Table of Contents
Definition / general | Essential features | Pathophysiology | Clinical features | Interpretation | Uses by pathologists | Prognostic factors | Microscopic (histologic) images | Positive staining - normal | Positive staining - disease | Negative staining | Sample pathology report | Board review style question #1 | Board review style answer #1Cite this page: Dusenbery A, Wick MR. SOX11. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/stainsSOX11.html. Accessed April 1st, 2025.
Definition / general
- SRY (sex determining region Y) related high mobility group (HMG) box 11 (SOX11) is a member of the SOX gene family; specifically a member of the SOXC group of transcription factors
- Located at chromosome 2p25.2 (Semin Cancer Biol 2020;67:3)
Essential features
- Generally considered to be a marker for mantle cell lymphoma (MCL), although a subset of SOX11 negative MCL cases have also been described
- Should be interpreted in the context of histomorphology and other immunohistochemical results (Appl Immunohistochem Mol Morphol 2014;22:720)
- Prognostic value is debated
Pathophysiology
- Involved in regulating embryonic development and progenitor / stem cell behavior, including neurogenesis and skeletogenesis (Semin Cancer Biol 2020;67:3)
- Context dependent posttranscriptional modifications, in addition to interactions with cofactors and microRNAs, may influence its function in disease (Semin Cancer Biol 2020;67:3, Exp Hematol 2018;58:27, Am J Cancer Res 2017;7:2305, Genes Chromosomes Cancer 2016;55:531)
- CCND1 and STAT3 may be involved in regulating SOX11 expression (Blood 2019;133:306)
- Role of SOX11 as an oncogene in MCL is currently an active area of research; proposed mechanisms of functioning include (Leukemia 2022;36:583, Ther Adv Med Oncol 2019;11:1758835919853449, Curr Oncol Rep 2017;19:43):
- Collaboration with cyclin D1 in the pathogenesis of MCL, generally together with genomic instability and the acquisition of secondary alterations (Curr Opin Hematol 2018;25:299, Curr Oncol Rep 2017;19:43)
- Alteration of terminal B cell differentiation, with PAX5 as one of its major targets (Blood 2013;121:2175)
- Repression of BCL6 transcription to block cell entrance into the germinal center (Leukemia 2016;30:1596)
- Regulation of CXCR4 and FAK expression to promote MCL tumor cell homing and invasion (Blood 2017;130:501, Blood 2017;130:389)
- Promotion of B cell receptor signaling (Blood 2018;131:2247)
- Association with an immunosuppressive microenvironment which could promote MCL progression (Blood 2021;138:2202)
- Driving proangiogenic signals via interaction with PDGFA (Blood 2014;124:2235)
- Via a distant superenhancer (Leukemia 2022;36:583)
- In contrast, the role of SOX11 as a tumor suppressor has also been observed in several studies (Leuk Lymphoma 2020;61:2068, Ther Adv Med Oncol 2019;11:1758835919853449, Tumour Biol 2015;36:6133, Oncogene 2015;34:1231, Mol Oncol 2011;5:527, Mol Cancer 2010;9:187, Cancer Res 2009;69:7953)
Clinical features
- SOX11 expression by qPCR has been proposed as a molecular marker for measuring minimal residual disease in MCL (Leuk Res 2019;78:1, Med Oncol 2018;35:49, Exp Hematol Oncol 2018;7:5, Eur J Haematol 2012;89:385)
- Limited preclinical work has evaluated the possibility of therapeutic targeting of SOX11 in MCL (Clin Cancer Res 2021;27:4652)
- Pathogenic variants have been implicated in Coffin-Siris syndrome or the related SWI / SNF related intellectual disability disorders (SSRIDDs) (Genes (Basel) 2021;12:937, Clin Dysmorphol 2021;30:44)
Interpretation
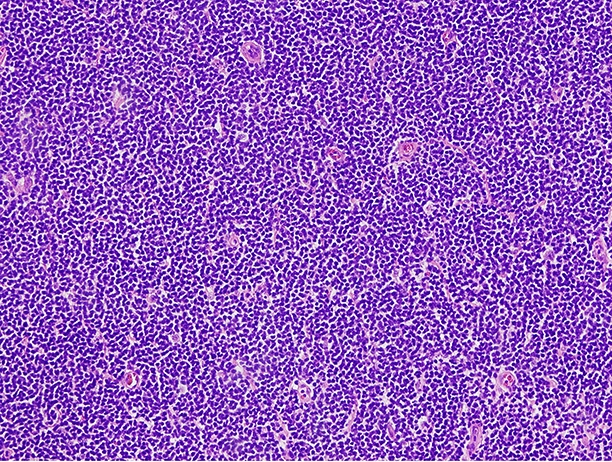
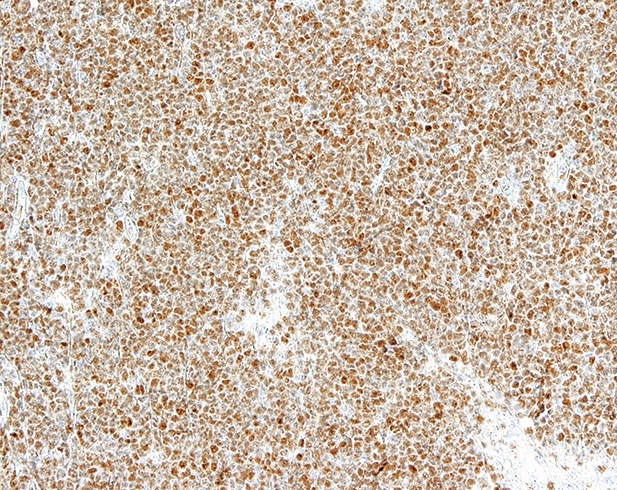
- Staining in MCL should be nuclear and is often intense (Blood 2008;111:800)
