Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Molecular / cytogenetics images | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3 | Board review style question #4 | Board review style answer #4Cite this page: Mansour B, Donati M. Invasive melanoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/skintumormelanocyticmelanoma.html. Accessed December 22nd, 2024.
Definition / general
- Malignant melanocytic tumor arising from melanocytes in the skin, mucosa and autochthonous (indigenous) melanocytes from numerous internal organs (i.e. GI tract, CNS, etc.)
Essential features
- Malignant melanocytic tumor arising from melanocytes
- Accounts for majority of mortality due to skin cancer
- Breslow depth is the most important prognostic factor
- BRAF mutation testing is recommended for patients with stage III - IV melanoma
Terminology
- Historically called melanose and fungoid disease (Melanoma Res 2012;22:114)
ICD coding
- ICD-O:
- 8743/3 - Superficial spreading melanoma
- 8742/3 - Lentigo maligna melanoma
- 8745/3 - Desmoplastic melanoma, malignant
- 8770/3 - Mixed epithelioid and spindle cell melanoma
- 8720/3 - Malignant melanoma, NOS
- 8746/3 - Mucosal lentiginous melanoma
- 8721/3 - Nodular melanoma
- 8780/3 - Blue nevus, malignant
- 8761/3 - Malignant melanoma in giant pigmented nevus
- 8771/3 - Epithelioid cell melanoma
- 8773/3 - Spindle cell melanoma, type A
- 8774/3 - Spindle cell melanoma, type B
- 8720/6 - Malignant melanoma, metastatic
- ICD-10: C43 - Malignant melanoma of skin
Epidemiology
- Incidence: 3 - 7% (Europe), 2.6% (U.S.)
- 1% of skin cancer
- Incidence has risen rapidly over the last 50 years
- Higher incidence in periequatorial zone
- M:F = 1.5:1
- Risk factors
- Fair skinned populations (Fitzpatrick scale I - II)
- Family and personal history of melanoma
- Intense intermittent sun exposure (or artificial UV radiation sources)
- Increased mole count (> 50)
- Dysplastic nevus phenotype
- Germline mutation in CDKN2A, CDK4, MITF, TERT, ACD, TERF2IP, POT1, MC1R or BAP1 genes (J Cutan Pathol 2020;47:606)
- Immunosuppression
Sites
- Cutaneous melanoma: anywhere on the skin's surface, including subungual location
- Frequent sites
- Lower extremities (female)
- Trunk (male)
- Extracutaneous
- Uvea
- Anorectal region
- Upper aerodigestive tract
- Sinonasal tract
- Leptomeninges
Pathophysiology
- Multistep process that involves interaction of genomic, environmental and host factors
- 4 step model proposed (Pigment Cell Melanoma Res 2016;29:122):
- Mitogenic driver mutation (i.e. BRAF mutation)
- Escaping primary senescence (i.e. CDKN2A loss)
- Overcoming apoptosis (i.e. TP53 mutation)
- Immortalization (i.e. TERT-p mutation)
- Main oncogenic signaling pathways
- Mitogen activated protein kinase (MAPK) pathway (RAS / RAF / MEK / ERK)
- PI3K / AKT / mTOR
- WNT / beta catenin signaling pathway (N Engl J Med 2015;373:1926)
Etiology
- Melanoma can occur de novo or develop on a pre-existent nevus, known as melanoma arising in nevus
- Ultraviolet exposure is the main etiological factor
- 2 main categories (Annu Rev Pathol 2014;9:239):
- Cumulative sun damage (CSD) (pathways I - III)
- Low CSD (superficial spreading melanoma / L CSD nodular melanoma)
- High CSD (lentigo maligna melanoma / H CSD nodular melanoma / desmoplastic melanoma)
- Not consistently associated with cumulative sun damage (pathways IV - IX)
- Spitz melanoma, acral melanoma, mucosal melanoma, melanoma arising in congenital nevus, melanoma arising in blue nevus and uveal melanoma
- Cumulative sun damage (CSD) (pathways I - III)
- Reference: Arch Pathol Lab Med 2020;144:500
Clinical features
- Flat, slightly elevated, nodular, polypoid or verrucous pigmented lesion
- May be achromic (amelanotic melanoma)
- ABCDE rule (superficial spreading melanoma, lentigo maligna melanoma, acral lentiginous melanoma)
- Asymmetry
- Irregular borders
- Variation in color
- Diameter (> 6 mm)
- Evolution
- Ugly duckling sign
- Hutchinson sign
- Blowfly sign (Donati: Clinical Dermatopathology - A Practical Guide to the Diagnosis of Skin Neoplasms, 1st Edition, 2019)
- Associated conditions
- Dysplastic nevus syndrome (BK mole syndrome)
- BAP1 inactivated tumor syndrome
- Xeroderma pigmentosum
- Parkinson disease
- Dermoscopic findings
- Atypical pigmented network
- Blue whitish veil
- Atypical vascular pattern
- Abrupt cutoff
- Atypical dots or globules
- Presence of pseudopods
- Radial steaming
- Milky red areas
- Shiny white structures
- Regression structures
- Scar-like depigmentation
- Multicomponent pattern
- More than 4 colors
- Dermoscopic scoring systems (J Am Acad Dermatol 2016;74:1093)
Diagnosis
- Total body skin examination for the identification of clinically suspicious lesions
- Histopathological diagnosis after wide surgical excision is the gold standard
- Correlation with clinical parameters including age, gender, anatomical location and dermoscopic findings
Laboratory
- Increased LDH level in advanced stage
Prognostic factors
- Favorable prognostic factors
- Young age
- Female
- Low risk sites: extremities
- Early staged melanomas
- Brisk tumor infiltrating lymphocytes
- Unfavorable prognostic factors
- Elderly patients
- Male
- High risk sites: back, upper arm, head and neck and acral sites
- High Breslow depth
- Positive sentinel lymph node biopsy
- High dermal mitotic rate
- Ulceration
- Absent or nonbrisk tumor infiltrating lymphocytes
- Presence of microsatellites
- Lymphatic invasion
- Neurotropism (local recurrence)
- Histologic subtype (pure desmoplastic melanoma and Spitz melanoma may have better prognosis) (Am J Surg Pathol 2004;28:1518, Ann Surg Oncol 2005;12:207, Mod Pathol 2020;33:1122, J Eur Acad Dermatol Venereol 2013;27:1214)
- Increasing angiogenesis
- 5 year relative survival rates
- Localized disease: 99%
- Regional lymph nodes involvement: 62.2%
- Metastatic disease: 27.3%
- Recurrence rate within 2 years
- 13% (for high risk melanoma) (JAMA Dermatol 2019;155:688)
Case reports
- 21 year old woman with a cutaneous lesion arising from the scalp (Am J Dermatopathol 2020;42:854)
- 34 year old man with a giant congenital nevus of the axilla (J Cutan Pathol 2020;47:1164)
- 61 year old woman with productive cough and chest pain (Medicine (Baltimore) 2017;96:e8772)
- 67 year old Caucasian woman with a tender subungual nodule (Am J Dermatopathol 2020;42:283)
- 67 year old man with progressive dysphagia (Am J Case Rep 2015;16:491)
- 70 year old woman with shortness of breath and wheezing (N Engl J Med 2018;379:e36)
- 72 year old man presented with a cutaneous lesion on the scalp (BMJ Case Rep 2018;2018:bcr2018224263)
- 73 year old man presented with a rapidly growing nodule on his lower left lateral thigh (J Cutan Pathol 2020;47:541)
- 79 year old Caucasian woman with a persistent nodule on her posterior neck and a slowly enlarging mass on the posterior scalp (J Cutan Pathol 2018;45:355)
- 82 year old man with unusual histopathological presentation (Am J Dermatopathol 2020;42:677)
- 85 year old man with a grayish nodule on the forehead (Am J Dermatopathol 2021;43:221)
Treatment
- Wide surgical excision with safety skin margins according to Breslow depth
- In situ: 0.5 cm safety skin margins
- Breslow depth up to 2 mm: 1 cm
- Breslow depth > 2 mm: 2 cm
- Sentinel lymph node biopsy (staging procedure and prognostic value)
- Adjuvant / systemic therapy starting from stage III melanomas
- Target therapy (BRAF and MEK inhibitors, KIT inhibitors)
- Checkpoint inhibitors (PD1 / PDL1 inhibitors, CTLA4 blockade)
- Chemotherapy
- Radiotherapy
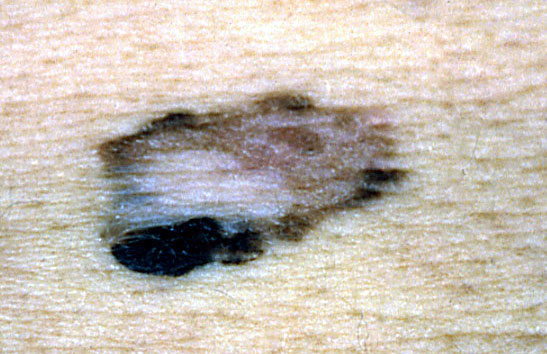
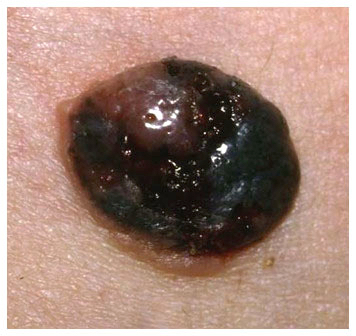
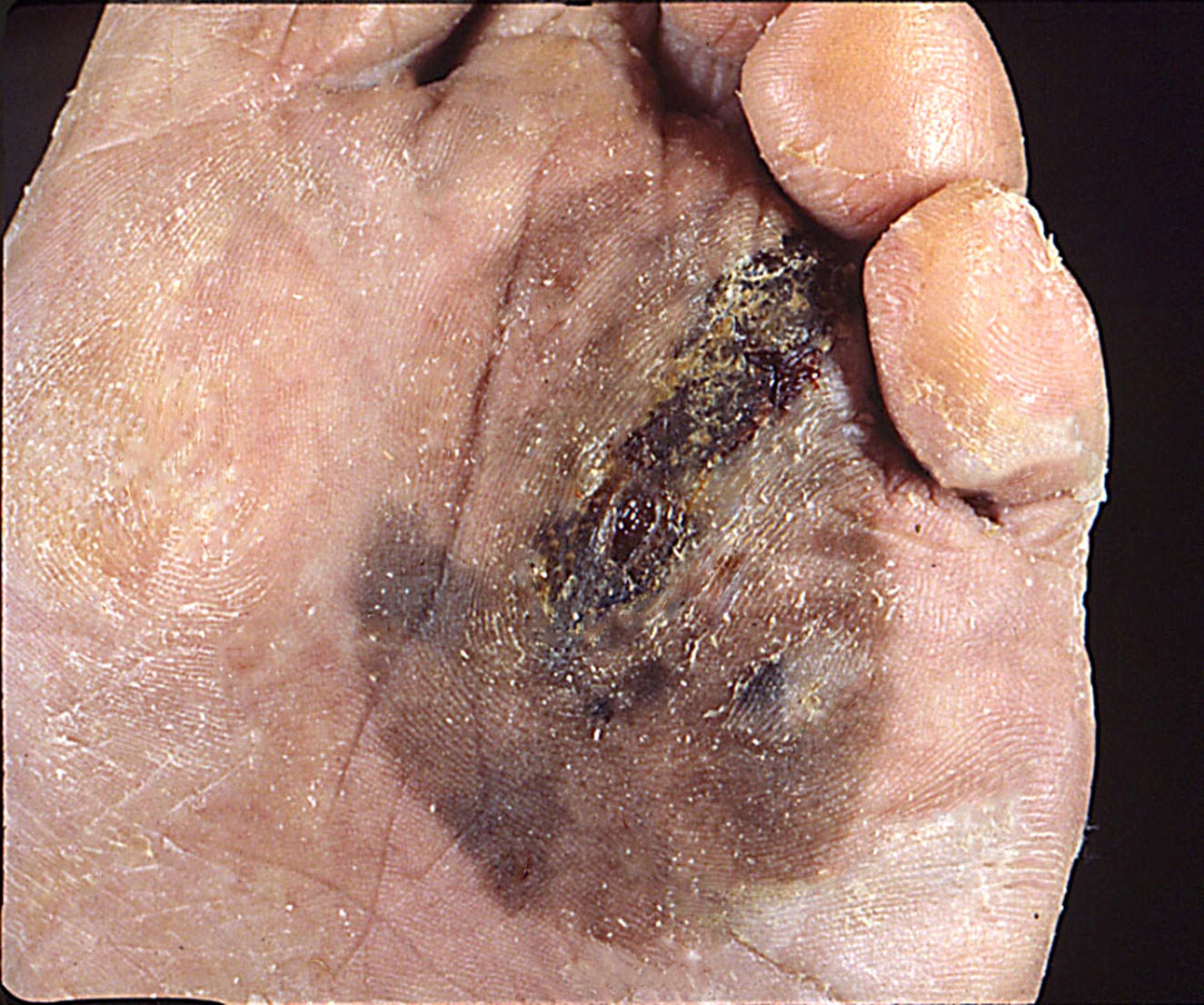
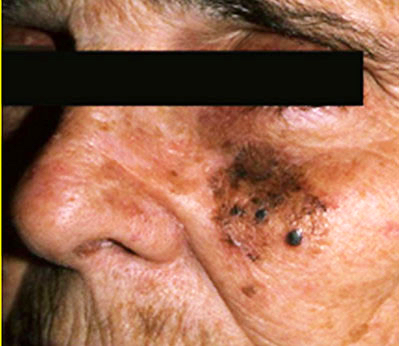
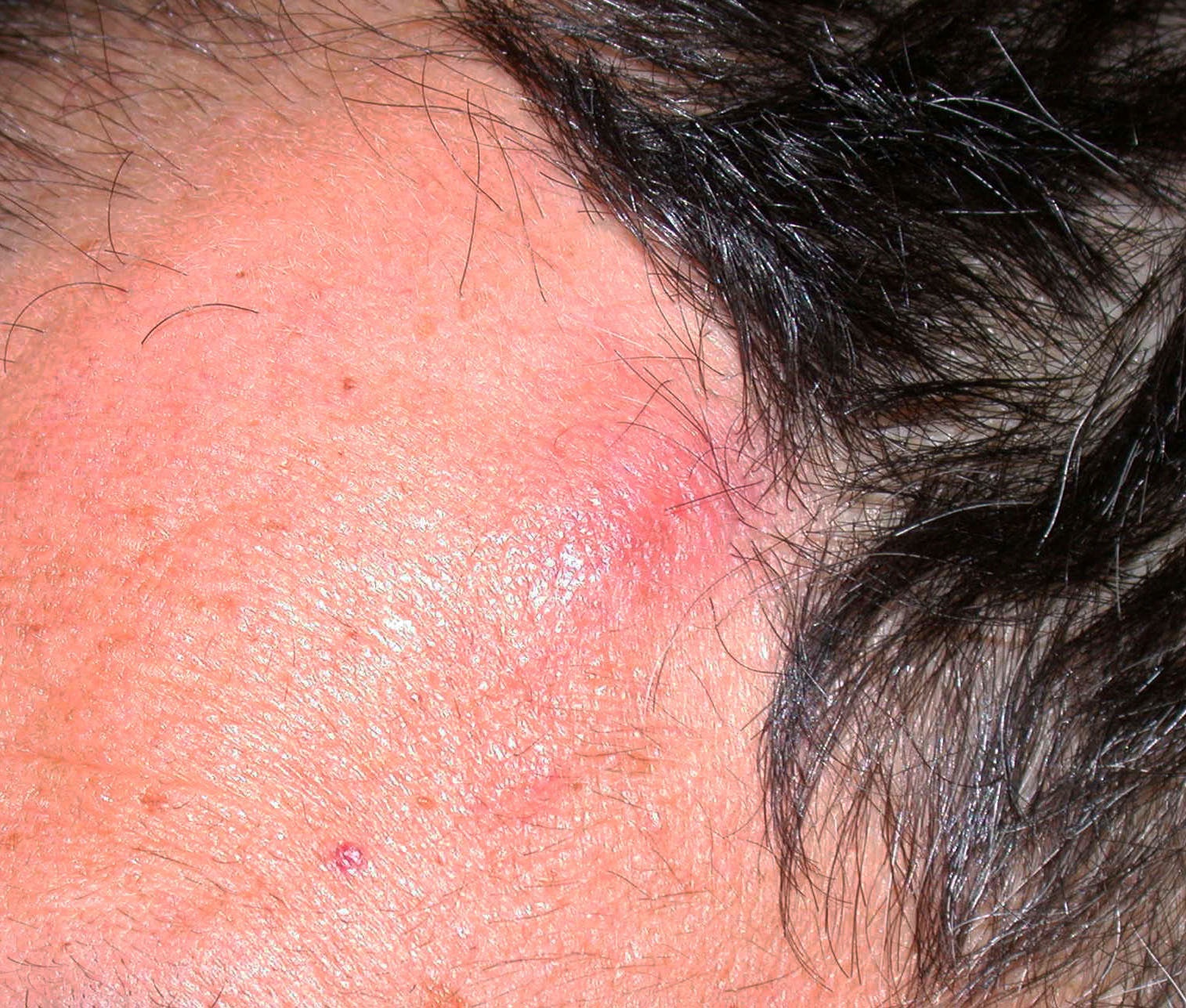
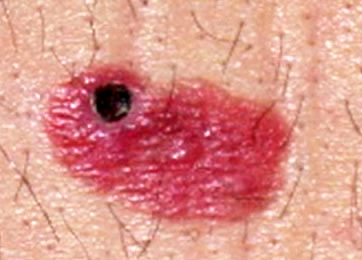
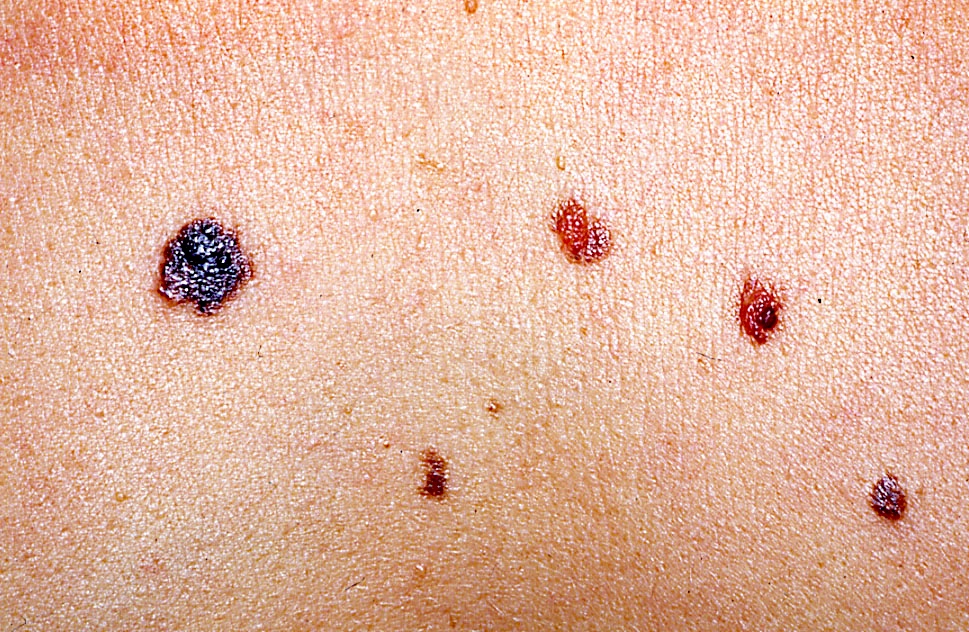
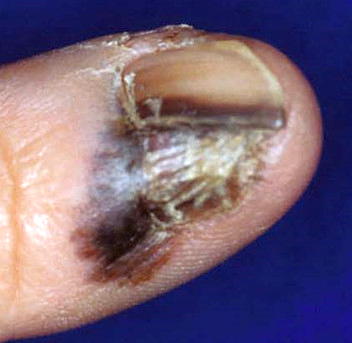
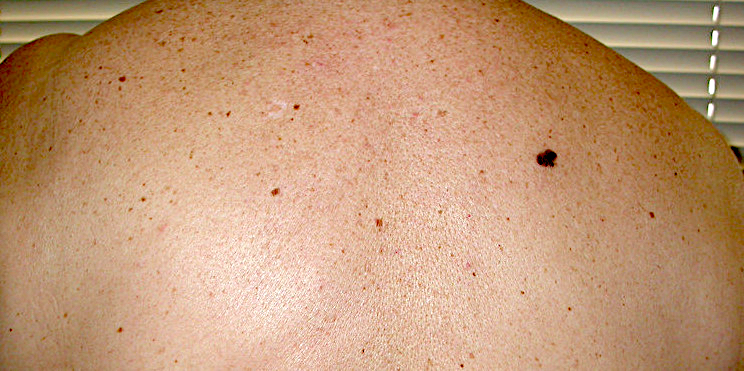
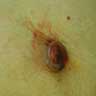
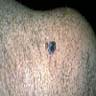
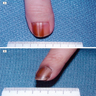
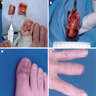
Clinical images
Contributed by Michele Donati, M.D.
