Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Kravtsov O, Mesa H. Sertoli cell tumor. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/testisSertoliNOS.html. Accessed December 22nd, 2024.
Definition / general
- Sex cord stromal tumor of testis composed of cells showing features of fetal, prepubertal, adult or atrophic Sertoli cells, at least focally
Essential features
- Second most common type of pure sex cord stromal tumor after Leydig cell tumor
- Most are small, unilateral tumors in adults and the vast majority are benign
- Characterized by tubular or cord pattern, at least focally
- Positive for SF1 and nuclear beta catenin
- Variable and inconsistent expression of calretinin, inhibin A, androgen receptor, CD56
- Negative for SALL4, OCT4 and other germ cell markers
- Poor response to therapy when metastatic
Terminology
- Androblastoma (obsolete)
Epidemiology
- < 1% of testicular tumors
- Rare before age 20; mean age 45 years
Etiology
- Risk factors: cryptorchidism, familial adenomatous polyposis (Virchows Arch 2012;461:713)
- No known environmental factors
Clinical features
- Slowly enlarging unilateral testicular mass
- Testicular discomfort, pain or incidental finding
- Hormonal manifestations very rare; more common in malignant tumors
- Exceptionally, metastases are initial manifestation
- References: Histopathology 2017;70:513, Ulbright: Tumors of the Testis and Adjacent Structures (AFIP Atlas of Tumor Pathology, Series 4), 1st Edition, 2014, Am J Surg Pathol 2015;39:1390
Diagnosis
- For suspected testicular mass, the first test is scrotal ultrasound (US) (sensitivity 95%, specificity 97%)
- If US confirms a lesion, the patient should be immediately referred to a urologist for evaluation and management
- Men 15 to 44 years old with retroperitoneal mass / metastases should undergo testicular examination and scrotal US
- Evaluate serum markers
- Biopsy of testicular tumors is in general not performed, to prevent tumor seeding
- Staging:
- In clinically stable patients, imaging may be performed before or after orchiectomy
- Imaging: computed tomography (CT) scan of the abdomen / pelvis with intravenous contrast, chest radiograph (CXR)
- Noncontrast chest CT should be performed if CXR is abnormal
- Reference: Med Clin North Am 2018;102:251
Laboratory
- Serum tumor markers:
- Beta human chorionic gonadotropin (hCG), alpha fetoprotein (AFP) should be normal and are used to exclude nonseminomatous germ cell tumors
- Lactate dehydrogenase (LDH) to assess global tumor burden
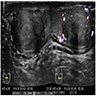
Radiology description
