Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Razzano D, Longacre TA. Well differentiated neuroendocrine tumor. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/stomachcarcinoid.html. Accessed January 10th, 2025.
Definition / general
- The 5th edition of the WHO 2019 Digestive Tumors Classification definition of neuroendocrine tumor (NET): well differentiated, grade 1, 2 or 3 based on mitotic rate per 2 mm2 (based on counting 10 mm2 and taking the average) and Ki67 immunohistochemical index (counted in ≥ 500 cells in the area of highest staining); grade assigned by whichever value is higher (see Diagrams / tables)
- Must lack features of carcinoma, which includes poorly differentiated morphology, tumoral necrosis, high N/C ratio and prominent nucleoli
Essential features
- Type I is the most common, followed by type III, with type II being very rare
- Type I is typically indolent and type II and III have a higher malignant potential (Surg Pathol Clin 2020;13:377)
- Grade 3 neoplasms are no longer automatically categorized as a carcinoma per the updated 2019 WHO classification
Terminology
- Historically referred to as carcinoid
- Can be a component of a mixed neuroendocrine nonneuroendocrine neoplasm (MiNEN); must represent ≥ 30% of tumor (Korean J Radiol 2019;20:5)
ICD coding
- ICD-10: D3A.092 - benign carcinoid tumor of the stomach