- Variation exists in thresholds for positivity (Haematologica 2015;100:e369)
Uses by pathologists
- Nuclear expression can aid in the diagnosis of MCL, especially in cyclin D1 negative cases (Haematologica 2009;94:1555)
- Can be evaluated on decalcified tissue (formalin or B5 fixed) (Hum Pathol 2017;59:94)
- Performance characteristics of SOX11 immunohistochemical staining appear to vary depending on the antibody used, with a generally superior performance noted with a monoclonal antibody (Am J Surg Pathol 2014;38:86, Am J Clin Pathol 2013;140:795, BMC Cancer 2012;12:269)
- Specifically, clone MRQ-58 mouse monoclonal antibody demonstrated robust performance (PLoS One 2019;14:e0225096, Appl Immunohistochem Mol Morphol 2014;22:720)
Prognostic factors
- The prognostic value of SOX11 status in MCL is debated and may vary depending on the clinical presentation (i.e., conventional versus leukemic nonnodal MCL) (BMC Cancer 2021;21:209, Blood 2012;119:4215)
- In varying MCL populations, higher levels of SOX11 expression have been shown to correlate both with a better and a worse prognosis (Blood 2018;131:417, Oncogene 2015;34:1231, Br J Haematol 2014;166:98, Histopathology 2019;75:704, Cancer Res 2010;70:1408)
- Absence of SOX11 expression may be characteristic of indolent MCL; however, it is also noted that some SOX11 negative tumors may progress rapidly, corresponding to a transformed phase (Cancer Res 2012;72:5307, Cancer Res 2010;70:1408, J Clin Invest 2012;122:3416)
- Other studies have found no prognostic value for SOX11 in MCL or report loss of significance in multivariate analysis (BMC Cancer 2021;21:209, Blood 2012;119:4215)
- Furthermore, studies evaluating SOX11 mRNA expression (rather than IHC) have also been performed; several have demonstrated higher SOX11 mRNA expression to be a good prognostic factor (J BUON 2019;24:1679, Leuk Lymphoma 2019;60:1420, Eur Rev Med Pharmacol Sci 2018;22:2556, Int J Hematol 2017;106:212)
- Several possible reasons have been suggested for these conflicting results:
- Compounding additional morphologic or genetic factors, notably including TP53 mutations or 17p deletions (Virchows Arch 2020;477:259, Haematologica 2020;105:754, Cancer Res 2012;72:5307, Leukemia 2012;26:1895, Blood 2012;119:4215)
- Nonuniform cut off values for SOX11 positivity or variable antibodies used (BMC Cancer 2021;21:209, PLoS One 2019;14:e0225096, J BUON 2019;24:1679, Haematologica 2015;100:e369)
- Varying methodologies for evaluating SOX11 expression (BMC Cancer 2021;21:209)
- Varying proportions of nodal and nonnodal MCL within studies (J BUON 2019;24:1679, Blood 2018;131:417)
- Among other hematologic neoplasms, select publications have demonstrated the following associations with SOX11 expression via immunohistochemistry:
- Favorable prognosis in B cell acute lymphoblastic leukemia (Sci Rep 2020;10:2043)
- No significant impact on prognosis in Burkitt lymphoma, in a small cohort (Leuk Lymphoma 2017;58:1760)
- Studies analyzing expression via mRNA in acute myeloid leukemia and chronic lymphocytic leukemia have demonstrated a worse prognosis associated with higher SOX11 expression (Leuk Res 2018;67:32, Tumour Biol 2015;36:4433)
- Among solid tumors, select publications have demonstrated the following associations with SOX11 expression via immunohistochemistry:
- Favorable prognosis in gastric cancer (Int J Oncol 2014;44:1512)
- Favorable prognosis in glioblastoma (Br J Cancer 2013;108:2142)
- Favorable prognosis in epithelial ovarian carcinoma, including high grade; of note, the first study referenced evaluated both nuclear and cytoplasmic staining (Eur J Cancer 2009;45:1510, BMC Cancer 2011;11:405)
