Table of Contents
Definition / general | Essential features | Pathophysiology | Clinical features | Interpretation | Uses by pathologists | Prognostic factors | Microscopic (histologic) images | Positive staining - normal | Positive staining - disease | Negative staining | Molecular / cytogenetics description | Sample pathology report | Additional references | Board review style question #1 | Board review style answer #1Cite this page: Nadeem U, Husain A. WT1. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/stainsWT1.html. Accessed March 31st, 2025.
Definition / general
- WT1 gene encodes for Wilms tumor protein located on chromosome 11p13
- Expressed / mutated in several different tumors
Essential features
- Interpretation is based on staining pattern and antibody used
- Staining patterns: nuclear and cytoplasmic
- Antibodies: against N and C terminal
- Expressed in a broad spectrum of tumors, including malignant mesothelioma, ovarian serous epithelial tumors and sex cord stromal tumors, astrocytoma, glioblastoma, benign and malignant vascular tumors and desmoplastic malignant melanoma
- Expression linked to poor prognosis of several tumors, including acute myeloid leukemia, pancreatic and colorectal cancers and astrocytoma
- Vaccination and immunotherapy directed against WT1 is in trials
Pathophysiology
- Transcription factor essential for normal development of the urogenital system
- Both tumor suppressing and tumorigenic role in several neoplasias, including breast, acute myeloid leukemia (AML) and mesothelioma
- Affects genes of the Wnt / β-catenin pathway and p53 tumor suppressor pathway
Clinical features
- WT1 mutations in Denys-Drash syndrome, diffuse mesangial sclerosis kidney, Fraiser syndrome, Wilms tumor in 15% of cases, congenital nephrotic syndrome, cytogenetically normal acute myeloid leukemia and prostate cancer (Adv Pediatr 2012;59:247)