Images hosted on other servers:
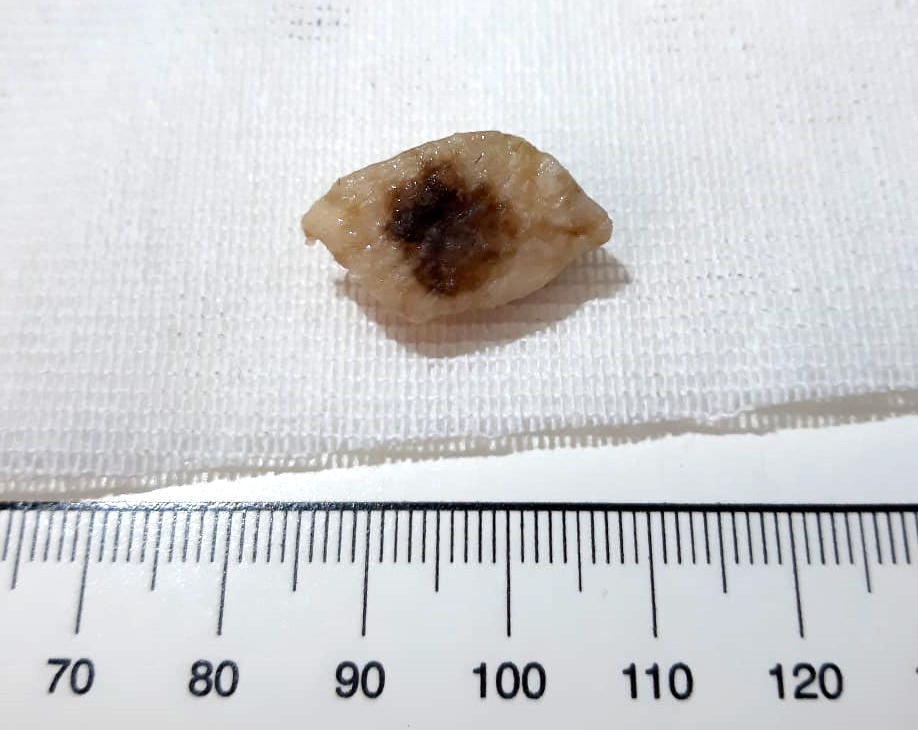
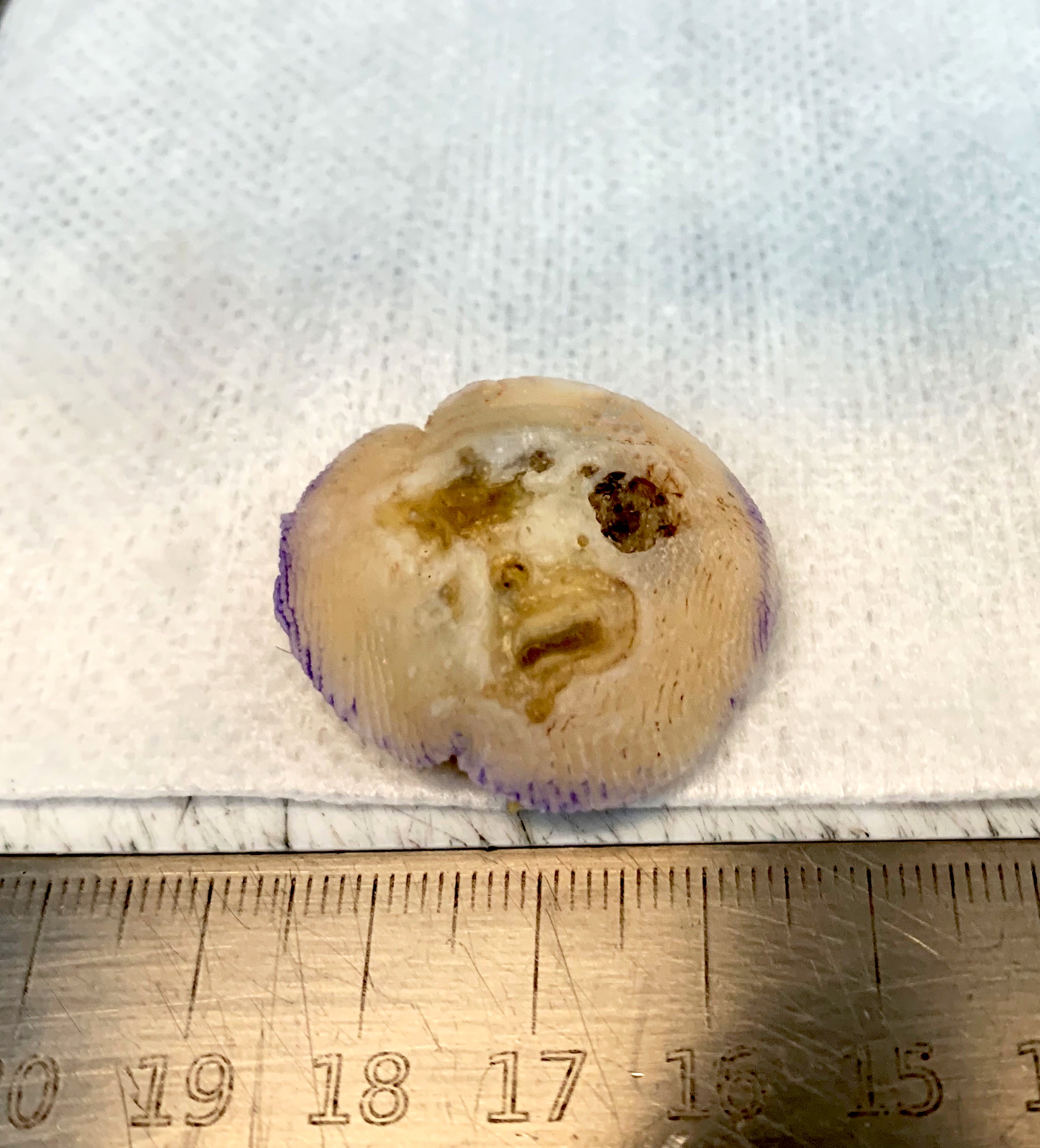
Gross description
- Skin ellipse with a lesion on the surface of variable presentation according to the clinical aspect (see Clinical features)
Gross images
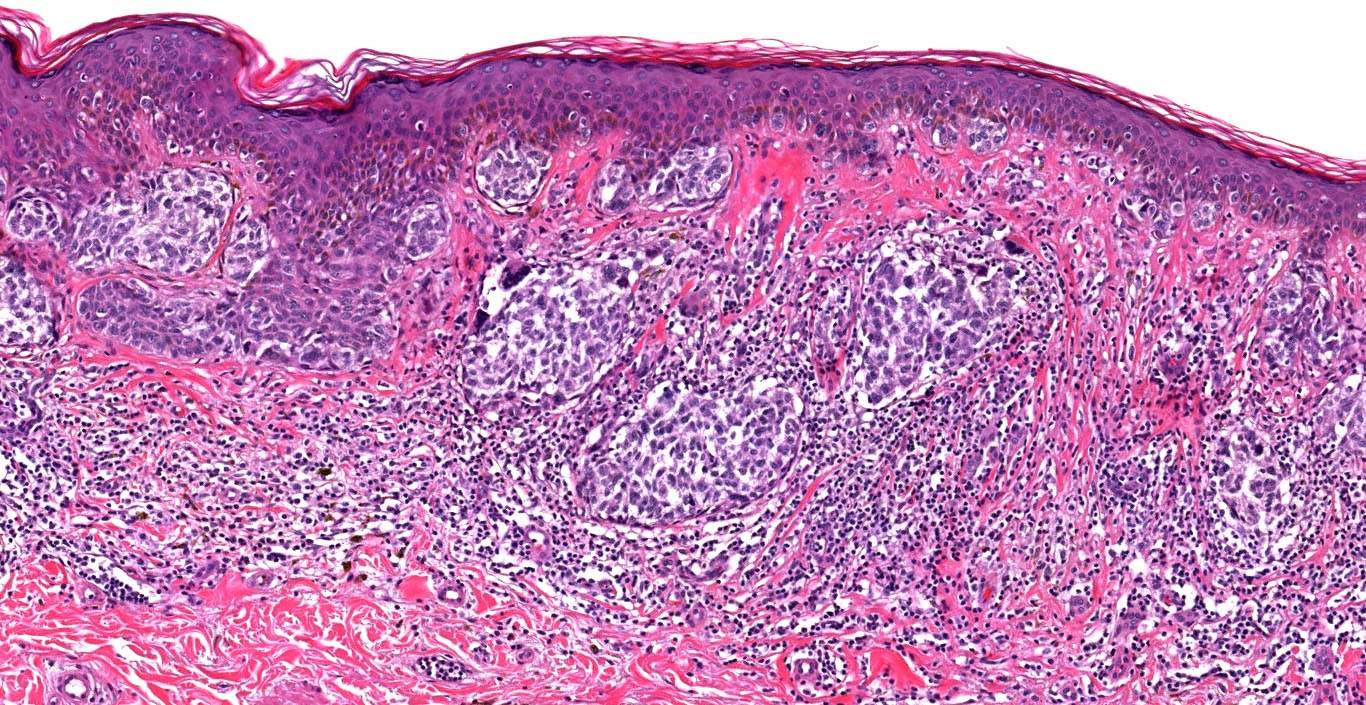
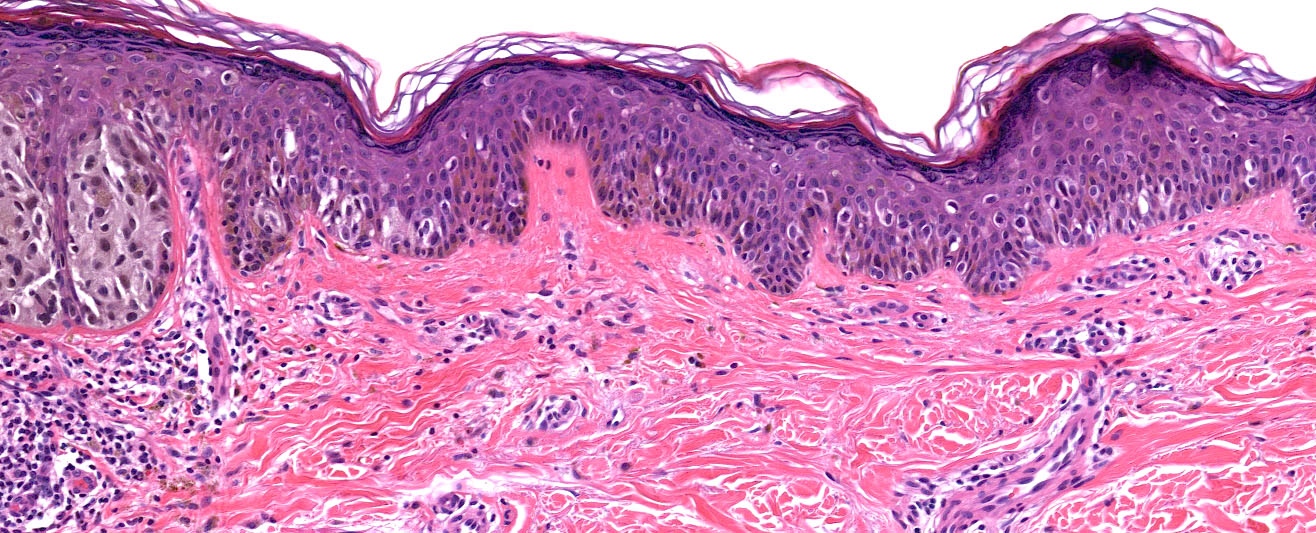
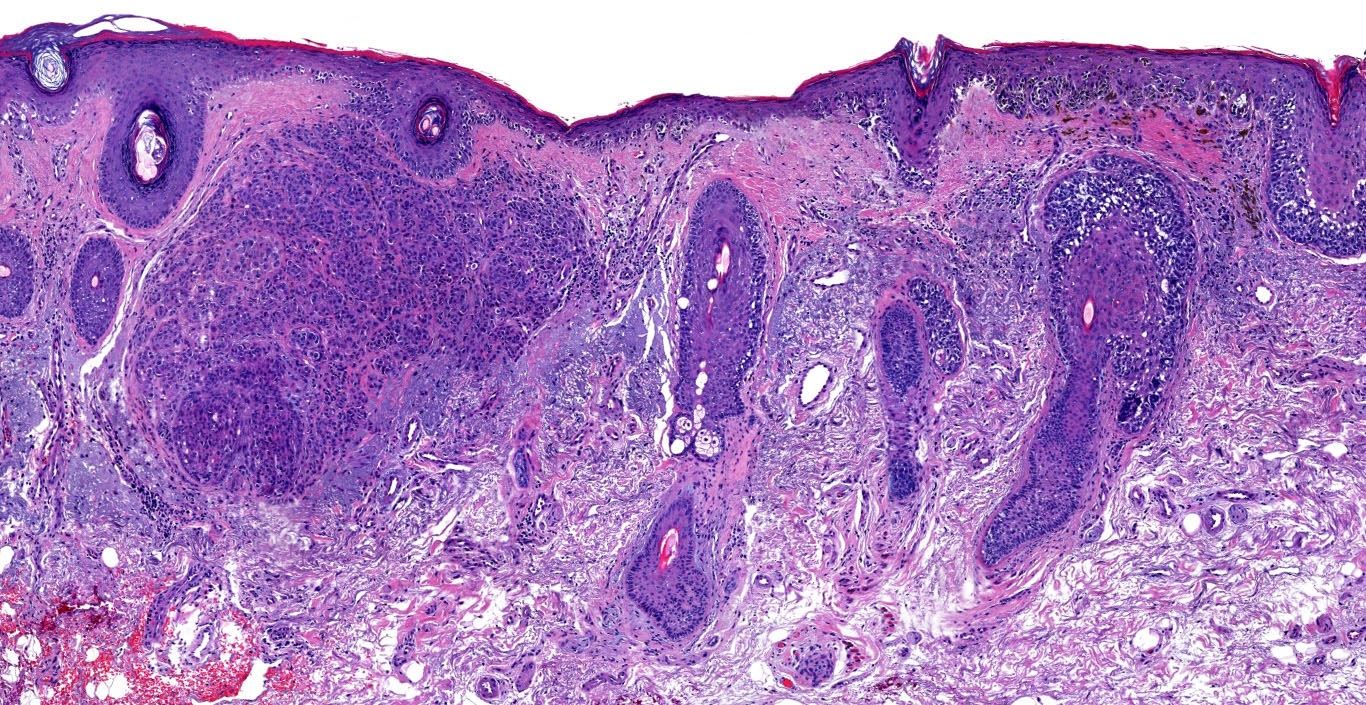
Microscopic (histologic) description
- Histologic features
- Dimension > 6 mm
- Asymmetry (assessed at scanning magnification)
- Epidermal findings / in situ melanoma
- Ill defined border
- Pagetoid melanocytes (single scattered melanocytes, especially in the upper layers of the epidermis)
- Epidermal consumption / ulceration
- Irregular distribution of junctional melanocytes
- Confluent growth
- Skip areas
- Nests of different size
- Nests of different shape
- Irregular distribution of nests
- Confluent nests
- Discohesive arrangement of melanocytes
- Dermal component
- Radial growth phase
- Invasion of single cells or small nests in the papillary dermis
- Vertical growth phase
- Early vertical growth phase: dominant nest within the papillary dermis (expansile nest larger than any junctional nests)
- Complex and asymmetrical growth pattern (irregular nests / fascicles)
- Expansile growth pattern (sheet-like)
- Absence of maturation (lack of decreasing size of melanocytes / nests from the top to the base of the lesion)
- Increased dermal mitotic activity (> 1/mm²)
- Tumor necrosis
- Radial growth phase
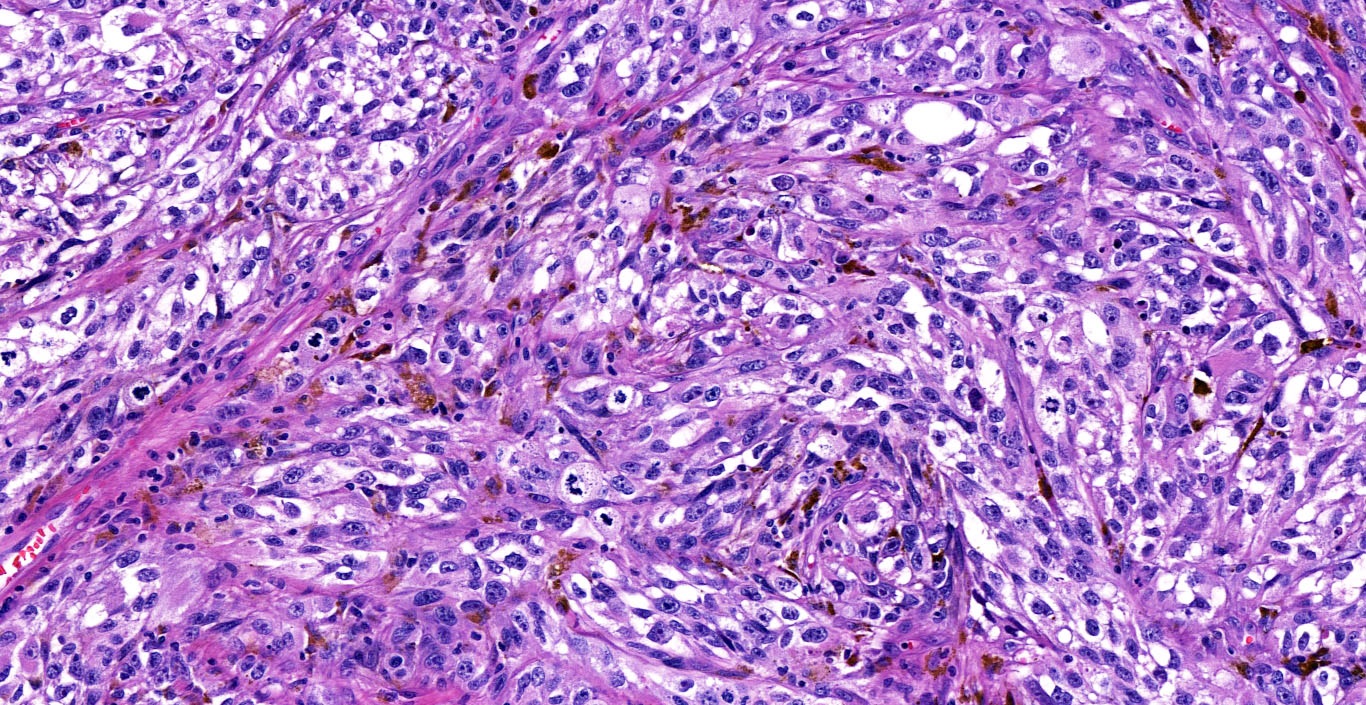
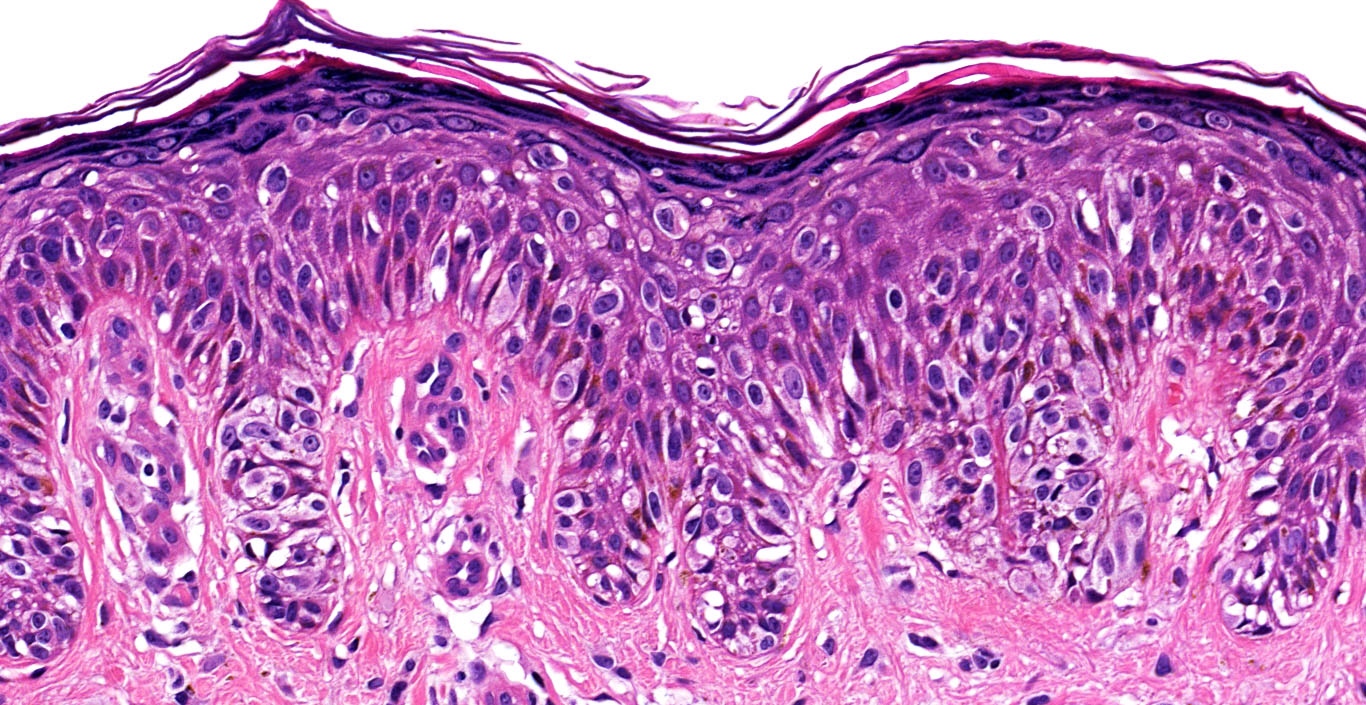
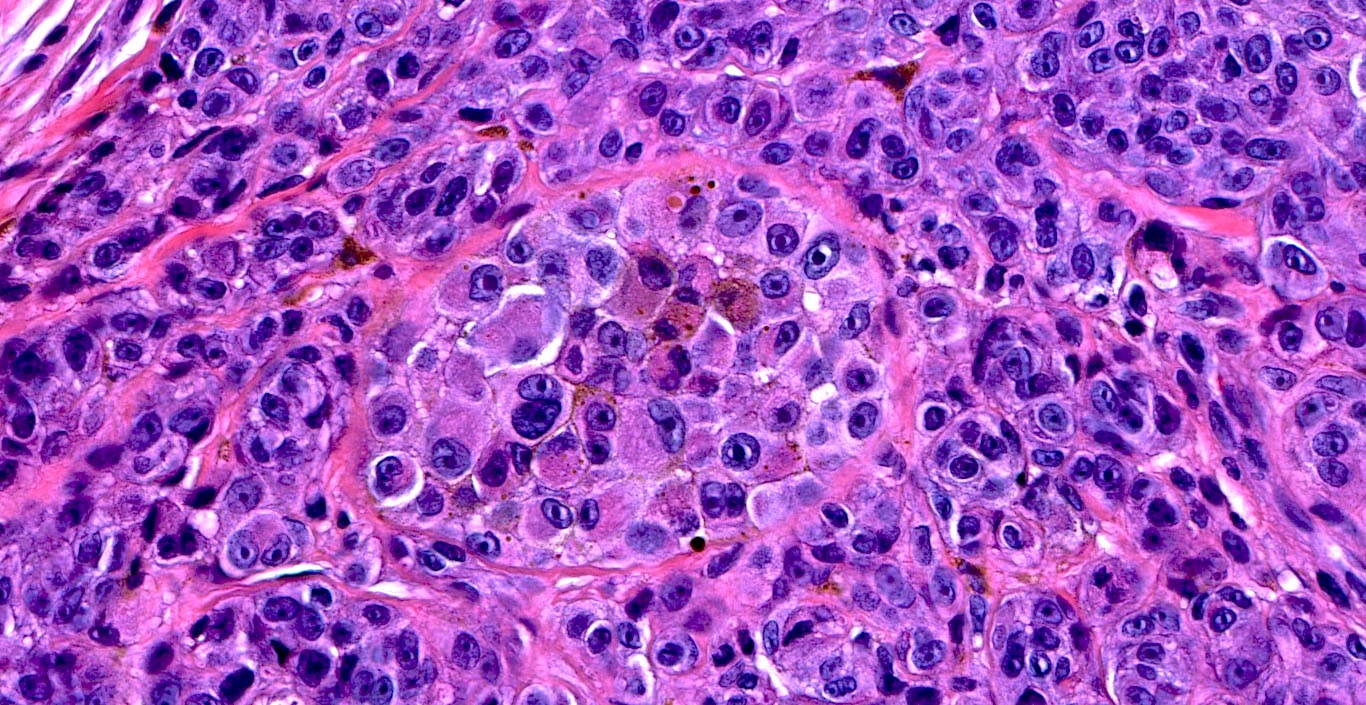
- Cytologic features
- Epithelioid / spindle shaped cell
- Nuclear pleomorphism
- Nuclear enlargement (> 1.5 basal keratinocytes)
- Nuclear hyperchromasia
- Coarse irregular chromatin pattern with peripheral condensation ("peppered moth" nuclei) (J Am Acad Dermatol 2016;75:1032)
- Prominent eosinophilic nucleoli
- Dusty pigmented cytoplasm
