Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Molecular / cytogenetics description | Molecular / cytogenetics images | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Warmke L, Meis J. Alveolar soft part sarcoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/softtissuealveolarsoft.html. Accessed March 30th, 2025.
Definition / general
- Alveolar soft part sarcoma (ASPS) is a rare sarcoma of uncertain histogenesis
- First formally described by Christopherson et al. in 1952 (Cancer 1952;5:100)
- Characterized by a specific translocation, der(17)t(X;17)(p11.2;q25), resulting in ASPSCR1-TFE3 gene fusion
Essential features
- Rare malignant mesenchymal neoplasm frequently composed of large, polygonal cells with abundant eosinophilic cytoplasm, a nested or pseudoalveolar growth pattern and PASD+ intracytoplasmic rhomboid or rod shaped crystals
- Predominantly affects the deep soft tissues of the extremities (thigh and buttock) in young adults and head and neck region (tongue and orbit) in children
- Characterized by ASPSCR1-TFE3 gene fusion
ICD coding
- ICD-O: 9581/3 - alveolar soft part sarcoma
- ICD-11: 2B5F.2 & XH8V95 - sarcoma, not elsewhere classified of other specified sites & alveolar soft part sarcoma
Epidemiology
- Accounts for < 1% of all soft tissue sarcomas
- Patient age range is 1 - 78 years (median: 25 years) (J Surg Oncol 2016;113:581)
- Most commonly seen in patients aged 15 - 35 years; 72% were < 30 years (J Surg Oncol 2016;113:581)
- Female predominance is well documented, with approximately 58% of patients being female (Cancer 1952;5:100, Cancer 1989;63:1, Oncology 2003;65:7, J Surg Oncol 2016;113:581)
- Female predominance is less pronounced in older patients (> 30 years) and children (Cancer 2001;91:585, Med Pediatr Oncol 1996;26:81, Histopathology 2004;45:526)
Sites