- Ultrasound: solitary hypoechoic lesion; not helpful in distinguishing from germ cell tumors
Prognostic factors
- Vast majority are benign, ~ 5% cases are malignant
- Tumors with sclerosis > 50% almost invariably benign (Am J Surg Pathol 2014;38:510)
- Features associated with malignancy: extratesticular spread, size > 5 cm, lymphovascular invasion, high grade cytological atypia, tumor necrosis, mitotic index > 5/10 high power fields (Am J Surg Pathol 1998;22:709)
- Very rarely tumors without malignant histology metastasize
Case reports
- 23 year old man with small, solid, well demarcated intraparenchymal tumor (Case #18)
- 23 year old man with sclerosing Sertoli cell tumor (Rev Urol 2014;16:191)
- 34 year old man with familial adenomatous polyposis and bilateral Sertoli cell tumors (Virchows Arch 2012;461:713)
- 13 examples of malignant Sertoli cell tumors (Am J Surg Pathol 2002;26:541)
Treatment
- Orchiectomy (total or partial) is treatment of choice
- Retroperitoneal lymph node dissection for malignant Sertoli cell tumors
- No specific therapy; poor response to chemotherapy or radiotherapy
- Reference: Med Clin North Am 2018;102:251
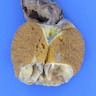
Gross description
- Cut surface usually homogenous white or yellowish
- Cystic component present in ~ 30% of cases (Am J Surg Pathol 1998;22:709)