- Stromal changes
- Variable inflammatory infiltrate (brisk, nonbrisk, absent)
- Dermal fibrosis
- Irregular distribution of pigment
- Histological classification
- Superficial spreading melanoma (SSM)
- Most common subtype
- Asymmetrical proliferation of atypical melanocytes
- Predominant junctional single units of melanocytes rather than nests
- Prominent pagetoid spread (area > 0.5 mm²)
- BRAF V600E mutation
- Lentigo maligna melanoma (LMM)
- Elderly patients on chronic sun damaged skin
- Confluent growth of solitary units of melanocytes along the dermoepidermal junction forming small nests (lentiginous pattern)
- Confluent horizontal arranged nests of variable size and shape (nevoid / dysplastic-like pattern)
- Extension into the hair follicles
- Prominent solar elastosis
- Dermal invasion of atypical melanocytes
- BRAF non-V600E, NRAS or KIT mutation
- Acral lentiginous melanoma (ALM)
- Most common in African Caribbeans and Asians
- Acral location (palms, soles and subungual)
- Asymmetrical lentiginous proliferation > 7 mm
- Melanocytes mainly at the tips of cristae profunda intermedia (Am J Dermatopathol 2011;33:468)
- Eccrine duct involvement
- KIT mutation
- Nodular melanoma (NM)
- No ABCDE rule (see Clinical features)
- No radial growth phase
- Junctional component not beyond the dermal component
- Nodular dermal proliferation of atypical melanocytes
- Superficial spreading melanoma (SSM)
- Uncommon variants
- Desmoplastic melanoma (DM)
- Elderly patients on chronic sun damaged skin
- Subtle scar-like paucicelluar dermal proliferation of spindle cells
- May be sarcoma-like pleomorphic spindle cell melanoma with only partial desmoplasia
- Atypical lentiginous junctional melanocytic proliferation in ~50%
- Perivascular lymphocytic aggregates
- Neurotropism
- May be pure or mixed (associated with conventional melanoma)
- Pure: 90% or more desmoplastic type
- Mixed: more than 10% conventional or spindle cell type
