Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Immunofluorescence description | Immunofluorescence images | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Alexiev BA. Desmoplastic small round cell tumor. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/softtissueDSRCT.html. Accessed April 2nd, 2025.
Definition / general
- Desmoplastic small round cell tumor (DSRCT) is a malignant mesenchymal neoplasm composed of small round tumor cells associated with prominent stromal desmoplasia, polyphenotypic differentiation and EWSR1-WT1 gene fusion
Essential features
- Nests of small round cells in a desmoplastic stroma
- Expression of cytokeratin, desmin and WT1 (antibody to carboxy terminus [C terminus])
- EWSR1-WT1 fusion transcripts
Terminology
- Intra-abdominal desmoplastic round cell tumor
ICD coding
- ICD-0: 8806/3 - desmoplastic small round cell tumor
- ICD-11: 2B5F.2 & XH5SN6 - sarcoma, not elsewhere classified of other specified sites & desmoplastic small round cell tumor
Epidemiology
- DSRCT primarily affects children and young adults
- Striking male predominance
- Wide age range (first to fifth decades), with peak incidence in the third decade of life
- Reference: Clin Cancer Res 2018;24:4865, Am J Surg Pathol 1991;15:499
Sites
- Vast majority of patients develop tumors in the abdominal cavity (Int J Surg Pathol 2016;24:672, Int J Surg Pathol 2017;25:158)
- Multiple serosal implants are common
- Clinical presentation outside the abdominal cavity is rare and mainly restricted to the thoracic cavity and paratesticular region (Am J Surg Pathol 1997;21:219)