- Commonly involves deep soft tissues of the extremities (61%; predominantly the lower extremity [51%]), trunk (20%), internal organs (8%) and head and neck (9%) (J Surg Oncol 2016;113:581)
- In adults, deep soft tissue of the thigh and buttock is common
- In children, there is a predilection for the head and neck region, especially the tongue and orbit (Hum Pathol 1982;13:569, Histopathology 2004;45:526)
- Uncommon locations include:
- Female genital tract (Int J Gynecol Pathol 1995;14:283, Am J Surg Pathol 2017;41:622)
- Bone (Histopathology 1999;35:411)
- Breast (Int J Surg Pathol 2005;13:81)
- Heart (Pediatr Dev Pathol 2008;11:142)
- Larynx (Head Neck 2008;30:1257)
- Urinary bladder (Am J Surg Pathol 2006;30:1322)
- Mediastinum (Histopathology 1997;31:469)
- Spine (World J Surg Oncol 2017;15:39)
Pathophysiology
- ASPSCR1-TFE3 fusion protein localizes to the nucleus, functioning as an aberrant transcription factor, resulting in c MET overexpression and activation of c MET signaling, making the tumor cells sensitive to c MET inhibition (Cancer Res 2007;67:919, J Pathol 2013;229:743, PLoS One 2017;12:e0185321)
- Mouse model demonstrated the dependance of ASPS on lactate for growth, which may relate to its common occurrence in muscle with the expression of lactate transporter, monocarboxylate transporter (MCT1) protein and CD147 (Cancer Cell 2014;26:851, Am J Pathol 2002;160:1215)
- Defined by a specific genetic alteration, der(17)t(X;17)(p11;q25) that results in the fusion of the TFE3 transcription factor gene (from Xp11) with ASPSCR1 at 17q25.3 (Oncogene 2001;20:48)
Etiology
- ASPSCR1-TFE3 translocation is an instigating genetic event (Oncogene 2001;20:48)
- No association with radiation or a cancer predisposition syndrome has been reported
Clinical features
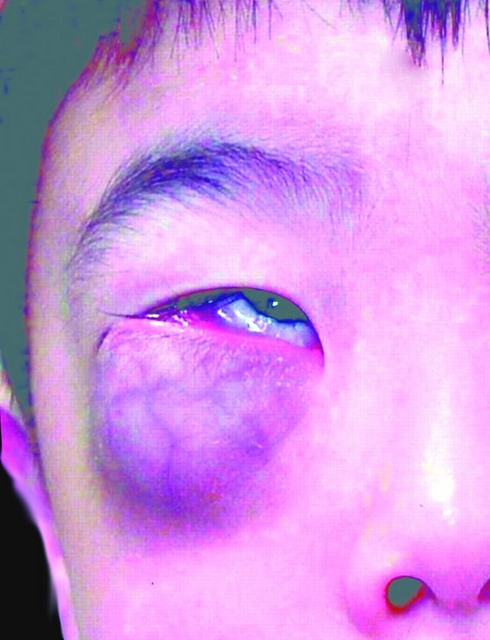
- Presents as a slow growing, painless mass (Clin Orthop Surg 2014;6:80)
- Proptosis can be seen with orbital tumors
- Vaginal bleeding can be seen with female genital tract tumors
