Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Electron microscopy images | Molecular / cytogenetics description | Molecular / cytogenetics images | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Warmke L. Ewing sarcoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/boneewing.html. Accessed December 23rd, 2024.
Definition / general
- Small round cell sarcoma showing gene fusions involving one member of the FET family of genes (usually EWSR1) and a member of the E26 transformation specific (ETS) family of transcription factors
- American pathologist James Ewing (1866 - 1943) first described the tumor as diffuse endothelioma of bone (CA Cancer J Clin 1972;22:95)
Essential features
- Small round cell sarcoma
- CD99 diffuse membranous expression
- Gene fusion involving FET family of genes (usually EWSR1) and member of ETS family of transcription factors, most commonly EWSR1-FLI1 (~85 - 90%)
Terminology
- Ewing sarcoma of bone
- Extraskeletal Ewing sarcoma
- Adamantinoma-like Ewing sarcoma
- Primitive neuroectodermal tumor (PNET), term no longer recommended
- Askin tumor (Ewing sarcoma arising in chest wall), term no longer recommended
ICD coding
Epidemiology
- Second most common malignant bone tumor in children / young adults after osteosarcoma (Cancer 1996;78:532)
- M:F = 1.5:1 (J Pediatr Hematol Oncol 2008;30:425)
- Peak incidence in second decade of life
- Nearly 80% patients < 20 years of age
