Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Clinical features | Tissue handling | Interpretation | Uses by pathologists | Prognostic factors | Microscopic (histologic) images | Positive staining - disease | Negative staining | Molecular / cytogenetics description | Molecular / cytogenetics images | Sample pathology report | Board review style question #1 | Board review style answer #1Cite this page: Tozbikian G. HER2 (c-erbB2) breast. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/stainsbreastHER2.html. Accessed April 1st, 2025.
Definition / general
- HER2 gene amplification and protein overexpression drive cancer progression
- HER2 status is an important prognostic and predictive biomarker in breast cancer
Essential features
- HER2 gene encodes a transmembrane growth factor receptor that regulates cell growth and survival
- HER2 is an oncogenic driver with negative prognostic impact in breast cancer
- HER2 testing identifies tumors that may respond to anti-HER2 directed targeted therapy
- Routinely assessed in all primary invasive breast cancer and metastatic / recurrent breast cancer to inform eligibility for anti-HER2 directed targeted therapy
- Scoring of HER2 IHC is tumor site specific (breast versus nonbreast)
Terminology
- Human epidermal growth factor receptor 2
- HER2 / neu
- ERBB2 (Erb-B2 receptor tyrosine kinase 2)
- c-erbB2
- CD340
Pathophysiology
- HER2 signaling activates multiple major signaling pathways (Ras / Raf / MEK / MAPK and PI3K / AKT) that promote cellular proliferation and antiapoptosis
- HER2 gene amplification results in HER2 protein overexpression
- HER2 alteration and dysregulation drives breast cancer progression (Cancer Treat Res 2000;103:57)
Clinical features
- Overexpressed in ~15% of breast tumors (J Clin Oncol 2023;41:3867)
