Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Kang A, Esnakula AK. Juvenile polyp. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/stomachjuvenile.html. Accessed April 2nd, 2025.
Definition / general
- Hamartomatous polyp characterized by hyperplastic foveolar epithelium, frequently associated with cystically dilated mucin filled glands lined by foveolar epithelium and inflamed edematous lamina propria
- Can be sporadic (rare) or syndromic associated with juvenile polyposis syndrome (JPS) (Clin Med Insights Gastroenterol 2016;9:3, Diagn Histopathol 2017;23:521, Diagn Histopathol 2020;26:39)
Essential features
- Hamartomatous polyps that may be sporadic but are more likely to be syndromic in the stomach
- Classic appearance is of excess edematous lamina propria with cystically dilated, mucin filled glands; however, an epithelial dominant variant can also be seen
- Presence of multiple such polyps should prompt consideration of syndromic polyposis, particularly JPS
- JPS patients have a 21 - 30% risk of developing gastric cancer and must undergo surveillance
- Germline mutations in SMAD4 and BMPR1A are associated with ~60% of JPS cases; other genes involved are PTEN and ENG
Terminology
- Retention polyps or inflammatory polyps when present as a solitary lesion (Gastroenterol Hepatol (N Y) 2013;9:640)
ICD coding
- ICD-10: K31.7 - polyp of stomach and duodenum
Epidemiology
- Uncommon; only ~1% of gastric polyps are hamartomatous (Intern Med 2008;47:259)