- Favorable prognosis in breast cancer (via immunohistochemistry) in one study; however, a worse prognosis was seen in another study (via immunohistochemistry), as well as in a third study (via mRNA) (Asian Pac J Cancer Prev 2014;15:5483, J Cell Physiol 2020;235:7295, Oncotarget 2016;7:13106)
- Favorable prognosis in aggressive fibromatosis (Cancer Med 2014;3:81)
- Adverse prognosis in endometrial cancer, using mRNA supported by IHC (Am J Cancer Res 2017;7:2305)
- Adverse prognosis in malignant melanoma (J Int Med Res 2013;41:1221)
- Hypermethylation in SOX11, including promoter hypermethylation, has been demonstrated across various tumor types, with some prognostic implications noted (Biochimie 2019;162:8, Oncol Rep 2019;41:2351, J Cancer 2018;9:2275, Int J Exp Pathol 2017;98:341, Cell Oncol (Dordr) 2015;38:183, BMC Cancer 2015;15:273, Cancer Cell Int 2013;13:109, Cancer Epidemiol Biomarkers Prev 2011;20:1483, PLoS One 2011;6:e21382, Mol Cancer 2010;9:187)
Positive staining - normal
- Not generally expressed in the nucleus of mature tissues; however, nuclear expression has been noted in Schwann cells, keratinocytes and other squamous or epithelial cells (Histopathology 2019;74:391, Blood 2008;111:800)
Positive staining - disease
- Mantle cell lymphoma (MCL; cyclin D1 positive and cyclin D1 negative): sensitivity ranged from 78 - 100% and specificity ranged from 72 - 100% in one meta analysis, with substantial heterogeneity in the reported specificities (PLoS One 2019;14:e0225096)
- Of note, nodal MCL is generally SOX11 positive, while leukemic nonnodal MCL is generally SOX11 negative; see Negative staining below (Am J Hematol 2019;94:710, Virchows Arch 2016;469:697)
- Specific subsets of MCL which have demonstrated SOX11 expression include pleomorphic MCL, secondary cutaneous MCL, MCL with mantle zone growth pattern (Am J Surg Pathol 2020;44:232, J Cutan Pathol 2016;43:354, Am J Clin Pathol 2019;152:132)
- Expression of SOX11 in in situ MCL is variable (Haematologica 2012;97:270)
- Expression of SOX11 in CD5 negative MCL ranged from 53 - 67% in the 2 studies referenced, with the latter noting no significant difference from its expression in CD5+ MCL (Leuk Lymphoma 2022;63:911, Am J Surg Pathol 2019;43:1052)
- 2 cases of SOX11+ MCL with loss of SOX11 expression after ibrutinib treatment have been reported (Leuk Lymphoma 2020;61:1769)
- Among other hematopoietic neoplasms, expression is most commonly noted in:
- Lymphoblastic leukemia / lymphoma (B or T cell), approximately 33 - 100% with various antibody types used; association with certain genetic subtypes has been identified in the third study listed (Haematologica 2009;94:1563, Haematologica 2009;94:1555, Sci Rep 2020;10:2043)
- Of note, 0% (0/7) cases of T ALL were positive in one study (Sci Rep 2020;10:2043)
- Burkitt lymphoma: 20 - 100% with various subpopulations and antibody types used (Br J Haematol 2022:196:681, Ann Diagn Pathol 2013;17:250, Haematologica 2009;94:1563, Haematologica 2009;94:1555)
- T cell prolymphocytic leukemia: 66% based on 3 cases (Haematologica 2009;94:1555)
- Hairy cell leukemia: 50% in the referenced publications with a correlation with cyclin D1 noted in both studies and weak nuclear staining noted in the latter study (Mod Pathol 2010;23:105, Haematologica 2009;94:1563)
- Lymphoblastic leukemia / lymphoma (B or T cell), approximately 33 - 100% with various antibody types used; association with certain genetic subtypes has been identified in the third study listed (Haematologica 2009;94:1563, Haematologica 2009;94:1555, Sci Rep 2020;10:2043)
- Expression has been noted in the following solid tumors (variable thresholds for positivity and scoring systems used in referenced publications):
- Solid pseudopapillary neoplasm of the pancreas: 92 - 100% (Cytopathology 2022;33:216, Am J Clin Pathol 2017;149:67, Cancer Cytopathol 2017;125:831)
