Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Cite this page: Slack JC, Warmke L. Arteriovenous malformation. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/softtissuearteriovenousmalformation.html. Accessed April 3rd, 2025.
Definition / general
- Arteriovenous malformation (AVM) is a fast flow vascular anomaly characterized by abnormal arteriovenous communications and malformed arteries, veins and capillaries; these are usually congenital but may be acquired later in life
- Subtypes include sporadic AVM, AVM occurring in Parkes Weber syndrome (PWS), AVM occurring in capillary malformation - arteriovenous malformation (CM AVM) and AVM occurring in hereditary hemorrhagic telangiectasia (HHT)
Essential features
- Essential to demonstrate malformed large vascular channels with an increased small vessel component in correlation with clinical and imaging features
- Clinically, the lesions are classically red, warm and pulsatile
- AVMs are usually sporadic but can be seen in several syndromic settings
- Histology demonstrates an admixture of malformed arteries, thickened veins and capillaries
Terminology
- Arteriovenous hemangioma (no longer recommended by International Society for the Study of Vascular Anomalies)
- Intramuscular hemangioma, Enzinger type (intramuscular fast flow vascular anomaly is now considered a subtype of AVM)
ICD coding
- ICD-O
- 9123/0 - arteriovenous malformation
- 9123/0 - sporadic arteriovenous malformation