- Uncommon in patients younger than 5 years and older than 30 years
- Predominantly Caucasians, rare in African Americans, Asians and Native Americans (Cancer Epidemiol Biomarkers Prev 2011;20:449)
- Overall incidence is 3 cases per million per year in United States population (J Pediatr Hematol Oncol 2008;30:425)
Sites
- Diaphyseal or metaphyseal - diaphyseal region of long bones (lower > upper extremity)
- Pelvic bones and ribs common
- Bone > soft tissue, approximately 12% extraskeletal (Nat Rev Dis Primers 2018;4:5)
Pathophysiology
- Primary mutation generates fusion gene between the FET and ETS families, altering gene expression and promoting tumorigenesis
- Additional, secondary mutations include STAG2 (15 - 22%), CDKN2A (12%) and TP53 (7%) (PLoS Genet 2014;10:e1004475, Cancer Discov 2014;4:1326, Cancer Discov 2014;4:1342, N Engl J Med 2015;373:2336)
- Majority of mutations are sporadic, while germline have been reported (N Engl J Med 2015;373:2336)
Etiology
- Chromosomal translocation commonly involving EWSR1 and ETS partner
- Cell of origin possibly mesenchymal stem cell (Cancer Cell 2007;11:421)
Clinical features
- Localized pain and swelling (J Bone Joint Surg Am 2000;82:667)
- Painful enlarging mass
- Associated pathologic fracture sometimes present
- Systemic findings (fever, weight loss, anemia, leukocytosis and increased sedimentation rate) can occur
Diagnosis
- Integration of clinical, radiographic, immunohistochemical and molecular information
