Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Clinical features | Interpretation | Uses by pathologists | Prognostic factors | Microscopic (histologic) images | Positive staining - normal | Positive staining - disease | Negative staining | Molecular / cytogenetics description | Molecular / cytogenetics images | Sample pathology report | Board review style question #1 | Board review style answer #1Cite this page: Liu Z, Tsang P. MyoD1. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/stainsMyoD1.html. Accessed April 2nd, 2025.
Definition / general
- Located on 11p15.1, MYOD1 (myogenic differentiation 1) encodes myogenic transcriptional regulatory protein expressed in early skeletal muscle differentiation during skeletal muscle development and regeneration
- MyoD1 is a sensitive immunostain for rhabdomyosarcoma (RMS) but lacks specificity
- Somatic recurrent mutation is associated with poor prognosis in spindle cell / sclerosing rhabdomyosarcomas (SRMS)
Essential features
- MYOD1 encodes a nuclear protein that promotes transcription of muscle specific target genes and regulates muscle cell differentiation
- MYOD1 p.L122R is a recurrent hotspot mutation in SRMS with unfavorable prognosis
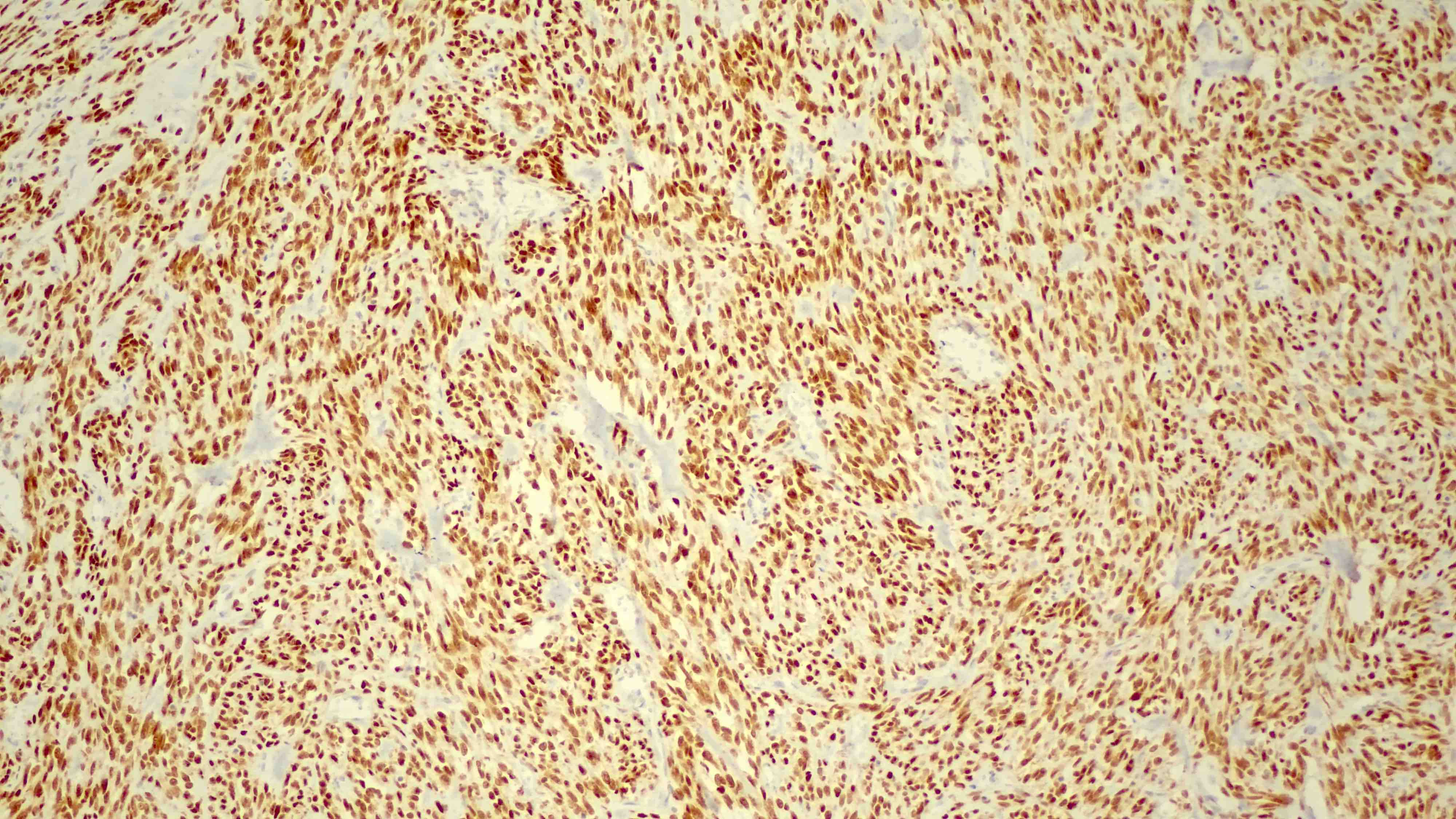
