Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Diagrams / tables | Clinical features | Interpretation | Uses by pathologists | Prognostic factors | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive staining - normal | Positive staining - disease | Negative staining | Sample pathology report | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Van Bockstal MR. MLH1. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/stainsmlh1.html. Accessed April 1st, 2025.
Definition / general
- Mismatch repair (MMR) protein
- Deficiency results in microsatellite instability (MSI)
- Germline mutations result in autosomal dominant hereditary Lynch syndrome
Essential features
- Mismatch repair protein whose deficiency results in high microsatellite instability (MSI high)
- Causes of deficiency include germline and somatic MLH1 mutations and somatic and constitutional epigenetic silencing through MLH1 promoter hypermethylation
- Germline MLH1 mutations result in the autosomal dominant hereditary Lynch syndrome, which predisposes to colorectal cancer and several other noncolorectal neoplasms, such as endometrial cancer (Mod Pathol 2019;32:1)
- Immunohistochemistry for the MMR proteins MLH1, PMS2, MSH2 and MSH6 is used for:
- Identification of MMR deficient, MSI high neoplasms
- Screening for Lynch syndrome
Terminology
- MutL homolog 1, colon cancer, nonpolyposis type 2 (E. coli)
- hMLH1
- HNPCC (Orphanet: MLH1 - MutL Homolog 1 [Accessed 7 April 2021])
- FCC2 (Orphanet: MLH1 - MutL Homolog 1 [Accessed 7 April 2021])
Pathophysiology
