Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1Cite this page: Iczkowski KA. Prostatic stromal sarcoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/prostatestromalsarcoma.html. Accessed November 27th, 2024.
Definition / general
- Prostatic stromal sarcoma (PSS) is a rare entity (Pathology 2013;45:104)
- Usually presents with urinary retention; also abnormal digital rectal examination, hematuria or hematospermia, palpable rectal mass
- Includes phyllodes tumors (like those in the breast) (Pathology 2021;53:12)
Essential features
- Cellular pleomorphism exceeds that of stromal proliferation of undetermined malignant potential (STUMP)
- Necrosis
- Mitotic activity
- Extension outside the prostate
Terminology
- Phyllodes tumor, cystic epithelial - stromal tumor, cystadenoleiomyofibroma, cystosarcoma phyllodes
ICD coding
- ICD-10: C61 - malignant neoplasm of prostate
Epidemiology
- Approximately 30 reported cases
- Mean age of 54 years but ranges from 14 - 86 (Am J Surg Pathol 1998;22:148)
- However, mean age in 1 study was 37, versus 61 for STUMP (Urol Int 2021;105:206)
Sites
- Prostate and periprostatic tissue
Etiology
- Unknown
Clinical features
- Most common presentation is bladder outlet obstruction, followed by abnormal digital rectal exam, hematuria and rectal fullness (Am J Surg Pathol 2006;30:694)
- Inclusion of phyllodes tumor is justified by its frequent early recurrence, infiltrative growth, extraprostatic extension and metastatic spread if incompletely excised (Clin Nucl Med 2021;46:348, J Urol 2004;172:894, Urol Case Rep 2019;28:101015, Mod Pathol 2007;20:175)
Diagnosis
- Cellular pleomorphism, necrosis, mitotic activity, extension outside the prostate and metastasis rule out benign mimics
Laboratory
- Serum PSA is usually normal