- Pure DM has higher local recurrence but lower regional lymph node involvement (Ann Surg 2010;252:1052)
- MelanA / MART1, tyrosinase, HMB45 negative
- NF1, TP53 mutation in ~50%
- Nevoid melanoma
- Verrucous or doom shaped silhouette
- Subtle asymmetry
- No radial growth phase
- Long thin rete ridges due to stuffed papillae: puffy shirt sign (J Cutan Pathol 2019;46:805)
- Pseudomaturation
- High mitotic activity
- Melanoma arising in blue nevus
- Presence of a pre-existing blue nevus at the periphery
- High cellular density with no intervening stroma
- Areas of necrosis
- BAP1 nuclear loss
- GNAQ, GNA11, CYSLTR2, EIF1AX mutation
- BAP1, SF3B1 mutation (secondary event)
- Desmoplastic melanoma (DM)
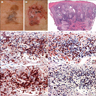
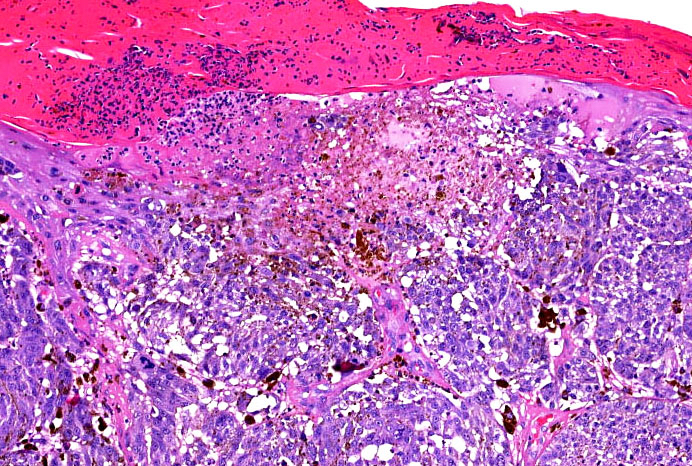
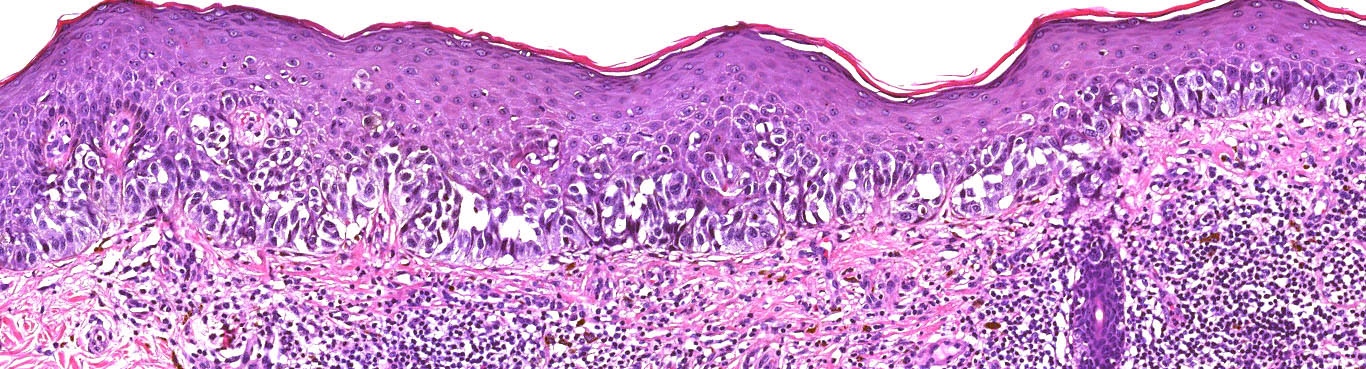
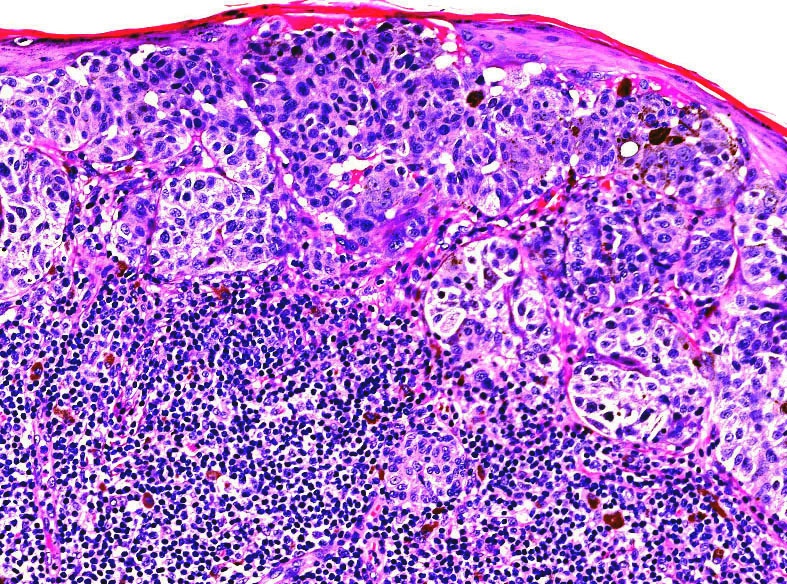
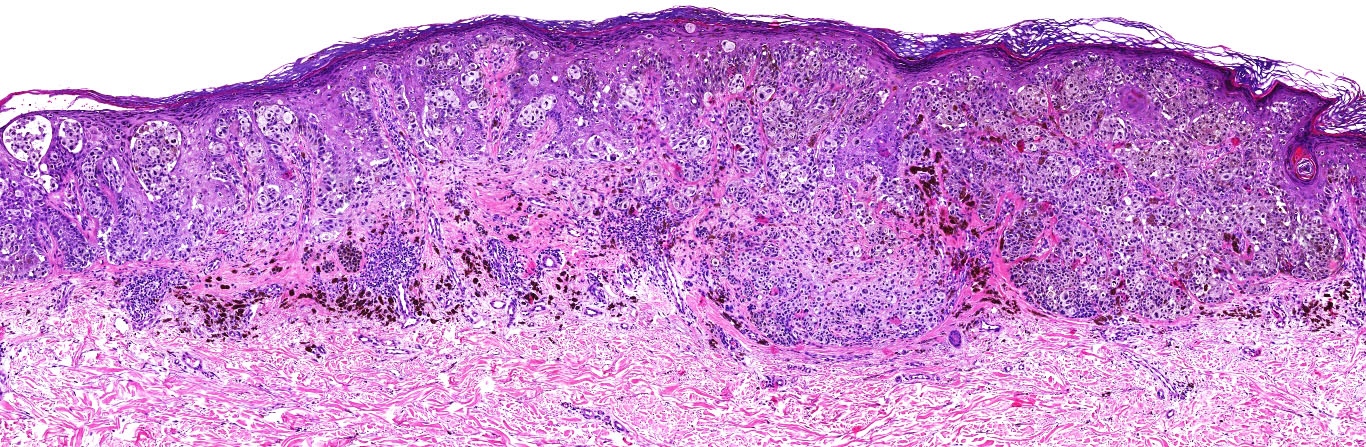
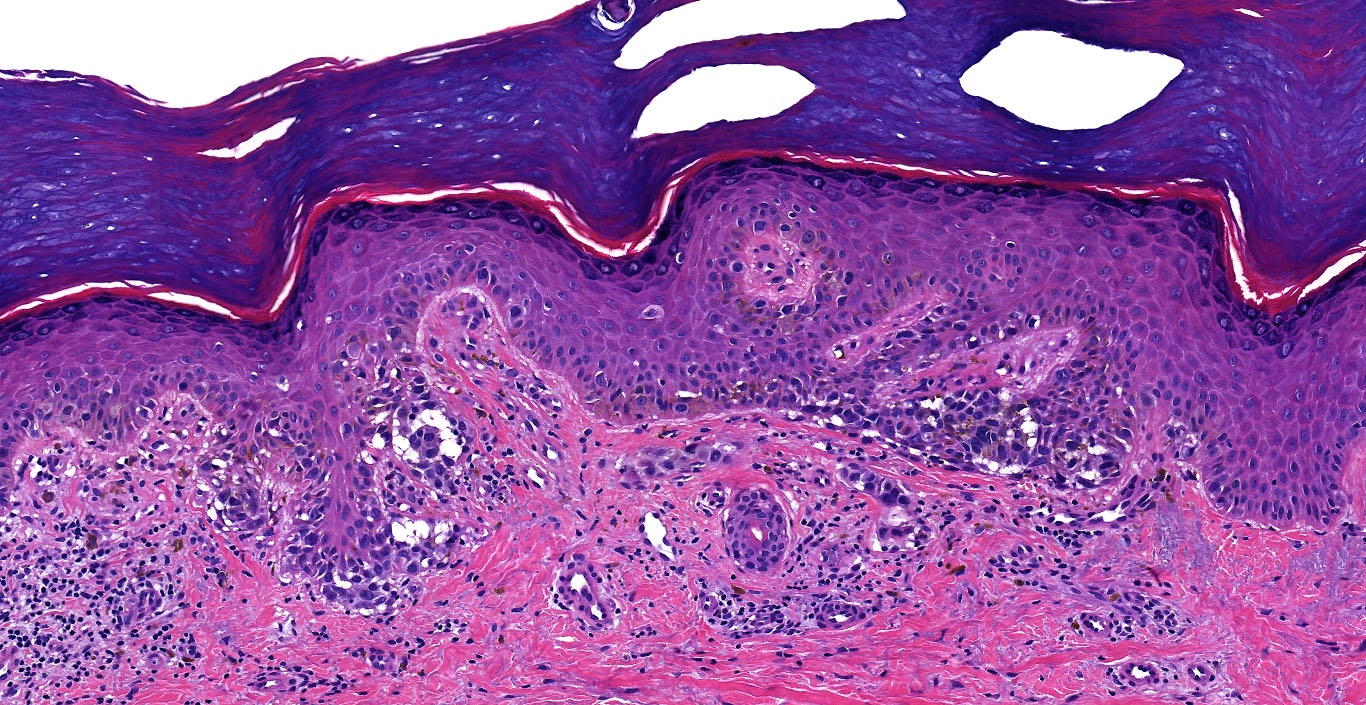
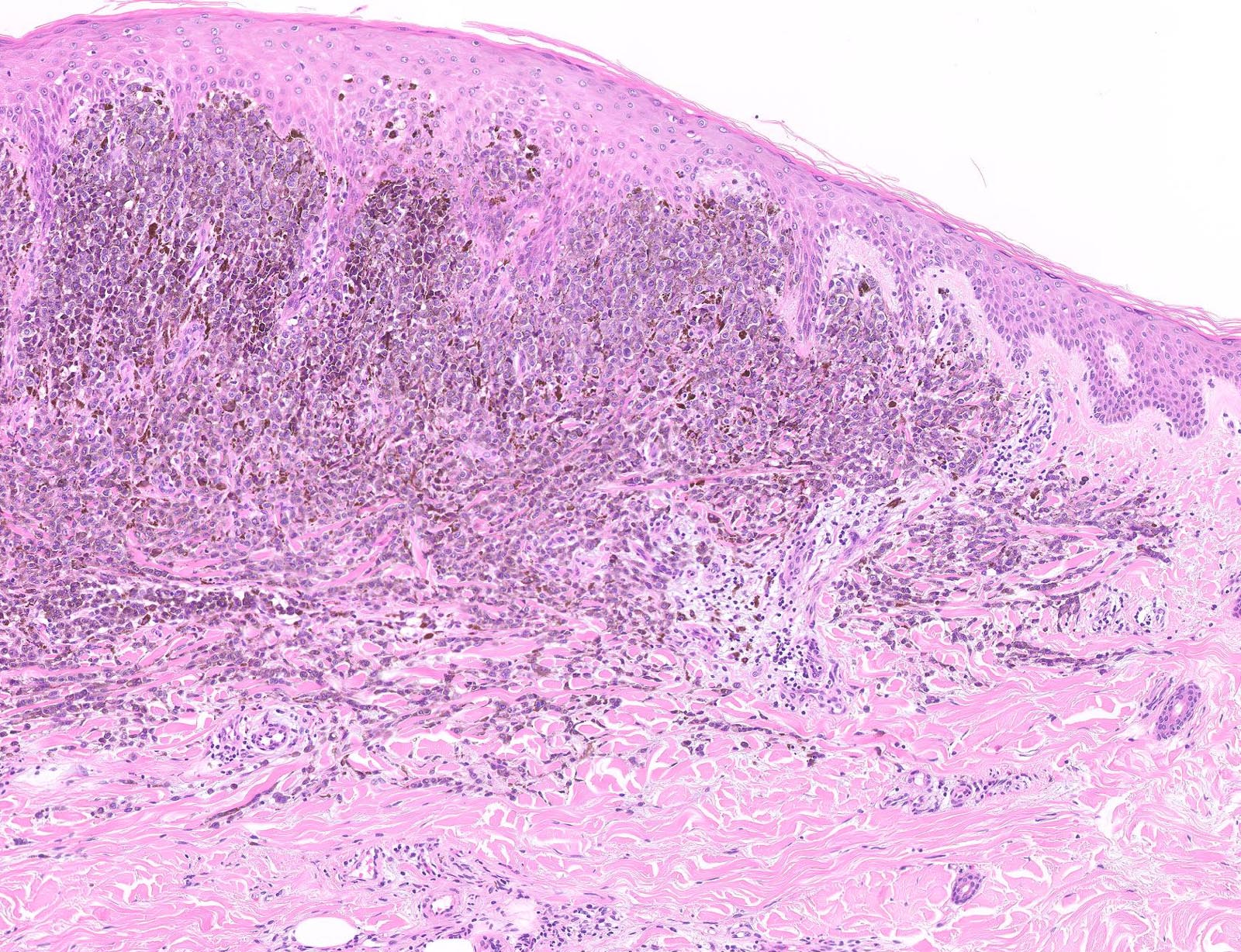
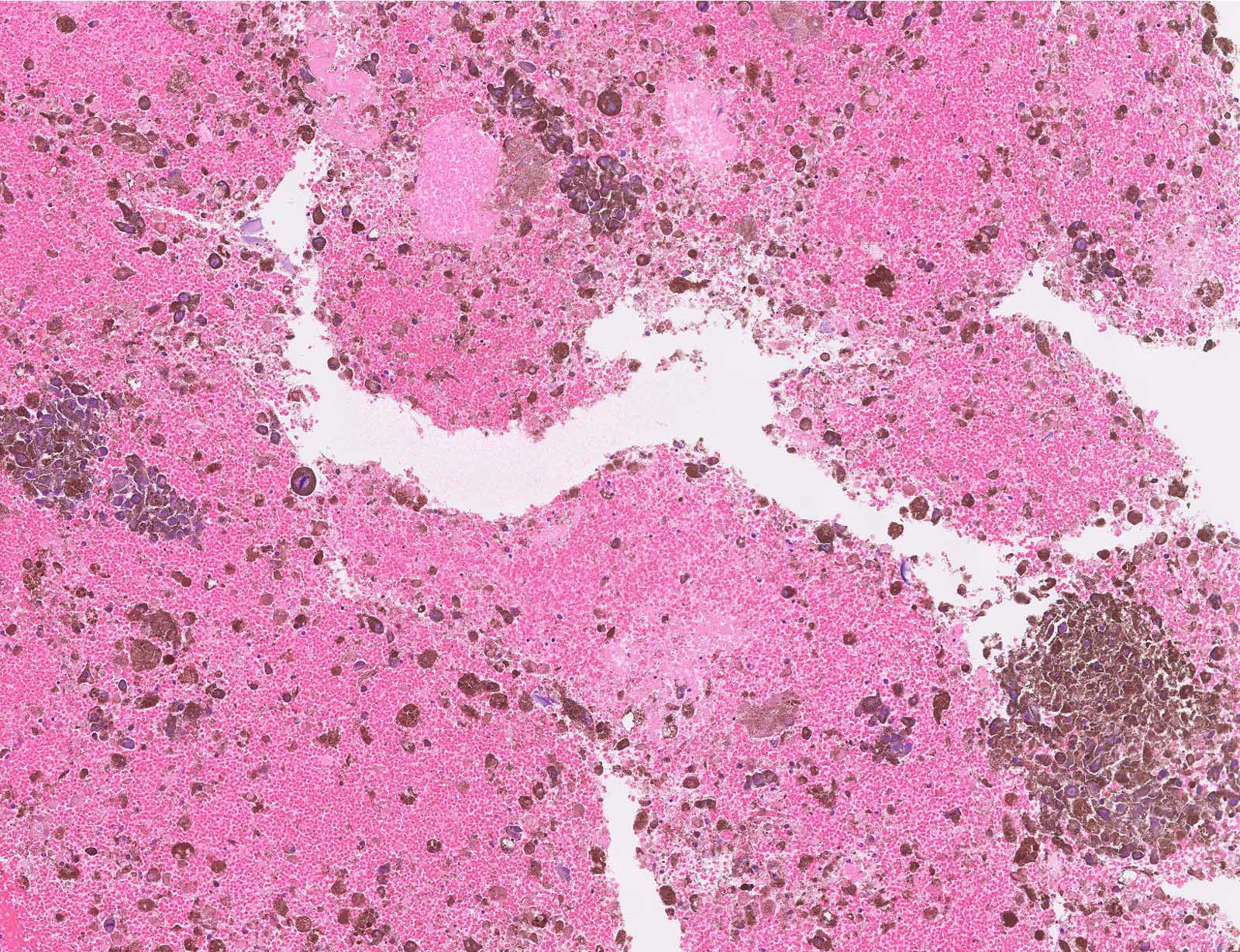
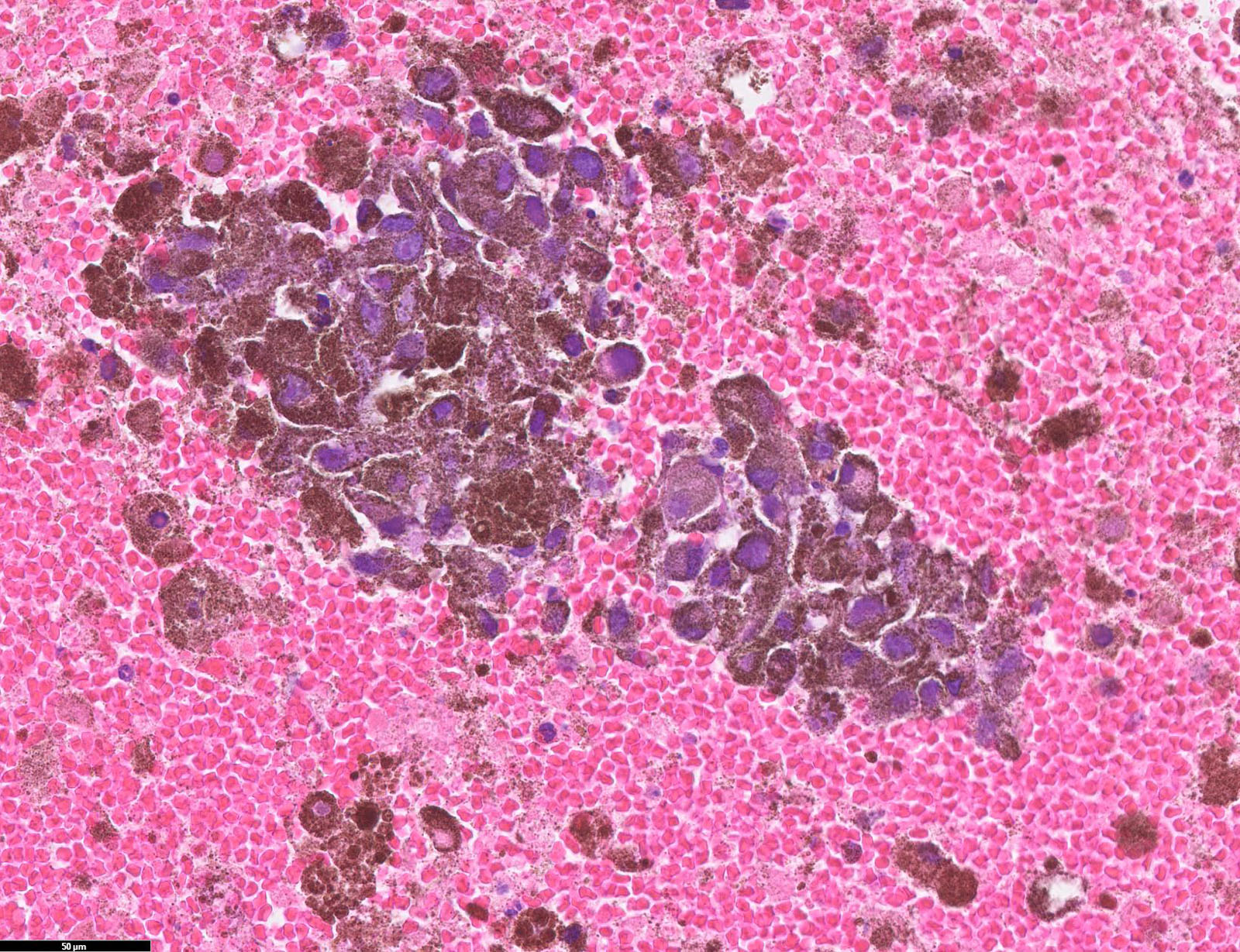
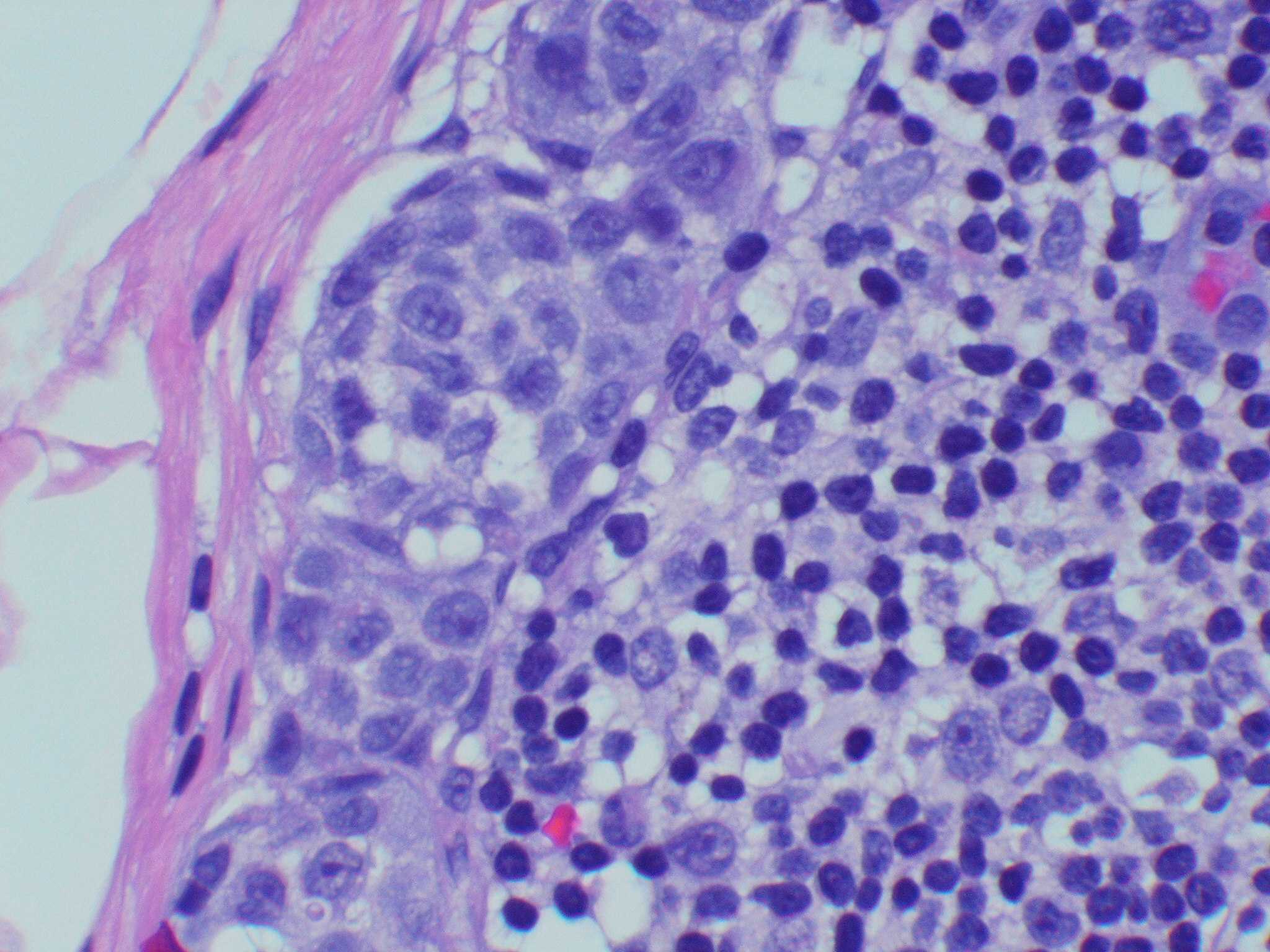
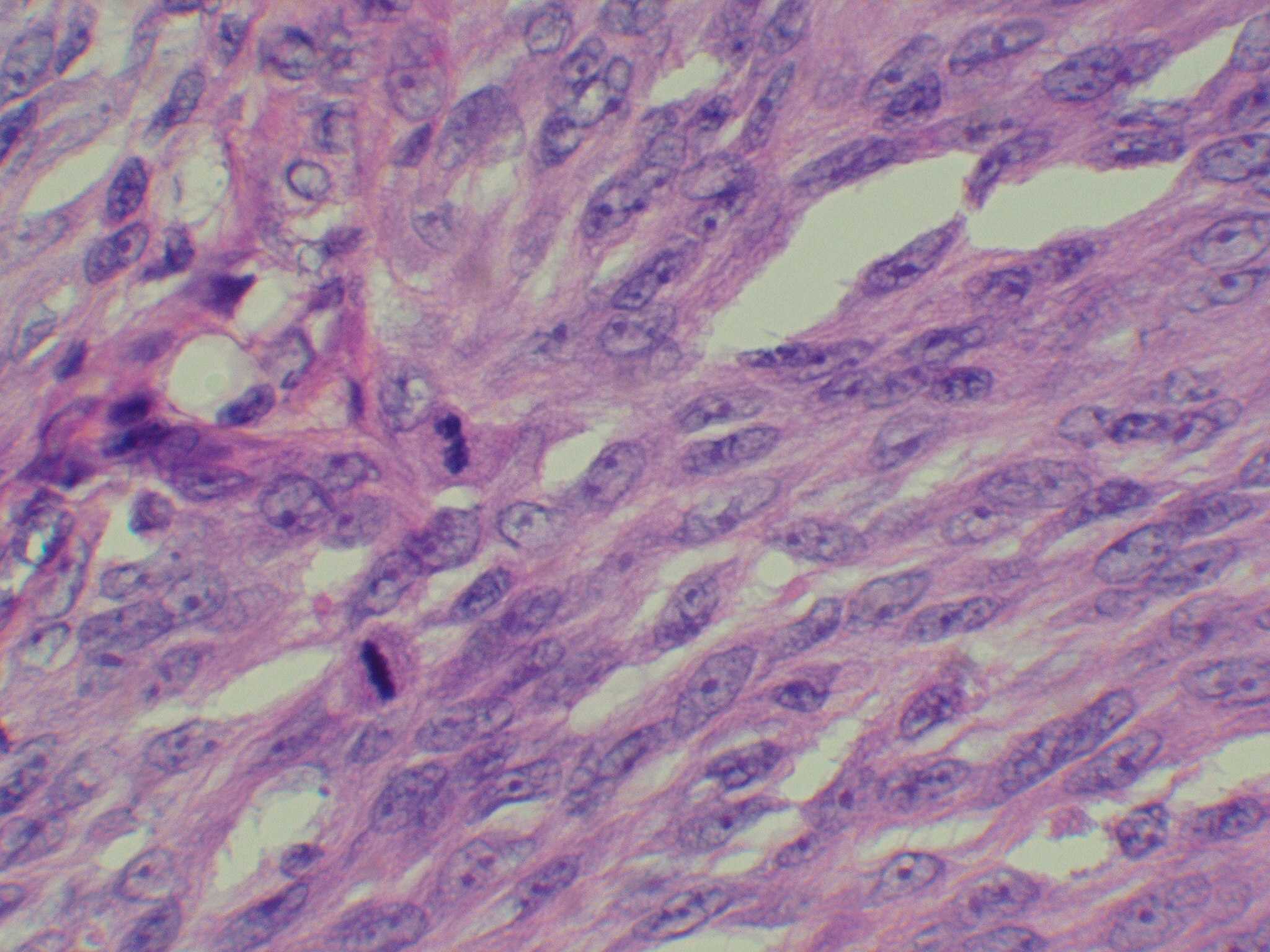
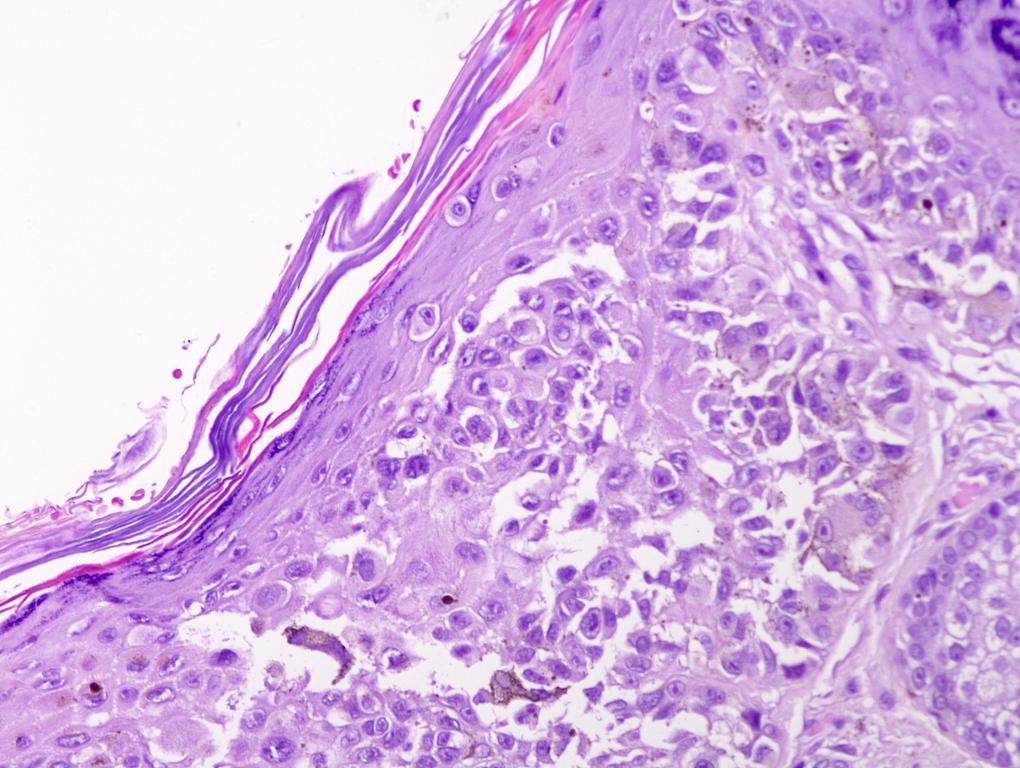
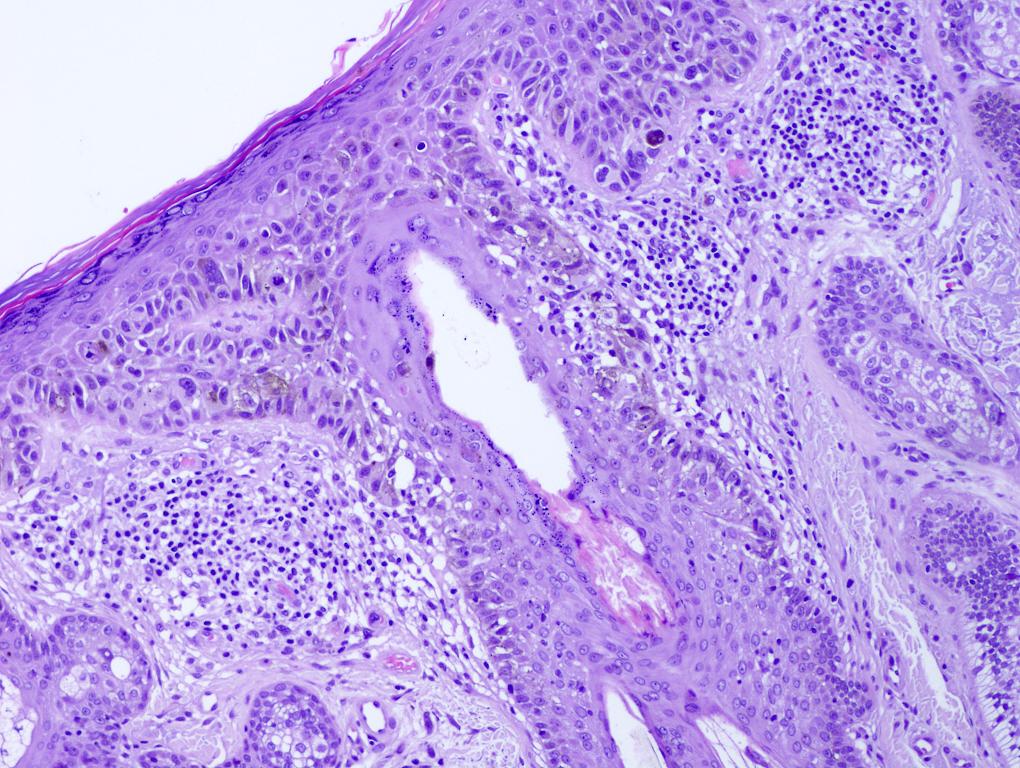
Microscopic (histologic) images
Contributed by Michele Donati, M.D.
Contributed by Jijgee Munkhdelger, M.D., Ph.D. and Andrey Bychkov, M.D., Ph.D.
Contributed anonymously
Virtual slides
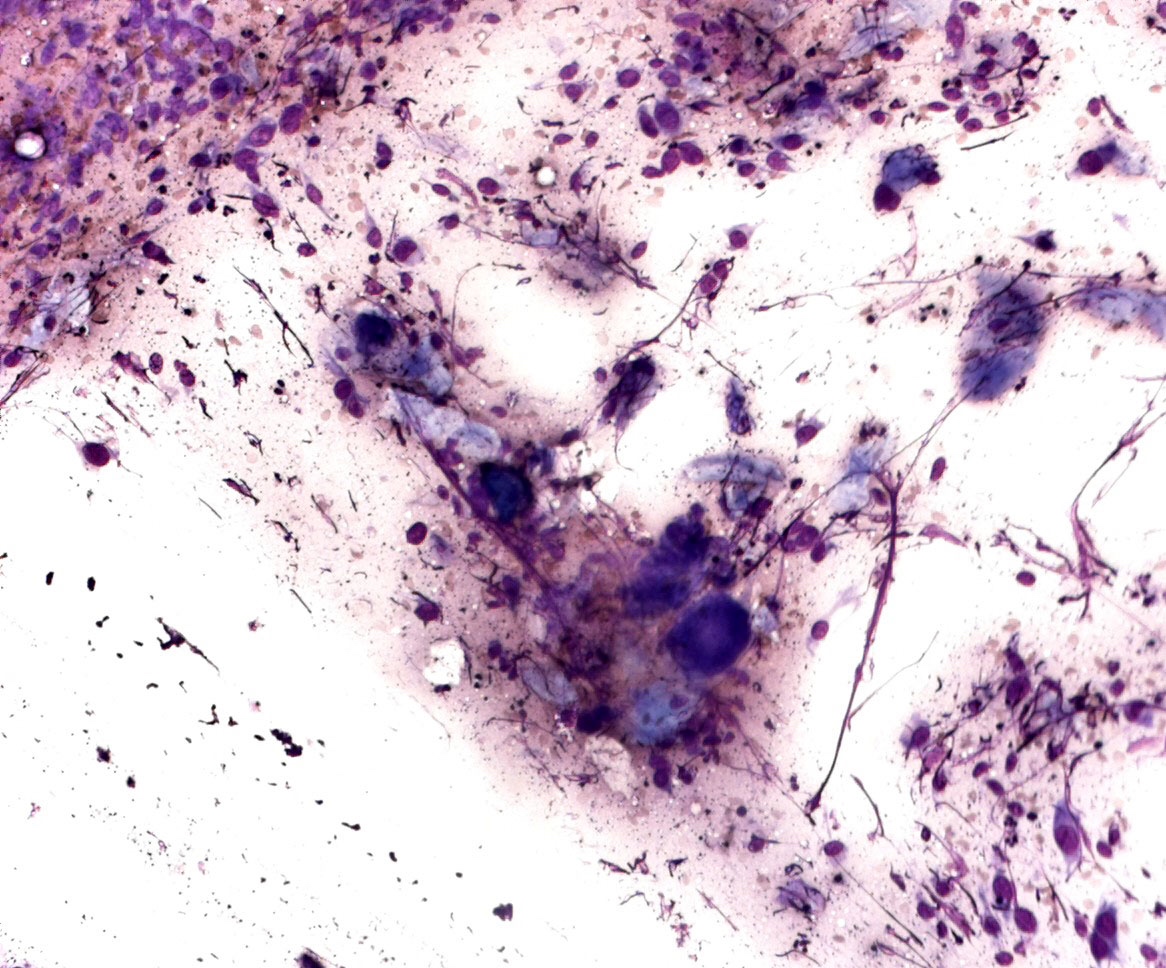
Cytology description
- Limitations
- Great variability of cytological presentation
- Can mimic almost any malignant tumor
- Variability in cluster configuration
- Cytological features
- Epithelioid, spindle cells or giant cells
- Dispersed and finely granular pigment (may be subtle or obscure other cytological details)
- Variable cell shape and size
- Large irregular nuclei
- Prominent eosinophilic nucleoli
- Nuclear pseudoinclusions
- Melanophages
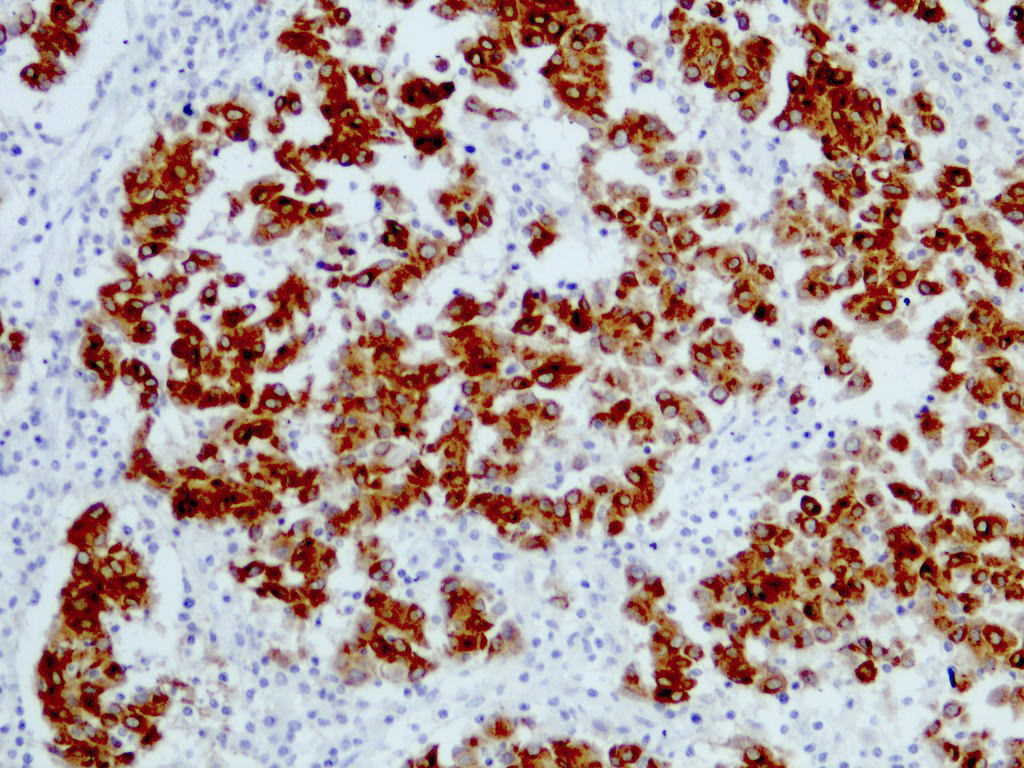
Positive stains
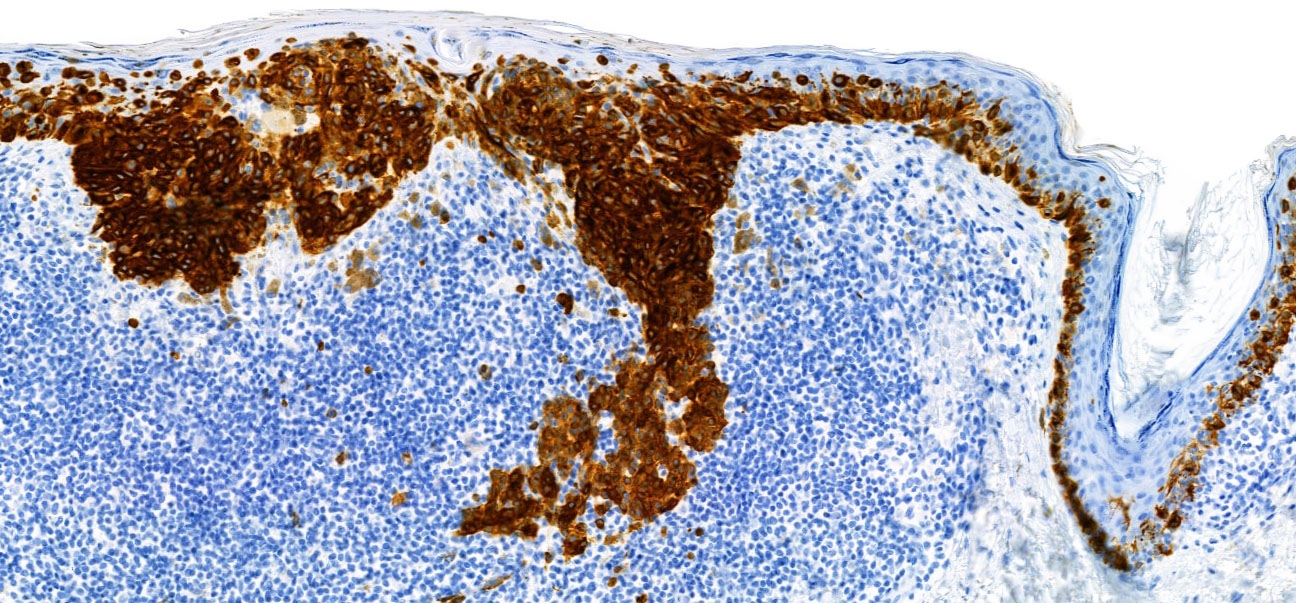