- Cell division requires accurate DNA replication; both daughter cells receive an identical DNA copy
- MLH1 gene encodes the MLH1 protein, which plays an essential role in postreplication DNA mismatch repair (Cell Res 2008;18:85)
- MLH1 forms a 2 protein complex with PMS2; this dimer, named MutL alpha, helps to repair 2 types of errors that occur during DNA replication:
- Base - base mismatches (Cell Res 2008;18:85)
- Insertion - deletion errors in segments of repetitive bases (known as microsatellites or short tandem repeats) (Cell Res 2008;18:85)
- MLH1-PMS2 dimer collaborates with the MSH2-MSH6 dimer to recognize mismatch errors
- Errors are targeted, excised, resynthesized and ligated (Cell Res 2008;18:85, The British Association of Gynaecological Pathologists: BAGP MMR IHC Interpretation June 2020 [Accessed 7 April 2021])
- MLH1 can also form heterodimers with the PMS1 or MLH3 protein
Diagrams / tables
Clinical features
- MLH1 deficiency results in microsatellite instability (MSI), characterized by accumulation of numerous replication errors within short nucleotide repeat sequences (i.e. microsatellites)
- MLH1 deficiency in cancer has 4 possible causes:
- Somatic MLH1 promoter hypermethylation
- Constitutional MLH1 promoter hypermethylation
- Germline MLH1 mutation
- Somatic MLH1 mutation
- Somatic MLH1 promoter hypermethylation is the cause of MLH1 deficiency in 10 - 15% of all colorectal and endometrial carcinomas; colorectal carcinomas fitting this description often also have BRAF V600E mutations (J Med Genet 2021 Jan 15 [Epub ahead of print])