Radiology description
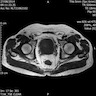
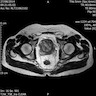
- Lesion that became metastatic was distinguished by intense uptake on 18F-FDG PET imaging (Clin Nucl Med 2021;46:348)
- This high intensity is in contrast to the benign entity of stromal hyperplasia with atypia, also called STUMP
- MRI shows a multinodular mass with homogeneous or heterogeneous low signal intensity on T1 weighted imaging and heterogenous high signal intensity on T2 weighted imaging (Oncol Lett 2016;11:2542)
Radiology images
Prognostic factors
- Low grade stromal sarcoma can invade locally, whereas high grade stromal sarcoma has the potential to metastasize
- Metastatic sites include:
- Lymph nodes (J Surg Case Rep 2016;2016:rjw065, Oncol Lett 2016;11:2542)
- Liver (Mod Pathol 2013;26:1536)
- Subcutaneous (Int J Surg Case Rep 2015;17:82)
- Bones (Clin Nucl Med 2021;46:348)
Case reports
- 14 year old boy with fatal case (Rare Tumors 2014;6:5607)
- 31 year old man with stromal sarcoma with rhabdoid features (Ann Diagn Pathol 2010;14:453)
- 32 year old man with an 8 cm prostatic mass protruding into the bladder (Oncol Lett 2016;11:2542)
- 40 year old man with tumor involving the rectum; no recurrence after exenteration (J Surg Case Rep 2020;2020:rjaa165)
- 42 year old man with subcutaneous metastasis of tumor 5 years after prostatectomy (Int J Surg Case Rep 2015;17:82)
- 52 year old man with recurrent tumor (J Clin Pathol 2007;60:330)
- 66 year old man with 8 cm mass extending to bladder (J Surg Case Rep 2016;2016:rjw065)
- 71 year old man with urinary obstruction (Case Rep Pathol 2011;2011:252805)
Treatment
- Can be managed by robotic procedure but surgery alone is inadequate (Korean J Urol 2014;55:620, J Surg Case Rep 2016;2016:rjw065)