- Benign: well circumscribed, most between 2 - 5 cm (Am J Surg Pathol 2014;38:510)
- Malignant: > 5 cm, poor circumscription, extratesticular extension, necrosis, hemorrhage
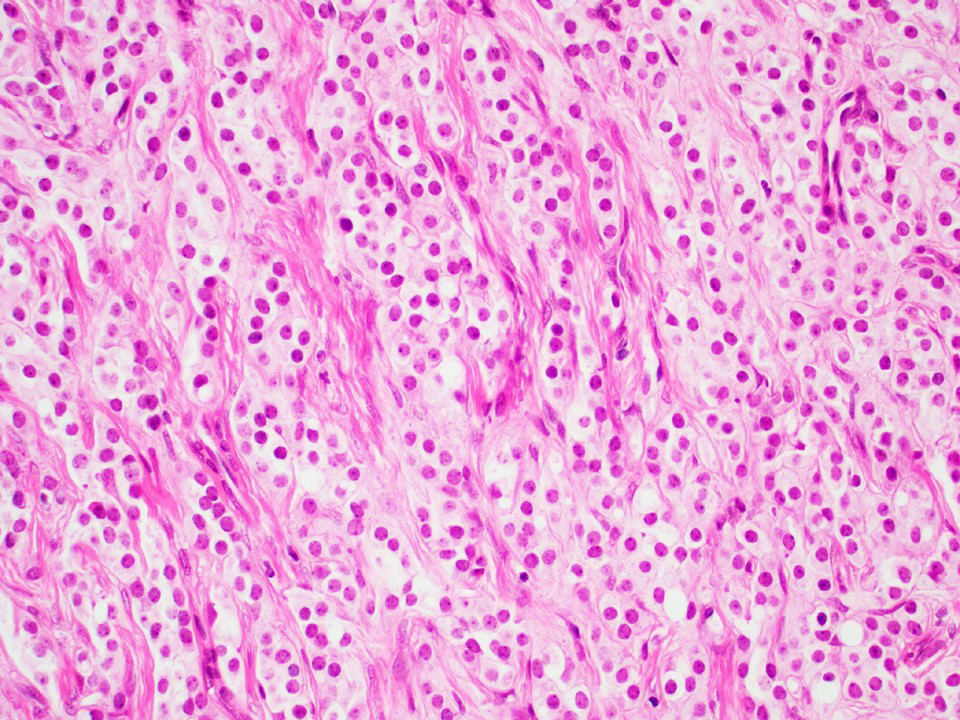
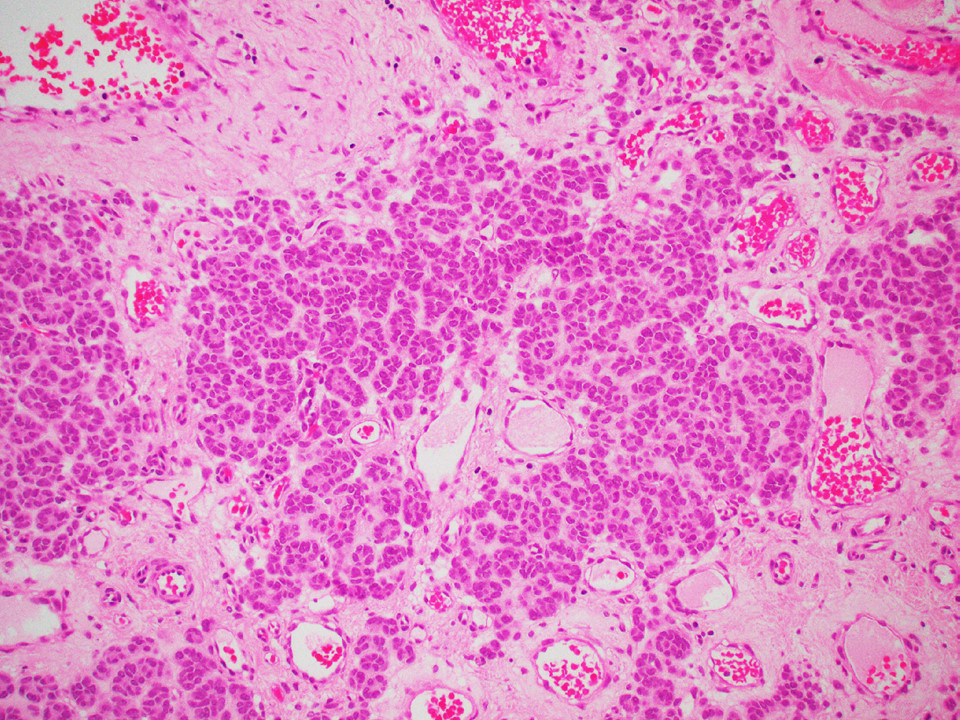
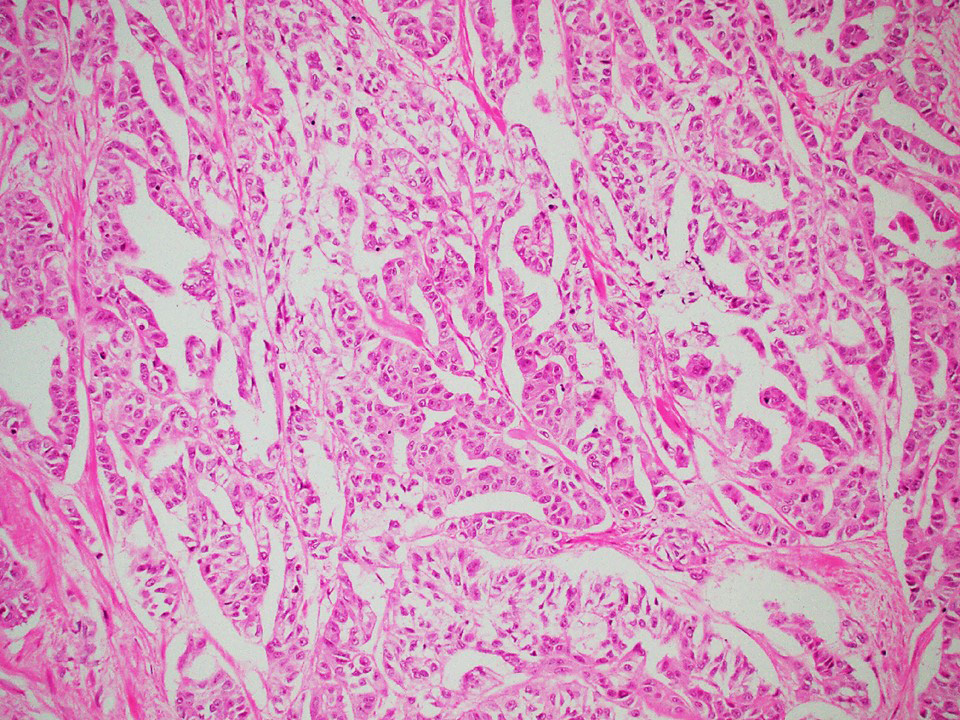
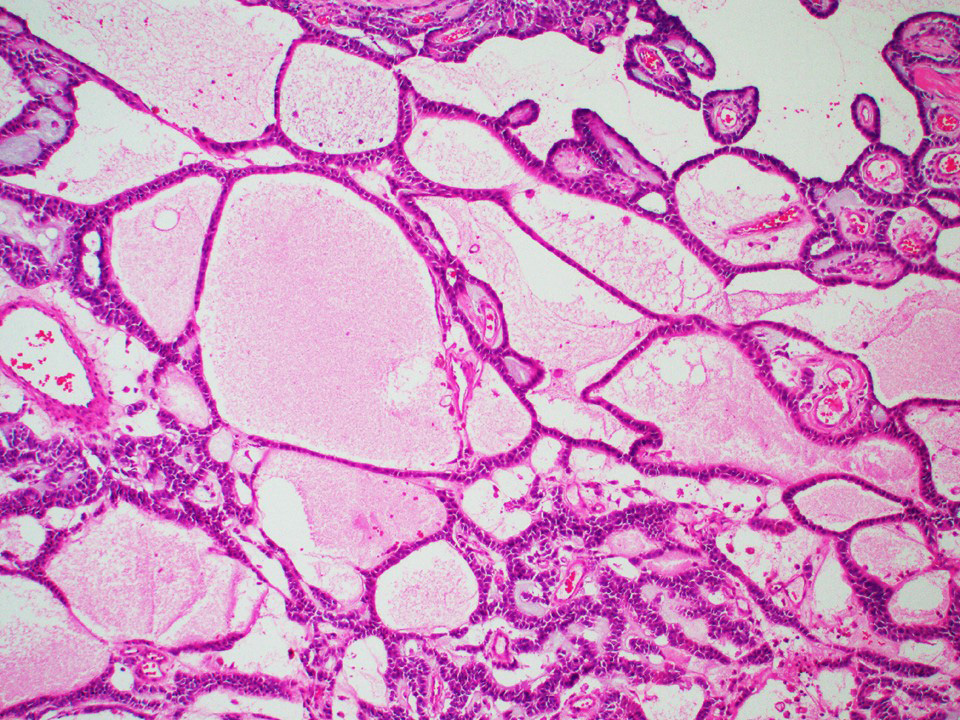
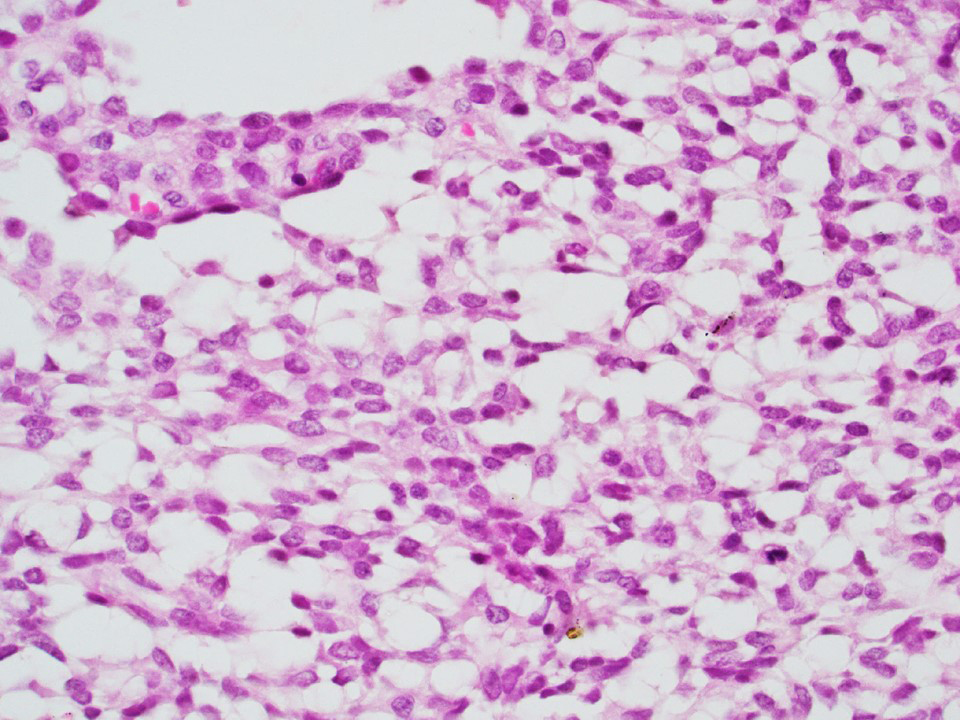
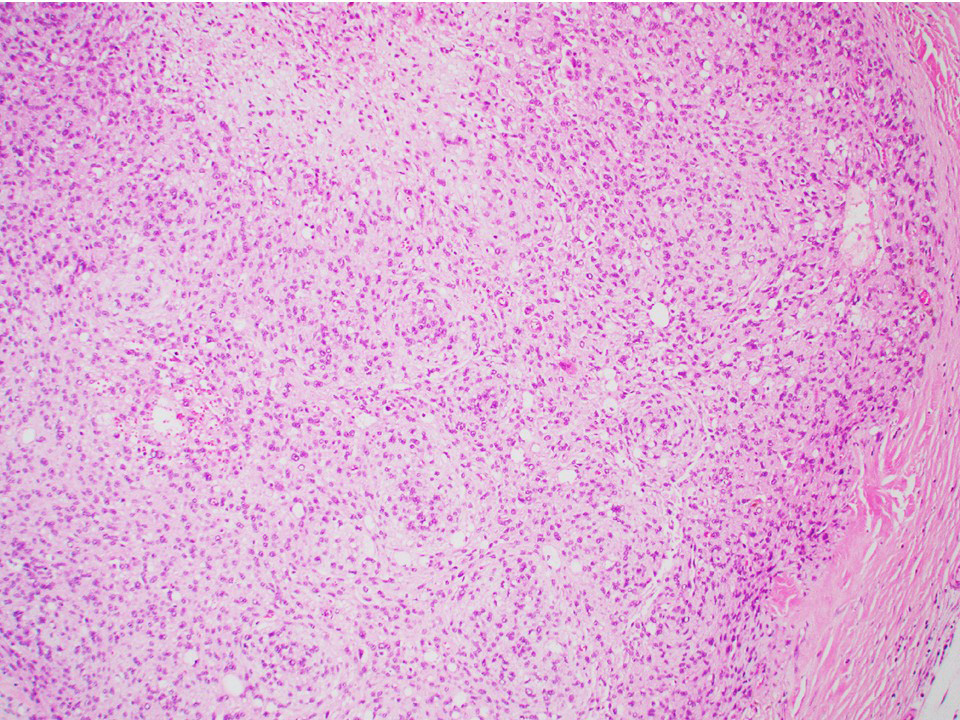
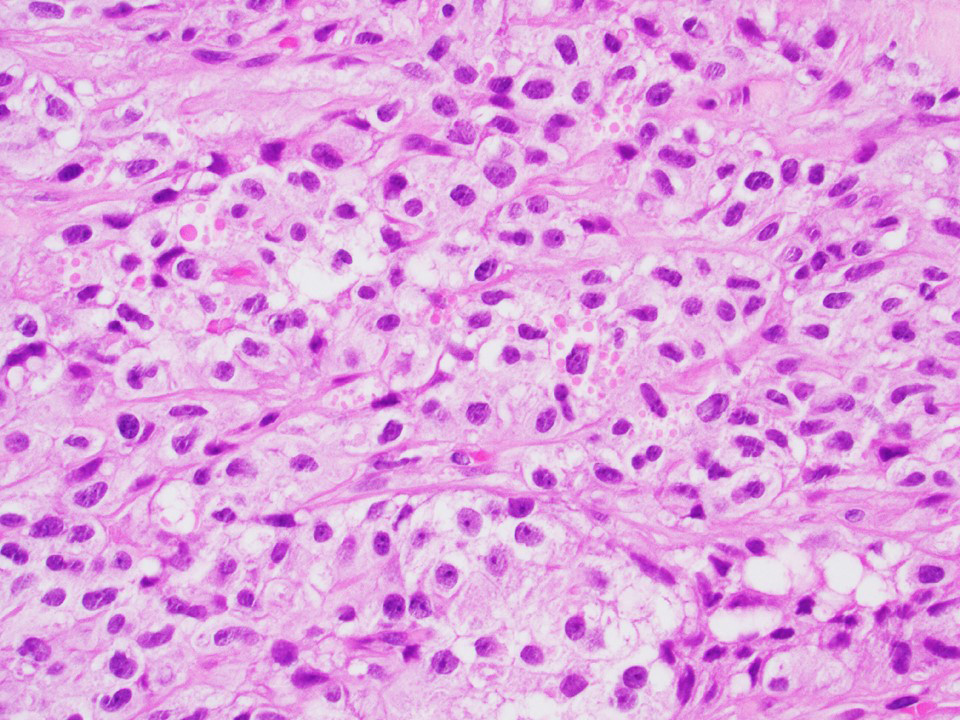
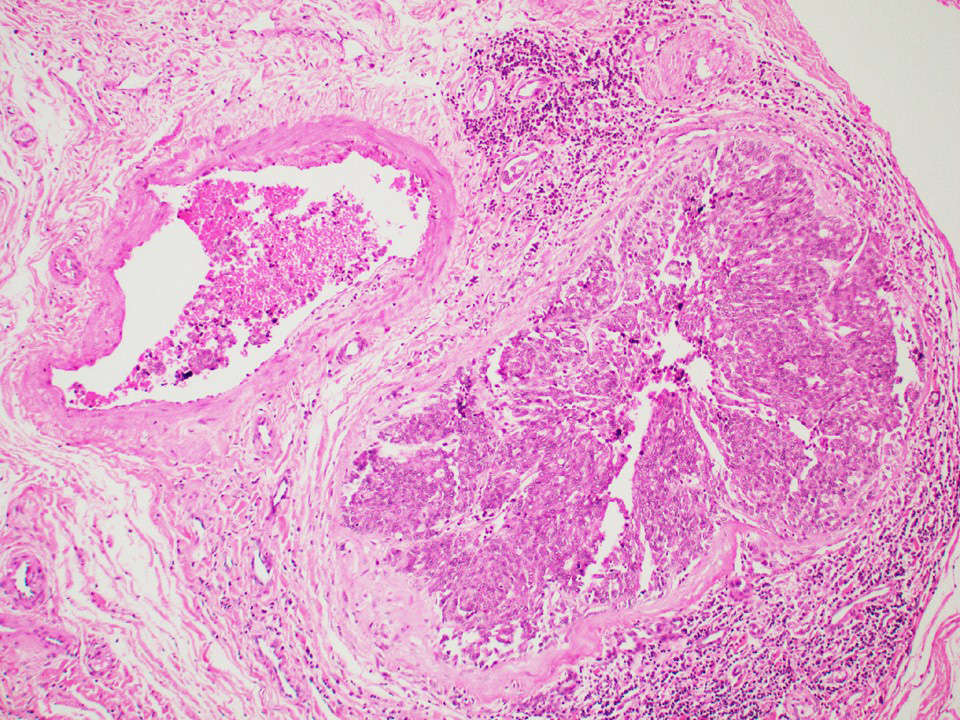
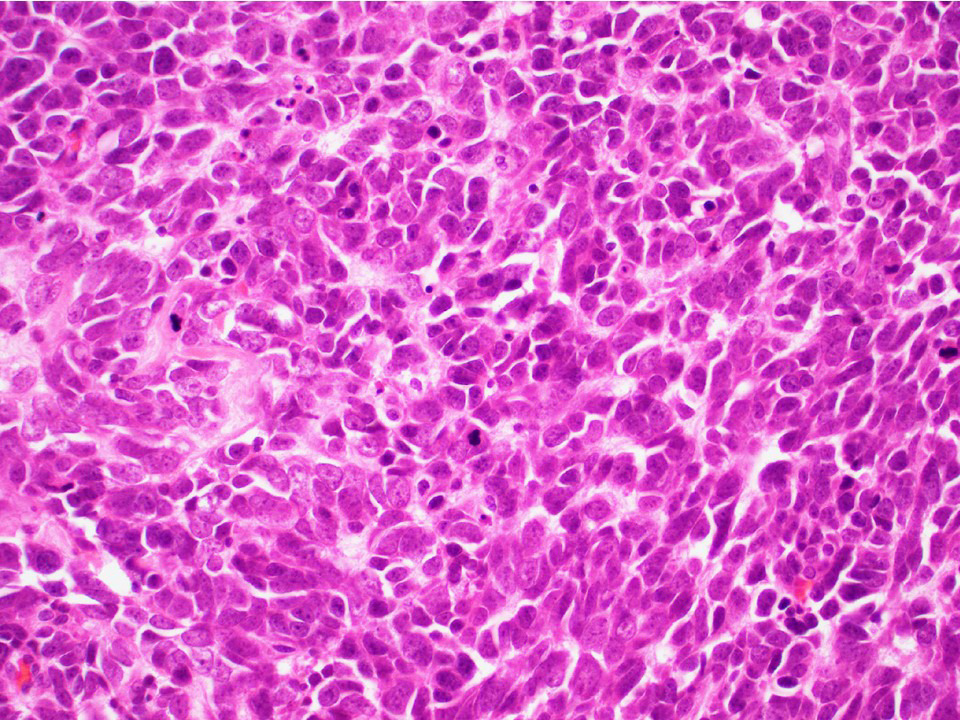
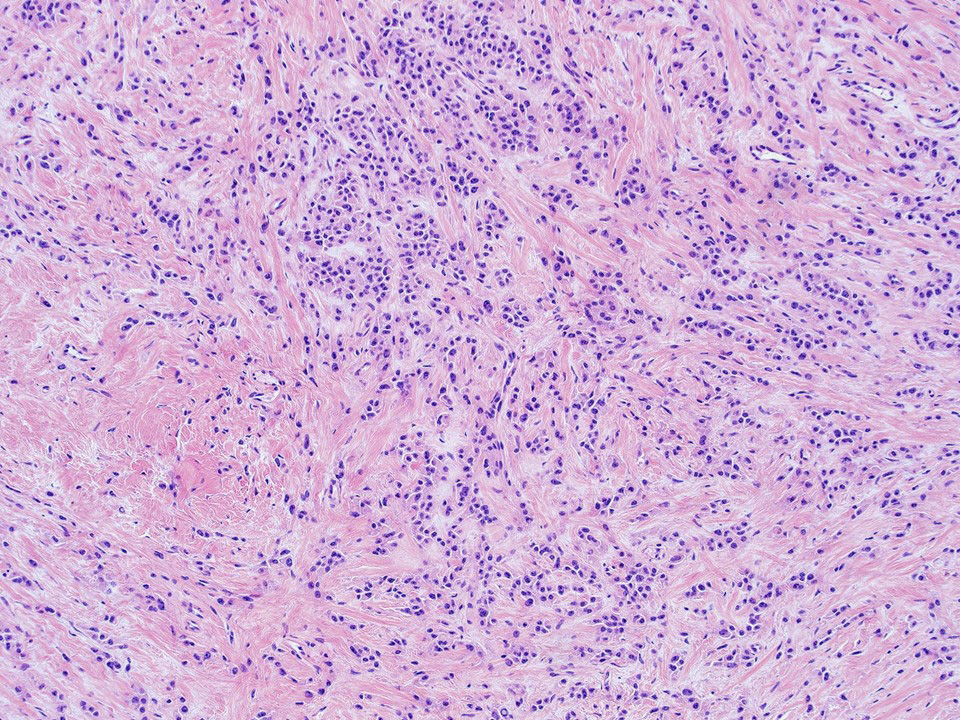
Microscopic (histologic) description
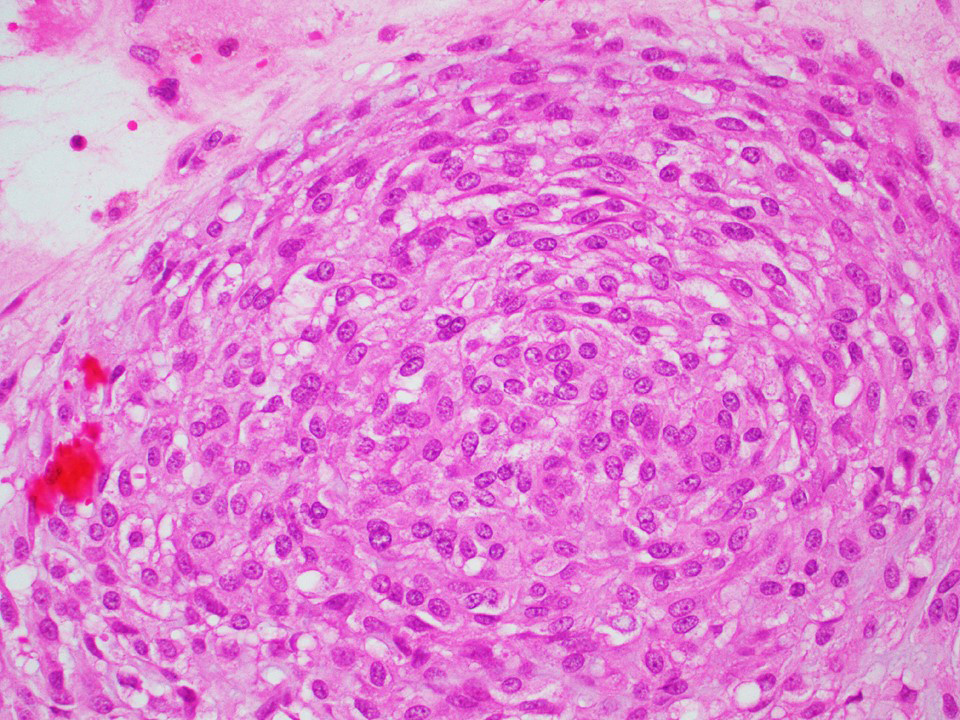
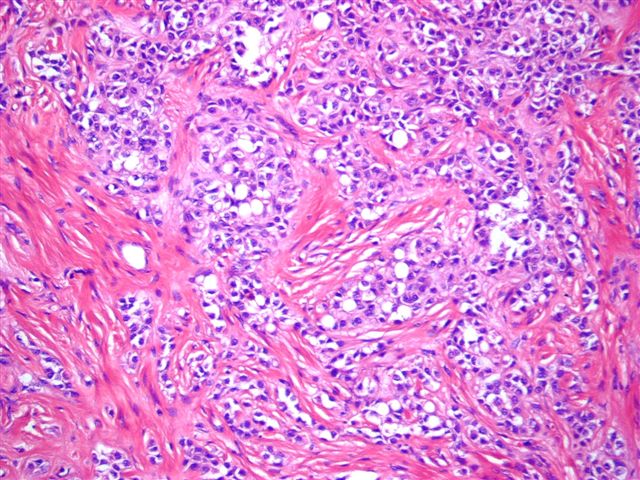
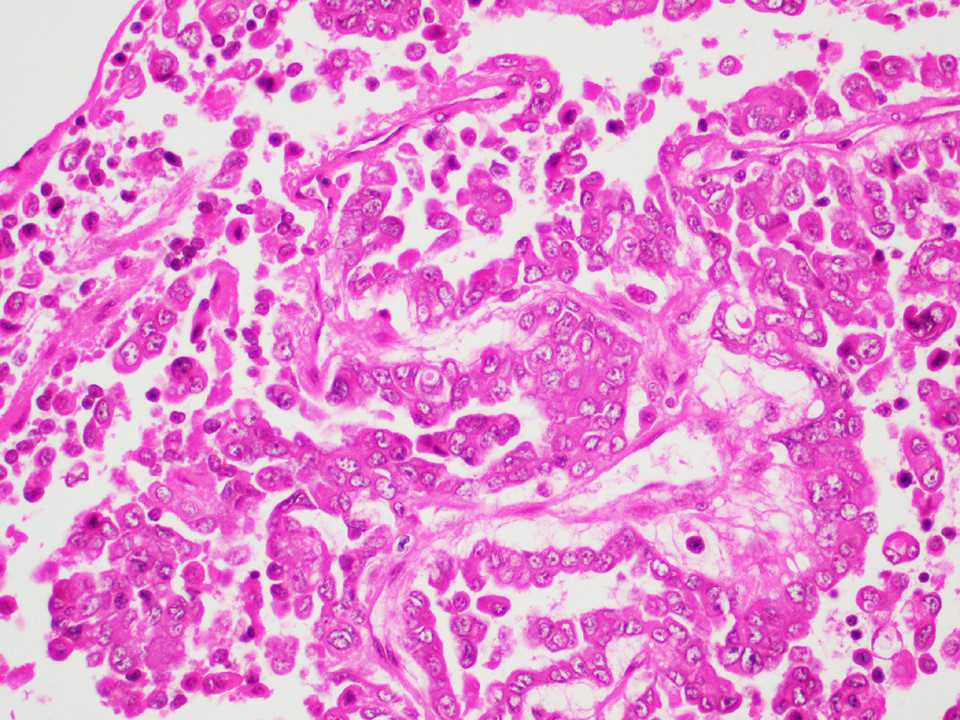
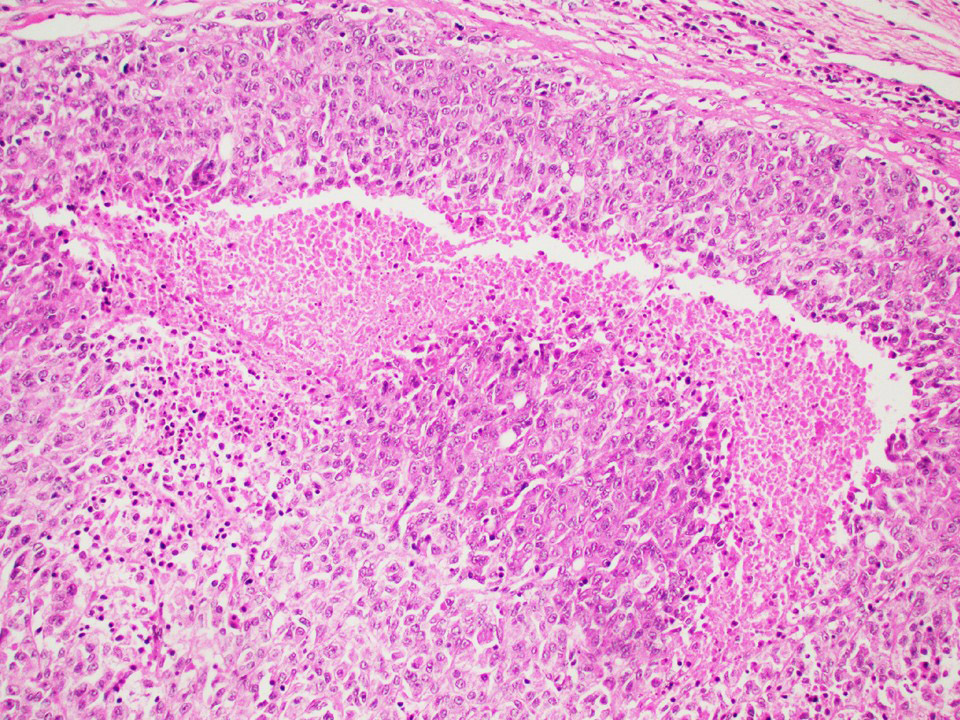
- Architecture: tubular (lumen), cords (no lumen), tubulopapillary present at least focally
- Other patterns: macro or microcystic, nested, trabecular, whorled, solid, retiform, pseudopapillary; most tumors show mixed patterns
- Nuclei: bland, uniform, round to ovoid in benign tumors; small hyperchromatic or large with prominent nucleoli in malignant tumors
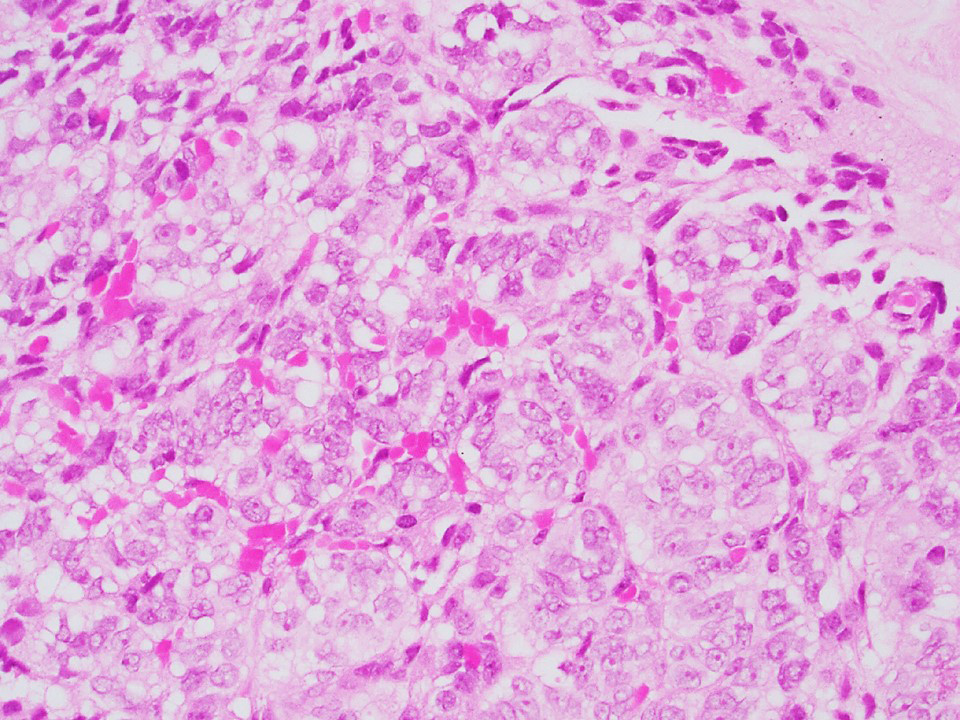
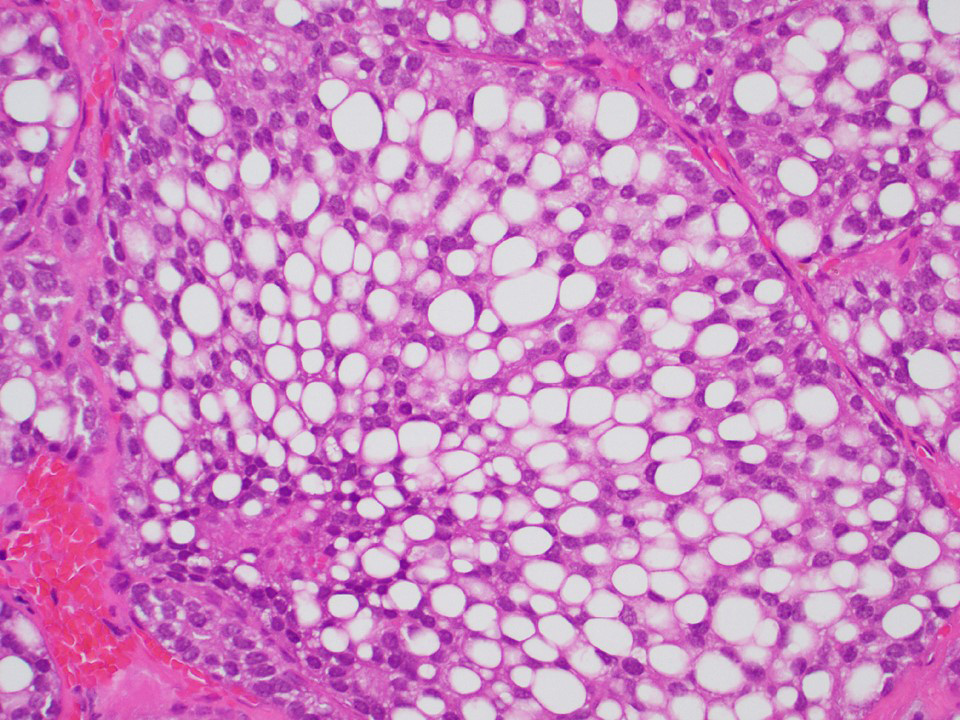
