Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Sun L, Chernock R. Neuroendocrine carcinoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/salivaryglandsLCNC.html. Accessed April 1st, 2025.
Definition / general
- Salivary neuroendocrine carcinomas are exceedingly rare (Am J Surg Pathol 2019;43:682)
- Divided into small cell carcinoma and large cell neuroendocrine carcinoma (Head Neck Pathol 2018;12:13, Head Neck Pathol 2013;7:295)
- Metastases, especially from the skin, are much more common than primary tumors (Am J Surg Pathol 2019;43:682)
- Many previously reported cases likely represent occult metastatic Merkel cell carcinoma of cutaneous origin (Am J Surg Pathol 2019;43:682)
Essential features
- Rare tumor type found predominantly in parotid gland
- 2 morphologies: small cell neuroendocrine carcinoma and large cell neuroendocrine carcinoma
- Positive for neuroendocrine IHC markers; also frequently CK20 positive (Am J Surg Pathol 1997;21:226)
- Can be difficult to distinguish from metastatic Merkel cell carcinoma (Am J Surg Pathol 2019;43:682)
Terminology
- Small cell neuroendocrine carcinoma of the salivary gland
- Large cell neuroendocrine carcinoma of the salivary gland
ICD coding
- ICD-10: C08.9 - malignant neoplasm of major salivary gland, unspecified
Epidemiology
- Adults
Sites
- Majority occur in the parotid gland, followed by the submandibular gland
Pathophysiology
- At least some arise as high grade transformation from a lower grade salivary tumor, such as pleomorphic adenoma and acinic cell carcinoma (Head Neck Pathol 2012;6:502, Head Neck Pathol 2016;10:152)
Etiology
- Unknown at this time
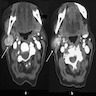
Clinical features
- Most common clinical presentation: rapidly growing neck mass
Diagnosis
- Fine needle aspiration or core biopsy of mass
Prognostic factors
- Unknown
Case reports
- 48 year old woman with a right cheek mass (small cell carcinoma ex pleomorphic adenoma) (Head Neck Pathol 2012;6:502)
