Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Molecular / cytogenetics description | Molecular / cytogenetics images | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Alexiev BA, Jennings LJ. Gastrointestinal stromal tumor (GIST). PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/softtissueGIST.html. Accessed December 4th, 2024.
Definition / general
- Gastrointestinal stromal tumor (GIST) is a mesenchymal neoplasm with variable behavior, characterized by differentiation toward the interstitial cells of Cajal based on IHC and molecular studies (Gastroenterol Clin North Am 2013;42:399)
Essential features
- Specific, generally KIT or PDGFRA mutation driven mesenchymal tumor (Arch Pathol Lab Med 2006;130:1466)
- Characteristic histologic features include spindle cell, epithelioid and rarely pleomorphic morphology
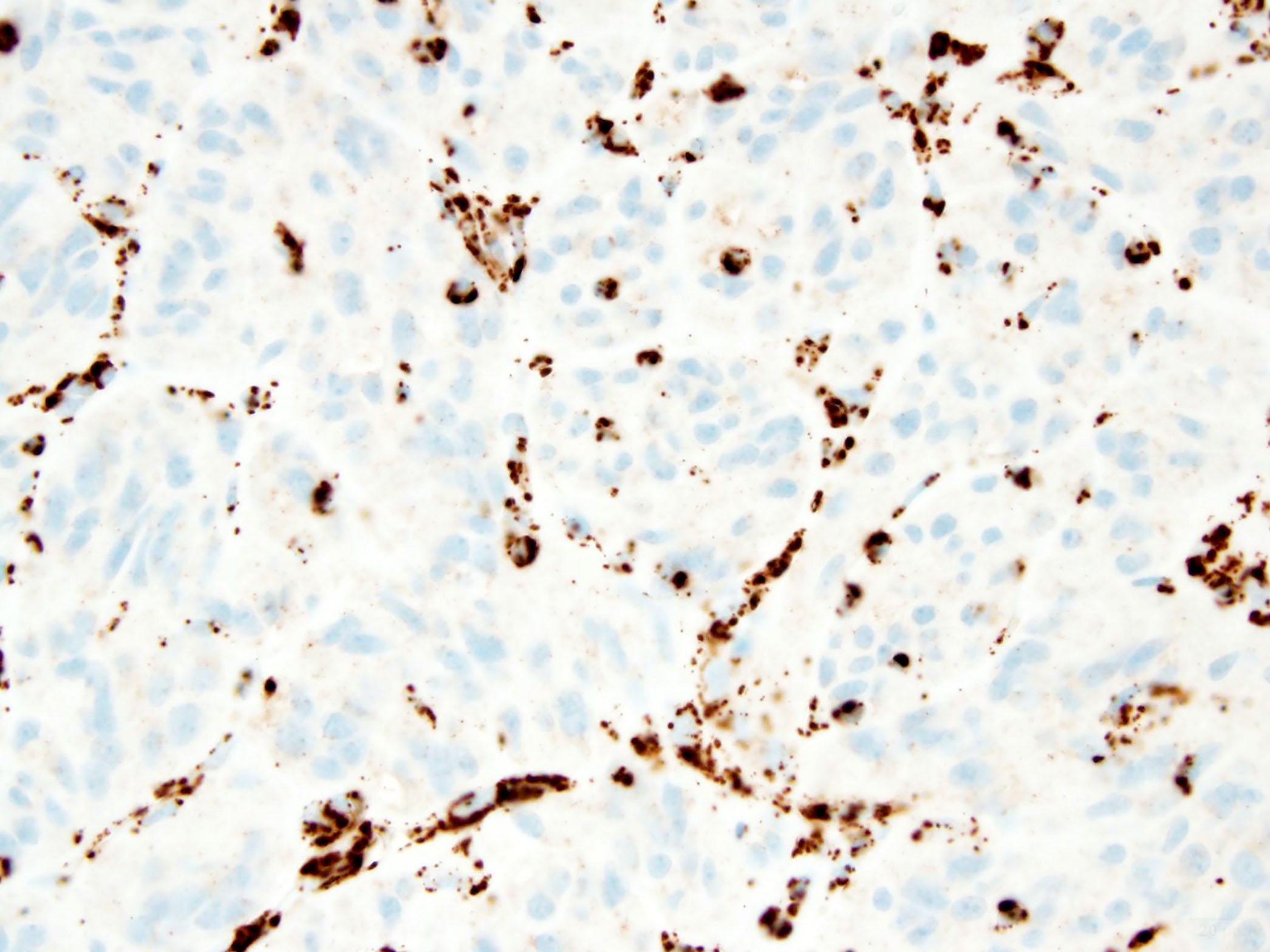
- KIT (CD117) or DOG1 immunopositivity; SDHB loss in succinate dehydrogenase (SDH) deficient GIST
- KIT and PDGFRA gene mutations in ~85% of tumors
- Many GISTs that are wild type for KIT and PDGFRA harbor alterations in SDH subunit genes
Terminology
- Not recommended: gastrointestinal autonomic nerve sheath tumor (GANT); gastrointestinal pacemaker cell tumor (GIPACT)
ICD coding
- ICD-O: 8936/3 - gastrointestinal stromal tumor
- ICD-11: 2B5B & XH9HQ1 - gastrointestinal stromal tumor, primary site & gastrointestinal stromal sarcoma
Epidemiology
- GISTs are the most common nonepithelial tumors of the gastrointestinal tract (Cancer 2005;103:821)
- Studies in Scandinavia indicate an incidence of 1.1 - 1.5 cases per 100,000 person years (Cancer 2005;103:821)
- Sporadic GISTs can occur at any age, with a peak incidence in the sixth decade of life (median age: 63 years) and a slight male predominance (55%) (Am J Surg Pathol 2005;29:52)
- GISTs are very rare in children; this group comprises no more than 1% of all GISTs (Lancet 2013;382:973)
- There is a female preponderance in the pediatric population for development of GISTs (70%) (Transl Gastroenterol Hepatol 2018;3:54)