Epidemiology
- Gastric NETs often occur in the setting of certain diseases (Surg Pathol Clin 2020;13:377):
- Type I:
- Derived from enterochromaffin-like (ECL) cells
- Occurs in a setting of chronic atrophic gastritis (type A) and hypergastrinemia
- More frequent in women
- Type II:
- Derived from ECL cells
- Occurs in a setting of hypergastrinemia due to Zollinger-Ellison syndrome
- Can be seen in patients with multiple endocrine neoplasia type 1 (MEN1) syndrome
- Affects men and women equally
- Type III:
- Sporadic
- More frequent in men
- Type IV:
- Discussed in the literature but not currently recognized by the WHO
- Serotonin producing EC cell NET:
- Rare and usually nonfunctional
- Gastrin producing G cell NET:
- Usually nonfunctional but can cause Zollinger-Ellison syndrome and is then referred to as a gastric gastrinoma
- Type I:
- All types tend to occur in the age range of 50 - 60 years
Sites
- Type I and II are more common in the gastric body and are often multifocal; usually < 2 cm
- Type III occurs anywhere in the stomach and is usually unifocal; usually > 2 cm
Pathophysiology
- May arise in the setting of autoimmune gastritis (type I), Zollinger-Ellison syndrome (type II) or in the absence of a known precursor (type III) (Surg Pathol Clin 2020;13:377)
Etiology
- See Epidemiology
