Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Wu B, Shinohara M. Reed nevus. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/skintumormelanocyticpigmentedspindlecellnevus.html. Accessed March 31st, 2025.
Definition / general
- Benign small, pigmented nevus that was first described in 1975 by Reed et al. and is a clinical and histopathological mimicker of melanoma (Semin Oncol 1975;2:119)
- Predominantly junctional melanocytic proliferation with plump spindled cells and abundant cytoplasm containing melanin
Essential features
- Small (< 1 cm) dark brown-black papule
- Most common on extremities of young adults
- Well circumscribed and symmetrical melanocytic proliferation that is predominately junctional with spindled cells and pigmented cytoplasm
- Dermal melanophages with inflammation often seen
Terminology
- Pigmented spindle cell nevus
- Pigmented spindle cell nevus of Reed
- Reed's nevus
ICD coding
- ICD-10: I78.1 - nevus, nonneoplastic
Epidemiology
- Predominately young adults
- Can occur in children and older adults; decreased incidence with age
- F > M
- Reference: J Am Acad Dermatol 1993;28:565
Sites
- Lower extremities (most common)
- Can also be found on upper extremities, trunk, head and neck
Pathophysiology
- Rapid early growth and subsequent senescence suggest it is fusion driven
- 78% of Reed nevi have kinase fusions; recent study shows 57% with NTRK3 fusions (Am J Surg Pathol 2018;42:1042)
- Other fusion include MERTK, ROS1 and RET
- Native NTRK3 has a role in melanocyte proliferation and migration
Etiology
- Independent of sun exposure
- Predisposition factors unknown
Clinical features
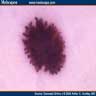
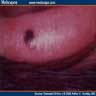
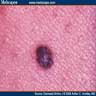
- Well circumscribed and deeply pigmented flat topped papule
- 3 common dermoscopic appearances
- Starburst pigmentation pattern (most common)
