Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Clinical features | Interpretation | Uses by pathologists | Microscopic (histologic) images | Virtual slides | Positive staining - normal | Positive staining - disease | Negative staining | Sample pathology report | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Zhou F. GMS. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/stainsGMS.html. Accessed April 2nd, 2025.
Definition / general
- Grocott-Gomori methenamine silver (GMS) is a special stain to detect fungi
- Also positive in some nonfungal organisms and nonorganisms
Essential features
- Outlines fungal organisms by staining polysaccharides in cell walls
- Fungal morphology on GMS usually not specific enough to allow definitive species identification
- Stains some nonfungal organisms: bacteria, mycobacteria, Strongyloides, viral inclusions
- Artifacts, mimickers and other pigments are common confounders to accurate interpretation
Terminology
- Other names for GMS:
- Grocott stain
- Grocott methenamine (hexamine) silver stain
- Disambiguation from other similar terms:
- Other silver stains (e.g. Warthin-Starry, Jones, Steiner)
- Giemsa stain
Pathophysiology
- Aldehyde groups form on polysaccharides (1,2-glycols) via oxidation by chromic acid (Suvarna: Bancroft's Theory and Practice of Histological Techniques, 8th Edition, 2019, IHC World: A Common Mistake When Staining for Fungi [Accessed 31 March 2021])
- Aldehyde groups are further oxidized over time
- Fungal cell walls (high polysaccharide density) retain more aldehyde groups
- Background structures, such as basement membranes, collagen and reticulin (lower polysaccharide density) retain fewer aldehyde groups