- Pediatric GISTs typically arise in the stomach as multifocal tumors with a multinodular growth pattern, epithelioid morphology, lymph node metastases and indolent behavior
- Such tumors are usually SDH deficient and KIT / PDGFRA wild type (Am J Surg Pathol 2005;29:1373, Proc Natl Acad Sci U S A 2011;108:314, Am J Surg Pathol 2011;35:495)
- ~25% of GISTs are clinically malignant
Sites
- GISTs usually occur throughout the gastrointestinal tract: 60% in stomach, 35% in small intestine and < 5% in rectum, esophagus, omentum and mesentery (Arch Pathol Lab Med 2006;130:1466)
Pathophysiology
- Early events in GIST development are activating mutations in KIT or PDGFRA, which occur in most GISTs and encode for mutated tyrosine receptor kinases that are therapeutic targets for tyrosine kinase inhibitors (TKI), including imatinib and sunitinib (Clin Med Insights Pathol 2012;5:23)
- Small minority of GISTs possessing neither KIT nor PDGFRA mutations may have germline mutations in SDH
Etiology
- Majority of GISTs are sporadic
- < 5% of GISTs are associated with 1 of the 3 tumor syndromes: neurofibromatosis type 1 (NF1), Carney triad / Carney-Stratakis syndrome and familial GIST syndrome, in order of decreasing frequency (Clin Med Insights Pathol 2012;5:23)
- In NF1 patients, GIST has a high predilection to small intestine, tumors are often multiple and the majority are small, mitotically inactive and clinically indolent (Arch Pathol Lab Med 2006;130:1466)
- SDH deficient GISTs may be part of distinct clinical syndromes, Carney-Stratakis syndrome (CSS) and Carney triad (gastric GIST, paraganglioma and pulmonary chondroma / hamartoma)
- Carney-Stratakis syndrome is also known as the dyad of GIST and paraganglioma
- Affects both genders equally
- Is inherited in an autosomal dominant manner with incomplete penetrance
