Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Srivastava S. Intestinal metaplasia. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/stomachintestinalmetaplasia.html. Accessed March 28th, 2025.
Definition / general
- Gastric intestinal metaplasia (IM) is a metaplastic condition wherein the normal gastric mucosa is replaced by intestinal type of mucosa replete with mucin producing goblet cells, with or without Paneth cells and absorptive cells
- Diagnosis of intestinal metaplasia in stomach is based on the presence of goblet cells
- Gastric intestinal metaplasia is a known high risk lesion for gastric carcinoma (GC)
Essential features
- In gastric intestinal metaplasia, native gastric mucosa is replaced with intestinal type of mucosa replete with goblet cells, with or without Paneth and absorptive cells
- It is considered to be a precancerous lesion and is associated with gastric carcinoma
- Prevalence of intestinal metaplasia is 25% according to a meta analysis; annually, 0.1 - 10% of gastric intestinal metaplasia can progress to gastric carcinoma
- Commonly occurs as a response to chronic H. pylori infection, bile acid reflux, smoking or high salt intake
- 2 types of gastric intestinal metaplasia are known: complete and incomplete
- Incomplete type is associated with gastric carcinoma
- Operative link for gastric intestinal metaplasia (OLGA / OLGIM) stages III / IV are considered high risk factors for gastric carcinoma
Terminology
- Gastric intestinal metaplasia (GIM)
ICD coding
Epidemiology
- Worldwide prevalence of gastric intestinal metaplasia is 25% according to a meta analysis; ranging from 24 - 84% in Eastern countries to 7 - 25% in Western countries (JGH Open 2020;4:569)
- Incidence increases with older age, male gender, Helicobacter pylori infection, family history of gastric cancer, bile reflux, smoking, high salt intake and a diet low in fruits, vegetables and vitamin C (Aliment Pharmacol Ther 2002;16:1209, Nat Rev Cancer 2017;17:594)