- Rare cases occur in the limbs, head and neck, kidney and brain (Diagn Pathol 2019;14:43, Am J Surg Pathol 2007;31:576, Am J Case Rep 2020;21:e922762, J Cutan Pathol 2020;47:768, J Neurosurg 2015;122:773, Int J Surg Pathol 2019;27:236)
Pathophysiology
- Recurrent t(11;22)(p13;q12) translocation leading to formation of the EWSR1-WT1 fusion gene, which generates a chimeric protein with transcriptional regulatory activity (Int J Surg Pathol 2016;24:672)
Etiology
- Unknown
Clinical features
- Clinical symptoms are usually related to the primary site of presentation, such as pain, palpable mass, abdominal distention, obstruction and ascites (J Gastrointest Cancer 2019;50:560)
- At initial presentation, 90% of patients have multifocal disease, nodular disease, diffuse peritoneal and omental disease or a combination of these conditions
- Substantial number of patients have diaphragmatic involvement
Diagnosis
- Tissue sampling is the gold standard for a definitive diagnosis
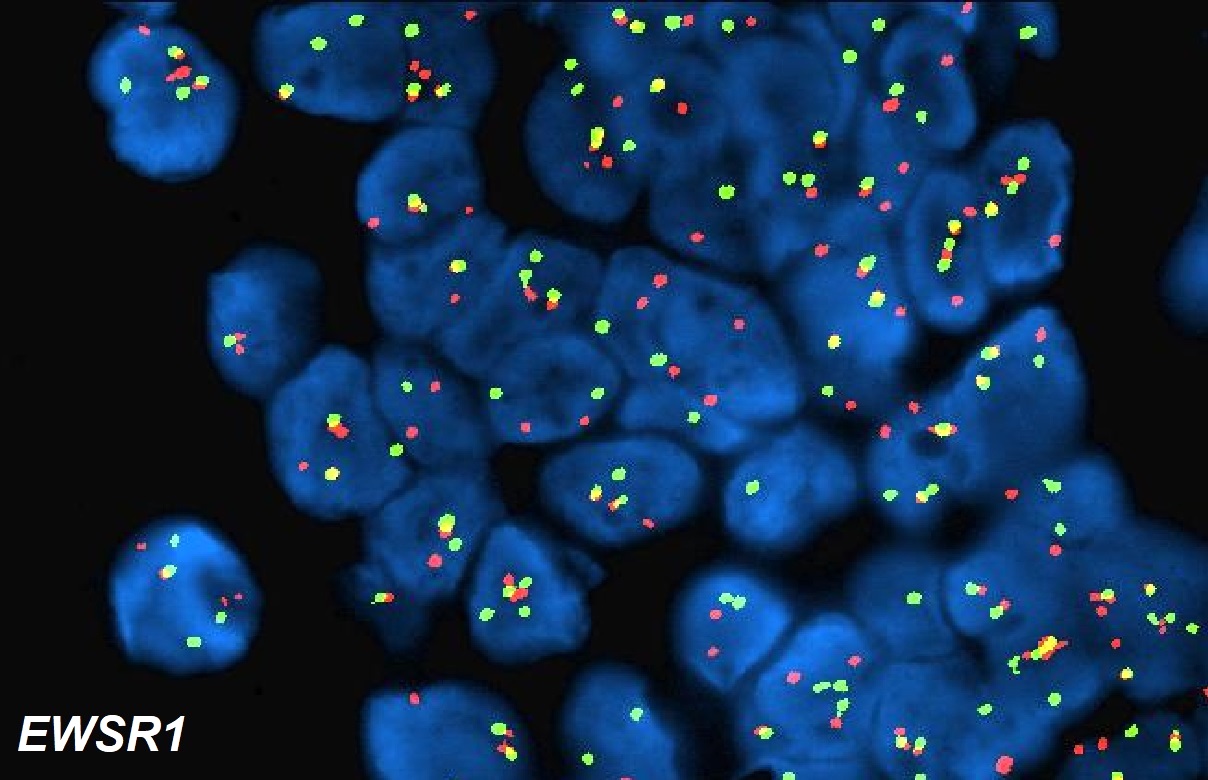
- Fluorescence in situ hybridization (FISH) to detect EWSR1 rearrangement and reverse transcription polymerase chain reaction (RT-PCR) to assess for EWSR1-WT1 fusion transcripts are routine diagnostic ancillary tools (Virchows Arch 2017;471:631)
- Both methods (FISH and RT-PCR) complement each other by confirming cases that the other method may not (Virchows Arch 2017;471:631)
Radiology description
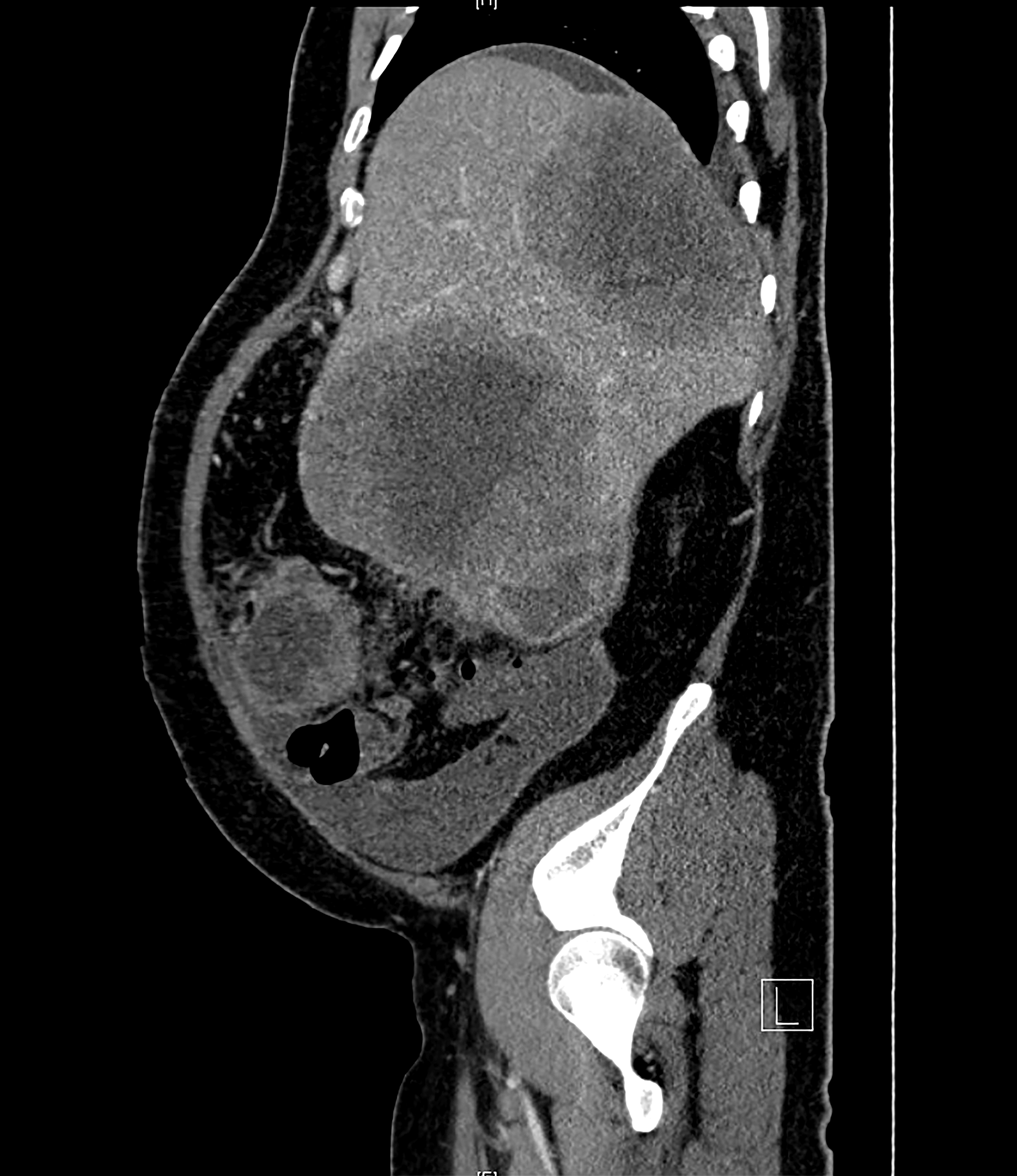
- On ultrasound, the DSRCTs appear as lobulated, hypoechoic and heterogeneous masses with increased vascularity (J Comput Assist Tomogr 2002;26:579, Radiology 1999;210:633, Curr Probl Diagn Radiol 2013;42:26)
- On unenhanced CT, DSRCTs appear as hypoattenuating masses involving the peritoneal surfaces, omentum and mesentery (AJR Am J Roentgenol 2019;212:W45)