- Chromosomal translocation (11;22)(p13;q12) EWSR1-WT1 gene fusion in desmoplastic round blue cell tumor
- Mutated in 1.45% of all carcinomas
Interpretation
- Can be exclusively nuclear or cytoplasmic according to the antibodies used (anti-C or N terminus WT1 antibody)
- Interpretation has to be based on the tissue being stained and the context
Uses by pathologists
- Nuclear stain against N terminus (disregard any cytoplasmic staining in these tumors) to differentiate:
- Malignant mesothelioma (WT1+) from non small cell lung carcinomas (WT1-) (Arch Pathol Lab Med 2018;142:89)
- Wilms tumor (WT1+ in blastemal and epithelial components) from mesoblastic nephroma, clear cell sarcoma, renal cell carcinoma and peripheral neuroectodermal tumor and angiomyolipoma (WT1-) (Indian J Med Res 2016;143:S59)
- Adenomatoid tumor (WT1+) from signet ring cell carcinoma and lymphangioma (WT1-) (Arch Pathol Lab Med 2015;139:39, Int J Gynecol Pathol 2004;23:123)
- Metanephric adenoma (WT1+) from solid variant of papillary renal cell carcinoma (WT1-) (Histopathology 2013;62:941, Mod Pathol 2015;28:1236)
- Fallopian tube and ovarian serous carcinoma (WT1+) from endometrioid, mucinous and clear cell adenocarcinoma (WT1-) (Int J Gynecol Pathol 2004;23:110)
- Transitional cell carcinoma of the ovary (WT1+) from malignant Brenner tumor (WT1-) (Int J Gynecol Pathol 2012;31:499)
- CIC-DUX4 sarcoma (WT1+) from BCOR-CCNB3 and Ewing sarcoma (WT1-) (Virchows Arch 2017;470:373)
- Desmoplastic melanoma (WT1+) from scar, fibromatosis and dermatofibroma (WT1-) (J Cutan Pathol 2016;43:313)
- Pleural endometriosis (WT1+) from biphasic pulmonary blastoma and lung adenocarcinoma (WT1-)