- 1 patient in a series of 25 acinic cell carcinomas with high grade transformation had small cell carcinoma ex acinic cell carcinoma of the parotid gland (Head Neck Pathol 2016;10:152)
Treatment
- Surgical: local resection and neck dissection
- Majority receive adjuvant radiation
- Subset receive chemotherapy
- Reference: Head Neck Pathol 2018;12:13
Gross description
- Mass lesion within the resected salivary gland
- Solid cut surface
- Areas of necrosis may be noted
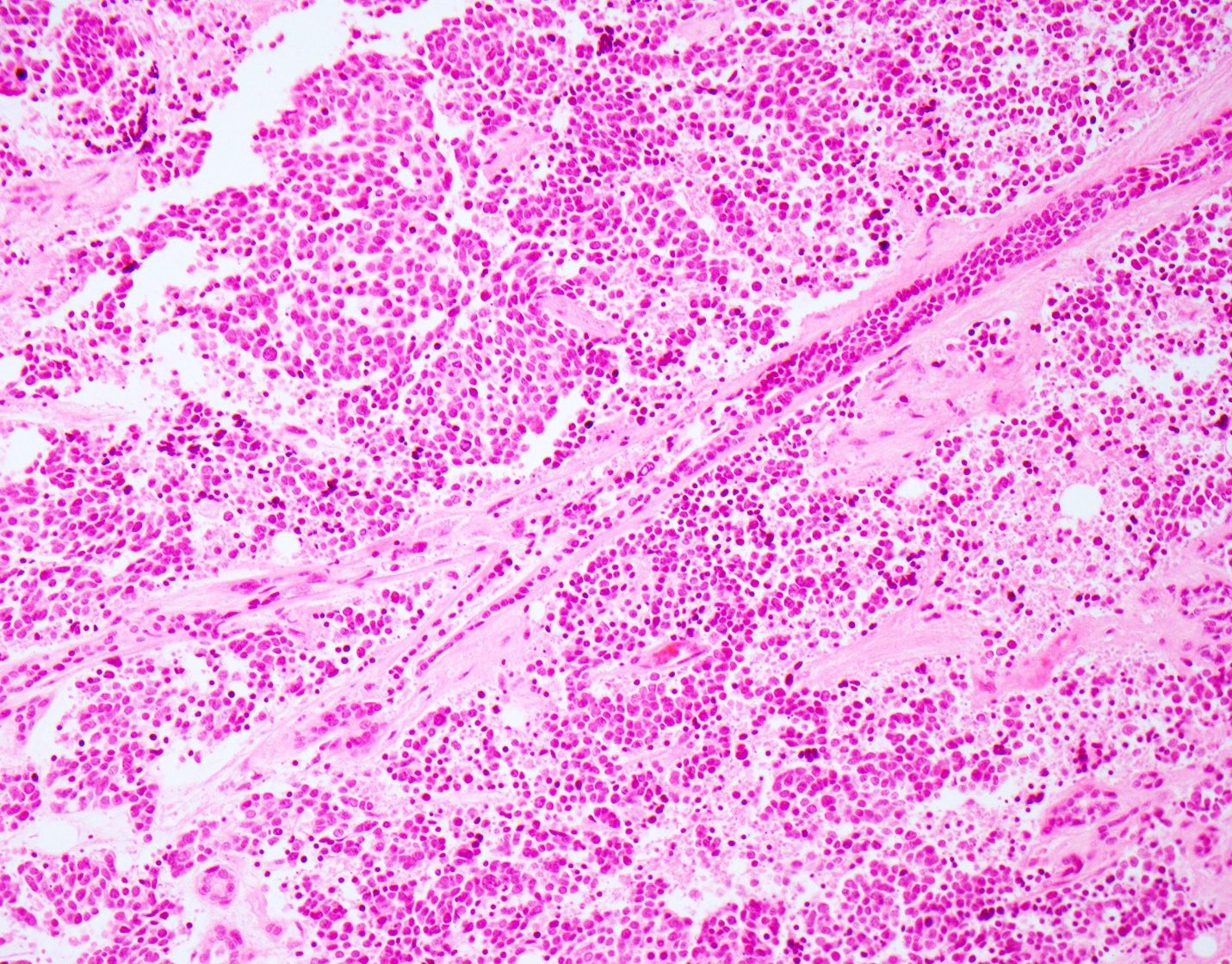
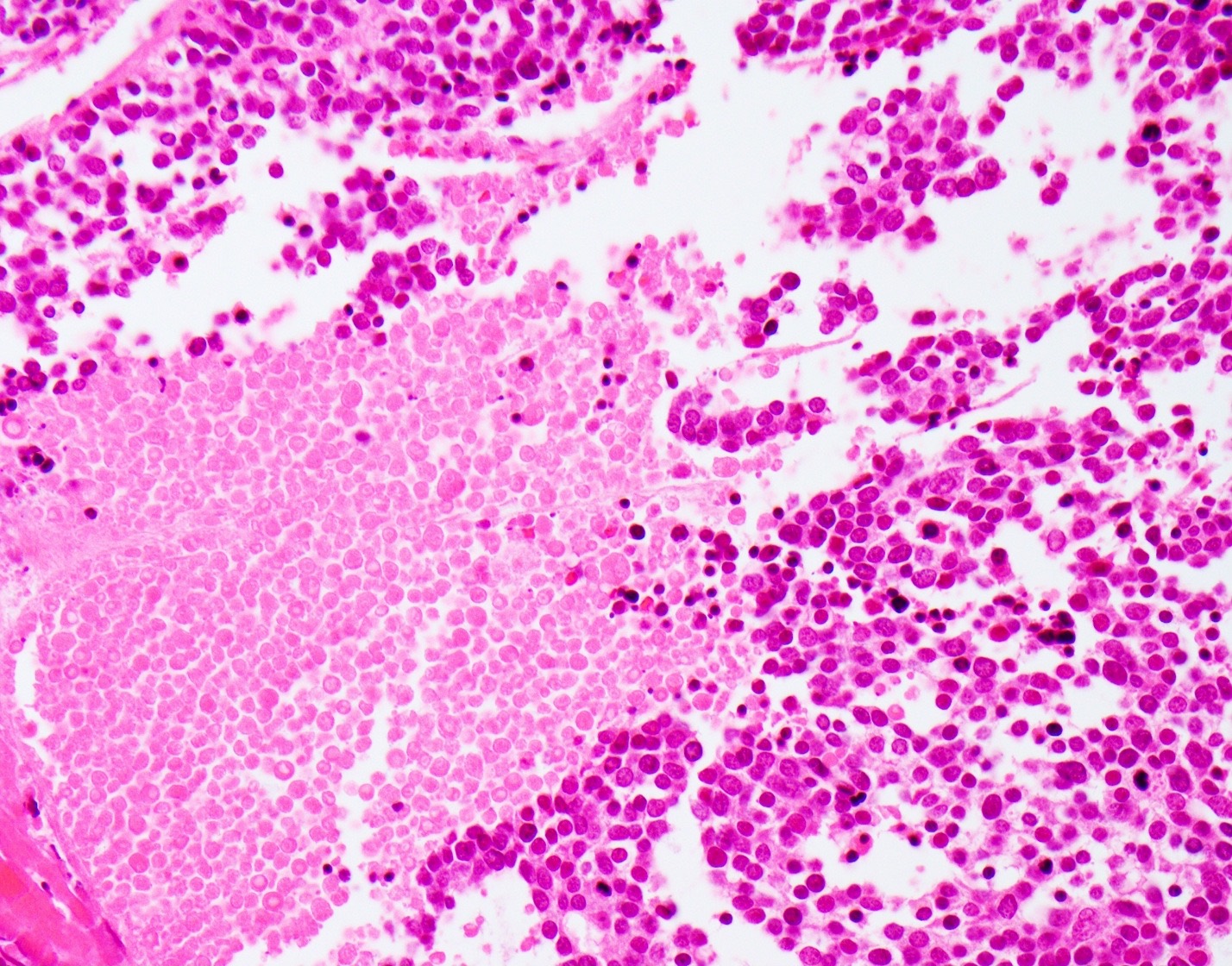
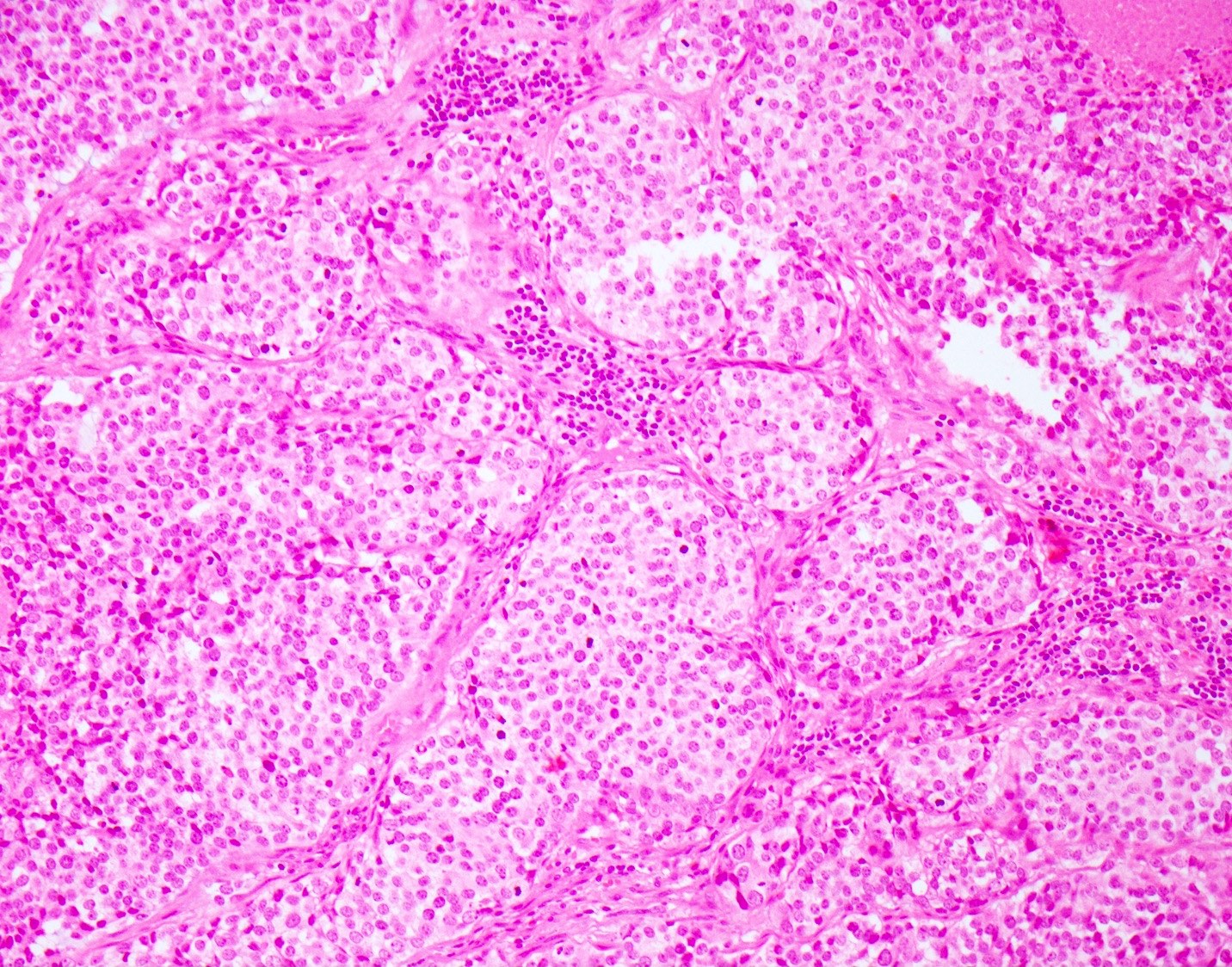
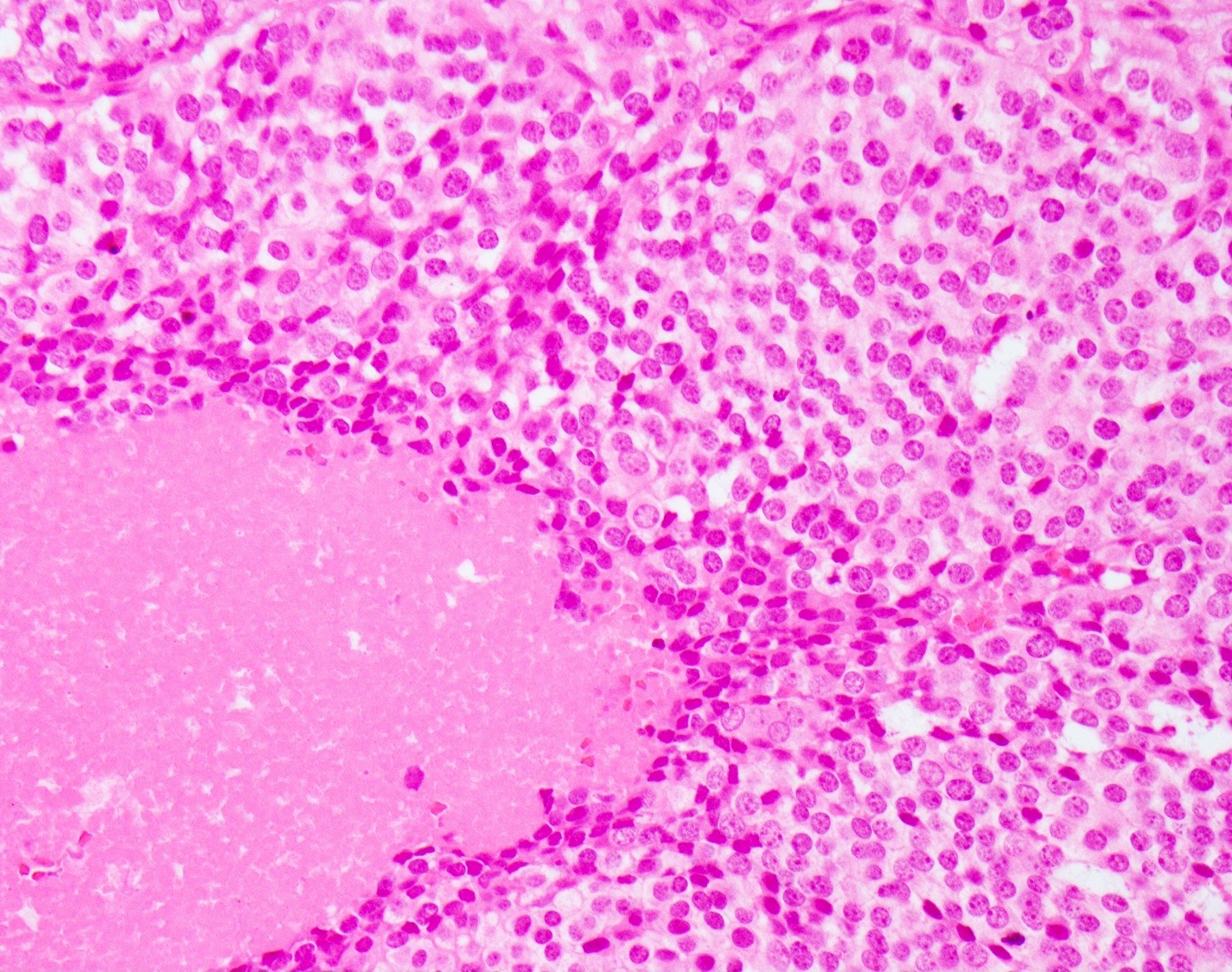
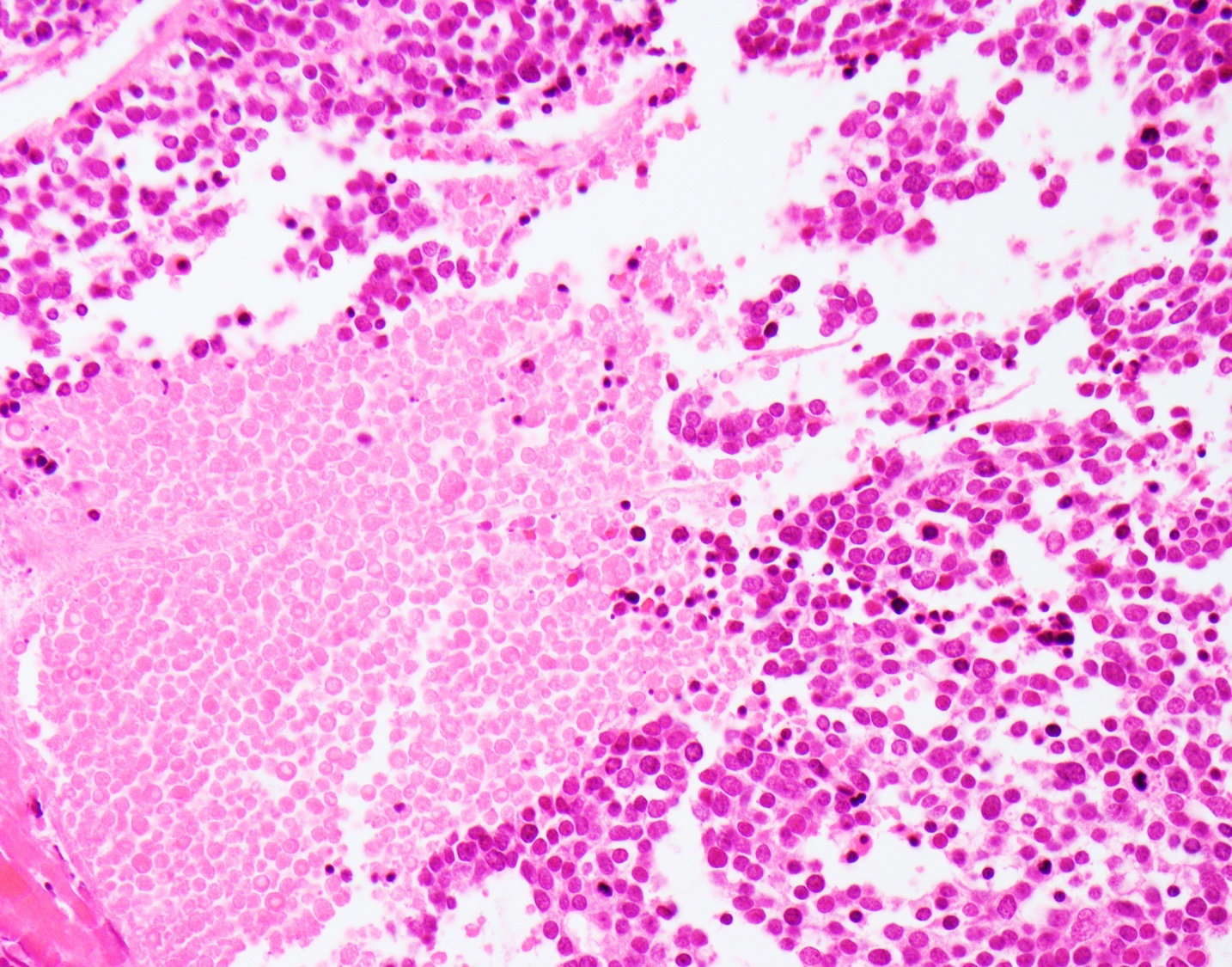
Microscopic (histologic) description
- Small cell neuroendocrine carcinoma (Head Neck Pathol 2018;12:13):
- Sheets, trabeculae or nests of small, blue tumor cells
- High nuclear to cytoplasmic ratios with scant cytoplasm
- Hyperchromatic, finely granular chromatin