- On MRI, DSRCTs appear as heterogeneous isointense to hypointense masses on T weighted images, compared with skeletal muscle (AJR Am J Roentgenol 2019;212:W45)
- Masses may show foci of calcification (AJR Am J Roentgenol 2019;212:W45)
- PET / CT images show intense FDG hypermetabolic activity in soft tissue masses (AJR Am J Roentgenol 2019;212:W45)
Prognostic factors
- Despite multimodal treatment, DSRCT is associated with dismal outcomes (J Surg Oncol 2018;117:1759)
- Approximately 60 - 70% of patients die due to disease progression, usually within 3 years after diagnosis (Cancers (Basel) 2021;13:498, J Surg Oncol 2018;117:1759)
- Median overall survival varies between 28 - 60 months, with a median disease free survival between 10 - 15 months (Cancers (Basel) 2021;13:498)
- Systemic chemotherapy and surgery are associated with reduced mortality (J Surg Oncol 2018;117:1759)
- Residual macroscopic disease after resection correlates with increased risk of mortality (J Surg Oncol 2018;117:1759)
Case reports
- 8 year old girl with tumor of the kidney (Diagn Pathol 2020;15:95)
- 23 year old woman with ovarian tumor (Am J Case Rep 2019;20:1675)
- 33 year old man with tumor of the tibia (J Bone Oncol 2019;20:100272)
- 35 year old man with tumor of the peritoneal cavity (Ann R Coll Surg Engl 2020;102:e77)
- 42 year old man with a painless nodule in his left inguinal region (World J Surg Oncol 2014;12:227)
Treatment
- Current management of DSRCT includes a combination of chemotherapy, radiation and aggressive cytoreductive surgery, plus intraperitoneal hyperthermic chemotherapy (Cancers (Basel) 2021;13:498)