- Overexpressed in ~30% of ductal carcinoma in situ (DCIS) but prognostic importance is unclear (BMC Cancer 2015;15:468, Med Oncol 2022;40:16)
- Prognostic biomarker, associated with higher histologic grade, advanced stage, brain and visceral organ metastases
- Overexpression can also be seen in nonbreast cancers (Mod Pathol 2007;20:192)
- Immunohistochemistry (IHC) detects evidence of protein overexpression via evaluation of the membranous staining in the tumor cells
American Society of Clinical Oncology (ASCO) / College of American Pathologists (CAP) recommendations for HER2 testing and result interpretation
Tissue handling
- Cytology specimens, needle biopsies and resection specimens can be used for testing
- Cold ischemia time must be limited, with the time to fixative within 1 hour
- Tissue fixed in 10% neutral buffered formalin between 6 and 72 hours
- Testing must be performed according to standardized analytically validated protocols
- Labs should show 95% concordance with another validated test (Arch Pathol Lab Med 2007;131:18, Mod Pathol 2008;21:S8)
- Similar recommendations in U.K. (J Clin Pathol 2008;61:818)
- Not validated in decalcified specimens
Interpretation
- HER2 classification in breast cancer is determined by ASCO / CAP guideline criteria (J Clin Oncol 2023;41:3867)
- Gastric / gastroesophageal junction cancers are assessed using different HER2 IHC scoring criteria
- In breast cancer, only membranous reactivity on the invasive tumor cells is counted towards scoring, expression in the in situ component is ignored
- Nonspecific cytoplasmic reactivity is ignored
HER2 IHC scoring
- Positive
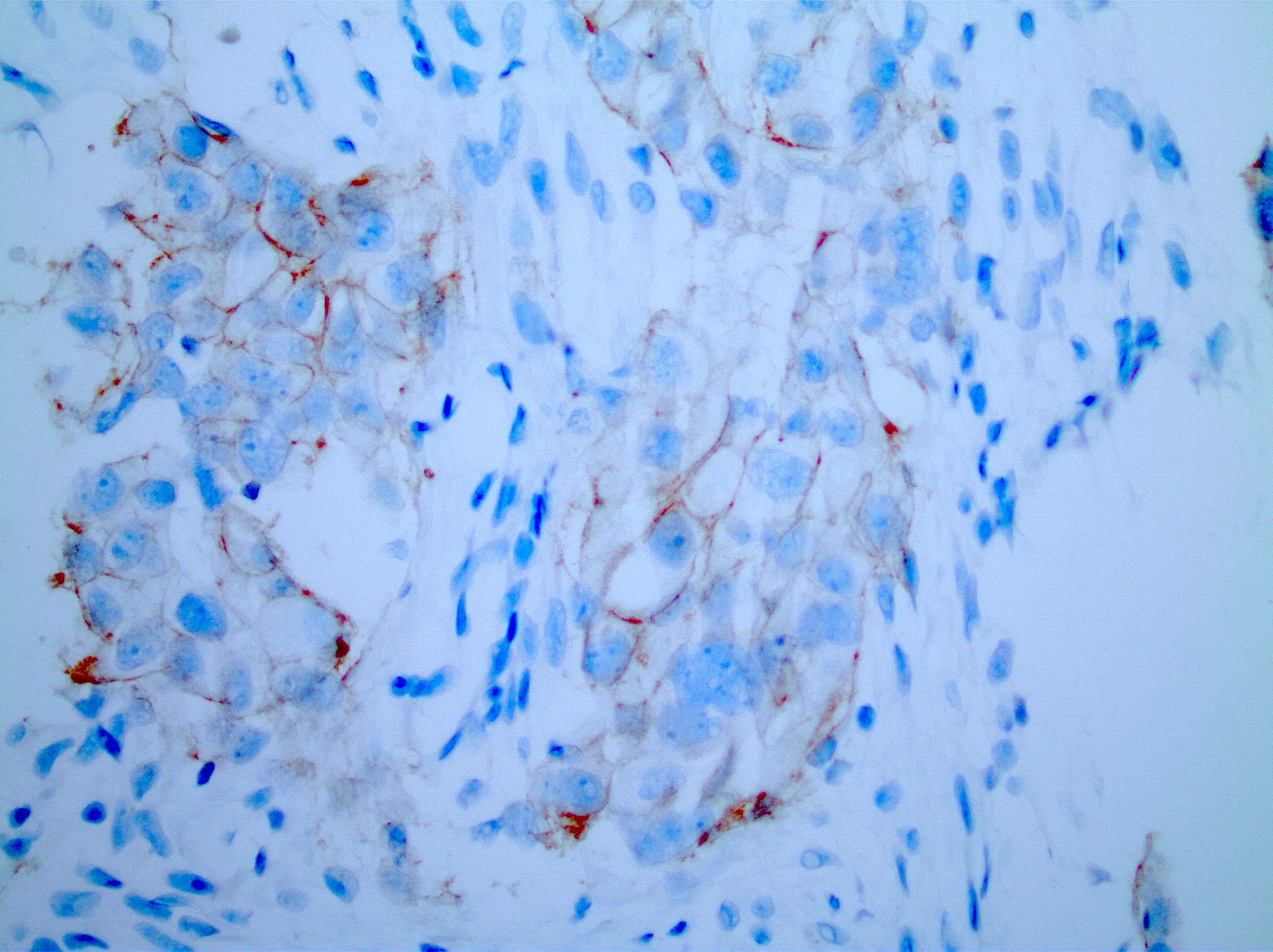
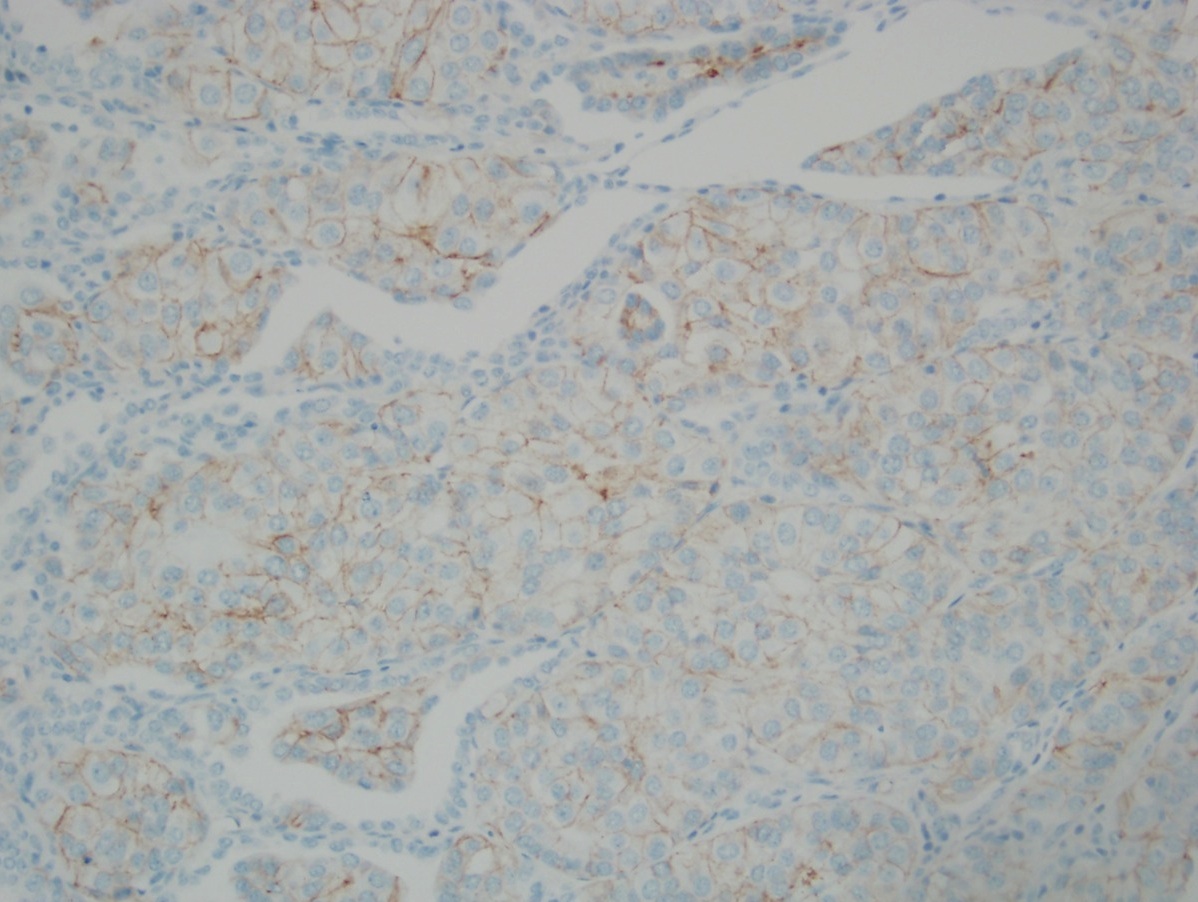