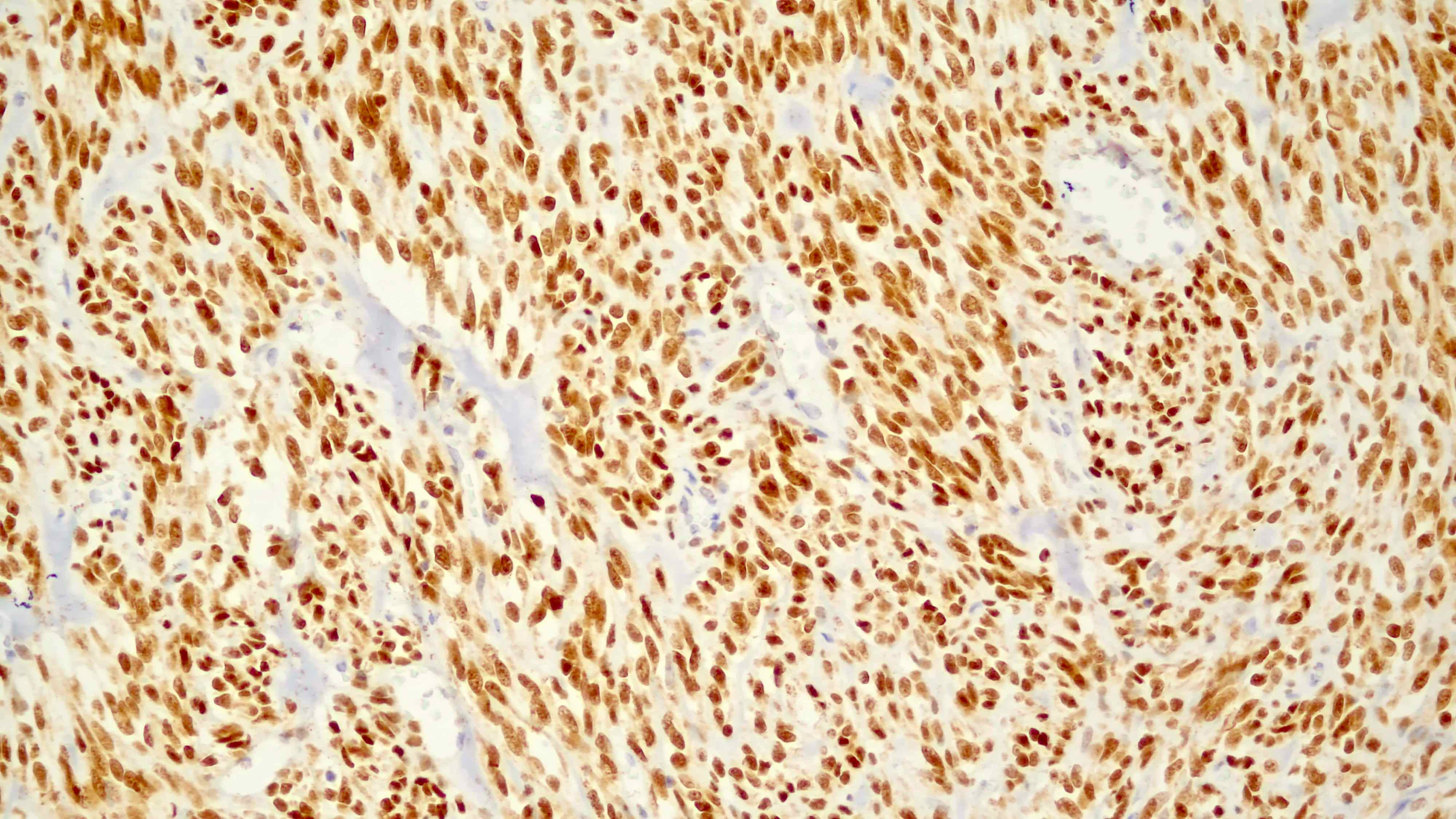
- Mutant cases typically show intense immunohistochemical (IHC) staining (Eur J Cancer 2022;172:367)
- Diffuse, strong nuclear staining in SRMS and variable expression in alveolar, embryonal and pleomorphic RMS
Terminology
- MYOD1 gene: myogenic differentiation 1 gene
- Myoblast determination protein 1
- MYOD1 c.365T>G, p.L122R substitution missense mutation (COSMIC: Mutation COSV51452486 [Accessed 15 November 2022])
- MyoD1 immunostain
- Class C basic helix - loop - helix protein 1 (BHLHC1)
- Myogenic factor 3 (MYF3)
Pathophysiology
- MYOD1 was discovered in 1987 as a gene that could convert fibroblasts to myoblasts (Cell 1987;51:987)
- Functions as a transcription factor of the basic helix - loop - helix family and the myogenic factor subfamily, regulating muscle cell differentiation by inducing cell cycle arrest, a prerequisite for myogenic initiation (Curr Opin Genet Dev 2013;23:568, J Biol Chem 2014;289:23417)
- Supports muscle repair and regeneration (Curr Opin Genet Dev 2013;23:568)
- MYOD1 p.L122R, a somatic hotspot transactivating mutation in exon 1, occurs in the conserved DNA binding domain, leading to reduced transcriptional activation at MYOD1 target genes and increased binding to MYC target sites (J Pathol 2014;232:300, Mod Pathol 2019;32:27)
