Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Negative stains | Flow cytometry description | Electron microscopy description | Molecular / cytogenetics description | Differential diagnosis | Additional referencesCite this page: Obeng R. Amyloid beta and amyloid beta precursor protein. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/stainsamyloidbetaapp.html. Accessed March 31st, 2025.
Definition / general
- Amyloid beta (A4) precursor protein (APP) is part of the type 1 transmembrane protein family
- The APP gene is located on chromosome 21 and encodes for a cell surface receptor and transmembrane precursor protein
- There are three homologs of APP: APP, APLP1 and APLP2 (Mol Neurodegener 2011;6:27)
- Cleavage of APP sequentially by beta secretase (rate limiting step) and gamma secretase produces beta amyloid (amyloid beta, A4, Abeta) peptides of 40 - 43 amino acids, as well as other peptides that have transcriptional, antimicrobial, or antifungal activities (Curr Opin Neurol 2000;13:377)
- The beta amyloid sequence is unique to APP and is not present in APLP1 or APLP2
- Cleavage of the precursor protein by gamma secretase is not precise and thus generates different forms of beta amyloid with Abeta40 being the most abundant, accounting for 80 - 90% of the beta amyloid that is produced (J Alzheimers Dis 2010;19:311)
- Beta amyloid peptides polymerize to form oligomers that then form fibrils or plaques
- Oligomers and fibrils of beta amyloid are deposited in the brain and in the cerebrovascular system; these deposits lead to the development of Alzheimer disease and cerebral amyloid angiopathy
- The Abeta42 form accounts for 5 - 10% of the amyloid beta peptides but is more hydrophilic and prone to fibril formation; it is the main form that is deposited in the brain
- Beta amyloid oligomers are also seen in inclusion body myositis and lewy body dementia
- Mutations in the APP gene lead to autosomal dominant / familial Alzheimer disease type 1, that accounts for less than 5% of Alzheimer cases and comprises 10 - 15% of early onset familial Alzheimer disease (Eur Neurol 1995;35:8, Am J Hum Genet 1999;65:664, Genet Med 2011;13:597)
- Mutations in APP also lead to autosomal dominant hereditary cerebral hemorrhage with amyloidosis-Dutch type (Neuropathology 2005;25:288)
Essential features
- Amyloid beta precursor protein is cleaved into several peptides with different functions, including beta amyloid
- There are several forms of beta amyloid peptides that aggregate to form oligomers and fibrils that are deposited in the brain and cerebral vasculature causing disease
- Studies suggest that beta amyloid oligomers, not the fibrils, are the neurotoxic form (J Neurosci 1999;19:8876, Nature 2002;418:291, PNAS 1998;95:6448)
- Mutations in APP lead to autosomal dominant Alzheimer disease and hereditary cerebral hemorrhage with amyloidosis-Dutch type, a type of cerebral amyloid angiopathy
- The association of beta amyloid deposits to the degree of dementia is weak at best
- Beta amyloid deposits in the brain and vasculature can be found in healthy, cognitively normal people
Terminology
- Amyloid beta precursor protein is also known as APP, AAA, AD1, PN2, ABPP, APPI, CVAP, ABETA, PN-II, CTFgamma
- Beta amyloid is also referred to as amyloid beta, Abeta, or A4
Epidemiology
- The prevalence of beta amyloid deposits in the brain ranges between 0 and 47% in cognitively normal elderly people and between 68 and 100% in those with Alzheimer disease (Neurology 2009;73:754, Alzheimers Dement 2010;6:221, Neurology 2012;79:1636, J Nucl Med 2009;50:878)
- Vascular beta amyloid has been detected in 27% of cognitively normal controls and 78% of patients with cerebral amyloid angiopathy (Mov Disord 2003;18:81, Folia Neuropathol 2013;51:120)
Sites
- The amyloid beta precursor protein is expressed in central nervous system as well as other normal tissues
- Beta amyloid oligomers and fibrils are generally found in the central nervous system and cerebral vasculature
Pathophysiology
- Beta amyloid peptides produced by cleavage of APP polymerize to form oligomers and then fibrils or plaques
- The accumulation of beta amyloid plaques in the brain (extracellular) and in the media and adventitia of medium and small arteries of the cerebral cortex and leptomeninges contributes to the development and progression of Alzheimer disease and cerebral amyloid angiopathy (Curr Med Chem 2009;16:2498)
- Recent studies have suggested that the oligomeric form of beta amyloid is the neurotoxic form that contributes to the pathology of beta amyloid
- The apolipoprotein E E4 allele is speculated to prevent the suppression of amyloid production or the clearance of amyloid
Etiology
- Some beta amyloid peptides are unstable in free form and thus polymerize to form fibrils and plaques
- Accumulation of amyloid plaques in the brain is dependent on the rate of production and clearance out of the brain