- Sporadic juvenile polyps rarely occur in the stomach
- Most are associated with JPS; found in 14% of JPS patients (Best Pract Res Clin Gastroenterol 2017;31:381, Clin Med Insights Gastroenterol 2016;9:3)
- JPS: most common heritable gastrointestinal (GI) polyposis syndrome; prevalence is as follows (Clin Med Insights Gastroenterol 2016;9:3, Cancer Syndromes: Juvenile Polyposis [Accessed 23 July 2024])
- 1 in 100,000 - 160,000
- M = F
- Higher in Northern European populations
- ~25% of JPS patients have a negative family history with a de novo mutation (Diagn Histopathol 2020;26:8)
Sites
- Typical location is the antrum of the stomach but may occur in the body and fundus (Gastroenterol Hepatol (N Y) 2013;9:640)
Pathophysiology
- Hamartomatous malformation of the layers above the muscularis mucosa (Cancer Syndromes: Juvenile Polyposis [Accessed 23 July 2024])
- JPS is an autosomal dominant syndrome that commonly (~60%) arises as the result of germline mutations in SMAD4 or BMPR1A, involved in the TGFβ signaling pathway (Hum Mol Genet 1998;7:1907, Am J Med Genet A 2005;138A:113, Clin Transl Gastroenterol 2019;10:e00054, J Med Genet 2007;44:702)
- Other genes associated with JPS are PTEN and ENG
- Rare cases of sporadic juvenile polyps with SMAD4 mutations have been reported (Clin J Gastroenterol 2024;17:23)
Etiology
- Sporadic or part of JPS
- JPS is commonly due to germline mutations in SMAD4 or BMPR1A genes
Clinical features
- Common presentation includes GI bleeding or anemia (Adv Anat Pathol 2018;25:1)