- Central pigmented zone with pigment globules at the edge
- Atypical pattern with asymmetry, diffuse irregular pigmentation and whitish blue veil
- Reference: Clin Dermatol 2002;20:259
Diagnosis
- Histopathological diagnosis with the following essential diagnostic criteria (WHO 5th edition)
- Circumscribed and generally symmetrical melanocytic proliferation
- Predominately intraepidermal with or without involvement of the superficial dermis
- Predominantly spindled morphology of melanocytes
- Significant melanin deposition in epidermal keratinocytes and dermal melanophages
- Circumscribed and generally symmetrical melanocytic proliferation
- Reference: J Am Acad Dermatol 1993;28:565
Prognostic factors
- Benign neoplasm with a very low risk of developing melanoma
- Can recur if incompletely excised
Case reports
- 3 year old boy with a rapidly growing, heavily pigmented left lower lid papule (Ocul Oncol Pathol 2017;3:176)
- 9 year old boy with acral Reed nevus with parallel ridge pattern (An Bras Dermatol 2023;98:246)
- 39 year old man with a rapidly growing pigmented shoulder macule found to have NTRK2 rearrangement (J Cutan Pathol 2021;48:1193)
Treatment
- Complete excision is recommended in partially sampled lesions
- Prevents recurrence
- Allows for complete evaluation of the lesion
Clinical images
Microscopic (histologic) description
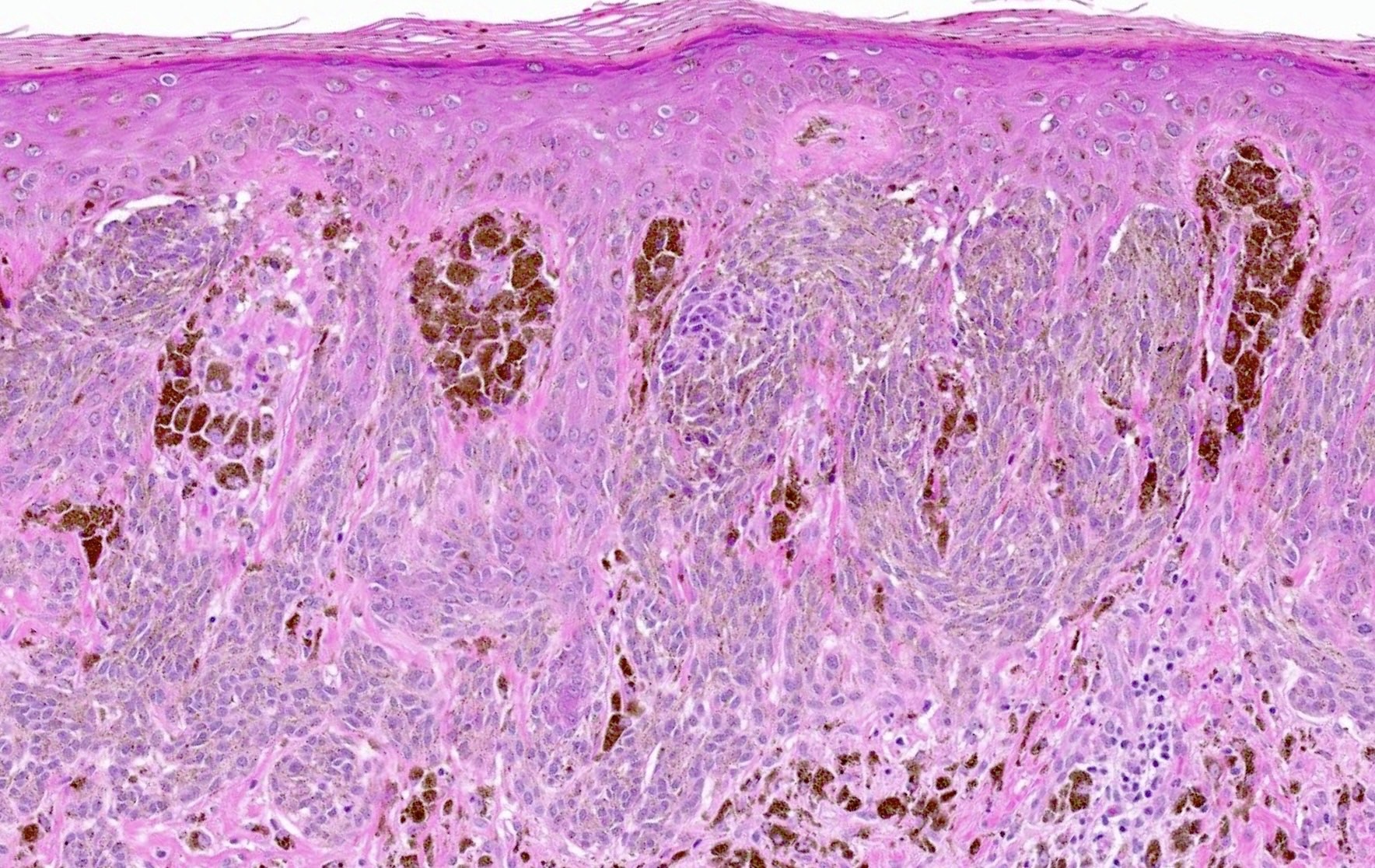
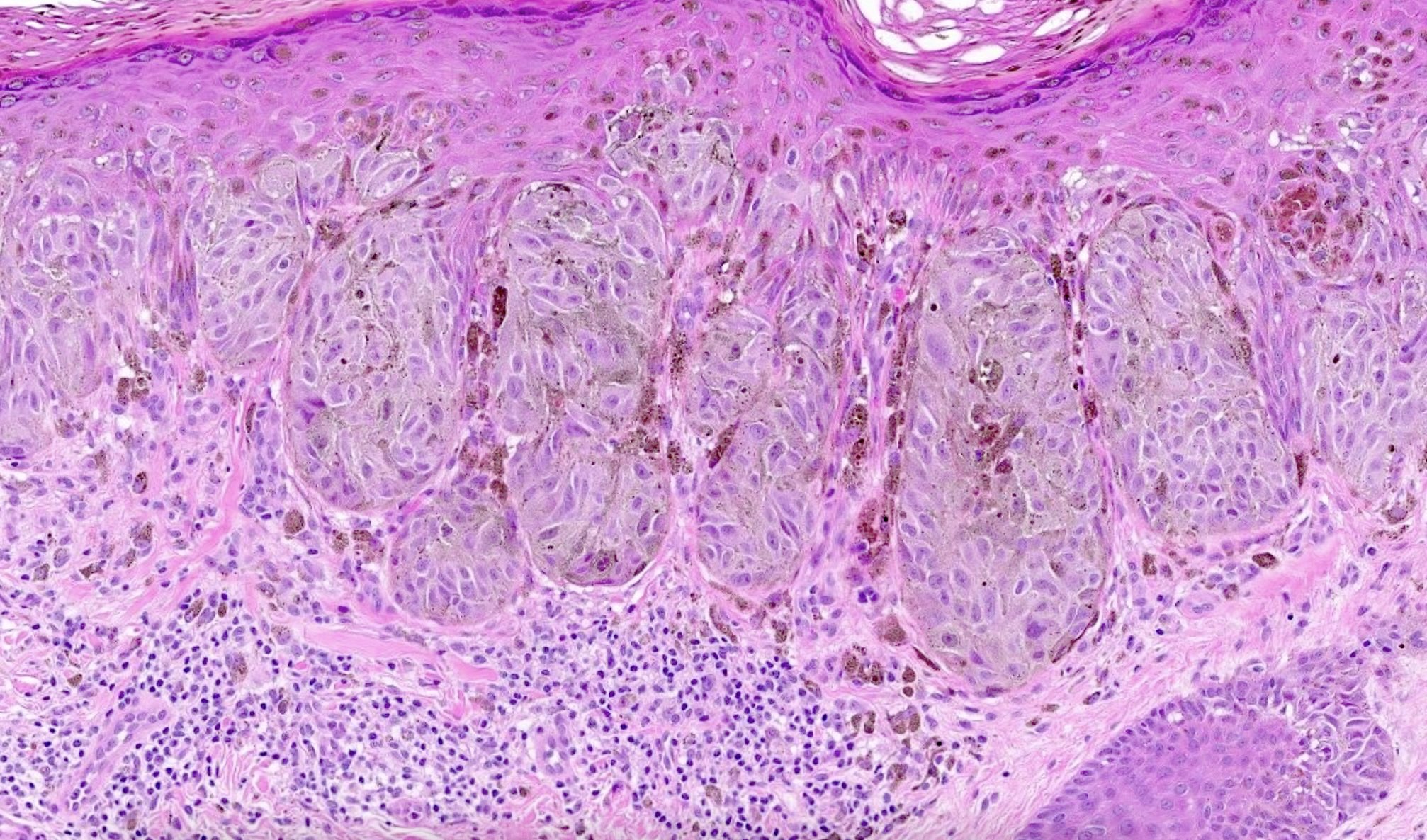
- Junctional (most common) or compound melanocytic proliferation
- Symmetrical and well circumscribed
- Large junctional nests that are vertical or horizontal with bridging across several rete ridges
- Pigmented Kamino bodies can be seen
- Pagetoid spread is common but is symmetrical, low level (less than half of the epidermis) and does not extend beyond the peripheral nests
- Can involve adnexal structures and expand into the papillary dermis
