Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Vanderbeck K, Torres-Cabala CA. WNT activated deep penetrating / plexiform melanocytoma (nevus). PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/skintumormelanocyticdeeppennevus.html. Accessed March 30th, 2025.
Definition / general
- Cellular melanocytic neoplasm with extension into deep dermis or subcutis (Arch Dermatol 1993;129:328)
- Wedge shaped appearance (inverted triangle) (Am J Surg Pathol 1989;13:39, J Cutan Pathol 1992;19:172, Arch Dermatol 1993;129:328, Biology (Basel) 2022;11:460)
Essential features
- Uncommon variant of acquired melanocytic nevus
- Mostly indolent; some may exhibit atypical histologic features
- Rare metastatic potential in lesions with atypical features (Eur J Dermatol 2014;24:594, Cancers (Basel) 2021;13:3066, J Cutan Pathol 2020;47:1150, Biology (Basel) 2022;11:460)
- Wedge shaped appearance on microscopy (Am J Surg Pathol 1989;13:39, J Cutan Pathol 1992;19:172, Arch Dermatol 1993;129:328, Biology (Basel) 2022;11:460)
- Cellular with deep extension into dermis and subcutis (Arch Dermatol 1993;129:328)
- Typically with abundant pigment
Terminology
- Deep penetrating nevus (DPN)
- Plexiform spindle cell melanocytoma / nevus and inverted type A nevus considered variants of WNT activated deep penetrating / plexiform melanocytoma (J Cutan Pathol 2018;45:254, Histopathology 1991;18:243, J Am Acad Dermatol 1994;30:724)