- At presentation, the tumor can be localized (38%), regional (11%) and metastatic (43%) (J Surg Oncol 2016;113:581)
Diagnosis
- Eosinophilic polygonal cells with a nested growth pattern, rich capillary network and intracytoplasmic rod shaped crystals
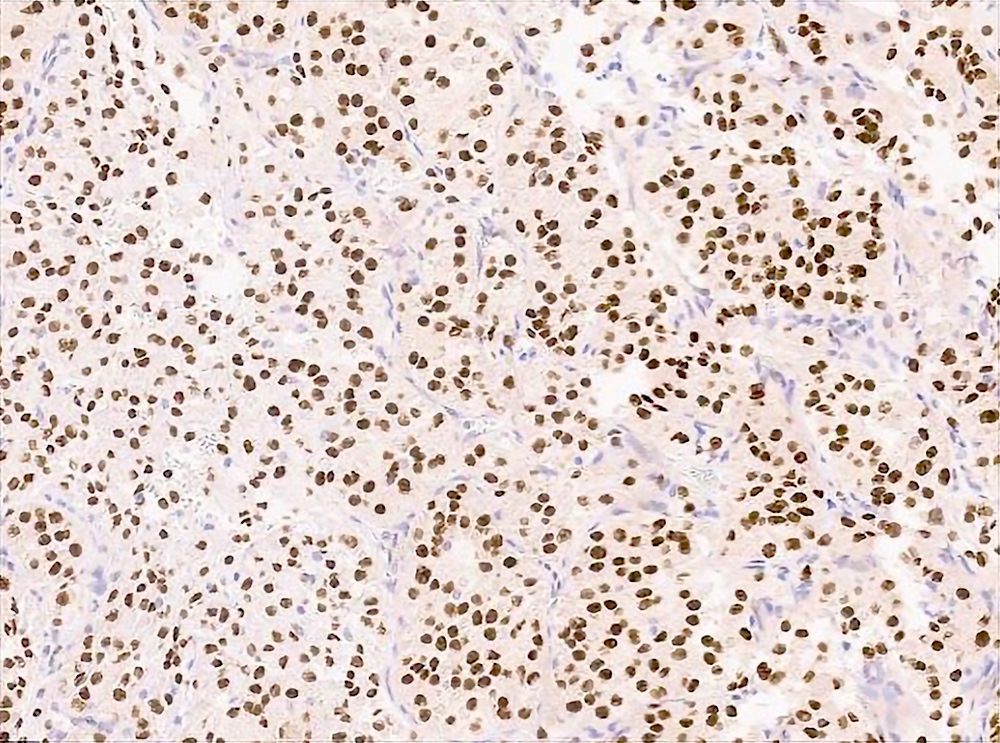
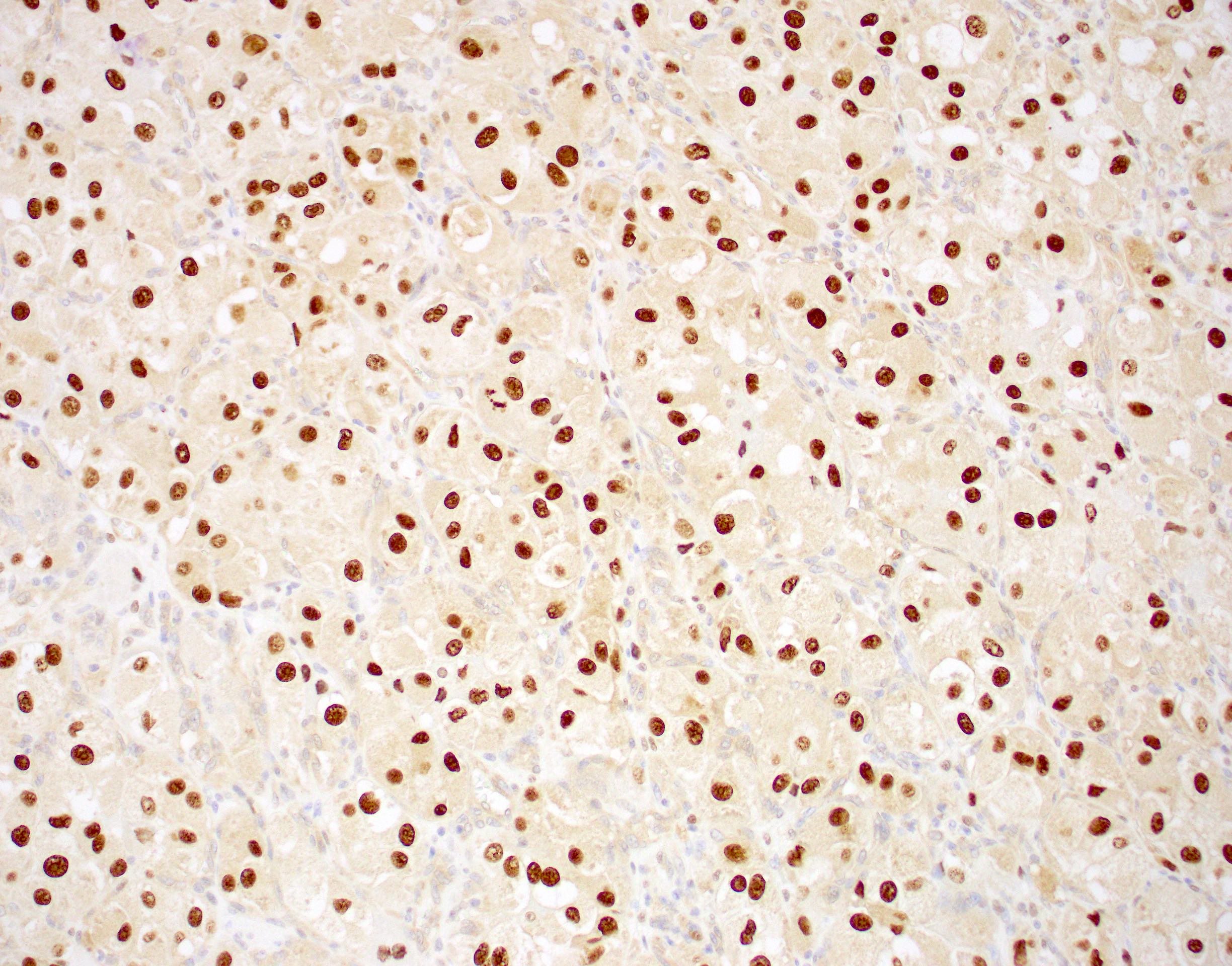
- TFE3 nuclear expression by immunohistochemistry
- Confirmation of TFE3 gene rearrangement or ASPSCR1-TFE3 gene fusion (Arch Pathol Lab Med 2015;139:1459)
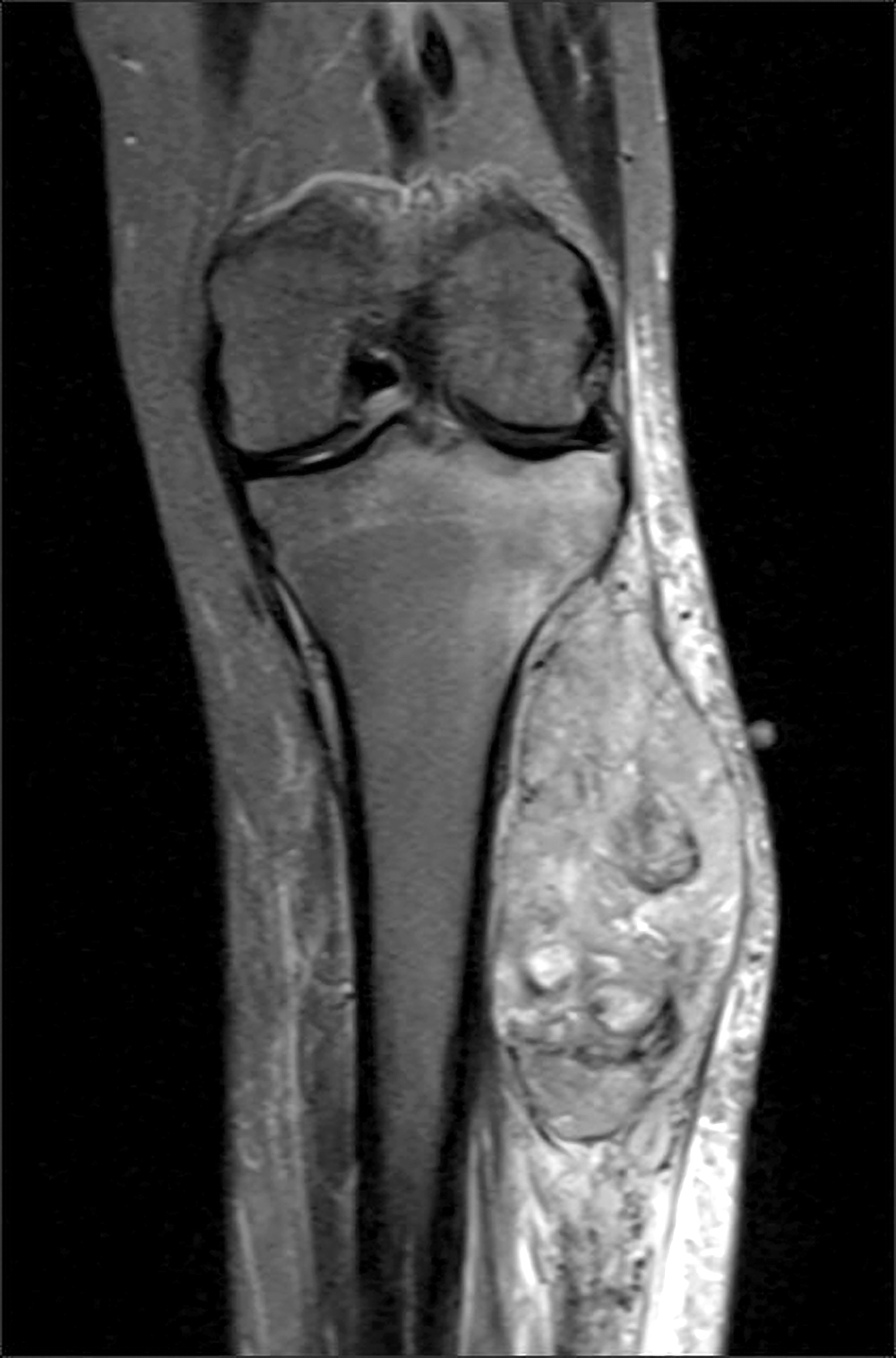
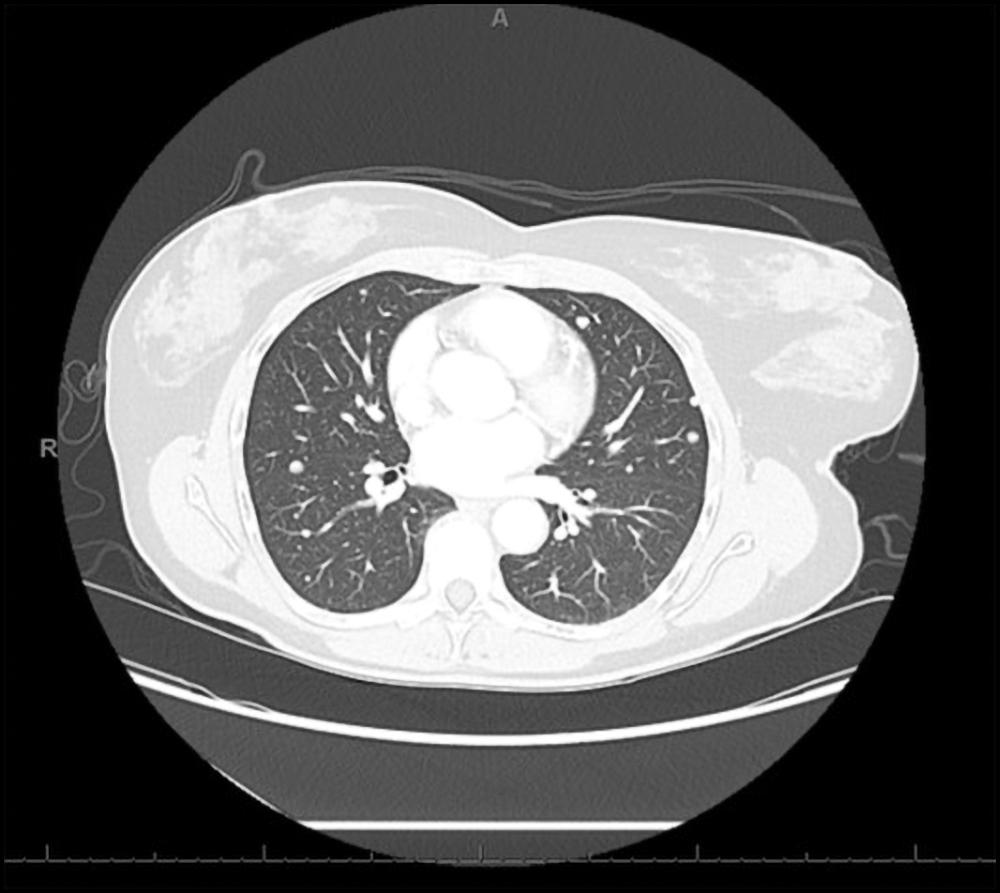
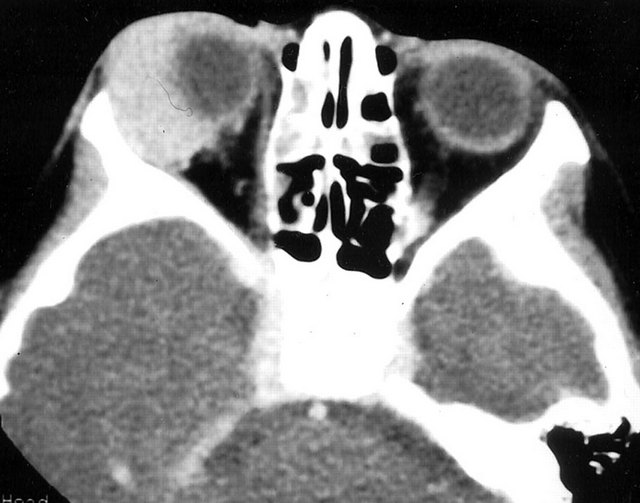
Radiology description
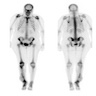
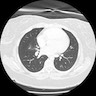
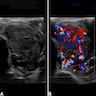
- CT scan and angiography of the tumor reveal its hypervascularity, prominent draining veins and prolonged capillary staining (Arch Pathol Lab Med 2007;131:488)
- MRI shows characteristic vascular pattern and moderate to intense postcontrast enhancement (Oncol Res Treat 2017;40:580, AJR Am J Roentgenol 2014;203:1345)
- ASPS can mimic an arteriovenous malformation on angiography (Arch Pathol Lab Med 2007;131:488)
Radiology images
Prognostic factors
- ASPS is not formally graded but considered to be high grade
- Initial course may seem indolent but overall prognosis is poor
- Local recurrence is 11 - 50% (J Surg Oncol 2016;113:581)
- Metastases often occur > 10 years after diagnosis and involve the lungs, liver, bone, brain and (rarely) lymph nodes (Pediatr Blood Cancer 2018;65:e26953, Ann Surg Oncol 2010;17:3229, Cancer 2001;91:585, Cancer 1989;63:1)
- Metastatic involvement of the brain is more common in ASPS than any other sarcoma
- Overall survival is 82% at 2 years and 56% at 5 years (J Surg Oncol 2016;113:581)