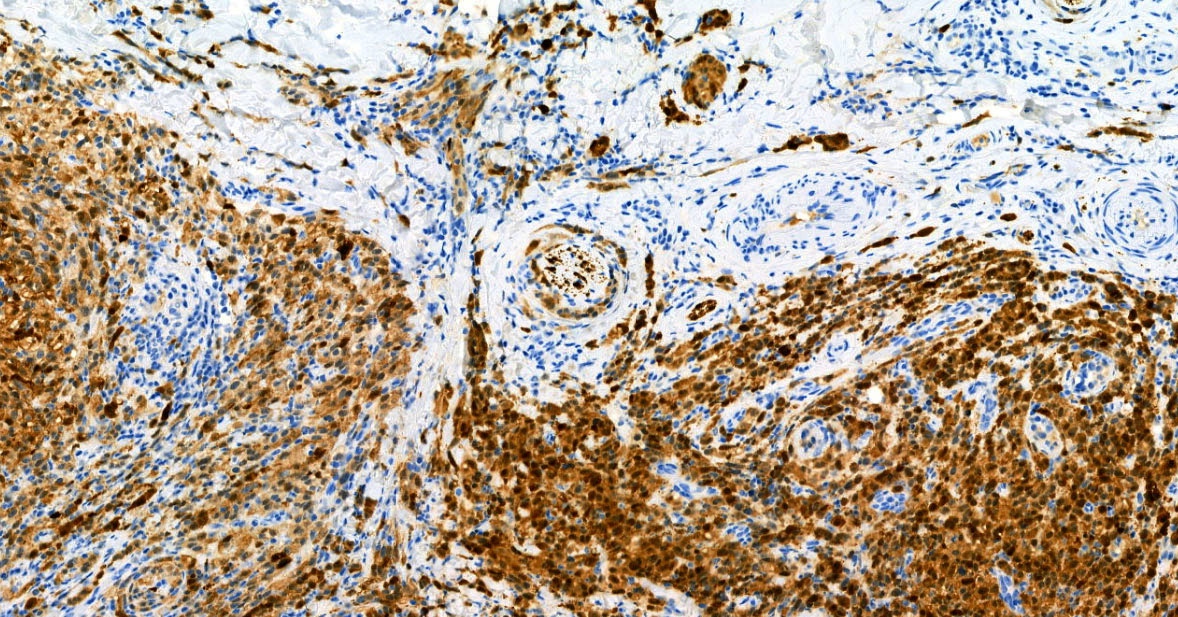
- S100 (nuclear and cytoplasmic)
- SOX10 (nuclear)
- MelanA / MART1 (cytoplasmic)
- HMB45 (cytoplasmic)
- Tyrosinase (cytoplasmic)
- MiTF (nuclear)
- PRAME (nuclear) (Am J Surg Pathol 2018;42:1456)
- PNL2 (cytoplasmic) (Mod Pathol 2003;16:481)
- KBA62 (cytoplasmic) (Am J Surg Pathol 2012;36:265)
- Ki67 / MIB1 > 10% (benign melanocytic nevi < 2%)
Negative stains
- p16 (nuclear loss in melanoma)
- Cytokeratin
- CD34
- LCA marker (CD45)
- CD68
Electron microscopy description
- Intracytoplasmic melanosomes and premelanosomes
- No longer used in clinical practice
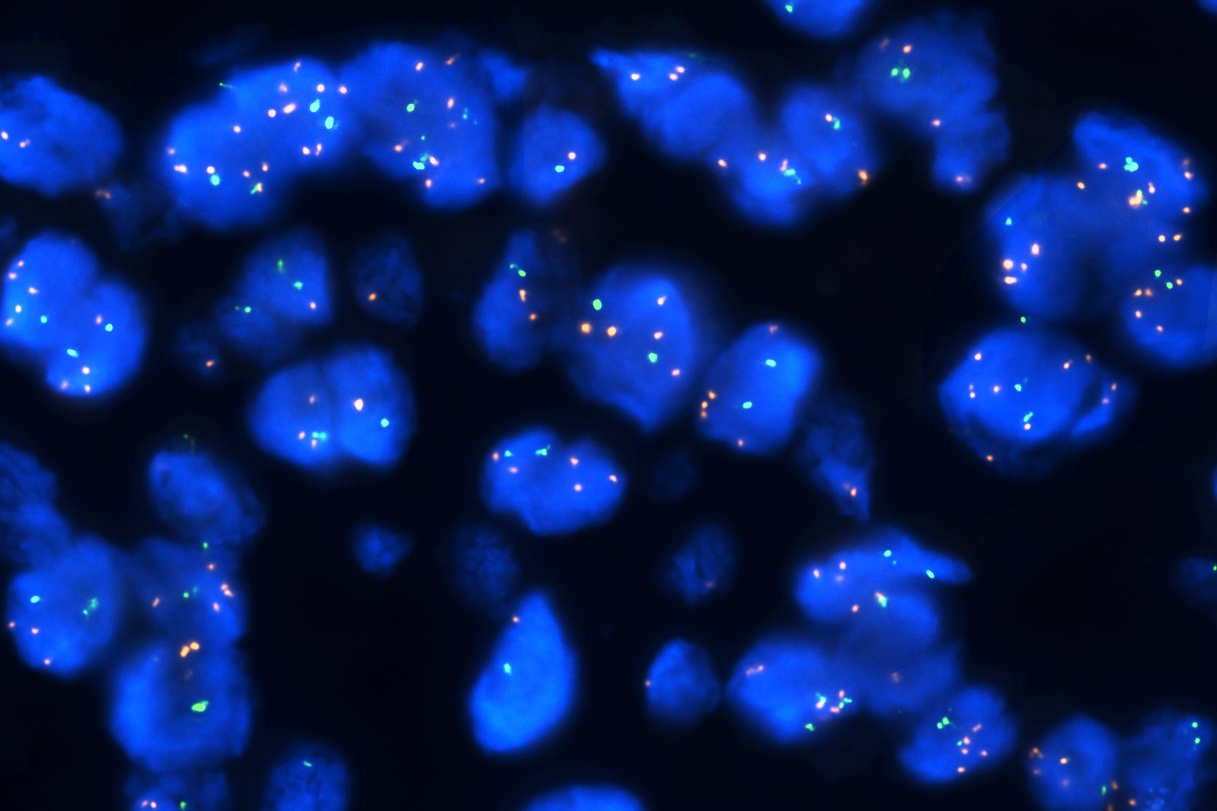
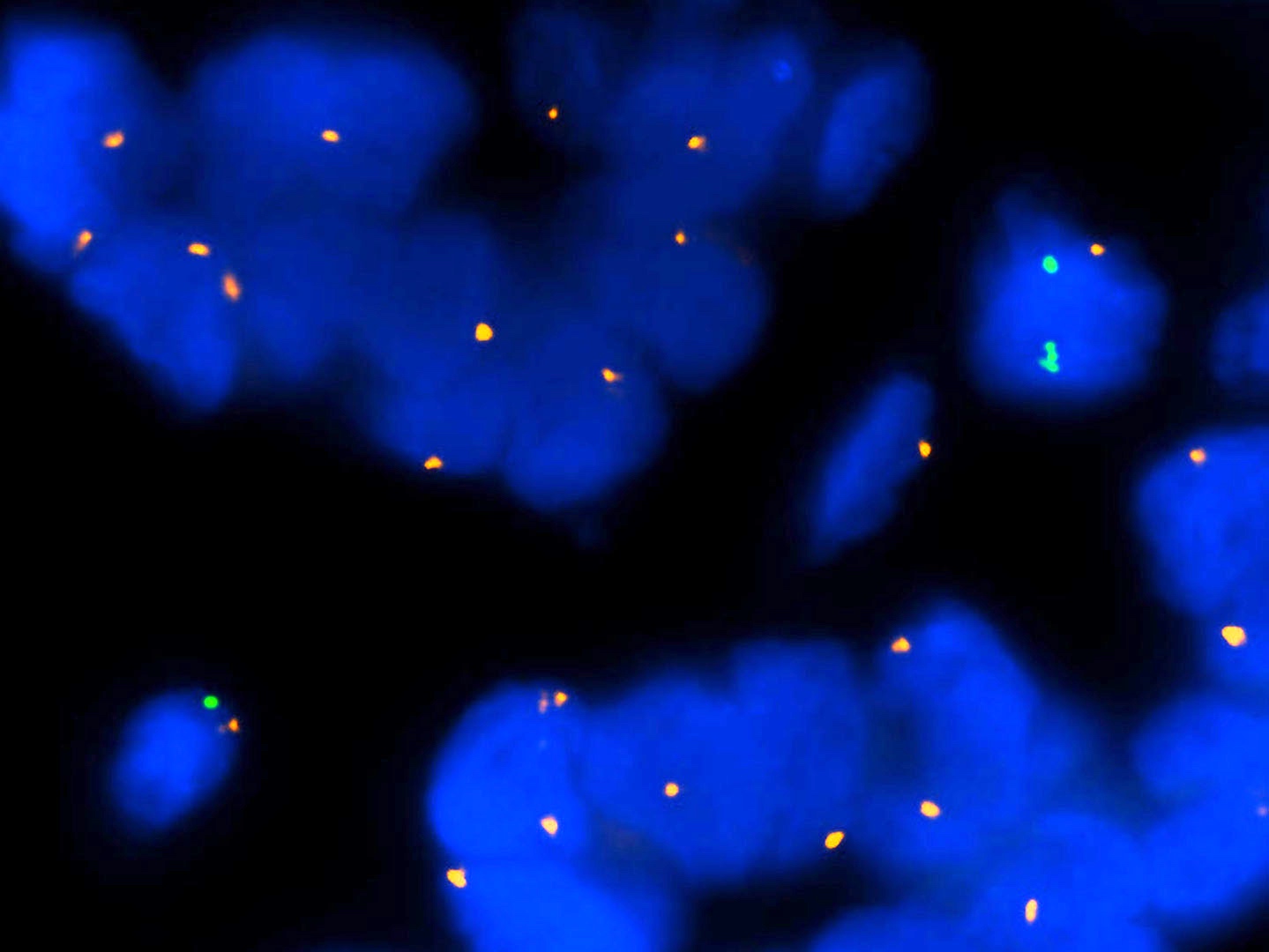
Molecular / cytogenetics description
- Molecular alterations do not constitute proof of malignancy per se and have to be interpreted in light of the clinical and histological findings
- In contrast with benign nevi, melanomas harbor multiple chromosomal copy number aberrations
- Main chromosomal copy number aberrations (detected by FISH, comparative genomic hybridization [CGH], array CGH and single nucleotide polymorphism array)
- Gain: 6p, 7q, 17q, 20q, 4q, 8q, 1q, 11q
- Loss: 9p21, 10, 21q
- Main chromosomal copy number aberrations (detected by FISH, comparative genomic hybridization [CGH], array CGH and single nucleotide polymorphism array)
- Main genetic driver alterations (detected by PCR, Sanger and next generation sequencing)
- BRAF, NRAS, NF1, KIT (Cell 2015;161:1681)
- Telomerase reverse transcriptase promoter (TERT-p) (Sci Rep 2015;5:11200)
- CDKN2A, PTEN, TP53
- Generally high tumor mutational burden (TMB > 10 mut/Mb)
- Gene expression profile (GEP), mRNA expression level of uveal and cutaneous melanoma related genes (Cancer Res 2004;64:7205, Clin Cancer Res 2015;21:175):
- PRAME
- S100A9 component
- 8 immune related genes
- 9 housekeeping genes
Videos
Melanoma
Superficial spreading melanoma
Acral lentiginous melanoma
Desmoplastic melanoma
Immunohistochemistry
Melanoma in situ