- p.L122R mutant cases typically show strong MyoD1 IHC staining (Eur J Cancer 2022;172:367)
Clinical features
- L122R mutated SRMS cases predominantly occur in the head and neck or paramengineal region (88%) (J Clin Oncol 2021;39:2859)
- Age range of 2 - 94 years, commonly in adolescents and young adults
- Generally absent in congenital / infantile cases which tend to harbor gene fusions without involving MYOD1 (Mod Pathol 2019;32:27)
- Mutation is associated with aggressive clinical course and typically a fatal outcome in both pediatric and adult patients
Interpretation
- IHC: strong nuclear staining of myoblasts (Surg Pathol Clin 2019;12:51)
Uses by pathologists
- IHC marker: diagnosis of rhabdomyosarcoma (Surg Pathol Clin 2019;12:51)
- Typically diffuse, strong nuclear expression of MyoD1 in SRMS with some exceptions showing absence of staining (Surg Pathol Clin 2019;12:51, Adv Anat Pathol 2002;9:198, Pediatr Blood Cancer 2021;68:e29085)
- Variable expression of MyoD1 in embryonal and pleomorphic RMS (Surg Pathol Clin 2019;12:51)
- MyoD1 expression in alveolar RMS can be diffuse or focal (Arch Pathol Lab Med 2022;146:47)
- More intense staining in alveolar RMS than in embryonal RMS