- Small round cell morphology
- CD99 diffuse membranous expression
- Genetic confirmation often required (all cases harbor FET-ETS fusion)
Laboratory
- Anemia, leukocytosis and elevated sedimentation rate can occur
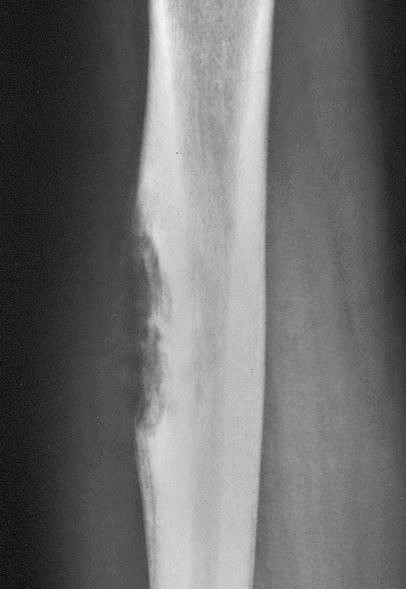
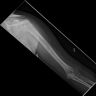
Radiology description
- Plain radiograph
- Osteolytic permeative lesion
- Poorly defined margins
- Moth eaten bone destruction
- Aggressive periosteal reaction (onion skin appearance)
- Sunburst periosteal reaction less common than osteosarcoma
- Saucerization, extraosseous tumor erodes cortex
- No evidence of tumor osteoid / matrix production
- Often underestimates extent of tumor
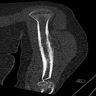
- CT / MRI / PET (Semin Musculoskelet Radiol 2019;23:36)
- Fully define primary lesion
- Evaluate soft tissue extension
- Assess for metastatic disease (~25%)
- T1 weighted MRI: low to intermediate signal intensity
- T2 weighted MRI: heterogeneous but predominantly high signal
Radiology images
Prognostic factors
- Presence of metastases main prognostic factor
- Localized, resectable disease with 5 year survival rate of 70%
- Metastatic disease with 5 year survival rate of < 30%
- Surgical removal of resectable lung metastases improves survival
- Favorable: complete pathologic response to neoadjuvant chemotherapy, small tumor size, superficial location, easily resectable (Bone Joint J 2016;98-B:1138)