- Score 3+: tumor displays complete, intense circumferential membranous staining in > 10% of tumor cells (readily appreciated using a low power objective and observed within a homogenous and contiguous invasive cell population)
- Equivocal
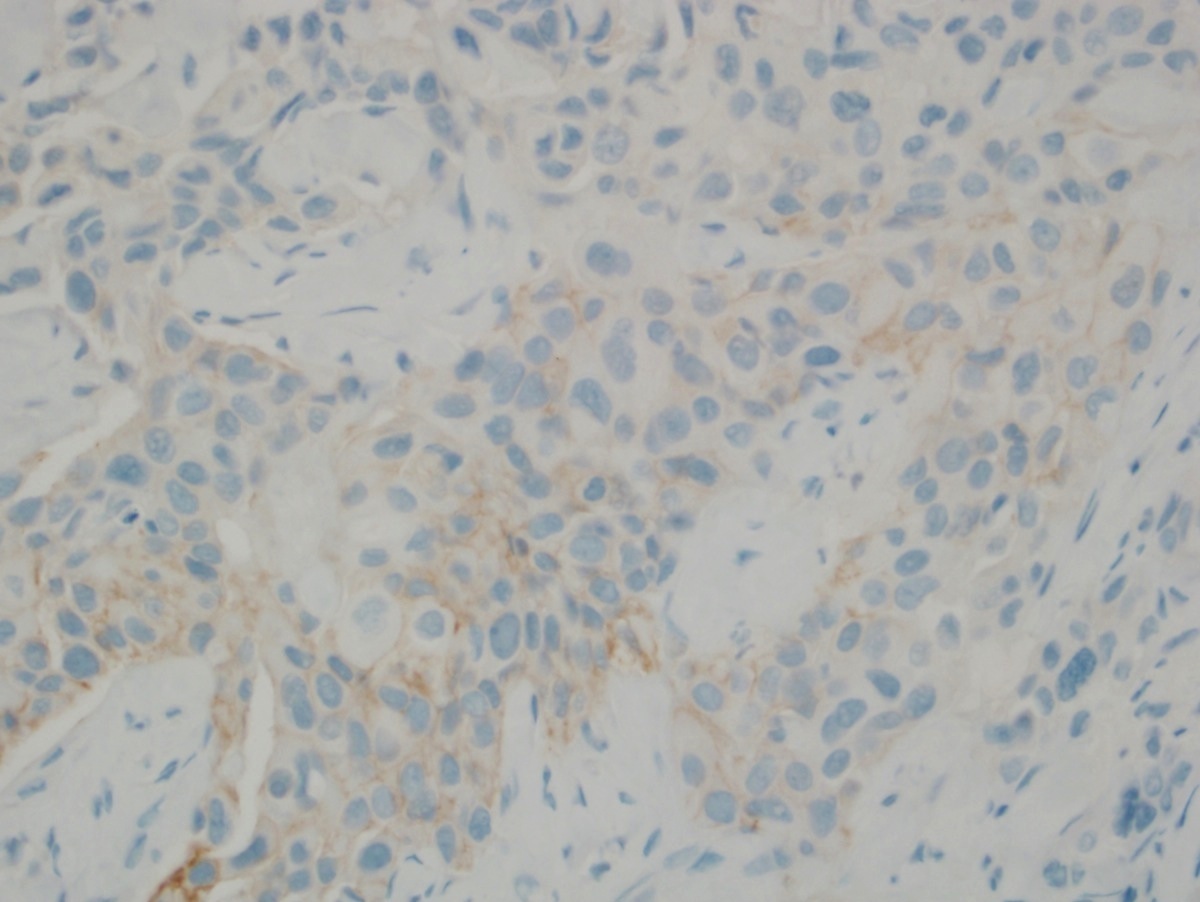
- Score 2+: weak to moderate complete membrane staining observed in > 10% of invasive tumor cells
- Other less common patterns also considered equivocal
- Circumferential membrane staining that is intense but within ≤ 10% of tumor cells
- IHC staining that is moderate to intense but incomplete (basolateral or lateral) often seen in micropapillary breast cancer
- Negative
- Score 1+: incomplete faint membrane staining and within > 10% of invasive tumor cells
- Score 0: no staining observed or incomplete faint / barely perceptible membrane staining within ≤ 10% of invasive tumor cells
- Indeterminate
- May be reported as indeterminate if technical problems preclude the reporting of a positive, negative or equivocal test result
- Reasons include improper specimen handling, crush artifact, edge artifacts and analytical testing failures
- In these cases, the reasoning for an indeterminate result should be reported and additional tissue should be acquired for testing
In situ hybridization (ISH) scoring
- Positive
- Dual probe HER2/CEP17 ratio ≥ 2.0 with average HER2 copy number ≥ 4.0
- Negative
- Dual probe HER2/CEP17 ratio < 2.0 with an average HER2 copy number < 4.0 signals/cell
- Additional workup required
- If a case has a HER2/CEP17 ratio ≥ 2.0 but the average HER2 signals/cell is < 4.0 (ISH group 2)
- If a case has an average of ≥ 6.0 HER2 signals/cell with a HER2/CEP17 ratio of < 2.0 (ISH group 3)
- If the case has an average HER2 signals/tumor cell of ≥ 4.0 and < 6.0 HER2 signals/cell and HER2/CEP17 ratio is < 2.0 (ISH group 4)
- Additional workup steps