- 9123/0 - arteriovenous malformation occurring in Parkes Weber syndrome
- 9123/0 - arteriovenous malformation occurring in capillary malformation - arteriovenous malformation
- 9123/0 - arteriovenous malformation occurring in hereditary hemorrhagic telangiectasia
- ICD-11: LA90.3Z - peripheral arteriovenous malformations, unspecified
Epidemiology
- Most AVMs are congenital lesions and are recognized at birth (Angiogenesis 2019;22:547, Circulation 2017;136:1037)
- Some do not become apparent until later in life
- They are rare, affecting < 1% of population
- M = F
- Syndrome associated AVMs, such as those in hereditary hemorrhagic telangiectasia, may not become apparent for several decades (Circulation 2017;136:1037)
- Syndromes associated with AVMs include Parkes Weber syndrome, capillary malformation - arteriovenous malformation 1 and 2 and hereditary hemorrhagic telangiectasia
Sites
- Sporadic AVM can involve any site in the body, although seem to be most common in the head and neck (Semin Pediatr Surg 2014;23:203)
- Those occurring in the setting of Parkes Weber syndrome almost invariably involve a lower extremity
- Multiple small AVMs are often seen in the setting of hereditary hemorrhagic telangiectasia
- Hereditary hemorrhagic telangiectasia may present with mucocutaneous telangiectasias as well as AVMs involving the central nervous system, lungs and liver (J Clin Med 2020;9:2714)
Pathophysiology