- Cytoplasmic stain against N terminus to differentiate:
- Benign and malignant vascular tumors (WT1+) from vascular malformations (WT1-) (J Am Acad Dermatol 2010;63:1052)
- Mammary myofibroblastoma, epithelioid type (WT1+) from lobular breast lobular carcinoma (WT1-) (Acta Histochem 2014;116:905)
- Young type fibromatosis, e.g. congenital / infantile fibrosarcoma, fibrous hamartoma of infancy, myofibroma / myofibromatosis and lipofibromatosis (WT1+) from adult type fibromatosis e.g. nodular fasciitis and desmoid type (WT1) (Acta Histochem 2014;116:1134)
- Nuclear stain against C terminus to differentiate:
- Desmoplastic round blue cell tumor (WT1+) from Ewing sarcoma / primitive neuroectodermal tumors, neuroblastomas or rhabdoid tumors of the kidney (WT1) (Am J Surg Pathol 2000;24:830)
Prognostic factors
- Acute myeloid leukemias: WT1+ have mutations in exon 7 and 9 of WT1 genes, have a worse prognosis and are resistant to chemotherapy
- Diffuse astrocytic tumors: WT1+ tumors have a higher WHO tumor grade, no IDH1 mutations and a poor outcome
- Colorectal and pancreatic ductal adenocarcinoma: WT1+ have a poorer prognosis
- High grade ovarian serous carcinomas: WT1+ have a better prognosis, especially when the tumor cells also express estrogen receptor (Gynecol Oncol 2016;140:494)
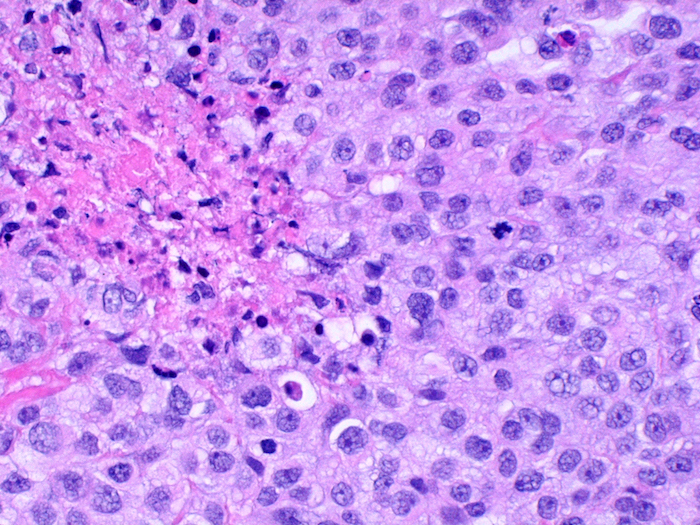
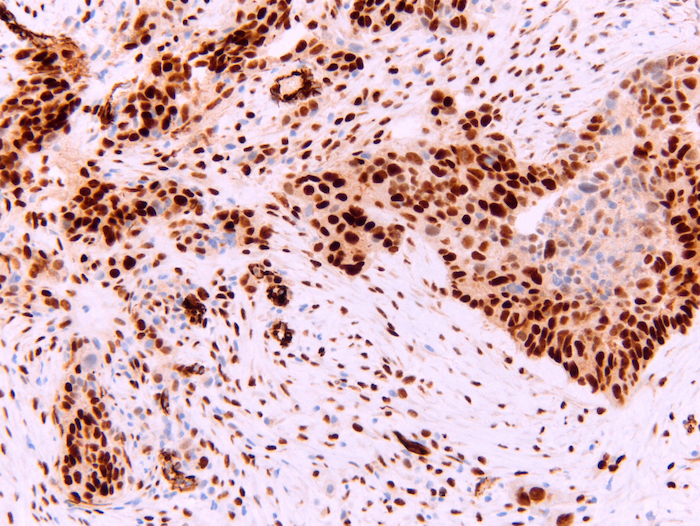
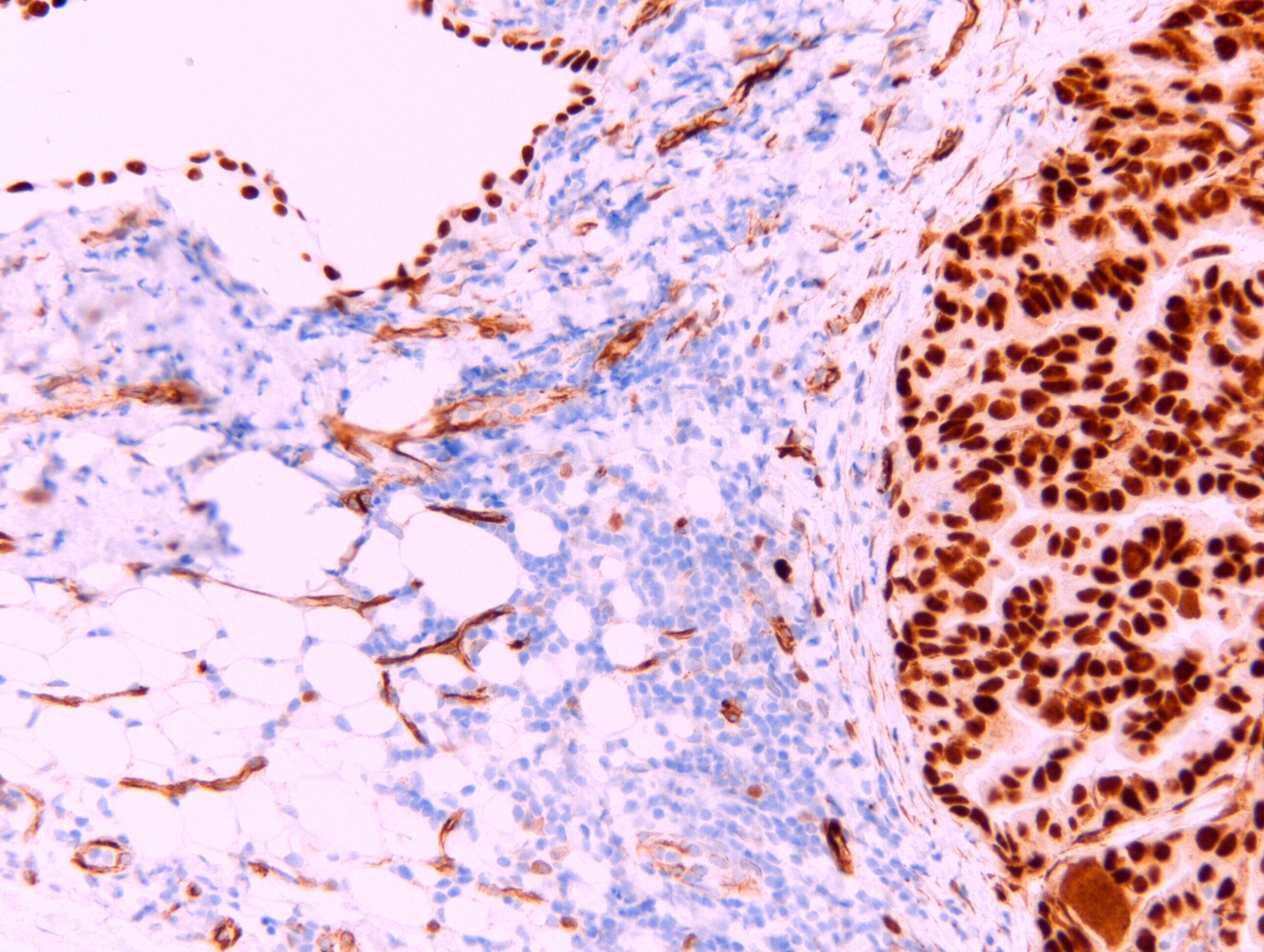
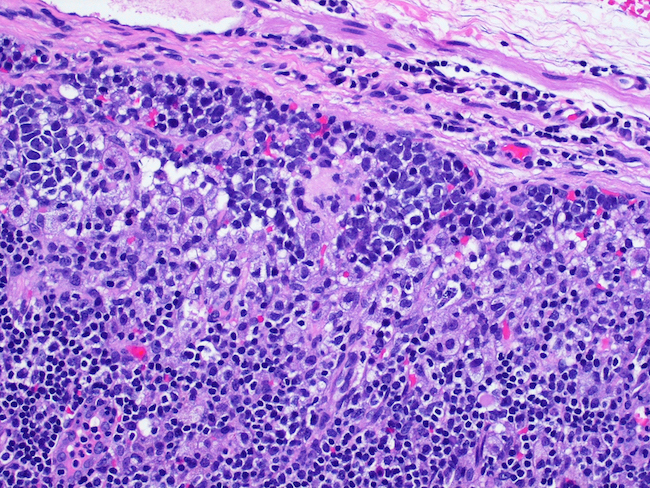
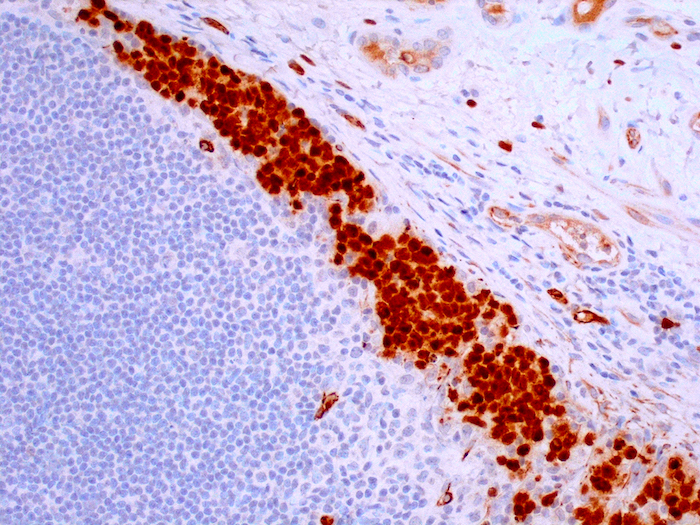
Microscopic (histologic) images
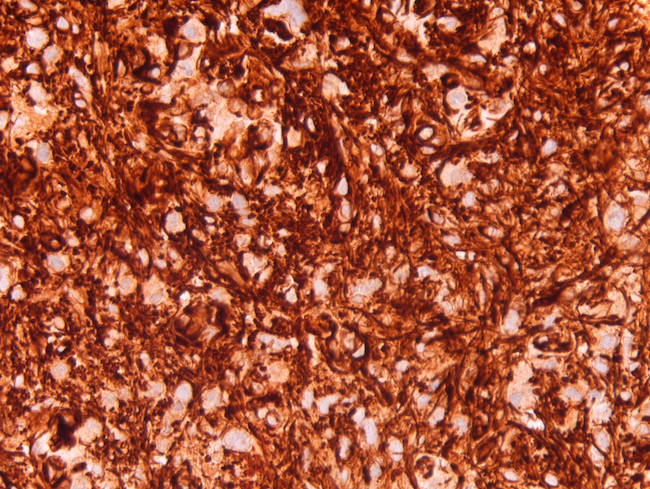
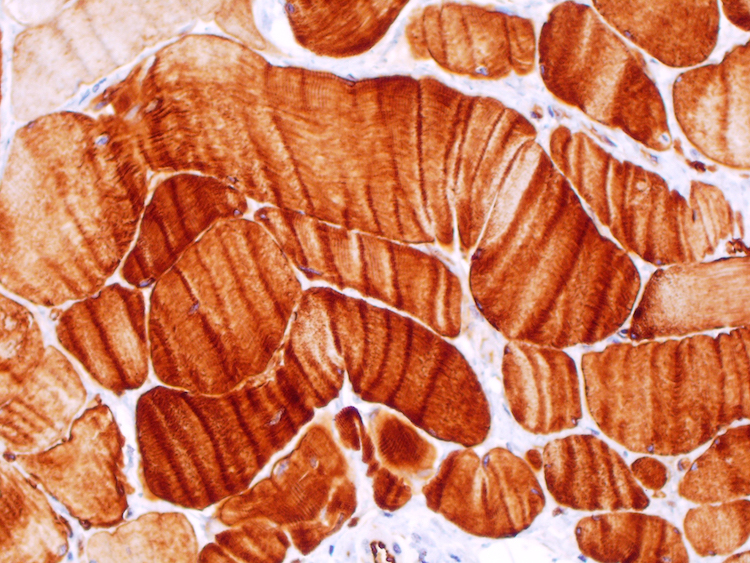
Positive staining - normal
- Nuclear
- Glomerular podocytes (Curr Biol 2001;11:1805)
- Sertoli cells of the testis (Mech Dev 1993;40:85)