- Cytoplasmic and nonspecific background staining should not be interpreted as positive (Am J Surg Pathol 2001;25:1150)
- Expression of MyoD1 may be seen in a variety of rare tumors with rhabdomyoblastic differentiation, including Wilms tumors, neuroendocrine carcinoma, malignant glial tumors, malignant peripheral nerve sheath tumor, teratoma and melanoma (Adv Anat Pathol 2002;9:198, Head Neck Pathol 2015;9:507)
- Focal expression of MyoD1 in a subset of fibroepithelial stromal polyps (Hum Pathol 2020;99:75)
- MYOD1 L122P activating hotspot mutation is found in 33 - 56% of spindle cell / sclerosing RMS, commonly in adolescents and young adults (Genes Chromosomes Cancer 2014;53:779, Oral Surg Oral Med Oral Pathol Oral Radiol 2022;134:354, Mod Pathol 2016;29:1532)
- May coexist with PIK3CA mutation
- Originally described in a subset of embryonal RMS cases which have since been reclassified as MYOD1 mutant SRMS (Mod Pathol 2016;29:1532)
Prognostic factors
- MYOD1 mutant SRMS: high mortality and unfavorable outcome (Mod Pathol 2016;29:1532, Mod Pathol 2019;32:27)
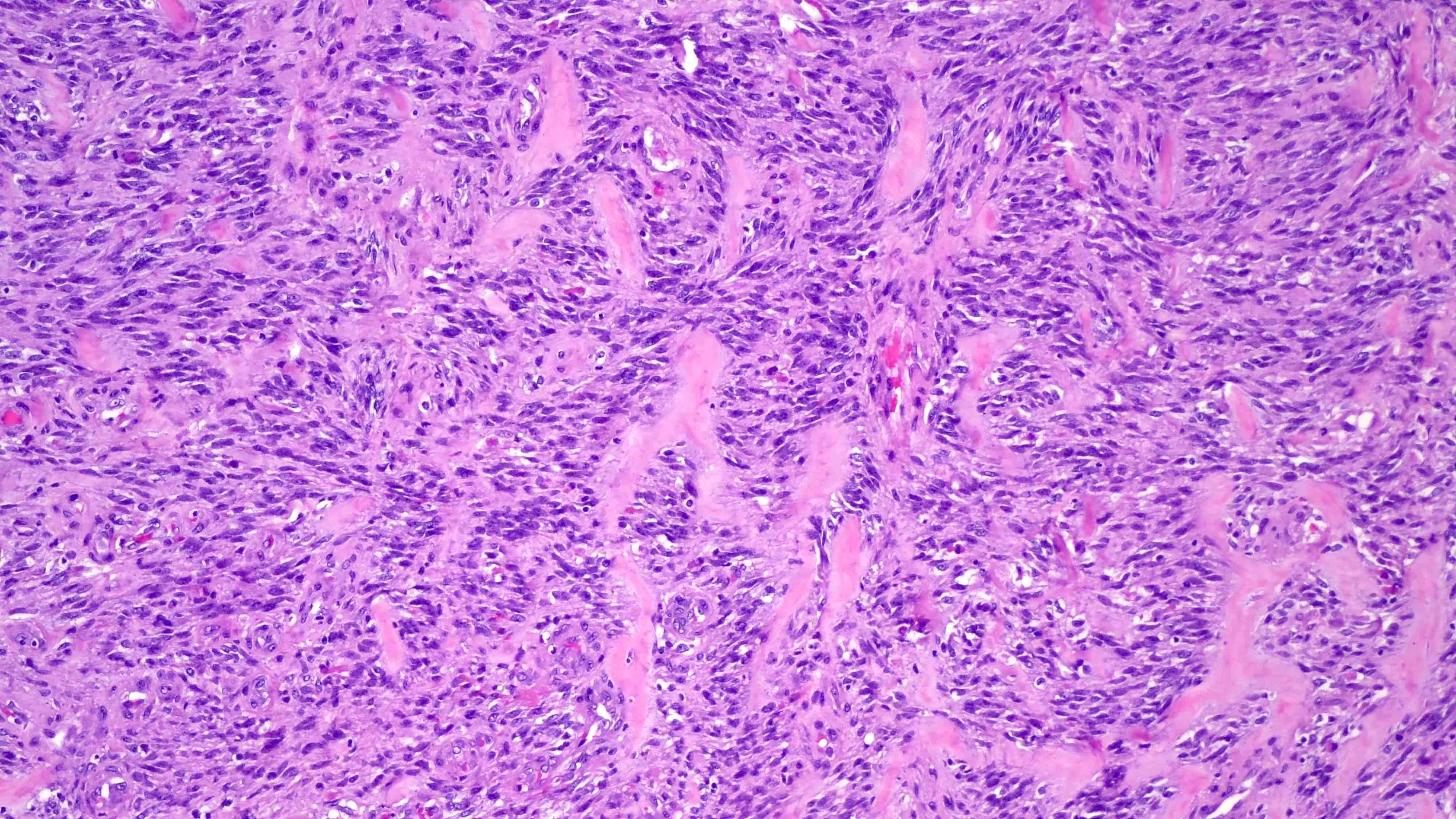
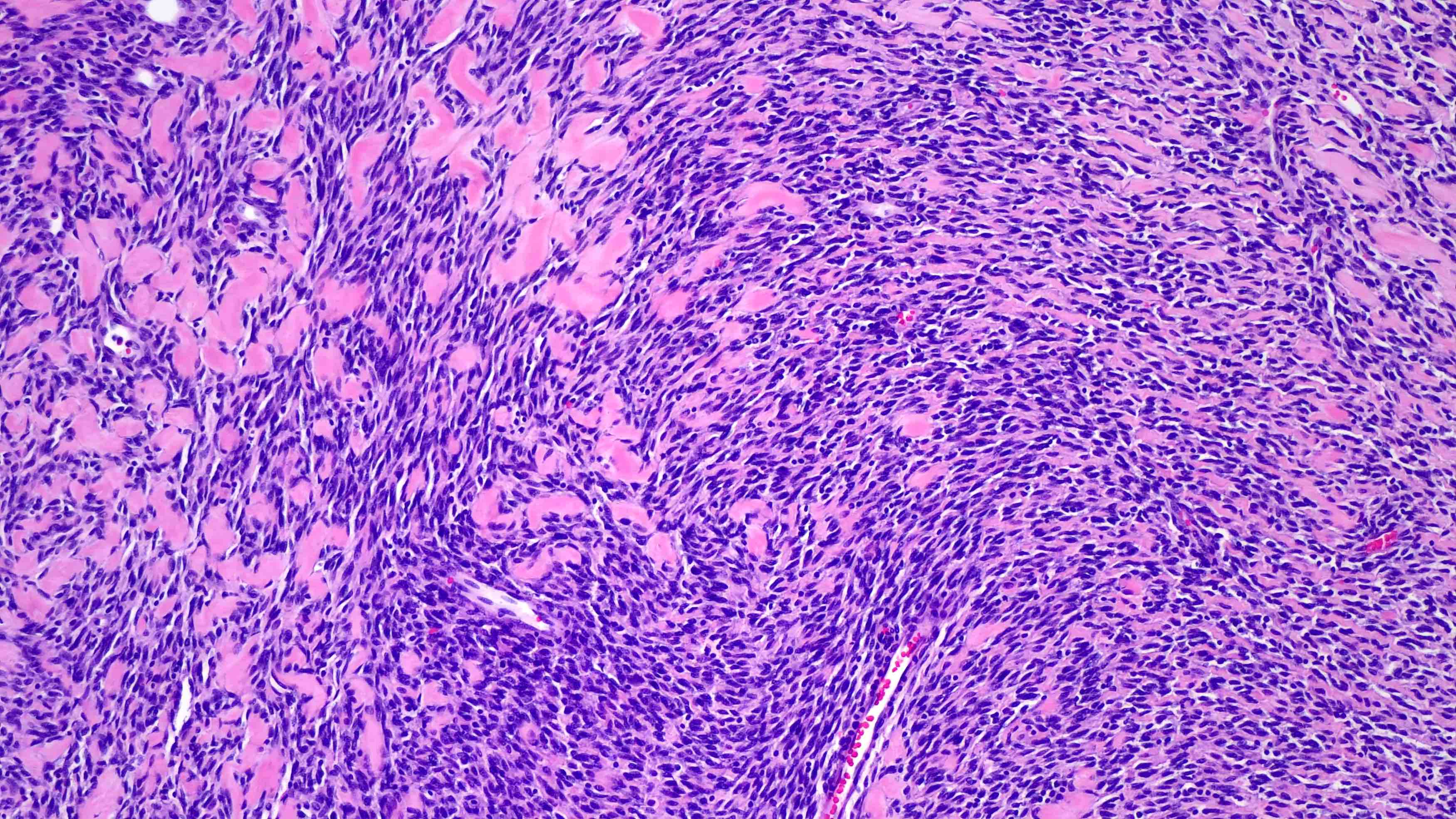
Microscopic (histologic) images
Positive staining - normal
- Normal fetal skeletal muscle (myoblasts of developing skeletal muscle)