- IHC testing for HER2 should be performed using sections from the same tissue sample used for ISH
- If the IHC result is 3+, diagnosis is HER2 positive
- If the IHC result is 2+, recount ISH by having an additional observer, blinded to previous ISH results, count at least 20 cells that include the area of invasion with IHC 2+ staining
- If reviewing the count by the additional observer changes the result into another ISH category, the result should be adjudicated per internal procedures to define the final category
- If the count remains an average of < 4.0 HER2 signals/cell and HER2/CEP17 ratio is ≥ 2.0, the diagnosis is HER2 negative with a comment
- If the HER2/CEP17 ratio remains < 2.0 with ≥ 6.0 HER2 signals/cell, the diagnosis is HER2 positive
- If the count remains an average of ≥ 4.0 and < 6.0 HER2 signals/cell with HER2/CEP17 ratio < 2.0, the diagnosis is HER2 negative with a comment
- If the IHC result is 0 / 1+, diagnosis is HER2 negative with comment
- IHC testing for HER2 should be performed using sections from the same tissue sample used for ISH
- Patient is considered HER2 positive if
- HER2 IHC score 3+ or
- IHC score 2+ and HER2 ISH positive or
- HER2 ISH positive with any IHC result
- Patient is considered HER2 negative if
- HER2 IHC score 0 or 1+ or
- IHC score 2+ and HER2 ISH negative
- If initial HER2 testing by immunohistochemistry results are equivocal, reflex testing should be performed on the same specimen using the alternative test or perform testing on a new specimen, if available, using the same or alternative test
- Breast cancers that are IHC score 1+ or 2+ and ISH nonamplified often informally referred to as having HER2 low status (this terminology is not accepted as a result category by current ASCO / CAP guidelines, however, can be referred to in a comment within the report) (J Clin Oncol 2023;41:3867)