- Annual rate of progression of intestinal metaplasia to gastric carcinoma varies from 0 - 10% (Helicobacter 2007;12:22)
- When associated with high grade dysplasia, progression to gastric carcinoma is reported in 60 - 85% of cases within 4 - 48 months; with low grade dysplasia (LGD), the risk is much lower (Gastroenterology 2008;134:945)
Sites
- All parts of the stomach can be affected; but it is most frequently observed in antrum and along the lesser curvature of stomach
Pathophysiology
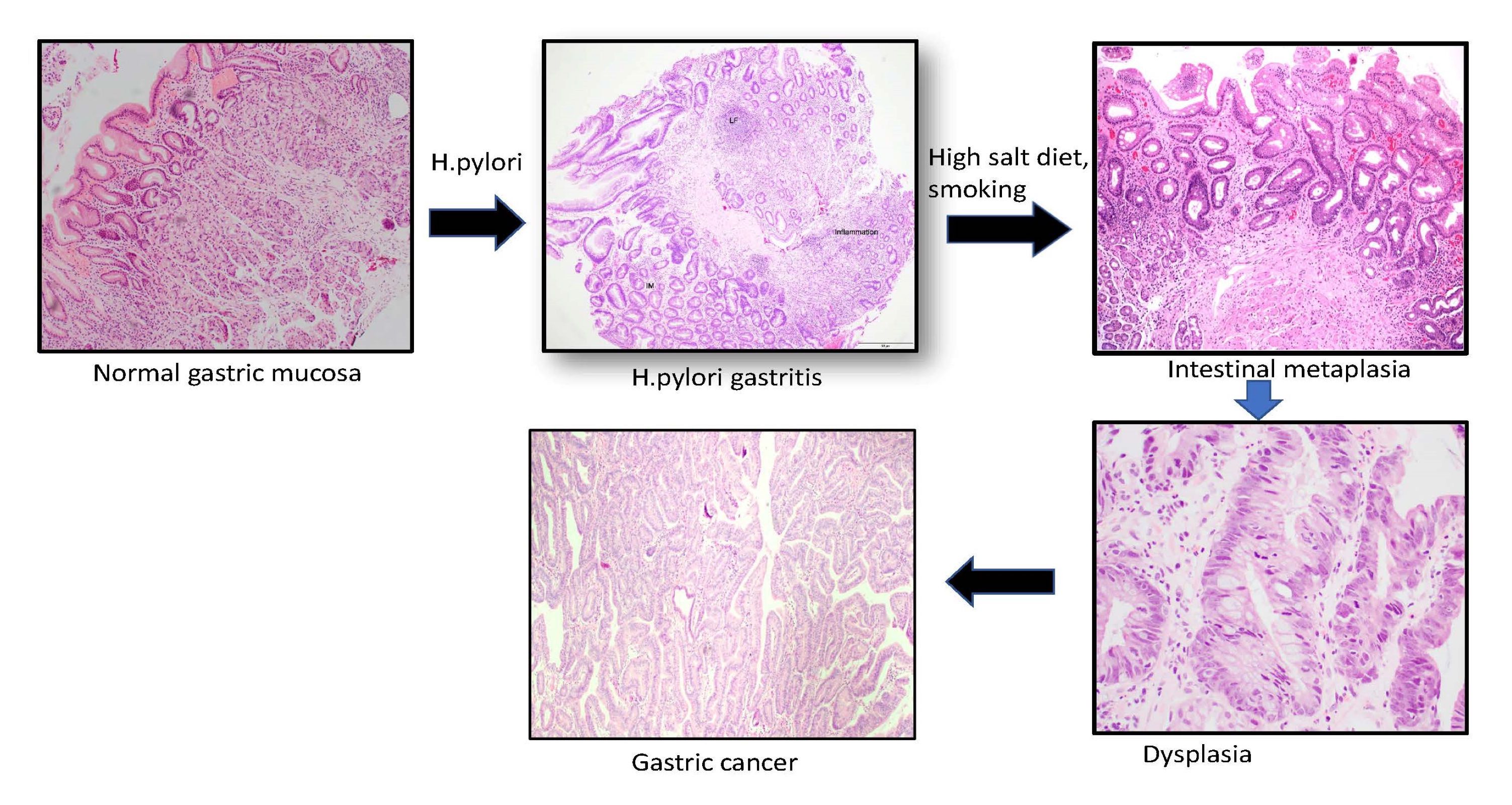
- Intestinal metaplasia results from a distinct interplay between host immune susceptibility, genetic response and environmental exposure; chronic mucosal inflammation leads to gastric atrophy, which leads to replacement of gastric mucosa with intestinal type epithelium
- Recent animal models have shown that spasmolytic polypeptide expressing metaplasia (SPEM) is a precursor of intestinal metaplasia (Gastroenterol Hepatol (N Y) 2018;14:92)
Etiology
- Helicobacter pylori infection, bile reflux, smoking, high salt intake
- Incidence increases with age > 50 years, male gender, positive family history of gastric cancer, immigrants from H. pylori endemic areas
Diagrams / tables
Clinical features
- Gastric intestinal metaplasia is usually asymptomatic; may present with acid reflux or heartburn due to underlying conditions like chronic acid reflux and H. pylori infection
- Associated with autoimmune gastritis and pernicious anemia
Diagnosis
- Gold standard for diagnosis of gastric intestinal metaplasia is histology
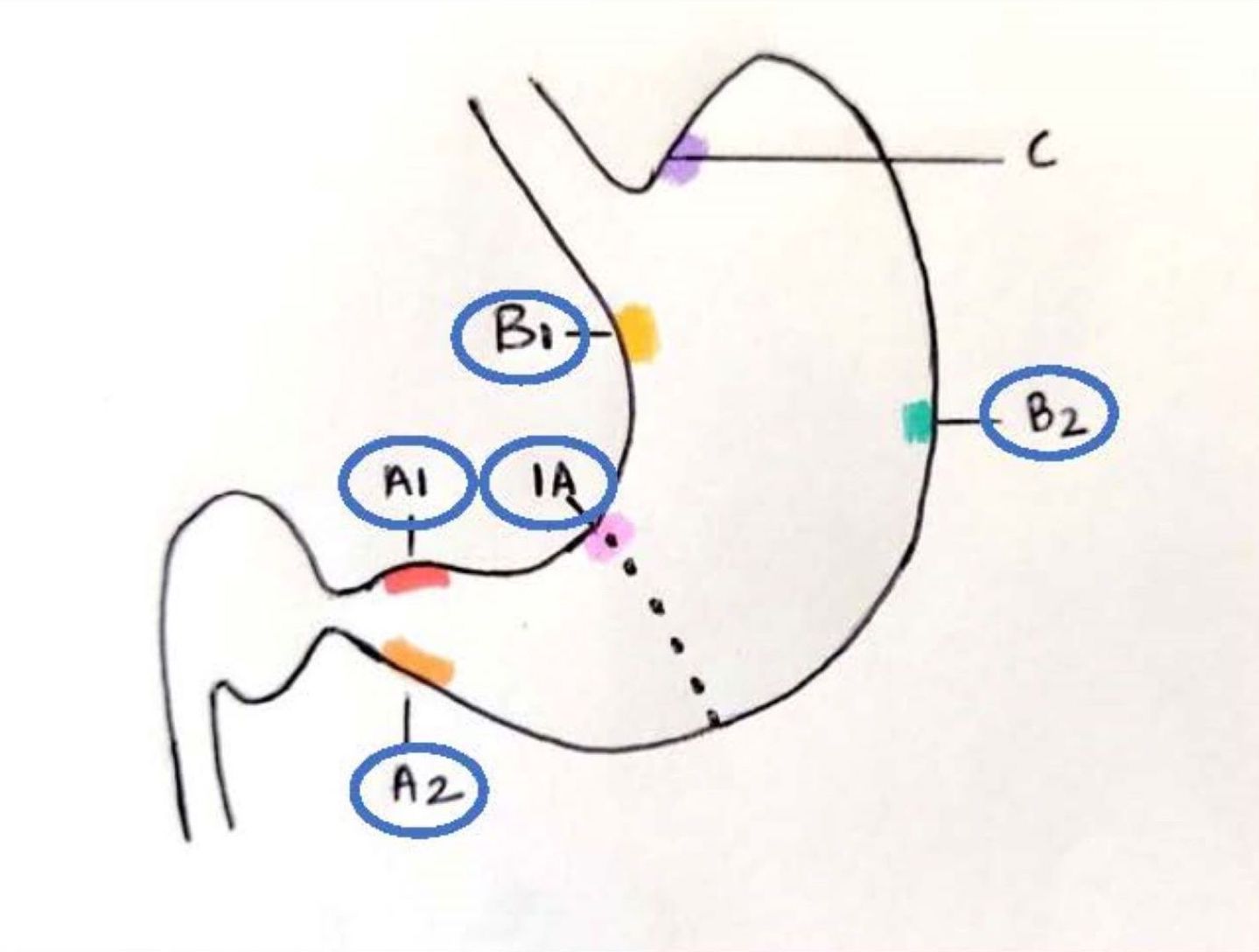
- According to updated Sydney protocol, endoscopic targeted biopsies were recommended initially for grading and recently for staging of intestinal metaplasia