Sample pathology report
- Trunk, excisional biopsy:
- Invasive melanoma, superficial spreading melanoma subtype
- Macroscopic: Skin ellipse 1.3 x 0.7 x 0.4 cm. On the surface, elevated darkly pigmented lesion 0.7 x 0.5 cm. The entire lesion submitted.
- Growth phase: vertical
- Breslow depth: 1.6 mm
- Ulceration: absent
- Dermal mitotic rate (mitoses/mm²): 2
- Regression: absent
- Neurotropism: absent
- Lymphatic invasion: absent
- Microsatellites: absent
- Tumor infiltrating lymphocytes (TILs): present (nonbrisk)
- Margin: minimal distance to the nearest peripheral margin 4 mm
- TNM staging: pT2a; N: x; M: x
Differential diagnosis
- Differential diagnosis of melanoma may be very broad
- Changes according to the histological subtype
- Invasive melanoma may mimic any undifferentiated malignancy (Am J Surg Pathol 2021;45:240)
- Benign melanocytic nevus and its histological variants:
- Dysplastic nevus:
- No asymmetry
- No florid pagetoid spread (< mm² in an area of 0.5)
- No confluence of expansile nests
- No epidermal consumption / ulceration
- Cytological maturation of dermal melanocytes
- Persistent / recurrent nevus:
- Atypical junctional melanocytic proliferation does not extend beyond the dermal scar
- Melanocytic nevi of special sites:
- Scalp, ear, skin folders, breast, genital area
- Variable degree of cytological and architectural atypia (Am J Dermatopathol 2016;38:867)
- Mitotically active nevi (posttrauma, postbiopsy, during pregnancy, halo reaction):
- Absence of an in situ component
- No cytological atypia
- Maturation of dermal melanocytes
- No atypical mitotic figures
- Spitz tumor:
- BAP1 inactivated melanocytic tumor (BIMT):
- No tumor necrosis
- Dermal mitoses < 1/mm²
- BAP1 nuclear loss in the epithelioid component
- BRAF mutation and BAP1 mutation / loss
- Pigmented epithelioid melanocytoma (PEM):
- No tumor necrosis
- Mitoses < 1/mm²
- BRAF or MAP2K1 mutation and PRKAR1a mutation (combined type)
- PRKC fusion (pure type)