Diagrams / tables
Clinical features
- Tumor functionality is based on clinical symptoms, not on immunohistochemical expression of the hormone (Surg Pathol Clin 2020;13:377)
- These tumors do not typically cause symptoms secondary to hormone secretion
- Serum chromogranin A is used as biomarker to assess the bulk of disease and monitor treatment (World J Gastrointest Oncol 2020;12:791)
Diagnosis
- Typically found on endoscopic exam
- Neuroendocrine neoplasms test (NETest) is a multianalyte liquid biopsy that measures neuroendocrine tumor gene expression in blood and can be used as a diagnostic and disease surveillance test (Endocrinol Metab Clin North Am 2018;47:485)
Laboratory
- Type I and type II will typically have elevated serum gastrin levels
Prognostic factors
- Dependent on tumor subtype, grade and stage at presentation
- Type I: excellent prognosis with a 5 year survival of 90 - 95% (World J Gastrointest Oncol 2020;12:791)
- Type II: good prognosis with a 5 year survival of 60 - 90% (World J Gastrointest Oncol 2020;12:791)
- Type III: worse prognosis with a 5 year survival rate of < 35% (World J Gastrointest Oncol 2020;12:791)
Case reports
- 37 year old woman presented with upper gastrointestinal bleed and epigastric pain (Int J Surg Case Rep 2016;25:62)