- It recommends at least 5 biopsies: 2 from antrum (1 each from lesser and greater curve, 3 cm proximal to the pylorus), 2 from body (lesser curve, 4 cm proximal to the incisura and midpoint of the greater curve) and 1 from the incisura (Am J Surg Pathol 1996;20:1161)
- According to updated Sydney protocol, endoscopic targeted biopsies were recommended initially for grading and recently for staging of intestinal metaplasia
- Japan, South Korea and Singapore are a few of the countries that have endoscopic screening programs for early detection of precancerous lesions and gastric carcinoma (Gut 2022;71:854, Dig Endosc 2022;34:412)
Laboratory
- Some studies have shown that serum TFF3 could be used as a marker to detect gastric intestinal metaplasia (Cancer Biomark 2017;19:231, J Pathol 2002;197:582)
Radiology description
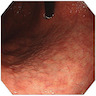
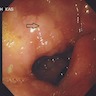
- Endoscopic findings: white light endoscopy shows grayish white, slightly elevated plaques surrounded by patchy pink and pale areas
- Image enhanced magnification endoscopy: groove type pattern for gastric body, light blue crest and marginal blue band for gastric antrum
- Narrow band imaging: tubular or tubulovillous glands with light blue crests
- Chromoendoscopy with methylene blue: blue colored irregular marks, blue round and tubular pits or blue villi or blue small pits
- Reference: Cancers (Basel) 2021;13:6242
Prognostic factors
- The following show high association with gastric carcinoma and warrant endoscopic surveillance (Endoscopy 2019;51:365):
- Incomplete (type III) type intestinal metaplasia
- OLGA / OLGIM stages III / IV
- Extensive intestinal metaplasia (intestinal metaplasia involving both antrum and body)
- Persistent H. pylori infection
- First degree family history of gastric cancer
- Immigrants from areas of high incidence of H. pylori
- Age > 50 years
- Smoking
Case reports
- 62 year old Chinese woman with intestinal metaplasia was diagnosed with multiple early gastric cancer after a surveillance of 7 years (Medicine (Baltimore) 2019;98:e15686)