- Other symptoms include diarrhea, abdominal pain due to gastric outlet obstruction
- JPS presents with polyps all over the GI tract, more commonly the colon and rectum
- JPS with SMAD4 mutations are also associated with hereditary hemorrhagic telangiectasia (HHT), which can present as telangiectasias of the skin, buccal mucosa and GI tract, epistaxis and arteriovenous malformations of various organs (Diagn Histopathol 2020;26:8)
Diagnosis
- Gold standard for evaluation is esophagogastroduodenoscopy (EGD) with polypectomy
- Rarely, it is possible to see very large polyps on noninvasive imaging (computed tomography [CT] or magnetic resonance imaging [MRI]) (StatPearls: Gastric Polyp [Accessed 24 July 2024])
- JPS diagnostic clinical criteria: 5 or more juvenile polyps in the colon, multiple juvenile polyps elsewhere in the GI tract or any number of juvenile polyps with a family history of juvenile polyposis (Am J Gastroenterol 2015;110:223)
Laboratory
- Iron deficiency anemia secondary to GI bleeding
Prognostic factors
- Solitary sporadic polyps usually have no malignant potential (Clin Med Insights Gastroenterol 2016;9:3)
- SMAD4 mutations are more likely to cause gastric polyps than BMPR1A mutations and twice as likely to cause anemia (JGH Open 2022;6:531)
- Patients with BMPR1A mutations are more likely to present at a younger age
- Patients with SMAD4 mutations are likely to have more aggressive polyps (Gastroenterol Hepatol (N Y) 2013;9:640)