- Tumor does respond to chemotherapy and radiotherapy (Rare Tumors 2014;6:5607)
Microscopic (histologic) description
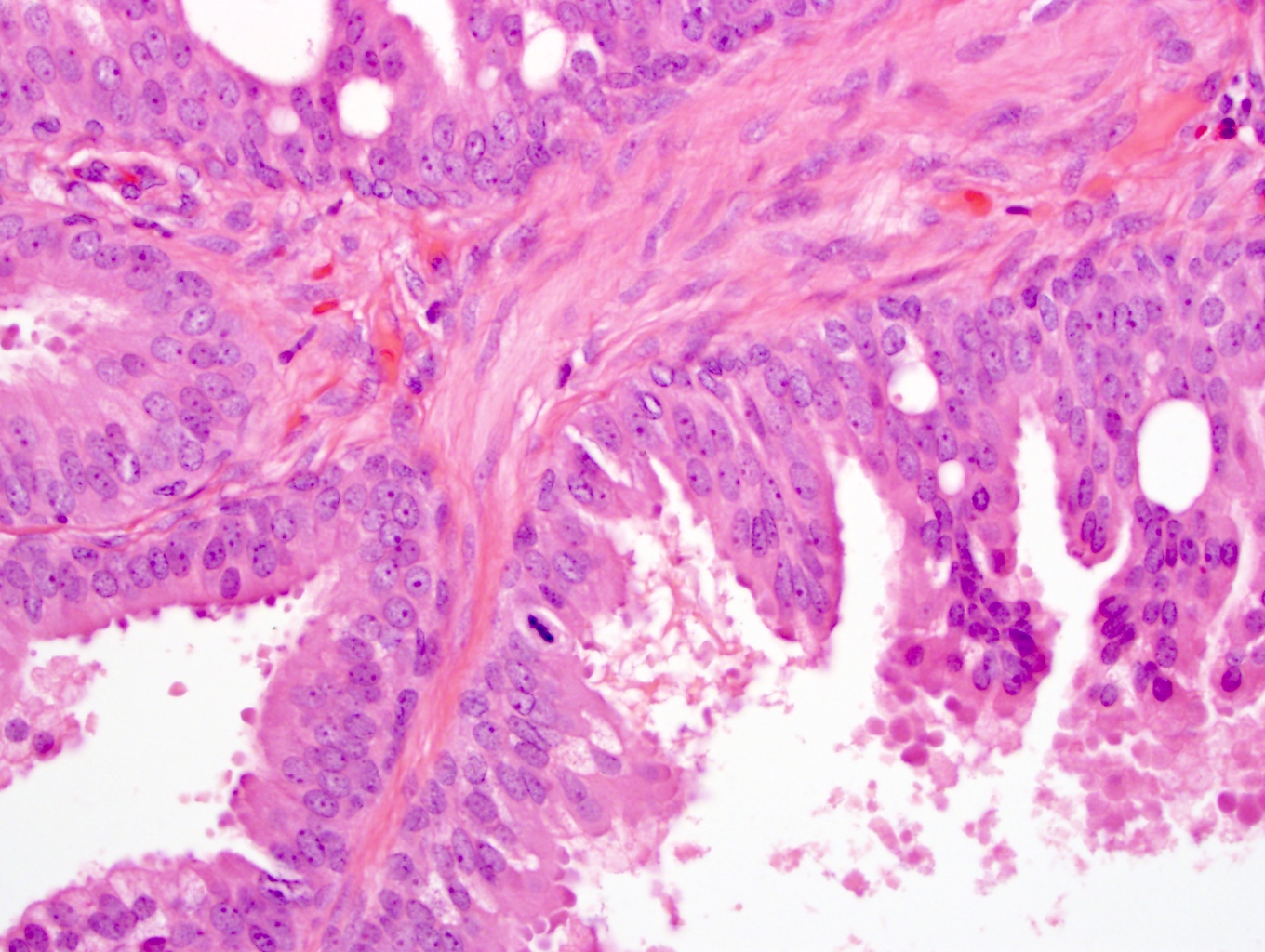
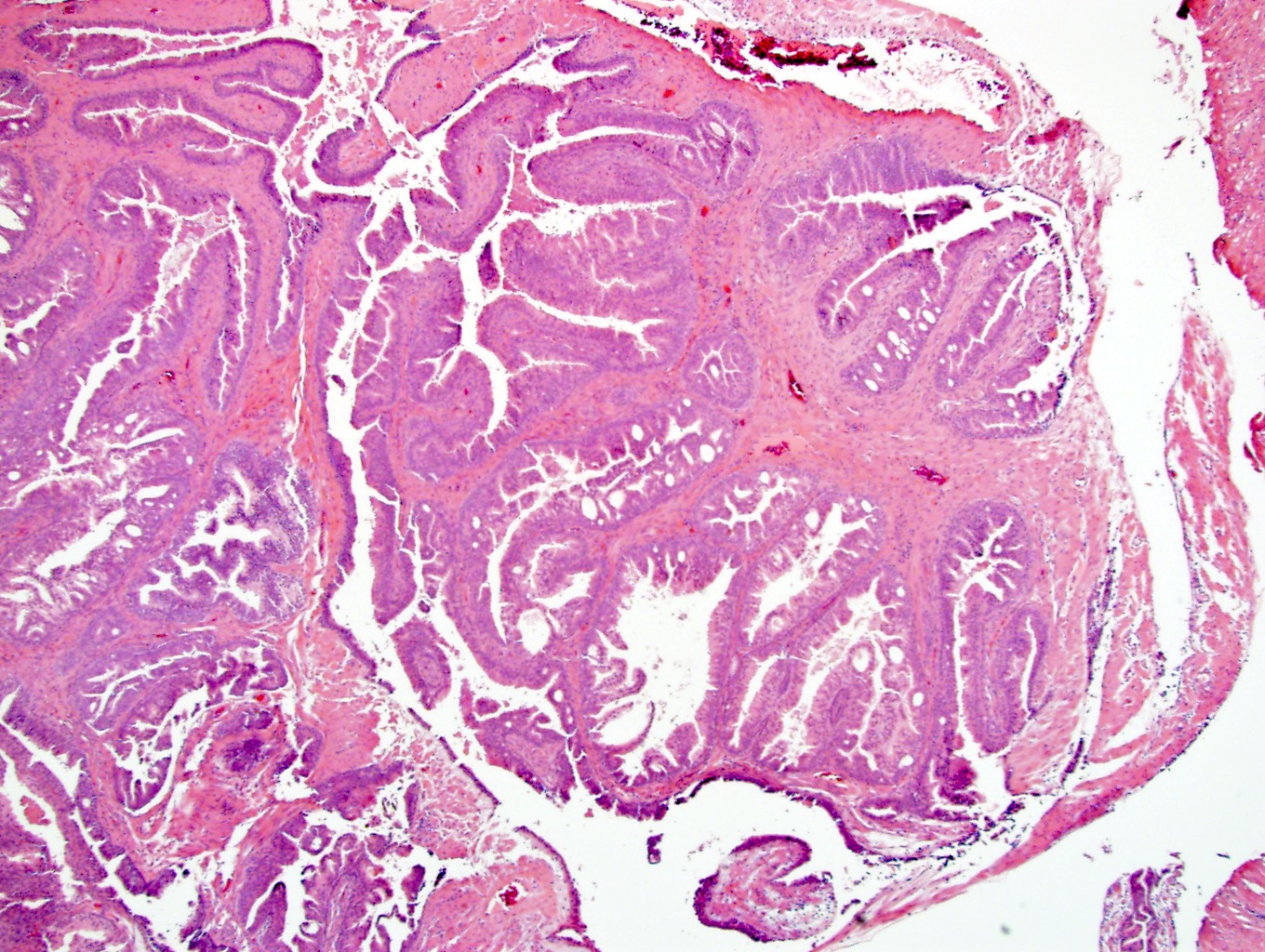
- Greater cellularity, mitotic activity, necrosis and stromal overgrowth than STUMP
- Storiform and infiltrative growth pattern
- Sarcomas are subdivided into low grade and high grade based on mitotic rate, necrosis and degree of atypia (Am J Surg Pathol 2006;30:694)
- There may be either stromal elements with benign glands resembling malignant breast phyllodes tumors or pure stromal elements
Microscopic (histologic) images
Positive stains
- Vimentin (100%), CD34 (100%), progesterone receptor (85%), desmin (50%)
- High Ki67 proliferation index stands in contrast to STUMP (Virchows Arch 2021;478:619)
Negative stains
- Smooth muscle actin (33%) and HHF35 (25%) may be positive
- S100 (100%), ER (usually), STAT6 (Virchows Arch 2021;478:619)
Molecular / cytogenetics description
- Both prostatic stromal sarcoma (all 4 cases) and stromal hyperplasia with atypia (also termed STUMP) (7 of 8 cases) shared chromosomal aberrations by array comparative genomic hybridization (aCGH)
- Most common was loss of chromosome 13 followed by losses of chromosomes 14 or 10 (Mod Pathol 2013;26:1536)
- Additional mutations, such as CHEK2 and KTM2D, favored benign entities, whereas TP53 and RB1 mutations favored malignant (Mod Pathol 2013;26:1536)
- This suggests 2 separate entities; however, either mutations or rearrangements were found via DNA sequencing in 10 of 10 cases of both STUMP and PSS, while only PSS included phyllodes pattern (Mod Pathol 2021;34:1763)
- Also, the specificity of chromosome 13 and 14 has been questioned and whole exome sequencing found a variety of changes in STUMP and PSS, implying these 2 entities are part of the same spectrum (Mod Pathol 2021;34:2082)