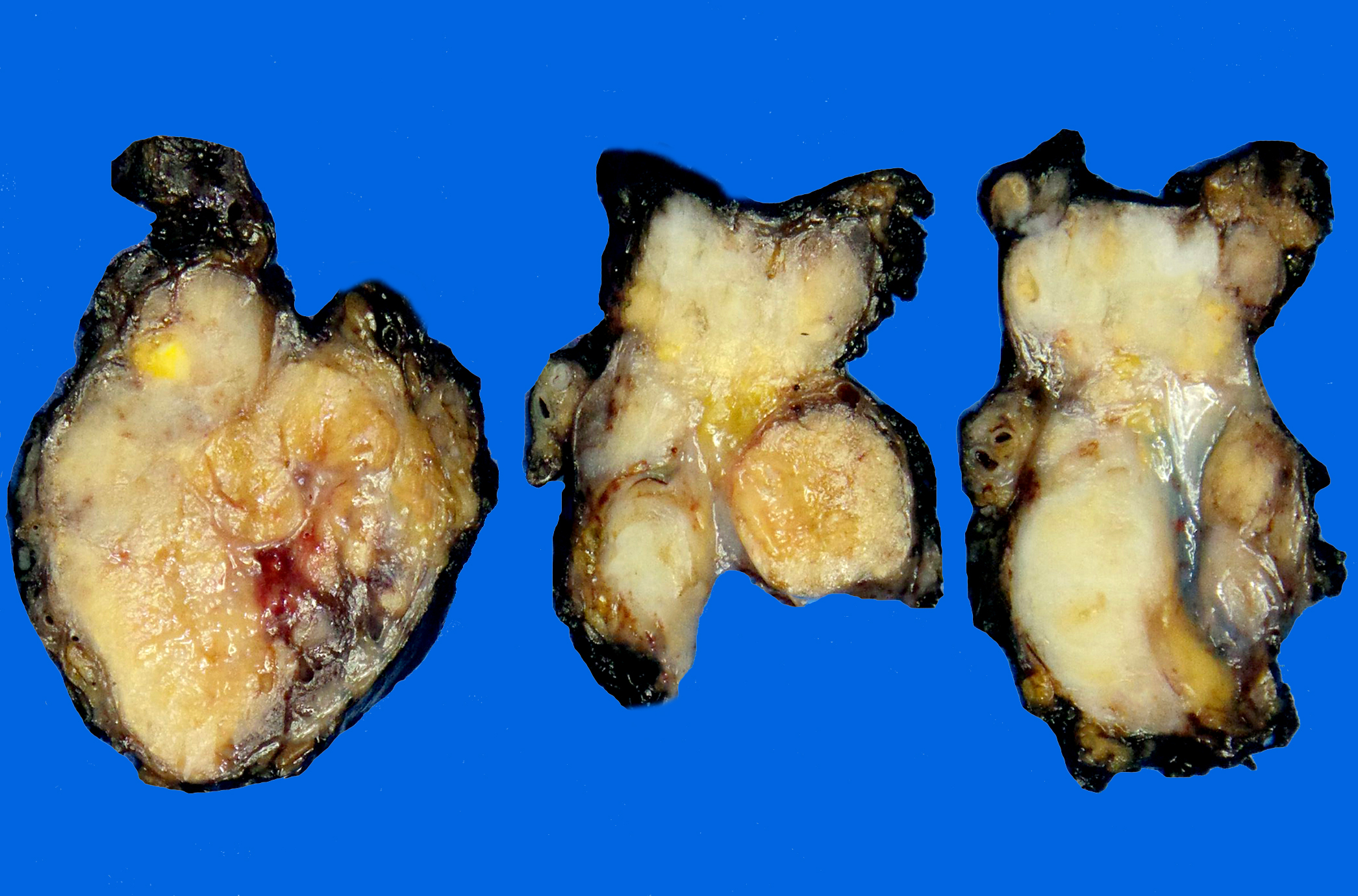
Gross description
- Typical gross appearance consists of multiple tumor nodules
- Cut surface is firm and grayish white, with foci of necrosis and hemorrhage
Frozen section description
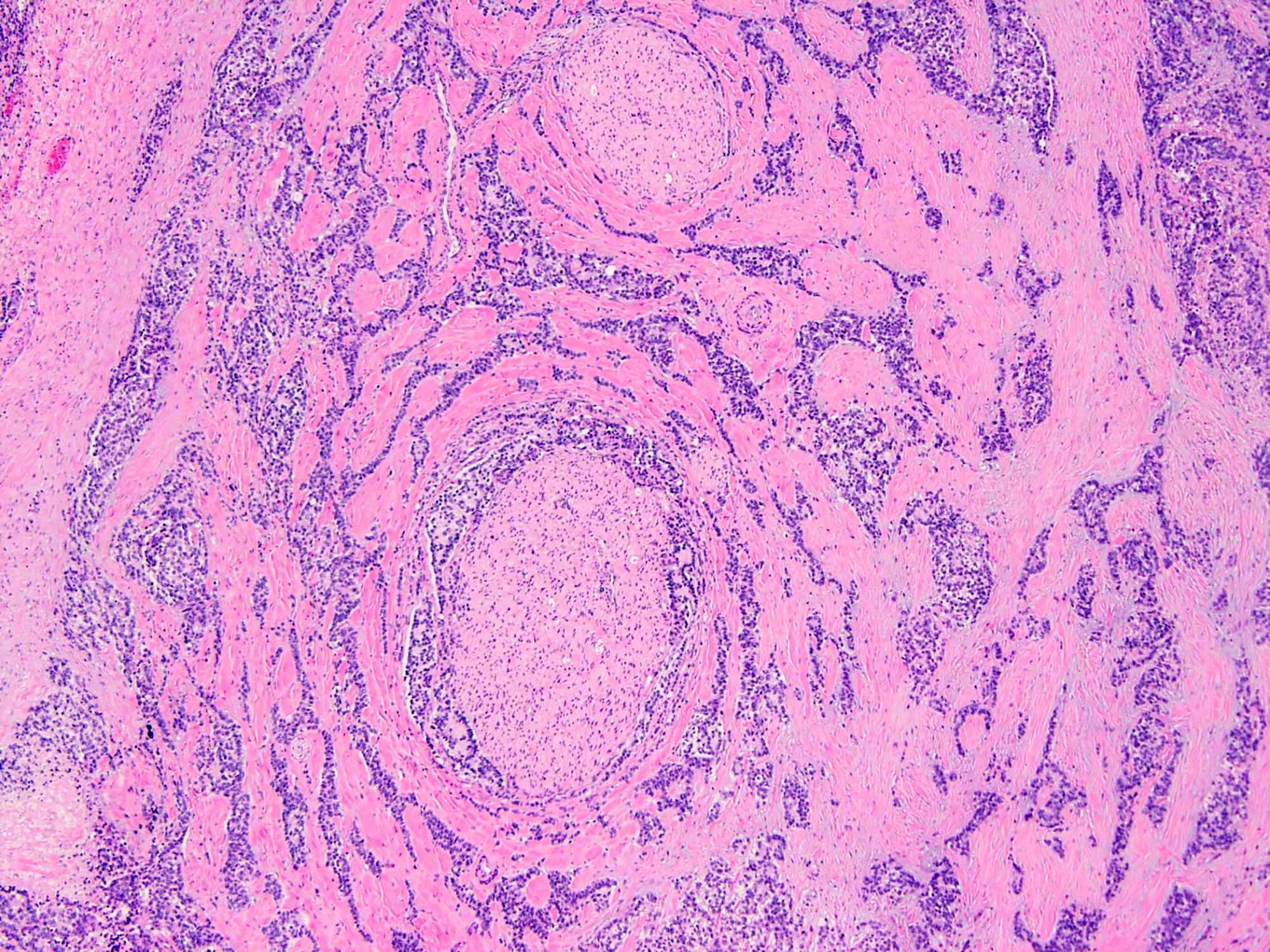
- Sharply outlined nests of small round, epithelioid or spindled cells surrounded by prominent desmoplastic stroma
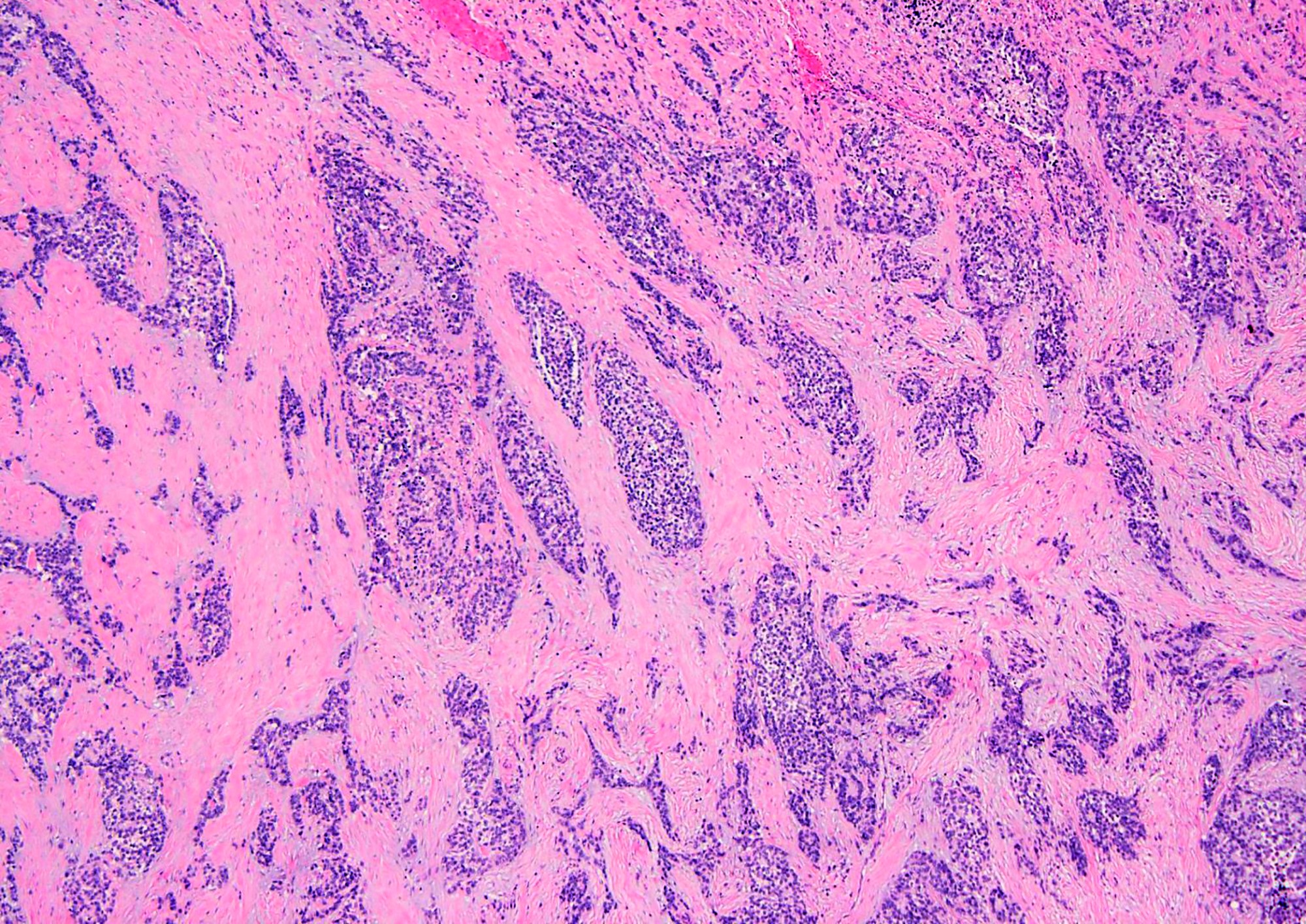
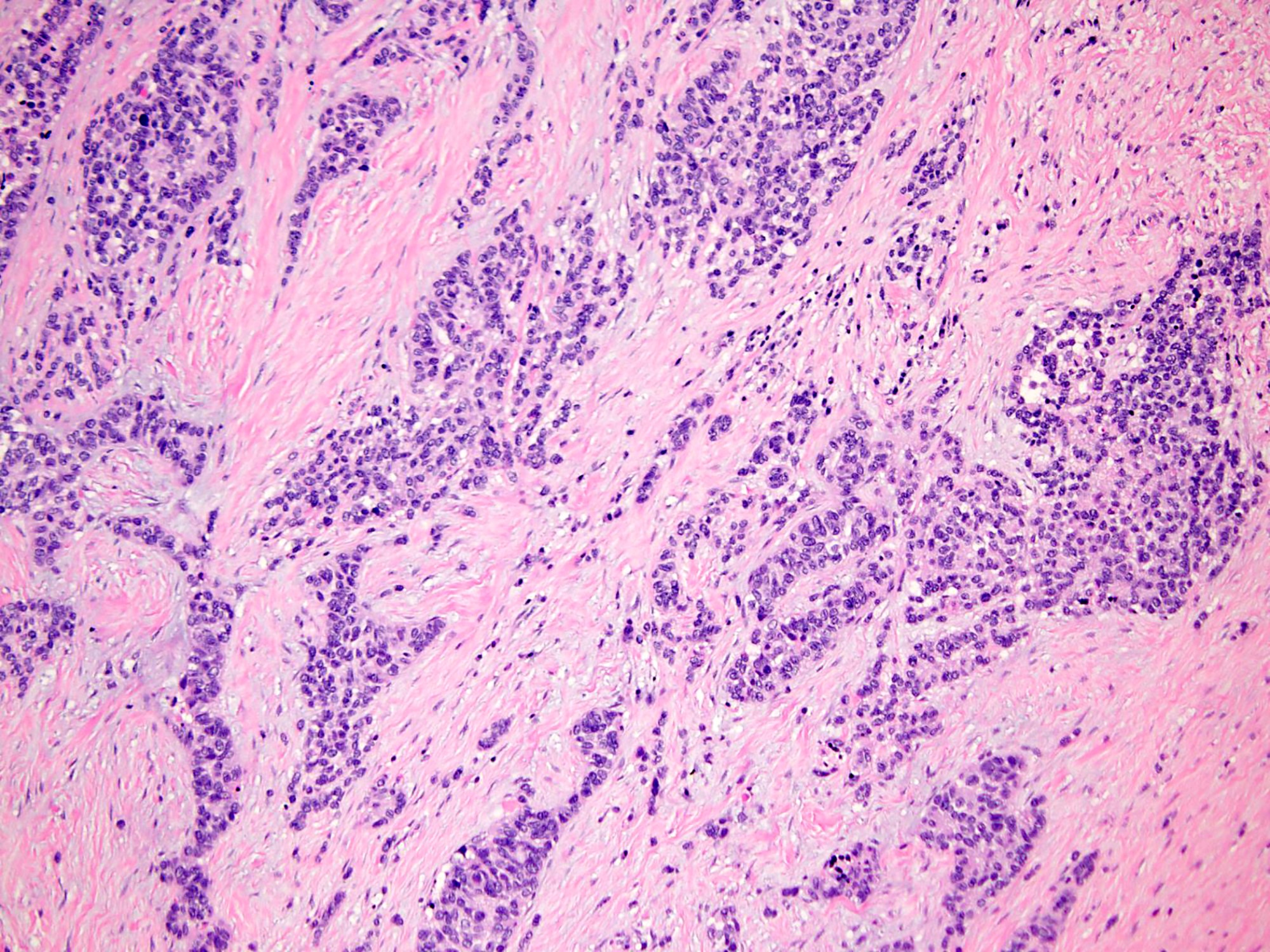
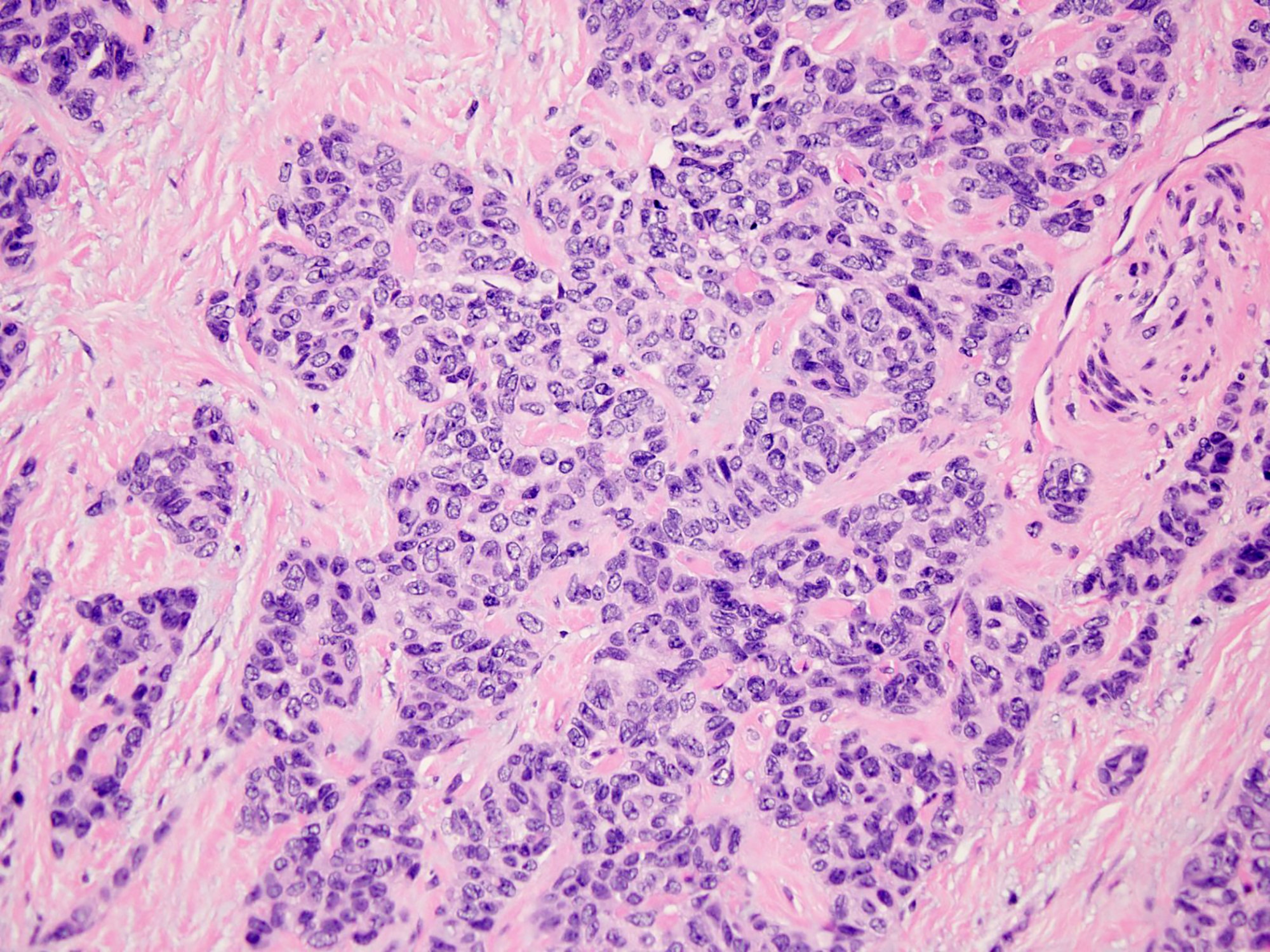
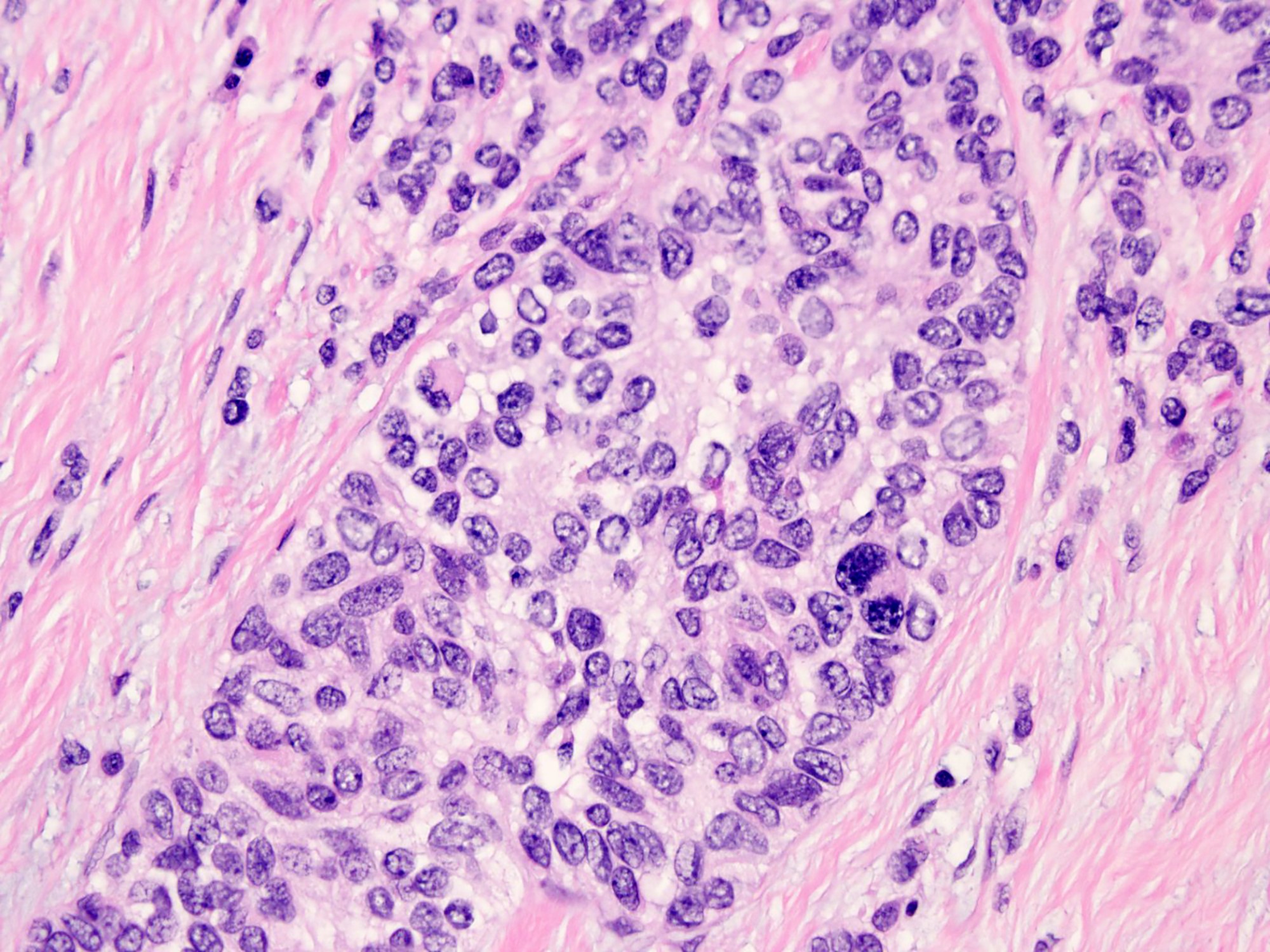
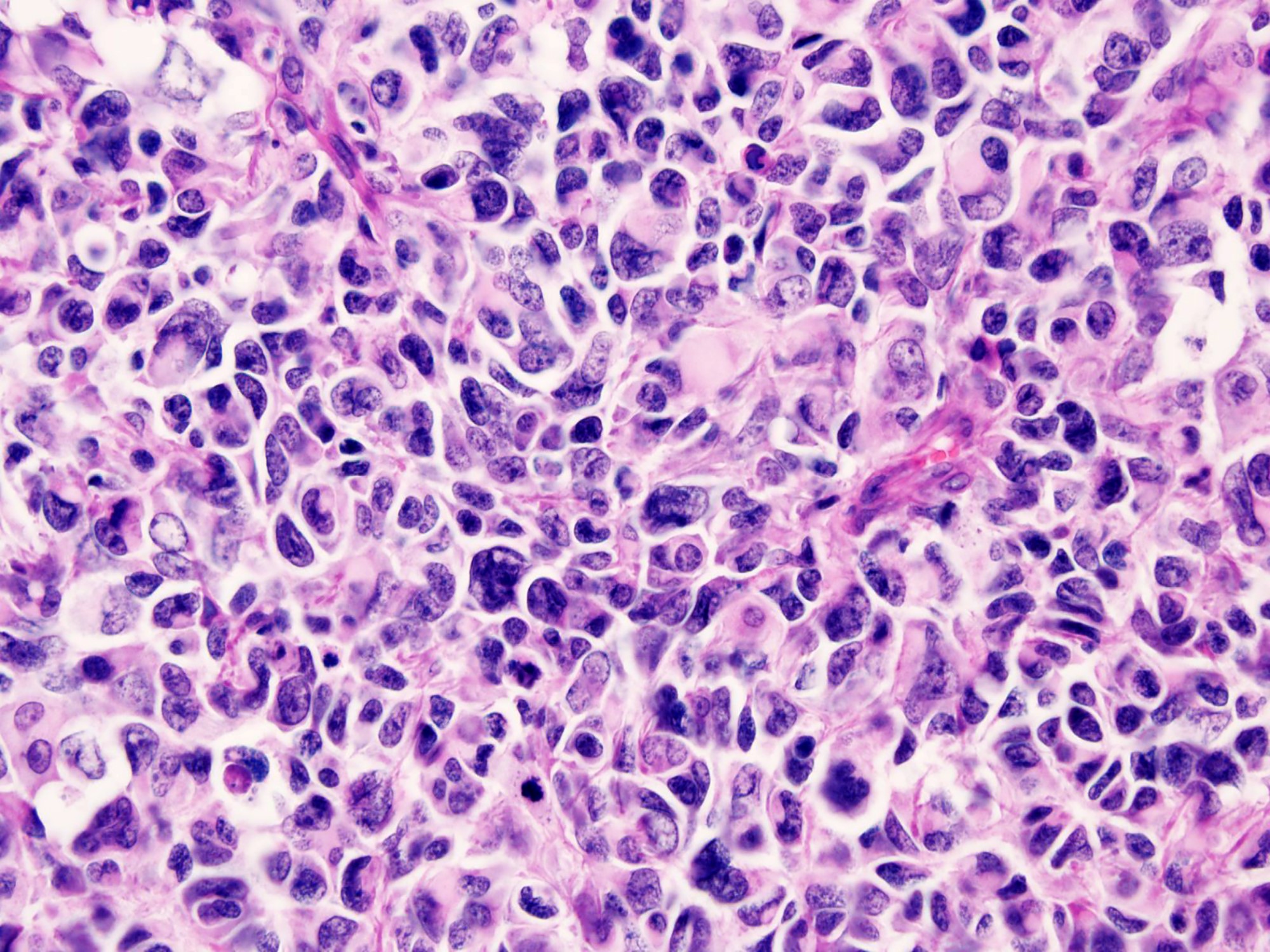
Microscopic (histologic) description
- Well defined nests of small round cells separated by desmoplastic stroma (Arch Pathol Lab Med 2006;130:728)
- In the more desmoplastic areas, tumor cells may be arranged as thin trabeculae or in a single file fashion (Arch Pathol Lab Med 2006;130:728)
- Uniform cells with small hyperchromatic nuclei, inconspicuous nucleoli, scant cytoplasm and indistinct cytoplasmic borders
- Some tumors have larger cells with greater anisonucleosis and dispersed chromatin
- Peripheral palisading is common
- Rosette-like structures and pseudopapillae may be observed (Am J Surg Pathol 1997;21:219, Am J Surg Pathol 1991;15:499)
- Rhabdoid cells or cells with clear cytoplasm (Diagn Pathol 2020;15:95)
- DSRCT with variant morphology:
- Solid pattern
- Entirely solid morphology, lacking evidence of desmoplastic stroma (Int J Surg Pathol 2017;25:158)
- Spindle cell pattern
- Nodules of spindle cells with scant cytoplasm and oval nuclei arranged in short, intersecting fascicles and set in a scant, nondesmoplastic stroma (Diagnostics (Basel) 2021;11:545)
- Glandular differentiation
