Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Ely KA. Intraductal carcinoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/salivaryglandsintraductalsalivary.html. Accessed April 2nd, 2025.
Definition / general
- Rare tumor usually involving the parotid, which is characterized by intracystic / intraductal proliferations of neoplastic epithelial cells
- Exists in at least 3 variations, each with unique histologic, immunophenotypic and molecular features
- While the apocrine variant is likely related to salivary duct carcinoma, the intercalated duct-like and oncocytic variants are not
Essential features
- Resembles ductal carcinoma in situ of the breast, with cribriform, micropapillary, solid, comedo or clinging patterns (Cancer 1996;78:958)
- At least 3 variants
- Intercalated duct-like (most common) (Am J Surg Pathol 2018;42:442)
- Apocrine (Am J Surg Pathol 2006;30:1014)
- Mixed / hybrid
- Possibly oncocytic but may be a variant of intercalated duct type (Histopathology 2018;73:314)
- Diagnosis requires thorough sampling to rule out invasion or presence of a myoepithelial layer by immunohistochemistry
- No purely intraductal carcinomas have definitively recurred or distantly metastasized
Terminology
- Low grade cribriform cystadenocarcinoma
- Low grade intraductal carcinoma
- Low grade salivary duct carcinoma
- Salivary duct carcinoma in situ
- All alternate terminologies are not recommended
ICD coding
Epidemiology
- Rare, with an estimated 93 cases reported (APMIS 2020;128:191)
- Affects a wide age range; F:M = 1.5:1 (Head Neck Pathol 2013;7:S59)
Sites
- Parotid (84.6%), intraparotid lymph nodes (5.1%), accessory parotid gland (2.6%), submandibular gland (2.6%) and minor salivary glands (5.1%) (Head Neck Pathol 2013;7:S59)
Clinical features
- Asymptomatic and slow growing mass (Head Neck Pathol 2013;7:S59)
Diagnosis
- Must exclude invasion by thorough sampling or presence of a myoepithelial layer by immunohistochemistry (Am J Surg Pathol 2004;28:266)
Prognostic factors
- No purely intraductal carcinomas have definitively recurred or distantly metastasized (Head Neck Pathol 2013;7:S59)
- Few show invasive growth, several with widespread invasion (Am J Surg Pathol 2019;43:1303)
Case reports
- 59 year old woman with a left parotid mass (Head Neck Pathol 2011;5:321)
- 61 year old man with a 1 month history of a painless mass in the left buccal mucosa (Int Cancer Conf J 2015;5:53)
- 75 year old woman with a 1 year history of a hard swelling of the left parotid gland (Pathol Res Pract 2017;213:706)
Treatment
- Complete tumor excision (Head Neck Pathol 2020;14:869)