- Aldehyde groups reduce silver nitrate (or Schiff molecules) to create a metallic silver precipitate which appears gray to black
- Weaker oxidizers like periodic acid (which is used in PAS and Jones stains) have more staining of background structures
Clinical features
- GMS stain cannot provide definitive organism identification
- Identification is preferably by other methods:
- Culture (which can also gather susceptibility data)
- Alternative testing of nontissue samples (e.g. antibody detection, antigen detection, galactomannan, β-D-glucan, PCR, blood culture, etc.)
- Molecular testing
- Few clinically validated tests are available for testing of formalin fixed, paraffin embedded (FFPE) blocks
- Many false negatives due to formalin crosslinking
- In a few instances, additional special stains on formalin fixed, paraffin embedded (FFPE) blocks
- Reference: Clin Microbiol Rev 2011;24:247
Interpretation
- Sharply outlines fungal cell walls
- Gray to black with peripheral outlines
- Organisms may be very few and pale
- Artifacts are common
- Irregular sizes and shapes
- No peripheral outline
- Not associated with tissue reaction
- Out of tissue plane
- Counterstain: light green
- Can also use light H&E (e.g. when a case sent for consult contains only one unstained slide)
- Signs of infection: inflammation with or without granulomas, necrosis, hemorrhage, angioinvasion
- Otherwise, it may be colonization rather than true infection; clinical correlation is required
- In comment, describe the fungal elements seen on GMS
- Hyphae: Septated or nonseptated? Branching or nonbranching? Acute or right angle branching?
- Yeasts: Budding or nonbudding? Broad based or narrow based budding?
- Presence of spherules or endospores