- 45 year old woman with autoimmune pernicious anemia and Hashimoto thyroiditis monitored by upper endoscopy (Cureus 2021;13:e13556)
- 56 year old woman with a history of cholelithiasis and irritable bowel syndrome presented with postprandial, colicky left upper quadrant pain radiating to the right shoulder lasting approximately 45 minutes (Cureus 2019;11:e4193)
- 66 year old man with gastric NET presented as a subepithelial tumor mimicking a gastrointestinal stromal tumor (Case Rep Oncol 2021;14:1271)
- 68 year old man with an incidental finding of a small nodule in the gastric fundus (Int J Surg Case Rep 2020;75:361)
Treatment
- Dependent on size at time of endoscopic evaluation
- Excision of tumors: endoscopic mucosal resection, local resection, antrectomy or total gastrectomy (World J Gastrointest Oncol 2020;12:791)
- Chemotherapy, less common (World J Gastrointest Oncol 2020;12:791)
- Somatostatin analogue therapy (World J Gastroenterol 2015;21:6785)
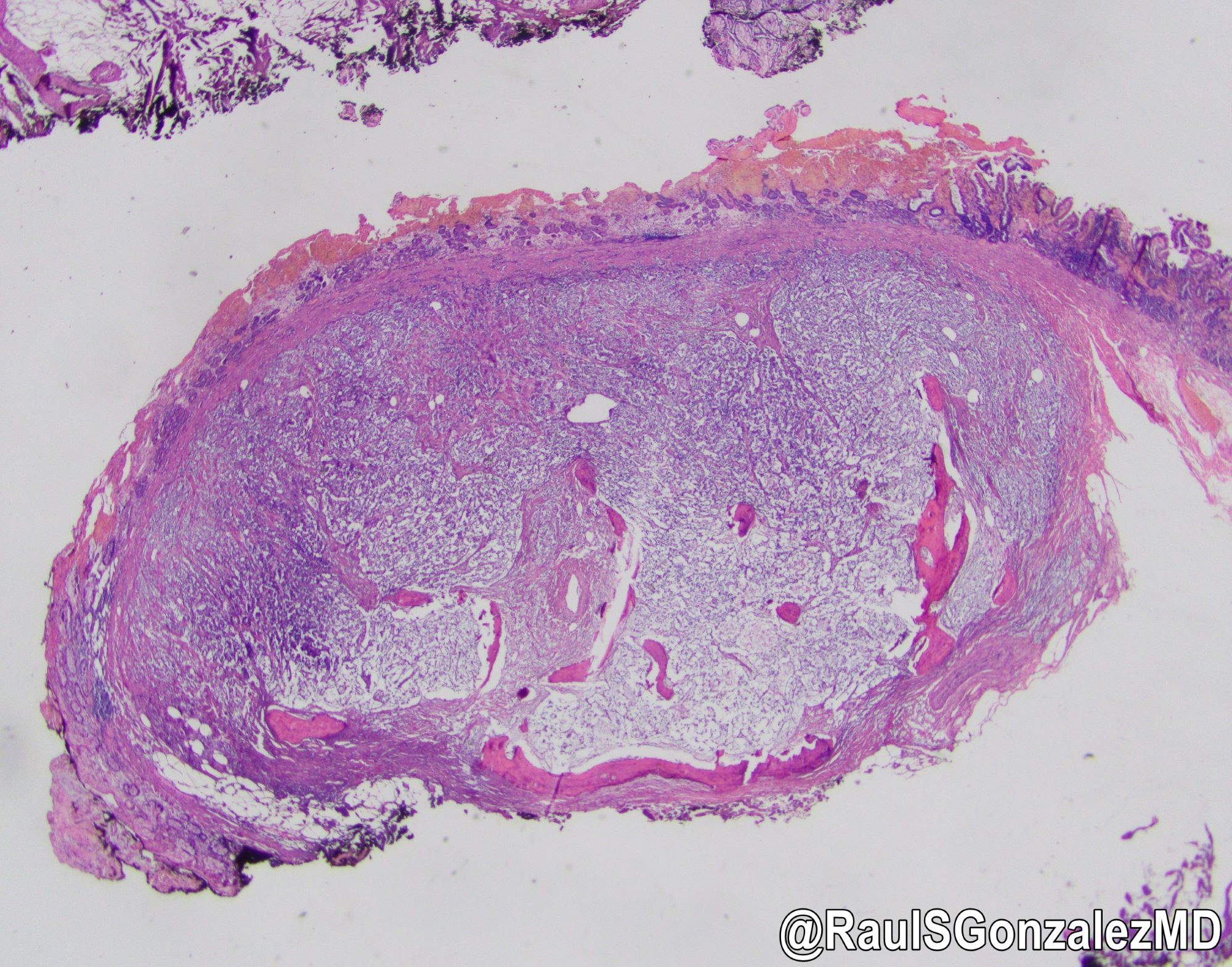
Gross description
- Small, sharply outlined, covered by flattened mucosa
- Resemble polyps or nodules (Medicine (Baltimore) 2022;101:e28550)
Microscopic (histologic) description
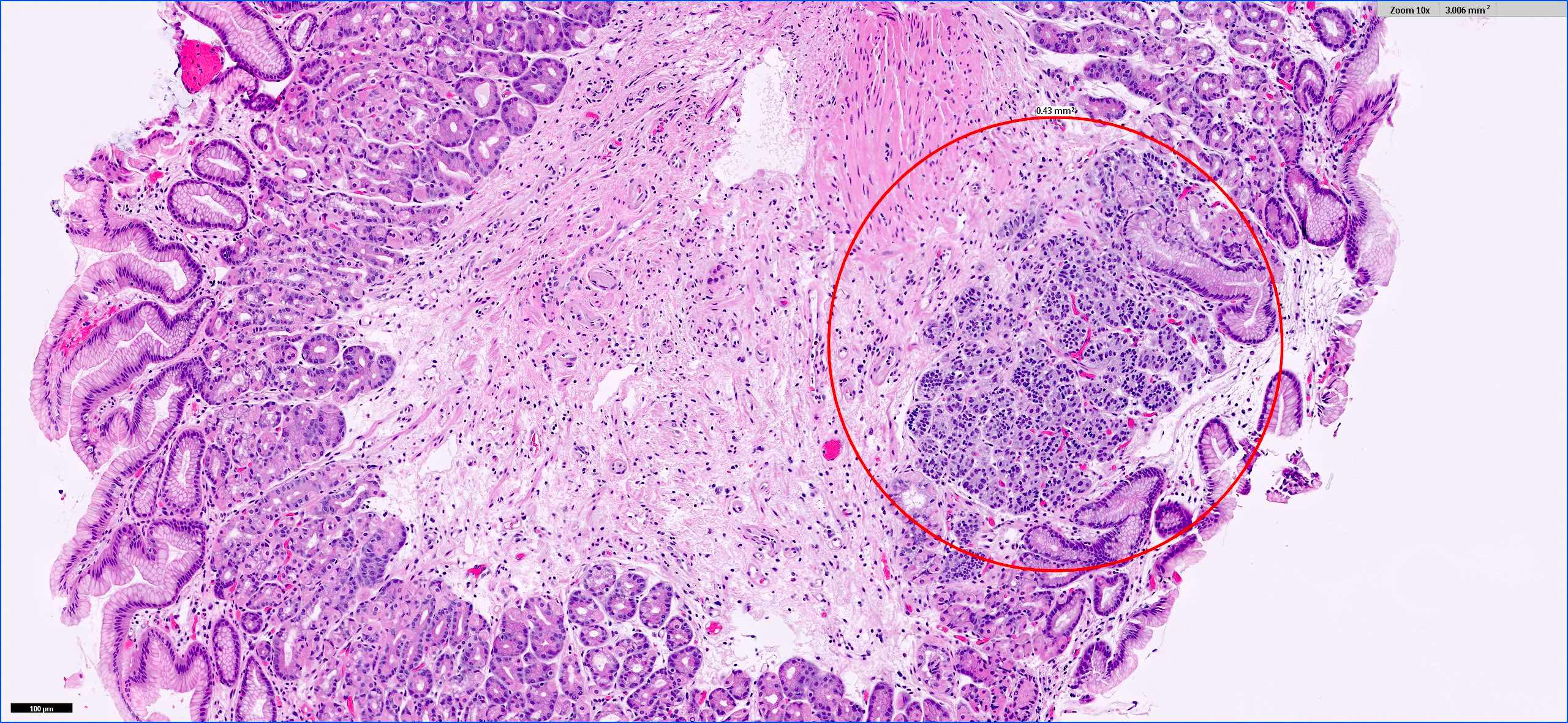
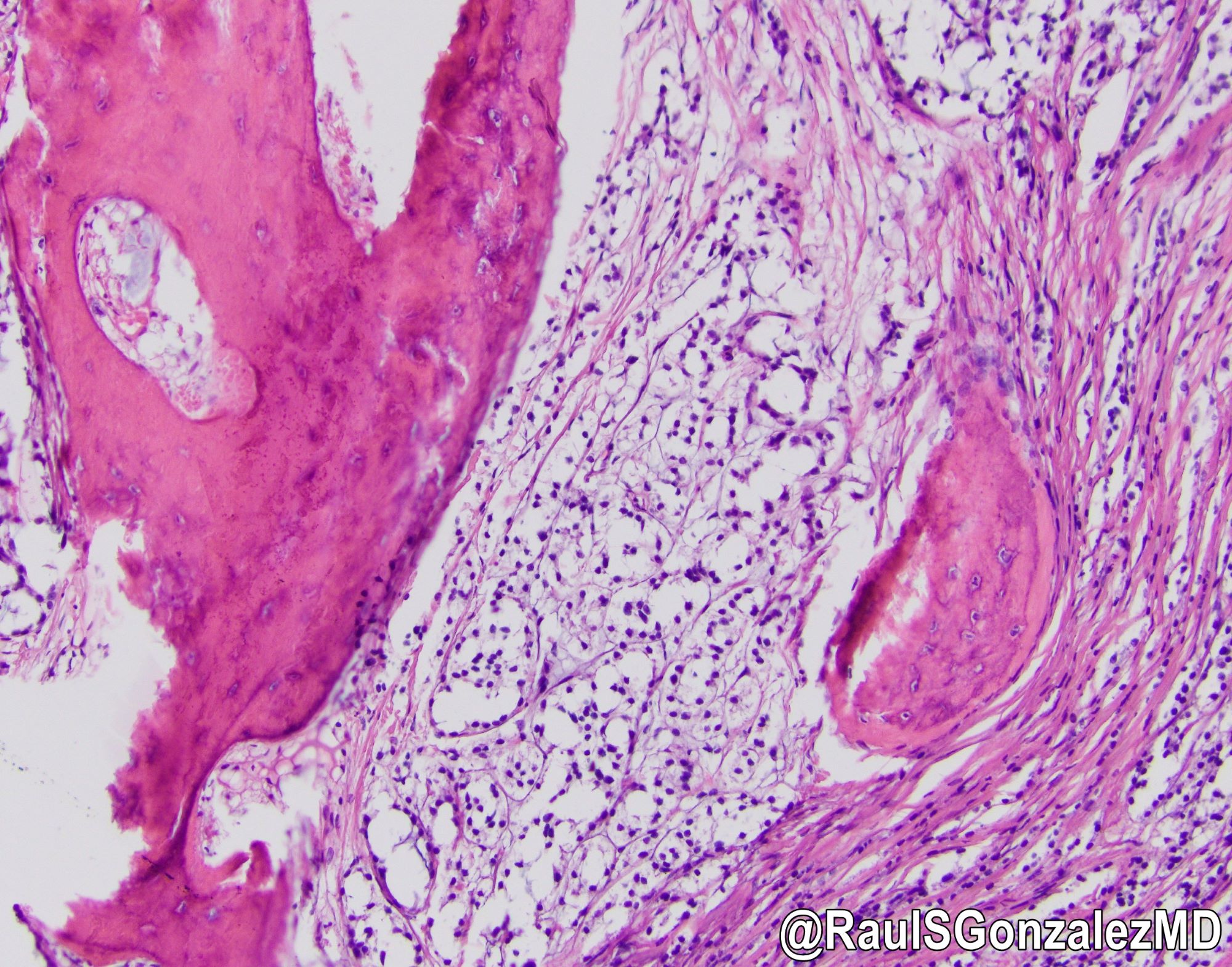
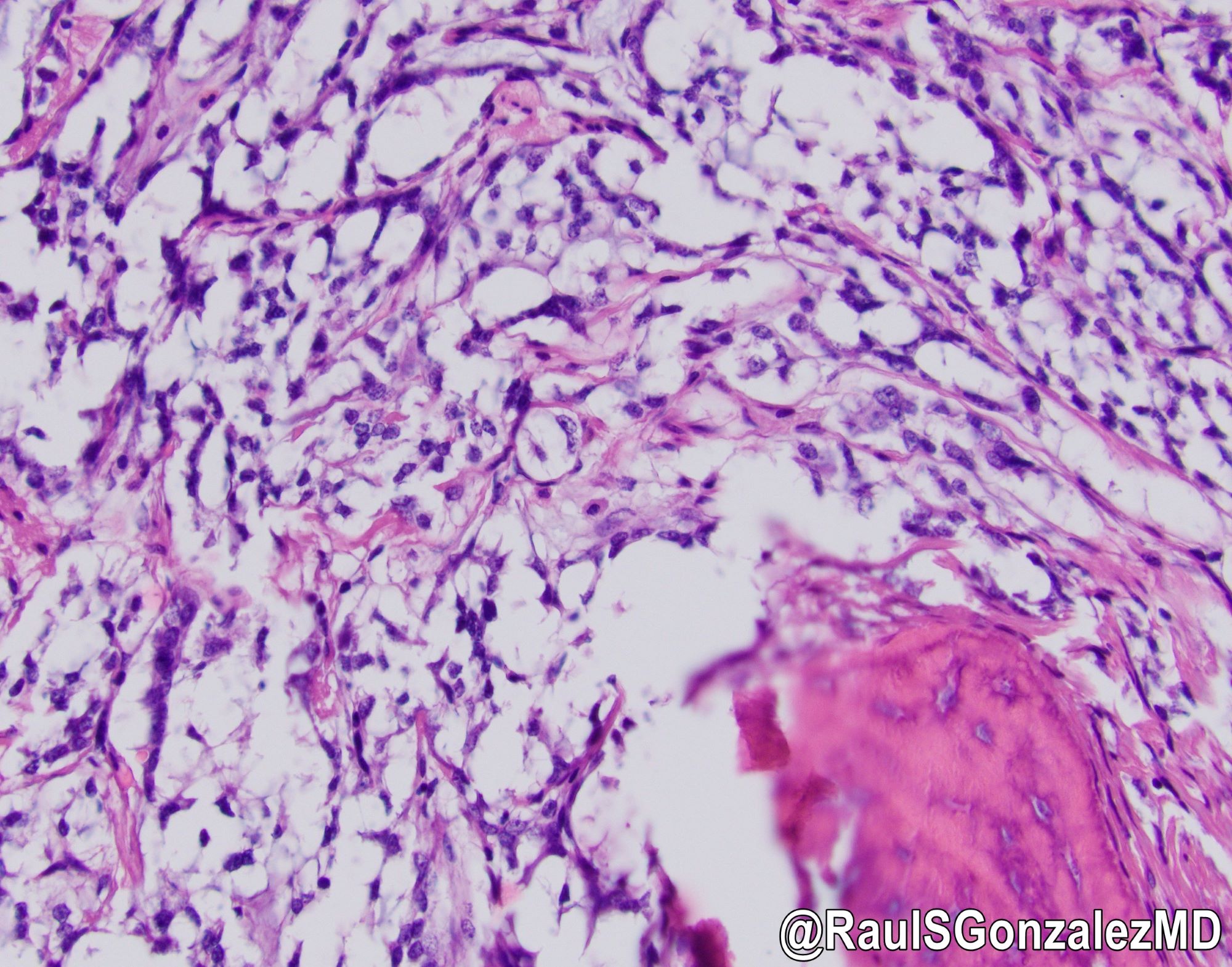
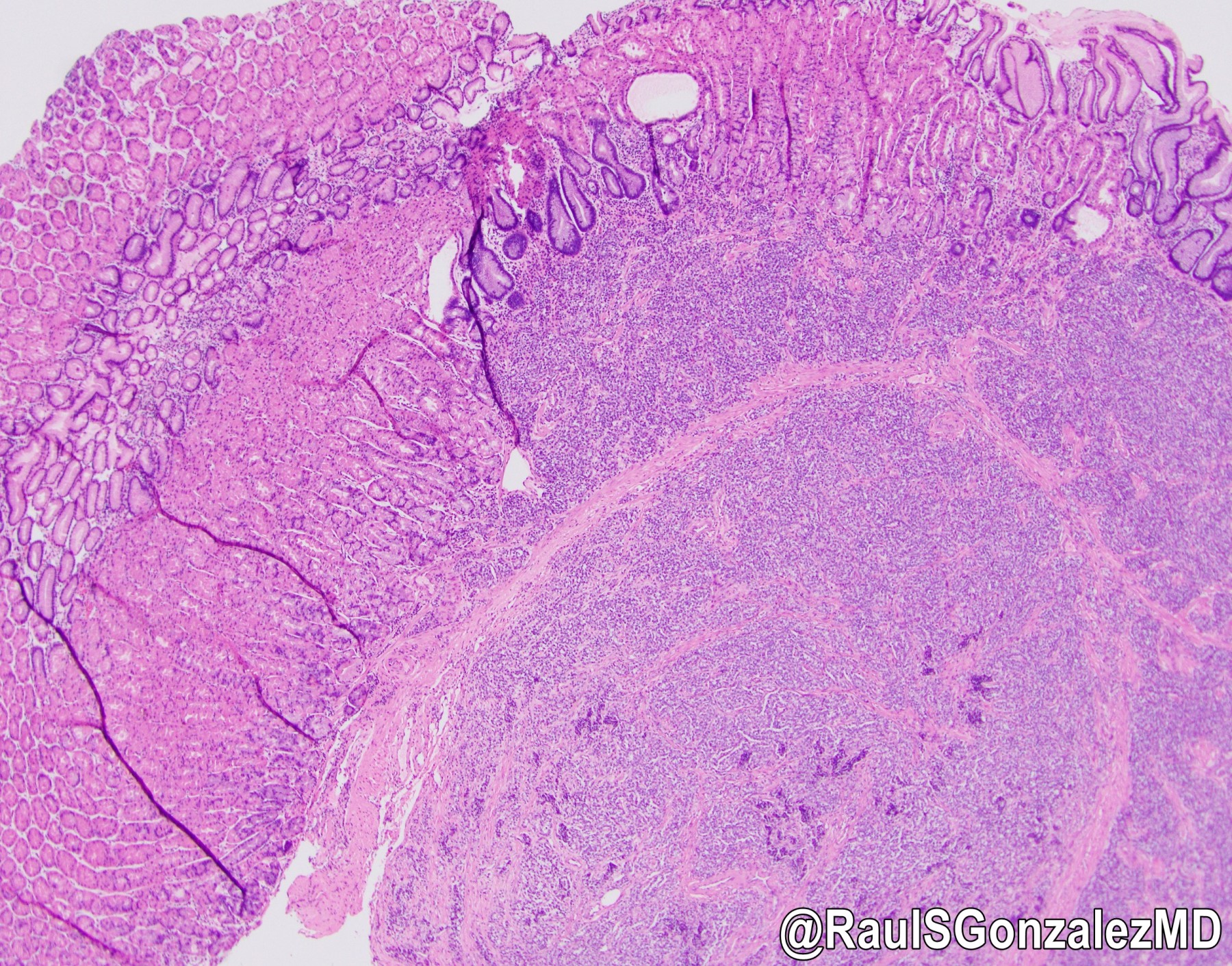
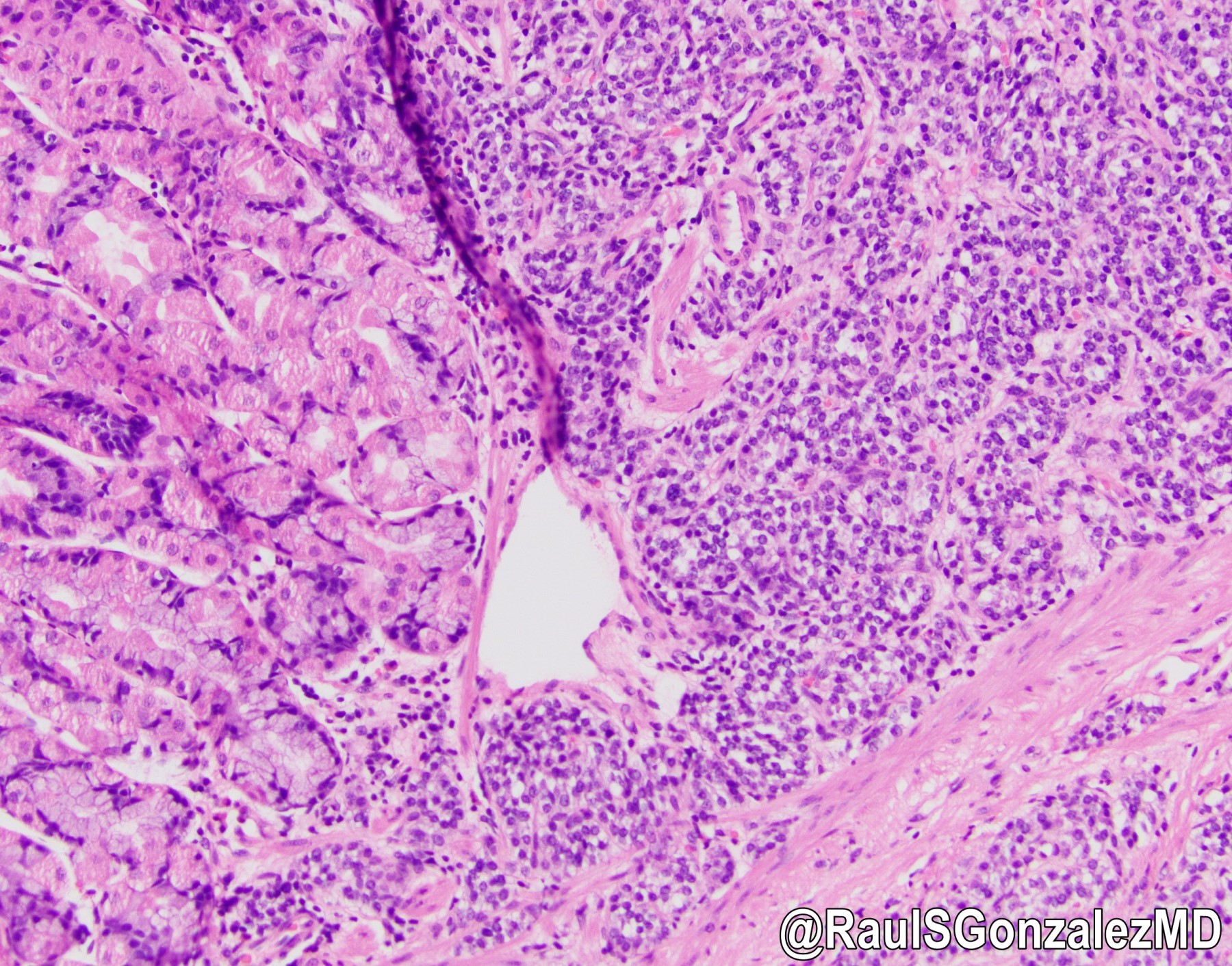
