have been demonstrated at autopsy in ~7.5% of infants without any pathological significance (Am J Pathol 1962;40:587) and in 15% of healthy neonates (BJU Int 2005;95:407, Eur Radiol 2000;10:1165)
- Probably under reported due to difficulty in identifying adrenal rests
- Tumors more common in patients with congenital adrenal hyperplasia who are noncompliant with treatment
- Tumor's adrenal origin and functional status has been inferred from in vitro and in vivo studies:
- Adrenal specific enzyme CYP11B1 (11b-hydroxylase) present in tumor tissue of CAH patient with TTAGS (J Clin Endocrinol Metab 1990;70:1408)
- Adrenal specific steroids present (J Steroid Biochem Mol Biol 2005;93:67)
- Adrenal specific 11b-hydroxylated steroids present in blood from gonadal veins (J Steroid Biochem Mol Biol 2005;93:67, J Clin Endocrinol Metab 1994;79:1390, J Clin Endocrinol Metab 1991;73:1129)
- mRNA expression seen of adrenal specific enzymes CYP11B1 (expressed by zona fasciculate and zona reticularis) and CYP11B2 (expressed by zona glomerulosa), as well as of ACTH and angiotensin II (AII) receptors (Best Pract Res Clin Endocrinol Metab 2009;23:209)
- However, persistence of tumors despite ACTH suppression indicates role played by other factors
- TTAGS also reported in patients with adequately controlled hormone levels (J Clin Endocrinol Metab 2001;86:3070)
- Angiotensin II may have role in growth of TTAGS (Regul Pept 2003;110:249)
- TTAGS have not been reported in late onset CAH without clearly elevated ACTH or Angiotensin II levels
- LH may have role in development and growth of TTAGS (Eur J Endocrinol 1999;141:231)
- Development of TTAGS is dependent on two factors:
- Effects of TTAGS:
- Mechanical effects: size and duration dependent blockage of rete testis with resulting atrophy of seminiferous tubules
- Paracrine effects: steroids produced by tumor cells may be toxic to Leydig cells or germ cells (Hum Reprod 2001;16:263)
- The irreversible end stage of longstanding TART is tubular hyalinization with obstruction of the lumen and complete loss of germ cells and Sertoli cells; differs from ischemic hyalinization by relative preservation of Interstitial Leydig cells
Clinical features
- Most tumors are detected on ultrasound screening of patients with Congenital adrenal hyperplasia
- Symptoms of congenital adrenal hyperplasia:
- Severe forms: critical adrenal insufficiency with salt losing form present in shock very early in childhood
- Milder forms: present with varying degrees of virilization and sexual precocity in a young child, adolescent or adult
- Most common presentation of TTAGS is bilateral presence of palpable nodules in both testes with testicular enlargement
- Though varying degree of testicular atrophy is common, presentation with infertility is rare (Actas Urol Esp 2003;27:234)
Diagnosis
- Diagnosis is suspected on clinical features and ultrasound examination of scrotum (Radiology 1996;198:99) and confirmed with a biopsy / surgical excision
Laboratory
- Increased blood levels of 11-β-OH steroids in effluent testicular blood
- Increased 11-β-hydroxylase activity in tumor tissue
- Low α-fetoprotein, LDH and β-HCG
Radiology description
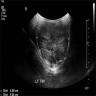
- Tumors are uniformly hypoechoic with well defined margins
- Multifocally and bilaterality is common (~75%)
- Mean diameter ~16 mm (range, 2 - 28 mm, Radiology 1992;183:425)
Prognostic factors
- Tumors are uniformly benign in behavior and many respond to ACTH suppression by glucocorticoid therapy
- No reports of malignant transformation or recurrence after complete excision
Case reports
- 19 year old man with testicular mass (Arch Pathol Lab Med 2000;124:785)
- 19 year old man with CAH, Leydig Cell Tumor and TART (Case Rep Urol 2012;2012:648643)
- 37 year old man with hyperplasia of adrenal rests in testis (Actas Urol Esp 2003;27:234)
- Bilateral tumors of testis in 21-alpha hydroxylase deficiency without adrenal hyperplasia (Urol Oncol 2005;23:178)
Treatment
- Early tumors are responsive to glucocorticoid therapy, which suppresses ACTH levels
- Surgery for tumors nonresponsive to glucocorticoid therapy, large tumors or if differential diagnosis with other tumors exists
- Testicular sparing surgery is preferred to preserve fertility, as tumor is benign
Staging / staging classifications
- Claahsen-van der Grinten et al (Best Pract Res Clin Endocrinol Metab 2009;23:209) proposed a staging system for TART development and progression, with an intent to guide treatment
- Stage 1: presence of adrenal rest cells within the rete testis, not detectable by scrotal ultrasound
- In healthy boys, probably regress in utero or in first year of life
- Stage 2: adrenal rest cells may proliferate in the presence of increased concentrations of ACTH (and possibly also of Angiotensin II)