- Extensive glandular differentiation with mucicarmine positive mucin content (Korean J Pathol 2013;47:182)
- Large cell pattern
- Predominantly large epithelioid cells with greater pleomorphism mimicking carcinoma (Ultrastruct Pathol 2000;24:333)
- Solid pattern
Microscopic (histologic) images
Cytology description
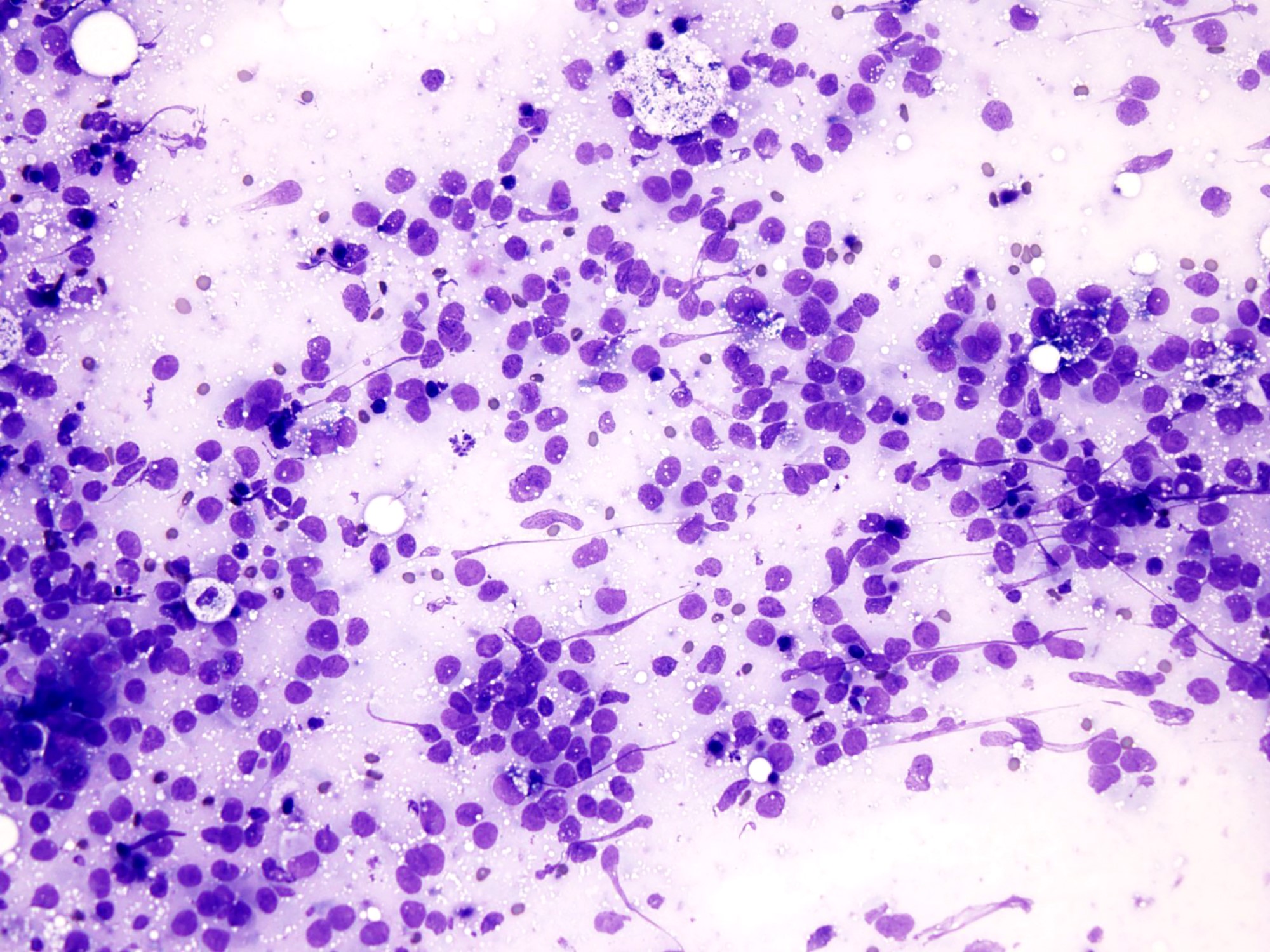
- Hypercellular smears of small round cells (Cancer Cytopathol 2014;122:386)
- Round or kidney shaped nuclei with inconspicuous nucleoli
- Nuclear molding usually present
- Scant cytoplasm
- Paranuclear cytoplasmic densities
- Numerous mitotic figures, crushed nuclei and apoptosis
- Connective tissue fragments (Acta Cytol 2012;56:576)
Immunofluorescence description
- EWSR1-WT1 break apart FISH demonstrates separation of signals indicative of translocation involving the EWS locus (Oncol Lett 2012;4:423)
- Paired locus specific EWS and WT1 probes show EWSR1-WT1 fusion signals in nuclei of DSRCT (Oncol Lett 2012;4:423)
Immunofluorescence images
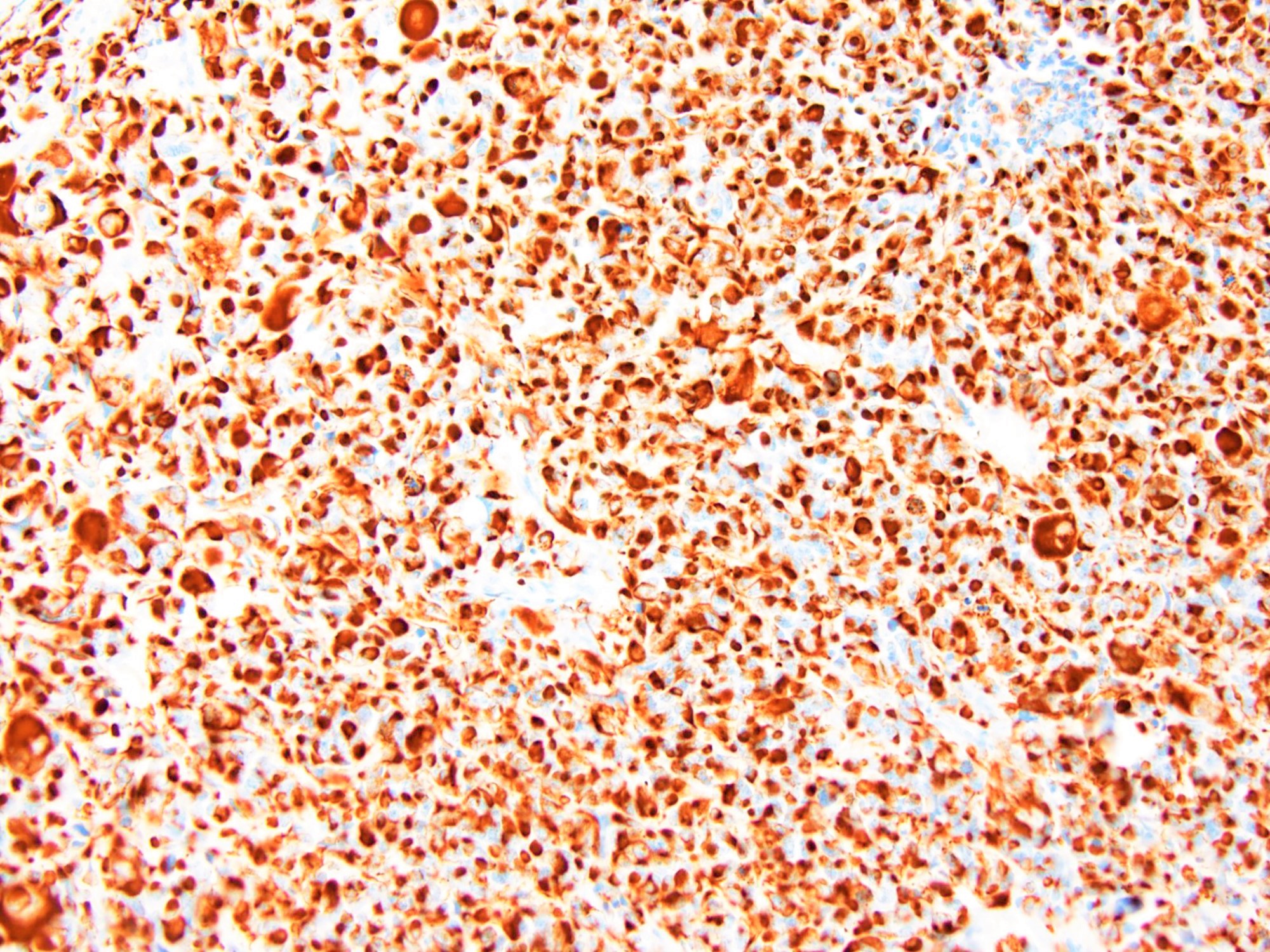
Positive stains
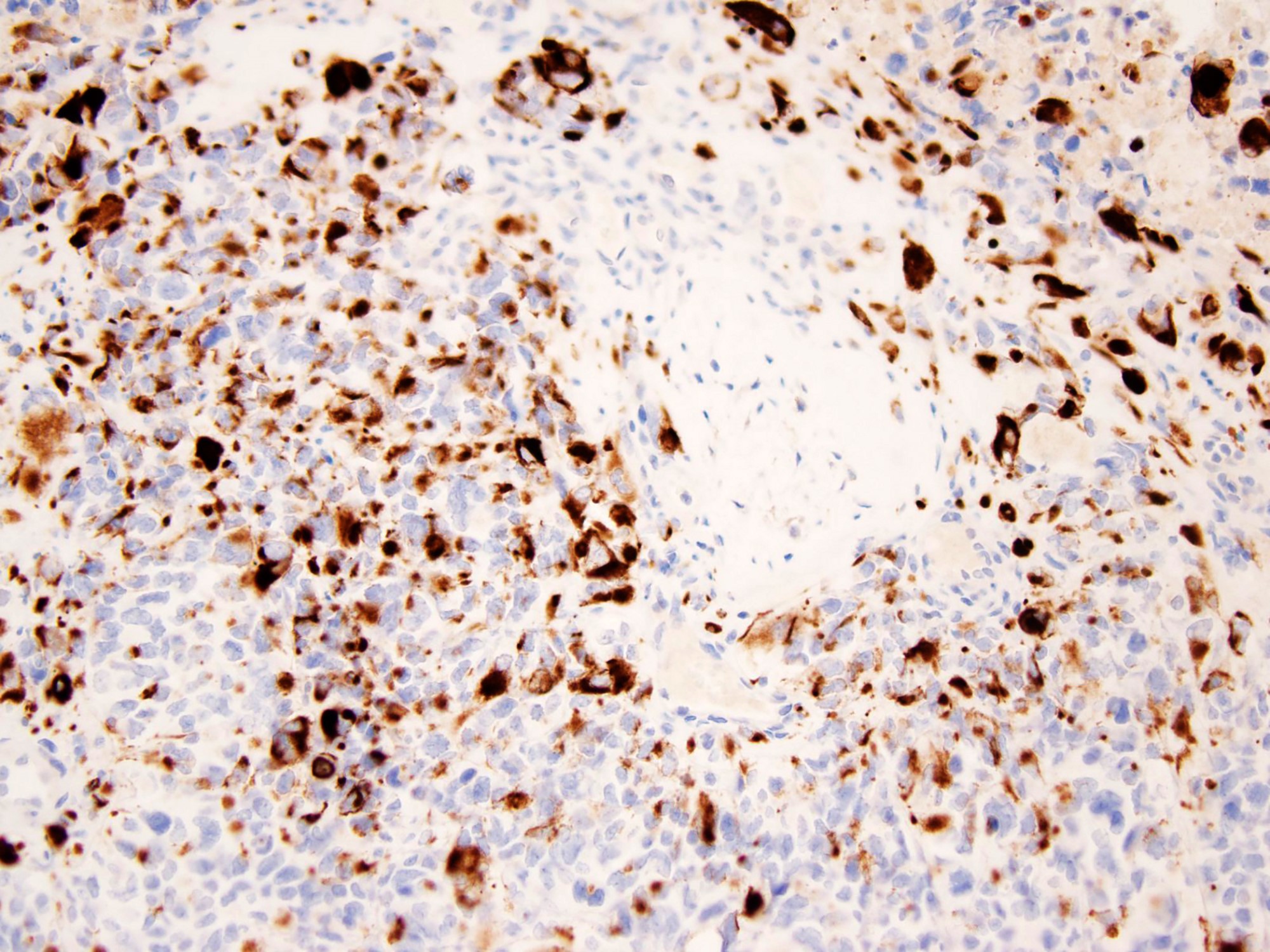
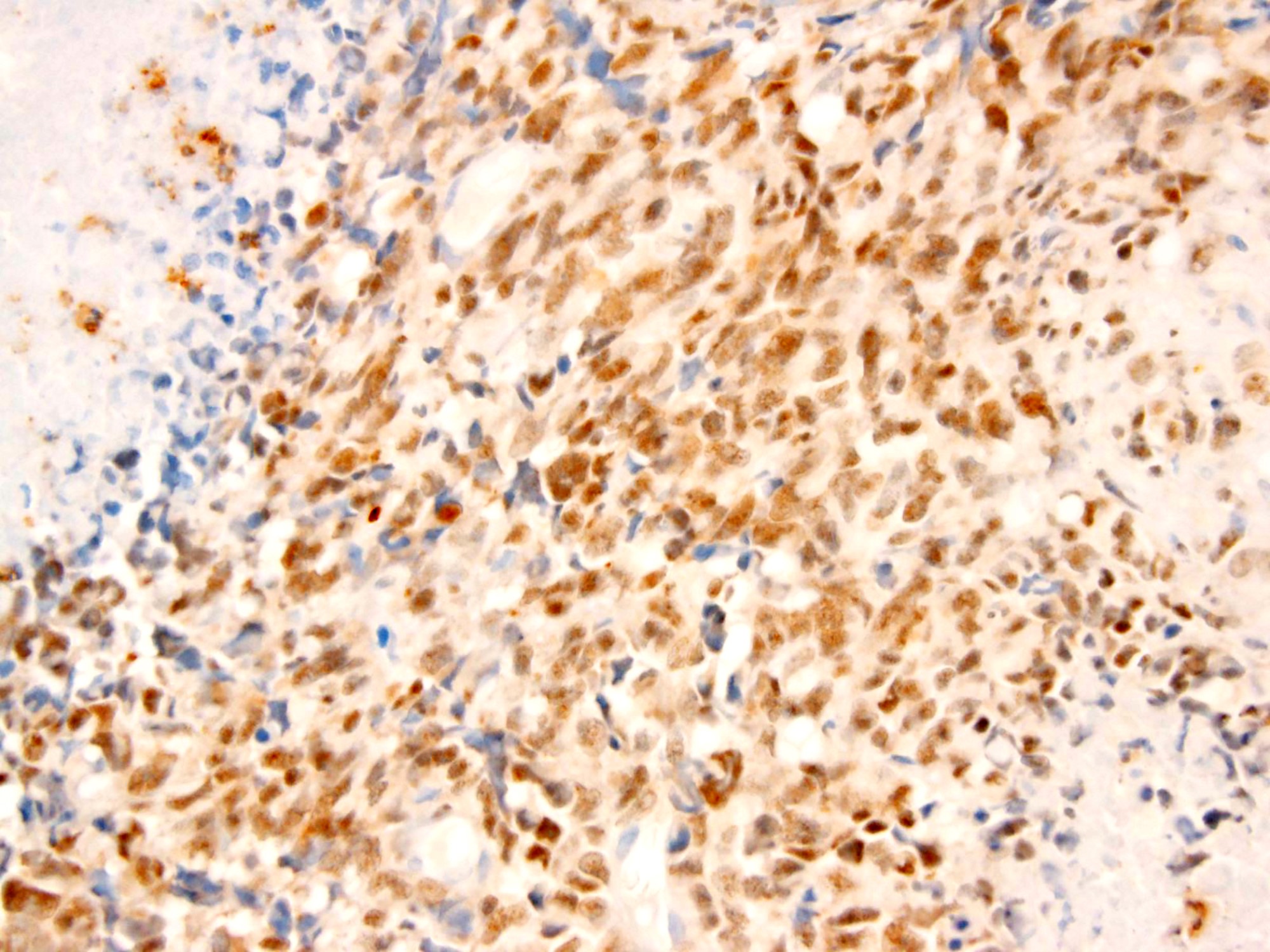
- WT1 (C terminus) (Am J Surg Pathol 2014;38:1220)
- Desmin (distinct dot-like and perinuclear cytoplasmic staining pattern) (Arch Pathol Lab Med 2006;130:728)
- Keratin (a distinctive dot-like cytoplasmic expression is seen occasionally), EMA (Am J Surg Pathol 1991;15:499)
- Vimentin (a distinctive dot-like cytoplasmic expression is seen occasionally), (Am J Surg Pathol 1997;21:219)
- GFAP, neurofilament protein, synaptophysin, NSE (Am J Surg Pathol 1992;16:998, Am J Surg Pathol 1997;21:219)