- Primitive neuroectodermal tumor / Ewing sarcoma: 100% (Histopathology 2019;74:391)
- Neuroblastoma: 100% (Histopathology 2019;74:391)
- Esthesioneuroblastoma: 100% (Histopathology 2019;74:391)
- Central neurocytoma: 100% (Histopathology 2019;74:391)
- Medulloblastoma: up to 100% but with some heterogeneity reported in the staining pattern of desmoplastic medulloblastomas (Histopathology 2019;74:391, Pathol Res Pract 2016;212:965, Neuro Oncol 2008;10:648, J Neurooncol 2002;57:201)
- Testicular germ cell tumor derived undifferentiated primitive neuroectodermal tumor: 100% (Arch Pathol Lab Med 2021;145:953)
- Immature teratoma: 100% (Histopathology 2019;74:391)
- Myxoid liposarcoma: 100% (Histopathology 2019;74:391)
- Salivary duct carcinoma: 97% (Histopathology 2019;74:391)
- Diffuse astrocytoma: approximately 50 - 98% in the listed publications with differences noted by grade in the former but not the latter (Histopathology 2019;74:391, Br J Cancer 2013;108:2142)
- Glioblastoma (Cell Oncol (Dordr) 2018;41:213)
- Rhabdomyosarcoma: 90% (Histopathology 2019;74:391)
- Pulmonary small cell carcinoma (68%) and large cell neuroendocrine carcinoma (39%) (Histopathology 2019;74:391)
- Benign prostatic hyperplasia (approximately 82%) and prostatic carcinoma (approximately 0 - 17%) (Histopathology 2019;74:391, Tumour Biol 2015;36:6133)
- Malignant melanoma: approximately 50 - 63% (Life (Basel) 2021;11:281, J Int Med Res 2013;41:1221)
- However, the publication referenced here noted staining in 0% (0/10) of malignant melanomas (Histopathology 2019;74:391)
- Gastric cancer: approximately 52% (Int J Oncol 2014;44:1512)
- Synovial sarcoma: 36% (Histopathology 2019;74:391)
- Breast cancer: nuclear staining in approximately 17 - 36% (Histopathology 2019;74:391, Asian Pac J Cancer Prev 2014;15:5483)
- Yolk sac tumor: 25% (Histopathology 2019;74:391)
- Malignant peripheral nerve sheath tumor: 24% (Histopathology 2019;74:391)
- Aggressive fibromatosis, although may be heterogeneous (Cancer Med 2014;3:81)
- Case report of papillary thyroid carcinoma with fibromatosis / fasciitis-like stroma with SOX11 expression exclusively in the mesenchymal component (Virchows Arch 2019;475:519)
- Serous ovarian carcinoma effusions, nuclear expression in any amount was identified in 37%; however, many of these cases demonstrated a limited extent of staining (Hum Pathol 2015;46:1)
- Another study demonstrated a mixture of nuclear and cytoplasmic staining in epithelial ovarian carcinoma (Eur J Cancer 2009;45:1510)
Negative staining
- Evolving literature suggests the division of MCL into 2 categories, conventional MCL and leukemic nonnodal MCL; the latter generally lacks SOX11 expression and has a more (though not uniformly) indolent disease course (Hum Pathol 2022;119:59, Exp Hematol Oncol 2021;10:41, Am J Hematol 2019;94:710, Am J Surg Pathol 2019;43:710, Genes Chromosomes Cancer 2016;55:531, Clin Cancer Res 2013;19:3121, Cancer Res 2012;72:5307, J Clin Invest 2012;122:3416, Cancer Res 2010;70:1408)
- One proposed explanation for some characteristics of these subtypes involves a functional effect of SOX11 on BCL6 (Leukemia 2016;30:1596)
- The following hematologic entities are generally negative, with rare exceptions:
- Normal hematopoietic cells (Blood 2008;111:800)
- Chronic lymphocytic leukemia / small lymphocytic lymphoma (CLL / SLL), including CLL / SLL with cyclin D1 positive proliferation centers (Hum Pathol 2022;121:29, Am J Clin Pathol 2012;138:132)
- Marginal zone lymphoma - nodal (0%), extranodal (0%) and splenic (3%) (Hum Pathol 2022;121:29)
- Lymphoplasmacytic lymphoma / Waldenström macroglobulinemia (LPL / WM) (Hum Pathol 2022;121:29)
- MCL with plasmacytic differentiation, based on 4 cases (Virchows Arch 2016;468:259)