- Abnormal RAS-MAPK and or PI3K-mTOR-AKT pathway signaling due to a mutation in 1 or more associated genes leads to abnormal vascular morphogenesis (Circulation 2017;136:1037, Vasc Med 2020;25:364)
Etiology
- Most sporadic AVMs, including intramuscular fast flow vascular anomaly, have somatic mutations in MAP2K1; less commonly, other genes involved (Angiogenesis 2019;22:547)
- AVMs associated with Parkes Weber syndrome and capillary malformation - arteriovenous malformation 1 generally have autosomal dominant mutations in RASA1; capillary malformation - arteriovenous malformation 2 has autosomal dominant mutations in EPHB4, while hereditary hemorrhagic telangiectasia is associated with mutations in a number of different genes (Eur J Hum Genet 2018;26:1521, Sci Rep 2023;13:11074, Genet Med 2019;21:2007, Circulation 2017;136:1037, Pediatr Rep 2023;15:129)
- Less commonly contains mutations in KRAS and HRAS (Clin Genet 2020;98:595, Hum Genet 2019;138:1419)
- Hereditary hemorrhagic telangiectasia has multiple subtypes classified according to genes involved: HHT1 (ENG), HHT2 (ACVRL1), HHT3 (5q31), HHT4 (7p14), HHT5 (GDF2) and juvenile polyposis / hereditary hemorrhagic telangiectasia syndrome (SMAD4) (J Clin Med 2020;9:2714)
Diagrams / tables
Table 1: Nomenclature for vascular anomalies
| Vascular tumors | Vascular malformations | |
|
Benign Locally aggressive or borderline Malignant | Simple | Combined |
| Capillary malformations Lymphatic malformations Venous malformations Arteriovenous malformations Arteriovenous fistula | Combination of capillary, venous, lymphatic or arterial vessels; for example