- Constitutional MLH1 promoter hypermethylation is a rare cause (< 1%) of Lynch syndrome (Genes Chromosomes Cancer 2009;48:737)
- In case of a heterozygous germline MLH1 mutation, a second hit can inactivate the remaining intact allele in a cell, resulting in deficient DNA mismatch repair and high microsatellite instability (J Mol Diagn 2004;6:308)
- Biallelic germline mutations in 1 of the 4 mismatch repair genes give rise to the constitutional mismatch repair deficiency syndrome (CMMRD), a rare but aggressive cancer predisposition syndrome with cancers in multiple organs, often presenting in childhood; phenotypic overlap with neurofibromatosis 1 is common (J Med Genet 2021 Feb 23 [Epub ahead of print], Genet Med 2020;22:2081, Clin Genet 2020;97:296)
- Somatic MLH1 mutation can occur either clonally or subclonally in a carcinoma (Am J Surg Pathol 2016;40:909)
- It explains around 71% of MLH1 deficiency in endometrial and colorectal carcinomas without germline pathogenic variants (J Med Genet 2021 Jan 15 [Epub ahead of print], Ann Oncol 2017;28:96)
- Somatic MLH1 mutation can co-occur with POLE mutations in endometrial carcinomas and ovarian endometrioid or clear cell carcinomas due to the hypermutated state and genomic instability of the tumor (Pathology 2021;53:179)
Interpretation
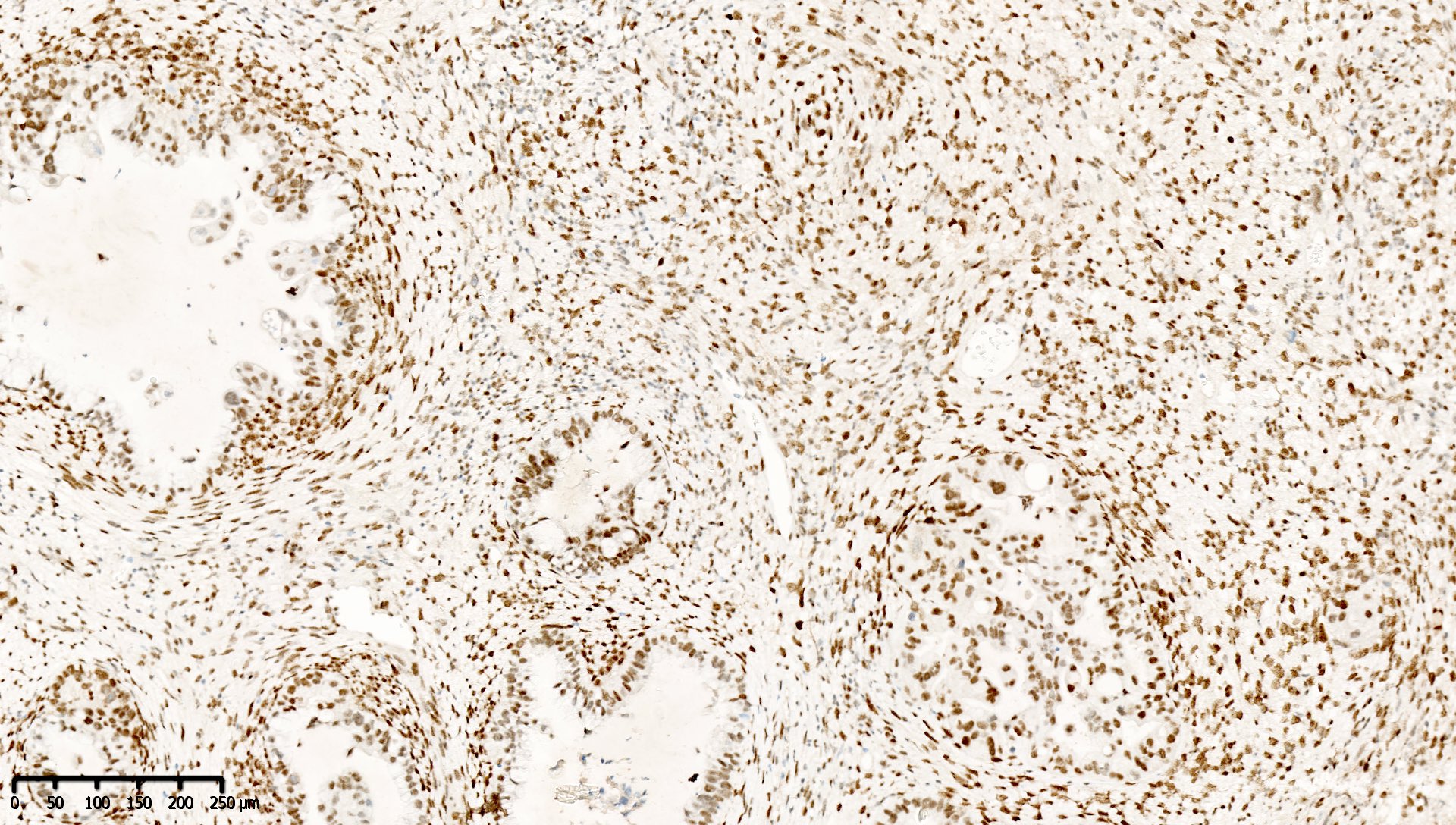
- Retained staining (i.e. MLH1 proficiency) is defined as diffuse nuclear immunoreactivity within neoplastic cells