Uses by pathologists
- HER2 IHC is the Food and Drug Administration (FDA) approved companion diagnostic for specific targeted therapies in certain clinical scenarios for
- Primary and metastatic HER2 positive breast cancer for anti-HER2 directed therapies (e.g., trastuzumab, pertuzumab)
- Patients with unresectable or metastatic breast cancer that is IHC score 1+ or 2+, HER2 ISH nonamplified (e.g., trastuzumab deruxtecan)
- Unresectable and metastatic HER2 positive solid tumors who have received prior systemic therapies and have no satisfactory alternative treatment options (e.g., trastuzumab deruxtecan) (FDA: FDA D.I.S.C.O. Burst Edition - FDA Approval of Enhertu (Fam-trastuzumab Deruxtecan-nxki) for Unresectable or Metastatic HER2-Positive Solid Tumors [Accessed 7 June 2024])
Prognostic factors
- Predictive biomarker used to identify patients with HER2 positive breast cancers that are most likely to respond to anti-HER2 targeted therapy (N Engl J Med 2005;353:1673)
- Patients with unresectable or metastatic breast cancer that is IHC score 1+ or 2+, HER2 ISH nonamplified (often referred to as having HER2 low expression) who received prior chemotherapy in the metastatic setting or recurred within 6 months of completing adjuvant chemotherapy are also eligible for trastuzumab deruxtecan therapy (N Engl J Med 2005;353:1673)
- Anti-HER2 therapies (e.g., trastuzumab, lapatinib) plus chemotherapy reduces recurrence, metastases and mortality in HER2 positive breast cancer patients (Int Semin Surg Oncol 2008;5:9, Acta Oncol 2008;47:1564, Biologics 2009;3:289)
- Anti-HER2 therapy improves survival in patients with HER2 positive metastatic disease (Am J Clin Oncol 2008;31:250, N Engl J Med 2007;357:1496)
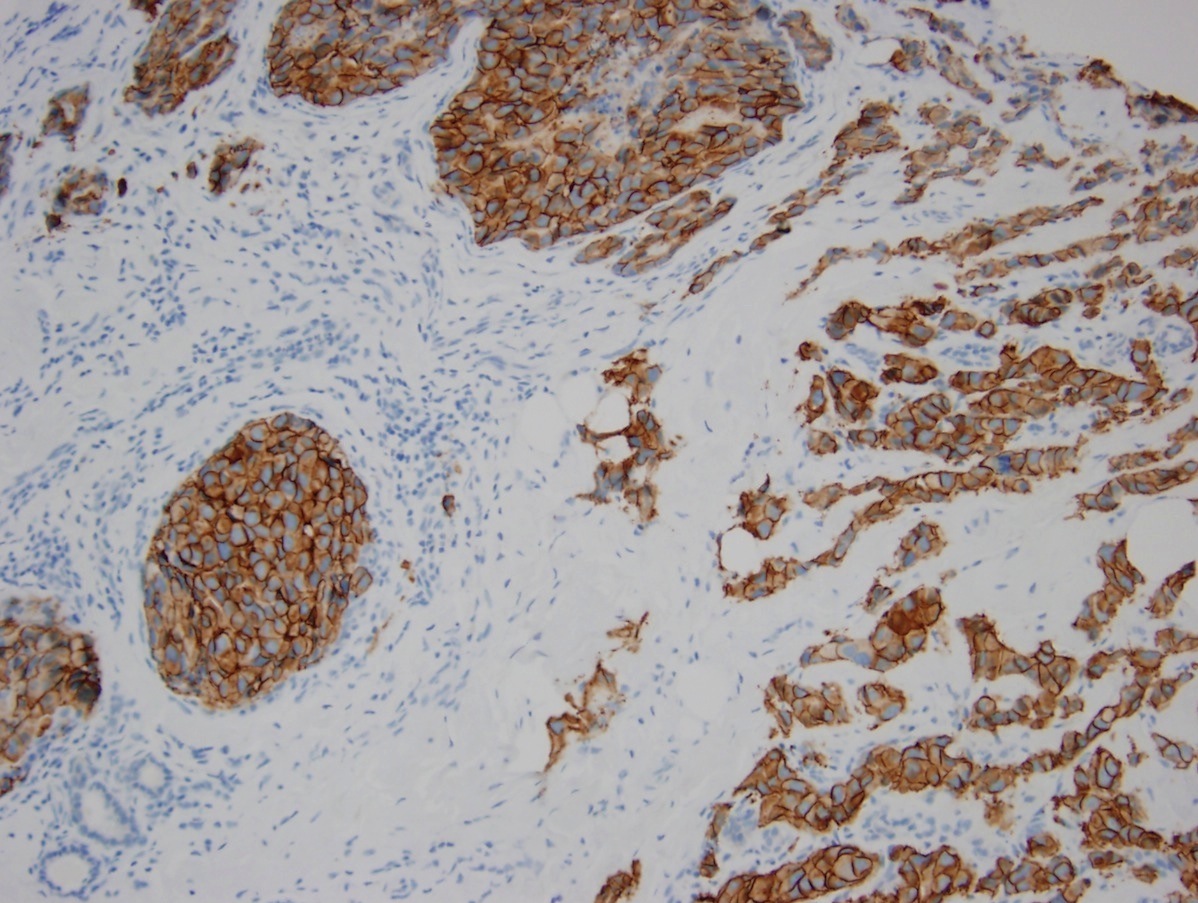