- One series reported a 5 year disease free survival of 71% in patients presenting with localized disease, compared to only 20% in patients presenting with metastasis (Cancer 2001;91:585)
- Positive prognostic factors: small tumor size (< 5 cm), absence of metastases at presentation, younger age at diagnosis (< 10 years), early detection at a completely resectable stage (Med Pediatr Oncol 1996;26:81, Histopathology 2004;45:526)
- Negative prognostic factors: older age, tumor size > 10 cm, distant metastasis at diagnosis, primary site in the trunk (J Surg Oncol 2016;113:581)
Case reports
- 13 year old girl with ASPS of the tongue (Cureus 2020;12:e11506)
- 19 year old pregnant woman presents with ASPS with multiple brain and lung metastases at 38 weeks gestation (Medicine (Baltimore) 2017;96:e8790)
- 24 year old woman with ASPS of uterine corpus (Int J Gynecol Pathol 2021;40:272)
- 71 year old man with primary thyroid gland ASPS (Head Neck Pathol 2020;14:701)
Treatment
- Radical surgical resection is the treatment of choice
- Excision of lung and brain metastasis may prolong survival (Cancer 1989;63:1)
- Adjuvant chemotherapy does not seem to be effective
- Radiation may reduce the risk of local recurrence (Cancer 2001;91:585)
- Clinical trial of crizotinib (c MET inhibitor) showed disease stabilization without much tumor shrinkage (Ann Oncol 2018;29:758)
- Multiple pulmonary metastases in select patients have responded to interferon alfa 2a (Br J Cancer 2003;89:243, Med Pediatr Oncol 2001;37:482)
- Long term clinical follow up is mandatory given the risk of metastasis > 10 years after diagnosis
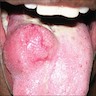
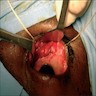
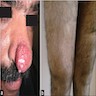
Clinical images
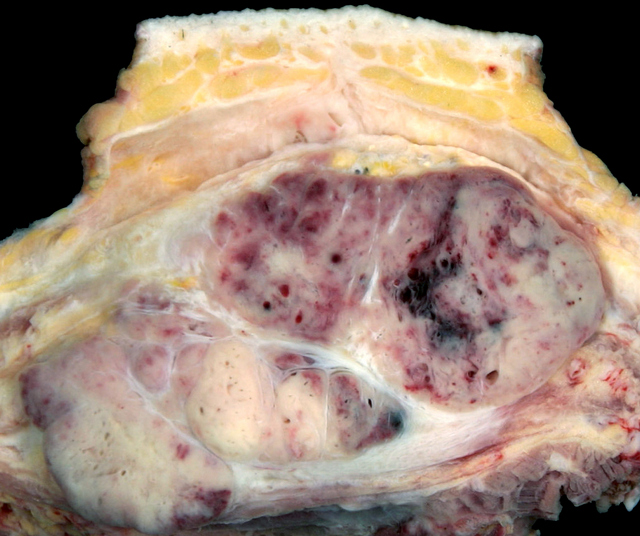
Gross description
- Solid, partially circumscribed mass with fleshy nodules and fibrotic bands
- Usually yellow to gray to white-tan
- Tumor size varies from 1.2 to 24 cm (median: 6.5 cm) (Cancer 2001;91:585)