- Cytoplasm: usually clear and abundant but extremely variable, ranging from scant, foamy, eosinophilic to markedly lipidized; hyaline globules are common
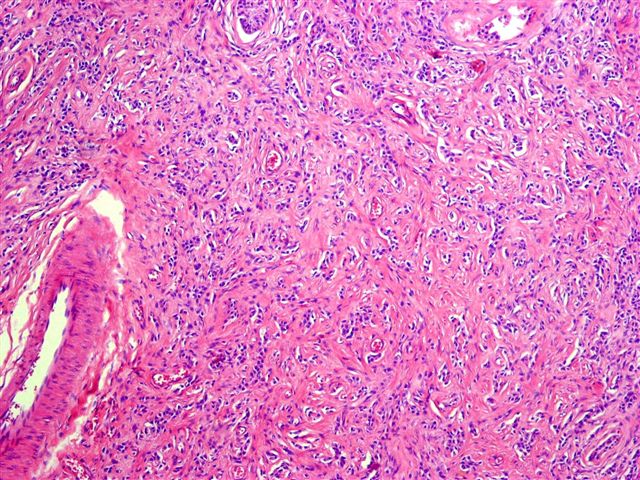
- Stroma: variable, basement membrane-like material around tubules, sclerotic, myxoid, edematous, angiomatous
- If sclerosis > 50% → sclerotic variant (now considered subtype of Sertoli cell tumor, NOS)
- Inflammatory cells usually absent, rarely prominent
- References: Histopathology 2017;70:513, Ulbright: Tumors of the Testis and Adjacent Structures (AFIP Atlas of Tumor Pathology, Series 4), 1st Edition, 2014, Am J Surg Pathol 2015;39:1390
Microscopic (histologic) images
Contributed by Thomas Ulbright, M.D. and Case #18
Architectural patterns
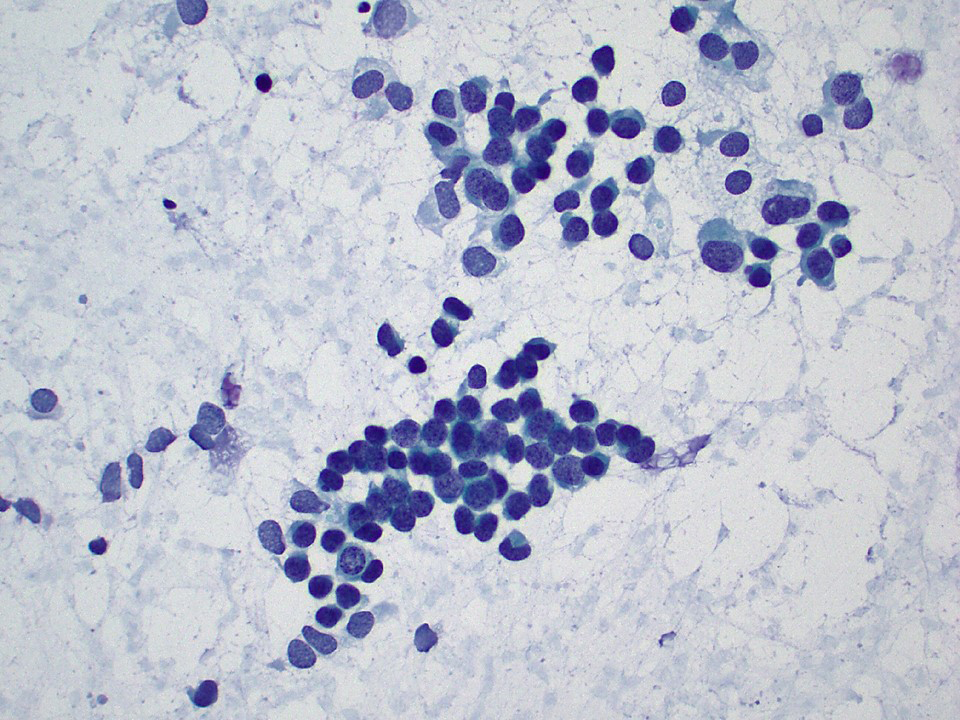
Cytology description
- Cytologic samples are from metastatic (malignant) Sertoli cell tumors
- Aspirates show branching tubules, microacinar and papillary groups
- Numerous individual cells are present in the background
- Cells are small, ovoid to plasmacytoid, relatively uniform
- Nuclei show smooth cell membranes and granular chromatin pattern
- Malignancy evident by nuclear overlap, discordant large nuclei and mitotic activity
- Aspirates resemble neuroendocrine tumors or well differentiated adenocarcinomas