Sample pathology report
- Prostate, transurethral resection:
- Prostatic stromal sarcoma (see comment)
- Comment: Cells have marked nuclear atypia, mitotic activity and pleomorphism. Necrosis is present. Tumor is positive for CD34 and has high Ki67 index, confirming the diagnosis.
Differential diagnosis
- Stromal hyperplasia with atypia (also termed STUMP):
- More common than stromal sarcoma
- No marked atypia or necrosis
- Degenerative nuclei
- Leiomyoma:
- Much more common
- Consistently positive for smooth muscle markers
- CD34 is negative
- Synovial sarcoma:
- Ruled out if CD34 is positive
- Gastrointestinal stromal tumor:
- Ruled out if CD117 is negative
Board review style question #1
If only 2 positive markers were chosen to rule in prostatic stromal sarcoma, what would they be?
- Desmin and S100 protein
- Estrogen receptor and CD34
- High Ki67 proliferation index and CD34
- S100 protein and STAT6
- Smooth muscle actin and estrogen receptor
Board review style answer #1
C. High Ki67 proliferation index and CD34. CD34 positivity rules out a smooth muscle tumor (leiomyoma, leiomyosarcoma) or synovial sarcoma, while a high Ki67 proliferation index rules out STUMP, which has a low index. Desmin (A) does not discriminate the sarcoma from STUMP. Estrogen receptor and CD34 (B) are mostly positive in both sarcoma and STUMP but do not distinguish them. S100 protein and STAT6 (D) are both negative in sarcoma and STUMP. STAT6 is specific for solitary fibrous tumor. Smooth muscle actin and estrogen receptor (E) do not discriminate sarcoma from STUMP.
Comment Here
Reference: Prostatic stromal sarcoma
Comment Here
Reference: Prostatic stromal sarcoma