- AR and KIT expression in 37% and 35% of DSRCTs, respectively (Cancer Chemother Pharmacol 2007;59:429)
Negative stains
Electron microscopy description
- Perinuclear whorls of intermediate filaments (Arch Pathol Lab Med 2006;130:728)
- Absence of specialized cell junctions, neurosecretory granules and long microvilli are pertinent negative ultrastructural findings (Arch Pathol Lab Med 2006;130:728)
- Rare dense core granules, scant organelles, occasional microtubules, glycogen particles (J Pathol Transl Med 2015;49:93)
Molecular / cytogenetics description
- Tumors associated with t(11;22)(p13;q12) (Semin Cancer Biol 2005;15:197)
- Chimeric EWS-WT1 RNA and DNA detected by PCR (J Clin Oncol 1998;16:3028)
Sample pathology report
- Peritoneum, biopsy:
- Desmoplastic small round cell tumor (see comment)
- Comment: The tumor is characterized by sharply outlined nests of small round cells surrounded by a prominent desmoplastic stroma. The tumor cells are uniform, with small hyperchromatic nuclei, inconspicuous nucleoli, scant cytoplasm and indistinct cytoplasmic borders. Mitoses are frequent and focal tumor cell necrosis is present. Immunohistochemically, the tumor cells are immunoreactive for keratin AE1 / AE3 and desmin and show strong nuclear expression for WT1 (C terminus), while all of the following are negative: myogenin, MYOD1, S100 and WT1 (N terminus). The findings support the above diagnosis. Despite multimodal treatment, DSRCT is associated with dismal outcomes.
Differential diagnosis
- Blastemal predominant Wilms tumor:
- Overlapping histologic features with DSRCT, including poorly differentiated morphology, desmoplastic stroma and immunoreactivity for desmin and cytokeratin (Am J Surg Pathol 2014;38:1220)