- Follicular lymphoma, with 2% of cases noted as positive in the first publication listed (Histopathology 2019;74:391, Haematologica 2009;94:1563, Haematologica 2009;94:1555)
- Diffuse large B cell lymphoma (Med Oncol 2012;29:1190, Mod Pathol 2010;23:105, Haematologica 2009;94:1563, Haematologica 2009;94:1555)
- However, the reference listed here noted staining in 46% (14/30) of diffuse large B cell lymphomas (Ann Diagn Pathol 2013;17:250)
- Plasma cell myeloma, including with t(11;14)(q13;q32) (J Clin Exp Hematop 2015;55:137, Mod Pathol 2010;23:105)
- Classic Hodgkin lymphoma, with only weak positivity noted in 3% of cases in the third publication listed (Histopathology 2019;74:391, Haematologica 2009;94:1563, Haematologica 2009;94:1555)
- MALT (Histopathology 2019;74:391)
- Several T cell lymphoma subtypes, excluding those listed above under Positive staining (Haematologica 2009;94:1563, Haematologica 2009;94:1555)
- The following solid tumors are noted to generally be negative, with rare exceptions (number of cases evaluated varies by tumor type) (Histopathology 2019;74:391)
- Low grade astrocytoma, pulmonary squamous cell carcinoma, pulmonary adenocarcinoma (2%), pulmonary large cell carcinoma (4%), pulmonary typical carcinoid, pulmonary atypical carcinoid (8%), esophageal squamous cell carcinoma, gastrointestinal adenocarcinoma (including stomach, colon, bile duct and pancreas), hepatocellular carcinoma, gastrointestinal neuroendocrine tumors, extrapulmonary small cell carcinoma / large cell neuroendocrine carcinoma (16%), adenocarcinoma of the female genital tract (cervix, uterus and ovary; 3%), cervical squamous carcinoma, certain germ cell tumors (including embryonic carcinoma, seminoma / dysgerminoma, choriocarcinoma and mature teratoma), renal cell carcinoma, urothelial carcinoma, prostatic adenocarcinoma, certain salivary gland tumors (including acinar cell carcinoma, adenoid cystic carcinoma (8%), mucoepidermoid carcinoma, epithelial myoepithelial carcinoma (18%) and myoepithelial carcinoma), nasopharyngeal carcinoma, adrenal cortical adenoma / carcinoma, thymoma / thymic carcinoma, papillary thyroid carcinoma, medullary thyroid carcinoma, parathyroid carcinoma, squamous cell carcinoma of the skin / mouth, angiosarcoma (10%), leiomyosarcoma, well differentiated / dedifferentiated liposarcoma, dermatofibrosarcoma protuberans, solitary fibrous tumor, alveolar soft part sarcoma, epithelioid sarcoma, gastrointestinal stromal tumor, chondrosarcoma, osteosarcoma (12%)
- There is conflicting information regarding the following entity:
- B cell lymphoma, unclassifiable, with features intermediate between diffuse large B cell lymphoma and Burkitt lymphoma (of note, this is a diagnostic entity from the 2008 WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, which is included given the publication dates of these studies)
- 83% (5/6) (Ann Diagn Pathol 2013;17:250)
- 0% (0/6) (Haematologica 2009;94:1563)
- B cell lymphoma, unclassifiable, with features intermediate between diffuse large B cell lymphoma and Burkitt lymphoma (of note, this is a diagnostic entity from the 2008 WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, which is included given the publication dates of these studies)
Sample pathology report
- See MCL-classic and MCL-aggressive variants
Board review style question #1
Which of the following would be expected to show positive staining for SOX11 in a similar expression pattern to that depicted in the image shown above?
- Chronic lymphocytic leukemia / small lymphocytic lymphoma
- Leukemic nonnodal mantle cell lymphoma
- Marginal zone lymphoma
- Nonneoplastic lymphocytes
- Solid pseudopapillary neoplasm of the pancreas
Board review style answer #1