- Neurotoxicity of beta amyloid and Tau (a microtubule-associated protein) deposits are thought to contribute significantly to Alzheimer disease, but the etiology of Alzheimer disease and cerebral amyloid angiopathy is not known
Clinical features
- Alzheimer disease: progressive memory loss and cognitive impairment, mood and personality changes, depression, anxiety – in part due to the accumulation of beta amyloid deposits in the brain
- Cerebral amyloid angiopathy: dementia, intracranial hemorrhage – due to beta amyloid deposits in the vasculature
Diagnosis
- Beta amyloid can be detected using Congo red staining, immunohistochemical staining or PET imaging
- Alzheimer disease: the presence of beta amyloid plaques and neurofibrillary tangles is essential for the diagnosis of Alzheimer disease (Alzheimer Dementia 2012;8:1) but is not pathognomonic for the disease – beta amyloid plaques may be seen in normal aging and neurofibrillary tangles can be present in other neurodegenerative disorders
- Cerebral amyloid angiopathy: clinical and MRI evidence of hemorrhage or hematomas with cortical biopsy demonstrating vascular amyloid deposition (Ann Neruol 2004;55:250)
- Histologic evaluation of vessels is required for definitive diagnosis
Laboratory
- Levels of beta amyloid are generally low in the cerebrospinal fluid of Alzheimer patients and cerebral amyloid angiopathic patients
Radiology description
- Guidelines for the use of PET imaging in differentiating Alzheimer disease from other types of dementia are set by the Amyloid Imaging Taskforce
- PET imaging uses 3 FDA approved beta amyloid tracers (florbetapir F 18, flutemetamol F18 and florbetaben F18) to detect beta amyloid deposits in the brain (Arch Neurol 2011;68:1404, JAMA 2011;305:275, Alzheimer Dis Assoc Disord 2012;26:8)
Prognostic factors
- Mutations in APP that lead to early onset Alzheimer disease result in a more aggressive disease and rapid course
- Cerebral amyloid angiopathy:
- Lobar intracranial hemorrhages have a better prognosis than hypertensive deep ganglionic bleeds
- 25 - 40% recurrence rate, which are associated with increased mortality (up to 40%); patients are at highest risk in the first year
Case reports
- Cerebral amyloid angiopathy-related inflammation (Case Rep Neurol Med 2015;2015:483020)
- Misdiagnosis of inclusion body myositis (J Med Case Rep 2015;9:169)
- Sporadic cerebral amyloid angiopathy with giant cell reaction (Acta Neuropathol 1990;81:95)
Treatment
- There are no effective therapies for APP or the accumulation of beta amyloid
- Symptomatic treatment of Alzheimer disease include drugs that modulate neurotransmitters (Ach and NMDA) and psychotropic medications
- Treatment of cerebral amyloid angiopathy includes standard therapy for intracranial hemorrhage, steroids and immunosuppressants for patients with co-existing vasculitis
Microscopic (histologic) description
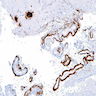
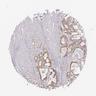
- Beta amyloid:
- Extracellular cortical deposits
- Pink amorphous extracellular material surrounded by a halo
- May also be surrounded by dystrophic / degenerating neurites and reactive glia, cotton wool plaques, amyloid lakes, subpial bands
- Homogenous, eosinophilic substance in vessel wall, may have a smudged appearance; fibrinoid necrosis in amyloid-laden vessels
- Yellow-green birefringence with Congo red stain and polarization
- Immunohistochemical staining for APP is cytoplasmic
- Subcellular localization in golgi apparatus of APP seen in human cell line U-2 OS
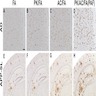
Microscopic (histologic) images
Virtual slides
Positive stains
- Weak to moderate APP staining in neuronal cells
- Weak positive APP staining has also been noted in other normal tissues such as the GI tract and male reproductive system
- Weak to moderate cytoplasmic APP staining is seen in many malignant tissues with strong staining noted in some testicular and urothelial cancer tissues
Negative stains
- APP: normal liver and skeletal tissue
Flow cytometry description
- Currently experimental for the detection of beta amyloid oligomers (J Alzheimers Dis 2007;11:117)
Electron microscopy description
- Beta amyloid plaques on EM appear as a dense core of star-like beta amyloid fibril bundles surrounded by dystrophic neurites, activated microglia and reactive astrocytes (Tosoni A., Barbiano di Belgiojoso G. and Nebuloni M.: Electron Microscopy in the Diagnosis of Amyloidosis, 2011)
Molecular / cytogenetics description
- Pathogenic mutations in APP are generally missense or nonsense mutations (but can also include deletions, insertions and splice site mutations) and mainly occur in exons 16 and 17
- Clinical sequence analysis or mutation scanning may be performed for the entire gene or restricted to exons 16 and 17 only
- Duplication mutations of APP are detected by FISH analysis and account for less than 1% of the pathogenic mutations in APP (Nat Genet 2006;38:24)
Differential diagnosis
Additional references