- Surface epithelium and granulosa cells of the ovary (Am J Surg Pathol 2008;32:884)
- Myometrium and endometrial stromal cells of the uterus (Genes Dev 1991;5:1345)
- Mesothelium (Cancer Cytopathol 2014;122:586)
- CD34 positive hematopoietic stem cells (Br J Haematol 2002;116:409)
- Cytoplasmic
- Skeletal muscle (Acta Histochem 2013;115:70)
- Endothelial cells (Acta Histochem 2013;115:70)
- Cardiac muscle cells (Acta Histochem 2013;115:70)
- Adrenal gland (Mol Pathol 1997;50:138)
- Plasma cells (Cancer Cytopathol 2014;122:586)
Positive staining - disease
- Nuclear N terminus
- Epithelioid mesothelioma (93%) (Am J Surg Pathol 2003;27:1031)
- Adenomatoid tumor and well differentiated papillary mesothelioma (Arch Pathol Lab Med 2015;139:39, Mod Pathol 2019;32:88)
- Wilms tumor: strong in blastemal and epithelial component and absent in the epithelial and stromal elements (Appl Sci 2020;10:40)
- Metanephric adenoma (Mod Pathol 2015;28:1236)
- Fallopian tube and ovarian serous carcinoma (Int J Gynecol Pathol 2004;23:110)
- Transitional cell carcinoma of the ovary (67%) (Int J Gynecol Pathol 2012;31:499)
- Ovarian Sertoli cell tumor (Am J Surg Pathol 2007;31:1378)
- Sex cord stromal tumors (78 - 100%) (Am J Surg Pathol 2009;33:354)
- Adult granulosa cell tumor (65 - 88%) (Am J Surg Pathol 2009;33:354)
- Small cell carcinoma of the ovary, hypercalcemic type (Rare Dis 2014;2:e967148)
- CIC-DUX4 sarcoma (Virchows Arch 2017;470:373)
- Pleural endometriosis (Suster: Diagnostic Pathology - Thoracic, 2nd Edition, 2017)
- Cutaneous Müllerian cyst (Case Rep Med 2016;2016:2487820)
- Breast adenocarcinoma mucinous (64%) and mixed mucinous (10%) (Mod Pathol 2008;21:1217)
- Cytoplasmic N terminus
- Vascular tumors, both benign and malignant (100%) (J Am Acad Dermatol 2010;63:1052)