- References: Histopathology 2017;70:513, Ulbright: Tumors of the Testis and Adjacent Structures (AFIP Atlas of Tumor Pathology, Series 4), 1st Edition, 2014, Am J Surg Pathol 2015;39:1390
Cytology images
Positive stains
- Consistent markers: SF1, nuclear beta catenin
- Inconsistent markers: calretinin, inhibin A, cytokeratins, MelanA, WT1, CD99, SOX9, vimentin, CD56, chromogranin, synaptophysin, nestin, PAX2, PAX8, androgen receptor (Am J Surg Pathol 2015;39:1390, Hum Pathol 2017;68:99, Hum Pathol 2017;61:181)
Electron microscopy description
- Prominent Golgi complex, variable smooth endoplasmic reticulum, lipid droplets, glycogen
- Cells interconnected by desmosomes, peripheral basement membrane
- References: Histopathology 2017;70:513, Ulbright: Tumors of the Testis and Adjacent Structures (AFIP Atlas of Tumor Pathology, Series 4), 1st Edition, 2014, Am J Surg Pathol 2015;39:1390
Molecular / cytogenetics description
- CTNNB1 gene mutations (Am J Surg Pathol 2014;38:66)
- Negative for isochromosome 12p
- Cytogenetics: gains of X chromosome (~ 40% cases), loss of chromosomes 2 and 19 (less common) (Virchows Arch 2007;450:425)
Sample pathology report
- Left testis, orchiectomy:
- Sertoli cell tumor, not otherwise specified (see comment and synoptic report)