- Unfavorable: early relapse, presence of metastases, anatomic location in trunk / pelvis
Case reports
- 4 year old girl with primary skull base Ewing sarcoma (Am J Case Rep 2021;22:e930384)
- 17 year old girl with primary superficial Ewing sarcoma of the left thigh (J Cutan Pathol 2020;47:970)
- 20 year old man with epiphyseal Ewing sarcoma involving medial femoral condyle (Radiol Case Rep 2021;16:1191)
- 26 year old woman with Ewing sarcoma of temporal bone with prominent myxoid stroma (Pathol Res Pract 2019;215:152665)
- 41 year old man with sinonasal adamantinoma-like Ewing sarcoma (Pathol Res Pract 2017;213:422)
Treatment
- Neoadjuvant chemotherapy and surgery
- Ewing specific protocol of alternating vincristine / doxorubicin / cyclophosphamide and ifosfamide / etoposide (VDC / IE)
- Chemotherapy improved prognosis to 75% for 5 year survival
- Radiotherapy for surgically inaccessible tumors, inadequate margins and palliation
- Limited efficacy of immunotherapeutic approaches (J Immunother Cancer 2020;8:e000653)
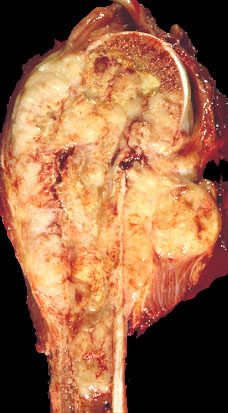
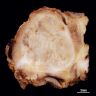
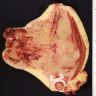
Gross description
- Gray-tan mass with infiltrative borders (Semin Diagn Pathol 2014;31:39)
- Intramedullary mass with soft tissue involvement
- Areas of hemorrhage and necrosis frequent
- Assess chemotherapy induced necrosis (≥ 90% good) with mapping
Gross images
Frozen section description
- Small round blue cell tumor
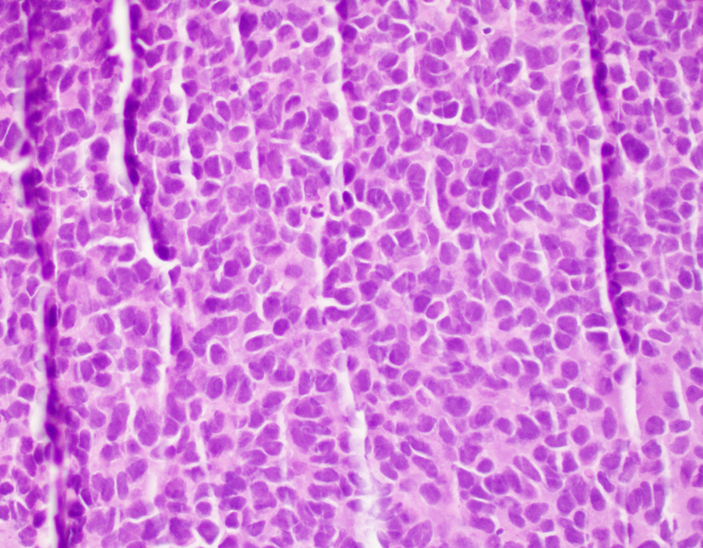