- Retained staining in neoplastic cells requires a similar or higher intensity than the nuclear immunoreactivity in normal epithelium, lymphocytes, fibroblasts and endothelium, which serve as internal control (Appl Immunohistochem Mol Morphol 2015;23:1)
- Aberrant loss of staining (i.e. MLH1 deficiency) is defined as either:
- Complete absence of nuclear immunoreactivity within neoplastic cells, in the presence of a positive internal control
- PMS2 expression is almost always lost as well
- Punctate, speckled, dot-like, nucleolar or granular heterogeneous nuclear MLH1 immunoreactivity within neoplastic cells, in the presence of a positive internal control (Histopathology 2019;74:795, Histopathology 2018;73:703)
- Punctate or dot-like staining pattern is most often observed with the MLH1 M1 clone
- Be aware of this pitfall to avoid erroneous reports of isolated PMS2 loss (Pathol Res Pract 2020;216:152581, Histopathology 2018;73:703)
- MMR panel should generally use MLH1, MSH2, MSH6 and PMS2
- 2 stain immunohistochemical screening (i.e. just PMS2 and MSH6) for Lynch syndrome may fail to detect MMR deficiency in rare cases and is not recommended (Mod Pathol 2018;31:1891)
- Rarely, germline MLH1 missense mutation results in loss of function with retained MLH1 expression
- If no germline PMS2 mutation is found in a carcinoma with isolated PMS2 loss, a germline MLH1 mutation should be excluded (Am J Surg Pathol 2016;40:e35)
- MLH1 immunohistochemistry is fixation dependent; poor fixation results in false negative staining
- MLH1 immunohistochemistry is preferentially performed on biopsies instead of resection specimens