- Frequently has large vessels at the periphery
Frozen section description
- Large polygonal cells with abundant eosinophilic cytoplasm
- Eccentric nuclei with prominent nucleoli
- Nests of tumor cells with surrounding vascular channels
- Scrape preparations can demonstrate cytomorphology (Cancer 2009;117:500)
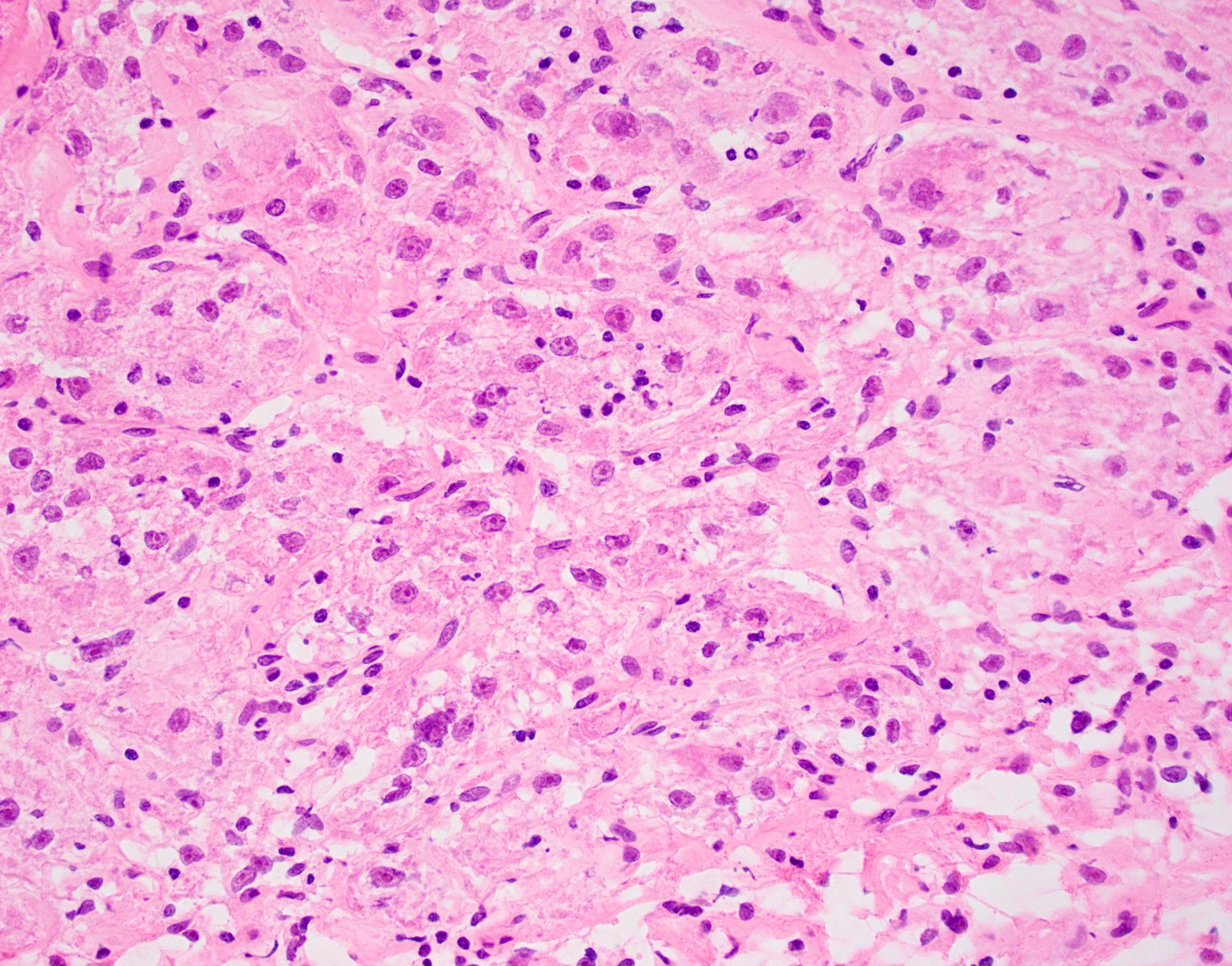
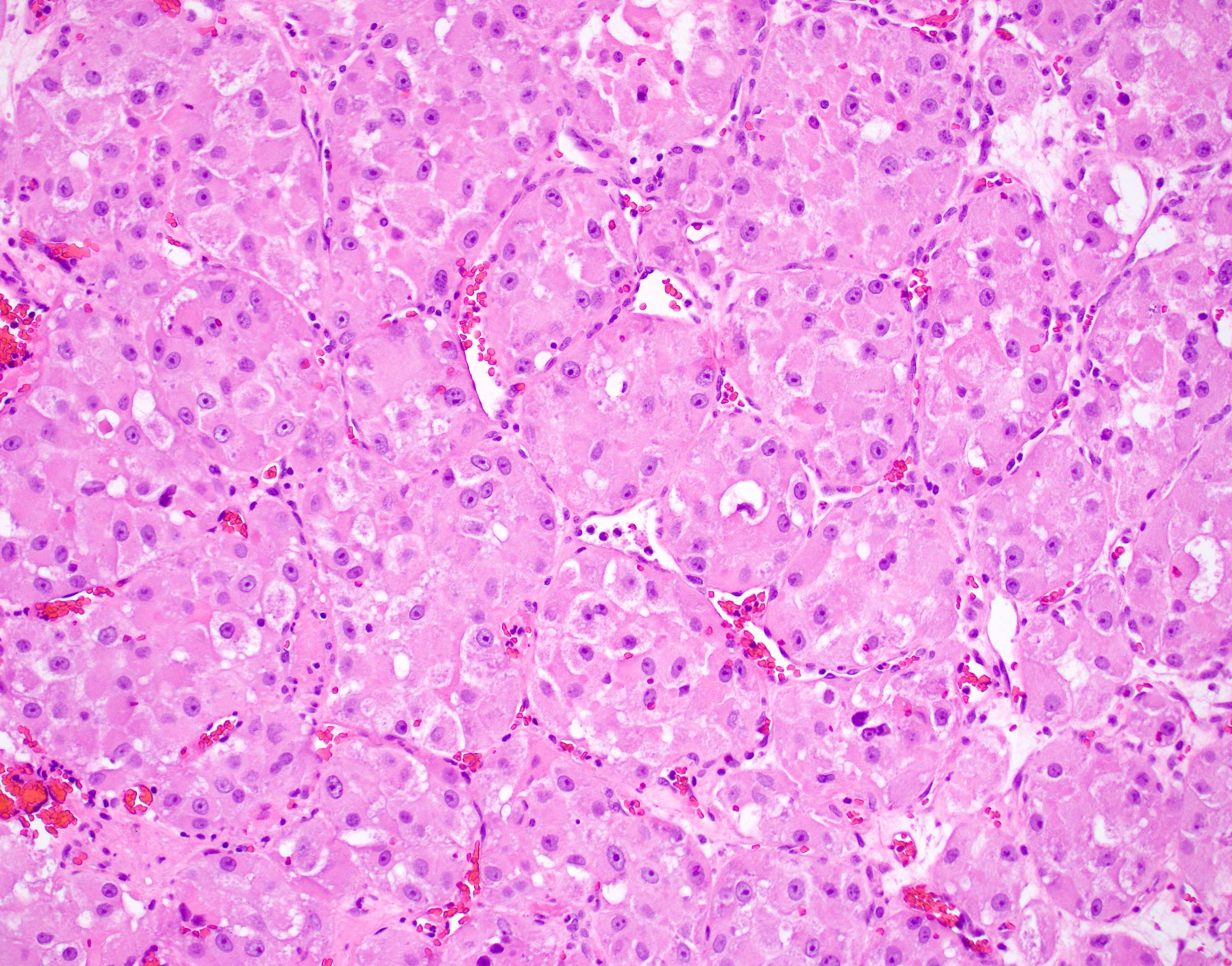
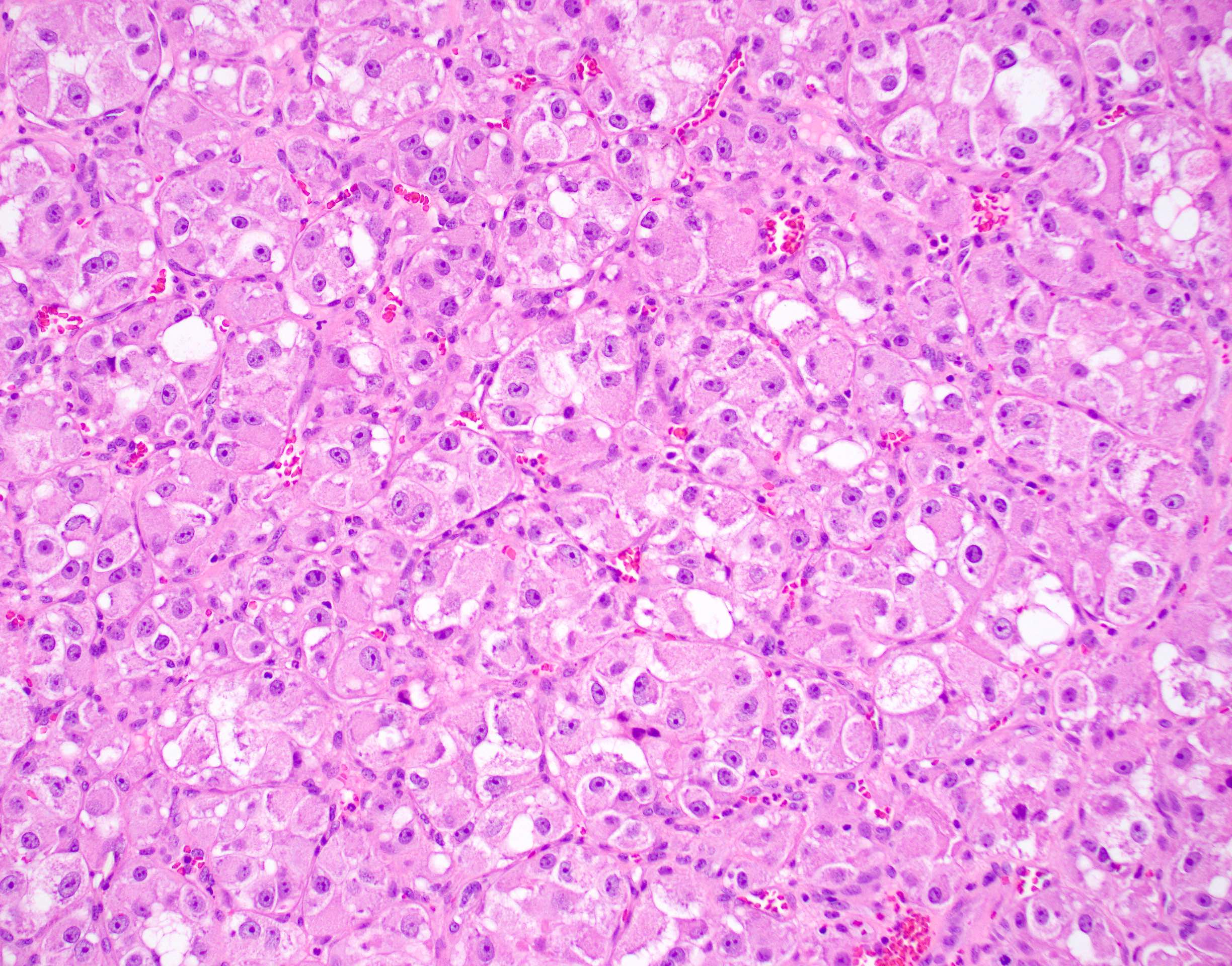
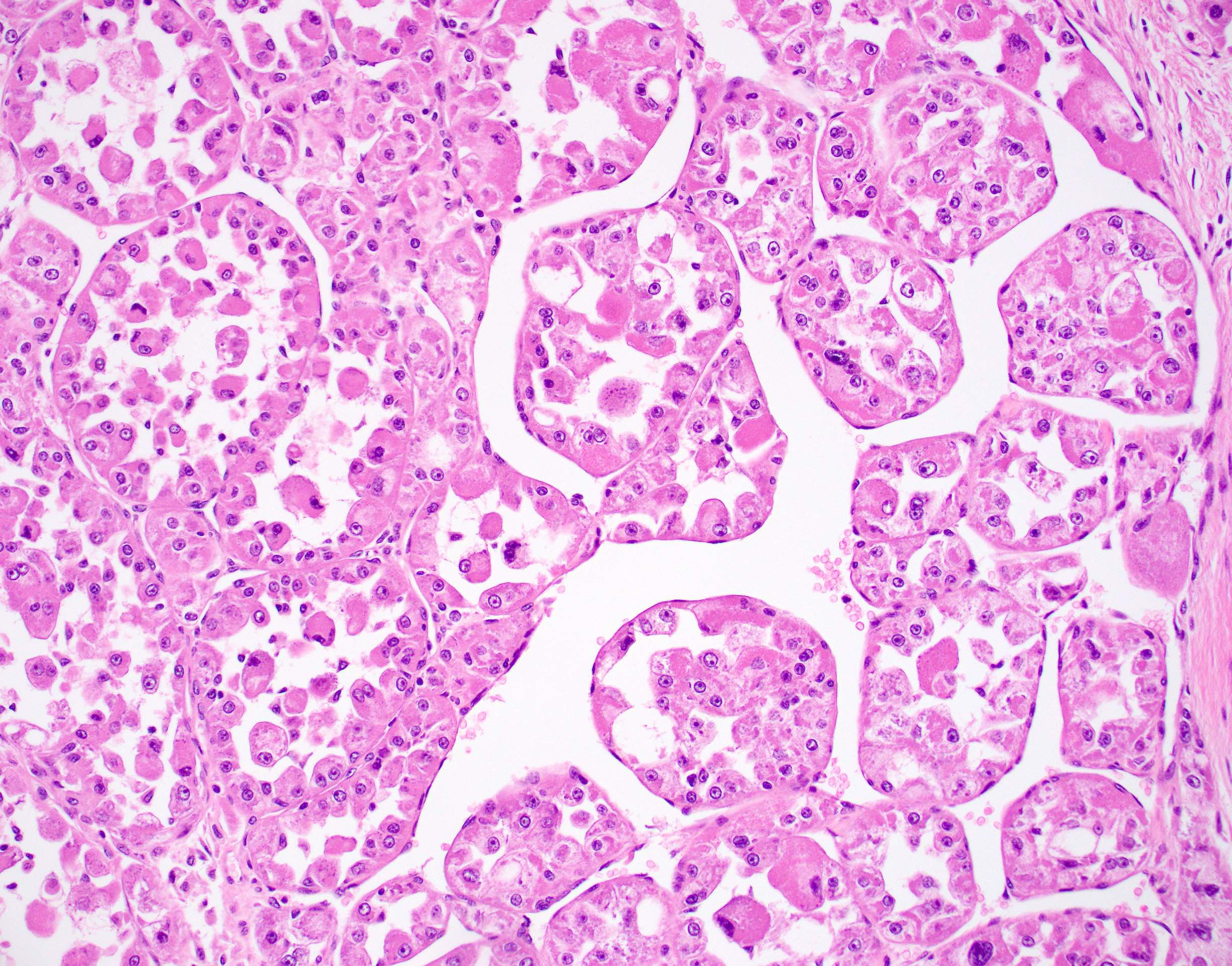
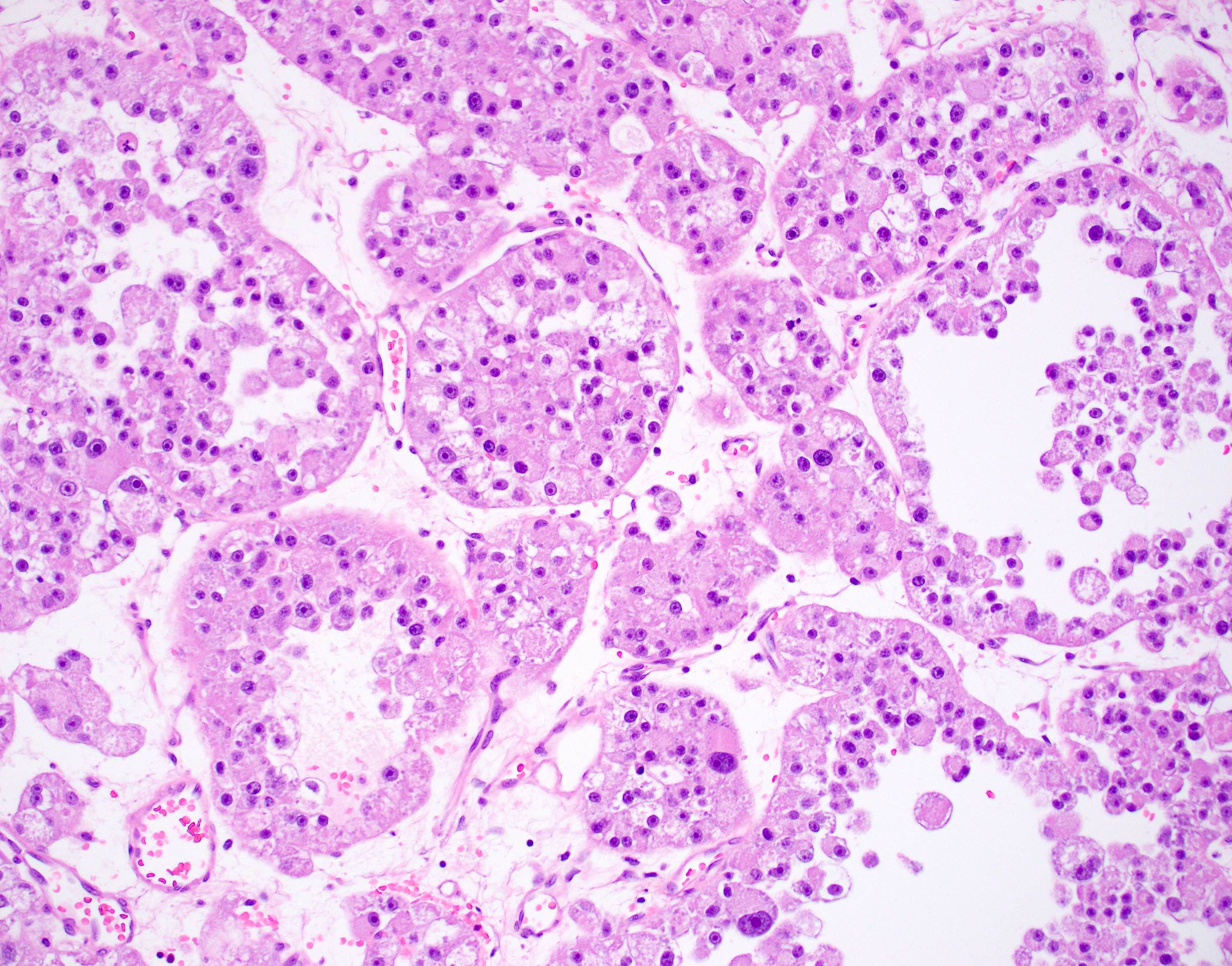
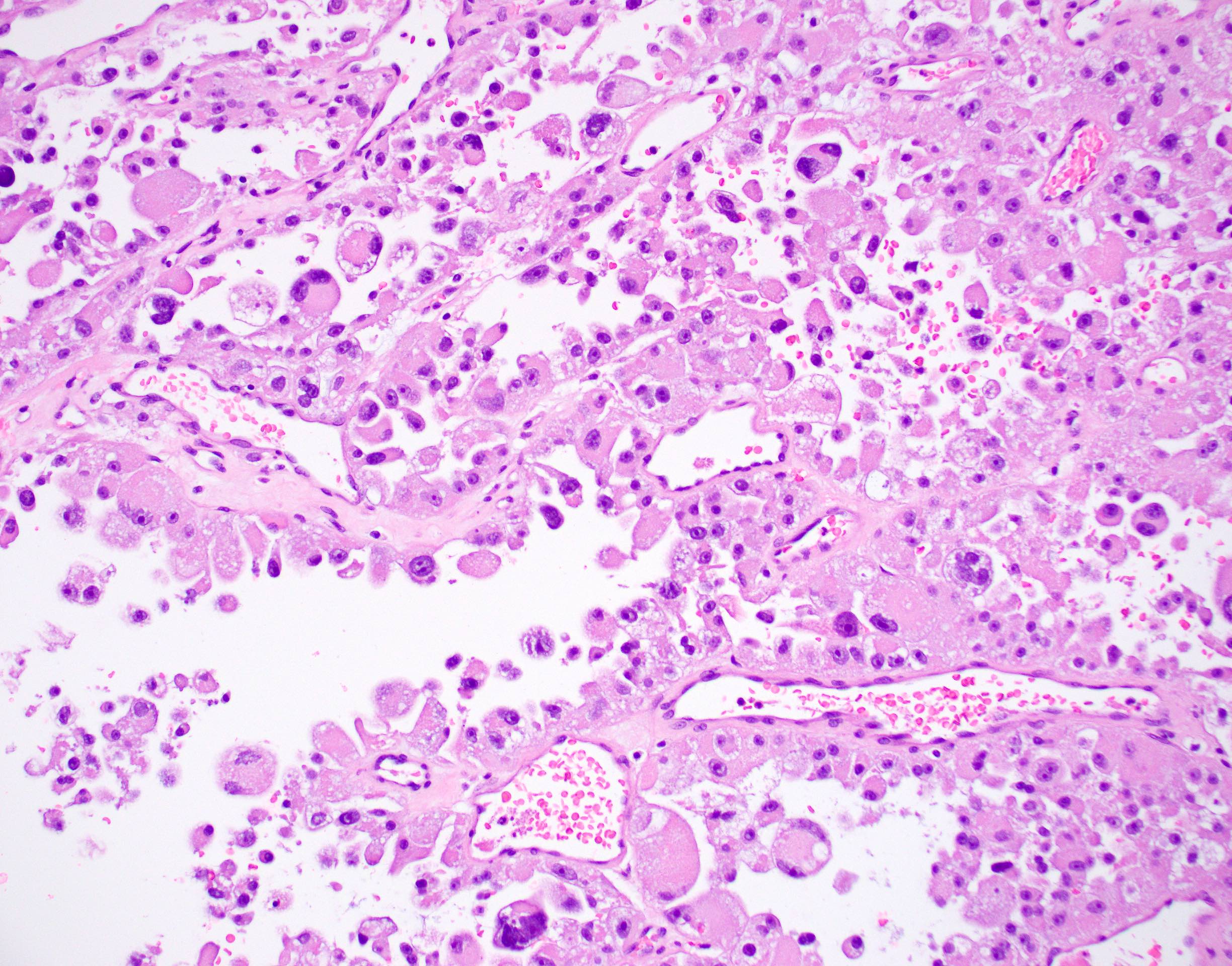
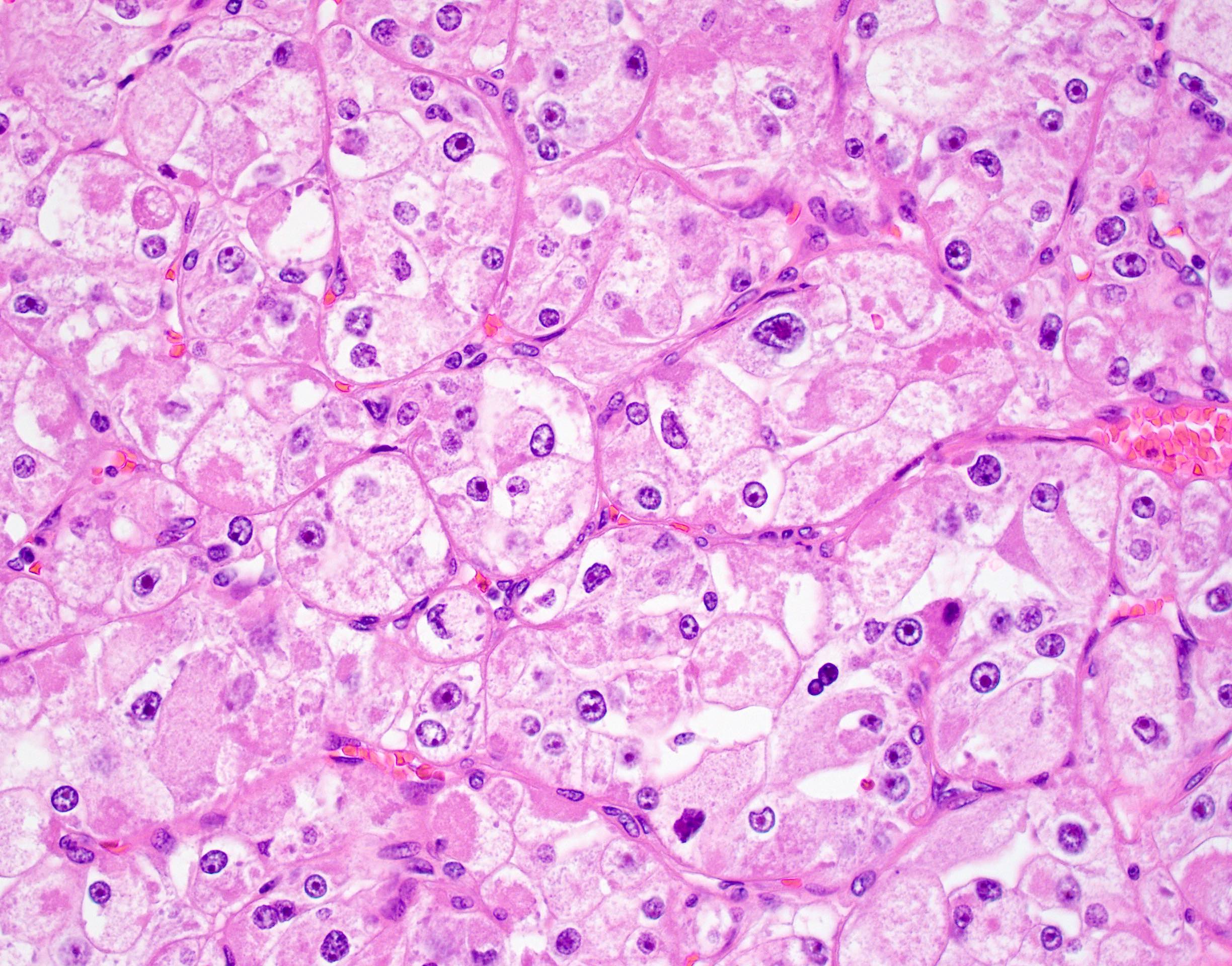
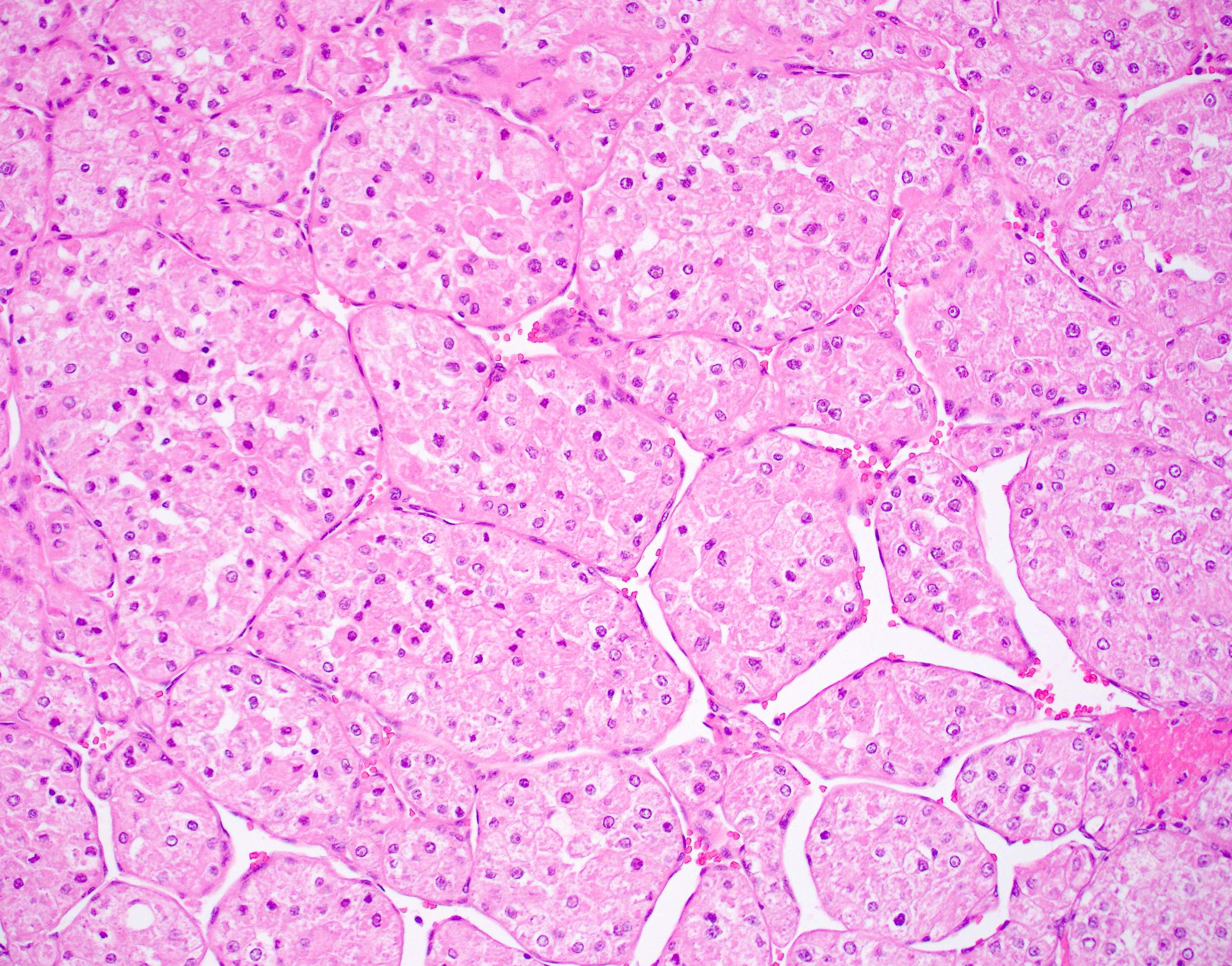
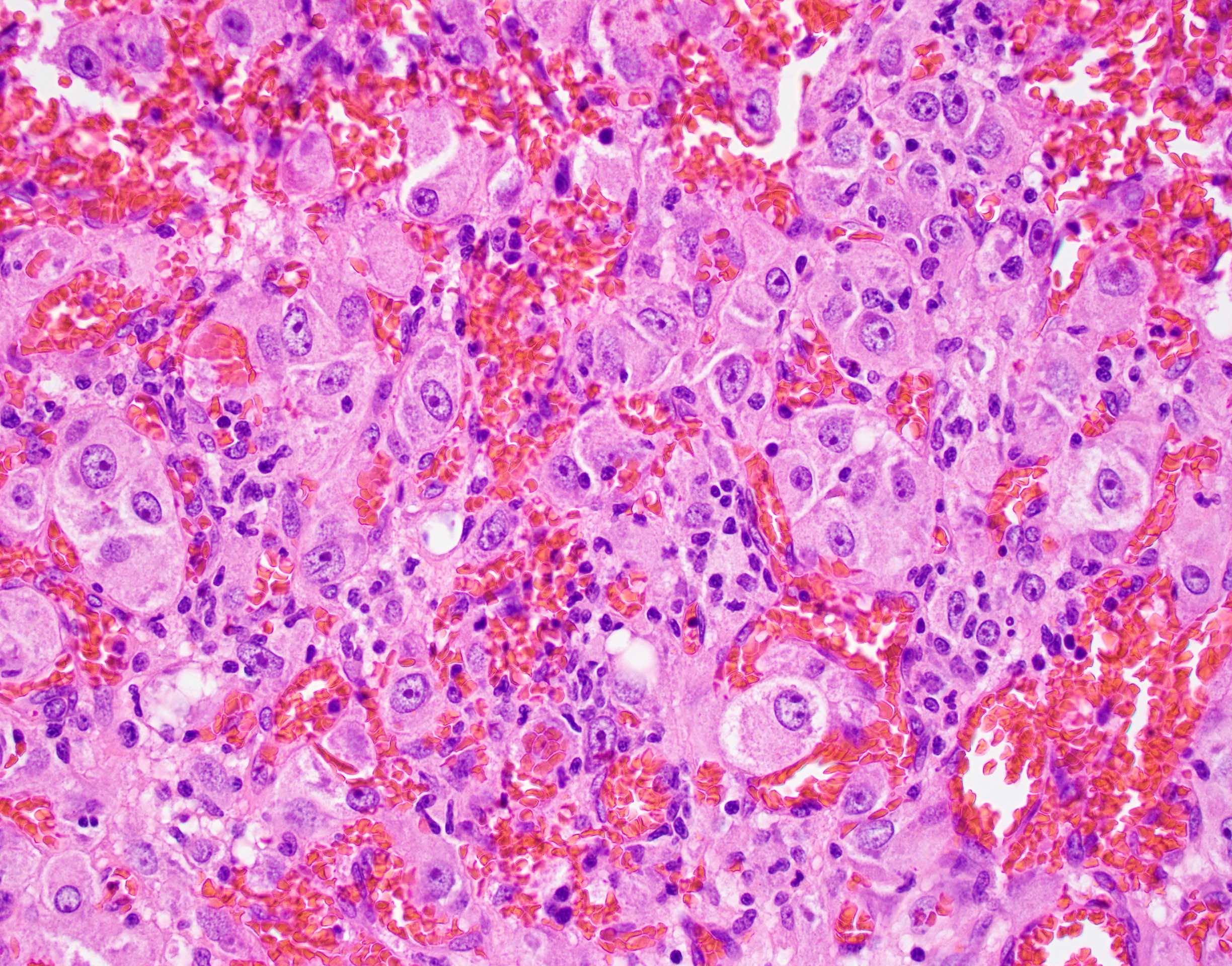
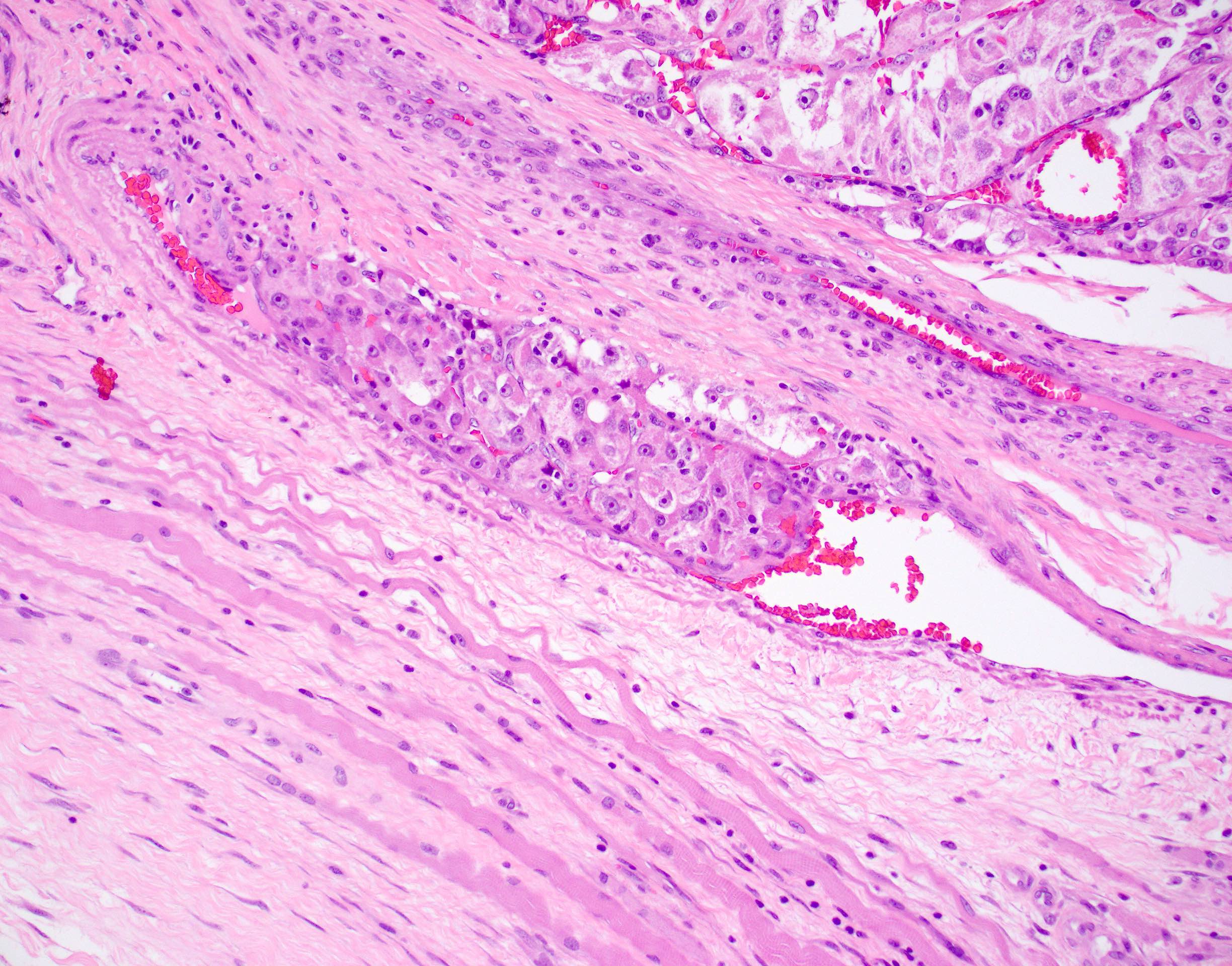
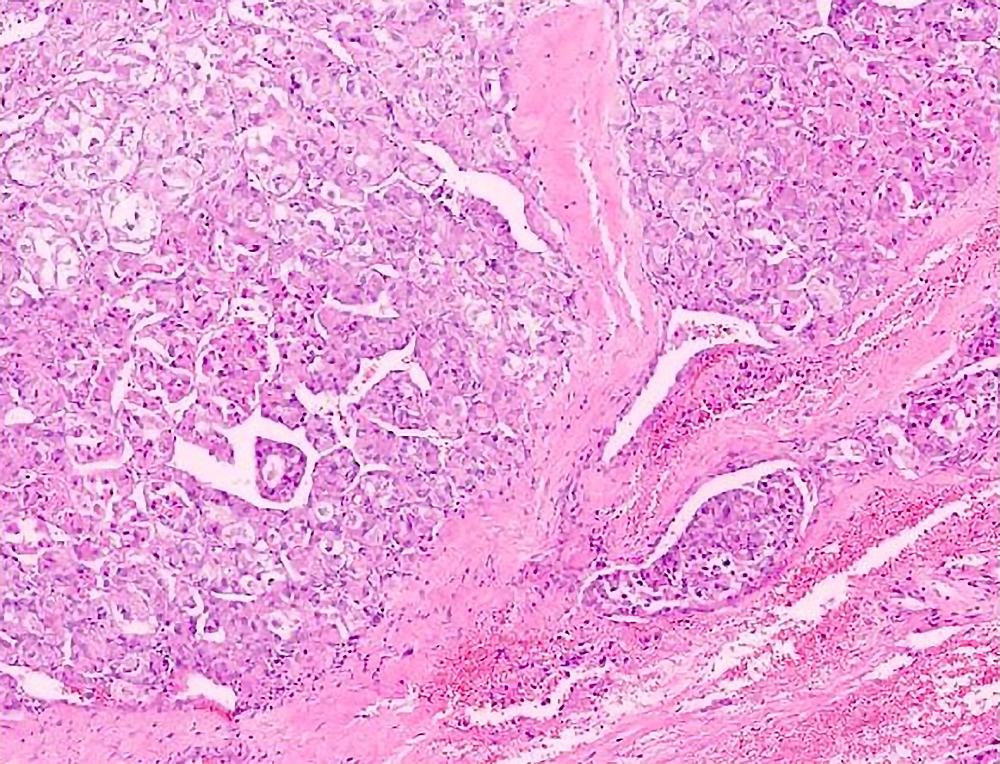
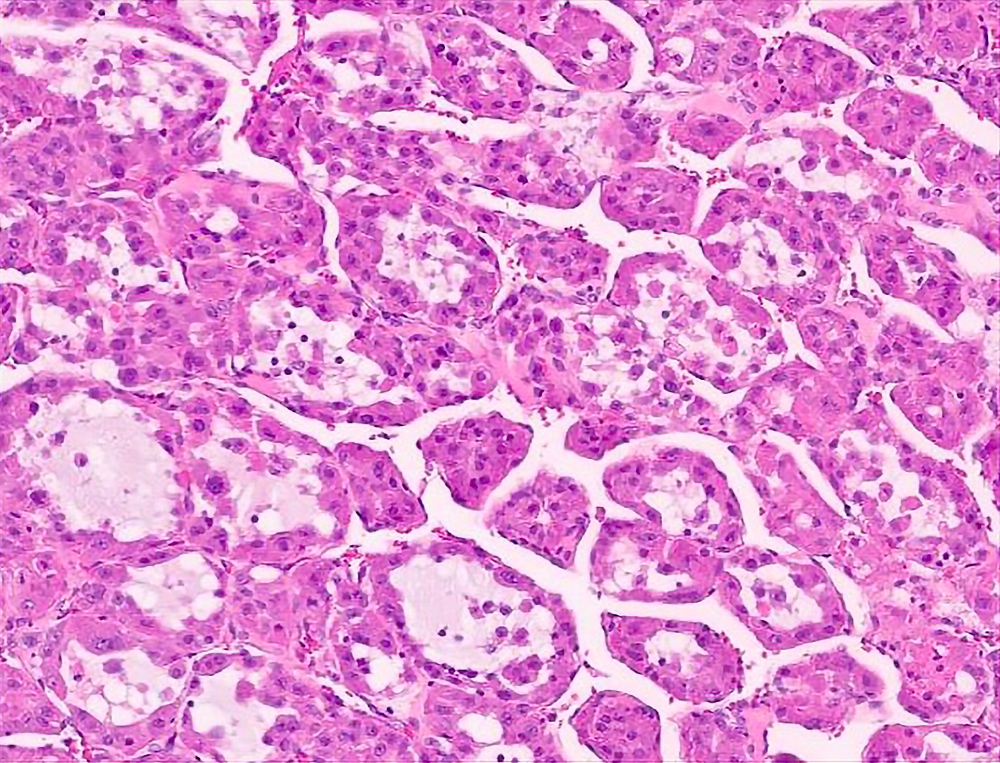
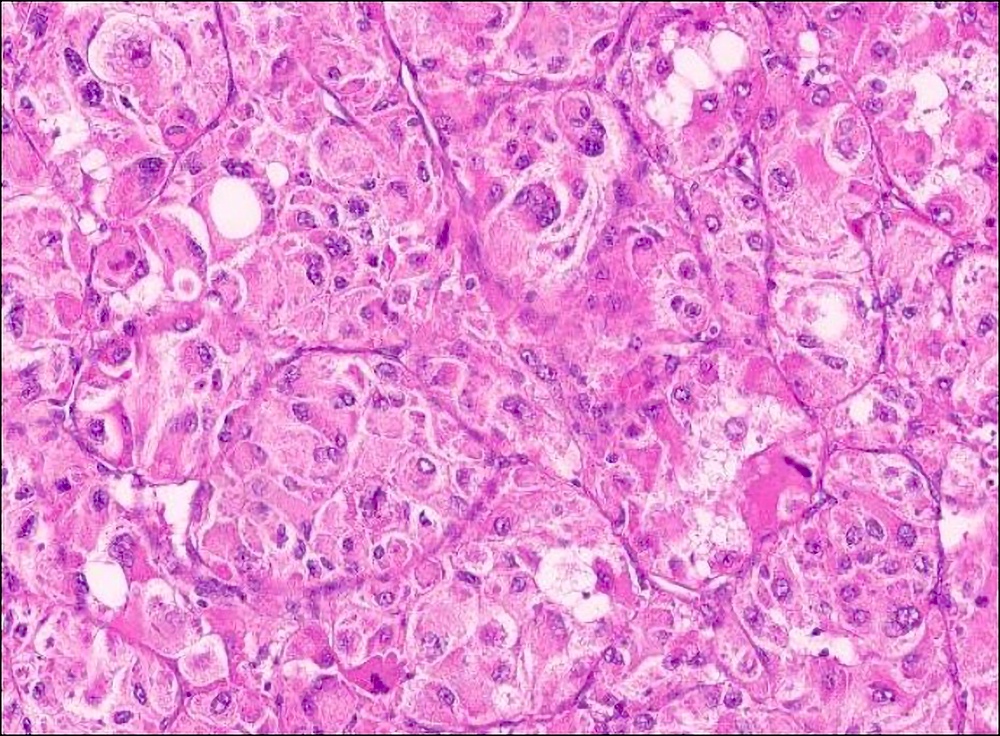
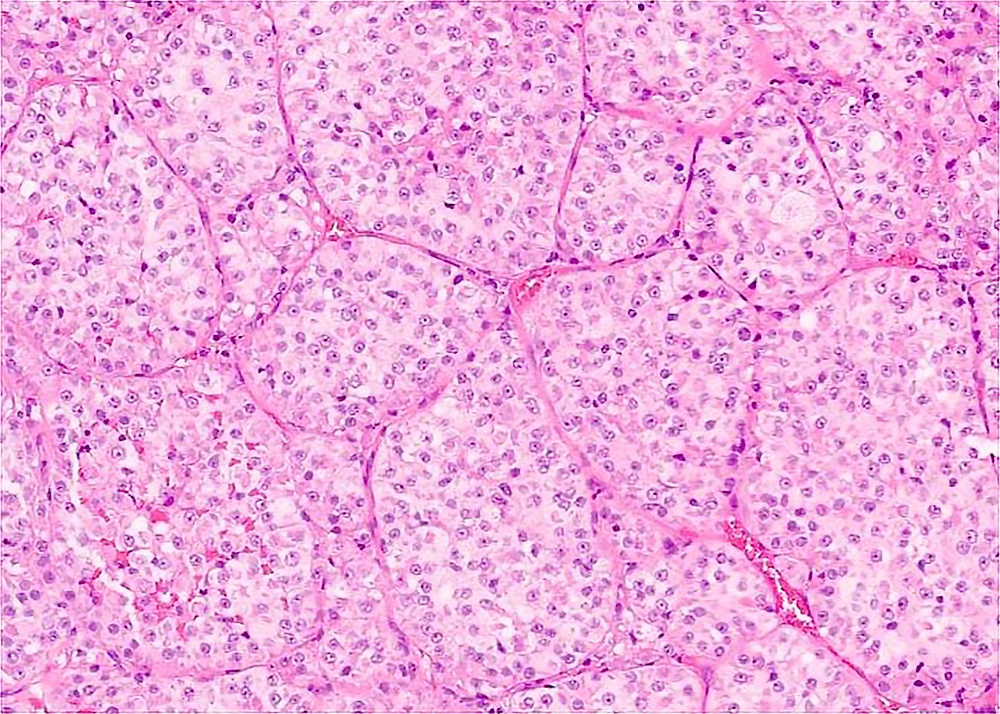
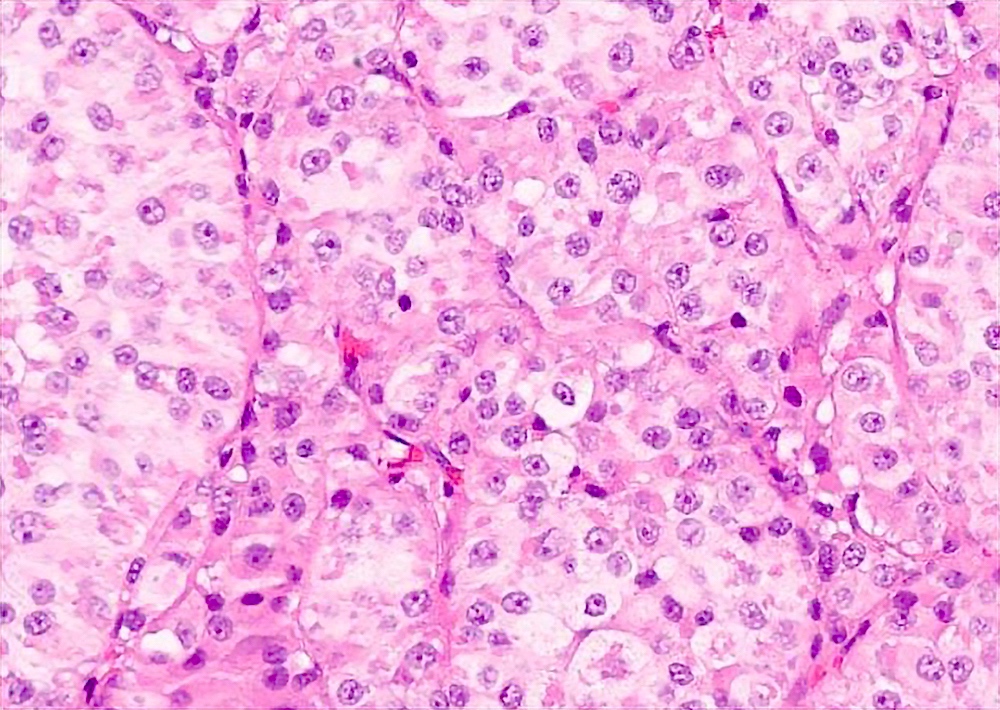
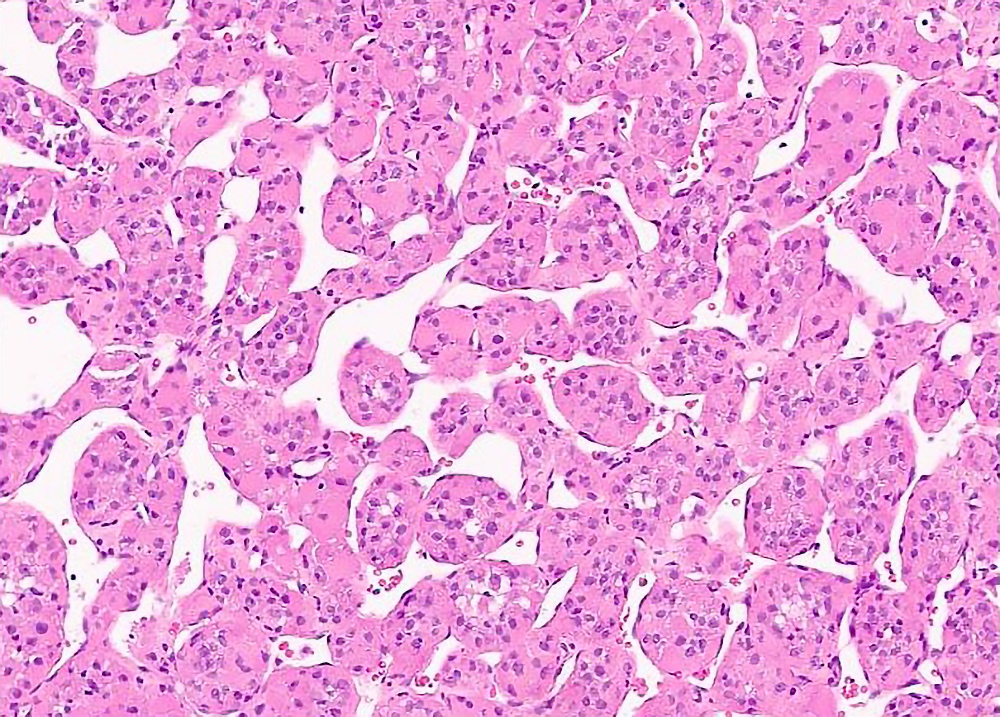
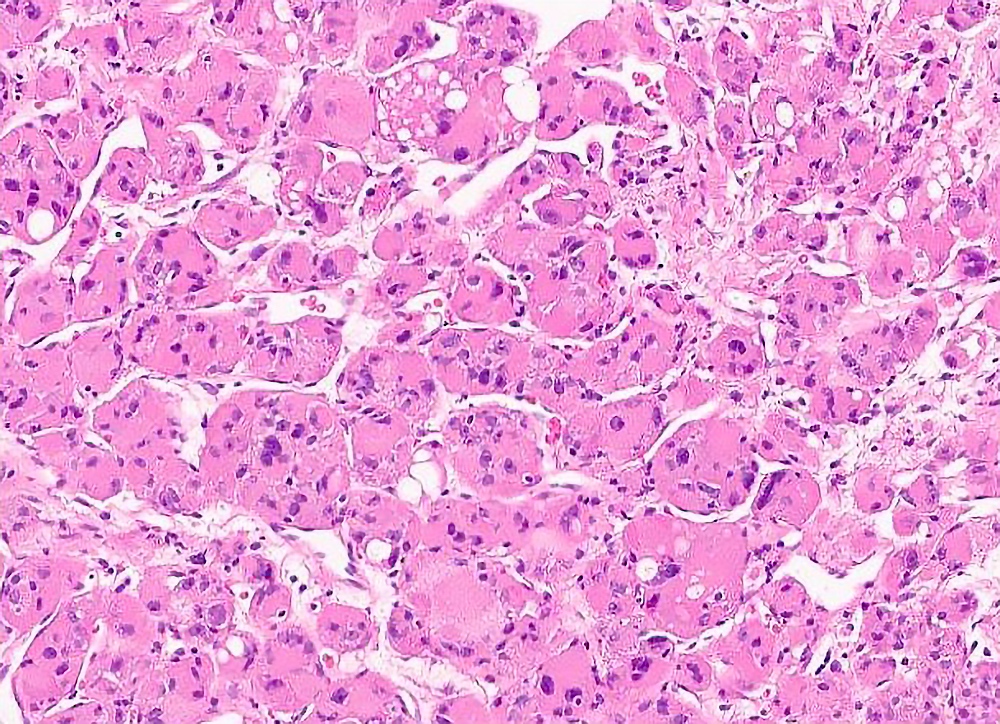
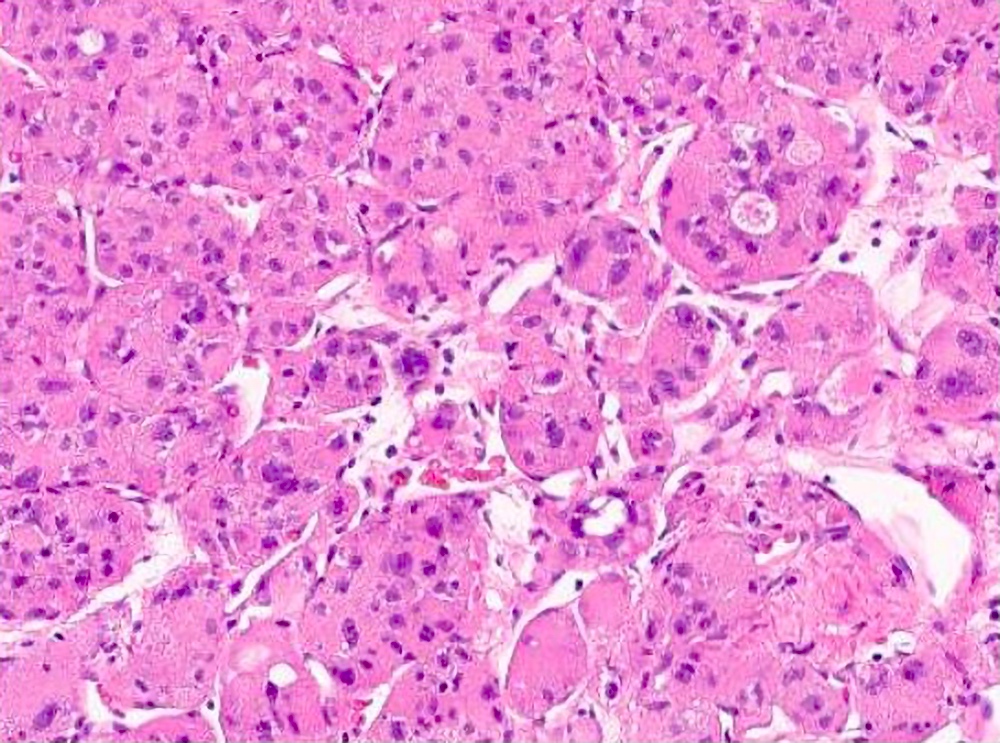
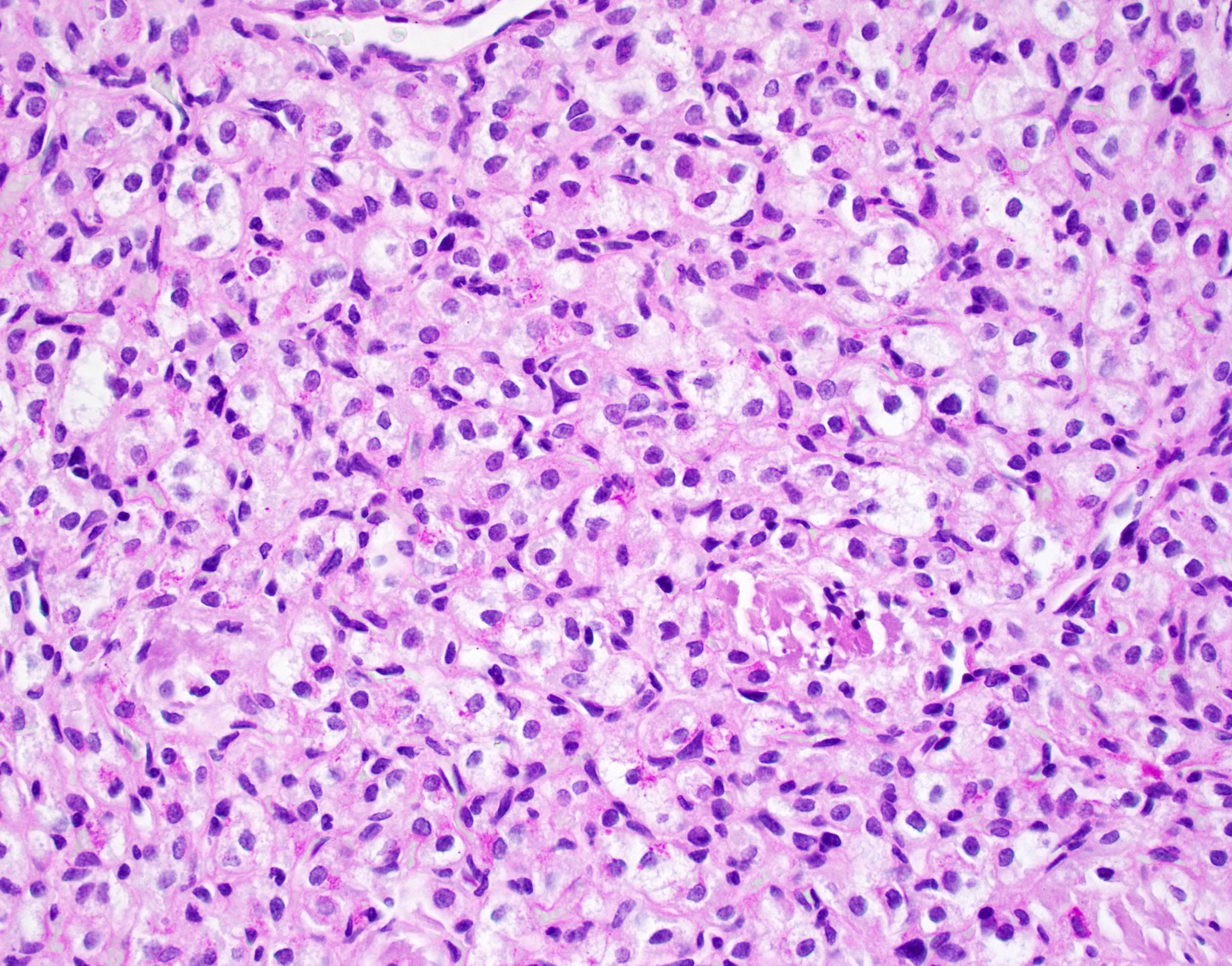
Microscopic (histologic) description
- Large, round to polygonal cells with well defined cell borders
- Usually little variation in individual tumor cell size
- Abundant eosinophilic granular cytoplasm
- Round, vesicular nucleus with a prominent nucleolus
- Organoid and nest-like growth pattern
- Central discohesion results in characteristic pseudoalveolar-like structures
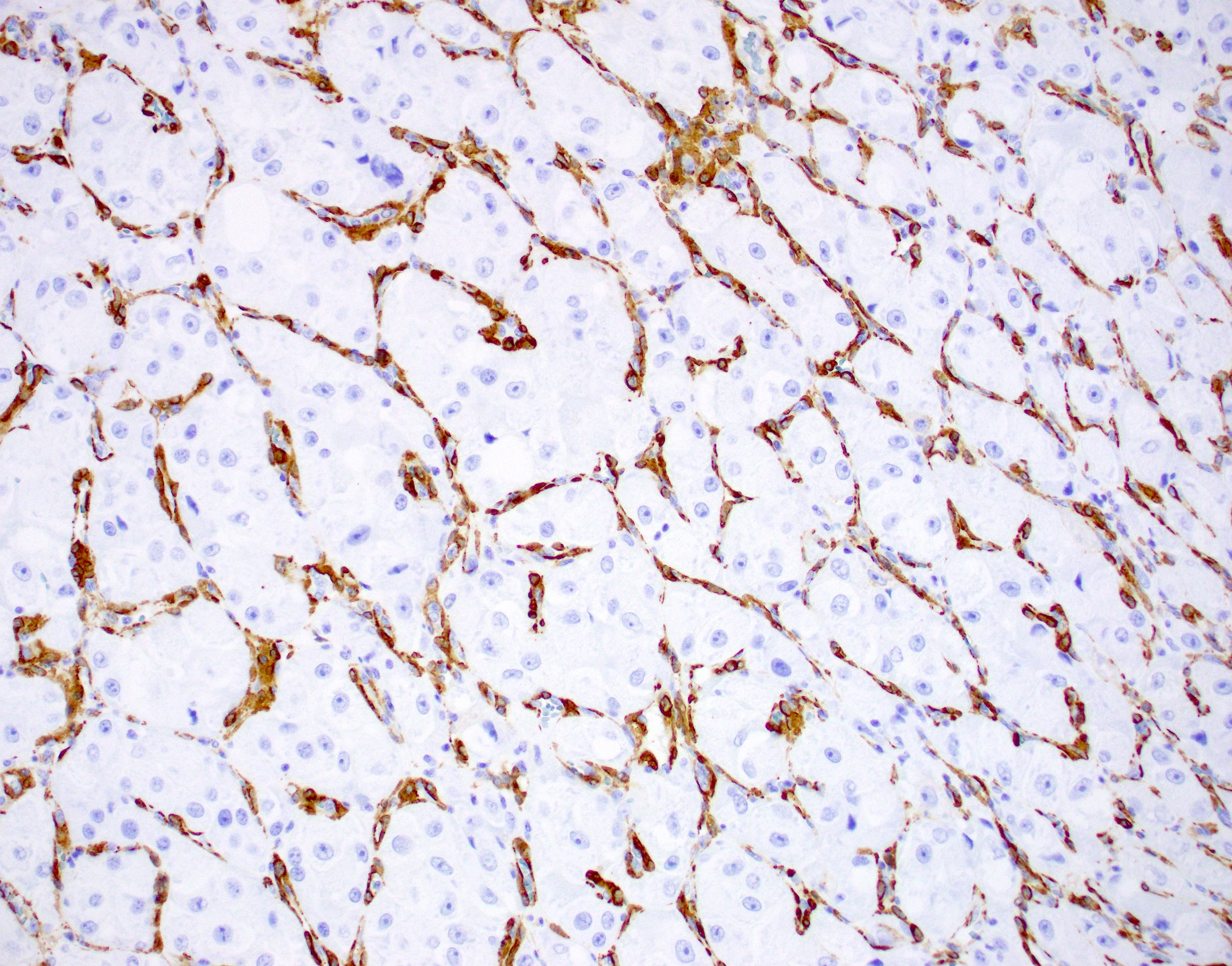
- Lobules of tumor are divided by thick fibrous septa and rich capillary vascular network
- Dilated veins usually present at the periphery of the mass
- Vascular invasion is common
- Cytoplasmic clearing, rhabdoid cells, pseudoglandular pattern and cystic / myxoid change may be seen (Pathology 2012;44:11, Histopathology 1998;32:63)