- is linked to (and probably caused by) germline loss of function alterations in the somatically encoded mitochondrial membrane protein genes for succinate hydrogenase subunits, especially SDHB (Mod Pathol 2011;24:147, Proc Natl Acad Sci U S A 2011;108:314)
Diagrams / tables
Clinical features
- There is no pathognomonic feature of GISTs; the symptoms vary a lot according to tumor size and more importantly, tumor location (Visc Med 2018;34:335)
- Diagnosis of GIST is commonly initiated through nonspecific symptoms such as abdominal pain, gastrointestinal bleeding or obstruction (Ther Adv Med Oncol 2023;15:17588359231192388)
- Most common clinical presentation is upper gastrointestinal bleeding and gastric discomfort or ulcer-like symptoms
- In many patients, GIST is an accidental finding and tumors are often detected with nonspecific symptoms
Diagnosis
- Tissue sampling is the gold standard for a definitive diagnosis
- Spindle cell, epithelioid or mixed morphology (Gastroenterol Clin North Am 2013;42:399)
- KIT (CD117) or DOG1 immunopositivity
- SDHB loss in SDH deficient GIST
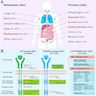
- Abdominal computerized tomography (CT) scan with contrast and image acquisitions of the arterial and portal phases is the first choice to study location and extension (Ther Adv Med Oncol 2023;15:17588359231192388)
- Pathology report must include information for risk assessment (anatomical location, tumor size, mitotic activity) and other important prognostic factors, such as tumor rupture and margin status
Radiology description
- Ultrasound studies show GISTs as submucosal and hypoechogenic lesions that, if enlarged, can displace neighboring structures and turn cystic, necrotic or hemorrhagic (Ther Adv Med Oncol 2023;15:17588359231192388)
- Abdominal CT scan is the first choice to study location and extension and for evaluating adjacent organ invasion, distant metastasis and peritoneal seeding
- Magnetic resonance imaging (MRI) is being used more frequently for the evaluation of abdominal diseases because of its high soft tissue contrast and multiplanar capability, both of which are helpful for determining the organ of origin of large tumors and the relation between the tumor and other organs and major blood vessels (AJR Am J Roentgenol 2014;203:980)