Uses by pathologists
- Immunohistochemistry for MMR proteins is used in carcinomas of the rectum, colon, small intestine and stomach to screen for:
- Lynch syndrome
- MSI high neoplasms
- Immunohistochemistry for MMR proteins and p53, combined with mutation analysis of POLE, is essential for the molecular classification of all endometrial carcinomas (including carcinosarcoma) and endometrioid, clear cell and mucinous carcinoma of the ovary (Clin Cancer Res 2020;26:5400, Int J Gynecol Pathol 2019;38:S64, Am J Surg Pathol 2014;38:1501, J Clin Oncol 2020;38:1222, The British Association of Gynaecological Pathologists: BAGP MMR IHC Interpretation June 2020 [Accessed 7 April 2021])
- MMR deficient tumors
- Polymerase E (POLE) mutant tumors
- Copy number high / p53 abnormal tumors
- Copy number low / p53 wild type tumors
- Immunohistochemistry for MMR proteins is part of the molecular classification of gastric cancer into 4 groups (Nature 2014;513:202, Ann Oncol 2017;28:1005)
- Microsatellite unstable cancers
- Epstein-Barr virus associated cancers
- Chromosomal unstable cancers
- Genomically stable cancers
- Additional methylation specific multiplex ligation dependent probe amplification distinguishes MLH1 promoter hypermethylation in sporadic neoplasms from neoplasms in individuals with Lynch syndrome (Mod Pathol 2020;33:1443)
- Germline mutation testing identifies individuals with Lynch syndrome
- Loss of MLH1 expression can help support the presence of dysplasia in sessile serrated lesions of the colon, especially when subtle (Gut 2017;66:97, Mod Pathol 2017;30:1728)