- If a fungal identity in the report is requested by clinician, then state the fungi most frequently associated with this morphology and that morphologic mimickers exist; include a statement about the importance of clinical correlation
- Reference: Clin Microbiol Rev 2011;24:247
Uses by pathologists
- GMS is ordered when fungi are suspected on H&E or cytology preparations
- Granulomatous reaction is most common
- Wide range of other reactions: exudative, necrotic or little cellular response (immunocompromised)
- On H&E and cytologic preparations, fungi can be stained at varying intensities, appear refractile (capsule) or may not be visible
- More than one organism may be identified in the same lesion
- Advantages
- Relatively quick and cost effective
- GMS positive fungus in tissue helps confirm a positive culture result for environmentally ubiquitous fungi (versus contaminant)
- Culture may be false negative
- Pneumocystis cannot be grown in culture (mBio 2018;9:e00939)
- Antibody testing can be negative in immunodeficient patients
- Antibody and antigen tests may crossreact between different organisms
- Disadvantages
- Low sensitivity
- Cannot definitively identify fungal species
- Many morphologic mimics (Clin Microbiol Rev 2011;24:247)
- Morphology may be changed by treatment, biopsy procedure or variant forms
- Cannot provide antifungal susceptibility data
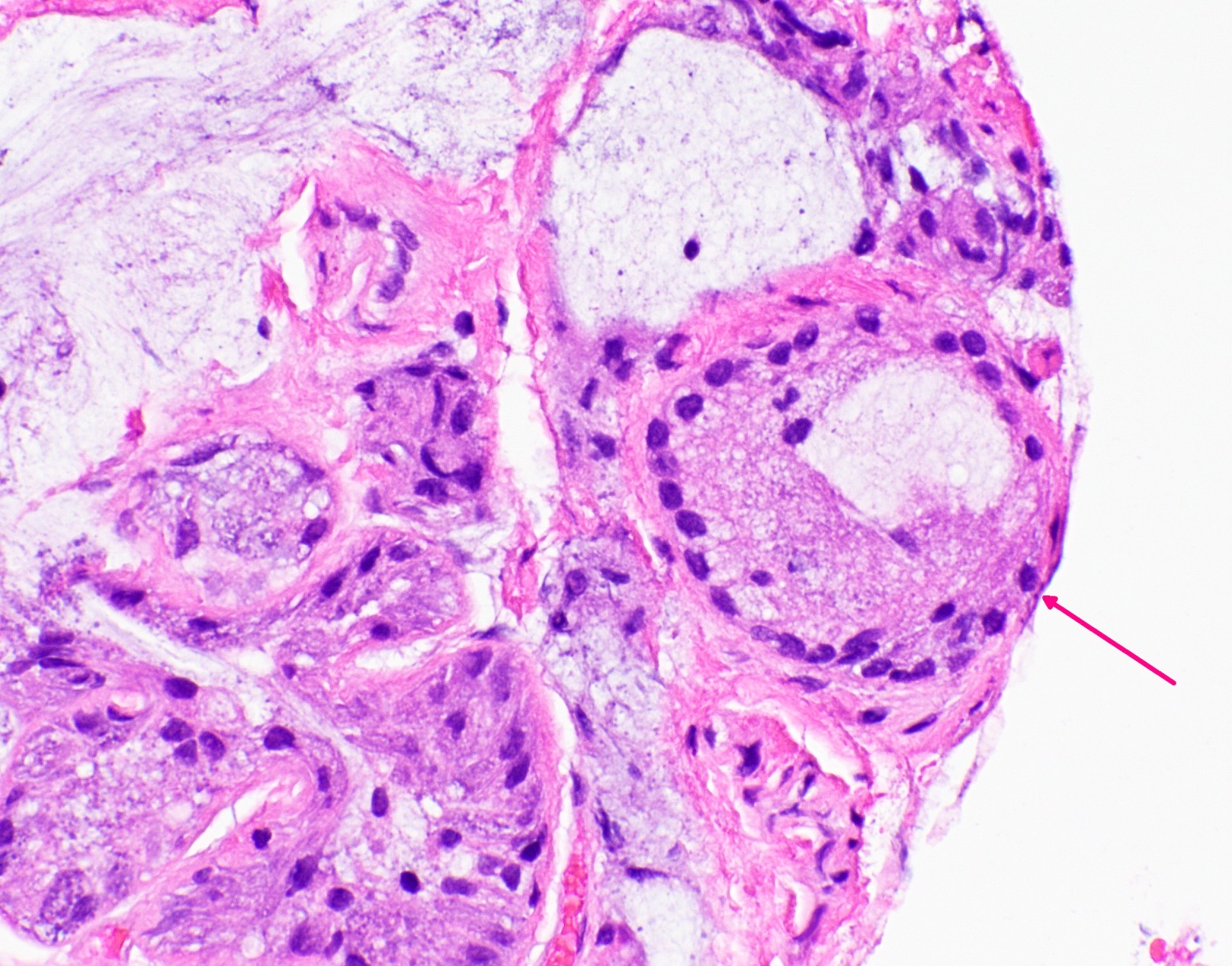
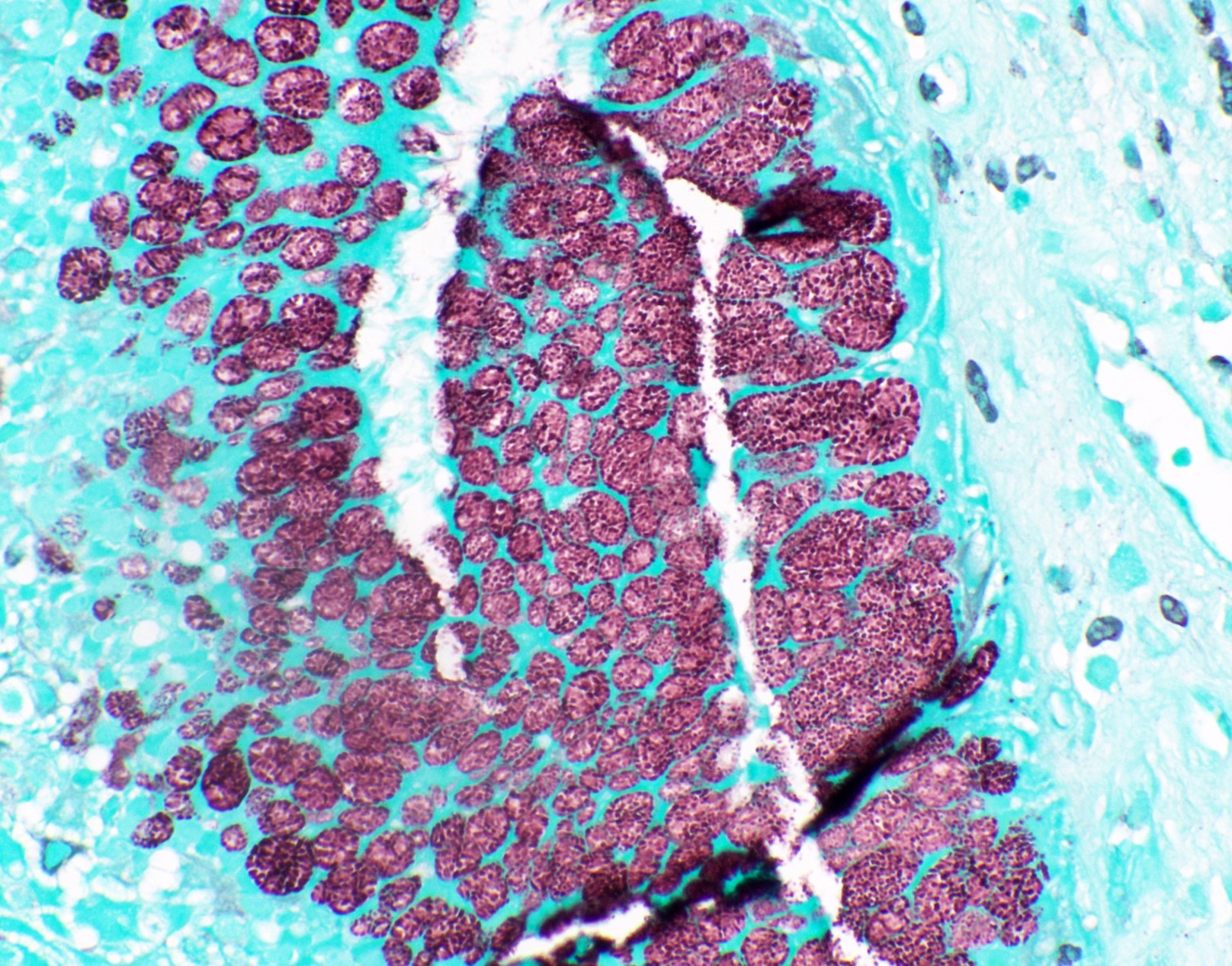
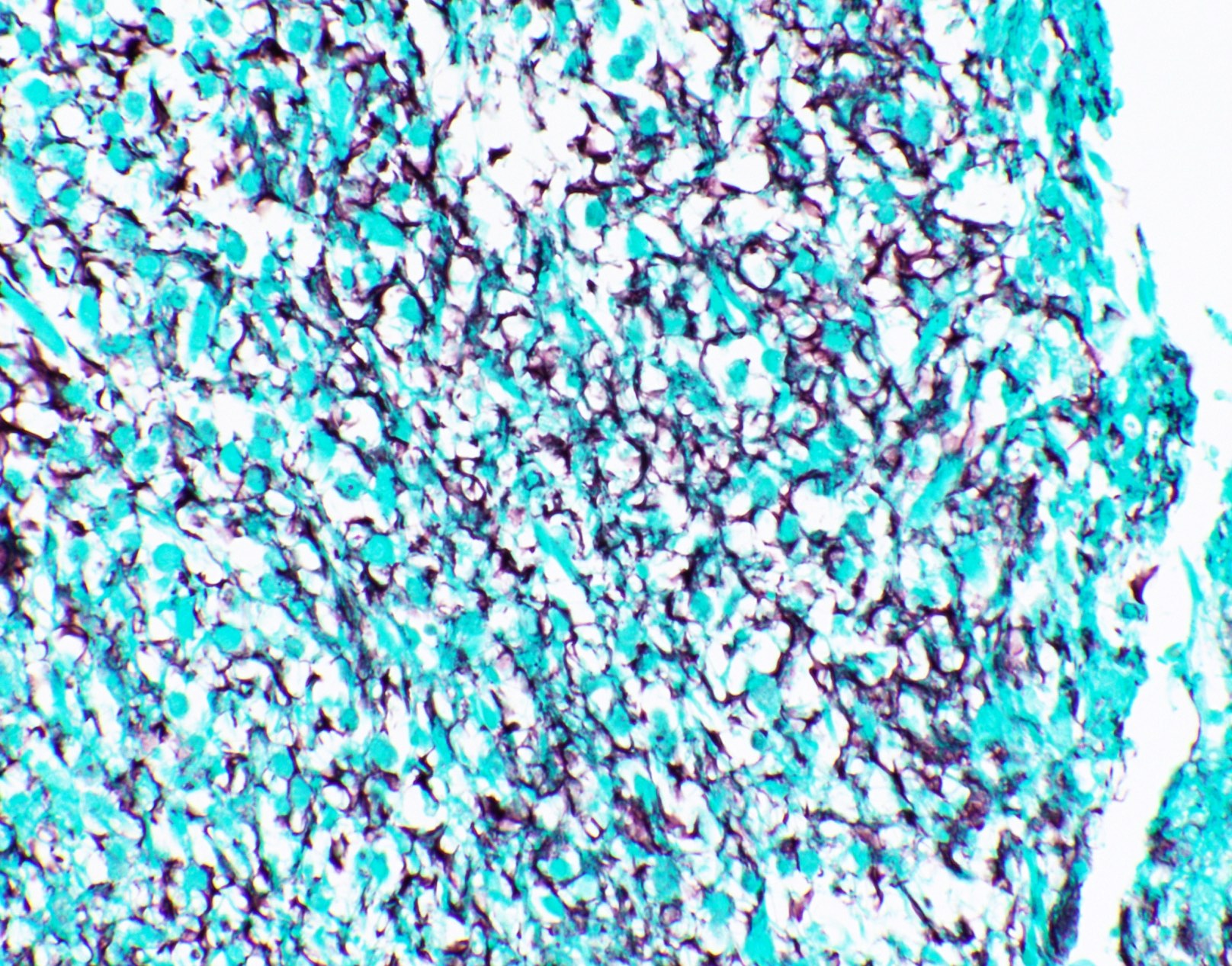
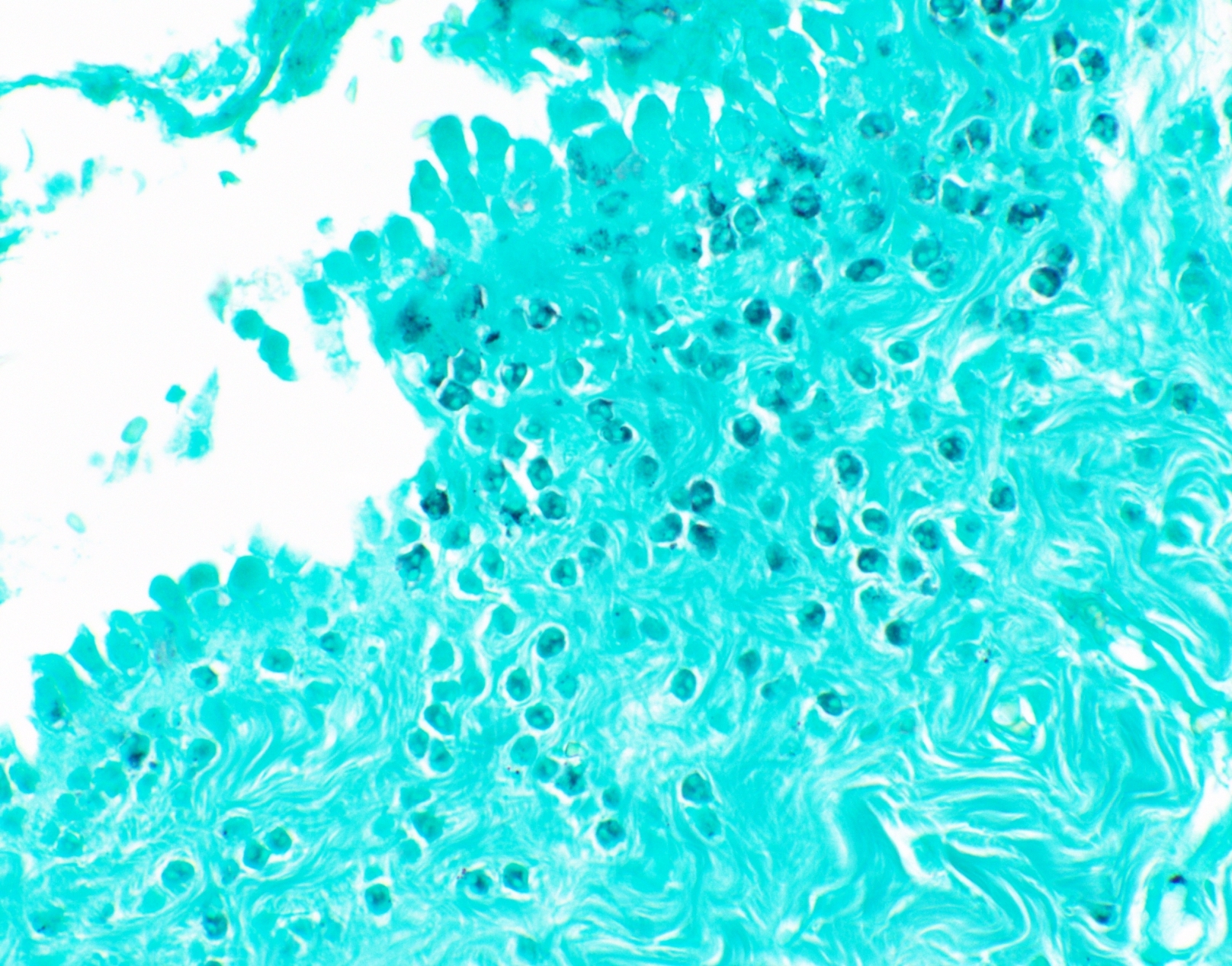
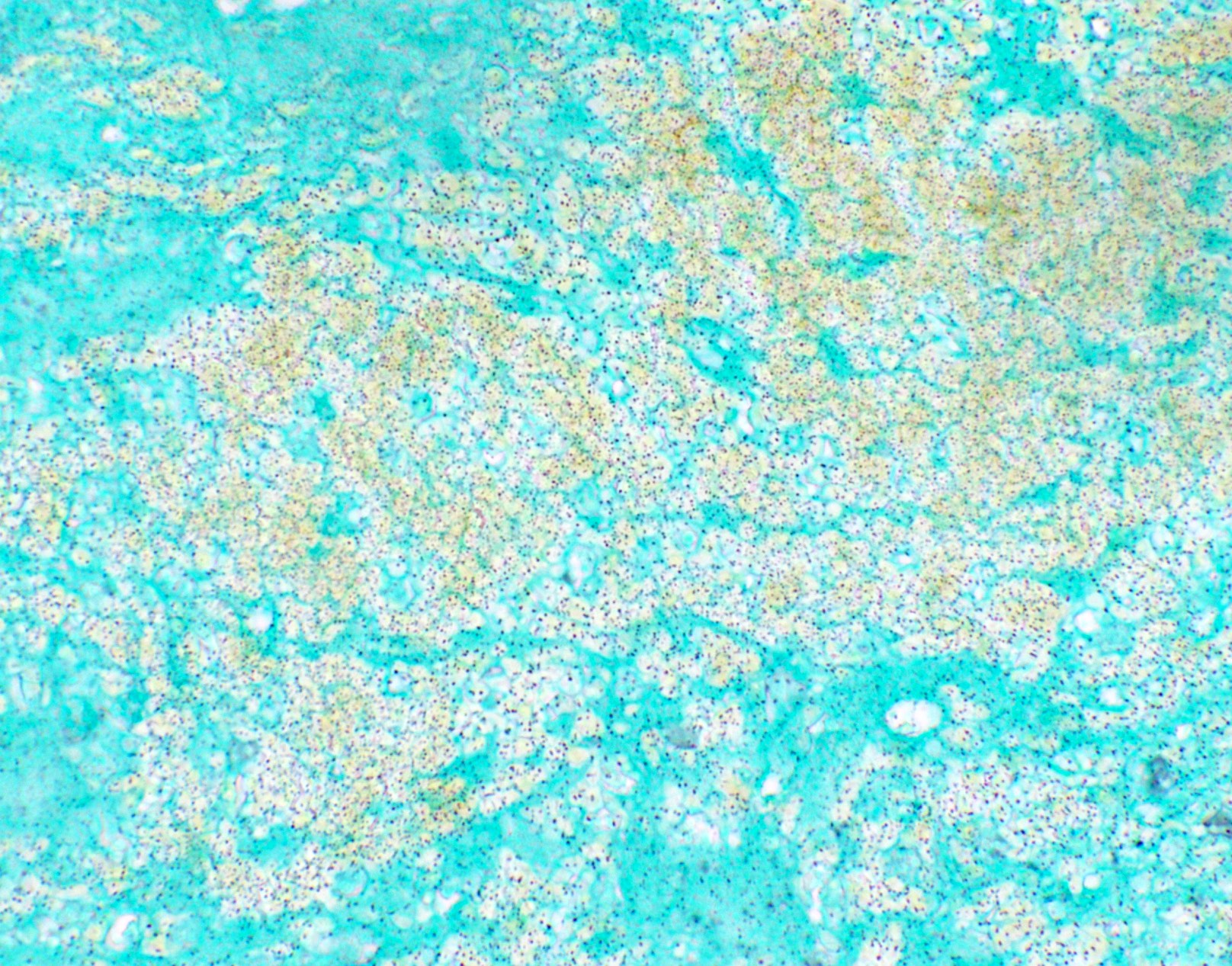
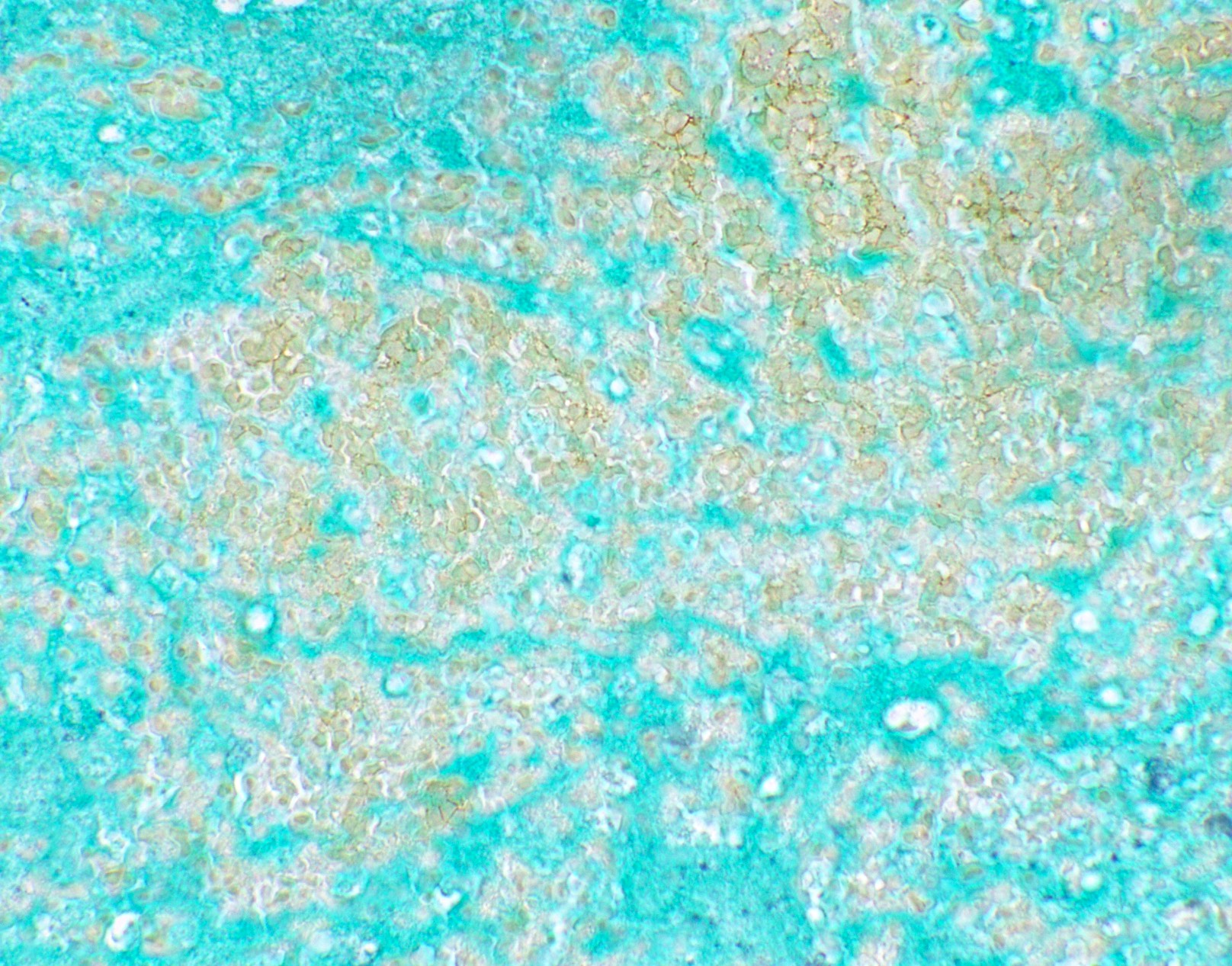
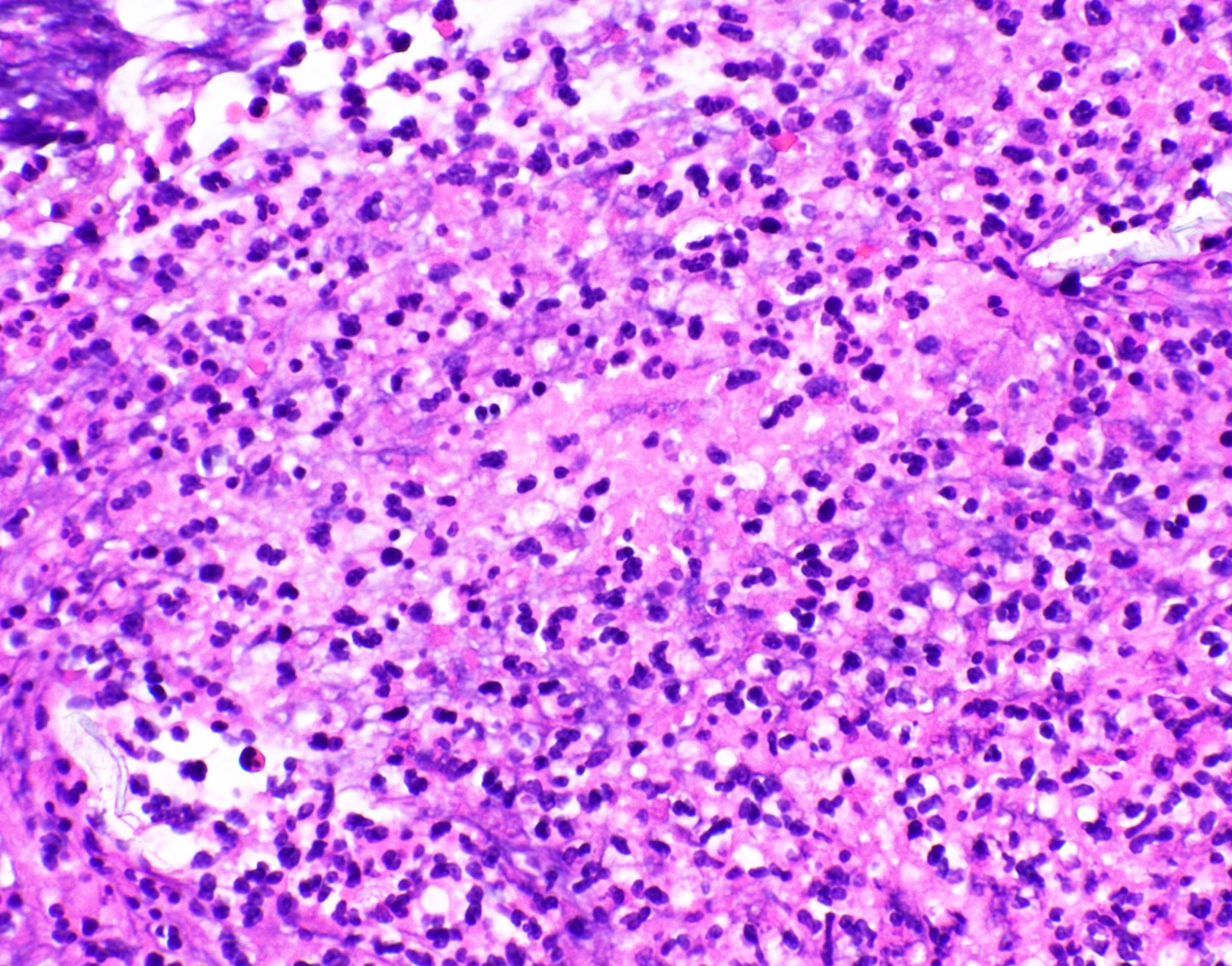
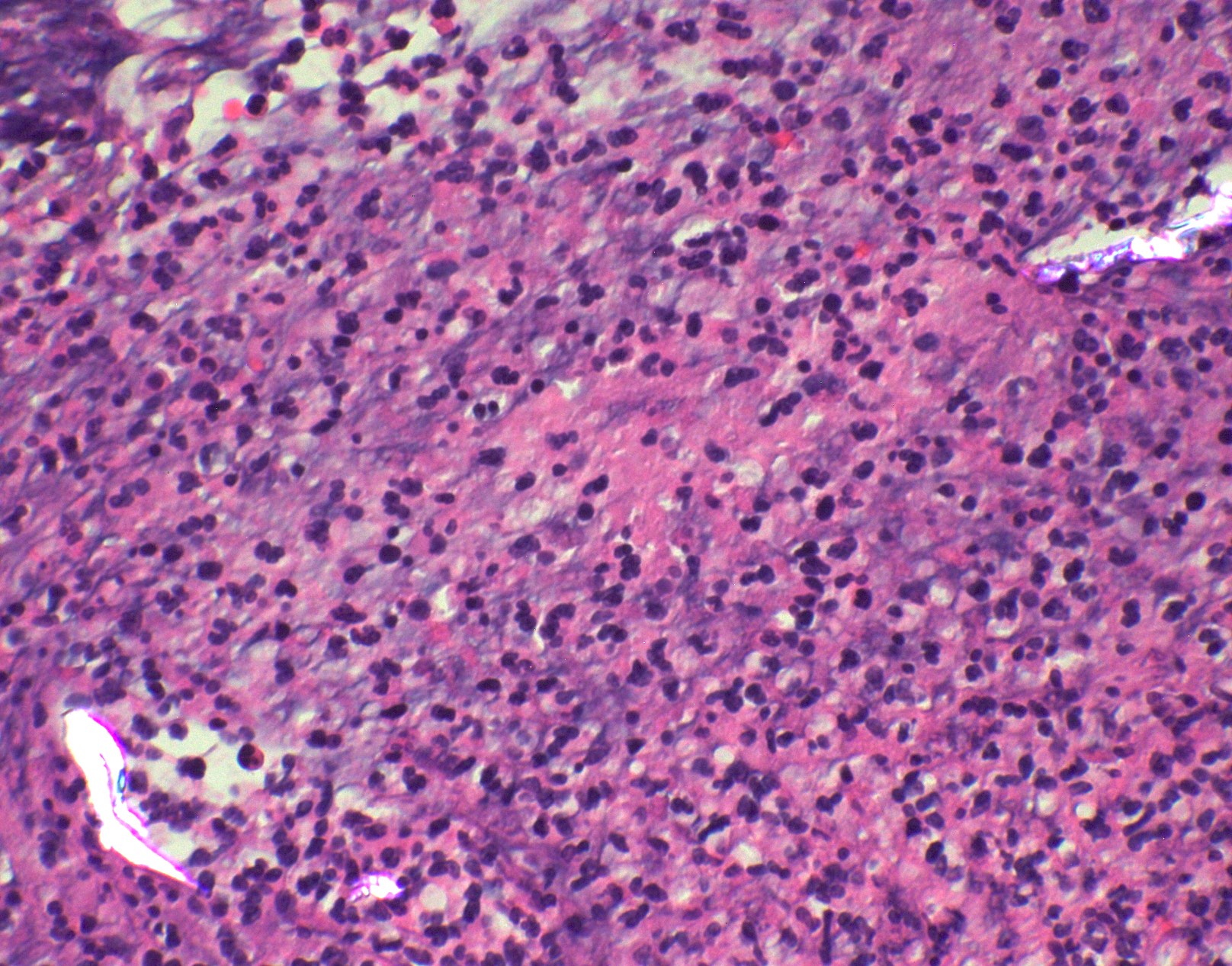