- Presence of necrotic, hemorrhagic and cystic change makes appearance variable with small (< 5 cm) and large (> 5 cm) lesions having differing imaging characteristics (Radiographics 2003;23:283)
Prognostic factors
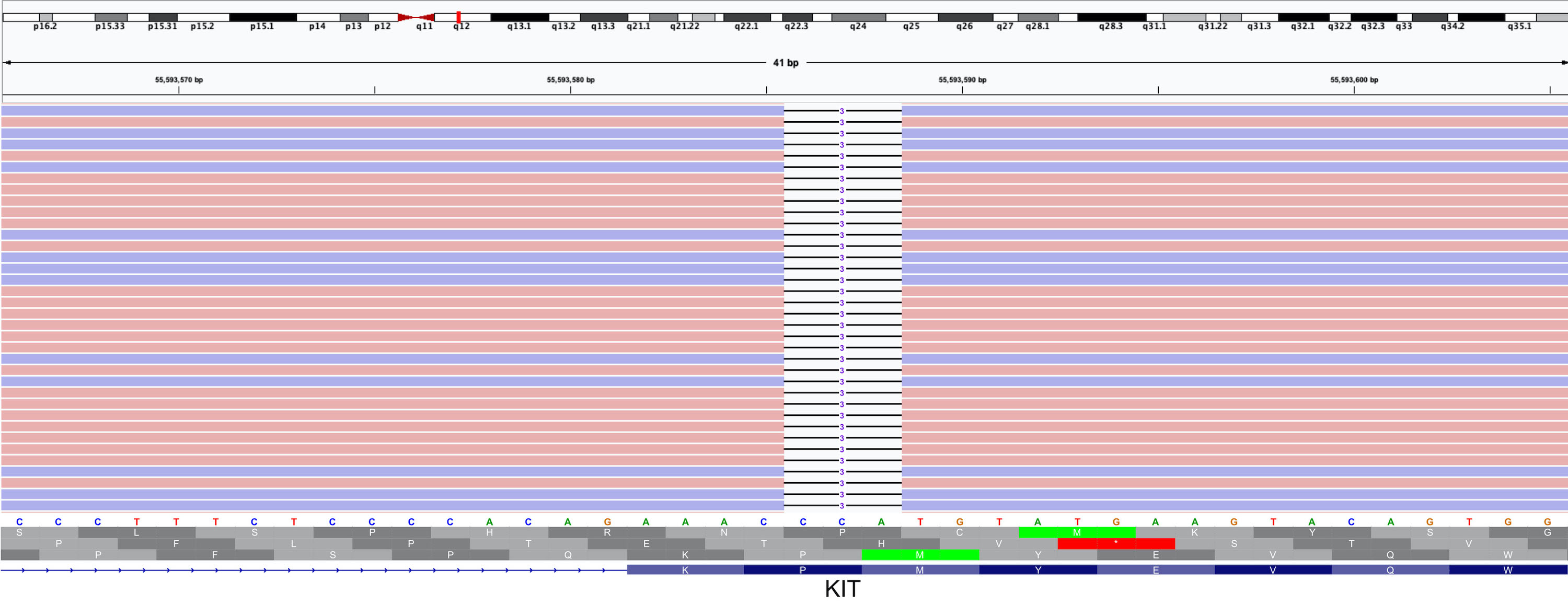
- See figure 2 in Diagrams / tables for guidelines for risk assessment of primary GIST
- Scheme includes location (anatomic site), mitotic count and size
- For anatomic sites not listed in this table, such as esophagus, mesentery and peritoneum (or in the case of insufficient data), it is best to use risk criteria for jejunum / ileum (Am J Surg Pathol 2005;29:52, Am J Surg Pathol 2001;25:1121, Am J Surg Pathol 2003;27:625, Semin Diagn Pathol 2006;23:70, Am J Surg Pathol 2006;30:477)
- Metastases are present at the time of diagnosis in ~15% of patients and are typically found in the peritoneum and liver (Ther Adv Med Oncol 2023;15:17588359231192388)
- Pediatric GISTs have a slow disease course and patients have a good prognosis despite the presence of metastasis to peritoneal cavity, lymph nodes and liver
- Rhabdoid gastric GISTs are associated with epithelioid morphology and PDGFRA mutations but do not imply aggressive behavior (Histopathology 2014;64:421)
Case reports
- 42 year old woman with history of NF1 and duodenal mass (Cureus 2021;13:e16034)
- 57 year old woman with asymptomatic tumor in the left rectus abdominis muscle (Int J Surg Case Rep 2011;2:253)
- 69 year old man with duodenal mass (Ann Ital Chir 2017;6:S2239253X17027220)
- 80 year old man with sigmoid tumor (Case Rep Gastroenterol 2014;8:257)
Treatment
- Complete surgical resection is the standard treatment for localized GIST (Ther Adv Med Oncol 2023;15:17588359231192388)
- Goal is an R0 surgery with complete removal of the tumor, including an intact pseudocapsule
- Recommended macroscopic resection margin is 1 cm