Prognostic factors
- Patients with a colorectal carcinoma with combined deficiency of MLH1 and PMS2, in the absence of somatic BRAF mutations and MLH1 promoter hypermethylation, should be referred for germline mutation testing (Mod Pathol 2019;32:1)
- MSI high neoplasms likely respond well to immune checkpoint inhibitors (Br J Cancer 2019;121:809, J Clin Oncol 2020;38:214, J Clin Oncol 2019;37:2786)
- MMR deficient endometrial cancers have:
- Better prognosis than copy number high / p53 abnormal endometrial cancers
- Similar prognosis to copy number low / p53 wild type endometrial cancers
- Worse prognosis than POLE mutant endometrial cancers (Nature 2013;497:67)
Microscopic (histologic) description
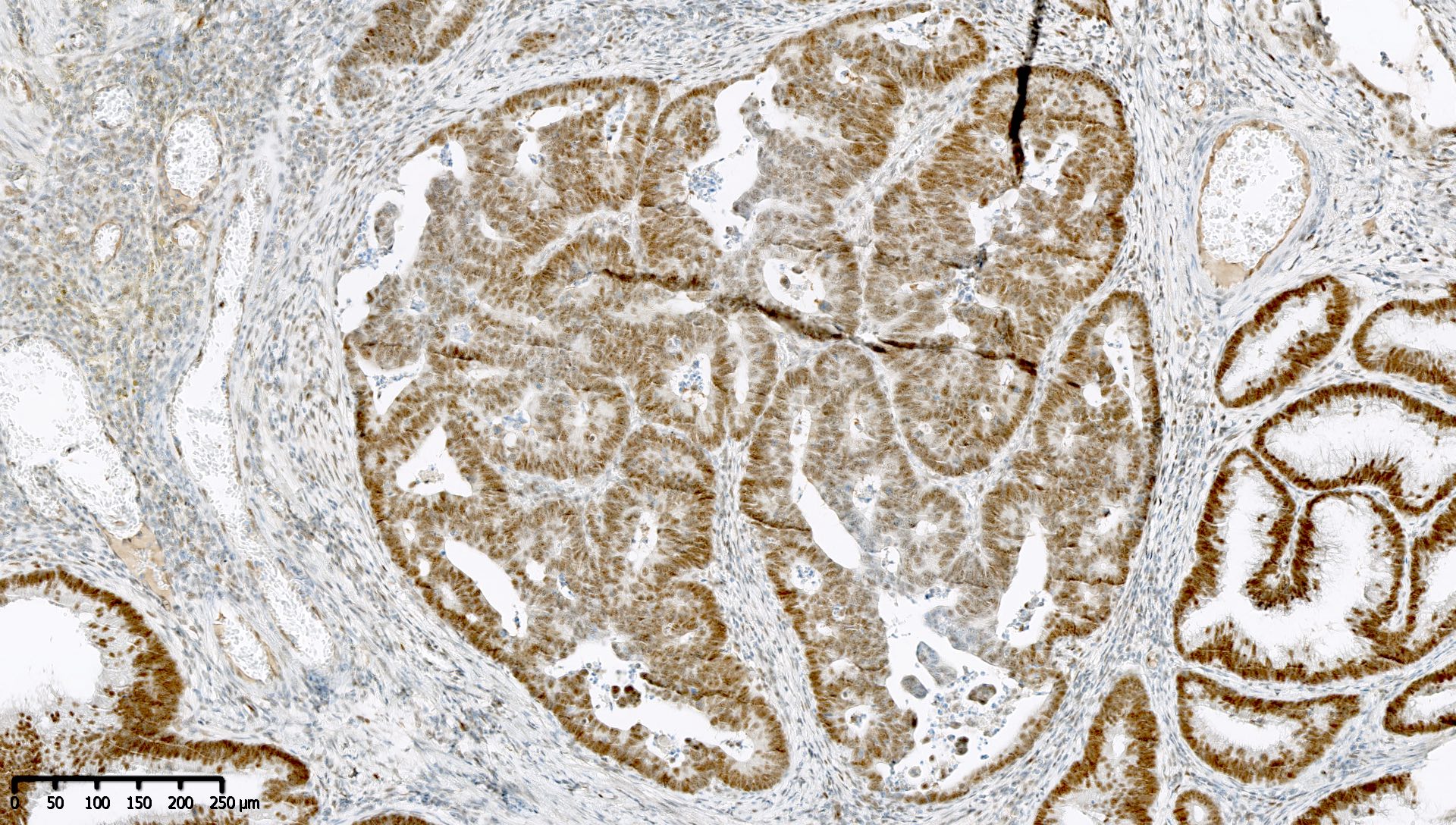
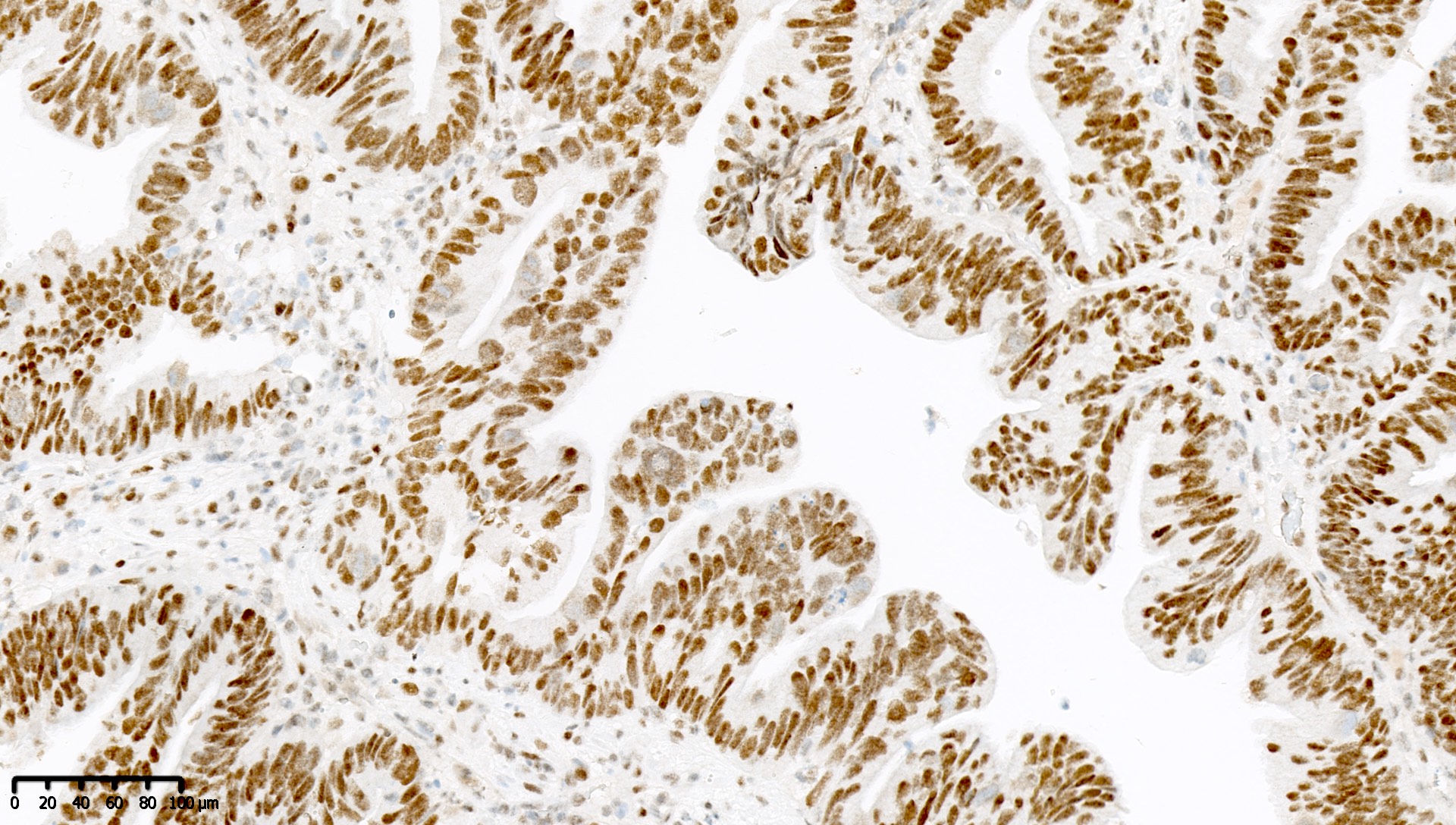
- Normal finding: nuclear stain in at least some but generally most / all tumor cells, in addition to nonneoplastic background cells
- Any other pattern could represent abnormality and should be further assessed (see Interpretation)
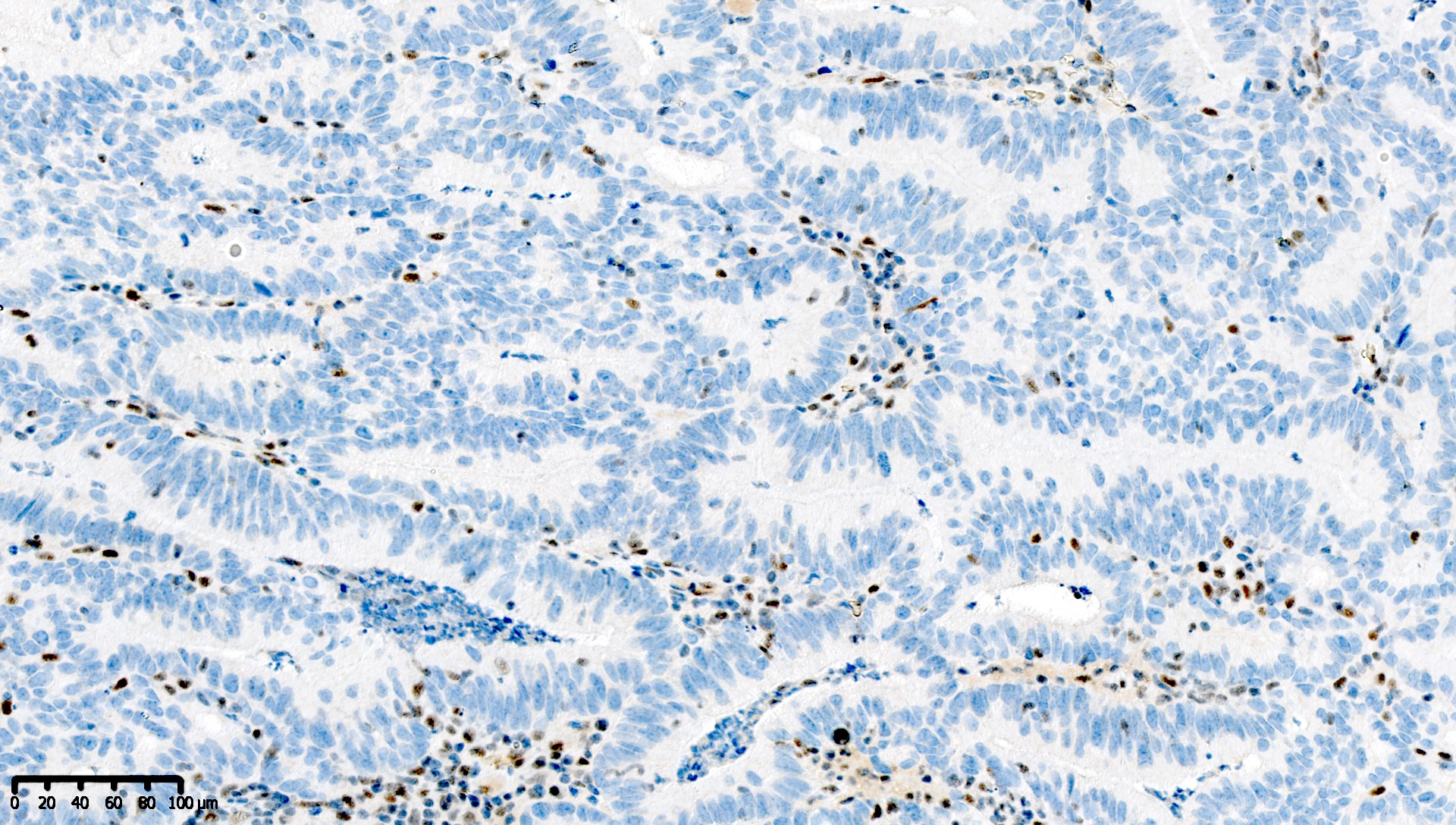
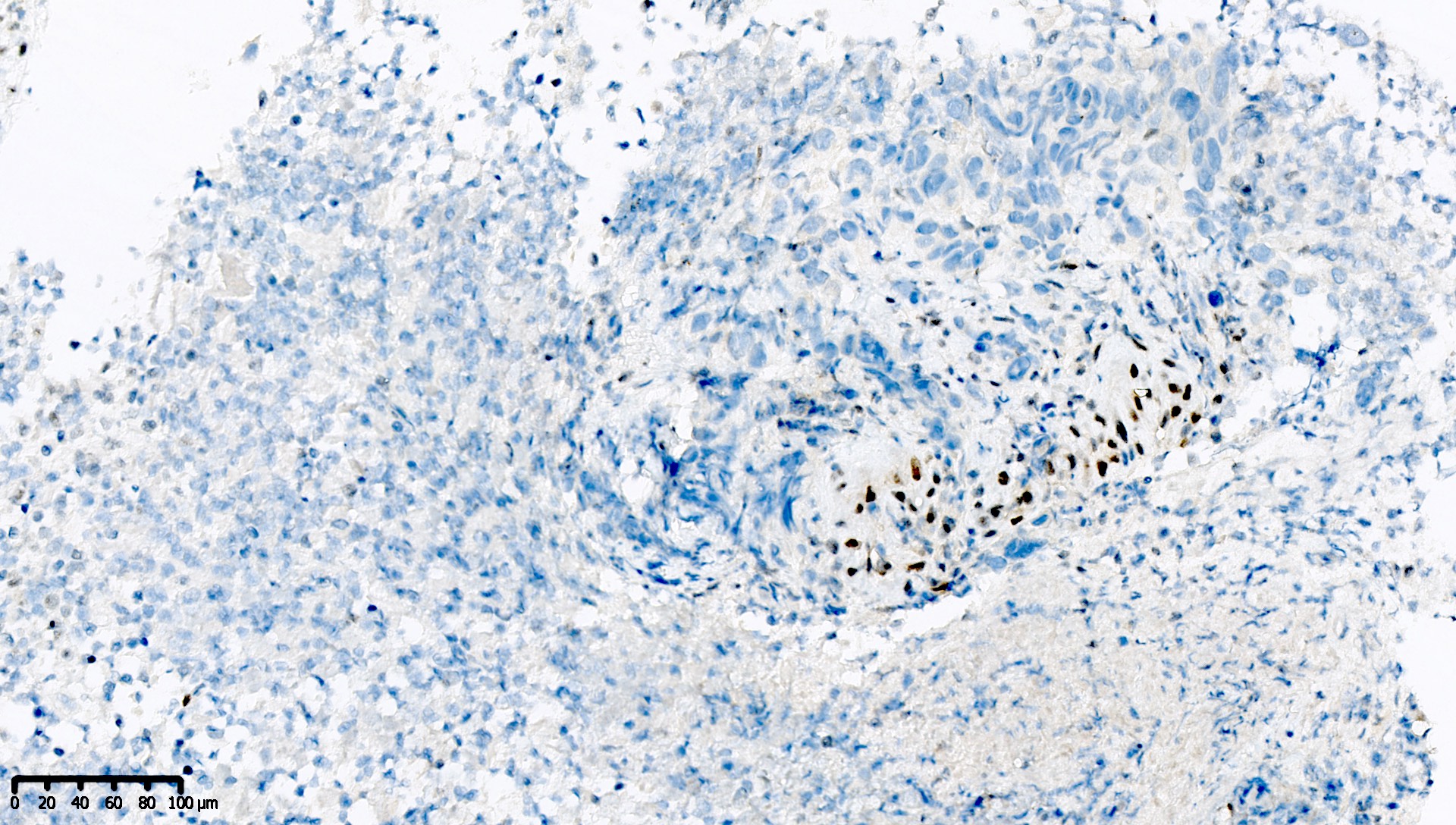
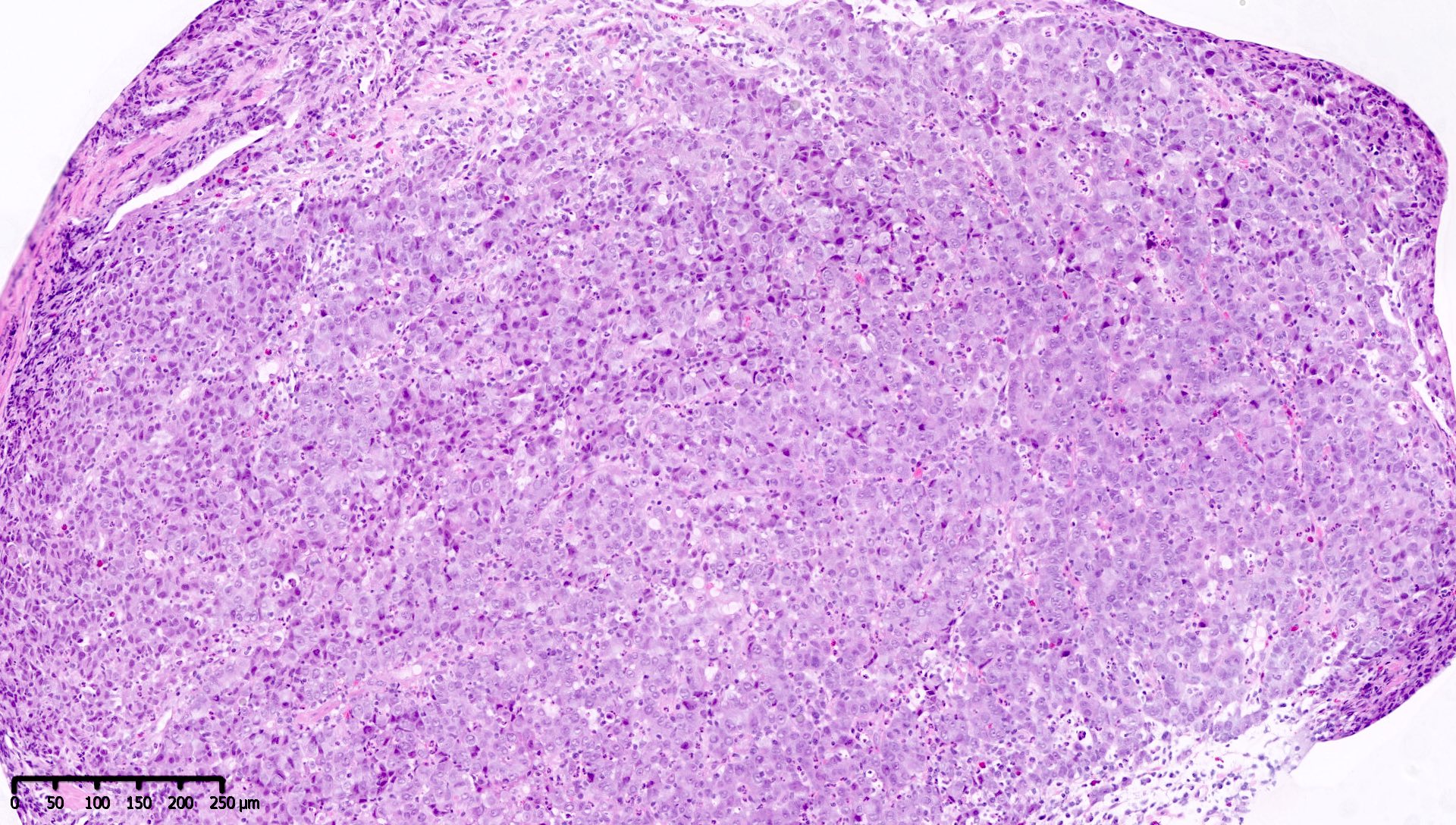
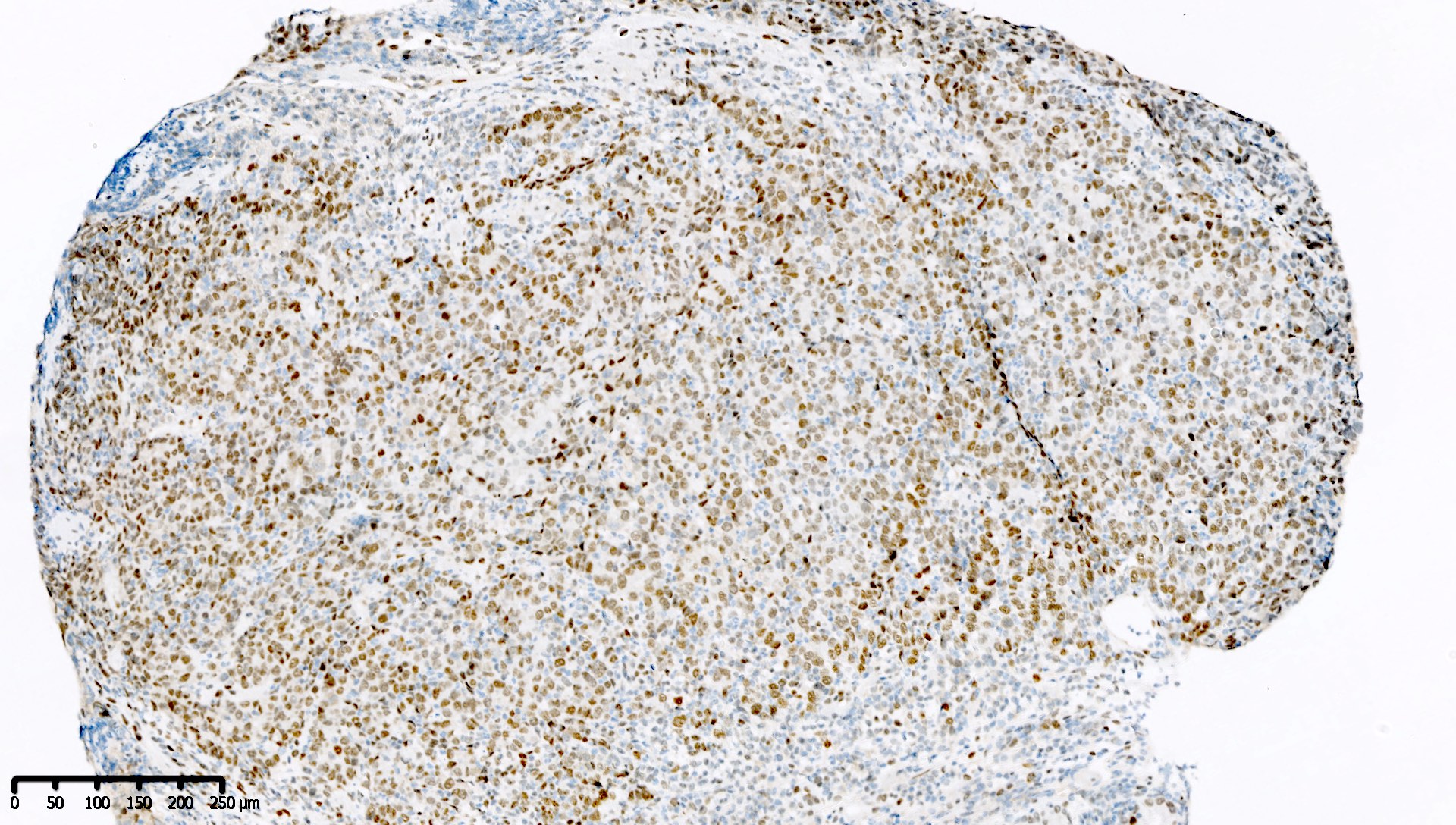
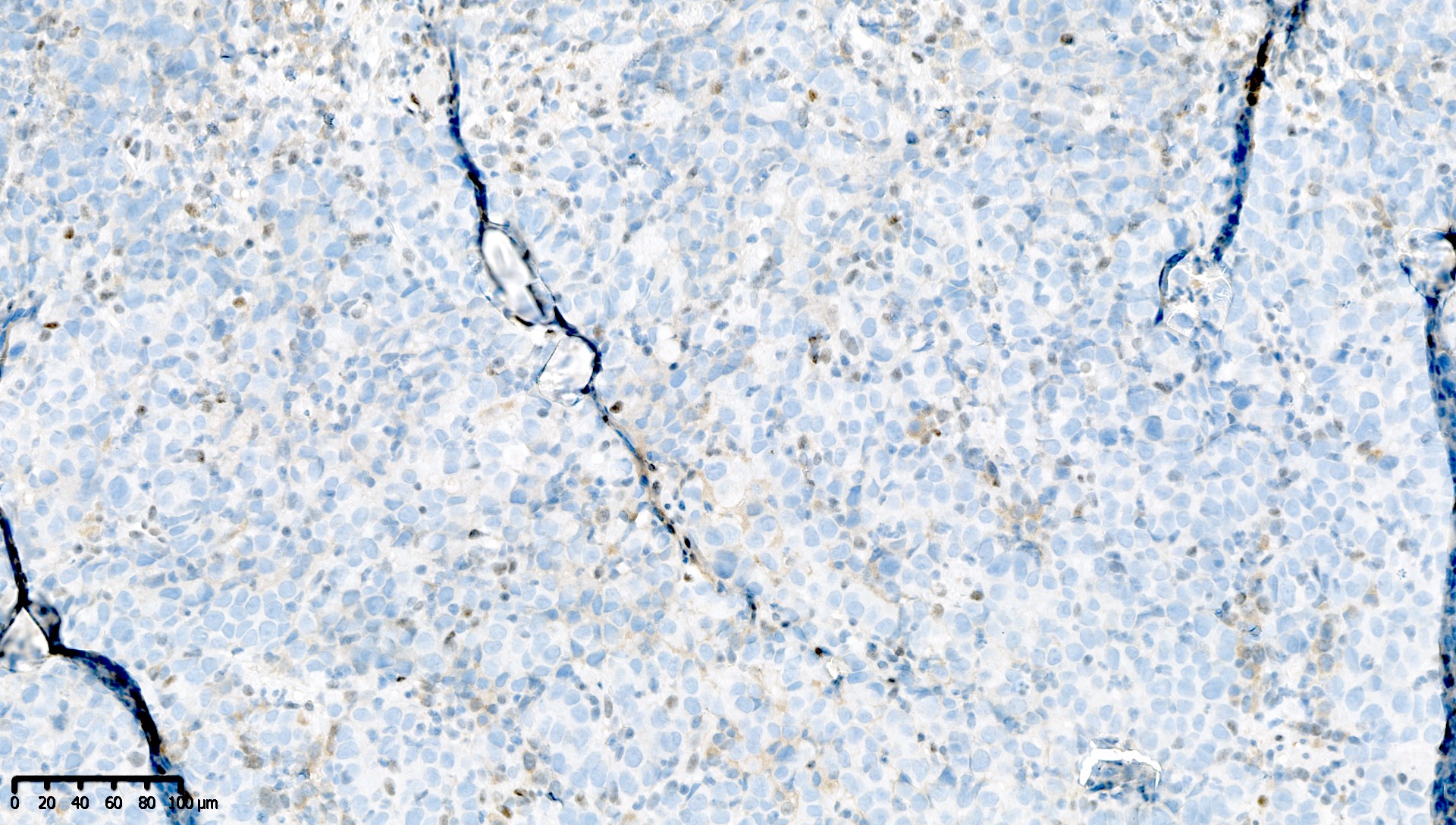
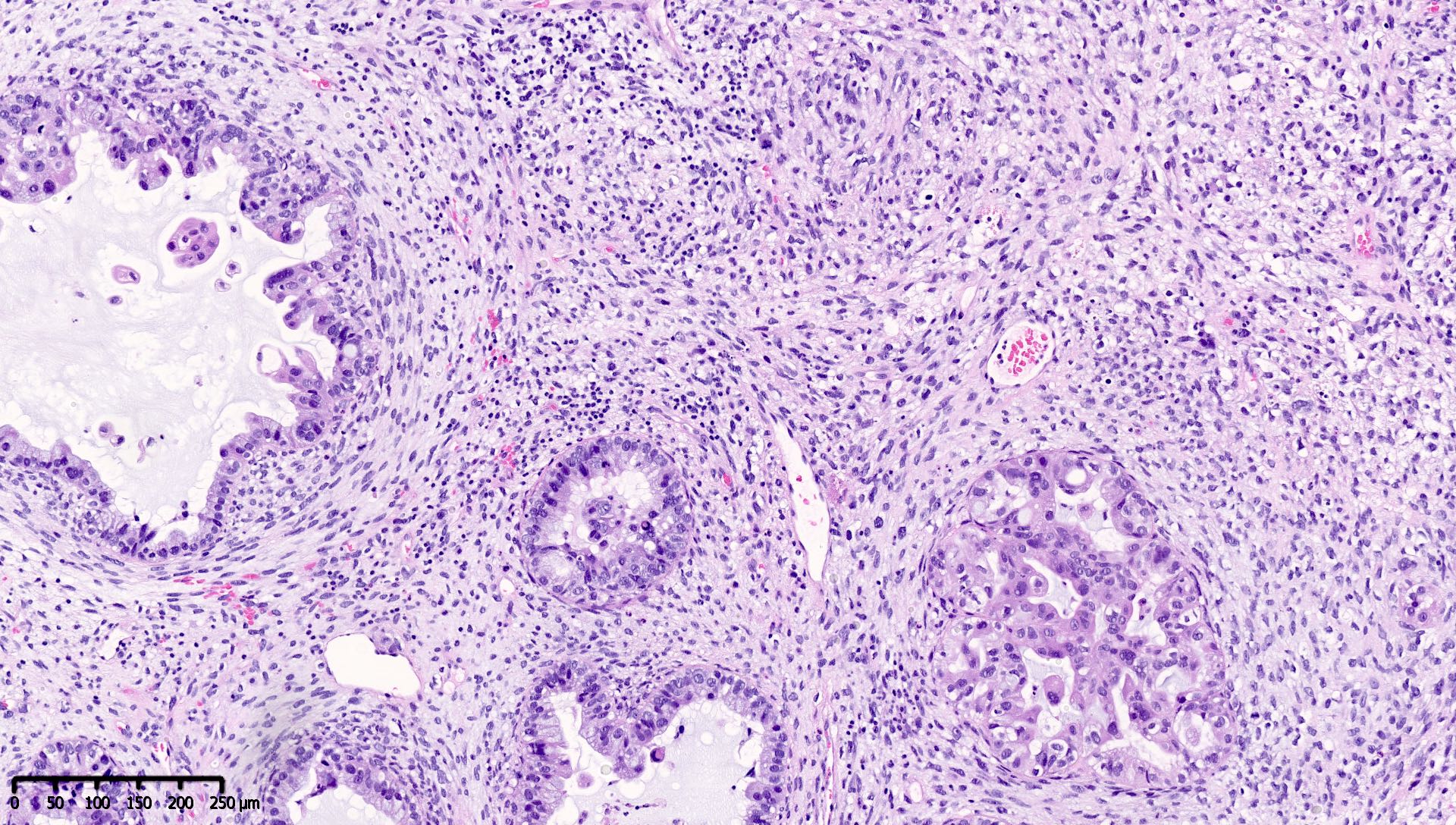
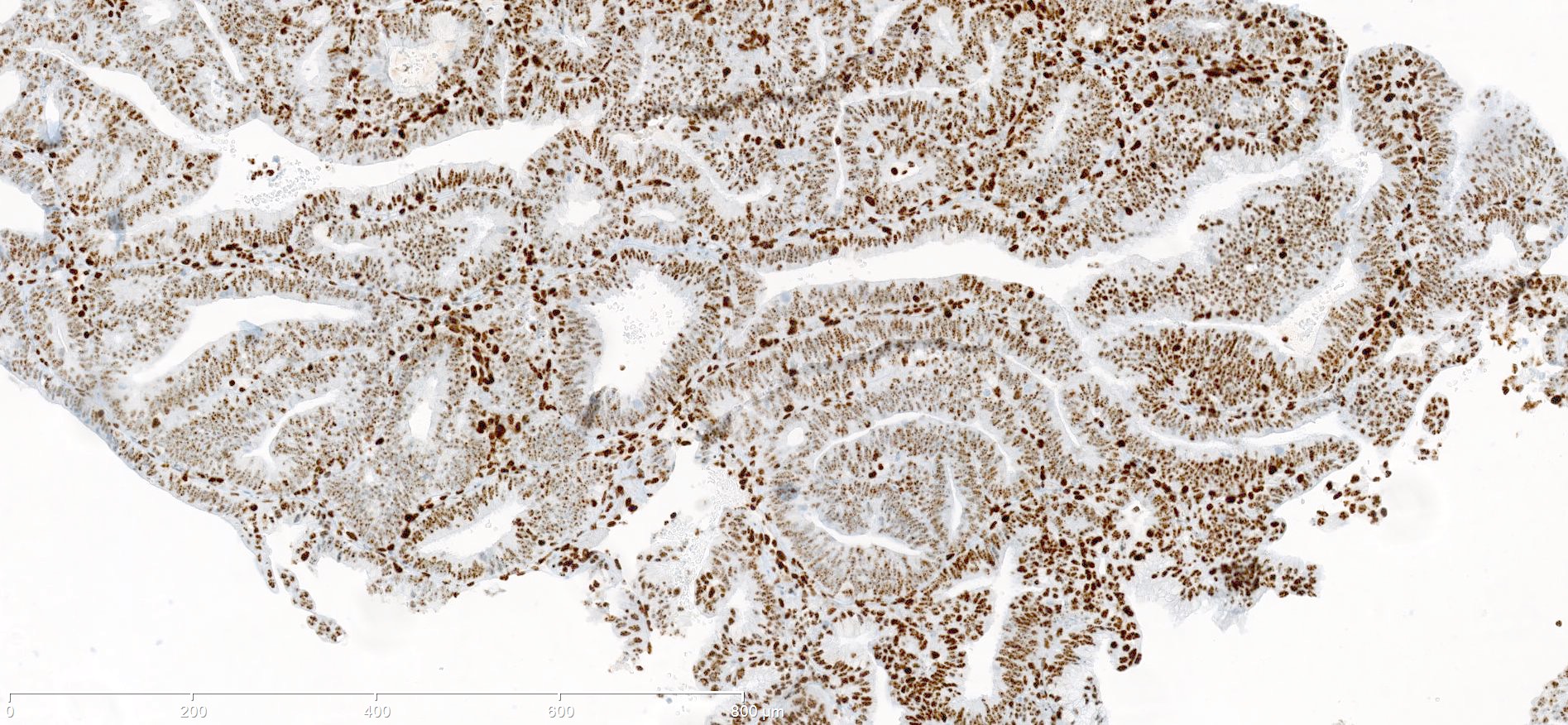
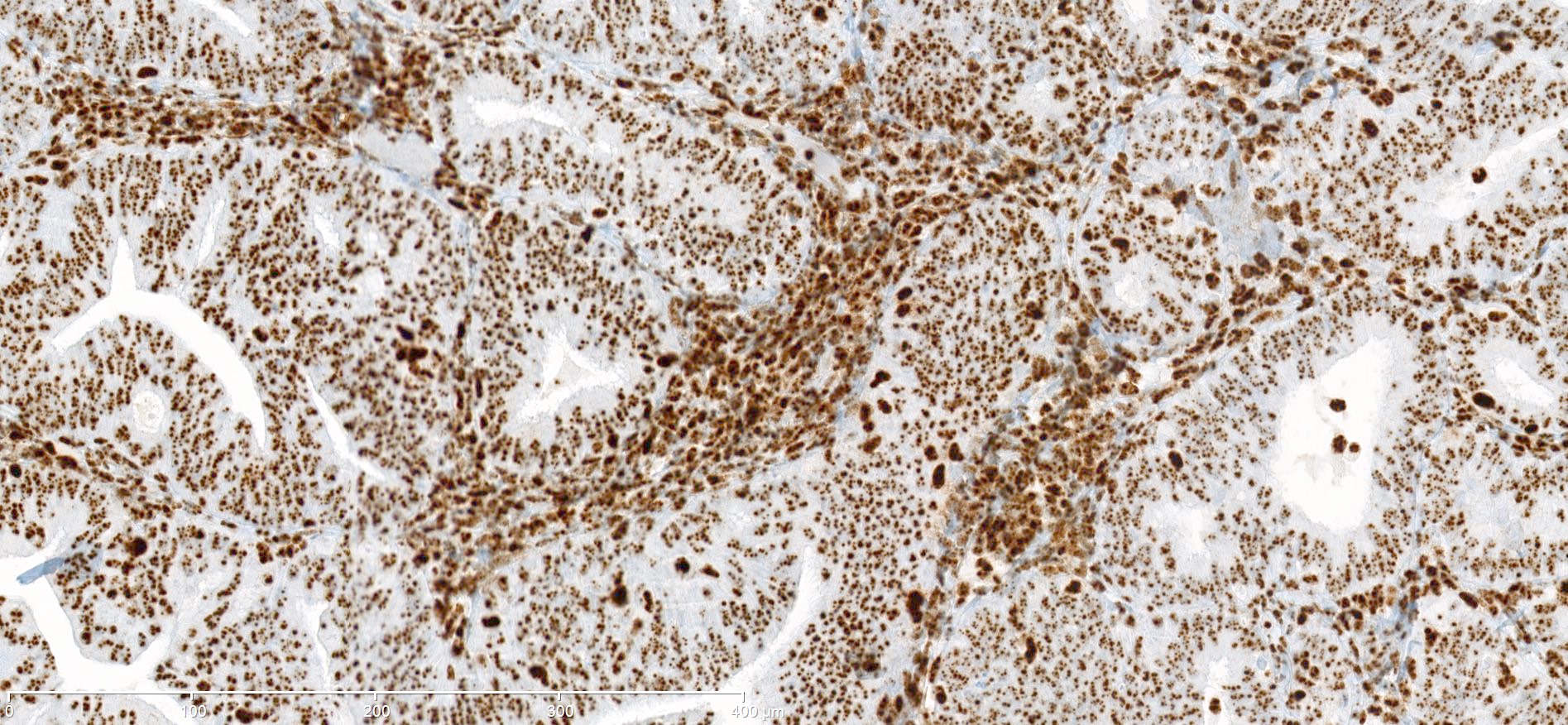
Microscopic (histologic) images
Contributed by Mieke R. Van Bockstal, M.D., Ph.D. and Shatavisha Dasgupta, M.D., Ph.D.