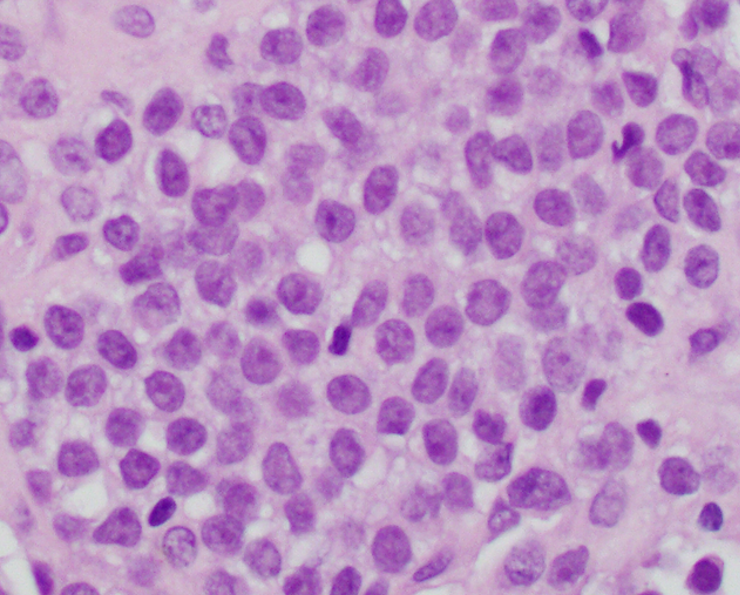
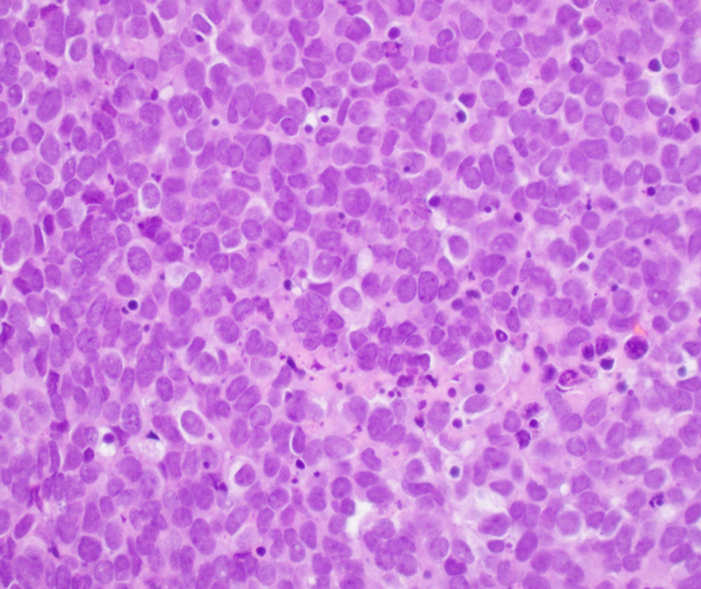
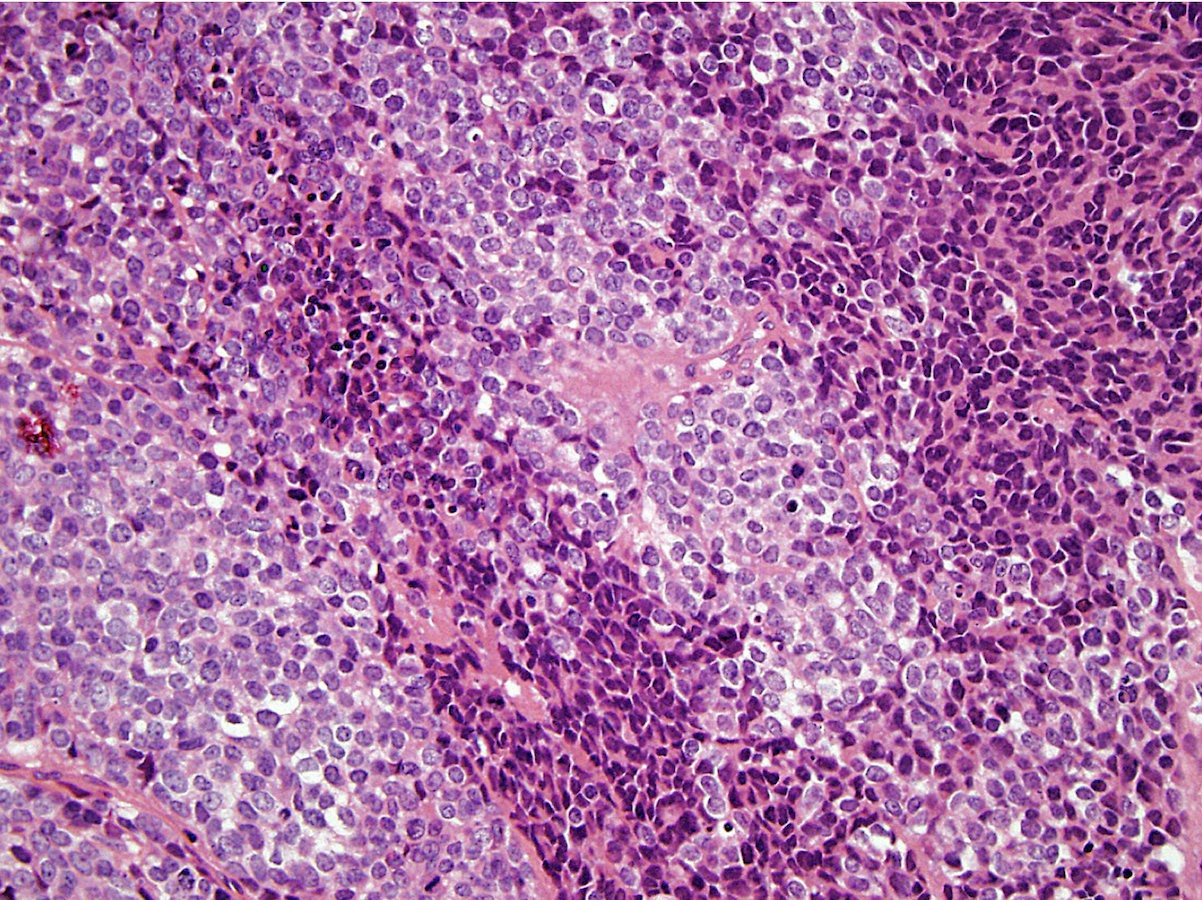
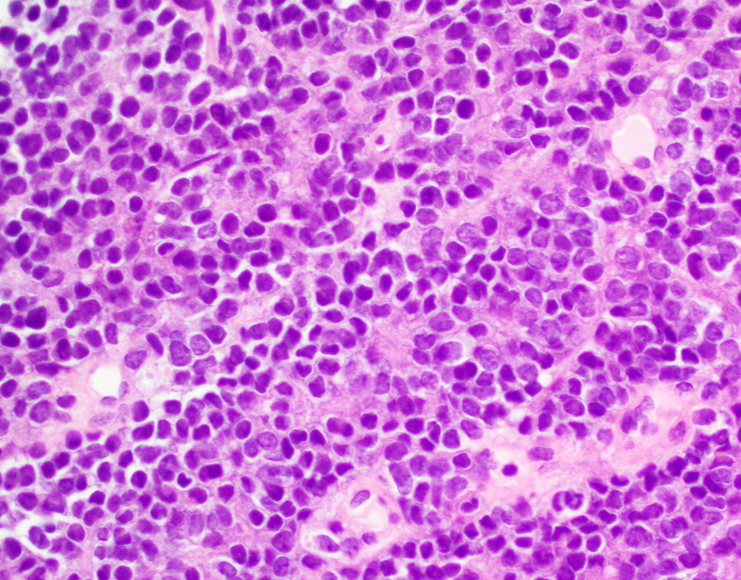
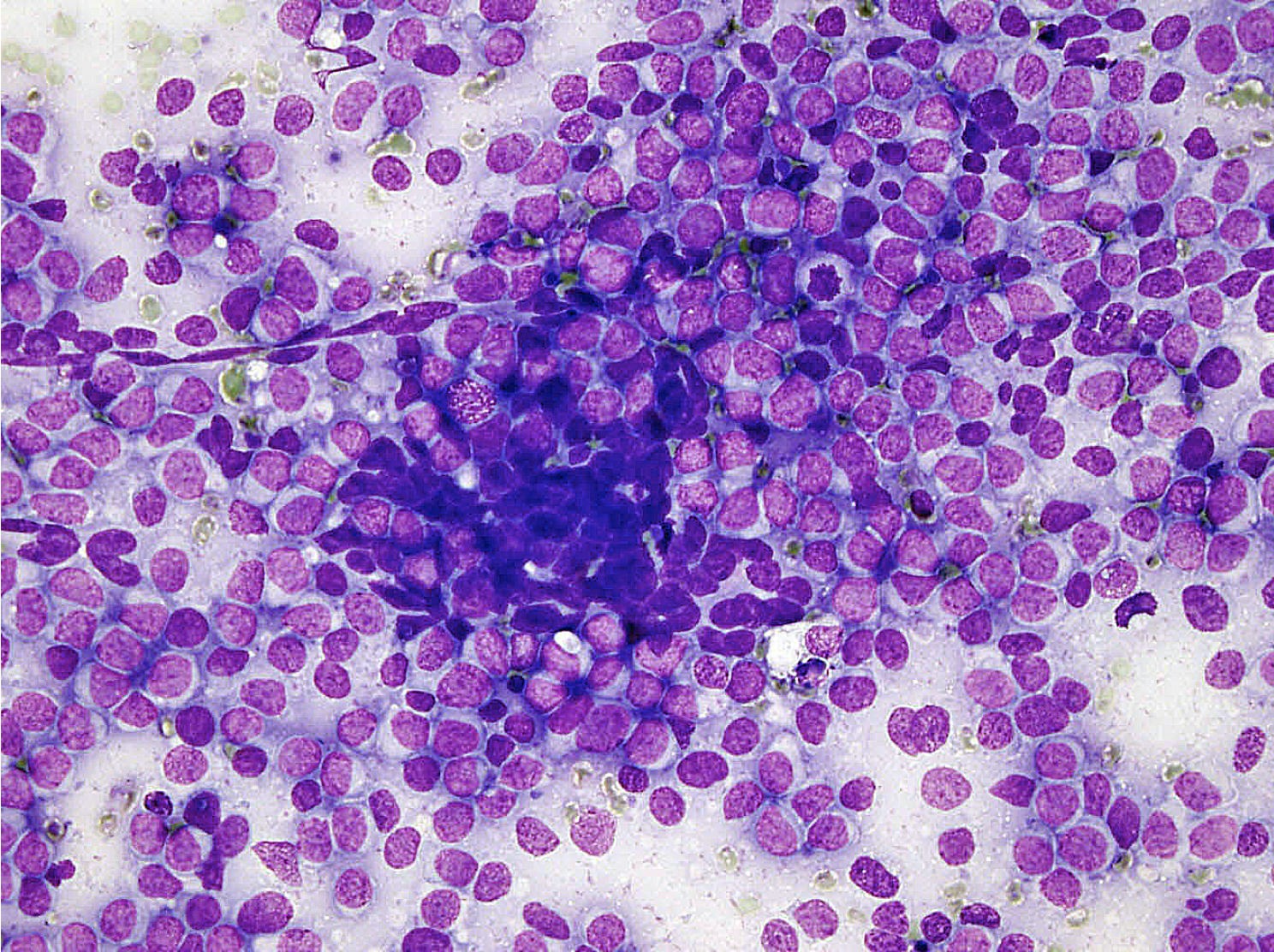
Microscopic (histologic) description
- Classical Ewing sarcoma (Virchows Arch 2009;455:397)
- Uniform small round cells
- Tumor cells 1 - 2x size of lymphocytes
- Round nuclei
- Finely stippled chromatin
- Inconspicuous nucleoli
- Scant clear to eosinophilic cytoplasm
- Indistinct cytoplasmic membranes
- Sheet-like growth pattern
- Islands separated by dense fibrous tissue
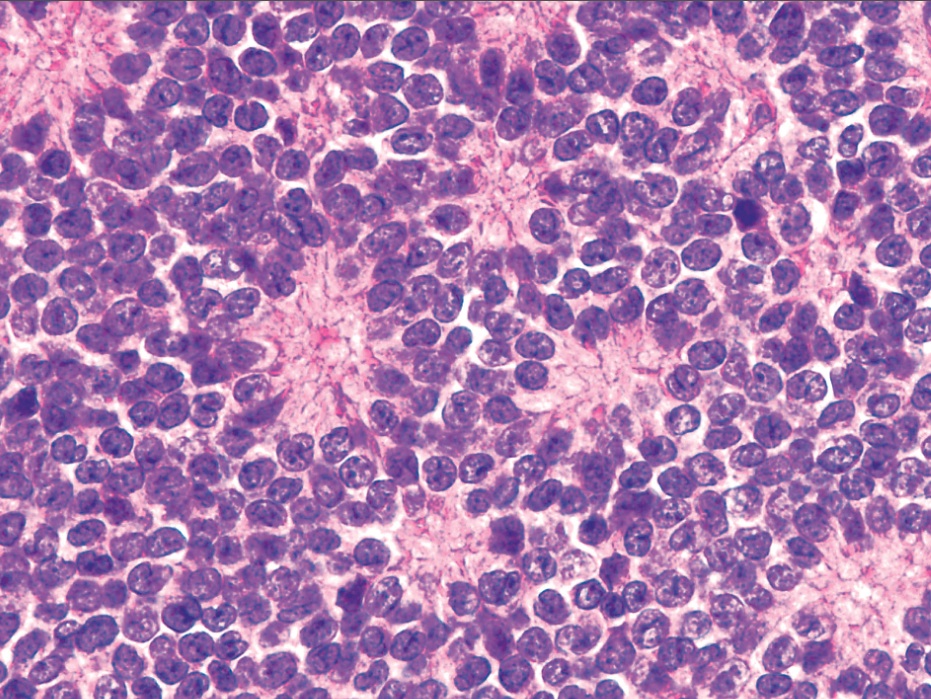
- Subset with neuroectodermal differentiation (Homer-Wright pseudorosettes)
- Atypical Ewing sarcoma (Virchows Arch 2011;458:281)
- Nuclear enlargement
- Irregular nuclear contours
- Vesicular or coarse chromatin
- Prominent nucleoli
- Adamantinoma-like Ewing sarcoma (Head Neck Pathol 2020;14:59, Am J Surg Pathol 1999;23:159, Am J Surg Pathol 2015;39:1267)
- Nests of basaloid cells
- Peripheral palisading and cording
- Prominent myxoid, fibromyxoid or hyalinized stroma
- Focal keratin pearl formation
- High grade features with minimal pleomorphism
Microscopic (histologic) images
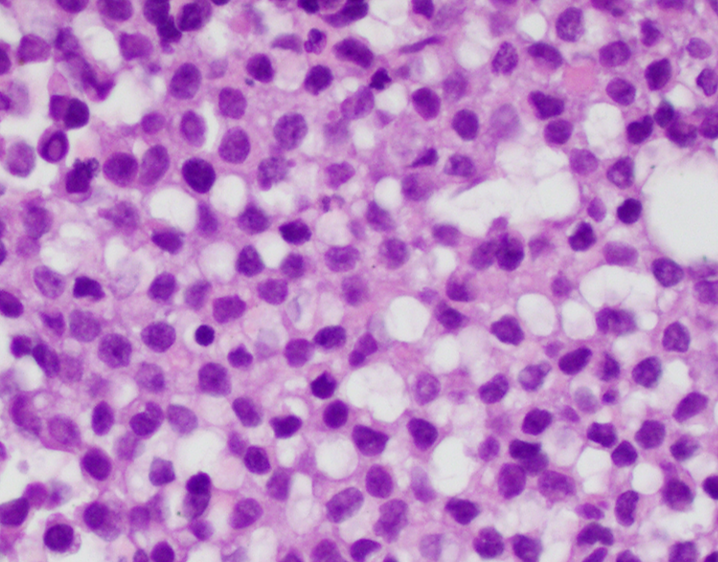
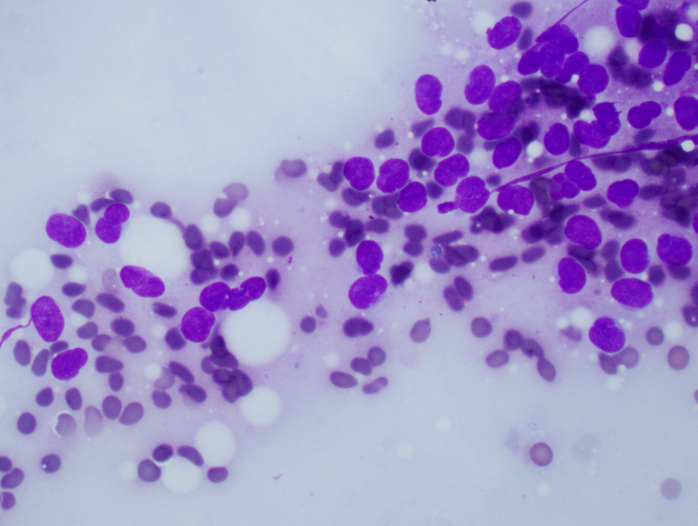
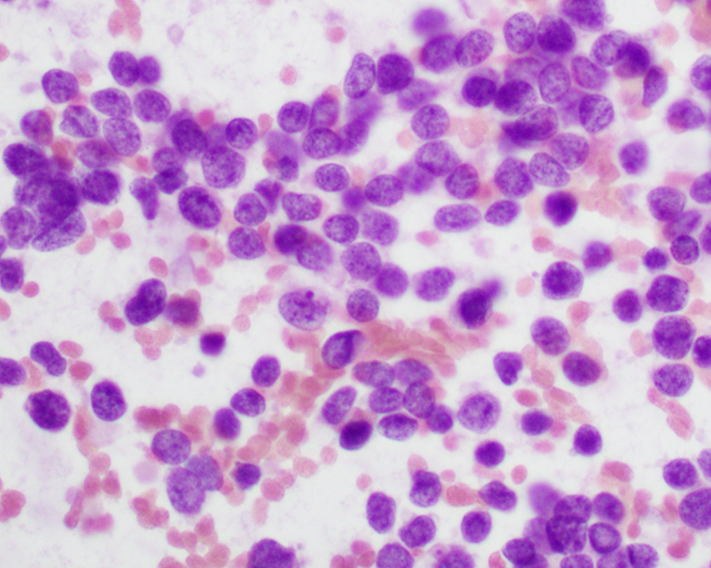
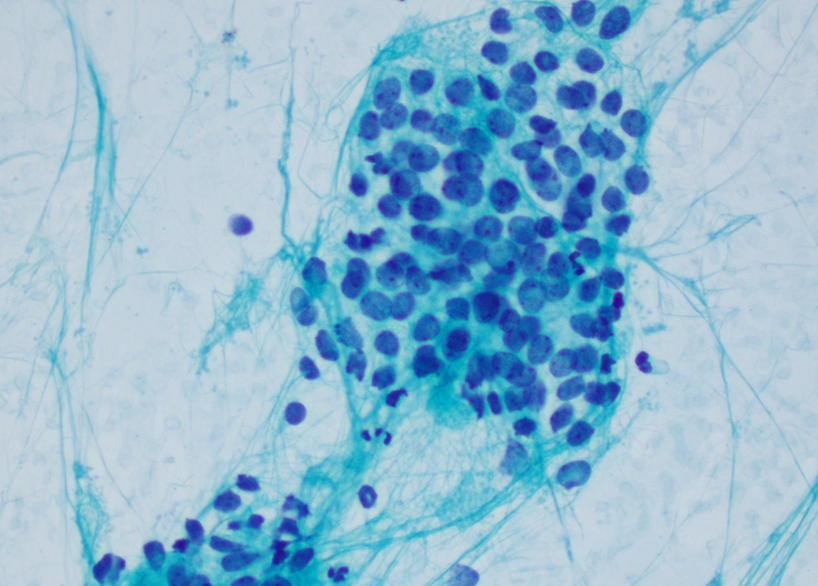
Cytology description
- Uniform small round cells (Diagn Cytopathol 2020;48:1098, Thorac Cancer 2016;7:602)