- Rhabdomyosarcoma, all histologic variants (100%) (Mod Pathol 2002;15:1080)
- Malignant peripheral nerve sheath tumor (71%) (World J Surg Oncol 2014;12:214)
- Mammary myofibroblastoma, epithelioid type only (Acta Histochem 2014;116:905)
- Endometrial sarcomas: leiomyosarcoma (76%), carcinosarcoma (44%), endometrial stromal sarcoma (47%), undifferentiated sarcoma (57%) (Eur J Cancer 2007;43:1630)
- Congenital / infantile fibrosarcoma (Am J Surg Pathol 1994;18:14)
- Fibrous hamartoma of infancy, myofibroma / myofibromatosis and lipofibromatosis (Acta Histochem 2015;117:367)
- Brain astrocytoma / glioblastoma (Neuropathol Appl Neurobiol 2009;35:69)
- Pancreatic ductal adenocarcinomas (75%) (Cancer Sci 2004;95:583)
- Colorectal adenocarcinomas (89%) (Cancer Sci 2003;94:712)
- Desmoplastic melanoma (71%) and nondesmoplastic melanoma (47%) (Am J Dermatopathol 2014;36:238)
- Compound / intradermal nevi (68.3%), congenital nevi (9.1%), Spitz nevi (62.5%), atypical nevi (30.4%) (J Cutan Pathol 2010;37:542)
- Nuclear C terminus
- Desmoplastic round blue cell tumor (90%)
Negative staining
- GU:
- Renal cell carcinoma, including papillary type (Arch Pathol Lab Med 2018;142:89, Mod Pathol 2015;28:1236)
- Congenital mesonephric tumor (Indian J Med Res 2016;143:S59)
- Gynecologic:
- Normal endometrium and cervical epithelium (Int J Gynecol Pathol 2000;19:158)
- Endometrioid adenocarcinoma (Int J Gynecol Pathol 2004;23:110)
- Mucinous and clear cell carcinomas of ovary (Arch Pathol Lab Med 2005;129:85)
- Lung:
- Non small cell lung carcinoma (Arch Pathol Lab Med 2018;142:89)
- Pleura:
- Sarcomatoid mesothelioma (33.3%) (Am J Cancer Res 2011;1:14)
- Soft tissue:
- Lymphangioma (Indian Dermatol Online J 2017;8:282)
- Vascular malformations (J Am Acad Dermatol 2010;63:1052)