- No current data to support radiation after surgical excision (Am J Surg Pathol 2006;30:1014)
Gross description
- Circumscribed and focally to predominantly cystic (Cancer 1996;78:958)
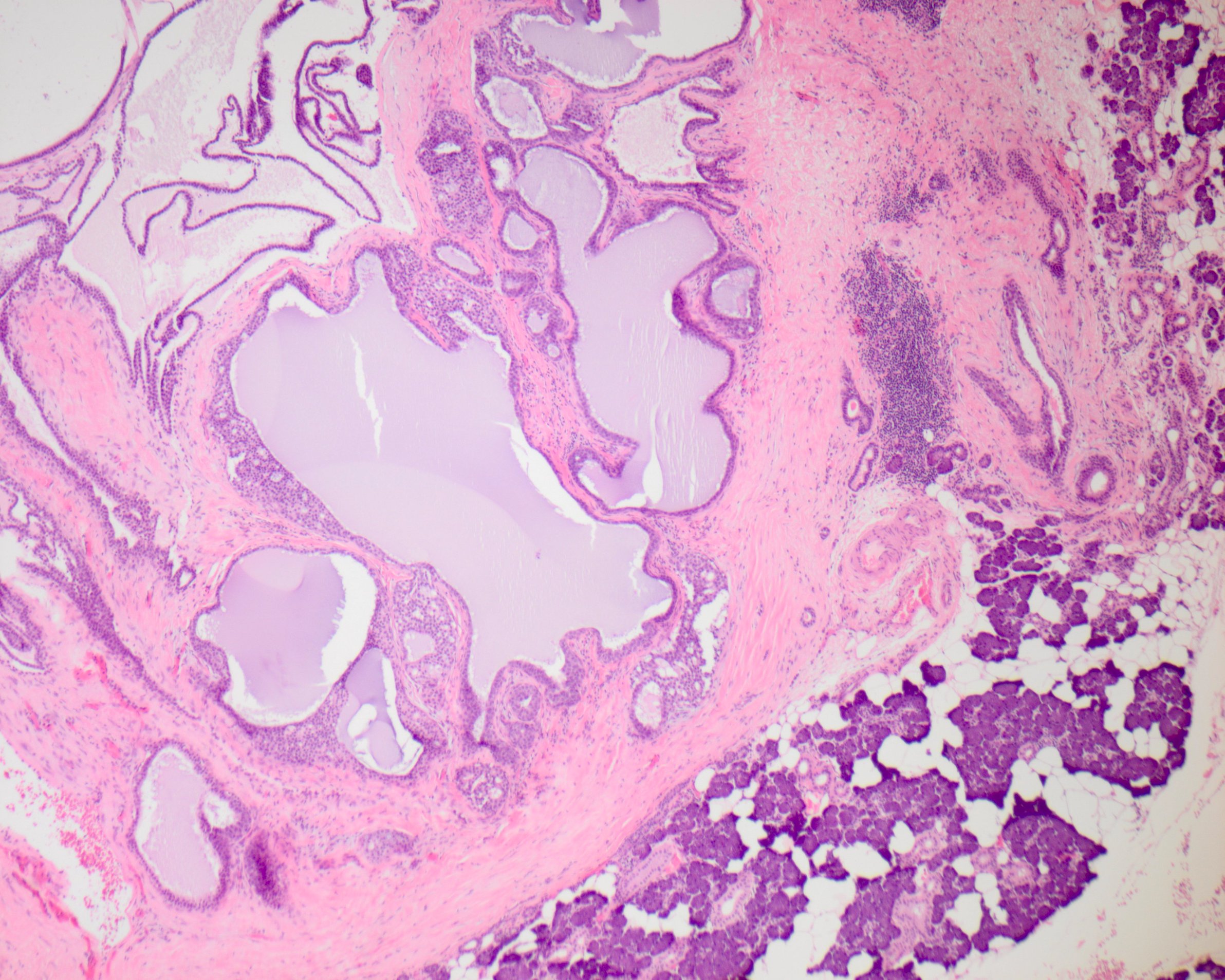
Microscopic (histologic) description
- General (criteria by Cheuk et al.) (Am J Surg Pathol 2004;28:266)
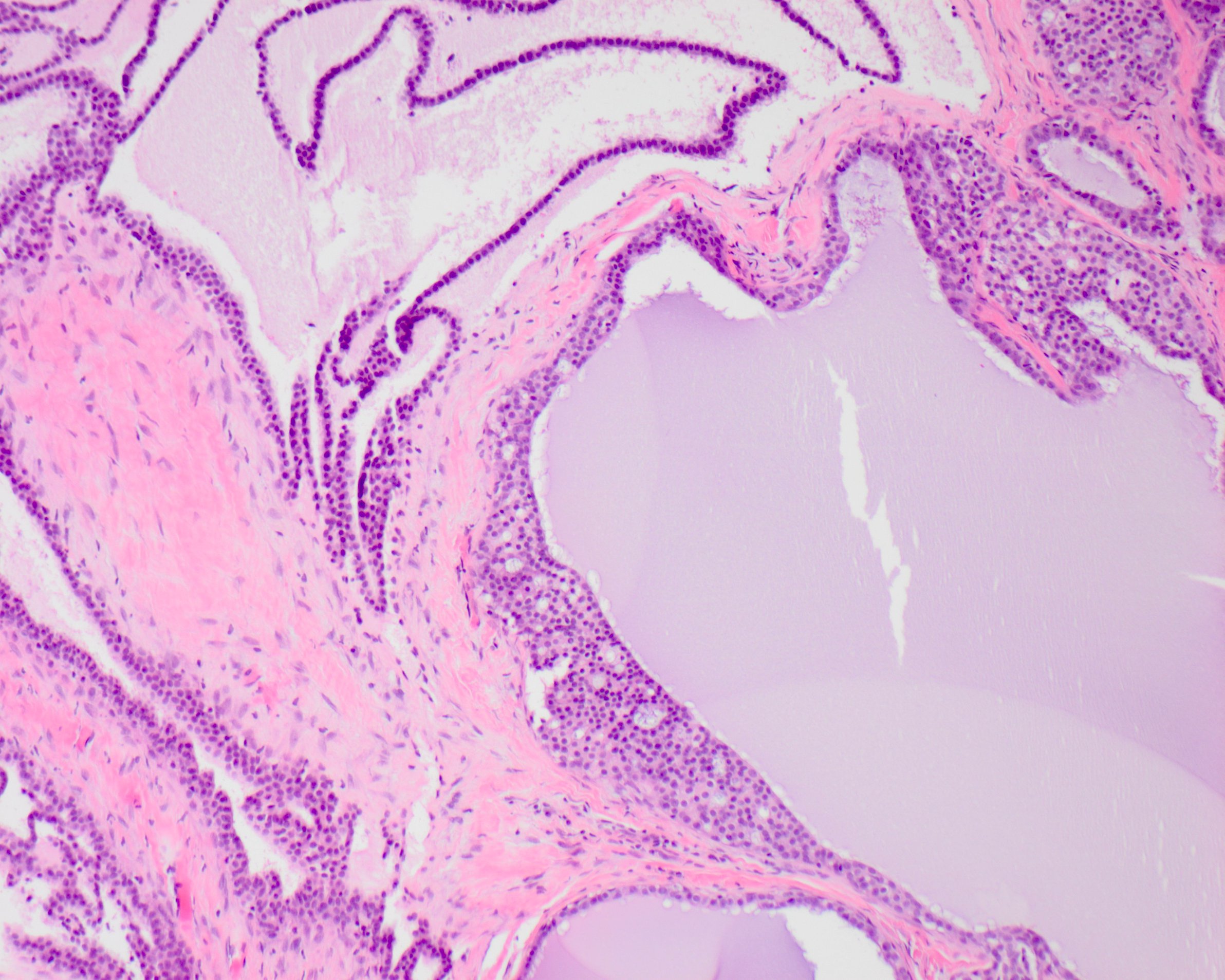
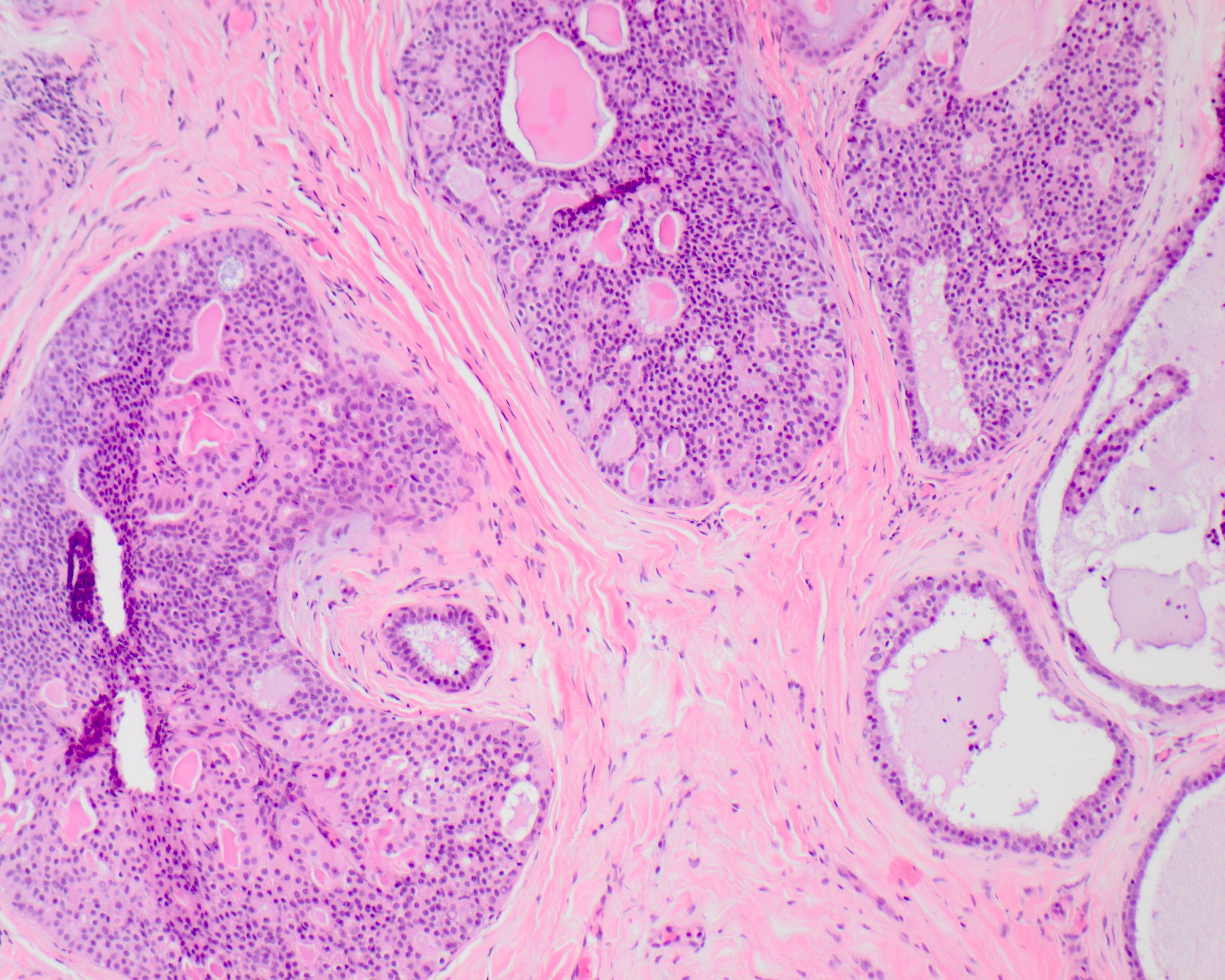
- Resembles mammary intraductal carcinoma with cribriform, micropapillary, solid, comedo or clinging patterns
- Exclusion of an invasive component by extensive sampling or demonstration by immunohistochemistry of a myoepithelial layer around epithelial nests
- Grade low, intermediate or high (Am J Surg Pathol 2004;28:266)
- No standardized criteria for grading (Head Neck Pathol 2020;14:869)
- Well circumscribed and unencapsulated at low power
- Variably sized cysts and ducts (Cancer 1996;78:958)
- May see a tumor associated lymphoid proliferation (Pathol Res Pract 2017;213:706)
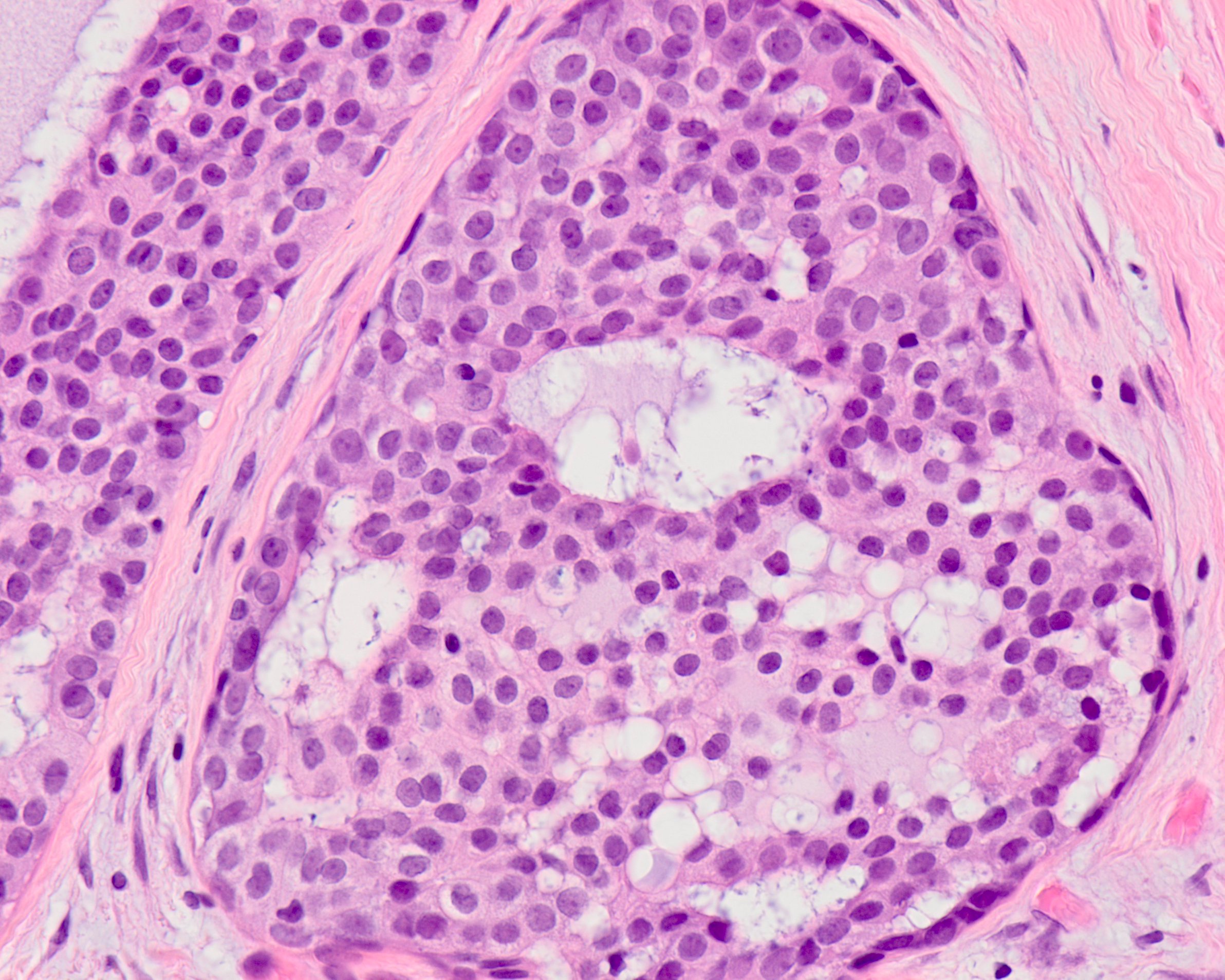
- Intercalated duct-like
- Small cuboidal cells with eosinophilic to amphophilic cytoplasm and small, oval nuclei with dispersed chromatin and inconspicuous nucleoli (Cancer 1996;78:958)
- Low grade nuclear features (Head Neck Pathol 2020;14:869)