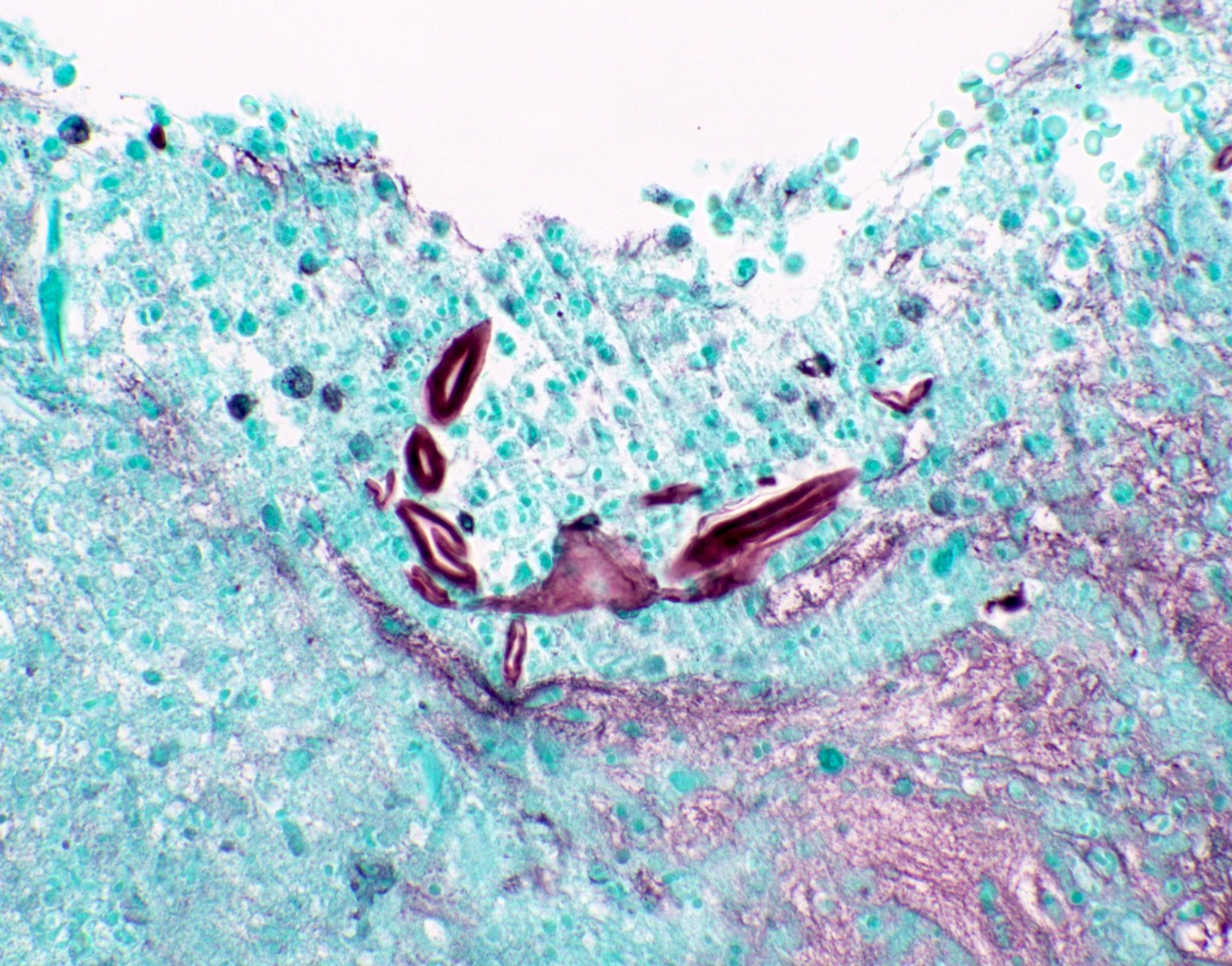
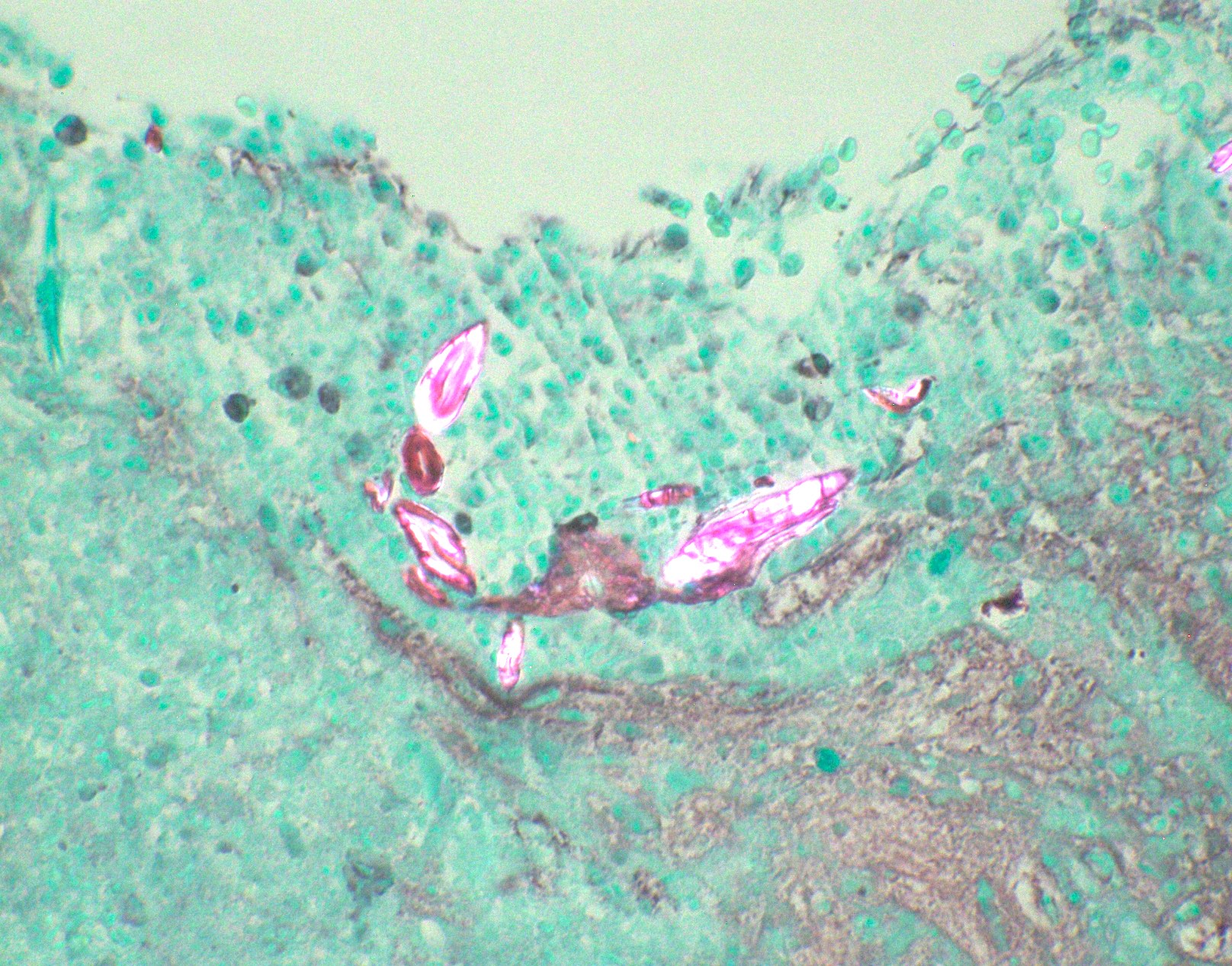
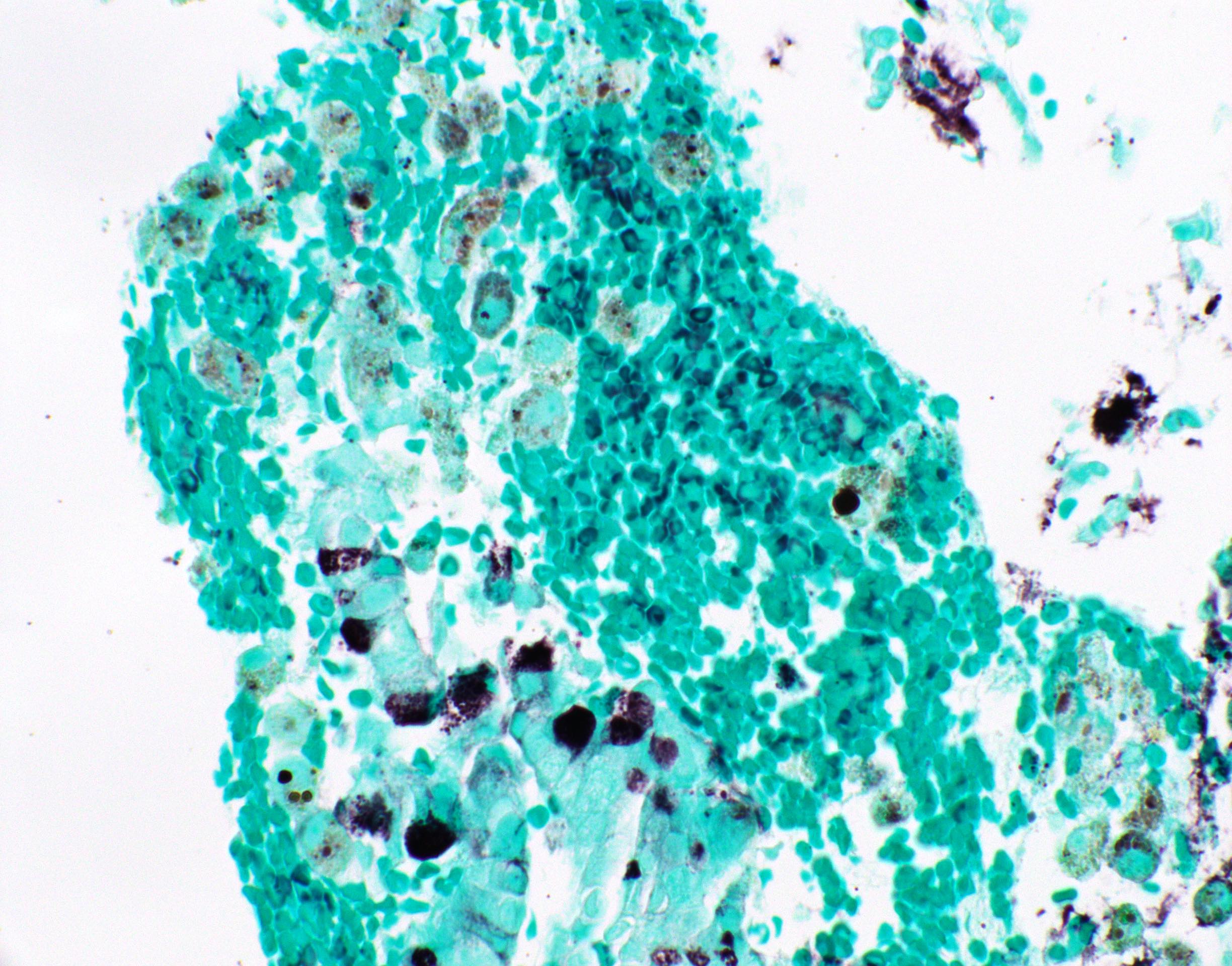
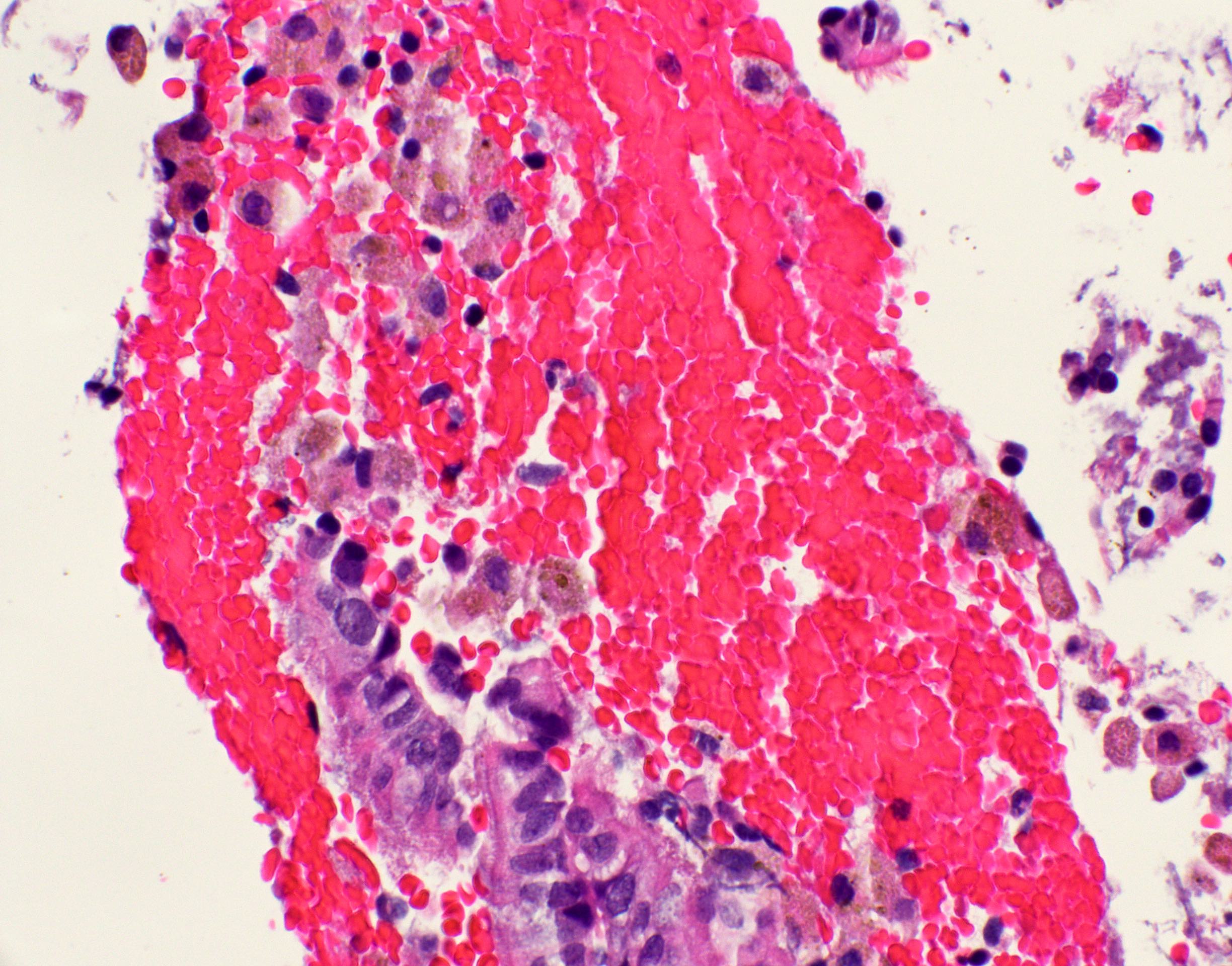
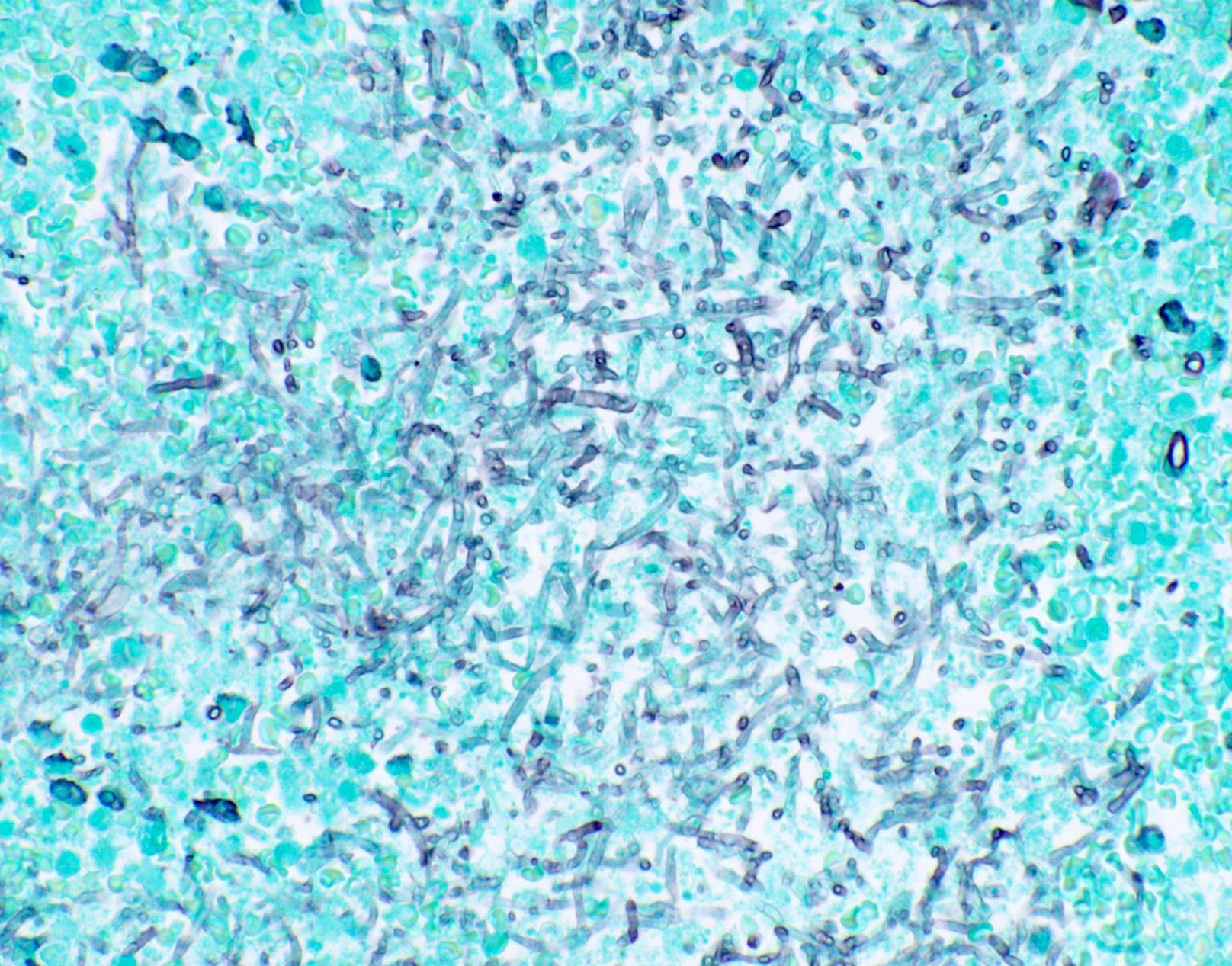
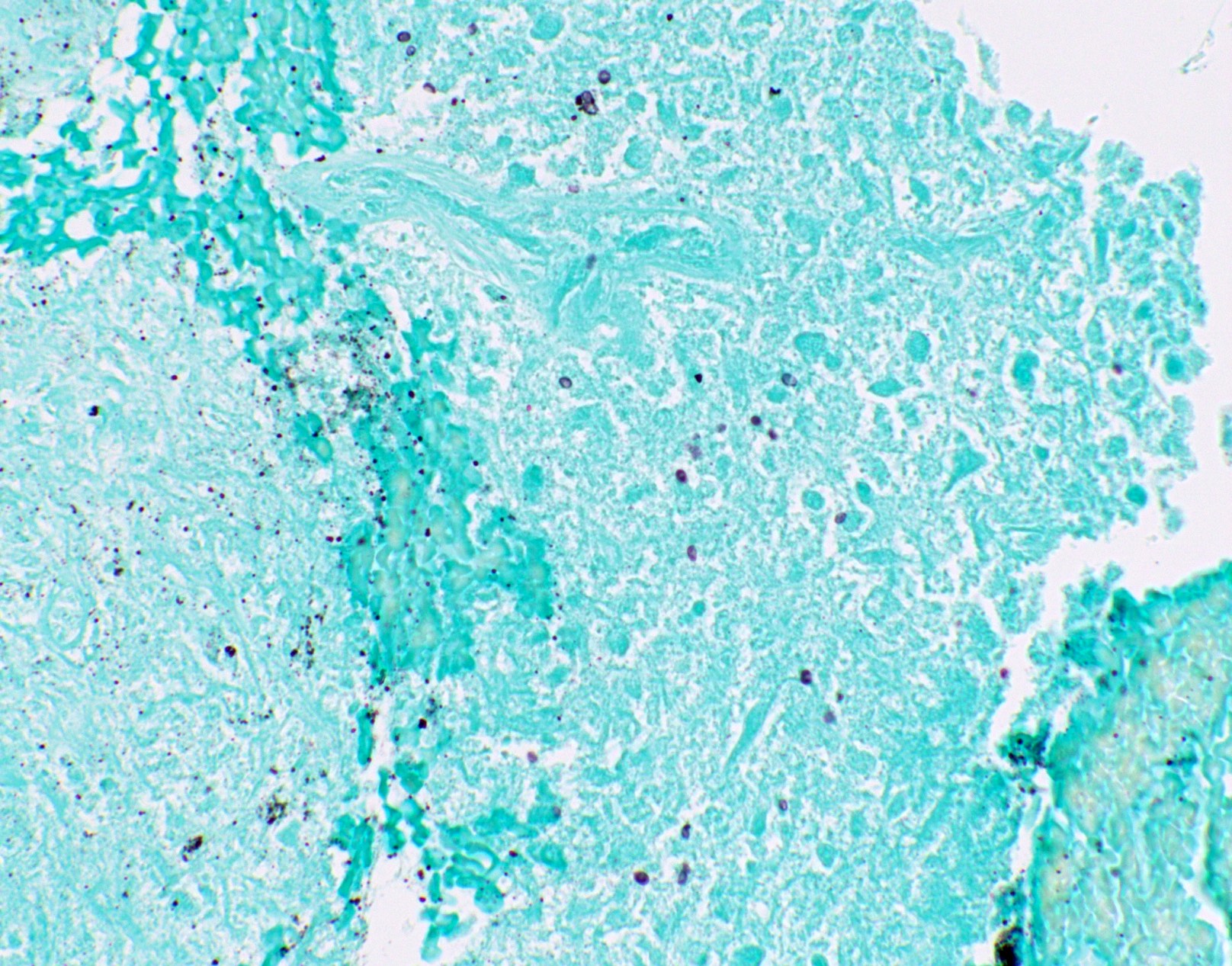
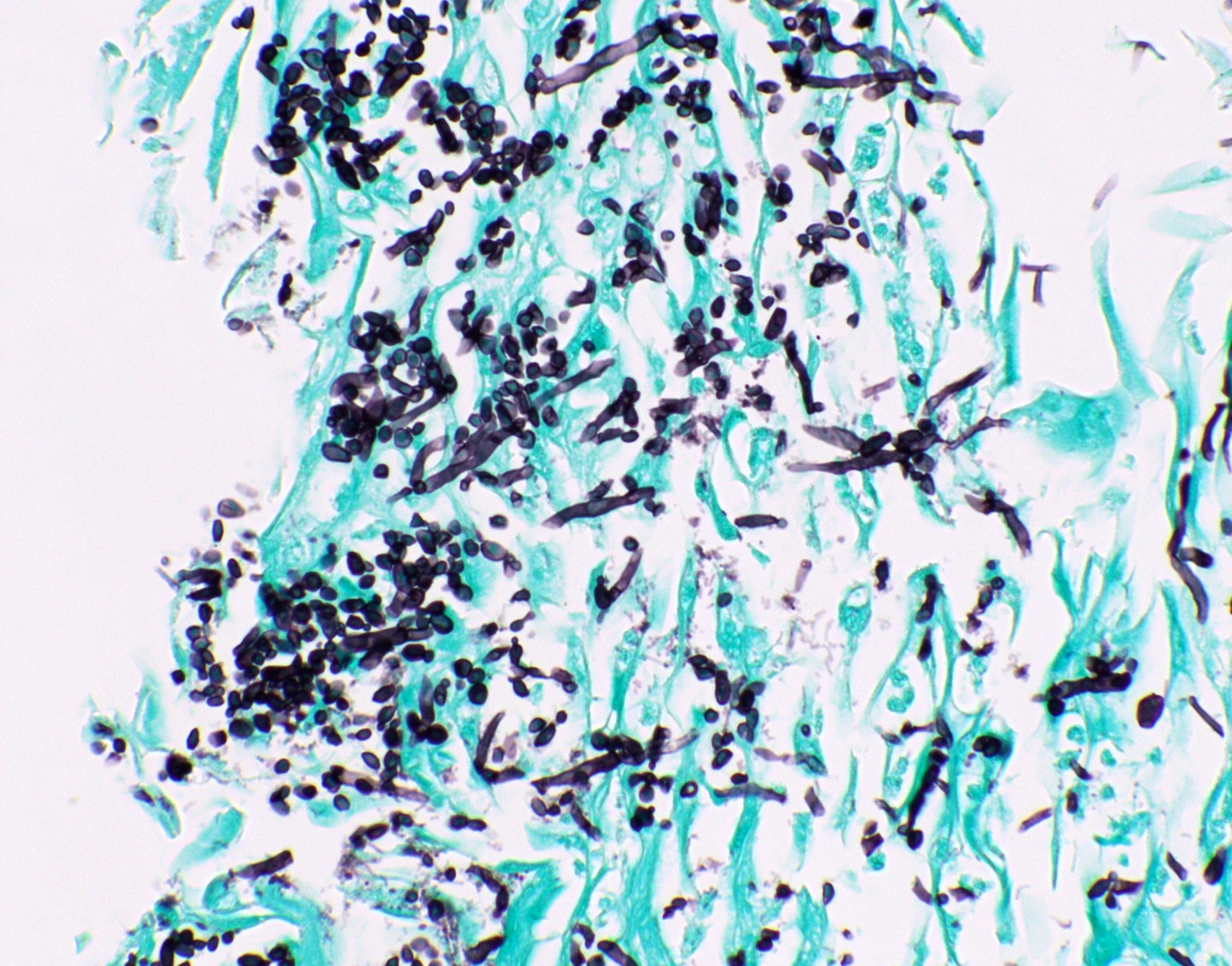
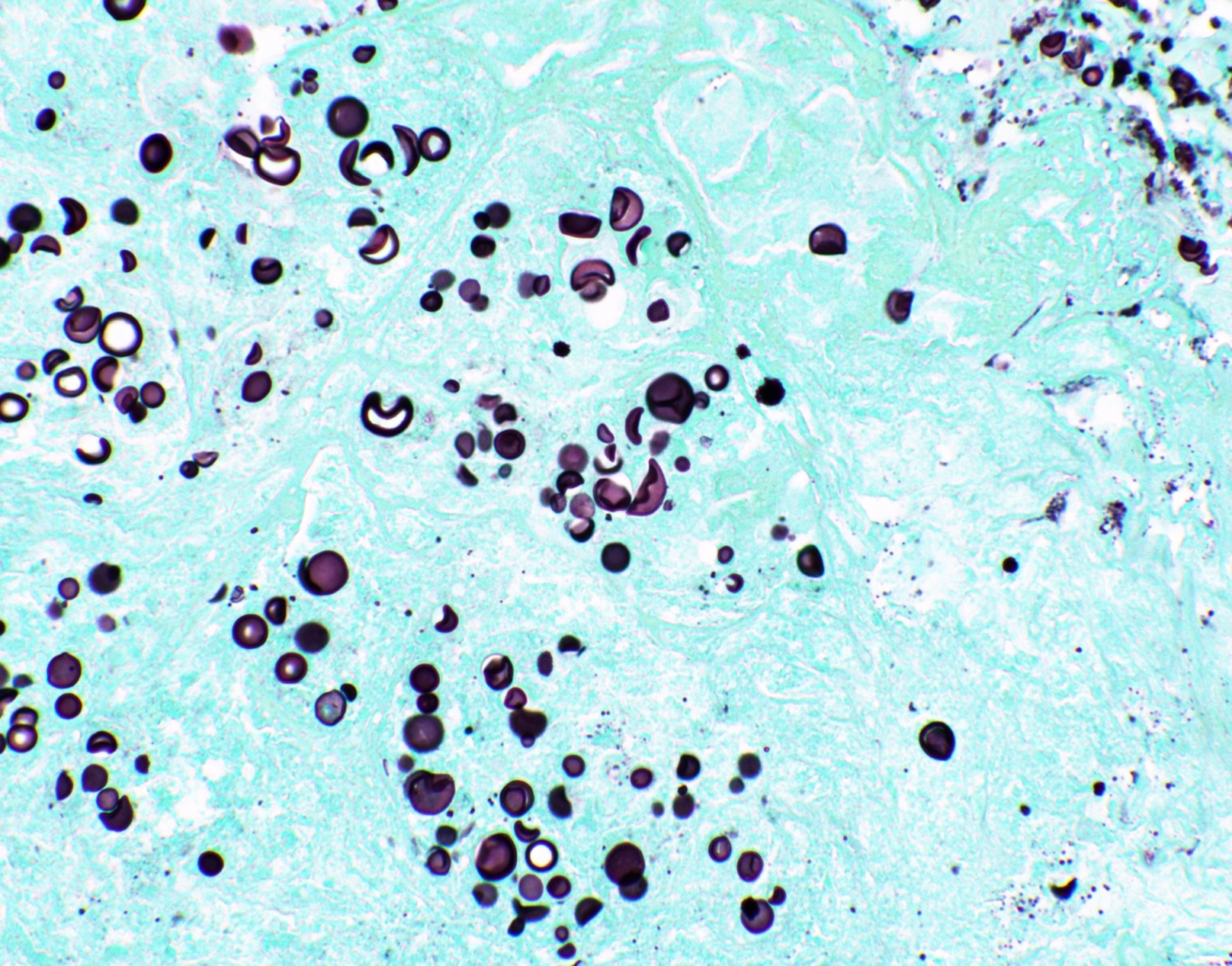
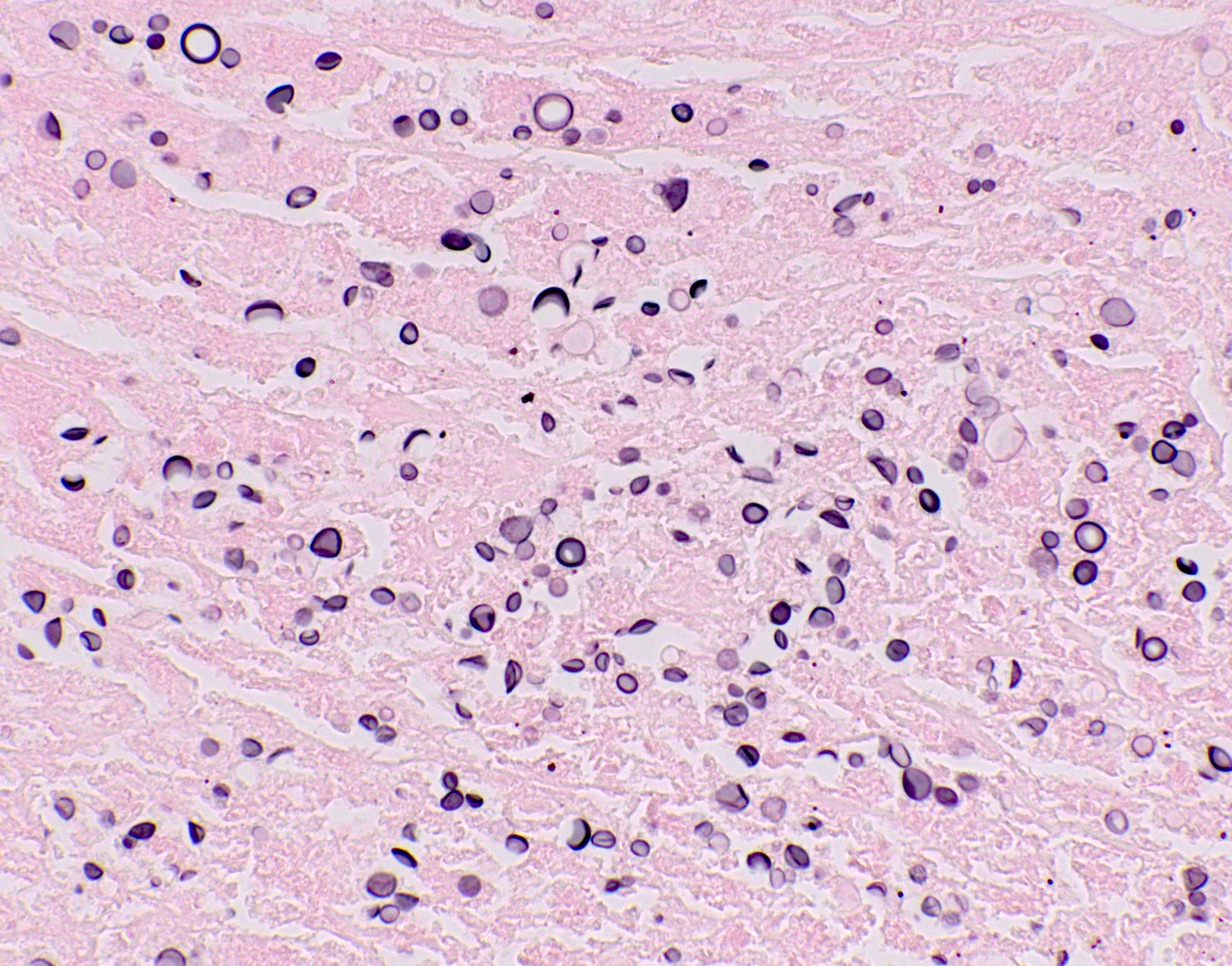
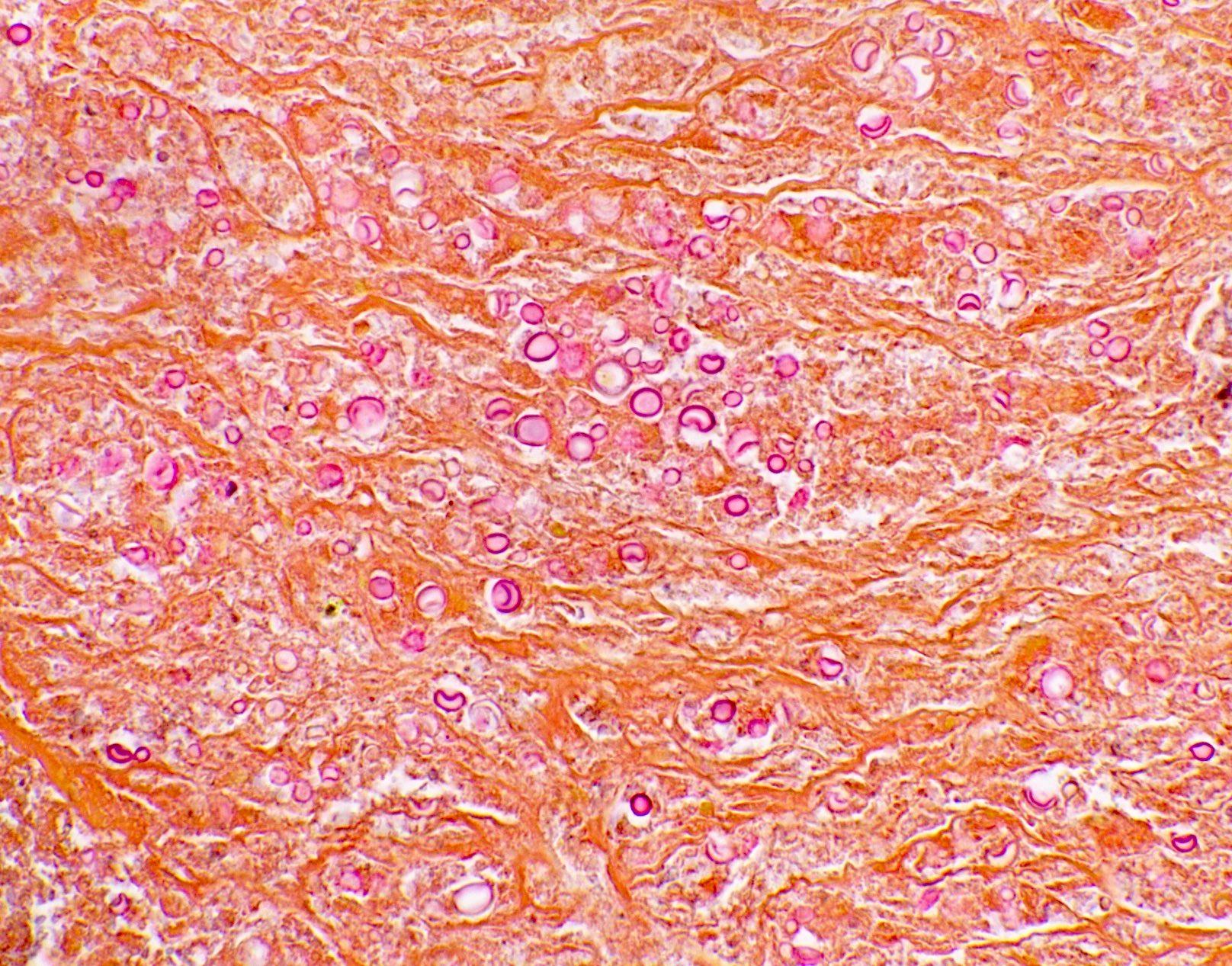
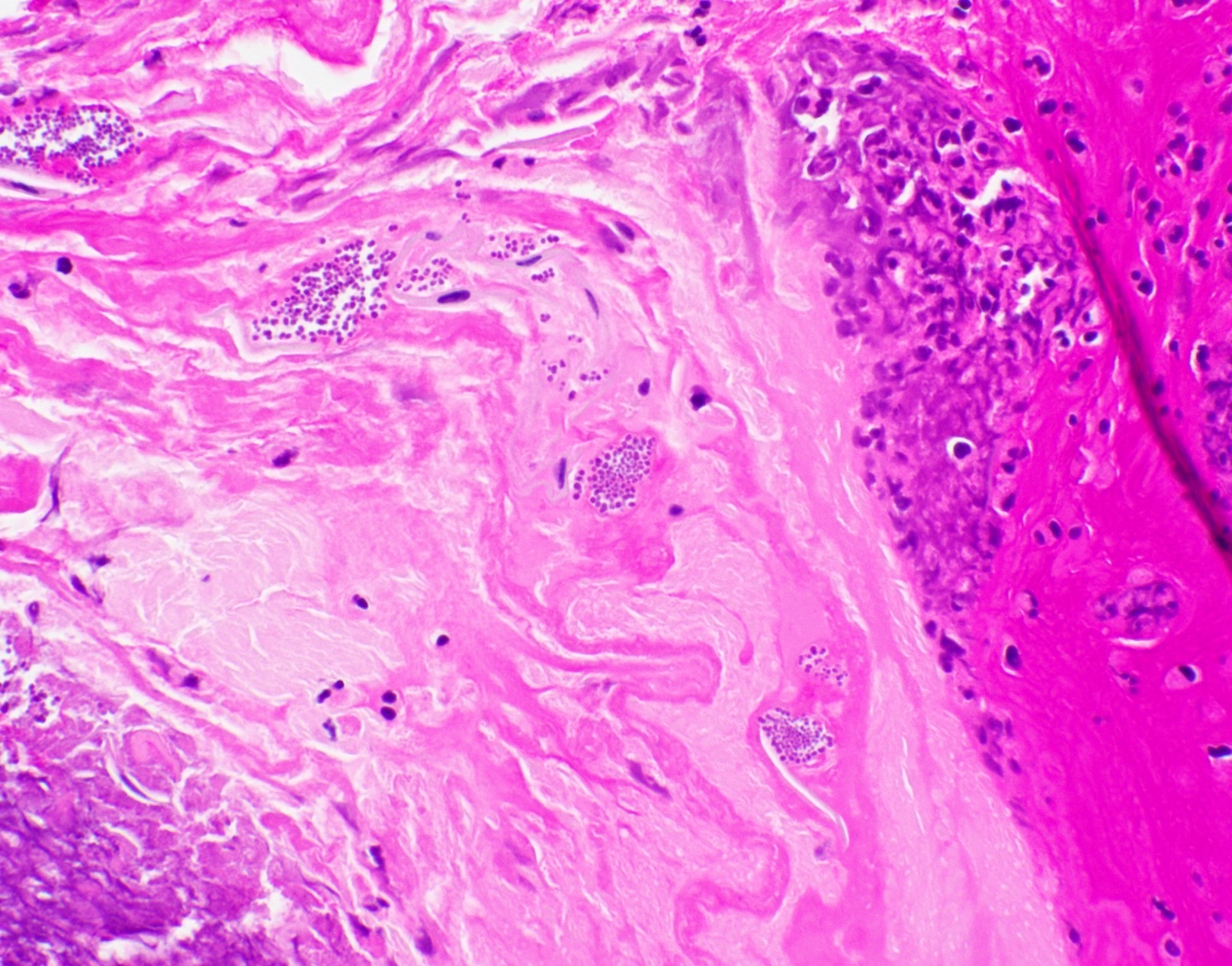
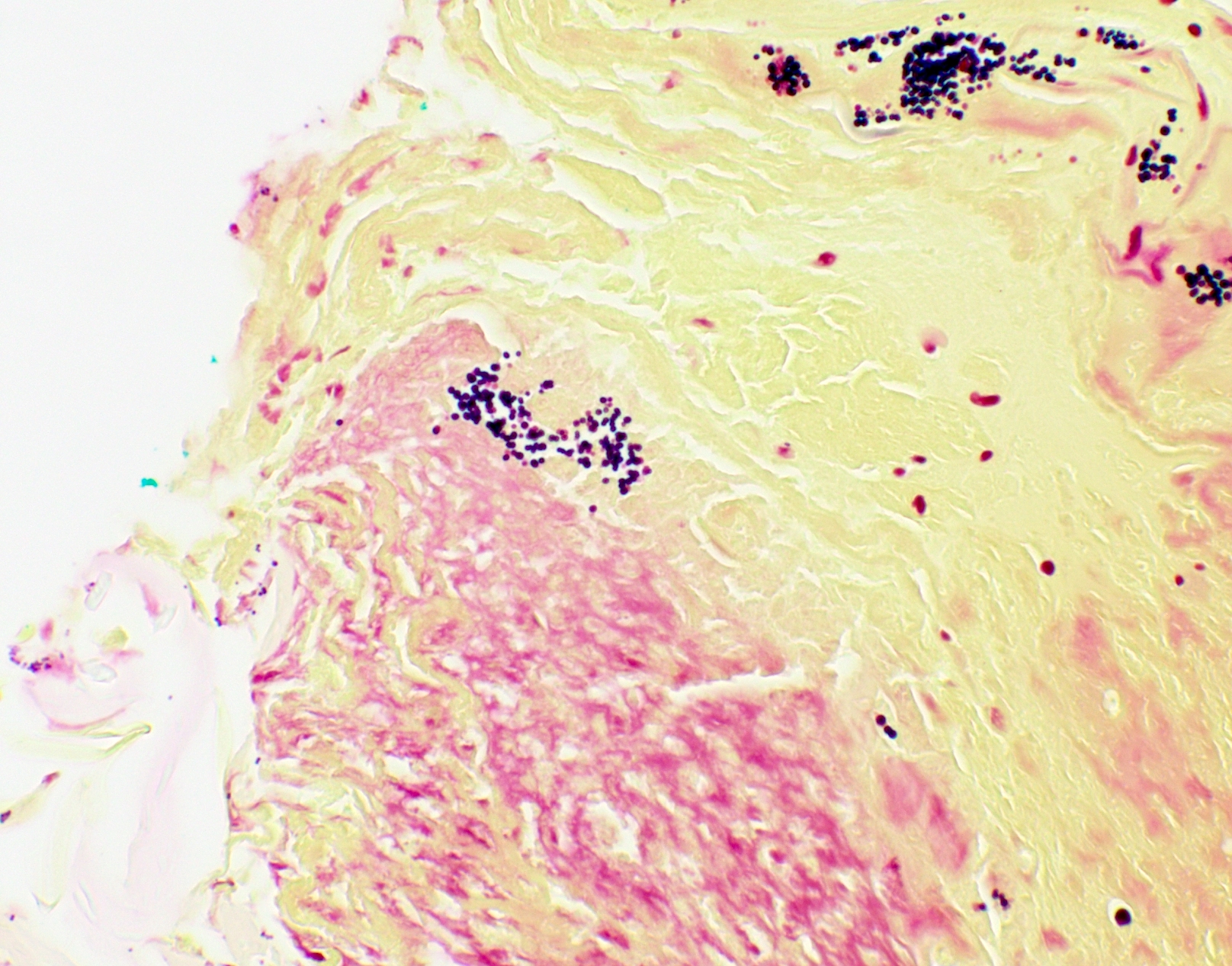
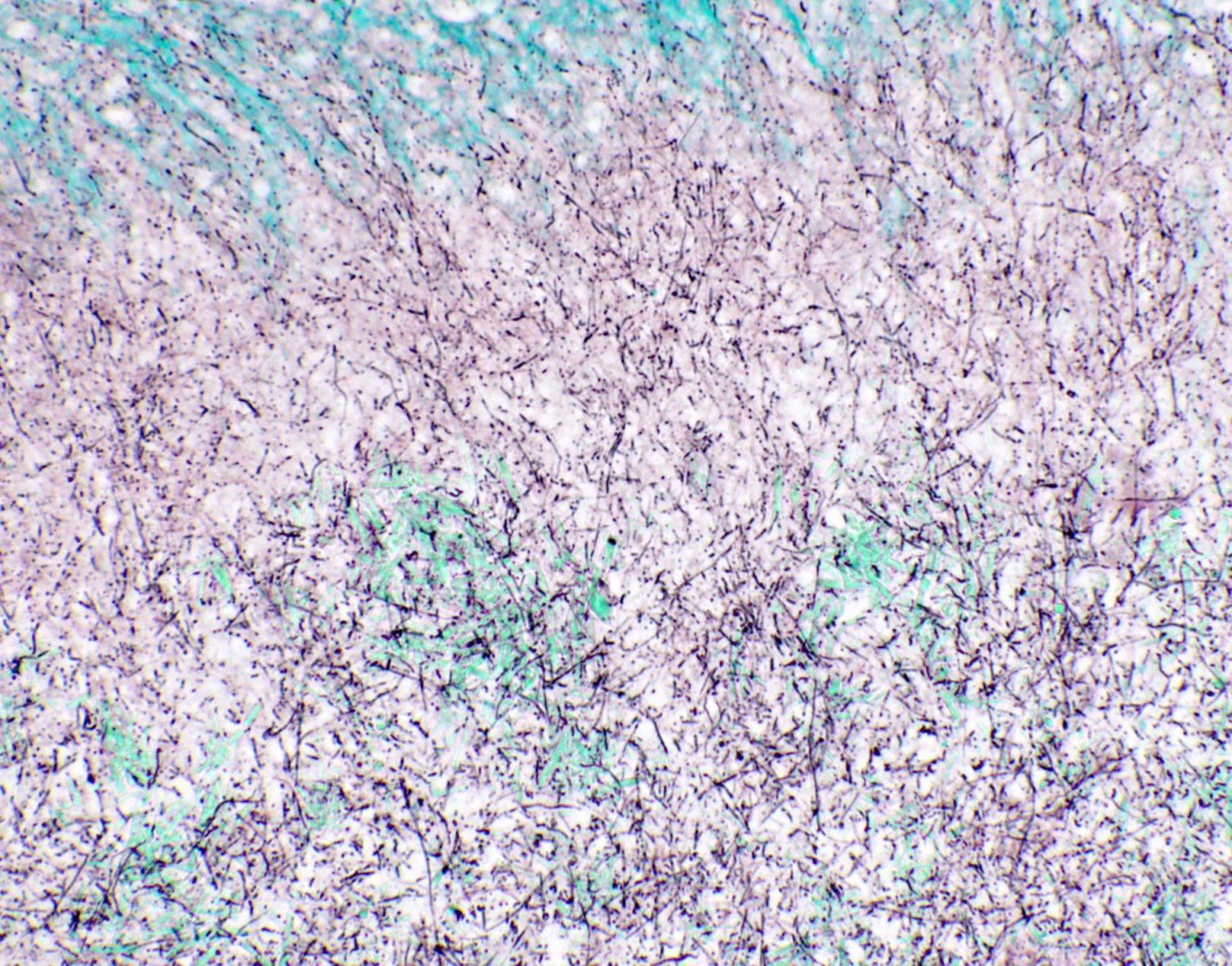
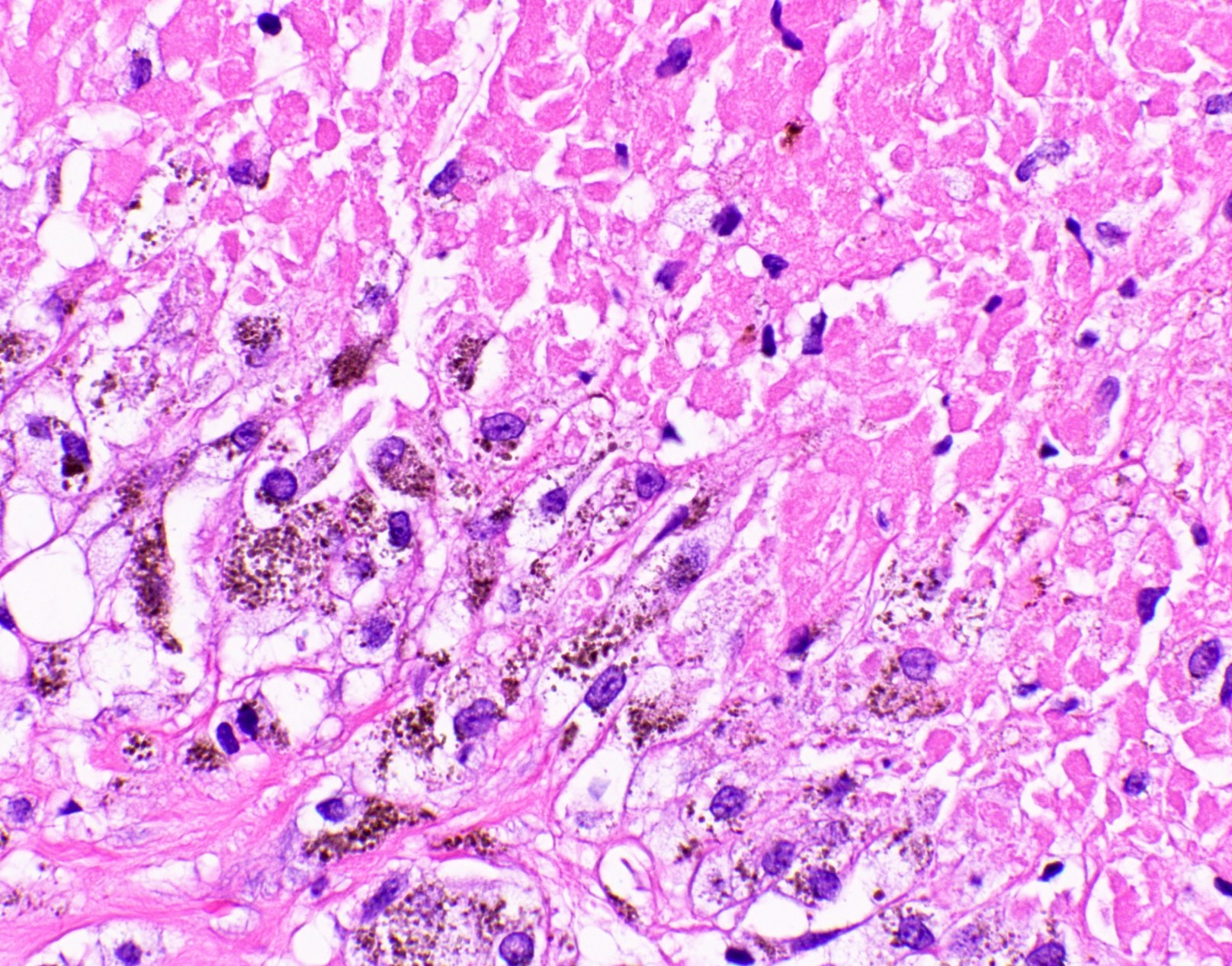
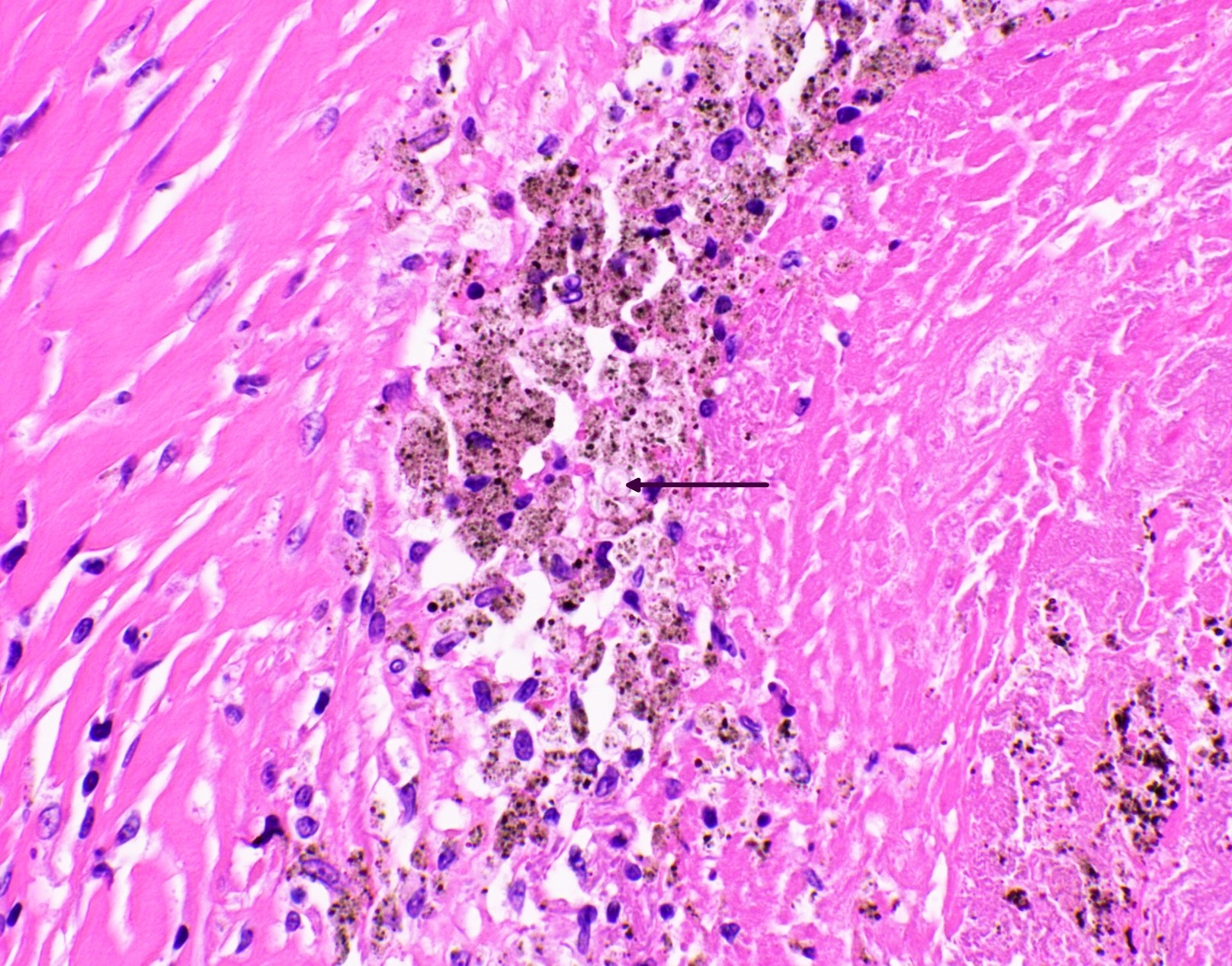
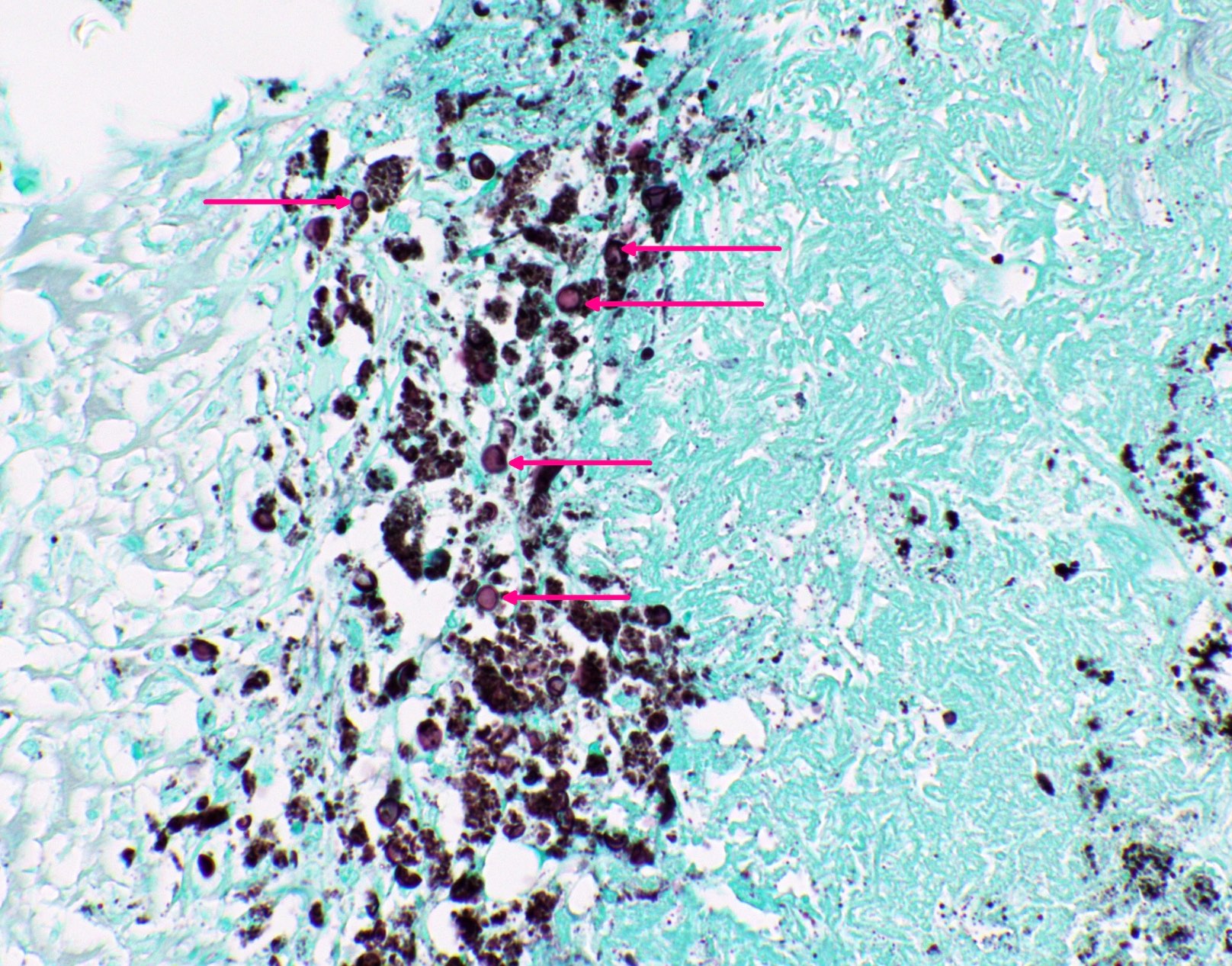
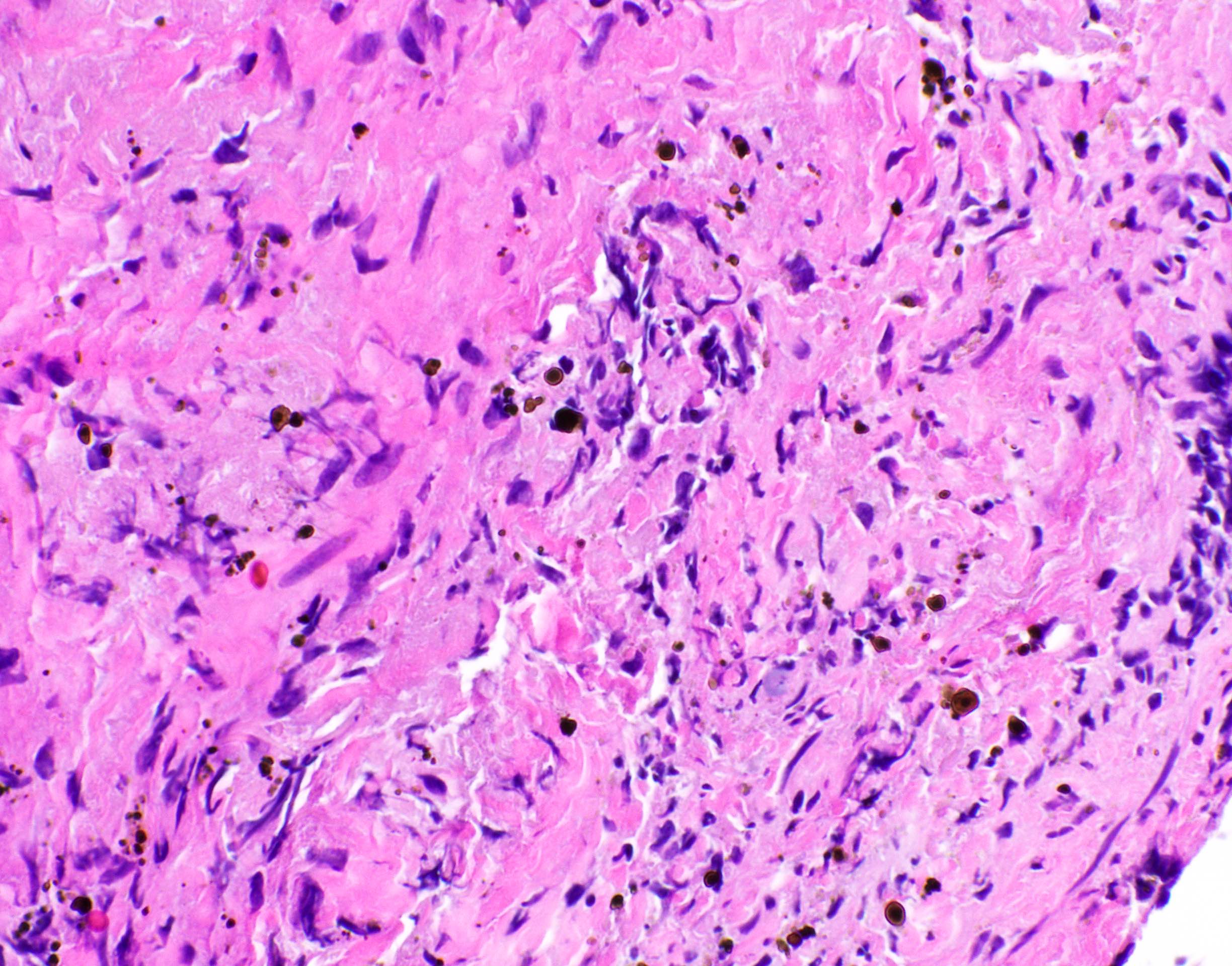
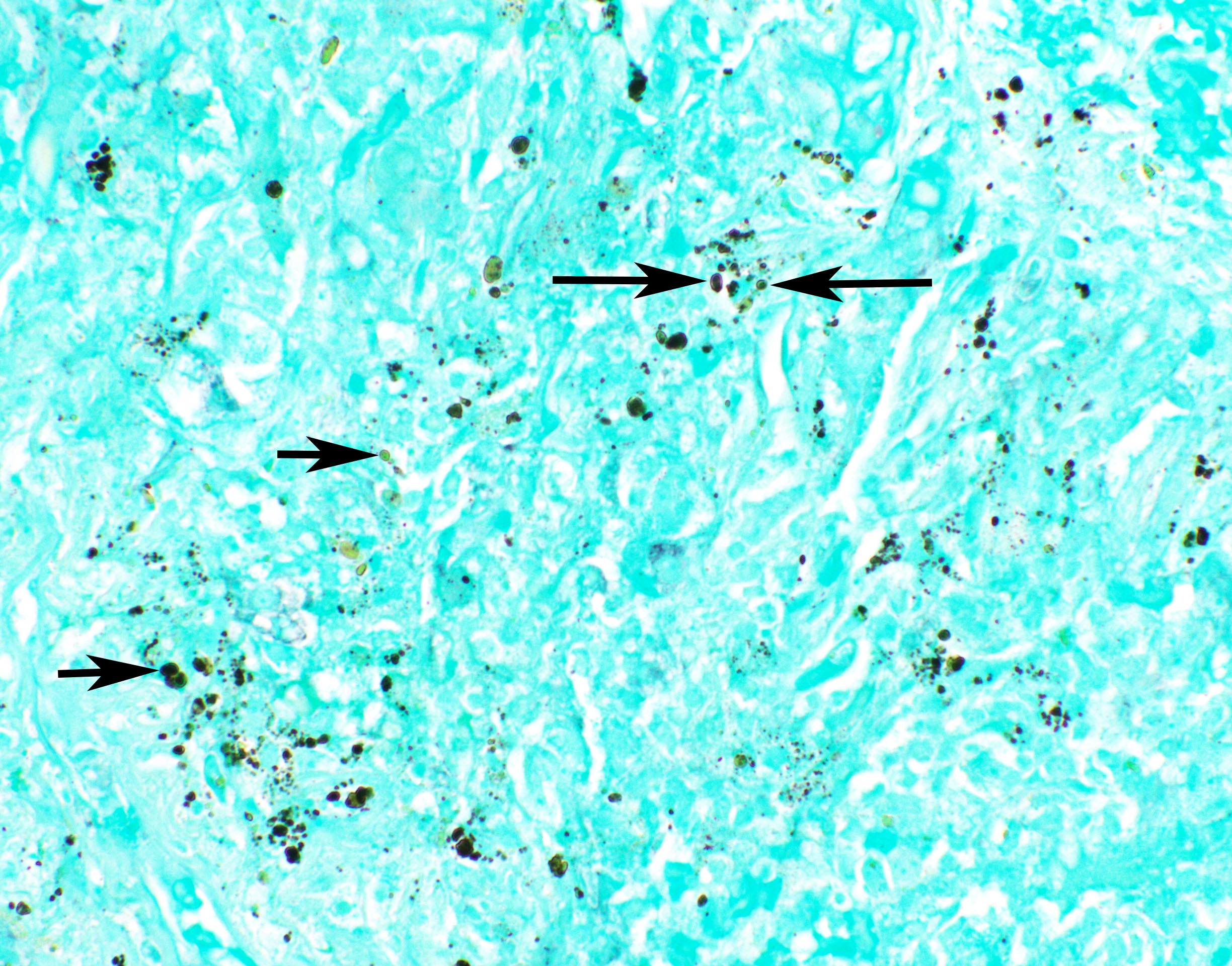
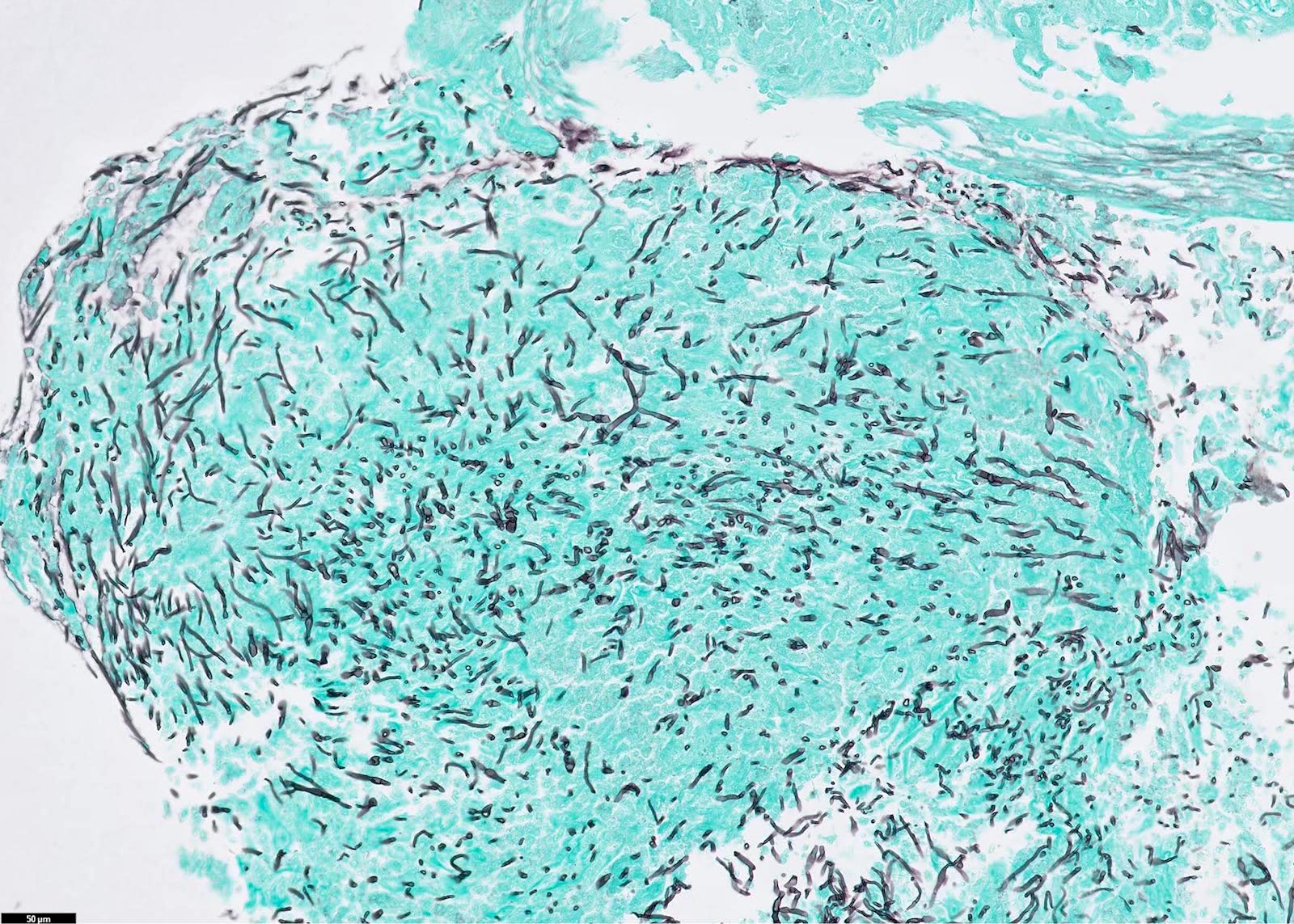
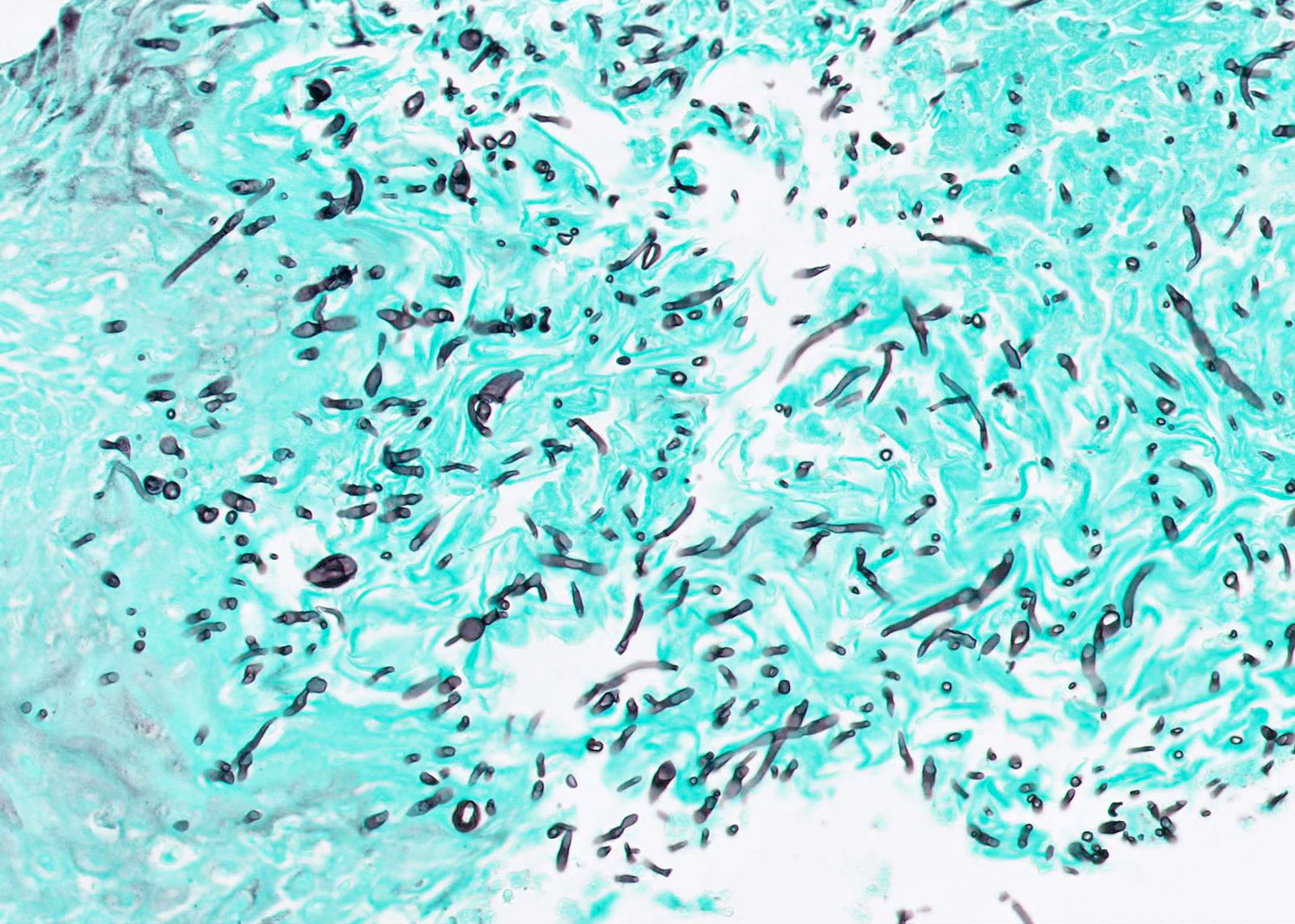
Microscopic (histologic) images
Contributed by Fang Zhou, M.D.