Epidemiology
- Uncommon lesion, first reported in 1989 (Am J Surg Pathol 1989;13:39)
- Usually appears before age 50 (Arch Pathol Lab Med 2011;135:321)
- Age range: 3 - 56 years (mean: 26 years) (Histopathology 2003;43:529)
- F > M
Sites
- Usually face, neck, upper back / trunk and proximal extremities (Arch Pathol Lab Med 2011;135:321)
Pathophysiology
- Lesions demonstrate Wnt pathway activation via gain of function mutation in CTNNB1, which encodes beta catenin protein (Biology (Basel) 2022;11:460, Zhonghua Jie He He Hu Xi Za Zhi 1988;11:362)
- Most arise from common acquired nevi and therefore may also exhibit mutations in the mitogen activated protein kinase (MAPK) pathway (BRAF [p.V600E], MEK1 / MAP2K1, HRAS) (Nat Commun 2017;8:644)
- Mutations in beta catenin and BRAF result in activation of LEF1 (transcription factor), which leads to epithelial mesenchymal transition and tumor growth and development (Biology (Basel) 2022;11:460)
- Rare cases may exhibit inactivation of APC (Biology (Basel) 2022;11:460)
- Increased melanocyte size and pigmentation derived from Wnt pathway activation
Clinical features
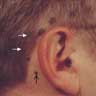
- < 1 cm in diameter
- Dark dome shaped papule with regular borders (Biology (Basel) 2022;11:460, Massi: Histological Diagnosis of Nevi and Melanoma, 2nd Edition, 2014)
- Often described as new or changing
- May resemble melanoma (J Plast Reconstr Aesthet Surg 2007;60:1252)