- Nuclear molding and crush artifact are common
- Occasional rosettes
- Brisk mitotic activity (> 10 mitoses / 10 HPF)
- Apoptotic debris and areas of geographic necrosis may be present
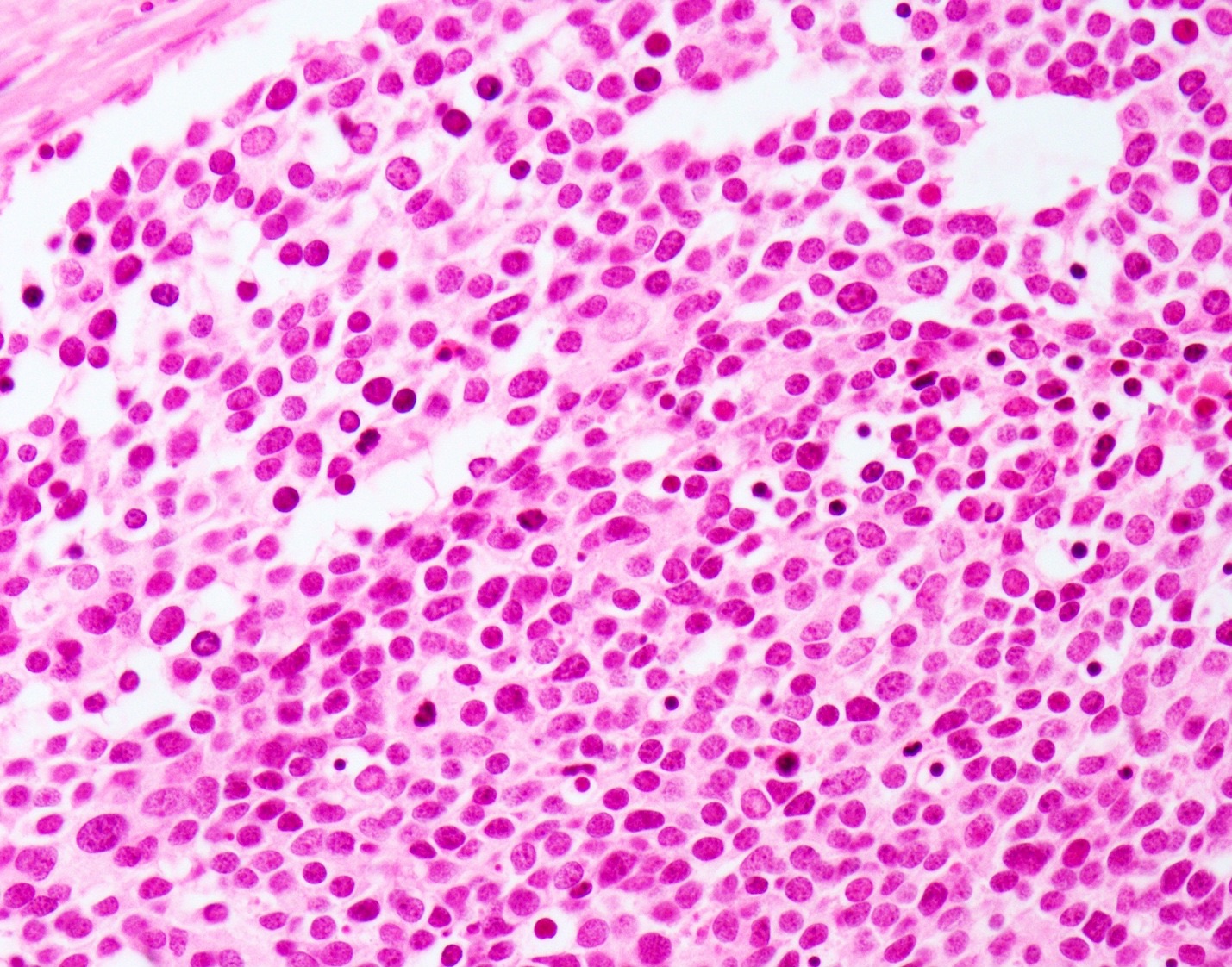
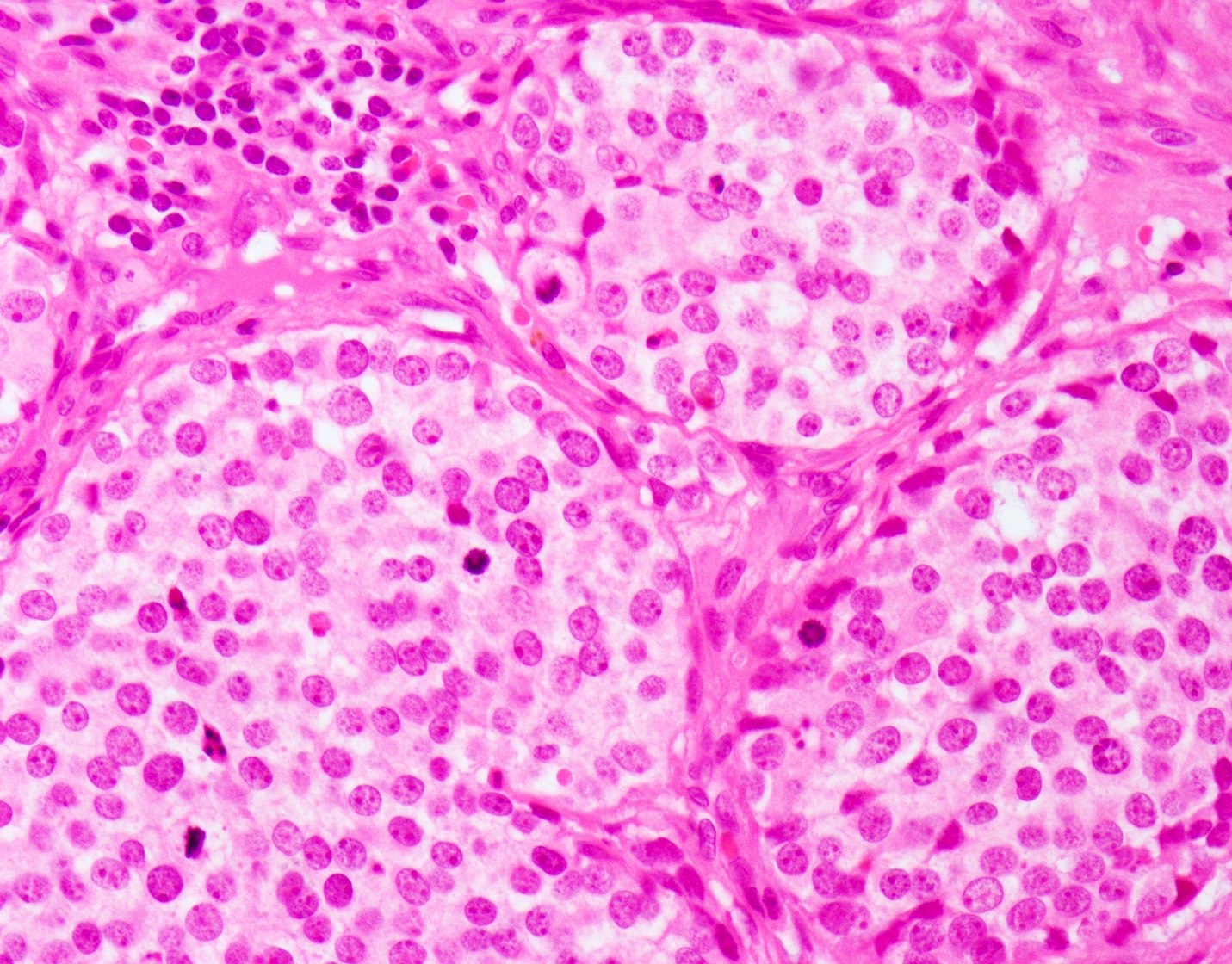
- Large cell neuroendocrine carcinoma (Head Neck Pathol 2018;12:13):
- More abundant cytoplasm
- Larger nuclei with course chromatin and often prominent nucleoli
- Palisading may be seen at the periphery of tumor nests
- Occasional rosettes
- Brisk mitotic activity (> 10 mitoses / 10 HPF)
- Apoptotic debris and areas of geographic necrosis may be present
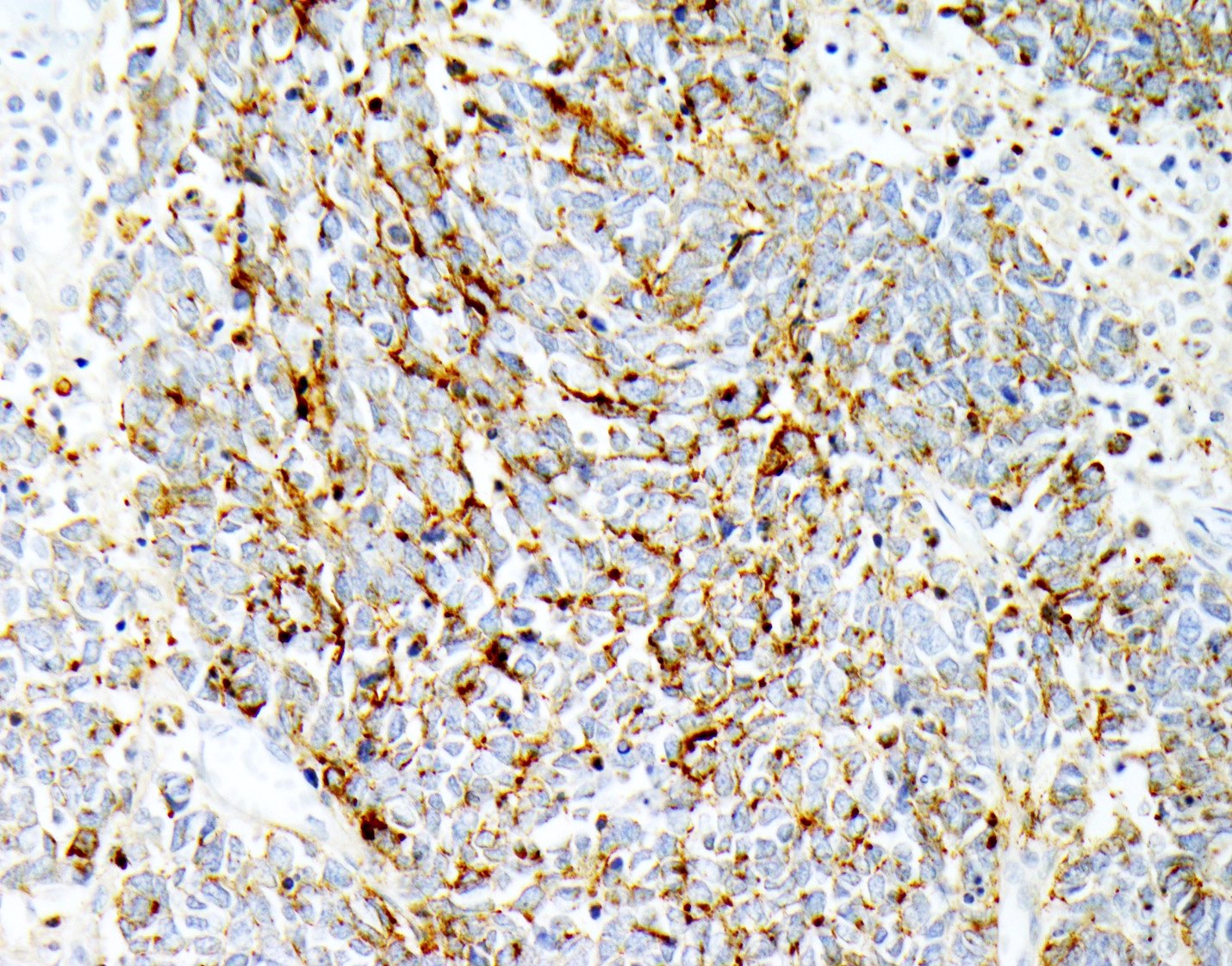
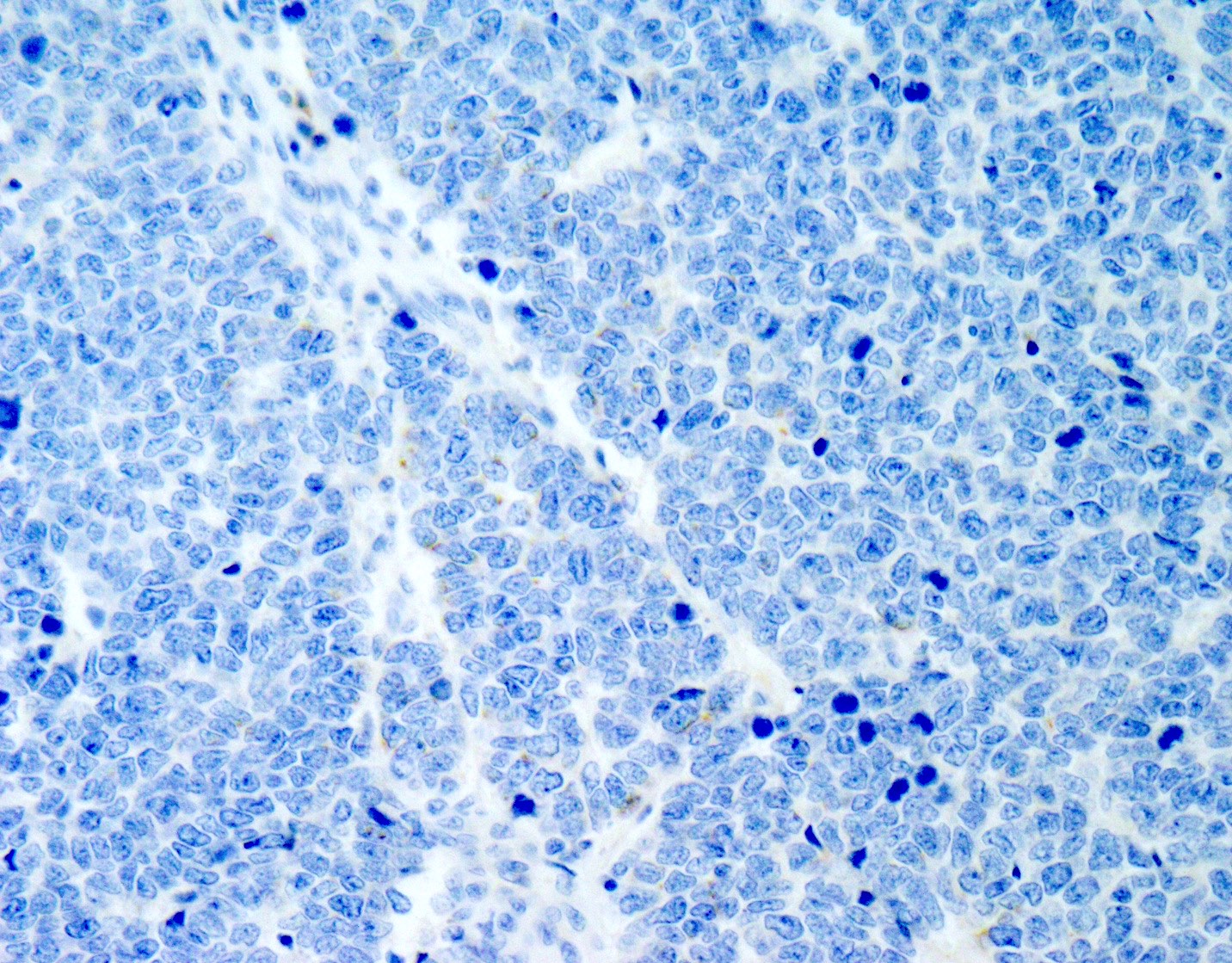
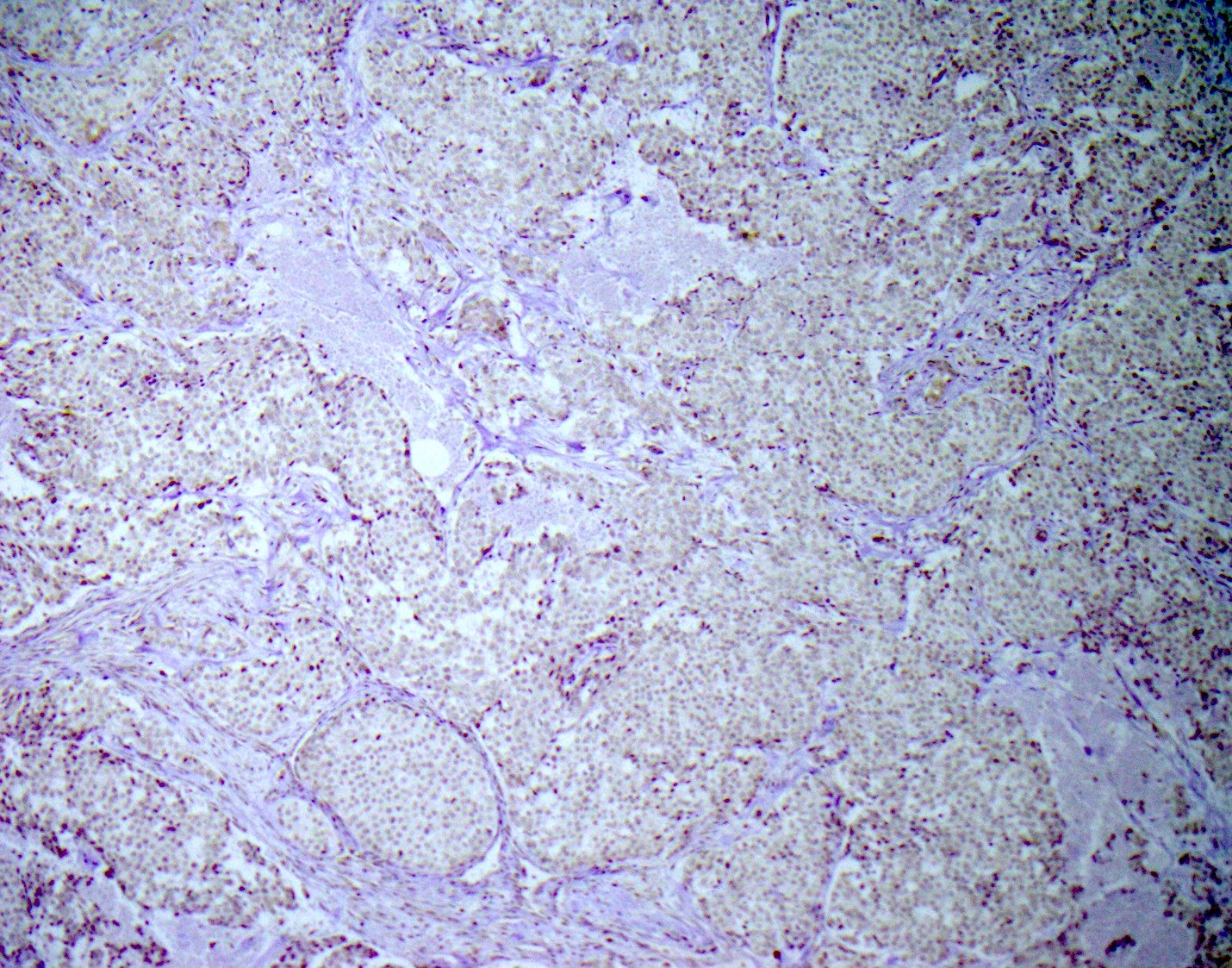
Microscopic (histologic) images
Cytology description
- Small cell neuroendocrine carcinoma (J Cytol 2016;33:34):
- Tumor cell clusters and single cells
- High nuclear to cytoplasmic ratios with scant cytoplasm