- Uncommon features: nuclear pleomorphism, hyperchromasia, giant cells, mitotic figures, necrosis, calcification, lymphocytic infiltrate and xanthomatous features (Cancer 1985;55:912, Histopathology 2004;45:526, J Clin Pathol 2006;59:1127, Pathology 2012;44:11, Int J Surg Pathol 2005;13:81)
- Lingual tumors can demonstrate small cells, nonalveolar growth and inconspicuous vessels (Histopathology 2004;45:526)
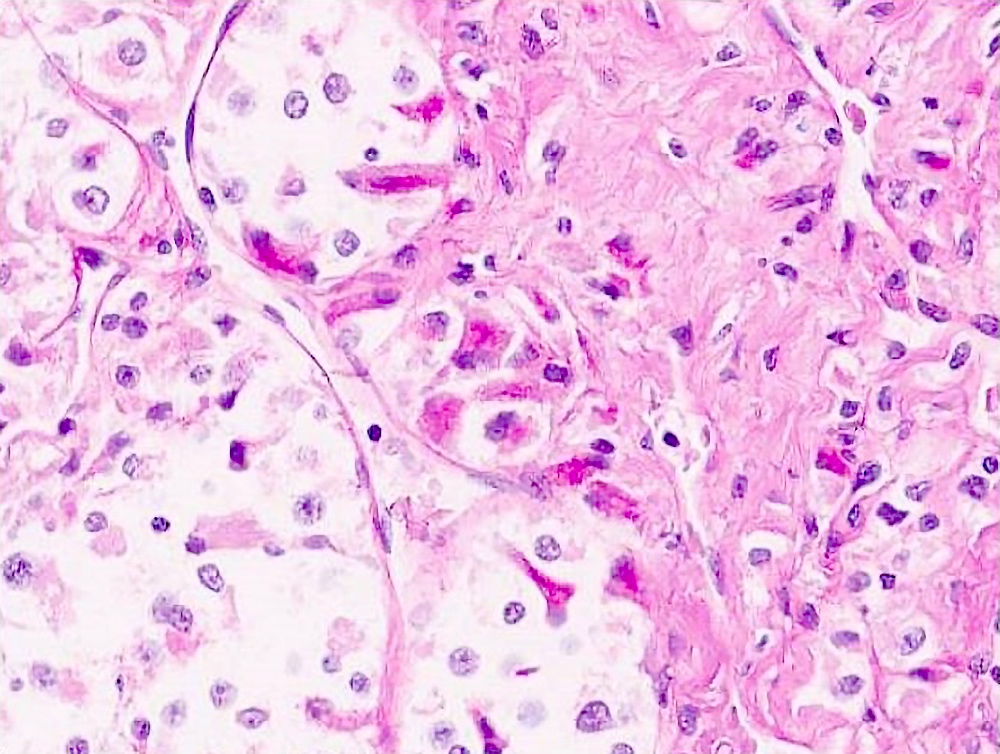
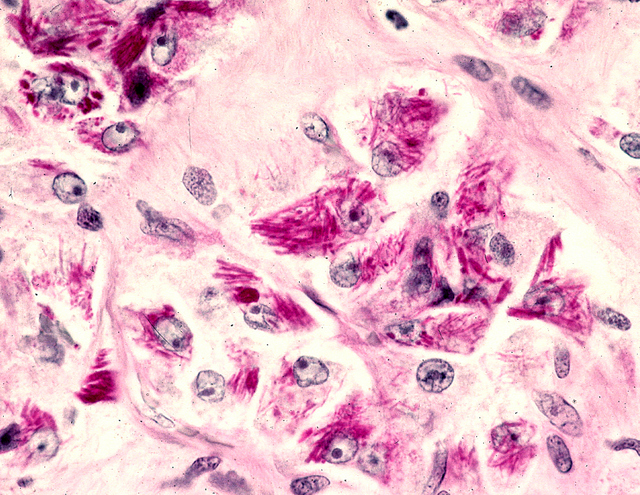
- Tumor frequently show intracytoplasmic rod-like or rhomboid crystalline structures, focally or diffusely (Histopathology 2004;45:526, J Clin Pathol 2006;59:1127, Pathology 2012;44:11)
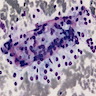
Microscopic (histologic) images
Contributed by Laura Warmke, M.D., Jeanne Meis, M.D. and Mark R. Wick, M.D.
Cytology description
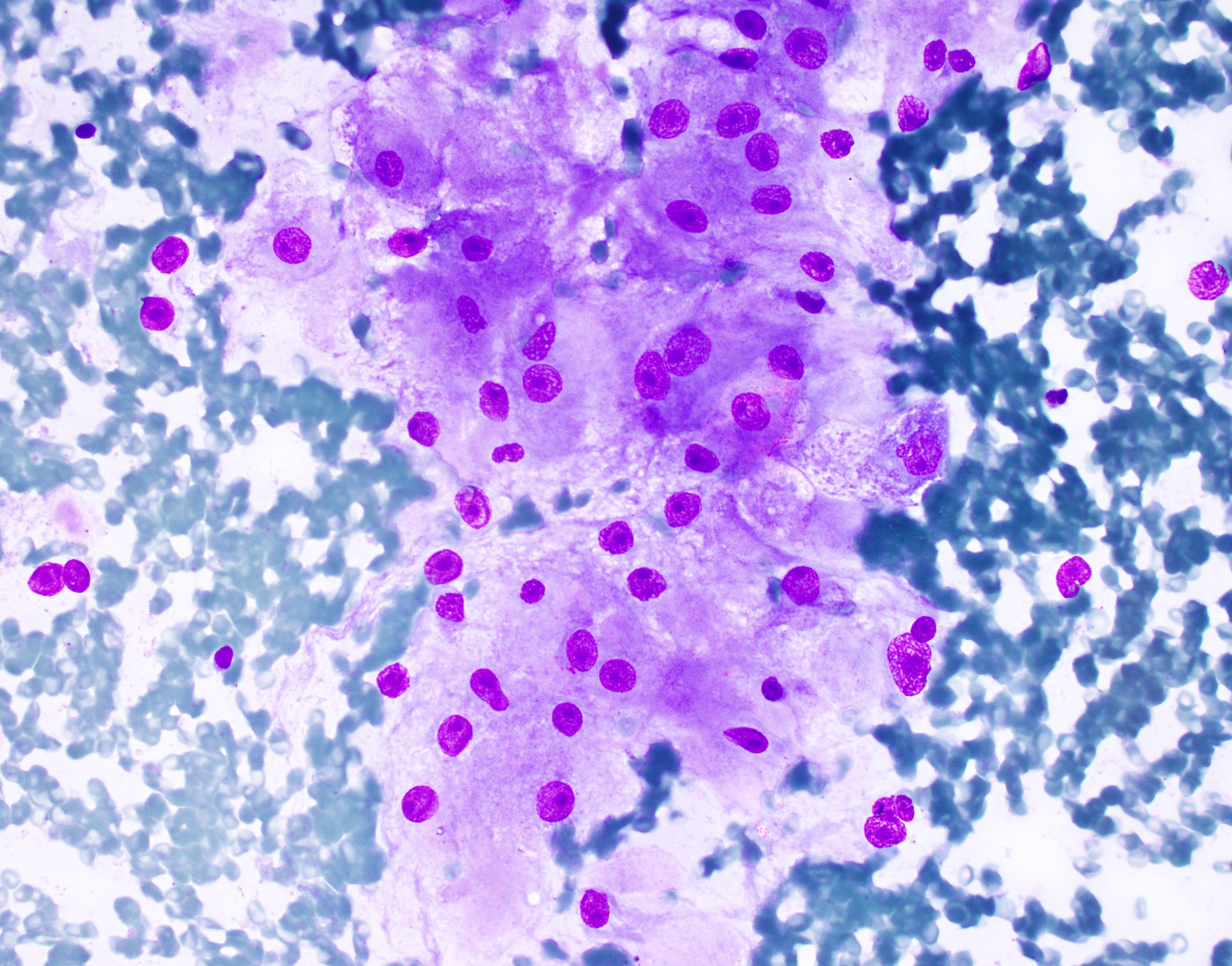
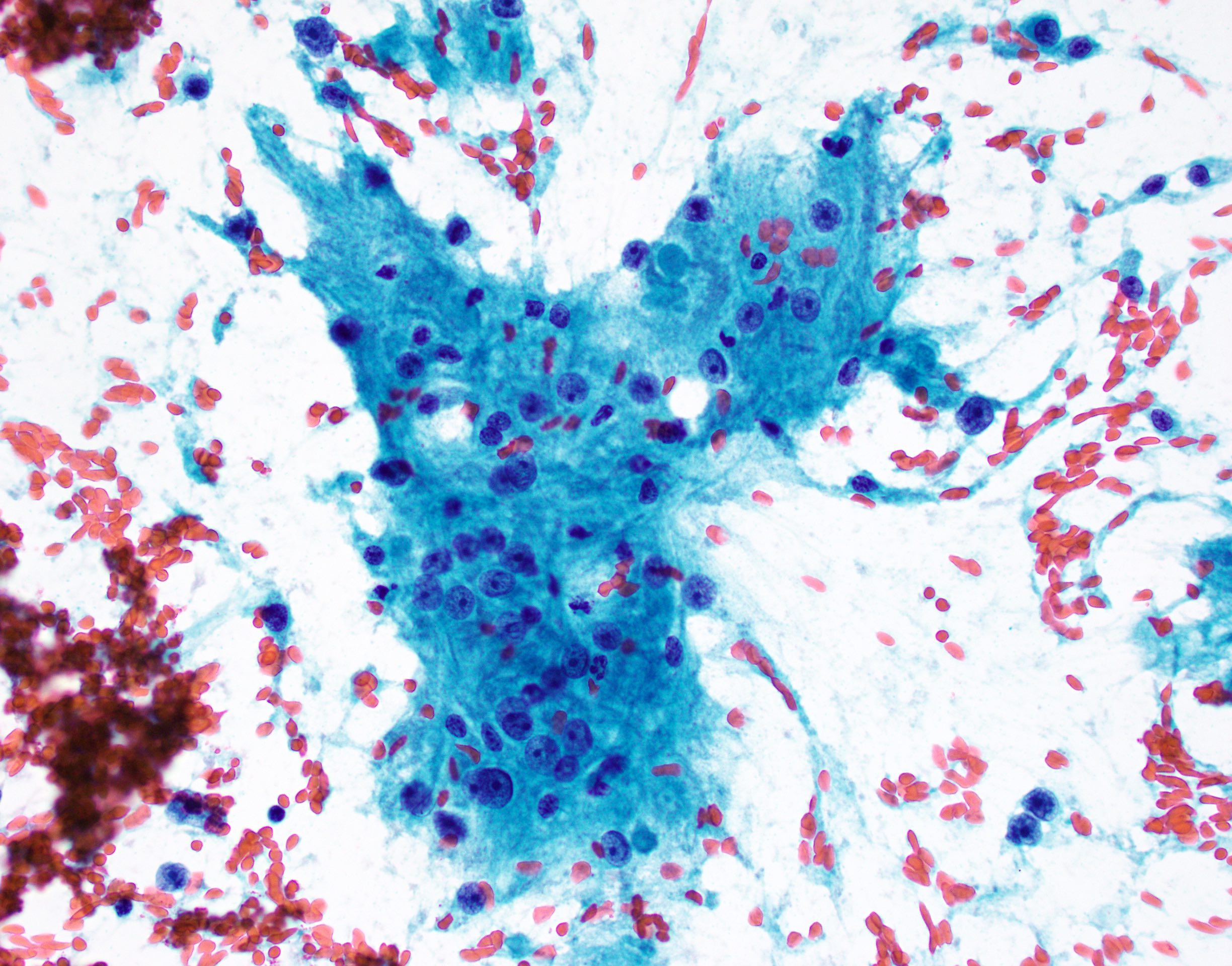
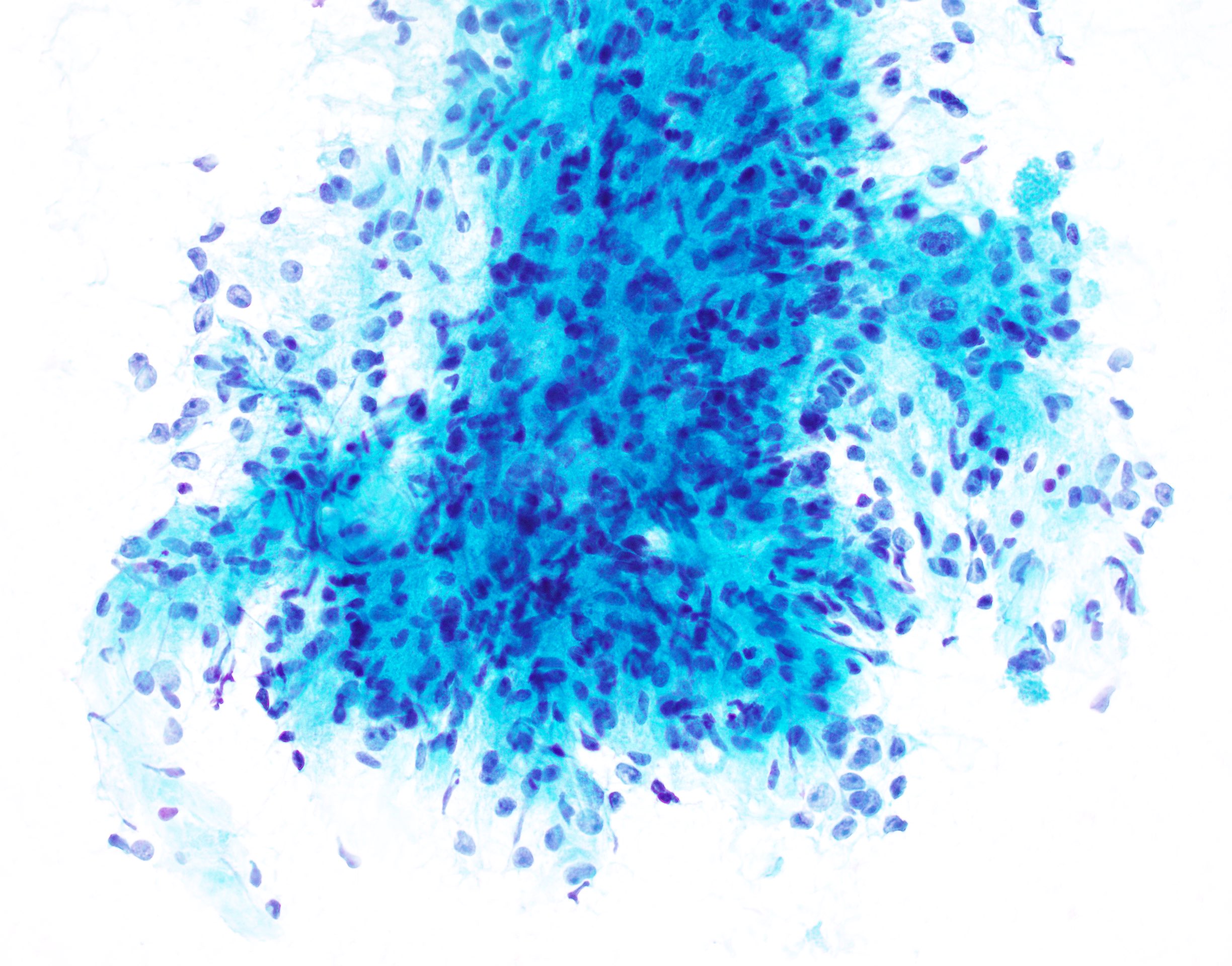
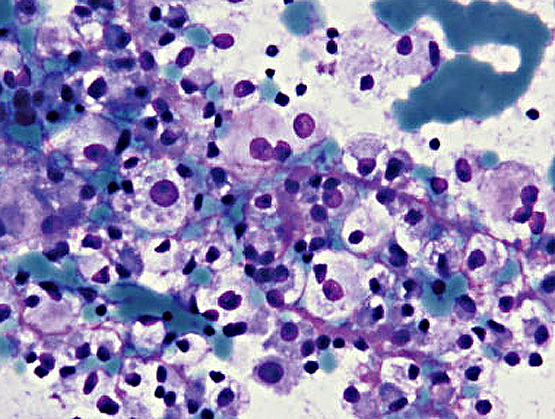
- Smears show large cells with cytoplasmic granularity and vacuoles (Cancer 2009;117:500, Diagn Cytopathol 1991;7:293)
Cytology images
Positive stains
- TFE3 demonstrates nuclear immunoreactivity (Am J Surg Pathol 2003;27:750, Diagn Mol Pathol 2008;17:245, Virchows Arch 2011;458:291)
- Cathepsin K is typically positive
- PAS highlights intracytoplasmic crystals and granules (PAS positive / diastase resistant)
- MCT1 and CD147 are positive in intracytoplasmic granules (Am J Pathol 2002;160:1215, Hum Pathol 2012;43:356)
Negative stains
- Pancytokeratin
- EMA
- Synaptophysin
- Chromogranin
- HMB45
- MelanA
- Calretinin (46%) may be positive (Mod Pathol 2011;24:1313, Hum Pathol 2014;45:1039)
- SMA may show nonspecific staining
- Caldesmon
- Desmin may be focally positive (J Clin Pathol 2006;59:1127)
- Vimentin and NSE may be positive in 30 - 50% of cases (J Clin Pathol 2006;59:1127)
- Myogenin
- MyoD1
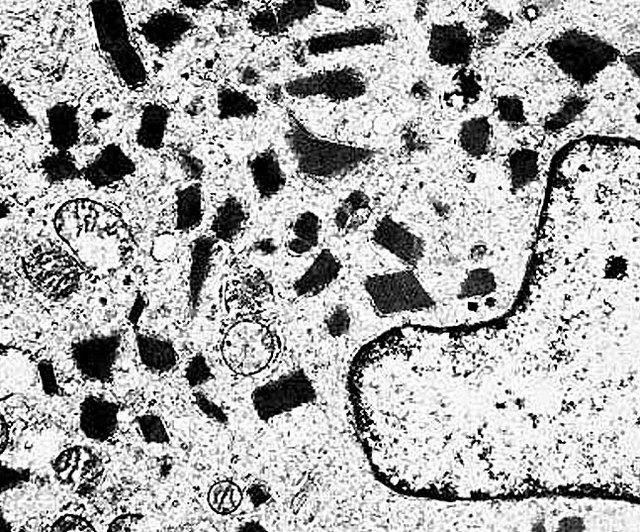