- Downregulated during later stages of differentiation
- Nuclear staining pattern
- Areas of skeletal muscle regeneration in myopathies and neurogenic muscle atrophy disorders (Acta Neuropathol 1994;87:605)
Positive staining - disease
- Spindle cell / sclerosing rhabdomyosarcoma (Surg Pathol Clin 2020;13:729)
- Alveolar rhabdomyosarcoma (J Clin Pathol 2003;56:412)
- Embryonal rhabdomyosarcoma (J Clin Pathol 2003;56:412)
- Pleomorphic rhabdomyosarcoma (Ann Diagn Pathol 2018;36:50)
- Rare tumors with rhabdomyoblastic differentiation (Adv Anat Pathol 2002;9:198)
Negative staining
- Normal adult muscle
Molecular / cytogenetics description
- Point mutation Leu122Arg (p.L122R) in exon 1 of the MYOD1 gene is seen in a subset (33 - 56%) of SRMS (Oral Surg Oral Med Oral Pathol Oral Radiol 2022;134:354)
- May be detected by PCR on genomic DNA extracted from tumor tissue, followed by Sanger sequencing of amplicon
Molecular / cytogenetics images
Sample pathology report
- Soft tissue mass, left thigh, needle core biopsy:
- Malignant spindle cell neoplasm, consistent with spindle cell rhabdomyosarcoma (see comment and addendum)