- Lymphadenectomy is unnecessary except for SDH deficient GIST or if macroscopic lymph node involvement is detected
- Treatment strategy based on frontline imatinib followed by surgery can be occasionally indicated in GIST patients with locally advanced disease
- Adjuvant treatment with imatinib in high risk patients with imatinib sensitive mutations in KIT or PDGFRA (Gastroenterol Clin North Am 2013;42:399)
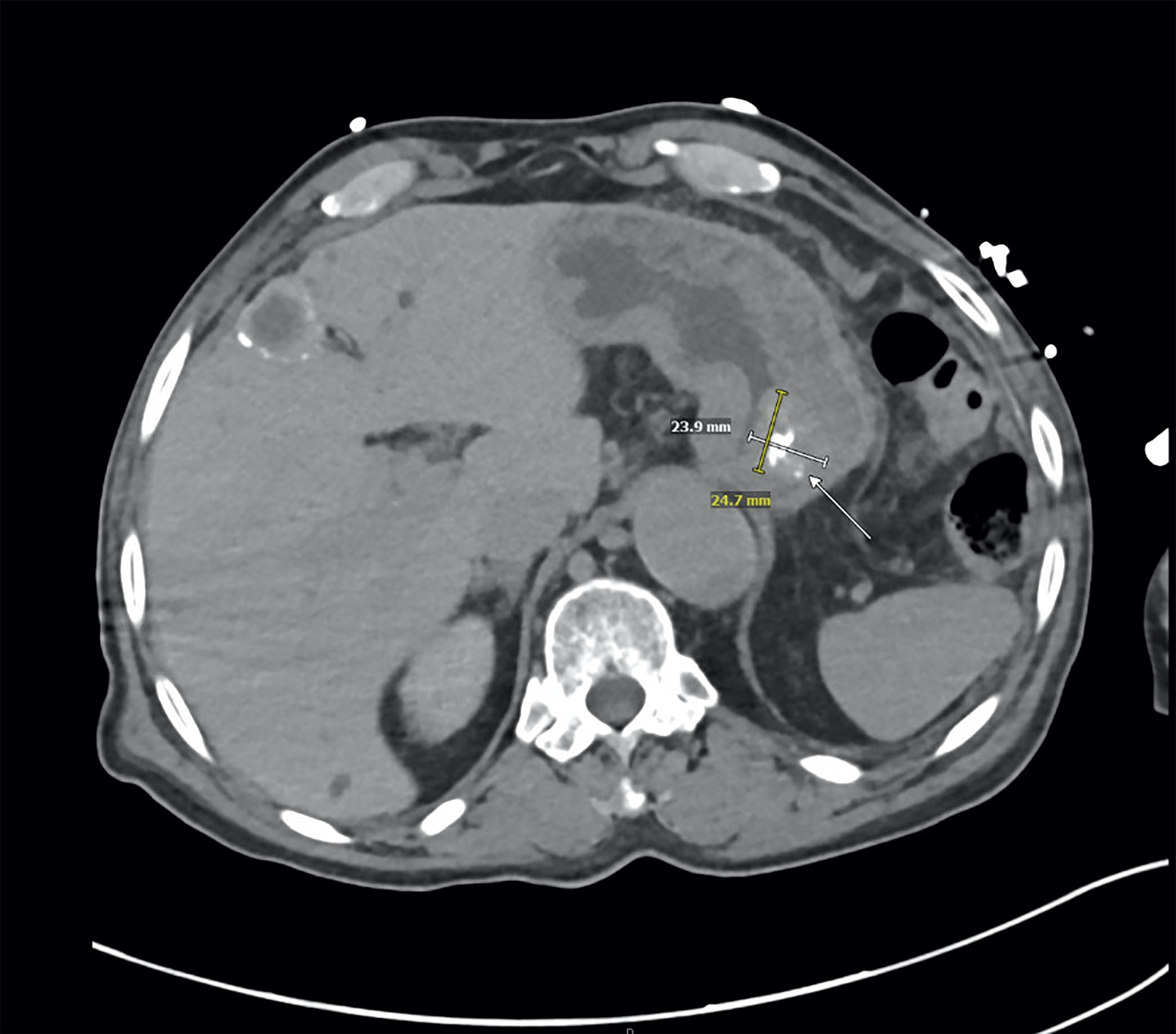
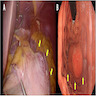
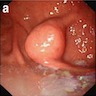
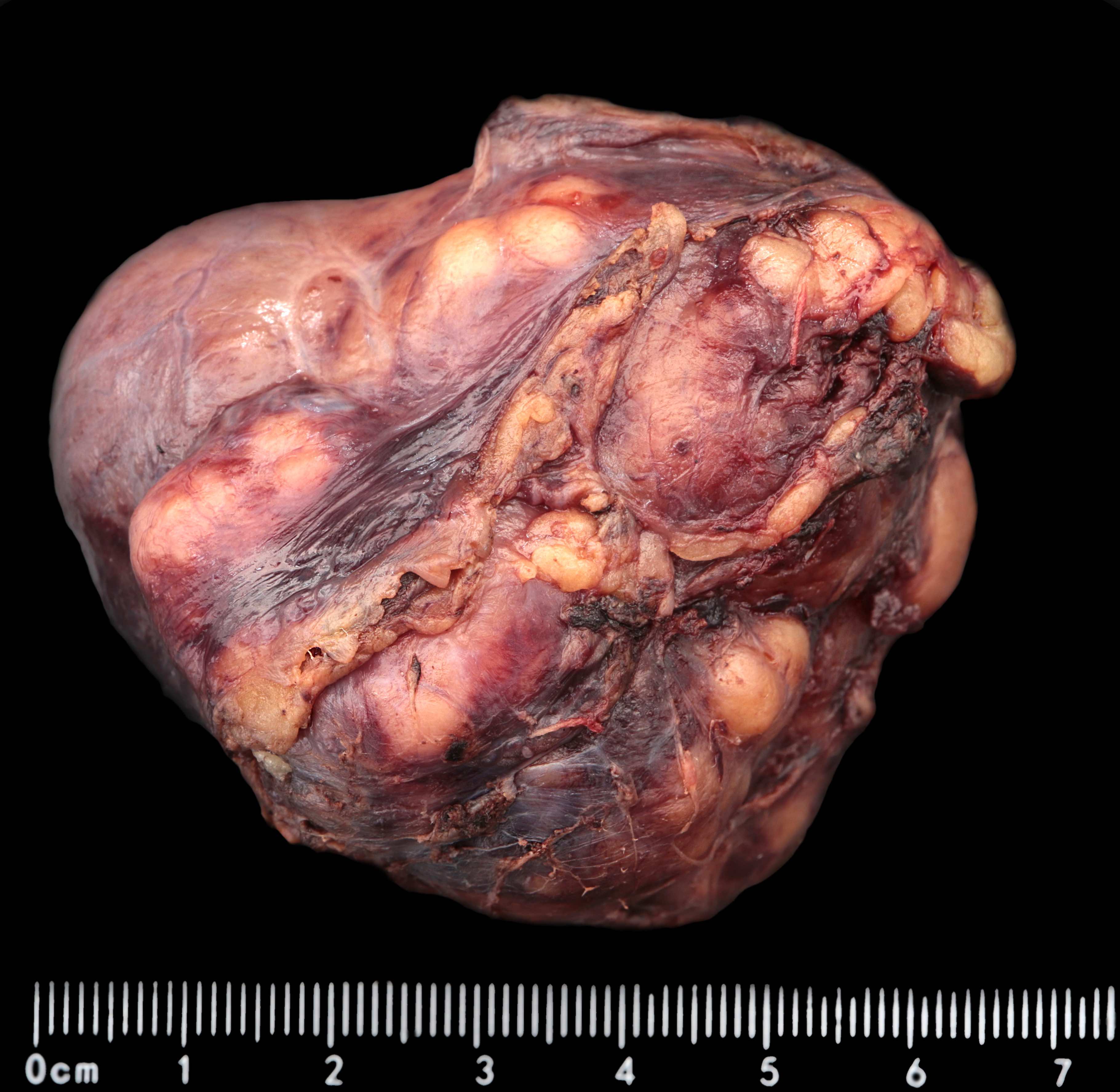
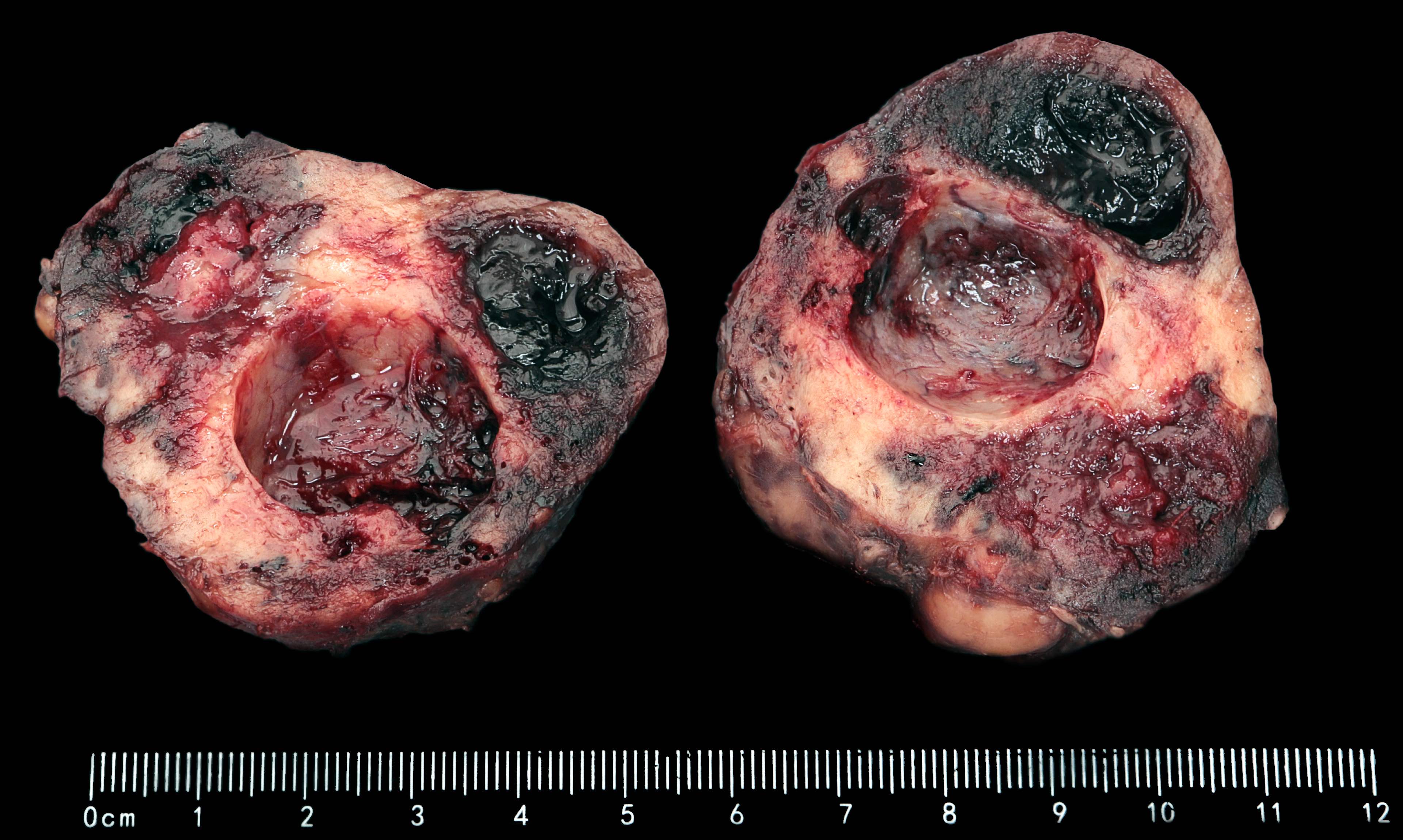
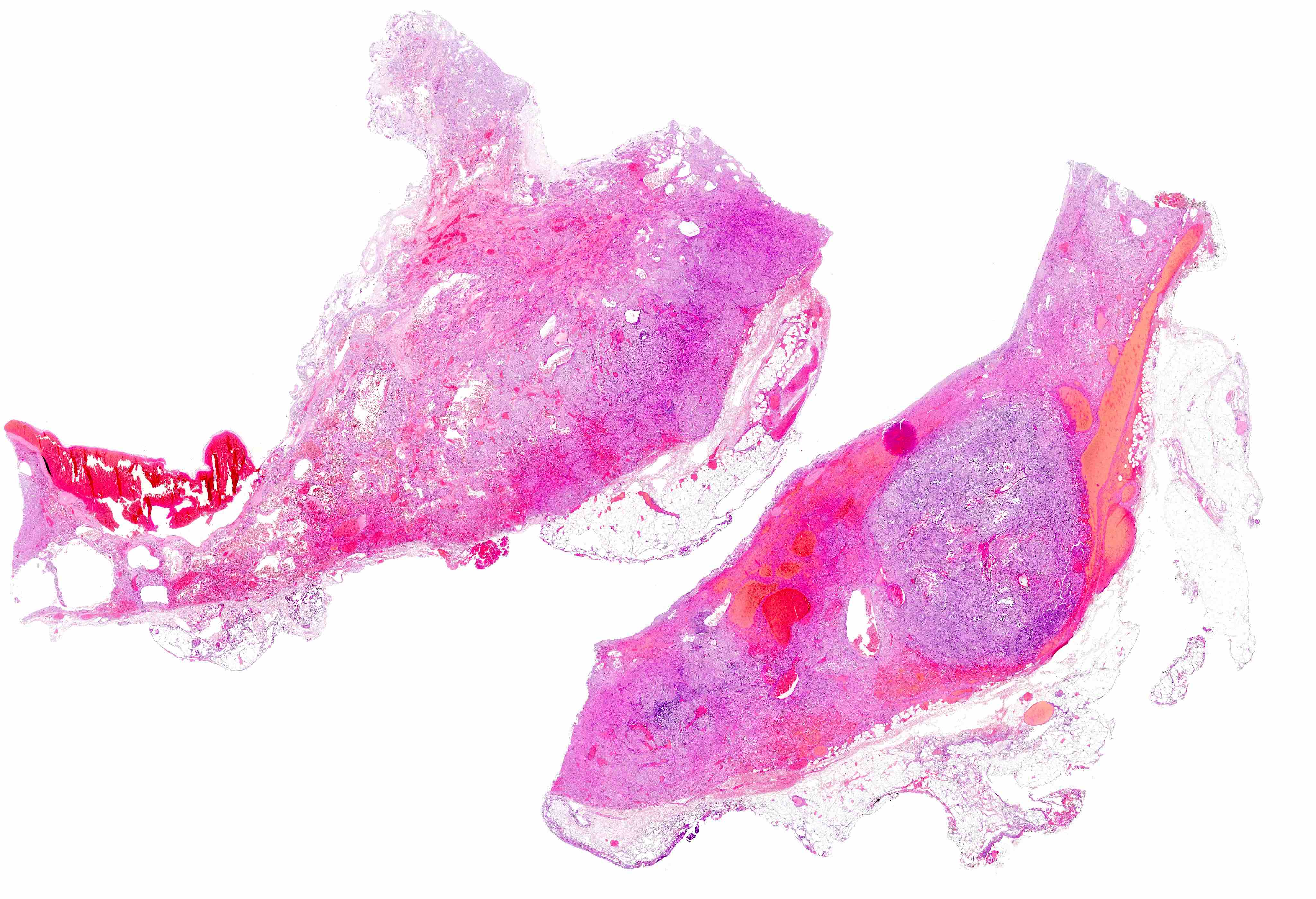
Gross description
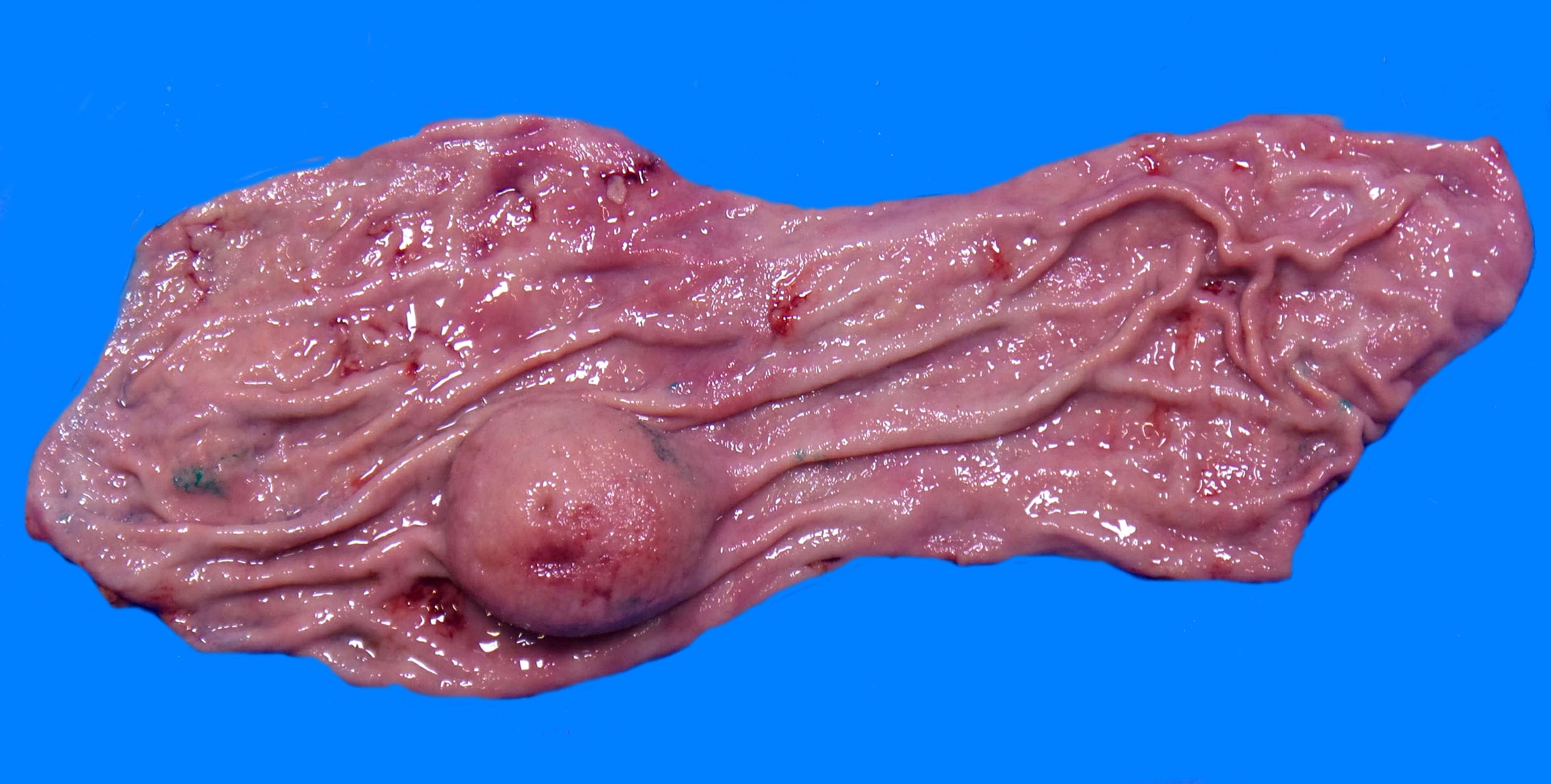
- Typically, GISTs arise from the wall of the digestive tract and extend inward toward the mucosa, outward to the serosa or in both directions (Virchows Arch 2001;438:1)
- Small GISTs often form solid subserosal, intramural or less commonly, polypoid intraluminal masses
- Majority of larger GISTs form external, sometimes pedunculated masses attached to the outer aspect of the gut and involve the muscular layers
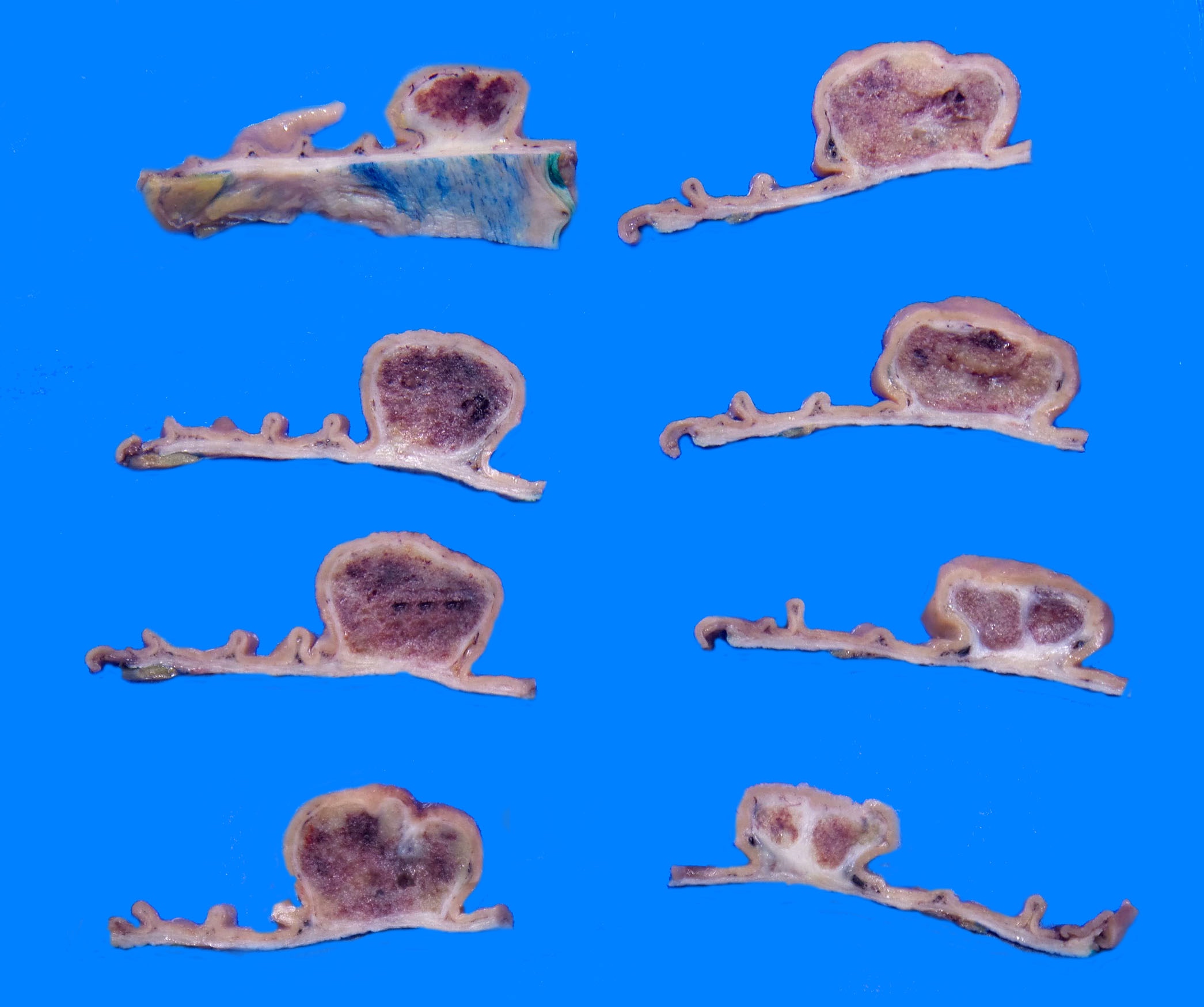
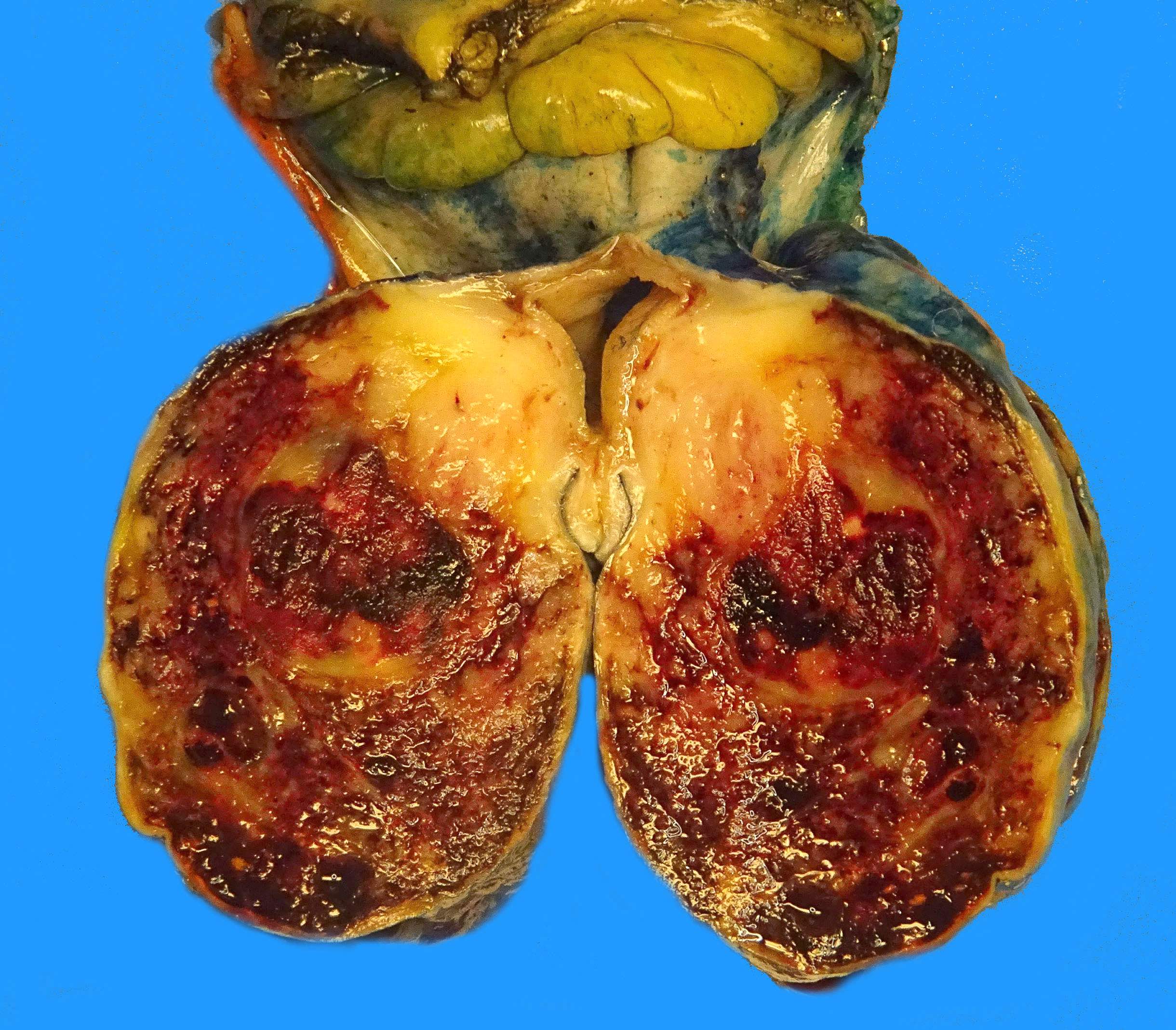
- Many larger tumors are centrally cystic (Arch Pathol Lab Med 2006;130:1466)
- Macroscopic areas of necrosis, hemorrhage and cystic degeneration may be seen (Virchows Arch 2001;438:1)
- Sporadic GISTs grow as solitary masses
- Multinodular patterns may raise suspicion for particular entities, such as SDH deficient GIST NF1 or familial GIST (Virchows Arch 2001;438:1)
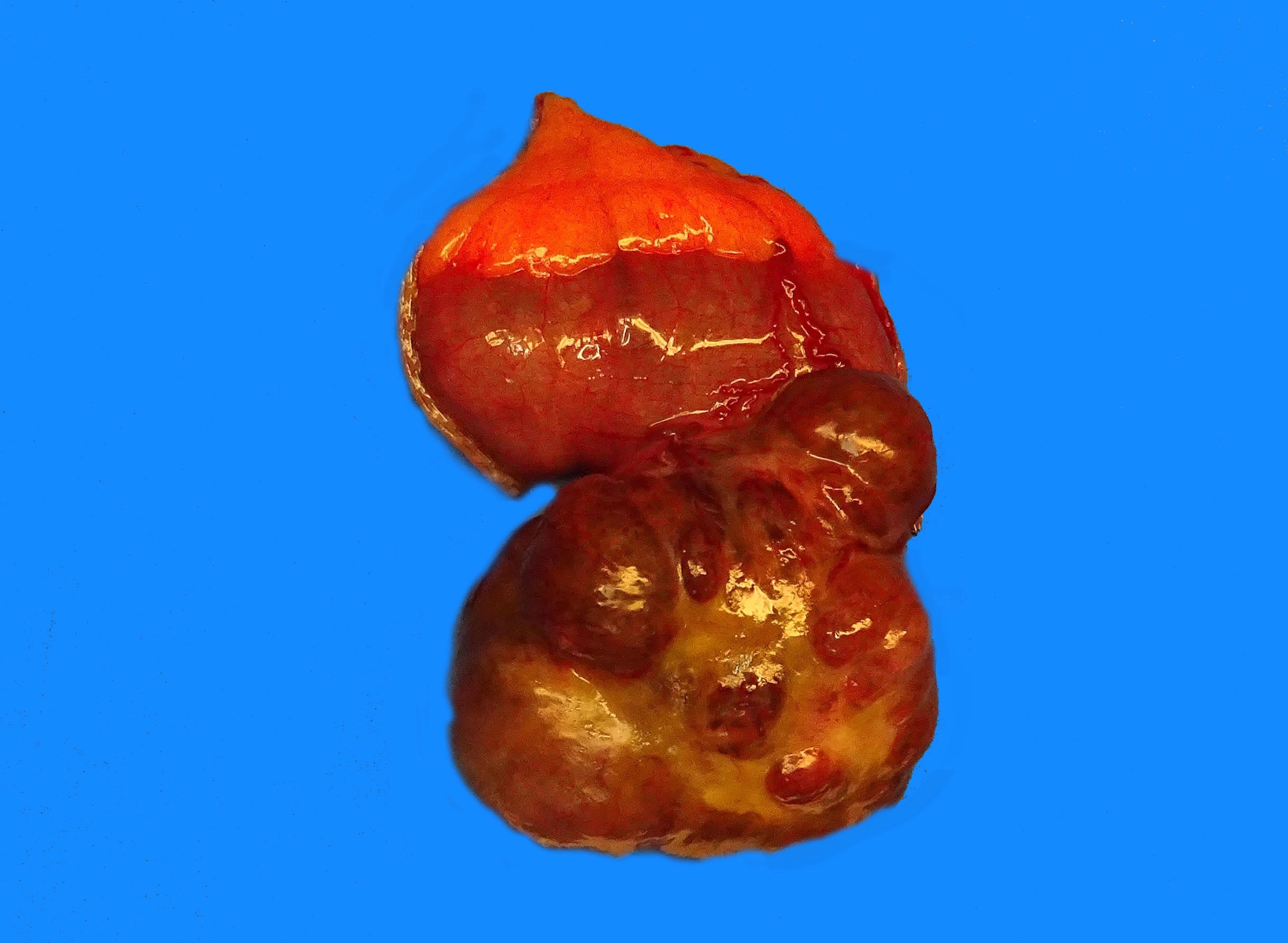
- Tumor is well circumscribed
- Fleshy, tan-pink or tan-brown cut surfaces, which may show hemorrhage, necrosis or cystic degeneration (J Korean Med Sci 2004;19:234)
Gross images
Frozen section description
- Spindle cell or epithelioid neoplasm
Microscopic (histologic) description
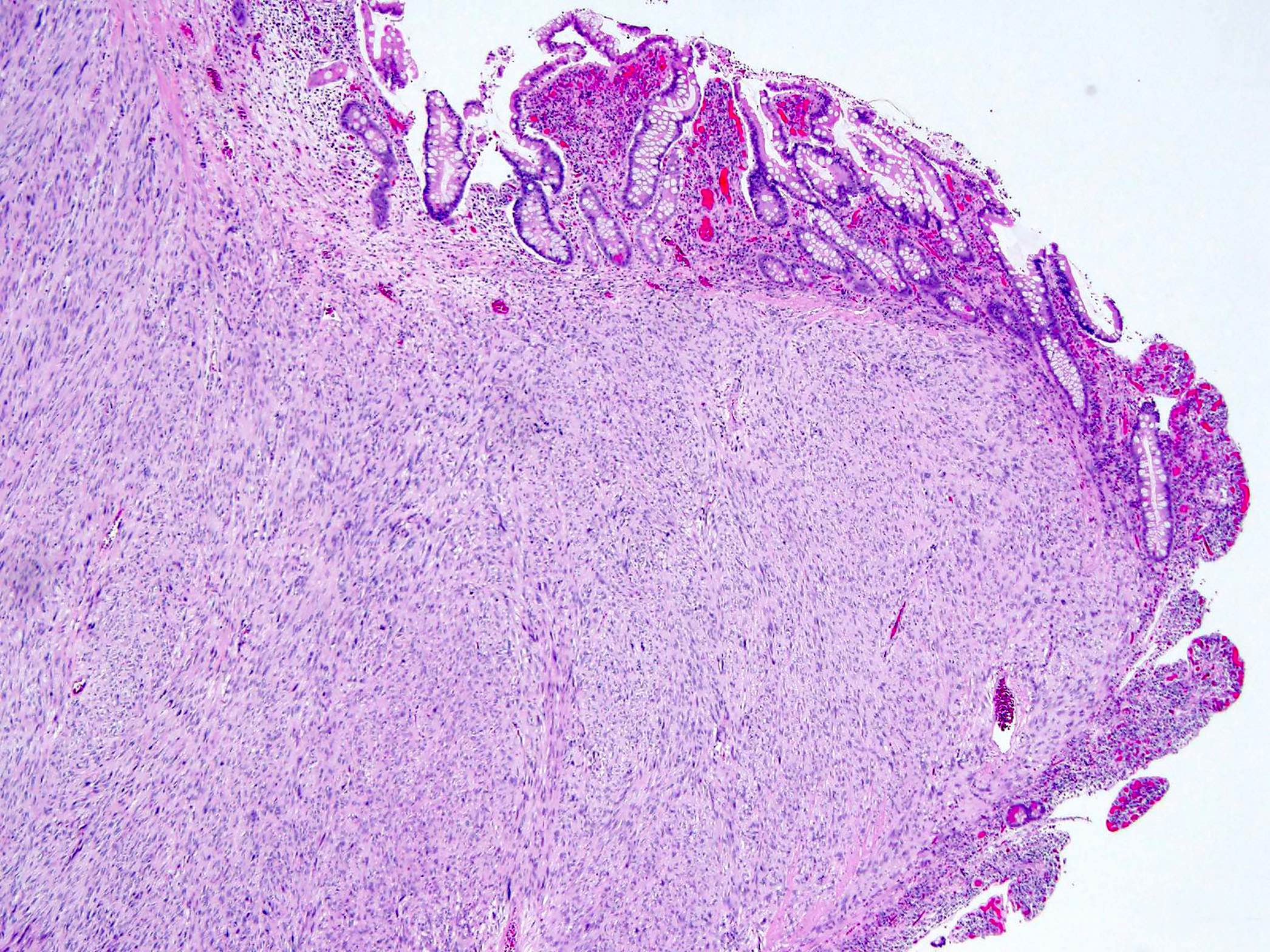
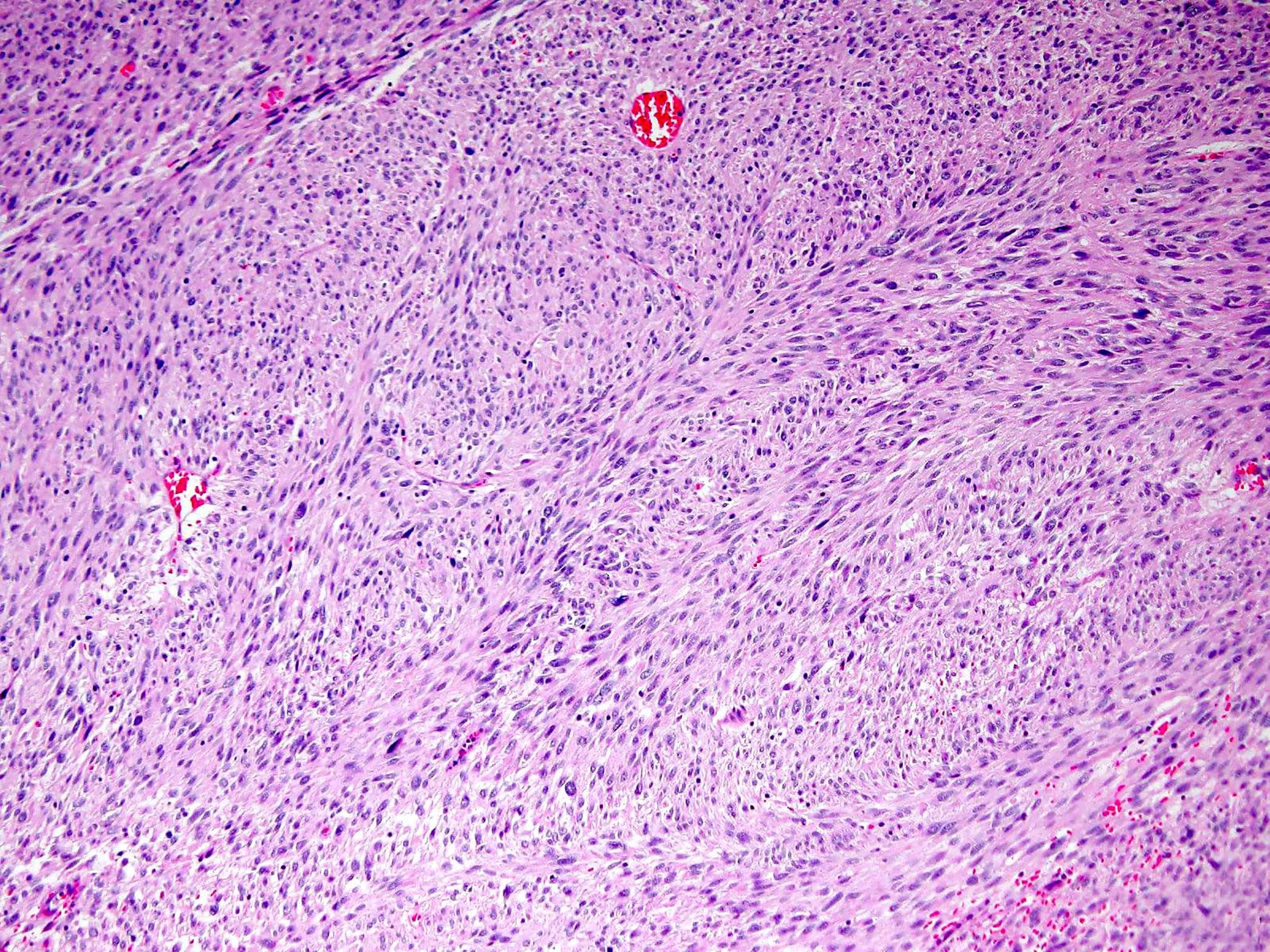