- Apocrine type
- Abundant granular eosinophilic cytoplasm
- Large, round, vesicular nuclei with macronucleoli (Am J Surg Pathol 2006;30:1014)
- Apocrine snouts and secretions (Am J Surg Pathol 2018;42:442)
- High grade nuclear features (Histopathology 2008;53:416)
- Hybrid / mixed, both intercalated duct-like and apocrine features (Am J Surg Pathol 2018;42:442)
- Oncocytic possible variant or oncocytic metaplasia of another intraductal carcinoma (Histopathology 2018;73:314)
- Medium sized round nuclei and abundant granular cytoplasm (Histopathology 2018;73:314)
Microscopic (histologic) images
Cytology description
- Overlapping sheets and groups of cells with tight intercellular connections (Diagn Cytopathol 2011;39:218)
- Cribriform / microcystic, solid, pseudopapillary configurations (Korean J Pathol 2013;47:592)
- Round to oval and slightly irregular nuclei
- Nucleoli may be evident
- Cytoplasm can be abundant, well delimited and apocrine
Positive stains
- Epithelial cells: AE1 / AE3, CAM 5.2, cytokeratin 7 and EMA (Am J Surg Pathol 2019;43:1303)
- Myoepithelial cells: p63, p40, calponin, smooth muscle actin (Histopathology 2020 Nov 1 [Epub ahead of print])
- Intercalated duct-like:
- Apocrine:
- Mixed / hybrid:
- Each component has the expected immunoprofile
- Oncocytic:
Negative stains
- Intercalated duct-like:
- Apocrine:
- Mixed / hybrid: each component has the expected immunoprofile
- Oncocytic:
Molecular / cytogenetics description
- Intercalated duct-like: frequent RET fusions, commonly NCOA4-RET (Am J Surg Pathol 2018;42:442)
- RET rearrangement is seen in epithelial and myoepithelial cells, suggesting that both populations are neoplastic (Am J Surg Pathol 2021;45:507)
- Apocrine: complex genetics including HRAS and PIK3CA hotspot mutations (Am J Surg Pathol 2018;42:442)
- Mixed / hybrid: RET fusions, especially TRIM27 (Am J Surg Pathol 2018;42:1445)
- Oncocytic: RET fusions TRIM33 and BRAF V600E mutations (Histopathology 2020 Nov 1 [Epub ahead of print])
Sample pathology report
- Parotid, right, complete excision:
- Intraductal carcinoma (see comment)
- Comment: The neoplasm is well circumscribed and consists of a complex intraductal proliferation of epithelial cells arranged in cribriform and papillary patterns. A p63 immunostain highlights the myoepithelial layer surrounding these intraductal nests. Comprising cells contain abundant granular eosinophilic cytoplasm and nuclei with prominent nucleoli. Mitoses are inconspicuous and necrosis absent. Additional immunohistochemical stains were performed and are positive for androgen receptor (AR), while negative for S100 and SOX10.