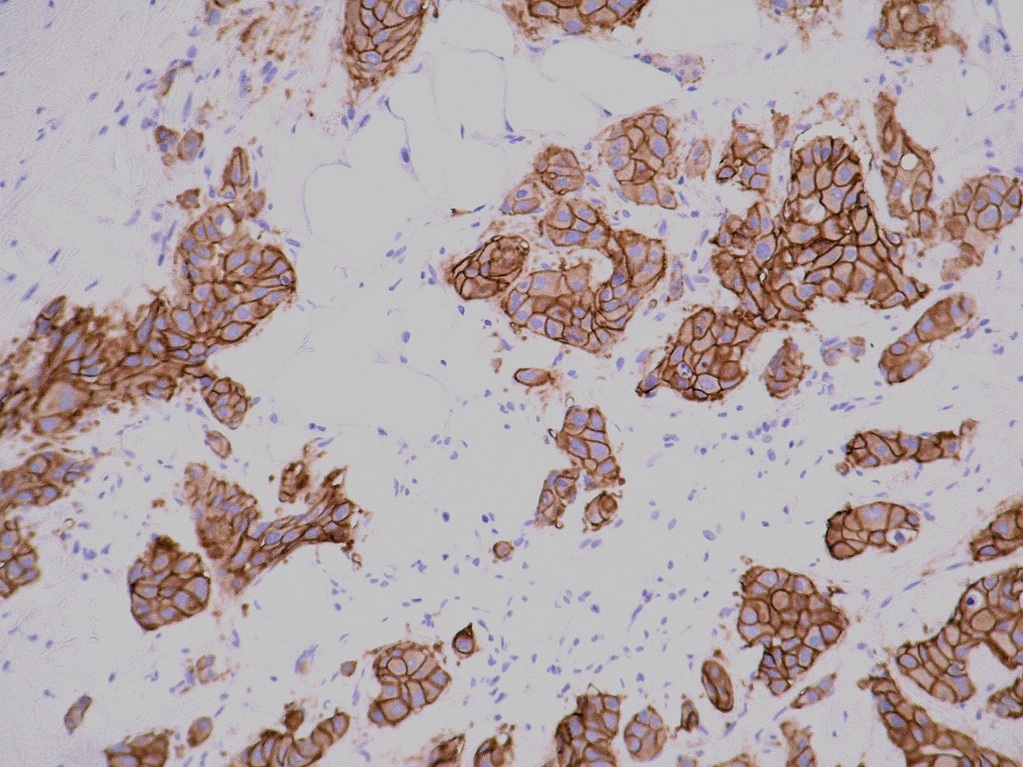
Microscopic (histologic) images
Positive staining - disease
- Positive in 12 - 20% of invasive breast cancer (NST) type
- Positive in 20 - 30% of invasive pleomorphic lobular carcinoma
Negative staining
- Low grade invasive breast cancer (NST) type (J Clin Oncol 2018;36:2105)
- Tubular carcinoma (J Clin Oncol 2010;28:99)
- Mucinous carcinoma (rare, unless micropapillary pattern) (Hum Pathol 2013;44:1577, Breast Cancer Res Treat 2015;151:443)
- Invasive lobular carcinoma (classic type) (rare) (Cancer 1991;68:331)
- Cribriform carcinoma (< 5%) (Medicine (Baltimore) 2015;94:e1309)
- Metaplastic carcinoma (Histopathology 2006;49:10, Oncologist 2018;23:481)
- Adenoid cystic carcinoma (J Minim Invasive Gynecol 2010;17:295)
- Secretory carcinoma (Am J Surg Pathol 2015;39:1458)
- Mucoepidermoid carcinoma (Medicine (Baltimore) 2017;96:e9385)
- Tall cell with reverse polarity (Am J Surg Pathol 2017;41:887)
- Neuroendocrine tumor (Histopathology 2016;68:422)
- Neuroendocrine carcinoma (Am J Surg Pathol 2000;24:1231)
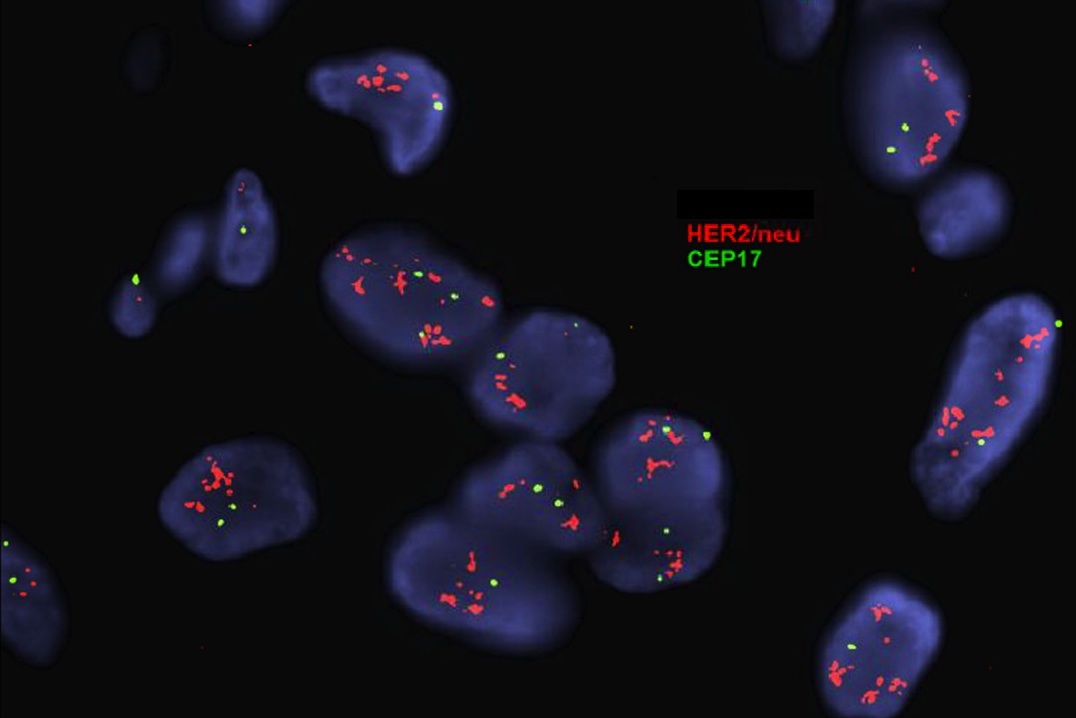
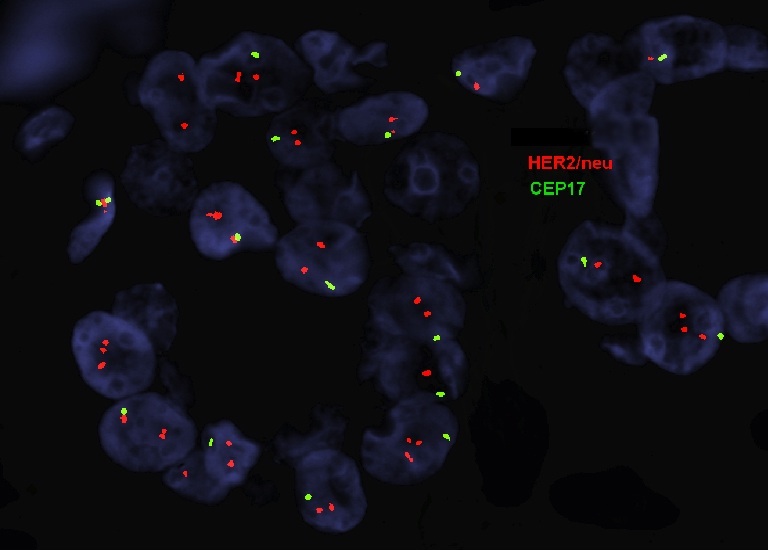
Molecular / cytogenetics description
- In situ hybridization (ISH) assay is used to detect HER2 gene amplification, generally using a dual probe fluorescent ISH assay (D-FISH) using fluorochrome labeled probes for (a) the HER2 locus on the long arm of chromosome 17 and (b) a site near the centromere of chromosome 17 (CEN17 or CEP17)
- In situ hybridization detects HER2 gene amplification as evaluated by counting at least 20 tumor cells and estimating both the HER2 copy number and the HER2/CEP17 ratio
- Amplification can also be detected with chromogenic ISH (CISH) and silver enhanced ISH (SISH) (Mod Pathol 2002;15:657, Mod Pathol 2005;18:1015, Mod Pathol 2006;19:481, Breast Cancer Res 2007;9:R68, Am J Clin Pathol 2009;132:514)