- Cytologic features: spindled to ovoid melanocytes, hyperchromatic to vesicular nuclei, inconspicuous nucleoli, moderate to abundant cytoplasm, prominent melanin pigmentation
- Dermal component superficial with single cells or small nests
- Often with dermal inflammation and numerous melanophages
- Atypical features
- > 6 mm in diameter, poor circumscription, asymmetry
- Single cell melanocytic hyperplasia along the junction peripherally
- Increased pagetoid scatter, mitotic activity (especially in junctional component) and cytologic atypia
- Dermal nodules of melanocytes failing to undergo maturation
- References: Hum Pathol 1991;22:52, Am J Surg Pathol 2011;35:1733, Dermatol Pract Concept 2016;6:37, Am J Surg Pathol 1984;8:645
Microscopic (histologic) images
Positive stains
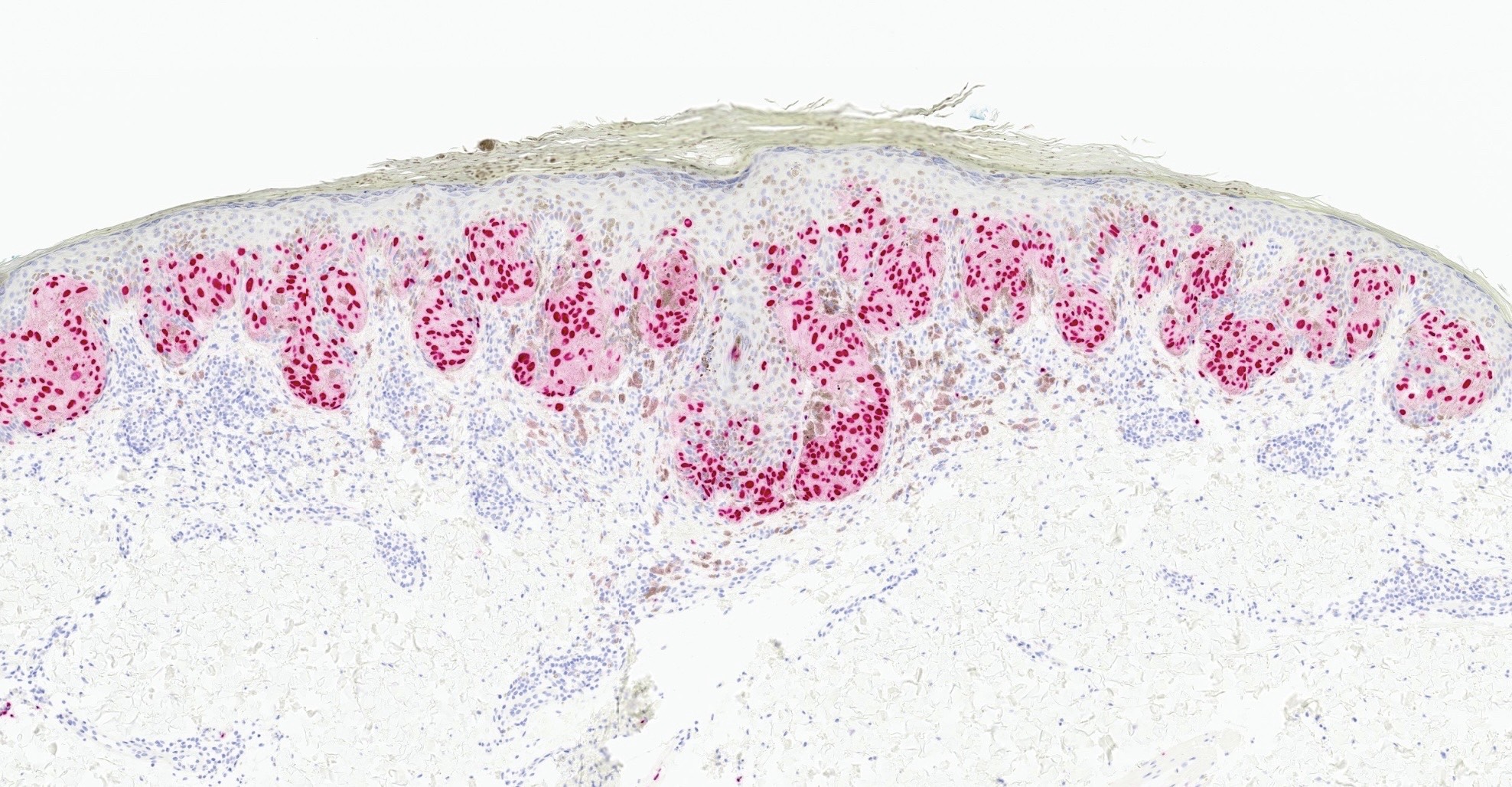
- Melanocytic markers (SOX10, MelanA, S100)
- Retained strong p16 expression
- HMB45 positive in intraepidermal component
- References: Am J Dermatopathol 1991;13:543, Am J Surg Pathol 2011;35:1733
Negative stains
- HMB45 negative in the dermal component (Am J Dermatopathol 1991;13:543, Am J Surg Pathol 2011;35:1733)
Molecular / cytogenetics description
- 78% of Reed nevi have kinase fusions, of which NTRK3 fusion is the most common
- Not necessary for diagnosis
Sample pathology report
- Skin, right thigh, punch biopsy:
- Pigmented spindle cell nevus (Reed nevus)
- Microscopic description: H&E stained sections show a symmetrical and circumscribed predominately junctional melanocytic proliferation. The junctional component consists of large, nested spindled to ovoid melanocytes with bridging across rete ridges and low level upward scatter limited to the center of the lesion. Associated prominent pigment and occasional Kamino bodies are seen. The dermal component consists of superficial, small nested melanocytes and single melanocytes with appropriate