- Reported risk of gastric cancer in JPS cases is 21 - 30%; the risk is much higher with SMAD4 mutations compared to BMPR1A mutations (Gastrointest Endosc 2023;97:407)
Case reports
- 32 year old man with a gastric mass and polyposis (BMJ Case Rep 2020;13:e236855)
- 46 year old woman with massive polyposis of the stomach (Fam Cancer 2019;18:165)
- 48, 52 and 54 year old women with gastric juvenile polyposis (JGH Open 2022;6:531)
- 50 year old woman with a familial history positive for GI cancers and other tumor types (Fam Cancer 2022;21:441)
- 50 year old woman was diagnosed with gastric hyperplastic polyps (Clin J Gastroenterol 2024;17:23)
Treatment
- Multiple gastric juvenile polyps (3 or more) should raise suspicion for JPS
- Genetic testing for SMAD4 and BMPR1A mutations is recommended; if positive, family members should also be tested
- Presence of SMAD4 mutations should also prompt evaluation for HHT
- If JPS is confirmed, surveillance with colonoscopy and gastroduodenal endoscopy with polypectomy (every 1 - 3 years) is recommended, starting at age 15 or when symptoms first appear
- Surgical resection is indicated in cases where polyps cannot be managed endoscopically (Am J Gastroenterol 2015;110:223, Clin Case Rep 2019;8:92)
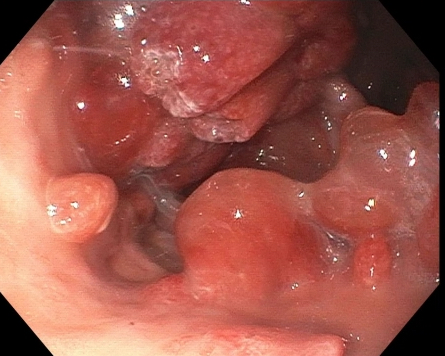
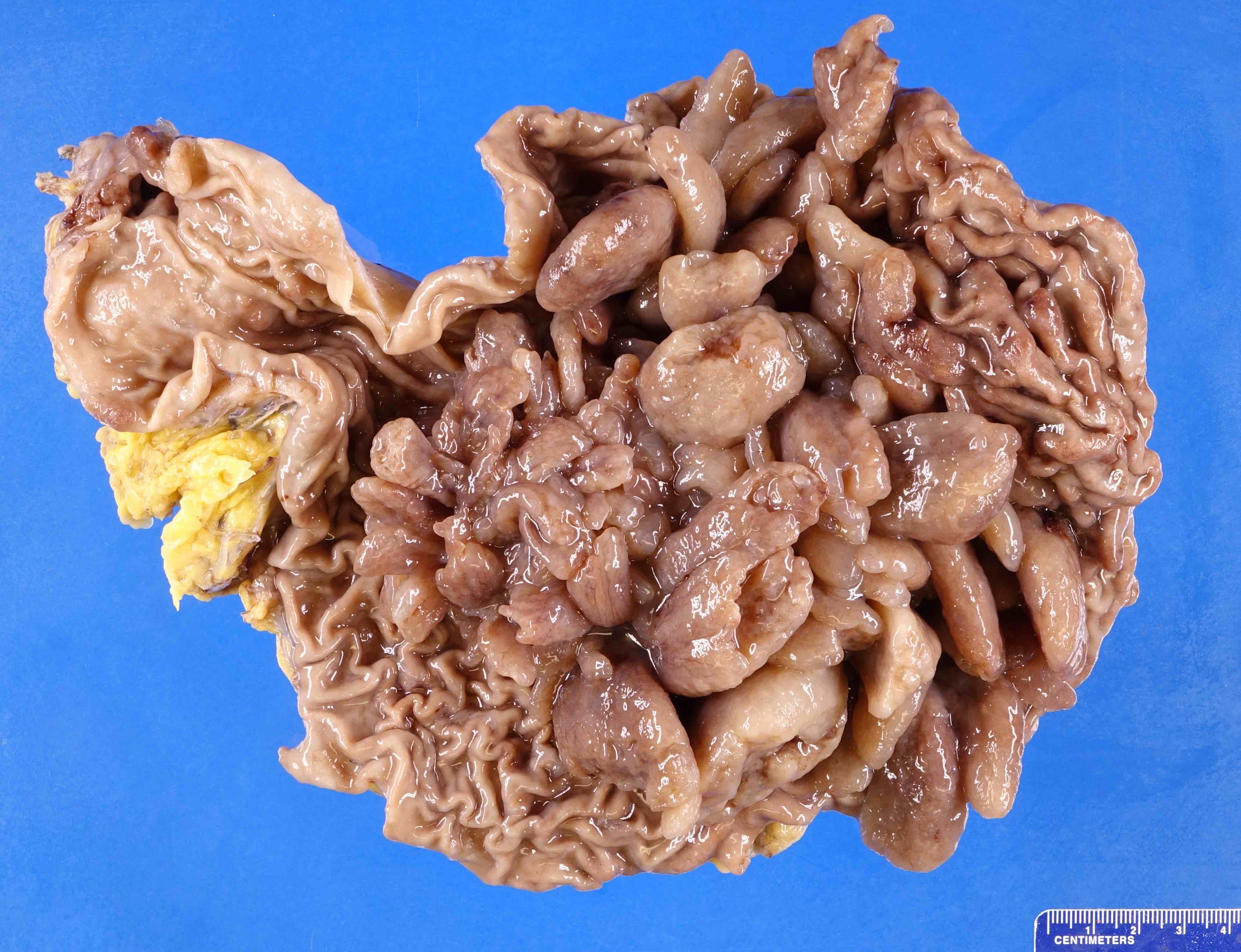
Gross description
- Usually pedunculated but can be sessile, especially the smaller gastric polyps
- May measure from 2 mm to 20 mm in size, rarely exceeding 30 mm (Cancer Syndromes: Juvenile Polyposis [Accessed 23 July 2024], Gastroenterol Hepatol (N Y) 2013;9:640)