- 76 year old woman presented with acute dysphagia with histology showing gastric intestinal metaplasia and Giardia duodenalis trophozoites (J Gastrointestin Liver Dis 2017;26:221)
- 81 year old man with a 1.2 cm nodule along the incisura of the stomach (Cureus 2020;12:e7427)
- 87 year old woman presented with a longstanding dyspeptic symptom along with a history of peptic ulcer disease and partial gastrectomy (BMJ Case Rep 2016;2016:bcr2016216556)
Treatment
- Currently no definitive treatment for gastric intestinal metaplasia
- American Association of Gastroenterologists (AGA 2020) recommends treatment and eradication of H. pylori to manage intestinal metaplasia; H. pylori eradication cannot reverse gastric intestinal metaplasia but it may slow down the progression to gastric carcinoma (Gastroenterology 2020;158:693)
- AGA further recommends endoscopic surveillance every 3 - 5 years in high risk gastric intestinal metaplasia (i.e., incomplete intestinal metaplasia, extensive intestinal metaplasia, family history of gastric carcinoma, racial / ethnic minorities and immigrants from high incidence areas)
- British Society of Gastroenterologists (BSG 2019) recommends endoscopic surveillance every 3 years for extensive intestinal metaplasia or intestinal metaplasia limited to antrum with a positive family history or persistent H. pylori infection, annually if low grade dysplasia or every 6 months if persistent high grade dysplasia (HGD) with no plan to treat (Gut Liver 2019;13:596)
- European Society of Gastrointestinal Endoscopy (ESGE 2019) recommends endoscopic surveillance as follows (Endoscopy 2019;51:365):
- Every 1 - 2 years with positive family history of gastric carcinoma plus either extensive intestinal metaplasia or OLGA / OLGIM stages III / IV
- Every 3 years if extensive intestinal metaplasia or OLGA / OLGIM stages III / IV