Differential diagnosis
- Cystadenoma:
- Papillae lined by oncocytes, similar to Warthin tumor
- Typically lacks complex internal architecture resembling atypical ductal hyperplasia
- Tends to be S100- and GCDFP-15- (Head Neck Pathol 2015;9:354)
- Cystadenocarcinoma:
- Prominent infiltrative growth and lacks a myoepithelial cell layer (Am J Surg Pathol 1996;20:1440)
- Sclerosing polycystic adenosis:
- Serous acini with prominent hypereosinophilic granules
- Lacks an invasive component (Am J Surg Pathol 1996;20:161)
- Salivary duct carcinoma:
- Comedonecrosis with widespread infiltrative growth
- Perineural and lymphovascular invasion (Oral Surg Oral Med Oral Pathol 1994;78:64)
- AR+ (Am J Surg Pathol 2000;24:579)
- GCDFP-15+ (Am J Surg Pathol 2015;39:705)
- S100- (Cancer 1996;77:223)
- SOX10- (Hum Pathol 2016;56:134)
- Papillary cystic variant of acinic cell carcinoma:
- Secretory carcinoma:
- Lacks a significant intraductal component (Am J Surg Pathol 2018;42:442)
- Lacks diffuse network of myoepithelial cells
- Contains ETV6-NTRK3 fusions (Am J Surg Pathol 2010;34:599)
Board review style question #1
Which of the following is true regarding this variant of intraductal carcinoma with the following immunophenotype: S100 and SOX10+; AR-?