- Fine chromatin
- Occasional nucleoli
- Scant to indistinct cytoplasm
- Occasional rosette-like structures
Cytology images
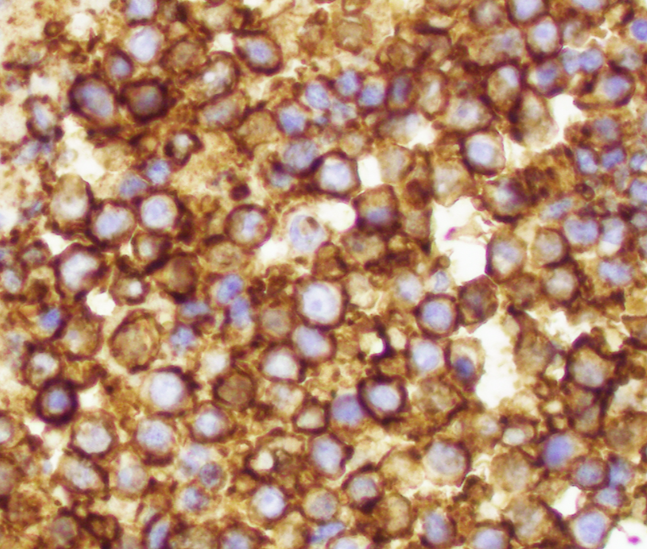
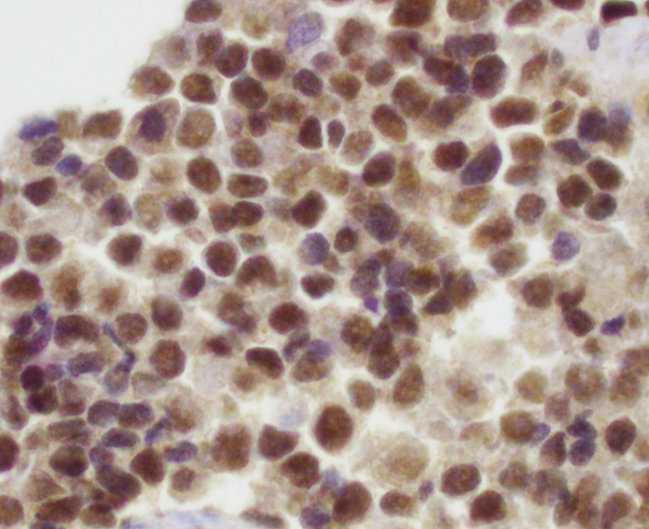
Positive stains
- CD99 (strong, diffuse membranous expression in ~90 - 95%) (Am J Surg Pathol 2005;29:1025)
- NKX2.2 (high specificity) (Am J Surg Pathol 2012;36:993)
- Vimentin (80 - 90%)
- FLI1 (nuclear staining with EWSR1-FLI1 fusion, ~90%) (Am J Surg Pathol 2000;24:1657, Appl Immunohistochem Mol Morphol 2001;9:255)
- ERG (nuclear staining with EWSR1-ERG fusion) (Mod Pathol 2012;25:1378)
- Cytokeratin (diffuse in adamantinoma-like variant)
- p63 or p40 (diffuse in adamantinoma-like variant)
- Perioidic acid-Schiff (PAS) stain (highlights cytoplasmic glycogen, diastase sensitive)
- NSE (~50%)
Negative stains
- LCA / CD45
- Desmin
- Myogenin
- MyoD1
- WT1
- ETV6
- CIC
- BCOR
- CCNB3
- SATB2
- SOX9
- NUT1
- TTF1
- Chromogranin
- TdT
- S100 protein (~30%)
- Synaptophysin (~30 - 40%)
- NCAM (CD56) (~30%)
- Cytokeratin (~25%, classical variant) (Am J Surg Pathol 2005;29:1025)
Molecular / cytogenetics description
- Most common translocation is t(11;22)(q24;q12), resulting in EWSR1-FLI1 fusion (~85 - 90%) (Cancer Genet 2011;204:351)
- Second most common is t(21;22)(q22;q12), resulting in EWSR1-ERG fusion (~5 - 10%) (Genes Chromosomes Cancer 2016;55:340)
- Rare fusions (< 1%) involve ETV1 (7p22), ETV4 (17q21) and FEV (2q35-36) (Adv Anat Pathol 2018;25:314)