- CISH and SISH use a peroxidase enzyme labeled probe with chromogenic detection, allowing results to be visualized with standard brightfield microscopy, so histologic features and HER2 status can be evaluated in parallel; signals do not decay over time, unlike FISH (Am J Clin Pathol 2009;132:539)
- Chromogenic in situ hybridization (CISH) is the only FDA approved single probe ISH test for HER2
- Next generation sequencing by Foundation One CDx is FDA approved for clinical HER2 assessment (FoundationOne®CDx: Technical Information [Accessed 7 June 2024])
- Testing must be performed in accredited laboratories
Molecular / cytogenetics images
Sample pathology report
- Left breast, 10:00 zone 3, ultrasound guided core needle biopsy:
- Invasive breast carcinoma, NST, grade 2, 0.8 cm in greatest length (see comment)
- Estrogen receptor: positive (90%, strong intensity)
- Progesterone receptor: negative (0%)
- HER2: equivocal (score 2+)
- HER2 FISH: pending
- Cold ischemia time: 0 minutes
- Total fixation time: 12 hours, 15 minutes
- Comment: HER2 protein expression is evaluated by manual quantitative immunohistochemistry on formalin fixed (for > 6 and < 72 hours if possible), paraffin embedded tissue using FDA approved clone 4B5 (rabbit monoclonal, Ventana) on a Ventana autostainer and scored according to ASCO / CAP criteria. Membrane staining of tumor cells is scored as readily appreciated using a low power objective as 0 (negative) no staining or membrane staining that is incomplete and faint / barely perceptible in ≤ 10% of invasive tumor cells; 1+ (negative) incomplete membrane staining that is faint / barely perceptible in > 10% of invasive tumor cells; 2+ (equivocal) weak