- The adrenal rest cells may become visible by ultrasound as one or more small hypoechogenic lesions
- Age of onset of cell growth may depend on the cumulative exposure to ACTH (and angiotensin II) concentrations over time and the number of ACTH (and angiotensin II) receptors on the adrenal rest cells
- Complete regression of tumors with ACTH suppression by glucocorticoid therapy is possible
- Stage 3: further growth of the adrenal rest cells will compress the rete testis
- In pubertal or postpubertal CAH patients, oligo or azoospermia may already be found due to obstruction of the seminiferous tubules
- Signs of gonadal dysfunction: decreased inhibin B and increased follicle stimulating hormone (FSH) and LH levels may also be present
- Tumors are responsive to ACTH suppression, however the tumors relapse after discontinuation of ACTH suppression
- Hence, glucocorticoid therapy is only a temporary solution
- Stage 4: further hypertrophy and hyperplasia of the adrenal rest cells with progressive obstruction of the rete testis, leads to induction of fibrosis within the tumor and focal lymphocytic infiltration
- Several small tumors within the rete testis will conflate, forming a single lobulated tumor, separated from the residual testicular tissue by fibrous strands
- Differentiation of adrenal cells with loss of ACTH and angiotensin II receptors
- Loss of response to ACTH suppression by glucocorticoids
- Surgical excision of TTAGS could prevent further decline of testicular function
- Stage 5: chronic obstruction subsequently will lead to destruction of the surrounding testicular parenchyma with irreversible damage of the testis
- Only indication for surgery is the relief of pain and discomfort caused by TTAGS
- Stage 1: presence of adrenal rest cells within the rete testis, not detectable by scrotal ultrasound
- This staging system has not been validated
- Early treatment of CAH with adequate suppression of ACTH levels by glucocorticoid treatment from childhood, may prevent development of TTAGS
- However, the benefit needs to be balanced against the effect of growth suppression by excess glucocorticoid therapy
Gross description
- Usually bilateral, commonly located in hilar region of testis
- Well circumscribed yellow to tan nodules separated by dense fibrous bands
Gross images
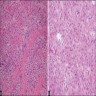
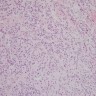
Microscopic (histologic) description
- Resemble adrenocortical cells
- Sheets, cords or lobules of large, polygonal cells with abundant, finely granular eosinophilic cytoplasm, often with lipochrome pigment
- Round, giant and hyperchromatic nuclei; nuclear pleomorphism can be prominent with presence of varying sized intranuclear inclusions
- Thin fibrovascular strands run through the tumor tissue but zonation is absent
- Mitoses are usually absent
- Reinke crystals are absent and help to differentiate from Leydig cell tumors to an extent
- Adjacent testicular tissue may be normal or may show evidence of atrophy in the form of reduced / absent spermatogenesis with reduced diameter of the seminiferous tubules, thickened basal membranes and deposition of fibrohyaline tissue
- The changes are more marked with tumors of long duration
Cytology description
- Cellular smears with large polygonal to round cells with foamy cytoplasm, round centrally placed nuclei with prominent nucleoli
- Abundant lipochrome pigment is characteristic
- Reinke crystals are absent
- No cytological feature to reliably distinguish from Leydig cell tumor in the absence of Reinke crystals
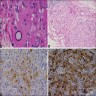
Immunohistochemistry & special stains
- Positive: CD56 strong, synaptophysin strong but can be focal or diffuse
- Inhibin positive but not useful to differentiate from Leydig cell tumor (Histopathology 2011;58:1013)
- Negative: androgen receptor
Electron microscopy description
- Abundant smooth endoplasmic reticulum, a moderate number of mitochondria with tubulovesicular cristae, lipid droplets and lipofuscin granules in the polygonal cells
- No Reinke crystals
Differential diagnosis
- Leydig cell tumor
- Less commonly bilateral (3% compared to 75% in TTAGS, Pathologica 1994;86:557, Urol Clin North Am 2000;27:519)
- Gynecomastia is more common (~30%), while it is rare in CAH (Urol Clin North Am 2000;27:519)
- Associated with Peutz-Jeghers syndrome, not associated with adrenal hyperplasia
- Nonresponsive to corticosteroid therapy
- Presence of Reinke crystals is diagnostic but seen in just 40 of cases.
- Malignancy related changes are seen in 10% of Leydig cell tumors while they never occur in TTAGS
- Absence of adipocyte metaplasia and extensive fibrosis
- Androgen receptor positive and weak reactivity / negative for CD56 and synaptophysin
- Biochemical features are NOT useful to differentiate (Urol Clin North Am 2000;27:519)