Positive staining - normal
- Tonsil is recommended as an on-slide external positive control:
- At least weak to moderate distinct nuclear staining in nearly all mantle zone B lymphocytes (Appl Immunohistochem Mol Morphol 2015;23:1)
- Moderate to strong nuclear staining in the germinal center B lymphocytes (Appl Immunohistochem Mol Morphol 2015;23:1, NordiQC: Mismatch Repair Protein MLH1 (MLH1) [Accessed 7 April 2021])
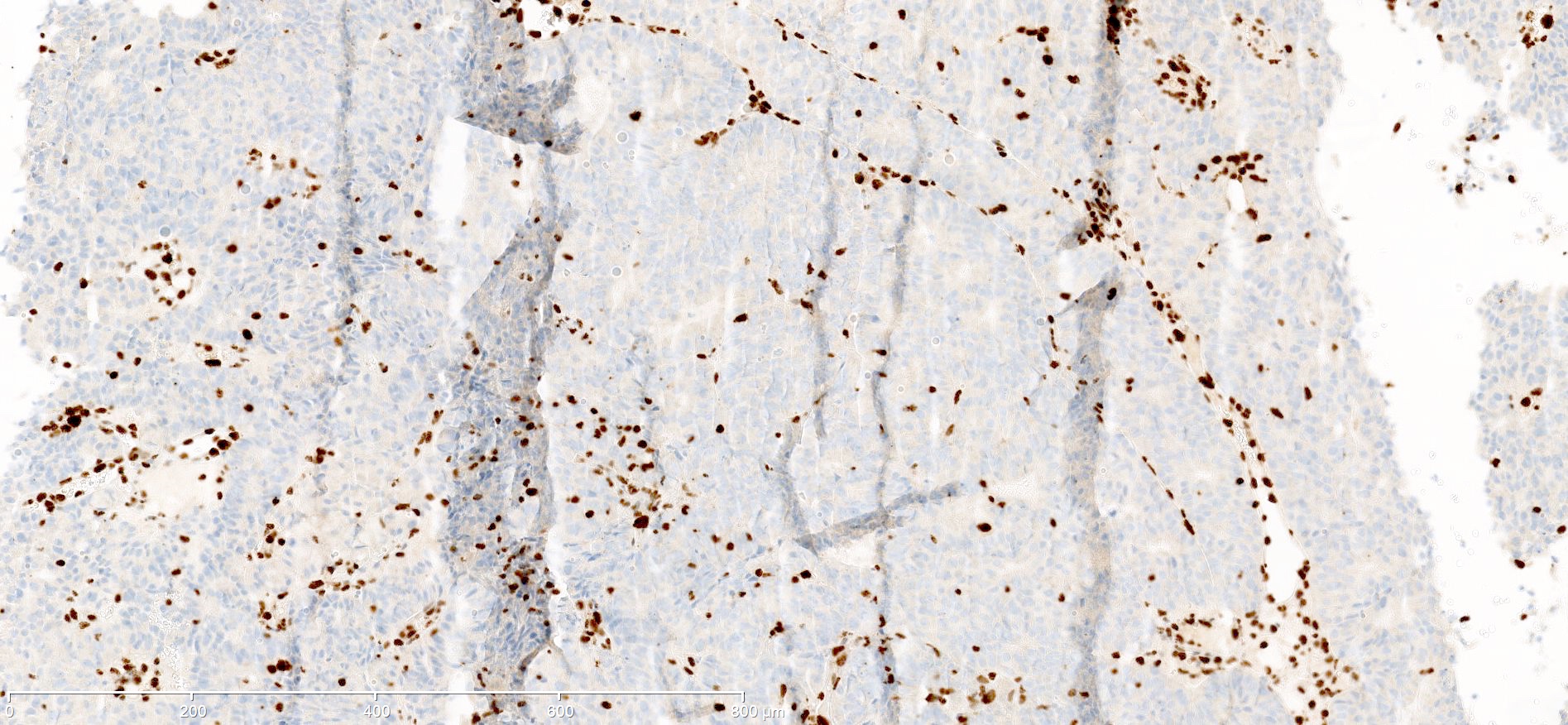
Positive staining - disease
- "Positive" result generally indicates aberrant loss of staining (see Interpretation), so report should be carefully worded to avoid miscommunication
Negative staining
- "Negative" result generally indicates retention of normal staining (see Interpretation), so report should be carefully worded to avoid miscommunication
Sample pathology report
| MMR immunohistochemistry result | Recommended sample report |
| Normal expression of all 4 MMR proteins MLH1, PMS2, MSH2 and MSH6 | Background nonneoplastic tissue / internal control shows intact nuclear expression of MLH1, PMS2, MSH2 and MSH6.
The tumor shows normal nuclear expression of MLH1, PMS2, MSH2 and MSH6. There is no immunohistochemical evidence of a mismatch repair deficiency or Lynch syndrome. However, referral for genetic counseling should be considered if there is a strong family / clinical history suggestive of Lynch and related syndromes, despite this immunohistochemical result. |
| Abnormal result; combined MLH1 and PMS2 loss with expression of MSH2 and MSH6 | Background nonneoplastic tissue / internal control shows intact nuclear expression of MLH1, PMS2, MSH2 and MSH6.
The tumor cells show loss of expression of MLH1 and PMS2, with normal nuclear expression of MSH2 and MSH6. This immunohistochemical profile suggests a mismatch repair deficiency, which requires MLH1 promoter hypermethylation testing.
|
| Abnormal result; isolated PMS2 loss with expression of MLH1, MSH2 and MSH6 | Background nonneoplastic tissue / internal control shows intact nuclear expression of MLH1, PMS2, MSH2 and MSH6.