Positive staining: normal
Positive staining: fungal organisms
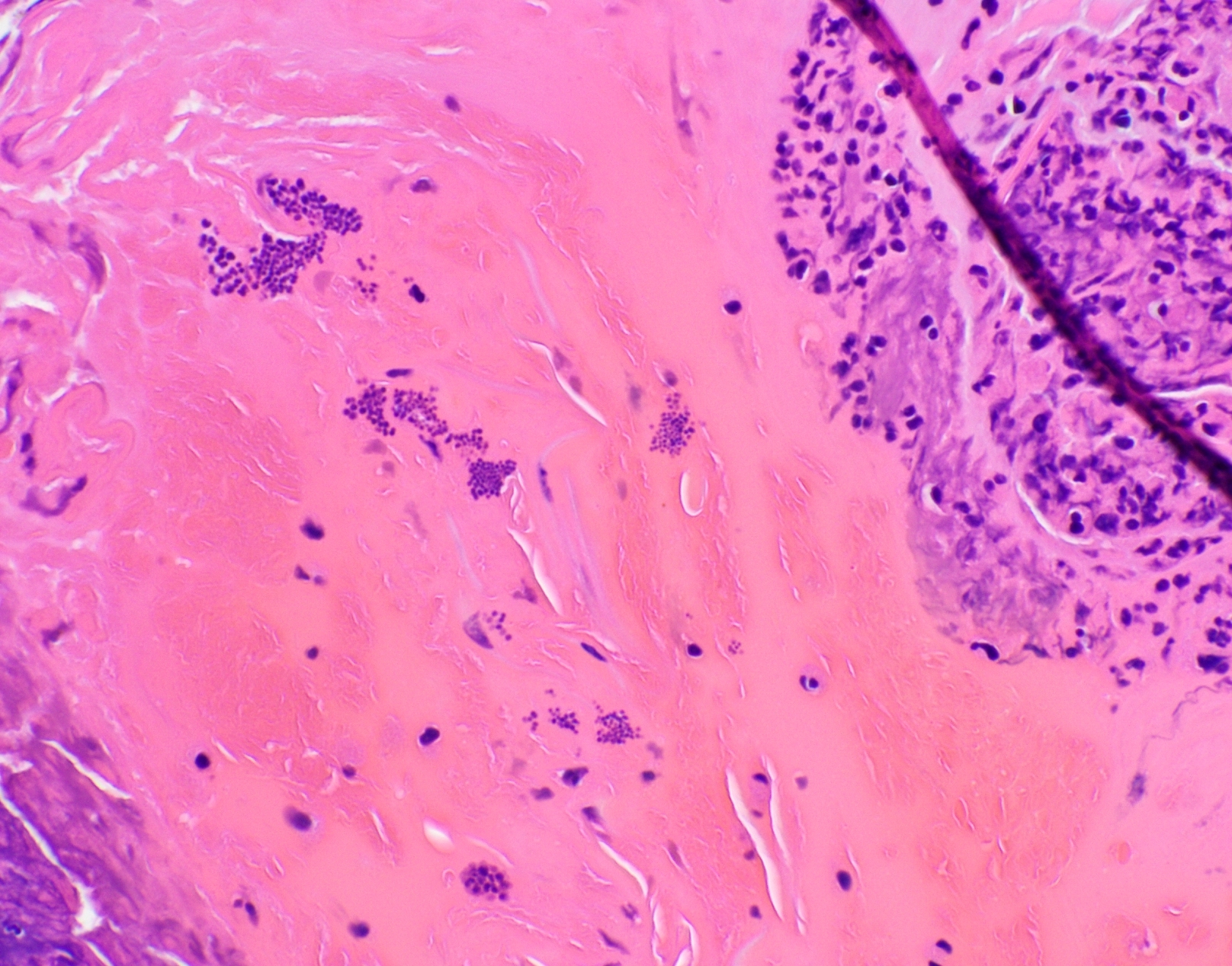
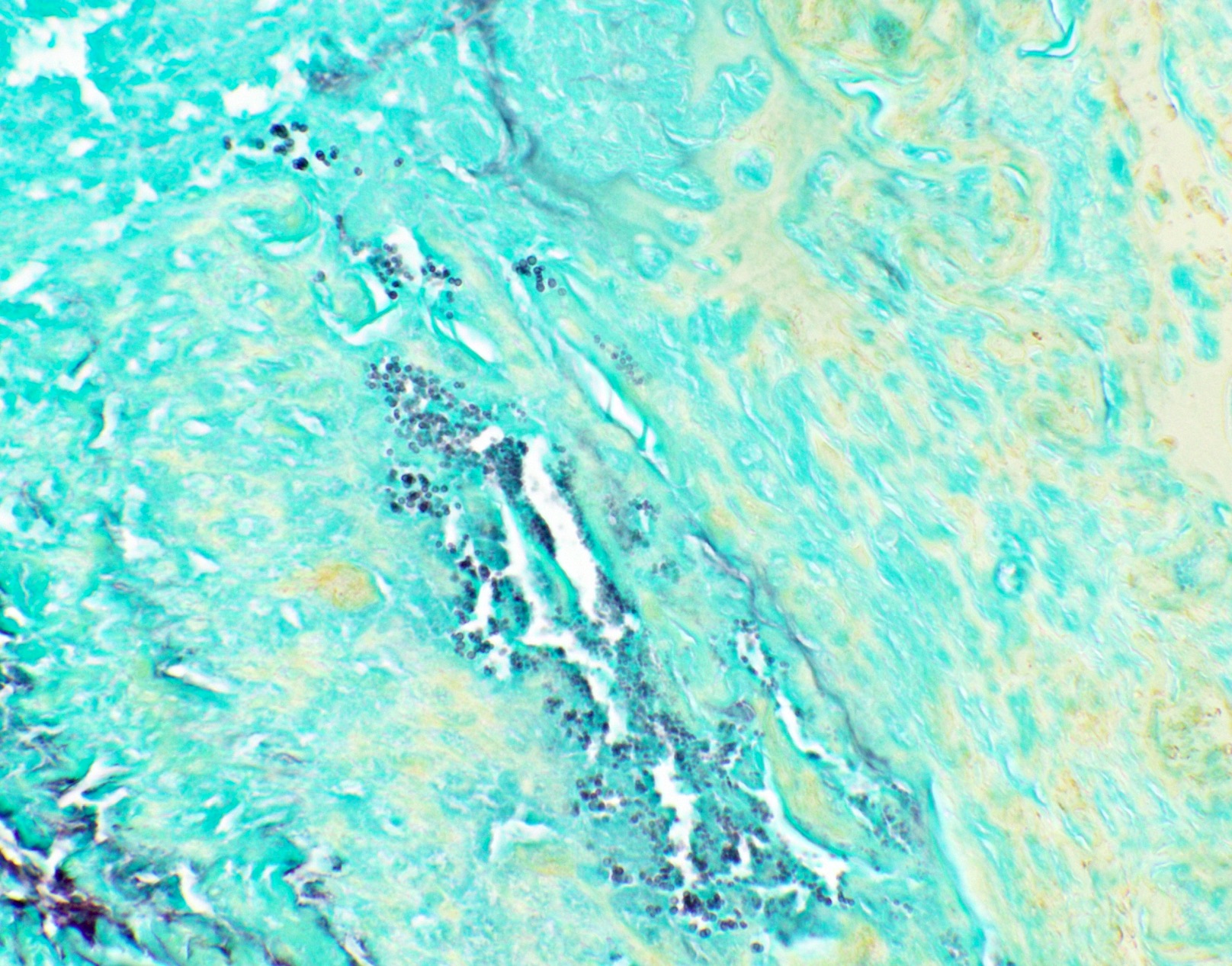
Positive staining: nonfungal organisms
Other confounding pigments
Contributed by Andrey Bychkov, M.D., Ph.D. and Jijgee Munkhdelger, M.D., Ph.D.
Virtual slides
Positive staining - normal
- Intracellular and extracellular mucin (J Am Soc Cytopathol 2017;6:223, Am J Clin Pathol 1946;10:177)
- Glycogen: can predigest with diastase but will also decrease sensitivity for fungi (IHC World: A Common Mistake When Staining for Fungi [Accessed 31 March 2021], Am J Clin Pathol 1946;10:177)
- Neutrophil cytoplasm (J Cytol 2019;36:184)
- Elastin: from overincubation (Suvarna: Bancroft's Theory and Practice of Histological Techniques, 8th Edition, 2019)
- Other pigments (Clin Microbiol Rev 2011;24:247)
- Anthracotic pigment: coarse granules, completely black, no cell wall outlines, remains black on GMS
- Melanin: fine to coarse brown granules on H&E, turns black on GMS
- Hamazaki-Wesenberg bodies: yellow-brown oval structures seen in sarcoidosis on H&E, turns black on GMS
- Red blood cells (aberrant staining)
Positive staining - disease
- Fungal yeasts (diameter 2 - 15 microns) (Clin Microbiol Rev 2011;24:247)
- Blastomyces dermatitidis
- Cryptococcus spp.
- Histoplasma capsulatum
- Candida glabrata: yeasts only (other Candida spp. show mixed yeasts and pseudohyphae)
- Pneumocystis jirovecii: will not grow in culture
- Penicillium marneffei
- Paracoccidioides brasiliensis
- Sporothrix schenckii
- Emmonsia crescens
- Lacazia loboi
- Other emerging opportunistic fungi, etc.
- Fungal hyphae (diameter 2 - 6 microns) (Clin Microbiol Rev 2011;24:247)
- Often ubiquitous in the environment (especially soil) and can contaminate cultures, so seeing hyphae in infected tissue is helpful to confirm culture results (Clin Microbiol Rev 2011;24:247)
- Aspergillus spp.
- Mucorales order: stains poorly with GMS; add PAS stain
- Mucor spp. and Rhizopus spp. (mucormycosis / zygomycosis)
- Entomophthorales spp.
- Dematiaceous (melanin+) fungi
- Madurella spp., Fonsecaea spp., Cladophialophora spp., Exophiala spp., Curvularia spp., Bipolaris spp., etc.
- Other hyphae seen in tissue infections: Trichosporon spp., Fusarium spp., Scedosporium spp., Trichoderma spp., Paecilomyces spp., Pseudoallescheria spp., Scopulariopsis spp., Acremonium spp., Schizophyllum spp., Phaeoacremonium spp., Trichophyton spp., Phialophora verrucosa, Bipolaris spicifera, Curvularia lunata, etc. (other emerging opportunistic fungi)
- Mixture of fungal yeasts and pseudohyphae:
- Candida spp. (except C. glabrata) (Clin Microbiol Rev 2011;24:247)
- Mixture of fungal spherules and endospores:
- Coccidioides spp.
- Spherules (10 - 100 microns) and endospores (2 - 5 microns)
- Endospores morphologically resemble yeasts
- Spherules are not always found
- Coccidioides immitis rarely infects tissues as spherules and hyphae (Clin Invest Infect 1994;105:P412, Clin Infect Dis 2000;30:349, Am J Trop Med Hyg 2019;100:772)
- Coccidioides spp.
- Nonfungal organisms (J Am Soc Cytopathol 2017;6:223, Diagn Cytopathol 2017;45:1105, Suvarna: Bancroft's Theory and Practice of Histological Techniques, 8th Edition, 2019, Clin Microbiol Rev 2011;24:247)
- Must tell them apart from fungi (use size, shape)
- May be helpful in challenging or sparse cases to prompt the ordering of additional validated confirmatory stains or clinical tests for the nonfungal organisms
- In absence of other confirmatory results, interpret the GMS with caution (contaminants, artifacts, mimickers)
- Mycobacterium
- Fatty capsule may be lost due to antimycobacterial therapy and tissue processing, causing acid fast stains to fail or stain fewer bacilli
- Sometimes, GMS can still stain residual cell wall carbohydrates
- Bacteria (Gram stain may be lost due to necrosis or antibiotics)
- Streptococcus spp.
- Staphylococcus spp.
- Filamentous: Nocardia and Actinomyces spp.
- Bacillus cereus
- Bartonella henselae
- Bartonella quintana
- Strongyloides stercoralis
- Schistosoma eggs
- Cytomegalovirus inclusions
- Toxoplasma
Negative staining
- Organisms mimicking fungi that are GMS negative (Clin Microbiol Rev 2011;24:247)
- Rhinosporidium seeberi
- Trypanosoma cruzi
- Leishmania spp.
- Nonorganisms (may resemble fungi on H&E but do not contain carbohydrates and are negative for GMS)
- Calcium phosphate and calcium carbonate deposits: associated with necrosis; mimic yeasts (Dako: Special Stains and H&E [Accessed 1 April 2021])
- Gamna-Gandy bodies: associated with blood - iron and calcium salts; mimic hyphae (Diagn Cytopathol 2020;48:670)
- Hemosiderin: mimics yeasts
- Frothy fibrin or edema: mimic yeasts
- Crystals and precipitates: mimic yeasts or hyphae
Sample pathology report
- Sinonasal mucosa, biopsy:
- Invasive fungal sinusitis (see comment)
- Comment: GMS stain on block A1 shows broad, nonseptate fungal hyphae with right angle branching, associated with tissue necrosis and angioinvasion, consistent with invasive fungal sinusitis. Dr. _ was notified at 10:00am on 4/19/2021 with repeat back verification.
Board review style question #1
Board review style answer #1
C. Candida glabrata, formerly known as Torulopsis glabrata, is the only Candida species that does not form pseudohyphae in tissue. It was formerly considered a saprophytic organism that is commonly found in normal human flora but it has emerged as an opportunistic organism with both innate and acquired antifungal resistance (Microorganisms 2019;7:39, J Clin Microbiol 2004;42:4419).
Comment Here
Reference: GMS
Comment Here
Reference: GMS
Board review style question #2
A biopsy from a solid lung nodule shows granulomatous inflammation and fungal hyphae are seen on GMS stain. Which of the following is the most likely causative organism?
- Blastomyces dermatitidis
- Cryptococcus
spp. - Histoplasma capsulatum
- Pneumocystis jirovecii
- Scedosporium
spp.
Board review style answer #2
E. Among the listed organisms, only Scedosporium spp. (though uncommon) are usually encountered as hyphae in infected tissue specimens (Clin Microbiol Rev 2008;21:157). It is an opportunistic fungus with intrinsic antifungal resistance (Med Mycol 2018;56:102). The other organisms in the answer choices are more commonly encountered dimorphic fungi that are usually seen as yeast forms in infected tissues.
Comment Here
Reference: GMS
Comment Here
Reference: GMS