Electron microscopy description
- Tumor cells have numerous mitochondria and well developed Golgi apparatus
- Characteristic rhomboid or rod shaped intracytoplasmic crystals with a regular lattice pattern (Arch Pathol Lab Med 2007;131:488)
- Ultrastructural immunohistochemistry has shown that the crystals consist of aggregates of MCT1 and CD147 (Am J Pathol 2002;160:1215)
Electron microscopy images
Molecular / cytogenetics description
- FISH can confirm TFE3 gene rearrangement (Am J Surg Pathol 2013;37:1150)
- Reverse transcription PCR can detect ASPSCR1-TFE3 fusion transcripts (J Mol Diagn 2018;20:653)
- While ASPSCR1-TFE3 gene fusions are highly specific and sensitive for ASPS among sarcomas, the same fusion has been reported in a small subset of renal cell carcinomas (Am J Pathol 2001;159:179)
- Other less common fusion partners with TFE3 have been identified, including HNRNPH3-TFE3, DVL2-TFE3 and PRCC-TFE3 (Genes Chromosomes Cancer 2019 Aug 21 [Epub ahead of print])
Molecular / cytogenetics images
Videos
Alveolar soft part sarcoma versus alveolar rhabdomyosarcoma, by Dr. Gardner
Sample pathology report
- Soft tissue, mass, anterior right thigh, core biopsy:
- Alveolar soft part sarcoma (see comment)
- Comment: This biopsy is composed of round to polygonal tumor cells with abundant eosinophilic cytoplasm and round nuclei with vesicular chromatin and prominent macronucleoli. The tumor cells grow in a nested or pseudoalveolar pattern with nests of cells separated by fibrous bands and delicate, thin walled blood vessels. Focal lymphovascular invasion is present. Immunohistochemical stains show that the tumor cells are positive for TFE3 (strong nuclear staining), while they are essentially negative for pancytokeratin, S100 protein, SMA, desmin, synaptophysin, MelanA and HMB45. Fluorescence in situ hybridization (FISH) confirms the presence of a TFE3 gene rearrangement. These results support the above diagnosis.