| |
Clinical features
- Clinical features depend on duration, anatomic location and size of the lesion
- Palpable pulsation or thrill may be felt overlying the lesion with or without an audible bruit
- Patients frequently present with a warm, red lesion that may be associated with pain, ulceration and bleeding
- Lesions grow proportionately with individual and do not spontaneously regress
- Parkes Weber syndrome can show an overgrown extremity with diffuse capillary stain
Diagnosis
- Per WHO, it is essential to demonstrate malformed large vascular channels with an increased small vessel component in correlation with clinical and imaging features
- Demonstration of somatic MAP2K1 (or other) mutations is desirable
Radiology description
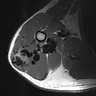
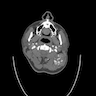
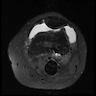
- Arteriovenous malformations are fast flow vascular malformations characterized by abnormal connections between feeding arteries and draining veins (Semin Pediatr Surg 2014;23:203)
- Absence of a well defined / solid mass
- Ultrasound can differentiate a high flow (fast flow) from a low flow (slow flow) lesion
- Ultrasound shows that AVM has a higher mean venous peak velocity than hemangiomas or other vascular malformations (Radiology 2000;214:747)
- MRI usually shows major feeding and draining vessels (Radiology 1986;158:475)
- CT is usually less informative than MRI
Radiology images
Prognostic factors
- Clinical significance largely predicated by degree of arteriovenous shunting
- Syndrome associated AVMs have unique clinical associations dictated by the specific syndrome (hereditary hemorrhagic telangiectasia, Parkes Weber syndrome, etc.)
- Schobinger clinical staging can be used (Plast Reconstr Surg 1998;102:643)
- Significant arteriovenous shunting can lead to cardiac failure (Indian Dermatol Online J 2020;11:367)
Case reports
- Newborn girl with AVM and Parkes Weber syndrome (Acta Med Litu 2020;27:90)
- 3 day old girl with capillary malformation - arteriovenous malformation with autosomal dominant family history (Cureus 2021;13:e12562)
- 11 year old girl with AVM secondary to a novel somatic HRAS mutation (Hum Genet 2019;138:1419)
- 60 year old man with pulmonary AVM and hereditary hemorrhagic telangiectasia (Cureus 2022;14:e32365)
- 4 generations of a family with capillary malformation - arteriovenous malformation 2 syndrome with confirmed alterations in EPHB4 (Acta Derm Venereol 2022;102:adv00662)
Treatment
- Asymptomatic lesions can be observed
- Compression garments can reduce swelling
- Transarterial catheter based embolization
- Surgical resection (Semin Pediatr Surg 2014;23:203)
- Transcutaneous sclerotherapy
- MEK1 inhibitor
Gross description
- Nonencapsulated
- Enlarged tortuous vascular channels admixed with native tissue
- Red-brown
- Typically firmer than venous malformations