- Architecturally, arranged in nests, cords or trabeculae
- Bland, round to oval cells with typical salt and pepper chromatin and amphophilic cytoplasm
- Focal endocrine atypia is acceptable
- Prominent vasculature is often seen (Front Oncol 2013;3:2)
- Type I NET background oxyntic mucosa is atrophic with metaplasia (intestinal type most commonly), with ECL cell hyperplasia
- Correlates with endoscopic impression of atrophy
- Type II NET background oxyntic mucosa is hyperplastic with ECL cell hyperplasia
- Correlates with endoscopic impression of hypertrophic mucosal folds
- Type III NET background oxyntic mucosa is normal without ECL cell hyperplasia (Medicine (Baltimore) 2022;101:e28550)
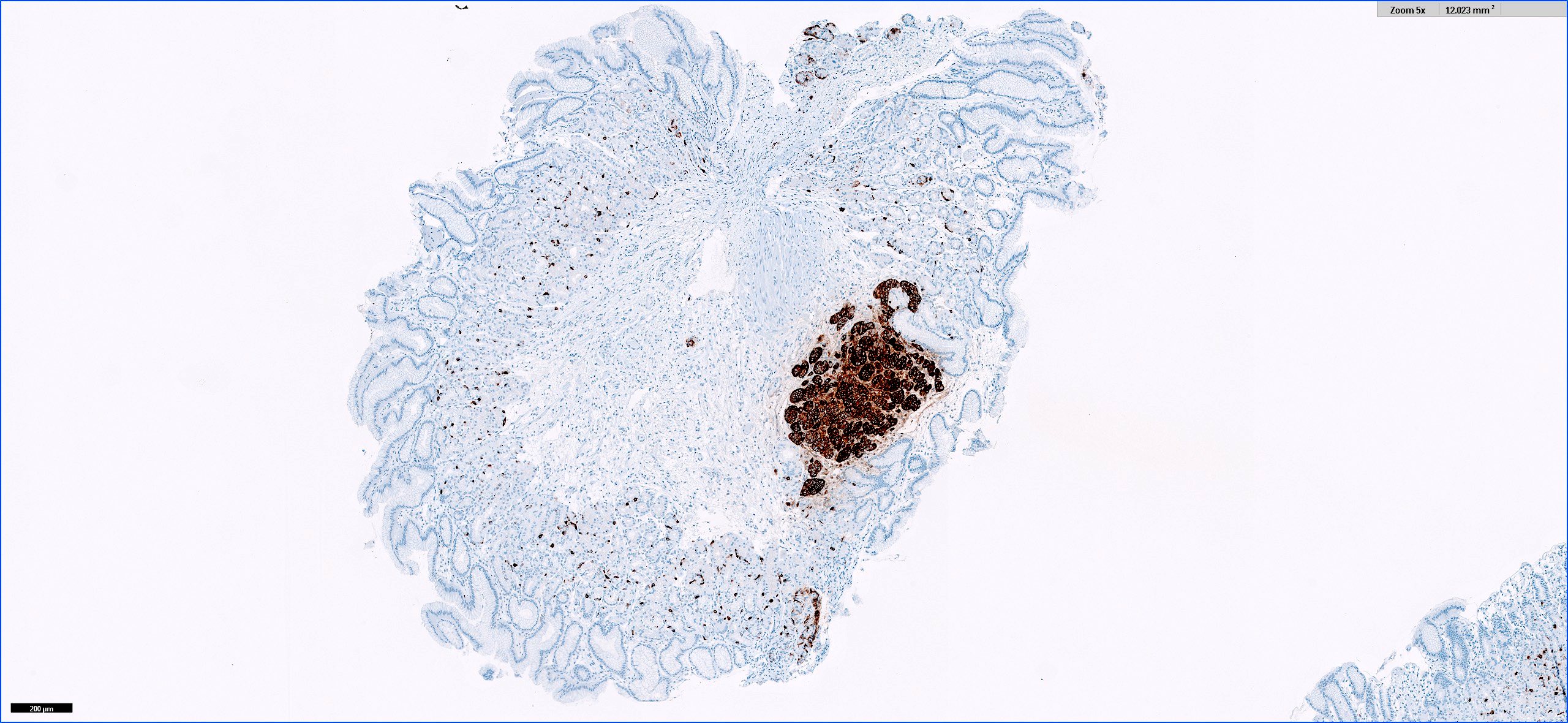
Microscopic (histologic) images
Positive stains
- Chromogranin, synaptophysin diffusely and strongly positive
- CD56, INSM1
- NSE, Leu7 / B3GAT1, protein gene product 9.5 (PGP9.5)
- Somatostatin receptors (SSTRs) (Exp Ther Med 2021;22:1179)
Negative stains
Molecular / cytogenetics description
- Mutations in MEN1, DAXX and ATRX are entity defining for well differentiated NETs (Histopathology 2020;76:182)
- Small cohort studies show associations with germline mutation in the ATP4A gene, with other studies demonstrating loss of heterozygosity at chromosome 11p13 (MEN1 locus) and hypermethylation at the CDKN2A locus as possibly frequent events in gastric NETs (Endocr Rev 2019;40:506)