- Consider every 3 years for focal intestinal metaplasia plus family history of gastric carcinoma, incomplete intestinal metaplasia or persistent H. pylori gastritis
- According to the latest Academy of Medicine, Singapore Clinical guidelines (2022), endoscopic surveillance is recommended every 3 years with OLGA / OLGIM stages III / IV and every 2 years with more than 2 risk factors, such as smoking, age > 50 years, incomplete intestinal metaplasia, persistent H. pylori infection, first degree family history of gastric cancer (Ann Acad Med Singap 2022;51:417)
- COX2 inhibitors are not recommended
- Use of dietary supplement with antioxidants is not supported as a therapy
- Endoscopic submucosal dissection (ESD) or endoscopic mucosal resection (EMR) are recommended for gastric intestinal metaplasia associated with visible dysplasia or early gastric carcinoma
Clinical images
Gross description
- Patchy erythema or slightly elevated, grayish white plaque
Microscopic (histologic) description
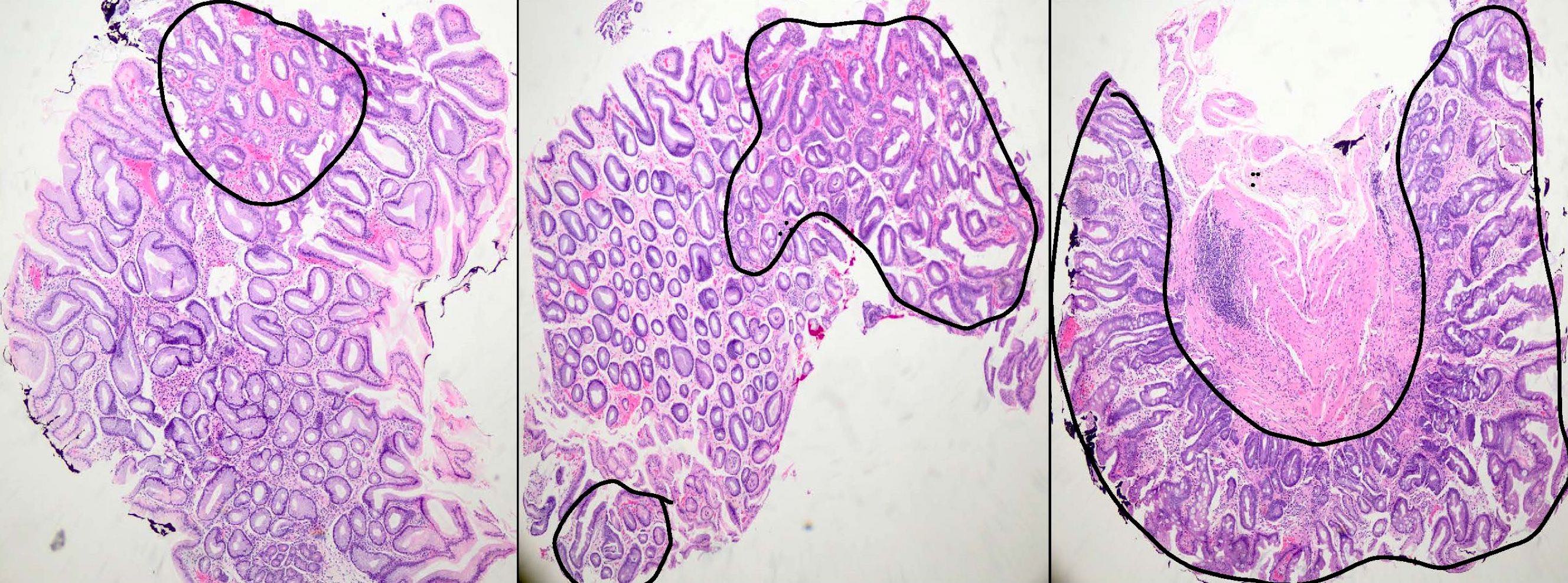
- Intestinal metaplasia can be identified by the replacement of gastric columnar mucosa with intestinal type of mucosa comprised of goblet cells, with or without Paneth cells and absorptive brush border
- Usually occurs in a background of chronic gastritis or H. pylori gastritis
- It can be classified as complete (type I) and incomplete (II and III) subtypes
- Complete (type I) intestinal metaplasia (Cancer Res 1999;59:1003):
- Resembles small intestinal mucosa with straight crypt architecture, well formed goblet cells, Paneth cells and absorptive brush border
- Goblet cells secrete sialomucins or occasionally sulphomucins
- It has been reported that the complete type of intestinal metaplasia shows weak or no expression of MUC1 and MUC5AC and the absorptive brush border express the enzyme sucrase, trehalase, maltase and intestinal type alkaline phosphatase
- Incomplete (type II and III):