Diagnosis
- Clinical presentation
- Dermoscopic criteria for DPN is not well established (J Am Acad Dermatol 2014;71:1234, Biology (Basel) 2022;11:460)
- Definite diagnosis is made on excision specimen through histologic examination
Case reports
- 4 year old boy with unconventional deep penetrating melanocytic nevus with microscopic involvement of regional lymph nodes (J Cutan Pathol 2012;39:25)
- 25 year old man with linear arrangement of multiple deep penetrating nevi (Arch Dermatol 2003;139:1608)
- 46 year old woman with penetrating nevus of cheek skin (Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007;104:e49)
- 49 year old woman with multiple oral and facial deep penetrating nevi (J Oral Maxillofac Surg 2017;75:2579)
- 51 year old woman with deep penetrating nevus of the left anterior leg (Diagn Pathol 2006;1:31)
Treatment
- Simple excision; only rarely recurs (Histopathology 2003;43:529, Cancers 2021;13:3066)
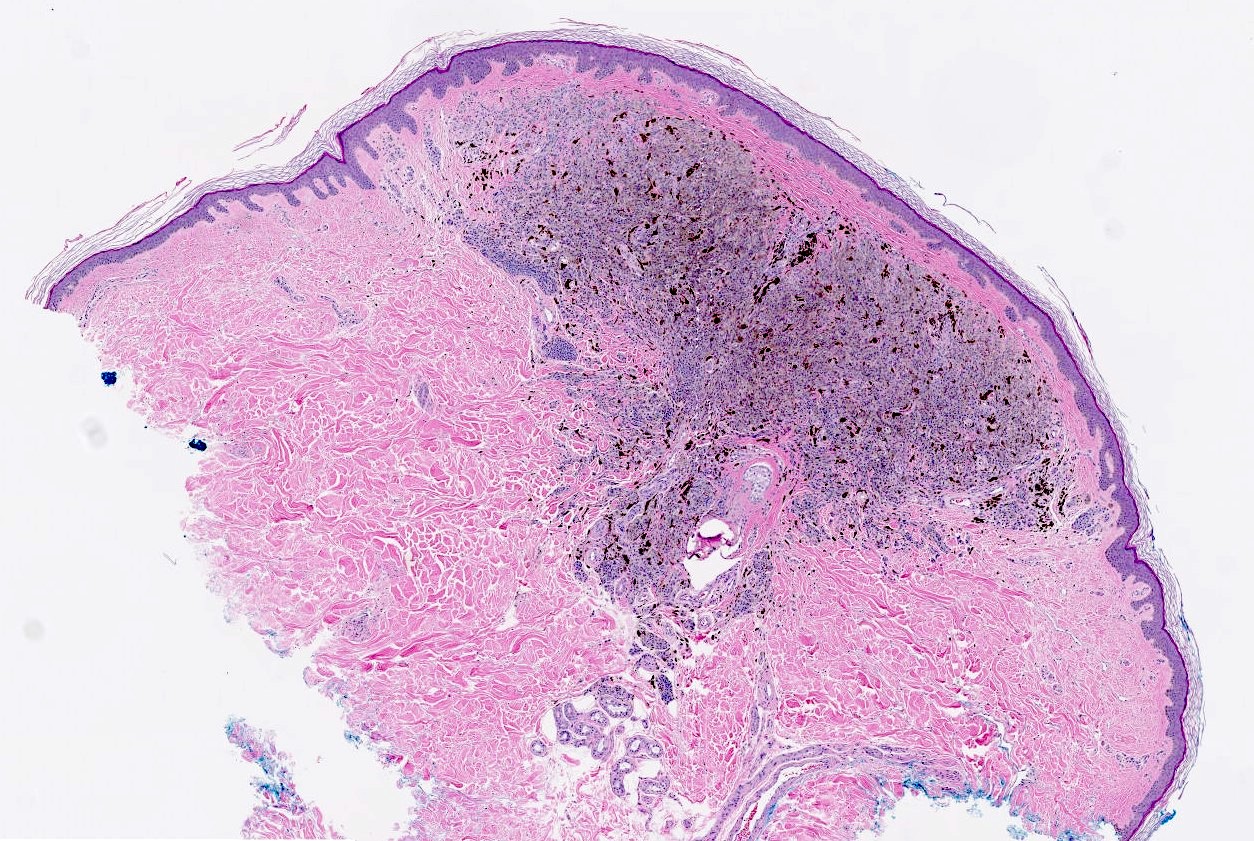
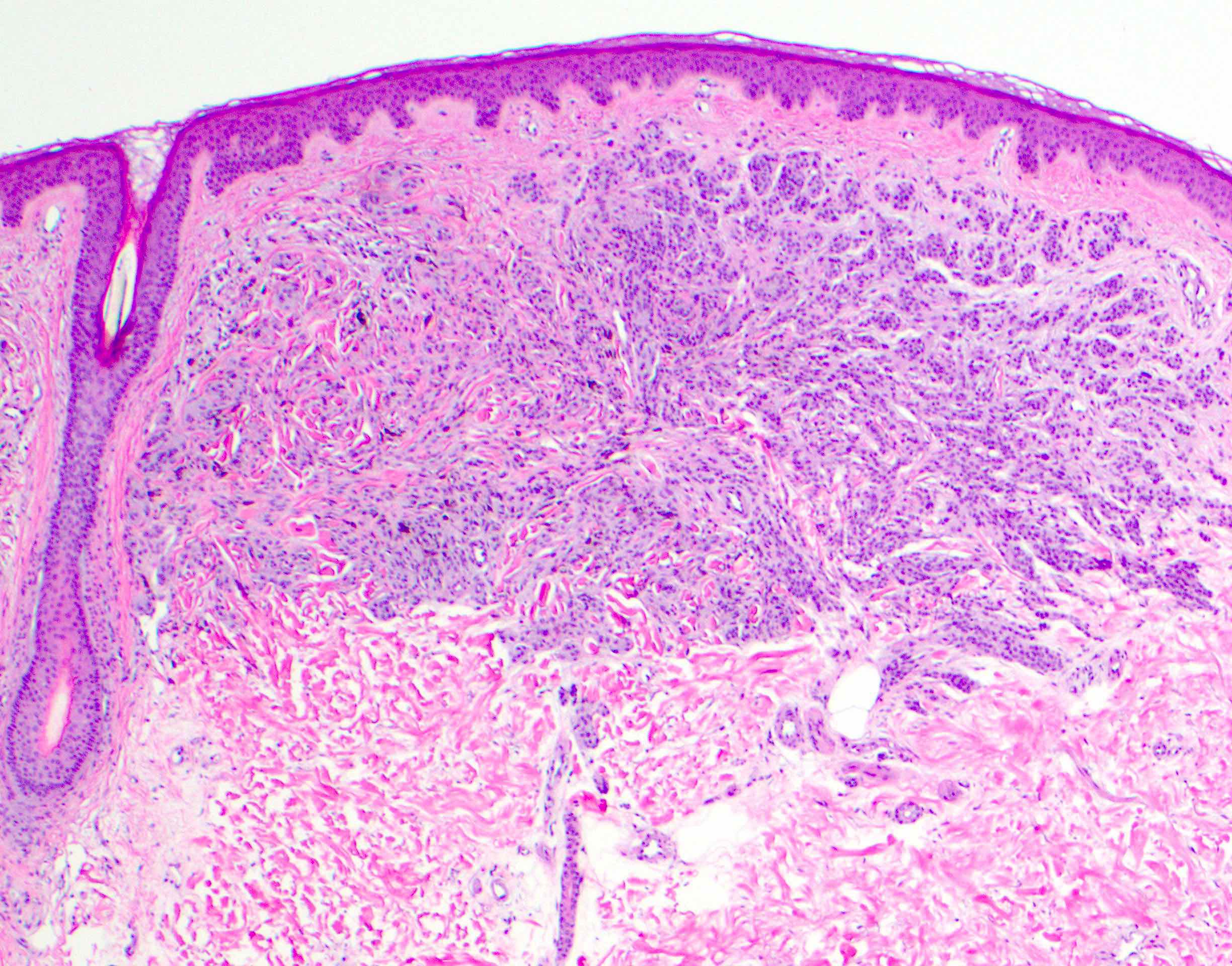
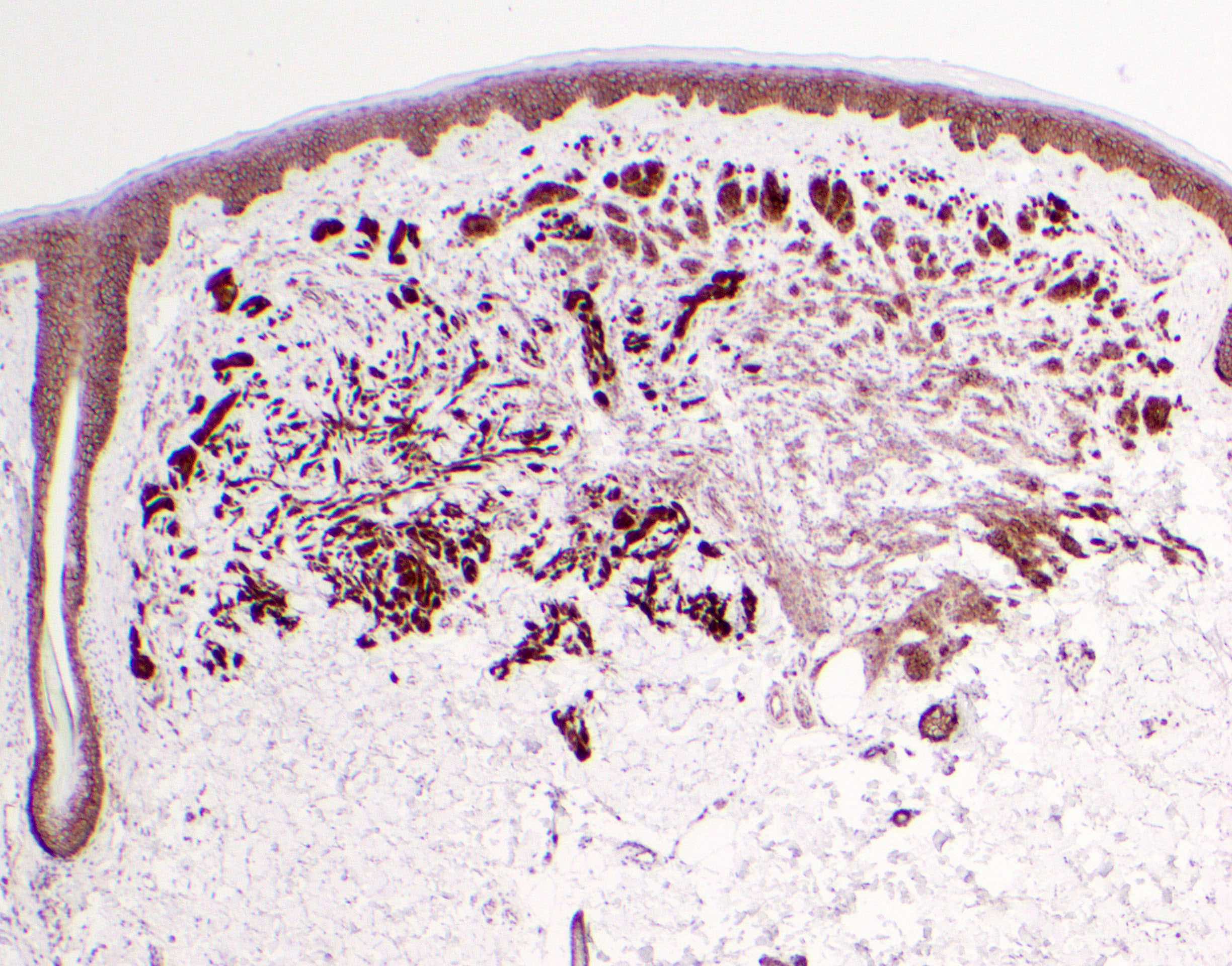
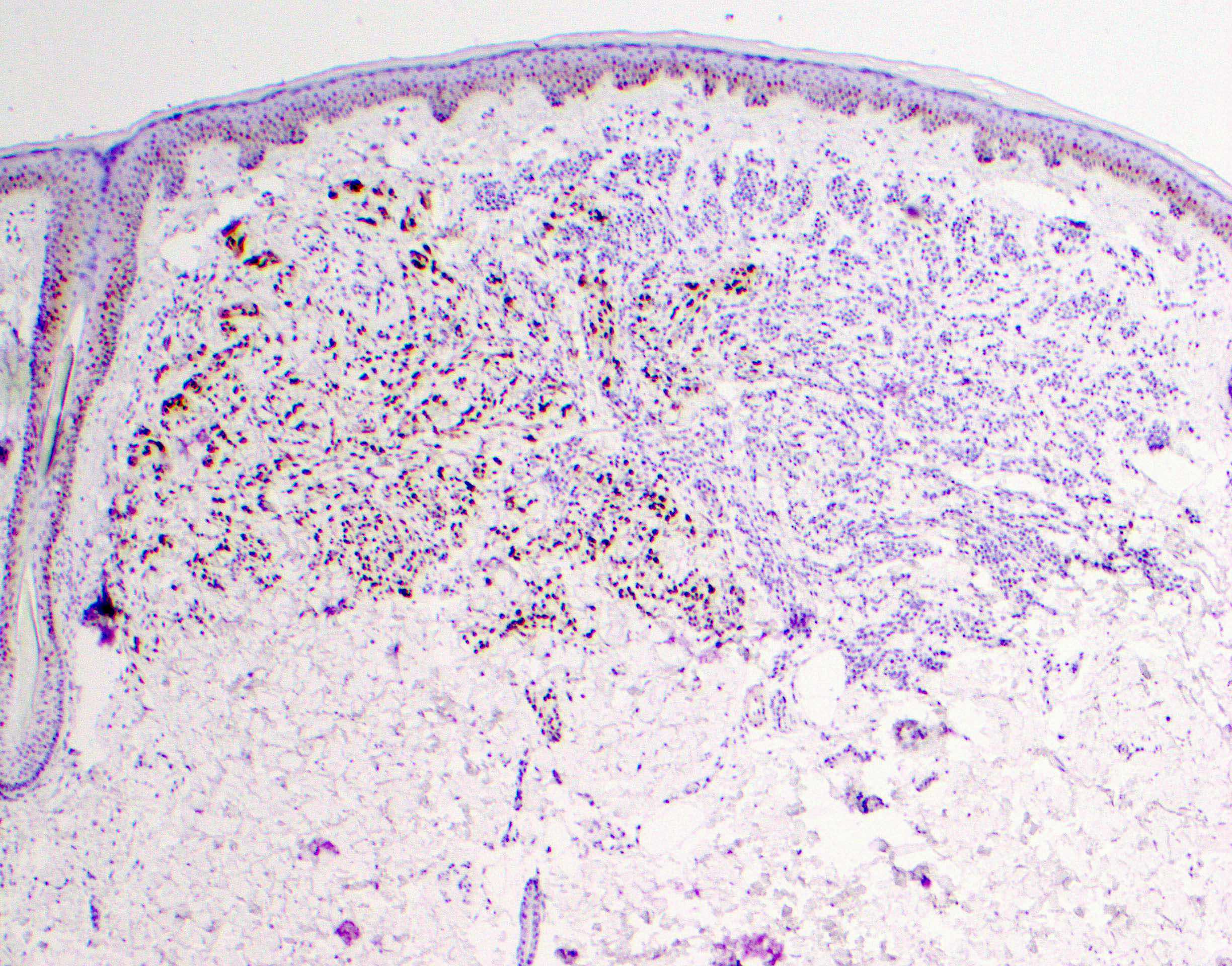
Microscopic (histologic) description
- Melanocytic neoplasm that exhibits a wedge shaped or plexiform growth pattern with melanin pigmentation (Am J Surg Pathol 1989;13:39, J Cutan Pathol 1992;19:172, Arch Dermatol 1993;129:328)
- Usually well circumscribed and symmetrical (Biology (Basel) 2022;11:460)