- No further genomic aberrations
- Deep penetrating nevus (DPN):
- No tumor necrosis
- Mitoses < 1/mm²
- Nuclear beta catenin+
- BRAF or MAP2K1 mutation and beta catenin mutation / APC loss
- No further genomic aberrations
- Cellular blue nevus:
- No nuclear atypia
- No tumor necrosis
- No overlapping nuclei
- Mitoses < 1/mm²
- GNAQ, GNA11 or CYSLTR2 mutation
- No further genomic aberrations
- Metastatic melanoma:
- Positive personal history of melanoma
- Absence of lymphocytic infiltrate
- Absence of junctional component (exceptionally rare epidermotropic metastases)
- Dysplastic nevus:
- Nonmelanocytic neoplasms:
- Bowen disease (squamous cell carcinoma in situ):
- Full thickness epithelial atypia
- Negative melanocytic markers in pagetoid cells
- Extramammary Paget disease (EMPD):
- Merkel cell carcinoma:
- Neuroendocrine markers (synaptophysin, chromogranin)+
- CK20+
- Atypical fibroxanthoma / pleomorphic dermal sarcoma:
- Absence of junctional component
- SMA+
- Poorly differentiated squamous cell carcinoma:
- Cytokeratin+, p63+
- Cutaneous leiomyosarcoma (atypical smooth muscle tumor):
- Absence of junctional component
- Smooth muscle markers (desmin, SMA, h-caldesmon)+
- Epithelioid angiosarcoma:
- Anaplastic lymphoma:
- LCA marker (CD45)+
- Bowen disease (squamous cell carcinoma in situ):
Additional references
Board review style question #1
Board review style answer #1
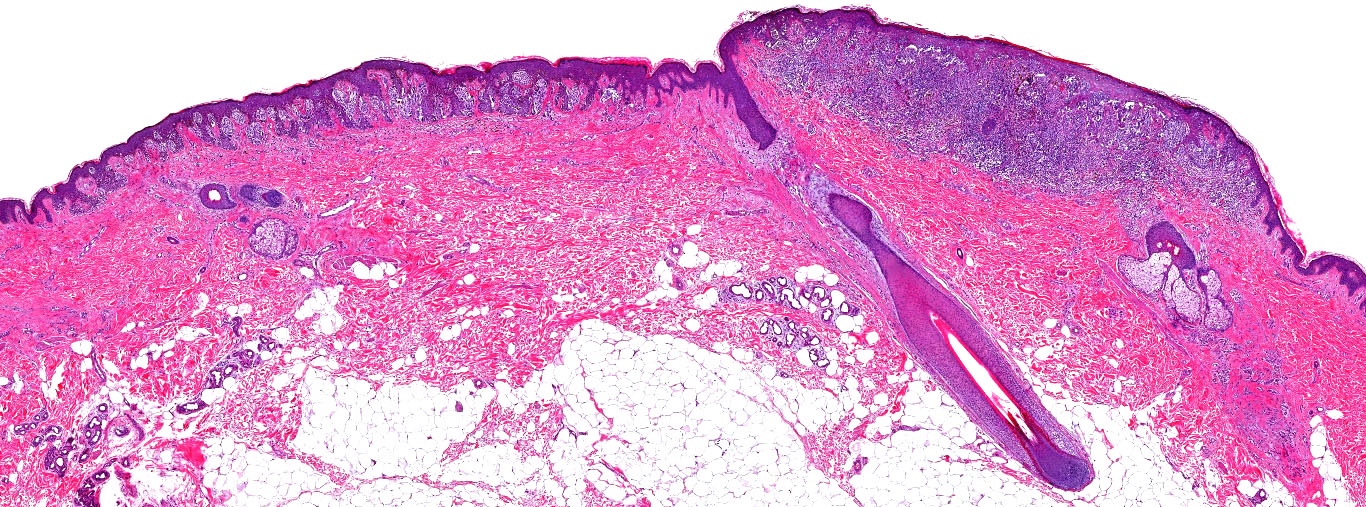
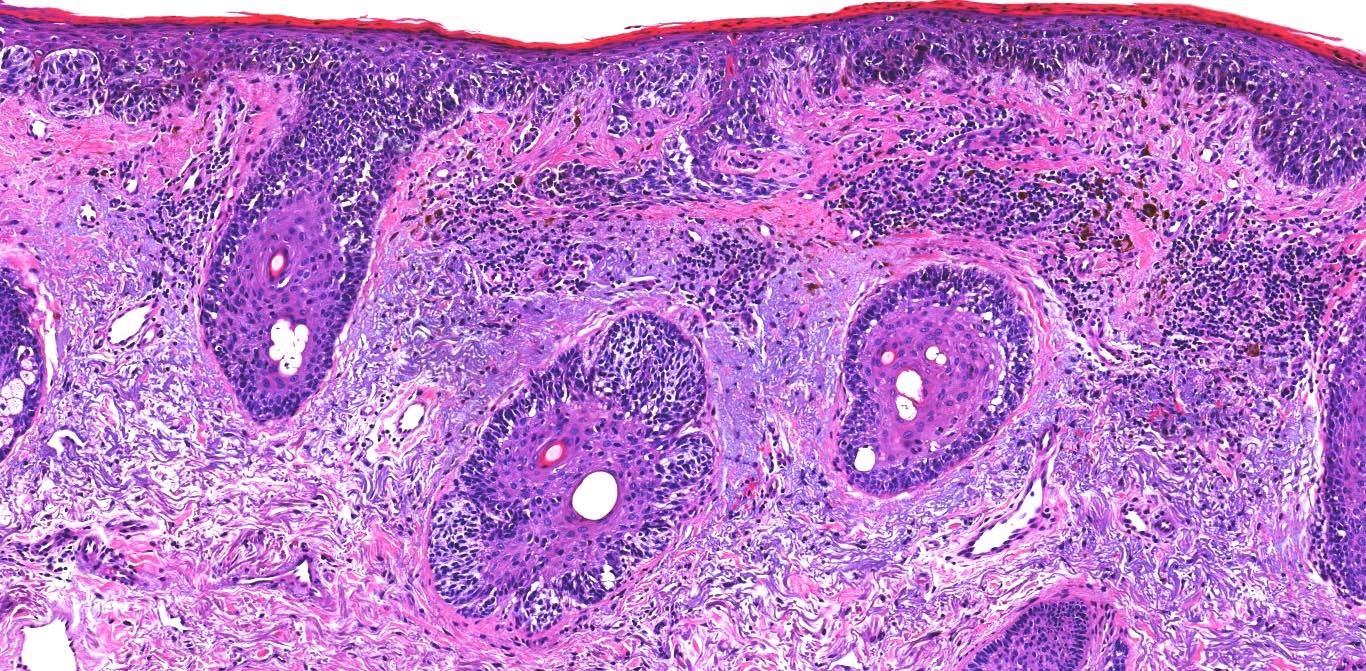
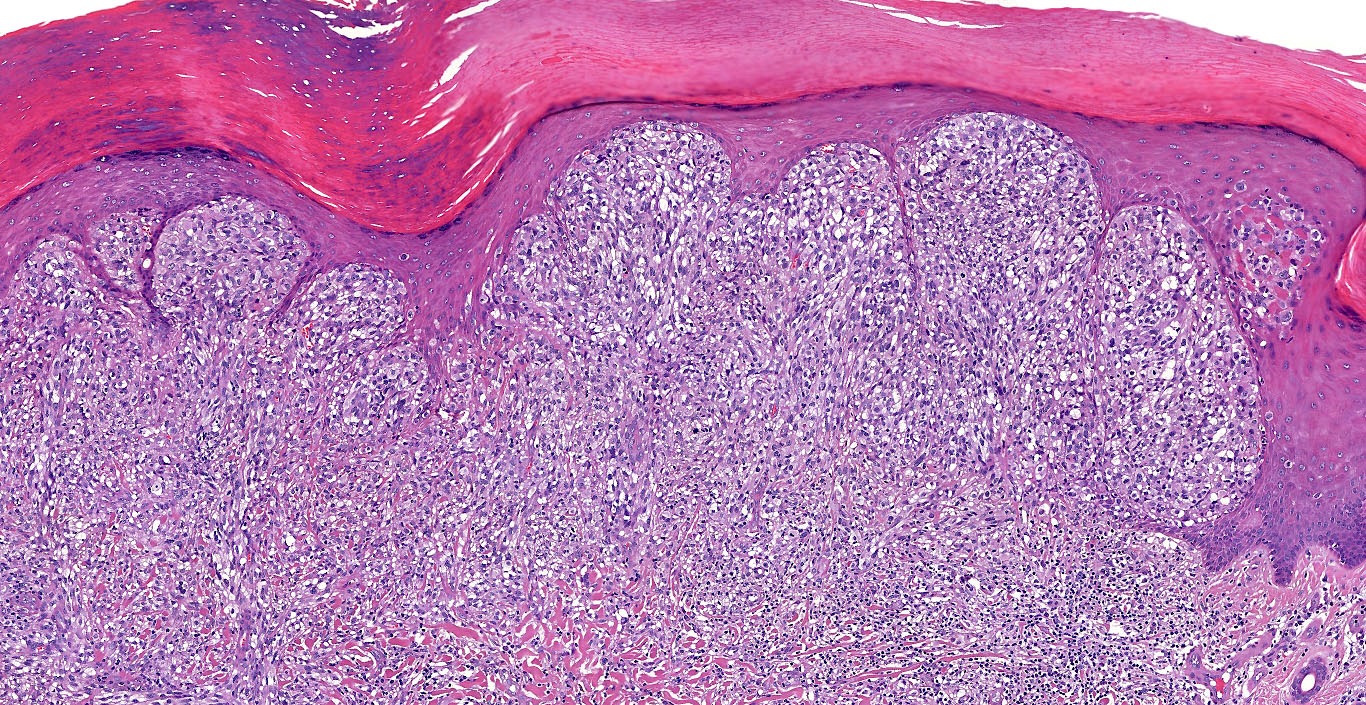
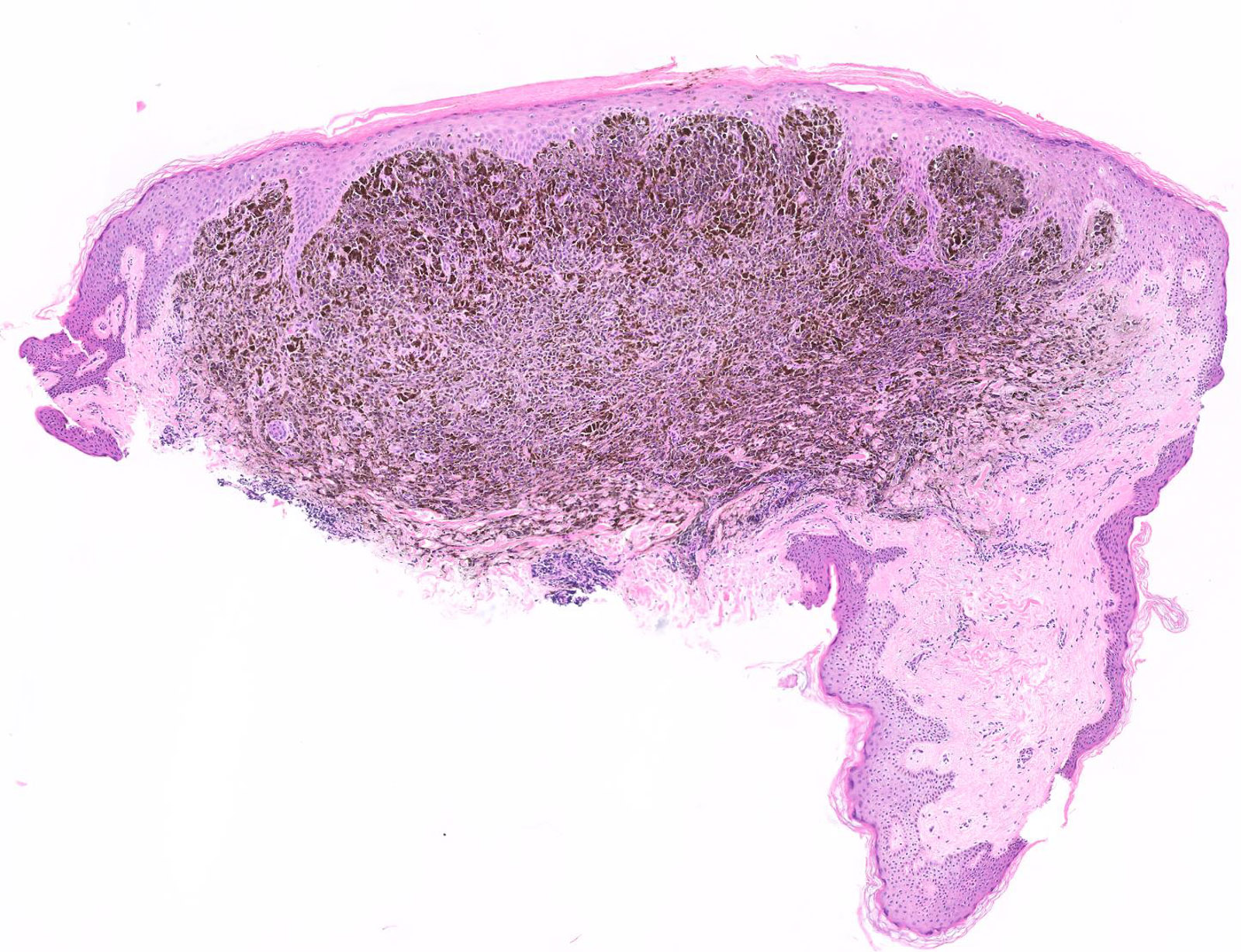
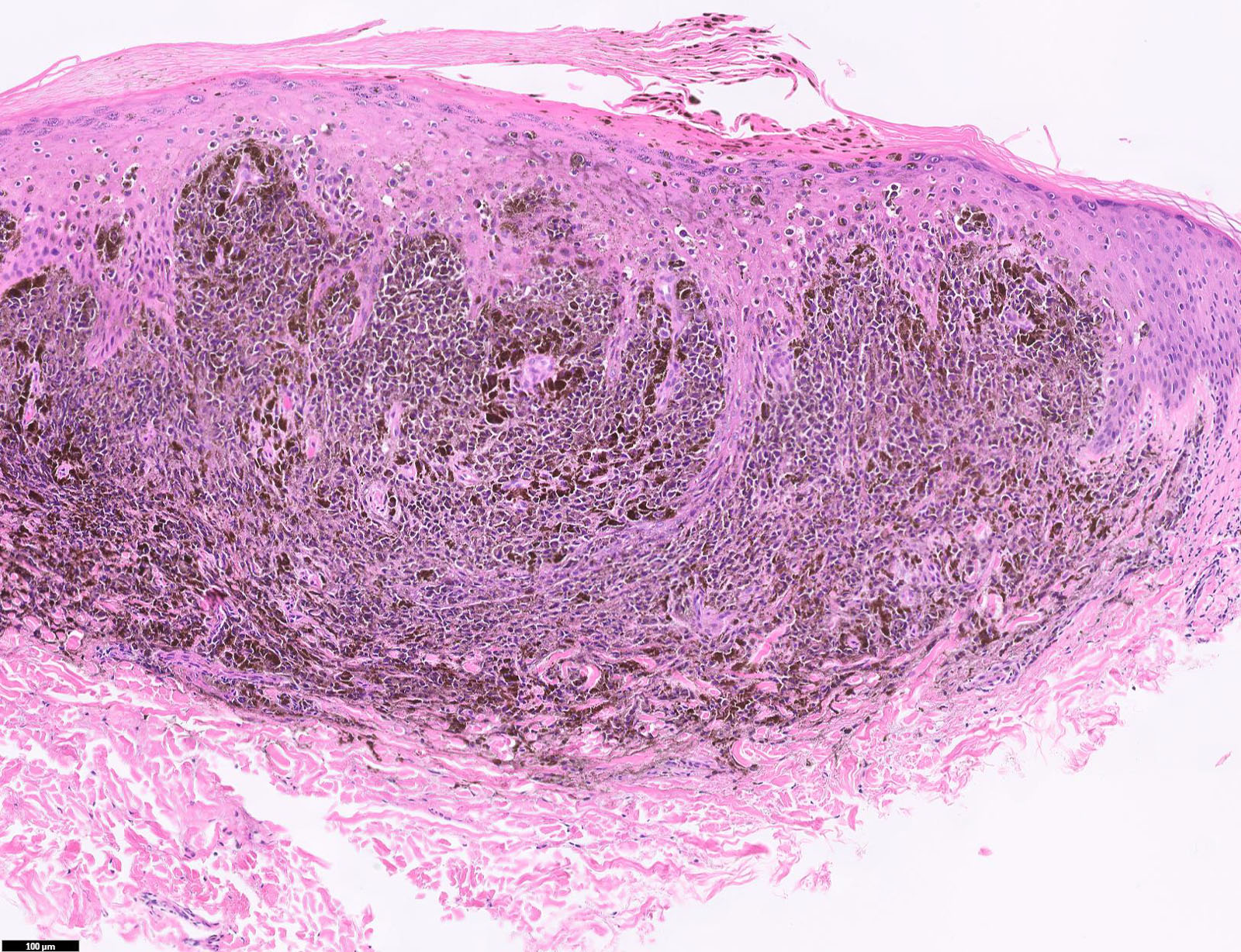
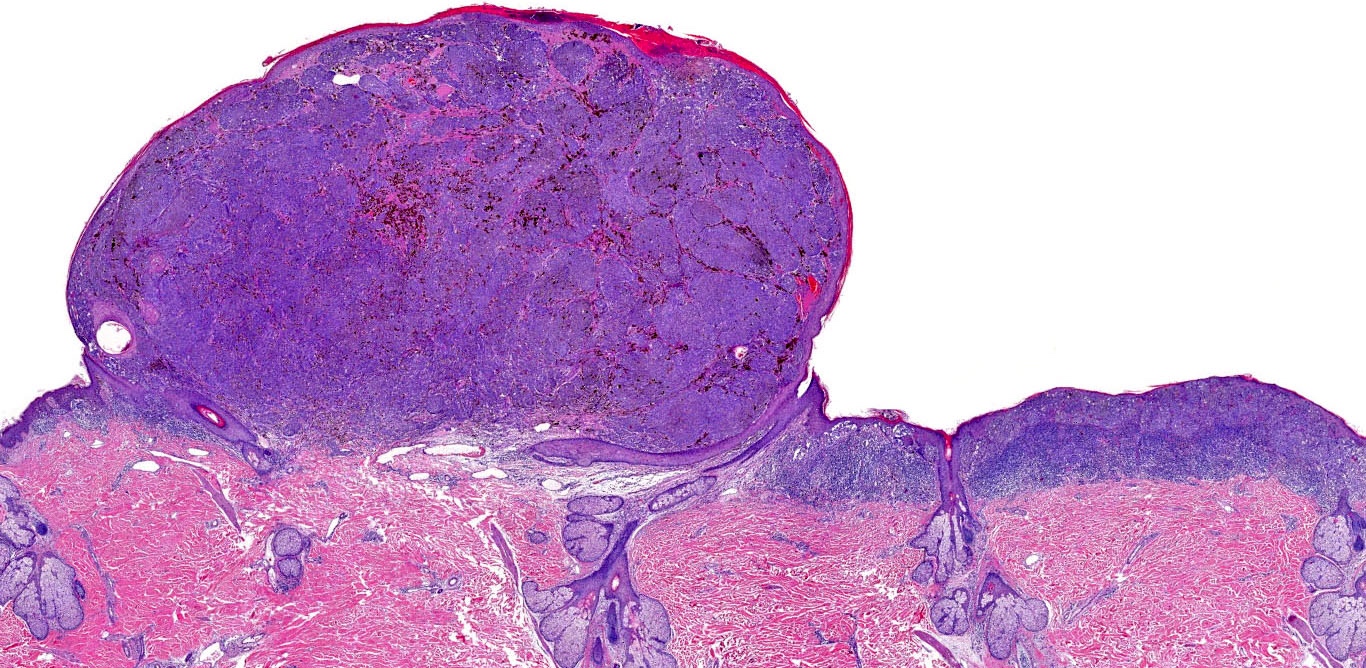
E. Superficial spreading melanoma. The picture shows the scanning of an invasive superficial spreading melanoma, vertical growth phase. Note the asymmetrical silhouette with a broad junctional component. The broad junctional component allows distinction between superficial spreading melanoma with prominent vertical growth phase and nodular melanoma.