- Histologically resembles either small or large intestinal mucosa and shows irregular architecture, variable size goblet cells and columnar mucosa in various stages of differentiation; Paneth cells and absorptive brush border may or may not be seen
- In type II intestinal metaplasia, goblet cells secrete sialomucins while the columnar mucosa may secrete sialomucins or neutral mucins
- In type III intestinal metaplasia, sulphomucins predominate in the columnar mucosa while sialo or sulphomucins predominate in goblet cells
- Incomplete intestinal metaplasia shows overexpression of MUC1 and MUC5AC and secretes sucrase, maltase but not trehalase and alkaline phosphatase
- Type III intestinal metaplasia shows highest association with gastric cancer
- Intestinal metaplasia is graded as mild (< 30%), moderate (31 - 60%) and marked (61 - 100%) according to the updated Sydney classification
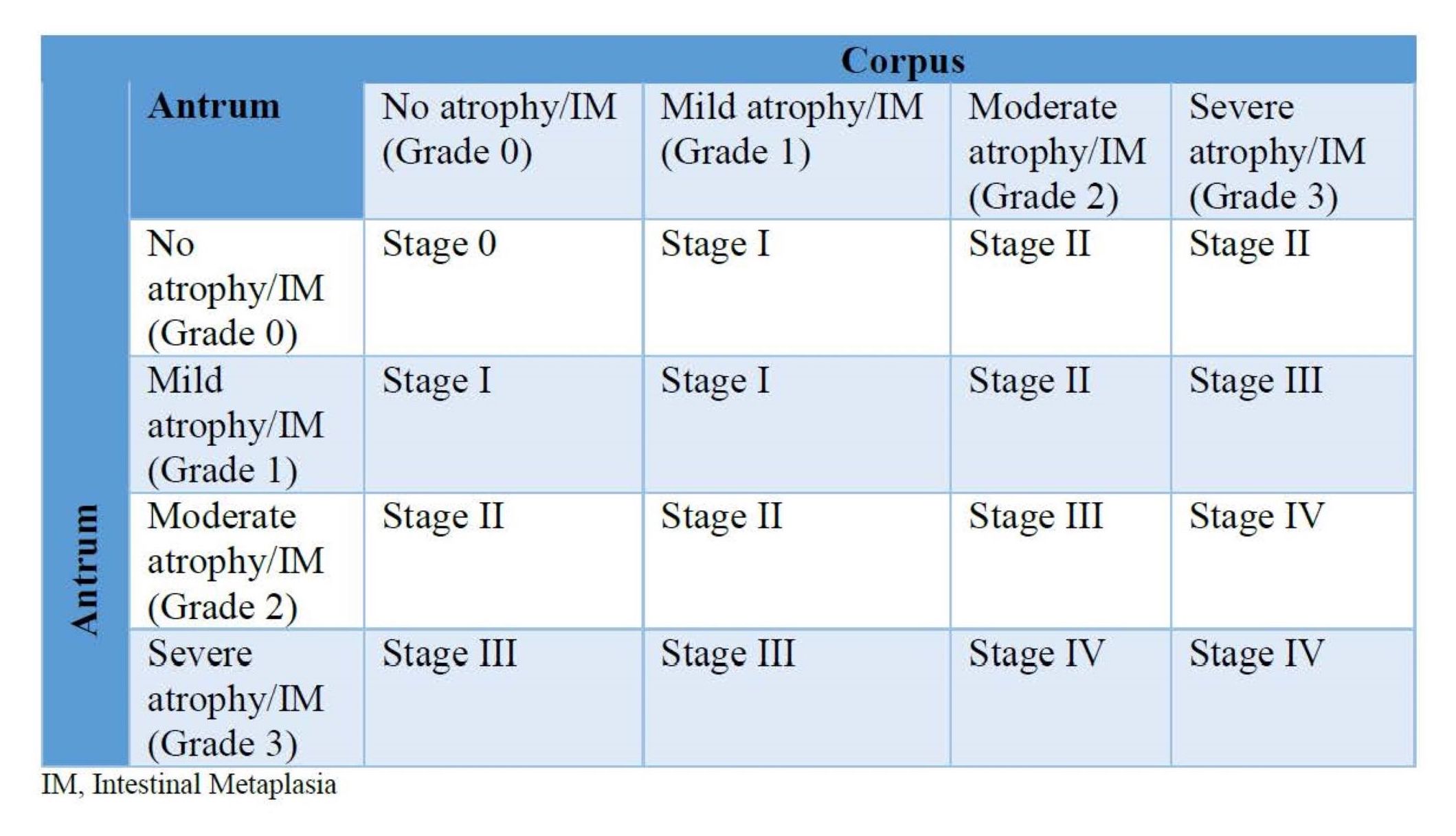
- Intestinal metaplasia is staged using the OLGA / OLGIM system; it is based on the grades of intestinal metaplasia in the antrum (including incisura) and body
- OLGA / OLGIM stages III and IV are considered as high risk for progression to gastric cancer (Gastrointest Endosc 2010;71:1150)
- In autoimmune gastritis, extensive (marked) gastric intestinal metaplasia is seen restricted to the body (oxyntic mucosa) in a background of moderate to marked chronic inflammation, loss of oxyntic glands and accompanied with SPEM or pseudopyloric metaplasia (presence of pseudopyloric or antral glands in body of stomach) (World J Gastroenterol 2015;21:12179)
Microscopic (histologic) images
Cytology description
- Goblet cells are visible in smears; in some smears, brush border can also be seen (J Clin Pathol 1961;14:132)
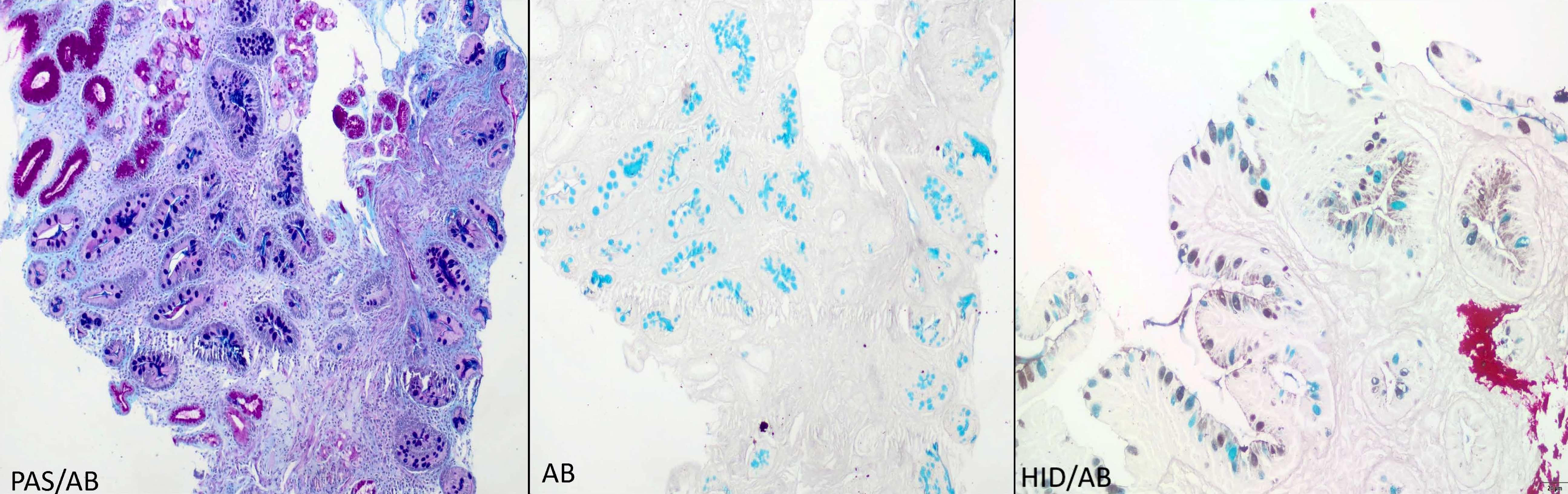
Positive stains
- Alcian blue (AB) at pH 2.5: diagnostic stain but not routinely needed as gastric intestinal metaplasia can be readily identified in H&E; AB stains goblet cells bright blue due to the presence of sialomucins