- Focal triphasic elements and glomeruloid bodies
- WT1 immunoreactivity with antibodies to both the amino terminus and carboxy terminus
- Negative for EWSR1-WT1 rearrangements
- Ewing sarcoma:
- Classic Ewing sarcoma:
- Composed of uniform small round cells with round nuclei containing finely stippled chromatin and inconspicuous nucleoli, and scant clear or eosinophilic cytoplasm
- Atypical Ewing sarcoma:
- Tumor cells are larger, with prominent nucleoli and irregular nuclear contours
- CD99 and NKX2.2 positive (Virchows Arch 2014;465:599)
- WT1 (C terminus) negative
- EWSR1-FLI1 and EWSR1-ERG fusions transcripts
- Negative for EWSR1-WT1 rearrangements
- Classic Ewing sarcoma:
- Rhabdomyosarcoma, embryonal and alveolar variants:
- Embryonal rhabdomyosarcoma
- Composed of primitive mesenchymal cells that show variable degrees of skeletal muscle differentiation
- Alveolar rhabdomyosarcoma
- Classic subtype: nests of neoplastic cells arranged in alveolar spaces
- Solid subtype: sheets of neoplastic cells; nests separated by thin fibrovascular septae but alveoli are not seen
- Mogenin and MyoD1 positive
- WT1 (C terminus) negative
- PAX3-FOXO1 and PAX7-FOXO1 fusion transcripts (alveolar rhabdomyosarcoma) (Mod Pathol 2021;34:748)
- Negative for EWSR1-WT1 rearrangements
- Embryonal rhabdomyosarcoma
- Lymphoma:
- Diffuse or nodular growth pattern, large atypical cells with abundant cytoplasm, nuclei with clumped chromatin and prominent nucleoli
- Positive for CD45, B cell and T cell markers (Appl Immunohistochem Mol Morphol 2013;21:116)
- WT1 (C terminus) negative
- Negative for EWSR1-WT1 rearrangements
- Small cell carcinoma:
- Small to medium sized, round to oval cells with minimal cytoplasm, nuclei with finely dispersed chromatic, no distinct nucleoli, nuclear molding, high mitotic rate
- TTF1, CD56, synaptophysin and chromogranin positive (J Thorac Oncol 2019;14:377)
- WT1 (C terminus) negative
- Negative for EWSR1-WT1 rearrangements
Board review style question #1
A 31 year old man presented with abdominal distention and multiple tumor nodules studding the peritoneal surface. Hematoxylin eosin stains demonstrate well defined nests of small round cells separated by desmoplastic stroma. The tumor cells are uniform, with small hyperchromatic nuclei, inconspicuous nucleoli and scant cytoplasm. Mitoses are frequent and focal tumor cell necrosis is present. Perineural invasion is identified. Immunohistochemically, the tumor cells are immunoreactive for keratin AE1 / AE3, desmin, NSE and WT1 (C terminus), while all of the following are negative: myogenin, MYOD1, S100 and WT1 (N terminus). Which of the following is most likely the correct diagnosis?
- Desmoplastic small round cell tumor
- Ewing sarcoma
- Lymphoma
- Small cell carcinoma
- Wilms tumor
Board review style answer #1
Board review style question #2
Which of the following is true about desmoplastic small round cell tumor?
- Desmoplastic stroma is always present
- Prognosis of DSRCT has improved considerably with current multimodal therapy
- Striking female predominance
- Tumor is positive for WT1 (N terminus)
- Widespread abdominal / peritoneal involvement is common
Board review style answer #2
E. Widespread abdominal / peritoneal involvement is common
Comment Here
Reference: Desmoplastic small round cell
Comment Here
Reference: Desmoplastic small round cell