- Extends into deep dermis or subcutis with no clear maturation with depth (Am J Surg Pathol 1989;13:39, J Cutan Pathol 1992;19:172, Arch Dermatol 1993;129:328, Arch Pathol Lab Med 2011;135:321)
- Can have encircling of blood vessels, adnexal structures, nerves (Am J Surg Pathol 1989;13:39, J Cutan Pathol 1992;19:172, Arch Dermatol 1993;129:328)
- Cellular, nested or fascicular with abundant pigment (Am J Surg Pathol 1989;13:39, Histopathology 2003;43:529)
- Lesions may be entirely intradermal, have an inconspicuous junctional component or uncommonly exhibit prominent junctional nests (Am J Surg Pathol 1989;13:39, Arch Pathol Lab Med 2011;135:321)
- Grenz zone may be present (Biology (Basel) 2022;11:460, Arch Pathol Lab Med 2011;135:321, J Am Acad Dermatol 2014;71:1234)
- May be combined with other nevi
- Melanocytes are epithelioid to spindled
- Nuclei are typically round to fusiform
- Cytoplasm may exhibit granular pigment and nuclear pseudoinclusions
- C shaped melanocytes surrounding epithelioid nests can be seen
- Occasional dermal mitotic figures (nonatypical, < 1 - 2/mm2) (J Cutan Pathol 1992;19:172, Arch Pathol Lab Med 2011;135:321, J Am Acad Dermatol 2014;71:1234)
- Occasional mild cytologic atypia can be seen including mild pleomorphism and hyperchromasia, variation in size, small nucleoli (Am J Surg Pathol 1989;13:39, Biology (Basel) 2022;11:460, J Cutan Pathol 1992;19:172, J Am Acad Dermatol 2014;71:1234)