- Periodic acid-Schiff Alcian blue (PAS AB): stains the acidic or sialomucins as purple and neutral mucins as magenta
- High iron diamine Alcian blue (HID AB): stains the acidic or sialomucins as blue and sulfated mucin as brownish black in color; not routinely performed due to toxic and carcinogenic reagents
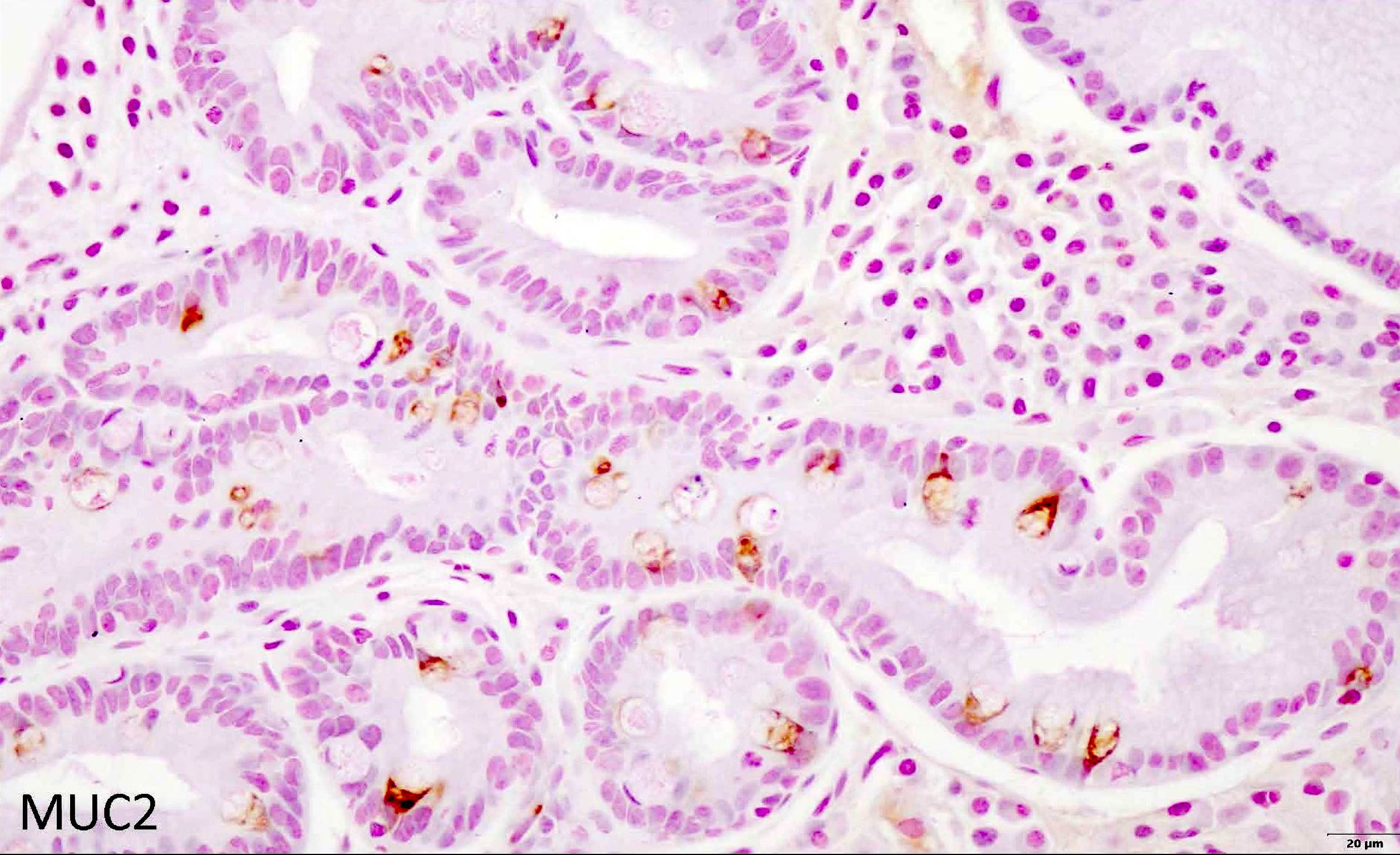
- MUC2 IHC: de novo expression in the cytoplasm of goblet cells (Virchows Arch 2002;440:311)
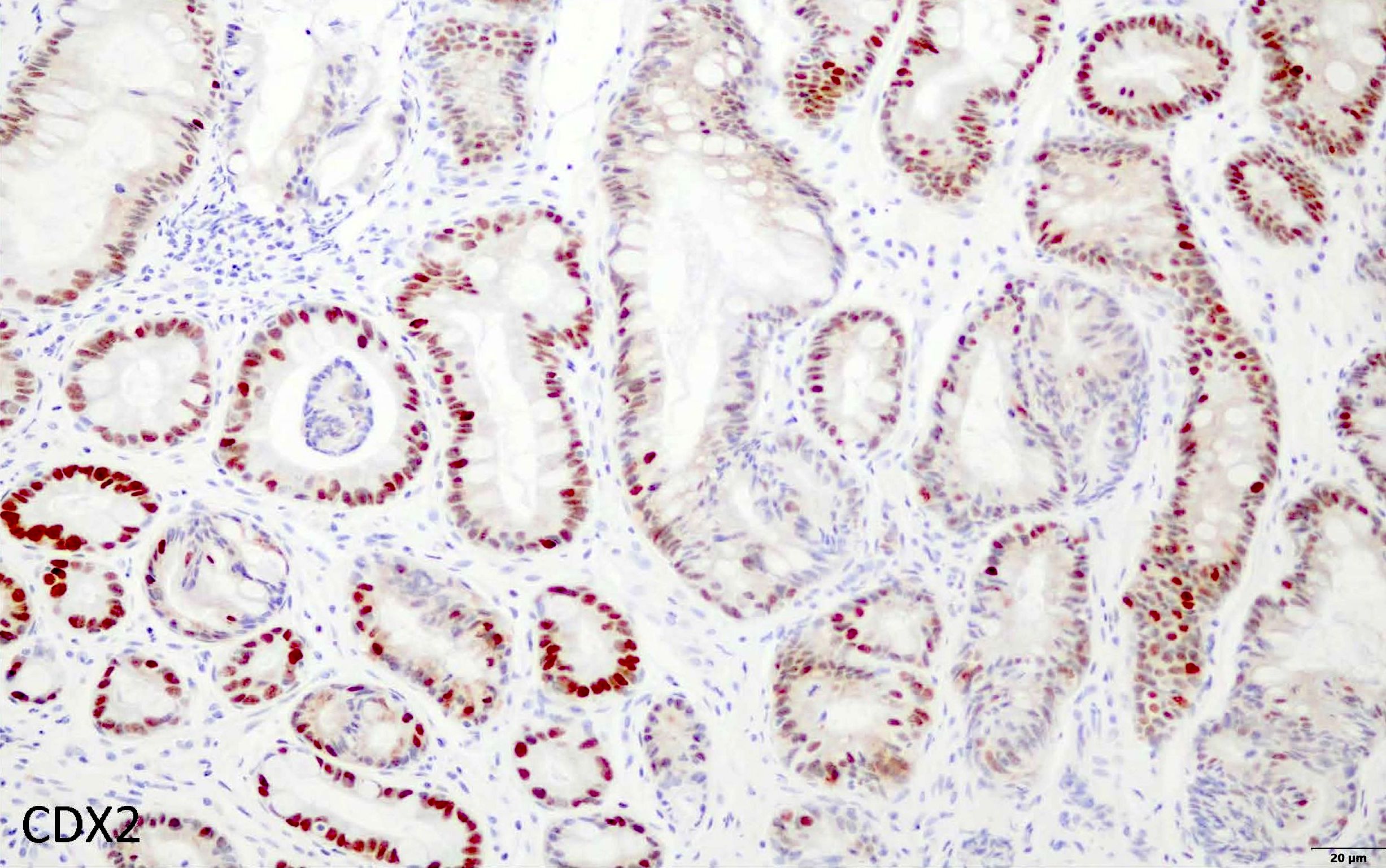
- CDX2: nuclear positivity of goblet cells and columnar mucosa (Gut Liver 2012;6:71)
- Intestinal markers like CD10, villin, Das1 are variably positive in gastric intestinal metaplasia
Negative stains
Electron microscopy description
- Microvilli border with interspersed goblet cells
Molecular / cytogenetics description
- Compared to gastric carcinoma, gastric intestinal metaplasia exhibits lower mutational burden, recurrent mutation in FBXW7 gene, chromosome 8q amplification, focal DNA hypermethylation and shortened telomeres (Cancer Cell 2018;33:137)
- CDX2 / CDX1, TFF3, villin, OCT1 and PDX1 genes are upregulated in intestinal metaplasia (J Gastroenterol Hepatol 2009;24:193)
Sample pathology report
- Stomach, biopsy:
- Gastric mucosa shows chronic gastritis with mild / moderate / marked intestinal metaplasia (see comment)
- Comment: No evidence of H. pylori, dysplasia or malignancy.
Differential diagnosis
- Pseudogoblet cells:
- These are columnar cells that are goblet or barrel shaped and arranged in a continuous linear fashion
- On the contrary, true goblet cells are rounded in shape and are dispersed singly
- AB stain can be used to distinguish pseudogoblet cells (bluish hue may be present sometimes) and true goblet cells (distinct blue colored goblet cells)
- Barrett esophagus (BE)
- Preneoplastic condition observed in the lower one - third of the esophagus