Differential diagnosis
- Metastatic renal cell carcinoma:
- Older patients (usually > 40 years)
- Clinical history of a renal mass
- Positive for pancytokeratin
- Positive for PAX8
- Xp11 translocation RCC can show nuclear expression with TFE3 (Mod Pathol 2014;27:875)
- Metastatic adrenal cortical carcinoma:
- Pleomorphic cells with frequent intranuclear inclusions
- Prominent mitotic activity with atypical mitotic figures
- Positive for inhibin, calretinin and MelanA
- Metastatic hepatocellular carcinoma:
- Tumor cells often resemble mature hepatocytes
- Bile production may be seen
- Positive for pancytokeratin and HepPar1
- Granular cell tumor:
- Paraganglioma:
- Small nests of cells (zellballen)
- Dense nuclei with inconspicuous nucleoli
- Surrounded by fibrous tissue
- Isolated pleomorphic cells present
- Positive for chromogranin and synaptophysin
- S100 protein highlights sustentacular cells
- Subset are positive for SDHB
- Can show nuclear expression with TFE3 (Acta Pathol Jpn 1986;36:895)
- Unusual in the extremities
- PEComa:
- Melanoma:
- Rhabdomyoma:
- Alveolar rhabdomyosarcoma:
Additional references
Board review style question #1
Board review style answer #1
C. PAS with diastase can highlight the characteristic rhomboid or rod shaped intracytoplasmic crystals within the tumor cells in approximately 80% of cases.
Comment Here
Reference: Alveolar soft part sarcoma
Comment Here
Reference: Alveolar soft part sarcoma
Board review style question #2
Board review style answer #2
B. Brain. While ASPS frequently metastasizes to the lungs and bone, as do many other sarcomas, metastases to the brain are much more common in ASPS than any other sarcoma. Metastatic involvement of the liver and lymph nodes is unusual in ASPS.
Comment Here
Reference: Alveolar soft part sarcoma
Comment Here
Reference: Alveolar soft part sarcoma