- Comment: The needle core biopsy demonstrates fascicles of spindle cell proliferation with a herringbone growth pattern. Rare rhabdomyoblasts are present. Mitoses are brisk. The neoplastic cells stain positively for MyoD1, desmin and myogenin (focal) and negatively for AE1 / AE3, S100, CD34 and SMA. The morphology and immunophenotypic profile are consistent with spindle cell rhabdomyosarcoma.
- Addendum: MYOD1 p.L122R mutation was detected. Notably, this somatic mutation is associated with an aggressive clinical course in spindle cell / sclerosing rhabdomyosarcoma. Per xxx (reference laboratory) report, PCR was performed on genomic DNA extracted from formalin fixed paraffin embedded tumor tissue. Sanger sequencing of the purified amplicon confirmed a missense substitution of MYOD1 (p.L122R, T>G), consistent with a hotspot mutation reported in a subset of spindle cell / sclerosing rhabdomyosarcoma.
Board review style question #1
Board review style answer #1
C. MYOD1 p.L122R is the most likely recurrent genetic abnormality in this MyoD1 expressing spindle cell / sclerosing rhabdomyosarcoma. This tumor commonly involves the head and neck region in adolescents and young adults. Congenital / infantile cases are more likely to harbor fusions involving the VGLL2, SRF, TEAD1, NCOA2 and CITED2 genes.
Comment Here
Reference: MyoD1
Comment Here
Reference: MyoD1