- Lack the common TP53 and Rb mutation found in most gastric neuroendocrine carcinomas (Histopathology 2020;76:182)
Videos
Neuroendocrine nuttiness in the digestive system - Dr. Raul S. Gonzalez
GI neuroendocrine tumors classification - Dr. Vikram Deshpande
Sample pathology report
- Stomach, partial gastrectomy:
- Well differentiated neuroendocrine tumor, WHO grade 1, 1.8 cm, excised (see comment and synoptic table)
- Oxyntic mucosa with moderate chronic gastritis
- Mild intestinal metaplasia, incomplete type
- Severe atrophy
- No Helicobacter organisms on immunohistochemistry
- Comment: Histologic sections of the stomach demonstrate involvement by nests of tumor cells with round nuclei, variably prominent nucleoli and abundant clear to eosinophilic cytoplasm, consistent with a well differentiated neuroendocrine tumor. Immunostains show that the tumor cells are positive for synaptophysin, chromogranin and INSM1. In accordance with recommended criteria for grading neuroendocrine tumors, mitotic activity assessed within 50 high power fields (10 mm2) on the resection specimen is < 1 mitotic figure per 10 high power fields (2 mm2); Ki67 index, assessed in 2,000 cells in the area of highest proliferation, is < 1%. Based on these proliferation indices, the tumor is best considered WHO grade 1.