- Typically have rounded and smooth contours (Clin Med Insights Gastroenterol 2016;9:3)
- Sectioning of larger polyps usually shows tan-pink, mucoid cut surfaces with multiple folds of involuted mucosa and focal cystic spaces filled with tan-white to translucent mucin
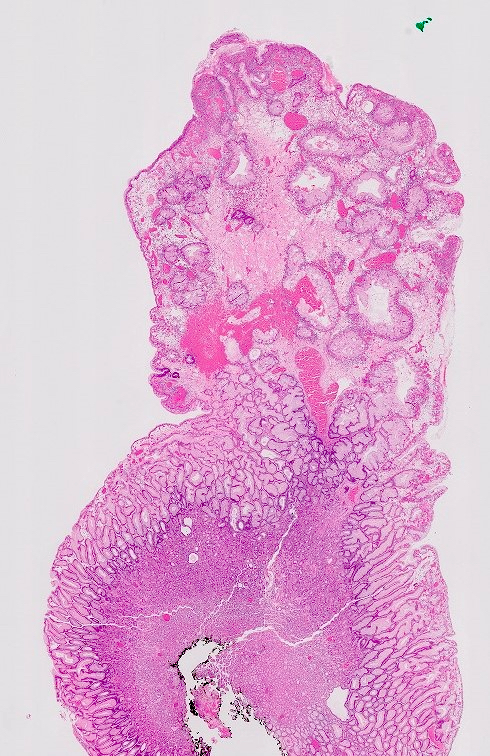
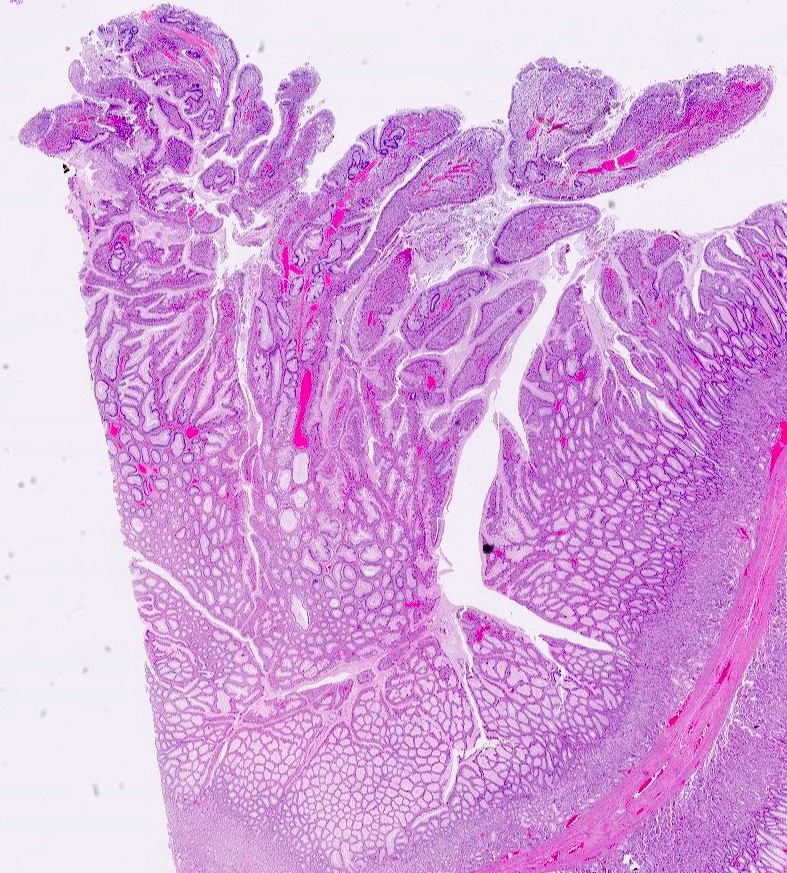
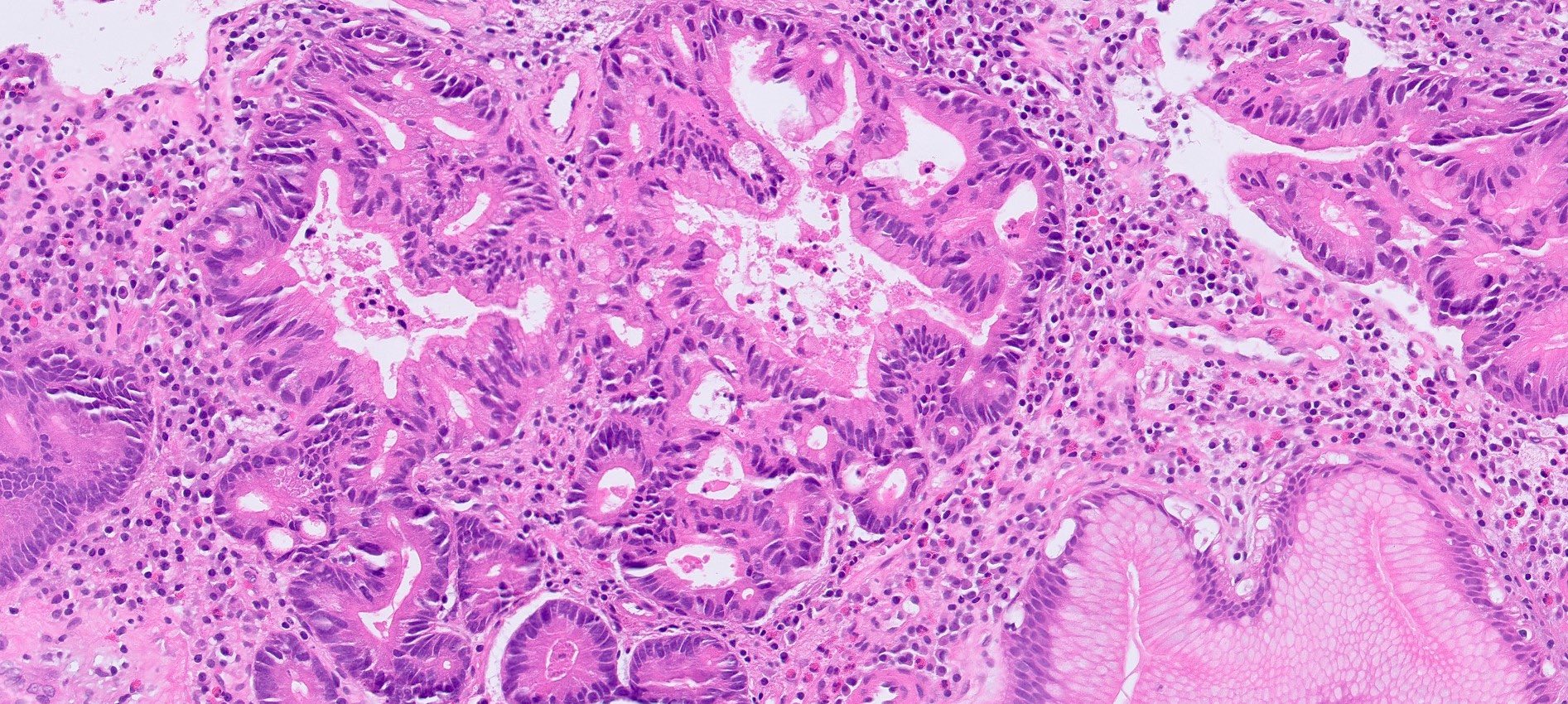
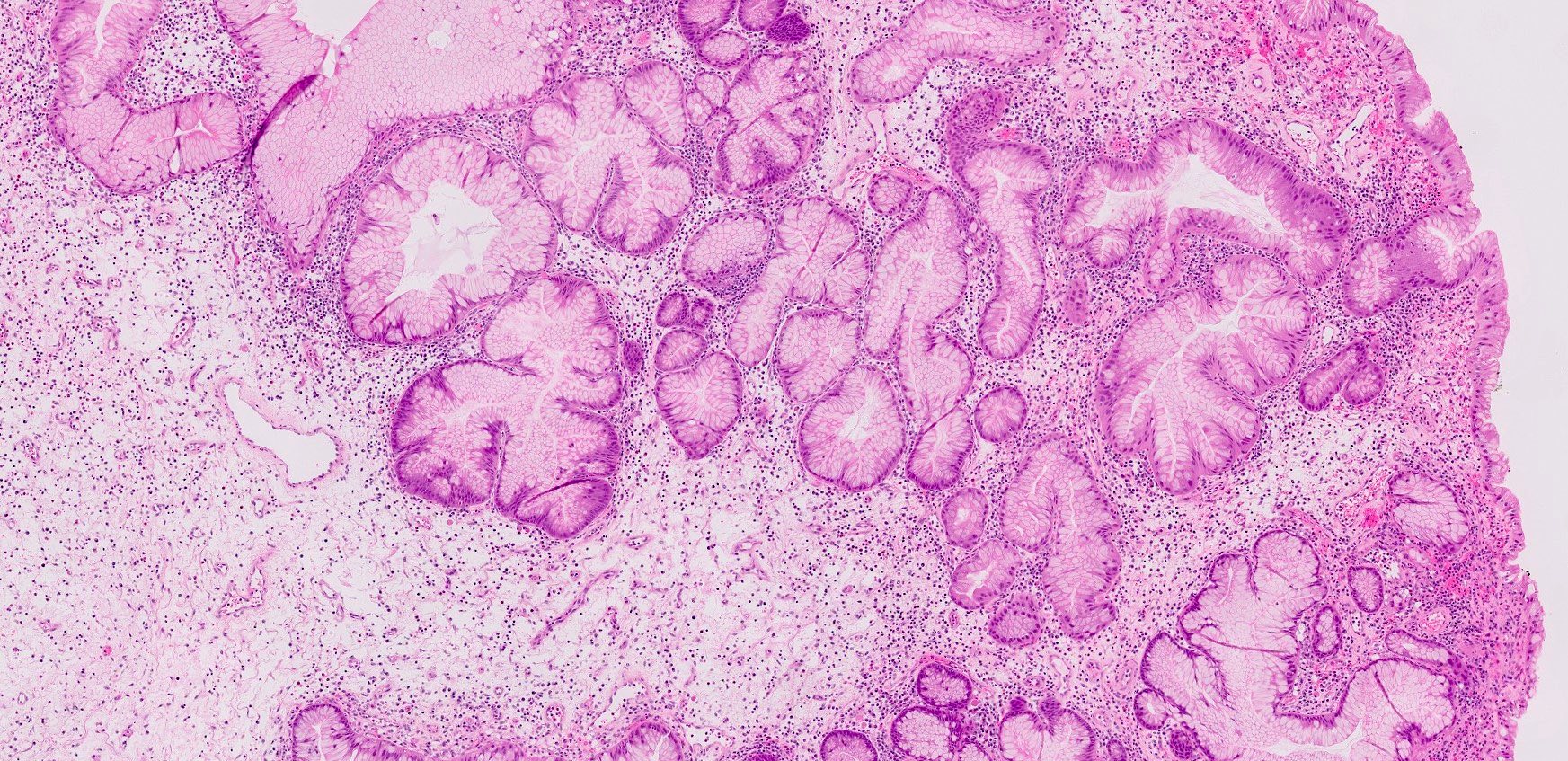
Microscopic (histologic) description
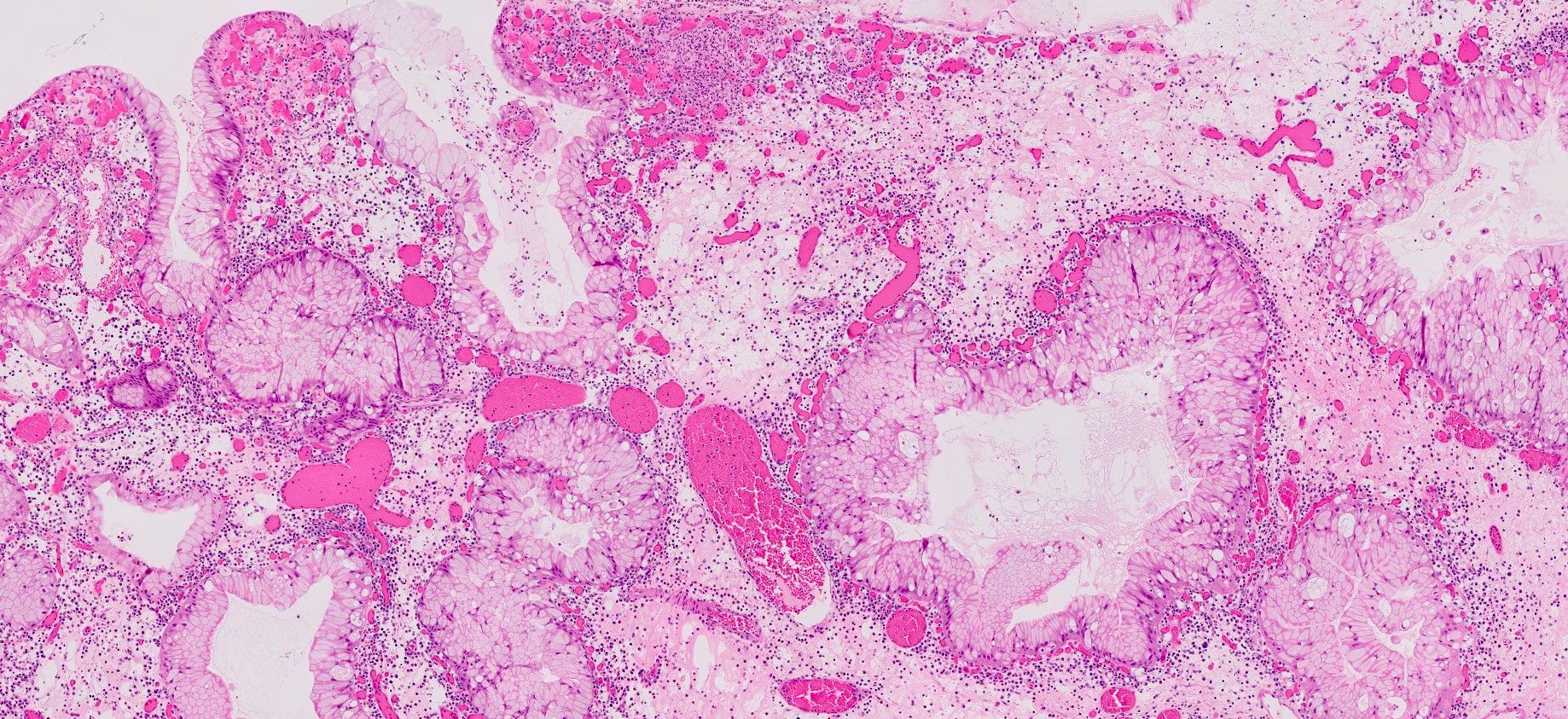
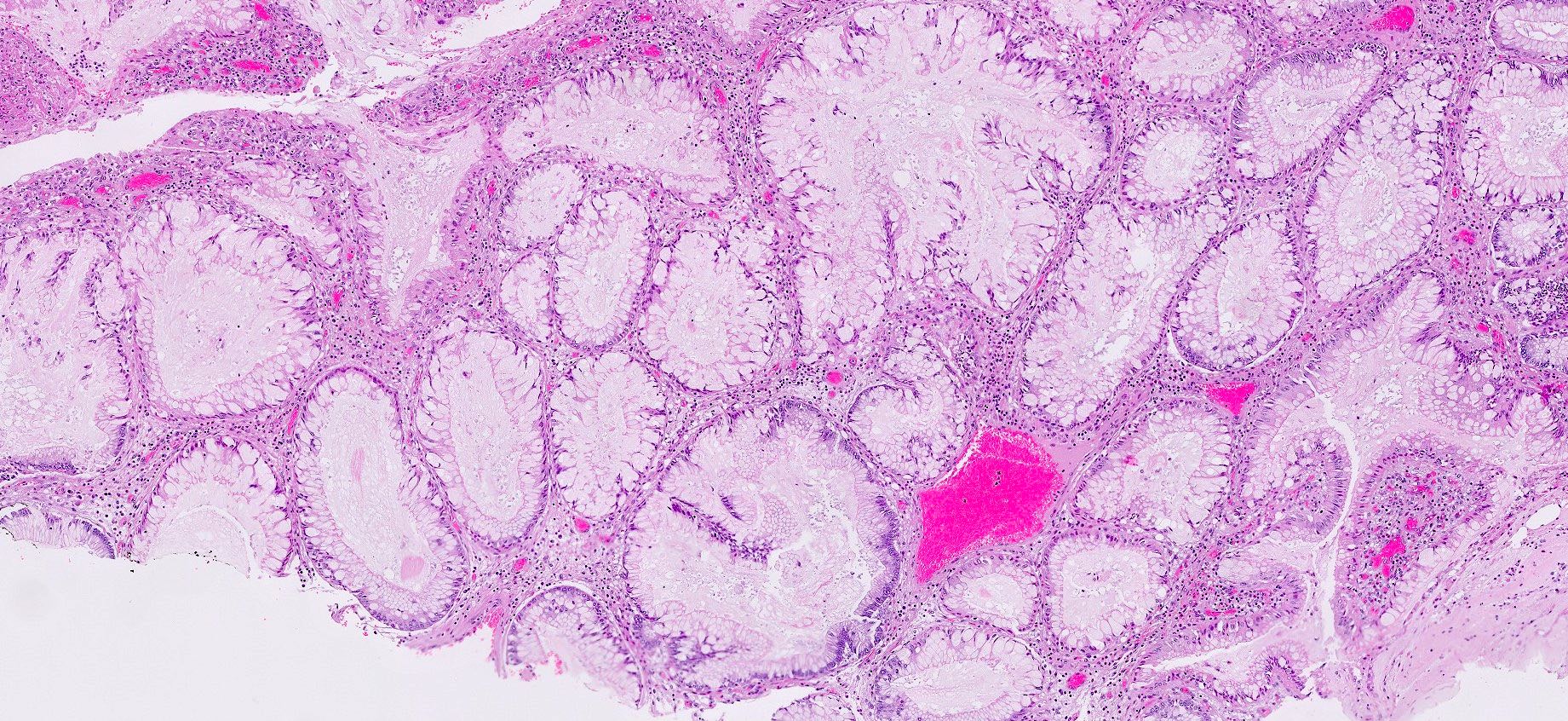
- Hyperplastic foveolar epithelium
- Frequent foveolar lined and mucin filled cystically dilated glands
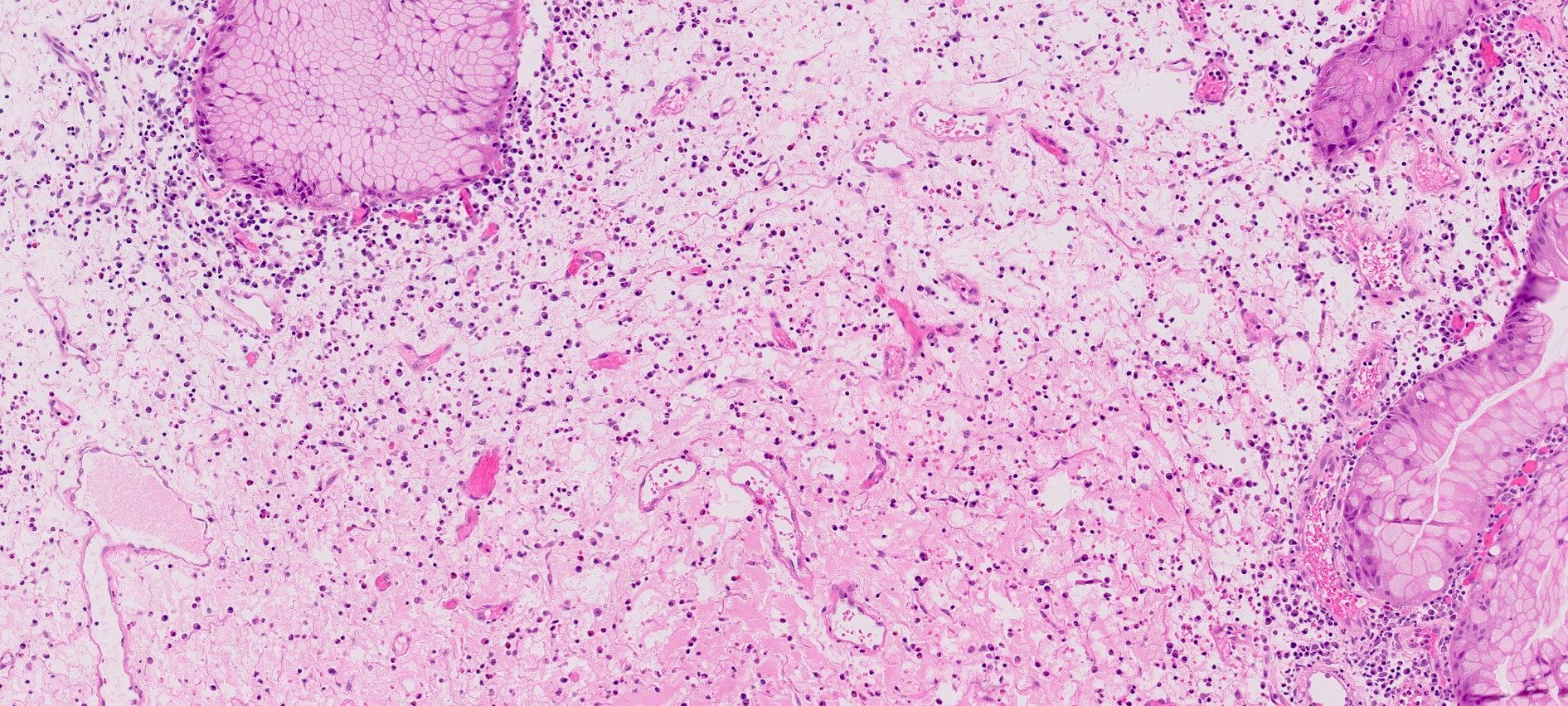
- Background stromal edema and prominent inflammation (neutrophils and eosinophils)
- Surface erosion with granulation tissue
- 2 distinct phenotypes based on crypt:stroma ratio (Am J Surg Pathol 2011;35:530)
- Classic / stromal dominant (crypt:stroma ratio < 1): spherical, prominent stromal compartment, low crypt density, eroded surface, inflammation and reactive changes (flattening) of the epithelium and distortion and dilation of the glands
- Epithelial dominant (crypt:stroma ratio ≥ 1): lobulated, no expanded stromal compartment, intact villous-like surface, prominent columnar hypermucinous epithelium
- Epithelial dominant variant more common with SMAD4 mutations
- Classic / stromal dominant variant more common with BMPR1A mutations