- 2 rare translocations of ERG or FEV with FUS: t(16;21)(p11;q22) FUS-ERG and t(2;16)(q35;p11) FUS-FEV (Adv Anat Pathol 2018;25:314)
Molecular / cytogenetics images
Videos
Ewing family of tumors
Sample pathology report
- Bone, right proximal humerus mass, core biopsy:
- Ewing sarcoma (see comment)
- Comment: This biopsy shows an undifferentiated small round cell sarcoma involving bone with focal areas of necrosis and scattered mitotic figures. Immunohistochemical studies show that the tumor cells are positive for CD99 (diffuse, membranous) and NKX2.2, while they are negative for cytokeratin cocktail, S100 protein, SMA, desmin and CD45. RT-PCR demonstrates the presence of an EWSR1-FLI1 fusion, confirming the above diagnosis.
Differential diagnosis
- Mesenchymal chondrosarcoma:
- Areas with hyaline cartilage differentiation
- Hemangiopericytoma-like vasculature
- HEY1-NCOA2 gene fusion
- Metastatic tumors with small round cell morphology, including neuroblastoma:
- CD99 negative or focal / patchy
- N-MYC amplified (neuroblastoma)
- Alveolar rhabdomyosarcoma:
- Primary bone lymphoma:
- Small cell osteosarcoma:
- Tumor osteoid / matrix present
- SATB2 positive
- Poorly differentiated synovial sarcoma:
- Cytokeratin variably positive
- Biphasic type has gland-like epithelial structures
- SS18-SSX gene fusion
- CIC rearranged sarcomas:
- CIC gene rearrangement, often CIC-DUX4
- CD99 patchy positive