- The background gastric mucosa shows moderate chronic gastritis with pseudopyloric and intestinal metaplasia. Linear and nodular endocrine cell hyperplasia are also present, confirmed by immunohistochemical stain for chromogranin. Gastrin immunostain is negative, indicating oxyntic mucosa with severe atrophy. The features are consistent with autoimmune gastritis in the appropriate clinical context. Correlation with clinical and laboratory findings is recommended.
Differential diagnosis
- Gastric neuroendocrine carcinoma:
- Poorly differentiated features, tumoral necrosis
- Ki67 > 20%
- Mixed neuroendocrine nonneuroendocrine neoplasm (MiNEN):
- Tumors with neuroendocrine (tumor or carcinoma) and adenocarcinoma components of ≥ 30% each
- Neuroendocrine cell hyperplasia (Odze: Surgical Pathology of the GI Tract, Liver, Biliary Tract and Pancreas, 4th Edition, 2022):
- No accepted definition
- Some use size criteria < 5 mm as a definition for hyperplasia and > 5 mm is the minimum size necessary to diagnose a NET
- Some may diagnose NET when grossly there is a polyp / nodule regardless of size in the absence of other explanatory findings
- Other criteria have been proposed for microscopic proliferations: if there is nodular growth of ECL cells > 150 microns or if there is a conglomeration of nodules, signs of microinfiltration or new stroma, then the lesion has been proposed to be classified as dysplasia or Tis
Additional references
Board review style question #1
A 51 year old symptomatic man was found to have multiple gastric polyps on screening esophagogastroduodenoscopy (EGD). One polyp showed an aggregate of nested bland tumor cells (seen in the figure above) that stained positive for synaptophysin and chromogranin. What is the appropriate way to grade this tumor?
- Ki67 and mitotic figure count, whichever is higher
- Ki67 and mitotic figure count, whichever is lower
- Ki67 index alone
- Mitotic figure count
Board review style answer #1
A. Ki67 and mitotic figure count, whichever is higher
Comment Here
Reference: Well differentiated neuroendocrine tumor
Comment Here
Reference: Well differentiated neuroendocrine tumor
Board review style question #2
What is the most common type of gastric neuroendocrine neoplasm?
- Gastric well differentiated NET type I
- Gastric well differentiated NET type II
- Gastric well differentiated NET type III
- Gastrin producing G cell NET
- Serotonin producing EC cell NET
Board review style answer #2
A. Gastric well differentiated NET type I
Comment Here
Reference: Well differentiated neuroendocrine tumor
Comment Here
Reference: Well differentiated neuroendocrine tumor