- It is associated with HRAS and PIK3CA hotspot mutations
- It is the most common variant
- It is the type most likely to exhibit high grade nuclear features
- It is usually GCDFP-15+
Board review style answer #1
B. It is the most common variant
The photomicrograph shows an intraductal proliferation of bland appearing cuboidal cells arranged in cribriform and clinging patterns. Nuclei are small and oval with a dispersed chromatin distribution. Immunohistochemical studies were positive for S100 and SOX10, while negative for AR (androgen receptor) and GCDFP-15. This constellation of findings is characteristic of the intercalated duct-like variant, which is the most common of the variants. It is frequently associated with RET fusions, commonly NCOA4-RET. HRAS and PIK3CA hotspot mutations are usually seen in the apocrine variant, which is the form more likely to exhibit higher grade atypia. It has the following immunophenotype: S100-, SOX10-, AR+ and GCDFP-15+. This staining pattern is similar to that of salivary duct carcinoma, leading to some to believe that apocrine intraductal carcinoma may be a precursor to conventional salivary duct carcinoma.
Comment Here
Reference: Intraductal carcinoma
The photomicrograph shows an intraductal proliferation of bland appearing cuboidal cells arranged in cribriform and clinging patterns. Nuclei are small and oval with a dispersed chromatin distribution. Immunohistochemical studies were positive for S100 and SOX10, while negative for AR (androgen receptor) and GCDFP-15. This constellation of findings is characteristic of the intercalated duct-like variant, which is the most common of the variants. It is frequently associated with RET fusions, commonly NCOA4-RET. HRAS and PIK3CA hotspot mutations are usually seen in the apocrine variant, which is the form more likely to exhibit higher grade atypia. It has the following immunophenotype: S100-, SOX10-, AR+ and GCDFP-15+. This staining pattern is similar to that of salivary duct carcinoma, leading to some to believe that apocrine intraductal carcinoma may be a precursor to conventional salivary duct carcinoma.
Comment Here
Reference: Intraductal carcinoma
Board review style question #2
Which of the following is true regarding intraductal carcinoma?
- A diagnosis can be made on core biopsy
- Has a marked predilection for women
- It is most common in the submandibular gland followed by the parotid
- No purely intraductal carcinomas have definitively recurred or distantly metastasized
Board review style answer #2
D. No purely intraductal carcinomas have definitively recurred or distantly metastasized.
Intraductal carcinoma involves the parotid (84.6%), intraparotid lymph nodes (5.1%), accessory parotid gland (2.6%), submandibular gland (2.6%) and minor salivary glands (5.1%). Women and men are almost equally affected, with a female to male ratio of 1.5:1. According to the diagnostic criteria outlined by Cheuk et al., a diagnosis of intraductal carcinoma can be made once an invasive component is excluded. This requires thorough sampling or the demonstration of a myoepithelial layer around the epithelial nest by immunohistochemistry. This is more confidently accomplished on an excisional specimen. When confined by a myoepithelial cell layer, no intraductal (or in situ) carcinomas have definitively recurred or distantly metastasized.
Comment Here
Reference: Intraductal carcinoma
Intraductal carcinoma involves the parotid (84.6%), intraparotid lymph nodes (5.1%), accessory parotid gland (2.6%), submandibular gland (2.6%) and minor salivary glands (5.1%). Women and men are almost equally affected, with a female to male ratio of 1.5:1. According to the diagnostic criteria outlined by Cheuk et al., a diagnosis of intraductal carcinoma can be made once an invasive component is excluded. This requires thorough sampling or the demonstration of a myoepithelial layer around the epithelial nest by immunohistochemistry. This is more confidently accomplished on an excisional specimen. When confined by a myoepithelial cell layer, no intraductal (or in situ) carcinomas have definitively recurred or distantly metastasized.
Comment Here
Reference: Intraductal carcinoma