- Atypical features in DPN include increased cytologic and architectural atypia
- 1 - 3 mitotic figures/mm2, increased nuclear pleomorphism, increased N:C ratio; cherry red nucleoli (Biology (Basel) 2022;11:460, Eur J Dermatol 2014;24:594, Cancers (Basel) 2021;13:3066, J Cutan Pathol 2020;47:1150)
- Asymmetrical, hypercellular, infiltrative borders (Biology (Basel) 2022;11:460, Eur J Dermatol 2014;24:594, Cancers (Basel) 2021;13:3066, J Cutan Pathol 2020;47:1150)
- These lesions have been associated with lymph node metastasis (Eur J Dermatol 2014;24:594, Cancers (Basel) 2021;13:3066, J Cutan Pathol 2020;47:1150, Biology (Basel) 2022;11:460)
- Can have increased Ki67 proliferation index (Biology (Basel) 2022;11:460)
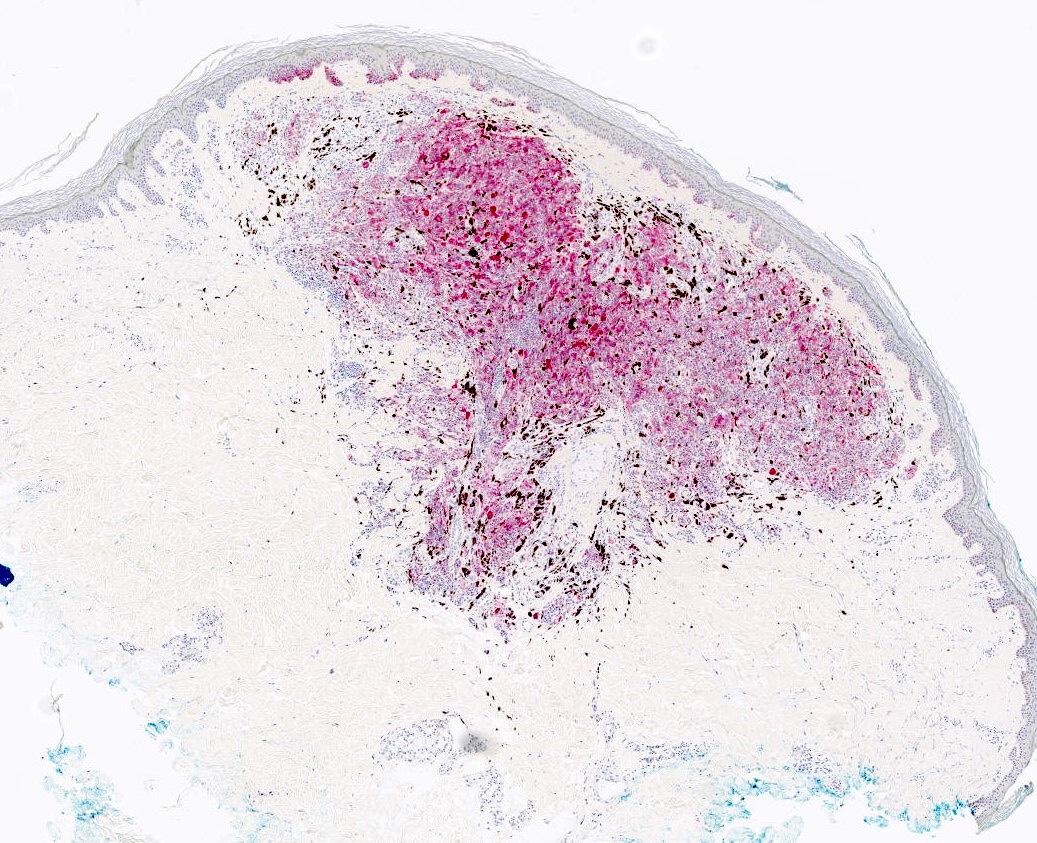
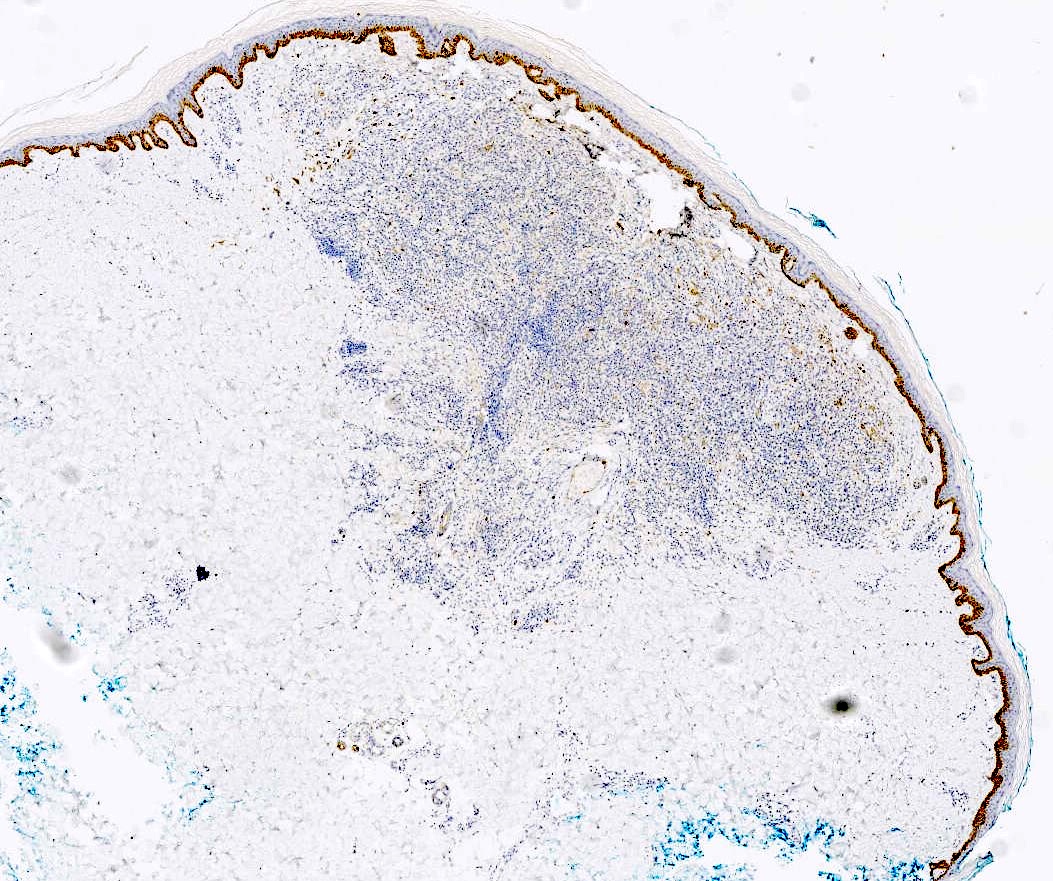
Microscopic (histologic) images
Positive stains
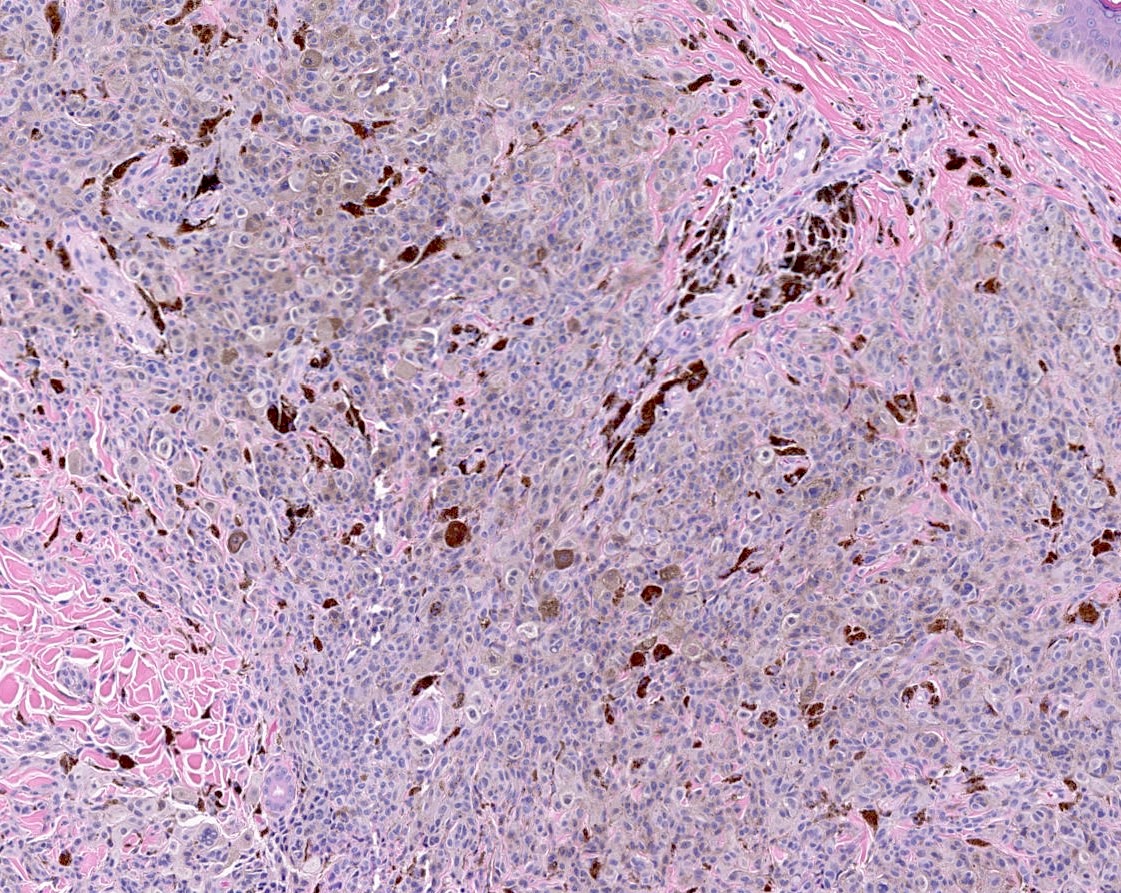
- HMB45 (diffuse), cyclin D1 (nuclear and cytoplasmic), beta catenin (nuclear, cytoplasmic and membranous) (Biology (Basel) 2022;11:460)
- LEF1 (nuclear)
- Ki67 proliferation index is typically low (Biology (Basel) 2022;11:460)
- p16: mosaic / checkerboard (J Cutan Pathol 2020;47:815)
Negative stains
- PRAME is typically negative (Am J Dermatopathol 2023;45:549)
Molecular / cytogenetics description
- Wnt / beta catenin pathway activation (Biology (Basel) 2022;11:460)