to moderate complete membrane staining observed in > 10% of tumor cells; 3+ (positive) circumferential, complete, intense membrane staining observed in a homogeneous contiguous population within > 10% of invasive tumor cells. Breast cancers with HER2 IHC score 3+ are usually eligible for HER2 targeted therapy. Breast cancers with HER2 IHC score 1+ or score 2+ and a negative ISH result are considered HER2 low and may also be eligible for HER2 targeted therapy.
Board review style question #1
A woman with a right breast mass and axillary lymphadenopathy was found to have invasive mammary breast carcinoma, no special type (NST) with a positive lymph node. HER2 by IHC was performed and resulted in an equivocal score (2+) on the core biopsy of the breast. What is the next step in management?
- Perform HER2 ISH on the breast core
- Report the HER2 status of the patient as indeterminate
- Start anti-HER2 treatment with neoadjuvant chemotherapy
- Surgical resection followed by radiation therapy
Board review style answer #1
A. Perform HER2 ISH on the breast core. The ASCO / CAP guidelines recommend reflex testing by ISH if the initial HER2 test by immunohistochemistry results in an equivocal score of 2+, which should be performed on the same specimen or a new test should be ordered on an alternative specimen using either IHC or ISH (in this case, the lymph node). Answer B is incorrect because an indeterminate result is given when technical issues preclude interpretation of the test result. Answer D is incorrect because HER2 status should be determined before next steps in care. Answer C is incorrect because anti-HER2 treatment should only begin if the patient is classified as HER2 positive.
Comment Here
Reference: HER2 (c-erbB2) breast
Comment Here
Reference: HER2 (c-erbB2) breast