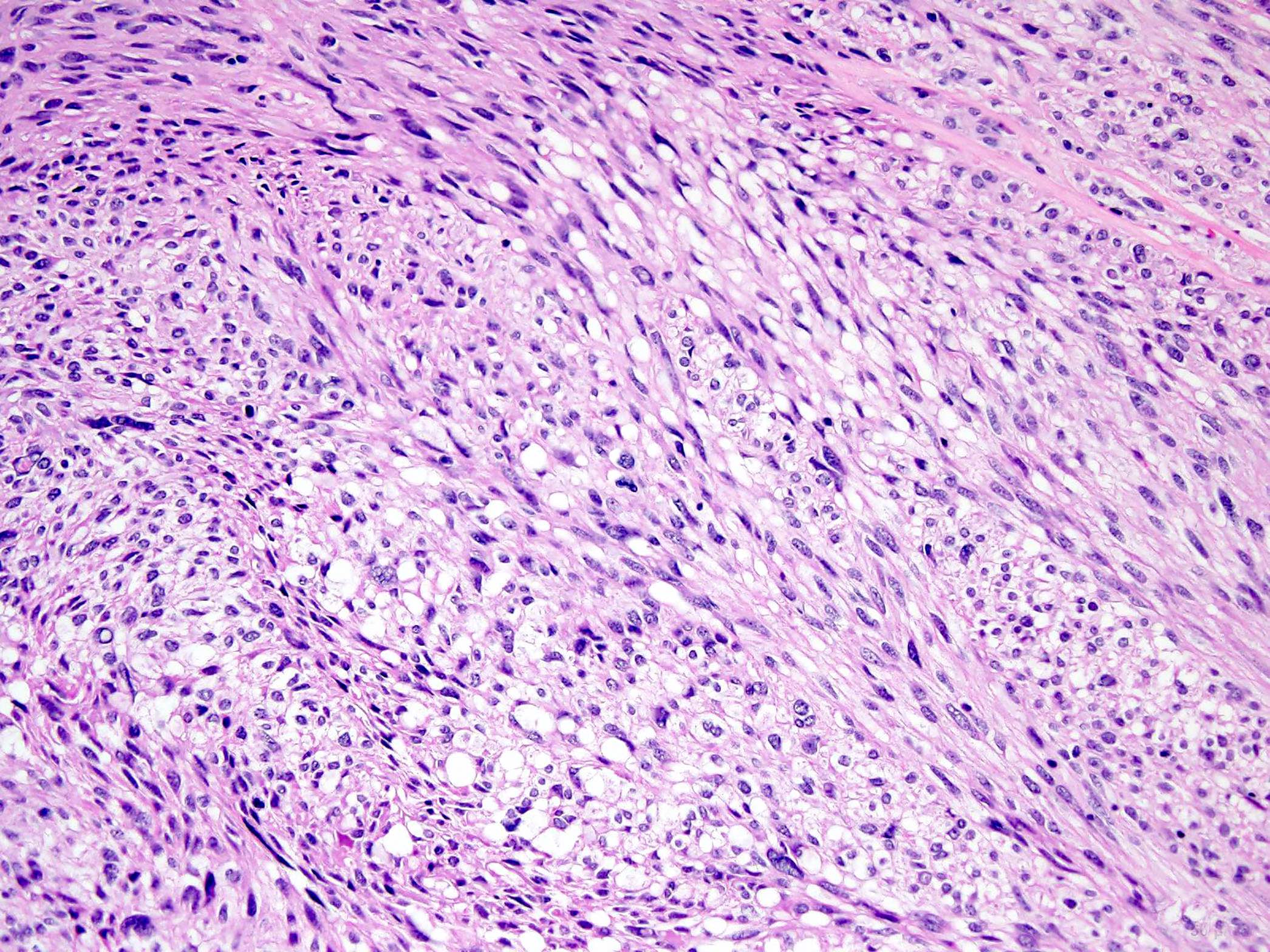
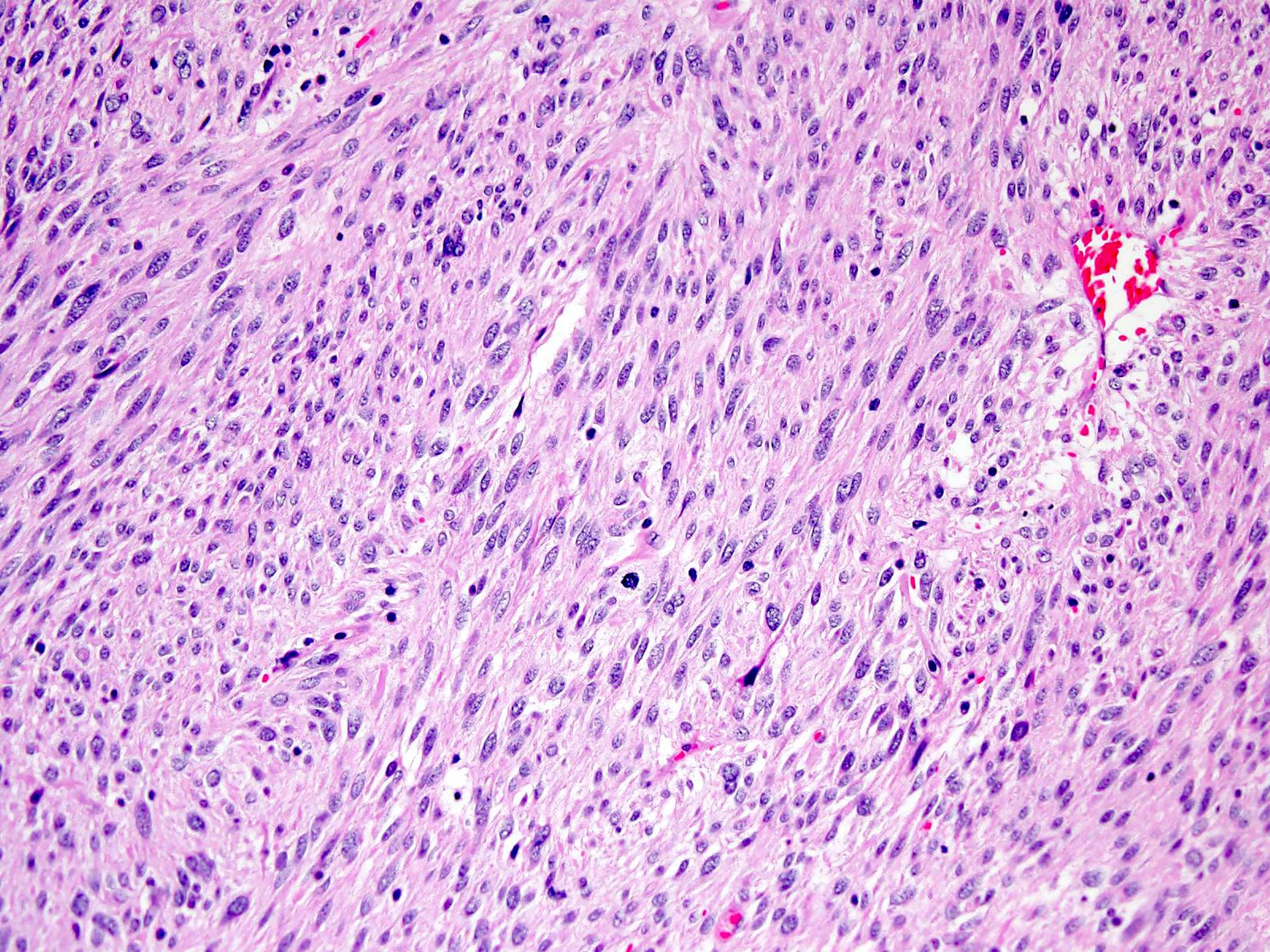
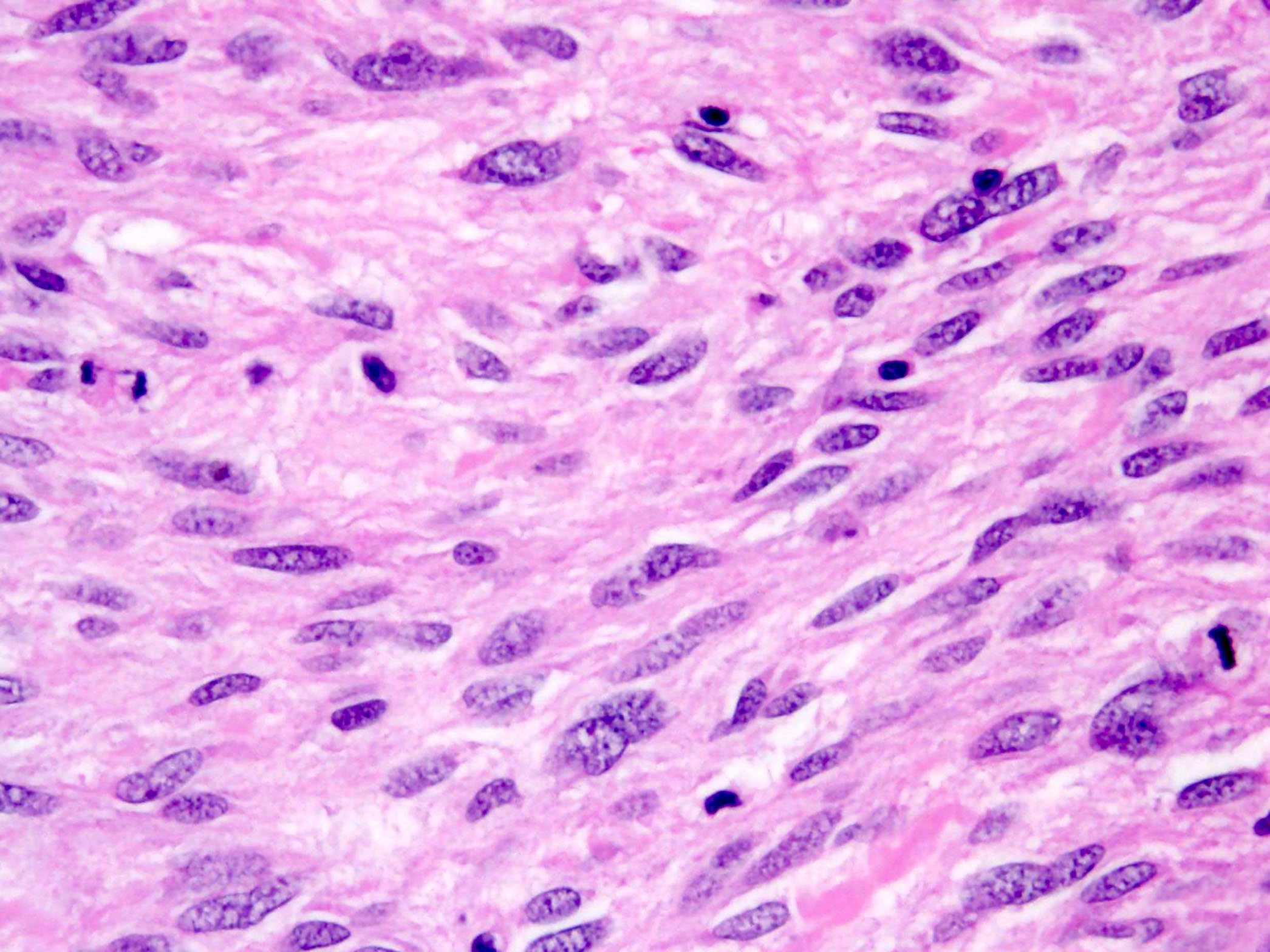
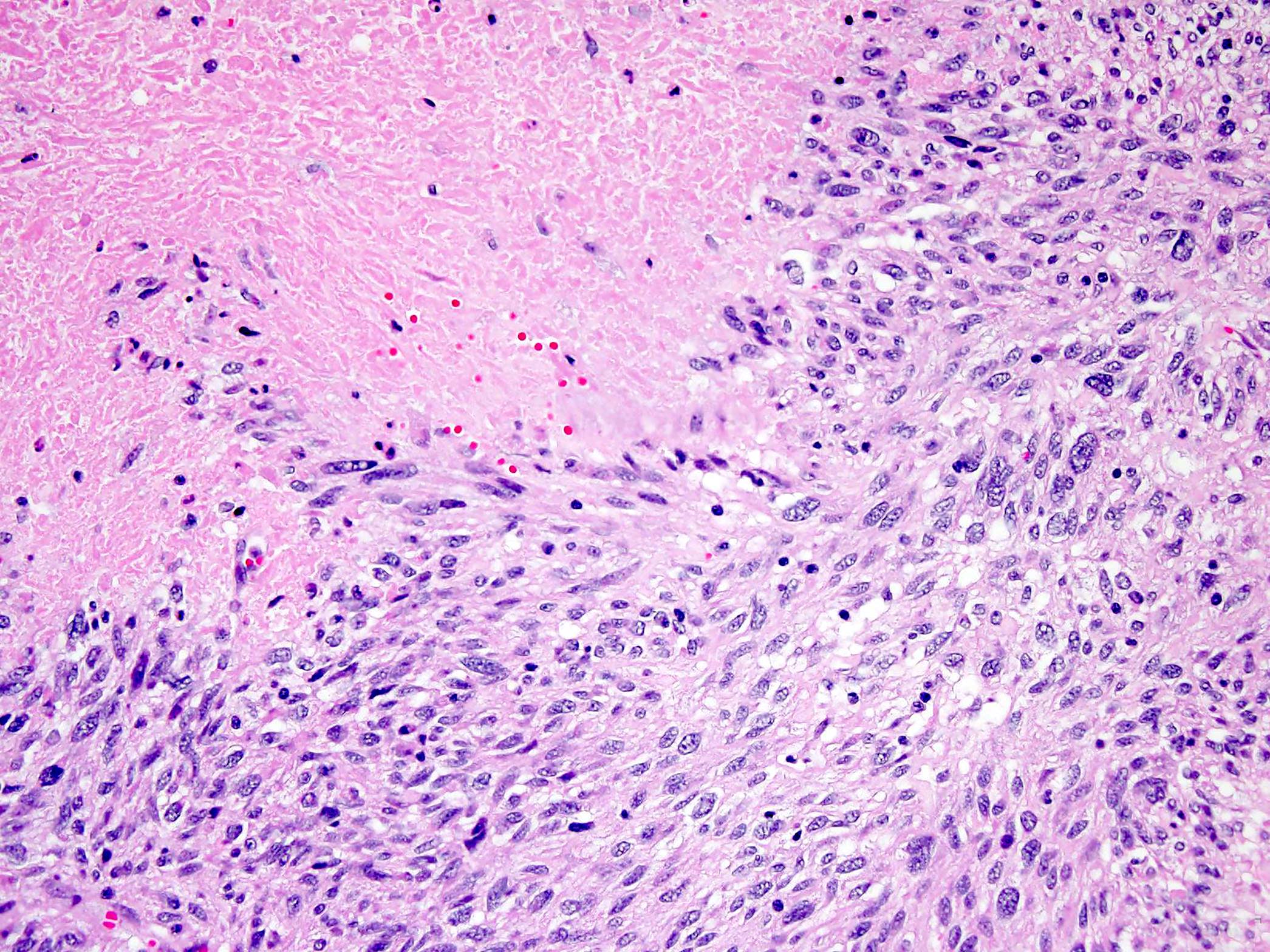
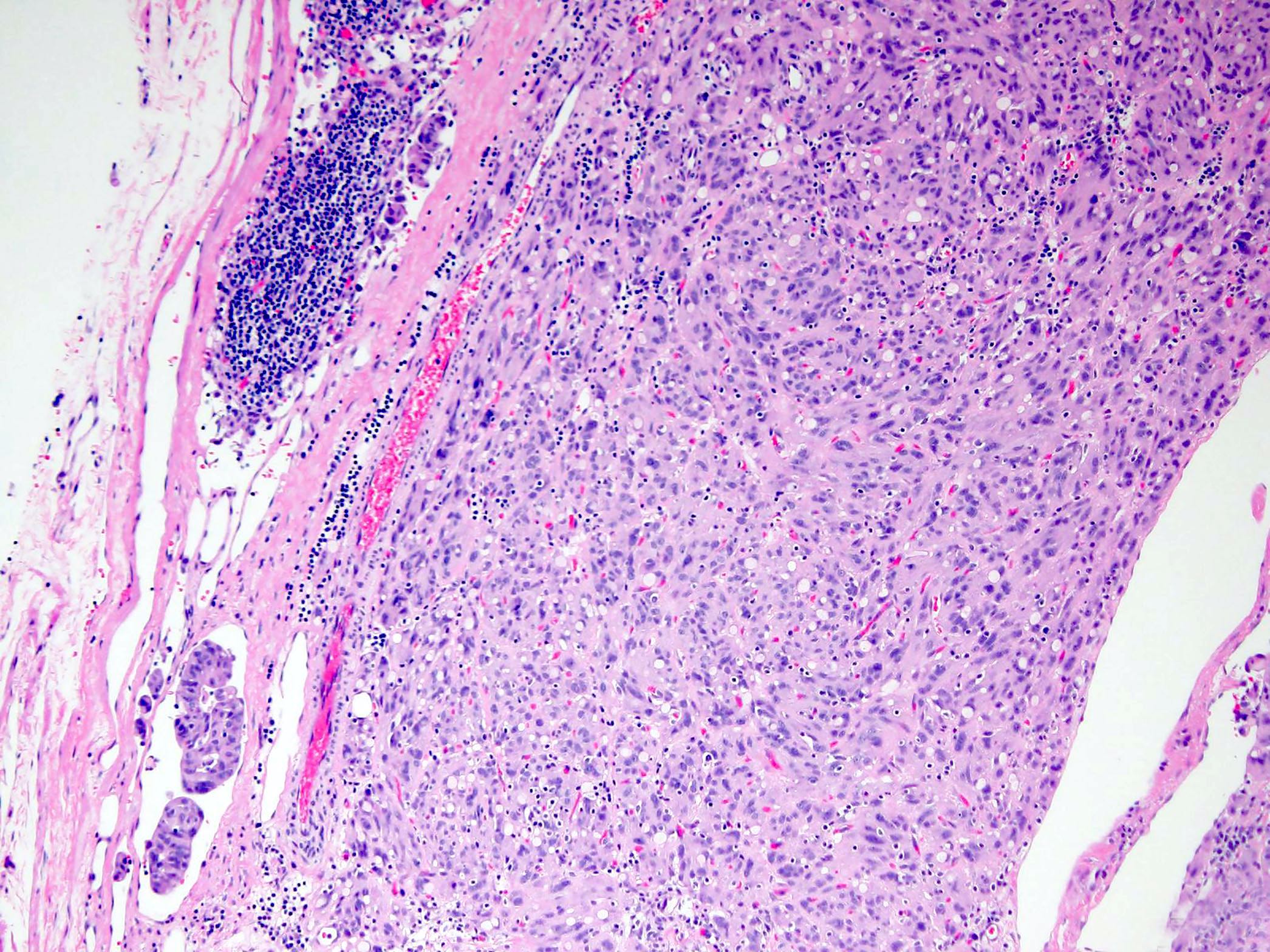
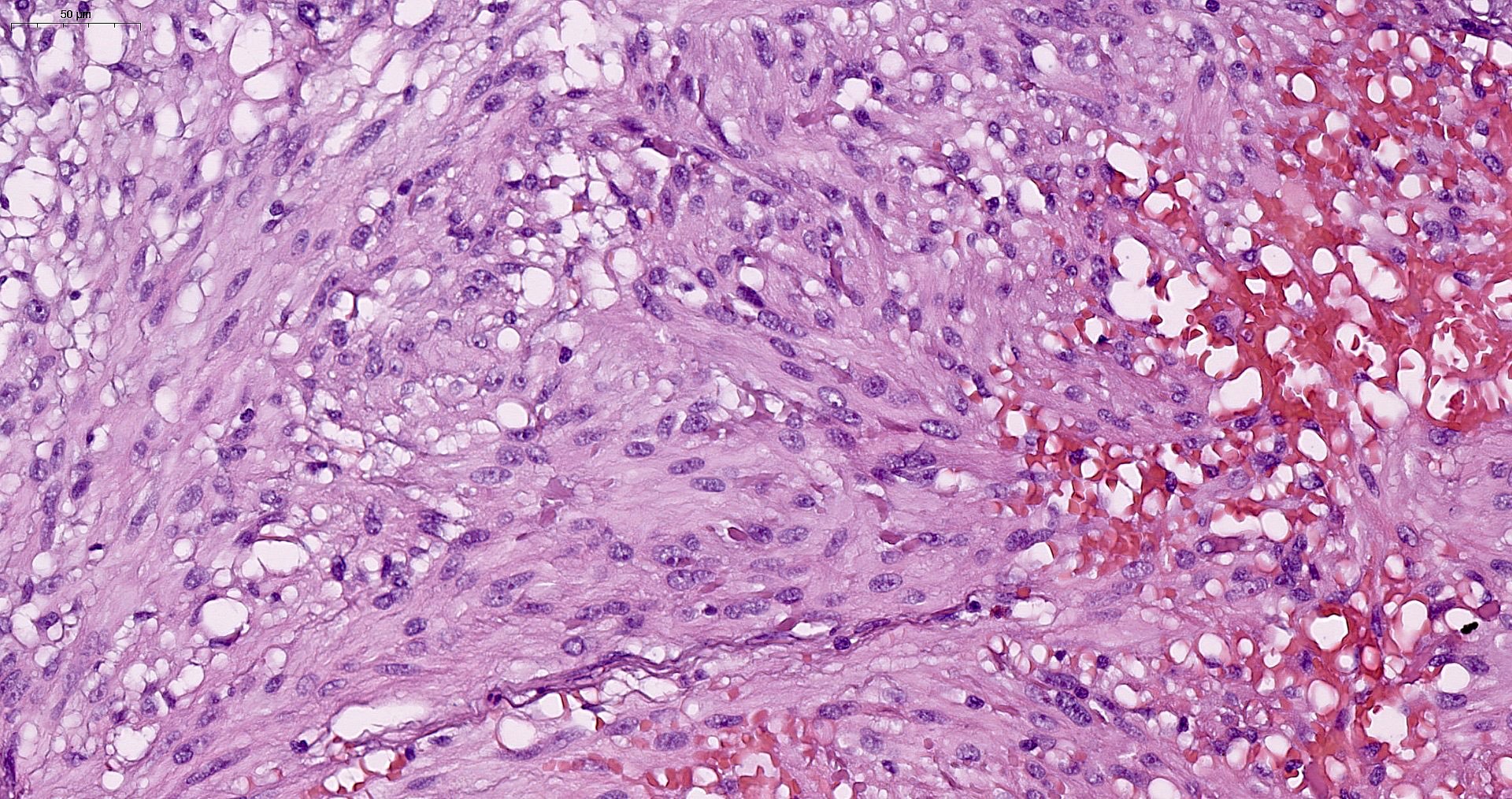
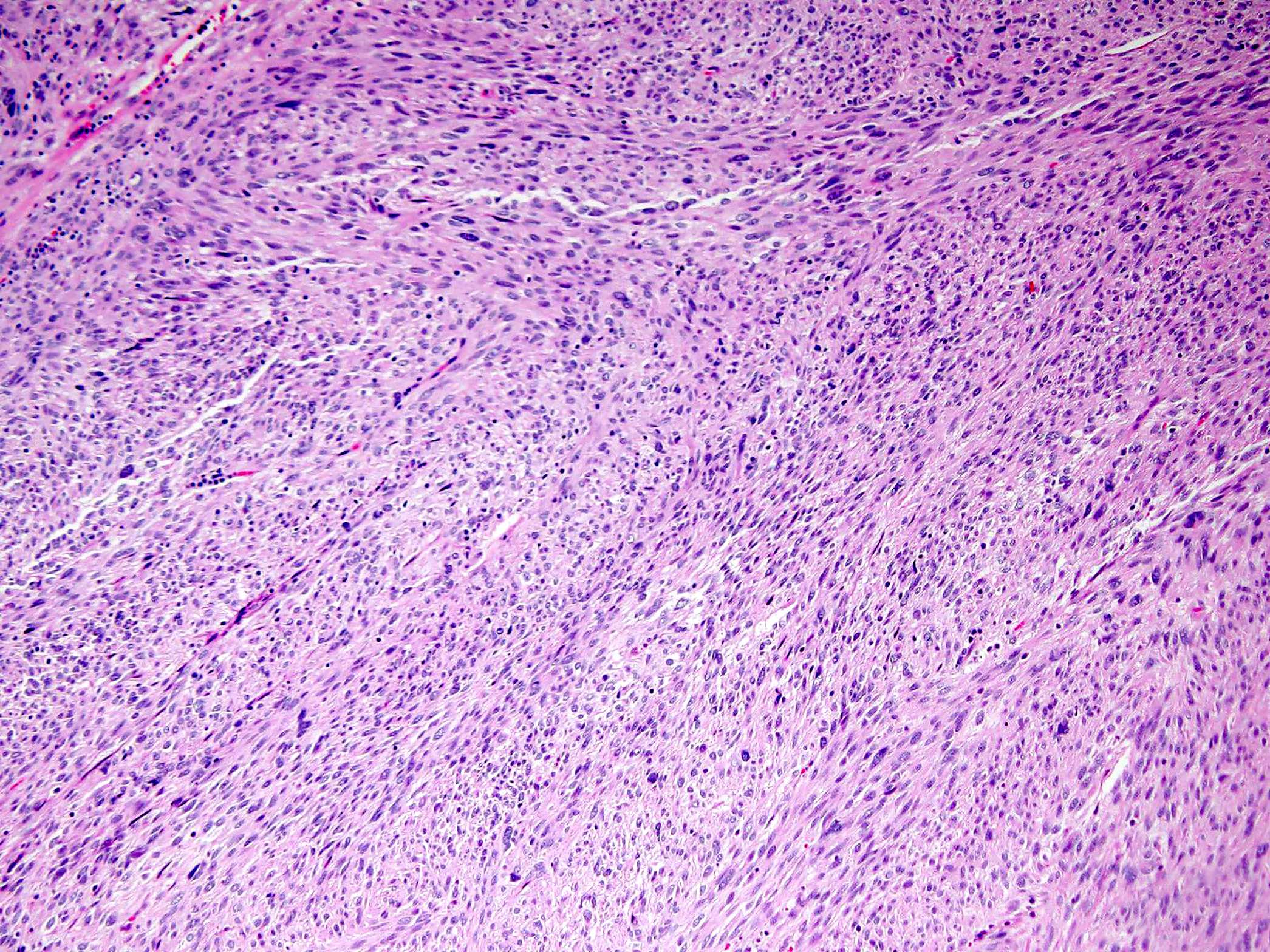
- GISTs exhibit a broad spectrum of morphology
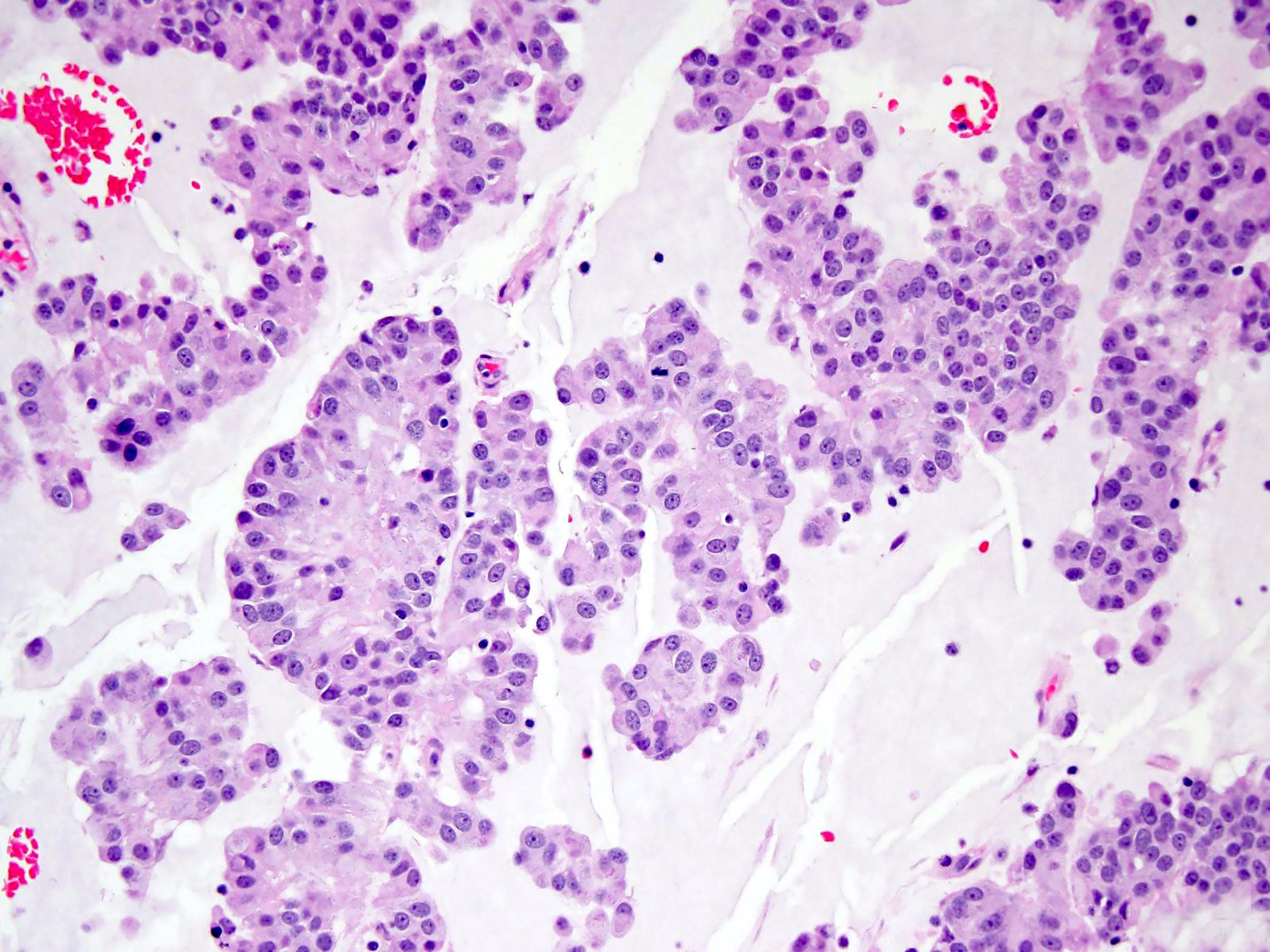
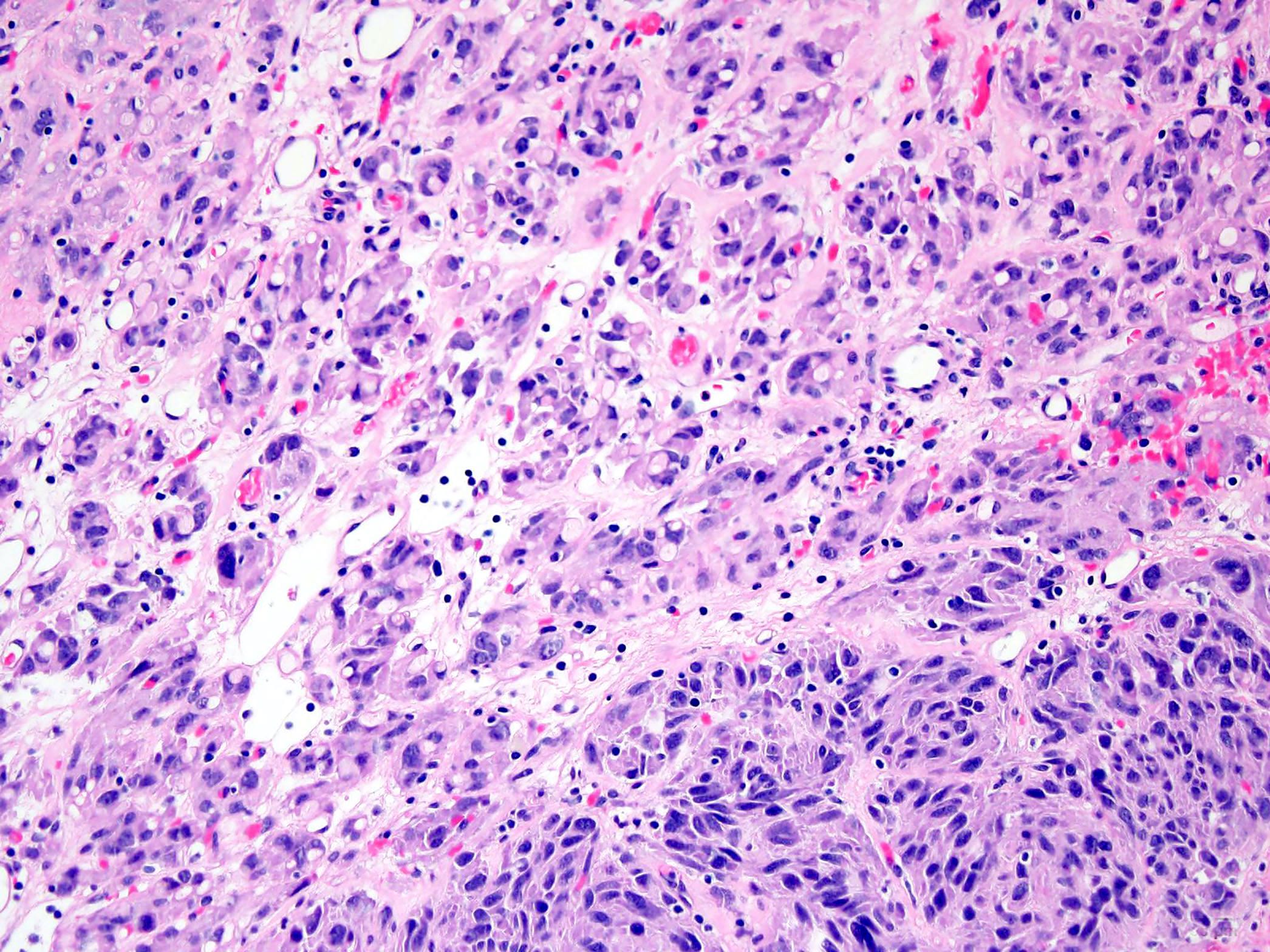
- Most GISTs are spindle cell tumors, with epithelioid morphology seen in ~25% of cases
- Some cases feature a combination of spindle cell and epithelioid morphology
- Nuclear pleomorphism is uncommon
- Tumor cells have round or elongated nuclei and abundant eosinophilic cytoplasm
- Perinuclear vacuolization is a distinctive feature
- Anuclear areas (somewhat mimicking Verocay bodies) composed of cell processes
- Nuclear palisading, perivascular hyalinization and regressive vascular changes similar to those in schwannomas can be seen
- Presence of skeinoid fibers (extracellular eosinophilic collagen globules)
- Myxoid stroma is rarely observed
- Mitotic activity or necrosis
- Diffuse Cajal cell hyperplasia is often present in patients with NF1
Histologic features of gastric GISTs
- Up to 70% of gastric GISTs can be histologically subclassified into 8 subtypes, 4 of them describing spindle cell and 4 of them describing epithelioid tumors (Arch Pathol Lab Med 2006;130:1466)
- Sclerosing spindle cell GISTs are usually small, mitotically inactive, relatively paucicellular tumors, in which the cells are dispersed in a prominently collagenous, sometimes calcified matrix
- Palisading vacuolated subtype shows nuclear palisading resembling that of peripheral schwannomas; prominent perinuclear vacuolization is a typical feature
- These tumors usually have low mitotic counts but can reach a size of > 10 cm
- This subtype is the most common among gastric GISTs
- Hypercellular subtype contains densely packed, uniform spindle cells lacking significant atypia and mitotic activity.