- Can demonstrate mitogen activated protein kinase (MAPK) mutations (i.e., BRAF, MAP2K1 / MEK1, HRAS) (Nat Commun 2017;8:644, Biology (Basel) 2022;11:460)
- Typically no chromosomal aberrations via FISH or comparative genomic hybridization (CGH) even in DPN with atypical features (J Cutan Pathol 2020;47:1150, Eur J Dermatol 2014;24:594, Biology (Basel) 2022;11:460)
- This may be helpful with other diagnostic considerations such as melanoma
- Appropriate clinical correlation is encouraged
Sample pathology report
- Skin, right arm, excisional biopsy:
- Deep penetrating nevus, margins appear free (see comment)
- Comment: Sections show skin and subcutis with a cellular melanocytic proliferation within dermis with extension into subcutis. Cytologically, the melanocytes are epithelioid to spindled with abundant melanin pigment appreciated. The melanocytes are arranged in a wedge shaped orientation with focal encircling on adnexal structures and nerves within predominantly deep dermis. Nuclear pleomorphism, conspicuous cherry red nucleoli, lymphovascular invasion and mitotic activity are not identified. By immunohistochemistry, the lesional cells of interest are positive for HMB45, cyclin D1 (nuclear and cytoplasmic), beta catenin (nuclear, cytoplasmic and membranous), LEF1 (nuclear) and p16. Ki67 proliferation index is relatively low. Overall, the findings are supportive of the above interpretation. Further clinical correlation and follow up are recommended as appropriate.