- Solitary, sporadic polyps are commonly classic / stromal dominant type
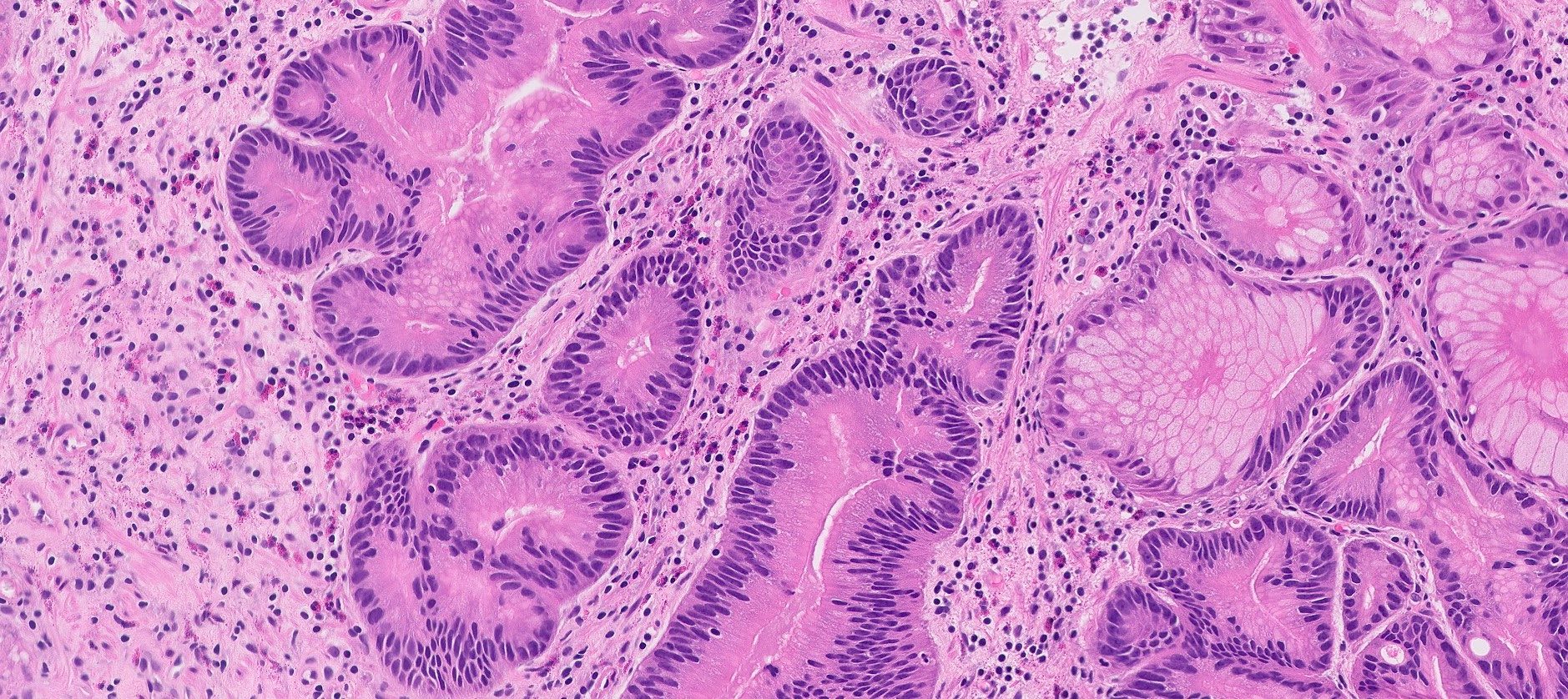
- Dysplasia (around 14%) is usually mixed with foveolar, intestinal or pyloric gland type (Am J Surg Pathol 2014;38:1618)
- Low grade: elongated hyperchromatic nuclei with maintained polarity
- High grade: complex cytoarchitectural features characterized by cribriform glands, back to back glands with enlarged rounded nuclei with frequent loss of polarity
Microscopic (histologic) images
Positive stains
- Loss of SMAD4 expression seen in 50 - 60% of cases
- Loss of SMAD4 does not correlate with the presence of dysplasia (Clin Cancer Res 2010;16:4126)
- Aberrant expression (loss or overexpression) of p53 frequently correlates with low grade dysplasia (Clin Cancer Res 2010;16:4126)
Molecular / cytogenetics description
- Germline mutations in SMAD4 and BMPR1A are associated with ~60% of JPS cases
- Other genes involved are PTEN and ENG
- References: Hum Mol Genet 1998;7:1907, Am J Med Genet A 2005;138A:113, Clin Transl Gastroenterol 2019;10:e00054, J Med Genet 2007;44:702
Sample pathology report
- Stomach, antrum, polypectomy:
- Gastric hyperplastic / juvenile polyp (see comment)
- Negative for intestinal metaplasia or dysplasia
- Comment: Gastric hyperplastic polyps and juvenile polyps show similar histologic features and cannot be reliably distinguished on histology alone. Gastric hyperplastic polyps arise in the setting of a background mucosal injury. Juvenile polyps are