- NKX2.2 negative
- ETV4 and WT1 positive (Mod Pathol 2016;29:1324)
- BCOR rearranged sarcomas:
- BCOR genetic alterations
- Round to spindle cell morphology
- Variable myxoid stromal change
- Round cell sarcoma with EWSR1-non-ETS fusion:
- EWSR1-NFATC2 and FUS-NFATC2 gene fusions
- EWSR1-PATZ1 gene fusion
- Desmoplastic small round cell tumor (DSRCT):
- Basaloid squamous cell carcinoma (adamantinoma-like variant):
- CD99 negative
- Lacks EWSR1 gene rearrangement
- High grade neuroendocrine carcinoma (adamantinoma-like variant):
- Synaptophysin and chromogranin positive
- CD99 negative
- Lacks EWSR1 gene rearrangement
Additional references
- WHO Classification of Tumours Editorial Board: Soft Tissue and Bone Tumours, 5th Edition, 2020, Greenspan: Radiology and Pathology Correlation of Bone Tumors - A Quick Reference and Review, 1st Edition, 2015, Nielsen: Diagnostic Pathology - Bone, 3rd Edition, 2021, Czerniak: Dorfman and Czerniak’s Bone Tumors, 2nd Edition, 2015
Board review style question #1
Board review style answer #1
Board review style question #2
A 12 year old boy injured his left arm while playing football. Radiograph demonstrated an aggressive lesion in the mid diaphyseal region of his left humerus with permeative appearance and lamellated periosteal reaction (onion skin). Biopsy shows a small round blue cell tumor. What is the most likely diagnosis?
- Chondrosarcoma
- Ewing sarcoma
- Lymphoma
- Osteosarcoma
Board review style answer #2