- Desmoid type fibromatosis (20%) (Acta Histochem 2014;116:1134)
- Nodular fasciitis (Acta Histochem 2014;116:1134)
- Ewing sarcoma (Virchows Arch 2017;470:373)
- Scar, fibromatoses, dermatofibroma (J Cutan Pathol 2016;43:313)
Molecular / cytogenetics description
- Desmoplastic small round cell tumor translocation in EWS1 and WT1 genes t(11;22)(p13;q12) (Med Pediatr Oncol 1996;27:434)
Sample pathology report
- Example:
- This pleural tumor is immunoreactive for WT1, suggestive of an mesothelial versus a lung origin.
Additional references
Board review style question #1
- A 60 year old man presents with recurrent pleural effusions. A 2.3 cm solid mass is found on the CT scan. H&E sections are shown in the images. Which stain would be most compatible with the diagnosis?
- WT1-, calretinin-, cytokeretain AE1 / AE3+, TTF1+, BAP1 lost
- WT1+, calretinin+, cytokeretain AE1 / AE3+, TTF1-, BAP1 lost
- WT1+, calretinin+, cytokeratin AE1 / AE3+, TTF1+, BAP1 lost
- WT1-, calretinin-, cytokeratin AE1 / AE3-, TTF1-, BAP1 retained
Board review style answer #1
B. WT1+, calretinin+, cytokeretain AE1 / AE3+, TTF1-, BAP1 lost. The diagnosis is malignant mesothelioma. The best panel to separate malignant mesothelioma from an adenocarcinoma should include at least 2 mesothelial markers and 2 carcinoma markers. The most specific mesothelial markers are WT1 and calretinin. WT1 staining is nuclear in malignant mesothelioma. Cytokeratin is positive in both mesotheliomas and carcinomas; however, it is important to rule out malignancies from other cell lineages, e.g. melanoma and vascular tumors. BAP1 is mutated / lost in 55 - 66% of malignant mesotheliomas and this can be demonstrated by IHC.
Comment Here
Reference: WT1
Comment Here
Reference: WT1