hamartomatous polyps, which can be sporadic or syndromic in a setting of juvenile polyposis syndrome. Further endoscopic examination for a sampling of background mucosa and any additional intestinal polyps will be helpful. If clinically indicated, genetic counseling and testing may be performed to evaluate the possibility of polyposis syndrome.
Differential diagnosis
- Hyperplastic polyp:
- Challenging to distinguish from gastric juvenile polyps on histology alone
- Background mucosa shows evidence of chronic gastritis, whereas the background gastric mucosa is normal in patients with juvenile polyposis
- Early age of onset and presence or history of multiple polyps is suggestive of JPS
- Peutz-Jeghers polyp:
- Challenging to distinguish from gastric juvenile polyps on histology alone
- Usually associated with small bowel polyps showing characteristic features of arborizing smooth muscle
- Concurrent mucocutaneous pigmentation is present
- Cronkite-Canada syndrome polyp:
- Challenging to distinguish from gastric juvenile polyps on histology alone
- Usually diffuse and involve the entire stomach
- Background mucosa is also abnormal with microcystic changes
Board review style question #1
A 21 year old man presents to a clinic with a history of black, tarry stools. Anemia is present on complete blood count (CBC). An endoscopy is performed and multiple sessile and pedunculated polyps with a smooth, eroded surface are found in the stomach. Multiple polyps are excised and a representative H&E is shown above. Germline mutations in which of the following genes is associated with the formation of such polyps?