Comment Here
Reference: Invasive melanoma
Comment Here
Reference: Invasive melanoma
Board review style question #2
Board review style answer #2
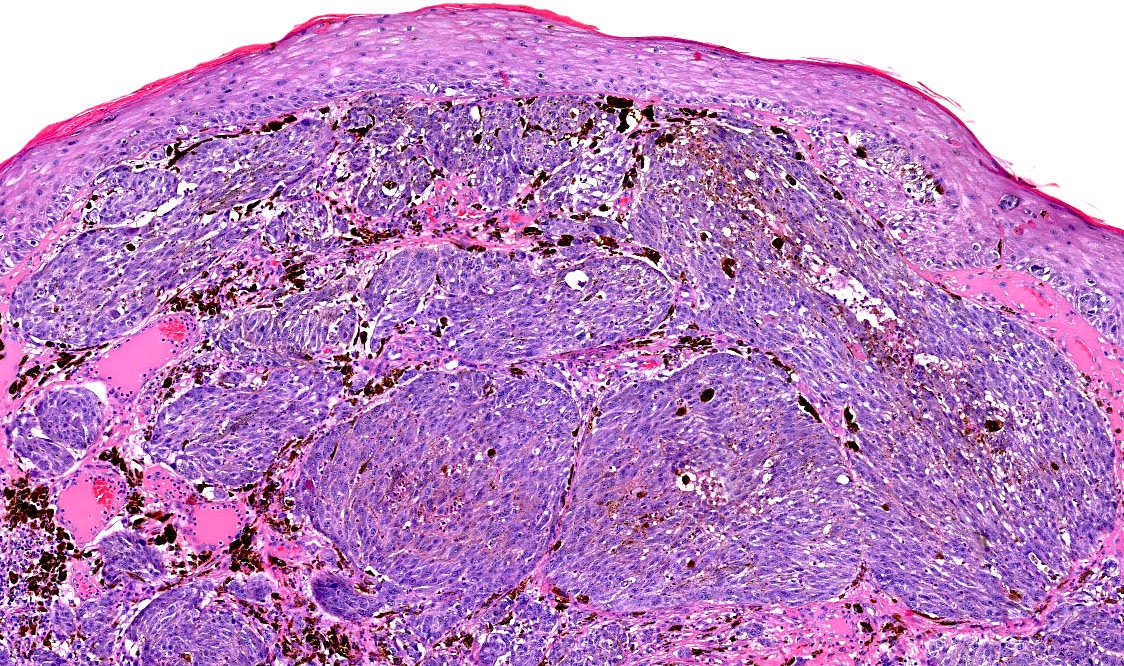
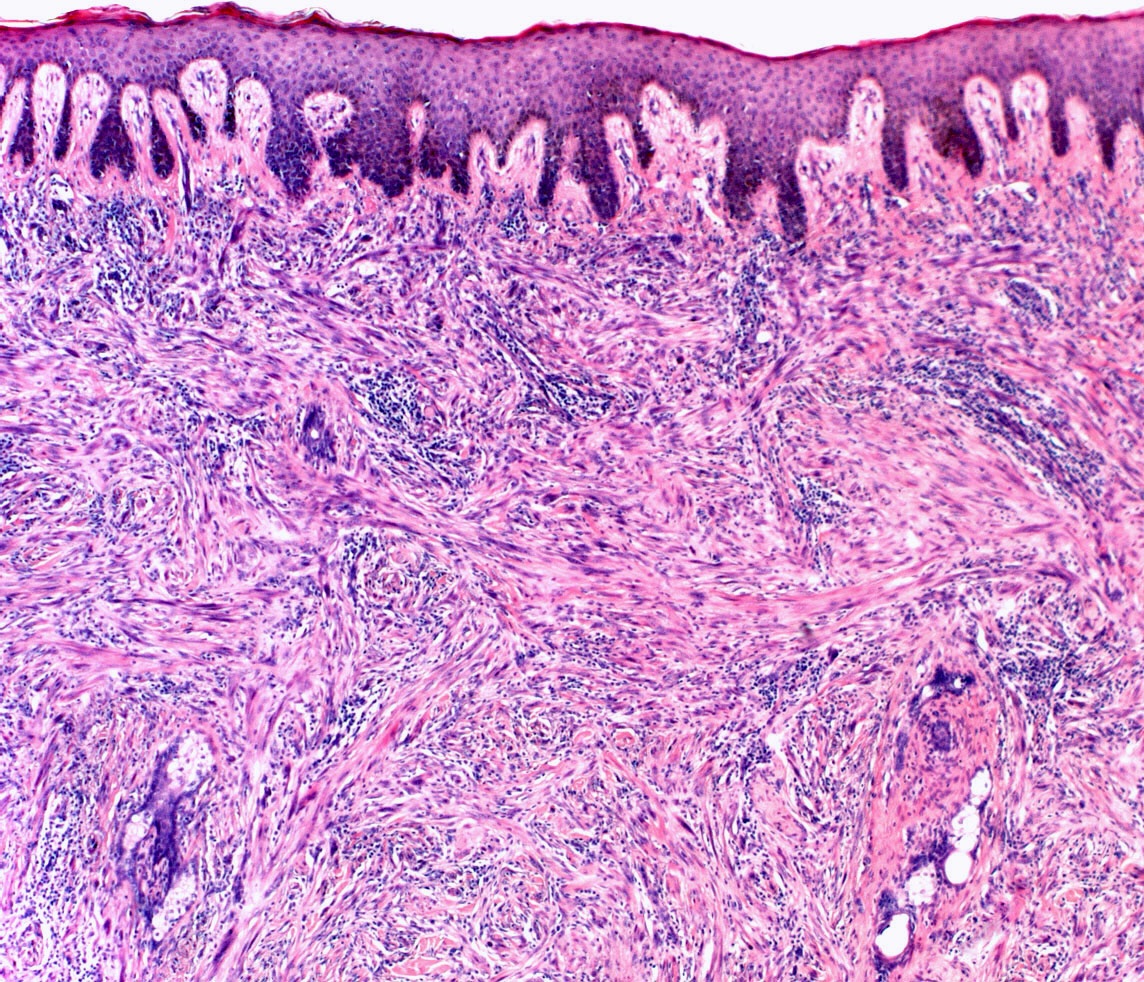
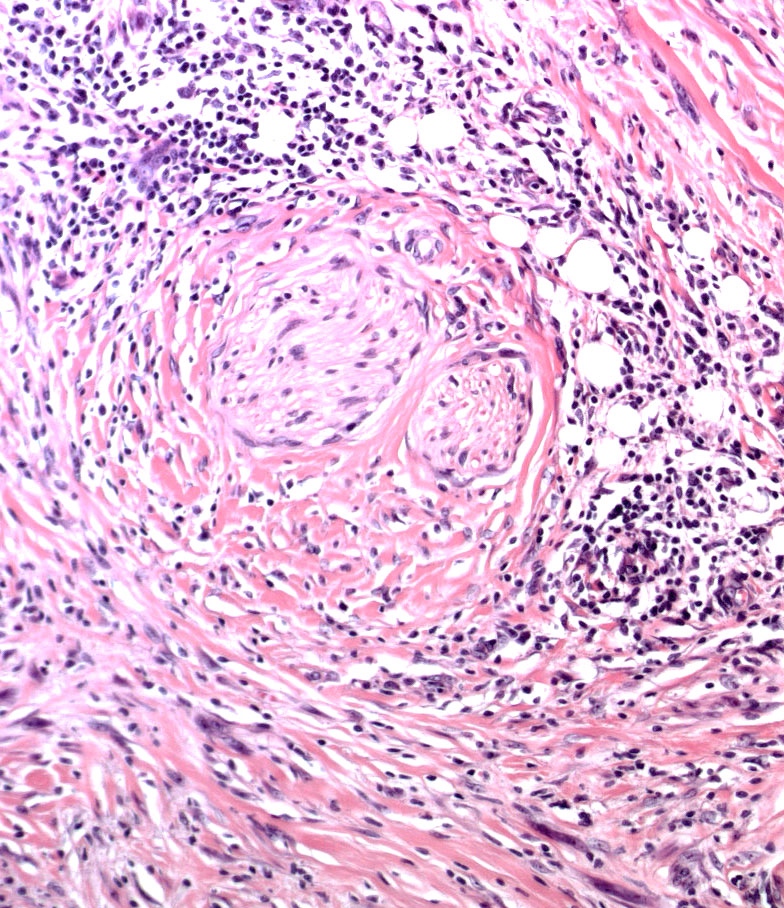
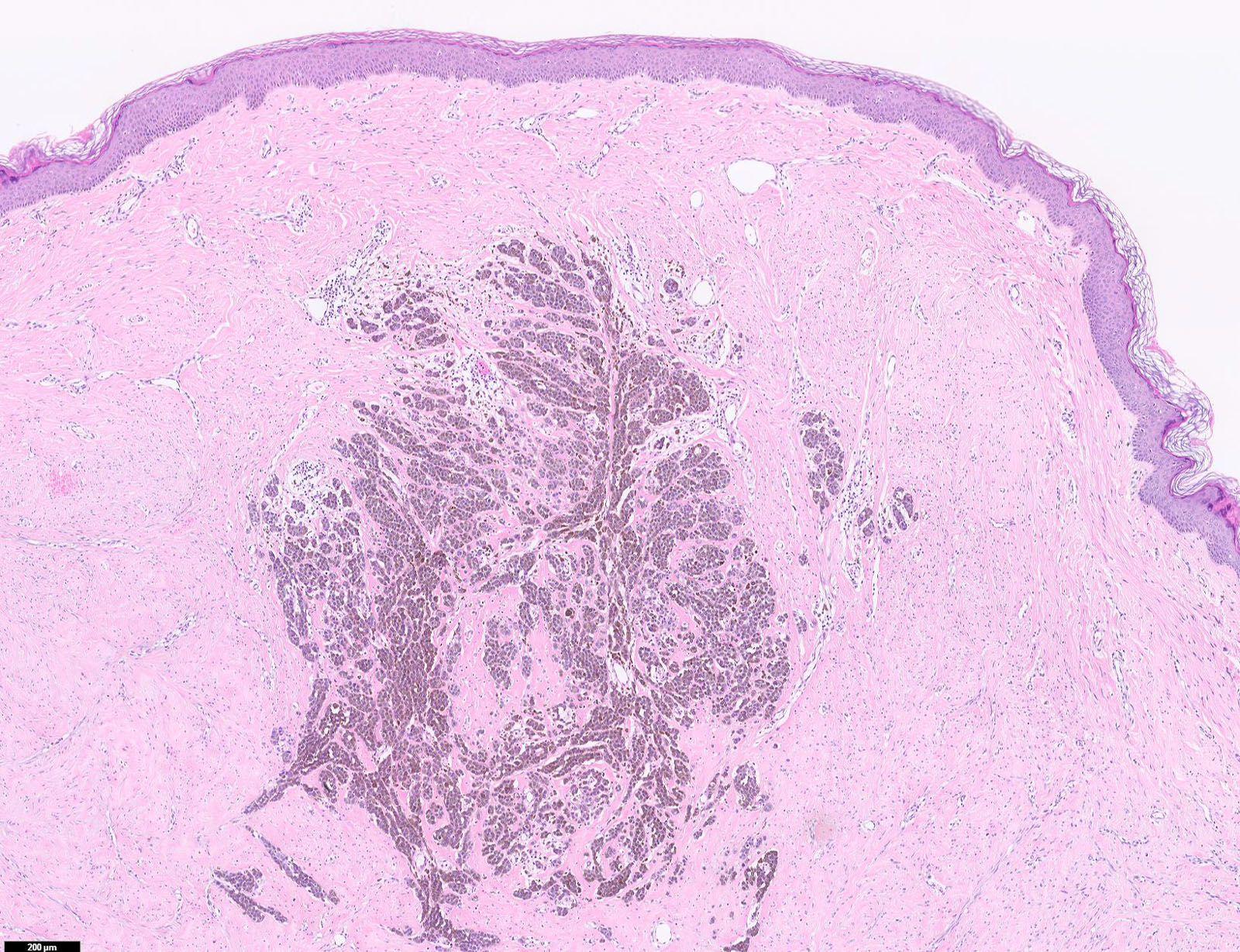
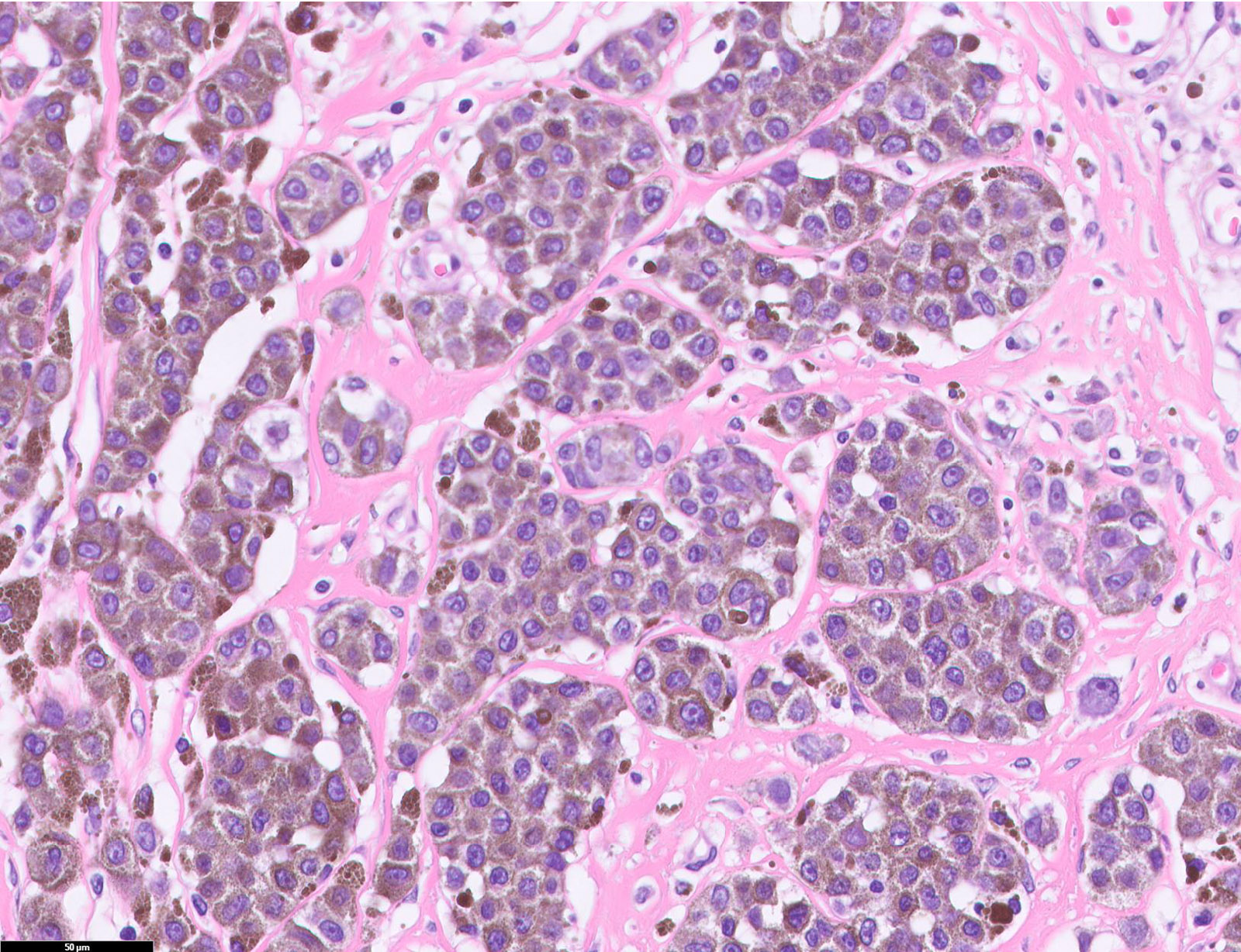
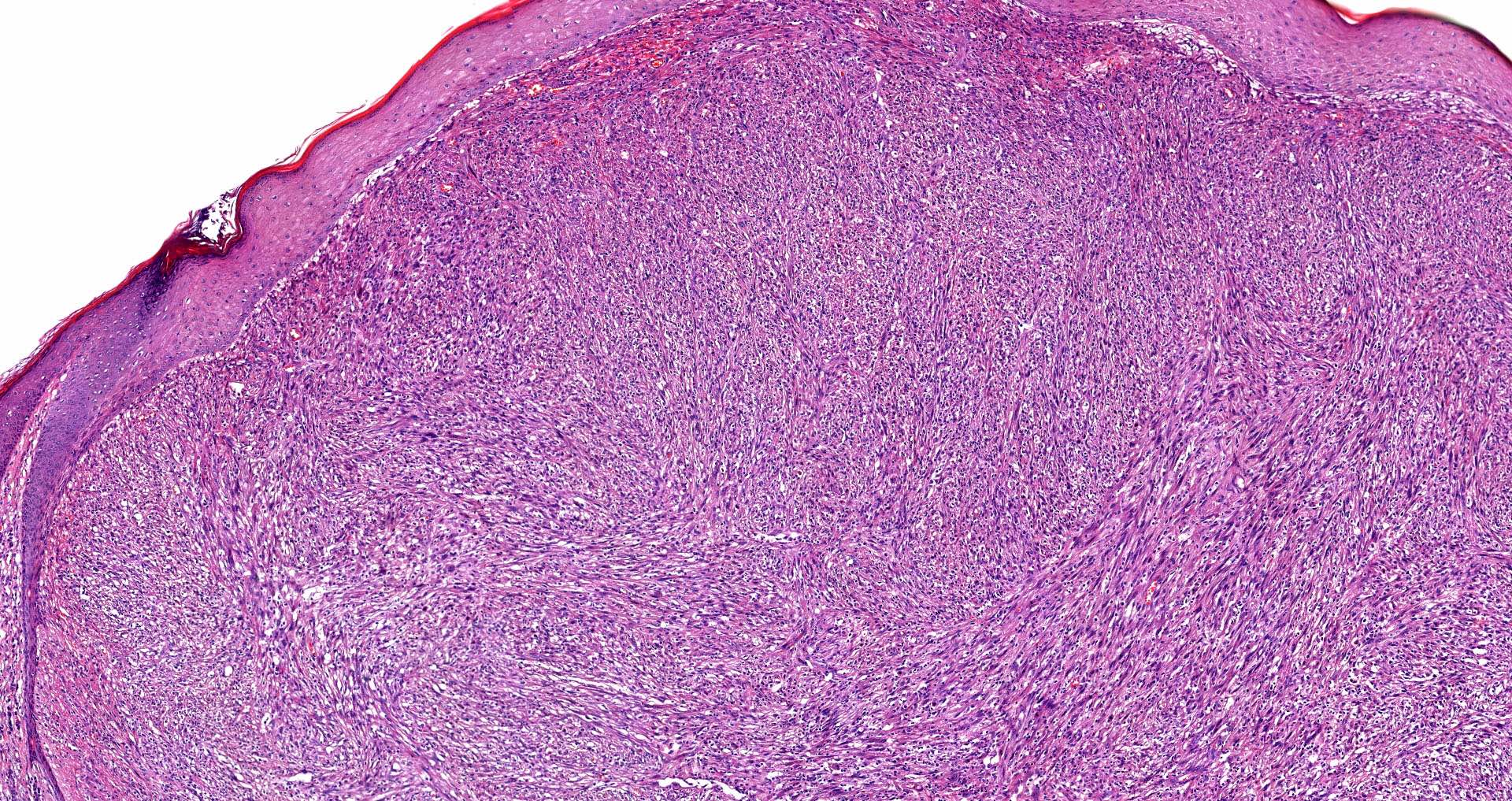
E. SOX10 and MelanA. The picture shows a nodular dermal proliferation of highly pleomorphic spindle cells; the main differential diagnosis includes poorly differentiated squamous cell carcinoma, spindle cell melanoma, atypical fibroxanthoma / pleomorphic dermal sarcoma (AFX / PDS), cutaneous leiomyosarcoma and angiosarcoma. Positive stain for SOX10 and MelanA confirms the diagnosis of invasive melanoma.
Comment Here
Reference: Invasive melanoma
Comment Here
Reference: Invasive melanoma
Board review style question #3
Which of the following mutations is most commonly observed in acral lentiginous melanoma?
- BRAF V600E
- KIT
- NF1
- NRAS
- TP53
Board review style answer #3
B. KIT. KIT mutation or amplification is the most common early genomic event observed in ALM. Secondary molecular events include loss of function mutation in key tumor suppressor genes (TP53 and CDKN2A).
Comment Here
Reference: Invasive melanoma
Comment Here
Reference: Invasive melanoma
Board review style question #4
Which of the following stains is useful to distinguish melanoma cells from melanocytes?
- HMB45
- MelanA
- PRAME
- S100
- SOX10
Board review style answer #4
C. PRAME. PRAME is a member of the cancer testis antigen family, normally expressed in testicular germ cells and occasionally placenta. It is present in a variety of cancers and is useful to support a diagnosis of melanoma, since positive stain is expected in melanoma but not in benign melanocytic nevi.
Comment Here
Reference: Invasive melanoma
Comment Here
Reference: Invasive melanoma