Gross images
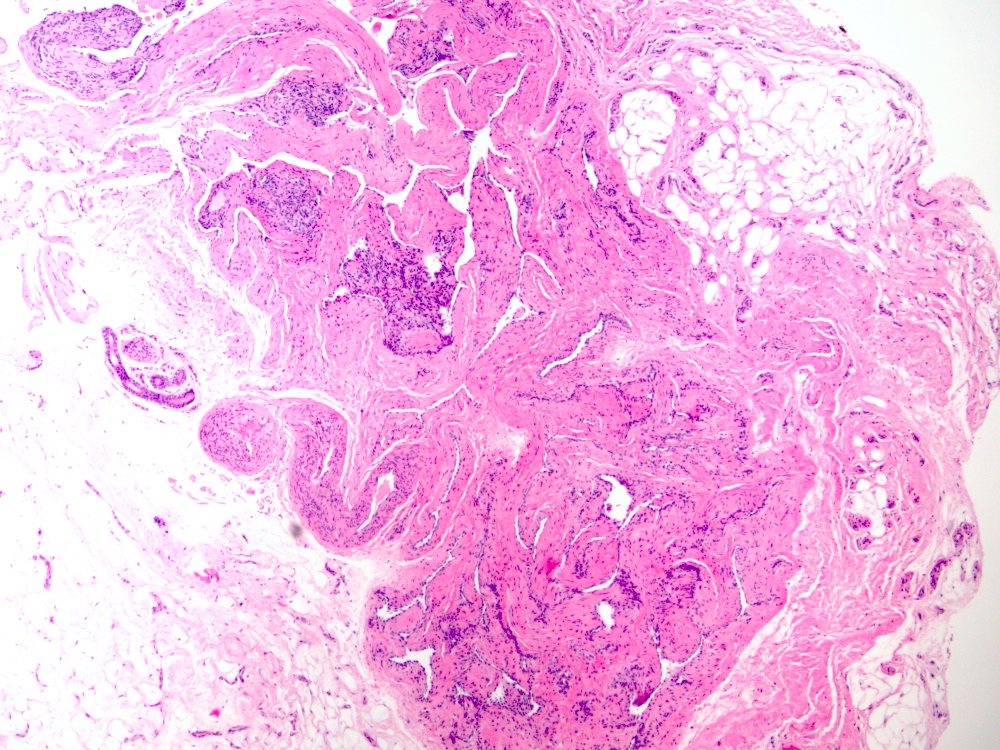
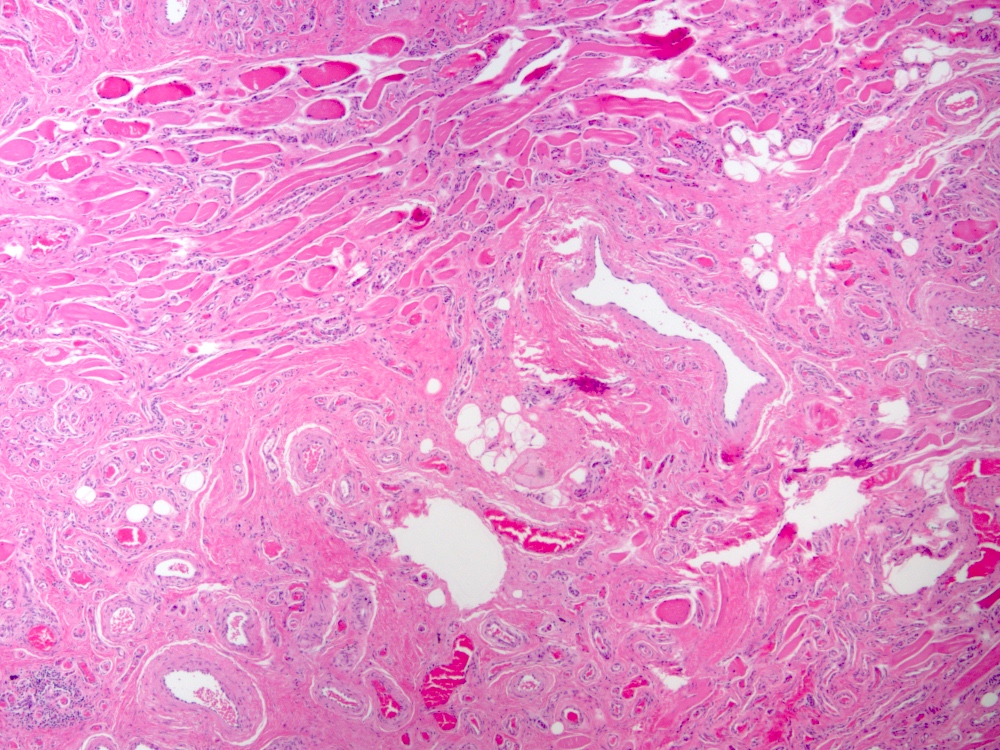
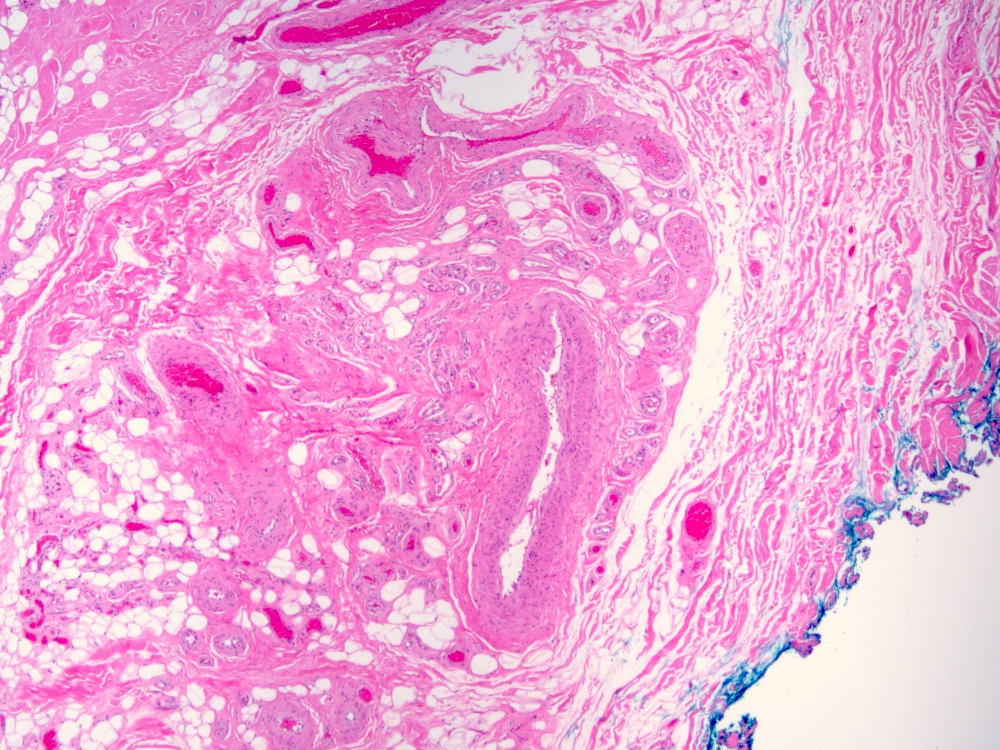
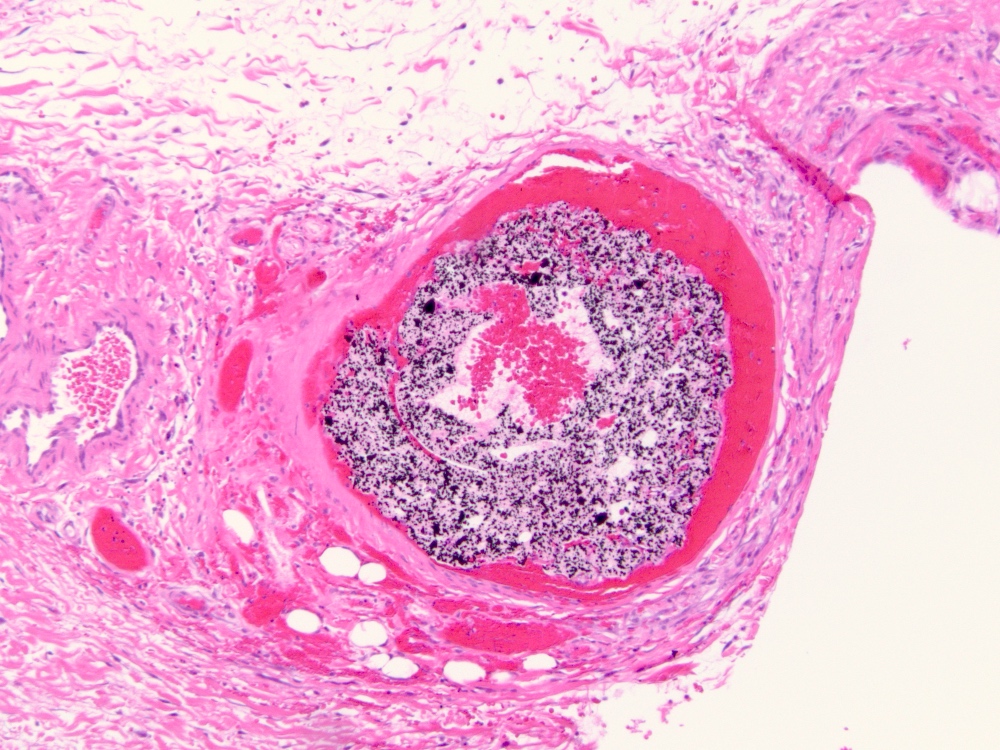
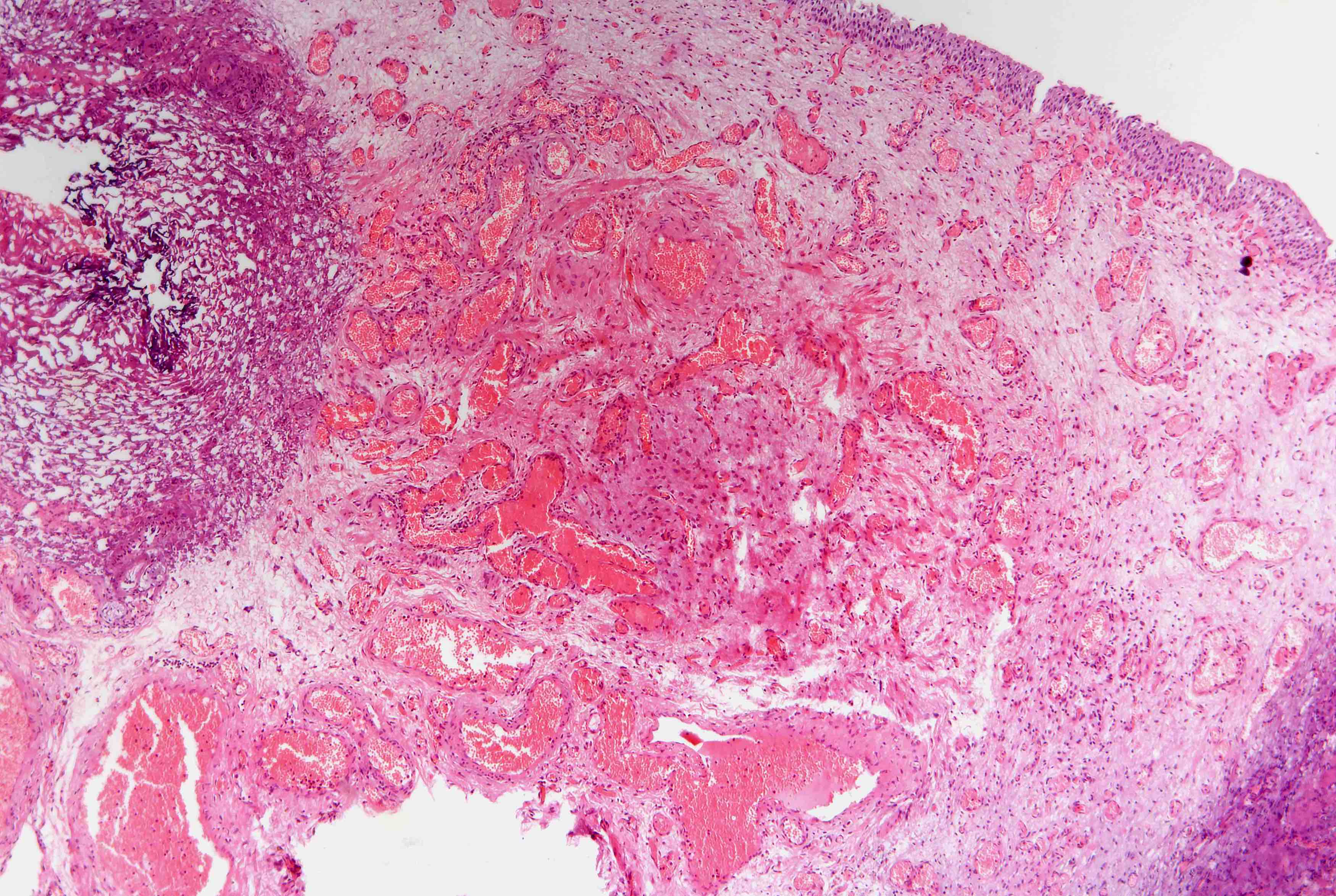
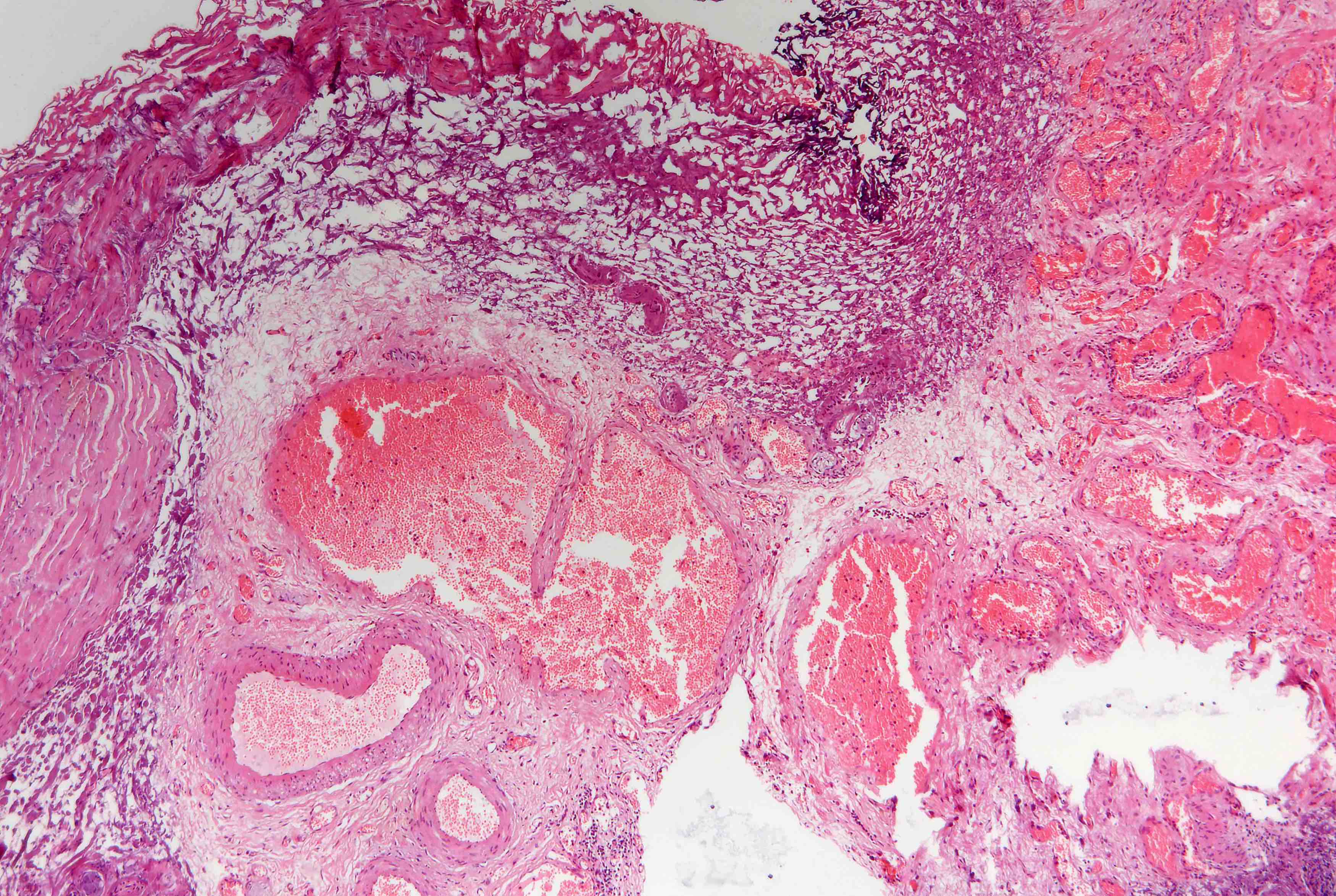
Microscopic (histologic) description
- Arteriovenous (AV) shunts are rarely demonstrated histologically as they generally require many serial sections
- AVMs vary in appearance
- Contain large tortuous thick walled arteries and veins, along with a small vessel component
- Small vessel component often has a capillary-like appearance
- Arteries may show focal loss of internal elastic lamina
- Veins may show thickened walls
Microscopic (histologic) images
Positive stains
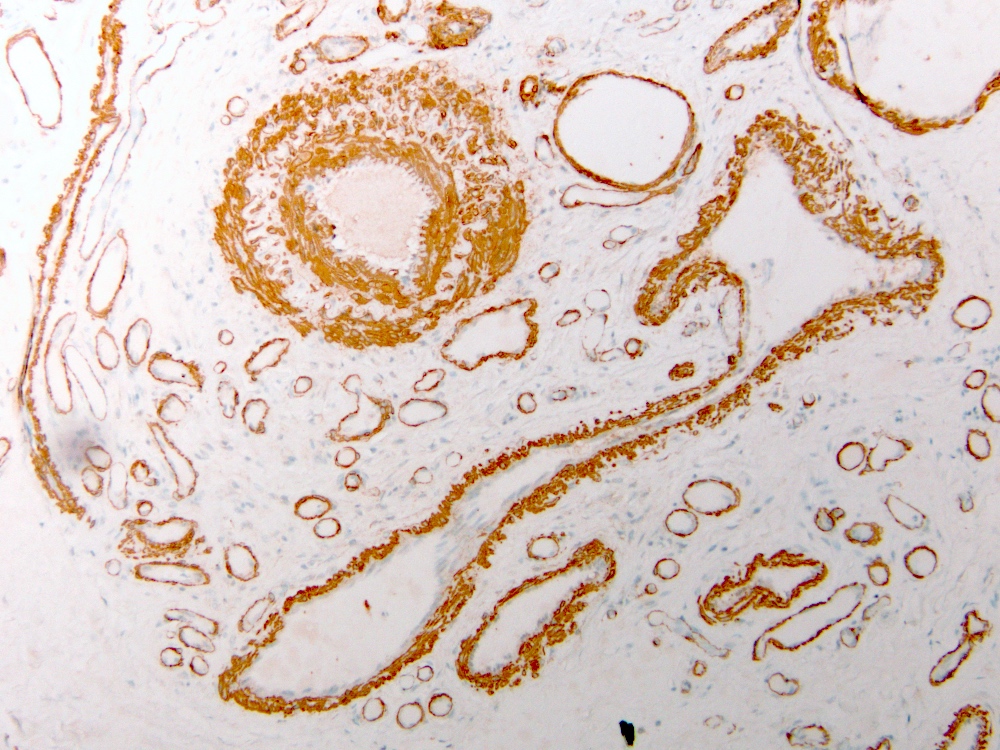
- Elastin stain (VVG) can highlight the internal elastic lamina in the arterial component
- ERG and CD31 are positive in endothelial cells
- SMA highlights abnormal muscularization of vascular channels
Negative stains
Molecular / cytogenetics description
- AVM has somatic MAP2K1, KRAS, HRAS or BRAF mutations
- Parkes Weber syndrome and capillary malformation - arteriovenous malformation 1 / 2 have mutations in RASA1 or EPHB4
- Hereditary hemorrhagic telangiectasia has germline mutations in ENG, ACVRL1, GDF2 or SMAD4 or genomic involvement of 5q31 or 7p14
Sample pathology report
- Soft tissue, left scalp, excision:
- Vascular anomaly, consistent with arteriovenous malformation in the appropriate clinical setting (see comment)