The tumor cells show loss of expression of PMS2, with normal nuclear expression of MLH1, MSH2 and MSH6. This immunohistochemical profile suggests a mismatch repair deficiency. The patient should be referred for germline genetic testing for Lynch syndrome. |
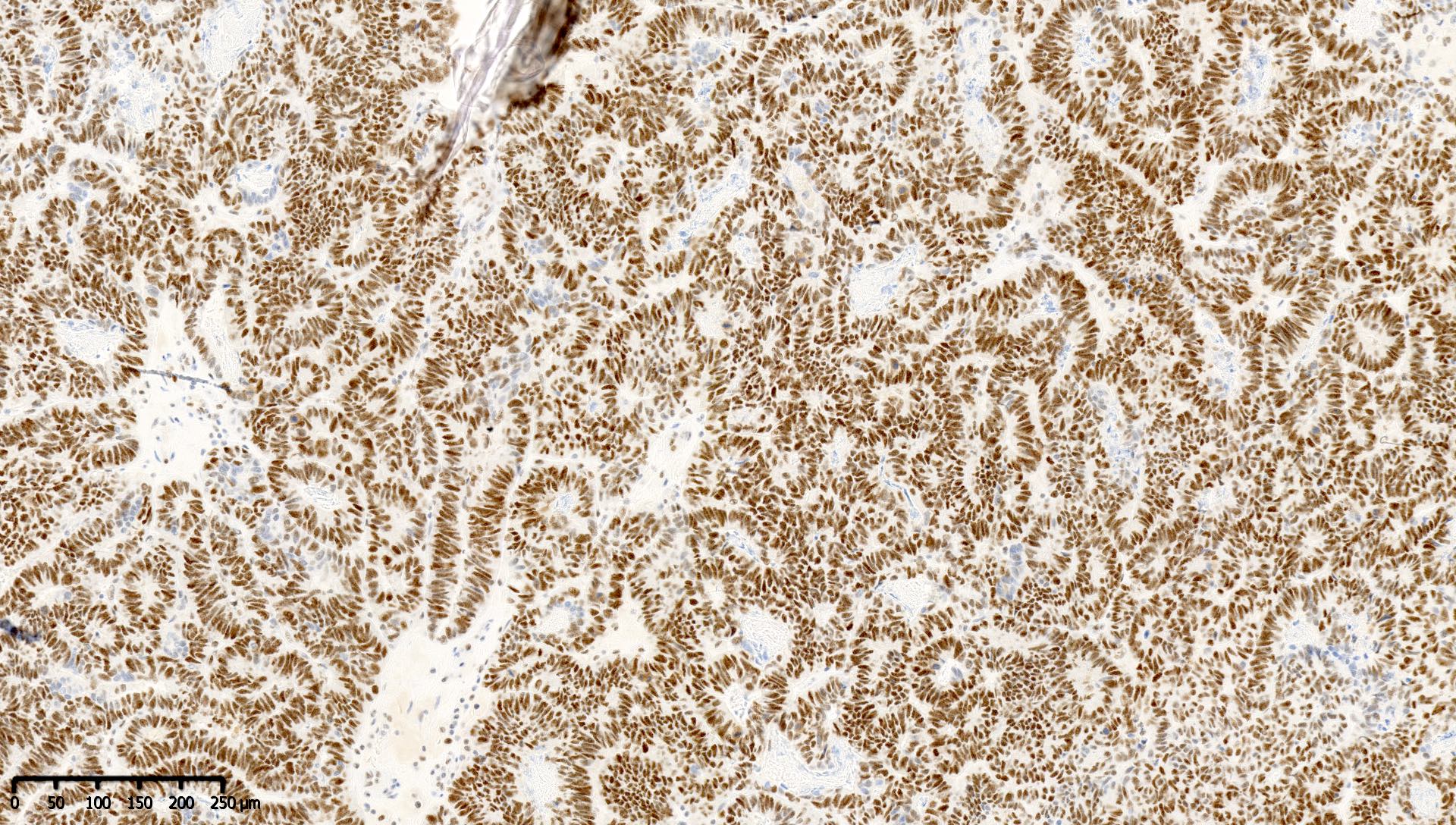
Board review style question #1
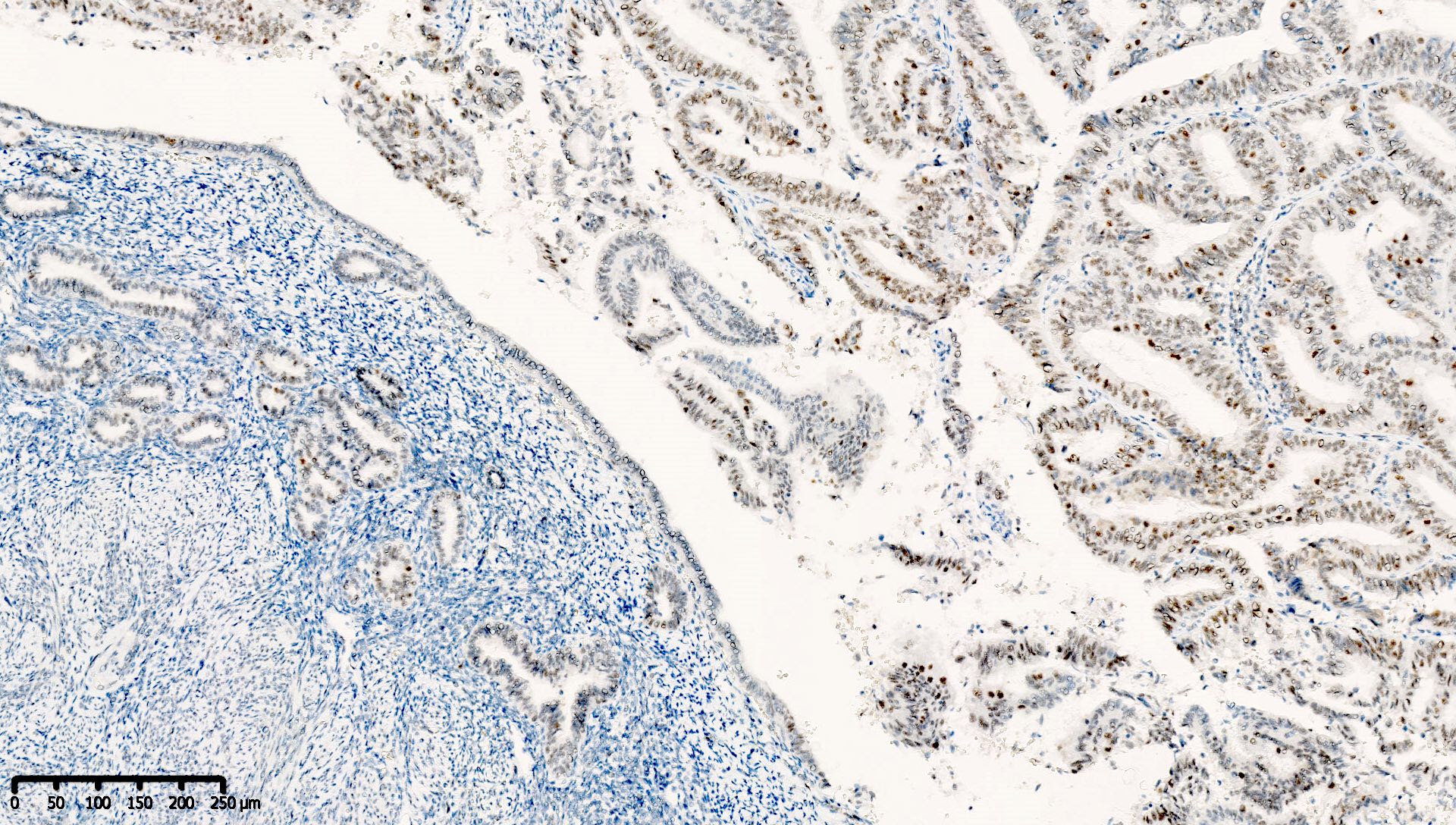
Which of the following is true about this MLH1 immunohistochemical stain, performed on a well differentiated endometrioid carcinoma of the ovary?
- Immunohistochemical stain for PMS2 should be performed to exclude PMS2 deficiency
- Mismatch repair protein MLH1 is deficient
- This immunoreactivity pattern is likely due to MLH1 promoter hypermethylation
- This patient likely has Lynch syndrome and should be referred for germline mutation testing
Board review style answer #1
A. Immunohistochemical stain for PMS2 should be performed to exclude PMS2 deficiency
Comment Here
Reference: MLH1
Comment Here
Reference: MLH1
Board review style question #2
Which of the following is true about mismatch repair immunohistochemistry?
- A patient with an MLH1 deficient, PMS2 deficient colon carcinoma harboring a BRAF mutation should be referred to genetics to exclude an underlying germline MLH1 mutation
- Colorectal carcinomas with isolated PMS2 deficiency likely present with MLH1 promoter hypermethylation
- Immunohistochemistry for mismatch repair proteins should be performed on each carcinosarcoma of the endometrium to allow molecular classification
- Microsatellite instable endometrioid carcinomas of the endometrium generally have a worse prognosis than MMR proficient, p53 mutant endometrioid carcinomas of the endometrium
Board review style answer #2
C. Immunohistochemistry for mismatch repair proteins should be performed on each carcinosarcoma of the endometrium to allow molecular classification
Comment Here
Reference: MLH1
Comment Here
Reference: MLH1