- Nuclear molding and crush artifact are common
- Granular salt and pepper chromatin with inconspicuous nucleoli
- Nuclear breakdown or apoptotic bodies / necrotic background
- Large cell neuroendocrine carcinoma (Auris Nasus Larynx 2005;32:89):
- Tumor cell clusters and single cells
- Larger cells with more abundant cytoplasm
- Granular salt and pepper chromatin with inconspicuous nucleoli
- Nuclear breakdown or apoptotic bodies / necrotic background
Positive stains
- Neuroendocrine markers (may not stain with every antibody, recommend panel of neuroendocrine markers): synaptophysin, chromogranin, CD56
- CK20 (perinuclear dot-like pattern, some cases)
Negative stains
- TTF1 (occasionally positive)
- CK7
- Merkel cell polyomavirus (MCPyV)
Molecular / cytogenetics description
- Absence of Merkel cell polyomavirus and UV signature mutations (Am J Surg Pathol 2011;35:1806, Am J Surg Pathol 2019;43:682, Head Neck Pathol 2018;12:13)
Sample pathology report
- Salivary gland, right parotid, parotidectomy:
- Small cell neuroendocrine carcinoma (see comment)
- Comment: Histologic sections show a small cell neuroendocrine carcinoma involving the parotid gland. The tumor cells are positive for synaptophysin, chromogranin and cytokeratin 20. TTF1 is negative. Immunohistochemistry for Merkel cell polyomavirus (MCPyV) is also negative. The majority of high grade neuroendocrine carcinomas located in the parotid gland represent metastases, usually from the head and neck skin, rather than primary salivary carcinomas. Correlation with clinical history and physical exam findings is recommended to exclude a metastasis. Even in the absence of an identifiable cutaneous primary, occult metastatic Merkel cell carcinoma is still more likely. Mutational signature analysis from next generation sequencing assays may aid in determining tumor origin as a UV signature would favor a metastasis from a cutaneous primary.