- Sarcomatous spindle cell tumors have marked mitotic activity (generally > 20 per high power field [HPF]; > 4 per 10 HPFs) and diffuse atypia manifested by nuclear enlargement and hyperchromasia without much pleomorphism
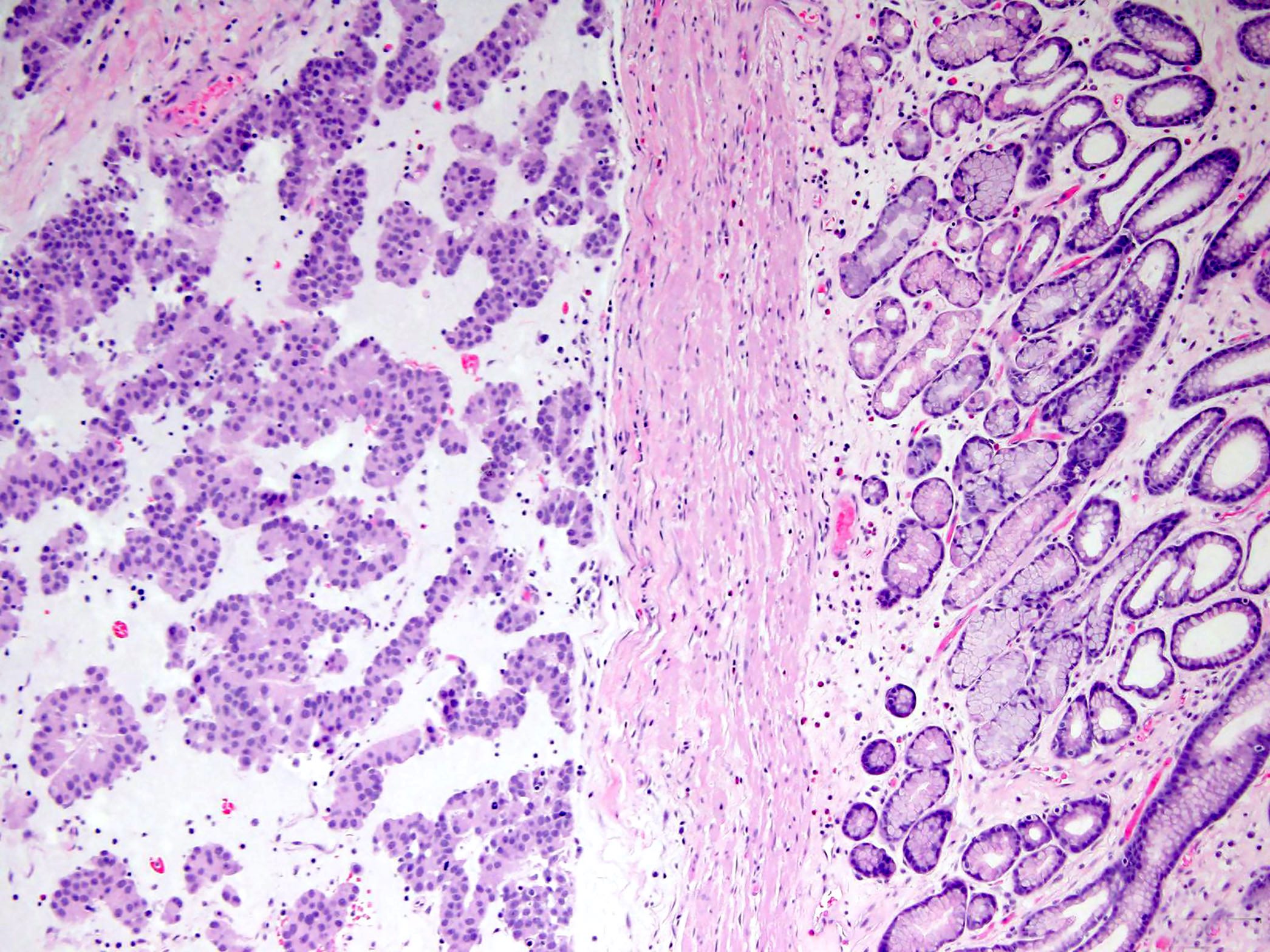
- Sclerosing epithelioid variant contains polygonal tumors cells set in a syncytial pattern in a variably sclerosing stroma without visible cell borders
- These tumors have a low mitotic rate but focal atypia and multinucleation is common
- Dyscohesive epithelioid subtype features epithelioid cells surrounded by a lacunar space and sharp cell borders
- Focal nuclear pleomorphism is common in these tumors and is not worrisome unless accompanied by an elevated mitotic rate
- Hypercellular and sarcomatous epithelioid subtypes represent highly cellular tumors with closely apposed cells, typically with well defined cell borders; the former is characterized by low mitotic activity, whereas the latter shows marked mitotic activity, often > 20 per 50 HPFs
Histologic features of small intestinal GISTs
- These tumors do not form distinctive histologic subtypes as gastric GISTs do
- Most small intestinal GISTs are composed of spindle cells and nearly half (40 - 50%) contain microscopically distinctive, round, oval or elongated eosinophilic and periodic acid-Schiff positive aggregates of extracellular collagen fibers; these have been named skeinoid fibers by their lamellar, concentric, ultrastructural appearance (Am J Surg Pathol 1992;16:145, Am J Surg Pathol 1997;21:407)
- Other patterns of small intestinal GISTs include tumors with anuclear areas resembling neuropil material
- Despite the commonly malignant course, only a minority of small intestinal GISTs have histologically sarcomatous features with high mitotic activity; pleomorphic forms are rare
- Epithelioid pattern in small intestinal GISTs is significantly linked with malignant tumors
- It differs both morphologically and clinically from the gastric epithelioid GISTs and probably represents a morphologic manifestation of tumor progression rather than a distinct histologic subtype
Histologic features of GISTs of other sites
- Most GISTs of sites other than stomach and small intestine are spindle cell tumors
- Appendiceal GISTs resemble the small intestinal tumors by the common content of skeinoid fibers, which are also seen in some colonic but not in rectal GISTs
- Rectal spindle cell GISTs can show hyalinized - calcified or palisading nuclear pattern somewhat similar to those seen in gastric tumors and malignant examples can have a leiomyosarcoma-like fascicular pattern
- Epithelioid GISTs similar to those often seen in the stomach are rarely observed in the rectum (Am J Surg Pathol 2001;25:1121)
- GISTs in the omentum can have spindle cell and epithelioid features resembling those of gastric GISTs, whereas mesenteric GISTs often have features resembling the small intestinal tumors, including the presence of skeinoid fibers in some examples (Am J Surg Pathol 2001;25:1121, J Surg Oncol 2011;104:865)
Histologic features of pediatric GISTs
- Pediatric GISTs typically arise in the stomach as multifocal tumors with a multinodular or plexiform growth pattern, epithelioid or mixed epithelioid and spindle cell morphology and lymph node metastases (Transl Gastroenterol Hepatol 2018;3:54, Am J Surg Pathol 2011;35:1245, Am J Surg Pathol 2011;35:495, J Surg Oncol 2011;104:865)
- Occasional GISTs in adults show similar features
Rhabdoid GISTs
- Rhabdoid GISTs are associated with epithelioid morphology (Histopathology 2014;64:421)
GISTs with prominent myxoid matrix
- Proliferation of round, spindle or stellate cells embedded in an abundant myxoid stroma (Am J Surg Pathol 1995;19:59)
Dedifferentiated GIST
- Small minority of GISTs exhibit morphologic and phenotypic changes and differentiate into an unusual phenotype through the process of dedifferentiation (Ann Diagn Pathol 2019;39:118, Am J Surg Pathol 2013;37:385)
- Dedifferentiation can occur either de novo or after prolonged treatment with imatinib, a selective tyrosine kinase inhibitor
- Dedifferentiated GISTs can present with various morphologies including rhabdomyosarcoma, angiosarcoma or undifferentiated pleomorphic sarcoma with high mitotic activity and necrosis
Microscopic (histologic) images
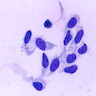
Cytology description
- Hypercellular sample composed predominantly of spindle shaped neoplastic cells (Cancer 2004;102:157)
- Loosely cohesive group of spindle shaped to ovoid neoplastic cells with moderate to marked nuclear atypia
- Coarse chromatin, prominent nucleoli and irregular nuclear contours
Cytology images
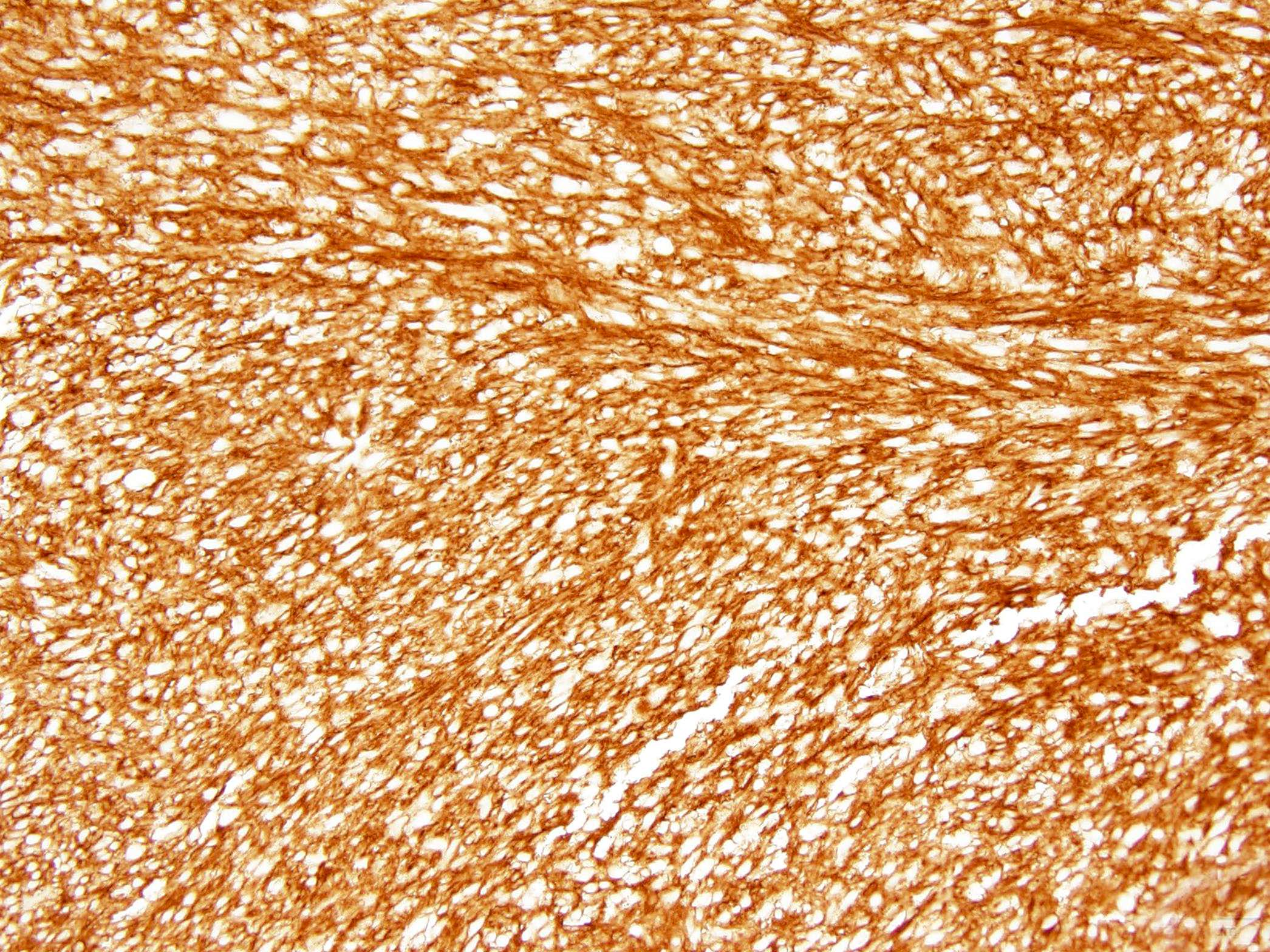
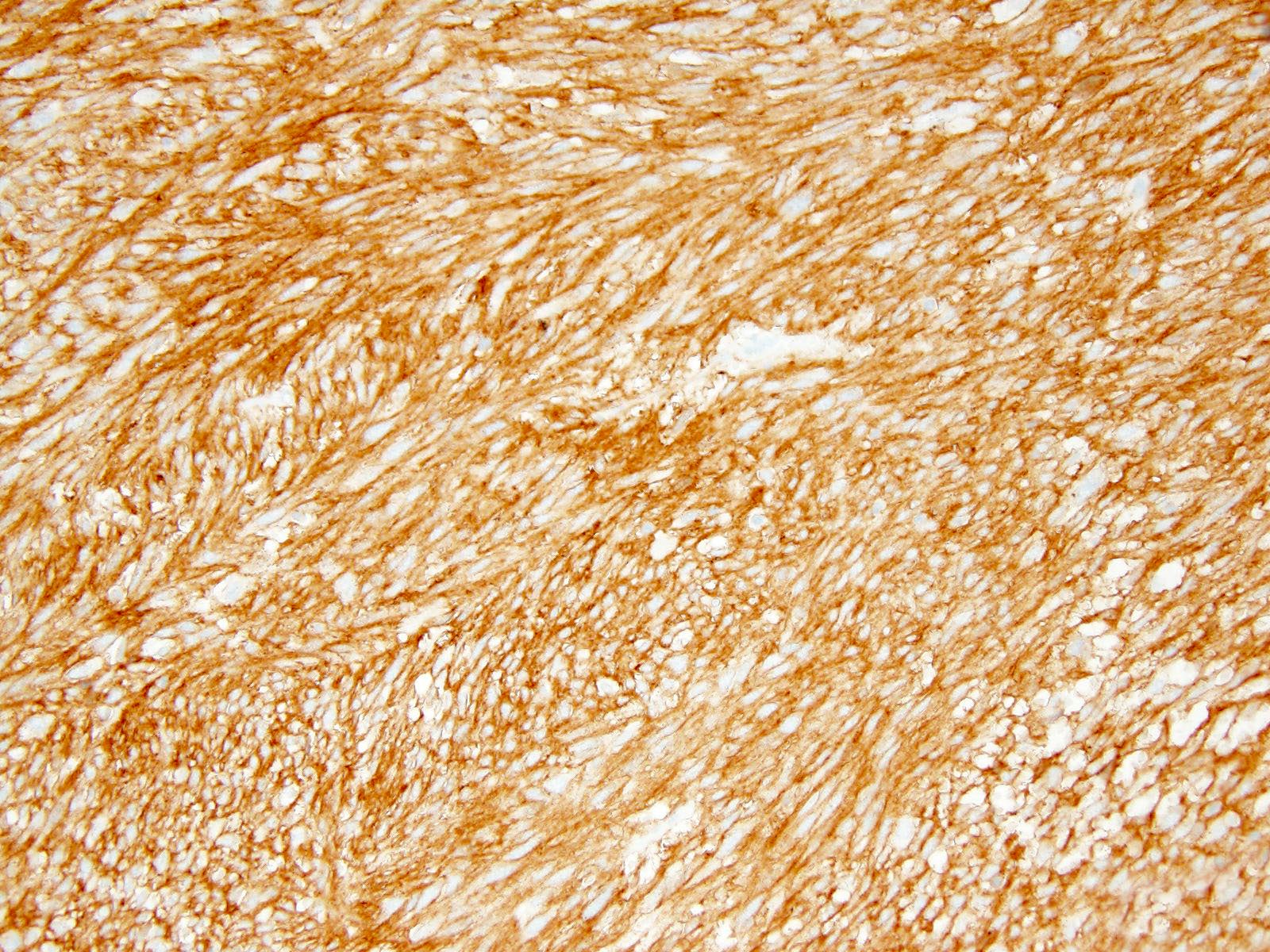
Positive stains
- KIT / CD117 (Mod Pathol 2000;13:1134)
- DOG1 is of great utility for the diagnosis of GIST subgroups lacking KIT or PDGFRA mutations (Am J Surg Pathol 2009;33:437, Appl Immunohistochem Mol Morphol 2010;18:333)
- CD34
Negative stains
- Desmin (Mod Pathol 2000;13:1134)
- S100
- Keratin AE1 / AE3
- ALK
- HMB45 / MelanA (Transl Gastroenterol Hepatol 2018;3:27)
- Nuclear beta catenin
- αSMA (47% positivity) (Histopathology 2017;71:805)
- h caldesmon (5% positivity) (Diagnostics (Basel) 2022;12:1563)
- STAT6
- Loss of SDHB in SDH deficient GIST (Am J Surg Pathol 2010;34:636)
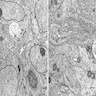
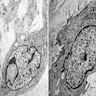
Electron microscopy description
- Neoplastic cells are polygonal or elongated in appearance (J Korean Med Sci 2004;19:234)
- Euchromatic or hyperchromatic nuclei with psedoinclusions and small nucleoli
- Abundant cytoplasmic organelles including smooth and rough endoplasmic reticulum, mitochondria, lysosomes and Golgi apparatus
- Slender cytoplasmic processes
- Hemidesmosome-like junctions and gap junctions
- Abundant intermediate filaments
- Extracellular skeinoid fibers
Molecular / cytogenetics description
- Most common gain of function mutations in GISTs are either in the KIT (60 - 70%) or platelet derived growth factor receptor alpha (PDGFRA) genes (10 - 15%), which are mutually exclusive (Cancers (Basel) 2022;14:5330)
- Smaller subset that lacks KIT and PDGFRA mutations is considered wild type GIST (Cancers (Basel) 2022;14:5330)
- Molecular classification of GISTs
- Until recently, GISTs were classified into 2 main groups: KIT / PDGFRA mutated GISTs (85% of cases) and KIT / PDGFRA wild type GIST; however, rare mutations or fusions have been described among the latter, which made the wild type description less precise (Cancers (Basel) 2022;14:5330)
- Among the wild type KIT / PDGFRA GISTs, subgroups with well defined molecular features have been described, including those harboring mutations in the BRAF, RAS or NF1 genes (Transl Gastroenterol Hepatol 2018;3:92)
- Around a third of wild type KIT / PDGFRA GISTs demonstrate the loss of function of the SDH complex, manifested by the loss of subunit B (SDHB) protein expression (Transl Gastroenterol Hepatol 2018;3:92)
- Pediatric GISTs differ from most adult ones in their lack of KIT and PDGFRA mutations
- They are linked to 2 syndromes (Carney-Stratakis syndrome and Carney triad), both of which include loss of SDHB expression in tumor (J Surg Oncol 2011;104:865, Clin Cancer Res 2008;14:3204)
- Therapeutic implications of GISTs with KIT or PDGFRA mutations
- Main therapeutic strategy to treat GISTs with either KIT or PDGFRA mutations has been the use of TKIs, especially imatinib (Cancers (Basel) 2022;14:5330)
- Tumors with deletions of codons 557 and 558 present more aggressive clinical behavior than those with exon 11 missense mutations
- GISTs with exon 9 mutations typically present lower response to imatinib when compared to those with exon 11 mutations
- Most PDGFRA mutations are sensitive to imatinib therapy (exons 12, 14 and 18)