- KRAS
- MSH2
- RET
- SMAD4
Board review style answer #1
D. SMAD4. The image shows a picture of the juvenile polyp. The endoscopic findings raise the possibility of juvenile polyposis syndrome (JPS), which is associated with germline mutations of SMAD4. Answers A, B and C are incorrect because these genes are not associated with JPS.
Comment Here
Reference: Juvenile polyp
Comment Here
Reference: Juvenile polyp
Board review style question #2
Which of the following statements is true about juvenile gastric polyps?
- Associated with SMAD4 and BMPR1A mutations
- Fundus is the most common site where juvenile gastric polyps occur
- Loss of SMAD4 correlates with the presence of dysplasia in juvenile polyps
- There is no associated increased risk of gastric cancer
Board review style answer #2
A. Associated with SMAD4 and BMPR1A mutations. Germline mutations in SMAD4 and BMPR1A are associated with ~60% of juvenile polyposis syndrome (JPS) cases. Answer C is incorrect because SMAD4 loss does not correlate with the presence / absence of dysplasia in these polyps. Aberrant p53 may correlate with the presence of low grade dysplasia. Answer B is incorrect because the most common site is the antrum. Answer D is incorrect because syndromic juvenile polyps are associated with a 21 - 30% risk of gastric cancer.
Comment Here
Reference: Juvenile polyp
Comment Here
Reference: Juvenile polyp