- Comment: The morphologic features are diagnostic of Sertoli cell tumor, not otherwise specified. No features of malignancy are identified in this tumor (i.e. extratesticular spread, size > 5 cm, high grade cytologic atypia, > 5 mitotic figures per 10 high power fields, necrosis or lymphovascular invasion).
Differential diagnosis
- Rete testis adenoma:
- Located at the rete testis
- Strong diffuse positivity for PAX2, PAX8 and pankeratin
- Adenomatoid tumor:
- Paratesticular location
- Uniformly positive mesothelial markers: pankeratin, CK7, WT1, CK5/6, calretinin
- Negative for SF1 or nuclear beta catenin
- Leydig cell tumor:
- Abundant eosinophilic cytoplasm, absent tubules / cords
- Consistent strong expression of calretinin, inhibin A, SF1
- Negative beta catenin (Am J Surg Pathol 2015;39:1390)
- Yolk sac tumor:
- Positive for SALL4, glypican 3, alpha fetoprotein, pankeratin
- Negative for OCT4, SF1, nuclear beta catenin, inhibin A, calretinin
- Seminoma:
- Positive for SALL4, OCT4, KIT, D2-40, PLAP
- Negative for SF1, nuclear beta catenin, inhibin A, calretinin
- Juvenile granulosa cell tumor:
- Most common in infants; 90% occur before age 10
- Follicles with mucicarmine+ basophilic or eosinophilic secretions
- Variable solid pattern, frequent brisk mitotic / apoptotic activity
- Consistent expression of inhibin A, calretinin, FOXL2
- Large cell calcifying Sertoli cell tumor:
- Large epithelioid cells, lamellated calcifications
- Associated with Carney complex
- Sertoli cell nodule (Pick adenoma):
- Small size, multifocal, monotonous tubular / cord architecture, associated with androgen insensitivity syndrome and cryptorchidism
Additional references
Board review style question #1
A 60 year old man presents with a 1.1 cm mass in the left testis. The tumor is positive for SF1, nuclear beta catenin, androgen receptor, variable for inhibin A and calretinin and negative for SALL4. What is the most likely diagnosis?
- Adenomatoid tumor
- Seminoma
- Sertoli cell tumor, NOS
- Yolk sac tumor
Board review style answer #1
Board review style question #2
What is the characteristic immunoprofile of a testicular Sertoli cell tumor, NOS?
- Positive for SF1, calretinin, chromogranin, synaptophysin; negative for SALL4 and beta catenin
- Positive for SALL4; negative for SF1, inhibin A and calretinin
- Positive for SF1, calretinin, inhibin A, beta catenin (nuclear); negative for SALL4, chromogranin, synaptophysin
- Positive for CD45; negative for SF1, inhibin A and SALL4
Board review style answer #2
C. Positive for SF1, calretinin, inhibin A, beta catenin (nuclear); negative for SALL4, chromogranin, synaptophysin
Comment Here
Reference: Sertoli cell, NOS
Comment Here
Reference: Sertoli cell, NOS