- Comment: The examined soft tissues are involved by an infiltrative vascular anomaly consisting of a haphazard proliferation of malformed arteries, veins, capillaries and indeterminate channels. In light of the radiologic findings of a fast flow vascular anomaly, the findings are consistent with the clinical impression of arteriovenous malformation. If clinically indicated, ancillary molecular studies could be pursued on remaining formalin fixed paraffin embedded tissue.
Differential diagnosis
- Pyogenic granuloma (lobular capillary hemangioma):
- Not present at birth
- No abnormal arteriovenous shunts
- Admixed inflammatory infiltrate may be striking
- Usually superficial and small
- Venous malformation:
- Also present at birth
- No abnormal arteriovenous shunts
- Cold, pale blue, nonpulsatile
- Slow flow on imaging studies
- Most common vascular malformation
- Lacks arterial component
- Associated with TEK / TIE2 and PIK3CA mutations
- Treated with sclerotherapy (first line treatment), surgery and mTOR inhibitor
- Glomuvenous malformation:
- Large, dilated thin walled veins
- Presence of glomus cells in the wall of the malformation
- SMA highlights the round perivascular glomus cells
- Slow flow lesion
- Verrucous venous malformation:
- Superficial vascular malformation
- Variable degree of hyperkeratosis
- Composed of capillary and veins in dermis and subcutaneous tissue
- Lymphatic malformation:
- Infantile hemangioma:
- Vascular tumors usually noticed in the first few days of life
- Proliferating and involuting phase
- Positive for GLUT1
- Lobules of vessels separated by normal tissue
- First line treatment with propranolol
- Congenital hemangioma:
- Benign vascular tumor fully grown at birth
- Somatic mutations involving GNAQ and GNAS
- Include rapidly involuting congenital hemangioma (RICH), noninvoluting congenital hemangioma (NICH), partially involuting congenital hemangioma (PICH) and RICH with fetal involution
- Lobules of vessels separated by abnormal dense fibrous tissue
- Negative for GLUT1
Additional references
Board review style question #1
Board review style answer #1
A. Arteriovenous malformation. The image shows an arteriovenous malformation (AVM) composed of an admixture of abnormal arteries, veins and small vessels. The clinical history of a red, warm, pulsatile congenital lesion is consistent with AVM. Answer C is incorrect because pyogenic granulomas lack abnormal arteriovenous connections, are not typically congenital and are nonpulsatile. Answer D is incorrect because venous malformations are nonpulsatile, cool to touch, white-blue and lack an arterial component. Answer B is incorrect because congenital hemangiomas are typically well circumscribed, may involute rapidly after birth, are not warm or pulsatile and lack abnormal arteriovenous connections.
Comment Here
Reference: Arteriovenous malformation
Comment Here
Reference: Arteriovenous malformation