- Metaplastic condition where the squamous epithelium of the distal esophagus is replaced by the intestinal epithelium with goblet cells
- Histologically, both gastric intestinal metaplasia and Barrett esophagus appear similar, the difference being the location (stomach versus distal esophagus)
- While gastric intestinal metaplasia occurs in a background of H. pylori commonly, Barrett esophagus has no causal association with H. pylori
- Incomplete type of intestinal metaplasia is more commonly observed in Barrett esophagus as compared to gastric intestinal metaplasia (Mod Pathol 2004;17:62)
- Predisposes to dysplasia and esophageal adenocarcinoma
Board review style question #1
A 65 year old man presented to clinic with complaints of dyspepsia and acid reflux. An endoscopic biopsy was done and the sample was sent for histopathological examination. Based on the histopathological image above, what is the diagnosis?
- Chronic gastritis only
- H. pylori gastritis with intestinal metaplasia
- Intestinal metaplasia
- Reactive gastropathy
Board review style answer #1
B. H. pylori gastritis with intestinal metaplasia. Intestinal metaplasia most commonly occurs in the background of H. pylori infection. Intestinal metaplasia can be recognized readily by the presence of well formed goblet cells.
Comment Here
Reference: Intestinal metaplasia
Comment Here
Reference: Intestinal metaplasia
Board review style question #2
Which of the following is a high risk factor for gastric intestinal metaplasia?
- Age < 50 years
- Complete intestinal metaplasia
- Extensive intestinal metaplasia
- Mild / focal intestinal metaplasia
Board review style answer #2
C. Extensive intestinal metaplasia is one of the high risk factors for gastric intestinal metaplasia. It refers to intestinal metaplasia present in both antrum and body.
Comment Here
Reference: Intestinal metaplasia
Comment Here
Reference: Intestinal metaplasia