Differential diagnosis
- Melanoma:
- May have atypical junctional components including pagetoid spread of melanocytes
- Obvious mitotic figures (including atypical), marked atypia, lymphovascular invasion, ulceration, necrosis
- Typically, increased Ki67 proliferation index
- Typically, PRAME positive, HMB45 positive
- Genetic aberrations common to melanoma can be identified
- References: Cancer Epidemiol Biomarkers Prev 2007;16:2486, Biology (Basel) 2022;11:460, Am J Surg Pathol 1989;13:39, J Am Acad Dermatol 2014;71:1234)
- Cellular blue nevus:
- Spindled melanocytes (ovoid to plump) within dermis with inconspicuous nucleoli
- Well circumscribed and pigmented
- Predominantly within superficial and mid dermis
- Extension into deep dermis and subcutis can be seen
- Typically, pushing borders
- Cyclin D1 negative, beta catenin demonstrates cytoplasmic staining (no nuclear staining), LEF1 negative in most cases (Am J Dermatopathol 2023;45:549)
- HMB45 positive
- GNAQ and GNA11 mutations (Biology (Basel) 2022;11:460)
- Inverted type A nevus:
- Considered a variant of DPN (J Cutan Pathol 2018;45:254)
- Melanocytic nevus with population of epithelioid melanocytes (type A nevus cells) with extension into dermis or subcutis
- More superficial than DPN (Biology (Basel) 2022;11:460)
- Mitoses absent to rare
- Often pigmented melanocytes
- Spitz nevus:
- Raining down pattern of epithelioid and spindled melanocytes
- Typically, compound
- Kamino bodies
- Younger patients
- HMB45 is typically negative
- ALK, ROS, NTRK fusions can be seen (Mod Pathol 2003;16:505)
- Plexiform spindle cell nevus:
- Considered a variant of DPN
- Fascicular plexiform architecture versus wedge shaped (Biology (Basel) 2022;11:460)
- Predominantly spindled cells splitting collagen fibers
- Typically, more superficial
- Pigmented epithelioid melanocytoma (PEM):
- Can be associated with Carney complex
- Epidermal hyperplasia is commonly seen
- Heavily pigmented; usually 3 melanocyte cell types (most demonstrate a mixed population) including small, large and spindled melanocytes
- Typically, abundant melanophages are present
- Can demonstrate PRKCA fusion or PRKAR1A mutation
Additional references
Board review style question #1
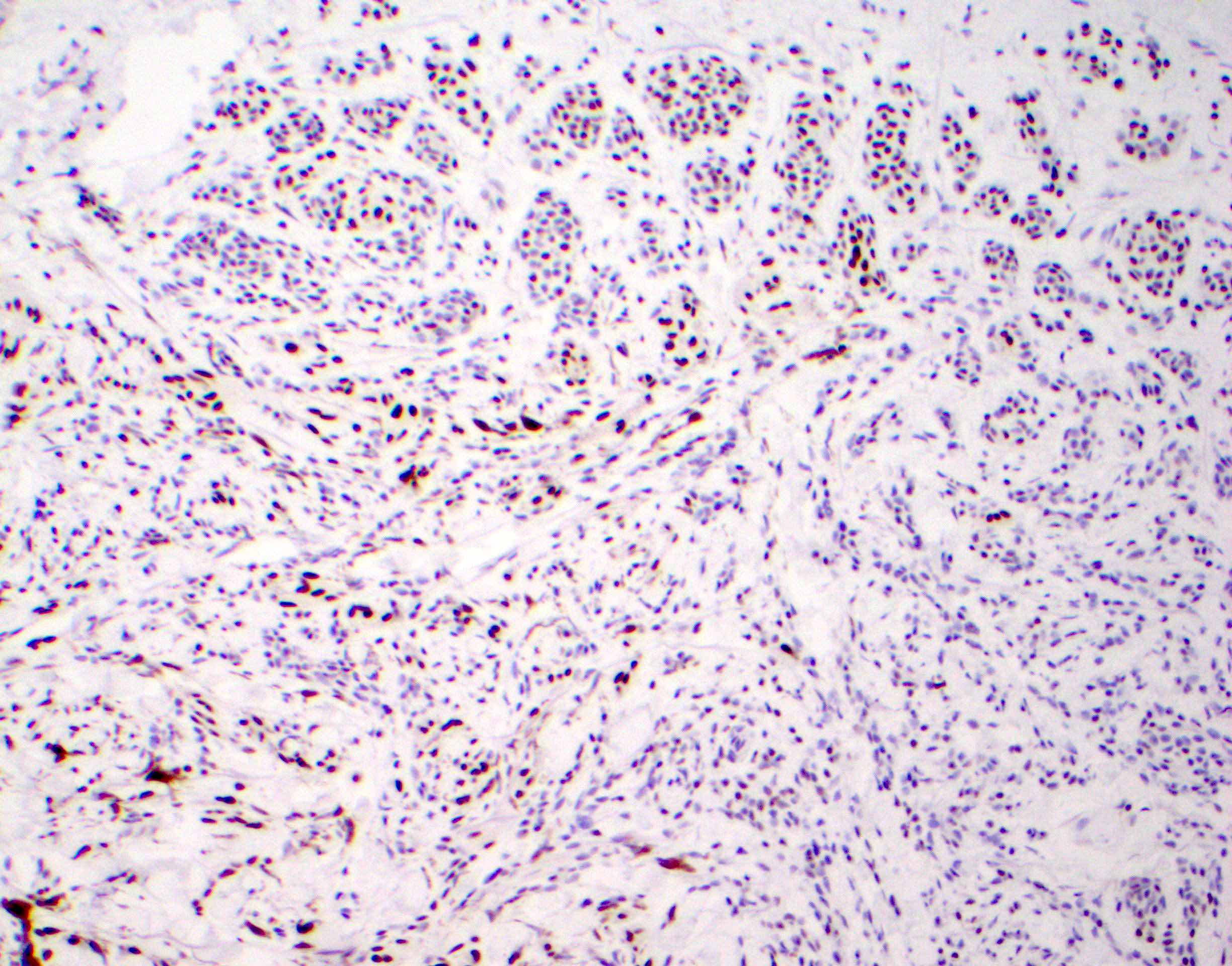
What immunohistochemical staining profile would you expect for the skin lesion pictured above?