- PDGFRA exon 18 D842V mutation can also present with primary resistance to imatinib, as well as other TKIs (Br J Cancer 2010;103:165)
- Therapeutic implications of NF1 mutant GISTs
- Since most NF1 mutant GISTs do not harbor KIT mutations, they are typically insensitive to imatinib and surgery remains the mainstay therapy for these patients
- Therapeutic implications of BRAF mutated GIST
- BRAF V600E mutation in GISTs has been shown to confer resistance to imatinib and sunitinib
- BRAF inhibitor is recommended for patients with unresectable or metastatic BRAF mutant GISTs
- Therapeutic implications of SDH deficient GISTs
- This subtype of GIST is known to be generally unresponsive to TKIs (Ann Oncol 2018;29:iv68)
Molecular / cytogenetics images
Sample pathology report
- Stomach, biopsy:
- Gastrointestinal stromal tumor, spindle cell type, low grade (see comment)
- Comment: There is a hypercellular proliferation of spindled cells with eosinophilic cytoplasm and elongated nuclei with small nucleoli. No mitotic activity and no tumor necrosis are seen. Immunohistochemically the tumor cells have strong expression of CD117 and DOG1 and are negative for SMA, desmin, S100 and keratin AE1 / AE3. This constellation of morphological and immunohistochemical features strongly supports the diagnosis of gastrointestinal stromal tumor (GIST).
- Biopsies are suboptimally positioned for GIST risk stratification as these may not include sufficient tumor (i.e., 5 mm2) for mitotic counting and may not sample mitotic hot spots.
- Molecular analysis / classification of the GIST is pending.
Differential diagnosis
- Leiomyoma:
- Spindle cells with blunt ended nuclei and moderate to abundant pale to brightly eosinophilic fibrillary cytoplasm (J Surg Oncol 2011;104:865)
- Immunohistochemical positivity for smooth muscle markers
- Negativity for KIT and DOG1
- Leiomyoma, Müllerian type:
- Estrogen and progesterone receptor positive leiomyomas, comparable to uterine smooth muscle tumors and representing their extrauterine counterparts (Mod Pathol 2014;27:S17)
- Negativity for KIT and DOG1
- Leiomyosarcoma:
- Schwannoma, conventional type:
- Spindle cells forming microtrabeculae or microfascicles (J Surg Oncol 2011;104:865)
- Compact area (Antoni A tissue) showing nuclear palisading (Verocay bodies), alternating with loosely arranged foci (Antoni B tissue)
- Immunohistochemical positivity for SOX10, S100 and GFAP
- Negativity for KIT and DOG1
- Schwannoma, epithelioid type:
- Inflammatory myofibroblastic tumor:
- Spindled cells and inflammatory cells form 3 basic histological patterns (myxoid, hypercellular, hypocellular fibrous) (Mod Pathol 2017;30:1489)
- Myofibroblasts with ganglion-like appearance
- Immunohistochemical positivity for ALK; variable staining for SMA, MSA, calponin and desmin
- Negativity for KIT and DOG1
- Positive for ALK rearrangement
- Epithelioid inflammatory myofibroblastic sarcoma:
- Plump polygonal and epithelioid cells with prominent nucleoli and amphophilic or eosinophilic cytoplasm (Am J Surg Pathol 2011;35:135)
- Neutrophilic infiltration is often present
- Abundant myxoid stroma
- Immunohistochemical positivity for ALK
- Negativity for KIT and DOG1
- Positive for ALK rearrangement
- Solitary fibrous tumor:
- Haphazardly arranged spindled to ovoid cells with indistinct pale eosinophilic cytoplasm (Diagn Pathol 2021;16:32, Pathol Res Pract 2021;224:153531)
- Variably collagenous stroma with branching and hyalinized staghorn shaped blood vessels
- Myxoid changes may be present
- Immunohistochemical positivity for STAT6
- Negativity for KIT and DOG1
- NAB2::STAT6 gene fusion
- Intra-abdominal desmoid tumor:
- Intra-abdominal desmoid tumor can involve the GI tract in GIST-like manner
- Histologically rich in collagen and mildly dilated, prominent vessels (Arch Pathol Lab Med 2006;130:1466)
- Sweeping fascicles of bland spindle cells with pale eosinophilic cytoplasm
- Lack of nuclear hyperchromasia and cytological atypia
- Focal expression of KIT / CD117 may be present (Am J Surg Pathol 2002;26:1296)
- Immunohistochemical demonstration of nuclear beta catenin positivity may be diagnostically helpful, although this is not seen in all cases
- Negativity for DOG1
- Synovial sarcoma:
- Spindle cell sarcoma showing variable epithelial differentiation (J Surg Oncol 2011;104:865)
- Biphasic synovial sarcoma has epithelial and spindle cell components
- Monophasic synovial sarcoma is composed of spindle cells only
- Delicate uniform spindle cells with sparse cytoplasm and ovoid hyperchromatic nuclei with inconspicuous nucleoli
- Epithelial cells are arranged in solid nests or cords, or glands
- Immunohistochemical positivity for SS18::SSX fusion specific antibody, TLE1, EMA, keratin AE1 / AE3, BCL2 and CD99 (Am J Surg Pathol 2020;44:922, Am J Clin Pathol 2011;135:839)
- Negativity for KIT and DOG1
- Specific SS18::SSX1 / 2 / 4 fusion gene
- Undifferentiated sarcoma:
- Undifferentiated sarcomas can involve any segment of the GI tract but they seem to be more common in the intestines than in the stomach (Arch Pathol Lab Med 2006;130:1466)
- Clinically, these tumors can resemble GISTs but they histologically often have greater pleomorphism and higher mitotic activity
- Lack of KIT and PDGFRA mutations
Additional references
Board review style question #1
A 51 year old man presented with gastrointestinal bleeding and a submucosal gastric mass. Hematoxylin eosin stains demonstrate a proliferation of spindled cells with eosinophilic cytoplasm and elongated nuclei with small nucleoli. No mitotic activity and no tumor necrosis are seen. Immunohistochemically the tumor cells have strong expression of CD117 and DOG1 and are negative for SMA, desmin, S100, SOX10, STAT6, TLE1, EMA and AE1 / AE3. Which of the following is most likely the correct diagnosis?
- Gastrointestinal stromal tumor
- Leiomyoma
- Schwannoma
- Solitary fibrous tumor
- Synovial sarcoma
Board review style answer #1
A. Gastrointestinal stromal tumor (GIST). Most GISTs are spindle cell tumors, with strong expression of CD117 / KIT and DOG1. Answer B is incorrect because leiomyomas express smooth muscle markers and are negative for CD117 and DOG1. Answer C is incorrect because schwannomas express S100 and SOX10 and are negative for CD117 and DOG1. Answer E is incorrect because synovial sarcomas express TLE1, EMA and keratin AE1 / AE3 and are negative for CD117 and DOG1. Answer D is incorrect because solitary fibrous tumors express STAT6 and are negative for CD117 and DOG1.
Comment Here
Reference: Gastrointestinal stromal tumor
Comment Here
Reference: Gastrointestinal stromal tumor
Board review style question #2
Which of the following is true regarding gastrointestinal stromal tumor (GIST)?
- GISTs are the second most common nonepithelial tumors of the gastrointestinal tract
- Majority of GISTs are clinically malignant
- Pediatric GIST patients have a poor prognosis
- Pediatric GISTs are usually KIT / PDGFRA mutated
- SDH deficient GISTs may be part of Carney-Stratakis syndrome
Board review style answer #2
E. SDH deficient GISTs may be part of Carney-Stratakis syndrome. Carney-Stratakis syndrome is a disease where people develop several types of tumors, including SDH deficient GIST. Many of the families with Carney-Stratakis syndrome carry mutations in SDH that can be passed on to children. Answer B is incorrect because only 25% of GISTs are clinically malignant. Answer D is incorrect because pediatric GISTS are usually SDH deficient and KIT / PDGFRA wild type. Answer C is incorrect because pediatric GISTs have a slow disease course and patients have a good prognosis despite the presence of metastasis to the peritoneal cavity, lymph nodes and liver. Answer A is incorrect because GISTs are the most common nonepithelial tumors of the gastrointestinal tract.
Comment Here
Reference: Gastrointestinal stromal tumor
Comment Here
Reference: Gastrointestinal stromal tumor