Differential diagnosis
- Metastatic neuroendocrine carcinoma:
- Parotid gland contains lymph nodes which are a favored site of metastasis from cutaneous head and neck cancer
- Metastatic Merkel cell carcinoma:
- Clinical history is most useful; however, there may not be an identifiable skin tumor and primary tumor regression also possible (Am J Dermatopathol 2016;38:e154)
- Absence of associated low grade salivary tumor (other than Warthin tumor)
- Also CK20 positive in perinuclear, dot-like pattern
- Merkel cell polyomavirus or UV signature mutations present in most cases and not found in primary salivary tumors (Am J Surg Pathol 2009;33:1771, Am J Surg Pathol 2019;43:682, Head Neck Pathol 2018;12:13)
- Metastatic lung neuroendocrine carcinoma:
- Lymphoma:
- CD45 positive
- Cytokeratin negative
Board review style question #1
A 70 year old man presented with an enlarging mass in the left cheek soft tissue. The mass was removed and shows the histology above. The tumor is positive for CK20 (dot-like) and synaptophysin and is negative for CK7. Which of the following features would favor a salivary primary over a metastasis from an occult cutaneous primary tumor?
- Adjacent pleomorphic adenoma
- History of multiple head and neck skin cancers
- Molecular analysis showing a UV signature
- Positive immunohistochemistry for MCPyV
Board review style answer #1
Board review style question #2
Which of the following is true about primary neuroendocrine carcinomas of the salivary gland?
- The most common site is in the sublingual gland
- They do not stain with classical neuroendocrine markers (synaptophysin and chromogranin)
- They have low grade morphology
- They often stain positively with CK20 in a perinuclear dot-like pattern
Board review style answer #2
D. They often stain positively with CK20 in a perinuclear dot-like pattern
Comment Here
Reference: Neuroendocrine carcinoma
Comment Here
Reference: Neuroendocrine carcinoma