- HMB45 negative; beta catenin negative; cyclin D1 shows cytoplasmic staining
- HMB45 negative; beta catenin shows nuclear, cytoplasmic and membranous staining; cyclin D1 negative
- HMB45 positive; beta catenin shows cytoplasmic staining; cyclin D1 negative
- HMB45 positive; beta catenin shows nuclear, cytoplasmic and membranous staining; cyclin D1 shows nuclear and cytoplasmic staining
Board review style answer #1
D. HMB45 positive; beta catenin shows nuclear, cytoplasmic and membranous staining; cyclin D1 shows nuclear and cytoplasmic staining. This is the expected staining profile for deep penetrating nevus / WNT activated deep penetrating / plexiform melanocytoma (DPN). DPN typically demonstrates positive staining for HMB45 and demonstrates nuclear, cytoplasmic and membranous staining for beta catenin and nuclear and cytoplasmic staining for cyclin D1. Of note, DPN typically demonstrates Wnt pathway activation via gain of function mutations of CTNNB1, which encodes beta catenin protein. Wnt activation results in the increased melanocyte size and pigmentation seen in DPN.
Answer B is incorrect because DPN is typically positive for HMB45 and demonstrates nuclear and cytoplasmic positivity for cyclin D1. DPN demonstrates nuclear, cytoplasmic and membranous staining for beta catenin. Answer C is incorrect because DPN typically demonstrates nuclear, cytoplasmic and membranous staining for beta catenin and nuclear and cytoplasmic staining for cyclin D1. DPN demonstrates positive staining for HMB45. Answer A is incorrect because DPN is typically positive for HMB45 and exhibits nuclear, cytoplasmic and membranous staining for beta catenin and nuclear and cytoplasmic staining for cyclin D1.
Comment Here
Reference: WNT activated deep penetrating / plexiform melanocytoma (nevus)
Answer B is incorrect because DPN is typically positive for HMB45 and demonstrates nuclear and cytoplasmic positivity for cyclin D1. DPN demonstrates nuclear, cytoplasmic and membranous staining for beta catenin. Answer C is incorrect because DPN typically demonstrates nuclear, cytoplasmic and membranous staining for beta catenin and nuclear and cytoplasmic staining for cyclin D1. DPN demonstrates positive staining for HMB45. Answer A is incorrect because DPN is typically positive for HMB45 and exhibits nuclear, cytoplasmic and membranous staining for beta catenin and nuclear and cytoplasmic staining for cyclin D1.
Comment Here
Reference: WNT activated deep penetrating / plexiform melanocytoma (nevus)
Board review style question #2
Board review style answer #2
B. WNT activated deep penetrating / plexiform melanocytoma (deep penetrating nevus). The image demonstrates a cellular wedge shaped melanocytic proliferation with abundant pigment and involvement of dermis and subcutis. A junctional component is not seen. These are hallmark features of deep penetrating nevus (DPN).
Answer D is incorrect. Typically compound, these nevi demonstrate a raining down appearance of melanocytes. Kamino bodies can be seen. These nevi are seen predominantly in younger / pediatric patients (see Spitz nevus). Answer A is incorrect because cellular blue nevus is usually more superficial and melanocytes are typically spindled in shape. Borders are typically pushing versus wedge shaped (see Blue nevus / cellular blue nevus). Answer C is incorrect because melanoma may have an atypical junctional component including pagetoid spread of melanocytes. Melanoma typically exhibits obvious mitotic figures (including atypical), marked atypia, lymphovascular invasion, ulceration, necrosis and other features of malignancy (see Melanoma).
Comment Here
Reference: WNT activated deep penetrating / plexiform melanocytoma (nevus)
Answer D is incorrect. Typically compound, these nevi demonstrate a raining down appearance of melanocytes. Kamino bodies can be seen. These nevi are seen predominantly in younger / pediatric patients (see Spitz nevus). Answer A is incorrect because cellular blue nevus is usually more superficial and melanocytes are typically spindled in shape. Borders are typically pushing versus wedge shaped (see Blue nevus / cellular blue nevus). Answer C is incorrect because melanoma may have an atypical junctional component including pagetoid spread of melanocytes. Melanoma typically exhibits obvious mitotic figures (including atypical), marked atypia, lymphovascular invasion, ulceration, necrosis and other features of malignancy (see Melanoma).
Comment Here
Reference: WNT activated deep penetrating / plexiform melanocytoma (nevus)