maturation. Dermal melanophages are prominent. The melanocytes are highlighted by SOX10 and MelanA immunostains. HMB45 immunostain is positive in junctional melanocytes and shows appropriate loss in the dermal component. There is retained p16 expression in the dermal melanocytes.
Differential diagnosis
- Melanoma:
- Older patients
- Most often in sun damaged skin
- Asymmetrical, lack of circumscription, severe cytologic atypia, increased mitotic activity and loss of p16 expression
- Spitz nevus:
- More common in head and neck region
- More often in children
- Mixed spindled and epithelioid cells, lack of prominent pigmentation and larger dermal nests
- Reference: Am J Surg Pathol 2011;35:1733
Additional references
Board review style question #1
Board review style answer #1
A. Asymmetrical lesion with increased junctional mitotic activity. Asymmetrical lesions that are poorly circumscribed with increased mitotic activity, particularly in the junctional component suggest an atypical Reed nevus. Answers B, C and D are incorrect because these are features of typical Reed nevi.
Comment Here
Reference: Reed nevus
Comment Here
Reference: Reed nevus
Board review style question #2
What is the most common known molecular alternation in Reed nevus?
- BRAF V600E mutation
- NTRK2 rearrangement
- NTRK3 fusion
- TERT promoter mutation
Board review style answer #2
C. NTRK3 fusion. 78% of Reed nevi have kinase fusions, with the most common being NTRK3 fusions.
Answer A is incorrect because BRAF V600E is more suggestive of atypical nevus with spitzoid features or melanoma.
Answer B is incorrect because although NTRK2 rearrangement has been reported in Reed nevus, it is not the most common molecular alternation identified.
Answer D is incorrect because TERT mutation is a common genetic alteration in melanoma.
Comment Here
Reference: Reed nevus
Comment Here
Reference: Reed nevus